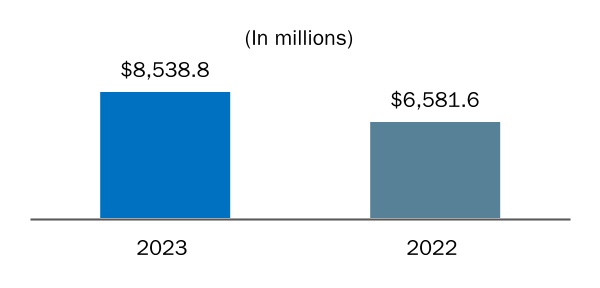|
|
For additional information on our collaboration arrangements with Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
GENENTECH RELATIONSHIPS
We have agreements with Genentech that entitle us to certain business and financial rights with respect to RITUXAN, RITUXAN HYCELA, GAZYVA, OCREVUS, LUNSUMIO, COLUMVI, which was granted accelerated approval by the FDA during the second quarter of 2023, and have the option to add other potential anti-CD20 therapies.
Our current anti-CD20 therapeutic programs and major markets are as follows:
| | | | | | | | | | | | | | |
| Product | | Indication | | Major Markets |
| | | | |
| | Non-Hodgkin's lymphoma
CLL
Rheumatoid arthritis
Two forms of ANCA-associated vasculitis
Pemphigus vulgaris | | U.S.
Canada |
| | | | |
| | Non-Hodgkin's lymphoma
CLL | | U.S. |
| | | | |
| | In combination with chlorambucil for previously untreated CLL
follicular lymphoma
In combination with chemotherapy followed by GAZYVA alone for previously untreated follicular lymphoma | | U.S. |
| | | | |
| | RMS
PPMS | | U.S. |
| | | | |
| | Relapsed or refractory follicular lymphoma | | U.S. |
| | | | |
| | Relapsed or refractory diffuse large B-cell lymphoma
Large B-cell lymphoma arising from follicular lymphoma | | U.S. |
For additional information on our collaboration arrangements with Genentech, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
OTHER
| | | | | | | | | | | | | | | | | | | | |
| Product | | Indication | | Collaborator | | Major Markets |
| | | | | | |
| | Moderate to severe plaque psoriasis | | None | | Germany |
PATIENT SUPPORT AND ACCESS
We interact with patients, advocacy organizations and healthcare societies in order to gain insights into unmet needs. The insights gained from these engagements help us support patients with services, programs and applications that are designed to help patients lead better lives. Among other things, we provide customer service and other related programs for our products, such as disease and product specific websites, insurance research services, financial assistance programs and the facilitation of the procurement of our marketed products.
We are dedicated to helping patients obtain access to our therapies. Our patient representatives have access to a suite of financial assistance tools. With those tools, we help patients understand their insurance coverage and, if needed, help patients compare insurance options and programs. In the U.S., we have established programs that provide co-pay assistance or free product for qualified uninsured or underinsured patients, based on specific eligibility criteria. We also provide charitable contributions to independent charitable organizations that assist patients with out-of-pocket expenses associated with their therapy.
We believe all healthcare stakeholders have a shared responsibility to ensure patients have equitable access to new, innovative medicines. We regularly review our pricing strategy and prioritize patient access to our therapies. We have a value-based contracting program designed to align the price of our therapies to the value our therapies deliver to patients. We also work with regulators, clinical researchers, ethicists, physicians and patient advocacy organizations and communities, among others, to determine how best to address requests for access to our investigational therapies in a manner that is consistent with our patient-focused values and compliant with regulatory standards and protocols. In appropriate situations, patients may have access to investigational therapies through Early Access Programs, single patient access or emergency use based on humanitarian or compassionate grounds.
MARKETING AND DISTRIBUTION
SALES FORCE AND MARKETING
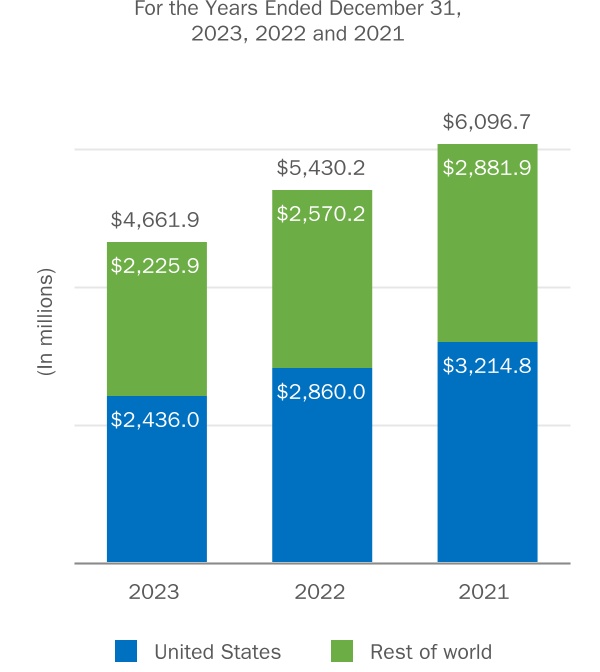
We promote our marketed products worldwide, including in the U.S., Europe and Japan, primarily through our own sales forces and marketing groups. In some countries, particularly in areas where we continue to expand into new geographic areas, we partner with third parties.
RITUXAN, RITUXAN HYCELA, GAZYVA, OCREVUS and LUNSUMIO are marketed by the Roche Group and its sublicensees.
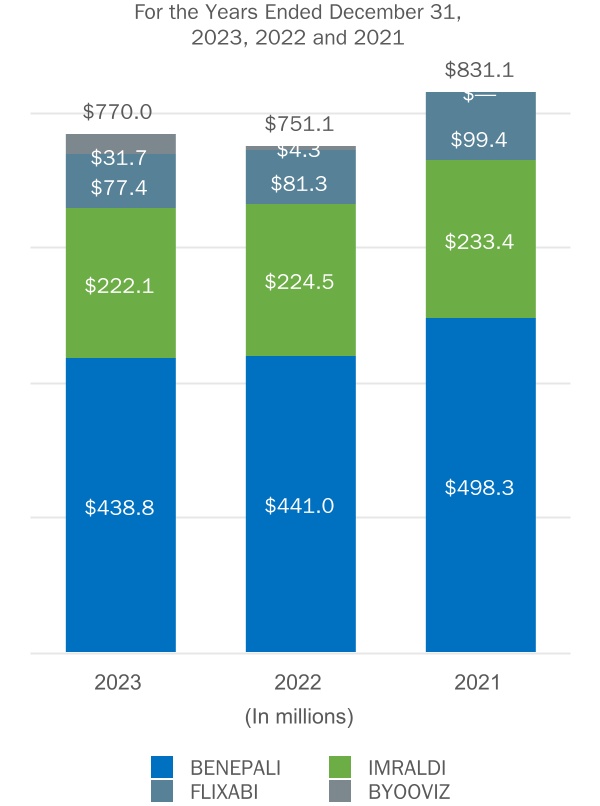
We commercialize BENEPALI, IMRALDI and FLIXABI pursuant to our agreement with Samsung Bioepis in certain countries in Europe, as well as BYOOVIZ in the U.S. and certain international markets.
We focus our sales and marketing efforts on physicians in private practice or at major medical centers. We use customary industry practices to market our products and to educate physicians. This includes our sales representatives calling on individual health care providers (in-person and virtually), advertisements, professional symposia, direct mail, digital marketing, point of care marketing, public relations and other methods. We focus on health care provider sales and marketing efforts on specialty providers in both private practice and at major medical centers.
DISTRIBUTION ARRANGEMENTS
We distribute our products in the U.S. principally through wholesale and specialty distributors of pharmaceutical products and specialty pharmacies, mail order specialty distributors or shipping service providers. In other countries, the distribution of our products varies from country to country, including through wholesale distributors of pharmaceutical products and third-party distribution partners who are responsible for most marketing and distribution activities.
RITUXAN, RITUXAN HYCELA, GAZYVA, OCREVUS and LUNSUMIO are distributed by the Roche Group and its sublicensees.
We distribute BENEPALI, IMRALDI and FLIXABI in certain countries in Europe and have an option to acquire exclusive rights to distribute these products in China, as well as BYOOVIZ in the U.S. and certain international markets.
Our product sales to two wholesale distributors each accounted for more than 10.0% of our total revenue for the years ended December 31, 2023, 2022 and 2021, and on a combined basis, accounted for approximately 36.9%, 37.9% and 38.9%, respectively, of our gross product revenue. For additional information, please read Note 5, Revenue, to our consolidated financial statements included in this report.
PATENTS AND OTHER PROPRIETARY RIGHTS
Patents are important for obtaining and protecting exclusive rights in our products and product candidates. We regularly seek patent protection in the U.S. and in selected countries outside the U.S. for inventions originating from our research and development efforts and those we license or acquire. In addition, we license rights to various patents and patent applications.
U.S. patents, as well as most foreign patents, are generally effective for 20 years from the date the earliest application was filed; however, U.S. patents on applications filed before June 8, 1995, may be effective until 17 years from the issue date, if that is later than the 20-year date. In some cases, the patent term may be extended to recapture a portion of the term lost during regulatory review of the claimed therapeutic or, in the case of the U.S., additional patent term may be awarded due to U.S. Patent and Trademark Office delays in prosecuting the application. In the U.S., under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, a patent that covers a drug approved by the FDA may be eligible for patent term extension (for up to 5 years, but not beyond a total of 14 years from the date of product approval) as compensation for patent term lost during the FDA regulatory review process. The duration and extension of the term of foreign patents vary, in accordance with local law. For example, in a number of European countries, SPCs can be granted to a product to compensate in part for delays in obtaining marketing approval.
Regulatory exclusivity, which may consist of regulatory data protection and market protection, can also provide meaningful protection for our products. Regulatory data protection provides to the holder of a drug or biologic marketing authorization, for a set period of time, the exclusive use of the proprietary pre-clinical and clinical data that it created at significant cost and submitted to the applicable regulatory authority to obtain approval of its product. After the period of exclusive use, third parties are permitted to reference such data in abbreviated applications for approval and to market (subject to any applicable market protection) their generic drugs and biosimilars. Market protection provides the holder of a drug or biologic marketing authorization the exclusive right to commercialize its product for a period of time, thereby preventing the commercialization of another product containing the same active ingredient(s) during that period. Although the World Trade Organization's agreement on trade-related aspects of intellectual property rights requires signatory countries to provide regulatory exclusivity to innovative pharmaceutical products, implementation and enforcement varies widely from country to country.
We also rely upon other forms of unpatented confidential information to remain competitive. We protect such information principally through refraining from public disclosure and utilizing confidentiality agreements with our employees, consultants, outside scientific collaborators, scientists whose research we sponsor and other advisers. In the case of our employees, these agreements also provide, in compliance with relevant law, that inventions and other intellectual property conceived by such employees during their employment are our exclusive property.
Our trademarks are important to us and are generally covered by trademark applications or registrations in the U.S. Patent and Trademark Office and the patent or trademark offices of other countries. We also use trademarks licensed from third parties. Trademark protection varies in accordance with local law, and continues in some countries as long as the trademark is used and in other countries as long as the trademark is registered. Trademark registrations generally are for fixed but renewable terms.
OUR PATENT PORTFOLIO
The following table describes certain patents in the U.S. and Europe that we currently consider of primary importance to our marketed products, including the territory, patent number, general subject matter and expected expiration dates. Except as otherwise noted, the expected expiration dates include any granted patent term extensions and issued SPCs. In some instances, there may be additional later-expiring patents relating to our products directed to, among other things, particular forms or compositions, methods of manufacturing or use of the drug in the treatment of particular diseases or conditions. We also continue to pursue additional patents and patent term extensions in the U.S. and other territories covering various aspects of our products that may, if issued, extend exclusivity beyond the expiration of the patents listed in the table.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Product | | Territory | | Patent No. | | General Subject Matter | | Patent Expiration(1) |
| TECFIDERA | | Europe | | 1,131,065 | | Formulations of dialkyl fumarates and their use for treating autoimmune diseases | | 2024(2) |
| | Europe | | 2,653,873 | | Methods of use | | 2028 |
| PLEGRIDY | | U.S. | | 8,017,733 | | Polymer conjugates of interferon beta-1a | | 2027 |
| | Europe | | 1,656,952 | | Polymer conjugates of interferon-beta-1a and uses thereof | | 2024(3) |
| | Europe | | 1,476,181 | | Polymer conjugates of interferon-beta-1a and uses thereof | | 2023(4) |
| TYSABRI | | U.S. | | 8,124,350 | | Methods of treatment | | 2027 |
| | U.S. | | 8,349,321 | | Formulation | | 2024 |
| | U.S. | | 8,815,236 | | Formulation | | 2024 |
| | U.S. | | 8,871,449 | | Methods of treatment | | 2026 |
| | U.S. | | 8,900,577 | | Formulation | | 2024 |
| | U.S. | | 9,316,641 | | Safety-related assay | | 2032 |
| | U.S. | | 9,493,567 | | Methods of treatment | | 2027 |
| | U.S. | | 9,709,575 | | Methods of treatment | | 2026 |
| | U.S. | | 10,119,976 | | Methods of evaluating patient risk | | 2034 |
| | U.S. | | 10,233,245 | | Methods of treatment | | 2027 |
| | U.S. | | 10,444,234 | | Safety-related assay | | 2031 |
| | U.S. | | 10,677,803 | | Methods of treatment | | 2034 |
| | U.S. | | 10,705,095 | | Methods of treatment | | 2026 |
| | U.S. | | 11,280,794 | | Methods of treatment | | 2034 |
| | U.S. | | 11,287,423 | | Safety-related assay | | 2031 |
| | U.S. | | 11,292,845 | | Methods of treatment | | 2027 |
| | Europe | | 2,170,390 | | Formulation | | 2028 |
| | Europe | | 2,236,154 | | Formulation | | 2024 |
| | Europe | | 3,339,865 | | Safety-related assay | | 2031 |
| | Europe | | 3,417,875 | | Formulation | | 2024 |
| | Europe | | 3,575,792 | | Safety-related assay | | 2032 |
| FAMPYRA | | Europe | | 1,732,548 | | Sustained-release aminopyridine compositions for increasing walking speed in patients with MS | | 2025(5) |
| | Europe | | 2,377,536 | | Sustained-release aminopyridine compositions for treating MS | | 2025(6) |
| VUMERITY | | U.S. | | 8,669,281 | | Compounds and pharmaceutical compositions | | 2033 |
| | U.S. | | 9,090,558 | | Methods of treatment | | 2033 |
| | U.S. | | 10,080,733 | | Crystalline forms, pharmaceutical compositions and methods of treatment | | 2033 |
| | Europe | | 2,970,101 | | Crystalline forms, pharmaceutical compositions and methods of treatment
Prodrugs of fumarates and their use in treating various diseases | | 2034 |
| SPINRAZA | | U.S. | | 7,838,657 | | SMA treatment via targeting of SMN2 splice site inhibitory sequences | | 2027 |
| | U.S. | | 8,110,560 | | SMA treatment via targeting of SMN2 splice site inhibitory sequences | | 2025 |
| | U.S. | | 8,361,977 | | Compositions and methods for modulation of SMN2 splicing | | 2030 |
| | U.S. | | 8,980,853 | | Compositions and methods for modulation of SMN2 splicing | | 2030 |
| | U.S. | | 9,717,750 | | Compositions and methods for modulation of SMN2 splicing | | 2030 |
| | U.S. | | 9,926,559 | | Compositions and methods for modulation of SMN2 splicing | | 2034 |
| | U.S. | | 10,266,822 | | SMA treatment via targeting of SMN2 splice site inhibitory sequences | | 2025 |
| | U.S. | | 10,436,802 | | Methods for Treating Spinal Muscular Atrophy | | 2035 |
| | Europe | | 1,910,395 | | Compositions and methods for modulation of SMN2 splicing | | 2026(7) |
| | Europe | | 2,548,560 | | Compositions and methods for modulation of SMN2 splicing | | 2026(8) |
| | Europe | | 3,305,302 | | Compositions and methods for modulation of SMN2 splicing | | 2030 |
| | Europe | | 3,308,788 | | Compositions and methods for modulation of SMN2 splicing | | 2026 |
| | Europe | | 3,449,926 | | Compositions and methods for modulation of SMN2 splicing | | 2030(10) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Product | | Territory | | Patent No. | | General Subject Matter | | Patent Expiration(1) |
| ADUHELM | | U.S. | | 8,906,367 | | Method of providing disease-specific binding molecules and targets | | 2032(11) |
| | U.S. | | 10,131,708 | | Methods of treating Alzheimer's disease | | 2028 |
| LEQEMBI | | U.S. | | 8,025,878 | | Protofibril selective antibodies and the use thereof | | 2027(1)(11) |
| QALSODY | | U.S. | | 10,385,341 | | Compositions for modulating SOD-1 expression | | 2035(11) |
| | U.S. | | 10,669,546 | | Compositions for modulating SOD-1 expression | | 2035 |
| | U.S. | | 10,968,453 | | Compositions for modulating SOD-1 expression | | 2035 |
| ZURZUVAE | | U.S. | | 9,512,165 | | 19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof | | 2034(9) |
| | U.S. | | 10,172,871 | | 19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof | | 2034(9) |
| | U.S. | | 10,342,810 | | 19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof | | 2034(9) |
| | U.S. | | 11,236,121 | | Crystalline 19-nor C3, 3-disubstituted C21-N-pyrazolyl steroid | | 2034(9) |
| SKYCLARYS | | U.S. | | 8,124,799 | | Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives with Amino and other Modifications at C-17 (Composition) | | 2029(9) |
| | U.S. | | 8,440,854 | | Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives with Amino and other Modifications at C-17 (Composition) | | 2029(9) |
| | U.S. | | 8,993,640 | | 2,2-Difluoropropionamide Derivatives of Bardoxolone Methyl, Polymorphic Forms and Methods of Use Thereof (Composition) | | 2033(9) |
| | U.S. | | 9,670,147 | | Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives with Amino and other Modifications at C-17 (Composition) | | 2029(9) |
| | U.S. | | 9,701,709 | | 2,2-Difluoropropionamide Derivatives of Bardoxolone Methyl, Polymorphic Forms and Methods of Use Thereof (Composition) | | 2033(9) |
| | U.S. | | 11,091,430 | | Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives with Amino and other Modifications at C-17 (Treatment Method) | | 2029(9) |
Footnotes follow on next page.
(1)In addition to patent protection, certain of our products are entitled to regulatory exclusivity in the U.S. and the E.U. expected until the dates set forth below:
| | | | | | | | | | | | | | |
| Product | | Territory | | Expected Expiration |
| TECFIDERA | | E.U. | | 2025 |
| PLEGRIDY | | U.S. | | 2026 |
| | E.U. | | 2024 |
| SPINRAZA | | E.U. | | 2029 |
| ADUHELM | | U.S. | | 2033 |
| LEQEMBI | | U.S. | | 2035 |
| QALSODY | | U.S. | | 2030 |
| ZURZUVAE | | U.S. | | 2028 |
| SKYCLARYS | | U.S. | | 2030 |
(2)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2024.
(3)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2024.
(4)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2028.
(5)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2026.
(6)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2026.
(7)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2031.
(8)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2031.
(9)A patent with this subject matter may be entitled to patent term extension in the U.S.
(10)This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2032.
The existence of patents does not guarantee our right to practice the patented technology or commercialize the patented product. Patents relating to pharmaceutical, biopharmaceutical and biotechnology products, compounds and processes, such as those that cover our existing products, compounds and processes and those that we will likely file in the future, do not always provide complete or adequate protection. Litigation, interferences, oppositions, inter partes reviews, administrative challenges or other similar types of proceedings are, have been and may in the future be necessary in some instances to determine the validity and scope of certain of our patents, regulatory exclusivities or other proprietary rights, and in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. We also face challenges to our patents, regulatory exclusivities or other proprietary rights covering our products by third-parties, such as manufacturers of generics, biosimilars, prodrugs and products approved under abbreviated regulatory pathways. A discussion of certain risks and uncertainties that may affect our patent position, regulatory exclusivities or other proprietary rights is set forth in Item 1A. Risk Factors included in this report, and the discussion of legal proceedings related to certain patents described above is set forth in Note 21, Litigation, to our consolidated financial statements included in this report.
COMPETITION
Competition in the biopharmaceutical industry and the markets in which we operate is intense. There are many companies, including biotechnology and pharmaceutical companies, engaged in developing products for the indications our approved products are approved to treat and the therapeutic areas we are targeting with our research and development activities. Some of our competitors may have substantially greater financial, marketing, research and development and other resources than we do.
We believe that competition and leadership in the industry is based on scientific, managerial and technological excellence and innovation as well as establishing patent and other proprietary positions through research and development. The achievement of a leadership position also depends largely upon our ability to maximize the approval, acceptance and use of our product candidates and the availability of adequate financial resources to fund facilities, equipment, personnel, clinical testing, manufacturing and marketing. Another key aspect of remaining competitive in the industry is recruiting and retaining leading scientists and technicians to conduct our research activities and advance our development programs, including with the regulatory and commercial expertise to effectively advance and market our products.
Competition among products approved for sale may be based, among other things, on patent position, product efficacy, safety, patient convenience, delivery devices, reliability, availability, reimbursement and price. In addition, early entry of a new pharmaceutical product into the market may have important advantages in gaining product acceptance and market share. Accordingly, the relative speed with which we can develop products, complete the testing and approval process and supply commercial quantities of products will have a significant impact on our competitive position.
The introduction of new products or technologies, including the development of new processes or technologies by competitors or new information about existing products or technologies, results in increased competition for our marketed products and pricing pressure on our marketed products. The development of new or improved treatment options or standards of care or cures for the diseases our products treat reduces and could eliminate the use of our products or may limit the utility and application of ongoing clinical trials for our product candidates.
In addition, the commercialization of certain of our own approved products, products of our collaborators and pipeline product candidates may negatively impact future sales of our existing products.
We believe our long-term competitive position depends upon our success in discovering and developing innovative, cost-effective products that serve unmet medical needs, along with our ability to manufacture products efficiently and to launch and market them effectively in a highly competitive environment.
Additional information about the competition that our marketed products face is set forth below and in Item 1A. Risk Factors included in this report.
NEUROLOGY
MULTIPLE SCLEROSIS
Our MS products and revenue streams continue to face increasing competition in many markets from the introduction of generic versions, prodrugs and biosimilars of existing products and products approved under abbreviated regulatory pathways. Such products are likely to be sold at substantially lower prices than branded products. Accordingly, the introduction of such products as well as other lower-priced competing products may significantly reduce both the price that we are able to charge for our products and the volume of products we sell, which will negatively impact our revenue. In some jurisdictions a decrease in reimbursed price is mandated by law. In addition, in some markets, when a generic or biosimilar version of one of our products is commercialized, it may be automatically substituted for our product and significantly reduce our revenue in a short period of time.
Competition in the MS market is intense. Along with us, a number of companies are working to develop additional treatments for MS that may in the future compete with our MS products. One such product that was approved in the U.S. in 2017 and in the E.U. in 2018 is OCREVUS, a treatment for RMS and PPMS that was developed by Genentech. While we have a financial interest in OCREVUS, future sales of our MS products may be adversely affected if OCREVUS continues to gain market share, or if other MS products that we or our competitors are developing are commercialized.
TECFIDERA, AVONEX, PLEGRIDY, TYSABRI and VUMERITY each compete with one or more of the following branded products as well as generic and biosimilar versions of some of these products:
| | | | | | | | |
| Competing Product | | Competitor |
| AUBAGIO (teriflunomide) | | Sanofi Genzyme |
| BAFIERTAM (monomethyl fumarate) | | Banner Life Sciences |
| BETASERON/BETAFERON (interferon-beta-1b) | | Bayer Group |
| BRIUMVI (ublituximab-xiiy) | | TG Therapeutics, Inc. |
| COPAXONE (glatiramer acetate) | | Teva Pharmaceuticals Industries Ltd. |
| EXTAVIA (interferon-beta-1b) | | Novartis AG |
| GILENYA (fingolimod) | | Novartis AG |
| GLATOPA (glatiramer acetate) | | Sandoz, a division of Novartis AG |
| KESIMPTA (ofatumumab) | | Novartis AG |
| LEMTRADA (alemtuzumab) | | Sanofi Genzyme |
| MAVENCLAD (cladribine) | | EMD Serono |
| MAYZENT (siponimod) | | Novartis AG |
| OCREVUS (ocrelizumab) | | Genentech |
| PONVORY (ponesimod) | | Janssen Pharmaceutical Companies of Johnson & Johnson |
| REBIF (interferon-beta-1) | | EMD Serono |
| TYRUKO (natalizumab-sztn) | | Sandoz, a division of Novartis AG |
| ZEPOSIA (ozanimod) | | Bristol Myers Squibb Company |
Multiple TECFIDERA generic entrants are now in North America, Brazil and certain E.U. countries and have deeply discounted prices compared to TECFIDERA.
Following a favorable March 2023 decision of the CJEU affirming TECFIDERA's right to regulatory data and marketing protection and the EC determination in May 2023 that TECFIDERA is entitled to an additional year of market protection for its pediatric indication, we believe that TECFIDERA is entitled to regulatory marketing protection in the E.U. until at least February 2, 2025, and are seeking to enforce this protection. In December 2023, the EC revoked all centralized marketing authorizations for generic versions of TECFIDERA. As of December 31, 2023, some of the TECFIDERA generics have not yet fully exited some E.U. markets and we expect removal of all generics from the market will take additional time. We are closely monitoring this situation and working to enforce our legal right to market protection. In addition, we will continue to enforce our EP 2 653 873 patent related to TECFIDERA, which expires in 2028.
The generic competition for TECFIDERA has significantly reduced our TECFIDERA revenue and we expect that TECFIDERA revenue will continue to decline in the future.
We are also aware of a biosimilar entrant of TYSABRI that was approved in the U.S. in August 2023 and the E.U. in September 2023. We believe that future sales of TYSABRI may be adversely affected by the entrance of this biosimilar.
For additional information on the U.S. patent litigation related to a TYSABRI biosimilar, please read Note 21, Litigation, to our consolidated financial statements included in this report.
ALZHEIMER'S DISEASE
The market for the treatment of Alzheimer's disease is undeveloped and could be subject to rapid change in the future. Most current treatments are symptomatic or intended to improve quality of life. Along with us, several companies are working to develop additional treatments. Most recently, we codeveloped LEQEMBI, a treatment to address a defining pathology of Alzheimer's disease and we and our collaborator Eisai are in the process of launching this product. We are aware of other products now in development that, if approved, may also compete with LEQEMBI.
RARE DISEASE
SPINAL MUSCULAR ATROPHY
We face competition from a gene therapy product ZOLGENSMA (onasemnogene abeparvovec-xioi) and an oral product EVRYSDI (risdiplam). We expect that we will experience competition from both products in additional jurisdictions in the future, which may adversely affect our sales of SPINRAZA.
Additionally, we are aware of other products now in development that, if launched, may also compete with SPINRAZA. Future sales of SPINRAZA may be adversely affected by the commercialization of competing products.
FRIEDREICH'S ATAXIA
SKYCLARYS is the first treatment on the market for this indication and could face future competition from pipeline programs under development.
BIOSIMILARS
BENEPALI, IMRALDI and FLIXABI, the three biosimilar products we currently commercialize in certain countries in Europe pursuant to an agreement with Samsung Bioepis, compete with their reference products, ENBREL, HUMIRA and REMICADE, respectively, as well as other biosimilars of those reference products.
BYOOVIZ, a biosimilar product we currently commercialize in the U.S. and certain international markets pursuant to an agreement with Samsung Bioepis, competes with its reference product LUCENTIS, as well as other biosimilars of this reference product.
GENENTECH RELATIONSHIPS IN OTHER INDICATIONS
RITUXAN, RITUXAN HYCELA, GAZYVA and LUNSUMIO in Oncology
RITUXAN, RITUXAN HYCELA, GAZYVA and LUNSUMIO compete with a number of therapies in the oncology market, including TREANDA (bendamustine HCL), ARZERRA (ofatumumab), IMBRUVICA (ibrutinib) and ZYDELIG (idelalisib) and other new innovative oncological therapies.
Biosimilar products referencing RITUXAN have launched in the U.S and are being offered at lower prices. This competition has had a significant adverse impact on the pre-tax profits of our collaboration arrangements with Genentech, as the sales of RITUXAN have decreased substantially compared to prior periods. We expect that biosimilar competition will continue to increase as these products capture additional market share and that this will have a significant adverse impact on our co-promotion profits in the U.S. in future years.
RITUXAN in Rheumatoid Arthritis
RITUXAN competes with several different types of therapies in the rheumatoid arthritis market, including, among others, traditional disease-modifying anti-rheumatic drugs such as steroids, methotrexate and cyclosporine, TNF inhibitors, ORENCIA (abatacept), ACTEMRA (tocilizumab) and XELJANZ (tofacitinib) and biosimilar versions of RITUXAN.
We are also aware of other products, including biosimilars, in development that, if approved, may compete with RITUXAN in the rheumatoid arthritis market.
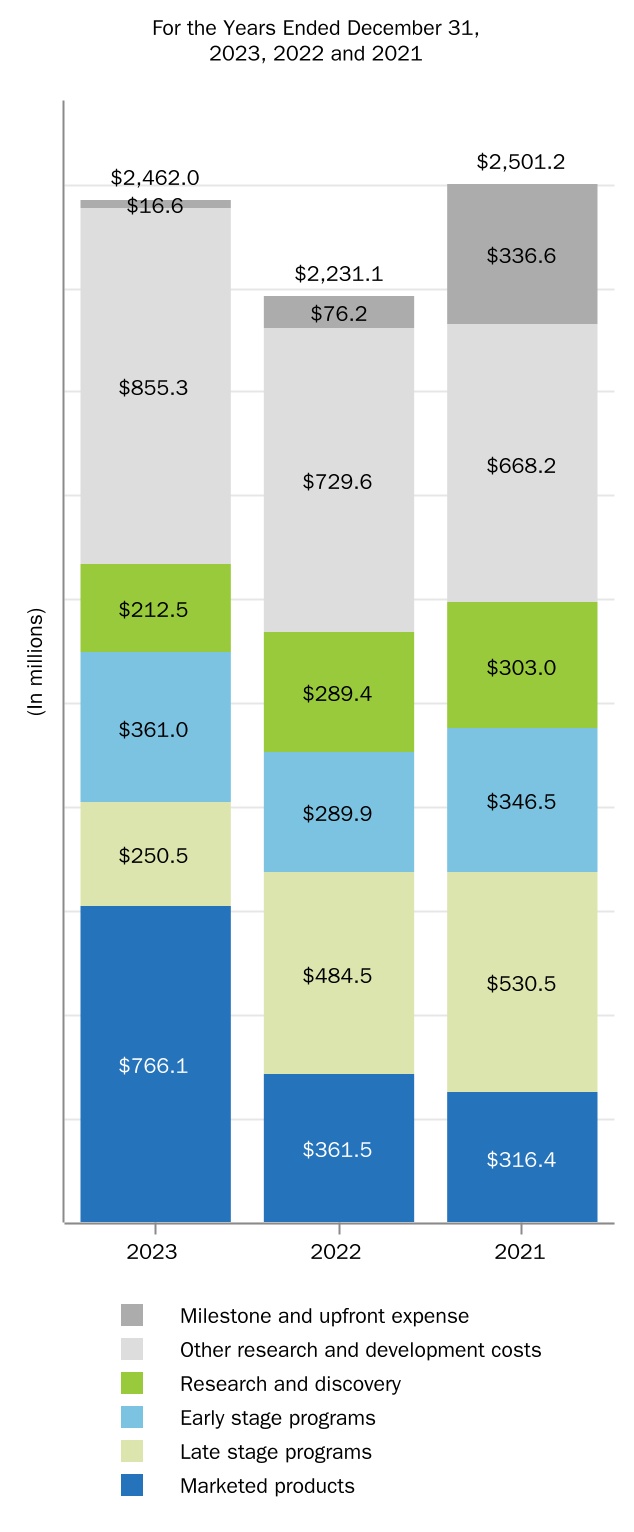
RESEARCH AND DEVELOPMENT PROGRAMS
A commitment to research is fundamental to our mission. Our research efforts are focused on better understanding the underlying biology of diseases so we can discover and deliver treatments that have the potential to make a real difference in the lives of patients with high unmet medical needs. By applying our expertise in biologics and our capabilities in small molecule, antisense, gene therapy and other technologies, we target specific medical needs where we believe new or better treatments are needed.
We intend to continue committing significant resources to targeted research and development opportunities where there is a significant unmet need and where a drug candidate has the potential to be highly differentiated. As part of our ongoing research and development efforts, we have devoted significant resources to conducting clinical studies to advance the development of new pharmaceutical products and technologies and to explore the utility of our existing products in treating disorders beyond those currently approved in their labels.
For additional information on our research and development expense included in our consolidated statements of income, please read Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations included in this report.
The table below highlights our current research and development programs that are in clinical trials and the current phase of such programs. Drug development involves a high degree of risk and investment, and the status, timing and scope of our development programs are subject to change. Important factors that could adversely affect our drug development efforts are discussed in Item 1A. Risk Factors included in this report.
| | | | | | | | | | | | | | | | | | | | | | | |
Alzheimer's Disease and
Dementia | | Lecanemab (Aβ mAb)(1)(2) - Alzheimer's | | Filed in the E.U. and Other Markets |
| | | | | | |
| Lecanemab (Aβ mAb)(1) - Preclinical Alzheimer's | | Phase 3 |
| | | | | | |
| BIIB080 (tau ASO)(1) - Alzheimer's | | Phase 2 | |
| | | | | | |
| BIIB113 (OGA inhibitor) - Alzheimer's | | Phase 1 | | | |
| | | | | | | |
| Neuropsychiatry | | Zuranolone (GABAA PAM)(1)(4) - MDD | | Phase 3 |
| | | | | | |
| Zuranolone (GABAA PAM)(1) - PPD | | Approved in the U.S. |
| | | | | | | |
| Specialized Immunology | | Dapirolizumab pegol (anti-CD40L)(1) - SLE | | Phase 3 |
| | | | | | |
| Litifilimab (anti-BDCA2) - SLE | | Phase 3 |
| | | | | | |
| Litifilimab (anti-BDCA2) - CLE | | Phase 2/3 |
| | | | | | | |
| Neuromuscular Disorders | | Omaveloxolone (Nrf2 activator) - FA | | Approved in the U.S. and the E.U. |
| | | | | | |
| Tofersen (SOD1 ASO)(1)(3) - SOD1 ALS | | Approved in the U.S.; Filed in the E.U. |
| | | | | | |
| BIIB105 (ataxin-2 ASO)# - ALS | | Phase 1b | | | |
| | | | | | |
| BIIB115 (SMN ASO)(1) - SMA | | Phase 1b | | | |
| | | | | | | |
Parkinson's and
Movement Disorders | | BIIB122 (LRRK2 inhibitor)(1) - Parkinson's | | Phase 2 | |
| | | | | | |
| BIIB124 (GABAA PAM)(1) - Essential Tremor | | Phase 2 | |
| | | | | | |
| BIIB094 (LRRK2 ASO)# - Parkinson's | | Phase 1b | | | |
| | | | | | |
| BIIB101 (a-syn ASO)# - Multiple System Atrophy | | Phase 1b | | | |
| | | | | | | |
| Multiple Sclerosis | | BIIB091 (peripheral BTK inhibitor) - MS | | Phase 2 | |
| | | | | | |
| BIIB107 (anti-VLA4) - MS | | Phase 1 | | | |
| | | | | | | |
| Genetic Neurodevelopmental Disorders | | BIIB121 (UBE3A ASO)# - Angelman Syndrome | | Phase 1b | | | |
| | | | | | | |
| Neuropathic Pain | | Cemdomespib (Hsp90 modulator) - DPNP | | Phase 2 | |
|
|
| |
|
|
|
|
| Education |
| l | Wellesley College, B.A. |
| l | Boston University School of Law, J.D. |
| | | | | |
| Michael R. McDonnell |
| Experience |
Mr. McDonnell has served as our Executive Vice President and Chief Financial Officer since August 2020. Prior to joining Biogen, Mr. McDonnell served as Executive Vice President and Chief Financial Officer of IQVIA Holdings Inc., a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry, from December 2015 until July 2020. Prior to that, Mr. McDonnell served as the Executive Vice President and Chief Financial Officer of Intelsat, a leading global provider of satellite services, from November 2008 to December 2015, as Executive Vice President and Chief Financial Officer of MCG Capital Corporation, a publicly-held commercial finance company, from September 2004 until October 2008 and as MCG Capital Corporation’s Chief Operating Officer from August 2006 until October 2008. Before joining MCG Capital Corporation, Mr. McDonnell served as Executive Vice President and Chief Financial Officer for EchoStar Communications Corporation (f/k/a DISH Network Corporation), a direct-to-home satellite television operator, from July 2004 until August 2004 and as its Senior Vice President and Chief Financial Officer from August 2000 to July 2004. Mr. McDonnell spent 14 years at PricewaterhouseCoopers LLP, including 4 years as a partner. Mr. McDonnell is a licensed certified public accountant (CPA). |
| Public Company Boards |
| l | Merit Medical Systems, Inc. |
| Education |
| l | Georgetown University, B.S. Accounting |
| | | | | |
| Nicole Murphy |
| Experience |
| Ms. Murphy has served as our Executive Vice President, Pharmaceutical Operations and Technology since February 2022. Prior to that, Ms. Murphy has held senior executive positions at Biogen, including most recently as our Senior Vice President, Head of Global Manufacturing & Technical Operations, from June 2019 to January 2022. In 2017, Ms. Murphy played a critical role during the successful spin-off of Biogen's hemophilia franchise, as the Vice President and Head of Technical Operations of Bioverativ responsible for clinical and commercial development, quality, regulatory, manufacturing and procurement. Prior to the spin-off Ms. Murphy was the General Manager and Head of Cambridge Site Operations at Biogen from May 2015 to December 2016. Prior to joining Biogen, Ms. Murphy was Executive Director, Head of Supply Chain at Amgen, a biopharmaceutical company, where her responsibilities included leadership of commercial manufacturing and technical operations. Ms. Murphy also held numerous technical and operational roles during her time at Amgen from 2001 to 2015 where she contributed significantly to various facility start-ups, business development integrations, strategic transformations and new product introductions. Prior to Amgen, Ms. Murphy held a variety of process development and engineering positions at Immunex Pharmaceuticals and the Monsanto Company. |
| Education |
| l | University of Massachusetts Amherst, B.S. Engineering |
| l | Rensselaer Polytechnic Institute, M.S. Engineering and a Masters of Business Administration |
| | | | | |
| Ginger Gregory, Ph.D. |
| Experience |
| Dr. Gregory has served as our Executive Vice President and Chief Human Resources Officer since July 2017. Prior to joining Biogen, Dr. Gregory served as Executive Vice President and Chief Human Resources Officer at Shire PLC, a global specialty biopharmaceutical company, from February 2014 to April 2017. Prior to that, Dr. Gregory held executive-level human resources positions for several multinational companies across a variety of industries, including Dunkin’ Brands Group Inc., a restaurant holding company, where she served as Chief Human Resource Officer, Novartis AG, a pharmaceutical company, where she was the division head of Human Resources for Novartis Vaccines and Diagnostics, Novartis Consumer Health and Novartis Institutes of BioMedical Research and Novo Nordisk A/S, a pharmaceutical company, where she served as Senior Vice President, Corporate People & Organization at the company’s headquarters in Copenhagen, Denmark. Earlier in her career, Dr. Gregory held a variety of human resources generalist and specialist positions at BMS, a pharmaceutical company, and served as a consultant with Booz Allen & Hamilton, an information technology consulting company, in the area of organization change and effectiveness. |
| Education |
| l | University of Massachusetts, B.A. Psychology |
| l | The George Washington University, Ph.D. Psychology |
| | | | | |
| Rachid Izzar |
| Experience |
Mr. Izzar has served as our Executive Vice President, Head of Global Product Strategy and Commercialization since July 2021. Prior to that Mr. Izzar served as our President for the Intercontinental Region, which includes Latin America, Australia, Asia, Japan, the Middle East and Africa, Turkey and Russia, and the Global Biogen Biosimilars Unit. Prior to joining Biogen, Mr. Izzar was a Country President for AstraZeneca in France, where his responsibilities included leadership for commercial and manufacturing operations. He held numerous roles at his time with AstraZeneca, including the position of Global Vice President of the Cardiovascular Franchise where he contributed significantly to the development of the franchise within the North American subsidiary, as well as in Europe and China. Prior to that, Mr. Izzar was Vice President Strategic Transformation, also, China Portfolio for CEO based in Shanghai and Vice President Commercial International covering China, Australia, Brazil, Russia, America Latin, Asia, Turkey, the Middle East and Africa. |
| Education |
| l | University of Sherbrooke, Masters of Business Administration |
| l | Harvard Business School, Enterprise Executive Transformation Program |
|
|
| | | | | |
| Priya Singhal, M.D., M.P.H. |
| Experience |
Dr. Singhal has served as our Executive Vice President and Head of Development since January 2023. Prior to that Dr. Singhal served as our Interim Head of Research and Development since 2021 in addition to serving as Head of Global Safety and Regulatory Sciences, including China and Japan Research and Development, since rejoining Biogen in 2020. Dr. Singhal was initially at Biogen from 2012 to 2018 and served in positions of increasing seniority as Vice President Clinical Trials Benefit-Risk Management, Global Head of Safety and Benefit Risk Management and as the Interim Co-lead and Senior Vice President of Global Development. Prior to her 2020 return to Biogen, Dr. Singhal served as Head of Research and Development and Manufacturing at Zafgen Inc. from 2019 to 2020. From 2008 to 2012 Dr. Singhal held roles at Vertex Pharmaceuticals, including Vice President, Medical Affairs. Dr. Singhal began her drug-development career at Millennium Pharmaceuticals, Inc. in 2005 and led benefit-risk management for Velcade and other compounds. |
| Education |
| l | Harvard School of Public Health, M.P.H. in International Health |
| l | University of Mumbai, Doctor of Medicine (M.D.) |
|
|
| | | | | |
| Jane Grogan, Ph.D. |
| Experience |
Dr. Grogan has served as our Executive Vice President and Head of Development since September 2023. Dr. Grogan most recently served as the Chief Scientific Officer at Graphite Bio from 2021 to 2023 and ArsenalBio from 2019 to 2021, both cell and gene therapy companies. From 2004 to 2019 Dr. Grogan held several roles in increasing seniority at Genentech across Immunology and Immuno-oncology, covering research strategies and drug development across Rheumatoid Arthritis, Lupus, MS, Inflammatory Bowel Disease and Cancer. |
| Education |
| l | Leiden University, Ph.D. in Immunology |
| l | University of Melbourne, B.Sc in Biochemistry and Pharmacology |
|
|
| | | | | |
| Adam Keeney, Ph.D. |
| Experience |
Dr. Keeney has served as our Executive Vice President and Head of Corporate Development since April 2023. Dr. Keeney brings more than 20 years of experience leading R&D, business development and strategy organizations at industry-leading companies within biotech and large pharma, Dr. Keeney most recently served as the Chief Executive Officer of NodThera, a clinical stage biotech company focused on chronic inflammation from 2018 to 2022. Prior to NodThera, Dr. Keeney was at Sanofi from 2014 to 2018 where he had responsibility for all of Sanofi Gezyme's business development activities, including early- and late-stage deals across therapeutic areas and modalities, successfully completing several significant transactions. From 2004 to 2013 Dr. Keeney worked at Johnson & Johnson where he held a number of business development roles with increasing responsibility and started his career at Lundbeck as a discovery scientist. |
| Education |
| l | University of Nottingham, UK, Ph.D. in Neuropharmacology |
| l | University of Leeds, UK, BSc (Hons) |
|
|
| | | | | |
| Robin C. Kramer |
| Experience |
| Ms. Kramer has served as our Senior Vice President, Chief Accounting Officer since December 2020. Prior to that, Ms. Kramer served as our Vice President, Chief Accounting Officer from November 2018 to December 2020. Prior to joining Biogen, Ms. Kramer served as the Senior Vice President and Chief Accounting Officer of Hertz Global Holdings, Inc., a car rental company, from May 2014 to November 2018. Prior to that, Ms. Kramer was an audit partner at Deloitte & Touche LLP (Deloitte), a professional services firm, from 2007 to 2014, including serving in Deloitte's National Office Accounting Standards and Communications Group from 2007 to 2010. From 2005 to 2007 Ms. Kramer served as Chief Accounting Officer of Fisher Scientific International, Inc., a laboratory supply and biotechnology company, and from 2004 to 2005 Ms. Kramer served as Director, External Reporting, Accounting and Control for the Gillette Company, a personal care company. Ms. Kramer also held partner positions in the public accounting firms of Ernst & Young LLP and Arthur Andersen LLP. Ms. Kramer is a licensed CPA in Massachusetts. She is a member of the Massachusetts Society of CPAs and the American Institute of CPAs. Ms. Kramer currently serves on the board of directors of the Center for Women and Enterprise. Ms. Kramer previously served as a Board Member for the Massachusetts State Board of Accountancy from September 2011 to December 2015 and Probus Insurance Company Europe DAC from 2016 to 2018. |
| Public Company Boards |
| l | Armata Pharmaceuticals, Inc., a biotechnology company |
| Education |
| l | Salem State University, B.B.A. Accounting |
AVAILABLE INFORMATION
Our principal executive offices are located at 225 Binney Street, Cambridge, MA 02142 and our telephone number is (617) 679-2000. Our website address is www.biogen.com. We make available free of charge through the Investors section of our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. We include our website address in this report only as an inactive textual reference and do not intend it to be an active link to our website. The contents of our website are not incorporated into this report.
