COOPER COMPANIES, INC. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________
FORM 10-K
________________________
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
FOR THE FISCAL YEAR ENDED OCTOBER 31, 2022
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
FOR THE TRANSITION PERIOD FROM TO
COMMISSION FILE NO. 001-08597
________________________
THE COOPER COMPANIES, INC.
(Exact name of registrant as specified in its charter)
________________________
| Delaware | 94-2657368 | ||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | ||||
Suite 500
San Ramon, California, 94583
(Address of principal executive offices) (Zip Code)
(925) 460-3600
(Registrant’s telephone number, including area code)
________________________
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||||||||||||
| Common Stock, $.10 par value | COO | The New York Stock Exchange | ||||||||||||
Securities registered pursuant to Section 12(g) of the Act:
None
________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☒ No ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of shares held by non-affiliates was $17.7 billion based on the closing price of the registrant’s common stock on the New York Stock Exchange on April 30, 2022.
Number of shares outstanding of the registrant's common stock, as of December 1, 2022: 49,354,384
Documents Incorporated by Reference:
| Document | Part of Form 10-K | |||||||
| Portions of the Proxy Statement for the Annual Meeting of Stockholders scheduled to be held in March 2023 | Part III | |||||||
Our independent registered public accounting firm is KPMG LLP, San Francisco, CA, Auditor ID: 185.
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Annual Report on Form 10-K
for the Fiscal Year Ended October 31, 2022
Table of Contents
| PART I | Page | |||||||
| Item 1. | Business | |||||||
| Item 1A. | Risk Factors | |||||||
| Item 1B. | Unresolved Staff Comments | |||||||
| Item 2. | Properties | |||||||
| Item 3. | Legal Proceedings | |||||||
| Item 4. | Mine Safety Disclosures | |||||||
| PART II | ||||||||
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |||||||
| Item 6. | Reserved | |||||||
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | |||||||
| Item 7A. | Quantitative and Qualitative Disclosure about Market Risk | |||||||
| Item 8. | Financial Statements and Supplementary Data | |||||||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |||||||
| Item 9A. | Controls and Procedures | |||||||
| Item 9B. | Other Information | |||||||
| Item 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | |||||||
| PART III | ||||||||
| Item 10. | Directors, Executive Officers and Corporate Governance | |||||||
| Item 11. | Executive Compensation | |||||||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |||||||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | |||||||
| Item 14. | Principal Accounting Fees and Services | |||||||
| PART IV | ||||||||
| Item 15. | Exhibits and Financial Statement Schedules | |||||||
| Item 16. | Form 10-K Summary | |||||||
3
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
PART I
Forward-Looking Statements
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These include statements relating to plans, prospects, goals, strategies, future actions, events or performance and other statements which are other than statements of historical fact, including: statements regarding the expected impact of global macroeconomic conditions, the COVID-19 pandemic and the war in Ukraine on our business; and statements regarding acquisitions (including the acquired companies' financial position, market position, product development and business strategy, expected cost synergies, expected timing and benefits of the transaction, difficulties in integrating entities or operations, as well as estimates of our and the acquired entities' future expenses, sales and earnings per share) that are forward-looking. In addition, all statements regarding anticipated growth in our net sales, anticipated effects of any product recalls, anticipated market conditions, planned product launches and expected results of operations and integration of any acquisition are forward-looking. To identify these statements, look for words like “believes,” “outlook,” “probable,” “expects,” “may,” “will,” “should,” “could,” “seeks,” “intends,” “plans,” “estimates” or “anticipates” and similar words or phrases. Forward-looking statements necessarily depend on assumptions, data or methods that may be incorrect or imprecise and are subject to risks and uncertainties. Among the factors that could cause our actual results and future actions to differ materially from those described in forward-looking statements are those described in our Securities and Exchange Commission filings, including the “Business,” “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” sections in this Annual Report on Form 10-K for the fiscal year ended October 31, 2022, as such Risk Factors may be updated in quarterly filings.
We caution investors that forward-looking statements reflect our analysis only on their stated date. We disclaim any intent to update them except as required by law.
4
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Summary Risk Factors
Our business faces significant risks. In addition to the summary below, you should carefully review the “Risk Factors” section of this Annual Report on Form 10-K. We may be subject to additional risks and uncertainties not presently known to us or that we currently deem immaterial. Our business, financial condition and results of operations could be materially adversely affected by any of these risks, and the trading prices of our common stock could decline by virtue of these risks. These risks should be read in conjunction with the other information in this report. Some of the more significant risks relating to our business include:
•Adverse changes in the global or regional general business, political and economic conditions, including the impact of continuing uncertainty and instability of certain countries, that could adversely affect our global markets, and the potential adverse economic impact and related uncertainty caused by these items, including but not limited to, the COVID-19 pandemic, inflation and escalating global trade barriers.
•The effects of the COVID-19 pandemic and related economic disruptions and new governmental regulations on our business, results of operations, cash flow and financial condition, including but not limited to the potential impact on our sales, operations and supply chain.
•The impact of Russia's invasion of Ukraine and the global response to this invasion on the global economy, European economy, financial markets, energy markets, currency rates and our ability to supply product to, or through, affected countries.
•Foreign currency exchange rate and interest rate fluctuations including the risk of fluctuations in the value of foreign currencies or interest rates that would decrease our net sales and earnings.
•Our existing and future variable rate indebtedness and associated interest expense is impacted by rate increases, which could adversely affect our financial health or limit our ability to borrow additional funds.
•Changes in tax laws, examinations by tax authorities, and changes in our geographic composition of income.
•Acquisition-related adverse effects including the failure to successfully achieve the anticipated net sales, margins and earnings benefits of acquisitions, integration delays or costs and the requirement to record significant adjustments to the preliminary fair value of assets acquired and liabilities assumed within the measurement period, required regulatory approvals for an acquisition not being obtained or being delayed or subject to conditions that are not anticipated, adverse impacts of changes to accounting controls and reporting procedures, contingent liabilities or indemnification obligations, increased leverage and lack of access to available financing (including financing for the acquisition or refinancing of debt owed by us on a timely basis and on reasonable terms).
•Compliance costs and potential liability in connection with U.S. and foreign laws and health care regulations pertaining to privacy and security of personal information, such as HIPAA and the California Consumer Privacy Act (CCPA) in the U.S. and the General Data Protection Regulation (GDPR) requirements in Europe, including but not limited to those resulting from data security breaches.
•A major disruption in the operations of our manufacturing, accounting and financial reporting, research and development, distribution facilities or raw material supply chain due to the COVID-19 pandemic, integration of acquisitions, man-made or natural disasters, cybersecurity incidents or other causes.
•A major disruption in the operations of our manufacturing, accounting and financial reporting, research and development or distribution facilities due to technological problems, including any related to our information systems maintenance, enhancements or new system deployments, integrations or upgrades.
•Market consolidation of large customers globally through mergers or acquisitions resulting in a larger proportion or concentration of our business being derived from fewer customers.
•Disruptions in supplies of raw materials, particularly components used to manufacture our silicone hydrogel lenses.
•New U.S. and foreign government laws and regulations, and changes in existing laws, regulations and enforcement guidance, which affect areas of our operations including, but not limited to, those affecting the health care industry, including the contact lens industry specifically and the medical device or pharmaceutical industries generally, including but not limited to the EU Medical Devices Regulation (MDR) and the EU In Vitro Diagnostic Medical Devices Regulation (IVDR).
•Legal costs, insurance expenses, settlement costs and the risk of an adverse decision, prohibitive injunction or settlement related to product liability, patent infringement or other litigation.
•Limitations on sales following product introductions due to poor market acceptance.
•New competitors, product innovations or technologies, including but not limited to, technological advances by competitors, new products and patents attained by competitors, and competitors' expansion through acquisitions.
•Reduced sales, loss of customers and costs and expenses related to product recalls and warning letters.
•Failure to receive, or delays in receiving, regulatory approvals or certifications for products.
•Failure of our customers and end users to obtain adequate coverage and reimbursement from third-party payors for our products and services.
•The requirement to provide for a significant liability or to write off, or accelerate depreciation on, a significant asset, including goodwill, other intangible assets and idle manufacturing facilities and equipment.
5
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
•The success of our research and development activities and other start-up projects.
•Dilution to earnings per share from acquisitions or issuing stock.
•Impact and costs incurred from changes in accounting standards and policies.
•Risks related to environmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability.
6
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 1. Business.
The Cooper Companies, Inc. (Cooper, we or the Company), a Delaware corporation organized in 1980, is a global medical device company publicly traded on the NYSE (NYSE: COO). Cooper operates through two business units, CooperVision and CooperSurgical.
CooperVision is a global manufacturer providing products for contact lens wearers. CooperVision develops, manufactures and markets a broad range of single-use, two-week and monthly contact lenses, featuring advanced materials and optics. CooperVision designs its products to solve vision challenges such as astigmatism, presbyopia and myopia; with a broad collection of spherical, toric and multifocal contact lenses. CooperVision offers contact lenses in a variety of materials including silicone hydrogel Aquaform® technology and phosphorylcholine technology (PC) Technology™. CooperVision also manufactures and markets myopia management and specialty eyecare products which it gained through a series of orthokeratology (ortho-k) and scleral lens acquisitions. In November 2019, CooperVision's internally developed MiSight® 1 day lens became first and only approved product by the United States Food and Drug Administration (FDA) for myopia control and is indicated to slow the progression of myopia in children when treatment is initiated between the ages of 8-12. The MiSight 1 day lens became available in the U.S. in fiscal 2020 and in August 2021, CooperVision received Chinese National Medical Products Administration (NMPA) approval for its MiSight® 1 day lens for use in China. CooperVision’s major manufacturing and distribution facilities are located in Belgium, Costa Rica, Hungary, Puerto Rico, the UK and the U.S., with other smaller locations also existing in multiple locations around the world.
CooperSurgical's business competes in the general health care market with a focus on advancing the health of women, babies and families through a diversified portfolio of products and services. Our fertility portfolio encompasses medical device coverage of the in vitro fertilization (IVF) cycle, egg and sperm donation, and cryopreservation. Our office and surgical platform encompasses more than 600 clinically-relevant medical devices used in gynecology and obstetrics, including contraception and labor & delivery, as well as cord blood and cord tissue storage services. CooperSurgical has established its market presence and distribution system by developing products and acquiring companies, products and services that complement its business model. We categorize CooperSurgical product sales based on the point of health care delivery, which includes products used in medical office and surgical procedures, primarily by Obstetricians/Gynecologists (OB/GYN); and fertility products/equipment and genetic testing services used primarily in fertility clinics and laboratories. CooperSurgical's major manufacturing, cryostorage and distribution facilities are located in Costa Rica, the Netherlands, the United Kingdom and the United States, with other smaller locations also existing in multiple locations around the world.
CooperVision and CooperSurgical each operate in highly competitive environments. Both of Cooper's businesses compete predominantly on the bases of product quality and differentiation, technological benefits, price, service levels and reliability.
COOPERVISION
CooperVision competes in the worldwide soft contact lens market and services three primary regions: the Americas, EMEA (Europe, Middle East and Africa) and Asia Pacific. The contact lens market has two major product categories:
•Spherical lenses including lenses that correct near- and farsightedness uncomplicated by more complex visual defects.
•Toric and multifocal lenses including lenses that, in addition to correcting near- and farsightedness, address more complex visual defects such as astigmatism, myopia and presbyopia by adding optical properties of cylinder and axis, which correct for irregularities in the shape of the cornea. Myopia management contact lenses slow the progression of myopia in children 8 to 12 years of age at initiation of the treatment.
In order to achieve comfortable and healthy contact lens wear, products are sold with recommended replacement schedules, often defined as modalities, with the primary modalities being single-use lenses and frequent replacement (FRP) lenses, which are designed for two-week and monthly replacement.
CooperVision offers spherical, toric, multifocal and toric multifocal lens products in most modalities. We believe that in order to compete successfully in the numerous categories of the contact lens market, companies must offer differentiated products that are priced competitively and manufactured efficiently. CooperVision uses different manufacturing processes, primarily cast molding, to produce its lenses. We believe this allows CooperVision to compete in its markets by:
•Producing high, medium and low volumes of lenses made with a variety of materials for a broader range of market niches: single-use, two-week and monthly disposable sphere, toric and multifocal lenses, custom toric lenses for patients with a high degree of astigmatism, and myopia management contact lenses.
•Offering a wide range of lens parameters, leading to a higher rate of successful fitting for practitioners and better visual acuity for patients.
7
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
The market for spherical lenses is growing with the addition of new value-added products, such as spherical lenses to alleviate dry eye symptoms, reduce eye fatigue from use of digital devices and add aspherical optical properties and/or higher oxygen permeable lenses such as silicone hydrogels, and myopia management contact lenses for 8 to 12 years (age-appropriate) children.
Sales of contact lenses utilizing silicone hydrogel materials continue to grow. Silicone hydrogel materials supply a higher level of oxygen to the cornea, as measured by the transmissibility of oxygen through a given thickness of material, or “dk/t,” than traditional hydrogel lenses. We believe our ability to compete successfully with a full range of silicone hydrogel products is an important factor to achieving success in our business. Silicone hydrogel lenses represent a significant portion of CooperVision's contact lens sales and our Biofinity® brand is CooperVision's leading product line in terms of sales. Under the Biofinity brand, CooperVision markets monthly silicone hydrogel spherical (including Biofinity Energys®), toric, extended range toric, multifocal and toric multifocal lens products.
CooperVision markets single-use silicone hydrogel lenses with a complete line of spherical, toric, extended range toric and multifocal lenses under our clariti® 1 day brand and single-use silicone hydrogel spherical, toric and multifocal lenses under our MyDay® brand. We also compete in the traditional single-use hydrogel product segment with branded lenses including our Proclear® and Biomedics® 1 day lenses. We believe the global market for single-use contact lenses will continue to grow and that our competitive silicone hydrogel and traditional hydrogel product offerings represent an opportunity for our business.
In addition to its silicone hydrogel product offerings, CooperVision competes in the contact lens market with other traditional hydrogel products.
CooperVision focuses on supporting the growth of all customers including key accounts (optical chains, global retailers, certain buying groups and mass merchandisers) by investing in selling, promotional and advertising activities. Further, we are increasing investment in our distribution and packaging capabilities to support the growth of our business and to continue providing quality service with our industry leading SKU range and customized offerings.
CooperVision believes that myopia management opens up an attractive new market for contact lenses. With MiSight, CooperVision offers the only FDA approved1 and first Chinese NMPA approved product to control the progression of myopia in age-appropriate children. CooperVision is investing to create this new market by educating eye care practitioners, patients and their families which increases awareness.
CooperVision is focused on greater worldwide market penetration of recently introduced products, and we continue to expand our presence in existing and emerging markets, both organically and through acquisitions. In fiscal 2022, CooperVision acquired a privately-held Denmark-based ortho-k contact lens distributor. In fiscal 2021, CooperVision acquired a privately-held medical device company and a privately-held UK contact lenses manufacturer. These acquisitions expanded CooperVision’s specialty eye care portfolio and its leadership in addressing the increasing severity and prevalence of myopia.
1 Indications for use of MiSight® 1 day (omafilcon A) soft (hydrophilic) contact lenses for daily wear are indicated for the correction of myopic ametropia and for slowing the progression of myopia in children with non-diseased eyes, who at the initiation of treatment are 8-12 years of age and have a refraction of -0.75 to -4.00 diopters (spherical equivalent) with ≤ 0.75 diopters of astigmatism. The lens is to be discarded after each removal.
8
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Contact Lens Product Sales

Single-use spheres – This includes Biomedics 1 day, clariti 1 day, MyDay, MiSight and Proclear 1 day
Toric – This includes Avaira Vitality toric, Biomedics toric, Biofinity toric, clariti 1 day toric, MyDay toric and Proclear toric
Multifocal – This includes Biofinity multifocal, Biofinity toric multifocal, clariti 1 day multifocal, MyDay multifocal and Proclear 1 day multifocal
Non single-use sphere, other – This includes our Avaira Vitality spheres, frequent replacement product (FRP) lens portfolio (Biofinity spheres, Biofinity Energys, Biomedics, Proclear spheres, clariti spheres), ortho-k, scleral and custom lenses, contact lens solutions and other
CooperVision Competition
The contact lens market is highly competitive. CooperVision's largest competitors in the worldwide market and its primary competitors in the spherical, toric and multifocal lens categories of that market are Johnson & Johnson Vision Care, Inc., Alcon Inc. and Bausch Health Companies Inc.
Certain of CooperVision's competitors may have greater financial resources, larger research and development budgets, larger sales forces, greater market penetration and/or larger manufacturing volumes. CooperVision seeks to offer a high level of customer service through its direct sales organizations around the world and through telephone sales and technical service representatives who consult with eye care professionals about the use of our lens products.
CooperVision also competes with manufacturers of eyeglasses and with refractive surgical procedures that correct visual defects including laser vision correction. CooperVision believes that laser vision correction is not a significant threat to its sales of contact lenses based on the growth of the contact lens market over the past decade.
CooperVision competes in the silicone hydrogel segment of the market with its following products: clariti 1 day brand of single-use sphere, toric and multifocal lenses; MyDay® single-use spherical, toric and multifocal lenses; Biofinity monthly spherical, toric, multifocal and toric multifocal lenses and Avaira Vitality® two-week spherical and toric lenses. CooperVision believes the clariti 1 day and MyDay brands of single-use contact lenses provide the broadest product portfolio in the single-use silicone hydrogel market. CooperVision offers both branded and private label/store brand options in contact lenses. Its private label option is frequently offered as part of a larger customized solution for its customers. It also competes in the specialty contact lens space with its FDA approved MiSight 1 day contact lens for myopia management in age-appropriate children as well as ortho-k and scleral lenses.
In addition to a broad offering of silicone hydrogel and specialty contact lenses, CooperVision competes with different manufacturing processes which allow it to produce a broad range of spheres, toric and multifocal lens parameters, which we believe provides wide choices for patient and practitioner and a high level of visual acuity. We also compete based on our customer and professional services. CooperVision believes that there are opportunities for contact lenses to gain market share, particularly in markets where the penetration of contact lenses in the vision correction market is low.
9
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
COOPERSURGICAL
CooperSurgical offers a broad array of products and services focused on advancing the health of women, babies and families through a diversified portfolio of products and services including medical devices, fertility, genomics, diagnostics, cryostorage, contraception and healthcare technology services (such as cord blood and cord tissue storage and genomic testing). We offer quality products, innovative technologies and superior services to health care professionals and patients worldwide. CooperSurgical collaborates with health care professionals to identify products and new technologies from disposable products to diagnostic tests to sophisticated instruments and equipment, to bring new products to market. The result is a broad portfolio of products and services that are intended to aid in the delivery of improved clinical outcomes for families and that health care professionals use routinely in the diagnosis and treatment of a wide spectrum of women's health and reproductive issues.
A focus area for CooperSurgical is key accounts, which include large group practices, integrated delivery networks and certain buying groups within the office/surgical business and fertility clinic networks within the fertility business. We believe our portfolio of offerings and focus on service, quality and clinical education will support the accelerated growth of our business in these key account groups.
Since its inception in 1990, CooperSurgical has established its market presence and distribution system by developing products and acquiring products and companies that complement its business model.
In fiscal 2022, CooperSurgical acquired both a private cryopreservation services company and Generate Life Sciences (Generate), a privately-held leading provider of donor egg and sperm for fertility treatments, fertility cryopreservation services and newborn stem cell storage (cord blood & cord tissue). In fiscal 2021, CooperSurgical acquired three privately-held medical device companies and one privately-held IVF cryostorage software solutions company. We intend to continue investing in CooperSurgical's business with the goal of expanding our integrated solutions model within the areas of family health, fertility and diagnostics.
In April 2022, CooperSurgical entered into an asset purchase agreement to acquire Cook Medical's Reproductive Health business, a manufacturer of minimally invasive medical devices focused on the fertility, obstetrics and gynecology markets. The aggregate consideration is $875.0 million in cash, with $675.0 million payable at the closing and the remaining $200.0 million payable in $50.0 million installments following each of the first, second, third and fourth anniversaries of the closing. The transaction is subject to customary closing conditions, such as receipt of required regulatory approvals.
Market for Women's and Family Health Care
CooperSurgical participates in the market for women's and family health care with its diversified product lines in three major categories based on the point of health care delivery: hospitals and surgical centers, OB/GYN medical offices and fertility clinics. In recent years, including with the acquisition of Generate in fiscal 2022, CooperSurgical’s business increasingly includes marketing and selling to end consumers of healthcare technology and reproductive planning products and services.
CooperSurgical expects patient visits to women’s health provider offices in the U.S. to increase over the next decade. From adolescent care to geriatrics, there is increasing global awareness of women’s health issues. During the reproductive years, fertility awareness and family planning and healthcare are key areas of focus. The attention in maternity care to improving access to safe, effective, and equitable obstetrical care continues. As we expect an increase in the population of women over the age of 65, office visits focused around abnormal bleeding, incontinence and menopause will likely increase.
Another trend in the market for women's health care includes the continued migration of OB/GYN health care professionals away from private practice ownership and toward aligning with group practices or employment with hospitals and health care systems. This overall trend of consolidation of healthcare systems includes the increasing influence of supply chain controls, such as value analysis committees, on product evaluation and procurement across these care-delivery systems. CooperSurgical believes that the market factors that are driving this trend will continue. We believe our broad product portfolio can be a benefit in this changing environment as health systems look to standardize and consolidate vendors.
Recent trends of patient-centered, value-based care include the development of more cost-effective health care delivery models, including moving treatment out of hospitals and surgery centers and into the office setting without compromising care. We expect to see continued changes in reimbursement and clinical best practices as payment models and policies continue to evolve.
10
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
The OB/GYN market encompasses the following significant points of healthcare delivery:
•Routine office visits: annual well-women checkups, preventative cancer screening and contraception.
•Evaluation and management (E/M) office visits: assessment of menstrual disorders, pelvic infections, urinary incontinence, abnormal Pap smears, fertility concerns, pregnancy and menopause. Approximately a third of gynecology office visits are related to abnormal uterine bleeding.
Trends in the OB/GYN market include:
•Office-based and outpatient procedures are increasing given high patient satisfaction, reduction of health system cost and comparative clinical outcomes.
•Hysterectomy and cesarean section remain common hospital surgical interventions in women worldwide.
•Pregnancy and childbirth complications are on the rise.
•The obstetrician is a key contributor to stem cell storage, facilitating the collection of cord blood and cord tissue following delivery in most markets.
•Initial evaluation and treatments for infertility, such as uterine assessment, ovulatory medications and intrauterine insemination (IUI), begin with the OB/GYN and then transition to fertility clinics.
With respect to fertility clinics, CooperSurgical expects growth in fertility treatments as:
•Infertility rates are increasing globally, and there is a significant unmet need for fertility services.
•Patient awareness of and access to services are increasing at a rapid pace.
•The number of fertility clinics is rising worldwide.
•The fertility market is fueled by dynamics such as increasing maternal age, single parents by choice, and LGBTQ+ identifying individuals starting families.
•Improved product offerings such as donor activity and cryopreservation services
•Technology improvements for both male and female infertility challenges
•Greater worldwide disposable income
Women's and Family Health Care Product Sales

11
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Office/Surgical – This includes Endosee endometrial imaging products, Fetal Pillow cephalic elevation devices for use in Cesarean sections, illuminated speculum products, Lone Star retractor systems, loop electrosurgical excision procedure (LEEP) products, Mara water ablation systems, newborn stem cell storage, PARAGARD contraceptive IUDs, point-of-care products and uterine positioning products.
Fertility – Our significant fertility products and services include cryostorage, donor gamete services, fertility consumables and equipment and genomic services (including preimplantation genetic testing).
CooperSurgical Competition
CooperSurgical focuses on selected segments of the women's and family health care market with a diversified portfolio of products and services including medical devices in outpatient and operating room settings, fertility, contraception and healthcare technology services.
Competitive factors in these segments in which CooperSurgical competes include technological and scientific advances, product quality and availability, price, customer service including response time and effective communication of product information to physicians, consumers, fertility clinics and hospitals. Competition in the medical device industry is dynamic and involves the search for technological and therapeutic innovations. CooperSurgical's strategy includes developing and acquiring new solutions.
CooperSurgical competes in categories across women’s health, including routine care, diagnostics and medical devices used in outpatient care, surgical procedures and labor and delivery. Larger companies such as Johnson & Johnson, Medtronic and Hologic have offerings that compete with our products.
CooperSurgical offers private cord blood and cord tissue cryostorage services in the U.S., Canada and Australia. In that field, we compete primarily with ViaCord, a division of Perkin Elmer, in the U.S., as well as other smaller companies globally.
CooperSurgical also offers PARAGARD, which is the only FDA approved, non-hormonal Intrauterine Device (IUD) contraceptive option in the U.S. and has a 10-year use indication. With PARAGARD, we compete with manufacturers of hormonal IUDs including Bayer and AbbVie, Long Acting Reversible Contraceptives (LARCs), including Organon, and other forms of birth control.
CooperSurgical also competes in the fertility category of the women's and family health care market. We have broad product offerings for fertility evaluations and IVF procedures by OB/GYN, reproductive endocrinologists and embryologists. These include products for use by the OB/GYN in their offices for initial evaluations with office-based hysteroscopy and first line treatments such as intrauterine insemination. In fertility clinics, our products include media, micro-tools and lab equipment. Additionally, services offered to clinics and families undergoing assisted reproductive technologies include genomics, donor gametes and cryostorage. CooperSurgical competes with a large number of competitors in the fertility market including Vitrolife Group, FujiFilm-Irvine Scientific, Cook Medical, Hamilton Thorne, Natera and Invitae.
RESEARCH AND DEVELOPMENT
The Company employs approximately 300 people in research and development. CooperVision's product development and clinical research is supported by internal and external specialists in lens design, formulation science, polymer chemistry, engineering, clinical trials, microbiology and biochemistry. CooperVision's research and development activities primarily include programs to develop new contact lens designs and manufacturing technology, along with improving formulations and existing products.
CooperSurgical conducts research and development in-house and has consulting agreements with external specialists in software, hardware and electrical engineering, genetic science and embryology. CooperSurgical's research and development activities include the design and improvement of surgical procedure devices, and the advancement and expansion of CooperSurgical's portfolio of fertility and general OB/GYN offerings.
GOVERNMENT REGULATION
Medical Device Regulation in the United States
Most of our products are medical devices subject to extensive regulation by the FDA in the U.S. and other regulatory bodies abroad. The Federal Food, Drug, and Cosmetic Act (FDCA) and FDA regulations govern, among other things, medical device design and development, testing, manufacturing, labeling, storage, record keeping, premarket clearance or approval, advertising and promotion, and sales and distribution. Unless an exemption applies, each medical device we
12
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
wish to distribute commercially in the U.S. will require either premarket notification to the FDA requesting clearance for commercial distribution under Section 510(k) of the FDCA, or premarket approval (PMA) from the FDA. A majority of the medical devices we currently market have received FDA clearance through the 510(k) process or approval through the PMA process. Because we cannot be assured that any new products we develop, or any product enhancements, will be exempt from the premarket clearance or approval requirements or will be subject to the shorter 510(k) clearance process rather than the PMA process, significant delays in the introduction of any new products or product enhancements may occur.
Device Classification
The FDA classifies medical devices into one of three classes—Class I, II or III—depending on the degree of risk associated with each medical device and the extent of control needed to ensure its safety and effectiveness. Both CooperVision and CooperSurgical develop and market medical devices subject to different levels of FDA regulation depending on the classification of the device. Class III devices, such as flexible and extended wear contact lenses, require extensive premarket testing and approval, while Class I and II devices require lower levels of regulation. The majority of CooperSurgical's products are Class II devices.
Class I devices are devices with the lowest risk and are those for which safety and effectiveness can be assured by adherence to the FDA's general regulatory controls for medical devices, which include compliance with the applicable portions of the FDA's Quality System Regulation (QSR), facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (General Controls). Some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process described below.
Class II devices are moderate risk devices, which are subject to the FDA's General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device, such as performance standards, post-market surveillance, FDA guidelines or particularized labeling requirements. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification procedure. Pursuant to the Medical Device User Fee and Amendments to the FDA Reauthorization Act (MDUFA IV), unless a specific exemption applies, 510(k) premarket notification submissions require payment of user fees. Certain Class II devices are exempt from this premarket review process.
Class III devices are those devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or certain implantable devices, or which have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device. The safety and effectiveness of Class III devices cannot be assured solely by the General Controls and other special controls such as those listed above. These devices almost always require formal clinical studies to demonstrate safety and effectiveness and must be approved through the PMA process described below. PMA applications (and supplemental PMA applications) are subject to substantially higher user fees under MDUFA IV than are 510(k) premarket notifications.
510(k) Clearance Pathway
When we are required to obtain a 510(k) clearance for a Class I or Class II device that we wish to market, we must submit a premarket notification to the FDA demonstrating that the device is substantially equivalent to a legally marketed predicate device. A predicate device is a legally marketed device that is not subject to a PMA, a device that was legally marketed in commercial distribution in the U.S. before May 28, 1976 (a pre-amendments device) and, for which the FDA has not yet called for the submission of a PMA, a device that has been reclassified from Class III to Class II or I, or a device that was found substantially equivalent through the 510(k) premarket notification process. The FDA aims to make substantial equivalence determinations following receipt of a 510(k) premarket notification within 90 days of submission of the notification, but as a practical matter, clearance can take significantly longer. Although many 510(k) pre-market notifications are cleared without clinical data, in some cases, the FDA requires additional information to support substantial equivalence. If the FDA agrees that the device is substantially equivalent to a predicate device, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is not substantially equivalent to a legally marketed predicate, the device is automatically designated as a Class III device. The device sponsor must fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the de novo process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) clearance or, depending on the modification, PMA approval or de novo classification. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer's determination. If
13
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
the FDA disagrees with a manufacturer's determination that a new clearance or approval is not required for a particular modification, the FDA may require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or until premarket approval is obtained or a de novo classification request is granted. In these circumstances, a manufacturer also may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements and modifications to our devices that we believe do not require new 510(k) clearances.
Over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products. For example, in November 2018, FDA officials announced steps that the FDA intended to take to modernize the premarket notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k) clearance pathway, and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. These proposals have not yet been finalized or adopted.
In September 2019, the FDA published updated guidance describing an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA maintains a list of device types appropriate for the “safety and performance based” pathway and continues to develop product-specific guidance documents that identify the performance criteria for each such device type, as well as recommended testing methods, where feasible.
Premarket Approval Pathway
A PMA application must be submitted if the device cannot be cleared through the 510(k) premarket notification procedures or if the device has been previously classified as Class III (unless otherwise 510(k) exempt). The PMA process is much more demanding than the 510(k) premarket notification process. A PMA application must be supported by extensive data including, but not limited to, technical, non-clinical, clinical trials, manufacturing and labeling to demonstrate to the FDA's satisfaction the safety and effectiveness of the device for its intended use.
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA, by statute and regulation, has 180 days to review an accepted PMA application, although the review generally occurs over a significantly longer period of time, and can take up to several years. During this review period, the FDA may request additional information, including clinical data, non-clinical data or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant's response to deficiencies communicated by the FDA. The FDA considers a PMA or PMA supplement to have been voluntarily withdrawn if an applicant fails to respond to an FDA request for information (e.g., major deficiency letter) within 180 days after the FDA issues such request. Also, during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, which, among other things requires manufacturers to implement and follow elaborate design, testing, control, documentation and other quality assurance procedures in the device design and manufacturing process.
The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution and collection of long-term follow-up data from patients in the clinical study that supported approval. The FDA may also condition approval of a PMA application on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in materially adverse enforcement action, including the loss or withdrawal of the approval. New PMA applications or PMA application supplements are required for significant modifications to the manufacturing process, labeling and design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes
14
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
from the device covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials for Medical Devices
A clinical trial is almost always required to support a PMA application and is sometimes required to obtain clearance of a 510(k) premarket notification. These trials may require submission of an application for an investigational device exemption (IDE) to the FDA depending on the device. If the device under evaluation does not present a significant risk to human health, then the device sponsor is not required to submit an IDE application to the FDA before initiating human clinical trials, but must still comply with abbreviated IDE requirements when conducting such trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. If the device presents a “significant risk” to human health, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application, which includes a clinical study protocol, must be supported by appropriate data, such as animal and laboratory testing results, showing that the potential benefits of testing the device in humans and the importance of the knowledge to be gained outweighs the risks to human subjects from the proposed investigation that the testing protocol is scientifically sound and there is reason to believe that the device as proposed for use will be effective. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies the company that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may permit a clinical trial to proceed under a conditional approval.
Regardless of the degree of risk presented by the medical device, clinical studies must be approved by, and conducted under the oversight of, an Institutional Review Board (IRB) for each clinical site. The IRB is responsible for the initial and continuing review of the study, and may pose additional requirements for the conduct of the study. There can be no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to unacceptable health risks that outweigh the benefits of participation in the study. During a study, we are required to comply with the FDA's IDE requirements for investigator selection, trial monitoring, reporting, record keeping and prohibitions on the promotion of investigational devices or making safety or efficacy claims for them. We are also responsible for the appropriate labeling and distribution of investigational devices.
Continuing FDA and Other Government Agency Regulation of Medical Devices
After a device is placed on the market, numerous regulatory requirements apply. These include: establishment registration and device listing with the FDA; the QSR, which requires manufacturers to follow design, testing, production, control, complaint handling, documentation and other quality assurance procedures during the manufacturing process; labeling regulations, which prohibit the promotion of products for uncleared or unapproved or “off-label” uses and impose other restrictions on labeling, advertising and promotion; new FDA unique device identifier regulations, which require changes to labeling and packaging; and medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections for cause by the FDA to determine our compliance with the QSR and other regulations.
Failure to comply with applicable regulatory requirements, which are subject to new legislation and change, can result in enforcement action by the FDA, or other federal and state government agencies which may include, but may not be limited to, any of the following sanctions or consequences: warning letters or untitled letters; fines, injunctions and civil penalties; recall, seizure or import holds of our products; operating restrictions, suspension or shutdown of production; refusing to issue certificates to foreign governments needed to export products for sale in other countries; refusing our request for 510(k) clearance or premarket approval of new or modified products; withdrawing 510(k) clearance or premarket approvals that are already granted; and criminal prosecution.
Laboratory Developed Tests
We provide certain genetic testing laboratory services. In the U.S., under the FDCA and the FDA’s regulatory framework, in vitro diagnostic devices (IVDs) are a type of medical device that can be used in the diagnosis or detection of diseases, such as cancer, or other conditions. The FDA considers Laboratory Developed Tests (LDTs) to be a subset of IVDs, which are intended for clinical use and are designed, manufactured, and used within a single laboratory. Although the FDA has statutory authority to assure that medical devices, including IVDs, are safe and effective for their intended uses, the FDA
15
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
has historically exercised its enforcement discretion and not enforced certain applicable provisions of the FDCA and regulations with respect to certain LDTs.
Even under its current enforcement discretion policy, the FDA has issued warning letters to IVD manufacturers for commercializing laboratory tests that were purported to be LDTs but that the FDA alleged failed to meet the definition of an LDT or otherwise were not subject to the FDA’s policy on enforcement discretion over LDTs because they presented a potential safety risk or because they were marketed directly to consumers without the involvement of a health care professional. Additionally, the FDA could change its policy of enforcement discretion for LDTs, even without legislation. For example, in recent years, the FDA has stated its intention to modify its enforcement discretion policy with respect to LDTs. Specifically, on July 31, 2014, the FDA notified Congress of its intent to modify, in a risk-based manner, its policy of enforcement discretion with respect to LDTs. On October 3, 2014, the FDA issued two draft guidance documents entitled “Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs),” or the Framework Guidance, and “FDA Notification and Medical Device Reporting for LDTs,” or the Reporting Guidance. The Framework Guidance stated that FDA intended to modify its policy of enforcement discretion with respect to LDTs in a risk-based manner consistent with the classification of medical devices generally in Classes I through III. The Reporting Guidance would have further enabled the FDA to collect information regarding the LDTs currently being offered for clinical use through a notification process, as well as to enforce its regulations for reporting safety issues and collecting information on any known or suspected adverse events related to the use of an LDT. The FDA halted finalization of this guidance in November 2016 to allow for further public discussion on an appropriate oversight approach for LDTs and to give congressional authorizing committees the opportunity to develop a legislative solution. In January 2017, the FDA issued a discussion paper on possible approaches to LDT regulation.
Legislative and administrative proposals proposing to amend the FDA’s oversight of LDTs have been introduced in recent years and we expect that new legislative and administrative proposals will continue to be introduced from time to time. For example, key congressional committees with jurisdiction over FDA matters have indicated an interest in continuing negotiations on potential legislation regarding LDTs. In March 2020, the VALID Act was introduced in the House and an identical version of the bill was introduced in the U.S. Senate. If passed in its current form, the VALID Act would create a new category of medical products separate from medical devices called “in vitro clinical tests,” or IVCTs. As proposed, the bill would establish a risk-based approach to imposing requirements related to premarket review, quality systems, and labeling requirements on all IVCTs, including LDTs, but would create exemptions for certain LDTs marketed before the effective date of the bill (though other regulatory requirements may apply, such as registration and adverse event reporting). In June 2021, a revised version of the VALID Act was reintroduced in both the House and the Senate. It is unclear whether the VALID Act or any other legislative proposals (including any proposals to reduce FDA oversight of LDTs) would be passed by Congress or signed into law by the President. Depending on the approach adopted under any potential legislation, certain LDTs could become subject to some form of premarket review, potentially with a transition period for compliance and a grandfathering provision.
If Congress does not take action in connection with the VALID Act or other LDT legislation, it is possible that the FDA could change its regulatory policy governing LDTs in a way that could require that our currently marketed genetic tests, and any future products that we anticipate marketing as LDTs, comply with certain additional FDA requirements.
As we operate a genetic testing laboratory, we are required to hold certain federal, state and local licenses, certifications and permits to conduct our business. Under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, we are required to hold a certificate applicable to the type of laboratory tests we perform and to comply with standards applicable to our operations, including test processes, personnel, facilities administration, equipment maintenance, recordkeeping, quality systems and proficiency testing. We have current certification under CLIA to perform testing at our New Jersey facility. To renew our CLIA certificate, we are subject to survey and inspection every two years to assess compliance with program standards. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business. Penalties for non-compliance with CLIA requirements include suspension, limitation or revocation of the laboratory’s CLIA certificate, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit or criminal penalties.
