BioNexus Gene Lab Corp - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2022
or
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________ to ________
Commission File Number: 333-269753
BIONEXUS GENE LAB CORP |
(Exact name of registrant as specified in its charter) |
Wyoming |
| 35-2604830 |
(State or Other Jurisdiction of Incorporation or Organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South 8 Jalan Kerinchi Kuala Lumpur, Malaysia |
| 59200 |
(Address of Principal Executive Offices) |
| (Zip Code) |
+60 1221-26512
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated Filer | ☒ | Smaller reporting company | ☒ |
|
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of December 31, 2022, the aggregate market value of shares held by non-affiliates of the registrant was approximately $ 48,487,449.
As of the date of this filing, there were 173,718,152 shares of common stock, no par value, outstanding.
| 2 |
| Table of Contents |
FORWARD-LOOKING STATEMENTS
Certain statements made in this Annual Report on Form 10-K are “forward-looking statements” (within the meaning of the Private Securities Litigation Reform Act of 1995) regarding the plans and objectives of management for future operations. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements of the Registrant to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations that involve numerous risks and uncertainties. The Company’s plans and objectives are based, in part, on assumptions involving the continued expansion of business. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the Company. Although the Company believes its assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and, therefore, there can be no assurance the forward-looking statements included in this Report will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by the Company or any other person that the objectives and plans of the Company will be achieved.
Unless stated otherwise, the words “we,” “us,” “our,” or “the Company” in this Annual Report collectively refers to BioNexus Gene Lab Corp., a Wyoming corporation and our wholly owned subsidiaries, BioNexus Gene Lab Sdn. Bhd., company registration no: 1139073-T (“BGL” or “BioNexus Malaysia”) and Chemrex Corporation Sdn. Bhd., company registration no: 667801-H (“Chemrex”), both Malaysian companies (“Subsidiaries”). “BGLC” or “BioNexus” refers to BioNexus Gene Lab Corp. “RM” refers to Malaysian Ringgit, the legal currency of Malaysia. “USD,” “US$,” or “$” refer to US dollars, the legal currency of the United States.
We acquired Chemrex on December 31, 2020, pursuant to a Share Exchange Agreement with Chemrex its shareholders. We acquired all of the issued and outstanding shares of capital stock of Chemrex from the Chemrex shareholders in exchange for 68,487,261 shares of common stock of BioNexus issued to the Chemrex shareholders.
Our principal corporate office is Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, 8 Jalan Kerinchi, Kuala Lumpur, Malaysia. The BioNexus Malaysia lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia and it also has a blood collection center located at 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. Chemrex offices and supply hub is located at 4 Jalan CJ 1/6 Kawasan Perusahaan Cheras Jaya, Selangor, Malaysia.
Our corporate and BioNexus Malaysia telephone number is (+60) 1221-26512 and its website is www.bionexusgenelab.com. Chemrex’s telephone number is (+60) 1922-23815 and its website is www.chemrex.com.my.
Item 1. Business.
Overview
BioNexus Gene Lab Corp., through our wholly owned subsidiary Chemrex Corporation Sdn. Bhd., focuses on the sale of chemical raw materials for the manufacture of industrial, medical, appliance, aero, automotive, mechanical, and electronic industries in the Southeast Asia region. These countries include Malaysia, Indonesia, Vietnam, and other countries in Southeast Asia.
Furthermore, the Company is also in the business of developing and providing safe, effective, and non-invasive liquid biopsy tests for the early detection of biomarkers that we believe are linked to diseases to minimize treatment costs and improve patient management. Our non-invasive blood tests provide analysis of changes in RNA to detect the potential risk of 11 different diseases.
| 3 |
| Table of Contents |
Corporate History
BioNexus was incorporated in the State of Wyoming on May 12, 2017. On August 23, 2017, the Company acquired all of the outstanding capital stock of BioNexus Gene Lab Sdn. Bhd. (formerly BGS Lab Sdn. Bhd.), a Malaysian corporation incorporated in Malaysia on April 7, 2015, which subsequently changed its name to BioNexus Gene Lab Sdn. Bhd.
On December 31, 2020, BioNexus consummated a Share Exchange Agreement with Chemrex and the Chemrex shareholders, pursuant to which we acquired all of the issued and outstanding shares of capital stock of Chemrex, which was incorporated in Malaysia on September 29, 2004, from the Chemrex shareholders in exchange for 68,487,261 shares of common stock of BioNexus issued to the Chemrex shareholders.
Corporate Structure
The corporate structure as of the date of this filing depicted below:
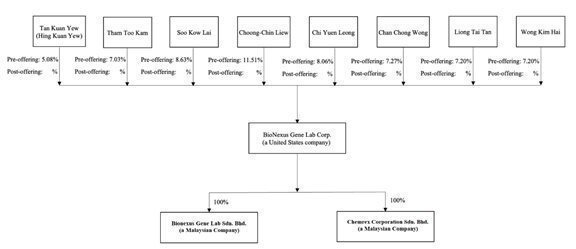
Chemical Raw Material Business
Our Products
Chemrex, our wholly owned subsidiary, is involved in the wholesale of chemical raw material products. We purchase raw chemical materials, mostly FRP, from domestic and international manufacturers and sell them to manufacturers in Southeast Asia. The FRP and other raw materials we offer are used to produce a wide variety of goods, including handrails, bench tops, automotive and aero parts, cleanroom panels, and covers for various instruments used in manufacturing.
The following table reflects Chemrex's five top selling products for FY2022, indicated by revenues, finished goods use, and percentage of total revenues of $10,832,891:
Raw Materials |
| Finished Goods |
| Revenue |
|
| % of total Revenue |
| ||
1. Resin, Stitch Mat, Roving |
| Chemical / water storage tanks |
| $ | 865,971 |
|
|
| 8.00 | % |
2. Chopped Strand Mat, Woven Roving |
| Bus & Car bodies, swimming pool |
| $ | 855,548 |
|
|
| 7.90 | % |
3. Resin, Pultrusion Roving |
| Floor grating, cable casing, electrical cable supporting arms |
| $ | 663,966 |
|
|
| 6.13 | % |
4. Resin, Stitch Mat |
| Oil & gas pipes and waste water pipes |
| $ | 576,908 |
|
|
| 5.33 | % |
5. Resin ATH Power |
| Laboratory table top and kitchen table top |
| $ | 393,260 |
|
|
| 3.63 | % |
| 4 |
| Table of Contents |
As can be seen from the table above, a substantial portion of the Company's revenue comes from the sale of FRP products. FRP products are highly sought after by our customers due to:
| · | The material's lightweight coupled with high strength. |
|
|
|
| · | The material's ability to be a good electrical insulator with no electro-magnetic behavior and no electric spark. |
|
|
|
| · | The material's rust-free nature and resistance to acid, alkali, organic dissolvents, and other gas and liquid mixtures. |
|
|
|
| · | The material's resistance to aging with more than 20 years of useful life under normal working conditions. |
|
|
|
| · | The material's ease of maintenance. |
Chemical Raw Material Product Examples
Listed below are some examples of FRP chemical raw material products the company sells. In addition, there are both general purpose and more specific use case materials.
|
Polyester Resin SHCP 268
SHCP 268 is a thixotropic, quick-curing unsaturated polyester resin suitable as a general-purpose resin. It can be used in generally all FRP products. However, it might not suffice depending on the customer’s needs since it might not have the required structural integrity, chemical resistance, or UV resistance properties a customer requires for their products. For example, one of the ways this material has been used is in the construction of train seats. |
|
|
|
Polyester Resin 9509
This is a premium raw material compared to Polyester Resin SHCP 268 and is priced higher. Like Polyester Resin SHCP 268, it is a general-purpose material but provides more structural integrity and is longer lasting. Customers have used this material to produce marine boats and water slides |
| 5 |
| Table of Contents |
| Polyester Resin 2802
This is also a more premium grade of resin. It has a niche use case and is generally used as a key component in the pultrusion process by certain manufacturers. |
Chemical Raw Material Product Applications
Our chemicals are used to produce a wide variety of finished goods. Common products utilizing our FRP materials include handrails, bench tops, automotive and aero parts, paneling for hospital/laboratory/industrial clean rooms, and covers for various instruments used in manufacturing. Some examples of FRP end-user products manufactured by our customers are displayed below:
Medical and Industrial Equipment

Platform, Handrail and Decking

Medical appliances

| 6 |
| Table of Contents |
Research and Development
The cost analysis of existing and planned research and development (“R&D”) efforts may be up to USD 0.8m. As part of our current research and development efforts, we are working closely with external R&D companies, such as Sift Center Sdn.Bhd. (www.siftcenter.com) and PCA Group Sdn.Bhd. (www.pcagroup.com), to produce and supply FRP products to Shell petrol stations. Sift Center Sdn. Bhd. and PCA Group Sdn. Bhd. are attempting to use the infusion vacuum process to produce Electrical Vehicle (EV) charging and hydrogen fueling stations. As part of our collaboration, we will provide the resin and fiberglass required to produce the infusion vacuum chamber and our technical expertise regarding the viability of the design.
Sales and Marketing
| · | Online Promotion. We market our product offerings through our website, www.chemrex.com.my. We utilize Google’s search engine optimization to drive traffic to our website. We also engaged Pan Pages, an internet marketing company, to further market our products to new consumers over the internet. New prospective customers can forward their inquiries via phone or our website. Our marketing and technical representatives will then contact the prospective customer and discuss how we can fulfill their order and accommodate any specific requests. Our marketing team also conducts online searches and look for new customers from time to time. |
|
|
|
| · | Product Display. We invite current and potential customers to examine our product range at our warehouse; thus, customers may get a more comprehensive assessment of our product's quality. |
|
|
|
| · | Marketing Personnel. Our product sales and marketing are performed internally by our Managing Director, Mr. Tham Too Kam, our Executive Director, Mr. Tan Liong Tai, and our Marketing Manager Mr. Chan Kwan Wah, together with three marketing and technical representatives. In addition, our marketing team visits our existing customers monthly, and we have several discussions with them to get information of new players in the market. |
|
|
|
| · | Business Introduction from Suppliers. We meet our suppliers regularly. From time to time, our suppliers will also provide us with the contact details of new potential customers to whom we can provide our products, and our marketing personnel will follow up on these new sales leads. |
Our Chemical Raw Material Customers
Most of our existing customers are well-established manufacturers and contractors with long-term relationships with Chemrex who regularly place orders. Typically, they would give us a forecast of the products they need and place their orders monthly. Our top five customers, based on revenue, accounted for approximately 30.92% of our revenue for the fiscal year ended December 31, 2022.
Chemrex Top 5 Customers |
|
| ||||||
A |
| $ | 858,990 |
|
|
| 7.93 | % |
B |
| $ | 855,710 |
|
|
| 7.90 | % |
C |
| $ | 577,023 |
|
|
| 5.33 | % |
D |
| $ | 664,098 |
|
|
| 6.13 | % |
E |
| $ | 393,339 |
|
|
| 3.63 | % |
Total |
|
| 3,349,159 |
|
|
| 30.92 | % |
From time to time, we assist customers with their new product development or projects with suitable and compatible raw materials. In addition, leveraging on our prior successful dealings with local and international raw materials manufacturers, we often collaborate with our customer's research teams to meet their new product needs, such as the various technical and aesthetic requirements of their new products or projects.
| 7 |
| Table of Contents |
Our Chemical Raw Material Suppliers
We consider our major vendors in each period to be those vendors that accounted for more than 10% of overall purchases in such period. We had four suppliers accounted for 15.56%, 14.82%, 14.81% and 12.18 of the Company’s total chemical raw material purchase, respectively. We had four major vendors during the fiscal year ended December 31, 2022, who collectively accounted for 57.37% of total purchases. We had four major vendors during the fiscal year ended December 31, 2021, who collectively accounted for 64.11% of total purchases. We purchase from a variety of suppliers and believe these raw materials are widely available. If we were unable to purchase from our primary suppliers, we do not expect we would face difficulties in locating another supplier at substantially the same price. We have secure and efficient access to all the raw materials necessary to produce customers’ products saving them the trouble of sourcing from several distributors. We believe our relationships with the suppliers of these raw materials are strong. While the prices of such raw materials may vary greatly from time to time, we believe we could hedge such risk by adjusting our price or absorb the higher cost at times if necessary.
Fiscal Year |
| 2022 |
| |||||
Vendor Name |
| Cost of Revenue (USD) |
|
| % of Cost of Revenue |
| ||
A |
|
| 1,497,142 |
|
|
| 15.56 | % |
B |
|
| 1,425,867 |
|
|
| 14.82 | % |
C |
|
| 1,424,476 |
|
|
| 14.81 | % |
D |
|
| 1,171,511 |
|
|
| 12.18 | % |
Total |
|
| 5,518,996 |
|
|
| 57.37 | % |
Fiscal Year |
| 2021 |
| |||||
Vendor Name |
| Cost of Revenue (USD) |
|
| % of Cost of Revenue |
| ||
C |
|
| 2,026,842 |
|
|
| 20.18 | % |
A |
|
| 1,815,817 |
|
|
| 18.08 | % |
B |
|
| 1,404,442 |
|
|
| 13.98 | % |
D |
|
| 1,191,344 |
|
|
| 11.86 | % |
Total |
|
| 6,438,444 |
|
|
| 64.11 | % |
Quality Control Policies
We have a strict quality control process centered around the handling, storage, and expiry dates of our chemical raw materials before they are delivered to our customers. All products supplied by us are attached with a Certificate of Analysis ("COA") issued by manufacturers. COA contains the batch numbers, test result data, and manufacturing date. There are also labels on the packaging of our products stating the production date and batch number.
Competition
Based on the information provided by our customers and suppliers, Malaysia's industrial chemical market size is approximately USD 50 million per annum, and our current market share is around 20% of the domestic market. In the wider Southeast Asian region, including Indonesia, Thailand, Vietnam, Philippines, Myanmar, and Cambodia, we rely on close relationships with our distributors to distribute our product to customers. As a result, the market size of the Southeast Asian market is USD 500 million per annum, and our current market share is around 2.0% of the Southeast Asian market.
As Chemrex's clients are primarily in Malaysia, we consider Chemrex's principal competitors to be in the Malaysian domestic market for selling chemical raw materials. Chemex's competitors include Kaliba Sdn.Bhd. ("Kaliba"), Myeast Sdn.Bhd. and RP Product Sdn.Bhd. Some of these competitors, such as Kaliba, may have greater resources than us. They are leading providers of Fibreglass reinforced materials such as Polyester Resin, Chopped Strand Mat, and Woven Roving, many of which overlap with our product offerings.
| 8 |
| Table of Contents |
Additionally, most of the chemical raw materials we distribute are made to industry standard specifications and either produced by or available from multiple sources. Our suppliers may also distribute directly or through multiple chemical distributors. Even for products that are unique in formulation or other characteristics, there are typically other products available that are functional substitutes, such as natural plant fiber green products, such that we face significant competition even where we are the exclusive distributors of a specialty product. Hence, our suppliers may also choose to limit their distribution outsourcing, particularly with respect to higher margin products, or to partner with other wholesalers or resellers for distribution, which could increase competition.
Competitive Advantages
Notwithstanding the competition, we are a well-established and reliable quality composite material distributor with professional services. In addition, we offer the following benefits to our existing and potential customers:
· | Technical Expertise: Our technical staff, comprising two chemists and one engineer, are highly competent and familiar with the technical advancements in the FRP industry. They provide technical know-how on mixing various products and offer product suggestions or modifications to our customers, which may involve strengthening or enhancing existing products sold by our customers.
|
· | Pricing Advantage: As a prominent reseller of FRP products in the domestic market with significant market share, we distribute our products at a relatively higher volume than our competitors. Hence, we enjoy the discounts we order from our suppliers in bulk which we then pass on to our customers. As a result, prospective customers could incur higher prices if they purchase from our competitors who do not transact at such a high volume.
|
· | Convenience: We provide a wide variety of over 100 FRP products. In contrast, some of our competitors might have a smaller product range. In addition, prospective customers could incur higher logistics if they purchase from many different sellers instead of relying on us as a one stop shop for all their business needs.
|
·
| Sourcing New Raw Materials for product development: We source a broad range of raw materials worldwide. This global reach greatly expands our potential customers and provides more opportunities for our customers to develop new products from a greater variety of raw materials. |
Growth strategy
The composite raw materials market is expected to reach an estimated $40.2 billion by 2024 and is forecasted to grow at a CAGR of 3.3% from 2019 to 2024. Furthermore, the composites end-user market is expected to reach an estimated $114.7 billion by 2024. The major drivers for growth in this market are the increasing demand for lightweight materials in the aerospace, defense, and automotive industries. Also, corrosion and chemical resistance materials are in demand in the construction and pipe and water tank industries. With our wide variety of product offerings, we are well-positioned to take advantage of this increase in chemical composite market demand. Source: Composites Market: Trends, Opportunities and Competitive Analysis (https://www.researchandmarkets.com/categories/chemicals-materials)
We are looking towards using automated warehousing and logistics powered by artificial intelligence to guide our inventory control/movement and business decisions more efficiently and quickly. We also want to deepen our ties with our major business partners, who have cooperated with us successfully for many years. Additionally, we are looking to hire more young and talented professionals to open more domestic and foreign markets, so our business growth strategy can be successfully implemented and sustained. We are also constantly looking for new products through various channels, such as trade shows to source new products to add to our product line. From 2023 to 2024, we are projecting 40% revenue growth, mainly driven by more orders for our raw materials from electric vehicle charging station manufacturers. From 2025 onwards, we are projecting that the growth rate will stabilize at 15%.
| 9 |
| Table of Contents |
Regulatory Matters
We are unaware of and do not anticipate spending significant resources to comply with governmental regulations. We are subject to the laws and regulations of those jurisdictions in Malaysia.
Listed below are the licenses Chemrex currently holds to conduct its business in Malaysia.
License/Permit/Approval | Holding entity | Issuing authority | Date of grant | Date of expiry |
Warehouse License | Chemrex | District Town Council of Selangor | September 22, 2022 | September 21, 2023 |
Importer Certificate | Chemrex | Department of Custom | October 14, 2020 | Expired once the goods cleared from the Custom |
Product Liability
Due to the nature of Chemrex's business, we may face claims for product liability resulting from any environmental or personal injury because of the chemical raw materials sold by Chemrex. We currently do not hold any insurance should a claim arise.
Diagnostics Business
Through our subsidiary BGL, we also engaged in applying genomic testing to enable early disease diagnosis and health management.
BGL's principal office address is Unit 02, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, 8 Jalan Kerinchi, Kuala Lumpur, Malaysia. Our molecular genomic lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia, and we have a colon cancer and infectious diseases screening lab located at 4th floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia. BGL's telephone number is (+60) 1221-26512 and website is www.bionexusgenelab.com.
Our Non-invasive Blood Tests
At BGL, we focus on developing and marketing safe, effective, and non-invasive blood tests to detect diseases in their early stages to minimize treatment costs and improve patient outcomes. Our non-invasive blood tests analyze changes in ribonucleic acid ("RNA") to detect specific risks and intricate of individuals' health conditions for eight cancers (nasopharyngeal, lung, liver, stomach, breast, cervical, prostate, and colon), two inflammatory bowel diseases (ulcerative colitis and crohn's) and osteoarthritis. In addition, heart attack, stroke, and mental disorders risk screening have also been included as of 2023/2024. To increase accuracy, we believe that genomic screening can be utilized in conjunction with conventional procedures for disease detection, such as imaging and biopsies.
We derive our revenue through screening patient samples utilizing the certain biomarkers we developed. We do not collect samples ourselves but rather market our screening service to healthcare providers, such as doctors, laboratories, and hospitals that collect the samples. BioNexus is the only commercial molecular lab in Malaysia that detects cancer, inflammatory bowel diseases, and osteoarthritis risk via RNA with a certified GeneChip from the Food and Drug Administration ("FDA"). Our screening process can also help guide personalized medicines and therapies for individual patients according to their needs and risks.
| 10 |
| Table of Contents |
Development of Screening Process
BGL's co-founder, late Dr. Choong-Chin Liew, developed and tested a novel approach in blood-based genomic analysis and screening by identifying biomarkers in Ribonucleic acid (RNA). Dr. Liew's research has determined that communication occurs between cells in blood and tissue as blood circulates throughout the body and subtle changes occur in cells communication occurs when a person suffers from an injury or disease. These cell-cell interactions induce manifest themselves in blood gene expression. Clinical studies performed by Dr. Liew and others have demonstrated that blood gene expression profiles can be used to develop personalized signatures capable of differentiating diseased patients from healthy ones.
We take advantage of profiling these changes, which enables us to identify unique molecular signatures (biomarkers) reflecting disease activity which can then be used to develop disease-specific molecular diagnostic assays. We use these biomarkers as the basis for screening tests for early disease detection and generate revenue from providing screening services.
The Screening Process
Our screening services begin with a blood sample from the patient. We do not conduct sample collection ourselves. Rather, a nurse or health care provider phlebotomist will draw 2.5 ml of blood from patients using an Paxgene tube. The blood and a completed company Blood Withdrawal Card are then sent to our lab via a third-party courier service.
A copy of the company form is shown below.
All blood samples delivered to us are labeled with the patient's name, personal identity number, and laboratory reference number on the tube where the blood sample is maintained for safekeeping.
| 11 |
| Table of Contents |
At our lab, the patient's RNA is extracted from the sample in a biosafety cabinet, followed by microcentrifuge and spectrophotometer to check the spectrophotometric concentration and quality of the extracted RNA. The RNA is purified, and biotinylated RNA will be mixed with purification beads and transferred to a U-bottom 96-well plate. Then, the plate will be placed onto a magnetic ring stand where labeled cRNA will be captured. The remaining solution will be removed, and the captured pellet will be cleaned-up to obtain cRNA with high purity. Then, purified cRNA will be fragmented for hybridization) and hybridized onto a genechip (we utilize the GeneChip 3' IVT PLUS Reagent Kit to prepare the biotinylated target from purified total RNA samples suitable for hybridization to GeneChip arrays). Double-stranded cDNA will be synthesized from the total RNA using reverse transcriptase and oligo-dT primers. An in-vitro transcription (IVT) reaction is then done to produce biotin-labeled cRNA from the cDNA (16 hours incubation) and scanned through the Affymetrix station. Once the overnight hybridization is completed, the Genechips will be washed with dedicated buffers and solutions to remove excess cRNAs and hybridization solutions. Washed chips will be stained with staining buffers to illuminate attached cRNAs. Specific experimental information is defined using AMDS software on a PC-compatible workstation. Stained chips are ready for scanning. The chips will be transferred into the scanner, and the image will be processed into data files. The data collected from microarray analysis are analyzed using our propriety software and algorithm to generate the disease risk score report for the individual patient.