USE OF WEBSITE TO PROVIDE INFORMATION
From time to time, we have used, and expect in the future to use, our website as a means of disclosing material information to the public in a broad, non-exclusionary manner, including for purposes of the SEC’s Regulation Fair Disclosure (Reg FD). Financial and other material information regarding the Company is routinely posted on our website and accessible at www.biogen.com. In order to receive notifications regarding new postings to our website, investors are encouraged to enroll on our website to receive automatic email alerts. None of the information on our website is incorporated into this report.
ITEM 1A. RISK FACTORS
Risks Related to Our Business
We are substantially dependent on revenue from our products.
Our revenue depends upon continued sales of our products as well as the financial rights we have in our anti-CD20 therapeutic programs. A significant portion of our revenue is concentrated on sales of our products in increasingly competitive markets. Any of the following negative developments relating to any of our products or any of our anti-CD20 therapeutic programs may adversely affect our revenue and results of operations or could cause a decline in our stock price:
•the introduction, greater acceptance or more favorable reimbursement of competing products, including new originator therapies, generics, prodrugs and biosimilars of existing products and products approved under abbreviated regulatory pathways;
•safety or efficacy issues;
•limitations and additional pressures on product pricing or price increases, including those relating to inflation and those resulting from governmental or regulatory requirements, including those relating to any future potential drug price negotiation under the IRA; increased competition, including from generic or biosimilar versions of our products; or changes in, or implementation of, reimbursement policies and practices of payors and other third-parties;
•adverse legal, administrative, geopolitical events, regulatory or legislative developments; or
•our ability to maintain a positive reputation among patients, healthcare providers and others, which may be impacted by our pricing and reimbursement decisions.
LEQEMBI and SKYCLARYS are in the early stages of commercial launch in the U.S. In addition to risks associated with new product launches and the other factors described in these Risk Factors, Biogen’s and Eisai’s ability to successfully commercialize LEQEMBI and our ability to successfully commercialize SKYCLARYS may be adversely affected due to:
•Eisai’s ability to obtain and maintain adequate reimbursement for LEQEMBI;
•the effectiveness of Eisai's and Biogen’s commercial strategy for marketing LEQEMBI;
•requirements such as participation in a registry and the use of imaging or other diagnostics for LEQEMBI;
•our ability to obtain approval in other markets;
•the approval of other new products for the same or similar indications;
•Eisai’s and Biogen’s ability to maintain a positive reputation among patients, healthcare providers and others in the Alzheimer’s disease community, which may be impacted by pricing and reimbursement decisions relating to LEQEMBI, which are made by Eisai;
•Biogen's ability to obtain and maintain adequate reimbursement for SKYCLARYS; and
•the effectiveness of Biogen's commercial strategy for marketing SKYCLARYS.
Our long-term success depends upon the successful development of new products and additional indications for our existing products.
Our long-term success will depend upon the successful development of new products from our research and development activities or our licenses or acquisitions from third-parties, as well as additional indications for our existing products.
Product development is very expensive and involves a high degree of uncertainty and risk and may not be successful. Only a small number of research and development programs result in the commercialization of a product. It is difficult to predict the success and the time and cost of product development of novel approaches for the treatment of diseases. The development of novel approaches for the treatment of diseases, including development efforts in new modalities such as those based on the antisense oligonucleotide platform and gene therapy, may present additional challenges and risks, including obtaining approval from regulatory authorities that have limited experience with the development of such therapies. For example, we are currently seeking approval of SKYCLARYS in Europe and any delays or challenges regarding its approval in Europe may adversely impact our ability to realize the anticipated benefits from the Reata acquisition.
Clinical trial data are subject to differing interpretations and even if we view data as sufficient to support the safety, effectiveness and/or approval of an investigational therapy, regulatory authorities may disagree and may require additional data, limit the scope of the approval or deny approval altogether. Furthermore, the approval of a product candidate by one regulatory agency does not mean that other regulatory agencies will also approve such product candidate.
Success in preclinical work or early-stage clinical trials does not ensure that later stage or larger scale clinical trials will be successful. Clinical trials may indicate that our product candidates lack efficacy, have harmful side effects, result in unexpected adverse events or raise other concerns that may significantly reduce or delay the likelihood of regulatory approval. This may result in terminated programs, significant restrictions on use and safety warnings in an approved label, adverse placement within the treatment paradigm or significant reduction in the commercial potential of the product candidate.
Even if we could successfully develop new products or indications, we may make a strategic decision to discontinue development of a product candidate or indication if, for example, we believe commercialization will be difficult relative to the standard of care or we prioritize other opportunities in our pipeline.
Sales of new products or products with additional indications may not meet investor expectations.
If we fail to compete effectively, our business and market position would suffer.
The biopharmaceutical industry and the markets in which we operate are intensely competitive. We compete in the marketing and sale of our products, the development of new products and processes, the acquisition of rights to new products with commercial potential and the hiring and retention of personnel. We compete with biotechnology and pharmaceutical companies that have a greater number of products on the market and in the product pipeline, substantially greater financial, marketing, research and development and other resources and other technological or competitive advantages.
Our products continue to face increasing competition from the introduction of new originator therapies, generics, prodrugs and biosimilars of existing products and products approved under abbreviated regulatory pathways. Some of these products are likely to be sold at substantially lower prices than our branded products. The introduction of such products as well as other lower-priced competing products has reduced, and may in the future, significantly reduce both the price that we are able to charge for our products and the volume of products we sell, which will negatively impact our revenue. For instance, demand and price for TECFIDERA declined significantly as a result of multiple TECFIDERA generic entrants entering the U.S. market in 2020. In addition, in some markets, when a generic or biosimilar version of one of our products is commercialized, it may be automatically substituted for our product and significantly reduce our revenue in a short period of time.
Our ability to compete, maintain and grow our business may also be adversely affected due to a number of factors, including:
•the introduction of other products, including products that may be more efficacious, safer, less expensive or more convenient alternatives to our products, including our own products and products of our collaborators;
•the off-label use by physicians of therapies indicated for other conditions to treat patients;
•patient dynamics, including the size of the patient population and our ability to identify, attract and maintain new and current patients to our therapies;
•the reluctance of physicians to prescribe, and patients to use, our products without additional data on the efficacy and safety of such products;
•damage to physician and patient confidence in any of our products, generic or biosimilars of our products or any other product from the same class as one of our products, or to our sales and reputation as a result of label changes, pricing and reimbursement decisions or adverse experiences or events that may occur with patients treated with our products or generic or biosimilars of our products;
•inability to obtain and maintain appropriate pricing and adequate reimbursement for our products compared to our competitors in key markets; or
•our ability to obtain and maintain patent, data or market exclusivity for our products.
Our business may be adversely affected if we do not successfully execute or realize the anticipated benefits of our strategic and growth initiatives.
The successful execution of our strategic and growth initiatives may depend upon internal development projects, commercial initiatives and external opportunities, which may include the acquisition and in-licensing of products,
technologies, companies, the entry into strategic alliances and collaborations or our Fit for Growth program, as well as our ability to execute on previously-announced initiatives such as the exploration of strategic options for our biosimilars business.
While we believe we have a number of promising programs in our pipeline, failure or delay of internal development projects to advance or difficulties in executing on our commercial initiatives could impact our current and future growth, resulting in additional reliance on external development opportunities for growth.
Supporting the further development of our existing products and potential new products in our pipeline will require significant capital expenditures and management resources, including investments in research and development, sales and marketing, manufacturing capabilities and other areas of our business. We have made, and may continue to make, significant operating and capital expenditures for potential new products prior to regulatory approval with no assurance that such investment will be recouped, which may adversely affect our financial condition, business and operations.
The availability of high quality, fairly valued external product development is limited and the opportunity for their acquisition is highly competitive. As such, we are not certain that we will be able to identify suitable candidates for acquisition or if we will be able to reach agreement to make any such acquisition if suitable candidates are identified.
We may fail to initiate or complete transactions for many reasons, including failure to obtain regulatory or other approvals as well as a result of disputes or litigation. Furthermore, we may not be able to achieve the full strategic and financial benefits expected to result from transactions, or the benefits may be delayed or not occur at all. We may also face additional costs or liabilities in completed transactions that were not contemplated prior to completion.
Any failure in the execution of a transaction, in the integration of an acquired asset or business or in achieving expected synergies could result in slower growth, higher than expected costs, the recording of asset impairment charges and other actions which could adversely affect our business, financial condition and results of operations. For example, we recently acquired Reata and are in the process of integrating Reata into our Company. The ultimate success of our acquisition of Reata and our ability to realize the anticipated benefits from the acquisition, including the SKYCLARYS product and anticipated synergies, depends on, among other things, how effective we are in integrating the Biogen and Reata operations.
We face risks associated with our Fit for Growth program that may impair our ability to achieve anticipated savings and operational efficiencies or that may otherwise harm our business. These risks include delays in implementation of cost optimization actions, loss of workforce capabilities, higher than anticipated separation expenses, litigation and the failure to meet financial and operational targets. In addition, the calculation of the anticipated cost savings and other benefits resulting from our Fit for Growth program are subject to many estimates and assumptions. These estimates and assumptions are subject to significant business, economic, competitive and other uncertainties and contingencies, many of which are beyond our control. if these estimates and assumptions are incorrect or if we experience delays or unforeseen events, our business and financial results could be adversely affected.
Sales of our products depend, to a significant extent, on adequate coverage, pricing and reimbursement from third-party payors, which are subject to increasing and intense pressure from political, social, competitive and other sources. Our inability to obtain and maintain adequate coverage, or a reduction in pricing or reimbursement, could have an adverse effect on our business, reputation, revenue and results of operations.
Sales of our products depend, to a significant extent, on adequate coverage, pricing and reimbursement from third-party payors. When a new pharmaceutical product is approved, the availability of government and private reimbursement for that product, diagnosis of the condition it treats and the cost to administer it may be uncertain, as is the pricing and amount for which that product will be reimbursed.
Pricing and reimbursement for our products may be adversely affected by a number of factors, including:
•changes in, and implementation of, federal, state or foreign government regulations or private third-party payors’ reimbursement policies;
•pressure by employers on private health insurance plans to reduce costs;
•consolidation and increasing assertiveness of payors seeking price discounts or rebates in connection with the placement of our products on their formularies and, in some cases, the imposition of restrictions on access or coverage of particular drugs or pricing determined based on perceived value;
•our ability to receive reimbursement for our products or our ability to receive comparable reimbursement to that of competing products; and
•our value-based contracting program pursuant to which we aim to tie the pricing of our products to their clinical values by either aligning price to patient outcomes or adjusting price for patients who discontinue therapy for any reason, including efficacy or tolerability concerns.
Our ability to set the price for our products varies significantly from country to country and, as a result, so can the price of our products. Governments may use a variety of cost-containment measures to control the cost of products, including price cuts, mandatory rebates, value-based pricing and reference pricing (i.e., referencing prices in other countries and using those reference prices to set a price). Drug prices are under significant scrutiny in the markets in which our products are prescribed; for example the IRA has certain provisions related to drug pricing. We expect drug pricing and other health care costs to continue to be subject to intense political and societal pressures on a global basis. Certain countries set prices by reference to the prices in other countries where our products are marketed. Our inability to obtain and maintain adequate prices in a particular country may not only limit the revenue from our products within that country but may also adversely affect our ability to secure acceptable prices in existing and potential new markets, which may limit market growth and result in reductions in revenue. This may create the opportunity for third-party cross-border trade or influence our decision to sell or not to sell a product, thus adversely affecting our geographic expansion plans and revenue. Additionally, in certain jurisdictions governmental health agencies may adjust, retroactively and/or prospectively, reimbursement rates for our products. Reimbursement for our products by governments, including the timing of any reimbursements, may also be affected by budgetary or political constraints, particularly in challenging economic environments. Government agencies often do not set their own budgets and therefore, have limited control over the amount of money they can spend. In addition, these agencies experience political pressure that may dictate the manner in which they spend money. There can be no assurance that the economic, budgeting or political issues will not worsen and adversely impact sales or reimbursements of our products.
Competition from current and future competitors may negatively impact our ability to maintain pricing and our market share. New products marketed by our competitors could cause our revenue to decrease due to potential price reductions and lower sales volumes. Additionally, the introduction of generic or biosimilar versions of our products, follow-on products, prodrugs or products approved under abbreviated regulatory pathways may significantly reduce the price that we are able to charge for our products and the volume of products we sell.
Many payors continue to adopt benefit plan changes that shift a greater portion of prescription costs to patients, including more limited benefit plan designs, higher patient co-pay or co-insurance obligations and limitations on patients' use of commercial manufacturer co-pay payment assistance programs (including through co-pay accumulator adjustment or maximization programs). Significant consolidation in the health insurance industry has resulted in a few large insurers and pharmacy benefit managers exerting greater pressure in pricing and usage negotiations with drug manufacturers, significantly increasing discounts and rebates required of manufacturers and limiting patient access and usage. Further consolidation among insurers, pharmacy benefit managers and other payors would increase the negotiating leverage such entities have over us and other drug manufacturers. Additional discounts, rebates, coverage or plan changes, restrictions or exclusions as described above could have a material adverse effect on sales of our affected products.
Our failure to obtain or maintain adequate coverage, pricing or reimbursement for our products could have an adverse effect on our business, reputation, revenue and results of operations.
We depend on relationships with collaborators and other third-parties for revenue, and for the development, regulatory approval, commercialization and marketing of certain of our products and product candidates, which are outside of our full control.
We rely on a number of collaborative and other third-party relationships for revenue and the development, regulatory approval, commercialization and marketing of certain of our products and product candidates. We also outsource certain aspects of our regulatory affairs and clinical development relating to our products and product candidates to third-parties. Reliance on third-parties subjects us to a number of risks, including:
•we may be unable to control the resources our collaborators or third-parties devote to our programs, products or product candidates, which may affect our ability to achieve development goals or milestones;
•disputes may arise under an agreement, including with respect to the achievement and payment of milestones, payment of development or commercial costs, ownership of rights to technology developed, and the underlying agreement may fail to provide us with significant protection or may fail to be effectively enforced if the collaborators or third-parties fail to perform;
•the interests of our collaborators or third-parties may not always be aligned with our interests, and such parties may not pursue regulatory approvals or market a product in the same manner or to the same extent
that we would, which could adversely affect our revenue, or may adopt tax strategies that could have an adverse effect on our business, results of operations or financial condition;
•third-party relationships require the parties to cooperate, and failure to do so effectively could adversely affect product sales or the clinical development or regulatory approvals of product candidates under joint control, could result in termination of the research, development or commercialization of product candidates or could result in litigation or arbitration;
•any failure on the part of our collaborators or third-parties to comply with applicable laws, including tax laws, regulatory requirements and/or applicable contractual obligations or to fulfill any responsibilities they may have to protect and enforce any intellectual property rights underlying our products could have an adverse effect on our revenue or reputation as well as involve us in possible legal proceedings; and
•any improper conduct or actions on the part of our collaborators or third-parties could subject us to civil or criminal investigations and monetary and injunctive penalties, require management attention, impact the accuracy and timing of our financial reporting and/or adversely impact our ability to conduct business, our operating results and our reputation.
Given these risks, there is considerable uncertainty regarding the success of our current and future collaborative efforts. If these efforts fail, our product development or commercialization of new products could be delayed, revenue from products could decline and/or we may not realize the anticipated benefits of these arrangements.
Our results of operations may be adversely affected by current and potential future healthcare reforms.
In the U.S., federal and state legislatures, health agencies and third-party payors continue to focus on containing the cost of health care. Legislative and regulatory proposals, enactments to reform health care insurance programs (including those contained in the IRA) and increasing pressure from social sources could significantly influence the manner in which our products are prescribed, purchased and reimbursed. For example, provisions of the PPACA have resulted in changes in the way health care is paid for by both governmental and private insurers, including increased rebates owed by manufacturers under the Medicaid Drug Rebate Program, annual fees and taxes on manufacturers of certain branded prescription drugs, the requirement that manufacturers participate in a discount program for certain outpatient drugs under Medicare Part D and the expansion of the number of hospitals eligible for discounts under Section 340B of the Public Health Service Act. These changes have had and are expected to continue to have a significant impact on our business.
We may face uncertainties as a result of efforts to repeal, substantially modify or invalidate some or all of the provisions of the PPACA. There is no assurance that the PPACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
There is substantial public attention on the costs of prescription drugs and we expect drug pricing and other health care costs to continue to be subject to intense political and societal pressures on a global basis. In addition, there have been (including elements of the IRA), and are expected to continue to be, legislative proposals to address prescription drug pricing. Some of these proposals could have significant effects on our business, including an executive order issued in September 2020 to test a “most favored nation” model for Part B and Part D drugs that tie reimbursement rates to international drug pricing metrics. These actions and the uncertainty about the future of the PPACA and healthcare laws may put downward pressure on pharmaceutical pricing and increase our regulatory burdens and operating costs.
There is also significant economic pressure on state budgets, that may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for our drugs. In recent years, some states have considered legislation and ballot initiatives that would control the prices of drugs, including laws to allow importation of pharmaceutical products from lower cost jurisdictions outside the U.S. and laws intended to impose price controls on state drug purchases. State Medicaid programs are requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Government efforts to reduce Medicaid expense may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding limitation on prices and reimbursement for our products.
In the E.U. and some other international markets, the government provides health care at low cost to consumers and regulates pharmaceutical prices, patient eligibility or reimbursement levels to control costs for the government-sponsored health care system. Many countries have announced or implemented measures, and may in the future implement new or additional measures, to reduce health care costs to limit the overall level of government
expenditures. These measures vary by country and may include, among other things, patient access restrictions, suspensions on price increases, prospective and possible retroactive price reductions and other recoupments and increased mandatory discounts or rebates, recoveries of past price increases and greater importation of drugs from lower-cost countries. These measures have negatively impacted our revenue and may continue to adversely affect our revenue and results of operations in the future.
Our success in commercializing biosimilars is subject to risks and uncertainties inherent in the development, manufacture and commercialization of biosimilars. If we are unsuccessful in such activities, our business may be adversely affected.
The development, manufacture and commercialization of biosimilar products require specialized expertise and are very costly and subject to complex regulation. Our success in commercializing biosimilars is subject to a number of risks, including:
•Reliance on Third-Parties. We are dependent, in part, on the efforts of collaboration partners and other third-parties over whom we have limited or no control in the development and manufacturing of biosimilars products. For example, a recently announced potential acquisition of a contract development and manufacturing organization by a third party. If these third-parties fail to perform successfully, or reduce their third party manufacturing production, our biosimilar product development or commercialization of biosimilar products could be delayed, revenue from biosimilar products could decline and/or we may not realize the anticipated benefits of these arrangements;
•Regulatory Compliance. Biosimilar products may face regulatory hurdles or delays due to the evolving and uncertain regulatory and commercial pathway of biosimilars products in certain jurisdictions;
•Ability to Provide Adequate Supply. Manufacturing biosimilars is complex. If we encounter any manufacturing or supply chain difficulties we may be unable to meet demand. We are dependent on a third-party for the manufacture of our biosimilar products and such third-party may not perform its obligations in a timely and cost-effective manner or in compliance with applicable regulations and may be unable or unwilling to increase production capacity commensurate with demand for our existing or future biosimilar products;
•Intellectual Property and Regulatory Challenges. Biosimilar products may face extensive intellectual property clearances and infringement litigation, injunctions or regulatory challenges, which could prevent the commercial launch of a product or delay it for many years or result in imposition of monetary damages, penalties or other civil sanctions and damage our reputation;
•Failure to Gain Market and Patient Acceptance. Market success of biosimilar products will be adversely affected if patients, physicians and/or payors do not accept biosimilar products as safe and efficacious products offering a more competitive price or other benefit over existing therapies; and
•Competitive Challenges. Biosimilar products face significant competition, including from innovator products and biosimilar products offered by other companies that may receive greater acceptance or more favorable reimbursement. Local tendering processes may restrict biosimilar products from being marketed and sold in some jurisdictions. The number of competitors in a jurisdiction, the timing of approval and the ability to market biosimilar products successfully in a timely and cost-effective manner are additional factors that may impact our success in this business area. The decision to explore strategic options related to our biosimilars business could adversely affect our operations related to our biosimilars business.
Risks Related to Intellectual Property
If we are unable to obtain and maintain adequate protection for our data, intellectual property and other proprietary rights, our business may be harmed.
Our success, including our long-term viability and growth, depends, in part, on our ability to obtain and defend patent and other intellectual property rights, including certain regulatory forms of exclusivity, that are important to the commercialization of our products and product candidates. Patent protection and/or regulatory exclusivity in the U.S. and other important markets remains uncertain and depends, in part, upon decisions of the patent offices, courts, administrative bodies and lawmakers in these countries. We may fail to obtain, defend or preserve patent and other intellectual property rights, including certain regulatory forms of exclusivity, or the protection we obtain may not be of sufficient breadth and degree to protect our commercial interests in all countries where we conduct business, which could result in financial, business or reputational harm to us or could cause a decline or volatility in our stock price. In addition, settlements of such proceedings often result in reducing the period of exclusivity and other protections, resulting in a reduction in revenue from affected products.
In many markets, including the U.S., manufacturers may be allowed to rely on the safety and efficacy data of the innovator's product and do not need to conduct clinical trials before marketing a competing version of a product after there is no longer patent or regulatory exclusivity. In such cases, manufacturers often charge significantly lower prices and a major portion of the company's revenue may be reduced in a short period of time. In addition, manufacturers of generics and biosimilars may choose to launch or attempt to launch their products before the expiration of our patent or other intellectual property protections.
Furthermore, our products may be determined to infringe patents or other intellectual property rights held by third-parties. Legal proceedings, administrative challenges or other types of proceedings are and may in the future be necessary to determine the validity, scope or non-infringement of certain patent rights claimed by third-parties to be pertinent to the manufacture, use or sale of our products. Legal proceedings may also be necessary to determine the rights, obligations and payments claimed during and after the expiration of intellectual property license agreements we have entered with third parties. Such proceedings are unpredictable and are often protracted and expensive. Negative outcomes of such proceedings could hinder or prevent us from manufacturing and marketing our products, require us to seek a license for the infringed product or technology or result in the assessment of significant monetary damages against us that may exceed amounts, if any, accrued in our financial statements. A failure to obtain necessary licenses for an infringed product or technology could prevent us from manufacturing or selling our products. Furthermore, payments under any licenses that we are able to obtain could reduce our profits from the covered products and services. Any of these circumstances could result in financial, business or reputational harm to us or could cause a decline or volatility in our stock price.
Risks Related to Development, Clinical Testing and Regulation of Our Products and Product Candidates
Successful preclinical work or early stage clinical trials does not ensure success in later stage trials, regulatory approval or commercial viability of a product.
Positive results in a clinical trial may not be replicated in subsequent or confirmatory trials. Additionally, success in preclinical work or early stage clinical trials does not ensure that later stage or larger scale clinical trials will be successful or that regulatory approval will be obtained. Even if later stage clinical trials are successful, regulatory authorities may delay or decline approval of our product candidates. Regulatory authorities may disagree with our view of the data, require additional studies, disagree with our trial design or endpoints or not approve adequate reimbursement. Regulatory authorities may also fail to approve the facilities or processes used to manufacture a product candidate, our dosing or delivery methods or companion devices. Regulatory authorities may grant marketing approval that is more restricted than anticipated, including limiting indications to narrow patient populations and the imposition of safety monitoring, educational requirements, requiring confirmatory trials and risk evaluation and mitigation strategies. The occurrence of any of these events could result in significant costs and expense, have an adverse effect on our business, financial condition and results of operations and/or cause our stock price to decline or experience periods of volatility.
Clinical trials and the development of biopharmaceutical products is a lengthy and complex process. If we fail to adequately manage our clinical activities, our clinical trials or potential regulatory approvals may be delayed or denied.
Conducting clinical trials is a complex, time-consuming and expensive process. Our ability to complete clinical trials in a timely fashion depends on a number of key factors, including protocol design, regulatory and institutional review board approval, patient enrollment rates and compliance with current Good Clinical Practices. If we or our third-party clinical trial providers or third-party CROs do not successfully carry out these clinical activities, our clinical trials or the potential regulatory approval of a product candidate may be delayed or denied.
We have opened clinical trial sites and are enrolling patients in a number of countries where our experience is limited. In most cases, we use the services of third-parties to carry out our clinical trial related activities and rely on such parties to accurately report their results. Our reliance on third-parties for these activities may impact our ability to control the timing, conduct, expense and quality of our clinical trials. One CRO has responsibility for a substantial portion of our activities and reporting related to our clinical trials and if such CRO does not adequately perform, many of our trials may be affected, including adversely affecting our expenses associated with such trials. We may need to replace our CROs, which may result in the delay of the affected trials or otherwise adversely affect our efforts to obtain regulatory approvals and commercialize our product candidates.
Adverse safety events or restrictions on use and safety warnings for our products can negatively affect our business, product sales and stock price.
Adverse safety events involving our marketed products, generic or biosimilar versions of our marketed products or products from the same class as one of our products may have a negative impact on our business. Discovery of safety issues with our products could create product liability and could cause additional regulatory scrutiny and
requirements for additional labeling or safety monitoring, withdrawal of products from the market and/or the imposition of fines or criminal penalties. Adverse safety events may also damage physician, patient and/or investor confidence in our products and our reputation. Any of these could result in adverse impacts on our results of operations.
Regulatory authorities are making greater amounts of stand-alone safety information directly available to the public through periodic safety update reports, patient registries and other reporting requirements. The reporting of adverse safety events involving our products or products similar to ours and public rumors about such events may increase claims against us and may also cause our product sales to decline or our stock price to experience periods of volatility.
Restrictions on use or safety warnings that may be required to be included in the label of our products may significantly reduce expected revenue for those products and require significant expense and management time.
Risks Related to Our Operations
A breakdown or breach of our information systems could subject us to liability or interrupt the operation of our business.
We are increasingly dependent upon information systems and data to operate our business. Changes in how we operate have caused us to modify our business practices in ways that heighten this dependence, including changing the requirement that most of our office-based employees in the U.S. and our other key markets work from the office, with many of our employees now working in hybrid or full-remote positions. As a result, we are increasingly dependent upon our information systems to operate our business and our ability to effectively manage our business depends on the security, reliability and adequacy of our information systems and data, which includes use of cloud technologies, including Software as a Service (SaaS), Platform as a Service (PaaS) and Infrastructure as a Service (IaaS). Breakdowns, invasions, corruptions, destructions and/or breaches, which impact may include, but not limited to, comprising the capacity, reliability or security of our information systems or those of our business partners, including our cloud tech
nologies, and/or unauthorized access to our data and information could subject us to significant liability, negatively impact our business operations, and/or require replacement of technology and/or sizeable ransom payments. Our information systems, including our cloud technologies, continue to increase in multitude and complexity, increasing our vulnerability when breakdowns, malicious intrusions and random attacks occur. Data privacy or security breaches also pose a risk that sensitive data, including intellectual property, trade secrets or personal information belonging to us, patients, customers or other business partners, may be exposed to unauthorized persons or to the public.
Cybersecurity threats and incidents are increasing in their frequency, sophistication and intensity, and are becoming increasingly difficult to detect, particularly when they impact vendors, customers or suppliers, and other companies in our supply chain. Cybersecurity threats and incidents are often carried out by motivated, well-resourced, skilled and persistent actors, including nation states, organized crime groups, “hacktivists” and may include or target employees or contractors acting with careless or malicious intent. Recent developments in the threat landscape include use of AI and machine learning, as well as an increased number of cyber extortion attacks, with higher financial ransom demand amounts and increasing sophistication and variety of ransomware techniques and methodology. Geopolitical instability, including that related to Russia's invasion of Ukraine or the conflict in the Middle East, may increase the risk of cybersecurity threats. Cybersecurity threats or incidents may include deployment of harmful malware and key loggers, ransomware, a denial-of-service attack, a malicious website, the use of social engineering and other means to affect the confidentiality, integrity and availability of our information systems and data. Cybersecurity threats and incidents also include manufacturing, hardware or software supply chain attacks, which could cause a delay in the manufacturing of products or products produced for contract manufacturing or lead to a data privacy or security breach. Our key business partners face similar risks and any security breach of their systems could adversely affect our security posture. In addition, our increased use of cloud technologies heightens these and other operational risks, and any failure by cloud or other technology service providers to adequately safeguard their systems and prevent cyber-attacks could disrupt our operations and result in misappropriation, corruption or loss of confidential or propriety information.
While we continue to build and improve our systems and infrastructure, including our business continuity plans, there can be no assurance that our efforts will prevent cybersecurity threats or incidents in our systems and any such incidents could materially adversely affect our business and operations and/or result in the loss of critical or sensitive information, which could result in material financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence. Our liability insurance may not be sufficient in type or amount to cover us against claims related to security breaches, cyber-attacks and other related breaches.
Regulations continue to change as regulators worldwide consider new rules. For example, the SEC has adopted additional disclosure rules regarding cyber security risk management, strategy, governance and incident reporting by public companies. These new regulations or other regulations being considered in Europe and around the world may impact the manner in which we operate.
Regulators are imposing new data privacy and security requirements, including new and greater monetary fines for privacy violations. For example, the E.U.’s General Data Protection Regulation established regulations regarding the handling of personal data, and provides an enforcement authority and imposes large penalties for noncompliance. New U.S. data privacy and security laws, such as the CCPA, and others that may be passed, similarly introduce requirements with respect to personal information, and non-compliance with the CCPA may result in liability through private actions (subject to statutorily defined damages in the event of certain data breaches) and enforcement. Failure to comply with these current and future laws, policies, industry standards or legal obligations or any security incident resulting in the unauthorized access to, or acquisition, release or transfer of personal information may result in governmental enforcement actions, litigation, fines and penalties or adverse publicity and could cause our customers to lose trust in us, which could have a material adverse effect on our business and results of operations.
Manufacturing issues could substantially increase our costs, limit supply of our products and/or reduce our revenue.
The process of manufacturing our products is complex, highly regulated and subject to numerous risks, including:
•Risks of Reliance on Third-Parties and Single Source Providers. We rely on third-party suppliers and manufacturers for many aspects of our manufacturing process for our products and product candidates. In some cases, due to the unique manner in which our products are manufactured, we rely on single source providers of raw materials and manufacturing supplies. These third-parties are independent entities subject to their own unique operational and financial risks that are outside of our control. For example, a recently announced potential acquisition of a contract development and manufacturing organization by a third party. These third-parties may not perform their obligations in a timely and cost-effective manner or in compliance with applicable regulations, and they may be unable or unwilling to increase production capacity commensurate with demand for our existing or future products. Finding alternative providers could take a significant amount of time and involve significant expense due to the specialized nature of the services and the need to obtain regulatory approval of any significant changes to our suppliers or manufacturing methods. We cannot be certain that we could reach agreement with alternative providers or that the FDA or other regulatory authorities would approve our use of such alternatives.
•Global Bulk Supply Risks. We rely on our manufacturing facilities for the production of drug substance for our large molecule products and product candidates. Our global bulk supply of these products and product candidates depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, labor or raw material shortages, geopolitical instability, public health epidemics, natural disasters, power failures, cyber-attacks and many other factors.
•Risks Relating to Compliance with current GMP (cGMP). We and our third-party providers are generally required to maintain compliance with cGMP and other stringent requirements and are subject to inspections by the FDA and other regulatory authorities to confirm compliance. Any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging or storage of our products as a result of a failure of our facilities or operations or those of third-parties to receive regulatory approval or pass any regulatory agency inspection could significantly impair our ability to develop and commercialize our products. Significant noncompliance could also result in the imposition of monetary penalties or other civil or criminal sanctions and damage our reputation.
•Risk of Product Loss. The manufacturing process for our products is extremely susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or manufacturing facilities, we may need to close our manufacturing facilities for an extended period of time to investigate and remediate the contaminant.
Any adverse developments affecting our manufacturing operations or the operations of our third-party suppliers and manufacturers may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls or other interruptions in the commercial supply of our products.
Furthermore, factors such as geopolitical events, global health outbreaks, weather events, labor or raw material shortages and other supply chain disruptions could result in difficulties and delays in manufacturing our products, which could have an adverse impact on our results in operations or result in product shortages. We may also have to take inventory write-offs and incur other charges and expense for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. Such developments could increase our
manufacturing costs, cause us to lose revenue or market share as patients and physicians turn to competing therapeutics, diminish our profitability or damage our reputation.
In addition, although we have business continuity plans to reduce the potential for manufacturing disruptions or delays and reduce the severity of a disruptive event, there is no guarantee that these plans will be adequate, which could adversely affect our business and operations.
Management, personnel and other organizational changes may disrupt our operations, and we may have difficulty retaining personnel or attracting and retaining qualified replacements on a timely basis for the management and other personnel who may leave the Company.
Changes in management, other personnel and our overall retention rate may disrupt our business, and any such disruption could adversely affect our operations, programs, growth, financial condition or results of operations. New members of management may have different perspectives on programs and opportunities for our business, which may cause us to focus on new opportunities or reduce or change emphasis on our existing programs.
Our success is dependent upon our ability to attract and retain qualified management and other personnel in a highly competitive environment. Qualified individuals are in high demand, and we may incur significant costs to attract or retain them. We may face difficulty in attracting and retaining talent for a number of reasons, including management changes, integration related to the Reata acquisition, the underperformance or discontinuation of one or more marketed, pre-clinical or clinical programs, recruitment by competitors or changes in the overall labor market. In addition, changes in our organizational structure or in our flexible working arrangements could impact employees' productivity and morale as well as our ability to attract, retain and motivate employees. We cannot ensure that we will be able to hire or retain the personnel necessary for our operations or that the loss of any personnel will not have a material impact on our financial condition and results of operations.
If we fail to comply with the extensive legal and regulatory requirements affecting the health care industry, we could face increased costs, penalties and a loss of business.
Our activities, and the activities of our collaborators, distributors and other third-party providers, are subject to extensive government regulation and oversight in the U.S. and in foreign jurisdictions, and are subject to change and evolving interpretations, which could require us to incur substantial costs associated with compliance or to alter one or more of our business practices. The FDA and comparable foreign agencies directly regulate many of our most critical business activities, including the conduct of preclinical and clinical studies, product manufacturing, advertising and promotion, product distribution, adverse event reporting, product risk management and our compliance with good practice quality guidelines and regulations. Our interactions with physicians and other health care providers that prescribe or purchase our products are also subject to laws and government regulation designed to prevent fraud and abuse in the sale and use of products and place significant restrictions on the marketing practices of health care companies. Health care companies are facing heightened scrutiny of their relationships with health care providers and have been the target of lawsuits and investigations alleging violations of laws and government regulation, including claims asserting submission of incorrect pricing information, impermissible off-label promotion of pharmaceutical products, payments intended to influence the referral of health care business, submission of false claims for government reimbursement, antitrust violations or violations related to environmental matters. There is also enhanced scrutiny of company-sponsored patient assistance programs, including testing, insurance premium and co-pay assistance programs and donations to third-party charities that provide such assistance. The U.S. government has challenged some of our donations to third-party charities that provide patient assistance. If we, or our vendors or donation recipients, are found to fail to comply with relevant laws, regulations or government guidance in the operation of these or other patient assistance programs, we could be subject to significant fines or penalties. Risks relating to compliance with laws and regulations may be heightened as we continue to expand our global operations and enter new therapeutic areas with different patient populations, which may have different product distribution methods, marketing programs or patient assistance programs from those we currently utilize or support.
Conditions and regulations governing the health care industry are subject to change, with possible retroactive effect, including:
•new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or judicial decisions, related to health care availability, pricing or marketing practices, compliance with employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements;
•changes in the FDA and foreign regulatory approval processes or perspectives that may delay or prevent the approval of new products and result in lost market opportunity;
•government shutdowns or relocations may result in delays to the review and approval process, slowing the time necessary for new drug candidates to be reviewed and/or approved, which may adversely affect our business;
•requirements that provide for increased transparency of clinical trial results and quality data, such as the EMA's clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception or legal action, which could harm our business; and
•changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or otherwise adversely affect the market for our products.
Violations of governmental regulation may be punishable by criminal and civil sanctions, including fines and civil monetary penalties and exclusion from participation in government programs, including Medicare and Medicaid, as well as against executives overseeing our business. We could also be required to repay amounts we received from government payors or pay additional rebates and interest if we are found to have miscalculated the pricing information we submitted to the government. In addition, legal proceedings and investigations are inherently unpredictable, and large judgments or settlements sometimes occur. While we believe that we have appropriate compliance controls, policies and procedures in place to comply with the laws or regulations of the jurisdictions in which we operate, there is a risk that acts committed by our employees, agents, distributors, collaborators or third-party providers might violate such laws or regulations. Whether or not we have complied with the law, an investigation or litigation related to alleged unlawful conduct could increase our expense, damage our reputation, divert management time and attention and adversely affect our business.
