DURECT CORP - Quarter Report: 2019 March (Form 10-Q)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2019
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-31615
DURECT CORPORATION
(Exact name of registrant as specified in its charter)
|
Delaware |
94-3297098 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
10260 Bubb Road
Cupertino, California 95014
(Address of principal executive offices, including zip code)
(408) 777-1417
(Registrant’s telephone number, including area code)
|
|
|
|
|
|
Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which Registered |
|
|
Common Stock $0.0001 par value per share Preferred Share Purchase Rights |
DRRX |
The NASDAQ Stock Market LLC (The Nasdaq Global Market) |
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by a check mark whether the registrant a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☒ |
|
|
|
|
|
|
Non-accelerated filer |
☐ |
Smaller reporting company |
☒ |
|
Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 3, 2019, there were 162,359,931 shares of the registrant’s Common Stock outstanding.
|
|
|
Page |
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. |
3 |
|
|
|
|
|
|
|
Condensed Balance Sheets as of March 31, 2019 and December 31, 2018 |
3 |
|
|
|
|
|
|
Condensed Statements of Comprehensive Loss for the three months ended March 31, 2019 and 2018 |
4 |
|
|
|
|
|
|
Condensed Statements of Stockholders’ Equity for the three months ended March 31, 2019 and 2018 |
5 |
|
|
|
|
|
|
Condensed Statements of Cash Flows for the three months ended March 31, 2019 and 2018 |
6 |
|
|
|
|
|
|
7 |
|
|
|
|
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
18 |
|
|
|
|
|
Item 3. |
35 |
|
|
|
|
|
|
Item 4. |
35 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. |
36 |
|
|
|
|
|
|
Item 1A. |
36 |
|
|
|
|
|
|
Item 2. |
58 |
|
|
|
|
|
|
Item 3. |
58 |
|
|
|
|
|
|
Item 4. |
58 |
|
|
|
|
|
|
Item 5. |
58 |
|
|
|
|
|
|
Item 6. |
58 |
|
|
|
|
|
|
59 |
||
2
DURECT CORPORATION
(in thousands)
|
|
|
March 31, 2019 |
|
|
December 31, 2018 |
|
||
|
|
|
(unaudited) |
|
|
(Note 1) |
|
||
|
A S S E T S |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
27,641 |
|
|
$ |
31,644 |
|
|
Short-term investments |
|
|
989 |
|
|
|
2,671 |
|
|
Accounts receivable (net of allowances of $68 at March 31, 2019 and $102 at December 31, 2018) |
|
|
2,221 |
|
|
|
1,757 |
|
|
Inventories, net |
|
|
3,410 |
|
|
|
3,421 |
|
|
Prepaid expenses and other current assets |
|
|
2,213 |
|
|
|
2,247 |
|
|
Total current assets |
|
|
36,474 |
|
|
|
41,740 |
|
|
Property and equipment, net |
|
|
589 |
|
|
|
605 |
|
|
Operating lease right-of-use assets |
|
|
7,028 |
|
|
|
— |
|
|
Goodwill |
|
|
6,399 |
|
|
|
6,399 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
150 |
|
|
Other long-term assets |
|
|
1,105 |
|
|
|
1,105 |
|
|
Total assets |
|
$ |
51,745 |
|
|
$ |
49,999 |
|
|
L I A B I L I T I E S A N D S T O C K H O L D E R S’ E Q U I T Y |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,933 |
|
|
$ |
1,589 |
|
|
Accrued liabilities |
|
|
4,322 |
|
|
|
4,668 |
|
|
Contract research liabilities |
|
|
1,489 |
|
|
|
1,405 |
|
|
Operating lease liabilities, current portion |
|
|
1,999 |
|
|
|
— |
|
|
Total current liabilities |
|
|
9,743 |
|
|
|
7,662 |
|
|
Deferred revenue, non-current portion |
|
|
812 |
|
|
|
812 |
|
|
Operating lease liabilities, non-current portion |
|
|
5,440 |
|
|
|
— |
|
|
Term loan, non-current portion, net |
|
|
20,670 |
|
|
|
20,533 |
|
|
Other long-term liabilities |
|
|
722 |
|
|
|
992 |
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Common stock |
|
|
16 |
|
|
|
16 |
|
|
Additional paid-in capital |
|
|
490,100 |
|
|
|
488,608 |
|
|
Accumulated other comprehensive loss |
|
|
(4 |
) |
|
|
— |
|
|
Accumulated deficit |
|
|
(475,754 |
) |
|
|
(468,624 |
) |
|
Stockholders’ equity |
|
|
14,358 |
|
|
|
20,000 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
51,745 |
|
|
$ |
49,999 |
|
The accompanying notes are an integral part of these condensed financial statements.
3
CONDENSED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands, except per share amounts)
(unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Collaborative research and development and other revenue |
|
$ |
1,500 |
|
|
$ |
1,096 |
|
|
Product revenue, net |
|
|
2,631 |
|
|
|
2,392 |
|
|
Total revenues |
|
|
4,131 |
|
|
|
3,488 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Cost of product revenues |
|
|
1,136 |
|
|
|
1,174 |
|
|
Research and development |
|
|
6,251 |
|
|
|
6,952 |
|
|
Selling, general and administrative |
|
|
3,454 |
|
|
|
3,194 |
|
|
Total operating expenses |
|
|
10,841 |
|
|
|
11,320 |
|
|
Loss from operations |
|
|
(6,710 |
) |
|
|
(7,832 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest and other income |
|
|
209 |
|
|
|
158 |
|
|
Interest expense |
|
|
(629 |
) |
|
|
(623 |
) |
|
Net other expense |
|
|
(420 |
) |
|
|
(465 |
) |
|
Net loss |
|
$ |
(7,130 |
) |
|
$ |
(8,297 |
) |
|
Net change in unrealized loss on available-for-sale securities, net of reclassification adjustments and taxes |
|
|
(4 |
) |
|
|
— |
|
|
Total comprehensive loss |
|
$ |
(7,134 |
) |
|
$ |
(8,297 |
) |
|
Net loss per share |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.04 |
) |
|
$ |
(0.05 |
) |
|
Diluted |
|
$ |
(0.04 |
) |
|
$ |
(0.05 |
) |
|
Weighted-average shares used in computing net loss per share |
|
|
|
|
|
|
|
|
|
Basic |
|
|
162,091 |
|
|
|
153,558 |
|
|
Diluted |
|
|
162,091 |
|
|
|
153,558 |
|
The accompanying notes are an integral part of these condensed financial statements.
4
CONDENSED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except per share amounts)
(unaudited)
|
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated Other Comprehensive |
|
|
Accumulated |
|
|
Total Stockholders’ |
|
|||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Deficit |
|
|
Equity |
|
||||||
|
Balance at December 31, 2018 |
|
|
162,060 |
|
|
$ |
16 |
|
|
$ |
488,608 |
|
|
$ |
— |
|
|
$ |
(468,624 |
) |
|
$ |
20,000 |
|
|
Issuance of common stock upon equity financings, net of issuance costs of $129 |
|
|
243 |
|
|
|
— |
|
|
|
61 |
|
|
|
— |
|
|
|
— |
|
|
|
61 |
|
|
Stock-based compensation expense from stock options and ESPP shares |
|
|
— |
|
|
|
— |
|
|
|
437 |
|
|
|
— |
|
|
|
— |
|
|
|
437 |
|
|
Fully vested options issued to settle accrued liabilities |
|
|
|
|
|
|
|
|
|
|
994 |
|
|
|
|
|
|
|
|
|
|
|
994 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7,130 |
) |
|
|
(7,130 |
) |
|
Change in unrealized gain on available-for-sale securities, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4 |
) |
|
|
— |
|
|
|
(4 |
) |
|
Balance at March 31, 2019 |
|
|
162,303 |
|
|
$ |
16 |
|
|
$ |
490,100 |
|
|
$ |
(4 |
) |
|
$ |
(475,754 |
) |
|
$ |
14,358 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2017 |
|
|
150,837 |
|
|
$ |
15 |
|
|
$ |
465,246 |
|
|
$ |
(1 |
) |
|
$ |
(443,772 |
) |
|
$ |
21,488 |
|
|
Adjustment due to changes in accounting policies |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
470 |
|
|
|
470 |
|
|
Issuance of common stock upon exercise of stock options |
|
|
515 |
|
|
|
— |
|
|
|
565 |
|
|
|
— |
|
|
|
— |
|
|
|
565 |
|
|
Issuance of common stock upon equity financings, net of issuance costs of $469 |
|
|
8,171 |
|
|
|
1 |
|
|
|
13,645 |
|
|
|
— |
|
|
|
— |
|
|
|
13,646 |
|
|
Stock-based compensation expense from stock options and ESPP shares |
|
|
— |
|
|
|
— |
|
|
|
663 |
|
|
|
— |
|
|
|
— |
|
|
|
663 |
|
|
Fully vested options issued to settle accrued liabilities |
|
|
|
|
|
|
|
|
|
|
1,860 |
|
|
|
|
|
|
|
|
|
|
|
1,860 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8,297 |
) |
|
|
(8,297 |
) |
|
Balance at March 31, 2018 |
|
|
159,523 |
|
|
$ |
16 |
|
|
$ |
481,979 |
|
|
$ |
(1 |
) |
|
$ |
(451,599 |
) |
|
$ |
30,395 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed financial statements
5
CONDENSED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(7,130 |
) |
|
$ |
(8,297 |
) |
|
Adjustments to reconcile net loss to net cash used in by operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
82 |
|
|
|
108 |
|
|
Stock-based compensation |
|
|
437 |
|
|
|
661 |
|
|
Amortization of debt issuance cost |
|
|
89 |
|
|
|
23 |
|
|
Net amortization on investments |
|
|
16 |
|
|
|
16 |
|
|
Changes in operating lease liabilities |
|
|
50 |
|
|
|
— |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(464 |
) |
|
|
557 |
|
|
Inventories |
|
|
11 |
|
|
|
(89 |
) |
|
Prepaid expenses and other assets |
|
|
33 |
|
|
|
259 |
|
|
Accounts payable |
|
|
344 |
|
|
|
(729 |
) |
|
Accrued and other liabilities |
|
|
792 |
|
|
|
1,396 |
|
|
Contract research liabilities |
|
|
84 |
|
|
|
(114 |
) |
|
Deferred revenue |
|
|
— |
|
|
|
(479 |
) |
|
Total adjustments |
|
|
1,474 |
|
|
|
1,609 |
|
|
Net cash used in operating activities |
|
|
(5,656 |
) |
|
|
(6,688 |
) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(66 |
) |
|
|
(24 |
) |
|
Purchases of available-for-sale securities |
|
|
(34 |
) |
|
|
(1,741 |
) |
|
Proceeds from maturities of available-for-sale securities |
|
|
1,696 |
|
|
|
4,300 |
|
|
Net cash provided by investing activities |
|
|
1,596 |
|
|
|
2,535 |
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Payments on equipment financing obligations |
|
|
(4 |
) |
|
|
(3 |
) |
|
Payment of additional issuance cost for term loan |
|
|
— |
|
|
|
(105 |
) |
|
Net proceeds from issuances of common stock |
|
|
61 |
|
|
|
14,211 |
|
|
Net cash provided by financing activities |
|
|
57 |
|
|
|
14,103 |
|
|
Net decrease in Cash, cash equivalents, and restricted cash |
|
|
(4,003 |
) |
|
|
9,950 |
|
|
Cash, cash equivalents, and restricted cash, beginning of the period |
|
|
31,794 |
|
|
|
29,525 |
|
|
Cash, cash equivalents, and restricted cash, end of the period (1) |
|
$ |
27,791 |
|
|
$ |
39,475 |
|
|
Supplementary disclosure of non-cash financing information |
|
|
|
|
|
|
|
|
|
Fully vested options issued to settle accrued liabilities |
|
$ |
994 |
|
|
$ |
1,860 |
|
|
Operating lease right-of-use assets obtained in exchange for operating lease obligations (2) |
|
$ |
7,329 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
(1) Includes restricted cash of $150,000 (in long term restricted investments) included in the condensed balance sheets at both March 31, 2019 and March 31, 2018. |
|
|
|
|
|
|
|
|
|
(2) Amounts for the three months ended March 31, 2019 include the transition adjustment for the adoption of Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842) (“Topic 842”). |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed financial statements.
6
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1. Summary of Significant Accounting Policies
Nature of Operations
DURECT Corporation (the Company) was incorporated in the state of Delaware on February 6, 1998. The Company is a biopharmaceutical company with research and development programs broadly falling into two categories: (i) new chemical entities derived from the Company’s Epigenetics Regulator Program, in which the Company attempts to discover and develop molecules which have not previously been approved and marketed as therapeutics, and (ii) proprietary pharmaceutical programs, in which the Company applies its formulation expertise and technologies largely to active pharmaceutical ingredients whose safety and efficacy have previously been established but which the Company aims to improve in some manner through a new formulation. The Company has several products under development by itself and with third party collaborators. The Company also manufactures and sells osmotic pumps used in laboratory research, and designs, develops and manufactures a wide range of standard and custom biodegradable polymers and excipients for pharmaceutical and medical device clients for use as raw materials in their products. In addition, the Company conducts research and development of pharmaceutical products in collaboration with third party pharmaceutical and biotechnology companies.
Basis of Presentation
These financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (SEC), and therefore do not include all the information and footnotes necessary for a complete presentation of the Company’s results of operations, financial position and cash flows in conformity with U.S. generally accepted accounting principles (U.S. GAAP). The unaudited financial statements reflect all adjustments (consisting only of normal recurring adjustments) which are, in the opinion of management, necessary for a fair presentation of the financial position at March 31, 2019, the operating results and comprehensive loss for the three months ended March 31, 2019 and 2018, stockholders’ equity for the three months ended March 31, 2019 and 2018, and cash flows for the three months ended March 31, 2019 and 2018. The balance sheet as of December 31, 2018 has been derived from audited financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These financial statements and notes should be read in conjunction with the Company’s audited financial statements and notes thereto, included in the Company’s annual report on Form 10-K for the fiscal year ended December 31, 2018 filed with the SEC.
The results of operations for the interim periods presented are not necessarily indicative of results that may be expected for any other interim period or for the full fiscal year.
Liquidity and Need to Raise Additional Capital
As of March 31, 2019, the Company had an accumulated deficit of $475.8 million as well as negative cash flows from operating activities for the three months ended March 31, 2019.
The Company historically has had negative cash flows from operating activities and expects its negative cash flows to continue. The Company will continue to require substantial funds to continue research and development, including clinical trials of its product candidates. Management’s plans in order to meet its operating cash flow requirements include seeking additional collaborative agreements for certain of its programs and achieving milestone and other payments under its collaboration and licensing agreements as well as financing activities such as public offerings and private placements of its common stock, preferred stock offerings, issuances of debt and convertible debt instruments.
There are no assurances that such additional funding will be obtained and that the Company will succeed in its future operations. If the Company cannot successfully raise additional capital and implement its strategic development plan, its liquidity, financial condition and business prospects will be materially and adversely affected.
Inventories
Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. Inventories, in part, include certain excipients that are sold to a customer for a currently marketed animal health product and included in several products in development, or awaiting regulatory approval or commercial launch. The Company may be required to expense previously capitalized inventory costs upon a change in management’s judgment due to, among other potential factors, a denial or delay of approval of a customer’s product by the necessary regulatory bodies, or new information that suggests that the inventory will not be saleable. If the Company is able to subsequently sell products made with raw materials that were previously written down, the Company will report an unusually high gross profit as there will be no associated cost of goods for these materials.
7
The Company’s inventories consist of the following (in thousands):
|
|
|
March 31, 2019 |
|
|
December 31, 2018 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Raw materials |
|
$ |
259 |
|
|
$ |
223 |
|
|
Work in process |
|
|
1,446 |
|
|
|
1,486 |
|
|
Finished goods |
|
|
1,705 |
|
|
|
1,712 |
|
|
Total inventories |
|
$ |
3,410 |
|
|
$ |
3,421 |
|
Leases
Effective January 1, 2019, the Company adopted Topic 842 using the modified retrospective transition method approach with a cumulative-effect adjustment as of January 1, 2019 in accordance with ASU No. 2018-11, Leases (Topic 842) - Targeted Improvements. Results for the three months ended March 31, 2019 are presented under Topic 842. Other prior period amounts are not adjusted and continue to be reported in accordance with our historic accounting under previous lease guidance, ASC Topic 840: Leases (Topic 840). The Company elected the package of practical expedients permitted under the transition guidance within the new standard, which among other things, allowed the Company to carry forward the historical lease classification of those leases in place as of January 1, 2019.
