Celsius Holdings, Inc. - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTIONS 13 OR 15(d)
OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2021
Commission File No. 001-34611
CELSIUS HOLDINGS, INC |
(Exact name of registrant as specified in its charter) |
|
Nevada |
|
20-2745790 |
(State or Other Jurisdiction of |
|
(I.R.S. Employer |
Incorporation or Organization) |
|
Identification No.) |
2424 N Federal Highway, Suite 208, Boca Raton, Florida 33431
(Address of Principal Executive Offices)
(561) 276-2239 |
(Registrant’s telephone number, including area code) |
|
(Former name, former address and former fiscal year, if changed since last report) |
Securities registered under Section 12(b) of the Exchange Act:
Title of Each Class |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which Registered |
Common Stock, $.001 par value |
|
CELH |
|
Nasdaq Capital Market |
Securities registered under Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☒ Yes ☐ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large Accelerated Filer |
☒ |
Accelerated Filer |
☐ |
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
The aggregate market value of the common stock held by non-affiliates of the Registrant was approximately $2,900,279,235 as of June 30, 2021 for the Company’s common stock on such date on the Nasdaq Capital Market. For purposes of the foregoing computation, all executive officers, directors, and 10% beneficial owners of the Registrant are deemed to be affiliates.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. There were 75,337,539 shares of common stock outstanding as of March 15, 2022.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Definitive Proxy Statement to be filed subsequent to the date hereof with the Securities and Exchange Commission (the “SEC”) pursuant to Regulation 14A in connection with the registrant’s 2022 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report. Such Definitive Proxy Statement will be filed with the SEC no later than 120 days after the conclusion of the registrant’s fiscal year ended December 31, 2021.
TABLE OF CONTENTS
|
|
Page |
|
|
|
|
1 |
|
|
|
|
Item 1. |
1 |
|
Item 1A. |
6 |
|
Item1B. |
16 |
|
Item 2. |
16 |
|
Item 3. |
16 |
|
Item 4. |
16 |
|
|
|
|
|
17 |
|
|
|
|
Item 5. |
17 |
|
Item 6. |
17 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
19 |
Item 7A. |
22 |
|
Item 8. |
22 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
22 |
Item 9A. |
23 |
|
Item 9B. |
24 |
|
|
|
|
|
25 |
|
|
|
|
Item 10. |
25 |
|
Item 11. |
25 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
25 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
25 |
Item 14. |
25 |
|
|
|
|
|
|
|
|
|
|
Item 15. |
25 |
|
Item 16. |
26 |
|
|
28 |
i
When used in this Annual Report on Form 10-K (this “Report”), unless otherwise indicated, the terms “the Company,” “Celsius,” “we,” “us” and “our” refers to Celsius Holdings, Inc. and its subsidiaries.
We have proprietary rights to trademarks used in this Report that are important to our business, many of which are registered under applicable intellectual property laws. Solely for convenience, the trademarks, service marks, logos and trade names referred to in this Report may sometimes appear without the ® and TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Report contains statements that are based on the current expectations of our Company and management about future events within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). You are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those projected in the forward-looking statements as a result of various factors.
Our forward-looking statements may include, but are not limited to, statements about:
|
● |
our expectations relating to expansion into additional geographic markets and product lines; |
|
● |
our expectations relating to revenue, operating costs and profitability; |
|
● |
our expectations regarding our strategy and investments; |
|
● |
our expectations regarding our business, including market opportunity, consumer demand and our competitive advantage; |
|
● |
our expectations regarding supply chains and distribution networks; |
|
● |
the impact of future and existing food and drug laws and regulations on our business; |
|
● |
our expectations regarding cost and availability of materials and ingredients; |
|
● |
our expectations regarding our future growth prospects and our ability manage our growth and hire capable personnel to support our growth; |
|
● |
the potential ongoing impact of the continuing COVID-19 pandemic on us directly, or on our distributors or suppliers; |
|
● |
expected competition from the functional energy drink and supplement industries and other sources; |
|
● |
our expectations relating to marketing and advertising expense; |
|
● |
the timing of our receipt and recognition of revenues and other payments; |
|
● |
our expectations about our trademarks and trade secrets; |
|
● |
our expectations relating to macroeconomic conditions; |
|
● |
our critical accounting policies and related estimates or changes in accounting practices; |
|
● |
liquidity and capital needs; |
|
● |
political, legislative, regulatory and legal challenges; |
|
● |
the merits or potential impact of any lawsuits filed against us or disputes we may be party to; and |
|
● |
other statements regarding our future operations, financial condition, prospects and business strategies. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including but not limited to: our ability to successfully make and integrate acquisitions; the impact on our operations of public health crises, including of the current coronavirus outbreak; and the performance, reliability and availability of our ecommerce platform and underlying network infrastructure.
The words “anticipate,” “assume,” “believe,” “could,” “designed,” “estimate,” “expect,” “forecast,” “goal,” “hope,” “intend,” “may,” “might,” “objective,” “plan,” “potential,” “project,” “seek,” “should,” “target,” or the negatives and variations of such words and similar expressions are intended to identify forward-looking statements, but are not the exclusive means of identifying such statements. Those statements appear in this Report, particularly in “Item 1. Business,” “Item 1A. Risk Factors” and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and include statements regarding the intent, belief or current expectations of the Company and management that are subject to known and unknown risks, uncertainties and assumptions.
ii
PART I
Item 1. Business
Overview
Celsius is a fast-growing company in the functional energy drink and liquid supplement categories in the United States and internationally. We engage in the development, processing, marketing, sale, and distribution of functional drinks and liquid supplements to a broad range of consumers. We believe that we provide differentiated products that offer clinically proven and innovative formulas meant to change the lives of our consumers for the better. We also believe that our brand is attractive to a broad range of customers including fitness enthusiasts.
Our core offerings include pre- and post-workout functional energy drinks, as well as protein bars. Our flagship functional energy drink and liquid supplement brands are backed by science, being clinically proven to deliver health benefits by six self-funded studies published in various journals including the Journal of the International Society of Sports Nutrition, the Journal of the American College of Nutrition, and the Journal of Strength and Conditioning Research. These studies have concluded that a single serving of CELSIUS® burns 100-140 calories (by increasing a consumer’s resting metabolism an average of 12%, while providing sustained energy for up to three hours.
Our flagship asset, CELSIUS®, is a fitness supplement drink which accelerates metabolism and burns calories and body fat while providing energy. This product line comes in two versions, a ready-to-drink supplement format and an on-the-go powder form. We also offer a CELSIUS HEAT and a BRANCH CHAIN AMINO ACIDS line, catered to both pre- and post-workout consumer needs. Our products are currently offered in major retail channels in the US including conventional grocery, natural, convenience, fitness, mass market, vitamin specialty and e-commerce.
An integral part of our value proposition is our focus on the functional energy drink and liquid supplement category, ensuring our products have clear and proven benefits. This is why we invest in research and development from the start and utilize our proprietary MetaPlus® formulation in our portfolio, a blend of ginger root, guarana seed extract, chromium, vitamins, and green tea extract.
Potential Effects of the COVID-19 Pandemic on the Company’s Business
The current COVID-19 pandemic has presented and continues to present a substantial public health and economic challenge around the world and is affecting our employees, communities and business operations, as well as the global economy and financial markets. The human and economic consequences of the COVID-19 pandemic as well as the measures taken or that may be taken in the future by governments, businesses (including the Company and our suppliers, bottlers/distributors, co-packers and other service providers) and the public at large to limit the COVID-19 pandemic, have and will directly and indirectly impact our business and results of operations, including, without limitation, the following:
1
Any of the negative impacts of the COVID-19 pandemic, including those described above, alone or in combination with others, may have a material adverse effect on our business, reputation, operating results and/or financial condition. Any of these negative impacts, alone or in combination with others, could exacerbate many of the risk factors discussed herein, any of which could materially affect our business, reputation, operating results and/or financial condition.
Corporate Information
We were incorporated in the State of Nevada in April 2005. Our principal executive offices are located at 2424 North Federal Highway, Suite 208, Boca Raton, Florida 33431, and our telephone number is (561) 276-2239. Our website is www.celsiusholdingsinc.com. Information contained on, or that can be accessed through, our website is not incorporated by reference into this Report.
Our Products
CELSIUS® calorie-burning functional energy drinks were first introduced to the marketplace in 2005.
According to multiple clinical studies we funded, a single serving (12 ounce can) of CELSIUS® burns 100 to 140 calories by increasing a consumer’s metabolism an average of 12% for up to a three-hour period. In addition, these studies have indicated that drinking a single serving of CELSIUS® prior to exercising may improve cardiovascular health and fitness and enhance the loss of fat and gain of muscle from exercise.
We seek to combine nutritional science with mainstream beverages by using our proprietary thermogenic (calorie-burning) MetaPlus® formulation, while fostering the goal of healthier everyday refreshment by being as natural as possible without the artificial preservatives often found in many energy drinks or sodas. CELSIUS® has no chemical preservatives, aspartame or high fructose corn syrup and is very low in sodium. CELSIUS® uses good-for-you ingredients and supplements such as green tea (EGCG), ginger, calcium, chromium, B vitamins and vitamin C. CELSIUS® is sweetened with sucralose, a sugar-derived sweetener that is found in Splenda®, which makes our functional energy drinks low-calorie and suitable for consumers whose sugar intake is restricted. Each 12 ounce can of CELSIUS® contains 200 milligrams of caffeine which is comparable to one 12-ounce cup of coffee from the leading coffeehouse.
We currently have five functional energy drink lines:
2
CELSIUS® ready-to drink products are packaged in a distinctive 12 ounce sleek can that uses vivid colors in abstract patterns to create a strong on-shelf impact. The cans are sold in various packaging units. We have recently redesigned our packaging to provide a cleaner, crisper and more modern look. In addition to being sugar free, our original ready-to -drink products line is non-GMO, kosher and vegan certified and soy and gluten free.
As a result of completion of the acquisition of Func Food in October 2019, we acquired the beverages, protein bars, supplements and superfoods marketed and distributed in Finland, Sweden, and Norway. These products are distributed under the FAST, FitFarm and CocoVi brands and which are owned by Func Food. FAST products is a market leader in Finland and has begun distribution into the Swedish market and in the US. FitFarm and CocoVi are well-established brands of superfoods and other supplements in the Nordic countries.
Clinical Studies
It is our belief that clinical studies substantiating product claims will become more important as more and more functional energy drinks and other beverages are marketed with health claims. CELSIUS® was one of the first functional energy drinks to be launched along with a clinical study. CELSIUS® is also one of very few functional energy drinks that has clinical research on the actual product itself. Some functional energy drink and supplement companies that do mention studies backing their claims are referencing independent studies conducted on one or more of the ingredients in the product. We believe that it is important and will become more important to have studies on the actual product.
We have funded seven U.S. based clinical studies for CELSIUS®. Each was conducted by a research organization and each studied the total CELSIUS® formula. The first study was conducted by the Ohio Research Group of Exercise Science & Sports Nutrition. The remaining studies were conducted by the Applied Biochemistry & Molecular Physiology Laboratory of the University of Oklahoma. We funded all of the studies and provided CELSIUS® functional energy drinks for the studies. However, none of our directors, executive officers or principal stockholders is in any way affiliated with either of the two research organizations which conducted the studies.
The first study was conducted in 2005 by the Ohio Research Group of Exercise Science & Sports Nutrition www.ohioresearchgroup.com. The Ohio Research Group of Exercise Science & Sports Nutrition is a multidisciplinary clinical research team dedicated to exploring the relationship between exercise, nutrition, dietary supplements and health. This placebo-controlled, double-blind cross-over study compared the effects of CELSIUS® and the placebo on metabolic rate. Twenty-two participants were randomly assigned to ingest a 12 ounce serving of CELSIUS® and on a separate day a serving of twelve ounces of Diet Coke®. All subjects completed both trials using a randomized, counterbalanced design. Randomized means that subjects were selected for each group randomly to ensure that the different treatments were statistically equivalent. Counterbalancing means that individuals in one group drank the placebo on the first day and drank CELSIUS® on the second day. The other group did the opposite. Counterbalancing is a design method that is used to control “order effects.” In other words, this was done to make sure that the order that subjects were served does not impact the results and analysis.
Metabolic rate (via indirect calorimetry, measurements taken from breaths into and out of calorimeter) and substrate oxidation (via respiratory exchange ratios) were measured at baseline (pre-ingestion) and for ten minutes at the end of each hour for three hours post-ingestion. The results showed an average increase of metabolism of twelve percent over the three-hour period, compared to a statistically insignificant change for the control group. Metabolic rate, or metabolism, is the rate at which the body expends energy. This is also referred to as the “caloric burn rate.” Indirect calorimetry calculates heat that living organisms produce from their production of carbon dioxide. It is called “indirect” because the caloric burn rate is calculated from a measurement of oxygen uptake. Direct calorimetry would involve the subject being placed inside the calorimeter for the measurement to determine the heat being produced. Respiratory Exchange Ratio is the ratio oxygen taken in a breath compared to the carbon dioxide breathed out in one breath or exchange. Measuring this ratio can be used for estimating which substrate (fuel such as carbohydrate or fat) is being metabolized or ‘oxidized’ to supply the body with energy.
The second study was conducted by the Applied Biochemistry & Molecular Physiology Laboratory of University of Oklahoma in 2007. This blinded, placebo-controlled study was conducted on a total of 60 men and women of normal weight. An equal number of participants were separated into two groups to compare one serving (a single 12 ounce can) of CELSIUS® to a placebo of the same amount. According to the study, those subjects consuming CELSIUS® burned significantly more calories versus those consuming the placebo, over a three-hour period. The study confirmed that over the three-hour period, subjects consuming a single serving of CELSIUS® burned 65% more calories than those consuming the placebo drink and burned an average of more than 100 to 140 calories compared to the placebo. These results were statistically significant.
The third study, conducted by the Applied Biochemistry & Molecular Physiology Laboratory of University of Oklahoma in 2007, extended our second study with the same group of 60 individuals and protocol for 28 days and showed the same statistical significance of increased calorie burn (minimal attenuation). While the University of Oklahoma study did extend for 28 days, more testing would be needed for long term analysis of the CELSIUS® calorie-burning effects. Also, although these studies were on relatively small numbers of subjects, they have statistically significant results. Additional studies on a larger number and wider range of body compositions can be considered to further the analysis.
Our fourth study, conducted by the Applied Biochemistry & Molecular Physiology Laboratory of University of Oklahoma in 2009, combined CELSIUS® use with exercise. This ten-week placebo-controlled, randomized and blinded study was conducted on a total of 37 subjects. Participants were randomly assigned into one of two groups: Group 1 consumed one serving of CELSIUS® per day, and Group 2 consumed one serving of an identically flavored and labeled placebo
3
beverage. Both groups participated in ten weeks of combined aerobic and weight training, following the American College of Sports Medicine guidelines of training for previously sedentary adults. The results showed that consuming a single serving of CELSIUS® prior to exercising may enhance the positive adaptations of exercise on body composition, cardio-respiratory fitness and endurance performance. According to the preliminary findings, subjects consuming a single serving of CELSIUS® lost significantly more fat mass and gained significantly more muscle mass than those subjects consuming the placebo — a 93.75% greater loss in fat and 50% greater gain in muscle mass, respectively. The study also confirmed that subjects consuming CELSIUS® significantly improved measures of cardio-respiratory fitness and the ability to delay the onset of fatigue when exercising to exhaustion.
Our fifth study was conducted by the Applied Biochemistry & Molecular Physiology Laboratory of University of Oklahoma in 2009. This ten-week placebo-controlled, randomized and blinded study was conducted on a total of 27 previously sedentary overweight and obese female subjects. Participants were randomly assigned into groups that consumed identically tasting treatment drinks with exercise or without exercise. All participants consumed one drink, either placebo or CELSIUS®, per day for 10 weeks. The exercise groups participated in ten weeks of combined aerobic and weight training, following the American College of Sports Medicine guidelines of training for previously sedentary adults. No changes were made to their diet. The results showed that consuming a single serving of CELSIUS® prior to exercising may improve cardiovascular health and fitness and enhance the positive adaptations of exercise on body composition. According to the preliminary findings, subjects consuming a single serving of CELSIUS® lost significantly more fat mass and gained significantly more muscle mass when compared to exercise alone — a 46% greater loss in fat, 27% greater gain in muscle mass, respectively. The study also confirmed that subjects consuming CELSIUS® significantly improved measures of cardio-respiratory fitness — 35% greater endurance performance with significant improvements to lipid profiles — total cholesterol decreases of 5 to 13% and bad LDL cholesterol 12 to 18%. Exercise alone had no effect on blood lipid levels.
Our sixth study was conducted by the Applied Biochemistry & Molecular Physiology Laboratory of University of Oklahoma in 2009. This ten-week placebo-controlled, randomized and blinded study was conducted on a total of 37 previously sedentary male subjects. Participants were randomly assigned into groups that consumed identically tasting treatment drinks with exercise or without exercise. All participants consumed one drink, either placebo or CELSIUS®, per day for 10 weeks. The exercise groups participated in ten weeks of combined aerobic and weight training, following the American College of Sports Medicine guidelines of training for previously sedentary adults. No changes were made to their diet. The results showed that consuming a single serving of CELSIUS® prior to exercising may improve cardiovascular health and fitness and enhance the positive adaptations of exercise on body composition. Significantly greater decreases in fat mass and percentage body fat and increases in VO2 were observed in the subjects that consumed CELSIUS® before exercise versus those that consumed the placebo before exercise. Mood was not affected. Clinical markers for hepatic, renal, cardiovascular and immune function, as determined by pre-and post-blood work revealed no adverse effects.
Our seventh study was conducted by Miami Research Institute in 2010 and demonstrated the efficacy and safety of the powders and the shots. This study allows the Company to make the same structure/function claims as its ready to drink products.
Manufacture and Supply of Our Products
Our functional energy drinks and supplements are produced by established third party beverage co-packers. A co-packer is a manufacturing plant that provides the service of filling bottles or cans for the brand owner. We believe one benefit of using co-packers is that we do not have to invest in the production facility and can focus our resources on brand development, sales and marketing. It also allows us produce in multiple locations strategically placed throughout the country. We purchase most of the ingredients and all packaging materials. The co-pack facility assembles our products and charges us a fee by the case. The shelf life of CELSIUS® products is specified as 15 to 18 months.
Substantially all of the raw materials used in the preparation, bottling and packaging of our products are purchased by us or by our co-packers in accordance with our specifications. Generally, we obtain the ingredients used in our products from domestic suppliers and some ingredients have several reliable suppliers. The ingredients in CELSIUS® include green tea (EGCG), ginger (from the root), caffeine, B vitamins, vitamin C, taurine, guarana, chromium, calcium, glucuronolactone, sucralose, natural flavors and natural colorings. CELSIUS® is labeled with a supplements facts panel. We have no major supply contracts with any of our suppliers. We single-source all our ingredients for purchasing efficiency; however, we have identified a second source for our critical ingredients and there are many suppliers of flavors, colorings and sucralose. In case of a supply restriction or interruption from any of the flavor and coloring suppliers, we would have to test and qualify other suppliers that may disrupt our production schedules.
Packaging materials, except for our distinctive sleek aluminum cans, are available from multiple sources in the United States; however, due to efficiencies we utilize single source vendor relationships. While the beverage industry has experienced some shortages of cans as a result of the COVID-19 pandemic, we have been able to secure adequate supply and have not experienced significant adverse effects from such shortages.
We believe that our co-packing arrangements and supply sources are adequate for our present needs.
Func Food similarly has its FAST and other products manufactured by independent third parties in the Nordic countries.
Distribution
Domestic
In the United States and elsewhere in North America, CELSIUS® is sold across many retail segments. They include supermarkets, convenience stores, drug stores, nutritional stores, and mass merchants. We also sell to health clubs, spas, gyms, the military, and e-commerce websites.
We distribute our products domestically through a hybrid of direct-store delivery (DSD) distributors and as well as sales direct to retailers (DTR).
We are continuing to emphasize the expansion of our DSD distribution network as we believe it has been a key element in opening additional domestic markets and retail segments and increasing sales.
4
Our products are sold online through e-commerce platforms such as Amazon. As a result of the shift to online purchasing by consumers, including additional growth arising from the effects of the COVID-19 pandemic as a result of the shutdown of various retail outlets, particularly gyms and health clubs in the fitness channel, e-commerce sales have accounted for a growing percentage of our domestic revenue.
International
We distribute our products in various foreign regions through regional and country-specific distribution partners. In October 2019, we acquired Func Food, our Nordic distribution partner, who markets both our products as well as other products under its own brands. CELSIUS® intends to use Func Food as a platform to expand product distribution elsewhere in Europe.
We market our products in the Asian market, which is one of the most dynamic and fastest-growing, through local distributors in Hong Kong and a license agreement with our partner in China, Qifeng Food Technology (Beijing) Co., Ltd. (“Qifeng”). While we initially began both local production and preliminary distribution of the CELSIUS® brand in 2018 through our partnership in Qifeng, effective January 1, 2019, we restructured our China distribution efforts by entering into two separate agreements as it relates to the commercialization of our CELSIUS® products (i.e., license agreement) and a repayment of investment agreement with Qifeng. Under the license agreement, Qifeng was granted the exclusive license rights to manufacture, market and commercialize CELSIUS® brand products in China. Qifeng will pay a minimum royalty fee of approximately $7.0 million for the five years of the term of the agreement, transitioning to a volume-based royalty fee, thereafter. Under a separate economic agreement, Qifeng Food will repay the marketing investments made by CELSIUS® into the China market through 2018, over the same five-year period. The repayment, which was formalized via a note receivable from Qifeng, will need to be serviced even if the licensing agreement is cancelled or terminated.
Customer Concentration
For the year ended December 31, 2021, Costco and Amazon accounted for approximately 12.7% and 10.1% of our revenue, respectively. For the year ended December 31, 2020, those customers accounted for approximately 2.9% and 15.1% of our revenues, respectively. Additionally, Amazon and Publix accounted for approximately 22.7% and 10.3% of our total accounts receivable as of December 31, 2021 and approximately11.3% and 6.0% of our accounts receivable. as of December 31, 2020.
Seasonality of Sales
As is typical in the functional energy drink and supplement industries, sales of our products are seasonal, with the highest sales volumes generally occurring in the second and third fiscal quarters, which correspond to the warmer months of the year in our major markets.
Competition
We believe that our CELSIUS® brand products are one of the few calorie-burning functional energy drink and supplement lines whose effectiveness is supported by clinical studies, which gives us a unique position in the functional energy drink and supplement markets. However, our products do compete broadly with not only functional energy drinks and supplements, but all categories of liquid refreshments. The functional energy drink, supplement and liquid refreshment markets are highly competitive, and include international, national, regional and local producers and distributors, most of whom have greater financial, management and other resources than us. Our direct competitors in the functional energy drink and supplement markets include, but are not limited to The Coca-Cola Company, Dr. Pepper Snapple Group, PepsiCo, Inc., Nestlé, Waters North America, Inc., Hansen Natural Corp., Vital Pharmaceuticals, Inc, Monster Energy, and Red Bull.
Proprietary Rights
We have registered the CELSIUS® and MetaPlus® trademarks with the United States Patent and Trademark Office, as well as a number of additional trademarks.
We have and will continue to take appropriate measures, such as entering into confidentiality agreements with our contract packers and ingredient suppliers, to maintain the secrecy and proprietary nature of our MetaPlus® formulation and product formulas.
We maintain our MetaPlus® formulation and product formulas as trade secrets. We believe that trade secrecy is a preferable method of protection for our formulas as patenting them might require their disclosure. Other than a company that is our outsourced production manager, no single member of the raw material supply chain or our co-packers has access to the complete formula.