Our sales and operations are subject to the risks of doing business internationally.
We are increasing our presence in international markets, subjecting us to many risks that could adversely affect our business and revenue. There is no guarantee that our efforts and strategies to expand sales in international markets will succeed. Emerging market countries may be especially vulnerable to periods of global and local political, legal, regulatory and financial instability and may have a higher incidence of corruption and fraudulent business practices. Certain countries may require local clinical trial data as part of the drug registration process in addition to global clinical trials, which can add to overall drug development and registration timelines. We may also be required to increase our reliance on third-party agents or distributors and unfamiliar operations and arrangements previously utilized by companies we collaborate with or acquire in emerging markets.
Our sales and operations are subject to the risks of doing business internationally, including:
•the impact of public health epidemics on the global economy and the delivery of healthcare treatments;
•less favorable intellectual property or other applicable laws;
•the inability to obtain necessary foreign regulatory approvals of products in a timely manner;
•limitations and additional pressures on our ability to obtain and maintain product pricing, reimbursement or receive price increases, including those resulting from governmental or regulatory requirements;
•increased cost of goods due to factors such as inflation and supply chain disruptions;
•additional complexity in manufacturing internationally, including materials manufactured in China;
•delays in clinical trials relating to geopolitical instability related to Russia's invasion of Ukraine and the military conflict in the Middle East;
•the inability to successfully complete subsequent or confirmatory clinical trials in countries where our experience is limited;
•longer payment and reimbursement cycles and uncertainties regarding the collectability of accounts receivable;
•fluctuations in foreign currency exchange rates that may adversely impact our revenue, net income and value of certain of our investments;
•the imposition of governmental controls;
•diverse data privacy and protection requirements;
•increasingly complex standards for complying with foreign laws and regulations that may differ substantially from country to country and may conflict with corresponding U.S. laws and regulations;
•the far-reaching anti-bribery and anti-corruption legislation in the U.K., including the U.K. Bribery Act 2010, and elsewhere and escalation of investigations and prosecutions pursuant to such laws;
•compliance with complex import and export control laws;
•changes in tax laws; and
•the imposition of tariffs or embargoes and other trade restrictions.
In addition, our international operations are subject to regulation under U.S. law. For example, the U.S. FCPA prohibits U.S. companies and their representatives from paying, offering to pay, promising to pay or authorizing the payment of anything of value to any foreign government official, government staff member, political party or political candidate for the purpose of obtaining or retaining business or to otherwise obtain favorable treatment or influence a person working in an official capacity. In many countries, the health care professionals we regularly interact with may meet the FCPA's definition of a foreign government official. Failure to comply with domestic or foreign laws could result in various adverse consequences, including possible delay in approval or refusal to approve a product, recalls, seizures or withdrawal of an approved product from the market, disruption in the supply or availability of our products or suspension of export or import privileges, the imposition of civil or criminal sanctions, the prosecution of executives overseeing our international operations and damage to our reputation. Any significant impairment of our ability to sell products outside of the U.S. could adversely impact our business and financial results. In addition, while we believe that we have appropriate compliance controls, policies and procedures in place to comply with the FCPA, there is a risk that acts committed by our employees, agents, distributors, collaborators or third-party providers might violate the FCPA and we might be held responsible. If our employees, agents, distributors, collaborators or third-party providers are found to have engaged in such practices, we could suffer severe penalties and may be subject to other liabilities, which could negatively affect our business, operating results and financial condition.
We built a large-scale biologics manufacturing facility and are building a gene therapy manufacturing facility, which will result in the incurrence of significant investment with no assurance that such investment will be recouped.
In order to support our future growth and drug development pipeline, we have expanded our large molecule production capacity by building a large-scale biologics manufacturing facility in Solothurn, Switzerland with no assurance that the additional capacity will be required or this investment will be recouped.
Although the Solothurn facility was approved by the FDA for ADUHELM and LEQEMBI, there can be no assurance that the regulatory authorities will approve the Solothurn facility for the manufacturing of other products.
Additionally, we are building a new gene therapy manufacturing facility in RTP, North Carolina with no assurance that this investment will be fully utilized. If we are unable to fully utilize this gene therapy manufacturing facility, charges from excess capacity may occur and would have a negative effect on our financial condition and results of operations.
If we are unable to fully utilize our manufacturing facilities, our business may be harmed. Charges resulting from excess capacity may continue to occur and would have a negative effect on our financial condition and results of operations.
The illegal distribution and sale by third-parties of counterfeit or unfit versions of our products or stolen products could have a negative impact on our reputation and business.
Third-parties might illegally distribute and sell counterfeit or unfit versions of our products, which do not meet our rigorous manufacturing, distribution and testing standards. A patient who receives a counterfeit or unfit drug may be at risk for a number of dangerous health consequences. Our reputation and business could suffer harm as a result of counterfeit or unfit drugs sold under our brand name. Inventory that is stolen from warehouses, plants or while in-transit, and that is subsequently improperly stored and sold through unauthorized channels, could adversely impact patient safety, our reputation and our business.
The increasing use of social media platforms and artificial intelligence based software presents new risks and challenges.
Social media is increasingly being used to communicate about our products and the diseases our therapies are designed to treat. Social media practices in the biopharmaceutical industry continue to evolve and regulations relating to such use are not always clear and create uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media channels to comment on the effectiveness
of a product or to report an alleged adverse event. When such disclosures occur, there is a risk that we fail to monitor and comply with applicable adverse event reporting obligations or we may not be able to defend the company or the public's legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our products. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on social media. We may also encounter criticism on social media regarding our company, management, product candidates or products. The immediacy of social media precludes us from having real-time control over postings made regarding us via social media, whether matters of fact or opinion. Our reputation could be damaged by negative publicity or if adverse information concerning us is posted on social media platforms or similar mediums, which we may not be able to reverse. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face restrictive regulatory actions or incur other harm to our business. Additionally, the use of AI based software is increasingly being used in the biopharmaceutical industry. Use of AI based software may lead to the release of confidential proprietary information which may impact our ability to realize the benefit of our intellectual property.
Risks Related to Holding Our Common Stock
Our operating results are subject to significant fluctuations.
Our quarterly revenue, expense and net income (loss) have fluctuated in the past and are likely to fluctuate significantly in the future due to the risks described in these Risk Factors as well as the timing of charges and expense that we may take. We have recorded, or may be required to record, charges that include:
•the cost of restructurings or other initiatives to streamline our operations and reallocate resources;
•the costs associated with decisions to terminate research and development programs;
•impairments with respect to investments, fixed assets and long-lived assets, including IPR&D and other intangible assets;
•inventory write-downs for failed quality specifications, charges for excess capacity, charges for excess or obsolete inventory and charges for inventory write-downs relating to product suspensions, expirations or recalls;
•changes in the fair value of contingent consideration or our equity investments;
•bad debt expense and increased bad debt reserves;
•outcomes of litigation and other legal or administrative proceedings, regulatory matters and tax matters;
•payments in connection with acquisitions, divestitures and other business development activities and under license and collaboration agreements;
•failure to meet certain contractual commitments; and
•the impact of public health epidemics, on employees, the global economy and the delivery of healthcare treatments.
Our revenue and certain assets and liabilities are also subject to foreign currency exchange rate fluctuations due to the global nature of our operations. Our efforts to mitigate the impact of fluctuating currency exchange rates may not be successful. As a result, currency fluctuations among our reporting currency, the U.S. dollar, and other currencies in which we do business will affect our operating results, often in unpredictable ways. Our net income may also fluctuate due to the impact of charges we may be required to take with respect to foreign currency hedge transactions. In particular, we may incur higher than expected charges from early termination of a hedge relationship.
Our operating results during any one period do not necessarily suggest the anticipated results of future periods.
Our investments in properties may not be fully realized.
We own or lease real estate primarily consisting of buildings that contain research laboratories, office space and manufacturing operations. We may decide to consolidate or co-locate certain aspects of our business operations or dispose of one or more of our properties, some of which may be located in markets that are experiencing high vacancy rates and decreasing property values. If we determine that the fair value of any of our owned properties is lower than their book value, we may not realize the full investment in these properties and incur significant impairment charges or additional depreciation when the expected useful lives of certain assets have been shortened due to the anticipated closing of facilities. If we decide to fully or partially vacate a property, we may incur significant cost, including facility closing costs, employee separation and retention expense, lease termination fees, rent expense in excess of sublease income and impairment of leasehold improvements and accelerated depreciation of assets. Any of these events may have an adverse impact on our results of operations.
Our investment portfolio is subject to market, interest and credit risk that may reduce its value.
We maintain a portfolio of marketable securities for investment of our cash as well as investments in equity securities of certain biotechnology companies. Changes in the value of our investment portfolio could adversely affect our earnings. The value of our investments may decline due to, among other things, increases in interest rates, downgrades of the bonds and other securities in our portfolio, negative company-specific news, biotechnology market sentiment, instability in the global financial markets that reduces the liquidity of securities in our portfolio, declines in the value of collateral underlying the securities in our portfolio and other factors. Each of these events may cause us to record charges to reduce the carrying value of our investment portfolio or sell investments for less than our acquisition cost. Although we attempt to mitigate these risks through diversification of our investments and continuous monitoring of our portfolio's overall risk profile, the value of our investments may nevertheless decline.
There can be no assurance that we will continue to repurchase shares or that we will repurchase shares at favorable prices.
From time to time our Board of Directors authorizes share repurchase programs. The amount and timing of share repurchases are subject to capital availability and our determination that share repurchases are in the best interest of our shareholders and are in compliance with all respective laws and our applicable agreements. Our ability to repurchase shares will depend upon, among other factors, our cash balances and potential future capital requirements for strategic transactions, our results of operations, our financial condition and other factors beyond our control that we may deem relevant. Additionally, the recently enacted IRA includes an excise tax on share repurchases, which will increase the cost of share repurchases. A reduction in repurchases under, or the completion of, our share repurchase programs could have a negative effect on our stock price. We can provide no assurance that we will repurchase shares at favorable prices, if at all.
We may not be able to access the capital and credit markets on terms that are favorable to us.
We may seek access to the capital and credit markets to supplement our existing funds and cash generated from operations for working capital, capital expenditure and debt service requirements and other business initiatives. The capital and credit markets are experiencing, and have in the past experienced, extreme volatility and disruption, which leads to uncertainty and liquidity issues for both borrowers and investors. In the event of adverse market conditions, we may be unable to obtain capital or credit market financing on favorable terms which could significantly increase our financing costs. Changes in credit ratings issued by nationally recognized credit rating agencies could also adversely affect our cost of financing and the market price of our securities.
Our indebtedness could adversely affect our business and limit our ability to plan for or respond to changes in our business.
Our indebtedness, together with our significant contingent liabilities, including milestone and royalty payment obligations, could have important consequences to our business; for example, such obligations could:
•increase our vulnerability to general adverse economic and industry conditions;
•limit our ability to access capital markets and incur additional debt in the future;
•require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow for other purposes, including business development, research and development and mergers and acquisitions; and
•limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate, thereby placing us at a disadvantage compared to our competitors that have less debt.
Some of our collaboration agreements contain change in control provisions that may discourage a third-party from attempting to acquire us.
Some of our collaboration agreements include change in control provisions that could reduce the potential acquisition price an acquirer is willing to pay or discourage a takeover attempt that could be viewed as beneficial to shareholders. Upon a change in control, some of these provisions could trigger reduced milestone, profit or royalty payments to us or give our collaboration partner rights to terminate our collaboration agreement, acquire operational control or force the purchase or sale of the programs that are the subject of the collaboration.
General Risk Factors
Our effective tax rate fluctuates, and we may incur obligations in tax jurisdictions in excess of accrued amounts.
As a global biopharmaceutical company, we are subject to taxation in numerous countries, states and other jurisdictions. As a result, our effective tax rate is derived from a combination of applicable tax rates, including
withholding taxes, in the various places that we operate. In preparing our financial statements, we estimate the amount of tax that will become payable in each of such places. Our effective tax rate may be different than experienced in the past or our current expectations due to many factors, including changes in the mix of our profitability from country to country, the results of examinations and audits of our tax filings, adjustments to the value of our uncertain tax positions, interpretations by tax authorities or other bodies with jurisdiction, the result of tax cases, changes in accounting for income taxes and changes in tax laws and regulations either prospectively or retrospectively and the effects of the integration of Reata.
Our inability to secure or sustain acceptable arrangements with tax authorities and future changes in the tax laws, among other things, may result in tax obligations in excess of amounts accrued in our financial statements.
The enactment of some or all of the recommendations set forth or that may be forthcoming in the OECD’s project on “Base Erosion and Profit Shifting” by tax authorities and economic blocs in the countries in which we operate, could unfavorably impact our effective tax rate. These initiatives focus on common international principles for the entitlement to taxation of global corporate profits and minimum global tax rates. Many countries have or are in the process of enacting legislation intended to implement the OECD GloBE Model Rules effective on January 1, 2024. The impact on the Company will depend on the timing of implementation, the exact nature of each country's GloBE legislation, guidance and regulations thereon and their application by tax authorities either prospectively or retrospectively.
Our business involves environmental risks, which include the cost of compliance and the risk of contamination or injury.
Our business and the business of several of our strategic partners involve the controlled use of hazardous materials, chemicals, biologics and radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials comply with state, federal and foreign standards, there will always be the risk of accidental contamination or injury. If we were to become liable for an accident, or if we were to suffer an extended facility shutdown, we could incur significant costs, damages and penalties that could harm our business. Manufacturing of our products and product candidates also requires permits from government agencies for water supply and wastewater discharge. If we do not obtain appropriate permits, including permits for sufficient quantities of water and wastewater, we could incur significant costs and limits on our manufacturing volumes that could harm our business. Additionally, regulators are considering new environmental disclosure rules. For example, the SEC and other regulators are considering environmental disclosure rules and California enacted new environmental disclosure laws in October 2023 that will generally require additional disclosure and reporting by 2026. The new California laws, the Climate Corporate Data Accountability Act and the Climate-Related Financial Risk Act, each impose additional climate-related reporting requirements on large companies conducting business in the state of California. We expect to be subject to these new laws, which impose extensive reporting obligations about greenhouse gas emissions and climate-related financial risks. These recently enacted and proposed regulations may require us to incur compliance and disclosure costs and require management attention.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
RISK MANAGEMENT AND STRATEGY
We maintain a technology and cybersecurity program, which includes information security, as part of our overall risk management process with the aim that our information systems, including those of our vendors and other third-parties, will be resilient, effective and capable of safeguarding against emerging risks and cybersecurity threats. We endeavor to assure our program is appropriately resourced and to attract and retain expert talent to execute it.
In designing, operating, evaluating and maintaining our program we use internal and external resources and frameworks, including cybersecurity expert consultants, industry working groups, the U.S. NIST Cybersecurity Framework and the U.S. Cybersecurity Agency's National Cyber Incident Scoring System model to benchmark, inform and evaluate the design of our program, our operational capabilities and our program maturity.
Consistent with NIST 800-53, our technology and cybersecurity program and controls include a third party and vendor risk management component. As part of our vendor risk management program, we conduct security assessments prior to engagement of high-risk vendors and other third-party providers and have a monitoring program to evaluate ongoing compliance with our cybersecurity standards.
A key element of our technology and cybersecurity program strategy is fostering training and awareness. Our training and awareness program includes annual cybersecurity awareness training and role-based phishing tests for our employees and for third parties with access to our systems.
Our technology and cybersecurity program focuses on the defense, rapid detection and rapid remediation of cybersecurity threats and incidents. Our program includes systems and processes designed based on defense-in-depth and zero-trust architectural principles and that are intended to provide the control capabilities set forth in NIST's 800-53 Rev 5, Security and Privacy Controls for Information Systems and Organizations. Our program also includes cybersecurity policies and a crisis response and management plan that is intended to allow rapid management and response and appropriate communication of cybersecurity threats and incidents.
We staff a cybersecurity operations center to respond to threats and incidents. Our cybersecurity crisis management plan sets forth the items, procedures and actions we expect to address and follow in the event of a cybersecurity incident, including detection, response, mitigation and remediation. In addition to the cybersecurity operations center and our designated cybersecurity response team, we maintain a cross-functional cybersecurity crisis core team, which includes our CISO and senior representatives from our Legal, Finance, IT and Corporate Security teams.
When a potential threat or incident is identified, our cyber security incident response team will assign a risk level classification and initiate the escalation and other steps called for by our plan. All incidents that are initially assessed by the cybersecurity incident response team as potentially high-risk are escalated promptly to our CISO. Our CISO, Chief Legal Officer and Chief Financial Officer, will determine whether and what elements of our cybersecurity crisis response and management plan should be activated, including escalation to other senior management or our Executive Committee. Our Executive Committee will inform our Board of Directors of cybersecurity incidents, as appropriate, considering a variety of factors, including financial, operational, legal or reputational impact.
Our program's maturity and operational readiness are regularly evaluated by independent experts using the U.S. NIST's CyberSecurity Framework and penetration tests. Our program, and the results of these independent evaluations and testing, are regularly reviewed by our senior management and members of our Board of Directors.
CYBERSECURITY RISK GOVERNANCE
We are committed to appropriate cybersecurity governance and oversight. Our technology and cybersecurity program is the principal responsibility of our Chief Information Officer and CISO, each of whom have over 20 years of experience in information systems, including cybersecurity training and experience. Additionally, we have a Cybersecurity steering committee that includes senior representatives from our Legal, Finance and IT departments, which meets regularly to discuss cybersecurity matters.
Our Board of Directors oversees management's processes for identifying and mitigating risks, including cybersecurity and information security risks. Our Audit Committee of our Board of Directors regularly reviews our technology and cybersecurity program and effectiveness, internal audits of our program, independent external expert evaluations of our program's maturity and operational readiness and the results of penetration testing. Our Audit Committee also receives regular cybersecurity updates and education on a broad range of topics, including:
•Current cybersecurity landscape and emerging threats;
•Status of ongoing cybersecurity initiatives and strategies;
•Incident report and learnings from any cybersecurity events; and
•Compliance with regulatory requirements and industry standards.
For additional information on our cybersecurity risks, please read Item 1A. Risk Factors - A breakdown or breach of our technology systems could subject us to liability or interrupt the operation of our business, included in this report.
ITEM 2. PROPERTIES
Below is a summary of our owned and leased properties as of December 31, 2023.
U.S.
MASSACHUSETTS
In Cambridge, Massachusetts we own approximately 263,000 square feet of real estate space, consisting of a building that houses a research laboratory and a cogeneration plant.
In addition, we lease a total of approximately 1,165,000 square feet in Massachusetts, which is summarized as follows:
•808,000 square feet in Cambridge, Massachusetts, which is comprised of offices for our corporate headquarters and other administrative and development functions and laboratories, of which 209,000 square feet is subleased by multiple companies for general office space, laboratories and manufacturing facilities; and
•357,000 square feet of office space in Weston, Massachusetts, of which 174,000 square feet is subleased through the remaining term of our lease agreement. Our lease expires in May 2025 and we do not intend on renewing the lease agreement.
Our Massachusetts lease agreements expire at various dates through the year 2028.
125 BROADWAY BUILDING SALE AND LEASEBACK
In September 2022 we completed the sale of our building and land parcel located at 125 Broadway. In connection with this sale, we simultaneously leased back the building for a term of approximately 5.5 years. The sale and immediate leaseback of this building qualified for sale and leaseback treatment and is classified as an operating lease. For additional information on our 125 Broadway sale and leaseback transaction, please read Note 11, Property, Plant and Equipment and Note 12, Leases, to our consolidated financial statements included in this report.
300 BINNEY STREET LEASE MODIFICATION
In September 2022 we entered into an agreement to partially terminate a portion of our lease located at 300 Binney Street, as well as to reduce the lease term for the majority of the remaining space. The agreement was driven by our 2022 efforts to reduce costs by consolidating real estate locations. For additional information on our 300 Binney Street lease modification, please read Note 12, Leases, to our consolidated financial statements included in this report.
NORTH CAROLINA
In RTP, North Carolina we own approximately 1,040,000 square feet of real estate space, which is summarized as follows:
•357,000 square feet of laboratory and office space;
•206,000 square foot multi-purpose facility, including an ASO manufacturing suite and administrative space;
•175,000 square feet related to a large-scale biologics manufacturing facility;
•105,000 square feet related to a small-scale biologics manufacturing facility;
•84,000 square feet of warehouse space and utilities;
•70,000 square feet related to a parenteral fill-finish facility; and
•43,000 square feet related to a large-scale purification facility.
In addition, we lease approximately 65,000 square feet of warehouse space in Durham, North Carolina. Our North Carolina lease agreements expire at various dates through the year 2025.
In the fourth quarter of 2021 we began construction of a new gene therapy manufacturing facility in RTP, North Carolina to support our gene therapy pipeline across multiple therapeutic areas. The new manufacturing facility will be approximately 197,000 square feet. As we continue to advance our research and development prioritization efforts, which includes refocusing our investment in gene therapy, we are evaluating several alternative uses for this facility.
TEXAS
As part of our acquisition of Reata in September 2023 we acquired leases totaling approximately 404,000 square feet of real estate space, which is summarized as follows:
•327,000 square feet in Plano, Texas, which is comprised of office and laboratory space, with an initial lease term through the year 2038. We do not intend to occupy this building and are evaluating opportunities to sublease this property;
•35,000 square feet in Irving, Texas, which is comprised of office and laboratory space and expires in 2024; and
•42,000 square feet in Plano, Texas, which is comprised of office and laboratory space and expires in 2024.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
INTERNATIONAL
SWITZERLAND
In order to support our future growth and drug development pipeline, we built a large-scale biologics manufacturing facility in Solothurn, Switzerland. This facility includes 393,000 square feet related to a large-scale biologics manufacturing facility, 290,000 square feet of warehouse, utilities and support space and 51,000 square feet of administrative space. In the second quarter of 2021 a portion of the facility (the first manufacturing suite) received a GMP multi-product license from the SWISSMEDIC and was placed into service. The second manufacturing suite became operational in January 2024. Solothurn has been approved for the manufacture of ADUHELM and LEQEMBI by the FDA.
For additional information on our Solothurn manufacturing facility, please read Note 11, Property, Plant and Equipment, to our consolidated financial statements included in this report.
OTHER INTERNATIONAL
We lease office space in Baar, Switzerland, our international headquarters; the U.K.; Germany; France; Japan; Canada and numerous other countries. Our international lease agreements expire at various dates through the year 2034.
ITEM 3. LEGAL PROCEEDINGS
For a discussion of legal matters as of December 31, 2023, please read Note 21, Litigation, to our consolidated financial statements included in this report, which is incorporated into this item by reference.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
MARKET AND STOCKHOLDER INFORMATION
Our common stock trades on The Nasdaq Global Select Market under the symbol “BIIB.” As of February 12, 2024, there were approximately 420 shareholders of record of our common stock.
DIVIDENDS
We have not paid cash dividends since our inception. While we historically have not paid cash dividends and do not have a current intention to pay cash dividends, we continually review our capital allocation strategies, including, among other things, payment of cash dividends, share repurchases and acquisitions.
ISSUER PURCHASES OF EQUITY SECURITIES
The following table summarizes our common stock repurchase activity during the fourth quarter of 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| Period | Total Number of
Shares Purchased
(#) | | Average Price
Paid per Share
($) | | Total Number of
Shares Purchased
as Part of Publicly
Announced Programs
(#) | | Approximate Dollar Value
of Shares That May Yet Be
Purchased Under
Our Programs
($ in millions) |
| October 2023 | — | | | $ | — | | | — | | | $ | 2,050.0 | |
| November 2023 | — | | | $ | — | | | — | | | $ | 2,050.0 | |
| December 2023 | — | | | $ | — | | | — | | | $ | 2,050.0 | |
Total(1) | — | | | $ | — | | | | | |
(1) There were no share repurchases during the fourth quarter of 2023.
In October 2020 our Board of Directors authorized our 2020 Share Repurchase Program, which is a program to repurchase up to $5.0 billion of our common stock. Our 2020 Share Repurchase Program does not have an expiration date. All share repurchases under our 2020 Share Repurchase Program will be retired. Under our 2020 Share Repurchase Program, we repurchased and retired approximately 3.6 million and 6.0 million shares of our common stock at a cost of approximately $750.0 million and $1.8 billion during the years ended December 31, 2022 and 2021, respectively. There were no share repurchases of our common stock during the year ended December 31, 2023. Approximately $2.1 billion remained available under our 2020 Share Repurchase Program as of December 31, 2023.
In August 2022 the IRA was signed into law. Among other things, the IRA levies a 1.0% excise tax on net stock repurchases after December 31, 2022. While we have historically made discretionary share repurchases, we had no share repurchases of our common stock during the year ended December 31, 2023.
PERFORMANCE GRAPH
The performance graph below compares the five-year cumulative total stockholder return on our common stock, the Nasdaq Pharmaceutical Index, the S&P 500 Index and the Nasdaq Biotechnology Index. The performance graph below assumes the investment of $100.00 on December 31, 2018, in our common stock and each of the three indexes, with dividends being reinvested.
The stock price performance in the graph below is not necessarily indicative of future price performance.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2018 | | 2019 | | 2020 | | 2021 | | 2022 | | 2023 |
| Biogen Inc. | | $100.00 | | $98.61 | | $81.37 | | $79.73 | | $92.01 | | $85.97 |
| Nasdaq Pharmaceutical Index | | $100.00 | | $114.51 | | $126.56 | | $157.42 | | $175.29 | | $182.08 |
| S&P 500 Index | | $100.00 | | $131.49 | | $155.68 | | $200.37 | | $164.08 | | $207.21 |
| Nasdaq Biotechnology Index | | $100.00 | | $125.11 | | $158.17 | | $158.20 | | $142.19 | | $148.72 |
The information included under the heading Performance Graph is “furnished” and not “filed” for purposes of Section 18 of the Securities Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed to be “soliciting material” subject to Regulation 14A or incorporated by reference in any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our consolidated financial statements and the accompanying notes beginning on page F-1 of this report.
For our discussion of the year ended December 31, 2022, compared to the year ended December 31, 2021, please read Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations located in our Annual Report on Form 10-K for the year ended December 31, 2022.
EXECUTIVE SUMMARY
INTRODUCTION
Biogen is a global biopharmaceutical company focused on discovering, developing and delivering innovative therapies for people living with serious and complex diseases worldwide. We have a broad portfolio of medicines to treat MS, have introduced the first approved treatment for SMA, co-developed treatments to address a defining pathology of Alzheimer’s disease and launched the first approved treatment to target a genetic cause of ALS. Through our 2023 acquisition of Reata we market the first and only drug approved in the U.S. and the E.U. for the treatment of Friedreich's Ataxia in adults and adolescents aged 16 years and older. We are focused on advancing our pipeline in neurology, specialized immunology and rare diseases. We support our drug discovery and development efforts through internal research and development programs and external collaborations.
Our marketed products include TECFIDERA, VUMERITY, AVONEX, PLEGRIDY, TYSABRI and FAMPYRA for the treatment of MS; SPINRAZA for the treatment of SMA; SKYCLARYS for the treatment of Friedreich's Ataxia; QALSODY for the treatment of ALS; and FUMADERM for the treatment of severe plaque psoriasis.
We also have collaborations with Eisai on the commercialization of LEQEMBI for the treatment of Alzheimer's disease and Sage on the commercialization of ZURZUVAE for the treatment of PPD and we have certain business and financial rights with respect to RITUXAN for the treatment of non-Hodgkin's lymphoma, CLL and other conditions; RITUXAN HYCELA for the treatment of non-Hodgkin's lymphoma and CLL; GAZYVA for the treatment of CLL and follicular lymphoma; OCREVUS for the treatment of PPMS and RMS; LUNSUMIO for the treatment of relapsed or refractory follicular lymphoma; COLUMVI, a bispecific antibody for the treatment of non-Hodgkin's lymphoma; and have the option to add other potential anti-CD20 therapies, pursuant to our collaboration arrangements with Genentech, a wholly-owned member of the Roche Group.
We commercialize a portfolio of biosimilars of advanced biologics including BENEPALI, an etanercept biosimilar referencing ENBREL, IMRALDI, an adalimumab biosimilar referencing HUMIRA, and FLIXABI, an infliximab biosimilar referencing REMICADE, in certain countries in Europe, as well as BYOOVIZ, a ranibizumab biosimilar referencing LUCENTIS, in the U.S. and certain international markets. We also have exclusive rights to commercialize TOFIDENCE, a tocilizumab biosimilar referencing ACTEMRA. We continue to develop potential biosimilar product SB15, a proposed aflibercept biosimilar referencing EYLEA. In February 2023 we announced that we are exploring strategic options for our biosimilars business.
For additional information on our collaboration arrangements, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
We seek to ensure an uninterrupted supply of medicines to patients around the world. To that end, we continually review our manufacturing capacity, capabilities, processes and facilities. In order to support our future growth and drug development pipeline, we expanded our large molecule production capacity and built a large-scale biologics manufacturing facility in Solothurn, Switzerland. In the second quarter of 2021 a portion of the facility (the first manufacturing suite) received a GMP multi-product license from the SWISSMEDIC and was placed into service. The second manufacturing suite became operational in January 2024. Solothurn has been approved for the manufacture of ADUHELM and LEQEMBI by the FDA. We believe that the Solothurn facility will support our anticipated near to mid-term needs for the manufacturing of biologic assets, including the commercial launch of LEQEMBI. The plant represents a significant increase in our overall manufacturing capacity and is not yet being fully utilized, resulting in our recording of excess capacity charges. If we are unable to fully utilize our manufacturing facilities, we will incur additional excess capacity charges which would have a negative effect on our financial condition and results of operations.
In the longer term, our revenue growth will depend upon the successful clinical development, regulatory approval and launch of new commercial products as well as additional indications for our existing products, our ability to obtain
and maintain patents and other rights related to our marketed products, assets originating from our research and development efforts and/or successful execution of external business development opportunities.
BUSINESS ENVIRONMENT
For a detailed discussion on our business environment, please read Item 1. Business, included in this report. For additional information on our competition and pricing risks that could negatively impact our product sales, please read Item 1A. Risk Factors, included in this report.
TECFIDERA
Multiple TECFIDERA generic entrants are now in North America, Brazil and certain E.U. countries and have deeply discounted prices compared to TECFIDERA. The generic competition for TECFIDERA has significantly reduced our TECFIDERA revenue and we expect that TECFIDERA revenue will continue to decline in the future.
Following a favorable March 2023 decision of the CJEU affirming TECFIDERA's right to regulatory data and marketing protection and the EC determination in May 2023 that TECFIDERA is entitled to an additional year of market protection for its pediatric indication, we believe that TECFIDERA is entitled to regulatory marketing protection in the E.U. until at least February 2, 2025, and are seeking to enforce this protection. In December 2023, the EC revoked all centralized marketing authorizations for generic versions of TECFIDERA. As of December 31, 2023, some of the TECFIDERA generics have not yet fully exited some E.U. markets and we expect removal of all generics from the market will take additional time. We are closely monitoring this situation and working to enforce our legal right to market protection. In addition, we will continue to enforce our EP 2 653 873 patent related to TECFIDERA, which expires in 2028.
For additional information, please read Note 21, Litigation, to our consolidated financial statements included in this report.
BUSINESS UPDATE REGARDING MACROECONOMIC CONDITIONS AND OTHER DISRUPTIONS
Significant portions of our business are conducted in Europe, Asia and other international geographies. Factors such as global health outbreaks, adverse weather events, geopolitical events, inflation, labor or raw material shortages and other supply chain disruptions could result in product shortages or other difficulties and delays or increased costs in manufacturing our products. Additionally, global disputes and interruptions in international relationships, including tariffs, trade protection measures, import or export licensing requirements and the imposition of trade sanctions or similar restrictions by the U.S. or other governments, affect our ability to do business. For example, tensions between the U.S. and China have led to a series of tariffs and sanctions being imposed by the U.S. on imports from China mainland, as well as other business restrictions.
CURRENT ECONOMIC CONDITIONS
Economic conditions remain vulnerable as markets continue to be impacted in part by elevated inflation, rising interest rates, global supply chain constraints and recent bank failures.
During 2023 concerns arose with respect to the financial condition of certain banking institutions in the U.S., in particular those with exposure to certain types of depositors and large portfolios of investment securities. In March 2023 two such banks were closed and taken over by the FDIC, which created significant market disruption. While we did not have any direct exposure to these institutions, we do maintain our cash at financial institutions, often in balances that exceed the current FDIC insurance limits, and will continue to monitor our cash, cash equivalents and investments and take steps to identify any potential impact and minimize any disruptions on our business.
If other banks and financial institutions enter receivership or become insolvent in the future due to financial conditions affecting the banking system and financial markets, our ability to access our cash, cash equivalents and investments, including transferring funds, making payments or receiving funds, may be threatened and could have a material adverse effect on our business and financial condition.
GEOPOLITICAL TENSIONS
The ongoing geopolitical tensions related to Russia's invasion of Ukraine and the recent military conflict in the Middle East have resulted in global business disruptions and economic volatility.
For example, sanctions and other restrictions have been levied on the government and businesses in Russia. Although we do not have affiliates or employees, in either Russia or Ukraine, we do provide various therapies to patients in Russia through a distributor. In addition, new government sanctions on the export of certain
manufacturing materials to Russia may delay or limit our ability to get new products approved. The impact of the conflict on our operations and financial performance remains uncertain and will depend on future developments, including the severity and duration of the conflict between Russia and Ukraine, its impact on regional and global economic conditions and whether the conflict spreads or has effects on countries outside Ukraine and Russia.
We will continue to monitor the ongoing conflict between Russia and Ukraine as well as the military conflict in the Middle East and assess any potential impacts on our business, supply chain, partners or customers, as well as any factors that could have an adverse effect on our results of operations. Revenue generated from sales in Russia and Ukraine represent less than 2.0% of total revenue for the years ended December 31, 2023, 2022 and 2021. Revenue generated from sales in the broader Middle East region represents less than 2.0% of total revenue for the years ended December 31, 2023, 2022 and 2021.
INFLATION REDUCTION ACT OF 2022
In August 2022 the IRA was signed into law in the U.S. The IRA introduced new tax provisions, including a 15.0% corporate alternative minimum tax and a 1.0% excise tax on stock repurchases. The provisions of the IRA are effective for periods after December 31, 2022. The IRA did not result in any material adjustments to our income tax provision or other income tax balances as of December 31, 2023 and 2022. Preliminary guidance has been issued by the IRS and we expect additional guidance and regulations to be issued in future periods. We will continue to assess its potential impact on our business and results of operations as further information becomes available.
The IRA also contains substantial drug pricing reforms that may have a significant impact on the pharmaceutical industry in the U.S. This includes allowing CMS to negotiate a maximum fair price for certain high-priced single source Medicare drugs, as well as redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, potentially resulting in higher contributions from plans and manufacturers. The IRA also establishes drug inflationary rebate requirements to penalize manufacturers from raising the prices of Medicare covered single-source drugs and biologics beyond the inflation-adjusted rate. Further, to incentivize biosimilar development, the IRA provides an 8.0% Medicare Part B add-on payment for qualifying biosimilar products for a five-year period.
The IRA's drug pricing controls and Medicare redesign may have an adverse impact on our sales (particularly for our products that are more substantially reliant on Medicare reimbursement), our business and our results of operations. However, the degree of impact from this legislation on our business depends on a number of implementation decisions. We will continue to assess as further information becomes available.
FINANCIAL HIGHLIGHTS
As described below under Results of Operations, our net income and diluted earnings per share attributable to Biogen Inc. for the year ended December 31, 2023, compared to the year ended December 31, 2022, reflects the following:
Decreased
$337.8 million or 3.3%
| | | | | |
DILUTED EARNINGS PER SHARE |
Decreased
$12.90 or 61.8%
Decreased
$741.1 million or 9.3%
•MS revenue decreased $768.3 million, or 14.1%
•Rare disease revenue increased $9.5 million, or 0.5%
•Biosimilars revenue increased $18.9 million, or 2.5%
•The decrease in MS product revenue was primarily due to a decrease in TECFIDERA demand as a result of multiple TECFIDERA generic entrants in North America, Brazil and certain E.U. countries, a decrease in Interferon demand due to competition as patients transition to higher efficacy therapies and a decrease in U.S. TYSABRI revenue primarily driven by increased competition and pricing pressure.
•The increase in rare disease revenue was primarily due to revenue from SKYCLARYS, which we began recognizing in the fourth quarter of 2023 as a result of our acquisition of Reata in September 2023. The increase was partially offset by a decrease in rest of world SPINRAZA revenue primarily due to the unfavorable impact of foreign currency exchange, increased competition, a decrease in pricing and the timing of shipments.
Increased
$1,957.2 million or 29.7%
•Cost of sales increased $255.1 million, or 11.2%
•R&D expense increased $230.9 million, or 10.3%
•SG&A expense increased $146.1 million, or 6.1%
•Restructuring expense increased $87.7 million, or 66.9%
•Other income decreased $423.7 million, net
•The increase in cost of sales was primarily due to unfavorable product mix from increased contract manufacturing revenue, MS product mix and higher idle capacity charges, partially offset by a decrease in excess and obsolescence inventory charges in 2023.
•The increase in research and development expense was primarily due to clinical trial close out costs incurred in 2023 of approximately $125.4 million and the recognition of $197.0 million in equity-based compensation expense related to our acquisition of Reata.
•The increase in selling, general and administrative expense was primarily due to the recognition of $196.4 million in equity-based compensation expense related to our acquisition of Reata.
•The increase in restructuring expense was primarily due to higher severance benefits associated with the 2023 cost savings initiatives as compared to 2022.
•The decrease in other income, net was primarily due to the pre-tax gain of $1.5 billion recorded in 2022 related to the sale of our equity interest in Samsung Bioepis, partially offset by a pre-tax charge of $900.0 million, plus settlement fees and expenses, related to a litigation settlement agreement
•Additionally, total cost and expense in 2022 was reduced by a pre-tax gain of approximately $503.7 million related to a sale of a building.
| | | | | |
| FINANCIAL CONDITION, LIQUIDITY AND CAPITAL RESOURCES |
•We generated $1,547.2 million of net cash flow from operations for the years ended December 31, 2023. Net cash flow from operations includes $393.4 million of equity-based compensation expense related to our acquisition of Reata in September 2023.
•Cash, cash equivalents and marketable securities totaled approximately $1.0 billion as of December 31, 2023, compared to approximately $5.6 billion as of December 31, 2022. The decrease was primarily due to consideration paid for our acquisition of Reata in September 2023.
•There were no share repurchases of our common stock during 2023 under our 2020 Share Repurchase Program. Approximately $2.1 billion remained available under our 2020 Share Repurchase Program as of December 31, 2023.
DEVELOPMENTS IN KEY COLLABORATIVE RELATIONSHIPS
LEQEMBI (lecanemab)
United States
In July 2023 the FDA granted traditional approval of LEQEMBI, an anti-amyloid antibody for the treatment of Alzheimer's disease, which was previously granted accelerated approval by the FDA in January 2023. Following the FDA's traditional approval of LEQEMBI, CMS confirmed broader coverage of LEQEMBI.
Additionally, in March 2023 Eisai announced that the U.S. Veteran's Health Administration will be providing coverage of LEQEMBI to veterans living with early stages of Alzheimer's disease.
Rest of World
Key developments related to LEQEMBI (lecanemab) in rest of world markets during 2023 consisted of the following:
•In January 2024 we and Eisai announced that the SAG will convene at the request of the CHMP to discuss the MAA of lecanemab that is currently under review by the EMA. The meeting of the SAG is expected to take place during the first quarter of 2024 and the EC decision for the MAA of lecanemab is expected during the first half of 2024.
•In January 2024 the NMPA approved LEQEMBI in China, with an expected launch date in 2024.
•In December 2023 we and Eisai announced that LEQEMBI intravenous infusion was launched in Japan.
•In September 2023 the Japanese Ministry of Health, Labor and Welfare approved LEQEMBI in Japan.
•In January 2023 the EMA accepted for review the MAA for lecanemab.
•In February 2023 the BLA for lecanemab was granted Priority Review by the NMPA of China.
•In May 2023 we and Eisai announced the submission of a MAA for lecanemab to the U.K. MHRA in Great Britain, which has been designated by the MHRA for the Innovative Licensing and Access Pathway. Additionally, in May 2023 Health Canada accepted for review the NDS for lecanemab.
•In June 2023 we and Eisai announced the submission of a MAA for lecanemab to the Ministry of Food and Drug Safety in South Korea.
ZURZUVAE (zuranolone)
In August 2023 the FDA approved ZURZUVAE for adults with PPD, pending DEA scheduling, which was completed in October 2023. Upon approval, ZURZUVAE for PPD became the first and only oral, once-daily, 14-day treatment that can provide rapid improvements in depressive symptoms by day 15 for women with PPD. ZURZUVAE for PPD became commercially available in the U.S. during the fourth quarter of 2023. Additionally, the FDA issued a CRL for the NDA for zuranolone in the treatment of adults with MDD. The CRL stated that the application did not provide substantial evidence of effectiveness to support the approval of zuranolone for the treatment of MDD and that an additional study or studies would be needed. We and Sage are continuing to seek feedback from the FDA and evaluating next steps.
