22nd Century Group, Inc. - Annual Report: 2020 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ Annual Report under Section 13 or 15(d) of the Securities
Exchange Act of 1934
For the fiscal year ended December 31, 2020
or
☐ Transitional Report under Section 13 or 15(d) of the
Securities Exchange Act of 1934
Commission File Number: 001-36338
22nd Century Group, Inc.
(Exact name of registrant as specified in its charter)
Nevada | 98-0468420 |
(State or other jurisdiction | (IRS Employer |
of incorporation) | Identification No.) |
8560 Main Street, Suite 4, Williamsville, New York 14221
(Address of principal executive offices)
(716) 270-1523
(Registrant’s telephone number, including area code)
Securities registered under Section 12(b) of the Act:
Title of Each Class |
| Trading Symbol |
| Name of Exchange on Which Registered |
Common Stock, $0.00001 par value |
| XXII | American |
Securities registered under Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act
Yes ◻ No ⌧
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act.
Yes◻ No ⌧
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days.
Yes ⌧ No ◻
Indicate by check mark whether the registrant has submitted electronically every Interactive Date File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files)
Yes ⌧ No ◻
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large Accelerated Filer ◻ | Accelerated Filer ◻ | Non-Accelerated Filer ⌧ | Smaller Reporting Company ☒ |
|
|
|
|
|
|
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No ⌧
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Act). Yes ☐ No ⌧
The aggregate market value of the registrant’s common stock as of June 30, 2020, the last day of the registrant’s most recently completed second fiscal quarter, was $106 million based upon the closing price reported for such date on the NYSE American. On March 9, 2021, the registrant had 149,615,010 shares of common stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2021 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K. Such Proxy Statement will be filed with the Securities and Exchange Commission within 120 days of December 31, 2020.
Table of Contents
2
Cautionary Note Regarding Forward-Looking Statements and Risk Factor Summary
This Annual Report on Form 10-K contains forward-looking statements concerning our business, operations and financial performance and condition as well as our plans, objectives and expectations for our business operations and financial performance and condition that are subject to risks and uncertainties. All statements other than statements of historical fact included in this Annual Report on Form 10-K are forward-looking statements. You can identify these statements by words such as “aim,” “anticipate,” “assume,” “believe,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “potential,” “positioned,” “predict,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends. These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate and our management’s beliefs and assumptions. These statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected, including the following summary of risk factors:
| ● | The coronavirus pandemic (COVID-19) or another pandemic may cause a variety of business disruptions and future business risks. |
| ● | We have had a history of losses, and we may be unable to achieve and sustain profitability. |
| ● | We have had a history of negative cash flow, and our ability to sustain positive cash flow is uncertain. |
| ● | If we fail to obtain FDA and foreign regulatory approvals for authorization to market our VLNC tobacco as a Modified Risk Cigarette, we will be unable to commercialize this potential product in and outside the U.S. |
| ● | Negative press from being in the hemp/cannabis space could have a material adverse effect on our business, financial condition, and results of operations. |
| ● | Any business-related cannabinoid production is dependent on laws pertaining to the hemp/cannabis industry. |
| ● | Our studies and testing of any of our potential products may produce negative or inconclusive results and we may decide, or regulators may require us, to conduct additional studies and/or testing for these potential products or cease our studies and testing. |
| ● | We may acquire or invest in other companies, which may divert our management’s attention, result in additional dilution to our stockholders, and consume resources that are necessary to sustain our business or result in losses. |
| ● | The loss of a significant customer for whom we manufacture tobacco products could have an adverse impact on our results of operation. |
| ● | Our principal competitors in the conventional or modified risk tobacco products market have, and any future competitors may have, greater financial and marketing resources than we do, and they may therefore develop products or other technologies similar or superior to ours, or otherwise compete more successfully than we do. |
| ● | Product liability claims, product recalls, or other claims could cause us to incur losses or damage our reputation. |
| ● | The failure of our information systems to function as intended or their penetration by outside parties with the intent to corrupt them could result in business disruption, litigation and regulatory action, and loss of revenue, assets, or personal or confidential data (cybersecurity). |
| ● | The manufacturing of tobacco products subjects us to significant governmental regulation and the failure to comply with such regulations could have a material adverse effect on our business and subject us to substantial fines or other regulatory actions. |
3
| ● | If regulations by the FDA requiring the reduction of nicotine to minimally or non-addictive levels in all cigarettes sold in the U.S. are delayed or do not become implemented, then the demand for our proprietary Very Low Nicotine Content tobacco may not occur. |
| ● | We may become subject to litigation related to cigarette smoking and/or exposure to environmental tobacco smoke, or ETS, which could severely impair our results of operations and liquidity. |
| ● | The FDA could force the removal of our products from the U.S. market. |
| ● | Certain of our proprietary rights have expired or may expire or may not otherwise adequately protect our intellectual property, products and potential products, and if we cannot obtain adequate protection of our intellectual property, products and potential products, we may not be able to successfully market our products and potential products. |
| ● | The ability to commercialize our potential products will depend on our ability to sell such products without infringing the patent or proprietary rights of third parties. If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and an unfavorable outcome could have a significant adverse effect on our business. |
| ● | Our patent applications may not result in issued patents, which may have a material adverse effect on our ability to prevent others from commercially exploiting products similar to ours. |
| ● | We license certain patent rights from third-party owners. If such owners do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects could be harmed. |
| ● | Our stock price may be highly volatile and could decline in value. |
| ● | We are named defendant in certain litigation matters, including federal securities class action lawsuits and derivative complaints; if we are unable to resolve these matters favorably, then our business, operating results and financial condition may be adversely affected. |
| ● | Future sales of our common stock will result in dilution to our common stockholders. |
| ● | We do not expect to declare any dividends on our common stock in the foreseeable future. |
For the discussion of these risks and uncertainties and others that could cause actual results to differ materially from those contained in our forward-looking statements, please refer to “Risk Factors” in this Annual Report on Form 10-K. The forward-looking statements included in this Annual Report on Form 10-K are made only as of the date hereof. We undertake no obligation to publicly update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
Unless the context otherwise requires, references to the “Company” “we” “us” and “our” refer to 22nd Century Group, Inc., a Nevada corporation, and its direct and indirect subsidiaries.
4
PART I
Item 1.Business.
Background
22nd Century Group, Inc. was incorporated under the laws of the State of Nevada on September 12, 2005 under the name Touchstone Mining Limited. On January 25, 2011, we entered into a reverse merger transaction with 22nd Century Limited, LLC, which we refer to herein as the “merger.” Upon the closing of the merger, 22nd Century Limited, LLC became our wholly-owned subsidiary. After the merger, we succeeded to the business of 22nd Century Limited, LLC as our sole line of business.
22nd Century Limited, LLC was originally formed as a New York limited liability company on February 20, 1998 as 21st Century Limited, LLC and subsequently merged with a newly-formed Delaware limited liability company, 22nd Century Limited, LLC, on November 29, 1999. Since inception, 22nd Century Limited, LLC has sponsored research and subsequently used biotechnology to regulate the nicotine content in tobacco plants.
Overview
22nd Century Group, Inc. is a biotechnology company developing disruptive, plant-based solutions for the life science, consumer product, and pharmaceutical markets. We are a focused on technology that allows us to modulate the level of nicotine and other nicotinic alkaloids in tobacco plants and the levels of cannabinoids and terpenes in hemp/cannabis plants through genetic engineering and modern plant breeding techniques. Our mission in tobacco is to reduce the harm caused by smoking by introducing adult smokers to our proprietary, Very Low Nicotine Content (“VLNC”) tobacco and cigarette products. Our mission in hemp/cannabis is to develop proprietary varieties of hemp with valuable cannabinoid and terpene profiles and other superior agronomic traits, with potential applications in life sciences and consumer products. We have a significant intellectual property portfolio of issued patents and patent applications relating to both tobacco and hemp/cannabis plants.
Tobacco
Our mission in tobacco is to reduce the harm caused by smoking by introducing adult smokers to our proprietary, VLNC tobacco and cigarettes, which contains 95% less nicotine than conventional tobacco and cigarettes. The Food and Drug Administration (“FDA “) publicly announced on July 28, 2017, that tobacco use remains the leading cause of preventable disease and death in the United States. The website for the U.S. Centers for Disease Control and Prevention (“CDC”) states that tobacco use causes more than 480,000 deaths per year and costs the United States economy nearly $300 billion annually in lost productivity and direct heath care costs. The CDC website also states that in 2015, nearly 7 in 10 (68.0%) adult cigarette smokers wanted to stop smoking and more than 5 in 10 (55.4%) adult cigarette smokers had made a quit attempt in the prior year.
We have developed unique and proprietary bright and burley VLNC tobaccos that grow with at least 95% less nicotine than tobacco used in conventional cigarettes. In the year 2011, we developed our SPECTRUM® research cigarettes in collaboration with independent researchers, officials from the FDA, the National Institute on Drug Abuse (“NIDA”), which is part of the National Institutes of Health (“NIH”), the National Cancer Institute (“NCI”), and the Centers for Disease Control and Prevention (“CDC”). Since 2011, we have provided more than 31.6 million variable nicotine research cigarettes for use in numerous independent clinical studies with agencies of the United States federal government. These independent clinical studies are estimated to have been performed at a cost of more than $125 million. The results of these independent clinical studies have been published in peer-reviewed publications (including the New England Journal of Medicine, the Journal of the American Medical Association, and many others). The results of these studies indicate that our VLNC tobaccos have been associated with reductions in smoking, nicotine exposure and nicotine dependence with little to no evidence of compensatory smoking and without serious adverse events. A list of completed and published clinical studies using cigarettes made with our VLNC tobaccos is shown on our website at https://www.xxiicentury.com/vln-clinical-studies/published-clinical-studies-on-very-low-nicotine-content-vlnc-cigarettes. A list of on-going clinical studies using our SPECTRUM® research cigarettes is shown on our website at
5
https://www.xxiicentury.com/vln-clinical-studies/on-going-clinical-studies-on-very-low-nicotine-content-vlnc-cigarettes. We do not incorporate third party studies or the information on our website into this Annual Report on Form 10-K.
The results of these numerous completed studies provide the independent scientific foundation for the public announcement on July 28, 2017 by the FDA that the FDA plans to enact a new rule to require that all combustible cigarettes sold in the United States contain only minimally or non-addictive levels of nicotine. On March 19, 2018, the FDA publicly announced its Advance Notice of Proposed Rulemaking (“ANPRM”) to solicit public comments on the FDA’s plan to enact a new nicotine reduction rule. On July 16, 2018, we publicly submitted to the FDA our formal written response to the ANPRM in which we described how (i) the FDA’s proposed new rule is supported by rigorous independent, published science, (ii) the FDA’s stated goal to render all cigarettes minimally or non-addictive is immediately feasible as evidenced by our production and delivery of millions of VLNC research cigarettes since the year 2011, and (iii) the FDA’s proposed new rule is exceedingly practical and urgently needed in the interests of public health. After we obtain all necessary regulatory approvals, which includes obtaining a Modified Risk designation, we plan to offer our proprietary VLNC cigarettes for domestic sale under the brand name of VLN®. Following approval, we also plan to offer VLN® for international sale and for licensing by third parties. Additional information regarding our regulatory activities with the FDA is described below.
FDAs proposed Mandate to Require Minimally or Non-Addictive Levels of Nicotine in all Cigarettes in the United States.
The Family Smoking Prevention and Tobacco Control Act of 2009 (“Tobacco Control Act”) granted the FDA authority over the regulation of all tobacco products in the United States. While the Tobacco Control Act prohibits the FDA from banning cigarettes outright, it allows the FDA to require the reduction of nicotine in tobacco and cigarette smoke. The Tobacco Control Act establishes procedures for the FDA to regulate the labeling and marketing of Modified Risk Tobacco Products, which includes cigarettes marketed to (i) reduce harm or the risk of tobacco-related disease or (ii) reduce or eliminate exposure to a substance (“Modified Risk Cigarettes”). The Tobacco Control Act required the FDA to issue specific regulations and/or guidance regarding applications submitted to the FDA for the authorization to label and market Modified Risk Tobacco Products. On March 30, 2012, the FDA issued Modified Risk Tobacco Product Applications Draft Guidance. We believe that our VLNC tobacco cigarettes will qualify as Modified Risk Cigarettes and have applied for an MRTP designation. The status of this application is discussed below.
In a June 16, 2010 press release, Dr. David Kessler, a former FDA Commissioner, recommended that “the FDA should quickly move to reduce nicotine levels in cigarettes to non-addictive levels. If we reduce the level of the stimulus, we reduce the craving. It is the ultimate harm reduction strategy.” Shortly thereafter in a Washington Post newspaper article, Dr. Kessler said that the amount of nicotine in a cigarette should drop from about 10 milligrams to less than 1 milligram.
In 2015, the World Health Organization (“WHO”) Study Group on Tobacco Product Regulation published an advisory note on a global nicotine reduction strategy of limiting the sale of cigarettes to brands with a nicotine content that is not sufficient to lead to the development and/or maintenance of addiction. The WHO study stated that no specific amount of nicotine has yet been identified by the WHO as the absolute threshold for addiction; however, the WHO report stated that it is likely to be equal to or possibly less than 0.4 mg/g of dry cigarette tobacco filler. The WHO report cites 22nd Century’s proprietary SPECTRUM® research cigarettes as meeting such a low level of nicotine of 0.4 mg/g of cigarette tobacco filler. The WHO report concluded that the evidence indicates that setting a maximum allowable nicotine content for all cigarettes could (i) reduce the acquisition of smoking and progression to addiction, (ii) reduce the prevalence of smoking in a proportion of addicted smokers as a result of behavioral extinction, (iii) increase the rate of quitting and reduce the number of smokers who relapse, and (iv) increase the development, availability, and use of alternative forms of nicotine, e.g. smokeless tobacco products, nicotine aerosol products, and medicinal nicotine, which have potential adverse health effects, including maintenance of addiction, but less than those of combusted products or conventional cigarettes. The WHO report stated that population benefits will result from decreased use of combusted tobacco by current cigarette smokers and from the prevention of addiction of non-smokers to cigarettes, especially among young people.
On July 28, 2017, then FDA Commissioner Scott Gottlieb, M.D., announced the FDA’s plan to exercise its authority under the Tobacco Control Act to require that all combustible cigarettes sold in the United States contain only
6
minimally or non-addictive levels of nicotine. In that public announcement, FDA Commissioner Gottlieb stated that (i) the overwhelming amount of death and disease attributable to tobacco is caused by addiction to cigarettes – the only legal consumer product that, when used as intended, will kill half of all long-term users, (ii) unless this course is changed, 5.6 million young people alive today will die prematurely later in life from tobacco use, (iii) envisioning a world where cigarettes would no longer create or sustain addiction, and where adults who still need or want nicotine could get it from alternative and less harmful sources, needs to be the cornerstone of the FDA’s efforts, and (iv) tobacco use remains the leading cause of preventable disease and death in the United States, causing more than 480,000 deaths per year and direct health care and lost productivity costs totaling nearly $300 billion each year.
On August 16, 2017, The New England Journal of Medicine published an article by FDA Commissioner Scott Gottlieb, M.D. and Mitchell Zeller, J.D., the Director of the FDA’s Center for Tobacco Products (“FDA/CTP”), entitled “A Nicotine-Focused Framework of Public Health.” In this article, FDA Commissioner Gottlieb and FDA/CTP Director Zeller stated that the Tobacco Control Act gives the FDA a regulatory tool called a tobacco “product standard” that can be used to alter the addictiveness of combustible cigarettes, and that such standards may set requirements related to an ingredient or constituent in a tobacco product, or related to any other aspect of product composition, construction, or other property, and that the establishment of the right product standard could alter the addictiveness of combustible cigarettes by setting maximum nicotine levels in such products. The article further stated that Section 907 of the Food, Drug, and Cosmetic Act authorizes the FDA to establish tobacco product standards that the FDA has determined to be appropriate for the protection of the public health, with the statute specifically noting that such a standard may address nicotine yields, among other characteristics. Although the statute prohibits the FDA from requiring the reduction of nicotine yields of a tobacco product to zero, the FDA stated in this article that the FDA has clear authority to otherwise reduce nicotine levels. The FDA concluded in this article that a nicotine-limiting standard could make cigarettes minimally addictive or non-addictive, helping current users of combustible cigarettes to quit and allowing most future users to avoid becoming addicted and proceeding to regular use, and that disrupting that progression – from experimentation to regular use to tobacco-related disease and even death – could save millions of American lives. In this article, the FDA also stated that the FDA will consider peer-reviewed, scientific studies in proposing a maximum nicotine level, but that rigorous studies of Very Low Nicotine Content cigarettes have evaluated the potential effects of various nicotine levels on smoking behaviors and biomarkers, and findings from such studies could inform decision-making on a possible maximum nicotine level in tobacco filler. The FDA stated that, as in all matters of public health policy, the FDA will be led by the science in this important area.
On December 5, 2018, the Company submitted to the FDA a new Premarket Tobacco Application (“PMTA”) and on December 27, 2018 we submitted to the FDA a new Modified Risk Tobacco Product application (“MRTPA”), in each case for our VLNC tobacco cigarettes. Through our applications, we requested a reduced exposure marketing authorization from the FDA to market these products as Modified Risk Cigarettes with product labeling that includes the proposed brand name of VLN® and states that VLN® has 95% less nicotine than conventional cigarettes.
On December 17, 2019, the FDA publicly announced that the FDA had authorized the Company to market in the U.S. our VLNC tobacco cigarettes under the product names of Moonlight® and Moonlight Menthol® that were the subject of our PMTA. Our related MRTPA remains pending with the FDA. Our Moonlight® and Moonlight Menthol® cigarettes are made with our proprietary VLNC tobacco and, as a result, contain very low levels of nicotine. Our Moonlight® and VLN® cigarettes are identical products except in name and were closely modeled after our VLNC SPECTRUM® research cigarettes. Our PMTA references more than 50 independent studies conducted using our proprietary SPECTRUM® research cigarettes. In its public announcement relating to our PMTA, the FDA stated that following a rigorous science-based review of our PMTA, the FDA had determined that authorizing these reduced nicotine products for sale in the U.S. is appropriate for the protection of the public health because of, among several key considerations, the potential to reduce nicotine dependence in addicted adult smokers, who may also benefit from decreasing nicotine exposure and cigarette consumption. The FDA further stated that the FDA had determined that non-smokers, including youth, are also unlikely to start using these products, and those who experiment are less likely to become addicted than people who experiment with conventional cigarettes.
On February 14, 2020, the FDA’s Tobacco Products Scientific Advisory Committee ("TPSAC") conducted its public hearing regarding our MRTPA for our VLNC cigarettes. This meeting was the first time that TPSAC considered an MRTP application for a modified exposure claim and also TPSAC’s first discussion of an application for a combustible tobacco product. We presented data in support of our product applications and the FDA also presented
7
perspectives on the applications and posed questions for the committee members to discuss. Recordings of the proceedings meeting materials, including briefing materials for the committee provided by the Company and FDA, and presentation given by the different stakeholders are publicly available for review via FDA’s website. The public comment period on the applications closed on May 18, 2020. The FDA continues to conduct a comprehensive evaluation of our applications; it is management’s belief, based on the process that FDA follows for MRTPAs, that we are in the final stages of the review and decision making process.
In summary, since 2011, the FDA, NIDA and other federal government agencies have invested an estimated $125 million in independent clinical studies utilizing 22nd Century’s proprietary tobaccos, with such studies being conducted by scientists at many different and well-known clinical study centers. During that same time, we have provided more than 31.6 million proprietary SPECTRUM® research cigarettes for use in such independent clinical studies. The results of these studies have been published in peer-reviewed articles and reflect the independent scientific support for the planned mandate by the FDA that all combustible cigarettes sold in the United States contain only minimally or non-addictive levels of nicotine.
We believe that our VLNC tobacco technology and our production and delivery of more than 31.6 million proprietary research cigarettes since 2011 reflects that the FDA’s plan to dramatically reduce nicotine in cigarettes is technically achievable. Cigarettes made with our proprietary VLNC tobacco have been the subject of numerous completed and on-going independent clinical studies. Accordingly, we are investigating the potential use of our VLNC tobacco in our own products that will be intended to comply with the new FDA regulations, as well as investigating the potential license of the use of our VLNC tobacco by third-parties. In the United States, we are focused on working with the FDA on its nicotine reduction mandate and on our Modified Risk Tobacco Product application (“MRTPA”). Outside the United States, we will focus on working with WHO-member countries that desire to utilize our proprietary VLNC tobacco to implement the WHO recommendation of limiting the sale of cigarettes to brands with a nicotine content that is not sufficient to lead to development and/or maintenance of addiction. Starting within 90-days of authorization of our MRTPA by the FDA, we are prepared to launch VLN® cigarettes in select markets. Our discussions with key distributors and retail chains have yielded multiple opportunities for initial sales and expansion. Our sales and marketing plans are well-developed and ready for implementation. Given that 60% of participants in our perception studies have indicated an intent to use VLN® cigarettes, we anticipate that there will be a robust market for VLN® cigarettes. We also believe that obtaining FDA designation of our VLN® cigarettes as Modified Risk Cigarettes will create opportunities for us to license our proprietary technology and/or our tobaccos to larger competitors.
SPECTRUM® Cigarettes and Government Research
NIDA, which is a part of NIH, provides the scientific community with controlled and uncontrolled research chemicals and drug compounds through its Drug Supply Program. In 2010, NIDA included an option to develop and produce research cigarettes with various levels of nicotine (from very low to conventional) in its request for proposals for a five-year contract for Preparation and Distribution of Research and Drug Products. We agreed, as a subcontractor to RTI International (“RTI”), to supply cigarettes with different nicotine contents (from very low to conventional) to NIDA. In August 2010, we met with officials from NIDA, FDA, RTI, CDC and the National Cancer Institute (“NCI”) to finalize certain aspects of the design of these research cigarettes. These cigarettes for government research produced by us under the mark SPECTRUM® have been, and continue to be, distributed by RTI for NIDA to independent researchers for scientific clinical studies. The SPECTRUM® research cigarette contract was renewed in 2019 for an additional five years.
Since 2011, the FDA, NIDA and other federal government agencies are estimated to have invested more than $125 million in independent clinical studies utilizing our proprietary tobaccos, with such studies being conducted at many well-known locations, including the Mayo Clinic, the MD Anderson Cancer Center at the University of Texas, the Johns Hopkins University, Duke University, the University of Pittsburgh, the University of Minnesota, the University of Vermont, the University of California, and others. Since 2011, we have provided more than 31.6 million SPECTRUM® research cigarettes for use in these independent clinical studies. The SPECTRUM® product line consists of a series of 24 cigarette styles (11 regular and 13 menthol versions) that have 8 different levels of nicotine – from very low to conventional. A list of the completed, independent clinical studies on our proprietary tobaccos can be found on our website as discussed above.
8
We expect that information from such government agency sponsored research studies will assist us, along with our own funded studies, in obtaining the necessary FDA authorizations to market our VLNC tobacco cigarettes under our proposed product name of VLN® as a Modified Risk Cigarette.
Hemp/Cannabis
On December 20, 2018, the Agricultural Improvement Act of 2018, which is also known as the “2018 Farm Bill,” was enacted and, among other things, further legalized hemp under U.S. federal law, but with compliance still being required with all applicable state hemp laws. The 2018 Farm Bill includes certain benefits for the hemp industry in the United States, including: (i) the extension of the protections for hemp research and researchers and the conditions in which hemp research can be done, (ii) the protection of hemp farmers and hemp production under federal crop insurance programs, (iii) the permitting of the cultivation, interstate transportation and sale of hemp and hemp products in the U.S. in compliance with all other applicable federal and state laws, and (iv) the removal of hemp and hemp derived products from Schedule 1 of the Controlled Substances Act (“CSA”).
As of January 1, 2021, (i) federal law and the laws of 47 states in the United States and the District of Columbia have legalized hemp, (ii) 36 states in the United States, the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands have enacted laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis/marijuana and consumer use of cannabis/marijuana in connection with medical treatment, and (iii) 15 states in the United States, the District of Columbia, Guam, and the Northern Mariana Islands have legalized cannabis/marijuana for adult recreational use. Many other states are considering similar legislation. Conversely, under the federal CSA, the policies and regulations of the federal government and its agencies are that cannabis/marijuana has no medical benefit and a range of activities are prohibited, including cultivation, possession, personal use and interstate distribution of cannabis/marijuana.
In hemp, we are developing proprietary hemp varieties with increased levels of certain cannabinoids and other desirable agronomic traits with the goal of generating new and valuable intellectual property and plant lines. Our activities in the United States involve only work with legal hemp in full compliance with U.S. federal and state laws. The hemp and the marijuana plants are both part of the same cannabis genus, except that hemp does not have more than 0.3% dry weight content of delta-9-tetrahydrocannabinol (“THC”). While 2018 Farm Bill legalized hemp and cannabinoids extracted from hemp in the U.S., such extracts remain subject to state laws and regulation by other U.S. federal agencies such as the FDA, U.S. Drug Enforcement Administration (“DEA”), and the U.S. Department of Agriculture (“USDA”). The same plant, with a higher THC content is marijuana, which is legal under certain state laws, but which is currently not legal under U.S. federal law. The similarities between these plants can cause confusion. To reflect this difference in law, sometimes we refer to legal hemp and the legal hemp industry as hemp/cannabis to distinguish this as being separate and apart from marijuana/cannabis which is not legal under U.S. federal law. Our activities with legal hemp have sometimes been incorrectly perceived as us being involved in federally illegal marijuana/cannabis. This is not the case. In the United States, we work only with legal hemp in full compliance with federal and state laws.
We have a license in the State of New York to research and grow hemp in response to the numerous public announcements by New York Governor Andrew Cuomo that New York State intends to become a leading grower and producer of hemp and hemp-derived products. In Canada, we previously conducted sponsored research on the hemp plant with Anandia in Vancouver, British Columbia, in full compliance with Canadian regulations.
In Europe and the United States, we are currently working with KeyGene NV (“Keygene”), a global leader in plant research involving high-value genetic traits and increased crop yields. In our exclusive, worldwide collaboration with Keygene, we are focused on developing hemp/cannabis plants with exceptional cannabinoid profiles and other superior agronomic traits for medical, therapeutic and agricultural uses, among many other applications. We also have an investment in Panacea Life Sciences (“Panacea” or “PLS”) described below.
We continue to review potential candidate companies in the hemp/cannabis field for strategic collaborations, affiliations, joint ventures, investments, and/or acquisitions.
9
Investment in Panacea Life Sciences
On December 3, 2019, we closed on an investment in Panacea for $13.2 million for a 15.8% ownership interest. Panacea is a vertically integrated developer, producer and seller of legal, hemp-derived, CBD products, with extraction, distillation, testing and manufacturing operations located in a 51,000 square foot facility in Golden, Colorado. Our investment consists of a $7 million convertible note receivable, 3,733,334 shares of Series B preferred stock, and a warrant to purchase additional shares of Series B preferred stock, which upon full exercise will provide us with a controlling equity position in Panacea. This investment was made at that time as the company was seeking to diversify its product line to augment its current and future sales of tobacco products with sales of branded consumer CBD products—produced and sold by Panacea.
We recently entered into a non-binding agreement with Panacea to potentially restructure our investment and business relationship. The non-binding agreement with Panacea generally provides for (i) the transfer of $7.2 million in operational assets, including an agricultural facility and various extraction and distillation equipment, from Panacea to us in exchange for the cancellation of the $7 million convertible note receivable plus accrued interest; (ii) an amendment of transaction documents to remove any future investment rights and obligations of the Company in Panacea, (iii) cancellation of the warrant to purchase additional Series B preferred stock; and (iv) various other amendments to Panacea’s charter to amend various investors rights therein. We believe this non-binding agreement to restructure our investment with Panacea will allow Panacea to optimize its focus on creating high-quality CBD and other hemp-based cannabinoid consumer products and provide us with an agricultural asset that will allow us to conduct further commercial research and development on various lines of hemp/cannabis genetics in our intellectual property portfolio. However, the agreement to restructure our investment with Panacea is preliminary, non-binding, subject to change and may not occur.
Research & Development (R&D) & Intellectual Property (IP)
Tobacco R&D
Since our inception, the majority of our research and development (“R&D”) efforts have been outsourced to highly qualified groups in their respective fields. Since 1998, we have had multiple R&D agreements with North Carolina State University (“NCSU”) and others resulting in exclusive worldwide licenses to various patented technologies. We have utilized the same model employed by many public-sector research organizations, which entails obtaining an exclusive option or license agreement to any invention arising out of funded research. In all cases, we fund and control all patent filings as the exclusive licensee. This model of contracting with public-sector researchers has enabled us to control R&D costs while achieving our desired results, including obtaining exclusive intellectual property rights relating to our outsourced R&D.
In August 2016, we opened our own laboratory on the Buffalo Niagara Medical Campus in Buffalo, New York where we are conducting our own proprietary research and development activities in tobacco and hemp. On October 30, 2017, we obtained a New York State hemp research and grower license to support our expanding hemp activities in New York.