Our software generates a report, which we forward to the healthcare provider for further consultation with the patient. This report can be used by the patient and the patient's physician to plan future tests and therapies and contains. The diagram below details the diagnostics and recommendations the report provided based on the screening results.
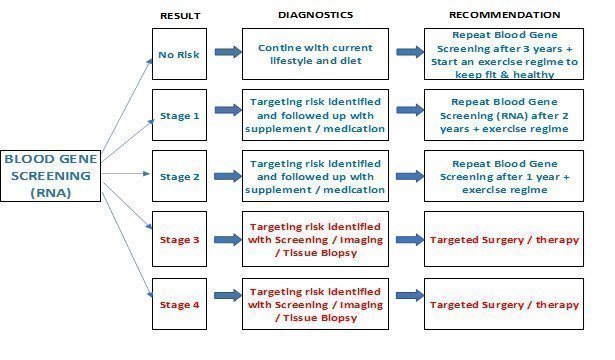
The process for effectuating RNA analysis depicted in the picture below.
| 12 |
| Table of Contents |
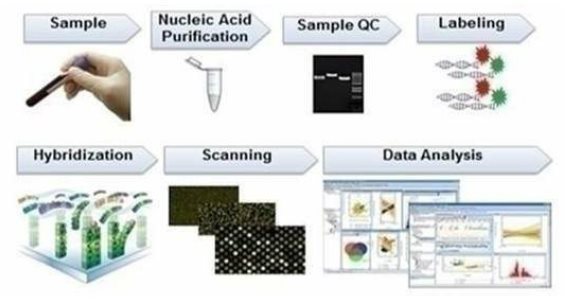
The raw data obtained will be analyzed and quality control processed by our lab in Malaysia using proprietary software to calculate the risk analysis of 11 different diseases. We simplify the result into a graph which is contained in the patient booklet provided to the health care professional. A sample graph is depicted below.

In the above chart, NPC is Nasopharyngeal Cancer, ATDS is Ascending, Transverse, Descending, and Sigmoid Colon Cancer, and OA is Osteoarthritis.
The following cautionary text is contained in the results booklet the Company provides to the healthcare provider and each patient. The results booklet contains recommendations to assist with a physician's final diagnosis and treatment plan and is not meant to be medical advice. Below is the disclaimer that is included in each result booklet.
This report/screening is not intended or implied as a substitute for professional medical advice, diagnostics, or treatment. The content, including text, graphics, and information in the report, illustrates the risk score only. BioNexus Gene Lab Sdn. Bhd. makes no representation and assumes no responsibility for the accuracy of the information, as such information and contents are subject to change without notice. You are encouraged to review any medical condition or treatment with your doctor.
| 13 |
| Table of Contents |
The key proprietary aspect of our process is our algorithm software, biomarkers, and the RNA extraction, preservation, quality control, hybridization, and data analysis processes developed by Dr. Liew. We acquired the software and the technological processes in June 2017. First, the gene expression from a reference population representing a specific disease condition is filtered using a quality assurance process based on repeatability data. Our proprietary algorithm software then analyzes this collected data and processes checked by the laboratory manager to ensure all the steps are followed in the deriving predictive model for each disease condition. Once these models have been established, they can be applied to the data from a new sample to make risk predictions for this individual. Each disease/disorder has a similar group of diseased/disordered genes identified through the Company's years of research and clinical trials in Malaysia.
Customer Service and Quality Control Policies
We envision this division of our business to provide high-quality screening tests. Our competitive advantage lies in our turnaround time, expert interpretation, and easy-to-understand reports with timely clinical decision-making. In addition, the company is dedicated to continuous quality improvement in our services and is committed to sensitivity and specificity priorities on each test.
We are committed to maintaining the confidentiality of patient information and to compliance with all privacy, security, and electronic transaction requirements of the Health Medical Act and Regulations and Code of Professional Conduct of the Malaysian Medical Council. Third parties requesting results, including any requests directly from the patient, are directed to the ordering facility. A copy of our screening test report includes reference ranges, interpretive comments, and footnotes. We submit test results electronically to healthcare providers, individual clients, and/or the Malaysian Health Ministry (HHS) regarding reportable diseases. Clients are responsible for compliance with CDC-specific statutes concerning reportable conditions. Patient test results are retained indefinitely.
All samples handled by our laboratory are treated as though they are infectious. The greatest dangers to healthcare workers exposed to blood and body fluids are hepatitis B, hepatitis C, and HIV viruses. Our laboratory turnaround time is monitored closely and compared to standardized laboratory metrics for continuous quality improvement. Laboratory scientists and technologists are all highly experienced in handling complex tests. Dr. Stephen Ponnampalam, the Chief Technology Officer, and our supervisors monitor performance indicators for all laboratory services. Performance improvement initiatives are regularly instituted and reviewed as part of an ongoing quality improvement program.
Business Development and Growth Strategy
In April 2017, we began marketing our screening services to healthcare providers, laboratories, and hospitals, all of which have licensed doctors or staff. As mentioned above, our screening service provides a risk analysis report of 11 diseases, of which eight are different forms of cancer. However, in Malaysia, the cost of the analysis is not covered by health insurance. Thus, patients are required to pay out of pocket for our services, which currently range from $200 for a single colon cancer screening to $975 for all 11 diseases under BGS screening based on the request of the patient/healthcare provider.
In November 2017, we expanded our marketing efforts to companies, business organizations, and insurance agents. As a result of these efforts, during November and December 2017, we entered arrangements with two companies in Kuala Lumpur to screen their employees for 11 diseases/disorders (lung cancer, colon cancer, nasopharyngeal cancer, liver cancer, stomach cancer, breast cancer, cervical cancer, prostate cancer, inflammatory bowel diseases (ulcerative colitis & Crohn’s disease), and osteoarthritis pursuant to which each company paid us $50,000. We completed the screening process of these two companies in the first quarter of 2019 and continue to market our services to other local companies in the Kuala Lumpur metropolitan area. In 2022, we had entered an arrangement with a clinical lab to conduct screening for the 11 diseases.
| 14 |
| Table of Contents |
Our pricing strategy is consistent with BGL's objectives, costs, competition, and demand for the product. BGL's management administers the policies to match the market needs. BGL plans to charge the following prices to individuals for its tests.
| · | $200 for Colon Cancer Screening (Single Colon Cancer screening per blood sample) |
|
|
|
| · | $975 for Blood-based Genomic Signature (BGS) Screening for 11 diseases/disorders (Molecular RNA Cancer Screening per blood sample) |
The price for each test charged to hospitals, clinics, and other healthcare operators is subject to an incentive-based rebate that ranges from 20% to 25% based on the monthly volume of tests conducted.
As of October 15, 2021, we work with 27 liquid biopsy sample collection centers, 12 in Klang Valley (comprised of our capital city Kuala Lumpur) and towns on the northern and southern fringes of the capital city), and 15 public hospitals and labs nationwide. These 27 locations account for approximately 90% of our patient population in 2022 and 2021.
We aim to have more healthcare providers in the Klang Valley referring patients to us for screening protocol. Once we have established our brand and reputation in Klang Valley, we will expand to other large cities in Malaysia. On August 25, 2022, we presented our mRNA screening service to the Health Ministry of Malaysia for nationwide implementation. Deputy Director Generals from Public Health and Cancer Divisions had scheduled another meeting on January 17, 2023. In the January’ meeting, the information we shared on the cost saving of $1.15 billion (RM 5.1 billion) in treatment expenses and $30.88m (RM135,861,660) in screening expenses for 68,617 persons (0.2% of the population) annually.
Some prevalent cancer cases in Malaysia from Globocan 2020 endorsed by WHO

| 15 |
| Table of Contents |
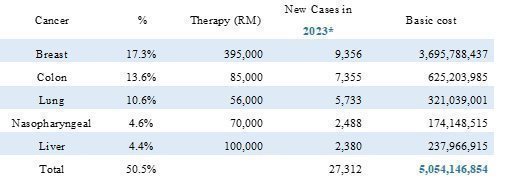
Cancer screening costs from diagnostic center is priced at $882 (RM3,880) as compared to mRNA screening at $432 (RM1,900), quantity genechip purchase would drastically reduce the screening cost. The proposed screening campaign is targeting 0.2% of the 34,308,525-population aged 40 years from 2023. A saving of $30.88m {68,617 persons x ($882-$432)}, a saving of $34,308,525.
| 16 |
| Table of Contents |
We believe that an increase in our marketing and promotional efforts will correlate to increased revenues and the expansion of our business. Our growth and expansion strategies are as follows:
| · | Continue to leverage our relationships with healthcare providers. To date, we have relied upon the efforts of management and their relationships with healthcare providers to create continued interest in our blood-based genomic screening. These relationships have been located primarily in the Klang Valley market. We will continue to use our relationships with providers in the Klang Valley market and elsewhere in Malaysia to increase sales and product awareness. |
| · | Allocate more capital resources to our marketing efforts. Apart from sales through existing relationships with healthcare providers, we intend to allocate more capital to marketing and promotion. As part of these efforts, we have appointed two commission-based Marketing Companies, Gloco and Yakin Healthcare, to bring awareness of our services in Malaysia. |
| · | Increase focus on corporate clients. To date, we have entered arrangements with six corporate clients to provide our 11 diseases/disorders screening services to their employees. In addition, we intend to solicit more corporate clients in the Klang Valley and major cities in Malaysia. We commenced these efforts last year and will continue in 2023. Our officers and the Marketing Companies will undertake these efforts. |
| · | Expand to other regions in Malaysia. We intend to expand to other large cities in Malaysia, such as Penang, Ipoh, Seremban, Melaka, Johor Bahru, and Kuantan. |
Competition
We believe that we have the only commercial molecular lab in Malaysia that provides liquid biopsy screenings that detect the risk of cancer, inflammatory diseases, and osteoarthritis risk via RNA biomarkers and provides a report which patients and physicians can use to plan for future tests and personalized therapies. Based on prior private conversations with the USA Thermo Fisher representative in Malaysia, there is a medical lab using similar equipment on DNA screening.
Competitive Strengths
We believe that we have several competitive strengths compared to these other health diagnostic tools. They are as follows:
| · | Our screening (a simple blood draw) is less invasive, unlike tissue biopsies. A tissue biopsy is a procedure in which a physician removes a piece of tissue or a sample of cells from a patient's body to be analyzed in a laboratory. If a patient experiences certain signs and symptoms or the physician has identified an area of concern, he may undergo a biopsy to determine whether the patient has cancer or another ailment. While biopsies can have higher accuracy, it is a more invasive procedure that is difficult to repeat and thus impractical for periodic monitoring. BGL's screening tests are a form of liquid biopsy which utilizes RNA biomarkers. Broadly speaking, a liquid biopsy is the collection of a body fluid sample to test for relevant biomarkers to inform patient management, most applied to the collection of peripheral blood for analysis of cell-free circulating tumor ribonucleic acids (RNA). Since liquid biopsies are performed on peripheral blood, which is easy to access, it allows for more widespread use, particularly in patients who cannot have surgery. As a result, liquid biopsies can reduce the time to treatment, improve the efficiency of medical staff and resources, and be used to screen more diseases. |
| 17 |
| Table of Contents |
| · | Non-DNA blood tests for diseases like cancer are not dispositive. There currently exist various examinations to detect diseases in patients. For example, abnormally high or low levels of certain substances in your body can be a sign of disease. Testing of blood, urine or other body fluids that measure these substances can help doctors make a diagnosis. However, abnormal lab results are not a sure sign of disease. Conventional blood tests are an important tool but are not always reliable because of low sensitivity, specificity, and predictive value. |
|
|
|
| · | Other Conventional tests could require a longer turnaround time. Imaging is a procedure in which physicians utilize pictures of areas inside the body that help the doctor see whether a disease is present. These images can be taken in several ways, including a CT scan, Nuclear Scan, MRI, PET Scan, and Ultrasound. Imaging is useful in providing physicians with real-time images to assist with diagnosis. However, imaging techniques can have longer turnaround times, the information provided can be limited, and the patient may be exposed to radiation. |
|
|
|
| · | Our screening provides a predictive risk assessment for developing the 11 diseases. Most other screening procedures detect diseases only when they are already present in the body and most cases, in the final stages of the disease, making it difficult to treat or reverse. Our screening can detect the 11 diseases at an earlier stage before any symptoms even appear. Early detection and targeted medical intervention could be crucial in saving patients' lives and financial resources. |
|
|
|
| · | Our screening measures the current risk of a specific individual rather than their lifetime risk. DNA tests measure a specific individual's lifetime risk based on their DNA. However, since DNA does not change with external factors, it cannot quantify an individual's specific risk of the disease materializing. However, our RNA-based test is highly specific since RNA expression changes with lifestyle and other external factors. Hence, at-risk patients can make timely adjustments to their lifestyles to reduce the potentiality of these diseases. Lifestyle adjustments may include reduction or changes to food, tobacco, and alcohol intake, change of working environment, and the implementation of exercise programs, among other changes. |
Seasonality
The nature of our business does not appear to be affected by seasonal variations.
Regulatory Matters
We are unaware of and do not anticipate spending significant resources to comply with governmental regulations. We are and will be subject to the laws and regulations of those jurisdictions in which we operate. Generally, business licensing requirements, income taxes, and payroll taxes apply to all business operations. The development and operation of our business are not subject to special regulatory and/or supervisory requirements. We only require an operating permit from the City Hall of Kuala Lumpur, Malaysia, which we have received. However, we cannot predict whether we would be able to comply with other regulations if implemented.
Product Liability
Due to the nature of BGL's business, BGL may face claims for product liability resulting from the inaccurate or erroneous diagnosis using our screening process. BGL does not currently have insurance against any such claims.
Research and Development
There are six persons in the R&D team consists of 3 scientists, YM Wong, Stephen PonnamPalam, HK Looi, and two medical doctors, Dr. YM Fong and Dr. Stephen PonnamPalam, and 2 laboratory managers, Sanggetha Periya and CH Yew.
| 18 |
| Table of Contents |
Our research and development budget over the years is listed in the following table:
Year |
| Research & Development (self-funded) |
| |
2017 |
| $ | 0 |
|
2018 |
| $ | 0 |
|
2019 |
| $ | 25,000 |
|
2020 |
| $ | 45,000 |
|
2021 |
| $ | 45,000 |
|
2022 |
| $ | 173,300 |
|
Our Properties
Our corporate office for BGL is located at Unit 2, Level 10, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, 8 Jalan Kerinchi, Bangsar South, 59200 Kuala Lumpur, Malaysia. The lease commenced on December 16, 2018 and terminates on December 15, 2024. The space consists of 1,300 square feet with an annual rent of approximately $13,500.
One of our laboratories is located at 4th Floor, Wisma Life Care, No. 5, Jalan Kerinchi, Bangsar South, 59200 Kuala Lumpur, Malaysia. The lease commenced on November 1, 2016 and terminates on October 31, 2023. The annual rent is approximately $6,800. Our other laboratory is located at Lab 353, University Science Malaysia, George Town, Penang, Malaysia. The lease commenced on December 1, 2017 and terminates on November 30, 2024. The space consists of 1,500 square feet with an annual rent of approximately $7,300.
On July 2, 2012, we purchased a 25,000 sq. ft wholesale distribution center at 4, Jalan CJ 1/6, Kawasan Perusahaan Cheras Jaya, 43200 Cheras, Selangor, Malaysia, and two investment properties for $1,395,210. The two investment properties are listed below.
| · | A 1,100 sqft condominium located at No. B-17-03, Duet Residence, Jalan Kinrara 6, Bandar Kinrara, 47180 Puchong, Selangor, purchased on August 26, 2020; |
| · | A 2,000 sqft commercial building located at First floor, No. 2B Pelangi Avenue, Jalan Kelicap 42A/KU1, Klang Bandar, Diraja, 41050 Klang, Selangor purchased on September 21, 2020. |
On January 18, 2023, we entered into a lease for the first-floor unit at No. 5-1, Jalan CJ3/13-2, Pusat Bandar Cheras Jaya, 43200 Cheras, Selangor. The lease commenced on 18 January 2023 and terminates on January 17, 2024. The purpose of this lease is to provide accommodation for our warehouse staff.
Intellectual Property
As of date of this filing, we have 1 trademark registered with the Intellectual Property Corporation of Malaysia. We do not have any patents, copyright, or licensing rights. Additionally, for BGL, we rely on trade secrets and know-how using the process developed by and assigned to the Company by Dr. Liew. Dr. Liew had previously on June 24, 2017 assigned the right to use the technical know-how and algorithm software owned by Golden Health DX Toronto, a Canadian company 100% owned by Dr. Liew to our subsidiary, BioNexus Gene Lab Sdn. Bhd. However, there is no assurance that others will not independently develop the same or similar technology or obtain unauthorized access to such trade secrets, know-how, and other unpatented technology. To protect our rights in these areas, we require all laboratory managers that work in our lab to enter into strict confidentiality agreements. Presently, we have two lab managers managing KL and Penang laboratories respectively and they are supported by a full-time lab technologist and 4 part-times lab technicians. Meanwhile, we are working on an updated algorithm software for data analysis and it would be patented around 3rd quarter, 2023. While we have attempted to protect the unpatented proprietary technology that we develop or acquire and will continue to attempt to protect future proprietary technology through patents, copyrights, and trade secrets, we believe that our success will depend, to a large extent, upon continued innovation and technological expertise.
| 19 |
| Table of Contents |
Employees
As of date of this filing, Chemrex has 18 full-time employees, and BGL has 9 full time employees and 6 part-time (2 director and 4 staff). Any collective bargaining agreement does not represent Chemrex's and BGL's employees, and we have never experienced a work stoppage. We believe we have good relations with our employees. The company presently is covered by social security insurance and contributes to the Employee Provident Fund of its employees, a compulsory pension scheme for all Malaysian citizens and permanent residents who are working in Malaysia.
The following table sets out the number of Chemrex's employees, excluding external experts, categorized by functions as of the date of this filing:
Function |
| Number of Employees |
| |
Director |
|
| 4 |
|
Sales & Marketing |
|
| 4 |
|
Warehouse |
|
| 6 |
|
Administration & Purchaser |
|
| 2 |
|
Finance |
|
| 2 |
|
Total |
|
| 18 |
|
The following table sets out the number of BGL's employees, excluding external experts, categorized by functions as of the date of this filing:
Function |
| Number of Employees |
| |
Director |
|
| 2 |
|
Finance |
|
| 1 |
|
Lab Operation |
|
| 4 |
|
Research & Development (1 full-time scientist & 4 Part-timers) |
|
| 5 |
|
General & Administration |
|
| 3 |
|
Total |
|
| 15 |
|
The following table sets out the number of BGLC's employees, excluding external experts, categorized by functions as of the date of this filing:
Function |
| Number of Employees |
| |
Director (3 independents & 1 assigned from Chemrex) |
|
| 4 |
|
Finance |
|
| 1 |
|
Research & Development |
|
| 1 |
|
Total |
|
| 6 |
|
Currently, we have entered into employment agreements with our officers. Therefore, we do not have stock options, profit sharing, or similar benefit plans. However, we may adopt plans in the future. We do not plan to hire additional employees currently.
Insurance
We maintain third-party liability insurance to cover claims in respect of personal injury or property or environmental damage arising from accidents on our chemical warehouse and office or relating to our operations. Our employees presently are covered by Social Security insurance (SOCSO) and retirement fund (EPF). We do not maintain business interruption insurance or key person insurance. Our insurance coverage is consistent with the industry and sufficient to cover our key assets, facilities, and liabilities. Also, as part of our Chemrex business, we maintain burglary and fire insurance for our property at 4, Jalan CJ 1/6, Kawasan Perusahaan Cheras Jaya, 43200 Cheras, Selangor, Malaysia, and fidelity guarantee insurance against our employees.
| 20 |
| Table of Contents |
Legal Proceedings
We are not subjected to nor engaged in any litigation, arbitration, or claim of material importance, and no litigation, arbitration, or claim of material importance is known to us to be pending or threatened by or against our Company that would have a material adverse effect on our Company's results of operations or financial condition.
Item 1A. Risk Factors
RISK FACTORS
An investment in our common stock involves a number of very significant risks. You should carefully consider the following known material risks and uncertainties in addition to other information in this Form 10-K in evaluating our company and its business before purchasing shares of our company’s common stock. You could lose all or part of your investment due to any of these risks.
Risk Factors Related to Our Financial Prospects and Capitalization
BioNexus is an early, commercial-stage company and have a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
BioNexus is an early commercial-stage company and have a limited operating history. BioNexus’ limited operating history may make it difficult to evaluate our current business and this makes predictions about our future success or viability subject to significant uncertainty. In combination with other anticipated increased operating expenses in connection with becoming a public company, these anticipated changes in our operating expenses may make it difficult to evaluate our current business, assess our future performance relative to prior performance and accurately predict BioNexus’ future performance.
BioNexus will continue to encounter risks and difficulties frequently experienced by early commercial-stage companies, including those associated with increasing the size of BioNexus’ organization and the prioritization of BioNexus’ commercial, research, and business development activities. If BioNexus does not address these risks successfully, BioNexus’ business could suffer.
BioNexus’ growth (organic and inorganic) may require substantial capital and long-term investments.
BioNexus’ competitiveness and growth depend on our ability to fund our capital expenditures. BioNexus cannot assure you that it will be able to fund our capital expenditures at reasonable costs due to adverse macroeconomic conditions, our performance or other external factors.
In the future, BioNexus expects to incur significant costs in connection with its operations. BioNexus intends to expand BioNexus’ business through increased marketing efforts of BioNexus Malaysia and Chemrex. These development activities generally require a substantial investment before BioNexus can determine commercial viability, and the proceeds of this offering will not be sufficient to fully fund these activities. BioNexus expects to need to raise additional funds through public or private equity or debt financings, collaborations or licensing arrangements to continue to fund or expand BioNexus’ operations.
| 21 |
| Table of Contents |
BioNexus’ actual liquidity and capital funding requirements will depend on numerous factors, including:
| · | the scope and duration of and expenditures associated with BioNexus’ discovery efforts and research and development programs; |
| · | the costs to fund BioNexus’ commercialization strategies for any product candidates for which BioNexus receive marketing authorization or otherwise launch and to prepare for potential product marketing authorizations, as required; |
| · | the costs of any acquisitions of complementary businesses or technologies that BioNexus may pursue; |
| · | potential licensing or partnering transactions, if any; |
| · | BioNexus’ facilities expenses, which will vary depending on the time and terms of any facility lease or sublease BioNexus may enter into, and other operating expenses; |
| · | the scope and extent of the expansion of BioNexus’ sales and marketing efforts; |
| · | the settlement of the government investigation described below, potential and pending litigation, potential payor recoupments of reimbursement amounts, and other contingencies; |
| · | the commercial success of BioNexus’ products; |
| · | BioNexus’ ability to obtain more extensive coverage and reimbursement for BioNexus’ tests and therapeutic products, if any, including in the general, average-risk patient population; and |
| · | BioNexus’ ability to collect its accounts receivable. |
The availability of additional capital, whether from private capital sources (including banks) or the public capital markets, fluctuates as BioNexus’ financial condition and market conditions in general change. There may be times when the private capital sources and the public capital markets lack sufficient liquidity or when BioNexus’ securities cannot be sold at attractive prices or at all, in which case BioNexus would not be able to access capital from these sources. In addition, a weakening of BioNexus’ financial condition or deterioration in its credit ratings could adversely affect BioNexus’ ability to obtain necessary funds. Even if available, additional financing could be costly or have adverse consequences.