BUSINESS COMBINATIONS
REATA ACQUISITION
On September 26, 2023, we completed the acquisition of all of the issued and outstanding shares of Reata, a biopharmaceutical company focused on developing therapeutics that regulate cellular metabolism and inflammation in serious neurologic diseases. As a result of this transaction we acquired SKYCLARYS (omaveloxolone), the first and only drug approved in the U.S. and the E.U. for the treatment of Friedreich's Ataxia in adults and adolescents aged 16 years and older, as well as other clinical and preclinical pipeline programs.
Under the terms of this acquisition, we paid Reata shareholders $172.50 in cash for each issued and outstanding Reata share, which totaled approximately $6.6 billion. In addition, we agreed to pay approximately $983.9 million in cash for Reata's outstanding equity awards, inclusive of employer taxes, of which approximately $590.5 million was attributable to pre-acquisition services and is therefore reflected as a component of total purchase price paid. Of the $983.9 million paid to Reata's equity award holders, we recognized approximately $393.4 million as compensation attributable to the post-acquisition service period, of which $196.4 million was recognized as a charge to selling, general and administrative expense with the remaining $197.0 million as a charge to research and development expense within our consolidated statements of income for the year ended December 31, 2023. These amounts were associated with the accelerated vesting of stock options and RSUs previously granted to Reata employees that required no future services to vest.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
OTHER KEY DEVELOPMENTS
SKYCLARYS (omaveloxolone)
In February 2024 the EC approved SKYCLARYS in the E.U. for the treatment of FA in adults and adolescents aged 16 years and older. SKYCLARYS is the first treatment approved within the E.U. for this rare, genetic, progressive neurodegenerative disease.
QALSODY (tofersen)
In April 2023 the FDA approved QALSODY for the treatment of ALS in adults who have a mutation in the SOD1 gene. This indication is approved under accelerated approval based on reduction in plasma neurofilament light chain observed in patients treated with QALSODY. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trial(s).
TECFIDERA
Following a favorable March 2023 decision of the CJEU affirming TECFIDERA's right to regulatory data and marketing protection and the EC determination in May 2023 that TECFIDERA is entitled to an additional year of market protection for its pediatric indication, we believe that TECFIDERA is entitled to regulatory marketing protection in the E.U. until at least February 2, 2025, and are seeking to enforce this protection. In December 2023, the EC revoked all centralized marketing authorizations for generic versions of TECFIDERA. As of December 31, 2023, some of the TECFIDERA generics have not yet fully exited some E.U. markets and we expect removal of all generics from the market will take additional time. We are closely monitoring this situation and working to enforce our legal right to market protection. In addition, we will continue to enforce our EP 2 653 873 patent related to TECFIDERA, which expires in 2028.
CORPORATE MATTERS
FIT FOR GROWTH
In 2023 we initiated additional cost saving measures as part of our Fit for Growth program to reduce operating costs, while improving operating efficiency and effectiveness. The Fit for Growth program is expected to generate approximately $1.0 billion in gross operating expense savings and $800.0 million in net operating expense savings by 2025, some of which will be reinvested in various initiatives. The Fit for Growth program is currently estimated to include net headcount reductions of approximately 1,000 employees and we expect to incur restructuring charges ranging from approximately $260.0 million to $280.0 million.
For additional information on our Fit for Growth program, please read Note 4, Restructuring, to our consolidated financial statements included in this report.
DISCONTINUED PROGRAMS AND STUDIES
ENVISION STUDY
In November 2023 we notified Neurimmune of our decision to terminate our collaboration and license agreement with Neurimmune, to discontinue the development and commercialization of ADUHELM and to terminate the ENVISION clinical study. In connection with this termination, we recorded close-out costs of approximately $60.0 million in research and development expense within our consolidated statements of income for the year ended December 31, 2023.
EMBARK STUDY
In September 2023 we discontinued our EMBARK study for aducanumab. In connection with this discontinuation we recorded termination costs of approximately $43.0 million in research and development expense within our consolidated statements of income for the year ended December 31, 2023.
ACORDA COLLABORATION
In January 2024 we notified Acorda of our decision to terminate our collaboration and license agreement, effective January 1, 2025. As a result of this termination, Acorda will regain global commercialization rights to FAMPYRA.
BIIB122
In June 2023 we and Denali announced plans to terminate the Phase 3 LIGHTHOUSE study for BIIB122, a small molecule inhibitor of LRRK2 in Parkinson's disease. The protocol for the Phase 2b LUMA study for BIIB122 in patients with early-stage Parkinson’s disease was amended to now include eligible patients with a LRRK2 genetic mutation in addition to continuing to enroll eligible patients with early-stage idiopathic Parkinson’s disease.
BIIB093
In April 2023 we announced that we would terminate the development of BIIB093 (glibenclamide IV), currently in a Phase 3 study for LHI and a Phase 2 study for brain contusion, due to operational challenges and other strategic considerations. In connection with this termination, we recorded close-out costs of approximately $13.2 million in research and development expense within our consolidated statements of income for the year ended December 31, 2023.
BIIB131
In April 2023 we announced that we will be pausing the initiation of a Phase 2b study for BIIB131 (TMS-007) for acute ischemic stroke and will continue to assess whether to initiate this study. We sold the rights to BIIB131 to a third-party biopharmaceutical company in exchange for an upfront with potential milestones and future royalties on global sales.
BIIB132
In April 2023 we announced that we would discontinue further development of BIIB132 in spinocerebellar ataxia type 3, as part of our ongoing research and development prioritization initiative.
RESULTS OF OPERATIONS
REVENUE
The following revenue discussion should be read in conjunction with Note 5, Revenue, to our consolidated financial statements included in this report.
Revenue is summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | % Change | | $ Change |
| | | 2023
vs.
2022 | | 2022
vs.
2021 | | 2023
vs.
2022 | | 2022
vs.
2021 |
| (In millions, except percentages) | | 2023 | | 2022 | | 2021 | | | |
| Product revenue, net: | | | | | | | | | | | | | | |
| United States | | $ | 3,141.4 | | | $ | 3,469.3 | | | $ | 3,805.7 | | | (9.5) | % | | (8.8) | % | | $ | (327.9) | | | $ | (336.4) | |
| Rest of world | | 4,105.3 | | | 4,518.5 | | | 5,041.2 | | | (9.1) | | | (10.4) | | | (413.2) | | | (522.7) | |
| Total product revenue, net | | 7,246.7 | | | 7,987.8 | | | 8,846.9 | | | (9.3) | | | (9.7) | | | (741.1) | | | (859.1) | |
| Revenue from anti-CD20 therapeutic programs | | 1,689.6 | | | 1,700.5 | | | 1,658.5 | | | (0.6) | | | 2.5 | | | (10.9) | | | 42.0 | |
| Contract manufacturing, royalty and other revenue | | 899.3 | | | 485.1 | | | 476.3 | | | 85.4 | | | 1.8 | | | 414.2 | | | 8.8 | |
| Total revenue | | $ | 9,835.6 | | | $ | 10,173.4 | | | $ | 10,981.7 | | | (3.3) | % | | (7.4) | % | | $ | (337.8) | | | $ | (808.3) | |
PRODUCT REVENUE
Product revenue is summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | % Change | | $ Change |
| | | 2023
vs.
2022 | | 2022
vs.
2021 | | 2023
vs.
2022 | | 2022
vs.
2021 |
| (In millions, except percentages) | | 2023 | | 2022 | | 2021 | | | |
| Multiple Sclerosis | | $ | 4,661.9 | | | $ | 5,430.2 | | | $ | 6,096.7 | | | (14.1) | % | | (10.9) | % | | $ | (768.3) | | | $ | (666.5) | |
| Rare disease | | 1,803.0 | | | 1,793.5 | | | 1,905.1 | | | 0.5 | | | (5.9) | | | 9.5 | | | (111.6) | |
| Biosimilars | | 770.0 | | | 751.1 | | | 831.1 | | | 2.5 | | | (9.6) | | | 18.9 | | | (80.0) | |
Other(1) | | 11.8 | | | 13.0 | | | 14.0 | | | (9.2) | | | (7.1) | | | (1.2) | | | (1.0) | |
| Total product revenue, net | | $ | 7,246.7 | | | $ | 7,987.8 | | | $ | 8,846.9 | | | (9.3) | % | | (9.7) | % | | $ | (741.1) | | | $ | (859.1) | |
(1) Other includes FUMADERM, ADUHELM and ZURZUVAE, which became commercially available in the U.S. during the fourth quarter of 2023.
•Global TECFIDERA revenue decreased $431.4 million, from $1,443.9 million in 2022 to $1,012.5 million in 2023, or 29.9%, driven by a decrease in demand as a result of multiple TECFIDERA generic entrants in North America, Brazil and certain E.U. countries.
•Global Interferon revenue decreased $199.7 million, from $1,305.4 million in 2022 to $1,105.7 million in 2023, or 15.3%, driven by a decrease in sales volumes as patients transition to higher efficacy therapies.
•Global VUMERITY revenue increased $22.9 million, from $553.4 million in 2022 to $576.3 million in 2023, or 4.1%, primarily due to an increase in global demand, partially offset by higher discounts and allowances in the U.S. driven by a favorable Medicaid-related sales adjustment in the first quarter of 2022.
•Global TYSABRI revenue decreased $154.0 million, from $2,030.9 million in 2022 to $1,876.9 million in 2023, or 7.6%, primarily due to a decrease in U.S. TYSABRI revenue driven by a decrease in demand, higher discounts and unfavorable channel dynamics.
MS revenue includes sales from TECFIDERA, VUMERITY, AVONEX, PLEGRIDY, TYSABRI and FAMPYRA. In 2024 we expect total MS revenue will continue to decline as a result of increasing competition for many of our MS products in both the U.S. and rest of world markets. We are also aware of a biosimilar entrant of TYSABRI that was approved in the U.S. in August 2023 and the E.U. in September 2023. We believe that future sales of TYSABRI may be adversely affected by the entrance of this biosimilar.
We believe that we have resolved previously reported manufacturing issues at our VUMERITY contract manufacturer. In addition, we are in the process of securing regulatory approval for a secondary source of supply. We do not anticipate a supply shortage in 2024 and are currently focused on rebuilding adequate inventory.
•U.S. SPINRAZA revenue increased $10.3 million, from $600.2 million in 2022 to $610.5 million in 2023, or 1.7%, primarily due to an increase in pricing, partially offset by higher discounts and allowances.
•Rest of world SPINRAZA revenue decreased $62.6 million, from $1,193.3 million in 2022 to $1,130.7 million in 2023, or 5.2%, primarily due to the unfavorable impact of foreign currency exchange, a decrease in demand in certain European markets driven by increased competition, a decrease in pricing and the timing of shipments in certain Asian markets.
•U.S. SKYCLARYS revenue was $55.9 million in 2023, which we began recognizing during the fourth quarter of 2023, subsequent to our acquisition of Reata.
Rare disease revenue includes sales from SPINRAZA, QALSODY, which became commercially available in the U.S. during the second quarter of 2023, and SKYCLARYS (omaveloxolone), which was obtained as part of our acquisition of Reata in September 2023.
SKYCLARYS became commercially available in the U.S. during the second quarter of 2023 and we began recognizing revenue from SKYCLARYS in the U.S. during the fourth quarter of 2023, subsequent to our acquisition of Reata. In February 2024 the EC approved SKYCLARYS in the E.U. for the treatment of FA in adults and adolescents aged 16 years and older.
In 2024 we expect growth in rare disease revenue as we continue to launch SKYCLARYS in the U.S. Despite competition from a gene therapy product and an oral product, we anticipate SPINRAZA revenue to be relatively flat in 2024. We expect moderate growth in SPINRAZA in the U.S. as well as continued access expansion in emerging markets to offset increased competition and the impact of loading dose dynamics.
•For 2023 compared to 2022, the increase in biosimilar revenue was primarily due to an increase in sales volumes related to the continued launch of BYOOVIZ in the U.S. and rest of world, partially offset by unfavorable BYOOVIZ pricing and the unfavorable impact of foreign currency exchange.
Biosimilars revenue includes sales from BENEPALI, IMRALDI, FLIXABI and BYOOVIZ. BYOOVIZ launched in the U.S. in June 2022 and became commercially available in July 2022 through major distributors in the U.S. In 2023 BYOOVIZ became commercially available in certain international markets. During the third quarter of 2023 the FDA approved TOFIDENCE, a tocilizumab biosimilar referencing ACTEMRA, which we expect to become commercially available during 2024.
In 2024 we anticipate modest growth in revenue from our biosimilars business driven by the continued launch of BYOOVIZ in the U.S. and rest of world, offset in part by lower pricing in certain markets.
We continue to work with our third-party contract manufacturers for IMRALDI and BENEPALI to address supply constraints. If not resolved these supply constraints could have an adverse impact on 2024 sales. In addition, one of our contract manufacturers for IMRALDI and BENEPALI entered into a proposed acquisition by a third party, which is expected to close at the end of 2024. We are currently evaluating the impact this will have on our biosimilars business.
In February 2023 we announced that we are exploring strategic options for our biosimilars business.
REVENUE FROM ANTI-CD20 THERAPEUTIC PROGRAMS
Our share of RITUXAN, including RITUXAN HYCELA, GAZYVA and LUNSUMIO collaboration operating profits in the U.S., royalty revenue on sales of OCREVUS and other revenue from anti-CD20 therapeutic programs are summarized in the table below. For purposes of this discussion, we refer to RITUXAN and RITUXAN HYCELA collectively as RITUXAN.
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| (In millions) | | 2023 | | 2022 | | 2021 |
| Royalty revenue on sales of OCREVUS | | $ | 1,266.2 | | | $ | 1,136.3 | | | $ | 991.7 | |
Biogen’s share of pre-tax profits in the U.S. for RITUXAN, GAZYVA and LUNSUMIO(1) | | 409.4 | | | 547.0 | | | 647.7 | |
| Other revenue from anti-CD20 therapeutic programs | | 14.0 | | | 17.2 | | | 19.1 | |
| Total revenue from anti-CD20 therapeutic programs | | $ | 1,689.6 | | | $ | 1,700.5 | | | $ | 1,658.5 | |
(1) LUNSUMIO became commercially available in the U.S. during the first quarter of 2023.
ROYALTY REVENUE ON SALES OF OCREVUS
For 2023 compared to 2022, the increase in royalty revenue on sales of OCREVUS was primarily due to sales growth of OCREVUS in the U.S.
OCREVUS royalty revenue is based on our estimates from third party and market research data of OCREVUS sales occurring during the corresponding period. Differences between actual and estimated royalty revenue will be adjusted for in the period in which they become known, which is generally expected to be the following quarter.
BIOGEN'S SHARE OF PRE-TAX PROFITS IN THE US. FOR RITUXAN, GAZYVA AND LUNSUMIO
The following table provides a summary of amounts comprising our share of pre-tax profits in the U.S. for RITUXAN, GAZYVA and LUNSUMIO:
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| (In millions) | | 2023 | | 2022 | | 2021 |
| Product revenue, net | | $ | 1,581.3 | | | $ | 1,729.2 | | | $ | 2,032.0 | |
| Cost and expense | | 419.9 | | | 253.6 | | | 291.8 | |
| Pre-tax profits in the U.S. | | $ | 1,161.4 | | | $ | 1,475.6 | | | $ | 1,740.2 | |
| Biogen's share of pre-tax profits | | $ | 409.4 | | | $ | 547.0 | | | $ | 647.7 | |
For 2023 compared to 2022, the decrease in U.S. product revenue, net was primarily due to a decrease in sales volumes of RITUXAN in the U.S. of 15.2%, resulting from competition from multiple biosimilar products, partially offset by an increase in sales volumes of GAZYVA of 17.9%.
For 2023 compared to 2022, the increase in collaboration costs and expense was primarily due to higher expense related to LUNSUMIO, which became commercially available in the first quarter of 2023.
In April 2023 our pre-tax profit share for RITUXAN, GAZYVA and LUNSUMIO decreased from 37.5% to 35.0%.
Prior to regulatory approval, we record our share of the expense incurred by the collaboration for the development of anti-CD20 products in research and development expense and pre-commercialization costs within selling, general and administrative expense in our consolidated statements of income. After an anti-CD20 product is approved, we record our share of the development and sales and marketing expense related to that product as a reduction of our share of pre-tax profits in revenue from anti-CD20 therapeutic programs.
We are aware of several other anti-CD20 molecules, including biosimilar products, that have been approved and are competing with RITUXAN and GAZYVA in the oncology and other markets. Biosimilar products referencing RITUXAN have launched in the U.S and are being offered at lower prices. This competition has had a significant adverse impact on the pre-tax profits of our collaboration arrangements with Genentech, as the sales of RITUXAN have decreased substantially compared to prior periods. We expect that biosimilar competition will continue to increase as these products capture additional market share and that this will have a significant adverse impact on our co-promotion profits in the U.S. in future years.
OTHER REVENUE FROM ANTI-CD20 THERAPEUTIC PROGRAMS
Other revenue from anti-CD20 therapeutic programs consists of our share of pre-tax co-promotion profits from RITUXAN in Canada, royalty revenue on sales of LUNSUMIO outside the U.S. and royalty revenue on net sales of COLUMVI in the U.S, which became commercially available during the second quarter of 2023.
For additional information on our collaboration arrangements with Genentech, including information regarding the pre-tax profit-sharing formula and its impact on future revenue from anti-CD20 therapeutic programs, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
CONTRACT MANUFACTURING, ROYALTY AND OTHER REVENUE
Contract manufacturing, royalty and other revenue is summarized as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| (In millions) | | 2023 | | 2022 | | 2021 |
| Contract manufacturing revenue | | $ | 848.2 | | | $ | 417.7 | | | $ | 427.7 | |
| Royalty and other revenue | | 51.1 | | | 67.4 | | | 48.6 | |
| Total contract manufacturing, royalty and other revenue | | $ | 899.3 | | | $ | 485.1 | | | $ | 476.3 | |
CONTRACT MANUFACTURING REVENUE
We record contract manufacturing revenue primarily from amounts earned under contract manufacturing agreements with our strategic customers.
For 2023 compared to 2022, the increase in contract manufacturing revenue was primarily driven by higher volumes due to the timing of batch production, which includes batches related to LEQEMBI that we began recognizing in the first quarter of 2023 upon the accelerated approval of LEQEMBI in the U.S.
As part of the 2020 sale of our Hillerød, Denmark manufacturing operations to FUJIFILM, we provided FUJIFILM with certain minimum batch production commitment guarantees, including batches related to our contract manufacturing arrangements. As of December 31, 2023, these batch commitments have been satisfied and we expect that our contract manufacturing revenue will be lower in 2024, compared to 2023, as we are no longer supplying contract manufacturing customers in this manner.
For additional information on our collaboration arrangements with Eisai, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
ROYALTY AND OTHER REVENUE
Royalty and other revenue primarily reflects the royalties we receive from net sales on products related to patents that we have out-licensed, as well as royalty revenue on biosimilar products from our license arrangements with Samsung Bioepis and our 50.0% share of LEQEMBI product revenue, net and cost of sales, including royalties, as we are not the principal.
For additional information on our collaborative arrangements with Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
RESERVES FOR DISCOUNTS AND ALLOWANCES
Revenue from product sales is recorded net of reserves established for applicable discounts and allowances, including those associated with the implementation of pricing actions in certain international markets where we operate.
These reserves are based on estimates of the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to our customer) or a liability (if the amount is payable to a party other than our customer). These estimates reflect our historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Actual amounts may ultimately differ from our estimates. If actual results vary, we adjust these estimates, which could have an effect on earnings in the period of adjustment.
Reserves for discounts, contractual adjustments and returns that reduced gross product revenue are summarized as follows:
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| (In millions) | | 2023 | | 2022 | | 2021 |
| Contractual adjustments | | $ | 2,681.7 | | | $ | 2,716.9 | | | $ | 2,852.6 | |
| Discounts | | 735.2 | | | 663.9 | | | 732.8 | |
| Returns | | 38.2 | | | 5.1 | | | 11.9 | |
| Total discounts and allowances | | $ | 3,455.1 | | | $ | 3,385.9 | | | $ | 3,597.3 | |
For the years ended December 31, 2023, 2022 and 2021, reserves for discounts and allowances as a percentage of gross product revenue were 32.0%, 30.1% and 28.6%, respectively.
CONTRACTUAL ADJUSTMENTS
Contractual adjustments primarily relate to Medicaid and managed care rebates in the U.S., pharmacy rebates, co-payment (copay) assistance, VA, 340B discounts, specialty pharmacy program fees and other government rebates or applicable allowances.
For 2023 compared to 2022, the decrease in contractual adjustments was primarily due to lower government rebates in the U.S. as a result of a contract pharmacy change made during the first quarter of 2023 related to our Interferons, partially offset by higher managed care and Medicaid rebates in the U.S. and higher government rebates in rest of world.
DISCOUNTS
Discounts include trade term discounts and wholesaler incentives.
For 2023 compared to 2022, the increase in discounts was primarily driven by higher purchase and volume discounts for biosimilars.
RETURNS
Product return reserves are established for returns made by wholesalers. In accordance with contractual terms, wholesalers are permitted to return product for reasons such as damaged or expired product. The majority of wholesaler returns are due to product expiration. Provisions for estimated product returns are recognized in the period the related revenue is recognized, resulting in a reduction to product sales.
For 2023 compared to 2022, the increase in returns was primarily driven by higher return rates in the U.S.
For additional information on our revenue reserves, please read Note 5, Revenue, to our consolidated financial statements included in this report.
COST AND EXPENSE
A summary of total cost and expense is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, | | % Change | | $ Change |
| | | 2023
vs.
2022 | | 2022
vs.
2021 | | 2023
vs.
2022 | | 2022
vs.
2021 |
| (In millions, except percentages) | | 2023 | | 2022 | | 2021 | | |
| Cost of sales, excluding amortization and impairment of acquired intangible assets | | $ | 2,533.4 | | | $ | 2,278.3 | | | $ | 2,109.7 | | | 11.2 | % | | 8.0 | % | | $ | 255.1 | | | $ | 168.6 | |
| Research and development | | 2,462.0 | | | 2,231.1 | | | 2,501.2 | | | 10.3 | | | (10.8) | | | 230.9 | | | (270.1) | |
| Selling, general and administrative | | 2,549.7 | | | 2,403.6 | | | 2,674.3 | | | 6.1 | | | (10.1) | | | 146.1 | | | (270.7) | |
| Amortization and impairment of acquired intangible assets | | 240.6 | | | 365.9 | | | 881.3 | | | (34.2) | | | (58.5) | | | (125.3) | | | (515.4) | |
| Collaboration profit sharing/(loss reimbursement) | | 218.8 | | | (7.4) | | | 7.2 | | | nm | | (202.8) | | | 226.2 | | | (14.6) | |
| (Gain) loss on fair value remeasurement of contingent consideration | | — | | | (209.1) | | | (50.7) | | | nm | | 312.4 | | | 209.1 | | | (158.4) | |
| Acquired in-process research and development | | — | | | — | | | 18.0 | | | — | | | nm | | — | | | (18.0) | |
| Restructuring charges | | 218.8 | | | 131.1 | | | — | | | 66.9 | | | nm | | 87.7 | | | 131.1 | |
| Gain on sale of building | | — | | | (503.7) | | | — | | | nm | | nm | | 503.7 | | | (503.7) | |
| Other (income) expense, net | | 315.5 | | | (108.2) | | | 1,095.5 | | | (391.6) | | | (109.9) | | | 423.7 | | | (1,203.7) | |
| Total cost and expense | | $ | 8,538.8 | | | $ | 6,581.6 | | | $ | 9,236.5 | | | 29.7 | % | | (28.7) | % | | $ | 1,957.2 | | | $ | (2,654.9) | |
nm Not meaningful
COST OF SALES, EXCLUDING AMORTIZATION AND IMPAIRMENT OF ACQUIRED INTANGIBLE ASSETS
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| (In millions) | | 2023 | | 2022 | | 2021 |
| Product | | $ | 1,787.2 | | | $ | 1,504.8 | | | $ | 1,281.2 | |
| Royalty | | 746.2 | | | 773.5 | | | 828.5 | |
| Total cost of sales | | $ | 2,533.4 | | | $ | 2,278.3 | | | $ | 2,109.7 | |
Cost of sales, as a percentage of total revenue, were 25.8%, 22.4% and 19.2% for the years ended December 31, 2023, 2022 and 2021, respectively.
PRODUCT COST OF SALES
For 2023 compared to 2022, the increase in product cost of sales was primarily due to unfavorable product mix from increased contract manufacturing revenue, MS product mix and higher idle capacity charges, partially offset by a decrease in excess and obsolescence inventory charges in 2023. Contract manufacturing revenue includes LEQEMBI inventory produced for Eisai, beginning in the first quarter of 2023 upon the accelerated approval of LEQEMBI in the U.S. Cost of sales as a percentage of revenue was adversely affected by LEQEMBI batches due to minimal margins. The increase was partially offset by a decrease in excess and obsolescence inventory charges as 2022 included a write-off of approximately $275.0 million during the first quarter of 2022 of excess inventory and contractual commitments related to ADUHELM.
As a result of our acquisition of Reata in September 2023 we recorded a fair value step-up adjustment related to the acquired inventory of SKYCLARYS of approximately $1.3 billion. This fair value step-up adjustment will be amortized to cost of sales within our consolidated statements of income when the inventory is sold, which is expected to be within approximately 3 years from the acquisition date. For the year ended December 31, 2023, amortization from the fair value step-up adjustment as a result of inventory sold during the fourth quarter was approximately $31.5 million. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
Write Downs and Other Charges
Inventory amounts written down as a result of excess, obsolescence or unmarketability totaled $124.4 million, $336.2 million and $167.6 million for the years ended December 31, 2023, 2022 and 2021, respectively.
For the year ended December 31, 2022, we recorded approximately $286.0 million of charges associated with the write-off of ADUHELM inventory and contractual commitments in excess of forecasted demand. We also recognized approximately $197.0 million related to Eisai's 45.0% share of inventory, idle capacity charges and contractual commitments in collaboration profit sharing/(loss reimbursement) within our consolidated statements of income for the year ended December 31, 2022.
For the years ended December 31, 2023 and 2022, we recorded approximately $165.2 million and $119.0 million, respectively, of aggregate gross idle capacity charges.
For additional information on our collaboration arrangements with Eisai, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
ROYALTY COST OF SALES
For 2023 compared to 2022, the decrease in royalty cost of sales was primarily due to lower royalties payable on lower sales of TYSABRI, partially offset by an increase in royalty cost of sales due to higher royalties payable on higher sales of VUMERITY and SKYCLARYS, which we began recognizing in the fourth quarter of 2023, subsequent to our acquisition of Reata.
RESEARCH AND DEVELOPMENT
Research and development expense, as a percentage of total revenue, was 25.0%, 21.9% and 22.8% for the years ended December 31, 2023, 2022 and 2021, respectively.
For 2023 compared to 2022, the increase in research and development was primarily due to approximately $197.0 million of equity-based compensation expense incurred as a result of our acquisition of Reata in 2023, an increase in spending for the development of LEQEMBI for the treatment of Alzheimer's disease, litifilimab for the treatment of CLE and SLE, and TOFIDENCE, a tocilizumab biosimilar referencing ACTEMRA, as well as clinical trial close out costs incurred in 2023 of approximately $125.4 million.
EARLY STAGE PROGRAMS
2023 vs. 2022
The increase in early stage programs was driven by an increase in costs associated with:
•development of BIIB121 for the treatment of Angelman syndrome;
•development of litifilimab for the treatment of CLE;
•development of BIIB115 for the treatment of SMA;
•development of BIIB091 for the treatment of MS; and
•development of BIIB080 for the treatment of Alzheimer's disease.
The increase was partially offset by a decrease in costs associated with:
•discontinuation of BIIB104 for the treatment of cognitive impairment associated with schizophrenia; and
•discontinuation of BIIB078 for the treatment of Alzheimer's disease.
LATE STAGE PROGRAMS
2023 vs. 2022
The decrease in late stage programs was driven by a decrease in costs associated with:
•advancement of LEQEMBI from late stage to marketed upon the accelerated approval of LEQEMBI in the U.S.;
•advancement of ZURZUVAE from late stage to marketed upon the approval of ZURZUVAE for PPD in the U.S.;
•advancement of QALSODY from late stage to marketed upon the accelerated approval of QALSODY in the U.S.; and
•advancement of LUNSUMIO from late stage to marketed upon the accelerated approval of LUNSUMIO in the U.S.
The decrease was partially offset by an increase in costs associated with:
•development of litifilimab for the treatment of SLE into late stage; and
•development of TOFIDENCE, a tocilizumab biosimilar referencing ACTEMRA.
MARKETED PROGRAMS
2023 vs. 2022
The increase in marketed programs was driven by an increase in costs associated with:
•advancement of LEQEMBI from late stage to marketed upon the accelerated approval of LEQEMBI in the U.S.;
•increased spend in ADUHELM primarily due to the change in our cost sharing arrangement with Eisai and clinical trial close out costs of approximately $103.0 million from the termination of our EMBARK and ENVISION studies;
•advancement of ZURZUVAE from late stage to marketed upon the approval of ZURZUVAE for PPD in the U.S.;
•increased spend in SKYCLARYS as a result of our acquisition of Reata in September 2023; and
•advancement of QALSODY from late stage to marketed upon the accelerated approval of QALSODY in the U.S.
MILESTONE AND UPFRONT EXPENSE
Research and development expense for 2023 includes:
•$7.5 million charge to research and development expense in connection with a milestone payment to Ionis; and
•$5.0 million charge to research and development expense in connection with exercising our option with Denali to license the ATV-enabled anti-amyloid beta program.
Research and development expense for 2022 includes:
•$37.0 million in charges to research and development expense in connection with milestone payments to Ionis;
•$15.0 million charge to research and development expense in connection with the upfront payment associated with entering into our collaboration with Alectos Therapeutics Inc. in the second quarter of 2022; and
•$10.0 million charge to research and development expense in connection with the upfront payment associated with entering into our collaboration with Alcyone Therapeutics in the fourth quarter of 2022.
Research and development expense is reported above based on the following classifications. The development stage reported is based upon the program status when incurred. Therefore, the same program could be reflected in different development stages in the same year.
•Research and discovery: represents costs incurred to support our discovery research and translational science efforts.
•Early stage programs: are programs in Phase 1 or Phase 2 development.
•Late stage programs: are programs in Phase 3 development or in registration stage.
•Marketed products: includes costs associated with product lifecycle management activities including, if applicable, costs associated with the development of new indications for existing products.
•Other research and development costs: A significant amount of our research and development costs consist of indirect costs incurred in support of overall research and development activities and non-specific programs, including activities that benefit multiple programs, such as management costs, as well as depreciation, information technology and facility-based expenses. These costs are considered other research and development costs in the table above and are not allocated to a specific program or stage. For several of our programs, the research and development activities are part of our collaborative and other relationships. Our costs reflect our share of the total costs incurred. For the year ended December 31, 2023, other research and development costs also includes approximately $197.0 million of equity-based compensation expense incurred as a result of our acquisition of Reata in September 2023.
Excluding any milestone and upfront payments, we expect our core research and development expense to decrease in 2024, while continuing to invest in our pipeline. This is primarily due to the continued realization of our cost savings initiatives and the one-time costs incurred from our acquisition of Reata in September 2023 of approximately $197.0 million. We intend to continue committing significant resources to targeted research and development opportunities where there is a significant unmet need and where a drug candidate has the potential to be highly differentiated.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
SELLING, GENERAL AND ADMINISTRATIVE
For 2023 compared to 2022, selling, general and administrative expense increased by approximately 6.1% primarily due to the recognition of approximately $196.4 million in equity-based compensation expense related to our acquisition of Reata in September 2023. Additionally, we incurred transaction and integration-related expense of approximately $34.6 million related to this acquisition. The increase in selling, general and administrative expense was also due to a $31.0 million obligation to Eisai related to the termination of the co-promotion agreement for our MS products in Japan during 2023 and approximately $11.5 million of accelerated depreciation, associated with exiting a leased property, recognized during the second quarter of 2023. The increases were partially offset by the impact of cost-reduction measures realized during 2023.
We expect selling, general and administrative costs to continue to decline in 2024 due to the continued realization of our cost savings initiatives and the one-time costs incurred from our acquisition of Reata in 2023 of approximately $196.4 million.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report. For additional information on our collaboration arrangements with Eisai, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
AMORTIZATION AND IMPAIRMENT OF ACQUIRED INTANGIBLE ASSETS
Our amortization expense is based on the economic consumption and impairment of intangible assets. Our most significant amortizable intangible assets are related to TYSABRI, AVONEX, SPINRAZA, VUMERITY and SKYCLARYS, which was obtained as part of our acquisition of Reata in September 2023. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
For 2023 compared to 2022, the decrease in amortization and impairment of acquired intangible assets was primarily due to higher impairment charges in 2022 of approximately $119.6 million, compared to no impairment charges in 2023.
For the year ended December 31, 2022, amortization and impairment of acquired intangible assets reflects the impact of a $119.6 million impairment charge related to vixotrigine (BIIB074) for the potential treatment of DPN.
Amortization of acquired intangible assets, excluding impairment charges, totaled $240.6 million, $246.3 million and $252.0 million for the years ended December 31, 2023, 2022 and 2021, respectively. The decrease in amortization of acquired intangible assets, excluding impairment charges, over the three years was primarily due to a lower rate of amortization for acquired intangible assets.
For additional information on the amortization and impairment of our acquired intangible assets, please read Note 7, Intangible Assets and Goodwill, to our consolidated financial statements included in this report.
COLLABORATION PROFIT SHARING/(LOSS REIMBURSEMENT)
Collaboration profit sharing/(loss reimbursement) primarily includes Samsung Bioepis' 50.0% share of the profit or loss related to our biosimilars 2013 commercial agreement with Samsung Bioepis. In the third quarter of 2023 we began recognizing collaboration profit sharing/(loss reimbursement) related to Sage's 50.0% share of income and expense in the U.S. related to ZURZUVAE for PPD. During 2022 we recognized Eisai's 45.0% share of income and expense in the U.S. related to the ADUHELM Collaboration Agreement. Beginning January 1, 2023, Eisai receives only a tiered royalty based on net sales of ADUHELM, and will no longer share global profits and losses.
For the years ended December 31, 2023 and 2022, we recognized net profit-sharing expense of $223.5 million and $217.4 million, respectively, to reflect Samsung Bioepis' 50.0% sharing of the net collaboration profits.
For the year ended December 31, 2023, we recognized net reductions to our operating expense of approximately $4.7 million to reflect Sage's 50.0% share of net collaboration losses in the U.S.
For the year ended December 31, 2022, we recognized net reductions to our operating expense of approximately $224.7 million to reflect Eisai's 45.0% share of net collaboration losses in the U.S. for ADUHELM.
For additional information on our collaboration and license arrangements with Samsung Bioepis, Sage and Eisai, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
(GAIN) LOSS ON FAIR VALUE REMEASUREMENT OF CONTINGENT CONSIDERATION
For the year ended December 31, 2022, the changes in fair value of our contingent consideration obligations were primarily due to the discontinuation of further development efforts related to vixotrigine for the potential treatment of TGN and DPN, resulting in a reduction of our contingent consideration obligations of approximately $195.4 million, reducing the remaining fair value of vixotrigine to zero, as well as changes in the interest rates used to revalue our contingent consideration liabilities.
For additional information on our IPR&D intangible assets, please read Note 7, Intangible Assets and Goodwill, to our consolidated financial statements included in this report.
RESTRUCTURING CHARGES
2023 FIT FOR GROWTH RESTRUCTURING PROGRAM
In 2023 we initiated additional cost saving measures as part of our Fit for Growth program to reduce operating costs, while improving operating efficiency and effectiveness. The Fit for Growth program is expected to generate approximately $1.0 billion in gross operating expense savings and $800.0 million in net operating expense savings by 2025, some of which will be reinvested in various initiatives. The Fit for Growth program is currently estimated to include net headcount reductions of approximately 1,000 employees and we expect to incur restructuring charges ranging from approximately $260.0 million to $280.0 million.
Total charges incurred from our 2023 cost saving initiatives are summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2023 |
| | |
| 23.3 | | | | |
| | | |
| | | |
| 212.5 | | | | |
| | | | | | | | | | | |
|
|
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Other Costs: includes costs associated with items such as asset abandonment and write-offs, facility closure costs, pretax gains and losses resulting from the termination of certain leases, employee non-severance expense, consulting fees and other costs.
REATA INTEGRATION
Following the close of our Reata acquisition, we implemented an integration plan designed to realize operating synergies through cost savings and avoidance. These amounts are primarily related to severance and are expected to be paid by the end of 2024. For the year ended December 31, 2023, we recognized approximately $30.4 million of net pre-tax restructuring charges related to employee severance costs.
2022 COST SAVING INITIATIVES
In December 2021 and May 2022 we announced our plans to implement a series of cost-reduction measures during 2022. These savings are being achieved through a number of initiatives, including reductions to our workforce, the substantial elimination of our commercial ADUHELM infrastructure, deprioritization of certain research and development programs, the consolidation of certain real estate locations and operating efficiencies across our selling, general and administrative and research and development functions. Charges related to our 2022 cost saving initiatives were substantially incurred during 2022 with remaining payments expected to be made through 2026.
Total charges incurred from our 2022 cost saving initiatives are summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2023 | | 2022 |
| (In millions) | | Severance
Costs | | Accelerated Depreciation and Other Costs | | Total | | Severance Costs | | Accumulated Depreciation and Other Costs(1) | | Total |
| Restructuring charges | | $ | (2.2) | | | $ | 2.6 | | | $ | 0.4 | | | $ | 112.6 | | | $ | 18.5 | | | $ | 131.1 | |
| Total charges | | $ | (2.2) | | | $ | 2.6 | | | $ | 0.4 | | | $ | 112.6 | | | $ | 18.5 | | | $ | 131.1 | |
(1) Amounts reflect a gain recorded during the third quarter of 2022 of approximately $5.3 million related to the partial termination of a portion of our lease located at 300 Binney Street. For additional information on our 300 Binney Street lease modification, please read Note 12, Leases, to these consolidated financial statements.
For additional information on our cost saving initiatives, please read Note 4, Restructuring, to our consolidated financial statements included in this report.
In September 2022 we completed the sale of our building and land parcel located at 125 Broadway for an aggregate sales price of approximately $603.0 million, which is inclusive of a $10.8 million tenant allowance. This sale resulted in a pre-tax gain on sale of approximately $503.7 million, net of transaction costs, which is reflected within gain on sale of building in our consolidated statements of income for the year ended December 31, 2022. Simultaneously, with the close of this transaction we leased back the building for a term of approximately 5.5 years, which resulted in the recognition of approximately $168.2 million in a new lease liability and right-of-use asset recorded within our consolidated balance sheets as of December 31, 2022. The sale and immediate leaseback of this building qualified for sale and leaseback treatment and is classified as an operating lease.
For additional information on our 125 Broadway sale and leaseback transaction, please read Note 11, Property, Plant and Equipment and Note 12, Leases, to our consolidated financial statements included in this report.
OTHER (INCOME) EXPENSE, NET
For 2023 compared to 2022, the change in other (income) expense, net primarily reflects a pre-tax gain recorded during 2022 of approximately $1.5 billion related to the sale of our 49.9% equity interest in Samsung Bioepis, partially offset by a pre-tax charge recorded during 2022 of approximately $900.0 million, plus settlement fees and expenses, related to a litigation settlement agreement to resolve a qui tam litigation relating to conduct prior to 2015. Additionally, other (income) expense, net for 2023 reflects higher interest income driven by higher interest rates in 2023.
NET (GAINS) LOSSES IN EQUITY SECURITIES
For the year ended December 31, 2023, net unrealized and realized losses on our holdings in equity securities were approximately $270.0 million and $5.2 million, respectively, compared to net unrealized losses and realized (gains) losses of approximately $264.7 million and zero, respectively, in 2022.
•The net unrealized losses recognized during the year ended December 31, 2023, primarily reflect a decrease in the aggregate fair value of our investments in Sage, Denali, Sangamo and Ionis common stock of approximately $248.5 million.
•The net unrealized losses recognized during the year ended December 31, 2022, primarily reflect a decrease in the aggregate fair value of our investments in Denali and Sangamo common stock of approximately $278.0 million, partially offset by an increase in the fair value of Ionis and Sage common stock of approximately $27.3 million.
INTEREST INCOME AND EXPENSE
For the year ended December 31, 2023, net interest income was $29.6 million, compared to net interest expense of $157.3 million in 2022. The increase was primarily due to higher interest rates leading to greater interest income earned on our investments in 2023, compared to 2022.
For 2024 compared to 2023, we anticipate an increase in net interest expense as a result of lower cash balances leading to lower interest income due to the funding of our acquisition of Reata.
For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to our consolidated financial statements included in this report.
For additional information on the litigation settlement agreement, please read Note 18, Other Consolidated Financial Statement Detail, to our consolidated financial statements included in this report.