The adjustments due to the adoption of Topic 842 primarily related to the recognition of an operating lease right-of-use asset and operating lease liability for our leased properties.
The impact of the adoption of Topic 842 on the accompanying Condensed Balance Sheet as of January 1, 2019 was as follows (in thousands):
|
|
|
As of March 31, 2019 |
|
|||||||||
|
|
|
December 31, 2018 |
|
|
Adjustments Due to the Adoption of Topic 842 |
|
|
January 1, 2019 |
|
|||
|
Condensed Balance Sheets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease right-of-use assets |
|
$ |
— |
|
|
$ |
7,329 |
|
|
$ |
7,329 |
|
|
Operating lease liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accrued liabilities (1) |
|
$ |
(92 |
) |
|
$ |
92 |
|
|
$ |
— |
|
|
Other long-term liabilities (1) |
|
|
(270 |
) |
|
|
270 |
|
|
|
— |
|
|
Lease liabilities, current portion |
|
|
— |
|
|
|
(1,972 |
) |
|
|
(1,972 |
) |
|
Lease liabilities, non-current portion |
|
|
— |
|
|
|
(5,719 |
) |
|
|
(5,719 |
) |
|
|
|
$ |
(362 |
) |
|
$ |
(7,329 |
) |
|
$ |
(7,691 |
) |
|
(1) Includes deferred rent, current and long-term portions of operating lease liabilities which was recorded against the operating lease right-of-use asset upon adoption of Topic 842 |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There was no effect from the adoption of Topic 842 on the Company’s condensed statement of cash flows.
Revenue Recognition
The Company enters into license and collaboration agreements under which the Company may receive upfront license fees, research funding and contingent milestone payments and royalties. Effective January 1, 2018, the Company adopted FASB ASC Topic 606, Revenue from Contracts with Customers, or ASC 606. In accordance with ASC 606, the Company changed certain characteristics of its revenue recognition accounting policy as described below. ASC 606 was applied using the modified retrospective method, where the cumulative effect of the initial application was recognized as an adjustment to opening retained earnings at January 1, 2018. The Company recorded a net increase to opening retained earnings of $470,000 with an offset entry to a contra liability account as of January 1, 2018 due to the cumulative impact of adopting Topic 606, with the impact relating to the Company’s deferred collaborative research and development revenues. There was no impact to reported total assets, revenues and operating expenses for the three months ended March 31, 2019 as a result of applying Topic 606.
Product Revenue, Net
The Company sells osmotic pumps used in laboratory research, and designs, develops and manufactures a wide range of standard and custom biodegradable polymers and excipients for pharmaceutical and medical device clients for use as raw materials in their products.
8
Revenues from product sales are recognized when the customer obtains control of the Company’s product, which occurs at a point in time, typically upon shipment to the customer. The Company expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that the Company would have recognized is one year or less.
Trade Discounts and Allowances: The Company provides certain customers with discounts that are explicitly stated in the Company’s contracts and are recorded as a reduction of revenue in the period the related product revenue is recognized.
Product Returns: Consistent with industry practice, the Company generally offers customers a limited right of return for products that have been purchased from the Company. The Company estimates the amount of its product sales that may be returned by its customers and records this estimate as a reduction of revenue in the period the related product revenue is recognized. The Company currently estimates product return liabilities using its historical sales information. The Company expects product returns to be minimal.
Collaborative Research and Development Revenues
The Company enters into license agreements which are within the scope of Topic 606, under which it licenses certain rights to its product candidates to third parties. The terms of these arrangements typically include payment to the Company of one or more of the following: non-refundable, up-front license fees; reimbursement of development costs incurred by the Company under approved work plans; development, regulatory and commercial milestone payments; payments for manufacturing supply services the Company provides through its contract manufacturers; and royalties on net sales of licensed products. Each of these payments results in collaborative research and development revenues, except for revenues from royalties on net sales of licensed products, which are classified as royalty revenues.
In determining the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. As part of the accounting for these arrangements, the Company must develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the contract. The Company uses key assumptions to determine the standalone selling price, which may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical and regulatory success. The Company expects to recognize revenue for the variable consideration currently being constrained when it is probable that a significant revenue reversal will not occur.
Licenses of intellectual property: If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenues from non-refundable, up-front fees allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Milestone Payments: At the inception of each arrangement that includes development milestone payments, the Company evaluates whether the milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of the Company or the licensee, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. The transaction price is then allocated to each performance obligation on a relative stand-alone selling price basis, for which the Company recognizes revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of such development milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaborative research and development revenues and net income (loss) in the period of adjustment.
Manufacturing Supply Services: Arrangements that include a promise for future supply of drug product for either clinical development or commercial supply at the customer’s discretion are generally considered as options. The Company assesses if these options provide a material right to the customer and if so, they are accounted for as separate performance obligations. If the Company is entitled to additional payments when the customer exercises these options, any additional payments are recorded in collaborative research and development revenue when the customer obtains control of the goods, which is upon delivery.
Royalties and Earn-outs: For arrangements that include sales-based royalties or earn-outs, including milestone payments based on the level of sales, and the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any significant royalty revenue
9
resulting from the Company’s collaborative arrangements or any earn-out revenue from the Company’s patent purchase agreement with Indivior.
The Company receives payments from its customers based on development cost schedules established in each contract. Up-front payments and fees are recorded as deferred revenue upon receipt or when due, and may require deferral of revenue recognition to a future period until the Company performs its obligations under these arrangements. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional. The Company does not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between payment by the customer and the transfer of the promised goods or services to the customer will be one year or less.
Total revenue by geographic region for the three months ended March 31, 2019 and 2018 are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
United States |
|
$ |
2,523 |
|
|
$ |
2,234 |
|
|
Europe |
|
|
827 |
|
|
|
756 |
|
|
Japan |
|
|
422 |
|
|
|
287 |
|
|
Other |
|
|
359 |
|
|
|
211 |
|
|
Total |
|
$ |
4,131 |
|
|
$ |
3,488 |
|
During the three months ended March 31, 2019, the Company did not recognize any revenue as a result of changes in the contract asset and the contract liability balances associated with the Company’s deferred research and development revenues for the Company’s collaboration agreements.
Comprehensive Loss
Components of other comprehensive loss are comprised entirely of unrealized gains and losses on the Company’s available-for-sale securities for all periods presented. Total comprehensive loss has been disclosed in the Company’s Statements of Comprehensive Loss.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding. Diluted net loss per share is computed using the weighted-average number of common shares outstanding and common stock equivalents (i.e., options to purchase common stock) outstanding during the period, if dilutive, using the treasury stock method for options.
The numerators and denominators in the calculation of basic and diluted net loss per share were as follows (in thousands except per share amounts):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Numerators: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(7,130 |
) |
|
$ |
(8,297 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted average shares used to compute basic net loss per share |
|
|
162,091 |
|
|
|
153,558 |
|
|
Dilutive common shares from stock options and ESPP |
|
|
— |
|
|
|
— |
|
|
Weighted average shares used to compute diluted net loss per share |
|
|
162,091 |
|
|
|
153,558 |
|
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.04 |
) |
|
$ |
(0.05 |
) |
|
Diluted |
|
$ |
(0.04 |
) |
|
$ |
(0.05 |
) |
Options to purchase approximately 30.2 million and 16.8 million shares of common stock were excluded from the denominator in the calculation of diluted net loss per share for the three ended March 31, 2019 and 2018, respectively, as the effect would be anti-dilutive.
Recently Adopted Accounting Standards
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). This ASU required the recognition of lease assets and liabilities for operating leases with terms of more than 12 months, in addition to the capital lease assets and liabilities currently recorded on the Company’s consolidated balance sheets. Presentation of leases within the consolidated statements of operations and cash flows are substantially consistent with current accounting guidance. The ASU, which was effective for annual reporting periods beginning after December 15, 2018, and interim periods within those annual periods, had a material impact on the Company’s
10
consolidated balance sheets. The Company adopted the ASU effective January 1, 2019 using the modified retrospective transition method and did not restate comparative periods. The modified retrospective transition method requires the cumulative effect, if any, of initially applying the guidance to be recognized as an adjustment to the Company’s accumulated deficit as of that adoption date. The Company elected the package of practical expedients permitted under the transition guidance within the ASU, which allowed it to carry forward prior conclusions about lease identification, classification and initial direct costs for leases entered into prior to adoption of Topic 842. Additionally, the Company did not separate lease and non-lease components for all of our leases. For leases with a term of 12 months or less, the Company elected the short-term lease exemption, which allowed it to not recognize right-of-use assets or lease liabilities for qualifying leases existing at transition and new leases we may enter into in the future. The Company recognized additional lease liabilities of approximately $7.7 million representing the present value of the remaining minimum lease payments at January 1, 2019 and corresponding right-of-use assets of approximately $7.3 million.
In June 2018, the FASB issued Accounting Standards Update (“ASU”) No. 2018-07, Compensation – Stock Compensation (Topic 718), which expands the scope of ASC 718 to include all share-based payment arrangements related to the acquisition of goods and services from both nonemployees and employees. Under the new standard, most of the guidance on stock compensation payments to nonemployees would be aligned with the requirements for share-based payments granted to employees. This standard was effective for fiscal years beginning after December 15, 2018, including interim reporting periods within those years, with early adoption permitted. The adoption of this standard did not have a material effect on the Company’s financial statements.
In February 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2018-02, “Income Statement – Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income.” The FASB issued the update to provide amended guidance to “allow a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act.” Additionally, under the new guidance an entity will be required to provide certain disclosures regarding stranded tax effects. The guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within those years, and the guidance may be applied either in the period of adoption or retrospectively to each period (or periods) in which the effect of the change in the U.S. federal income tax rate in the Tax Cuts and Jobs Act is recognized. Early adoption is permitted. The adoption of this standard did not have a material effect on the Company’s financial statements.
Recently Issued Accounting Standards
In November 2018, the Financial Accounting Standards Board (the FASB) issued ASU No. 2018-18, Collaborative Arrangements (Topic 808): Clarifying the Interaction between Topic 808 and Topic 606 (ASU 2018-18). ASU 2018-18 clarifies that certain transactions between collaborative arrangement participants should be accounted for as revenue under Topic 606 when the counterparty is a customer for a distinct good or service (i.e. a unit of account). For units of account that are in the scope of Topic 606, all of the guidance in Topic 606 should be applied, including the guidance on recognition, measurement, presentation and disclosure. ASU 2018-18 also adds a reference in Topic 808 to the unit of account guidance in ASC 606 and requires that it be applied only to assess whether transactions in a collaborative arrangement are in the scope of Topic 606. ASU 2018-18 will preclude entities from presenting amounts related to transactions with a counterparty in a collaborative arrangement that is not a customer as revenue from contracts with customers. ASU 2018-18 is effective for the Company for all interim and annual reporting periods beginning after December 15, 2019. Early adoption is permitted. The Company is in the process of assessing the impact of ASU 2018-18 on its financial statements.
In August 2018, the FASB issued Accounting Standards Update (“ASU”) No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement, which eliminates certain disclosure requirements for fair value measurements for all entities, requires public entities to disclose certain new information and modifies some disclosure requirements. This standard is effective for fiscal years beginning after December 15, 2019, including interim reporting periods within those years, with early adoption permitted. The Company does not expect the adoption of this standard to have a material effect on its financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles—Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment, (ASU 2017-04). ASU 2017-04 eliminated Step 2 from the goodwill impairment test. Instead, under the amendments in ASU 2017-04, an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. ASU 2017-04 is effective for all interim and annual reporting periods beginning after December 15, 2019. Early adoption is permitted. The Company does not expect the adoption of ASU 2017-04 to have a material impact on its financial statements.
In June 2016, the FASB issued Accounting Standards Update No. 2016-13 (ASU 2016-13) “Financial Instruments - Credit Losses: Measurement of Credit Losses on Financial Instruments.” ASU 2016-13 requires measurement and recognition of expected
11
credit losses for financial assets. This standard is effective for fiscal years beginning after December 15, 2019, including interim reporting periods within those years and must be adopted using a modified retrospective approach, with certain exceptions. Early adoption is permitted. The Company does not expect the adoption of this standard to have a material effect on its financial statements.
Note 2. Strategic Agreements
The collaborative research and development and other revenues associated with the Company’s major collaborators or counterparties are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Collaborator/Counterparty |
|
|
|
|
|
|
|
|
|
Indivior UK Limited (Indivior) (1) |
|
$ |
7 |
|
|
$ |
— |
|
|
Santen Pharmaceutical Co. Ltd. (Santen) (2) |
|
|
14 |
|
|
|
1 |
|
|
Others (3) |
|
|
1,479 |
|
|
|
1,095 |
|
|
Total collaborative research and development and other revenue |
|
$ |
1,500 |
|
|
$ |
1,096 |
|
|
(1) |
Amount related to $7,000 earn-out revenue from PERSERIS net sales for the three months ended March 31, 2019, compared to zero for the corresponding period in 2018. |
|
(2) |
Amounts related to ratable recognition of upfront fees were zero for each of the three months ended March 31, 2019 and 2018. In January 2018, we were notified by Santen that due to a shift in near term priorities, Santen has elected to reallocate research and development resources and put our program on pause until further notice. While the main program is on pause, the parties are working together on a limited set of research and development activities funded by Santen. |
(3) Includes revenue recognized associated with the Company’s feasibility agreements for each of the three months ended March 31, 2019 and 2018.
Patent Purchase Agreement with Indivior
On September 26, 2017, the Company entered into a Patent Purchase Agreement (the “Agreement”) with Indivior. Pursuant to the Agreement, the Company assigned to Indivior certain patents that may provide further intellectual property protection for PERSERIS™ (risperidone), Indivior’s extended-release injectable suspension for the treatment of schizophrenia in adults. In consideration for such assignment, Indivior made an upfront non-refundable payment to the Company of $12.5 million. Indivior also agreed to make an additional $5.0 million payment to the Company contingent upon NDA approval of PERSERIS, as well as quarterly earn-out payments that are based on a single digit percentage of U.S. net sales for certain products covered by the assigned patent rights, including PERSERIS. The assigned patent rights include granted patents extending through at least 2026. The Company also receives a non-exclusive right under the assigned patents to develop and commercialize certain risperidone-containing products and products that do not contain risperidone or buprenorphine. The agreement contains customary representations, warranties and indemnities of the parties. The Company received the payment of $12.5 million from Indivior in September 2017 and recognized the $12.5 million as revenue from sale of intellectual property rights in 2017 as the Company did not have any continuing obligations under the purchase agreement. On July 27, 2018, Indivior announced that the FDA had approved the NDA for PERSERIS thereby triggering the $5.0 million payment to the Company; this payment was received by the Company in August 2018. The Company recognized the $5.0 million as milestone revenue during the twelve months ended December 31, 2018 as there is no further performance obligation associated with this milestone payment. The Company recognized earn-out revenue of approximately $7,000 and zero from PERSERIS net sales in the three months ended March 31, 2019 and 2018, respectively.
Agreement with Pain Therapeutics, Inc.
In December 2002, the Company entered into an agreement with Pain Therapeutics, amended in December 2005, under which the Company granted Pain Therapeutics the exclusive, worldwide right to develop and commercialize selected long-acting oral opioid products using its ORADUR technology incorporating four specified opioid drugs. The cumulative aggregate payments received by the Company from Pain Therapeutics as of March 31, 2019 were $40.4 million under this agreement.
On March 20, 2019, the Company received written notice from Pain Therapeutics that, effective June 18, 2019, Pain Therapeutics is terminating this agreement. As a result of this termination, Pain Therapeutics will be returning its exclusive, worldwide commercialization rights to develop and market REMOXY ER to the Company.
Agreement with Santen Pharmaceutical Co., Ltd.
12
On December 11, 2014, the Company and Santen Pharmaceutical Co., Ltd. (Santen) entered into a definitive agreement (the Santen Agreement). Pursuant to the Santen Agreement, the Company granted Santen an exclusive worldwide license to the Company’s proprietary SABER formulation platform and other intellectual property to develop and commercialize a sustained release product utilizing the Company’s SABER technology to deliver an ophthalmology drug. Santen controls and funds the development and commercialization program, and the parties established a joint management committee to oversee, review and coordinate the development activities of the parties under the Santen Agreement.