In addition to federal certification requirements of laboratories under CLIA, licensure is required and maintained for our laboratory under state law. Such laws establish standards for the day-to-day operation of a clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, state laws mandate proficiency testing, which involves testing of specimens that have been specifically prepared for the laboratory. In addition, certain states require licensing of out-of-state laboratories in order to receive and test specimens from those tests. If a laboratory is out of compliance with such statutory or regulatory standards, the state may suspend, limit, revoke or annul the laboratory’s license, censure the holder of the license or assess civil money penalties.
16
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
FDA Regulation of HCT/Ps
We currently operate a provider of donor egg and sperm for fertility treatments, fertility cryopreservation services and newborn stem cell storage. Donated reproductive tissue (eggs or sperm) are regulated by the FDA as human cells, tissues, and cellular and tissue-based products (HCT/Ps). In addition, Section 361 of the Public Health Service Act (PHSA) authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease with respect to HCT/Ps. HCT/Ps regulated as “361 HCT/Ps” are subject to requirements relating to registering facilities and listing products with the FDA, and stringent requirements for processing, storing, labeling and distributing HCT/Ps, including required labeling information, screening and testing for tissue donor eligibility, record keeping and adverse event reporting, among other applicable requirements and laws. 361 HCT/Ps do not require 510(k) clearance, PMA approval, submission of a Biologics License Application, or other premarket authorization from the FDA before marketing. However, to be regulated as a 361 HCT/P, the product must, among other things, be “minimally manipulated,” which for structural tissue products means that the manufacturing processes do not alter the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction, repair, or replacement, and for cells or nonstructural tissue products, means that the manufacturing processes do not alter the relevant biological characteristics of cells or tissues. A 361 HCT/P must also be intended for “homologous use,” which refers to use in the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor. HCT/Ps that do not meet the criteria of Section 361 are regulated under Section 351 of the PHSA. Unlike 361 HCT/Ps, HCT/Ps regulated as “351 HCT/Ps” are subject to premarket review and/or approval by the FDA, as required. We believe our HCT/Ps are regulated as 361 HCT/Ps.
Pharmaceutical Regulation in the United States
FDA has determined that the primary mode of action for PARAGARD is the drug component and is therefore regulated by FDA’s Center for Drug Evaluation and Research as a drug product.
In the U.S., the FDA regulates drugs under the FDCA and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending New Drug Applications (NDA), withdrawal of an approval, imposition of a clinical hold, untitled letters, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including manufacturing, periodic reporting, product sampling and distribution, advertising, promotion, drug shortage reporting, compliance with any post-approval requirements imposed as a conditional of approval such as Phase 4 clinical trials, a Risk Evaluation and Mitigation Strategy (REMS), and surveillance, recordkeeping and reporting requirements, including adverse experiences.
After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to further testing to new clinical investigation requirements and prior FDA review and approval. There also are continuing, annual program fee requirements for any approved products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and to list their drug products and are subject to periodic announced and unannounced inspections by the FDA and these state agencies for compliance with Good Manufacturing Practices, or cGMPs, and other requirements, which impose procedural and documentation requirements upon us and our third-party manufacturers.
Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented, or FDA notification. FDA regulations also require investigation and correction of any deviations from cGMPs specifications and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in withdrawal of marketing approval, mandatory revisions to the approved labeling to add new safety information or other limitations, imposition of post-market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a REMS program, among other consequences.
17
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA. Physicians, in their independent professional medical judgement, may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. We, however, are prohibited from marketing or promoting drugs for uses outside of the approved labeling. In addition, the distribution of prescription pharmaceutical products, including samples, is subject to the Prescription Drug Marketing Act (PDMA), which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution. The Drug Supply Chain Security Act also imposes obligations on manufacturers of pharmaceutical products related to product and tracking and serialization.
Failure to comply with any of the FDA’s requirements, which are subject to new legislation and change, could result in significant adverse enforcement actions. These include a variety of administrative or judicial sanctions, such as refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, imposition of a clinical hold or termination of clinical trials, warning letters, untitled letters, cyber letters, modification of promotional materials or labeling, product recalls, product seizures or detentions, refusal to allow imports or exports, total or partial suspension of production or distribution, debarment, injunctions, fines, consent decrees, corporate integrity agreements, refusals of government contracts and new orders under existing contracts, exclusion from participation in federal and state healthcare programs, restitution, disgorgement or civil or criminal penalties, including fines and imprisonment. It is also possible that failure to comply with the FDA’s requirements relating to the promotion of prescription drugs may lead to investigations alleging violations of federal and state healthcare fraud and abuse and other laws, as well as state consumer protection laws. Any of these sanctions could result in adverse publicity, among other adverse consequences.
Foreign Regulation
Health authorities in foreign countries regulate Cooper's clinical studies and medical device sales. The regulations vary widely from country to country. Even if the FDA has cleared or approved a product in the U.S., the regulatory agencies or notified bodies in other countries must approve or certify new products before they may be marketed there. The time required to obtain approval or certification in another country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ. There is a trend towards harmonization of quality system standards among the European Union (EU), U.S., Canada and various other industrialized countries. Japan has one of the most rigorous regulatory systems in the world and requires in-country clinical trials. The Japanese quality and regulatory standards remain stringent even with the more recent harmonization efforts and updated Japanese regulations. China is also updating its regulations and is requiring rigorous in-country product testing. These regulatory procedures require a considerable investment in time and resources and usually result in a substantial delay between new product development and marketing. If the Company does not maintain compliance with regulatory standards or if problems occur after marketing, product approval may be withdrawn.
In addition to FDA regulatory requirements, CooperVision maintains ISO 13485 certification and CE marks for its products and CooperSurgical maintains ISO 13485 certification for medical devices and ISO 15189 certification for the Genomics laboratories. The ISO 13485 Quality Measurement System registration is now also required for registration of products in Asia Pacific and Latin American countries. In order to maintain these quality benchmarks, the Company is subjected to rigorous biannual reassessment audits of its quality systems and procedures.
Regulation of Medical Devices and In Vitro Diagnostic Medical Devices in the European Union
The EU has adopted specific directives and regulations regulating the design, manufacture, clinical investigations, conformity assessment, labeling and adverse event reporting for medical devices (including IVDs).
In the EU, until May 25, 2021, medical devices were regulated by the Council Directive 93/42/EEC (the EU MDD) which has been repealed and replaced by Regulation (EU) No 2017/745 (the EU MDR). Similarly, until May 25, 2022, IVDs were regulated by Directive 98/79/EC (the EU IVDD) which has been repealed and replaced by Regulation (EU) 2017/746 of the European Parliament and of the Council (the EU IVDR). However, on October 14, 2021, the European Commission proposed a “progressive” roll-out of the EU IVDR to prevent disruption in the supply of IVDs. The European Parliament and Council adopted the proposed regulation on December 15, 2021. The EU IVDR became applicable on May 26, 2022 but there is a tiered system extending the grace period for many devices (depending on their risk classification) before they have to be fully compliant with the regulation. Unlike directives, regulations are directly applicable in all EU member states without the need for member states to implement into national law. Both regulations were adopted to establish a modernized and more robust EU legislative framework, with the aim of ensuring better protection of public health and patient safety.
18
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Our current certificates have been granted under the EU MDD and the EU IVDD. Devices lawfully placed on the market pursuant to the EU MDD prior to May 26, 2021 may generally continue to be made available on the market or put into service until May 26, 2025, provided that the requirements of the transitional provisions are fulfilled. In particular, the certificate in question must still be valid. Similarly, with respect to IVDs, they may generally continue to be made available provided that the requirements of the transitional provisions are fulfilled. However, respectively as of May 26, 2021 and May 26, 2022 some of the EU MDR and EU IVDR requirements apply in place of the corresponding requirements of the former directives with regard to registration of economic operators and of devices, post-market surveillance and vigilance requirements. Pursuing marketing of medical devices (including IVDs) in the EU will notably require that our devices be certified under the new regimes set forth in the EU MDR and the EU IVDR when our current certificates expire.
Both the EU MDR and the EU IVDR seek to:
•strengthen the rules on placing devices on the market and reinforce surveillance once they are available;
•establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market;
•establish explicit provisions on importers’ and distributors’ obligations and responsibilities;
•impose an obligation to identify a responsible person who is ultimately responsible for all aspects of compliance with the requirements of the new regulation;
•improve the traceability of medical devices throughout the supply chain to the end-user or patient through the introduction of a unique identification number, to increase the ability of manufacturers and regulatory authorities to trace specific devices through the supply chain and to facilitate the prompt and efficient recall of medical devices that have been found to present a safety risk;
•set up a central database (Eudamed) to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and
•strengthen rules for the assessment of certain high-risk devices that may have to undergo an additional check by experts before they are placed on the market.
In the EU, there is currently no premarket government review of medical devices (including IVDs). However, all medical devices (including IVDs) placed on the EU market must meet general safety and performance requirements , including that a medical device must be designed and manufactured in such a way that, during normal conditions of use, it is suitable for its intended purpose. Medical devices must be safe and effective and must not compromise the clinical condition or safety of patients, or the safety and health of users and, where applicable, other persons, provided that any risks which may be associated with their use constitute acceptable risks when weighed against the benefits to the patient and are compatible with a high level of protection of health and safety, taking into account the generally acknowledged state of the art.
Compliance with the general safety and performance requirements is a prerequisite for European Conformity Marking (CE mark) without which medical devices cannot be marketed or sold in the EU. To demonstrate compliance with the general safety and performance requirements medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risk) classification. Except for low-risk medical devices (Class I) or general IVDs (Class A), where the manufacturer can self-assess the conformity of its products with the general safety and performance requirements (except for any parts which relate to sterility, metrology or reuse aspects of a medical device), a conformity assessment procedure requires the intervention of a notified body. Notified bodies are independent organizations designated by EU member states to assess the conformity of devices before being placed on the market. A notified body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality system (notified body must presume that quality systems which implement the relevant harmonized standards—ISO 13485:2016 for Quality Management Systems—conform to these requirements). If satisfied that the relevant product conforms to the general safety and performance requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE mark to the device, which allows the device to be placed on the market throughout the EU.
Throughout the term of the certificate of conformity, the manufacturer will be subject to periodic surveillance audits to verify continued compliance with the applicable requirements. In particular, there will be a new audit by the notified body before it will renew the relevant certificate(s).
All manufacturers placing medical devices on the market in the EU must comply with the EU medical device vigilance system. Under this system, serious incidents and Field Safety Corrective Actions (FSCAs) must be reported to the relevant authorities of the EU member states. Manufacturers are required to take FSCAs to prevent or reduce a risk of a serious incident associated with the use of a medical device that is made available on the market. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device.
19
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
The advertising and promotion of medical devices is subject to some general principles set forth by EU directives. Only devices that are CE marked may be marketed and advertised in the EU in accordance with their intended purpose. Directive 2006/114/EC concerning misleading and comparative advertising and Directive 2005/29/EC on unfair commercial practices, while not specific to the advertising of medical devices, also apply to the advertising thereof and contain general rules, for example requiring that advertisements are evidenced, balanced and not misleading. Specific requirements are defined at the national level. EU member states laws related to the advertising and promotion of medical devices, which vary between jurisdictions, may limit or restrict the advertising and promotion of products to the general public and may impose limitations on promotional activities with healthcare professionals.
In the EU, regulatory authorities have the power to carry out announced and, if necessary, unannounced inspections of companies, as well as suppliers and/or sub-contractors and, where necessary, the facilities of professional users. Failure to comply with regulatory requirements (as applicable) could require time and resources to respond to the regulatory authorities’ observations and to implement corrective and preventive actions, as appropriate. Regulatory authorities have broad compliance and enforcement powers, and if such issues cannot be resolved to their satisfaction can take a variety of actions, including untitled or warning letters, fines, consent decrees, injunctions, or civil or criminal penalties.
Regulation of Laboratory Developed Tests in the European Union
In the EU, laboratory developed tests (LDTs) are exempt from the regulations that govern medical devices and IVDs under certain conditions. According to the former Article 1(5) of the EU IVDD, “[this Directive] shall not apply to devices manufactured and used only within the same health institution and on the premises of their manufacture or used on premises in the immediate vicinity without having been transferred to another legal entity.” Article 1(5) further provided that this exemption did not affect the right of an EU member state from imposing “appropriate protection requirements.” In order to fall within this exemption under the EU IVDD, medical devices, including laboratory developed tests, had to be designed and used within such health institution (which may include hospitals, laboratories and public health institutions that support the healthcare system and/or address patient needs but do not treat or care for patients directly) on a non-industrial scale, without being released into the market. However, the legal framework for applying the exemption under the EU IVDD to laboratory developed tests was not entirely clear, as the EU IVDD did not specify what non-industrial scale would be.
Since the EU IVDR became applicable, it may provide greater clarity on the regulation of LDTs. Under the EU IVDR, the general safety and performance requirements set out in Annex I of the EU IVDR are also applicable to devices manufactured and used only within health institutions. Manufacturers of such devices are required to demonstrate conformity with the general safety and performance requirements set out in Annex I of the EU IVDR through performance evaluations in accordance with Article 56 of the EU IVDR and the manufacturer’s quality management system framework.
The EU IVDR provides that the relevant general safety and performance requirements set out in Annex I of the EU IVDR do not generally apply to devices manufactured and used only within health institutions established in the EU, provided that the conditions set out in Article 5 of the EU IVDR are met. Under the EU IVDR, health institutions may manufacture, modify and use medical devices within such institutions, thereby addressing the specific needs of target patient groups on a non-industrial scale. Under such circumstances, where the LDTs are manufactured and used strictly within health institutions (which may include hospitals, laboratories, public health institutions that support the healthcare system and/or address patient needs but do not treat or care for patients directly), LDTs would continue to be exempt from regulation. However, compared to the previous regulatory regime, the exemptions for LDTs have, overall, been narrowed, as even in relation to LDTs, health institutions, among others, are required to provide information upon request on the use of such devices to their competent authority and each health institution will have to draw up a declaration which it will make publicly available. If these conditions are not met and/or diagnostic tests are manufactured and used only within health institutions but “on an industrial scale,” such tests will qualify as IVDs with the full applicability of the EU IVDR. LDTs regulated by the EU IVDR are subject to conformity assessments and inspections by the relevant competent authority, who will also review the declarations and statements made by the health institutions in relation to their LDTs. Our current and future tests will need to be analyzed as to whether any or all of them would qualify for an exemption under Article 5 of the EU IVDR or otherwise we will be required to comply with various certification and documentation criteria, and we may be subject to conformity assessments and inspections.
The aforementioned EU rules are generally applicable in the European Economic Area (EEA) which consists of the 27 EU member states plus Norway, Liechtenstein and Iceland.
The Impact of Brexit
Following a national referendum and enactment of legislation by the government of the UK, the UK formally withdrew from the EU on January 31, 2020, commonly referred to as “Brexit”, and, following the expiry of the Brexit transitional period on December 31, 2020, the UK now operates under a distinct regulatory regime and certain EU laws now only
20
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
apply to the UK in respect of Northern Ireland (as laid out in the Protocol on Ireland and Northern Ireland). The Medicines and Healthcare products Regulatory Agency (MHRA) is now the UK’s standalone regulator. Although the UK and EU have now reached an agreement on its future trading relationship (implemented in the EU-UK Trade and Cooperation Agreement from January 1, 2021, or the TCA), the agreement does not cover all regulatory areas regarding medical devices (including IVDs), which may be subject to future bilateral discussions going forward and could further change the relationship between the UK and the EU in this regard.
EU laws which were directly applicable before the end of the transitional period or have been transposed into UK law through secondary legislation continue to be applicable as “retained EU law.” However, new legislation such as the EU MDR and EU IVDR will not be applicable. The UK government has introduced a new Medicines and Medical Devices Act which seeks to address regulatory gaps through implementing regulations and delegated powers covering the fields of human medicines, clinical studies of human medicines, and medical devices.
Significantly, under the TCA there is no mutual recognition of regulatory regimes and certifications between the EU and the UK. Post-Brexit, amendments have been made to the existing UK medical devices legislation which require medical devices to be registered with the MHRA before being placed on the Great Britain market. Manufacturers based outside of the UK need to appoint a UK Responsible Person to register devices with the MHRA. Following a government consultation on changes to the UK’s medical device regulations, the response to which was published on June 26, 2022, it is anticipated that amendments to the legislation will soon be published by the government and should become applicable by July 1, 2023 so that medical devices placed on the market in Great Britain (England, Scotland, and Wales) will require a UK Conformity Assessment (UKCA) mark. However, within the government’s consultation response, it has proposed that transition periods will be included in the revised legislation so that products with existing and valid conformity assessments (CE mark or UKCA mark) could continue to be placed on the Great Britain market for a maximum of 3-5 years after July 1, 2023, depending on which legislation the medical device has been certified under. IVDs with valid certification can continue to be placed on the market until the earlier of certificate expiry or for a period of five years following publication of the new regulations. However, UKCA marking alone will not be recognized in the EU. The rules for placing medical devices on the Northern Ireland market will differ from those in the Great Britain. These modifications may have an effect on the way we intend to conduct our business in these countries.
Other Health Care Regulation
We may be subject to various federal, state and foreign laws pertaining to health care fraud and abuse, including anti-kickback laws and physician self-referral, false claims laws and physician payment transparency laws and regulations.
In the U.S., the federal Anti-Kickback Statute prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting, or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing, or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The federal physician self-referral prohibitions, commonly known as the Stark Law, generally prohibit entities from billing a patient or the Medicare or Medicaid programs for certain designated health services, including clinical laboratory services, when the physician ordering the service, or any member of such physician’s immediate family, has a financial interest, such as an ownership or investment interest in or compensation arrangement with us, unless the arrangement meets an exception to the prohibition. These prohibitions apply regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral.
The federal False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to, or approval by, the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute or Stark Law constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
21
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
The federal Health Insurance Portability and Accountability Act of 1996 also created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
In addition, the federal government, as part of the Patient Protection and Affordable Care Act (the ACA), as well as certain state governments have enacted laws aimed at increasing transparency in relationships between medical device companies and health care professionals. We are now required by the federal Physician Payments Sunshine Act and similar state and foreign laws to report annually many types of payments made and items of value provided to licensed health care professionals and teaching hospitals, as well as certain ownership and investment interests held by physicians (as defined by statute) and their immediate family members. Certain states also mandate implementation of commercial compliance programs, impose restrictions on device manufacturer marketing practices and tracking and/or require the reporting of gifts, compensation and other remuneration to physicians. In addition, certain foreign jurisdictions have adopted, or are currently acting to implement, similar laws.
In the EU, many member states have adopted specific anti-gift statutes that further limit commercial practices for medical devices (including IVDs), in particular vis-à-vis healthcare professionals and organizations. Additionally, there has been a recent trend of increased regulation of payments and transfers of value provided to healthcare professionals or entities. In addition, many EU member states have adopted national “Sunshine Acts” which impose reporting and transparency requirements (often on an annual basis), similar to the requirements in the U.S., on medical device manufacturers. Certain countries also mandate implementation of commercial compliance programs.
Violations of these laws may be punishable by criminal and civil sanctions, including fines and civil monetary penalties, the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid), disgorgement and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on companies. Similar sanctions and penalties, as well as imprisonment, also can be imposed upon executive officers and employees of such companies.
Coverage and Reimbursement
Market acceptance and sales of our CooperSurgical products to our customers, who primarily consist of hospitals and surgical centers, OB/GYN medical offices and fertility clinics, will depend on the availability of payor coverage and the adequacy of reimbursement, for the procedures using our products, by government insurance programs and other third-party payors. Payor coverage and reimbursement for procedures using medical devices in the U.S. and international markets vary significantly by country.
In the U.S., our currently approved products are commonly treated as general supplies utilized in surgical procedures and if covered by third-party payors, are paid for as part of the procedure. Outside of the U.S., there are many reimbursement programs through private payors as well as government programs. In some countries, government reimbursement is the predominant program available to patients and hospitals. Our commercial success depends in part on the extent to which governmental authorities, private health insurers and other third-party payors provide coverage for and establish adequate reimbursement levels for the procedures during which our products are used. Failure by physicians, hospitals, surgery centers, fertility clinics and other users of our products to obtain sufficient coverage and reimbursement from third-party payors for procedures in which our products are used, or adverse changes in government and private third-party payors’ coverage and reimbursement policies.
We believe the overall escalating cost of medical products and services being paid for by the government and private health insurance has led to, and will continue to lead to, increased pressures on the healthcare and medical device industry to reduce the costs of products and services. All third-party reimbursement programs are developing increasingly sophisticated methods of controlling healthcare costs through prospective reimbursement and capitation programs, group purchasing, redesign of benefits, requiring second opinions prior to major surgery, careful review of bills, encouragement of healthier lifestyles and other preventative services and exploration of more cost-effective methods of delivering healthcare. In addition to uncertainties surrounding coverage policies, there are periodic changes to reimbursement levels. Third-party payors regularly update reimbursement amounts and also from time to time revise the methodologies used to determine reimbursement amounts. This includes routine updates to payments to physicians, hospitals and ambulatory surgery centers for procedures during which our products are used. These updates could directly impact the demand for our products.
22
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
With respect to drug coverage and reimbursement, third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of drugs, in addition to their safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of PARAGARD or any other drug product that receives approval. Third-party payors may not consider our products to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development. Additionally, decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product.
In international markets including the EU, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific product lines and procedures. There can be no assurance that procedures using our products will be covered for a specific indication, that our products will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be available or that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably. For example, in the EU, member states impose controls on whether products are reimbursable by national or regional health service providers and on the prices at which devices are reimbursed under state-run healthcare schemes. More and more, local, product specific reimbursement law is applied as an overlay to medical device regulation, which has provided an additional layer of clearance requirement.
Healthcare Reform
In the U.S, there has been, and continues to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect the profitable sale of product candidates.
Among policy makers and payors in the U.S, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. Passed in March 2010, the ACA substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical, medical device and clinical laboratory industries. Among other things, the ACA increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1%; required collection of rebates for drugs paid by Medicaid managed care organizations; required manufacturers to participate in a coverage gap discount program, in which manufacturers must agree to offer point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell certain “branded prescription drugs” to specified federal government programs, introduced a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; expanded eligibility criteria for Medicaid programs; created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and established a Center for Medicare & Medicaid Innovation at the CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
Since its enactment, there have been judicial and political challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court rejected a challenge by a group of states and individuals to the constitutionality of the ACA.
Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. Most recently, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). Individual states in the U.S. have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
In foreign countries where we market our products, recent healthcare reform has taken place as well. For instance in December 2021, the EU Regulation No 2021/2282 on Health Technology Assessment (HTA) amending Directive
23
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
2011/24/EU, was adopted. This regulation, which became effective in January 2022, intends to boost cooperation among EU member states in assessing health technologies, including some medical devices, and providing the basis for cooperation at the EU level for joint clinical assessments in these areas. The regulation foresees a three-year transitional period and will permit EU member states to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU member states will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement.
We expect that additional state, federal and foreign healthcare reform measures will be adopted in the future, any of which could limit the amounts that state, federal and foreign governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressures.
Data Privacy and Security Laws and Regulations
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the U.S., numerous federal and state laws and regulations, including data breach notification laws, health information privacy laws, and federal and state consumer protection laws and regulations, that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. For example, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder (collectively, HIPAA) imposes privacy, security and breach notification obligations on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Further, entities that knowingly obtain, use, or disclose individually identifiable health information maintained by a HIPAA covered entity in a manner that is not authorized or permitted by HIPAA may be subject to criminal penalties.
Even when HIPAA does not apply, according to the Federal Trade Commission (FTC), violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
In addition, certain state and non-U.S. laws, such as the EU General Data Protection Regulation (GDPR), govern the privacy and security of personal data, including health-related data in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, the California Consumer Privacy Act (CCPA) went into effect on January 1, 2020. The CCPA, among other things, creates data privacy obligations for covered companies and provides certain privacy rights to California residents, including the right to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Further, the California Privacy Rights Act (CPRA), which will go into effect on January 1, 2023, significantly amends the CCPA and will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. In Europe, the GDPR went into effect in May 2018 and imposes strict requirements for processing the personal data in the context of an establishment in the EEA or the processing of personal data of individuals within the EEA. The GDPR imposes comprehensive data privacy compliance obligations in relation to collection, processing, sharing, disclosure, transfer and other use of data relating to an identifiable living individual or “personal data,” including a principal of accountability and the obligation to demonstrate compliance through policies, procedures, training and audit. In addition, the GDPR increases the scrutiny of transfers of personal data from the EEA, including clinical trial sites located in the EEA to the U.S. and other jurisdictions that the European Commission does not recognize as having “adequate” data protection laws; in July 2020, the Court of Justice of the EU (CJEU) limited how organizations could lawfully transfer personal data from the EEA to the U.S. by invalidating the EU-US Privacy Shield and imposing further
24
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
restrictions on use of the standard contractual clauses, which could increase our costs and our ability to efficiently process personal data from the EEA. Indeed, while the CJEU upheld the adequacy of the standard contractual clauses (a standard form of contract approved by the European Commission as an adequate personal data transfer mechanism, and potential alternative to the Privacy Shield), it made clear that reliance on them alone may not necessarily be sufficient in all circumstances. Use of the standard contractual clauses must now be assessed on a case-by-case basis taking into account the legal regime applicable in the destination country, in particular applicable surveillance laws and rights of individuals and additional measures and/or contractual provisions may need to be put in place. Subsequent European court and regulator decisions have taken a restrictive approach to international data transfers.
Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant undertaking, whichever is greater.
Additionally, following the UK’s withdrawal from the EU, and the expiry of the transition period, companies will have to comply with the GDPR and the GDPR as incorporated into the UK national law, the latter regime having the ability to separately fine up to the greater of £17.5 million or 4% of annual global turnover. The European Commission has adopted an adequacy decision in favor of the UK, enabling data transfers from EU member states to the UK without additional safeguards. However, the UK adequacy decision will automatically expire in June 2025 unless the European Commission re-assesses and renews/extends that decision, and remains under review by the Commission during this period. The relationship between the UK and the EU in relation to certain aspects of data protection law remains unclear, and it is unclear how UK data protection laws and regulations will develop in the medium to long term, and how data transfers to and from the UK will be regulated in the long term.
RAW MATERIALS
Our businesses utilize various chemicals, packaging materials, components, parts and raw materials which are generally available from more than one source. However, in certain instances we acquire components and materials from sole suppliers to make our silicone hydrogel contact lens, certain medical devices and IVF products. Supply of these materials is protected by contractual agreements and safety stocks. However, if current raw material suppliers fail to supply sufficient materials on a timely basis, or at all for any reason, we could experience inventory shortages and disruption in the supply of products if we were required to use an alternative supplier on short notice.
MARKETING AND DISTRIBUTION
CooperVision markets our products through our own field sales representatives, distributors and eye care practitioners, including optometrists, ophthalmologists, opticians and optical chains. CooperVision also sells to distributors and to mass merchandisers who offer eye care services. To support the sale and use of CooperVision products, CooperVision engages in various activities and offers a variety of services. These include clinical training, digital marketing for the customer, e-commerce, telemarketing, social media, and journal advertisements. CooperVision also invested in tools that allow our customers to offer their patients monthly purchase and delivery subscriptions. In certain smaller countries, CooperVision often uses distributors and leverages our distributors' sales and marketing resources to attract major customers to CooperVision. With the addition of MiSight, CooperVision has expanded the breadth and depth of its sales support by adding myopia management specialists while it has expanded awareness campaigns to include direct to consumer elements including print, internet/social media, radio and television.
CooperSurgical's products are marketed by a network of dedicated field sales representatives, independent agents and distributors. CooperSurgical augments its sales and marketing activities by participating in national and regional industry trade shows, professional educational programs and internet promotions including e-commerce, social media and collaborative efforts with professional organizations, telemarketing, direct mail and advertising in professional journals. Since the addition of PARAGARD and cord blood and cord tissue storage services, CooperSurgical has also expanded its awareness campaigns to include direct to consumer elements including print, internet/social media, radio and television.
PATENTS, TRADEMARKS AND LICENSING AGREEMENTS
Cooper owns or licenses a variety of domestic and foreign patents, which, in total, are material to our overall business. The names of certain Cooper's products are protected by trademark registrations in the U.S. Patent and Trademark Office and, in some cases, also in foreign trademark offices. Applications are pending for additional trademark and patent registrations. Cooper intends to protect our intellectual property rights aggressively.
In addition to trademarks and patent licenses, we own certain trade secrets, copyrights, know-how and other intellectual property.
25
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Aquaform®, Avaira®, Avaira Vitality®, Biofinity®, Biofinity Energys®, MyDay®, MiSight®, ActivControl®, Proclear® and Biomedics® are registered trademarks of The Cooper Companies, Inc., its affiliates and/or subsidiaries. PC Technology™ and FIPS™ are trademarks of The Cooper Companies, Inc., its affiliates and/or subsidiaries. The clariti® mark is a registered trademark of The Cooper Companies, Inc., its affiliates and/or subsidiaries worldwide except in the U.S. where the use of clariti® is licensed. PARAGARD®, Mara®, Fetal Pillow® and Endosee® are registered trademarks of CooperSurgical, Inc.
DEPENDENCE ON CUSTOMERS
No customer accounted for 10% or more of our consolidated net revenue in fiscal 2022 and 2021. See Note 12. Business Segment Information of the Consolidated Financial Statements for additional information.
GOVERNMENT CONTRACTS
Neither of our business units is materially subject to profit renegotiation or termination of contracts or subcontracts at the election of the U.S. government.
SEASONALITY
CooperVision and CooperSurgical net sales in the fiscal first quarter, which runs from November 1 through January 31, are typically lower than subsequent quarters, as patient traffic to practitioners' offices, fertility clinics, and hospitals/surgical centers for surgical procedures is relatively light during the holiday season.
COMPLIANCE WITH ENVIRONMENTAL LAWS
Federal, state and local provisions that regulate the discharge of materials into the environment, or relate to the protection of the environment, do not currently materially affect Cooper's capital expenditures, earnings or competitive position.
In addition, the Company continues to monitor and comply with environmental health and safety regulations in countries in which it operates throughout the world, in particular, EU and China Restrictions on the use of certain Hazardous Substances in electrical and electronic equipment (RoHS) and Registration, Evaluation, Authorization and Restriction of Chemical substances (REACH).
HUMAN CAPITAL RESOURCES
As of October 31, 2022, we had a workforce of more than 14,000. We believe we have good relations with our workforce. Our employees are located around the world, with 52% in Americas, 43% in EMEA and 5% in Asia Pacific. Human capital management areas of focus include a people-focused culture; embedding diversity and inclusion; fostering an environment of health, safety, and well-being; investing in and developing our employees through training and engagement. In addition, we regularly conduct an employee survey to gauge employee engagement.
The Chart below shows percentage of employees located in Americas, EMEA and Asia Pacific as of October 31, 2022.
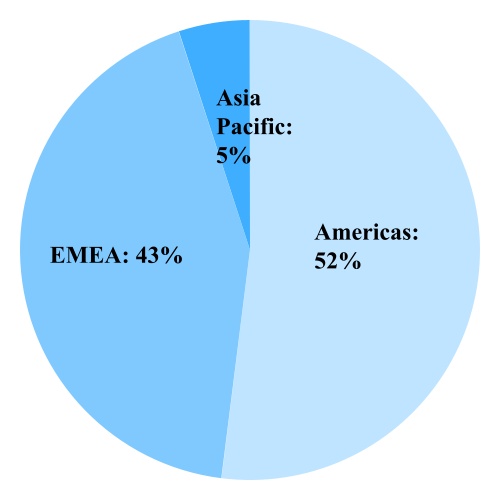
26
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Additional information is included in our annual ESG Report (located on our website at www.coopercos.com/esg). Information on our website, including the ESG Report, shall not be deemed incorporated by reference into this Annual Report.
CYBERSECURITY
In the normal course of business, we may collect and store personal information and other sensitive information, including proprietary and confidential business information, trade secrets, intellectual property, information regarding trial participants in connection with clinical trials, sensitive third-party information and employee information. To protect this information, our existing cybersecurity policies require continuous monitoring and detection programs, network security precautions, and in-depth security assessment of vendors. We maintain various protections designed to safeguard against cyberattacks, including firewalls and virus detection software. We have established and regularly test our disaster recovery plan and we protect against business interruption by backing up our major systems. In addition, we periodically scan our environment for any vulnerabilities, perform penetration testing and engage third parties to assess effectiveness of our data security practices. A third-party security consultant conducts regular network security reviews, scans and audits. In addition, we maintain insurance that includes cybersecurity coverage.
Our cybersecurity program is led by a team of highly skilled cybersecurity professionals, including 10 dedicated internal cybersecurity resources. Four members of the security team currently have CISSP credentials, five members hold one or more GIAC/SANS cybersecurity certificates, and in total the team has over 40 security and network certifications. In addition to our internal security staff, we partner with various third-party security service providers, including two 24/7 SOC teams, to augment our staffing, expertise, and hours of operation. The program incorporates industry-standard frameworks, policies and practices designed to protect the privacy and security of our sensitive information. The program also includes a suite of best-in-breed security technologies and tools to implement and automate security protections for our networks, employees, and customers. Our cybersecurity team reports to the Audit Committee quarterly on information security and cybersecurity matters, or as needed. Our Audit Committee, which is comprised of several members from our Board of Directors, has oversight responsibility for our data security practices and we believe the committee has the requisite skills and visibility into the design and operation of our data security practices to fulfill this responsibility effectively.
Despite the implementation of our cybersecurity program, our security measures cannot guarantee that a significant cyberattack will not occur. A successful attack on our information technology systems could have significant consequences to the business. While we devote resources to our security measures to protect our systems and information, these measures cannot provide absolute security. See “Risk Factors – Risks Relating to Our Business” for additional information about the risks to our business associated with a breach or compromise to our information security systems.
AVAILABLE INFORMATION
The Cooper Companies, Inc. Internet address is http://www.coopercos.com. The information on the Company's website is not part of this or any other report we file with, or furnish to, the Securities and Exchange Commission (SEC). Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, along with all other reports and amendments filed with or furnished to the SEC, are publicly available free of charge on our website as soon as reasonably practicable. The SEC maintains a website that contains such reports, proxy and information statements and other information whose Internet address is http://www.sec.gov. The Company's Corporate Governance Principles, Code of Conduct and charters of each standing committee of the Board of Directors are also posted on the Company's website.
27
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 1A. Risk Factors.
Our business faces significant risks. These risks include those described below and may include additional risks and uncertainties not presently known to us or that we currently deem immaterial. Our business, financial condition and results of operations could be materially adversely affected by any of these risks, and the trading prices of our common stock could decline by virtue of these risks. These risks should be read in conjunction with the other information in this report.
Risks Relating to Our Business
Current market conditions and recessionary pressures in one or more of our markets could impact our ability to grow our business.
Over the last few years in the U.S. and globally, market and economic conditions have been challenging, particularly in light of the COVID-19 pandemic. Foreign countries, in particular the Euro zone, have experienced recessionary pressures and face continued concerns about the systemic impacts of adverse economic conditions and geopolitical issues. Any negative impact on economic conditions and international markets, continued volatility or deterioration in the debt and equity capital markets, inflation, deflation or other adverse economic conditions may adversely affect our liquidity and financial condition. It may limit our ability to replace maturing liabilities and to access the capital markets to meet liquidity needs, which could have a material adverse effect on our financial condition and results of operations.
Ongoing uncertain economic and financial market conditions may also adversely affect the financial condition of our customers, suppliers and other business partners. When our customers’ financial conditions are adversely affected, customers may reduce their purchases of our products or we may not be able to collect accounts receivable, each of which could have a material adverse impact on our business operations or financial results. Our global business has been negatively affected by local economic conditions, including inflation, increasing labor costs, recession, and currency exchange rate fluctuations, which has adversely affected our cost to manufacture and provide our products and services and revenues generated through sales of such products and services. There is no guarantee that we will be able to fully absorb any such additional costs or revenue declines in the prices for our products and services.
CooperVision and CooperSurgical are encountering consolidation in their customer bases and emergence of more centralized large customer groups and retail chains. Due to this trend, global and regional key account customers now represent a larger proportion or concentration of our business and any disruption to these relationships may have a material adverse impact on our business, financial condition and results of operations.
Inflation could materially adversely affect our business and operations.
Our operating results could be materially impacted by changes in the overall macroeconomic environment and other economic factors that impact our cost structure and revenue results. Changes in economic conditions, supply chain constraints, logistics challenges, labor shortages, the war in Ukraine, and steps taken by governments and central banks, particularly in response to the COVID-19 pandemic, as well as other stimulus and spending programs, have led to higher inflation, which is likely to lead to an increase in costs and may cause changes in fiscal and monetary policy, including increased interest rates. In a higher inflationary environment, we may be unable to raise the prices of our products and services sufficiently to keep up with the rate of inflation.
Our substantial and expanding international operations are subject to uncertainties which could affect our operating results.
A significant portion of our current operations are conducted and located outside the U.S., and our growth strategy involves expanding our existing foreign operations and entering into new foreign jurisdictions. We have significant manufacturing and distribution sites in North America, Latin America and Europe. More than half of our net sales for the fiscal years ended October 31, 2022 and 2021, were derived from the sale of products outside the United States. We believe that sales outside the United States will continue to account for a material portion of our total net sales for the foreseeable future. International operations and business expansion plans are subject to numerous additional risks, including the following:
•difficulty managing a large organization spread throughout various countries;
•fluctuations in currency exchange rates adversely affecting our results;
•difficulty managing the effects of the COVID-19 pandemic on our ability to operate internationally and for our employees to travel internationally;
•challenges associated with enforcing intellectual property rights in some foreign countries;
28
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
•difficulty gaining market share in countries such as China because of regulatory restrictions and customer preferences;
•difficulty growing our sales in emerging markets such as China, India, Russia, Brazil and other developing nations due to, among other things, customer acceptance, undeveloped and/or unfamiliar distribution channels, regulatory restrictions and changes, and business knowledge of these new markets;
•foreign earnings being subject to withholding requirements or the imposition of tariffs, exchange controls or other restrictions, including the tariffs enacted by the Chinese government on certain U.S. goods, the scope and duration of which remain uncertain;
•challenges in complying with a variety of international legal, compliance and regulatory requirements such as the Foreign Corrupt Practices Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the UK Bribery Act, international data security and privacy laws, EU MDR and EU IVDR;
•foreign customers creating longer payment cycles than customers in the U.S.;
•failure to comply with U.S. Department of Commerce and other nations' import-export controls may result in fines and/or penalties;
•general economic and political conditions in the countries where we operate having an adverse effect on our operations in those countries or being unfavorable to our growth strategy;
•natural disasters, pandemics, war, terrorism, labor disruptions and international conflicts may cause significant economic disruption and political and social instability, resulting in decreased demand for our products, adversely affecting our manufacturing and distribution capabilities, or causing interruptions in our supply chain;
•foreign governments adopting regulations, including those similar to the EU MDR and EU IVDR or take other actions that would have a direct or indirect adverse impact on our business and market opportunities, including but not limited to increased enforcement of potentially conflicting and ambiguous anti-bribery and privacy laws;
•challenges enforcing agreements and collecting receivables through some foreign legal systems; and
•unforeseen economic or political events in certain countries that may have an impact on our customers' ability or preferences to buy our products.
