Azenta, Inc. - Annual Report: 2013 (Form 10-K)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One) | ||
þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For fiscal year ended September 30, 2013 | ||
or | ||
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from to . | ||
Commission File Number: 0-25434
Brooks Automation, Inc.
(Exact name of Registrant as Specified in Its Charter)
Delaware | 04-3040660 | |
(State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification No.) | |
15 Elizabeth Drive Chelmsford, Massachusetts (Address of Principal Executive Offices) | 01824 (Zip Code) | |
978-262-2400
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on Which Registered | |
Common Stock, $0.01 par value | The NASDAQ Stock Market LLC | |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Rule 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to the Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer þ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ |
(Do not check if a smaller reporting company) | |||
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes ¨ No þ
The aggregate market value of the registrant's Common Stock, $0.01 par value, held by nonaffiliates of the registrant as of March 28, 2013, was approximately $662,787,700 based on the closing price per share of $10.18 on that date on the Nasdaq Stock Market. As of March 31, 2013, 66,490,810 shares of the registrant's Common Stock, $0.01 par value, were outstanding. As of November 5, 2013, 66,577,235 shares of the registrant's Common Stock, $0.01, par value, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's Proxy Statement involving the election of directors, which is expected to be filed within 120 days after the end of the registrant's fiscal year, are incorporated by reference in Part III of this Report.
BROOKS AUTOMATION, INC.
TABLE OF CONTENTS
PAGE NUMBER | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Item 15. | ||
2
PART I
Item 1. | Business |
Brooks Automation, Inc. (“Brooks”, “we”, “us”, or “our”), a Delaware corporation, is a leading worldwide provider of automation, vacuum and instrumentation solutions for multiple markets including semiconductor manufacturing, technology device manufacturing and life sciences. Our technologies, engineering competencies and global service capabilities provide customers speed to market and ensure high uptime and rapid response, which equate to superior value in their mission-critical controlled environments. Since 1978, we have been a leading partner to the global semiconductor manufacturing markets and through product development initiatives and strategic business acquisitions we have expanded our reach to meet the needs of customers in technology markets adjacent to semiconductor and life sciences. Brooks is headquartered in Chelmsford, MA with full service operations in North America, Europe and Asia.
Our company initially developed and marketed automated handling equipment for front-end semiconductor manufacturing tools and became a publicly traded company in February 1995. Through both internal product development and significant business acquisition activity we became the leading provider of these automation solutions in this market. Since that time, we have expanded both the markets we serve as well as our core product capabilities. A notable step in our diversification was the acquisition of Helix Technology Corporation in 2005 which provided us with leading technology solutions in vacuum and instrumentation equipment and allowed us to serve a broader set of markets. In 2011, we identified life sciences as a strategic market that was underserved that provided favorable growth opportunities where Brooks' core competencies of automation and cold temperature management of a controlled environment could provide enabling technology solutions. During 2011 we made two strategic acquisitions to penetrate this market. In April 2011, we acquired RTS Life Sciences (“RTS”), a Manchester, UK-based business, and in July 2011 we acquired Nexus Biosystems, Inc. (“Nexus”), a Poway, CA-based business. We further enhanced our life sciences offering in August 2013 by acquiring substantially all of the assets of Matrical, Inc. ("Matrical"), a Spokane, WA-based business.
Although we have made acquisitions to expand the non-semiconductor portions of our business, we have continued to make investments to maintain and grow our semiconductor product and service offerings. In January 2012, we acquired intangible assets from Intevac, Inc. for $3.0 million, for use in the development of next generation semiconductor automation tools. In October 2012, we acquired Crossing Automation Inc., a Fremont, CA-based provider of automation solutions for the global semiconductor front-end market, for $59.0 million in cash.
During the period 2006 through June 2011 we acquired and then further developed a significant contract manufacturing business in order to provide leading wafer front-end equipment manufacturers with an extension of their own assembly and test capability, enabling them to better focus on their core processes and to offer flexibility during industry cycles. In June 2011, we divested this business in order to focus on our core technology solutions.
Markets
Our fiscal 2013, 2012 and 2011 revenue by end market was as follows:
2013 | 2012 | 2011 | ||||||
Semiconductor capital equipment | 53 | % | 54 | % | 65 | % | ||
Service and spares | 20 | % | 17 | % | 13 | % | ||
Industrial capital equipment | 10 | % | 11 | % | 11 | % | ||
Other adjacent technology markets | 7 | % | 8 | % | 9 | % | ||
Life sciences | 10 | % | 10 | % | 2 | % | ||
100 | % | 100 | % | 100 | % | |||
The proportion of our revenue by end market is changing as a result of our internal product and sales initiatives, our acquisitions and divestitures and the cyclical nature of the semiconductor capital equipment market. Our divested contract manufacturing business exclusively served semiconductor product end markets, and the RTS, Nexus and Matrical acquisitions serve life sciences markets. Although we have entered into transactions to expand the non-semiconductor portions of our business, we will continue to make investments to maintain and grow our semiconductor product and service offerings.
Semiconductor capital equipment
The global semiconductor capital equipment industry is cyclical with a long term growth profile driven by the expanded use of semiconductor devices and the increase in device complexity, each necessitating incremental equipment purchases. This growth is increasingly focused in Asia. The production of advanced semiconductor chips is a complex and logistically challenging manufacturing activity. To create the tens of millions of microscopic transistors and connect them both horizontally and in vertical layers in order to produce a functioning integrated circuit, or IC chip, the silicon wafers must go through hundreds of process steps performed by complex processing equipment, or tools, to create the integrated circuits. A large
3
production fab may have more than 70 different types of process and metrology tools, totaling as many as 500 tools or more. Up to 40% of these tools perform processes in a vacuum, such as removing, depositing, or measuring material on wafer surfaces. Wafers can go through as many as 400 different process steps before fabrication is complete. These steps, which comprise the initial fabrication of the integrated circuit and are referred to in the industry as front-end processes, are repeated many times to create the desired pattern on the silicon wafer. As the complexity of semiconductors continues to increase, the number of process steps that occur in a vacuum environment also increases, resulting in a greater need for both automation and vacuum technology solutions due to the sensitive handling requirements and increased number of tools. The requirement for efficient, higher throughput and extremely clean manufacturing for semiconductor wafer fabs and other high performance electronic-based products has created a substantial market for substrate handling automation (moving the wafers around and between tools in a semiconductor fab), tool automation (the use of robots and modules used in conjunction with and inside process tools that move wafers from station-to-station), and vacuum systems technology to create and sustain the environment necessary to fabricate various products. Advanced processing used to form three dimensional structures of the previously patterned integrated circuits is emerging. This processing, often referred to as Wafer Level Packaging ("WLP"), is typically performed at what would be considered the back-end processing of an IC chip. Some front-end processes are being used in this back-end advance packaging, thereby increasing the market for automation solutions.
Service and spares
Whereas sales for production equipment are typically made to original equipment manufacturers (“OEMs”), the service and spares support of that equipment is more typically a relationship with the end-user manufacturer who is using that equipment in a productive capacity. While the majority of the market that we currently address with our service and spares activities is the semiconductor manufacturing market, we are actively looking to increase our service offerings in the life science market.
Industrial capital equipment
There are a variety of industrial manufacturing operations that require either a vacuum or significant cooling for effective deposition of films or coatings. The expansion of technologies such as touch screen manufacturing equipment is driving greater application of these operations and the requirement for the associated vacuum and instrumentation solutions that we provide. These deposition processes are typically performed on equipment that cycles from an uncontrolled atmospheric environment for loading and unloading to a controlled vacuum environment for processing. The transition to the controlled vacuum environment requires removal of large amounts of moisture inherent from the air in a typical operation. This moisture removal is accomplished by deep cooling of coils within the vacuum chamber and the increased need for the equipment necessary to deliver refrigerant supply to those coils results in increased demand for our products.
Other adjacent technology markets
There are a variety of markets that have adopted, or are adopting, similar manufacturing methods to those utilized by the semiconductor industry. Frequently, these markets have common customers but technology applications in the end markets are still maturing. We serve a variety of these evolving markets including light emitting diode (“LED”) applications. High Brightness LED (“HBLED”) is a potential clean energy solution replacing incandescent lighting sources. We believe that the application of HBLED solutions to these general illumination applications will expand as manufacturing processes for these products advance, resulting in lower costs of production and more attractive pricing for these products. Organic LED (“OLED”) solutions provide lower power consumption for high clarity still and video images. OLED applications are gaining traction in the mobile computing and telecommunications device markets. Other evolving markets which utilize our products include Micro-Electro-Mechanical Systems (“MEMS”) manufacturing and solar panel manufacturing. MEMS applications, which include accelerometers, self tuning antennae and pressure gauges, are expanding in automotive, mobile computing and telecommunications device markets. We believe that solar panel production is also expanding, and our products are used in the production of thin film solar panels which require cooling to effectuate deposition and adhesion.
Life Sciences
There is a broad market of devices, systems and consumables that support the pharmaceutical, biotechnology, healthcare research and diagnostics industries in the advanced handling, processing, storage and distribution of biological and compound samples. At the core of these activities is sample storage. Facilities that store biological samples are commonly called biobanks or biorepositories. Automated sample stores are generally more effective in maintaining a controlled environment, tracking samples, reliably processing and quickly handling samples, than are manual systems. These automated sample management systems are at the center of the complete sample handling process. With the advent of personalized medicine linking DNA to optimal treatment regimens, the expansion of mass storage of key biological material to support rapidly expanding comparative and longitudinal studies, and the accumulation of samples taken from surgical and other procedures, we believe that the numbers of samples in storage is expanding between 25 and 30% per annum on a global basis. We believe that this expansion,
4
together with manual stores that become overwhelmed by the numbers of samples they accumulate, will drive consistent growth in automated sample management equipment.
Products
In the semiconductor industry, wafer handling robotics have emerged as a critical technology in determining the efficacy and productivity of complex production tools in the world's most advanced 300mm wafer fabs. A tool is built around a process chamber using automation technology to move wafers into and out of the chamber. Today, OEMs build their tools using a cluster architecture, whereby several process chambers are mounted to one central transfer module. High wafer throughput and new materials require advanced automation solutions to address the challenging equipment needs for multiple substrate sizes, including the emerging 450mm wafer size transition and advanced technology nodes. We specialize in developing and building the handling systems, as well as the vacuum technologies used in these tools. Our products can be utilized as individual components or as complete integrated handling systems. Automation products are provided to support both atmospheric and vacuum based processes while our focus remains on improving performance and productivity. The majority of our product revenue is derived from sales to OEMs and semiconductor device manufacturers.
We provide high vacuum pumps and instrumentation which are required in certain process steps to create and to optimize the process environment by maintaining pressure consistency of the known process gas. To achieve optimal production yields, semiconductor manufacturers must ensure that each process operates at carefully controlled pressure levels. Impurities or incorrect pressure levels can lower production yields, thereby significantly increasing the cost per usable semiconductor chip produced. We provide various pressure measurement instruments that form part of this pressure control loop on production processing equipment. Some key vacuum processes include: dry etching and dry stripping, chemical vapor deposition ("CVD"), physical vapor deposition ("PVD"), and ion implantation. Our cryogenic vacuum pumps are considered the industry standard by many leading semiconductor device manufacturers for ion implant and PVD applications.
In the HBLED market we have worked with leading manufacturers to develop advanced automation solutions that improve the productivity of processes that were previously manual. These LEDs are also made using vacuum processes for certain production steps, very similar to the semiconductor manufacturing flow. We have been successful in capturing market share for our vacuum product offerings and for high payload automated tool architectures. In other adjacent markets, such as MEMS and WLP applications, unique wafer handling and automation solutions are required to accommodate increasingly thinner and sometimes bowed substrates. We are developing differentiated solutions to address the requirements in these high growth market segments.
For the life science markets we provide automated sample management platforms that store samples (e.g., nucleic acid, blood, drug compounds, biological tissue, etc.) in a controlled environment and automate the process (vials are typically stored in racks or plates) of the subsequent retrieval of specifically selected samples from those racks or plates. The storage environments ensure that samples are preserved within a narrow temperature band to maintain their integrity for long periods while providing absolute accuracy in the identification and selection of samples. We are an early pioneer in bringing to market stores that operate as low as minus 80OC.
In providing comprehensive solutions to the life science markets we also provide equipment for sealing and de-sealing samples stored on plates and automated cappers and de-cappers for samples stored in tubes. We also provide consumables in the form of sample plates, micro-plates and tubes and support services for many of the customers who have purchased our equipment.
Segments
We report financial results in four segments: Brooks Product Solutions; Brooks Global Services; Brooks Life Science Systems; and Contract Manufacturing.
The Brooks Product Solutions segment provides a variety of products critical to technology equipment productivity and availability. Those products include atmospheric and vacuum tool automation systems, atmospheric and vacuum robots and robotic modules and cryogenic vacuum pumping, thermal management and vacuum measurement solutions which are used to create, measure and control critical process vacuum applications.
The Brooks Global Services segment provides an extensive range of support services including on-and off-site repair services, on-and off-site diagnostic support services, and installation services that enable our customers to maximize process tool uptime and productivity. This segment also provides end-user customers with spare parts support services that maximize tool productivity.
The Brooks Life Science Systems segment provides automated sample management systems for automated cold sample storage, equipment for sample preparation and handling, consumables, and parts and support services to a wide range of life science customers including pharmaceutical companies, biotechnology companies, biobanks, national laboratories, research institutes and research universities.
5
The Contract Manufacturing segment provided services to build equipment front-end modules, vacuum transport modules and other subassemblies which enabled our customers to effectively source high quality and high reliability process tools for semiconductor market applications. We sold this business unit to Celestica Inc. on June 28, 2011.
Customers
Within the semiconductor industry, we sell our products and services to most of the major semiconductor chip manufacturers and semiconductor equipment OEMs in the world. Our customers outside the semiconductor industry are broadly diversified. We have major customers in North America, Europe and Asia. Additionally, although much of our equipment sales ship to United States OEMs, many of those products ultimately are utilized outside of North America. See Part I, Item 1A, “Risk Factors” for a discussion of the risks related to foreign operations. The Brooks Global Services business provides support to leading fabs and foundries across the globe.
Our life sciences systems solutions are used by pharmaceutical customers, national laboratories, biological drug development companies, research institutes and research hospitals. There is no continuing concentration of customers for the Brooks Life Science Systems segment although given the size of particular projects, an individual customer may be significant to the life science segment in a given quarter or fiscal year.
Relatively few customers account for a substantial portion of our revenue, with the top 10 customers accounting for approximately 39% of our business in fiscal 2013. We have one customer, Applied Materials, Inc., that accounted for 11% of our overall revenue for the year.
In our assessment of customer concentration, we primarily consider the OEM who designs the proprietary tool as our customer since they make the design-in decision rather than an intermediary contract manufacturer who is the entity to whom we invoice. For fiscal 2013, no contract manufacturer represented more than 10% of revenue.
Sales, Marketing and Customer Support
We market and sell most of our semiconductor, industrial and other adjacent technology market products and services in Asia, Europe, the Middle East and North America through our direct sales organization. The sales process for our products is often multilevel, involving a team comprised of individuals from sales, marketing, engineering, operations and senior management. In many cases we assign a team to a customer and that team engages the customer at different levels of its organization to facilitate planning, provide product customization when required, and to ensure open communication and support. Some of our vacuum and instrumentation products and services are sold through local country distributors. Additionally, we serve the Japanese market for our robotics and automation products through Yaskawa Brooks Automation, our joint venture with Yaskawa Electric Corporation of Japan.
We market to most of our life sciences customers through our direct Brooks Life Science Systems sales force. In regions with emerging life science industries such as China, India and the Middle East, we leverage local distributors to assist in the sales process. The sales process for our larger sample management systems may take 6-18 months to complete and it involves a team comprised of individuals from sales, marketing, engineering and senior management.
We typically provide warranties from one to two years, depending upon the type of product, with the average of our products at 15 months.
Our marketing activities include participation in trade shows, delivery of seminars, participation in industry forums, distribution of sales literature, publication of press releases and articles in business and industry publications. To enhance communication and support, particularly with our international customers, we maintain sales and service centers in Asia, Europe, the Middle East and North America. These facilities, together with our headquarters, maintain local support capability and demonstration equipment for customers to evaluate. Customers are encouraged to discuss features and applications of our demonstration equipment with our engineers located at these facilities.
Net revenue for the years ended September 30, 2013, 2012 and 2011 based upon the source of the order by geographic area is as follows (in thousands):
Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
North America | $ | 193,222 | $ | 230,735 | $ | 349,456 | |||||
Asia/Pacific | 165,452 | 194,711 | 244,524 | ||||||||
Europe | 92,278 | 94,005 | 94,125 | ||||||||
$ | 450,952 | $ | 519,451 | $ | 688,105 | ||||||
The geographic location of an OEM is not indicative of where our products will eventually be used. This is determined by the onward sale of an OEM system which incorporates our sub-systems and/or components.
6
Our property, plant and equipment by geographic area is as follows (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
North America | $ | 38,869 | $ | 51,546 | |||
Asia/Pacific | 1,646 | 2,123 | |||||
Europe | 7,355 | 10,809 | |||||
$ | 47,870 | $ | 64,478 | ||||
Competition
We operate in a variety of niches of varying breadth and with differing competitors and competitive dynamics. The semiconductor and adjacent technology markets, and process equipment manufacturing industries are highly competitive and characterized by continual changes and improvements in technology. A significant portion of equipment automation is still done in-house by OEMs. Our competitors among external vacuum automation suppliers are primarily Japanese companies such as Daihen, Daikin and Rorze. Our competitors among vacuum components suppliers include Sumitomo Heavy Industries, Genesis and Telemark. We have a significant share of the market for vacuum cryogenic pumps and mixed gas cryo-chillers. Competitors in markets for our instrumentation products include MKS Instruments and Inficon. Atmospheric tool automation is typically less demanding and has a larger field of competitors. We compete directly with other equipment automation suppliers of atmospheric modules and systems such as Hirata, Kawasaki, Genmark, Rorze, Sankyo, TDK and Symphonia. Contract manufacturers such as Celestica and Flextronics are also providing assembly and manufacturing services for atmospheric systems.
Our Life Science Systems business unit competes with a number of private companies in providing automated sample management systems. These competitors include Hamilton, HighRes Biosolutions, Liconic and TTP LabTech.
We believe our customers will purchase our equipment automation products and vacuum subsystems as long as our products continue to provide the necessary throughput, reliability, contamination control and accuracy at an acceptable price point. We believe that we have competitive offerings with respect to all of these factors; however, we cannot guarantee that we will be successful in selling our products to OEMs who currently satisfy their automation needs in-house or from other independent suppliers, regardless of the performance or price of our products.
Research and Development
Our research and development efforts are focused on developing new products and also enhancing the functionality, degree of integration, reliability and performance of our existing products. Our engineering, marketing, operations and management personnel leverage their close collaborative relationships with many of their counterparts in customer organizations in an effort to proactively identify market demands with an ability to refocus our research and development investment to meet our customer demands. With the rapid pace of change that characterizes the markets we serve, it is essential for us to provide high-performance and reliable products in order for us to maintain our leadership position.
Our research and development spending for fiscal years 2013, 2012 and 2011 was $49.0 million, $47.5 million and $39.8 million, respectively.
Manufacturing
Our manufacturing operations are used for product assembly, integration and testing. We have adopted quality assurance procedures that include standard design practices, component selection procedures, vendor control procedures and comprehensive reliability testing and analysis to ensure the performance of our products. Our major manufacturing facilities are located in Chelmsford, Massachusetts; Poway, California; Petaluma, California; Longmont, Colorado; Monterrey, Mexico; Yongin-City, South Korea and Manchester, UK. We also provide service and spare parts support to end users throughout the world. Many of our service customers are based outside of the U.S., with many in Asia. We have service and support locations close to these customers to provide rapid response to their service needs. We have service and support locations in Chelmsford, Massachusetts; Chu Bei City, Taiwan; Yongin-City, South Korea; Yokohama, Japan; Shanghai, China; Singapore; Jena, Germany; Oberdiessbach, Switzerland; and, Kiryat-Gat, Israel.
We utilize lean manufacturing techniques for a large portion of our manufacturing process. We believe that this strategy, coupled with the outsourcing of non-critical components such as machined parts, wire harnesses and PC boards, reduces our fixed operating costs, improves our working capital efficiency, reduces our manufacturing cycle times and improves our flexibility to rapidly adjust production capacities. While we often use single source suppliers for certain key components and common assemblies to achieve quality control and the benefits of economies of scale, we believe that these parts and materials are readily available from other supply sources. We expect to continue to broaden the sourcing of our components to low cost
7
regions, including Asia. We also outsource the production of certain product lines to contract manufacturers, most of which are located in Asia.
Patents and Proprietary Rights
We rely on patents, trade secret laws, confidentiality procedures, copyrights, trademarks and licensing agreements to protect our technology. Our United States patents expire at various times through 2030. Due to the rapid technological change that characterizes the life sciences, semiconductor, adjacent technology markets and related process equipment industries, we believe that the improvement of existing technology, reliance upon trade secrets and unpatented proprietary know-how and the development of new products may be as important as patent protection in establishing and maintaining a competitive advantage. To protect trade secrets and know-how, it is our policy to require all technical and management personnel to enter into proprietary information and nondisclosure agreements. We cannot guarantee that these efforts will meaningfully protect our trade secrets.
We have successfully licensed our front-opening unified pod load port technology to significant load port manufacturers.
Backlog
Backlog for our products as of September 30, 2013, totaled $107.2 million as compared to $81.1 million at September 30, 2012. Backlog consists of purchase orders for which a customer has scheduled delivery within the next 12 months. Backlog consists of orders principally for hardware and service agreements. Orders included in the backlog may be canceled or rescheduled by customers without significant penalty. Backlog as of any particular date should not be relied upon as indicative of our revenue for any future period. A substantial percentage of current business generates no backlog because we deliver our products and services in the same period in which the order is received.
Environmental Matters
We are subject to federal, state, and local environmental laws and regulations, as well as the environmental laws and regulations of the foreign national and local jurisdictions in which we have manufacturing facilities. We believe we are in material compliance with all such laws and regulations.
Compliance with foreign, federal, state, and local laws and regulations has not had, and is not expected to have, an adverse effect on our capital expenditures, competitive position, financial condition, or results of operations.
Employees
At September 30, 2013, we had 1,471 full time employees. In addition, we utilized 185 part time employees and contractors. Approximately 45 employees in our facility in Jena, Germany are covered by a collective bargaining agreement. We consider our relationships with these and all employees to be good.
Available Information
We file annual, quarterly, and current reports, proxy statements, and other documents with the Securities and Exchange Commission (“SEC”) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The public may read and copy any materials that we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Also, the SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers, including Brooks Automation, Inc., that file electronically with the SEC. The public can obtain any documents that we file with the SEC at www.sec.gov.
Our internet website address is http://www.brooks.com. Through our website, we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after such materials are electronically filed, or furnished to, the SEC. These SEC reports can be accessed through the investors section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
Item 1A. | Risk Factors |
Factors That May Affect Future Results
You should carefully consider the risks described below and the other information in this report before deciding to invest in shares of our common stock. These are the risks and uncertainties we believe are most important for you to consider. Additional risks and uncertainties not presently known to us, which we currently deem immaterial or which are similar to those faced by other companies in our industry or business in general, may also impair our business operations. If any of the following risks or uncertainties actually occurs, our business, financial condition and operating results would likely suffer. In that event, the market price of our common stock could decline and you could lose all or part of your investment.
8
Risks Relating to Our Industry
Due in part to the cyclical nature of the semiconductor manufacturing industry and related industries, as well as due to volatility in worldwide capital and equity markets, we have previously incurred operating losses and may have future losses.
Our business is largely dependent on capital expenditures in the semiconductor manufacturing industry and other businesses employing similar manufacturing technology. The semiconductor manufacturing industry in turn depends on current and anticipated demand for integrated circuits and the products that use them. In recent years, these businesses have experienced unpredictable and volatile business cycles due in large part to rapid changes in demand and manufacturing capacity for semiconductors, and these cycles have had an impact on our business, sometimes causing declining revenue and operating losses. We could experience future operating losses during an industry downturn. If an industry downturn continues for an extended period of time, our business could be materially harmed. Conversely, in periods of rapidly increasing demand, we could have insufficient inventory and manufacturing capacity to meet our customer needs on a timely basis, which could result in the loss of customers and various other expenses that could reduce gross margins and profitability.
We face competition which may lead to price pressure and otherwise adversely affect our sales.
We face competition throughout the world in each of our product areas. This comes from competitors as discussed in Part I, Item 1, “Business - Competition” as well as internal robotic capabilities at larger OEMs. Many of our competitors have substantial engineering, manufacturing, marketing and customer support capabilities. We expect our competitors to continue to improve the performance of their current products and to introduce new products and technologies that could adversely affect sales of our current and future products and services. New products and technologies developed by our competitors or more efficient production of their products could require us to make significant price reductions or decide not to compete for certain orders. If we fail to respond adequately to pricing pressures or fail to develop products with improved performance or developments with respect to the other factors on which we compete, we could lose customers or orders. If we are unable to compete effectively, our business and prospects could be materially harmed.
Risks Relating to Brooks
Our operating results could fluctuate significantly, which could negatively impact our business.
Our revenue, operating margins and other operating results could fluctuate significantly from quarter to quarter depending upon a variety of factors, including:
• | demand for our products as a result of the cyclical nature of the semiconductor manufacturing industry and the markets upon which it depends or otherwise; |
• | changes in the timing and terms of product orders by our customers as a result of our customer concentration or otherwise; |
• | changes in the mix of products and services that we offer; |
• | changes in the demand for the mix of products and services that we offer; |
• | timing and market acceptance of our new product introductions; |
• | delays or problems in the planned introduction of new products, or in the performance of any such products following delivery to customers; |
• | new products, services or technological innovations by our competitors, which can, among other things, render our products less competitive due to the rapid technological change in our industry; |
• | the timing and related costs of any acquisitions, divestitures or other strategic transactions; |
• | our ability to reduce our costs in response to decreased demand for our products and services; |
• | our ability to accurately estimate customer demand, including the accuracy of demand forecasts used by us; |
• | disruptions in our manufacturing process or in the supply of components to us; |
• | write-offs for excess or obsolete inventory; and |
• | competitive pricing pressures. |
As a result of these risks, we believe that quarter-to-quarter comparisons of our revenue and operating results may not be meaningful, and that these comparisons may not be an accurate indicator of our future performance.
9
If we do not continue to introduce new products and services that reflect advances in technology in a timely and effective manner, our products and services may become obsolete and our operating results will suffer.
Our success is dependent on our ability to respond to the technological change present in the markets we serve. The success of our product development and introduction depends on our ability to:
• | accurately identify and define new market opportunities and products; |
• | obtain market acceptance of our products; |
• | timely innovate, develop and commercialize new technologies and applications; |
• | adjust to changing market conditions; |
• | differentiate our offerings from our competitors' offerings; |
• | obtain intellectual property rights where necessary; |
• | continue to develop a comprehensive, integrated product and service strategy; |
• | properly price our products and services; and |
• | design our products to high standards of manufacturability such that they meet customer requirements. |
If we cannot succeed in responding in a timely manner to technological and/or market changes or if the new products that we introduce do not achieve market acceptance, it could diminish our competitive position which could materially harm our business and our prospects.
The global nature of our business exposes us to multiple risks.
For the fiscal years ended September 30, 2013 and 2012, approximately 57% and 56%, respectively, of our revenue was derived from sales outside North America. We expect that international sales, including increased sales in Asia, will continue to account for a significant portion of our revenue. We maintain a global footprint of sales, service and repair operations. As a result of our international operations, we are exposed to many risks and uncertainties, including:
• | longer sales-cycles and time to collection; |
• | tariff and international trade barriers; |
• | fewer or less certain legal protections for intellectual property and contract rights abroad; |
• | different and changing legal and regulatory requirements in the jurisdictions in which we operate; |
• | government currency control and restrictions on repatriation of earnings; |
• | fluctuations in foreign currency exchange and interest rates, particularly in Asia and Europe; and |
• | political and economic instability, changes, hostilities and other disruptions in regions where we operate. |
Negative developments in any of these areas in one or more countries could result in a reduction in demand for our products, the cancellation or delay of orders already placed, threats to our intellectual property, difficulty in collecting receivables, and a higher cost of doing business, any of which could materially harm our business and profitability.
Our business could be materially harmed if we fail to adequately integrate the operations of the businesses that we have acquired or may acquire.
We have made in the past, and may make in the future, acquisitions or significant investments in businesses with complementary products, services and/or technologies. Our acquisitions present numerous risks, including:
• | difficulties in integrating the operations, technologies, products and personnel of the acquired companies and realizing the anticipated synergies of the combined businesses; |
• | defining and executing a comprehensive product strategy; |
• | managing the risks of entering markets or types of businesses in which we have limited or no direct experience; |
• | the potential loss of key employees, customers and strategic partners of ours or of acquired companies; |
• | unanticipated problems or latent liabilities, such as problems with the quality of the installed base of the target company's products or infringement of another company's intellectual property by a target company's activities or products; |
• | problems associated with compliance with the acquired company's existing contracts; |
• | difficulties in managing geographically dispersed operations; and |
• | the diversion of management's attention from normal daily operations of the business. |
10
If we acquire a new business, we may be required to expend significant funds, incur additional debt or issue additional securities, which may negatively affect our operations and be dilutive to our stockholders. In periods following an acquisition, we will be required to evaluate goodwill and acquisition-related intangible assets for impairment. When such assets are found to be impaired, they will be written down to estimated fair value, with a charge against earnings. The failure to adequately address these risks could materially harm our business and financial results.
Entering new markets introduces new competitors and commercial risks.
A key part of our growth strategy is to continue expanding beyond the semiconductor manufacturing market into semiconductor adjacent and life sciences markets. As part of this strategy, we expect to diversify our product sales by leveraging our core technologies, which requires investments and resources which may not be available as needed. We cannot guarantee that we will be successful in leveraging our capabilities into the life sciences market to meet all the needs of these new customers and to compete favorably. Because a significant portion of our growth potential may be dependent on our ability to increase sales to markets beyond semiconductor manufacturing, an inability to successfully enter new markets may adversely impact future financial results.
Changes in key personnel could impair our ability to execute our business strategy.
The continuing service of our executive officers and essential engineering, technical and management personnel, together with our ability to attract and retain such personnel, is an important factor in our continuing ability to execute our strategy. There is substantial competition to attract such employees and the loss of any such key employees could have a material adverse effect on our business and operating results. The same could be true if we were to experience a high turnover rate among engineering and technical personnel and we were unable to replace them.