BioNexus may incur net losses in the near future.
BioNexus has devoted substantial resources to the development and commercialization of the products of BioNexus Malaysia and Chemrex. BioNexus might not remain profitable for any period. BioNexus’ failure to achieve profitability would negatively affect BioNexus’ business, financial condition, results of operations, and cash flows. If BioNexus is unable to execute BioNexus’ sales and marketing strategy and BioNexus’ products are unable to gain sufficient acceptance in the market, BioNexus may be unable to generate sufficient revenues to sustain BioNexus’ business.
Any additional capital BioNexus raises may not be available on satisfactory terms and may adversely affect stockholders’ holdings or rights.
Additional capital, if needed, may not be available on satisfactory terms or at all. In addition, the terms of any financing may adversely affect stockholders’ holdings or rights. Debt financing, if available, may include restrictive covenants. To the extent that BioNexus raises additional funds through collaborations and licensing arrangements, it may be necessary to relinquish some rights to BioNexus’ technologies or grant licenses on terms that may not be favorable to us.
| 22 |
| Table of Contents |
If BioNexus is not able to obtain adequate funding when needed, BioNexus may be required to delay development programs or sales and marketing initiatives. If BioNexus is unable to raise additional capital in sufficient amounts or on satisfactory terms, BioNexus may have to make reductions in BioNexus’ workforce and may be prevented from continuing BioNexus’ discovery, development, and commercialization efforts and exploiting other corporate opportunities. In addition, it may be necessary to work with a partner on one or more of BioNexus’ tests or products under development, which could lower the economic value of those products to us. Each of the foregoing may harm BioNexus’ business, operating results, and financial condition and may impact BioNexus’ ability to continue as a going concern.
Raising additional capital may lead to dilution of shareholdings by BioNexus’ existing shareholders, restrict BioNexus’ operations, and may further result in fair value loss, adversely affecting BioNexus’ financial results.
BioNexus may seek additional funding through a combination of equity and debt financings and collaborations. To the extent that BioNexus raises additional capital through the sale of equity or convertible debt securities, the ownership interest of existing holders of BioNexus’ shares will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of BioNexus’ existing shareholders.
The incurrence of additional indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on BioNexus’ ability to incur additional debt or issue additional equity, limitations on BioNexus’ ability to acquire or license IP rights and other operating restrictions that could adversely impact BioNexus’ ability to conduct its business.
Risk Factors Related to Our Business and Industry
General Business and Industry Risks
BioNexus is unable to predict the duration of current economic conditions.
Future economic downturns, prolonged slow growth or stagnation in the economy could materially adversely affect BioNexus’ business, results of operations, financial condition and cash flows.
Global economic conditions could materially adversely impact demand for BioNexus’ products and services.
BioNexus’ operations and performance depend significantly on economic conditions. Global financial conditions continue to be subject to volatility arising from international geopolitical developments and global economic phenomenon, as well as general financial market turbulence and natural phenomena such as the COVID-19 pandemic. Uncertainty about global economic conditions could result in
| · | customers postponing purchases of its products and services in response to tighter credit, unemployment, negative financial news and/or declines in income or asset values and other macroeconomic factors, which could have a material negative effect on demand for its products and services; and |
|
|
|
| · | third-party suppliers being unable to produce devices for its products or raw materials in the same quantity or on the same timeline or being unable to deliver such parts and components as quickly as before or subject to price fluctuations, which could have a material adverse effect on the services and products provided by BGL; and accordingly, on its business, results of operations or financial condition. |
| 23 |
| Table of Contents |
Access to public financing and credit can be negatively affected by the effect of these events on Malaysian, U.S. and global credit markets. The health of the global financing and credit markets may affect its ability to obtain equity or debt financing in the future and the terms at which financing or credit is available to us. These instances of volatility and market turmoil could adversely affect its operations and the trading price of its common stock.
BioNexus’ risk management programs, processes, or procedures for identifying and addressing risks in BGL’s business may not be adequate or effectively applied, and this may adversely impact its businesses.
BGL relies on a combination of technical and human factors to protect us against risks. BGL policies, procedures and practices are used to identify, monitor and control a variety of risks, including risks related to human error and hardware and software errors. The administration and results of each test are reviewed by a physician and a scientist in Malaysia before the results are released to the patient. The Company’s standard of operations has been developed internally primarily by Dr. Liew. These risk-management methods may not adequately prevent losses and may not protect us against all risks, in which case BioNexus’ business, economic conditions, operations and cash flows may be materially adversely affected.
BioNexus has risk-management policies, control systems and compliance manuals in place; however, there is no guarantee that such policies, systems, and manuals will be effectively applied in every circumstance by BioNexus’ staff. For example, employees could override the system technology and theoretically waive requirements, thereby exposing the company accurately conduct its quality control.
BioNexus may be adversely impacted by changes in laws and regulations, or in their application.
Currently, there are no governmental regulations that materially restrict BioNexus’s screening business in Malaysia. BGL’s laboratory in Malaysia was established through an invitation by the Malaysian Health Minister alongside a government grant of $1,250,000. BGL’s screening tests have gone through preclinical and clinical trials involving private hospitals and government agencies including the Institute of Medical Research (IMR), Malaysian Biotechnology Corporation (BiotechCorp) and the Clinical Research Centre (CRC). The findings of the preclinical and clinical trials are published in peer reviewed journals such as the Journal of Molecular and Cellular Cardiology, and Physiological Genomics. Once published, BGL would do confirmational tests before applying for commercialization. BGL’s Malaysian lab is currently national operating under an operating license granted by the city of Kuala Lumpur.
The Malaysian government passed the Pathology Laboratory Bill of 2007 (“Pathology Act”). However, since 2007, the government has not implemented the regulations underlying the legislation nor has the government enforced the Pathology Act. Any such regulations could establish criteria for the various classes and specialties of laboratories, the organization and management system of the laboratory, the qualification and experience of the person-in-charge, the qualification and competence of pathologists, scientific and technical staff engaged to conduct tests, and the standards of laboratory practice. BGL cannot predict whether it would be able to comply with the Pathology Act and its regulations, if implemented. In addition, there also is a risk that the regulations arising from the Pathology Act or new legislation or regulations could increase BGL’ costs of doing business or otherwise prevent BioNexus from carrying out the expansion of its business. Accordingly, BioNexus’ business may be harmed if BioNexus is not able to comply with any future governmental legislation or regulations, including the Pathology Act.
BGL is currently operating under an operating license granted by the City Hall of Kuala Lumpur, Malaysia. Under Malaysian and local laws, BioNexus may continue to operate under its current operating license which BioNexus Malaysia currently has. BioNexus cannot predict whether there will be future regulations which may impact its ability to conduct its business.
Currently, there are no governmental regulations that affect Chemrex’s business in Malaysia and it may continue to operate under an operating license granted by the Kajang Town Hall of Selangor, Malaysia. Future legislation or regulations could increase Chemrex’s costs of doing business or otherwise prevent BioNexus from carrying out the expansion of its business.
| 24 |
| Table of Contents |
Business disruptions could seriously harm BioNexus’ future revenue and financial condition and increase its costs and expenses.
BioNexus’ operations could be subject to power shortages, telecommunications failures, wildfires, water shortages, floods, earthquakes, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions. The occurrence of any of these business disruptions could seriously harm BGL’ operations and financial condition and increase BGL’ costs and expenses. Unfavorable global economic conditions could adversely affect BioNexus’ business, financial condition, or results of operations.
BioNexus do not carry insurance for all categories of risk that BioNexus’ business may encounter. Although BGL intend to obtain some form of business interruption insurance in the future, there can be no assurance that BioNexus will secure adequate insurance coverage or that any such insurance coverage will be sufficient to protect BioNexus operations to significant potential liability in the future. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect BioNexus’ financial position and results of operations.
Our lack of insurance could expose us to significant costs and business disruption.
We currently do not have any product liability or disruption insurance to cover our operations in Malaysia or overseas. We have determined that the costs of insuring for these risks and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical for us to have such insurance. If we suffer any losses, damages or liabilities in the course of our business operations, we may not have adequate insurance coverage to provide sufficient funds to cover any such losses, damages or product claim liabilities. Therefore, there may be instances when we will sustain losses, damages and liabilities because of our lack of insurance coverage, which may in turn materially and adversely affect our financial condition and results of operations.
Our internal controls are progressively improved with additional independent directors coming on board and audit committee appointed which could cause our financial reporting to be reasonably reliable.
Our management, including our chief executive officer and chief financial officer, is responsible for establishing and maintaining adequate internal control over our financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America and includes those policies and procedures that: pertain to the maintenance of records in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and/or directors of the Company; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
In connection with the audits of our consolidated financial statements as of December 31, 2022 and 2021, we identified these “material weaknesses,” were rectified with independent directors and audit committee be included in 2022 significant improvement in our internal control over financial reporting.
| · | We maintained segregation of duties within our business operations and reliance on several individuals fulfilling the role of officers and directors; |
|
|
|
| · | A functioning audit committee and a majority of independent members and outside directors on our board of directors, resulting in better oversight in the establishment and monitoring of required internal control and procedures; |
| 25 |
| Table of Contents |
We will expand our current board of directors to include additional individuals from US public company in the near term due to our limited financial resources. Until such remedial actions can be fully realized, we will continue to rely on the advice of outside professionals and consultants.
As a public company, we may become subject to the Section 404 of the Sarbanes-Oxley Act, or SOX 404, which requires that we include a report from management on the effectiveness of our internal control over financial reporting in our annual report on Form 10-K and in our quarterly report on Form 10-Q if we are qualified as an accelerated filer.
We are currently a “smaller reporting company”, meaning that we are not an investment company, an asset- backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and annual revenues of less than $50.0 million during the most recently completed fiscal year. In the event that we are still considered a “smaller reporting company,” at such time as we cease being an “emerging growth company,” we will be required to provide additional disclosure in our SEC filings. However, similar to an “emerging growth companies”, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
Our independent registered public accounting firm may be required to attest to and report on the effectiveness of our internal control over financial reporting. Our management may conclude that our internal control over financial reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. In addition, after we become a public company, our reporting obligations may place a significant strain on our management, operational and financial resources and systems for the foreseeable future. We may be unable to timely complete our evaluation testing and any required remediation.
During the course of documenting and testing our internal control procedures, in order to satisfy the requirements of SOX 404, we may identify other weaknesses and deficiencies in our internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with SOX 404. If we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of our shares. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions. We may also be required to restate our financial statements from prior periods.
Fluctuations in foreign currency exchange rates could have a material adverse effect on our financial results.
We earn revenues, pay expenses, own assets and incur liabilities in countries using Malaysian Ringgit (“RM”) other than the U.S. dollar (“$”). Since our consolidated financial statements are presented in U.S. dollars, we must translate revenues, income and expenses, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. Therefore, increases or decreases in the value of the U.S. dollar against Malaysian currency affect our net operating revenues, operating income and the value of balance sheet items denominated in foreign currencies. We cannot assure you that fluctuations in foreign currencies exchange rates, particularly the strengthening or weakening of the U.S. dollar against Malaysian currency would not materially affect our financial results.
| 26 |
| Table of Contents |
Risk Related to BGL’s Business and Industry
Exponential growth in Biotechnology
Biotechnology is a rapidly changing field that continues to transform both in scope and impact. Well-funded established molecular labs are gathering big data on health records, genomics, lifestyle information that led to new health solutions. Digitization is revolutionizing health care, allowing for patient reported symptoms, health outcome to be captured as mineable data. BGL could lose out to its competitors’ exponential growth if we unable to establish network with medical centers, pharmaceutical groups and other molecular laboratories synergistically in sharing customers and big data.
BGL’s inability to manage growth could harm its business.
BGL expects to continue to add personnel in the areas of sales and marketing, research & development, laboratory operations, finance, quality assurance and compliance. As BGL builds its commercialization efforts and expands research and development activities, operating expenses and capital requirements will increase, and BGL expects that they will continue to increase, significantly. BGL’s ability to manage its growth effectively requires us to forecast expenses accurately, and to properly forecast and expand operational and testing facilities, if necessary, to expend funds to improve our operational, financial and management controls, reporting systems and procedures. As BGL moves forward in marketing our tests and developing our test portfolio, the company will also need to effectively manage its growing manufacturing, laboratory operations and sales and marketing needs. If BGL is unable to manage its anticipated growth effectively, BGL’s future business could be harmed.
BGL’s financial prospects depend substantially upon the successful commercialization of the Company’s services and products in the future, which may fail or experience significant delays.
BGL’s future success depends upon BGL’s ability to continuously develop technologies and successfully market its existing cancer genetic offerings to customers within Malaysia and expand overseas. BGL’s ability to generate significant revenue in the next several years will depend primarily on the successes of each key stage of its business, including pre-clinical research and development, clinical trials, regulatory approval, manufacture, marketing and commercialization of its services and products, which is subject to significant uncertainty. BGL’s ability to generate sales revenue from its products and services and its future profitability depends on several factors, including its ability to:
| · | obtain regulatory approvals and marketing authorizations for BGL’s services and products; |
|
|
|
| · | obtain market acceptance by patients, hospitals, clinicians, biopharmaceutical companies and others in the medical community; |
| · | establish sufficient testing capacity and commercial capabilities, either by expanding BGL’s current facility or making arrangements with third parties; |
| · | develop and maintain BGL’s sales network to launch and commercialize its new cancer genomic testing services and products; |
| · | set appropriate and favorable prices for BGL’s genomic testing services and products and obtaining adequate reimbursement from third-party payers; |
| 27 |
| Table of Contents |
| · | maintain commercially viable supply relationships with third parties and maintaining sufficient research and development capabilities and infrastructure; |
| · | address any competing technological and market developments; and |
| · | maintain, protect, and expand BGL’s portfolio of intellectual property rights including trade secrets and know-how. |
The marketing, sale and use of BGL’s products and services could result in substantial damages arising from products or service liability or professional liability claims, that exceed BGL’s resources.
Due to the nature of BGL’s business, it may face claims for products or service liability. These claims may arise from the inaccurate or erroneous diagnosis of patient information or the mix-up of patient information whereby a patient receives the wrong diagnostic information. While the company feels confident in its quality control measures to ensure the safeguard of patient and client information, it cannot provide assurances that products or service liability claims will arise in the future.
Moreover, litigation or adverse publicity resulting from these allegations could materially and adversely affect BGL’s business, regardless of whether the allegations are valid or whether the company is liable. Currently BGL has no products and service liability insurance coverage, and even if there was such coverage, such coverage might not be sufficient to properly protect BGL. Further, claims of this type, whether substantiated or not, may divert BGL’s financial and management resources from revenue generating activities and the business operation.
BGL may face technology transfer challenges and expenses in adding new tests to its portfolio and in expanding its reach into new geographical areas.
BGL’s plan for expanding its business includes developing and acquiring additional tests or additional biomarkers that can be transferred into its current and future diagnostic product portfolio and distributed in target markets. Due to differences in the hardware and software platforms available at different laboratories for running molecular tests, BGL’s may need to adjust the configuration of the reagents and there may be changes to the related software in order for the tests to be performed on particular hardware platforms. Making any such adjustments could take a considerable amount of time and expense, and BGL’s might not will succeed in running its tests on the hardware and software that it may encounter in different laboratories. To manage this issue, BGL’s may license or acquire additional instruments and software from another company that will be compatible with its tests. This may include additional licenses and license fees needed for reagents or components required hereto as well.
BGL’s biomarkers have not undergone clinical trials.
As there are no governmental regulations that materially restrict our screening business in Malaysia, BGL has not conducted clinical trials on its biomarkers. While BGL believes that its tests help detect the potential risk of different diseases, the specificity and sensitivity of those tests have not been determined in clinical trials let alone those that meet the scope or standards of clinical trials that would satisfy regulators in the United States or the European Union. If BGL were to conduct such clinical trials, the results might prove to be less successful than we anticipate, and such tests might not be approved for sale in markets that require such clinical trials.
BGL currently receives and expects to continue to receive a significant portion of its revenues from its genomic screening products, and if its efforts to further increase the use and adoption of these products fail, its business will be harmed.
BGL currently receives and expects to continue to receive a significant portion of its revenues from its screening tests. BGL undertakes efforts to increase the awareness and adoption of its tests among laboratories, clinics, clinicians, physicians, payors, and patients in new markets. Continued and additional market acceptance and its ability to attract new customers are key elements to its future success.
| 28 |
| Table of Contents |
BGL’s ability to increase sales of its services and establish greater levels of adoption and reimbursement for its tests is uncertain for many reasons, including, among others:
| · | BGL may be unable to demonstrate to laboratories, clinics, clinicians, physicians, payors, and patients that its services are superior to alternatives with respect to value, convenience, specificity, sensitivity, scope of coverage, and other factors; |
| · | third-party coverage and reimbursement are currently primarily limited to high-risk pregnancies and may not gain acceptance for use in the average-risk pregnancy population or for the screening of microdeletions, limiting the overall addressable market; |
| · | third-party payors may set the amounts of reimbursement at prices that reduce its profit margins or do not allow us to cover its expenses; |
| · | BGL may not be able to maintain and grow effective sales and marketing capabilities; |
| · | its sales and marketing efforts may fail to effectively reach customers or communicate the benefits of its services; |
|
|
|
| · | superior alternatives to its services may be developed and commercialized; |
| · | BGL may experience supply constraints, including due to the failure of its key suppliers to provide required sequencing instruments and reagents; |
| · | regulatory or legislative bodies may adopt new regulations or policies or take other actions that impose significant restrictions on its ability to market its services. |
If the market and its market share for its genomic products fail to grow or grow more slowly than expected, its business, operating results, and financial condition would be adversely affected.
BGL’s success depends on their ability to improve and enhance its current tests and new test candidates, which is complex and costly, and the results are uncertain.
Effective execution of research and development activities and the timely introduction of enhanced, improved, or new tests and test candidates to the market are important elements of BGL’s business strategy. For example, BGL is currently collaborating with the National Heart Institute in Malaysia to identify genomic signatures in acute myocardial infarctions. However, the development of enhanced, improved, or new heart attack risks is complex, costly, and uncertain and requires us to, among other factors, accurately anticipate patients’, clinicians’, and payors’ needs, and emerging technology trends.
In the development of enhanced, improved, or new test and test candidates, BioNexus can provide no assurance that:
| · | BGL will develop any tests that meet its desired target product profile and address the relevant clinical need or commercial opportunity; |
| · | any tests that BGL develop will prove to be effective in clinical trials, platform validations, or otherwise; |
| 29 |
| Table of Contents |
| · | BGL will obtain necessary regulatory authorizations, in a timely manner or at all; |
| · | any tests that BGL develop will be successfully marketed to and ordered by healthcare providers; |
| · | any tests that BGL develop will be produced at an acceptable cost and with appropriate quality; |
| · | its current or future competitors will not introduce tests similar to ours that have superior performance, lower prices, or other characteristics that cause healthcare providers to recommend, and consumers to choose, such competitive tests over ours; or |
| · | third parties do not or will not hold patents in any key jurisdictions that would be infringed by its tests. |
These and other factors beyond BGL’s control could delay its launch of enhanced, improved, or new test and test candidates.
The research and development process in the biotechnology industry generally requires a significant amount of time from the research and design stage through commercialization. The launch of such new test requires the completion of certain clinical development and/or assay validations in the commercial laboratory. This process is conducted in various stages, and each stage presents the risk that BGL will not achieve its goals and will not be able to complete clinical development for any planned test in a timely manner. Such development and/or validation failures could prevent or significantly delay its ability to obtain FDA clearance or approval as may be necessary or desired, obtain approval by entities that provide oversight over laboratory diagnostic tests in the localities BGL operate in, or launch any of its planned tests and test candidates. At times, it may be necessary for us to abandon a product in which BGL has invested substantial resources. Without the timely introduction of new test candidates and improvements or enhancements of its current tests, its tests may become obsolete over time and its competitors may develop tests that are more competitive, in which case its business, operating results, and financial condition will be harmed.
BGL faces challenges from the evolving regulatory environment and increasing public awareness on privacy, personal data protection and cyber security. Actual or alleged failure to comply with privacy, cybersecurity and data protection-related laws and regulations could adversely affect BGL’s business and reputation.
BGL face risks inherent in handling large volumes of data and in protecting the security of such data. In particular, BGL face a number of challenges relating to data inter-connected with regional labs, including:
| · | protecting the data in and hosted on BGL’s system, including against hacking on BGL’s system by outside parties or its employees; |
|
|
|
| · | addressing concerns related to privacy and sharing, safety, security and others; |
|
|
|
| · | complying with applicable laws, rules and regulations relating to the collection, use, disclosure of personal information, including any requests from regulatory and government authorities relating to such data; |
|
|
|
| · | Any systems failure or security breach or lapse those results in the release of user data could harm BGL’s reputation and brand and, consequently, BGL’s business, in addition to exposing us to potential legal liability. |
As the company’s operations expand, it may be subject to these laws in other jurisdictions where its customers and other participants are located. The laws, rules and regulations of other jurisdictions may impose more stringent or conflicting requirements and penalties than those in Malaysia, compliance with which could require significant resources and costs. BGL’s privacy policies and practices concerning the collection, use and disclosure of user data are posted on its websites. Any failure, or perceived failure, by us to comply with BGL’s posted privacy policies or with any regulatory requirements or privacy protection-related laws, rules and regulations could result in proceedings or actions against us by authorities or others. These proceedings or actions may subject us to significant penalties and negative publicity, require BGL to change its business practices, increase its costs and severely disrupt its business.
| 30 |
| Table of Contents |
BGL’s software is highly complex and may contain undetected errors.
BGL’s proprietary software underlying its diagnosis is highly complex and may contain undetected errors or vulnerabilities, some of which may only be discovered after a diagnosis. This may result in an inaccurate diagnosis which could expose us to substantial liability due to the misdiagnosis. Any errors or vulnerabilities discovered in BGL’ software could result in damage to BioNexus’ reputation, loss of clients, loss of revenue or liability for damages, any of which could adversely affect BioNexus’ growth prospects and its business.
