BioNexus Gene Lab Corp - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2021
or
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________ to ________
Commission File Number: 333-229399
BIONEXUS GENE LAB CORP |
(Exact name of registrant as specified in its charter) |
Wyoming |
| 35-2604830 |
(State or Other Jurisdiction of Incorporation or Organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
Unit 02, Level 10, Tower B, Avenue 3,The Vertical Business Suite II,Bangsar South No. 8 Jalan Kerinchi Kuala Lumpur, Malaysia |
| 59200 |
(Address of Principal Executive Offices) |
| (Zip Code) |
+60 1221-26512
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated Filer | ☐ | Smaller reporting company | ☒ |
|
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. At June 30, 2021, the aggregate market value of shares held by non-affiliates of the registrant was $41,737,068.
As of March 30, 2022 there were 171,218,152 shares of common stock, no par value, outstanding.
| 2 |
| Table of Contents |
FORWARD-LOOKING STATEMENTS
Certain statements made in this Annual Report on Form 10-K are “forward-looking statements” (within the meaning of the Private Securities Litigation Reform Act of 1995) regarding the plans and objectives of management for future operations. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements of the Registrant to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations that involve numerous risks and uncertainties. The Company’s plans and objectives are based, in part, on assumptions involving the continued expansion of business. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the Company. Although the Company believes its assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and, therefore, there can be no assurance the forward-looking statements included in this Report will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by the Company or any other person that the objectives and plans of the Company will be achieved.
Unless stated otherwise, the words “we,” “us,” “our,” “the Company” or “BioNexus” in this Annual Report collectively refers to BioNexus Gene Lab Corp., a Wyoming corporation and our wholly owned subsidiaries, BioNexus Gene Lab Sdn. Bhd. (“BGL” or BioNexus Malaysia”) and Chemrex Corporation Sdn. Bhd. (“CCSB” or “Chemrex”), both Malaysian companies (“Subsidiaries”).
We acquired Chemrex on December 31, 2020 pursuant to a Share Exchange Agreement (“Agreement”) with Chemrex its shareholders. We acquired all of the issued and outstanding shares of capital stock of Chemrex from the Chemrex shareholders in exchange for 68,487,261 shares of common stock of the Company issued to the Chemrex shareholders.
Our principal corporate office is Unit 02, Level 10, Tower B Vertical Business Suite, Bangsar South, No. 8 Jalan Kerinchi, Kuala Lumpur, Malaysia, the BioNexus Malaysia lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia and it also has a blood collection center located at 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. Chemrex offices and supply hub is located at 4 Jalan CJ 1/6 Kawasan Perusahaan Cheras Jaya, Selangor, Malaysia.
Our corporate and BioNexus Malaysia telephone number is (+60) 1221-26512 and its website is www.bionexusgenelab.com. Chemrex’s telephone number is (+60) 1922-23815 and its website is www.chemrex.com.my.
| 3 |
| Table of Contents |
Item 1. Business.
BUSINESS DESCRIPTION
Company Overview
BioNexus Gene Lab Corp., a Wyoming corporation, was incorporated on May 12, 2017.
On August 23, 2017, we acquired all of the outstanding capital stock of Bionexus Gene Lab Sdn. Bhd., a Malaysian corporation (“BGL” or “BioNexus Malaysia”). BioNexus Malaysia was incorporated in Malaysia on April 7, 2015.
On December 31, 2020, we acquired all of the outstanding capital stock of Chemrex Corporation Sdn. Bhd. (“CCSB” or “Chemrex”). Chemrex was incorporated in Malaysia on September 29, 2004.
Our corporate structure is depicted below:
The corporate structure as at December 31, 2021 is depicted below:
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
|
| ||
100% owned |
|
| 100% owned | ||
Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| Chemrex Corporation Sdn. Bhd., a Malaysian Company | ||
BioNexus Malaysia
BioNexus Malaysia is an emerging molecular diagnostics company focused on the application of functional genomics to enable early diagnosis and personalized health management. Our focus is on developing and marketing safe, effective and non-invasive blood tests for early detection of diseases in order to minimize treatment cost and improve patient management. Our non-invasive blood tests analyze changes in ribonucleic acid (or RNA) to detect the risk potentiality of 11 different diseases. These diseases include eight cancers (nasopharyngeal, lung, liver, stomach, breast, cervical, prostate and colon), two bowel diseases (colitis and Crohn) and osteoarthritis. This unique blood based genomic biomarker approach is based on the scientific observation that circulating blood reflects, in a detectable way, what is occurring throughout the body currently.
The Company believes that its blood based genomic screening protocol for the risk of disease detection can be utilized in conjunction with other medical procedures for disease detection including blood tests, imaging and biopsies. We market our blood based genomic screening process to health care providers, such as doctors, laboratories and hospitals, which began in July 2017.
Our principal office address is Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, No. 8 Jalan Kerinchi, Kuala Lumpur, Malaysia, our lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia, and we have a blood collection center located at 1st Floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. Our telephone number is (+60) 1221-26512 and web-site is www.bionexusgenelab.com
| 4 |
| Table of Contents |
Acquisition of BGS Lab Sdn. Bhd.
On August 23, 2017, BioNexus Gene Lab Corp., a Wyoming company, acquired all of the capital stock of Bionexus Gene Lab Sdn. Bhd., a Malaysian company (“BioNexus Malaysia”), from its then existing shareholders. In connection with the transaction, the following shareholders of BioNexus Malaysia received the corresponding number of shares of our common stock; Soo Kow Lai, our Chairman, received 10,000,000 shares; Chi Yuen Leong, our President received 10,000,000 shares; Mr. Chan Chong Wong, our Chief Executive Officer received 10,000,000 shares; and late Dr. Choong Chin Liew, our Scientific Adviser received 20,000,000 shares. In exchange, we received certain software, equipment, know-how, related inventory and technology relating to blood based genomic analysis and screening developed by Dr. Liew which had enabled us to conduct our current operations. The technology and related assets were previously acquired by the BioNexus Malaysia from Dr. Liew in June 2017 in exchange for Dr. Liew receiving 40% of the equity of BioNexus Malaysia and the obligation to pay Dr. Liew the sum of approximately $354,930. The Company paid Dr. Liew the sum of $83,664 on January 23, 2018 and on February 15, 2018, Dr. Liew agreed to waive the remaining balance due to him by the Company which amounted to $271,266.
Development of the Blood Analysis Process.
Our company’s major shareholder, Dr. Liew, developed and tested a novel approach in blood based genomic analysis and screening by identifying biomarkers in RNA as opposed to deoxyribonucleic acid (or DNA) analysis. Through his extensive research and clinical trials, he had determined that communication occurs between cells in blood and tissue as blood circulates throughout the body and subtle changes occur in cells over time due to injury or disease. These cell-cell interactions induce changes in blood gene expression. Profiling these changes enables the Company to identify unique molecular signatures reflecting disease activity which can then be used to develop disease-specific molecular diagnostic assays. The Company uses disease-specific blood-based biomarkers as the basis for molecular diagnostics tests and to enable personalized health management through the development of systems biology tools and algorithms.
The Company focuses on developing and commercializing proprietary molecular diagnostic tests for early detection of diseases and personalized health management, with a primary focus on cardiovascular, diabetes and cancer-related indications. There is a constant and dynamic interaction of blood with cells, tissues and organs of the human body. Many clinical studies performed by Dr. Liew and others have demonstrated that blood gene expression profiles can be used to develop personalized signatures capable of differentiating patients with cancer from healthy patients across a broad spectrum of pathologies interaction between tumor cells and the immune system that has been referred to as immunoediting. Immunoediting is the response of the immune system to a tumor and comprises three stages: elimination (in which the immune system identifies cancerous and/or precancerous cells and attempts to eradicate them), equilibrium (in which the surviving tumor cells begin mutating rapidly), and escape (in which tumor cells proliferate uncontrollably, leading to tumor progression). Each of these stages induces leukocyte gene expression changes that constitute a unique, detectable molecular signature.
Dr. Liew began his research in 1962 and has published numerous articles in medical and peer review journals. His publications include the following;
Peripheral Blood Transcriptome Dynamically Reflects System-wide Biology: A Potential Diagnostic Tool. Liew CC, Ma J, Tang HC, Zheng R, Dempsey AA. Journal of Laboratory and Clinical Medicine (March 2006).
The Peripheral Blood Transcriptome: New Insights into Disease and Risk Assessment. Mohr, S Liew CC. Trends in Molecular Medicine (October 2007).
| 5 |
| Table of Contents |
DNA and RNA each consist of a single molecule and both are present in the blood. DNA is the carrier of human genetic information and is passed down from generation to generation. At conception, a person receives DNA from both parents. All of the cells in our bodies, except red blood cells, contain a copy of our DNA. Humans share about 99% of the same genetic code. However, it is the 1% of the genetic code that makes us all very distinct individuals. Historically, the study of DNA has been used to detect ancestry and inherited characteristics, including certain inherited diseases like Huntington Disease, Cystic Fibrosis and Down Syndrome, among others. It also is believed there is a genetic (DNA) pre-disposition to certain cancers, like breast cancer, colon cancer and gastric cancer.
While DNA is relatively static, RNA conversely is subject to change and is affected by an individual’s lifestyle, such as diet, alcohol, tobacco and/or drug use, excessive or ongoing stress from work/family and exercise, along with exposure to environmental influences, such as pollutants and chemicals.
The distinctions between the characteristics of RNA and DNA are illustrated in the below table.

· Static | · Dynamic |
|
|
· Measures lifetime risk | · Measure current risk |
|
|
· Repeated test does not provide different result | · Repeated test provides different result |
|
|
· DNA does not change with external factors | · Lifestyle and external factors affect RNA expression |
The Collection and Analysis Process.
Our process involves a simple blood draw. A nurse or technician of the health care provider draws 2.5 ml of blood from the patient using a Paxgene blood tube. The blood and a completed company form are couriered to our lab located at 353, Chemical Science Centre, USM, George Town. All blood samples are labelled with name and personal identity number and laboratory reference number on the tube where the blood sample is maintained for safekeeping.
| 6 |
| Table of Contents |
At our lab, RNA is extracted in biosafety cabinet followed by microcentrifuge and spectrophotometer to check spectrophotometric concentration and quality of the extracted RNA. The RNA then is purified (Biotinylated RNA will be mixed with purification beads and transferred to a U-bottom 96-well plate. Then, the plate will be placed onto a magnetic ring stand. Labeled cRNA will be captured when placed on the magnetic stand. The remaining solution will be removed and the captured pellet will be cleaned-up to obtain cRNA with high purity. Then, purified cRNA will be fragmented for hybridization) and hybridized onto a genechip (GeneChip 3’ IVT PLUS Reagent Kit will be used for preparing biotinylated target from purified total RNA samples suitable for hybridization to GeneChip arrays. Double-stranded cDNA will be synthesized from the total RNA using reverse transcriptase and oligo-dT primers. An in-vitro transcription (IVT) reaction is then done to produce biotin-labeled cRNA from the cDNA in 16 hours incubation) and scanned through the affymetrix station (Once the overnight hybridization is completed, the Genechips will be washed with dedicated buffers and solutions to remove excess cRNAs and hybridization solutions. Washed chips will be stained with staining buffers to illuminate attached cRNAs. All these processes will be conducted in the fluidic station by following the given instructions. Specific experimental information is defined using AMDS software on a PC-compatible workstation. Stained chips are ready for scanning. The chips will be transferred into the scanner. The scan is automatically completed and the image is processed into data files. The data collected from microarray analysis is analysed using our propriety software and algorithm calculation to generate the disease risk score report for the individual patient. A report is generated by our software and is forwarded to the health care provider for further consultation with the patient. This report can be used by patient and physician to plan future tests and therapies.
The process for effectuating RNA analysis depicted in the below pictures.
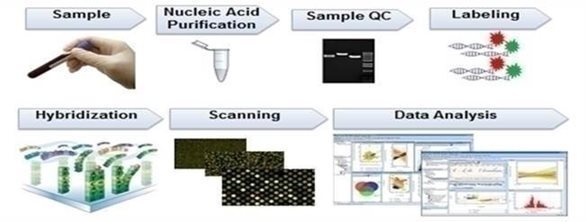
| 7 |
| Table of Contents |
The raw data obtained will be analyzed and quality control processed by our lab in Malaysia using our proprietary software to calculate the risk analysis of 11 different diseases. We simplify the result into a graph which is contained in the patient booklet provided to the health care professional. A sample graph is depicted below.
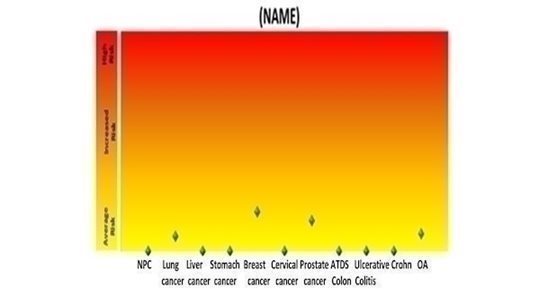
In the above chart, NPC is Nasopharyngeal Cancer, ATDS is Ascending, Transverse, Descending and Sigmoid Colon Cancer, and OA is Osteoarthritis. |
The following cautionary text in contained in the results booklet which the Company provides to each patient:
This report/screening is not intended or implied to be substitute for professional medical advice, diagnostics or treatment. The content, including text, graphics and information in the report illustrate the risk score only. Bionexus Gene Lab Sdn Bhd makes no representation and assumes no responsibility for the accuracy of the information as such information and contents are subject to change without notice. You are encouraged to review any medical condition or treatment with your doctor.
The key proprietary aspect of our process is our proprietary algorithm software and the RNA extraction, preservation, quality control, hybridization, data analysis processes which was developed by Dr. Liew. We acquired the software and the technological processes in June 2017. The gene expression from a reference population representing a specific disease condition is filtered according to a quality assurance process based on repeatability data. This collected data is then analyzed by our proprietary algorithm software and processes checked by the laboratory manager to ensure all the steps are followed in the deriving predictive model for each disease condition. Once these models have been established, they then can be applied to the data from a new sample to make risk prediction for this individual. Each disease/disorder has similar group of diseased/disordered genes which were identified through the years of research and clinical trials in Malaysia.
Business Development.
As mentioned above, the screening provides a risk analysis of 11 diseases, of which eight are different forms of cancer. In Malaysia, the cost for the analysis is not covered by health insurance. Thus, patients are required to pay for the costs of the services, which under our current pricing of $975 for the 11-disease panel for an individual plan. We do have different pricing for groups, like companies and associations.
| 8 |
| Table of Contents |
As of December 31, 2021, we have 10 swab and blood sample collection centers, 8 in Klang Valley (which is comprised of our capital city Kuala Lumpur, together with towns on the northern and southern fringes of the capital city) recommending our blood based genomic screening protocol to their patients. Of these centers, Lifecare Medical Centre, Iheal Medical Centre, National Heart Centre, 1-Utama Clinic, Clinic Q, LYC Healthcare, Gene Therapy Centre, Nexus Reality Lab in Klang Valley, Sandakan hospital and Tawau Hospital and account for approximately 90% of our patient population from Jan 2020 through December 31, 2021. We believe that there are over 500 clinics and hospitals located in the Kuala Valley metropolitan area.
Our goal is to have a large percentage of health care providers in the Klang Valley refer patients to us for our screening protocol. Once we have established our brand and reputation in Klang Valley, we intend to expend to other large cities in Malaysia, followed by an expansion to large metropolitan areas other countries in Asia Pacific, such as Indonesia, Taiwan and Singapore. However, we do not foresee expansion beyond Malaysia until fiscal year 2022 and beyond.
Our existing equipment and personnel are sufficient to handle up to 94 patients a day. If our daily patient count increases above 200 patients, we will be required to hire another laboratory technician and purchase and install an additional semi-automatic Affymetrix station equipment estimate to be $200,000.
For health care providers, we pay a referral fee of between 20% to 25%. Typically, the patient, while in the offices of the health care provider, completes a form which identifies the name, address and other contact information of the patient.
During the 3rd fiscal quarter of 2020, we began testing for COVID 19 using our RNA platform apply qPCR process. During this period, the Ministry of Health began providing us with COVID samples taken at hospitals for testing using our laboratory. To date, we had screened more than 5,000 samples reported less than 100 positive covid-19 cases to the Health Ministry In addition, during 3rd quarter of 2020, we began promoting this q-PCR test through the Malaysian General & Life Insurance Association, various banks, airports and small and medium enterprises (SME) in Malaysia. The response from these markets have slow in developing. Our Covid screening generates substantially lower per ticket charges at $55 per test compared with $975 per customer for our RNA screening. They also generate lower margins than our RNA screenings.
Competition and Competitive Strengths.
While the Company believes that there is no similar commercialized blood based genomic screening for 11 diseases using RNA analysis, it believes that its blood based genomic screening protocol for disease detection could be utilized in conjunction with other medical procedures for disease diagnostics including lab (blood, urine or other body fluids) tests, imaging and biopsies. As such, the Company does not consider it to be in competition with these other medical procedures which have been industry standards for many years.
Disease detection for cancer, for example, are numerous and is dependent on the type of cancer tested. Information from the National Cancer Institute provides the following information;
· | Genetic testing, also known as DNA testing, allows the determination of bloodlines and the genetic diagnosis of vulnerabilities to inherited diseases. In humans, genetic testing can be used to determine a child’s parentage or in general a person’s ancestry; |
|
|
· | Lab tests. High or low levels of certain substances in your body can be a sign of cancer. So, lab tests of the blood, urine, or other body fluids that measure these substances can help doctors make a diagnosis. However, abnormal lab results are not a sure sign of cancer. Lab tests are an important tool, but doctors cannot rely on them alone to diagnose cancer. Current tumour markers available in many countries including Malaysia are CEA, CA 19-9, CA 125, PSA, AFP, β-hCG, CA 27.29. All are NOT suitable for screening and diagnostic use because of low sensitivity, specificity and predictive value. Source: American Family Physician (2003) Vol 68 (6) |
|
|
· | Imaging Procedures. Imaging procedures create pictures of areas inside your body that help the doctor see whether a tumor is present. These pictures can be made in several ways, including a CT Scan, Nuclear Scan, MRI, PET Scan, among others. |
|
|
· | Biopsies. In most cases, doctors need to do a biopsy to make a diagnosis of cancer. A biopsy is a procedure in which the doctor removes a sample of tissue. A pathologist then looks at the tissue under a microscope to see if it is cancer. |
| 9 |
| Table of Contents |
We however believe that we have a number of competitive strengths compared these other health diagnostic tools are as follows:
| · | Our screening is non-invasive (other than a simple blood draw), unlike biopsies; |
| · | In one test, we can screen for 11 diseases unlike conventional diagnostics; |
| · | Non-DNA blood tests for diseases like cancer are not dispositive and detect only elevated levels of proteins or other substances which are caused by cancer; |
| · | DNA blood tests are limited to certain types of inherited diseases, Huntington Disease, Cystic Fibrosis and Down Syndrome, among others. Such inherited disease(s) may or may not happen in a person’ life time; |
| · | MRI exams are uncomfortable and require fasting prior to testing, and implants in the body will distort result; |
| · | Most importantly, our screening provides a predictive risk assessment for developing the 11 diseases. Most other disease detection procedures detect diseases already present in the body, and in most cases in the final stages of the disease making it difficult to treat or reverse. With our screening, patients are able to monitor the development of these diseases in the future through further medical testing, including using our protocol. In addition, patients are able to make adjustments to their lifestyles in an effort to reduce the potentiality of these diseases. Lifestyle adjustments may include reduction or changes to food, tobacco and alcohol intake, change of working environment and the implementation of exercise programs, among other changes. |
Our Growth Strategy
We will look to grow and expand our business by further penetrating the Klang Valley market and expand our marketing efforts elsewhere in Malaysia. We believe that an increase in our marketing and promotional efforts will correlate to increased revenues and the expansion of our business. Our growth and expansion strategy for the next 6-12 months are as follows:
• Continue to leverage our relationships with healthcare providers
To date, we have relied upon the efforts of management and their relationships with health care providers to create the initial interest in our blood based genomic screening. These relationships have been located primarily in the Kuala Lumpur market. We will continue to use our relationships with providers in the Kuala Lumpur market and elsewhere in Malaysia to increase sales and product awareness.
