Cogent Biosciences, Inc. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2022
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-38443
COGENT BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
Delaware |
46-5308248 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
|
|
275 Wyman Street, 3rd Floor Waltham, Massachusetts |
02451 |
(Address of principal executive offices) |
(Zip Code) |
(617) 945-5576
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol |
|
Name of exchange on which registered |
Common Stock, $0.001 Par Value |
|
COGT |
|
The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☒ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing
reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by
any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of Common Stock held by non-affiliates of the registrant computed by reference to the price of the registrant’s Common Stock as of June 30, 2022, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $567.8 million (based on the last reported sale price on the Nasdaq Global Select Market as of such date).
As of March 10, 2023, there were 69,946,790 shares of the registrant’s Common Stock, $0.001 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The information required by Part III of this report, to the extent not set forth herein, is incorporated herein by reference from our definitive proxy statement relating to the 2023 Annual Meeting of Stockholders, which definitive proxy statement shall be filed with the Securities and Exchange Commission within 120 days after the end of the annual period to which this report relates.
Cogent Biosciences, Inc.
Index
|
|
|
Page |
PART I4 |
|
||
|
|
|
|
Item 1. |
|
6 |
|
Item 1A. |
|
38 |
|
Item 1B. |
|
53 |
|
Item 2. |
|
53 |
|
Item 3. |
|
53 |
|
Item 4. |
|
53 |
|
|
|
||
PART II |
|
||
|
|
|
|
Item 5. |
|
54 |
|
Item 6. |
|
54 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
55 |
Item 7A. |
|
66 |
|
Item 8. |
|
67 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
91 |
Item 9A. |
|
91 |
|
Item 9B. |
|
92 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
|
92 |
|
|
||
PART III |
|
||
|
|
||
Item 10. |
|
93 |
|
Item 11. |
|
93 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
93 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
|
93 |
Item 14. |
|
93 |
|
|
|
||
PART IV |
|
||
|
|
|
|
Item 15. |
|
94 |
|
Item 16. |
|
96 |
|
94 |
|||
97 |
|||
2
Summary of the Material Risks Associated with Our Business
Our business is subject to numerous risks and uncertainties that you should be aware of in evaluating our business. These risks include, but are not limited to, the following:
The summary risk factors described above should be read together with the text of the full risk factors in Item 1A. “Risk Factors” and the other information set forth in this Annual Report on Form 10-K, including our consolidated financial statements and the related notes, as well as in other documents that we file with the SEC. The risks summarized above or described in full below are not the only risks that we face. Additional risks and uncertainties not precisely known to us, or that we currently deem to be immaterial may also materially adversely affect our business, financial condition, results of operations and future growth prospects.
3
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements, which reflect our current views with respect to, among other things, our operations and financial performance. All statements other than statements of historical facts contained in this Annual Report on Form 10-K, including statements regarding our future results of operations and financial position, business strategy and plans, and objectives of management for future operations, are forward-looking statements. These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “should,” “expects,” “might,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,” “seek,” “would” or “continue,” or the negative of these terms or other similar expressions. The forward-looking statements in this Annual Report on Form 10-K are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K and are subject to a number of risks, uncertainties and assumptions described in the “Risk Factors” section and elsewhere in this Annual Report on Form 10-K. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Some of the key factors that could cause actual results to differ from our expectations include:
4
While we may elect to update these forward-looking statements at some point in the future, whether as a result of any new information, future events, or otherwise, we have no current intention of doing so except to the extent required by applicable law.
5
PART I
Unless the context otherwise requires, we use the terms “Cogent,” “company,” “we,” “us,” and “our” to refer to Cogent Biosciences, Inc. and, where appropriate, our subsidiaries.
ITEM 1. BUSINESS
Overview
We are a biotechnology company focused on developing precision therapies for genetically defined diseases. Our approach is to design rational precision therapies that treat the underlying cause of disease and improve the lives of patients. Our most advanced program is bezuclastinib (also known as CGT9486), a selective tyrosine kinase inhibitor designed to target exon 17 mutations found within the KIT receptor tyrosine kinase, including KIT D816V. When KIT D816V remains in a perpetual ‘on’ state it causes mast cells, a type of white blood cell, to accumulate in various internal organs including the bone marrow. This mast cell accumulation results in an orphan disease called Systemic Mastocytosis (“SM”). Exon 17 mutations have also been found in advanced Gastrointestinal Stromal Tumors (“GIST”), a type of cancer with strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a new treatment option for these patient populations. In addition to bezuclastinib, the Cogent Research Team is developing a portfolio of novel targeted therapies to help patients fighting serious, genetically driven diseases, and is initially targeting FGFR2 and ErbB2.
We have assembled a management team with extensive experience in the research, development, manufacturing and commercialization of pharmaceutical products, specifically including numerous successful precision medicines for genetically defined diseases. With the support of our board of directors and their expertise, we believe that the Company is well positioned to develop and commercialize novel precision medicines. Beginning with bezuclastinib, our mission is to develop and commercialize pharmaceutical products that improve the lives of patients fighting rare, genetically driven diseases.
Our Strategy
Our vision is to discover, develop, and commercialize best-in-class therapies that have a meaningful impact for patients with genetically defined diseases. The principal components of our strategy include:
6
Our Pipeline

Bezuclastinib Overview
Bezuclastinib is designed to target mutations within the KIT receptor tyrosine kinase, including KIT D816V. As a Type I inhibitor, bezuclastinib is designed to selectively bind the active conformation of mutant KIT. In preclinical studies, bezuclastinib has demonstrated comparable potency relative to other FDA-approved KIT mutant inhibitors, and clear selectivity for KIT mutations versus other kinase targets frequently associated with other KIT inhibitors including, but not limited to, FLT3, VEGFR, PDGFRα and CSF1R. In preclinical studies of bezuclastinib, limited blood-brain-barrier penetration was observed, and there have been no clinically significant CNS toxicities identified either preclinically or clinically. This preclinical profile of selectivity against kinases that have been associated with off-target toxicities and limited blood-brain-barrier penetration differentiate bezuclastinib from other KIT mutant inhibitors, and support the potential for a best-in-class clinical profile. The figures below provide a summary of potency and selectivity preclinical data.
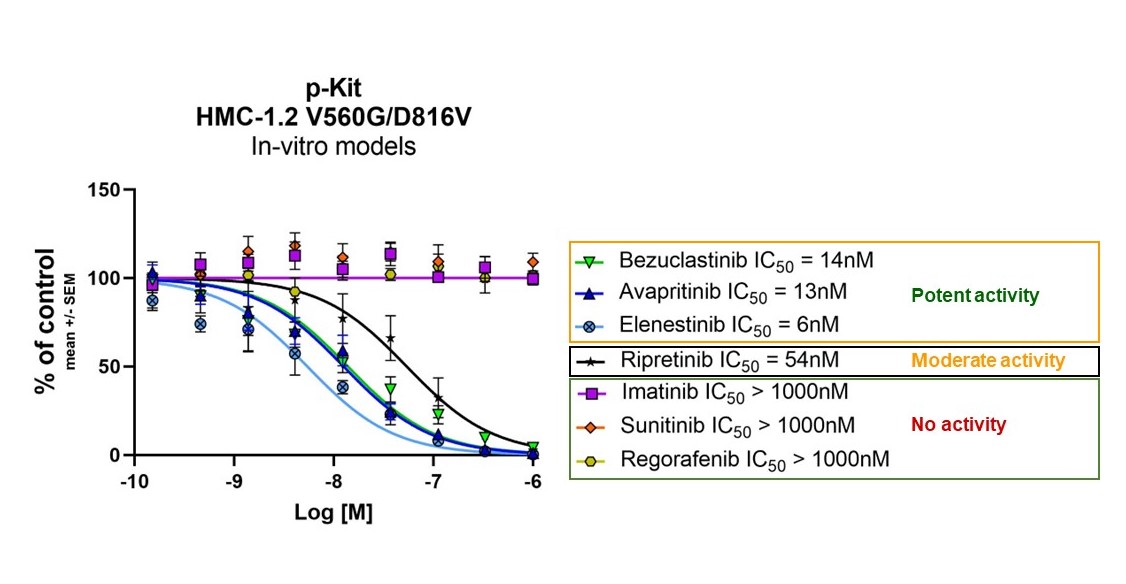
Figure 1. Potent Inhibitor of KIT Activation Loop Mutants, Including D816V
HMC-1.2 human mast cells were treated with indicated inhibitors for 1 hour (n = 3 biological replicates) Readout is phosphorylated c-Kit (Human Phospho c-Kit ELISA, R&D Systems)
7
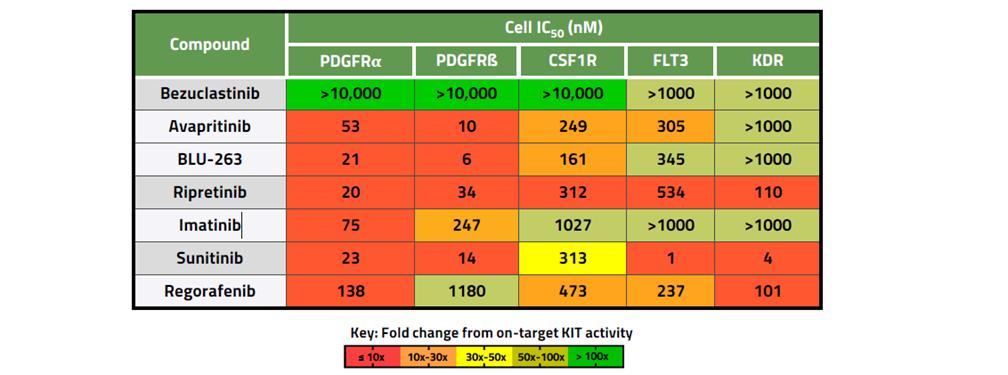
Figure 2. Selectivity Against Related Kinases
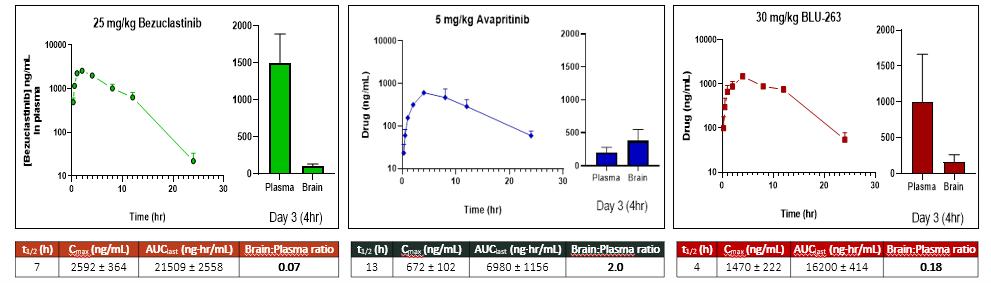
Figure 3. Bezuclastinib Demonstrates Minimal Brain Penetration
We licensed the exclusive worldwide rights to develop and commercialize bezuclastinib from Plexxikon Inc., a member of the Daiichi Sankyo Group (“Plexxikon”). Under the terms of the license agreement, Plexxikon received an upfront payment and is eligible for additional development and regulatory milestone payments along with mid- to high- single-digit royalty payments.
Bezuclastinib, a potential best-in-class KIT mutant inhibitor, has demonstrated promising clinical activity and safety results in a completed Phase 1/2 clinical trial in patients with GIST and in an on-going Phase 2 clinical trial in patients with AdvSM, supporting accelerated timelines for further development. In 2021, we initiated three clinical trials designed to explore the safety and efficacy of bezuclastinib in patients with AdvSM, Non-AdvSM and GIST. Currently, all three clinical trials are actively recruiting patients.
In 2022, we introduced a new formulation of bezuclastinib, which is currently being used in our clinical trial for patients with GIST and which we expect to incorporate into our SM trials in 2023. In 2022, we also filed a provisional patent application seeking to protect the new formulation of bezuclastinib, which could potentially provide exclusivity through at least 2043.
8
Clinical Trials and Disease Overviews
Bezuclastinib – SM
SM is driven by KIT D816V mutations causing a perpetual ‘on’ state within mast cells, a type of white blood cell, leading to proliferation and accumulation in various internal organs and bone marrow. Key biomarkers of SM include but are not limited to, elevated serum tryptase, high mass cell burden in bone marrow and the KIT D816V variant allele frequency. As a highly selective and potent KIT inhibitor, bezuclastinib has the potential to provide a new treatment option for patients with SM. SM occurs when mast cells inappropriately accumulate in various internal organs in the body. Approximately 90% of patients present with Non-AdvSM and 10% of patients present with AdvSM a rare, very aggressive form of SM. There are three subtypes of AdvSM: aggressive SM (“ASM”), SM with associated hematologic neoplasm (“SM-AHN”) and mast cell leukemia (“MCL”).
Patients diagnosed with Non-AdvSM, a life-long illness with chronic symptoms including headaches, urticaria pigmentosa, skin lesions, skin redness and warmth (flushing), abdominal pain, bloating, vomiting, diarrhea, and gastroesophageal reflux (“GERD”), that significantly impact the patient’s quality of life. Many patients are also at high risk for severe, life-threatening anaphylactic reactions to various triggers such as insect bites or stings. Patients with Non-AdvSM suffer from a poor quality of life and without any currently approved therapies, are in need of new treatment options.
Patients with AdvSM may suffer from a multitude of debilitating symptoms such as anemia, thrombocytopenia, ascites, bone fractures, gastrointestinal abnormalities, and enlargement of the liver, spleen, and lymph nodes, which ultimately lead to organ failure and early death. Patients with AdvSM have a significantly diminished lifespan with a median survival of less than 3.5 years.
Based on the characteristics of bezuclastinib, we are pursuing development of the compound in both patients living with AdvSM and patients with Non-AdvSM, the vast majority of whom have a KIT D816V mutation. Emerging clinical data for other kinase inhibitors with activity against KIT D816V have shown that SM patients are highly sensitive to inhibition of the target. Bezuclastinib was specifically designed to selectively inhibit KIT mutations, including KIT D816V.
The underlying SM patient population is not yet well understood. The prevalence of SM in the United States is estimated to be up to 30,000 patients, with the prevalence of Non-AdvSM being approximately 25,000 patients. We believe there is a significant unmet medical need for clinically active, well tolerated treatment options for this patient population. We believe bezuclastinib is well suited to meet this need and target the direct underlying cause of SM.
APEX (AdvSM)
We are currently enrolling Part 1 of APEX, our global, open-label, multi-center, Phase 2 clinical trial in patients with AdvSM evaluating the safety, efficacy, pharmacokinetic, and pharmacodynamic profiles of bezuclastinib. We expect to provide an update on the planned initiation of APEX Part 2 based on clinical data from approximately 25-30 patients in APEX Part 1 in mid-2023.
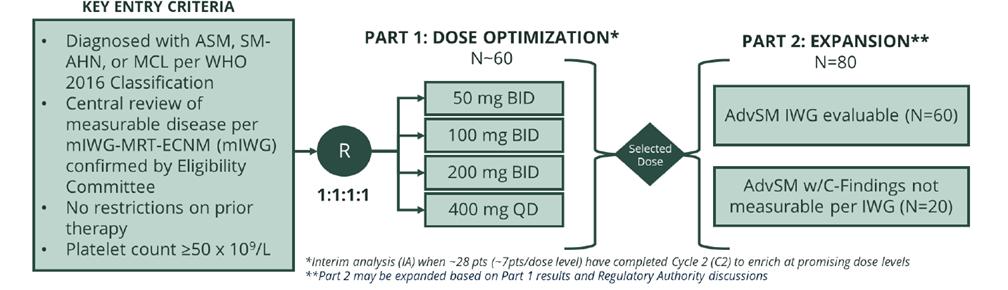
Figure 4. APEX study design graphic
9
In December 2022, at the 64th American Society of Hematology (ASH) Annual Meeting, we reported positive updated clinical data from the ongoing APEX trial. As of the data cutoff date of October 26, 2022, 16 patients had been treated in Part 1 at one of four dose levels (50 mg BID, 100 mg BID, 200 mg BID or 400 mg QD). Eleven patients were evaluable for response per the modified IWG-MRT-ECNM criteria, and 12 patients were evaluable for response using pure pathological response (PPR) criteria. An objective response rate (ORR) of 89% (including centrally adjudicated confirmed and unconfirmed responses) was achieved in TKI therapy naïve patients, including 67% of patients achieving complete remission (CR), CR with partial hematologic remission (CRh), partial remission (PR) and 22% achieving CR or CRh. An ORR of 73% was achieved in all patients, regardless of prior treatment, and 75% ORR was achieved by PPR criteria, regardless of prior treatment. Additionally, results of key markers of clinical activity were reported from 16 patients.
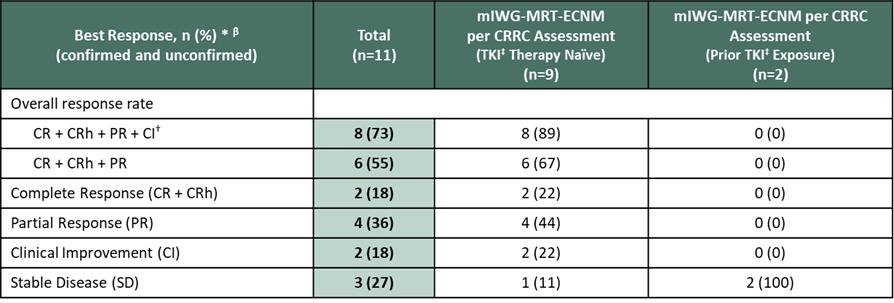
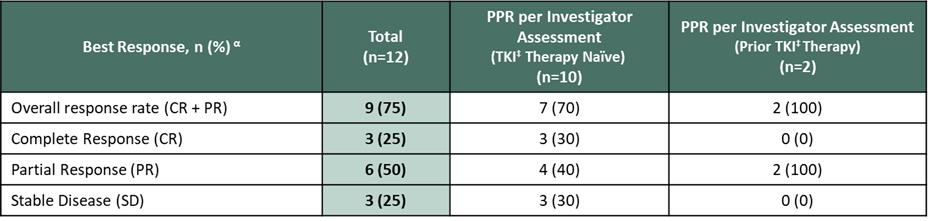
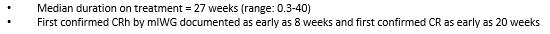
Figure 5. Early Responses Observed by mIWG-MRT-ECNM and PPR Criteria (Source: ASH conference 2022)
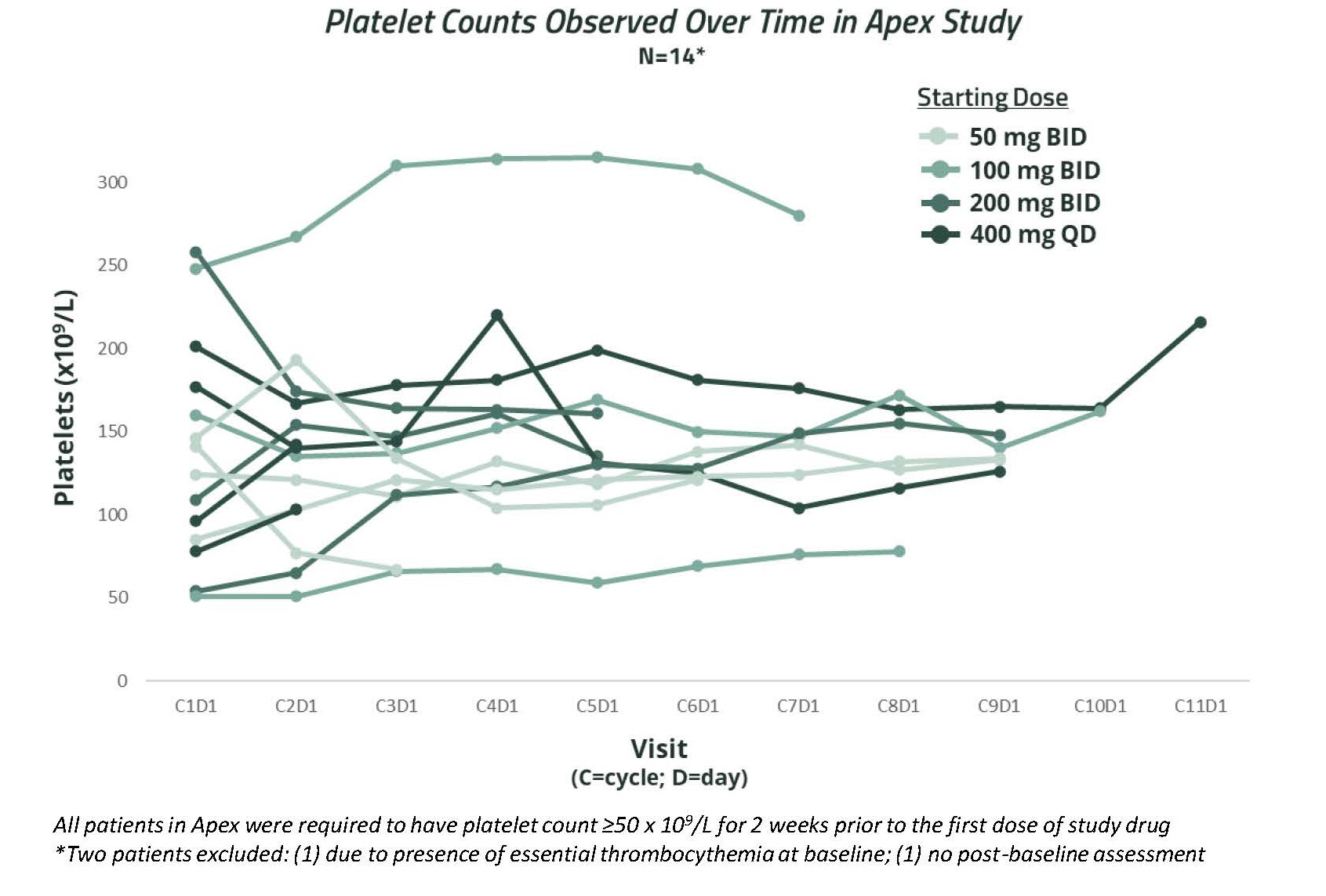
Figure 6. Platelet counts observed over time in APEX study (Source: ASH conference 2022)
10
As of the cut-off date of October 26, 2022, 14 out of 16 patients treated with bezuclastinib achieved at least a 50% reduction in serum tryptase, with a median reduction of 85%, regardless of prior KIT D816V inhibitor treatment; 13 of 13 patients with at least two cycles of treatment achieved at least a 50 % reduction in bone marrow mast cell aggregates, with 10 of these patients achieving complete clearance of bone marrow mast cell aggregates. Also, 11 of 12 patients with baseline D816V mutation and at least two cycles of treatment achieved at least a 50% reduction in blood KIT D816V variant allele fraction by droplet digital polymerase chain reaction. Bezuclastinib was generally well-tolerated at all doses. The majority of adverse events were Grade 1/2 and occurred in no more than one patient. Grade 3 events reported as at least possibly related to bezuclastinib were neutropenia (2 patients), thrombocytopenia (1 patient), anemia (1 patient) and hypersensitivity/mediator flare (1 patient). Importantly, there were no related cognitive effects or bleeding events reported, which have been associated with other KIT inhibitors. Limited low-grade edema was observed, and analysis of platelet counts in bezuclastinib-treated patients showed a limited effect of bezuclastinib on platelet counts.
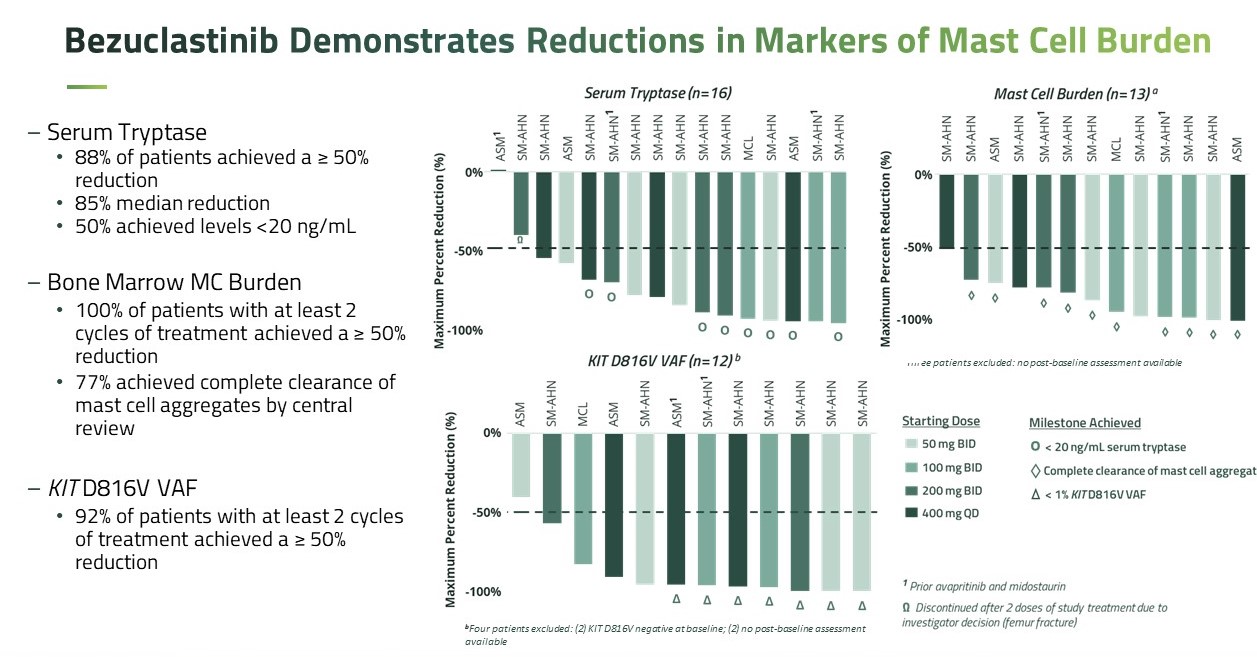
Figure 7. Reductions in markers of mast cell burden (Source: ASH conference 2022)
SUMMIT (Non-AdvSM)
We are also currently enrolling Part 1 of SUMMIT, a randomized, double-blind, placebo-controlled, global Phase 2 clinical trial. The study is designed to explore the safety and efficacy of bezuclastinib in patients with moderate to severe Indolent Systemic Mastocytosis (“ISM”) or Smoldering Systemic Mastocytosis (“SSM”), collectively considered as Non-AdvSM. Based on the performance of bezuclastinib’s new formulation in the PEAK lead-in trial, as well as in a healthy normal volunteer study, the SUMMIT trial protocol has been amended to allow for the new formulation to be introduced during the dose optimization phase. We expect to present initial clinical data in patients with Non-AdvSM in the second half of 2023. Clinical data is expected to include safety/tolerability, pharmacokinetics and measures of clinical activity. The below figure shows the current SUMMIT clinical trial design. In March 2023, Cogent received approvals from European regulatory authorities to initiate the SUMMIT trial in patients with Non-AdvSM. Beginning in April 2023, we expect to start activating clinical trial sites across major countries in the European Union.