BGL’s use of “open source” software could subject its proprietary software to general release, adversely affect its ability to sell its tests and subject the company to possible litigation.
A portion of the screenings by BGL incorporate so-called “open-source” software and BGL may incorporate open-source software into other tests and technologies in the future. Such open-source software generally is licensed by its authors or other third parties under open-source licenses. Some open-source licenses may contain certain unfavorable conditions, such as requirements that BGL disclose source code for modifications or derivative works that the company makes to the open-source software and that the company license such modifications or derivative works to third parties at no cost or under the terms of the particular open-source license. BGL monitors its use of open-source software in an effort to avoid uses in a manner that would require it to disclose or grant licenses under its proprietary source code; however, there can be no assurance that such efforts will be successful. Open-source license terms are often ambiguous and such use could inadvertently occur. There is little legal precedent governing the interpretation of many of the terms of these licenses, and the potential impact of these terms on BioNexus’ business may result in unanticipated obligations regarding its technologies. If an author or other third party that distributes such open-source software were to allege that BGL had not complied with the conditions of an open-source license, the company could incur significant legal costs defending itself against such allegations. In the event such claims were successful, BGL could be subject to significant damages or be enjoined from the distribution of the infringing product. These risks could be difficult to eliminate or manage, and, if not addressed, could harm BioNexus’ business, financial condition and results of operations.
BGL currently only uses open-source software for Covid- 19, HPV, HIV, and Dengue screenings. For screening process on cancers, inflammatory diseases and osteoarthritis, BGL uses company proprietary algorithm software for data analysis and interpretation established by Co-founder Professor CC Liew.
BGL may face competition from other biotechnology competitors and its operating results will suffer if BGL fail to compete effectively.
BGL competes with companies worldwide that specialize in RNA blood analysis to detect disease. Laboratories in universities and research institutions that are attempting to extend their research from DNA into RNA screening could become competitors if they succeed. Many of BGL’ competitors and potential competitors may have stronger financial resources than the company. Their discovery and development of novel protocols could make BGL’s screening obsolete. As a result of these factors, BGL’s competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing screening process for cancer, inflammation, osteoarthritis and many more indications.
| 31 |
| Table of Contents |
In addition, smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. In addition, many universities and private and public research institutes may become active in BGL’s target disease areas.
If BGL’s competitors market products that are more effective, safer or less expensive or that reach the market sooner than BGL’s future tests, if any, BioNexus may not achieve commercial success. In addition, because of BGL’s limited resources, it may be difficult for us to stay abreast of the rapid changes in each technology. If BGL fails to stay at the forefront of technological change, BGL may be unable to compete effectively. Technological advances or products developed by BGL’s competitors may render BGL’s technologies or test candidates obsolete, less competitive or not economical.
Security breaches, loss of data, and other disruptions could compromise sensitive information related to BGL’s business or prevent us from accessing critical information and expose us to liability, which could adversely affect BGL’s business and its reputation.
In the ordinary course of BGL’s business, BGL collect and store sensitive data, including protected health information, personally identifiable information, financial information, intellectual property, and proprietary business information owned or controlled by the company or its customers, payers, and other parties. BGL manages and maintains its applications and data utilizing a combination of on-site systems and cloud-based data centers. The company utilize external security and infrastructure vendors to manage parts of its data centers. BGL also communicates sensitive data, including patient data, electronically, and through relationships with multiple third-party vendors and their subcontractors. These applications and data encompass a wide variety of business-critical information, including research and development information, patient data, commercial information, and business and financial information. BGL faces a number of risks relative to protecting this critical information, including loss of access risk, inappropriate use or disclosure, inappropriate modification, and the risk of the company being unable to adequately monitor, audit, and modify its controls over critical information. This risk extends to the third-party vendors and subcontractors BGL uses to manage this sensitive data.
The secure processing, storage, maintenance, and transmission of this critical information are vital to BGL’s operations and business strategy, and BGL devote significant resources to protecting such information. Although BGL takes measures to protect sensitive data from unauthorized access, use or disclosure, BGL’s information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance, or other malicious or inadvertent disruptions. In addition, while BGL has implemented security measures and a formal, dedicated enterprise security program to prevent unauthorized access to patient data, such data is currently accessible through multiple channels, and there is no guarantee BGL can protect its data from breach. Unauthorized access, loss, or dissemination could also result in delays of BGL’s services and tests development and commercialization as well as damage BGL’s reputation, including BGL’s ability to conduct its analysis, deliver test results, process claims and appeals, provide customer assistance, conduct research and development activities, collect, process, and prepare company financial information, provide information about BGL’s tests and other patient and physician education and outreach efforts through its website, and manage the administrative aspects of its business.
Any such unauthorized access, loss, or dissemination of information could also result in legal claims or proceedings, liabilities under Malay laws and regulations in relation to the protection of personal information and cybersecurity as well as those specifically governing patient and medical data. BGL shall establish, maintain and execute internal systems to safeguard relevant personal healthcare data. Any failure to comply with above-mentioned regulation would result in administrative liabilities including but not limited to informed criticism.
| 32 |
| Table of Contents |
BGL plans to expand its tests and services to multiple countries exposes us to risks associated with doing business outside of Malaysia. The expansion may not be successful, which could limit BGL’s ability to grow its revenue, net income, and profitability.
As BGL plan to set up RNA screening labs operations in Indonesia, Middle East, USA, China and Germany, if approved, its businesses are subject to risks associated with doing business outside Malaysia including an increase in BioNexus’ expenses, diversion of BioNexus’ management’s attention from the research and development of additional diseases/disorders risk detection or forgoing profitable licensing opportunities in these economies. Additionally, Chemrex currently offers and sells chemical raw materials to customers in Southeast Asia markets outside of Malaysia.
Accordingly, the Company’s business and financial results in the future could be adversely affected due to a variety of factors including the risks associated with expanding into markets in which the Company has limited or no experience and in which the company may be less well-known. The Company may be unable to attract a sufficient number of customers and other participants, fail to anticipate competitive conditions or face difficulties in operating effectively in these new markets. The expansion of the Company’s cross-border business will also expose us to risks relating to staffing and managing cross-border operations, increased costs to protect intellectual property, tariffs and other trade barriers, differing and potentially adverse tax consequences, increased and conflicting regulatory compliance requirements, lack of acceptance of the Company’s product and service offerings, challenges caused by distance, language and cultural differences, exchange rate risk and political instability. Accordingly, any efforts the Company make to expand its cross-border operations may not be successful, which could limit the Company’s ability to grow its revenue, net income and profitability.
Risk Related to Chemrex’s Business and Industry
The chemical raw material industry is cyclical and both recessions and prolonged periods of slow economic growth could have an adverse effect on Chemrex’s business.
Demand for most of Chemrex’s products is cyclical in nature and sensitive to general economic conditions. Chemrex’s business supports cyclical industries such as the construction, energy, appliance and medical devices. As a result, downturns in the Malaysian economy, the global economy or any of these industries could materially adversely affect Chemrex’s results of operations, financial condition and cash flows. The global economy is recovering from its lows during the third quarter of 2022, but the pace of the recovery in 2023 will likely depend on how quickly normal activities can resume as well as government stimulus programs or infrastructure spending. We expect that Chemrex can do better in 2023 if investments and visitors (especially from China) resume their entry into Malaysia as was the same pre-pandemic. The boosts in the tourism and public transportation industry will push up the FRP material usage for industrial needs. Nonetheless, even with this economic recovery, challenges from ongoing uncertainties, both in Malaysia and in other regions of the world, remain. However, we believed that Chemrex's customers in the manufacturing, construction, and oil and gas sectors would resume their normal operations from the second or third quarter of 2023.
BioNexus is unable to predict the duration of current economic conditions. Future economic downturns, prolonged slow growth or stagnation in the economy, or a sector-specific slowdown in one of its key end-use markets, such as non-residential construction, could materially adversely affect Chemrex’s business, results of operations, financial condition and cash flows, especially considering the capital-intensive nature of Chemrex’s business.
The results of Chemrex’s operations are sensitive to volatility in the cost of raw materials, particularly fibre reinforced plastics.
Chemrex, as a reseller, rely on outside vendors to supply us with raw materials, including fibre reinforced plastics. Chemrex purchase most of its primary raw material, from numerous other sources located throughout Malaysia and internationally.
Prices of these chemical raw materials are volatile and are influenced by changes exports in response to demands of Chemrex’s global competitors and customers, as well as currency fluctuations. At any given time, BioNexus may be unable to obtain an adequate supply of these chemical raw materials with price and other terms acceptable to us. The availability and prices of raw materials may also be negatively affected by new laws and regulations, allocation by suppliers, interruptions in production, accidents or natural disasters, changes in exchange rates, worldwide price fluctuations, and the availability and cost of transportation.
| 33 |
| Table of Contents |
If Chemrex’s suppliers increase the prices of its chemical raw materials, BioNexus may not have alternative sources of supply. In addition, to the extent that Chemrex has quoted prices to its customers and accepted customer orders for its products prior to purchasing necessary raw materials, it may be unable to raise the price of its products to cover all or part of the increased cost of the materials. Also, if Chemrex are unable to obtain adequate and timely deliveries of its chemical raw materials, it may be unable to timely deliver orders of its products. This could cause Chemrex to lose sales, incur additional costs or suffer harm to its reputation.
Disruptions in the supply of chemicals that we distribute or in the operations of our customers could adversely affect our business.
Our business depends on access to adequate supplies of the chemicals that our customers purchase from us. From time to time, we may be unable to access adequate quantities of certain chemicals because of supply disruptions due to natural disasters (including hurricanes and other extreme weather), industrial accidents, scheduled production outages, high demand leading to allocation, port closures and other transportation disruptions and other circumstances beyond our control, or we may be unable to purchase chemicals that we are obligated to deliver to our customers at prices that enable us to earn a profit. In addition, unpredictable events may have a significant impact on the industries in which many of our customers operate, reducing demand for products that we normally distribute in significant volumes.
Significant changes in the business strategies of our suppliers could also disrupt our supply. Large chemicals manufacturers may elect to distribute certain products (or products in certain regions) directly to end user customers, instead of relying on independent distributors such as us. While we do not believe that our results depend materially on access to any individual producer’s products, a reversal of the trend toward more outsourced distribution of chemicals would likely result in increasing margin pressure or products becoming unavailable to us. Any of these developments could have a material adverse effect on our business, financial condition and results of operations.
We have non-written contracts with suppliers and customers, which are generally terminable upon notice, and the termination of our relationships with suppliers and customers contracts could negatively affect our business.
Our purchases and sales of chemicals are typically made pursuant to verbal purchase orders rather than written contracts. Many of our contracts with both customers and suppliers are terminable without cause upon 30 days’ notice to us from the supplier or customer. Our business relationships and reputation may suffer if we are unable to meet our delivery obligations to customers which may occur because many of our suppliers are not subject to contracts or can terminate contracts on short notice. In addition, renegotiation of purchase or sales terms to our disadvantage could reduce our sales margins. Any of these developments could adversely affect our business, financial condition, and results of operations.
We may lose customers and suffer damage to our reputation if we are unable to meet customer demand for a particular product.
We face the risk of dissatisfied customers and damage to our reputation if we cannot meet customer demand for a particular chemical because we are short on inventories. In addition, particularly in cases of pronounced cyclicality in the end market, it can be difficult to anticipate our customers’ requirements for particular chemicals, and we could be asked to deliver larger-than-expected quantities of a particular chemical on short notice. If for any reason we experience widespread, systemic difficulties in filling customer orders, our customers may be dissatisfied and discontinue their relationship with us or we may be required to pay a higher price to obtain the needed chemical on short notice, thereby adversely affecting our margins.
| 34 |
| Table of Contents |
We may be exposed to product returns and product liability claims and latent defect liability claims.
Our FRP and other raw materials are used to produce a wide variety of goods including handrails, bench tops, automotive and aero parts, cleanroom panels, and covers for various instruments used in manufacturing. We are exposed to potential product returns and latent defect liability claims from our customers and the end-users of goods and products. Although we have put in place stringent quality control measures, including the setting up of different teams for incoming quality control, quality control and quality assurance which monitor the quality of the raw material, semi-finished products as well as finished products, there may be undetected flaws or manufacturing defects or other irregularities that may be subsequently detected at any point in the life of our products. We have adopted return policy on products with manufacturing defects to accommodate our customers. If after any checkup or analysis by our laboratory the defect of a product is found to be manufacturing defect, return and replacement of products will be made. Therefore, if undetected flaws or manufacturing defects or other irregularities from either the design or manufacture of our products are to occur, additional costs and expenses which we may not recoup may incur, and our revenue and costs control can be negatively impacted.
In addition, if our defective or sub-standard products cause bodily injuries or property damage, our suppliers may face latent defect liability claims from our customers or the end-users of goods and products made with our products and regardless of the merits or the outcome of these claims, we may be required to address and, if necessary, and divert management attention and other resources from our business and operations. We may also face adverse publicity associated with such claims, which could have an adverse effect on our business, results of operations and financial condition.
Risks Related to Its Operations
BioNexus’ officers and directors may in future have outside business activities. As a result, there may be potential conflicts of interest and negatively impact the amount of time they will be able to dedicate to the company.
Currently BioNexus’ officers, who are also directors, have been working on promoting business for the Company. A potential conflict of interest may arise in the future that may cause BioNexus’ business to fail, including conflicts of interest in allocating their time to the company and their other business interests. While the company’s officers have verbally agreed to devote sufficient time and attention to the affairs of the Company, it has no written arrangement with BioNexus’ officers regarding this matter. As a result, BioNexus may face conflicts between business decisions that they may have to make regarding its operations and that of their other business interests.
BioNexus may not be able to attract and retain key senior management members and research and development personnel.
BioNexus’ future success depends upon the continuing services of members of its senior management team and key research and development personnel and consultants. Although BioNexus typically requires BioNexus’ key personals to enter into non-compete and confidentiality agreement with us, BioNexus cannot prevent they join the company’s competitor after the non-compete period. The loss of their services could adversely impact its ability to achieve its business objectives. If one or more of BioNexus’ senior management or key clinical and scientific personnel are unable or unwilling to continue in their present positions or joins a competitor or forms a competing company, the company may not be able to replace them in a timely manner or at all, which will have a material and adverse effect on its business, financial condition and results of operations.
In addition, the continued growth of BioNexus’ business depends on its ability to hire additional qualified personnel with expertise in molecular biology, chemistry, biological information processing, software, engineering, sales, marketing, and technical support. BioNexus compete for qualified management and scientific personnel with other life science and technology companies, universities, and research institutions in Malaysia and overseas. Competition for these individuals is intense, and the turnover rate can be high. Failure to attract and retain management and scientific and engineering personnel could prevent the company from pursuing collaborations or developing its services and products or technologies.
| 35 |
| Table of Contents |
BioNexus may be unable to protect the company’s intellectual property adequately.
BioNexus’ software intellectual property is an essential asset of its business. To establish and protect its intellectual property rights, BioNexus relies primarily upon trade secrets, and to a lesser extent, contractual provisions with current and future employees. As a result, BioNexus’ efforts to protect its intellectual property may not be sufficient or effective. If these measures do not protect its intellectual property rights, third parties could use the Company’s technology, and its ability to compete in the market would be reduced significantly.
In addition, BioNexus may not be effective in policing unauthorized use of the company’s intellectual property. Even if BioNexus does detect violations, BioNexus may need to engage in litigation to enforce its intellectual property rights. Any enforcement efforts BioNexus undertake, including litigation, could be time-consuming and expensive and could divert BioNexus’ management’s attention. In addition, BioNexus’ efforts may be met with defenses and counterclaims challenging the validity and enforceability of its intellectual property rights or may result in a court determining that its intellectual property rights are unenforceable. If BioNexus is unable to cost-effectively protect its intellectual property rights, then its business could be harmed.
BioNexus may be subject to intellectual property claims, which are extremely costly to defend, could require us to pay significant damages and could limit the company’s ability to use certain technologies in the future.
Companies in bio-medical or bio-technology industries are frequently subject to litigation based on allegations of infringement or other violations of intellectual property rights. To the extent BioNexus gain greater public recognition, BioNexus may face a higher risk of being the subject of intellectual property claims. Third-party intellectual property rights may cover significant aspects of BioNexus’ technologies or business methods or block us from expanding its offerings. Any intellectual property claims against us, with or without merit, could be time consuming and expensive to settle or litigate and could divert the attention of its management. Litigation regarding intellectual property rights is inherently uncertain due to the complex issues involved, and the company may not be successful in defending itself in such matters.
In addition, some of BioNexus’ competitors have extensive portfolios of issued patents. Many potential litigants, including some of BioNexus’ competitors and patent holding companies, have the ability to dedicate substantial resources to enforcing their intellectual property rights. Any claims successfully brought against us could subject us to significant liability for damages and BioNexus may be required to stop using technology or other intellectual property alleged to be in violation of a third party’s rights. BioNexus also might be required to seek a license for third-party intellectual property. Even if a license is available, BioNexus could be required to pay significant royalties or submit to unreasonable terms, which would increase its operating expenses. BioNexus may also be required to develop alternative non-infringing technology, which could require significant time and expense. If BioNexus cannot license or develop technology for any allegedly infringing aspect of its business, BioNexus would be forced to limit its service and may be unable to compete effectively. Any of these results could harm BioNexus’ business.
BioNexus may pursue collaborations, in-licensing or out-license arrangements, joint ventures, strategic alliances, partnerships or other strategic investments or arrangements, which may fail to produce anticipated benefits and adversely affect the company’s operations.
BioNexus may pursue opportunities for collaboration, in-licensing, out-license, joint ventures, acquisitions of products, assets or technology, strategic alliances, or partnerships that BioNexus believes would be complementary to or promote BioNexus’ existing business. Proposing, negotiating and implementing these opportunities may be a lengthy and complex process. Other companies, including those with substantially greater financial, marketing, sales, technology, or other business resources, may compete with us for these opportunities or arrangements. BioNexus may not be able to identify, secure, or complete any such transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms, or at all.
| 36 |
| Table of Contents |
BioNexus has limited experience with respect to these business development activities. Management and integration of a licensing arrangement, collaboration, joint venture or other strategic arrangement may disrupt BioNexus’ current operations, decrease its profitability, result in significant expenses, or divert management resources that otherwise would be available for its existing business. BioNexus may not realize the anticipated benefits of any such transaction or arrangement.
Furthermore, partners, collaborators, or other parties to such transactions or arrangements may fail to fully perform their obligations or meet its expectations or cooperate with us satisfactorily for various reasons and subject us to potential risks, including the followings:
| · | partners, collaborators, or other parties have significant discretion in determining the efforts and resources that they will apply to a transaction or arrangement; |
| · | partners, collaborators, or other parties could independently develop, or develop with third parties, services and products that compete directly or indirectly with its services and products; |
| · | partners, collaborators, or other parties may stop, delay or discontinue research and development, and commercialization efforts; |
| · | partners, collaborators, or other parties may not properly maintain or defend BioNexus’ intellectual property rights or may use its intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate its intellectual property or proprietary information or expose us to potential liability; |
|
|
|
| · | disputes may arise between us and partners, collaborators, or other parties that cause the delay or termination of the research, development or commercialization of BioNexus’ services and products, or that result in costly litigation or arbitration that diverts management attention and resources; |
| · | partners, collaborators, or other parties may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable services and products; and |
| · | partners, collaborators, or other parties may own or co-own intellectual property covering BioNexus’ services and products that results from BioNexus’ collaborations with them, and in such cases, BioNexus would not have the exclusive right to commercialize such intellectual property. |
Any such transactions or arrangements may also require actions, consents, approval, waiver, participation or involvement of various degrees from third parties, such as regulators, government authorities, creditors, licensors or licensees, related individuals, suppliers, distributors, shareholders or other stakeholders or interested parties. There is no assurance that such third parties will be cooperative as BioNexus desires, or at all, in which case BioNexus may be unable to carry out the relevant transactions or arrangements.
| 37 |
| Table of Contents |
Risks Related to Doing Business in the Southeast Asia Region
Changes in policies in Malaysia and other Southeast Asian countries could have a significant impact upon the company’s ability to operate profitably in Malaysia and the Southeast Asia region.
Changes in the political and economic policies of Malaysia and other governments in Southeast Asia may materially and adversely affect BioNexus’ business, financial condition and results of operations and may result in its inability to sustain its growth and expansion strategies. Accordingly, BioNexus’ financial condition and results of operations are affected to a significant extent by economic, political and legal developments in Southeast Asia region.
The Southeast Asia economy differs from the economies of most developed countries in many respects, including the extent of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. In addition, the government continues to play a significant role in regulating industry development by imposing industrial policies. The government also exercises significant control over economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy, regulating financial services and institutions and providing preferential treatment to particular industries or companies.
Local governments have implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall economy, but may also have a negative effect on us. BioNexus’ financial condition and results of operation could be materially and adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. In addition, the government has implemented in the past certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity, which in turn could lead to a reduction in demand for its services and consequently have a material adverse effect on its businesses, financial condition and results of operations.
Developments in the social, political, regulatory and economic environment in Malaysia may have a material adverse impact on us.
BioNexus’ business, prospects, financial condition and results of operations may be adversely affected by social, political, regulatory and economic developments in Malaysia. Such political and economic uncertainties include, but are not limited to, the risks of war, terrorism, nationalism, nullification of contract, changes in interest rates, imposition of capital controls and methods of taxation.
All sectors of the economy in 2022 across Malaysia saw their supply chains interrupted, demand for their products and services decline, shortages in supplies and inputs. We will emerge in a very different world compared to the one before the outbreak. All organizational functions are intended to prioritize and optimize spending or postpone tasks that will not bring value in the current environment. It created serious consequences because various businesses are facing massive losses due to their declining activities and the accompanying unpredictable future of many businesses. A substantial decrease has been observed in overall spending, which resulted in an array of estimated long-term uncertainty impacts. Consequently, many businesses and firms closed, and employees were dismissed. Towards a new recovery phase in 2022, most businesses and organizational functions were prioritizing our spending or postpone any tasks and events that do not bring any value to the current situation because even when the challenges are successfully addressed, this will not guarantee any promising future. Hence, we were alerted about the available survival strategies to sustain us throughout this unforeseen circumstance and in the future. A “new normal” indicates how we should digest the current situation and initiate a business growth pattern. Returning to the pre-pandemic business pattern will take time and depends on the government’s response to the population health and socioeconomic demands arising due to the pandemic.
Although the overall Malaysian economic environment (in which BioNexus predominantly operate) appears to be positive, there can be no assurance that this will continue to prevail in the future. Economic growth is determined by countless factors, and it is extremely difficult to predict with any level of absolute certainty.
| 38 |
| Table of Contents |
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in Malaysia against the company or its management based on foreign laws, and the ability of U.S. authorities to bring actions in Malaysia may also be limited.