INCOME TAX PROVISION
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| (In millions, except percentages) | | 2023 | | 2022 | | 2021 |
| Income before income tax (benefit) expense | | $ | 1,296.8 | | | $ | 3,591.8 | | | $ | 1,745.2 | |
| Income tax (benefit) expense | | 135.3 | | | 632.8 | | | 52.5 | |
| Effective tax rate | | 10.4 | % | | 17.6 | % | | 3.0 | % |
Our effective tax rate fluctuates from year to year due to the global nature of our operations. The factors that most significantly impact our effective tax rate include changes in tax laws, variability in the allocation of our taxable earnings among multiple jurisdictions, the amount and characterization of our research and development expense, the levels of certain deductions and credits, acquisitions and licensing transactions.
For 2023 compared to 2022, the decrease in our effective tax rate was driven by the impact of the non-cash changes in the value of our equity investments, the impact of Fit for Growth related expenses and Reata acquisition-related expenses, as well as the combined net unfavorable tax rate impacts in 2022 related to a litigation settlement agreement, the sale of our equity interest in Samsung Bioepis, the impact of a Neurimmune valuation allowance and an international reorganization to align with global tax developments. The change also benefits from the resolution of an uncertain tax matter during the first quarter of 2023 related to tax credits.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
For additional information on the litigation settlement agreement, please read Note 18, Other Consolidated Financial Statement Detail, to our consolidated financial statements included in this report.
For additional information on our income taxes, uncertain tax positions and income tax rate reconciliation, please read Note 17, Income Taxes, to our consolidated financial statements included in this report.
EQUITY IN (INCOME) LOSS OF INVESTEE, NET OF TAX
In February 2012 we entered into a joint venture agreement with Samsung BioLogics establishing an entity, Samsung Bioepis, to develop, manufacture and market biosimilar products.
In April 2022 we completed the sale of our 49.9% equity interest in Samsung Bioepis to Samsung BioLogics. Following the sale of Samsung Bioepis we no longer recognize gains or losses associated with Samsung Bioepis' results of operations and amortization related to basis differences.
Prior to this sale, we recognized our share of the results of operations related to our investment in Samsung Bioepis under the equity method of accounting one quarter in arrears when the results of the entity became available, which was reflected as equity in (income) loss of investee, net of tax in our consolidated statements of income. We also recognized amortization on certain basis differences resulting from our November 2018 investment.
For the year ended December 31, 2022, we recognized net income on our investment of $2.6 million, reflecting our share of Samsung Bioepis' operating profits, net of tax, totaling $17.0 million offset by amortization of basis differences totaling $14.4 million. This amount reflects our share of results prior to the sale of Samsung Bioepis as the results are recognized one quarter in arrears.
For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to our consolidated financial statements included in this report.
For additional information on our collaboration arrangements with Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
NONCONTROLLING INTERESTS, NET OF TAX
Our consolidated financial statements include the financial results of a variable interest entity, Neurimmune, as we determined that we were the primary beneficiary.
In November 2023 we notified Neurimmune of our decision to terminate the Neurimmune Agreement. Subsequent to the termination, we reconsidered our relationship with Neurimmune and determined that we were no longer the primary beneficiary of the variable interest entity. As a result, we recorded a net gain on the deconsolidation of
Neurimmune of approximately $3.0 million, which was recorded in other (income) expense, net within our consolidated statements of income for the year ended December 31, 2023.
For 2023 compared to 2022, the change in net income (loss) attributable to noncontrolling interests, net of tax, was primarily due to an increase in a valuation allowance of approximately $85.0 million recorded in the first quarter of 2022.
For additional information on the valuation allowance, deconsolidation and our collaboration agreement with Neurimmune, please read Note 20, Investments in Variable Interest Entities, to our consolidated financial statements included in this report.
FINANCIAL CONDITION, LIQUIDITY AND CAPITAL RESOURCES
Our financial condition is summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | | | | |
| (In millions, except percentages) | | 2023 | | 2022 | | % Change | | $ Change |
| Financial assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 1,049.9 | | | $ | 3,419.3 | | | (69.3) | % | | $ | (2,369.4) | |
| Marketable securities — current | | — | | | 1,473.5 | | | nm | | (1,473.5) | |
| Marketable securities — non-current | | — | | | 705.7 | | | nm | | (705.7) | |
| Total cash, cash equivalents and marketable securities | | $ | 1,049.9 | | | $ | 5,598.5 | | | (81.2) | % | | $ | (4,548.6) | |
| Borrowings: | | | | | | | | |
| Current portion of term loan | | $ | 150.0 | | | $ | — | | | nm | | $ | 150.0 | |
| Notes payable and term loan | | 6,788.2 | | | 6,281.0 | | | 8.1 | | | 507.2 | |
| Total borrowings | | $ | 6,938.2 | | | $ | 6,281.0 | | | 10.5 | % | | $ | 657.2 | |
| Working Capital: | | | | | | | | |
| Current assets | | $ | 6,859.3 | | | $ | 9,791.2 | | | (29.9) | % | | $ | (2,931.9) | |
| Current liabilities | | (3,434.3) | | | (3,272.8) | | | 4.9 | | | (161.5) | |
| Total working capital | | $ | 3,425.0 | | | $ | 6,518.4 | | | (47.5) | % | | $ | (3,093.4) | |
nm Not meaningful
OVERVIEW
We have historically financed and expect to continue to fund our operating and capital expenditures primarily through cash flow earned through our operations, as well as our existing cash resources. We believe that generic and biosimilar competition for many of our key products, the continued overall decline of our MS business and our investments in the launch of key new products and the development of our pipeline will have a significant adverse impact on our future cash flow from operations.
We believe that our existing funds, when combined with cash generated from operations and our access to additional financing resources, if needed, are sufficient to satisfy our operating, working capital, strategic alliance, milestone payment, capital expenditure and debt service requirements for the foreseeable future. In addition, we may choose to opportunistically return cash to shareholders and pursue other business initiatives, including acquisition and licensing activities. We may also seek additional funding through a combination of new collaborative agreements, strategic alliances and additional equity and debt financings or from other sources should we identify a significant new opportunity.
On September 26, 2023, we completed the acquisition of all of the issued and outstanding shares of Reata for $6.6 billion and $983.9 million for outstanding equity awards. This transaction was funded with cash on hand and the issuance of a $1.0 billion term loan. Additionally, we paid approximately $459.9 million to settle outstanding debt obligations acquired as part of our acquisition of Reata. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
For additional information on certain risks that could negatively impact our financial position or future results of operations, please read Item 1A. Risk Factors and Item 7A. Quantitative and Qualitative Disclosures About Market Risk included in this report.
LIQUIDITY
WORKING CAPITAL
Working capital is defined as current assets less current liabilities. Our working capital was $3.4 billion as of December 31, 2023, compared to $6.5 billion as of December 31, 2022. The change in working capital reflects a decrease in total current assets of approximately $2,931.9 million and an increase in total current liabilities of approximately $161.5 million. The changes in total current assets and total current liabilities were primarily driven by the following:
CURRENT ASSETS
•$3,842.9 million decrease in cash, cash equivalents and current marketable securities primarily due to consideration paid for our acquisition of Reata as well as the early repayment of $350.0 million in outstanding debt obligations associated with our Reata acquisition;
•$235.6 million decrease in other current assets primarily due to the receipt of $812.5 million from Samsung BioLogics related to the sale of Samsung Bioepis, partially offset by the final deferred payment of $437.5 million now due within one year; and
•$1,183.0 million increase in inventory primarily due to the fair value step-up adjustment for acquired inventory from our acquisition of Reata.
CURRENT LIABILITIES
•$88.2 million decrease in accounts payable primarily due to timing of payments;
•$102.2 million increase in accrued expense and other primarily reflecting accrued costs related to our acquisition of Reata; and
•$150.0 million increase in current portion of debt due to the short-term portion of our outstanding 2023 Term Loan related to our acquisition of Reata.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
For additional information on our 2023 Term Loan, please read Note 13, Indebtedness, to our consolidated financial statements included in this report.
For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to our consolidated financial statements included in this report.
CASH, CASH EQUIVALENTS AND MARKETABLE SECURITIES
As of December 31, 2023, we had cash, cash equivalents and marketable securities totaling approximately $1.0 billion compared to approximately $5.6 billion as of December 31, 2022. The decrease in the balance was primarily due to the use of cash, cash equivalents and marketable securities to fund our acquisition of Reata. In connection with our acquisition of Reata we paid approximately $6.6 billion for the issued and outstanding shares of Reata and $983.9 million related to Reata's outstanding equity awards, inclusive of employer taxes. Additionally, we assumed a payable to Blackstone of approximately $300.0 million related to a one-time contract termination fee to eliminate potential future royalty obligations related to SKYCLARYS, which was triggered as part of the change in control provision under Reata's funding agreement with Blackstone. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements included in this report.
Until required for another use in our business, we typically invest our cash reserves in bank deposits, certificates of deposit, commercial paper, corporate notes, U.S. and foreign government instruments, overnight reverse repurchase agreements and other interest-bearing marketable debt instruments in accordance with our investment policy. It is our policy to mitigate credit risk in our cash reserves and marketable securities by maintaining a well-diversified portfolio that limits the amount of exposure as to institution, maturity and investment type. We have experienced no significant limitations in our liquidity resulting from uncertainties in the banking sector.
The following table summarizes the fair value of our significant common stock investments in our strategic investment portfolio:
| | | | | | | | | | | | | | |
| | As of December 31, |
| (In millions) | | 2023 | | 2022 |
Denali(1) | | $ | 273.6 | | | $ | 370.2 | |
| Sage | | 135.3 | | | 238.0 | |
Sangamo(1) | | 7.9 | | | 74.3 | |
Ionis(2) | | — | | | 108.6 | |
| Total | | $ | 416.8 | | | $ | 791.1 | |
(1) During 2023 we sold a portion of our Sangamo and Denali common stock.
(2) During 2023 we sold our remaining shares of Ionis common stock.
Our ability to liquidate our investments in Denali, Sage and Sangamo may be limited by the size of our interest, the volume of market related activity, our concentrated level of ownership and potential restrictions resulting from our status as a collaborator. Therefore, we may realize significantly less than the current value of such investments.
For additional information on our collaboration arrangements, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
CASH FLOW
The following table summarizes our cash flow activity:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | % Change |
| | | For the Years Ended December 31, | | 2023
vs.
2022 | | 2022
vs.
2021 |
| (In millions, except percentages) | | 2023 | | 2022 | | 2021 | | |
| Net cash flow provided by (used in) operating activities | | $ | 1,547.2 | | | $ | 1,384.3 | | | $ | 3,639.9 | | | 11.8 | % | | (62.0) | % |
| Net cash flow provided by (used in) investing activities | | (4,101.0) | | | 1,576.6 | | | (563.7) | | | (360.1) | | | 379.7 | |
| Net cash flow provided by (used in) financing activities | | 149.3 | | | (1,747.3) | | | (2,086.2) | | | 108.5 | | | (16.2) | |
OPERATING ACTIVITIES
Operating cash flow is derived by adjusting our net income for:
•non-cash operating items such as depreciation and amortization, impairment charges, unrealized (gain) loss on strategic investments and share-based compensation;
•changes in operating assets and liabilities, which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in results of operations; and
•(gains) losses on the disposal of assets, deferred income taxes, changes in the fair value of contingent payments associated with our acquisitions of businesses and acquired IPR&D.
For 2023 compared to 2022, the change in net cash flow provided by operating activities was driven by changes in operating assets and liabilities primarily related to a lower inventory build in 2023 as compared to 2022, the favorable timing of customer payment receipts in 2023 and the unfavorable timing of U.S. federal tax payments in 2023. Operating cash flow in 2023 was also negatively impacted by the $393.4 million in equity-based compensation payments associated with the Reata acquisition. Operating cash flow in 2022 was also negatively impacted by the payment of of approximately $900.0 million, plus settlement fees and expenses, related to a litigation settlement agreement to resolve a qui tam litigation relating to conduct prior to 2015.
INVESTING ACTIVITIES
For 2023 compared to 2022, the change in net cash flow provided by (used in) investing activities was primarily due to a $6.9 billion payment made in 2023 for our acquisition of Reata, net of cash acquired, partially offset by higher net proceeds from the sales of marketable securities in the current period. Additionally, we received $582.6 million in 2022 related to the sale of one of our buildings.
FINANCING ACTIVITIES
For 2023 compared to 2022, the change in net cash flow provided by (used in) financing activities was primarily due to the issuance of our 2023 Term Loans totaling $1.0 billion under our $1.5 billion term loan credit agreement which were used to partially fund our acquisition of Reata, partially offset by repayments of borrowings and debt premiums paid totaling $809.9 million. We had debt repayments of approximately $1.0 billion and share repurchases of $750.0 million during the same period in 2022.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
CAPITAL RESOURCES
DEBT AND CREDIT FACILITIES
LONG-TERM DEBT AND TERM LOAN CREDIT AGREEMENTS
Our long-term obligations primarily consist of long-term debt related to our Senior Notes with final maturity dates ranging between 2025 and 2051. As of December 31, 2023, our outstanding balance related to long-term debt was $6,788.2 million.
In connection with our acquisition of Reata in September 2023 we entered into a $1.5 billion term loan credit agreement. On the closing date of the Reata acquisition we drew $1.0 billion from the 2023 Term Loan, comprised of a $500.0 million floating rate 364-day tranche and a $500.0 million floating rate three-year tranche. The remaining unused commitment of $500.0 million was terminated. During the fourth quarter of 2023 we repaid $350.0 million of the 364-day tranche. As of December 31, 2023, we had $650.0 million outstanding under the 2023 Term Loan, of which $150.0 million was outstanding under the 364-day tranche and $500.0 million was outstanding under the three-year tranche.
2020 REVOLVING CREDIT FACILITY
In January 2020 we entered into a $1.0 billion, five-year senior unsecured revolving credit facility under which we are permitted to draw funds for working capital and general corporate purposes. The terms of the revolving credit facility include a financial covenant that requires us not to exceed a maximum consolidated leverage ratio. As of December 31, 2023, we had no outstanding borrowings and were in compliance with all covenants under this facility.
For a summary of the fair values of our outstanding borrowings as of December 31, 2023 and 2022, please read Note 8, Fair Value Measurements, to our consolidated financial statements included in this report.
For additional information on our Senior Notes, 2023 Term Loan and credit facility please read, Note 13, Indebtedness, to our consolidated financial statements included in this report.
SHARE REPURCHASE PROGRAMS
In October 2020 our Board of Directors authorized our 2020 Share Repurchase Program, which is a program to repurchase up to $5.0 billion of our common stock. Our 2020 Share Repurchase Program does not have an expiration date. All share repurchases under our 2020 Share Repurchase Program will be retired. Under our 2020 Share Repurchase Program, we repurchased and retired approximately 3.6 million and 6.0 million shares of our common stock at a cost of approximately $750.0 million and $1.8 billion during the years ended December 31, 2022 and 2021, respectively. There were no share repurchases of our common stock during the year ended December 31, 2023. Approximately $2.1 billion remained available under our 2020 Share Repurchase Program as of December 31, 2023.
CAPITAL EXPENDITURES
In the fourth quarter of 2021 we began construction of a new gene therapy manufacturing facility in RTP, North Carolina to support our gene therapy pipeline across multiple therapeutic areas. The new manufacturing facility will be approximately 197,000 square feet with an estimated total investment of approximately $195.0 million. As we continue to advance our research and development prioritization efforts, which includes refocusing our investment in gene therapy, we are evaluating several alternative uses for this facility.
CONTRACTUAL OBLIGATIONS AND OFF-BALANCE SHEET ARRANGEMENTS
CONTRACTUAL OBLIGATIONS
The following table summarizes our contractual obligations as of December 31, 2023, excluding amounts related to uncertain tax positions, funding commitments, contingent development, regulatory and commercial milestone payments, contingent payments and contingent consideration related to our business combinations, as described below. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period |
| (In millions) | | Total | | Less than
1 Year | | 1 to 3
Years | | 3 to 5
Years | | After
5 Years |
Non-cancellable operating leases (1)(2)(3) | | $ | 524.6 | | | $ | 85.1 | | | $ | 152.3 | | | $ | 116.7 | | | $ | 170.5 | |
Long-term debt obligations (4) | | 10,756.0 | | | 400.3 | | | 2,685.5 | | | 323.7 | | | 7,346.5 | |
Purchase and other obligations (5) | | 807.7 | | | 524.9 | | | 277.5 | | | 0.8 | | | 4.5 | |
| Defined benefit obligation | | 98.0 | | | — | | | — | | | — | | | 98.0 | |
| Total contractual obligations | | $ | 12,186.3 | | | $ | 1,010.3 | | | $ | 3,115.3 | | | $ | 441.2 | | | $ | 7,619.5 | |
(1) We lease properties and equipment for use in our operations. Amounts reflected within the table above detail future minimum rental commitments under non-cancelable operating leases as of December 31 for each of the periods presented. In addition to the minimum rental commitments, these leases may require us to pay additional amounts for taxes, insurance, maintenance and other operating expenses.
(2) Obligations are presented net of sublease income expected to be received for our vacated portions of our Weston, Massachusetts facility and other facilities throughout the world.
(3) In connection with our acquisition of Reata in September 2023 we assumed operating lease commitments, including the responsibility for a single-tenant, built-to-suit building with a total net present value of rental expense of approximately $154.4 million over the next 15 years. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
(4) Long-term debt obligations are related to our 2015 Senior Notes, our 2020 Senior Notes and our 2021 Exchange Offer Senior Notes, including principal and interest payments, and our 2023 Term Loan. For additional information on our long-term debt obligations, please read Note 13, Indebtedness, to our consolidated financial statements included in this report.
(5) Purchase and other obligations include $419.5 million related to the remaining payments on the Transition Toll Tax and $31.6 million related to the fair value of net liabilities on derivative contracts.
ROYALTY PAYMENTS
TYSABRI
We are obligated to make contingent payments of 18.0% on annual worldwide net sales of TYSABRI up to $2.0 billion and 25.0% on annual worldwide net sales of TYSABRI that exceed $2.0 billion. Royalty payments are recognized as cost of sales in our consolidated statements of income.
SPINRAZA
We make royalty payments to Ionis on annual worldwide net sales of SPINRAZA using a tiered royalty rate between 11.0% and 15.0%, which are recognized as cost of sales in our consolidated statements of income.
For additional information on our collaboration arrangements with Ionis, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
QALSODY
We make royalty payments to Ionis on annual worldwide net sales of QALSODY using a tiered royalty rate between 11.0% and 15.0%, which are recognized as cost of sales in our consolidated statements of income.
For additional information on our collaboration arrangements with Ionis, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
VUMERITY
We make royalty payments to Alkermes on worldwide net sales of VUMERITY using a royalty rate of 15.0%, which are recognized as cost of sales in our consolidated statements of income. Royalties payable on net sales of VUMERITY are subject, under certain circumstances, to tiered minimum annual payment requirements for a period of five years following FDA approval.
In October 2019 we entered into a new supply agreement and amended our license and collaboration agreement with Alkermes for VUMERITY. We have elected to initiate a technology transfer and, following a transition period, to manufacture VUMERITY or have VUMERITY manufactured by a third party we have engaged in exchange for paying an increased royalty rate to Alkermes on any portion of future worldwide net commercial sales of VUMERITY that is manufactured by us or our designee.
For additional information on our collaboration arrangement with Alkermes, please read Note 19, Collaborative and Other Relationships, to our consolidated financial statements included in this report.
SKYCLARYS
In connection with our acquisition of Reata in September 2023 we assumed additional contractual obligations related to royalty payments. Reata entered into agreements to pay royalties on future sales of SKYCLARYS, which will cumulatively range in the low- to mid-single digits.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
CONTINGENT DEVELOPMENT, REGULATORY AND COMMERCIAL MILESTONE PAYMENTS
Based on our development plans as of December 31, 2023, we could trigger potential future milestone payments to third parties of up to approximately $5.1 billion, including approximately $0.9 billion in development milestones, approximately $0.4 billion in regulatory milestones and approximately $3.8 billion in commercial milestones, as part of our various collaborations, including licensing and development programs. Payments under these agreements generally become due and payable upon achievement of certain development, regulatory or commercial milestones. Because the achievement of these milestones was not considered probable as of December 31, 2023, such contingencies have not been recorded in our financial statements. Amounts related to contingent milestone payments are not considered contractual obligations as they are contingent on the successful achievement of certain development, regulatory or commercial milestones.
During the fourth quarter of 2023 we accrued a milestone payment due to Sage of $75.0 million upon the first commercial sale of ZURZUVAE for PPD in the U.S., which was recorded within intangible assets, net in our consolidated balance sheets, and subsequently paid in January 2024. If certain clinical and commercial milestones are met, we may pay up to approximately $109.0 million in milestones in 2024 under our current agreements.
OTHER FUNDING COMMITMENTS
As of December 31, 2023, we have several ongoing clinical studies in various clinical trial stages. Our most significant clinical trial expenditures are to CROs. The contracts with CROs are generally cancellable, with notice, at our option. We recorded accrued expense of approximately $47.2 million in our consolidated balance sheets for expenditures incurred by CROs as of December 31, 2023. We have approximately $669.0 million in cancellable future commitments based on existing CRO contracts as of December 31, 2023.
TAX RELATED OBLIGATIONS
We exclude liabilities pertaining to uncertain tax positions from our summary of contractual obligations as we cannot make a reliable estimate of the period of cash settlement with the respective taxing authorities. As of December 31, 2023, we have approximately $172.0 million of liabilities associated with uncertain tax positions.
As of December 31, 2023 and 2022, we have accrued income tax liabilities of approximately $419.5 million and $558.0 million, respectively, under the Transition Toll Tax. Of the amounts accrued as of December 31, 2023, approximately $185.4 million is expected to be paid within one year. The Transition Toll Tax is being paid in installments over an eight--year period, which started in 2018, and will not accrue interest.
OTHER OFF-BALANCE SHEET ARRANGEMENTS
We do not have any relationships with entities often referred to as structured finance or special purpose entities that were established for the purpose of facilitating off-balance sheet arrangements. As such, we are not exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in such relationships. We consolidate variable interest entities if we are the primary beneficiary.
NEW ACCOUNTING STANDARDS
For a discussion of new accounting standards please read Note 1, Summary of Significant Accounting Policies, to our consolidated financial statements included in this report.
LEGAL MATTERS
For a discussion of legal matters as of December 31, 2023, please read Note 21, Litigation, to our consolidated financial statements included in this report.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of our consolidated financial statements, which have been prepared in accordance with U.S. GAAP, requires us to make estimates, judgments and assumptions that may affect the reported amounts of assets, liabilities, equity, revenue and expense and related disclosure of contingent assets and liabilities. On an ongoing basis we evaluate our estimates, judgments and assumptions. We base our estimates on historical experience and on various other assumptions that we believe are reasonable, the results of which form the basis for making judgments about the carrying values of assets, liabilities and equity and the amount of revenue and expense. Actual results may differ from these estimates. Other significant accounting policies are outlined in Note 1, Summary of Significant Accounting Policies, to our consolidated financial statements included in this report.
REVENUE RECOGNITION
We recognize revenue when our customer obtains control of promised goods or services, in an amount that reflects the consideration which we expect to receive in exchange for those goods or services. We recognize revenue following the five-step model prescribed under FASB ASC 606, Revenue from Contracts with Customers: (i) identify contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) we satisfy the performance obligations.
PRODUCT REVENUE
In the U.S., we sell our products primarily to wholesale and specialty distributors and specialty pharmacies. In other countries, we sell our products primarily to wholesale distributors, hospitals, pharmacies and other third-party distribution partners. These customers subsequently resell our products to health care providers and patients. In addition, we enter into arrangements with health care providers and payors that provide for government-mandated or privately-negotiated discounts and allowances related to our products.
Product revenue is recognized when the customer obtains control of our product, which occurs at a point in time, typically upon delivery to the customer. We expense incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that we would have recognized is one year or less or the amount is immaterial.
RESERVES FOR DISCOUNTS AND ALLOWANCES
Product revenue is recorded net of reserves established for applicable discounts and allowances that are offered within contracts with our customers, health care providers or payors, including those associated with the implementation of pricing actions in certain of the international markets in which we operate. Our process for estimating reserves established for these variable consideration components do not differ materially from our historical practices.
Product revenue reserves, which are classified as a reduction in product revenue, are generally characterized in the following categories: discounts, contractual adjustments and returns.
These reserves are based on estimates of the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to our customer) or a liability (if the amount is payable to a party other than our customer). Our estimates of reserves established for variable consideration are calculated based upon a consistent application of our methodology utilizing the expected value method. These estimates reflect our historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. The transaction price, which includes variable consideration reflecting the impact of discounts and allowances, may be subject to constraint and is included in the net sales price only to the extent that it is probable that a significant reversal of the amount of the
cumulative revenue recognized will not occur in a future period. Actual amounts may ultimately differ from our estimates. If actual results vary, we adjust these estimates, which could have an effect on earnings in the period of adjustment.
As of December 31, 2023 and 2022, a 10.0% change in our discounts, contractual adjustments and reserves would have resulted in a decrease of our pre-tax earnings by approximately $345.5 million and $338.6 million, respectively.
In addition to discounts, rebates and product returns, we also maintain certain customer service contracts with distributors and other customers in the distribution channel that provide us with inventory management, data and distribution services, which are generally reflected as a reduction of revenue. To the extent we can demonstrate a separable benefit and fair value for these services we classify these payments in selling, general and administrative expense in our consolidated statements of income.
For additional information on our revenue, please read Note 5, Revenue, to our consolidated financial statements included in this report.
ACQUIRED INTANGIBLE ASSETS, INCLUDING IPR&D
When we purchase a business, the acquired IPR&D is measured at fair value, capitalized as an intangible asset and tested for impairment at least annually, as of October 31, until commercialization, after which time the IPR&D is amortized over its estimated useful life. If we acquire an asset or group of assets that do not meet the definition of a business under applicable accounting standards, the acquired IPR&D is expensed on its acquisition date. Future costs to develop these assets are recorded to research and development expense as they are incurred.
We have acquired, and expect to continue to acquire, intangible assets through the acquisition of biotechnology companies or through the consolidation of variable interest entities. These intangible assets primarily consist of technology associated with human therapeutic products, IPR&D product candidates and priority review vouchers. When significant identifiable intangible assets are acquired, we generally engage an independent third-party valuation firm to assist in determining the fair values of these assets as of the acquisition date. Management will determine the fair value of less significant identifiable intangible assets acquired. Discounted cash flow models are typically used in these valuations, and these models require the use of significant estimates and assumptions including but not limited to:
•estimating the timing of and expected costs to complete the in-process projects;
•projecting regulatory approvals;
•estimating future cash flow from product sales resulting from completed products and in process projects; and
•developing appropriate discount rates and probability rates by project.
We believe the fair values assigned to the intangible assets acquired are based upon reasonable estimates and assumptions given available facts and circumstances as of the acquisition dates.
If these projects are not successfully developed, the sales and profitability of the company may be adversely affected in future periods. Additionally, the value of the acquired intangible assets may become impaired. No assurance can be given that the underlying assumptions used to estimate expected project sales, development costs or profitability, or the events associated with such projects, will transpire as estimated.
INVENTORY
At each reporting period we review our inventories for excess or obsolescence and write-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. The determination of obsolete or excess inventory requires management to make estimates based on assumptions about the future demand of our products, product expiration dates, estimated future sales and our general future plans. If customer demand subsequently differs from our forecasts, we may be required to record additional charges for excess inventory.
Although we believe that the assumptions we use in estimating inventory write-downs are reasonable, no assurance can be given that significant future changes in these assumptions or changes in future events and market conditions could result in different estimates.
During 2022 and 2021 we wrote-off excess inventory of $275.0 million and $120.0 million, respectively, related to ADUHELM.
IMPAIRMENT AND AMORTIZATION OF LONG-LIVED ASSETS
Long-lived assets to be held and used include property, plant and equipment as well as intangible assets, including IPR&D and trademarks. Property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. We review our intangible assets with indefinite lives for impairment annually, as of October 31, and whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable.
When performing our impairment assessment, we calculate the fair value using the same methodology as described above under Acquired Intangible Assets, including IPR&D. If the carrying value of our acquired IPR&D exceeds its fair value, then the intangible asset is written down to its fair value. Changes in estimates and assumptions used in determining the fair value of our acquired IPR&D could result in an impairment. Impairments are recorded within amortization and impairment of acquired intangible assets in our consolidated statements of income.
Based on our most recent impairment assessment we incurred impairment charges of approximately $119.6 million for the year ended December 31, 2022, mainly related to the discontinuation of IPR&D programs. For the year ended December 31, 2023, we had no impairment charges. For additional information on our impairments, please read Note 7, Intangible Assets and Goodwill, to our consolidated financial statements included in this report.
Our most significant intangible assets are our acquired and in-licensed rights and patents. Acquired and in-licensed rights and patents primarily relate to our acquisition of all remaining rights to TYSABRI. We amortize the intangible assets related to our marketed products using the economic consumption method based on revenue generated from the products underlying the related intangible assets. An analysis of the anticipated lifetime revenue of our marketed products is performed annually during our long-range planning cycle and whenever events or changes in circumstances would significantly affect anticipated lifetime revenue of the relevant products.
For additional information on the impairment charges related to our long-lived assets during 2023, 2022 and 2021, please read Note 7, Intangible Assets and Goodwill, to our consolidated financial statements included in this report.
INCOME TAXES
We prepare and file income tax returns based on our interpretation of each jurisdiction’s tax laws and regulations. In preparing our consolidated financial statements, we estimate our income tax liability in each of the jurisdictions in which we operate by estimating our actual current tax expense together with assessing temporary differences resulting from differing treatment of items for tax and financial reporting purposes. These differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheets. Upon our election in the fourth quarter of 2018 to record deferred taxes for GILTI, we have included amounts related to GILTI taxes within temporary difference.
Significant management judgment is required in assessing the realizability of our deferred tax assets. In performing this assessment, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. In making this determination, under the applicable financial accounting standards, we are allowed to consider the scheduled reversal of deferred tax liabilities, projected future taxable income and the effects of tax planning strategies. In the event that actual results differ from our estimates, we adjust our estimates in future periods and we may need to establish a valuation allowance, which could materially impact our consolidated financial position and results of operations.
We account for uncertain tax positions using a “more likely than not” threshold for recognizing and resolving uncertain tax positions. We evaluate uncertain tax positions on a quarterly basis and consider various factors including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, information obtained during in process audit activities and changes in facts or circumstances related to a tax position. We adjust the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. Our liabilities for uncertain tax positions can be relieved only if the contingency becomes legally extinguished, through either payment to the taxing authority or the expiration of the statute of limitations, the recognition of the benefits associated with the position meet the “more likely than not” threshold or the liability becomes effectively settled through the examination process. We consider matters to be effectively settled once the taxing authority has completed all of its required or expected examination procedures, including all appeals and administrative reviews, we have no plans to appeal or litigate any aspect of the tax position and we believe that it is highly unlikely that the taxing authority would examine
or re-examine the related tax position. We also accrue for potential interest and penalties related to unrecognized tax benefits in income tax expense.
BUSINESS COMBINATIONS
Business combinations are recorded using the acquisition method of accounting. The results of operations of the acquired company are included in our results of operations beginning on the acquisition date, and assets acquired and liabilities assumed are recognized on the acquisition date at their respective fair values. Any excess of consideration transferred over the net carrying value of the assets acquired and liabilities assumed as of the acquisition date is recognized as goodwill.
We use the multi-period excess earnings method, which is a form of the income approach, utilizing post-tax cash flow and discount rates in estimating the fair value of identifiable intangible assets acquired when allocating the purchase consideration paid for the acquisition. The estimates of the fair value of identifiable intangible assets involve significant judgment by management and include assumptions with measurement uncertainty, such as the amount and timing of projected cash flow, long-term sales forecasts, discount rates and additionally for IPR&D intangible assets, the timing and probability of regulatory and commercial success.
We use the net realizable value method in estimating the fair value of acquired finished goods and work-in-process inventory. Raw materials acquired are valued using the replacement cost method.
Transaction and restructuring costs related to business combinations are expensed as incurred. The fair value of assets acquired and liabilities assumed in certain cases may be subject to revision based on the final determination of fair value during a period of time not to exceed 12 months from the acquisition date. If we determine the assets acquired do not meet the definition of a business, the transaction will be accounted for as an asset acquisition rather than a business combination.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are subject to certain risks that may affect our results of operations, cash flow and fair values of assets and liabilities, including volatility in foreign currency exchange rates, interest rate movements and equity price exposure as well as changes in economic conditions in the markets in which we operate as a result of the conflict between Russia and Ukraine and the military conflict in the Middle East. We manage the impact of foreign currency exchange rates and interest rates through various financial instruments, including derivative instruments such as foreign currency forward contracts, foreign currency options, interest rate lock contracts and interest rate swap contracts. We do not enter into financial instruments for trading or speculative purposes. The counterparties to these contracts are major financial institutions, and there is no significant concentration of exposure with any one counterparty.
FOREIGN CURRENCY EXCHANGE RISK
Our results of operations are subject to foreign currency exchange rate fluctuations due to the global nature of our operations. As a result, our consolidated financial position, results of operations and cash flow can be affected by market fluctuations in foreign currency exchange rates, primarily with respect to the Euro, British pound sterling, Canadian dollar and Swiss franc.
While the financial results of our global activities are reported in U.S. dollars, the functional currency for most of our foreign subsidiaries is their respective local currency. Fluctuations in the foreign currency exchange rates of the countries in which we do business will affect our operating results, often in ways that are difficult to predict. In particular, as the U.S. dollar strengthens versus other currencies, the value of the non-U.S. revenue will decline when reported in U.S. dollars. The impact to net income as a result of a strengthening U.S. dollar will be partially mitigated by the value of non-U.S. expense, which will also decline when reported in U.S. dollars. As the U.S. dollar weakens versus other currencies, the value of the non-U.S. revenue and expense will increase when reported in U.S. dollars.
We have established revenue and operating expense hedging and balance sheet risk management programs to protect against volatility of future foreign currency cash flow and changes in fair value caused by volatility in foreign currency exchange rates.
During the second quarter of 2018 the International Practices Task Force of the Center for Audit Quality categorized Argentina as a country with a projected three-year cumulative inflation rate greater than 100.0%, which indicated that Argentina’s economy is highly inflationary. This categorization did not have a material impact on our results of operations or financial position as of December 31, 2023, and is not expected to have a material impact on our results of operations or financial position in the future. In December 2023 the Argentinian Peso experienced a substantial devaluation following a presidential election. The devaluation resulted in a $16.0 million charge recorded
during the fourth quarter of 2023 in other (income) expense, net within our consolidated statements of income for the year ended December 31, 2023.
REVENUE AND OPERATING EXPENSE HEDGING PROGRAM
Our foreign currency hedging program is designed to mitigate, over time, a portion of the impact resulting from volatility in exchange rate changes on revenue and operating expense. We use foreign currency forward contracts and foreign currency options to manage foreign currency risk, with the majority of our forward contracts and options used to hedge certain forecasted revenue and operating expense transactions denominated in foreign currencies in the next 12 months. We do not engage in currency speculation. For a more detailed disclosure of our revenue and operating expense hedging program, please read Note 10, Derivative Instruments, to our consolidated financial statements included in this report.
Our ability to mitigate the impact of foreign currency exchange rate changes on revenue and net income diminishes as significant foreign currency exchange rate fluctuations are sustained over extended periods of time. In particular, devaluation or significant deterioration of foreign currency exchange rates are difficult to mitigate and likely to negatively impact earnings. The cash flow from these contracts are reported as operating activities in our consolidated statements of cash flow.
BALANCE SHEET RISK MANAGEMENT HEDGING PROGRAM
We also use forward contracts to mitigate the foreign currency exposure related to certain balance sheet items. The primary objective of our balance sheet risk management program is to mitigate the exposure of foreign currency denominated net monetary assets and liabilities of foreign affiliates. In these instances, we principally utilize currency forward contracts. We have not elected hedge accounting for the balance sheet related items. The cash flow from these contracts are reported as operating activities in our consolidated statements of cash flow.
The following quantitative information includes the impact of currency movements on forward contracts used in our revenue, operating expense and balance sheet hedging programs. As of December 31, 2023 and 2022, a hypothetical adverse 10.0% movement in foreign currency exchange rates compared to the U.S. dollar across all maturities would result in a hypothetical decrease in the fair value of forward contracts of approximately $249.4 million and $293.7 million, respectively. The estimated fair value change was determined by measuring the impact of the hypothetical exchange rate movement on outstanding forward contracts. Our use of this methodology to quantify the market risk of such instruments is subject to assumptions and actual impact could be significantly different. The quantitative information about market risk is limited because it does not take into account all foreign currency operating transactions.
INTEREST RATE RISK
Our investment portfolio includes cash equivalents and short-term investments. The fair value of our marketable securities is subject to change as a result of potential changes in market interest rates. The potential change in fair value for interest rate sensitive instruments has been assessed on a hypothetical 100 basis point adverse movement across all maturities. As of December 31, 2022, we estimate that such hypothetical 100 basis point adverse movement would result in a hypothetical loss in fair value of approximately $11.7 million to our interest rate sensitive instruments. The fair values of our investments were determined using third-party pricing services or other market observable data.
We partially funded our Reata acquisition through available cash, cash equivalents and marketable securities. As of December 31, 2023, we have sold all of our marketable debt securities. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to our consolidated financial statements included in this report.
CREDIT RISK
Financial instruments that potentially subject us to concentrations of credit risk include cash and cash equivalents, investments, derivatives and accounts receivable. We attempt to minimize the risks related to cash and cash equivalents and investments by investing in a broad and diverse range of financial instruments. We have established guidelines related to credit ratings and maturities intended to safeguard principal balances and maintain liquidity. Our investment portfolio is maintained in accordance with our investment policy, which defines allowable investments, specifies credit quality standards and limits the credit exposure of any single issuer. We minimize credit risk resulting from derivative instruments by choosing only highly rated financial institutions as counterparties.
We operate in certain countries where weakness in economic conditions, including the effects of the conflict between Russia and Ukraine and the military conflict in the Middle East, can result in extended collection periods. We continue to monitor these conditions, including the volatility associated with international economies and the relevant financial markets, and assess their possible impact on our business. To date, we have not experienced any significant losses with respect to the collection of our accounts receivable.
We believe that our allowance for doubtful accounts was adequate as of December 31, 2023 and 2022.
EQUITY PRICE RISK
Our strategic investment portfolio includes investments in equity securities of certain biotechnology companies. While we are holding such securities, we are subject to equity price risk, and this may increase the volatility of our income in future periods due to changes in the fair value of equity investments. We may sell such equity securities based on our business considerations, which may include limiting our price risk.
Changes in the fair value of these equity securities are impacted by the volatility of the stock market and changes in general economic conditions, among other factors. The potential change in fair value for equity price sensitive instruments has been assessed on a hypothetical 10.0% adverse movement. As of December 31, 2023 and 2022, a hypothetical adverse 10.0% movement would result in a hypothetical decrease in fair value of approximately $41.7 million and $79.1 million, respectively.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is contained on pages F-1 through F-85 of this report and is incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROL OVER FINANCIAL REPORTING
CONTROLS AND PROCEDURES
We have carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) under the Securities Exchange Act of 1934, as amended), as of December 31, 2023. Based upon that evaluation, our principal executive officer and principal financial officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures are effective in ensuring that:
(a) the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the U.S. Securities and Exchange Commission's rules and forms; and
(b) such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
MANAGEMENT'S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in its 2013 Internal Control — Integrated Framework.
Based on our assessment, our management has concluded that, as of December 31, 2023, our internal control over financial reporting is effective based on those criteria.
We excluded Reata from our assessment of internal control over financial reporting as of December 31, 2023, as Reata was acquired by our Company in a business combination during 2023. The total assets and total revenue of Reata represents 1.0% and 0.6%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2023.
The effectiveness of our internal control over financial reporting as of December 31, 2023, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their attestation report, which is included herein.
ITEM 9B. OTHER INFORMATION
RULE 10b5-1 TRADING ARRANGEMENTS
| Total Shares to be Sold | | Expiration Date | () | | | | | | X | | — | | | | 11/10/2025 |
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not Applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information concerning our executive officers is set forth under the heading Information about our Executive Officers in Item 1 of this report. The text of our code of business conduct, which includes the code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, and persons performing similar functions, is posted on our website, www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the site. We intend to make all required disclosures regarding any amendments to, or waivers from, provisions of our code of business conduct at the same location of our website.
The response to the remainder of this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Proposal 1 - Election of Directors,” “Corporate Governance” and “Miscellaneous - Stockholder Proposals” contained in the proxy statement for our 2024 annual meeting of stockholders.