On June 22, 2018, the Company entered into an amendment to its existing license agreement with NCSU under which the Company exclusively licensed several bright and burley tobacco plant lines with Very Low Nicotine Content that are not genetically modified (non-GMO) plants. The amendment provides for the Company to pay NCSU a total exclusive license fee of $1.2 million—refer to Note 8 to our consolidated financial statements for additional information. The Company will also pay running royalties to NCSU based on a portion of the net sales revenue received by the Company from sales of products that contain any portions of the plant materials that have been received by the Company from NCSU.
On October 22, 2018, the Company entered into a license agreement with the University of Kentucky (“UK”) to license on a non-exclusive basis a next-generation very low nicotine content burley tobacco plant lines that are not genetically modified (non-GMO) plants. The UK license agreement provides for the Company to pay UK a total license fee of $1.2 million—refer to Note 8 to our consolidated financial statements for additional information. The Company
10
will also pay running royalties to UK based on a portion of the net sales revenue received by the Company from sales of products that contain any portions of the plant materials that have been received by the Company from UK.
After several field trial evaluations throughout the United States, the Company has produced new non-GMO burley and bright tobacco varieties with 95% reduced nicotine and enhanced flavor notes. These new varieties include technology previously licensed from NCSU, which is protected with a patent portfolio.
Our research also showed a successful introduction of the non-GMO VLNC technology on several oriental lines and we expect to have 95% reduced nicotine oriental tobacco in the future.
We are currently developing new versions of our VLNC cigarettes utilizing these non-GMO tobacco lines for future commercialization in the U.S. and globally.
Tobacco IP
Our intellectual property enables us to alter the level of nicotine and other nicotinic alkaloids in tobacco plants through genetic engineering and modern plant breeding. The basic techniques include, but are not limited to, those that are used in the production of genetically modified (“GM”) and gene-edited varieties of other crops, which are also known as “biotech crops.”
We have extensive patent protection and exclusive rights covering tobacco plants with altered nicotine content produced from modifying expression of certain genes in the tobacco plant. Our patent families related to nicotine biosynthesis are expected to expire between 2021 and 2036, with certain extensions of terms in the U.S. applications resulting from patent term adjustments at the U.S. Patent and Trademark Office. (A “patent family” is a set of patent applications and patents, filed in various countries, that relate back to at least one common earlier application.). Our Vector 21-41 VLNC tobacco plants with the QPT modification are also protected by plant variety protection (“PVP”) through 2023, which further restricts third-parties from using such plants.
The creation and production of unique tobacco plants with VLNC levels, with sufficiently high germination rates and sufficiently large plant yields at harvest, among many other desirable qualities, are necessary for the plants to be sufficiently reliable to be planted at commercial scale. The expiration of a portion of the QPT patent family in 2018 provides third parties with the freedom to target the QPT gene in the tobacco plant, but such targeting of the QPT gene alone does not mean that a third party will be successful in creating a tobacco plant with altered levels of nicotine. The freedom to target the QPT gene means that a third party may conduct scientific experiments to try to discover how to alter or affect the QPT gene in ways that may or may not result in a change in nicotine levels in the tobacco plant. If a third party is able subsequently to learn, over time, how to utilize the QPT gene to alter nicotine levels in the tobacco plant, then such third party would still need to develop and create a unique tobacco plant with very low levels of nicotine (not just a “low nicotine” or “reduced nicotine” plant), which would involve, among many other things, multiple plantings over multiple generations of the plants to try to create stable and reliable VLNC plants, with no assurance that any third party could be successful in such efforts.
We believe that targeting of the QPT gene alone will not result in a VLNC tobacco plant and, hence, that other genes will have to be targeted in the tobacco plant, likely including genes and other intellectual property for which we have continuing patent protection that would need to be used.
We also have exclusive plant variety rights in the United States and may other countries. Plant variety protection certificates are issued in the United States by the U.S. Department of Agriculture (“PVP”). A PVP certificate prevents anyone other than the owner/licensee from planting, propagating, selling, importing or exporting a plant variety for twenty (20) years in the U.S. and, generally, for twenty (20) years in other member countries of the International Union for the Protection of New Varieties of Plants, known as UPOV, an international treaty concerning plant breeders’ rights. There are currently more than 70 countries that are members of UPOV. Our current VLNC tobaccos are protected by our patent portfolio and our Vector 21-41 VLNC tobacco is additionally protected by PVP.
In addition to our patents, patent applications, and PVP certificates, we own various registered trademarks in the United States and around the world.
11
Hemp/Cannabis R&D and IP
Our intellectual property enables us to alter the level the levels of cannabinoids in cannabis plants through genetic engineering and modern plant breeding. The basic techniques include, but are not limited to, those that are used in the production of genetically modified (“GM”) and gene-edited varieties of other crops, which are also known as “biotech crops.” We have developed various types of cannabis plants with agronomically desirable traits for commercial uses and/or unique cannabinoid/terpenoid levels. We believe that we have many types of superior and unique cannabis plant varieties in development, including (i) plants with low to no amounts of THC and other desirable agronomic traits for the legal hemp industry and (ii) plants with high levels of cannabinoids (including THC, CBD and many minor cannabinoids) for use in legal cannabinoid markets.
In September 2014, we entered into a Sublicense Agreement with Anandia (the “Anandia Sublicense”). Under the terms of the Anandia Sublicense, we were granted an exclusive sublicense in the United States and a co-exclusive sublicense in the remainder of the world, excluding Canada, to four U.S. patents and 26 patent applications relating to genes in the cannabis plant that are required for the production of cannabinoids in the cannabis plant or any microorganism, including yeast or bacteria. Three of these patents are essential for all the cannabinoids’ core biosynthesis and one is specific for CBC and derivatives. The Anandia Sublicense continues through the life of the last-to-expire patent, which is expected to be in 2035.
In December 2016, we entered into a sponsored research agreement with the University of Virginia (“UVA”) and an exclusive license agreement with the University of Virginia Patent Foundation d/b/a University of Virginia Licensing & Ventures Group (“UVA LVG”) pursuant to which we invested approximately $1 million over a three-year period with UVA to work on the creation of unique industrial hemp plants with guaranteed levels of THC below the legal limits and to optimize other desirable hemp plant characteristics to improve the plant’s suitability for growing in Virginia and other legacy tobacco regions in the United States.
On October 19, 2017, we announced that UVA had completed its first harvest of our hemp plants and identified several promising hemp varieties that could form the foundation for commercial hemp production throughout the legacy tobacco regions of the United States. The 22nd Century-UVA hemp field trials used multiple varieties of hemp. In 2018 and 2019, we continued to use our proprietary hemp plants for plantings with UVA in Virginia. UVA and 22nd Century conducted all activities in this scientific collaboration within the parameters of state and federal licenses and permits held by UVA for such work. The agreements with UVA and UVA LVG grant us exclusive rights to commercialize all results of the collaboration in consideration of royalty payments by our Company to UVA LVG. This project with UVA completed in December 2019 and all seeds and plants were transferred to Keygene for further research and development.
Through our partnership with KeyGene, we have completed a deep analysis of several hemp/cannabis lines, established and expanded a proprietary cannabis genomic database, began the sequencing and development of high-quality de novo assemblies of several hemp/cannabis plant lines, and developed novel laboratory analysis techniques. These activities will facilitate our on-going hemp/cannabis research efforts focused on developing hemp/cannabis plants with exceptional cannabinoid profiles and other superior agronomic traits for medical, therapeutic and agricultural uses. Towards this end, we have identified several lines with superior cannabinoids and terpenoids profiles using standard genomics and molecular breeding technologies. Also, we have developed metabolomics methods, male-female flower induction, and rapid cycle breeding.
On February 10, 2021, we announced that we have developed and launched a new, cutting-edge technology platform that will enable us and our strategic partners to quickly identify and incorporate commercially valuable traits of hemp/cannabis plants to create new, stable hemp/cannabis lines. The platform incorporates a suite of proprietary molecular tools and a large library of genomic markers and gene-trait correlations. The platform was developed in collaboration with researchers at KeyGene, a global leader in plant research involving high-value genetic traits and increased crop yields. Using this new breeding technology, we have already characterized millions of high-value single nucleotide polymorphisms (SNPs). SNPs are molecular markers or guideposts within a plant’s genome that indicate important variations in Deoxyribonucleic acid (DNA) sequences. Targeting these newly identified SNPs, we were able to locate and isolate specific sections of genetic code from genome assemblies present in our state-of-the-art hemp/cannabis bioinformatics database.
12
Our bioinformatics database continues to grow and already contains hundreds of hemp/cannabis genomes and thousands expression datapoints across a wide array of hemp/cannabis varieties and phenotypes. The ability to identify specific genetic variations allows researchers to isolate high-value traits, like increased CBD or tetrahydrocannabinol (THC) production, and then introduce those traits in new plant lines using modern plant breeding techniques, including trait tracking using molecular marker profiles and proprietary accelerated breeding.
Tobacco Master Settlement Agreement
In September 2013, we entered into a Membership Interest Purchase Agreement (the “NASCO Acquisition”) to purchase all of the issued and outstanding membership interests of NASCO, a federally licensed tobacco product manufacturer and subsequent participating manufacturer under the Master Settlement Agreement (“MSA”). The MSA is an accord reached in November 1998 between the State Attorneys General of 46 states, five U.S. territories, the District of Columbia and the five largest tobacco companies in the United States concerning the advertising, marketing and promotion of tobacco products. The MSA also set standards for, and imposes restrictions on, the sale and marketing of cigarettes by participating cigarette manufacturers. On August 29, 2014, we entered into an Amended Adherence Agreement with the 46 Settling States under the MSA pursuant to which the Company was approved to acquire NASCO and become a subsequent participating manufacturer under the MSA. On that same date, we closed the NASCO Acquisition and became a subsequent participating manufacturer under the MSA. NASCO has since been our wholly-owned subsidiary.
Manufacturing
We lease a cigarette manufacturing facility and warehouse located in Mocksville, North Carolina. In 2013, we purchased certain (i) cigarette manufacturing equipment, and (ii) equipment parts, factory items, office furniture and fixtures, vehicles and computers from the bankruptcy estate of PTM Technologies, Inc. for approximately $3.2 million.
The facility was primarily in a pre-manufacturing stage during 2014 as we sought approval during that time for us to become a subsequent participating manufacturer under the MSA. Since August 29, 2014, the Company has been a subsequent participating manufacture under the MSA. Since 2015, we have manufactured and sold our SPECTRUM® government research cigarettes, as well as third-party MSA-compliant cigarette and filtered cigar brands, at our factory in North Carolina.
Our strategic acquisition of our factory has allowed us to become vertically integrated so that we can control production priorities/timing and maintain the required high quality of our products, including our SPECTRUM® research cigarettes and our PMTA-designation VLNC cigarettes. In the future, our factory will also allow us to produce our own VLNC cigarette brands in the event they comply with the FDA mandate for reduced nicotine in cigarettes and if/when the FDA authorizes our MRTPA submissions under our proposed product name of VLN®.
Sources of Raw Materials
We obtain our reduced nicotine tobacco leaf from farmers in multiple states in the United States who are under direct contracts with us. These contracts prohibit the transfer of our proprietary tobaccos, seeds and plant materials to other parties. We purchase other tobacco through third parties. In anticipation of the FDA’s authorization of our MRTPA for our VLN® cigarettes, and in the event the FDA mandates that all combustible cigarettes contain only minimally or non-addictive levels of nicotine, we have increased the amount of tobacco leaf we obtain directly from growers under contract. In 2021, we plan to increase our growing quantities of VLNC tobacco to supply the potential launch and expansion of VLN® cigarette sales. Our North Carolina-based manufacturing facility is prepared to manufacture quantities of VLN® cigarettes to supply the anticipated launch and roll-out of VLN®.
Government Regulation
The development, testing, manufacturing, and marketing of our potential products are subject to extensive regulation by governmental authorities in the United States and throughout the world.
13
Tobacco
The Tobacco Control Act, which became law in June 2009, granted the FDA authority over the regulation of all tobacco products in the United States. While the Tobacco Control Act prohibits the FDA from banning cigarettes outright, or mandating that nicotine levels be reduced to zero, it does allow the FDA to require the reduction of nicotine or other compounds in tobacco and cigarette smoke. The FDA has authority to restrict marketing and advertising, impose regulations on packaging, mandate warnings and disclosure of flavors or other ingredients, prohibit the sale of tobacco products with certain flavors or other characteristics, limit or prohibit the sale of tobacco products by certain retail establishments and the sale of tobacco products in certain packaging sizes, and seek to hold retailers and distributors responsible for the adverse health effects associated with both smoking and exposure to environmental tobacco smoke. In 2009, the Tobacco Control Act also banned all sales in the United States of cigarettes with flavored tobacco (other than menthol). As of June 2010, all cigarette companies were required to cease use of the terms “low tar,” “light” and “ultra light” in describing cigarettes sold in the United States.
Manufacturers of tobacco products must comply with FDA regulations which require, among other things, compliance with the FDA’s evolving regulations on Current Good Manufacturing Practices (“cGMP(s)”), which are enforced by the FDA through its facilities inspection program. The manufacture of products is subject to strict quality control, testing and record keeping requirements, and continuing obligations regarding the submission of safety reports and other post-market information.
We expect significant regulatory developments will take place over the next few years in many markets, driven principally by the World Health Organization’s Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging cessation.
Hemp/Cannabis
On December 20, 2018, the Agricultural Improvement Act of 2018, which is also known as the “2018 Farm Bill,” was enacted and legalized hemp and hemp products under U.S. federal law, but with compliance still being required with all applicable state hemp laws and all regulations developed by the USDA. In addition, the FDA is regulating products derived from hemp, including CBD, for compliance under the Federal Food, Drug and Cosmetic Act and has issued several warning letters to firms marketing CBD products to treat disease or for other therapeutic uses. Under the Federal Food, Drug and Cosmetic Act, any product intended to affect the structure or function of the body of humans or animals is considered a drug that must receive premarket approval by the FDA through its new drug application process.
As of January 1, 2021, (i) federal law and the laws of 47 states in the United States and the District of Columbia have legalized hemp, (ii) 36 states in the United States, the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands have enacted laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis/marijuana and consumer use of cannabis/marijuana in connection with medical treatment, and (iii) 15 states in the United States, the District of Columbia, Guam, and the Northern Mariana Islands have legalized cannabis/marijuana for adult recreational use. Many other states are considering similar legislation. Conversely, under the federal Controlled Substance Act (the “CSA”), the policies and regulations of the federal government and its agencies are that marijuana has no medical benefit and a range of activities are prohibited, including cultivation, possession, personal use, and interstate distribution of marijuana. In the event the U.S. Department of Justice begins strict enforcement of the CSA in states that have laws legalizing medical and/or adult recreational marijuana, there may be a direct and adverse impact to any future business or prospects that we may have in the marijuana business. Even in those jurisdictions in which the manufacture and use of medical marijuana has been legalized at the state level, the possession, use, and cultivation of marijuana all remain violations of federal law that are punishable by imprisonment and substantial fines. Moreover, individuals and entities may violate federal law if they intentionally aid and abet another in violating these federal controlled substance laws or conspire with another to violate them.
We currently conduct sponsored research on hemp in Maryland and the Netherlands with third parties that possess all necessary permits and licenses to engage legally in such activities. We have conducted hemp research in Virginia, Oregon, and Canada with third-parties possessing all necessary permits and licenses to engage legally in such
14
activities. In order to carry out research in other countries, similar licenses are required to be issued by the relevant authority in each country. We also conduct hemp research in our laboratories in New York under a license granted to us by the State of New York.
Environmental Regulations
We are subject to a variety of federal, state and local environmental laws and regulations. We have developed specific programs across our business units for ensuring high standards of environmental compliance, including, standard operating practices and procedures at our manufacturing facility as well at our research and development centers. We believe that our manufacturing facility complies with all federal, state, and local environmental regulations, including the Clean Air Act, the Clean Water Act, and the Resource Conservation and Recovery Act.
In addition, any new products introduced by us are subject to a comprehensive environmental assessment by an independent third-party expert, including an assessment of how such products may create environmental risks. For our PMTA product, the FDA prepared a programmatic environmental assessment (PEA), based on our submitted data in accordance with the Council on Environmental Quality's regulations (40 CFR 1500-1508) implementing the National Environmental Policy Act (NEPA) and FDA’s NEPA regulations (21 CFR 25.40). The PEA concluded that the marketing orders would have no significant impact and that environmental impact statements would not be required.
Competition
We are not currently aware of any competition to our VLNC tobaccos inside or outside of the United States. It is possible that our VLNC tobacco cigarettes may compete with FDA-approved smoking cessation aids. In the market for FDA-approved smoking cessation aids, our principal competitors would include Pfizer Inc., GlaxoSmithKline plc, Perrigo Company plc, Novartis International AG, and Niconovum AB, a subsidiary of Reynolds American Inc. The industry consists of major domestic and international companies, most of which have existing relationships in the markets into which we plan to sell, as well as financial, technical, marketing, sales, manufacturing, scaling capacity, distribution and other resources, and name recognition substantially greater than ours. We are also aware that several domestic cigarette competitors are continuing to research very low nicotine tobacco and have filed patent applications. However, our understanding is that these competitors continue to have a long development horizon to obtain a 95% reduction in nicotine.
We are also not aware of any competition in the creation and provision of VLNC tobacco research cigarettes for use in independent clinical studies.
Cigarette and filtered cigar companies compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, innovation, packaging, service, marketing, advertising, retail shelf space, and price. Cigarette sales can be significantly influenced by weak economic conditions, erosion of consumer confidence, competitors’ introduction of low-price products or innovative products, higher taxes, higher absolute prices and larger gaps between price categories, and product regulation that diminishes the ability to differentiate tobacco products. Domestic cigarette competitors included Philip Morris USA Inc., Reynolds American Inc., ITG Brands, and Vector Group Ltd. International competitors included Philip Morris International Inc., British American Tobacco, JT International SA, Imperial Brands plc, and regional and local tobacco companies; and in some instances, government-owned tobacco enterprises such as the China National Tobacco Corporation.
Human Capital Resources
As of December 31, 2020, we had 67 employees and we consider our employee relations to be good. Our human capital resource objectives are designed to attract, and retain, highly motivated and well qualified employees. We believe that we offer a competitive compensation package and have also worked diligently to provide a flexible and safe work environment—especially during the unforeseen COVID-19 global pandemic. The health and safety of our employees and clientele is of the upmost importance to us. We have taken significant steps to protect our workforce during COVID-19 including but not limited to, working remotely, increased cleaning and sanitization of facilities, and social distancing protocols consistent with guidelines issued by federal, state, and local governments.
15
Corporate Information
We are a Nevada corporation and our corporate headquarters is located at 8560 Main Street, Suite 4, Williamsville, New York 14221. Our telephone number is (716) 270-1523. Our internet address is www.xxiicentury.com. All of our filings with the Securities and Exchange Commission can be accessed free of charge through our website promptly after filing; however, in the event that the website is inaccessible, we will provide paper copies of our most recent Annual Report on Form 10-K, the most recent Quarterly Report on Form 10-Q, Current Reports filed or furnished on Form 8-K, and all related amendments, excluding exhibits, free of charge upon request. These filings are also accessible on the SEC’s website at www.sec.gov. We do not incorporate the information on our website into this Annual Report on Form 10-K.
Item 1A.Risk Factors-
You should carefully consider the risk factors set forth below and in other reports that we file from time to time with the Securities and Exchange Commission and the other information in this Annual Report on Form 10-K. The matters discussed in the risk factors, and additional risks and uncertainties not currently known to us or that we currently deem immaterial, could have a material adverse effect on our business, financial condition, results of operation and future growth prospects and could cause the trading price of our common stock to decline.
Risks Related to the COVID-19 Pandemic or Another Pandemic
The coronavirus pandemic (COVID-19) or another pandemic may cause a variety of business disruptions and future business risks.
The COVID-19 pandemic has adversely impacted the U.S. economy and supply chains and created volatility in U.S. financial markets. The COVID-19 pandemic has disrupted our business operations to a limited degree already, and there is a risk that state and federal authorities’ responses to the COVID-19 pandemic or another pandemic may disrupt our business in the future. The COVID-19 pandemic also poses a risk to our business which includes delays by third party providers of goods or services to our business, inability to operate in-person at our offices, interruptions to our sales, research and development, and administrative activities, and disruptions to our manufacturing operations, including the ability to staff our manufacturing operations at full capacity or at all. Similarly, state or federal authorities may also be affected in their capacity or capability to operate as normal and may impact the timeline of product authorizations which may disrupt our business plans. Our corporate office, laboratory in Buffalo, and production facility remain open and are operating under strict safety protocols. We continue to encourage remote work arrangements by our employees where job duties permit. At times during 2020, we were unable to have our full staff (or any staff) in our laboratory in Buffalo and some of our external research and development partners operated (or are still operating) on a modified or limited schedule, which slowed our research activities. These interruptions have had a minimal impact on our research operations. The future extent of the impact of the COVID-19 pandemic or another pandemic, including our ability to execute our business strategies as planned, will depend on future developments, including the duration and severity of the pandemic, which are highly uncertain and cannot be predicted.
The COVID-19 pandemic may also impact our ability to access the U.S. capital markets on a timetable and under terms that are advantageous to the Company.
Our executive leadership team and staff are monitoring this evolving situation and its impacts on our business. We will continue to monitor the local, state and federal guidance regarding our business practices.
Risks Related to Our Business and Operations
We have had a history of losses, and we may be unable to achieve and sustain profitability.
We have experienced net losses of approximately $19.7 million, $26.6 million and $8.0 million during the years ended December 31, 2020, 2019, and 2018, respectively. While our current balance of cash and cash equivalents and short-term investment securities is adequate to sustain our current planned operations, generating net income in the future will depend on our ability to successfully operate our cigarette manufacturing facility, create, sell and market our
16
proprietary tobacco and hemp products, and generate royalty revenue from the licensing of our intellectual property. There is no guarantee that we will be able to achieve or sustain profitability in the future. An inability to successfully achieve profitability will decrease our long-term viability.
We have had a history of negative cash flow, and our ability to sustain positive cash flow is uncertain.
As a result of our extensive research and development activities and regulatory expenses seeking FDA approvals, we have had a history of negative cash flow from operating activities, before cash used in investing activities and cash provided by financing activities, including approximately $15.6 million during the year ended December 31, 2020. We believe our current position of cash and cash equivalents and short-term investment securities is adequate to sustain operations and to meet all current planned obligations as they come due for over one year. Generation of positive cash flow from operations will depend on our ability to successfully implement income generating activities discussed in the previous risk factor discussion. An inability to successfully implement positive cash flows from operating activities will decrease our long-term viability.
If we fail to obtain FDA and foreign regulatory approvals for authorization to market our VLNC tobacco as a Modified Risk Cigarette, we will be unable to commercialize this potential product in and outside the U.S.
Although our PMTA for our VLNC tobacco cigarettes has been authorized by the FDA for marketing and sale in the United States, there can be no assurance that our MRTPA for our VLNC tobacco cigarettes will be authorized by the FDA and/or that our VLNC tobacco cigarettes will be approved by foreign regulators. In addition, there can be no assurance that all necessary approvals will be granted for our potential products or that review or actions will not involve delays caused by requests for additional information or testing that could adversely affect the time and cost to market and sell our potential products.
The development, testing, manufacturing, and marketing of our potential products are subject to extensive regulation by governmental authorities in the United States and throughout the world. In particular, the process of obtaining approvals by the FDA and other international FDA equivalent agencies in targeted countries is costly and time consuming, and the time required for such approvals is uncertain, as are the ultimate decisions of these agencies. Our MRTPA for our VLNC tobacco cigarettes must undergo an extensive regulatory approval process mandated by the FDA in the U.S. and any other approval processes required by FDA-equivalent agencies in foreign countries where we want to introduce our potential products.
The scope of review, including product testing and exposure studies, to be required by the FDA under the Tobacco Control Act in order for cigarettes to be marketed as Modified Risk Cigarettes has not yet been fully established, even though the FDA issued Modified Risk Tobacco Product Applications Draft Guidance on March 30, 2012. Additionally, as a combustible product, albeit one pursuing a modified exposure order, our product may be subject to different considerations or requirements by regulators than products undergoing similar regulatory reviews.
Delays or the denial of our MRTPA for our VLNC tobacco cigarettes or another future application by the FDA or another regulatory authority would negatively impact our business and planned operations and could result in a loss of time and expense pursuing such approvals and the inability to commercialize such potential products at all.
Our competitors may develop products that are less expensive, safer or otherwise more appealing, which may diminish or eliminate the commercial success of any potential product that we may commercialize.
If our competitors market products that are less expensive, safer or otherwise more appealing than our potential products, or that reach the market before our potential products, we may not achieve commercial success. The market may choose to continue utilizing existing products for any number of reasons, including familiarity with or pricing of these existing products. The failure of our PMTA product or our MRTPA product to compete with products marketed by our competitors would impair our ability to generate revenue, which would have a material adverse effect on our future business, financial condition, results of operations, and cash flows. Our competitors may:
| ● | develop and market products that are less expensive, safer, or otherwise more appealing than our products; |
| ● | commercialize competing products before we or our partners can launch our products; and |
17
| ● | initiate or withstand substantial price competition more successfully than we can. |
If we fail to stay at the forefront of technological change, we may be unable to compete effectively.
Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages that we believe we derive from our research approach and proprietary technologies. Our competitors may:
| ● | operate larger research and development programs or have substantially greater financial resources than we do; |
| ● | have greater success in recruiting skilled technical and scientific workers from the limited pool of available talent; |
| ● | more effectively negotiate third-party licenses and strategic relationships; and |
| ● | take advantage of acquisition or other opportunities more readily than we can. |
Negative press from being in the hemp/cannabis space could have a material adverse effect on our business, financial condition, and results of operations.
The hemp plant and the marijuana plant are both part of the same cannabis genus of plant, except that hemp, by definition, has not more than 0.3% THC content and is legal under the federal 2018 Farm Bill and certain state laws, but the same plant with a higher THC content is defined as marijuana, which is legal under certain state laws, is not legal under federal law. The similarities between these plants can cause confusion, and our activities with legal hemp may be incorrectly perceived as us being involved in federally illegal marijuana. Also, despite growing support for the marijuana industry and legalization of marijuana in certain U.S. states, many individuals and businesses remain opposed to the marijuana industry. Any negative press resulting from the incorrect perception that we have entered into the marijuana space could result in a loss of current or future business. It could also adversely affect the public’s perception of us and lead to reluctance by new parties to do business with us or to own our common stock. We cannot assure you that additional business partners, including but not limited to financial institutions, banking institutions and customers, will not attempt to end or curtail their relationships with us. Any such negative press or cessation of business could have a material adverse effect on our business, financial condition, and results of operations.
Any business-related cannabinoid production is dependent on laws pertaining to the hemp/cannabis industry.
On December 20, 2018, the Agricultural Improvement Act of 2018, which is also known as the “2018 Farm Bill,” was enacted and legalized hemp and hemp products under U.S. federal law, but with compliance still being required with all applicable state hemp laws and all regulations developed by the United States Department of Agriculture (“USDA”). In addition, the FDA is regulating products derived from hemp, including cannabidiol (“CBD”), for compliance under the Federal Food, Drug and Cosmetic Act and has issued several warning letters to firms marketing CBD products to treat disease or for other therapeutic uses. Under the Federal Food, Drug and Cosmetic Act, any product intended to affect the structure or function of the body of humans or animals is considered a drug that must receive premarket approval by the FDA through its new drug application process. Thus, participants in the hemp industry will need to comply with all applicable federal and state laws, rules and regulations in the cultivation, transportation, and sale of hemp and hemp derived products, including the Federal Food, Drug and Cosmetic Act.
As of January 1, 2021, (i) federal law and the laws of 47 states in the United States and the District of Columbia have legalized hemp, (ii) 36 states in the United States, the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands have enacted laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis/marijuana and consumer use of cannabis/marijuana in connection with medical treatment, and (iii) 15 states in the United States, the District of Columbia, Guam, and the Northern Mariana Islands have legalized cannabis/marijuana for adult recreational use. Many other states are considering similar legislation. Conversely, under the federal Controlled Substance Act (the “CSA”), the policies and regulations of the federal government and its agencies are that marijuana has no medical benefit and a range of activities are prohibited, including cultivation, possession, personal use, and interstate distribution of marijuana. In the event the U.S. Department of Justice begins strict enforcement of the CSA in states that have laws legalizing medical and/or adult recreational marijuana, there may be a direct and adverse impact to
18
any future business or prospects that we may have in the marijuana business. Even in those jurisdictions in which the manufacture and use of medical marijuana has been legalized at the state level, the possession, use, and cultivation of marijuana all remain violations of federal law that are punishable by imprisonment and substantial fines. Moreover, individuals and entities may violate federal law if they intentionally aid and abet another in violating these federal controlled substance laws or conspire with another to violate them.
We currently conduct sponsored research on hemp in Maryland and the Netherlands with third parties that possess all necessary permits and licenses to engage legally in such activities. We have conducted hemp research in Virginia, Oregon, and Canada with third-parties possessing all necessary permits and licenses to engage legally in such activities. In order to carry out research in other countries, similar licenses are required to be issued by the relevant authority in each country. We also conduct hemp research in our laboratories in New York under a license granted to us by the State of New York.