We may be subject to claims of infringement of third-party intellectual property rights, or demands that we license third-party technology, which could result in significant expense and prevent us from using our technology.
We rely upon patents, trade secret laws, confidentiality procedures, copyrights, trademarks and licensing agreements to protect our technology. Due to the rapid technological change that characterizes the semiconductor and adjacent technology markets, we believe that the improvement of existing technology, reliance upon trade secrets and unpatented proprietary know-how and the development of new products may be as important as patent protection in establishing and maintaining competitive advantage. To protect trade secrets and know-how, it is our policy to require all technical and management personnel to enter into nondisclosure agreements. We cannot guarantee that these efforts will meaningfully protect our trade secrets.
There has been substantial litigation regarding patent and other intellectual property rights in the semiconductor-related industries. We have in the past been, and may in the future be, notified that we may be infringing intellectual property rights possessed by third parties. We cannot guarantee that infringement claims by third parties or other claims for indemnification by customers or end users of our products resulting from infringement claims will not be asserted in the future or that such assertions, if proven to be true, will not materially and adversely affect our business, financial condition and results of operations.
We cannot predict the extent to which we might be required to seek licenses or alter our products so that they no longer infringe the rights of others. We also cannot guarantee that licenses will be available or the terms of any licenses we may be required to obtain will be reasonable. Similarly, changing our products or processes to avoid infringing the rights of others may be costly or impractical and could detract from the value of our products. If a judgment of infringement were obtained against us, we could be required to pay substantial damages and a court could issue an order preventing us from selling one or more of our products. Further, the cost and diversion of management attention brought about by such litigation could be substantial, even if we were to prevail. Any of these events could result in significant expense to us and may materially harm our business and our prospects.
Our failure to protect our intellectual property could adversely affect our future operations.
Our ability to compete is significantly affected by our ability to protect our intellectual property. Existing trade secret, trademark and copyright laws offer only limited protection. Our success depends in part on our ability to obtain and enforce patent protection for our products both in the United States and in other countries. We own numerous U.S. and foreign patents, and we intend to file additional applications, as appropriate, for patents covering our products and technology. Any issued patents owned by or licensed to us may be challenged, invalidated or circumvented, and the rights under these patents may not provide us with competitive advantages. In addition, the laws of some countries in which our products are or may be developed, manufactured or sold may not fully protect our products. We cannot guarantee that the steps we have taken to protect our intellectual property will be adequate to prevent the misappropriation of our technology. Other companies could independently develop similar or superior technology without violating our intellectual property rights. In the future, it may be necessary to engage in litigation or like activities to enforce our intellectual property rights, to protect our trade secrets or to
11
determine the validity and scope of proprietary rights of others, including our customers. This could require us to incur significant expenses and to divert the efforts and attention of our management and technical personnel from our business operations.
The expiration of our patents over time could lead to competitors entering the market for our products and a decline in our revenue.
One of our main competitive strengths is our technology and we are dependent on our patent rights and other intellectual property rights to maintain our competitive position. Our current patents will expire from time to time through 2030 and pending or future patent applications are subject to issuance at the discretion of the relevant governmental authorities, and the claims allowed under any issued patents may not be sufficiently broad to protect our technology. The expiration of a patent could result in increased competition and a decline in revenue for the expiring patent holder. In addition, we license certain of our patents to third parties in exchange for agreed upon royalties which will terminate upon the expiration of those patents and could therefore adversely impact our revenue.
If our manufacturing sites were to experience a significant disruption in operations, our business could be materially harmed.
We have a limited number of manufacturing facilities for our products. If the operations of these facilities were disrupted as a result of a natural disaster, fire, power or other utility outage, work stoppage or other similar event, our business could be seriously harmed because we may be unable to manufacture and ship products and parts to our customers in a timely fashion.
Our business could be materially harmed if one or more key suppliers fail to continuously deliver key components of acceptable cost and quality.
We currently obtain many of our key components on an as-needed, purchase order basis from numerous suppliers. In some cases we have only a single source of supply for necessary components and materials used in the manufacturing of our products. Further, we are increasing our sourcing of products in Asia, and particularly in China, and we do not have a previous course of dealing with many of these suppliers. We do not generally have long-term supply contracts with any of these suppliers, and many of them underwent cost-containment measures in light of the last industry downturn. As the industry has recovered, these suppliers have faced challenges in delivering components on a timely basis. This volatility in demand has led some of our vendors to exit the semiconductor market, and other vendors may also decide to exit this market. Our inability to obtain components or materials in required quantities or of acceptable cost and quality and with the necessary continuity of supply could result in delays or reductions in product shipments to our customers. In addition, if a supplier or sub-supplier suffers a production stoppage or delay for any reason, including natural disasters like the ones that affected Japan and Thailand, this could result in a delay or reduction in product shipments to our customers. Any of these contingencies could cause us to lose customers, result in delayed or lost revenue and otherwise materially harm our business.
Our outsource providers may fail to perform as we expect.
Outsource providers have played and will continue to play a key role in our manufacturing operations and in many of our transactional and administrative functions, such as information technology and facilities management. Although we attempt to select reputable providers and secure their performance on terms documented in written contracts, it is possible that one or more of these providers could fail to perform as we expect and such failure could have an adverse impact on our business.
Our business relies on certain critical information systems and a failure or breach of such a system could harm our business and results of operations and, in the event of unauthorized access to a customer’s data or our data, incur significant legal and financial exposure and liabilities.
We maintain and rely upon certain critical information systems for the effective operation of our business. These information systems include telecommunications, the internet, our corporate intranet, various computer hardware and software applications, network communications, and e-mail. These information systems may be owned and maintained by us, our outsource providers or third parties such as vendors and contractors. These information systems are subject to attacks, failures, and access denials from a number of potential sources including viruses, destructive or inadequate code, power failures, and physical damage to computers, hard drives, communication lines, and networking equipment. To the extent that these information systems are under our control, we have implemented security procedures, such as virus protection software and emergency recovery processes, to mitigate the outlined risks. However, security procedures for information systems cannot be guaranteed to be failsafe and our inability to use or access these information systems at critical points in time, or unauthorized releases of confidential information, could unfavorably impact the timely and efficient operation of our business.
Confidential information stored on these information systems could also be compromised. If a third party gains unauthorized access to our data, including any regarding our customers, such security breach could expose us to a risk of loss of this information, loss of business, litigation and possible liability. These security measures may be breached as a result of third-party action, including intentional misconduct by computer hackers, employee error, malfeasance or otherwise.
12
Additionally, third parties may attempt to fraudulently induce employees or customers into disclosing sensitive information such as user names, passwords or other information in order to gain access to our customers' data or our data, including our intellectual property and other confidential business information, or our information technology systems. Because the techniques used to obtain unauthorized access, or to sabotage systems, change frequently and generally are not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. Any security breach could result in a loss of confidence by our customers, damage our reputation, disrupt our business, lead to legal liability and negatively impact our future sales.
Our intangible assets may become impaired.
As of September 30, 3013, we had approximately $122 million of goodwill and $60 million in net intangible assets, which has been identified as a result of our acquisitions. We periodically review our goodwill and the estimated useful lives of our identifiable intangible assets, taking into consideration any events or circumstances that might result in either a diminished fair value, or for intangible assets, a revised useful life. The events and circumstances include significant changes in the business climate, legal factors, operating performance indicators, advances in technology and competition. Any impairment or revised useful life could have a material and adverse effect on our financial position and results of operations, and could harm the trading price of our common stock.
Changes in tax rates or tax regulation could affect results of operations.
As a global company, we are subject to taxation in the United States and various other countries. Significant judgment is required to determine and estimate worldwide tax liabilities. Our future annual and quarterly effective tax rates could be affected by numerous factors, including changes in the: applicable tax laws; composition of pre-tax income in countries with differing tax rates; and/or valuation of our deferred tax assets and liabilities. In addition, we are subject to regular examination by the Internal Revenue Service, state, local and foreign tax authorities. We regularly assess the likelihood of favorable or unfavorable outcomes resulting from these examinations to determine the adequacy of our provision for income taxes. Although we believe our tax estimates are reasonable, there can be no assurance that any final determination will not be materially different from the treatment reflected in our historical income tax provisions and accruals, which could materially and adversely affect our financial condition and results of operations.
We are subject to numerous governmental regulations.
We are subject to federal, state, local and foreign regulations, including environmental regulations and regulations relating to the design and operation of our products and control systems. We might incur significant costs as we seek to ensure that our products meet safety and emissions standards, many of which vary across the states and countries in which our products are used. In the past, we have invested significant resources to redesign our products to comply with these directives. Compliance with future regulations, directives, and standards could require us to modify or redesign some products, make capital expenditures, or incur substantial costs. If we do not comply with current or future regulations, directives, and standards:
• | we could be subject to fines; |
• | our production or shipments could be suspended; and |
• | we could be prohibited from offering particular products in specified markets. |
If we were unable to comply with current or future regulations, directives and standards our business, financial condition and results of operations could be materially and adversely affected.
Our stock price is volatile.
The market price of our common stock has fluctuated widely. From the beginning of fiscal year 2012 through the end of fiscal year 2013, our stock price fluctuated between a high of $12.65 per share and a low of $7.00 per share. Consequently, the current market price of our common stock may not be indicative of future market prices, and we may be unable to sustain or increase the value of an investment in our common stock. Factors affecting our stock price may include:
• | variations in operating results from quarter to quarter; |
• | changes in earnings estimates by analysts or our failure to meet analysts' expectations; |
• | changes in the market price per share of our public company customers; |
• | market conditions in the semiconductor and other industries into which we sell products; |
• | economic conditions in Europe and general economic conditions; |
• | political changes, hostilities or natural disasters such as hurricanes and floods; |
• | low trading volume of our common stock; and |
• | the number of firms making a market in our common stock. |
13
In addition, the stock market has in the past experienced significant price and volume fluctuations. These fluctuations have particularly affected the market prices of the securities of high technology companies like ours. These market fluctuations could adversely affect the market price of our common stock.
Unfavorable currency exchange rate fluctuations may lead to lower operating margins, or may cause us to raise prices, which could result in reduced sales.
Currency exchange rate fluctuations could have an adverse effect on our sales and results of operations and we could experience losses with respect to forward exchange contracts into which we may enter. Unfavorable currency fluctuations could require us to increase prices to foreign customers, which could result in lower net sales by us to such customers. Alternatively, if we do not adjust the prices for our products in response to unfavorable currency fluctuations, our results of operations could be materially and adversely affected. In addition, most sales made by our foreign subsidiaries are denominated in the currency of the country in which these products are sold and the currency they receive in payment for such sales could be less valuable at the time of receipt as a result of exchange rate fluctuations. From time to time, we enter into forward exchange contracts to reduce currency exposure arising from intercompany sales of inventory. However, we cannot be certain that our efforts will be adequate to protect us against significant currency fluctuations or that such efforts will not expose us to additional exchange rate risks, which could materially and adversely affect our results of operations.
Risks Relating to Our Customers
Because we rely on a limited number of customers for a large portion of our revenue, the loss of one or more of these customers could materially harm our business.
We receive a significant portion of our revenue in each fiscal period from a relatively limited number of customers, and that trend is likely to continue. Sales to our ten largest customers accounted for approximately 39%, 43% and 55% of our total revenue in the fiscal years ended September 30, 2013, 2012 and 2011, respectively. While we expect this percentage to continue to decrease due to the sale of our contract manufacturing business and our life sciences acquisitions, the loss of one or more of these major customers, a significant decrease in orders from one of these customers, or the inability of one or more customers to make payments to us when they are due could materially affect our revenue, business and reputation. In addition, there has been and may continue to be significant consolidation among some of our largest OEM customers, which could lead to increased pressure to reduce the price of our products and/or decreased market share with the combined companies.
Because of the lengthy sales cycles of many of our products, we may incur significant expenses before we generate any revenue related to those products.
Our customers may need several months to test and evaluate our products. This increases the possibility that a customer may decide to cancel or change plans, which could reduce or eliminate our sales to that customer. The impact of this risk can be magnified during the periods in which we introduce a number of new products, as has been the case in recent years. As a result of this lengthy sales cycle, we may incur significant research and development expenses, and selling, general and administrative expenses before we generate the related revenue for these products, and we may never generate the anticipated revenue if our customer cancels or changes its plans.
In addition, many of our products will not be sold directly to the end-user but will be components of other products. As a result, we rely on OEMs to select our products from among alternative offerings to be incorporated into their equipment at the design stage; so-called design-ins. The OEMs' decisions often precede the generation of volume sales, if any, by a year or more. Moreover, if we are unable to achieve these design-ins from an OEM, we would have difficulty selling our products to that OEM because changing suppliers involves significant cost, time, effort and risk on the part of that OEM.
Customers generally do not make long term commitments to purchase our products and our customers may cease purchasing our products at any time.
Sales of our products are often made pursuant to individual purchase orders and not under long-term commitments and contracts. Our customers frequently do not provide any assurance of minimum or future sales and are not prohibited from purchasing products from our competitors at any time. Accordingly, we are exposed to competitive pricing pressures on each order. Our customers also engage in the practice of purchasing products from more than one manufacturer to avoid dependence on sole-source suppliers for certain of their needs. The existence of these practices makes it more difficult for us to increase price, gain new customers and win repeat business from existing customers.
Item 1B. | Unresolved Staff Comments |
None.
14
Item 2. | Properties |
Our corporate headquarters and primary manufacturing/research and development facilities are currently located in two buildings in Chelmsford, Massachusetts, which we purchased in January 2001. We lease a third building in Chelmsford adjacent to the two that we own. In September 2013, we sold an underutilized building that we owned on the Chelmsford, Massachusetts campus, to a real estate investment trust. In summary, we maintain the following active principal facilities:
Location | Functions | Square Footage (Approx.) | Ownership Status/Lease Expiration | ||||
Chelmsford, Massachusetts | Corporate headquarters, training, manufacturing and R&D | 201,000 | Owned | ||||
Chelmsford, Massachusetts | Manufacturing | 97,000 | October 2014 | ||||
Petaluma, California | Manufacturing and R&D | 72,300 | February 2016 | ||||
Poway, California | Manufacturing and R&D | 67,600 | July 2015 | ||||
Longmont, Colorado | Manufacturing and R&D | 60,900 | February 2015 | ||||
Yongin-City, South Korea | Manufacturing, R&D and sales & support | 49,600 | February 2022 | ||||
Fremont, California | R&D and sales and support | 44,900 | August 2018 | ||||
Manchester, UK | Manufacturing, R&D and sales & support | 42,000 | December 2019 | ||||
Jena, Germany | Manufacturing, R&D and sales & support | 30,100 | January 2017 | ||||
Chu Bei City, Taiwan | Sales & support | 28,600 | June 2014 | ||||
Our Brooks Product Solutions segment utilizes the facilities in Massachusetts, California, Colorado and South Korea as well as a smaller manufacturing and R&D facility in Germany. Our Brooks Global Services segment utilizes the facilities in Massachusetts, South Korea, Germany and Taiwan. Our Brooks Life Science Systems segment utilizes the facilities in Poway, California and the UK.
We maintain additional sales and support and training offices in California and Texas and overseas in Europe (France, Germany and Switzerland), as well as in Asia (Japan, China, Singapore and Taiwan) and the Middle East (Israel).
We utilize a third party to manage our manufacturing operation in Mexico. As part of our arrangement with this third party, we guarantee a lease for a 56,100 square foot manufacturing facility. The remaining payments under this lease, which expires in 2015, are approximately $0.5 million.
Item 3. | Legal Proceedings |
We are subject to various legal proceedings, both asserted and unasserted, that arise in the ordinary course of business. We cannot predict the ultimate outcome of such legal proceedings or in certain instances provide reasonable ranges of potential losses. However, as of the date of this report, we believe that none of these claims will have a material adverse effect on our consolidated financial condition or results of operations. In the event of unexpected subsequent developments and given the inherent unpredictability of these legal proceedings, there can be no assurance that our assessment of any claim will reflect the ultimate outcome and an adverse outcome in certain matters could, from time-to-time, have a material adverse effect on our consolidated financial condition or results of operations in particular quarterly or annual periods.
15
Item 4. | Mine Safety Disclosures |
Not applicable.
PART II
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is traded on the NASDAQ Stock Market LLC under the symbol “BRKS.” The following table sets forth the high and low intraday sales prices per share of our common stock as reported by the NASDAQ Stock Market LLC and the cash dividends declared per common share for the periods indicated:
Market Price | Dividends Declared | ||||||||||
High | Low | ||||||||||
Fiscal year ended September 30, 2013 | |||||||||||
First quarter | $ | 8.24 | $ | 7.00 | $ | 0.08 | |||||
Second quarter | 10.50 | 8.23 | 0.08 | ||||||||
Third quarter | 10.97 | 8.78 | 0.08 | ||||||||
Fourth quarter | 10.56 | 8.74 | 0.08 | ||||||||
Fiscal year ended September 30, 2012 | |||||||||||
First quarter | $ | 11.05 | $ | 7.40 | $ | 0.08 | |||||
Second quarter | 12.49 | 9.98 | 0.08 | ||||||||
Third quarter | 12.65 | 8.82 | 0.08 | ||||||||
Fourth quarter | 9.89 | 7.78 | 0.08 | ||||||||
Number of Holders
As of October 31, 2013, there were 758 holders of record of our common stock.
Dividend Policy
Dividends are declared at the discretion of our Board of Directors and depend on actual cash from operations, our financial condition and capital requirements and any other factors our Board of Directors may consider relevant. Future dividend declarations, as well as the record and payment dates for such dividends, will be determined by our Board of Directors on a quarterly basis.
On November 12, 2013, our Board of Directors approved a cash dividend of $0.08 per share payable on December 27, 2013 to common stockholders of record on December 6, 2013.
16
Comparative Stock Performance
The following graph compares the cumulative total shareholder return (assuming reinvestment of dividends) from investing $100 on September 30, 2008, and plotted at the last trading day of each of the fiscal years ended September 30, 2009, 2010, 2011, 2012 and 2013, in each of (i) the Company's Common Stock; (ii) the NASDAQ/NYSE MKT/NYSE Index of companies; (iii) an old peer group comprised of: Advanced Energy Industries, Inc., Entegris, Inc., FEI Company, LAM Research Corporation, Mattson Technology, Inc., MKS Instruments, Inc., Photronics, Inc., Ultra Clean Technology, Inc. and Veeco Instruments Inc; and (iv) a new peer group comprised of: Advanced Energy Industries, Inc., ATMI Inc., Bruker Corp., Entegris, Inc., FEI Company, Formfactor Inc., LTX-Credence Corp., MKS Instruments, Inc., Photronics, Inc., Teradyne Inc., Ultra Clean Technology, Inc. and Veeco Instruments Inc. The new peer group index will replace the old peer group index in future filings. We believe this graph best represents our peer groups in the end markets that we serve. The stock price performance on the graph below is not necessarily indicative of future price performance.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Brooks Automation, Inc., the NASDAQ/NYSE MKT/NYSE Index,
Old Peer Group and New Peer Group
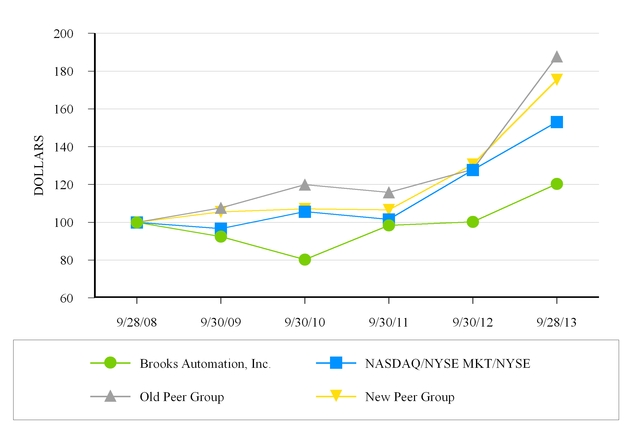
* $100 invested on September 30, 2008 in stock or index, including reinvestment of dividends.
Fiscal year ending September 30.
9/28/08 | 9/30/09 | 9/30/10 | 9/30/11 | 9/30/12 | 9/28/13 | ||||||||||||
Brooks Automation, Inc. | 100.00 | 92.46 | 80.26 | 98.36 | 100.23 | 120.26 | |||||||||||
NASDAQ/NYSE MKT/NYSE | 100.00 | 96.59 | 105.63 | 101.53 | 127.73 | 153.11 | |||||||||||
Old Peer Group | 100.00 | 107.58 | 119.92 | 115.79 | 127.99 | 187.73 | |||||||||||
New Peer Group | 100.00 | 105.51 | 107.06 | 106.59 | 130.64 | 175.47 | |||||||||||
The information included under the heading “Comparative Stock Performance” in Item 5 of this Annual Report on Form 10-K shall not be deemed to be “soliciting material” or subject to Regulation 14A, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act.
17
Unregistered Sales of Securities
Not applicable.
Issuer's Purchases of Equity Securities
As part of our equity compensation program, we offer recipients of restricted stock awards the opportunity to elect to sell their shares at the time of vesting to satisfy tax obligations in connection with such vesting. The following table provides information concerning shares of our Common Stock, $0.01 par value, purchased in connection with the forfeiture of shares to satisfy the employees' obligations with respect to withholding taxes in connection with the vesting of certain shares of restricted stock during the three months ended September 30, 2013. Upon purchase, these shares are immediately retired.
Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number (or Approximate Dollar Value) of Shares that May Yet be Purchased Under the Plans or Programs | ||||||||||
July 1 - 31, 2013 | — | $ | — | — | $ | — | ||||||||
August 1 - 31, 2013 | 12,851 | 9.79 | 12,851 | — | ||||||||||
September 1 - 30, 2013 | — | — | — | — | ||||||||||
Total | 12,851 | $ | 9.79 | 12,851 | $ | — | ||||||||
Item 6. | Selected Financial Data |
The selected consolidated financial data set forth below should be read in conjunction with our consolidated financial statements and notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” appearing elsewhere in this report.
Year Ended September 30, | |||||||||||||||||||
2013(8)(9) | 2012(5)(6)(7) | 2011(3)(4) | 2010(2) | 2009(1) | |||||||||||||||
(In thousands, except per share data) | |||||||||||||||||||
Revenue | $ | 450,952 | $ | 519,451 | $ | 688,105 | $ | 592,972 | $ | 218,706 | |||||||||
Gross profit (loss) | $ | 145,982 | $ | 173,486 | $ | 223,021 | $ | 166,295 | $ | (5,996 | ) | ||||||||
Income (loss) before income taxes and equity in earnings (losses) of joint ventures | $ | (6,762 | ) | $ | 11,420 | $ | 127,576 | $ | 56,064 | $ | (226,917 | ) | |||||||
Net income (loss) | $ | (2,150 | ) | $ | 136,835 | $ | 130,437 | $ | 59,884 | $ | (227,527 | ) | |||||||
Net income (loss) attributable to Brooks Automation, Inc. | $ | (2,215 | ) | $ | 136,789 | $ | 130,385 | $ | 59,841 | $ | (227,612 | ) | |||||||
Basic net income (loss) per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.10 | $ | 2.02 | $ | 0.94 | $ | (3.62 | ) | |||||||
Diluted net income (loss) from continuing operations per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.08 | $ | 2.01 | $ | 0.93 | $ | (3.62 | ) | |||||||
Shares used in computing basic earnings (loss) per share | 65,912 | 65,128 | 64,549 | 63,777 | 62,911 | ||||||||||||||
Shares used in computing diluted earnings (loss) per share | 65,912 | 65,722 | 65,003 | 64,174 | 62,911 | ||||||||||||||
Cash dividends per share | $ | 0.32 | $ | 0.32 | $ | 0.08 | $ | — | $ | — | |||||||||
As of September 30, | |||||||||||||||||||
2013 | 2012 | 2011 | 2010 | 2009 | |||||||||||||||
(In thousands) | |||||||||||||||||||
Total assets | $ | 736,763 | $ | 741,960 | $ | 636,958 | $ | 517,040 | $ | 411,569 | |||||||||
Working capital | $ | 237,558 | $ | 266,298 | $ | 224,785 | $ | 219,176 | $ | 150,700 | |||||||||
Total Brooks Automation, Inc. stockholders’ equity | $ | 631,956 | $ | 648,666 | $ | 518,347 | $ | 387,631 | $ | 317,376 | |||||||||
18
Year Ended September 30, 2013 | |||||||||||||||
First Quarter(8) | Second Quarter(8) | Third Quarter(8) | Fourth Quarter(8)(9) | ||||||||||||
(In thousands, except per share data) | |||||||||||||||
Revenue | $ | 98,025 | $ | 116,619 | $ | 118,072 | $ | 118,236 | |||||||
Gross profit | $ | 29,158 | $ | 36,612 | $ | 39,686 | $ | 40,526 | |||||||
Net income (loss) | $ | (9,219 | ) | $ | (511 | ) | $ | 1,568 | $ | 6,012 | |||||
Net income (loss) attributable to Brooks Automation, Inc. | $ | (9,236 | ) | $ | (538 | ) | $ | 1,544 | $ | 6,015 | |||||
Basic net income (loss) per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.14 | ) | $ | (0.01 | ) | $ | 0.02 | $ | 0.09 | |||||
Diluted net income (loss) per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.14 | ) | $ | (0.01 | ) | $ | 0.02 | $ | 0.09 | |||||
Year Ended September 30, 2012 | |||||||||||||||
First Quarter | Second Quarter(5) | Third Quarter(5) | Fourth Quarter(5)(6)(7) | ||||||||||||
(In thousands, except per share data) | |||||||||||||||
Revenue | $ | 120,228 | $ | 139,337 | $ | 140,437 | $ | 119,449 | |||||||
Gross profit | $ | 40,357 | $ | 48,282 | $ | 46,240 | $ | 38,607 | |||||||
Net income | $ | 2,831 | $ | 9,726 | $ | 8,025 | $ | 116,253 | |||||||
Net income attributable to Brooks Automation, Inc. | $ | 2,823 | $ | 9,721 | $ | 8,028 | $ | 116,217 | |||||||
Basic net income per share attributable to Brooks Automation, Inc. common stockholders | $ | 0.04 | $ | 0.15 | $ | 0.12 | $ | 1.78 | |||||||
Diluted net income per share attributable to Brooks Automation, Inc. common stockholders | $ | 0.04 | $ | 0.15 | $ | 0.12 | $ | 1.77 | |||||||
(1) | Gross profit (loss) includes a $20.9 million impairment of long-lived assets. Income (loss) before income taxes and equity in earnings of joint ventures, net income (loss) and net income (loss) attributable to Brooks Automation, Inc. includes a $71.8 million charge for the impairment of goodwill and a $35.5 million charge for the impairment of long-lived assets. |
(2) | Income (loss) before income taxes and equity in earnings of joint ventures, net income (loss) and net income (loss) attributable to Brooks Automation, Inc. includes a $7.8 million gain on the sale of certain patents and patents pending related to a legacy product line. |
(3) | Amounts include results of operations of RTS Life Science Limited (acquired effective April 1, 2011) and Nexus Biosystems, Inc. (acquired effective July 25, 2011) for the periods subsequent to their acquisition. |
(4) | On June 28, 2011, we disposed of our contract manufacturing business which did not qualify as discontinued operations. As such, the operations prior to the divestiture are included in our results of operations. Income (loss) before income taxes and equity in earnings of joint ventures, net income (loss) and net income (loss) attributable to Brooks Automation, Inc. includes a $45.0 million pre-tax gain on the sale of our contract manufacturing business. |
(5) | Amounts include results of operations of the Celigo® product line (acquired December 30, 2011) for the period subsequent to the acquisition. |
(6) | Net income (loss) and net income (loss) attributable to Brooks Automation, Inc. includes a $121.8 million deferred income tax benefit in connection with a reversal of a majority of the valuation allowance against our net deferred tax assets. |
(7) | Income (loss) before income taxes and equity in earnings of joint ventures, net income (loss) and net income (loss) attributable to Brooks Automation, Inc. includes an $8.9 million charge in connection with the settlement of our U.S. defined benefit pension plan. |
(8) | Amounts include results of operations of Crossing Automation Inc. (acquired October 29, 2012) for the period subsequent to the acquisition. |
(9) | Amounts include results of operations of the Matrical, Inc. net assets (acquired August 1, 2013) for the period subsequent to the acquisition. |
19
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Certain statements in this Form 10-K, and in particular in “Management’s Discussion and Analysis of Financial Condition and Results of Operation,” constitute forward-looking statements, which are subject to the safe harbor provisions created by the Private Securities Litigation Reform Act of 1995. Certain, but not all, of the forward-looking statements in this report are specifically identified as forward-looking, by use of phrases and words such as “we believe,” “we anticipate,” “we expect,” “may,” “should,” “could,” and other future-oriented terms. The identification of certain statements as “forward-looking” is not intended to mean that other statements not specifically identified are not forward-looking. Forward-looking statements include, but are not limited to, statements that relate to our future revenue, margin, expense levels, shipments, costs, earnings, product development, demand, acceptance and market share, competitiveness, market opportunities and performance, levels of research and development (R&D), the success of our marketing, sales and service efforts, outsourced activities and operating expenses, anticipated manufacturing, customer and technical requirements, the ongoing viability of the solutions that we offer and our customers’ success, tax expenses, our management’s plans and objectives for our current and future operations and business focus, the levels of customer spending, general economic conditions, the sufficiency of financial resources to support future operations, and capital expenditures. Such statements are based on current expectations and are subject to risks, uncertainties, and changes in condition, significance, value and effect, including without limitation those discussed above under the heading “Risk Factors” within Item 1A and elsewhere in this report and other documents we file from time to time with the Securities and Exchange Commission (the “SEC”), such as our quarterly reports on Form 10-Q and our current reports on Form 8-K. Such risks, uncertainties and changes in condition, significance, value and effect could cause our actual results, performance or achievements to differ materially from those expressed in this report and in ways we cannot readily foresee. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof and are based on information currently and reasonably known to us. We do not undertake any obligation to release the results of any revisions to these forward-looking statements, which may be made to reflect events or circumstances that occur after the date of this report or to reflect the occurrence or effect of anticipated or unanticipated events. Precautionary statements made herein should be read as being applicable to all related forward-looking statements wherever they appear in this report.