In connection with the Santen agreement, Santen agreed to pay the Company an upfront fee of $2.0 million in cash and to make contingent cash payments to the Company of up to $76.0 million upon the achievement of certain milestones, of which $13.0 million are development-based milestones and $63.0 million are commercialization-based milestones including milestones requiring the achievement of certain product sales targets (none of which has been achieved as of March 31, 2019). Santen will also pay for certain Company costs incurred in the development of the licensed product. If the product is commercialized, the Company would also receive a tiered royalty on annual net product sales ranging from single-digit to the low double digits, determined on a country-by-country basis. In January 2018, the Company was notified by Santen that due to a shift in near term priorities, Santen elected to reallocate research and development resources and put the Company’s program on pause until further notice. As of March 31, 2019, the cumulative aggregate payments received by the Company under this agreement were $3.3 million.
The following table provides a summary of collaborative research and development revenue recognized under the Santen Agreement (in thousands).
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Research and development expenses reimbursable by Santen |
|
$ |
14 |
|
|
$ |
1 |
|
|
Total collaborative research and development revenue |
|
$ |
14 |
|
|
$ |
1 |
|
Agreement with Sandoz AG
In May 2017, the Company and Sandoz AG (“Sandoz”) entered into a license agreement to develop and market POSIMIR (bupivacaine extended release solution) in the United States. Following expiration of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (HSR), the agreement became effective in June 2017.
The cumulative aggregate payments received by the Company from Sandoz as of March 31, 2019 were $20.0 million under this agreement.
On January 2, 2019, the Company received written notice from Sandoz that effective January 27, 2019, Sandoz AG is terminating this agreement. As a result of this termination, Sandoz returned its exclusive commercialization rights to develop and market POSIMIR in the United States. The parties are in dispute with regard to Sandoz AG’s obligation to pay a termination fee to the Company. The Company has initiated a formal dispute resolution process related to the termination fee.
Note 3. Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company’s valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company follows a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. These levels of inputs are the following:
|
|
• |
Level 1—Quoted prices in active markets for identical assets or liabilities. |
|
|
• |
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
|
|
• |
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
13
The Company’s financial instruments are valued using quoted prices in active markets or based upon other observable inputs. Money market funds are classified as Level 1 financial assets. Certificates of deposit, commercial paper, corporate debt securities, and U.S. Government agency securities are classified as Level 2 financial assets. The fair value of the Level 2 assets is estimated using pricing models using current observable market information for similar securities. The Company’s Level 2 investments include U.S. government-backed securities and corporate securities that are valued based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications. The fair value of commercial paper is based upon the time to maturity and discounted using the three-month treasury bill rate. The average remaining maturity of the Company’s Level 2 investments as of March 31, 2019 is less than twelve months and these investments are rated by S&P and Moody’s at AAA or AA- for securities and A1, A2, P1 or P2 for commercial paper.
The following is a summary of available-for-sale securities as of March 31, 2019 and December 31, 2018 (in thousands):
|
|
|
March 31, 2019 |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Estimated Fair Value |
|
||||
|
Money market funds |
|
$ |
547 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
547 |
|
|
Certificates of deposit |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
Commercial paper |
|
|
26,658 |
|
|
|
1 |
|
|
|
(5 |
) |
|
|
26,654 |
|
|
|
|
$ |
27,355 |
|
|
$ |
1 |
|
|
$ |
(5 |
) |
|
$ |
27,351 |
|
|
Reported as: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
26,217 |
|
|
$ |
— |
|
|
$ |
(5 |
) |
|
$ |
26,212 |
|
|
Short-term investments |
|
|
988 |
|
|
|
1 |
|
|
|
— |
|
|
|
989 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
|
|
$ |
27,355 |
|
|
$ |
1 |
|
|
$ |
(5 |
) |
|
$ |
27,351 |
|
|
|
|
December 31, 2018 |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Estimated Fair Value |
|
||||
|
Money market funds |
|
$ |
502 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
502 |
|
|
Certificates of deposit |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
Commercial paper |
|
|
32,224 |
|
|
|
— |
|
|
|
— |
|
|
|
32,224 |
|
|
|
|
$ |
32,876 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
32,876 |
|
|
Reported as: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
30,055 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
30,055 |
|
|
Short-term investments |
|
|
2,671 |
|
|
|
— |
|
|
|
— |
|
|
|
2,671 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
|
|
$ |
32,876 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
32,876 |
|
The following is a summary of the cost and estimated fair value of available-for-sale securities at March 31, 2019, by contractual maturity (in thousands):
|
|
|
March 31, 2019 |
|
|||||
|
|
|
Amortized Cost |
|
|
Estimated Fair Value |
|
||
|
Mature in one year or less |
|
$ |
26,808 |
|
|
$ |
26,804 |
|
|
|
|
$ |
26,808 |
|
|
$ |
26,804 |
|
There were no securities that have had an unrealized loss for more than 12 months as of March 31, 2019.
As of March 31, 2019, unrealized losses on available-for-sale investments are not attributed to credit risk and are considered to be temporary. The Company believes that it is more-likely-than-not that investments in an unrealized loss position will be held until maturity or the recovery of the cost basis of the investment. To date, the Company has not recorded any impairment charges on marketable securities related to other-than-temporary declines in market value.
14
Note 4. Stock-Based Compensation
As of March 31, 2019, the Company has three stock-based compensation plans. The stock-based compensation cost that has been included in the statements of comprehensive loss is shown as below (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Cost of product revenues |
|
$ |
21 |
|
|
$ |
25 |
|
|
Research and development |
|
|
174 |
|
|
|
353 |
|
|
Selling, general and administrative |
|
|
242 |
|
|
|
283 |
|
|
Total stock-based compensation |
|
$ |
437 |
|
|
$ |
661 |
|
As of March 31, 2019 and 2018, $12,000 and $14,000 of stock-based compensation cost was capitalized in inventory on the Company’s balance sheets, respectively.
The Company uses the Black-Scholes option pricing model to value its stock options. The expected life computation is based on historical exercise patterns and post-vesting termination behavior. The Company considered its historical volatility in developing its estimate of expected volatility.
The Company used the following assumptions to estimate the fair value of stock options granted and shares purchased under its employee stock purchase plan for the three months ended March 31, 2019 and 2018:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Stock Options |
|
|
|
|
|
|
|
|
|
Risk-free rate |
|
2.3-2.7% |
|
|
2.7-2.9% |
|
||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
Expected life of option (in years) |
|
7.5-10.0 |
|
|
7.0-10.0 |
|
||
|
Volatility |
|
79-83% |
|
|
80-86% |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Employee Stock Purchase Plan |
|
|
|
|
|
|
|
|
|
Risk-free rate |
|
2.5% |
|
|
1.3% |
|
||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
Expected life of option (in years) |
|
0.5 |
|
|
0.5 |
|
||
|
Volatility |
|
60% |
|
|
146% |
|
||
Note 5. Term Loan
In July 2016, the Company entered a $20.0 million secured single-draw term loan with Oxford Finance LLC (Oxford Finance). The Loan Agreement provides for interest only payments for the first 18 months, followed by consecutive monthly payments of principal and interest in arrears starting on March 1, 2018 and continuing through the maturity date of the term loan of August 1, 2020. The Loan Agreement also provides for a floating interest rate (7.95% initially and 9.84% as of March 31, 2019) based on an index rate plus a spread, a $150,000 facility fee that was paid at closing and an additional payment equal to 9.25% of the principal amount of the term loan, which is due when the term loan becomes due or upon the prepayment of the facility. If the Company elects to prepay the loan, there is also a prepayment fee between 1% and 3% of the principal amount of the term loan depending on the timing of prepayment. The facility fee and other debt offering/issuance costs have been recorded as debt discount on the Company’s balance sheet and together with the final $1.9 million payment are being amortized to interest expense during the life of the term loan using the effective interest rate method.
The term loan is secured by substantially all of the assets of the Company, except that the collateral does not include any intellectual property (including licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The Loan Agreement contains customary representations, warranties and covenants by the Company, which covenants limit the Company’s ability to convey, sell, lease, transfer, assign or otherwise dispose of certain assets of the Company; engage in any business other than the businesses currently engaged in by the Company or reasonably related thereto; liquidate or dissolve; make certain management changes; undergo certain change of control events; create, incur, assume, or be liable with respect to certain indebtedness; grant certain liens; pay dividends and make certain other restricted payments; make certain investments; and make payments on any subordinated debt.
15
The Loan Agreement also contains customary indemnification obligations and customary events of default, including, among other things, the Company’s failure to fulfill certain obligations of the Company under the Loan Agreement and the occurrence of a material adverse change which is defined as a material adverse change in the Company’s business, operations, or condition (financial or otherwise), a material impairment of the prospect of repayment of any portion of the loan, or a material impairment in the perfection or priority of lender’s lien in the collateral or in the value of such collateral. In the event of default by the Company under the Loan Agreement, the lender would be entitled to exercise its remedies thereunder, including the right to accelerate the debt, upon which the Company may be required to repay all amounts then outstanding under the Loan Agreement, which could harm the Company’s financial condition. The conditionally exercisable call option related to the event of default is considered to be an embedded derivative which is required to be bifurcated and accounted for as a separate financial instrument. In the periods presented, the value of the embedded derivative is not material, but could become material in future periods if an event of default became more probable than is currently estimated.
In February 2018, the Company and Oxford Finance entered into a First Amendment of the Loan Agreement, which modified the terms of the Loan Agreement to change the first principal payment date from March 1, 2018 to December 1, 2018 and to increase the additional payment due when the term loan becomes due or upon the prepayment of the facility from 9.25% of the principal amount of the term loan to 10% of such amount. The interest rate and the maturity date remain unchanged, and the Company paid Oxford Finance a loan modification fee of $100,000.
On November 1, 2018, the Company and Oxford Finance entered into a Second Amendment of the Loan Agreement, which modified the terms of the Loan Agreement to change the first principal payment date from December 1, 2018 to June 1, 2020 and the final maturity date from August 1, 2020 to November 1, 2022. If the Company elects to prepay the loan, there is also a prepayment fee of between 0.75% and 2.5% of the principal amount of the term loan depending on the timing of prepayment. The interest rate and the final payment remained unchanged, however the Company paid Oxford Finance a Second Amendment fee of $900,000. This amount was recorded as additional debt discount and presented within net non-current portion of term loan on the Company’s balance sheet as of December 31, 2018 and will be amortized as interest expense using the effective interest method over the revised term of the loan.
The fair value of the term loan approximates the carrying value. Future maturities and interest payments due under the term loan as of March 31, 2019, are as follows (in thousands):
|
Nine months ended December 31, 2019 |
|
$ |
1,215 |
|
|
2020 |
|
|
6,824 |
|
|
2021 |
|
|
8,885 |
|
|
2022 |
|
|
8,911 |
|
|
Total minimum payments |
|
|
25,835 |
|
|
Less amount representing interest |
|
|
(4,304 |
) |
|
Gross balance of term loan |
|
|
21,531 |
|
|
Less unamortized debt discount |
|
|
(861 |
) |
|
Carrying value of term loan |
|
|
20,670 |
|
|
Less term loan, current portion, net |
|
|
— |
|
|
Term loan, non-current portion, net |
|
$ |
20,670 |
|
As of March 31, 2019, the Company was in compliance with all material covenants under the Loan Agreement and there had been no material adverse change.
Note 6. Commitments
Operating Leases
16
The Company has lease arrangements for its facilities in California and Alabama as follows.
Under these leases, the Company is required to pay certain maintenance expenses in addition to monthly rent. Rent expense is recognized on a straight-line basis over the lease term for leases that have scheduled rental payment increases. Rent expense under all operating leases was $537,000 and $464,000 for the three ended March 31, 2019 and 2018 respectively.
Future minimum payments under these noncancelable leases are as follows (in thousands):
|
|
|
Operating Leases |
|
|
|
Nine months ended December 31, 2019 |
|
$ |
1,607 |
|
|
2020 |
|
|
2,200 |
|
|
2021 |
|
|
2,126 |
|
|
2022 |
|
|
1,991 |
|
|
Thereafter |
|
|
2,245 |
|
|
|
|
$ |
10,169 |
|
Note 7. Stockholders’ Equity
In August 2018, the Company filed a new shelf registration statement on Form S-3 with the SEC, which upon being declared effective in October 2018, terminated the November 2015 registration statement and allowed the Company to offer up to $175.0 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of additional shares of common stock which the Company may sell, subject to certain limitations, under the 2015 Sales Agreement through Cantor Fitzgerald, acting as agent.
During the three months ended March 31, 2019, the Company raised net proceeds (net of commissions) of approximately $184,000 from the sale of 242,750 shares of the Company’s common stock in the open market at a weighted average price of $0.78 per share, through its Controlled Equity Offering sales agreement with Cantor Fitzgerald, entered into in November 2015 (Controlled Equity Offering).
17
This Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three months ended March 31, 2019 and 2018 should be read in conjunction with our annual report on Form 10-K for the year ended December 31, 2018 filed with the Securities and Exchange Commission and “Risk Factors” section included elsewhere in this Form 10-Q. This Form 10-Q contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. When used in this report, the words “believe,” “anticipate,” “intend,” “plan,” “estimate,” “expect,” “may,” “will,” “could,” “potentially” and similar expressions are forward-looking statements. Such forward-looking statements are based on current expectations and beliefs. Any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements as a result of various factors.
Forward-looking statements made in this report include, for example, statements about:
|
|
• |
the clinical trial plans for DUR-928; |
|
|
• |
potential regulatory filings for or approval of DUR-928, POSIMIR, or any of our or any third parties’ other product candidates; |
|
|
• |
the potential earn-out payments we may receive from Indivior related to the commercialization of PERSERIS; |
|
|
• |
the progress of our third-party collaborations, including estimated milestones; |
|
|
• |
our intention to seek, and ability to enter into and maintain strategic alliances and collaborations; |
|
|
• |
the potential benefits and uses of our products; |
|
|
• |
responsibilities of our third-party collaborators, including the responsibility to make cost reimbursement, milestone, royalty and other payments to us, and our expectations regarding our collaborators’ plans with respect to our products and continued development of our products; |
|
|
• |
our responsibilities to our third-party collaborators, including our responsibilities to conduct research and development, clinical trials and manufacture products; |
|
|
• |
our ability to protect intellectual property, including intellectual property licensed to our collaborators; |
|
|
• |
market opportunities for products in our product pipeline; |
|
|
• |
the progress and results of our research and development programs and our evaluation of additional development programs; |
|
|
• |
requirements for us to purchase supplies and raw materials from third parties, and the ability of third parties to provide us with required supplies and raw materials; |
|
|
• |
the results and timing of clinical trials, including for DUR-928 and POSIMIR, the possible commencement of future clinical trials and announcements of the findings of our clinical trials; |
|
|
• |
conditions for obtaining regulatory approval of our product candidates; |
|
|
• |
submission and timing of applications for regulatory approval; |
|
|
• |
the impact of FDA, DEA, EMEA and other government regulation on our business; |
|
|
• |
the impact of potential Risk Evaluation and Mitigation Strategies (REMS) on our business; |
|
|
• |
uncertainties associated with obtaining, asserting and protecting patents and other intellectual property rights, as well as avoiding the intellectual property rights of others; |
|
|
• |
products and companies that will compete with the products we license to third-party collaborators; |
|
|
• |
the possibility we may commercialize our own products and build up our commercial, sales and marketing capabilities and other required infrastructure; |
|
|
• |
the possibility that we may develop additional manufacturing capabilities; |
|
|
• |
our employees, including the number of employees and the continued services of key management, technical and scientific personnel; |
18
|
|
• |
sufficiency of our cash resources, anticipated capital requirements and capital expenditures, our ability to comply with covenants of our term loan, and our need for additional financing, including potential sales under our shelf registration statement; |
|
|
• |
our expectations regarding marketing expenses, research and development expenses, and selling, general and administrative expenses; |
|
|
• |
the composition of future revenues; and |
|
|
• |
accounting policies and estimates. |
Forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements as a result of various factors. For a more detailed discussion of such forward looking statements and the potential risks and uncertainties that may impact upon their accuracy, see the “Risk Factors” section and “Overview” section of this Management’s Discussion and Analysis of Financial Condition and Results of Operations. These forward-looking statements reflect our view only as of the date of this report. We undertake no obligations to update any forward-looking statements. You should also carefully consider the factors set forth in other reports or documents that we file from time to time with the Securities and Exchange Commission.