As we continue to expand our business globally, our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our international operations. However, any of these factors could adversely affect our international operations and, consequently, our operating results.
The war between Russia and Ukraine could adversely affect our business, financial condition and results of operations.
On February 24, 2022, Russian military forces launched a military action in Ukraine, and sustained conflict and disruption in the region is likely. The length, impact, and outcome of this ongoing military conflict is highly unpredictable and could lead to significant market and other disruptions, including significant volatility in commodity prices and supply of energy resources, instability in financial markets, supply chain interruptions, political and social instability, trade disputes or trade barriers, changes in consumer or purchaser preferences, as well as an increase in cyberattacks and espionage.
The war has led to significant sanctions programs imposed by the U.S., the European Union, the UK, Canada, Switzerland, Japan, and other countries against Russia, Belarus, the Crimea Region of Ukraine, the so-called Donetsk People’s Republic, and the so-called Luhansk People’s Republic, including, among others:
•blocking sanctions against some of the largest state-owned and private Russian financial institutions (and their subsequent removal from the Society for Worldwide Interbank Financial Telecommunication payment system) and certain Russian businesses, some of which have significant financial and trade ties to the European Union;
•blocking sanctions against Russian and Belarusian individuals, including the Russian President, other politicians, and those with government connections or involved in Russian military activities;
•blocking of Russia’s foreign currency reserves as well as expansion of sectoral sanctions and export and trade restrictions, limitations on investments and access to capital markets, and bans on various Russian imports; and
•enhanced export controls and trade sanctions targeting Russia’s imports of technological goods as a whole, including tighter controls on exports and reexports of dual-use items, stricter licensing policy with respect to issuing export licenses, and/or increased use of “end-use” controls to block or impose licensing requirements on exports, as well as higher import tariffs and a prohibition on exporting luxury goods to Russia and Belarus.
In retaliation against new international sanctions and as part of measures to stabilize and support the volatile Russian financial and currency markets, the Russian authorities also imposed significant currency control measures aimed at restricting the outflow of foreign currency and capital from Russia, imposed various restrictions on transacting with non-Russian parties, banned exports of various products, and imposed other economic and financial restrictions. The situation is rapidly evolving, and additional sanctions by Russia on the one hand, and by the other countries on the other hand, could adversely affect the global economy, financial markets, energy supply and prices, certain critical materials and metals, supply chains, and global logistics and could adversely affect our business, financial condition, and results of operations.
29
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Our business must be conducted in compliance with applicable economic and trade sanctions laws and regulations, including those administered and enforced by the U.S. Department of Treasury’s Office of Foreign Assets Control, the U.S. Department of State, the U.S. Department of Commerce, the United Nations Security Council, and other relevant governmental authorities. If we are found to be in violation of U.S. sanctions or export control laws, it could result in substantial fines and penalties for us and for individuals working for us. We are actively monitoring the situation in Ukraine and Russia and assessing its impact on our business, including our business partners, employees and customers. To date, we have not experienced any material interruptions in our infrastructure, supplies, technology systems, or networks needed to support our operations. The conflict has caused us to modify our operations in Russia and could lead to additional modifications in Russia. We cannot predict the progress or outcome of the war or its impacts in the territories where we operate. The extent and duration of the military action, sanctions, other consequences, such as Russia imposing restrictions on transactions or banning the export of energy products, including natural gas, and the resulting market disruptions could be significant and could potentially have substantial impact on the global economy and our business for an unknown period of time. Any such disruption may also magnify the impact of other risks described in this section.
Our results of operations have been adversely affected, and our results of operations, cash flow and financial condition could be materially adversely affected in the future, by the global COVID-19 pandemic and related economic disruptions.
The COVID-19 pandemic has negatively impacted business and healthcare activity globally and has created significant volatility, uncertainty and economic disruption within the markets in which we operate. The pandemic has adversely affected and is likely to further adversely affect nearly all aspects of our business and markets, including our sales, operations, cash flow and workforce and the operations of our customers, suppliers, vendors and business partners. Among other things, many optical practitioners and retailers, hospitals, medical offices and fertility clinics closed their facilities, restricted access, or delayed or canceled patient visits, exams and elective medical procedures in response to the pandemic, and many customers that have reopened are experiencing reduced patient visits, which has resulted in reduced demand for and sales of our products and services.
To the extent the COVID-19 pandemic persists, with surges in infection and associated government responses, our results of operations, cash flow and financial condition could be materially adversely affected in numerous ways, including, but not limited to, decreased net sales from sales of our products and services due to customer facility closures, restricted access and reduced patient visits, exams and elective medical procedures; disruption in the manufacture and distribution of our products, including increased manufacturing and distribution costs, reduced manufacturing capacity and inadequate inventory levels; increased risk of inventory that may expire; write-offs or obsolescence of inventory, equipment or other assets; disruptions to or increased costs from our raw material and product suppliers and broader supply chain and distribution systems; delays in our clinical trials which could negatively impact our new product pipeline milestones and regulatory clearances, approvals or certifications; extended delays in or defaults on payments of outstanding receivables; insolvency of customers, suppliers, vendors and business partners; an inability to access lending, capital markets and other sources of liquidity when needed on reasonable terms or at all; an inability to comply with financial covenants in our debt agreements; and future restructuring, impairment and other charges.
The extent to which the COVID-19 pandemic and related economic disruptions impact our business, results of operations, cash flow and financial condition will depend on future developments, which are highly uncertain, difficult to predict and largely outside of our control, including, but not limited to, the continued spread, duration and severity of the pandemic outbreak; the occurrence, spread, duration and severity of any subsequent wave or waves of outbreaks, including the emergence and spread of variants of the COVID-19 virus; the impact on our customers and suppliers; the actions taken by the U.S. and foreign governments to contain the pandemic, address its impact or respond to the reduction in global and local economic activity; the occurrence, duration and severity of a global, regional or national recession, depression or other sustained adverse market event; and how quickly and to what extent normal economic and operating conditions can resume. Even after the COVID-19 pandemic has subsided, we may continue to experience materially adverse effects on our results of operations and financial condition.
Acquisitions that we have made and may make in the future involve numerous risks.
We have a history of acquiring businesses and products that have significantly contributed to our growth in recent years. As part of our growth strategy, we intend to continue to consider acquiring complementary technologies, products and businesses. Future acquisitions could result in potentially dilutive issuances of equity securities, the incurrence of debt and contingent liabilities and an increase in amortization and/or impairments of goodwill and other intangible assets, which could have a material adverse effect upon our business, financial condition and results of operations. In fiscal 2022, CooperVision acquired a privately-held Denmark-based ortho-k contact lens distributor. In fiscal 2021, CooperVision acquired a privately held medical device company and a privately-held UK contact lenses manufacturer. In fiscal 2022, CooperSurgical acquired a private cryopreservation services company and Generate Life Sciences (Generate), a privately-held provider of donor egg and sperm for fertility treatments, fertility cryopreservation services and newborn stem cell storage (cord blood & cord tissue). In fiscal 2021, CooperSurgical acquired three privately-held medical device companies
30
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
and one privately-held IVF cryostorage software solutions company. Risks we could face with respect to these acquisitions include:
•failure to successfully obtain the anticipated revenues, margins and earnings benefits;
•difficulties in, and expenses related to, the integration of the operations, technologies, products and personnel of the acquired company and establishment of appropriate accounting controls and reporting procedures and other regulatory compliance procedures, including but not limited to third-party compliance and due diligence;
•increased leverage and the risk of lack of access to available financing, including financing for the acquisition or refinancing of debt owed by us on a timely basis and on reasonable terms;
•risks of entering markets in which we have no or limited prior experience;
•potential loss of employees;
•an inability to identify and consummate future acquisitions on favorable terms or at all;
•diversion of management's attention away from other business concerns;
•risks of the acquired company’s noncompliance with applicable laws or regulations;
•expenses of any undisclosed or potential liabilities, contingent liabilities or indemnification obligations of the acquired company;
•expenses, including restructuring expenses, to shut-down our own locations or terminate our employees;
•application of and compliance with new and unfamiliar regulatory frameworks such as regulation applicable to our newly acquired fertility-related businesses;
•Failure to successfully obtain or maintain reimbursements under the third-party payor plans, including but not limited to governmental programs, due to complex reporting and payment obligations;
•a dilution of earnings per share; and
•risks inherent in accounting allocations and the risk that we are required to record significant adjustments to the preliminary fair value of assets acquired and liabilities assumed within the measurement period.
We face risks associated with disruption of our manufacturing, distribution and storage operations, including possible failure to develop necessary manufacturing processes, or constrained, idle or excess capacity, which could adversely affect our profitability or competitive position.
We manufacture a significant portion of the medical device products we sell. Any prolonged disruption in the operations of our existing manufacturing or distribution facilities or our fertility and stem cell storage facilities, whether due to the effects of the COVID-19 pandemic and related work stoppages, technical or labor difficulties, integration difficulties, destruction of or damage to any facility (as a result of natural disaster, use and storage of hazardous materials or other events), enforcement action by the FDA or other regulatory body if we are found to be in non-compliance with current Good Manufacturing Practices (cGMP) or similar foreign requirements or other reasons, could have a material adverse effect on our business, financial condition and results of operations. In addition, materials such as silicone hydrogel require improvements to our manufacturing processes to make them cost effective. While we have improved our manufacturing capabilities for our silicone hydrogel products, our failure to continue to develop improvements to our manufacturing processes and reduce our cost of goods could significantly impact our ability to compete. Conversely, constrained, excess or idle capacity, which could result from acquisitions, unexpected demand, inaccurate sales forecasting or unexpected manufacturing efficiencies, could significantly impact our profitability, capital investments, customer service levels and near-term financial condition.
CooperVision manufactures molded contact lenses, which represent the majority of our contact lens revenues, primarily at our facilities in Costa Rica, Hungary, Puerto Rico, the UK and the U.S., with other smaller facilities also existing in multiple locations around the world. CooperSurgical manufactures the majority of its products in Costa Rica, the UK and the U.S., with other smaller locations also existing in multiple locations around the world. In November 2017, CooperSurgical purchased a manufacturing facility in Costa Rica to consolidate a portion of global manufacturing. We manufacture certain products at only one manufacturing site for certain markets, and certain of our products are approved for manufacturing only at one site. If there were any prolonged disruption in the operations of the approved facility, it could take a significant amount of time to obtain required regulatory approvals, validate a second site and replace lost product, which could result in lost customers and thereby reduce sales, profitability and market share.
CooperVision distributes products out of Belgium, Hungary, the UK and the U.S. and various smaller international distribution sites. CooperSurgical primarily distributes products out of its facilities in the U.S. and the Netherlands and
31
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
operates fertility and stem cell storage facilities in the U.S., Canada and Australia. Any prolonged disruption in the operations of our existing distribution or storage facilities, whether due to technical or labor difficulties, challenges related to system implementation, destruction of or damage to any facility (as a result of natural disaster, use and storage of hazardous materials or other events) or other reasons, could have a material adverse effect on our business, financial condition and results of operations.
Cybersecurity threats continue to increase in frequency and sophistication; a successful cybersecurity attack could interrupt or disrupt our information technology systems, or those of our third-party service providers, or cause the loss of confidential or protected data which could disrupt our business, force us to incur excessive costs or cause reputational harm.
Security breaches, computer malware and computer hacking attacks have become more prevalent across industries and may occur on our systems or those of our third-party service providers or partners. The size and complexity of our information systems make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from attacks by malicious third parties. Such attacks are increasing in their frequency, levels of persistence, levels of sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise, especially given increased vulnerability of corporate information technology systems as distributed work environments have become prevalent (including as a result of the COVID-19 pandemic). In addition to unauthorized access to or acquisition of personal data, confidential information, intellectual property or other sensitive information, such attacks could include the deployment of harmful malware and ransomware, and may use a variety of methods, including denial-of-service attacks, social engineering and other means, to attain such unauthorized access or acquisition or otherwise affect service reliability and threaten the confidentiality, integrity and availability of information. Like many other companies, we experience attempted cybersecurity actions on a frequent basis, and the frequency of such attempts could increase in the future. While we have invested in the protection of data and information technology, there can be no assurance that our efforts will prevent or quickly identify service interruptions or security breaches. The techniques used by cybercriminals change frequently, may not be recognized until launched and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations or hostile foreign governments or agencies. We cannot assure that our data protection efforts and our investment in information technology will prevent significant breakdowns, data leakages or breaches in our systems or those of our third-party services providers or partners. Any such interruption or breach of our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us. We maintain cyber liability insurance; however, this insurance may not be sufficient to cover the financial, legal, business or reputational losses that may result from an interruption or breach of our systems.
We manage our businesses utilizing complex integrated software and hardware information technology operating systems that are regularly maintained and upgraded; an interruption or disruption to these systems could disrupt our business or force us to incur excessive costs.
We utilize complex integrated software and hardware operating systems, including enterprise resource planning and warehouse management systems, to support our business units and we have a continuous improvement strategy in place to keep our systems and overarching technology stable and in line with business needs and growth. Regular upgrades of our computer hardware and software revisions are typical and expected. We employ controlled change management methodologies to plan, test and execute all such system upgrades and improvements, and we believe that we assign adequate staffing and other resources to projects to ensure successful implementation. However, we cannot assure that our systems will meet our future business needs or that upgrades will operate as designed. We cannot assure that there will not be associated excessive costs or disruptions in portions of our business in the course of our maintenance, support and/or upgrade of these systems.
We are in the midst of a multiyear process of implementing new enterprise resource planning (ERP) systems at CooperVision and CooperSurgical. Implementing a new ERP system is not only costly but complex and difficult. Implementing a new ERP system can negatively affect not only financial accounting and reporting processes but also external commercial activities such as order receipt and product delivery. There can be no assurance that we will successfully implement our new ERP system or that we will avoid these and other negative impacts from our implementation efforts.
Pricing pressure from our competitors, customers and changes in third-party coverage and reimbursement may adversely affect demand for our products and negatively impact our operating results.
Competition in our industry has increased as a result of new market entrants, new technologies and as more established companies have intensified competitive pricing pressure. As a result of these competitive forces, we believe there will continue to be pricing pressure in the future. Because our CooperSurgical products are generally purchased by hospitals and surgical centers, OB/GYN medical offices and fertility clinics, and billed to various third-party payors, changes in the
32
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
purchasing behavior of such customers or the amount such payors are willing to reimburse our customers for procedures using our products, including as a result of healthcare reform initiatives, could create additional pricing pressure on us. In addition to these competitive forces, we continue to see pricing pressure as our customers introduce new pricing structures into their contracts and agreements, including fixed price formulas, capitated pricing and structured pricing intended to contain healthcare costs. Such trends may adversely affect demand for our products and may drive down the prices we are able to charge for our products, both of which would negatively affect our operating results.
We rely on independent suppliers in our supply chain for raw materials, packaging materials and components, mechanical equipment and some finished goods; we could experience inventory shortages if any of these suppliers encounter a manufacturing or distribution disruption
Our businesses utilize various chemicals, packaging materials, components, parts and raw materials which are generally available from more than one source. However, in certain instances we acquire components and materials from sole or primary suppliers to make our silicone hydrogel contact lens, certain medical devices and IVF products. We also source mechanical equipment and in certain instances finished goods from OEM suppliers. Supply of these goods, items and materials is protected by contractual agreements, availability of alternative suppliers and/or safety stocks. However, if current suppliers fail to supply sufficient goods, items or materials to us on a timely basis, or at all for any reason, we could experience inventory shortages and disruption in our supply of products. For example, among other situations, some of the primary material used to make our silicone hydrogel contact lens products, including MyDay, Biofinity, Avaira and clariti, are supplied by few or sole suppliers, and the failure of a key or sole supplier to timely supply sufficient items and materials necessary for the manufacture of our silicone hydrogel contact lenses could in turn disrupt our supply of those lenses to the market, which would have a material adverse effect on our business, financial condition and results of operations.
Our supply chain and our cost of goods also may be negatively impacted by unanticipated price increases due to factors such as inflation, including wage inflation, or to supply restrictions beyond our control or the control of our suppliers.
If we fail to protect our intellectual property adequately, our business could suffer.
We consider our intellectual property rights, including patents, trade secrets, trademarks and licensing agreements, to be an integral component of our business. We attempt to protect our intellectual property rights through a combination of patent, trademark, copyright and trade secret laws, as well as licensing agreements and third-party nondisclosure and assignment agreements. Our failure to obtain or maintain adequate protection of our intellectual property rights for any reason could have a material adverse effect on our business, financial condition and results of operations.
We also may seek to enforce our intellectual property rights on others through litigation. Our claims, even if meritorious, may be found invalid or inapplicable to a party we believe infringes or has misappropriated our intellectual property rights. In addition, litigation can:
•be expensive and time consuming to prosecute or defend;
•result in a finding that we do not have certain intellectual property rights or that such rights lack sufficient scope or strength;
•divert management's attention and resources; or
•require us to license our intellectual property.
We have applied for patent protection in the U.S., the UK and other foreign jurisdictions relating to certain existing and proposed processes and products. We cannot assure that any of our patent applications will be approved. Patent applications in the U.S., the UK and other foreign jurisdictions are maintained in secrecy for a period of time, which may last until patents are issued, and since publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several months, we cannot be certain that we will be the first creator of inventions covered by any patent application we make or the first to file patent applications on such inventions. The patents we own could be challenged, invalidated or circumvented by others and may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage. We also cannot assure that we will have adequate resources to enforce our patents.
Both CooperVision and CooperSurgical also rely on proprietary technology which is unpatented. It is possible that others will independently develop the same or similar technology or otherwise obtain access to our unpatented technology. To protect our trade secrets and other proprietary information, we require employees, consultants, advisors and collaborators to enter into confidentiality agreements and assignment agreements, which generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, we cannot assure that these
33
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
confidentiality agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use, misappropriation or disclosure of such trade secrets, know-how or other proprietary information. Enforcing a claim that a party illegally obtained and is using our trade secrets is difficult, expensive and time consuming and the outcome is unpredictable.
We rely on trademarks to establish a market identity for our products. To maintain the value of our trademarks, we might have to file lawsuits against third parties to prevent them from using trademarks confusingly similar to or dilutive of our registered or unregistered trademarks. We also might not obtain registrations for our pending or future trademark applications and might have to defend our registered trademark and pending applications from challenge by third parties. Enforcing or defending our registered and unregistered trademarks might result in significant litigation costs and damages, including the inability to continue using certain trademarks.
The laws of foreign countries in which we do business or contemplate doing business in the future may not recognize intellectual property rights or protect them to the same extent as do the laws of the U.S. Adverse determinations in a judicial or administrative proceeding could prevent us from manufacturing and selling our products or prevent us from stopping others from manufacturing and selling competing products, and thereby have a material adverse effect on our business, financial condition and results of operations.
Our products or processes could be subject to claims of infringement of the intellectual property of others.
Our competitors in both the U.S. and foreign countries, some of which have substantially greater resources and have made substantial investments in competing technologies, as well as other third parties, may have applied for or obtained, or may in the future apply for and obtain, patents that will prevent, limit or otherwise interfere with our ability to make and sell our existing and planned products. In the contact lens industry, CooperVision, its competitors and other third parties hold patents covering contact lens designs, business methods, processes and materials. Claims that our products, business methods or processes infringe upon the proprietary rights of others often are not asserted until after commencement of commercial sales of products incorporating our technology.
Significant litigation regarding intellectual property rights exists in our industries. For example, CooperVision in the past faced significant patent litigation over its silicone hydrogel contact lens products. Third parties have made, and may make in the future, claims of infringement against us or our contract manufacturers in connection with the use of our technology. Any claims, even those without merit, could:
•be expensive and time consuming to defend;
•cause us to cease making, licensing or selling products that incorporate the challenged intellectual property;
•require us to redesign or re-engineer our products, if feasible;
•divert management's attention and resources; or
•require us to enter into royalty or licensing agreements in order to obtain the right to use a necessary product, component or process.
We cannot be certain of the outcome of any litigation. Any royalty or licensing agreement, if required, may not be available to us on acceptable terms or at all. Our failure to obtain the necessary licenses or other rights could prevent the sale, manufacture, or distribution of some of our products and, therefore, could have a material adverse effect on our business.
A successful claim of infringement against us or our contract manufacturers in connection with the use of our technology, in particular if we are unable to manufacture or sell any of our planned products in any major market, could adversely affect our business.
We could experience losses from product liability claims or legal claims relating to our service offerings, including such claims and other losses resulting from sales of counterfeit and other infringing products.
We face an inherent risk of exposure to product liability claims in the event that the use of our products results in personal injury. We also face the risk that defects in the design or manufacture of our products or sales of counterfeit or other infringing products might necessitate a product recall and other actions by manufacturers, distributors or retailers in order to safeguard the health of consumers and protect the integrity of the subject brand. Additionally, we face the inherent risk of exposure to legal claims, including negligence, relating to our provision of certain service offerings, including the accuracy and quality of our genetic testing, fertility cryopreservation, fertility donor gamete supply, and stem cell storage services. These risks may be heightened due to our direct-to-consumer marketing efforts for some of our products and services (e.g., stem cell storage and Paragard IUDs). Consumers may halt or delay purchases of a product or service that is
34
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
the subject of a claim or recall or has been counterfeited. We handle some risk with third-party carrier policies that are subject to deductibles and limitations. These insurance policies may become more expensive (or not be available) for new risks we may assume when we acquire new businesses. For example, professional liability insurance for our genomics, gamete and tissue storage businesses could add significant cost. There can be no assurance that we will not experience material losses due to product liability claims or recalls, legal claims relating to our service offerings, or a decline in sales resulting from sales of counterfeit or other infringing products, in the future.
If our products are not accepted by the market, we will not be able to sustain or expand our business.
Certain of our proposed products have not yet been clinically tested or commercially introduced, and some of our existing products are marketed and sold on the basis of potential future medical or therapeutic value (assuming technology advances), and we cannot be sure that any of them will achieve market acceptance or generate revenues or operating profits. The development of a market for our products may be influenced by many factors, some of which are out of our control, including:
•acceptance of our products by eye care and health care practitioners;
•the cost competitiveness of our products;
•consumer reluctance to try and use a new product;
•regulatory and legislative requirements;
•adequate coverage and reimbursement by third-party payors;
•lack of scientific advancements to validate the medical value of certain products, such as stored cord blood or cord tissue (or scientific advancements in other medical approaches that reduce or eliminate the value of such products); and
•the earlier release of competitive products, such as new silicone hydrogel products or contraceptive technologies, into the market by our competitors; and the emergence of newer and more competitive products.
We operate in the highly competitive health care industry and there can be no assurance that we will be able to compete successfully.
Each of our businesses operates within a highly competitive environment. In our soft contact lens business, CooperVision faces intense competition from competitors' products, in particular silicone hydrogel contact lenses, and may face increasing competition as other new products enter the market. Our largest competitors in the contact lens business, Johnson & Johnson Vision Care, Inc., Alcon Inc. and Bausch Health Companies Inc. may have substantially greater financial resources, larger research and development budgets, larger sales forces, greater market penetration and/or larger manufacturing volumes than CooperVision. They offer competitive products and differentiated materials, plus a variety of other eye care products including lens care products and ophthalmic pharmaceuticals, which may give them a competitive advantage in marketing their lenses. The market for contact lenses is intensely competitive and is characterized by declining sales volumes for older product lines and growing demand for silicone hydrogel-based products. Our ability to respond to these competitive pressures will depend on our ability to decrease our costs and maintain gross margins and operating results and to introduce new products successfully, on a timely basis in the Americas, EMEA and Asia Pacific, and to achieve manufacturing efficiencies and sufficient manufacturing capacity and capabilities for such products. Any significant decrease in our costs per lens will depend, in part, on our ability to increase sales volume and production capabilities and our ability to secure adequate supply of materials used in production at reasonable costs. Our failure to respond to competitive pressures in a timely manner could have a material adverse effect on our business, financial condition and results of operations.
To a lesser extent, CooperVision also competes with manufacturers of eyeglasses and providers of other forms of vision correction including ophthalmic surgery.
There can be no assurance that we will not encounter increased competition in the future, for example with increased product entries from Asia Pacific contact lens manufacturers, or that our competitors' newer contact lens products will not successfully erode CooperVision's contact lens business, which could have a material adverse effect on our business, financial condition and results of operations.
The contact lens industry also continues to evolve with respect to the introduction of new distribution and fulfillment models and service technologies which may conflict with CooperVision’s strategy or interfere with its customers’ relationships and loyalty. For example, more contact lenses are being fulfilled directly to the consumer by manufacturers and wholesalers via online platforms, telemedicine is gaining popularity and more vision correction prescriptions are being provided through online refractive exams rather than in office by an eye care practitioner. CooperVision’s failure to adapt
35
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
to the threats posed by these new and emerging distribution models and Internet driven services may have a material adverse impact on our business, financial condition and results of operations.
CooperSurgical focuses on selected segments of the family and women's health care market with a diversified portfolio of products and services including medical devices in outpatient and operating room settings, fertility, contraception and healthcare technology services. Competitive factors in these segments in which CooperSurgical competes include technological and scientific advances, product quality and availability, price, customer service including response time and effective communication of product information to physicians, consumers, fertility clinics and hospitals. Competition in the medical device industry is dynamic and involves the search for technological and therapeutic innovations.
CooperSurgical competes with a number of manufacturers and service providers in its women’s family health care market areas. Some of these competitors have substantially greater financial and personnel resources and sell a broader range of products, which may give them an advantage in marketing competitive products. In addition, some of CooperSurgical’s markets, such as genomics, contraception and cord blood and cord tissue storage, are characterized by rapid technological advancement. We face the risk that demand for our products will not grow or will decline if our competitors are more successful than us at innovating in these and other areas. There is also risk that emerging technologies or technology advancements could reduce the medical value of certain of our products and services, such as cord blood and cord tissue storage, which could adversely affect our business. In recent years, CooperSurgical has also expanded direct-to-consumer products and services, which requires implementing new competitive strategies and increases the importance of customer service and consumer reputation as competitive factors.
New medical and technological developments may reduce the need for our products.
Technological developments in the eye care, family and women's health care, and diagnostics testing industries, such as new surgical procedures or medical devices, and genetic testing technology may limit demand for our products and services. For example, corneal refractive surgical procedures such as Lasik surgery and the development of new pharmaceutical products may decrease the demand for our optical products. If these new advances provide a practical alternative to traditional vision correction, the demand for contact lenses and eyeglasses may materially decrease. We cannot assure that medical advances and technological developments will not have a material adverse effect on our businesses.
Product innovations are important in the industry in which we operate, and we face the risk of product obsolescence if we are unable to develop new products or gain regulatory approvals or certifications or if our competitors introduce new products.
Product innovations are important in the contact lens market in which CooperVision competes and in the areas of the health care industry in which CooperSurgical competes. CooperVision, both internally and externally with third parties, invests in new product development, including the development of silicone hydrogel-based contact lenses. While much of CooperVision’s research and development activities are performed internally, it also uses external research and development investment in collaborations and joint development with third parties. CooperSurgical has historically purchased, leveraged or licensed the technology developments of others. CooperSurgical also has invested in expanding the internal research and development function with the goal of organic growth and to complement our acquisitions strategy. Research and development time commitments, higher feasibility risk with longer term projects, greater dependence on, and reduced control over, third-party deliverables, the cost of obtaining necessary regulatory approval or certification and other costs related to product innovations can be substantial. There can be no assurance that we will successfully obtain necessary regulatory approvals, certifications or clearances for our new products or that our new products will successfully compete in the marketplace and, as a result, justify the expense involved in their development and regulatory approval or certification. In addition, our competitors may have developed or may in the future develop new products or technologies. With respect to our CooperVision products, these developments could include contact lenses with anti-microbial or anti-allergenic features, or “smart” contact lenses that incorporate electronics, which could lead to the obsolescence of one or more of our products. Competitors may also introduce new uses for contact lenses, such as for drug delivery or the control of myopia. With respect to CooperSurgical products and services, novel contraceptive devices or methods, genetic testing methods and disease treatments could reduce or eliminate demand for certain of our products and services. Failure to develop new product offerings and technological changes and to offer products that provide performance that is at least comparable to competing products could have a material adverse effect on our business, financial condition and results of operations.
36
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
We face risks related to environmental matters.
Our facilities are subject to a broad range of U.S federal, state, local and foreign environmental laws and requirements, including those governing discharges to the air and water, the handling or disposal of solid and hazardous substances and wastes, remediation of contamination associated with the release of hazardous substances at our facilities and offsite disposal locations and occupational safety and health. We have made, and will continue to make, expenditures to comply with such laws and requirements. Future events, such as changes in existing laws and regulations, or the enforcement thereof, or the discovery of contamination at our facilities, may give rise to additional compliance or remediation costs that could have a material adverse effect on our business, financial condition and results of operations. Such laws and requirements are constantly changing, are different in every jurisdiction and can impose substantial fines and sanctions for violations. As a manufacturer of various products, we are exposed to some risk of claims with respect to environmental matters, and there can be no assurance that material costs or liabilities will not be incurred in connection with any such claims.
We continue to evaluate the necessary steps for compliance with regulations as they are enacted. These regulations include, for example, regulations enacted in the European Union such as the Registration, Evaluation, Authorization and Restriction of Chemical Substances, which requires the registration of and regulates use of certain chemicals, the Restriction on the Use of Certain Hazardous Substances in Electrical and Electronic Equipment Directive, which regulates the use of certain hazardous substances in certain products our CooperSurgical division manufactures. These and similar legislation that has been or is in the process of being enacted in Japan, China and various states of the U.S. may require us to re-design certain products to ensure compliance with the applicable laws and regulations.
Environmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. If our ESG practices fail to meet regulatory requirements or investor, customer, consumer, employee or other stakeholders' evolving expectations and standards for responsible corporate citizenship in areas including environmental stewardship, support for local communities, Board of Director and employee diversity, human capital management, employee health and safety practices, product quality, supply chain management, corporate governance and transparency, our reputation, brand and employee retention may be negatively impacted, and our customers and suppliers may be unwilling to continue to do business with us.
Customers, consumers, investors and other stakeholders are increasingly focusing on environmental issues, including climate change, energy and water use, plastic waste and other sustainability concerns. Concern over climate change or plastics and packaging materials, in particular, may result in new or increased legal and regulatory requirements to reduce or mitigate impacts to the environment. Changing customer and consumer preferences or increased regulatory requirements may result in increased demands or requirements regarding plastics and packaging materials, including single-use and non-recyclable plastic products and packaging, other components of our products and their environmental impact on sustainability, or increased customer and consumer concerns or perceptions (whether accurate or inaccurate) regarding the effects of substances present in certain of our products. Complying with these demands or requirements could cause us to incur additional manufacturing, operating or product development costs.
If we do not adapt to or comply with new regulations, or fail to meet evolving investor, industry or stakeholder expectations and concerns regarding ESG issues, investors may reconsider their capital investment in our Company, and customers and consumers may choose to stop purchasing our products, which could have a material adverse effect on our reputation, business or financial condition.
If we do not retain our key personnel and attract and retain other highly skilled employees, our business could suffer.
If we fail to recruit, develop and retain the necessary personnel, our business and our ability to obtain new customers, develop new products and provide acceptable levels of customer service could suffer. The success of our business is heavily dependent on the leadership of our key management personnel. Our success also depends on our ability to recruit, develop and retain and motivate highly skilled sales, marketing, manufacturing engineering and scientific personnel. Competition for these persons in our industry is intense, and we may not be able to successfully recruit, train or retain qualified personnel. We are experiencing increasing challenges in building and retaining our workforce in certain markets, where pressure from inflation and competition have exacerbated turnover and retention trends continuing from the COVID-19 pandemic. Labor shortages and competition for qualified personnel could cause disruptions in our business operations.
37
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Provisions of our governing documents and Delaware law may have anti-takeover effects.
Certain provisions of our Second Restated Certificate of Incorporation and Amended and Restated By-Laws may inhibit changes in control of the Company not approved by our Board of Directors. These provisions include advance notice requirements for stockholder proposals and nominations. We also have the protections of Section 203 of the Delaware General Corporation Law, which could have anti-takeover effects.
Risks Relating to Government Regulation of Manufacture and Sale of Our Products and Services.
The costs of complying with the requirements of federal, state and foreign laws pertaining to the privacy and security of personal information, including health related information and the potential liability associated with failure to do so could materially adversely affect our business and results of operations.
Numerous laws and regulations govern the collection, dissemination, use, privacy, confidentiality, security, availability and integrity of individually identifiable information, including protected health information (PHI). We collect and process individually identifiable information in multiple ways in our various business lines and are subject to risk associated with compliance with many of these laws and regulations. Some of our businesses expose us to increasingly stringent regulations for handling personal information (where, for example, we collect or process personal data deemed to be sensitive by regulatory authorities, such as PHI).
Under U.S. law, HIPAA establishes national privacy and security standards for protection of PHI by covered entities, such as our genetics testing subsidiaries, and the business associates with whom such entities contract for services, including another one of our subsidiaries, Eye Care Prime LLC, which offers value-added software solutions for eye care professionals. HIPAA requires both covered entities and business associates to develop and maintain policies and procedures for PHI that is used or disclosed, and to adopt administrative, physical and technical safeguards to protect PHI. When we are acting as a business associate, our clients that are covered entities are mandated by HIPAA to enter into written agreements with us - known as business associate agreements - that require us to safeguard PHI in accordance with HIPAA. Our genetics testing subsidiaries are likewise required to enter into business associate agreements with any of their business associates.
Mandatory penalties for HIPAA violations can be significant. A single breach incident can result in violations of multiple standards. If a person knowingly or intentionally obtains or discloses PHI in violation of HIPAA requirements, criminal penalties may also be imposed.
We maintain safeguards that we believe are reasonable and appropriate to protect the privacy and security of PHI and other personally identifiable information consistent with applicable law and our contractual obligations; however, our systems may be vulnerable to physical break-ins, viruses, hackers, and other potential sources of security breaches. In addition, we may not be able to prevent incidences of inappropriate use or unauthorized access to PHI by our employees or contractors, despite the safeguards. Any such breaches could result in exposure to liability under federal and state laws and/or under our contractual arrangements and could adversely impact our business.
Even when HIPAA does not apply, according to the FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
Further, California enacted the CCPA, which went into effect on January 1, 2020. The CCPA gives California residents certain rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Although there are limited exemptions for health-related information, including PHI and clinical trial data, the CCPA may increase our compliance costs and potential liability. Further, the CPRA, which will go into effect on January 1, 2023, significantly amends the CCPA and will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk processing, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. Similar laws have been proposed or enacted in other states and at the federal level, and when passed, such laws may have potentially conflicting requirements that would make compliance challenging.
We are also subject to laws and regulations in countries other than U.S. covering data privacy and the protection of health-related and other personal information. EU and EEA member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the GDPR went into effect on May
38
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
25, 2018, and imposes stringent operational requirements for processors and controllers of personal data in the context of an establishment in the EEA or the processing of personal data of individuals within the EEA, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information, increased requirements pertaining to health data and pseudonymized (e.g. key-coded) data, mandatory data breach notification requirements, handling data subject access requests and higher standards for data controllers to demonstrate that they have obtained valid consent for certain data processing activities. In addition, countries of the EEA may impose further obligations relating to the processing of genetic, biometric or health data, which could further add to our compliance costs and limit how we process this information. Some of the personal data we process in respect of clinical trial participants is special category or sensitive personal data under the GDPR, and subject to additional compliance obligations and to local law derogations. We may be subject to diverging requirements under EU member state laws and UK law, such as whether consent can be used as the legal basis for processing in clinical trials and the roles, responsibilities and liabilities as between contract research organizations and sponsors. As these laws develop, we may need to make operational changes to adapt to these diverging rules, which could increase our costs and adversely affect our business.
In addition, the GDPR increases the scrutiny of transfers of personal data from the EEA, including from clinical trial sites located in the EEA, to the U.S. and other jurisdictions that the European Commission does not recognize as having “adequate” data protection laws. In July 2020, the Court of Justice of the European Union issued its judgment in the Schrems II case and limited how organizations could lawfully transfer personal data from the EEA to the U.S. by invalidating the EU-US Privacy Shield and imposing further restrictions on use of the standard contractual clauses, which could increase our costs and our ability to efficiently process personal data from the EEA. Indeed, while the CJEU upheld the adequacy of the standard contractual clauses (a standard form of contract approved by the European Commission as an adequate personal data transfer mechanism, and potential alternative to the Privacy Shield), it made clear that reliance on them alone may not necessarily be sufficient in all circumstances. Use of the standard contractual clauses must now be assessed on a case-by-case basis taking into account the legal regime applicable in the destination country, in particular applicable surveillance laws and rights of individuals and additional measures and/or contractual provisions may need to be put in place. Subsequent European court and regulator decisions have taken a restrictive approach to international data transfers. On June 4, 2021, the European Commission adopted the new standard contractual clauses for the transfer of personal data to third countries (New SCCs). The New SCCs combine general clauses applicable in all cases with four modules that are adapted to different transfer scenarios. While the New SCCs better address the Schrems II requirements and the modern digital economy, with its diverse processing realities and often complex processing chains with multiple parties and sometimes changing roles, they are generally more complicated to prepare and adopt. On June 18, 2021, the European Data Protection Board issued Recommendations 01/2020 on measures that supplement transfer tools to ensure compliance with the EU level of protection of personal data.
The European Commission has adopted an adequacy decision in favor of the UK, enabling data transfers from EU member states to the UK without additional safeguards. However, the UK adequacy decision will automatically expire in June 2025 unless the European Commission re-assesses and renews/extends that decision, and remains under review by the Commission during this period. The relationship between the UK and the EU in relation to certain aspects of data protection law remains unclear, and it is unclear how UK data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the UK will be regulated in the long term, which exposes us to further compliance risk.