The company’s operating subsidiaries are incorporated in Malaysia and conduct substantially all of its operations in Southeast Asia. All of BioNexus’ executive officers and directors reside outside the United States, and all their assets are located outside of the United States. As a result, it may be difficult or impossible for shareholders to bring an action against us or against these individuals in Malaysia in the event that you believe that your rights have been infringed under the securities laws of the United States or otherwise. Even if you are successful in bringing an action of this kind, the laws of Malaysia may render you unable to enforce a judgment against BioNexus’ assets or the assets of BioNexus’ directors and officers. There is no statutory recognition in Malaysia of judgments obtained in the United States, although the courts of Malaysia will generally recognize and enforce a non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits. The rights of shareholders to take legal action against us and BioNexus’ directors, actions by minority shareholders and the fiduciary responsibilities of its directors are to a large extent governed by the common law of Malaysia. The common law of Malaysia is derived in part from comparatively limited judicial precedent in Malaysia as well as from English common law, which provides persuasive, but not binding, authority in a court in Malaysia. The rights of BioNexus’ shareholders and the fiduciary responsibilities of its directors under Malaysian law are not as clearly established as they would be under statutes or judicial precedents in the United States. Malaysia has a less developed body of securities laws than the United States and provides significantly less protection to investors. As a result, BioNexus’ public shareholders may have more difficulty in protecting their interests through actions against us, its management, its directors or its major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States. In addition, to receive any form of remedy, the shareholders would have to engage Malaysian counsel regarding the process to receive any such remedy.
BioNexus is subject to foreign exchange control policies in Malaysia.
The ability of BioNexus’ subsidiaries to pay dividends or make other payments may be restricted by the foreign exchange control policies in the countries where they operate. For example, there are foreign exchange policies in Malaysia which support the monitoring of capital flows into and out of the country in order to preserve its financial and economic stability. The foreign exchange policies are administered by the Foreign Exchange Administration, an arm of Bank Negara Malaysia (“BNM”), the central bank of Malaysia. The foreign exchange policies monitor and regulate both residents and non-residents. Under the current Foreign Exchange Administration rules issued by BNM, non-residents are free to repatriate any amount of funds from Malaysia in foreign currency other than the currency of Israel at any time (subject to limited exceptions), including capital, divestment proceeds, profits, dividends, rental, fees and interest arising from investment in Malaysia, subject to any withholding tax. In the event BNM or any other country where BioNexus operates introduces any restrictions in the future, it may be affected in its ability to repatriate dividends or other payments from BioNexus’ subsidiaries in Malaysia or in such other countries. Since BioNexus rely principally on dividends and other payments from its subsidiaries for its cash requirements, any restrictions on such dividends or other payments could materially and adversely affect its liquidity, financial condition and results of operations.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate office for BGL is located at Unit 2, level, Tower B, Avenue 3, The Vertical Business Suite II, Bangsar South, 8 Jalan Kerinchi, 59200 Kuala Lumpur, Malaysia. The lease commenced on December 16, 2018 and terminates on December 15, 2024. The space consists of 1,300 square feet with an annual rent of approximately $13,500.
One of our labs is located at 4th Floor, Wisma Life Care, No. 5, Jalan Kerinchi, Bangsar South, 59200 Kuala Lumpur, Malaysia. The lease commenced on November 1, 2016 and terminates on October 31, 2023. The annual rent is approximately $6,800. Our other laboratory is located at Lab 353, University Science Malaysia, George Town, Penang, Malaysia. The lease commenced on December 1, 2017 and terminates on November 30, 2024. The space consists of 1,500 square feet with an annual rent of approximately $7,300.
| 39 |
| Table of Contents |
On July 2, 2012, we purchased a 25,000 sq. ft wholesale distribution center located at 4, Jalan CJ 1/6, Kawasan Perusahaan Cheras Jaya, 43200 Cheras, Selangor, Malaysia, and two investment properties for $1,506,969. The two investment properties are listed below.
| · | A 1,100 sqft condominium located at No. Unit 2B-17-03, Duet Residence, Jalan Kinrara 6, Bandar Kinrara, 47180 Puchong, Selangor, purchased on August 26, 2020; |
| · | A 2,000 sqft commercial building located at First floor, No. 2B Pelangi Avenue, Jalan Kelicap 42A/KU1, Klang Bandar, Diraja, 41050 Klang, Selangor purchased on September 21, 2020. |
On January 18, 2023, we entered into a lease for the first-floor unit at No. 5-1, Jalan CJ3/13-2, Pusat Bandar Cheras Jaya, 43200 Cheras, Selangor. The lease commenced on 18 January 2023 and terminates on January 17, 2024. The purpose of this lease is to provide accommodation for our warehouse staff.
Item 3. Legal Proceedings.
We are not subjected to nor engaged in any litigation, arbitration, or claim of material importance, and no litigation, arbitration, or claim of material importance is known to us to be pending or threatened by or against our Company that would have a material adverse effect on our Company's results of operations or financial condition.
Item 4. Mine Safety Disclosures.
None.
| 40 |
| Table of Contents |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
On March 11, 2020, FINRA authorized the trading of BioNexus’ common stock under the symbol “BGLC”, however, we began trading on the OTC-QB markets in September 2020. The table below sets forth, for the fiscal quarters indicated, the high and low bid prices per share of our common stock as reflected on the OTC-QB markets. The quotations represent inter-dealer prices without adjustment for retail markups, markdowns or commissions, and may not necessarily represent actual transactions.
The bid prices set forth below reflect inter-dealer quotations, do not include retail markups, markdowns or commissions and do not necessarily reflect actual transactions.
|
| High |
|
| Low |
| ||
Fiscal Year Ended December 31, 2022 |
|
|
|
|
|
| ||
First Quarter |
| $ | 2.23 |
|
| $ | 1.10 |
|
Second Quarter |
| $ | 1.73 |
|
| $ | 1.01 |
|
Third Quarter |
| $ | 1.18 |
|
| $ | 0.16 |
|
Fourth quarter |
| $ | 1.08 |
|
| $ | 0.72 |
|
Fiscal Year Ended December 31, 2021 |
|
|
|
|
|
|
|
|
Third Quarter |
| $ | 1.86 |
|
| $ | 1.00 |
|
Fourth Quarter |
| $ | 2.25 |
|
| $ | 1.00 |
|
The OTC-QB is a quotation system and not a national securities exchange, and many companies have experienced limited liquidity when traded through this quotation system. Any trading has been sporadic and there has been no meaningful trading volume.
Capital Stock:
Our authorized capital stock consists of 300,000,000 shares of common stock, no par value per share, and 30,000,000 shares of preferred stock, no par value per share. As of the date of this filing, there are 173,718,152 shares of our common stock issued and outstanding that was held by 316 stockholders of record and no shares of preferred stock issued and outstanding. The shares of preferred stock are “blank check’ meaning the Company’s board of directors can issue shares of preferred stock in such series with such rights, privileges and preferences as determined from time to time by the board of directors without shareholder approval.
Dividend Policy
The Company has not declared or paid any cash dividends on its Common Stock and does not intend to declare or pay any cash dividend in the foreseeable future. The payment of dividends, if any, is within the discretion of the board of directors and will depend on the Company’s earnings, if any, its capital requirements and financial condition and such other factors as the board of directors may consider.
Securities Authorized for Issuance under Equity Compensation Plans
The Company does not have any equity compensation plans or any individual compensation arrangements with respect to its Common Stock or Preferred Stock. The issuance of any of our Common Stock or Preferred Stock is within the discretion of our board of directors, which has the power to issue any or all of our authorized but unissued shares without stockholder approval.
Recent Sales of Unregistered Securities.
None
Issuer Purchases of Equity Securities
None
Item 6. Selected Financial Data.
As a “smaller reporting company” as defined by Rule 12b-2 of the Exchange Act, the Company is not required to provide this information.
| 41 |
| Table of Contents |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
General.
BioNexus was incorporated in the State of Wyoming on May 12, 2017 and operations of our Malaysian company began operations in July 2017. Consequently, the following discussion and analysis of the results of operations and financial condition of the Company is for fiscal years ended December 31, 2022 and December 31, 2021, respectively. This information should be read in conjunction with the notes to the financial statements that are included elsewhere herein. The consolidated financial statements presented herein (and to which this discussion relates) reflect the results of operations of the Company and its Malaysian subsidiaries. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements. We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this report, except as required by law. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this quarterly report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
Company Overview
BioNexus Gene Lab Corp., through our wholly owned subsidiary Chemrex Corporation Sdn. Bhd., focuses on the sale of chemical raw materials for the manufacture of industrial, medical, appliance, aero, automotive, mechanical, and electronic industries in the Southeast Asia region. These countries include Malaysia, Indonesia, Vietnam, and other countries in Southeast Asia.
Furthermore, the Company is also in the business of developing and providing safe, effective, and non-invasive liquid biopsy tests for the early detection of biomarkers that we believe are linked to diseases to minimize treatment costs and improve patient management. Our non-invasive blood tests provide analysis of changes in RNA to detect the potential risk of 11 different diseases.
Result of Operations
Results of Operations for the Year Ended December 31, 2022 Compared to the Year Ended December 31, 2021 (Audited).
The following table sets forth key components of the results of operations for fiscal years ended December 31, 2022 and 2021, respectively. As stated herein, on December 31, 2022, BioNexus consummated its acquisition of Chemrex Corporation Sdn. Bhd. (“Chemrex”), pursuant to a Share Exchange Agreement by and among BioNexus and Chemrex and the Chemrex shareholders. Accordingly, the audited financial information for the period ended December 31, 2022 includes the accounts of Chemrex and BioNexus Malaysia and the (audited) financial information for the period ended December 31, 2021 only includes the accounts of BioNexus Malaysia.
| 42 |
| Table of Contents |
The discussion following the table addresses these results.
Consolidated |
| Year ended |
| |||||
|
| December 31 (Audited) |
| |||||
|
| 2022 |
|
| 2021 |
| ||
|
|
|
|
|
|
| ||
REVENUE |
| $ | 10,928,707 |
|
| $ | 13,362,567 |
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (9,669,678 | ) |
|
| (11,095,626 | ) |
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 1,259,029 |
|
|
| 2,266,941 |
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 179,283 |
|
|
| 66,491 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
General and administrative |
|
| (1,729,489 | ) |
|
| (1,277,605 | ) |
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT FROM OPERATIONS |
|
| (291,177 | ) |
|
| 1,055,827 |
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (12,479 | ) |
|
| (12,973 | ) |
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT BEFORE TAX |
|
| (303,656 | ) |
|
| 1,042,854 |
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
Deferred tax |
|
| (3,898 | ) |
|
| (26,736 | ) |
Income tax |
|
| (48,412 | ) |
|
| (264,547 | ) |
Total tax expenses |
|
| (52,310 | ) |
|
| (291,283 | ) |
|
|
|
|
|
|
|
|
|
NET (LOSS)/PROFIT |
| $ | (355,966 | ) |
| $ | 751,571 |
|
|
|
|
|
|
|
|
| |
Other comprehensive income: |
|
|
|
|
|
|
|
|
Foreign currency translation (loss)/gain |
|
| (308,800 | ) |
|
| (233,946 | ) |
|
|
|
|
|
|
|
|
|
COMPREHENSIVE (LOSS)/ INCOME |
| $ | (664,766 | ) |
| $ | 517,625 |
|
Revenues. For the year ended December 31, 2022, we had revenues of $10,928,707 as compared to revenues of $13,362,567 for the year ended December 31, 2021, a decrease of approximately 18.2% due to after effect of Covid pandemic. The 2021’ revenue of $13,362,567 was partly contributed from Covid-19 screening amounting to $1,515,673 outsourced by Health Ministry to BGL.
In 2022, all sectors of the economy across the country saw their supply chains interrupted, demand for our products and services decline, shortages and delay in supplies and inputs, we emerged in a very different world compared to the one before the outbreak. Our business was affected because various businesses are facing massive losses due to their declining activities and the accompanying unpredictable future of many businesses. A substantial decrease has been observed in overall spending, which resulted in an array of estimated long-term uncertainty impacts. Consequently, many businesses and firms closed, and employees were dismissed. Towards a new recovery phase in 2022, most businesses and organizational functions were prioritizing our spending or postpone any tasks and events that do not bring any value to the current situation because even when the challenges are successfully addressed, this will not guarantee any promising future.
Cost of revenues. For the year ended December 31, 2022 we had cost of revenues of $9,669,678 as compared to cost of revenues of $11,095,626 for the year ended December 31, 2021, a decrease of approximately 12.9% due to lower sales caused by the above reasons.
Other Income. For the year ended December 31, 2022, we had other income of $179,283 as compared to other income of $66,491 for the year ended December 31, 2021, an increase of 169.6% for current year. The increase in other income for the current annual period was due to dividend income from Chemrex’ equity investment and additional fund deposited with the bank resulting from the revenue generated by Covid screening.
| 43 |
| Table of Contents |
Operating Expenses. For the year ended December 31, 2022, we had operating expenses of $1,729,489 as compared to operating expenses of $1,277,605 for the year ended December 31, 2021, an increase of 35.4% for the current year was due to depreciation on new equipment purchased, equipment maintenance expenses, employee compensation and benefits, professional and directors’ fees, marketing, travel expenses and adjustment of unrealized loss on foreign exchange for subsidiaries BGL and Chemrex.
Profit/(loss) from operations. We had a loss from operations of $291,177 for the year ended December 31, 2022 compared to a profit from operations of $1,055,827 for the year ended December 31, 2021, a decrease of 127.6% for the reasons discussed above.
Tax expense. For the year ended December 31, 2022, we had the total tax expense of $52,310 from deferred tax of $3,898 and tax provision of $48,412. The year ended December 31, 2021 the total tax expenses were $291,283 from deferred tax of $26,736 and income tax provision of $264,547. The higher tax expense for 2021 was also due to higher profit in 2021
Foreign currency translation (loss)/gain. We are exposed to fluctuations in foreign exchange rates on the revaluation of monetary assets and liabilities denominated in currencies other than the US Dollar. Therefore, any change in the relevant exchange rate will require us to recognize a transaction gain or loss on revaluation. For the annual period ended December 31, 2022, we had foreign currency translation loss of $308,800 compared with foreign currency translation loss of $233,946 for the prior annual period.
BioNexus Malaysia and Chemrex
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BioNexus Malaysia |
|
| Chemrex |
| ||||
|
| Year ended December 31, 2022 |
|
| Year ended December 31, 2021 |
| ||||||||||
REVENUE |
| $ | 95,816 |
|
| $ | 10,832,891 |
|
| $ | 1,515,673 |
|
| $ | 11,846,894 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (51,465 | ) |
|
| (9,618,213 | ) |
|
| (1,052,938 | ) |
|
| (10,042,688 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 44,351 |
|
|
| 1,214,678 |
|
|
| 462,735 |
|
|
| 1,804,206 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 8,830 |
|
|
| 170,453 |
|
|
| 7,467 |
|
|
| 59,024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (286,753 | ) |
|
| (1,051,855 | ) |
|
| (160,094 | ) |
|
| (989,617 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (5,657 | ) |
|
| (6,822 | ) |
|
| (4,158 | ) |
|
| (8,815 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT BEFORE TAX |
|
| (239,229 | ) |
|
| 326,454 |
|
|
| 305,950 |
|
|
| 864,798 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense : |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| (1,428 | ) |
|
| (2,470 | ) |
|
| (11,997 | ) |
|
| (14,739 | ) |
Income tax |
|
| - |
|
|
| (48,412 | ) |
|
| (30,482 | ) |
|
| (234,065 | ) |
Total tax expense |
|
| (1,428 | ) |
|
| (50,882 | ) |
|
| (42,479 | ) |
|
| (248,804 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET (LOSS)/PROFIT |
| $ | (240,657 | ) |
| $ | 275,572 |
|
| $ | 263,471 |
|
| $ | 615,994 |
|
Revenue. For the year ended December 31, 2022, Chemrex contributed $10,832,891 (99.1%) of total combined revenue of $10,928,707 compared to its contribution of $11,846,894 (88.66%) of total combined revenue of $13,362,567 for the year ended December 31, 2021, a decrease of 8.56%. The revenue decreased in 2022 was due to the lowering of selling price in view of competition. Some competitors were clearing their stock to improve their cash flow position after 1 ½ years of movement controls imposed by the government.
BioNexus had a revenue of $95,816 (0.9%) for the year ended December 31, 2022 as compared to revenues of $1,515,673 (11.34%) from the same period ended December 31, 2021, a decrease of 93.7%. The revenue decreased in 2022 was due to the outsource contract for Covid19 PCR test from Ministry of Health (HHS) had ended in December 2021.
| 44 |
| Table of Contents |
Cost of revenues. For the year period ended December 31, 2022, Chemrex had incurred $9,618,213 (99.5%) of the total combined cost of revenue of $9,669,678 as compared to the year ended December 31, 2021 wherein Chemrex had incurred $10,042,688 (90.5%) of the total combined cost of revenue of $11,095,626. The decrease of 4.23% in Chemrex’s cost of revenues was due to its decreased in revenues and reasons stated above.
BioNexus had incurred $51,465 (0.5%) on cost of revenues for the year ended December 31, 2022 as compared to cost of revenues of $1,052,938 (9.5%) for the same year ended December 31, 2021. The decrease of 95.1% was due to the buying less extract kits, reagents, laboratory consumables for covid-19 samples processing.
Other Income. For the year ended December 31, 2022, Chemrex contributed $170,453 (95.1%) of total other combined income of $179,283 as compared to the year ended December 31, 2021, 59,024 (88.8%) The increase of 188.79% is due to dividend income from equity investment.
BioNexus had other income of $8,830 (4.9%) for the year ended December 31, 2022 as compared $7,467 (11.2%) for the year ended December 31, 2021, an increase of 18.3% due to bank interest generated from Covid-19 screening’s revenue.
Operating Expenses. For the year ended December 31, 2022, Chemrex had incurred $1,051,855 (78.6%) of the total combined operating expenses of $1,338,608 for the year ended December 31, 2022 as compared to the operating expenses of $989,617 (86.1%) for the year ended December 31, 2021. The increase of 6.29% in Chemrex operating expenses for the 2022 due to increase in director's remuneration and loss on fair value of equity investment.
BioNexus Malaysia had incurred $286,753 (21.4%) on operating expenses for the year ended December 31, 2022 as compared to the operating expenses of $160,094 (13.9%) for the year ended December 31, 2021, an increase of 79.1%. The increase of $126,659 in operating costs was due to increase in marketing expenses, hiring and training new lab staffs, writing off investment in Genenews Diagnostic (company has been closed) and expired covid19 test kits. In addition, expenses incurred in heart attack research/clinical test and also year-end adjustment for unrealized loss on foreign exchange of $52,129 due to weakening of Malaysia ringgit against US dollar.
Profit /(loss) before tax. Chemrex had a profit before tax of $326,454 for the year ended December 31, 2022 as compared to $864,798 for the year ended December 31, 2021, a decrease of 62.25% while Bionexus Malaysia incurred a loss of $239,229 for the year ended December 31, 2022, a decrease of 178.2% compared to the year ended December 31, 2021, for the reasons discussed above.
Income tax expense. Chemrex had total tax expenses of $50,882 (97.3%) from deferred tax of $2,470 and tax provision of $48,412 for the year ended December 31, 2022 as compared to total tax expense of $248,804 (85.42%) for the last year ended December 31, 2021 from deferred tax of $14,739 and tax provision of $234,065.
BioNexus Malaysia had deferred tax of $1,428 (2.7%) and no tax provision for current year 2022 as compared to total tax expense of $42,479 (14.58%) for the last year ended December 31, 2021 from deferred tax of $11,997 and tax provision of $30,482.
LIQUIDITY AND CAPITAL RESOURCES
As of December 31, 2022, we had working capital of $4,017,749 compared with working capital of $4,821,100 as of December 31, 2021. The decreased in working capital as of December 31, 2022 from December 31, 2021 is due principally to the operating loss the Company experienced for the year 2022.
Our primary uses of cash have been for operations. The main sources of cash have been from operational revenues and the private placement of our common stock. The following trends are reasonably likely to result in a material decrease in our liquidity over the near to long term:
· | Addition of administrative and marketing personnel as the business grows, |
· | Development and patenting data analysis algorithm software, |
· | Increases in advertising and marketing in order to attempt to generate more revenues, and |
· | The cost of being a public company. |
| 45 |
| Table of Contents |
The Company believes that cash flow from operations together will be sufficient to sustain its current level of operations for at least the next 12 months of operations.
The following is a summary of the Company’s cash flows provided by (used in) / generated from operating, investing, and financing activities for the year ended December 31, 2022 and 2021:
|
| Year ended |
| |||||
|
| December 31, |
| |||||
|
| 2022 |
|
| 2021 |
| ||
Net Cash generated from operating activities |
| $ | 544,028 |
|
| $ | 9,161 |
|
Net cash used in investing activities |
|
| (450,498 | ) |
|
| (490,574 | ) |
Net cash generated from/(used in) financing activities |
|
| 115,962 |
|
|
| (28,222 | ) |
Foreign currency translation adjustment |
|
| (214,547 | ) |
|
| (154,138 | ) |
Net Change in Cash and Cash Equivalents |
| $ | (5,055 | ) |
| $ | (663,773 | ) |
Operating Activities
During the year ended December 31, 2022, the Company incurred a net loss of $355,966 which, after adjusting for amortization, depreciation, dividend income, fair value on investment, a decrease in inventories, trade receivables and deposits, a substantial reduction in trade payables, operating lease liabilities, resulted in net cash of $544,028 being generated from operating activities during the period. By comparison, during the year ended December 31, 2021, the Company had a net profit of $751,571 which, after adjusting for amortization, depreciation, dividend income, fair value on investment, an increase in inventories, trade receivables and deposits, a substantial reduction in trade payables, operating lease liabilities, resulted in net cash of $9,161 being generated from operating activities during the period.
Investing Activities
During the year ended December 31, 2022, the Company had net cash of $450,498 used in investment activities from acquisition of share investment of $511,706, purchase of plant & equipment of $54,171 and cash generated from dividend income of $115,379. During the year ended December 31, 2021, the Company had net cash from acquisition of share investment of $515,840, purchase of plant and equipment and disposal of other investments, resulting in net cash used in financing activities of $490,574.
Financing Activities
During the year ended December 31, 2022, Company had net cash of $115,962 generated from financing activities for 2.5 million shares subscriptions of $150,000 and fully repayment of a finance lease of $34,038. By comparison, during the year ended December 31, 2021, we had net cash used in financing activities of $28,222 for continued the repayment of a finance lease $26,302 and repayment of director $1,920.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable to smaller reporting companies.
Item 8. Financial Statements and Supplementary Data.