We consider our trademarks and trade secrets to be of considerable value and importance to our business. No successful challenges to our registered trademarks have arisen and we have no reason to believe that any such challenges will arise in the future.
As a result of the acquisition of Func Food in October 2019, we have acquired additional brands and tradenames.
Government Regulation
The production, distribution and sale of our products in the United States is subject to the Federal Food, Drug and Cosmetic Act, the Dietary Supplement Health and Education Act of 1994, the Occupational Safety and Health Act, various environmental statutes and various other federal, state and local statutes and regulations applicable to the production, transportation, sale, safety, advertising, labeling and ingredients of such products. California law requires that a specific warning appear on any product that contains a component listed by California as having been found to cause cancer or birth defects. The law exposes all food and beverage producers to the possibility of having to provide warnings on their products because the law recognizes no generally applicable quantitative thresholds below which a warning is not required. Consequently, even trace amounts of listed components can expose affected products to the prospect of warning labels. Products containing listed substances that occur naturally in the product or that are contributed to the product solely by a municipal water supply are generally exempt from the warning requirement. While none of our products are required to display warnings under this law, we cannot predict whether an
5
important component of any of our products might be added to the California list in the future. We also are unable to predict whether or to what extent a warning under this law would have an impact on costs or sales of our products.
Measures have been enacted in various localities and states that require that a deposit be charged for certain non-refillable beverage containers. The precise requirements imposed by these measures vary. Other deposit, recycling or product stewardship proposals have been introduced in certain states and localities and in Congress, and we anticipate that similar legislation or regulations may be proposed in the future at the local, state and federal levels, both in the United States and elsewhere.
Our facilities in the United States are subject to federal, state and local environmental laws and regulations. Compliance with these provisions has not had, and we do not expect such compliance to have, any material adverse effect upon our business, financial condition and results of operations.
The marketing and sale of our products internationally is similarly subject to compliance with applicable laws, rules and regulations in those foreign countries where our products are sold.
Employees
As of the date of this Report, the Company employs 225 people, including its executive officers.
Information about our Executive Officers
The following is a description of the business experience and background of Edwin Negron-Carballo, our Chief Financial Officer, who is our only executive officer who is not a member of our board of directors.
Edwin Negron-Carballo, 60, became our Chief Financial Officer in July 2018. He is well versed in US GAAP and IFRS as a Certified Public Accountant and has significant experience in mergers and acquisitions. Mr. Negron-Carballo served as the Chief Financial Officer of Concurrent Manufacturing Solutions, LLC from October 2012 to December 2017. Mr. Negron-Carballo’s prior experience also includes working for major companies such as KPMG, Sodexo, S.A., Tyco Healthcare-Latin America, Energizer Battery and Frito-Lay.
Item 1A. Risk Factors
In addition to the other information contained in this Report, including in “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statement and notes, you should carefully consider the following risks. The risks and uncertainties discussed below and elsewhere in this Report may not be the only risks we face. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also materially adversely affect our business, financial condition, operating results and prospects.
Summary of Principal Risk Factors
The following is a summary of the principal risks that could materially adversely affect our business, financial condition, operating results and prospects. You should read this summary together with the more detailed description of each risk set forth below:
|
● |
The COVID-19 pandemic, and newly emerging variants of COVID-19, have had, and we expect will continue to have, certain impacts on our business and operations, and such impacts may have a material adverse or other effect on our business and results of operations. |
|
● |
We rely on third party co-packers to manufacture our products. If we are unable to maintain good relationships with our co-packers and/or their ability to manufacture our products becomes constrained or unavailable to us, our business could suffer. |
|
● |
We rely on distributors to distribute our products in the Direct Store Delivery sales channel and in international markets. If we are unable to secure such distributors and/or we are unable to maintain good relationships with our existing distributors, our business could suffer. |
|
● |
Our customers are material to our success. If we are unable to maintain good relationships with our existing customers, our business could suffer. |
|
● |
Increases in costs or shortages of raw materials, increases in co-packing costs and challenges in our distribution efforts could adversely impact our business. |
|
● |
We are subject to significant competition in the functional energy drink and supplement industries. |
|
● |
Our inability to innovate successfully and to provide new cutting-edge products could adversely affect our business and financial results. |
|
● |
Changes in consumer product and shopping preferences may reduce demand for some of our products. |
|
● |
We derive virtually all of our revenues from functional energy drinks and supplements, and competitive pressure in the functional energy drink and supplement category could adversely affect our business and operating results. |
|
● |
We compete in an industry that is brand-conscious, so brand name recognition and acceptance of our products are critical to our success and significant marketing and advertising could be needed to achieve and sustain brand recognition. |
|
● |
Product safety and quality concerns, or other negative publicity (whether or not warranted) could damage our brand image and corporate reputation and may cause our business to suffer. |
6
Risk Factors Relating to Our Business
Our business and results of operations may be adversely affected by the COVID-19 pandemic
The current COVID-19 pandemic has presented and continues to present a substantial public health and economic challenge around the world and is affecting our employees, communities and business operations, as well as the global economy and financial markets. The human and economic consequences of the COVID-19 pandemic as well as the measures taken or that may be taken in the future by governments, businesses (including the Company and our suppliers, bottlers/distributors, co-packers and other service providers) and the public at large to limit the COVID-19 pandemic, have and will directly and indirectly impact our business and results of operations, including, without limitation, the following:
7
Any of the negative impacts of the COVID-19 pandemic, including those described above, alone or in combination with others, may have a material adverse effect on our business, reputation, operating results and/or financial condition. Any of these negative impacts, alone or in combination with others, could exacerbate many of the risk factors discussed herein, any of which could materially affect our business, reputation, operating results and/or financial condition.
Consolidation of retailers, wholesalers and distributors in the industry may result in downward pressure on sales prices.
Consolidation can cause significant downward pricing pressure and can impose additional costs on us. If we are unable to successfully manage the potential impact of these commercial changes, our financial results may be materially and adversely affected.
We rely on third party co-packers to manufacture our products. If we are unable to maintain good relationships with our co-packers and/or their ability to manufacture our products becomes constrained or unavailable to us, our business could suffer.
We do not directly manufacture our products, but instead outsource such manufacturing to established third party co-packers. These third-party co-packers may not be able to fulfill our demand as it arises, could begin to charge rates that make using their services cost inefficient or may simply not be able to or willing to provide their services to us on a timely basis or at all. In the event of any disruption or delay, whether caused by a rift in our relationship or the inability of our co-packers to manufacture our products as required, we would need to secure the services of alternative co-packers. We may be unable to procure alternative packing facilities at commercially reasonable rates and/or within a reasonably short time period and any such transition could be costly. In such case, our business, financial condition and results of operations would be adversely affected.
We rely on distributors to distribute our products in the DSD sales channel and in international markets. If we are unable to secure such distributors and/or we are unable to maintain good relationships with our existing distributors, our business could suffer.
We distribute CELSIUS® in the DSD sales channel by entering into agreements with direct-to-store delivery distributors having established sales, marketing and distribution organizations. We similarly are seeking to expand our international distribution, particularly in the Far East and elsewhere in Asia by entering into agreements with large established distributors who service those markets. Many of our distributors are affiliated with and manufacture and/or distribute other beverage products. In many cases, such products compete directly with our products. The marketing efforts of our distributors are important for our success. If CELSIUS® proves to be less attractive to our distributors and/or if we fail to attract distributors, and/or our distributors do not market and promote our products with greater focus in preference to the products of our competitors, our business, financial condition and results of operations could be adversely affected.
Our customers are material to our success. If we are unable to maintain good relationships with our existing customers, our business could suffer.
Unilateral decisions could be taken by our distributors, grocery chains, convenience chains, drug stores, nutrition stores, mass merchants, club warehouses and other customers to discontinue carrying all or any of our products that they are carrying at any time, which could cause our business to suffer.
Increases in cost or shortages of raw materials or increases in costs of co-packing could harm our business.
The principal raw materials used by us are flavors and ingredient blends as well as aluminum cans, the prices of which are subject to fluctuations. We are uncertain whether the prices of any of the above or any other raw materials or ingredients we utilize will rise in the future and whether we will be able to pass any of such increases on to our customers. We do not use hedging agreements or alternative instruments to manage the risks associated with securing sufficient ingredients or raw materials. In addition, some of these raw materials, such as our distinctive sleek 12 ounce can, are available from a single or a limited number of suppliers. As alternative sources of supply may not be available, any interruption in the supply of such raw materials might materially harm us.
While the functional energy drink and supplement industries have experienced some shortages of cans as a result of the COVID-19 pandemic, Celsius has been able to secure adequate supply, though potentially at higher cost. To address the industry-wide shortage of aluminum cans, we have imported cans manufactured abroad which affected our margins in 2021 and will continue to affect our margins in 2022. We believe the increased prices, combined with a requirement to prepay for raw materials, will negatively impact our margins. We are unable to accurately predict how these cost trends might evolve prospectively. Such industry-wide shortages of raw materials, including aluminum cans, could from time to time in the future be encountered, which could interfere with and/or delay production of certain of our products and negatively impact our financial performance.
Our failure to accurately estimate demand for our products could adversely affect our business and financial results.
We may not correctly estimate demand for our existing products and/or new products. Our ability to estimate demand for our products is imprecise, particularly with regard to new products, and may be less precise during periods of rapid growth, including in new markets. If we materially underestimate demand for our products or are unable to secure sufficient ingredients, flavors, sleek aluminum cans and other raw materials for our supplements or experience difficulties with our co-packing arrangements, including production shortages or quality issues, we might not be able to satisfy demand on a short-term basis. Moreover, industry-wide shortages of certain ingredients have occurred and could occur, from time to time in the future, resulting in production fluctuations and/or product shortages. We generally do not use hedging agreements or alternative instruments to manage this risk. Such shortages could interfere with and/or delay production of certain of our products and could have a material adverse effect on our business and financial results.
8
If we do not accurately anticipate the future demand for a particular product or the time it will take to obtain new inventory, our inventory levels may be inadequate and our results of operations may be negatively impacted. If we fail to meet our shipping schedules, we could damage our relationships with distributors and/or retailers, increase our distribution costs and/or cause sales opportunities to be delayed or lost. In order to be able to deliver our products on a timely basis, we need to maintain adequate inventory levels of the desired products. If the inventory of our products held by our distributors and/or retailers is too high, they will not place orders for additional products, which could unfavorably impact our future sales and adversely affect our operating results.
Changes in the retail landscape or the loss of key retail or foodservice customers could adversely affect our financial results.
Our industry is being affected by the trend toward consolidation in and blurring of the lines between retail channels, particularly in Europe and the United States. Larger retailers may seek lower prices from us, may demand increased marketing or promotional expenditures, and may be more likely to use their distribution networks to introduce and develop private-label brands, any of which could negatively affect our profitability. In addition, discounters and value stores are growing at a rapid pace. Our industry is also being affected by the rapid growth in sales through e-commerce retailers, e-commerce websites, mobile commerce applications and subscription services, which may result in a shift away from physical retail operations to digital channels. As we build our e-commerce capabilities, we may not be able to develop and maintain successful relationships with existing and new e-commerce retailers without experiencing a deterioration of our relationships with key customers operating physical retail channels. If we are unable to successfully adapt to the rapidly changing retail landscape, including the rapid growth in digital commerce, our share of sales, volume growth and overall financial results could be negatively affected. In addition, our success depends in part on our ability to maintain good relationships with key retail customers. The loss of one or more of our key retail customers could have an adverse effect on our financial performance.
Significant additional labeling or warning requirements or limitations on the marketing or sale of our products may inhibit sales of affected products.
Various jurisdictions may seek to adopt significant additional product labeling or warning requirements or limitations on the marketing or sale of our products as a result of what they contain or allegations that they cause adverse health effects. If these types of requirements become applicable to one or more of our products under current or future environmental or health laws or regulations, they may inhibit sales of such products.
For example, under one such law in California, known as Proposition 65, if the state has determined that a substance causes cancer or harms human reproduction, a warning must be provided for any product sold in the state that exposes consumers to that substance, unless the exposure falls under an established safe harbor level or another exemption is applicable. If we were required to add Proposition 65 warnings on the labels of one or more of our supplement products produced for sale in California, the resulting consumer reaction to the warnings and possible adverse publicity could negatively affect our sales both in California and in other markets.
Our continued expansion outside of the United States exposes us to uncertain conditions and other risks in international markets.
We have continued expanding our sales of our products internationally into a variety of new markets and are currently seeking to expand our international distribution, particularly in East Asia and elsewhere in Asia by entering into agreements with large established distributors who service those markets. As our growth strategy includes further expanding our international business, if we are unable to continue to expand distribution of our products outside the United States, our growth rate could be adversely affected. Although we intend to sell through established distributors in international markets, we have limited or no operating experience in many of such markets and it may be costly to promote our brands in international markets. We face and will continue to face substantial risks associated with foreign distribution and sale of our products, including: economic and/or political instability in various international markets; fluctuations in foreign currency exchange rates; restrictions on or costs relating to the repatriation of foreign profits to the United States, including possible taxes and/or withholding obligations on any repatriations; and tariffs and/or trade restrictions. These risks could have a significant impact on our ability to distribute and sell our products on a competitive basis in international markets and could have a material adverse effect on our business, financial condition and results of operations. Also, distribution and sale of products outside of the United States are subject to risks relating to appropriate compliance with legal and regulatory requirements in local jurisdictions, potentially higher product damage rates if our products are shipped long distances, potentially higher incidence of fraud and/or corruption, credit risk of distributors and potentially adverse tax consequences.
Numerous U.S. and International laws including export and import controls affect our ability to compete in international markets.
U.S. export control laws and economic and trade sanctions prohibit the provision of certain products and services to U.S. embargoed or sanctioned countries, governments and persons. Even though we take precautions to prevent our products from being shipped or provided to embargoed countries and U.S. sanctions targets, they could be shipped, or provided by our distributors, to those countries and targets despite such precautions. The provision of goods in violation of U.S. export controls and/or sanctions could have negative consequences for our business, including government investigations, penalties and reputational harm. We must also comply with U.S. import laws.
U.S. laws such as the Foreign Corrupt Practices Act (the “FCPA”) also impact our international activities. We are subject to the FCPA and other laws that prohibit improper payments and offers to foreign officials and political parties for the purpose of obtaining or retaining business. Selling products into international markets, including through distributors, creates the risk of unauthorized payments or offers, for which we may be held responsible. Violations of the FCPA or other applicable anti-corruption and anti-bribery laws may result in severe criminal or civil sanctions, or other liabilities, which could negatively affect our business, operating results and financial condition.
Changes in export and import regulations, economic sanctions and related laws, shifts in the enforcement or scope of existing regulations, changes in the countries, governments or persons targeted by such regulations and the imposition of tariffs may create delays in the introduction and sale of our products in international markets, result in decreased ability to export or sell our products to existing or potential customers with international operations or in some cases, prevent the export or import of our products to certain countries, governments or persons.
Actions taken with respect to tariffs or trade relations between the United States and other countries, the products subject to such actions, and actions taken by other countries in retaliation may also have an adverse impact on us. The failure to comply with applicable current or future U.S. import, export control, sanctions
9
and anti-corruption laws, including U.S. Customs regulations, could expose us and our employees to substantial civil or criminal penalties, fines and in extreme cases, incarceration. In addition, if our distributors fail to obtain appropriate import, export or re-export licenses or authorizations, or otherwise act in accordance with applicable laws, we may be adversely affected through reputational harm and penalties.
Global or regional catastrophic events could impact our operations and affect our ability to grow our business.
Because of our increasingly global presence, our business could be affected by unstable political conditions, civil unrest, protests and demonstrations, large-scale terrorist acts, especially those directed against the United States or other major industrialized countries where our products are distributed, the outbreak or escalation of armed hostilities, major natural disasters and extreme weather conditions, such as hurricanes, wildfires, tornados, earthquakes or floods, or widespread outbreaks of infectious diseases (such as the COVID-19 pandemic). Such catastrophic events could impact our operations and our supply chain, including the production and/or distribution of our products. Materials and/or personnel may need to mobilize to other locations. Our headquarters and a large part of our operations are located in Florida, a state at greater risk of hurricanes. Some of the raw materials we use, including certain sizes of cans, are available from limited suppliers, and a regional catastrophic event impacting such suppliers could adversely impact our operations. In addition, such events could disrupt global or regional economic activity, which could affect consumer purchasing power and consumers’ ability to purchase our products, thereby reducing demand for our products. If our operations are disrupted or we are unable to grow our business as a result of these factors, our growth rate could decline and our business, financial condition and results of operations could be adversely affected.
Climate change and natural disasters may affect our business.
There is concern that a gradual increase in global average temperatures due to increased carbon dioxide and other greenhouse gases in the atmosphere could cause significant changes in weather patterns around the globe and an increase in the frequency and severity of natural disasters. Changing weather patterns could result in decreased agricultural productivity in certain regions, and/or outbreaks of diseases or other health issues, which may limit availability and/or increase the cost of certain ingredients used in our products and could impact the food security of communities around the world. Increased frequency or duration of extreme weather conditions could also impair production capabilities, disrupt our supply chain and/or impact demand for our products.
Natural disasters and extreme weather conditions, such as hurricanes, wildfires, earthquakes or floods, and outbreaks of diseases (such as the COVID-19 pandemic) or other health issues may affect our operations and the operation of our supply chain, impact the operations of our distributors and unfavorably impact our consumers’ ability to purchase our products. In addition, public expectations for reductions in greenhouse gas emissions could result in increased energy, transportation and raw material costs, and may require us to make additional investments in facilities and equipment. Changes in applicable laws, regulations, standards or practices related to greenhouse gas emissions, packaging and water scarcity, as well as initiatives by advocacy groups in favor of certain climate change-related laws, regulations, standards or practices, may result in increased compliance costs, capital expenditures and other financial obligations, which could affect our business, financial condition and results of operations. Sales of our products may also be influenced to some extent by weather conditions in the markets in which we operate. Our third-party co-packers use a number of key ingredients in the manufacture of our liquid supplement products and powder packets that are derived from agricultural commodities. Increased demand for food products and decreased agricultural productivity in certain regions of the world as a result of changing weather patterns and other factors may limit the availability or increase the cost of such agricultural commodities and could impact the food security of communities around the world. Weather conditions may influence consumer demand for certain of our supplements, which could have an effect on our operations, either positively or negatively.
We depend upon our trademarks and proprietary rights, and any failure to protect our intellectual property rights or any claims that we are infringing upon the rights of others may adversely affect our competitive position.
Our success depends, in large part, on our ability to protect our current and future brands and products and to defend our intellectual property rights. We cannot be sure that trademarks will be issued with respect to any future trademark applications or that our competitors will not challenge, invalidate or circumvent any existing or future trademarks issued to, or licensed by, us.
Our products are manufactured using our proprietary blends of ingredients. These blends are created by third-party suppliers to our specifications and then supplied to our co-packers. Although all of the third parties in our supply and manufacture chain execute confidentiality agreements, there can be no assurance that our trade secrets, including our proprietary ingredient blends will not become known to competitors.
We believe that our competitors, many of whom are more established and have greater financial and personnel resources than we do, may be able to replicate or reverse engineer our processes, brands, flavors, or our products in a manner that could circumvent our protective safeguards. Therefore, we cannot give you any assurance that our confidential business information will remain proprietary. Any such loss of confidentiality could diminish or eliminate any competitive advantage provided by our proprietary information.
We must continually maintain, protect and/or upgrade our information technology systems, including protecting us from internal and external cybersecurity threats.
Information technology enables us to operate efficiently, interface with customers, maintain financial accuracy and efficiency and accurately produce our financial statements. If we do not appropriately allocate and effectively manage the resources necessary to build and sustain the proper technology infrastructure, we could be subject to transaction errors, processing inefficiencies, the loss of customers, business disruptions, and/or the loss of and/or damage to intellectual property through security breaches, including internal and external cybersecurity threats. Cybersecurity attacks are evolving and include, but are not limited to, malicious software (malware, ransomware and viruses), phishing and social engineering, attempts to gain unauthorized access to networks, computer systems and data, malicious or negligent actions of employees (including misuse of information they are entitled to access) and other forms of electronic security breaches that could lead to disruptions in business systems, an inability to process customer orders and/or lost customer orders, unauthorized release of confidential or otherwise protected information and corruption of data.
10
We rely on relationships with third parties, including suppliers, distributors, co-packers, contractors, cloud data storage and other information technology service providers and other external business partners, for certain functions or for services in support of our operations. These third-party service providers and partners, with whom we may share data, are subject to similar risks as we are relating to cybersecurity, privacy violations, business interruption, and systems, as well as employee failures. While we have procedures in place for selecting and managing our relationships with third-party service providers and other business partners, we do not have control over their business operations or governance and compliance systems, practices and procedures, which increases our financial, legal, reputational and operational risk. These third parties may experience cybersecurity incidents that may involve data we share with them or rely on them to provide to us, and the need to coordinate with such third-parties, including with respect to timely notification and access to personnel and information concerning an incident, may complicate our efforts to resolve any issues that arise.
We believe that we have adopted appropriate measures including ongoing cybersecurity risk assessments to mitigate potential risks to our technology and our operations from these information technology-related disruptions. However, given the unpredictability of the timing, nature and scope of such disruptions, we could potentially be subject to operational interruption, damage to our brand image and private data exposure.
Moreover, if our data management systems do not effectively collect, store, process and report relevant data for the operation of our business (whether due to equipment malfunction or constraints, software deficiencies, cybersecurity attack and/or human error), our ability to effectively plan, forecast and execute our business plan and comply with applicable laws and regulations will be impaired, perhaps materially. Any such impairment could materially and adversely affect our financial condition, results of operations, cash flows and the timeliness with which we report our internal and external operating results.
If we fail to comply with data privacy and personal data protection laws, we could be subject to adverse publicity, government enforcement actions and/or private litigation, which may negatively impact our business and operating results.
We receive, process, transmit and store information relating to certain identified or identifiable individuals (“personal data”), including current and former employees, in the ordinary course of business. As a result, we are subject to various U.S. federal and state and foreign laws and regulations relating to personal data. These laws are subject to change, and new personal data legislation may be enacted in other jurisdictions at any time. In the European Union, the General Data Protection Regulation (the “GDPR”) became effective in May 2018 for all member states. The GDPR includes operational requirements for companies receiving or processing personal data of residents of the European Union different from those that were previously in place and also includes significant penalties for noncompliance. Additionally, the California Consumer Privacy Act of 2018 (the “CCPA”), which was enacted in June 2018 and came into effect on January 1, 2020, provides a new private right of action and statutory damages for certain data breaches and imposes operational requirements on companies that process personal data of California residents, including making new disclosures to consumers about data collection, processing and sharing practices and allowing consumers to opt out of certain data sharing with third parties.
Changes introduced by the GDPR and the CCPA, as well as other changes to existing personal data protection laws and the introduction of such laws in other jurisdictions, subject the Company to, among other things, additional costs and expenses and may require costly changes to our business practices and security systems, policies, procedures and practices. There can be no assurances that our security controls over personal data, training of personnel on data privacy and data security, vendor management processes, and the policies, procedures and practices we implement will prevent the improper processing or breaches of personal data. Data breaches or improper processing, or breaches of personal data in violation of the GDPR, the CCPA and/or of other personal data protection or privacy laws and regulations, could harm our reputation, cause loss of consumer confidence, subject us to government enforcement actions (including fines), or result in private litigation against us, which may result in potential loss of revenue, increased costs, liability for monetary damages or fines and/or criminal prosecution, thereby negatively impacting our business and operating results.
If we fail to manage future growth effectively, our business could be materially adversely affected.