Compliance with U.S. and foreign privacy and security laws, rules and regulations could require us to take on more onerous obligations in our contracts, require us to engage in costly compliance exercises, restrict our ability to collect, use and disclose data, or in some cases, impact our or our partners’ or suppliers’ ability to operate in certain jurisdictions. Each of these constantly evolving laws can be subject to varying interpretations. Any failure or perceived failure by us to comply with privacy or security laws, policies, legal obligations or industry standards or any security incident that results in the unauthorized release or transfer of personally identifiable information may result in governmental enforcement actions and investigations including by European Data Protection Supervisory Authorities, fines and penalties, litigation and/or adverse publicity, including by consumer advocacy groups, and could cause our customers to lose trust in us, which could have an adverse effect on our reputation and business. For example, companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant undertaking, whichever is greater. Additionally, following the UK’s withdrawal from the EEA and the EU, and the expiry of the transition period, companies will have to comply with the GDPR and the GDPR as incorporated into the UK national law (the UK GDPR), the latter regime having the ability to separately fine up to the greater of £17.5 million or 4% of annual global turnover. In addition to fines, a breach of the GDPR and/or UK GDPR may result in regulatory investigations, reputational damage, orders to cease or change our data processing activities, enforcement notices, assessment notices for a compulsory audit and/or civil claims (including class actions). Such failures could have a material adverse effect on our financial condition and operations. If the third parties we work with violate applicable laws, contractual obligations or suffer a security breach, such violations may also put us in breach of our obligations under privacy laws and regulations and could in turn have a material adverse effect on our business.
When we acquire companies or business that engage personal data processing, we may become subject to additional regulation or scrutiny, particularly if such activity is different in nature from what we have done in the past. For example,
39
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
with the recent addition of cord blood and cord tissue storage (and other cryostorage) businesses, we interact directly with our customers and collect and maintain personal information regarding our customers and donors. Acquisitions like this could subject us to additional regulatory and consumer liability risk and the cost of analyzing and integrating new privacy compliance programs.
Changes in legislation and government regulation of the health care industry both in the United States and internationally, as well as third-party payors' efforts to control the costs of health care could materially adversely affect our business.
The ACA made extensive changes to the delivery of health care in the U.S. Among the provisions of the ACA, of greatest importance to the medical device industry and pharmaceutical industry are the following:
•Establishment of the Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
•Payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain health care services through bundled payment models;
•Establishment of a Center for Medicare & Medicaid Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending; and
•An increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively.
These measures could result in decreased net revenues or increased expenses from our fertility, office and surgical products and decrease potential returns from our development efforts.
Other legislative changes have been proposed and adopted since the ACA was enacted. The Budget Control Act of 2011, among other things, included aggregate reductions to Medicare payments to providers. In addition, the Medicare Access and CHIP Reauthorization Act of 2015, among other things, repealed the formula by which Medicare made annual payment adjustments to physicians and replaced the former formula with fixed annual updates and a new system of incentive payments began in 2019 that are based on various performance measures and physicians’ participation in alternative payment models such as accountable care organizations.
Other legislative changes have been proposed and adopted in the U.S. since the ACA was enacted. For example, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price (AMP), beginning January 1, 2024. Most recently, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. For that and other reasons, it is currently unclear how the IRA will be effectuated and the impact of the IRA on the pharmaceutical industry cannot yet be fully determined.
In foreign countries where we market our products, recent healthcare reform has taken place as well. For instance, in December 2021, the EU Regulation No 2021/2282 on Health Technology Assessment (HTA) amending Directive 2011/24/EU was adopted. This regulation, which became effective in January 2022, intends to boost cooperation among EU member states in assessing health technologies, including some medical devices, and providing the basis for cooperation at the EU level for joint clinical assessments in these areas. The regulation foresees a three-year transitional period and will permit EU member states to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU member states will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement.
We expect that additional state, federal and foreign health care reform measures will be adopted in the future, including those initiatives affecting coverage and reimbursement for our products, any of which could limit the amounts that federal state and foreign governments will pay for health care products and services, which could adversely affect the growth of the market for our products or demand for our products, or result in additional pricing pressures. Also, any adoption of
40
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
health care reform proposals on a state-by-state basis could require us to develop state-specific marketing and sales approaches. We cannot predict the effect such reforms or the prospect of their enactment may have on our business.
In addition, third-party payors, whether governmental or commercial, whether inside the U.S. or abroad, increasingly attempt to contain or reduce the costs of health care. These cost-control methods include prospective payment systems, capitated rates, group purchasing, redesign of benefits, requiring pre-authorizations or second opinions prior to certain medical procedures, encouragement of healthier lifestyles and exploration of more cost-effective methods of delivering health care. Although cost controls or other requirements imposed by third-party payors have not historically had a significant effect on contact lens prices or distribution practices, this could change in the future and could adversely affect our business, financial condition and results of operations.
We may enroll as in-network providers and suppliers with certain payors. Although, becoming an in-network provider or enrolling as a supplier means that we have agreed with these payors to provide certain of our tests at negotiated rates, it does not obligate any physicians to order our tests or guarantee that we will receive reimbursement for our tests from these or any other payors at adequate levels. Thus, these payor relationships, or any similar relationships we may establish in the future, may not result in acceptable levels of reimbursement for our tests or meaningful increases in our physician customer base. We cannot predict whether, under what circumstances, or at what payment levels payors will cover and reimburse for our tests. If we fail to establish and maintain broad coverage and reimbursement for our tests, our ability to generate increased revenue and grow our test volume and customer base could be limited and our future prospects and our business could suffer.
Laws pertaining to health care fraud and abuse could materially adversely affect our business, financial condition and results of operations.
We may be subject to various federal, state and foreign laws pertaining to health care fraud and abuse, including anti-kickback, physician self-referral false claims and physician payment transparency laws and regulations. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including our commercial laboratory operations and how we research, market, sell and distribute any products for which we obtain marketing approval. Such laws include:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or providing any remuneration (including any kickback, bribe or certain rebates), directly or indirectly, overtly or covertly, in cash or in kind, in return for, either the referral of an individual or the purchase, lease, or order, or arranging for or recommending the purchase, lease, or order of any good, facility, item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation;
•the federal physician self-referral prohibitions, commonly known as the Stark Law, which generally prohibit entities from billing a patient or the Medicare or Medicaid programs for certain designated health services, including clinical laboratory services, when the physician ordering the service, or any member of such physician’s immediate family, has a financial interest, such as an ownership or investment interest in or compensation arrangement with us, unless the arrangement meets an exception to the prohibition. These prohibitions apply regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral;
•the federal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval that are false or fraudulent, or knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or knowingly making or causing to be made a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute or Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
•the federal Health Insurance Portability and Accountability Act of 1996, which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
41
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
•the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the CMS, information related to payments and other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by such healthcare professionals and their immediate family members. Beginning in 2022, applicable manufacturers also will be required to report such information regarding payments and transfers of value provided during the previous year to physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists and certified nurse-midwives; and
•analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers and self-pay patients; some state laws that require biotechnology companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug and device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; some state laws that require biotechnology companies to report information on the pricing of certain drug products; and some state and local laws that require the registration of sales representatives.
In addition, federal government price reporting laws, among other things, require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our marketed drugs. Participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs and potentially limit our ability to offer certain marketplace discounts.
Violations of these laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state health care programs, including Medicare, Medicaid, Veterans Administration health programs and TRICARE. Similarly, if the physicians or other providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could indirectly have a negative impact on our business, financial condition and results of operations. Because of the complex and far-reaching nature of these laws, there can be no assurance that we would not be required to alter one or more of our practices to be in compliance with these laws. Any violations of these laws or regulations could result in a material adverse effect on our business, financial condition and results of operations. In addition, changes in these laws, regulations, or administrative or judicial interpretations, may require us to further change our business practices or subject our existing business practices to legal challenges, which could have a material adverse effect on our business, financial condition and results of operations.
Legislative or regulatory reforms in the United States or Europe may make it more difficult and costly for us to obtain regulatory clearances, approvals or certifications for our products or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. For example, over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain clearance or approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
In addition to traditional regulatory controls on medical devices, our business could be affected by emerging laws or regulations limiting our ability offer certain of our products and services. For example, in the U.S., the reversal by the U.S. Supreme Court of Roe v. Wade has raised concerns in the fertility industry that more restrictive laws could limit access to various reproductive services. New and emerging laws may be interpreted to limit access to contraceptive technologies or cryostorage services, which could adversely affect certain aspects of CooperSurgical’s business.
42
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
In addition, the EU landscape concerning medical devices (including IVDs) has recently evolved. A new set of two EU regulations have been adopted on April 5, 2017. On May 26, 2021, the EU MDR became applicable and replaced previous directives. As to the EU IVDR, it became applicable on May 26, 2022. However, on October 14, 2021, the European Commission proposed a “progressive” roll-out of the EU IVDR to prevent disruption in the supply of IVDs. The European Parliament and Council adopted the proposed regulation on December 15, 2021. The EU IVDR fully applies since May 26, 2022 but there will be a tiered system extending the grace period for many devices (depending on their risk classification) before they have to be fully compliant with the regulation. Both regulations have been adopted to establish a uniform, transparent, predictable and sustainable regulatory framework across the EU for medical devices (including IVDs) and ensure a high level of safety and health while supporting innovation.
These modifications may have an effect on the way we intend to develop our business in the EU and EEA. For example, as a result of the transition towards the new regimes, notified body review times have lengthened, and product introductions could be delayed or canceled. Additionally, only a few notified bodies have been designated for EU IVDR certification, which could adversely affect our ability to grow our business.
Following the end of the “Brexit” transitional period, from January 1, 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) became the UK’s independent regulatory agency for medical devices. Post-Brexit, amendments have been made to the existing UK medical devices legislation which require medical devices to be registered with the MHRA before being placed on the Great Britain market. Manufacturers based outside of the UK need to appoint a UK Responsible Person to register devices with the MHRA. Following a government consultation on changes to the UK’s medical device regulations, the response to which was published on June 26, 2022, it is anticipated that amendments to the legislation will soon be published by the government and should become applicable by July 1, 2023 so that medical devices placed on the market in Great Britain (England, Scotland, and Wales) will require a UK Conformity Assessment (UKCA) mark. However, within the government’s consultation response, it has proposed that transition periods will be included in the revised legislation so that products with existing and valid conformity assessments (CE mark or UKCA mark) could continue to be placed on the Great Britain market for a maximum of 3-5 years after July 1, 2023, depending on which legislation the medical device has been certified under. IVDs with valid certification can continue to be placed on the market until the earlier of certificate expiry or for a period of five years following publication of the new regulations. However, UKCA marking alone will not be recognized in the EU. The rules for placing medical devices on the Northern Ireland market will differ from those in Great Britain. These modifications may have an effect on the way we intend to conduct our business in these countries.
Our medical device products are subject to reporting requirements and recalls, even after receiving regulatory clearance, approval or certification, which could harm our reputation, business and financial results.
After a device is placed on the market, numerous regulatory requirements apply, including the FDA's QSR regulations, which require manufacturers to follow, among other things, design, testing, production, control, documentation and other quality assurance procedures during the manufacturing process; labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and medical device reporting regulations that require us to report to FDA or similar governmental bodies in other countries if our products may have caused or contributed to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar governmental bodies in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Medical device manufacturers, such as CooperVision and CooperSurgical, may, under their own initiative, recall a product if a reasonable possibility of serious injury or any material deficiency in a device is found, or withdraw a product to improve device performance or for other reasons. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. Recalls of any of our products may divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. A recall could harm our reputation with customers and consumers which could reduce the sales of our products. In addition, the FDA or other foreign governmental agencies may implement enforcement actions in connection with a recall which could impair our product offerings and be harmful to our business and financial results.
If our manufacturing operations fail to comply with applicable regulations, our manufacturing could be delayed or disrupted, our products could be subject to recall, and sales and profitability could suffer.
Our manufacturing operations and processes are required to comply with numerous federal, state and foreign regulatory requirements, including the FDA's cGMP for medical devices, known as the QSR regulations, which govern the procedures related to the design, testing, production processes, controls, quality assurance, labeling, packaging, storage, importing, exporting and shipping of our products. We also are subject to state requirements and licenses applicable to manufacturers of medical devices. In addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facilities and records for periodic unscheduled inspections by governmental agencies, including the FDA, state authorities and comparable agencies (as well as audits by notified bodies) in other countries.
43
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Failure to comply with QSR requirements and other applicable domestic or international regulatory requirements or to respond to any adverse inspectional observations or product safety issues could result in disruption of our operations and manufacturing delays in addition to, among other things, warning letters, significant fines, injunctions, suspension of approvals, seizures, recalls or import holds of products, operating restrictions and criminal prosecutions. As a result, any failure to comply with applicable requirements could adversely affect our product sales and profitability.
Our failure to comply with regulatory requirements or to receive regulatory clearance, approval or certification for our products or operations could adversely affect our business.
Our products and operations are subject to rigorous regulation by the FDA, and numerous other federal, state and foreign governmental authorities. In the U.S., the FDA regulates virtually all aspects of medical device and pharmaceutical design, development, testing, manufacture, safety, labeling (including, for example, unique device identifier regulations), storage, recordkeeping, reporting, marketing, promotion, advertising and distribution, as well as product import and export. Our failure to comply with FDA regulations could lead to the imposition of administrative or judicial sanctions, including injunctions, fines, warning letters, suspensions or the loss of regulatory approvals, product recalls, termination of distribution or product seizures. In the most egregious cases, criminal sanctions or closure of our manufacturing facilities are possible.
Our medical devices and pharmaceutical products require clearance or approval by the FDA before they can be commercially distributed in the U.S. and may require similar approvals by foreign regulatory agencies before distribution in foreign jurisdictions. Medical devices and drug products may only be marketed for the indications for which they are approved or cleared. The process of obtaining, renewing and maintaining regulatory clearances and approvals to market a medical device, particularly from the FDA, can be costly and time consuming. There can be no assurance that such clearances and approvals will be granted on a timely basis, if at all, and significant delays in the introduction of any new products or product enhancements may occur, which could adversely affect our competitive position and results of operations. In addition, the FDA and authorities in foreign jurisdictions may change their policies, adopt additional regulations or revise existing regulations, each of which could prevent or delay premarket approval or clearance of our products, increase the cost of compliance, impose additional regulatory requirements on us, or otherwise impact our ability to market our currently approved or cleared products.
Modifications and enhancements to medical devices also require a new FDA clearance or approval if they could significantly affect its safety or effectiveness or would constitute a major change in its intended use, design or manufacture. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer's decision. We have made modifications and enhancements to our medical devices that we do not believe require a new clearance or application, but we cannot confirm that the FDA will agree with our decisions. If the FDA requires us to seek clearance or approval for a modification of a previously cleared product for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties, which could have a material adverse effect on our financial results and competitive position. We also cannot assure that we will be successful in obtaining clearances or approvals for our modifications, if required.
Our efforts to promote some of our products and services via direct-to-consumer marketing initiatives may subject us to additional scrutiny by the FDA, FTC or other agencies. For example, we promote Paragard and cord blood and cord tissue storage directly to end consumers. Regulatory agencies may scrutinize our practices with respect to effective communication of risk information, benefits or claims with respect to such products.
Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products and product candidates. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our Company and our operating results may be adversely affected.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may be subject to enforcement action and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations. We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the U.S. or abroad.
Subject to transitional provisions and in order to sell our products in the EU, our products must respectively comply with general safety and performance requirements of the EU MDR and the EU IVDR. Compliance with these requirements is a prerequisite to be able to affix the European Conformity (CE) mark to our products, without which they cannot be sold or marketed in the EU. All medical devices placed on the market in the EU must meet the general safety and performance
44
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
requirements laid down in the Annexes to the EU MDR and EU IVDR including that a medical device must be designed and manufactured in such a way that, during normal conditions of use, it is suitable for its intended purpose. Medical devices must be safe and effective and must not compromise the clinical condition or safety of patients, or the safety and health of users and, where applicable, other persons, provided that any risks which may be associated with their use constitute acceptable risks when weighed against the benefits to the patient and are compatible with a high level of protection of health and safety, taking into account the generally acknowledged state of the art. To demonstrate compliance with the general safety and performance requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risk) classification. Except for low-risk medical devices (Class I) or general IVDs (Class A), where the manufacturer can self-assess the conformity of its products with the general safety and performance requirements (except for any parts which relate to sterility, metrology or reuse aspects of a medical device), a conformity assessment procedure requires the intervention of a notified body. Notified bodies are independent organizations designated by EU member states to assess the conformity of devices before being placed on the market. A notified body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality system (notified body must presume that quality systems which implement the relevant harmonized standards—ISO 13485:2016 for Quality Management Systems—conform to these requirements). If satisfied that the relevant product conforms to the general safety and performance requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE mark to the device, which allows the device to be placed on the market throughout the EU. If we fail to remain in compliance with applicable EU laws, directives or regulations, we would be unable to continue to affix the CE mark to our products, which would prevent us from selling them within the EU and EEA.
The EU regulatory landscape concerning medical devices (including IVDs) has recently evolved and the new requirements may have a significant effect on the way we conduct our business in the EU and the EEA. Following Brexit, the UK regulatory landscape concerning medical devices (including IVDs) is evolving and may have a significant effect on the way we conduct our business in the UK. See Risk Factors – “Legislative or regulatory reforms in the United States or Europe may make it more difficult and costly for us to obtain regulatory clearances, approvals or certifications for our products or to manufacture, market or distribute our products after clearance or approval is obtained”.
Development and marketing of our products are subject to strict governmental regulation by foreign regulatory agencies, and failure to receive, or delay in receiving, foreign qualifications or certifications could have a material adverse effect on our business.
In many of the foreign countries in which we market our products, we are subject to regulations affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, the reporting of certain payments to health care practitioners in certain markets (for example, the French anti-gift legislation), duties and tax requirements. Many of the regulations applicable to our devices and products in such countries are similar to those of the FDA.
In many countries, the national health or social security organizations require our products to be qualified before they can be marketed with the benefit of reimbursement eligibility. To date, we have not experienced difficulty in complying with these regulations. However, our failure to receive, or delays in the receipt of, relevant foreign qualifications could have a material adverse effect on our business, financial condition and results of operations.
Increased regulatory scrutiny of genetic testing may adversely affect our business through increased costs and risks associated with gaining marketing approvals or certifications and potential decreased demand for our genetic testing services.
We offer certain genetic testing services to help identify the likelihood of pregnancy as well as identify possible disorders or diseases of a child prior to birth. Regulatory and legislative proposals addressing oversight of genetic testing have been introduced in the U.S., and we expect that new proposals will be introduced from time to time both in the U.S. and in foreign countries in the future. Although the FDA has statutory authority to assure that medical devices, including IVDs, are safe and effective for their intended uses, the FDA has historically exercised its enforcement discretion and not enforced applicable provisions of the FDCA and regulations with respect to laboratory developed tests (LDTs). We believe our tests fall within the definition of an LDT. As a result, we believe our tests are not currently subject to the FDA’s enforcement of its medical device regulations and the applicable FDCA provisions. However, if there are changes in the FDA’s policy, or if the FDA disagrees that our marketed tests are LDTs or that we are marketing our tests outside the scope of the FDA’s current policy of enforcement discretion, we may become subject to extensive regulatory requirements and may be required to stop selling our existing tests or launching any other tests we may develop and to conduct additional clinical trials or take other actions prior to continuing to market our tests. This could significantly increase the costs and expenses of conducting, or otherwise harm, our business.
Legislative proposals addressing the FDA’s oversight of LDT have been introduced by Congress in the past and new legislative proposals may be introduced from time to time in the future. The likelihood that Congress will pass such legislation and the extent to which such legislation may affect the FDA’s ability to enforce its medical device regulations
45
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
with respect to certain LDT is difficult to predict at this time. If the FDA ultimately begins to enforce its medical device requirements with respect to LDT, our genetic tests may be subject to additional regulatory requirements imposed by the FDA, the nature and extent of which would depend upon applicable final guidance or regulation by the FDA or instruction by Congress. If the FDA imposes significant changes to the regulation of LDT it could reduce our revenue or increase our costs and adversely affect our business, prospects, results of operations or financial condition.
Any new FDA enforcement policies affecting LDT or new legislation, regulations such as the EU IVDR regulation may result in increased regulatory burdens on our ability to continue marketing our genetic products and to develop and introduce new products in the future, which could reduce our revenue or increase our costs and adversely affect our business, prospects, results of operations or financial condition.
In addition, changes in the way the EU regulates LDTs could result in additional expenses for offering our current and any future tests or possibly delay or suspend development or commercialization of such tests. In the EU, the regulatory landscape has recently evolved and the general safety and performance requirements set out in Annex I are also applicable to devices manufactured and used only within health institutions. The exemptions provided under the EU IVDR for LDTs remain to be further interpreted and clarified. If our tests do not qualify for an exemption, we may be subject to the full application of the EU IVDR with respect to some or all of our existing, as well as future, tests, and we would be required to expend additional time and resources to complying with the requirements of the EU IVDR.
If we fail to comply with applicable federal, state, local and foreign laboratory licensing requirements, we could lose the ability to perform our genetic tests or experience disruptions to our business.
We are subject to the Clinical Laboratory Improvement Amendments of 1988 (CLIA), UK Human Fertilization & Embryology Association (HFEA) regulating IVF, a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. Our clinical laboratories must be certified under CLIA and ISO 15189 in order for us to perform testing on human specimens. In addition, our proprietary tests must also be recognized as part of our accredited programs under CLIA so that we can offer them in our laboratory. CLIA is intended to ensure the quality and reliability of clinical laboratories in the U.S. by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. The law also requires us to maintain a state laboratory license to conduct testing in that state. Our laboratories are located in Japan, the UK and U.S., and we must maintain the requisite licenses in each jurisdiction.
Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our failure to renew a CLIA certificate, a state license or accreditation, could have a material and adverse effect on our diagnostic testing business, operating results and financial condition. The CMS also has the authority to impose a wide range of sanctions, including revocation of the CLIA certification along with a bar on the ownership or operation of a CLIA-certified laboratory by any owners or operators of the deficient laboratory. If we were to lose our CLIA certification or required state licensure, we would not be able to operate our clinical laboratory and conduct our tests, in full or in particular states, which would adversely impact our diagnostic testing business, operating results, and financial condition.
Our HCT/P products are subject to extensive government regulation and our failure to comply with these requirements could cause our business to suffer.
In the U.S., we provide donor egg and sperm for fertility treatments, in addition to fertility cryopreservation services and newborn stem cell storage (cord blood and cord tissue). Donated reproductive tissues, including eggs and sperm, as well as cord blood and cord tissue, are regulated to by the FDA as human cells, tissues and cellular or tissue-based products (HCT/Ps). In the U.S., we are marketing these HCT/Ps pursuant to Section 361 of the Public Health Service Act (PHSA) and 21 C.F.R. Part 1271 of FDA’s regulations. The so-called “361 HCT/Ps” are not currently subject to the FDA requirements to obtain marketing authorizations, so long as they meet certain criteria set forth in FDA regulations. However, HCT/Ps regulated as 361 HCT/Ps are currently subject to requirements relating to registering facilities and listing products with the FDA, as well as stringent requirements relating to processing, storing, labeling and distributing HCT/Ps, including, screening and testing for tissue donor eligibility, providing required labeling information, record keeping and adverse event reporting. If we fail to comply with these requirements, we could be subject to FDA allegations of noncompliance or enforcement action, including, for example, warning letters, fines, injunctions, product recalls or seizures, and, in the most serious cases, criminal penalties. To be regulated as 361 HCT/Ps, these products must meet the FDA’s criteria to be considered “minimally manipulated” and intended for “homologous use,” among other requirements. HCT/Ps that do not meet the criteria to be considered 361 HCT/Ps are subject to the FDA’s regulatory requirements applicable to medical devices, biologics or drugs, including, importantly, the requirement for premarket review and approval or clearance prior to marketing.
46
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
We believe our HCT/Ps are regulated solely under Section 361 of the PHSA, and therefore, we have not sought or obtained 510(k) clearance, PMA approval, or licensure through a Biologics License Application (BLA) for such HCT/Ps. However, the FDA could disagree with our determination that these human tissue products are 361 HCT/Ps and could determine that these products are biologics requiring a BLA or medical devices requiring 510(k) clearance or PMA approval, and could require that we cease marketing such products and/or recall them pending appropriate clearance, approval or licensure from the FDA, which would adversely affect our business, results of operations and financial condition.
In addition, the FDA may in the future modify the scope of its enforcement discretion with respect to 361 HCT/Ps or change its position on which current or future products qualify as 361 HCT/Ps, or determine that some or all of our HCT/P products may not be lawfully marketed without a marketing authorization. Any regulatory changes could have adverse consequences for us and make it more difficult or expensive for us to conduct our business by requiring pre-market clearance or approval and compliance with additional post-market regulatory requirements with respect to those products.
Disruptions at the FDA and other government agencies or notified bodies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, cleared or approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA, foreign agencies and notified bodies to review and clear, approve or certify new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory, and policy changes, the FDA’s, foreign agencies’ and notified bodies’ ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s, foreign agencies’ and notified bodies’ ability to perform routine functions. Average review times at the FDA, foreign agencies and notified bodies have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA, foreign agencies and notified bodies and other agencies may also slow the time necessary for new drugs and medical devices or modifications to cleared or approved drugs and medical devices to be reviewed, approved and/or certified by necessary government agencies or notified bodies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical employees and stop critical activities.
Separately, in response to the COVID-19 pandemic, starting in March 2020, the FDA postponed most inspections of foreign and domestic manufacturing facilities. Subsequently, in July 2020, the FDA resumed certain on-site inspections of domestic manufacturing facilities subject to a risk-based prioritization system. The FDA utilized this risk-based system to assist in determining when and where it was safest to conduct prioritized domestic inspections. In May 2021, the FDA outlined a detailed plan to move toward a more consistent state of inspectional operations, and in July 2021, the FDA resumed standard inspectional operations of domestic facilities. Since that time, the FDA has continued to monitor and implement changes to its inspectional activities to ensure the safety of its employees and those of the firms it regulates as it adapts to the evolving COVID-19 pandemic. Regulatory authorities outside the U.S. have adopted similar restrictions or other policy measures in response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA, other regulatory authorities or notified bodies from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA, other regulatory authorities or notified bodies to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
In the EU, notified bodies must be officially designated to certify products and services in accordance with the EU MDR and EU IVDR. Several notified bodies have been designated under the EU MDR but only a few notified bodies have been designated under the EU IVDR so far. The COVID-19 pandemic has significantly slowed down their designation process and the current designated notified bodies are facing a large amount of requests with the new regulations, as a consequence of which review times may have lengthened. This situation may impact the ability of our notified body to timely review and process our regulatory submissions and perform its audits.
Ethical, legal and social concerns related to the use of genetic information, sperm and egg selection services and stem cells could reduce demand for our service offerings.
Genetic testing, sperm and egg selection services and the use of stem cells have raised ethical, legal and social issues regarding privacy and the appropriate uses of information related to these services. Government authorities could, for social or other purposes, limit or regulate the use of genetic information or genetic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. They also could limit, regulate or prohibit (1) sperm and egg selection services or (2) the use of stem cells. Ethical, legal or social concerns may lead patients to refuse to use, or physicians to be reluctant to order or recommend, genetic tests, sperm and egg selection services and stem cell storage services even if permissible. These and other ethical, legal and social concerns may limit market
47
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
acceptance and adoption of our service offerings or reduce the potential markets for our service offerings, either of which could have an adverse effect on our business, financial condition and results of operations.
Risks Relating to Interest and Foreign Exchange Rates, Debt and Equity
Exchange rate fluctuations and foreign currency hedges could adversely affect our financial results.
As a result of our international operations, currency exchange rate fluctuations may affect our results of operations and financial position. Our most significant currency exposures are the British pound sterling, euro and Japanese yen. We expect to generate an increasing portion of our revenue and incur a significant portion of our expenses in currencies other than U.S. dollars. To the extent we are unable to materially offset non-functional currency flows, exchange rate fluctuations could have a positive or negative impact on our financial condition and results of operations. Because our consolidated financial results are reported in U.S. dollars, if we generate sales or earnings in other currencies, the translation of those results into U.S. dollars can result in a significant increase or decrease in the amount of those sales or earnings and can make it more difficult for our shareholders to understand the relative strengths or weaknesses of the underlying business on a period-over-period comparative basis. Although we may enter into foreign exchange agreements with financial institutions to reduce our net exposure to fluctuations in foreign currency values relative to our non-functional currency obligations or balances, they would not eliminate that risk entirely.
We are vulnerable to interest rate risk with respect to our debt.
We are subject to interest rate risk in connection with the issuance of variable and fixed-rate debt. In order to maintain a desired mix of fixed-rate and variable-rate debt, from time to time we may use interest rate swap agreements to fix a portion of our variable-rate debt as further described in Note 13. Financial Derivatives and Hedging of the Consolidated Financial Statements. We may not be successful in structuring such swap agreements to manage our risks effectively, which could adversely affect our business, earnings and financial condition.
The UK’s Financial Conduct Authority (FCA), which regulates the London Interbank Offered Rate (LIBOR), announced in July 2017 that it will no longer persuade or require banks to submit rates for LIBOR after 2021. In March 2021, the FCA confirmed its intention to stop requiring banks to submit rates required to calculate LIBOR after 2021. However, for U.S. dollar-denominated (USD) LIBOR, only one-week and two-month USD LIBOR will cease to be published after 2021, and all remaining USD LIBOR tenors will continue being published until June 2023. We have multiple debt facilities which bear interest at a variable rate based on the Eurodollar LIBOR rate in effect from time to time. A change or transition away from LIBOR as a common reference rate in the global financial market could have a material adverse effect on our business. Our management continues to monitor the status and discussions regarding LIBOR. We do not expect a material impact on our financial statements related to this transition.
Our indebtedness could adversely affect our financial health and prevent us from fulfilling our debt obligations.
We have now and expect to continue to have a significant amount of indebtedness.
Our indebtedness could:
•increase our vulnerability to general adverse economic and industry conditions;
•require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions, research and development efforts and other general corporate purposes;
•limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate;
•place us at a competitive disadvantage compared to our competitors that have less debt;
•result in greater interest rate risk and volatility;
•limit our ability to borrow additional funds; and
•make it more difficult for us to satisfy our obligations with respect to our debt, including our obligation to repay our credit facilities under certain circumstances, or refinance our indebtedness on favorable terms or at all.
Our credit facilities contain financial and other restrictive covenants that could limit our ability to engage in activities that may be in our long-term best interests. Our failure to comply with those covenants could result in an event of default which, if not cured or waived, could result in the acceleration of all of our debt, which could adversely affect our business, earnings and financial condition.
48
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Volatility in the securities markets, interest rates, and other factors could substantially increase our defined benefit plan costs.
We sponsor a defined benefit plan for certain employees in the U.S. This defined benefit plan is funded with trust assets invested in a diversified portfolio of securities and other investments. Changes in interest rates, mortality rates, early retirement rates, investment returns, discount rates and the market value of plan assets can affect the funded status of our defined benefit plan and cause volatility in the net periodic benefit cost and future funding requirements of the plan. A significant increase in our obligations or future funding requirements could have a negative impact on our results of operations and cash flows from operations.
Risks Relating to Taxes
Changes in tax laws, examinations by tax authorities, and changes in our geographic composition of income could adversely affect our cash flows, results of operations, and financial condition
Income taxes and other taxes are based on enacted tax laws and the results of operations in each jurisdiction. Taxes could significantly increase due to changes in tax laws or changes in our interpretation of those laws. Changes in tax laws could result from a framework being developed by the Organisation for Economic Co-operation and Development (OECD), a global policy forum, that, if implemented, includes a global minimum tax rate of 15%. Taxes could also significantly increase due to changes in accounting guidance.
Income taxes and other taxes could significantly increase based on the resolution of tax authority examinations. Tax authorities could challenge our interpretations of tax laws and estimates we use to calculate taxes. Tax authorities could also challenge our positions related to transfer pricing and intercompany transactions, including the valuation of intangible assets. Tax examinations can result in costly litigation with significant interest and penalties and ultimate settlement can take several years. For example, we have engaged (and expect to continue to engage) with tax authorities over tax positions we have taken in connection with acquisitions, and such examinations could cause us to incur significant expense (and adverse determinations by the tax authority could result in penalties).
Our effective tax rate could fluctuate based on the geographic composition of income, which could significantly change based on our business results and acquisitions. Our effective tax rate could also fluctuate based on changes in estimates, changes in excess tax benefits from share-based compensation, changes in non-deductible expenses, and the valuation of deferred tax assets and liabilities. These fluctuations could have an adverse effect on our cash flows, results of operations, and financial condition.
Item 1B. Unresolved Staff Comments.
None.
49
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 2. Properties.
The following is a summary of Cooper's principal facilities as of October 31, 2022. We generally lease our office and operations facilities but own several manufacturing and research and development facilities, including 298,852 square feet in the United Kingdom, 350,600 square feet in Costa Rica, 78,767 square feet in New York and 33,630 square feet in Texas. Our lease agreements expire at various dates through the year 2045. We believe our properties are suitable and adequate for our businesses.
| Location | Approximate Square Feet | Operations | |||||||||
| AMERICAS | |||||||||||
| United States: | |||||||||||
| California | 201,143 | Executive offices; CooperVision manufacturing, research & development and administrative offices; CooperSurgical research & development, distribution and administrative offices | |||||||||
| New York | 349,047 | CooperVision and CooperSurgical distribution and administrative offices | |||||||||
| New Jersey | 37,700 | CooperSurgical research and development, distribution and administrative offices | |||||||||
| Connecticut | 285,538 | CooperSurgical distribution and administrative offices | |||||||||
| Arizona | 45,000 | CooperVision manufacturing | |||||||||
| Puerto Rico | 563,284 | CooperVision manufacturing, research and development and distribution | |||||||||
| Canada | 60,035 | CooperVision manufacturing and administrative office; CooperSurgical research & development, distribution and administrative offices | |||||||||
| Brazil | 38,623 | CooperVision distribution and administrative office | |||||||||
| Other Americas | 45,135 | CooperVision distribution and administrative offices; CooperSurgical research & development, distribution and administrative offices | |||||||||
| EMEA | |||||||||||
| United Kingdom | 691,131 | CooperVision manufacturing, distribution, research & development and administrative offices; CooperSurgical research & development, administrative offices | |||||||||
| Hungary | 330,149 | CooperVision manufacturing | |||||||||
| Belgium | 279,967 | CooperVision distribution | |||||||||
| Spain | 181,145 | CooperVision distribution and administrative office; CooperSurgical administrative office | |||||||||
| Netherlands | 86,027 | CooperVision administrative offices; CooperSurgical research & development and distribution | |||||||||
| Other EMEA | 125,005 | CooperVision distribution and administrative offices; CooperSurgical administrative offices | |||||||||
| ASIA PACIFIC | |||||||||||
| Japan | 103,533 | CooperVision distribution and administrative offices; CooperSurgical laboratory/research & development | |||||||||
| Australia | 32,844 | CooperVision marketing and distribution; CooperSurgical research & development and distribution | |||||||||
| Other Asia Pacific | 91,244 | CooperVision distribution, marketing and administrative offices; CooperSurgical marketing and administrative office | |||||||||
50
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 3. Legal Proceedings.
Information regarding legal proceedings is included in Note 11. Contingencies of the Consolidated Financial Statements.
Item 4. Mine Safety Disclosures.
Not applicable.
51
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Cooper's common stock, par value $0.10 per share, is traded on the New York Stock Exchange under the symbol “COO.” At December 1, 2022, there were 268 common stockholders of record.
Dividend Policy
Our current policy is to pay annual cash dividends on our common stock of $0.06 per share, in two semiannual payments of $0.03 per share each. In dollar terms, we paid cash for dividends of $3.0 million in each of fiscal 2022 and 2021. Dividends are paid when, as and if declared at the discretion of our Board of Directors from funds legally available for that purpose. Our Board of Directors periodically reviews our dividend policy and considers the Company's earnings, financial condition, liquidity needs, business plans and opportunities and other factors in making and setting dividend policy.
Performance Graph
The following graph compares the cumulative total return on Cooper's common stock with the cumulative total return of the Standard & Poor 500 and the Standard & Poor's Health Care Equipment Index for the five-year period ended October 31, 2022. The graph assumes that the value of the investment in Cooper and in each index was $100 on October 31, 2017 and assumes that all dividends were reinvested.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among The Cooper Companies, Inc.,
the S&P 500 Index and the S&P Health Care Equipment Index
*$100 invested on October 31, 2017 in stock or index, including reinvestment of dividends.
Fiscal year ending October 31.
Copyright© 2022 Standard & Poor's, a division of S&P Global. All rights reserved.
Issuer Purchases of Equity Securities
There was no share repurchase activity during the three-month period ended October 31, 2022.
52
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Equity Compensation Plan Information
The following table sets forth certain information as of October 31, 2022, concerning the shares of our Common Stock that may be issued under any form of award granted under our equity compensation plans in effect as of October 31, 2022:
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights(1) (A) | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (B) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column A) (C) | ||||||||||||||
Equity compensation plans approved by shareholders(2) | 1,432,459 | $264.85 | 1,671,522 | ||||||||||||||
| Equity compensation plans not approved by shareholders | — | — | — | ||||||||||||||
| Total | 1,432,459 | $264.85 | 1,671,522 | ||||||||||||||
(1) The amount of total securities to be issued under Company equity plans upon exercise of outstanding options, warrants and rights shown in Column A includes 289,238 Restricted Stock Units granted pursuant to the Company's equity plans. These awards allow for the distribution of shares to the grant recipient upon the completion of time-based vesting periods. The total also includes 79,378 shares representing the maximum number of shares that may be issued subject to Performance Share Awards outstanding as of the end of the fiscal year. Restricted Stock Units and Performance Share Awards do not have an associated exercise price. Accordingly, these awards are not reflected in the weighted-average exercise price disclosed in Column B.