11
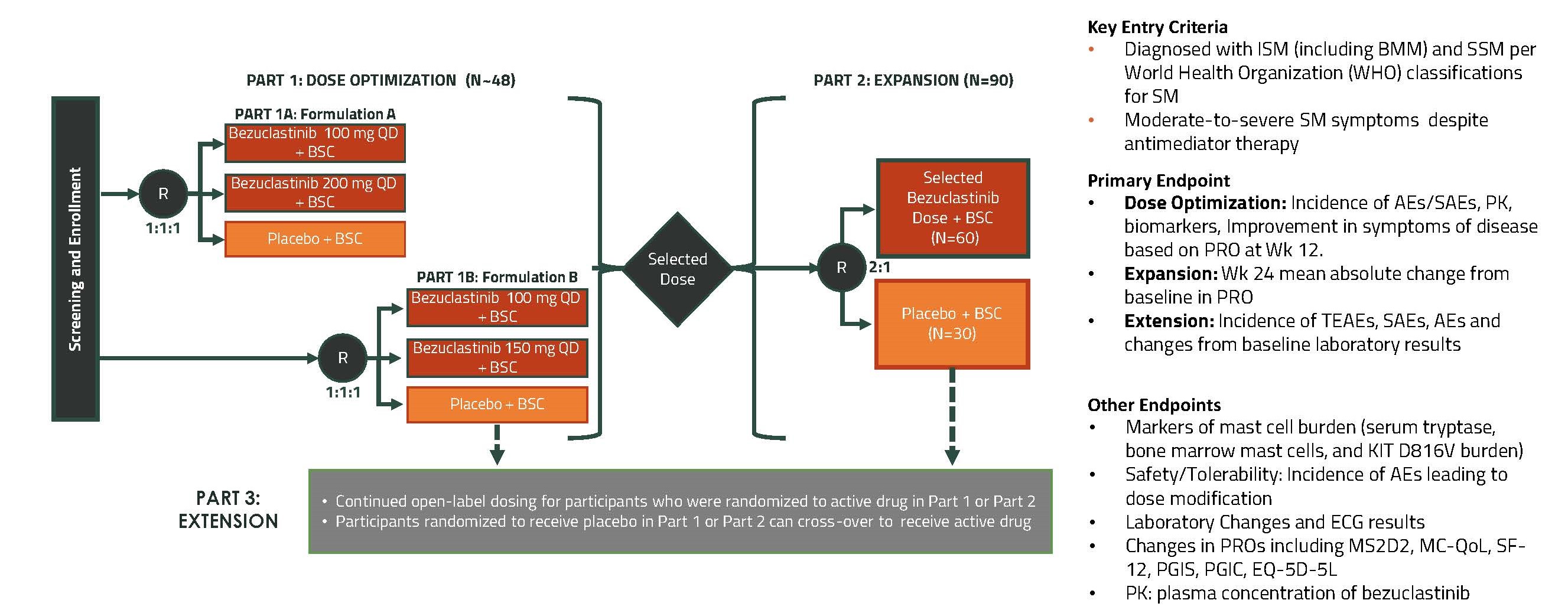
Figure 8. SUMMIT study design graphic
Bezuclastinib – GIST
GIST is characterized by uncontrolled cell growth in the interstitial cells of the gastrointestinal (“GI”) tract. At diagnosis, about 80% of GIST patients’ tumors are the result of primary KIT mutations. Imatinib is the current standard of care for treating GIST patients in the first line setting, with a median PFS of 19 months. However, the majority of GIST patients eventually develop resistance to imatinib due to secondary KIT mutations, most notably in exon 17 and exon 13. There are an estimated 2,000 to 3,500 patients with imatinib-resistant GIST eligible for treatment each year in the United States. We believe there is a significant unmet medical need for clinically active, well tolerated treatment options for this patient population and results from our clinical trial of bezuclastinib in combination with sunitinib demonstrated the potential for this novel combination to address the underlying drivers of imatinib resistance. The FDA has granted orphan drug designation to bezuclastinib for the treatment of GIST.
Bezuclastinib is designed to be a potent and selective inhibitor of KIT exon 17 mutations. By combining bezuclastinib with sunitinib, a tyrosine kinase inhibitor known to inhibit KIT exon 13 mutations, we believe this combination has the potential to offer a new, active treatment option for imatinib resistant GIST patients.
The safety profile of bezuclastinib was clinically evaluated in approximately 50 GIST patients both as a single agent and as part of a combination therapy. Clinical data from this trial were published in the Journal of American Medical Association (“JAMA”) and were presented at several scientific conferences, including most recently by us at the 2020 annual Connective Tissue Oncology Society (“CTOS”) meeting, and previously by Plexxikon at the 2018 annual American Society of Clinical Oncology (“ASCO”) meeting and the 2017 annual CTOS meeting. In November 2020, we presented final results from a Phase 1/2 trial testing the combination of bezuclastinib with sunitinib in 18 heavily pre-treated GIST patients at 2020 CTOS. In the subset of 15 patients who had not been previously treated with bezuclastinib as a single-agent, the estimated mPFS reached 12 months , the confirmed ORR was 20% and the clinical benefit rate (CR+PR+SD) was 80%, with 27% of patients remaining on therapy out 27-34 months. Importantly, there were no dose limiting toxicities in the three dose levels tested, and the most common Treatment Emergent Adverse Events that were grade 3 or higher included anemia (5 patients, 27.8%), hypophosphatemia (3 patients, 16.7%), diarrhea, fatigue, hypertension, and lymphopenia (each 2 patients, 11.1%). Four subjects continued to receive bezuclastinib via individual patient INDs beyond the conclusion of the trial.
12
Demographics and Prior Therapy: Heavily Pretreated GIST Patients treated in Phase 1/2 Trial Testing the Combination of Bezuclastinib with Sunitinib
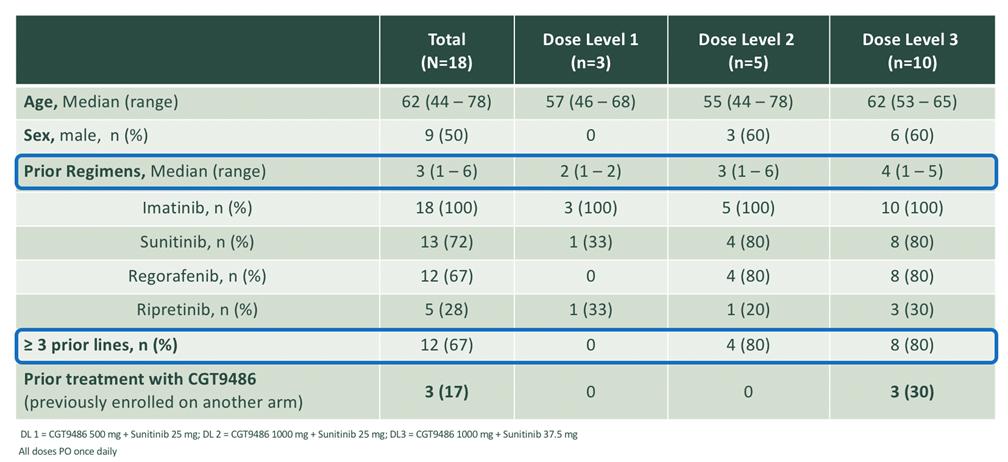
Figure 9. GIST Phase 1/2 trial demographics (Source: 2020 CTOS annual meeting)
Durable Responses in Patients Treated with Bezuclastinib + Sunitinib
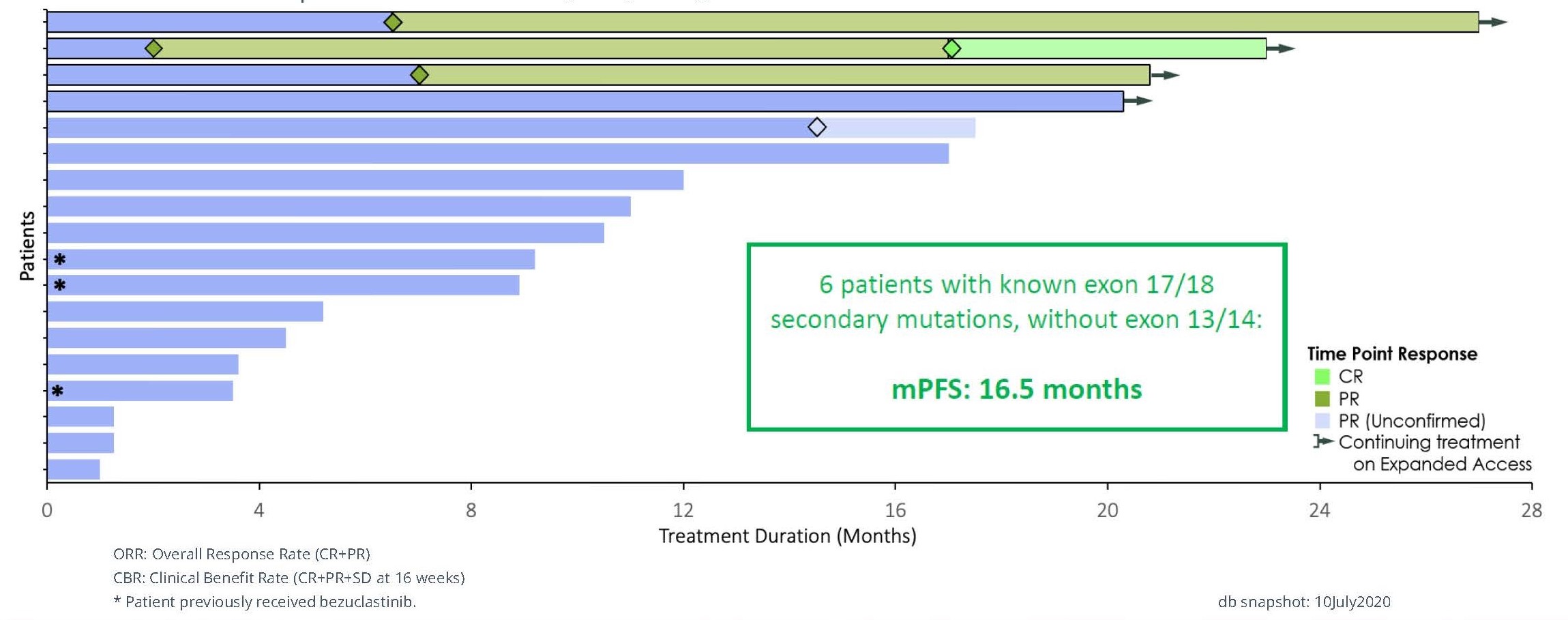
Figure 10. Patient Responses in GIST Phase 1/2 trial ( Source: 2020 CTOS annual meeting)
13
PEAK (GIST)
We are currently enrolling Part 2 of PEAK, our randomized open-label, global Phase 3 clinical trial designed to evaluate the safety, tolerability, and efficacy of bezuclastinib in combination with sunitinib compared to sunitinib alone in patients with locally advanced, unresectable or metastatic GIST who have received prior treatment with imatinib.
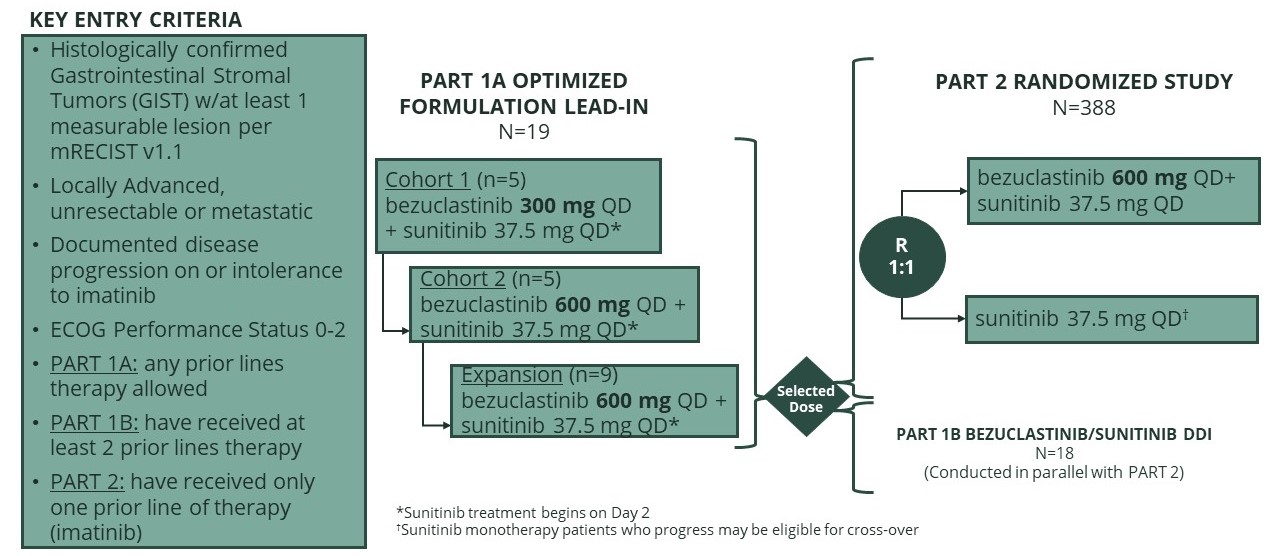
Figure 11. PEAK study design graphic
Based on the data from the PEAK lead-in study we initiated the randomized portion of PEAK using a 600 mg dose of our new formulation of bezuclastinib, supplied as 75 mg tablets, which in the lead-in portion of the study demonstrated clinical exposure comparable to the 1,000 mg original formulation used in our GIST Phase 1/2 clinical trial. Initial safety and pharmacokinetic data from the PEAK lead-in study was presented at the CTOS annual meeting in November 2022. We expect to present updated clinical data from refractory GIST patients in the lead-in cohort of the Phase 3 PEAK trial of bezuclastinib plus sunitinib during the first half of 2023.
Research Programs
During the second quarter of 2021, we announced the formation of the Cogent Research Team, a highly experienced discovery and research group. Based in Boulder, Colorado, the Cogent Research Team is focused on pioneering best-in-class, small molecule therapeutics to expand our pipeline and deliver novel precision therapies for patients living with unmet medical needs. Dr. John Robinson, our Chief Scientific Officer, leads the Cogent Research team composed of highly experienced scientists with deep expertise across a broad range of functional specialties including medicinal chemistry, computational chemistry, biology, enzymology and pharmacology.
Our research team is building a pipeline of small molecule inhibitors, with our first efforts aimed toward targeting currently undrugged mutations in fibroblast growth factor receptor (“FGFR”). FGFR mutations are well-established oncogenic drivers in multiple diseases, but approved medicines fail to capture the full landscape of FGFR altered tumor types, with FGFR1-mediated hyperphosphatemia serving as the most common dose-limiting toxicity for pan-FGFR inhibitors. In October 2022, we reported preclinical data at EORTC-NCI-AACR (“ENA”) annual meeting on a next-generation fibroblast growth factor receptor 2 (“FGFR2”) program, which retains potency across all primary, gatekeeper and molecular brake resistance mutations, including N549K and V564I, while sparing FGFR1 inhibition.
Our research team is also advancing a novel, ErbB2 mutant program, which is focused on actionable and underserved mutations in a variety of solid tumor indications. Currently available oral ErbB2 inhibitors struggle to provide broad mutant coverage while sparing EGFR activity. In October 2022, presented preclinical data at ENA on a novel ErbB2 mutant selective program which demonstrates robust cellular inhibition of all key resistance and primary driver mutations, including L755S, V842I and S310F/Y, while sparing wild type EGFR target engagement.
For both FGFR and ErBB2, we see an opportunity to provide a more robust molecular response compared to existing therapies. We expect to initiate clinical trials for both of these programs in 2024.
14
Intellectual Property
One key to our success will be our ability to establish and maintain protection for our product candidates and know-how, in order to enforce and defend our intellectual property rights and to operate without infringing on the rights of others. We rely on our know-how, trade secrets and continuing technological innovation as well as on in-licensing of third-party intellectual property to develop and maintain our proprietary position. Our patent portfolio consists of U.S. patents and foreign patents and patent applications that we in-licensed exclusively from Plexxikon, as well as additional patent applications we have filed on our own.
With the acquisition of Kiq Bio LLC (formerly Kiq LLC) (“Kiq”) on July 6, 2020, we obtained an exclusive, sublicensable, worldwide license to patents and applications owned by Plexxikon pursuant to a license agreement between Plexxikon and Kiq (the “License Agreement”). The licensed patents and applications under the License Agreement cover bezuclastinib, its therapeutic uses, and methods of making bezuclastinib and intermediates. These patents and applications include issued patents in multiple territories, including, but not limited to, Australia, Brazil, Canada, China, Colombia, Europe (validated in Germany, Spain, France, Great Britain, Italy, the Netherlands, as well as various other EU countries), Hong Kong, India, Indonesia, Israel, Japan, Mexico, New Zealand, Peru, the Philippines, Republic of Korea, Russia, Singapore, South Africa, Taiwan, and the United States. The pending applications also include patent applications pending in Australia, Brazil, Canada, China, Egypt, Europe, India, Indonesia, Israel, Japan, Korea, Mexico, Philippines, Singapore, South Africa, and the United States. The issued U.S. patents covering bezuclastinib and its therapeutic uses are expected to expire in 2033 and 2034, and the issued foreign patents covering bezuclastinib and its therapeutic uses are expected to expire in 2033, without consideration of potential patent term extensions. Patent applications covering methods of making bezuclastinib and intermediates could potentially provide exclusivity through at least 2041. In 2022, we filed a provisional patent application seeking to protect our new formulation of bezuclastinib, which could potentially provide exclusivity through at least 2043.We may seek to obtain rights under additional patent applications relating to bezuclastinib and its use to treat SM and GIST in the United States and in other countries as we proceed with this development program.
We are not currently a party to and have not been a party to any legal proceedings involving patent rights.
In addition to the protection afforded by patents, we seek to protect our technology and product candidates, in part, by trade secret and confidentiality agreements with those who have access to our confidential information, including our employees, contractors, consultants, collaborators, and advisors. We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations, and systems, agreements or security measures may be breached and we may not have adequate remedies for any breach. Furthermore, the laws of some foreign countries may not protect proprietary rights to the same extent or in the same manner as the laws of the United States.
In addition, our trade secrets may otherwise become known or may be independently discovered by competitors. To the extent that our employees, contractors, consultants, collaborators, and advisors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Moreover, we may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property. Disputes regarding ownership or inventorship of our patents or other intellectual property can arise in various contexts, including collaborations and sponsored research. If we are subject to a dispute challenging our rights in or to patents or other intellectual property, such a dispute could be expensive and time consuming. If we are unsuccessful, we could lose valuable rights in intellectual property that we regard as our own.
For more comprehensive risks related to our proprietary technology, inventions, improvements and products, please see the section on “Risk Factors—Risks Related to Intellectual Property.”
15
Licenses and Third-Party Research Collaborations
License Agreement with Plexxikon Inc.
In July 2020, we obtained an exclusive, sublicensable, worldwide license to certain patents and other intellectual property rights to research, develop, and commercialize bezuclastinib. Under the terms of the License Agreement, we are required to pay Plexxikon aggregate payments of up to $7.5 million upon the satisfaction of certain clinical milestones and up to $25.0 million upon the satisfaction of certain regulatory milestones. During the second quarter of 2022, as a result of the progression of the PEAK study, the first clinical milestone was achieved, resulting in payment of $2.5 million to Plexxikon in June 2022. As of December 31, 2022, no other milestone payments have been made or are considered probable of occurring.
We are also required to pay Plexxikon tiered royalties ranging from a low-single digit percentage to a high-single digit percentage on annual net sales of products. These royalty obligations last on a product-by-product basis and country-by-country basis until the latest of (i) the date on which there is no valid claim of a licensed Plexxikon patent covering a subject product in such country or (ii) the 10th anniversary of the date of the first commercial sale of the product in such country. In addition, if we sublicense the rights under the License Agreement, we are required to pay a certain percentage of the sublicense revenue to Plexxikon ranging from mid-double digit percentages to mid-single digit percentages, depending on whether the sublicense is entered into prior to or after certain development and regulatory milestones.
The License Agreement will expire on a country-by-country and licensed product-by-licensed product basis until the later of the last to expire of the patents covering such licensed products or services or the 10-year anniversary of the date of first commercial sale of the licensed product in such country. Plexxikon may terminate the License Agreement within 30 days after written notice in the event of a breach of contract that remains uncured. Plexxikon may also terminate the agreement upon written notice in the event of our bankruptcy, liquidation, or insolvency. In addition, we have the right to terminate the License Agreement in its entirety at will upon 90 days’ advance written notice to Plexxikon.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary drugs. While we believe that our technology, development experience, and scientific knowledge provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions governmental agencies, and public and private research institutions. Any drug candidates that we successfully develop and commercialize will compete with existing drugs and new drugs that may become available in the future.
We compete in the segments of the pharmaceutical, biotechnology, and other related markets that address precision medicines for patients with genetically defined diseases. There are several other companies working to develop therapies in this field using a similar strategy. These companies include divisions of large pharmaceutical companies and biotechnology companies of various sizes.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical, biotechnology, and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any drugs that we or our collaborators may develop. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we or our collaborators are able to enter the market. The key competitive factors affecting the success of all of our drug candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of companion diagnostics, the level of generic competition, and the availability of reimbursement from government and other third-party payors.
16
Bezuclastinib, if approved for the indications for which we are currently enrolling clinical trials, will compete with the drugs discussed below and will likely compete with other drugs that are currently in development.
In SM, the only approved drugs for the treatment of AdvSM are Blueprint Medicines Corporation’s ("Blueprint") avapritinib and Novartis AG’s midostaurin. Additionally, Novartis AG’s imatinib is approved for AdvSM patients without the KIT D816V mutation or mutational status unknown. There are currently no approved drugs for the treatment of Non-AdvSM. The most advanced drug candidate for the treatment of Non-AdvSM is Blueprint’s avapritinib, for which Blueprint has submitted a supplement new drug application to the FDA and has a PDUFA date of May 22, 2023. We may also face competition from other drug candidates in pre-clinical or clinical development for SM.
In GIST, the current approved standards of care for unresectable or metastatic patients are first-line imatinib, followed by second-line sunitinib upon imatinib progression, followed by third-line regorafenib upon sunitinib progression, followed by fourth-line ripretinib for patients who have received three or more prior kinase inhibitors. In addition, avapritinib was approved by the FDA in January 2020 for patients with GIST harboring a PDGFRα exon 18 mutation, including PDGFRA D842V mutations only. We may face competition from other drug candidates in pre-clinical or clinical development including, Celldex Therapeutics, Inc., Deciphera Pharmaceuticals, Inc., Taiho Pharmaceutical Co. Ltd, Xencor, Inc., Theseus Pharmaceuticals, Inc. and IDRx.
Manufacturing and Supply
We do not own or operate, and have no current plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on third parties to manufacture our drug candidates for preclinical and clinical testing, as well as for future commercial supply of any drugs that we may commercialize. To date, we have obtained API and drug product from third-party manufacturers for bezuclastinib to support preclinical and clinical testing. We obtain our supplies from these manufacturers on a purchase-order basis and do not have any long-term supply arrangements. We do not currently have a validated manufacturing process in place for any product candidate which would be required to support commercialization of any of our drug candidates, if approved.
Our drug candidates are compounds of low molecular weight, generally called small molecules. They can be manufactured from readily available starting materials in reliable and reproducible synthetic processes. The manufacturing process is amenable to scale-up. As we continue our clinical development of bezuclastinib, we expect to continue to enhance our manufacturing process to allow for drug candidates that are safer, more effective, have superior dosing regimens and are cost-effective.
Government Regulation
Government authorities in the United States, at the federal, state and local levels, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, product approval, manufacture, quality control, manufacturing changes, packaging, storage, recordkeeping, labeling, promotion, advertising, sales, distribution, marketing, and import and export of drugs and biologic products. Our current product candidates are expected to be regulated as drugs. The processes for obtaining regulatory approval in the United States and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory authorities both pre- and post-commercialization, are a significant factor in the production and marketing of our products and our research and development activities and require the expenditure of substantial time and financial resources.
17
Review and Approval of Drugs in the United States
In the United States, the FDA and other government entities regulate drugs under the Federal Food, Drug, and Cosmetic Act, or the FDCA and the regulations promulgated thereunder, as well as other federal and state statutes and regulations. Failure to comply with applicable legal and regulatory requirements in the United States at any time during the product development process, approval process, or after approval, may subject us to a variety of administrative or judicial sanctions, such as a delay in approving or refusal by the FDA to approve pending applications, withdrawal of approvals, delay or suspension of clinical trials, issuance of warning letters and other types of regulatory letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil monetary penalties, refusals of or debarment from government contracts, exclusion from the federal healthcare programs, restitution, disgorgement of profits, civil or criminal investigations by the FDA, U.S. Department of Justice, State Attorneys General, and/or other agencies, False Claims Act suits and/or other litigation, and/or criminal prosecutions.
An applicant seeking approval to market and distribute a new drug in the United States must typically undertake the following:
18
Preclinical Studies and an IND
Preclinical studies can include in vitro and animal studies to assess the potential for adverse events and, in some cases, to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. Other studies include laboratory evaluation of the purity, stability and physical form of the manufactured drug substance or active pharmaceutical ingredient and the physical properties, stability and reproducibility of the formulated drug or drug product. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical studies, among other things, to the FDA as part of an IND. Some preclinical testing, such as longer-term toxicity testing, animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Following commencement of a clinical trial under an IND, the FDA may place a clinical hold on that trial. A clinical hold is an order issued by the FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. A partial clinical hold is a delay or suspension of only part of the clinical work requested under the IND. For example, a specific protocol or part of a protocol is not allowed to proceed, while other protocols may do so. No more than 30 days after imposition of a clinical hold or partial clinical hold, the FDA will provide the sponsor a written explanation of the basis for the hold. Following issuance of a clinical hold or partial clinical hold, an investigation may only resume after the FDA has notified the sponsor that the investigation may proceed. The FDA will base that determination on information provided by the sponsor correcting the deficiencies previously cited or otherwise satisfying the FDA that the investigation can proceed.