We have experienced rapid growth, and anticipate such growth may continue. During the year ended December 31, 2021, we grew from 154 to 225 employees, and expect to continue expanding our hiring and marketing efforts with no assurance that our business or revenue will continue to grow. This growth may place significant demands on management and our operational infrastructure. As we continue to grow, we must manage such growth effectively by successfully integrating, developing and motivating a large number of new employees, while maintaining the beneficial aspects of our company culture. If we do not manage the growth of our business and operations effectively, the quality of our products and efficiency of our operations could suffer and we may not be able to execute on our business plan, which could harm our brand, results of operations and overall business. Accordingly, we cannot guarantee that we will achieve our planned growth, or even if we are able to grow as planned, that we will continue to sustain such growth or performance.
We may incur material losses as a result of product recall and product liability.
We may be liable if the consumption of any of our products causes injury, illness or death. We also may be required to recall some of our products if they become contaminated or are damaged or mislabeled. A significant product liability judgment against us, or a widespread product recall, could have a material adverse effect on our business, financial condition and results of operations. The amount of the insurance we carry is limited, and that insurance is subject to certain exclusions and may or may not be adequate.
We rely on our management team and other key personnel.
We depend on the skills, experience, relationships, and continued services of key personnel, including our experienced management team. In addition, our ability to achieve our operating goals also depends on our ability to recruit, train, and retain qualified individuals. We compete with other companies both within and outside of our industry for talented personnel, and we may lose key personnel or fail to attract and retain additional talented personnel. Any such loss or failure could adversely affect our product sales, financial condition, and operating results.
In particular, our continued success will depend in part, on our ability to retain the talents and dedication of key employees. If key employees finalize their employment, become ill as a result of the COVID-19 pandemic, or if an insufficient number of employees is retained to maintain effective operations, our business may be adversely affected, and our management team may be distracted. Furthermore, we may not be able to locate suitable replacements for any of
11
our key employees who leave or be able to offer employment to potential replacements on reasonable terms, all of which could adversely affect our procurement and distribution processes, sales and marketing activities, financial processes & condition and results of operations.
The U.S. Food and Drug Administration (the “FDA”) has not passed on the efficacy of our products or the accuracy of any claim we make related to our products.
Although six independent clinical studies have been conducted relating to the calorie-burning and related effects of our products, the results of these studies have not been submitted to or reviewed by the FDA.
Further, the FDA has not passed on the efficacy of any of our products nor has it reviewed or passed on any claims we make related to our products, including the claim that our products aid consumers in burning calories or enhancing their metabolism.
The Federal Trade Commission (the “FTC”) regulates advertising and may review the truthfulness of and substantiation for any claim we make related to our products.
Our advertising activities are subject to regulation by the FTC under the Federal Trade Commission Act. In recent years, the FTC and state attorneys general have initiated numerous investigations of dietary and nutritional supplement companies and products. Any actions or investigations initiated against the Company by governmental authorities or private litigants could have a material adverse effect on our business, financial condition and results of operations.
The shifting regulatory environment through the various jurisdictions in which are products are sold necessitates building and maintaining robust systems to achieve and maintain compliance in multiple jurisdictions and increases the possibility that we may violate one or more of the legal requirements. If our operations are found to be in violation of any applicable laws or regulations, we may be subject to, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, injunctions, or product withdrawals, recalls or seizures, any of which could adversely affect our ability to operate our business, our financial condition and results of operations.
Risk Factors Relating to Our Industry
We are subject to significant competition in the functional energy drink and supplement industries.
The functional energy drink and supplement industries are highly competitive. The principal areas of competition are pricing, packaging, distribution channel penetration, development of new products and flavors and marketing campaigns. Our products compete with a wide range of drinks produced by a relatively large number of manufacturers, most of which have substantially greater financial, marketing and distribution resources and name recognition than we do.
Important factors affecting our ability to compete successfully include the taste and flavor of our products, trade and consumer promotions, rapid and effective development of new, unique cutting-edge products, attractive and different packaging, branded product advertising and pricing. Our products compete with all liquid refreshments and with products of much larger and substantially better financed competitors, including the products of numerous nationally and internationally known producers, such as The Coca Cola Company, Dr. Pepper Snapple Group, PepsiCo, Inc., Nestlé, Waters North America, Inc., Hansen Natural Corp., Vital Pharmaceuticals, Inc., Monster Energy and Red Bull. We also compete with companies that are smaller or primarily local in operation. Our products also compete with private label brands such as those carried by supermarket chains, convenience store chains, drug store chains, mass merchants and club warehouses. New competitors continue to emerge, some of which target specific markets of ours as well as the health and wellness space. This may require additional marketing expenditures on our part to remain competitive.
The rapid growth in sales through e-commerce retailers, e-commerce websites, mobile commerce applications and subscription services, and closures of physical retail operations, particularly during, and potentially following, the COVID-19 pandemic, may result in a shift away from physical retail operations to digital channels and a reduction in impulse purchases. Further, the ability of consumers to compare prices on a real-time basis using digital technology puts additional pressure on us to maintain competitive prices. Sales in gas chains may also be affected by improvements in fuel efficiency and increased consumer preferences for electric or alternative fuel-powered vehicles, which may result in fewer trips by consumers to gas stations and a corresponding reduction in purchases by consumers in convenience gas retailers. We have been growing our e-commerce sales by using Amazon and leveraging our retail partners e-commerce platforms, rather than building our own internal platform. However, if we are unable to successfully adapt to the rapidly changing retail landscape, our share of sales, volume growth and overall financial results could be negatively affected.
Due to competition in the functional energy drink and supplement industries, there can be no assurance that we will not encounter difficulties in maintaining our current revenues, market share or position in the functional energy drink and supplement industries. If our revenues decline, our business, financial condition and results of operations could be adversely affected.
Our inability to innovate successfully and to provide new cutting-edge products could adversely affect our business and financial results.
Our ability to compete in the highly competitive functional energy drink and supplement industries and to achieve our business growth objectives depends, in part, on our ability to develop new flavors, products and packaging. The success of our innovation, in turn, depends on our ability to identify consumer trends and cater to consumer preferences. If we are not successful in our innovation activities, our business, financial condition and results of operation could be adversely affected.
Changes in consumer product and shopping preferences may reduce demand for some of our products.
The functional energy drink and supplement categories are subject to changing consumer preferences and shifts in consumer preferences may adversely affect us. There is increasing awareness of and concern for health, wellness and nutrition considerations, including concerns regarding caloric intake associated with sugar-sweetened drinks and the perceived undesirability of artificial ingredients. Our products do not contain the artificial preservatives often found in many
12
energy drinks and sodas. CELSIUS® has no artificial preservatives, aspartame or high fructose corn syrup and is very low in sodium. The main CELSIUS® line of products are sweetened with sucralose, a sugar-derived sweetener that is found in Splenda®, which makes our liquid supplements low-calorie. However, consumer preferences may shift away from the trend towards healthier options that we have observed, and as such, there can be no assurance that our current products and product lines will maintain their current levels of demand. There are also changes in demand for different packages, sizes and configurations. This may reduce demand for our functional energy drinks and liquid supplements, which could reduce our revenues and adversely affect our results of operations.
Consumers are seeking greater variety in their functional energy drinks and supplements. Our future success will depend, in part, upon our continued ability to develop and introduce different and innovative drinks and supplements that appeal to consumers. In order to retain and expand our market share, we must continue to develop and introduce different and innovative supplements and be competitive in the areas of efficacy, taste, quality and price, although there can be no assurance of our ability to do so. There is no assurance that consumers will continue to purchase our products in the future. Product lifecycles for some functional energy drink and supplement brands, products and/or packages may be limited to a few years before consumers’ preferences change. The functional energy drink and supplements we currently market are in varying stages of their product lifecycles, and there can be no assurance that such products will become or remain profitable for us. We may be unable to achieve volume growth through product and packaging initiatives. We may also be unable to penetrate new markets. Additionally, as shopping patterns are being affected by the digital evolution, with consumers embracing shopping by way of mobile device applications, e-commerce retailers and e-commerce websites or platforms, we may be unable to address or anticipate changes in consumer shopping preferences or engage with our consumers on their preferred platforms. If our revenues decline, our business, financial condition and results of operations could be adversely affected.
We derive virtually all of our revenues from functional energy drinks and supplements, and competitive pressure in the functional energy drink and supplement category could adversely affect our business and operating results.
Our focus is in the functional energy drink and supplement categories, and our business is vulnerable to adverse changes impacting the fitness supplement category and business, which could adversely impact our business and the trading price of our common stock.
Virtually all of our sales are derived from our functional energy drink and supplements, including our CELSIUS® Originals, CELSIUS HEAT, CELSIUS BCCA+ENERGY, CELSIUS® On-the-Go and CELSIUS® product lines. Any decrease in the sales of our functional energy drinks and supplements could significantly adversely affect our future revenues and net income. Historically, we have experienced substantial competition from new entrants in the functional energy drink and supplement categories.
The increasing number of competitive products and limited amount of shelf space, including in coolers, in retail stores may adversely impact our ability to gain or maintain our share of sales in the marketplace. In addition, certain actions of our competitors, including unsubstantiated and/or misleading claims, false advertising claims and tortious interference in our business, as well as competitors selling misbranded products, could impact our sales. Competitive pressures in the functional energy drink and supplement categories could impact our revenues, cause price erosion and/or lower market share, any of which could have a material adverse effect on our business and results of operations.
We compete in an industry that is brand-conscious, so brand name recognition and acceptance of our products are critical to our success and significant marketing and advertising could be needed to achieve and sustain brand recognition.
Our business is substantially dependent upon awareness and market acceptance of our products and brands by our targeted consumers. Our business depends on acceptance by our independent distributors of our brand as one that has the potential to provide incremental sales growth rather than reduce distributors’ existing beverage sales. The development of brand awareness and market acceptance is likely to require significant marketing and advertising expenditures. There can be no assurance that CELSIUS® will achieve and maintain satisfactory levels of acceptance by independent distributors and retail consumers. Any failure of the CELSIUS® brand to maintain or increase acceptance or market penetration would likely have a material adverse effect on business, financial condition and results of operations.
If we are unable to successfully manage new product launches, our business and financial results could be adversely affected.
Due to the highly competitive nature of the global functional energy drink and supplement sectors, we expect and intend to continue to introduce new products and evolve existing products to better match consumer demand. The success of new and evolved products depends on a number of factors, including timely and successful development and consumer acceptance. Such endeavors may also involve significant risks and uncertainties, including distraction of management from current operations, greater than expected liabilities and expenses, inadequate return on capital, exposure to additional regulations and reliance on the performance of third parties.
Our sales are affected by seasonality.
As is typical in the functional energy drink and supplement industries, our sales are seasonal. Our highest sales volumes generally occur in the second and third quarters, which correspond to the warmer months of the year in our major markets. Consumer demand for our products is also affected by weather conditions. Cool, wet spring or summer weather could result in decreased sales of our products and could have an adverse effect on our results of operations.
Our business is subject to many regulations and noncompliance is costly.
The production, marketing and sale of our functional energy drinks and supplements are subject to the rules and regulations of various federal, state and local health agencies. The marketing and sale of our products internationally is similarly subject to compliance with applicable laws, rules and regulations in those foreign countries where our products are sold. If a regulatory authority finds that a current or future product or production run is not in compliance with any of these regulations, we may be fined, or production may be stopped, thus adversely affecting our business, financial condition and results of operations. Similarly, any adverse publicity associated with any noncompliance may damage our reputation and our ability to successfully market our products. Furthermore, the rules and regulations are subject to change from time to time and while we closely monitor developments in this area, we have no way of anticipating whether changes
13
in these rules and regulations will impact our business adversely. Additional or revised regulatory requirements, whether labeling, environmental, tax or otherwise, could have an adverse effect on our business, financial condition and results of operations.
Product safety and quality concerns, or other negative publicity (whether or not warranted) could damage our brand image and corporate reputation and may cause our business to suffer.
Our success depends in large part on our ability to maintain consumer confidence in the safety and quality of all of our products. We have rigorous product safety and quality standards, which we expect our operations as well as our suppliers to meet. However, despite our strong commitment to product safety and quality, we or our suppliers may not always meet these standards, particularly as we expand our product offerings through innovation or acquisitions into product categories that are beyond our traditional range of functional energy drinks and supplements. If we or our suppliers fail to comply with applicable product safety and quality standards, or if our supplement products taken to the market are or become contaminated or adulterated by any means, we may be required to conduct costly product recalls and may become subject to product liability claims and negative publicity, which could cause our business to suffer.
Our success also depends on our ability to build and maintain the brand image for our existing products, new products and brand extensions and maintain our corporate reputation. There can be no assurance that our advertising, marketing and promotional programs and our commitment to product safety and quality, human rights and environmental sustainability will have the desired impact on our products’ brand image and on consumer preferences and demand. Claims regarding product safety, quality and/or ingredient content issues, efficacy or lack thereof (real or imagined), our culture and our workforce, our environmental impact and the sustainability of our operations, or allegations of product contamination, even if false or unfounded, could tarnish the image of our brands and may cause consumers to choose other products. Consumer demand for our products could diminish significantly if we, our employees, distributors, suppliers or business partners fail to preserve the quality of our products, act or are perceived to act in an unethical, illegal, discriminatory, unequal or socially irresponsible manner, including with respect to the sourcing, content or sale of our products, service and treatment of our customers, or the use of customer data. For example, Celsius’ former managing director of Asia, since leaving the Company, has been charged in connection with 1Malaysia Development Bhd.-related matters. Furthermore, our brand image or perceived product quality could be adversely affected by litigation, unfavorable reports in the media (internet or elsewhere), studies in general and regulatory or other governmental inquiries (in each case whether involving our products or those of our competitors) and proposed or new legislation affecting our industry. For example, we are currently in arbitration proceedings with McGovern Capital, Inc. and Kevin McGovern about a fee dispute emerging from a representative agreement among the parties and Celsius Holdings in connection with sales of our products in the People’s Republic of China for four years starting from September 1, 2017. Negative postings or comments on social media or networking websites about the Company or any one of our brands, even if inaccurate or malicious, could generate adverse publicity that could damage the reputation of our brands or the Company. Business incidents, whether isolated or recurring and whether originating from us, our distributors, suppliers or business partners, that erode consumer trust can significantly reduce brand value or potentially trigger boycotts of our products and can have a negative impact on consumer demand for our products as well as our reputation and financial results. The impact of such incidents may be exacerbated if they receive considerable publicity, including rapidly through social or digital media (including for malicious reasons) or result in litigation.
In addition, from time to time, there are public policy endeavors that are either directly related to our products and packaging or to our business. These public policy debates can occasionally be the subject of backlash from advocacy groups that have a differing point of view and could result in adverse media and consumer reaction, including product boycotts. Similarly, our sponsorship relationships could subject us to negative publicity as a result of actual or alleged misconduct by individuals or entities associated with organizations we sponsor or support. Likewise, campaigns by activists connecting us, or our supply chain, with human and workplace rights, environmental or animal rights issues could adversely impact our corporate image and reputation. Allegations, even if untrue, that we are not respecting the human rights found in the United Nations Universal Declaration of Human Rights; actual or perceived failure by our suppliers or other business partners to comply with applicable labor and workplace rights laws, including child labor laws, or their actual or perceived abuse or misuse of migrant workers; adverse publicity surrounding obesity and health concerns related to our products, our environmental impact and the sustainability of our operations, labor relations, our culture and our workforce or the like could negatively affect our Company’s overall reputation and brand image, which in turn could have a negative impact on our products’ acceptance by consumers.
Litigation regarding our products could expose us to significant liabilities and reduce demand for our products.
We have been and are a party, from time to time, to various litigation claims and legal proceedings, including, but not limited to, intellectual property, false advertising, product liability, breach of contract claims and others. Other lawsuits have been filed against us claiming that certain statements made in our advertisements and/or on the labels of our products were false and/or misleading or otherwise not in compliance with food standards under local law. Putative class action lawsuits have also been filed against us, alleging that certain claims in our marketing promotional amount to false advertising. We do not believe any statements made by us in our promotional materials or set forth on our product labels are false or misleading or noncompliant with local law, and we have been defending, and will continue to vigorously defend such lawsuits.
Any of the foregoing matters or other litigation, the threat thereof, or unfavorable media attention arising from pending or threatened litigation could consume significant financial and managerial resources and result in diminished operational efficiency of the Company, significant monetary awards against us, an injunction barring the sale of any of our products and injury to our reputation. Our failure to successfully defend or settle any litigation or legal proceedings could result in liabilities that, to the extent not covered by our insurance, could have a material adverse effect on our financial condition, revenue and profitability, and could cause the market value of our common stock to decline.
Risk Factors Relating to our Status as a Reporting Public Company
We are subject to the periodic reporting requirements of the Exchange Act that require us to incur audit fees and legal fees in connection with the preparation of such reports. These additional costs could reduce or eliminate our ability to earn a profit.
We are subject to the periodic reporting requirements of the Exchange Act and as a result, we are now required to file periodic reports with the Securities and Exchange Commission (the “SEC”) pursuant to the Exchange Act and the rules and regulations promulgated thereunder. In order to comply with these requirements, our independent registered public accounting firm has to review our financial statements on a quarterly basis and audit our financial statements on
14
an annual basis. Moreover, our legal counsel has to review and assist in the preparation of such reports. The costs charged by these professionals for such services cannot be accurately predicted at this time because factors such as the number and type of transactions that we engage in and the complexity of our reports cannot be determined at this time and will have a major effect on the amount of time to be spent by our auditors and attorneys. However, the incurrence of such costs will obviously be an expense to our operations and thus have a negative effect on our ability to meet our overhead requirements and earn a profit. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
Our periodic filings as required under the Exchange Act may be subject to review and comment by the SEC, which may result in changes to our public disclosure.
Our periodic filings with the SEC in compliance with the reporting requirements of the Exchange Act, may be subject to review and comment by the SEC. Should the SEC conduct such review and comment, we may be required to revise such periodic filings, including but not limited to the financial statements contained therein.
If we do not maintain an effective internal control environment as well as adequate control procedures over our financial reporting, investor confidence may be adversely affected thereby affecting the value of our stock price
We are required to maintain proper internal control over our financial reporting and adequate controls related to our disclosures. As defined in Rule 13a-15(f) under the Exchange Act, internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officers and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. If we fail to maintain adequate controls, our business, the results of operations, financial condition and/or the value of our stock may be adversely impacted.
The Company has identified material weaknesses in its internal control over financial reporting that, if not remediated, could result in additional material misstatements in its consolidated financial statements.
As described in Part II, Item 9A — Controls and Procedures, management has identified material weaknesses in the Company's internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company's annual or interim financial statements will not be prevented or detected on a timely basis.
The Company is in the process of developing and implementing a remediation plan to address the material weaknesses. If the Company’s remediation efforts are insufficient or if additional material weaknesses in internal control over financial reporting are discovered or occur in the future, the Company's consolidated financial statements may contain material misstatements and it could be required to revise or restate its financial results, which could materially and adversely affect the Company’s business, results of operations and financial condition, restrict its ability to access the capital markets, require it to expend significant resources to correct the material weakness, subject it to fines, penalties or judgments, harm its reputation or otherwise cause a decline in investor confidence.
We face investigation from the SEC, the timeline for which and the results of which are currently unknown.
On January 8, 2021, we received a letter from the SEC Division of Enforcement seeking the production of documents in connection with a non-public fact-finding inquiry by the SEC to determine whether violations of the federal securities laws have occurred. On August 20, 2021, the SEC issued a subpoena for production of documents in connection with the matter. Neither the January 8, 2021 SEC letter nor the August 20, 2021 subpoena means that the SEC has concluded that the Company or anyone else has violated the federal securities laws. We have cooperated and will continue to cooperate with the SEC staff in its investigation. At this time, however, we cannot predict the length, scope, or results of the investigation or the impact, if any, of the investigation on our results of operations.
We may also be subject to further or other examinations, investigations, proceedings and orders by the SEC or other regulators. Any such further or other actions could be expensive and damaging to our business, results of operations and financial condition.
Risk Factors Related to our Common Stock
We cannot guarantee the continued existence of an active established public trading market for our common stock.
Our common stock currently is listed for trading on the Nasdaq Capital Market. Trading in stock quoted on the Nasdaq Capital Market may often experience wide fluctuations in trading prices, due to many factors that may have little to do with our operations or business prospects. This volatility could depress the market price of our common stock for reasons unrelated to operating performance.
Market prices for our common stock may also be influenced by a number of other factors, including:
15
Our board of directors has the authority, without stockholder approval, to issue preferred stock with terms that may not be beneficial to common stockholders and with the ability to affect adversely stockholder voting power and perpetuate their control over us.
Our Articles of Incorporation allows our board of directors to issue shares of preferred stock without any vote or further action by our stockholders. Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock.
Our principal stockholders can exert significant influence on the Company’s corporate affairs.
Our principal stockholder owns approximately 25% of our issued and outstanding common stock. Accordingly, they will be able to effectively control the election of directors, as well as all other matters requiring stockholder approval. The interests of our principal stockholders may differ from the interests of other stockholders with respect to the issuance of shares, business transactions with or sales to other companies, selection of other directors and other business decisions.
We do not expect to pay cash dividends in the foreseeable future.
We have never paid cash dividends on our common stock. We do not expect to pay cash dividends on our common stock at any time in the foreseeable future. The future payment of dividends directly depends upon our future earnings, capital requirements, financial requirements and other factors that our board of directors will consider. Since we do not anticipate paying cash dividends on our common stock, return on your investment, if any, will depend solely on an increase, if any, in the market value of our common stock.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We do not own any real property. We lease our principal executive offices located at 2424 N Federal Highway, Boca Raton, Florida 33431. Our premises are leased for a monthly cost of $24,494. The current lease expires on January 2024. The Company has no warehouses or other facilities as we store our product at third party contract warehouse facilities.
We also have leased premises in Europe which have an aggregate monthly cost of approximately $11,600. These leases have different periods and extend until August 2023.
Item 3. Legal Proceedings.
In addition to other matters previously reported in our periodic filings under the Exchange Act, from time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business.
Item 4. Mine Safety Disclosures
Not applicable.
16
PART II
Item 5. Market Price of and Dividends on the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock has been listed on the Nasdaq Capital Market under the symbol “CELH.”
Holders
As of March 15, 2022, there were 33 holders of record of our common stock and in excess of 5,000 beneficial owners of our common stock.
Dividends
We have never paid cash dividends on our common stock. We do not expect to pay cash dividends on our common stock at any time in the foreseeable future. The future payment of dividends directly depends upon our future earnings, capital requirements, financial requirements and other factors that our board of directors will consider.
Securities Authorized for Issuance under Equity Compensation Plans
Plan category |
|
Number of |
|
|
|
Weighted-average |
|
|
Number of |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Equity compensation plans approved by security holders |
|
|
3,599,499 |
|
(1) |
|
$ |
7.47 |
|
|
|
4,263,879 |
|
(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Equity compensation plans not approved by security holders |
|
|
— |
|
|
|
n/a |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Total |
|
|
3,599,499 |
|
(1) |
|
$ |
7.47 |
|
|
|
4,263,879 |
|
(1) |
Recent Sales of Unregistered Securities
None.