Overview
We are a leading provider of automation, vacuum and instrumentation solutions for multiple markets and are a valued business partner to original equipment manufacturers (“OEMs”) and equipment users throughout the world. We serve markets where equipment productivity and availability is a critical factor for our customers’ success, typically in demanding temperature and/or pressure environments. Our largest served market is the semiconductor capital equipment industry, which represented approximately 53%, 54% and 65% of our consolidated revenue for fiscal years 2013, 2012 and 2011, respectively. These decreases are the result of a cyclical downturn in the semiconductor capital equipment market, combined with our efforts to target certain non-semiconductor revenue opportunities, including acquisitions and a divestiture. The non-semiconductor markets served by us include life sciences, industrial capital equipment and other adjacent technology markets.
The demand for semiconductors and semiconductor manufacturing equipment is cyclical, resulting in periodic expansions and contractions of this market. Demand for our products has been impacted by these cyclical industry conditions. Our revenue for fiscal year 2011 benefited from an upturn in the semiconductor capital equipment market. Revenue for fiscal year 2012 was impacted by our acquisitions and divestitures and weakness in demand from semiconductor capital equipment customers. Revenue for fiscal year 2013 was also impacted by weakness in demand for semiconductor capital equipment, particularly in the first half of our fiscal year. We have observed indications of improved demand from the semiconductor capital equipment market and we believe our revenue will improve in fiscal 2014 as compared to fiscal 2013.
We expect the semiconductor equipment market will continue to be a key end market for our products, however, we intend to acquire and develop technologies that will create opportunities outside of the semiconductor market. We completed the following acquisitions and investments to expand our life sciences product offering:
• | RTS Life Sciences (“RTS”), a United Kingdom based provider of automation solutions to the life sciences markets, acquired on April 1, 2011 for $3.4 million, net of cash on hand; |
• | Nexus Biosystems, Inc. (“Nexus”), a California based provider of automation solutions and consumables to the life sciences markets, acquired on July 25, 2011 for $84.9 million, net of cash on hand; and |
• | Matrical, Inc. ("Matrical"), a Washington based provider of biological sample preparation, management and storage solutions to the life sciences markets, acquired on August 1, 2013 for $9.3 million, net of cash on hand. |
Although we have entered into transactions to expand the non-semiconductor portions of our business, we continue to make investments to maintain and grow our semiconductor product and service offerings. On January 6, 2012, we acquired intangible assets from Intevac, Inc. for $3.0 million. We acquired these assets for use in the development of a next generation of semiconductor automation tools. We expensed essentially all of this asset purchase as an in-process research and development cost in the three months ended March 31, 2012. On October 29, 2012, we acquired Crossing Automation Inc. (“Crossing”), a
20
U.S. based provider of automation solutions for the global semiconductor front-end markets. The purchase price was $59.0 million, net of cash on hand. The acquisition of Crossing provides us with the opportunity to enhance our existing capabilities with respect to manufacturing of atmospheric and vacuum automation solutions within the semiconductor front-end market.
On April 20, 2011, we entered into an agreement with affiliates of Celestica Inc. (“Celestica”) to sell the assets of our Contract Manufacturing segment (the “Business”). The sale was completed on June 28, 2011 for a purchase price of $78.2 million in cash. In addition, Brooks and Celestica entered into certain commercial supply and license agreements at the closing of the sale. Pursuant to those agreements, Brooks supplies Celestica with certain products and has licensed to Celestica certain intellectual property needed to run the Business and Celestica supplies certain products to Brooks. Due to the significance of these ongoing commercial arrangements, the sale did not qualify for discontinued operations treatment. Therefore, historical financial results of the divested business are not segregated within our consolidated financial statements for the historical periods during which this business was part of us.
In December 2012, we committed to a restructuring plan that would achieve cost synergies associated with our acquisition of Crossing and further reduce costs and improve profitability in light of the continued near-term macro-economic environment. We eliminated redundant positions across the Company and have substantially completed our restructuring plan as of September 30, 2013. However, we continually review our cost structure and could take further cost saving actions in the future. For a discussion of our restructuring actions, please refer to “Restructuring and Other Charges” below.
We report financial results in four segments: Brooks Product Solutions, Brooks Global Services, Brooks Life Science Systems and Contract Manufacturing. This financial reporting structure was implemented effective as of the beginning of our fourth quarter of fiscal year 2011 in response to changes in our management structure as well as our acquisitions of RTS and Nexus in the second half of fiscal 2011.
The Brooks Product Solutions segment provides a variety of products critical to technology equipment productivity and availability. Those products include atmospheric and vacuum tool automation systems, atmospheric and vacuum robots and robotic modules and cryogenic vacuum pumping, thermal management and vacuum measurement solutions which are used to create, measure and control critical process vacuum applications.
The Brooks Global Services segment provides an extensive range of support services including on-and off-site repair services, on-and off-site diagnostic support services, and installation services that enable our customers to maximize process tool uptime and productivity. This segment also provides end-user customers with spare parts support services that maximize tool productivity.
The Brooks Life Science Systems segment provides automated sample management systems for automated cold sample storage, equipment for sample preparation and handling, consumables, and parts and support services to a wide range of life science customers including pharmaceutical companies, biotechnology companies, biobanks, national laboratories, research institutes and research universities.
The Contract Manufacturing segment provided services to build equipment front-end modules, vacuum transport modules and other subassemblies which enabled our customers to effectively source high quality and high reliability process tools for semiconductor market applications. We sold this business unit to Celestica Inc. on June 28, 2011.
Critical Accounting Policies and Estimates
The preparation of the consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue, bad debts, inventories, derivative instruments, intangible assets, goodwill, income taxes, warranty obligations, pensions and stock-based compensation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, including current and anticipated worldwide economic conditions both in general and specifically in relation to the semiconductor and life science industries, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. As discussed in the year over year comparisons below, actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue
Product revenue is associated with the sale of hardware systems, components and spare parts as well as product license revenue. Service revenue is associated with service contracts, repairs, upgrades and field service. Shipping and handling fees billed to customers, if any, are recognized as revenue. The related shipping and handling costs are recognized in cost of revenue.
21
Revenue from product sales that do not include significant customization is recorded upon delivery and transfer of risk of loss to the customer provided there is evidence of an arrangement, fees are fixed or determinable, collection of the related receivable is reasonably assured and, if applicable, customer acceptance criteria have been successfully demonstrated. Customer acceptance provisions are included in certain arrangements to ensure that the product meets the published specification requirements. Customer acceptance provisions in our arrangements generally consist of final testing procedures that are carried out either in one of our facilities or at the customer location. When an arrangement includes acceptance criteria to be carried out in one of our facilities, we recognize revenue when the acceptance criteria have been successfully demonstrated and delivery and transfer of risk of loss have occurred. When significant on site customer acceptance provisions are present in the arrangement, revenue is recognized upon completion of customer acceptance testing.
Revenue from product sales that does include significant customization, which primarily include life science automation systems, is recorded using the percentage of completion method whereby revenue is recorded as work progresses based on a percentage that incurred labor effort to date bears to total projected labor effort. Payments collected from customers in advance of recognizing the related revenue is recorded as deferred revenue. These arrangements are reviewed on a regular basis to determine whether a probable future loss exists. If such a loss is estimated by comparing total estimated contract revenue to the total estimated contract costs, a loss is accrued in the same period we determine that the loss is probable.
Revenue associated with service agreements is generally recognized ratably over the term of the contract, with payments from customers being recorded as deferred revenue. Revenue from repair services or upgrades of customer-owned equipment is recognized upon completion of the repair effort and upon the shipment of the repaired item back to the customer. In instances where the repair or upgrade includes installation, revenue is recognized when the installation is completed.
A portion of the revenue arrangements for sales of life science automation systems are multiple element arrangements that can include product, service, as well as other elements. For revenue arrangements with multiple elements, arrangement consideration is allocated to each element based upon their relative selling price using vendor-specific objective evidence (“VSOE”), or third-party evidence (“TPE”) or based upon the relative selling price using estimated selling prices if VSOE or TPE does not exist. We rely primarily on relative selling prices when we do not have VSOE for a specific element. We recognize revenue on each element of the arrangement in accordance with our policies for revenue recognition. The fair value of any undelivered elements is deferred until the undelivered element is delivered and all other criteria for revenue recognition have been met.
Intangible Assets, Goodwill and Other Long-Lived Assets
As a result of our acquisitions, we have identified intangible assets other than goodwill and generated significant goodwill. Intangible assets other than goodwill are valued based on estimates of future cash flows and amortized over their estimated useful life. Goodwill is subject to annual impairment testing as well as testing upon the occurrence of any event that indicates a potential impairment. Intangible assets other than goodwill and other long-lived assets are subject to an impairment test if there is an indicator of impairment. We conduct our annual goodwill impairment test as of our fiscal year end, or September 30th.
The testing of goodwill for impairment is to be performed at a level referred to as a reporting unit. A reporting unit is either the “operating segment level” or one level below, which is referred to as a “component.” The level at which the impairment test is performed requires an assessment as to whether the operations below the operating segment constitute a self-sustaining business, in which case testing is generally required to be performed at this level. We currently have five reporting units that have goodwill, including three components that are part of our Brooks Product Solutions operating segment and sole reporting units that are our Brooks Global Services and Brooks Life Science Systems operating segments.
Goodwill impairment testing is a two-step process. The first step of the goodwill impairment test, used to identify potential impairment, compares the fair value of each reporting unit to its respective carrying amount, including goodwill. If the fair value of the reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered impaired. If the reporting unit’s carrying amount exceeds the fair value, the second step of the goodwill impairment test must be completed to measure the amount of the impairment loss, if any. The second step compares the implied fair value of goodwill with the carrying value of goodwill. The implied fair value is determined by allocating the fair value of the reporting unit to all of the assets and liabilities of that unit, the excess of the fair value over amounts assigned to its assets and liabilities is the implied fair value of goodwill. The implied fair value of goodwill determined in this step is compared to the carrying value of goodwill. If the implied fair value of goodwill is less than the carrying value of goodwill, an impairment loss is recognized equal to the difference.
We determine the fair value of our reporting units using an Income Approach, specifically the Discounted Cash Flow Method (“DCF Method”). The DCF Method includes future cash flow projections, which are discounted to present value, and an estimate of terminal values, which are also discounted to present value. Terminal values represent the present value an investor would pay today for the rights to the cash flows of the business for the years subsequent to the discrete cash flow projection period. We consider the DCF Method to be the most appropriate valuation indicator as the DCF analyses are based
22
on management’s long-term financial projections. Given the dynamic nature of the cyclical semiconductor equipment market, management's projections as of the valuation date are considered more objective since other market metrics for peer companies fluctuate over the cycle. However, we also use market-based valuation techniques to test the reasonableness of the reporting unit fair values determined by the DCF Method. In addition, we compare the aggregate fair value of our reporting units plus our net corporate assets to our overall market capitalization.
For our annual goodwill impairment test as of September 30, 2013, we determined that the estimated fair value of each reporting unit exceeded its carrying value by at least 10% and that no impairment existed. Our tests of goodwill as of September 30, 2012 and 2011 indicated that we did not have any impairment to goodwill.
The fair values of the reporting units in the Brooks Product Solutions and Brooks Global Services segments exceeded their carrying values by amounts ranging from 18% to 114% at September 30, 2013.
The goodwill allocated to the Brooks Life Science Systems reporting unit at September 30, 2013 was $47.4 million. The fair value of the Brooks Life Science Systems reporting unit exceeded its carrying value by 10% at September 30, 2013. The observable inputs used in our DCF for analysis for the Brooks Life Science Systems reporting unit include a discount rate of 18%. In addition, we determined the terminal value using the Gordon growth method and a terminal growth rate of 3%. The Gordon growth method assumes that the reporting unit will grow and generate free cash flow at a constant rate. We believe that the Gordon growth method is the most appropriate method for determining the terminal value because the terminal value was calculated at the point in which we have assumed that the Brooks Life Science Systems reporting unit has reached a stable growth rate.
In fiscal 2013, we experienced a decline in revenue and operating profit for the products in the Brooks Life Science Systems reporting unit. The cash flow assumptions in our DCF Method analysis for the Brooks Life Science Systems reporting unit project growth in our current automated sample management systems and the development of new automated sample management systems that would allow us to address a broader automated sample management market than we can address with our current products. While we believe our assumptions are reasonable, our actual results could differ from our projections. To the extent that the operating results of the Brooks Life Science Systems reporting unit do not improve as expected and new life science products being designed to expand the markets we serve are not introduced in a timely manner or accepted by the market, we may be required to write down all or a portion of the goodwill and other long-lived assets associated with this reporting unit, which would adversely impact our earnings.
We are required to test long-lived assets, other than goodwill, when indicators of impairment are present. For purposes of this test, long-lived assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities. When we determine that indicators of potential impairment exist, the next step of the impairment test requires that the potentially impaired long-lived asset group is tested for recoverability. The test for recoverability compares the undiscounted future cash flows of the long-lived asset group to its carrying value. If the carrying values of the long-lived asset group exceed the future cash flows, the assets are potentially impaired. The next step in the impairment process is to determine the fair value of the individual net assets within the long-lived asset group. If the aggregate fair values of the individual net assets of the group are less than the carrying values, an impairment charge is recorded equal to the excess of the aggregate carrying value of the group over the aggregate fair value. The loss is allocated to each asset within the group based on their relative carrying values, with no asset reduced below its fair value.
We determined that impairment indicators were present for long-lived assets related to the Celigo product line as of September 30, 2013. Indicators of impairment for this asset group included declining sales in the trailing twelve months and negative cash flows from the asset group. We tested the recoverability of the asset group by comparing the sum of the expected future undiscounted cash flows directly attributable to the assets to their carrying values, which resulted in the conclusion that the carrying amounts of the assets were not recoverable. The fair value of the long-lived assets related to the Celigo products was based primarily on market-based valuation techniques reflecting the view of a market participant using the assets in the group to their best possible use. We determined that the carrying value of the asset group exceeded the fair value of the asset group by approximately $2.0 million and we recorded this amount as an impairment charge in the fourth quarter of 2013.
Except as described above, we have not tested any other long-lived assets, other than goodwill, since 2009 because no events have occurred that would require an impairment assessment.
Accounts Receivable
We record trade accounts receivable at the invoiced amount. Trade accounts receivables do not bear interest. We maintain an allowance for doubtful accounts representing our best estimate of the amount of probable credit losses in our existing accounts receivable. If the financial results of our customers deteriorate, reducing their ability to make payments, additional allowances would be required, resulting in a charge to operations. We do not have any off-balance-sheet credit exposure related to our customers.
23
Warranty
We provide for the estimated cost of product warranties at the time revenue is recognized. While we engage in extensive product quality programs and processes, including actively monitoring and evaluating the quality of our component suppliers, our warranty obligation is affected by product failure rates, material usage and service delivery costs incurred in correcting a product failure. Should actual product failure rates, material usage or service delivery costs differ from our estimates, revisions to the estimated warranty liability would be required and may result in additional benefits or charges to operations.
Inventory
We provide reserves for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. We fully reserve for inventories and noncancelable purchase orders for inventory deemed obsolete. We perform periodic reviews of all inventory items to identify excess inventories on hand by comparing on-hand balances to anticipated usage using recent historical activity as well as anticipated or forecasted demand, based upon sales and marketing inputs through our planning systems. If estimates of demand diminish further or actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Deferred Taxes
We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. We consider recent historical income, estimated future taxable income, carry-forward periods of tax attributes, the volatility of the semiconductor industry and ongoing tax planning strategies in assessing the need for the valuation allowance. We maintain a valuation allowance against certain deferred tax assets in the U.S. and in certain foreign jurisdictions. We will continue to assess the need for a valuation allowance in future periods. If future operating results of the U.S. or these foreign jurisdictions deviate from long-term expectations, it is reasonably possible that there could be a change in the valuation allowance in the future. A change in the valuation allowance, in whole or in part, would result in a non-cash income tax expense or benefit during the period of change.
Pension Plans
We sponsor defined benefit pension plans in Switzerland and Taiwan. The cost and obligations of these arrangements are calculated using many assumptions to estimate the benefits that the employee earns while working, the amount of which cannot be completely determined until the benefit payments cease. Major assumptions used in the accounting for these employee benefit plans include the discount rate, expected return on plan assets and rate of increase in employee compensation levels. Assumptions are determined based on company data and appropriate market indicators in consultation with third-party actuaries, and are evaluated each year as of the plans’ measurement date. Should any of our assumptions change, they would have an effect on net periodic pension costs and the unfunded benefit obligation.
Derivative Financial Instruments
We record all derivative instruments as assets or liabilities at their fair value, which is determined by estimating future cash flows of the instrument. Subsequent changes in a derivative's fair value are recognized in income, unless specific hedge accounting criteria are met. Changes in the fair value of a derivative that is highly effective and designated as a cash flow hedge are recognized in accumulated other comprehensive income until the forecasted transaction occurs or it becomes probable that the forecasted transaction will not occur. We perform an assessment at the inception of the hedge and on a quarterly basis thereafter, to determine whether our derivatives are highly effective in offsetting changes in the value of the hedged items. Any changes in the fair value resulting from hedge ineffectiveness are immediately recognized as income or expense.
Stock-Based Compensation
We measure compensation cost for all employee stock awards at fair value on the date of grant and recognize compensation expense over the service period for awards expected to vest. The fair value of restricted stock is determined based on the number of shares granted and the excess of the quoted price of our common stock over the exercise price of the restricted stock on the date of grant, if any, and the fair value of stock options is determined using the Black-Scholes valuation model. Such value is recognized as expense over the service period, net of estimated forfeitures. The estimation of stock awards that will ultimately vest requires significant judgment. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience. In addition, for stock-based awards where vesting is dependent upon achieving certain operating performance goals, we estimate the likelihood of achieving the performance goals. Actual results, and future changes in estimates, may differ from our current estimates.
24
Year Ended September 30, 2013, Compared to Year Ended September 30, 2012
Revenue
We reported revenue of $451.0 million for fiscal year 2013, compared to $519.5 million in the previous year, a decrease of 13%. This decrease was due to reduced demand for our products, primarily due to weakness in demand for semiconductor capital equipment which led to a reduction of $96.4 million in revenue from our Brooks Product Solutions segment and a $4.2 million reduction in revenue from our Brooks Global Services segment. These decreases were partially offset by the acquisition of Crossing which added $33.5 million of revenue to our Brooks Product Solutions segment and $8.0 million of revenue to our Brooks Global Services segment for fiscal year 2013. Our Brooks Life Science Systems segment had lower sales of $9.3 million for fiscal year 2013 as compared to the previous year due to lower demand.
Our Brooks Product Solutions segment reported revenue of $317.9 million for fiscal year 2013, a decrease of 17% from $380.9 million in the prior year. These decreases were mostly attributable to lower volumes of shipments to semiconductor capital equipment and semiconductor adjacent customers, which decreased by $96.4 million for fiscal year 2013, as compared to the prior year. This decrease was partially offset by $33.5 million of product revenue for fiscal year 2013, contributed by our acquisition of Crossing.
Our Brooks Global Services segment reported revenue of $89.7 million for fiscal year 2013, a 4% increase from $86.0 million in the prior year. Excluding the acquisition of Crossing, revenue for this segment declined $4.2 million for fiscal year 2013 as compared to the prior year period primarily due to weakness in demand. Total service revenue for this segment was $76.6 million for fiscal year 2013, including revenue contributed by the acquisition of Crossing. For the prior year period, service contract and repair revenue was $74.6 million. Total spare parts revenue was $13.1 million for fiscal year 2013, including revenue contributed by the acquisition of Crossing. For the prior year period, spare parts revenue was $11.3 million.
Our Brooks Life Science Systems segment reported revenue of $43.3 million for fiscal year 2013, a decrease of 18% from $52.6 million in the same prior year period. These decreases were the result of delays in customer decisions to purchase automated sample management systems during the first half of fiscal year 2013.
Revenue outside the United States was $259.0 million, or 57% of total revenue, and $290.8 million, or 56% of total revenue, for fiscal years 2013 and 2012, respectively. We expect that revenue outside the United States will continue to account for a significant portion of total revenue.
Gross Margin
Gross margin percentage decreased to 32.4% for fiscal year 2013, compared to 33.4% for the prior year. Gross margin in fiscal 2013 includes $2.7 million of charges related to the acquisition of Crossing, related primarily to the sale of acquired inventory to which a step-up in value was applied in our purchase accounting, which reduced gross margin by 0.6 percentage points. Gross margin in fiscal 2013 also includes $2.4 million of impairment charges and inventory reserves related to the Celigo product line which reduced gross margin by 0.5 percentage points. Gross margin was also negatively impacted by lower production which resulted in reduced absorption of our fixed costs. However, the decrease in absorption was offset by lower charges for excess and obsolete inventories, warranty costs and other manufacturing costs which increased gross margin by approximately 1.1 percentage points in fiscal year 2013.
Our gross margin percentage for our Brooks Products Services segment decreased to 32.8% for fiscal year 2013 as compared to 33.5% in the prior year. The decrease is due primarily to reduced demand for our products, which resulted in reduced absorption of our fixed costs. In addition, gross margin for the Brooks Products Services segment includes $1.4 million related primarily to the sale of acquired inventory to which a step-up in value was applied in our purchase accounting, which reduced gross margin by 0.4 percentage points. The decreases in gross margin were partially offset by lower charges for excess and obsolete inventories and warranty costs which increased gross margin by 0.8 percentage points in fiscal year 2013.
Our gross margin percentage for our Brooks Global Services segment increased to 30.6% for fiscal year 2013 as compared to 29.8% in the prior year. The increase is due to lower charges for excess and obsolete inventories which increased gross margin by 1.3 percentage points in fiscal year 2013 and higher absorption of fixed costs resulting from an increase in revenue. The increase was partially offset by $1.3 million of charges related to the acquisition of Crossing, related primarily to the sale of acquired inventory to which a step-up in value was applied in our purchase accounting, which reduced gross margin by 1.4 percentage points. In addition, amortization expense associated with Crossing reduced gross margin by 0.2 percentage points in fiscal year 2013.
Our gross margin percentage for our Brooks Life Science Systems segment decreased to 32.7% for fiscal year 2013 as compared to 38.8% in the prior year. The decrease is due primarily to $2.4 million of impairment charges and inventory reserves related to the Celigo product line. The charges reduced gross profit margin by 5.5 percentage points for fiscal year 2013. The remaining decrease relates to lower production and as a result, reduced absorption of our fixed costs.
25
Research and Development
Research and development, or R&D, expenses for fiscal year 2013 were $49.0 million, an increase of $1.5 million, compared to $47.5 million in the previous year. Lower labor and material costs resulting from restructuring actions and other cost saving initiatives were more than offset by our Crossing acquisition, which increased R&D expenses by $6.8 million and were not included in the prior year.
Selling, General and Administrative
Selling, general and administrative, or SG&A, expenses were $99.5 million for fiscal year 2013, a decrease of $1.7 million compared to $101.2 million in the prior year. The decrease is the result of $5.2 million of lower consulting costs and other professional service fees which decreased primarily because a project focused on improving operating efficiencies was completed in the prior year. We also reduced labor costs by $5.0 million through restructuring actions taken over the last twelve months and incurred $1.2 million less stock-based compensation expense as a result of performance-based vesting criteria that we no longer expect to achieve. Lower SG&A costs for fiscal 2013 were partially offset by $6.9 million of SG&A expenses for Crossing, which were not included in the prior year. In addition, in the second quarter of fiscal 2012, we received $3.3 million of insurance proceeds as reimbursement of previously incurred litigation related costs.
Restructuring and Other Charges
We recorded a restructuring charge of $6.5 million for fiscal 2013. These charges relate primarily to workforce reductions implemented to consolidate the operations of Crossing and the Company, to transition manufacturing of the Polycold product line (certain of our cryopump and cryochiller products) to a third party contract manufacturer and other programs designed to improve our cost structure. Restructuring charges also included facility related costs incurred in connection with the consolidation of Crossing facilities with our facilities.
Restructuring costs recorded in fiscal 2013 consist of $5.6 million of severance costs and $0.8 million of facility related costs. Severance costs relate to a series of workforce reductions implemented to improve our cost structure by eliminating approximately 200 positions.
Total severance charges related to the outsourcing of the Polycold manufacturing operation are expected to be $1.3 million, including severance and retention bonuses. This charge is being amortized over the period from notification of the closing to the actual service end date. In the fourth quarter of 2013, we extended the service end date from September 2013 to September 2014 as a result of changes in the transition plan with the third party contract manufacturer. We expensed $0.6 million of the total charge as of September 30, 2013, and will expense the balance ratably through fiscal 2014.
Unpaid severance charges of $0.8 million as of September 30, 2013 are expected to be paid during fiscal year 2014 and the remaining $0.5 million is expected to be paid during fiscal year 2015.
We recorded a restructuring charge of $3.3 million for fiscal year 2012. These charges are related primarily to a series of workforce reductions implemented to improve the Company’s cost structure by eliminating 118 employees.
Pension Settlement
During fiscal year 2012, we advised participants of our frozen U.S. defined benefit pension plan that we intended to settle this pension obligation. This settlement occurred in the quarter ended September 30, 2012 and resulted in accelerated cash payments of approximately $6.4 million to fully satisfy the pension liability, and resulted in an accelerated amortization of approximately $8.9 million of prior pension losses that were previously reported in accumulated other comprehensive income.
In-process Research and Development
During the three months ended March 31, 2012, we acquired primarily intellectual property from Intevac, Inc. for $3.0 million. Management evaluated this asset purchase to determine if this acquisition would be considered an acquisition of a business. Since only a limited amount of assets were acquired, management concluded that the inputs and processes required to meet the definition of a business were not acquired in this transaction, therefore, this transaction was treated as the purchase of an asset group. This asset group includes primarily intellectual property that is used in the development of a next generation of semiconductor automation tools. We expensed essentially all of this asset purchase as an in-process research and development cost in the three months ended March 31, 2012.
Interest Income
Interest income was $1.0 million and $1.2 million, respectively, for fiscal year 2013 and 2012. The reduction is due to lower cash balances available for investing due to the cash acquisitions of Crossing in October 2012 and Matrical in August 2013.
26
Other Income, net
Other income, net of $1.2 million for fiscal year 2013 consists primarily of a $1.4 million gain on the sale of certain underutilized buildings in Chelmsford, MA and Oberdiessbach, Switzerland and $0.6 million of joint venture management fee income which was partially offset by foreign exchange losses of $0.9 million. Other income, net of $0.7 million for fiscal year 2012 consists primarily of $1.0 million of joint venture management fee income partially offset by foreign exchange losses of $0.4 million.
Income Tax Provision (Benefit)
We recorded an income tax benefit of $2.2 million for fiscal year 2013. This benefit consists of deferred tax benefits in the U.S. generated by pre-tax losses and tax credits of $2.5 million. We recorded the benefit in the U.S. because there is no valuation allowance against the deferred tax assets generated in the current year. Despite the current year loss in the U.S. we are reporting taxable income due to the reversal of book to taxable income differences. As a result, we have not changed any estimates with regard to long-term utilization of our net operating losses and credits. This benefit is partially offset by foreign taxes on profits of our foreign subsidiaries. Additionally, we recorded $1.0 million of tax benefits for the reversal of tax reserves resulting from the expiration of statutes of limitations in certain foreign jurisdictions. The U.S. tax benefit includes $0.9 million of U.S. tax credits from fiscal year 2012 that were recognized in fiscal year 2013. These credits were reinstated under The American Taxpayer Relief Act of 2012 that was signed into law on January 2, 2013.
We recorded an income tax benefit of $123.3 million for fiscal year 2012. This benefit includes a $121.8 million deferred income tax benefit, primarily resulting from a significant reduction in the valuation allowance against deferred tax assets. We considered the weight of both positive and negative evidence as of September 30, 2012 and concluded that a substantial portion of the deferred tax assets would be realized. The tax benefit for fiscal year 2012 was partially offset by U.S. state income taxes and foreign taxes.
Equity in Earnings of Joint Ventures
Income associated with our 50% interest in ULVAC Cryogenics, Inc., a joint venture with ULVAC Corporation of Japan, was $2.6 million for fiscal year 2013 as compared to $2.0 million for fiscal year 2012. Loss associated with our 50% interest in Yaskawa Brooks Automation, Inc., a joint venture with Yaskawa Electric Corporation of Japan was $(0.2) million for fiscal year 2012 as compared to a gain of $0.1 million for fiscal year 2012.
Year Ended September 30, 2012, Compared to Year Ended September 30, 2011
Revenue
We reported revenue of $519.5 million for fiscal year 2012, compared to $688.1 million in the previous year, a 25% decrease. The total decrease in revenue of $168.6 million was the result of the sale of our Contract Manufacturing segment, which had revenue of $137.3 million in the prior year, decreased demand for our Brooks Product Solutions products which resulted in $70.4 million of decreased revenue and reduced demand for products and services from our Brooks Global Services segment which resulted in $2.9 million of lower revenue. These decreases were partially offset by $42.0 million of increased revenue from our Brooks Life Science Systems segment due to the acquisitions of RTS and Nexus in the last half of fiscal 2011.
Our Brooks Product Solutions segment reported revenue of $380.9 million for fiscal year 2012, a decrease of 16% from $451.3 million in the prior year. These decreases were attributable to lower volumes of shipments to most of the end markets served by this segment due to a decline in demand for semiconductor capital equipment.
Our Brooks Global Services segment reported revenue of $86.0 million for fiscal year 2012, a 3% decrease from $88.8 million in the prior year. The decrease included a $3.4 million decrease in product revenue, which was comprised mostly of spare part sales to end users. This decrease, which was primarily attributable to decreased demand from our semiconductor customers, was partially offset by a $0.6 million increase in revenue from services.
Our Brooks Life Science Systems segment reported revenue of $52.6 million for fiscal year 2012, a 396% increase from $10.6 million in the prior year. This increase was primarily the result of the timing of our acquisitions. We acquired RTS on April 1, 2011, Nexus on July 25, 2011 and the Celigo product line on December 31, 2011. Revenue for this segment for fiscal year 2012 included $39.7 million of product revenue, which included sales of automated systems and consumables. The balance of the fiscal year 2012 revenue, or $12.9 million, was comprised of service revenue to support deployed automation systems.
Our Contract Manufacturing segment reported revenue of $137.3 million for fiscal year 2011. This segment was sold on June 28, 2011.
27
Revenue from the Brooks Product Solutions segment for the fiscal year 2011 included intercompany sales of $49.2 million from this segment to the Contract Manufacturing segment. This intercompany revenue was eliminated from the revenue of Contract Manufacturing.