• Allocate more capital resources to our marketing efforts
Apart from sales through existing relationships with health care providers, we intend to allocate more capital resources to marketing and promotion. As part of these efforts, we have appointed two commission-based Marketing Companies last year and they managed to bring some business after creating awareness of our services nationwide.
• Increase focus on corporate clients.
To date, we have entered into arrangements with two corporate clients for screening on their employees. We intend to solicit more corporate clients in the Klang Valley and major cities in Malaysia. We commenced these efforts last year and is continued in 2022. These efforts will be undertaken by the officers and the Marketing Companies.
• Expand to other regions in Malaysia.
We intend to reach other large cities in Malaysian, such as Penang, Ipoh, Seremban, Melaka, Johor Bahru and Kuantan.
Regulatory Matters
We are unaware of and do not anticipate having to expend significant resources to comply with any governmental regulations. We are subject to the laws and regulations of those jurisdictions in wellness operation and advertising materials circulation. Generally, business licensing requirements, income taxes and payroll taxes are applicable to all types of business operations. The development and operation of our business is not subject to special regulatory and/or supervisory requirements. In 2007, the Malaysian Parliament passed the Pathology Laboratory Bill of 2007 (“Pathology Act”), subject to the finalization of the underlying regulations. Since the passage of the Pathology Act, the Malaysian government has not implemented the legislation. Currently, we are only required an operating permit from local council, which we have received. However, we cannot predict whether we would be able to comply with the Pathology Act and its regulations, if implemented.
| 10 |
| Table of Contents |
Employees
As of the date of this this filing, BioNexus Malaysia has eight full-time employee and five directors/officers who work part-time. Management does not plan to hire additional employees at this time. Our employees are not represented by any collective bargaining agreement, and we have never experienced a work stoppage. We believe we have good relations with our employees.
Currently, BioNexus Malaysia has not entered into an employment agreement with any of our officers. The Company presently does not have pension, health, annuity, insurance, stock options, profit sharing or similar benefit plans; however, the Company may adopt plans in the future. Management does not plan to hire additional employees at this time.
Intellectual Property.
BioNexus Malaysia does not have any patents protecting our blood based genomic screening process. Instead, we rely on trade secrets and know-how using the process developed by Dr. Liew. There is no assurance that others will not independently develop the same or similar technology or obtain unauthorized access to our trade secrets, know-how and other unpatented technology. To protect our rights in these areas, we require all employees that work in our lab to enter into strict confidentiality agreements. Presently, we have one lab manager and 2 casual lab technicians. These agreements may not provide meaningful protection for our unpatented technology in the event of an unauthorized use, misappropriation or disclosure. While we have attempted to protect the unpatented proprietary technology that we develop or acquire, and will continue to attempt to protect future proprietary technology through patents, copyrights and trade secrets, we believe that our success will depend, to a large extent, upon continued innovation and technological expertise.
In general, the level of protection afforded by a patent is directly proportional to the ability of the patent owner to protect and enforce those rights through legal action. Since our financial resources are limited, and patent litigation can be both expensive and time consuming, there can be no assurance that we will be able to successfully prosecute an infringement claim in the event that a competitor develops a technology or introduces a product that infringes on one or more of our patents or patent applications. There can be no assurance that our competitors will not independently develop other technologies that render our proposed products obsolete. In general, we believe the best protection of our proprietary technology will come from market position, technical innovation, speed-to-market, and product performance. There is no assurance that we will realize any benefit from our intellectual property rights.
Product Liability.
Due to nature of the Company’s business, the Company may face claims for product liability resulting from the inaccurate or erroneous diagnosis using our screening process. Presently, the Company does not maintain any product liability insurance to cover any claims for an erroneous diagnosis.
Chemrex
Chemrex is a wholesaler of industrial chemicals for the manufacture of industrial, medical, appliance, aero, automotive, mechanical and electronic industries in South East Asia region. These countries include Malaysia, Indonesia, Vietnam and numerous other countries. These superior graded industrial chemicals come with JIS, ANSI / IPC and ISO 9002 certification since 2004.
Chemrex’s corporate office and distribution and storage center is located at 4 Jalan CJ 1/6 Kawasan Perusahaan Cheras Jaya, Selangor, Malaysia. Its phone number is (+60) 1922-23815 and web-site is www.chemrex.com.my.
Acquisition of Chemrex Sdn. Bhd.
On December 31, 2020, BioNexus completed a Share Exchange Agreement with Chemrex, and the shareholders of the Chemrex ("Chemrex Shareholders") pursuant to which the Company acquired all of the issued and outstanding shares of capital stock of Chemrex from the Chemrex Shareholders in exchange for 68,487,261 shares of common stock of the Company issued to the Chemrex Shareholders on a pro-rata basis.
Liong Tai Tan, our Chief Operating Officer (appointed on November 27, 2020), who is also the Chief Operating Officer of Chemrex, was a Chemrex shareholder and received 14,553,543 shares of common stock in connection with the transaction.
| 11 |
| Table of Contents |
Business of Chemrex
Chremrex buys raw materials (chemicals) from domestic and international manufacturers. Chemrex wholesales these Fibre Reinforced Polymer (FRP) materials to various manufacturers in South East Asia. The chemicals are used to produce a wide variety of finished goods. Common products using FRP are handrails, bench tops, automotive and aero parts, paneling for hospital/laboratory/industrial clean rooms, covers for various instruments under in manufacturing, among other uses. FRP materials have strong design and technology. According to the product shape technical requirements, use and quantity flexible choice of molding process. The process is simple, can be shaped at one time, the economic effect is outstanding, especially for the complex shape, not easy to shape the number of products, more outstanding its process superiority.
Examples of FRP products manufactured by Chemrex customers are displayed below:
Medical and Industrial Equipment.

Platform, Handrail and Decking.

| 12 |
| Table of Contents |
Medical appliances.
|
|
|
|
Key features of FRP products are as follows:
- Lightweight with high strength.
- Good electrical insulator with no electro-magnetic behavior and no electric spark.
- Rust free and resistant to acid, alkali, organic dissolvents and other gas and liquid mixtures.
- Anti-aging with more than 20 years of useful life under normal working condition.
- Ease of maintenance.
The following table reflects Chemrex’s five top selling products for fiscal 2021, indicated by revenues, finished goods use and percentage of total revenues of $11,846,894:
Raw Materials |
| Finished Goods |
| Revenue |
|
| % of total Revenue |
| ||
1. Chopped Strand Mat 450gm |
| Chemical tanks |
| $ | 1,600,000 |
|
|
| 13.51 |
|
2. Laminating Resin 268 |
| Public transport bodies, water resistant items |
| $ | 1,200,000 |
|
|
| 10.13 |
|
3. High Viscosity polyester Resin 9509 |
| Clean room, ICU flooring & wall and marine boat |
| $ | 820,000 |
|
|
| 6.92 |
|
4. Centrifugal Resin 8452A |
| pipes for oil & gas industry, sewerage |
| $ | 650,000 |
|
|
| 5.49 |
|
5. Casting Resin 2720 |
| hospital and laboratory counter coating |
| $ | 610,000 |
|
|
| 5.15 |
|
Sales and Marketing.
Our product sales and marketing are performed internally by Mr. Tham Too Kam, Managing Director, Mr. Tan Liong Tai, Executive Director and Mr. Chan Kwan Wah, Marketing Manager and 3 other marketing and technical representatives.
New customers would check through our website for products and forward their enquiries to us. Our marketing and technical representatives would contact the prospective customer and discuss a range of company products to suit their needs.
Most of our existing customers also are well-established manufacturers and contractors who know us well and place their orders regular basis. Typically, the provide us with a forecast and accordingly place their orders with us on a monthly basis. If they need an item in small quantity, they would normally get it from hardware retailer. Chemrex has distinct advantage of having full range with competitive prices fulfilling customers’ complete requirement.
We also assist customers on their new product development or projects with suitable and compatible raw materials. We jointly explore with customers all technical and aesthetic requirements of the new products or projects. In view of our years of successful dealings with local and international raw materials manufacturers, we often collaborate with their research teams to meet the new product needs of our customers.
Competition and Competitive Strengths.
Chemrex is a well-established, reliable quality composite material distributor with professional services. As a dominant, well-stocked company, it has complete range of products couple with strong technical supports as compared to its competitors’ incomplete range resulting in higher logistics and purchasing costs.
| 13 |
| Table of Contents |
Chemrex has a number of competitors in Malaysia, some of which have greater resources than us. However, we believe that we offer the following competitive advantages to our existing and potential customers:
| - | Our Technical Staff. Our technical staff, comprised of two chemists and one engineer, are highly competent and familiar with the trending advancements in the FRP industry. They provide technical know-how on mixing various products and offer product suggestions/modifications to our customers which can involve strengthening or enhancing existing products sold by our customers. |
|
|
|
| - | Sourcing Raw Materials. We source a broad range of raw materials on a world-wide basis. This feature greatly expands our reach to potential customers. It also assists our customers in developing new products. |
Regulatory Matters
We are unaware of and do not anticipate having to expend significant resources to comply with any governmental regulations. We are subject to the laws and regulations of those jurisdictions in Malaysia
Intellectual Property.
Chemrex does not have any patent, trademark, copyright or licensing rights.
Employees
As of the date of this this filing, Chemrex has 19 full-time employees, including 12 Directors/Executives and 7 Warehouse Staff for the Company. Chemrex believes its relationship with employees to be excellent.
In total, Chemrex and Bionexus Malaysia have 27 full-time employees, including 4 Chemrex’ Directors, 13 Technical and Administrative Executives and 10 warehouse staff. On the holding level, BGLC has 5 Directors/Officers on part-time basis
Item 1A. Risk Factors
RISK FACTORS
An investment in our common stock involves a number of very significant risks. You should carefully consider the following known material risks and uncertainties in addition to other information in this Form 10-K in evaluating our company and its business before purchasing shares of our company’s common stock. You could lose all or part of your investment due to any of these risks.
| 14 |
| Table of Contents |
Risk Factors Relating to Our Business
BIONEXUS MALAYSIA HAS A LIMITED OPERATING HISTORY AND LIMITED BUSINESS GROWTH. BioNexus Malaysia has been operational since April 2017; therefore, it has had limited operations which makes it difficult to evaluate its business and our prospects. In addition, to date, they have not experienced substantial growth in our business. The likelihood of its success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered by a small operating company trying to expand its business enterprise and the highly competitive environment in which we will operate. Consequently, there can be no assurance that the business of BioNexus Malaysia will grow in the future. Moreover, because of its limited operating history, it is difficult to extrapolate any meaningful projections about its future.
THE EFFICACY OF BIONEXUS MALAYSIA BLOOD SCREENING PROCESS HAS NOT SUPPORTED BY ANY INDEPENDENT STUDIES OR TESTS. BioNexus Malaysia blood screening process, which was commercially launched in July 2017, has been developed by late Dr. Choong Chin Liew, our largest shareholder. Dr. Liew has spent many years developing and testing various aspects of his current protocols and has published numerous articles concerning his blood screening protocols. Nonetheless, these protocols and procedures have not been the subject of a wide scale independent study or studies proving the efficacy of our testing protocols.
As a result, it is conceivable, that despite Dr. Liew’s efforts, our current blood based genomic screening process may not be as efficacious as we believe, which in effect would yield false positive or false negative test results. Inaccurate test results in turn could lead to significant financial exposure to the Company. The exposure would arise from claims by patients for a misdiagnosis of current or perceived current medical conditions. Claims for a false positive diagnosis could include increased medical costs arising from more medical tests and physician examinations in response to the false positive diagnosis. Claims for false negative diagnosis could include claims for loss of life and pain and suffering arising from the failure to diagnose a current medical condition. While we inform patients that our diagnostics are merely one of many tools employed in a health care diagnoses, these claims could be substantial and cause a material adverse impact on our business.
BIONEXUS MALAYSIA MAY FACE PRODUCT LIABILITY CLAIMS. Due to the nature of our business, we may face claims for product liability. These claims may arise from the inaccurate or erroneous diagnosis of patient information or the mix-up of patient information whereby a patient receives the wrong diagnostic information. While we feel confident in our accuracy of our diagnostic analysis and the procedures which we have implemented to ensure the safeguard of patient information, we cannot provide assurances that product liability claims will arise in the future.
Moreover, litigation or adverse publicity resulting from these allegations could materially and adversely affect our business, regardless of whether the allegations are valid or whether we are liable. Currently we have no product liability insurance coverage, and even if there was such coverage, there would be no assurance that such coverage would be sufficient to properly protect us. Further, claims of this type, whether substantiated or not, may divert our financial and management resources from revenue generating activities and the business operation.
Presently, we do not have insurance to cover any product liability claims. This lack of insurance may cause a material adverse impact on the Company if product liability claims arise.
INEFFECTIVE RISK MANAGEMENT POLICIES AND PROCEDURES. The Company relies on a combination of technical and human factors to protect the Company against risks. Its policies, procedures and practices are used to identify, monitor and control a variety of risks, including risks related to human error and hardware and software errors. The administration and results of each test are reviewed by a physician and a scientist in Malaysia before the results are released to the patient. The Company’s standard of operations has been developed internally primarily by Dr. Liew. These risk-management methods may not adequately prevent losses and may not protect us against all risks, in which case our business, economic conditions, operations and cash flows may be materially adversely affected.
| 15 |
| Table of Contents |
We have risk-management policies, control systems and compliance manuals in place; however, there is no guarantee that such policies, systems, and manuals will be effectively applied in every circumstance by our staff for example; employees could override the system technology and theoretically waive requirements, thereby exposing our company to the risk of compromised test result.
WE WILL NEED ADDITIONAL FINANCING IN ORDER TO GROW OUR BUSINESS. We do not have significant assets with which to expand our business. We intend to expand our business through increased marketing efforts in Malaysia and elsewhere for both of our business line, the blood based genomic screening process and chemical distribution business. These additional expenditures are intended to be funded from cash on hand and, if necessary, third-party sources, including the incurring of debt and/or the sale of additional equity securities. In addition to requiring additional financing to fund expansion, the Company may require additional financing to fund working capital and operating losses in the future should the need arise. The incurrence of debt creates additional financial leverage and therefore an increase in the financial risk of the Company’s operations. The sale of additional equity securities will be dilutive to the interests of current equity holders. In addition, there can be no assurance that such additional financing, whether debt or equity, will be available to the Company or that it will be available on acceptable commercial terms. Any inability to secure such additional financing on appropriate terms could have a materially adverse impact on the business, financial condition and operating results of the Company.
AS WE UNDERTAKE BIONEXUS MALAYSIA’S BUSINESS, WE WILL BE SUBJECT TO COMPLIANCE WITH POTENTIAL GOVERNMENT REGULATION THAT MAY INCREASE IN THE FUTURE. Currently, there are no governmental regulations that materially restrict our screening business. Pathology Laboratory Bill of 2007 (“Pathology Act”) has been passed by the Malaysian Parliament, however, since 2007, the government has not implemented the regulations underlying the legislation nor has the government enforced the Pathology Act. Any such regulations could establish criteria for the various classes and specialties of laboratories, the organization and management system of the laboratory, the qualification and experience of the person-in-charge, the qualification and competence of pathologists, scientific and technical staff engaged to conduct tests, and the standards of laboratory practice. We cannot predict whether we would be able to comply with the Pathology Act and its regulations, if implemented. In addition, there also is a risk that the regulations arising from the Pathology Act or new legislation or regulations could increase our costs of doing business or otherwise prevent us from carrying out the expansion of our business. Accordingly, our business may be harmed if we are no able to comply with any future governmental legislation or regulations, including the Pathology Act.
OUR BLOOD BASED GENOMIC SCREENING PROCESS MAY NOT ACHIEVE COMMERCIAL SUCCESSES IN THE MARKETPLACE. Our blood based genomic screening process may not be acceptable in the marketplace for a variety of factors. One factor may be that doctors and hospitals may be loathed to recommend our screening process as it may be deemed competitive to existing health care services that are offered by doctors and hospitals. Another factor may be that patients could be fearful of learning potentially negative health results and as a consequence, may not subscribe to our screening process. The occurrence of either of these factors may impact the successful reception of our product in the marketplace and negatively impair our further revenue potential.
BUSINESS DISRUPTIONS COULD SERIOUSLY HARM OUR FUTURE REVENUE AND FINANCIAL CONDITION AND INCREASE OUR COSTS AND EXPENSES. Our operations could be subject to power shortages, telecommunications failures, wildfires, water shortages, floods, earthquakes, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. Unfavorable global economic conditions could adversely affect both of our business segments, financial condition, or results of operations.
We do not carry insurance for all categories of risk that our business may encounter. Although we intend to obtain some form of business interruption insurance in the future, there can be no assurance that we will secure adequate insurance coverage or that any such insurance coverage will be sufficient to protect our operations to significant potential liability in the future. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our financial position and results of operations.
| 16 |
| Table of Contents |
OUR SOFTWARE IS HIGHLY COMPLEX AND MAY CONTAIN UNDETECTED ERRORS. Our proprietary software underlying our diagnosis is highly complex and may contain undetected errors or vulnerabilities, some of which may only be discovered after a diagnosis. This may result in an inaccurate diagnosis which could expose us to substantial liability due to the misdiagnosis. Any errors or vulnerabilities discovered in our software could result in damage to our reputation, loss of clients, loss of revenue or liability for damages, any of which could adversely affect our growth prospects and our business.
WE MAY BE UNABLE TO PROTECT OUR INTELLECTUAL PROPERTY ADEQUATELY. Our proprietary software is an essential asset of our business. To establish and protect our intellectual property rights, we rely primarily upon a trade secret, and to a lesser extent, contractual provisions with current and future employees. Further, our software is not patent protected nor is it copyrighted. Resultantly, our efforts to protect our intellectual property may not be sufficient or effective. If these measures do not protect our intellectual property rights, third parties could use the Company’s technology, and its ability to compete in the market would be reduced significantly.
In addition, we may not be effective in policing unauthorized use of our intellectual property. Even if we do detect violations, we may need to engage in litigation to enforce our intellectual property rights. Any enforcement efforts we undertake, including litigation, could be time-consuming and expensive and could divert our management’s attention. In addition, our efforts may be met with defenses and counterclaims challenging the validity and enforceability of our intellectual property rights or may result in a court determining that our intellectual property rights are unenforceable. If we are unable to cost-effectively protect our intellectual property rights, then our business could be harmed.
WE MAY BE SUBJECT TO INTELLECTUAL PROPERTY CLAIMS, WHICH ARE EXTREMELY COSTLY TO DEFEND, COULD REQUIRE US TO PAY SIGNIFICANT DAMAGES AND COULD LIMIT OUR ABILITY TO USE CERTAIN TECHNOLOGIES IN THE FUTURE. Companies in bio-medical or bio-technology industries are frequently subject to litigation based on allegations of infringement or other violations of intellectual property rights. To the extent we gain greater public recognition, we may face a higher risk of being the subject of intellectual property claims. Third-party intellectual property rights may cover significant aspects of our technologies or business methods or block us from expanding our offerings. Any intellectual property claims against us, with or without merit, could be time consuming and expensive to settle or litigate and could divert the attention of our management. Litigation regarding intellectual property rights is inherently uncertain due to the complex issues involved, and we may not be successful in defending ourselves in such matters.