(2) Includes information with respect to the Third Amended and Restated 2007 Long Term Incentive Plan for Employees (2007 Plan) and the 2019 Employee Stock Purchase Plan (2019 ESPP), as discussed in Note 9. Stock Plans of the Consolidated Financial Statements. Also includes information from Second Amended and Restated 2006 Long Term Incentive Plan for Non-Employee Directors, which expired by its terms in March 2019, and the 2020 Long Term Incentive Plan for Non-Employee Directors (2020 Directors' Plan), which was approved by stockholders on March 18, 2020 and provides for the issuance of up to 50,000 shares of Common Stock. As of October 31, 2022, up to 690,596 shares of Common Stock may be issued pursuant to the 2007 Plan, up to 948,090 shares of Common Stock may be issued pursuant to the 2019 ESPP and up to 32,836 shares of Common Stock may be issued pursuant to the 2020 Directors' Plan.
Item 6. Reserved
53
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
Note numbers refer to “Notes to Consolidated Financial Statements” in Item 8. Financial Statements and Supplementary Data.
Results of Operations
In this section, we discuss the results of our operations for fiscal 2022 compared with fiscal 2021. We discuss our cash flows and current financial condition under “Capital Resources and Liquidity.” For a discussion related to fiscal 2021 compared with fiscal 2020, please refer to Item 7 of Part II, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the Year Ended October 31, 2021, which was filed with the United States Securities and Exchange Commission (SEC) on December 10, 2021, and is available on the SEC's website at www.sec.gov and our Investor Relations website at investor.coopercos.com.
Within the tables presented, percentages are calculated based on the underlying whole-dollar amounts and, therefore, may not recalculate exactly from the rounded numbers used for disclosure purposes.
Outlook
We are optimistic about the long-term prospects for the worldwide contact lens and general health care markets, and the resilience of and growth prospects for our businesses and products. However, we face significant risks and uncertainties in our global operating environment as further described in the “Risk Factors” section in Part I, Item 1A of this filing. These risks include uncertain global and regional business, political and economic conditions, including but not limited to those associated with the COVID-19 pandemic, Russia’s invasion of Ukraine, inflation, foreign exchange rate fluctuations, regulatory developments, supply chain disruptions, and escalating global trade barriers. These risks and uncertainties have adversely affected our sales, cash flow and current performance in the past and are likely to further adversely affect our future sales, cash flow and performance.
Global Market and Economic Conditions - Over the last few years in the U.S. and globally, market and economic conditions have been challenging, particularly in light of the COVID-19 pandemic. Foreign countries, in particular the Euro zone, have experienced recessionary pressures and face continued concerns about the systemic impacts of adverse economic conditions and geopolitical issues. In addition, changes in economic conditions, supply chain constraints, logistics challenges, labor shortages, the war in Ukraine, and steps taken by governments and central banks, particularly in response to the COVID-19 pandemic, as well as other stimulus and spending programs, have led to higher inflation, which is likely to lead to an increase in costs and may cause changes in fiscal and monetary policy, including increased interest rates. In a higher inflationary environment, we may be unable to raise the prices of our products and services sufficiently to keep up with the rate of inflation. These economic conditions could have a material adverse effect on our results of operations and financial condition.
COVID-19 Considerations - The COVID-19 pandemic and health crisis led to ongoing economic and societal disruptions and uncertainties that have negatively impacted business and healthcare activity globally. As a result of healthcare systems responding to the demands of managing the pandemic, governments around the world imposing measures designed to reduce the transmission of the COVID-19 virus, and individuals responding to the concerns of contracting the COVID-19 virus, many optical practitioners and retailers, hospitals, medical offices and fertility clinics closed their facilities, restricted access, or delayed or canceled patient visits, exams and elective medical procedures, and many customers that have reopened are experiencing reduced patient visits. These factors have had, and in the future may continue to have, an adverse effect on our sales, operating results and cash flows.
We have taken an active role in addressing the pandemic’s impact on our employees, suppliers, distribution channels, operations and customers, including taking precautionary measures and developing contingency plans with respect to our operations and to help ensure the safety of our personnel in all our facilities, and we have endeavored and continue to follow recommended actions of government and health authorities to protect our employees worldwide. As of the date of this filing, we have not experienced any significant disruption at our manufacturing facilities or in our access to necessary raw materials and other supplies or with our distribution network; however, we have experienced higher unabsorbed fixed overhead costs, labor inefficiencies, delays in receiving certain raw materials, higher cost of production and higher freight charges as a result of the COVID-19 pandemic.
At this time, future developments with respect to the COVID-19 pandemic remain highly uncertain and largely outside of our control. We cannot predict the spread, duration and severity of the pandemic or any subsequent outbreaks, potential actions taken by governments to respond to the pandemic, or potential impacts on global and local economic activity. We
54
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
will continue to closely monitor the developments relating to the COVID-19 pandemic and the responses from governments and private sector participants.
For more information on the risks associated with the COVID-19 pandemic, refer to Part I, Item 1A, "Risk Factors" herein.
CooperVision - We compete in the worldwide contact lens market with our spherical, toric, multifocal, toric multifocal contact lenses offered in a variety of materials including using silicone hydrogel Aquaform® technology and PC Technology™. We believe that there will be lower contact lens wearer dropout rates as technology improves and enhances the wearing experience through a combination of improved designs and materials and the growth of preferred modalities such as single-use and monthly wearing options. CooperVision also competes in the myopia management and specialty eye care contact lens markets with myopia management contact lenses using its ActivControl® technology and with products such as orthokeratology (ortho-k) and scleral lenses. In November 2019, CooperVision received U.S. Food and Drug Administration (FDA) approval for its MiSight® 1 day lens, which is the first and only FDA-approved product indicated to slow the progression of myopia in children with treatment initiated between the ages of 8-12 and became available in the United States during fiscal 2020. In August 2021, CooperVision received Chinese National Medical Products Administration (NMPA) approval for its MiSight® 1 day lens for use in China. CooperVision is focused on greater worldwide market penetration using recently introduced products, and we continue to expand our presence in existing and emerging markets, including through acquisitions.
CooperVision acquired the following entity during fiscal 2022:
• A privately-held Denmark-based ortho-k contact lens distributor in May 2022
CooperVision acquired the following entities during fiscal 2021:
•A privately-held UK contact lens manufacturer in April 2021
•A privately-held medical device company (SightGlass Vision Inc. (SGV), a developer of spectacle lenses for myopia management) in January 2021
During the second quarter of fiscal 2022, the Company initiated a plan to exit its contact lens care business, a non-core business unit of the CooperVision segment. We expect the exit activity to be substantially completed in the first half of fiscal 2023. Exit charges recognized in the three and twelve months ended October 31, 2022, were $9.2 million and $33.2 million, of which $26.7 million is recognized in cost of sales and $6.5 million is recognized in selling, general, and administrative expense in the Consolidated Statements of Income. Exit costs primarily related to inventory write-down, asset impairments and employee-related costs. Total exit costs are expected to be in a range of $30.0 million to $40.0 million.
In March 2022, CooperVision and Essilor International SAS (Essilor) entered into a Contribution Agreement and a Stock Purchase Agreement under which Essilor paid CooperVision $52.1 million in exchange for a 50% interest in SGV and a proportionate share of certain revenue-based milestone payments related to the January 2021 acquisition of SGV by CooperVision. As part of these agreements, each party contributed their interest in SGV and $10 million in cash to form a new joint venture. CooperVision then remeasured the fair value of its retained equity investment in the joint venture at $90.0 million which resulted in a $56.9 million gain in Other (income) expense on deconsolidation of SGV.
On November 1, 2022, subsequent to the fiscal year ended October 31, 2022, CooperVision closed an Agreement and Plan of Merger (the “Merger Agreement”) to acquire a U.S. based privately held leading expert in specialty contact lenses for both normal and irregular corneal conditions. The Company is in the process of finalizing purchase accounting information.
Our ability to compete successfully with a full range of silicone hydrogel products is an important factor to achieving our desired future levels of sales growth and profitability. CooperVision manufactures and markets a wide variety of silicone hydrogel contact lenses. Our single-use silicone hydrogel product franchises, clariti® and MyDay®, remain a focus as we expect increasing demand for these products as well as future single-use products as the global contact lens market continues to shift to this modality. Outside of single-use, the Biofinity® and Avaira Vitality® product families comprise our focus in the FRP, or frequent replacement product, market which encompasses the 2-week and monthly modalities. Included in this segment are unique products such as Biofinity Energys®, which helps individuals with digital eye fatigue.
CooperSurgical - Our CooperSurgical business competes in the general health care market with a commitment to advancing the health of women, babies and families through its diversified portfolio of products and services focusing on women's health and fertility. CooperSurgical has established its market presence and distribution system by developing products and acquiring companies, products and services that complement its business model.
55
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
CooperSurgical acquired the following entities during fiscal 2022:
• A private cryopreservation services company in April 2022
• Generate Life Sciences (Generate), a privately-held leading provider of donor egg and sperm for fertility treatments, fertility cryopreservation services and newborn stem cell storage (cord blood & cord tissue) in December 2021
CooperSurgical acquired the following entities during fiscal 2021:
•A privately-held medical device company that develops single-use illuminating medical devices in May 2021
•A privately-held medical device company in March 2021
•A privately-held medical device company in February 2021
•A privately-held in vitro fertilization (IVF) cryostorage software solutions company in December 2020
On April 6, 2022, CooperSurgical entered into an asset purchase agreement to acquire Cook Medical's Reproductive Health business, a manufacturer of minimally invasive medical devices focused on the fertility, obstetrics and gynecology markets. The aggregate consideration is $875.0 million in cash, with $675.0 million payable at the closing and the remaining $200.0 million payable in $50.0 million installments following each of the first, second, third and fourth anniversaries of the closing. The transaction is subject to customary closing conditions, such as receipt of required regulatory approvals.
Transition from LIBOR
The UK’s Financial Conduct Authority (FCA), which regulates the London Interbank Offered Rate (LIBOR), announced in July 2017 that it will no longer persuade or require banks to submit rates for LIBOR after 2021. In March 2021, the FCA confirmed its intention to stop requiring banks to submit rates required to calculate LIBOR after 2021. However, for U.S. dollar-denominated (USD) LIBOR, only one-week and two-month USD LIBOR will cease to be published after 2021, and all remaining USD LIBOR tenors will continue being published until June 2023. Further, in March 2020, the Financial Accounting Standards Board (FASB) issued ASU 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting. This guidance provides optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. We have material contracts that are indexed to LIBOR and are continuing to monitor this activity and evaluate the related risk. We are continuing to evaluate the scope of impacted contracts and the potential impact. We are also monitoring the developments regarding alternative rates and may amend certain contracts to accommodate those rates if the contract does not already specify a replacement rate. While the notional value of agreements potentially indexed to LIBOR is material, we do not expect a material impact on our financial statements related to this transition.
We believe that current cash, cash equivalents and future cash flow from operating activities will be sufficient to meet our anticipated cash needs, including working capital needs, capital expenditures and contractual obligations for at least 12 months from the issuance date of the financial statements included in this annual report. To the extent additional funds are necessary to meet our liquidity needs such as that for acquisitions, share repurchases, cash dividends or other activities as we execute our business strategy, we anticipate that additional funds will be obtained through the incurrence of additional indebtedness, additional equity financings or a combination of these potential sources of funds; however, such financing may not be available on favorable terms, or at all.
56
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
2022 Compared with 2021
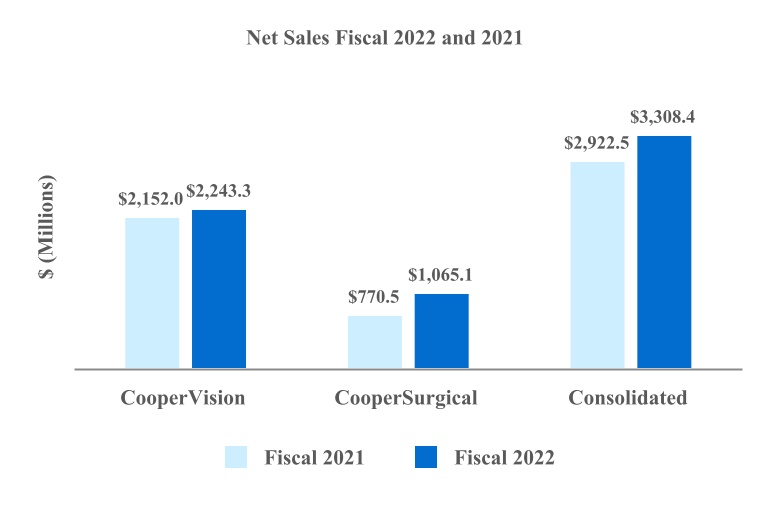
CooperVision Net Sales
The contact lens market has two major product categories:
•Spherical lenses including lenses that correct near- and farsightedness uncomplicated by more complex visual defects; and
•Toric and multifocal lenses including lenses that, in addition to correcting near- and farsightedness, address more complex visual defects such as astigmatism and presbyopia by adding optical properties of cylinder and axis, which correct for irregularities in the shape of the cornea.
CooperVision Net Sales by Category
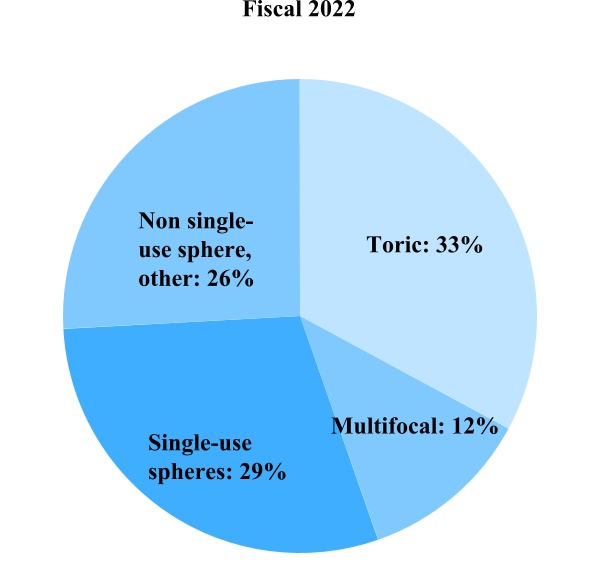
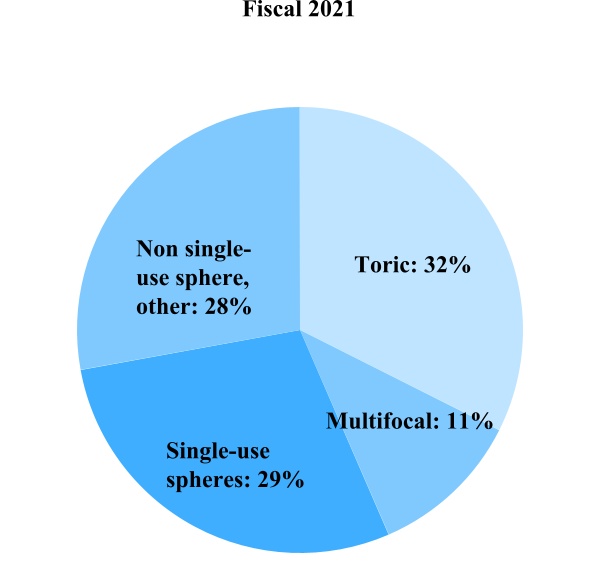
Single-use spheres – This includes Biomedics 1 day, clariti 1 day, MyDay, MiSight and Proclear 1 day
Toric – This includes Avaira Vitality toric, Biomedics toric, Biofinity toric, clariti 1 day toric, MyDay toric and Proclear toric
Multifocal – This includes Biofinity multifocal, Biofinity toric multifocal, clariti 1 day multifocal, MyDay multifocal and Proclear 1 day multifocal
Non single-use sphere, other – This includes our Avaira Vitality spheres, frequent replacement product (FRP) lens portfolio (Biofinity spheres, Biofinity Energys, Biomedics, Proclear spheres, clariti spheres), ortho-k, scleral and custom lenses, contact lens solutions and other
57
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
| ($ in millions) | 2022 | 2021 | 2022 vs. 2021 % Change | ||||||||||||||
| Toric | $ | 737.4 | $ | 697.5 | 6 | % | |||||||||||
| Multifocal | 264.4 | 238.6 | 11 | % | |||||||||||||
| Single-use spheres | 661.6 | 616.3 | 7 | % | |||||||||||||
| Non single-use sphere, other | 579.9 | 599.6 | (3) | % | |||||||||||||
| $ | 2,243.3 | $ | 2,152.0 | 4 | % | ||||||||||||
In the fiscal year ended October 31, 2022, the growth experienced across all categories (except for "Other" as mentioned below) was partially offset by unfavorable foreign exchange rate fluctuations, which approximated $149.5 million. Sales growth was primarily driven by an increase in the volume of lenses sold across our core portfolio due to a recovery in demand from the impact of the COVID-19 pandemic.
•Toric and multifocal lenses grew primarily through the success of MyDay and Biofinity.
•Single-use sphere lenses grew primarily through MyDay, clariti and MiSight lenses.
•Non single-use sphere lenses grew primarily through Biofinity and ortho-k.
•"Other" products decreased primarily due to exit of the contact lens care business. Contact lens care represented approximately 1% and 2% of net sales in fiscal 2022 and 2021.
•Total silicone hydrogel products increased by 7%, representing 78% of net sales in fiscal 2022 compared to 76% in fiscal 2021.
CooperVision Net Sales by Geography
CooperVision competes in the worldwide soft contact lens market and services in three primary regions: the Americas, EMEA (Europe, Middle East and Africa) and Asia Pacific.
| ($ in millions) | 2022 | 2021 | 2022 vs. 2021 % Change | ||||||||||||||
| Americas | $ | 887.2 | $ | 832.1 | 7 | % | |||||||||||
| EMEA | 843.7 | 819.5 | 3 | % | |||||||||||||
| Asia Pacific | 512.4 | 500.4 | 2 | % | |||||||||||||
| $ | 2,243.3 | $ | 2,152.0 | 4 | % | ||||||||||||
CooperVision's growth in net sales across all regions was primarily attributable to market gains of silicone hydrogel contact lenses. Refer to CooperVision Net Sales by Category above for further discussion.
CooperSurgical Net Sales by Category
CooperSurgical supplies the family health care market with a diversified portfolio of products and services. Our office and surgical offerings include products that facilitate surgical and non-surgical procedures that are commonly performed primarily by obstetricians and gynecologists in hospitals, surgical centers, fertility clinics and medical offices. Fertility offerings include highly specialized products and services that target the IVF process, including diagnostics testing with a goal to make fertility treatment safer, more efficient and convenient.
58
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The chart below shows the percentage of net sales of office and surgical products and fertility.
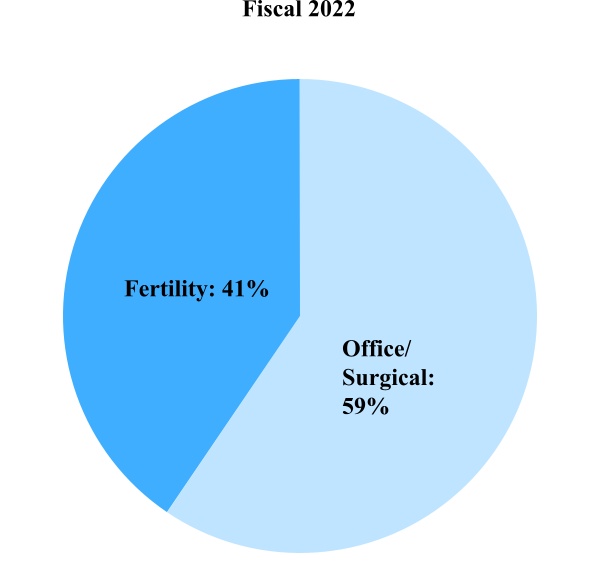
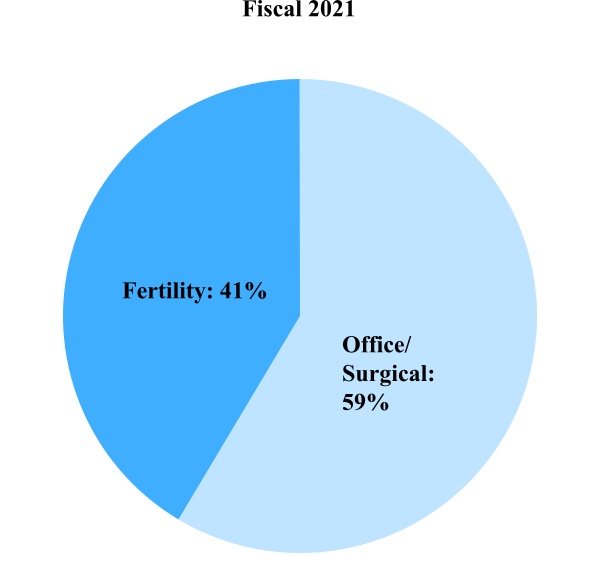
Office/Surgical – This includes Endosee endometrial imaging products, Fetal Pillow cephalic elevation devices for use in Cesarean sections, illuminated speculum products, Lone Star retractor systems, loop electrosurgical excision procedure (LEEP) products, Mara water ablation systems, newborn stem cell storage, PARAGARD contraceptive IUDs, point-of-care products and uterine positioning products.
Fertility – Our significant fertility products and services include cryostorage, donor gamete services, fertility consumables and equipment and genomic services (including preimplantation genetic testing).
($ in millions) | 2022 | 2021 | 2022 vs. 2021 % Change | |||||||||||||||||
| Office and surgical products | $ | 633.6 | $ | 451.3 | 40 | % | ||||||||||||||
| Fertility | 431.5 | 319.2 | 35 | % | ||||||||||||||||
| $ | 1,065.1 | $ | 770.5 | 38 | % | |||||||||||||||
In the fiscal year ended October 31, 2022, net sales increase in both categories was mainly due to the Generate acquisition. The increase was offset by unfavorable foreign exchange rate fluctuations, which approximated $33.4 million.
Gross Margin
Consolidated Gross Margin decreased in fiscal 2022 to 65% compared to 67% in fiscal 2021 primarily driven by unfavorable currency and contact lens care exit costs.
Selling, General and Administrative Expense (SGA)
| ($ in millions) | 2022 | % Net Sales | 2021 | % Net Sales | 2022 vs. 2021 % Change | ||||||||||||||||||||||||
| CooperVision | $ | 826.7 | 37 | % | $ | 843.9 | 39 | % | (2) | % | |||||||||||||||||||
| CooperSurgical | 461.7 | 43 | % | 320.0 | 42 | % | 44 | % | |||||||||||||||||||||
| Corporate | 53.8 | — | 47.3 | — | 14 | % | |||||||||||||||||||||||
| 1,342.2 | 41 | % | $ | 1,211.2 | 41 | % | 11 | % | |||||||||||||||||||||
CooperVision's SGA decreased in fiscal 2022 compared to fiscal 2021 primarily due to the $56.8 million increase in fair value of the contingent consideration related to SGV acquisition in fiscal 2021, partially offset by increase in SGA to support sales growth.
59
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
CooperSurgical's SGA increased in fiscal 2022 compared to fiscal 2021 primarily due to the addition of Generate's SGA and acquisition and integration expenses.
Corporate SGA increased in fiscal 2022 compared to fiscal 2021 primarily due to share-based compensation related expenses.
Research and Development Expense (R&D)
| ($ in millions) | 2022 | % Net Sales | 2021 | % Net Sales | 2022 vs. 2021 % Change | ||||||||||||||||||||||||
| CooperVision | $ | 62.4 | 3 | % | $ | 61.6 | 3 | % | 1 | % | |||||||||||||||||||
| CooperSurgical | 47.9 | 4 | % | 31.1 | 4 | % | 54 | % | |||||||||||||||||||||
| $ | 110.3 | 3 | % | $ | 92.7 | 3 | % | 19 | % | ||||||||||||||||||||
CooperVision's R&D expense increased in fiscal 2022 compared to fiscal 2021 primarily due to myopia management programs and timing of R&D projects. CooperVision's R&D activities are primarily focused on the development of contact lenses, manufacturing technology and process enhancements.
CooperSurgical's R&D expense increased in fiscal 2022 compared to fiscal 2021 mainly due to the addition of Generate's R&D expense. CooperSurgical's R&D activities are focused on developing and refining diagnostic and therapeutic products including medical interventions, surgical devices and fertility solutions.
Amortization Expense
| ($ in millions) | 2022 | % Net Sales | 2021 | % Net Sales | 2022 vs. 2021 % Change | ||||||||||||||||||||||||
| CooperVision | $ | 32.3 | 1 | % | $ | 35.7 | 2 | % | (10) | % | |||||||||||||||||||
| CooperSurgical | 147.2 | 14 | % | 110.4 | 14 | % | 33 | % | |||||||||||||||||||||
| $ | 179.5 | 5 | % | $ | 146.1 | 5 | % | 23 | % | ||||||||||||||||||||
CooperVision's amortization expense decreased in absolute dollars in fiscal 2022 compared to fiscal 2021, primarily due to the deconsolidation of SGV.
CooperSurgical's amortization expense increased in absolute dollars in fiscal 2022 compared to fiscal 2021, primarily due to the amortization of intangible assets newly acquired through acquisitions.
Operating Income
| ($ in millions) | 2022 | % Net Sales | 2021 | % Net Sales | 2022 vs. 2021 % Change | ||||||||||||||||||||||||
| CooperVision | $ | 494.3 | 22 | % | $ | 481.3 | 22 | % | 3 | % | |||||||||||||||||||
| CooperSurgical | 67.1 | 6 | % | 71.8 | 9 | % | 7 | % | |||||||||||||||||||||
| Corporate | (53.8) | — | % | (47.3) | — | (14) | % | ||||||||||||||||||||||
| $ | 507.6 | 15 | % | $ | 505.8 | 17 | % | — | % | ||||||||||||||||||||
CooperVision's operating income increased in fiscal 2022 compared to fiscal 2021, primarily due to an increase in net sales partially offset by net changes in operating expenses.
CooperSurgical's operating income decreased in fiscal 2022 compared to fiscal 2021, primarily due to an increase in SGA and amortization expenses, partially offset by an increase in net sales.
Corporate operating loss increased in fiscal 2022 compared to fiscal 2021, primarily due to higher share-based compensation expense.
60
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
On a consolidated basis, operating income increased in fiscal 2022 compared to fiscal 2021, primarily due to an increase in consolidated net sales.
Interest Expense
| ($ in millions) | 2022 | % Net Sales | 2021 | % Net Sales | 2022 vs. 2021 % Change | ||||||||||||||||||||||||
| Interest expense | $ | 57.3 | 2 | % | $ | 23.1 | 1 | % | 148 | % | |||||||||||||||||||
Interest expense increased during fiscal 2022 compared to the prior year, primarily due to higher average debt balances and higher interest rates.
Other (Income) Expense, Net
| ($ in millions) | 2022 | 2021 | |||||||||
| Investment gain | $ | (47.7) | $ | (11.6) | |||||||
| Foreign exchange loss | 22.0 | 5.5 | |||||||||
| Other expense (income), net | 0.7 | (2.7) | |||||||||
| $ | (25.0) | $ | (8.8) | ||||||||
Investment gain primarily consists of a gain on remeasurement of the fair value of retained equity investment in SGV as a result of deconsolidation.
Foreign exchange loss is primarily associated with the strengthening of the US dollar against foreign currencies and the effect on intercompany receivables.
Other expense (income), net increased in fiscal 2022, primarily due to a loss on minority investments, partially offset by defined benefit plan related income.
Provision for Income Taxes
The effective tax rates for fiscal 2022 and 2021 were 18.8% and (499.1)%, respectively. The increase was primarily due to an intra-group transfer of intellectual property in fiscal 2021 and UK tax rate change in fiscal 2021, as discussed below. The increase was also due to changes in the geographic composition of pre-tax earnings and changes in excess tax benefits from share-based compensation.
The effective tax rate for fiscal 2022 was lower than the US federal statutory rate primarily due to foreign earnings in jurisdictions with lower tax rates and changes in unrecognized tax benefits, partially offset by foreign earnings subject to US tax. The effective tax rate for fiscal 2021 was lower than the US federal statutory tax rate primarily due to the intra-group transfer, UK tax rate change, and earnings in foreign jurisdictions with lower tax rates partially offset by foreign earnings subject to US tax.
In November 2020, the Company completed an intra-group transfer of certain intellectual property and related assets of CooperVision to a UK subsidiary as part of a group restructuring to establish headquarters operations in the UK. Determining fair value involved significant judgment related to future revenue growth, operating margins, and discount rates. The transfer resulted in a step-up of the UK tax-deductible basis in the intellectual property and goodwill, creating a temporary difference between the book basis and the tax basis of these assets. As a result, the Company recognized a deferred tax asset of $1,987.9 million, with a corresponding income tax benefit, during the first quarter of fiscal 2021. During the third quarter of fiscal 2021, the Company recognized a $536.7 million tax benefit related primarily to the remeasurement of this deferred tax asset caused by the UK enactment of a 25% corporate tax rate.
See Note 6. Income Taxes for additional information.
61
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
CAPITAL RESOURCES AND LIQUIDITY
Working capital at October 31, 2022 and October 31, 2021, was $253.4 million and $733.2 million, respectively. The decrease in working capital is primarily due to an increase in accounts payable as a result of timing of payment to vendors, and an increase in short-term debt due to the 364-day term loan agreement entered into during fiscal 2022. See Note 5. Financing Arrangements for further information.
The $43.1 million increase in inventories was primarily due to higher sales, and the buildup of inventory for future product launches.
Cash Flow
| ($ in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Operating activities | $ | 692.4 | $ | 738.6 | $ | 486.6 | |||||||||||
| Investing activities | (1,831.2) | (450.3) | (364.5) | ||||||||||||||
| Financing activities | 1,193.7 | (311.4) | (95.5) | ||||||||||||||
| Effect of exchange rate changes on cash, cash equivalents, restricted cash and restricted cash equivalents | (12.9) | 2.9 | 0.7 | ||||||||||||||
| Increase (decrease) in cash, cash equivalents, restricted cash and restricted cash equivalents | $ | 42.0 | $ | (20.2) | $ | 27.3 | |||||||||||
Operating cash flow
Cash provided by operating activities in fiscal 2022 was lower than cash provided by operating activities in fiscal 2021, primarily due to settlement of contingent consideration of $52.3 million.
Investing Cash Flow
Cash used in investing activities in fiscal 2022 was higher than cash used in investing activities in fiscal 2021, primarily attributable to $1.6 billion cash paid, net of cash acquired, for the Generate acquisition, partially offset by $52.1 million proceeds from the sale of a 50% interest in SGV. See Note 3. Acquisitions and Joint Venture for further information.
Financing Cash Flow
Cash was provided by financing activities in fiscal 2022 compared to used in financing activities in fiscal 2021, primarily due to a decrease in repayments of long-term debt obligations by $854.5 million, and net proceeds from short-term debt of $329.3 million in fiscal 2022, compared to net repayments of short-term debt obligations of $321.3 million in fiscal 2021.
The following is a summary of the maximum commitments and the net amounts available to us under different credit facilities as of October 31, 2022:
| (In millions) | Facility Limit | Outstanding Borrowings | Outstanding Letters of Credit | Total Amount Available | Maturity Date | |||||||||||||||||||||||||||
| Revolving Credit: | ||||||||||||||||||||||||||||||||
| 2020 Revolving Credit | $ | 1,290.0 | $ | — | $ | 1.3 | $ | 1,288.7 | April 1, 2025 | |||||||||||||||||||||||
| Term Loan: | ||||||||||||||||||||||||||||||||
| 2021 364-Day Term Loan | 840.0 | 338.0 | n/a | — | November 1, 2022 | |||||||||||||||||||||||||||
| 2020 Term Loan | 850.0 | 850.0 | n/a | — | April 1, 2025 | |||||||||||||||||||||||||||
| 2021 Term Loan | 1,500.0 | 1,500.0 | n/a | — | December 17, 2026 | |||||||||||||||||||||||||||
| Total | $ | 4,480.0 | $ | 2,688.0 | $ | 1.3 | $ | 1,288.7 | ||||||||||||||||||||||||
As of October 31, 2022, the Company was in compliance with all debt covenants. See Note 5. Financing Arrangements for additional information.
Considering recent market conditions and the COVID-19 pandemic crisis, we have re-evaluated our operating cash flows and cash requirements and continue to believe that current cash, cash equivalents, future cash flow from operating activities and cash available under our 2020 Credit Agreement will be sufficient to meet our anticipated cash needs, including working capital needs, capital expenditures and contractual obligations for at least 12 months from the issuance date of the
62
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Consolidated Financial Statements included in this quarterly report. To the extent additional funds are necessary to meet our liquidity needs such as that for acquisitions, share repurchases, cash dividends or other activities as we execute our business strategy, we anticipate that additional funds will be obtained through the incurrence of additional indebtedness, additional equity financings or a combination of these potential sources of funds; however, such financing may not be available on favorable terms, or at all.
Share Repurchases
In December 2011, the Company's Board of Directors authorized the 2012 Share Repurchase Program and through subsequent amendments, the most recent in March 2017, the total repurchase authorization was increased from $500.0 million to $1.0 billion of the Company's common stock. The program has no expiration date and may be discontinued at any time. Purchases under the 2012 Share Repurchase Program are subject to a review of the circumstances in place at the time and may be made from time to time as permitted by securities laws and other legal requirements.
In fiscal 2022, we repurchased 191,165 shares of our common stock for $78.5 million. At October 31, 2022, $256.4 million remained authorized for repurchase under the program. See Note 8. Stockholders’ Equity for additional information.
Dividends
In fiscal 2022 and 2021, the Company declared regular dividends of 6 cents per share (a semiannual dividend of 3 cents per share) and paid a total of $3.0 million in each fiscal year.
CONTRACTUAL OBLIGATIONS
As of October 31, 2022, we had the following contractual obligations:
Payments Due by Fiscal Year (In millions) | Total | 2023 | 2024 & 2025 | 2026 & 2027 | 2028 & Beyond | ||||||||||||||||||||||||
| Interest payments | $ | 319.3 | $ | 95.1 | $ | 169.7 | $ | 54.5 | $ | — | |||||||||||||||||||
Transition tax on unremitted foreign earnings and profits (1) | 100.4 | 11.8 | 51.7 | 36.9 | — | ||||||||||||||||||||||||
Purchase obligations (2) | 270.9 | 181.2 | 87.4 | 2.3 | — | ||||||||||||||||||||||||
| Total contractual obligations | $ | 690.6 | $ | 288.1 | $ | 308.8 | $ | 93.7 | $ | — | |||||||||||||||||||
(1) As of October 31, 2022, we had $100.4 million of income tax liabilities related to the one-time transition tax that resulted from the enactment of the 2017 US Tax Act, which is payable in annual installments through fiscal 2026. The installment for fiscal 2022 is classified as a current income tax payable on our consolidated balance sheet.
We are unable to reliably estimate the timing of future payments related to uncertain tax positions and have excluded $25.4 million of long-term income taxes payable from the table above. See Note 6. Income Taxes for additional information.
(2) Purchase obligations consist of agreements to purchase goods and services that are enforceable and legally binding and includes obligations for inventory, capital expenditures and other operating expense commitments.
The table above excludes future payments for operating leases, long-term debt, and our defined benefit plan. The minimum future payments for operating leases are disclosed in Note 2. Operating Leases and future maturities of long-term debt are disclosed in Note 5. Financing Arrangements. The expected future benefit payments for our Retirement Income Plan through 2032 are disclosed in Note 10. Employee Benefits.
63
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Critical Accounting Estimates
Management estimates and judgments are an integral part of financial statements prepared in accordance with GAAP. We believe that the critical accounting policies described in this section address the more significant estimates required of management when preparing the Consolidated Financial Statements in accordance with GAAP. We consider an accounting estimate critical if changes in the estimate may have a material impact on our financial condition or results of operations. We believe that the accounting estimates employed are appropriate and resulting balances are reasonable; however, actual results could differ from the original estimates, requiring adjustment to these balances in future periods.
We believe the followings represent our critical accounting policies and estimates used in the preparation of our consolidated financial statements:
•Revenue recognition - We recognize revenue from product sales when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of the goods to customers and/or when services are rendered. Our payment terms are typically between 30 to 120 days. Provisions for certain rebates, sales incentives, volume discounts, contractual pricing allowances and product returns are accounted for as variable consideration and recorded as a reduction in sales.
Product discounts, including certain rebates, sales incentives, and volume discounts are granted based on terms of the arrangement with direct distribution customers and at times the indirect end consumer. We evaluate contractual terms, historical experience, and perform internal analysis to estimate total product discounts at the time revenue is recognized. CooperSurgical rebates are predominately related to the Medicaid rebate provision that is estimated based upon contractual terms, historical experience, and trend analysis.
Sales returns are estimated and recorded based on historical sales return data. Promotional programs, such as cooperative advertising arrangements, are recorded in the same period as related sales. Reasonably likely changes to assumptions used to calculate the accruals for rebates, sales incentives, volume discounts, contractual pricing allowances and product returns are not anticipated to have a material effect on the financial statements. We currently disclose the impact of changes to assumptions in the quarterly or annual filing in which there is a material financial statement impact.
•Business combinations - We routinely consummate business combinations. Results of operations for acquired companies are included in our consolidated results of operations from the date of acquisition. We recognize separately from goodwill, the identifiable assets acquired, including acquired in-process research and development, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date fair values as defined by accounting standards related to fair value measurements. Key assumptions routinely utilized the allocation of purchase price to intangible assets include discount rates, and projected financial information such as revenue projections for companies acquired. As of the acquisition date, goodwill is measured as the excess of consideration given, over the net of the acquisition date fair values of the identifiable assets acquired and the liabilities assumed. Direct acquisition costs are expensed as incurred.
•Income taxes - Income taxes are estimated based on enacted income tax laws and the results of operations in each jurisdiction. Deferred tax assets and liabilities are estimated based on temporary differences between the financial reporting basis and income tax basis of assets and liabilities. Deferred tax assets are reduced by a valuation allowance to the extent it is more likely than not they are not expected to be realized. Long-term tax payable is estimated income tax to be paid for unrecognized tax benefits. A tax benefit is recognized if it is more likely than not a tax position will be sustained based on its technical merits in a tax authority examination, based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority.
Accounting Pronouncements
Information regarding new accounting pronouncements is included in Note 1. Organization and Significant Accounting Policies.
64
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 7A. Quantitative and Qualitative Disclosure About Market Risk.
We are exposed to market risks that relate principally to changes in interest rates and foreign currency fluctuations. We do not enter into derivative financial instrument transactions for speculative purposes.