Human Clinical Studies in Support of an NDA
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations.
Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on its ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness.
Phase 2: The product candidate is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: The product candidate is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
19
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2, and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. The FDA will typically inspect one or more clinical sites in late-stage clinical trials to assure compliance with GCP and the integrity of the clinical data submitted.
Submission of an NDA to the FDA
Assuming successful completion of required clinical testing and other requirements, the results of the preclinical and clinical studies, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to an application user fee, currently $2.876 million for fiscal year 2021, for applications requiring clinical data, and the sponsor of an approved NDA is also subject to an annual program fee, currently $336,432 for fiscal year 2021. These fees are adjusted annually.
Under certain circumstances, the FDA will waive the application fee for the first human drug application that a small business, defined as a company with less than 500 employees, including employees of affiliates, submits for review. An affiliate is defined as a business entity that has a relationship with a second business entity if one business entity controls, or has the power to control, the other business entity, or a third-party controls, or has the power to control, both entities. In addition, an application to market a prescription drug product that has received orphan designation is not subject to a prescription drug user fee unless the application includes an indication for other than the rare disease or condition for which the drug was designated. Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a disease or condition that affects fewer than 200,000 individuals in the U.S., or for which there is no reasonable expectation that U.S. sales will be sufficient to recoup the development and production costs.
The FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after the FDA’s receipt of the submission to determine whether the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review process of NDAs. Most such applications are meant to be reviewed within ten months from the date of filing, and most applications for “priority review” products are meant to be reviewed within six months of filing. The review process may be extended by the FDA for three additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with GMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with cGCP.
The FDA also may require submission of a REMS plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA is required to refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
20
The FDA’s Decision on an NDA
On the basis of the FDA’s evaluation of the NDA and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
If the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies be conducted to further assess the drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, which can materially affect the potential market and profitability of the product. After approval, the FDA may seek to prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. Some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Expedited Review and Accelerated Approval Programs
A sponsor may seek approval of its product candidate under programs designed to accelerate the FDA’s review and approval of NDAs. For example, Fast Track Designation may be granted to a drug intended for treatment of a serious or life-threatening disease or condition and data demonstrate its potential to address unmet medical needs for the disease or condition. The key benefits of Fast Track Designation are the eligibility for priority review, rolling review (submission of portions of an application before the complete marketing application is submitted), and accelerated approval, if relevant criteria are met. The FDA may grant the NDA a priority review designation, which sets the target date for FDA action on the application at six months after the FDA accepts the application for filing. Priority review is granted where there is evidence that the proposed product would be a significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of a serious condition. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
The FDA may approve an NDA under the accelerated approval program if the drug treats a serious condition, provides a meaningful advantage over available therapies, and demonstrates an effect on either (1) a surrogate endpoint that is reasonably likely to predict clinical benefit, or (2) on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Post-marketing studies or completion of ongoing studies after marketing approval are generally required to verify the drug’s clinical benefit in relationship to the surrogate endpoint or ultimate outcome in relationship to the clinical benefit.
In addition, the Food and Drug Administration Safety and Innovation Act of 2012, or FDASIA, established the Breakthrough Therapy designation. A sponsor may seek FDA designation of its product candidate as a breakthrough therapy if the drug is intended, alone or in combination with one or more other drugs, to treat a serious or life- threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. If a drug is designated as breakthrough therapy, FDA will provide more intensive guidance on the drug development program and expedite its review.
Fast track designation, breakthrough therapy designation, priority review, and accelerated approval do not change the standards for approval, but may expedite the development or approval process. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or that the time period for FDA review or approval will not be shortened. We may explore some of these opportunities for our product candidates as appropriate.
21
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented.
FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events or problems with manufacturing processes of unanticipated severity or frequency, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant criminal and civil liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
22
Hatch-Waxman Patent Certification and the 30 Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or a method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval.
Specifically, the applicant must certify with respect to each patent that:
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
To the extent that a Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Legislative Developments
The 21st Century Cures Act, or the Cures Act, which was signed into law in December 2016, includes provisions to accelerate the development and delivery of new treatments. For example, the Cures Act requires the FDA to establish a program to evaluate the potential use of real world evidence to help to support the approval of a new indication for an approved drug and to help to support or satisfy post-approval study requirements, to issue guidance on adaptive and novel clinical trial designs for new drugs, and to establish a process for qualifying drug development tools used to support FDA approval for marketing or investigational use of a drug. The Cures Act also permits the FDA to rely on qualified data summaries to support the approval of a supplemental application for an already approved drug. The FDA is in the process of implementing the Cures Act requirements.
23
Review and Approval of Drug Products in the European Union
In order to market any pharmaceutical product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions governing, among other things, research and development, testing, manufacturing, quality control, safety, efficacy, labeling, clinical trials, marketing authorization, packaging, storage, record keeping, reporting, export and import, advertising, marketing and other promotional practices involving pharmaceutical products, as well as commercial sales, distribution, authorization, approval and post-approval monitoring and reporting of our products. Whether or not it obtains FDA approval for a pharmaceutical product, the company would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the pharmaceutical product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Drug Development Process
The conduct of clinical trials is currently governed by the EU Clinical Trials Directive 2001/20/EC, or Clinical Trials Directive, and will be replaced by the EU Clinical Trials Regulation (EU) No. 536/2014 (“CTR”) once the latter comes into effect. The CTR introduces a complete overhaul of the existing regulation of clinical trials for medicinal products in the EU. It entered into force on January 31, 2022. Under the current regime, which will expire after a transition period of one or three years, respectively, as outlined below in more detail, before a clinical trial can be initiated it must be approved in each EU Member State where there is a site at which the trial is to be conducted. The approval must be obtained from two separate entities: the National Competent Authority (“NCA”) and one or more Ethics Committees. The NCA of the EU Member States in which the clinical trial will be conducted must authorize the conduct of the trial, and the independent Ethics Committee must grant a positive opinion in relation to the conduct of the clinical trial in the relevant EU Member State before the commencement of the trial. Any substantial changes to the trial protocol or other information submitted with the clinical trial applications must be submitted to or approved by the relevant NCA and Ethics Committees. Under the current regime all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial must be reported to the NCA and to the Ethics Committees of the EU Member State where they occur.
A more unified procedure will apply under the new CTR. A sponsor will be able to submit a single application for approval of a clinical trial through a centralized EU clinical trials portal. One national regulatory authority (the reporting EU Member State proposed by the applicant) will take the lead in validating and evaluating the application consult and coordinate with the other concerned Member States. If an application is rejected, it may be amended and resubmitted through the EU clinical trials portal. If an approval is issued, the sponsor may start the clinical trial in all concerned Member States. However, a concerned EU Member State may in limited circumstances declare an “opt-out” from an approval and prevent the clinical trial from being conducted in such Member State. The CTR also aims to streamline and simplify the rules on safety reporting, and introduces enhanced transparency requirements such as mandatory submission of a summary of the clinical trial results to the EU Database. The CTR foresees a three-year transition period. Member States will work in CTIS immediately after the system has gone live. For one year, until 31 January 2023, clinical trial sponsors can still choose whether to submit an initial clinical trial application in line with the current system (Clinical Trials Directive) or via CTIS. From 31 January 2023, submission of initial clinical trial applications via CTIS becomes mandatory, and by 31 January 2025, all ongoing trials approved under the current Clinical Trials Directive will be governed by the new Regulation and have to be transitioned to CTIS.
Under both the current regime and the new CTR, national laws, regulations, and the applicable Good Clinical Practice and Good Laboratory Practice standards must also be respected during the conduct of the trials, including the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (“ICH”) guidelines on Good Clinical Practice (“GCP)” and the ethical principles that have their origin in the Declaration of Helsinki.
24
During the development of a medicinal product, the European Medical Agency (“EMA”) and national regulators within the EU provide the opportunity for dialogue and guidance on the development program. At the EMA level, this is usually done in the form of scientific advice, which is given by the Committee for Medicinal Products for Human Use (“CHMP”) on the recommendation of the Scientific Advice Working Party (“SAWP”). A fee is incurred with each scientific advice procedure, but is significantly reduced for designated orphan medicines. Advice from the EMA is typically provided based on questions concerning, for example, quality (chemistry, manufacturing and controls testing), nonclinical testing and clinical studies, and pharmacovigilance plans and risk-management programs. Advice is not legally binding with regard to any future Marketing Authorization Application (“MAA”) of the product concerned.
Marketing Authorization Procedures
In the EU and in Iceland, Norway and Liechtenstein (together the European Economic Area or “EEA”), after completion of all required clinical testing, pharmaceutical products may only be placed on the market after obtaining a Marketing Authorization (“MA”). To obtain an MA of a drug under European Union regulatory systems, an applicant can submit an MAA through, amongst others, a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single MA by the European Commission (EC) that is valid for all EU Member States and, after respective national implementing decisions, in the three additional EEA Member States. The centralized procedure is compulsory for specific medicinal products, including for medicines developed by means of certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products (“ATMP”) and medicinal products with a new active substance indicated for the treatment of certain diseases (AIDS, cancer, neurodegenerative disorders, diabetes, auto- immune and viral diseases). For medicinal products containing a new active substance not yet authorized in the EEA before May 20, 2004 and indicated for the treatment of other diseases, medicinal products that constitute significant therapeutic, scientific or technical innovations or for which the grant of a MA through the centralized procedure would be in the interest of public health at EU level, an applicant may voluntarily submit an application for a marketing authorization through the centralized procedure.
Under the centralized procedure, the Committee for Medicinal Products for Human Use (“CHMP”), established at the EMA, is responsible for conducting the initial assessment of a drug. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure, the timeframe for the evaluation of an MAA by the EMA’s CHMP is, in principle, 210 days from receipt of a valid MAA. However, this timeline excludes clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP, so the overall process typically takes a year or more, unless the application is eligible for an accelerated assessment. Accelerated assessment might be granted by the CHMP in exceptional cases when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. On request, the CHMP can reduce the time frame to 150 days if the applicant provides sufficient justification for an accelerated assessment. The CHMP will provide a positive opinion regarding the application only if it meets certain quality, safety and efficacy requirements. However, the EC has final authority for granting the MA within 67 days after receipt of the CHMP opinion.
The decentralized procedure permits companies to file identical MA applications for a medicinal product to the competent authorities in various EU Member States simultaneously if such medicinal product has not received marketing approval in any EU Member State before. This procedure is available for pharmaceutical products not falling within the mandatory scope of the centralized procedure. The competent authority of a single EU Member State, known as the reference EU Member State, is appointed to review the application and provide an assessment report. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference EU Member State and concerned EU Member States. The reference EU Member State prepares a draft assessment report and drafts of the related materials within 120 days after receipt of a valid application. Subsequently each concerned EU Member State must decide whether to approve the assessment report and related materials.
If an EU Member State cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the EC, whose decision is binding for all EU Member States.
All new MAAs must include a Risk Management Plan (“RMP”), describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. RMPs and Periodic Safety Update Reports (“PSURs”) are routinely available to third parties requesting access, subject to limited redactions.
25
Marketing Authorizations have an initial duration of five years. After these five years, the authorization may subsequently be renewed on the basis of a reevaluation of the risk-benefit balance. Once renewed, the MA is valid for an unlimited period unless the EC or the national competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with only one additional five-year renewal. Applications for renewal must be made to the EMA at least nine months before the five-year period expires.
Data and Market Exclusivity in the European Union
As in the United States, it may be possible to obtain a period of market and/or data exclusivity in the EU that would have the effect of postponing the entry into the marketplace of a competitor’s generic, hybrid or biosimilar product (even if the pharmaceutical product has already received a MA) and prohibiting another applicant from relying on the MA holder’s pharmacological, toxicological and clinical data in support of another MA for the purposes of submitting an application, obtaining MA or placing the product on the market. New Chemical Entities (“NCE”) approved in the EU qualify for eight years of data exclusivity and 10 years of marketing exclusivity.
An additional non-cumulative one-year period of marketing exclusivity is possible if during the data exclusivity period (the first eight years of the 10-year marketing exclusivity period), the MA holder obtains an authorization for one or more new therapeutic indications that are deemed to bring a significant clinical benefit compared to existing therapies.
The data exclusivity period begins on the date of the product’s first MA in the EU. After eight years, a generic product application may be submitted and generic companies may rely on the MA holder’s data. However, a generic product cannot launch until two years later (or a total of 10 years after the first MA in the EU of the innovator product), or three years later (or a total of 11 years after the first MA in the EU of the innovator product) if the MA holder obtains MA for a new indication with significant clinical benefit within the eight-year data exclusivity period. Additionally, another noncumulative one -year period of data exclusivity can be added to the eight years of data exclusivity where an application is made for a new indication for a well-established substance, provided that significant pre-clinical or clinical studies were carried out in relation to the new indication. Another year of data exclusivity may be added to the eight years, where a change of classification of a pharmaceutical product has been authorized on the basis of significant pre-trial tests or clinical trials (when examining an application by another applicant for or holder of market authorization for a change of classification of the same substance the competent authority will not refer to the results of those tests or trials for one year after the initial chance was authorized).
Products may not be granted data exclusivity since there is no guarantee that a product will be considered by the European Union’s regulatory authorities to include a NCE. Even if a compound is considered to be a NCE and the MA applicant is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the medicinal product if such company can complete a full MAA with their own complete database of pharmaceutical tests, preclinical studies and clinical trials and obtain MA of its product.
Orphan Designation and Exclusivity
The criteria for designating an orphan medicinal product in the European Union are similar in principle to those in the United States. The EMA grants orphan drug designation if the medicinal product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the European Union (prevalence criterion). In addition, Orphan Drug Designation can be granted if, for economic reasons, the medicinal product would be unlikely to be developed without incentives and if there is no other satisfactory method approved in the European Union of diagnosing, preventing, or treating the condition, or if such a method exists, the proposed medicinal product is a significant benefit to patients affected by the condition. An application for orphan drug designation (which is not a marketing authorization, as not all orphan-designated medicines reach the authorization application stage) must be submitted first before an application for marketing authorization of the medicinal product is submitted. The applicant will receive a fee reduction for the marketing authorization application if the orphan drug designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted, and sponsors must submit an annual report to EMA summarizing the status of development of the medicine. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. Designated orphan medicines are eligible for conditional marketing authorization.
26
The EMA’s Committee for Orphan Medicinal Products reassesses the orphan drug designation of a product in parallel with the review for a marketing authorization; for a product to benefit from market exclusivity it must maintain its orphan drug designation at the time of marketing authorization review by the EMA and approval by the EC. Additionally, any marketing authorization granted for an orphan medicinal product must only cover the therapeutic indication(s) that are covered by the orphan drug designation. Upon the grant of a marketing authorization, orphan drug designation provides up to ten years of market exclusivity in the orphan indication.
During the 10-year period of market exclusivity, with a limited number of exceptions, the regulatory authorities of the EU Member States and the EMA may not accept applications for marketing authorization, accept an application to extend an existing marketing authorization or grant marketing authorization for other similar medicinal products for the same therapeutic indication. A similar medicinal product is defined as a medicinal product containing a similar active substance or substances as contained in a currently authorized orphan medicinal product, and which is intended for the same therapeutic indication. An orphan medicinal product can also obtain an additional two years of market exclusivity for an orphan-designated condition when the results of specific studies are reflected in the Summary of Product Characteristics (“SmPC”), addressing the pediatric population and completed in accordance with a fully compliant Pediatric Investigation Plan (“PIP”). No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications.
The 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, i.e. the condition prevalence or financial returns criteria under Article 3 of Regulation (EC) No. 141/2000 on orphan medicinal products. When the period of orphan market exclusivity for an indication ends, the orphan drug designation for that indication expires as well. Orphan exclusivity runs in parallel with normal rules on data exclusivity and market protection. Additionally, a marketing authorization may be granted to a similar medicinal product (orphan or not) for the same or overlapping indication subject to certain requirements.
Pediatric Development
In the European Union, companies developing a new medicinal product are obligated to study their product in children and must therefore submit a PIP together with a request for agreement to the EMA. The EMA issues a decision on the PIP based on an opinion of the EMA’s Pediatric Committee, or PDCO. Companies must conduct pediatric clinical trials in accordance with the PIP approved by the EMA, unless a deferral (e.g., until enough information to demonstrate its effectiveness and safety in adults is available) or waiver (e.g., because the relevant disease or condition occurs only in adults) has been granted by the EMA. The marketing authorization application for the product must include the results of all pediatric clinical trials performed and details of all information collected in compliance with the approved PIP, unless a waiver or a deferral has been granted, in which case the pediatric clinical trials may be completed at a later date. Medical products that are granted a marketing authorization on the basis of the pediatric clinical trials conducted in accordance with the approved PIP are eligible for a six month extension of the protection under a supplementary protection certificate (if any is in effect at the time of approval) or, in the case of orphan medicinal products, a two year extension of the orphan market exclusivity. This pediatric reward is subject to specific conditions and is not automatically available when data in compliance with the approved PIP are developed and submitted. An approved PIP is also required when a marketing-authorization holder wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized and covered by intellectual property rights.
Post-Approval Regulation
Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the EC and/or the competent regulatory authorities of the EU Member States. This oversight applies both before and after grant of manufacturing licenses and marketing authorizations. It includes control of compliance with EU good manufacturing practices rules, manufacturing authorizations, pharmacovigilance rules and requirements governing advertising, promotion, sale, and distribution, recordkeeping, importing and exporting of medicinal products.
Failure by us or by any of our third-party partners, including suppliers, manufacturers and distributors to comply with EU laws and the related national laws of the individual EU Member States governing the conduct of clinical trials, manufacturing approval, MA of pharmaceutical products and marketing of such products, both before and after grant of MA, manufacturing of pharmaceutical products, statutory health insurance, bribery and anti-corruption or with other applicable regulatory requirements may result in administrative, civil or criminal penalties. These penalties could include delays or refusal to authorize the conduct of clinical trials or to grant MA, product withdrawals and recalls, product seizures, suspension, withdrawal or variation of the MA, total or partial suspension of production, distribution, manufacturing or clinical trials, operating restrictions, injunctions, suspension of licenses, fines and criminal penalties.
27
The holder of a MA for a medicinal product must also comply with EU pharmacovigilance legislation and its related regulations and guidelines, which entail many requirements for conducting pharmacovigilance, or the assessment and monitoring of the safety of medicinal products.
These pharmacovigilance rules can impose on holders of MAs the obligation to conduct a labor intensive collection of data regarding the risks and benefits of marketed medicinal products and to engage in ongoing assessments of those risks and benefits, including the possible requirement to conduct additional clinical studies or post-authorization safety studies to obtain further information on a medicine’s safety, or to measure the effectiveness of risk-management measures, which may be time consuming and expensive and could impact our profitability. MA holders must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance, who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of PSURs in relation to medicinal products for which they hold MAs. The EMA reviews PSURs for medicinal products authorized through the centralized procedure. If the EMA has concerns that the riskbenefit profile of a product has varied, it can adopt an opinion advising that the existing MA for the product be suspended, withdrawn or varied. The agency can advise that the MA holder be obliged to conduct post-authorization Phase IV safety studies. If the EC agrees with the opinion, it can adopt a decision varying the existing MA. Failure by the MA holder to fulfill the obligations for which the EC’s decision provides can undermine the ongoing validity of the MA.
More generally, non-compliance with pharmacovigilance obligations can lead to the variation, suspension or withdrawal of the MA for the product or imposition of financial penalties or other enforcement measures.
The manufacturing process for pharmaceutical products in the European Union is highly regulated and regulators may shut down manufacturing facilities that they believe do not comply with regulations. Manufacturing requires a manufacturing authorization, and the manufacturing authorization holder must comply with various requirements set out in the applicable EU laws, regulations and guidance, including Directive 2001/83/EC, Directive 2003/94/EC, Regulation (EC) No 726/2004 and the European Commission Guidelines for Good Manufacturing Practice (“GMP”). These requirements include compliance with EU GMP standards when manufacturing pharmaceutical products and active pharmaceutical ingredients, including the manufacture of active pharmaceutical ingredients outside of the European Union with the intention to import the active pharmaceutical ingredients into the European Union.
Similarly, the distribution of pharmaceutical products into and within the European Union is subject to compliance with the applicable EU laws, regulations and guidelines, including the requirement to hold appropriate authorizations for distribution granted by the competent authorities of the EU Member States. The manufacturer or importer must have a qualified person who is responsible for certifying that each batch of product has been manufactured in accordance with GMP, before releasing the product for commercial distribution in the European Union or for use in a clinical trial. Manufacturing facilities are subject to periodic inspections by the competent authorities for compliance with GMP.
Advertising and Promotion
The advertising and promotion of our products is also subject to EU laws concerning promotion of medicinal products, interactions with physicians, misleading and comparative advertising and unfair commercial practices. In addition, other national legislation of individual EU Member States may apply to the advertising and promotion of medicinal products and may differ from one country to another. These laws require that promotional materials and advertising in relation to medicinal products comply with the product’s SmPC as approved by the competent regulatory authorities. The SmPC is the document that provides information to physicians concerning the safe and effective use of the medicinal product. It forms an intrinsic and integral part of the marketing authorization granted for the medicinal product. Promotion of a medicinal product that does not comply with the SmPC is considered to constitute off-label promotion. All advertising and promotional activities for the product must be consistent with the approved SmPC and therefore all off-label promotion is prohibited. Direct-to-consumer advertising of prescription-only medicines is also prohibited in the EU. Violations of the rules governing the promotion of medicinal products in the European Union could be penalized by administrative measures, fines and imprisonment. These laws may further limit or restrict the advertising and promotion of our products to the general public and may also impose limitations on its promotional activities with healthcare professionals.
28
Pricing and Reimbursement Environment
Even if a pharmaceutical product obtains a marketing authorization in the European Union, there can be no assurance that reimbursement for such product will be secured on a timely basis or at all. The EU Member States are free to restrict the range of pharmaceutical products for which their national health insurance systems provide reimbursement, and to control the prices and reimbursement levels of pharmaceutical products for human use. An EU Member State may approve a specific price or level of reimbursement for the pharmaceutical product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the pharmaceutical product on the market, including volume-based arrangements, caps and reference pricing mechanisms.
Reference pricing used by various EU Member States and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we may be required to conduct a clinical study or other studies that compare the cost-effectiveness of our product candidates, if any, to other available therapies in order to obtain or maintain reimbursement or pricing approval. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, pharmaceutical products launched in the European Union do not follow price structures of the United States and generally published and actual prices tend to be significantly lower. Publication of discounts by third party payers or authorities and public tenders may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries.
The so-called health technology assessment (“HTA”), of pharmaceutical products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including France, Germany, Ireland, Italy and Sweden. The HTA process, which is governed by the national laws of these countries, is the procedure according to which the assessment of the public health impact, therapeutic impact, and the economic and societal impact of use of a given pharmaceutical product in the national healthcare systems of the individual country is conducted. HTA generally focuses on the clinical efficacy and effectiveness, safety, cost, and cost- effectiveness of individual pharmaceutical products as well as their potential implications for the healthcare system. Those elements of pharmaceutical products are compared with other treatment options available on the market. The outcome of HTA regarding specific pharmaceutical products will often influence the pricing and reimbursement status granted to pharmaceutical products by the regulatory authorities of individual EU Member States. A negative HTA of one of our products by a leading and recognized HTA body could not only undermine our ability to obtain reimbursement for such product in the EU Member State in which such negative assessment was issued, but also in other EU Member States. For example, EU Member States that have not yet developed HTA mechanisms could rely to some extent on the HTA performed in other countries with a developed HTA framework, when adopting decisions concerning the pricing and reimbursement of a specific pharmaceutical product.
29
On January 31, 2018, the European Commission adopted a proposal for a regulation on health technology assessment. This legislative proposal is intended to boost EU level cooperation among EU Member States in assessing health technologies, including new pharmaceutical products, and providing the basis for cooperation at the EU level for joint clinical assessments in these areas. The proposal provides that EU Member States will be able to use common HTA tools, methodologies and procedures across the European Union, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU Member States will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technology, and making decisions on pricing and reimbursement. While EU Member States could choose to delay participation in the joint work until three years after the rules enter into force, it will become mandatory after six years. The European Commission has stated that the role of the HTA regulation is not to influence pricing and reimbursement decisions in the individual EU Member States, but there can be no assurance that the HTA regulation will not have effects on pricing and reimbursement decisions. The HTA entered into force on January 11, 2022 and applies as of January 2025 followed by a further three-year transitional period during which EU member states must fully adapt to the new system.
To obtain reimbursement or pricing approval in some countries, including the EU Member States, we may be required to conduct studies that compare the cost-effectiveness of our product candidates to other therapies that are considered the local standard of care. There can be no assurance that any country will allow favorable pricing, reimbursement and market access conditions for any of our products, or that we will be feasible to conduct additional cost-effectiveness studies, if required.
In certain of the EU Member States, pharmaceutical products that are designated as orphan pharmaceutical products may be exempted or waived from having to provide certain clinical, cost-effectiveness and other economic data in connection with their filings for pricing/reimbursement approval.
European Data Laws
The collection and use of personal health data and other personal information in the European Union is governed by the provisions of the European General Data Protection Regulation (EU) 2016/679) (“GDPR”), which came into force in May 2018 and related implementing laws in individual EU Member States.
The GDPR imposes a number of strict obligations and restrictions on the ability to process (processing includes collection, analysis and transfer of) personal data of individuals within the European Union and in the EEA, including health data from clinical trials and adverse event reporting. The GDPR also includes requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals prior to processing their personal data or personal health data, notification of certain data processing activities to the national data protection authorities and the security and confidentiality of the personal data. EU Member States may also impose additional requirements in relation to health, genetic and biometric data through their national implementing legislation.
Under the GDPR, personal data can only be transferred within the EU Member States and the three additional European Economic Area countries (Norway, Iceland and Liechtenstein) that have adopted a national law implementing the GDPR. Appropriate safeguards are required to enable cross-border transfers of personal data from the EU and EEA Member States to a “third country” (a country outside the EU or EEA). This status has a number of significant practical consequences, in particular for international data transfers, competent supervisory authorities and enforcement of the GDPR.