In addition, some of our competitors have extensive portfolios of issued patents. Many potential litigants, including some of our competitors and patent holding companies, have the ability to dedicate substantial resources to enforcing their intellectual property rights. Any claims successfully brought against us could subject us to significant liability for damages and we may be required to stop using technology or other intellectual property alleged to be in violation of a third party’s rights. We also might be required to seek a license for third-party intellectual property. Even if a license is available, we could be required to pay significant royalties or submit to unreasonable terms, which would increase our operating expenses. We may also be required to develop alternative non-infringing technology, which could require significant time and expense. If we cannot license or develop technology for any allegedly infringing aspect of our business, we would be forced to limit our service and may be unable to compete effectively. Any of these results could harm our business.
WE FACE COMPETITION FROM OTHER LABORATORIES AND OUR OPERATING RESULTS WILL SUFFER IF WE FAIL TO COMPETE EFFECTIVELY. We believe there are a limited number of companies worldwide that specialize in RNA blood analysis to detect disease. However, there are a few laboratories in universities and research institutions that are attempting to extend their researches from DNA into RNA screening. If they have some breakthrough and they could be our potential competitors. Many of our potential competitors may have strong financial and resources, such as sophisticated research capabilities and development staff than we do. Their discovery and development of novel protocol that could make our genomic screening obsolete even though with FDA and European Union certification. As a result of these factors, our competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing screening process for the cancer, inflammation, osteoarthritis and many more indications.
| 17 |
| Table of Contents |
In addition, smaller or early-stage companies also may prove to be significant competitors, particularly through collaborative arrangements with large, established companies. In addition, many universities and private and public research institutes may become active in our target disease areas.
If our competitors market products that are more effective, safer or less expensive or that reach the market sooner than our future products, if any, we may not achieve commercial success. In addition, because of our limited resources, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our technologies or product candidates obsolete, less competitive or not economical.
WE ALSO FACE COMPETITION FROM OTHER CHEMICAL DISTRIBUTORS AND OUR OPERATING RESULTS WILL SUFFER IF WE FAIL TO COMPETE EFFECTIVELY. We believe there are a limited number of companies in Malaysia that provide the broad range of chemicals that we provide to customers. Many of our existing and potential competitors may have stronger financial, product development and marketing capabilities than our existing capabilities. As a result of these factors, our competitors may succeed in attracting new customers which we may need to further develop our business.
WE MAY INCUR SIGNIFICANT COSTS TO BE A PUBLIC COMPANY TO ENSURE COMPLIANCE WITH U.S. CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS AND WE MAY NOT BE ABLE TO ABSORB SUCH COSTS. We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect these costs to approximate $50,000 per year, consisting of $30,000 in legal, $50,000 in audit and $10,000 for EDGAR filing and transfer agent fees. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We may not be able to cover these costs from our operations and may need to raise or borrow additional funds. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. In addition, we may not be able to absorb these costs of being a public company which will negatively affect our business operations.
OUR OFFICERS AND DIRECTORS MAY HAVE A CONFLICT OF INTEREST WITH THE MINORITY SHAREHOLDERS AT SOME TIME IN THE FUTURE. SINCE THE MAJORITY OF OUR SHARES OF COMMON STOCK ARE OWNED BY OUR OFFICERS AND DIRECTORS AND A KEY CONSULTANT, OUR OTHER STOCKHOLDERS MAY NOT BE ABLE TO INFLUENCE CONTROL OF THE COMPANY OR DECISION MAKING BY MANAGEMENT OF THE COMPANY. Our Officers and Directors beneficially own approximately 35% of our outstanding common stock. The interests of our Officers and Directors may not be, at all times, the same as that of our other shareholders. Our Officers and Directors are not simply passive investors but are also executives of the Company, their interests as executives may, at times be averse to those of passive investors. Where those conflicts exist, our shareholders will be dependent upon our directors exercising, in a manner fair to all of our shareholders, their fiduciary duties as officers or as member of the Company’s Board of Directors. Also, our directors will have the ability to control the outcome of most corporate actions requiring shareholder approval, including the sale of all or substantially all of our assets and amendments to our articles of incorporation. This concentration of ownership may also have the effect of delaying, deferring or preventing a change of control of us, which may be disadvantageous to minority shareholders.
BECAUSE OUR OFFICERS AND DIRECTORS MAY IN FUTURE HAVE OUTSIDE BUSINESS ACTIVITIES, THERE IS A POTENTIAL CONFLICT OF INTEREST, INCLUDING THE AMOUNT OF TIME THEY WILL BE ABLE TO DEDICATE TO THE COMPANY. Currently our officers, who are also directors, have been working on promoting business for the Company. A potential conflict of interest may arise in the future that may cause our business to fail, including conflicts of interest in allocating their time to our company and their other business interests. While our officers have verbally agreed to devote sufficient time and attention to the affairs of the Company, we have no written arrangement with our officers regarding this matter. As a result, we may face conflicts between business decisions that they may have to make regarding our operations and that of their other business interests.
| 18 |
| Table of Contents |
BECAUSE OUR MANAGEMENT DOES NOT HAVE PRIOR EXPERIENCE RUNNING A PUBLIC COMPANY, WE MAY HAVE TO HIRE INDIVIDUALS OR SUSPEND OR CEASE OPERATIONS. Because our management has limited prior experience in running a public company, including the preparation of reports under the Securities Act of 1934, we may have to hire additional experienced personnel to assist us with the preparation thereof. If we need the additional experienced personnel and we do not hire them, we could fail in our plan of operations and have to suspend operations or cease operations entirely.
INDEPENDENT AUDIT COMMITTEE. Although the common stock is not listed on any national securities exchange, for purposes of independence we use the definition of independence applied by NASDAQ. Currently, BGLC has three independent directors and they served as audit committee members. They are considered to be “independent” in accordance with the requirements set forth in NASDAQ Listing Rule 5605(a)(2). An independent audit committee plays a crucial role in the corporate governance process, assessing our Company’s processes relating to our risks and control environment, overseeing financial reporting, and evaluating internal and independent audit processes.
POTENTIAL DATA BREACHES. If we are successful, our services will generate and process a large quantity of personal health condition data. We face risks inherent in handling large volumes of data and in protecting the security of such data. In particular, we face a number of challenges relating to data inter-connected with regional labs, including:
· | protecting the data in and hosted on our system, including against hacking on our system by outside parties or our employees; |
· | addressing concerns related to privacy and sharing, safety, security and others; |
· | complying with applicable laws, rules and regulations relating to the collection, use, disclosure of personal information, including any requests from regulatory and government authorities relating to such data; |
· | Any systems failure or security breach or lapse those results in the release of user data could harm our reputation and brand and, consequently, our business, in addition to exposing us to potential legal liability. |
As we expand our operations, we may be subject to these laws in other jurisdictions where our customers and other participants are located. The laws, rules and regulations of other jurisdictions may impose more stringent or conflicting requirements and penalties than those in Malaysia, compliance with which could require significant resources and costs. Our privacy policies and practices concerning the collection, use and disclosure of user data are posted on our websites. Any failure, or perceived failure, by us to comply with our posted privacy policies or with any regulatory requirements or privacy protection-related laws, rules and regulations could result in proceedings or actions against us by authorities or others. These proceedings or actions may subject us to significant penalties and negative publicity, require us to change our business practices, increase our costs and severely disrupt our business.
| 19 |
| Table of Contents |
CROSS-BORDER OPERATIONS. As we plan to continue expanding our existing cross-border operations into existing and other markets, we will face risks associated with expanding into markets in which we have limited or no experience and in which our company may be less well-known. We may be unable to attract a sufficient number of customers and other participants, fail to anticipate competitive conditions or face difficulties in operating effectively in these new markets. The expansion of our cross-border business will also expose us to risks relating to staffing and managing cross-border operations, increased costs to protect intellectual property, tariffs and other trade barriers, differing and potentially adverse tax consequences, increased and conflicting regulatory compliance requirements, lack of acceptance of our service offerings, challenges caused by distance, language and cultural differences, exchange rate risk and political instability. Accordingly, any efforts we make to expand our cross-border operations may not be successful, which could limit our ability to grow our revenue, net income and profitability.
RISKS RELATED TO DOING BUSINESS IN ASIA PACIFIC REGION. Changes in the political and economic policies of the local government may materially and adversely affect our business, financial condition and results of operations and may result in our inability to sustain our growth and expansion strategies. Accordingly, our financial condition and results of operations are affected to a significant extent by economic, political and legal developments in Asia Pacific region.
The Asia Pacific economy differs from the economies of most developed countries in many respects, including the extent of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. In addition, the government continues to play a significant role in regulating industry development by imposing industrial policies. The government also exercises significant control over economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy, regulating financial services and institutions and providing preferential treatment to particular industries or companies.
The local government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall economy, but may also have a negative effect on us. Our financial condition and results of operation could be materially and adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. In addition, the government has implemented in the past certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity, which in turn could lead to a reduction in demand for our services and consequently have a material adverse effect on our businesses, financial condition and results of operations.
YOU MAY EXPERIENCE DIFFICULTIES IN EFFECTING SERVICE OF LEGAL PROCESS, ENFORCING FOREIGN JUDGMENTS OR BRINGING ORIGINAL ACTIONS IN MALAYSIA BASED ON UNITED STATES OR OTHER FOREIGN LAWS AGAINST US OR OUR MANAGEMENT. Our operating subsidiary is incorporated in Malaysia and conducts substantially all of our operations in Asia Pacific. All of our executive officers and directors reside outside the United States and all of their assets are located outside of the United States. As a result, it may be difficult or impossible for shareholders to bring an action against us or against these individuals in Malaysia in the event that you believe that your rights have been infringed under the securities laws of the United States or otherwise. Even if you are successful in bringing an action of this kind, the laws of Malaysia may render you unable to enforce a judgment against our assets or the assets of our directors and officers. There is no statutory recognition in Malaysia of judgments obtained in the United States, although the courts of Malaysia will generally recognize and enforce a non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits. The rights of shareholders to take legal action against us and our directors, actions by minority shareholders and the fiduciary responsibilities of our directors are to a large extent governed by the common law of Malaysia. The common law of Malaysia is derived in part from comparatively limited judicial precedent in Malaysia as well as from English common law, which provides persuasive, but not binding, authority in a court in Malaysia. The rights of our shareholders and the fiduciary responsibilities of our directors under Malaysian law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, Malaysia has a less developed body of securities laws than the United States and provides significantly less protection to investors. As a result, our public shareholders may have more difficulty in protecting their interests through actions against us, our management, our directors or our major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
| 20 |
| Table of Contents |
Risks Related to Our Common Stock
SALES OF OUR COMMON STOCK IN RELIANCE ON RULE 144 MAY REDUCE PRICES IN THAT MARKET BY A MATERIAL AMOUNT. A significant number of the outstanding shares of our common stock are “restricted securities” within the meaning of Rule 144 under the Securities Act. As restricted securities, those shares may be resold only pursuant to an effective registration statement or pursuant to the requirements of Rule 144 or other applicable exemptions from registration under the Securities Act and as required under applicable state securities laws. Rule 144 provides in essence that an affiliate (i.e., an officer, director or control person) who has held restricted securities for a prescribed period may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed 1.0% of the issuer’s outstanding common stock. The alternative limitation on the number of shares that may be sold by an affiliate, which is related to the average weekly trading volume during the four calendar weeks prior to the sale is not available to stockholders of companies whose securities are not traded on an “automated quotation system” because the OTC-QB Market is not such a system, market-based volume limitations are not available for holders of our securities selling under Rule 144.
Pursuant to the provisions of Rule 144, there is no limit on the number of restricted securities that may be sold by a non-affiliate (i.e., a stockholder who has not been an officer, director or control person for at least 90 consecutive days before the date of the proposed sale) after the restricted securities have been held by the owner for a prescribed period, although there may be other limitations and/or criteria to satisfy. A sale pursuant to Rule 144 or pursuant to any other exemption from the Securities Act, if available, or pursuant to registration of shares of our common stock held by our stockholders, may reduce the price of our common stock in any market that may develop.
YOU MAY NOT BE ABLE TO LIQUIDATE YOUR INVESTMENT SINCE THERE IS NO ASSURANCE THAT A PUBLIC MARKET WILL DEVELOP FOR OUR COMMON STOCK OR THAT OUR COMMON STOCK WILL EVER BE APPROVED FOR TRADING ON A RECOGNIZED EXCHANGE. There is no established public trading market for our securities. Although we intend to be quoted on the OTC-QB Market in the United States, our shares are not and have not been quoted on any exchange or quotation system. We cannot assure you that a market maker will agree to file the necessary documents with the FINRA, nor can there be any assurance that such an application for quotation will be approved or that a regular trading market will develop or that if developed, will be sustained. In the absence of a trading market, an investor may be unable to liquidate its investment, which will result in the loss of your investment.
OUR COMMON STOCK IS SUBJECT TO THE “PENNY STOCK” RULES OF THE SEC AND THE TRADING MARKET IN OUR SECURITIES IS LIMITED, WHICH MAKES TRANSACTIONS IN OUR STOCK CUMBERSOME AND MAY REDUCE THE VALUE OF AN INVESTMENT IN OUR STOCK. Under U.S. federal securities legislation, our common stock will constitute “penny stock”. Penny stock is any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a potential investor’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. In order to approve an investor’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prepared by the Commission relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination. Brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock. Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
| 21 |
| Table of Contents |
IN THE FUTURE, WE MAY ISSUE ADDITIONAL COMMON AND PREFERRED SHARES, WHICH WOULD REDUCE INVESTORS’ PERCENT OF OWNERSHIP AND MAY DILUTE OUR SHARE VALUE. Our Articles of Incorporation authorize the issuance of 300,000,000 shares of common stock. As of the date of this filing, the Company had 171,218,152 shares of common stock outstanding. Accordingly, we may issue up to an additional 128,781,848 shares of common stock. In addition, we have the right to issue 30,000,000 shares of preferred stock. The preferred stock is known as “blank check” as the Board of Directors is authorized to set the rights, privileges and preference of the preferred stock. The future issuance of common stock and preferred may result in substantial dilution in the percentage of our common stock held by our then existing shareholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock or preferred stock for future services or acquisitions or other corporate actions may have the effect of diluting the value of the shares held by our investors, and might have an adverse effect on any trading market for our common stock.
UPON EFFECTIVENESS OF THIS REGISTRATION STATEMENT, WE WILL NOT BE A FULLY REPORTING COMPANY UNDER SECTION 12(G) OF THE SECURITIES EXCHANGE ACT OF 1934, RATHER WE WILL BE SUBJECT TO THE REPORTING REQUIREMENTS OF SECTION 15(D) OF THE EXCHANGE ACT WHICH IS LESS RESTRICTIVE ON US AND OUR INSIDERS. In order for us to become a fully reporting company under Section 12(g) of the Exchange Act, we will have to file a Registration Statement on Form 8-A. If we do not become subject to Section 12 of the Exchange Act, we will be subject to Section 15(d) of the Exchange Act, and as such we will not be required to comply with (i) the proxy statement requirements which means shareholders may have less notice of pending matters, and (ii) the Williams Act which requires disclosure of persons or groups that acquire 5% of a company’s publicly traded stock and also regulates tender offers. In addition, our officer, director and 10% stockholder will not be required to submit reports to the SEC on their stock ownership and stock trading activity. These reports include Form 3, 4 and 5. Therefore, as a shareholder, less information and disclosure concerning these matters will be available to you.
WE DO NOT INTEND TO PAY ANY CASH DIVIDENDS ON OUR COMMON STOCK, OUR STOCKHOLDERS WILL NOT BE ABLE TO RECEIVE A RETURN ON THEIR SHARES UNLESS THEY SELL THEM. We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
OUR COMMON STOCK PRICE IS LIKELY TO BE HIGHLY VOLATILE WHICH MAY SUBJECT US TO SECURITIES LITIGATION THEREBY DIVERTING OUR RESOURCES WHICH MAY AFFECT OUR PROFITABILITY AND RESULTS OF OPERATION. The market price for our common stock is likely to be highly volatile as the stock market in general and the market for Internet-related stocks.
The following factors will add to our common stock price’s volatility:
| - | fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us; |
| - | changes in estimates of our financial results or recommendations by securities analysts; |
| - | changes in market valuations of similar companies; |
| 22 |
| Table of Contents |
| - | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| - | regulatory developments in Malaysia or other countries wherein we expect to conduct business; |
| - | litigation involving our company, our general industry or both; |
| - | investors’ general perception of us; and |
| - | changes in general economic, industry and market conditions. |
Many of these factors are beyond our control. These factors may decrease the market price of our common stock, regardless of our operating performance. In the past, plaintiffs have initiated securities class action litigation against a company following periods of volatility in the market price of its securities. In the future, we may be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
REDUCED DISCLOSURE REQUIREMENTS APPLICABLE TO EMERGING GROWTH COMPANIES MAY MAKE OUR COMMON STOCK LESS ATTRACTIVE TO INVESTORS. We qualify as an “emerging growth company” under the JOBS Act. As a result, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, we will not be required to:
· | have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
· | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
· | submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay” and “say-on-frequency;” and |
· | disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. |
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We will remain an emerging growth company for up to five full fiscal years, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any January 31 before that time, we would cease to be an emerging growth company as of the following December 31, or if our annual revenues exceed $1 billion, we would cease to be an emerging growth company the following fiscal year, or if we issue more than $1 billion in non-convertible debt in a three-year period, we would cease to be an emerging growth company immediately.
Notwithstanding the above, we are also currently a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, nor a majority-owned subsidiary of a parent company that is not a smaller reporting company, and has a public float of less than $75 million and annual revenues of less than $50 million during the most recently completed fiscal year. If we are still considered a “smaller reporting company” at such time as we cease to be an “emerging growth company,” we will be subject to increased disclosure requirements. However, the disclosure requirements will still be less than they would be if we were not considered either an “emerging growth company” or a “smaller reporting company.” Specifically, similar to “emerging growth companies”, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; are not required to conduct say-on-pay and frequency votes until annual meetings occurring on or after January 21, 2015; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in its SEC filings due to its status as an “emerging growth company” or “smaller reporting company” may make us less attractive to investors given that it will be harder for investors to analyze the Company’s results of operations and financial prospects and, as a result, it may be difficult for us to raise additional capital as and when we need it.
| 23 |
| Table of Contents |
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate offices and the offices of BioNexus Malaysia are located at Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, No. 8 Jalan Kerinchi, Kuala Lumpur, Malaysia. The lease commenced in December 15, 2020 and terminates in December 15, 2023. The space consists of 1,300 square feet with an annual rent of approximately $13,200 USD.
Our laboratory is located at Lab 353, University Science Malaysia, George Town, Penang, Malaysia. The lease commenced on May 2017 and terminates on April 2023. The space consists of 1,500 square feet with an annual rent of approximately $8,400 USD.
We also have a blood collection center located on 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. As mentioned above, Lifecare Medical Centre refers their patients to us and as a result, they have allowed to maintain a blood collection center on their premises on a rent-free basis.
Chemrex’s office and wholesale distribution center is located at 4, Jalan CJ 1/6, Kawasan Perusahaan Cheras Jaya, 43200 Cheras, Selangor, Malaysia. The space consists of 25,000 square feet. Chemrex is the owner of the premises.
Chemrex also maintains two investment properties;
1. A condominium located at No. B-17-03, Duet Residence, Jalan Kinrara 6, Bandar Kinrara, 47180 Puchong, Selangor. The premises is 1,100 square feet and is leased to a third party at a rate of $488 per month. The lease is for 2 years terminating on February 2022.