Performance Graph
The following graph shows a five-year comparison of cumulative total returns
17
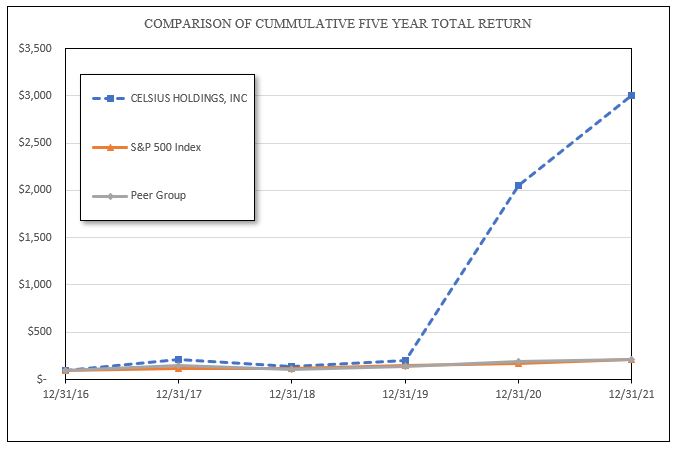
18
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion in conjunction with the audited financial statements and the corresponding notes, the unaudited financial statements and the corresponding notes included elsewhere in this information statement. This Item 7 contains forward-looking statements. The matters discussed in these forward-looking statements are subject to risk, uncertainties, and other factors that could cause actual results to differ materially from those made, projected or implied in the forward-looking statements. Please refer to “Item 1A. Risk Factors” for a discussion of the uncertainties, risks and assumptions associated with these statements.
Results of Operations
Year ended December 31, 2021 compared to year ended December 31, 2020
Revenue
For the year ended December 31, 2021, revenue was approximately $314.3 million, an increase of $183.5 million or 140% from $130.7 million for the year ended December 31, 2020. Approximately 97% of this growth was as a result of increased revenues from North America where 2021 revenues were $273.0 million, an increase of $177.5 million or 186% from 2020. The balance of the increase was largely attributable to a 13% growth in European revenues to $38.1 million in 2021 from $33.7 million in 2020. Asian revenues (which primarily consist of royalty revenues from our China licensee) for the year ended December 31, 2021 were $2.5 million, an increase of $1.4 million from $1.1 million for the year ended December 31, 2020. Other international markets generated $0.6 million in revenues during the year ended December 31, 2021, an increase of approximately 48.5% from $0.4 million for the year ended December 31, 2020.
The total increase in revenue from 2020 to 2021 was largely attributable to increases in sales volume, as opposed to increases in product pricing. The primary factors behind the increase in North American sales volume were related to continued strong triple-digit growth in traditional distribution channels, combined with an increase in and optimization of our products’ presence in world class retailers. Additionally, the continued expansion of DSD network resulted in significant growth of our distributor revenues when compared to the prior year period. Furthermore, our fitness and vending channels also reflected triple digit growth of incremental revenue when compared to the prior year period, during which many fitness facilities were closed due to the COVID-19 pandemic.
Additionally, e-commerce results also contributed to the increase in revenues for the year ended December 31, 2021. Furthermore, the favorable currency fluctuations accounted for approximately 29% of the increase in European revenue in the 2021 period, when compared to the 2020 period.
The following table sets forth the amount of revenues by category and changes therein for the years ended December 31, 2021 and December 31, 2020:
|
|
Year Ending December 31, |
|
|||||
Revenue Source |
|
2021 |
|
|
2020 |
|
||
Total Revenue |
|
$ |
314,271,559 |
|
|
$ |
130,725,777 |
|
|
|
|
|
|
|
|
||
North American Revenue |
|
$ |
273,004,795 |
|
|
$ |
95,480,310 |
|
|
|
|
|
|
|
|
||
European Revenue |
|
$ |
38,097,016 |
|
|
$ |
33,727,441 |
|
|
|
|
|
|
|
|
||
Asian Revenue |
|
$ |
2,538,099 |
|
|
$ |
1,092,703 |
|
|
|
|
|
|
|
|
||
Other Revenue |
|
$ |
631,649 |
|
|
$ |
425,323 |
|
Gross profit
For the year ended December 31, 2021, gross profit increased by approximately $67.2 million or 110% to $128.2 million, from $61.0 million for the year ended December 31, 2020. Gross profit margins decreased to 40.8% for the year ended December 31, 2021 from 46.6% for the year ended December 31, 2020. The increase in gross profit dollars is related to increases in sales volumes, while the decrease in gross profit margins from the 2020 period to the 2021 period is mainly related to increases in raw material costs (primarily aluminum cans), ocean freight costs, distribution costs, repackaging costs and processing costs. These incremental costs are directly related to added complexities in the supply chain as a result of the COVID-19 pandemic. The increase in gross profit dollars of approximately $67.2 million from the year ended December 31, 2020 to the year ended December 31, 2021, includes approximately $85.0 million related to volume increases, as well as a favorable currency impact of $1.3 million, which was offset in part by unfavorable increases in costs of approximately $18.3 million.
Sales and marketing expenses
Sales and marketing expenses for the year ended December 31, 2021 were approximately $74.7 million, an increase of approximately $39.9 million or 114% from approximately $34.9 million for the year ended December 31, 2020. This increase was largely a result of incremental marketing investment activities of approximately $22.5 million from the 2020 period. Additionally, employee costs increased by approximately $5.6 million from the year ago period as we continued to invest in this area in order to have the proper infrastructure to support our growth and incurred additional travel and business expenses now that we are able to resume in-person marketing events and selling activities. Similarly, we experienced increases in other sales expense in the amount of approximately $1.3 million mainly related to trade-marketing activities to support our continued expansion of our DSD network. Lastly, storage and distribution as well as broker costs accounted for the remainder of the increase in this area in the amount of $10.4 million when compared to the prior year period, mainly related to the increase in business volume.
19
General and administrative expenses
General and administrative expenses for the year ended December 31, 2021 were approximately $57.5 million, an increase of $39.3 million or 216% from $18.2 million for the year ended December 31, 2020. This increase was primarily attributable to stock option expense which amounted to $36.5 million for the year ended December 31, 2021, an increase from $6.3 million in the prior year period. This increase in stock option expense when compared to the prior year, was mainly attributable the revaluation of certain share-based payment awards that were modified during 2021. Management deems it very important to motivate employees by providing them ownership in the business in order to promote over performance which translates into the continued success of our business based on key performance attributes. Additionally, employee costs for the year ended December 31, 2021 reflect an increase of $3.1 million or 68.9%, as investments in this area were also required to properly support our higher business volume and the commercial and operational areas of the business, as well as travel expenses are now being incurred. Administrative expenses amounted to $11.0 million or an increase of $5.4 million or 96.4%, when compared to the prior year period. This variance is mainly related to an increase in bad debt reserve of $1.5 million and increases in audit costs, legal expenses, insurance costs and office rent account for the majority of the remaining fluctuation of $3.9 million. Depreciation and amortization had an increase of approximately $0.2 million when compared to the prior year due to investments in operational equipment (e.g., coolers). All other administrative expenses which were mainly composed of research, development and quality control testing, increased by approximately $0.5 million from the 2020 period.
Other Income/(expense)
Total net other income for the year ended December 31, 2021 was $31,069 which reflects a decrease of $697,957 when compared to net total other income of $0.7 million for the year ended December 31, 2020. The variance of $0.7 million is mainly related to a decrease in foreign currency exchange fluctuation of $1.6 million, and a decrease in interest income of $39,000 related to the note receivable from our China licensee, which are offset by the reductions of bond amortization and net interest expense, as well as other all other non-operational charges which provided a total net increase of $0.9 million.
Income tax benefit/(expense)
An income tax benefit of approximately $8.0 million was obtained during the 2021 year, mainly related to the release of our US valuation allowance and windfall benefits on stock-based compensation awards. In 2020 our Sweden subsidiary incurred approximately $116,000 of income tax expense due to the inability to fully utilize the NOLs generated from prior periods.
Net Income/Loss
Net income for the year ended December 31, 2021, was $3.9 million or $0.05 per share based on a weighted average of 73,781,130 shares outstanding and dilutive earnings per share of $0.05 based on a fully-dilutive weighted average of 77,688,501 shares outstanding, which includes the dilutive impact of outstanding stock options to purchase 3,907,371 shares. In comparison, for the year ended December 31, 2020 the Company had net income of $8.5 million or $0.12 per share based on a weighted average of 70,195,085 shares outstanding, and a dilutive earnings per share of $0.11 based on a weighted average of 74,443,601 shares outstanding.
Liquidity and Capital Resources
General
As of December 31, 2021, and December 31, 2020, we had cash of approximately $16.3 million and $43.2 million, respectively, and working capital of approximately $169.2 million and $66.8 million, respectively.
In addition to cash flow from operations, our primary sources of working capital have been private placements and public offerings of our securities, including an underwritten public offering of 1,133,953 shares at an offering price of $62.50 per share completed on June 14, 2021 and a private placement of 1,437,909 shares at a price of $15.30 completed on August 25, 2020.
Our current operating plan for the next twelve (12) months reflects sufficient financial resources, notwithstanding the potential effects of the COVID-19 pandemic.
Cash flows (used in) provided by operating activities
Cash flows used in operating activities totaled $96.6 million in 2021, which compares to $3.4 million net cash provided by operating activities for the year ended December 31, 2020. The use of cash was primarily driven by higher inventory levels in order to properly service the demand for our products and to mitigate the current inefficiencies of the supply chain. Additionally, the significant increase in business volume or revenue resulted in an increase in our accounts receivable based on the credit terms offered to our clients. Pre-payments or deposits to procure inventory reflected a reduction and therefore were a source of cash. We also leveraged the use of our accounts payable to partially offset the increase in working capital by efficiently utilizing the terms offered by our vendors as it relates to the commitments and disbursements for the goods and services that are needed for our operations.
Cash flows provided by (used in) investing activities
Cash flows used in investing activities totaled $1.3 million in 2021, which compares to cash provided by investing activities of $0.8 million for the year ended December 31, 2020. The change in the cash used in investing activities when compared to the 2020 period was primarily due to the investment of approximately $3.1 million mainly pertaining to operational equipment which was partially offset by note receivable payments from our China licensee of approximately $1.9 million.
Cash flows (used in) provided by financing activities
20
Cash flows provided by financing activities totaled $71.4 million for 2021, representing a $55.8 million increase from $15.6 million in 2020. The increase of cash provided by financing activities is mainly related to the net proceeds of $67.8 million from our June 2021 public offering and proceeds from stock option exercises of $3.7 million, which was offset in part by payments of approximately $94,000 pertaining to financial leases.
Off Balance Sheet Arrangements
As of December 31, 2021 and 2020, we had no off-balance sheet arrangements.
Critical Accounting Estimates
Our consolidated financial statements are prepared in accordance with U.S. GAAP. In connection with the preparation of our consolidated financial statements, we are required to make judgments and estimates that significantly affect the reported amounts of assets, liabilities, revenues and expenses and related disclosures. Our estimates are based on historical experience, current trends and various other assumptions we believe to be relevant under the circumstances. We review the underlying factors used in our estimates regularly, including reviewing the significant accounting policies impacting the estimates, to ensure compliance with U.S. GAAP. However, due to the uncertainty inherent in our estimates, actual results may be materially different. We have identified the accounting estimates below as critical to understanding and evaluating the financial results reported in our consolidated financial statements.
For a complete description of our significant accounting policies, see Item 8 Financial Statements and Supplementary Data, Note 2, "Basis of Presentation and Summary of Significant Accounting Policies".
Valuation Allowances for Deferred Tax Assets
Deferred tax assets arise when we recognize expenses in our financial statements that will be allowed as income tax deductions in future periods. Deferred tax assets also include unused tax net operating losses that we are allowed to carry forward to future years. Accounting rules permit us to carry deferred tax assets on the balance sheet at full value as long as it is “more likely than not” that the deductions, losses or credits will be used in the future. A valuation allowance must be recorded against a deferred tax asset if this test cannot be met. Our determination of valuation allowances is based upon a number of assumptions, judgments and estimates, including forecasted earnings, future taxable income and the relative proportions of revenue and income before taxes in the various jurisdictions in which we operate. Concluding that a valuation allowance is not required is difficult when there is significant negative evidence that is objective and verifiable, such as cumulative losses in recent years.
We have deferred tax assets related to foreign net operating losses, primarily in China, Hong Kong, Sweden, Finland and Norway, against which we have recorded valuation allowances as it is currently not more likely than not that the foreign net operating losses will be realized in the future.
We have deferred tax assets related to US Federal and state net operating losses on which we retained a full valuation allowance through September 30, 2021. During the fourth quarter of 2021, we concluded that it is more likely than not that the US deferred tax assets would be realized. This conclusion was based on US profitability and NOL utilization in 2021 and 2020 as well as future forecasts of US profitability. This positive evidence supported the release of the Federal and State valuation allowance. For the year ended December 31, 2021, the Company reported a release of its US valuation allowance for deferred tax assets of approximately $6.0 million
Goodwill and Intangible Assets Valuation
We evaluate the carrying value of our goodwill and indefinite-lived intangible assets for impairment at least annually or when an interim triggering event occurs that would indicate impairment may have taken place. We evaluate our other definite-lived intangible assets for impairment when evidence exists that certain events or changes in circumstances indicate that the carrying amount of these assets may not be recoverable. Judgments and assumptions are required in such impairment evaluations. Our annual impairment test of indefinite-lived intangible assets was performed as of October 1, the first day of our last fiscal quarter. Our annual impairment test of indefinite-lived intangible assets was performed as of October 1, the first day of the last fiscal quarter.
Our annual impairment evaluation of goodwill involves both qualitative and quantitative aspects, including comparing our single reporting unit's fair value to its respective carrying value. Some of the qualitative aspects evaluated included macro-economic conditions, industry and market considerations, cost factors regarding our raw materials and operations and overall financial performance of our business. If the fair value exceeds carrying value, then we conclude that no goodwill impairment has occurred. If the reporting unit's carrying value exceeds its fair value, we would recognize an impairment loss in an amount equal to the excess up to the total amount of goodwill.
We use the income approach to determine the fair value of our definite-lived customer relationships intangible asset. Our projections used in the income approach to determine the fair value of our definite-lived customer relationships intangible asset include assumptions for growth rates of sales, costs, and profits, which are based on various long-range financial and operational plans. Additionally, discount rates used in the definite-lived intangible analysis are based on a weighted-average cost of capital, driven by the prevailing interest rates in the geographies where the company operates, as well as credit ratings, financing abilities and opportunities, among other factors. Discount rates may differ slightly to adjust for country or market specific risk, among other factors.
Changes in economic and operating conditions impacting these assumptions could result in goodwill and intangible assets being impaired in future periods. Additionally, disruptions to our business such as prolonged recessionary periods or unexpected significant declines in operating results could result in impairment of goodwill and other intangible assets in future periods.
Changes in the factors used in our fair value estimates, including the impacts of the COVID-19 pandemic, cost inflation or other margin erosion, and discount rates used, could have a significant impact on the fair values of the reporting unit and indefinite-lived intangible assets.
21
Item 7. Quantitative and Qualitative Disclosures about Market Risk.
While the Company is a newly designated “large accelerated” filer as of the fiscal year ended December 31, 2021, it is not required to satisfy the larger reporting company disclosure requirements until the first quarter after the fiscal year in which this determination is made. As such, the Company may continue to apply the scaled disclosure requirements described in Item 305(e) of Regulation S-K, which permits a “smaller reporting company” to omit Quantitative and Qualitative Disclosures about Market Risk.
Item 8. Financial Statements and Supplementary Data
The information required by this Item 8 is set forth following the signature page to this Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
22
Item 9A. Controls and Procedures.
Disclosure controls and procedures
Background
Subsequent to filing the Company’s Quarterly Reports on Form 10-Q for the periods ended June 30, 2021, and September 30, 2021, the Company determined that the calculation of expense of share based compensation related to grants of stock options and restricted stock units (“RSUs”) issued to former employees and retired directors was materially understated during the three-and six-month periods ended June 30, 2021 and three- and nine-month periods ended September 30, 2021 (the “Affected Periods”), based on the application of U.S. generally accepted accounting principles. During the Affected Periods, the stock options and RSUs were modified and the expense should have been calculated and recognized using the fair market value of the awards as of the date of modification and recognized over the remaining service period. In order to properly account for the modifications, the Company has withdrawn reliance on previously reported unaudited consolidated financial statements for the three- and six- months ended June 30, 2021 and three- and nine-months ended September 30, 2021 and has restated the unaudited consolidated financial statements for those periods in this Annual Report on Form 10-K. See the Company's Current Report on Form 8-K filed on March 1, 2022 and "Unaudited Supplementary Information" included in "Item 8. Financial Statements and Supplementary Data" of this Report.
The conclusions that management reached in its evaluations of the effectiveness of our disclosure controls and procedures and internal control over financial reporting as of December 31, 2021, are described below in detail.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) are designed to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms adopted by the SEC, including to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is accumulated and communicated to the Company’s management, including our President and Chief Executive Officer (our principal executive officer) and our Chief Financial Officer (our principal financial and accounting officer), or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Our President and Chief Executive Officer as well as our Chief Financial Officer, have evaluated the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of the end of the period covered by this Report. Based on that evaluation, our President and Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this annual report because of the material weaknesses in internal control over financial reporting described below.
Notwithstanding the conclusion by our President and Chief Executive Officer and our Chief Financial Officer that our disclosure controls and procedures as of December 31, 2021 were not effective, and notwithstanding the material weaknesses in our internal control over financial reporting described below, management believes that the consolidated financial statements and related financial information included in this Annual Report on Form 10-K fairly present in all material respects our financial condition, results of operations and cash flows as of the dates presented, and for the periods ended on such dates, in conformity with U.S. GAAP.
Management’s report on internal control over financial reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process designed by, or under the supervision of, the Company’s principal executive and financial officers and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our President and Chief Executive Officer and our Chief Financial Officer conducted an evaluation of the effectiveness of internal control over financial reporting as of December 31, 2021 based on the criteria set forth in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013) (the “COSO Framework”).
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company's annual or interim financial statements will not be prevented or detected on a timely basis.
23
As of December 31, 2021, management has identified the following material weaknesses in internal controls:
As a result of the material weaknesses described above, the Company’s management has concluded that, as of December 31, 2021, our internal control over financial reporting was ineffective.
Remediation Plan
As of the date of this Annual Report on Form 10-K, management is re-assessing the design of controls and modifying processes designed to improve our internal control over financial reporting and remediate the control deficiencies that led to the material weaknesses, including but not limited to, (a) enhancing monitoring and oversight controls in the application of U.S. GAAP guidance pertaining to modifications of share-based payments, (b) hiring additional accounting and IT personnel with appropriate technical skillsets, (c) improving consistency in change management supported by standard operating procedures to govern the authorization, testing and approval of changes to information technology systems supporting all of the Company’s internal control processes, (d) enhancing design and implementation of our control environment, including the expansion of formal accounting and IT policies and procedures and financial reporting controls, and (e) implementing appropriate timely review and oversight responsibilities within the accounting and financial reporting functions.
Changes in Internal Controls Over Financial Reporting
Except for the determination by management of the material weaknesses in internal controls over financial reporting described above, there have been no other changes in our internal controls over financial reporting that occurred during the fourth quarter of the year ended December 31, 2021, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Attestation Report of the Registered Public Accounting Firm
Effective with the filing of this Report, with respect to the Company's fiscal year ended December 31, 2021, the Company is deemed to be a “large-accelerated filer” as defined in Rule 12b-2 under the Exchange Act. Accordingly, for only the fiscal year ended December 31, 2021 we have included the Report of Independent Registered Public Accounting Firm on the Company’s internal control over financial reporting in accordance with Item 308 (b) of Regulation S-K.
With respect to the Company's fiscal year ended December 31, 2020, the Company was deemed to be a “non-accelerated filer” as defined in Rule 12b-2 under the Exchange Act. Accordingly, this Report is not required to include an attestation report of the Company's registered public accounting firm on the Company’s internal control over financial reporting for the year ended December 31, 2020.
Item 9B. Other Information.
None.
24
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item regarding our directors is included under the caption “Proposal One – Election of Directors” in our Proxy Statement for our 2022 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2021 (the “2022 Proxy Statement”) and is incorporated herein by reference.
Information concerning compliance with Section 16(a) of the Exchange Act is included under the caption “Delinquent Section 16(a) Reports” in our 2022 Proxy Statement and is incorporated herein by reference. Information concerning the Audit Committee and the Audit Committee Financial Expert is reported under the caption “Audit Committee; Report of the Audit Committee; Duties and Responsibilities” in our 2022 Proxy Statement and is incorporated herein by reference.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all our directors, officers (including our principal executive officer and principal financial officer) and employees. The Code of Business Conduct and Ethics and any amendment thereto, as well as any waivers that are required to be disclosed by the rules of the SEC or NASDAQ, may be obtained at no cost to you by writing or telephoning us at the following address or telephone number:
Celsius Holdings, Inc.
2424 North Federal Hwy., Suite 208
Boca Raton FL 33431
(561) 276-2239
Item 11. Executive Compensation
Information concerning the compensation of our directors and executive officers and Compensation Committee Interlocks and Insider Participation is reported under the captions “Compensation Discussion and Analysis” and “Compensation Committee,” respectively, in our 2022 Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The disclosure set forth in “Item 5. “Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Repurchases of Equity Securities - Securities Authorized for Issuance under Equity Compensation Plans” of this Report is incorporated herein.
Information concerning the beneficial ownership of the Company’s common stock by (a) those persons known to the Company to be the beneficial owners of more than 5% of the Company’s common stock; (b) each of the Company’s directors and nominees for director; and (c) the Company’s executive officers and all of the Company’s current directors and executive officers as a group is reported under the caption “Principal Stockholders and Security Ownership of Management” in our 2022 Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions and Director Independence.
Information concerning certain relationships and related transactions will be reported under the caption “Certain Relationships and Related Transactions and Director Independence” in our 2022 Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
Information concerning our accountant fees and our audit committee’s pre-approval of audit and permissible non-audit services of our independent registered public accounting firm is reported under the captions “Principal Accountant Fees” and “Pre-Approval of Audit and Non-Audit Services,” respectively, in our 2022 Proxy Statement and is incorporated herein by reference.
Item 15. Financial Statements and Exhibits
Reports of Independent Registered Public Accounting Firms
Consolidated Balance Sheets as of December 31, 2021 and 2020
Consolidated Statements of Operations and Comprehensive Income for the years ended December 31, 2021 and 2020
Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2021 and 2020
25
Consolidated Statements of Cash Flows for the years ended December 31, 2021 and 2020
Notes to Consolidated Financial Statements
Financial Statement Schedules are omitted because the information required is not applicable or the required information is shown in the financial statements or notes thereto.
Item 16. Form 10-K Summary
None
26
Exhibit No. |
|
Description |
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
10.3 |
|
|
|
|
|
10.4 |
|
|
|
|
|
10.5 |
|
|
|
|
|
10.6 |
|
|
|
|
|
10.7 |
|
|
|
|
|
10.8 |
|
|
|
|
|
10.16 |
|
|
|
|
|
10.20 |
|
Employment Agreement between the Company and John Fieldly dated August 1, 2020****+ |
|
|
|
21.1 |
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm******* |
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
|
32.1 |
|
|
|
|
|
32.2 |
|
|
+ |
Management compensation plan or arrangement. |
|
* |
Previously filed as an Exhibit to the Company’s Registration Statement on Form 10 and incorporated herein by reference, except for an amendment thereto filed as an Exhibit to the Company’s Current Report on Form 8-K dated May 15, 2020 and incorporated herein by reference. |
|
** |
Previously filed as an Exhibit to the Company’s Registration Statement on Form 10 and incorporated herein by reference. |
|
*** |
Previously filed as an Exhibit to the Company’s Current Report on Form 8-K dated December 19, 2018 and incorporated herein by reference. |
|
**** |
Previously filed as an Exhibit to the Company’s Current Report on Form 8-K dated August 4, 2020 and incorporated herein by reference. |
|
***** |
Previously filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 and incorporated herein by reference. |
|
******* |
Filed herewith. |
Item 16. Form 10-K Summary
None.