Foreign Currency Exchange Risk
We operate multiple foreign subsidiaries that manufacture and market our products worldwide. As a result, our earnings, cash flow and financial position are exposed to foreign currency risk from foreign currency denominated receivables and payables, sales transactions, capital expenditures and net investment in certain foreign operations. Most of our operations outside the United States have their local currency as their functional currency. We are exposed to risks caused by changes in foreign exchange, principally our British pound sterling, euro and Japanese yen denominated debt and receivables denominated in currencies other than the United States dollar, and from operations in other foreign currencies. We did not have any cross-currency swaps or foreign currency forward contracts as of October 31, 2022. At October 31, 2022, a uniform hypothetical 5% increase or decrease in the foreign currency exchange rates in comparison to the United States dollar would have resulted in a corresponding increase or decrease of approximately $35.0 million in operating income for the fiscal year ended October 31, 2022. For additional information, see Item 1A. Risk Factors - "Our substantial and expanding international operations are subject to uncertainties which could affect our operating results." and Note 1. Organization and Significant Accounting Policies for additional information.
Interest Rate Risk
We are exposed to risks associated with changes in interest rates, as the interest rates on our revolving lines of credit and term loans may vary with the federal funds rate and LIBOR. As of October 31, 2022, we had outstanding debt for an aggregate carrying amount of $2.7 billion. We have entered, and in the future may enter, into interest rate swaps to manage interest rate risk.
Our ultimate realized gain or loss with respect to interest rate fluctuations will depend on interest rates, the exposures that arise during the period and our hedging strategies at that time. If interest rates were to increase or decrease by 1% or 100 basis points, annual interest expense would increase or decrease by approximately $4.6 million based on average debt outstanding, after consideration of our interest rate swap contracts, during the fourth quarter of fiscal 2022. For further information about our debt, see Item 1A. Risk Factors - "We are vulnerable to interest rate risk with respect to our debt.", and Note 5. Financing Arrangements for additional information.
65
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 8. Financial Statements and Supplementary Data.
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors
The Cooper Companies, Inc.:
Opinions on the Consolidated Financial Statements and Internal Control Over Financial Reporting
We have audited the accompanying consolidated balance sheets of The Cooper Companies, Inc. and subsidiaries (the Company) as of October 31, 2022 and 2021, the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the years in the three-year period ended October 31, 2022, and the related notes and financial statement Schedule II (collectively, the consolidated financial statements). We also have audited the Company’s internal control over financial reporting as of October 31, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of October 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the three-year period ended October 31, 2022, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of October 31, 2022 based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
The Cooper Companies, Inc., acquired Generate Life Sciences (Generate) on December 17, 2021, and management excluded from its assessment of the effectiveness of the Company’s internal control over financial reporting as of October 31, 2022, Generate’s internal control over financial reporting associated with total assets of $2.1 billion and total revenues of $249.5 million included in the consolidated financial statements of the Company as of and for the year ended October 31, 2022. Our audit of internal control over financial reporting of the Company also excluded an evaluation of the internal control over financial reporting of Generate.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s consolidated financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and
66
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Fair value of acquired customer relationships intangible asset
As discussed in Notes 1 and 3 to the consolidated financial statements, the Company consummated the acquisition of Generate Life Sciences (Generate) for $1.663 billion during the year ended October 31, 2022. The acquisition-date fair value of Generate’s customer relationships intangible assets was $718.3 million, which included a customer relationships intangible asset related to newborn stem cell storage contracts (stem cell customer relationships intangible asset).
We identified the evaluation of the acquisition-date fair value of the stem cell customer relationships intangible asset in the acquisition of Generate as a critical audit matter. We performed sensitivity analyses to determine the key assumptions used to value the stem cell customer relationships intangible asset which required challenging auditor judgment. The fair value of the acquired intangible assets were sensitive to possible changes in the forecasted revenue and discount rate assumptions, requiring a high degree of auditor judgment and the assistance of valuation professionals with specialized skills and knowledge.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls related to the Company’s acquisition-date valuation process, including controls over the development of the key assumptions identified above. We evaluated forecasted revenue by comparing it to the historical performance of peer companies, the Company and the acquired business. We also assessed the Company’s ability to accurately forecast by comparing forecasted revenue of the acquired business to actual results since the acquisition date. We involved valuation professionals with specialized skills and knowledge, who assisted in (1) evaluating the discount rate by comparing it against a discount rate range that was independently developed based on publicly available market data for comparable entities, and (2) developing a fair value estimate of the stem cell customer relationships intangible asset using the Company’s cash flow projections and independently developed range of discount rates and comparing it to the Company’s estimate.
/s/ KPMG LLP
We have served as the Company’s auditor since 1982.
San Francisco, California
December 9, 2022
67
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Consolidated Statements of Income
Years Ended October 31, (In millions, except for earnings per share) | 2022 | 2021 | 2020 | ||||||||||||||
| Net sales | $ | 3,308.4 | $ | 2,922.5 | $ | 2,430.9 | |||||||||||
| Cost of sales | 1,168.8 | 966.7 | 896.1 | ||||||||||||||
| Gross profit | 2,139.6 | 1,955.8 | 1,534.8 | ||||||||||||||
| Selling, general and administrative expense | 1,342.2 | 1,211.2 | 992.5 | ||||||||||||||
| Research and development expense | 110.3 | 92.7 | 93.3 | ||||||||||||||
| Amortization of intangibles | 179.5 | 146.1 | 137.2 | ||||||||||||||
| Operating income | 507.6 | 505.8 | 311.8 | ||||||||||||||
| Interest expense | 57.3 | 23.1 | 36.8 | ||||||||||||||
| Other (income) expense, net | (25.0) | (8.8) | 8.5 | ||||||||||||||
| Income before income taxes | 475.3 | 491.5 | 266.5 | ||||||||||||||
| Provision for income taxes (Note 6) | 89.5 | (2,453.2) | 28.1 | ||||||||||||||
| Net income | $ | 385.8 | $ | 2,944.7 | $ | 238.4 | |||||||||||
| Earnings per share (Note 7) | |||||||||||||||||
| Basic | $ | 7.83 | $ | 59.80 | $ | 4.85 | |||||||||||
| Diluted | $ | 7.76 | $ | 59.16 | $ | 4.81 | |||||||||||
| Number of shares used to compute earnings per share: | |||||||||||||||||
| Basic | 49.3 | 49.2 | 49.1 | ||||||||||||||
| Diluted | 49.7 | 49.8 | 49.6 | ||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
68
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Net income | $ | 385.8 | $ | 2,944.7 | $ | 238.4 | |||||||||||
| Other comprehensive (loss) income: | |||||||||||||||||
Cash flow hedges, net of tax of $26.1, $8.2 and $(4.1), respectively | 81.3 | 26.1 | (13.0) | ||||||||||||||
Change in minimum pension liability, net of tax of $8.7, $7.2 and $(4.0), respectively | 27.9 | 22.6 | (12.8) | ||||||||||||||
| Foreign currency translation adjustment | (234.7) | 82.0 | 0.9 | ||||||||||||||
| Other comprehensive (loss) income | (125.5) | 130.7 | (24.9) | ||||||||||||||
| Comprehensive income | $ | 260.3 | $ | 3,075.4 | $ | 213.5 | |||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
69
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
October 31, (In millions) | 2022 | 2021 | |||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | 138.2 | $ | 95.9 | |||||||
Trade accounts receivable, net of allowance for credit losses of $20.7 at October 31, 2022 and $9.2 at October 31, 2021 | 557.8 | 515.3 | |||||||||
| Inventories (Note 1) | 628.7 | 585.6 | |||||||||
| Prepaid expense and other current assets | 208.9 | 179.3 | |||||||||
| Assets held-for-sale | — | 89.2 | |||||||||
| Total current assets | 1,533.6 | 1,465.3 | |||||||||
| Property, plant and equipment, net | 1,432.9 | 1,347.6 | |||||||||
| Goodwill (Note 4) | 3,609.7 | 2,574.0 | |||||||||
| Other intangibles, net (Note 4) | 1,885.1 | 1,271.5 | |||||||||
| Deferred tax assets | 2,443.1 | 2,546.6 | |||||||||
| Other assets | 587.9 | 401.2 | |||||||||
| Total assets | $ | 11,492.3 | $ | 9,606.2 | |||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities: | |||||||||||
| Short-term debt (Note 5) | $ | 412.6 | $ | 83.4 | |||||||
| Accounts payable | 248.8 | 161.4 | |||||||||
| Employee compensation and benefits | 152.1 | 148.7 | |||||||||
| Deferred revenue | 93.6 | 19.0 | |||||||||
| Other current liabilities | 373.1 | 317.9 | |||||||||
| Liabilities held-for-sale | — | 1.7 | |||||||||
| Total current liabilities | 1,280.2 | 732.1 | |||||||||
| Long-term debt (Note 5) | $ | 2,350.8 | $ | 1,397.6 | |||||||
| Deferred tax liabilities | 149.9 | 24.1 | |||||||||
| Long-term tax payable | 113.2 | 139.6 | |||||||||
| Deferred revenue | 198.3 | $ | 0.1 | ||||||||
| Accrued pension liability and other | 225.2 | 370.7 | |||||||||
| Total liabilities | $ | 4,317.6 | $ | 2,664.2 | |||||||
| Contingencies (Note 12) | |||||||||||
| Stockholders’ equity: | |||||||||||
Preferred stock, $10 cents par value, 1.0 shares authorized, zero shares issued or outstanding | — | — | |||||||||
Common stock, $10 cents par value, 120.0 shares authorized, 53.8 issued and 49.3 outstanding at October 31, 2022 and 53.7 issued and 49.3 outstanding at October 31, 2021 | 5.4 | 5.4 | |||||||||
| Additional paid-in capital | 1,765.5 | 1,715.2 | |||||||||
| Accumulated other comprehensive loss | (466.8) | (341.3) | |||||||||
| Retained earnings | 6,584.9 | 6,202.1 | |||||||||
Treasury stock at cost: 4.5 shares at October 31, 2022 and 4.4 shares at October 31, 2021 | (714.5) | (639.6) | |||||||||
| Total Cooper stockholders' equity | 7,174.5 | 6,941.8 | |||||||||
| Noncontrolling interests | 0.2 | 0.2 | |||||||||
| Stockholders’ equity (Note 8) | 7,174.7 | 6,942.0 | |||||||||
| Total liabilities and stockholders’ equity | $ | 11,492.3 | $ | 9,606.2 | |||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
70
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders' Equity
| Common Shares | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Income (Loss) | Retained Earnings | Treasury Stock | Noncontrolling Interests | Total Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| (In millions) | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at October 31, 2019 | 49.1 | $ | 4.9 | 4.1 | $ | 0.4 | $ | 1,615.0 | $ | (447.1) | $ | 3,026.4 | $ | (571.2) | $ | 0.2 | $ | 3,628.6 | |||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 238.4 | — | — | 238.4 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | — | — | — | — | — | (24.9) | — | — | — | (24.9) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for stock plans, net and employee stock purchase plan | 0.2 | — | — | — | (5.0) | — | — | 1.7 | — | (3.3) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Treasury stock repurchase | (0.2) | — | 0.2 | — | — | — | — | (47.8) | — | (47.8) | |||||||||||||||||||||||||||||||||||||||||||||||||
Dividends on common stock ($0.03 per share) | — | — | — | — | — | — | (3.0) | — | — | (3.0) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | 36.8 | — | — | — | — | 36.8 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at October 31, 2020 | 49.1 | $ | 4.9 | 4.3 | $ | 0.4 | $ | 1,646.8 | $ | (472.0) | $ | 3,261.8 | $ | (617.3) | $ | 0.2 | $ | 3,824.8 | |||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 2,944.7 | — | — | 2,944.7 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — | — | — | 130.7 | — | — | — | 130.7 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for stock plans, net and employee stock purchase plan | 0.3 | 0.1 | — | — | 24.6 | — | — | 2.5 | $ | — | 27.2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Treasury stock repurchase | (0.1) | — | 0.1 | — | — | — | — | (24.8) | — | (24.8) | |||||||||||||||||||||||||||||||||||||||||||||||||
Dividends on common stock ($0.03 per share) | — | — | — | — | — | — | (3.0) | — | — | (3.0) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | 43.8 | — | — | — | — | 43.8 | |||||||||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | — | — | (1.4) | — | — | (1.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at October 31, 2021 | 49.3 | $ | 5.0 | 4.4 | $ | 0.4 | $ | 1,715.2 | $ | (341.3) | $ | 6,202.1 | $ | (639.6) | $ | 0.2 | $ | 6,942.0 | |||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 385.8 | — | — | 385.8 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — | — | — | (125.5) | — | — | — | (125.5) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for stock plans, net and employee stock purchase plan | 0.1 | — | — | — | (2.1) | — | — | 3.6 | — | 1.5 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Treasury stock repurchase | (0.1) | — | 0.1 | — | — | — | — | (78.5) | — | (78.5) | |||||||||||||||||||||||||||||||||||||||||||||||||
Dividends on common stock ($0.03 per share) | — | — | — | — | — | — | (3.0) | — | — | (3.0) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | 52.4 | — | — | — | — | 52.4 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at October 31, 2022 | 49.3 | $ | 5.0 | 4.5 | $ | 0.4 | $ | 1,765.5 | $ | (466.8) | $ | 6,584.9 | $ | (714.5) | $ | 0.2 | $ | 7,174.7 | |||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
71
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Cash flows from operating activities: | |||||||||||||||||
| Net income | $ | 385.8 | $ | 2,944.7 | $ | 238.4 | |||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||||||||
| Depreciation and amortization | 346.1 | 309.3 | 287.1 | ||||||||||||||
| Impairment of intangibles | 2.3 | — | — | ||||||||||||||
| Share-based compensation expense | 54.2 | 43.8 | 37.6 | ||||||||||||||
| Non-cash operating lease expense | 32.2 | 31.8 | 32.5 | ||||||||||||||
| Impairment and loss on disposal of property, plant and equipment, and other | 2.2 | (5.0) | 17.7 | ||||||||||||||
| Change in fair value of contingent consideration | (10.3) | 66.1 | — | ||||||||||||||
| Deferred income taxes | 53.9 | (2,502.2) | (0.9) | ||||||||||||||
| Change in assets and liabilities: | |||||||||||||||||
| Accounts receivable | (33.8) | (75.5) | 8.5 | ||||||||||||||
| Inventories | (40.4) | (9.2) | (62.3) | ||||||||||||||
| Other assets | (16.9) | (69.1) | (41.1) | ||||||||||||||
| Operating lease right-of-use assets and liabilities, net | (51.3) | (27.5) | (20.0) | ||||||||||||||
| Accounts payable | 49.9 | (16.0) | 23.2 | ||||||||||||||
| Accrued liabilities | 32.4 | 59.1 | (9.3) | ||||||||||||||
| Accrued income taxes | (27.4) | 10.0 | (12.4) | ||||||||||||||
| Other long-term liabilities | (34.2) | (21.7) | (12.4) | ||||||||||||||
| Settlement of contingent consideration | (52.3) | — | — | ||||||||||||||
| Net cash provided by operating activities | 692.4 | 738.6 | 486.6 | ||||||||||||||
| Cash flows from investing activities: | |||||||||||||||||
| Purchases of property, plant and equipment | (242.0) | (214.4) | (310.4) | ||||||||||||||
| Acquisitions of businesses and assets, net of cash acquired | (1,641.3) | (235.9) | (54.1) | ||||||||||||||
| Proceeds from sale of interest in a subsidiary | 52.1 | — | — | ||||||||||||||
| Net cash used in investing activities | (1,831.2) | (450.3) | (364.5) | ||||||||||||||
| Cash flows from financing activities: | |||||||||||||||||
| Proceeds from long-term debt, net of issuance costs | 1,511.0 | 1,427.4 | 3,199.8 | ||||||||||||||
| Repayments of long-term debt | (561.5) | (1,416.0) | (3,235.9) | ||||||||||||||
| Net proceeds from (repayments of) short-term debt, other | 329.3 | (321.3) | (4.5) | ||||||||||||||
| Repurchase of common stock | (78.5) | (24.8) | (47.8) | ||||||||||||||
| Proceeds related to share-based compensation awards | 8.9 | 33.7 | 13.5 | ||||||||||||||
| Payments related to share-based compensation awards | (16.8) | (13.2) | (20.3) | ||||||||||||||
| Dividends on common stock | (3.0) | (3.0) | (3.0) | ||||||||||||||
| Issuance of common stock for employee stock purchase plan | 7.2 | 5.8 | 2.7 | ||||||||||||||
| Settlement of contingent consideration | (2.9) | — | — | ||||||||||||||
| Net cash provided by (used in) financing activities | 1,193.7 | (311.4) | (95.5) | ||||||||||||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (12.9) | 2.9 | 0.7 | ||||||||||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash | 42.0 | (20.2) | 27.3 | ||||||||||||||
| Cash, cash equivalents, restricted cash and cash held for sale at beginning of year | 96.6 | 116.8 | 89.5 | ||||||||||||||
| Cash, cash equivalents and restricted cash at end of year | $ | 138.6 | $ | 96.6 | $ | 116.8 | |||||||||||
| Supplemental disclosures of cash flow information: | |||||||||||||||||
| Cash paid for: | |||||||||||||||||
| Interest | $ | 49.1 | $ | 28.4 | $ | 46.5 | |||||||||||
72
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Income taxes | $ | 66.6 | $ | 63.2 | $ | 51.1 | |||||||||||
| Operating lease liabilities | $ | 45.3 | $ | 37.4 | $ | 40.6 | |||||||||||
| Operating lease ROU assets obtained in exchange for lease obligations | $ | 29.8 | $ | 26.5 | $ | 17.7 | |||||||||||
| Reconciliation of cash flow information: | |||||||||||||||||
| Cash and cash equivalents | $ | 138.2 | $ | 95.9 | $ | 115.9 | |||||||||||
| Restricted cash included in other current assets | 0.4 | 0.4 | 0.9 | ||||||||||||||
| Cash held for sale | — | 0.3 | — | ||||||||||||||
| Total cash, cash equivalents, restricted cash and cash held for sale | $ | 138.6 | $ | 96.6 | $ | 116.8 | |||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
73
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 1. Organization and Significant Accounting Policies
Organization
The Cooper Companies, Inc. (Cooper, we or the Company) is a global medical device company publicly traded on the NYSE (NYSE:COO). Cooper operates through two business units, CooperVision and CooperSurgical.
•CooperVision primarily develops, manufactures and markets a broad range of soft contact lenses for the worldwide vision correction market.
•CooperSurgical primarily develops, manufactures, markets medical devices and procedures solutions, and provides services to improve health care delivery to women, babies and families.
Principles of Consolidation
The financial statements in this report include the results of all of Cooper's consolidated entities. All significant intercompany transactions and balances are eliminated on consolidation.
Use of Estimates
The preparation of Consolidated Financial Statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of net sales and expenses during the reporting period. Actual results could differ from those estimates.
In particular, the COVID-19 pandemic negatively impacted business and healthcare activity globally. As a result of healthcare systems responding to the demands of managing the pandemic, governments around the world imposing measures designed to reduce the transmission of the COVID-19 virus, and individuals responding to the concerns of contracting the COVID-19 virus, many optical practitioners and retailers, hospitals, medical offices and fertility clinics closed their facilities, restricted access, or delayed or canceled patient visits, exams and elective medical procedures, and many customers that have reopened are experiencing reduced patient visits. These factors have had, and in the future may have, an adverse effect on our sales, operating results and cash flows.
The full extent to which the pandemic will directly or indirectly impact the Company's business, results of operations, and financial condition, including sales, expenses, manufacturing, clinical trials, research and development costs, reserves and allowances, fair value measurements, asset impairment charges, contingent consideration obligations, and the effectiveness of the Company's hedging instruments, will depend on future developments that are highly uncertain and difficult to predict. These developments include, but are not limited to, the duration and spread of the outbreak (including new and more contagious variants of COVID-19), its severity, the actions to contain the virus or address its impact, the timing, distribution, public acceptance and efficacy of vaccines and other treatments, United States and foreign government actions to respond to the reduction in global economic activity, and how quickly and to what extent normal economic and operating conditions can resume. There was not a material impact to the estimates in the Company’s Consolidated Financial Statements for fiscal 2022.
The Company continually monitors and evaluates the estimates used as additional information becomes available. Adjustments will be made to these provisions periodically to reflect new facts and circumstances that may indicate that historical experience may not be indicative of current and/or future results. The Company’s future assessment of the magnitude and duration of COVID-19, as well as other factors, could result in material changes to the estimates and material impacts to the Company’s Consolidated Financial Statements in future reporting periods.
Revenue recognition
Net Sales
The Company sells its products principally to a limited number of distributors, group purchasing organizations, eye care or health care professionals including independent practices, corporate retailers, hospitals and clinics or authorized resellers (collectively, its Customers). These Customers may subsequently resell the Company’s products to eye care or health care providers and patients. In addition to product supply and distribution agreements with Customers, the Company enters into arrangements with health care providers and payors that provide for government-mandated and/or privately negotiated rebates, chargebacks and discounts with respect to the purchase of the Company’s products. The Company considers purchase orders, which in some cases are governed by master sales agreements, to be contracts with a customer. As part of its consideration of the contract, the Company evaluates certain factors including the customer’s ability to pay (or credit risk). For each contract, the Company considers the promise to transfer products, each of which is distinct, to be the identified performance obligations.
74
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Revenues from product sales are recognized when the Customer obtains control of the Company’s product, which occurs at a point in time, typically upon shipment or delivery to the Customer. Taxes collected from Customers relating to product sales and remitted to governmental authorities are excluded from revenues. The Company expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that the Company would have recognized is one year or less. See Note 12. Business Segment Information for disaggregation of revenue.
Reserves for Variable Consideration
Revenues from product sales are recorded at the net sales price (transaction price), which includes estimates of variable consideration for which reserves are established and which result from discounts, returns, chargebacks, rebates and other allowances that are offered within contracts between the Company and its Customers, health care providers, payors and other indirect customers relating to the Company’s sales of its products. These reserves are based on the amounts earned or to be claimed on the related sales and are classified primarily in current liabilities. Variable consideration is estimated based on the most likely amount or expected value approach, depending on which method the Company expects to better predict the amount of consideration to which it will be entitled. Once the Company elects one of the methods to estimate variable consideration for a particular type of performance obligation, the Company applies that method consistently.
Where appropriate, these estimates take into consideration a range of possible outcomes which are probability-weighted for relevant factors such as the Company’s historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, these reserves reflect the Company’s best estimates of the amount of consideration to which it is entitled based on the terms of the contract.
Trade Discounts and Allowances
The Company generally provides Customers with discounts, which include incentive fees that are stated in the Company’s contracts and are recorded as a reduction of revenue in the period the related product revenue is recognized.
Product Returns
Consistent with industry practice, the Company generally offers Customers a limited right of return for a product that has been purchased from the Company. The Company estimates the amount of its product sales that may be returned by its Customers and records this estimate as a reduction of revenue in the period the related product revenue is recognized. Historically, returns have been infrequent and insignificant relative to our total sales. Our refund liability for product returns was $14.5 million and $13.7 million at October 31, 2022 and 2021, respectively, which is included in Accrued Liabilities on our Consolidated Balance Sheets and represents the expected value of the aggregate refunds that will be due to our customers.
Rebates and Chargebacks
Rebates are estimated based on contractual terms, historical experience, customer mix, trend analysis and projected market conditions in the various markets served.
Chargebacks for fees and discounts to providers represent the estimated obligations resulting from contractual commitments to sell products to qualified healthcare providers at prices lower than the list wholesale prices charged to the Company’s direct customers. For certain office and surgical products in CooperSurgical, customers charge the Company for the difference between what they pay for the product and the ultimate selling price to the qualified healthcare providers. These reserves are established in the same period that the related revenue is recognized, resulting in a reduction of product revenue. Chargeback amounts are generally determined at the time of resale to the qualified healthcare provider by customers. CooperSurgical rebates are predominately related to the Medicaid rebate provision that is estimated based upon contractual terms, historical experience, and trend analysis.
Contract Liabilities
Deferred revenue primarily represents prepaid stem cell storage as part of the CooperSurgical business unit. Revenue related to stem cell storage is recognized over the service period, which can range from one year to the lifetime of a customer.
Share-Based Compensation
We grant various share-based compensation awards, including stock options, performance unit shares, restricted stock and restricted stock units. The Company accounts for share-based compensation expense based on estimated grant-date fair value, and expenses the amount over the vesting period of the award. Determining the fair value of share-based awards at the grant date requires judgment, including estimating Cooper's stock price volatility, employee exercise behaviors and related employee forfeiture rates.
75
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The expected life of the share-based awards is based on the observed and expected time to post-vesting forfeiture and/or exercise. Groups of employees that have similar historical exercise behavior are considered separately for valuation purposes. In determining the expected volatility, management considers implied volatility from publicly-traded options on Cooper's common stock at the date of grant, historical volatility and other factors. The risk-free interest rate is based on the continuous rates provided by the United States Treasury with a term equal to the expected life of the award. The dividend yield is based on the projected annual dividend payment per share, divided by the stock price at the date of grant.
Forfeitures are estimated at the time of grant, based on historical experience, and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Foreign Currency Translation
Most of our operations outside the United States use their local currency as their functional currency. We translate these assets and liabilities into United States dollars at year-end exchange rates. We translate income and expense accounts at average rates for each month. We record gains and losses from the translation of financial statements in foreign currencies into United States dollars in other comprehensive income. We record gains and losses from changes in exchange rates on transactions denominated in currencies other than each reporting location's functional currency in net income for each period. We recorded in other expense and income a net foreign exchange loss of $22.0 million for fiscal 2022, $5.5 million for fiscal 2021 and $1.2 million for fiscal 2020.
Financial Derivatives and Hedging
Derivatives are recorded on the Consolidation Balance Sheets at fair value. Accounting for gains or losses resulting from changes in the values of those derivatives depends on the use of the derivative instrument and whether it qualifies for hedge accounting.
The gain or loss on derivative instruments designated and qualifying for cash flow hedge accounting is deferred in other comprehensive income. The changes in fair value for all trades that are not designated for hedge accounting are recognized in current period earnings. Deferred gains or losses from designated cash flow hedges are reclassified into earnings in the period that the hedged interest expense affects earnings. The effectiveness of cash flow hedges is assessed at inception and quarterly thereafter. The Company does not offset fair value amounts recognized for derivative instruments in its Consolidated Balance Sheets for presentation purposes.
Fair Value Measurements
Accounting standards define fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy prioritizes the inputs to valuation techniques used to measure fair value. An asset’s or liability’s level is based on the lowest level of input that is significant to the fair value measurement. Assets and liabilities carried at fair value are valued and disclosed in one of the following three levels of the valuation hierarchy:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market-based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs reflecting the reporting entity’s own assumptions.
The carrying value of cash and cash equivalents, accounts receivable, prepaid expense and other current assets, lines of credit, accounts payable and other current liabilities approximate fair value due to the short-term nature of such instruments and the ability to obtain financing on similar terms.
The carrying value of the Company's revolving credit facility and term loans approximates fair value based on current market rates (Level 2).
The fair value of the Company's interest rate swap contracts is measured on a recurring basis by netting the discounted future fixed cash payments and the discounted expected variable cash receipts. The variable cash receipts are based on the expectation of future interest rates (forward curves) derived from observable market interest rate curves. The interest rate swap contracts were categorized as Level 2 in the fair value hierarchy, as the inputs to the derivative pricing model are generally observable and do not contain a high level of subjectivity. The gain or loss on the derivatives is recorded as a component of accumulated other comprehensive income and subsequently reclassified into interest expense in the same period during which the hedged transaction affects earnings. Refer to Note 13. Financial Derivatives and Hedging for further information.
The Company uses fair value measures when determining assets and liabilities acquired in an acquisition, which are considered a Level 3 measurement. The fair value of the Company's contingent consideration for which a liability is recorded and the initial measurement of the joint venture interest are a Level 3 measurement, and the change in fair value is recognized in selling,
76
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
general and administrative expense in the Consolidated Statements of Income. Refer to Note 3. Acquisitions and Joint Venture for further information.
Income taxes
Income taxes are estimated based on enacted income tax laws and the results of operations in each jurisdiction. Deferred tax assets and liabilities are estimated based on temporary differences between the financial reporting basis and income tax basis of assets and liabilities. Deferred tax assets are also estimated based on net operating loss and tax credit carryforwards. Deferred tax assets are reduced by a valuation allowance to the extent it is more likely than not they are not expected to be realized. Adjustments to deferred tax assets and liabilities due to changes in tax laws, changes in jurisdiction from intra-group transfers of assets, and changes in judgment regarding a valuation allowance are recognized in provision for income taxes in the quarter in which such changes occur. Long-term tax payable is estimated income tax to be paid for unrecognized tax benefits. A tax benefit is recognized if it is more likely than not a tax position will be sustained based on its technical merits in a tax authority examination, based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority. Adjustments to unrecognized tax benefits due to changes in judgment are recognized in provision for income taxes in the quarter in which such changes occur. Interest and penalties related to unrecognized tax benefits are recognized in provision for income taxes.
Earnings Per Share
We determine basic earnings per share (EPS) by using the weighted average number of shares outstanding. We determine diluted EPS by increasing the weighted average number of shares outstanding in the denominator by the number of outstanding dilutive equity awards using the treasury stock method.
Cash and Cash Equivalents
The Company considers all short-term, highly liquid investments purchased with maturities of three months or less to be cash equivalents.
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is computed using standard cost that approximates actual cost, on a first-in, first-out basis.
October 31, (In millions) | 2022 | 2021 | |||||||||
| Raw materials | $ | 173.7 | $ | 137.7 | |||||||
| Work-in-process | 15.2 | 14.0 | |||||||||
| Finished goods | 439.8 | 433.9 | |||||||||
| $ | 628.7 | $ | 585.6 | ||||||||
In assessing the value of inventories, we make estimates and judgments regarding aging of inventories and other relevant issues potentially affecting the salable condition of products and estimated prices at which those products will sell. On an ongoing basis, we review the carrying value of our inventory, measuring number of months on hand and other indications of salability. We reduce the value of inventory if there are indications that the carrying value is greater than net realizable value, resulting in a new, lower-cost basis for that inventory. Subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis. While estimates are involved, historically, obsolescence has not been a significant factor due to long product dating and lengthy product life cycles.
Property, Plant and Equipment
We record property, plant, and equipment at cost. We compute depreciation expense using the straight-line method over the estimated useful lives of the assets. Useful lives are generally 3 to 15 years except for buildings which are depreciated over 30 to 40 years and leasehold improvements, which we amortize over the shorter of the useful life or the lease term. We charge maintenance and repairs to expense as we incur them.
77
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
October 31, (In millions) | 2022 | 2021 | |||||||||
| Land and improvements | $ | 18.7 | $ | 20.3 | |||||||
| Buildings and improvements | 415.6 | 388.0 | |||||||||
| Machinery and equipment | 1,973.6 | 1,863.6 | |||||||||
| Construction in progress | 393.0 | 383.8 | |||||||||
| Property, plant and equipment, at cost | $ | 2,800.9 | $ | 2,655.7 | |||||||
| Less: Accumulated depreciation | 1,387.2 | 1,308.1 | |||||||||
| Property, plant and equipment, net | $ | 1,413.7 | $ | 1,347.6 | |||||||
| Finance lease ROU assets, net | 19.2 | — | |||||||||
| $ | 1,432.9 | $ | 1,347.6 | ||||||||
Leases
We consider an arrangement a lease if the arrangement transfers the right to control the use of an identified asset in exchange for consideration. We have operating leases, but do not have material financing leases. The Company primarily has operating leases for office, manufacturing and warehouse space, vehicles, and office equipment.
Lease right-of-use assets represent the right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make payments arising from the lease agreement. These assets and liabilities are recognized at the commencement of the lease based upon the present value of the future minimum lease payments over the lease term. The lease term reflects the noncancellable period of the lease together with periods covered by an option to extend or terminate the lease when management is reasonably certain that it will exercise such option. Changes in the lease term assumption could impact the right-of-use assets and lease liabilities recognized on the Consolidated Balance Sheets. As our leases typically do not contain a readily determinable implicit rate, we determine the present value of the lease liability using our incremental borrowing rate at the lease commencement date based on the lease term on a collateralized basis.
The Company’s operating leases typically include non-lease components such as common-area maintenance costs. The Company has elected to include non-lease components with lease payments for the purpose of calculating lease right-of-use assets and liabilities, to the extent that they are fixed. Non-lease components that are not fixed are expensed as incurred as variable lease payments.
Leases with a term of one year or less are not recognized on the Consolidated Balance Sheets, while the associated lease payments are expensed in the Consolidated Statements of Income and Comprehensive Income on a straight-line basis over the lease term.
Operating leases are classified in “Other current liabilities”, “Accrued pension liability and other”, and “Other assets” on our consolidated balance sheets. Operating lease expense is recognized on a straight-line basis over the expected lease term and included in selling, general and administrative expenses in the Consolidated Statements of Income. Financing leases are classified in "Property, plant and equipment", "Short-term debt", and "Long-term debt" on our consolidated balance sheets. See Note 2. Operating Leases and Note 5. Financing Arrangements for further information.
Cloud Computing Arrangements
The Company capitalizes certain costs related to the acquisition and development of internal use software, including implementation costs incurred in a cloud computing arrangement, during the application development stages of projects. Capitalized implementation costs are amortized on a straight-line basis over the expected term of the hosting arrangement, which includes consideration of the non-cancellable contractual term and reasonably certain renewals. Costs incurred during the preliminary project or the post-implementation/operation stages of the project are expensed as incurred. Implementation costs are included in “Other assets” in the Consolidated Balance Sheets. Amortization of capitalized implementation costs is included in the same line item in the Consolidated Statements of Income as the expense for fees for the associated hosting arrangement.
Valuation of goodwill
We evaluate goodwill for impairment annually during the fiscal third quarter and when an event occurs or circumstances change such that it is reasonably possible that impairment may exist. Goodwill is tested for impairment at the reporting unit level by performing a qualitative assessment to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value. We perform a qualitative assessment to test each reporting unit's goodwill for impairment, which
78
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
includes industry and market considerations, overall financial performance and other relevant events and factors affecting each reporting unit. Based on our qualitative assessment, if we determine that the fair value of a reporting unit is more likely than not to be less than its carrying amount, the fair value of a reporting unit will be compared with its carrying amount and an impairment charge will be recognized for the amount that the carrying value exceeds the fair value of the reporting unit.
Long-lived Assets
We review long-lived assets held and used, intangible assets with definite useful lives and assets held for sale for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If an evaluation of recoverability is required, the estimated undiscounted future cash flows associated with the asset group are compared to the asset group's carrying amount to determine if a write-down is required. If the undiscounted cash flows are less than the carrying amount, an impairment loss is recorded to the extent that the carrying amount exceeds the fair value. If management has committed to a plan to dispose of long-lived assets, the assets to be disposed of are reported at the lower of carrying amount or fair value less estimated costs to sell.
Indefinite-lived Intangible Assets
We assess indefinite-lived intangible assets annually in the third quarter of the fiscal year, or whenever events or changes in circumstances indicate that the carrying amount of an indefinite-lived intangible asset (asset group) may not be recoverable. We evaluate whether the indefinite-lived intangible asset is impaired by comparing its carrying value to its fair value. If the carrying value of an indefinite-lived intangible asset is not recoverable, an impairment loss is recognized based on the amount by which the carrying value exceeds the fair value.
Business combinations
We routinely consummate business combinations. Results of operations for acquired companies are included in our consolidated results of operations from the date of acquisition. We recognize separately from goodwill, the identifiable assets acquired, including acquired in-process research and development, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date fair values as defined by accounting standards related to fair value measurements. Key assumptions routinely utilized in allocation of purchase price to intangible assets include discount rates and projected financial information such as revenue projections for companies acquired. As of the acquisition date, goodwill is measured as the excess of consideration given, over the net of the acquisition date fair values of the identifiable assets acquired and the liabilities assumed. Direct acquisition costs are expensed as incurred.
Litigation
We are subject to various legal proceedings, claims, litigation, investigations and contingencies arising out of the ordinary course of business. If we believe the likelihood of an adverse legal outcome is probable and the amount is estimable, we accrue a liability in accordance with accounting guidance for contingencies. We consult with legal counsel on matters related to litigation and seek input both within and outside the Company.
Treasury Stock
We record treasury stock purchases under the cost method whereby the entire cost of the acquired stock is recorded as treasury stock.
Exit costs
During the second quarter of fiscal 2022, the Company initiated a plan to exit its contact lens care business, a non-core business unit of the CooperVision segment. We expect the exit activity to be substantially completed in the first half of fiscal 2023. Exit charges recognized in the three and twelve months ended October 31, 2022, were $9.2 million and $33.2 million, of which $26.7 million is recognized in cost of sales and $6.5 million is recognized in selling, general, and administrative expense in the Consolidated Statements of Income. Exit costs primarily related to inventory write-down, asset impairments and employee-related costs. Total exit costs are expected to be in a range of $30.0 million to $40.0 million.
Accounting Pronouncements Recently Adopted
On November 1, 2021, we prospectively adopted ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers to the business combinations entered into during fiscal 2022. This update requires that an acquirer recognize and measure contract assets and contract liabilities acquired in a business combination in accordance with Topic 606, Revenue from Contracts with Customers.
79
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Accounting Pronouncements Issued Not Yet Adopted
In March 2020, the FASB issued ASU 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting and subsequent amendment to the initial guidance: ASU 2021-01, Reference Rate Reform (Topic 848): Scope (collectively, “Topic 848”). Topic 848 provides optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. The amendments apply only to contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate expected to be discontinued because of reference rate reform. The guidance generally can be applied from March 12, 2020 through December 31, 2022. The Company is currently evaluating the impact of ASU 2020-04 on the Consolidated Condensed Financial Statements.
In November 2021, the FASB issued ASU 2021-10, Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance. This update requires annual disclosures about transactions with a government that are accounted for by applying a grant or contribution accounting model by analogy. This standard is effective for fiscal years beginning after December 15, 2021, and should be applied either prospectively or retrospectively. Early adoption is permitted. The Company is currently evaluating the impact of ASU 2021-10 on the Consolidated Condensed Financial Statements.
No other recently issued accounting pronouncements had or are expected to have a material impact on our Consolidated Financial Statements.