2. A commercial building located at First floor, No. 2B Pelangi Avenue, Jalan Kelicap 42A/KU1, Klang Bandar, Diraja, 41050 Klang, Selangor. The premises is 2,000 square feet and is not leased as of the date of this filing.
Item 3. Legal Proceedings.
There are presently no pending legal proceedings to which the Company or any of its property is subject, or any material proceedings to which any director, officer or affiliate of the Company, any owner of record or beneficially of more than five percent of any class of voting securities is a party or has a material interest adverse to the Company, and no such proceedings are known to the Company to be threatened or contemplated against it.
Item 4. Mine Safety Disclosures.
None.
| 24 |
| Table of Contents |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
On March 11, 2020, FINRA authorized the trading of our common stock under the symbol “BGLC”, however, we began trading on the OTC-QB markets in September 2020. The table below sets forth, for the fiscal quarters indicated, the high and low bid prices per share of our common stock as reflected on the OTC-QB markets. The quotations represent inter-dealer prices without adjustment for retail markups, markdowns or commissions, and may not necessarily represent actual transactions.
The bid prices set forth below reflect inter-dealer quotations, do not include retail markups, markdowns or commissions and do not necessarily reflect actual transactions.
|
| High |
|
| Low |
| ||
Fiscal Year Ended December 31, 2021 |
|
|
|
|
|
| ||
First Quarter |
| $ | 2.69 |
|
| $ | 0.70 |
|
Second Quarter |
| $ | 2.10 |
|
| $ | 1.00 |
|
Third Quarter |
| $ | 1.86 |
|
| $ | 1.00 |
|
Fourth quarter |
| $ | 2.25 |
|
| $ | 1.00 |
|
Fiscal Year Ended December 31, 2020 |
|
|
|
|
|
|
|
|
Third Quarter |
| $ | 2.50 |
|
| $ | 0.36 |
|
Fourth Quarter |
| $ | 3.45 |
|
| $ | 1.06 |
|
The OTC-QB is a quotation system and not a national securities exchange, and many companies have experienced limited liquidity when traded through this quotation system. Any trading has been sporadic and there has been no meaningful trading volume.
Capital Stock:
Our authorized capital stock consists of 300,000,000 shares of common stock, no par value per share, and 30,000,000 shares of preferred stock, no par value per share. As of March 30, 2022, there are 171,218,152 shares of our common stock issued and outstanding that was held by 319 stockholders of record and no shares of preferred stock issued and outstanding. The shares of preferred stock are “blank check’ meaning the Company’s Board of Directors can issue shares of preferred stock in such series with such rights, privileges and preferences as determined from time to time by the Board of Directors without shareholder approval.
Dividend Policy
The Company has not declared or paid any cash dividends on its Common Stock and does not intend to declare or pay any cash dividend in the foreseeable future. The payment of dividends, if any, is within the discretion of the Board of Directors and will depend on the Company’s earnings, if any, its capital requirements and financial condition and such other factors as the Board of Directors may consider.
Securities Authorized for Issuance under Equity Compensation Plans
The Company does not have any equity compensation plans or any individual compensation arrangements with respect to its Common Stock or Preferred Stock. The issuance of any of our Common Stock or Preferred Stock is within the discretion of our Board of Directors, which has the power to issue any or all of our authorized but unissued shares without stockholder approval.
Recent Sales of Unregistered Securities.
None
Issuer Purchases of Equity Securities
None
Item 6. Selected Financial Data.
As a “smaller reporting company” as defined by Rule 12b-2 of the Exchange Act, the Company is not required to provide this information.
| 25 |
| Table of Contents |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
General.
Our Company was incorporated on April 5, 2017 and operations of our Malaysian company began operations in July 2017. Consequently, the following discussion and analysis of the results of operations and financial condition of the Company is for fiscal years ended December 31, 2021 and December 31, 2020, respectively. This information should be read in conjunction with the notes to the financial statements that are included elsewhere herein. The consolidated financial statements presented herein (and to which this discussion relates) reflect the results of operations of the Company and its Malaysian subsidiaries. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements. We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this report, except as required by law. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this quarterly report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
COMPANY OVERVIEW
Our financial statements are prepared in US Dollars and in accordance with accounting principles generally accepted in the United States. See information immediately below for information concerning the exchange rates at the Malaysian translated into US Dollars (“USD”) at various pertinent dates and for pertinent periods.
Translation of amounts from the local currency of the Company into US$1 has been made at the following exchange rates for the respective years:
|
| As of and for the year ended December 31, |
| |||||
|
| 2021 |
|
| 2020 |
| ||
Year-end MYR : US$1 exchange rate |
|
| 4.1650 |
|
|
| 4.0170 |
|
Yearly average MYR : US$1 exchange rate |
|
| 4.1456 |
|
|
| 4.2010 |
|
| 26 |
| Table of Contents |
Summary of Business
We have two operating subsidiaries located in Malaysia, Bionexus Gene Lab Sdn. Bhd. (“Bionexus Malaysia”) and Chemrex Corporation Sdn. Bhd. (“Chemrex”).
BioNexus Malaysia is an emerging molecular diagnostics company focused on the application of functional genomics to enable early diagnosis and personalized health management. It was incorporated in the State of Wyoming on May 12, 2017. On August 23, 2017, we acquired all of the outstanding capital stock of BioNexus Malaysia, which was incorporated in Malaysia on April 7, 2015. BioNexus Malaysia owns algorithm software, technology and know-how related to the detection of common diseases through blood analysis which we use in our business.
Our principal office address is Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, No. 8 Jalan Kerinchi, Kuala Lumpur, Malaysia., our lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia. We also have a blood collection center located at 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. Our telephone number is (+60) 122126512 and web-site is www.bionexusgenelab.com.
Chemrex is a wholesaler of industrial chemicals for the manufacture of industrial, medical, appliance, aero, automotive, mechanical and electronic industries in Asia Pacific region. On December 31, 2020, we acquired all of the outstanding capital stock of Chemrex, which was incorporated in Malaysia on September 29, 2004.
Chemrex’s corporate offices and distribution and storage center is located at 4 Jalan CJ 1/6 Kawasan Perusahaan Cheras Jaya, Selangor, Malaysia. Its phone number is (+60) 1922-23815 and web-site is www.chemrex.com.my.
Our corporate structure is depicted below:
The corporate structure as at December 31, 2021 is depicted below:
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
100% owned |
|
| 100% owned | ||
Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| Chemrex Corporation Sdn. Bhd., a Malaysian Company | ||
Recent Events.
Collaboration with Malaysia’s National Heart Institute
On August 1, 2020, our wholly owned Malaysian subsidiary, Bionexus Gene Lab Sdn Bhd (“BioNexus Malaysia”), entered into an Agreement for The Development Blood-Based Genomic Signatures in Acute Myocardial Infarction Risk Prediction Research Proposal with National Heart Centre (“NHI”) and Dato Dr. Amin Ariff Bin Nuruddin (“Principal Investigator”) (“Development Agreement”).
| 27 |
| Table of Contents |
The NHI is a Malaysian private limited company operating the business of Institut Jantung Negara, Malaysia’s National Heart Institute. NHI is the national referral center for acute myocardial infraction diseases (“AMI”) which provide diagnostic, medical and surgical services. NHI is under the direction of the Malaysian Ministry of Health. The Principal Investigator is an employee of the Institution and he is an experienced head of cardiologist and an interventionist of NHI.
Pursuant to the Development Agreement, the Institute and NHI, through the Principal Investigator, have agreed to collaborate in a research project with BioNexus Malaysia to provide information for purposes of utilizing and further developing our RNA technology. Blood samples will be collected from consenting AMI patients within two hours of being admitted to the NHI and prior to the administering any medication, so as to provide a true picture of the changes in the patient’s RNA by the microarray process. The blood samples will be provided to our lab where the RNA will be extracted for analysis. Similar to our other disease analysis process, the isolated RNA will be hybridized, probe array washed and stained and array scanned. We will then use the data from the research project to create a gene expression profile for the likelihood of an AMI event in patients using our RNA analysis. We expect to develop a gene panel from this project within the next 3 to 6 months.
Among other terms and conditions, the research project has been extended beyond on March 31, 2022 and BioNexus Malaysia is required to pay the approximately $1,100 per month in fees to NHI for the usage of its freezer and allowance for nurses and doctors who are required to explain to each participating patient about the research, his consent and patient consultation on the blood- based gene expression report for each patient.
During 2021, we have spent substantial time and effort on planning and preparing documentation for the Institute’s Ethics Committee review and approval such as establishing the objectives and benefits of the research and process protocols and other procedural items. Following the successful conclusion of the study by 3rd quarter of 2022, we are hopeful that NHI will take the lead to incorporate our RNA screening into their regular screening processes for patients. Industry information from the Institute indicates that Malaysians are developing heart disease at a younger age compared with their peers in other countries.
RESULTS OF OPERATIONS
Results of Operations for the Year Ended December 31, 2021 Compared to the Year Ended December 31, 2020 (Audited).
The following table sets forth key components of the results of operations for fiscal year ended December 31, 2021 and 2020, respectively. As stated herein, on December 31, 2021, the Company consummated its acquisition of Chemrex Corporation Sdn. Bhd. (“Chemrex”), pursuant to a Share Exchange Agreement by and among the Company and Chemrex and the Chemrex shareholders. Accordingly, the audited financial information for the period ended December 31, 2021 includes the accounts of Chemrex and BioNexus Malaysia and the (audited) financial information for the period ended December 31, 2020 only includes the accounts of BioNexus Malaysia.
| 28 |
| Table of Contents |
The discussion following the table addresses these results.
Consolidated |
| Year ended |
| |||||
|
| December 31 (Audited) |
| |||||
|
| 2021 |
|
| 2020 |
| ||
|
|
|
|
|
|
| ||
REVENUE |
| $ | 13,362,567 |
|
| $ | 11,390,440 |
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (11,168,747 | ) |
|
| (9,670,617 | ) |
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 2,193,820 |
|
|
| 1,719,823 |
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 66,491 |
|
|
| 886,942 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
General and administrative |
|
| (1,204,484 | ) |
|
| (1,332,943 | ) |
|
|
|
|
|
|
|
|
|
PROFIT FROM OPERATIONS |
|
| 1,055,827 |
|
|
| 1,273,822 |
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (12,973 | ) |
|
| (11,313 | ) |
|
|
|
|
|
|
|
|
|
PROFIT BEFORE TAX |
|
| 1,042,854 |
|
|
| 1,262,509 |
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
Deferred tax |
|
| (26,736 | ) |
|
| 1,238 |
|
Income tax |
|
| (264,547 | ) |
|
| (169,649 | ) |
Total tax expenses |
|
| (291,283 | ) |
|
| (168,411 | ) |
|
|
|
|
|
|
|
|
|
NET PROFIT |
| $ | 751,571 |
|
| $ | 1,094,098 |
|
|
|
|
|
|
|
|
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
Foreign currency translation (loss)/gain |
|
| (233,946 | ) |
|
| 150,787 |
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
| $ | 517,625 |
|
| $ | 1,244,885 |
|
Revenues. For the annual period ended December 31, 2021, we had revenues of $13,362,567 as compared to revenues of $11,390,440 for the annual period ended December 31, 2020, an increase of approximately 17.3% from the prior period due to qPCR screening on Covid-19 50,000 samples outsourced by public hospitals and clinics
Cost of revenues. For the annual period ended December 31, 2021 we had cost of revenues of $11,168,747 as compared to cost of revenues of $9,670,617 for the annual period ended December 31, 2020, an increase of approximately 15.5% from the prior period due to increase cost from qPCR kits and reagents purchases.
Other Income. For the annual period ended December 31, 2021, we had other income of $66,491 as compared to other income of $886,942 for the annual period ended December 31, 2020, a decrease of 92.5% for current year. The decrease in other income for the current annual period is due last year primarily Chemrex’s disposal of property which resulted in income of $706,953.
Operating Expenses. For the annual period ended December 31, 2021, we had operating expenses of $1,204,484, as compared to operating expenses of $1,332,943 for the annual period ended December 31, 2020, a decrease of 9.6% for the current year. Operating expenses include depreciation of fixed assets, equipment maintenance expenses, transportation, employee compensation and benefits, professional fees and marketing and travel expenses.
| 29 |
| Table of Contents |
Profit/(loss) from operations. We had a profit from operations of $1,055,827 for the annual period ended December 31, 2021 compared to a profit from operations of $1,273,822 for the annual period ended December 31, 2020, a decrease of 17.1% for the reasons discussed above.
Tax expense. For the year ended December 31, 2021, we had a total tax expense of $291,283 from deferred tax of $26,736 and income tax provision of $264,547. Last year ended December 31, 2020 the total tax expenses were $168,411 from an adjustment for over provision of deferred tax liabilities in prior year for credit amount of $1,238 and income tax provision of $169,649.
Foreign currency translation (loss)/gain. We are exposed to fluctuations in foreign exchange rates on the revaluation of monetary assets and liabilities denominated in currencies other than the US Dollar. Therefore, any change in the relevant exchange rate will require us to recognize a transaction gain or loss on revaluation. For the annual period ended December 31, 2021, we had foreign currency translation loss of $233,946 compared with foreign currency translation gain $150,787 for the prior annual period.
BioNexus-Malaysia and Chemrex
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BioNexus Malaysia |
|
| Chemrex |
| ||||
|
| Year ended December 31, 2021 |
|
| Year ended December 31, 2020 |
| ||||||||||
REVENUE |
| $ | 1,515,673 |
|
| $ | 11,846,894 |
|
| $ | 134,095 |
|
| $ | 11,256,345 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (1,126,059 | ) |
|
| (10,042,688 | ) |
|
| (113,042 | ) |
|
| (9,557,576 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 389,614 |
|
|
| 1,804,206 |
|
|
| 21,053 |
|
|
| 1,698,769 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 7,467 |
|
|
| 59,024 |
|
|
| 13,148 |
|
|
| 873,793 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (86,973 | ) |
|
| (989,617 | ) |
|
| (88,789 | ) |
|
| (1,107,002 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (4,158 | ) |
|
| (8,815 | ) |
|
| (2,482 | ) |
|
| (8,831 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFIT/(LOSS) BEFORE TAX |
|
| 305,950 |
|
|
| 864,798 |
|
|
| (57,070 | ) |
|
| 1,456,729 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense : |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| (11,997 | ) |
|
| (14,739 | ) |
|
| - |
|
|
| 1,238 |
|
Income tax |
|
| (30,482 | ) |
|
| (234,065 | ) |
|
| (881 | ) |
|
| (168,768 | ) |
Total tax expense |
|
| (42,479 | ) |
|
| (248,804 | ) |
|
| (881 | ) |
|
| (167,530 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET PROFIT/(LOSS) |
| $ | 263,471 |
|
| $ | 615,994 |
|
| $ | (57,951 | ) |
| $ | 1,289,199 |
|
Revenue. For the annual period ended December 31, 2021, Chemrex contributed $11,846,894 (88.66%) of total combined revenue of $13,362,567 compared to its contribution of $11,256,345 (98.82%) of total combined revenue of $11,390,440 for the same period last year, an increase of 17.31% on the total combined revenues from both subsidiaries. The increase was due the material selling prices have been increased by Chemrex and the government outsourcing Covid’ qPCR screening to Bionexus Malaysia in 2021.
BioNexus had a revenue of $1,515,673 (11.34%) for the current year compared to revenues of $134,095 (1.18%) from the last year, an increase of 1,030.30%. BioNexus revenues for the current year resulted from the outsource of Covid19 PCR test from Health Ministry which began on May 2021 for contract value of $549,133 which had been completed in July 2021 and an additional contract in July, 2021 for contract value of $859,804 which had been completed as of Dec 31, 2021
Cost of revenues. For the annual period ended December 31, 2021, Chemrex had incurred $10,042,688 (89.92%) of the total combined cost of revenue of $11,168,747 as compared to the same period last year wherein Chemrex had incurred $9,557,576 (98.83%) of the total combined cost of revenue of $9,670,617. The increase of 5.08% in Chemrex’s cost of revenues for the current year was due to its increased revenues for the current period and reasons as stated above.
BioNexus had incurred $1,126,059 (10.08%) on cost of revenues for the current year as compared to cost of revenues of $113,042 (1.17%) for the same period last year. The increase of 896% in cost of revenue for the current period reflects the costs associated with the outsource of Covid19 PCR test from the Health Ministry which began in May 2021 till December 2021.
| 30 |
| Table of Contents |
Other Income. For the annual period ended December 31, 2021, Chemrex contributed $59,024 (88.77%) of total other combined income of $66,491 as compared to the last year period $873,793 (98.52%). A reduction of 93.25% from the prior year is due principally the net income of $707,618 from disposal of a property which occurred during financial year 2020. We did not have a similar gain during the current year.
BioNexus had other income of $7,467 (11.23%) for current year as compared $13,148 (1.48%) for the last year, a decrease of 43.21% for current year due to reduced bank interest rate.
Operating Expenses. For the annual period ended December 31, 2021, Chemrex had incurred $989,617 (91.92%) of the total combined operating expenses of $1,076,590 for the current annual period in 2021 as compared to the operating expenses of $1,107,002 (92.57%) for the same period last year. The decrease of 10.60% in Chemrex operating expenses for the current period due to major expenses reduced were the commission payout to property agent and website development fee which had been completed
BioNexus Malaysia had incurred $86,973 (8.08%) on operating expenses during for the current annual period in 2021 as compared to the operating expenses of $88,789 (7.43%) for the same period last year, a decrease of 2.05% for current year. Due to a $9,890 write-off on an investment in Genenews Diagnostics Sdn. Bhd,
Profit from Operations. Chemrex had a profit from operations of $864,798 (73.87%) during the current annual period in 2021 compared to a profit from operations of $1,456,729 while Bionexus Malaysia incurred a loss of $57,070 for the same period in 2020, for the reasons discussed above. In addition, during last year 2020 period, Chemrex had one-time property disposal gain of $742,487.
Income tax expense. Chemrex had tax provision of $234,065 and deferred tax of $14,739, total tax expense of $248,804 (85.42%) for current year ended December 31, 2021 as compared to total tax expense of $167,530 for the last year ended December 31, 2020 from deferred tax credit of $1,238 and tax provision of $168,768.
BioNexus Malaysia had tax provision of $30,482 and deferred tax of $11,997, total tax expense of $42,479 (14.58%) for current year 2021 as compared with $881 for the last year ended December 31, 2020.
| 31 |
| Table of Contents |
LIQUIDITY AND CAPITAL RESOURCES
As of December 31, 2021, we had working capital of $4,821,100 compared with working capital of $4,611,896 as of December 31, 2020. The increased in working capital as of December 31, 2021 from December 31, 2020 is due principally to the operating profit the Company experienced for the current year end period.
Our primary uses of cash have been for operations. The main sources of cash have been from operational revenues and the private placement of our common stock. The following trends are reasonably likely to result in a material decrease in our liquidity over the near to long term:
· | Addition of administrative and marketing personnel as the business grows, |
· | Development of a Company website, |
· | Increases in advertising and marketing in order to attempt to generate more revenues, and |
· | The cost of being a public company. |
The Company believes that cash flow from operations together will be sufficient to sustain its current level of operations for at least the next 12 months of operations.