27
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: March 16, 2022 |
CELSIUS HOLDINGS, INC. |
|
|
|
|
|
By: |
/s/ John Fieldly |
|
|
John Fieldly, Chief Executive Officer (Principal executive officer) |
|
By: |
/s/ Edwin Negron-Carballo |
|
|
Edwin Negron-Carballo, Chief Financial Officer (Principal financial and accounting officer) |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signatures |
|
Title(s) |
|
Date |
|
|
|
|
|
/s/ John Fieldly |
|
President, Chief Executive Officer and Director |
|
March 16, 2022 |
John Fieldly |
|
(Principal executive officer) |
|
|
|
|
|
|
|
/s/ Edwin F. Negron-Carballo |
|
Chief Financial Officer |
|
March 16, 2022 |
Edwin F. Negron-Carballo |
|
(Principal financial and accounting officer) |
|
|
|
|
|
|
|
/s/ Cheryl S. Miller |
|
Director |
|
March 16, 2022 |
Cheryl S. Miller |
|
|
|
|
|
|
|
|
|
/s/ Hal Kravitz |
|
Director |
|
March 16, 2022 |
Hal Kravitz |
|
|
|
|
|
|
|
|
|
/s/ Joyce Russell |
|
Director |
|
March 16, 2022 |
Joyce Russell |
|
|
|
|
|
|
|
|
|
/s/ Damon DeSantis |
|
Director |
|
March 16, 2022 |
Damon DeSantis |
|
|
|
|
|
|
|
|
|
/s/ Nicholas Castaldo |
|
Director |
|
March 16, 2022 |
Nicholas Castaldo |
|
|
|
|
|
|
|
|
|
/s/ Caroline Levy |
|
Director |
|
March 16, 2022 |
Caroline Levy |
|
|
|
|
|
|
|
|
|
/s/ Alexandre Ruberti |
|
Director |
|
March 16, 2022 |
Alexandre Ruberti |
|
|
|
|
28
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page |
|
|
Reports of Independent Registered Public Accounting Firms Ernst & Young LLP PCAOB ID: 42 |
F-7 |
|
|
Reports of Independent Registered Public Accounting Firms Assurance Dimensions PCAOB ID: 5036 |
F-7 |
|
|
Consolidated Balance Sheets as of December 31, 2021 and 2020 |
F-7 |
|
|
F-8 |
|
|
|
F-9 |
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2021 and 2020 |
F-10 |
|
|
F-11 |
|
|
|
F-11 |
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Celsius Holdings, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited Celsius Holdings, Inc.’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weaknesses described below on the achievement of the objectives of the control criteria, Celsius Holdings, Inc. (the Company) has not maintained effective internal control over financial reporting as of December 31, 2021, based on the COSO criteria.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment. Management has identified material weaknesses related to a) the Company’s failure to design effective controls related to the Company’s evaluation of modifications to share-based payment arrangements; b) ineffectively designed and operating information technology general controls (ITGCs) throughout a substantial portion of the year ended December 31, 2021, which resulted in ineffective business process controls (automated and IT-dependent manual controls); and c) the Company’s failure to fully implement components of the COSO framework, including elements of the control environment, information and communication, control activities and monitoring activities components, relating to: (i) providing sufficient and timely management oversight and ownership over the internal control evaluation process; (ii) hiring and training sufficient personnel to timely support the Company’s internal control objectives; (iii) performing timely monitoring and oversight to ascertain whether the components of internal control are present and functioning effectively.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the 2021 consolidated financial statements of the Company. The material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the 2021 consolidated financial statements, and this report does not affect our report dated March 16, 2022, which expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/Ernst & Young LLP
We have served as the Company’s auditor since 2021.
Boca Raton, Florida March 16, 2022
F-2
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Celsius Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Celsius Holdings, Inc. (the Company) as of December 31, 2021, the related consolidated statements of operations and comprehensive income, changes in stockholders’ equity, and cash flows for the year ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021, and the results of its operations and its cash flows for the year ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 16, 2022 expressed an adverse opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
F-3
|
Valuation of indefinite-lived intangible assets
|
Description of the Matter |
At December 31, 2021, the Company’s indefinite-lived intangible assets related to the acquired brands from the 2019 Func Foods Acquisition were $3.2M. As discussed in Note 2 to the consolidated financial statements, intangibles with indefinite lives are tested for impairment on an annual basis, or more frequently if the Company believes indicators of impairment exist. The Company measured the fair value of these indefinite-lived intangible assets using a relief from royalty method.
Auditing the Company's annual impairment test related to these indefinite-lived intangible assets was complex due to the estimation uncertainty in determining their fair values. The significant assumptions used to estimate the fair value of these intangible assets included forecasted sales, discount rates and royalty rates. These significant assumptions are forward-looking and could be affected by future economic and market conditions, which can vary significantly and depend on market forces and events outside of the Company’s control. |
How We Addressed the Matter in Our Audit
|
To test the estimated fair value of these indefinite-lived intangible assets, our audit procedures included, among others, evaluating the valuation methodology used, the significant assumptions discussed above, and the underlying data used by the Company. Such data includes historical sales, future business plans, as well as data from comparable companies. We compared the significant assumptions to current industry or economic trends, the Company’s business model, and other relevant factors. We involved our valuation specialists to assist in our procedures to test the discount rate, which included comparison of the selected discount rate to the weighted average cost of capital of the Company and other comparable companies and the risk associated with the projected cash flows, and the royalty rate, which included comparing management’s selected royalty rate to royalty rates for similar intangible assets in the Company’s industry. In addition, we assessed the historical accuracy of management’s estimates by comparing them to actual operating results and performed sensitivity analyses of the significant assumptions described above to evaluate the impact on the fair value of these indefinite-lived intangible assets.
|
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2021.
Boca Raton, Florida
March 16, 2022
F-4

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Celsius Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Celsius Holdings, Inc. (the Company) as of December 31, 2020, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for the year ended December 31, 2020, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the year ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Collectability of Note Receivable
Description of the Matter
Effective January 1, 2019, the Company entered into a separate economic agreement for the repayment of its marketing investment in China with Qifeng. Under the repayment agreement, Qifeng agreed to refund the Company over a five-year period. The repayment was formalized via a Note Receivable (“Note”) from Qifeng. The initial outstanding principal under the Note was approximately $12.2 million and was denominated in the Chinese Renminbi (CNY). Installment payments under the Note are due on March 31 each year and the first installment payment on the Note was due March 31, 2020. This payment was delayed and paid in September 2020 due to the COVID 19 pandemic. The next installment payment due March 31, 2021 will be paid via a release of 62,000 shares of Celsius common stock owned by Qifeng. This release will provide sufficient funds to pay the full amount that is due under the second installment payment. Under a separate guarantee agreement the Company will maintain control over 275,079 Celsius common shares which serves as collateral for the payment on the remaining amounts that will become due over the next three-years.
We determined based on the fact pattern noted above the long term collectability of the remaining Note balance was as a critical audit matter. The Note is collateralized by the Company’s shares, however we noted that there is volatility relating to the value of the collateral which is based on the share price of the Company’s stock.
How We Addressed the Matter in Our Audit
The primary procedures we performed to address this critical audit matter included the following: 1. Obtaining and testing for reasonableness of management’s analysis to support the collectability of the remaining Note balance. 2. Obtained, a confirmation from Qifeng of the remaining balance due under the Note as of December 31, 2020 as well as confirming their intent and ability to pay the next installment keeping them current with the agreement. 3. Reviewed a signed guarantee letter from Qifeng over the Company shares that are being used as collateral associated with the remainder of the Note. 4. Obtained the Qifeng account
F-5
statement from the Company’s transfer agent verifying the number of shares owned by Qifeng. The value of the collateral shares at December 31, 2020 was in excess of the total note receivable balance as of December 31, 2020. Based on the procedures performed and evidence obtained we concluded that no allowance for collectability was required related to the Note as of December 31, 2020.
|
|
/s/ Assurance Dimensions |
|
We have served as the Company’s auditor from 2017 to 2021 |
|
Margate, Florida March 10, 2021 |
|
F-6
Celsius Holdings, Inc.
Consolidated Balance Sheets
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
|
|
|
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash |
|
$ |
16,254,708 |
|
|
$ |
43,248,021 |
|
Accounts receivable-net (note 2) |
|
|
38,741,049 |
|
|
|
14,986,213 |
|
Note receivable-current (note 6) |
|
|
2,587,905 |
|
|
|
1,885,887 |
|
Inventories-net (note 4) |
|
|
191,221,851 |
|
|
|
18,403,622 |
|
Prepaid expenses and other current assets (note 5) |
|
|
13,555,037 |
|
|
|
14,626,922 |
|
Total current assets |
|
|
262,360,550 |
|
|
|
93,150,665 |
|
|
|
|
|
|
|
|
||
Note receivable (note 6) |
|
|
7,116,738 |
|
|
|
9,429,437 |
|
Property and equipment-net (note 8) |
|
|
3,180,058 |
|
|
|
579,377 |
|
Deferred tax asset (note 14) |
|
|
9,019,241 |
|
|
|
— |
|
Right of use assets-operating leases (note 7) |
|
|
1,128,151 |
|
|
|
836,038 |
|
Right of use assets-financial leases (note 7) |
|
|
85,953 |
|
|
|
162,119 |
|
Long term security deposits |
|
|
299,828 |
|
|
|
122,733 |
|
Intangibles (note 9) |
|
|
16,301,326 |
|
|
|
16,590,083 |
|
Goodwill (note 9) |
|
|
14,526,583 |
|
|
|
10,419,321 |
|
Total Assets |
|
$ |
314,018,428 |
|
|
$ |
131,289,773 |
|
|
|
|
|
|
|
|
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable and accrued expenses (note 10) |
|
$ |
91,478,612 |
|
|
$ |
25,412,753 |
|
Lease liability obligation-operating leases (note 7) |
|
|
511,764 |
|
|
|
321,283 |
|
Lease liability obligation-financial leases (note 7) |
|
|
157,046 |
|
|
|
205,824 |
|
Other current liabilities (note 11) |
|
|
976,072 |
|
|
|
425,232 |
|
Total current liabilities |
|
|
93,123,494 |
|
|
|
26,365,092 |
|
|
|
|
|
|
|
|
||
Long-term liabilities: |
|
|
|
|
|
|
||
Lease liability obligation-operating leases (note 7) |
|
|
657,935 |
|
|
|
514,948 |
|
Lease liability obligation-financial leases (note 7) |
|
|
45,408 |
|
|
|
82,290 |
|
Deferred tax liability (note 14) |
|
|
3,146,394 |
|
|
|
|
|
Total Liabilities |
|
|
96,973,231 |
|
|
|
26,962,330 |
|
|
|
|
|
|
|
|
||
Stockholders’ Equity: |
|
|
|
|
|
|
||
Common stock, $0.001 par value; 100,000,000 shares authorized, 74,908,845 and 72,262,829 shares |
|
|
74,909 |
|
|
|
72,263 |
|
Additional paid-in capital |
|
|
267,846,196 |
|
|
|
159,884,154 |
|
Accumulated other comprehensive income (loss) |
|
|
613,651 |
|
|
|
(202,142 |
) |
Accumulated deficit |
|
|
(51,489,559 |
) |
|
|
(55,426,832 |
) |
Total Stockholders’ Equity |
|
|
217,045,197 |
|
|
|
104,327,443 |
|
Total Liabilities and Stockholders’ Equity |
|
$ |
314,018,428 |
|
|
$ |
131,289,773 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-7
Celsius Holdings, Inc.
Consolidated Statements of Operations and Comprehensive Income
|
|
For the year |
|
|||||
|
|
ended December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
Revenue (note 3) |
|
$ |
314,271,559 |
|
|
$ |
130,725,777 |
|
Cost of revenue (note 2) |
|
|
186,103,035 |
|
|
|
69,752,032 |
|
Gross profit |
|
|
128,168,524 |
|
|
|
60,973,745 |
|
|
|
|
|
|
|
|
||
Selling and marketing expenses |
|
|
74,738,295 |
|
|
|
34,875,339 |
|
General and administrative expenses |
|
|
57,519,745 |
|
|
|
18,187,406 |
|
Total operating expense |
|
|
132,258,040 |
|
|
|
53,062,745 |
|
|
|
|
|
|
|
|
||
Income/(loss) from operations |
|
|
(4,089,516 |
) |
|
|
7,911,000 |
|
|
|
|
|
|
|
|
||
Other Income/(Expense): |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Interest income on note receivable (note 6) |
|
|
314,833 |
|
|
|
355,821 |
|
Interest expense on bonds |
|
|
— |
|
|
|
(431,813 |
) |
Interest on other obligations |
|
|
(7,505 |
) |
|
|
(16,004 |
) |
Amortization of discount on bonds payable |
|
|
|
|
|
(576,415 |
) |
|
Other miscellaneous expense |
|
|
|
|
|
(49,100 |
) |
|
Gain on lease cancellations |
|
|
|
|
|
152,112 |
|
|
Foreign exchange gain/(loss) |
|
|
(276,259 |
) |
|
|
1,375,925 |
|
European deferred tax (note 14) |
|
|
|
|
|
(81,500 |
) |
|
Total other income |
|
|
31,069 |
|
|
|
729,026 |
|
|
|
|
|
|
|
|
||
Net income (loss) before income taxes |
|
|
(4,058,447 |
) |
|
|
8,640,026 |
|
|
|
|
|
|
|
|
||
Income tax benefit (expense) (note 14) |
|
|
7,995,720 |
|
|
|
(116,177 |
) |
|
|
|
|
|
|
|
||
Net income |
|
|
3,937,273 |
|
|
|
8,523,849 |
|
|
|
|
|
|
|
|
||
Other comprehensive income: |
|
|
|
|
|
|
||
Foreign currency translation gain |
|
|
815,793 |
|
|
|
551,378 |
|
Comprehensive Income |
|
$ |
4,753,066 |
|
|
$ |
9,075,227 |
|
|
|
|
|
|
|
|
||
Income per share: |
|
|
|
|
|
|
||
Basic |
|
$ |
0.05 |
|
|
$ |
0.12 |
|
Diluted |
|
$ |
0.05 |
|
|
$ |
0.11 |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
||
Basic |
|
|
73,781,130 |
|
|
|
70,195,085 |
|
Diluted1 |
|
|
77,688,501 |
|
|
|
74,443,601 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-8
Celsius Holdings, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
For the Years Ended December 31, 2021 and 2020
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
|
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Total |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance at December 31, 2019 |
|
|
68,941,311 |
|
|
$ |
68,942 |
|
|
$ |
127,552,998 |
|
|
$ |
(753,520 |
) |
|
$ |
(63,409,431 |
) |
|
$ |
63,458,989 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Issuance of common stock from private placement |
|
|
1,437,909 |
|
|
|
1,438 |
|
|
|
21,890,878 |
|
|
|
|
|
|
|
|
|
21,892,316 |
|
||
Stock option expense |
|
|
|
|
|
|
|
|
6,340,000 |
|
|
|
|
|
|
|
|
|
6,340,000 |
|
||||
Issuance of common stock pursuant to exercise of stock |
|
|
567,559 |
|
|
|
567 |
|
|
|
(567 |
) |
|
|
|
|
|
|
|
|
— |
|
||
Issuance of common stock pursuant to exercise of stock |
|
|
1,316,050 |
|
|
|
1,316 |
|
|
|
4,125,475 |
|
|
|
|
|
|
|
|
|
4,126,791 |
|
||
Cash paid for taxes on restricted stock awards |
|
|
|
|
|
|
|
|
(115,430 |
) |
|
|
|
|
|
(541,250 |
) |
|
|
(656,680 |
) |
|||
Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
551,378 |
|
|
|
|
|
|
551,378 |
|
||||
Short swing payment |
|
|
|
|
|
|
|
|
90,800 |
|
|
|
|
|
|
|
|
|
90,800 |
|
||||
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8,523,849 |
|
|
|
8,523,849 |
|
||||
Balance at December 31, 2020 |
|
|
72,262,829 |
|
|
$ |
72,263 |
|
|
$ |
159,884,154 |
|
|
$ |
(202,142 |
) |
|
$ |
(55,426,832 |
) |
|
$ |
104,327,443 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Issuance of common stock |
|
|
1,133,953 |
|
|
|
1,133 |
|
|
|
67,768,253 |
|
|
|
|
|
|
|
|
|
67,769,386 |
|
||
Stock option expense |
|
|
|
|
|
|
|
|
36,475,161 |
|
|
|
|
|
|
|
|
|
36,475,161 |
|
||||
Issuance of common stock pursuant to exercise of stock |
|
|
539,572 |
|
|
|
540 |
|
|
|
(540 |
) |
|
|
|
|
|
|
|
|
— |
|
||
Issuance of common stock pursuant to exercise of stock |
|
|
972,491 |
|
|
|
973 |
|
|
|
3,719,168 |
|
|
|
|
|
|
|
|
|
3,720,141 |
|
||
Foreign currency translation |
|
|
|
|
|
|
|
|
|
|
|
815,793 |
|
|
|
|
|
|
815,793 |
|
||||
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,937,273 |
|
|
|
3,937,273 |
|
||||
Balance at December 31, 2021 |
|
|
74,908,845 |
|
|
$ |
74,909 |
|
|
$ |
267,846,196 |
|
|
$ |
613,651 |
|
|
$ |
(51,489,559 |
) |
|
$ |
217,045,197 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-9
Celsius Holdings, Inc.
Consolidated Statements of Cash Flows
|
|
For the year ended |
|
|||||
|
|
December 31, |
|
|
December 31, |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net income |
|
$ |
3,937,273 |
|
|
$ |
8,523,849 |
|
Adjustments to reconcile net income to net cash (used in)/provided by operating activities: |
|
|
|
|
|
|
||
Depreciation |
|
|
549,689 |
|
|
|
127,263 |
|
Amortization |
|
|
713,532 |
|
|
|
1,484,303 |
|
Bad debt expense |
|
|
1,493,567 |
|
|
|
257,177 |
|
Inventory excess and obsolescence |
|
|
2,354,837 |
|
|
|
747,507 |
|
Stock-based compensation expense |
|
|
36,475,161 |
|
|
|
6,340,000 |
|
Deferred tax asset-net |
|
|
(9,019,241 |
) |
|
|
|
|
Un-realized exchange gain |
|
|
879,846 |
|
|
|
|
|
Gain on China transaction |
|
|
|
|
|
(322,936 |
) |
|
Gain on lease cancellations |
|
|
(27,917 |
) |
|
|
(152,112 |
) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable-net |
|
|
(25,248,403 |
) |
|
|
(7,468,772 |
) |
Inventory-net |
|
|
(175,173,066 |
) |
|
|
(3,858,780 |
) |
Prepaid expenses and other current assets |
|
|
1,071,885 |
|
|
|
(10,456,787 |
) |
Accounts payable and accrued expenses |
|
|
65,186,015 |
|
|
|
8,120,106 |
|
Deferred tax liability-net |
|
|
(182,156 |
) |
|
|
|
|
Deposits and other current liabilities |
|
|
373,744 |
|
|
|
(96,281 |
) |
Change in right of use and lease obligation-net |
|
|
|
|
|
150,547 |
|
|
Net cash (used)/provided by operating activities |
|
|
(96,586,324 |
) |
|
|
3,395,084 |
|
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Proceeds from note receivable |
|
|
1,885,724 |
|
|
|
1,331,011 |
|
Purchase of property and equipment |
|
|
(3,150,370 |
) |
|
|
(573,751 |
) |
Net cash provided/(used) in investing activities |
|
|
(1,264,646 |
) |
|
|
757,260 |
|
|
|
|
|
|
|
|
||
Cash flows from financing activities: |
|
|
|
|
|
|
||
Payments on bonds payable |
|
|
|
|
|
(9,601,531 |
) |
|
Principal payments on financial lease obligations |
|
|
(94,102 |
) |
|
|
(280,208 |
) |
Proceeds from exercise of stock options |
|
|
3,720,141 |
|
|
|
4,126,791 |
|
Cash paid on taxes on restricted stock awards |
|
|
|
|
|
(656,680 |
) |
|
Net proceeds from collection of section 16b short swing profit |
|
|
|
|
|
90,800 |
|
|
Net proceeds from sale of common stock |
|
|
|
|
|
21,892,316 |
|
|
Net cash provided by financing activities |
|
|
71,395,426 |
|
|
|
15,571,488 |
|
Effect on exchange rate changes on cash and cash equivalents |
|
|
(537,769 |
) |
|
|
433,507 |
|
Net (decrease)/increase in cash |
|
|
(26,993,313 |
) |
|
|
20,157,339 |
|
Cash and cash equivalents at beginning of the period |
|
|
43,248,021 |
|
|
|
23,090,682 |
|
|
|
|
|
|
|
|
||
Cash at end of the period |
|
$ |
16,254,708 |
|
|
$ |
43,248,021 |
|
Supplemental disclosures: |
|
|
|
|
|
|
||
Cash paid during period for: |
|
|
|
|
|
|
||
Interest |
|
$ |
7,431 |
|
|
$ |
447,816 |
|
European Acquisition Adjustment: |
|
|
|
|
|
|
||
Goodwill |
|
$ |
|
|
$ |
395,515 |
|
|
Other liabilities |
|
$ |
|
|
$ |
(395,515 |
) |
|
The accompanying notes are an integral part of these consolidated financial statements
F-10
Business —Celsius Holdings, Inc. (the “Company” or “Celsius Holdings”) was incorporated under the laws of the State of Nevada on April 26, 2005. On January 24, 2007, the Company entered into a merger agreement and plan of reorganization with Elite FX, Inc., a Florida corporation. Under the terms of the Merger Agreement, Elite FX, Inc. was merged into the Company’s subsidiary, Celsius, Inc. and became a wholly-owned subsidiary of the Company on January 26, 2007. In addition, on March 28, 2007 the Company established Celsius Netshipments, Inc. a Florida corporation as a subsidiary of the Company.
On February 7, 2018, the Company established Celsius Asia Holdings Limited a Hong Kong corporation as a wholly-owned subsidiary of the Company. On February 7, 2018 Celsius China Holdings Limited a Hong Kong corporation became a wholly-owned subsidiary of Celsius Asia Holdings Limited and on May 9, 2018, Celsius Asia Holdings Limited established Celsius (Beijing) Beverage Limited, a China corporation as a wholly-owned subsidiary of Celsius Asia Holdings Limited.
On October 25, 2019, the Company acquired 100% of Func Food Group, Oyj (“Func Food”). The Acquisition was structured as a purchase of all of Func Food’s equity shares and a restructuring of Func Food’s pre-existing debt. Func Food was the Nordic distributor for the Company since 2015. Func Food is a marketer and distributor of nutritional supplements, health food products, and beverages.
The Company is engaged in the development, marketing, sale and distribution of “functional” calorie-burning functional energy drinks and liquid supplements under the Celsius® brand name.
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and the rules and regulations of the SEC.
Consolidation Policy — The accompanying consolidated financial statements include the accounts of Celsius Holdings, Inc. and its subsidiaries. All inter-company balances and transactions have been eliminated in consolidation.
Significant Estimates — The preparation of consolidated financial statements and accompanying disclosures in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses and disclosure of contingent assets and liabilities at the date of the financial statements. Although these estimates are based on management's best knowledge of current events and actions that the Company may undertake in the future, actual results may differ from those estimates. Significant estimates include the allowance for doubtful accounts, allowance for inventory obsolescence, the useful lives of property, fixtures and equipment, impairment of intangible assets & goodwill, valuation of stock-based compensation, and deferred tax asset valuation allowance.
Segment Reporting — Operating segments are defined as components of an enterprise that engage in business activities, have discrete financial information, and whose operating results are regularly reviewed by the chief operating decision maker (CODM) to make decisions about allocating resources and to assess performance. Even though we have operations in several geographies, we operate as a single enterprise. Our operations and strategies are centrally designed and executed given that our geographical components are very similar. Our CODM, the CEO, reviews operating results primarily from a consolidated perspective, and makes decisions and allocates resources based on that review. The reason our CODM focuses on consolidated results in making decisions and allocating resources is because of the significant economic interdependencies between our geographical operations and the Company’s U.S. entity.