Local, state, federal, and international hemp and marijuana laws and regulations are broad in scope and subject to evolving interpretations, which could require us to incur substantial costs associated with compliance requirements. In addition, violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our operations. In addition, it is possible that regulations may be enacted in the future that will be directly applicable to our proposed business regarding cannabinoid production. It is also possible that the federal government will begin strictly enforcing existing laws, which may limit the legal uses of the hemp plant and its derivatives and extracts, such as cannabinoids. However, our work in hemp would continue since hemp research, development, and commercialization activities are permitted under applicable federal and state laws, rules, and regulations. We cannot predict the nature of any future laws, regulations, interpretations, or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on our activities in the legal hemp industry.
A key aspect of the revised strategy for cannabis is to reach an agreement with third parties to research characteristics of the cannabis plant and commercialize patented products through the value chain.
Any inability to produce hemp/cannabis products due to regulatory restrictions or otherwise would have a material adverse impact on our business and operations.
Our studies and testing of any of our potential products may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional studies and/or testing for these potential products or cease our studies and testing.
We do not know whether further studies and testing of our potential products will demonstrate safety and efficacy sufficient to result in marketable products. We may not be able to obtain approval or marketing authorization for these potential products or our anticipated time of bringing these potential products to the market may be substantially delayed. We may also experience significant additional development costs and be required to undertake additional studies and/or testing if we change our potential products. Furthermore, the development of new products is costly, time-consuming, and has no guarantee of success. Any such delays or the inability to effectively develop new products in a cost-effective manner would have a material adverse effect on our business.
We have no experience marketing and selling Modified Risk Tobacco products and any potential hemp/cannabis products and our working capital and inventory estimates based on demand expectations may be incorrect, which could harm our operating results and financial condition.
While members of management are experienced in the selling of conventional cigarette products, we have no experience in selling Modified Risk Tobacco Products or any hemp/cannabis derived products on a commercial basis. As work towards potentially commercializing one or more of our potential products for sale, including our VLN cigarettes, we intend to base our working capital and inventory decisions on management’s estimates of future demand. If demand for such potential new products does not increase as quickly as we have estimated, our inventory costs and working capital expenses could rise, and our business and operating results could suffer. Alternatively, if we experience sales in excess of our estimates, our working capital and inventory needs may be higher than those currently anticipated. Since our Modified Risk Tobacco is not widely available and must be grown specifically for our potential products, any
19
shortage in such tobacco could prevent us from increasing sales to meet demand and any surplus could result in inventory obsolescence and become a total loss. Similarly, for any potential hemp/cannabis products to be marketed and sold, our management must make estimates for inventory, distribution, marketing and compliance with regulations, which could provide to be incorrect. Our ability to meet any demand for our products may depend on our ability to arrange for additional financing for any ongoing working capital shortages, since it is likely that cash flow from sales will lag behind our investment requirements.
Any inability to incorrectly estimate demand for future products could negatively harm our operating results and financial condition.
We may acquire or invest in other companies, which may divert our management’s attention, result in additional dilution to our stockholders, and consume resources that are necessary to sustain our business or result in losses.
We may acquire or invest in complementary solutions, services, technologies, or businesses in the future. We may also enter into relationships with other businesses to expand our intellectual property portfolio, which could involve preferred or exclusive licenses or investments in other companies. Negotiating these transactions can be time-consuming, difficult and expensive, and our ability to complete these transactions may often be subject to conditions or approvals that are beyond our control. Consequently, these transactions, even if undertaken and announced, may not close.
Acquisitions may also disrupt our business, divert our resources, and require significant management attention that would otherwise be available for the development of our business. Moreover, the anticipated benefits of any acquisition, investment, or business relationship may not be realized or we may be exposed to unknown liabilities, including litigation against the companies that we may acquire.
We may be unable to restructure our investment in Panacea which could result in further impairment or losses.
As a result of increased competition and other macroeconomic factors, our investment in Panacea has been impaired since the date of our initial investment—see Note 6 to our consolidated financial statements for further information. Although we recently entered into a non-binding agreement with Panacea to potentially restructure our investment and business relationship, the agreement is preliminary, non-binding, subject to change, and may not occur. The failure to restructure our investment with Panacea could result in further impairment of our investment, additional capital investments to fund operations, losses as a result of a default on their $7 million principal convertible note receivable with us, litigation, or further losses.
We may require additional capital before we can complete the FDA authorization process for our Modified Risk Tobacco Product Application (“MRTPA”).
We may require additional capital in the future before we can complete the FDA authorization process for our MRTPA and to commercialize and market VLN® successfully. The cost of completing the FDA authorization process for our MRTPA is difficult to estimate since it is currently unknown exactly what the FDA will require; there is no precedent for the authorization of a combusted product under the MRTP requirements. If we raise additional funds through the issuance of equity securities to complete the FDA authorization process for our MRTPA, our stockholders may experience substantial dilution, or the equity securities may have rights, preferences or privileges senior to those of existing stockholders. If we raise additional funds through debt financings, these financings may involve significant cash payment obligations and covenants that restrict our ability to operate our business and make distributions to our stockholders. We could also wait for our own revenues and profits to be sufficient for us to provide such funding, which could delay our completion of the FDA authorization process for our MRTPA. We also could elect to seek funds through arrangements with collaborators or licensees. To the extent that we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or our potential products or grant licenses on terms that are not favorable to us.
If we cannot raise additional capital on acceptable terms, we may not be able to, among other things:
| ● | undertake the steps necessary to seek FDA authorization of our MRTPA; |
20
| ● | develop or enhance our potential products or introduce new products; |
| ● | expand our development, sales and marketing, and general and administrative activities; |
| ● | attract tobacco growers, customers, or manufacturing and distribution partners; |
| ● | acquire complementary technologies, products, or businesses; |
| ● | expand our operations in the United States or internationally; |
| ● | hire, train, and retain employees; or |
| ● | respond to competitive pressures or unanticipated working capital requirements. |
The loss of a significant customer for whom we manufacture tobacco products could have an adverse impact on our results of operation.
Currently, a significant portion of our revenues (and corresponding accounts receivable) from manufacturing tobacco products are derived from our three largest customers, and we do not have an agreement with such customers requiring them to purchase a minimum amount of products from us or guaranteeing any minimum future purchase amounts from us. Such customers may, at any time, delay or decrease their level of purchases from us or cease doing business with us altogether. Since many of our manufacturing costs are fixed, if sales to such customers cease or are reduced, we may not obtain sufficient purchase orders from other customers necessary to offset any such losses or reductions, which could have a negative impact on our results of operations.
Our principal competitors in the conventional or modified risk tobacco products market have, and any future competitors may have, greater financial and marketing resources than we do, and they may therefore develop products or other technologies similar or superior to ours, or otherwise compete more successfully than we do.
While there is significant management experience in selling conventional products, we have no experience in selling modified risk tobacco products. As of December 31, 2020, the only companies that have received authorization from the FDA to market modified risk tobacco products in the United States are Swedish Match and Phillip Morris SA, which both have significantly greater resources than us. The industry consists of major domestic and international companies, most of which have existing relationships in the markets in which we plan to sell, as well as financial, technical, research and development, marketing, sales, manufacturing, scaling capacity, distribution and other resources and name recognition substantially greater than ours. In addition, we expect new competitors will enter the markets for our products in the future and the nature and extent of this market entrance cannot be quantified at this time. Potential customers may choose to do business with our more established competitors because of their perception that our competitors are more stable, are more likely to complete various projects, can scale operations more quickly, have greater manufacturing capacity, are more likely to continue as a going concern, and lend greater credibility to any joint venture. If we are unable to compete successfully against manufacturers of other modified risk tobacco products for the custom of adult who smoke, our business could suffer, and we could lose or be unable to obtain market share.
Product liability claims, product recalls, or other claims could cause us to incur losses or damage our reputation.
The risk of product liability claims or product recalls, and associated adverse publicity, is inherent in the development, manufacturing, marketing, and sale of modified risk tobacco products. Any product recall or lawsuit seeking significant monetary damages may have a material adverse effect on our business and financial condition. A successful product liability claim against us could require us to pay a substantial monetary award. Though we currently have no pending product liability claims against us, we cannot assure you that such claims will not be made in the future and any such claim could cause us to incur substantial losses or damage our reputation.
The failure of our information systems to function as intended or their penetration by outside parties with the intent to corrupt them could result in business disruption, litigation and regulatory action, and loss of revenue, assets, or personal or confidential data (cybersecurity).
We use information systems to help manage business processes, collect and interpret business data and communicate internally and externally with employees, suppliers, customers and others. Some of these information
21
systems are managed by third-party service providers. We have backup systems and business continuity plans in place, and we take care to protect our systems and data from unauthorized access. However, a failure of our systems to function as intended, or penetration of our systems by outside parties intent on extracting or corrupting information or otherwise disrupting business processes, could place us at a competitive disadvantage, result in a loss of revenue, assets or personal or other sensitive data, litigation and regulatory action, cause damage to our reputation and that of our brands and result in significant remediation and other costs. Any cybersecurity incident could cause substantial harm to our business and result in regulatory action, fines, and/or substantial costs.
We have limited experience in managing growth. If we fail to manage our growth effectively, we may be unable to execute our business plan or to address competitive challenges adequately.
From 2013 to December 31, 2020, we grew from nine (9) employees to sixty-seven (67) employees. Any future growth in our business will place a significant strain on our managerial, administrative, operational, financial, information technology and other resources. We intend to further expand our overall business, customer base, employees and operations, which will require substantial management effort and significant additional investment in our infrastructure. We will be required to continue to improve our operational, financial and management controls and our reporting procedures and we may not be able to do so effectively. As such, we may be unable to manage our growth effectively and such failure would have a material adverse impact on our operations.
Business interruptions, whether caused by natural disaster, terrorism, economic downturns, global pandemics or other events, could negatively impact our business.
A natural disaster (such as an earthquake, hurricane, fire, or flood), pandemics (including the COVID-19 pandemic), or an act of terrorism could cause substantial delays in our operations, damage or destroy our equipment or facilities, and cause us to incur additional expenses and lose revenue. The insurance we maintain against natural disasters may not be adequate to cover our losses in any particular case, which would require us to expend significant resources to replace any destroyed assets, thereby materially and adversely affecting our financial condition and prospects. Other global incidents could have a similar effect of disrupting our business to the extent they reach and impact the areas in which we operate, the availability of inventory we need, the customers we serve, the partners on whom we rely for products or services or the employees who operate our businesses. For example, the outbreak of COVID-19 or another pandemic could disrupt our supply chain for tobacco, as well as negatively impact employee productivity, including affecting the availability of employees reporting for work. Any business interruption caused by such unforeseen events could have a material adverse impact on our business and operations.
Risks Related to the Tobacco Industry
The manufacturing of tobacco products subjects us to significant governmental regulation and the failure to comply with such regulations could have a material adverse effect on our business and subject us to substantial fines or other regulatory actions.
As of December 31, 2020, most of the revenues of our manufacturing business are from the production of tobacco cigarettes and filtered cigars made for third-party brand owners of such products and we anticipate generating future revenue from the sales of our VLNC cigarettes and other modified risk tobacco products, assuming we obtain regulatory approvals. Companies that manufacture and/or sell tobacco products face significant governmental regulation, especially in the United States pursuant to the Tobacco Control Act, including but not limited to efforts aimed at reducing the incidence of tobacco use, restricting marketing and advertising, imposing regulations on packaging, mandating warnings and disclosure of flavors or other ingredients, prohibiting the sale of tobacco products with certain flavors or other characteristics, requiring compliance with certain environmental standards, limiting or prohibiting the sale of tobacco products by certain retail establishments and the sale of tobacco products in certain packaging sizes, and seeking to hold retailers and distributors responsible for the adverse health effects associated with both smoking and exposure to environmental tobacco smoke.
Manufacturers of tobacco products must comply with FDA regulations which require, among other things, compliance with the FDA’s evolving regulations on Current Good Manufacturing Practices (“cGMP(s)”), which are enforced by the FDA through its facilities inspection program. The manufacture of products is subject to strict quality
22
control, testing and record keeping requirements, and continuing obligations regarding the submission of safety reports and other post-market information. We cannot guarantee that our current manufacturing facility will pass FDA inspections and/or similar inspections in foreign countries to produce our tobacco products, or that future changes to cGMP manufacturing standards will not also negatively affect the cost or sustainability of our manufacturing facility.
We and our customers for whom we manufacture tobacco products also face significant governmental regulation, including efforts aimed at reducing the incidence of tobacco use. Actions by the FDA and other federal, state or local governments or agencies may impact the adult tobacco consumer acceptability of or access to tobacco products (for example, through product standards proposed by the FDA for nicotine and flavors including menthol), delay or prevent the launch of new or modified tobacco products or products with claims of reduced risk, require the recall or other removal of tobacco products from the marketplace, impose additional manufacturing, labeling or packing requirements, interrupt manufacturing or otherwise significantly increase the cost of doing business. Any one or more of these actions may have a material adverse impact on us or the business of our customers for whom we make tobacco products, which could have a negative impact on our results of operations.
Significant regulatory developments will take place over the next few years in many markets, driven principally by the World Health Organization’s Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging cessation. In addition, the FCTC has led to increased efforts by tobacco control advocates and public health organizations to reduce the appeal of tobacco products. Partly because of some or a combination of these efforts, unit sales of conventional tobacco products in certain markets, principally Western Europe and Japan, have been in general decline and we expect this trend to continue. Our operating results could be significantly affected by any significant increase in the cost of complying with new regulatory requirements.
Compliance with current and future regulations regarding tobacco could have a material impact on our business and operations and could result in fines, government actions to restrict or prevent sales of products, as well as result in substantial costs and expenses.
If the FDA’s proposed rule regarding graphic health warnings on cigarette packaging and in cigarette advertising is finalized, the final rule is likely to have a negative impact on sales of our third-party customers’ products and potential Company products.
On August 16, 2019, as required by the Federal Cigarette Labeling and Advertising Act, as amended by the Family Smoking Prevention and Tobacco Control Act, the FDA issued a proposed rule to modify the required warnings that appear on cigarette packages and in cigarette advertisements. The FDA proposes to require tobacco product manufacturers to display one of thirteen pairs of text warnings and photorealistic images depicting negative health consequences of smoking. If finalized, the rule would require, within 15 months of finalization, the graphic health warnings to appear directly on the cigarette package and be directly visible beneath the cellophane wrapping and comprise the top 50 percent of the front and rear panels of cigarette packages. FDA first promulgated a final rule in September 2012, which would have imposed graphic health warning requirements to those proposed in the FDA’s August 2019 proposed rule. However, a federal appellate court vacated the September 2012 rules after holding that the rule was unconstitutional. If the FDA successfully implements these revised regulations announced in its August 2019 proposed rule, and any reviewing federal court does not strike them down on constitutional or other grounds, then all cigarettes manufactured for sale or distribution in the United States will need to include these new graphic health warnings on their packages consistent with the effective date(s) included in such regulations. Any resulting reduction in the number of smokers will probably reduce the demand for the products manufactured by our factory for our potential Company products and for third-party brand owners of such products.
If regulations by the FDA requiring the reduction of nicotine to minimally or non-addictive levels in all cigarettes sold in the U.S. are delayed or not implemented, then the anticipated resulting demand for our proprietary Very Low Nicotine Content tobacco may not occur.
On July 28, 2017, the FDA publicly announced that it intends to implement new regulations that will mandate minimally or non-addictive levels of nicotine in all cigarettes sold in the U.S.
23
There can be no assurance that the FDA will implement such new regulations or, if implemented, when such regulations would take effect. In the event the FDA does not implement such new regulations or implementation is delayed, then the anticipated resulting demand for our proprietary VLNC tobacco may not occur and such action would have a material adverse effect on our future business and operations.
We may become subject to litigation related to cigarette smoking and/or exposure to environmental tobacco smoke, or ETS, which could severely impair our results of operations and liquidity.
Although we are not currently subject to legal proceedings related to cigarette smoking or ETS, we may become subject to litigation related to the sale of our Modified Risk Cigarettes or other tobacco products we sell or manufacture. Legal proceedings covering a wide range of matters related to tobacco use are pending or threatened in various U.S. and foreign jurisdictions. Various types of claims are raised in these proceedings, including product liability, consumer protection, antitrust, tax, contraband shipments, patent infringement, employment matters, claims for contribution, and claims of competitors and distributors.
Litigation is subject to uncertainty and it is possible that there could be adverse developments in pending cases. An unfavorable outcome or settlement of pending tobacco related litigation could encourage the commencement of additional litigation. The variability in pleadings, together with the actual experience of management in litigating claims, demonstrates that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome.
Damages claimed in some tobacco-related litigation are significant and, in certain cases, range into the billions of dollars. We anticipate that new cases will continue to be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our results of operations, cash flows, or financial position could be materially affected by an unfavorable outcome or settlement of litigation.
Cigarettes are subject to substantial taxes. Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions. These tax increases may affect the sales of our potential Company products and our third-parties customers’ tobacco products manufactured at our factory, which could result in decreased sales and profitability of our manufacturing business.
Tax regimes, including excise taxes, sales taxes, and import duties, can disproportionately affect the retail price of manufactured cigarettes versus other tobacco products, or disproportionately affect the relative retail price of our Modified Risk Cigarettes versus lower-priced cigarette brands manufactured by our competitors. Increases in cigarette taxes are expected to continue to have an adverse impact on sales of cigarettes resulting in (i) lower consumption levels, (ii) a shift in sales from manufactured cigarettes to other tobacco products or to lower-price cigarette categories, (iii) a shift from local sales to legal cross-border purchases of lower price products, and (iv) illicit products such as contraband and counterfeit.
Government mandated prices or taxes, production control programs, shifts in crops driven by economic conditions, climatic or adverse weather patterns may increase the cost or reduce the quality of the tobacco and other agricultural products used to manufacture our products.
We depend on independent tobacco farmers to grow our specialty proprietary tobaccos with specific nicotine contents for our potential products. As with other agricultural commodities, the price of tobacco leaf can be influenced by imbalances in supply and demand, and crop quality can be influenced by variations in weather patterns, diseases, and pests. We must also compete with other tobacco companies for contract production with independent tobacco farmers. Tobacco production in certain countries is subject to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for agricultural products could cause farmers to plant less tobacco. Any significant change in tobacco leaf prices or taxes, quality and quantity could affect our profitability and our business.
We intend to distribute and sell our potential products outside of the U.S., which will subject us to other regulatory risks.
In addition to seeking authorization from the FDA to market our VLNC tobacco cigarettes as a Modified Risk Tobacco Product in the U.S., we intend to seek governmental approvals required to market our VLNC tobacco cigarettes
24
and our other products in other countries. Marketing of our products is not permitted in certain countries until we have obtained required approvals or exemptions in these individual countries. The regulatory review process varies from country to country, and approval by foreign governmental authorities is unpredictable, uncertain, and generally expensive. Our ability to market our potential products could be substantially limited due to delays in receipt of, or failure to receive, the necessary approvals or clearances. We anticipate commencing the applications required in some or all of these countries following authorization by the FDA; however, we may decide to file applications in advance of the FDA authorization if we determine such filings to be both time and cost effective. If we export any of our potential products, or products that have not yet been cleared for commercial distribution in the U.S., then such products may be subject to FDA export restrictions. Failure to obtain necessary regulatory approvals could impair our ability to generate revenue from international sources.
We may become subject to governmental investigations on a range of matters.
Tobacco companies are often subject to investigations, including allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within certain markets, allegations of underpayment of custom duties and/or excise taxes, and allegations of false and misleading usage of descriptors such as “lights” and “ultra-lights.” We cannot predict the outcome of any investigations to which we may become subject, but we may be materially affected by an unfavorable outcome of potential future investigations.
The FDA could force the removal of our products from the U.S. market.
The FDA has broad authority over the regulation of tobacco products. The FDA could, among other things, force us to remove from the U.S. market our VLNC tobacco cigarettes even after the FDA authorization on December 17, 2019 of our PMTA or the potential future authorization of our MRTPA for us to market in the U.S. our VLNC tobacco cigarettes, as well as the FDA could levy fines or change their regulations on advertising. Any adverse action by the FDA could have a material adverse impact on our business.
Risks Related to Intellectual Property
Certain of our proprietary rights have expired or may expire or may not otherwise adequately protect our intellectual property, products and potential products, and if we cannot obtain adequate protection of our intellectual property, products and potential products, we may not be able to successfully market our products and potential products.
Our commercial success will depend in part on obtaining and maintaining intellectual property protection for our technologies, products, and potential products. We will only be able to protect our technologies, products, and potential products from unauthorized use by third parties to the extent that valid and enforceable patents cover them, or to the extent that other market exclusionary rights apply.
The patent positions of life sciences companies, like ours, can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. The general patent environment outside the United States also involves significant uncertainty. Accordingly, we cannot predict the breadth of claims that may be allowed or that the scope of these patent rights could provide a sufficient degree of future protection that could permit us to gain or keep our competitive advantage with respect to these products and technology. Additionally, life science companies like ours are often dependent on creating a pipeline of products. We may not be able to develop additional potential products or proprietary technologies that produce commercially viable products or that are themselves patentable.
Although there are currently no challenges to any portion of our intellectual property, our issued patents may be subject to challenge and potential invalidation by third parties. Changes in either the patent laws or in the interpretations of patent laws in the United States, or in other countries, may diminish the value of our intellectual property. In addition, others may independently develop similar or alternative products and technologies that may be outside the scope of our intellectual property. Should third parties obtain patent rights to similar products or technology, this may have an adverse effect on our business.
25
The expiration of a portion of the QPT patent family in 2018 may provide third parties with the freedom to target the QPT gene in the tobacco plant. This could result in experiments to try to reduce nicotine levels in tobacco plants to levels that may satisfy the planned new nicotine reduction regulations coming from the FDA. There can be no assurance about whether any third-parties will or will not be successful in such efforts, how long or short in time such efforts will entail and/or if such efforts will or will not infringe other genes and other intellectual property on which we have continuing patent protection that would need to be used, in combination with QPT, to result in VLNC tobacco. If our competitors are able to successfully reduce nicotine levels in tobacco plants without violating our patent protections, our ability to license our technology would be negatively impacted and we would likely face increased competition. We note however, that even if achieved, competitors would still be required to pursue FDA regulatory pathways, such as PMTA and MRTP, which in themselves are lengthy, complicated, and costly activities.
We also rely on trade secrets to protect our technology, products, and potential products, especially where we do not believe patent protection is appropriate or obtainable. Trade secrets, however, are difficult to protect. While we believe that we use reasonable efforts to protect our trade secrets, our own, our licensees’ or our strategic partners’ employees, consultants, contractors or advisors may unintentionally or willfully disclose our information to competitors. We seek to protect this information, in part, through the use of non-disclosure and confidentiality agreements with employees, consultants, advisors, and others. These agreements may be breached, and we may not have adequate remedies for a breach. In addition, we cannot ensure that those agreements will provide adequate protection for our trade secrets, know-how, or other proprietary information, or prevent their unauthorized use or disclosure.
To the extent that consultants or key employees apply technological information independently developed by them or by others to our products and potential products, disputes may arise as to the proprietary rights of the information, which may not be resolved in our favor. Key employees are required to assign all intellectual property rights in their discoveries to us. However, these key employees may terminate their relationship with us, and we cannot preclude them indefinitely from dealing with our competitors. If our trade secrets become known to competitors with greater experience and financial resources, the competitors may copy or use our trade secrets and other proprietary information in the advancement of their products, methods, or technologies. If we were to prosecute a claim that a third party had illegally obtained and was using our trade secrets, it could be expensive and time consuming and the outcome could be unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets than courts in the United States. Moreover, if our competitors independently develop equivalent knowledge, we would lack any contractual claim to this information, and our business could be harmed.
The ability to commercialize our potential products will depend on our ability to sell such products without infringing the patent or proprietary rights of third parties. If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and an unfavorable outcome could have a significant adverse effect on our business.
The ability to commercialize our potential products will depend on our ability to sell such products without infringing the patents or other proprietary rights of third parties. Third-party intellectual property rights in our field are complicated, and third-party intellectual property rights in these fields are continuously evolving. While we have conducted searches for such third-party intellectual property rights, we have not performed specific searches for third-party intellectual property rights that may raise freedom-to-operate issues, and we have not obtained legal opinions regarding commercialization of our potential products. As such, there may be existing patents that may affect our ability to commercialize our potential products.
In addition, because patent applications are published up to 18 months after their filing, and because patent applications can take several years to issue, there may be currently pending third-party patent applications and freedom-to-operate issues that are unknown to us, which may later result in issued patents.
If a third-party claims that we infringe on its patents or other proprietary rights, we could face a number of issues that could seriously harm our competitive position, including:
| ● | infringement claims that, with or without merit, can be costly and time consuming to litigate, can delay the regulatory approval process, and can divert management’s attention from our core business strategy; |
26
| ● | substantial damages for past infringement which we may have to pay if a court determines that our products or technologies infringe upon a competitor’s patent or other proprietary rights; |
| ● | a court order prohibiting us from commercializing our potential products or technologies unless the holder licenses the patent or other proprietary rights to us, which such holder is not required to do; |
| ● | if a license is available from a holder, we may have to pay substantial royalties or grant cross licenses to our patents or other proprietary rights; and |
| ● | redesigning our process so that it does not infringe the third-party intellectual property, which may not be possible, or which may require substantial time and expense including delays in bringing our potential products to market. |
Such actions could harm our competitive position and our ability to generate revenue and could result in increased costs.
Our patent applications may not result in issued patents, which may have a material adverse effect on our ability to prevent others from commercially exploiting products similar to ours.
We own or exclusively control many issued patents and pending patent applications. We cannot be certain that these patent applications will issue, in whole or in part, as patents. Patent applications in the United States are maintained in secrecy until the patents are published or are issued. Since publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several months, we cannot be certain that we are the first creator of inventions covered by pending patent applications or the first to file patent applications on these inventions. We also cannot be certain that our pending patent applications will result in issued patents or that any of our issued patents will afford protection against a competitor. In addition, patent applications filed in foreign countries are subject to laws, rules and procedures that differ from those of the United States, and thus we cannot be certain that foreign patent applications related to U.S. patents will be issued. Furthermore, if these patent applications issue, some foreign countries provide significantly less effective patent enforcement than in the United States.
The status of patents involves complex legal and factual questions and the breadth of claims allowed is uncertain. Accordingly, we cannot be certain that the patent applications that we or our licensors file will result in patents being issued, or that our patents and any patents that may be issued to us in the near future will afford protection against competitors with similar technology. In addition, patents issued to us may be infringed upon or designed around by others and others may obtain patents that we need to license or design around, either of which would increase costs and may adversely affect our operations.
We license certain patent rights from third-party owners. If such owners do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects could be harmed.
We license rights to third-party intellectual property that is necessary or useful for our business, and we may enter into additional licensing agreements in the future. Our success could depend in part on the ability of some of our licensors to obtain, maintain, and enforce patent protection for their intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents are issued with respect to these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we could. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects.
Our worldwide exclusive licenses relating to tobacco from NCSU involve multiple patent families. The exclusive rights under the NCSU agreements expire on the date on which the last patent or registered plant variety covered by the subject license expires in the country or countries where such patents or registered plant varieties are in effect. The NCSU licenses relate predominately to issued patents, and our exclusive rights in the NCSU licenses are expected to expire in 2036.
27
Our worldwide sublicense from Anandia, a plant biotechnology company based in Vancouver, Canada, grants us exclusive rights in the United States and co-exclusive rights with Anandia everywhere else in the world (except not in Canada where Anandia retains exclusive rights) to certain patents and patent applications relating to certain genes in the hemp/cannabis plant that are required for the production of cannabinoids, the “active ingredients” in the cannabis plant. The Anandia sublicense continues through the life of the last-to-expire patent, which is expected to be in 2035.
Risks Related to Ownership of Our Common Stock
An active trading market for our common stock may not be sustained, and you may not be able to resell your shares at or above the price at which you purchased them.
An active trading market for our shares may not be sustained. In the absence of an active trading market for our common stock, shares of common stock may not be able to be resold at or above the purchase price of such shares. Although there can be no assurances, we expect that our common stock will continue to be quoted on the New York Stock Exchange American market (“NYSE American”). However, even if our common stock continues to be quoted on the NYSE American, there is no assurance that an active market for our common stock will continue in the foreseeable future. There also can be no assurance that we can maintain such listing on the NYSE American. If we are ever no longer listed on the NYSE American or other national stock exchange in the future, then it would be more difficult to dispose of shares or to obtain accurate quotations as to the market value of our common stock compared to securities of companies whose shares are traded on national stock exchanges.
Our stock price may be highly volatile and could decline in value.