The following is a summary of the Company’s cash flows provided by (used in) / generated from operating, investing, and financing activities for the year ended December 31, 2021 and 2020:
|
| Year ended |
| |||||
|
| December 31, |
| |||||
|
| 2021 |
|
| 2020 |
| ||
Net Cash generated from Operating Activities |
| $ | 9,161 |
|
| $ | 552,680 |
|
Net cash (used in) /generated from investing activities |
|
| (490,574 | ) |
|
| 1,319,990 |
|
Net cash used in generated from financing activities |
|
| (28,222 | ) |
|
| (4,947 | ) |
Foreign currency translation adjustment |
|
| (154,138 | ) |
|
| 60,893 |
|
Net Change in Cash and Cash Equivalents |
| $ | (663,773 | ) |
| $ | 1,928,616 |
|
Operating Activities
During the year ended December 31, 2021, the Company incurred a net profit of 751,571 which, after adjusting for amortization, depreciation, dividend income, fair value on investment, an increase in inventories, trade receivables and deposits, a substantial reduction in trade payables, operating lease liabilities, resulted in net cash of $9,161 being generated from operating activities during the period. By comparison, during the year ended December 31, 2020, the Company had a net profit of $1,094,098 which, after adjusting for amortization, depreciation, dividend income, gain on disposal of property, plant and equipment $701,447, a decrease in inventories, a reduce in receivables and deposits, a substantial reduction in trade payables, operating lease liabilities, resulted in net cash of $552,680 being generated from operating activities during the period.
Investing Activities
During the year ended December 31, 2021, the Company had net cash of $490,574 used in investment activities. During the year ended December 31, 2020, the Company had net cash from acquisition of business under common control and disposed of property, plant and equipment of $1,467,865, resulting in net cash generated from investment activities of $1,319,990
Financing Activities
During the year ended December 31, 2021, Company continued the repayment of a finance lease resulting in net cash used in financing activities of $28,222. By comparison, during the year ended December 31, 2020, we had financing activities of $4,947 due to 6 months moratorium on leasing and loan instalments as initiated by the Malaysian Prime Minister in order to lessen the hardship faced by individual borrowers and Small and Medium Enterprises to enjoy an automatic monthly loan instalments moratorium for year, starting from April 1, 2020 till September 30, 2020
| 32 |
| Table of Contents |
Summary of Significant Accounting Policies.
The accompanying consolidated financial statements reflect the application of certain significant accounting policies as described in this note and elsewhere in the accompanying consolidated financial statements and notes.
• | Basis of presentation |
These accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”).
• | Basis of consolidation |
The consolidated financial statements include the accounts of Bionexus Gene Lab Corp. and its subsidiaries. All significant inter-company balances and transactions within the Company have been eliminated upon consolidation.
• | Use of estimates |
In preparing these financial statements, management makes estimates and assumptions that affect the reported amounts of assets and liabilities in the balance sheets and revenues and expenses during the years reported. Actual results may differ from these estimates.
• | Cash and cash equivalents |
Cash and cash equivalents represent cash on hand, demand deposits placed with banks or other financial institutions and all highly liquid investments with an original maturity of three months or less as of the purchase date of such investments.
| 33 |
| Table of Contents |
• | Trade receivables |
Trade receivables are recorded at the invoiced amount and do not bear interest. Management reviews the adequacy of the allowance for doubtful accounts on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to make adjustments in the allowance when it is considered necessary. Trade balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
• | Inventories |
Inventories consisting of products available for sell, are stated at the lower of cost or market value. Cost of inventory is determined using the first-in, first-out (FIFO) method. Inventory reserve is recorded to write down the cost of inventory to the estimated market value due to slow-moving merchandise and damaged goods, which is dependent upon factors such as historical and forecasted consumer demand, and promotional environment. The Company takes ownership, risks and rewards of the products purchased. Write downs are recorded in cost of revenues in the Condensed Statements of Operations and Comprehensive Income.
• | Leases |
In February 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2016-02, Leases, which was subsequently amended in 2018 by ASU 2018-10, ASU 2018-11 and ASU 2018-20 (collectively, Topic 842). Topic 842 will require the recognition of a right-of-use asset and a corresponding lease liability, initially measured at the present value of the lease payments, for all leases with terms longer than 12 months. For operating leases, the asset and liability will be expensed over the lease term on a straight-line basis, with all cash flows included in the operating section of the statement of cash flows. For finance leases, interest on the lease liability will be recognized separately from the amortization of the right-of-use asset in the statement of comprehensive income and the repayment of the principal portion of the lease liability will be classified as a financing activity while the interest component will be included in the operating section of the statement of cash flows. Topic 842 is effective for annual and interim reporting periods beginning after December 15, 2018. Early adoption is permitted. Upon adoption, leases will be recognized and measured at the beginning of the earliest period presented using a modified retrospective approach. Topic 842 allows for a cumulative-effect adjustment in the period the new lease standard is adopted and will not require restatement of prior periods.
Prior to January 1, 2019, the Company accounted for leases under ASC 840, Accounting for Leases. Effective January 1,2019, the Company adopted the guidance of ASC 842, Leases, which requires an entity to recognize a right-of-use asset and a lease liability for virtually all leases. The Company adopted ASC 842 using a modified retrospective approach. As a result, the comparative financial information has not been updated and the required disclosures prior to the date of adoption have not been updated and continue to be reported under the accounting standards in effect for those periods.
| 34 |
| Table of Contents |
• | Property, Plant and equipment |
Property, plant and equipment are stated at cost less accumulated depreciation and accumulated impairment losses, if any. Depreciation is calculated on the straight-line basis to write off the cost over the following expected useful lives of the assets concerned. The principal annual rates used are as follows:
Categories |
| Principal Annual Rates/Expected Useful Life | |
Air conditioner |
|
| 20% |
Buildings |
|
| 2% |
Computer and software |
|
| 33% |
Equipment |
|
| 20% |
Furniture and fittings |
| 10% to 20% | |
Lab Equipment |
|
| 10% |
Motor vehicle |
| 10% to 20% | |
Office equipment |
|
| 20% |
Renovation |
| 10% to 20% | |
Signboard |
|
| 10% |
Leasehold lands are depreciated over the period of lease term. Leased assets are depreciated over the shorter of the lease term and their useful lives unless it is reasonably certain that the Company will obtain ownership by the end of the lease term. Freehold land is not depreciated. Property, plant and equipment under construction are not depreciated until the assets are ready for their intended use
Maintenance and repairs are charged to operations as incurred. Expenditures which substantially increase the useful lives of the related assets are capitalized. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place.
• | Impairment of long-lived assets |
Long-lived assets primarily include goodwill, intangible assets and property, plant and equipment. In accordance with the provision of ASC Topic 360, “Impairment or Disposal of Long-Lived Assets”, the Company generally conducts its annual impairment evaluation to its long-lived assets, usually in the fourth quarter of each fiscal year, or more frequently if indicators of impairment exist, such as a significant sustained change in the business climate. The recoverability of long-lived assets is measured at the lowest level group. If the total of the expected undiscounted future net cash flows is less than the carrying amount of the asset, a loss is recognized for the difference between the fair value and carrying amount of the asset. There has been no impairment charge for the years presented.
| 35 |
| Table of Contents |
• | Finance lease |
Leases that transfer substantially all the rewards and risks of ownership to the lessee, other than legal title, are accounted for as finance leases. Substantially all of the risks or benefits of ownership are deemed to have been transferred if any one of the four criteria is met: (i) transfer of ownership to the lessee at the end of the lease term, (ii) the lease containing a bargain purchase option, (iii) the lease term exceeding 75% of the estimated economic life of the leased asset, (iv) the present value of the minimum lease payments exceeding 90% of the fair value. At the inception of a finance lease, the Company as the lessee records an asset and an obligation at an amount equal to the present value of the minimum lease payments. The leased asset is amortized over the shorter of the lease term or its estimated useful life if title does not transfer to the Company, while the leased asset is depreciated in accordance with the Company’s depreciation policy if the title is to eventually transfer to the Company. The periodic rent payments made during the lease term are allocated between a reduction in the obligation and interest element using the effective interest method in accordance with the provisions of ASC Topic 835-30, “Imputation of Interest”.
• | Revenue recognition |
Revenue recognized when it is probable that the economic benefits associated with the transaction will flow to the enterprise and the amount of the revenue can be measured reliably. Revenue is measured at the fair value of consideration received or receivable.
a. The Company follows the guidance of Accounting Standards Codification (ASC) 606, Revenue from Contracts, ASC 606 creates a five-step model that requires entities to exercise judgment when considering the terms of contracts, which includes
i. Identifying the contracts or agreements with a customer,
ii. Identifying our performance obligations in the contract or agreement,
iii. Determining the transaction price,
iv. Allocating the transaction price to the separate performance obligations, and
v. Recognizing revenue as each performance obligation is satisfied.
The Company only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the services it transfers to its clients.
b. Interest income
Interest is recognized on receipt basis.
• | Cost of revenues |
Cost of revenue includes the purchase cost of retail goods for re-sale to customers and packing materials (such as boxes). It excludes purchasing and receiving costs, inspection costs, warehousing costs, internal transfer costs and other costs of distribution network in cost of revenues.
| 36 |
| Table of Contents |
• | Shipping and handling fees |
Shipping and handling fees, if billed to customers, are included in revenue. Shipping ang handling fees associated with inbound and outbound freight are expensed as incurred and included in selling and distribution expenses.
• | Comprehensive income |
ASC Topic 220, “Comprehensive Income” establishes standards for reporting and display of comprehensive income, its components and accumulated balances. Comprehensive income as defined includes all changes in equity during a period from non-owner sources. Accumulated other comprehensive income, as presented in the accompanying statements of stockholders’ equity consists of changes in unrealized gains and losses on foreign currency translation and cumulative net change in the fair value of available-for-sale investments held at the balance sheet date. This comprehensive income is not included in the computation of income tax expense or benefit.
• | Income taxes |
Income taxes are determined in accordance with the provisions of ASC Topic 740, “Income Taxes” (“ASC Topic 740”). Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Any effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
ASC 740 prescribes a comprehensive model for how companies should recognize, measure, present, and disclosed in their financial statements uncertain tax positions taken or expected to be taken on a tax return. Under ASC 740, tax positions must initially be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. Such tax positions must initially and subsequently be measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority assuming full knowledge of the position and relevant facts.
The Company conducts major businesses in Malaysia and is subject to tax in their own jurisdictions. As a result of its business activities, the Company will file separate tax returns that are subject to examination by the foreign tax authorities.
• | Net loss per share |
The Company calculates net loss per share in accordance with ASC Topic 260 “Earnings per share”. Basic loss per share is computed by dividing the net loss by the weighted average number of common shares outstanding during the period. Diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common stock equivalents had been issued and if the additional common shares were dilutive.
• | Foreign currencies translation |
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the statement of operations.
The functional currency of the Company is the United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$. In addition, the Company maintains its books and record in a local currency, Malaysian Ringgit (“MYR” or “RM”), which is functional currency as being the primary currency of the economic environment in which the entity operates.
| 37 |
| Table of Contents |
In general, for consolidation purposes, assets and liabilities of its subsidiaries whose functional currency is not US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of foreign subsidiary are recorded as a separate component of accumulated other comprehensive income.
Translation of amounts from the local currency of the Company into US$1 has been made at the following exchange rates for the respective years:
|
| As of and for the year ended December 31, |
| |||||
|
| 2021 |
|
| 2020 |
| ||
Year-end MYR: US$1 exchange rate |
|
| 4.1650 |
|
|
| 4.0170 |
|
Yearly average MYR: US$1 exchange rate |
|
| 4.1456 |
|
|
| 4.2010 |
|
• | Related parties |
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
• | Fair value of financial instruments |
The carrying value of the Company’s financial instruments: cash and cash equivalents, trade receivable, deposits and other receivables, amount due to related parties and other payables approximate at their fair values because of the short-term nature of these financial instruments.
The Company also follows the guidance of the ASC Topic 820-10, “Fair Value Measurements and Disclosures” (“ASC 820-10”), with respect to financial assets and liabilities that are measured at fair value. ASC 820-10 establishes a three-tier fair value hierarchy that prioritizes the inputs used in measuring fair value as follows:
• | Level 1: Observable inputs such as quoted prices in active markets; |
• | Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
• | Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions |
As of December 31, 2021, and December 31, 2020, the Company did not have any non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements, at least annually, on a recurring basis, nor did the Company have any assets or liabilities measured at fair value on a non-recurring basis.
• | Recent accounting pronouncements |
The Company has reviewed all recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements may be expected to cause a material impact on its financial condition or the results of its operations.
| 38 |
| Table of Contents |
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable to smaller reporting companies.
Item 8. Financial Statements and Supplementary Data.
Our financial statements are contained in pages F-1 through F-19, which appear at the end of this Form 10-K Annual Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
In connection with the preparation of this annual report, an evaluation was carried out by the Company’s management, with the participation of the principal executive officer and the principal financial officer, of the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act (“Exchange Act”) as of December 31, 2021. Disclosure controls and procedures are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the Commission’s rules and forms, and that such information is accumulated and communicated to management, including the principal executive officer and the principal financial officer, to allow timely decisions regarding required disclosures.
Based on that evaluation, the Company’s management concluded, as of the end of the period covered by this report, that the Company’s disclosure controls and procedures were not effective in recording, processing, summarizing, and reporting information required to be disclosed, within the time periods specified in the Commission’s rules and forms, and that such information was not accumulated and communicated to management, including the principal executive officer and the principal financial officer, to allow timely decisions regarding required disclosures.
Management’s Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control over financial reporting is a process, under the supervision of the principal executive officer and the principal financial officer, designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company’s financial statements for external purposes in accordance with United States generally accepted accounting principles (GAAP). Internal control over financial reporting includes those policies and procedures that:
● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the Company’s assets; |
● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and the board of directors; and |
● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
| 39 |
| Table of Contents |
The Company’s management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013) as set forth in its Internal Control - Integrated Framework. This assessment identified material weaknesses in internal control over financial reporting. A material weakness is a control deficiency, or a combination of deficiencies in internal control over financial reporting that creates a reasonable possibility that a material misstatement in annual or interim financial statements will not be prevented or detected on a timely basis. Since the assessment of the effectiveness of our internal control over financial reporting did identify a material weakness, management considers its internal control over financial reporting to be ineffective.
Management has concluded that our internal control over financial reporting had the following material deficiencies:
| ● | We were unable to maintain segregation of duties within our business operations due to our reliance on a single individual fulfilling the role of sole officer and director. |
|
|
|
| ● | Lack of a functioning audit committee due to a lack of a majority of independent members and a lack of a majority of outside directors on our Board of Directors, resulting in ineffective oversight in the establishment and monitoring of required internal control and procedures. |
These control deficiencies to our 2020 or 2019 interim or annual financial statements could have resulted in a material misstatement that might have been prevented or detected by a segregation of duties. Accordingly, we have determined that this control deficiency constitutes a material weakness.
To the extent reasonably possible, given our limited resources, our goal is, upon consummation of a merger with a private operating company, to separate the responsibilities of principal executive officer and principal financial officer, intending to rely on two or more individuals. We will also seek to expand our current board of directors to include additional individuals willing to perform directorial functions. Since the recited remedial actions will require that we hire or engage additional personnel, this material weakness may not be overcome in the near term due to our limited financial resources. Until such remedial actions can be realized, we will continue to rely on the advice of outside professionals and consultants.
This annual report does not include an attestation report of our registered public accounting firm regarding our internal controls over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to Section 404(c) of the Sarbanes-Oxley Act that permit us to provide only management’s report in this annual report.
Changes in Internal Controls over Financial Reporting
During the year ended December 31, 2021, other than the change in ownership, there has been no change in internal control over financial reporting that has materially affected or is reasonably likely to materially affect our internal control over financial reporting.
Item 9B. Other Information.
None
| 40 |
| Table of Contents |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table sets forth the name, age, and position of sole executive officers and directors. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
NAME |
| AGE |
| POSITION |
Soo Kow (Kenny) Lai |
| 53 |
| Chairman |
Chi Yuen (George) Leong |
| 70 |
| President and Director |
Chan Chong Wong |
| 59 |
| Chief Executive Officer and Director |
Wei Li Leong |
| 32 |
| Chief Financial Officer |
Liong Tai Tan |
| 63 |
| Chief Operating Officer and Director |
Soo Kow (Kenny) Lai has been our Chairman of the Board since inception. Mr. Lai received his Bachelor of Jurisprudence from University of Malaya and Bachelor of Business Administration, Wharton Management School, Malaya. Mr. Lai had served as Managing Director, CEO for several Malaysian companies for the past 25 years. From 2014 until the present, he has been serving as the Director General of Oversea Investment Union of The Investment Association of China. He was the Operation Director of Tower Regency Hotel from 2010 to 2014. Mr. Lai brings a wide range of business experience to our board of directors.
Chi Yuen (George) Leong has been our President since inception. He was the Chief Executive Officer of Network Food Australia from February 1, 2006 until August 20, 2008. He joined the US’ Arem Pacific Corporation the Chief Executive Officer from March 1, 2009 until Sept 30, 2015. Mr. Leong received a Bachelor of Behavioral Sciences from the University of East Asia, Malaysia. Mr. Leong brings a wide range of business experience, including experience with public companies, to our board of directors.
Chan Chong Wong has been our Chief Executive Officer since inception. From 1990 to the present, Mr. Wong is the Managing Partner for Handy & Trendy Trading in Malaysia. Besides, he started the Modern Mum, a lady fashion retail and wholesale chain stores in Malaysia from 2008 to 2014. The latest venture is with from 2014 until today, he has been serving as the Chief of Staff for the Oversea Investment Union of The Investment Association of China. He received a Bachelor’s degree from University Asia Institute of Management Science and received an Executive Master in Business Administration, Beijing University.
Wei Li Leong has been Chief Financial Officer and Principal Accounting Officer of the Company since inception. Ms. Leong is a Certified Practising Accountant and graduated from RMIT University, Melbourne with a Bachelor’s degree in Business (Accountancy). She interned at Russell Bedford LC & Company as an audit vacation trainee during her summer break in 2009. From September 1, 2010 to February 22, 2012, she worked as trainee and later as accountant with SJ Accounting Services firm in Melbourne, Australia. From March 2013 to February 2015, she was engaged with BDO Malaysia in the tax compliance department and from March 2015 to February 2017, she was employed by Ernst & Young (Malaysia) specializing in international tax. Ms. Leong is currently self employed as a tax consultant. Ms. Leong is the daughter of the Company’s President.
Liong Tai Tan has been the Company’s Chief Operating Officer of the Company since inception. He obtained a diploma in Business Management from Vanto Academy, Malaysia. He has been Chief Operating Officer and Director of the Company since November 27, 2020. Mr. Tan has been the marketing director of our subsidiary Chemrex Corporation Sdn Bhd, since 2006. Mr. Tan was elected to the Company’s Board of Directors due to his significant amount of business experience, particularly in the chemicals industry.
Sook Keng Yeoh has been appointed as an Independent Non-Executive Director and a member of the Audit Committee effective March 7, 2022. Prior to Mr. Yeoh appointment in the Company, he was the Director of ADS Sentral Sdn Bhd. and Chief Financial Officer/Finance Director of TRC Synergy Bhd, from 1999 until 2019. During his tenure with TRC Synergy Bhd, he was also the CEO of TRC Energy Sdn Bhd and Executive Director of PetroBru (B) Sdn Bhd. Mr. Yeoh had been Group Internal Auditor at Lingui Corporation Bhd from 1995 to 1999. Prior to Lingui Corporation Bhd, Mr. Yeoh served at Paramount Corporation Bhd from 1991 to 1995 as an internal auditor and as an Audit Manager at Asia Commercial Finance Bhd from 1988 to 1991. Mr. Yeoh started his career as a Bank Officer (Audit) at Kwong Yik Bank in 1981 before joining Metroplex Bhd as a Chief Internal Auditor in 1985. Mr. Yeoh holds a Bachelor’s of Commerce in Accounting, Finance, and Systems from the University of New South Wales (Australia). Mr. Yeoh is also admitted to the Malaysian Institute of Accountants as a Chartered Accountant.
| 41 |
| Table of Contents |
Teng Fook Fong has been appointed as an Independent Non-Executive Director and a member of the Audit Committee effective March 7, 2022. Mr. Fong is currently the Independent Non-Executive Chairman of MDT Innovations Sdn Bhd, a position he has held since 2018. He has also served as the Non-Executive Director of Sierramas Homeowners Berhad since 2017. From 1985 to 1989, Mr. Fong was a Manager at BCCI Leasing (M) Sdn Bhd. Prior to his time as a manager, Mr. Fong was an international trainee at the Bank of Credit & Commerce International’s regional office in Hong Kong in 1984. Mr. Fong started his career as an accountant at Sherwood Medical Supplies Pty. Ltd from 1982 to 1983. Mr. Fong holds a Bachelor’s of Commerce and a Master of Commerce from the University of New South Wales. He also holds a Bachelor’s of Laws from the University of London. Since 1991, Mr. Fong has been practicing as a member of the Malaysia Bar Council as an Advocate and Solicitor at Yusuf Khan & Fong.