Overview
We are a biopharmaceutical company with research and development programs broadly falling into two categories: (i) new chemical entities derived from our Epigenetic Regulator Program, in which we attempt to discover and develop molecules which have not previously been approved and marketed as therapeutics, and (ii) proprietary pharmaceutical programs, in which we apply our formulation expertise and technologies largely to active pharmaceutical ingredients whose safety and efficacy have previously been established but which we aim to improve in some manner through a new formulation. We also manufacture and sell osmotic pumps used in laboratory research and design, develop and manufacture a wide range of standard and custom biodegradable polymers and excipients for pharmaceutical and medical device clients for use as raw materials in their products. In addition, we conduct research and development of pharmaceutical products in collaboration with third party pharmaceutical and biotechnology companies.
Our product pipeline currently consists of multiple investigational drug candidates in development. DUR‑928, a new chemical entity in Phase 1 and 2 development, is the lead candidate in DURECT’s Epigenetic Regulator Program. An endogenous, orally bioavailable small molecule, DUR-928 has been shown in preclinical studies to play an important regulatory role in lipid homeostasis, inflammation, and cell survival. Human applications may include acute organ injury such as alcoholic hepatitis (AH) and acute kidney injury (AKI), chronic metabolic diseases such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH) and other liver diseases, and inflammatory skin conditions such as psoriasis and atopic dermatitis. DURECT’s proprietary oral and injectable delivery technologies are designed to enable new indications and enhanced attributes for small-molecule and biologic drugs. One late-stage development program in this category is POSIMIR® (bupivacaine extended-release solution), an investigational analgesic product intended to deliver bupivacaine to provide up to three days of pain relief after surgery.
A central aspect of our business strategy involves advancing multiple product candidates at one time, which is enabled by leveraging our resources with those of corporate collaborators. Thus, certain of our programs are currently licensed to corporate collaborators on terms which typically call for our collaborator to fund all or a substantial portion of future development costs and then pay us milestone payments based on specific development or commercial achievements plus a royalty on product sales. At the same time, we have retained the rights to other programs, which are the basis of future potential collaborations and which over time may provide a pathway for us to develop our own biopharmaceutical commercial, sales and marketing organization.
Additional details of these programs and related strategic agreements are contained in our annual report on Form 10-K for the year ended December 31, 2018 and in Note 2 of the financial statements included in Item 1 above.
Epigenetic Regulator Program and New Chemical Entities
Epigenetic regulation involves biochemical modification of either DNA itself or of proteins that intimately associate with DNA. These modifications lead to changes to gene expression that facilitate downstream biological effects.
|
NOTE: |
POSIMIR®, SABER®, CLOUD®, ORADURTM, ALZET® and LACTEL® are trademarks of DURECT Corporation. Other trademarks referred to belong to their respective owners. |
19
DURECT’s Epigenetic Regulator Program involves a multi-year collaborative effort with the Department of Internal Medicine at Virginia Commonwealth University (VCU), the VCU Medical Center and the McGuire VA Medical Center. The knowledge base supporting this program is a result of more than 30 years of lipid research by Shunlin Ren, M.D., Ph.D., Professor of Internal Medicine at the VCU Medical Center and a recipient of multiple grants from the National Institutes of Health (NIH) and other agencies for metabolic disease research. The lead compound from this program, DUR-928, is an endogenous, orally available small molecule that modulates the activity of various nuclear receptors that play important regulatory roles in lipid homeostasis, inflammation and cell survival. Under a license with VCU, we hold the exclusive royalty-bearing worldwide right to develop and commercialize DUR-928 and related molecules discovered in the program.
The biological activity of DUR-928 has been demonstrated in over a dozen different animal disease models involving three animal species. Several of these disease models represent chronic metabolic disorders of hepatic lipid accumulation and dysfunction (e.g., nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH)) and several represent acute organ injuries (e.g., endotoxin shock, acute oxidative damage, ischemic-reperfusion kidney injury, and stroke models).
Our major product research and development efforts planned for DUR-928 in 2019 are set forth in the following table:

In pharmacokinetic and/or toxicity studies conducted in mice, hamsters, rats, rabbits, dogs, minipigs and monkeys, DUR-928 has been found to be orally available, locally tolerable and safe by all routes tested to date. These non-clinical results support the use of DUR-928 in the completed, ongoing and planned human safety, pharmacokinetics (PK) and proof-of-concept trials.
Acute Organ Injury Program with Injectable DUR-928
Market Opportunity. Acute organ injury is another area of major unmet medical need for which effective pharmaceutical treatment is often lacking. Alcoholic liver disease (ALD) is a syndrome characterized by progressive inflammatory liver injury associated with long-term heavy intake of alcohol, and involves a spectrum that ranges from mild injury to severe, life threatening injury. Alcoholic hepatitis (AH), is an acute, inflammatory form of ALD for which there are no effective therapeutics available. The prevalence of AH is believed to be 10-35% of heavy drinkers. There were over 320,000 hospitalizations related to alcoholic hepatitis in 2010, and the hospitalization costs amounted to nearly $50,000 per patient. The cost of a liver transplant exceeds $800,000. Acute kidney injury (AKI), a sudden loss of kidney function due to renal failure or injury, affects approximately 2.8 million patients per year in the United States and is associated with increased mortality, prolonged hospital stays, kidney dialysis and progression to chronic kidney disease. There are various forms of acute organ injury affecting the liver, the kidney or other organs for which we are or may seek to develop DUR-928.
20
Clinical Program. In 2018, we began a Phase 2a clinical trial to evaluate intravenously infused DUR-928 in patients with moderate and severe alcoholic hepatitis (AH). This is an open label, dose escalation (30, 90 and 150 mg), multi-center U.S. study, originally designed to be conducted in two sequential parts. Part A includes patients with moderate AH and Part B includes patients with severe AH (as determined by the Model of End-Stage Liver Disease (MELD) scores, a common scoring system to assess the severity and prognosis of AH patients). Dose escalation may occur following review of safety and pharmacokinetic (PK) results of the prior dose level by a Dose Escalation Committee (DEC). The target number of patients for the study is 4 per dose group. The objectives of this study include assessment of safety, PK and pharmacodynamic (PD) signals, including liver biochemistry and biomarkers. After completing dosing for the low-dose 30 mg cohort (n=4) of Part A (moderate AH patients), the DEC approved commencement of the 90 mg cohort in Part A while simultaneously commencing recruitment for Part B (severe AH patients) with the 30 mg dose. Upon completion of dosing for the 30 mg cohort (n=4) of Part B, the enrollment of which was more rapid than Part A, the DEC approved commencement of the 90 mg cohort in Part B. In parallel with our ongoing trial, we are supporting Dr. Craig McClain’s efforts to initiate an NIH-funded study of DUR-928 in AH patients at the University of Louisville.
Ten patients have completed dosing with DUR-928 to date in the ongoing open label, dose-escalation, multi-center U.S. trial. Eight patients (4 moderate and 4 severe) have been treated with DUR-98 at the 30 mg dose, and two patients (1 moderate and 1 severe) at the 90 mg dose.
Lille scores are used in clinical practice to help determine the prognosis for AH patients after 7 days of treatment. Patients with a Lille score below 0.45 have an 85% 6-month survival rate vs. those with Lille scores of above 0.45, have only a 25% 6-month survival rate (Louvet A et al. Hepatology 2007; 45: 1348-54). The lower the Lille score, the better the prognosis is for the AH patient. In our study, the median Lille score for the 9 AH patients treated with DUR-928 who returned for their Day 7 visit is 0.04, with a range of 0.01 to 0.19. The median Lille score among a cohort of 15 patients treated with either supportive care or supportive care with corticosteroids at the University of Louisville (UL) is 0.41 (shown as historical control). 1
The chart below shows individual patient Lille scores plotted as a function of their initial MELD scores.

|
|
1) |
Our advisor, Dr. Craig McClain from the University of Louisville (UL), shared anonymized data from his study, in which 15 AH patients with initial MELD scores ranging from 15-30 received either supportive care alone (n=8) or supportive care with corticosteroids (n=7). |
|
|
2) |
Of the 10 AH patients dosed to date with DUR-928, one patient did not return for the day 7 visit, so Lille scores could only be calculated for 9 of 10 patients. |
|
|
3) |
Lille scores in the DUR-928 patients were significantly lower than that of the UL patients (p=0.002; Wilcoxon's Rank Sum Test). |
21
Mathurin et al. proposed three prognostic classifications of AH patients in response to treatment based on Lille scores and their correlation with 28-day survival rates from a meta-analysis of four randomized controlled trials evaluating the effectiveness of corticosteroids in an aggregate of 324 patients with severe AH. (Methurin P. et al. Gut 2011; 60:255-260) The following table shows the percentage of patients from three AH data sets, including DUR-928, in each Mathurin classification based on patients’ Lille scores. Seventy-eight percent (7/9) of the DUR-928 treated AH patients with Lille scores are classified as complete responders, 22% (2/9) are partial responders and none (0/9) were null responders.
|
|
Percent of AH Patients in each Classification (data from separate studies) |
||||
|
Lille Score |
Classification a |
28-Day Survival Rate a |
Prednisolone (n=51)b |
UL (n=15)c |
DUR-928 (n=9)d |
|
≤0.16 |
Complete Responder |
91.1% ± 2.7% |
49% |
33% |
78% |
|
0.16-0.56 |
Partial Responder |
79.4%± 3.8% |
43% |
20% |
22% |
|
≥0.56 |
Null Responder |
53.3%±5.1% |
8% |
47% |
0% |
|
|
|
P<0.0001 (a) |
|
P=0.002 (e) |
|
|
|
a) |
Mathurin, et. al., Gut 2011;60:255-260 |
|
|
b) |
Mathurin, “Selonsertib in Combination with Prednisolone for the Treatment of Severe Alcoholic Hepatitis: A Phase 2 Randomized Controlled Trial” presented at AASLD San Francisco November 2018. The table presents patients from the control group – all treated with corticosteroids (prednisolone + placebo). Initial MELD scores in this study ranged from 19 to 24. |
|
|
c) |
See footnote 1 on page 1. d) See footnote 2 on page 1. e) See footnote 3 on page 1. |
Bilirubin is formed by the breakdown of red blood cells in the body. The level of total bilirubin in the blood is an indication of how the liver is functioning. Compared to baseline (n=10), the median reduction in total bilirubin in the DUR-928 treated patients was 16% at Day 7 (n=9) and 41% at Day 28 (n=8) compared to 3% at Day 7 and 35% at Day 28 in the UL patients. The chart below shows the percent change in total bilirubin at Day 7 and 28 compared to baseline (Day 0) for both the separate UL (as historical control) and DUR-928 studies.

P-Values calculated with Wilcoxon's Signed Rank Test
22
Model of End-Stage Liver Disease (MELD) score is another common scoring system used to assess the severity and prognosis of AH patients. Patients with MELD scores of 11-19 are classified as having moderate AH and patients with MELD scores of 20-30 are classified as having severe AH. As with Lille scores, the lower the MELD score, the better the prognosis for the AH patient. In our study (shown in the chart below), the median reduction from baseline (Day 0, prior to treatment) (n=10) in MELD in the DUR-928 treated patients was 4% at Day 7 (n=9) and 21% at Day 28 (n=8) compared to a 4% increase at Day 7 and 6% reduction at Day 28 in the UL patients (as historical control).
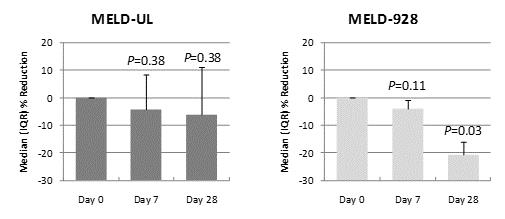
MELD is calculated based on (a) bilirubin, (b) serum creatinine (sCr), and (c) International Normalized Ratio (INR), which is a measure of prothrombin time. P-Values calculated with Wilcoxon's Signed Rank Test
To date there is no statistical difference in the pharmacokinetic profiles between moderate and severe AH patients treated with DUR-928. There have been no drug-related adverse events in the DUR-928 treated patients to date. The data presented are preliminary and will be finalized upon completion of the trial. There can be no assurance that additional patients treated with DUR-928 will have similar results as those reported here.
Phase 1 trials of DUR-928 administered through injection have supported the development of DUR-928 in AH. The initial Phase 1 trial in healthy subjects was a single-site, randomized, double-blinded, placebo-controlled, single-ascending-dose study that evaluated the safety, tolerability and PK of intramuscular (IM) injected DUR-928. The 24-subject study (16 healthy volunteers on the drug and 8 on placebo) of four escalating dose levels resulted in dose proportional systemic exposure of DUR-928 with peak plasma concentrations greater than 1000-fold higher than endogenous levels. DUR-928 was well-tolerated at all dose levels, with no serious treatment-related adverse events reported. We also conducted a multiple-dose study involving 10 healthy subjects, in which participants received IM-injected DUR-928 for 5 consecutive days (8 subjects on the drug, 2 on placebo) using the next to highest dose from the single dose study. No serious treatment related adverse events were reported, no subjects withdrew from the study, no accumulation in plasma concentrations were observed with repeat dosing, and the pain scores and injection site reactions were minimal. We also conducted a single-ascending dose intravenous infusion (IV) study with 16 healthy subjects and observed no treatment-related serious adverse events. The systemic exposure following IV infusion was dose proportional.
A Phase 1 drug-drug interaction study conducted in healthy subjects demonstrated that neither orally administered nor intravenously injected DUR-928 at doses tested affected the safety and PK of midazolam, a drug metabolized by CYP3A4, which is one of the important enzymes associated with clinically relevant drug-drug interactions.
We have also conducted a Phase 1b study with injected DUR-928 in patients with impaired kidney function (stage 3 and 4 chronic kidney disease (CKD)) and matched control subjects (MCS), matched by age, body mass and gender with normal kidney function. This study was a single-site, open-label, single-ascending-dose study. in two successive cohorts (first a low dose of 30 mg and then a high dose of 120 mg) evaluating safety and PK of intramuscular injected DUR-928. The low dose cohort consisted of 6 patients with CKD and 3 MCS; the high dose cohort consisted of 5 CKD patients and 3 MCS. In this trial, DUR-928 was well tolerated among all subjects and the PK parameters between the kidney function impaired patients and the MCS were comparable. While the number of subjects involved was small and not designed to assess efficacy, we did observe decreases in bilirubin and CK-18 when those levels were elevated at baseline, although the results were not statistically significant. The results of this Phase 1b trial were presented at Kidney Week 2018 in San Diego, CA.
Skin Inflammatory Disorder Program with Topical DUR-928
Market opportunity. Psoriasis is an inflammatory skin disease and an immune-mediated condition that causes the body to make new skin cells in days rather than weeks. In the United States, there are about 150,000 new cases of psoriasis every year and it affects more than 8 million Americans. According to the International Federation of Psoriasis Associations (IFPA), nearly 3% of the world’s
23
population, or about 125 million people, has some form of psoriasis. Psoriasis causes itchiness and irritation and may be painful. There is no cure for psoriasis, but treatment can ease symptoms.
Approximately 80% of patients with psoriasis have localized disease. Topical agents remain the mainstay of localized psoriasis treatment. Other inflammatory skin disorders, such as atopic dermatitis, which affects approximately 32 million Americans, represent significant unmet medical needs. Most currently available topical treatments, such as steroids, are typically employed as first-line therapy and either slow down excessive skin cell proliferation or reduce inflammation.
Clinical program. We have conducted an exploratory Phase 1b trial in psoriasis patients (9 evaluable patients) in Australia. The double-blinded and placebo-controlled trial was conducted using a micro-plaque assay with intralesional injections of DUR-928. We believe that the initial results were encouraging and warrant further investigation. As a result, we are conducting a Phase 2a, randomized, double-blind, vehicle-controlled proof-of-concept clinical trial, in which DUR-928 is applied topically once-daily for 28 days in patients with mild to moderate plaque psoriasis. The trial is being be conducted at multiple clinical sites in the U.S. Twenty patients are planned to be enrolled to obtain approximately 15 evaluable patients. Patients serve as their own controls, applying DUR-928 to the plaque on one arm and the vehicle (placebo) to a similar plaque on the other arm. After the treatment period, patients will be followed for an additional four weeks. The primary efficacy endpoint will be change in local psoriasis scores from baseline in the DUR-928-treated plaques compared to that in the vehicle-treated plaques. We began dosing patients in March 2019 and expect to announce top-line data from this study in the second half of 2019.