Our common stock is currently traded on the NYSE American and the market price for our common stock has been volatile. Further, the market prices for securities in general have been highly volatile and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| ● | failure or discontinuation of any of our research programs; |
| ● | delays in establishing new strategic relationships; |
| ● | delays in the development of our potential products and commercialization of our potential products; |
| ● | market conditions in our sector and issuance of new or changed securities analysts’ reports or recommendations; |
| ● | general economic conditions, including adverse changes in the global financial markets; |
| ● | actual and anticipated fluctuations in our quarterly financial and operating results; |
| ● | developments or disputes concerning our intellectual property or other proprietary rights; |
| ● | introduction of technological innovations or new commercial products by us or our competitors; |
| ● | issues in manufacturing or distributing our products or potential products; |
| ● | market acceptance of our products or potential products; |
| ● | FDA or other United States or foreign regulatory actions affecting us or our industry; |
| ● | litigation or public concern about the safety of our products or potential products; |
| ● | negative press or publicity regarding us or our common stock; |
| ● | the announcement of litigation against us or the results of on-going litigation; |
| ● | additions or departures of key personnel; |
| ● | third-party sales of large blocks of our common stock or third party short-selling activity; |
| ● | third-party articles regarding us or our securities; |
28
| ● | pending or future shareholder litigation; |
| ● | future developments with respect to our investment in Panacea; |
| ● | sales of our common stock by our executive officers, directors, or significant stockholders; and |
| ● | equity sales by us of our common stock or securities convertible into common stock to fund our operations. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock, such as the current class action and derivative lawsuits. Such lawsuits and any future related lawsuits could cause us to incur substantial costs defending the lawsuit and can also divert the time and attention of our management, which would have a negative adverse impact on our business. See the risk factor below entitled: “We are named defendant in certain litigation matters, including federal securities class action lawsuits and derivative complaints; if we are unable to resolve these matters favorably, then our business, operating results and financial condition may be adversely affected.”
We are named defendant in certain litigation matters, including federal securities class action lawsuits and derivative complaints; if we are unable to resolve these matters favorably, then our business, operating results and financial condition may be adversely affected.
We are currently involved in certain litigation matters, including securities class action and derivative litigation. See "Item 3 – Legal Proceedings" included in this Annual Report on Form 10-K. We cannot at this time predict the outcome of these matters or any future litigations matters (whether related or unrelated) or reasonably determine the probability of a material adverse result or reasonably estimate range of potential exposure, if any, that these matters or any future matters might have on us, our business, our financial condition or our results of operations, although such effects, including the cost to defend, any judgements or indemnification obligations, among others, could be materially adverse to us. In addition, in the future, we may need to record litigation reserves with respect to these matters. Further, regardless of how these matters proceed, it could divert our management’s attention and other resources away from our business.
Future sales of our common stock will result in dilution to our common stockholders.
Sales of a substantial number of shares of our common stock in the public market may depress the prevailing market price for our common stock and could impair our ability to raise capital through the future sale of our equity securities. Additionally, if any of the holders of outstanding options or warrants exercise or convert those shares, as applicable, our common stockholders will incur dilution in their relative percentage ownership. The prospect of this possible dilution may also impact the price of our common stock.
We do not expect to declare any dividends on our common stock in the foreseeable future.
We have not paid cash dividends to date on our common stock. We currently intend to retain our future earnings, if any, to fund the development and growth of our business, and we do not anticipate paying any cash dividends on our common stock for the foreseeable future. Additionally, the terms of any future debt facilities may preclude us from paying dividends on the common stock. As a result, capital appreciation, if any, of our common stock could be the sole source of gain for the foreseeable future.
Anti-takeover provisions contained in our articles of incorporation and bylaws, as well as provisions of Nevada law, could impair a takeover attempt.
Our amended and restated articles of incorporation and bylaws currently contain provisions that, together with Nevada law, could have the effect of rendering more difficult or discouraging an acquisition deemed undesirable by our board of directors. Our corporate governance documents presently include the following provisions:
| ● | providing for a “staggered” board of directors in which only one-third (1/3) of the directors can be elected in any year; |
29
| ● | authorizing blank check preferred stock, which could be issued with voting, liquidation, dividend, and other rights superior to our common stock; and |
| ● | limiting the liability of, and providing indemnifications to, our directors and officers. |
These provisions, alone or together, could delay hostile takeovers and changes in control of our Company or changes in our management.
As a Nevada corporation, we also may become subject to the provisions of Nevada Revised Statutes Sections 78.378 through 78.3793, which prohibit an acquirer, under certain circumstances, from voting shares of a corporation’s stock after crossing specific threshold ownership percentages, unless the acquirer obtains the approval of the stockholders of the issuer corporation. The first such threshold is the acquisition of at least one-fifth, but less than one-third of the outstanding voting power of the issuer. We may become subject to the above referenced Statutes if we have 200 or more stockholders of record, at least 100 of whom are residents of the State of Nevada and do business in the State of Nevada directly or through an affiliated corporation.
As a Nevada corporation, we are subject to the provisions of Nevada Revised Statutes Sections 78.411 through 78.444, which prohibit an “interested stockholder” from entering into a combination with the corporation, unless certain conditions are met. An “interested stockholder” is a person who, together with affiliates and associates, beneficially owns (or within the prior two years did own) 10 percent or more of the corporation’s voting stock.
Any provision of our amended and restated articles of incorporation, our bylaws or Nevada law that has the effect of delaying or deterring a change in control of our Company could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
Item 1BUnresolved Staff Comments.
None.
Item 2.Properties.
As of December 31, 2020, we had four leases located in the following locations:
|
| City |
| State | ||
Corporate Headquarters | Williamsville | NY | ||||
Research and Development Laboratory | Buffalo | NY | ||||
Manufacturing Facility and Warehouse | Mocksville | NC | ||||
Tobacco Storage Warehouse | Wilson | NC |
In January 2021, we announced that our corporate headquarters will be relocated to downtown Buffalo, NY. The new leased office space is in a state-of-the-art facility where we will join other multinational technology and professional services companies. The facility will accommodate all current Williamsville, NY employees and will provide significant room for expansion and we are expecting to move in during March 2021. Refer to Note 4 and Note 17 to our consolidated financial statements for additional information.
We believe that all facilities are adequate for our current needs.
Item 3.Legal Proceedings.
See Note 12 - Commitments and Contingencies – Litigation - to our consolidated financial statements included in this Annual Report for information concerning our on-going litigation. In addition to the lawsuits described in Note 12 to our consolidated financial statements, from time to time we may be involved in claims arising in the ordinary course of business. To our knowledge other than the cases described in Note 12 to our consolidated financial statements, no material legal proceedings, governmental actions, investigations or claims are currently pending against us or involve us
30
that, in the opinion of our management, could reasonably be expected to have a material adverse effect on our business and financial condition.
Item 4.Mine Safety Disclosures.
Not applicable
31
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is listed on the NYSE American under the symbol “XXII.” As of December 31, 2020, there were 86 holders of record of shares of our common stock.
Dividend Policy
We have not previously and do not plan to declare or pay any dividends on our common stock. Our current policy is to retain all funds and any earnings for use in the operation and expansion of our business. Payment of future dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including current financial condition, operating results and current and anticipated cash needs.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Shares authorized for issuance under equity compensation plans
On April 12, 2014, our stockholders approved the 22nd Century Group, Inc. 2014 Omnibus Incentive Plan (the “OIP”) and the authorization of 5,000,000 shares thereunder. On April 29, 2017, the stockholders approved an amendment to the OIP to increase the number of shares available for issuance by an additional 5,000,000 shares and on May 3, 2019, the stockholders approved an additional amendment to the OIP to increase the number of shares available for issuance by an additional 5,000,000 shares. The OIP allows for the granting of equity and cash incentive awards to eligible individuals over the life of the OIP. The OIP has a term of ten years and is administered by the Compensation Committee of our Board of Directors to determine the various types of incentive awards that may be granted to recipients under this plan and the number of shares of common stock to underlie each such award under the OIP. As of December 31, 2020, we had available 3,987,167 shares remaining for future awards under the OIP.
The following table summarizes the number of shares of common stock to be issued upon exercise of outstanding options and vesting of restricted stock units under the OIP and our prior 2010 Equity Incentive Plan, the weighted-average exercise price of such stock options, and the number of securities available to be issued under the OIP as of December 31, 2020:
|
|
| Number of securities |
| ||||
Number of securities to | remaining available for |
| ||||||
be issued upon exercise | issuance under equity |
| ||||||
of outstanding options, | Weighted average | compensation plans |
| |||||
and restricted stock | exercise price of | (excluding securities |
| |||||
units, | outstanding options | reflected in column (a)) |
| |||||
(a) | (b) | (c) |
| |||||
Equity compensation plans approved by security holders |
| 9,519,247 | $ | 1.50 |
| 3,987,167 | ||
Equity compensation plans not approved by security holders |
| — |
| N/A |
| — | ||
Total |
| 9,519,247 |
| — |
| 3,987,167 | (1) | |
| (1) | Consists of shares available for award under the OIP. No future awards are available under the 2010 Equity Incentive Plan. |
32
Stock Performance Graph
The following information in this Item of the Annual Report on Form 10-K is not deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or to the liabilities of Section 18 of the Exchange Act, and will not be deemed to be incorporated by reference to any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent that we specifically incorporate such information into such filing.
The performance graph shown below compares the cumulative total shareholder return on the Company’s common stock, based on the market price of the common stock, with the total return of the NYSE American Composite Index and the NASDAQ US Small Cap Biotechnology Index for the period covering December 31, 2015 through December 31, 2020. The comparison of total return assumes that a fixed investment of $100 was invested on December 31, 2015 in the Company’s common stock and in each of the foregoing indices and further assumes the reinvestment of dividends. The stock price performance shown on the graph is not necessarily indicative of future price performance.
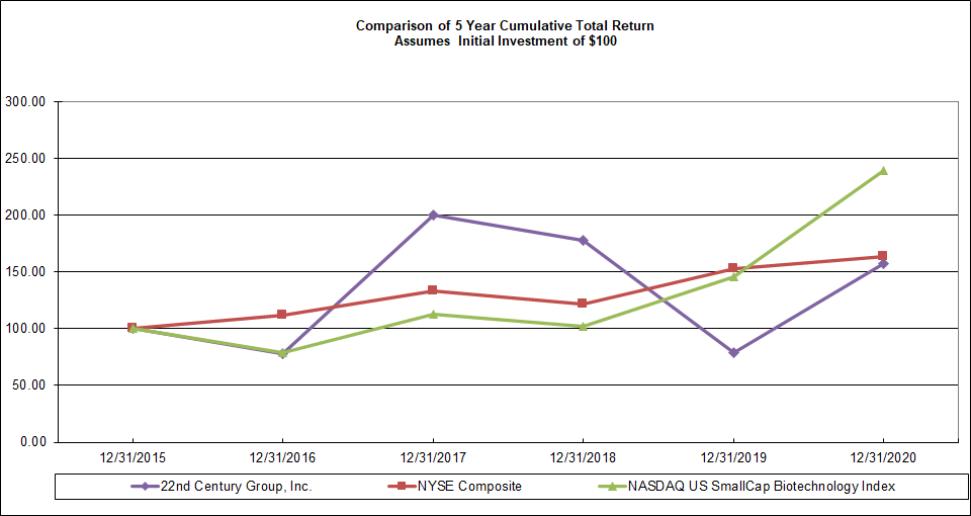
Item 6.Selected Financial Data
Not applicable for a smaller reporting company, as defined by Item 10(f)(1) of Regulation S-K.
33
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations.
This discussion should be read in conjunction with the other sections of this Form 10-K, including “Risk Factors,” and the Financial Statements and notes thereto. This section of the Form 10-K generally discusses 2020 and 2019 items and year-to-year comparisons of 2020 to 2019. Discussions of 2018 items and year-to-year comparisons of 2019 and 2018 that are not included in this Form 10-K can be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7 on our Annual Report on Form 10-K for the year ended December 31, 2019. The various sections of this discussion contain a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risk factors described throughout this Annual Report on Form 10-K. See “Cautionary Note Regarding Forward-Looking Statements and Risk Factor Summary.” Our actual results may differ materially. For purposes of this Management’s Discussion and Analysis of Financial Condition and Results of Operations, references to the “Company,” “we,” us” or “our” refer to the operations of 22nd Century Group, Inc. and its direct and indirect subsidiaries for the periods described herein.
Executive Overview of Full Year 2020 Results
Key Business and Financial Highlights
| ● | Securing MRTP authorization for VLN® remains our number one priority and we are confident that the FDA is in the final stages of the review process related to our application. We are working to ensure that the launch of VLN® King and VLN® Menthol King cigarettes occurs within 90 days of receiving MRTP designation. |
| ● | Since reporting third quarter 2020 earnings, we have refocused our hemp/cannabis strategy to target the upstream segment of the cannabinoid value chain: a shift away from the already saturated U.S. consumer market of cannabidiol (CBD) and hemp-based products. |
| ● | We have now secured four out of the five key partnerships needed to maximize each component in the upstream segment of the hemp/cannabis value chain. These partnerships will enable us to accelerate the new development of valuable, commercial hemp/cannabis lines and intellectual property to market. |
| ● | Net sales revenue of $7.3 million for the fourth quarter of 2020 was consistent with the prior year period and improved by 8.8% to $28.1 million for the full year, compared to the full year of 2019. |
| ● | Gross profit for the fourth quarter of 2020 improved by $364 thousand and by $1.4 million for the full year, compared to each respective prior year period. |
| ● | Net loss improved by $207 thousand and $6.8 million for the fourth quarter of 2020 and full year, respectively. |
Corporate Business Highlights
| ● | On January 13, 2020, we appointed Roger O’Brien as a new member of our Board of Directors and appointed Nora Sullivan, an existing director of the Board, as the new Board Chair. Since 2000, Mr. O’Brien has served as President of O’Brien Associates, LLC, a general management consulting firm providing advisory and implementation services to companies in a variety of competitive industries, with a special focus on general management, technology commercialization, marketing, and strategy development. Ms. Sullivan has served as a director of 22nd Century Group since May 18, 2015. Ms. Sullivan is also currently President of Sullivan Capital Partners, LLC, a financial services company providing investment banking and consulting services to businesses seeking growth through acquisitions, strategic partnerships, and joint venture relationships. Her experience includes the development and advancement of strategic initiatives and the implementation of best practice governance policies. |
34
| ● | On June 3, 2020, we appointed James A. Mish as our Chief Executive Officer and John Franzino as our Chief Financial Officer. Mr. Mish brings extensive global executive leadership experience in science-driven organizations with a recent focus on the development, manufacturing, and commercialization of active pharmaceutical ingredients (“API”), including cannabinoids, and related consumer products. He has an outstanding track record of delivering profitable growth at both privately held and publicly traded companies. Prior to joining 22nd Century Group, Mr. Franzino served as Chief Financial Officer of the West Point Association of Graduates. Additionally, he has extensive strategic financial leadership experience serving as Vice President of Finance and Controller at Bard College; as Chief Financial Officer of Santa Fe Natural Tobacco Company, a subsidiary of Reynolds American, Inc.; and as Chief Financial Officer of Labatt USA. Franzino is a Certified Public Accountant (CPA) and holds a Master of Business Administration degree from Fairleigh Dickinson University. |
| ● | On September 17, 2020, we appointed Michael Koganov Sc.D., Ph.D., to our Board of Directors. Dr. Koganov is recognized as a leading expert in the development of natural products using plant biotechnology and has achieved considerable accomplishments in physico-chemistry, biochemistry, bioelectrochemistry, and biotechnology. He is credited with developing Electro-Membrane technology for the comprehensive processing of plants to produce protein concentrates and secondary metabolites. |
| ● | On January 19, 2021, we announced the dismissal with prejudice of the federal securities class action lawsuit captioned Noto. V. 22nd Century Group, Inc., 19-CV-1285 by a federal district court in the Western District of New York on January 14, 2021. The case was initially filed in the Eastern District of New York, where it was captioned Bull v. 22nd Century Group, Inc. 1:19-CV-00409. In denying the Plaintiffs’ request for an opportunity to file another amended Complaint, the Court held that “further amendment would be futile.” |
| ● | On January 28, 2021, we announced that we are moving our corporate headquarters to the up-and-coming Larkinville District in downtown Buffalo in March of 2021. Our new Buffalo office space is in a state-of-the-art, restored manufacturing facility located at 500 Seneca Street, joining other multinational technology and professional services companies. our new headquarters will accommodate all of our staff from our current office location in nearby Williamsville and has significant room for expansion. |
| ● | During February and March of 2021, our warrant holders exercised 9,577,612 warrants for cash in exchange for common stock. In connection with these exercises, we received net proceeds of $10.0 million. On March 10, 2021, our warrant holders exercised for cash the remaining 1,715,599 outstanding warrants and no outstanding warrants remain. |
Tobacco Franchise Highlights and Notable Accomplishments
| ● | We believe that our proprietary, reduced nicotine content cigarettes, VLN®, have massive global market opportunity. In 2018, the global tobacco market was worth $817 billion and of that, $714 billion, or approximately 90% of the global tobacco market is comprised of combustible cigarettes. There are more than 1 billion global and 34 million U.S. adult smokers. More than two-thirds of adult smokers want to quit, yet less than ten percent of them are able to quit successfully. We believe that smokers are actively seeking alternatives to addictive combustible cigarettes. Based on our consumer perception studies, 60% of adult smokers indicate a likelihood to use VLN®. |
| ● | Our VLN® cigarettes contain 95% less nicotine than conventional cigarettes and are a familiar combustible product format that replicates the conventional cigarette experience, including the sensory and experiential elements of taste, scent, smell, and “hand-to-mouth” behavior. VLN® contains 0.5 milligrams of nicotine per gram of tobacco, an amount cited by the FDA, based on clinical studies, to be “minimally or non-addictive”. The lack of reward from nicotine creates a dissociation between the act of smoking and nicotine which helps adult smokers reduced the harm caused by smoking. |
| ● | Since 2011, our reduced nicotine content cigarettes have been used in more than 50 independent scientific clinical studies by universities and institutions. The studies, using our reduced nicotine content tobacco cigarettes, show that smokers who use our products: (i) reduce their nicotine exposure and dependence, (ii) smoke fewer cigarettes per day, (iii) increase their number of smoke-free days, and (iv) double their quit attempts – all with minimal or no evidence of nicotine withdrawal or compensatory smoking. |
35
| ● | In December 2019, the FDA granted a PMTA authorization for our reduced nicotine content cigarettes, giving us the ability to sell the product. In order to market our reduced nicotine content cigarettes under the brand name VLN®, with pack and advertising claims stating that the product contains 95% less nicotine than conventional tobacco cigarettes, as well as related claims regarding nicotine exposure, we will need to secure an MRTP authorization from the FDA. |
| ● | As a part of the MRTP application process, on February 14, 2020, we presented our MRTP application for our reduced nicotine content cigarettes, VLN® to the FDA’s Tobacco Products Scientific Advisory Committee (TPSAC) and passed one of the final regulatory milestones. On April 17, 2020, the FDA set May 18, 2020, as the deadline for the submission of public comments on our MRTP application. The public comment period is now closed and we believe that we are in the final stages of the review and decision making process. |
| ● | Throughout 2020, we were and remain focused on our primary mission and highest, near-term priority of securing MRTP authorization for our proprietary, reduced nicotine content tobacco cigarettes, VLN®. We are maintaining our dialogue with the FDA and are confident that the Agency is in the final stages of the review process related to our application. In addition to our ongoing contact with the FDA, we have been and are working with various legal advisors, regulatory consultants, and government affairs specialists to highlight the public health importance of our MRTP application to encourage a near-term authorization of our application. |
| ● | We are prepared to launch VLN® within 90 days of receiving MRTP authorization from the FDA and are in advanced discussions with potential partners in the independent, regional, and national retail trade. We anticipate a phased rollout of VLN® in select geographies and plan to position VLN® in the premium pricing segment of the cigarette market. On January 11, 2021, we announced that we will expand our growing program and increase planting in the 2021 crop year for VLN® reduced nicotine content tobacco. This new planting for VLN® tobacco is in addition to our current VLN® inventory, which we plan to use for initial sales of VLN®. |
| ● | We believe that recent political changes will likely be favorable to our business prospects from a policy priority and regulatory standpoint. Under the new administration, we believe that the FDA will refocus on implementing its ground-breaking Comprehensive Plan for Tobacco and Nicotine Regulation, in particular the agency’s plan to cap the amount of nicotine in combustible cigarettes to a “minimally or non-addictive” level. We believe that the MRTP authorization and the launch of VLN® will serve as a starting point for the FDA’s proposed mandate. |
| ● | Our research cigarettes, SPECTRUM®, continue to fuel numerous independent, scientific studies to validate the enormous public health benefit identified by the FDA and others of implementing a national standard requiring all cigarettes to contain “minimally or non-addictive” levels of nicotine. In December 2020, the FDA in coordination with the National Institute on Drug Abuse (NIDA) and others, submitted an order to us for 3.6 million variable nicotine research cigarettes. |
| ● | We believe that our next generation, non-GMO, plant research is the key to commercializing our reduced nicotine content tobacco and technology in international markets where non-GMO products are preferred, or GMO products are banned. We continue to make progress in our non-GMO tobacco research. In partnership with North Carolina State University, have completed successful research field trials that have validated new non-GMO methodologies for reducing nicotine in tobacco plants and have consistently achieved reductions in nicotine levels by as much as 99%. During the fourth quarter of 2020, we announced that we were granted a new U.S. patent, No. 10,669,552, entitled “Up-regulation of auxin response factor NbTF7”, related to the reduction of nicotine in the tobacco plant. The new technology provides us with a rapid pathway to introduce very low nicotine traits into virtually any variety of tobacco, including bright, burley, and oriental tobacco varieties. We have successfully applied our non-GMO technology to bright and burley varieties of tobacco and have developed a VLN® 2.0 prototype cigarette. We are also using our non-GMO technology to introduce reduced nicotine traits into oriental varieties of tobacco. |
Hemp/Cannabis Franchise Highlights and Notable Accomplishments
| ● | We continue to place an emphasis on our hemp/cannabis strategy to target the upstream segments of the cannabinoid value chain in the areas of plant biotechnology research, gene modification and engineering, modern plant breeding and development, and extraction. We believe that we can differentiate ourselves in the hemp/cannabis industry by building upon our core strength and expertise in plant science and the ingredient value chain and through our strategic, operational partnerships, including the addition of our new partner, CannaMetrix. |
36
| We continue to shift our focus away from the already saturated U.S. consumer market of cannabidiol (CBD) and hemp-based products and expect to gain ingredient cultivation capabilities and extraction and purification services through a non-binding agreement with Panacea, which is expected to provide us with operational assets, including a farm and various extraction and distillation equipment. |
| ● | We developed and launched a new, cutting-edge technology platform that will enable us and our strategic partners to quickly identify and incorporate commercially valuable traits of hemp/cannabis plants to create new, stable hemp/cannabis lines. The platform, developed in collaboration with researchers at KeyGene, incorporates a suite of proprietary molecular tools and a large library of genomic markers and gene-trait correlations. We have already characterized millions of high-value single nucleotide polymorphisms (SNPs). By targeting these newly identified SNPs, we have been able to locate and isolate specific sections of genetic code from genome assemblies present in our state-of-the-art hemp/cannabis bioinformatics database. This breakthrough enables us to quickly and easily identify the genes responsible for specific traits in a plant and is a powerful tool for us and the hemp/cannabis industry. We have already begun discussions to license this platform to strategic partners to help them improve their plant breeding techniques and optimize their hemp/cannabis lines. |
| ● | We continue to secure commercially, valuable patents and intellectual property through our internal research capabilities and external research partnerships. We were recently granted a new U.S. Patent No. 10,787,674 B2 entitled “Trichome specific promoters for the manipulation of cannabinoids and other compounds in glandular trichomes”. This new intellectual property enables us to develop and deliver new hemp/cannabis plants that are designed to produce cannabinoids more efficiently by activating the molecular promoters, “on/off switches,” specifically and only in the plant’s trichomes where the majority of cannabinoids are produced. The patent application describes eight promoters, which are essentially molecular on/off switches, covering all of the major steps in the cannabinoid biosynthesis pathway and is related to the control of cannabinoid and terpene production. |
| ● | We have secured an exclusive agreement with CannaMetrix, LLC for the use of their proprietary, human cell-based testing CannaMetrix EC50Array™ technology that will enable us to accelerate the commercialization of new, disruptive hemp/cannabis plant lines and intellectual property. CannaMetrix’s proprietary CannaMetrix EC50Array™ technology serves as a high-throughput roadmap for developing new hemp/cannabis plant lines with tailor-made cannabinoid and terpenoid profiles for use in the life science, consumer product, and pharmaceutical markets. The human cell-based assay has the ability to measure and validate the potency and efficacy of cannabinoids and/or terpenoids through defined biomarkers and receptor activity and can rapidly identify optimum plant profiles by measuring the potency and effect on the human cell system. |
| ● | We believe that we can accelerate the development of commercially, valuable hemp/cannabis lines and related intellectual property through selective partnerships and have now secured four out of the five key partnerships needed to maximize each component in the upstream segment of the cannabinoid value chain. |
2021 Priorities and Areas of Focus
| 1. | We remain focused on securing FDA authorization for VLN®. Starting within 90-days of authorization of our MRTPA by the FDA, we are prepared to launch VLN® cigarettes in select markets. |
| 2. | We believe that an equally important first priority initiative is for us to support and advance the FDA’s plan to require that all cigarettes sold in the U.S. be made “minimally or non-addictive” by limiting their nicotine content to just 0.5 milligrams of nicotine per gram of tobacco. |
| 3. | We continue to target the upstream segment of the cannabinoid value chain; creating proprietary, commercially valuable new plant lines and related intellectual property with stabilized genetics to harness and optimize hemp/cannabis plant potential. We will seek to monetize a portion of our existing hemp/cannabis IP in 2021 and will continue to bring disruptive technology forward. |
| 4. | We will turn attention to the development of a third, plant-based franchise after securing MRTP authorization for VLN®. We will leverage our plant science expertise to develop and secure valuable intellectual property and sign lucrative strategic partnerships to support the development of this franchise. |
| 5. | We will maintain diligent financial execution, efficient operating structure, and balance sheet strength to support our growth initiatives. |
37
Results of Operations
Year Ended December 31, 2020 compared to Year Ended December 31, 2019.
Amounts in thousands, except for share and per-share data
Revenue - Sale of products, net
| Year Ended | |||||
| December 31 | December 31 | ||||
|
| 2020 |
| 2019 | ||
Sale of products, net | $ | 28,111 | $ | 25,833 | ||
Dollar Change from Prior Year | $ | 2,278 | ||||
The increase in sales revenue for the year ended December 31, 2020, compared to the year ended December 31, 2019, was primarily the result of an increase in sales revenue of contract manufactured cigarettes. Filtered cigar sales revenue increased by $3 compared to the prior year, while cigarette sales revenue increased $2,275 compared to the prior year. The increase in filtered cigar sales was primarily driven by a volume decrease offset by price increases, compared to the prior period. The increase in cigarette sales revenue was driven by both volume and price increases compared to the prior period.
Costs of goods sold – Products / Gross profit (loss)
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Cost of goods sold | $ | 26,673 | $ | 25,818 | |||
Percent of Product Sales | 94.9 | % | 99.9 | % | |||
Dollar Change from Prior Year | $ | 855 | |||||
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Gross profit (loss) | $ | 1,438 | $ | 15 | |||
Percent of Product Sales | 5.1 | % | 0.1 | % | |||
Dollar Change from Prior Year | $ | 1,423 | |||||
For the year ended December 31, 2020, the increase in gross profit was primarily driven by increased cigarette sales volume, increased cigarette pricing, and lower labor and overhead costs per unit driven by factory efficiencies implemented during 2020. In addition, gross margin was impacted favorably due to a CARES Act employee retention credit which reduced payroll taxes by $112.
Research and development expense
| Year Ended | ||||||
| December 31 | December 31 | |||||
| 2020 |
| 2019 | ||||
Research and Development | $ | 4,090 | $ | 6,381 | |||
Percent of Product Sales |
| 14.5 | % | 24.7 | % | ||
Dollar Change from Prior Year |
| $ | (2,291) | ||||
Lower R&D expense in the year ended December 31, 2020, as compared to the prior year, was primarily driven by a decrease in R&D personnel expense and a decrease in tobacco leaf inventory impairment. Personnel expense decreased by $1,285 year over year, due to more focused R&D headcount to accomplish our strategies. Tobacco leaf inventory impairment decreased by $625 year over year. Additionally, license and contract costs decreased $213
38
compared to prior year. The lower license and contract spending is primarily a result of 2019 milestone payments for certain research agreements which were not due in the current year. We continue to prioritize our R&D activities to achieve our strategic objectives.
Research and development expense—MRTP
| Year Ended | ||||||
| December 31 | December 31 | |||||
| 2020 |
| 2019 | ||||
Research and Development - MRTP | $ | 38 | $ | 1,679 | |||
Percent of Product Sales |
| 0.1 | % | 6.5 | % | ||
Dollar Change from Prior Year |
| $ | (1,641) | ||||
MRTP expenses for the year ended December 31, 2020 declined significantly, as we submitted our MRTP application to the FDA during 2019. MRTP-related expenses for 2020 are primarily related to our February 14, 2020 Tobacco Products Scientific Advisory Committee (TPSAC) hearing, offset by the finalization of a 2019 cigarette cessation study which resulted in a reimbursement of $124.