Chee Keong Yap has been appointed as an Independent Non-Executive Director and a member of the Audit Committee effective March 7, 2022. Mr. Yap age 66 obtained a Bachelor of Arts (First Class Honours) degree in Economics from the University of Leeds, England in 1978. Member of the Institute of Chartered Accountants of Scotland (“ICAS”), UK in 1981. Upon his graduation, he was articled to an international firm of chartered accountants now known as KPMG Peat Marwick in London, UK. He has now been a member of ICAS for over 40 years.
He joined Bumiputra Merchant Bankers Berhad (BMB) in 1981 as corporate finance officer and was promoted to Head of Corporate Finance in 1987 and the Executive Director/Chief Executive Officer of BMB in July 1992. He resigned from BMB in September 1997.
Mr. Yap has served as a director, both executive and non-executive roles in 9 public listed companies in Malaysia. In addition, he has served as confidential financial adviser to numerous public listed companies. During his sixteen (16) years distinguished career with BMB, Mr. Yap had advised on many significant corporate transactions. At BMB, Mr. Yap has also successfully raised multimillion Ringgit financial facilities for clients in all the industries. Mr. Yap has also been involved in over 22 successful public listings.
Board Committees
We plan to establish three committees under the board of directors: an audit committee, a compensation committee and a nominating committee. We plan to adopt a charter for each of the three committees.
Audit Committee
Our Audit Committee is currently composed of three members: Sook Keng Yeoh, Teng Fook Fong and Chee Keong Yap. Our Board of Directors determined that each member of the Audit Committee meets the independence criteria prescribed by applicable regulation and the rules of the SEC for Audit Committee membership and is an “independent” director within the meaning of the NASDAQ Marketplace Rules. Each Audit Committee member also meets NASDAQ’s financial literacy requirements. Mr. Sook Keng Yeoh currently serves as Chairman of the Audit Committee.
Our Audit Committee oversees our accounting and financial reporting processes and the audits of our financial statements. Our Audit Committee is responsible for, among other things:
| · | selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors; |
|
|
|
| · | reviewing with our independent auditors any audit problems or difficulties and management’s response; |
|
|
|
| · | reviewing and approving all proposed related-party transactions; |
|
|
|
| · | discussing the annual audited financial statements with management and our independent auditors; |
|
|
|
| · | reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of significant internal control deficiencies; |
|
|
|
| · | annually reviewing and reassessing the adequacy of our Audit Committee charter; |
|
|
|
| · | meeting separately and periodically with management and our internal and independent auditors; |
|
|
|
| · | reporting regularly to the full Board of Directors; and |
|
|
|
| · | such other matters that are specifically delegated to our Audit Committee by our Board of Directors from time to time. |
Our Board of Directors has determined that Mr. Sook Keng Yeoh is the “Audit Committee Financial Expert” as such term is defined in Item 407(d) of Regulation S-K promulgated by the SEC and also meets NASDAQ’s financial sophistication requirements.
| 42 |
| Table of Contents |
Dr. Liew began his research in 1962 while doing his Doctorate study supervised by Dr. Best in Banting and Best Institute, Toronto (Insulin was discovered by Banting and Best, the key to preventing diabetes and controlling normal metabolism. In 1923 Nobel Prize was awarded for one of the most important, and most controversial, breakthroughs in modern medical history).
Professor Liew is a pioneer in the emerging field of molecular medicine and globally recognized as a leader in disease-specific genomics research. He has received some 14 Honorary Professorships in universities including The Chinese University of Hong Kong, and Peking University, Beijing. In 2002 he won the Society of Chinese Bio scientists in America (SCBA) Distinguished Scientist Award (Ontario Chapter) and the Makoto Nagano Award for Achievements in Cardiovascular Education, and in 2005 the Nanyang Distinguished Alumni Award.
To date, he has published more than 300 original scientific papers, abstracts, and monographs. His 1997 landmark publication in Circulation, a pre-eminent U.S. peer reviewed journal, reported his work in cardiovascular genomics. This report is widely acknowledged to represent the most comprehensive analysis of genes expressed in a single human organ. More recently Professor Liew and Dr Victor Dzau, Chancellor for Health Affairs of Duke University, published “Molecular Genetics and Genomics of Heart Failure in Nature Reviews Genetics (2004) and co-edited Cardiovascular Genetics and Genomics for the Cardiologist, published in July 2007 by Blackwell’s, Oxford.
Family Relationships
Except as stated herein above, there are no family relationships among our directors or officers.
Involvement in Legal Proceedings
To the best of our knowledge, none of our directors or executive officers, during the past ten years, has been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past five years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the Securities and Exchange Commission.
Director Independence
Our Board of Directors is currently composed of three members, whom do not qualify as an independent director in accordance with the published listing requirements of the NASDAQ Global Market (the Company has no plans to list on the NASDAQ Global Market). The NASDAQ independence definition includes a series of objective tests, such as that the directors are not, and have not been for at least three years, one of our employees and that neither the Director, nor any of their family members have engaged in various types of business dealings with us. In addition, our board of directors has not made a subjective determination as to our director that no relationship exist which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, though such subjective determination is required by the NASDAQ rules. Had our board of directors made these determinations, our board of directors would have reviewed and discussed information provided by our director and us with regard to our director’s business and personal activities and relationships as they may relate to us and our management.
Code of Ethics
We currently do not have a code of ethics that applies to our officers, employees and directors, including our Chief Executive Officer and Chief Financial Officer; however, we intend to adopt one in the near future.
| 43 |
| Table of Contents |
Conflicts of Interest
Since we do not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees are performed by our directors. The Board of Directors has not established an audit committee and does not have an audit committee financial expert, nor has the Board established a nominating committee. The Board is of the opinion that such committees are not necessary since the Company is an early-stage company, and to date, such directors have been performing the functions of such committees. Thus, there is a potential conflict of interest in that our Directors and Officers have the authority to determine issues concerning management compensation, nominations, and audit issues that may affect management decisions.
In addition, our Officers have committed to spend a sufficient amount of time and attention to the affairs of the Company to fulfill their respective officer responsibilities. In this regard, generally, each Officer spends between 15 to 40 hours per week on the affairs of the Company, depending on the circumstances. Therefore, we may face conflicts of interest between the time and attention each Officer devotes to the Company and that of their other business interests.
Other than as described above, we are not aware of any other conflicts of interest of our executive Officers and Directors.
Involvement in Certain Legal Proceedings
There are no legal proceedings that have occurred since our incorporation concerning our Director, or control persons which involved a criminal conviction, a criminal proceeding, an administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
Item 11. Executive Compensation.
Summary Executive Compensation Table
The following table reflects the Summary Compensation for our named executive officers for fiscal years ended December 31, 2021, 2020 and 2018, respectively. For such periods, there were no bonus, non-equity plan compensation, nonqualified compensation earnings or other compensation other than as stated below for the named executive officers. Further, we have not entered into an employment agreement with any of our officers, directors or any other persons and no such agreements are anticipated in the immediate future.
| 44 |
| Table of Contents |
|
|
|
|
| Stock |
|
| Other |
|
|
| |||||||
|
|
|
|
| Award |
|
| Compensation |
|
| Total |
| ||||||
Name and Position |
| Year |
| Salary |
|
| $ |
|
| $ |
|
| $ |
| ||||
Soo Kow Lai |
| 2019 |
|
| 0 |
|
| $ | 5,000 | (2) |
|
| 0 |
|
| $ | 5,000 |
|
Chairman |
| 2020 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 2021 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chan Chong Wong |
| 2019 |
|
| 0 |
|
| $ | 5,000 | (2) |
|
| 0 |
|
| $ | 5,000 |
|
Chief (Principal) |
| 2020 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Executive Officer |
| 2021 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chan Chong Wong |
| 2019 |
|
| 0 |
|
| $ | 5,000 | (2) |
|
| 0 |
|
| $ | 5,000 |
|
Chief (Principal) |
| 2020 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Executive Officer |
| 2021 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wei Li Leong |
| 2019 |
|
| 0 |
|
| $ | 2,000 | (2) |
|
| 0 |
|
| $ | 2,000 |
|
Chief (Principal) |
| 2020 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Financial Officer |
| 2021 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liong Tai Tan |
| 2019 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Chief |
| 2020 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Operating Officer |
| 2021 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
(1). On October 10, 2017, the named officer received 1,000,000 shares of common stock of the Company. These shares were valued at $1,000. Ms. Leong received 1,000,000 shares of common stock in connection with her appointment as Chief Financial Officer of the Company.
(2). On October 1, 2020, the Company and each officer set forth below entered into a Stock Grant Agreement pursuant to which the Company made the following common stock grants to the respective officer; Soo Kow Lai-5,000,000 shares, Chi Yuen Leong-5,000,000 shares, Chan Chong Wong-5,000,000 shares and Wei Li Leong-2,000,000. The shares were allocated at $0.001 per share each.
Employment Agreements
The Company does not have any employment or other compensation agreement with its executive officers. Moreover, there are no agreements or understandings for any of our executive officers or directors to resign at the request of another person and no officer or director is acting on behalf of nor will any of them act at the direction of any other person.
Grants of Plan-Based Awards
Except as stated above, no plan-based awards were granted to any of our named executive officers during the interim fiscal year ended December 31, 2021.
| 45 |
| Table of Contents |
Outstanding Equity Awards at Interim Fiscal Year End
The equity awards reflected in the Summary Compensation Table above represents all restricted stock awards issued to our executive officers at December 31, 2021. No other stock or stock option awards were granted to any other officer of the Company as at December 31, 2021.
Option Exercises and Stock Vested
No option to purchase our capital stock was exercised by any of our named executive officers, nor was any restricted stock held by such executive officers vested during the interim fiscal period ended December 31, 2021.
Pension Benefits
No named executive officers received or held pension benefits during the interim fiscal period ended December 31, 2021.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information, as of the date hereof, with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five percent (5%); (ii) each of our executive officers and directors; and (iii) our directors and executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned. The information is based on 171,218,152 shares of common stock issued and outstanding as of this March 30, 2022.
Name and Address of |
| Amount and Nature of |
|
|
|
| ||
Beneficial Owner |
| Beneficial Ownership (1) |
|
| Percent of Class |
| ||
Officers and Directors |
| |||||||
Soo Kow (Kenny) Lai(2) Chairman of Board |
|
| 15,000,000 |
|
|
| 8.76 | % |
Chi Yuen (George) Leong (2) President and Director |
|
| 14,000,000 |
|
|
| 8.18 | % |
Chan Chong Wong(2) Chief Executive Officer and Director |
|
| 12,998,329 |
|
|
| 7.59 | % |
Wei Li Leong (2) Chief Financial Officer |
|
| 4,797,709 |
|
|
| 2.80 | % |
Liong Tai Tan (2) Chief Operating Officer |
|
| 12,500,460 |
|
|
| 7.30 | % |
All officers and directors as a group (5 persons) |
|
| 59,882,836 |
|
|
| 34.97 | % |
5% or greater shareholders | ||||||||
Dr. Choong-Chin Liew (3) |
|
| 20,000,000 |
|
|
| 11.68 | % |
|
|
|
|
|
|
|
|
|
Tan Kuan Yew (Hing Kuan Yew) (4) |
|
| 8,830,917 |
|
| 5.1%6 |
| |
Tham Too Kam (5) |
|
| 12,210,460 |
|
|
| 7.13 | % |
Wong Kim Hai (6) |
|
| 12,510,460 |
|
|
| 7.31 | % |
———————
(1). Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has ownership of and voting power and investment power with respect to our Common stock or Preferred Shares. For each beneficial owner above, any options exercisable within 60 days have been included in the denominator.
(2). The address of each shareholder is the address of the Company.
(3). The address of the shareholder is 81 Millersgrove Dr., Toronto, Canada M2R 3S1.
(4). The address of the shareholder is 32 Jalan Putra Mahkota 7/2H, Putra Heights Subang Jaya, Selangor Malaysia 47650.
(5). The address of the shareholder is 12A Jalan Sl 15/1 Bandar Sungai Long Kajang, Selangor Malaysia 43000.
(6). The address of the Shareholder is 24 Jalan Molek 3/8, Taman Molek Johor Baru, Johore Malaysia 81100.
| 46 |
| Table of Contents |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
In connection with our acquisition of Chemrex from the Chemrex shareholders, Liong Tai Tan, our Chief Operating Officer (appointed on November 27, 2020), was a Chemrex shareholder and received 14,553,543 shares of common stock in connection with the transaction.
Other than as stated herein, there have been no related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
Item 14. Principal Accountant Fees and Services.
JP Centurion & Partner PLT is the Company’s current independent registered public accounting firm.
(1) Audit Fees
The aggregate fees billed for each of the last two fiscal years for professional services rendered by the principal accountant for our audit of annual financial statements and review of financial statements included in our quarterly reports or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for those fiscal years were:
2021 |
| $ | 31,530 |
|
2020 |
| $ | 25,429 |
|
(2) Audit-Related Fees
The aggregate fees billed in each of the last two fiscal years for assurance and related services by the principal accountants that are reasonably related to the performance of the audit or review of our financial statements and are not reported in the preceding paragraph:
2021 |
| $ | 934 |
|
2020 |
| $ | 592 |
|
(3) Tax Fees
The aggregate fees billed in each of the last two fiscal years for professional services rendered by the principal accountant for tax compliance, tax advice, and tax planning were:
2021 |
| $ | 209 |
|
2020 |
| $ | 2,075 |
|
(4) All Other Fees
The aggregate fees billed in each of the last two fiscal years for the products and services provided by the principal accountant, other than the services reported in paragraphs (1), (2), and (3) were:
2021 |
| $ | 10,400 |
|
2020 |
| $ | 0 |
|
The percentage of hours expended on the principal accountant’s engagement to audit our financial statements for the most recent fiscal year that were attributed to work performed by persons other than the principal accountant’s full time, permanent employees was 0%.
Audit Committee’s Pre-Approval Process
The Audit Committee of the Company, and accordingly, all services are approved by all the members of the Committee
| 47 |
| Table of Contents |
PART IV.
Item 15. Exhibits, Financial Statement Schedules.
EXHIBIT INDEX
Exhibit |
| Description |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
101.INS |
| XBRL INSTANCE DOCUMENT* |
101.SCH |
| XBRL TAXONOMY EXTENSION SCHEMA DOCUMENT* |
101.CAL |
| XBRL TAXONOMY CALCULATION LINKBASE DOCUMENT* |
101.DEF |
| XBRL TAXONOMY DEFINITION LINKBASE DOCUMENT* |
101.LAB |
| XBRL TAXONOMY LABEL LINKBASE DOCUMENT* |
101.PRE |
| XBRL TAXONOMY PRESENTATION LINKBASE DOCUMENT* |
___________
(1) Previously filed as an exhibit to the Company’s Form S-1 Registration Statement filed on January 29, 2020.
(2) Previously filed as an exhibit to the Company’s Form 8-K filed on January 7, 2021.
(3) Previously filed as an exhibit to the Company’s Form 8-K filed on October 1, 2020.
* Filed herewith
+ In accordance with SEC Release 33-8238, Exhibits 32.1 is being furnished and not filed.
| 48 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BioNexus Gene Lab Corporation |
|
|
|
|
|
(Registrant) |
|
|
|
|
|
/s/ Chan Chong Wong |
| Dated: March 31, 2022 |
Chan Chong Wong |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
/s/ Chan Chong Wong |
| Dated: March 31, 2022 |
Chan Chong Wong |
|
|
Chief Executive Officer and Director |
|
|
(Principal Executive Officer) |
|
|
/s/ Wei Li Leong |
| Dated: March 31, 2022 |
Wei Li Leong |
|
|
Chief Financial Officer |
|
|
(Principal Financial and Accounting Officer) |
|
|
/s/ Soo Kow (Kenny) Lai |
| Dated: March 31, 2022 |
Soo Kow (Kenny) Lai |
|
|
Chairman |
|
|
|
|
|
/s/ Chi Yuen (George) Leong |
| Dated: March 31, 2022 |
Chi Yuen (George) Leong |
|
|
President and Director |
|
|
/s/ Liong Tai Tan |
| Dated: March 31, 2022 |
Liong Tai Tan |
|
|
Chief Operating |
|
|
Officer and Director | ||
| 49 |
| Table of Contents |
TABLE OF CONTENTS
|
| Page |
| ||
|
|
|
F-2 | ||
| F-2 | |
| Consolidated Balance Sheets as of December 31, 2021 and 2020 | F-3 - F-4 |
| F-5 | |
| F-6 | |
| Consolidated Statement of Cash Flows for the Year Ended December 31, 2021 and 2020 | F-7 - F-8 |
| F-9 - F-22 |
| F-1 |
PART I — FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
BioNexus Gene Lab Corp.
Unit 02 Level 10, Tower B, Avenue 3,
Vertical Business Suite,
No. 8, Jalan Kerinchi, Bangsar South,
59200 Kuala Lumpur, Malaysia
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioNexus Gene Lab Corp. (the ‘Company’) as of December 31, 2021 and 2020, and the related consolidated statements of operations and comprehensive income, stockholders’ equity, and cash flows for the each of two years in the year ended of December 31, 2021 and 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of two years in the year ended December 31, 2021 and 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to those charged with governance that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgements. We determined that there are no critical matters.