Note 2. Operating Leases
The following table presents information about leases on the Consolidated Balance Sheets:
| October 31, (In millions) | 2022 | 2021 | ||||||||||||
| Operating Leases | ||||||||||||||
| $ | 230.1 | $ | 257.0 | |||||||||||
| 35.5 | 35.7 | |||||||||||||
| 205.5 | 231.7 | |||||||||||||
| Total operating lease liabilities | $ | 241.0 | $ | 267.4 | ||||||||||
| Weighted average remaining lease term (in years) | 9.8 | 10.6 | ||||||||||||
| Weighted average discount rate | 3% | 3% | ||||||||||||
Operating lease expense for the fiscal years ended October 31, 2022, 2021 and 2020 was $45.0 million, $44.1 million and $41.2 million.
Maturity of Lease Liabilities
The minimum rental payments required under operating leases that have initial or remaining noncancellable lease terms in excess of one year as of October 31, 2022 are:
| (In millions) | ||||||||
| 2023 | $ | 42.2 | ||||||
| 2024 | 36.6 | |||||||
| 2025 | 32.8 | |||||||
| 2026 | 30.2 | |||||||
| 2027 | 26.6 | |||||||
| Thereafter | 115.8 | |||||||
| Total lease payments | $ | 284.2 | ||||||
| Less: interest | 43.2 | |||||||
| Present value of lease liabilities | $ | 241.0 | ||||||
80
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 3. Acquisitions and Joint Venture
The following is a summary of the allocation of the total purchase consideration for business and asset acquisitions that the Company completed during fiscal 2022, 2021, and 2020:
| (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Technology | $ | 1.9 | $ | 178.6 | $ | — | |||||||||||
| In-Process Research & Development (IPR&D) | — | 20.0 | — | ||||||||||||||
| Customer relationships | 729.2 | 7.5 | 11.4 | ||||||||||||||
| Trademarks | 55.4 | 1.3 | 5.1 | ||||||||||||||
| Other | — | 0.6 | 3.9 | ||||||||||||||
| Total identifiable intangible assets | $ | 786.5 | $ | 208.0 | $ | 20.4 | |||||||||||
| Goodwill | 1,184.8 | 91.6 | 15.3 | ||||||||||||||
| Net tangible liabilities | (286.5) | (10.8) | (0.3) | ||||||||||||||
| Fair value of contingent consideration | (1.5) | (39.1) | — | ||||||||||||||
| Total closing purchase price | $ | 1,683.3 | $ | 249.7 | $ | 35.4 | |||||||||||
All acquisitions were funded by cash generated from operations or facility borrowings.
For business acquisitions, the Company recorded tangible and intangible assets acquired and liabilities assumed at their fair values as of the applicable date of acquisition. For asset acquisitions, the Company recorded tangible and intangible assets acquired and liabilities assumed at their estimated and relative fair values as of the applicable date of acquisition.
The Company believes these acquisitions strengthen CooperSurgical's and CooperVision's businesses through the addition of new distributors or complementary products and services.
Fiscal Year 2022
On May 31, 2022, CooperVision completed the acquisition of a privately-held Denmark-based contact lens distributor focusing on orthokeratology and scleral contact lenses. This acquisition expands CooperVision's ortho-k eye care portfolio in the Nordic market.
On April 6, 2022, CooperSurgical completed the acquisition of a private cryopreservation services company that specializes in cryogenic services. The purchase price allocation is preliminary, and the Company is in the process of finalizing information primarily related to the effect on taxes and the corresponding impact on goodwill.
Refer to "Fiscal Year 2021" below for details on formation of a joint venture with Essilor International and related activities that occurred in fiscal year 2022 following the acquisition of SightGlass Vision, Inc. (SGV) in fiscal year 2021.
On April 6, 2022, CooperSurgical entered into an asset purchase agreement to acquire Cook Medical's Reproductive Health business, a manufacturer of minimally invasive medical devices focused on the fertility, obstetrics and gynecology markets. The aggregate consideration is $875.0 million in cash, with $675.0 million payable at the closing and the remaining $200.0 million payable in $50.0 million installments following each of the first, second, third and fourth anniversaries of the closing. The transaction is subject to customary closing conditions, such as receipt of required regulatory approvals.
Generate Life Sciences®
On December 17, 2021, CooperSurgical completed the acquisition of 100% of the equity interests in Generate Life Sciences (Generate), a privately held leading provider of donor egg and sperm for fertility treatments, fertility cryopreservation services and newborn stem cell storage (cord blood & cord tissue), and paid an aggregate purchase consideration of approximately $1.663 billion, reflecting working capital, and other adjustments. The cash consideration was funded through a combination of $1.5 billion in proceeds from the issuance of a senior unsecured term loan and available cash on hand.
The Company has accounted for the acquisition of Generate as a business combination, in accordance with ASC Topic 805, Business Combinations. The following table summarizes the preliminary fair values of assets acquired and liabilities assumed as of the acquisition date:
81
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
(In millions) | |||||
| Current assets: | |||||
Cash and cash equivalents | $ | 58.6 | |||
Trade accounts receivable, net | 18.1 | ||||
Inventories | 3.3 | ||||
Prepaid expense and other current assets | 33.5 | ||||
| Total current assets | 113.5 | ||||
Property, plant and equipment | 42.6 | ||||
| Goodwill | 1,177.3 | ||||
| Customer relationships | 718.3 | ||||
| Trademarks | 54.9 | ||||
Other assets | 21.5 | ||||
Total assets acquired | $ | 2,128.1 | |||
| Current liabilities: | |||||
Accounts payable | $ | 12.6 | |||
Employee compensation and benefits | 12.3 | ||||
| Deferred revenue | 68.0 | ||||
Other current liabilities | 12.4 | ||||
| Total current liabilities | 105.3 | ||||
Deferred tax liabilities | 147.3 | ||||
Lease liabilities | 16.6 | ||||
Deferred revenue | 192.2 | ||||
Other long-term liabilities | 3.6 | ||||
Total liabilities assumed | $ | 465.0 | |||
Total purchase price | $ | 1,663.1 | |||
The Company is in the process of finalizing purchase accounting information primarily related to deferred tax adjustments and the corresponding impact on goodwill. The Company recorded measurement period adjustments of $115.3 million to goodwill in fiscal 2022.
Deferred revenue was recognized in accordance with ASC Topic 606, Revenue from Contracts with Customers, as a result of the adoption of ASU 2021-08. See Note 1. Organization and Significant Accounting Policies for additional information.
The Company currently estimates that customer relationships will be amortized over 20 years and trademarks will be amortized over 15 years. Goodwill is primarily attributable to assembled workforce and expected synergies to be achieved. The goodwill recognized is not deductible for tax purposes.
The transaction costs associated with the acquisition consisted primarily of legal, regulatory and financial advisory fees, which were expensed as incurred as selling, general and administrative expense.
Generate's revenue and net income for the period from the acquisition date to October 31, 2022, were $249.5 million and $27.8 million, respectively. The following unaudited pro forma information summarizes the combined results of operations of the Company and Generate as if the acquisition had been completed at the beginning of the Company’s fiscal 2021:
| (In millions) | 2022 | 2021 | |||||||||||||||
| Revenue | $ | 3,344.3 | $ | 3,183.2 | |||||||||||||
| Net income | $ | 370.7 | $ | 2,959.8 | |||||||||||||
The unaudited pro forma information for fiscal 2022 and 2021 was calculated after applying the Company's accounting policies and the impact of acquisition date fair value adjustments. The adjustments primarily include increased amortization for the fair value of acquired intangible assets, increased depreciation for the fair value of acquired property, plant, and equipment,
82
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
increased revenue as a result of the ASU 2021-08 deferred revenue adjustments, decreased interest expense as a result of the reversal of Generate's historical interest expense partially offset by additional interest expense on the debt obtained to finance the transaction.
The pro forma information does not reflect the effect of costs or synergies that would have been expected to result from the integration of the acquisition. The pro forma information does not purport to be indicative of the results of operations that actually would have resulted had the acquisition occurred at the beginning of fiscal 2021, or of future results of the consolidated entities.
Subsequent Event
On November 1, 2022, subsequent to the fiscal year ended October 31, 2022, CooperVision closed an Agreement and Plan of Merger (the “Merger Agreement”) to acquire a U.S. based privately held leading expert in specialty contact lenses for both normal and irregular corneal conditions. The Company is in the process of finalizing purchase accounting information.
Fiscal Year 2021
On May 3, 2021, CooperSurgical completed the acquisition of a privately-held medical device company that develops single-use illuminating medical devices.
On April 26, 2021, CooperVision completed the acquisition of a privately-held UK contact lens manufacturer focusing on specialty contact lenses. This acquisition expands CooperVision’s specialty eye care portfolio and accelerates its development of myopia management solutions in the UK.
On March 1, 2021, CooperSurgical completed the acquisition of a privately-held medical device company that designed and developed an innovative obstetric product for use in urgent obstetrics to reduce risks associated with childbirth.
On February 1, 2021, CooperSurgical acquired all of the remaining equity interests of a privately-held medical device company that developed the Mara® Water Vapor Ablation System, which is used for endometrial ablation. The Company accounted for this acquisition as an asset acquisition, whereby the Company allocated the total cost of the acquisition to the net assets acquired on the basis of their estimated relative fair values on the acquisition date with no goodwill recognized. The primary asset acquired in this asset acquisition is Technology.
On January 19, 2021, CooperVision acquired all of the remaining equity interests of SGV, a privately-held medical device company that developed spectacle lenses for myopia management. The transaction included potential payments of future consideration that were contingent upon the achievement of the regulatory approval milestone (the regulatory approval payment) and the acquired business reaching certain revenue thresholds over a specified period (the revenue payments). The undiscounted range of the contingent consideration was zero to $139.1 million payable to the other former equity interest owners.
The fair value of the regulatory approval payment was determined using an option pricing framework based on the expected payment under the contractual terms and the estimates of the probability of achieving the regulatory approval. The fair value of the revenue payments was determined using a Monte Carlo simulation based on the revenue projections and the expected payment for each simulation.
In March 2022, the entities amended the terms of the contingent consideration, which resulted in CooperVision paying $42.9 million to the former equity interest owners in exchange for the elimination of the revenue payments. CooperVision recognized a net gain of $12.2 million during fiscal 2022. As of October 31, 2022, the remaining contingent liability related to regulatory approval payment was $31.8 million.
In March 2022, CooperVision and Essilor International SAS (Essilor) entered into a Contribution Agreement and a Stock Purchase Agreement under which Essilor paid CooperVision $52.1 million in exchange for a 50% interest in SGV and a proportionate share of certain revenue-based milestone payments related to the January 2021 acquisition of SGV by CooperVision. As part of these agreements, each party contributed their interest in SGV and $10 million in cash to form a new joint venture. CooperVision then remeasured the fair value of its retained equity investment in the joint venture at $90.0 million which resulted in a $56.9 million gain in Other (income) expense on deconsolidation of SGV.
The fair value of the joint venture was determined using the income valuation approach. Under the income approach, we used a discounted cash flow model (“DCF”) in which cash flows anticipated over several periods, plus a terminal value at the end of that time horizon, are discounted to their present value using an appropriate expected rate of return. The discount rate used for cash flows reflects capital market conditions and the specific risks associated with the business. This valuation approaches is considered a Level 3 fair value measurement. Fair value determination requires complex assumptions and judgment by management in projecting future operating results, selecting guideline companies for comparisons, determining appropriate
83
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
market value multiples, selecting the discount rate to measure the risks inherent in the future cash flows. Any material changes in key assumptions, including failure to meet business plans, deterioration in the financial market, an increase in interest rate or an increase in the cost of equity financing by market participants within the industry or other unanticipated events and circumstances, may affect such estimates.
On December 31, 2020, CooperSurgical completed the acquisition of a privately-held in vitro fertilization (IVF) cryostorage software solutions company.
The pro forma results of operations of these acquisitions have not been presented because the effect of the business combinations described above was not material to the consolidated results of operations.
Fiscal Year 2020
On August 7, 2020, CooperVision completed the acquisition of a privately-held U.S contact lens manufacturer focusing on ortho-k lenses. This acquisition expands CooperVision’s specialty eye care portfolio and its leadership in addressing the increasing severity and prevalence of myopia.
On December 13, 2019, CooperSurgical completed the acquisition of a privately-held distributor of IVF medical devices and systems.
The pro forma results of operations of these acquisitions have not been presented because the effect of the business combinations described above was not material to the consolidated results of operations.
Contingent Consideration
Certain of the Company’s business combinations involve potential payments of future consideration that are contingent upon the achievement of regulatory milestones and/or the acquired business reaching certain revenue thresholds. A liability is recorded for the estimated fair value of the contingent consideration on the acquisition date. The fair value of the contingent consideration is remeasured at each reporting period, and the change in fair value is recognized in selling, general and administrative expense in the Consolidated Statements of Income.
The following table provides a reconciliation of the beginning and ending balances of contingent consideration:
| (In millions) | 2022 | 2021 | |||||||||
| Beginning balance | $ | 97.4 | $ | — | |||||||
| Purchase price contingent consideration | 1.5 | 31.3 | |||||||||
| Payments | (55.2) | — | |||||||||
| (10.3) | 66.1 | ||||||||||
| Ending balance | $ | 33.4 | $ | 97.4 | |||||||
Note 4. Intangible Assets
Goodwill
The Company has three reporting units: CooperVision and within the CooperSurgical segment, Office/Surgical and Fertility, reflecting the current way the Company manages its business. There was no impairment of goodwill in its reporting units in fiscal 2022, 2021, and 2020.
| (In millions) | CooperVision | CooperSurgical | Total | ||||||||||||||
| Balance at October 31, 2021 | $ | 1,841.0 | $ | 733.0 | $ | 2,574.0 | |||||||||||
| Net additions | 0.9 | 1,183.9 | 1,184.8 | ||||||||||||||
| Foreign currency translation adjustment | (131.6) | (17.5) | (149.1) | ||||||||||||||
| Balance at October 31, 2022 | $ | 1,710.3 | $ | 1,899.4 | $ | 3,609.7 | |||||||||||
Of the October 31, 2022 goodwill balance, $214.1 million for CooperSurgical and $22.4 million for CooperVision is expected to be deductible for tax purposes. Of the October 31, 2021 goodwill balance, $137.2 million for CooperSurgical and $24.6 million for CooperVision was expected to be deductible for tax purposes.
84
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Other Intangible Assets
| October 31, 2022 | October 31, 2021 | ||||||||||||||||||||||||||||
| (In millions) | Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | Weighted Average Amortization Period (in years) | ||||||||||||||||||||||||
| Intangible assets with definite lives: | |||||||||||||||||||||||||||||
| Trademarks | $ | 209.6 | $ | 62.4 | $ | 156.7 | $ | 49.1 | 15 | ||||||||||||||||||||
| Composite intangible asset | 1,061.9 | 354.0 | 1,061.8 | 283.2 | 15 | ||||||||||||||||||||||||
| Technology | 504.1 | 317.5 | 513.0 | 287.9 | 12 | ||||||||||||||||||||||||
| Customer relationships | 1,092.7 | 287.0 | 378.4 | 240.1 | 19 | ||||||||||||||||||||||||
| License and distribution rights and other | 50.7 | 23.8 | 33.4 | 21.6 | 11 | ||||||||||||||||||||||||
| 2,919.0 | $ | 1,044.7 | 2,143.3 | $ | 881.9 | 16 | |||||||||||||||||||||||
| Less: accumulated amortization and translation | 1,044.7 | 881.9 | |||||||||||||||||||||||||||
| Intangible assets with definite lives, net | $ | 1,874.3 | $ | 1,261.4 | |||||||||||||||||||||||||
Intangible assets with indefinite lives, net (1) | 10.8 | 10.1 | |||||||||||||||||||||||||||
| Total other intangibles, net | $ | 1,885.1 | $ | 1,271.5 | |||||||||||||||||||||||||
(1) Intangible assets with indefinite lives include technology and trademarks.
Balances include foreign currency translation adjustments.
Intangible assets with definite lives are amortized over the estimated useful life of the assets. As of October 31, 2022, the estimate of future amortization expenses for intangible assets with definite lives is as follows:
| Fiscal years: | (In millions) | ||||
| 2023 | $ | 183.2 | |||
| 2024 | 179.2 | ||||
| 2025 | 169.3 | ||||
| 2026 | 162.0 | ||||
| Thereafter | 1,180.6 | ||||
| Total remaining amortization for intangible assets with definite lives | $ | 1,874.3 | |||
The Company considered the impact on its near and long-term forecasts from the general deterioration of economic and market conditions as a result of higher inflation, regional and global conflict, supply chain disruption, and the ongoing disruptions of the COVID-19 pandemic and determined that it was not more likely than not that the fair value of reporting units or relevant asset groups was below carrying amounts. Therefore, the Company determined that there was no impairment to either its definite-lived or indefinite-lived intangible assets during fiscal 2022, 2021 and 2020. There was an immaterial impairment charge related to our exit from the contact lens care business.
85
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 5. Financing Arrangements
The Company had outstanding debt as follows:
October 31, (In millions) | 2022 | 2021 | |||||||||
| Overdraft and other credit facilities | $ | 57.7 | $ | 83.0 | |||||||
| Term loans | 338.0 | — | |||||||||
| Less: unamortized debt issuance cost | — | (0.1) | |||||||||
| Short-term debt, excluding financing leases | 395.7 | 82.9 | |||||||||
| 16.9 | 0.5 | ||||||||||
| Short-term debt | $ | 412.6 | $ | 83.4 | |||||||
| Revolving credit | $ | — | $ | 546.1 | |||||||
| Term loans | 2,350.0 | 850.0 | |||||||||
| Other | 0.2 | 0.2 | |||||||||
| Less: unamortized debt issuance cost | (3.1) | (0.2) | |||||||||
| Long-term debt, excluding financing leases | 2,347.1 | 1,396.1 | |||||||||
| 3.7 | 1.5 | ||||||||||
| Long-term debt | $ | 2,350.8 | $ | 1,397.6 | |||||||
| Total debt | $ | 2,763.4 | $ | 1,481.0 | |||||||
As of October 31, 2022, the Company was in compliance with all debt covenants.
Term Loan Agreement on December 17, 2021
On December 17, 2021, the Company entered into a Term Loan Agreement (the 2021 Credit Agreement) by and among the Company, the lenders from time to time party thereto, and PNC Bank, National Association, as administrative agent. The 2021 Credit Agreement provides for a term loan facility (the 2021 Term Loan Facility) in an aggregate principal amount of $1.5 billion, which, unless terminated earlier, matures on December 17, 2026. In addition, the Company has the ability from time to time to request an increase to the commitments under the 2021 Term Loan Facility or to establish a new term loan facility under the 2021 Credit Agreement in an aggregate principal amount not to exceed $1.125 billion, upon prior written notice to the administrative agent and subject to the discretionary participation of the lenders funding such term loans and certain limitations set forth in the 2021 Credit Agreement.
Amounts outstanding under the 2021 Term Loan Facility will bear interest, at the Company’s option, at either (i) the alternate base rate, which is a rate per annum equal to the greatest of (a) the administrative agent’s prime rate, (b) one-half of one percent in excess of the federal funds effective rate and (c) one percent in excess of the adjusted London interbank offered rate (“LIBOR”) for a one-month interest period on such day, or (ii) the adjusted LIBOR, plus, in each case, an applicable rate of, initially, zero basis points, in respect of base rate loans, and 75 basis points, in respect of adjusted LIBOR loans. Following a specified period after the closing date, the applicable rates will be determined quarterly by reference to a grid based upon the Company’s ratio of consolidated net indebtedness to consolidated EBITDA, each as defined in the 2021 Credit Agreement.
The Company may prepay loan balances from time to time, in whole or in part, without premium or penalty (other than any related breakage costs).
On December 17, 2021, the Company borrowed $1.5 billion under the 2021 Term Loan Facility and used the proceeds to fund the acquisition of Generate. Refer to Note 3. Acquisitions and Joint Venture for more details.
The interest rate on the 2021 Term Loan Facility was 4.44% at October 31, 2022.
The 2021 Credit Agreement contains customary restrictive covenants, as well as financial covenants that require the Company to maintain a certain Total Leverage Ratio and Interest Coverage Ratio, each as defined in the 2021 Credit Agreement, consistent with the 2020 Credit Agreement discussed below.
86
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Term Loan Agreement on November 2, 2021
On November 2, 2021, the Company entered into a 364-day, $840.0 million, term loan agreement by and among the Company, the lenders party thereto and The Bank of Nova Scotia, as administrative agent, which matured subsequent to year end on November 1, 2022. The Company used part of the funds to partially repay outstanding borrowings under the 2020 Revolving Credit Facility and for general corporate purposes.
We repaid $502.0 million during fiscal 2022. Amounts outstanding under the 2021 364-Day Term Loan Agreement will bear interest, at the Company’s option, at either the alternate base rate, or the adjusted LIBOR (each as defined in the 2021 364-Day Term Loan Agreement), plus, in the case of adjusted LIBOR loans, an applicable rate of 60 basis points.
The 2021 364-Day Term Loan Agreement contains customary restrictive covenants, as well as financial covenants that require the Company to maintain a certain total leverage ratio and interest coverage ratio, each as defined in the 2021 364-Day Term Loan Agreement, consistent with the 2020 Credit Agreement discussed below.
Revolving Credit and Term Loan Agreement on April 1, 2020
On April 1, 2020, the Company entered into a Revolving Credit and Term Loan Agreement (the 2020 Credit Agreement), among the Company, CooperVision International Holding Company, LP, CooperSurgical Netherlands B.V., CooperVision Holding Kft. the lenders from time to time party thereto, and KeyBank National Association, as administrative agent. The 2020 Credit Agreement provides for (a) a multicurrency revolving credit facility (the 2020 Revolving Credit Facility) in an aggregate principal amount of $1.29 billion and (b) a term loan facility (the 2020 Term Loan Facility) in an aggregate principal amount of $850.0 million, each of which, unless terminated earlier, mature on April 1, 2025. The Company used $850.0 million under the 2020 Term Loan Facility and $445.0 million under the 2020 Revolving Credit Facility to fully repay all borrowings outstanding under a previously existing term loan agreement and transfer all letters of credit and borrowings outstanding under a previously existing credit agreement to the 2020 Credit Agreement. The Company has an uncommitted option to increase the revolving credit facility or establish a new term loan in an aggregate amount up to $1.605 billion.
On October 30, 2020, the Company entered into Amendment No. 1 to the 2020 Credit Agreement, adding CooperVision International Limited as a revolving borrower and releasing certain borrowers in the 2020 Credit Agreement.
On December 17, 2021, the Company entered into Amendment No.2 to the 2020 Credit Agreement, modifying the 2020 Credit Agreement by, among other things, adding CooperSurgical Holdings Limited as a revolving borrower, releasing CooperVision Holding Kft as a borrower, and updating the benchmark replacement language in the 2020 Credit Agreement.
The 2020 Credit Agreement will bear interest, at the Company’s option, at either the base rate, or the adjusted LIBOR or adjusted foreign currency rate, plus, in each case, an applicable rate of between 0.00% and 0.50% in respect of base rate loans, and between 0.75% and 1.50% in respect of adjusted LIBOR or adjusted foreign currency rate loans, in each case in accordance with a pricing grid tied to the Total Leverage Ratio, as defined in the 2020 Credit Agreement. The Company may borrow, repay and re-borrow amounts available under the Revolving Credit Facility, subject to voluntary reduction of the revolving commitment.
The Company pays an annual commitment fee that ranges from 0.10% to 0.20% of the unused portion of the 2020 Revolving Credit Facility based upon the Company’s Total Leverage Ratio, as defined in the 2020 Credit Agreement.
At October 31, 2022, the Company had $850.0 million outstanding under the 2020 Term Loan Facility and none outstanding under the 2020 Revolving Credit Facility. The interest rate on the 2020 Term Loan Facility was 4.13% at October 31, 2022. The interest rate on the 2020 Revolving Credit Facility was 4.13% at October 31, 2022.
Payments on the outstanding long-term debt balance of $850.0 million are due in the fiscal year ending October 31, 2025.
European and Asian Pacific Credit Facilities
The Company maintains European credit facilities. The aggregate facility limit was $30.7 million and $35.8 million at October 31, 2022 and 2021, respectively. At October 31, 2022, $12.3 million of the facilities was utilized and the weighted average interest rate on the outstanding balances was 2.46%.
The Company maintains Yen-denominated credit facilities in Japan. The aggregate facility limit was $73.0 million and $95.0 million at October 31, 2022 and 2021, respectively. At October 31, 2022, $45.4 million of the combined facilities was utilized and the weighted average interest rate on the outstanding balances was 0.40%.
Each facility is supported by a continuing and unconditional guaranty.
87
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 6. Income Taxes
Effective Tax Rate
The effective tax rates for fiscal 2022 and 2021 were 18.8% and (499.1)%, respectively. The increase was primarily due to an intra-group transfer of intellectual property in fiscal 2021 and UK tax rate change in fiscal 2021, as discussed below. The increase was also due to changes in the geographic composition of pre-tax earnings and changes in excess tax benefits from share-based compensation.
The effective tax rate for fiscal 2022 was lower than the US federal statutory rate primarily due to foreign earnings in jurisdictions with lower tax rates and changes in unrecognized tax benefits, partially offset by foreign earnings subject to US tax. The effective tax rate for fiscal 2021 was lower than the US federal statutory tax rate primarily due to the intra-group transfer, UK tax rate change, and earnings in foreign jurisdictions with lower tax rates partially offset by foreign earnings subject to US tax.
In November 2020, the Company completed an intra-group transfer of certain intellectual property and related assets of CooperVision to a UK subsidiary as part of a group restructuring to establish headquarters operations in the UK. Determining fair value involved significant judgment related to future revenue growth, operating margins, and discount rates. The transfer resulted in a step-up of the UK tax-deductible basis in the intellectual property and goodwill, creating a temporary difference between the book basis and the tax basis of these assets. As a result, the Company recognized a deferred tax asset of $1,987.9 million, with a corresponding income tax benefit, during the first quarter of fiscal 2021. During the third quarter of fiscal 2021, the Company recognized a $536.7 million tax benefit related primarily to the remeasurement of this deferred tax asset caused by the UK enactment of a 25% corporate tax rate.
Components of income before income taxes:
| Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Income before income taxes: | |||||||||||||||||
| United States | $ | 31.4 | $ | (31.0) | $ | (88.0) | |||||||||||
| Foreign | 443.9 | 522.5 | 354.5 | ||||||||||||||
| $ | 475.3 | $ | 491.5 | $ | 266.5 | ||||||||||||
88
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Components of provision for income taxes:
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Current: | |||||||||||||||||
| Federal | $ | 10.2 | $ | 21.0 | $ | 1.4 | |||||||||||
| State | 3.8 | 1.3 | 1.1 | ||||||||||||||
| Foreign | 21.7 | 26.7 | 26.5 | ||||||||||||||
| 35.7 | 49.0 | 29.0 | |||||||||||||||
| Deferred: | |||||||||||||||||
| Federal | 10.5 | (8.8) | 3.2 | ||||||||||||||
| State | (2.2) | (0.5) | 0.8 | ||||||||||||||
| Foreign | 45.6 | (2,492.9) | (4.9) | ||||||||||||||
| 53.9 | (2,502.2) | (0.9) | |||||||||||||||
| Provision for income taxes | $ | 89.5 | $ | (2,453.2) | $ | 28.1 | |||||||||||
Reconciliation between the expected provision for income taxes at the US federal statutory rate and the provision for income taxes:
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Provision for income taxes at United States statutory tax rate | $ | 99.8 | $ | 103.2 | $ | 56.0 | |||||||||||
| (Decrease) increase in taxes resulting from: | |||||||||||||||||
| Foreign earnings in jurisdictions with lower tax rates | (22.3) | (43.6) | (54.7) | ||||||||||||||
| Foreign earnings subject to United States tax | 20.7 | 25.4 | 32.0 | ||||||||||||||
| Excess tax benefits from share-based compensation | (2.6) | (13.0) | (6.2) | ||||||||||||||
| Deferred tax asset step-up | (3.4) | 3.2 | (9.0) | ||||||||||||||
| United States provision-to-return | 0.5 | (1.2) | 7.0 | ||||||||||||||
| Intra-group transfer to UK subsidiary | — | (1,987.8) | — | ||||||||||||||
| Remeasurement of deferred tax assets from UK rate change | — | (536.7) | — | ||||||||||||||
| Change in unrecognized tax benefits | (12.7) | (7.6) | (0.1) | ||||||||||||||
| State tax provision | 5.0 | 0.8 | 1.9 | ||||||||||||||
| Other, net | 4.5 | 4.1 | 1.2 | ||||||||||||||
| Provision for income taxes | $ | 89.5 | $ | (2,453.2) | $ | 28.1 | |||||||||||
89
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Components of deferred tax assets and liabilities:
Years Ended October 31, (In millions) | 2022 | 2021 | |||||||||
| Deferred tax assets: | |||||||||||
| Accounts receivable | $ | 4.9 | $ | 3.4 | |||||||
| Inventories | 6.3 | 6.1 | |||||||||
| Accrued liabilities, reserves and compensation accruals | 79.9 | 78.1 | |||||||||
| Foreign deferred tax assets | 2,500.5 | 2,531.5 | |||||||||
| Share-based compensation | 14.5 | 28.6 | |||||||||
| Net operating loss and tax credit carryforwards | 19.6 | 19.3 | |||||||||
| Intangible assets | — | 6.8 | |||||||||
| Capitalized research and experimental expenses | 15.4 | 13.5 | |||||||||
| Total gross deferred tax assets | 2,641.1 | 2,687.3 | |||||||||
| Less: valuation allowance | (60.1) | (51.8) | |||||||||
| Deferred tax assets | 2,581.0 | 2,635.5 | |||||||||
| Deferred tax liabilities: | |||||||||||
| Tax deductible goodwill | (39.7) | (34.0) | |||||||||
| Intangible assets | (153.8) | — | |||||||||
| Plant and equipment | (48.8) | (46.5) | |||||||||
| Foreign deferred tax liabilities | (45.5) | (32.5) | |||||||||
| Total gross deferred tax liabilities | (287.8) | (113.0) | |||||||||
| Net deferred tax assets | $ | 2,293.2 | $ | 2,522.5 | |||||||
In assessing the realizability of deferred tax assets, the Company analyzes whether some or all deferred tax assets will not be realized. This analysis considers historical taxable income, the projected reversal of deferred tax liabilities, projected taxable income and tax planning strategies. Based upon this analysis, it is more likely than not the deferred tax assets, net of valuation allowance, will be realized. The increase in valuation allowance is primarily related to foreign tax attributes.
At October 31, 2022, the Company had federal net operating loss carryforwards of $65.4 million and state net operating loss carryforwards of $97.3 million. Federal net operating loss carryforwards of $17.1 million expire on various dates between 2025 and 2037 and $48.3 million do not expire. The state net operating loss carryforwards expire on various dates between 2026 through 2042.
A tax benefit is recognized if it is more likely than not that a tax position will be sustained on its technical merits, based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority.
90
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Changes in unrecognized tax benefits:
| (In millions) | |||||
| Balance at October 31, 2020 | $ | 58.5 | |||
| Decrease based on tax positions in prior fiscal years | (8.3) | ||||
| Increase based on tax positions in current fiscal year | 307.2 | ||||
| Settlements | (1.9) | ||||
| Lapses of statutes of limitations | (1.7) | ||||
| Balance at October 31, 2021 | $ | 353.8 | |||
| Decrease based on tax positions in prior fiscal years | (12.5) | ||||
| Settlements | (0.2) | ||||
| Lapses of statutes of limitations | (4.2) | ||||
| Balance at October 31, 2022 | $ | 336.9 | |||
These tax benefits, if recognized, would reduce provision for income taxes for 2022, 2021 and 2020, by $324.3 million, $336.5 million, and $46.0 million, respectively. Interest and penalties related to unrecognized tax benefits are recognized in provision for income taxes. As of October 31, 2022, 2021 and 2020, accrued gross interest and penalties related to unrecognized tax benefits was $5.4 million, $6.4 million, and $7.3 million, respectively.
Included in the balance of unrecognized tax benefits at October 31, 2022 is $9.1 million related to tax positions for which it is reasonably possible that the total amounts could change during the next twelve months.
Filed tax returns are subject to examination by tax authorities in major tax jurisdictions after fiscal 2016, including the UK and the US.
Note 7. Earnings Per Share
| Years Ended October 31, | |||||||||||||||||
| (In millions, except for earnings per share) | 2022 | 2021 | 2020 | ||||||||||||||
| Net income | $ | 385.8 | $ | 2,944.7 | $ | 238.4 | |||||||||||
| Basic: | |||||||||||||||||
| Weighted average common shares | 49.3 | 49.2 | 49.1 | ||||||||||||||
| Basic earnings per share | $ | 7.83 | $ | 59.80 | $ | 4.85 | |||||||||||
| Diluted: | |||||||||||||||||
| Weighted average common shares | 49.3 | 49.2 | 49.1 | ||||||||||||||
| Effect of dilutive stock plans | 0.4 | 0.6 | 0.5 | ||||||||||||||
| Diluted weighted average common shares | 49.7 | 49.8 | 49.6 | ||||||||||||||
| Diluted earnings per share | $ | 7.76 | $ | 59.16 | $ | 4.81 | |||||||||||
The following table sets forth stock options to purchase our common stock and restricted stock units that were not included in the diluted earnings per share calculation because their effect would have been antidilutive for the periods presented:
| Years Ended October 31, | |||||||||||||||||
| (In thousands, except exercise prices) | 2022 | 2021 | 2020 | ||||||||||||||
| Stock option shares excluded | 227 | 107 | 207 | ||||||||||||||
| Exercise prices | $300.12 - $406.17 | $ | 345.74 | $ | 304.54 | ||||||||||||
| Restricted stock units excluded | 87 | 2 | 1 | ||||||||||||||
91
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 8. Stockholders’ Equity
Analysis of Changes in Accumulated Other Comprehensive Income (Loss):
| (In millions) | Foreign Currency Translation Adjustment | Derivatives | Minimum Pension Liability | Total | |||||||||||||||||||
| Balance at October 31, 2019 | $ | (403.2) | $ | — | $ | (43.9) | $ | (447.1) | |||||||||||||||
| Gross change in value | 0.9 | (17.1) | (16.8) | (33.0) | |||||||||||||||||||
| Tax effect | — | 4.1 | 4.0 | 8.1 | |||||||||||||||||||
| Balance at October 31, 2020 | $ | (402.3) | $ | (13.0) | $ | (56.7) | $ | (472.0) | |||||||||||||||
| Gross change in value | $ | 82.2 | $ | 34.3 | $ | 29.8 | $ | 146.3 | |||||||||||||||
| Tax effect | (0.2) | (8.2) | (7.2) | (15.6) | |||||||||||||||||||
| Balance at October 31, 2021 | $ | (320.3) | $ | 13.1 | $ | (34.1) | $ | (341.3) | |||||||||||||||
| Gross change in value | $ | (234.7) | $ | 107.4 | $ | 36.6 | $ | (90.7) | |||||||||||||||
| Tax effect | — | (26.1) | (8.7) | (34.8) | |||||||||||||||||||
| Balance at October 31, 2022 | $ | (555.0) | $ | 94.4 | $ | (6.2) | $ | (466.8) | |||||||||||||||
Share Repurchases
In December 2011, the Company's Board of Directors authorized the 2012 Share Repurchase Program and through subsequent amendments, the most recent in March 2017, the total repurchase authorization was increased from $500.0 million to $1.0 billion of the Company's common stock. This program has no expiration date and may be discontinued at any time. Purchases under the 2012 Share Repurchase Program are subject to a review of the circumstances in place at the time and may be made from time to time as permitted by securities laws and other legal requirements.
For the years ended October 31, 2022 and 2021, the Company's share repurchases were as follow:
| Years Ended October 31, | 2022 | 2021 | |||||||||
| Number of shares | 191,165 | 69,622 | |||||||||
| Average repurchase price per share | $ | 410.4 | $ | 356.6 | |||||||
| Total costs of shares repurchased (in millions) | $ | 78.5 | $ | 24.8 | |||||||
At October 31, 2022, $256.4 million remained authorized for repurchase under the program.
Note 9. Stock Plans
2007 Long-Term Incentive Plan (2007 Plan)
In March 2007, we received stockholder approval of the 2007 Plan. The 2007 Plan was subsequently amended and restated, and granted stockholder approval in March 2009, March 2011, and March 2016.
The Third Amended and Restated 2007 Plan is designed to increase our stockholder value by attracting, retaining and motivating key employees and consultants who directly influence our profitability. The Third Amended and Restated 2007 Plan authorizes either our Board of Directors, or a designated committee thereof composed of two or more Non-Employee Directors, to grant to eligible individuals during the period ending December 31, 2026, up to 6,930,000 shares in the form of specified equity awards including stock options, restricted stock units and performance share awards, subject to adjustment for future stock splits, stock dividends, expirations, forfeitures and similar events. RSUs have no dividend or voting rights prior to vesting.
As of October 31, 2022, 690,596 shares remained available under the Third Amended and Restated 2007 Plan for future grants. The amount of available shares includes shares which may be distributed under performance share awards.
92
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Share-Based Compensation
Compensation expense and the related tax benefit recognized in our Consolidated Statements of Income for share-based awards, including the Employee Stock Purchase Plan, were as follows:
| October 31, | |||||||||||||||||
| (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Selling, general and administrative expense | $ | 46.7 | $ | 38.4 | $ | 32.2 | |||||||||||
| Cost of sales | 4.5 | 3.9 | 4.0 | ||||||||||||||
| Research and development expense | 3.0 | 2.4 | 2.4 | ||||||||||||||
| Total compensation expense | $ | 54.2 | $ | 44.7 | $ | 38.6 | |||||||||||
| Related income tax benefit | $ | 5.0 | $ | 5.6 | $ | 4.8 | |||||||||||
Stock Options
The fair value of each stock option award granted is estimated on the date of grant using the Black-Scholes option valuation model and assumptions noted in the following table.
| Years Ended October 31, | 2022 | 2021 | 2020 | ||||||||||||||
| Expected life | 4.1 years | 4.0 years | 4.4 years | ||||||||||||||
| Expected volatility | 25.8 | % | 30.3 | % | 24.5 | % | |||||||||||
| Risk-free interest rate | 1.1 | % | 0.3 | % | 1.6 | % | |||||||||||
| Dividend yield | 0.02 | % | 0.02 | % | 0.02 | % | |||||||||||
The activity and status of our stock option plans are summarized below:
| Number of Shares | Weighted- Average Exercise Price Per Share | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | ||||||||||||||||||||
| Outstanding at October 31, 2021 | 972,692 | $ | 245.09 | ||||||||||||||||||||
| Granted | 122,760 | $ | 403.44 | ||||||||||||||||||||
| Exercised | (26,866) | $ | 171.87 | ||||||||||||||||||||
| Forfeited or expired | (4,743) | $ | 325.21 | ||||||||||||||||||||
| Outstanding at October 31, 2022 | 1,063,843 | $ | 264.85 | 5.75 | |||||||||||||||||||
| Vested and expected to vest at October 31, 2022 | 1,038,594 | $ | 262.91 | 5.70 | $ | 38,806,329 | |||||||||||||||||
| Vested and exercisable at October 31, 2022 | 624,512 | $ | 223.45 | 4.57 | $ | 35,586,767 | |||||||||||||||||
The weighted-average fair value of options granted during fiscal 2022, 2021 and 2020, estimated as of the grant date using the Black-Scholes option pricing model, for the 2007 Plan was $90.41, $84.10 and $70.45, respectively. The total intrinsic value of options exercised during the fiscal years ended October 31, 2022, 2021 and 2020 was $6.6 million, $64.7 million and $22.6 million, respectively.