Revenue for the Contract Manufacturing segment for the fiscal year 2011 excluded intercompany sales of $10.7 million from this segment to the Brooks Product Solutions segment.
Revenue outside the United States was $290.8 million, or 56% of total revenue, and $339.9 million, or 49% of total revenue, for fiscal years 2012 and 2011, respectively.
Gross Margin
Gross margin percentage increased to 33.4% for fiscal year 2012, compared to 32.4% for the prior year. This increase was primarily attributable to a more favorable product mix with the addition of our higher margin Brooks Life Science Systems segment and because we had sold our lower margin Contract Manufacturing segment. This increase in gross margin percentage was partially offset by weaker margins in our Brooks Product Solutions and Brooks Global Services segments.
Gross margin percentage of our Brooks Product Solutions segment decreased to 33.5% for fiscal year 2012 as compared to 38.1% in the prior year. The decrease was due in part to a less favorable product mix, which decreased gross margin by 1.8 percentage points, plus increased charges for excess and obsolete inventory which reduced gross margin by 0.6 percentage points. The balance of the reduction in gross margin was mostly attributable to reduced production and lower absorption of our fixed costs. The reduced cost absorption was the result of weakening demand for semiconductor capital equipment.
Gross margin percentage of our Brooks Global Services segment decreased to 29.8% for fiscal year 2012 as compared to 35.7% in the prior year. This decrease was due to lower sales of higher margin spare parts, which reduced gross margin by 3.1 percentage points, higher charges for excess and obsolete inventory which reduced gross margin by 0.8 percentage points, with the balance of the decrease primarily attributable to increased costs to expand our service capacity.
Gross margin percentage for our Brooks Life Science Systems segment increased to 38.8% for fiscal year 2012 as compared to 21.2% in the prior year. The increase was due to the reduction of purchase accounting related adjustments that impacted the valuation of deferred revenue obligations and the step-up in value of acquired inventory, which reduced gross margin percentage by 1.6 percentage points for fiscal year 2012 as compared to a reduction of 11.9 percentage points for the prior year. In addition, reduced charges for excess and obsolete inventories for fiscal year 2012 as compared to the prior year resulted in a 5.5 percentage points improvement in gross margin percentage. The balance of the increase related to the increased production and improved absorption of our fixed costs. These increases in gross margin percentage were partially offset by increased amortization for completed technology intangible assets which decreased gross margin percentage by 1.0 percentage points as compared to the prior year.
Gross margin percentage for our Contract Manufacturing segment for fiscal year 2011 was 12.5%. The sale of this lower gross margin segment at the end of our third quarter of fiscal year 2011 led to an overall increase in our consolidated post-sale gross margin percentage.
Research and Development
Research and development, or R&D, expenses for fiscal year 2012 were $47.5 million, an increase of $7.7 million, compared to $39.8 million in the previous year. This increase related primarily to our life sciences acquisitions, which increased R&D expenses by $7.0 million over the prior year.
Selling, General and Administrative
Selling, general and administrative, or SG&A, expenses were $101.2 million for fiscal year 2012, a decrease of $1.3 million compared to $102.5 million in the prior year. The decrease was attributable to $3.3 million of net insurance proceeds as reimbursement of litigation related costs previously incurred which was recorded as a reduction to SG&A during the three months ended March 31, 2012, a $5.1 million reduction of labor-related costs as a result of lower accruals for incentive based compensation due to the Company’s reduced financial performance and $1.4 million of lower labor costs due to cost reduction programs implemented in the latter half of fiscal year 2012. These decreases were partially offset by the acquisitions of Nexus and RTS, which increased SG&A costs by $7.4 million, including $2.2 million of amortization of intangible assets. Other cost increases included $1.6 million of higher consulting costs in connection with our initiatives to improve certain operating efficiencies.
Restructuring Charges
We recorded a restructuring charge of $3.3 million for fiscal year 2012. These charges were related primarily to a series of workforce reductions implemented to improve the Company’s cost structure by eliminating 118 employees.
28
We recorded a restructuring charge of $1.0 million for fiscal year 2011. These charges included severance related costs of $0.7 million, which related to the elimination of approximately 20 positions. We also incurred $0.3 million of facility-related costs for facilities exited in previous years. The costs for these exited facilities ended as of September 30, 2011.
Pension Settlement
During the quarter ended March 31, 2012, we advised participants of our frozen U.S. defined benefit pension plan that we intended to settle this pension obligation during fiscal year 2012. This settlement occurred in the quarter ended September 30, 2012 and resulted in accelerated cash payments of approximately $6.4 million to fully satisfy the pension liability, and resulted in an accelerated amortization of approximately $8.9 million of prior pension losses that were previously reported in accumulated other comprehensive income.
In-process Research and Development
During the three months ended March 31, 2012, we acquired primarily intellectual property from Intevac, Inc. for $3.0 million. Management evaluated this asset purchase to determine if this acquisition would be considered an acquisition of a business. Since only a limited amount of assets were acquired, management concluded that the inputs and processes required to meet the definition of a business were not acquired in this transaction, therefore, this transaction was treated as the purchase of an asset group. We expensed essentially all of this asset purchase as an in-process research and development cost in the three months ended March 31, 2012.
Interest Income
Interest income was $1.2 million for both fiscal year 2012 and 2011.
Sale of Contract Manufacturing Business in 2011
We closed the sale of our extended factory contract manufacturing business on June 28, 2011 with affiliates of Celestica Inc. The gross proceeds on this transaction were $81.8 million, of which $79.3 million was received on the closing. The balance of $2.5 million represents a working capital normalizing adjustment, and was received during our fourth quarter of 2011. The gross proceeds included the reimbursement of $1.3 million of cash on hand at the closing offset by $2.3 million of transaction expenses. The pre-tax gain on the sale was $45.0 million.
We also entered into certain commercial supply and license agreements with Celestica which would govern the ongoing relationship between Celestica and us. Pursuant to those agreements we supply Celestica with certain products and have licensed to Celestica certain intellectual property needed to run the business and Celestica supplies certain products to us. Due to the significance of these ongoing commercial arrangements, the sale did not qualify for discontinued operations treatment. Therefore, historical financial results of the divested business is not segregated within our consolidated financial statements for the historical periods in which this business was part of us.
Other Income, net
Other income, net of $0.7 million for fiscal year 2012 consists primarily of joint venture management fee income of $1.0 million which was partially offset by foreign exchange losses of $0.4 million. Other income, net of $1.9 million for fiscal year 2011 consists primarily of joint venture management fee income of $1.1 million and $0.7 million from a litigation settlement.
Income Tax Provision (Benefit)
We recorded an income tax benefit of $123.3 million for fiscal year 2012. This benefit included a $121.8 million deferred income tax benefit, primarily resulting from a significant reduction in the valuation allowance against deferred tax assets. At September 30, 2012, we considered the weight of both positive and negative evidence and we concluded that a substantial portion of the deferred tax assets would be realized. The tax benefit for fiscal year 2012 was partially offset by U.S. state income taxes and foreign taxes.
We recorded an income tax provision of $2.0 million for fiscal year 2011, which included $2.5 million of foreign and U.S. state income taxes, and an additional $2.4 million of income taxes relating to the sale of our Contract Manufacturing segment. These provisions were reduced by net favorable adjustments of $3.9 million to our liability for unrecognized tax benefits, primarily due to the expiration of certain statutes and a favorable audit outcome. We provided a full valuation allowance for our net deferred tax assets at September 30, 2011. At September 30, 2011, we considered the weight of both positive and negative evidence and we concluded that it was more likely than not that the future tax benefits from accumulated net operating losses and other temporary differences would not be realized.
29
Equity in Earnings of Joint Ventures
Income associated with our 50% interest in ULVAC Cryogenics, Inc., a joint venture with ULVAC Corporation of Japan, was $2.0 million for fiscal year 2012 as compared to $4.3 million for fiscal year 2011, due to the decline in demand for semiconductor capital equipment. Income associated with our 50% interest in Yaskawa Brooks Automation, Inc., a joint venture with Yaskawa Electric Corporation of Japan was $0.1 million for fiscal year 2012 as compared to $0.5 million for fiscal year 2011.
Liquidity and Capital Resources
A considerable portion of our revenue is dependent on the demand for semiconductor capital equipment. Demand for this equipment has historically experienced periodic downturns. This cyclicality makes estimates of a considerable portion of our future revenue and cash flows inherently uncertain.
At September 30, 2013, we had cash, cash equivalents and marketable securities aggregating $173.4 million. This amount was comprised of $83.0 million of cash and cash equivalents, $45.9 million of investments in short-term marketable securities and $44.5 million of investments in long-term marketable securities. Our marketable securities are generally readily convertible to cash without an adverse impact.
Cash and cash equivalents were $83.0 million at September 30, 2013, an increase of $28.3 million from September 30, 2012. The increase in cash was primarily due to $54.4 million of cash flow from operations, $53.3 million of net sales of marketable securities and $14.1 million of proceeds from the sale of a portion of our Chelmsford, Massachusetts campus and a portion of our Switzerland campus. These sources of cash were partially offset by the $68.3 million we used to acquire Crossing and Matrical and $21.3 million we used to pay cash dividends to our shareholders.
Cash provided by operating activities was $54.4 million for fiscal year 2013, and was composed of $28.4 million for non-cash related charges and $28.1 million of working capital improvements which were partially offset by a net loss of $2.2 million. Non-cash related charges consisted of items such as $24.2 million of depreciation and amortization, $7.8 million of stock-based compensation and $2.0 million of impairment charges, partially offset by a deferred tax benefit of $2.9 million. The decrease in working capital is primarily due to a $15.5 million decrease in inventory, $9.0 million increase in deferred revenue and $6.4 million decrease in accounts receivable, partially offset by a $3.9 million decrease in accrued expenses and other current liabilities.
Cash used in investing activities was $7.1 million for fiscal year 2013 and is composed of $68.3 million used in connection with the acquisitions of Crossing and Matrical, $3.6 million of capital expenditures and $3.1 million of deferred leasing costs we paid in connection with the lease of one of our buildings in our Chelmsford, MA campus. Cash used in investing activities was partially offset by net sales of marketable securities of $53.3 million and $14.1 million from the sale of buildings within our Chelmsford, MA campus and our Switzerland campus.
Cash used in financing activities was $19.5 million for fiscal year 2013 and includes $21.3 million for our quarterly cash dividends, which was partially offset by $1.8 million of cash generated from our employee stock purchase plans.
At September 30, 2012, we had cash, cash equivalents and marketable securities aggregating $200.2 million. This amount was comprised of $54.6 million of cash and cash equivalents, $85.7 million of investments in short-term marketable securities and $59.9 million of investments in long-term marketable securities.
Cash and cash equivalents were $54.6 million at September 30, 2012, a decrease of $4.2 million from September 30, 2011. The significant uses of cash during fiscal year 2012 included a payment of cash dividends of $21.0 million, a cash outflow of $9.2 million in connection with the acquisition of Celigo, $8.7 million of capital expenditures and an investment of $3.0 million. These uses of cash were mostly offset by cash provided by operating activities of $36.0 million and $1.7 million of proceeds from the issuance of common stock under our employee stock purchase plans.
Cash provided by operating activities was $36.0 million for fiscal year 2012, and was comprised of net income of $136.8 million, plus non-cash charges of $39.4 million, such as $21.6 million of depreciation and amortization, an $8.9 million non-cash charge related to our pension settlement and $8.6 million of stock-based compensation. These sources of cash were partially offset by a $121.8 million of a non-cash tax benefit related to the reversal of a significant portion of our deferred tax asset valuation allowance, and a $18.1 million of increase in working capital. Increases to working capital included an $11.2 million decrease in accounts payable, a $5.8 million decrease in accrued pension costs due primarily to the settlement of the U.S. defined benefit pension plan and a $4.9 million decrease in accrued compensation.
Cash used in investing activities was $21.0 million for fiscal year 2012 and was principally comprised of $9.2 million in connection with the acquisition of Celigo, $8.7 million of capital expenditures, an investment of $3.0 million and net purchases of marketable securities of $0.7 million. Cash used in investing activities was partially offset by a $0.5 million decrease in restricted cash related to expirations of certain cash collateralized international letters of credit. Capital expenditures include $1.4 million for the implementations of software, including the completion of our international implementations of our Oracle
30
ERP system, the implementation of the Oracle system for Brooks Life Science Systems and a new global Human Resource Information System.
Cash used in financing activities was $19.2 million for fiscal year 2012 and included $21.0 million for our quarterly cash dividends, which was partially offset by $1.7 million of cash generated from our employee stock purchase plans.
At September 30, 2013, we had approximately $5.7 million of letters of credit outstanding.
Our contractual obligations consist of the following at September 30, 2013 (in thousands):
Total | Less than One Year | One to Three Years | Four to Five Years | Thereafter | |||||||||||||||
Operating leases | $ | 19,552 | $ | 7,199 | $ | 9,368 | $ | 2,047 | $ | 938 | |||||||||
Pension funding | 1,111 | 199 | 398 | 200 | 314 | ||||||||||||||
Purchase commitments and other | 69,116 | 67,081 | 2,035 | — | — | ||||||||||||||
Total contractual obligations | $ | 89,779 | $ | 74,479 | $ | 11,801 | $ | 2,247 | $ | 1,252 | |||||||||
As of September 30, 2013, the total amount of net unrecognized tax benefits for uncertain tax positions and the accrual for the related interest was $6.9 million, all of which represents a potential future cash outlay. We are unable to make a reasonably reliable estimate of the timing of the cash settlement for this liability since the timing of future tax examinations by various tax jurisdictions and the related resolution is uncertain.
We are a guarantor on a lease in Mexico that expires in January 2015. The remaining payments under this lease at September 30, 2013 are approximately $0.5 million.
On June 25, 2013, we filed a registration statement on Form S-3 with the SEC to sell up to $200 million of securities, before any fees or expenses of the offering. Securities that may be sold include common stock, preferred stock, warrants, debt securities, depository shares, purchase contracts and purchase units. Any such offering, if it does occur, may happen in one or more transactions. Specific terms of any securities to be sold will be described in supplemental filings with the SEC. This registration statement will expire on July 1, 2016.
Our Board of Directors declared the following dividends (in thousands, except per share data):
Declaration Date | Dividend per Share | Record Date | Total | Payment Date | ||||||||
Year Ended September 30, 2013 | ||||||||||||
November 7, 2012 | $ | 0.08 | December 7, 2012 | $ | 5,311 | December 28, 2012 | ||||||
January 30, 2013 | 0.08 | March 8, 2013 | 5,361 | March 29, 2013 | ||||||||
May 8, 2013 | 0.08 | June 7, 2013 | 5,316 | June 28, 2013 | ||||||||
August 7, 2013 | 0.08 | September 6, 2013 | 5,340 | September 27, 2013 | ||||||||
Year Ended September 30, 2012 | ||||||||||||
November 8, 2011 | $ | 0.08 | December 9, 2011 | $ | 5,184 | December 30, 2011 | ||||||
February 8, 2012 | 0.08 | March 9, 2012 | 5,258 | March 30, 2012 | ||||||||
May 9, 2012 | 0.08 | June 8, 2012 | 5,277 | June 29, 2012 | ||||||||
August 8, 2012 | 0.08 | September 7, 2012 | 5,234 | September 28, 2012 | ||||||||
On November 12, 2013, our Board of Directors approved a cash dividend of $0.08 per share of the Company’s common stock. The total dividend of approximately $5.3 million will be paid on December 27, 2013 to shareholders of record at the close of business on December 6, 2013.
Dividends are declared at the discretion of our Board of Directors and depend on actual cash from operations, our financial condition and capital requirements and any other factors our Board of Directors may consider relevant. We intend to pay quarterly cash dividends in the future; however, the amount and timing of these dividends may be impacted by the cyclical nature of certain markets that we serve. We may reduce, delay or cancel a quarterly cash dividend based on the severity of a cyclical downturn.
We believe that we have adequate resources to fund our currently planned working capital and capital expenditure requirements for the next twelve months. The cyclical nature of our served markets and uncertainty with the current global economic environment makes it difficult for us to predict longer-term liquidity requirements with certainty. We may be unable to obtain any required additional financing on terms favorable to us, if at all. If adequate funds are not available on acceptable terms, we may be unable to successfully develop or enhance products, respond to competitive pressure or take advantage of acquisition opportunities, any of which could have a material adverse effect on our business.
31
Recent Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board ("FASB") issued an amendment to the accounting guidance for presentation of comprehensive income. Under the amended guidance, a company may present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. This authoritative guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. On October 1, 2012 we adopted this guidance and elected to present two separate but consecutive statements.
In February 2013, the FASB issued guidance to provide information about the amounts reclassified out of accumulated other comprehensive income (“AOCI”) by component. In addition, an entity is required to present, either on the face of the financial statements or in the notes, significant amounts reclassified out of AOCI by the respective line items of net income, but only if the amount reclassified is required to be reclassified in its entirety in the same reporting period. For amounts that are not required to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures that provide additional details about those amounts. On January 1, 2013 we adopted this standard, which had no impact on our financial position or results of operations.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to a variety of market risks, including changes in interest rates affecting the return on our cash and cash equivalents, short-term and long-term investments and fluctuations in foreign currency exchange rates.
Interest Rate Exposure
Our cash and cash equivalents consist principally of money market securities which are short-term in nature. Our short-term and long-term investments consist mostly of highly rated corporate debt securities, U.S. Treasury securities, and obligations of U.S. Government Agencies and other municipalities. At September 30, 2013, the unrealized loss position on marketable securities was $13,000, which is included in “Accumulated other comprehensive income” in the consolidated balance sheets. A hypothetical 100 basis point change in interest rates would result in an annual change of approximately $1.5 million in interest income earned.
Currency Rate Exposure
We have transactions and balances denominated in currencies other than the U.S. dollar. Most of these transactions or balances are denominated in Euros, British Pounds and a variety of Asian currencies. Sales in currencies other than the U.S. dollar were 25% of our total sales for the year ended September 30, 2013. These foreign sales were made primarily by our foreign subsidiaries, which have cost structures that substantially align with the currency of sale.
In the normal course of our business, we have short-term advances between our legal entities that are subject to foreign currency exposure. These short-term advances were approximately $10.2 million at September 30, 2013, and relate to the Euro, British Pound and a variety of Asian currencies. We mitigate the impact of potential currency translation losses on these short-term intercompany advances by the timely settlement of each transaction, generally within 30 days. We also utilize forward contracts to hedge our exposures. We incurred a foreign currency loss of $0.9 million for the year ended September 30, 2013, which relates to the currency fluctuation on these advances between the time the transaction occurs and the ultimate settlement of the transaction. A hypothetical 10% change in foreign exchange rates at September 30, 2013 would result in a $0.6 million change in our net income.
32
Item 8. | Financial Statements and Supplementary Data |
33
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Brooks Automation, Inc.
Chelmsford, Massachusetts
We have audited the accompanying consolidated balance sheet of Brooks Automation, Inc. as of September 30, 2013 and the related consolidated statements of operations, comprehensive income (loss), changes in equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements and schedules. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Brooks Automation, Inc. at September 30, 2013 and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Brooks Automation, Inc.’s internal control over financial reporting as of September 30, 2013, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated November 22, 2013 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Boston, Massachusetts
November 22, 2013
34
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Brooks Automation, Inc.:
In our opinion, the consolidated balance sheet as of September 30, 2012 and the related consolidated statements of operations, comprehensive income (loss), changes in equity and cash flows for each of the two years in the period ended September 30, 2012 present fairly, in all material respects, the financial position of Brooks Automation, Inc. and its subsidiaries at September 30, 2012, and the results of their operations and their cash flows for each of the two years in the period ended September 30, 2012, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Boston, Massachusetts
November 21, 2012
35
BROOKS AUTOMATION, INC.
CONSOLIDATED BALANCE SHEETS
September 30, 2013 | September 30, 2012 | ||||||
(In thousands, except share and per share data) | |||||||
Assets | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | 82,971 | $ | 54,639 | |||
Restricted cash | 177 | 763 | |||||
Marketable securities | 45,900 | 85,646 | |||||
Accounts receivable, net | 77,483 | 78,855 | |||||
Inventories | 97,719 | 102,985 | |||||
Deferred tax assets | 16,839 | 15,531 | |||||
Prepaid expenses and other current assets | 9,030 | 9,070 | |||||
Total current assets | 330,119 | 347,489 | |||||
Property, plant and equipment, net | 47,870 | 64,478 | |||||
Long-term marketable securities | 44,491 | 59,946 | |||||
Long-term deferred tax assets | 99,146 | 104,626 | |||||
Goodwill | 122,030 | 88,440 | |||||
Intangible assets, net | 60,088 | 39,400 | |||||
Equity investment in joint ventures | 25,687 | 31,428 | |||||
Other assets | 7,332 | 6,153 | |||||
Total assets | $ | 736,763 | $ | 741,960 | |||
Liabilities and equity | |||||||
Current liabilities | |||||||
Accounts payable | $ | 35,392 | $ | 28,988 | |||
Deferred revenue | 19,653 | 9,986 | |||||
Accrued warranty and retrofit costs | 7,349 | 7,329 | |||||
Accrued compensation and benefits | 14,225 | 14,118 | |||||
Accrued restructuring costs | 1,412 | 2,098 | |||||
Accrued income taxes payable | 1,077 | 1,699 | |||||
Accrued expenses and other current liabilities | 13,453 | 16,973 | |||||
Total current liabilities | 92,561 | 81,191 | |||||
Long-term tax liabilities | 7,036 | 6,356 | |||||
Long-term pension liability | 815 | 1,688 | |||||
Other long-term liabilities | 3,695 | 3,424 | |||||
Total liabilities | 104,107 | 92,659 | |||||
Commitments and contingencies (Note 21) | |||||||
Equity | |||||||
Preferred stock, $0.01 par value, 1,000,000 shares authorized, no shares issued or outstanding | — | — | |||||
Common stock, $0.01 par value, 125,000,000 shares authorized, 80,039,104 shares issued and 66,577,235 shares outstanding at September 30, 2013, 79,790,557 shares issued and 66,328,688 shares outstanding at September 30, 2012 | 800 | 798 | |||||
Additional paid-in capital | 1,825,499 | 1,817,706 | |||||
Accumulated other comprehensive income | 22,604 | 23,642 | |||||
Treasury stock at cost, 13,461,869 shares | (200,956 | ) | (200,956 | ) | |||
Accumulated deficit | (1,015,991 | ) | (992,524 | ) | |||
Total Brooks Automation, Inc. stockholders’ equity | 631,956 | 648,666 | |||||
Noncontrolling interest in subsidiaries | 700 | 635 | |||||
Total equity | 632,656 | 649,301 | |||||
Total liabilities and equity | $ | 736,763 | $ | 741,960 | |||
The accompanying notes are an integral part of these consolidated financial statements.
36
BROOKS AUTOMATION, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
(In thousands, except per share data) | |||||||||||
Revenue | |||||||||||
Product | $ | 362,404 | $ | 431,961 | $ | 611,117 | |||||
Services | 88,548 | 87,490 | 76,988 | ||||||||
Total revenue | 450,952 | 519,451 | 688,105 | ||||||||
Cost of revenue | |||||||||||
Product | 243,709 | 283,377 | 411,610 | ||||||||
Services | 61,261 | 62,588 | 53,474 | ||||||||
Total cost of revenue | 304,970 | 345,965 | 465,084 | ||||||||
Gross profit | 145,982 | 173,486 | 223,021 | ||||||||
Operating expenses | |||||||||||
Research and development | 48,991 | 47,464 | 39,846 | ||||||||
Selling, general and administrative | 99,545 | 101,223 | 102,542 | ||||||||
Restructuring and other charges | 6,465 | 3,275 | 1,036 | ||||||||
Pension settlement | — | 8,937 | — | ||||||||
In-process research and development | — | 3,026 | — | ||||||||
Total operating expenses | 155,001 | 163,925 | 143,424 | ||||||||
Operating income (loss) | (9,019 | ) | 9,561 | 79,597 | |||||||
Interest income | 1,032 | 1,213 | 1,153 | ||||||||
Interest expense | (2 | ) | (14 | ) | (65 | ) | |||||
Gain on sale of contract manufacturing business | — | — | 45,009 | ||||||||
Other income, net | 1,227 | 660 | 1,882 | ||||||||
Income (loss) before income taxes and equity in earnings of joint ventures | (6,762 | ) | 11,420 | 127,576 | |||||||
Income tax provision (benefit) | (2,170 | ) | (123,282 | ) | 1,954 | ||||||
Income (loss) before equity in earnings of joint ventures | (4,592 | ) | 134,702 | 125,622 | |||||||
Equity in earnings of joint ventures | 2,442 | 2,133 | 4,815 | ||||||||
Net income (loss) | $ | (2,150 | ) | $ | 136,835 | 130,437 | |||||
Net income attributable to noncontrolling interests | (65 | ) | (46 | ) | (52 | ) | |||||
Net income (loss) attributable to Brooks Automation, Inc. | $ | (2,215 | ) | $ | 136,789 | 130,385 | |||||
Basic net income (loss) per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.10 | $ | 2.02 | ||||
Diluted net income (loss) per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.08 | $ | 2.01 | ||||
Dividend declared per share | $ | 0.32 | $ | 0.32 | $ | 0.08 | |||||
Weighted-average shares used in computing earnings (loss) per share | |||||||||||
Basic | 65,912 | 65,128 | 64,549 | ||||||||
Diluted | 65,912 | 65,722 | 65,003 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
37
BROOKS AUTOMATION, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
(In thousands) | |||||||||||
Net income (loss) | $ | (2,150 | ) | $ | 136,835 | $ | 130,437 | ||||
Comprehensive income (loss), net of tax: | |||||||||||
Cumulative translation adjustment | (2,113 | ) | (2,406 | ) | 947 | ||||||
Unrealized gain (loss) on marketable securities | (135 | ) | 393 | (445 | ) | ||||||
Unrealized gain on cash flow hedge | 14 | — | — | ||||||||
Actuarial gain (loss) | 1,109 | (606 | ) | (1,044 | ) | ||||||
Pension settlement | 87 | 8,937 | — | ||||||||
Comprehensive income (loss), net of tax | (3,188 | ) | 143,153 | 129,895 | |||||||
Comprehensive income attributable to noncontrolling interests | (65 | ) | (46 | ) | (52 | ) | |||||
Comprehensive income (loss) attributable to Brooks Automation, Inc., net of tax | $ | (3,253 | ) | $ | 143,107 | $ | 129,843 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
38
BROOKS AUTOMATION, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
(In thousands) | |||||||||||
Cash flows from operating activities | |||||||||||
Net income (loss) | $ | (2,150 | ) | $ | 136,835 | $ | 130,437 | ||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||
Depreciation and amortization | 24,155 | 21,620 | 17,249 | ||||||||
Impairment of assets | 1,960 | — | — | ||||||||
Stock-based compensation | 7,757 | 8,647 | 6,752 | ||||||||
Amortization of premium on marketable securities | 1,274 | 2,401 | 2,283 | ||||||||
Undistributed earnings of joint ventures | (2,442 | ) | (2,133 | ) | (4,815 | ) | |||||
Deferred income tax benefit | (2,936 | ) | (122,136 | ) | (276 | ) | |||||
Pension settlement | 87 | 8,937 | — | ||||||||
Gain on sale of contract manufacturing business | — | — | (45,009 | ) | |||||||
Loss (gain) on disposal of long-lived assets | (1,394 | ) | (63 | ) | 10 | ||||||
Changes in operating assets and liabilities, net of acquisitions and disposals: | |||||||||||
Accounts receivable | 6,422 | (784 | ) | 9,916 | |||||||
Inventories | 15,490 | 5,874 | (19,131 | ) | |||||||
Prepaid expenses and other current assets | 4,359 | 5,801 | 4,205 | ||||||||
Accounts payable | 3,123 | (11,182 | ) | (15,099 | ) | ||||||
Deferred revenue | 8,971 | (4,684 | ) | 1,841 | |||||||
Accrued warranty and retrofit costs | (1,806 | ) | (123 | ) | (1,420 | ) | |||||
Accrued compensation and benefits | (2,625 | ) | (4,878 | ) | 1,717 | ||||||
Accrued restructuring costs | (972 | ) | 1,930 | (3,212 | ) | ||||||
Accrued pension | (950 | ) | (5,772 | ) | 2,014 | ||||||
Accrued expenses and other current liabilities | (3,934 | ) | (4,252 | ) | 188 | ||||||
Net cash provided by operating activities | 54,389 | 36,038 | 87,650 | ||||||||
Cash flows from investing activities | |||||||||||
Purchases of property, plant and equipment | (3,635 | ) | (8,653 | ) | (6,455 | ) | |||||
Purchases of marketable securities | (91,740 | ) | (132,015 | ) | (186,718 | ) | |||||
Sale/maturity of marketable securities | 145,023 | 131,317 | 120,095 | ||||||||
Acquisitions, net of cash acquired | (68,331 | ) | (9,216 | ) | (88,309 | ) | |||||
Decrease (increase) in restricted cash | 586 | 530 | (1,293 | ) | |||||||
Proceeds from the sale of the contract manufacturing business | — | — | 78,249 | ||||||||
Other investment | — | (3,000 | ) | — | |||||||
Proceeds from the sale of property, plant and equipment | 14,082 | — | — | ||||||||
Payment of deferred leasing cost | (3,134 | ) | — | — | |||||||
Other | — | — | 181 | ||||||||
Net cash used in investing activities | (7,149 | ) | (21,037 | ) | (84,250 | ) | |||||
Cash flows from financing activities | |||||||||||
Proceeds from issuance of common stock, net of issuance costs | 1,851 | 1,705 | 1,358 | ||||||||
Common stock dividend paid | (21,328 | ) | (20,953 | ) | (5,180 | ) | |||||
Net cash used in financing activities | (19,477 | ) | (19,248 | ) | (3,822 | ) | |||||
Effects of exchange rate changes on cash and cash equivalents | 569 | 53 | (568 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | 28,332 | (4,194 | ) | (990 | ) | ||||||
Cash and cash equivalents, beginning of year | 54,639 | 58,833 | 59,823 | ||||||||
Cash and cash equivalents, end of year | $ | 82,971 | $ | 54,639 | $ | 58,833 | |||||
Supplemental disclosures: | |||||||||||
Cash paid during the year for interest | $ | 2 | $ | 14 | $ | 65 | |||||
Cash paid (refunded) during the year for income taxes, net | $ | (762 | ) | $ | 4,282 | $ | 1,042 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
39
BROOKS AUTOMATION, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
Common Stock Shares | Common Stock at Par Value | Additional Paid-In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Treasury Stock | Total Brooks Automation, Inc. Stockholders’ Equity | Noncontrolling Interests in Subsidiaries | Total Equity | ||||||||||||||||||||||||||
(In thousands, except share data) | ||||||||||||||||||||||||||||||||||
Balance September 30, 2010 | 78,869,331 | $ | 789 | $ | 1,803,121 | $ | 17,866 | $ | (1,233,188 | ) | $ | (200,956 | ) | $ | 387,632 | $ | 537 | $ | 388,169 | |||||||||||||||
Shares issued under stock option, restricted stock and purchase plans, net | 867,858 | 8 | (586 | ) | (578 | ) | (578 | ) | ||||||||||||||||||||||||||
Stock-based compensation | 6,752 | 6,752 | 6,752 | |||||||||||||||||||||||||||||||
Common stock dividend declared | (5,302 | ) | (5,302 | ) | (5,302 | ) | ||||||||||||||||||||||||||||
Net income | 130,385 | 130,385 | 52 | 130,437 | ||||||||||||||||||||||||||||||
Currency translation adjustments | 947 | 947 | 947 | |||||||||||||||||||||||||||||||
Changes in unrealized loss on marketable securities | (445 | ) | (445 | ) | (445 | ) | ||||||||||||||||||||||||||||
Actuarial loss arising in the year | (1,044 | ) | (1,044 | ) | (1,044 | ) | ||||||||||||||||||||||||||||
Balance September 30, 2011 | 79,737,189 | 797 | 1,809,287 | 17,324 | (1,108,105 | ) | (200,956 | ) | 518,347 | 589 | 518,936 | |||||||||||||||||||||||
Shares issued under stock option, restricted stock and purchase plans, net | 53,368 | 1 | (228 | ) | (227 | ) | (227 | ) | ||||||||||||||||||||||||||
Stock-based compensation | 8,647 | 8,647 | 8,647 | |||||||||||||||||||||||||||||||
Common stock dividend declared | (21,208 | ) | (21,208 | ) | (21,208 | ) | ||||||||||||||||||||||||||||
Net income | 136,789 | 136,789 | 46 | 136,835 | ||||||||||||||||||||||||||||||
Currency translation adjustments | (2,406 | ) | (2,406 | ) | (2,406 | ) | ||||||||||||||||||||||||||||
Changes in unrealized gain on marketable securities | 393 | 393 | 393 | |||||||||||||||||||||||||||||||
Actuarial loss arising in the year | (606 | ) | (606 | ) | (606 | ) | ||||||||||||||||||||||||||||
Recognition of pension settlement in earnings | 8,937 | 8,937 | 8,937 | |||||||||||||||||||||||||||||||
Balance September 30, 2012 | 79,790,557 | 798 | 1,817,706 | 23,642 | (992,524 | ) | (200,956 | ) | 648,666 | 635 | 649,301 | |||||||||||||||||||||||
Shares issued under stock option, restricted stock and purchase plans, net | 248,547 | 2 | 186 | 188 | 188 | |||||||||||||||||||||||||||||
Stock-based compensation | 7,607 | 7,607 | 7,607 | |||||||||||||||||||||||||||||||
Common stock dividend declared | (21,252 | ) | (21,252 | ) | (21,252 | ) | ||||||||||||||||||||||||||||
Net loss | (2,215 | ) | (2,215 | ) | 65 | (2,150 | ) | |||||||||||||||||||||||||||
Currency translation adjustments | (2,113 | ) | (2,113 | ) | (2,113 | ) | ||||||||||||||||||||||||||||
Changes in unrealized loss on marketable securities, net of tax of $79 | (135 | ) | (135 | ) | (135 | ) | ||||||||||||||||||||||||||||
Changes in unrealized gain on cash flow hedges, net of tax of $9 | 14 | 14 | 14 | |||||||||||||||||||||||||||||||
Actuarial gain arising in the year, net of tax of $360 | 1,109 | 1,109 | 1,109 | |||||||||||||||||||||||||||||||
Recognition of pension settlement in earnings | 87 | 87 | 87 | |||||||||||||||||||||||||||||||
Balance September 30, 2013 | 80,039,104 | $ | 800 | $ | 1,825,499 | $ | 22,604 | $ | (1,015,991 | ) | $ | (200,956 | ) | $ | 631,956 | $ | 700 | $ | 632,656 | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
40
BROOKS AUTOMATION, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of the Business
Brooks Automation, Inc. (“Brooks” or the “Company”) is a leading worldwide provider of automation, vacuum and instrumentation solutions for multiple markets including semiconductor manufacturing, technology device manufacturing and life sciences. The Company's technologies, engineering competencies and global service capabilities provide customers speed to market and ensure high uptime and rapid response, which equate to superior value in their mission-critical controlled environments. Since 1978, the Company has been a leading partner to the global semiconductor manufacturing markets and through product development initiatives and strategic business acquisitions Brooks has expanded its reach to meet the needs of customers in technology markets adjacent to semiconductor and life sciences.