Chronic Liver Disease Program with Orally Administered DUR-928
Market Opportunity. Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in both children and adults. It is estimated that NAFLD affects approximately 20% to 30% of adults and 10% of children in the United States. Non-alcoholic steatohepatitis (NASH), a more severe and progressive form of NAFLD, is one of the most common chronic liver diseases worldwide, with an estimated prevalence of more than 10% of adults in the United States, Europe, Japan and other developed countries. No drug is currently approved for treatment of NAFLD or NASH. Moreover, alcoholic fatty liver disease (AFLD), including its more advanced stage, alcoholic steatohepatitis (ASH), develops in approximately 90% of individuals who drink more than 60 grams/day of alcohol, but may occur in individuals who drink less, and is a major contributor to the global burden of liver cirrhosis. In addition to these liver diseases, there are a number of orphan liver diseases for which we may seek to develop DUR-928.
Clinical Program. In March 2019 we began enrolling patients in a Phase 1b randomized and open-label clinical study being conducted in the U.S. to evaluate safety, pharmacokinetics and signals of biological activity of DUR-928 in NASH patients with stage 1-3 fibrosis. Three doses of DUR-928 (50 mg QD, 150 mg QD and 300 mg BID) will be administered orally for 28 consecutive days with approximately 20 patients per dose group for a total of approximately 60 patients in the trial as shown below. Key endpoints include safety and pharmacokinetics (PK), clinical chemistry and biomarkers (e.g., bilirubin, lipids, liver enzymes, CK-18s, and inflammatory cytokines) as well as liver imaging. We expect to announce initial data from this study in the second half of 2019.
DUR-928 Phase 1b 28-Day Daily Oral Dosing Clinical Trial Schema
Screening
Run-in 2 weeks
(baseline data)
28-day dosing
28-day follow-up
DURECT has completed multiple single-site Phase I trials in healthy subjects with orally administered DUR-928. One of the studies was a randomized, double-blinded, placebo-controlled, single-ascending-dose study that evaluated the safety, tolerability and PK of DUR-928. The 30-subject study evaluated DUR-928 in five cohorts of healthy volunteers receiving DUR-928 (n=20 on drug, 10 on placebo) at escalating doses that resulted in peak plasma concentrations greater than 100-fold higher than endogenous levels. DUR-928 was well-tolerated at all dose levels, with no serious treatment-related adverse events reported. Dose related increases in plasma concentrations were observed with peak plasma concentration at approximately 2-6 hours after dosing. We subsequently conducted a randomized, double-blinded, placebo-controlled, multiple-ascending-dose study in 20 healthy subjects (n=16 on drug, 4 on placebo). Following multiple dosing, DUR-928 was well-tolerated at all doses, with no serious drug-related adverse events reported and no accumulation in plasma concentrations observed with repeat dosing. We also conducted a food effect study with 8 healthy subjects and observed no food effect on DUR-928 absorption.
We also conducted a Phase 1b trial in cirrhotic and non-cirrhotic NASH patients and matched control subjects (MCS) (matched by age, body mass index and gender with normal liver function) utilizing orally administered DUR-928. This was an open-label,
24
single-ascending-dose safety and PK study conducted in Australia in two successive dose cohorts (first a low dose of 50 mg and then a high dose of 200 mg). Both cohorts consisted of 10 NASH patients and 6 MCS. Data from this study was presented at the International Liver Congress™ 2017 organized by the European Association for the Study of the Liver (EASL) in Amsterdam on April 22, 2017. All patients and MCS in this study tolerated DUR-928 well. One patient (with a prior history of arrhythmia and an ongoing viral infection) in the high dose cohort experienced a serious adverse event (shortness of breath), which occurred without unusually abnormal biochemical changes and resolved without intervention but was considered possibly treatment related by the physician due to its temporal association with dosing. In both the low and high dose cohorts, the PK parameters were comparable between the NASH patients and the MCS. In addition, the systemic exposure following the low and high doses of DUR-928 was dose dependent.
While this study was not designed to assess efficacy, we observed significant reductions from baseline of several biomarkers after both doses of DUR-928. A single oral dose of DUR-928 statistically significantly reduced the levels of both full-length and cleaved cytokeratin-18 (CK-18), bilirubin, hsCRP, and IL-18 in these subjects. The mean decrease of full-length CK-18 (a generalized cell death marker) at the measured time point of greatest effect (12 hours after dosing) was 33% in the low dose cohort and 41% in the high dose cohort. The mean decrease of cleaved CK-18 (a cell apoptosis marker) at the measured time point of greatest effect (12 hours after dosing) was 37% in the low dose cohort and 47% in the high dose cohort. The mean reduction of total bilirubin (a liver function marker) at the measured time point of greatest effect (12 hours after dosing) was 27% in the low dose cohort and 31% in the high dose cohort. The mean decrease of high sensitivity C-Reactive Protein (hsCRP) (a marker of inflammation) at the measured time point of greatest effect (24 hours after dosing) was 8% in the low dose cohort and 13% in the high dose cohort. The mean decrease of IL-18 (an inflammatory mediator) at the measured time point of greatest effect (8 hours after dosing) was 4% in the low dose cohort and 8% in the high dose cohort.
Collectively, the reduction of these biomarkers plus results from our animal and cell culture studies suggest potential therapeutic activity of DUR-928 for patients with liver diseases. However, additional studies are required to evaluate the safety and efficacy of DUR-928, and there is no assurance that these biomarker effects will be associated with clinically relevant benefits, or that DUR-928 will demonstrate safety or efficacy in treating liver diseases in future controlled trials.
Additional Proprietary Pharmaceutical Programs
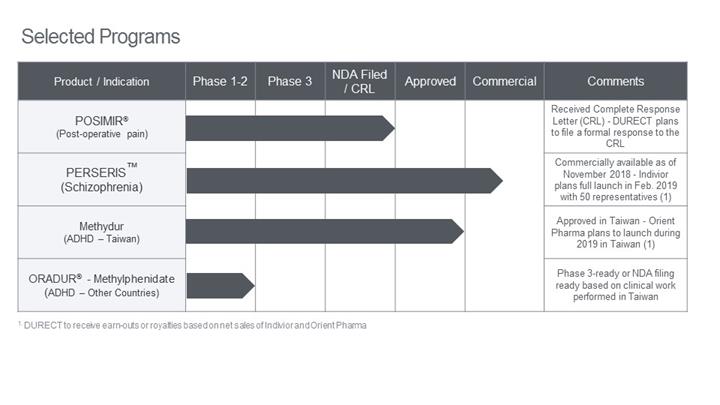 POSIMIR® (Extended Release Bupivacaine)
POSIMIR® (Extended Release Bupivacaine)
Our post-operative pain relief depot, POSIMIR, is a sustained release injectable using our proprietary SABER® delivery system to deliver bupivacaine, an off-patent pharmaceutical agent. SABER is a controlled drug delivery technology that is administered via the parenteral (i.e., injectable) route to deliver drugs that act systemically or locally. POSIMIR is designed to be administered to a
25
surgical site at the end of surgery for post-operative pain relief and is intended to provide local analgesia for up to 3 days, which we believe coincides with the time period of the greatest need for post-surgical pain control in most patients.
Status. In April 2013, we submitted an NDA as a 505(b)(2) application, which relied in part on the FDA’s findings of safety and effectiveness of a reference drug. In February 2014, we received a Complete Response Letter from the FDA. Based on the Complete Response Letter and subsequent communications with the FDA, we conducted a new POSIMIR Phase 3 clinical trial (the PERSIST trial) consisting of patients undergoing laparoscopic cholecystectomy (gallbladder removal) surgery to further evaluate the benefits and risks of POSIMIR. We began recruiting patients for this trial in November 2015 comparing POSIMIR to placebo. Based on advice from the FDA received subsequent to the start of the trial, in April 2016 we decided to amend the PERSIST trial. Starting in August 2016, we began implementing Part 2 of the PERSIST trial to evaluate POSIMIR against standard bupivacaine HCl rather than placebo as we had been doing initially in the study. In October 2017, we reported that the PERSIST trial did not meet its primary efficacy endpoint of reduction in pain on movement over the first 48 hours after surgery as compared to standard bupivacaine HCl. While results trended in favor of POSIMIR versus the comparator, they did not achieve statistical significance.
In total, we have completed 16 clinical studies with POSIMIR, in seven different surgical procedures, including inguinal hernia repair, shoulder surgery (primarily subacromial decompression), appendectomy, abdominal hysterectomy, open laparotomy, laparoscopic cholecystectomy, and laparoscopic colectomy. The incision lengths treated ranged from a few centimeters for laparoscopic portals, to open laparotomy incisions of up to 35 cm. The seriousness of the surgery ranged from day surgery hernia repair in relatively healthy patients to major abdominal surgery for colon cancer in elderly patients with substantial co-morbidity who were often hospitalized for a week or more. The safety experience from this variety of procedures and patients was designed to allow for extrapolation of the safety and efficacy data to a broad surgical population. Our POSIMIR clinical development program has been devised to establish the safety and efficacy of POSIMIR for the treatment of post-surgical pain for 3 days.
26
We believe that two of the completed clinical trials support the efficacy of POSIMIR in post-operative pain and meet the requirements to be considered as adequate and well-controlled pivotal clinical trials:
Hernia pivotal efficacy trial
The hernia pivotal efficacy clinical trial was designed to evaluate the tolerability, activity, dose response and pharmacokinetics of POSIMIR in patients undergoing open inguinal hernia repair. The trial was conducted in Australia and New Zealand as a multi-center, randomized, double blind, placebo-controlled study in 122 patients. Study patients were randomized into three treatment groups: patients that were treated with POSIMIR 2.5 mL (n=43), POSIMIR 5 mL (n=47) and SABER placebo (n=32). The co-primary efficacy endpoints for the study were Mean Pain Intensity on Movement area under the curve (AUC), a measure of pain over a period of 1-72 hours post-surgery, and the proportion of patients requiring supplemental opioid analgesic medication during the study (defined as 0-15 days).
In relation to the co-primary endpoint of pain reduction as measured by Mean Pain Intensity on Movement AUC 1-72 hours post-surgery, the patient group treated with POSIMIR 5 mL reported thirty-one percent (31%) less pain versus placebo, and the result was statistically significant (p=0.0031). Fifty-three percent (53%) of the study patients in the POSIMIR 5 mL group took supplemental opioid analgesic medications versus seventy-two percent (72%) of the placebo patients (p=0.0909). Although this positive trend for this co-primary endpoint in favor of the POSIMIR 5 mL group was not statistically significant, both secondary endpoints measuring opioid analgesic medication consumption were met at a statistically significant level. During the periods of 1-24 hours, 24-48 hours and 48-72 hours after surgery, placebo patients consumed approximately 3.5 (p=0.0009), 2.9 (p=0.0190) and 3.6 (p=0.0172) times more supplemental opioid analgesic medications (mean total daily consumption of opioid analgesic medication in morphine equivalents), respectively, than the POSIMIR 5 mL treatment group. The median decrease in supplemental opioid analgesics taken over the first three days after surgery was 80% (p=0.0085) for the POSIMIR 5 mL group as compared to the placebo group.
Shoulder pivotal efficacy trial
The shoulder pivotal efficacy trial was a multicenter, randomized, double-blind, active- and placebo-controlled, parallel-group, dose-response trial conducted at 9 investigational centers in Europe. In this study, 107 patients were randomly assigned to one of three treatment groups prior to undergoing elective arthroscopic shoulder surgery: POSIMIR 5 mL (n=53), SABER-Placebo (n=25) or bupivacaine HCl solution (n=29). All patients were given a background pain treatment consisting of a daily dose of two or four grams (depending on the patient’s weight) of paracetamol (acetaminophen). In addition, each patient was provided supplemental opioid rescue medication, if needed. With respect to efficacy, the primary endpoints of the study were to demonstrate: (1) an improvement in terms of pain intensity on movement area under the curve (AUC) during the period 1–72 hours post-surgery, and (2) a decrease in the total use of opioid rescue analgesia 0–72 hours post-surgery.
Results from this study demonstrate that the POSIMIR group experienced a statistically significant reduction in pain intensity of approximately 20% (p=0.012) versus SABER-Placebo. Applying the appropriate statistical test given the data distribution, the POSIMIR group showed a statistically significant reduction of approximately 67% (p=0.013) in median opioid use in favor of POSIMIR. No statistical differences were found when POSIMIR was compared to bupivacaine HCl.
Safety
Bupivacaine is a well-known drug with an extensive understanding of its risks and benefits. DURECT has completed 16 clinical trials involving POSIMIR. A total of 1463 patients participated in these trials, 1144 of whom were exposed to POSIMIR or SABER-Placebo in volumes ranging from 2.5 to 10 mL. A total of 876 patients were exposed to POSIMIR with the dose of bupivacaine ranging from 330 to 990 mg. Our planned commercial dose is 5 mL (660 mg). In addition, a total of 272 patients were treated with bupivacaine HCl in control groups and 268 patients received SABER-Placebo in control groups.
Overall, the POSIMIR patient groups showed a similar systemic safety profile as the patient groups treated with SABER-Placebo and bupivacaine HCl. Local site reactions were observed more frequently in the POSIMIR and SABER-Placebo groups than in the active comparator groups, most frequently in abdominal surgeries; most of these observations were discolorations (e.g., surgical bruising), the majority of which resolved without treatment during the observation period. There was little difference in the incidence of severe or serious adverse events between the POSIMIR, SABER-Placebo and bupivacaine HCl treatment groups. Most of the serious adverse events seen in these trials appear to be due to complications of surgery, anesthesia, analgesics, or co-morbidity and not POSIMIR-related. The clinical history for serious adverse events has been reviewed, and we do not believe POSIMIR was associated with any bupivacaine toxicity. The adverse event data was analyzed in a variety of ways to detect any evidence of bupivacaine central nervous system or cardiac toxicity or other unexpected effects. No patients treated with POSIMIR had an instance of a severe central nervous system or cardiac adverse event traditionally associated with bupivacaine toxicity.
27
After carefully reviewing the existing POSIMIR data and evaluating the feedback we have received from the FDA, including the CRL and other correspondence, we plan to submit a response to the CRL to the FDA in the second quarter of 2019. The submission will request FDA approval of POSIMIR based on what the Company and its advisors believe is adequate evidence of both safety and efficacy. As the submission is intended to be a response to a CRL, we expect a 6-month FDA review period.
Market Opportunity. According to data published by the Center for Disease Control and Prevention, there are approximately 72 million ambulatory and inpatient surgical procedures performed annually in the U.S. Insufficient postoperative pain control remains a significant problem, with studies indicating that roughly 65% of patients experience moderate-to-extreme pain after surgery. The current standard of care for post-surgical pain includes a variety of opiate and non-opiate analgesics and muscle relaxants. While systemic opioids can effectively reduce post-surgical pain, they commonly cause side effects including drowsiness, constipation, nausea and vomiting, and cognitive impairment. Effective pain management can be compromised if patients fail to adhere to recommended dosing regimens because they are suffering from these side effects. Post-surgical pain also can be treated effectively with local anesthetics; however, their usefulness often is limited by their short duration of action.
In September 2017, we entered into an agreement with Indivior, under which we assigned to Indivior certain patents that may provide further intellectual property protection for PERSERIS, Indivior’s extended-release injectable suspension for the treatment of schizophrenia in adults. In consideration for such assignment, Indivior made an upfront non-refundable payment to DURECT of $12.5 million. Indivior also paid a $5 million milestone payment to DURECT in August 2018 following the FDA approval of PERSERIS. Under the terms of the agreement with Indivior, DURECT receives quarterly earn-out payments that are based on a single digit percentage of U.S. net sales of PERSERIS.
Indivior has announced the commercial launch of PERSERIS in February 2019 with a 50-person field force. While Indivior has projected a potential peak net revenue goal of $200 to $300 million per year for PERSERIS, there can be no assurance such revenues will be achieved.
ORADUR-ADHD Program
Market Opportunity. Attention Deficit Hyperactivity Disorder (ADHD) is a neurobehavioral condition that is estimated to affect over 6 million (approximately 9%) of U.S. children ages 2-17, according to the U.S. Department of Health and Human Services. The principal characteristics of ADHD are inattention, hyperactivity, and impulsivity. The condition presents itself in childhood and can be life long as a significant number of children with ADHD continue to present symptoms as adults. 62% of children with ADHD are estimated to be under treatment with medication, utilizing stimulants such as amphetamine or methylphenidate as first-line treatments. U.S. sales of ADHD treatments were approximately $10.4 billion in 2016. The 2016 National Survey on Drug Use & Health estimates that 612,000 Americans over the age of 12 abuse stimulants for euphoric highs and increased performance or wakefulness.