Sales, general and administrative expense
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Sales, general and administrative | $ | 14,971 | $ | 12,954 | |||
Percent of Product Sales | 53.3 | % | 50.1 | % | |||
Dollar Change from Prior Year | $ | 2,017 | |||||
The increase in sales, general and administrative (“SG&A”) expense during the year ended December 31, 2020, as compared to the prior year, was driven by a $1,613 increase in insurance expenses, a $1,227 increase in consulting and professional services expenses, a $1,385 increase in personnel expense, and a $131 increase in marketing expenses. We have deployed this incremental SG&A spending to place new members of the management team, including our CEO, to support our efforts in governmental affairs related to the approval of our MRTP application, as well as to conduct pre-market research in anticipation of the authorization of our MRTP filing for VLN®. These increases in SG&A were partially offset by decreased one-time severance expenses of $415, and decreased equity compensation expense of $1,639—both driven primarily by management changes in the third quarter of 2019. Additionally, legal fees decreased by $435 compared to the prior year.
Impairment of intangible assets
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Impairment of intangible assets | $ | 176 | $ | 1,142 | |||
Percent of Product Sales |
| 0.6 | % | 4.4 | % | ||
Dollar Change from Prior Year |
| $ | (966) | ||||
During the years ended December 31, 2020 and 2019, management conducted a review of intellectual property assets and determined that impairment was required for certain patent and trademark costs. As such, we recorded impairment charges of approximately $176 and $1,142 for the current year and prior year, respectively. Refer to Note 5 to our consolidated financial statements for additional information.
39
Depreciation expense
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Depreciation | $ | 688 | $ | 589 | |||
Percent of Product Sales | 2.4 | % | 2.3 | % | |||
Dollar Change from Prior Year | $ | 99 | |||||
The increase in depreciation expense was primarily due to additional machinery and equipment acquisitions during 2020 and 2019 to for our production facility in North Carolina.
Amortization expense
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Amortization | $ | 658 | $ | 836 | |||
Percent of Product Sales |
| 2.3 | % | 3.2 | % | ||
Dollar Change from Prior Year |
| $ | (178) | ||||
Amortization expense relates to amortization taken on capitalized patent costs and license fees. The decrease was primarily due to amortization expense on a lower patent base which resulted from one-time impairment charges as described in the “Impairment expense” section above.
Unrealized (loss) gain on investments
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Unrealized gain (loss) on investments | $ | (434) | $ | (2,419) | |||
Percent of Product Sales |
| (1.5) | % | (9.4) | % | ||
Dollar Change from Prior Year |
| $ | 1,985 | ||||
The warrants to purchase 81,164 shares of Aurora Cannabis, Inc (“Aurora”) common stock are considered an equity security and are recorded at fair value. We recorded the fair value of the stock warrants of $239 at December 31, 2020, using the Black-Scholes pricing model, and recorded an unrealized loss on the warrants in the amount of $434 for the year ended December 31, 2020.
Impairment of Panacea Investment
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Impairment of Panacea Investment | $ | (1,741) | $ | — | |||
Percent of Product Sales |
| (6.2) | % | — | % | ||
Dollar Change from Prior Year |
| $ | (1,741) | ||||
During 2020, we incurred impairment charges on our investment in Panacea. Refer to Note 6 to our consolidated financial statements for additional information regarding our investment in Panacea.
40
Realized gain (loss) on short-term investment securities
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Realized gain (loss) on short-term investment securities | $ | 5 | $ | 221 | |||
Percent of Product Sales | 0.0 | % | 0.9 | % | |||
Dollar Change from Prior Year | $ | (216) | |||||
The realized gain on short-term investment securities for the year ended December 31, 2020 was $5, a decrease of $216 from the gain of $221 realized for the year ended December 31, 2019. The realized gain on short-term investment securities was the result of the maturity of various debt instruments held in the short-term investment account. Investments in the short-term investment account are managed in accordance with our investment policy.
Litigation settlement expense
We incurred an expense relating to the settlement agreement in the Crede litigation in the amount of $1,891 for the year ended December 31, 2019. We had no litigation settlement expenses for the year ended December 31, 2020.
Gain on the sale of machinery and equipment
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Gain on the sale of machinery and equipment | $ | 1 | $ | 87 | |||
Percent of Product Sales | — | % | 0.3 | % | |||
Dollar Change from Prior Year | $ | (86) | |||||
We recognized a small gain on equipment sold during the year ended December 31, 2020. During the year ended December 31, 2019, we sold a piece of machinery and equipment no longer required in our factory operations in North Carolina and recorded a gain on the sale in the amount of $87.
Interest income, net
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Interest Income, net | $ | 1,751 | $ | 1,066 | |||
Percent of Product Sales | 6.2 | % | 4.1 | % | |||
Dollar Change from Prior Year | $ | 685 | |||||
Interest income, net (interest income less investment fees) for the year ended December 31, 2020 is comprised of cash interest on our convertible note receivable and short-term investment securities, and non-cash interest accretion on our convertible note, preferred stock investment in Panacea, and short-term investment securities purchased at a discount or premium. During 2020, the increase in interest income, net was driven by an increase in non-cash interest income, net of $347 and an increase in cash interest income, net of $338.
Interest expense
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Interest Expense | $ | (72) | $ | (56) | |||
Percent of Product Sales |
| (0.3) | % | (0.2) | % | ||
Dollar Change from Prior Year |
| $ | (16) | ||||
41
Interest expense for 2020 was $16 higher than the prior year. Interest expense for the year ended December 31, 2020 and 2019 related to interest accretion on notes payable to NCSU and the University of Kentucky, and interest accretion on accrued severance. 2020 also included cash interest expense of $29 on notes payable for D&O insurance.
Net loss
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Net Loss | $ | (19,711) | $ | (26,558) | |||
Percent of Product Sales |
| (70.1) | % | (102.7) | % | ||
Dollar Change from Prior Year |
| $ | 6,847 | ||||
The decrease in net loss for the year ended December 31, 2020 compared to the prior year ended December 31, 2019, was primarily the result of improved gross profit, lower total operating expenses, one-time 2019 litigation settlement costs that did not repeat in 2020, lower unrealized loss on our Aurora warrants, and increased interest income primarily related to the Panacea investment. These impacts were somewhat offset by 2020 impairment charges on our Panacea investment (see Note 6 to our consolidated financial statements).
Other comprehensive income
| Year Ended | ||||||
| December 31 | December 31 | |||||
|
| 2020 |
| 2019 | |||
Other Comprehensive Income (Loss) | $ | 67 | $ | (14) | |||
Percent of Product Sales |
| 0.2 | % | (0.1) | % | ||
Dollar Change from Prior Year |
| $ | 81 | ||||
We maintain an account for short-term investment securities that are classified as available-for-sale securities and consist of money market funds and corporate bonds with maturities greater than three months at the time of acquisition. Unrealized gains and losses on short-term investment securities (the adjustment to fair value) are recorded as other comprehensive income or loss. We recorded an unrealized gain on short-term investment securities in the amount of $72 and a reclassification of gains to net loss of $5, resulting in other comprehensive income of $67 for the year ended December 31, 2020. For the year ended December 31, 2019, we recorded an unrealized gain on short-term investment securities of $207 and a reclassification of gains to net loss of $221, resulting in other comprehensive loss of $14.
Liquidity and Capital Resources
| Year-to-Date | |||||
| December 31 | December 31 | ||||
|
| 2020 |
| 2019 | ||
Net cash used in operating activities | $ | (15,621) | $ | (14,588) | ||
Net cash provided by investing activities |
| 16,469 |
| 4,552 | ||
Net cash provided by (used) in financing activities |
| (304) |
| 9,916 | ||
Net increase (decrease) in cash and cash equivalents |
| 544 |
| (120) | ||
Cash and cash equivalents - beginning of period |
| 485 |
| 605 | ||
Cash and cash equivalents - end of period | $ | 1,029 |
| $ | 485 | |
Short-term investment securities | $ | 21,313 |
| $ | 38,477 | |
Working Capital
As of December 31, 2020, we had working capital of approximately $20,998 compared to working capital of approximately $36,962 as of December 31, 2019, a decrease of $15,964. This decrease in working capital was primarily due to a decrease in net current assets of approximately $14,402, as well as an increase in net current liabilities of approximately $1,562. Cash, cash equivalents and short-term investment securities decreased by approximately $16,620
42
and the remaining net current assets increased by approximately $2,218. We used approximately $15,621 of cash in operating activities during the year ended December 31, 2020. Our cash balance as of December 31, 2020 (inclusive of cash and cash equivalents and short-term investments) was $22,342, a decrease of $16,620 from the cash balance of $38,962 as of December 31, 2019. This equates to an average monthly cash usage of $1,385 for the year ended December 31, 2020.
Net cash used in operating activities
The increase in cash used in operations in the amount of $1,033 was due to an increase in cash used for working capital components related to operations in the amount of $2,270 which was partially offset by a decrease in the cash used from the portion of the net loss in the amount of $1,237 for the year ended December 31, 2020 as compared to the year ended December 31, 2019. The increase in cash used for working capital components was primarily due to an increase in pre-paid D&O insurance, which is described further in Note 8 to our consolidated financial statements, and an increase in accounts receivable due to timing of collections.
Net cash provided by (used in) investing activities
The increase in cash provided by investing activities, in the amount of $11,917 when compared to the prior year was primarily attributed to the 2019 acquisition of Panacea which totaled $12,000. In addition, cash flows decreased for the acquisition of patents and trademarks, machinery and equipment in the net amount of $570. These increased cash inflows were partially offset by a decrease in the net proceeds received from our short term investments in the amount of $493 and decreased proceeds from the sale of machinery and equipment in the amount of $160.
Net cash provided by (used in) financing activities
During the year ended December 31, 2020, cash used in by financing activities was $304 primarily due to principal payments on our notes payable of $2,549 which was partially offset by $2,195 of proceeds received from a note payable issuance. Refer to Note 8 to our consolidated financial statements for additional information.
During the year ended December 31, 2019, we realized $9,916 in proceeds from our financing activities resulting from net proceeds from the exercise of warrants in the amount of $10,616, partially offset by payments on notes payable in the amount of $700.
Cash demands on operations
Our principal sources of liquidity are our cash and cash equivalents, short-term investment securities, and cash generated from our contract manufacturing business. As of December 31, 2020, we had approximately $22,342 of cash and cash equivalents and short-term investments which is a decline of $16,620 from December 31, 2019. During 2020, our average monthly cash usage was approximately $1,385, compared to $1,449 in 2019. Our short-term investment securities along with sustained contract manufacturing sales provide sufficient resources for estimated contractual commitments for 2021, described further in Note 12 to our consolidated financial statements, and normal cash requirements for operations. In addition to the commitments described in Note 12 to our consolidated financial statements, we are currently in the process of securing contracts with select tobacco farmers to assist with the 2021 growing of our VLNC tobacco. These contacts, once finalized and executed, will increase the quantity of our current leaf inventory which will help support expected demand of VLN®, if MTRP authorization is granted by the FDA. The cost of such growing efforts is dependent on the final agricultural yields and leaf quality, but we expect the cost to range between $1.5 million and $1.9 million.
We also believe that we have appropriate liquidity to successfully manufacture and distribute our VLN® cigarette within 90 days of MRTP authorization by the FDA, if granted in 2021.
During February and March of 2021, our warrant holders exercised 9,577,612 warrants for cash in exchange for common stock. In connection with these exercises, we received net proceeds of $9,993. On March 10, 2021, our warrant holders exercised for cash the remaining 1,715,599 outstanding warrants and no outstanding warrants remain. We also have an effective S-3 shelf registration statement on file with the U.S. Securities and Exchange Commission (SEC),
43
which provides us flexibility and optionality to raise capital, however there can be no assurance that capital will be available to us on acceptable terms or at all.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) which requires management to make estimates, judgements, and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. For a discussion of our significant accounting policies, refer to Note 1 to our consolidated financial statements. We believe that our most critical accounting estimates relate to investment valuation and impairment of long-lived intangible assets.
Investment Valuation –We have two investments—our investment in Panacea Life Sciences, Inc. (“Panacea”) and Aurora Cannabis, Inc. (“ACB”)—that require us to estimate fair value and assess for impairment indicators as of each period-end. Our investment in Aurora consists of stock warrants that are exercisable into common stock at a predetermined exercise price. We estimate the Aurora stock warrant fair value using the Black-Scholes pricing model noting one unobservable input—an estimated volatility factor based on the underlying stock price of ACB. Therefore, changes in market volatility will impact the fair value measurement for our investment.
Our investment in Panacea includes three instruments: Series B preferred stock (“preferred stock”), a convertible note receivable, and a stock warrant to purchase additional shares of Series B preferred stock. The preferred stock and convertible note receivable are recorded as available-for-sale debt securities, while the stock warrant is recorded at its cost basis in accordance with the practicability exception under ASU 2016-01. We consider the current market conditions of the cannabidiol (“CBD”) consumer product industry, competition, governmental regulation developments, our preferred stock priority equity preferences and rights, and the overall finance performance of Panacea to assess if fair value supports our investment carrying value.
Refer to Note 6 and Note 7 to our consolidated financial statements for additional information regarding our investment valuations.
Impairment of Long-Lived Assets – Our intangible asset portfolio consists of both definite-lived and indefinite-lived intangible assets which include patents, trademarks, licenses, and our inclusion within the tobacco master settlement agreement (“MSA”). Our intangible assets subject to amortization are reviewed for strategic importance and commercialization opportunity prior to expiration. If it is determined that the asset no longer supports the Company’s strategic objectives and/or will not be commercially viable prior to expiration, the asset is impaired. To determine if an asset’s carrying value is appropriate, we are required to estimate the expected commercialization of our tobacco and cannabis intellectual property—either through future product sales or potential license opportunities. This estimate process includes expected future cash flow projections, industry market assessments, and assumptions around positive regulatory developments from government agencies—including authorization of our MRTPA.
For our indefinite-lived intangible assets—MSA and cigarette brand predicate—we consider current and future sales projections, strategic objectives, future market and economic conditions, competition, and federal and state regulations to determine if it is more likely than not that that the asset is impaired. If it is more likely than note that the asset is impaired, we will compare the asset carrying value to fair value.
Management has discussed these critical accounting policies and estimates with the Audit Committee of the Company’s Board of Directors. While our estimates and assumptions are based on our knowledge of current events and future actions, actual results may ultimately differ from these estimates and assumptions.
Inflation
Inflation did not have a material effect on our operating results for the years ended December 31, 2020 and 2019.
Off-Balance Sheet Arrangement
We do not have any off-balance sheet arrangements as defined by Item 303(a)(4) of Regulation S-K.
44
Item 8.Financial Statements and Supplementary Data.
The required financial statements and the notes thereto are contained in a separate section of this Form 10-K beginning with the page following Item 15 (Exhibits and Financial Statement Schedules, including Selected Quarterly Financial Data).
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None
Item 9A.Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the "Exchange Act"). Based on this evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report on Form 10-K to ensure information required to be disclosed in the reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported, within the time period specified in the SEC’s rules and forms. These disclosure controls and procedures include controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit is accumulated and communicated to management, including our president and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework (2013), our management concluded that our internal control over financial reporting was effective as of December 31, 2020.
This Annual Report on Form 10-K does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting due to the change in definition of accelerated filer.
Our system of internal control over financial reporting was designed to provide reasonable assurance regarding the preparation and fair presentation of published financial statements in accordance with accounting principles generally accepted in the United States. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Controls over Financial Reporting
There was no change in the Company’s internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) during the quarter ended December 31, 2020 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
45
Item 9B.Other Information.
None.
PART III
Item 10.Directors, Executive Officers and Corporate Governance.
Information concerning our executive officers, directors and corporate governance is incorporated herein by reference to our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Form 10-K with respect to its 2021 Annual Meeting of Stockholders.
Set forth below is information regarding our directors, executive officers and key personnel as of March 11, 2021:
Name |
| Age |
| Position | |
James A. Mish | 57 | Chief Executive Officer | |||
Michael Zercher | 50 | President and Chief Operating Officer | |||
John Franzino |
| 64 |
| Chief Financial Officer & Treasurer | |
Dr. Michael Koganov |
| 70 |
| Director | (1) |
Richard M. Sanders |
| 68 |
| Director | (2) |
Nora B. Sullivan |
| 63 |
| Director | (3) |
Clifford B. Fleet |
| 50 |
| Director | (4) |
Roger D. O’Brien |
| 72 |
| Director | (5) |
| (1) | Since 2019, Dr. Koganov has served as President and Co-Founder of Intellebio LLC, a consulting and testing firm focused on the development of novel technologies, advanced test methods, and breakthrough products in the life science field. |
| (2) | Since August 2009, Mr. Sanders has served as a General Partner of Phase One Ventures, LLC, a venture capital firm which focuses on nanotechnology and biotechnology start-up opportunities in New Mexico and surrounding states. Mr. Sanders is also an active Angel and private placement investor. |
| (3) | Since May 18, 2015, Ms. Sullivan is President of Sullivan Capital Partners, LLC, a financial services company providing investment banking and consulting services to businesses seeking growth through acquisitions or strategic partnerships. Ms. Sullivan focuses on due diligence, deal structure, strategic planning and governance matters. |
| (4) | Since January 2020, Mr. Fleet has served as President and CEO of the Colonial Williamsburg Foundation. Prior to that, Mr. Fleet previously served as the President and Chief Executive Officer of the Company from August 3, 2019 until December 13, 2019 and served as a strategic advisor consultant to the Company from December 2018 to August 3, 2019. Prior to that, Mr. Fleet served as President and CEO of Philip Morris USA. |
| (5) | Since 2000 Mr. O’Brien has been the President of O’Brien Associates, LLC, a general management consulting firm providing advisory and implementation services to companies in a variety of competitive industries, with special focus on general management, technology commercialization, organizational development and strategy. Mr. O’Brien has also served as an officer of several publicly held companies, including Sun Microsystems and Ultralife Batteries, Inc. |
Code of Ethics
In 2006, we adopted a Code of Ethics that applies to all our employees. A copy of our Code of Ethics is available on our website at http://www.xxiicentury.com and will be provided to any person requesting same without charge. To request a copy of our Code of Ethics, please make a written request to our General Counsel, c/o 22nd Century Group, Inc., 8560 Main Street, Suite 4, Williamsville, New York 14221. Future material amendments or waivers relating
46
to the Code of Ethics will be disclosed on our website within four business days following the date of such amendment or waiver.
Item 11.Executive Compensation.
Information is incorporated herein by reference to our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Form 10-K with respect to its 2021 Annual Meeting of Stockholders.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information is incorporated herein by reference to our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Form 10-K with respect to its 2021 Annual Meeting of Stockholders.
Item 13.Certain Relationships and Related Transactions, and Director Independence.
Information is incorporated herein by reference to our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Form 10-K with respect to its 2021 Annual Meeting of Stockholders.
Item 14.Principal Accounting Fees and Services.
Information is incorporated herein by reference to our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the end of the fiscal year covered by this Form 10-K with respect to its 2021 Annual Meeting of Stockholders.
47
PART IV
Item 15.Exhibits and Financial Statement Schedules.
| (a) | Financial Statements |
| (b) | Financial Statement Schedules |
|
| Page |
| F-2 | |
|
|
|
Consolidated Financial Statements: |
|
|
|
|
|
| F-5 | |
|
|
|
Consolidated Statements of Operations and Comprehensive Loss |
| F-6 |
|
|
|
| F-7 | |
|
|
|
| F-8 | |
|
|
|
| F-9 – F-30 |
Item 16. Form 10-K Summary
None.
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
22nd Century Group, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of 22nd Century Group, Inc. and Subsidiaries (the "Company") as of December 31, 2020 and 2019, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2020, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Valuation of Intangible Assets – impairment analysis
Critical Audit Matter Description
As discussed in Note 1 to the consolidated financial statements, intangible assets are recorded at cost and consist primarily of (1) expenditures incurred with third-parties related to the processing of patent claims and trademarks with government authorities, as well as costs to acquire patents from third parties (2) license fees paid for third-party intellectual property (3) costs to become a signatory under the tobacco Master Settlement Agreement and (4) license fees paid to acquire a predicate cigarette brand. The Company reviews the carrying value of its amortizing long-lived assets whenever events or changes in circumstances indicate that the historical cost-carrying value of an asset may no longer be recoverable. On at least an annual basis, the Company assesses whether events or changes in circumstances indicate that the carrying amount may not be recoverable. If any such indicators are present, the Company will test for recoverability. Intangible assets subject to amortization are reviewed for strategic importance and commercialization opportunity prior to expiration. If it is determined that the asset no longer supports the Company’s strategic objectives and/or will not be commercially viable prior to expiration, the asset is impaired. In addition, the Company will assess the expected future undiscounted
F-2
cash flows for its intellectual property based on consideration of future market and economic conditions, competition, federal and state regulations, and licensing opportunities. If the carrying value of such assets are not recoverable, the carrying value will be reduced to fair value. Indefinite-lived intangible asset carrying values are reviewed at least annually or more frequently if events or changes in circumstances indicate that it is more likely than not that an impairment exists. The Company first performs a qualitative assessment and considers its current strategic objectives, future market and economic conditions, competition, and federal and state regulations to determine if an impairment is more likely than not.
Because the Company has not fully commercialized its core intellectual property and the markets for those products are not yet developed, the Company assesses recoverability by reviewing the strategic importance and commercialization opportunities and making assumptions related to future market and economic conditions, competition, federal and state regulations, and licensing opportunities. Due to the magnitude of intangible assets and subjectivity of these assumptions, we identified the impairment analysis of intangible assets as a critical audit matter, which required a high degree of auditor judgment.
Addressing the matter involved performing subjective procedures and evaluating audit evidence in connection with forming our overall opinion on the financial statements. The primary procedures we performed include: obtaining an understanding of the process and assumptions used by management to perform the impairment test; testing the completeness and accuracy of the gross and net capitalized costs by asset group used in the analysis; evaluating the reasonableness and consistency of the methodology and assumptions applied by management.
Accounting for investment instruments of Panacea Life Sciences, Inc. (“Panacea”)
Critical Audit Matter Description
As discussed in Notes 1 and 6, the investment in Panacea reported as of December 31, 2020 includes three instruments: Series B preferred stock (“preferred stock”), a convertible note receivable, and a stock warrant to purchase additional shares of Series B preferred stock. The preferred stock and convertible note receivable are recorded at fair value as available-for-sale debt securities, while the stock warrant is an equity instrument recorded at its cost basis less impairment in accordance with the practicability exception under ASU 2016-01. Panacea is a private company that is not traded in active markets.
To assess fair value and impairment, the Company must use qualitative factors when considering change to fair value for the debt securities and potential impairment to all three instruments. The Company considers the current market conditions of the cannabidiol (“CBD”) consumer product industry, competition, governmental regulation developments, preferred stock priority equity preferences and rights, and the overall financial performance of Panacea to assess if fair value supports the investment’s carrying value.
Additionally, as discussed in Note 6 to the consolidated financial statements, the Company entered into a non-binding agreement to restructure the investment and business relationship with Panacea. Under the terms of this agreement being negotiated, the convertible note receivable, accrued interest on the note receivable, and the equity warrant would be exchanged for a combination of certain operational assets. Certain other amendments to the securities purchase agreement are also being considered. Management included consideration of this non-binding agreement when estimating fair value and potential impairment of the Panacea investment.
Due to the magnitude of this investment and subjectivity of the qualitative factors used to value the investment, we identified fair value accounting of the Panacea investment instruments as a critical audit matter, which required a high degree of auditor judgment.
F-3
Addressing the matter involved performing subjective procedures and evaluating audit evidence in connection with forming our overall opinion on the financial statements. The primary procedures we performed include: evaluating assumptions related to change in fair value, evaluating the reasonableness and consistency of the methodology and assumptions applied by management in their impairment analysis including understanding the terms of the non-binding agreement and consideration of fair value of assets to be acquired in the exchange, how management gained comfort over forecasts used in the preferred shares impairment model; and consideration of the preferred shares equity preferences and rights, along with reasonableness of other assumptions and qualitative factors used in Management’s impairment analysis.
/s/ Freed Maxick, CPAs, P.C. | |
We have served as the Company’s auditor since 2011. | |
Buffalo, New York | |
March 11, 2021 |
F-4
22nd CENTURY GROUP, INC. AND SUBSIDIARIES | |
CONSOLIDATED BALANCE SHEETS | |
($ in thousands) | |
December 31, | December 31, | |||||
|
| 2020 |
| 2019 | ||
ASSETS |
|
|
|
| ||
Current Assets: |
|
|
|
| ||
Cash and cash equivalents | $ | 1,029 | $ | 485 | ||
Short-term investment securities |
| 21,313 |
| 38,477 | ||
Accounts receivable, net |
| 2,159 |
| 867 | ||
Inventory, net |
| 2,034 |
| 2,266 | ||
Prepaid expenses and other assets |
| 1,806 |
| 648 | ||
Total current assets |
| 28,341 |
| 42,743 | ||
Property, plant and equipment: |
|
|
|
| ||
Machinery and equipment, net |
| 2,483 |
| 3,120 | ||
Operating leases right-of-use assets, net |
| 247 |
| 602 | ||
Total property, plant and equipment |
| 2,730 |
| 3,722 | ||
Other assets: |
|
|
| |||
Intangible assets, net |
| 8,211 |
| 8,494 | ||
Investments |
| 6,536 |
| 8,403 | ||
Convertible note | 5,876 | 5,589 | ||||
Total other assets |
| 20,623 |
| 22,486 | ||
Total assets | $ | 51,694 | $ | 68,951 | ||
|
|
|
| |||
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
| ||
Current liabilities: |
|
|
|
| ||
Notes payable | $ | 539 | $ | 581 | ||
Operating lease obligations |
| 247 |
| 220 | ||
Accounts payable |
| 1,116 |
| 1,997 | ||
Accrued expenses |
| 4,830 |
| 2,619 | ||
Accrued severance |
| 339 |
| 359 | ||
Deferred income |
| 272 |
| 5 | ||
Total current liabilities |
| 7,343 |
| 5,781 | ||
Long-term liabilities: |
|
|
|
| ||
Notes payable |
| — |
| 292 | ||
Operating lease obligations |
| — |
| 382 | ||
Severance obligations | 241 | 446 | ||||
Total liabilities | 7,584 | 6,901 | ||||
Commitments and contingencies (Note 12) |
|
| ||||
Shareholders' equity |
|
|
|
| ||
10,000,000 preferred shares, $.00001 par value |
|
|
|
| ||
300,000,000 common shares, $.00001 par value |
|
|
|
| ||
Capital stock and : |
|
|
|
| ||
139,061,690 common shares (138,362,809 at December 31, 2019) |
|
| ||||
Common stock value | 1 | 1 | ||||
Capital in excess of par value |
| 189,439 |
| 187,735 | ||
Accumulated other comprehensive (loss) income |
| 74 |
| 7 | ||
Accumulated deficit |
| (145,404) |
| (125,693) | ||
Total shareholders' equity |
| 44,110 |
| 62,050 | ||
Total liabilities and shareholders’ equity | $ | 51,694 | $ | 68,951 | ||
See accompanying notes to consolidated financial statements.
F-5
22nd CENTURY GROUP, INC. AND SUBSIDIARIES | |
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS ($ in thousands except per-share data) | |
Year Ended | |||||||||
December 31, | |||||||||
| 2020 |
| 2019 |
| 2018 | ||||
Revenue: |
|
|
|
| |||||
Sale of products, net | $ | 28,111 | $ | 25,833 | $ | 26,426 | |||
Cost of goods sold (exclusive of depreciation shown separately below): |
|
|
|
|
|
| |||
Products |
| 26,673 |
| 25,818 |
| 25,527 | |||
Gross profit (loss) |
| 1,438 |
| 15 |
| 899 | |||
Operating expenses: |
|
|
|
|
|
| |||
Research and development |
| 4,090 |
| 6,381 |
| 5,215 | |||
Research and development - MRTP | 38 | 1,679 | 9,775 | ||||||
Sales, general and administrative |
| 14,971 |
| 12,954 |
| 8,585 | |||
Impairment of intangible assets | 176 | 1,142 | — | ||||||
Depreciation |
| 688 |
| 589 |
| 523 | |||
Amortization |
| 658 |
| 836 |
| 820 | |||
Total operating expenses |
| 20,621 |
| 23,581 |
| 24,918 | |||
Operating loss |
| (19,183) |
| (23,566) |
| (24,019) | |||
Other income (expense): |
|
|
|
|
|
| |||
Unrealized gain (loss) on investments |
| (434) |
| (2,419) |
| 284 | |||
Impairment of Panacea investment | (1,741) | — | — | ||||||
Realized gain on investments | — | — | 14,493 | ||||||
Realized gain (loss) on short-term investment securities |
| 5 |
| 221 |
| (54) | |||
Litigation settlement | — | (1,891) | — | ||||||
Gain on the sale of machinery and equipment |
| 1 |
| 87 |
| — | |||
Dividend income and other | — | — | 271 | ||||||
Interest income, net |
| 1,751 |
| 1,066 |
| 1,069 | |||
Interest expense |
| (72) |
| (56) |
| (11) | |||
Total other income (expense) |
| (490) |
| (2,992) |
| 16,052 | |||
Loss before income taxes |
| (19,673) |
| (26,558) | (7,967) | ||||
Income taxes |
| 38 |
| — | — | ||||
Net loss | $ | (19,711) | $ | (26,558) | $ | (7,967) | |||
Other comprehensive income (loss): |
|
|
|
|
|
| |||
Unrealized gain (loss) on short-term investment securities |
| 72 |
| 207 |
| (22) | |||
Reclassification of (gain) loss to net loss |
| (5) |
| (221) |
| 43 | |||
Other comprehensive income (loss) | 67 | (14) | 21 | ||||||
Comprehensive loss | $ | (19,644) | $ | (26,572) | $ | (7,946) | |||
Net loss per common share - basic and diluted | $ | (0.14) | $ | (0.21) | $ | (0.06) | |||
Weighted average common shares outstanding - basic and diluted (in thousands) |
| 138,813 |
| 125,883 |
| 124,299 | |||
See accompanying notes to consolidated financial statements.