Our financial statements are contained in pages F-1 through F-19, which appear at the end of this Form 10-K Annual Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
| 46 |
| Table of Contents |
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
In connection with the preparation of this annual report, an evaluation was carried out by the Company’s management, with the participation of the principal executive officer and the principal financial officer, of the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act (“Exchange Act”) as of December 31, 2022. Disclosure controls and procedures are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the Commission’s rules and forms, and that such information is accumulated and communicated to management, including the principal executive officer and the principal financial officer, to allow timely decisions regarding required disclosures.
Based on that evaluation, the Company’s management concluded, as of the end of the period covered by this report, that the Company’s disclosure controls and procedures were not effective in recording, processing, summarizing, and reporting information required to be disclosed, within the time periods specified in the Commission’s rules and forms, and that such information was not accumulated and communicated to management, including the principal executive officer and the principal financial officer, to allow timely decisions regarding required disclosures.
Management’s Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control over financial reporting is a process, under the supervision of the principal executive officer and the principal financial officer, designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company’s financial statements for external purposes in accordance with United States generally accepted accounting principles (GAAP). Internal control over financial reporting includes those policies and procedures that:
· | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the Company’s assets; |
· | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and the board of directors; and |
· | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013) as set forth in its Internal Control - Integrated Framework. This assessment identified material weaknesses in internal control over financial reporting. A material weakness is a control deficiency, or a combination of deficiencies in internal control over financial reporting that creates a reasonable possibility that a material misstatement in annual or interim financial statements will not be prevented or detected on a timely basis. Since the assessment of the effectiveness of our internal control over financial reporting did identify a material weakness, management considers its internal control over financial reporting to be ineffective.
| 47 |
| Table of Contents |
Management has concluded that our internal control over financial reporting had the following material deficiencies:
| · | We were unable to maintain segregation of duties within our business operations due to our reliance on a single individual fulfilling the role of sole officer and director. |
|
|
|
| · | Lack of a functioning audit committee due to a lack of a majority of independent members and a lack of a majority of outside directors on our board of directors, resulting in ineffective oversight in the establishment and monitoring of required internal control and procedures. |
These control deficiencies to our 2020 or 2019 interim or annual financial statements could have resulted in a material misstatement that might have been prevented or detected by a segregation of duties. Accordingly, we have determined that this control deficiency constitutes a material weakness.
To the extent reasonably possible, given our limited resources, our goal is, upon consummation of a merger with a private operating company, to separate the responsibilities of principal executive officer and principal financial officer, intending to rely on two or more individuals. We will also seek to expand our current board of directors to include additional individuals willing to perform directorial functions. Since the recited remedial actions will require that we hire or engage additional personnel, this material weakness may not be overcome in the near term due to our limited financial resources. Until such remedial actions can be realized, we will continue to rely on the advice of outside professionals and consultants.
This annual report does not include an attestation report of our registered public accounting firm regarding our internal controls over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to Section 404(c) of the Sarbanes-Oxley Act that permit us to provide only management’s report in this annual report.
Changes in Internal Controls over Financial Reporting
During the year ended December 31, 2022, other than the change in ownership, there has been no change in internal control over financial reporting that has materially affected or is reasonably likely to materially affect our internal control over financial reporting.
Item 9B. Other Information.
None
| 48 |
| Table of Contents |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table set forth the name, age, and position of sole executive officers and directors. Executive officers were elected annually by our board of directors. Each executive officer held his office until he resigned, was removed by the Board, or his successor was elected and qualified. Directors were elected annually by our stockholders at the annual meeting. Each director held his office until his successor was elected and qualified or his earlier resignation or removal.
NAME |
| AGE |
| POSITION |
Yeat Min Fong |
| 44 |
| Chairman / Director |
Yee Meng Wong |
| 38 |
| President / Director |
Sook Keng Yeoh |
| 65 |
| Chief Executive Officer / Director |
Liong Tai Tan |
| 65 |
| Chief Operating Officer / Director |
Wei Li Leong |
| 34 |
| Chief Financial Officer |
Dr. Yeat Min Fong served as our Chairman and Director since August 9, 2022. Dr. Fong joined Doctor Anywhere in 2020 as the Country General Manager of Malaysia and was responsible for all business activities in the country. Prior to joining Doctor Anywhere, he was the General Manager of iHeal Medical Centre, a medical center, Kuala Lumpur, Malaysia, from 2014 to 2020. From 2014 to 2018, Dr. Fong was the Deputy Medical Director prior to being entrusted as the Medical Director in the medical center. Before entering the private medical industry, Dr. Fong served for seven years in various Ministry of Health’s hospitals in Malaysia, where he practiced his clinical and surgical years. He graduated from University of Cardiff Metropolitan, Cardiff, UK and obtained the Master of Business Administration (MBA) degree in 2018, and he obtained India Bachelor of Medicine and Bachelor of Surgery (MBBS) from Manipal Academy in 2006.
Dr. Yee Meng Wong served as our President and Director since August 9, 2022. She is a detailed oriented and a driven team player with 6 years of consultation experience and more than 10 years of research experience in various microbiology and analytical chemistry sector. In BGL, she supervises R&D projects, public presentation and liaison with healthcare association, medical centres and research institutions She is highly skilled in research activities, troubleshooting and problem solving, comprehensive data analysis and evaluation, presentation, technical and scientific reporting. Dr Wong obtained her Biotechnology (Microbiology) PhD degree from Monash University after her BSc. (Hon) Biotechnology.
Mr. Sook Keng Yeoh served as our CEO and Director since August 9, 2022. Mr. Yeoh is currently a Director of ADS Sentral Sdn. Bhd. Prior to ADS Sentral Sdn. Bhd., Mr. Yeoh served as a Chief Financial Officer/Finance Director of TRC Synergy Bhd, from 1999 until 2019. From 2019 to 2022, Mr. Yeoh had been the Group Internal Auditor, CFO and Director of public companies in Malaysia. While Mr. Yeoh was with TRC Synergy Bhd, he was also CEO of TRC Energy Sdn. Bhd and an Executive Director of PetroBru (B) Sdn. Bhd. Prior to those positions, Mr. Yeoh was a Group Internal Auditor at Lingui Corporation Bhd from 1995 to 1999. Prior to Lingui Corporation Bhd, Mr. Yeoh served at Paramount Corporation Bhd from 1991 to 1995 as an internal auditor and as an Audit Manager at Asia Commercial Finance Bhd from 1988 to 1991. Mr. Yeoh started his career as a Bank Officer (Audit) at Kwong Yik Bank in 1981 before joining Metroplex Bhd as a Chief Internal Auditor in 1985. Mr. Yeoh holds a Bachelor of Commerce in Accounting, Finance, and Systems from the University of New South Wales (Australia). Mr. Yeoh is also admitted to the Malaysian Institute of Accountants as a Chartered Accountant.
Mr. Liong Tai Tan served as our Chief Operating Officer since November 27, 2020. Mr. Tan has been the marketing director of our subsidiary Chemrex Corporation Sdn. Bhd., since 2006. Mr. Tan has significant amount of business experience, particularly in the chemicals industry. He obtained a diploma in Business Management from Vanto Academy, Malaysia.
| 49 |
| Table of Contents |
Ms. Wei Li Leong served as our Chief Financial Officer and Principal Accounting Officer since inception. Her role as the CFO is on a part-time basis. Separately, Ms. Leong is currently self employed as a tax consultant. From March 2015 to February 2017, she was employed by Ernst & Young (Malaysia) specializing in international tax. From March 2013 to February 2015, she was engaged with BDO Malaysia in the tax compliance department. From September 1, 2010 to February 22, 2012, she worked as trainee and later as accountant with SJ Accounting Services firm in Melbourne, Australia. Ms. Leong interned at Russell Bedford LC & Company as an audit vacation trainee in 2009. Ms. Leong is a Certified Practicing Accountant and graduated from RMIT University, Melbourne with a bachelor’s degree in Business (Accountancy).
Employment Agreements
We have entered into employment agreements with all our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate employment for cause, at any time, without advance notice or remuneration, for certain acts of the executive officer, such as conviction or plea of guilty to a felony or any crime involving moral turpitude, negligent or dishonest acts to our detriment, or misconduct or a failure to perform agreed duties. We may also terminate an executive officer’s employment without cause upon advance written notice or payment in-lieu of notice. In such case of termination by us, we will provide severance payments to the executive officer as expressly required by applicable law of the jurisdiction where the executive officer is based. The executive officer may resign at any time upon advance written notice.
Each executive officer has agreed to hold, both during and after the termination or expiry of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment or pursuant to applicable law, any of our confidential information or trade secrets, any confidential information or trade secrets of our clients or prospective clients, or the confidential or proprietary information of any third party received by us and for which we have confidential obligations.
Terms of Directors and Officers
Our officers are elected by and serve at the discretion of the board of directors and the stockholders voting by ordinary resolution.
Compensation of Directors and Executive Officers
For the year ended December 31, 2022, we paid an aggregate of approximately $30,000 including 25,000 shares valued at $25,000, respectively, in cash and benefits to our executive officers. We do not have a share incentive program to provide for grants of awards to our directors and executive officers. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. We have no service contracts with any of our directors providing for benefits upon termination of employment.
Board of Directors and Committees
Our board of directors will consist of four directors, including five independent directors: an audit committee, a compensation committee and a nominating and corporate governance committee. We intend to adopt and approve a charter for each of the three committees prior to consummation of this offering. Each of the committees of the board of directors shall have the composition and responsibilities described below.
| 50 |
| Table of Contents |
Audit Committee
Our Audit Committee is currently composed of three members:
NAME |
| AGE |
| POSITION |
Chee Keong Yap |
| 67 |
| Independent non-executive director |
Chak Hua Yew |
| 38 |
| Independent non-executive director |
Teng Fook Fong |
| 65 |
| Independent non-executive director |
Mr. Chee Keong Yap is Chairing the Audit Committee meeting. Our board of directors determined that each member of the Audit Committee meets the independence criteria prescribed by applicable regulation and the rules of the SEC for Audit Committee membership and is an “independent” director within the meaning of the NASDAQ Marketplace Rules. Each Audit Committee member also meets NASDAQ’s financial literacy requirements. The compensation committee’s responsibilities include:
| ·
| evaluating the performance of our chief executive officer in light of our company’s corporate goals and objectives and, based on such evaluation: (i) recommending to the board of Directors the cash compensation of our chief executive officer, and (ii) reviewing and approving grants and awards to our chief executive officer under equity-based plans; |
| · | reviewing and recommending to the board of Directors the cash compensation of our other executive officers; |
| · | reviewing and establishing our overall management compensation, philosophy and policy; |
| · | overseeing and administering our compensation and similar plans; |
| · | reviewing and approving the retention or termination of any consulting firm or outside advisor to assist in the evaluation of compensation matters and evaluating and assessing potential and current compensation advisors in accordance with the independence standards identified in the applicable Nasdaq rules; |
| · | retaining and approving the compensation of any compensation advisors; |
| · | reviewing and approving our policies and procedures for the grant of equity-based awards; |
| · | reviewing and recommending to the board of directors the compensation of our directors; and |
| · | preparing the compensation committee report required by SEC rules, if and when required. |
It is determined that [Independent Director *] possesses accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC.
Compensation Committee
Our compensation committee will consist of:
NAME |
| AGE |
| POSITION |
Teng Fook Fong |
| 65 |
| Independent non-executive director |
Boon Teong Teoh |
| 42 |
| Independent non-executive director |
Chai Ping Lin |
| 41 |
| Independent non-executive director |
| 51 |
| Table of Contents |
Chai Ping Lin who has more than 15 years of laboratory equipment and consumables distribution, financial management, manpower recruitment, compensation and training and she will be the chairperson of our compensation committee. The compensation committee will be responsible for, among other things:
| · | reviewing and approving, or recommending to the board for its approval, the compensation for our chief executive officer and other executive officers; |
| · | reviewing and recommending to the shareholders for determination with respect to the compensation of our directors; |
| · | reviewing periodically and approving any incentive compensation or equity plans, programs or similar arrangements; and |
| · | selecting compensation consultant, legal counsel or other adviser only after taking into consideration all factors relevant to that person’s independence from management. |
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee will consist of Teng Fook Fong, Chai Peng Lin and Dr. Chak Hua Yew upon the effectiveness of their appointments. Teng Fook Fong will be the chair of our nominating committee. We have determined that Teng Fook Fong, Chai Peng Lin, and Dr. Chak Hua Yew satisfy the “independence” requirements under NASDAQ Rule 5605. The nominating committee will assist the board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating committee will be responsible for, among other things:
| · | selecting and recommending to the board nominees for election by the shareholders or appointment by the board; |
| · | reviewing annually with the board the current composition of the board with regards to characteristics such as independence, knowledge, skills, experience and diversity; |
| · | making recommendations on the frequency and structure of board meetings and monitoring the functioning of the committees of the board; and |
| · | advising the board periodically with regards to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations and making recommendations to the board on all matters of corporate governance and on any remedial action to be taken. |
Family Relationships
Except as stated herein above, there are no family relationships among our directors or officers.
Involvement in Legal Proceedings
To the best of our knowledge, none of our directors or executive officers, during the past ten years, had been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or had been a party to any judicial or administrative proceeding during the past five years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Related Party Transactions,” none of our directors, director nominees or executive officers had been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which were required to be disclosed pursuant to the rules and regulations of the Securities and Exchange Commission.
| 52 |
| Table of Contents |
Director Independence
Our board of directors is currently composed of five members, one of whom qualifies as an independent director in accordance with the published listing requirements of NASDAQ. The NASDAQ independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the Director, nor any of his family members have engaged in various types of business dealings with us. In addition, our board of directors had not made a subjective determination as to our director that no relationship existed which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, though such subjective determination is required by the NASDAQ rules. Had our board of directors made these determinations, our board of directors would have reviewed and discussed information provided by our director and us with regard to our director’s business and personal activities and relationships as they may relate to us and our management.
Code of Ethics
We currently do not have a code of business conduct and ethics applicable to our directors, officers, and employees; however, we intend to adopt one in the near future in connection with our application to list on The Nasdaq Capital Market.
Conflicts of Interest
Since we do not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees are performed by our directors. The board of directors is establishing an audit committee and meanwhile, the existing directors have been performing the functions of such committees. Thus, there is a potential conflict of interest in that our Directors and Officers have the authority to determine issues concerning management compensation, nominations, and audit issues that may affect management decisions.
In addition, our Officers have committed to spend a sufficient amount of time and attention to the affairs of the Company to fulfill their respective officer responsibilities. In this regard, generally, each Officer spends between 15 to 40 hours per week on the affairs of the Company, depending on the circumstances. Therefore, we may face conflicts of interest between the time and attention each Officer devotes to the Company and that of their other business interests.
Other than as described above, we are not aware of any other conflicts of interest of our executive Officers and Directors.
Item 11. Executive Compensation.
Summary Executive Compensation Table
The following table reflects the Summary Compensation for our named executive officers for fiscal years ended December 31, 2022 and 2021, respectively. For such periods, there were no bonus, non-equity plan compensation, nonqualified compensation earnings or other compensation other than as stated below for the named executive officers. Further, we have not entered into an employment agreement with any of our officers, directors or any other persons and no such agreements are anticipated in the immediate future.
|
|
|
|
| Stock |
|
| Other |
|
|
| |||||||
|
|
|
|
| Award |
|
| Compensation |
|
| Total |
| ||||||
Name and Position |
| Year |
| Salary |
|
| $ |
|
| $ |
|
| $ |
| ||||
Yeat Min Fong |
| 2022 |
| $ | 5,000 |
|
| $ | 10,417 |
|
| $ | 0 |
|
| $ | 15,417 |
|
Chairman |
| 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yee Meng Wong |
| 2022 |
| $ | 5,000 |
|
| $ | 10,417 |
|
| $ | 0 |
|
| $ | 15,417 |
|
President |
| 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sook Keng Yeoh |
| 2022 |
| $ | 5,000 |
|
| $ | 10,417 |
|
| $ | 0 |
|
| $ | 15,417 |
|
Chief Executive Officer |
| 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wei Li Leong |
| 2022 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
Chief Financial Officer and Principal Accounting Officer |
| 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liong Tai Tan |
| 2022 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
Chief Operating Officer |
| 2021 |
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| $ | 0 |
|
| 53 |
| Table of Contents |
Employment Agreements
Employment Agreement between Mr. Yeat Min Fong and BioNexus
Effective as of August 9, 2022, BioNexus entered into an employment agreement with Mr. Yeat Min Fong. The agreement provides for an annual base salary, together with such additional discretionary bonus. Mr. Yeat Min Fong’s employment will continue automatically, unless either party gives written notice to the other party 60 days prior to the next anniversary of the employment agreement, subject to termination by either party to the agreement upon 30 days’ prior written notice. The agreement also provides that Mr. Yeat Min Fong shall not, during the term of the agreement and for 24 months after cessation of employment, carry on business in competition with the Group.
Employment Agreement between Mr. Yee Meng Wong and BioNexus
Effective as of August 9, 2022, BioNexus entered into an employment agreement with Mr. Yee Meng Wong. The agreement provides for an annual base salary, together with such additional discretionary bonus. Mr. Yee Meng Wong’s employment will continue automatically, unless either party gives written notice to the other party 60 days prior to the next anniversary of the employment agreement, subject to termination by either party to the agreement upon 30 days’ prior written notice. The agreement also provides that Mr. Yee Meng Wong shall not, during the term of the agreement and for 24 months after cessation of employment, carry on business in competition with the Group.
Employment Agreement between Mr. Sook Keng Yeoh and BioNexus
Effective as of August 9, 2022, BioNexus entered into an employment agreement with Mr. Sook Keng Yeoh. The agreement provides for an annual base salary, together with such additional discretionary bonus. Mr. Sook Keng Yeoh’s employment will continue automatically, unless either party gives written notice to the other party 60 days prior to the next anniversary of the employment agreement, subject to termination by either party to the agreement upon 30 days’ prior written notice. The agreement also provides that Mr. Sook Keng Yeoh shall not, during the term of the agreement and for 24 months after cessation of employment, carry on business in competition with the Group.
Employment Agreement between Wei Li Leong and BioNexus
Effective as of June 19, 2017, BioNexus entered into an employment agreement with Mr. Wei Li Leong. The agreement provides for a compensation of 5 million shares over the five-year term of the agreement. Mr. Wei’s employment will last for a term of 5 years and can be extended automatically for a 1-year term at the request of the company. During the term of the agreement, either BioNexus or Mr. Wei can terminate the employment for whatever reason upon giving 3 months’ prior written notice. The agreement also provides that Mr. Wei Li Leong shall not, during the term of the agreement and for 24 months after cessation of employment, carry on business in competition with the Group.
Grants of Plan-Based Awards
Except as stated above, no plan-based awards were granted to any of our named executive officers during the interim fiscal year ended December 31, 2022.
Outstanding Equity Awards at Interim Fiscal Year End
The equity awards reflected in the Summary Compensation Table above represents all restricted stock awards issued to our executive officers as of December 31, 2022. No other stock or stock option awards were granted to any other officer of the Company as of December 31, 2022.
| 54 |
| Table of Contents |
Option Exercises and Stock Vested
No option to purchase our capital stock was exercised by any of our named executive officers, nor was any restricted stock held by such executive officers vested during the interim fiscal period ended December 31, 2022.
Pension Benefits
No named executive officers received or held pension benefits during the interim fiscal period ended December 31, 2022.
Item 12. Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information, as of the date hereof, with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five percent (5%); (ii) each of our executive officers and directors; and (iii) our directors and executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned. The information is based on 173,718,152 shares of common stock issued and outstanding as of this filing.
Executive Officers and Directors |
| Amount and nature of Beneficial Ownership of (1) |
|
| Percent of Class (2) |
| ||
Directors and Named Executive Officers: |
|
|
|
|
|
| ||
Yeat Min Fong |
|
| - |
|
|
| - |
|
Yee Meng Wong |
|
| - |
|
|
| - |
|
Sook Keng Yeoh |
|
| - |
|
|
| - |
|
Wei Li Leong |
|
| 4,797,709 |
|
|
| 2.76 | % |
Liong Tai Tan |
|
| 12,500,460 |
|
|
| 7.20 | % |
Teng Fook Fong (2) |
|
| - |
|
|
| - |
|
Chee Keong Yap (2) |
|
| - |
|
|
| - |
|
Chak Hua Yew (2) |
|
| - |
|
|
| - |
|
Boon Teong Teoh (2) |
|
| - |
|
|
| - |
|
Chai Ping Lin (2) |
|
| - |
|
|
| - |
|
All executive officers and directors as a group (10 persons) |
|
| 17,298,169 |
|
|
| 9.96 | % |
|
|
|
|
|
|
|
|
|
5% or Greater Stockholders |
|
|
|
|
|
|
|
|
Soo Kow Lai |
|
| 15,000,000 |
|
|
| 8.63 | % |
Chi Yuen Leong |
|
| 14,000,000 |
|
|
| 8.06 | % |
Chan Chong Wong |
|
| 12,498,529 |
|
|
| 7.19 | % |
Liong Tai Tan |
|
| 12,500,460 |
|
|
| 7.20 | % |
Choong-Chin Liew (3) |
|
| 20,000,000 |
|
|
| 11.51 | % |
Tan Kuan Yew (Hing Kuan Yew) (4) |
|
| 8,830,917 |
|
|
| 5.08 | % |
Tham Too Kam (5) |
|
| 12,210,460 |
|
|
| 7.03 | % |
Wong Kim Hai (6) |
|
| 12,510,460 |
|
|
| 7.20 | % |
(1) | Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has ownership of and voting power and investment power with respect to our Common stock or Preferred Shares. For each beneficial owner above, any options exercisable within 60 days have been included in the denominator. |
(2) | The individual is an independent director of BGLC. |
(3) | Choong-Chin Liew (Deceased) is the record holder of the shares. To the best of our knowledge, as of the date hereof, Galina Liew is the Administer of the Estate of Dr. Choong-Chin Liew. |
(4) | The address of the shareholder is 32 Jalan Putra Mahkota 7/2H, Putra Heights Subang Jaya, Selangor Malaysia 47650. |
(5) | The address of the shareholder is 12A Jalan Sl 15/1 Bandar Sungai Long Kajang, Selangor Malaysia 43000. |
(6) | The address of the Shareholder was 24 Jalan Molek 3/8, Taman Molek Johor Baru, Johore Malaysia 81100. |
| 55 |
| Table of Contents |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
In connection with our acquisition of Chemrex from the Chemrex shareholders, Liong Tai Tan, our Chief Operating Officer (appointed on November 27, 2020), was a Chemrex shareholder and received 14,553,543 shares of common stock in connection with the transaction.
Other than as stated herein, there have been no related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
Item 14. Principal Accountant Fees and Services.
JP Centurion & Partner PLT is the Company’s current independent registered public accounting firm.