/s/ JP CENTURION & PARTNERS PLT |
|
We have served as the Company’s auditor since 2020. | |
| |
JP CENTURION & PARTNERS PLT (PCAOB: 6723) |
|
Kuala Lumpur, Malaysia |
|
March 31, 2022 |
|
| F-2 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
|
|
| As of |
| |||||||
|
| Note |
|
| December 31, 2021 |
|
| December 31, 2020 |
| |||
ASSETS |
|
|
|
|
|
|
|
|
| |||
CURRENT ASSETS |
|
|
|
|
|
|
|
|
| |||
Cash and bank balances |
|
|
|
| $ | 578,511 |
|
| $ | 699,585 |
| |
Fixed deposits placed with financial institutions |
|
|
|
|
| 1,545,408 |
|
|
| 2,088,107 |
| |
Trade receivables |
|
| 4 |
|
|
| 3,356,898 |
|
|
| 3,996,802 |
|
Other receivables, deposits and prepayments |
|
|
|
|
|
| 79,517 |
|
|
| 22,640 |
|
Deferred cost of revenue |
|
|
|
|
|
| 67,606 |
|
|
| - |
|
Tax recoverable |
|
| 5 |
|
|
| - |
|
|
| 2,190 |
|
Inventories |
|
|
|
|
|
| 1,521,915 |
|
|
| 1,176,170 |
|
Total current assets |
|
|
|
|
|
| 7,149,855 |
|
|
| 7,985,494 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease right of use assets |
|
| 6 |
|
|
| 41,090 |
|
|
| 62,529 |
|
Property, plant and equipment, net |
|
| 7 |
|
|
| 1,634,418 |
|
|
| 1,785,602 |
|
Other investments |
|
| 8 |
|
|
| 749,027 |
|
|
| 281,668 |
|
Total non-current assets |
|
|
|
|
|
| 2,424,535 |
|
|
| 2,129,799 |
|
TOTAL ASSETS |
|
|
|
|
| $ | 9,574,390 |
|
| $ | 10,115,293 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
Trade payables |
|
| 9 |
|
| $ | 2,012,266 |
|
| $ | 3,170,653 |
|
Other payables and accrued liabilities |
|
|
|
|
|
| 71,814 |
|
|
| 95,882 |
|
Current portion of obligation under finance lease |
|
| 10 |
|
|
| 21,235 |
|
|
| 25,048 |
|
Current portion of operating lease liabilities |
|
| 6 |
|
|
| 18,272 |
|
|
| 20,702 |
|
Advance payment from customer |
|
|
|
|
|
| 30,307 |
|
|
| - |
|
Deferred revenue |
|
|
|
|
|
| 77,276 |
|
|
| - |
|
Tax payables |
|
| 5 |
|
|
| 97,585 |
|
|
| 61,313 |
|
Total current liabilities |
|
|
|
|
|
| 2,328,755 |
|
|
| 3,373,598 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
Non-current portion of operating lease liabilities |
|
| 6 |
|
|
| 12,803 |
|
|
| 42,377 |
|
Non-current portion of obligation under finance lease |
|
| 10 |
|
|
| 24,637 |
|
|
| 35,292 |
|
Deferred tax liabilities |
|
| 5 |
|
|
| 28,416 |
|
|
| 1,872 |
|
Total non-current liabilities |
|
|
|
|
|
| 65,856 |
|
|
| 79,541 |
|
TOTAL LIABILITIES |
|
|
|
|
| $ | 2,394,611 |
|
| $ | 3,453,139 |
|
See accompanying notes to the consolidated financial statements.
| F-3 |
| Table of Contents |
PART I — FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (CONT’D)
BIONEXUS GENE LAB CORP.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
|
|
| As of |
| |||||||
|
| Note |
|
| December 31, 2021 |
|
| December 31, 2020 |
| |||
|
|
|
|
|
|
|
|
|
| |||
STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
| |||
As at December 31, 2021, common stock, no par value; 300,000,000 shares authorized and 171,218,152 shares outstanding, and preferred stock, no par value; 30,000,000 shares authorized and no shares outstanding. As at December 31, 2020, common stock, no par value; 300,000,000 shares authorized and 171,218,152 shares outstanding, and preferred stock, no par value; 30,000,000 shares authorized and no shares outstanding. |
|
| 11 |
|
| $ | 10,779,574 |
|
| $ | 10,779,574 |
|
Additional paid in capital |
|
|
|
|
|
| (5,011,891 | ) |
|
| (5,011,891 | ) |
Accumulated surplus |
|
|
|
|
|
| 1,512,358 |
|
|
| 760,787 |
|
Other comprehensive (losses)/income |
|
|
|
|
|
| (100,262 | ) |
|
| 133,684 |
|
TOTAL STOCKHOLDERS’ EQUITY |
|
|
|
|
|
| 7,179,779 |
|
|
| 6,662,154 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
| $ | 9,574,390 |
|
| $ | 10,115,293 |
|
See accompanying notes to the consolidated financial statements.
| F-4 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF OPERATIONS AND COMPREHENSIVE INCOME/(LOSS)
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Year ended |
| |||||
|
| December 31, |
| |||||
|
| 2021 |
|
| 2020 |
| ||
|
|
|
|
|
|
| ||
REVENUE |
| $ | 13,362,567 |
|
| $ | 11,390,440 |
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (11,168,747 | ) |
|
| (9,670,617 | ) |
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 2,193,820 |
|
|
| 1,719,823 |
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 66,491 |
|
|
| 886,942 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
General and administrative |
|
| (1,204,484 | ) |
|
| (1,332,943 | ) |
|
|
|
|
|
|
|
|
|
PROFIT FROM OPERATIONS |
|
| 1,055,827 |
|
|
| 1,273,822 |
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (12,973 | ) |
|
| (11,313 | )) |
|
|
|
|
|
|
|
|
|
PROFIT BEFORE TAX |
|
| 1,042,854 |
|
|
| 1,262,509 |
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
Deferred tax |
|
| (26,736 | ) |
|
| 1,238 |
|
Income tax |
|
| (264,547 | ) |
|
| (169,649 | ) |
Total tax expense |
|
| (291,283 | ) |
|
| (168,411 | ) |
|
|
|
|
|
|
|
|
|
NET PROFIT |
| $ | 751,571 |
|
| $ | 1,094,098 |
|
|
|
|
|
|
|
|
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
Foreign currency translation (loss)/gain |
|
| (233,946 | ) |
|
| 150,787 |
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
| $ | 517,625 |
|
| $ | 1,244,885 |
|
|
|
|
|
|
|
|
|
|
Earnings per share - Basic and diluted |
|
| 0.003 |
|
|
| 0.012 |
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding – Basic and diluted |
|
| 171,218,152 |
|
|
| 102,918,015 |
|
See accompanying notes to the consolidated financial statements.
| F-5 |
| Table of Contents |
BIONEXUS GENE LAB CORP
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Common stock |
|
| Additional |
|
|
|
| Accumulated other comprehensive |
| |||||||||||||
|
| Number of shares |
|
| Amount |
|
| paid in capital |
|
| Accumulated surplus/(loss) |
|
| income/ (loss) |
|
| Total Equity |
| ||||||
Balance as of January 1, 2020 |
|
| 102,730,891 |
|
| $ | 6,484,669 |
|
| $ | (5,011,891 | ) |
| $ | (333,311 | ) |
| $ | (17,103 | ) |
| $ | 1,122,364 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares |
|
| 68,487,261 |
|
|
| 4,294,905 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 4,294,905 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit for the year |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,094,098 |
|
|
| - |
|
|
| 1,094,098 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation gain |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 150,787 |
|
|
| 150,787 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2020 |
|
| 171,218,152 |
|
| $ | 10,779,574 |
|
| $ | (5,011,891 | ) |
| $ | 760,787 |
|
| $ | 133,684 |
|
| $ | 6,662,154 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit for the year |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 751,571 |
|
|
| - |
|
|
| 751,571 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (233,946 | ) |
|
| (233,946 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2021 |
|
| 171,218,152 |
|
| $ | 10,779,574 |
|
| $ | (5,011,891 | ) |
| $ | 1,512,358 |
|
| $ | (100,262 | ) |
| $ | 7,179,779 |
|
See accompanying notes to the consolidated financial statements. |
| F-6 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Year Ended |
| |||||
|
| December 31, |
| |||||
|
| 2021 |
|
| 2020 |
| ||
Cash flows from operating activities: |
|
|
|
|
|
| ||
Net profit |
| $ | 751,571 |
|
| $ | 1,094,098 |
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net profit to net cash generated from operating activities: |
|
|
|
|
|
|
|
|
Amortization of right of use asset |
|
| 16,933 |
|
|
| 10,816 |
|
Bad debts |
|
| 3,809 |
|
|
| 2,658 |
|
Depreciation of property, plant and equipment |
|
| 91,282 |
|
|
| 90,787 |
|
Dividend income |
|
| (22,036 | ) |
|
| (6,310 | ) |
Fair value gain on other investments |
|
| 29,850 |
|
|
| (38,742 | ) |
Loss on disposal of other investments |
|
| - |
|
|
| 3,965 |
|
Gain on disposal of property, plant and equipment |
|
| - |
|
|
| (706,953 | ) |
Operating profit before working capital changes |
|
| 871,409 |
|
|
| 450,319 |
|
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Inventories |
|
| (345,745 | ) |
|
| 147,038 |
|
Trade and other receivables |
|
| 579,217 |
|
|
| 411,069 |
|
Deferred cost of revenue |
|
| (67,606 | ) |
|
| - |
|
Trade and other payables |
|
| (1,180,535 | ) |
|
| (524,240 | ) |
Advance payment from customer |
|
| 30,307 |
|
|
| - |
|
Deferred revenue |
|
| 77,276 |
|
|
| - |
|
Operating lease liabilities |
|
| (20,169 | ) |
|
| 38,931 |
|
Tax recoverable |
|
| 65,007 |
|
|
| 29,563 |
|
Cash generated from operating activities |
|
| 9,161 |
|
|
| 552,680 |
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
Acquisition of other investment |
|
| (515,840 | ) |
|
| (108,631 | ) |
Dividend income |
|
| 22,036 |
|
|
| 6,310 |
|
Net cash from acquisition of business under common control |
|
| - |
|
|
| 346,008 |
|
Purchase of plant and equipment |
|
| (3,162 | ) |
|
| (421,621 | ) |
Proceeds from disposal of other investments |
|
| 6,392 |
|
|
| 30,059 |
|
Proceeds from disposal of property, plant and equipment |
|
| - |
|
|
| 1,467,865 |
|
Net cash (used in)/generated from investing activities |
|
| (490,574 | ) |
|
| 1,319,990 |
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
Repayment of finance lease |
|
| (26,302 | ) |
|
| (4,947 | ) |
Repayments to directors |
|
| (1,920 | ) |
|
| - |
|
Net cash used in financing activities |
|
| (28,222 | ) |
|
| (4,947 | ) |
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
| (154,138 | ) |
|
| 60,893 |
|
NET CHANGE IN CASH AND CASH EQUIVALENTS |
|
| (663,773 | ) |
|
| 1,928,616 |
|
CASH AND CASH EQUIVALENTS, BEGINNING OF FINANCIAL YEAR |
|
| 2,787,692 |
|
|
| 859,076 |
|
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS, END OF FINANCIAL YEAR |
| $ | 2,123,919 |
|
| $ | 2,787,692 |
|
| F-7 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))(CONT’D)
(Audited)
CASH AND CASH EQUIVALENTS INFORMATION: |
|
|
|
|
|
| ||
Fixed deposits placed with financial institutions |
| $ | 1,545,408 |
|
| $ | 2,088,107 |
|
Cash and bank balances |
|
| 578,511 |
|
|
| 699,585 |
|
Cash and cash equivalents, end of financial year |
|
| 2,123,919 |
|
|
| 2,787,692 |
|
Supplementary cash flow information: |
|
|
|
|
|
|
|
|
Interest paid |
| $ | (12,973 | ) |
| $ | (11,313 | ) |
Income tax refunded |
|
| - |
|
|
| 11,697 |
|
Income tax paid |
|
| (226,770 | ) |
|
| (143,496 | ) |
See accompanying notes to the consolidated financial statements.
| F-8 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 1 – ORGANIZATION AND BUSINESS BACKGROUND
BioNexus Gene Lab Corp. was incorporated in the State of Wyoming on May 12, 2017. On August 23, 2017, the Company acquired all of the outstanding capital stock of BGS Lab Sdn. Bhd., a Malaysian corporation (“BioNexus Malaysia”). BioNexus Malaysia was incorporated in Malaysia on April 7, 2015 which it then subsequently changed its name to Bionexus Gene Lab Sdn. Bhd.
The principal office address is Unit 02 Level 10, Tower B, Vertical Business Suite, No. 8 Jalan Kerinchi, Bangsar South, 59200 Kuala Lumpur, Malaysia, our lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia. We also have a blood collection center located at 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia.
On December 31, 2021, the Company consummated its acquisition of Chemrex Corporation Sdn. Bhd. (“Chemrex”), pursuant to a Share Exchange Agreement by and among the Company, Chemrex and the Chemrex shareholders wherein the Company acquired all of the issued and outstanding shares of capital stock of Chemrex from the Chemrex shareholders in exchange for 68,487,261 shares of common stock of the Company.
The acquisition of Chemrex has been accounted for as a common control transaction as there is no change in the control over the assets acquired and liabilities assumed. The net assets are derecognized by the transferring entity (i.e. Chemrex) and recognized by the receiving entity (i.e. the Company). The difference between the consideration transferred and the carrying amounts of the net assets is recognized in equity.
The financial statements of the receiving entity report the results of operations for the period in which the transfer occurs as though the transfer of net assets or exchange of equity interests had occurred at the beginning of the period. Results of operations for that period will thus comprise those of the previously separate entities combined from the beginning of the period to the date the transfer is completed and those of the combined operations from that date to the end of the period. The comparative financial statements were not adjusted retrospectively as Chemrex was not under common control during the comparative period.
The corporate structure as at December 31, 2021 is depicted below:
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
100% owned |
|
| 100% owned | ||
Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| Chemrex Corporation Sdn. Bhd., a Malaysian Company | ||
| F-9 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The accompanying consolidated financial statements reflect the application of certain significant accounting policies as described in this note and elsewhere in the accompanying consolidated financial statements and notes.
☐ | Basis of presentation |
The accompanying consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”).
☐ | Basis of consolidation |
The consolidated financial statements include the accounts of Bionexus Gene Lab Corp. and its subsidiaries. All significant inter-company balances and transactions within the Company have been eliminated upon consolidation.
☐ | Use of estimates |
In preparing these consolidated financial statements, management makes estimates and assumptions that affect the reported amounts of assets and liabilities in the balance sheets and revenues and expenses during the periods reported. Actual results may differ from these estimates.
☐ | Cash and cash equivalents |
Cash and cash equivalents represent cash on hand, demand deposits placed with banks or other financial institutions and all highly liquid investments with an original maturity of three months or less as of the purchase date of such investments.
☐ | Trade receivables |
Trade receivables are recorded at the invoiced amount and do not bear interest. Management reviews the adequacy of the allowance for expected credit losses on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to make adjustments in the allowance when it is considered necessary. Trade balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
☐ | Inventories |
Inventories consisting of products available for sell, are stated at the lower of cost or market value. Cost of inventory is determined using the first-in, first-out (FIFO) method. Inventory reserve is recorded to write down the cost of inventory to the estimated market value due to slow-moving merchandise and damaged goods, which is dependent upon factors such as historical and forecasted consumer demand, and promotional environment. The Company takes ownership, risks and rewards of the products purchased. Write downs are recorded in cost of revenues in the Statement of Operations and Comprehensive Income.
| F-10 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Leases |
In February 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2016-02, Leases, which was subsequently amended in 2018 by ASU 2018-10, ASU 2018-11 and ASU 2018-20 (collectively, Topic 842). Topic 842 will require the recognition of a right-of-use asset and a corresponding lease liability, initially measured at the present value of the lease payments, for all leases with terms longer than 12 months. For operating leases, the asset and liability will be expensed over the lease term on a straight-line basis, with all cash flows included in the operating section of the statement of cash flows. For finance leases, interest on the lease liability will be recognized separately from the amortization of the right-of-use asset in the statement of comprehensive income and the repayment of the principal portion of the lease liability will be classified as a financing activity while the interest component will be included in the operating section of the statement of cash flows. Topic 842 is effective for annual and interim reporting periods beginning after December 15, 2018. Early adoption is permitted. Upon adoption, leases will be recognized and measured at the beginning of the earliest period presented using a modified retrospective approach. Topic 842 allows for a cumulative-effect adjustment in the period the new lease standard is adopted and will not require restatement of prior periods.
Prior to January 1, 2019, the Company accounted for leases under ASC 840, Accounting for Leases. Effective January 1, 2019, the Company adopted the guidance of ASC 842, Leases, which requires an entity to recognize a right-of-use asset and a lease liability for virtually all leases. The Company adopted ASC 842 using a modified retrospective approach. As a result, the comparative financial information has not been updated and the required disclosures prior to the date of adoption have not been updated and continue to be reported under the accounting standards in effect for those periods.
☐ | Property, plant and equipment |
Property, plant and equipment are stated at cost less accumulated depreciation and accumulated impairment losses, if any. Depreciation is calculated on the straight-line basis to write off the cost over the following expected useful lives of the assets concerned. The principal annual rates used are as follows:
Categories |
| Principal Annual Rates | |
Air conditioner |
|
| 20% |
Buildings |
|
| 2% |
Computer and software |
|
| 33% |
Equipment |
|
| 20% |
Furniture and fittings |
| 10% to 20% | |
Lab Equipment |
|
| 10% |
Motor vehicle |
| 10% to 20% | |
Office equipment |
|
| 20% |
Renovation |
| 10% to 20% | |
Signboard |
|
| 10% |
Leasehold lands are depreciated over the period of lease term. Leased assets are depreciated over the shorter of the lease term and their useful lives unless it is reasonably certain that the Company will obtain ownership by the end of the lease term. Freehold land is not depreciated. Property, plant and equipment under construction are not depreciated until the assets are ready for their intended use
Maintenance and repairs are charged to operations as incurred. Expenditures which substantially increase the useful lives of the related assets are capitalized. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place.
Fully depreciated plant and equipment are retained in the financial statements until they are no longer in use.
| F-11 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Impairment of long-lived assets |
Long-lived assets primarily include goodwill, intangible assets and property, plant and equipment. In accordance with the provision of ASC Topic 360, “Impairment or Disposal of Long-Lived Assets”, the Company generally conducts its annual impairment evaluation to its long-lived assets, usually in the fourth quarter of each fiscal year, or more frequently if indicators of impairment exist, such as a significant sustained change in the business climate. The recoverability of long-lived assets is measured at the lowest level group. If the total of the expected undiscounted future net cash flows is less than the carrying amount of the asset, a loss is recognized for the difference between the fair value and carrying amount of the asset. There has been no impairment charge for the years presented.
☐ | Revenue recognition |
Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services.
The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services.
The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
| · | identify the contract with a customer; |
| · | identify the performance obligations in the contract; |
| · | determine the transaction price; |
| · | allocate the transaction price to performance obligations in the contract; and |
| · | recognize revenue as the performance obligation is satisfied. |
The Company records revenue at point in time which is recognized upon goods delivered or services rendered.
☐ | Shipping and handling fees |
Shipping and handling fees, if billed to customers, are included in revenue. Shipping ang handling fees associated with inbound and outbound freight are expensed as incurred and included in selling and distribution expenses.
☐ | Comprehensive income |
ASC Topic 220, “Comprehensive Income” establishes standards for reporting and display of comprehensive income, its components and accumulated balances. Comprehensive income as defined includes all changes in equity during a period from non-owner sources. Accumulated other comprehensive income, as presented in the accompanying statements of stockholders’ equity consists of changes in unrealized gains and losses on foreign currency translation and cumulative net change in the fair value of available-for-sale investments held at the balance sheet date. This comprehensive income is not included in the computation of income tax expense or benefit.
| F-12 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Income taxes |
Income taxes are determined in accordance with the provisions of ASC Topic 740, “Income Taxes” (“ASC Topic 740”). Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Any effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
ASC 740 prescribes a comprehensive model for how companies should recognize, measure, present, and disclosed in their financial statements uncertain tax positions taken or expected to be taken on a tax return. Under ASC 740, tax positions must initially be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. Such tax positions must initially and subsequently be measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority assuming full knowledge of the position and relevant facts.
The Company conducts major businesses in Malaysia and is subject to tax in their own jurisdictions. As a result of its business activities, the Company will file separate tax returns that are subject to examination by the foreign tax authorities.
☐ | Net earnings or loss per share |
The Company calculates net earnings or loss per share in accordance with ASC Topic 260 “Earnings per share”. Basic earnings or loss per share is computed by dividing the net earnings or loss by the weighted average number of common shares outstanding during the period. Diluted earnings or loss per share is computed similar to basic earnings or loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common stock equivalents had been issued and if the additional common shares were dilutive.
☐ | Foreign currencies translation |
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the statement of operations.