30
In conclusion, the GDPR prohibits the transfer of personal data to countries outside of the European Union/EEA (including the United States) that are not considered by the European Commission to provide an adequate level of data protection, except if the data importer meets very specific requirements such as the use of standard contractual clauses (“SCCs”), issued by the European Commission. In this respect recent legal developments in Europe have created complexity and compliance uncertainty regarding certain transfers of personal data from the EU/EEA. For example, on June 4, 2021 the EU Commission issued a new set of SCCs for data transfers from controllers or processors in the EU/EEA to controllers or processors established outside the EU/EEA. These SCCs replace the old sets of SCCs that were adopted under the previous European Data Protection Directive 95/46. There were various implementation deadlines linked to the use of these new SCCs, and all contracts incorporating SCCs had to be updated to include the new SCCs by December 27, 2022. On November 11, 2021, the European Data Protection Board has adopted recommendations on such appropriate safeguards that supplement transfer mechanisms (like the SCCs). These recommendations aim to assist data exporters with their duty to identify and implement appropriate supplementary measures where they are needed to ensure an essentially equivalent level of protection to the personal data they transfer to third countries. However, the European Commission published a set of Questions and Answers on May 25, 2022 which provides that the new 2021 SCCs do not work for data importers whose processing operations are subject to the GDPR as they would duplicate, and in part deviate from, obligations that already follow directly from the GDPR. The Commission indicated that it is in the processing of developing an additional set of SCCs for this scenario. This has created a situation where very limited transfer mechanisms exist for use by data importers in third countries such as the United States.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EU Member States may result in significant monetary fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater, other administrative penalties and a number of criminal offenses (punishable by uncapped fines) for organizations and in certain cases their directors and officers as well as civil liability claims from individuals whose personal data was processed. Data protection authorities from the different EU Member States may still implement certain variations, enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing personal data in the European Union. Guidance developed at both EU level and at the national level in individual EU Member States concerning implementation and compliance practices are often updated or otherwise revised.
There is, moreover, a growing trend towards required public disclosure of clinical trial data in the European Union which adds to the complexity of obligations relating to processing health data from clinical trials. Such public disclosure obligations are provided in the new EU CTR, EMA disclosure initiatives and voluntary commitments by industry. Failing to comply with these obligations could lead to government enforcement actions and significant penalties against us, harm to our reputation, and adversely impact our business and operating results. The uncertainty regarding the interplay between different regulatory frameworks, such as the Clinical Trials Regulation and the GDPR, further adds to the complexity that we face with regard to data protection regulation.
On June 28, 2021 the European Commission adopted two adequacy decisions for the United Kingdom – one under the GDPR and the other for the Law Enforcement Directive. Personal data may now freely flow from the European Union to the United Kingdom since the United Kingdom is deemed to have an adequate data protection level. Additionally, following the UK’s withdrawal from the European Union and the EEA, companies also have to comply with the UK’s data protection laws (including the UK GDPR, which is based on the EU GDPR), which has the ability to separately fine up to the greater of £17.5 million or 4% of global turnover. The adequacy decisions include a ‘sunset clause’ which entails that the decisions will automatically expire four years after their entry into force.
Promotional Activities
In the European Union, interactions between pharmaceutical companies and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct both at EU level and in the individual EU Member States (at a national or regional level). The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of pharmaceutical products is prohibited in the EU. The provision of benefits or advantages to physicians is also governed by the national anti-bribery laws of the EU Member States. Violation of these laws could result in substantial fines and imprisonment.
31
Payments made to physicians in certain EU Member States must be publicly disclosed. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer, his/her regulatory professional organization, and/or the competent authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the individual EU Member States (at a national or regional level). Failure to comply with these requirements could result in reputational risk, public reprimands, exclusion from public tenders, administrative penalties, fines or imprisonment.
While the UK has left the EU, as mentioned above, it should be noted that the UK still has the strictest anti-bribery regime in Europe, the UK Bribery Act 2010. The Act is applicable English law and continues to apply to any company incorporated in or “carrying on business” in the United Kingdom, irrespective of where in the world the alleged bribery activity occurs.
Other Legislation Regarding Marketing, Authorization and Pricing of Pharmaceutical Products in the European Union
Other core legislation relating to the marketing, authorization and pricing of pharmaceutical products in the European Union includes the following:
32
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in the European Union, its member states and other states of Europe that could significantly change the statutory provisions governing the testing, approval, manufacturing, marketing, coverage and reimbursement of pharmaceutical products. In addition to new legislation, pharmaceutical regulations and policies are often revised or interpreted by the EMA and national agencies in ways that may significantly affect our business and our products.
The United Kingdom (“UK”) formally left the EU on January 31, 2020 and the transition period, during which EU laws continued to apply to the UK, expired on December 31, 2020. This means EU laws now only apply to the UK in respect of Northern Ireland as laid out in the Protocol on Ireland and Northern Ireland. Following the end of the transition period, the EU and the UK concluded a trade and cooperation agreement (“TCA”), which applied provisionally from January 1, 2021 and entered into force on May 1, 2021.
The TCA includes provisions affecting the life sciences sector (including on customs and tariffs) but areas for further discussion between the EU and the UK remain. In addition, there are some specific provisions concerning pharmaceuticals. These include the mutual recognition of Good Manufacturing Practice (“GMP”) and issued GMP documents. The TCA does not, however, contain wholesale mutual recognition of UK and EU pharmaceutical regulations and product standards.
Since January 1, 2021, the EU laws which have been transposed into UK law through secondary legislation continue to be applicable in the UK as “retained EU law”. As there is no general power to amend these regulations, the UK government has enacted the Medicines and Medical Devices Act 2021. The purpose of the act is to enable the existing regulatory frameworks in relation to human medicines, clinical trials of human medicines, veterinary medicines and medical devices to be updated. The powers under the act may only be exercised in relation to specified matters and must safeguard public health.
Specified provisions of the Medicines and Medical Devices Act 2021 entered into force on February 11, 2021. The remaining provisions came into effect within two months of February 11, 2021 or will otherwise come into effect as stipulated in subsequent statutory instruments. The Medicines and Medical Devices Act 2021 supplements the UK Medical Devices Regulations 2002 (“UK Regulations”), which are based on the EU Medical Devices Directive as amended to reflect the UK’s post-Brexit regulatory regime. Notably, the UK Regulations do not include any of the revisions that have been made by the EU Medical Devices Regulation (EU) 2017/745, which, since May 26, 2021, now applies in all EU Member States.
The UK’s Medicines and Healthcare products Regulatory Agency (“MHRA”) conducted a comprehensive consultation between September and November 2021 on proposals to develop a new UK regime for medical devices in the UK. The proposals include more closely aligning definitions for medical devices and in vitro medical devices with internationally recognised definitions and changing the classification of medical devices according to levels or risk. The proposals are intended to improve patient and public safety and increase the appeal of the UK market. The new regime is planned to come into force on July 1, 2023, which will align with the date from which the UK is due to stop accepting CE marked medical devices and require UKCA (“UK Conformity Assessed”) marking. It is envisaged that, in Northern Ireland, the amended regime could run in parallel with any existing or future EU rules in accordance with the Protocol on Ireland and Northern Ireland.
33
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as, in the United States, Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost- effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor’s decision to provide coverage for a drug product does not necessarily imply that an adequate reimbursement rate will be approved. Third-party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on our investment in product development. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs, which may impact physician utilization.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third-party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider a product to be cost effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, risk sharing, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals. As a result, the marketability of any product which receives regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement.
In addition, an increasing emphasis on managed care in the United States has increased and will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, the ACA, contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs.
Since its enactment, there have been continual judicial and Congressional challenges to certain aspects of the ACA. It is unclear how these efforts to repeal, replace or otherwise modify the ACA will impact the law on reimbursement. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level, particularly as a result of the recent presidential election, or how any future legislation or regulation may affect us. Even if favorable coverage and reimbursement status is attained for one or more products that receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert
34
competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements for any of our products.
Healthcare Laws and Regulations
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with healthcare providers, physicians, third-party payors and customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
35
Various state and foreign laws also govern the privacy and security of health information in some circumstances; many of these laws differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. Violation of any of these laws or any other current or future governmental laws and regulations that may apply to drug manufacturers include significant civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm, diminished profits and future earnings, additional reporting obligations and oversight if the manufacturer becomes subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of its operations, any of which could substantially disrupt its operations. If any of the physicians or other healthcare providers or entities with whom we do business is found to be not in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Additional Regulation
In addition to the foregoing, state, and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservation and Recovery Act, and the Toxic Substances Control Act, affect our business. These and other laws govern the use, handling, and disposal of various biologic, chemical, and radioactive substances used in, and wastes generated by, operations. If our operations result in contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and governmental fines. Equivalent laws have been adopted in third countries that impose similar obligations.
Human Capital
As of December 31, 2022, we had 138 employees, approximately 62% of whom have an M.D., Ph.D., or other advanced degree. We believe that our future success largely depends upon our continued ability to attract and retain a diverse group of highly skilled employees. We provide our employees with competitive salaries and bonuses, opportunities for equity ownership, development programs that enable continued learning and growth and a robust employment package that promotes well-being across all aspects of their lives, including health care, retirement planning and paid time off. None of our employees are represented by a labor union or covered under a collective bargaining agreement. We consider our employee relations to be good.
Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. We are not currently a party to any material legal proceedings.
36
Corporate Information
We were incorporated under the laws of the State of Delaware in March 2014 under the name Unum Therapeutics Inc. On April 3, 2018, we completed our initial public offering (IPO) of our common stock under the ticker “UMRX.” On October 2, 2020, we filed an amendment to our certificate of incorporation to change our name to Cogent Biosciences, Inc. The name change became effective on October 6, 2020. In connection with the name change, our common stock began trading under the ticker symbol “COGT.”
As of December 31, 2022, we had 90,761,994 shares outstanding on a fully diluted and as-converted basis, including the 69,893,434 shares of common stock outstanding, the 606,060 pre-funded warrants that are exercisable for shares of common stock, and the 81,050 shares of Series A Preferred stock, which are convertible into 20,262,500 shares of common stock.
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended (JOBS Act). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of our IPO; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission (SEC). We may choose to take advantage of some but not all of these exemptions. We have taken advantage of the reduced reporting requirements in this Annual Report on Form 10-K. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock.
We have irrevocably elected to “opt out” of the exemption for the delayed adoption of certain accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Available Information
Our Internet address is www.cogentbio.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, including exhibits, proxy and information statements and amendments to those reports filed or furnished pursuant to Sections 13(a), 14, and 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available through the “Investors” portion of our website free of charge as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information on our website is not part of this Annual Report on Form 10-K or any of our other securities filings unless specifically incorporated herein by reference. In addition, our filings with the SEC may be accessed through the SEC’s Interactive Data Electronic Applications system at http://www.sec.gov. All statements made in any of our securities filings, including all forward-looking statements or information, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
37
ITEM 1A. RISK FACTORS
The following risk factors and other information included in this Annual Report on Form 10-K should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations. You should carefully consider the risks described below, as well as the other information in this Annual Report on Form 10-K, including our financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as our other filings with the Securities and Exchange Commission, before deciding whether to invest in our common stock. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected.
Risks Related to the Discovery and Development of Our Drug Candidates
Our business is highly dependent on the success of our bezuclastinib program and our ability to discover and develop additional product candidates. We may not be successful in our efforts to develop bezuclastinib or expand our pipeline of drug candidates.
Our business and future success depend on our ability to develop, obtain regulatory approval for and then successfully commercialize bezuclastinib and any other product candidates that we may discover and develop. We are pursuing clinical development of bezuclastinib to target SM and GIST through our APEX, SUMMIT and PEAK clinical trials. There is no guarantee that any or all of these trials will be successful. Even if our trials are successful, bezuclastinib will require regulatory review and approval, substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before we are able to generate any revenue from product sales, if ever.
Through the development of the research team, we are also working to build a pipeline of other product candidates. Researching, developing, obtaining regulatory approval for and commercializing additional product candidates will require substantial additional funding beyond the net proceeds from the public offering and private placement of our securities and consideration received from our collaborative agreements and is prone to the risks of failure inherent in medical product development. Even if we are successful in continuing to build and expand our pipeline, we cannot provide you any assurance that we will be able to successfully advance any of these additional product candidates through the development process, or that any such product candidates will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives.
If unacceptable side effects are identified during the development of our drug candidates, we may need to abandon or limit such development.
If our drug candidates are associated with unacceptable side effects in preclinical or clinical trials or have characteristics that are unexpected, we may need to abandon their development, limit development to more narrow uses or subpopulations in which the unacceptable side effects or other characteristics are less prevalent, less severe, or more acceptable from a risk-benefit perspective or highlight these risks, side effects, or other characteristics in the approved product label. In pharmaceutical development, many drugs that initially show promise in early-stage testing may later be found to cause side effects that prevent further development of the drug. Currently marketed therapies for the treatment of AdvSM and cancer are generally limited to some extent by their toxicity. In addition, some of our drug candidates would be chronic therapies, for which safety concerns may be particularly important. Use of our drug candidates as monotherapies may also result in adverse events consistent in nature with other marketed therapies. In addition, when used in combination with other therapies, our drug candidates could exacerbate adverse events associated with the other therapy. If unexpected side effects are identified during development, we may be required to develop a Risk Evaluation and Mitigation Strategy (“REMS”) to mitigate those serious safety risks, which could impose significant distribution and/or use restrictions on our products.
38
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The development and commercialization of new pharmaceutical and biotechnology products is highly competitive. We face competition with respect to our current clinical-stage drug candidates and will face competition with respect to any drug candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our drug candidates. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing, and commercialization.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have superior dosing regimens, have fewer or less severe side effects, are approved for broader indications or patient populations, are approved for specific sub-populations, are more convenient or are less expensive than bezuclastinib or any other products that we may develop. Our competitors also may obtain FDA or other marketing approval for their products more rapidly than any approval we may obtain for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic products.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing approvals, and marketing and selling approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. For further information, see “Business-Competition,” which discusses the pharmaceutical and biotechnology companies developing or marketing treatments for cancer and hematologic diseases that are competitive with bezuclastinib and the drug candidates we are developing.
As difficulties arise enrolling patients in our clinical trials, clinical development activities could be delayed or otherwise adversely affected.
We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons. As an example, clinical trial site start-up and patient enrollment in our Phase 2 SUMMIT trial has been slower than originally forecast. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. The enrollment of patients depends on many factors, including: the patient eligibility criteria defined in the protocol; the size of the patient population required for analysis of the trial’s primary endpoints; and our ability to recruit clinical trial investigators with the appropriate competencies and experience.
In addition, our clinical trials compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition reduces the number and types of patients available to us because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Additional delays in patient enrollment may result in increased costs or may affect the timing or outcome of our ongoing and planned clinical trials.
The incidence and prevalence for target patient populations of our drug candidates have not been established with precision. If the market opportunities for our drug candidates are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the patient population, our revenue potential and ability to achieve profitability will be adversely affected.
The precise incidence and prevalence for GIST and SM are unknown. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our drug candidates, are based on estimates, which are inherently uncertain. The total addressable market opportunity for bezuclastinib, and any other drug candidates we may produce will ultimately depend upon, among other things, the diagnosis criteria included in the final label for our future approved drugs for sale for these indications, acceptance by the medical community and patient access, drug pricing, and reimbursement. The number of patients in our targeted commercial markets and elsewhere may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our drug, or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
39
Clinical trials are expensive, time-consuming, and difficult to design and implement.
Human clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. We are unable to predict when or if any of our drug candidates will prove effective or safe in humans or will obtain marketing approval. Before obtaining marketing approval from regulatory authorities for the sale of any drug candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our drug candidates in humans. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, interim or preliminary results of a clinical trial do not necessarily predict final results, and results for one indication may not be predictive of the success in additional indications. In particular, the small number of patients in our early clinical trials may make the results of these trials less predictive of the outcome of later clinical trials. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy, insufficient durability of efficacy, or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that commence clinical trials are never approved as products.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to obtain marketing approval or commercialize our drug or drug candidates. Our product development costs will increase if we experience delays in preclinical studies or clinical trials or in obtaining marketing approvals. We do not know whether any of our planned preclinical studies or clinical trials will begin on a timely basis or at all, will need to be restructured, or will be completed on schedule, or at all. Significant preclinical study or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our drug candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our drug candidates and may harm our business and results of operations.
Since the number of patients that we have dosed to date in our clinical trials is small, the results from such clinical trials may be less reliable than results achieved in larger clinical trials.
A study design that is considered appropriate for regulatory approval includes a sufficiently large sample size with appropriate statistical power, as well as proper control of bias, to allow a meaningful interpretation of the results. The preliminary results of trials with smaller sample sizes can be disproportionately influenced by the impact the treatment had on a few individuals, which limits the ability to generalize the results across a broader community, thus making the study results less reliable than studies with a larger number of patients. As a result, there may be less certainty that such product candidates would achieve a statistically significant effect in any future clinical trials. In our current and any future clinical trials, we may not achieve a statistically significant result or the same level of statistical significance, if any, that we may have seen in prior clinical trials or preclinical studies.
Interim, “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available, may be interpreted differently if additional data are disclosed, and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we publicly disclose preliminary or “top-line” data from our clinical trials, which is based on a preliminary analysis of then-available data in a summary or “top-line” format, and the results and related findings may change as more patient data become available, may be interpreted differently if additional data are disclosed at a later time and are subject to audit and verification procedures that could result in material changes in the final data. If additional results from our clinical trials are not viewed favorably, our ability to obtain approval for and commercialize our drug candidates, our business, operating results, prospects, or financial condition may be harmed and our stock price may decrease.
We may choose not to develop a potential product candidate, or we may suspend, deprioritize or terminate one or more discovery programs or preclinical or clinical product candidates or programs.
At any time and for any reason, we may determine that one or more of our discovery programs or preclinical or clinical product candidates or programs does not have sufficient potential to warrant the allocation of resources toward such program or product candidate. Accordingly, we may choose not to develop a potential product candidate or elect to suspend, deprioritize or terminate one or more of our discovery programs or preclinical or clinical product candidates or programs. If we suspend, deprioritize or terminate a program or product candidate in which we have invested significant resources, we will have expended resources on a program or product candidate that will not provide a full return on our investment and may have
40
missed the opportunity to have allocated those resources to potentially more productive uses, including existing or future programs or product candidates.
We may form or seek collaborations or strategic alliances or enter into additional licensing arrangements in the future, but we may not realize any resulting benefits.
We may form or seek strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. In particular, we may seek to enter into collaborations with our bezuclastinib program and other collaborations to progress the clinical development of the bezuclastinib program. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business.
We may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy and obtain marketing approval. Further, collaborations involving our product candidates are subject to numerous technical, business, and legal risks. Even if we are successful in entering into a collaboration with respect to the development and/or commercialization of one or more product candidates, there is no guarantee that the collaboration will be successful.
We may not be able to file investigational new drug applications (“IND”s) or IND amendments or clinical trial authorization applications (“CTA”s) to commence additional clinical trials on the timelines we expect, and even if we are able to, the FDA or other regulatory authorities may not permit us to proceed.
Our timing of filing INDs or CTAs on our product candidates is dependent on further research. We cannot be sure that submission of an IND or CTA will result in the FDA or other regulatory authority allowing further clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such clinical trials.
We have limited experience as a company conducting clinical trials.
We have limited experience as a company in conducting clinical trials. In part because of this lack of experience, we cannot be certain that our ongoing clinical trials will be completed on time or if the planned clinical trials will begin or be completed on time, if at all.
Our updated bezuclastinib formulation is unproven and may not work as intended in clinical trials.
In November 2021, we announced an updated formulation of bezuclastinib which is intended to reduce the number of daily tablets required, thereby potentially improving the overall patient experience. This formulation is currently being used in our PEAK trial. The formulation is unproven to date, and there is no guarantee that it will be successful.
The commercial success of any future approved drugs, including bezuclastinib, will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
If bezuclastinib and any future approved drugs do not achieve an adequate level of acceptance by physicians, patients, third-party payors, and others in the medical community, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of bezuclastinib and of any current or future drug candidates, if approved for commercial sale, will depend on a number of factors, including the availability, perceived advantages, and relative cost, safety, and efficacy of alternative and competing treatments; and the prevalence and severity of any side effects, adverse reactions, misuse, or any unfavorable publicity in these areas, in particular compared to alternative treatments. Even if a potential drug displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the drug will not be known until after it is launched.
A variety of risks associated with marketing our product candidates internationally could materially adversely affect our business.
We plan to seek regulatory approval of our product candidates outside of the United States and, accordingly, we expect that we will be subject to additional risks and regulatory requirements related to operating in foreign countries if we obtain the
41
necessary approvals. Risks associated with our international operations may materially adversely affect our ability to attain or maintain profitable operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products.
The COVID-19 pandemic, and the future outbreak of other highly infectious or contagious diseases, could seriously harm our development efforts, increase our costs and expenses and have a material adverse effect on our business, financial condition and results of operations.
The extent to which the COVID-19 pandemic, or the future outbreak of any other highly infectious or contagious diseases, impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the scope, severity and duration of such pandemic, the actions taken to contain the pandemic or mitigate its impact, and the direct and indirect economic effects of the pandemic and containment measures, among others. The ongoing development and fluidity of this situation precludes any prediction as to the full adverse impact of the COVID-19 pandemic. Nevertheless, the COVID-19 pandemic has already affected and may continue to adversely affect our business, financial condition and results of operations, including the below:
Risks Related to Our Reliance on Third Parties
We currently rely and for the foreseeable future will continue to rely on third parties to conduct our clinical trials and to assist with various research, discovery, manufacturing and supply activities. If these third parties do not properly and successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval of or commercialize our product candidates or discover new product candidates.
We depend and will depend upon independent investigators and collaborators, such as medical institutions, contract research organizations (“CROs”), contract manufacturing organizations (“CMO”s) and strategic partners to conduct our preclinical studies and clinical trials under agreements with us. We will rely heavily on these third parties over the course of our clinical trials, and we control only certain aspects of their activities. As a result, we have less direct control over the conduct, timing and completion of these clinical trials and the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. We and these third parties are required to comply with good clinical practices (“GCP”s), which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for product candidates in clinical development. If we or any of these third parties fail to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. In addition, failure or any failure by these third parties to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could affect their performance on our behalf.
42
If any CMO with whom we contract fails to perform its obligations, we may be forced to manufacture the materials ourselves, for which we may not have the capabilities or resources, or enter into an agreement with a different CMO, which we may not be able to do on reasonable terms, if at all. In either scenario, our clinical trials supply could be delayed significantly as we establish alternative supply sources. In some cases, the technical skills required to manufacture our products or product candidates may be unique or proprietary to the original CMO and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills to a back-up or alternate supplier, or we may be unable to transfer such skills at all. Furthermore, a CMO may possess technology related to the manufacture of our product candidate that such CMO owns independently. This would increase our reliance on such CMO or require us to obtain a license from such CMO in order to have another CMO manufacture our product candidates. In addition, changes in manufacturers often involve changes in manufacturing procedures and processes, which could require that we conduct bridging studies between our prior clinical supply used in our clinical trials and that of any new manufacturer. We may be unsuccessful in demonstrating the comparability of clinical supplies which could require the conduct of additional clinical trials.
We also rely on third party vendors and collaborators to support our research and discovery efforts and to help expand our drug candidate pipeline, including certain third parties located in China, and we expect to continue to use such third parties. A natural disaster, epidemic or pandemic disease outbreaks, including the COVID-19 pandemic, trade war, political unrest or other local events could disrupt the business or operations of these third parties and thus negatively impact our research and discovery capabilities.
We contract with third parties for the manufacture of our drug candidates for preclinical development and clinical trials. This reliance on third parties increases the risk that we will not have sufficient quantities of our drug candidates or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not currently own or operate any manufacturing facilities. We rely, and expect to continue to rely, on third parties for the manufacture of our drug candidates for preclinical development and clinical testing, as well as for the commercial manufacture of our current and future drugs. This reliance on third parties increases the risk that we will not have sufficient quantities of our drug candidates or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts.
We do not have long-term supply agreements with our contract manufacturers, and purchase our required drug supply, including the API and drug product used in our drug candidates, on a purchase order basis with certain contract manufacturers. In addition, we may be unable to establish or maintain any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish and maintain agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks. In addition, our drug candidates may compete with other drug candidates for access to manufacturing facilities. As a result, we may not obtain access to these facilities on a priority basis or at all. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
For our other potential products, if we are not able to negotiate commercial supply terms with any such third-party manufacturers, we may be unable to commercialize our products if they were to be approved, and our business and financial condition would be materially harmed. If we are forced to accept unfavorable terms for our relationships with any such third-party manufacturer, our business and financial condition would be materially harmed.
Third-party manufacturers may not be able to comply with the FDA’s cGMP regulations or similar regulatory requirements outside of the U.S. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, seizures or voluntary recalls of drug candidates or products, operating restrictions, and criminal prosecutions, any of which could significantly and adversely affect supplies of our products. Third-party manufacturers’ failure to achieve and maintain high manufacturing standards, in accordance with applicable regulatory requirements, or the incidence of manufacturing errors, also could result in patient injury or death, product shortages, delays or failures in product testing or delivery, cost overruns, or other problems that could seriously harm our business. Third-party manufacturers often encounter difficulties involving production yields, quality control, and quality assurance, as well as shortages of qualified personnel.
43
The third parties upon whom we rely for the supply of the API and drug product used in bezuclastinib are our sole source of supply, and the loss of any of these suppliers could significantly harm our business.
The API and drug product used in bezuclastinib are currently supplied to us from single-source suppliers. Our ability to successfully develop our drug candidates and supply our drug candidates for clinical trials, depends in part on our ability to obtain the API and drug product for these drugs in accordance with regulatory requirements and in sufficient quantities for clinical testing. We will need to enter into arrangements to establish redundant or second-source supply of some of the API and drug product. If any of our suppliers ceases its operations for any reason or is unable or unwilling to supply API or drug product in sufficient quantities or on the timelines necessary to meet our needs, including as a result of the COVID-19 pandemic, it could significantly and adversely affect our business, the supply of our current or future drug candidates or any future approved drugs and our financial condition.
For bezuclastinib and any other product candidates, we intend to identify and qualify additional manufacturers to provide such API and drug product prior to submission of a New Drug Application (“NDA”) to the FDA and/or a Marketing Authorization Application (“MAA”) to the EMA. We are not certain, however, that our single-source suppliers will be able to meet our demand for their products, either because of the nature of our agreements with those suppliers, our limited experience with those suppliers or our relative importance as a customer to those suppliers. It may be difficult for us to assess their ability to timely meet our demand in the future based on past performance and they may subordinate our needs in the future to their other customers.
While we seek to maintain adequate inventory of the API and drug product used in our current or future drug candidates and any future approved drugs, any interruption or delay in the supply of components or materials, or our inability to obtain such API and drug product from alternate sources at acceptable prices in a timely manner could impede, delay, limit or prevent our development efforts, which could harm our business, results of operations, financial condition and prospects.