2. Summary of Significant Accounting Policies
Principles of Consolidation and Basis of Presentation
The consolidated financial statements include the accounts of the Company and all majority-owned subsidiaries. All intercompany accounts and transactions are eliminated. Equity investments in which the Company exercises significant influence but does not control and is not the primary beneficiary are accounted for using the equity method.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant estimates are associated with accounts receivable, inventories, intangible assets, goodwill, deferred income taxes, warranty obligations, revenue recognized using the percentage of completion method and stock-based compensation expense on performance-based awards. Although the Company regularly assesses these estimates, actual results could differ from those estimates. Changes in estimates are recorded in the period in which they become known.
Foreign Currency Translation
Some transactions of the Company and its subsidiaries are made in currencies different from their functional currency. Foreign currency gains (losses) on these transactions or balances are recorded in “Other (income) expense, net” when incurred. Net foreign currency transaction losses included in income (loss) before income taxes and equity in earnings of joint ventures totaled $0.9 million, $0.4 million and $0.1 million for the years ended September 30, 2013, 2012 and 2011, respectively. For non-U.S. subsidiaries, assets and liabilities are translated at period-end exchange rates, and statements of operations items are translated at the average exchange rates for the period. The local currency is considered to be the functional currency for all of our foreign subsidiaries and, accordingly, translation adjustments are reported in “Accumulated other comprehensive income.” Foreign currency translation adjustments are one of the components of comprehensive net income (loss).
Derivative Financial Instruments
All derivatives, whether designated in a hedging relationship or not, are recorded on the consolidated balance sheets at fair value. The accounting for changes in fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and further, on the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, the Company must designate the hedging instrument, based on the exposure being hedged, as a fair value hedge, cash flow hedge or a hedge of a net investment in a foreign operation. Certain derivatives held by the Company are not designated as hedges but are used in managing exposure to changes in foreign exchange rates.
A fair value hedge is a derivative instrument designated for the purpose of hedging the exposure of changes in fair value of an asset or a liability resulting from a particular risk. If the derivative is designated as a fair value hedge, the changes in the fair value of the derivative and of the hedged item attributable to the hedged risk are both recognized in the same caption in the consolidated statements of operations and comprehensive income.
A cash flow hedge is a derivative instrument designated for the purpose of hedging the exposure to variability in future cash flows resulting from a particular risk. If the derivative is designated as a cash flow hedge, the effective portions of changes in the fair value of the derivative are recorded in accumulated other comprehensive income and are recognized in the results of operations when the hedged item affects earnings. Ineffective portions of changes in the fair value of cash flow hedges are recognized in the results of operations.
A hedge of a net investment in a foreign operation is achieved through a derivative instrument designated for the purpose of hedging the exposure of changes in value of investments in foreign subsidiaries. If the derivative is designated as a hedge of
41
a net investment in a foreign operation, the effective portions of changes in the fair value of the derivative are recorded in other comprehensive income as a part of the currency translation adjustment. Ineffective portions of net investment hedges are recognized in the results of operations.
For derivative instruments not designated as hedging instruments, changes in fair value are recognized in the Consolidated Statements of Operations as gains and losses consistent with the classification of the underlying risk.
Cash and Cash Equivalents
Cash and cash equivalents include cash and highly liquid investments with original maturities of three months or less. At September 30, 2013 and 2012, cash equivalents were $7.8 million and $17.5 million, respectively. Cash equivalents are held at cost which approximates fair value due to their short-term maturities and varying interest rates.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist primarily of trade receivables and temporary and long-term cash investments in treasury bills and commercial paper. The Company restricts its investments to U.S. government and its agencies, municipalities, corporate securities and mutual funds that invest in U.S. government securities. The Company's customers are concentrated in the semiconductor industry, and relatively few customers account for a significant portion of the Company's revenue. The Company's top ten largest customers account for approximately 39% of revenue for the year ended September 30, 2013. One of the Company's customers accounted for 11% of revenue for the year ended September 30, 2013. The Company regularly monitors the creditworthiness of its customers and believes that it has adequately provided for exposure to potential credit losses.
Accounts Receivable and Allowance for Doubtful Accounts and Sales Returns
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company's best estimate of the amount of probable credit losses in its existing accounts receivable. The Company determines the allowance based on a number of factors, including an evaluation of customer credit worthiness, the age of the outstanding receivable, economic trends and historical experience. The Company reviews its allowance for doubtful accounts on a quarterly basis and changes in estimates are reflected in the period in which they become known. Accounts receivable balances are written-off against the allowance for doubtful accounts when the Company determines that the receivable is not recoverable. Provisions for doubtful accounts are recorded in "General and Administrative Expenses" in the Consolidated Statements of Operations. The allowance for sales returns is the Company's best estimate of probable returns from one of its semiconductor customers. Provisions for sales returns are recorded in "Revenue" in the Consolidated Statements of Operations. The Company does not have any off-balance-sheet credit exposure related to its customers.
Inventories
Inventories are stated at the lower of cost or market, cost being determined using a standard costing system which approximates cost based on a first-in, first-out method. The Company provides inventory reserves for excess, obsolete or damaged inventory based on changes in customer demand, technology and other economic factors.
Fixed Assets, Intangible Assets and Impairment of Long-lived Assets
Property, plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method. Depreciable lives are summarized below:
Buildings | 20 - 40 years |
Computer equipment and software | 2 - 7 years |
Machinery and equipment | 2 - 10 years |
Furniture and fixtures | 3 - 10 years |
Leasehold improvements are amortized over the shorter of their estimated useful lives or the term of the respective leases. Equipment used for demonstrations to customers is included in machinery and equipment and is depreciated over its estimated useful life. Repair and maintenance costs are expensed as incurred.
The Company has developed software for internal use. Internal and external labor costs incurred during the application development stage of a project are capitalized. Costs incurred prior to application development and post implementation are expensed as incurred. Training and data conversion costs are expensed as incurred.
When an asset is retired, the cost of the asset disposed of and the related accumulated depreciation are removed from the accounts, and any resulting gain or loss is included in the determination of operating profit (loss).
42
As a result of the Company's acquisitions, the Company has identified finite-lived intangible assets other than goodwill. Finite-lived intangible assets are valued based on estimates of future cash flows and amortized over their estimated useful life using methods that approximate the pattern in which the economic benefits are expected to be realized.
Finite-lived intangibles assets and fixed assets are tested for impairment when indicators of impairment are present. For purposes of this test, long-lived assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities. When the Company determines that indicators of potential impairment exist, the next step of the impairment test requires that the potentially impaired long-lived asset group is tested for recoverability. The test for recoverability compares the undiscounted future cash flows of the long-lived asset group to its carrying value. The future cash flow period is based on the future service life of the primary asset within the long-lived asset group. If the carrying values of the long-lived asset group exceed the future cash flows, the assets are considered to be potentially impaired. The next step in the impairment process is to determine the fair value of the individual net assets within the long-lived asset group. If the aggregate fair values of the individual net assets of the group are less than their carrying values, an impairment is recorded equal to the excess of the aggregate carrying value of the group over the aggregate fair value. The loss is allocated to each asset within the group based on their relative carrying values, with no asset reduced below its fair value.
The amortizable lives of intangible assets, including those identified as a result of purchase accounting, are summarized as follows:
Patents | 7 - 15 years |
Completed technology | 2 - 10 years |
License agreements | 5 years |
Trademarks and trade names | 2 - 6 years |
Non-competition agreements | 3 - 5 years |
Customer relationships | 4 - 13 years |
Goodwill
Goodwill represents the excess of purchase price over the fair value of net tangible and identifiable intangible assets of the businesses the Company acquired. The Company performs an annual impairment test of its goodwill on September 30 of each fiscal year unless interim indicators of impairment exist.
The testing of goodwill for impairment is performed at a level referred to as a reporting unit. A reporting unit is either the “operating segment level” or one level below, which is referred to as a “component.” The level at which the impairment test is performed requires an assessment as to whether the operations below the operating segment constitute a self-sustaining business, testing is generally required to be performed at this level. The Company currently has five reporting units that have goodwill, including three components that are part of the Brooks Product Solutions operating segment, one reporting unit that is the Brooks Global Services operating segment and one reporting unit that is the Brooks Life Science Systems operating segment.
The Company determines the fair value of its reporting units using an Income Approach, specifically the Discounted Cash Flow Method (“DCF Method”). The DCF Method includes future cash flow projections, which are discounted to present value, and an estimate of terminal values, which are also discounted to present value. Terminal values represent the present value an investor would pay today for the rights to the cash flows of the business for the years subsequent to the discrete cash flow projection period. The Company considers the DCF Method to be the most appropriate valuation indicator as the DCF analyses are based on management's long-term financial projections. Given the dynamic nature of the cyclical semiconductor equipment market, management's projections as of the valuation date are considered more objective since other market metrics for peer companies fluctuate over the cycle. However, the Company also uses market-based valuation techniques to test the reasonableness of the reporting unit fair values determined by the DCF Method and compares the aggregate fair value of its reporting units plus its net corporate assets to its overall market capitalization.
Goodwill impairment testing is a two-step process. The first step of the goodwill impairment test, used to identify potential impairment, compares the fair value of each reporting unit to its respective carrying amount, including goodwill. If the fair value of the reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered impaired. If the reporting unit's carrying amount exceeds the fair value, the second step of the goodwill impairment test must be completed to measure the amount of the impairment loss, if any. The second step compares the implied fair value of goodwill with the carrying value of goodwill. The implied fair value is determined by allocating the fair value of the reporting unit to all of the assets and liabilities of that unit, the excess of the fair value over amounts assigned to its assets and liabilities is the implied fair value of goodwill. The implied fair value of goodwill determined in this step is compared to the carrying value of goodwill. If the implied fair value of goodwill is less than the carrying value of goodwill, an impairment loss is recognized equal to the difference.
43
Pension Plans
The cost and obligations of the Company's defined benefit pension plans are calculated using many assumptions to estimate the benefits that the employee earns while working, the amount of which cannot be completely determined until the benefit payments cease. Major assumptions used in the accounting for these employee benefit plans include the discount rate, expected return on plan assets and rate of increase in employee compensation levels. Assumptions are determined based on Company data and appropriate market indicators in consultation with third-party actuaries, and are evaluated each year as of the plans' measurement date.
Revenue Recognition
Product revenue is associated with the sale of hardware systems, components and spare parts as well as product license revenue. Service revenue is associated with service contracts, repairs, upgrades and field service. Shipping and handling fees, billed to customers, if any, are recognized as revenue. The related shipping and handling costs are recognized in cost of revenue.
Revenue from product sales that do not include significant customization is recorded upon delivery and transfer of risk of loss to the customer provided there is evidence of an arrangement, fees are fixed or determinable, collection of the related receivable is reasonably assured and, if applicable, customer acceptance criteria have been successfully demonstrated. Customer acceptance provisions are included in certain arrangements to ensure that the product meets the published specification requirements. Customer acceptance provisions in the Company’s arrangements generally consist of final testing procedures that are carried out either in one of the Company’s facilities or at the customer location. When an arrangement includes acceptance criteria to be carried out in one of the Company’s facilities, the Company recognizes revenue when the acceptance criteria have been successfully demonstrated and delivery and transfer of risk of loss have occurred. When significant on site customer acceptance provisions are present in the arrangement, revenue is recognized upon completion of customer acceptance testing.
Revenue from product sales that does include significant customization, which primarily include life science automation systems, is recorded using the percentage of completion method whereby revenue is recorded as work progresses based on a percentage that incurred labor effort to date bear to total projected labor effort. Payments collected from customers in advance of recognizing the related revenue is recorded as deferred revenue. In addition, contracts are reviewed on a regular basis to determine whether a probable future loss exists. If such a loss is estimated by comparing total estimated contract revenue to the total estimated contract costs, a loss will be accrued in the same period the Company determines that the loss is probable.
Revenue associated with service agreements is generally recognized ratably over the term of the contract, with payments from customers being recorded as deferred revenue. Revenue from repair services or upgrades of customer-owned equipment is recognized upon completion of the repair effort and upon the shipment of the repaired item back to the customer. In instances where the repair or upgrade includes installation, revenue is recognized when the installation is completed.
A portion of the revenue arrangements for sales of life science automation systems are multiple element arrangements that can include product, service, as well as other elements. For revenue arrangements with multiple elements, arrangement consideration is allocated to each element based upon their relative selling price using vendor-specific objective evidence (“VSOE”), or third-party evidence (“TPE”) or based upon the relative selling price using estimated selling prices if VSOE or TPE does not exist. We rely primarily on relative selling prices when we do not have VSOE for a specific element. The Company recognizes revenue on each element of the arrangement in accordance with its policies for revenue recognition. The fair value of any undelivered elements is deferred until the undelivered element is delivered and all other criteria for revenue recognition have been met.
Warranty
The Company offers warranties on the sales of certain of its products and records an accrual for estimated future claims. Such accruals are based upon historical experience and management's estimate of the level of future claims.
Research and Development Expenses
Research and development costs are charged to expense when incurred.
Stock-Based Compensation
The Company measures compensation cost for all employee stock awards at fair value on the date of grant and recognizes compensation expense over the service period for awards expected to vest. The fair value of restricted stock is determined based on the number of shares granted and the quoted price of the Company's common stock on the date of grant, and the fair value of stock options is determined using the Black-Scholes valuation model. Such value is recognized as expense over the service period, net of estimated forfeitures. The estimation of stock awards that will ultimately vest requires significant judgment. The Company considers many factors when estimating expected forfeitures, including types of awards, employee
44
class, and historical experience. In addition, for stock-based awards where vesting is dependent upon achieving certain operating performance goals, the Company estimates the likelihood of achieving the performance goals. Actual results, and future changes in estimates, may differ substantially from the Company's current estimates.
During the year ended September 30, 2013, the Company granted 1,394,000 shares of restricted stock to members of senior management of which 716,625 shares vest over the service period and the remaining 677,375 shares vest upon the achievement of certain financial performance goals which were measured at the end of fiscal year 2013. Total compensation expense on these awards is a maximum of $15.1 million, net of cancellations. During the year ended September 30, 2012, the Company granted 1,527,000 shares of restricted stock to members of senior management of which 406,750 shares vest over the service period and the remaining 1,120,250 shares vest upon the achievement of certain financial performance goals which will be measured at the end of fiscal year 2014. Total compensation expense on these awards is a maximum of $14.4 million, net of cancellations. Awards subject to service criteria are being recorded to expense ratably over the vesting period. Awards subject to performance criteria are expensed over the related service period when attainment of the performance condition is considered probable. The total amount of compensation recorded depends on the Company's achievement against performance targets. Changes to the projected attainment against performance targets during the vesting period may result in an adjustment to the amount of cumulative compensation recorded as of the date the estimate is revised.
During the three months ended December 31, 2011, the Company's Chief Executive Officer was granted an award of 100,000 cash settled phantom units, which are subject to the same vesting terms as the performance-based restricted stock units from fiscal year 2012. The Company's consolidated balance sheet at September 30, 2013 and 2012 includes a liability of approximately $0 and $78,000, respectively, for this potential cash payment. The Company recorded an expense of $78,000 related to this award during the year ended September 30, 2012, but was reversed during the year ended September 30, 2013 because the performance criteria was not met.
The following table reflects compensation expense recorded during the years ended September 30, 2013, 2012 and 2011 (in thousands):
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Restricted stock | $ | 7,261 | $ | 8,098 | $ | 6,248 | |||||
Employee stock purchase plan | 496 | 549 | 504 | ||||||||
$ | 7,757 | $ | 8,647 | $ | 6,752 | ||||||
Valuation Assumptions for Stock Options and Employee Stock Purchase Plans
No stock options were granted for the years ended September 30, 2013, 2012 or 2011.
The fair value of shares issued under the employee stock purchase plan was estimated on the commencement date of each offering period using the Black-Scholes option-pricing model with the following assumptions:
Year ended September 30, | ||||||||
2013 | 2012 | 2011 | ||||||
Risk-free interest rate | 0.1 | % | 0.1 | % | 0.2 | % | ||
Volatility | 32 | % | 45 | % | 50 | % | ||
Expected life | 6 months | 6 months | 6 months | |||||
Dividend yield | 3.30% - 3.40% | 2.75% - 3.30% | 0% - 3% | |||||
The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected life of the option; expected volatilities are based on historical volatilities of the Company's common stock; and the expected life represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and the Company's historical exercise patterns. Dividend yields are projected based on the Company's history of dividends declared, and management's intention for future dividend declarations.
Equity Incentive Plans
The Company's equity incentive plans are intended to attract and retain employees and to provide an incentive for them to assist the Company to achieve long-range performance goals and to enable them to participate in the long-term growth of the Company. The equity incentive plans consist of plans under which employees may be granted options to purchase shares of the Company's stock, restricted stock and other equity incentives. Stock options generally had a vesting period of four years and are exercisable for a period not to exceed seven years from the date of issuance. Restricted stock awards generally vest over two to four years. At September 30, 2013, a total of 3,259,558 shares were reserved and available for the issuance of awards under the plans.
45
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company's consolidated financial statements contain certain deferred tax assets which have arisen primarily as a result of operating losses, as well as other temporary differences between financial and tax accounting. A valuation allowance is established if the likelihood of realization of the deferred tax assets is not considered more likely than not based on an evaluation of positive and negative evidence and the extent to which that evidence is objectively verifiable. Significant management judgment is required in determining the Company's provision for income taxes, the Company's deferred tax assets and liabilities and any valuation allowance recorded against those net deferred tax assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
The calculation of the Company's tax liabilities involves dealing with uncertainties in the application of complex tax regulations. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. If we determine that a tax position will more likely than not be sustained on audit, the second step requires us to estimate and measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as we have to determine the probability of various possible outcomes. We re-evaluate these uncertain tax positions on a quarterly basis. This evaluation is based on factors such as changes in facts or circumstances, changes in tax law, new audit activity, and effectively settled issues. Determining whether an uncertain tax position is effectively settled requires judgment. Such a change in recognition or measurement would result in the recognition of a tax benefit or an additional charge to the tax provision.
Earnings Per Share
Basic earnings per share is calculated based on the weighted average number of common shares outstanding during the period. Diluted earnings per share is calculated based on the weighted average number of common shares and dilutive common equivalent shares assumed outstanding during the period. Shares used to compute diluted earnings per share exclude common share equivalents if their inclusion would have an anti-dilutive effect.
Fair Value of Financial Instruments
The Company's financial instruments include cash and cash equivalents, restricted cash, marketable securities, derivative instruments, accounts receivable and accounts payable. The carrying amounts of these items reported in the balance sheets approximate their fair value at September 30, 2013 and 2012 because of their short-term nature. In the case of marketable securities and derivative instruments, measurement is based on quoted market prices for identical or similar securities or instruments.
Reclassifications
In order to conform with the current year presentation, cash distributions received from joint ventures of $5.1 million and $2.4 million in the years ended September 30, 2012 and 2011, respectively, were reclassified from “Undistributed Earnings of Joint Ventures” to “Prepaid Expenses and Other Current Assets” on the Consolidated Statements of Cash Flows. The reclassification of these amounts had no impact on previously reported net cash provided by operating activities, results of operations or financial position for the years ended September 30, 2012 and 2011.
Recent Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board ("FASB") issued an amendment to the accounting guidance for presentation of comprehensive income. Under the amended guidance, a company may present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. This authoritative guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. On October 1, 2012 the Company adopted this guidance and elected to present two separate but consecutive statements.
In February 2013, the FASB issued guidance to provide information about the amounts reclassified out of accumulated other comprehensive income (“AOCI”) by component. In addition, an entity is required to present, either on the face of the financial statements or in the notes, significant amounts reclassified out of AOCI by the respective line items of net income, but only if the amount reclassified is required to be reclassified in its entirety in the same reporting period. For amounts that are not required to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures that
46
provide additional details about those amounts. On January 1, 2013 the Company adopted this standard, which had no impact on its financial position or results of operations.
3. Acquisitions and Divestiture
Acquisition of Matrical
On August 1, 2013, the Company acquired certain assets and assumed certain liabilities of Matrical, Inc.’s life science businesses (collectively “the Matrical assets”) for cash consideration of approximately $9.3 million, net of cash acquired. Matrical, Inc. is a Spokane, Washington-based, privately held company that provides biological sample preparation, management and storage solutions to customers in agricultural biotechnology, biotechnology, life science and pharmaceutical markets. The acquisition of the Matrical assets provides the Company with the opportunity to enhance its existing product offerings in biobanking and sample management.
The assets and liabilities associated with the purchase of the Matrical assets were recorded at their fair values as of the acquisition date. The amounts recorded follow (in thousands):
Accounts receivable | $ | 636 | |
Inventory | 2,095 | ||
Prepaid and other current assets | 103 | ||
Property, plant and equipment | 534 | ||
Completed technology | 500 | ||
Customer relationships | 1,500 | ||
Goodwill | 7,137 | ||
Debt | (902 | ) | |
Accounts payable | (294 | ) | |
Deferred revenue | (412 | ) | |
Customer deposits | (1,249 | ) | |
Other current liabilities | (322 | ) | |
Total purchase price, net of cash acquired | $ | 9,326 | |
In performing the purchase price allocation, the Company considered, among other factors, its intention for future use of the acquired assets, analyses of historical financial performance, and estimates of future cash flows from Matrical’s products and services. The purchase price was allocated based upon the fair value of the identified assets acquired and liabilities assumed as of the acquisition date from a market participant’s perspective.
The Company used the relief-from-royalty method to value the completed technology and the excess earnings method to value the customer relationships. Cash flows were discounted at a rate of 18%. The weighted-average amortization periods are 4.6 years for completed technologies and 7.0 years for customer relationships. The intangible assets acquired will be amortized using the straight-line method because it approximates the pattern in which the economic benefits are expected to be realized.
Goodwill represents the excess of the purchase price over the fair values of the net tangible and intangible assets acquired and is primarily the result of expected synergies from combining the Matrical products with the Company's other life science products. Goodwill arising from the acquisition of the Matrical assets is deductible for tax purposes.
The operating results from the Matrical assets have been included in the results of operations for the Brooks Life Science Systems segment from the date of acquisition. Pro forma results are not provided as the results of operations were not material.
Transaction costs incurred by the Company related to this acquisition were $0.3 million and are included in selling, general and administrative expense.
Acquisition of Crossing
On October 29, 2012, the Company acquired all the outstanding stock of Crossing Automation Inc. (“Crossing”), a U.S. based provider of automation solutions and services primarily to global semiconductor front-end markets. The Company paid, in cash, an aggregate merger consideration of $59.0 million net of cash acquired. Crossing is based in Fremont, California. The acquisition of Crossing provides the Company with the opportunity to enhance its existing capabilities with respect to manufacturing of atmospheric and vacuum automation solutions within the semiconductor front-end market.
47
The assets and liabilities associated with Crossing were recorded at their fair values as of the acquisition date. The amounts recorded follow (in thousands):
Accounts receivable | $ | 5,356 | |
Inventory | 8,668 | ||
Prepaid expenses | 1,968 | ||
Property, plant and equipment | 2,270 | ||
Completed technology | 10,530 | ||
Customer relationships | 20,010 | ||
Goodwill | 26,453 | ||
Other long term assets | 885 | ||
Accounts payable | (3,024 | ) | |
Accrued liabilities | (5,172 | ) | |
Deferred revenue | (319 | ) | |
Other current liabilities | (388 | ) | |
Other long-term liabilities | (8,232 | ) | |
Total purchase price, net of cash acquired | $ | 59,005 | |
In performing the purchase price allocation, the Company considered, among other factors, its intention for future use of the acquired assets, analyses of historical financial performance, and estimates of future cash flows from Crossing’s products and services. The purchase price was allocated based upon the fair value of the identified assets acquired and liabilities assumed as of the acquisition date from a market participant’s perspective.
The Company used the relief-from-royalty method to value the completed technology and the excess earnings method to value the customer relationships. Cash flows were discounted at a rate of 15%. The weighted-average amortization periods are 7.7 years for completed technologies and 8.0 years for customer relationships. The intangible assets acquired will be amortized using methods that approximate the pattern in which the economic benefits are expected to be realized, including variable declining balance and straight-line methods.
Goodwill is primarily the result of expected synergies from combining the operations of Crossing with the Company. Goodwill arising from the acquisition of Crossing is not deductible for tax purposes.
Crossing’s operating results have been included in the results of operations for the Brooks Product Solutions and Brooks Global Services segments from the acquisition date. Revenue from Crossing for the year ended September 30, 2013 was $41.5 million, and the after-tax net loss attributable to Crossing was $2.6 million. The net loss includes the impact of step-up in value of acquired inventories sold in fiscal 2013 which increased the net loss by $2.7 million. The operating results for Crossing also include amortization expense of $3.7 million for the year ended September 30, 2013.
The following pro forma summary presents consolidated information of the Company as if the acquisition of Crossing occurred on October 1, 2011 (in thousands):
Year ended September 30, | |||||||
2013 | 2012 | ||||||
Revenue | $ | 453,045 | $ | 570,864 | |||
Net income (loss) attributable to Brooks Automation, Inc. | (3,216 | ) | 135,245 | ||||
The pro forma net income (loss) has been adjusted to reflect additional amortization from adjustments to intangible assets as if those adjustments had been applied as of October 1, 2011.
Transaction costs of $3.6 million incurred by Crossing prior to the closing of the acquisition have been eliminated from pro forma net income (loss) as presented above. These costs include banker fees of $1.5 million and one-time incentive compensation payments related to the transaction of $1.2 million. Transaction costs incurred by the Company related to this acquisition were $0.6 million for the year ended September 30, 2013, and are included in selling, general and administrative expense.