The functional currency of the Company is the United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$ as being the primary currency of the economic environment in which the Company operates. The functional currency of the subsidiaries is Malaysian Ringgit (“MYR”) as being the primary currency of the economic environment in which the subsidiaries operate.
In general, for consolidation purposes, assets and liabilities of its subsidiaries whose functional currency is not US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of foreign subsidiaries are recorded as a separate component of accumulated other comprehensive income.
| F-13 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
Translation of amounts from MYR into US$1.00 has been made at the following exchange rates for the respective years:
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
|
|
|
|
|
|
| ||
Year-end US$1.00: MYR exchange rate |
|
| 4.1650 |
|
|
| 4.0170 |
|
|
|
|
|
|
|
|
|
|
|
| January 1, 2021 to December 31, 2021 |
|
| January 1, 2020 to December 31, 2020 |
| ||
|
|
|
|
|
|
|
|
|
Yearly average US$1.00: MYR exchange rate |
|
| 4.1456 |
|
|
| 4.2010 |
|
☐ | Related parties |
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
☐ | Fair value of financial instruments |
The carrying value of the Company’s financial instruments: cash and cash equivalents, trade receivable, deposits and other receivables, amount due to related parties and other payables approximate at their fair values because of the short-term nature of these financial instruments
The Company also follows the guidance of the ASC Topic 820-10, “Fair Value Measurements and Disclosures” ("ASC 820-10"), with respect to financial assets and liabilities that are measured at fair value. ASC 820-10 establishes a three-tier fair value hierarchy that prioritizes the inputs used in measuring fair value as follows:
· | Level 1 : Observable inputs such as quoted prices in active markets; |
· | Level 2 : Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
· | Level 3 : Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions |
As of December 31, 2021, and December 31, 2020, the Company did not have any non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements, at least annually, on a recurring basis, nor did the Company have any assets or liabilities measured at fair value on a non-recurring basis.
☐ | Recent accounting pronouncements |
The Company has reviewed all recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements may be expected to cause a material impact on its financial condition or the results of its operations.
| F-14 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 4 – TRADE RECEIVABLES
The Company has performed an analysis on all its trade receivables and determined that all amounts are collectible by the Company. As such, trade receivables are reflected as a current asset and no allowance for expected credit loss has been recorded as of December 31, 2021 and December 31, 2020. Total of $3,809 and $2,658 of bad debts were written off for the year ended December 31, 2021 and December 31, 2020, respectively. The Company’s trade receivables consist of receivable from customers which are unrelated to the Company. The account receivables are non-interest bearing and is generally on 30 days to 90 days term.
NOTE 5 – INCOME TAXES
The Company provides for income taxes under ASC 740, “Income Taxes. ASC 740 requires the use of an asset and liability approach in accounting for income taxes. Deferred tax assets and liabilities are recorded based on the differences between the financial statements and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse. It also requires the reduction of deferred tax assets by a valuation allowance if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Provision for income taxes consisted of the following:
United States of America
The Company is registered in the State of Wyoming and is subject to the tax laws of the United States of America.
Malaysia
BioNexus Malaysia and Chemrex are both subject to Malaysia Corporate Tax, which is charged at the statutory income tax rate range is 24% on its assessable income. Under the amendment of Income Tax Act 1967 by the Finance Act 2020 and with effect from year of assessment 2020, companies with paid-up capital of MYR2.5 million or less, and with annual business income of not more than RM50 million are subject to Small and Medium Enterprise Corporate Tax at 17% on chargeable income up to MYR600,000 (2020: MYR600,000) except for companies with investment holding nature or companies does not have gross income from business sources are subject to corporate tax at 24% on chargeable income.
|
| As of |
| |||||
|
| December 31, |
|
| December 31, |
| ||
|
| 2021 |
|
| 2020 |
| ||
|
|
|
|
|
|
| ||
Tax Recoverable |
|
|
|
|
|
| ||
Local |
| $ | - |
|
| $ | - |
|
Foreign, representing Malaysia |
|
| - |
|
|
| (2,190 | ) |
Tax Recoverable |
|
| - |
|
|
| (2,190 | ) |
|
|
|
|
|
|
|
|
|
Income tax liabilities: |
|
|
|
|
|
|
|
|
Local |
|
| - |
|
|
| - |
|
Foreign, representing Malaysia |
|
| 97,585 |
|
|
| 61,313 |
|
Income tax liabilities |
|
| 97,585 |
|
|
| 61,313 |
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Local |
|
| - |
|
|
| - |
|
Foreign, representing Malaysia |
|
| 28,416 |
|
|
| 1,872 |
|
Deferred tax liabilities |
|
| 28,416 |
|
|
| 1,872 |
|
Total |
| $ | 126,001 |
|
| $ | 60,995 |
|
| F-15 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 6 – OPERATING LEASE RIGHT OF USE ASSET AND LEASE LIABILITIES
Lease liabilities are measured at present value of the sum of remaining rental payment as of recognition with discount rate of 5.40% per annum adopted from Malayan Banking (Maybank) Berhad's base rate as a reference for discount rate, as this bank is the largest bank and national bank of Malaysia.
A single lease cost is recognized over the lease term on a generally straight-line basis. All cash payments of operating lease cost are classified within operating activities in the statement of cash flows.
As of December 31, 2021 and 2020 operating lease right of use assets as follows:
|
| As of |
|
| As of |
| ||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
Balance as of December 31, 2020 |
| $ | 62,529 |
|
| $ | 23,542 |
|
Add: Addition of right of use assets |
|
| - |
|
|
| 61,128 |
|
Reduction due to discount on rental |
|
| (913 | ) |
|
| - |
|
Less: accumulated amortization |
|
| (18,305 | ) |
|
| (22,587 | ) |
Foreign translation differences |
|
| (2,221 | ) |
|
| 446 |
|
Balance as of December 31, 2021 |
| $ | 41,090 |
|
| $ | 62,529 |
|
As of December 31, 2021 and 2020 operating lease liabilities as follows:
|
| As of |
|
| As of |
| ||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
Balance as of beginning of the year |
| $ | 63,079 |
|
| $ | 24,148 |
|
Add: Addition of lease liabilities |
|
| - |
|
|
| 61,128 |
|
Less: Discount on rental |
|
| (972 | ) |
|
| - |
|
Less: gross repayment |
|
| (16,856 | ) |
|
| (26,036 | ) |
Add: imputed interest |
|
| 2,704 |
|
|
| 3,380 |
|
Foreign translation differences |
|
| (5,046 | ) |
|
| 459 |
|
Balance as of end of the year |
|
| 42,909 |
|
|
| 63,079 |
|
Less: lease liabilities current portion |
|
| (18,272 | ) |
|
| (20,702 | ) |
Lease liabilities non-current portion |
| $ | 24,637 |
|
| $ | 42,377 |
|
As of December 31, 2021 and 2020, the maturities of the operating lease obligation are as follows:
|
| As of |
|
| As of |
| ||
Years ending December 31: |
| December 31, 2021 |
|
| December 31, 2020 |
| ||
2021 |
| $ | - |
|
| $ | 20,702 |
|
2022 |
|
| 18,272 |
|
|
| 18,945 |
|
2023 |
|
| 11,987 |
|
|
| 12,428 |
|
2024 |
|
| 12,650 |
|
|
| 11,004 |
|
Total |
| $ | 42,909 |
|
| $ | 63,079 |
|
The amortization of the operating lease right of use asset for the year ended December 31, 2021 and 2020 were $16,933 and $10,816, respectively. | ||||||||
| F-16 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
Other information:
|
| As of |
|
| As of |
| ||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
| ||
Operating cash flow from operating leases |
| $ | (20,169 | ) |
| $ | 38,931 |
|
Right of use assets obtained in exchange for operating lease liabilities |
|
| 41,090 |
|
|
| 65,529 |
|
Remaining lease term for operating leases (years) |
|
| 2 |
|
|
| 4 |
|
Weighted average discount rate for operating leases |
| $ | 5.40 | % |
| $ | 5.40 | % |
Lease expenses for the year ended December 31, 2021 and 2020 were $2,704 and $3,380 respectively.
NOTE 7 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following:
|
| As of |
| |||||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
|
|
|
|
|
|
| ||
Air conditioner |
| $ | 1,124 |
|
| $ | 1,124 |
|
Computer and software |
|
| 1,814 |
|
|
| 1,371 |
|
Equipment |
|
| 43,010 |
|
|
| 42,830 |
|
Furniture and fittings |
|
| 86,961 |
|
|
| 86,961 |
|
Lab equipment |
|
| 284,822 |
|
|
| 284,822 |
|
Land and buildings |
|
| 1,506,969 |
|
|
| 1,506,969 |
|
Motor vehicle |
|
| 137,914 |
|
|
| 137,914 |
|
Office equipment |
|
| 37,700 |
|
|
| 35,160 |
|
Renovation |
|
| 107,414 |
|
|
| 107,414 |
|
Signboard |
|
| 704 |
|
|
| 704 |
|
|
|
| 2,208,432 |
|
|
| 2,205,269 |
|
(Less): Accumulated depreciation |
|
| (525,631 | ) |
|
| (441,541 | ) |
Add: Foreign translation differences |
|
| (48,383 | ) |
|
| 21,874 |
|
Property, plant and equipment, net |
| $ | 1,634,418 |
|
| $ | 1,785,602 |
|
During the year ended December 31, 2021 and 2020, the Company recorded depreciation of $91,282 and $90,787, respectively.
| F-17 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 8 – OTHER INVESTMENTS
|
| As of |
|
| As of |
| ||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
As of beginning of the year |
| $ | 281,668 |
|
| $ | 12,215 |
|
Acquisition of business under common control |
|
| - |
|
|
| 147,882 |
|
Addition during the year |
|
| 515,840 |
|
|
| 108,631 |
|
Disposal during the year |
|
| (6,392 | ) |
|
| (34,923 | ) |
Fair value gain |
|
| (29,850 | ) |
|
| 38,742 |
|
Foreign exchange translation |
|
| (12,239 | ) |
|
| 9,121 |
|
As of end of the year |
| $ | 749,027 |
|
| $ | 281,668 |
|
The other investments consists of the following shares:
|
| As of |
| |||||
|
| December 31, |
|
| December 31, |
| ||
|
| 2021 |
|
| 2020 |
| ||
Investment in quoted shares: |
|
|
|
|
|
| ||
Malaysia |
|
| 590,788 |
|
|
| 279,724 |
|
Singapore |
|
| 97,780 |
|
|
| - |
|
Hong Kong |
|
| 58,584 |
|
|
| - |
|
|
| $ | 747,152 |
|
| $ | 279,724 |
|
Investment in unquoted shares: |
|
|
|
|
|
|
|
|
Malaysia |
|
| 1,875 |
|
|
| 1,944 |
|
|
| $ | 749,027 |
|
| $ | 281,668 |
|
NOTE 9 – TRADE PAYABLES
Trade payables are amounts billed to the Company by suppliers for goods and services in the ordinary course of business. All amounts have short-term repayment terms and vary by supplier.
NOTE 10 – FINANCE LEASE
The Company purchased motor vehicles under finance lease agreements with the effective interest rate 5.70% of per annum, with principal and interest payable monthly. The obligations under the finance lease are as follows:
|
| As of |
| |||||
|
| December 31, 2021 |
|
| December 31, 2020 |
| ||
|
|
|
|
|
|
| ||
Finance lease |
| $ | 35,254 |
|
| $ | 63,703 |
|
Less: interest expense |
|
| (1,216 | ) |
|
| (3,363 | ) |
Net present value of finance lease |
|
| 34,038 |
|
|
| 60,340 |
|
|
|
|
|
|
|
|
|
|
Current portion |
|
| 21,235 |
|
|
| 25,048 |
|
Non-current portion |
|
| 12,803 |
|
|
| 35,292 |
|
Total |
| $ | 34,038 |
|
| $ | 60,340 |
|
| F-18 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
As of December 31, 2021 and 2020, the maturities of the finance lease obligations are as follows:
|
| As of |
|
| As of |
| ||
Years ending December 31: |
| December 31, 2021 |
|
| December 31, 2020 |
| ||
2021 |
| $ | - |
|
| $ | 25,048 |
|
2022 |
|
| 21,235 |
|
|
| 22,017 |
|
2023 and after |
|
| 12,803 |
|
|
| 13,275 |
|
Total |
| $ | 34,038. |
|
| $ | 60,340 |
|
NOTE 11 – CONCENTRATION OF RISKS
a) Major customers
There are no major customers who accounted for 10% or more of the Company’s revenue for the financial year ended December 31, 2021 and 2020.
b) Major suppliers
For year ended December 31, 2021, the suppliers who accounted for 10% or more of the Company’s cost of sales and their balances at year ended are presented as follows:
|
| 2021 |
|
| 2020 |
|
| 2021 |
|
| 2020 |
|
| 2021 |
|
| 2020 |
| ||||||
|
| Purchase |
|
| Percentage of purchases |
|
| Accounts payable trade |
| |||||||||||||||
Vendor A |
| $ | 2,026,842 |
|
| $ | 2,090,090 |
|
|
| 18.17 | % |
|
| 21.61 | % |
| $ | 640,827 |
|
| $ | 1,031,417 |
|
Vendor B |
| $ | 1,815,817 |
|
| $ | 1,319,232 |
|
|
| 16.28 | % |
|
| 13.64 | % |
| $ | 397,636 |
|
| $ | 335,215 |
|
Vendor C |
| $ | 1,404,442 |
|
| $ | 860,177 |
|
|
| 12.59 | % |
|
| 8.89 | % |
| $ | 405,999 |
|
| $ | 249,429 |
|
Vendor D |
| $ | 1,191,344 |
|
| $ | 1,350,069 |
|
|
| 10.68 | % |
|
| 13.96 | % |
| $ | 269,966 |
|
| $ | 572,938 |
|
|
| $ | 6,438,445 |
|
| $ | 5,619,568 |
|
|
| 57.72 | % |
|
| 58.10 | % |
| $ | 1,714,428 |
|
| $ | 2,188,999 |
|
NOTE 12– STOCKHOLDERS’ EQUITY
As at December 31, 2021 and 2020, the Company issued and outstanding, common stock is 171,218,152 and 171,218,152 shares respectively.
| F-19 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 13 – SEGMENTED INFORMATION
At December 31, 2021, the Company (“BGLC”) operates in the biochemical industry segment through its two Malaysian subsidiaries, BioNexus Malaysia and Chemrex.
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
100% owned |
|
| 100% owned | ||
Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| Chemrex Corporation Sdn. Bhd., a Malaysian company | ||
For year ended December 31, 2021, segmented revenue and net profit/(loss) (Currency expressed in United States Dollars (“US$”) are as follows:
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BGLC |
|
| Total |
| ||||
|
| Year ended December 31, 2021 |
| |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
REVENUE |
| $ | 1,515,673 |
|
| $ | 11,846,894 |
|
| $ | - |
|
| $ | 13,362,567 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (1,126,059 | ) |
|
| (10,042,688 | ) |
|
| - |
|
|
| (11,168,747 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 389,614 |
|
|
| 1,804,206 |
|
|
| - |
|
|
| 2,193,820 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 7,467 |
|
|
| 59,024 |
|
|
| - |
|
|
| 66,491 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (86,973 | ) |
|
| (989,617 | ) |
|
| (127,894 | ) |
|
| (1,204,484 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (4,158 | ) |
|
| (8,815 | ) |
|
| - |
|
|
| (12,973 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFIT/(LOSS) BEFORE TAX |
|
| 305,950 |
|
|
| 864,798 |
|
|
| (127,894 | ) |
|
| 1,042,854 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| (11,997 | ) |
|
| (14,739 | ) |
|
|
|
|
|
| (26,736 | ) |
Income tax |
|
| (30,482 | ) |
|
| (234,065 | ) |
|
|
|
|
|
| (264,547 | ) |
Total tax expense |
|
| (42,479 | ) |
|
| (248,804 | ) |
|
| - |
|
|
| (291,283 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET PROFIT/(LOSS) |
| $ | 263,471 |
|
| $ | 615,994 |
|
| $ | (127,894 | ) |
| $ | 751,571 |
|
| F-20 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2021 AND 2020
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BGLC |
|
| Total |
| ||||
|
| Year ended December 31, 2020 |
| |||||||||||||
REVENUE |
| $ | 134,095 |
|
| $ | 11,256,345 |
|
| $ | - |
|
| $ | 11,390,440 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (113,042 | ) |
|
| (9,557,576 | ) |
|
| - |
|
|
| (9,670,617 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 21,053 |
|
|
| 1,698,769 |
|
|
| - |
|
|
| 1,719,823 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 13,148 |
|
|
| 873,793 |
|
|
| 0 |
|
|
| 886,942 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (88,789 | ) |
|
| (1,107,002 | ) |
|
| (137,152 | ) |
|
| (1,332,943 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (2,482 | ) |
|
| (8,831 | ) |
|
| - |
|
|
| (11,313 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFIT/(LOSS) BEFORE TAX |
|
| (57,070 | ) |
|
| 1,456,729 |
|
|
| (137,150 | ) |
|
| 1,262,509 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| - |
|
|
| 1,238 |
|
|
| - |
|
|
| 1,238 |
|
Income tax |
|
| (881 | ) |
|
| (168,768 | ) |
|
| - |
|
|
| (169,649 | ) |
Total tax expense |
|
| (881 | ) |
|
| (167,530 | ) |
|
| - |
|
|
| (168,411 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET PROFIT/(LOSS) |
| $ | (57,951 | ) |
| $ | 1,289,199 |
|
| $ | (137,150 | ) |
| $ | 1,094,098 |
|
|
| As of December 31, 2021 and 2020 |
| |||||||||||||
|
| Total Assets |
|
| Total Liabilities |
| ||||||||||
|
| 2021 |
|
| 2020 |
|
| 2021 |
|
| 2020 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
BGLC & Bionexus |
| $ | 1,167,214 |
|
| $ | 1,107,048 |
|
| $ | 121,586 |
|
| $ | 169,325 |
|
Chemrex |
|
| 8,407,176 |
|
|
| 9,008,245 |
|
|
| 2,273,025 |
|
|
| 3,283,814 |
|
TOTAL |
|
| 9,574,390 |
|
|
| 10,115,293 |
|
|
| 2,394,611 |
|
|
| 3,453,139 |
|
| F-21 |
| Table of Contents |
NOTE 14 – SIGNIFICANT EVENTS
a) Acquisition of business under common control
During the financial year, the Company consummated its acquisition of Chemrex Corporation Sdn. Bhd., pursuant to a Share Exchange Agreement. The transfer of net assets was completed on 31 December 2020.
b) Covid-19 pandemic outbreak
On 11 March 2020, the World Health Organization declared the Coronavirus (“Covid-19”) outbreak to be a pandemic, which has caused severe global social and economic disruptions and uncertainties, including markets where the Company operates or intends to operate. The Company is actively monitoring and managing its operations to respond to these changes, the Company does not consider it practicable to provide any quantitative estimate on the potential impact it may have on the Company as the outbreak continue to evolve as of the date of this report.
NOTE 15 – SUBSEQUENT EVENTS
In accordance with ASC Topic 855, “Subsequent Events”, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued, the Company has evaluated all events or transactions that occurred after December 31, 2021 up through March 24, 2022 of these consolidated financial statements. During the period, the Company did not have any material recognizable subsequent events.
| F-22 |
Similar companies
See also LABCORP HOLDINGS INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also QUEST DIAGNOSTICS INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-30)
See also EXACT SCIENCES CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Guardant Health, Inc. - Annual report 2023 (10-K 2023-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Natera, Inc. - Annual report 2023 (10-K 2023-12-31) Annual report 2024 (10-Q 2024-06-30)