(1) Audit Fees
The aggregate fees billed for each of the last two fiscal years for professional services rendered by the principal accountant for our audit of annual financial statements and review of financial statements included in our quarterly reports or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for those fiscal years were:
2022 |
| $ | 32,500 |
|
2021 |
| $ | 31,530 |
|
| 56 |
| Table of Contents |
(2) Audit-Related Fees
The aggregate fees billed in each of the last two fiscal years for assurance and related services by the principal accountants that are reasonably related to the performance of the audit or review of our financial statements and are not reported in the preceding paragraph:
2022 |
| $ | 2,301 |
|
2021 |
| $ | 934 |
|
(3) Tax Fees
The aggregate fees billed in each of the last two fiscal years for professional services rendered by the principal accountant for tax compliance, tax advice, and tax planning were:
2022 |
| $ | 11,441 |
|
2021 |
| $ | 209 |
|
(4) All Other Fees
The aggregate fees billed in each of the last two fiscal years for the products and services provided by the principal accountant, other than the services reported in paragraphs (1), (2), and (3) were:
2022 |
| $ | 3,000 |
|
2021 |
| $ | 10,400 |
|
The percentage of hours expended on the principal accountant’s engagement to audit our financial statements for the most recent fiscal year that were attributed to work performed by persons other than the principal accountant’s full time, permanent employees was 0%.
Audit Committee’s Pre-Approval Process
The Audit Committee of the Company, and accordingly, all services are approved by all the members of the Committee.
| 57 |
| Table of Contents |
PART IV.
Item 15. Exhibits, Financial Statement Schedules.
EXHIBIT INDEX
Exhibit |
| Description |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
101.INS |
| INLINE XBRL INSTANCE DOCUMENT* |
101.SCH |
| INLINE XBRL TAXONOMY EXTENSION SCHEMA DOCUMENT* |
101.CAL |
| INLINE XBRL TAXONOMY CALCULATION LINKBASE DOCUMENT* |
101.DEF |
| INLINE XBRL TAXONOMY DEFINITION LINKBASE DOCUMENT* |
101.LAB |
| INLINE XBRL TAXONOMY LABEL LINKBASE DOCUMENT* |
101.PRE |
| INLINE XBRL TAXONOMY PRESENTATION LINKBASE DOCUMENT* |
104 |
| COVER PAGE INTERACTIVE DATA FILE (FORMATTED AS INLINE XBRL AND CONTAINED IN EXHIBIT 101)* |
___________
(1) Previously filed as an exhibit to the Company’s Form S-1 Registration Statement filed on January 29, 2020.
(2) Previously filed as an exhibit to the Company’s Form 8-K filed on January 7, 2021.
(3) Previously filed as an exhibit to the Company’s Form 8-K filed on October 1, 2020.
(4) Previously filed as an exhibit to the Company’s Form S-1 Registration Statement filed on February 14, 2023
* Filed herewith
| 58 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BioNexus Gene Lab Corporation |
|
|
|
|
|
/s/ Sook Keng Yeoh |
| Dated: March 31, 2023 |
Sook Keng Yeoh |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
/s/ Sook Keng Yeoh |
| Dated: March 31, 2023 |
Sook Keng Yeoh |
|
|
Chief Executive Officer and Director |
|
|
(Principal Executive Officer) |
|
|
/s/ Wei Li Leong |
| Dated: March 31, 2023 |
Wei Li Leong |
|
|
Chief Financial Officer |
|
|
(Principal Financial and Accounting Officer) |
|
|
/s/ Yeat Min Fong |
| Dated: March 31, 2023 |
Yeat Min Fong |
|
|
Chairman |
|
|
|
|
|
/s/ Yee Meng Wong |
| Dated: March 31, 2023 |
Yee Meng Wong |
|
|
President and Director |
|
|
/s/ Liong Tai Tan |
| Dated: March 31, 2023 |
Liong Tai Tan |
|
|
Chief Operating |
|
|
Officer and Director | ||
| 59 |
| Table of Contents |
|
| Page |
| ||
|
|
|
| ||
| F-2 | |
| Consolidated Balance Sheets as of December 31, 2022 and 2021 | F-3 to F-4 |
| F-5 | |
| F-6 | |
| Consolidated Statement of Cash Flows for the Year Ended December 31, 2022 and 2021 | F-7 to F-8 |
| F-9 to F-21 |
| F-1 |
| Table of Contents |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
Bionexus Gene Lab Corp.
Unit 02 Level 10, Tower B, Avenue 3,
Vertical Business Suite,
No. 8, Jalan Kerinchi, Bangsar South,
59200 Kuala Lumpur, Malaysia.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Bionexus Gene Lab Corp. (the ‘Company’) as of December 31, 2022, and the related consolidated statements of operations and comprehensive income (loss), stockholders’ equity, and cash flows for the year ended of December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year ended of December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to those charged with governance that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgements. We determined that there are no critical matters.
/s/ JP CENTURION & PARTNERS PLT |
| |
JP CENTURION & PARTNERS PLT (ID: 6723) |
| |
|
| |
We have served as the Company’s auditor since 2020. |
| |
|
| |
Kuala Lumpur, Malaysia |
| |
March 31, 2023 |
| |
| F-2 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
|
|
| As of |
| |||||||
|
| Note |
|
| December 31, 2022 |
|
| December 31, 2021 |
| |||
ASSETS |
|
|
|
|
|
|
|
|
| |||
CURRENT ASSETS |
|
|
|
|
|
|
|
|
| |||
Cash and bank balances |
|
|
|
| $ | 611,849 |
|
| $ | 578,511 |
| |
Fixed deposits placed with financial institutions |
|
|
|
|
| 1,507,015 |
|
|
| 1,545,408 |
| |
Trade receivables |
|
| 3 |
|
|
| 2,868,364 |
|
|
| 3,356,898 |
|
Other receivables, deposits and prepayments |
|
|
|
|
|
| 25,240 |
|
|
| 79,517 |
|
Deferred cost of revenue |
|
|
|
|
|
| - |
|
|
| 67,606 |
|
Tax recoverable |
|
| 4 |
|
|
| 31,551 |
|
|
| - |
|
Inventories |
|
|
|
|
|
| 977,807 |
|
|
| 1,521,915 |
|
Total current assets |
|
|
|
|
|
| 6,021,826 |
|
|
| 7,149,855 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease right of use assets |
|
| 5 |
|
|
| 55,730 |
|
|
| 41,090 |
|
Property, plant and equipment, net |
|
| 6 |
|
|
| 1,511,708 |
|
|
| 1,634,418 |
|
Other investments |
|
| 7 |
|
|
| 1,150,898 |
|
|
| 749,027 |
|
Total non-current assets |
|
|
|
|
|
| 2,718,336 |
|
|
| 2,424,535 |
|
TOTAL ASSETS |
|
|
|
|
| $ | 8,740,162 |
|
| $ | 9,574,390 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
Trade payables |
|
| 8 |
|
| $ | 1,861,015 |
|
| $ | 2,012,266 |
|
Other payables and accrued liabilities |
|
|
|
|
|
| 103,370 |
|
|
| 71,814 |
|
Current portion of obligation under finance lease |
|
|
|
|
|
| - |
|
|
| 21,235 |
|
Current portion of operating lease liabilities |
|
| 5 |
|
|
| 16,569 |
|
|
| 18,272 |
|
Advance payment from customer |
|
|
|
|
|
| 23,123 |
|
|
| 30,307 |
|
Deferred revenue |
|
|
|
|
|
| - |
|
|
| 77,276 |
|
Tax payables |
|
| 4 |
|
|
| - |
|
|
| 97,585 |
|
Total current liabilities |
|
|
|
|
|
| 2,004,077 |
|
|
| 2,328,755 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
Non-current portion of obligation under finance lease |
|
|
|
|
|
| - |
|
|
| 12,803 |
|
Non-current portion of operating lease liabilities |
|
| 5 |
|
|
| 40,206 |
|
|
| 24,637 |
|
Deferred tax liabilities |
|
| 4 |
|
|
| 30,866 |
|
|
| 28,416 |
|
Total non-current liabilities |
|
|
|
|
|
| 71,072 |
|
|
| 65,856 |
|
TOTAL LIABILITIES |
|
|
|
|
| $ | 2,075,149 |
|
| $ | 2,394,611 |
|
See accompanying notes to the consolidated financial statements.
| F-3 |
| Table of Contents |
PART I — FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (CONT’D)
BIONEXUS GENE LAB CORP.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
|
|
| As of |
| |||||||
|
| Note |
|
| December 31, 2022 |
|
| December 31, 2021 |
| |||
|
|
|
|
|
|
|
|
|
| |||
STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
| |||
As at December 31, 2022, common stock, no par value; 300,000,000 shares authorized and 173,718,152 shares outstanding, and preferred stock, no par value; 30,000,000 shares authorized and no shares outstanding. As at December 31, 2021, common stock, no par value; 300,000,000 shares authorized and 171,218,152 shares outstanding, and preferred stock, no par value; 30,000,000 shares authorized and no shares outstanding. |
|
| 10 |
|
| $ | 10,929,574 |
|
| $ | 10,779,574 |
|
Additional paid in capital |
|
|
|
|
|
| (5,011,891 | ) |
|
| (5,011,891 | ) |
Accumulated surplus |
|
|
|
|
|
| 1,156,392 |
|
|
| 1,512,358 |
|
Accumulated other comprehensive losses |
|
|
|
|
|
| (409,062 | ) |
|
| (100,262 | ) |
TOTAL STOCKHOLDERS’ EQUITY |
|
|
|
|
|
| 6,665,013 |
|
|
| 7,179,779 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
| $ | 8,740,162 |
|
| $ | 9,574,390 |
|
See accompanying notes to the consolidated financial statements.
| F-4 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF OPERATIONS AND COMPREHENSIVE INCOME/(LOSS)
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Year ended |
| |||||
|
| December 31, |
| |||||
|
| 2022 |
|
| 2021 |
| ||
|
|
|
|
|
|
| ||
REVENUE |
| $ | 10,928,707 |
|
| $ | 13,362,567 |
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (9,669,678 | ) |
|
| (11,095,626 | ) |
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 1,259,029 |
|
|
| 2,266,941 |
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 179,283 |
|
|
| 66,491 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
General and administrative |
|
| (1,729,489 | ) |
|
| (1,277,605 | ) |
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT FROM OPERATIONS |
|
| (291,177 | ) |
|
| 1,055,827 |
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (12,479 | ) |
|
| (12,973 | ) |
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT BEFORE TAX |
|
| (303,656 | ) |
|
| 1,042,854 |
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
Deferred tax |
|
| (3,898 | ) |
|
| (26,736 | ) |
Income tax |
|
| (48,412 | ) |
|
| (264,547 | ) |
Total tax expense |
|
| (52,310 | ) |
|
| (291,283 | ) |
|
|
|
|
|
|
|
|
|
NET (LOSS)/PROFIT |
| $ | (355,966 | ) |
| $ | 751,571 |
|
|
|
|
|
|
|
|
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
Foreign currency translation loss |
|
| (308,800 | ) |
|
| (233,946 | ) |
|
|
|
|
|
|
|
|
|
COMPREHENSIVE (LOSS)/INCOME |
| $ | (664,766 | ) |
| $ | 517,625 |
|
|
|
|
|
|
|
|
|
|
Earnings per share - Basic and diluted |
|
| (0.002 | ) |
|
| 0.004 |
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding – Basic and diluted |
|
| 172,916,782 |
|
|
| 171,218,152 |
|
See accompanying notes to the consolidated financial statements.
| F-5 |
| Table of Contents |
BIONEXUS GENE LAB CORP
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Common stock |
|
|
|
|
|
|
|
|
| |||||||||||||
|
| Number of shares |
|
| Amount |
|
| Additional paid in capital |
|
| Accumulated surplus |
|
| Accumulated other comprehensive income/ (loss) |
|
| Total Equity |
| ||||||
Balance as of January 1, 2021 |
|
| 171,218,152 |
|
| $ | 10,779,574 |
|
| $ | (5,011,891 | ) |
| $ | 760,787 |
|
| $ | 133,684 |
|
| $ | 6,662,154 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit for the year |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 751,571 |
|
|
| - |
|
|
| 751,571 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (233,946 | ) |
|
| (233,946 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2021 |
|
| 171,218,152 |
|
| $ | 10,779,574 |
|
| $ | (5,011,891 | ) |
| $ | 1,512,358 |
|
| $ | (100,262 | ) |
| $ | 7,179,779 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares |
|
| 2,500,000 |
|
|
| 150,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 150,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (355,966 | ) |
|
| - |
|
|
| (355,966 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (308,800 | ) |
|
| (308,800 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2022 |
|
| 173,718,152 |
|
| $ | 10,929,574 |
|
| $ | (5,011,891 | ) |
| $ | 1,156,392 |
|
| $ | (409,062 | ) |
| $ | 6,665,013 |
|
See accompanying notes to the consolidated financial statements. |
| F-6 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| Year Ended |
| |||||
|
| December 31, |
| |||||
|
| 2022 |
|
| 2021 |
| ||
Cash flows from operating activities: |
|
|
|
|
|
| ||
Net (loss)/profit |
| $ | (355,966 | ) |
| $ | 751,571 |
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net profit to net cash (used in)/generated from operating activities: |
|
|
|
|
|
|
|
|
Amortization of right of use asset |
|
| 13,992 |
|
|
| 16,933 |
|
Bad debts |
|
| 4,165 |
|
|
| 3,809 |
|
Depreciation of property, plant and equipment |
|
| 91,427 |
|
|
| 91,282 |
|
Dividend income |
|
| (115,379 | ) |
|
| (22,036 | ) |
Fair value gain on other investments |
|
| 70,628 |
|
|
| 29,850 |
|
Loss on written off of other investments |
|
| 1,776 |
|
|
| - |
|
Operating (loss)/profit before working capital changes |
|
| (289,357 | ) |
|
| 871,409 |
|
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Inventories |
|
| 544,108 |
|
|
| (345,745 | ) |
Trade and other receivables |
|
| 538,646 |
|
|
| 579,217 |
|
Deferred cost of revenue |
|
| 67,606 |
|
|
| (67,606 | ) |
Trade and other payables |
|
| (119,695 | ) |
|
| (1,180,535 | ) |
Advance payment from customer |
|
| (7,184 | ) |
|
| 30,307 |
|
Deferred revenue |
|
| (77,276 | ) |
|
| 77,276 |
|
Operating lease liabilities |
|
| (126,686 | ) |
|
| (20,169 | ) |
Tax recoverable |
|
| 13,866 |
|
|
| 65,007 |
|
Cash generated from operating activities |
|
| 544,028 |
|
|
| 9,161 |
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
Acquisition of other investment |
|
| (511,706 | ) |
|
| (515,840 | ) |
Dividend income |
|
| 115,379 |
|
|
| 22,036 |
|
Purchase of plant and equipment |
|
| (54,171 | ) |
|
| (3,162 | ) |
Proceeds from disposal of other investments |
|
| - |
|
|
| 6,392 |
|
Net cash used in investing activities |
|
| (450,498 | ) |
|
| (490,574 | ) |
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
Repayment of finance lease |
|
| (34,038 | ) |
|
| (26,302 | ) |
Repayments to directors |
|
| - |
|
|
| (1,920 | ) |
Shares subscriptions |
|
| 150,000 |
|
|
| - |
|
Net cash generated from /(used in) financing activities |
|
| 115,962 |
|
|
| (28,222 | ) |
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
| (214,547 | ) |
|
| (154,138 | ) |
NET CHANGE IN CASH AND CASH EQUIVALENTS |
|
| (5,055 | ) |
|
| (663,773 | ) |
CASH AND CASH EQUIVALENTS, BEGINNING OF FINANCIAL YEAR |
|
| 2,123,919 |
|
|
| 2,787,692 |
|
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS, END OF FINANCIAL YEAR |
| $ | 2,118,864 |
|
| $ | 2,123,919 |
|
| F-7 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))(CONT’D)
(Audited)
CASH AND CASH EQUIVALENTS INFORMATION: |
|
|
|
|
|
| ||
Fixed deposits placed with financial institutions |
| $ | 1,507,015 |
|
| $ | 1,545,408 |
|
Cash and bank balances |
|
| 611,849 |
|
|
| 578,511 |
|
Cash and cash equivalents, end of financial year |
|
| 2,118,864 |
|
|
| 2,123,919 |
|
Supplementary cash flow information: |
|
|
|
|
|
|
|
|
Interest paid |
| $ | (12,479 | ) |
| $ | (12,973 | ) |
Income tax paid |
|
| (170,447 | ) |
|
| (226,770 | ) |
See accompanying notes to the consolidated financial statements.
| F-8 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 1 – ORGANIZATION AND BUSINESS BACKGROUND
BioNexus Gene Lab Corp. was incorporated in the State of Wyoming on May 12, 2017. On August 23, 2017, the Company acquired all the outstanding capital stock of BGS Lab Sdn. Bhd., a Malaysian corporation (“BioNexus Malaysia”). BioNexus Malaysia was incorporated in Malaysia on April 7, 2015 which it then subsequently changed its name to Bionexus Gene Lab Sdn. Bhd.
The principal office address is Unit 02 Level 10, Tower B, Vertical Business Suite, No. 8 Jalan Kerinchi, Bangsar South, 59200 Kuala Lumpur, Malaysia, our lab is located at Lab 353, Chemical Science Centre, University Science Malaysia, George Town, Penang, Malaysia. We also have a blood collection center located at 1st floor, Lifecare Medical Centre, Kuala Lumpur, Malaysia.
On December 31, 2020, the Company consummated its acquisition of Chemrex Corporation Sdn. Bhd. (“Chemrex”), pursuant to a Share Exchange Agreement by and among the Company, Chemrex and the Chemrex shareholders wherein the Company acquired all the issued and outstanding shares of capital stock of Chemrex from the Chemrex shareholders in exchange for 68,487,261 shares of common stock of the Company.
The acquisition of Chemrex has been accounted for as a common control transaction as there is no change in the control over the assets acquired and liabilities assumed. The net assets are derecognized by the transferring entity (i.e. Chemrex) and recognized by the receiving entity (i.e. the Company). The difference between the consideration transferred and the carrying amounts of the net assets is recognized in equity.
The financial statements of the receiving entity report the results of operations for the period in which the transfer occurs as though the transfer of net assets or exchange of equity interests had occurred at the beginning of the period. Results of operations for that period will thus comprise those of the previously separate entities combined from the beginning of the period to the date the transfer is completed and those of the combined operations from that date to the end of the period. The comparative financial statements were not adjusted retrospectively as Chemrex was not under common control during the comparative period.
On April 12, 2022, the Company entered into Sales & Purchase Agreement with Keith Wong pursuant to which the Company agreed to the sales of 2,500,000 shares of its common stock at $0.06/share. The issuance was exempt under Section 4(a)(2) of the Securities Act as the recipient was a sophisticated investor, the shares were restricted securities and he represented that he is acquiring the shares for investment purposes.
The corporate structure as at December 31, 2022 is depicted below:
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
100% owned |
|
| 100% owned | ||
Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| Chemrex Corporation Sdn. Bhd., a Malaysian Company | ||
| F-9 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The accompanying consolidated financial statements reflect the application of certain significant accounting policies as described in this note and elsewhere in the accompanying consolidated financial statements and notes.
☐ | Basis of presentation |
The accompanying consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”).
☐ | Basis of consolidation |
The consolidated financial statements include the accounts of Bionexus Gene Lab Corp. and its subsidiaries. All significant inter-company balances and transactions within the Company have been eliminated upon consolidation.
☐ | Use of estimates |
In preparing these consolidated financial statements, management makes estimates and assumptions that affect the reported amounts of assets and liabilities in the balance sheets and revenues and expenses during the periods reported. Actual results may differ from these estimates.
☐ | Cash and cash equivalents |
Cash and cash equivalents represent cash on hand, demand deposits placed with banks or other financial institutions and all highly liquid investments with an original maturity of three months or less as of the purchase date of such investments.
☐ | Trade receivables |
Trade receivables are recorded at the invoiced amount and do not bear interest. Management reviews the adequacy of the allowance for impairment on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to adjust in the allowance when it is considered necessary. Trade balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
☐ | Inventories |
Inventories consisting of products available for sell, are stated at the lower of cost or market value. Cost of inventory is determined using the first-in, first-out (FIFO) method. Inventory reserve is recorded to write down the cost of inventory to the estimated market value due to slow-moving merchandise and damaged goods, which is dependent upon factors such as historical and forecasted consumer demand, and promotional environment. The Company takes ownership, risks and rewards of the products purchased. Write downs are recorded in cost of revenues in the Statement of Operations and Comprehensive Income.
| F-10 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Leases |
Prior to January 1, 2019, the Company accounted for leases under ASC 840, Accounting for Leases. Effective January 1, 2019, the Company adopted the guidance of ASC 842, Leases, which requires an entity to recognize a right-of-use asset and a lease liability for virtually all leases. The Company adopted ASC 842 using a modified retrospective approach. As a result, the comparative financial information has not been updated and the required disclosures prior to the date of adoption have not been updated and continue to be reported under the accounting standards in effect for those periods.
☐ | Property, plant and equipment |
Property, plant and equipment are stated at cost less accumulated depreciation and accumulated impairment losses, if any. Depreciation is calculated on the straight-line basis to write off the cost over the following expected useful lives of the assets concerned. The principal annual rates used are as follows:
Categories |
| Principal Annual Rates |
| |
Air conditioner |
|
| 20 | % |
Buildings |
|
| 2 | % |
Computer and software |
|
| 33 | % |
Equipment |
|
| 20 | % |
Furniture and fittings |
| 10% to 20 | % | |
Lab Equipment |
|
| 10 | % |
Motor vehicle |
| 10% to 20 | % | |
Office equipment |
|
| 20 | % |
Renovation |
| 10% to 20 | % | |
Signboard |
|
| 10 | % |
Leasehold lands are depreciated over the period of lease term. Leased assets are depreciated over the shorter of the lease term and their useful lives unless it is reasonably certain that the Company will obtain ownership by the end of the lease term. Freehold land is not depreciated. Property, plant and equipment under construction are not depreciated until the assets are ready for their intended use
Maintenance and repairs are charged to operations as incurred. Expenditures which substantially increase the useful lives of the related assets are capitalized. When properties are disposed of, the related costs and accumulated depreciation are removed from the accounts and any gain or loss is reported in the period the transaction takes place.