Acquisition of Intellectual Property from Intevac, Inc.
During the three months ended March 31, 2012, the Company acquired primarily intellectual property from Intevac, Inc. for $3.0 million. Management evaluated this asset purchase to determine if this acquisition would be considered an acquisition of a business. Since only a limited amount of assets were acquired, management concluded that the inputs and processes
48
required to meet the definition of a business were not acquired in this transaction, therefore, this transaction was treated as the purchase of an asset group. This asset group includes primarily intellectual property that we use in the development of the Company's next generation of semiconductor automation tools that resides within the Brooks Product Solutions segment. The Company expensed essentially all of this asset purchase as an in-process research and development cost in the three months ended March 31, 2012.
Acquisition of Celigo
On December 30, 2011, the Company acquired the Celigo® automated Cell Cytometer product line (“Celigo”) from Cyntellect, Inc., for $8.7 million in cash, plus a deferred cash payment of $0.5 million that was paid in July 2012. The Celigo product line provides life science customers with cellular imaging in a high-throughput and easy-to-use platform. Celigo's operations were based in San Diego, California, and were integrated into the Company's nearby Poway, California-based life sciences operation shortly after the acquisition. The Celigo product line resides in the Brooks Life Science Systems segment. The acquisition of Celigo provides a complementary analysis tool for customers currently using the Company's automated sample management systems.
The assets and liabilities associated with Celigo were recorded at their fair values as of the acquisition date. The amounts recorded follow (in thousands):
Accounts receivable | $ | 897 | |
Inventory | 1,139 | ||
Property, plant and equipment | 202 | ||
Completed technology | 3,540 | ||
Trademarks and trade names | 70 | ||
Goodwill | 3,713 | ||
Accounts payable | (13 | ) | |
Deferred revenue | (326 | ) | |
Other current liabilities | (6 | ) | |
Total purchase price, net of cash acquired | $ | 9,216 | |
The estimated fair value attributed to the completed technologies was determined based upon a discounted cash flow forecast. Cash flows were discounted at a rate of 25%.
In 2013, the Company recorded an impairment charge of $2.0 million in the fourth quarter of fiscal 2013, related to long-lived assets acquired with the Celigo product line. The impairment charge is described in "Note 7. Goodwill and Intangible Assets."
Goodwill is primarily the result of expected synergies from combining the Celigo product line with the Company's other Life Science products. Goodwill arising from the acquisition will be deductible for tax purposes.
Celigo's operating results have been included in the Company's results of operations from the acquisition date. Pro forma results are not provided as Celigo's results of operations were not material. Transaction costs related to this acquisition were $0.1 million for the first quarter ended December 31, 2011, and are included in selling, general and administrative expense. There were no transaction costs subsequent to December 31, 2011.
Acquisition of Nexus
On July 25, 2011, the Company acquired all of the outstanding stock of Nexus Biosystems, Inc. (“Nexus”), a privately held company, for $84.9 million, net of cash acquired. Nexus is a U.S. based provider of automation solutions and consumables to the life sciences market, with a product development, service and support operation located in Switzerland, and service and support locations in Japan and Germany. The acquisition significantly enhances the breadth of the Company’s product offering for its main target market within the life sciences industry, specifically biobanking and compound sample management. Shortly after completing the Nexus acquisition, the Company reorganized the management of Nexus and RTS into one operating segment, Brooks Life Science Systems.
49
The assets and liabilities associated with Nexus were recorded at their fair values as of the acquisition date. The amounts recorded follow (in thousands):
Accounts receivable | $ | 5,708 | |
Inventory | 7,481 | ||
Other current assets | 4,522 | ||
Property, plant and equipment | 12,527 | ||
Completed technology | 6,000 | ||
Customer relationships | 31,000 | ||
Trademarks and trade names | 100 | ||
Goodwill | 33,033 | ||
Accounts payable and accrued expenses | (6,563 | ) | |
Deferred revenue | (3,692 | ) | |
Other current liabilities | (1,534 | ) | |
Deferred tax liabilities | (2,584 | ) | |
Other long-term liabilities | (1,070 | ) | |
Total purchase price, net of cash acquired | $ | 84,928 | |
The estimated fair value attributed to the completed technologies was determined based upon a discounted cash flow forecast utilizing the relief from royalty method. The royalty rate was determined to be 6% based on a review of comparable royalty arrangements. Cash flows were discounted at a rate of 17%. The fair value of the completed technologies will be amortized over a period of 6 years on a straight-line basis, which approximates the pattern in which the economic benefits of the completed technologies are expected to be realized.
The estimated fair value attributed to the customer relationships was determined based upon a discounted forecast of estimated net future cash flows to be generated from the relationships discounted at a rate of 17% - 18%. The fair value of customer relationships for systems will be amortized over a period of 6 years, while the estimated fair value of customer relationships for consumables and service are expected to be amortized over a period of 13 years. The amortization will be amortized on a straight-line basis, which approximates the pattern in which the economic benefits of the customer relationships are expected to be realized.
The fair value of the trade name will be amortized over 2 years on a straight-line basis, which approximates the pattern in which the economic benefits of the trade names will be realized.
Goodwill represents the excess of the purchase price over the fair values of the net tangible and intangible assets acquired. Goodwill arising from the acquisition will not be deductible for tax purposes.
Nexus operating results have been included in the Company’s results of operations from the acquisition date. Nexus revenues and net loss for the period from July 26, 2011 to September 30, 2011 was $4.9 million and $(3.2) million, respectively. The net loss includes charges to expense from the step-up of acquired inventories of $0.7 million and $0.6 million of charges for excess and obsolete inventory based on an assessment of inventory performed in the fourth quarter of fiscal year 2011.
The following unaudited pro forma summary presents consolidated information of the Company as if the acquisition of Nexus occurred on October 1, 2010 (in thousands):
Year Ended September 30, | |||
2011 | |||
Revenue | $ | 720,989 | |
Net income attributable to Brooks Automation, Inc. | 124,114 | ||
The pro forma net income has been adjusted to reflect additional amortization and depreciation expense from the adjustments to intangible assets and property, plant and equipment as if those adjustments had been applied as of October 1, 2010.
Transaction costs related to this acquisition were $719,000 for fiscal year 2011, and are included in selling, general and administrative expense.
50
Acquisition of RTS
On April 1, 2011, the Company acquired all of the outstanding stock of RTS Life Science Limited (“RTS”), a privately held company, for $3.4 million, net of cash acquired. RTS is a provider of automation solutions to the life sciences market, located in Manchester, United Kingdom. The acquisition provides the Company with biobanking and compound sample management, and the ability to leverage the Company’s existing automation technologies with those of RTS.
The assets and liabilities associated with RTS were recorded at their fair values as of the acquisition date. The amounts recorded follow (in thousands):
Accounts receivable | $ | 3,156 | |
Inventory | 1,668 | ||
Other current assets | 1,008 | ||
Property, plant and equipment | 860 | ||
Completed technology | 1,524 | ||
Customer relationships | 577 | ||
Trademarks and trade names | 64 | ||
Goodwill | 3,556 | ||
Accounts payable | (1,397 | ) | |
Deferred revenue | (5,232 | ) | |
Other current liabilities | (2,403 | ) | |
Total purchase price, net of cash acquired | $ | 3,381 | |
The completed technology will be amortized to cost of revenue over its estimated useful life of 5 to 7 years, the customer relationships will be amortized to operating expense over 7 years and the trademarks and trade names will be amortized to operating expense over 3 years. Goodwill arising from the acquisition will not be deductible for tax purposes.
RTS’s operating results have been included in the Company’s results of operations from the acquisition date, and were not material. Pro forma results are not provided as RTS’s results of operations were not material. Transaction costs related to this acquisition were $188,000 for fiscal year 2011, and are included in selling, general and administrative expense.
Divestiture
On April 20, 2011, the Company entered into an agreement with affiliates of Celestica Inc. (the “Buyers”) to sell the assets of its extended factory contract manufacturing business (the “Business”). The Buyers also agreed to assume certain liabilities related to the Business (the “Asset Sale”). The Asset Sale was completed on June 28, 2011 (the “Closing”). At the Closing, the Buyers paid the Company a total purchase price of $78 million in cash, plus $1.3 million as consideration for cash acquired in the Asset Sale. An additional $2.5 million of proceeds was paid during the Company’s fourth quarter of 2011, which represents a working capital normalizing adjustment. The Company paid $2.3 million of transaction expenses. During the three months ended June 30, 2011, the Company recorded a gain on this sale of $45.0 million, before income taxes. Income taxes directly attributable to this gain of $2.4 million were also recorded during the three months ended June 30, 2011.
The Company and the Buyers also entered into certain commercial supply and license agreements at the Closing which will govern the ongoing relationship between the Buyers and the Company. Pursuant to those agreements, the Company will supply the Buyers with certain products and has licensed to the Buyers certain intellectual property needed to run the Business and the Buyers will supply certain products to the Company. Due to the significance of these ongoing commercial arrangements, the sale did not qualify for discontinued operations treatment. Therefore, historical financial results of the divested business will not be segregated in the Company’s consolidated financial statements for the historical periods in which this business was part of the Company.
4. Marketable Securities
The Company invests in marketable securities and classifies them as available-for-sale. The Company records these securities at fair value. Marketable securities reported as current assets represent investments that mature within one year from the balance sheet date. Long-term marketable securities represent investments with maturity dates greater than one year from the balance sheet date. At the time that the maturity dates of these investments become one year or less, the securities are reclassified to current assets. Unrealized gains and losses are excluded from earnings and reported in a separate component of stockholders’ equity until the security is sold or matures. At the time of sale, any gains or losses, calculated by the specific identification method, will be recognized as a component of operating results.
51
The following is a summary of marketable securities (included in short and long-term marketable securities in the Consolidated Balance Sheets), including accrued interest receivable, as of September 30, 2013 and 2012 (in thousands):
Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | ||||||||||||
September 30, 2013: | |||||||||||||||
U.S. Treasury securities and obligations of U.S. government agencies | $ | 19,528 | $ | 6 | $ | (13 | ) | $ | 19,521 | ||||||
Corporate securities | 35,045 | 11 | (47 | ) | 35,009 | ||||||||||
Mortgage-backed securities | 1,093 | 25 | (1 | ) | 1,117 | ||||||||||
Other debt securities | 88 | — | — | 88 | |||||||||||
Municipal securities | 25,199 | 15 | (7 | ) | 25,207 | ||||||||||
Bank certificate of deposits | 9,451 | — | (2 | ) | 9,449 | ||||||||||
$ | 90,404 | $ | 57 | $ | (70 | ) | $ | 90,391 | |||||||
September 30, 2012: | |||||||||||||||
U.S. Treasury securities and obligations of U.S. government agencies | $ | 41,202 | $ | 15 | $ | (2 | ) | $ | 41,215 | ||||||
Corporate securities | 64,102 | 138 | (16 | ) | 64,224 | ||||||||||
Mortgage-backed securities | 1,310 | 42 | (1 | ) | 1,351 | ||||||||||
Other debt securities | 13 | — | — | 13 | |||||||||||
Municipal securities | 34,777 | 25 | (1 | ) | 34,801 | ||||||||||
Bank certificate of deposits | 3,987 | 1 | — | 3,988 | |||||||||||
$ | 145,391 | $ | 221 | $ | (20 | ) | $ | 145,592 | |||||||
Gross realized gains on sales of available-for-sale marketable securities included in “Other income, net” in the Consolidated Statements of Operations was $57,000, $15,000 and $24,000 for the years ended September 30, 2013, 2012 and 2011, respectively. A gross realized loss of $36,000 for the year ended September 30, 2013 was also recorded in "Other income, net." There were no gross realized losses for the years ended September 30, 2012 or 2011.
The fair value of the marketable securities at September 30, 2013 by contractual maturity, are shown below (in thousands). Expected maturities will differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
Fair Value | |||
Due in one year or less | $ | 45,900 | |
Due after one year through five years | 41,194 | ||
Due after ten years | 3,297 | ||
$ | 90,391 | ||
5. Fair Value Measurements
The fair value measurement guidance establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1: Quoted prices in active markets for identical assets or liabilities as of the reporting date. Active markets are those in which transactions for the asset and liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis.
Level 2: Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
52
Assets and liabilities of the Company measured at fair value on a recurring basis as of September 30, 2013 and 2012 are summarized as follows (in thousands):
Fair Value Measurements at Reporting Date Using | ||||||||||||||||
Description | September 30, 2013 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||||||
Assets | ||||||||||||||||
Cash equivalents | $ | 7,754 | $ | 6,152 | $ | 1,602 | $ | — | ||||||||
Available-for-sale securities | 90,391 | 2,199 | 88,192 | — | ||||||||||||
Foreign exchange contracts | 31 | — | 31 | |||||||||||||
Total Assets | $ | 98,176 | $ | 8,351 | $ | 89,825 | $ | — | ||||||||
Liabilities | ||||||||||||||||
Foreign exchange contracts | $ | 5 | $ | — | $ | 5 | $ | — | ||||||||
Fair Value Measurements at Reporting Date Using | ||||||||||||||||
Description | September 30, 2012 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||||||
Assets | ||||||||||||||||
Cash equivalents | $ | 17,508 | $ | 17,508 | $ | — | $ | — | ||||||||
Available-for-sale securities | 145,592 | 60,231 | 85,361 | — | ||||||||||||
Foreign exchange contracts | 10 | — | 10 | — | ||||||||||||
Total Assets | $ | 163,110 | $ | 77,739 | $ | 85,371 | $ | — | ||||||||
Liabilities | ||||||||||||||||
Foreign exchange contracts | $ | 13 | $ | — | $ | 13 | $ | — | ||||||||
Cash Equivalents
Cash equivalents of $6.2 million and $17.5 million at September 30, 2013 and 2012, respectively, consisting primarily of Money Market Funds, are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices in active markets. Cash equivalents of $1.6 million at September 30, 2013 consisting of Bank Certificate of Deposits and Municipal Securities, are classified within Level 2 of the hierarchy because they are not actively traded.
Available-For-Sale Securities
Available-for-sale securities of $2.2 million and $60.2 million at September 30, 2013 and 2012, respectively, consisting of highly rated Corporate Bonds, are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices in active markets of identical assets or liabilities. Available-for-sale securities of $88.2 million and $85.4 million at September 30, 2013 and 2012, respectively, consisting of Mortgage-Backed Securities, Municipal Securities, Bank Certificate of Deposits, Commercial Paper and U.S. Treasury Securities and Obligations of U.S. Government Agencies are classified within Level 2 of the fair value hierarchy because they are not actively traded and are valued using matrix pricing and benchmarking. Matrix pricing is a mathematical technique used to value securities by relying on the securities’ relationship to other benchmark quoted prices.
Foreign Exchange Contracts
Foreign exchange contract assets and liabilities are classified within Level 2 of the fair value hierarchy because there may not be an active market for each contract. However, the inputs used to calculate the value of the contract were obtained from an active market.
53
6. Property, Plant and Equipment
Property, plant and equipment as of September 30, 2013 and 2012 were as follows (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Buildings and land | $ | 38,539 | $ | 55,352 | |||
Computer equipment and software | 72,927 | 70,719 | |||||
Machinery and equipment | 55,375 | 51,813 | |||||
Furniture and fixtures | 10,298 | 10,908 | |||||
Leasehold improvements | 16,522 | 18,938 | |||||
Capital projects in progress | 2,098 | 2,614 | |||||
195,759 | 210,344 | ||||||
Less accumulated depreciation and amortization | (147,889 | ) | (145,866 | ) | |||
Property, plant and equipment, net | $ | 47,870 | $ | 64,478 | |||
Depreciation expense was $14.0 million, $13.4 million and $12.6 million for the years ended September 30, 2013, 2012 and 2011, respectively.
7. Goodwill and Intangible Assets
The Company performed its goodwill impairment test as of September 30, 2013 and 2012, and determined that no adjustment to goodwill was necessary. As of September 30, 2013, the fair value of all reporting units exceeded the respective carrying values by at least 10%.
The fair values of the reporting units in the Brooks Product Solutions and Brooks Global Services segments exceeded their carrying values by amounts ranging from 18% to 114% at September 30, 2013.
The fair value of the Brooks Life Science Systems reporting unit exceeded its carrying value by 10% at September 30, 2013. The observable inputs used in the Company's DCF for analysis for the Brooks Life Science Systems reporting unit include a discount rate 18%. In addition, the Company determined the terminal value using the Gordon growth method and a terminal growth rate of 3%. The Gordon growth method assumes that the reporting unit will grow and generate free cash flow at a constant rate. The Company believes that the Gordon growth method is the most appropriate method for determining the terminal value because the terminal value was calculated at the point in which the Company has assumed that the Brooks Life Science Systems reporting unit has reached a stable growth rate.
In fiscal 2013, the Company experienced a decline in revenue and operating profit for the products in the Brooks Life Science Systems reporting unit. The cash flow assumptions in the Company's DCF Method analysis for the Brooks Life Science Systems reporting unit project growth in the Company's current automated sample management systems and the development of new automated sample management systems that would allow the Company to address a broader automated sample management market than it can address with its current products. While the Company believes its assumptions are reasonable, actual results could differ from projections. To the extent that the operating results of the Brooks Life Science Systems reporting unit do not improve as expected and new life science products being designed to expand the markets the Company serve are not introduced in a timely manner or accepted by the market, the Company may be required to write down all or a portion of the goodwill and other long-lived assets associated with this reporting unit, which would adversely impact its earnings.
54
The components of the Company’s goodwill by business segment at September 30, 2013 and 2012 are as follows (in thousands):
Brooks Product Solutions | Brooks Global Services | Brooks Life Science Systems | Contract Manufacturing | Other | Total | ||||||||||||||||||
Gross goodwill at September 30, 2011 | $ | 485,844 | $ | 151,238 | $ | 36,589 | $ | 18,593 | $ | 7,421 | $ | 699,685 | |||||||||||
Acquisitions and adjustments during fiscal 2012 | — | — | 3,713 | — | — | 3,713 | |||||||||||||||||
Gross goodwill at September 30, 2012 | 485,844 | 151,238 | 40,302 | 18,593 | 7,421 | 703,398 | |||||||||||||||||
Acquisitions and adjustments during fiscal 2013 | 20,899 | 5,554 | 7,137 | — | — | 33,590 | |||||||||||||||||
Gross goodwill at September 30, 2013 | $ | 506,743 | $ | 156,792 | $ | 47,439 | $ | 18,593 | $ | 7,421 | $ | 736,988 | |||||||||||
Accumulated goodwill impairments at September 30, 2011 | $ | (437,706 | ) | $ | (151,238 | ) | $ | — | $ | (18,593 | ) | $ | (7,421 | ) | $ | (614,958 | ) | ||||||
Impairments recorded during fiscal 2012 | — | — | — | — | — | — | |||||||||||||||||
Accumulated goodwill impairments at September 30, 2012 | (437,706 | ) | (151,238 | ) | — | (18,593 | ) | (7,421 | ) | (614,958 | ) | ||||||||||||
Impairments recorded during fiscal 2013 | — | — | — | — | — | — | |||||||||||||||||
Accumulated goodwill impairments at September 30, 2013 | $ | (437,706 | ) | $ | (151,238 | ) | $ | — | $ | (18,593 | ) | $ | (7,421 | ) | $ | (614,958 | ) | ||||||
Goodwill, less accumulated impairments at September 30, 2012 | $ | 48,138 | $ | — | $ | 40,302 | $ | — | $ | — | $ | 88,440 | |||||||||||
Goodwill, less accumulated impairments at September 30, 2013 | $ | 69,037 | $ | 5,554 | $ | 47,439 | $ | — | $ | — | $ | 122,030 | |||||||||||
The Company is required to test certain long-lived assets when indicators of impairment are present. The Company determined that impairment indicators were present for the long-lived assets related to the Celigo product line as of September 30, 2013. The long-lived assets in question were tested for recoverability by comparing the sum of the undiscounted cash flows directly attributable to the assets to their carrying values, which resulted in the conclusion that the carrying amounts of the assets were not recoverable. The fair values of the assets were then evaluated to determine the amount of the impairment, if any. The fair value of the assets was based primarily on market-based valuation techniques. As a result of this analysis, management determined that an impairment loss of $2.0 million had occurred as of September 30, 2013, and allocated the loss amount to the long-lived assets in the impaired asset group based on the carrying value of each asset, with no asset reduced below its respective fair value. The impairment loss was recorded in the Brooks Life Science Systems segment. The impairment charge was allocated as follows (in thousands):
Year Ended September 30, 2013 | |||
Reported as cost of revenue: | |||
Completed technology intangible asset impairment | $ | 1,910 | |
Reported as selling, general and administrative expense: | |||
Trademarks and trade name intangible asset impairment | 50 | ||
Total impairment charges | $ | 1,960 | |
55
Components of the Company’s identifiable intangible assets are as follows (in thousands):
September 30, 2013 | September 30, 2012 | ||||||||||||||||||||||
Cost | Accumulated Amortization | Net Book Value | Cost | Accumulated Amortization | Net Book Value | ||||||||||||||||||
Patents | $ | 7,808 | $ | 7,196 | $ | 612 | $ | 7,808 | $ | 7,093 | $ | 715 | |||||||||||
Completed technology | 65,594 | 48,898 | 16,696 | 54,583 | 42,751 | 11,832 | |||||||||||||||||
Trademarks and trade names | 4,013 | 4,003 | 10 | 4,014 | 3,880 | 134 | |||||||||||||||||
Customer relationships | 70,490 | 27,720 | 42,770 | 48,654 | 21,935 | 26,719 | |||||||||||||||||
$ | 147,905 | $ | 87,817 | $ | 60,088 | $ | 115,059 | $ | 75,659 | $ | 39,400 | ||||||||||||
In connection with the acquisition of Crossing during fiscal year 2013, the Company allocated a portion of the purchase price to the following intangible assets: Completed Technology - $10.5 million and Customer Relationships - $20.0 million. These intangible assets support the products and services provided by the Brooks Products Solutions segment of $24.6 million and the Brooks Global Services segment of $5.9 million. In connection with the acquisition of Matrical during fiscal year 2013, the Company allocated a portion of the purchase price to the following intangible assets: Completed Technology - $0.5 million and Customer Relationships - $1.5 million. These intangible assets support the products and services provided by the Brooks Life Science Systems segment.
In connection with the acquisition of Celigo during fiscal year 2012, the Company allocated a portion of the purchase price to the following intangible assets: Completed Technology - $3.5 million and Trademarks and Trade Names - $0.1 million. These intangible assets support the products and services provided by the Brooks Life Science Systems segment.
For details regarding these intangible assets see "Note 3. Acquisitions."
Amortization expense for intangible assets was $10.1 million, $8.2 million and $4.6 million for the years ended September 30, 2013, 2012 and 2011, respectively.
Estimated future amortization expense for the intangible assets recorded by the Company as of September 30, 2013 is as follows (in millions):
Year ended September 30, | |
2014 | $10.1 |
2015 | 9.4 |
2016 | 8.7 |
2017 | 8.0 |
2018 | 6.1 |
Thereafter | 17.8 |
$60.1 | |
8. Investment in Affiliates
Joint Ventures
The Company participates in a 50% joint venture, ULVAC Cryogenics, Inc. (“UCI”) with ULVAC Corporation of Chigasaki, Japan. UCI manufactures and sells cryogenic vacuum pumps, principally to ULVAC Corporation. At September 30, 2013 and 2012, the carrying value of the Company's investment in UCI was $22.7 million and $27.4 million, respectively. For the years ended September 30, 2013, 2012 and 2011, the Company recorded income associated with UCI of $2.6 million, $2.0 million and $4.3 million, respectively. For the years ended September 30, 2013, 2012 and 2011, management fee payments received by the Company from UCI were $0.6 million, $1.0 million and $1.1 million, respectively. For the years ended September 30, 2013, 2012 and 2011, the Company incurred charges from UCI for products or services of $0.5 million, $0.8 million and $0.4 million, respectively. At September 30, 2013 and 2012 the Company owed UCI $26,000 and $73,000, respectively, in connection with accounts payable for unpaid products and services. During the fiscal years ended September 30, 2013 and 2012, the Company received $5.0 million and $5.1 million, respectively, of cash dividends from UCI.
The Company participates in a 50% joint venture with Yaskawa Electric Corporation (“Yaskawa”) called Yaskawa Brooks Automation, Inc. (“YBA”) to exclusively market and sell Yaskawa's semiconductor robotics products and Brooks' automation hardware products to semiconductor customers in Japan. At September 30, 2013 and 2012, the carrying value of the Company's investment in YBA was $3.0 million and $4.0 million, respectively. For the years ended September 30, 2013, 2012 and 2011, the Company recorded income (expense) associated with YBA of $(0.2) million, $0.1 million and $0.5 million,
56
respectively. For the years ended September 30, 2013, 2012 and 2011, the Company earned revenue for sales to YBA of $6.3 million, $8.0 million and $9.6 million, respectively. The amount due from YBA included in accounts receivable at September 30, 2013 and 2012 was $2.3 million and $2.0 million, respectively. For the years ended September 30, 2013, 2012 and 2011, the Company incurred charges from YBA for products and services of $0.5 million, $0.5 million and $0.3 million, respectively. At September 30, 2013 and 2012 the Company owed YBA $47,000 and $58,000, respectively, in connection with accounts payable for unpaid products and services.
These investments are accounted for using the equity method. Under this method of accounting, the Company records in income its proportionate share of the earnings (losses) of the joint ventures with a corresponding increase (decrease) in the carrying value of the investment.
9. Earnings per Share
Below is a reconciliation of weighted average common shares outstanding for purposes of calculating basic and diluted earnings per share (in thousands, except per share data):
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Net (loss) income attributable to Brooks Automation, Inc. | $ | (2,215 | ) | $ | 136,789 | $ | 130,385 | ||||
Weighted average common shares outstanding used in computing basic earnings per share | 65,912 | 65,128 | 64,549 | ||||||||
Dilutive common stock options and restricted stock awards | — | 594 | 454 | ||||||||
Weighted average common shares outstanding used in computing diluted earnings per share | 65,912 | 65,722 | 65,003 | ||||||||
Basic net (loss) income per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.10 | $ | 2.02 | ||||
Diluted net (loss) income per share attributable to Brooks Automation, Inc. common stockholders | $ | (0.03 | ) | $ | 2.08 | $ | 2.01 | ||||
Options to purchase approximately 43,000, 238,000 and 387,000 shares of common stock and 3,006,000, 2,000 and 413,000 shares of restricted stock were excluded from the computation of diluted earnings per share attributable to Brooks Automation, Inc. common stockholders for the years ended September 30, 2013, 2012 and 2011, respectively, as their effect would be anti-dilutive. All outstanding stock options and unvested shares of restricted stock were excluded from the computation of diluted earnings per share for the year ended September 30, 2013 as a result of the net loss for that period.
10. Investment in Variable Interest Entity
In 2012, the Company provided a strategic partner (the “Borrower”) in the life science industry a loan of $3.0 million to support their future product development and other working capital requirements. The loan bears interest at a rate of 9%. The outstanding principal and interest under the loan is payable in May 2015. The Company also received warrants to purchase the Borrower's common stock in the event of an equity offering by the Borrower. The Company determined that the loan and the warrants had fair values of $2.8 million and $0.2 million, respectively, as of September 30, 2012. At September 30, 2013, the loan and warrants had carrying values of $2.9 million and $0.2 million, respectively. The loan and the warrants are recorded as long-term other assets in the Consolidated Balance Sheets. The loan agreement also provides the Company with certain other rights related to conversion of the loan, first refusal to acquire the Borrower and a redemption premium. The loan is secured by a security agreement that gives the Company the first interest in all of the assets of the Borrower.
The Company determined that the level of equity investment at risk is not sufficient for the entity to finance its activities without additional financial support and as a result, represents a variable interest entity. However, the Company does not have the power to direct the activities that most significantly impact the Borrower's economic performance and would not absorb the majority of the expected losses from the Borrower, and therefore does not qualify as the primary beneficiary. The Company has no future contractual funding commitments to the Borrower and as a result, the Company's exposure to loss is limited to the outstanding principal and interest under the loan.
57
11. Derivative Instruments
The Company has transactions and balances denominated in currencies other than the U.S. dollar. Most of these transactions or balances are denominated in Euros, British Pounds and a variety of Asian currencies. These transactions and balances, including short-term advances between the Company and its subsidiaries, subject the Company's operations to exposure from exchange rate fluctuations. The impact of currency exchange rate movement can be positive or negative in any period. The Company mitigates the impact of potential currency translation gains and losses on short-term intercompany advances through timely settlement of each transaction, generally within 30 days. The Company also enters into foreign exchange contracts to reduce its exposure to currency translation. Under forward contract arrangements, the Company typically agrees to purchase a fixed amount of U.S. Dollars in exchange for a fixed amount of a foreign currency on specified dates with maturities of three months or less. These transactions do not qualify for hedge accounting.
The Company had the following notional amounts outstanding under foreign currency contracts that do not qualify for hedge accounting at September 30, 2013 and 2012 (in thousands):
September 30, 2013:
Buy Currency | Notional Amount of Buy Currency in U.S. Dollars | Sell Currency | Maturity | Notional Amount of Sell Currency in U.S. Dollars | Fair Value of Assets | Fair Value of Liabilities | ||||||||||||||
U.S. Dollar | $ | 2,770 | Japanese Yen | October 2013 to December 2013 | $ | 2,762 | $ | 8 | $ | — | ||||||||||
Korean Won | 686 | U.S. Dollar | October 2013 | 688 | — | 2 | ||||||||||||||
U.S. Dollar | 301 | Israeli Shekel | October 2013 | 304 | — | 3 | ||||||||||||||
U.S. Dollar | 231 | Singapore Dollar | October 2013 | 231 | — | — | ||||||||||||||
$ | 3,988 | $ | 3,985 | $ | 8 | $ | 5 | |||||||||||||
September 30, 2012:
Buy Currency | Notional Amount of Buy Currency in U.S. Dollars | Sell Currency | Maturity | Notional Amount of Sell Currency in U.S. Dollars | Fair Value of Assets | Fair Value of Liabilities | ||||||||||||||
U.S. Dollar | $ | 2,791 | Taiwan Dollar | October 2012 | $ | 2,786 | $ | 5 | $ | — | ||||||||||
U.S. Dollar | 1,842 | Israeli Shekel | October 2012 | 1,854 | — | 12 | ||||||||||||||
U.S. Dollar | 1,058 | Singapore Dollar | October 2012 | 1,056 | 2 | — | ||||||||||||||
U.S. Dollar | 806 | Korean Won | October 2012 | 803 | 3 | — | ||||||||||||||
U.S. Dollar | 611 | Japanese Yen | October 2012 | 611 | — | — | ||||||||||||||
U.S. Dollar | 524 | British Pound | October 2012 | 525 | — | 1 | ||||||||||||||
$ | 7,632 | $ | 7,635 | $ | 10 | $ | 13 | |||||||||||||
The Company recorded net gains (losses) of $0.1 million and $(0.2) million related to these contracts during the years ended September 30, 2013 and 2012, respectively. These amounts are recorded in the Company's Consolidated Statements of Operations as a component of other income, net.