Concentrations of Risk — Substantially all of the Company’s revenue derives from the sale of Celsius ® functional energy drinks and liquid supplements.
The Company uses single supplier relationships for its raw materials purchases and filling capacity, which potentially subjects the Company to a concentration of business risk. If a supplier had operational problems or ceased making product available to the Company, operations could be adversely affected.
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company places its cash and cash equivalents with high-quality financial institutions. At times, balances in the Company’s cash accounts may exceed the Federal Deposit Insurance Corporation limit. At December 31, 2021 and 2020, the Company had approximately $16.3 million and $43.2 million, respectively, in excess of the Federal Deposit Insurance Corporation limit.
For the years ended December 31, 2021 and 2020, the Company had the following 10 percent or greater concentrations of revenue with its customers. Specifically, there are two customers that have accounted for approximately 12.7% and 10.1% of our revenue, for the year ended December 31, 2021. Those two customers accounted for approximately 2.9% and 15.1% of our revenue for the year ended December 31, 2020. The below table reflects this customer’s evolution as a percentage of our total revenue.
|
|
2021 |
|
|
2020 |
|
||
Amazon |
|
|
10.1 |
% |
|
|
15.1 |
% |
Costco |
|
|
12.7 |
% |
|
|
2.9 |
% |
All other |
|
|
77.2 |
% |
|
|
82.0 |
% |
Total |
|
|
100.0 |
% |
|
|
100.0 |
% |
At December 31, 2021 and 2020, the Company had the following 10 percent or greater concentrations of accounts receivable with its customers:
F-11
|
|
2021 |
|
|
2020 |
|
||
Amazon |
|
|
22.7 |
% |
|
|
11.3 |
% |
Publix |
|
|
10.3 |
% |
|
|
6.0 |
% |
All other |
|
|
67.0 |
% |
|
|
82.7 |
% |
Total |
|
|
100.0 |
% |
|
|
100.0 |
% |
Cash Equivalents — The Company considers all highly liquid instruments with an original maturity of three months or less when purchased to be cash equivalents. At December 31, 2021 and 2020, the Company did not have any investments with original maturities of three months or less.
Accounts Receivable — Accounts receivable are reported at net realizable value. The Company establishes an allowance for doubtful accounts based upon factors pertaining to the credit risk of specific customers, historical trends, and other information. Delinquent accounts are written-off when it is determined that the amounts are uncollectible. At December 31, 2021 and 2020, there was an allowance for doubtful accounts of approximately $0.8 million and $0.5 million, respectively.
Inventories — Inventories include only the purchase cost and are stated at the lower of cost or net realizable value. Cost is determined using the FIFO method. Inventories consist of raw materials and finished products. The Company establishes an inventory reserve to reduce the value of the inventory during the period in which such materials and products are no longer usable or marketable. Specifically, the Company reviews inventory utilization during the past twelve months and also customer orders for subsequent months. If there has been no utilization during the last 12 months and there are no orders in-place in future months which will require the use of inventory item, then inventory item will be included as part of the reserve during the period being evaluated. Management will then specifically evaluate whether these items may be utilized within a reasonable time frame (e.g., 3 to 6 months). At December 31, 2021 and December 31, 2020, the Company recorded an allowance of approximately $2.6 million and $1.6 million respectively. The changes in the allowance are included in cost of revenue.
Property and Equipment — Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation of property and equipment is calculated using the straight-line method over the estimated useful life of the asset generally ranging from to seven years.
Impairment of Long-Lived Assets — In accordance with ASC Topic 360, “Property, Plant, and Equipment” the Company reviews the carrying value of long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An impairment loss is determined regarding a long-lived asset if its carrying amount is not recoverable and exceeds its fair value. The carrying amount is not recoverable when it exceeds the sum of the undiscounted cash flows expected to result from use of the asset over its remaining useful life and final disposition. The Company did not record any impairments during the years ended December 31, 2021 and December 31, 2020.
Long-lived Asset Geographic Data
The following table sets forth long-lived asset information which includes property and equipment and lease right of use assets and excludes goodwill and intangibles, where individual countries represent a material portion of the total:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
United States |
|
$ |
3,043,105 |
|
|
$ |
694,697 |
|
|
|
|
|
|
|
|
||
Sweden |
|
|
1,050,476 |
|
|
|
431,959 |
|
Finland |
|
|
300,581 |
|
|
|
450,878 |
|
Long-lived assets related to foreign operations |
|
|
1,351,057 |
|
|
|
882,837 |
|
Total long-lived assets-net |
|
$ |
4,394,162 |
|
|
$ |
1,577,534 |
|
Goodwill — The Company records goodwill when the consideration paid for an acquisition exceeds the fair value of net tangible and intangible assets acquired, including related tax effects. Goodwill is not amortized; instead, goodwill is tested for impairment on an annual basis, or more frequently if the Company believes indicators of impairment exist. The Company first assesses qualitative factors such as macro-economic conditions, industry and market conditions, cost factors as well as other relevant events, to determine whether it is more-likely-than-not that the fair value of a reporting unit is less than its carrying value. If the Company determines that the fair value is less than the carrying value, the Company will recognize an impairment charge based on the excess of a reporting unit’s carrying value over its fair value.
During the fourth quarter of 2021, we changed the date of our annual impairment test for goodwill and indefinite-lived intangible assets from December 31st to October 1st. This voluntary change was made to better align the timing of the assessment with the Company’s planning and forecasting process and to give the Company, which is a newly designated large accelerated SEC filer as of December 31, 2021, additional time to complete the annual assessment in advance of year-end reporting. We believe this change in accounting principle measurement date is preferable under the circumstances. This change in assessment date was applied prospectively and did not delay, accelerate, or avoid a potential impairment charge. As of October 1, 2021, there were no indicators of impairment.
Intangible assets – Intangible assets are comprised of customer relationships and brands acquired in a business combination. The Company amortizes intangible assets with a definitive life over their respective useful lives. Assets with indefinite lives are tested for impairment on an annual basis, or more frequently if the Company believes indicators of impairment exist. We utilize both qualitative and quantitative aspects to evaluate the impairment of our intangible assets. The Company measured the fair value of these indefinite-lived intangible assets using a relief from royalty method. The fair value was estimated by projections to determine the present value of future cash flows that the asset is expected to generate over its lifetime. Our projections used in the valuation included assumptions regarding future growth rates of sales, which are based on various long-range financial and operational plans, the royalty
F-12
rate, and the discount rate. Discount rates used in the indefinite-lived intangible analysis are based on a weighted-average cost of capital, driven by the prevailing interest rates in the geographies where the company operates, as well as credit ratings, financing abilities and opportunities, among other factors. Discount rates may differ slightly to adjust for country or market specific risk, among other factors. We believe our evaluations are consistent with those a market participant would utilize.
Revenue Recognition — The Company recognizes revenue in accordance with ASC Topic 606 “Revenue from Contracts with Customers.” The Company recognizes revenue when performance obligations under the terms of a contract with the customer are satisfied. Product sales occur once control or title is transferred based on the commercial terms. Revenue is measured as the amount of consideration the Company expects to receive in exchange for transferring goods. Product sales are recorded net of variable consideration, such as provisions for returns, discounts and allowances. Such provisions are calculated using historical averages and adjusted for any expected changes due to current business conditions. Consideration given to customers for cooperative advertising is recognized as a reduction of revenue except to the extent that there is a distinct good or service, in which case the expense is classified as selling or marketing expense. Provisions for customer volume rebates are based on achieving a certain level of purchases and other performance criteria that are established on a situation basis. These rebates are estimated based on the expected amount to be provided to the customers and are recognized as a reduction of revenue. The amount of consideration the Company receives and revenue the Company recognizes varies with changes in customer incentives the Company offers to its customers and their customers. Additionally, for any agreements which are 1 year or less, the practical expedient under ASC 340-40-25-4 is applied to expense costs when incurred if the amortization period of the contract asset would have otherwise been recognized in one year or less. Sales taxes and other similar taxes are excluded from revenue.
Customer Advances — From time to time the Company requires deposits in advance of delivery of products and/or production runs. Such amounts are initially recorded as customer advances liability within other current liabilities. The Company recognizes such revenue as it is earned in accordance with revenue recognition policies. As of December 31, 2021 and 2020, the Company did not have any customer advances.
Advertising Costs — Advertising costs are expensed as incurred. The Company uses mainly radio, local sampling events, sponsorships, endorsements, and digital advertising. The Company incurred advertising expenses of approximately $36.7 million and $14.2 million, during years ending December 31, 2021 and 2020, respectively.
Research and Development — Research and development costs are charged to general and administrative expenses as incurred and consist primarily of consulting fees, raw material usage and test productions of functional energy drinks and liquid supplements. The Company incurred expenses of approximately $1.0 million and $0.5 million during years ending December 31, 2021 and 2020, respectively.
Foreign Currency Gain/Losses — Foreign subsidiaries’ functional currency is the local currency of operations and the net assets of foreign operations are translated into U.S. dollars using current exchange rates. The foreign subsidiaries perform re-measurements of their assets and liabilities denominated in non-functional currencies on a periodic basis and the gain or losses from these adjustments are included in the Statement of Operations as foreign exchange gains or losses. For the year ended December 31, 2021 exchange losses have amounted to approximately $0.3 million while during the year ended December 31, 2020, we recognized foreign currency gains of approximately $1.4 million mainly related to fluctuations in exchange rates. Translation gain and losses that arise from the translation of net assets, as well as exchange gains and losses on intercompany balances of long-term investment nature, are included in other comprehensive income. The Company incurred foreign currency translation net gain during the year ended December 31, 2021 of approximately $0.8 million and a gain of approximately $0.6 million during the year ended December 31, 2020. Our operations in different countries required that we transact in the following currencies:
Chinese-Yuan
Norwegian-Krone
Swedish-Krona
Finland-Euro
Fair Value of Financial Instruments — The carrying value of cash, accounts receivable, accounts payable, accrued expenses and other current liabilities approximate fair value due to their relative short-term maturity and market interest rates.
Fair Value Measurements - ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Additionally, ASC 820 requires the use of valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs. These inputs are prioritized below:
Level 1: Observable inputs such as quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market-based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs for which there is little or no market data, which require the use of the reporting entity’s own assumptions.
Other than these noted previously, the Company did not have any other assets or liabilities measured at fair value at December 31, 2021 and 2020.
Income Taxes — The Company accounts for income taxes pursuant to the provisions of ASC 740-10, “Accounting for Income Taxes,” which requires, among other things, an asset and liability approach to calculating deferred income taxes. The asset and liability approach require the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. A valuation allowance is provided to offset any net deferred tax assets for which management believes it is more likely than not that the net deferred asset will not be realized. The Company follows the provisions of the ASC 740 -10 related to, Accounting for Uncertain Income Tax Positions. When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. In accordance with the guidance
F-13
of ASC 740-10, the benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any.
Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above should be reflected as a liability for uncertain tax benefits in the accompanying balance sheet along with any associated interest and penalties that would be payable to the taxing authorities upon examination.
The Company has adopted ASC 740-10-25 Definition of Settlement, which provides guidance on how an entity should determine whether a tax position is effectively settled for the purpose of recognizing previously unrecognized tax benefits and provides that a tax position can be effectively settled upon the completion of an examination by a taxing authority without being legally extinguished. For tax positions considered effectively settled, an entity would recognize the full amount of tax benefit, even if the tax position is not considered more likely than not to be sustained based solely on the basis of its technical merits and the statute of limitations remains open.
The Company’s tax returns for tax years in 2018 through 2020 remain subject to potential examination by the taxing authorities.
Earnings per Share — Basic earnings per share are calculated by dividing net income available to stockholders by the weighted-average number of common shares outstanding during each period. Diluted earnings per share are computed using the weighted average number of common and dilutive common share equivalents outstanding during the period. Please refer to the below table for additional details:
|
|
For the years ended December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
Net income |
|
$ |
3,937,273 |
|
|
$ |
8,523,849 |
|
Income per share: |
|
|
|
|
|
|
||
Basic |
|
$ |
0.05 |
|
|
$ |
0.12 |
|
Diluted |
|
$ |
0.05 |
|
|
$ |
0.11 |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
||
Basic |
|
|
73,781,130 |
|
|
|
70,195,085 |
|
Effect of dilutive share based awards |
|
|
3,907,371 |
|
|
|
4,248,516 |
|
Diluted |
|
|
77,688,501 |
|
|
|
74,443,601 |
|
Share-Based Payments —The Company follows the provisions of ASC Topic 718 “Compensation — Stock Compensation” and related interpretations. As such, compensation cost is measured on the date of grant at the fair value of the share-based payments. Such compensation amounts, if any, are amortized over the respective vesting periods of the grants. On April 30, 2015, the Company adopted the 2015 Stock Incentive Plan. This plan is intended to provide incentives which will attract and retain highly competent persons at all levels as employees of the Company, as well as independent contractors providing consulting or advisory services to the Company, by providing them opportunities to acquire the Company’s common stock. The 2015 Plan permits the grant of options and shares for up to 5,000,000 shares. In addition, there is a provision for an annual increase of 15% to the shares included under the plan, with the shares to be added on the first day of each calendar year, beginning on January 1, 2017 (note 15). As of December 31, 2021, and 2020, total shares available are 4.3 million and 2.0 million, respectively.
Cost of Sales — Cost of sales consists of the cost of concentrates and or liquid bases, the costs of raw materials utilized in the manufacture of products, co-packing fees, repacking fees, in-bound & out-bound freight charges, as well as certain internal transfer costs, warehouse expenses incurred prior to the manufacture of the Company’s finished products, inventory allowance for excess and obsolete products, and certain quality control costs. Raw materials account for the largest portion of the cost of sales. Raw materials include cans, bottles, other containers, flavors, ingredients and packaging materials.
Operating Expenses — Operating expenses include selling expenses such as warehousing expenses after manufacture, as well as expenses for advertising, samplings and in-store demonstrations costs, costs for merchandise displays, point-of-sale materials and premium items, sponsorship expenses, other marketing expenses and design expenses. Operating expenses also include such costs as payroll costs, travel costs, professional service fees (including legal fees), depreciation and other general and administrative costs.
Shipping and Handling Costs — Shipping and handling costs for freight expense on goods shipped are included in cost of sales. Freight expense on goods shipped for the years ended December 31, 2021 and 2020 was approximately $26.9 million and $9.5 million, respectively.
Recent Accounting Pronouncements
The Company adopts all applicable, new accounting pronouncements as of the specified effective dates.
In September 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326) (“ASU 2016-13”), which requires the immediate recognition of management’s estimates of current and expected credit losses. In November 2018, the FASB issued ASU 2018-19, which makes certain improvements to Topic 326. In April and May 2019, the FASB issued ASUs 2019-04 and 2019-05, respectively, which adds codification improvements and transition relief for Topic 326. In November 2019, the FASB issued ASU 2019-10, which delays the effective date of Topic 326 for Smaller Reporting Companies to interim and annual periods beginning after December 15, 2022, with early adoption permitted. We have elected the relief provided. In November 2019, the FASB issued ASU 2019-11, which makes improvements to certain areas of Topic 326. In February 2020, the FASB issued ASU 2020-02, which adds an SEC paragraph, pursuant to the issuance of SEC Staff Accounting Bulletin No. 119, to Topic 326. Topic 326 is effective for the Company for fiscal years and interim reporting periods within those years beginning after December 15, 2022.
F-14
All new accounting pronouncements issued but not yet effective are not expected to have a material impact on our results of operations, cash flows or financial position except for the previously disclosed above, there have been no new accounting pronouncements not yet effective that have significance to our consolidated financial statements.
Liquidity — These financial statements have been prepared assuming the Company will be able to continue as a going concern. At December 31, 2021, the Company had an accumulated deficit of approximately $51.5 million which includes a net income available to common stockholders of approximately $3.9 million for year ended December 31, 2021. During the year ending December 31, 2021 the Company’s net cash used in operating activities totaled approximately $96.6 million. As of December 31, 2020, the Company had an accumulated deficit of $55.4 million which includes a net income available to common stockholders of $8.5 million for year ended December 31, 2020. During the year ending December 31, 2020 the Company’s net cash provided by operating activities totaled approximately $3.4 million.
If our sales volumes do not meet our projections, expenses exceed our expectations, our plans change, we may be unable to generate enough cash flow from operations to cover our working capital requirements. In such case, we may be required to adjust our business plan, by reducing marketing, lower our working capital requirements and reduce other expenses or seek additional financing. Furthermore, our business and results of operations may be adversely affected by changes in the global macro-economic environment related to the pandemic and public health crises related to the COVID-19 outbreak.
Correction of Immaterial Errors — The company performed immaterial corrections to the previously reported consolidated financial statements related to the Func Foods acquisition in 2019. During the third quarter of 2021, goodwill increased by $3.7 million and deferred tax liabilities increased by $3.5 million attributable to tax implications of acquired intangible assets that had not been recorded in the purchase accounting treatment acquisition. The impact on the consolidated statements of operations and comprehensive income for the year ended December 31, 2021, resulted in a $0.2 million deferred tax benefit.
Correction of previously issued financial statements — Subsequent to filing the Company’s Quarterly Reports on Form 10-Q for the periods ended June 30, 2021, and September 30, 2021, the Company determined that the calculation of expense of share based compensation related to grants of stock options and restricted stock units (“RSUs”) issued to former employees and retired directors was materially understated during the three-and six-month periods ended June 30, 2021 and three- and nine-month periods ended September 30, 2021 (the “Affected Periods”), based on the application of U.S. generally accepted accounting principles. During the Affected Periods, the stock options and RSUs were modified and the expense should have been calculated and recognized using the fair market value of the awards as of the date of modification and recognized over the remaining service period.
In accordance with Staff Accounting Bulletin ("SAB") No. 99, Materiality, and SAB No. 108, Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements, the Company evaluated the misstatements and based on an analysis of quantitative and qualitative factors determined that the impact of the misstatement to its interim reporting periods ending June 30, 2021, and September 30, 2021, was material. Accordingly, the Company has restated its interim consolidated financial statements for the three- and six-months ended June 30, 2021, and three- and nine- months ended September 30, 2021, respectively, and included that restated financial information within this annual report.
See unaudited Selected Quarterly Financial Results for restatements to the Company's previously reported unaudited interim consolidated financial statements that were impacted by this misstatement.
The Company recognizes revenue in accordance with ASC Topic 606 “Revenue from Contracts with Customers.” The Company recognizes revenue when performance obligations under the terms of a contract with the customer are satisfied. Product sales occur once control is transferred, , based on the commercial terms. Revenue is measured as the amount of consideration the Company expects to receive in exchange for transferring goods. Product sales are recorded net of variable consideration, such as provisions for returns, discounts and allowances. Such provisions are calculated using historical averages and adjusted for any expected changes due to current business conditions. Consideration given to customers for cooperative advertising is recognized as a reduction of revenue except to the extent that there is a distinct good or service, in which case the expense is classified as selling or marketing expense. Provisions for customer volume rebates are based on achieving a certain level of purchases and other performance criteria that are established on a situation basis. These rebates are estimated based on the expected amount to be provided to the customers and are recognized as a reduction of revenue. The amount of consideration the Company receives and revenue the Company recognizes varies with changes in customer incentives the Company offers to its customers and their customers. Additionally, for any agreements which are 1 year or less, the practical expedient under ASC 340-40-25-4 is applied to expense costs when incurred if the amortization period of the contract asset would have otherwise been recognized in one year or less.
Information about the Company’s net sales by geographical location for the years ended December 31, 2021 and 2020 is as follows:
|
|
For the years ended |
|
|||||
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
|
|
|
|
|
|
|
||
North America |
|
$ |
273,004,795 |
|
|
$ |
95,480,310 |
|
Europe |
|
|
38,097,016 |
|
|
|
33,727,441 |
|
Asia |
|
|
2,538,099 |
|
|
|
1,092,703 |
|
Other |
|
|
631,649 |
|
|
|
425,323 |
|
Net sales |
|
$ |
314,271,559 |
|
|
$ |
130,725,777 |
|
All of the Company’s North America revenue is derived from the United States, which is the Company’s country of domicile. Of the Company’s total foreign revenues of approximately $41.3 million and $35.2 million for the years ended December 31, 2021 and 2020, respectively, Sweden represented the largest foreign portion of total consolidated revenue of approximately $26.9 million and $23.1 million for the years ended December 31, 2021 and 2020, respectively.
F-15
License Agreement
In January 2019, the Company entered into a license and repayment of investment agreement with Qifeng Food Technology (Beijing) Co., Ltd (“Qifeng”). Under the agreement, Qifeng was granted the exclusive license rights to manufacture, market and commercialize Celsius branded products in China. The term of the agreement is 50 years, with annual royalty fees due from Qifeng after the end of each calendar year. The royalty fees are based on a percentage of Qifeng’s sales of Celsius branded products; however, the fees are fixed for the first five years of the agreement, totaling approximately $6.9 million, and then are subject to annual guaranteed minimums over the remaining term of the agreement.
Under the agreement, the Company grants Qifeng exclusive license rights and provides ongoing support in product development, brand promotion and technical expertise. The ongoing support is integral to the exclusive license rights and, as such, both of these represent a combined, single performance obligation. The transaction price consists of the guaranteed minimums and the variable royalty fees, all of which are allocated to the single performance obligation.
The Company recognizes revenue from the agreement over time because the customer simultaneously receives and consumes the benefits from the services. The Company uses the passage of time to measure progress towards satisfying its performance obligation because of its ongoing efforts in providing the exclusive license rights including providing continuous access, updates and support. Total revenue recognized under the agreement was approximately $1.6 million for the year ended December 31, 2021 and approximately $0.8 million for the year ended December 31, 2020, which is reflected in the revenues from Asia.
Inventories consist of the following at:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Finished goods |
|
$ |
123,594,177 |
|
|
$ |
15,334,386 |
|
Raw Materials |
|
|
70,200,702 |
|
|
|
4,682,291 |
|
Less: Inventory reserve |
|
|
(2,573,028 |
) |
|
|
(1,613,055 |
) |
Inventories |
|
$ |
191,221,851 |
|
|
$ |
18,403,622 |
|
Prepaid expenses and other current assets total approximately $13.6 million and $14.6 million, at December 31, 2021 and 2020, respectively, and consist mainly of prepaid advances to co-packers related to inventory production, advertising, prepaid insurance, prepaid slotting fees, value added tax payments and deposits on purchases.
Note receivable consists of the following at:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Note Receivable-current |
|
$ |
2,587,905 |
|
|
$ |
1,885,887 |
|
Note Receivable-non-current |
|
|
7,116,738 |
|
|
|
9,429,437 |
|
Total Note Receivable |
|
$ |
9,704,643 |
|
|
$ |
11,315,324 |
|
Effective January 1, 2019, we restructured our China distribution efforts by entering into two separate economic agreements as it relates to the commercialization of our Celsius products (i.e., license agreement) and a repayment of investment agreement with Qifeng. Under the license agreement, Qifeng was granted the exclusive license rights to manufacture, market and commercialize Celsius® brand products in China. Qifeng will pay a minimum royalty fee of approximately $6.9 million for the five years of the term of the agreement, transitioning to a volume-based royalty fee, thereafter. Under a separate economic agreement, Qifeng Food will repay the marketing investments made by Celsius into the China market through 2018, over the same five-year period. The repayment, which was formalized via a Note Receivable from Qifeng, will need to be serviced even if the licensing agreement is cancelled or terminated. The note receivable is denominated in Chinese-Yuan.