Development Strategy. In collaboration with Orient Pharma, we have developed a drug candidate (ORADUR- Methylphenidate ER) based on our ORADUR Technology for the treatment of ADHD. This drug candidate is intended to provide once-a-day dosing with added tamper resistant characteristics to address common methods of abuse and misuse of these types of drugs. In August 2009, we entered into a development and license agreement with Orient Pharma, a diversified multinational pharmaceutical, healthcare and consumer products company with headquarters in Taiwan, under which we granted to Orient Pharma development and commercialization rights in certain defined Asian and South Pacific countries to ORADUR-Methylphenidate ER. We retain rights to North America, Europe, Japan and all other countries not specifically licensed to Orient Pharma.
Orient Pharma conducted a Phase 3, multi-center, randomized, double-blind, placebo-controlled, two-way cross-over study designed to demonstrate the efficacy and safety of ORADUR-Methylphenidate ER (referred to as Methydur Sustained Release Capsules or Methydur) in children and adolescents with ADHD between the ages of 6 and 18 years. According to Orient Pharma, the study was conducted in Taiwan and enrolled 110 subjects, of which 100 evaluable subjects completed the study. For the primary efficacy endpoint, Orient Pharma observed a statistically significant difference between Methydur and Placebo treatments in the mean change of total score for the Swanson, Nolan, and Pelham-IV (SNAP-IV) teacher form (p=0.0044 for the intent to treat population and p=0.0104 for the per protocol population). Orient Pharma’s safety analysis indicates that the incidence of adverse events with Methydur was similar to other approved methylphenidate products.
In September 2018, Orient Pharma informed us that it had obtained marketing authorization for Methydur from the Ministry of Health and Welfare in Taiwan. Methydur is indicated for the treatment of ADHD and will be available in three strengths (22 mg, 33 mg and 44 mg) in Taiwan. Orient Pharma also has stated that it expects to make Methydur commercially available in Taiwan in 2019, while seeking a partner in China and pursuing regulatory approvals in selected other countries in Southeast Asia where it has
28
commercialization rights and a commercialization presence. We are seeking potential development and commercialization partners for ORADUR-Methylphenidate ER Capsules for major markets not licensed to Orient Pharma.
REMOXY® ER
In December 2002, we entered into an agreement with Pain Therapeutics, amended in December 2005, under which we granted Pain Therapeutics the exclusive, worldwide right to develop and commercialize selected long-acting oral opioid products using our ORADUR technology incorporating four specified opioid drugs.
On March 20, 2019, we received written notice from Pain Therapeutics that, effective June 18, 2019, Pain Therapeutics is terminating their agreement with us. As a result of this termination, Pain Therapeutics will have no license under DURECT’s technology and will be returning its exclusive, worldwide commercialization rights to develop and market REMOXY ER to the Company.
Drug Delivery Technologies and Programs
Our drug delivery technologies are designed to deliver the right drug to the right place, in the right amount and at the right time to treat a variety of chronic, acute and episodic diseases and conditions. We aim to improve therapy for a given disease or patient population by controlling the rate and duration of drug administration. In addition, if advantageous for the therapy, our technologies can target the delivery of the drug to its intended site of action.
Our technologies are suitable for providing long-term drug therapy because they can often store highly concentrated, stabilized drugs in a small volume and protect the drug from degradation by the body. This, in combination with the ability to continuously deliver desired doses of a drug, can extend the therapeutic value of a wide variety of drugs, including, in some cases, those which would otherwise be ineffective, too unstable, too potent or cause adverse side effects. In some cases, delivering the drug directly to the intended site of action can improve efficacy while minimizing unwanted side effects elsewhere in the body, which often limit the long-term use of many drugs. Our pharmaceutical systems may thus provide better therapy for chronic diseases or conditions, or for certain acute conditions where longer drug dosing is required or advantageous, by replacing multiple injection therapy or oral dosing, improving drug efficacy, reducing side effects and ensuring dosing compliance. Our technology may thereby improve patients’ quality of life by eliminating more repetitive treatments, reducing dependence on caregivers and allowing patients to lead more independent lives.
We currently have several major active drug delivery technology platforms:
The SABER and CLOUD Bioerodible Injectable Depot Systems
Our bioerodible injectable depot systems include our SABER and CLOUD platform technologies. SABER uses a high viscosity base component, such as sucrose acetate isobutyrate (SAIB), to provide controlled release of a drug. When the high viscosity SAIB is formulated with drug, biocompatible excipients and other additives, the resulting formulation is easily injectable with standard syringes and needles. After injection of a SABER formulation, the excipients diffuse away, leaving a viscous depot which provides controlled sustained release of drug. CLOUD is a class of bioerodible injectable depot technology which generally does not contain SAIB but includes various other release rate modifying excipients and/or bioerodible polymers to achieve the delivery of drugs for periods of days to months from a single injection. We are researching and developing a variety of controlled-release products based on the SABER and CLOUD technologies. Based on research and development work to date, our bioerodible injectable depot technologies have shown the following advantages:
|
|
• |
Peptide/Protein/Small Molecule Delivery—The chemical nature of our bioerodible injectable depot systems tends to repel water and body enzymes and thereby stabilizes proteins and peptides. For this reason, we believe that bioerodible injectable depot systems are well suited as a platform for biotechnology therapeutics based on proteins and peptides. |
|
|
• |
Controlled Onset and Release—Typically, controlled release injections are associated with an initial higher release of drug immediately after injection (also called “burst”). Animal and human studies have shown that our bioerodible injectable depots can be associated with less post-injection burst than is typically associated with other commercially available injectable controlled release technologies, while still achieving controlled rapid onset of drug concentration. |
|
|
• |
High Drug Loading—Drug loading in our bioerodible injectable depot formulations can be as high as 30%, considerably greater than is typical with other commercially available injectable controlled release technologies. As a result, smaller injection volumes are possible with this technology. |
|
|
• |
Ease of Administration—Prior to injection, our bioerodible injectable depot formulations are fairly liquid and therefore can be injected through small needles. Additionally, because of the higher drug concentration of our bioerodible injectable depot formulations, less volume is required to be injected. In some cases, small injection volumes and more liquid solutions can result in easier, less painful administration. |
29
|
|
• |
Patent Protection—Our bioerodible injectable depot technology is covered by United States and foreign patents. See “Patents, Licenses and Proprietary Rights” below. |
|
|
• |
Ease of Manufacture—Compared to microspheres and other polymer-based controlled release injectable systems, in some cases, our bioerodible injectable depot formulations are readily manufacturable at low cost. |
The SABER technology is the basis of POSIMIR (described above). The SABER technology is also utilized in our ophthalmic program with Santen Pharmaceutical Co., Ltd. (Santen), as well as multiple feasibility programs. In our clinical studies thus far, our bioerodible injectable depot formulations have been observed to be generally safe and well-tolerated.
The SABER technology is also the basis for SucroMate™ Equine, an injectable animal health drug utilizing our SABER technology to deliver the peptide deslorelin. This was the first FDA approved SABER injectable product when it was launched in 2011 by CreoSalus, Inc.
The ORADUR Sustained Release Gel Cap Technology
We believe that our ORADUR sustained release technology can transform short-acting oral capsule dosage forms into sustained release oral products. Products based on our ORADUR technology can take the form of an easy to swallow capsule that uses a high-viscosity base component such as sucrose acetate isobutyrate (SAIB) to provide controlled release of active ingredients for an extended period of time. Oral dosage forms based on the ORADUR gel-cap may also have the added benefit of being less prone to abuse (e.g., by crushing and then snorting, smoking, injecting or extracting by mixing with alcohol or water). These properties have the potential to make ORADUR-based products an attractive option for pharmaceutical companies that seek to develop abuse-deterrent oral products.
The ORADUR technology is the basis of our ORADUR-Methylphenidate ER and REMOXY ER programs (described above).
Depot Injectable Programs
In addition to biologic drugs, many traditional small molecule drugs have to be given by frequent injections, which is costly, inconvenient and may result in either unwanted side effects or suboptimal efficacy. We have active programs underway to improve our depot injectable systems and to apply those systems to various drugs and drug candidates, and have entered into a number of feasibility studies with biotechnology and pharmaceutical companies to test their products in our systems. The ophthalmic program with Santen is a project which started as a depot injectable feasibility project and then matured into a development and license agreement.
Product Revenues
We also currently generate product revenue from the sale of the following three products:
|
|
• |
ALZET® osmotic pumps which are used for animal research; |
|
|
• |
LACTEL® biodegradable polymers which are used by our customers as raw materials in their pharmaceutical and medical products; and |
|
|
• |
to a much lesser extent, certain key excipients that are included in Methydur and one excipient that is included in a currently marketed animal health product. |
Because we consider our core business to be developing and commercializing pharmaceuticals, we do not intend to significantly increase our investments in or efforts to sell or market any of our existing product lines. However, we expect that we will continue to make efforts to increase our revenue related to collaborative research and development by entering into additional research and development agreements with third-party collaborators to develop product candidates based on our drug delivery technologies.
Operating Results
Since our inception in 1998, we have had a history of operating losses. At March 31, 2019, we had an accumulated deficit of $475.8 million. Our net loss was $7.1 million for the three months ended March 31, 2019. Our net losses were $25.3 million and $3.7 million for the years ended December 31, 2018 and 2017, respectively. These losses have resulted primarily from costs incurred to research and develop our product candidates and to a lesser extent, from selling, general and administrative costs associated with our operations and product sales. We expect our research and development expenses in the near future to increase compared to the first quarter of 2019 as we experience higher research and development expenses related to DUR-928. We expect selling, general and administrative expenses in the near future to be lower compared to the first quarter of 2019 due to lower patent related expenses. We do not anticipate meaningful revenues from our products in development, should they be approved, for at least the next twelve months. Therefore, we expect to incur continuing losses and negative cash flows from operations for the foreseeable future.
30
Depending on whether we enter into additional collaborative agreements in the near term and the extent to which we earn milestone revenues, we may be required to raise additional capital through a variety of sources.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. The most significant estimates and assumptions relate to revenue recognition, the recoverability of our long-lived assets, including goodwill and other intangible assets, accrued liabilities, contract research liabilities, inventories and stock-based compensation. Actual amounts could differ significantly from these estimates. There have been no material changes to our other critical accounting policies and estimates as compared to the disclosures in our annual report on Form 10-K for the year ended December 31, 2018.
Results of Operations
Three months ended March 31, 2019 and 2018
Collaborative research and development and other revenue
We recognize revenues from collaborative research and development activities and service contracts. Collaborative research and development revenue primarily represents reimbursement of qualified expenses related to collaborative agreements with various third parties to research, develop and commercialize potential products using our drug delivery technologies, and revenue recognized from ratable recognition of upfront fees and milestone payments in connection with our collaborative agreements.
We expect our collaborative research and development and other revenue in the near future to be comparable to the first quarter of 2019. We expect our collaborative research and development and other revenue to fluctuate in future periods pending our efforts to enter into potential new collaborations, our existing third party collaborators’ commitment to and progress in the research and development programs, and any royalty or earn-out revenue recognized from collaborators or counterparties. The collaborative research and development and other revenues associated with our major collaborators or counterparties are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Collaborator/Counterparty |
|
|
|
|
|
|
|
|
|
Indivior UK Limited (Indivior) (1) |
|
$ |
7 |
|
|
$ |
— |
|
|
Santen Pharmaceutical Co. Ltd. (Santen) (2) |
|
|
14 |
|
|
|
1 |
|
|
Others (3) |
|
|
1,479 |
|
|
|
1,095 |
|
|
Total collaborative research and development and other revenue |
|
$ |
1,500 |
|
|
$ |
1,096 |
|
|
(1) |
Amount related to earn-out revenue from PERSERIS net sales was $7,000 for the three months ended March 31, 2019, compared to zero for the corresponding period in 2018. |
|
(2) |
Amounts related to ratable recognition of upfront fees were zero for each of the three months ended March 31, 2019 and 2018. In January 2018, we were notified by Santen that due to a shift in near term priorities, Santen had elected to reallocate research and development resources and put our program on pause until further notice. While the main program is on pause, the parties are working together on a limited set of research and development activities funded by Santen. |
|
(3) |
Includes revenue recognized associated with our feasibility agreements for each of the three and three months ended March 31, 2019 and 2018. |
Product revenue
A portion of our revenues is derived from product sales, which include our ALZET mini pump product line, our LACTEL biodegradable polymer product line and certain excipients that are included in Methydur and in a currently marketed animal health product. Net product revenues were $2.6 million and $2.4 million in the three months ended March 31, 2019 and 2018, respectively. The increase in the three months ended March 31, 2019 was primarily attributable to higher revenue from our LACTEL product line as a result of higher units sold, partially offset by lower revenue from our ALZET mini pump product line as a result of lower units sold compared to the corresponding period in 2018.
31
Cost of product revenues was $1.1 million and $1.2 million for the three months ended March 31, 2019 and 2018, respectively. The decrease in the cost of product revenue in the three months ended March 31, 2019 were primarily the result of lower cost of goods sold related to our LACTEL product line arising from lower manufacturing costs for units sold as well as lower cost of goods sold related to our ALZET product line arising from lower units sold compared to the corresponding period in 2018. Cost of product revenues and gross profit margin will fluctuate from period to period depending upon the product mix in a particular period and unit volumes sold. Stock-based compensation expense recognized related to cost of product revenues was $21,000 and $25,000 for the three months ended March 31, 2019 and 2018, respectively.
We had 22 manufacturing employees as of March 31, 2019 compared with 21 as of March 31, 2018. We expect the number of employees involved in manufacturing will remain comparable in the near future.
Research and development
Research and development expenses are primarily comprised of salaries, benefits, stock-based compensation and other compensation cost associated with research and development personnel, overhead and facility costs, preclinical and non-clinical development costs, clinical trial and related clinical manufacturing costs, contract services, and other outside costs.
Research and development expenses were $6.3 million and $7.0 million for the three months ended March 31, 2019 and 2018, respectively. The decrease in the three months ended March 31, 2019 was primarily attributable to lower research and development costs associated with POSIMIR, ORADUR-ADHD, REMOXY ER and other research programs, partially offset by higher research and development costs associated with depot injectable programs, DUR-928 and the Santen ophthalmic program compared to the corresponding period in 2018, as more fully discussed below. Stock-based compensation expense recognized related to research and development personnel was $174,000 and $353,000 for the three months ended March 31, 2019 and 2018, respectively. As of March 31, 2019, we had 44 research and development employees compared with 48 as of March 31, 2018. We expect research and development expenses in the near future to increase compared to the first quarter of 2019 as we expect to incur higher research and development expenses for DUR-928.
Research and development expenses associated with our major development programs approximate the following (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
DUR-928 |
|
$ |
4,196 |
|
|
$ |
4,088 |
|
|
Depot injectable programs |
|
|
1,265 |
|
|
|
973 |
|
|
POSIMIR |
|
|
722 |
|
|
|
1,738 |
|
|
ORADUR-ADHD |
|
|
17 |
|
|
|
32 |
|
|
Santen ophthalmic program (1) |
|
|
12 |
|
|
|
8 |
|
|
REMOXY ER (1) |
|
|
2 |
|
|
|
4 |
|
|
Others |
|
|
37 |
|
|
|
109 |
|
|
Total research and development expenses |
|
$ |
6,251 |
|
|
$ |
6,952 |
|
|
(1) |
See Note 2 Strategic Agreements in the financial statements for more details about our agreements with Pain Therapeutics, and Santen. |
DUR-928
Our research and development expenses for DUR-928 were $4.2 million in the three months ended March 31, 2019 compared to $4.1 million for the corresponding period in 2018. The increase in the three months ended March 31, 2019 was primarily due to higher clinical trial expenses and higher employee-related expenses incurred for this drug candidate compared with the corresponding period in 2018.
Depot injectable programs
Our research and development expenses for depot injectable programs were $1.3 million in the three months ended March 31, 2019 compared to $973,000 for the corresponding period in 2018. The increase in the three months ended March 31, 2019 was primarily due to higher employee-related costs for these programs compared with the corresponding period in 2018.
POSIMIR
Our research and development expenses for POSIMIR were $722,000 in the three months ended March 31, 2019 compared to $1.7 million for the corresponding period in 2018. The decrease in the three months ended March 31, 2019 was primarily due to lower outside expenses and lower employee-related expenses for POSIMIR compared with the corresponding period in 2018.