F-6
22nd CENTURY GROUP, INC. AND SUBSIDIARIES | |
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY | |
($ in thousands) | |
|
|
|
|
|
|
|
|
|
|
|
| ||||||
| Years Ended December 31, 2020, 2019, and 2018 | ||||||||||||||||
Common | Par Value | Capital in | Other | ||||||||||||||
Shares | of Common | Excess of | Comprehensive | Accumulated | Shareholders' | ||||||||||||
| Outstanding |
| Shares |
| Par Value |
| Income |
| Deficit |
| Equity | ||||||
Balance at December 31, 2017 |
| 123,569,367 | $ | 1 | $ | 166,591 | $ | — | $ | (91,168) | $ | 75,424 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with warrant exercises |
| 490,012 |
| — |
| — |
| — |
| — |
| — | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with option exercises |
| 583,214 |
| — |
| 445 |
| — |
| — |
| 445 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Equity-based compensation |
| — |
| — |
| 3,187 |
| — |
| — |
| 3,187 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Reclassification of warrant liability to capital in excess of par |
| — |
| — |
| 168 |
| — |
| — |
| 168 | |||||
Unrealized gain (loss) on short-term investment securities | — | — | — | (22) | — | (22) | |||||||||||
Reclassification of losses (gains) to net loss | — | — | — | 43 | — | 43 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Net loss |
| — |
| — |
| — |
| — |
| (7,967) |
| (7,967) | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance at December 31, 2018 |
| 124,642,593 |
| 1 |
| 170,391 |
| 21 |
| (99,135) |
| 71,278 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with warrant exercises |
| 11,293,211 |
| — |
| 10,616 |
| — |
| — |
| 10,616 | |||||
|
|
|
|
|
|
|
|
|
|
| |||||||
Stock issued in connection with option exercises |
| 39,988 |
| — |
| — |
| — |
| — |
| — | |||||
|
|
|
|
|
|
|
|
|
|
| |||||||
Stock issued in connection with RSU vesting | 100,000 | — | — | — | — | — | |||||||||||
Equity-based compensation |
| — |
| — |
| 3,540 |
| — |
| — |
| 3,540 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with litigation expense |
| 990,000 |
| — |
| 1,891 |
| — |
| — |
| 1,891 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with Panacea investment |
| 1,297,017 |
| — |
| 1,297 |
| — |
| — |
| 1,297 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Unrealized gain (loss) on short-term investment securities |
| — |
| — |
| — |
| 207 |
| — |
| 207 | |||||
Reclassification of losses (gains) to net loss | — | — | — | (221) | — | (221) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Net loss |
| — |
| — |
| — |
| — |
| (26,558) |
| (26,558) | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance at December 31, 2019 |
| 138,362,809 |
| 1 |
| 187,735 |
| 7 |
| (125,693) |
| 62,050 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with RSU vesting |
| 552,800 |
| — |
| — |
| — |
| — |
| — | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Stock issued in connection with option exercises |
| 146,081 |
| — |
| 50 |
| — |
| — |
| 50 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Equity-based compensation |
| — |
| — |
| 1,654 |
| — |
| — |
| 1,654 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Unrealized gain (loss) on short-term investment securities |
| — |
| — |
| — |
| 72 |
| — |
| 72 | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Reclassification of losses (gains) to net loss |
| — |
| — |
| — |
| (5) |
| — |
| (5) | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Net loss |
| — |
| — |
| — |
| — |
| (19,711) |
| (19,711) | |||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance at December 31, 2020 |
| 139,061,690 | $ | 1 | $ | 189,439 | $ | 74 | $ | (145,404) | $ | 44,110 | |||||
See accompanying notes to consolidated financial statements.
F-7
22nd CENTURY GROUP, INC. AND SUBSIDIARIES | |
CONSOLIDATED STATEMENTS OF CASH FLOWS | |
($ in thousands) | |
Year Ended | |||||||||
December 31, | |||||||||
| 2020 |
| 2019 |
| 2018 | ||||
Cash flows from operating activities: |
|
|
|
| |||||
Net loss | $ | (19,711) | $ | (26,558) | $ | (7,967) | |||
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
| |||
Impairment of intangible assets | 176 | 1,142 | — | ||||||
Impairment of Panacea investment | 1,741 | — | — | ||||||
Amortization and depreciation |
| 1,094 |
| 1,186 |
| 1,200 | |||
Amortization of license fees |
| 251 |
| 239 |
| 143 | |||
Amortization of ROU Asset |
| 182 |
| 212 |
| — | |||
Realized gain on the sale of investments | — | — | (14,493) | ||||||
Unrealized (gain) loss on investment |
| 434 |
| 2,419 |
| (284) | |||
Realized (gain) loss on short-term investment securities | (5) | (221) | 54 | ||||||
Litigation settlement |
| — |
| 1,891 |
| — | |||
Gain on the sale of machinery and equipment |
| (1) |
| (87) |
| — | |||
Warrant liability (gain) loss |
| — |
| — |
| (49) | |||
Accretion of non cash interest expense | 43 | 48 | 11 | ||||||
Accretion of non cash interest income |
| (369) |
| (22) |
| — | |||
Equity-based employee compensation expense |
| 1,654 |
| 3,540 |
| 3,187 | |||
Inventory write-off | 521 | 985 | — | ||||||
(Decrease) increase in inventory reserves | — | — | (95) | ||||||
(Increase) decrease in assets: |
|
|
|
|
| — | |||
Accounts receivable |
| (1,292) |
| 4 |
| 86 | |||
Inventory |
| (289) |
| (207) |
| 334 | |||
Prepaid expenses and other assets |
| (787) |
| 280 |
| (182) | |||
Increase (decrease) in liabilities: |
| — |
|
|
| — | |||
Operating lease obligations |
| (552) |
| (212) |
| — | |||
Accounts payable |
| (936) |
| (732) |
| 322 | |||
Accrued expenses |
| 2,183 |
| 792 |
| (167) | |||
Accrued severance | (225) | 792 | — | ||||||
Deferred income |
| 267 |
| (78) |
| 55 | |||
Net cash provided by (used in) operating activities |
| (15,621) |
| (14,588) |
| (17,845) | |||
Cash flows from investing activities: |
|
|
|
|
| ||||
Acquisition of patents, trademarks, and licenses |
| (468) |
| (565) |
| (657) | |||
Acquisition of machinery and equipment |
| (54) |
| (527) |
| (449) | |||
Proceeds from the sale of machinery and equipment |
| 6 |
| 166 |
| — | |||
Investment in Panacea | — | (12,000) | — | ||||||
Proceeds from the sale of investments | — | — | 13,052 | ||||||
Sales and maturities of short-term investment securities |
| 39,728 |
| 19,320 |
| 37,415 | |||
Purchase of short-term investment securities |
| (22,743) |
| (1,842) |
| (34,216) | |||
Net cash provided by (used in) investing activities |
| 16,469 |
| 4,552 |
| 15,145 | |||
Cash flows from financing activities: |
|
|
|
|
| ||||
Payment on note payable | (2,549) | (700) | (800) | ||||||
Proceeds from note payable issuance | 2,195 | — | — | ||||||
Net proceeds from option exercise | 50 | — | 445 | ||||||
Net proceeds from warrant exercise | — | 10,616 | — | ||||||
Proceeds from SBA loan | 1,183 | — | — | ||||||
Repayment of SBA loan | (1,183) | — | — | ||||||
Net cash provided by (used in) financing activities |
| (304) |
| 9,916 |
| (355) | |||
Net increase (decrease) in cash and cash equivalents |
| 544 |
| (120) |
| (3,055) | |||
Cash and cash equivalents - beginning of period |
| 485 |
| 605 |
| 3,660 | |||
Cash and cash equivalents - end of period | $ | 1,029 | $ | 485 | $ | 605 | |||
Supplemental disclosures of cash flow information: |
|
|
|
|
|
| |||
Net cash paid for: |
|
|
|
|
|
| |||
Cash paid during the period for interest | $ | 29 | $ | 3 | $ | — | |||
Non-cash transactions: |
|
|
|
|
|
| |||
Patent and trademark additions included in accounts payable | $ | 55 | $ | 155 | $ | 152 | |||
Machinery and equipment additions included in accounts payable | $ | 2 | $ | — | $ | 19 | |||
Right-of-use assets and corresponding operating lease obligations | $ | 198 | $ | 814 | $ | — | |||
Patent and trademark additions included in accrued expenses | $ | 28 | $ | — | $ | — | |||
Licenses acquired with notes payable | $ | — | $ | — | $ | 2,326 | |||
Reclassification of warrant liability to capital in excess of par | $ | — | $ | — | $ | 168 | |||
Stock issued in connection with investment | $ | — | $ | 1,297 | $ | — | |||
See accompanying notes to consolidated financial statements.
F-8
22nd CENTURY GROUP, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
Amounts in thousands, except for share and per share data
NOTE 1. – NATURE OF BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation – The accompanying consolidated financial statements include the accounts of 22nd Century Group, Inc. (“22nd Century Group”), its three wholly-owned subsidiaries, 22nd Century Limited, LLC (“22nd Century Ltd”), NASCO Products, LLC (“NASCO”), and Botanical Genetics, LLC (“Botanical Genetics”), and two wholly-owned subsidiaries of 22nd Century Ltd, Goodrich Tobacco Company, LLC (“Goodrich Tobacco”) and Heracles Pharmaceuticals, LLC (“Heracles Pharma”) (collectively, “the Company”). All intercompany accounts and transactions have been eliminated.
Nature of Business – 22nd Century Group is a biotechnology company developing disruptive plant-based solutions for the life science, consumer product, and pharmaceutical markets. The Company is focused on technology that allows it to alter the level of nicotine and other nicotinic alkaloids in tobacco plants and the levels of cannabinoids and terpenes in hemp/cannabis plants through genetic engineering and modern plant breeding techniques. Goodrich Tobacco and Heracles Pharma are business units for the Company’s potential modified risk tobacco products. NASCO is a federally licensed tobacco products manufacturer, a subsequent participating member under the tobacco Master Settlement Agreement (“MSA”) between the tobacco industry and the settling states under the MSA and operates the Company’s tobacco products manufacturing business in North Carolina. Botanical Genetics is a wholly-owned subsidiary of 22nd Century Group that performs research and development related to the Company’s hemp business.
Reclassifications – Certain items in the 2019 and 2018 financial statements have been reclassified to conform to the 2020 classification. During 2020, the Company expanded its research and development expense line item into two line items in the operating expense section of the Company’s Consolidated Statements of Operations and Comprehensive Loss: (i) Research and development and (ii) Research and development—MRTP. The comparative classifications for 2019 and 2018 have been reclassified to conform to the new presentation.
Preferred stock authorized – The Company is authorized to issue “blank check” preferred stock, which could be issued with voting, liquidation, dividend and other rights superior to our common stock.
Concentration of Credit Risk – Financial instruments that potentially subject the Company to concentration of credit risk consist of cash accounts in financial institutions. Although the cash accounts exceed the federally insured deposit amount, management does not anticipate nonperformance by the financial institutions. Management reviews the financial viability of these institutions on a periodic basis.
Cash and cash equivalents – The Company considers all highly liquid investments with maturities of three months or less at the date of acquisition to be cash equivalents. However, the Company has elected to classify money market mutual funds related to its short-term investment portfolio as short-term investment securities. There are no restrictions on the Company’s cash and cash equivalents.
Short-term investment securities – The Company’s short-term investment securities are classified as available-for-sale securities and consist of money market funds, corporate bonds, U.S. government agency bonds, U.S. treasury securities, and commercial paper with maturities greater than three months at the time of acquisition. The Company’s short-term investment securities are carried at fair value within current assets on the Company’s Consolidated Balance Sheets. The Company views its available-for-sale securities as available for use in current operations regardless of the stated maturity date of the security. The Company’s investment policy states that all investment securities must have a maximum maturity of (24) months or less and the maximum weighted maturity of the investment securities must not exceed twelve (12) months. All the Company’s short-term investment securities are fixed-income debt instruments, and accordingly, all unrealized gains and losses incurred on the short-term investment securities (the adjustment to fair value) are recorded in other comprehensive income or loss on the Company’s Consolidated Statements of Operations and Comprehensive Loss. Realized gains and losses on short-term investment securities are recorded in
F-9
the other income (expense) portion of the Company’s Consolidated Statements of Operations and Comprehensive Loss. Interest income is recorded on the accrual basis and presented net of investment related fees.
Accounts receivable – The Company periodically reviews aged account balances for collectability. The Company concluded that an allowance for doubtful accounts was not required at both December 31, 2020 and December 31, 2019.
Inventory – Inventories are valued at the lower of historical cost or net realizable value. Cost is determined using (i) an average cost method for tobacco leaf inventory and raw materials inventory, and (ii) a standard cost method, approximating average costs, for finished goods inventory. Inventories are evaluated to determine whether any amounts are not recoverable based on slow moving or obsolete condition and are written off or reserved as appropriate.
Machinery and equipment – Machinery and equipment are recorded at their acquisition cost and depreciated on a straight-line basis over their estimated useful lives, generally ranging from ranging from to . Leasehold improvements are depreciated on a straight-line basis over the term of the lease or the estimate useful life of the asset, whichever is shorter. Depreciation commences when the asset is placed in service.
Intangible Assets – Intangible assets are recorded at cost and consist primarily of (1) expenditures incurred with third-parties related to the processing of patent claims and trademarks with government authorities, as well as costs to acquire patent rights from third-parties, (2) license fees paid for third-party intellectual property, (3) costs to become a signatory under the tobacco MSA, and (4) license fees paid to acquire a predicate cigarette brand. The amounts capitalized relate to intellectual property that the Company owns or to which it has exclusive rights.
The Company’s capitalized intellectual property costs are amortized using the straight-line method over the remaining statutory life of the granted patent assets in each of the Company’s patent families, which have estimated expiration dates ranging from 2026 to 2041. Periodic maintenance or renewal fees are expensed as incurred. Annual minimum license fees are charged to expense. License fees paid for third-party intellectual property are amortized on a straight-line basis over the last to expire patents, which patent expiration dates are expected to range from 2019 through 2036. The Company believes costs associated with becoming a signatory to the MSA and acquiring a predicate cigarette brand have an indefinite life and as such, no amortization is taken. At each reporting period, the Company evaluates whether events and circumstances continue to support the indefinite-lived classification.
Impairment of Long-Lived Assets – The Company reviews the carrying value of its amortizing long-lived assets whenever events or changes in circumstances indicate that the historical cost-carrying value of an asset may no longer be recoverable. On at least an annual basis, the Company assesses whether events or changes in circumstances indicate that the carrying amount may not be recoverable. If any such indicators are present, the Company will test for recoverability in accordance with ASC 360-Property, plant, and equipment or ASC 350- Intangibles, Goodwill, and Other.
Intangible assets subject to amortization are reviewed for strategic importance and commercialization opportunity prior to expiration. If it is determined that the asset no longer supports the Company’s strategic objectives and/or will not be commercially viable prior to expiration, the asset is impaired. In addition, the Company will assess the expected future undiscounted cash flows for its intellectual property based on consideration of future market and economic conditions, competition, federal and state regulations, and licensing opportunities. If the carrying value of such assets are not recoverable, the carrying value will be reduced to fair value.
Indefinite-lived intangible asset carrying values are reviewed at least annually or more frequently if events or changes in circumstances indicate that it is more likely than not that an impairment exists. The Company first performs a qualitative assessment and considers its current strategic objectives, future market and economic conditions, competition, and federal and state regulations to determine if an impairment is more likely than not. If it is determined that an impairment is more likely than not, a quantitative assessment is performed to compare the asset carrying value to fair value.
Right-of-use assets (ROU) and Lease Obligations – On January 1, 2019, the Company adopted ASU 2016-02, Subtopic ASC 842, Leases (the “new guidance”). Under the new guidance, the Company was required to evaluate its
F-10
leases and record a Right-of-use (“ROU”) asset and a corresponding lease obligation for leases that qualified as either finance or operating leases. The Company elected to use the practical expedient which allowed the Company to carry forward the historical lease classifications of the existing leases. The Company determined that its leases contained (1) no variable lease expenses, (2) no termination options, (3) no residual lease guarantees, and (4) no material restrictions or covenants. All remaining renewal options were included in the computation of the ROU assets and lease obligations upon adoption.
The Company reviews any lease arrangements in accordance with ASU 2016-02, Subtopic ASC 842, Leases. Any lease having a lease term greater than twelve months will be recognized on the Consolidated Balance Sheets as a ROU asset with an associated lease obligation—all other leases are considered short-term in nature and will be expensed on a month-to-month basis. The ROU assets and lease obligations are recognized as of the commencement date at the net present value of the fixed minimum lease payments for the lease term. The discount rate used is the interest rate implicit in the lease, if available, or the Company’s incremental borrowing rate which is determined using a base line rate plus an applicable spread.
All financial results and disclosures for periods beginning after January 1, 2019 are presented in accordance with Subtopic ASC 842. 2018 results have not been adjusted continue to be reported under ASC 840. Refer to Note 4 for additional information regarding our ROU assets and liabilities.
Income Taxes – The Company recognizes deferred tax assets and liabilities for any basis differences in its assets and liabilities between tax and U.S. GAAP reporting, and for operating loss and credit carry-forwards.
As a result of the Company’s history of cumulative net operating losses and the uncertainty of their future utilization, the Company has established a valuation allowance to fully offset its net deferred tax assets as of December 31, 2020 and December 31, 2019. Additionally, the Company has elected to present other comprehensive income items relating to net unrealized gains on short-term investment securities gross and not net of taxes.
The Company’s federal and state tax returns for the years ended December 31, 2017 through December 31, 2019 are currently open to audit under the statutes of limitations. There are no pending audits as of December 31, 2020.
Stock Based Compensation – The Company uses a fair-value based method to determine compensation for all arrangements under which Company employees and others receive shares, restricted stock units or options to purchase common shares of the Company. Stock based compensation expense is recorded over the requisite service period based on estimates of probability and time of achieving milestones and vesting. For accounting purposes, the shares will be considered issued and outstanding. Forfeitures are accounted for when the occur.
Revenue Recognition – The Company recognizes revenue when it satisfies a performance obligation by transferring control of the product to a customer. For additional discussion on revenue recognition, refer to Note 16.
Derivatives – The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. The Company evaluates all our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. Derivative financial instruments are initially recorded at fair market value and then are revalued at each reporting date, with changes in fair value reported in the Consolidated Statements of Operations and Comprehensive Loss. The classification of derivative instruments is evaluated at the end of each reporting period. Derivative instruments are classified on the balance sheet as current or non-current based on if the net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date.
Research and Development – Research and development costs are expensed as incurred.
Loss Per Common Share – Basic loss per common share is computed using the weighted-average number of common shares outstanding. Diluted loss per share is computed assuming conversion of all potentially dilutive securities. Potential common shares outstanding are excluded from the computation if their effect is anti-dilutive. Refer to Note 13 for additional information.
F-11
Use of Estimates – The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of income and expenses during the reporting period. Actual results could differ from those estimates.
Fair Value of Financial Instruments – The Company’s financial instruments include cash and cash equivalents, short-term investment securities, accounts receivable, investments, a convertible note receivable, accounts payable, accrued expenses, and notes payable. Other than for cash equivalents, short-term investment securities, investments, and convertible note receivable, fair value is assumed to approximate carrying values for these financial instruments, since they are short term in nature, they are receivable or payable on demand, or had stated interest rates that approximate the interest rates available to the Company as of the reporting date. The determination of the fair value of cash equivalents, short-term investment securities, investments (stock warrants and equity investment), and convertible note receivable are discussed in Note 7.
Investments – The Company accounts for investments in equity securities of other entities under the equity method of accounting if the Company’s investment in the stock of the other entity provides the Company with the ability to have significant influence over the operating and financial policies of the investee. If the Company does not have significant influence over the operating or financial policies of the entity, and such equity investment does not have a readily determinable market value, then the Company accounts for such equity investments in accordance with FASB ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assts and Financial Liabilities, which the Company adopted in the first quarter of 2018 with respect to the Company’s investments. Under ASU 2016-01 equity securities are recorded at fair value, with changes in fair value recorded through the statement of operations. Equity securities without a readily determinable market value are carried at cost less impairment, adjusted for observable price changes in orderly transactions for an identical or similar investment of the same issuer. The Company considers debt instruments as available-for-sale securities, and accordingly, all unrealized gains and losses incurred on the short-term investment securities (the adjustment to fair value) are recorded in other comprehensive income or loss on the Company’s Consolidated Statements of Operations and Comprehensive Loss. Refer to Note 6 for additional disclosures relating to the Company’s investments.
Loss Contingencies – The Company establishes an accrued liability for litigation and regulatory matters when those matters present loss contingencies that are both probable and estimable. In such cases, there may be an exposure to loss in excess of any amounts accrued. When a loss contingency is not both probable and estimable, the Company does not establish an accrued liability. As a litigation or regulatory matter develops, the Company, in conjunction with any outside counsel handling the matter, evaluates on an ongoing basis whether such matter presents a loss contingency that is probable and estimable. If, at the time of evaluation, the loss contingency related to a litigation or regulatory matter is not both probable and estimable, the matter will continue to be monitored for further developments that would make such loss contingency both probable and estimable. When a loss contingency related to a litigation or regulatory matter is deemed to be both probable and estimable, the Company will establish an accrued liability with respect to such loss contingency and record a corresponding amount of related expenses. The Company will then continue to monitor the matter for further developments that could affect the amount of any such accrued liability. Our current legal matters are discussed further in Note 12.
Recent Accounting Pronouncement(s) –
In June 2016, the FASB issued ASU 2016-13, “Measurement of Credit Losses on Financial Instruments.” The standard replaces the incurred loss model with the current expected credit loss (CECL) model to estimate credit losses for financial assets measured at amortized cost and certain off-balance sheet credit exposures. The CECL model requires companies to estimate credit losses expected over the life of the financial assets based on historical experience, current conditions and reasonable and supportable forecasts. The provisions of the ASU are effective for fiscal years beginning after December 15, 2019 and interim periods within those fiscal years—excluding small reporting companies (SRCs) which have an effective date beginning after December 15, 2022 and interim periods within those fiscal years. The Company plans to adopt ASU 2016-13 on January 1, 2023 and is evaluating the expected impacts.
We consider the applicability and impact of all ASUs. If the ASU is not listed above, it was determined that the ASU was either not appliable or would have an immaterial impact on our financial statements and related disclosures.
F-12
.
NOTE 2. – INVENTORY
Inventories at December 31, 2020 and December 31, 2019 consisted of the following:
| December 31, |
| December 31, | |||
| 2020 |
| 2019 | |||
Inventory - tobacco leaf | $ | 821 | $ | 1,178 | ||
Inventory - finished goods |
| |||||
Cigarettes and filtered cigars |
| 171 |
| 106 | ||
Inventory - raw materials |
|
| ||||
Cigarette and filtered cigar components | 1,142 | 1,082 | ||||
Less: inventory reserve |
| (100) |
| (100) | ||
$ | 2,034 | $ | 2,266 | |||
During the year ended December 31, 2020, the Company wrote off inventory totaling $521 on the Company’s Consolidated Statement of Operations and Comprehensive Loss ($361 included within research and development expenses and $161 included within cost of goods sold). During the year ended December 31, 2019, the Company wrote off inventory totaling $985 which is included within research and development expenses on the Company’s Consolidated Statement of Operations and Comprehensive Loss.
NOTE 3. – MACHINERY AND EQUIPMENT
Machinery and equipment at December 31, 2020 and December 31, 2019 consisted of the following:
December 31, | December 31, | |||||||
| Useful Life |
| 2020 |
| 2019 | |||
Cigarette manufacturing equipment | or | $ | 4,893 | $ | 4,870 | |||
Office furniture, fixtures and equipment |
| 20 |
| 152 | ||||
Laboratory equipment |
| 117 |
| 125 | ||||
Leasehold improvements |
| 123 |
| 257 | ||||
|
| |||||||
Less: accumulated depreciation |
|
| (2,670) |
| (2,284) | |||
Machinery and equipment, net |
| $ | 2,483 | $ | 3,120 | |||
Depreciation expense was $688, $589, and $523 for the years ended December 31, 2020, 2019 and 2018, respectively.
NOTE 4. – RIGHT-OF-USE ASSETS, LEASE OBLIGATIONS, AND OTHER LEASES
The Company leases a manufacturing facility and warehouse in North Carolina, a corporate headquarters in Williamsville, New York, and a laboratory space in Buffalo, New York.
During the fourth quarter of 2020, the Company made the decision to not renew its corporate headquarters lease which initial term expired on January 1, 2021. Upon adoption of ASC 842-Leases, the Company assumed all three optional one-year renewal options which created a lease term expiration of all initial renewal options within the lease agreement—which assumed a lease term expiration in 2023. With the non-renewal decision, the Company’s lease will operate month-to-month beginning on January 1, 2021. As such, the ROU asset and lease obligation was removed from the Consolidated Balance Sheets as of December 31, 2020. The remaining ROU assets and lease obligations relate to the laboratory space in New York and the manufacturing facility and warehouse in North Carolina.
F-13
The following table summarized the Company’s discount rate and remaining lease terms as of December 31, 2020:
Weighted average remaining lease term in years | 1.1 | |||
Weighted average discount rate |
| 4.5 | % |
Future minimum lease payments as of December 31, 2020 are as follows:
2021 | $ | 245 | |
2022 |
| 9 | |
Total lease payments |
| 254 | |
Less: imputed interest |
| (6) | |
Total | $ | 248 |
The Company also leases a warehouse space in North Carolina to store and operate tobacco leaf processing equipment as well as proprietary tobacco leaf inventory. This lease is month-to-month and is not included within our Consolidated Balance Sheets.
Operating lease costs for the years ended December 31, 2020 and 2019 were $335 and $250, respectively.
In January 2021, the Company announced that it would relocate its corporate headquarters to the Larkinville District in downtown Buffalo and executed a new lease agreement for such space. Refer to Note 17 for additional information on this Type II subsequent event.
NOTE 5. – INTANGIBLE ASSETS
Our intangible assets at December 31, 2020 and December 31, 2019 consisted of the following:
December 31, | December 31, | |||||
| 2020 |
| 2019 | |||
Intangible assets, net |
|
|
|
| ||
Patent and trademark costs | $ | 5,667 | $ | 5,712 | ||
Less: accumulated amortization |
| (2,936) |
| (2,839) | ||
Patent and trademark costs, net |
| 2,731 |
| 2,873 | ||
License fees |
| 3,876 |
| 3,777 | ||
Less: accumulated amortization |
| (948) |
| (709) | ||
License fees, net |
| 2,928 |
| 3,069 | ||
MSA signatory costs |
| 2,202 |
| 2,202 | ||
| ||||||
License fee for predicate cigarette brand |
| 350 |
| 350 | ||
$ | 8,211 | $ | 8,494 | |||
Amortization expense relating to the above intangible assets for the years ended December 31, 2020, 2019 and 2018 amounted to $658, $836, and $820, respectively. During years ended December 31, 2020 and 2019, the Company incurred an impairment related to patent intellectual property that would be expired prior to expected commercialization. Impairment expense the year ended December 31, 2020 amounted to $176 (cost of approximately $448 less accumulated amortization of approximately $302).
Impairment expense for the year ended December 31, 2019 amounted to $1,142 (cost of $2,092 less accumulated amortization of approximately $950) and related to tobacco intellectual property (patents, patent applications, and trademarks) that did not align to the Company’s very low nicotine strategic objectives.
F-14
The impairment charges are included as a separate line item in operating expenses on the Company’s Consolidated Statements of Operations and Comprehensive Loss. There was no impairment recorded during for the year ended December 31, 2018.
The estimated for the next five years is approximately $350 for and $244 for .
NOTE 6. – INVESTMENTS & CONVERTIBLE NOTE RECEIVABLE
Investment in Panacea Life Sciences, Inc.