Risks Related to Regulatory Approval of Our Drug Candidates and Other Legal Compliance Matters
The FDA regulatory approval process is lengthy and time-consuming, and we may experience significant delays in the clinical development and regulatory approval of our product candidates.
We currently have one drug candidate in clinical development and its risk of failure is high. We are unable to predict when or if any of our drug candidates will prove effective or safe in humans or will obtain marketing approval. Before obtaining marketing approval from regulatory authorities for the sale of any drug candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our drug candidates in humans.
While bezuclastinib is a highly potent and selective KIT D816V inhibitor that is being developed to treat SM and GIST patients, we may find that patients treated with bezuclastinib have or develop mutations that confer resistance to treatment. If patients have or develop resistance to treatment with our drug candidates, we may be unable to successfully complete our clinical trials, and may not be able to obtain regulatory approval of, and commercialize, our drug candidates.
Our product development costs will increase if we experience delays in preclinical studies or clinical trials or in obtaining marketing approvals. We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to obtain marketing approval or commercialize our drug candidates. We may utilize companion diagnostics in our planned clinical trials in the future in order to identify appropriate patient populations for our drug candidates. If a satisfactory companion diagnostic is not commercially available, we may be required to create or obtain one that would be subject to regulatory approval requirements. The process of obtaining or creating such diagnostic is time consuming and costly.
44
Regulatory authorities, including the FDA, may disagree with our regulatory plan and we may fail to obtain regulatory approval of our product candidates.
We are conducting clinical trials with our lead product candidate, bezuclastinib, in patients with GIST, AdvSM and Non-AdvSM. The FDA may not agree with our regulatory plans for initial registration of bezuclastinib in some or all of these indications and may require additional clinical trials to be conducted prior to approval. Our clinical trial results may also not support approval.
In addition, our product candidates could fail to receive regulatory approval for many reasons, including if we are unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that our product candidates are safe and effective for any of their proposed indications, or that our product candidates’ clinical and other benefits outweigh their safety risks.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. We may also submit marketing applications in other countries. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
The impact of healthcare legislation and other changes in the healthcare industry and in healthcare spending on us is currently unknown, and may adversely affect our business model.
Our revenue prospects could be affected by changes in healthcare spending and policy in the United States and abroad. We operate in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to healthcare availability, the method of delivery or payment for healthcare products and services could negatively impact our business, operations and financial condition.
There has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. In fact, both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation, regulations, and policies affecting coverage and reimbursement rates, which are designed to contain or reduce the cost of health care. Further federal and state proposals and healthcare reforms are likely, which could limit the prices that can be charged for the product candidates that we develop and may further limit our commercial opportunity. There may be future changes that result in reductions in potential coverage and reimbursement levels for our product candidates, if approved and commercialized, and we cannot predict the scope of any future changes or the impact that those changes would have on our operations. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect us.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. If we obtain FDA approval of any of our product candidates and begin commercializing those products in the United States, our potential exposure under the regulations of the FDA and other similar foreign regulatory bodies will increase significantly, and our costs associated with compliance with such laws and regulations are also likely to increase. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business.
45
We face potential liability related to the privacy of health information we obtain from clinical trials sponsored by us.
Most healthcare providers, including research institutions from which we obtain patient health information, are subject to privacy and security regulations promulgated under HIPAA, as amended by the HITECH. We are not currently classified as a covered entity or business associate under HIPAA and thus are not directly subject to its requirements or penalties. However, any person may be prosecuted under HIPAA’s criminal provisions either directly or under aiding-and-abetting or conspiracy principles. Consequently, depending on the facts and circumstances, we could face substantial criminal penalties if we knowingly receive individually identifiable health information from a HIPAA-covered healthcare provider or research institution that has not satisfied HIPAA’s requirements for disclosure of individually identifiable health information. In addition, we may maintain sensitive personally identifiable information, including health information, that we receive throughout the clinical trial process, in the course of our research collaborations, and directly from individuals (or their healthcare providers) who enroll in our patient assistance programs. As such, we may be subject to state laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA.
Furthermore, certain health privacy laws, data breach notification laws, consumer protection laws and genetic testing laws may apply directly to our operations and/or those of our collaborators and may impose restrictions on our collection, use and dissemination of individuals’ health information. Patients about whom we or our collaborators obtain health information, as well as the providers who share this information with us, may have statutory or contractual rights that limit our ability to use and disclose the information. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws. Claims that we have violated individuals’ privacy rights or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business. Additionally, we are subject to state and foreign equivalents of each of the healthcare laws described above, among others, some of which may be broader in scope and may apply regardless of the payor.
If we are unable to successfully develop companion diagnostic tests for our drug candidates that require such tests, or experience significant delays in doing so, we may not realize the full commercial potential of these drug candidates.
We may develop, either by ourselves or with collaborators, in vitro companion diagnostic tests for our drug candidates for certain indications. To be successful, we or our collaborators will need to address a number of scientific, technical, regulatory, and logistical challenges. The FDA regulates in vitro companion diagnostics as medical devices that will likely be subject to clinical trials in conjunction with the clinical trials for our drug candidates, and which will require regulatory clearance or approval prior to commercialization. We may rely on third parties for the design, development, and manufacture of companion diagnostic tests for our therapeutic drug candidates that require such tests. If these parties are unable to successfully develop companion diagnostics for these therapeutic drug candidates, or experience delays in doing so, the development of these therapeutic drug candidates may be adversely affected or may not obtain marketing approval, and we may not realize the full commercial potential of any of these therapeutics that obtain marketing approval.
Risks Related to Our Intellectual Property
If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our market.
We rely upon a combination of patents, confidentiality agreements, trade secret protection and license agreements to protect the intellectual property related to our technologies. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
Currently, we have patents issued from our in-licensed portfolio under our license agreement with Plexxikon. in multiple territories, including but not limited to, Australia, Brazil, Canada, China, Colombia, Europe (validated in Germany, Spain, France, Great Britain, Italy, the Netherlands, as well as various other EU countries), Hong Kong, India, Indonesia, Israel, Japan, Mexico, New Zealand, Peru, the Philippines, Republic of Korea, Russia, Singapore, South Africa, Taiwan, and the United States. We also have patent applications pending in Australia, Brazil, Canada, China, Egypt, Europe, India, Indonesia, Israel, Japan, Korea, Mexico, Philippines, Singapore, South Africa, and the United States. We anticipate additional patent applications will be filed both in the United States and in other countries, as appropriate. There is no guarantee that patent applications will provide meaningful protection or result in patents being issued and granted.
46
Third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patent applications we hold with respect to our product candidates is threatened, it could dissuade companies from collaborating with us to develop, and threaten our ability to commercialize, our product candidates. Further, if we encounter delays in our clinical trials, the period of time during which we could market our product candidates under patent protection would be reduced.
We depend on intellectual property licensed from third parties and termination of any of these licenses could result in the loss of significant rights, which would harm our business.
We are dependent on patents, know-how and proprietary technology, both our own and licensed from others. In particular, bezuclastinib and other molecules are subject to a license from Plexxikon. We expect in the future to be party to additional material license or collaboration agreements. Any termination of our current or future licenses could result in the loss of significant rights and could harm our ability to commercialize our product candidates. These licenses do and future licenses may include provisions that impose obligations and restrictions on us. This could delay or otherwise negatively impact a transaction that we may wish to enter into. Disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement. If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates. We are generally also subject to all of the same risks with respect to protection of intellectual property that we license, as we are for intellectual property that we own, which are described below. If we or our licensors fail to adequately protect this intellectual property, our ability to commercialize products could suffer.
We may not be successful in obtaining or maintaining necessary rights to product components and processes for our development pipeline through acquisitions and in-licenses.
Presently we have rights to certain intellectual property, through licenses from third parties and under patent applications that we own or will own, related to bezuclastinib , and certain other product candidates. Because additional product candidates may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license or use these proprietary rights. Our product candidates may also require specific formulations to work effectively and efficiently and these rights may be held by others. Similarly, efficient production or delivery of our product candidates may also require specific compositions or methods, and the rights to these may be owned by third parties. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology.
Third-party claims of intellectual property infringement may prevent or delay our product discovery and development efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others. Third parties may assert that we are employing their proprietary technology without authorization. While we do not believe that any claims that could materially adversely affect commercialization of our product candidates, if approved, are valid and enforceable, we may be incorrect in this belief, or we may not be able to prove it in litigation. There may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may infringe. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
47
We may fail to identify relevant patents or incorrectly conclude that a patent is invalid, not enforceable, exhausted, or not infringed by our activities. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our product candidates, molecules used in or formed during the manufacturing process, or any final product itself, the holders of any such patents may be able to block our ability to commercialize the product candidate unless we obtained a license under the applicable patents, or until such patents expire or they are finally determined to be held invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy or patient selection methods, the holders of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtained a license or until such patent expires or is finally determined to be held invalid or unenforceable. In either case, if we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, could involve substantial litigation expense and would be a substantial diversion of employee resources from our business. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
An unfavorable outcome of any post-grant proceedings, including interference proceedings, provoked by third parties or brought by the USPTO could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or post-grant proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court or the USPTO.
If we or one of our licensing partners initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate, as applicable, is invalid and/or unenforceable. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business.
48
Risks Related to Employee Matters and Managing Growth
We are highly dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our inability or failure to successfully attract and retain qualified personnel, particularly at the management level, could adversely affect our ability to execute our business plan and harm our operating results. We are highly dependent on our management, scientific and medical personnel, including our Chief Executive Officer and President, our Chief Financial Officer, our Chief Technology Officer, our Chief Scientific Officer, our Chief Medical Officer and our Chief Legal Officer. The loss of the services of any of our executive officers, other key employees and other scientific and medical advisors, and an inability to find suitable replacements could result in delays in product development and harm our business.
Competition for skilled personnel in our market is intense and may limit our ability to hire and retain highly qualified personnel on acceptable terms or at all. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical personnel. Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. The employment agreements with our key employees provide for at-will employment, which means that any of our employees could leave our employment at any time, with or without notice.
We have undergone significant growth across both locations over the past year and we may face challenges in managing our growth.
During the year ended December 31, 2022, we increased our headcount from 77 to 138 full time employees through the expansion of our research, development, manufacturing and G&A infrastructure, and we moved into new offices and labs in Massachusetts and Colorado, respectively. To manage these organizational changes and growth, we must continue to enhance our operational, financial and management controls and systems, reporting systems and infrastructure, and policies and procedures. We may not be able to implement enhancements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls. We must also continue to recruit, train and retain qualified personnel and we may be unable to do so effectively. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our development timelines may be delayed, our ability to generate revenue could be reduced, and we may not be able to implement our business strategy.
Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems.
We are increasingly dependent on our information technology systems and infrastructure for our business. We collect, store and transmit sensitive information including intellectual property, proprietary business information and personal information in connection with business operations. The secure maintenance of this information is critical to our operations and business strategy. Some of this information could be an attractive target of criminal attack or unauthorized access and use by third parties with a wide range of motives and expertise, including organized criminal groups, “hacktivists,” patient groups, disgruntled current or former employees and others. Cyber-attacks are of ever-increasing levels of sophistication, and despite our security measures, our information technology and infrastructure may be vulnerable to such attacks or may be breached, including due to employee error or malfeasance.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage or interruption from computer viruses, unauthorized or inappropriate access or use, natural disasters, pandemics (including COVID-19), terrorism, war, and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts, as well as delays in the commercialization of our products, and significantly increase our costs. To the extent that any disruption, security breach or unauthorized or inappropriate use or access to our systems were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, including but not limited to patient, employee or vendor information, we could incur notification obligations to affected individuals and government agencies, liability, including potential lawsuits from patients, collaborators, employees, stockholders or other third parties and liability under foreign, federal and state laws that protect the privacy and security of personal information, and the development and potential commercialization of our product candidates could be delayed.
49
Risks Related to Our Financial Position and Need for Additional Capital
We will require substantial additional funding. If we fail to obtain additional financing when needed, or on attractive terms, we may be unable to complete the development and commercialization of our product candidates.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to continue the clinical and preclinical development of our product candidates, including our clinical trials for bezuclastinib. If approved, we will require significant additional amounts in order to launch and commercialize our product candidates. We cannot be certain that additional funding will be available on acceptable terms, or at all. Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidates or other research and development initiatives. Our license agreements may also be terminated if we are unable to meet the payment and other obligations under the agreements. We could be required to seek collaborators for our product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available or relinquish or license on unfavorable terms our rights to our product candidates in markets where we otherwise would seek to pursue development or commercialization ourselves.
Any of the above events could significantly harm our business, prospects, financial condition and results of operations and cause the price of our common stock to decline.
We have incurred net losses in every year since our inception and anticipate that we will continue to incur net losses in the future.
We are a clinical-stage biopharmaceutical company with a limited operating history. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate effect or an acceptable safety profile, gain regulatory approval and become commercially viable. We have no products approved for commercial sale and have not generated any revenue from product sales to date, and we continue to incur significant research and development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in each period since our inception in March 2014. For further information, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
There can be no assurance that the product candidates under development by us will be approved for sale in the United States or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will be successfully commercialized, which would have an adverse effect on our business prospects, financial condition and results of operation. Even if we succeed in commercializing one or more of our product candidates, we will continue to incur substantial research and development and other expenditures to develop and market additional product candidates. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
50
Our ability to use net operating losses and research and development credits to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating losses or tax credits, or NOLs or credits, to offset future taxable income or taxes. As a result of the shares issued in July 2020 related to the acquisition of Kiq and the sale of Series A convertible preferred stock, the Company has experienced a change in ownership, as defined by Section 382. As a result of the ownership change, utilization of the federal and state net operating loss carryforwards and research and development tax credit carryforwards is subject to annual limitation under Section 382. Under Section 382, the annual limitation is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term tax-exempt rate, and then could be subject to additional adjustments, as required. This limitation resulted in the expiration of federal and state net operating loss carryforwards before utilization of $26.9 million and $79.5 million, respectively, and federal and state research and development tax credit carryforwards before utilization of $6.6 million and $2.0 million, respectively. We have written off the deferred tax assets related to these attributes, which were previously fully reserved for, in 2020. As of December 31, 2022, approximately $66.4 million and $2.8 million of federal and state net operating losses, respectively, as well as $7.1 million of future amortization for federal purposes, were subject to the July 6 limitation of $0.3 million per year. A second ownership change occurred in December 2020 as a result of the underwritten public offering of common stock which resulted in a limitation of tax attributes generated from July 2020 to December 2020. The December 1, 2020 ownership change is not expected to have a material impact to the Company’s net operating loss carryforwards or research and development tax credit carryforwards as these net operating losses and tax credit carryforwards may be utilized, subject to annual limitation, assuming sufficient taxable income is generated before expiration. The Company has not performed a Section 382 analysis since December 2020.
Risks Related to Ownership of our Common Stock
The price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock is likely to continue to be highly volatile. Market prices for our common stock could be subject to wide fluctuations in response to various factors. In addition, the stock market in general, and The Nasdaq Global Select Market and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. If the market price of our common stock does not exceed your purchase price, you may not realize any return on your investment in us and may lose some or all of your investment. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results, or financial condition.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant influence over matters subject to stockholder approval.
Our executive officers, directors, and 5% stockholders beneficially owned approximately 56% of our outstanding common stock as of December 31, 2022. These stockholders will have the ability to influence us through this ownership position. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of our directors, amendments to our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that may be in the best interests of our stockholders.
An active trading market for our common stock may not be sustained.
Given the low trading volumes of our common stock, there is a risk that an active trading market for our shares may not be sustained, which could put downward pressure on the market price of our common stock and thereby affect the ability of our stockholders to sell some or all of their shares at attractive prices, at the times and in the volumes that they would like to sell them, or at all.
51
Future sales and issuances of our common stock or rights to purchase common stock could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, research and development activities, and incurring costs associated with operating as a public company. To raise capital, we may sell common stock, convertible securities, or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities, or other equity securities, investors may be materially diluted by such sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences, and privileges senior to the holders of our common stock.
Anti-takeover provisions under our charter documents and Delaware law could delay or prevent a change of control, which could limit the market price of our common stock and may prevent or frustrate attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporate Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These anti-takeover provisions and other provisions in our amended and restated certificate of incorporation and amended and restated bylaws could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by the then-current board of directors and could also delay or impede a merger, tender offer, or proxy contest involving our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing or cause us to take other corporate actions you desire. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our amended and restated certificate of incorporation or our amended and restated bylaws, any action to interpret, apply, enforce, or determine the validity of our certificate of incorporation or bylaws or any action asserting a claim against us that is governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
52
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Waltham, Massachusetts
Our corporate headquarters are located in Waltham, Massachusetts, where we sublease approximately 17,749 square feet of office space pursuant to a sublease agreement that commenced in June 2022 and expires in September 2026. This facility primarily houses our clinical, regulatory, and administrative personnel.
Boulder, Colorado
We lease approximately 44,657 square feet of office and laboratory space in Boulder, Colorado. The Boulder Lease has an initial term of 12 years with the option to extend for three successive five-year terms. This facility primarily houses our research and other administrative personnel.
Cambridge, Massachusetts
We also lease approximately 33,500 square feet of office and laboratory space in Cambridge, Massachusetts pursuant to a lease agreement that commenced in July 2015 and expires in April 2023. This served as our corporate headquarters before moving to our new location in Waltham, Massachusetts. We sublease approximately 70% of this space under the terms of a sublease agreement.
We believe that our current facilities are adequate to meet our immediate needs.
ITEM 3. LEGAL PROCEEDINGS
We are not currently a party to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Regardless of the outcome, litigation can have a material adverse effect on us because of defense and settlement costs, diversion of management resources, and other factors.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
53
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Certain Information Regarding the Trading of Our Common Stock
Our common stock trades under the symbol “COGT” on the Nasdaq Global Select Market and has been publicly traded since March 29, 2018. On October 2, 2020, we filed an amendment to our certificate of incorporation to change our name from Unum Therapeutics Inc. to Cogent Biosciences, Inc. The name change became effective on October 6, 2020. In connection with the name change, our common stock began trading under the ticker symbol “COGT.” Our common stock previously traded under the ticker symbol “UMRX.” Prior to March 29, 2018, there was no public market for our common stock.
Holders of Our Common Stock
As of March 12, 2023, there were approximately 4 holders of record of shares of our common stock. This number does not include stockholders for whom shares are held in “nominee” or “street” name.
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
Securities authorized for issuance under equity compensation plans
Information about our equity compensation plans will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
Recent Sales of Unregistered Equity Securities
None.
Issuer Purchases of Equity Securities
None.
ITEM 6. Reserved
54
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes appearing at the end of this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, includes forward looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report on Form 10-K, our actual results could differ materially from the results described in, or implied by, the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biotechnology company focused on developing precision therapies for genetically defined diseases. Our approach is to design rational precision therapies that treat the underlying cause of disease and improve the lives of patients. Our lead drug candidate, bezuclastinib, is designed to target exon 17 mutations found within the KIT receptor tyrosine kinase, including KIT D816V. When KIT D816V remains in a perpetual ‘on’ state in mast cells, a type of white blood cell, it causes them to accumulate in various internal organs including the bone marrow. The result is an orphan disease called Systemic Mastocytosis (“SM”). Exon 17 mutations have also been found in advanced Gastrointestinal Stromal Tumors (“GIST”), which have a strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a new treatment option for patients with both of these diseases. In addition to bezuclastinib, the Cogent Research Team is developing a portfolio of novel targeted therapies to help patients fighting serious, genetically driven diseases and is initially targeting FGFR2 and ErbB2.
Bezuclastinib
In October 2021, we presented preclinical data in a virtual poster at the 2021 AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics that identified bezuclastinib as a differentiated, potent and selective KIT mutant inhibitor with unique selectivity for KIT D816V and minimal evidence of brain penetration that avoids targeting PDGFR isoforms. In April 2022, we presented additional preclinical data at the 2022 American Associated for Cancer Research annual meeting (“AACR”) demonstrating that bezuclastinib potently inhibits A loop-mutations exquisitely selective against other closely related kinases, and differentiates bezuclastinib by its lack of brain penetration. We also presented data supporting that bezuclastinib inhibits KIT downstream signaling and may drive tumor regressions at clinically achievable doses.
We are continuing the development of bezuclastinib in patients living with Advanced Systemic Mastocytosis (“AdvSM”) and Non-Advanced Systemic Mastocytosis (“Non-AdvSM”). The vast majority of AdvSM and Non-AdvSM patients have a KIT D816V mutation. Patients with AdvSM have a significantly diminished lifespan with a median survival of less than 3.5 years. For patients with Non-AdvSM, there are no available approved therapies, and while their lifespan is not impacted by the disease, these patients suffer from a poor quality of life and new treatment options are badly needed.
APEX is our global, open-label, multi-center, Phase 2 clinical trial in patients with AdvSM evaluating the safety, efficacy, pharmacokinetic, and pharmacodynamic profiles of bezuclastinib. In June 2022, we reported positive initial clinical data from the ongoing APEX trial at the 2022 European Hematology Association Annual Congress and we presented updated positive clinical data in an oral presentation at the American Society of Hematology Annual Meeting in December 2022. We expect to provide an update on the planned initiation of APEX Part 2 based on clinical data from approximately 25-30 patients in APEX Part 1 in mid-2023.
SUMMIT is our randomized, double-blind, placebo-controlled, global multi-center Phase 2 clinical trial for patients with Non-AdvSM. The study is designed to evaluate the safety and efficacy of bezuclastinib in patients with moderate to severe Indolent Systemic Mastocytosis or Smoldering Systemic Mastocytosis. Based on the performance of bezuclastinib’s new formulation in the PEAK lead-in trial, as well as in a healthy normal volunteer study, the SUMMIT trial protocol was amended in 2022 to allow for the new formulation to be introduced during the dose exploration phase. We expect to report initial clinical data in patients with Non-AdvSM in the second half of 2023. In March 2023, Cogent received approvals from European regulatory authorities to initiate the SUMMIT trial in patients with Non-AdvSM. Beginning in April 2023, we expect to start activating clinical trial sites across major countries in the European Union.
55
We are also pursuing the development of bezuclastinib in patients living with GIST based on our study of more than 50 advanced solid tumor and GIST patients in a Phase 1/2 clinical trial, with the vast majority of those patients living with advanced GIST. GIST is a disease frequently driven by KIT mutations, and resistance to currently available therapeutics is frequently associated with the emergence of other KIT mutations. Anti-tumor activity for bezuclastinib was observed in both single agent and combination settings, including in combination with sunitinib, an approved treatment option for GIST patients. Clinical data from the Phase 1/2 clinical trial were published in the Journal of American Medical Association and were presented at several scientific conferences, including most recently by Cogent at the 2020 annual Connective Tissue Oncology Society (“CTOS”) meeting, and previously by Plexxikon Inc., a member of the Daiichi Sankyo Group (“Plexxikon”), at the 2018 annual American Society of Clinical Oncology meeting and the 2017 annual CTOS meeting. Within the group of 15 heavily pre-treated GIST patients who received the combination of bezuclastinib and sunitinib, and who had not received prior treatment with bezuclastinib, the confirmed objective response rate was twenty percent, including two partial responses and one complete response, while the estimated median progression free survival (“mPFS”) for this group was twelve months. Four subjects continued to receive bezuclastinib via individual patient INDs beyond the conclusion of the trial.
PEAK is our randomized open-label, global Phase 3 clinical trial designed to evaluate the safety, tolerability, and efficacy of bezuclastinib in combination with sunitinib compared to sunitinib alone in patients with locally advanced, unresectable or metastatic GIST who have received prior treatment with imatinib. The FDA has granted orphan drug designation to bezuclastinib for the treatment of GIST.
In November 2021, through a partnership with Serán Biosciences, we announced the development of an optimized formulation of bezuclastinib, which was used in the PEAK lead-in study. Based on the data from the PEAK lead-in study we have initiated the randomized portion of PEAK using a 600 mg dose of a new formulation of bezuclastinib, which in the lead-in portion of the study demonstrated clinical exposure equivalent to the 1,000 mg original formulation used in our GIST Phase 1/2 clinical trial. Initial safety and pharmacokinetic data from the PEAK lead-in study was presented at the CTOS annual meeting in November 2022. We expect to present updated clinical data from refractory GIST patients in the lead-in cohort of the Phase 3 PEAK trial of bezuclastinib plus sunitinib during the first half of 2023.
Worldwide rights to develop and commercialize bezuclastinib are exclusively licensed from Plexxikon. Under the terms of the license agreement, Plexxikon received an upfront payment and is eligible for additional development milestones of up to $7.5 million upon the satisfaction of certain clinical milestones and up to $25.0 million upon the satisfaction of certain regulatory milestones. During the second quarter of 2022, as a result of the progression of the Peak study, the first clinical milestone was achieved, resulting in payment of $2.5 million to Plexxikon in June 2022.
Patents protecting bezuclastinib include composition of matter claims which have been issued in the US and other key territories and provide exclusivity through 2033 and potentially beyond through patent term extensions. In addition, we intend to file a provisional patent application seeking to protect our new formulation of bezuclastinib, which could potentially provide exclusivity through at least 2043.
Research programs
During the second quarter of 2021, we announced the formation of the Cogent Research Team, a highly experienced discovery and research group. Based in Boulder, Colorado, the Cogent Research Team is focused on pioneering best-in-class, small molecule therapeutics to expand our pipeline and deliver novel precision therapies for patients living with unmet medical needs. Our research team is building a pipeline of small molecule inhibitors, with our first efforts aimed toward targeting currently undrugged mutations in fibroblast growth factor receptor ("FGFR"). FGFR mutations are well-established oncogenic drivers in multiple diseases, but approved medicines fail to capture the full landscape of FGFR altered tumor types, with FGFR1-mediated hyperphosphatemia serving as the most common dose-limiting toxicity for pan-FGFR inhibitors. In October 2022, we reported preclinical data at EORTC-NCI-AACR (ENA) annual meeting on a next-generation fibroblast growth factor receptor 2 (“FGFR2”) program, which retains potency across all primary, gatekeeper and molecular brake resistance mutations, including N549K and V564I, while sparing FGFR1 inhibition.
56
Our research team is also advancing a novel, ErbB2 mutant program, which is focused on actionable and underserved mutations in a variety of solid tumor indications. Currently available oral ErbB2 inhibitors struggle to provide broad mutant coverage while sparing EGFR activity. In October 2022, presented preclinical data at ENA on a novel ErbB2 mutant selective program which demonstrates robust cellular inhibition of all key resistance and primary driver mutations, including L755S, V842I and S310F/Y, while sparing wild type EGFR target engagement.