32
Our research and development expenses for ORADUR-ADHD were $17,000 in the three months ended March 31, 2019 compared to $32,000 for the corresponding period in 2018. The decrease in the three months ended March 31, 2019 was primarily due to lower employee-related costs for this drug candidate compared with the corresponding period in 2018.
Santen ophthalmic program
Our research and development expenses for the Santen ophthalmic program were $12,000 in the three months ended March 31, 2019 compared to $8,000 for the corresponding period in 2018. The increase in the three months ended March 31, 2019 was primarily due to higher employee-related costs associated with this drug candidate compared with the corresponding period in 2018.
REMOXY ER
Our research and development expenses for REMOXY ER were $2,000 in the three months ended March 31, 2019 compared to $4,000 for the corresponding period in 2018. The decreases in the three months ended March 31, 2019 was primarily due to lower employee-related costs for REMOXY ER compared with the corresponding period in 2018.
Other DURECT research programs
Our research and development expenses for all other programs were $37,000 in the three months ended March 31, 2019 compared to $109,000 for the corresponding period in 2018. The decrease in the three months ended March 31, 2019 was primarily due to lower employee-related costs incurred as well as lower outside expenses associated with these programs compared with the corresponding period in 2018.
We expect our research and development expenses in the near future to increase compared to the first quarter of 2019 as we expect to incur higher research and development expenses for DUR-928. The duration of development of our research and development programs may span as many as ten years or more, and estimation of completion dates or costs to complete are speculative and subjective due to the numerous risks and uncertainties associated with developing pharmaceutical products, including significant and changing government regulation, the uncertainties of future preclinical and clinical study results, the uncertainties with our collaborators’ commitment to and progress in the programs and the uncertainties associated with process development and manufacturing as well as sales and marketing. In addition, with respect to our development programs subject to third-party collaborations, the timing and expenditures to complete the programs are subject to the control of our collaborators. Therefore, we cannot reasonably estimate the timing and estimated costs of the efforts necessary to complete the research and development programs. For additional information regarding these risks and uncertainties, see “Risk Factors” below.
Selling, general and administrative. Selling, general and administrative expenses are primarily comprised of salaries, benefits, stock-based compensation and other compensation cost associated with finance, legal, business development, sales and marketing and other administrative personnel, overhead and facility costs, and other general and administrative costs.
Selling, general and administrative expenses were $3.5 million for the three months ended March 31, 2019 compared to $3.2 million for the corresponding period in 2018. The increase in selling, general and administrative expenses in the three months ended March 31, 2019 was primarily due to higher patent related expenses compared with the corresponding period in 2018. Stock-based compensation expense recognized related to selling, general and administrative personnel was $242,000 and $283,000 for the three months ended March 31, 2019 and March 31, 2018, respectively.
We had 22 selling, general and administrative employees as of March 31, 2019 compared with 23 as of March 31, 2018. We expect selling, general and administrative expenses in the near future to be lower compared to the first quarter of 2019 due to lower patent related expenses.
Other income (expense). Interest and other income was $209,000 for the three months ended March 31, 2019 compared to $158,000 for the corresponding period in 2018. The increase in interest and other income in the three months ended March 31, 2019 was primarily the result of higher interest income generated from our investments as a result of higher yields in the three months ended March 31, 2019 compared with the corresponding period in 2018.
Interest and other expense was $629,000 for the three months ended March 31, 2019 compared to $623,000 for the corresponding period in 2018. The increase in interest and other expense in the three months ended March 31, 2019 was primarily due to higher interest rates associated with the term loan compared with the corresponding period in 2018.
Liquidity and Capital Resources
33
We had cash, cash equivalents and investments totaling $28.8 million at March 31, 2019 compared to $34.5 million at December 31, 2018. These balances include $150,000 of interest-bearing marketable securities classified as restricted investments on our balance sheets as of March 31, 2019 and December 31, 2018. The decrease in cash, cash equivalents and investments during the three months ended March 31, 2019 was primarily the result of ongoing operating expenses and interest payments, partially offset by payments received from collaboration partners and customers and cash received from the sale of our common stock.
We used $5.7 million of cash in operating activities in the three months ended March 31, 2019 compared to $6.7 million received for the corresponding period in 2018. The cash used for operations was primarily to fund operations as well as our working capital requirements which involved a decrease in net loss of $1.2 million, partially offset by the changes in account receivable, prepaid expenses and other assets, and accrued and other liabilities.
We received $1.6 million of cash from investing activities for the three months ended March 31, 2019 compared to $2.5 million for the corresponding period in 2018. The decrease in cash received from investing activities was primarily due to a decrease in proceeds from maturities of available-for-sale securities for the three months ended March 31, 2019 compared to the corresponding period in 2018. We anticipate incurring capital expenditures of approximately $100,000 in 2019 to purchase research and development and other capital equipment.
We received $57,000 of cash from financing activities for the three months ended March 31, 2019 compared to $14.1 million for the corresponding period in 2018. The decrease in cash received from financing activities was primarily due to lower net proceeds received from issuances of common stock in the three months ended March 31, 2019 compared with the corresponding period in 2018. During the three months ended March 31, 2019, we raised net proceeds (net of commission) of approximately $184,000 from the sale of 243,000 shares of common stock at a weighted average price of $0.78 per share in the open market pursuant to the October 2018 registration statement. The net proceeds were further reduced by net issuance costs of approximately $123,000.
We anticipate that cash used in operating activities in the near future will increase compared to the first quarter of 2019 due to higher research and development expenses related to DUR-928.
In November 2015, we entered into the 2015 Sales Agreement with Cantor Fitzgerald, acting as agent. As of December 31, 2018, approximately 24.6 million registered shares of common stock were sold through the 2015 Sales Agreement for total net proceeds (net of commissions) of approximately $38.3 million. In August 2018, we filed a shelf registration statement on Form S-3 with the SEC, which, upon being declared effective in October 2018, allowed us to offer up to $175.0 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of additional shares of common stock which we may sell, subject to certain limitations, under the 2015 Sales Agreement through Cantor Fitzgerald, acting as agent. As of May 3, 2019, we raised net proceeds (net of commissions) of approximately $184,000 from the sale of 242,750 shares of the Company’s common stock in the open market at a weighted average price of $0.78 per share pursuant to the October 2018 registration statement. Any material sales in the public market of our common stock, under the Controlled Equity Offering program with Cantor Fitzgerald or otherwise under the October 2018 shelf registration statement, could adversely affect prevailing market prices for our common stock.
During the three months ended March 31, 2019, there were no significant changes in our commercial commitments and contractual obligations as compared with the information presented in our Annual Report on Form 10-K for the year ended December 31, 2018.
We believe that our existing cash, cash equivalents and investments and anticipated revenues will be sufficient to fund our planned operations, existing debt and contractual commitments and planned capital expenditures through at least the next 12 months from the date the financial statements are filed. We may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. Additionally, we do not expect to generate significant revenues from our pharmaceutical products currently under development for at least the next twelve months, if at all. Depending on whether we enter into additional collaborative agreements in the near term and the extent to which we earn milestone revenues, we may be required to raise additional capital through a variety of sources, including:
|
|
• |
the public equity markets; |
|
|
• |
private equity financings; |
|
|
• |
collaborative arrangements; and/or |
|
|
• |
public or private debt. |
There can be no assurance that we will enter into additional collaborative agreements in the near term, will earn milestone revenues or that additional capital will be available on favorable terms, if at all. If adequate funds are not available, we may be required to significantly reduce or refocus our operations or to obtain funds through arrangements that may require us to relinquish rights to certain of our products, technologies or potential markets, either of which could have a material adverse effect on our
34
business, financial condition and results of operations. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in ownership dilution to our existing stockholders (assuming convertible debt securities were converted into shares).
Our cash and investments policy emphasizes liquidity and preservation of principal over other portfolio considerations. We select investments that maximize interest income to the extent possible given these two constraints. We satisfy liquidity requirements by investing excess cash in securities with different maturities to match projected cash needs and limit concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers.
Off-Balance Sheet Arrangements
As of March 31, 2019, we did not have any off-balance sheet arrangements, as defined under SEC Regulation S-K Item 303(a)(4)(ii).
Item 3.Quantitative and Qualitative Disclosures about Market Risk
During the three months ended March 31, 2019, there have been no significant changes in market risks as disclosed in our Annual Report on Form 10-K for the year ended December 31, 2018.
Evaluation of Disclosure Controls and Procedures: The Company’s principal executive and principal financial officers reviewed and evaluated the Company’s disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) as of the end of the period covered by this Form 10-Q. Based on that evaluation, the Company’s principal executive and principal financial officers concluded that the Company’s disclosure controls and procedures are effective at ensuring that information required to be disclosed by the Company in reports that the Company files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to management, including the Company’s principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting: There were no significant changes in the Company’s internal control over financial reporting (as defined in Exchange Act Rule 13a-15(f)) during the Company’s most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
35
We are not a party to any material legal proceedings.
In addition to the other information in this Form 10-Q, a number of factors may affect our business and prospects. These factors include but are not limited to the following, which you should consider carefully in evaluating our business and prospects. If any of the following risks actually occur, our business, financial condition, results of operations and growth prospects may be materially and adversely affected.
Risks Related To Our Business
New chemical entities derived from our Epigenetic Regulator Program, which is in the early stages of development, may require more time and resources for development, testing and regulatory approval than our Drug Delivery Program product candidates, and may not result in any approval or viable commercial products
Our Epigenetic Regulator Program is in the early stages of development, involves a novel therapeutic approach and new chemical entities, requires significant further research and development and regulatory approvals and is subject to the risks of failure inherent in the development of products based on innovative approaches. New chemical entities derived from our Epigenetic Regulator Program are molecules that have not previously been approved and marketed as therapeutics, unlike product candidates in our Drug Delivery Programs, in which we apply our formulation expertise and technologies largely to active pharmaceutical ingredients whose safety and efficacy have previously been established but which we aim to improve in some manner through a new formulation. As a result, the product candidates from our Epigenetic Regulator Program may face greater risk of unanticipated safety issues or other side-effects, or may not demonstrate efficacy. Further, the regulatory pathway for our new chemical entities may be more demanding than that for product candidates under our Drug Delivery Programs, for which we may be able to leverage existing data under Section 505(b)(2) of the Act to reduce development risk, time and cost. For example, we have yet to define the therapeutic dose or dosing regimen in any indication for DUR-928, the first drug candidate in our Epigenetic Regulator Program.
Also, because our Epigenetic Regulator Program is in early stages, we have not defined with precision those indications we wish to pursue, each of which may have unique challenges. If the first indications pursued do not show positive results, the credibility of any product candidate from this program may be tarnished, even if the molecule might be effective for other indications. Our decisions regarding which indications to pursue may cause us to fail to capitalize on indications that could have given rise to viable commercial products and profitable market opportunities.
Early indications of activity from Phase 1 and 2 clinical trials of DUR-928 may not predict therapeutic efficacy
Although Phase 1 and Phase 2 clinical trials of DUR-928 have demonstrated that DUR-928 can lead to the reduction of certain biomarkers, such as statistically significant reductions from baseline in the levels of both full-length and cleaved cytokeratin-18 (CK-18), bilirubin, high sensitivity C-Reactive Protein (hsCRP) and IL-18 in NASH patients, and non-statistically significant reductions in CK-18 and bilirubin in CKD patients, and have also shown signs of activity in psoriasis patients, and encouraging initial data from the 30 mg dose group in AH patients, such biomarkers changes and initial indications of activity may ultimately not be correlated with treatment or improvement in the associated disease, and there is a risk that DUR-928 may not demonstrate therapeutic efficacy, despite apparent improvements in biomarker levels and encouraging initial data. Additional controlled clinical trials will be required to evaluate the safety and efficacy of DUR-928 to treat any indication, including NASH, psoriasis, AH and CKD. There can be no assurance that these studies will demonstrate the safety or efficacy of DUR-928 in a statistically significant manner. The failure of DUR-928 to show efficacy, or if safety signals emerge in ongoing and future clinical trials, would significantly harm our business.
Ongoing and planned clinical trials for DUR-928 may be delayed and may not demonstrate efficacy or safety in the indications tested.
Clinical trials of orally administered DUR-928 in NASH patients, IV infusion of DUR-928 in moderate and severe AH patients and topically administered DUR-928 in patients with psoriasis are ongoing, with preliminary, initial or top line data expected in the second half of 2019. There can be no assurance that these trials will enroll at a rate to generate data in the second half of 2019. In parallel with our ongoing AH trial, we are supporting Dr. Craig McClain’s efforts to initiate an NIH-funded study of DUR-928 in AH patients at the University of Louisville. There can be no assurance that this trial will ever begin or that the grant funding required to support this trial will provide sufficient funding. Additionally, there can be no assurance that the results from Dr. McClain’s trial will be similar to the results from our AH trial(s). If the results from Dr. McClain’s AH trial and the Company’s AH trial are sufficiently different, it could make it difficult to interpret the results from either trail, which could cause material harm to the Company. With respect to all of the above ongoing and contemplated clinical trials, there can be no assurance that biological activity demonstrated in previous animal disease models or earlier clinical trials will also be seen in these additional patients in ongoing trials or future clinical
36
trials, or that any clinically relevant biological activity will be observed, or that enrollment rates will be favorable or that these additional trials or additional patients in ongoing trials will not identify safety issues. Failure of these trials to achieve desired results in their anticipated timeframes would negatively impact our business and ability to raise additional capital.
The path to regulatory approval of DUR-928 is uncertain
We are currently developing DUR-928 in several indications, including AH, psoriasis and NASH. In several of these indications, there are no currently approved drugs. Accordingly, we will have to interact with the FDA and other regulatory agencies regarding important aspects of the clinical development program, including the size of clinical trials, the specific primary and secondary endpoints for the clinical trials, inclusion and exclusion criteria, stopping rules, duration of follow up, size of the safety database and other matters. This uncertainty may make it difficult to predict the timing or expense required to obtain regulatory approval for DUR-928. We also may need to revise our clinical development plans after trials have commenced or been completed, which could add to the time and expense associated with the clinical development of DUR-928. If we are unable to reach agreement with the FDA or other regulatory agencies regarding clinical development plans for DUR-928, we may curtail or limit our development activities for this product candidate.
Prospects for POSIMIR are uncertain following the failure of the PERSIST trial to achieve its primary efficacy endpoint and the termination of our agreement with Sandoz
The failure of the PERSIST trial for POSIMIR to achieve its primary efficacy endpoint may reduce the prospects of obtaining FDA approval for POSIMIR. In January 2019, Sandoz elected to terminate our licensing agreement for POSIMIR, as a result of which we will not receive any milestone or royalty payments from Sandoz and we or a potential future partner will be responsible for commercialization of POSIMIR in the United States, if approved. We intend to seek a new collaboration partner for POSIMIR in the United States, but there is no assurance we will be successful in that effort or that any terms offered will be attractive to the Company. We may elect to terminate development of POSIMIR at any time. If we elect to continue to develop POSIMIR, we may be required to make a larger investment than previously planned, which would limit the funds available for other product development activities or require us to raise additional capital.
The FDA may not agree with our response to its Complete Response Letter (CRL) to the NDA submission for POSIMIR
After carefully reviewing the existing POSIMIR data and evaluating the feedback we have received from the FDA, including the CRL and other correspondence, we have made the decision to prepare and submit to FDA a response to the CRL. The submission will request FDA approval of POSIMIR based on what the Company and its advisors believe is adequate evidence of both safety and efficacy. As the submission is intended to be a response to a CRL, we expect a 6-month FDA review period. There can be no assurance that the FDA will agree with our assumption of a 6-month review period, will complete their review in a timely manner, or will agree with our response to the CRL and approve POSIMIR for marketing. The FDA may require additional studies or additional information regarding POSIMIR. We would need to review any such requests to determine whether we believe that a viable path for regulatory approval of POSIMIR remains available. It is also possible that the FDA will require us to participate in a public advisory committee meeting whereby FDA would obtain feedback from a panel of third-party experts and advisors related to POSIMIR. If such an advisory committee meeting were to occur, it is possible that the FDA or the committee or public speakers would raise issues about POSIMIR’s safety or efficacy, make disparaging remarks about POSIMIR, vote against the product’s approval or vote for limitations in the product’s labeled indication or for the inclusion of additional warnings and precautions against certain aspects of the product’s use, or for other requirements in the product labeling that would be potentially harmful to the product’s commercial value or viability. Any of these outcomes would harm the value of POSIMIR and the value of our business.