On December 3, 2019, the Company entered into a securities purchase agreement with Panacea Life Sciences, Inc. (“Panacea”) for consideration valued at $13,297 ($12,000 cash and $1,297 of the Company’s shares of common stock valued at $1 per share) in exchange for a 15.8% ownership interest. The Company’s investment consists of three instruments: shares of Series B preferred stock (“preferred stock”); a convertible note receivable with a $7,000 face value; and a warrant (“stock warrant”) to purchase additional shares of Series B preferred stock, to obtain 51% ownership of Panacea, at an exercise price of $2.344 per share. The convertible note receivable has a term of five years, bears interest of 10% per annum, and can be converted to shares of Series B preferred stock at the Company’s discretion. The embedded conversion option is not considered a derivative instrument for accounting purposes The preferred stock carries an annual 10% cumulative dividend, compounded annually, and has an implicit put option after the fifth anniversary date so long that the stock warrants have not been exercised. The put option is not considered a derivative instrument for accounting purposes. The stock warrant may be exercised at any time after the fifth anniversary date and would be accelerated if Panacea achieves certain sales targets for consecutive years. The Series B preferred stock also has first priority equity preferences in the event of a liquidation, sale, or transfer of Panacea assets. These rights entitle the Company to the original Series B issuance price of $7,000 plus any unpaid accrued dividends.
To allocate the cost of the stock warrant, the Company calculated a fair value based on the following assumptions: volatility of 70%, discount of 25% for lack of marketability, and a risk-free rate of 2%. The value of the stock warrant was allocated to the preferred stock and the convertible note receivable, equally, as a discount to the acquisition price. The discount on the preferred stock was determined to be for lack of control and the discount on the convertible note receivable was determined to be related to issuing the note at a below market interest rate for similar instruments. The discount on the convertible note receivable amounted to $1,433 and is being amortized into interest income over the term of the note. The discount on the preferred stock amounted to $1,433 and is being amortized into interest income over the term of the implicit put option.
The convertible note receivable and the preferred stock investment are considered available for sale debt securities with a private company that is not traded in active markets. Since observable price quotations were not available at acquisition, fair value was estimated based on cost less an appropriate discount upon acquisition. The discount of each instrument is accreted into interest income over the respective term as shown within the Company’s Consolidated Statements of Operations and Comprehensive Loss. See Note 7 for additional information on these fair value measurements. The stock warrant was recorded at its cost basis in accordance with the practicability exception under ASU 2016-01.
Impairment of Panacea Investment:
As a result of increased competition and other macroeconomic factors, the Company recognized an impairment of $1,062 on the Panacea stock warrant during the second quarter of 2020. The impairment is recorded within the Consolidated Statements of Operations and Comprehensive Loss as “Impairment of Panacea Investment.”
The Company recently entered into a non-binding agreement with Panacea to potentially restructure the investment and business relationship. The non-binding agreement with Panacea generally provides for (i) the transfer of $7,170 in operational assets, including an agricultural facility and various extraction and distillation equipment, from Panacea to the Company in exchange for the cancellation of the $7,000 convertible note receivable plus accrued interest; (ii) an amendment of transaction documents to remove any future investment rights and obligations of the Company in
F-15
Panacea, (iii) cancellation of the stock warrant to purchase additional Series B preferred stock; and (iv) various other amendments to Panacea’s charter to amend various investors rights therein.
As a result of the expected outcome of this non-binding agreement, the Company determined that the carrying value of the stock warrant and the convertible note receivable plus accrued interest exceeded the fair value outlined in the non-binding agreement. As such, the Company recorded an impairment of $679, which reduced the stock warrant carrying value, so that the carrying value of the stock warrant, and convertible note receivable plus accrued interest amounted to a value of $7,170 as of December 31, 2020. The impairment is recorded within the Consolidated Statements of Operations and Comprehensive Loss as “Impairment of Panacea Investment.”
In accordance with ASC 326- Financial Instruments-Credit Losses, the Company reviewed the fair value of its preferred stock investment and considered the following: (i) increased competition in the cannabinoid industry; (ii) the Company’s preferred stock priority equity preferences; and (iii) other macroeconomic factors. Based on the assessment performed, it was determined that no credit loss existed for the preferred stock available-for-sale debt security.
As of December 31, 2020, the total carrying value of the Company’s investment in Panacea is outlined below, net of 2020 impairment expense:
December 31, | |||
2020 | |||
Panacea preferred stock |
| $ | 5,173 |
Panacea stock warrant | 1,124 | ||
Accrued interest on convertible note receivable (included within prepaid expenses and other assets) | 170 | ||
Convertible note receivable | 5,876 | ||
Total | $ | 12,343 | |
Investment in Anandia
The Company (through its wholly-owned subsidiary, Botanical Genetics) previously held an equity investment in Anandia, a Canadian plant biotechnology company.
Effective January 1, 2018, the Company adopted Financial Accounting Standards Board ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. This guidance changed how entities account for equity investments that do not result in consolidation and are not accounted for under the equity method of accounting. Under ASU 2016-01, the Company was required to measure its investment in Anandia at fair value at the end of each reporting period and recognize changes in fair value in net income. As allowed by ASU 2016-01, since the Company’s investment in Anandia did not have readily determinable fair value, the Company elected to account for its investment at cost. The cost basis is required to be adjusted in the event of impairment, if any, and for any observable price changes in orderly transactions for the identical or a similar investment of the same issuer. As result of an equity issuance by Anandia in January of 2018, the Company recorded an unrealized gain on its investment in Anandia in the amount of $6,147 during the first quarter of 2018.
On August 8, 2018, all of Anandia’s outstanding common stock was acquired by Aurora Cannabis, Inc. (“Aurora”), a Canadian company (NYSE: ACB and TSX: ACB), and as a result the Company received in exchange for its Anandia equity: (i) 1,947,943 free trading shares of Aurora common stock, and (ii) Aurora stock warrants to purchase 973,971 shares of Aurora common stock (the “Anandia transaction”). The stock warrants have a contractual term, an exercise price of $9.37 Canadian Dollars (CAD) per share. The fair value of the Aurora common stock and Aurora stock warrant was $9,222 and $2,808, respectively. The Company recorded a realized gain on the transaction in the amount of $4,516 during the third quarter of 2018. Additionally, the $6,147 unrealized gain on the Company’s investment in Anandia became a realized gain at time of the Anandia transaction. Subsequent to the transaction, the Company sold all of its Aurora common stock resulting in net sales proceeds to the Company of $13,052 and realized a gain on the sale of $3,830 during the year ended December 31, 2018.
F-16
Investment in Aurora Cannabis, Inc.
The Company’s investment in Aurora Cannabis Inc. (“Aurora”) stock warrant, discussed in the Anandia section above, are considered equity securities in accordance with ASC 321 – Investments – Equity Securities and a derivative instrument under ASC 815 – Derivatives and Hedging. The stock warrants are not designated as a hedging instrument, and in accordance with ASC 815, the Company’s investment in stock warrants are recorded at fair value with changes in fair value recorded to unrealized gain/loss as shown within the Company’s Consolidated Statements of Operations and Comprehensive Loss. See Note 7 for additional information on the fair value measurements.
Reverse stock split- During the second quarter of 2020, Aurora announced a 12-to-1 reverse stock which adjusted our total warrant to purchase 81,164 shares of Aurora common stock (from 973,971) at an exercise price of $112.44 CAD per share (from $9.37 CAD per share).
The carrying value of the Company’s investments at December 31, 2020 and December 31, 2019 consisted of the following:
December 31, | December 31, | |||||
2020 | 2019 | |||||
Aurora stock warrants |
| $ | 239 |
| $ | 673 |
Panacea preferred stock | 5,173 | 4,865 | ||||
Panacea stock warrant | 1,124 | 2,865 | ||||
Total Investments | $ | 6,536 | $ | 8,403 | ||
Convertible Note Receivable | $ | 5,876 | $ | 5,589 | ||
NOTE 7. – FAIR VALUE MEASUREMENTS AND SHORT-TERM INVESTMENTS
FASB ASC 820 - “Fair Value Measurements and Disclosures” establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows:
| ● | Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| ● | Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full term of the financial instrument; and |
| ● | Level 3 inputs are unobservable inputs based on the Company’s own assumptions used to measure assets and liabilities at fair value. |
A financial asset’s or a financial liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
F-17
The following table presents information about our assets and liabilities measured at fair value at December 31, 2020 and December 31, 2019, and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
Fair Value | ||||||||||||
December 31, 2020 | ||||||||||||
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |||||
Assets |
|
|
|
|
|
|
|
| ||||
Short-term investment securities: |
|
|
|
|
|
|
|
| ||||
Money market funds | $ | 8,636 | $ | — | $ | — | $ | 8,636 | ||||
Corporate bonds |
| — |
| 12,677 |
| — |
| 12,677 | ||||
Total short-term investment securities | $ | 8,636 | $ | 12,677 | $ | — | $ | 21,313 | ||||
Investment - Aurora stock warrants | $ | — | $ | — | $ | 239 | $ | 239 | ||||
Investment - Panacea preferred stock | $ | — | $ | — | $ | 5,173 | $ | 5,173 | ||||
Convertible note receivable | $ | — | $ | — | $ | 5,876 | $ | 5,876 | ||||
Fair Value | ||||||||||||
December 31, 2019 | ||||||||||||
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |||||
Assets |
|
|
|
|
|
|
|
| ||||
Short-term investment securities: |
|
|
|
|
|
|
|
| ||||
Money market funds | $ | 12,146 | $ | — | $ | — | $ | 12,146 | ||||
Corporate bonds |
| — |
| 26,331 |
| — |
| 26,331 | ||||
Total short-term investment securities | $ | 12,146 | $ | 26,331 | $ | — | $ | 38,477 | ||||
Investment - Aurora stock warrants | $ | — | $ | — | $ | 673 | $ | 673 | ||||
Investment - Panacea preferred stock | $ | — | $ | — | $ | 4,865 | $ | 4,865 | ||||
Convertible note receivable | $ | — | $ | — | $ | 5,589 | $ | 5,589 | ||||
Money market mutual funds are valued at their daily closing price as reported by the fund. Money market mutual funds held by the Company are open-end mutual funds that are registered with the SEC that generally transact at a stable $1.00 Net Asset Value (“NAV”) representing its estimated fair value. On a daily basis the fund’s NAV is determined by the fund based on the amortized cost of the funds underlying investments.
Corporate bonds are valued using pricing models maximizing the use of observable inputs for similar securities.
The investment in the Aurora stock (ACB) warrants is measured at fair value using the Black-Scholes pricing model and is classified within Level 3 of the valuation hierarchy. The unobservable input is an estimated volatility factor of 137% and 83% as of December 31, 2020 and December 31, 2019, respectively. Therefore, changes in market volatility will impact the fair value measurement of our ACB investment.
A 20% increase or decrease in the volatility factor used as of December 31, 2020 would have the impact of increasing or decreasing the fair value measurement of the stock warrants by approximately $115. A 20% increase or decrease in the volatility factor used at December 31, 2019 would have the impact of increasing or decreasing the fair value measurement of the stock warrants by approximately $260.
The Panacea convertible note receivable and the preferred stock investment are considered available-for-sale debt securities with a private company that is not traded in active markets. Since observable price quotations were not available, fair value was estimated based on cost less an appropriate discount upon acquisition. The discount of each instrument is accreted into interest income over the respective term and will adjust the amortized cost basis of the investments. See Note 6 for further information regarding the Company’s investment in Panacea.
F-18
The following table sets forth a summary of the changes in fair value of the Company’s Level 3 investments for the year ended December 31, 2020.
Fair Value at December 31, 2018 |
| $ | 3,092 |
Unrealized gain as a result of change in fair value |
| (2,419) | |
Accretion of interest income on convertible note receivable | 22 | ||
Panacea convertible note receivable | 5,567 | ||
Preferred stock in Panacea | 4,865 | ||
Fair Value at December 31, 2019 | $ | 11,127 | |
Unrealized loss as a result of change in fair value | (434) | ||
Accretion of interest on Panacea investment | 595 | ||
Fair Value at December 31, 2020 | $ | 11,288 |
The following tables set forth a summary of the Company’s available-for-sale debt securities from amortized cost basis to fair value as of December 31, 2020 and December 31, 2019:
Available for Sale Debt Securities | ||||||||||||
December 31, 2020 | ||||||||||||
Amortized | Gross | Gross | ||||||||||
Cost | Unrealized | Unrealized | Fair | |||||||||
| Basis |
| Gains |
| Losses |
| Value | |||||
Corporate bonds | $ | 12,603 | $ | 74 | $ | — | $ | 12,677 | ||||
Convertible note receivable |
| 5,876 |
| — |
| — |
| 5,876 | ||||
Investment - Panacea preferred stock |
| 5,173 |
| — |
| — |
| 5,173 | ||||
$ | 23,652 | $ | 74 | $ | — | $ | 23,726 | |||||
Available for Sale Debt Securities | ||||||||||||
December 31, 2019 | ||||||||||||
Amortized | Gross | Gross | ||||||||||
Cost | Unrealized | Unrealized | Fair | |||||||||
| Basis |
| Gains |
| Losses |
| Value | |||||
Corporate bonds | $ | 26,324 | $ | 64 | $ | (57) | $ | 26,331 | ||||
Convertible note receivable |
| 5,589 |
| — |
| — |
| 5,589 | ||||
Investment - Panacea preferred stock |
| 4,865 |
| — |
| — |
| 4,865 | ||||
$ | 36,778 | $ | 64 | $ | (57) | $ | 36,785 | |||||
The following table sets forth a summary of the Company’s available-for-sale securities from amortized cost basis and fair value by contractual maturity as of December 31, 2020 and December 31, 2019:
Available for Sale Debt Securities | ||||||||||||
December 31, 2020 | December 31, 2019 | |||||||||||
Amortized | Amortized | |||||||||||
| Cost Basis |
| Fair Value |
| Cost Basis |
| Fair Value | |||||
Due in one year or less | $ | 11,692 | $ | 11,753 | $ | 16,823 | $ | 16,851 | ||||
Due after one year through five years |
| 11,960 |
| 11,973 |
| 9,501 |
| 9,480 | ||||
Due in five years | — | — | 10,454 | 10,454 | ||||||||
$ | 23,652 | $ | 23,726 | $ | 36,778 | $ | 36,785 | |||||
NOTE 8. – NOTES PAYABLE
License Fees
On June 22, 2018, the Company entered into the Second Amendment to the License Agreement (the “Second Amendment”) with North Carolina State University (“NCSU”) that amended an original License Agreement between the Company and NCSU, dated December 8, 2015, and the First Amendment, dated February 14, 2018, to the original
F-19
License Agreement. Under the terms of the Second Amendment, the Company was obligated to pay NCSU milestone payments totaling $1,200, which originally amounted to a present value of $1,175. As of June 30, 2020 the Company paid the final milestone payment of $300. The cost of the of acquired license amounted to $1,175 and is included in Intangible assets, net on the Company’s Consolidated Balance Sheets, and is amortized on a straight-line basis over the last-to-expire patent, which is expected to be in 2036.
On October 22, 2018, the Company entered into a License Agreement with the University of Kentucky. Under the terms of the License Agreement, the Company is obligated to pay the University of Kentucky milestone payments totaling $1,200, of which $300 was payable upon execution, and $300 will be payable annually over on the anniversary of the execution of the License Agreement. The Company has recorded the present value of the obligations under the License Agreement as a note payable that originally amounted to $1,151. The cost of the of acquired licenses amounted to $1,151 and is included in Intangible assets, net on the Company’s Consolidated Balance Sheets and will be amortized on a straight-line basis over the last-to-expire patent, which is expected to be in 2033.
CARES Act Paycheck Protection Program Loan
On May 1, 2020, the Company received an U.S. Small Business Administration Loan (“SBA Loan”) from Bank of America, N.A. related to the COVID-19 crisis in the amount of $1,200. On May 12, 2020, the Company repaid the SBA loan in full.
D&O Insurance
During the second quarter of 2020, the Company renewed its Director and Officer (“D&O”) insurance for a policy premium totaling $2,744. The Company paid $549 as a premium down payment and financed the remaining $2,195 of policy premiums over at a 3.19% annual percentage rate. The financed amount is recorded within current notes payable on the Company’s Consolidated Balance Sheets.
The table below outlines our notes payable balances as of December 31, 2020 and December 31, 2019:
December 31, | December 31, |
| |||||
|
| 2020 |
| 2019 |
| ||
License Fees | $ | 293 | $ | 581 | |||
D&O Insurance |
| 246 |
| — | |||
Total current notes payable | $ | 539 | $ | 581 | |||
|
| ||||||
Long term license fees | $ | — | $ | 292 | |||
Accretion of non-cash interest expense amounted to $20 , $36, and $11 for the years ended December 31, 2020, 2019 and 2018, respectively.
NOTE 9. – SEVERANCE LIABILITY
During the second quarter of 2020, the Company recorded an accrual for severance benefits for $306 in accordance with FASB ASC 712 - “Compensation – Nonretirement Postemployment Benefits.” Consistent with certain contractual obligations related to a resignation, the Company will provide these severance benefits over a period.
During 2019, the Company recorded an accrual for severance benefits for $881 in accordance with FASB ASC 712 - “Compensation – Nonretirement Postemployment Benefits.” Consistent with certain contractual obligations, $771 of the related accrual will be paid via a monthly consulting fee for a period of .
The current and long-term accrued severance balance remaining as of December 31, 2020 was $339 and $241, respectively. The current and long-term accrued severance balance remaining as of December 31, 2019 was $359 and $446, respectively.
F-20
NOTE 10. – WARRANTS FOR COMMON STOCK
During 2018, the Company’s warrant holders exercised 794,869 outstanding warrants on a cashless basis resulting in the issuance of 490,012 shares of the Company’s common stock and the recording of a $168 reclassification of the remaining warrant liability to capital in excess of par. As of December 31, 2018, the outstanding warrants did not have an associated warrant liability.
On November 25, 2019, the Company entered into Warrant Exercise Agreements (the "2019 Exercise Agreements") with all of the holders (the "Holders") of its outstanding warrants to purchase up to 11,293,211 shares of common stock of the Company with an exercise price of $2.15 per share (the "Warrants") whereby the Holders and the Company agreed that the Holders would immediately exercise for cash 7,350,000 of the Warrants at a reduced exercise price of $1.00 per share, generating proceeds to the Company before expenses of approximately $7,400. In addition, the Holders agreed to exercise the remaining 3,943,211 Warrants for cash on or prior to January 27, 2020 provided that the Holders are in compliance with the beneficial ownership limitation provisions contained in the Warrants. The Holders exercised all of the Warrants for cash during December 2019 and the Company received net proceeds of approximately $10,600 from the exercise of all of the Warrants, after deducting expenses associated with the transaction.
In consideration for the Holders exercising their Warrants for cash, the Company issued to each Holder a new warrant (each, a "2019 Warrant") to purchase shares of common stock equal to the number of shares of common stock underlying the Warrants that shall be exercisable to the extent such Holder exercises for cash such Holder's Warrants pursuant to the 2019 Exercise Agreements. The terms of the 2019 are (i) exercisable from first issuance of the 2019 Warrants for a period of five years and (ii) had an initial exercise price equal to $1.25 per share. On December 22, 2019, the Company entered in to a Warrant Amendment Agreement with the holders of the 2019 Warrants (the "Amendment") whereby the Company agreed to amend the Warrants to (i) reduce the exercise price of the Warrants to $1.11 and (ii) to add a call provision whereby the Company may call the Warrants with prior notice to the holders for $0.001 per Warrant (during which time the holders may exercise the Warrants) provided that the Company's volume weighted average stock price exceeds $3.00 per share for consecutive trading days and certain other conditions are satisfied.
The Company’s outstanding warrants for the year ended December 31, 2020 and December 31, 2019 do not include anti-dilution features and therefore are not considered derivative instruments and do not have an associated warrant liability. The following table summarizes the Company’s warrant activity since December 31, 2018:
Number of | ||
Warrants | ||
Warrants outstanding at December 31, 2018 | 11,293,211 | |
Warrants exercised in Q4 2019 |
| (11,293,211) |
Warrants issued in Q4 2019 | 11,293,211 | |
Warrants outstanding at December 31, 2020 |
| 11,293,211 |
See Note 17 “Subsequent Events” for information regarding the cash exercise of the outstanding warrants subsequent to December 31, 2020.
NOTE 11. – RETIREMENT PLAN
The Company sponsors a defined contribution plan under IRC Section 401(k). The plan covers all employees who meet the minimum eligibility requirements. Under the 401(k) plan eligible employees are allowed to make voluntary deferred salary contribution to the plan, subject to statutory limits. The Company has elected to make Safe Harbor Non-Elective Contributions to the plan for eligible employees in the amount of three percent (3%) of the employee’s compensation. Total employer contributions to the plan for the years ended December 31, 2020, 2019 and 2018 amounted to $150, $157 and $141, respectively.
F-21
NOTE 12. – COMMITMENTS AND CONTINGENCIES
License agreements and sponsored research – The Company has entered into various license, sponsored research, collaboration, and other agreements (the “Agreements”) with various counter parties in connection with the Company’s plant biotechnology business relating to tobacco and hemp/cannabis. The schedule below summarizes the Company’s commitments, both financial and other, associated with each Agreement. Costs incurred under the Agreements are generally recorded as research and development expenses on the Company’s Consolidated Statements of Operations and Comprehensive Loss.
Future Commitments | |||||||||||||||||||||||||
Commitment |
| Counter Party |
| Product Relationship |
| Commitment Type |
| 2021 |
| 2022 |
| 2023 |
| 2024 |
| 2025 & After |
| Total |
| ||||||
Research Agreement | KeyGene | Hemp / Cannabis | Contract fee | $ | 1,200 | $ | 1,200 | $ | 1,200 | $ | 1,200 | $ | 300 | $ | 5,100 | (1) | |||||||||
License Agreement | NCSU | Tobacco | Annual royalty fee | 225 | 225 | — | — | — | 450 | (2), (3) | |||||||||||||||
License Agreement | NCSU | Tobacco | Minimum annual royalty | 25 | 50 | 50 | 50 | 600 | 775 | (3) | |||||||||||||||
License Agreement | NCSU | Tobacco | Minimum annual royalty | 50 | 50 | 50 | 50 | 500 | 700 | (3) | |||||||||||||||
Research Agreement | NCSU | Tobacco | Contract fee | 121 | — | — | — | — | 121 | (4) | |||||||||||||||
Sublicense Agreement | Anandia Laboratories, Inc. | Hemp / Cannabis | Annual license fee | 10 | 10 | 10 | 10 | 110 | 150 | (5) | |||||||||||||||
Growing Agreement | Various | Various | Contract fee | 115 | — | — | — | — | 115 | (6) | |||||||||||||||
$ | 1,746 | $ | 1,535 | $ | 1,310 | $ | 1,310 | $ | 1,510 | $ | 7,411 | ||||||||||||||
| (1) | Exclusive agreement with the Company with respect to the Cannabis Sativa L. plant (the "Field"). The initial term of the agreement is five years with an option for an additional two years. The aggregate cost of the agreement over the initial term is $6,000. The Company will exclusively own all results and all intellectual property relating to the results of the collaboration with KeyGene (the "Results”). The Company will pay royalties in varying amounts to KeyGene relating to the Company's commercialization in the Field of certain Results. The Company has granted KeyGene a license to commercialize the Results outside of the Field and KeyGene will pay royalties in varying amounts to the Company relating to KeyGene's commercialization outside of the Field of the Results. |
| (2) | The license agreement also requires a milestone payment of $150 upon FDA approval or clearance of a product that uses the NCSU licensed technology. The annual royalty fee is credited against running royalties on sales of licensed products. |
| (3) | The Company is also responsible for reimbursing NSCU for actual third-party patent costs incurred, including capitalized patent costs and patent maintenance costs. These costs vary from year to year and the Company has certain rights to direct the activities that result in these costs. |
| (4) | On September 11, 2020, the Company entered into a Sponsored Project Agreement with NCSU for continued research of tobacco alkaloid formation. |
| (5) | The Company is also responsible for the payment of certain costs, including, capitalized patent costs and patent maintenance costs, a running royalty on future net sales of products made from the sublicensed intellectual property, and a sharing of future sublicensing consideration received from sublicensing to third parties. |
| (6) | Various R&D growing agreements for hemp / cannabis and tobacco. |
Investment in Panacea - On December 3, 2019, the Company entered into an agreement to obtain a 15.8% ownership in Panacea. The Company paid Panacea $12,000 in cash and issued shares of 22nd Century common stock with a fair value of $1,297. The agreement with Panacea also requires the Company to purchase 5,333,334 shares of puttable preferred stock at $1.875 when Panacea achieves certain twelve-month sales targets—payable in cash of $8,500 and the remainder in common stock of the Company. The Company recently entered into a non-binding agreement with Panacea which would include an amendment of transaction documents to remove any future investment rights and obligations of the Company. See Note 6 for further information regarding the Company’s investment in Panacea.
Modified Risk Tobacco Product Application (“MRTP Application”) – In connection with the Company’s MRTP Application for its Very Low Nicotine Content (“VLNC”) cigarettes with the FDA, the Company has entered in various contracts with third-party service providers to fulfill various requirements of the MRTP Application. Such contracts include services for clinical trials, perception studies, legal guidance, product testing, and consulting expertise. During the years ended December 31, 2020, 2019 and 2018 the Company incurred expenses relating to these contracts in
F-22
the approximate amount of $38, $1,679, and $9,775, respectively. The Company will continue to incur consulting and legal expenses as the MRTP Application continues through the FDA review process. The Company cannot currently quantify the additional expenses that the Company will incur in the FDA review process because it will involve various factors that are within the discretion and control of the FDA.
Litigation -
Crede Settlement
On June 19, 2019, the Company, Crede CG III, LTD. (“Crede”) and Terren Peizer (“Peizer”) participated in a settlement conference meeting as required by the United States District Court for the Southern District of New York (the “SDNY Court”) entitled Crede CG III, LTD. v. 22nd Century Group, Inc. Subsequently, the Company, Crede and Peizer entered into a settlement agreement that settled this case, with the effective date of the settlement agreement being on July 22, 2019. Under the terms of the settlement agreement: (i) the Company issued to Crede on July 25, 2019 an aggregate of Nine Hundred Ninety Thousand (990,000) shares of common stock of the Company in full satisfaction of the cashless exchange of the Tranche 1A warrant and in settlement of all disputes between Crede, Peizer and the Company; (ii) Crede granted a proxy to the Company for a period of (5) years for the Company to vote all of the shares of common stock of the Company owned by Crede in favor of the recommendations by the Company’s Board of Directors (excluding any extraordinary transactions); (iii) Crede agreed to not purchase, borrow or short any securities of the Company; and (iv) the Company, Crede and Peizer agreed to mutual releases of all claims between the parties and the dismissal of all the litigation claims and counterclaims with prejudice.
The Company accrued an expense related to the settlement of this case during the second quarter of 2019 in the amount of $1,891, which is equal to the fair value of the 990,000 shares of Company common stock on July 22, 2019. The accrual was reclassified to capital upon the issuance of the common stock during the third quarter of 2019.
Class Action
On January 21, 2019, Matthew Jackson Bull, a resident of Denver, Colorado, filed a Complaint against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United States District Court for the Eastern District of New York entitled: Matthew Bull, Individually and on behalf of all others similarly situated, v. 22nd Century Group, Inc., Henry Sicignano III, and John T. Brodfuehrer, Case No. 1:19-cv-00409.
On January 29, 2019, Ian M. Fitch, a resident of Essex County Massachusetts, filed a Complaint against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United States District Court for the Eastern District of New York entitled: Ian Fitch, Individually and on behalf of all others similarly situated, v. 22nd Century Group, Inc., Henry Sicignano III, and John T. Brodfuehrer, Case No. 2:19-cv-00553.
On May 28, 2019, the plaintiff in the Fitch case voluntarily dismissed that action. On August 1, 2019, the Court in the Bull case issued an order designating Joseph Noto, Garden State Tire Corp, and Stephens Johnson as lead plaintiffs.
On September 16, 2019, pursuant to a joint motion by the parties, the Court in the Bull case transferred the class action to federal district court in the Western District of New York, where it remains pending as Case No. 1:19-cv-01285.
Plaintiffs in the Bull case filed an Amended Complaint on November 19, 2019 that alleges three counts: Count I sues the Company and Messrs. Sicignano and Brodfuehrer and alleges that the Company's quarterly and annual reports, SEC filings, press releases and other public statements and documents contained false statements in violation of Section 10(b) of the Securities Exchange Act and Rule 10b-5; Count II sues Messrs. Sicignano and Brodfuehrer pursuant to Section 10(b) of the Securities Exchange Act and Rule 10b5(a) and (c); and Count III sues Messrs. Sicignano and Brodfuehrer for the allegedly false statements pursuant to Section 20(a) of the Securities Exchange Act. The Amended Complaint seeks to certify a class, and unspecified compensatory and punitive damages, and attorney's fees and costs.
F-23
On January 29, 2020, the Company and Messrs. Sicignano and Brodfuehrer filed a Motion to Dismiss the Amended Complaint. On July 31, 2020, the Court heard oral arguments on the motion to dismiss. On January 14, 2021, the Court granted motion, dismissing all claims with prejudice. The Plaintiffs filed a notice of appeal on February 12, 2021 to the Second Circuit Court of Appeals. The Second Circuit has granted an expedited briefing schedule and Plaintiff’s/Appellant’s must be filed no later than April 12, 2021.