ITEM 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Executive Compensation Tables,” "Compensation Discussion and Analysis" and “Corporate Governance” contained in the proxy statement for our 2024 annual meeting of stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Stock Ownership” and “Equity Compensation Plan Information” contained in the proxy statement for our 2024 annual meeting of stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Certain Relationships and Related Person Transactions” and “Corporate Governance” contained in the proxy statement for our 2024 annual meeting of stockholders.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The response to this item is incorporated by reference from the discussion responsive thereto in the section entitled “Proposal 2 - Ratification of the Selection of our Independent Registered Public Accounting Firm” contained in the proxy statement for our 2024 annual meeting of stockholders.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
a. (1) Consolidated Financial Statements:
The following financial statements are filed as part of this report:
| | | | | | | | |
| Financial Statements | | Page Number |
| Consolidated Statements of Income | | F-2 |
| Consolidated Statements of Comprehensive Income | | F-3 |
| Consolidated Balance Sheets | | F-4 |
| Consolidated Statements of Cash Flow | | F-5 |
| Consolidated Statements of Equity | | F-6 |
| Notes to Consolidated Financial Statements | | F-9 |
Report of Independent Registered Public Accounting Firm (PCAOB ID ) | F-83 |
Certain totals may not sum due to rounding.
(2) Exhibits
The exhibits listed on the Exhibit Index beginning on page 98, which is incorporated herein by reference, are filed or furnished as part of this report or are incorporated into this report by reference.
(3) Financial Statement Schedules
Schedules are omitted because they are not applicable, or are not required, or because the information is included in the consolidated financial statements and notes thereto.
ITEM 16. FORM 10-K SUMMARY
Not applicable.
EXHIBIT INDEX
| | | | | | | | |
| Exhibit No. | | Description |
| 2.1 | | |
| 3.1 | | |
| 3.2 | | |
| 3.3 | | |
| 3.4 | | |
| 4.1 | | |
| 4.2 | | |
| 4.3 | | |
| 4.4 | | |
| 4.5 | | |
| 4.6+ | | |
10.1 | | |
10.2 | | |
10.3 | | |
10.4† | | |
10.5† | | |
10.6* | | |
10.7* | | |
10.8* | | |
10.9* | | |
10.10* | | |
10.11* | | |
10.12* | | |
10.13* | | |
10.14* | | |
10.15* | | |
10.16* | | |
| | | | | | | | |
| Exhibit No. | | Description |
10.17* | | |
10.18* | | |
10.19* | | |
10.20* | | |
10.21* | | |
10.22* | | |
10.23* | | |
10.24* | | |
10.25* | | |
10.26* | | |
10.27* | | |
10.28* | | |
10.29* | | |
10.30* | | |
10.31* | | |
10.32*+ | | |
10.33*+ | | |
10.34* | | |
10.35+ | | |
10.36 | | |
10.37 | | |
| 21+ | | |
| 23+ | | |
| 31.1+ | | |
| 31.2+ | | |
| 32.1++ | | |
97.1+ | | |
| 101++ | | The following materials from Biogen Inc.’s Annual Report on Form 10-K for the year ended December 31, 2023, formatted in iXBRL (Inline Extensible Business Reporting Language): (i) the Consolidated Statements of Income, (ii) the Consolidated Statements of Comprehensive Income, (iii) the Consolidated Balance Sheets, (iv) the Consolidated Statements of Cash Flow, (v) the Consolidated Statements of Equity and (vi) Notes to Consolidated Financial Statements. |
| | | | | |
| * | Management contract or compensatory plan or arrangement. |
| | | | | |
| † | Confidential treatment has been granted or requested with respect to portions of this exhibit. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| BIOGEN INC. |
| |
| By: | /S/ CHRISTOPHER A. VIEHBACHER |
| Christopher A. Viehbacher |
| Chief Executive Officer |
Date: February 13, 2024
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Capacity | | Date |
| | | | |
/S/ CHRISTOPHER A. VIEHBACHER | | Director and Chief Executive Officer (principal executive officer) | | February 13, 2024 |
| Christopher A. Viehbacher | | |
| | | | |
/S/ MICHAEL R. MCDONNELL | | Executive Vice President and Chief Financial Officer (principal financial officer) | | February 13, 2024 |
| Michael R. McDonnell | | |
| | | | |
/S/ ROBIN C. KRAMER | | Senior Vice President, Chief Accounting Officer (principal accounting officer) | | February 13, 2024 |
| Robin C. Kramer | | |
| | | | |
/S/ CAROLINE D. DORSA | | Director and Chair of the Board of Directors | | February 13, 2024 |
| Caroline D. Dorsa | | |
| | | | |
/S/ MARIA C. FREIRE | | Director | | February 13, 2024 |
| Maria C. Freire | | |
| | | | |
/S/ WILLIAM A. HAWKINS | | Director | | February 13, 2024 |
| William A. Hawkins | | |
| | | | |
/S/ SUSAN LANGER | | Director | | February 13, 2024 |
| Susan Langer | | |
| | | | |
/S/ JESUS B. MANTAS | | Director | | February 13, 2024 |
| Jesus B. Mantas | | |
| | | | |
/S/ MONISH PATOLAWALA | | Director | | February 13, 2024 |
| Monish Patolawala | | |
| | | | |
/S/ ERIC K. ROWINSKY | | Director | | February 13, 2024 |
| Eric K. Rowinsky | | |
| | | | |
/S/ STEPHEN A. SHERWIN | | Director | | February 13, 2024 |
| Stephen A. Sherwin | | |
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
| | | | | | | | |
| | | Page Number |
| Consolidated Statements of Income | | F-2 |
| Consolidated Statements of Comprehensive Income | | F-3 |
| Consolidated Balance Sheets | | F-4 |
| Consolidated Statements of Cash Flow | | F-5 |
| Consolidated Statements of Equity | | F-6 |
| Notes to Consolidated Financial Statements | | F-9 |
| Report of Independent Registered Public Accounting Firm (PCAOB ID 238) | F-83 |
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
(In millions, except per share amounts)
| | | | | | | | | | | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Revenue: | | | | | | |
Product revenue, net | | $ | | | | $ | | | | $ | | |
| Revenue from anti-CD20 therapeutic programs | | | | | | | | | |
| Contract manufacturing, royalty and other revenue | | | | | | | | | |
| Total revenue | | | | | | | | | |
| Cost and expense: | | | | | | |
| Cost of sales, excluding amortization and impairment of acquired intangible assets | | | | | | | | | |
| Research and development | | | | | | | | | |
| Selling, general and administrative | | | | | | | | | |
| Amortization and impairment of acquired intangible assets | | | | | | | | | |
| Collaboration profit sharing/(loss reimbursement) | | | | | () | | | | |
| |
| (Gain) loss on fair value remeasurement of contingent consideration | | | | | () | | | () | |
| Acquired in-process research and development | | | | | | | | | |
| Restructuring charges | | | | | | | | | |
| Gain on sale of building | | | | | () | | | | |
| Other (income) expense, net | | | | | () | | | | |
| Total cost and expense | | | | | | | | | |
| Income before income tax (benefit) expense and equity in loss of investee, net of tax | | | | | | | | | |
| Income tax (benefit) expense | | | | | | | | | |
| Equity in (income) loss of investee, net of tax | | | | | () | | | () | |
| Net income | | | | | | | | | |
| Net income (loss) attributable to noncontrolling interests, net of tax | | | | | () | | | | |
| Net income attributable to Biogen Inc. | | $ | | | | $ | | | | $ | | |
| | | | | | |
| Net income per share: | | | | | | |
| Basic earnings per share attributable to Biogen Inc. | | $ | | | | $ | | | | $ | | |
| Diluted earnings per share attributable to Biogen Inc. | | $ | | | | $ | | | | $ | | |
| | | | | | |
| Weighted-average shares used in calculating: | | | | | | |
| Basic earnings per share attributable to Biogen Inc. | | | | | | | | | |
| Diluted earnings per share attributable to Biogen Inc. | | | | | | | | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In millions)
| | | | | | | | | | | | | | | | | | | | |
| | | For the Years Ended December 31, |
| | | 2023 | | 2022 | | 2021 |
| Net income attributable to Biogen Inc. | | $ | | | | $ | | | | $ | | |
| Other comprehensive income: | | | | | | |
| Unrealized gains (losses) on securities available for sale, net of tax | | | | | () | | | () | |
| Unrealized gains (losses) on cash flow hedges, net of tax | | () | | | () | | | | |
| Gains (losses) on net investment hedges, net of tax | | | | | () | | | | |
| Unrealized gains (losses) on pension benefit obligation, net of tax | | () | | | | | | | |
| Currency translation adjustment | | | | | () | | | () | |
| Total other comprehensive income (loss), net of tax | | | | | () | | | | |
| Comprehensive income (loss) attributable to Biogen Inc. | | | | | | | | | |
| Comprehensive income (loss) attributable to noncontrolling interests, net of tax | | | | | () | | | | |
| Comprehensive income (loss) | | $ | | | | $ | | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In millions, except per share amounts)
| | | | | | | | | | | |
| As of December 31, |
| 2023 | | 2022 |
| ASSETS |
| Current assets: | | | |
| Cash and cash equivalents | $ | | | | $ | | |
| Marketable securities | | | | | |
Accounts receivable, net of allowance for doubtful accounts of $ and $, respectively | | | | | |
| Due from anti-CD20 therapeutic programs | | | | | |
| Inventory | | | | | |
| Other current assets | | | | | |
| Total current assets | | | | | |
| Marketable securities | | | | | |
| Property, plant and equipment, net | | | | | |
| Operating lease assets | | | | | |
| Intangible assets, net | | | | | |
| Goodwill | | | | | |
| Deferred tax asset | | | | | |
| Investments and other assets | | | | | |
| Total assets | $ | | | | $ | | |
| LIABILITIES AND EQUITY |
| Current liabilities: | | | |
| Current portion of term loan | $ | | | | $ | | |
| Taxes payable | | | | | |
| Accounts payable | | | | | |
| Accrued expense and other | | | | | |
| Total current liabilities | | | | | |
| Notes payable and term loan | | | | | |
| Deferred tax liability | | | | | |
| Long-term operating lease liabilities | | | | | |
| Other long-term liabilities | | | | | |
| Total liabilities | | | | | |
| Commitments, contingencies and guarantees (Notes 22 and 23) | | | |
| Equity: | | | |
| Biogen Inc. shareholders’ equity | | | |
Preferred stock, par value $ per share | | | | | |
Common stock, par value $ per share | | | | | |
| Additional paid-in capital | | | | | |
| Accumulated other comprehensive income (loss) | () | | | () | |
| Retained earnings | | | | | |
Treasury stock, at cost; million and million shares, respectively | () | | | () | |
| Total Biogen Inc. shareholders’ equity | | | | | |
| Noncontrolling interests | | | | () | |
| Total equity | | | | | |
| Total liabilities and equity | $ | | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOW
(In millions)
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Cash flow from operating activities: | | | | | |
| Net income | $ | | | | $ | | | | $ | | |
| Adjustments to reconcile net income to net cash flow from operating activities: | | | | | |
| Depreciation and amortization | | | | | | | | |
| Impairment of intangible assets | | | | | | | | |
| Excess and obsolescence charges related to inventory | | | | | | | | |
| Amortization of inventory step-up | | | | | | | | |
| Acquired in-process research and development | | | | | | | | |
| Share-based compensation | | | | | | | | |
| Contingent consideration | | | | () | | | () | |
| Deferred income taxes | () | | | () | | | () | |
| (Gain) loss on strategic investments | | | | | | | | |
| (Gain) loss on equity method investment | | | | () | | | () | |
| Gain on sale of equity interest in Samsung Bioepis | | | | () | | | | |
| Gain on sale of building | | | | () | | | | |
| Other | | | | | | | | |
Changes in operating assets and liabilities, net of effects of business acquired: | | | | | |
| Accounts receivable | | | | () | | | | |
| Due from anti-CD20 therapeutic programs | () | | | () | | | | |
| Inventory | () | | | () | | | () | |
| Accrued expense and other current liabilities | () | | | () | | | () | |
| Income tax assets and liabilities | () | | | () | | | | |
| Other changes in operating assets and liabilities, net | () | | | () | | | () | |
| Net cash flow provided by (used in) operating activities | | | | | | | | |
| Cash flow from investing activities: | | | | | |
| Purchases of property, plant and equipment | () | | | () | | | () | |
| Proceeds from sales and maturities of marketable securities | | | | | | | | |
| Purchases of marketable securities | () | | | () | | | () | |
| Acquisition of Reata, net of cash acquired | () | | | — | | | — | |
| Proceeds from sale of equity interest in Samsung Bioepis | | | | | | | | |
| Proceeds from sale of building | | | | | | | | |
| Proceeds from divestiture of Hillerød, Denmark manufacturing operations | | | | | | | | |
| Acquired in-process research and development | — | | | — | | | () | |
| Acquisitions of intangible assets | () | | | () | | | () | |
| Proceeds from sales of strategic investments | | | | | | | | |
| Other | () | | | | | | | |
| Net cash flow provided by (used in) investing activities | () | | | | | | () | |
| Cash flow from financing activities: | | | | | |
| Purchase of treasury stock | | | | () | | | () | |
| Payments related to issuance of stock for share-based compensation arrangements, net | () | | | () | | | () | |
| Repayments of borrowings and premiums paid | () | | | () | | | () | |
| Proceeds from borrowings | | | | | | | | |
|
| Net (distribution) contribution to noncontrolling interest | | | | | | | () | |
|
| Other | () | | | () | | | () | |
| Net cash flow provided by (used in) financing activities | | | | () | | | () | |
| Net increase (decrease) in cash and cash equivalents | () | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | | | () | | | () | |
| Cash and cash equivalents, beginning of the year | | | | | | | | |
| Cash and cash equivalents, end of the year | $ | | | | $ | | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Preferred stock | | Common stock | | Additional
paid-in
capital | | Accumulated other comprehensive income (loss) | | Retained
earnings | | Treasury stock | | Total
Biogen Inc.
shareholders’
equity | | Noncontrolling
interests | | Total
equity |
| | Shares | | Amount | | Shares | | Amount | | | | | Shares | | Amount | | | |
| Balance, December 31, 2022 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | () | | | $ | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | | — | | | — | | | | | | | | | | |
| Other comprehensive income (loss), net of tax | — | | | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | | | | — | | | | |
| Capital contribution from noncontrolling interest | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | | | | | |
| Deconsolidation of noncontrolling interest | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | () | | | () | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Issuance of common stock under stock option and stock purchase plans | — | | | — | | | | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Issuance of common stock under stock award plan | — | | | — | | | | | | — | | | () | | | — | | | — | | | — | | | — | | | () | | | — | | | () | |
| Compensation expense related to share-based payments | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Other | — | | | — | | | — | | | — | | | () | | | — | | | — | | | — | | | — | | | () | | | — | | | () | |
| Balance, December 31, 2023 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY - (Continued)
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Preferred stock | | Common stock | | Additional
paid-in
capital | | Accumulated other comprehensive income (loss) | | Retained
earnings | | Treasury stock | | Total
Biogen Inc.
shareholders’
equity | | Noncontrolling
interests | | Total
equity |
| Shares | | Amount | | Shares | | Amount | | | | | Shares | | Amount | | | |
| Balance, December 31, 2021 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | | | | $ | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | | — | | | — | | | | | | () | | | | |
| Other comprehensive income (loss), net of tax | — | | | — | | | — | | | — | | | — | | | () | | | — | | | — | | | — | | | () | | | — | | | () | |
| | | | | | | | | | | | | | | | | | |
| Capital contribution from noncontrolling interest | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | | | | | |
| Repurchase of common stock pursuant to the 2020 Share Repurchase Program, at cost | — | | | — | | | — | | | — | | | — | | | — | | | — | | | () | | | () | | | () | | | — | | | () | |
| Retirement of common stock pursuant to the 2020 Share Repurchase Program, at cost | — | | | — | | | () | | | — | | | () | | | — | | | () | | | | | | | | | — | | | — | | | | |
| Issuance of common stock under stock option and stock purchase plans | — | | | — | | | | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Issuance of common stock under stock award plan | — | | | — | | | | | | — | | | () | | | — | | | — | | | — | | | — | | | () | | | — | | | () | |
| Compensation expense related to share-based payments | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Other | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Balance, December 31, 2022 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | () | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY - (Continued)
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Preferred stock | | Common stock | | Additional
paid-in
capital | | Accumulated other comprehensive income (loss) | | Retained
earnings | | Treasury stock | | Total
Biogen Inc.
shareholders’
equity | | Noncontrolling
interests | | Total
equity |
| Shares | | Amount | | Shares | | Amount | | | | | Shares | | Amount | | | |
| Balance, December 31, 2020 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | () | | | $ | | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | | | | — | | | — | | | | | | | | | | |
| Other comprehensive income (loss), net of tax | — | | | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | | | | | | | | |
| Distribution to noncontrolling interest | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | () | | | () | |
| Capital contribution from noncontrolling interest | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | | | | | |
| Repurchase of common stock pursuant to the 2020 Share Repurchase Program, at cost | — | | | — | | | — | | | — | | | — | | | — | | | — | | | () | | | () | | | () | | | — | | | () | |
| Retirement of common stock pursuant to the 2020 Share Repurchase Program, at cost | — | | | — | | | () | | | — | | | () | | | — | | | () | | | | | | | | | — | | | — | | | | |
| Issuance of common stock under stock option and stock purchase plans | — | | | — | | | | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Issuance of common stock under stock award plan | — | | | — | | | | | | — | | | () | | | — | | | () | | | — | | | — | | | () | | | — | | | () | |
| Compensation expense related to share-based payments | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Other | — | | | — | | | — | | | — | | | | | | — | | | — | | | — | | | — | | | | | | — | | | | |
| Balance, December 31, 2021 | — | | | $ | — | | | | | | $ | | | | $ | | | | $ | () | | | $ | | | | () | | | $ | () | | | $ | | | | $ | | | | $ | | |
See accompanying notes to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
% of the economics, we record net income (loss) attributable to noncontrolling interests, net of tax in our consolidated statements of income equal to the percentage of the economic or ownership interest retained in such entities by the respective noncontrolling parties. Intercompany balances and transactions are eliminated in consolidation. In determining whether we are the primary beneficiary of a variable interest entity, we apply a qualitative approach that determines whether we have both (1) the power to direct the economically significant activities of the entity and (2) the obligation to absorb losses of, or the right to receive benefits from, the entity that could potentially be significant to that entity. We continuously assess whether we are the primary beneficiary of a variable interest entity as changes to existing relationships or future transactions may result in us consolidating or deconsolidating one or more of our collaborators or partners. In November 2023 we terminated the Neurimmune Agreement, which resulted in the deconsolidation of our variable interest entity, Neurimmune.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For additional information on our collaboration arrangements with Genentech, Eisai, Sage and Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We do not adjust our receivables for the effects of a significant financing component at contract inception if we expect to collect the receivables in one year or less from the time of sale.
We provide reserves against accounts receivable for estimated losses that may result from a customer's inability to pay. Amounts determined to be uncollectible are charged or written-off against the reserve.
Receivables from Samsung BioLogics
In April 2022 we completed the sale of our % equity interest in Samsung Bioepis to Samsung BioLogics, which resulted in a receivable of approximately $ billion in cash to be deferred over payments. The first deferred payment of $ million was received in April 2023 and the second deferred payment of $ million is due at the second anniversary of the closing of this transaction in April 2024. The payments due to us from Samsung BioLogics have been recorded at their estimated fair values through the use of risk-adjusted discount rates. For additional information on the accounting for the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| Leasehold Improvements | Lesser of the useful life or the term of the respective lease |
| Furniture and Fixtures | to years |
| Machinery and Equipment | to years |
| Computer Software and Hardware | to years |
When we dispose of property, plant and equipment, we remove the associated cost and accumulated depreciation from the related accounts in our consolidated balance sheets and include any resulting gain or loss in our consolidated statements of income.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For additional information on our leases, please read Note 12, Leases, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As described in Note 25, Segment Information, to these consolidated financial statements, we operate as operating segment, which is our only reporting unit.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For additional information on our derivative instruments and hedging activities, please read Note 10, Derivative Instruments, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For the years ended December 31, 2023, 2022 and 2021, advertising costs totaled $ million, $ million and $ million, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We funded this acquisition through available cash, cash equivalents and marketable securities, supplemented by the issuance of a $ billion term loan under our term loan credit agreement. For additional information on our term loan credit agreement, please read Note 13, Indebtedness, to these consolidated financial statements.
We accounted for this acquisition as a business combination using the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations, and recorded assets acquired and liabilities assumed at their respective fair values as of the acquisition date.
Purchase Price Consideration
| Fair value of Reata equity compensation pre-acquisition services and related taxes(2) | | | |
| Total consideration | | $ | | |
(1) Represents cash consideration transferred of $ per outstanding Reata ordinary share based on million Reata shares outstanding at closing.
(2) Represents the fair value of Reata stock options and stock units issued to Reata equity award holders and the related taxes attributable to pre-acquisition vesting services.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | — | | | $ | | | Accounts receivable | | | | | — | | | | |
| Inventory | | | | | () | | | | |
| Other current assets | | | | | — | | | | |
| Intangible assets: | | | | | | |
| Completed technology for SKYCLARYS (U.S.) | | | | | | | | | |
| In-process research and development (omaveloxolone) | | | | | | | | | |
| Priority review voucher | | | | | — | | | | |
| Other clinical programs | | | | | | | | | |
| Operating lease assets | | | | | () | | | | |
| Accrued expense and other | | () | | | () | | | () | |
| Debt payable | | () | | | — | | | () | |
Contingent payable to Blackstone(1) | | () | | | — | | | () | |
| Deferred tax liability | | () | | | | | | () | |
Operating lease liabilities | | () | | | — | | | () | |
| Other assets and liabilities, net | | () | | | () | | | () | |
| Total identifiable net assets | | | | | | | | | |
| Goodwill | | | | | () | | | | |
| Total assets acquired and liabilities assumed | | $ | | | | $ | — | | | $ | | |
(1) For additional information on the contingent payable to Blackstone, please read Note 18, Other Consolidated Financial Statement Detail, to these consolidated financial statements.
Inventory: Total inventory acquired was approximately $ billion, which reflects a step-up in the fair value of finished goods and work-in-process inventory for SKYCLARYS. The fair value was determined based on the estimated selling price of the inventory, less the remaining manufacturing and selling costs and a normal profit margin on those manufacturing and selling efforts. This fair value step-up adjustment will be amortized to cost of sales within our consolidated statements of income when the inventory is sold, which is expected to be within approximately years from the acquisition date. For the year ended December 31, 2023, amortization from the fair value step-up adjustment as a result of inventory sold during the fourth quarter was approximately $ million.
Intangible assets: Intangible assets are comprised of $ billion related to SKYCLARYS commercialization rights in the U.S., $ billion of IPR&D related to the omaveloxolone program outside the U.S., which had not yet received regulatory approval in the E.U. as of the acquisition date, $ million related to a rare pediatric disease priority voucher which may be used to obtain priority review by the FDA for a future regulatory submission or sold to a third party and $ million related to other clinical programs. The estimated fair values of the program related intangible assets were determined using a multi-period excess earnings method, a form of the income approach, utilizing a discount rate of % and the estimated fair value of the priority review voucher was based on recent external purchase and sale transactions of similar vouchers.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
These fair value measurements were based on significant inputs not observable in the market and thus represent Level 3 fair value measurements.
Leases: We assumed responsibility for a single-tenant, build-to-suit building of approximately square feet of office and laboratory space located in Plano, Texas, with an initial lease term of years. We recorded a lease liability of approximately $ million, which represents the net present value of rental expense over the remaining lease term of approximately years, with a corresponding right-of-use asset of approximately $ million, which represents our estimate of the fair value for a market participant of the current rental market in the Dallas, Texas area. Included in our estimate of the market rental rate is the value of any leasehold improvements or tenant allowances related to the building. We do not intend to occupy this building and are evaluating opportunities to sublease the property.
Goodwill: Goodwill was calculated as the excess of the consideration transferred over the net assets recognized and represents the future economic benefits arising from the other assets acquired that could not be individually identified and separately recognized. We recognized goodwill of approximately $ million, which is not deductible for tax purposes. The goodwill recognized from our acquisition of Reata is primarily the result of the deferred tax consequences from the transaction recorded for financial statement purposes.
Acquisition-related expenses: Acquisition-related expenses, which were comprised primarily of regulatory, advisory and legal fees, and other transaction costs, totaled approximately $ million and are included within selling, general and administrative expense within our consolidated statements of income for the year ended December 31, 2023.
Assumptions in the Allocations of Purchase Price
The results of operations of Reata, along with the estimated fair values of the assets acquired and liabilities assumed in the Reata acquisition, have been included in our consolidated financial statements since the closing of the Reata acquisition on September 26, 2023.
Our preliminary estimate of the fair value of the specifically identifiable assets acquired and liabilities assumed as of the date of acquisition is subject to the finalization of management's analysis related to certain matters, such as finalizing our assessment of income taxes. The final determination of these fair values will be completed as additional information becomes available but no later than one year from the acquisition date. The final determination may result in asset and liability fair values that are different than the preliminary estimates.
Subsequent to the acquisition date, our results of operations include the results of operations of Reata. Due to the immateriality of Reata's revenue and expense, additional pro forma information combining the results of operations of Biogen and Reata have not been included.
% equity interest in Samsung Bioepis to Samsung BioLogics in exchange for total consideration of approximately $ billion. Under the terms of this transaction, we received approximately $ billion in cash at closing, with approximately $ billion in cash to be deferred over payments. The first deferred payment of $ million was received in April 2023 and the second deferred payment of $ million is due at the second anniversary of the closing of this transaction in April 2024. Prior to the sale, the carrying value of our investment in Samsung Bioepis totaled $ million. For the year ended December 31, 2022, we recognized a pre-tax gain of approximately $ billion related to this transaction, which was recorded in other (income) expense, net in our consolidated statements of income. This pre-tax gain included reclassifications from AOCI to net income of approximately $ million in cumulative translation losses, partially offset by approximately $ million in gains resulting from the termination of our net investment hedge.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
For the year ended December 31, 2023, we recognized a gain of approximately $ million to reflect the change in fair value related to the first deferred payment due to us, which was received in April 2023. Additionally, for the year ended December 31, 2023, we recognized a gain of approximately $ million to reflect the change in fair value related to the second deferred payment due to us. For the year ended December 31, 2022, we recognized a gain of approximately $ million and a loss of approximately $ million to reflect the changes in fair value related to the first and second deferred payments due to us, respectively. These changes were recorded in other (income) expense, net within our consolidated statements of income.
As part of this transaction, we are also eligible to receive up to an additional $ million upon the achievement of certain commercial milestones. Our policy for contingent payments of this nature is to recognize the payments in the period the payments become realizable, which is generally the same period in which the payments are earned.
If any payments due to us remain outstanding after the second anniversary of the closing of this transaction, we may elect to receive shares of Samsung BioLogics common stock at a % discount in lieu of a cash payment for the remaining amount due. Currently, we believe that the likelihood of Samsung BioLogics failing to make the second deferred payment due to us is remote.
Additionally, for the year ended December 31, 2022, we recorded a discrete tax expense of approximately $ million related to this transaction, which is reflected in income tax (benefit) expense in our consolidated statements of income.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | Research and development | | | | | | | | | |
| Restructuring charges | | | | | | | | | |
| Total charges | | $ | | | | $ | | | | $ | | |
| |
|
|
| |
| |
| |
| |
| | Other Costs: includes costs associated with items such as asset abandonment and write-offs, facility closure costs, pretax gains and losses resulting from the termination of certain leases, employee non-severance expense, consulting fees and other costs.
Reata Integration
Following the close of our Reata acquisition, we implemented an integration plan designed to realize operating synergies through cost savings and avoidance. These amounts are primarily related to severance and are expected to be paid by the end of 2024. For the year ended December 31, 2023, we recognized approximately $ million of net pre-tax restructuring charges related to employee severance costs.
2022 Cost Saving Initiatives
In December 2021 and May 2022 we announced our plans to implement a series of cost-reduction measures during 2022. These savings are being achieved through a number of initiatives, including reductions to our workforce, the substantial elimination of our commercial ADUHELM infrastructure, deprioritization of certain research and development programs, the consolidation of certain real estate locations and operating efficiencies across our selling, general and administrative and research and development functions. Charges related to our 2022 cost saving initiatives were substantially incurred during 2022 with remaining payments expected to be made through 2026.
) | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
| Total charges | | $ | () | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
(1) Amounts reflect a gain recorded during the third quarter of 2022 of approximately $ million related to the partial termination of a portion of our lease located at 300 Binney Street. For additional information on our 300 Binney Street lease modification, please read Note 12, Leases, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | Expense | | | |
| Payment | | () | |
| Foreign currency and other adjustments | | | |
| Restructuring reserve, December 31, 2022 | | | |
| Expense | | | |
| Payment | | () | |
| Foreign currency and other adjustments | | () | |
| Restructuring reserve, December 31, 2023 | | $ | | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
| VUMERITY | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Fumarate | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| AVONEX | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| PLEGRIDY | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Interferon | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TYSABRI | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| FAMPYRA | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subtotal: Multiple Sclerosis | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Rare Disease: | | | | | | | | | | | | | | | | | | |
| SPINRAZA | | | | | | | | | | | | | | | | | | | | | | | | | | | |
QALSODY(1) | | | | | | | | | | | | | | | | | | | | | | | | | | | |
SKYCLARYS(2) | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subtotal: Rare Disease | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Biosimilars: | | | | | | | | | | | | | | | | | | |
| BENEPALI | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| IMRALDI | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| FLIXABI | | | | | | | | | | | | | | | | | | | | | | | | | | | |
BYOOVIZ(3) | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subtotal: Biosimilars | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
Other(4) | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total product revenue | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
(1) QALSODY became commercially available in the U.S. during the second quarter of 2023.
(2) SKYCLARYS was obtained as part of our acquisition of Reata in September 2023. SKYCLARYS became commercially available in the U.S. during the second quarter of 2023 and we began recognizing revenue from SKYCLARYS in the U.S. during the fourth quarter of 2023, subsequent to our acquisition.
(3) BYOOVIZ became commercially available in the U.S. during the third quarter of 2022 and commercially available in certain international markets in 2023.
(4) Other includes FUMADERM, ADUHELM and ZURZUVAE, which became commercially available in the U.S. during the fourth quarter of 2023.
We recognized revenue from wholesalers accounting for % and % of gross product revenue in 2023, % and % of gross product revenue in 2022 and % and % of gross product revenue in 2021, respectively.
As of December 31, 2023, wholesale distributors individually accounted for approximately % and % of net accounts receivable associated with our product sales, as compared to % and % as of December 31, 2022, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | $ | | | | Current provisions relating to sales in current year | | | | | | | | | | | | |
| Adjustments relating to prior years | | () | | | () | | | | | | () | |
| Payments/credits relating to sales in current year | | () | | | () | | | () | | | () | |
| Payments/credits relating to sales in prior years | | () | | | () | | | () | | | () | |
| Ending balance | | $ | | | | $ | | | | $ | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2022 |
| (In millions) | | Discounts | | Contractual
Adjustments | | Returns | | Total |
| Beginning balance | | $ | | | | $ | | | | $ | | | | $ | | |
| Current provisions relating to sales in current year | | | | | | | | | | | | |
| Adjustments relating to prior years | | () | | | | | | () | | | () | |
| Payments/credits relating to sales in current year | | () | | | () | | | () | | | () | |
| Payments/credits relating to sales in prior years | | () | | | () | | | () | | | () | |
| Ending balance | | $ | | | | $ | | | | $ | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2021 |
| (In millions) | | Discounts | | Contractual
Adjustments | | Returns | | Total |
| Beginning balance | | $ | | | | $ | | | | $ | | | | $ | | |
| Current provisions relating to sales in current year | | | | | | | | | | | | |
| Adjustments relating to prior years | | () | | | () | | | () | | | () | |
| Payments/credits relating to sales in current year | | () | | | () | | | () | | | () | |
| Payments/credits relating to sales in prior years | | () | | | () | | | () | | | () | |
| Ending balance | | $ | | | | $ | | | | $ | | | | $ | | |
| | $ | | | | Component of accrued expense and other | | | | | | |
| Total revenue-related reserves | | $ | | | | $ | | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | Biogen's share of pre-tax profits in the U.S. for RITUXAN, GAZYVA and LUNSUMIO(1) | | | | | | | | | |
| Other revenue from anti-CD20 therapeutic programs | | | | | | | | | |
| Total revenue from anti-CD20 therapeutic programs | | $ | | | | $ | | | | $ | | |
(1) LUNSUMIO became commercially available in the U.S. during the first quarter of 2023.
Approximately %, % and % of our total revenue in 2023, 2022 and 2021, respectively, was derived from our collaboration arrangements with Genentech. For additional information on our collaboration arrangements with Genentech, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
Contract Manufacturing, Royalty and Other Revenue
Contract manufacturing, royalty and other revenue
| | $ | | | | $ | | | | Royalty and other revenue | | | | | | | | | |
| Total contract manufacturing, royalty and other revenue | | $ | | | | $ | | | | $ | | |
Contract Manufacturing Revenue
Contract manufacturing revenue primarily reflects amounts earned under contract manufacturing agreements with our strategic customers. During the first quarter of 2023 we began recognizing contract manufacturing revenue for LEQEMBI, upon accelerated approval of LEQEMBI in the U.S. Prior to accelerated approval, our share of contract manufacturing amounts related to LEQEMBI were recognized in research and development expense within our consolidated statements of income.
During the third quarter of 2019, we amended our agreement with a contract manufacturing customer pursuant to which we licensed certain of our manufacturing-related intellectual property to the customer. In the second quarter of 2020, the customer received regulatory approval for its product that is being manufactured using certain of our manufacturing-related intellectual property. As a result we were entitled to $ million in a series of payments. The first payment became due upon a regulatory approval of such product and was received during the second quarter of 2020. The second payment became due upon the first anniversary of the regulatory approval and was received during the second quarter of 2021. The third payment became due upon the second anniversary of the regulatory approval and was received during the second quarter of 2022.
Royalty and Other Revenue
Royalty and other revenue primarily reflects the royalties we receive from net sales on products related to patents that we have out-licensed, as well as royalty revenue on biosimilar products from our license arrangements with Samsung Bioepis and our % share of LEQEMBI product revenue, net and cost of sales, including royalties, as we are not the principal.
For additional information on our collaboration arrangements with Eisai and our license arrangements with Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | Work in process | | | | | | |
| Finished goods | | | | | | |
| Total inventory | | $ | | | | $ | | |
| | | | |
| Balance Sheet Classification: | | | | |
| Inventory | | $ | | | | $ | | |
| Investments and other assets | | | | | | |
| Total inventory | | $ | | | | $ | | |
We recorded approximately $ billion of acquired inventory, which includes measurement period adjustments, related to SKYCLARYS as a result of our acquisition of Reata in September 2023. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
Long-term inventory is included in investments and other assets in our consolidated balance sheets.
Write Downs and Other Charges
Inventory amounts written down as a result of excess, obsolescence or unmarketability are charged to cost of sales, and totaled $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively.
During the first quarter of 2022 we wrote-off approximately $ million of inventory related to ADUHELM, as a result of the final NCD, which was recognized in cost of sales within our consolidated statements of income for the year ended December 31, 2022. We recognized approximately $ million related to Eisai's % share of these charges in collaboration profit sharing/(loss reimbursement) within our consolidated statements of income for the year ended December 31, 2022.
During the fourth quarter of 2021 we wrote-off approximately $ million of inventory in excess of forecasted demand related to ADUHELM, which was recognized in cost of sales within our consolidated statements of income for the year ended December 31, 2021. We have recognized approximately $ million related to Eisai's % share of these charges in collaboration profit sharing/(loss reimbursement) within our consolidated statements of income for the year ended December 31, 2021.
As of December 31, 2023 and 2022, the carrying value of ADUHELM inventory was zero. In November 2023 we notified Neurimmune of our decision to terminate our collaboration and license agreement with Neurimmune and to discontinue the development and commercialization of ADUHELM.
For additional information on our collaboration with Eisai, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements. For additional information on the discontinuation of ADUHELM, please read Note 20, Investments in Variable Interest Entities, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| $ | | | | $ | () | | | $ | | | | $ | | | | $ | () | | | $ | | | Developed technology and other | | - years | | | | | () | | | | | | | | | () | | | | |
| | | | | | | | | |
Total completed technology | | | | | | | () | | | | | | | | | () | | | | |
| In-process research and development | | Indefinite until commercialization | | | | | — | | | | | | | | | — | | | | |
| Priority review voucher | | Indefinite | | | | | — | | | | | | | | | — | | | | |
| Trademarks and trade names | | Indefinite | | | | | — | | | | | | | | | — | | | | |
| Total intangible assets | | | | $ | | | | $ | () | | | $ | | | | $ | | | | $ | () | | | $ | | |
Amortization and Impairments
Amortization and impairment of acquired intangible assets totaled $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively. For the year ended December 31, 2023, we had no impairment charges.
Amortization of acquired intangible assets, excluding impairment charges, totaled $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively. The decrease in amortization of acquired intangible assets, excluding impairment charges, over the three years was primarily due to a lower rate of amortization for acquired intangible assets.
For the year ended December 31, 2022, amortization and impairment of acquired intangible assets reflects the impact of a $ million impairment charge related to vixotrigine (BIIB074) for the potential treatment of DPN.
For the year ended December 31, 2021, amortization and impairment of acquired intangible assets reflects the impact of a $ million impairment charge related to BIIB111 (timrepigene emparvovec), a $ million impairment charge related to BIIB112 (cotoretigene toliparvovec) and a $ million impairment charge related to vixotrigine for the potential treatment of TGN.
We monitor events and expectations regarding product performance. If new information indicates that the assumptions underlying our most recent analysis are substantially different than those utilized in our current estimates, our analysis would be updated and may result in a significant change in the anticipated lifetime revenue of the relevant products. The occurrence of an adverse event could substantially increase the amount of amortization expense related to our acquired intangible assets as compared to previous periods or our current expectations, which may result in a significant negative impact on our future results of operations.
Completed Technology
Completed technology primarily relates to our other marketed products and programs acquired through asset acquisitions, licenses and business combinations. Completed technology intangible assets are amortized over their estimated useful lives, which range between to years, with a remaining weighted average useful life of years for acquired and in-licensed rights and patents and years for developed technology and other. In connection with our acquisition of Reata in September 2023 we acquired SKYCLARYS, a commercially-approved product in the U.S., with an estimated fair value of approximately $ billion, which includes measurement period adjustments.
IPR&D Related to Business Combinations
IPR&D represents the fair value assigned to research and development assets that we acquired as part of a business combination and had not yet reached technological feasibility at the date of acquisition. Included in IPR&D balances are adjustments related to foreign currency exchange rate fluctuations. We review amounts capitalized as
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Priority Review Voucher
In connection with our acquisition of Reata in September 2023 we acquired a rare pediatric disease priority review voucher that may be used to obtain priority review by the FDA for a future regulatory submission or sold to a third party. We recorded the priority review voucher based on its estimated fair value of $ million as an intangible asset. The estimated fair value was based on recent external purchase and sale transactions of similar vouchers.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
Vixotrigine
In connection with our acquisition of Convergence, we recognized $ million of acquired IPR&D intangible assets for vixotrigine. In the periods following our acquisition of vixotrigine, there were numerous delays in the initiation of Phase 3 studies for the potential treatment of TGN and for the potential treatment of DPN, another form of neuropathic pain. We engaged with the FDA regarding the design of the potential Phase 3 studies of vixotrigine for the potential treatment of TGN and DPN and performed an additional clinical trial of vixotrigine, which was completed during 2022.
The performance of this additional clinical trial delayed the initiation of the Phase 3 studies of vixotrigine for the potential treatment of TGN, and, as a result, we recognized an impairment charge of $ million related to vixotrigine for the potential treatment of TGN during the first quarter of 2021.
During the fourth quarter of 2022 we discontinued further development of vixotrigine based on regulatory, development and commercialization challenges. For the year ended December 31, 2022, we recognized an impairment charge of approximately $ million related to vixotrigine for the potential treatment of DPN, reducing the remaining book value of this IPR&D intangible asset to zero. We also adjusted the value of our contingent consideration obligations related to this asset resulting in a pre-tax gain of approximately $ million, which was recognized in (gain) loss on fair value remeasurement of contingent consideration within our consolidated statements of income for the year ended December 31, 2022.
BIIB111 and BIIB112
In connection with our acquisition of Nightstar Therapeutics plc, we recognized $ million and $ million of acquired IPR&D intangible assets for BIIB111 and BIIB112, respectively. During the second quarter of 2021 we announced that our Phase 3 STAR study of BIIB111 and our Phase 2/3 XIRIUS study of BIIB112 did not meet their primary endpoints. In the third quarter of 2021 we suspended further development on these programs based on the decision by management as part of its strategic review process. For the year ended December 31, 2021, we recognized an impairment charge of $ million related to BIIB111 and an impairment charge of $ million related to BIIB112, reducing the remaining book values of these IPR&D intangible assets to .