Scheduled principal payments plus accrued interest are due annually on March 31 of each year starting in 2020. The note receivable is recorded at amortized cost and accrues interest at a rate per annum equal to the weighted average of 5% of the outstanding principal up to $5 million and 2% of the outstanding principal above $5 million. On June 12, 2020, it was agreed to fix the interest rate at 3.21% which reflected the weighted average interest rate for the 5-year period of the Note. For the year ended December 31, 2021 and 2020, interest income was approximately $0.3 million and $0.4 million, respectively.
The Company assesses the note receivable for impairment at each reporting period, by evaluating whether it is probable that the Company will be unable to collect all the contractual principal and interest payments as scheduled in the Note agreement, based on historical experience of Qifeng’s ability to pay, the current economic environment and other factors. If the Note is determined to be impaired, the impairment is measured based on the present value of the expected future cash flows under the Note, discounted at the Note’s effective interest rate. At December 31, 2021, the Note was not deemed to be impaired. As of December 31, 2021, Qifeng is current on all amounts due under the Note and the license agreement.
As collateral for the Note, a stock certificate in Celsius Holdings, Inc., which amounts to 122,830 of shares owned by an affiliate under common control with Qifeng is being held at a brokerage account. These shares were originally issued on April 20, 2015 via a private transaction which involved Risejoy Services
F-16
Limited an affiliate under the common control of Qifeng, our Chinese licensee. Payment in-full was received timely pertaining to the amounts due on March 31, 2021. Furthermore, a letter of guarantee was executed with several restrictions regarding these shares. In particular, it was agreed that the stock would not be sold or transferred without the prior written consent from Celsius Holdings, Inc. There are other restrictions and agreements, which include that a Statement of Account will be provided to Celsius on a Quarterly basis to confirm and validate the existence of the shares. These shares serve only as collateral and is a component of management’s consideration when evaluating impairment indicators.
The Company’s leasing activities include an operating lease of its corporate office space from a related party (see Note 12) and several other operating and finance leases of vehicles and office space for the Company’s European operations.
At the inception of a contract, the Company assesses whether the contract is, or contains, a lease. The Company’s assessment is based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether the Company obtains the right to substantially all the economic benefit from the use of the asset throughout the term, and (3) whether the Company has the right to direct the use of the asset. The Company allocates the consideration in the contract to each lease and non-lease component based on the component’s relative stand-alone price to determine the lease payments. Lease and non-lease components are accounted for separately.
Leases are classified as either finance leases or operating leases based on criteria in ASC Topic 842, “Leases”. The Company’s operating leases are generally comprised of real estate and vehicles, and the Company’s finance leases are generally comprised of vehicles.
At lease commencement, the Company records a lease liability equal to the present value of the remaining lease payments, discounted using the rate implicit in the lease or, if that rate cannot be readily determined, the Company’s incremental borrowing rate. A corresponding right-of-use asset (“ROU asset”) is recorded, measured based on the initial measurement of the lease liability. ROU assets also include any lease payments made and exclude lease incentives. Lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
Lease expense for operating leases, consisting of lease payments, is recognized on a straight-line basis over the lease term. Included in lease expense are any variable lease payments incurred in the period that were not included in the initial lease liability. Lease expense for finance leases consists of the amortization of the ROU asset on a straight-line basis over the shorter of the useful life of the asset or the lease term, and interest expense is calculated using the effective interest rate method.
The following is a summary of lease cost recognized in the Company’s consolidated statements of operations and comprehensive income:
|
|
Year ended |
|
|
Year ended |
|
||||||||||
|
|
December 31, |
|
|
December 31, |
|
||||||||||
|
|
Operating |
|
|
Finance |
|
|
Operating |
|
|
Finance |
|
||||
|
|
Leases |
|
|
Leases |
|
|
Leases |
|
|
Leases |
|
||||
Lease cost in general and administrative expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Operating lease expense |
|
$ |
476,815 |
|
|
$ |
— |
|
|
$ |
384,503 |
|
|
$ |
— |
|
Amortization of finance lease ROU assets |
|
|
|
|
|
124,971 |
|
|
|
|
|
|
328,505 |
|
||
Total lease cost in general and administrative expenses |
|
|
476,815 |
|
|
|
124,971 |
|
|
|
384,503 |
|
|
|
328,505 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Lease cost in other expense: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest on finance lease liabilities |
|
|
|
|
|
7,504 |
|
|
|
|
|
|
16,004 |
|
||
Total lease cost in other expense |
|
|
|
|
|
7,504 |
|
|
|
|
|
|
16,004 |
|
||
Total lease cost |
|
$ |
476,815 |
|
|
$ |
132,475 |
|
|
$ |
384,503 |
|
|
$ |
344,509 |
|
The following is a summary of the impact of the Company’s leases on the consolidated statements of cash flows:
|
|
Years ended |
|
|||||
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
Leasing activity in cash flows from operating activities: |
|
|
|
|
|
|
||
Payments under operating leases |
|
|
(470,544 |
) |
|
|
(389,831 |
) |
Interest payments on finance lease liabilities |
|
|
(7,431 |
) |
|
|
(16,004 |
) |
Total leasing activity in cash flows from operating activities |
|
|
(477,975 |
) |
|
|
(405,835 |
) |
|
|
|
|
|
|
|
||
Leasing activity in cash flows from financing activities: |
|
|
|
|
|
|
||
Principal payments on finance lease liabilities |
|
|
(94,102 |
) |
|
|
(280,208 |
) |
Total leasing activity in cash flows from financing activities: |
|
|
(94,102 |
) |
|
|
(280,208 |
) |
The weighted-average remaining lease terms and weighted-average discount rates for operating and finance leases at December 31, 2021 and December 31, 2020 were as follows:
F-17
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Weighted average remaining lease term (years) - operating leases |
|
|
2.3 |
|
|
|
2.6 |
|
Weighted average remaining lease term (years) - finance leases |
|
|
0.7 |
|
|
|
1.1 |
|
Weighted average discount rate - operating leases |
|
|
5.78 |
% |
|
|
6.52 |
% |
Weighted average discount rate - finance leases |
|
|
3.01 |
% |
|
|
3.95 |
% |
The future annual minimum lease payments required under the Company’s leases as of December 31, 2021 are as follows:
|
|
Operating |
|
|
Finance |
|
|
|
|
|||
Future minimum lease payments |
|
Leases |
|
|
Leases |
|
|
Total |
|
|||
2022 |
|
$ |
561,648 |
|
|
$ |
160,239 |
|
|
$ |
721,887 |
|
2023 |
|
|
513,462 |
|
|
|
45,955 |
|
|
|
559,417 |
|
2024 |
|
|
162,538 |
|
|
|
— |
|
|
|
162,538 |
|
2025 |
|
|
5,212 |
|
|
|
— |
|
|
|
5,212 |
|
Total future minimum lease payments |
|
|
1,242,860 |
|
|
|
206,194 |
|
|
|
1,449,054 |
|
Less: Amount representing interest |
|
|
(73,161 |
) |
|
|
(3,740 |
) |
|
|
(76,901 |
) |
Present value of lease liabilities |
|
|
1,169,699 |
|
|
|
202,454 |
|
|
|
1,372,153 |
|
Less: current portion |
|
|
(511,764 |
) |
|
|
(157,046 |
) |
|
|
(668,810 |
) |
Long-term portion |
|
$ |
657,935 |
|
|
$ |
45,408 |
|
|
$ |
703,343 |
|
Property and equipment consist of the following at:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Merchandising equipment - coolers |
|
$ |
3,051,938 |
|
|
$ |
394,418 |
|
Office equipment |
|
|
890,695 |
|
|
|
630,759 |
|
Vehicles |
|
|
304,210 |
|
|
|
78,124 |
|
Less: accumulated depreciation |
|
|
(1,066,785 |
) |
|
|
(523,924 |
) |
Total |
|
$ |
3,180,058 |
|
|
$ |
579,377 |
|
Depreciation expense amounted to approximately $0.5 million and $0.1 million during years ended December 31, 2021 and 2020, respectively.
Goodwill consists of approximately $14.5 million resulting from the excess of the consideration paid and the fair value of net tangible and intangible assets acquired from the Func Food Acquisition, including an immaterial correction further detailed in Note 2 above.
Intangible assets consist of acquired customer relationships and brands from the Func Food Acquisition. The gross carrying amount and accumulated amortization of intangible assets were as follows as of December 31, 2021 and December 31, 2020:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Definite-lived intangible assets |
|
|
|
|
|
|
||
Customer relationships |
|
$ |
14,248,342 |
|
|
$ |
14,050,000 |
|
Less: accumulated amortization |
|
|
(1,139,868 |
) |
|
|
(582,917 |
) |
Definite-lived intangible assets, net |
|
$ |
13,108,474 |
|
|
$ |
13,467,083 |
|
|
|
|
|
|
|
|
||
Indefinite-lived intangible assets |
|
|
|
|
|
|
||
Brands |
|
$ |
3,192,852 |
|
|
$ |
3,123,000 |
|
Total Intangibles |
|
$ |
16,301,326 |
|
|
$ |
16,590,083 |
|
Customer relationships are amortized over an estimated useful life of 25 years and brands have an indefinite life. Amortization expense for the year ended December 31, 2021 and 2020 was approximately $0.6 million and $0.6 million, respectively and is reflected in general and administrative expenses.
Other fluctuations in the amounts of intangible assets are due to currency translation adjustments.
The following is the future estimated amortization expense related to customer relationships as of December 31, 2021:
F-18
Year ending December 31, |
|
|
|
|
2022 |
|
$ |
570,000 |
|
2023 |
|
|
570,000 |
|
2024 |
|
|
570,000 |
|
2025 |
|
|
570,000 |
|
2026 |
|
|
570,000 |
|
Thereafter |
|
|
10,258,474 |
|
|
|
$ |
13,108,474 |
|
10. ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consist of the following at:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Accounts payable |
|
$ |
35,820,120 |
|
|
$ |
11,854,421 |
|
Promotional allowances |
|
|
19,036,986 |
|
|
|
5,561,063 |
|
Freight |
|
|
15,871,800 |
|
|
|
673,930 |
|
Accrued expenses |
|
|
15,311,390 |
|
|
|
7,282,906 |
|
Unbilled purchases |
|
|
5,438,316 |
|
|
|
40,433 |
|
Total |
|
$ |
91,478,612 |
|
|
$ |
25,412,753 |
|
11. OTHER LIABILITIES
Other current liabilities consist of the following at:
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
Other Liabilities-State Beverage Container Deposit |
|
$ |
976,072 |
|
|
$ |
425,232 |
|
Total |
|
$ |
976,072 |
|
|
$ |
425,232 |
|
12. RELATED PARTY TRANSACTIONS
The Company’s office is rented from a company affiliated with CD Financial, LLC which is controlled by one of our major stockholders. The current lease expires on January 2024 with monthly rent of $24,494.
13. STOCKHOLDERS’ EQUITY
Issuance of common stock pursuant to incentive stock plan exercises
During the year ended December 31, 2021, the Company issued an aggregate of 1,512,063 shares of its common stock pursuant to the exercise of stock options granted under the Company’s 2015 Stock Incentive Plan. The Company received aggregate proceeds of approximately $3.7 million for 972,491 options exercised for cash, with the balance of the options having been exercised on a "cashless” basis.
During the year ended December 31, 2020, the Company issued an aggregate of 1,883,609 shares of its common stock pursuant to the exercise of grants under the Company’s Stock Incentive Plans. The Company received aggregate proceeds of $4,126,791 for 1,316,050 cash exercises, with the balance of the grants having been exercised on a “cashless” basis.
June 2021 Public Offering
On June 9, 2021, the Company and certain selling stockholders (the "Selling Stockholders”) entered into an underwriting agreement (the "Underwriting Agreement”) with UBS Securities LLC and Jefferies LLC, as representatives (the "Representatives”) of the several underwriters (the "Underwriters”), relating to the sale of 6,518,267 shares of common stock, par value $0.001 per share, of the Company at a public offering price of $62.50 per share less underwriting discounts and commissions in a registered public offering (the "Offering”). The Company and certain Selling Stockholders also granted the Underwriters an option, exercisable for 30 days, to purchase up to an additional 977,740 shares of its Common Stock. The Underwriters partially exercised their option to purchase 873,141 shares of the Company’s Common Stock on June 11, 2021; 1,133,953 of which were sold by the Company and 739,188 of which were sold by certain of the Selling Stockholders. The Offering closed on June 14, 2021. The Company issued and sold 1,133,953 shares of Common Stock, and the Selling Stockholders sold 6,257,455 shares, in the aggregate, of Common Stock in the Offering. The Offering generated net proceeds for the Company of $67,769,386 and net proceeds for the Selling Stockholders of $375,447,300. The Company intends to use the proceeds for general corporate purposes. The Company did not receive any proceeds from the sale of shares by the Selling Stockholders.
The Underwriting Agreement contains customary representations and warranties of the parties, and indemnification and contribution provisions under which the Company and the Selling Stockholders have agreed to indemnify the Underwriters against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the "Securities Act”). Pursuant to the Underwriting Agreement, the Company has agreed, subject to certain exceptions, not to sell or transfer any shares of Common Stock or any securities convertible into or exercisable or exchangeable for Common Stock for 90 days after June 9, 2021 without first obtaining the written consent of the Representatives.
F-19
Issuance of common stock pursuant to private placement
On August 25, 2020 the Company issued 1,437,909 shares of its common stock and obtained approximately $22,000,000 of cash as part of a private placement.
Cash paid for taxes on restricted stock awards
On October 29, 2020, the CEO and CFO elected to have a portion of their vested restricted stock awards withheld by the Company, in order to cover their personal income taxes. This election was permitted by the underlying restricted stock award agreements that were provided to both the CEO and CFO. The total cash paid by the Company was $656,680, corresponding to the applicable statutory tax amounts required to be withheld. The payment was recorded as a reduction to stockholders’ equity, in accordance with ASC-505-30, Equity-Treasury Stock. On the consolidated statements of cash flows, the payment is presented as a cash outflow from financing activities.
14. INCOME TAXES
The domestic and foreign components of the Company's income before provision for income taxes are as follows:
|
|
2021 |
|
|
2020 |
|
||
Domestic |
|
$ |
(4,175,915 |
) |
|
$ |
8,110,620 |
|
Foreign |
|
|
117,468 |
|
|
|
529,406 |
|
Income (loss) before provision for income taxes |
|
$ |
(4,058,447 |
) |
|
$ |
8,640,026 |
|
The provision (benefit) for income taxes consists of the following:
Current: |
|
2021 |
|
|
2020 |
|
||
Domestic |
|
$ |
— |
|
|
$ |
|
|
State |
|
|
1,522,955 |
|
|
|
|
|
Foreign(1) |
|
|
(37,871 |
) |
|
|
116,177 |
|
|
|
$ |
1,485,084 |
|
|
$ |
116,177 |
|
(1)
Deferred |
|
|
|
|
|
|
||
Domestic |
|
$ |
(7,141,704 |
) |
|
$ |
|
|
State and local |
|
|
(1,877,538 |
) |
|
|
|
|
Foreign |
|
|
(461,562 |
) |
|
|
|
|
|
|
|
(9,480,804 |
) |
|
$ |
|
|
|
|
|
|
|
|
|
||
Total |
|
|
(7,995,720 |
) |
|
|
116,177 |
|
The reconciliation of the U.S. federal statutory rate to the Company's effective rate on income before provision (benefit) for income taxes is as follows:
|
|
2021 |
|
|
2020 |
|
||
U.S. Statutory federal rate |
|
|
% |
|
|
21.0 |
% |
|
State taxes, net of federal benefit |
|
|
(12.5 |
)% |
|
|
4.4 |
% |
Stock based compensation |
|
|
50.4 |
% |
|
|
6.7 |
% |
Change in valuation allowance |
|
|
219.8 |
% |
|
|
(29.8 |
)% |
Change in deferred balances |
|
|
(80.6 |
)% |
|
|
|
|
Other |
|
|
(0.9 |
)% |
|
|
0.0 |
% |
Effective tax rate |
|
|
197.2 |
% |
|
|
2.3 |
% |
The Tax Cuts and Jobs Act introduced a provision to tax global intangible low-taxed income ("GILTI") of foreign subsidiaries and a measure to tax certain intercompany payments under the base erosion anti-abuse tax "BEAT" regime. For the years ended December 31, 2021 and 2020, the Company did not generate intercompany transactions that met the BEAT threshold but does have to include GILTI relating to the Company's foreign subsidiaries. The Company elected to account for GILTI as a current period cost.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts for income tax purposes. Deferred tax assets and liabilities consisted of the following:
F-20
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
6,685,036 |
|
|
$ |
12,051,503 |
|
Charitable Contributions |
|
|
17,339 |
|
|
|
— |
|
Fixed Assets |
|
|
(627,075 |
) |
|
|
(50,411 |
) |
Right of Use Liability |
|
|
134,102 |
|
|
|
— |
|
Right of Use Asset |
|
|
(121,895 |
) |
|
|
|
|
FIN48 |
|
|
130,298 |
|
|
|
|
|
Stock-based compensation |
|
|
6,190,115 |
|
|
|
389,816 |
|
Inventory allowance |
|
|
800,443 |
|
|
|
548,118 |
|
Intangibles |
|
|
(3,316,817 |
) |
|
|
— |
|
Total deferred tax assets |
|
|
9,891,546 |
|
|
|
12,939,026 |
|
|
|
|
|
|
|
|
||
Valuation allowance |
|
|
(4,018,699 |
) |
|
|
(12,939,026 |
) |
|
|
|
|
|
|
|
||
Net Deferred Tax Assets |
|
|
5,872,847 |
|
|
|
— |
|
At December 31, 2021, the Company has approximately $9.6 million of Federal net operating loss carryforwards and $14.9 million of state net operating loss carryforwards, which will begin to expire in 2031. The Federal and State NOLs are subject to limitation under Section 382 due to a December 2008 ownership change of greater than 50% over a three-year testing period. The ownership change resulted in approximately $4.5 million of NOLs that will not be utilized prior to expiration. The $4.5 million has been removed from the available NOL carryforward and US NOL deferred tax asset. The Company had foreign NOL carryforwards of approximately $22.4 million, some of which will begin to expire in 2023.
At December 31, 2021, undistributed earnings, as well as outside basis differences, of our foreign subsidiaries was determined to be immaterial and such earnings are considered to be indefinitely reinvested. Accordingly, no taxes have been provided thereon. Determination of the amount of unrecognized deferred tax liability is not practicable due to the complexities associated with this hypothetical calculation.
As required by the authoritative guidance on accounting for income taxes. the Company evaluates the realizability of deferred tax assets on a jurisdictional basis at each reporting date. Accounting for income taxes requires that a valuation allowance be established when it is more likely than not that all or a portion of the deferred taxes will not be realized. In circumstances where there is sufficient negative evidence indicating that the deferred tax assets are not more likely than not realizable, the Company establishes a valuation allowance. Through the year ended December 31, 2020, the Company has maintained a full valuation allowance on its worldwide net deferred tax assets. During the fourth quarter of 2021, the Company concluded that it is more likely than not that its US deferred tax assets would be realized. This conclusion was based on the US profitability and NOL utilization in 2021 and 2020 as well as future forecasts of US profitability. For the year ended December 31, 2021, the Company released its US valuation allowance for deferred tax assets of approximately $6.0 million. The Company continues to maintain a valuation allowance on its foreign net operating losses as it is not more likely than not that the losses in those specific jurisdictions will be realized.
A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows:
|
|
2021 |
|
|
2020 |
|
||
Gross unrecognized tax benefit, beginning of period |
|
$ |
81,500 |
|
|
$ |
|
|
Additions based on tax positions related to the current year |
|
|
|
|
|
81,500 |
|
|
Additions based on tax positions related to the prior years |
|
|
998,691 |
|
|
|
|
|
Reductions due to lapse in statute of limitations and settlements |
|
|
— |
|
|
|
— |
|
Gross unrecognized tax benefit, end of period |
|
$ |
1,080,191 |
|
|
$ |
81,500 |
|
The Company recognizes only those tax positions that meet the more-likely-than-not recognition threshold and establishes tax reserves for uncertain tax positions that do not meet this threshold. To the extent these unrecognized tax benefits are ultimately recognized, approximately $1.1 million will impact the Company's effective tax rate in future periods. Unrecognized tax benefits are likely to decrease by approximately $0.8 million, inclusive of interest and penalties, within the next twelve months as a result of the Company's intention to remediate certain income tax positions via voluntary disclosure. Interest and penalties associated with income tax matters are included in the provision for income taxes. As of December 31, 2021, the Company had uncertain tax positions of approximately $1.3 million, inclusive of $0.2 million of interest and penalties.
The Company files U.S., state, and foreign income tax returns in jurisdictions with various statutes of limitations. Below is a summary of the filing jurisdictions and open tax years:
|
|
Open Years |
U.S. Federal |
|
2018-2020 |
U.S State and local |
|
2017-2020 |
Non-U.S. |
|
2015-2020 |
15. STOCK-BASED COMPENSATION
F-21
The Company adopted an Incentive Stock Plan on January 18, 2007. This plan is intended to provide incentives which will attract and retain highly competent persons at all levels as employees of the Company, as well as independent contractors providing consulting or advisory services to the Company, by providing them opportunities to acquire the Company’s common stock. While the plan terminates 10 years after the adoption date, issued options have their own schedule of termination. During 2013, the majority of the stockholders approved to increase the total available shares in the plan from 2.5 million to 3.5 million shares of common stock. During May 2014, the majority of the stockholders approved to increase the total available shares in the plan from 3.5 million to 4.25 million shares of common stock, during February 2015, the majority of the stockholders approved to increase the total available shares in the plan from 4.25 million to 4.6 million shares of common stock and during April 2015, the majority of the stockholders approved to increase the total available shares in the plan from 4.6 million to 5.1 million shares of common stock. Upon exercise, shares of new common stock are issued by the Company.
The Company adopted the 2015 Stock Incentive Plan on April 30, 2015. This plan is intended to provide incentives which will attract and retain highly competent persons at all levels as employees of the Company, as well as independent contractors providing consulting or advisory services to the Company, by providing them opportunities to acquire the Company’s common stock or to receive monetary payments based on the value of such shares pursuant to Awards issued. The 2015 Plan permits the grant of options and shares for up to 5,000,000 shares. In addition, there is a provision for an annual increase of 15% of the shares pertaining to the 2015 plan that are outstanding as of the last day of the prior year. As of December 31, 2021, approximately 4.3 million shares are available.
Under the 2015 Stock Incentive Plan, the Company has issued options to purchase approximately 3.6 million shares at an average price of $7.56 with a fair value of $241.5 million. For the years ended December 31, 2021 and 2020, the Company issued options to purchase 304,750 and 620,535 shares, respectively. Upon exercise, shares of new common stock are issued by the Company.
For the years ended December 31, 2021 and 2020, the Company recognized an expense of approximately $36.5 million and $6.3 million, respectively, of non-cash compensation expense (included in General and Administrative expense in the accompanying Consolidated Statement of Operations) determined by application of a Black-Scholes option pricing model with the following inputs: exercise price, dividend yields, risk-free interest rate, and expected annual volatility. As of December 31, 2021, the Company had approximately $7.7 million of unrecognized pre-tax non-cash compensation expense related to options to purchase shares, which the Company expects to recognize, based on a weighted-average period of 1.9 years. The Company used straight-line amortization of compensation expense over the to three-year requisite service or vesting period of the grant. The maximum contractual term of the Company's stock options is 10 years. The Company recognizes forfeitures as they occur. There are options to purchase approximately 2.31 million shares that have vested as of December 31, 2021.