We believe that the claims are frivolous, meritless and that the Company and Messrs. Sicignano and Brodfuehrer have substantial legal and factual defenses to the claims. We intend to vigorously defend the Company and Messrs. Sicignano and Brodfuehrer against such claims.
Shareholder Derivative Cases
On February 6, 2019, Melvyn Klein, a resident of Nassau County New York, filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of Directors in the United States District Court for the Eastern District of New York entitled: Melvyn Klein, derivatively on behalf of 22nd Century Group v. Henry Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B. Sullivan, James W. Cornell, John T. Brodfuehrer and 22nd Century Group, Inc., Case No. 1:19-cv-00748. Mr. Klein brings this action derivatively alleging that (i) the director defendants supposedly breached their fiduciary duties for allegedly allowing the Company to make false statements; (ii) the director defendants supposedly wasted corporate assets to defend this lawsuit and the other related lawsuits; (iii) the defendants allegedly violated Section 10(b) of the Securities Exchange Act and Rule 10b-5 promulgated thereunder for allegedly approving or allowing false statements regarding the Company to be made; and (iv) the director defendants allegedly violated Section 14(a) of the Securities Exchange Act and Rule 14a-9 promulgated thereunder for allegedly approving or allowing false statements regarding the Company to be made in the Company’s proxy statement.
On February 11, 2019, Stephen Mathew filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of Directors in the Supreme Court of the State of New York, County of Erie, entitled: Stephen Mathew, derivatively on behalf of 22nd Century Group, Inc. v. Henry Sicignano, III, John T. Brodfuehrer, Richard M. Sanders, Joseph Alexander Dunn, James W. Cornell, Nora B. Sullivan and 22nd Century Group, Inc., Index No. 801786/2019. Mr. Mathew brings this action derivatively generally alleging the same allegations as in the Klein case. The Complaint seeks declaratory relief, unspecified monetary damages, corrective corporate governance actions, and attorney’s fees and costs. On August 15, 2019, the Court consolidated the Mathew and Klein actions pursuant to a stipulation by the parties (Western District of New York, Case No. 1-19-cv-0513). We believe that the claims are frivolous, meritless and that the Company and the individual defendants have substantial legal and factual defenses to the claims. We intend to vigorously defend the Company and the individual defendants against such claims.
On June 10, 2019, Judy Rowley filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of Directors in the Supreme Court of the State of New York, County of Erie, entitled: Judy Rowley, derivatively on behalf of 22nd Century Group, Inc. v. Henry Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B. Sullivan, James W. Cornell, John T. Brodfuehrer, and 22nd Century Group, Inc., Index No. 807214/2019. Ms. Rowley brings this action derivatively alleging that the director defendants supposedly breached their fiduciary duties by allegedly allowing the Company to make false statements. The Complaint seeks declaratory relief, unspecified monetary damages, corrective corporate governance actions, and attorney’s fees and costs. We believe that the claims are frivolous, meritless and that the Company and the individual defendants have substantial legal and factual defenses to the claims. We intend to vigorously defend the Company and the individual defendants against such claims. On September 13, 2019, the Court ordered the litigation stayed pursuant to a joint stipulation by the parties.
On January 15, 2020, Kevin Broccuto filed a shareholder derivative claim against the Company, the Company's then Chief Executive Officer, Henry Sicignano III, the Company's Chief Financial Officer, John T. Brodfuehrer, and certain members of the Company's prior Board of Directors in the District Court of the State of Nevada, County of Clark, entitled: Kevin Broccuto, derivatively on behalf of 22nd Century Group, Inc. v. James W. Cornell, Richard M.
F-24
Sanders, Nora B. Sullivan, Henry Sicignano, III, and John T. Brodfuehrer, Case No. A-20-808599. Mr. Broccuto brings this action derivatively alleging three counts: Count I alleges that the defendants breached their fiduciary duties; Count II alleges they committed corporate waste; and Count III that they were unjustly enriched, by allegedly allowing the Company to make false statements.
On February 11, 2020, Jerry Wayne filed a shareholder derivative claim against the Company, the Company's then Chief Executive Officer, Henry Sicignano III, the Company's Chief Financial Officer, John T. Brodfuehrer, and certain members of the Company's prior Board of Directors in the District Court of the State of Nevada, County of Clark, entitled: Jerry Wayne, derivatively on behalf of 22nd Century Group, Inc. v. James W. Cornell, Richard M. Sanders, Nora B. Sullivan, Henry Sicignano, III, and John T. Brodfuehrer, Case No. A-20-808599. Mr. Wayne brings this action derivatively alleging generally the same allegations as the Brocutto case. The Complaint seeks unspecified monetary damages, corrective corporate governance actions, disgorgement of alleged profits and imposition of constructive trusts, and attorney's fees and costs. The Complaint also seeks to declare as unenforceable the Company's Bylaw requiring derivative lawsuits to be filed in Erie County, New York, where the Company is headquartered.
On March 25, 2020, the Court ordered the Brocutto and Wayne cases consolidated and stayed pursuant to a joint stipulation from the parties. We believe that the claims are frivolous, meritless and that the Company and the individual defendants have substantial legal and factual defenses to the claims. We intend to vigorously defend the Company and the individual defendants against such claims.
NOTE 13. – EARNINGS PER COMMON SHARE
The following table sets forth the computation of basic and diluted earnings (loss) per common share for the years ended December 31, 2020, 2019 and 2018, respectively. Outstanding warrants, options, and restricted stock units were excluded from the calculation of diluted EPS as the effect was antidilutive.
Year Ended | |||||||||
December 31, | |||||||||
| 2020 |
| 2019 | 2018 | |||||
(in thousands, except for per-share data) | |||||||||
Net loss | $ | (19,711) | $ | (26,558) | $ | (7,967) | |||
Weighted average common shares outstanding - basic and diluted | 138,813 | 125,883 | 124,299 | ||||||
Net loss per common share - basic and diluted | $ | (0.14) | $ | (0.21) | $ | (0.06) | |||
Anti-dilutive shares are as follows: | |||||||||
Warrants | 11,293 | 11,293 | 11,293 | ||||||
Options | 6,581 | 7,837 | 8,672 | ||||||
Restricted stock units | 2,938 | 951 | — | ||||||
20,812 | 20,081 | 19,965 | |||||||
NOTE 14. – EQUITY BASED COMPENSATION
On April 12, 2014, the stockholders of the Company approved the 22nd Century Group, Inc. 2014 Omnibus Incentive Plan (the “OIP”) and the authorization of 5,000,000 shares to be reserved for issuance thereunder. On April 29, 2017, the stockholders approved an amendment to the OIP to increase the number of shares available for issuance by an additional 5,000,000 shares and on May 3, 2019, the stockholders approved an additional amendment to the OIP to increase the number of shares available for issuance by an additional 5,000,000 shares. The OIP allows for the granting of equity and cash incentive awards to eligible individuals over the life of the OIP, including the issuance of up to an aggregate of 15,000,000 shares of the Company’s common stock pursuant to awards under the OIP. The OIP has a term of ten years and is administered by the Compensation Committee of the Company’s Board of Directors to determine the various types of incentive awards that may be granted to recipients under the OIP and the number of shares of common stock to underlie each such award under the OIP. As of December 31, 2020, the Company had available 3,987,167 shares remaining for future awards under the OIP.
F-25
Restricted Stock Units (“RSU”). We grant restricted stock units to employees and non-employee directors which are valued based on the Company’s stock price on the award grant date. The following table summarizes the changes in unvested restricted stock from December 31, 2018 through December 31, 2020.
Unvested RSUs | |||||
Weighted | |||||
Average | |||||
Number of | Grant-date | ||||
| Shares |
| Fair Value | ||
in thousands | $ per share | ||||
Unvested at December 31, 2018 | — | $ | — | ||
Granted | 1,301 | $ | 2.21 | ||
Vested | (100) | $ | 2.02 | ||
Forfeited | (250) | $ | 2.02 | ||
Unvested at December 31, 2019 |
| 951 | $ | 2.15 | |
Granted |
| 2,885 | $ | 0.71 | |
Vested | (325) | $ | 1.07 | ||
Forfeited | (573) | $ | 1.90 | ||
Unvested at December 31, 2020 | 2,938 | $ | 0.85 | ||
The fair value of RSUs that vested during the years ended December 31, 2020 and 2019 was approximately $601 and $202, respectively, based on the stock price at the time of vesting.
Stock Options. Our outstanding stock options were valued using the Black-Scholes option-pricing model on the date of the award. A summary of all stock option activity since December 31, 2017 is as follows:
Weighted | |||||||||||
Weighted | Average | ||||||||||
Average | Remaining | Aggregate | |||||||||
Number of | Exercise | Contractual | Intrinsic | ||||||||
| Options |
| Price |
| Term |
|
| Value | |||
in thousands | $ per share | ||||||||||
Outstanding at December 31, 2017 |
| 8,157 | $ | 1.28 |
|
|
|
|
| ||
Granted |
| 1,632 | $ | 2.64 |
|
|
|
|
| ||
Exercised |
| (613) | $ | 0.87 |
|
|
|
|
| ||
Expired / cancelled |
| (504) | $ | 1.71 |
|
|
|
|
| ||
Outstanding at December 31, 2018 |
| 8,672 | $ | 1.54 |
|
|
|
|
| ||
Granted |
| 600 | $ | 2.07 |
|
|
|
|
| ||
Exercised | (75) | $ | 0.93 | ||||||||
Forfeited | (1,360) | $ | 2.09 | ||||||||
Outstanding at December 31, 2019 | 7,837 | $ | 1.49 | ||||||||
Exercised | (399) | $ | 1.04 | ||||||||
Forfeited | (169) | $ | 1.83 | ||||||||
Expired | (688) | $ | 1.51 | ||||||||
Outstanding December 31, 2020 |
| 6,581 | $ | 1.50 |
| 3.9 | years |
| $ | 3,983 | |
Exercisable at December 31, 2020 |
| 5,737 | $ | 1.51 |
| 3.7 | years |
| $ | 4,325 | |
The intrinsic value of a stock option is the amount by which the current market value or the market value upon exercise of the underlying stock exceeds the exercise price of the option.
F-26
The fair value of each option grant is estimated on the date of grant using the Black-Scholes option-pricing model. No option awards were granted in 2020. The following assumptions were used for the years ended December 31, 2019 and 2018:
| 2019 |
| 2018 |
| |
Risk-free interest rate (weighted average) |
| 1.54 | % | 2.77 | % |
Expected dividend yield |
| — | % | — | % |
Expected stock price volatility |
| 70 | % | 90 | % |
Expected life of options (weighted average) |
| 5.15 | years | 5.61 | years |
The Company estimated the expected volatility of the Company’s stock to be 70%. The expected term was estimated using the contract life of the option. The risk-free interest rate assumption was determined using yield of the equivalent U.S. Treasury bonds over the expected term. The Company has never paid any cash dividends and does not anticipate paying any cash dividends in the foreseeable future. Therefore, the Company assumed an expected dividend yield of zero.
Restricted Stock and Stock Option Compensation Expense. The Company recognized the following compensation costs, net of actual forfeitures, related to restricted stock and stock options:
Year Ended | |||||||||
December 31, | |||||||||
| 2020 |
| 2019 |
| 2018 | ||||
Sales, general, and administrative | $ | 1,526 | $ | 3,166 | $ | 1,341 | |||
Research and Development |
| 128 |
| 374 |
| 1,846 | |||
Total restricted stock and stock option compensation | $ | 1,654 | $ | 3,540 | $ | 3,187 | |||
As of December 31, 2020, unrecognized compensation expense amounted to $1,528 which is expected to be recognized over a weighted average period of approximately 0.9 years. In addition, there is approximately $637 of unrecognized stock option compensation expense that requires the achievement of certain milestones which have yet to be obtained.
NOTE 15. – INCOME TAXES
The following is a summary of the components giving rise to the income tax provision (benefit) ended years ended December 31, 2020, 2019 and 2018:
| 2020 |
| 2019 |
| 2018 | ||||
Current: |
|
|
|
|
|
| |||
Federal | $ | — | $ | — | $ | — | |||
State |
| — |
| — |
| — | |||
Total current | $ | — | $ | — | $ | — | |||
Deferred: |
|
|
|
|
|
| |||
Federal | (3,932) |
| (5,607) |
| (1,446) | ||||
State |
| (200) |
| 55 |
| (14) | |||
Total deferred |
| (4,132) |
| (5,552) |
| (1,460) | |||
Change in valuation allowance |
| 4,170 |
| 5,552 |
| 1,460 | |||
Total income taxes | $ | 38 | $ | — | $ | — | |||
F-27
The provision (benefit) for income tax varies from that which would be expected based on applying the statutory federal rate to pre-tax accounting loss, including the effect of the change in the U.S. corporate income tax rates, as follows:
| 2020 |
| 2019 |
| 2018 |
| |
Statutory federal rate |
| (21.0) | % | (21.0) | % | (21.0) | % |
Other items |
| (0.3) |
| (0.1) |
| 0.1 | |
Litigation Settlement |
| — |
| 1.5 |
|
| |
Derivative liability |
| — |
| — |
| (0.1) | |
Stock based compensation |
| 0.7 |
| 1.8 |
| 2.5 | |
Research and development credit carryforward |
| 0.2 |
| (3.3) |
| (1.2) | |
State tax provision, net of federal benefit |
| (0.8) |
| 0.2 |
| (0.1) | |
Equity investment |
| — |
| — |
| 1.5 | |
Federal tax rate change |
| — |
| — |
| — | |
Valuation allowance |
| 21.4 |
| 20.9 |
| 18.3 | |
Effective tax rate (benefit) provision |
| 0.2 | % | — | % | — | % |
Individual components of deferred taxes consist of the following as of December 31:
| 2020 |
| 2019 |
| 2018 | ||||
Deferred tax assets: |
|
|
|
|
|
| |||
Net operating loss carry-forward | $ | 18,498 | $ | 14,996 | $ | 11,527 | |||
Inventory |
| 115 |
| 52 |
| 21 | |||
Stock-based compensation |
| 1,099 |
| 1,049 |
| 813 | |||
Start-up expenditures |
| 199 |
| 221 |
| 243 | |||
Research and development credit carryforward |
| 1,171 |
| 1,209 |
| 326 | |||
Loss on equity investment |
| — |
| — |
| — | |||
Accrued bonus |
| 423 |
| 200 |
| 101 | |||
Severance liability |
| 122 |
| 134 |
| — | |||
Investment in Panacea |
| 360 |
| 40 |
| — | |||
Operating lease obligations |
| 52 |
| 127 |
| — | |||
Other |
| 29 |
| 22 |
| 20 | |||
$ | 22,068 | $ | 18,050 | $ | 13,051 | ||||
Deferred tax liabilities: |
|
|
|
|
|
| |||
Machinery and equipment |
| (237) |
| (239) |
| (215) | |||
Patents and trademarks |
| (358) |
| (351) |
| (562) | |||
Gain on investment |
| (13) |
| (104) |
| (612) | |||
Accrued expense |
| (24) |
| (51) |
| (71) | |||
Operating lease right-of-use assets |
| (52) |
| (126) |
| — | |||
Other intangible assets |
| (224) |
| (189) |
| (153) | |||
| (908) |
| (1,060) |
| (1,613) | ||||
Valuation allowance |
| (21,198) |
| (16,990) |
| (11,438) | |||
Net deferred taxes | $ | (38) | $ | — | $ | — | |||
The Company generated net operating losses (“NOL”) of approximately $15,500, $16,800, and $7,700 for the years ended December 31, 2020, 2019 and 2018 respectively, and these NOL carryforward losses do not expire. The Company had accumulated an NOL carryforward of approximately $46,900 through December 31, 2017 and this NOL carryforward begins to expire in 2031. As of December 31, 2020, the Company has a research and development credit carryforward of approximately $1,171 that begins to expire in 2031. Utilization of these NOL carryforwards may be subject to an annual limitation in the case of equity ownership changes, as defined by law. Due to the uncertainty of the Company’s ability to generate sufficient taxable income in the future, the Company has recorded a valuation allowance
F-28
to reduce the net deferred tax asset to zero. These carryforwards are included in the net deferred tax asset that has been fully offset by the valuation allowance.
ASC 740 provides guidance on the financial statement recognition and measurement for uncertain income tax positions that are taken or expected to be taken in a company’s income tax return. The Company has evaluated its tax positions and believes there are no uncertain tax positions as of December 31, 2020.
NOTE 16. – REVENUE RECOGNITION
The Company recognizes revenue when it satisfies a performance obligation by transferring control of the product to a customer. The Company’s customer contracts consist of obligations to manufacture the customer’s branded filtered cigars and cigarettes. For certain contracts, the performance obligation is satisfied over time as the Company determines, due to contract restrictions, it does not have an alternative use of the product, and it has an enforceable right to payment as the product is manufactured. The Company recognizes revenue under those contracts at the unit price stated in the contract based on the units manufactured. Revenue from the sale of the Company’s products is recognized net of cash discounts, sales returns and allowances. There was no allowance for discounts or returns and allowances at December 31, 2020 and December 31, 2019.
Contract Assets and Liabilities
Unbilled receivables (contract assets) represent revenues recognized for performance obligations that have been satisfied but have not been billed. These receivables are included as Accounts receivable, net on the Consolidated Balance Sheets. Customer payment terms vary depending on the terms of each customer contract, but payment is generally due prior to product shipment or within extended credit terms up to (21) days after shipment. Deferred Revenue (contract liabilities) relate to down payments received from customers in advance of satisfying a performance obligation. This deferred revenue is included as Deferred income on the Consolidated Balance Sheets.
Total contract assets and contract liabilities are as follows:
December 31, | December 31, | |||||
| 2020 |
| 2019 | |||
Unbilled receivables |
| $ | 349 |
| $ | 406 |
Deferred Revenue | (272) | (5) | ||||
Net contract assets | $ | 77 | $ | 401 | ||
Disaggregation of Revenue
The Company’s net sales revenue is derived from customers located primarily in the United States of America and is disaggregated by the timing of revenue recognition—net sales transferred over time and net sales transferred at a point in time. All revenue is related to contract manufacturing.
Year Ended | |||||||||
December 31, | |||||||||
| 2020 |
| 2019 |
| 2018 | ||||
Net sales-over time | $ | 16,326 | $ | 16,466 | $ | 16,785 | |||
Net sales-point in time |
| 11,785 |
| 9,367 |
| 9,641 | |||
Total Revenue | $ | 28,111 | $ | 25,833 | $ | 26,426 | |||
The Company had certain customers whose revenue individually represented 10% or more the Company’s total revenue. For the year ended December 31, 2020, two customers accounted for approximately 91% of total revenue. For the years ended December 31, 2019, and 2018, three customers accounted for approximately for 92.7% and 92.0% of total revenue, respectively.
F-29
NOTE 17. – SUBSEQUENT EVENT
Corporate Office Relocation
On January 15, 2021, the Company signed a lease agreement to relocate its corporate headquarters to the Larkinville District in downtown Buffalo, NY. Details on the lease are described below ($ in thousands):
Key Lease Terms | |||
$ in thousands | |||
Commencement Date | February 15, 2021 | ||
Monthly Base Rent (Year 1) | $ | 6 | |
Base Rent Annual Increase (Year 2 - onward) | 2.5 | % | |
Initial Term (months) | 36 | ||
In addition, the lease includes two optional, extensions at the Company’s discretion. During the first quarter of 2021, the Company will recognize the respective ROU asset and lease liability for the above operating lease.
Warrant Exercises
During February and March of 2021, the Company’s warrant holders exercised 9,577,612 warrants for cash in exchange for common stock. In connection with these exercises, the Company received net proceeds of $9,993. On March 10, 2021, the Company’s warrant holders exercised for cash the remaining 1,715,599 outstanding warrants and no outstanding warrants remain.
NOTE 18. – SELECTED QUARTERLY FINANCIAL DATA (unaudited)
Below is selected quarterly financial data for the years ended December 31, 2020 and 2019:
| Three Months Ended | |||||||||||
March 31, | June 30, | September 30, | December 31, | |||||||||
| 2020 |
| 2020 |
| 2020 |
| 2020 | |||||
Revenue, net | $ | 7,058 | $ | 6,435 | $ | 7,310 | $ | 7,308 | ||||
Gross (loss) profit | $ | 287 | $ | 201 | $ | 362 | $ | 588 | ||||
Loss from operations | $ | (4,142) | $ | (4,753) | $ | (4,040) | $ | (6,248) | ||||
Net loss | $ | (4,028) | $ | (5,057) | $ | (4,221) | $ | (6,405) | ||||
Loss per common share – basic and diluted | $ | (0.03) | $ | (0.04) | $ | (0.03) | $ | (0.04) | ||||
| Three Months Ended | |||||||||||
| March 31, |
| June 30, |
| September 30, |
| December 31, | |||||
| 2019 |
| 2019 |
| 2019 |
| 2019 | |||||
Revenue, net | $ | 6,294 | $ | 5,815 | $ | 6,462 | $ | 7,262 | ||||
Gross profit | $ | (103) | $ | (86) | $ | (21) | $ | 225 | ||||
Loss from operations | $ | (5,379) | $ | (5,029) | $ | (7,606) | $ | (5,552) | ||||
Net income (loss) | $ | (2,073) | $ | (8,042) | $ | (10,245) | $ | (6,198) | ||||
Loss per common share – basic and diluted | $ | (0.02) | $ | (0.06) | $ | (0.08) | $ | (0.05) | ||||
F-30
Item 15(b).Financial Statement Schedules
All schedules have been omitted as they are not required, not applicable, or the required information is otherwise included.
Item 15(c).Exhibits
In reviewing the agreements included as exhibits to this report, please remember they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about the Company, its subsidiaries or other parties to the agreements. The agreements contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the other parties to the applicable agreement and:
· | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
· | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
· | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and |
· | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. We acknowledge that, notwithstanding the inclusion of the foregoing cautionary statements, we are responsible for considering whether additional specific disclosures of material information regarding material contractual provisions are required to make the statements in this report not misleading. Additional information about the Company may be found elsewhere in this report and the Company’s other public files, which are available without charge through the SEC’s website at http://www.sec.gov.
52
Exhibit No. |
| Description |
|
|
|
| Amended and Restated Certificate of Incorporation of the Company (incorporated herein by reference to Exhibit 3.2 of the Company’s Annual Report on Form 10-K for the year ended September 30, 2010 filed with the Commission on December 1, 2010). | |
|
|
|
| Amendment to Certificate of Incorporation of the Company (incorporated by reference to Appendix A to the Company’s Definitive Proxy Statement filed with the Commission on March 4, 2014). | |
|
|
|
| Amended and Restated Bylaws of the Company (incorporated herein by reference to Exhibit 3.2 of the Company’s Annual Report on Form 10-K for the year ended December 31, 2014 filed with the Commission on January 30, 2014). | |
|
|
|
| Amendment No. 1 to Amended and Restated Bylaws of the Company (incorporated herein by reference to Exhibit 3.2 of the Company’s Form 8-K filed with the Commission on April 28, 2015). | |
|
|
|
| Form of New Warrant Agreement (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the Commission on November 25, 2019) | |
|
|
|
| Description of Securities Registered Pursuant to Section 12 | |
|
|
|
| 2010 Equity Incentive Plan (incorporated herein by reference to Exhibit 4.3 to the Company’s Form S-8 filed with the Commission on March 30, 2011). | |
|
|
|
| Employment Agreement between the Company and Michael J. Zercher (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the Commission on September 13, 2019) | |
|
|
|
| License Agreement dated March 6, 2009 between North Carolina State University and 22nd Century Limited, LLC (incorporated by reference to Exhibit 10.21 to the Company’s Form S-1 registration statement filed with the Commission on August 26, 2011). | |
|
|
|
| Amendment dated August 9, 2012 to License Agreement dated March 6, 2009 between North Carolina State University and 22nd Century Limited, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Commission on August 20, 2012). | |
|
|
|
| Letter Agreement between the Company and North Carolina State University dated November 22, 2011 (incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed with the Commission on November 23, 2011). | |
|
|
|
| Form of Restricted Stock Award Agreement (incorporated by reference to Form 10-Q filed on May 10, 2013). | |
|
|
|
| Form of Stock Option Award Agreement (incorporated by reference to Form 10-Q filed on May 10, 2013). | |
|
|
|
| Form of Restricted Stock Award Agreement under 22nd Century Group, Inc. 2014 Omnibus Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed with the Commission on April 14, 2014). | |
|
|
|
| Form of Stock Option Award Agreement under 22nd Century Group, Inc. 2014 Omnibus Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K filed with the Commission on April 14, 2014). | |
|
|
|
53
| 22nd Century Group, Inc. 2014 Omnibus Incentive Plan, as amended and restated (incorporated by reference from Appendix A to the Company’s definitive proxy statement filed on March 22, 2019). | |
|
|
|
| Form of Executive Restricted Stock Unit Award under 2014 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.15 of the Company’s Annual Report on Form 10-K filed with the Commission on March 6, 2019). | |
|
|
|
| Form of Director Restricted Stock Unit Award under 2014 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.16 of the Company’s Annual Report on Form 10-K filed with the Commission on March 6, 2019). | |
|
|
|
| Series B Preferred Stock Purchase Agreement, dated as of December 3, 2019, between Panacea Life Sciences., Inc and 22nd Century Group, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Commission on December 3, 2019). | |
|
|
|
| Convertible Note of Panacea Life Sciences., Inc., dated December 3, 2019, issued to 22nd Century Group, Inc. (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the Commission on December 3, 2019). | |
|
|
|
| Warrant to purchase shares of Series B Preferred Stock of Panacea Life Sciences., Inc., dated December 3, 2019, issued to 22nd Century Group, Inc. (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K filed with the Commission on December 3, 2019). | |
|
|
|
| Side Agreement, dated as of December 3, 2019, Panacea Life Sciences, Inc., [EMPLOYEE], [EMPLOYEE], [EMPLOYEE], [EMPLOYEE], Quintel-MC Incorporated, and 22nd Century Group, Inc. (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K filed with the Commission on December 3, 2019). | |
|
|
|
| Framework Collaborative Research Agreement, dated as of April 3, 2019, between KeyGene N.V. and 22nd Century Group, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q filed with the Commission on May 7, 2019). | |
Employment Agreement between the Company and James Mish (incorporated by reference to exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Commission on June 3, 2020). | ||
Employment Agreement between the Company and John Franzino (incorporated by reference to exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the Commission on June 3, 2020). | ||
|
|
|
| Consent of Freed Maxick CPAs, P.C. | |
|
|
|
| Section 302 Certification. | |
|
|
|
| Section 302 Certification. | |
|
|
|
| Written Statement of Principal Executive Officer and Chief Financial Officer pursuant to 18.U.S.C §1350. | |
|
|
|
101* |
| Interactive data files formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Cash Flows, and (iv) the Notes to the Consolidated Financial Statements. |
|
|
|
101.INS XBRL |
| Instance Document* |
54
|
|
|
101.SCH XBRL |
| Taxonomy Extension Schema Document* |
|
|
|
101.CAL XBRL |
| Taxonomy Extension Calculation Linkbase Document* |
|
|
|
101.DEF XBRL |
| Taxonomy Extension Definition Linkbase Document* |
|
|
|
101.LAB XBRL |
| Taxonomy Extension Label Linkbase Document* |
|
|
|
101.PRE XBRL |
| Taxonomy Extension Presentation Linkbase Document* |
Exhibit 104 | Cover Page Interactive Data File – The cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document* |
* Filed herewith.
† Management contract or compensatory plan, contract or arrangement.
†† Certain portions of the exhibit have been omitted pursuant to a confidential treatment order. An unredacted copy of the exhibit has been filed separately with the United States Securities and Exchange Commission pursuant to the request for confidential treatment.
†††Certain portions of the exhibit have been omitted pursuant Regulation S-K Item 601(b) because it is both (i) not material to investors and (ii) likely to cause competitive harm to the Company is publicly disclosed.
55
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
| 22nd CENTURY GROUP, INC. |
|
|
|
|
Date: | March 11, 2021 | By: | /s/ James A. Mish |
|
|
| James A. Mish |
|
|
| Chief Executive Officer |
|
|
| (Principal Executive Officer) |
|
|
|
|
Date: | March 11, 2021 | By: | /s/ John Franzino |
|
|
| John Franzino |
|
|
| Chief Financial Officer |
|
|
| (Principal Accounting and Financial Officer) |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: | March 11, 2021 | By: | /s/ Nora B. Sullivan |
|
|
| Nora B. Sullivan |
|
|
|
|
Date: | March 11, 2021 | By: | /s/ Richard M. Sanders |
|
|
| Richard M. Sanders |
|
|
|
|
Date: | March 11, 2021 | By: | /s/ Clifford B. Fleet |
|
|
| Clifford B. Fleet |
|
|
|
|
Date: | March 11, 2021 | By: | /s/ Roger D. O’Brien |
|
| Roger D. O’Brien | |
Date: | March 11, 2021 | By: | /s/ Dr. Michael Koganov |
|
| Dr. Michael Koganov Director | |
56
Similar companies
See also Philip Morris International Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also British American Tobacco p.l.c.
See also ALTRIA GROUP, INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also SWEDISH MATCH CORP
See also RLX Technology Inc.