In addition, as a result of our decision to suspend further development of BIIB111 and BIIB112, we recorded charges of approximately $ million during the third quarter of 2021 related to our manufacturing arrangements and other costs that we expect to incur as a result of suspending these programs. These charges were recognized in research and development expense in our consolidated statements of income for the year ended December 31, 2021.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | 2025 | | | |
| 2026 | | | |
| 2027 | | | |
| 2028 | | | |
Goodwill
| | $ | | | | Goodwill resulting from Reata acquisition | | | | | | |
|
| Other | | | | | () | |
| Goodwill, end of year | | $ | | | | $ | | |
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
accumulated impairment losses related to goodwill. Other includes adjustments related to foreign currency exchange rate fluctuations.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | $ | | | | | | |
| | | |
| | | |
| | | |
| Marketable equity securities | | | | | | | | | | | | |
| Other current assets: | | | | | | | | |
Receivable from Samsung BioLogics(1) | | | | | | | | | | | | |
| Derivative contracts | | | | | | | | | | | | |
| Other non-current assets: | | | | | | | | |
| Plan assets for deferred compensation | | | | | | | | | | | | |
| | | |
| Total | | $ | | | | $ | | | | $ | | | | $ | | |
| Liabilities: | | | | | | | | |
| Derivative contracts | | $ | | | | $ | | | | $ | | | | $ | | |
| | | |
| Total | | $ | | | | $ | | | | $ | | | | $ | | |
(1) Represents the fair value of the current payment due from Samsung BioLogics as a result of the sale of our % equity interest in Samsung Bioepis to Samsung BioLogics during the second quarter of 2022, for which we elected the fair value option. For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
During the third quarter of 2023 we sold all of our marketable debt securities and used the proceeds to partially fund our acquisition of Reata. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | $ | | | | Marketable debt securities: | | | | | | | | |
| Corporate debt securities | | | | | | | | | | | | |
| Government securities | | | | | | | | | | | | |
| Mortgage and other asset backed securities | | | | | | | | | | | | |
| Marketable equity securities | | | | | | | | | | | | |
| Other current assets: | | | | | | | | |
Receivable from Samsung BioLogics(1) | | | | | | | | | | | | |
| Other non-current assets: | | | | | | | | |
| Derivative contracts | | | | | | | | | | | | |
| Plan assets for deferred compensation | | | | | | | | | | | | |
Receivable from Samsung BioLogics(1) | | | | | | | | | | | | |
| Total | | $ | | | | $ | | | | $ | | | | $ | | |
| Liabilities: | | | | | | | | |
| Derivative contracts | | $ | | | | $ | | | | $ | | | | $ | | |
| | | |
|
|
|
) |
|
| | |
For the year ended December 31, 2022, the changes in fair value of our contingent consideration obligations were primarily due to the discontinuation of further development efforts related to vixotrigine for the potential treatment of TGN and DPN, resulting in a reduction of our contingent consideration obligations of approximately $ million, reducing the remaining fair value of vixotrigine to zero, as well as changes in the interest rates used to revalue our contingent consideration liabilities.
Changes in the fair values of our contingent consideration obligations are recorded in (gain) loss on fair value remeasurement of contingent consideration in our consolidated statements of income. The fair values of the contingent consideration liabilities were based on a probability-adjusted discounted cash flow calculation using Level 3 fair value measurements and inputs. For additional information on the valuation techniques and inputs utilized in the valuation of our financial assets and liabilities, please read Note 1, Summary of Significant Accounting Policies, to these consolidated financial statements.
Convergence Pharmaceuticals Holdings Limited
In connection with our acquisition of Convergence in February 2015 we recorded a contingent consideration obligation of $ million. As of December 31, 2021, the fair value of this contingent consideration obligation was $ million. During the fourth quarter of 2022 we discontinued further development of vixotrigine based on regulatory, development and commercialization challenges. As a result, the fair value of the contingent consideration obligations related to Convergence has been adjusted to , resulting in a pre-tax gain of approximately $ million for the year ended December 31, 2022. This pre-tax gain was recorded in (gain) loss on fair value remeasurement of contingent consideration within our consolidated statements of income.
Nonrecurring Fair Value Measurements
For the year ended December 31, 2022, we recorded impairment charges of $ million related to vixotrigine. As a result, the remaining book values associated with these programs were reduced to zero. For the year ended December 31, 2021, we recorded impairment charges of $ million related to BIIB111 and $ million related to BIIB112. As a result, the remaining book values associated with these programs were reduced to .
For additional information on our impairments for intangible assets, please read Note 7, Intangible Assets and Goodwill, to these consolidated financial statements.
Financial Instruments Not Carried at Fair Value
Other Financial Instruments
Due to the short-term nature of certain financial instruments, the carrying value reflected in our consolidated balance sheets for current accounts receivable, due from anti-CD20 therapeutic programs, other current assets, accounts payable and accrued expense and other, approximates fair value.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | Current portion of notes payable and term loan | | | | | | |
| | | | |
| Non-current portion: | | | | |
2023 Term Loan three-year tranche(1) | | | | | | |
% Senior Notes due September 15, 2025 | | | | | | |
% Senior Notes due May 1, 2030 | | | | | | |
% Senior Notes due September 15, 2045 | | | | | | |
% Senior Notes due May 1, 2050 | | | | | | |
% Senior Notes due February 15, 2051 | | | | | | |
| Non-current portion of notes payable and term loan | | | | | | |
| Total notes payable and term loan | | $ | | | | $ | | |
(1) In connection with our acquisition of Reata we drew $ billion from our 2023 Term Loan, comprised of a $ million floating rate 364-day tranche and a $ million floating rate three-year tranche. For additional information on our 2023 Term Loan, please read Note 13, Indebtedness, to these consolidated financial statements.
The fair values of each of our series of Senior Notes were determined through market, observable and corroborated sources. The changes in the fair values of our Senior Notes as of December 31, 2023, compared to 2022, are primarily related to increases in U.S. treasury yields partially offset by a decrease in credit spreads used to value our Senior Notes since December 31, 2022. For additional information related to our Senior Notes, please read Note 13, Indebtedness, to these consolidated financial statements.
| | $ | | | | Overnight reverse repurchase agreements | | | | | | |
| Money market funds | | | | | | |
| Short-term debt securities | | | | | | |
| Total | | $ | | | | $ | | |
The carrying values of our commercial paper, including accrued interest, overnight reverse repurchase agreements, money market funds and short-term debt securities approximate fair value due to their short-term maturities.
We partially funded our Reata acquisition through available cash, cash equivalents and marketable securities. As of December 31, 2023, we have sold all of our marketable debt securities. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | () | | | $ | | | | Marketable equity securities, non-current | | | | | | | | () | | | | |
| Total marketable equity securities | | $ | | | | $ | | | | $ | () | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | As of December 31, 2022 |
| (In millions) | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value |
| Marketable debt securities: | | | | | | | | |
| Corporate debt securities: | | | | | | | | |
| Current | | $ | | | | $ | | | | $ | () | | | $ | | |
| Non-current | | | | | | | | () | | | | |
| Government securities: | | | | | | | | |
| Current | | | | | | | | () | | | | |
| Non-current | | | | | | | | () | | | | |
| Mortgage and other asset backed securities: | | | | | | | | |
| Current | | | | | | | | | | | | |
| Non-current | | | | | | | | () | | | | |
| Total marketable debt securities | | $ | | | | $ | | | | $ | () | | | $ | | |
| | | | | | | | |
| Marketable equity securities: | | | | | | | | |
| | | |
|
| | | |
|
| () | | | $ | () | |
(1) Beginning in the second quarter of 2022 we no longer held net investment hedges as they were closed with the sale of our % equity interest in Samsung Bioepis in April 2022. For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
For additional information on our collaboration arrangements with Samsung Bioepis, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
Foreign Currency Forward Contracts - Other Derivative Instruments
We also enter into other foreign currency forward contracts, usually with durations of one month or less, to mitigate the foreign currency risk related to certain balance sheet positions. We have not elected hedge accounting for these transactions.
The aggregate notional amount of these outstanding foreign currency forward contracts was $ million and $ million as of December 31, 2023 and 2022, respectively. Net gains of $ million, net losses of $ million and net losses of $ million related to these contracts were recorded as a component of other (income) expense, net for the years ended December 31, 2023, 2022 and 2021, respectively.
Summary of Derivative Instruments
While certain of our derivative instruments are subject to netting arrangements with our counterparties, we do not offset derivative assets and liabilities in our consolidated balance sheets. The amounts in the table below would not be substantially different if the derivative assets and liabilities were offset.
| | $ | | | | |
| Liability derivative instruments | | Accrued expense and other | | | | | | |
| |
| | | | | | |
| Other Derivative Instruments: | | | | | | |
| Asset derivative instruments | | Other current assets | | | | | | |
| Liability derivative instruments | | Accrued expense and other | | | | | | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | Buildings | | | | | | |
| Leasehold improvements | | | | | | |
| Machinery and equipment | | | | | | |
| Computer software and hardware | | | | | | |
| Furniture and fixtures | | | | | | |
| Construction in progress | | | | | | |
| Total cost | | | | | | |
| Less: accumulated depreciation | | () | | | () | |
| Total property, plant and equipment, net | | $ | | | | $ | | |
Depreciation expense totaled $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively.
For the years ended December 31, 2023, 2022 and 2021, we capitalized interest costs related to construction in progress totaling approximately $ million, $ million and $ million, respectively.
Solothurn, Switzerland Manufacturing Facility
In order to support our future growth and drug development pipeline, we built a large-scale biologics manufacturing facility in Solothurn, Switzerland. This facility includes square feet related to a large-scale biologics manufacturing facility, square feet of warehouse, utilities and support space and square feet of administrative space. As of December 31, 2023 and 2022, we had approximately $ million and $ million, respectively, capitalized as construction in progress related to this facility. In the second quarter of 2021 a portion of this facility (the first manufacturing suite) received a GMP multi-product license from the SWISSMEDIC, resulting in approximately $ billion of fixed assets being placed into service during the second quarter of 2021. The second manufacturing suite became operational in January 2024, resulting in approximately $ million of fixed assets being placed into service during the first quarter of 2024. Solothurn has been approved for the manufacture of ADUHELM and LEQEMBI by the FDA.
125 Broadway Building Sale
In September 2022 we completed the sale of our building and land parcel located at 125 Broadway for an aggregate sales price of approximately $ million, which is inclusive of a $ million tenant allowance. This sale resulted in a pre-tax gain on sale of approximately $ million, net of transaction costs, which is reflected within gain on sale of building in our consolidated statements of income for the year ended December 31, 2022. This transaction included approximately $ million of property, plant and equipment, net, which comprised of approximately $ million for buildings, approximately $ million for land and approximately $ million for machinery and equipment.
to . Certain leases include one or more options to renew, exercised at our sole discretion, with renewal terms that can extend the lease term from to .In addition, we sublease certain real estate to third parties. Our sublease portfolio consists of operating leases, with remaining lease terms ranging from to .
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | | | | | |
| Liabilities | | | | | |
| Current operating lease liabilities | Accrued expense and other | | $ | | | | $ | | |
| Non-current operating lease liabilities | Long-term operating lease liabilities | | | | | | |
| Total operating lease liabilities | | | $ | | | | $ | | |
| | $ | | | | $ | | | | | Selling, general and administrative | | | | | | | | | |
| Variable lease cost | | Research and development | | | | | | | | | |
| | Selling, general and administrative | | | | | | | | | |
| Sublease income | | Selling, general and administrative | | () | | | () | | | () | |
| | Other (income) expense, net | | () | | | () | | | () | |
| Net lease cost | | | | $ | | | | $ | | | | $ | | |
Variable lease cost primarily related to operating expense, taxes and insurance associated with our operating leases. As these costs are generally variable in nature, they are not included in the measurement of the operating lease asset and related lease liability.
| | 2025 | | | |
| 2026 | | | |
| 2027 | | | |
| 2028 | | | |
| Thereafter | | | |
| Total lease payments | | $ | | |
| Less: interest | | | |
| Present value of operating lease liabilities | | $ | | |
| | | Weighted average discount rate | | | % | | | % |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | |
| Operating lease assets obtained in exchange for lease obligations | | | | | | | | | |
6100 Legacy Drive Lease
In connection with our acquisition of Reata we assumed responsibility for a single-tenant, build-to-suit building of approximately square feet of office and laboratory space located in Plano, Texas, with an initial lease term of years. We recorded a lease liability of approximately $ million, which represents the net present value of rental expense over the remaining lease term of approximately years, with a corresponding right-of-use asset of approximately $ million, which represents our estimate of the fair value for a market participant of the current rental market in the Dallas, Texas area. Included in our estimate of the market rental rate is the value of any leasehold improvements or tenant allowances related to the building. We do not intend to occupy this building and are evaluating opportunities to sublease the property.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
125 Broadway Building Sale and Leaseback Transaction
In connection with the sale of our 125 Broadway building during the third quarter of 2022, we simultaneously leased back the building for a term of approximately years, which resulted in the recognition of approximately $ million in a new lease liability and right-of-use asset recorded within our consolidated balance sheets as of December 31, 2022. The sale and immediate leaseback of this building qualified for sale and leaseback treatment and is classified as an operating lease. For additional information on the sale of our 125 Broadway building, please read Note 11, Property, Plant and Equipment, to these consolidated financial statements.
300 Binney Street Lease Modification
In September 2022 we entered into an agreement to partially terminate a portion of our lease located at 300 Binney Street, as well as to reduce the lease term for the majority of the remaining space. The agreement was driven by our 2022 efforts to reduce costs by consolidating real estate locations. The transaction was treated as a lease modification as of the effective date and resulted in the derecognition of a right-of-use asset of approximately $ million and a lease liability of approximately $ million, which resulted in a gain of approximately $ million, which was recorded within restructuring charges in our consolidated statements of income for the year ended December 31, 2022.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | Current portion of notes payable and term loan | | $ | | | | $ | | |
| Non-current portion: | | | | |
2023 Term Loan three-year tranche(1) | | $ | | | | $ | | |
% Senior Notes due September 15, 2025 | | | | | | |
% Senior Notes due May 1, 2030 | | | | | | |
% Senior Notes due September 15, 2045 | | | | | | |
% Senior Notes due May 1, 2050 | | | | | | |
% Senior Notes due February 15, 2051 | | | | | | |
| Non-current portion of notes payable and term loan | | $ | | | | $ | | |
(1) In connection with our acquisition of Reata we drew $ billion from our 2023 Term Loan, comprised of a $ million floating rate 364-day tranche and a $ million floating rate three-year tranche.
As of December 31, 2023, we were in compliance with our senior note covenants and term loan covenants.
2023 Term Loan Credit Agreement
In connection with our acquisition of Reata in September 2023 we entered into a $ billion term loan credit agreement (2023 Term Loan). On the closing date of the Reata acquisition we drew $ billion from the 2023 Term Loan, comprised of a $ million floating rate 364-day tranche and a $ million floating rate three-year tranche. The remaining unused commitment of $ million was terminated. During the fourth quarter of 2023 we repaid $ million of the 364-day tranche. As of December 31, 2023, we had $ million outstanding under the 2023 Term Loan, of which $ million was outstanding under the 364-day tranche and $ million was outstanding under the three-year tranche.
2021 Exchange Offer
In February 2021 we completed our private offer to exchange (Exchange Offer) our tendered % Senior Notes due September 15, 2045 (2045 Senior Notes) for a new series of % Senior Notes due February 15, 2051 (2051 Senior Notes) and cash, and an offer to purchase our tendered 2045 Senior Notes for cash.
An aggregate principal amount of approximately $ million of our 2045 Senior Notes was exchanged for an aggregate principal amount of approximately $ million of our 2051 Senior Notes and aggregate cash payments of approximately $ million. Our Exchange Offer has been accounted for as a debt modification; as such, the cash component has been reflected as additional debt discount and is amortized as an adjustment to interest expense over the term of our 2051 Senior Notes.
In addition, we redeemed an aggregate principal amount of approximately $ million of our 2045 Senior Notes for aggregate cash payments of approximately $ million, excluding accrued and unpaid interest. The redemption has been accounted for as a debt extinguishment; as such, we recognized a pre-tax charge of $ million upon the extinguishment of such 2045 Senior Notes. This charge, which was recognized in interest expense in other (income) expense, net in our consolidated statements of income for the year ended December 31, 2021, reflects the payment of an early call premium and the write-off of the remaining unamortized original debt issuance costs and discount balances associated with such 2045 Senior Notes.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
2020 Senior Notes
On April 30, 2020, we issued senior unsecured notes for an aggregate principal amount of $ billion (2020 Senior Notes), consisting of the following:
•$ billion aggregate principal amount of % Senior Notes due May 1, 2030, valued at % of par; and
•$ billion aggregate principal amount of % Senior Notes due May 1, 2050, valued at % of par.
Our 2020 Senior Notes are senior unsecured obligations and may be redeemed at our option at any time at % of the principal amount plus accrued interest and, until a specified period before maturity, a specified make-whole amount. Our 2020 Senior Notes contain a change-of-control provision that, under certain circumstances, may require us to purchase our 2020 Senior Notes at a price equal to % of the principal amount plus accrued and unpaid interest to the date of repurchase.
The original costs associated with this offering of approximately $ million have been recorded as a reduction to the carrying amount of the debt in our consolidated balance sheets. These costs along with the discounts will be amortized as additional interest expense using the effective interest rate method over the period from issuance through maturity. Interest on our 2020 Senior Notes is payable May 1 and November 1 of each year, commencing November 1, 2020.
2015 Senior Notes
The following is a summary of our currently outstanding senior unsecured notes issued in 2015 (the 2015 Senior Notes), consisting of the following:
•$ billion aggregate principal amount of % Senior Notes due September 15, 2025, valued at % of par; and
•$ billion aggregate principal amount of % Senior Notes due September 15, 2045, valued at % of par.
Our 2015 Senior Notes are senior unsecured obligations and may be redeemed at our option at any time at % of the principal amount plus accrued interest and a specified make-whole amount. Our 2015 Senior Notes contain a change of control provision that may require us to purchase the notes at a price equal to % of the principal amount plus accrued and unpaid interest to the date of purchase under certain circumstances.
The original costs associated with this offering of approximately $ million have been recorded as a reduction to the carrying amount of the debt in our consolidated balance sheets. These costs along with the discounts will be amortized as additional interest expense using the effective interest rate method over the period from issuance through maturity.
3.625% Senior Notes due September 15, 2022
On September 15, 2015, we issued $ billion aggregate principal amount of our % Senior Notes due September 15, 2022, at % of par. Our % Senior Notes were senior unsecured obligations. In July 2022 we redeemed our % Senior Notes prior to their maturity and recognized a net pre-tax charge of approximately $ million upon the extinguishment of these Senior Notes, which primarily reflects the payment of an early call premium as well as the write-off of remaining unamortized original debt issuance costs and discount balances. These charges were recognized as interest expense in other (income) expense, net in our consolidated statements of income for the year ended December 31, 2022.
2020 Revolving Credit Facility
In January 2020 we entered into a $ billion, senior unsecured revolving credit facility under which we are permitted to draw funds for working capital and general corporate purposes. The terms of the revolving credit facility
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Debt Maturity
| | 2025 | | | |
| 2026 | | | |
| 2027 | | | |
| 2028 | | | |
| 2029 and thereafter | | | |
Total current and non-current debt | | $ | | |
| Less: debt discount and issuance fees | | () | |
Total current and non-current debt, net | | $ | | |
The fair value of our debt is disclosed in Note 8, Fair Value Measurements, to these consolidated financial statements.
million shares of Preferred Stock authorized, of which million shares are authorized as Series A, million shares are authorized as Series X junior participating and million shares are undesignated. Shares may be issued without a vote or action of shareholders from time to time in classes or series with the designations, powers, preferences and the relative, participating, optional or other special rights of the shares of each such class or series and any qualifications, limitations or restrictions thereon as set forth in the instruments governing such shares. Any such Preferred Stock may rank prior to common stock as to dividend rights, liquidation preference or both, and may have full or limited voting rights and may be convertible into shares of common stock. shares of Preferred Stock were issued and outstanding during 2023, 2022 and 2021.Common Stock
| | | | | | | | | | | | | | | | | | | | | | | | | Share Repurchases
In October 2020 our Board of Directors authorized our 2020 Share Repurchase Program, which is a program to repurchase up to $ billion of our common stock. Our 2020 Share Repurchase Program does not have an expiration date. All share repurchases under our 2020 Share Repurchase Program will be retired. Under our 2020 Share Repurchase Program, we repurchased and retired approximately million and million shares of our common stock at a cost of approximately $ million and $ billion during the years ended December 31, 2022 and 2021, respectively. There were share repurchases of our common stock during the year ended December 31, 2023. Approximately $ billion remained available under our 2020 Share Repurchase Program as of December 31, 2023.
Amounts paid to repurchase shares in excess of their par value are allocated between additional paid-in capital and retained earnings, with payments in excess of our additional paid-in-capital balance recorded as a reduction to retained earnings.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | () | | | $ | () | | | $ | () | | | Other comprehensive income (loss) before reclassifications | | | | | () | | | () | | | | | | | |
| Amounts reclassified from AOCI | | | | | () | | | | | | | | | | |
| Net current period other comprehensive income (loss) | | | | | () | | | () | | | | | | | |
| Balance, December 31, 2023 | | $ | | | | $ | () | | | $ | () | | | $ | () | | | $ | () | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2022 |
| (In millions) | | Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax | | Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax | | Gains (Losses) on Net Investment Hedges, Net of Tax(1) | | Unrealized Gains (Losses) on Pension Benefit Obligation, Net of Tax | | Currency Translation Adjustments | | Total |
| Balance, December 31, 2021 | | $ | () | | | $ | | | | $ | | | | $ | () | | | $ | () | | | $ | () | |
| Other comprehensive income (loss) before reclassifications | | () | | | | | | | | | | | | () | | | | |
| Amounts reclassified from AOCI | | | | | () | | | () | | | | | | | | | () | |
| Net current period other comprehensive income (loss) | | () | | | () | | | () | | | | | | () | | | () | |
| Balance, December 31, 2022 | | $ | () | | | $ | | | | $ | | | | $ | () | | | $ | () | | | $ | () | |
(1) Beginning in the second quarter of 2022 we no longer held net investment hedges as they were closed with the sale of our % equity interest in Samsung Bioepis in April 2022. For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2021 |
| (In millions) | | Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax | | Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax | | Gains (Losses) on Net Investment Hedges, Net of Tax | | Unrealized Gains (Losses) on Pension Benefit Obligation, Net of Tax | | Currency Translation Adjustments | | Total |
| Balance, December 31, 2020 | | $ | | | | $ | () | | | $ | () | | | $ | () | | | $ | () | | | $ | () | |
| Other comprehensive income (loss) before reclassifications | | () | | | | | | | | | | | | () | | | | |
| Amounts reclassified from AOCI | | | | | | | | | | | | | | | | | | |
| Net current period other comprehensive income (loss) | | () | | | | | | | | | | | | () | | | | |
| Balance, December 31, 2021 | | $ | () | | | $ | | | | $ | | | | $ | () | | | $ | () | | | $ | () | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | () | | | $ | () | | | Other (income) expense | | | | | | | | | | | | Income tax (benefit) expense |
| | | | | | | | |
| Gains (losses) on cash flow hedges | | | | | | | | () | | | Revenue |
| | | | | () | | | () | | | Operating expense |
| | () | | | () | | | | | | Other (income) expense |
| | () | | | () | | | | | | Income tax (benefit) expense |
| | | | | | | | |
Gains (losses) on net investment hedges(1) | | | | | | | | () | | | Other (income) expense |
| | | | | | | | |
| Currency translation adjustments | | | | | () | | | | | | Other (income) expense |
| | | | | | | | |
| Total reclassifications, net of tax | | $ | () | | | $ | | | | $ | () | | | |
(1) Beginning in the second quarter of 2022 we no longer held net investment hedges as they were closed with the sale of our % equity interest in Samsung Bioepis in April 2022. For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
| | $ | | | | $ | | | | Denominator: | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | | | |
| Effect of dilutive securities: | | | | | | |
| |
| Time-vested restricted stock units | | | | | | | | | |
| Market stock units | | | | | | | | | |
| Performance stock units settled in stock | | | | | | | | | |
| Dilutive potential common shares | | | | | | | | | |
| Shares used in calculating diluted earnings per share | | | | | | | | | |
Amounts excluded from the calculation of net income per diluted share because their effects were anti-dilutive were insignificant.
Earnings per share for the years ended December 31, 2022 and 2021, reflects the repurchase of approximately million shares and million shares of our common stock, respectively, under our share repurchase programs. There were share repurchases of our common stock during the year ended December 31, 2023. For additional information on our share repurchase programs, please read Note 14, Equity, to these consolidated financial statements.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | Selling, general and administrative | | | | | | | | | |
| Subtotal | | | | | | | | | |
| Capitalized share-based compensation costs | | () | | | () | | | () | |
| Share-based compensation expense included in total cost and expense | | | | | | | | | |
| Income tax effect | | () | | | () | | | () | |
| Share-based compensation expense included in net income attributable to Biogen Inc. | | $ | | | | $ | | | | $ | | |
In connection with our acquisition of Reata in September 2023 we recognized Reata equity-based compensation expense, inclusive of employer taxes, of approximately $ million attributable to the post-acquisition service period, of which $ million was recognized as a charge to selling, general and administrative expense with the remaining $ million as a charge to research and development expense within our consolidated statements of income for the year ended December 31, 2023. These amounts were associated with the accelerated vesting of stock options and RSUs previously granted to Reata employees that required no future services to vest. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
| | $ | | | | $ | | | | Time-vested restricted stock units | | | | | | | | | |
| Performance stock units settled in stock | | | | | | | | | |
| Performance stock units settled in cash | | | | | | | | | |
| Employee stock purchase plan | | | | | | | | | |
| Stock options | | | | | | | | | |
Reata equity awards(1) | | | | | | | | | |
| Subtotal | | | | | | | | | |
| Capitalized share-based compensation costs | | () | | | () | | | () | |
| Share-based compensation expense included in total cost and expense | | $ | | | | $ | | | | $ | | |
(1) Relates to the Reata equity-based compensation expense attributable to the post-acquisition service period, associated with the accelerated vesting of stock options and RSUs previously granted to Reata employees that required no future services to vest. For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
As of December 31, 2023, unrecognized compensation cost related to unvested share-based compensation was approximately $ million, net of estimated forfeitures. We expect to recognize the cost of these unvested awards over a weighted-average period of years.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Directors Plan
In May 2006 our shareholders approved the 2006 Directors Plan for share-based awards to our directors. Awards granted from the 2006 Directors Plan may include stock options, shares of restricted stock, RSUs, stock appreciation rights and other awards in such amounts and with such terms and conditions as may be determined by a committee of our Board of Directors, subject to the provisions of the 2006 Directors Plan. We have reserved a total of million shares of common stock for issuance under the 2006 Directors Plan. The 2006 Directors Plan provides that awards other than stock options and stock appreciation rights will be counted against the total number of shares reserved under the plan in a -to-1 ratio. In June 2015 our shareholders approved an amendment to extend the term of the 2006 Directors Plan until June 2025.
Omnibus Plan
In June 2017 our shareholders approved the 2017 Omnibus Equity Plan for share-based awards to our employees. Awards granted from the 2017 Omnibus Equity Plan may include stock options, shares of restricted stock, RSUs, performance shares, stock appreciation rights and other awards in such amounts and with such terms and conditions as may be determined by a committee of our Board of Directors, subject to the provisions of the 2017 Omnibus Equity Plan. Shares of common stock available for grant under the 2017 Omnibus Equity Plan consist of million shares reserved for this purpose, plus shares of common stock that remained available for grant under the Biogen Idec Inc. 2008 Omnibus Equity Plan (2008 Omnibus Equity Plan) as of June 7, 2017, or that could again become available for grant if outstanding awards under the 2008 Omnibus Equity Plan as of June 7, 2017, are cancelled, surrendered or terminated in whole or in part. The 2017 Omnibus Equity Plan provides that awards other than stock options and stock appreciation rights will be counted against the total number of shares available under the plan in a -to-1 ratio.
We have not made any awards pursuant to the 2008 Omnibus Equity Plan since our shareholders approved the 2017 Omnibus Equity Plan, and do not intend to make any awards pursuant to the 2008 Omnibus Equity Plan in the future, except that unused shares under the 2008 Omnibus Equity Plan have been carried over for use under the 2017 Omnibus Equity Plan.
Stock Options
In 2022 we granted approximately stock options to our CEO (2022 CEO Grant) under the 2017 Omnibus Plan with a grant date fair value of $ per option for a total of approximately $ million. The fair value of the stock option grant is estimated as of the date of grant using a Black-Scholes option valuation model. The estimated fair value of the stock option is then expensed over the options' vesting periods. The 2022 CEO Grant is eligible to vest in equal annual installments over a period from the grant date, subject to the CEO’s continued employment. The outstanding stock option has a term and is exercisable at a price per share not less than the fair market value of the underlying common stock on the date of grant.
| | $ | | | | years | | Granted | | | | | | | | |
Exercised | | | | | | | | |
| Forfeited | | | | | | | | |
Outstanding at December 31, 2023 | | | | | $ | | | | years |
Exercisable at December 31, 2023 | | | | | $ | | | | years |
| | | |
|
%
% - %
% - %
- $
|
The fair values of MSUs vested in 2023, 2022 and 2021 totaled $ million, $ million and $ million, respectively.
Performance Stock Units
PSUs Settled in Stock
During the first quarter of 2018 we began granting awards for performance-vested RSUs that will settle in stock. PSUs awarded to employees have a performance period and vest on the third anniversary of the grant date. The vesting of these awards is subject to the respective employee’s continued employment. The number of PSUs granted represents the target number of units that are eligible to be earned based on the achievement of cumulative performance measures established at the beginning of the performance period, which ends on December 31 of the third year of the performance period.
Participants may ultimately earn between and % of the target number of PSUs granted based on the degree of achievement of the applicable performance metric. Accordingly, additional PSUs may be issued or currently outstanding PSUs may be cancelled upon final determination of the number of units earned.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | Granted (1) | | | | | |
| Vested | () | | | | |
| Forfeited | () | | | | |
| Unvested at December 31, 2023 | | | | $ | | |
PSUs settled in stock granted in 2022 and 2021 had weighted average grant date fair values of $ and $, respectively.
We value grants of PSUs using a lattice model with a Monte Carlo simulation. This valuation methodology utilizes several key assumptions, the calendar day average closing stock price on the date of grant for PSUs, expected volatility of our stock price, risk-free rates of return and expected dividend yield.
% | % | | Range of expected stock price volatility | | % | | % - % |
| Range of risk-free interest rates | | % | | % - % |
| 30 calendar day average stock price on grant date | | $ | | $ - $ |
| Weighted-average per share grant date fair value | | $ | | $ | The fair values of PSUs settled in stock that vested in 2023, 2022 and 2021 totaled $ million, $ million and $ million, respectively.
PSUs Settled in Cash
During the first quarter of 2018 we began granting awards for performance-vested restricted stock units that will settle in cash. PSUs awarded to employees have three performance periods and vest on the third anniversary of the grant date. The vesting of these awards is subject to the respective employee’s continued employment. The number of PSUs granted represents the target number of units that are eligible to be earned based on the achievement of three annual performance measures established when the performance objectives are defined, which will be at the beginning of each year and will end on December 31 of such year.
Participants may ultimately earn between and % of the target number of PSUs granted based on the degree of achievement of the applicable performance metric. Accordingly, additional PSUs may be issued or currently outstanding PSUs may be cancelled upon final determination of the number of units earned. PSUs are classified as liability awards and will be settled in cash based on the calendar day average closing stock price through the vesting date, once the actual vested and earned number of PSUs is determined. Since no shares are issued, these awards do not dilute equity.
Beginning in 2022 we no longer grant this type of PSUs as part of our long term incentive program and have replaced with granting time-vested RSUs.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
|
Granted(1) | | |
| Vested | () | |
| Forfeited | () | |
| Unvested at December 31, 2023 | | |
(1) PSUs settled in cash granted in 2023 represent the adjustment recorded to reflect the total number of units earned based on the finalization of the related performance multiplier for awards previously granted in 2020.
The fair values of PSUs settled in cash that vested in 2023, 2022 and 2021 totaled $ million, $ million and $ million, respectively.
Time-Vested Restricted Stock Units
RSUs awarded to employees generally vest no sooner than one-third per year over on the anniversary of the date of grant, or upon the third anniversary of the date of the grant, provided the employee remains continuously employed with us, except as otherwise provided in the plan. Shares of our common stock will be delivered to the employee upon vesting, subject to payment of applicable withholding taxes. RSUs awarded to directors for service on our Board of Directors vest on the first anniversary of the date of grant, provided in each case that the director continues to serve on our Board of Directors through the vesting date. Shares of our common stock will be delivered to the director upon vesting and are not subject to any withholding taxes.
| | $ | | | Granted (1) | | | | | |
| Vested | () | | | | |
| Forfeited | () | | | | |
| Unvested at December 31, 2023 | | | | $ | | |
(1) RSUs granted in 2023 primarily represent RSUs granted in conjunction with our annual awards made in February 2023 and awards made in conjunction with the hiring of new employees. RSUs granted in 2023 also include approximately RSUs granted to our Board of Directors.
RSUs granted in 2022 and 2021 had weighted average grant date fair values of $ and $, respectively.
The fair values of RSUs vested in 2023, 2022 and 2021 totaled $ million, $ million and $ million, respectively.
Employee Stock Purchase Plan
In June 2015 our shareholders approved the 2015 ESPP. The maximum aggregate number of shares of our common stock that may be purchased under the 2015 ESPP is million.
| | | | | | | | Cash received under the 2015 ESPP | | $ | | | | $ | | | | $ | | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | Foreign | | | | | | | | | |
| Total income before income tax (benefit) expense | | $ | | | | $ | | | | $ | | |
| Income tax (benefit) expense: | | | | | | |
| Current: | | | | | | |
| Federal | | $ | | | | $ | | | | $ | | |
| State | | | | | | | | | |
| Foreign | | | | | | | | | |
| Total current | | | | | | | | | |
| Deferred: | | | | | | |
| Federal | | () | | | () | | | () | |
| State | | () | | | | | | () | |
| Foreign | | | | | | | | () | |
| Total deferred | | () | | | () | | | () | |
| Total income tax (benefit) expense | | $ | | | | $ | | | | $ | | |
Transition Toll Tax
The Tax Cuts and Jobs Act of 2017 eliminated the deferral of U.S. income tax on the historical unrepatriated earnings by imposing the one-time mandatory deemed repatriation tax on accumulated foreign subsidiaries' previously untaxed foreign earnings. The Transition Toll Tax was assessed on our share of our foreign corporations' accumulated foreign earnings that were not previously taxed. Earnings in the form of cash and cash equivalents were taxed at a rate of 15.5% and all other earnings were taxed at a rate of 8.0%.
As of December 31, 2023 and 2022, we have accrued income tax liabilities of $ million and $ million, respectively, under the Transition Toll Tax. Of the amounts accrued as of December 31, 2023, approximately $ million is expected to be paid within one year. The Transition Toll Tax is being paid in installments over an eight--year period, which started in 2018, and will not accrue interest.
Unremitted Earnings
At December 31, 2023, we considered our earnings not to be permanently reinvested outside the U.S. and therefore recorded deferred tax liabilities associated with an estimate of the total withholding taxes expected as a result of our repatriation of earnings. Other than for earnings, we are permanently reinvested for book/tax basis differences of approximately $ billion as of December 31, 2023, primarily arising through the impacts of purchase accounting. These permanently reinvested basis differences could reverse through sales of the foreign subsidiaries, as well as various other events, none of which were considered probable as of December 31, 2023. The residual U.S. tax liability, if these differences reverse, would be between $ million and $ million as of December 31, 2023.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | Inventory, other reserves and accruals | | | | | | |
| Intangibles, net | | | | | | |
| Neurimmune's tax basis in ADUHELM | | | | | | |
| IRC Section 174 capitalized research and development | | | | | | |
| Net operating loss | | | | | | |
| Share-based compensation | | | | | | |
| Other | | | | | | |
| Valuation allowance | | () | | | () | |
| Total deferred tax assets | | $ | | | | $ | | |
| Deferred tax liabilities: | | | | |
Purchased inventory valuation step-up and intangible assets | | $ | () | | | $ | () | |
| Samsung Bioepis investment installments | | () | | | () | |
| GILTI | | () | | | () | |
| Tax credits | | | | | () | |
| Depreciation, amortization and other | | () | | | () | |
| Total deferred tax liabilities | | $ | () | | | $ | () | |
As of December 31, 2023, 2022, 2021 and 2020, we had a valuation allowance of $ million, $ million, $ million and $ million, respectively, related to net operating losses in Switzerland and Neurimmune's tax basis in ADUHELM. The change in the valuation allowance between December 31, 2023 and 2022, was primarily driven by a reduction of approximately $ million related to the elimination of Neurimmune's tax basis in ADUHELM as a result of its deconsolidation and reduction of approximately $ million due to movements in net operating loss deferred tax assets in Switzerland. The net income tax impact of the changes in the valuation allowance was an expense of approximately $ million for the year ended December 31, 2023. The change in the valuation allowance between December 31, 2022 and 2021, and between December 31, 2021 and 2020, was primarily driven by additions of $ million and $ million, respectively, related to Neurimmune's tax basis in ADUHELM. For additional information on the deconsolidation and our collaboration arrangement with Neurimmune, please read Note 20, Investments in Variable Interest Entities, to these consolidated financial statements.
In addition to deferred tax assets and liabilities, we have recorded deferred charges related to intra-entity sales of inventory. As of December 31, 2023 and 2022, the total deferred charges were $ million and $ million, respectively.
Inflation Reduction Act
In August 2022 the IRA was signed into law in the U.S. The IRA introduced new tax provisions, including a 15.0% corporate alternative minimum tax and a 1.0% excise tax on stock repurchases. The provisions of the IRA are effective for periods after December 31, 2022. The IRA did not result in any material adjustments to our income tax provision or income tax balances as of December 31, 2023 and 2022. Preliminary guidance has been issued by the IRS and we expect additional guidance and regulations to be issued in future periods. We will continue to assess its potential impact on our business and results of operations as further information becomes available.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
% | | | % | | | % | | State taxes | | | | | | | | | |
| Taxes on foreign earnings | | () | | | () | | | () | |
| Tax credits | | () | | | () | | | () | |
Purchased inventory valuation step-up and intangible assets | | | | | | | | () | |
| |
| GILTI | | () | | | | | | | |
| Sale of Samsung Bioepis | | | | | () | | | | |
| Litigation settlement agreement | | | | | | | | | |
| |
| Neurimmune tax impacts | | | | | | | | () | |
| Internal reorganization | | () | | | () | | | | |
| Other, including permanent items | | | | | () | | | | |
| Effective tax rate | | | % | | | % | | | % |
Changes in Tax Rate
For the year ended December 31, 2023, compared to 2022, the decrease in our effective tax rate was driven by the impact of the non-cash changes in the value of our equity investments, the impact of Fit for Growth related expenses and Reata acquisition-related expenses, as well as the combined net unfavorable tax rate impacts in 2022 related to a litigation settlement agreement, the sale of our equity interest in Samsung Bioepis, the impact of a Neurimmune valuation allowance, as discussed below, and an international reorganization to align with global tax developments. The change also benefits from the resolution of an uncertain tax matter during the first quarter of 2023 related to tax credits.
For the year ended December 31, 2022, compared to 2021, the increase in our effective tax rate, excluding the impact of the net Neurimmune deferred tax asset, as discussed below, includes the tax impacts of the litigation settlement agreement and the sale of our building at 125 Broadway. These increases were partially offset by the impact of the current year tax benefits related to an international reorganization to align with global tax developments, the impacts of the sale of our equity interest in Samsung Bioepis and the tax impacts of the decision to discontinue development of vixotrigine. Further in 2021, our effective tax rate benefited from the tax effects of the BIIB111 and BIIB112 impairment charges and the non-cash tax effects of changes in the value of our equity instruments.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
For additional information on the litigation settlement agreement, please read Note 18, Other Consolidated Financial Statement Detail, to these consolidated financial statements.
Neurimmune Deferred Tax Asset
During 2021 we recorded a net deferred tax asset in Switzerland of approximately $ million on Neurimmune's tax basis in ADUHELM, the realization of which was dependent on future sales of ADUHELM.
During the first quarter of 2022, upon issuance of the final NCD related to ADUHELM, we recorded an increase in a valuation allowance of approximately $ million to reduce the net value of this deferred tax asset to zero.
These adjustments to our net deferred tax asset were each recorded with an equal and offsetting amount assigned to net income (loss) attributable to noncontrolling interests, net of tax in our consolidated statements of income, resulting in a zero net impact to net income attributable to Biogen Inc.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In assessing the realizability of our deferred tax assets, we have considered whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. In making this determination, under the applicable financial reporting standards, we are allowed to consider the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies. Based upon the level of historical taxable income and income tax liability and projections for future taxable income over the periods in which the deferred tax assets are utilizable, we believe it is more likely than not that we will realize the net benefits of the deferred tax assets of our wholly owned subsidiaries, net of the recorded valuation allowance. In the event that actual results differ from our estimates or we adjust our estimates in future periods, we may need to adjust or establish a valuation allowance, which could materially impact our consolidated financial position and results of operations.