For both FGFR and ErBB2, we see an opportunity to provide a more robust molecular response compared to existing therapies. We expect to initiate clinical trials for both of these programs in 2024.
Since our inception in 2014, we have focused significant efforts and financial resources on establishing and protecting our intellectual property portfolio, conducting research and development of our product candidates, manufacturing drug product material for use in preclinical studies and clinical trials, staffing our company, and raising capital. We do not have any products approved for sale and have not generated any revenue from product sales. Our ability to generate product revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of our product candidates. Our net losses were $140.2 million for the year ended December 31, 2022 compared to net losses of $72.3 million for the year ended December 31, 2021. As of December 31, 2022, we had an accumulated deficit of $411.2 million. We expect to continue to incur significant expenses and operating losses for at least the next several years. We expect that our expenses and capital requirements will increase substantially in connection with our ongoing activities, particularly if and as we:
We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for our product candidates. If we obtain regulatory approval for any of our product candidates and do not enter into a commercialization partnership, we expect to incur significant expenses related to developing our internal commercialization capability to support product sales, marketing, and distribution.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back, or discontinue the development and commercialization of one or more of our product candidates.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
57
As of December 31, 2022, we had cash, cash equivalents and marketable securities of $259.3 million. Based on our current plans, we expect that our current cash, cash equivalents and marketable securities will be sufficient to fund our operating expenses and capital expenditure requirements into 2025.
Components of Our Results of Operations
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts, and the development of our product candidates, which include:
We expense research and development costs as incurred. Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. The prepaid amounts are expensed as the related goods are delivered or the services are performed.
Certain of our direct research and development expenses are tracked on a program-by-program basis and consist of costs, such as fees paid to consultants, contractors, CMOs, and CROs in connection with our discovery, preclinical and clinical development activities. We do not allocate employee costs, costs associated with the manufacture of bezuclastinib, costs associated with our discovery efforts, laboratory supplies, and facilities, including depreciation or other indirect costs, to specific product development programs because these costs are deployed across multiple product development programs and, as such, are not separately classified.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will increase substantially in connection with our planned clinical and preclinical development activities in the near term and in the future. At this time, we cannot reasonably estimate or know the nature, timing, and costs of the efforts that will be necessary to complete the preclinical and clinical development of any of our product candidates. The successful development and commercialization of our product candidates is highly uncertain. This is due to the numerous risks and uncertainties associated with product development and commercialization, including the following:
58
A change in the outcome of any of these variables with respect to the development of any of our product candidates could significantly change the costs and timing associated with the development of that product candidate. We may never succeed in obtaining regulatory approval for any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs, including stock-based compensation, for personnel in executive, finance, and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, patent, consulting, investor and public relations, accounting, and audit services. We anticipate that our general and administrative expenses will increase in the future as a result of the costs associated with the expansion of operations to support our on-going discovery, preclinical and clinical activities.
Interest Income
Interest income consists of interest earned on our cash equivalents and marketable securities balances.
Sublease Income
Sublease income consists of income from subleasing a portion of our former headquarters facilities in Cambridge, Massachusetts.
Other Income, Net
Other income consists of miscellaneous income and expense unrelated to our core operations.
Change in Fair Value of the CVR liability
This consists of changes in the fair value of the CVR liability.
59
Income Taxes
Since our inception, we have not recorded any current or deferred tax benefit for the net losses we have incurred in each year or for our research and development tax credits generated, as we believe, based upon the weight of available evidence, that it is more likely than not that our net operating loss carryforwards and tax credits will not be realized. Accordingly, a full valuation allowance has been established against the deferred tax assets as of December 31, 2022. We reevaluate the utilization of net operating loss carryforwards and tax credits at each reporting period. As of December 31, 2022, we had U.S. federal and state net operating loss carryforwards of $151.9 million and $65.1 million, respectively, which may be available to offset future taxable income and begin to expire in 2035. Of the federal net operating loss carryforwards at December 31, 2022, $148.7 million is available to be carried forward indefinitely but we are permitted to offset a maximum of 80% of taxable income per year. As of December 31, 2022, we also had U.S. federal and state research and development tax credit carryforwards of $10.7 million and $1.9 million, respectively, which may be available to offset future income tax liabilities and begin to expire in 2040 and 2035, respectively.
Utilization of the U.S. federal and state net operating loss carryforwards and research and development tax credit carryforwards may be subject to annual limitation under Section 382 of the Internal Revenue Code of 1986, and corresponding provisions of state law, due to ownership changes that have occurred previously or that could occur in the future. These ownership changes may limit the amount of carryforwards that can be utilized annually to offset future taxable income. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50% over a three-year period.
We have recorded a full valuation allowance against our net deferred tax assets at each balance sheet date.
Results of Operations
Comparison of the Years Ended December 31, 2022 and 2021
The following table summarizes our results of operations for the years ended December 31, 2022 and 2021:
|
|
Year Ended December 31, |
|
|
|
|
||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|||
|
|
(in thousands) |
|
|
|
|
|
|
|
|||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Research and development |
|
$ |
121,627 |
|
|
$ |
55,913 |
|
|
$ |
65,714 |
|
General and administrative |
|
|
26,212 |
|
|
|
19,638 |
|
|
|
6,574 |
|
Total operating expenses |
|
|
147,839 |
|
|
|
75,551 |
|
|
|
72,288 |
|
Loss from operations |
|
|
(147,839 |
) |
|
|
(75,551 |
) |
|
|
(72,288 |
) |
Other income: |
|
|
|
|
|
|
|
|
|
|||
Interest income |
|
|
3,989 |
|
|
|
467 |
|
|
|
3,522 |
|
Other income, net |
|
|
2,249 |
|
|
|
2,468 |
|
|
|
(219 |
) |
Change in fair value of CVR liability |
|
|
1,360 |
|
|
|
343 |
|
|
|
1,017 |
|
Total other income, net |
|
|
7,598 |
|
|
|
3,278 |
|
|
|
4,320 |
|
Net loss |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
|
$ |
(67,968 |
) |
60
Research and Development Expenses
The following table summarizes our research and development expenses for the year ended December 31, 2022 and 2021:
|
|
Year Ended December 31, |
|
|
|
|
||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|||
|
|
(in thousands) |
|
|
|
|
||||||
Direct external research and development expenses: |
|
|
|
|
|
|
|
|
|
|||
Bezuclastinib |
|
$ |
61,270 |
|
|
$ |
29,526 |
|
|
$ |
31,744 |
|
Preclinical research and discovery |
|
|
12,957 |
|
|
|
4,121 |
|
|
|
8,836 |
|
Unallocated expenses: |
|
|
|
|
|
|
|
|
|
|||
Personnel related (including stock-based compensation) |
|
|
35,506 |
|
|
|
16,809 |
|
|
|
18,697 |
|
Laboratory supplies, facility related and other |
|
|
11,894 |
|
|
|
5,457 |
|
|
|
6,437 |
|
Total research and development expenses |
|
$ |
121,627 |
|
|
$ |
55,913 |
|
|
$ |
65,714 |
|
Total research and development expense increased by $65.7 million for the year ended December 31, 2022 compared to the year ended December 31, 2021 and the increase was driven by higher external research and development costs associated with the manufacture and development of bezuclastinib, including costs associated with the APEX, SUMMIT and PEAK trials, and the continued development of our research pipeline. Additionally, there was an increase in unallocated expenses driven by higher personnel costs due to an increase in headcount, including stock-based compensation expense which increased by $4.1 million for the year ended December 31, 2022 compared to the year ended December 31, 2021. This is further driven by increased lab supplies and other facilities costs to support the build-out of the Cogent Research Team.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2022 were $26.2 million, compared to $19.6 million for the year ended December 31, 2021. The increase in general and administrative expenses was primarily due to higher personnel costs driven by an increase in headcount, including stock-based compensation expense which increased by $2.6 million for the year ended December 31, 2022 compared to the year ended December 31, 2021.
Interest Income
Interest income for the year ended December 31, 2022 was $4.0 million, compared to $0.5 million for the year ended December 31, 2021. The increase in interest income was primarily due to higher average invested balances and higher interest rates in the current year compared to the prior period.
Other Income, Net
Other income, net was $2.2 million in the year ended December 31, 2022, compared to $2.5 million for the year ended December 31, 2021. Other income represents sublease income recognized resulting from the sublease of a portion of our former corporate headquarters space, partially offset by the right-of-use asset impairment charge of $0.4 million recorded for this space in 2022.
Change in fair value of CVR liability
The change in fair value of CVR liability for year ended December 31, 2022 was $1.4 million, compared to $0.3 million for the year ended December 31, 2021. The increase in the change in fair value of CVR liability was a result of a decrease in the probability of receiving the milestone payments from Sotio prior to the expiration of the CVR.
Liquidity and Capital Resources
Since our inception, we have incurred significant operating losses. We have generated limited revenue to date from funding arrangements with our former collaboration partner. We have not yet commercialized any of our product candidates and we do not expect to generate revenue from sales of any product candidates for several years, if at all. We have historically funded our operations primarily through the public offering and private placement of our securities and consideration received from our collaborative agreements.
61
On May 6, 2022, we filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows us to sell from time-to-time up to $300.0 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for our own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
Additionally, on May 6, 2022, pursuant to the Form S-3, we entered into a Sales Agreement (the “Sales Agreement”) with Guggenheim Securities, LLC (“Guggenheim Securities”), pursuant to which we may issue and sell, from time to time, shares of our common stock having an aggregate offering price of up to $75.0 million through Guggenheim Securities, as the sales agent. As of December 31, 2022, no shares have been sold under the Sales Agreement.
On June 13, 2022, we completed an underwritten public offering of 17,899,698 shares of our common stock at a public offering price of $8.25 per share (including the exercise in full by the underwriters of their 30-day option to purchase up to 2,730,000 additional shares of common stock) and, in lieu of common stock to certain investors, pre-funded warrants to purchase 3,030,302 shares of our common stock at a purchase price of $8.24 per underlying share. The net proceeds from the offering were approximately $161.9 million, after deducting the underwriting discounts and commissions and estimated offering expenses.
On February 10, 2023, we filed a Form S-3ASR with the SEC (“2023 Shelf Registration”) for the issuance of common stock, preferred stock, warrants, rights, debt securities and units, which became effective immediately upon filing. At the time any of the securities covered by the 2023 Shelf Registration Statement are offered for sale, a prospectus supplement will be prepared and filed with the SEC containing specific information about the terms of any such offering.
As of December 31, 2022, we had 90,761,994 shares outstanding on a fully diluted and as-converted basis, including the 69,893,434 shares of common stock outstanding, the 606,060 pre-funded warrants that are exercisable for shares of common stock, and the 81,050 shares of Series A Preferred stock, which are convertible into 20,262,500 shares of common stock.
As of December 31, 2022, we had cash, cash equivalents and marketable securities of $259.3 million, which we believe will be sufficient to fund our operating expenses and capital expenditure requirements into 2025. Cogent holds a de minimis amount of cash related balances at Silicon Valley Bank.
Cash Flows
The following table summarizes our sources and uses of cash for each of the periods presented:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
(in thousands) |
|
|||||
Net cash used in operating activities |
|
$ |
(118,638 |
) |
|
$ |
(58,763 |
) |
Net cash used in investing activities |
|
|
(124,718 |
) |
|
|
(1,719 |
) |
Net cash provided by financing activities |
|
|
163,558 |
|
|
|
37,976 |
|
Net decrease in cash, cash equivalents and restricted cash |
|
$ |
(79,798 |
) |
|
$ |
(22,506 |
) |
Operating Activities
During the year ended December 31, 2022, operating activities used $118.6 million of cash, primarily resulting from our net loss of $140.2 million, partially offset by changes in our operating assets and liabilities of $0.8 million and net non-cash charges of $20.9 million. Net cash used by changes in our operating assets and liabilities for the year ended December 31, 2022 consisted primarily of a $12.0 million increase in accounts payable and accrued expenses and other current liabilities, partially offset by a $8.7 million decrease in operating lease liabilities, a $1.5 million increase in prepaid expenses and other current assets and a $1.0 million decrease in other assets.
During the year ended December 31, 2021, operating activities used $58.8 million of cash, primarily resulting from our net loss of $72.3 million, partially offset by changes in our operating assets and liabilities of $0.2 million and net non-cash charges of $13.3 million. Net cash used by changes in our operating assets and liabilities for the year ended December 31, 2021 consisted primarily of a $0.2 million decrease in prepaid expenses and other current assets, a $2.1 million decrease in operating lease liabilities and a $3.7 million decrease in other assets, partially offset by $6.2 million increase in accounts payable and accrued expenses and other current liabilities.
62
Changes in accounts payable, accrued expenses, and prepaid expenses and other current assets and other assets in all periods were generally due to changes in our business, the advancement of our product candidates, and the timing of vendor invoicing and payments.
Investing Activities
During the year ended December 31, 2022, net cash used in investing activities was $124.7 million, consisting of purchases of marketable securities and property and equipment, partially offset by maturities and sales of marketable securities.
During the year ended December 31, 2021, net cash used in investing activities was $1.7 million, consisting of purchases of property and lab equipment.
Financing Activities
During the year ended December 31, 2022, net cash provided by financing activities was $163.6 million which consisted of $161.9 million in proceeds from the issuance of common stock and pre-funded warrants in an underwritten public offering, net of paid offering costs, $1.6 million from the issuance of common stock upon stock option exercises, from the issuance of common stock under the Employee Stock Purchase Plan and from pre-funded warrant exercises.
During the year ended December 31, 2021, net cash provided by financing activities was $38.0 million which consisted of $38.0 million in net proceeds from the issuance of common stock under the ATM, $0.1 million from the issuance of common stock upon stock option exercises and from the issuance of common stock under the Employee Stock Purchase Plan. This is offset by $0.1 million in payments to CVR holders.
63
Funding Requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we advance the clinical development of our current and any future product candidates and conduct additional research, development and preclinical activities. The timing and amount of our operating expenditures will depend largely on:
Based on our current plans, we believe that our existing cash, cash equivalents and marketable securities of $259.3 million as of December 31, 2022 will enable us to fund our operating expenses and capital expenditure requirements into 2025. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. We will require additional funding to complete the critical activities planned to support ongoing research and development programs.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing ownership interests will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures, or declaring dividends. If we raise additional funds through collaborations, strategic alliances, or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce, or terminate our research, product development, or future commercialization efforts, or grant rights to develop and market drug candidates that we would otherwise prefer to develop and market ourselves.
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of our consolidated financial statements and related disclosures requires us to make estimates, assumptions and judgments that affect the reported amount of assets, liabilities, revenue, costs and expenses, and related disclosures. Actual results may differ from these estimates under different assumptions or conditions and could have a material impact on our reported results. While our significant accounting policies are more fully described in the Notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements.
64
Accrued Research and Development Expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing open contracts and purchase orders, communicating with our applicable personnel to identify services that have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual costs. The majority of our service providers invoice us in arrears for services performed, on a pre-determined schedule or when contractual milestones are met; however, some require advance payments. We make estimates of our accrued expenses as of each balance sheet date in the consolidated financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of the estimates with the service providers and make adjustments if necessary. Examples of estimated accrued research and development expenses include fees paid to:
We base our expenses related to preclinical studies and clinical trials on our estimates of the services received and efforts expended pursuant to quotes and contracts with multiple CMOs and CROs that supply, conduct, and manage preclinical studies and clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract, and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we adjust the accrual or prepaid expense accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are too high or too low in any particular period.
Stock-Based Compensation
We measure stock options and other stock-based awards granted to employees, non-employees and directors based on their fair value on the date of the grant and recognize compensation expense of those awards over the requisite service period, which is generally the vesting period of the respective award. We apply the straight-line method of expense recognition to all awards with only service-based vesting conditions and apply the graded-vesting method to all awards with performance-based vesting conditions or to awards with both service-based and performance-based vesting conditions.
For performance-based stock options, we begin to recognize expense when we determine that the achievement of such performance conditions is deemed probable. This determination requires significant judgment by management. At the date achievement becomes probable, we record a cumulative expense catch-up, with remaining expense amortized over the remaining service period.
We estimate the fair value of our stock-based awards to employees and non-employees using the Black-Scholes option-pricing model, which requires the input of highly subjective assumptions, including (a) the expected volatility of our stock, (b) the expected term of the award, (c) the risk-free interest rate, and (d) expected dividends. Due to the lack of a sufficient history of public trading of our common stock and a lack of sufficient company-specific historical and implied volatility data, we have based our estimate of expected volatility on the historical volatility of a group of companies in the pharmaceutical and biotechnology industries in a similar stage of development as us and that are publicly traded. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available. We have estimated the expected term of our employee stock options using the "simplified" method, whereby, the expected term equals the average of the vesting term and the original contractual term of the option. The risk-free interest rates for periods within the expected life of the option are based on the U.S. Treasury yield curve in effect during the period the options were granted. The expected dividend yield of zero is based on the fact that we have never paid cash dividends and do not expect to pay any cash dividends in the foreseeable future. We account for forfeitures as they occur.
65
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to our consolidated financial statements appearing elsewhere in this Annual Report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company, as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, for this reporting period and are not required to provide the information required under this item.
66
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
COGENT BIOSCIENCES, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page |
Report of Independent Registered Public Accounting Firm (PCAOB ID 238) |
68 |
69 |
|
Consolidated Statements of Operations and Comprehensive Loss |
70 |
71 |
|
72 |
|
73 |
67
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Cogent Biosciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Cogent Biosciences, Inc. and its subsidiaries (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations and comprehensive loss, of stockholders’ equity, and of cash flows for the years then ended, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 14, 2023
We have served as the Company’s auditor since 2015.
68
COGENT BIOSCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
139,886 |
|
|
$ |
219,684 |
|
Marketable securities |
|
|
119,390 |
|
|
|
— |
|
Prepaid expenses and other current assets |
|
|
4,435 |
|
|
|
2,949 |
|
Restricted cash |
|
|
1,255 |
|
|
|
— |
|
Total current assets |
|
|
264,966 |
|
|
|
222,633 |
|
Operating lease, right-of-use asset |
|
|
23,316 |
|
|
|
2,771 |
|
Property and equipment, net |
|
|
7,783 |
|
|
|
1,706 |
|
Restricted cash |
|
|
— |
|
|
|
1,255 |
|
Other assets |
|
|
4,745 |
|
|
|
3,727 |
|
Total assets |
|
$ |
300,810 |
|
|
$ |
232,092 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
5,842 |
|
|
$ |
3,483 |
|
Accrued expenses and other current liabilities |
|
|
17,884 |
|
|
|
8,210 |
|
CVR liability (Note 3) |
|
|
1,700 |
|
|
|
3,060 |
|
Operating lease liability |
|
|
1,423 |
|
|
|
2,324 |
|
Total current liabilities |
|
|
26,849 |
|
|
|
17,077 |
|
Operating lease liability, net of current portion |
|
|
18,226 |
|
|
|
831 |
|
Total liabilities |
|
|
45,075 |
|
|
|
17,908 |
|
|
|
|
|
|
|
|||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $0.001 par value; 9,000,000 shares authorized; no shares |
|
|
— |
|
|
|
— |
|
Series A non-voting convertible preferred stock, $0.001 par value; |
|
|
65,830 |
|
|
|
85,400 |
|
Common stock, $0.001 par value; 150,000,000 shares authorized; |
|
|
70 |
|
|
|
44 |
|
Additional paid-in capital |
|
|
601,153 |
|
|
|
399,713 |
|
Accumulated other comprehensive loss |
|
|
(104 |
) |
|
|
— |
|
Accumulated deficit |
|
|
(411,214 |
) |
|
|
(270,973 |
) |
Total stockholders’ equity |
|
|
255,735 |
|
|
|
214,184 |
|
Total liabilities and stockholders’ equity |
|
$ |
300,810 |
|
|
$ |
232,092 |
|
The accompanying notes are an integral part of these consolidated financial statements.
69
COGENT BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share amounts)
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
$ |
121,627 |
|
|
$ |
55,913 |
|
General and administrative |
|
|
26,212 |
|
|
|
19,638 |
|
Total operating expenses |
|
|
147,839 |
|
|
|
75,551 |
|
Loss from operations |
|
|
(147,839 |
) |
|
|
(75,551 |
) |
Other income: |
|
|
|
|
|
|
||
Interest income |
|
|
3,989 |
|
|
|
467 |
|
Other income, net |
|
|
2,249 |
|
|
|
2,468 |
|
Change in fair value of CVR liability |
|
|
1,360 |
|
|
|
343 |
|
Total other income, net |
|
|
7,598 |
|
|
|
3,278 |
|
Net loss |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(2.39 |
) |
|
$ |
(1.87 |
) |
Weighted average common shares outstanding, basic and diluted |
|
|
58,739,713 |
|
|
|
38,730,813 |
|
Comprehensive loss: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
Other comprehensive loss |
|
|
|
|
|
|
||
Net unrealized losses on marketable securities |
|
|
(104 |
) |
|
|
— |
|
Total other comprehensive loss |
|
|
(104 |
) |
|
|
— |
|
Comprehensive loss |
|
$ |
(140,345 |
) |
|
$ |
(72,273 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
70
COGENT BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
|
|
Series A Non-Voting Convertible |
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated |
|
|
|
|
|
Total |
|
|||||||||||
|
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid-in |
|
|
Other Comprehensive |
|
|
Accumulated |
|
|
Stockholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Loss |
|
|
Deficit |
|
|
Equity |
|
||||||||
Balance at December 31, 2020 |
|
|
132,244 |
|
|
$ |
110,881 |
|
|
|
32,347,905 |
|
|
$ |
32 |
|
|
$ |
322,454 |
|
|
$ |
— |
|
|
$ |
(198,700 |
) |
|
$ |
234,667 |
|
Conversion of Series A non-voting preferred stock into common stock |
|
|
(28,955 |
) |
|
|
(25,481 |
) |
|
|
7,238,750 |
|
|
|
8 |
|
|
|
25,473 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock for services |
|
|
— |
|
|
|
— |
|
|
|
31,683 |
|
|
|
— |
|
|
|
260 |
|
|
|
— |
|
|
|
— |
|
|
|
260 |
|
Issuance of common stock upon exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
15,758 |
|
|
|
— |
|
|
|
24 |
|
|
|
— |
|
|
|
— |
|
|
|
24 |
|
Issuance of common stock under Employee Stock Purchase Plan |
|
|
— |
|
|
|
— |
|
|
|
4,497 |
|
|
|
— |
|
|
|
31 |
|
|
|
— |
|
|
|
— |
|
|
|
31 |
|
Issuance of common stock under ATM, net of issuance costs of $1.2 million |
|
|
— |
|
|
|
— |
|
|
|
3,954,900 |
|
|
|
4 |
|
|
|
38,002 |
|
|
|
— |
|
|
|
— |
|
|
|
38,006 |
|
Issuance of common stock to settle CVR liability |
|
|
— |
|
|
|
— |
|
|
|
212,429 |
|
|
|
— |
|
|
|
2,043 |
|
|
|
— |
|
|
|
— |
|
|
|
2,043 |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11,426 |
|
|
|
— |
|
|
|
— |
|
|
|
11,426 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(72,273 |
) |
|
|
(72,273 |
) |
Balances at December 31, 2021 |
|
|
103,289 |
|
|
$ |
85,400 |
|
|
|
43,805,922 |
|
|
$ |
44 |
|
|
$ |
399,713 |
|
|
$ |
— |
|
|
$ |
(270,973 |
) |
|
$ |
214,184 |
|
Issuance of common stock in underwritten public offering, net of offering costs of $10.8 million |
|
|
— |
|
|
|
— |
|
|
|
17,899,698 |
|
|
|
18 |
|
|
|
161,897 |
|
|
|
— |
|
|
|
— |
|
|
|
161,915 |
|
Pre-funded warrant exercise |
|
|
— |
|
|
|
— |
|
|
|
2,424,242 |
|
|
|
2 |
|
|
|
22 |
|
|
|
— |
|
|
|
— |
|
|
|
24 |
|
Conversion of Series A non-voting preferred stock into common stock |
|
|
(22,239 |
) |
|
|
(19,570 |
) |
|
|
5,559,750 |
|
|
|
6 |
|
|
|
19,564 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock under Employee Stock Purchase Plan |
|
|
— |
|
|
|
— |
|
|
|
49,000 |
|
|
|
— |
|
|
|
351 |
|
|
|
— |
|
|
|
— |
|
|
|
351 |
|
Unrealized losses on marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(104 |
) |
|
|
— |
|
|
|
(104 |
) |
Issuance of common stock from exercises |
|
|
— |
|
|
|
— |
|
|
|
154,822 |
|
|
|
— |
|
|
|
1,238 |
|
|
|
— |
|
|
|
— |
|
|
|
1,238 |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
18,368 |
|
|
|
— |
|
|
|
— |
|
|
|
18,368 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(140,241 |
) |
|
|
(140,241 |
) |
Balances at December 31, 2022 |
|
|
81,050 |
|
|
$ |
65,830 |
|
|
|
69,893,434 |
|
|
$ |
70 |
|
|
$ |
601,153 |
|
|
$ |
(104 |
) |
|
$ |
(411,214 |
) |
|
$ |
255,735 |
|
The accompanying notes are an integral part of these consolidated financial statements.