The Company also periodically enters into contracts to provide products and services that are denominated in non-functional currencies. In June 2013, the Company entered into foreign exchange contracts to reduce its exposure to changes in foreign exchange rates associated with an order for multiple automated sample management systems. The Company concluded that these foreign currency contracts meet the criteria to qualify as a cash flow hedge. Accordingly, the Company reflected changes in the fair value of the effective portion of these foreign currency contracts in accumulated other comprehensive income. Amounts recorded in accumulated other comprehensive income will be reclassified to revenue in the consolidated statement of income when the forecasted transaction occurs.
The Company had the following notional amounts outstanding under foreign currency contracts that qualify for cash flow hedge accounting at September 30, 2013 (in thousands):
Buy Currency | Notional Amount of Buy Currency in U.S. Dollars | Sell Currency | Maturity | Notional Amount of Sell Currency in U.S. Dollars | Fair Value of Assets | Fair Value of Liabilities | ||||||||||||||
U.S. Dollar | $ | 2,037 | Japanese Yen | April 2014 | $ | 2,014 | $ | 23 | $ | — | ||||||||||
58
The Company expects to classify the accumulated gain into revenue in April 2014. The Company did not recognize any amounts related to ineffectiveness in the results of operations for the year ended September 30, 2013.
There were no cash flow hedges outstanding at September 30, 2012.
The fair values of the forward contracts described above are recorded in the Company's Consolidated Balance Sheets as prepaid expenses and other current assets and accrued expenses and other current liabilities.
12. Income Taxes
The components of the income tax provision (benefit) are as follows (in thousands):
Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Current income tax provision (benefit): | |||||||||||
Federal | $ | 15 | $ | 15 | $ | 16 | |||||
State | 70 | 213 | 1,356 | ||||||||
Foreign | 681 | (1,374 | ) | 858 | |||||||
Total current income tax provision (benefit) | 766 | (1,146 | ) | 2,230 | |||||||
Deferred income tax (benefit): | |||||||||||
Federal | (2,523 | ) | (118,432 | ) | — | ||||||
State | (90 | ) | (291 | ) | — | ||||||
Foreign | (323 | ) | (3,413 | ) | (276 | ) | |||||
Total deferred income tax (benefit) | (2,936 | ) | (122,136 | ) | (276 | ) | |||||
Income tax provision (benefit) | $ | (2,170 | ) | $ | (123,282 | ) | $ | 1,954 | |||
The components of income (loss) before income taxes and equity in earnings of joint ventures are as follows (in thousands):
Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Domestic | $ | (6,968 | ) | $ | 2,204 | $ | 111,053 | ||||
Foreign | 206 | 9,216 | 16,523 | ||||||||
$ | (6,762 | ) | $ | 11,420 | $ | 127,576 | |||||
The differences between the income tax provision (benefit) and income taxes computed using the applicable U.S. statutory federal tax rate is as follows (in thousands):
Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Income tax provision computed at federal statutory rate | $ | (1,535 | ) | $ | 4,728 | $ | 45,736 | ||||
State income taxes, net of federal benefit | (8 | ) | 260 | 1,848 | |||||||
Foreign income taxed at different rates | 493 | (832 | ) | (1,546 | ) | ||||||
Dividends | 115 | 956 | (219 | ) | |||||||
Change in deferred tax asset valuation allowance | 523 | (125,479 | ) | (42,608 | ) | ||||||
Reduction in uncertain tax positions | (1,022 | ) | (3,732 | ) | (3,719 | ) | |||||
Nondeductible compensation | 474 | 1,339 | 295 | ||||||||
Tax credits generated | (2,002 | ) | (1,195 | ) | (1,179 | ) | |||||
Travel and entertainment | 124 | 139 | 119 | ||||||||
Merger costs | 251 | — | 315 | ||||||||
Other | 417 | 534 | 2,912 | ||||||||
Income tax provision (benefit) | $ | (2,170 | ) | $ | (123,282 | ) | $ | 1,954 | |||
59
The Company has not provided for U.S. income taxes on the unremitted earnings of certain foreign subsidiaries as these earnings are considered to be indefinitely reinvested. These earnings amounted to approximately $17.5 million at September 30, 2013. It is not practicable to compute the estimated deferred tax liability on these earnings.
The significant components of the net deferred tax assets and liabilities are as follows (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Accruals and reserves not currently deductible | $ | 11,050 | $ | 10,489 | |||
Federal, state and foreign tax credits | 20,084 | 18,963 | |||||
Other assets | 1,859 | 1,655 | |||||
Net operating loss carryforwards | 101,717 | 101,713 | |||||
Inventory reserves and valuation | 9,052 | 8,790 | |||||
Deferred tax assets | 143,762 | 141,610 | |||||
Depreciation and intangible amortization | 12,208 | 5,418 | |||||
Deferred tax liabilities | 12,208 | 5,418 | |||||
Valuation allowance | (16,509 | ) | (16,035 | ) | |||
Net deferred tax asset | $ | 115,045 | $ | 120,157 | |||
Management has considered the weight of all available evidence in determining whether a valuation allowance is required against its deferred tax assets at September 30, 2013. After consideration of both positive and negative evidence management has concluded that it is more likely than not that a substantial portion of its deferred tax assets will be realized. The positive evidence considered was three year U.S. historical cumulative profitability, projected future taxable income and length of carry-forward periods of net operating losses and tax credits. The primary negative evidence considered is the volatile semiconductor industry in which the Company operates.
The Company recorded a tax benefit of $121.8 million resulting from the reduction in the valuation allowance during the fiscal year ended September 30, 2012. The Company has continued to maintain a valuation allowance in the U.S. against certain tax credits and state net operating losses due to the uncertainty of their realization based on long-term Company forecasts and the expiration dates on these attributes. The Company has also continued to maintain a valuation allowance in certain jurisdictions that have not generated historical cumulative profitability.
If future operating results of the U.S. or these foreign jurisdictions deviate from expectations, it is reasonably possible that there could be a further change in the valuation allowance in the future. A change in the valuation allowance, in whole or in part, would result in a non-cash income tax expense or benefit during the period of change.
As of September 30, 2013, the Company had federal, state and foreign net operating loss carryforwards of approximately $242.0 million, $122.2 million and $37.3 million, respectively, and federal and state research and development tax credit carryforwards of approximately $24.0 million available to reduce future tax liabilities, which expire at various dates through 2033. The net operating loss carryforward includes excess deductions related to stock compensation in the amount of $11.7 million which have not been recognized for financial statement purposes. The benefits of these tax deductions will be credited to additional paid-in capital upon being realized.
The Company has performed studies to determine if there are any annual limitations on the federal net operating losses under section 382 of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”). As a result of these studies, the Company has determined that ownership changes have occurred primarily in connection with acquisitions when the Company has issued stock to the sellers, as well as ownership changes in the subsidiaries acquired by the Company. Certain limitations have been calculated and the benefits of the net operating losses that will expire before utilization have not been recorded as deferred tax assets in the financial statements. The Company's U.S. net operating losses expire at various dates through 2030.
60
A reconciliation of the beginning and ending amount of the consolidated liability for unrecognized income tax benefits during the fiscal years ended September 30, 2013, 2012 and 2011 is as follows (in thousands):
Unrecognized Tax Benefit | Interest and Penalties | Total | |||||||||
Balance at October 1, 2010 | $ | 12,816 | $ | 2,152 | $ | 14,968 | |||||
Additions for tax positions of prior years | 184 | 447 | 631 | ||||||||
Additions for tax positions related to current year | 242 | — | 242 | ||||||||
Reductions from lapses in statutes of limitations | (961 | ) | — | (961 | ) | ||||||
Reductions from settlements with taxing authorities | (3,392 | ) | (610 | ) | (4,002 | ) | |||||
Foreign exchange rate adjustment | 122 | — | 122 | ||||||||
Balance at September 30, 2011 | 9,011 | 1,989 | 11,000 | ||||||||
Additions for tax positions of prior years | 242 | 247 | 489 | ||||||||
Reductions from lapses in statutes of limitations | (3,125 | ) | (607 | ) | (3,732 | ) | |||||
Foreign exchange rate adjustment | (167 | ) | 15 | (152 | ) | ||||||
Balance at September 30, 2012 | 5,961 | 1,644 | 7,605 | ||||||||
Additions for tax positions of prior years | — | 228 | 228 | ||||||||
Additions for tax positions related to acquired entities | 116 | — | 116 | ||||||||
Reductions from lapses in statutes of limitations | (944 | ) | (78 | ) | (1,022 | ) | |||||
Foreign exchange rate adjustment | 14 | — | 14 | ||||||||
Balance at September 30, 2013 | $ | 5,147 | $ | 1,794 | $ | 6,941 | |||||
As of September 30, 2013 all of the Company's unrecognized tax benefits, if recognized, would affect the effective tax rate. The Company recognizes interest related to unrecognized benefits as a component of tax expense, of which $0.2 million, $0.2 million and $0.4 million was recognized for the years ended September 30, 2013, 2012 and 2011, respectively.
The Company is subject to U.S. federal income tax and various state, local and international income taxes in various jurisdictions. The amount of income taxes paid is subject to the Company's interpretation of applicable tax laws in the jurisdictions in which it files. In the normal course of business, the Company is subject to examination by taxing authorities throughout the world. The statute of limitations lapsed on several uncertain tax positions in the foreign jurisdictions related to transfer pricing and income tax withholding during fiscal year 2013 that resulted in a $1.0 million reduction in gross unrecognized tax benefits that impacted the effective tax rate. The Company is subject to income tax audits in various global jurisdictions in which it operates. In the Company's U.S. and international jurisdictions, the years that may be examined vary, with the earliest tax year being 2007. Based on the outcome of these examinations, or the expiration of statutes of limitations for specific jurisdictions, it is reasonably possible that the related unrecognized tax benefits could change from those recorded in the Company's statement of financial position. The Company currently anticipates that it is reasonably possible that the unrecognized tax benefit will be reduced by approximately $1.3 million during the next twelve months primarily as the result of statutes of limitations expiring.
13. Postretirement Benefits
Defined Benefit Pension Plans
The Company has two active defined benefit pension plans (collectively, the “Plans”). The Plans cover substantially all of the Company’s employees in Switzerland and Taiwan. Retirement benefits are generally earned based on years of service and compensation during active employment; however, the level of benefits varies within the Plans. Eligibility is determined in accordance with local statutory requirements.
In connection with actions taken under the Company’s restructuring programs, the number of employees accumulating benefits under the Switzerland Plan has declined significantly. As a result, a partial settlement event occurred and resulted in accelerated amortization of approximately $0.1 million of prior pension losses. The settlement loss, recorded in the quarter ended December 31, 2012, is included in restructuring and other charges in the Consolidated Statements of Operations.
In addition, the Company had a plan that was acquired through its purchase of Helix Technology Corporation that covered certain employees in the United States. The Company settled its pension obligation with the participants in the quarter ended September 30, 2012 which resulted in accelerated cash payments of approximately $6.4 million and $8.9 million of
61
accelerated amortization of prior pension losses which is included as a pension settlement charge in the Consolidated Statements of Operations.
The Company uses a September 30th measurement date in the determination of net periodic benefit costs, benefit obligations and the value of plan assets for all plans. The following tables set forth the funded status and amounts recognized in the Company’s Consolidated Balance Sheets at September 30, 2013 and 2012 (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Benefit obligation at beginning of year | $ | 10,181 | $ | 28,068 | |||
Service cost | 604 | 787 | |||||
Interest cost | 148 | 984 | |||||
Actuarial (gain) loss | (670 | ) | 1,235 | ||||
Benefits paid | (1,421 | ) | (2,203 | ) | |||
Settlement loss | — | 1,040 | |||||
Settlements paid | (1,383 | ) | (18,928 | ) | |||
Curtailment gain | (500 | ) | — | ||||
Foreign currency translation | 148 | (802 | ) | ||||
Benefit obligation at end of year | $ | 7,107 | $ | 10,181 | |||
September 30, | |||||||
2013 | 2012 | ||||||
Fair value of assets at beginning of year | $ | 8,015 | $ | 20,173 | |||
Actual return on plan assets | 304 | 1,446 | |||||
Disbursements | (1,573 | ) | (2,463 | ) | |||
Employer contributions | 292 | 7,684 | |||||
Employee contributions | 194 | 334 | |||||
Settlements paid | (1,383 | ) | (18,928 | ) | |||
Foreign currency translation | 147 | (231 | ) | ||||
Fair value of assets at end of year | $ | 5,996 | $ | 8,015 | |||
September 30, | |||||||
2013 | 2012 | ||||||
Funded status/accrued benefit liability | $ | (1,111 | ) | $ | (2,166 | ) | |
The following table provides pension amounts recorded within the account line items of the Company’s consolidated balance sheets (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Accrued compensation and benefits | $ | 296 | $ | 478 | |||
Long-term pension liability | 815 | 1,688 | |||||
In addition, accumulated other comprehensive income at September 30, 2013 and 2012 includes unrecognized net actuarial gains (losses) of $0.5 million and $(1.1) million, respectively.
62
The components of the Company’s net pension cost for the years ended September 30, 2013, 2012 and 2011 is as follows (in thousands):
Year ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Service cost | $ | 604 | $ | 787 | $ | 216 | |||||
Interest cost | 148 | 984 | 796 | ||||||||
Expected return on assets | (247 | ) | (1,072 | ) | (764 | ) | |||||
Amortization of losses | 4 | 620 | 458 | ||||||||
Other | 160 | — | — | ||||||||
Net periodic pension cost | 669 | 1,319 | 706 | ||||||||
Settlement loss | 87 | 8,937 | — | ||||||||
Total pension cost | $ | 756 | $ | 10,256 | $ | 706 | |||||
Other changes in Plan assets and benefit obligations recognized in other comprehensive loss (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Net (gain) loss | $ | (791 | ) | $ | 1,226 | ||
Amortization of net loss | (3 | ) | (620 | ) | |||
Curtailment loss | (675 | ) | — | ||||
Settlement loss | (87 | ) | (8,937 | ) | |||
Total recognized in other comprehensive income (loss) | (1,556 | ) | (8,331 | ) | |||
Total recognized in net periodic pension cost and other comprehensive income (loss) | $ | (887 | ) | $ | (7,012 | ) | |
Certain information for the Plans with respect to accumulated benefit obligations follows (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Projected benefit obligation | $ | 7,107 | $ | 10,181 | |||
Accumulated benefit obligation | 6,272 | 8,906 | |||||
Fair value of plan assets | 5,996 | 8,015 | |||||
Weighted-average assumptions used to determine net cost at September 30, 2013, 2012 and 2011 follows:
Year Ended September 30, | ||||||||
2013 | 2012 | 2011 | ||||||
Discount rate | 2.15 | % | 3.51 | % | 3.99 | % | ||
Expected return on plan assets | 2.17 | % | 2.18 | % | 4.68 | % | ||
Rate of compensation increase | 1.89 | % | 1.84 | % | 1.79 | % | ||
The expected return on plan assets is based on an evaluation of fixed income yield curves and equity return assumption studies applied to the asset allocation.
Weighted-average assumptions used to determine the pension obligation at September 30, 2013, 2012 and 2011 follows:
Year Ended September 30, | ||||||||
2013 | 2012 | 2011 | ||||||
Discount rate | 2.15 | % | 1.89 | % | 3.76 | % | ||
Rate of compensation increase | 1.89 | % | 1.84 | % | 1.79 | % | ||
Compensation increase assumptions for the periodic pension cost and pension obligation apply to the Switzerland Plan and Taiwan Plan only.
The Company bases its determination of pension expense or benefit on a market-related valuation of assets, which reduces year-to-year volatility. This market-related valuation recognizes investment gains or losses over a five-year period from
63
the year in which they occur. Investment gains or losses for this purpose are the difference between the expected return calculated using the market-related value of assets and the actual return on assets. Since the market-related value of assets recognizes gains or losses over a five-year period, the future value of assets will be impacted as previously deferred gains or losses are recognized. As of September 30, 2013, under the Plans, the Company had cumulative investment gains of approximately $0.1 million, which remain to be recognized in the calculation of the market-related value of assets. The Company also had cumulative other actuarial gains of $0.4 million at September 30, 2013, which are amortized into net periodic benefit costs over the average remaining service period of active participants in the plans.
Plan Assets
The fair value of plan assets for the Switzerland Plan and Taiwan Plan were $5.5 million and $0.5 million, respectively, at September 30, 2013. As is customary with Swiss pension plans, the assets of the Switzerland Plan are invested in a collective fund with multiple employers through a Swiss insurance company. The Company does not have any rights to the assets of the plan. Investment holdings are primarily in highly rated debt securities. The assets of the Taiwan Plan are invested with a trustee that has been selected by the Taiwan government. The Company has no investment authority over the assets of either the Switzerland Plan or the Taiwan Plan. The asset allocation of the plan assets at September 30, 2013 was as follows:
Percentage of Plan Assets at September 30, 2013 | ||
Debt securities | 75 | % |
Equity securities | 5 | |
Cash | 2 | |
Other | 18 | |
100 | % | |
The fair value of pension assets by asset category and by level at September 30, 2013 were as follows (in thousands):
As of September 30, 2013 | |||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Swiss Life collective foundation | $ | — | $ | 5,474 | $ | — | $ | 5,474 | |||||||
Taiwan collective trust | — | 522 | — | 522 | |||||||||||
Total | $ | — | $ | 5,996 | $ | — | $ | 5,996 | |||||||
See "Note 5. Fair Value Measurements" for a description of the levels of inputs used to determine fair value measurements.
During the first quarter of fiscal year 2013, the Company made contributions of $0.1 million to the Switzerland Plan in connection with the settlement, which was in addition to the $0.2 million of required minimum contributions made to both Plans throughout fiscal year 2013. The Company made required minimum contributions throughout fiscal year 2013 to all of its plans of $0.3 million. The Company expects to contribute $0.2 million to its plans in fiscal 2014 to meet minimum funding targets.
Expected benefit payments over the next ten years are anticipated to be paid as follows (in thousands):
2014 | $ | 232 | |
2015 | 56 | ||
2016 | 57 | ||
2017 | 58 | ||
2018 | 59 | ||
2019-2023 | 583 | ||
Defined Contribution Plans
The Company sponsors defined contribution plans that meet the requirements of Section 401(k) of the Internal Revenue Code. All United States employees of the Company who meet minimum age and service requirements are eligible to participate in the plan. The plan allows employees to invest, on a pre-tax basis, a percentage of their annual salary subject to statutory limitations.
The Company’s contribution expense for worldwide defined contribution plans was $3.2 million, $3.1 million and $2.9 million for the years ended September 30, 2013, 2012 and 2011, respectively.
64
14. Stockholders’ Equity
Preferred Stock
At September 30, 2013 and 2012 there were one million shares of preferred stock, $0.01 par value per share authorized; no shares were issued or outstanding at September 30, 2013 or 2012. Preferred stock may be issued at the discretion of the Board of Directors without stockholder approval with such designations, rights and preferences as the Board of Directors may determine.
Accumulated Other Comprehensive Income
The following is a summary of the components of accumulated other comprehensive income, net of tax, at September 30 (in thousands):
Currency Translation Adjustments | Unrealized Gains (Losses) on Available-for-Sale Securities | Unrealized Gains on Cash Flow Hedges | Pension Liability Adjustments | Total | ||||||||||||||||
Balance at September 30, 2010 | $ | 25,970 | $ | 253 | $ | — | $ | (8,357 | ) | $ | 17,866 | |||||||||
Other comprehensive income before reclassifications | 947 | (421 | ) | — | (1,044 | ) | (518 | ) | ||||||||||||
Amounts reclassified from accumulated other comprehensive income | — | (24 | ) | — | — | (24 | ) | |||||||||||||
Balance at September 30, 2011 | 26,917 | (192 | ) | — | (9,401 | ) | 17,324 | |||||||||||||
Other comprehensive income before reclassifications | (2,406 | ) | 408 | — | (606 | ) | (2,604 | ) | ||||||||||||
Amounts reclassified from accumulated other comprehensive income | — | (15 | ) | — | 8,937 | 8,922 | ||||||||||||||
Balance at September 30, 2012 | 24,511 | 201 | — | (1,070 | ) | 23,642 | ||||||||||||||
Other comprehensive income before reclassifications | (2,113 | ) | (114 | ) | 14 | 1,109 | (1,104 | ) | ||||||||||||
Amounts reclassified from accumulated other comprehensive income | — | (21 | ) | — | 87 | 66 | ||||||||||||||
Balance at September 30, 2013 | $ | 22,398 | $ | 66 | $ | 14 | $ | 126 | $ | 22,604 | ||||||||||
Reclassifications from AOCI to net income related to available-for-sale securities result from sale of these securities as described in “Note 4. Marketable Securities.” Reclassifications from AOCI to net income related to the Company’s pension plans relate to settlement losses under defined benefit pension plans as described in “Note 13. Postretirement Benefits.”
15. Stock Plans
Amended and Restated 2000 Equity Incentive Plan
The purposes of the Amended and Restated 2000 Equity Incentive Plan (the “2000 Plan”), are to attract and retain employees and to provide an incentive for them to assist the Company to achieve long-range performance goals and to enable them to participate in the long-term growth of the Company. Under the 2000 Plan the Company may grant (i) incentive stock options intended to qualify under Section 422 of the Internal Revenue Code, and (ii) options that are not qualified as incentive stock options (“nonqualified stock options”) and (iii) stock appreciation rights, performance awards and restricted stock. All employees of the Company or any affiliate of the Company, independent directors, consultants and advisors are eligible to participate in the 2000 Plan. Options under the 2000 Plan generally vest over four years and expire seven years from the date of grant. A total of 9,000,000 shares of common stock was reserved for issuance under the 2000 Plan. As of September 30, 2013, no options are outstanding and 2,792,393 shares remain available for grant.
During the year ended September 30, 2013, the Company issued 392,931 shares of restricted stock or units under the Amended and Restated 2000 Equity Incentive Plan, net of cancellations. These restricted stock awards generally have the following vesting schedules: immediate; three year vesting in which one-third vest at the end of Year 1, one-third vest at the end of Year 2 and one-third vest at the end of Year 3; and three year vesting in which one-half vest at the end of Year 2 and one-
65
half vest at the end of Year 3. Compensation expense related to these awards is being recognized on a straight line basis over the vesting period, based on the fair market value of the Company’s common stock on the date of grant. In addition, in fiscal 2013, the Company granted 562,125 restricted stock awards net of cancellations to senior management, the number of shares ultimately issued was measured at the end of fiscal year 2013 and was dependent upon the achievement of certain financial performance goals. These awards are expensed over the related service period when attainment of the performance condition is considered probable. The total amount of compensation recorded will depend on the Company’s achievement of performance targets. Changes to the projected attainment of performance targets during the vesting period may result in an adjustment to the amount of cumulative compensation recorded as of the date the estimate is revised.
Stock Options of Acquired Companies
In connection with the acquisition of Helix on October 26, 2005, the Company assumed the outstanding options of multiple stock option plans that were adopted by Helix. At acquisition, 689,622 options to purchase Helix common stock were outstanding and converted into 765,480 options to purchase the Company’s common stock. A total of 15,540 options are outstanding and 467,165 shares remain available for grant under the Helix plans as of September 30, 2013. The Company does not intend to issue any additional options under the Helix stock option plan.
Stock Option Activity
The following table summarizes stock option activity for all the above plans for the year ended September 30, 2013:
2013 | ||||||||||||
Shares | Weighted- Average Remaining Contractual Term | Weighted Average Price | Aggregate Intrinsic Value (In Thousands) | |||||||||
Options outstanding at September 30, 2012 | 193,182 | $ | 13.11 | |||||||||
Exercised | (8,600 | ) | $ | 7.75 | ||||||||
Forfeited/expired | (169,042 | ) | $ | 13.13 | ||||||||
Options outstanding at September 30, 2013 | 15,540 | 0.8 years | $ | 15.86 | $ | — | ||||||
Vested at September 30, 2013 | 15,540 | 0.8 years | $ | 15.86 | $ | — | ||||||
Options exercisable at September 30, 2013 | 15,540 | 0.8 years | $ | 15.86 | $ | — | ||||||
Options available for future grant | 3,259,558 | |||||||||||
The aggregate intrinsic value in the table above represents the total intrinsic value, based on the Company’s closing stock price of $9.31 as of September 30, 2013, which would have been received by the option holders had all option holders exercised their options as of that date.
No stock options were granted in fiscal 2013, 2012 or 2011. The total intrinsic value of options exercised during fiscal 2013, 2012 or 2011 was $19,000, $56,000 and $15,000, respectively. The total cash received from participants as a result of stock option exercises during fiscal 2013, 2012 or 2011 was $67,000, $103,000 and $6,000, respectively.
As of September 30, 2013, there was no future compensation cost related to stock options as all outstanding stock options have vested.
The Company settles employee stock option exercises with newly issued common shares.
66
Restricted Stock Activity
A summary of the status of the Company’s restricted stock as of September 30, 2013 and changes during the year is as follows:
2013 | ||||||
Shares | Weighted Average Grant-Date Fair Value | |||||
Outstanding at September 30, 2012 | 2,732,448 | $ | 10.47 | |||
Awards granted | 1,471,977 | $ | 9.33 | |||
Awards vested | (755,187 | ) | $ | 9.67 | ||
Awards canceled | (533,825 | ) | $ | 11.41 | ||
Outstanding at September 30, 2013 | 2,915,413 | $ | 11.25 | |||
The weighted average grant date fair value of restricted stock granted during fiscal 2012 and fiscal 2011 was $11.80 and $11.27 per share, respectively. The fair value of restricted stock awards vested during fiscal 2013, 2012 and 2011 was $7.3 million, $5.6 million and $7.4 million, respectively.
As of September 30, 2013, the unrecognized compensation cost related to restricted stock that is expected to vest is $5.6 million and will be recognized over an estimated weighted average amortization period of 1.5 years.
1995 Employee Stock Purchase Plan
On February 22, 1996, the stockholders approved the 1995 Employee Stock Purchase Plan (the “1995 Plan”) which enables eligible employees to purchase shares of the Company’s common stock. Under the 1995 Plan, eligible employees may purchase up to an aggregate of 3,000,000 shares during six-month offering periods commencing on February 1 and August 1 of each year at a price per share of 85% of the lower of the fair market value price per share on the first or last day of each six-month offering period. On February 8, 2012, the stockholders approved an amendment to the 1995 Plan to increase the number of shares of the Company’s common stock available for issuance by 1,000,000 shares, from 3,000,000 to 4,000,000 shares. Participating employees may elect to have up to 10% of their base pay withheld and applied toward the purchase of such shares. The rights of participating employees under the 1995 Plan terminate upon voluntary withdrawal from the plan at any time or upon termination of employment. As of September 30, 2013, 3,134,074 shares of common stock have been purchased under the 1995 Plan and 865,926 shares remain available for purchase.
16. Restructuring and Other Charges
Fiscal 2013 Activities
The Company recorded a restructuring charge of $6.5 million for fiscal 2013. These charges relate primarily to workforce reductions implemented to consolidate the operations of Crossing and the Company, to transition internal manufacturing of the Polycold product line (certain of the Company’s cryopump and cryochiller products) to a third party contract manufacturer and other programs designed to improve the Company’s cost structure. Restructuring charges also included facility related costs incurred in connection with the consolidation of Crossing facilities with the Company’s facilities.
Restructuring costs recorded in fiscal 2013 consist of $5.6 million of severance costs and $0.8 million of facility related costs. Severance costs incurred in fiscal 2013 relate to the elimination of approximately 200 positions. The Brooks Product Solutions segment incurred a severance charge of $2.6 million; the Brooks Global Services segment incurred a severance charge of $1.1 million; and the Company incurred $1.5 million related to the elimination of corporate positions. The Brooks Life Science Systems segment incurred severance charges of $0.4 million, mainly due to the consolidation of administrative functions. Restructuring and other charges recorded in fiscal 2013 also include $0.1 million related to a partial settlement of a defined benefit pension plan that covers substantially all of the Company’s Swiss employees.
Total severance charges related to the outsourcing of the Polycold manufacturing operation are expected to be $1.3 million, including severance and retention bonuses. This charge is being amortized over the period from notification of the closing to the actual service end date. In the fourth quarter of 2013, the Company extended the service end date from September 2013 to September 2014 as a result of changes in the transition plan with the third party contract manufacturer. The Company has expensed $0.6 million of the total charge as of September 30, 2013, and will expense the balance ratably through fiscal 2014.
67
Fiscal 2012 Activities
The Company recorded a restructuring charge of $3.3 million for fiscal year 2012. These charges are related primarily to a series of workforce reductions implemented to improve the Company’s cost structure by eliminating 118 employees. The Brooks Product Solutions segment incurred a severance charge of $1.3 million; the Brooks Global Services segment incurred a severance charge of $1.0 million; and the Company incurred $0.7 million to eliminate corporate support positions. These workforce reductions were implemented to better align resources with future requirements. The Brooks Life Science Systems segment incurred severance charges of $0.3 million to eliminate 14 positions, mainly due to the consolidation of administrative functions.
Fiscal 2011 Activities
The Company recorded a charge to operations of $1.0 million in the year ended September 30, 2011 for restructuring costs. Of this amount, $0.7 million related to workforce reductions and $0.3 million related to facility costs. The severance costs are comprised of $0.3 million of severance for the elimination of 19 employees, and $0.4 million of adjustments for contingent severance arrangements for corporate management positions eliminated in prior periods. The facility costs relate to facilities exited in previous years. The costs for these exited facilities ended as of September 30, 2011.