Accounting for Uncertainty in Income Taxes
| | $ | | | | $ | | | | Additions based on tax positions related to the current period | | | | | | | | | |
| Additions for tax positions of prior periods | | | | | | | | | |
| Reductions for tax positions of prior periods | | () | | | () | | | () | |
| Statute expirations | | () | | | () | | | () | |
| Settlement refund (payment) | | () | | | () | | | () | |
| Ending balance | | $ | | | | $ | | | | $ | | |
During the year ended December 31, 2021, we increased our gross unrecognized tax benefits by approximately $ million, related to a deferred tax asset for Swiss tax purposes for Neurimmune's tax basis in ADUHELM. This unrecognized tax benefit was recorded as a reduction to the gross deferred tax asset, resulting in the net deferred tax asset, as discussed above, and not as a separate liability on our consolidated balance sheets. As of December 31, 2022, the unrecognized tax benefits related to Neurimmune was approximately $ million. During the year ended December 31, 2023, we decreased our gross unrecognized tax benefits by approximately $ million related to this item as a result of the deconsolidation of Neurimmune.
We file income tax returns in various U.S. states and in U.S. federal and other foreign jurisdictions. With few exceptions, we are no longer subject to U.S. federal tax examination for years before 2019 or state, local or non-U.S. income tax examinations for years before 2013.
The U.S. Internal Revenue Service and other national tax authorities routinely examine our intercompany transfer pricing with respect to intellectual property related transactions and it is possible that they may disagree with one or more positions we have taken with respect to such valuations.
Included in the balance of unrecognized tax benefits as of December 31, 2023, 2022 and 2021, are $ million, $ million and $ million (net of the federal benefit on state issues), respectively, of unrecognized tax benefits that, if recognized, would affect the effective income tax rate in future periods.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
It is reasonably possible that we will adjust the value of our uncertain tax positions related to certain transfer pricing, collaboration matters, withholding taxes and other issues as we receive additional information from various taxing authorities, including reaching settlements with such authorities.
We estimate that it is reasonably possible that our gross unrecognized tax benefits, exclusive of interest, could decrease by up to approximately $ million in the next 12 months as a result of various audit closures, settlements and expiration of the statute of limitations.
| | $ | | | | $ | | | | Income taxes | | | | | | | | | |
Other (Income) Expense, Net
| | $ | () | | | $ | | | | Litigation settlement agreement and settlement fees | | | | | | | | | |
| Interest income | | () | | | () | | | () | |
| Interest expense | | | | | | | | | |
| (Gains) losses on investments, net | | | | | | | | | |
| Foreign exchange (gains) losses, net | | | | | | | | | |
| Other, net | | | | | | | | | |
| Total other (income) expense, net | | $ | | | | $ | () | | | $ | | |
(1) Reflects the pre-tax gain, net of transaction costs, recognized from the sale of our % equity interest in Samsung Bioepis to Samsung BioLogics in April 2022. For additional information on the sale of our equity interest in Samsung Bioepis, please read Note 3, Dispositions, to these consolidated financial statements.
The (gains) losses on investments, net, as reflected in the table above, relate to debt securities, equity securities of certain biotechnology companies, venture capital funds where the underlying investments are in equity securities of certain biotechnology companies and non-marketable equity securities.
During the second quarter of 2022 we recorded a pre-tax charge of $ million, plus settlement fees and expenses, related to a litigation settlement agreement to resolve a qui tam litigation relating to conduct prior to 2015. This charge is included within other (income) expense, net in our consolidated statements of income for the year ended December 31, 2022.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | Less: Net (gains) losses realized on equity securities | | | | | | | | () | |
| Net unrealized (gains) losses recognized on equity securities | | $ | | | | $ | | | | $ | | |
The net unrealized losses recognized during the year ended December 31, 2023, primarily reflect a decrease in the aggregate fair value of our investments in Sage, Denali, Sangamo and Ionis common stock of approximately $ million.
The net unrealized losses recognized during the year ended December 31, 2022, primarily reflect a decrease in the aggregate fair value of our investments in Denali and Sangamo common stock of approximately $ million, partially offset by an increase in the fair value of Ionis and Sage common stock of approximately $ million.
Accrued Expense and Other
| | $ | | | | Employee compensation and benefits | | | | | | |
| Collaboration expense | | | | | | |
| Royalties and licensing fees | | | | | | |
|
|
|
| Reata acquisition-related accrued expense | | | | | | |
| Other | | | | | | |
| Total accrued expense and other | | $ | | | | $ | | |
Other long-term liabilities were $ million and $ million as of December 31, 2023 and 2022, respectively, and included accrued income taxes totaling $ million and $ million, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
OCREVUS
Pursuant to the terms of our collaboration arrangements with Genentech, we receive a tiered royalty on U.S. net sales from % and increasing up to % if annual net sales exceed $ million. There will be a % reduction to these royalties if a biosimilar to OCREVUS is approved in the U.S.
In addition, we receive a gross % royalty on net sales of OCREVUS outside the U.S., with the royalty period lasting years from the first commercial sale of OCREVUS on a country-by-country basis.
The commercialization of OCREVUS does not impact the percentage of the co-promotion profits we receive for RITUXAN, LUNSUMIO or GAZYVA. Genentech is solely responsible for development and commercialization of OCREVUS and funding future costs. Genentech cannot develop OCREVUS in CLL, non-Hodgkin's lymphoma or rheumatoid arthritis.
OCREVUS royalty revenue is based on our estimates from third party and market research data of OCREVUS sales occurring during the corresponding period. Differences between actual and estimated royalty revenue will be adjusted for in the period in which they become known, which is generally expected to be the following quarter.
If we undergo a change in control, as defined in our collaboration agreement, Genentech will be deemed to have purchased our rights to OCREVUS in exchange for the continued payment of the current royalties on net sales (as defined in our collaboration agreement and summarized above) in the U.S. only, until the year anniversary of the first commercial sale of OCREVUS in the U.S.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Under our collaboration with Genentech, we were responsible for % of development costs for LUNSUMIO prior to FDA approval and will be entitled to a tiered share of co-promotion operating profits and losses in the U.S., as summarized in the table below. In addition, we receive low single-digit royalties on sales of LUNSUMIO outside the U.S. In December 2022 LUNSUMIO was granted accelerated approval by the FDA for the treatment of relapsed or refractory follicular lymphoma.
If we undergo a change in control, as defined in our collaboration agreement, Genentech will be deemed to have purchased our rights to LUNSUMIO in exchange for % of the U.S. co-promotion operating profits or losses until the 11 year anniversary of the first commercial sale of LUNSUMIO in the U.S.
COLUMVI (glofitamab)
In December 2022 we entered into an agreement with Genentech related to the commercialization and sharing of economics for COLUMVI, a bispecific antibody for the treatment of B-cell non-Hodgkin's lymphoma, which was subsequently granted accelerated approval by the FDA in June 2023. Under the terms of this agreement, we will have no payment obligations. Genentech will have sole decision-making rights on the commercialization of COLUMVI within the U.S. and we will receive tiered royalties in the mid-single digit range on net sales of COLUMVI in the U.S. The commercialization of COLUMVI does not impact the percentage of the co-promotion profits we receive for RITUXAN, LUNSUMIO or GAZYVA.
If we undergo a change in control, as defined in our collaboration agreement, Genentech will be deemed to have purchased our rights to COLUMVI in exchange for a mid-single digit royalty on net sales (as defined in our collaboration agreement) in the U.S. only, until the 11 year anniversary of the first commercial sale of the product in the U.S.
Profit-sharing Formulas
RITUXAN and LUNSUMIO Profit Share
Our current pretax co-promotion profit-sharing formula for RITUXAN and LUNSUMIO in the U.S. provides for a % share on the first $ million of combined co-promotion operating profits earned each calendar year. million varies upon the following events, as summarized in the table below:
% | | After First Threshold Date until the Second Threshold Date | | % |
| After Second Threshold Date | | % |
First Threshold Date means the earlier of (i) the first day of the calendar quarter following the date U.S. gross sales of GAZYVA within any consecutive 12-month period have reached $ million or (ii) the first date in any calendar year in which U.S. gross sales of LUNSUMIO have reached $ million.
Second Threshold Date means the later of (i) the first date the gross sales in any calendar year in which U.S. gross sales of LUNSUMIO reach $ million or (ii) January 1 of the calendar year following the calendar year in which the First Threshold Date occurs.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
GAZYVA Profit Share
Our current pretax profit-sharing formula for GAZYVA provides for a % share on the first $ million of operating profits earned each calendar year. million varies upon the following events, as summarized in the table below:
% | | After Second GAZYVA Threshold Date | | % |
|
|
| | | |
ADUHELM Co-promotion Profits and Losses
Under our initial ADUHELM Collaboration Agreement, we recognized revenue on sales of ADUHELM in the U.S. to third parties as a component of product revenue, net in our consolidated statements of income. We also recorded the related cost of revenue and sales and marketing expense in our consolidated statements of income as these costs were incurred. Payments made to and received from Eisai for its % share of the co-promotion profits or losses in the U.S. were recognized in collaboration profit sharing/(loss reimbursement) in our consolidated statements of income. For the years ended December 31, 2022 and 2021, we recognized net reductions to our operating expense of approximately $ million and $ million, respectively, to reflect Eisai's % share of net collaboration losses in the U.S. for ADUHELM.
For the year ended December 31, 2021, we recognized a net reduction to our operating expense of $ million to reflect Eisai's % share of the $ million milestone payment made to Neurimmune related to the launch of ADUHELM in the U.S., which was recorded in collaboration profit sharing/(loss reimbursement) in our consolidated statements of income.
During the fourth quarter of 2021 we recorded approximately $ million of charges associated with the write-off of inventory and purchase commitments in excess of forecasted demand related to ADUHELM. During the first quarter of 2022, as a result of the final NCD, we recorded approximately $ million of charges associated with the write-off of inventory and purchase commitments in excess of forecasted demand related to ADUHELM. Additionally, for the years ended December 31, 2022 and 2021, we recorded approximately $ million and $ million, respectively, of aggregate gross idle capacity charges related to ADUHELM. These charges were recorded in cost of sales within our consolidated statements of income for the years ended December 31, 2022 and 2021.
We recognized approximately $ million and $ million related to Eisai's % share of inventory, idle capacity charges and contractual commitments in collaboration profit sharing/(loss reimbursement) within our consolidated statements of income for the years ended December 31, 2022 and 2021, respectively.
Amounts receivable from Eisai related to the agreements discussed above were approximately $ million and $ million as of December 31, 2023 and 2022, respectively. Amounts payable to Eisai related to the agreements discussed above were approximately $ million and $ million as of December 31, 2023 and 2022, respectively.
UCB
We have a collaboration agreement with UCB, effective November 2003, to jointly develop and commercialize dapirolizumab pegol, an anti-CD40L pegylated Fab, for the potential treatment of SLE and other future agreed
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | $ | | | | $ | | | | Biogen's share of the UCB collaboration development expense reflected in research and development expense in our consolidated statements of income | | | | | | | | | |
Alkermes
In November 2017 we entered into an exclusive license and collaboration agreement with Alkermes Pharma Ireland Limited, a subsidiary of Alkermes, for VUMERITY, a novel fumarate for the treatment of RMS. In October 2019 the FDA approved VUMERITY in the U.S. for the treatment of RMS. During the fourth quarter of 2021 VUMERITY was approved for the treatment of RRMS in certain international markets.
Under this agreement, we received an exclusive, worldwide license to develop and commercialize VUMERITY and we pay Alkermes royalties of % on worldwide net commercial sales of VUMERITY, which are recognized in cost of sales within our consolidated statements of income. Royalties payable on net commercial sales of VUMERITY are subject, under certain circumstances, to tiered minimum annual payment requirements for a period of following FDA approval. Royalty cost of sales related to sales of VUMERITY for the years ended December 31, 2023, 2022 and 2021, totaled approximately $ million, $ million and $ million, respectively.
Alkermes is eligible to receive royalties in the high-single digits to sub-teen double digits of annual net commercial sales upon successful development and commercialization of new product candidates, other than VUMERITY, developed under the exclusive license from Alkermes.
Alkermes currently supplies both VUMERITY and FAMPYRA to us pursuant to separate supply agreements. In October 2019 we entered into a new supply agreement and amended our license and collaboration agreement with Alkermes for VUMERITY. We have elected to initiate a technology transfer and, following a transition period, to manufacture VUMERITY or have VUMERITY manufactured by a third party we have engaged in exchange for paying an increased royalty rate to Alkermes on any portion of future worldwide net commercial sales of VUMERITY that is manufactured by us or our designee. In January 2023 we entered into a new supply agreement with Alkermes for FAMPYRA through January 2025. In December 2023 Alkermes entered into a definitive agreement to sell its development and manufacturing facility to Novo Nordisk, which is expected to close in mid-2024. Alkermes and Novo Nordisk plan to enter into subcontracting arrangements to continue work currently performed at the facility for a period of time after closing the transaction, which may continue through the end of 2025.
Acorda Therapeutics, Inc.
In June 2009 we entered into a collaboration and license agreement with Acorda to develop and commercialize products containing fampridine, such as FAMPYRA, in markets outside the U.S.
Under this agreement, we pay tiered royalties based on the level of ex-U.S. net sales and we may pay potential milestone payments based on the successful achievement of certain regulatory and commercial milestones.
In January 2024 we notified Acorda of our decision to terminate our collaboration and license agreement, effective January 1, 2025. As a result of this termination, Acorda will regain global commercialization rights to FAMPYRA.
For the years ended December 31, 2023, 2022 and 2021, total cost of sales related to royalties and commercial supply of FAMPYRA reflected in our consolidated statements of income were approximately $ million, $ million and $ million, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In August 2023 the FDA approved ZURZUVAE for adults with PPD, pending DEA scheduling, which was completed in October 2023. Upon approval, ZURZUVAE became the first and only oral, once-daily, 14-day treatment that can provide rapid improvements in depressive symptoms by day 15 for women with PPD. ZURZUVAE for PPD became commercially available in the U.S. during the fourth quarter of 2023. Additionally, the FDA issued a CRL for the NDA for zuranolone in the treatment of adults with MDD. The CRL stated that the application did not provide substantial evidence of effectiveness to support the approval of zuranolone for the treatment of MDD and that an additional study or studies would be needed. We and Sage are continuing to seek feedback from the FDA and evaluating next steps.
Under this collaboration, both companies will share equal responsibility and costs for development as well as profits and losses for commercialization in the U.S. Outside of the U.S., we are responsible for development and commercialization, excluding Japan, Taiwan and South Korea, with respect to zuranolone and may pay Sage potential tiered royalties in the high teens to low twenties. During the fourth quarter of 2023 we accrued a milestone payment due to Sage of $ million upon the first commercial sale of ZURZUVAE for PPD in the U.S., which was recorded within intangible assets, net in our consolidated balance sheets, and subsequently paid in January 2024.
For the year ended December 31, 2023, we recognized net reductions to our operating expense of approximately $ million to reflect Sage's % share of net collaboration losses in the U.S., which is recognized in collaboration profit sharing/(loss reimbursement) in our consolidated statements of income.
| | $ | | | | $ | | | | Biogen's share of the Sage collaboration development expense reflected in research and development expense in our consolidated statements of income | | | | | | | | | |
| Total sales and marketing expense incurred by the Sage collaboration | | | | | | | | | |
| Biogen's share of the Sage collaboration sales and marketing expense reflected in selling, general and administrative expense and collaboration profit sharing/(loss reimbursement) in our consolidated statements of income | | | | | | | | | |
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In April 2023 we exercised our option with Denali to license the ATV-enabled anti-amyloid beta program. In connection with this exercise, we assumed responsibility for all development and commercial activities and associated expenses related to this program. In addition, we made a one-time option exercise payment to Denali and, should certain milestones be achieved, may pay Denali additional development and commercial milestone payments and royalties based on future net sales. Our agreement with Denali was amended in August 2023, whereby certain milestone criteria were changed, while the total amount of development, regulatory and commercial milestones remains the same. In addition, we agreed to waive our option right to the second option program.
Under the LRRK2 Collaboration, both companies share responsibility and costs for global development based on specified percentages as well as profits and losses for commercialization in the U.S. and China. Outside the U.S. and China we are responsible for commercialization and may pay Denali potential tiered royalties.
| | $ | | | | $ | | | | Biogen's share of the Denali collaboration development expense reflected in research and development expense in our consolidated statements of income | | | | | | | | | |
Sangamo Therapeutics, Inc.
In February 2020 we entered into a collaboration and license agreement with Sangamo to pursue certain neurological targets leveraging Sangamo’s proprietary zinc finger protein technology delivered via adeno-associated virus to modulate the expression of key genes involved in neurological diseases.
In connection with the closing of this transaction in April 2020 we purchased $ million of Sangamo common stock, or approximately million shares at approximately $ per share, which were initially subject to transfer restrictions. These restrictions have now lapsed.
In March 2023 we terminated our collaboration and license agreement with Sangamo.
| | $ | | | | $ | | | | Biogen's share of the Sangamo collaboration development expense reflected in research and development expense in our consolidated statements of income | | | | | | | | | |
InnoCare Pharma Limited
In July 2021 we entered into a collaboration and license agreement with InnoCare Pharma Limited (InnoCare) for orelabrutinib, an oral small molecule Bruton's tyrosine kinase inhibitor for the potential treatment of MS. In connection with the closing of this transaction in August 2021 we made an upfront payment of $ million that was recorded as research and development expense within our consolidated statements of income for the year ended December 31, 2021.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Samsung Bioepis Co., Ltd.
Joint Venture Agreement
In February 2012 we entered into a joint venture agreement with Samsung BioLogics establishing an entity, Samsung Bioepis, to develop, manufacture and market biosimilar products. Samsung BioLogics contributed billion South Korean won (approximately $ million) for an % ownership interest in Samsung Bioepis and we contributed billion South Korean won (approximately $ million) for the remaining % ownership interest. In June 2018 we exercised our option under our joint venture agreement to increase our ownership percentage in Samsung Bioepis from approximately %, which reflected the effect of previous equity financings in which we did not participate, to approximately %. The share purchase transaction was completed in November 2018 and, upon closing, we paid billion South Korean won ($ million) to Samsung BioLogics.
In April 2022 we completed the sale of our % equity interest in Samsung Bioepis to Samsung BioLogics in exchange for total consideration of approximately $ billion. Under the terms of this transaction, we received approximately $ billion in cash at closing, with approximately $ billion in cash to be deferred over payments. The first deferred payment of $ million was received in April 2023 and the second deferred payment of $ million is due at the second anniversary of the closing of this transaction in April 2024.
As part of this transaction, we are also eligible to receive up to an additional $ million upon the achievement of certain commercial milestones. Our policy for contingent payments of this nature is to recognize the payments in the period that they become realizable, which is generally the same period in which the payments are earned.
Prior to this sale, we recognized our share of the results of operations related to our investment in Samsung Bioepis under the equity method of accounting one quarter in arrears when the results of the entity became available, which was reflected as equity in (income) loss of investee, net of tax in our consolidated statements of income.
Upon our November 2018 investment, the equity method of accounting required us to identify and allocate differences between the fair value of our investment and the carrying value of our interest in the underlying net assets of the investee. These basis differences were being amortized over their economic life, until the completion of the sale in April 2022, as discussed above. The total basis difference was approximately $ million and related to inventory, developed technology, IPR&D and deferred tax balances. The basis differences related to inventory were amortized, net of tax, over their estimated useful lives of years, and the basis differences related to developed technology and IPR&D for marketed products were being amortized, net of tax, over their estimated useful lives of years.
For the year ended December 31, 2022, we recognized net income on our investment of $ million, reflecting our share of Samsung Bioepis' operating profits, net of tax, totaling $ million offset by amortization of basis differences totaling $ million. Following the sale of Samsung Bioepis we no longer recognize gains or losses associated with Samsung Bioepis' results of operations and amortization related to basis differences.
For the year ended December 31, 2021, we recognized net income on our investment of $ million, reflecting our share of Samsung Bioepis' operating profits, net of tax, totaling $ million offset by amortization of basis differences totaling $ million.
Net income on our investment for the year ended December 31, 2021, reflects a $ million benefit related to the release of a valuation allowance on deferred tax assets associated with Samsung Bioepis. The valuation allowance was released in the second quarter of 2021 based on a consideration of the positive and negative evidence, including the historic earnings of Samsung Bioepis.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
During the third quarter of 2021 we accrued $ million in milestone payments related to the approval of BYOOVIZ in the U.S., the E.U. and the U.K., that were capitalized within intangible assets, net in our consolidated balance sheets. We may also pay Samsung Bioepis up to approximately $ million in additional development, regulatory and sales-based milestones.
2013 Commercial Agreement
In December 2013 we entered into an agreement with Samsung Bioepis to commercialize, over a term, 3 anti-TNF biosimilar product candidates which includes IMRALDI, an adalimumab biosimilar referencing HUMIRA, FLIXABI, an infliximab biosimilar referencing REMICADE, and BENEPALI, an etanercept biosimilar referencing ENBREL, in Europe, and in the case of BENEPALI, Japan. We have an option to extend this agreement by an additional , subject to payment of an option exercise fee of $ million by August 2024. We also have an option to acquire exclusive rights to commercialize these products in China.
We reflect revenue on sales of BENEPALI, IMRALDI and FLIXABI to third parties in product revenue, net in our consolidated statements of income and record the related cost of revenue and sales and marketing expense in our consolidated statements of income to their respective line items when these costs are incurred. Royalty payments to AbbVie on sales of IMRALDI are recognized in cost of sales within our consolidated statements of income.
We share % of the profit or loss related to our commercial agreement with Samsung Bioepis, which is recognized in collaboration profit sharing/(loss reimbursement) in our consolidated statements of income. For the years ended December 31, 2023, 2022 and 2021, we recognized net profit-sharing expense of $ million, $ million and $ million, respectively, to reflect Samsung Bioepis' % sharing of the net collaboration profits.
Other Services
Simultaneous with the formation of Samsung Bioepis, we also entered into a license agreement with Samsung Bioepis.
Under this license agreement, we granted Samsung Bioepis an exclusive license to use, develop, manufacture and commercialize biosimilar products created by Samsung Bioepis using Biogen product-specific technology. In exchange, we receive single digit royalties on biosimilar products developed and commercialized by Samsung Bioepis. Royalty revenue under the license agreement is recognized as a component of contract manufacturing, royalty and other revenue in our consolidated statements of income.
For the years ended December 31, 2023, 2022 and 2021, we recognized $ million, $ million and $ million, respectively, as a component of contract manufacturing, royalty and other revenue in our consolidated statements of income related to the license agreement and other services performed under our collaboration with Samsung Bioepis.
Amounts receivable from Samsung Bioepis related to the agreements discussed above were $ million and $ million as of December 31, 2023 and 2022, respectively. Amounts payable to Samsung Bioepis related to the agreements discussed above were $ million and $ million as of December 31, 2023 and 2022, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In November 2023 we notified Neurimmune of our decision to terminate the Neurimmune Agreement. Subsequent to the termination, we reconsidered our relationship with Neurimmune and determined that we were no longer the primary beneficiary of the variable interest entity. As a result, we recorded a net gain on the deconsolidation of Neurimmune of approximately $ million, which was recorded in other (income) expense, net within our consolidated statements of income for the year ended December 31, 2023.
In June 2021 ADUHELM was granted accelerated approval by the FDA. Under the terms of the Neurimmune Agreement, we were required to pay Neurimmune a milestone payment of $ million related to the launch of ADUHELM in the U.S. During the second quarter of 2021 we made this $ million payment, which was recognized as a charge to net income (loss) attributable to noncontrolling interests, net of tax in our consolidated statements of income. In addition, during the second quarter of 2021 we recognized net profit-sharing income of $ million to reflect Eisai's % share of the $ million milestone payment, which was recognized in collaboration profit sharing/(loss reimbursement) in our consolidated statements of income.
During 2021 we recorded a net deferred tax asset in Switzerland of approximately $ million on Neurimmune's tax basis in ADUHELM, the realization of which was dependent on future sales of ADUHELM. During the first quarter of 2022, upon issuance of the final NCD related to ADUHELM, we recorded an increase in a valuation allowance of approximately $ million to reduce the net value of this deferred tax asset to zero. These adjustments to our net deferred tax asset were each recorded with an equal and offsetting amount assigned to net income (loss) attributable to noncontrolling interests, net of tax in our consolidated statements of income, resulting in a zero net impact to net income attributable to Biogen Inc.
Excluding the impact of the Neurimmune deferred tax asset, the assets and liabilities of Neurimmune are not significant to our consolidated financial position or results of operations as it is a research and development organization. We have provided no financing to Neurimmune other than contractually required amounts.
Research and development costs for which we reimbursed Neurimmune are reflected in research and development expense in our consolidated statements of income. For the years ended December 31, 2023, 2022 and 2021, amounts reimbursed were immaterial.
For additional information on our collaboration arrangements with Eisai, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
Unconsolidated Variable Interest Entities
We have relationships with various variable interest entities that we do not consolidate as we lack the power to direct the activities that significantly impact the economic success of these entities. These relationships include investments in certain biotechnology companies and research collaboration agreements.
As of December 31, 2023 and 2022, the carrying value of our investments in certain biotechnology companies representing potential unconsolidated variable interest entities totaled $ million and $ million, respectively. Our maximum exposure to loss related to these variable interest entities is limited to the carrying value of our investments.
We have also entered into research collaboration agreements with certain variable interest entities where we are required to fund certain development activities. These development activities are included in research and
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
ERISA Class Action Litigation
In 2020 participants in the Biogen 401(k) Savings Plan filed actions against us in the U.S. District Court for the District of Massachusetts alleging breach of fiduciary duty under ERISA and seeking a declaration of the actions as class actions and monetary and other relief. In January 2024 the Court granted final approval of a settlement under which we agreed to pay $ million without any admission of liability and dismissed the actions with prejudice.
Humana Patient Assistance Litigation
In March 2023 the U.S. District Court for the District of Massachusetts dismissed the previously disclosed action filed against us by Humana in September 2020. Humana had alleged damages related to our providing MS patients with free medications and making charitable contributions to non-profit organizations that assist MS patients and had alleged violations of the federal RICO Act and state laws. In December 2023 Humana appealed to the United States Court of Appeals for the First Circuit and the appeal is pending.
Genentech Litigation
In February 2023 Genentech, Inc. filed suit against us in the U.S. District Court for the Northern District of California, alleging that it is owed royalties on sales of TYSABRI that occurred after the expiration of a patent licensed by Genentech to Biogen, together with interest and costs. The Company estimates that the royalties claimed total approximately $ million.
Bardoxolone Securities Litigation
In 2021 and 2022 putative stockholders of Reata (later acquired by Biogen) filed litigation in the United States District Court for the Eastern District of Texas alleging violations of the federal securities laws by Reata, certain former officers and directors and certain underwriters under 15 U.S.C. §78j(b) and §78t(a), 17 C.F.R. §240.10b-5, and 15 U.S.C. §§77k, 77l(a)(2) and 77o in connection with Reata's asset bardoxolone. Plaintiffs seek declaration of the actions as a class actions and monetary relief. In October 2023 the parties reached a settlement providing for payment by Reata of $ million without any admission of liability. The Court preliminarily approved the settlement in November 2023 and has set a final fairness hearing for March 2024. We expect a portion of the payment to be reimbursed under Reata's insurance coverage.
Lender Dispute
One of Reata's lenders claims that approximately $ million is owing under a loan agreement with Reata.
Other Matters
Government Investigations
The Company has received subpoenas from the SEC seeking information relating to ADUHELM and its launch. The Company has received a subpoena from the DOJ seeking information relating to our business operations in several foreign countries. The Company is also providing information relating to our business operations in several foreign countries to the SEC.
TYSABRI Biosimilar Patent Matter
In September 2022 we filed an action in the U.S. District Court for the District of Delaware against Sandoz Inc., other Sandoz entities and Polpharma Biologics S.A. under the Biologics Price Competition and Innovation Act, 42 U.S.C. §262, seeking a declaratory judgment of patent infringement.
Annulment Proceedings in the General Court of the European Union relating to TECFIDERA
In November 2020 Mylan Ireland filed an action in the General Court of the European Union to annul the EMA's decision not to validate its applications to market generic versions of TECFIDERA on the grounds that TECFIDERA benefits from regulatory data protection.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
SPINRAZA
We make royalty payments to Ionis on annual worldwide net sales of SPINRAZA using a tiered royalty rate between % and %, which are recognized as cost of sales in our consolidated statements of income. For additional information on our collaboration arrangements with Ionis, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
QALSODY
We make royalty payments to Ionis on annual worldwide net sales of QALSODY using a tiered royalty rate between % and %, which are recognized as cost of sales in our consolidated statements of income.
For additional information on our collaboration arrangements with Ionis, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
VUMERITY
We make royalty payments to Alkermes on worldwide net sales of VUMERITY using a royalty rate of %, which are recognized as cost of sales in our consolidated statements of income. Royalties payable on net sales of VUMERITY are subject, under certain circumstances, to tiered minimum annual payment requirements for a period of following FDA approval.
In October 2019 we entered into a new supply agreement and amended our license and collaboration agreement with Alkermes for VUMERITY. We have elected to initiate a technology transfer and, following a transition period, to manufacture VUMERITY or have VUMERITY manufactured by a third party we have engaged in exchange for paying an increased royalty rate to Alkermes on any portion of future worldwide net commercial sales of VUMERITY that is manufactured by us or our designee. For additional information on our collaboration arrangement with Alkermes, please read Note 19, Collaborative and Other Relationships, to these consolidated financial statements.
SKYCLARYS
In connection with our acquisition of Reata in September 2023 we assumed additional contractual obligations related to royalty payments. Reata entered into agreements to pay royalties on future sales of SKYCLARYS, which will cumulatively range in the low- to mid-single digits.
For additional information on our acquisition of Reata, please read Note 2, Acquisitions, to these consolidated financial statements.
Regulatory and Commercial Milestone Payments
Based on our development plans as of December 31, 2023, we could trigger potential future milestone payments to third parties of up to approximately $ billion, including approximately $ billion in development milestones, approximately $ billion in regulatory milestones and approximately $ billion in commercial milestones, as part of our various collaborations, including licensing and development programs. Payments under these agreements generally become due and payable upon achievement of certain development, regulatory or commercial milestones. Because the achievement of these milestones was not considered probable as of December 31, 2023, such contingencies have not been recorded in our financial statements. Amounts related to contingent milestone payments are not considered contractual obligations as they are contingent on the successful achievement of certain development, regulatory or commercial milestones.
During the fourth quarter of 2023 we accrued a milestone payment due to Sage of $ million upon the first commercial sale of ZURZUVAE for PPD in the U.S., which was recorded within intangible assets, net in our
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
In June 2021 ADUHELM was granted accelerated approval by the FDA. Under the terms of the Neurimmune Agreement, we were required to pay Neurimmune a milestone payment of $ million related to the launch of ADUHELM in the U.S. During the second quarter of 2021 we made this $ million payment, which was recognized as a charge to net income (loss) attributable to noncontrolling interests, net of tax in our consolidated statements of income.
Other Funding Commitments
As of December 31, 2023, we have several ongoing clinical studies in various clinical trial stages. Our most significant clinical trial expenditures are to CROs. The contracts with CROs are generally cancellable, with notice, at our option. We recorded accrued expense of approximately $ million in our consolidated balance sheets for expenditures incurred by CROs as of December 31, 2023. We have approximately $ million in cancellable future commitments based on existing CRO contracts as of December 31, 2023.
Tax Related Obligations
We exclude liabilities pertaining to uncertain tax positions from our summary of contractual obligations as we cannot make a reliable estimate of the period of cash settlement with the respective taxing authorities. As of December 31, 2023, we have approximately $ million of liabilities associated with uncertain tax positions.
As of December 31, 2023 and 2022, we have accrued income tax liabilities of approximately $ million and $ million, respectively, under the Transition Toll Tax. Of the amounts accrued as of December 31, 2023, approximately $ million is expected to be paid within one year. The Transition Toll Tax is being paid in installments over an eight--year period, which started in 2018, and will not accrue interest. For additional information on the Transition Toll Tax, please read Note 17, Income Taxes, to these consolidated financial statements.
. Participants may make voluntary contributions. We make matching contributions according to the 401(k) Savings Plan’s matching formula. All matching contributions and participant contributions vest immediately. The 401(k) Savings Plan also holds certain transition contributions on behalf of participants who previously participated in the Biogen, Inc. Retirement Plan. The expense related to our 401(k) Savings Plan primarily consists of our matching contributions.Expense related to our 401(k) Savings Plan totaled approximately $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Pension Plans
Our retiree benefit plans include defined benefit plans for employees in our affiliates in Switzerland and Germany as well as other insignificant defined benefit plans in certain other countries where we maintain an operating presence.
Our Swiss plan is a government-mandated retirement fund that provides employees with a minimum investment return. The minimum investment return is determined annually by the Swiss government and was % in 2023, % in 2022 and % in 2021. Under the Swiss plan, both we and certain of our employees with annual earnings in excess of government determined amounts are required to make contributions into a fund managed by an independent investment fiduciary. Employer contributions must be in an amount at least equal to the employee’s contribution. Minimum employee contributions are based on the respective employee’s age, salary and gender. As of December 31, 2023 and 2022, the Swiss plan had an unfunded net pension obligation of $ million and $ million, respectively, and plan assets that totaled $ million and $ million, respectively. In 2023, 2022 and 2021 we recognized net expense totaling $ million, $ million and $ million, respectively, related to our Swiss plan, of which $ million, $ million and $ million, respectively, was included in other (income) expense, net in our consolidated statements of income.
The obligations under the German plans are unfunded and totaled $ million and $ million as of December 31, 2023 and 2022, respectively. Net periodic pension cost related to the German plans totaled $ million, $ million and $ million for the years ended December 31, 2023, 2022 and 2021, respectively, of which $ million, $ million and $ million, respectively, was included in other (income) expense, net in our consolidated statements of income.
BIOGEN INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Enterprise-wide disclosures about product revenue, other revenue and long-lived assets by geographic area are presented below. Revenue is primarily attributed to individual countries based on location of the customer or licensee.
Geographic Information
| | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | Revenue from anti-CD20 therapeutic programs | | | | | | | | | | | | | | | | | | |
| Contract manufacturing, royalty and other revenue | | | | | | | | | | | | | | | | | | |
| Long-lived assets | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | December 31, 2022 |
| (In millions) | | U.S. | | Europe(1) | | Germany | | Asia | | Other | | Total |
| Product revenue from external customers | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
| Revenue from anti-CD20 therapeutic programs | | | | | | | | | | | | | | | | | | |
| Contract manufacturing, royalty and other revenue | | | | | | | | | | | | | | | | | | |
| Long-lived assets | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | December 31, 2021 |
| (In millions) | | U.S. | | Europe(1) | | Germany | | Asia | | Other | | Total |
| Product revenue from external customers | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | | | $ | | |
| Revenue from anti-CD20 therapeutic programs | | | | | | | | | | | | | | | | | | |
| Contract manufacturing, royalty and other revenue | | | | | | | | | | | | | | | | | | |
| Long-lived assets | | | | | | | | | | | | | | | | | | |
(1) Represents amounts related to Europe less those attributable to Germany.
Long-Lived Assets
As of December 31, 2023, 2022 and 2021, approximately $ million, $ million and $ million, respectively, of our long-lived assets were related to the construction of our large-scale biologics manufacturing facility in Solothurn, Switzerland.
For additional information on our Solothurn manufacturing facility, please read Note 11, Property, Plant and Equipment, to these consolidated financial statements.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Biogen Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Biogen Inc. and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of income, of comprehensive income, of equity and of cash flow for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Annual Report on Internal Control over Financial Reporting, management has excluded Reata Pharmaceuticals, Inc. (Reata) from its assessment of internal control over financial reporting as of December 31, 2023, because it was acquired by the Company in a purchase business combination during 2023. We have also excluded Reata from our audit of internal control over financial reporting. Reata is a wholly-owned subsidiary whose total assets and total revenue excluded from management’s assessment and our audit of internal control over financial reporting represent 1.0% and 0.6%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2023.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in
accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Reserves for Medicaid and Managed Care Rebates in the U.S.
As described in Notes 1 and 5 to the consolidated financial statements, the Company recognized revenue from product sales, net of reserves, including contractual adjustments related to Medicaid and managed care rebates in the U.S. Within accrued expense and other, revenue-related reserves amounted to $926.5 million as of December 31, 2023. A portion of this balance includes contractual adjustments for Medicaid and managed care rebates in the U.S. Medicaid rebates relate to the Company’s estimated obligations to states under established reimbursement arrangements. The Company’s liability for Medicaid rebates consists of estimates for claims that a state will make for the current quarter, claims for prior quarters that have been estimated for which an invoice has not been received, invoices received for claims from the prior quarters that have not been paid and an estimate of potential claims that will be made for inventory that exists in the distribution channel at period end. Managed care rebates in the U.S. represent the Company’s estimated obligations to third-parties, primarily pharmacy benefit managers. These rebates result from performance-based goals, formulary position and price increase limit allowances (price protection). The calculation of the accrual for these rebates is based on an estimate of the coverage patterns and the resulting applicable contractual rebate rate(s) to be earned over a contractual period. Rebate accruals for Medicaid and managed care in the U.S. are recorded in the same period the related revenue is recognized, resulting in a reduction of product revenue and the establishment of a liability which is included in accrued expense and other current liabilities. The estimates of the reserves for Medicaid and managed care in the U.S. reflect historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns.
The principal considerations for our determination that performing procedures relating to reserves for Medicaid and managed care rebates in the U.S. is a critical audit matter are (i) the significant judgment by management due to the significant measurement uncertainty when developing the estimate of the reserves and (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating assumptions related to historical experience, current contractual requirements, specific known market events, and forecasted customer buying and payment patterns.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management's estimate of the reserves for Medicaid and managed care rebates in the U.S. These procedures also included, among others (i) developing an independent estimate of the reserves for Medicaid and managed care rebates in the U.S. by utilizing third-party data related to product demand, data related to price changes, the terms of the specific rebate programs, the historical trend of actual rebate claims paid and consideration of contractual requirement changes and market events; (ii) comparing the independent estimate to management’s estimate to evaluate the reasonableness of management's estimate; and (iii) testing, on a sample basis, rebate claims paid by the Company, including evaluating the claims for consistency with the contractual terms of the Company’s rebate agreements.
Acquisition of Reata - Valuation of Completed Technology and In-Process Research and Development Intangible Assets
As described in Notes 1 and 2 to the consolidated financial statements, the Company completed the acquisition of Reata for total consideration of $7,193.4 million. Of the acquired intangible assets, $4,200.0 million of completed technology and $2,300.0 million of in-process research and development (IPR&D) were recorded. Management uses the multi-period excess earnings method, which is a form of the income approach, utilizing post-tax cash flows and discount rates in estimating the fair value of identifiable intangible assets acquired when allocating the purchase consideration paid for the acquisition. The estimates of the fair value of identifiable intangible assets involve significant judgment by management and include assumptions with measurement uncertainty, such as the amount and timing of projected cash flows, long-term sales forecasts, discount rate, and additionally for the IPR&D intangible asset, the timing and probability of regulatory and commercial success.
The principal considerations for our determination that performing procedures relating to the valuation of completed technology and IPR&D intangible assets acquired in the acquisition of Reata is a critical audit matter are (i) the significant judgment by management due to the significant measurement uncertainty when developing the fair value estimate of the completed technology and IPR&D intangible assets acquired; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to long-term sales forecasts and discount rate for the completed technology intangible asset and long-term sales forecasts, discount rate, and probability of regulatory and commercial success for the IPR&D intangible asset; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the acquisition accounting, including controls over management’s valuation of the completed technology and IPR&D intangible assets acquired. These procedures also included, among others (i) reading the purchase agreement; (ii) testing management’s process for developing the fair value estimate of the completed technology and IPR&D intangible assets acquired; (iii) evaluating the appropriateness of the multi-period excess earnings method used by management; (iv) testing the completeness and accuracy of certain of the data used in the multi-period excess earnings method; and (v) evaluating the reasonableness of the significant assumptions used by management related to long-term sales forecasts and discount rate for the completed technology intangible asset and long-term sales forecasts, discount rate, and probability of regulatory and commercial success for the IPR&D intangible asset. Evaluating management’s assumptions related to long-term sales forecasts for the completed technology and IPR&D intangible assets and probability of regulatory and commercial success for the IPR&D intangible asset involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the acquired business and (ii) whether the assumptions were consistent with evidence obtained in other areas of the audit. Evaluating management’s assumption related to long-term sales forecasts also involved considering the consistency with external market and industry data. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the multi-period excess earnings method and (ii) the reasonableness of the discount rate significant assumption for the completed technology and IPR&D intangible assets.
/s/
February 13, 2024
We have served as the Company's auditor since 2003.

Similar companies
See also AMGEN INC -
Annual report 2022 (10-K 2022-12-31)
Annual report 2023 (10-Q 2023-09-30)
See also GILEAD SCIENCES, INC. -
Annual report 2022 (10-K 2022-12-31)
Annual report 2023 (10-Q 2023-09-30)
See also Moderna, Inc. -
Annual report 2022 (10-K 2022-12-31)
Annual report 2023 (10-Q 2023-09-30)
See also BioNTech SE
See also Seagen Inc. -
Annual report 2022 (10-K 2022-12-31)
Annual report 2023 (10-Q 2023-09-30)
 Open main menu
Open main menu
 Open main menu
Open main menu