71
COGENT BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization expense |
|
|
842 |
|
|
|
147 |
|
Stock-based compensation expense |
|
|
18,368 |
|
|
|
11,686 |
|
Amortization of right-of-use operating lease assets |
|
|
5,036 |
|
|
|
1,844 |
|
Change in fair value of CVR liability |
|
|
(1,360 |
) |
|
|
(343 |
) |
Net amortization (accretion) of premiums (discounts) on marketable securities |
|
|
(1,638 |
) |
|
|
— |
|
Right-of-use asset impairment |
|
|
(396 |
) |
|
|
— |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
(1,486 |
) |
|
|
(227 |
) |
Other assets |
|
|
(1,018 |
) |
|
|
(3,727 |
) |
Accounts payable |
|
|
2,359 |
|
|
|
2,751 |
|
Accrued expenses and other current liabilities |
|
|
9,586 |
|
|
|
3,431 |
|
Operating lease liability |
|
|
(8,690 |
) |
|
|
(2,052 |
) |
Net cash used in operating activities |
|
|
(118,638 |
) |
|
|
(58,763 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
(6,863 |
) |
|
|
(1,719 |
) |
Purchases of marketable securities |
|
|
(177,855 |
) |
|
|
— |
|
Maturities and sales of marketable securities |
|
|
60,000 |
|
|
|
— |
|
Net cash used in investing activities |
|
|
(124,718 |
) |
|
|
(1,719 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
||
Proceeds from issuance of common stock under ATM, net of issuance costs of $1.2 million |
|
|
— |
|
|
|
38,006 |
|
Proceeds from issuance of shares of common stock and pre-funded warrants, net of offering costs of $10.8 million |
|
|
161,945 |
|
|
|
— |
|
Proceeds from issuance of common stock upon stock option exercises |
|
|
1,238 |
|
|
|
24 |
|
Proceeds from pre-funded warrant exercises |
|
|
24 |
|
|
|
— |
|
Proceeds from issuance of stock from employee stock purchase plan |
|
|
351 |
|
|
|
31 |
|
Payments to CVR Holders |
|
|
— |
|
|
|
(85 |
) |
Net cash provided by financing activities |
|
|
163,558 |
|
|
|
37,976 |
|
Net decrease in cash, cash equivalents and restricted cash |
|
|
(79,798 |
) |
|
|
(22,506 |
) |
Cash, cash equivalents and restricted cash at beginning of period |
|
|
220,939 |
|
|
|
243,445 |
|
Cash, cash equivalents and restricted cash at end of period |
|
$ |
141,141 |
|
|
$ |
220,939 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
||
Right-of-use assets obtained in exchange for new operating lease liabilities |
|
|
25,184 |
|
|
|
— |
|
Supplemental disclosure of noncash investing and financing information: |
|
|
|
|
|
|
||
Conversion of Series A non-voting convertible preferred stock into common stock |
|
|
19,570 |
|
|
|
25,481 |
|
Offering costs included in accounts payable and accrued expenses |
|
|
30 |
|
|
|
— |
|
Property & equipment included in accounts payable and accrued expenses |
|
|
58 |
|
|
|
— |
|
Issuance of common shares in partial settlement of CVR liability |
|
|
— |
|
|
|
2,043 |
|
|
|
|
|
|
|
|
||
The accompanying notes are an integral part of these consolidated financial statements.
72
COGENT BIOSCIENCES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of the Business and Basis of Presentation
Cogent Biosciences, Inc. (“Cogent” or the “Company”) is a biotechnology company focused on developing precision therapies for genetically defined diseases. Cogent’s approach is to design rational precision therapies that treat the underlying cause of disease and improve the lives of patients. Cogent’s most advanced program is bezuclastinib, also known as CGT9486, a highly selective tyrosine kinase inhibitor designed to potently inhibit the KIT D816V mutation as well as other mutations in KIT exon 17. In the vast majority of cases, KIT D816V is responsible for driving Systemic Mastocytosis (“SM”), a serious disease caused by unchecked proliferation of mast cells. Exon 17 mutations are also found in patients with advanced gastrointestinal stromal tumors (“GIST”), a type of cancer with strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a new treatment option for these patient populations. In addition to bezuclastinib, the Company’s research team is developing a portfolio of novel targeted therapies to help patients fighting serious, genetically driven diseases, and is initially targeting FGFR2 and ErbB2.
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations and the ability to secure additional capital to fund operations. Product candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel and infrastructure and extensive compliance-reporting capabilities. Even if the Company’s drug development efforts are successful, it is uncertain when, if ever, the Company will realize revenue from product sales.
The accompanying consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business. The Company has incurred recurring losses since inception, including a net loss of $140.2 million for the year ended December 31, 2022. As of December 31, 2022, the Company had an accumulated deficit of $411.2 million. The Company expects to continue to generate operating losses in the foreseeable future. As of the issuance date of the consolidated financial statements, the Company expects that its cash, cash equivalents and marketable securities will be sufficient to fund its operating expenses and capital expenditure requirements for at least the next 12 months from issuance of the consolidated financial statements.
The Company expects that it will continue to incur significant expenses in connection with its ongoing business activities. The Company will need to seek additional funding through equity offerings, debt financings, collaborations, licensing arrangements and other marketing and distribution arrangements, partnerships, joint ventures, combinations or divestitures of one or more of its assets or businesses. The Company may not be able to obtain financing on acceptable terms, or at all, and the Company may not be able to enter into collaborative arrangements or divest its assets. The terms of any financing may adversely affect the holdings or the rights of the Company’s stockholders. Arrangements with collaborators or others may require the Company to relinquish rights to certain of its technologies or product candidates. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate its research and development programs or commercialization efforts, which could adversely affect its business prospects, or the Company may be unable to continue operations.
The Company’s consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
73
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Mono, Inc. and Kiq Bio LLC. All intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reporting periods. Significant estimates and assumptions reflected in these consolidated financial statements include, but are not limited to, the accrual of research and development expenses, the valuation of the CVR liability and the valuation of stock-based awards. The Company bases its estimates on historical experience, known trends and other market-specific or other relevant factors that it believes to be reasonable under the circumstances. On an ongoing basis, management evaluates its estimates, as there are changes in circumstances, facts and experience. Actual results may differ from those estimates or assumptions.
Concentrations of Credit Risk and of Significant Suppliers
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash, cash equivalents and marketable securities. Periodically, the Company maintains deposits in accredited financial institutions in excess of federally insured limits. The Company maintains most of its cash and cash equivalents at two accredited financial institutions. The Company has not experienced any losses on such accounts and does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships. Such deposits have and will continue to exceed federally insured limits.
The Company is dependent on third-party vendors for its product candidates. In particular, the Company relies, and expects to continue to rely, on a small number of vendors to manufacture supplies and process its product candidates for its development programs. These programs could be adversely affected by a significant interruption in the manufacturing process.
Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents.
Restricted Cash
Restricted cash consists of security deposits in separate restricted bank accounts as required under the terms of the Company’s lease agreement for its former corporate headquarters in Cambridge, Massachusetts, which expires in April 2023.
Marketable Securities
The Company’s marketable securities, consisting of debt securities, are classified as available-for-sale. Available-for-sale marketable debt securities are carried at fair value with the unrealized gains and losses included in other comprehensive income (loss) as a component of stockholders’ equity until realized. Any premium or discount arising at purchase is amortized and/or accreted to interest income and/or expense over the life of the instrument. Realized gains and losses are determined using the specific identification method and are included in other income (expense). The Company reviews its portfolio of available-for-sale debt securities, using both quantitative and qualitative factors, to determine if declines in fair value below cost have resulted from a credit-related loss or other factors. If the decline in fair value is due to credit-related factors, a loss is recognized in net income, and if the decline in fair value is not due to credit-related factors, the loss is recorded in other comprehensive income (loss).
74
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization expense is recognized using the straight-line method over the estimated useful life of each asset as follows:
|
|
Estimated Useful Life |
Laboratory equipment |
|
5 years |
Computer equipment and software |
|
3 years |
Furniture and fixtures |
|
5 years |
Leasehold improvements |
|
Shorter of life of lease or 10 years |
Costs for capital assets not yet placed into service are capitalized as construction-in-progress and depreciated in accordance with the above guidelines once placed into service. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation and amortization are removed from the accounts and any resulting gain or loss is included in loss from operations. Expenditures for repairs and maintenance are charged to expense as incurred.
Impairment of Long-Lived Assets
Long-lived assets consist of property and equipment. Long-lived assets to be held and used are tested for recoverability whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Factors that the Company considers in deciding when to perform an impairment review include significant underperformance of the business in relation to expectations, significant negative industry or economic trends and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate a long-lived asset group for recoverability, the Company compares forecasts of undiscounted cash flows expected to result from the use and eventual disposition of the long-lived asset group to its carrying value. An impairment loss would be recognized in loss from operations when estimated undiscounted future cash flows expected to result from the use of an asset group are less than its carrying amount. The impairment loss would be based on the excess of the carrying value of the impaired asset group over its fair value.
Fair Value Measurements
Certain assets and liabilities are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
The Company’s cash equivalents and marketable securities are carried at fair value, determined according to the fair value hierarchy described above (see Note 3). The carrying values of the Company’s accounts payable and accrued expenses approximate their fair values due to the short-term nature of these liabilities.
Segment Information
The Company manages its operations as a segment for the purposes of assessing performance and making operating decisions. The Company’s singular focus is the development and commercialization of precision therapies for genetically defined diseases. All of the Company’s tangible assets are held in the United States.
75
Leases
The Company accounts for a contract as a lease when it has the right to control the asset for a period of time while obtaining substantially all of the assets’ economic benefits. The Company determines the initial classification and measurement of its operating right-of-use assets and operating lease liabilities at the lease commencement date, and thereafter if modified. The lease term includes any renewal options that the Company is reasonably assured to exercise. The Company’s policy is to not record leases with an original term of twelve months or less on its consolidated balance sheets. The Company’s only existing leases are for office and laboratory space.
The right-of-use asset represents the right to use the leased asset for the lease term. The lease liability represents the present value of the lease payments under the lease. The present value of lease payments is determined by using the interest rate implicit in the lease, if that rate is readily determinable; otherwise, the Company uses its estimated secured incremental borrowing rate for that lease term.
Lease payments included in the measurement of the lease liability consist of the following: the fixed noncancelable lease payments, payments for optional renewal periods where it is reasonably certain the renewal period will be exercised, and payments for early termination options unless it is reasonably certain the lease will not be terminated early.
Leases may contain rent escalation clauses and variable lease payments that require additional rental payments in later years of the term, including payments based on an index or inflation rate. Payments based on the change in an index or inflation rate, or payments based on a change in the Company’s portion of the operating expenses, including real estate taxes and insurance, are not included in the initial lease liability and are recorded as a period expense when incurred. The operating leases may include an option to renew the lease term for various renewal periods and/or to terminate the leases early. These options to exercise the renewal or early termination clauses in the Company’s operating leases were not reasonably certain of exercise as of the date of adoption and these have not been included in the determination of the initial lease liability or operating lease expense.
Rent expense for operating leases is recognized on a straight-line basis over the reasonably assured lease term based on the total lease payments and is included in operating expense in the consolidated statements of operations and comprehensive loss. For finance leases, any interest expense is recognized using the effective interest method and is included within interest expense. The Company has no financing leases.
Research and Development Costs
Research and development costs are expensed as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries, stock-based compensation and benefits, facilities costs and laboratory supplies, depreciation, manufacturing expenses and external costs of outside vendors engaged to conduct preclinical development activities and clinical trials as well as the cost of licensing technology.
Upfront payments and milestone payments made for the licensing of technology are expensed as research and development in the period in which they are incurred. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. The prepaid amounts are expensed as the related goods are delivered or the services are performed.
Research Contract Costs and Accruals
The Company has entered into various research and development contracts with companies both inside and outside of the United States. These agreements are generally cancelable, and related payments are recorded as research and development expenses as incurred. The Company records accruals for estimated ongoing research costs. When evaluating the adequacy of the accrued liabilities, the Company analyzes progress of the studies or trials, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period. Actual results could differ from the Company’s estimates.
Patent Costs
All patent-related costs incurred in connection with filing and prosecuting patent applications are expensed as incurred due to the uncertainty about the recovery of the expenditure. Amounts incurred are classified as general and administrative expenses.
76
Stock-Based Compensation
The Company measures stock options and other stock-based awards granted to employees, non-employees and directors based on their fair value on the date of the grant and recognizes compensation expense of those awards over the requisite service period, which is generally the vesting period of the respective award. The Company applies the straight-line method of expense recognition to all awards with only service-based vesting conditions and applies the graded-vesting method to all awards with performance-based vesting conditions or to awards with both service-based and performance-based vesting conditions.
For performance-based stock options, the Company begins to recognize expense when it determines that the achievement of such performance conditions is deemed probable. This determination requires significant judgment by management. At the date achievement becomes probable, the Company records a cumulative expense catch-up, with remaining expense amortized over the remaining service period.
The Company estimates the fair value of stock-based awards to employees and non-employees using the Black-Scholes option-pricing model, which requires the input of highly subjective assumptions, including (a) the expected volatility of its stock, (b) the expected term of the award, (c) the risk-free interest rate, and (d) expected dividends. Due to the lack of a sufficient history of public trading of the Company’s common stock and a lack of sufficient company-specific historical and implied volatility data, the Company has based the estimate of expected volatility on the historical volatility of a group of companies in the pharmaceutical and biotechnology industries in a similar stage of development and that are publicly traded. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available. The Company estimates the expected life of employee stock options using the "simplified" method, whereby, the expected life equals the average of the vesting term and the original contractual term of the option. The risk-free interest rates for periods within the expected life of the option are based on the U.S. Treasury yield curve in effect during the period the options were granted. The expected dividend yield of zero is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future. The Company accounts for forfeitures as they occur.
Comprehensive Loss
Comprehensive loss includes net loss as well as other changes in stockholders’ equity that result from transactions and economic events other than those with stockholders. For the year ended December 31, 2022, the Company’s only element of other comprehensive loss was unrealized gains (losses) on marketable securities. For the year ended December 31, 2021, there were no elements of other comprehensive loss.
Net Income (Loss) per Share
Basic net income (loss) per common share attributable to common stockholders is computed by dividing the net income (loss) attributable to common stockholders by the weighted average number of shares of common stock outstanding for the period.
Diluted net income (loss) per common share attributable to common stockholders is computed by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding for the period, including potential dilutive common shares assuming the dilutive effect of outstanding stock options. Accordingly, in periods in which the Company reported a net loss, dilutive common shares were not assumed to have been issued as their affect was anti-dilutive, and as a result, diluted net loss per common share was the same as basic net loss per common share.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined on the basis of the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
77
The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06 Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40) related to the measurement and disclosure requirements for convertible instruments and contracts in an entity’s own equity. The pronouncement simplifies and adds disclosure requirements for the accounting and measurement of convertible instruments and the settlement assessment for contracts in an entity’s own equity. The Company adopted ASU 2020-06 on January 1, 2022. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
3. Fair Value Measurements of Financial Assets and Liabilities
The following table summarizes the Company’s marketable securities (in thousands):
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Amortized |
|
|
Gross |
|
|
Gross |
|
|
Fair |
|
||||
U.S. Treasury bills and notes (due within one year) |
|
$ |
119,494 |
|
|
$ |
— |
|
|
$ |
(104 |
) |
|
$ |
119,390 |
|
|
|
$ |
119,494 |
|
|
$ |
— |
|
|
$ |
(104 |
) |
|
$ |
119,390 |
|
As of December 31, 2022, the Company held seven securities that were in an unrealized loss position. The aggregate fair value of securities held by the Company in an unrealized loss position for less than twelve months as of December 31, 2022 was $99.5 million and there were no securities held by the Company in an unrealized loss position for more than twelve months. The Company has the intent and ability to hold such securities until recovery. As a result, the Company did not record any charges for credit-related impairments for its marketable debt securities for the year ended December 31, 2022. The Company did not hold any marketable securities as of December 31, 2021.
The following tables present the Company’s fair value hierarchy for its financial assets and liabilities, which are measured at fair value on a recurring basis (in thousands):
|
|
Fair Value Measurements at December 31, 2022 Using: |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
108,829 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
108,829 |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. Treasury bills and notes |
|
$ |
— |
|
|
$ |
119,390 |
|
|
$ |
— |
|
|
$ |
119,390 |
|
Total Assets |
|
$ |
108,829 |
|
|
$ |
119,390 |
|
|
$ |
— |
|
|
$ |
228,219 |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
CVR Liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,700 |
|
|
$ |
1,700 |
|
Total Liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,700 |
|
|
$ |
1,700 |
|
|
|
Fair Value Measurements at December 31, 2021 Using: |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
CVR Liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,060 |
|
|
$ |
3,060 |
|
Total Liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,060 |
|
|
$ |
3,060 |
|
78
Money market funds were valued using quoted prices in active markets, which represent a Level 1 measurement in the fair value hierarchy. U.S. Treasury bills and notes were valued by the Company using quoted prices in active markets for similar securities, which represent a Level 2 measurement within the fair value hierarchy.
On July 6, 2020, the Company issued a non-transferrable contingent value right (“CVR”), which was distributed to stockholders of record as of the close of business on July 6, 2020, and prior to the issuance of any shares to acquire Kiq Bio LLC (“Kiq”) (the “Kiq Acquisition”) or sold to the Private Investment in Public Equity (“PIPE”) investors. Holders of the CVR are entitled to receive common shares and/or cash payments from proceeds received by the Company, if any, related to the disposition of its legacy cell therapy assets for a period of three years from July 2020. In accordance with the terms of the CVR agreement, the payment to CVR holders will be made in shares or cash, depending on the timing of the receipt of the sales proceeds by the Company. For sales proceeds received by the Company prior to December 31, 2020, CVR holders were entitled to receive payment in the form of common shares of the Company. For sales proceeds received by the Company after December 31, 2020 and prior to July 2023, CVR holders are entitled to receive payment in cash.
The Company classifies the CVR as a liability on its consolidated balance sheet. The fair value of the CVR liability was determined using the probability weighted discounted cash flow method to estimate future cash flows associated with the sale of the legacy cell therapy assets, including the Bolt-on Chimeric Receptor (“BOXR”) technology and Autologous Cell Therapy Industrial Automation technology (collectively, the “BOXR Platform”), Antibody-Coupled T cell Receptor technology and other fixed assets based on assumptions at the date of the CVR issuance and each subsequent quarterly period end, less certain permitted deductions. For sales proceeds received by the Company prior to December 31, 2020, the number of common shares to be received by CVR holders was determined by dividing the proceeds received by the Company by the closing price of the Company’s common stock on July 6, 2020 of $8.80. The closing price of the Company’s common stock at each measurement date through February 2021 was used to determine the fair value of the share payments included in the CVR liability. The liability measured at the date of CVR issuance was recorded as a common stock dividend, returning capital to the legacy stockholders of record as of the close of business on July 6, 2020. Changes in fair value of the liability are recognized as a component of other income (expense) in the consolidated statement of operations and comprehensive loss. The CVR liability was valued based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy.
On August 28, 2020, the Company sold its assets, rights and interests relating to its BOXR Platform, to Sotio. Pursuant to the BOXR Platform Purchase Agreement, Sotio has agreed to pay the Company total cash consideration of up to $11.5 million, consisting of an upfront payment of $8.1 million and potential milestone payments of up to $3.4 million in the aggregate upon the achievement of certain milestones related to the issuance of Specified Claims (as described in the BOXR Platform Purchase Agreement) by the U.S. Patent and Trademark Office and the European Patent Office. The upfront payment was received in 2020. In 2020, the Company also sold additional fixed assets used in the legacy business. Both transactions triggered payment to the CVR holders. In November 2020, the Company issued 707,938 shares of common stock in partial settlement of the CVR liability. In February 2021, the Company issued an additional 212,429 shares of common stock and paid $0.1 million in partial settlement of the CVR liability. Any settlement of the remaining CVR liability will be a cash settlement.
In the fourth quarter of 2022, the Company updated the probability weighted discounted cash flow assumptions to reflect the current probability of receiving the milestone payments from Sotio prior to the expiration of the CVR. Based on the Company’s assessment of the available information, this update resulted in a decrease in the probability of receiving the milestone payment prior to the expiration of the CVR and a corresponding decrease in the CVR liability. The change in fair value of the liability of $1.4 million was recognized as a component of other income (expense) in 2022.
79
The following table sets forth a summary of the changes in the fair value of the Company’s CVR liability (in thousands):
|
|
|
|
|
Balance at December 31, 2020 |
|
$ |
5,531 |
|
ue |
|
|
(343 |
) |
CVR settlement |
|
|
(2,128 |
) |
Balance at December 31, 2021 |
|
$ |
3,060 |
|
|
|
(1,360 |
) |
|
Balance at December 31, 2022 |
|
$ |
1,700 |
|
During the years ended December 31, 2022 and 2021, there were no transfers between Level 1, Level 2 and Level 3.
4. Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Laboratory equipment |
|
$ |
5,507 |
|
|
$ |
1,073 |
|
Computer equipment and software |
|
|
546 |
|
|
|
53 |
|
Furniture and fixtures |
|
|
873 |
|
|
|
85 |
|
Leasehold improvements |
|
|
1,776 |
|
|
|
408 |
|
Construction-in-progress |
|
|
482 |
|
|
|
646 |
|
Total property and equipment |
|
|
9,184 |
|
|
|
2,265 |
|
Accumulated depreciation and amortization |
|
|
(1,401 |
) |
|
|
(559 |
) |
Property and equipment, net |
|
$ |
7,783 |
|
|
$ |
1,706 |
|
Depreciation and amortization expense was $0.8 million and $0.1 million for the years ended December 31, 2022 and 2021, respectively.
5. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Accrued employee compensation and benefits |
|
$ |
6,063 |
|
|
$ |
3,389 |
|
Accrued external research and development expense |
|
|
5,898 |
|
|
|
1,953 |
|
Accrued external manufacturing costs |
|
|
3,741 |
|
|
|
1,556 |
|
Accrued professional and consulting services |
|
|
1,778 |
|
|
|
1,077 |
|
Other |
|
|
404 |
|
|
|
235 |
|
|
|
$ |
17,884 |
|
|
$ |
8,210 |
|
6. Preferred Stock, Series A Non-Voting Convertible Preferred Stock and Common Stock
The Company’s authorized capital stock consists of 150,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share, 1,000,000 of which are designated as Series A Preferred Stock and 9,000,000 of which shares of preferred stock are undesignated.
Series A Non-Voting Convertible Preferred Stock
On July 6, 2020, the Company filed a Certificate of Designation of Preferences, Rights and Limitations of the Series A Non-Voting Convertible Preferred Stock (“Series A Preferred Stock”) with the Secretary of State of the State of Delaware (the “Certificate of Designation”) in connection with the 2020 Kiq Acquisition and the PIPE. The Certificate of Designation provides for the issuance of shares of Series A Preferred Stock, par value $0.001 per share.
80
Holders of Series A Preferred Stock are entitled to receive dividends on shares of Series A Preferred Stock equal, on an as-if-converted-to-common-stock basis, and in the same form as dividends actually paid on shares of the common stock. Except as otherwise required by law, the Series A Preferred Stock does not have voting rights. However, as long as any shares of Series A Preferred Stock are outstanding, the Company will not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series A Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock, (b) alter or amend the Certificate of Designation, (c) amend its certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series A Preferred Stock, (d) increase the number of authorized shares of Series A Preferred Stock, (e) prior to the stockholder approval of the Conversion Proposal or at any time while at least 40% of the originally issued Series A Preferred Stock remains issued and outstanding, consummate a Fundamental Transaction (as defined in the Certificate of Designation) or (f) enter into any agreement with respect to any of the foregoing. The Series A Preferred Stock does not have a preference upon any liquidation, dissolution or winding-up of the Company.
Each share of Series A Preferred Stock is convertible at any time at the option of the holder thereof, into 250 shares of common stock, subject to certain limitations, including that a holder of Series A Preferred Stock is prohibited from converting shares of Series A Preferred Stock into shares of common stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own more than a specified percentage (to be established by the holder between 4.9% and 19.9%) of the total number of shares of common stock issued and outstanding immediately after giving effect to such conversion. Cumulatively, through December 31, 2022, 82,275 shares of Series A Preferred Stock, or 50.4% of the issued Series A Preferred Stock, have been converted into 20,568,750 shares of common stock. The 81,050 shares of Series A Preferred Stock outstanding as of December 31, 2022 are convertible into 20,262,500 shares of common stock. Subsequent to December 31, 2022, an additional 4,000 shares of Series A Preferred Stock were converted into 1,000,000 shares of common stock.
No other classes of preferred stock have been designated and no other preferred shares have been issued or are outstanding as of December 31, 2022 or 2021.
Common Stock
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are not entitled to receive dividends, unless declared by the board of directors. In the event of the Company’s liquidation, dissolution or winding up, holders of the Company’s common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
On February 8, 2021, the Company filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows the Company to sell from time-to-time up to $200.0 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for its own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering. On May 6, 2022, the Company filed an Amendment to its February 8, 2021 Form S-3 Registration Statement to terminate the effectiveness of the registration statement and to remove from registration all securities registered but not sold under the registration statement.
Additionally, on February 8, 2021, pursuant to the Form S-3, the Company entered into a Sales Agreement (the “SVB Sales Agreement”) with SVB Leerink LLC (“SVB Leerink”), pursuant to which the Company may issue and sell, from time to time, shares of its common stock having an aggregate offering price of up to $75.0 million through SVB Leerink as the sales agent. Cumulatively, the Company has sold 3,954,900 shares of common stock under the SVB Sales Agreement with offering prices ranging between $9.25 and $10.30 per share for net proceeds of approximately $38.0 million. The Company terminated the existing SVB Sales Agreement, effective as of May 5, 2022. The Company did not incur any termination penalties as a result of the termination of the SVB Sales Agreement.
81
On May 6, 2022, the Company filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows the Company to sell from time-to-time up to $300.0 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for its own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
Additionally, on May 6, 2022, pursuant to the Form S-3, the Company entered into a Sales Agreement (the “Sales Agreement”) with Guggenheim Securities, LLC (“Guggenheim Securities”), pursuant to which the Company may issue and sell, from time to time, shares of its common stock having an aggregate offering price of up to $75.0 million through Guggenheim Securities, as the sales agent. As of December 31, 2022, no shares have been sold under the Sales Agreement.
On June 13, 2022, the Company completed an underwritten public offering of 17,899,698 shares of its common stock at a public offering price of $8.25 per share (including the exercise in full by the underwriters of their 30-day option to purchase up to 2,730,000 additional shares of common stock) and, in lieu of common stock to certain investors, pre-funded warrants to purchase 3,030,302 shares of its common stock at a purchase price of $8.24 per underlying share. The net proceeds from the offering were approximately $161.9 million, after deducting the underwriting discounts and commissions of $10.4 million and offering expenses of $0.4 million.
Each pre-funded warrant entitles the holder to purchase shares of common stock at an exercise price of $0.01 per share and is exercisable at any time beginning on the date of issuance. These warrants were recorded as a component of stockholders’ equity within additional paid-in capital. In accordance with the terms of the warrant agreement, a holder of the outstanding warrant is not entitled to exercise any portion of the pre-funded warrant if, upon giving effect to such exercise, would cause the aggregate number of shares of common stock beneficially owned by such holder (together with its affiliates and any other person whose beneficial ownership of common stock would be aggregated with the holder) to exceed 9.99% of the total number of then issued and outstanding shares of common stock, as such percentage ownership is determined in accordance with the terms of the pre-funded warrant and subject to such holder’s rights under the pre-funded warrant to increase or decrease such percentage to any other percentage not in excess of 19.99% upon at least 61 days’ prior notice from such holder. As of December 31, 2022, 2,424,242 pre-funded warrants have been exercised and 606,060 pre-funded warrants remain outstanding.