The activity related to the Company’s restructuring and other charges are below (in thousands):
Fiscal 2013 Activity | |||||||||||||||
Balance September 30, 2012 | Expense | Utilization | Balance September 30, 2013 | ||||||||||||
Facilities and other | $ | — | $ | 818 | $ | (663 | ) | $ | 155 | ||||||
Workforce-related | 2,098 | 5,560 | (6,401 | ) | 1,257 | ||||||||||
$ | 2,098 | $ | 6,378 | $ | (7,064 | ) | $ | 1,412 | |||||||
Fiscal 2012 Activity | |||||||||||||||
Balance September 30, 2011 | Expense | Utilization | Balance September 30, 2012 | ||||||||||||
Workforce-related | $ | 293 | $ | 3,275 | $ | (1,470 | ) | $ | 2,098 | ||||||
Fiscal 2011 Activity | |||||||||||||||
Balance September 30, 2010 | Expense | Utilization | Balance September 30, 2011 | ||||||||||||
Facilities and other | $ | 3,509 | $ | 310 | $ | (3,819 | ) | $ | — | ||||||
Workforce-related | — | 726 | (433 | ) | 293 | ||||||||||
$ | 3,509 | $ | 1,036 | $ | (4,252 | ) | $ | 293 | |||||||
Unpaid severance charges as of September 30, 2013 of $0.8 million are expected to be paid during fiscal year 2014 and the remaining $0.5 million is expected to be paid during fiscal year 2015.
17. Segment and Geographic Information
The Company reports financial results in four segments: Brooks Product Solutions, Brooks Global Services, Brooks Life Science Systems and Contract Manufacturing. This financial reporting structure was implemented effective as of the beginning of the Company’s fourth quarter of fiscal year 2011 in response to changes in its management structure as well as the acquisition of two life science companies in the second half of fiscal 2011.
The Brooks Product Solutions segment provides a variety of products critical to technology equipment productivity and availability. Those products include atmospheric and vacuum tool automation systems, atmospheric and vacuum robots and robotic modules and cryogenic vacuum pumping, thermal management and vacuum measurement solutions which are used to create, measure and control critical process vacuum applications.
The Brooks Global Services segment provides an extensive range of support services including on-and off-site repair services, on-and off-site diagnostic support services, and installation services that enable our customers to maximize process tool uptime and productivity. This segment also provides end-user customers with spare parts support services that maximize tool productivity.
68
The Brooks Life Science Systems segment provides automated sample management systems for automated cold sample storage, equipment for sample preparation and handling, consumables, and parts and support services to a wide range of life science customers including pharmaceutical companies, biotechnology companies, biobanks, national laboratories, research institutes and research universities.
The Contract Manufacturing segment provided services to build equipment front-end modules, vacuum transport modules and other subassemblies which enabled the Company's customers to effectively source high quality and high reliability process tools for semiconductor market applications. The Company sold this business unit to Celestica Inc. on June 28, 2011.
The Company evaluates performance and allocates resources based on revenue, operating income (loss) and returns on invested assets. Operating income (loss) for each segment includes selling, general and administrative expenses directly attributable to the segment. The profits reported on intercompany transactions are based on the transfer prices charged which approximates fair value to third parties. Other unallocated corporate expenses, amortization of acquired intangible assets (excluding completed technology), restructuring and other charges, pension settlement and in-process research and development are excluded from the segments’ operating income (loss). The Company’s indirect overhead costs, which include various general and administrative expenses, are allocated among the segments based upon various cost drivers associated with the respective administrative function, including segment revenue, segment headcount, or an analysis of the segments that benefit from a specific administrative function. Segment assets exclude cash, cash equivalents, restricted cash, marketable securities, deferred tax assets and investments in joint ventures.
69
Financial information for the Company’s business segments is as follows (in thousands):
Brooks Product Solutions | Brooks Global Services | Brooks Life Science Systems | Contract Manufacturing | Total | |||||||||||||||
Year ended September 30, 2013 | |||||||||||||||||||
Revenue | |||||||||||||||||||
Product | $ | 317,916 | $ | 13,152 | $ | 31,336 | $ | — | $ | 362,404 | |||||||||
Services | — | 76,596 | 11,952 | — | 88,548 | ||||||||||||||
$ | 317,916 | $ | 89,748 | $ | 43,288 | $ | — | $ | 450,952 | ||||||||||
Gross profit | $ | 104,350 | $ | 27,492 | $ | 14,140 | $ | — | $ | 145,982 | |||||||||
Segment operating income (loss) | $ | 8,509 | $ | 10,172 | $ | (12,380 | ) | $ | — | $ | 6,301 | ||||||||
Depreciation expense | $ | 9,022 | $ | 2,746 | $ | 2,256 | $ | — | $ | 14,024 | |||||||||
Assets | $ | 254,424 | $ | 59,875 | $ | 105,221 | $ | — | $ | 419,520 | |||||||||
Year ended September 30, 2012 | |||||||||||||||||||
Revenue | |||||||||||||||||||
Product | $ | 380,888 | $ | 11,324 | $ | 39,749 | $ | — | $ | 431,961 | |||||||||
Services | — | 74,628 | 12,862 | — | 87,490 | ||||||||||||||
$ | 380,888 | $ | 85,952 | $ | 52,611 | $ | — | $ | 519,451 | ||||||||||
Gross profit | $ | 127,472 | $ | 25,599 | $ | 20,415 | $ | — | $ | 173,486 | |||||||||
Segment operating income (loss) | $ | 21,319 | $ | 9,404 | $ | (3,139 | ) | $ | — | $ | 27,584 | ||||||||
Depreciation expense | $ | 8,952 | $ | 2,344 | $ | 2,111 | $ | — | $ | 13,407 | |||||||||
Assets | $ | 218,799 | $ | 56,120 | $ | 107,530 | $ | — | $ | 382,449 | |||||||||
Year ended September 30, 2011 | |||||||||||||||||||
Revenue | |||||||||||||||||||
Product | $ | 451,287 | $ | 14,786 | $ | 7,715 | $ | 137,329 | $ | 611,117 | |||||||||
Services | — | 74,058 | 2,930 | $ | — | 76,988 | |||||||||||||
$ | 451,287 | $ | 88,844 | $ | 10,645 | $ | 137,329 | $ | 688,105 | ||||||||||
Gross profit | $ | 171,801 | $ | 31,750 | $ | 2,260 | $ | 17,210 | $ | 223,021 | |||||||||
Segment operating income (loss) | $ | 64,921 | $ | 13,293 | $ | (4,684 | ) | $ | 10,649 | $ | 84,179 | ||||||||
Depreciation expense | $ | 8,597 | $ | 2,481 | $ | 543 | $ | 1,000 | $ | 12,621 | |||||||||
Assets | $ | 235,322 | $ | 52,354 | $ | 101,331 | $ | — | $ | 389,007 | |||||||||
Revenue from the Brooks Product Solutions segment for the fiscal year ended September 30, 2011 includes intercompany sales of $49.2 million from this segment to the Contract Manufacturing segment. This intercompany revenue has been eliminated from the revenue of Contract Manufacturing.
Revenue for the Contract Manufacturing segment for the fiscal year ended September 30, 2011 excludes intercompany sales of $10.7 million from this segment to the Brooks Product Solutions segment.
70
A reconciliation of the Company’s reportable segment operating income and segment assets to the corresponding consolidated amounts as of and for the years ended September 30, 2013, 2012 and 2011 is as follows (in thousands):
As of and for the Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
Segment operating income | $ | 6,301 | $ | 27,584 | $ | 84,179 | |||||
Other unallocated corporate expenses(1) | 3,002 | (1,833 | ) | 1,135 | |||||||
Amortization of acquired intangible assets | 5,853 | 4,618 | 2,411 | ||||||||
Restructuring and other charges | 6,465 | 3,275 | 1,036 | ||||||||
Pension settlement | — | 8,937 | — | ||||||||
In-process research and development | — | 3,026 | — | ||||||||
Total operating income (loss) | $ | (9,019 | ) | $ | 9,561 | $ | 79,597 | ||||
Segment assets | $ | 419,520 | $ | 382,449 | |||||
Cash, cash equivalents, restricted cash and marketable securities | 173,539 | 200,994 | |||||||
Deferred tax assets | 115,985 | 120,157 | |||||||
Investments in joint ventures | 25,687 | 31,428 | |||||||
Other unallocated corporate net assets | 2,032 | 6,932 | |||||||
Total assets | $ | 736,763 | $ | 741,960 | |||||
______________
(1) | Other unallocated corporate expenses for the year ended September 30, 2012 includes a credit of $3.3 million related to insurance proceeds received as reimbursement of litigation costs previously incurred. |
Net revenue based upon the source of the order by geographic area is as follows (in thousands):
Year Ended September 30, | |||||||||||
2013 | 2012 | 2011 | |||||||||
North America | $ | 193,222 | $ | 230,735 | $ | 349,456 | |||||
Asia/Pacific | 165,452 | 194,711 | 244,524 | ||||||||
Europe | 92,278 | 94,005 | 94,125 | ||||||||
$ | 450,952 | $ | 519,451 | $ | 688,105 | ||||||
Property, plant and equipment by geographic area are as follows (in thousands):
September 30, | |||||||||
2013 | 2012 | ||||||||
North America | $ | 38,869 | $ | 51,546 | |||||
Asia/Pacific | 1,646 | 2,123 | |||||||
Europe | 7,355 | 10,809 | |||||||
$ | 47,870 | $ | 64,478 | ||||||
18. Significant Customers
The Company had one customer that accounted for more than 10% of revenue, at 11%, in the year ended September 30, 2013. The Company had one customer that accounted for more than 10% of revenue, at 13%, in the year ended September 30, 2012. The Company had two customers that each accounted for more than 10% of revenue, at 15% and 13%, respectively, in the year ended September 30, 2011. The Company did not have any customers that accounted for more than 10% of its accounts receivable balance at September 30, 2013 or 2012.
71
19. Other Balance Sheet Information
The following is a summary of accounts receivable at September 30, 2013 and 2012 (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Accounts receivable | $ | 78,460 | $ | 79,706 | |||
Less allowance for doubtful accounts | (863 | ) | (851 | ) | |||
Less allowance for sales returns | (114 | ) | — | ||||
$ | 77,483 | $ | 78,855 | ||||
The allowance for doubtful accounts activity for the years ended September 30, 2013, 2012 and 2011 were as follows (in thousands):
Description | Balance at Beginning of Period | Provisions | Reversals of Bad Debt Expense | Write-offs and Adjustments | Balance at End of Period | |||||||||||||||
2013 Allowance for doubtful accounts | $ | 851 | $ | 48 | $ | (143 | ) | $ | 107 | $ | 863 | |||||||||
2012 Allowance for doubtful accounts | 617 | 367 | (130 | ) | (3 | ) | 851 | |||||||||||||
2011 Allowance for doubtful accounts | 491 | — | — | 126 | 617 | |||||||||||||||
As part of the acquisition of Crossing in fiscal year 2013, the Company acquired a contract in which a certain customer has a right of return on the purchase of spare parts. The allowance for returns activity for the year ended September 30, 2013 was as follows (in thousands):
Description | Balance at Beginning of Period | Provisions | Write-offs and Adjustments | Balance at End of Period | |||||||||||
2013 Allowance for sales returns | $ | — | $ | 72 | $ | 42 | $ | 114 | |||||||
The following is a summary of inventories at September 30, 2013 and 2012 (in thousands):
September 30, | |||||||
2013 | 2012 | ||||||
Inventories | |||||||
Raw materials and purchased parts | $ | 60,106 | $ | 64,732 | |||
Work-in-process | 20,256 | 20,800 | |||||
Finished goods | 17,357 | 17,453 | |||||
$ | 97,719 | $ | 102,985 | ||||
Reserves for excess and obsolete inventory were $24.3 million and $23.2 million at September 30, 2013 and 2012, respectively. The Company recorded charges to reserves for excess and obsolete inventory of $5.4 million, $4.3 million and $2.2 million in fiscal 2013, 2012 and 2011, respectively. The Company reduced the reserves for excess and obsolete inventory by $4.3 million, $5.8 million and $3.5 million, in fiscal 2013, 2012 and 2011, respectively, for disposals of inventory.
72
The Company provides for the estimated cost of product warranties, primarily from historical information, at the time product revenue is recognized and retrofit accruals at the time retrofit programs are established. The Company’s warranty obligation is affected by product failure rates, utilization levels, material usage, service delivery costs incurred in correcting a product failure, and supplier warranties on parts delivered to the Company. Product warranty and retrofit activity on a gross basis for the years ended September 30, 2013, 2012 and 2011 is as follows (in thousands):
Balance at September 30, 2010 | $ | 8,195 | |
Adjustments for acquisitions and divestitures | 698 | ||
Accruals for warranties during the year | 11,299 | ||
Costs incurred during the year | (12,754 | ) | |
Balance at September 30, 2011 | 7,438 | ||
Adjustments for acquisitions and divestitures | 7 | ||
Accruals for warranties during the year | 13,751 | ||
Costs incurred during the year | (13,867 | ) | |
Balance at September 30, 2012 | 7,329 | ||
Adjustments for acquisitions and divestitures | 1,187 | ||
Accruals for warranties during the year | 10,111 | ||
Costs incurred during the year | (11,278 | ) | |
Balance at September 30, 2013 | $ | 7,349 | |
20. Sale of Building and Land
On September 27, 2013, the Company completed a Purchase and Sale Agreement ("Agreement") to sell a portion of its Chelmsford, Massachusetts campus to a real estate investment trust for $11.3 million. The property sold is an underutilized building and the related land. The components of the gain on the sale is as follows (in thousands):
Sale proceeds | $ | 11,275 | |
Net book value of building and land | (6,095 | ) | |
Deferred leasing costs and other | (3,718 | ) | |
Direct transaction costs | (437 | ) | |
Gain on the sale of building and land | $ | 1,025 | |
In December 2012, the Company entered into an agreement to lease this property to an unrelated third party. Unamortized deferred costs of $3.7 million, consisting of primarily of commissions and tenant allowances, were written off and included in the determination of the gain on the sale. Direct transaction costs, consisting of broker commissions and legal fees were also included in the determination of the gain on the sale.
In addition, in fiscal year 2013, the Company sold certain buildings in Oberdiessbach, Switzerland for total proceeds of $3.2 million. The sale of these assets resulted in a gain of $0.2 million.
Gains related to the sale of these buildings are recorded in the Company's Consolidated Statements of Operations as a component of other income (expense), net.
73
21. Commitments and Contingencies
Lease Commitments
The Company leases manufacturing and office facilities and certain equipment under operating leases that expire through 2022. Rental expense under operating leases, excluding expense recorded as a component of restructuring, for the years ended September 30, 2013, 2012 and 2011 was $8.4 million, $4.8 million and $4.9 million, respectively. Future minimum lease commitments on non-cancelable operating leases are as follows (in thousands):
Operating Leases | |||
Year ended September 30, 2014 | $ | 7,199 | |
2015 | 5,084 | ||
2016 | 2,728 | ||
2017 | 1,557 | ||
2018 | 1,359 | ||
Thereafter | 1,625 | ||
$ | 19,552 | ||
The Company is a guarantor on a lease in Mexico that expires in January 2015. As of September 30, 2013, the remaining payments under this lease are approximately $0.5 million.
Letters of Credit
At September 30, 2013, the Company had $5.7 million of outstanding letters of credit.
Purchase Commitments
The Company has non-cancelable contracts and purchase orders for inventory of $68.6 million at September 30, 2013.
Contingencies
The Company is subject to various legal proceedings, both asserted and unasserted, that arise in the ordinary course of business. The Company cannot predict the ultimate outcome of such legal proceedings or in certain instances provide reasonable ranges of potential losses. However, as of the date of this report, the Company believes that none of these claims will have a material adverse effect on its consolidated financial condition or results of operations. In the event of unexpected subsequent developments and given the inherent unpredictability of these legal proceedings, there can be no assurance that the Company's assessment of any claim will reflect the ultimate outcome and an adverse outcome in certain matters could, from time to time, have a material adverse effect on the Company's consolidated financial condition or results of operations in particular quarterly or annual periods.
22. Subsequent Events
On November 12, 2013, the Company’s Board of Directors declared a cash dividend of $0.08 per share payable on December 27, 2013 to common stockholders of record on December 6, 2013. Dividends are declared at the discretion of the Company’s Board of Directors and depend on actual cash from operations, the Company’s financial condition and capital requirements and any other factors the Company’s Board of Directors may consider relevant. Future dividend declarations, as well as the record and payment dates for such dividends, will be determined by the Company’s Board of Directors on a quarterly basis.
74
Item 9. | Changes In and Disagreements With Accountants on Financial Accounting and Financial Disclosure |
As previously reported, effective November 20, 2012, the Audit Committee of the Company’s Board of Directors elected not to retain PricewaterhouseCoopers LLP as its independent registered public accounting firm and engaged BDO USA, LLP as the Company’s independent registered public accounting firm for fiscal 2013. This change in accounting firms was reported in the Company’s Form 8-K filed with the United States Securities and Exchange Commission on November 27, 2012.
Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act. Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported on a timely basis and that such information is accumulated and communicated to management, including the chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. Based upon this evaluation, our chief executive officer and our chief financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, as a process designed by, or under the supervision of our chief executive and chief financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
• | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
• | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
• | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an assessment of the effectiveness of our internal control over financial reporting as of September 30, 2013. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) an Internal Control-Integrated Framework. Based on our assessment, we concluded that, as of September 30, 2013, our internal control over financial reporting was effective.
The effectiveness of our internal control over financial reporting as of September 30, 2013 has been audited by BDO USA LLP, an independent registered public accounting firm, as stated in the following report:
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Brooks Automation, Inc.
Chelmsford, Massachusetts
We have audited Brooks Automation Inc.’s internal control over financial reporting as of September 30, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Brooks Automation, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
75
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Brooks Automation, Inc. maintained, in all material respects, effective internal control over financial reporting as of September 30, 2013, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of Brooks Automation, Inc. as of September 30, 2013 and the related consolidated statements of operations, comprehensive income (loss), changes in equity, and cash flows for the year then ended and our report dated November 22, 2013 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Boston, Massachusetts
November 22, 2013
Changes in Internal Control Over Financial Reporting
There were no changes in internal control over financial reporting during the fiscal fourth quarter ended September 30, 2013, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. | Other Information |
None.
PART III
Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this Item 10 is contained in our definitive proxy statement for our 2014 annual meeting of shareholders to be filed by us within 120 days after the close of our fiscal year (the "2014 Proxy Statement") and is incorporated herein by reference.
Item 11. | Executive Compensation |
The information required by this Item 11 is contained in the 2014 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this Item 12 is contained in the 2014 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
76
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item 13 is contained in the 2014 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 14. | Principal Accountant Fees and Services |
The information required by this Item 14 is contained in the 2014 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
PART IV
Item 15. | Exhibits and Financial Statement Schedules |
(a) Financial Statements and Financial Statement Schedules
The consolidated financial statements of the Company are listed in the index under Part II, Item 8, in this Form 10-K.
Other financial statement schedules are omitted because of the absence of conditions under which they are required or because the required information is given in the supplementary consolidated financial statements or notes thereto.
(b) Exhibits
Exhibit No. | Description | |
3.01 | Restated Certificate of Incorporation of the Company (incorporated herein by reference to Exhibit 3.01 to the Company’s registration statement on Form S-3 (Reg. No. 333-189582), filed on June 25, 2013). | |
3.02 | Amended and Restated Bylaws (incorporated herein by reference to Exhibit 3.01 of the Company's current report on Form 8-K, filed on February 11, 2008). | |
4.01 | Specimen Certificate for shares of the Company's common stock (incorporated herein by reference to the Company's registration statement on Form S-3 (Reg. No. 333-88320), filed on May 15, 2002). | |
10.01 | Shareholders’ Agreement, dated as of June 30, 2006, among Yaskawa Electric Corporation, Brooks Automation, Inc. and Yaskawa Brooks Automation, Inc. (incorporated herein by reference to Exhibit 10.01 to the Company's 2010 10-K). | |
10.02 | U.S. Robot Supply Agreement, made as of June 30, 2006, by and between Brooks Automation, Inc. and Yaskawa Electric Corporation (incorporated herein by reference to Exhibit 10.02 to the 2010 10-K). | |
10.03 | Brooks Japan Robot Supply Agreement, made as of June 30, 2006, by and between Yaskawa Brooks Automation, Inc. and Brooks Automation, Inc. (incorporated herein by reference to Exhibit 10.03 to the Company's 2010 10-K). | |
10.04 | Basic agreement between the Company and Ulvac Corporation dated August 17, 1981 (incorporated by reference to Exhibit 10.13 of the registration statement on Form S-2 (Reg. No. 2-84880) filed by Helix Technology Corporation)). | |
10.05 | Form of Indemnification Agreement for directors and officers of the Company (incorporated herein by reference to the Company's registration statement on Form S-1 (Reg. No. 333-87296), filed on December 13, 1994 (the “Brooks S-1”)). | |
10.06 | Retention Agreement dated March 5, 2013 between Brooks Automation, Inc. and Martin S. Headley (incorporated herein by reference to Exhibit 10.01 to the Company's current report on Form 8-K, filed on March 11, 2013). | |
10.07 | Letter Agreement dated June 6, 2013 between Brooks Automation, Inc. and Martin S. Headley (incorporated herein by reference to Exhibit 10.01 to the Company's current report on Form 8-K, filed on June 11, 2013). | |
10.08 | Employment Agreement, effective as of April 5, 2010, by and between Brooks Automation, Inc. and Stephen S. Schwartz (incorporated herein by reference to Exhibit 10.01 to the Company's quarterly report on Form 10-Q for the fiscal quarter ended March 31, 2010, filed on May 6, 2010). | |
10.09 | Offer letter dated December 1, 2011 between the Company and Mark D. Morelli (incorporated herein by reference to Exhibit 10.08 to the Company's annual report on Form 10-K for the fiscal year ended September 30, 2012, as filed on November 21, 2012 (“2012 10-K”)). | |
10.10 | Separation Agreement dated November 8, 2012 between the Company and Steven Michaud (incorporated herein by reference to Exhibit 10.09 to the Company's 2012 10-K). | |
77
10.11 | Offer letter dated September 5, 2013 between the Company and Lindon G. Robertson. | |
10.12 | Transition Agreement entered into on September 25, 2013 between Brooks Automation, Inc. and Thomas R. Leitzke (incorporated herein by reference to Exhibit 10.01 to the Company's current report on Form 8-K, filed on September 30, 2013). | |
10.13 | 1995 Employee Stock Purchase Plan, as amended (incorporated herein by reference to Exhibit 10.13 to the Company's 2010 10-K). | |
10.14 | Second Amended and Restated 2000 Equity Incentive Plan, restated as of May 7, 2013 (incorporated herein by reference to Exhibit 10.01 to the Company's current report on Form 8-K, filed on May 9, 2013). | |
10.15 | Helix Technology Corporation 1996 Equity Incentive Plan (incorporated herein by reference to Exhibit 4.1 of the Company's registration statement on Form S-8 (Reg. No. 333-129724), filed on November 16, 2005). | |
10.16 | Helix Technology Corporation Amended and Restated Stock Option Plan for Non-Employee Directors (incorporated herein by reference to Exhibit 4.2 of the Company's registration statement on Form S-8 (Reg. No. 333-129724), filed on November 16, 2005). | |
10.17 | Form of 2000 Equity Incentive Plan New Employee Nonqualified Stock Option Agreement (incorporated herein by reference to Exhibit 10.18 to the Company's 2010 10-K). | |
10.18 | Form of 2000 Equity Incentive Plan Existing Employee Nonqualified Stock Option Agreement (incorporated herein by reference to Exhibit 10.19 to the Company's 2010 10-K). | |
10.19 | Form of 2000 Equity Incentive Plan Director Stock Option Agreement (incorporated herein by reference to Exhibit 10.20 to the Company's 2010 10-K). | |
10.20 | Form of Restricted Stock Agreement (incorporated herein by reference to Exhibit 10.21 to the Company's 2010 10-K). | |
10.21 | Form of Restricted Stock Unit Award Notice (incorporated herein by reference to Exhibit 10.18 to the Company's annual report on Form 10-K for the fiscal year ended September 30, 2011, as filed on November 28, 2011 (“2011 10-K”)) . | |
10.22 | Non-Employee Directors Stock Grant/Restricted Stock Unit Election Form (incorporated herein by reference to Exhibit 10.40 to the Company's 2010 10-K). | |
10.23 | Brooks Automation, Inc. Deferred Compensation Plan, as amended (incorporated herein by reference to Exhibit 10.25 to the Company's 2010 10-K). | |
10.24 | Amendment No. 2008-01 to the Brooks Automation, Inc. Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.01 to the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2008, filed on August 8, 2008). | |
10.25 | Lease between the Company and BerCar II, LLC for 12 Elizabeth Drive, Chelmsford, Massachusetts dated October 23, 2002 (incorporated herein by reference to Exhibit 10.28 to the Company's 2008 10-K). | |
10.26 | First Amendment to Lease between the Company and BerCar II, LLC for 12 Elizabeth Drive, Chelmsford, Massachusetts dated November 1, 2002 (incorporated herein by reference to Exhibit 10.29 to the Company's 2008 10-K). | |
10.27 | Lease, dated May 14, 1999, between MUM IV, LLC as Lessor and the Company as Lessee (incorporated herein by reference to Exhibit 10.30 to the 2010 10-K). | |
10.28 | Factory Lease Advanced Agreement among Sang Chul Park, Young Ja Kim, Joon Ho Park, Brooks Automation Asia, Ltd. and Brooks Automation Korea, Inc. (incorporated herein by reference to Exhibit 10.36 to the Company's 2010 10-K). | |
10.29 | Lease dated September 6, 2001 between The Harry Friedman and Edith B. Friedman Revocable Living Trust Dated May 15, 1986 et al as Lessor and the Company (IGC - Polycold Systems Inc.) as Lessee (incorporated herein by reference to Exhibit 10.37 to the Company's 2010 10-K). | |
10.30 | Lease dated August 8, 2008 between the Company and Koll/Intereal Bay Area for 4051 Burton Drive, Santa Clara, CA (incorporated herein by reference to Exhibit 10.38 to the Company's 2008 10-K). | |
78
10.31 | Standard Industrial lease dated May 31, 2010 by and between Brooks Automation, Inc. (formerly Nexus Biosystems, Inc.) and Crest Partners-Poway One Danielson for 14100 Danielson Street, Building 100, Poway, California (incorporated herein by reference to Exhibit 10.29 to the Company's 2011 10-K). | |
10.32 | Purchase Agreement dated July 31, 2013 between Brooks Automation, Inc. and Ram Management Co., Inc. (incorporated herein by reference to Exhibit 10.01 to the Company's current report on Form 8-K filed on August 5, 2013). | |
10.33 | Agreement and Plan of Merger among Nexus Biosystems, Inc., the Company, Spurs Acquisition, Inc., a wholly-owned subsidiary of the Company, and Telegraph Hill Partners Management Company LLC, dated as of July 25, 2011 (incorporated herein by reference to Exhibit 2.1 to the Company's current report on Form 8-K, filed on July 29, 2011). | |
10.34 | Master Purchase and Sale Agreement by and among Brooks Automation, Inc., Celestica Oregon LLC, 2281392 Ontario Inc., and, for the limited purposes set forth therein, Celestica, Inc., dated as of April 20, 2011 (incorporated herein by reference to Exhibit 2.1 to the Company's current report on Form 8-K, filed on April 26, 2011). | |
10.35 | Agreement and Plan of Merger among Crossing Automation Inc., the Company, Niners Acquisition Corporation, a wholly-owned subsidiary of the Company, and Shareholder Representative Services LLC, dated as of October 28, 2012 (incorporated herein by reference to Exhibit 2.1 to the Company's current report on Form 8-K, filed on October 31, 2012). | |
16.1 | Letter of PricewaterhouseCoopers LLP dated November 27, 2012 (incorporated herein by reference to Exhibit 16.1 to the Company's current report on Form 8-K filed on November 27, 2012). | |
21.01 | Subsidiaries of the Company. | |
23.01 | Consent of BDO (Independent registered public accounting firm for the Company). | |
23.02 | Consent of PricewaterhouseCoopers LLP (Independent registered public accounting firm for the Company). | |
31.01 | Certification of the Company's Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
31.02 | Certification of the Company's Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
32 | Certification of the Company's Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
101 | The following material from the Company's Annual Report on Form 10-K, for the year ended September 30, 2013, formatted in XBRL (Xtensible Business Reporting Language): (i) the Consolidated Balance Sheets; (ii) the Consolidated Statements of Operations; (iii) the Consolidated Statements of Comprehensive Income (Loss) (iv) the Consolidated Statements of Cash Flows; (v) the Consolidated Statements of Changes in Equity; and (vi) the Notes to Consolidated Financial Statements. | |
79
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BROOKS AUTOMATION, INC. | |
By: | /S/ STEPHEN S. SCHWARTZ |
Stephen S. Schwartz Chief Executive Officer | |
Date: November 22, 2013
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date |
/S/ STEPHEN S. SCHWARTZ | Director and Chief Executive Officer | November 22, 2013 |
Stephen S. Schwartz | (Principal Executive Officer) | |
/S/ LINDON G. ROBERTSON | Executive Vice President and | November 22, 2013 |
Lindon G. Robertson | Chief Financial Officer | |
(Principal Financial Officer) | ||
/S/ DAVID PIETRANTONI | Vice President - Finance and | November 22, 2013 |
David Pietrantoni | Corporate Controller | |
(Principal Accounting Officer) | ||
/S/ A. CLINTON ALLEN | Director | November 22, 2013 |
A. Clinton Allen | ||
/S/ ROBYN C. DAVIS | Director | November 22, 2013 |
Robyn C. Davis | ||
/S/ JOSEPH R. MARTIN | Director | November 22, 2013 |
Joseph R. Martin | ||
/S/ JOHN K. MCGILLICUDDY | Director | November 22, 2013 |
John K. McGillicuddy | ||
/S/ KRISHNA G. PALEPU | Director | November 22, 2013 |
Krishna G. Palepu | ||
/S/ KIRK P. POND | Director | November 22, 2013 |
Kirk P. Pond | ||
/S/ ALFRED WOOLLACOTT III | Director | November 22, 2013 |
Alfred Woollacott III | ||
/S/ MARK S. WRIGHTON | Director | November 22, 2013 |
Mark S. Wrighton | ||
/S/ ELLEN M. ZANE | Director | November 22, 2013 |
Ellen M. Zane | ||
80
Similar companies
See also ASML HOLDING NVSee also LAM RESEARCH CORP - Annual report 2023 (10-K 2023-06-25) Annual report 2025 (10-Q 2025-03-30)
See also AXCELIS TECHNOLOGIES INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Cricut, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Energy Recovery, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