Fully depreciated plant and equipment are retained in the financial statements until they are no longer in use.
| F-11 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Impairment of long-lived assets |
Long-lived assets primarily include goodwill, intangible assets and property, plant and equipment. In accordance with the provision of ASC Topic 360, “Impairment or Disposal of Long-Lived Assets”, the Company generally conducts its annual impairment evaluation to its long-lived assets, usually in the fourth quarter of each fiscal year, or more frequently if indicators of impairment exist, such as a significant sustained change in the business climate. The recoverability of long-lived assets is measured at the lowest level group. If the total of the expected undiscounted future net cash flows is less than the carrying amount of the asset, a loss is recognized for the difference between the fair value and carrying amount of the asset. There has been no impairment charge for the years presented.
☐ | Revenue recognition |
Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services.
The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services.
The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements:
| · | identify the contract with a customer; |
| · | identify the performance obligations in the contract; |
| · | determine the transaction price; |
| · | allocate the transaction price to performance obligations in the contract; and |
| · | recognize revenue as the performance obligation is satisfied. |
The Company records revenue at point in time which is recognized upon goods delivered or services rendered.
☐ | Shipping and handling fees |
Shipping and handling fees, if billed to customers, are included in revenue. Shipping ang handling fees associated with inbound and outbound freight are expensed as incurred and included in selling and distribution expenses.
☐ | Comprehensive income |
ASC Topic 220, “Comprehensive Income” establishes standards for reporting and display of comprehensive income, its components and accumulated balances. Comprehensive income as defined includes all changes in equity during a period from non-owner sources. Accumulated other comprehensive income, as presented in the accompanying statements of stockholders’ equity consists of changes in unrealized gains and losses on foreign currency translation and cumulative net change in the fair value of available-for-sale investments held at the balance sheet date. This comprehensive income is not included in the computation of income tax expense or benefit.
☐ | Income taxes |
Income taxes are determined in accordance with the provisions of ASC Topic 740, “Income Taxes” (“ASC Topic 740”). Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Any effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
| F-12 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
ASC 740 prescribes a comprehensive model for how companies should recognize, measure, present, and disclosed in their financial statements uncertain tax positions taken or expected to be taken on a tax return. Under ASC 740, tax positions must initially be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. Such tax positions must initially and subsequently be measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority assuming full knowledge of the position and relevant facts.
The Company conducts major businesses in Malaysia and is subject to tax in their own jurisdictions. As a result of its business activities, the Company will file separate tax returns that are subject to examination by the foreign tax authorities.
☐ | Net earnings or loss per share |
The Company calculates net earnings or loss per share in accordance with ASC Topic 260 “Earnings per share”. Basic earnings or loss per share is computed by dividing the net earnings or loss by the weighted average number of common shares outstanding during the period. Diluted earnings or loss per share is computed similar to basic earnings or loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common stock equivalents had been issued and if the additional common shares were dilutive.
☐ | Foreign currencies translation |
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the statement of operations.
The functional currency of the Company is the United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$ as being the primary currency of the economic environment in which the Company operates. The functional currency of the subsidiaries is Malaysian Ringgit (“MYR”) as being the primary currency of the economic environment in which the subsidiaries operate.
In general, for consolidation purposes, assets and liabilities of its subsidiaries whose functional currency is not US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of foreign subsidiaries are recorded as a separate component of accumulated other comprehensive income.
Translation of amounts from RM (MYR) into US$1.00 has been made at the following exchange rates for the respective years:
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
|
|
|
|
|
|
| ||
Year-end US$1.00: MYR exchange rate |
|
| 4.3900 |
|
|
| 4.1650 |
|
|
|
|
|
|
|
|
|
|
|
| January 1, 2022 to December 31, 2022 |
|
| January 1, 2021 to December 31, 2021 |
| ||
|
|
|
|
|
|
|
|
|
Yearly average US$1.00: MYR exchange rate |
|
| 4.3996 |
|
|
| 4.1456 |
|
| F-13 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
☐ | Related parties |
Parties, which can be a corporation or individual, are related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
☐ | Fair value of financial instruments |
The carrying value of the Company’s financial instruments: cash and cash equivalents, trade receivable, deposits and other receivables, amount due to related parties and other payables approximate at their fair values because of the short-term nature of these financial instruments
The Company also follows the guidance of the ASC Topic 820-10, “Fair Value Measurements and Disclosures” ("ASC 820-10"), with respect to financial assets and liabilities that are measured at fair value. ASC 820-10 establishes a three-tier fair value hierarchy that prioritizes the inputs used in measuring fair value as follows:
· | Level 1 : Observable inputs such as quoted prices in active markets; |
· | Level 2 : Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
· | Level 3 : Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions |
As of December 31, 2022, and December 31, 2021, the Company did not have any non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements, at least annually, on a recurring basis, nor did the Company have any assets or liabilities measured at fair value on a non-recurring basis.
☐ | Recent accounting pronouncements |
The Company has reviewed all recently issued, but not yet effective, accounting pronouncements and do not believe the future adoption of any such pronouncements may be expected to cause a material impact on its financial condition or the results of its operations.
In May 2019, the FASB issued ASU 2019-05, which is an update to ASU Update No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, which introduced the expected credit losses methodology for the measurement of credit losses on financial assets measured at amortized cost basis, replacing the previous incurred loss methodology. The amendments in Update 2016-13 added Topic 326, Financial Instruments—Credit Losses, and made several consequential amendments to the Codification. The amendments in this Update address those stakeholders’ concerns by providing an option to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost basis. For those entities, the targeted transition relief will increase comparability of financial statement information by providing an option to align measurement methodologies for similar financial assets. Furthermore, the targeted transition relief also may reduce the costs for some entities to comply with the amendments in Update 2016-13 while still providing financial statement users with decision-useful information. In November 2019, the FASB issued ASU No. 2019-10, which to update the effective date of ASU No. 2016-13 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. ASU 2019-05 is effective for the Company for annual and interim reporting periods beginning January 1, 2023 as the Company is qualified as a smaller reporting company. The Company is currently evaluating the impact ASU 2019-05 may have on its consolidated financial statements.
FASB issues various Accounting Standards Updates relating to the treatment and recording of certain accounting transactions. On June 10, 2014, the Financial Accounting Standards Board issued Accounting Standards Update (ASU) No. 2014-10, Development Stage Entities (Topic 915) Elimination of Certain Financial Reporting Requirements, including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation, which eliminates the concept of a development stage entity (DSE) entirely from current accounting guidance. The Company has elected adoption of this standard, which eliminates the designation of DSEs and the requirement to disclose results of operations and cash flows since inception.
NOTE 3 – TRADE RECEIVABLES
The Company has performed an analysis on all its trade receivables and determined that all amounts are collectible by the Company. As such, trade receivables are reflected as a current asset and no allowance for impairment has been recorded as of December 31, 2022 and December 31, 2021. Total of $12,600 and $3,809 of bad debts were written off for the year ended December 31, 2022 and December 31, 2021, respectively. The Company’s trade receivables consist of receivable from customers which are unrelated to the Company. The account receivables are non-interest bearing and is generally on 30 days to 90 days term.
| F-14 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 4 – INCOME TAXES
The Company provides for income taxes under ASC 740, “Income Taxes. ASC 740 requires the use of an asset and liability approach in accounting for income taxes. Deferred tax assets and liabilities are recorded based on the differences between the financial statements and tax basis of assets and liabilities and the tax rates in effect when these differences are expected to reverse. It also requires the reduction of deferred tax assets by a valuation allowance if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Provision for income taxes consisted of the following:
United States of America
The Company is registered in the State of Wyoming and is subject to the tax laws of the United States of America.
Malaysia
BioNexus Malaysia and Chemrex are both subject to Malaysia Corporate Tax, which is charged at the statutory income tax rate range is 24% on its assessable income. Under the amendment of Income Tax Act 1967 by the Finance Act 2020 and with effect from year of assessment 2020, companies with paid-up capital of RM2.5 million or less, and with annual business income of not more than RM50 million are subject to Small and Medium Enterprise Corporate Tax at 17% on chargeable income up to RM600,000 (2021: RM600,000) except for companies with investment holding nature or companies does not have gross income from business sources are subject to corporate tax at 24% on chargeable income.
|
| As of |
| |||||
|
| December 31, |
|
| December 31, |
| ||
|
| 2022 |
|
| 2021 |
| ||
|
|
|
|
|
|
| ||
Tax Recoverable |
|
|
|
|
|
| ||
Local |
| $ | - |
|
| $ | - |
|
Foreign, representing Malaysia |
|
| (31,551 | ) |
|
| - |
|
Tax Recoverable |
|
| (31,551 | ) |
|
| - |
|
|
|
|
|
|
|
|
|
|
Income tax liabilities: |
|
|
|
|
|
|
|
|
Local |
|
| - |
|
|
| - |
|
Foreign, representing Malaysia |
|
| - |
|
|
| 97,585 |
|
Income tax liabilities |
|
| - |
|
|
| 97,585 |
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Local |
|
| - |
|
|
| - |
|
Foreign, representing Malaysia |
|
| 30,866 |
|
|
| 28,416 |
|
Deferred tax liabilities |
|
| 30,866 |
|
|
| 28,416 |
|
Total |
| $ | (685 | ) |
| $ | 126,001 |
|
| F-15 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 5 – OPERATING LEASE RIGHT OF USE ASSET AND LEASE LIABILITIES
Lease liabilities are measured at present value of the sum of remaining rental payment as of recognition with discount rate of 6.40% per annum adopted from Malayan Banking (Maybank) Berhad's base rate as a reference for discount rate, as this bank is the largest bank and national bank of Malaysia.
A single lease cost is recognized over the lease term on a generally straight-line basis. All cash payments of operating lease cost are classified within operating activities in the statement of cash flows.
As of December 31, 2022 and 2021 operating lease right of use assets as follows:
|
| As of |
|
| As of |
| ||
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
Balance as of December 31, 2021 |
| $ | 41,090 |
|
| $ | 62,529 |
|
Add: Addition of right of use assets |
|
| 32,281 |
|
|
| - |
|
Reduction due to discount on rental |
|
| - |
|
|
| (913 | ) |
Less: accumulated amortization |
|
| (15,534 | ) |
|
| (18,305 | ) |
Foreign translation differences |
|
| (2,107 | ) |
|
| (2,221 | ) |
Balance as of December 31, 2022 |
| $ | 55,730 |
|
| $ | 41,090 |
|
As of December 31, 2022 and 2021 operating lease liabilities as follows:
|
| As of |
|
| As of |
| ||
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
Balance as of beginning of the year |
| $ | 42,909 |
|
| $ | 63,079 |
|
Add: Addition of lease liabilities |
|
| 30,770 |
|
|
| - |
|
Less: Discount on rental |
|
| - |
|
|
| (972 | ) |
Less: gross repayment |
|
| (19,618 | ) |
|
| (16,856 | ) |
Add: imputed interest |
|
| 4,913 |
|
|
| 2,704 |
|
Foreign translation differences |
|
| (2,199 | ) |
|
| (5,046 | ) |
Balance as of end of the year |
|
| 56,775 |
|
|
| 42,909 |
|
Less: lease liabilities current portion |
|
| (16,569 | ) |
|
| (18,272 | ) |
Lease liabilities non-current portion |
| $ | 40,206 |
|
| $ | 24,637 |
|
As of December 31, 2022 and 2021, the maturities of the operating lease obligation are as follows:
|
| As of |
|
| As of |
| ||
Years ending December 31: |
| December 31, 2022 |
|
| December 31, 2021 |
| ||
2022 |
|
| - |
|
|
| 18,272 |
|
2023 |
|
| 16,569 |
|
|
| 11,987 |
|
2024 |
|
| 17,048 |
|
|
| 12,650 |
|
2025 |
|
| 11,209 |
|
|
| - |
|
2026 |
|
| 11,949 |
|
|
| - |
|
Total |
| $ | 56,775 |
|
| $ | 42,909 |
|
The amortization of the operating lease right of use asset for the year ended December 31, 2022 and 2021 were $13,992 and $16,933, respectively.
| F-16 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
Other information:
|
| As of |
|
| As of |
| ||
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
| ||
Operating cash flow from operating leases |
| $ | (126,686 | ) |
| $ | (20,169 | ) |
Right of use assets obtained in exchange for operating lease liabilities |
|
| 55,730 |
|
|
| 41,090 |
|
Remaining lease term for operating leases (years) |
|
| 4 |
|
|
| 2 |
|
Weighted average discount rate for operating leases |
| $ | 6.40 | % |
| $ | 5.40 | % |
Lease expenses for the year ended December 31, 2022 and 2021 were $4,913 and $2,704 respectively.
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following:
|
| As of |
| |||||
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
|
|
|
|
|
|
| ||
Air conditioner |
| $ | 1,124 |
|
| $ | 1,124 |
|
Computer and software |
|
| 2,516 |
|
|
| 1,814 |
|
Equipment |
|
| 60,525 |
|
|
| 43,010 |
|
Furniture and fittings |
|
| 87,122 |
|
|
| 86,961 |
|
Lab equipment |
|
| 320,102 |
|
|
| 284,822 |
|
Land and buildings |
|
| 1,506,969 |
|
|
| 1,506,969 |
|
Motor vehicle |
|
| 137,914 |
|
|
| 137,914 |
|
Office equipment |
|
| 38,213 |
|
|
| 37,700 |
|
Renovation |
|
| 107,414 |
|
|
| 107,414 |
|
Signboard |
|
| 704 |
|
|
| 704 |
|
|
|
| 2,262,603 |
|
|
| 2,208,432 |
|
(Less): Accumulated depreciation |
|
| (616,913 | ) |
|
| (525,631 | ) |
Add: Foreign translation differences |
|
| (133,982 | ) |
|
| (48,383 | ) |
Property, plant and equipment, net |
| $ | 1,511,708 |
|
| $ | 1,634,418 |
|
During the year ended December 31, 2022 and 2021, the Company recorded depreciation of $91,427 and $91,282, respectively.
| F-17 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 7 – OTHER INVESTMENTS
|
| As of |
|
| As of |
| ||
|
| December 31, 2022 |
|
| December 31, 2021 |
| ||
As of beginning of the year |
| $ | 749,027 |
|
| $ | 281,668 |
|
Acquisition of business under common control |
|
| - |
|
|
| - |
|
Addition during the year |
|
| 511,706 |
|
|
| 515,840 |
|
Disposal during the year |
|
| - |
|
| (6,392 | ) | |
Written off during the year |
|
| (1,776 | ) |
|
| - |
|
Fair value gain |
|
| (70,628 | ) |
|
| (29,850 | ) |
Foreign exchange translation |
|
| (37,431 | ) |
|
| (12,239 | ) |
As of end of the year |
| $ | 1,150,898 |
|
| $ | 749,027 |
|
The other investments consist of the following shares:
|
| As of |
| |||||
|
| December 31, |
|
| December 31, |
| ||
|
| 2022 |
|
| 2021 |
| ||
Investment in quoted shares: |
|
|
|
|
|
| ||
Malaysia |
|
| 659,970 |
|
|
| 590,788 |
|
Singapore |
|
| 101,426 |
|
|
| 97,780 |
|
Hong Kong |
|
| 389,502 |
|
|
| 58,584 |
|
|
| $ | 1,150,898 |
|
| $ | 747,152 |
|
Investment in unquoted shares: |
|
|
|
|
|
|
|
|
Malaysia |
|
| - |
|
|
| 1,875 |
|
|
| $ | 1,150,898 |
|
| $ | 749,027 |
|
NOTE 8 – TRADE PAYABLES
Trade payables are amounts billed to the Company by suppliers for goods and services in the ordinary course of business. All amounts have short-term repayment terms and vary by supplier.
| F-18 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
NOTE 9 – CONCENTRATION OF RISKS
a) Major customers
There are no major customers who accounted for 10% or more of the Company’s revenue for the financial year ended December 31, 2022 and 2021.
b) Major suppliers
For year ended December 31, 2022, the suppliers who accounted for 10% or more of the Company’s cost of sales and their balances at year ended are presented as follows:
|
| 2022 |
|
| 2021 |
|
| 2022 |
|
| 2021 |
|
| 2022 |
|
| 2021 |
| ||||||
|
| Purchase |
|
| Percentage of purchases |
|
| Accounts payable trade |
| |||||||||||||||
Vendor A |
| $ | 1,497,142 |
|
| $ | 1,815,817 |
|
|
| 15.48 | % |
|
| 16.37 | % |
| $ | 147,376 |
|
| $ | 397,636 |
|
Vendor B |
| $ | 1,425,867 |
|
| $ | 1,404,442 |
|
|
| 14.74 | % |
|
| 12.66 | % |
| $ | 389,697 |
|
| $ | 405,999 |
|
Vendor C |
| $ | 1,424,476 |
|
| $ | 2,026,842 |
|
|
| 14.73 | % |
|
| 18.27 | % |
| $ | 509,031 |
|
| $ | 640,827 |
|
Vendor D |
| $ | 1,171,511 |
|
| $ | 1,191,344 |
|
|
| 12.11 | % |
|
| 10.74 | % |
| $ | 366,764 |
|
| $ | 269,966 |
|
|
| $ | 5,518,996 |
|
| $ | 6,438,445 |
|
|
| 57.06 | % |
|
| 58.03 | % |
| $ | 1,412,868 |
|
| $ | 1,714,428 |
|
NOTE 10– STOCKHOLDERS’ EQUITY
As at December 31, 2022 and 2021, the Company issued and outstanding, common stock is 173,718,152 and 171,218,152 shares respectively.
On April 12, 2022, the Company entered into Sales & Purchase Agreement with Keith Wong pursuant to which the Company agreed to the sales of 2,500,000 shares of its common stock at $0.06/share. The issuance was exempt under Section 4(a)(2) of the Securities Act as the recipient was a sophisticated investor, the shares were restricted securities and he represented that he is acquiring the shares for investment purposes.
NOTE 11 – SEGMENTED INFORMATION
At December 31, 2022, the Company (“BGLC”) operates in the biochemical industry segment through its two Malaysian subsidiaries, BioNexus Malaysia and Chemrex.
|
| BioNexus Gene Lab Corp. a Wyoming company |
|
| |
|
|
|
|
|
|
|
|
| |||
100% owned Bionexus Gene Lab Sdn. Bhd., a Malaysian company |
|
| 100% owned Chemrex Corporation Sdn. Bhd., a Malaysian company | ||
|
| ||||
| F-19 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
For year ended December 31, 2022, segmented revenue and net profit/(loss) (Currency expressed in United States Dollars (“US$”) are as follows:
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BGLC |
|
| Total |
| ||||
|
| Year ended December 31, 2022 |
| |||||||||||||
REVENUE |
| $ | 95,816 |
|
| $ | 10,832,891 |
|
| $ | - |
|
| $ | 10,928,707 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (51,465 | ) |
|
| (9,618,213 | ) |
|
| - |
|
|
| (9,669,678 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 44,351 |
|
|
| 1,214,678 |
|
|
| - |
|
|
| 1,259,029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 8,830 |
|
|
| 170,453 |
|
|
| - |
|
|
| 179,283 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (286,753 | ) |
|
| (1,051,855 | ) |
|
| (390,881 | ) |
|
| (1,729,489 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (5,657 | ) |
|
| (6,822 | ) |
|
| - |
|
|
| (12,479 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(LOSS)/PROFIT BEFORE TAX |
|
| (239,229 | ) |
|
| 326,454 |
|
|
| (390,881 | ) |
|
| (303,656 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| (1,428 | ) |
|
| (2,470 | ) |
|
| - |
|
|
| (3,898 | ) |
Income tax |
|
| - |
|
|
| (48,412 | ) |
|
| - |
|
|
| (48,412 | ) |
Total tax expense |
|
| (1,428 | ) |
|
| (50,882 | ) |
|
| - |
|
|
| (52,310 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET (LOSS)/PROFIT |
| $ | (240,657 | ) |
| $ | 275,572 |
|
| $ | (390,881 | ) |
| $ | (355,966 | ) |
|
| BioNexus Malaysia |
|
| Chemrex |
|
| BGLC |
|
| Total |
| ||||
|
| Year ended December 31, 2021 |
| |||||||||||||
REVENUE |
| $ | 1,515,673 |
|
| $ | 11,846,894 |
|
| $ | - |
|
| $ | 13,362,567 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUE |
|
| (1,052,938 | ) |
|
| (10,042,688 | ) |
|
| - |
|
|
| (11,095,626 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 462,735 |
|
|
| 1,804,206 |
|
|
| - |
|
|
| 2,266,941 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME |
|
| 7,467 |
|
|
| 59,024 |
|
|
| - |
|
|
| 66,491 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
| (160,094 | ) |
|
| (989,617 | ) |
|
| (127,894 | ) |
|
| (1,277,605 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FINANCE COSTS |
|
| (4,158 | ) |
|
| (8,815 | ) |
|
| - |
|
|
| (12,973 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFIT/(LOSS) BEFORE TAX |
|
| 305,950 |
|
|
| 864,798 |
|
|
| (127,894 | ) |
|
| 1,042,854 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax |
|
| (11,997 | ) |
|
| (14,739 | ) |
|
| - |
|
|
| (26,736 | ) |
Income tax |
|
| (30,482 | ) |
|
| (234,065 | ) |
|
| - |
|
|
| (264,547 | ) |
Total tax expense |
|
| (42,479 | ) |
|
| (248,804 | ) |
|
| - |
|
|
| (291,283 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET PROFIT/(LOSS) |
| $ | 263,471 |
|
| $ | 615,994 |
|
| $ | (127,894 | ) |
| $ | 751,571 |
|
| F-20 |
| Table of Contents |
BIONEXUS GENE LAB CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2022 AND 2021
(Currency expressed in United States Dollars (“US$”))
(Audited)
|
| As of December 31, 2022 and 2021 |
| |||||||||||||
|
| Total Assets |
|
| Total Liabilities |
| ||||||||||
|
| 2022 |
|
| 2021 |
|
| 2022 |
|
| 2021 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
BGLC & Bionexus |
| $ | 677,477 |
|
| $ | 1,167,214 |
|
| $ | 108,390 |
|
| $ | 121,586 |
|
Chemrex |
|
| 8,062,685 |
|
|
| 8,407,176 |
|
|
| 1,966,759 |
|
|
| 2,273,025 |
|
TOTAL |
|
| 8,740,162 |
|
|
| 9,574,390 |
|
|
| 2,075,149 |
|
|
| 2,394,611 |
|
NOTE 12 – SUBSEQUENT EVENTS
In accordance with ASC Topic 855, “Subsequent Events”, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued, the Company has evaluated all events or transactions that occurred after December 31, 2022 up through March 30, 2023 of these consolidated financial statements. During the period, the Company did not have any material recognizable subsequent events.
Similar companies
See also LABCORP HOLDINGS INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also QUEST DIAGNOSTICS INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-30)
See also EXACT SCIENCES CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Guardant Health, Inc. - Annual report 2023 (10-K 2023-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Natera, Inc. - Annual report 2023 (10-K 2023-12-31) Annual report 2024 (10-Q 2024-06-30)