Stock options outstanding under our current plans have been granted at prices which are either equal to or above the market value of the common stock on the date of grant. Options granted under the 2007 Plan generally vest over a range of to five years based on service conditions and expire no later than ten years after the grant date. Options granted under the 2020 Directors' Plan generally vest in one year and expire no later than ten years after the grant date. We generally recognize compensation expense ratably over the vesting period. As of October 31, 2022, there was $21.0 million of total unrecognized compensation cost related to nonvested options, which is expected to be recognized over a remaining weighted-average vesting period of 2.3 years.
93
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Restricted Stock Units
RSUs granted under the 2007 Plan generally vest over to five years. The grant-date fair value of RSUs is estimated based on the market price of our common stock. We recognize compensation expense ratably over the vesting period. As of October 31, 2022, there was $64.4 million of total unrecognized compensation cost related to nonvested RSUs, which is expected to be recognized over a remaining weighted-average vesting period of 2.5 years.
The status of our non-vested RSUs is summarized below:
| Number of Shares | Weighted- Average Grant Date Fair Value Per Share | ||||||||||
| Non-vested RSUs at October 31, 2021 | 317,335 | $ | 293.80 | ||||||||
| Granted | 113,079 | $ | 399.21 | ||||||||
| Vested and issued | (113,301) | $ | 271.74 | ||||||||
| Forfeited or expired | (27,875) | $ | 324.67 | ||||||||
| Non-vested RSUs at October 31, 2022 | 289,238 | $ | 340.68 | ||||||||
Performance Units
Performance units may be granted to selected key employees with vesting contingent upon meeting future reported earnings per share goals over a defined performance cycle, usually three years. Performance units, if earned, may be paid in cash or shares of common stock. We granted performance unit awards on December 8, 2020 under the 2007 Plan. The performance shares actually earned will range from zero to 200% of the target number of performance shares for performance periods ending in fiscal 2021 through fiscal 2024. Subject to limited exceptions set forth in the performance share plan, any shares earned will be distributed in the subsequent fiscal year after the performance period. The fair value of performance unit awards is estimated on the date of grant based on the current market price of our common stock and the estimate of probability of award achievement. This estimate is reviewed each fiscal quarter and adjustments are recorded if it is determined that the estimate of probability of award achievement has changed.
We recognize compensation expense ratably over the vesting period. As of October 31, 2022, there was $11.2 million of total unrecognized compensation cost related to non-vested performance units, which is expected to be recognized over a remaining weighted-average vesting period of 1.8 years.
Employee Stock Purchase Plan
On March 18, 2019, the Company received stockholder approval for the Employee Stock Purchase Plan (ESPP). The first offering period began on November 4, 2019 and offerings are generally made on a quarterly basis. The purpose of the ESPP is to provide eligible employees of the Company with the opportunity to acquire shares of common stock at 85% of the market price on the last business day of each offering period by means of accumulated payroll deductions. The ESPP initially authorized the issuance of 1,000,000 shares of common stock. These shares will be made available from shares of common stock reacquired by the Company as Treasury Stock. During fiscal 2022 and 2021, we issued 22,695 and 17,575 shares to our employees under the ESPP, respectively. At October 31, 2022, the number of shares remaining available for future issuance under the ESPP was 948,090 shares. Total ESPP share-based compensation recognized during fiscal 2022 and 2021 was $1.1 million and $1.0 million, respectively.
Note 10. Employee Benefits
Cooper's Retirement Income Plan
The Company's Retirement Income Plan (Plan), a defined benefit plan, is only available to full-time United States employees, subject to the soft freeze mentioned below. The Company's contributions are designed to fund normal cost on a current basis and to fund the estimated prior service cost of benefit improvements. The unit credit actuarial cost method is used to determine the annual cost. The Company pays the entire cost of the Plan and funds such costs as they accrue. Virtually all of the assets of the Plan are comprised of equities and participation in equity and fixed income funds.
94
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The Company uses individual spot rates along the yield curve that correspond with the timing of each benefit payment to determine the service and interest costs of components of its net periodic benefit cost utilizing the correlation of projected cash outflows and corresponding spot rates on the yield curve.
The following table sets forth the Plan's benefit obligations and fair value of the Plan assets at October 31, 2022, 2021 and 2020 and the funded status of the Plan and net periodic pension costs for each of the years in the three-year periods ended October 31, 2022. The net amounts recognized in the Consolidated Balance Sheets consist of noncurrent liabilities. The accumulated benefit obligation was $134.9 million, $207.6 million and $195.8 million for the years ended October 31, 2022, 2021 and 2020.
Retirement Income Plan
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Change in benefit obligation | |||||||||||||||||
| Benefit obligation, beginning of year | $ | 230.9 | $ | 218.8 | $ | 189.7 | |||||||||||
| Service cost | 18.3 | 17.2 | 13.9 | ||||||||||||||
| 5.1 | 4.4 | 5.2 | |||||||||||||||
| Benefits paid | (13.1) | (11.5) | (10.0) | ||||||||||||||
| Actuarial (gain)/loss | (93.2) | 2.0 | 20.0 | ||||||||||||||
| Benefit obligation, end of year | $ | 148.0 | $ | 230.9 | $ | 218.8 | |||||||||||
| Change in plan assets | |||||||||||||||||
| Fair value of plan assets, beginning of year | $ | 199.5 | $ | 159.5 | $ | 136.0 | |||||||||||
| Actual return on plan assets | (43.5) | 38.8 | 10.1 | ||||||||||||||
| Employer contributions | — | 12.7 | 23.4 | ||||||||||||||
| Benefits paid | (13.1) | (11.5) | (10.0) | ||||||||||||||
| Fair value of plan assets, end of year | $ | 142.9 | $ | 199.5 | $ | 159.5 | |||||||||||
| Funded status at end of year | $ | (5.1) | $ | (31.4) | $ | (59.3) | |||||||||||
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Amounts recognized in accumulated other comprehensive income consist of: | |||||||||||||||||
| Net loss | $ | 8.0 | $ | 44.4 | $ | 74.2 | |||||||||||
| Accumulated other comprehensive income | $ | 8.0 | $ | 44.4 | $ | 74.2 | |||||||||||
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Reconciliation of (prepaid) accrued pension cost: | |||||||||||||||||
| (Prepaid)/Accrued pension cost at prior fiscal year end | $ | (13.0) | $ | (14.8) | $ | (3.7) | |||||||||||
| Net periodic benefit cost | 10.1 | 14.5 | 12.3 | ||||||||||||||
| Contributions made during the year | — | (12.7) | (23.4) | ||||||||||||||
| (Prepaid)/Accrued pension cost at fiscal year end | $ | (2.9) | $ | (13.0) | $ | (14.8) | |||||||||||
95
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Components of net periodic benefit cost and other amounts recognized in the Consolidated Statements of Income: | |||||||||||||||||
| Net periodic benefit cost: | |||||||||||||||||
| Service cost | $ | 18.3 | $ | 17.2 | $ | 13.9 | |||||||||||
| Interest cost | 5.1 | 4.4 | 5.2 | ||||||||||||||
| (15.5) | (12.5) | (10.8) | |||||||||||||||
| 2.2 | 5.4 | 4.0 | |||||||||||||||
| Net periodic pension cost | $ | 10.1 | $ | 14.5 | $ | 12.3 | |||||||||||
Years Ended October 31, (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Other changes in plan assets and benefit obligations recognized in other comprehensive income: | |||||||||||||||||
| Net (gain) loss | $ | (34.1) | $ | (24.4) | $ | 20.8 | |||||||||||
| Amortizations of net gain | (2.5) | (5.4) | (4.0) | ||||||||||||||
| Total recognized in other comprehensive (income) loss | $ | (36.6) | $ | (29.8) | $ | 16.8 | |||||||||||
| Total recognized in net periodic benefit cost and other comprehensive (income) loss | $ | (26.2) | $ | (15.2) | $ | 29.0 | |||||||||||
| Years Ended October 31, | 2022 | 2021 | 2020 | ||||||||||||||
| Weighted-average assumptions used in computing the net periodic pension cost and projected benefit obligation at year end: | |||||||||||||||||
| Discount rate for determining net periodic pension cost: | |||||||||||||||||
| Projected Benefit Obligation | 2.76 | % | 2.78 | % | 3.13 | % | |||||||||||
| Service Cost | 2.79 | % | 2.86 | % | 3.18 | % | |||||||||||
| Interest Cost | 2.28 | % | 2.07 | % | 2.78 | % | |||||||||||
| Discount rate for determining benefit obligations at year end | 5.74 | % | 2.76 | % | 2.78 | % | |||||||||||
| Rate of compensation increase for determining expense | 3.60 | % | 3.60 | % | 3.60 | % | |||||||||||
| Rate of compensation increase for determining benefit obligations at year end | 3.60 | % | 3.60 | % | 3.60 | % | |||||||||||
| Expected rate of return on plan assets for determining net periodic pension cost | 8.00 | % | 8.00 | % | 8.00 | % | |||||||||||
| Expected rate of return on plan assets at year end | 8.00 | % | 8.00 | % | 8.00 | % | |||||||||||
| Measurement date for determining assets and benefit obligations at year end | 10/31/2022 | 10/31/2021 | 10/31/2020 | ||||||||||||||
The discount rate enables us to state expected future cash flows at a present value on the measurement date. The discount rate used for the Plan is based primarily on the yields of a universe of high-quality corporate bonds rated AA or above, with durations corresponding to the expected durations of the benefit obligations. A change in the discount rate will cause the present value of benefit obligations to change in the opposite direction. If a discount rate of 2.76%, which is 0.02% lower than prior fiscal year, had been used, the projected benefit obligation would have been $217.2 million, and the accumulated benefit obligation would have been $194.2 million.
The expected rate of return on plan assets was determined based on a review of historical returns, both for this plan and for medium- to large-sized defined benefit pension funds with similar asset allocations. This review generated separate expected returns for each asset class listed below. These expected future returns were then blended based on this Plan's target asset allocation.
Reasons for Significant Liability Gains and Losses
The projected benefit obligation experienced a net gain of approximately $93.2 million during the year. This net gain is primarily due to gains from assumption changes of approximately $97.1 million, offset by losses of approximately $3.9 million
96
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
due to demographic experience. The key assumption changes were the increase in the discount rate (gain of $72.7 million), a change in the assumed payment form election probabilities (gain of $0.3 million), and changes in assumptions for lump sum determination (gain of $24.1 million). The primary reasons for demographic losses were the net effect of retirement rates, termination rates, salary increases and other experience that was different from assumed.
Plan Assets
Weighted-average asset allocations at year end, by asset category are as follows:
| Years Ended October 31, | 2022 | 2021 | 2020 | ||||||||||||||
| Asset category | |||||||||||||||||
| Cash and cash equivalents | 2.0 | % | 5.0 | % | 11.8 | % | |||||||||||
| Equity mutual funds | 65.8 | % | 62.8 | % | 57.7 | % | |||||||||||
| Hedging Strategy Funds | 5.2 | % | 4.7 | % | 4.3 | % | |||||||||||
| Bond mutual funds | 27.0 | % | 27.5 | % | 26.2 | % | |||||||||||
| Total | 100.0 | % | 100.0 | % | 100.0 | % | |||||||||||
The Plan invests in a diversified portfolio of assets intended to minimize risk of poor returns while maximizing expected portfolio returns. To achieve the long-term rate of return, plan assets will be invested in a mixture of instruments, including but not limited to, corporate common stock (may include the Company's stock), investment grade bond funds, cash, balanced funds, real estate funds, small or large cap equity funds and international equity funds. The allocation of assets will be determined by the investment manager and will typically include 50% to 70% equities with the remainder invested in fixed income, hedging strategy funds and cash. Presently, this diversified portfolio is expected to return roughly 8% in the long run.
As of the measurement date of October 31, 2022, the fair value measurement of plan assets is as follows:
| (In millions) | Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||||||||
| Asset category | |||||||||||||||||||||||
| Cash and cash equivalents | $ | 2.9 | $ | 2.9 | $ | — | $ | — | |||||||||||||||
| Equity mutual funds | 93.9 | 93.9 | — | — | |||||||||||||||||||
| Hedging Strategy Funds | 7.5 | 7.5 | — | — | |||||||||||||||||||
| Bond mutual funds | 38.6 | 14.5 | 24.1 | — | |||||||||||||||||||
| Total | $ | 142.9 | $ | 118.8 | $ | 24.1 | $ | — | |||||||||||||||
The Plan has an established process for determining the fair value of plan assets. For investments in equity and bond mutual funds, and real estate funds, fair value is based on observable, Level 1 inputs.
While the Company believes its valuation methods are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
Plan Cash Flows
Contributions
The Company made no contributions to the Plan in fiscal 2022. The Company contributions to the Plan were $12.7 million for fiscal 2021 and, $23.4 million for fiscal 2020. The Company closely monitors the funded status of the Plan with respect to legislative and accounting rules. The Company does not expect to make a contribution to the Plan during fiscal 2023.
97
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Estimated Future Benefit Payments
Years (In millions) | |||||
| 2023 | $ | 9.4 | |||
| 2024 | $ | 11.0 | |||
| 2025 | $ | 11.9 | |||
| 2026 | $ | 11.4 | |||
| 2027 | $ | 11.9 | |||
| 2028-2032 | $ | 66.1 | |||
Plan Soft Freeze
On June 18, 2019 the Board of Directors of the Company approved a soft freeze of the Plan effective August 1, 2019. The Plan was closed to employees hired on or after August 1, 2019, including former participants or employees rehired on or after August 1, 2019 and employees hired in connection with a stock or asset acquisition, merger or other similar transaction on or after August 1, 2019. Existing employees already covered by the Plan, continue to accrue their benefits.
Cooper's 401(k) Savings Plan
Cooper's 401(k) savings plan provides for the deferral of compensation as described in the Internal Revenue Code and is available to substantially all United States employees. Employees who participate in the 401(k) plan may elect to have up to 75% of their pre-tax salary or wages deferred and contributed to the trust established under the Plan. Cooper's contributions on account of participating employees, were $9.0 million, $7.2 million and $6.8 million for the years ended October 31, 2022, 2021 and 2020, respectively.
Note 11. Contingencies
The Company is involved in various lawsuits, claims and other legal matters from time to time that arise in the ordinary course of conducting business, including matters involving our products, intellectual property, supplier relationships, distributors, competitor relationships, employees and other matters. The Company does not believe that the ultimate resolution of these proceedings or claims pending against it could have a material adverse effect on its financial condition or results of operations. At each reporting period, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under ASC 450, Contingencies. Legal fees are expensed as incurred.
Note 12. Business Segment Information
The Company discloses information about its operating segments, which were established based on the way that management organizes segments within the Company for making operating decisions and assessing financial performance. The Company's two operating segments are described below.
•CooperVision. Competes in the worldwide contact lens market by developing, manufacturing and marketing a broad range of products for contact lens wearers, featuring advanced materials and optics.
•CooperSurgical. Competes in the general health care market with a focus on advancing the health of women, babies and families through a diversified portfolio of products and services focusing on women's health and fertility.
The Company uses operating income, as presented in our financial reports, as the primary measure of segment profitability. The Company does not allocate costs from corporate functions to segment operating income. The Company uses the same accounting policies to generate segment results as it does for consolidated results.
No customers accounted for 10% or more of our consolidated net revenue in fiscal 2022, 2021 and 2020.
Total identifiable assets are those used in continuing operations except cash and cash equivalents, which the Company includes as corporate assets.
98
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
The following table presents a summary of our business segment net sales:
| (In millions) | 2022 | 2021 | 2020 | ||||||||||||||
| CooperVision net sales by category: | |||||||||||||||||
| Toric lens | $ | 737.4 | $ | 697.5 | $ | 598.2 | |||||||||||
| Multifocal lens | 264.4 | 238.6 | 197.0 | ||||||||||||||
| Single-use sphere lens | 661.6 | 616.3 | 529.0 | ||||||||||||||
| Non single-use sphere, other | 579.9 | 599.6 | 518.8 | ||||||||||||||
| Total CooperVision net sales | 2,243.3 | 2,152.0 | 1,843.0 | ||||||||||||||
| CooperSurgical net sales by category: | |||||||||||||||||
| Office and surgical products | 633.6 | 451.3 | 358.8 | ||||||||||||||
| Fertility | 431.5 | 319.2 | 229.1 | ||||||||||||||
| Total CooperSurgical net sales | 1,065.1 | 770.5 | 587.9 | ||||||||||||||
| Total net sales | $ | 3,308.4 | $ | 2,922.5 | $ | 2,430.9 | |||||||||||
99
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Information by business segment for each of the years in the three-year period ended October 31, 2022, follows:
| (In millions) | CooperVision | CooperSurgical | Corporate | Consolidated | |||||||||||||||||||
| 2022 | |||||||||||||||||||||||
| Net sales | $ | 2,243.3 | $ | 1,065.1 | $ | — | $ | 3,308.4 | |||||||||||||||
| Operating income (loss) | $ | 494.3 | $ | 67.1 | $ | (53.8) | $ | 507.6 | |||||||||||||||
| Interest expense | 57.3 | ||||||||||||||||||||||
| Other (income), net | (25.0) | ||||||||||||||||||||||
| Income before income taxes | $ | 475.3 | |||||||||||||||||||||
| Identifiable assets | $ | 6,778.9 | $ | 4,407.8 | $ | 305.6 | $ | 11,492.3 | |||||||||||||||
| Depreciation expense | $ | 144.5 | $ | 22.1 | $ | — | $ | 166.6 | |||||||||||||||
| Amortization expense | $ | 32.3 | $ | 147.2 | $ | — | $ | 179.5 | |||||||||||||||
| Capital expenditures | $ | 223.0 | $ | 19.0 | $ | — | $ | 242.0 | |||||||||||||||
| 2021 | |||||||||||||||||||||||
| Net sales | $ | 2,152.0 | $ | 770.5 | $ | — | $ | 2,922.5 | |||||||||||||||
| Operating income (loss) | $ | 481.3 | $ | 71.8 | $ | (47.3) | $ | 505.8 | |||||||||||||||
| Interest expense | 23.1 | ||||||||||||||||||||||
| Other (income), net | (8.8) | ||||||||||||||||||||||
| Income before income taxes | $ | 491.5 | |||||||||||||||||||||
| Identifiable assets | $ | 6,965.9 | $ | 2,395.6 | $ | 244.7 | $ | 9,606.2 | |||||||||||||||
| Depreciation expense | $ | 148.3 | $ | 14.9 | $ | — | $ | 163.2 | |||||||||||||||
| Amortization expense | $ | 35.7 | $ | 110.4 | $ | — | $ | 146.1 | |||||||||||||||
| Capital expenditures | $ | 190.0 | $ | 24.4 | $ | — | $ | 214.4 | |||||||||||||||
| 2020 | |||||||||||||||||||||||
| Net sales | $ | 1,843.0 | $ | 587.9 | $ | — | $ | 2,430.9 | |||||||||||||||
| Operating income (loss) | $ | 375.7 | $ | (14.7) | $ | (49.2) | $ | 311.8 | |||||||||||||||
| Interest expense | 36.8 | ||||||||||||||||||||||
| Other expense, net | 8.5 | ||||||||||||||||||||||
| Income before income taxes | $ | 266.5 | |||||||||||||||||||||
| Identifiable assets | $ | 4,236.3 | $ | 2,293.8 | $ | 207.4 | $ | 6,737.5 | |||||||||||||||
| Depreciation expense | $ | 138.2 | $ | 11.7 | $ | — | $ | 149.9 | |||||||||||||||
| Amortization expense | $ | 32.4 | $ | 104.8 | $ | — | $ | 137.2 | |||||||||||||||
| Capital expenditures | $ | 260.3 | $ | 50.1 | $ | — | $ | 310.4 | |||||||||||||||
100
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Information by geographical area by country of domicile for each of the years in the three-year period ended October 31, 2022, follows:
| (In millions) | United States | Europe | Rest of World, Other Eliminations & Corporate | Consolidated | |||||||||||||||||||
| 2022 | |||||||||||||||||||||||
| Net sales to unaffiliated customers | $ | 1,638.5 | $ | 987.2 | $ | 682.7 | $ | 3,308.4 | |||||||||||||||
| Sales between geographic areas | 514.4 | 897.3 | (1,411.7) | — | |||||||||||||||||||
| Net sales | $ | 2,152.9 | $ | 1,884.5 | $ | (729.0) | $ | 3,308.4 | |||||||||||||||
| Operating income | $ | 71.8 | $ | 403.8 | $ | 32.0 | $ | 507.6 | |||||||||||||||
| Long-lived assets | $ | 856.1 | $ | 310.8 | $ | 266.0 | $ | 1,432.9 | |||||||||||||||
| 2021 | |||||||||||||||||||||||
| Net sales to unaffiliated customers | $ | 1,339.2 | $ | 957.9 | $ | 625.4 | $ | 2,922.5 | |||||||||||||||
| Sales between geographic areas | 494.9 | 815.1 | (1,310.0) | — | |||||||||||||||||||
| Net sales | $ | 1,834.1 | $ | 1,773.0 | $ | (684.6) | $ | 2,922.5 | |||||||||||||||
| Operating (loss) income | $ | (26.8) | $ | 416.2 | $ | 116.4 | $ | 505.8 | |||||||||||||||
| Long-lived assets | $ | 737.5 | $ | 377.2 | $ | 232.9 | $ | 1,347.6 | |||||||||||||||
| 2020 | |||||||||||||||||||||||
| Net sales to unaffiliated customers | $ | 1,103.6 | $ | 789.8 | $ | 537.5 | $ | 2,430.9 | |||||||||||||||
| Sales between geographic areas | 391.7 | 327.1 | (718.8) | — | |||||||||||||||||||
| Net sales | $ | 1,495.3 | $ | 1,116.9 | $ | (181.3) | $ | 2,430.9 | |||||||||||||||
| Operating (loss) income | $ | (14.5) | $ | 21.9 | $ | 304.4 | $ | 311.8 | |||||||||||||||
| Long-lived assets | $ | 721.3 | $ | 363.0 | $ | 197.6 | $ | 1,281.9 | |||||||||||||||
101
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 13. Financial Derivatives and Hedging
As part of the Company’s overall risk management practices the Company enters into financial derivatives, interest rate swaps designated as cash flow hedges, to hedge the Company's exposure to changes in cash flows associated with its variable rate debt.
Credit risk related to derivative transactions reflects the risk that a party to the transaction could fail to meet its obligation under the derivative contracts. Therefore, the Company’s exposure to the counterparty’s credit risk is generally limited to the amounts, if any, by which the counterparty’s obligations to the Company exceed the Company’s obligations to the counterparty. The Company’s policy is to enter into contracts only with financial institutions which meet certain minimum credit ratings to help mitigate counterparty credit risk.
On April 6, 2020 the Company entered into six interest rate swap contracts which were used to hedge its exposure to changes in cash flows associated with its variable rate debt and were designated as derivatives in a cash flow hedge. The payment streams were based on a total notional amount of $1.5 billion at the inception of the contracts. As of October 31, 2022, three of the six interest rate swap contracts have matured and the outstanding contracts have a total notional amount of $1.0 billion and remaining maturities of five years or less..
The Company did not have any cross-currency swaps or foreign currency forward contracts as of October 31, 2022.
The pre-tax impact of gain on derivatives designated for hedge accounting recognized in other comprehensive income (loss) was $124.5 million ($30.1 million, net of tax) as of October 31, 2022. The pre-tax impact of gain on derivatives designated for hedge accounting recognized in other comprehensive income (loss) was $17.2 million ($13.1 million, net of tax) as of October 31, 2021. The fair value of derivative instruments are classified in "Other non-current assets" on our consolidated balance sheets.
The following table summarizes the amounts recognized with respect to our derivative instruments within the accompanying Consolidated Statements of Income:
| Periods Ended October 31, | |||||||||||||||||||||||
| (In millions) | 2022 | 2021 | 2020 | ||||||||||||||||||||
| Derivatives designated as cash flow hedges | Location of Loss (Income) Recognized on Derivatives | ||||||||||||||||||||||
| Interest rate swap contracts | Interest expense (income) | $ | (2.3) | $ | 8.0 | $ | 3.7 | ||||||||||||||||
The Company expects that ($38.3 million) recorded as a component of accumulated other comprehensive income (loss) will be realized in the Consolidated Statements of Income over the next twelve months and the amount will vary depending on prevailing interest rates.
The following table details the changes in accumulated other comprehensive income:
| (In millions) | Amount | |||||||
| Balance as of October 31, 2020 | $ | (17.1) | ||||||
Amount recognized in other comprehensive income on interest rate swap contracts, gross ($20.0, net of tax) | 26.3 | |||||||
Amount reclassified from other comprehensive income into earnings, gross ($6.1, net of tax) | 8 | |||||||
| Balance gain as of October 31, 2021 | $ | 17.2 | ||||||
Amount recognized in other comprehensive income on interest rate swap contracts, gross ($79.7, net of tax) | 105.1 | |||||||
Amount reclassified from other comprehensive income into earnings, gross ($1.7, net of tax) | 2.2 | |||||||
| Balance gain as of October 31, 2022 | $ | 124.5 | ||||||
102
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
The Company has established and currently maintains disclosure controls and procedures designed to ensure that information required to be disclosed in its reports filed under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified by the Securities and Exchange Commission's rules and forms and that such information is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosures. In designing and evaluating the disclosure controls and procedures, management recognizes that controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving desired control objectives. In reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
In conjunction with the close of each fiscal quarter, the Company conducts a review and evaluation, under the supervision and with the participation of the Company's management, including the Chief Executive Officer (our Principal Executive Officer) and Chief Financial Officer (our Principal Financial Officer), of the effectiveness of the design and operation of the Company's disclosure controls and procedures. The Company's Chief Executive Officer and Chief Financial Officer based upon their evaluation as of October 31, 2022, the end of the fiscal period covered in this report, concluded that the Company's disclosure controls and procedures were effective at the reasonable assurance level.
Management's Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements, errors or fraud.
Management assessed the effectiveness of the Company's internal control over financial reporting as of October 31, 2022, based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control - Integrated Framework (2013). Based on this assessment, management, under the supervision and with the participation of the Company's Chief Executive Officer and Chief Financial Officer, concluded that the Company's internal control over financial reporting was effective as of October 31, 2022. The Company acquired Generate Life Sciences on December 17, 2021, and management excluded it from its assessment of the effectiveness of internal control over financial reporting as of October 31, 2022. Generate’s and its subsidiaries’ internal control over financial reporting associated with total assets of $2.1 billion and total revenues of $249.5 million included in the consolidated financial statements of The Cooper Companies, Inc. as of and for the year ended October 31, 2022.
The Company's independent registered public accounting firm, KPMG LLP, has audited the effectiveness of the Company's internal control over financial reporting as of October 31, 2022, as stated in their report in Part II, Item 8 of this Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
There have been no changes in the Company's internal control over financial reporting during the Company's fiscal quarter ended October 31, 2022, that materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not Applicable.
103
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item is incorporated by reference to the Company’s Proxy Statement for the Annual Meeting of Stockholders scheduled to be held in March 2023 (the 2023 Proxy Statement).
Item 11. Executive Compensation.
The information required by this item is incorporated by reference to the 2023 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
See Item 5. Market for Registrant's Common Equity and Related Stockholder Matters - Equity Compensation Plan Information. Additional information required by this item is incorporated by reference to the 2023 Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference to the 2023 Proxy Statement.
Item 14. Principal Accounting Fees and Services.
The information required by this item is incorporated by reference to “Report of the Audit Committee” section of the 2023 Proxy Statement.
104
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a) 1. Financial Statements
The following financial statements are filed as a part of this report:
Report of KPMG LLP, Independent Registered Public Accounting Firm
Consolidated Financial Statements:
Statements of Income for the years ended October 31, 2022, 2021 and 2020
Statements of Comprehensive Income for the years ended October 31, 2022, 2021 and 2020
Balance Sheets as of October 31, 2022 and 2021
Statements of Stockholders' Equity for the years ended October 31, 2022, 2021 and 2020
Statements of Cash Flows for the years ended October 31, 2022, 2021 and 2020
Notes to Consolidated Financial Statements
2. Financial Statement Schedules of the Company.
Schedule Number Description
Schedule II Valuation and Qualifying Accounts
(b) Exhibits.
The exhibits listed on the accompanying Exhibit Index are filed as part of this report.
All other schedules which are included in the applicable accounting regulations of the Securities and Exchange Commission are not required here because they are not applicable.
105
Schedule II
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS
Three Years Ended October 31, 2022
| (In millions) | Balance Beginning of Year | Additions | Reductions/ Charges | Balance at End of Year | |||||||||||||||||||
| Deferred income tax valuation allowance: | |||||||||||||||||||||||
| Year Ended October 31, 2022 | 51.8 | 13.3 | (5.0) | 60.1 | |||||||||||||||||||
| Year Ended October 31, 2021 | 45.3 | 8.8 | (2.3) | 51.8 | |||||||||||||||||||
| Year Ended October 31, 2020 | 41.5 | 5.9 | (2.1) | 45.3 | |||||||||||||||||||
106
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
EXHIBIT INDEX
| Exhibit Number | Description of Document | ||||
| 3.1 | |||||
| 3.2 | |||||
| 4.1 | |||||
| 10.1# | |||||
| 10.2# | |||||
| 10.3# | |||||
| 10.4# | |||||
| 10.5# | |||||
| 10.6# | |||||
| 10.7# | |||||
| 10.8# | |||||
| 10.9# | |||||
| 10.10# | |||||
| 10.11# | |||||
| 10.12# | Form of Restricted Stock Unit Agreement pursuant to the 2020 Long Term Incentive Plan for Non-Employee Directors of The Cooper Companies, Inc., incorporated by reference to Exhibit 10.13 to the Company's Annual Report on Form 10-K for the fiscal year ended October 31, 2020, incorporated by reference to Exhibit 10.1 | ||||
10.13(a) | |||||
10.14(a) | |||||
| 10.15 | |||||
| 10.16 | |||||
| 10.17 | |||||
107
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
| Exhibit Number | Description of Document | ||||
| 10.18 | |||||
| 10.19 | |||||
| 10.20 | |||||
| 10.21 | |||||
| 10.22 | Amendment No.2 and Joinder, dated as of December 17, 2021, to Revolving Credit and Term Loan Agreement, dated as of April 1, 2020, among the Company, CooperVision International Limited, CooperVision Holding Kft., CooperSurgical Holdings Limited, the lenders party thereto, and KeyBank, National Association, as administrative agent, Exhibit 10.3 of the Company's Current Report on Form 8-K filed December 10, 2021 | ||||
| 10.23# | |||||
| 10.24 | |||||
| 21 | |||||
| 23 | |||||
| 24 | Power of Attorney (included on signature page hereto) | ||||
| 31.1 | |||||
| 31.2 | |||||
| 32.1* | |||||
| 32.2* | |||||
| 101 | The following materials from the Company's Annual Report on Form 10-K for the year ended October 31, 2022, formatted in Inline XBRL (Extensible Business Reporting Language):(i) Consolidated Statements of Income for the years ended October 31, 2022, 2021 and 2020 (ii) Consolidated Statements of Comprehensive Income for the years ended October 31, 2022, 2021 and 2020 (iii) Consolidated Balance Sheets at October 31, 2022 and 2021, (iv) Consolidated Statements of Stockholders' Equity for the years ended October 31, 2022, 2021 and 2020 (v) Consolidated Statements of Cash Flows for the years ended October 31, 2022, 2021 and 2020, (vi) related notes to consolidated financial statements and (vii) Schedule II Valuation and Qualifying Accounts | ||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | ||||
(a) The agreement received confidential treatment from the Securities and Exchange Commission with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Commission.
# Indicates management contract or compensatory plan.
* The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K are not deemed filed with the SEC and are not to be incorporated by reference into any filing of The Cooper Companies, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.
108
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Item 16. Form 10-K Summary.
None.
109
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on December 9, 2022.
THE COOPER COMPANIES, INC.
By: /s/ Albert G. White, III
Albert G. White, III
President & Chief Executive Officer
110
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated on the dates set forth opposite their respective names.
| Signature | Capacity | Date | ||||||||||||
| /s/ ALBERT G. WHITE, III | President, Chief Executive Officer and Director (Principal Executive Officer) | December 9, 2022 | ||||||||||||
| (Albert G. White, III) | ||||||||||||||
| /s/ ROBERT S. WEISS | Chairman of the Board | December 9, 2022 | ||||||||||||
| (Robert S. Weiss) | ||||||||||||||
| /s/ WILLIAM A. KOZY | Vice Chairman of the Board and Lead Director | December 9, 2022 | ||||||||||||
| (William A. Kozy) | ||||||||||||||
| /s/ BRIAN G. ANDREWS | Executive Vice President, Chief Financial Officer and Treasurer | December 9, 2022 | ||||||||||||
| (Brian G. Andrews) | (Principal Financial Officer) | |||||||||||||
| /s/ AGOSTINO RICUPATI | Senior Vice President and Chief Accounting Officer | December 9, 2022 | ||||||||||||
| (Agostino Ricupati) | (Principal Accounting Officer) | |||||||||||||
| /s/ COLLEEN E. JAY | Director | December 9, 2022 | ||||||||||||
| (Colleen E. Jay) | ||||||||||||||
| /s/ JODY S. LINDELL | Director | December 9, 2022 | ||||||||||||
| (Jody S. Lindell) | ||||||||||||||
| /s/ GARY S. PETERSMEYER | Director | December 9, 2022 | ||||||||||||
| (Gary S. Petersmeyer) | ||||||||||||||
| /s/ MARIA RIVAS M.D. | Director | December 9, 2022 | ||||||||||||
| (Maria Rivas M.D.) | ||||||||||||||
| /s/ TERESA S. MADDEN | Director | December 9, 2022 | ||||||||||||
| (Teresa S. Madden) | ||||||||||||||
/s/ CYNTHIA L. LUCCHESE | Director | December 9, 2022 | ||||||||||||
| (Cynthia L. Lucchese) | ||||||||||||||
111
THE COOPER COMPANIES, INC. AND SUBSIDIARIES
CORPORATE INFORMATION
BOARD OF DIRECTORS Robert S. Weiss Chairman of the Board William A. Kozy Vice Chairman and Lead Director Colleen E. Jay Director Jody S. Lindell President and Chief Executive Officer, S.G. Management, Inc. Cynthia L. Lucchese Chief Strategy Officer, Penske Entertainment Corp. Teresa S. Madden Director Gary S. Petersmeyer Director Maria Rivas M.D. Global Chief Medical Affairs Officer and Head of Evidence Generation, Pfizer, Inc. Albert G. White, III President & Chief Executive Officer COMMITTEES OF THE BOARD Audit Committee Teresa S. Madden (Chairman) Jody S. Lindell Cynthia L. Lucchese Gary S. Petersmeyer Maria Rivas M.D. Corporate Governance and Nominating Committee William A. Kozy (Chairman) Colleen E. Jay Jody S. Lindell Cynthia L. Lucchese Maria Rivas M.D. Organization and Compensation Committee Colleen E. Jay (Chairman) William A. Kozy Teresa S. Madden Gary S. Petersmeyer | EXECUTIVE OFFICERS Albert G. White, III President and Chief Executive Officer Daniel G. McBride Executive Vice President and Chief Operating Officer Brian G. Andrews Executive Vice President, Chief Financial Officer and Treasurer Agostino Ricupati Senior Vice President and Chief Accounting Officer Nicholas S. Khadder Vice President, General Counsel and Corporate Secretary Holly R. Sheffield President of CooperSurgical, Inc. Gerard H. Warner III President of CooperVision, Inc. PRINCIPAL SUBSIDIARIES CooperVision, Inc. 6101 Bollinger Canyon Road Suite 500 San Ramon, CA 94583 925-460-3600 www.coopervision.com CooperSurgical, Inc. 75 Corporate Drive Trumbull, CT 06611 203-601-5200 www.coopersurgical.com CORPORATE OFFICES The Cooper Companies, Inc. 6101 Bollinger Canyon Road Suite 500 San Ramon, CA 94583 925-460-3600 www.coopercos.com | INVESTOR INFORMATION Recent news releases, the annual report on Securities and Exchange Commission Form 10-K, information about the Company's corporate governance program, recent investor presentations, replays of quarterly conference calls and historical stock quotes are available on our Web site at www.coopercos.com. INVESTOR RELATIONS CONTACT Kim Duncan Vice President, Investor Relations and Risk Management 6101 Bollinger Canyon Road Suite 500 San Ramon, CA 94583 Voice: 925-460-3663 E-mail: ir@cooperco.com ANNUAL MEETING The Cooper Companies will hold its Annual Stockholders' Meeting in March 2023. TRANSFER AGENT American Stock Transfer & Trust Company 6201 15th Avenue Brooklyn, NY 11219 800-937-5449 TRADEMARKS The Cooper Companies, Inc., its subsidiaries or affiliates own, license or distribute the registered trademarks, common law trademarks and trade names referenced in this report. INDEPENDENT AUDITORS KPMG LLP STOCK EXCHANGE LISTING The New York Stock Exchange Ticker Symbol “COO” | ||||||||||||
112
Similar companies
See also ALCON INCSee also STAAR SURGICAL CO - Annual report 2022 (10-K 2022-12-30) Annual report 2023 (10-Q 2023-09-29)
See also National Vision Holdings, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Warby Parker Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also RxSight, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-06-30)