7. Stock-Based Compensation
2018 Stock Option and Incentive Plan
The Company’s 2018 Stock Option and Incentive Plan, (the “2018 Plan”), which became effective on March 27, 2018, provides for the grant of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock units, restricted stock awards, unrestricted stock awards, cash-based awards and dividend equivalent rights. The number of shares initially reserved for issuance under the 2018 Plan was 700,180. Additionally, the shares of common stock that remained available for issuance under the previously outstanding 2015 Stock Incentive Plan (the “2015 Plan”) became available under the 2018 Plan. The number of shares reserved for the 2018 Plan automatically increases on each January 1 by 4% of the number of shares of the Company’s common stock outstanding on the immediately preceding December 31 or a lesser number of shares determined by the Company’s board of directors. As of December 31, 2022, 686,585 shares of common stock remain available for issuance under the 2018 Plan.
The number of authorized shares reserved for issuance under the 2018 Plan was increased by 2,795,737 shares effective as of January 1, 2023. The shares of common stock underlying any awards that are forfeited, canceled, held back upon exercise or settlement of an award to satisfy the exercise price or tax withholding, repurchased or are otherwise terminated by the Company under the 2018 Plan or the 2015 Plan will be added back to the shares of common stock available for issuance under the 2018 Plan.
On June 16, 2021, at the Company’s 2021 annual stockholder meeting, the Company’s stockholders approved the amendment and restatement of the 2018 Stock Plan to increase the number of shares of common stock issuable under the 2018 Plan by 6,000,000 shares. Upon stockholder approval, in accordance with ASC 718- Compensation- Stock Compensation, a grant date was established for accounting purposes with respect to 3,402,768 options previously granted to employees and non-employee directors during the year ended December 31, 2021, which were subject to stockholder approval of the amendment and restatement of the 2018 Plan.
82
Inducement Plan
On October 22, 2020, the board of directors adopted the Cogent Biosciences, Inc. 2020 Inducement Plan (the “Inducement Plan”). The board of directors also adopted a form of non-qualified stock option agreement for use with the Inducement Plan. A total of 3,750,000 shares of common stock have been reserved for issuance under the Inducement Plan, subject to adjustment for stock dividends, stock splits, or other changes in the Company's common stock or capital structure. On November 5, 2020, the Company filed a Registration Statement on Form S-8 related to the 3,750,000 shares of its common stock reserved for issuance under the Inducement Plan. As of December 31, 2022, 677,995 shares of common stock remain available for issuance under the Inducement Plan.
2018 Employee Stock Purchase Plan
The Company’s 2018 Employee Stock Purchase Plan (the “ESPP”) became effective on March 28, 2018, at which time a total of 78,500 shares of common stock were reserved for issuance. In addition, the number of shares of common stock that may be issued under the ESPP automatically increases on each January 1 through January 1, 2027, by the least of (i) 125,000 shares of common stock, (ii) 1% of the number of shares of the Company’s common stock outstanding on the immediately preceding December 31 or (iii) such lesser number of shares as determined by the ESPP administrator. As of December 31, 2022, 404,268 shares remain available for issuance under the ESPP. The number of authorized shares reserved for issuance under the ESPP was increased by 125,000 shares effective as of January 1, 2023. In January 2023, 39,228 shares were issued to employees under the ESPP.
Stock Option Valuation
The following table presents, on a weighted average basis, the assumptions used in the Black-Scholes option-pricing model to determine the fair value of stock options granted to employees and directors:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Risk-free interest rate |
|
|
2.2 |
% |
|
|
1.3 |
% |
Expected volatility |
|
|
72.4 |
% |
|
|
75.3 |
% |
Expected dividend yield |
|
|
— |
|
|
|
— |
|
Expected life (in years) |
|
|
6.22 |
|
|
|
6.21 |
|
Stock Option Activity
The following table summarizes the activity of our 2018 Stock Option and Incentive Plan and the Inducement Plan, excluding performance-based stock options:
|
|
Number |
|
|
Weighted |
|
|
Weighted |
|
|
Aggregate |
|
||||
|
|
|
|
|
|
|
|
(in years) |
|
|
(in thousands) |
|
||||
Outstanding as of December 31, 2021 |
|
|
8,793,626 |
|
|
$ |
9.57 |
|
|
|
|
|
|
|
||
Granted |
|
|
4,724,584 |
|
|
|
8.36 |
|
|
|
|
|
|
|
||
Exercised |
|
|
(154,822 |
) |
|
|
7.99 |
|
|
|
|
|
|
|
||
Forfeited |
|
|
(531,617 |
) |
|
|
8.55 |
|
|
|
|
|
|
|
||
Outstanding as of December 31, 2022 |
|
|
12,831,771 |
|
|
$ |
9.19 |
|
|
|
8.49 |
|
|
$ |
32,864 |
|
Vested and expected to vest as of December 31, |
|
|
12,831,771 |
|
|
$ |
9.19 |
|
|
|
8.49 |
|
|
$ |
32,864 |
|
Options exercisable as of December 31, 2022 |
|
|
4,639,104 |
|
|
$ |
9.31 |
|
|
|
8.15 |
|
|
$ |
11,134 |
|
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had strike prices lower than the fair value of the Company’s common stock.
83
The aggregate intrinsic value of options exercised during the years ended December 31, 2022 and 2021 was $1.0 million and $0.1 million, respectively. The weighted average grant-date fair value of awards granted during the years ended December 31, 2022 and 2021 was $5.52 per share and $5.93 per share, respectively.
Employee Stock Purchase Plan
The Company estimates the fair value of shares to be issued under the 2018 Employee Stock Purchase Plan using the Black-Scholes option-pricing model on the date of grant, or first day of the offering period. The following table summarizes information pertaining to stock purchase rights granted under the employee stock purchase plan, during the years indicated:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Risk-free interest rate |
|
|
1.3 |
% |
|
|
0.1 |
% |
Expected volatility |
|
|
64.1 |
% |
|
|
66.9 |
% |
Expected dividend yield |
|
|
— |
|
|
|
— |
|
Expected life (in years) |
|
|
0.50 |
|
|
|
0.50 |
|
Stock-Based Compensation
The following table summarizes stock-based compensation expense during the years ended December 31, 2022, in thousands:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Stock-based compensation expense by type of award: |
|
|
|
|
|
|
||
Time-based stock options |
|
$ |
18,144 |
|
|
$ |
11,361 |
|
Employee stock purchase plan |
|
|
224 |
|
|
|
65 |
|
Non-employee stock options |
|
|
— |
|
|
|
260 |
|
Total |
|
$ |
18,368 |
|
|
$ |
11,686 |
|
The Company recorded stock-based compensation expense in the following expense categories of its consolidated statements of operations and comprehensive loss (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Research and development expenses |
|
$ |
8,510 |
|
|
$ |
4,392 |
|
General and administrative expenses |
|
|
9,858 |
|
|
|
7,294 |
|
Total |
|
$ |
18,368 |
|
|
$ |
11,686 |
|
As of December 31, 2022, total unrecognized compensation cost related to the unvested stock-based options was $47.5 million, which is expected to be recognized over a weighted average period of 2.57 years.
8. Income Taxes
During the years ended December 31, 2022 and 2021, the Company recorded no current or deferred income tax benefits due to its full valuation allowance. Also, the Company had no foreign operations.
84
A reconciliation of the U.S. federal statutory income tax rate to the Company’s effective income tax rate is as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Federal statutory income tax rate |
|
|
(21.0 |
)% |
|
|
(21.0 |
)% |
State taxes, net of federal benefit |
|
|
(4.4 |
) |
|
|
(2.9 |
) |
Federal and state research and development tax |
|
|
(6.1 |
) |
|
|
(4.0 |
) |
Nondeductible stock compensation |
|
|
1.1 |
|
|
|
1.4 |
|
Other items |
|
|
(0.2 |
) |
|
|
0.4 |
|
Change in valuation allowance |
|
|
30.6 |
|
|
|
26.1 |
|
Effective income tax rate |
|
|
0.0 |
% |
|
|
0.0 |
% |
The Company's net deferred tax assets as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Deferred tax assets (liabilities): |
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
36,161 |
|
|
$ |
30,147 |
|
Research and development and investment tax |
|
|
12,202 |
|
|
|
3,719 |
|
Accrued expenses |
|
|
1,676 |
|
|
|
734 |
|
Capitalized research and development expense |
|
|
33,155 |
|
|
|
8,529 |
|
Operating lease right-of-use assets |
|
|
(5,922 |
) |
|
|
(703 |
) |
Operating lease liabilities |
|
|
4,991 |
|
|
|
800 |
|
Contingent consideration |
|
|
864 |
|
|
|
862 |
|
Stock compensation |
|
|
4,758 |
|
|
|
2,257 |
|
Other |
|
|
1,659 |
|
|
|
342 |
|
Total deferred tax assets |
|
|
89,544 |
|
|
|
46,687 |
|
Valuation allowance |
|
|
(89,544 |
) |
|
|
(46,687 |
) |
Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
As of December 31, 2022, the Company had U.S. federal and state net operating loss carryforwards of $151.9 million and $65.1 million, respectively, which may be available to offset future taxable income and begin to expire in 2035. Of the federal net operating loss carryforwards at December 31, 2022, $148.7 million is available to be carried forward indefinitely but can only offset 80% of taxable income per year. As of December 31, 2022, the Company also had U.S. federal and state research and development tax credit carryforwards of $10.7 million and $1.9 million, respectively, which may be available to offset future income tax liabilities and begin to expire in 2040 and 2035, respectively.
On December 22, 2017, the Tax Cuts and Jobs Act (the "TCJA") was signed into law. Under the TCJA provisions, effective with tax years beginning on or after January 1, 2022, taxpayers can no longer immediately expense research and development expenditures. Taxpayers are now required to capitalize and amortize these costs over 5 years for research conducted within the United States or 15 years for research conducted abroad. As a result, the Company capitalized $111.2 million of research and development expenses for the year ended December 31, 2022.
Utilization of the U.S. federal and state net operating loss carryforwards and research and development tax credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986, and corresponding provisions of state law, due to ownership changes that have occurred previously or that could occur in the future. These ownership changes may limit the amount of carryforwards that can be utilized annually to offset future taxable income. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50% over a three-year period.
85
As a result of the shares issued in July 2020 related to the acquisition of Kiq and the sale of Series A convertible preferred stock, the Company experienced a change in ownership, as defined by Section 382. As a result of the ownership change, utilization of the federal and state net operating loss carryforwards and research and development tax credit carryforwards is subject to annual limitation under Section 382. Under Section 382, the annual limitation is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term tax-exempt rate, and then could be subject to additional adjustments, as required. This limitation resulted in the expiration of federal and state net operating loss carryforwards before utilization of $26.9 million and $79.5 million, respectively, and federal and state research and development tax credit carryforwards before utilization of $6.6 million and $2.0 million, respectively. The Company has written off the deferred tax assets related to these attributes, which were previously fully reserved for, in 2020. As of December 31, 2022, approximately $66.4 million and $2.8 million of federal and state net operating losses, respectively, as well as $7.1 million of future amortization for federal purposes were subject to the July 6 limitation of $0.3 million per year. A second ownership change occurred in December 2020 as a result of the underwritten public offering of common stock which resulted in a limitation of tax attributes generated from July 2020 to December 2020. The December 2020 ownership change is not expected to have a material impact to the Company’s net operating loss carryforwards or research and development tax credit carryforwards as these net operating losses and tax credit carryforwards may be utilized, subject to annual limitation, assuming sufficient taxable income is generated before expiration. The Company has not performed a Section 382 analysis since December 2020.
The Company has not performed a research and development tax credit study. Any change to the Company’s credits as a result of a study would be offset by a change in the valuation allowance.
The Company has evaluated the positive and negative evidence bearing upon its ability to realize its net deferred tax assets. Management has considered the Company’s history of cumulative net losses incurred since inception and its lack of commercialization of any products or generation of any revenue from product sales since inception and has concluded that it is more likely than not that the Company will not realize the benefits of its net deferred tax assets. Accordingly, a full valuation allowance has been established against the deferred tax assets as of December 31, 2022 and 2021. Management reevaluates the positive and negative evidence at each reporting period.
The changes in the valuation allowance during the year ended December 31, 2022 primarily related to net operating loss carryforwards and capitalized research and development expenses and the change in the valuation allowance during the year ended December 31, 2021 primarily related to the operating loss carryforwards and were as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Valuation allowance as of beginning of year |
|
$ |
46,687 |
|
|
$ |
27,799 |
|
Decreases recorded to income tax provision |
|
|
— |
|
|
|
— |
|
Increases recorded to income tax provision |
|
|
42,857 |
|
|
|
18,888 |
|
Valuation allowance as of end of year |
|
$ |
89,544 |
|
|
$ |
46,687 |
|
As of December 31, 2022 and 2021, the Company had not recorded any amounts for unrecognized tax benefits. The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. The statute of limitations for assessment by the Internal Revenue Service remains open for all years since 2019, with certain states open since 2018. 'The Company's tax attributes related to years prior to 2019 can still be adjusted under audit. No federal or state tax audits are currently in process.
9. Commitments and Contingencies
Operating Leases
Corporate Headquarters- Waltham, MA
On March 19, 2022, the Company and Cimpress USA Incorporated (the “Cimpress”) entered into a sublease agreement (the “Waltham Sublease”) pursuant to which the Company subleases approximately 17,749 square feet of office space in Waltham, Massachusetts, which serves as the Company’s corporate headquarters. The Waltham Sublease became effective on May 5, 2022.
86
The Waltham Sublease has a term of four years and four months, commencing June 1, 2022 and expiring September 30, 2026. The Company will pay Cimpress base rent at an initial rate of $42.50 per square foot per year. Rent is payable in equal monthly installments and subject to $1.00 per square foot annual increases over the term. Additionally, the Company is responsible for reimbursing Cimpress for the Company’s share of the building’s property taxes and operating expenses. In connection with the Waltham Sublease, the Company provided a cash security deposit to the landlord in an amount of $0.4 million which is recorded in Other Assets in the consolidated balance sheet as of December 31, 2022.
The lease commencement date occurred in May 2022, following landlord consent, as the Company gained access to the space under the terms of the lease. The Company recorded a right-of-use asset and lease liability for this lease of $2.9 million at the lease commencement date.
Research Facility- Boulder, CO
On July 6, 2021, the Company entered into a lease agreement (the “Original Lease”) pursuant to which the Company leases approximately 38,075 square feet (the “Initial Premises”) in Boulder, Colorado, which includes office and laboratory space. Subsequently, on March 29, 2022, the Company entered into the First Amendment to the lease agreement (the “First Amendment” and together with the Original Lease, the “Boulder Lease”) pursuant to which the Company leases approximately 6,582 square feet of additional office space on the second floor (the “Expansion Premises”).
In accordance with the terms of the Original Lease, the landlord will contribute an aggregate of approximately $6.9 million toward the cost of landlord assets (the “Improvements”), as well as an additional amount of up to approximately $2.3 million in the form of a tenant improvement loan at an annual interest rate of 6%. Any monies borrowed under the tenant improvement loan are required to be repaid over the Boulder Lease term. Additionally, under the terms of the First Amendment, the landlord will provide an additional tenant improvement allowance (the “Additional Allowance”) of $0.6 million, of which $0.3 million will be used in the Initial Premises toward the cost of landlord assets. The remaining $0.3 million Additional Allowance is to be used for work to be performed in the Expansion Premises for the construction of lessee assets. The Company incurred net construction costs of approximately $7.0 million for the development of the Initial Premises at the Boulder location.
The Boulder Lease has an initial term of 12 years with the option to extend for three successive five-year terms. Boulder Lease payments will begin in June 2023 after an initial free rent period. Rent will be payable in equal monthly installments and subject to annual increases over the term. Additionally, the Company is responsible for reimbursing the landlord for its share of the building’s property taxes and operating expenses. The Boulder Lease is an operating lease. In connection with the Boulder Lease, the Company provided a cash security deposit to the landlord in an amount of $0.7 million which is recorded in Other Assets in the consolidated balance sheet as of December 31, 2022.
The Company recorded the initial right-of-use assets and lease liabilities for the lease of $22.3 million as of the lease commencement dates.
87
Former Corporate Headquarters- Cambridge, MA
The Company leases office and laboratory space in Cambridge, Massachusetts under a non-cancelable operating lease (the “Cambridge Lease”) that expires in April 2023.
In August 2020, the Company entered into a sublease (the “Cambridge Sublease Agreement”) for a significant portion of the leased premises for the remaining term of the lease. Under the terms of the Cambridge Sublease Agreement, the sublessee leased approximately 70% of the facility and is responsible for the corresponding percentage of operating lease costs and variable lease costs. Variable lease costs include common area maintenance and other operating charges. The Company recorded a right-of-use impairment charge of $0.4 million during year ended December 31, 2022, following the move of the Corporate Headquarters from Cambridge, Massachusetts to Waltham, Massachusetts.
The elements of the lease expense, net of sublease income, were as follows (in thousands):
|
|
Year Ended |
|
Year Ended |
|
||
Lease cost |
|
|
|
|
|
||
Operating lease cost |
|
$ |
4,052 |
|
$ |
2,424 |
|
Variable lease cost (1) |
|
|
991 |
|
|
825 |
|
Sublease income |
|
|
(2,621 |
) |
|
(2,468 |
) |
Total lease cost |
|
$ |
2,422 |
|
$ |
781 |
|
|
|
|
|
|
|
||
Other information |
|
|
|
|
|
||
Cash paid for amounts included in the measurement of |
|
$ |
8,413 |
|
$ |
3,250 |
|
Weighted average remaining lease term |
|
|
10.84 |
|
|
1.33 |
|
Weighted average discount rate |
|
|
8.04 |
% |
|
9.50 |
% |
Future minimum lease payments under the Company’s operating leases as of December 31, 2022 are as follows (in thousands):
Year Ending December 31, |
|
|
|
|
2023 |
|
$ |
2,544 |
|
2024 |
|
|
2,780 |
|
2025 |
|
|
2,841 |
|
2026 |
|
|
2,697 |
|
2027 |
|
|
2,132 |
|
Thereafter |
|
|
17,546 |
|
Total future minimum lease payments |
|
|
30,540 |
|
Less: imputed interest |
|
|
10,628 |
|
Less: tenant improvement allowance receivable |
|
|
263 |
|
Total operating lease liability |
|
$ |
19,649 |
|
Included in the consolidated balance sheet: |
|
|
|
|
Current operating lease liability |
|
$ |
1,423 |
|
Operating lease liability, net of current portion |
|
|
18,226 |
|
Total operating lease liability |
|
$ |
19,649 |
|
88
Under the terms of the Cambridge Lease, the Company issued a $1.3 million letter of credit to the landlord as collateral for the leased facility. The underlying cash collateralizing this letter of credit has been classified as current restricted cash in the accompanying consolidated balance sheets. This is a refundable deposit and not a lease payment. Under the terms of the Cambridge Sublease Agreement, the sublessee obtained a letter of credit for $1.3 million for the benefit of the Company. This has been excluded from the undiscounted cash flows above.
License Agreements
Plexxikon License Agreement
In July 2020, the Company obtained an exclusive, sublicensable, worldwide license (the “License Agreement”) to certain patents and other intellectual property rights to research, develop and commercialize bezuclastinib. Under the terms of the License Agreement, the Company is required to pay Plexxikon Inc. (“Plexxikon”) aggregate payments of up to $7.5 million upon the satisfaction of certain clinical milestones and up to $25.0 million upon the satisfaction of certain regulatory milestones. During the second quarter of 2022, as a result of the progression of the PEAK study, the first clinical milestone was achieved, resulting in payment of $2.5 million to Plexxikon in June 2022. As of December 31, 2022, no other milestone payments have been made or are considered probable of occurring.
The Company is also required to pay Plexxikon tiered royalties ranging from a low-single digit percentage to a high-single digit percentage on annual net sales of products. These royalty obligations last on a product-by-product basis and country-by-country basis until the latest of (i) the date on which there is no validate claim of a licensed Plexxikon patent covering a subject product in such country or (ii) the 10th anniversary of the date of the first commercial sale of the product in such country. In addition, if the Company sublicenses the rights under the License Agreement, the Company is required to pay a certain percentage of the sublicense revenue to Plexxikon ranging from mid-double digit percentages to mid-single digit percentages, depending on whether the sublicense is entered into prior to or after certain clinical trial events.
The license agreement will expire on a country-by-country and licensed product-by-licensed product basis until the later of the last to expire of the patents covering such licensed products or services or the 10-year anniversary of the date of first commercial sale of the licensed product in such country. The Company may terminate the license agreement within 30 days after written notice in the event of a material breach. The Company may also terminate the agreement upon written notice in the event of the Company’s bankruptcy, liquidation or insolvency. In addition, the Company has the right to terminate this agreement in its entirety at will upon 90 days’ advance written notice to Plexxikon.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnification of varying scope and terms to vendors, lessors, business partners and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and its executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. To date, the Company has not incurred any material costs as a result of such indemnifications. The Company is not aware of any claims under indemnification arrangements that will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its consolidated financial statements as of December 31, 2022 or 2021.
Legal Proceedings
The Company is not currently party to any material legal proceedings. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies. The Company expenses as incurred the costs related to such legal proceedings.
89
10. Net Loss per Share
Basic and diluted net loss per common share was calculated as follows (in thousands, except share and per share amounts):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Numerator: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
Net loss attributable to common stockholders |
|
$ |
(140,241 |
) |
|
$ |
(72,273 |
) |
Denominator: |
|
|
|
|
|
|
||
Weighted average common shares outstanding, |
|
|
58,739,713 |
|
|
|
38,730,813 |
|
Net loss per common share, basic and diluted |
|
$ |
(2.39 |
) |
|
$ |
(1.87 |
) |
The Company’s potential dilutive securities have been excluded from the computation of diluted net loss per share as the effect would be anti-dilutive and would result in a reduction to net loss per share. The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common stockholders for the periods indicated above because including them would have had an anti-dilutive effect:
|
|
December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Stock options to purchase common stock |
|
|
12,831,771 |
|
|
|
8,793,626 |
|
Series A Preferred Stock |
|
|
20,262,500 |
|
|
|
25,822,250 |
|
|
|
|
33,094,271 |
|
|
|
34,615,876 |
|
In accordance with ASC Topic 260, Earnings Per Share, the outstanding pre-funded warrants are included in the computation of basic and diluted net loss per share because the exercise price is negligible ($0.01 per share) and they are fully vested and exercisable at any time after the original issuance date.
11. Retirement Plan
The Company has a defined-contribution plan under Section 401(k) of the Internal Revenue Code (the “401(k) Plan”). The 401(k) Plan covers all employees who meet defined minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pre-tax basis. The 401(k) Plan allows for discretionary matching contributions of 100% of the first 4% of elective contributions, which vest immediately. Contributions under the plan were approximately $0.8 million and $0.4 million for the years ended December 31, 2022 and December 31, 2021, respectively.
90
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and President and our Chief Financial Officer (our principal executive officer and principal financial officer, respectively), evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2022. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2022, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Internal Control Over Financial Reporting
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d‑15(f) under the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of Cogent’s internal control over financial reporting as of December 31, 2022. In making this assessment, it used the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on such assessment, our management has concluded that Cogent’s internal control over financial reporting was effective, as of December 31, 2022.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting due to an exemption established by the Jumpstart Our Business Startups Act of 2012 for “emerging growth companies.”
Changes in Internal Control Over Financial Reporting
No change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the three months ended December 31, 2022 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
91
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
92
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
The information required by this Item 13 will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 14 will be included in our definitive proxy statement to be filed with the SEC with respect to our 2023 Annual Meeting of Stockholders and is incorporated herein by reference.
93
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) 1. Financial Statements
For a list of the financial statements included herein, see Index to the Financial Statements on page 64 of this Annual Report on Form 10-K, incorporated into this Item by reference.
Financial statement schedules have been omitted because they are either not required or not applicable or the information is included in the financial statements or the notes thereto.
See the Exhibit Index in Item 15(b) below.
Exhibit Number |
|
Description |
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
3.3 |
|
|
|
|
|
3.4 |
|
|
|
|
|
3.5 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
10.1 |
|
|
|
|
|
10.2# |
|
|
|
|
|
10.3# |
|
|
|
|
|
10.4# |
|
|
|
|
|
10.5(1) |
|
|
|
|
|
10.6 |
|
94
|
|
|
10.7 |
|
|
|
|
|
10.8 |
|
|
|
|
|
10.9 |
|
|
|
|
|
10.10 |
|
|
|
|
|
10.11 |
|
|
|
|
|
10.12# |
|
|
|
|
|
10.13# |
|
|
|
|
|
10.14# |
|
|
|
|
|
10.15# |
|
|
|
|
|
10.16 |
|
|
|
|
|
10.17 |
|
|
|
|
|
21.1* |
|
|
|
|
|
23.1* |
|
Consent of PricewaterhouseCoopers LLP, independent registered public accounting firm. |
|
|
|
31.1* |
|
|
|
|
|
31.2* |
|
|
|
|
|
32.1*† |
|
|
|
|
|
32.2*† |
|
|
|
|
|
101INS* |
|
Inline XBRL Instance Document. |
|
|
|
101SCH* |
|
Inline XBRL Taxonomy Extension Schema Document. |
|
|
|
101CAL* |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
101LAB* |
|
Inline XBRL Taxonomy Extension Labels Linkbase Document. |
|
|
|
101PRE* |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
95
101DEF* |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
104* |
|
Coverage Page Interative Data File (formatted as inline XRBL with applicable taxonomy extensive information contained in Exhibits 101.) |
* Filed herewith.
# Indicates management contract or compensation plan.
† The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K, are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Cogent Biosciences, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing.
ITEM 16. FORM 10-K SUMMARY
None.
96
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: March 14, 2023 |
|
COGENT BIOSCIENCES, INC. |
|
|
|
|
|
|
|
By: |
/s/ Andrew Robbins |
|
|
|
Andrew Robbins |
|
|
|
Chief Executive Officer and President |
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated on March 14, 2023:
Signature |
|
Title(s) |
|
|
|
/s/ Andrew Robbins
Andrew Robbins |
|
Chief Executive Officer, President and Director (Principal Executive Officer) |
|
|
|
/s/ John Green
John Green |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
|
|
/s/ Chris Cain
Chris Cain |
|
Director |
|
|
|
/s/ Karen Ferrante
Karen Ferrante, M.D. |
|
Director |
|
|
|
/s/ Peter Harwin
Peter Harwin |
|
Director |
|
|
|
/s/ Arlene Morris
Arlene Morris |
|
Director |
|
|
|
/s/ Matthew Ros
Matthew Ros |
|
Director |
|
|
|
/s/ Todd Shegog
Todd Shegog |
|
Director |
97
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-29)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