The Company uses the Black-Scholes option-pricing model to estimate the fair value of its stock option awards and warrant issuances. The calculation of the fair value of the awards using the Black-Scholes option-pricing model is affected by the Company’s stock price on the date of grant as well as assumptions regarding the following:
|
|
Year ended December 31, |
||
|
|
2021 |
|
2020 |
Expected volatility |
|
69.2% - 81.1% |
|
69.2-81.1% |
Expected term |
|
4.5 - 5.0 Years |
|
4.8-5.0 Years |
Risk-free interest rate |
|
0.3% - 1.4% |
|
0.2% - 1.4% |
Forfeiture Rate |
|
0.0% |
|
0.0% |
The expected volatility was determined with reference to the historical volatility of the Company’s stock. The Company uses historical data to estimate option exercise and employee termination within the valuation model. The expected term of options granted represents the period of time that options granted are expected to be outstanding. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury rate in effect at the time of grant.
A summary of the status of the Company’s outstanding stock options as of December 31, 2021 and 2020 and changes during the periods ending on that date is as follows:
|
|
|
|
|
Weighted Average |
|
|
Aggregate |
|
|
Weighted |
|
||||||||
|
|
Shares |
|
|
Exercise |
|
|
Grant Date |
|
|
Intrinsic |
|
|
Average |
|
|||||
|
|
(000’s) |
|
|
Price |
|
|
Value |
|
|
(000’s) |
|
|
Term (Yrs) |
|
|||||
Options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2019 |
|
|
6,528 |
|
|
$ |
3.54 |
|
|
|
|
|
$ |
8,978 |
|
|
|
6.58 |
|
|
Granted |
|
|
575 |
|
|
|
7.14 |
|
|
$ |
4.66 |
|
|
|
|
|
|
|
||
Exercised |
|
|
(1,806 |
) |
|
|
2.83 |
|
|
$ |
20.27 |
|
|
|
31,480 |
|
|
|
|
|
Forfeiture and cancelled |
|
|
(99 |
) |
|
|
3.93 |
|
|
|
|
|
|
|
|
|
|
|||
At December 31, 2020 |
|
|
5,198 |
|
|
$ |
4.23 |
|
|
|
|
|
$ |
240,866 |
|
|
|
6.89 |
|
|
Granted |
|
|
305 |
|
|
$ |
42.37 |
|
|
$ |
30.32 |
|
|
|
|
|
|
|
||
Exercised |
|
|
(1,460 |
) |
|
|
3.85 |
|
|
$ |
80.58 |
|
|
|
84,371 |
|
|
|
|
|
Forfeiture and cancelled |
|
|
(443 |
) |
|
|
5.01 |
|
|
|
|
|
|
|
|
|
|
|||
At December 31, 2021 |
|
|
3,600 |
|
|
$ |
7.47 |
|
|
|
|
|
$ |
241,515 |
|
|
|
6.37 |
|
|
Exercisable at December 31, 2021 |
|
|
2,314 |
|
|
$ |
4.06 |
|
|
|
|
|
$ |
163,151 |
|
|
|
5.49 |
|
|
The following table summarizes information about employee stock options outstanding at December 31, 2021:
F-22
|
|
Outstanding Options |
|
|
Vested Options |
|
||||||||||||||||||
|
|
Number |
|
|
|
|
|
|
|
|
Number |
|
|
|
|
|
|
|
||||||
|
|
Outstanding |
|
|
Weighted |
|
|
Weighted |
|
|
Exercisable |
|
|
Weighted |
|
|
Weighted |
|
||||||
|
|
at |
|
|
Averaged |
|
|
Averaged |
|
|
at |
|
|
Averaged |
|
|
Averaged |
|
||||||
|
|
December 31, |
|
|
Remaining |
|
|
Exercise |
|
|
December 31, |
|
|
Exercise |
|
|
Remaining |
|
||||||
Range of Exercise Price |
|
2021 (000’s) |
|
|
Life |
|
|
Price |
|
|
2021 (000’s) |
|
|
Price |
|
|
Life |
|
||||||
$0.20 - $0.53 |
|
|
20 |
|
|
|
2.08 |
|
|
$ |
0.34 |
|
|
|
20 |
|
|
$ |
0.34 |
|
|
|
2.08 |
|
$0.65 - $1.80 |
|
|
100 |
|
|
|
3.15 |
|
|
$ |
1.05 |
|
|
|
100 |
|
|
$ |
1.05 |
|
|
|
3.15 |
|
$1.83 - $2.84 |
|
|
107 |
|
|
|
4.02 |
|
|
$ |
1.97 |
|
|
|
107 |
|
|
$ |
1.97 |
|
|
|
4.02 |
|
$3.20 - $6.20 |
|
|
2,993 |
|
|
|
6.27 |
|
|
$ |
4.16 |
|
|
|
2,062 |
|
|
$ |
4.21 |
|
|
|
5.68 |
|
$7.20-$60.00 |
|
|
380 |
|
|
|
8.93 |
|
|
$ |
37.15 |
|
|
|
25 |
|
|
$ |
15.93 |
|
|
|
8.63 |
|
Outstanding options |
|
|
3,600 |
|
|
|
6.37 |
|
|
$ |
7.47 |
|
|
|
2,314 |
|
|
$ |
4.06 |
|
|
|
5.49 |
|
As of December 31, 2021, the Company had approximately $7.7 million of unrecognized pre-tax non-cash compensation expense related to options to purchase shares, which the Company expects to recognize, based on a weighted-average period of 1.9 years.
Restricted Stock Awards
Restricted stock awards are awards of common stock that are subject to restrictions on transfer and to a risk of forfeiture if the holder leaves the Company before the restrictions lapse. The holders of a restricted stock award are generally entitled after the release to transact and obtain the same rights as rights of a stockholder of the Company, including the right to vote the shares. The holders of unvested restricted stock awards do not have the same rights as stockholders including but not limited to any dividends which may be declared by the Company, and do not have the right to vote. The value of restricted stock awards that vest over time is established by the market price on the date of its grant and generally vests over a period of 3 years. A summary of the Company’s restricted stock activity for the years ended December 31, 2021 and 2020 is presented in the following table:
|
|
For the twelve months ended |
|
|||||||||||||
|
|
December 31, 2021 |
December 31, 2020 |
|
||||||||||||
|
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
||||
|
|
|
|
|
Average |
|
|
|
|
|
Average |
|
||||
|
|
|
|
|
Grant Date |
|
|
|
|
|
Grant Date |
|
||||
|
|
Shares |
|
|
Fair Value |
|
|
Shares |
|
|
Fair Value |
|
||||
Unvested at beginning of period |
|
|
66,229 |
|
|
$ |
28.11 |
|
|
|
90,000 |
|
|
$ |
3.23 |
|
Transfers to restricted stock units |
|
|
(45,871 |
) |
|
|
34.02 |
|
|
|
— |
|
|
|
— |
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
175,737 |
|
|
|
17.46 |
|
Vested |
|
|
(18,913 |
) |
|
|
14.79 |
|
|
|
(170,145 |
) |
|
|
8.08 |
|
Forfeited and cancelled |
|
|
1,187 |
|
|
|
14.72 |
|
|
|
(29,363 |
) |
|
|
4.15 |
|
Unvested at end of period |
|
|
258 |
|
|
$ |
14.72 |
|
|
|
66,229 |
|
|
$ |
28.11 |
|
The total fair value of shares vested during the year ended December 31, 2021 and 2020 was approximately $1.3 million and $2.9 million, respectively. Unrecognized compensation expense related to outstanding restricted stock awards to employees and directors as of December 31, 2021 was approximately $1,096 and is expected to be expensed over the next 0.6 years. Unrecognized compensation expense related to outstanding restricted stock awards to employees and directors as of December 31, 2020 was $0.2 million.
Restricted Stock Units
Restricted stock units are awards that give the holder the right to receive one share of common stock for each restricted stock unit upon meeting service-based vesting conditions (typically annual vesting in three equal annual installments, with a requirement that the holder remains in the continuous employment of the Company). The holders of unvested units do not have the same rights as stockholders including but not limited to any dividends which may be declared by the Company, and do not have the right to vote. The value of restricted stock units that vest over time is established by the market price on the date of its grant. A summary of the Company’s restricted stock unit activity for the year ended December 31, 2021 and 2020 is presented in the following table:
|
|
For the twelve months ended |
|
|||||||||||||
|
|
December 31, 2021 |
December 31, 2020 |
|
||||||||||||
|
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
||||
|
|
|
|
|
Average |
|
|
|
|
|
Average |
|
||||
|
|
|
|
|
Grant Date |
|
|
|
|
|
Grant Date |
|
||||
|
|
Shares |
|
|
Fair Value |
|
|
Shares |
|
|
Fair Value |
|
||||
Unvested at beginning of period |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
Transfers to restricted stock awards |
|
|
45,871 |
|
|
|
34.02 |
|
|
|
— |
|
|
|
— |
|
Granted |
|
|
573,428 |
|
|
|
54.40 |
|
|
|
— |
|
|
|
— |
|
Vested |
|
|
(18,758 |
) |
|
|
64.58 |
|
|
|
— |
|
|
|
— |
|
Forfeited and cancelled |
|
|
(35,000 |
) |
|
|
50.46 |
|
|
|
— |
|
|
|
— |
|
Unvested at end of period |
|
|
565,541 |
|
|
$ |
52.66 |
|
|
$ |
— |
|
|
$ |
— |
|
F-23
The total fair value of shares vested during the year ended December 31, 2021 was approximately $1.4 million. Unrecognized compensation expense related to outstanding restricted stock units to employees and directors as of December 31, 2021 was approximately $19.8 million and is expected to be expensed over the next 2.4 years.
Stock-based Awards Issued to Non-employee Consultants
The Company issues stock-based awards to third-party consultants for providing marketing, sales, and general business development services related to Celsius products. The stock-based awards are in the form of restricted stock units with performance vesting conditions (“performance stock units” or “PSUs”). The holders of unvested PSUs do not have the same rights as stockholders including but not limited to any dividends which may be declared by the Company, and do not have the right to vote. The PSU performance vesting conditions are linked to the consultants obtaining specified incremental earnings for the Company in a given year over the performance vesting period, typically five years. The fair value of PSUs is based on the market price of the underlying stock on the grant date. The Company recognizes compensation cost for performance stock awards issued to non-employees in the same manner and periods as though cash had been paid for services received. A summary of the Company’s PSU activity for the years ended December 31, 2021 and 2020 is presented in the following table:
|
|
For the twelve months ended |
|
|||||||||||||
|
|
December 31, 2021 |
December 31, 2020 |
|
||||||||||||
|
|
|
|
|
Weighted |
|
|
|
|
|
Weighted |
|
||||
|
|
|
|
|
Average |
|
|
|
|
|
Average |
|
||||
|
|
|
|
|
Grant Date |
|
|
|
|
|
Grant Date |
|
||||
|
|
Shares |
|
|
Fair Value |
|
|
Shares |
|
|
Fair Value |
|
||||
Unvested at beginning of period |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
Granted |
|
|
15,468 |
|
|
|
64.65 |
|
|
|
— |
|
|
|
— |
|
Vested |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Forfeited and cancelled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Unvested at end of period |
|
|
15,468 |
|
|
$ |
64.65 |
|
|
$ |
— |
|
|
$ |
— |
|
Unrecognized compensation expense related to outstanding PSUs issued to non-employee consultants as of December 31, 2021 was approximately $0.9 million and is expected to be expensed over the next 4.6 years.
Modifications
There were certain Board of Directors members and employees whose service was terminated during the year. In connection with their terminations, the vesting conditions of the previously granted awards were modified to accelerate the vesting of specified un-vested awards pursuant to Board resolutions or severance agreements. Pursuant ASC 718, these were Type III modifications requiring re-valuation of un-vested awards to modification date fair value with recognition of compensation expense over the remaining service period. The Company modified awards for nine grantees resulting in approximately $19.3 million in incremental compensation cost during the year ended December 31, 2021. See Note 2 and Unaudited Supplementary Information for the impact of these modifications on the previously reported unaudited 2021 quarterly consolidated statements of operations and comprehensive income (loss) for the periods ending June 30, 2021 and September 30, 2021.
16. COMMITMENTS AND CONTINGENCIES
In November of 2020, McGovern Capital, Inc. and Kevin McGovern (collectively "McGovern”) filed a claim in arbitration related to its Representative Agreement with Celsius Holdings, Inc. as amended by the first amendment dated August 6, 2016. Pursuant to the Representative Agreement, McGovern is entitled to receive a fee of three percent (3%) of "Net Revenues” received by the Company from sales of the Company’s Products in the People’s Republic of China for a period of four years from Initial Commercial Sale (which was September 1, 2017). "Net Revenues” are defined in the Representative Agreement as "the Company’s revenues net of actual discounts applied, credits and returns.” Effective January 1, 2019, the Company restructured its China operations from a distribution arrangement with Qifeng Food Technology (Beijing) Co. Ltd. ("Qifeng”), to a license and royalty arrangement and a note, pursuant to which Qifeng will market and distribute the Company’s products in China, and Celsius will receive an annual royalty payment. The Company intends to pay McGovern its percentage of the annual royalty payment, but McGovern has objected claiming that McGovern is entitled to be paid commissions on the entire royalty payment and the amount of the loan to Qifeng. The Company intends to defend against McGovern’s claims vigorously and has filed a counterclaim related to McGovern’s failure to comply with the covenant of good faith and fair dealing in the Representative Agreement. At this stage in the matter, the Company has fully participated in the producing all documents as required by the discovery process and continues to defend itself vigorously. Based on the current status the Company is unable to predict the outcome at this time.
In March of 2019, Daniel Prescod filed a putative class action lawsuit against the Company in the Superior Court for the State of California, County of Los Angeles, Case Number 19STCV09321, filed on March 19, 2019, (the "Prescod Litigation”). Daniel Prescod asserts that the Company’s use of citric acid in its products while simultaneously claiming "no preservatives” violates California Consumer Legal Remedies Act, California Business and Professions Code Section 17200, et seq., and California Business and Professions Code Section 17500, et seq., because citric acid acts as a preservative. The Company does not use citric acid as a preservative in its products, but rather as a flavoring, and therefore it believes that its "no preservatives” claim is fair and not deceptive. A motion to certify the case as a class action was filed and on August 2, 2021, that motion was granted. However, the Company also has a motion for summary adjudication pending and that motion would be dispositive of plaintiff’s claims if granted. No fact discovery has been conducted on the merits and this matter is still in its initial stages. The Company intends to contest the claims vigorously on the merits. Since merits discovery is still in its initial stages, we are unable to predict the outcome at this time.
On November 23, 2021, a case related to the Prescod Litigation, Amit Heli and Joseph Nina v. Celsius Holdings, was filed in the United States District Court for the Southern District of New York, Case No. 1:21-cv-09892. Like the Prescod Litigation, the plaintiffs in this case allege that the Company’s use of citric
F-24
acid in its products while simultaneously claiming “no preservatives” constitutes false advertising and unfair or deceptive trade practices. Unlike the Prescod Litigation, in this case the violations alleged are of New York’s General Business Law. Celsius answered the complaint on February 11, 2022. As with the Prescod Litigation, the Company does not use citric acid as a preservative in its products, but rather as a flavoring, and therefore it believes that its “no preservatives” claim is fair and not deceptive. No discovery has been conducted. and this matter is still in its initial stages. The Company intends to contest the claims vigorously on the merits. As a result, we are unable to predict the outcome at this time.
On January 8, 2021, we received a letter from the SEC Division of Enforcement seeking the production of documents in connection with a non-public fact-finding inquiry by the SEC to determine whether violations of the federal securities laws have occurred. On August 20, 2021, the SEC issued a subpoena for production of documents in connection with the matter. Neither the January 8, 2021 SEC letter nor the August 20, 2021 subpoena means that the SEC has concluded that the Company or anyone else has violated the federal securities laws. We have cooperated and will continue to cooperate with the SEC staff in its investigation. At this time, however, we cannot predict the length, scope, or results of the investigation or the impact, if any, of the investigation on our results of operations.
In addition to the foregoing, from time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business.
The Company has entered into distribution agreements with liquidated damages in case the Company cancels the distribution agreements without Cause. Cause has been defined in various ways. It is management’s belief that no such agreement has created any liability as of December 31, 2021.
Additionally, our business and results of operations may be adversely affected by the pandemic and public health crises related to the COVID-19 outbreak which is affecting the macro-economic environment.
17. SUBSEQUENT EVENTS
None
F-25
Celsius Holdings, Inc.
UNAUDITED SUPPLEMENTARY INFORMATION
December 31, 2021 and 2020
Selected Quarterly Financial Results
Subsequent to filing the Company’s Quarterly Reports on Form 10-Q for the periods ended June 30, 2021, and September 30, 2021, the Company determined that certain amounts reported in the Company's previously issued unaudited consolidated statements of operations and comprehensive income (loss) and consolidated balance sheets contained misstatements (see Note 2 for further details). In accordance with Staff Accounting Bulletin No. 99, Materiality, management evaluated the materiality of the misstatements from a qualitative and quantitative perspective and concluded that the misstatements were material to the three- and six- months ended June 30, 2021 and the three- and nine- months ended September 30, 2021 interim consolidated financial statements. Accordingly, the Company has restated its interim consolidated financial statements for the three- and six- months ended June 30, 2021, and three- and nine- months ended September 30, 2021, respectively.
The effects of the adjustments to the Company's previously reported unaudited 2021 quarterly consolidated statements of operations and comprehensive income (loss) on a standalone quarter basis are as follows:
|
As Reported |
|
|
Adjustments |
|
|
As Restated |
|
|||||||||||||||
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Revenue |
$ |
65,073,323 |
|
|
$ |
94,909,100 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
65,073,323 |
|
|
$ |
94,909,100 |
|
Gross Profit |
|
28,249,369 |
|
|
|
37,693,372 |
|
|
|
— |
|
|
|
— |
|
|
|
28,249,369 |
|
|
|
37,693,372 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
General and administrative expenses1 |
|
9,119,532 |
|
|
|
11,140,030 |
|
|
|
3,180,353 |
|
|
|
12,116,438 |
|
|
|
12,299,885 |
|
|
|
23,256,468 |
|
Total operating expense |
|
24,650,520 |
|
|
|
33,761,092 |
|
|
|
3,180,353 |
|
|
|
12,116,438 |
|
|
|
27,830,873 |
|
|
|
45,877,530 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Income (loss) from operations |
|
3,598,849 |
|
|
|
3,932,280 |
|
|
|
(3,180,353 |
) |
|
|
(12,116,438 |
) |
|
|
418,496 |
|
|
|
(8,184,158 |
) |
Net income (loss) before income taxes |
|
3,960,344 |
|
|
|
3,579,610 |
|
|
|
(3,180,353 |
) |
|
|
(12,116,438 |
) |
|
|
779,991 |
|
|
|
(8,536,828 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net income (loss) |
$ |
3,960,344 |
|
|
$ |
2,745,791 |
|
|
$ |
(3,180,353 |
) |
|
$ |
(12,116,438 |
) |
|
$ |
779,991 |
|
|
$ |
(9,370,647 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Basic |
$ |
0.05 |
|
|
$ |
0.04 |
|
|
$ |
(0.04 |
) |
|
$ |
(0.17 |
) |
|
$ |
0.01 |
|
|
$ |
(0.13 |
) |
Diluted |
$ |
0.05 |
|
|
$ |
0.03 |
|
|
$ |
(0.04 |
) |
|
$ |
(0.15 |
) |
|
$ |
0.01 |
|
|
$ |
(0.12 |
) |
1
The effects of the adjustments to the Company's previously reported unaudited 2021 quarterly consolidated statements of operations and comprehensive income (loss) on a year-to-date basis are as follows:
|
As Reported |
|
|
Adjustments |
|
|
As Restated |
|
|||||||||||||||
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Revenue |
$ |
115,108,202 |
|
|
$ |
210,217,302 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
115,108,202 |
|
|
$ |
210,217,302 |
|
Gross Profit |
|
48,828,464 |
|
|
|
123,495,466 |
|
|
|
— |
|
|
|
— |
|
|
|
48,828,464 |
|
|
|
123,495,466 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
General and administrative expenses1 |
$ |
16,926,198 |
|
|
$ |
28,066,228 |
|
|
$ |
3,180,353 |
|
|
$ |
15,296,791 |
|
|
$ |
20,106,551 |
|
|
$ |
43,363,019 |
|
Total operating expense |
|
44,416,239 |
|
|
|
78,177,331 |
|
|
|
3,180,353 |
|
|
|
15,296,791 |
|
|
|
47,596,592 |
|
|
|
93,474,122 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Income (loss) from operations |
|
4,412,225 |
|
|
|
8,344,505 |
|
|
|
(3,180,353 |
) |
|
|
(15,296,791 |
) |
|
|
1,231,872 |
|
|
|
(6,952,286 |
) |
Net income (loss) before income taxes |
|
4,545,768 |
|
|
|
8,215,378 |
|
|
|
(3,180,353 |
) |
|
|
(15,296,791 |
) |
|
|
1,365,415 |
|
|
|
(7,171,413 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net income (loss) |
$ |
4,545,768 |
|
|
$ |
7,251,559 |
|
|
$ |
(3,180,353 |
) |
|
$ |
(15,296,791 |
) |
|
$ |
1,365,415 |
|
|
$ |
(8,005,232 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Basic |
$ |
0.06 |
|
|
$ |
0.10 |
|
|
$ |
(0.04 |
) |
|
$ |
(0.21 |
) |
|
$ |
0.02 |
|
|
$ |
(0.11 |
) |
Diluted |
$ |
0.06 |
|
|
$ |
0.09 |
|
|
$ |
(0.04 |
) |
|
$ |
(0.19 |
) |
|
$ |
0.02 |
|
|
$ |
(0.10 |
) |
1
The effects of the adjustments to the Company's previously reported unaudited 2021 quarterly consolidated balance sheets are as follows:
F-26
|
As Reported |
|
|
Adjustments |
|
|
As Restated |
|
|||||||||||||||
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Current assets |
$ |
205,305,787 |
|
|
$ |
252,561,960 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
205,305,787 |
|
|
$ |
252,561,960 |
|
Total assets |
|
241,548,125 |
|
|
|
294,978,175 |
|
|
|
— |
|
|
|
— |
|
|
|
241,548,125 |
|
|
|
294,978,175 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Current liabilities |
$ |
54,256,877 |
|
|
$ |
93,421,330 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
54,256,877 |
|
|
$ |
93,421,330 |
|
Total liabilities |
|
54,708,765 |
|
|
|
97,579,964 |
|
|
|
— |
|
|
|
— |
|
|
|
54,708,765 |
|
|
|
97,579,964 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Additional paid-in capital |
$ |
237,763,609 |
|
|
$ |
244,293,710 |
|
|
$ |
3,180,353 |
|
|
$ |
15,296,791 |
|
|
$ |
240,943,962 |
|
|
$ |
259,590,501 |
|
Accumulated deficit |
|
(50,881,064 |
) |
|
|
(48,135,273 |
) |
|
|
(3,180,353 |
) |
|
|
(15,296,791 |
) |
|
|
(54,061,417 |
) |
|
|
(63,432,064 |
) |
Total Stockholder's Equity |
$ |
186,839,360 |
|
|
$ |
197,398,211 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
186,839,360 |
|
|
$ |
197,398,211 |
|
These corrections had no effect on the Company's previously reported net cash flows from operating activities, investing activities or financing activities for the six months ended June 30, 2021, and the nine months ended September 30, 2021, respectively.
F-27
Similar companies
See also Monster Beverage Corp - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also MEXICAN ECONOMIC DEVELOPMENT INC
See also COCA-COLA EUROPACIFIC PARTNERS plc
See also COCA COLA FEMSA SAB DE CV
See also Coca-Cola Consolidated, Inc. - Annual report 2023 (10-K 2023-12-31) Annual report 2023 (10-Q 2023-09-29)