If we experience delays or difficulties in the enrollment of subjects in clinical trials, our product development expenses may increase, clinical trial data could be delayed and receipt of necessary regulatory approvals could be delayed or prevented
Successful and timely completion of clinical trials will require that we enroll a sufficient number of subjects. Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population and the ability of clinical sites to successfully recruit subjects to participate in clinical trials. Trials may be subject to delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. We may not be able to initiate or continue clinical trials for our drug candidates if we are unable to locate and enroll a sufficient number of eligible subjects to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. It is possible that the specific requirements by the FDA for our patients to be included in these trials may make the trials more difficult to conduct or may significantly extend the time required for enrollment of these trials.
We cannot predict how successful we will be at enrolling subjects in our clinical trials. Subject enrollment is affected by other factors including:
|
|
• |
the eligibility criteria for the trial in question; |
|
|
• |
the prevalence and incidence of the conditions being studied in the clinical trials; |
37
|
|
• |
clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs or therapeutic biologics that may be approved for the indications we are investigating; |
|
|
• |
the efforts to facilitate timely enrollment in clinical trials; |
|
|
• |
competition for patients from other clinical trials; |
|
|
• |
the willingness of potential clinical trial subjects to provide informed consent to participate in the trial; |
|
|
• |
the patient referral practices of physicians; |
|
|
• |
the ability to monitor subjects adequately during and after treatment; and |
|
|
• |
the proximity and availability of clinical trial sites for prospective subjects. |
Our inability to enroll a sufficient number of subjects for clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether. Enrollment delays in these clinical trials may result in increased development costs for our drug candidates or delays in regulatory filings and progression, which would cause the value of our company to decline and limit our ability to obtain additional financing.
The FDA may require more information or clinical studies for all of our product candidates, and our product candidates may never be approved
The failure to adequately demonstrate the safety and effectiveness of a pharmaceutical product candidate under development to the satisfaction of FDA and other regulatory agencies will result in delays to the regulatory approval or non-approvability of our product candidates, and could materially harm our business. Clinical trials may not demonstrate the sufficient levels of safety and efficacy necessary to obtain the requisite regulatory approvals for our product candidates, or may require such significant numbers of patients or additional costs to make it impractical to satisfy the FDA’s requirements, and thus our product candidates may not be approved for marketing. For example, the Phase 3 PERSIST trial for POSIMIR did not meet its primary efficacy endpoint. In addition, during the review process, the FDA may request more information regarding the safety of our product candidates, as they have in their Complete Response Letter for POSIMIR, and answering such questions could require significant additional work and expense, and take a significant amount of time, resulting in a material delay of approval or the failure to obtain approval or lead the company to abandon the development of that product candidate. During the review process, the FDA may also request more information regarding the chemistry, manufacturing or controls related to our product candidates or to abuse deterrent properties of certain product candidates, as they have in their Complete Response Letters for REMOXY ER, and answering such questions could require significant additional work and expense, and take a significant amount of time, resulting in a material delay of approval or the failure to obtain approval or abandonment of the product candidate. Additionally, even if our product candidates receive FDA approval, the FDA may require that we conduct additional clinical studies after such approval, place limitations on our products in applicable labels, require marketing under a REMS program, delay approval to market our products or limit the use of our products, which may harm our business and results of operations.
We currently have a significant amount of debt. Compliance with repayment obligations and other covenants may be difficult, and failure by us to fulfill our obligations under the applicable loan agreements may cause the repayment obligations to accelerate
In July 2016, we entered into a Loan and Security Agreement (the Loan Agreement) with Oxford Finance LLC (Oxford Finance), pursuant to which Oxford Finance provided a $20 million secured single-draw term loan to us with an initial maturity date of August 1, 2020. The term loan was fully drawn at close and the proceeds may be used for working capital and general business requirements. The term loan repayment schedule provided initially for interest only payments for the first 18 months, followed by consecutive monthly payments of principal and interest in arrears starting on March 1, 2018 and continuing through the maturity date of August 1, 2020. Following two amendments, we make interest only payments under the amended Loan Agreement until June 1, 2020 and the final maturity date of the loan is November 1, 2022. The Loan Agreement provides for a floating interest rate (7.95% initially and 9.84% as of March 31, 2019) based on an index rate plus a spread and an additional payment equal to 10% of the principal amount of the term loan, which is due when the term loan becomes due or upon the prepayment of the facility. If we elect to prepay the loan, there is also a prepayment fee between 0.75% and 2.5% of the principal amount of the term loan depending on the timing of prepayment. Our debt repayment obligations under the Loan Agreement, as amended, may prove a burden to the Company as they become due, particularly following the expiration of the interest-only period.
The Loan Agreement contains customary events of default, including, among other things, our failure to fulfill certain of our obligations under the Loan Agreement and the occurrence of a material adverse change in our business, operations or condition (financial or otherwise), a material impairment of the prospect of repayment of any portion of the loan, the failure to deliver an unqualified audit report and board approved financial projections within time periods set forth in the Loan Agreement, or a material impairment in the perfection or priority of lender’s lien in the collateral or in the value of such collateral. In the event of default by us
38
under the Loan Agreement, the lender would be entitled to exercise its remedies thereunder, including the right to accelerate the debt, upon which we may be required to repay all amounts then outstanding under the Loan Agreement, which could harm our business, operations and financial condition.
In addition, the term loan is secured by substantially all of our assets, except that the collateral does not include any equity interests in the Company, any intellectual property (including all licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The Loan Agreement contains customary representations, warranties and covenants by us, which covenants limit our ability to convey, sell, lease, transfer, assign or otherwise dispose of certain of our assets; engage in any business other than the businesses currently engaged in by us or reasonably related thereto; liquidate or dissolve; make certain management changes; undergo certain change of control events; create, incur, assume, or be liable with respect to certain indebtedness; grant certain liens; pay dividends and make certain other restricted payments; make certain investments; make payments on any subordinated debt; and enter into transactions with any of our affiliates outside of the ordinary course of business or permit our subsidiaries to do the same. Complying with these covenants may make it more difficult for us to successfully execute our business strategy.
We will require and may have difficulty raising needed capital in the future
Our business currently does not generate sufficient revenues to meet our capital requirements and we do not expect that it will do so in the near future. We have expended and will continue to expend substantial funds to complete the research, development and clinical testing of our product candidates. We will require additional funds for these purposes, to establish additional clinical- and commercial-scale manufacturing arrangements and facilities, and to provide for the marketing and distribution of our product candidates. Additional funds may not be available on acceptable terms, if at all, and such availability will depend on a number of factors, some of which are outside of our control, including general capital markets conditions and investors’ view of our prospects and valuation. If adequate funds are unavailable from operations or additional sources of financing, we may have to delay, reduce the scope of or eliminate one or more of our research or development programs which would materially harm our business, financial condition and results of operations.
We believe that our cash, cash equivalents and investments and anticipated revenues will be adequate to satisfy our capital needs for at least the next 12 months from the date the financial statements are filed. However, our independent auditors may not agree with this assessment, and our actual capital requirements will depend on many factors, including:
|
|
• |
success in entering into collaboration agreements and achieving milestones under such agreements; |
|
|
• |
the continuation of our collaborative agreements that provide financial funding for our activities; |
|
|
• |
regulatory actions with respect to our and our collaborators’ product candidates; |
|
|
• |
continued progress and cost of our research and development programs; |
|
|
• |
progress with preclinical studies and clinical trials; |
|
|
• |
the time and costs involved in obtaining regulatory clearance; |
|
|
• |
costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; |
|
|
• |
costs of developing sales, marketing and distribution channels and our ability and that of our collaborators to sell our products, products we have a financial interest in and eventually, product candidates; |
|
|
• |
costs involved in establishing manufacturing capabilities for pre-clinical, non-clinical, clinical and commercial quantities of our product candidates; |
|
|
• |
competing technological and market developments; |
|
|
• |
market acceptance of our products, products we have a financial interest in and eventually, product candidates; |
|
|
• |
any failure to comply with the covenants in our debt instruments that results in acceleration of repayment obligations; |
|
|
• |
costs for recruiting and retaining employees and consultants; and |
|
|
• |
unexpected legal, accounting and other costs and liabilities related to our business. |
We may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. We may seek to raise additional funds through equity or debt financings, convertible debt financings, collaborative arrangements with corporate collaborators or other sources, which may be dilutive to existing stockholders and may cause the price of our common stock to decline. In addition, in the event that additional funds are obtained through arrangements with collaborators or other sources, we may have to relinquish rights to some of our technologies or pharmaceutical product candidates that we would otherwise seek to
39
develop or commercialize ourselves. If adequate funds are not available, we may be required to significantly reduce or refocus our product development efforts, resulting in delays in generating future product revenue.
We do not control the commercialization of PERSERIS or Methydur
We rely on Indivior for the commercialization of PERSERIS. Indivior has stated that it launched PERSERIS in February 2019 in the U.S. with a sales force consisting of approximately 50 representatives and expects peak year net revenue goal for PERSERIS of $200 to $300 million. There can be no assurance that Indivior will obtain market acceptance and meaningful sales. If Indivior does not successfully commercialize PERSERIS, the earn-out payments we receive under our agreement with them may be limited. We rely on Orient Pharma for the commercialization of Methydur in the territories licensed to Orient Pharma. If Orient Pharma does not successfully commercialize Methydur, the royalty payments we receive under our agreement with them may be limited.
Development of our pharmaceutical product candidates is not complete, and we cannot be certain that our product candidates will be able to be commercialized
To be profitable, we or our third-party collaborators must successfully research, develop, obtain regulatory approval for, manufacture, introduce, market and distribute our pharmaceutical product candidates under development. For each product candidate that we or our third-party collaborators intend to commercialize, we must successfully meet a number of critical developmental milestones for each disease or medical condition targeted, including:
|
|
• |
with respect to each product candidate based on a new chemical entity, determining appropriate indication(s); |
|
|
• |
with respect to our Proprietary Pharmaceutical Programs based on our drug delivery technologies, selecting and developing a drug delivery technology to deliver the proper dose of drug over the desired period of time; |
|
|
• |
determining the appropriate route of administration and drug dosage for each product candidate in each indication; |
|
|
• |
developing product candidates that will be tolerated, safe and effective and that will be compatible with the active pharmaceutical agent; |
|
|
• |
demonstrating each product candidate will be stable for commercially reasonable time periods; |
|
|
• |
demonstrating through clinical trials that each product candidate is safe and effective in patients for the intended indication at an achievable dose and that the product candidate’s benefits outweigh its risks; |
|
|
• |
demonstrating abuse deterrent properties to the satisfaction of the FDA for certain product candidates for which abuse-deterrence is considered an important feature by the FDA, and |
|
|
• |
completing the manufacturing development and scale-up to permit manufacture of the product candidate in commercial quantities and at acceptable cost. |
The time frame necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we may not successfully complete these milestones for any of our product candidates in development. Except for marketing authorization for Orient Pharma’s distribution of Methydur Sustained Release Capsules in Taiwan, development is incomplete for all product candidates in our development programs, including DUR-928. We may not be able to finalize the design or formulation of any of these product candidates. Further, although we believe our design and formulation of POSIMIR and ORADUR-Methylphenidate ER to be substantially complete, there can be no assurance that additional development will not be required prior to regulatory approval of these products (in addition to the marketing authorization for Orient Pharma’s distribution of Methydur Sustained Release Capsules in Taiwan). In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not been previously approved for use in pharmaceutical products, which may require us or our collaborators to perform additional studies and may delay clinical testing and regulatory approval of our product candidates. Even after we complete the design of a product candidate, the product candidate must still complete required clinical trials and additional safety testing in animals before approval for commercialization. We are continuing testing and development of our product candidates and may explore possible design or formulation changes to address issues of safety, manufacturing efficiency, stability and performance. We or our collaborators may not be able to complete development of any product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. If we or our third-party collaborators are unable to complete development of DUR-928, POSIMIR, ORADUR-Methylphenidate ER or other product candidates, we will not be able to earn revenue from them, which would materially harm our business.
We depend to a large extent on third-party collaborators, and we have limited or no control over the development, sales, distribution and disclosure for our product candidates which are the subject of third-party collaborative or license agreements
Our performance depends to a large extent on the ability of our third-party collaborators to successfully develop and obtain approvals for our pharmaceutical product candidates. We have entered into agreements with Indivior, Santen, Orient Pharma and others under which we granted such third parties the right to develop, apply for regulatory approval for, market, promote or distribute
40
certain product candidates, subject to payments to us in the form of product royalties, earn-out and other payments. We have limited or no control over the expertise or resources that any collaborator may devote to the development, clinical trial strategy, regulatory approval, marketing or sale of these product candidates, or the timing of their activities. Any of our present or future collaborators may not perform their obligations as expected. These collaborators may breach or terminate their agreement with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Enforcing any of these agreements in the event of a breach by the other party could require the expenditure of significant resources and consume a significant amount of management time and attention. Our collaborators may also conduct their activities in a manner that is different from the manner we would have chosen, had we been developing such product candidates ourselves. Further, our collaborators may elect not to develop or commercialize product candidates arising out of our collaborative arrangements or not devote sufficient resources to the development, clinical trials, regulatory approval, manufacture, marketing or sale of these product candidates. If any of these events occur, we may not recognize revenue from the commercialization of our product candidates based on such collaborations. In addition, these third parties may have similar or competitive products to the ones which are the subject of their collaborations with us, or relationships with our competitors, which may reduce their interest in developing or selling our product candidates. We may not be able to control public disclosures made by some of our third-party collaborators, which could negatively impact our stock price.
Cancellation of collaborations regarding our product candidates may adversely affect potential economic benefits
Third-party collaboration agreements typically allow the third party to terminate the agreement (or a specific program within an agreement) by providing notice. For example in January 2019, Sandoz notified us that they were terminating our agreement with respect to POSIMIR, and in March 2019, Pain Therapeutics notified us that they were terminating our agreement with respect to REMOXY ER. In these cases, the product rights revert to us. If there have been payments under such agreements that are being recognized over time, termination of such agreements (or programs) can lead to a near-term increase in our reported revenues resulting from the immediate recognition of the balance of such payments. Termination deprives us of potential future economic benefits under such agreements, and may make it more difficult to enter into agreements with other third parties for use of the assets that were subject to the terminated agreement. Termination of our agreements with Santen or Orient Pharma could have similar effects.
A significant component of our revenues depend on collaboration agreements with other companies. If we are unable to enter into new agreements or meet our obligations or manage our relationships with our collaborators under these agreements our revenues may decrease. Acquisitions of our collaborators can be disruptive
Our revenues are based to a significant extent on collaborative arrangements with third parties, pursuant to which we receive payments based on our performance of research and development activities set forth in these agreements. We have seen periodic declines in revenues associated with our existing collaboration agreements, which reflect the current development stage of the product candidates subject to those agreements, and our collaborator’s decreased needs for our services. We do not expect our collaboration revenues to increase materially unless we enter into new collaboration agreements, and there can be no assurance that we will do so. Even if we enter into new collaboration agreements, we may not be able to fulfill our obligations or attain milestones set forth in any specific agreement, which could cause our revenues to fluctuate or be less than anticipated and may expose us to liability for contractual breach. In addition, these agreements may require us to devote significant time and resources to communicating with and managing our relationships with such collaborators and resolving possible issues of contractual interpretation which may detract from time our management would otherwise devote to managing our operations. Such agreements are generally complex and contain provisions that could give rise to legal disputes, including potential disputes concerning ownership of intellectual property under collaborations. Such disputes can delay or prevent the development of potential new product candidates, or can lead to lengthy, expensive litigation or arbitration. In general, our collaboration agreements, including our agreements with Orient Pharma with respect to Methydur, and Santen with respect to an ophthalmic product may be terminated by the other party at will or upon specified conditions including, for example, if we fail to satisfy specified performance milestones or if we breach the terms of the agreement. Acquisitions of our collaborators or strategic changes or re-organizations or re-prioritizations of our collaborators can lead to turnover of program staff, a review of development programs and strategies by the acquirer, and other events that can disrupt a program, resulting in program delays or discontinuations.
If we do not enter into new collaboration agreements, and if any of our collaborative agreements are terminated or delayed, our anticipated revenues may be reduced or not materialize, and our products in development related to those agreements may not be commercialized.
We have an ongoing dispute with Sandoz AG related to a disputed termination fee
The Company and Sandoz are in dispute with regard to Sandoz’s obligation to pay a termination fee to DURECT. DURECT has initiated a formal dispute resolution process related to the termination fee. The Company may file for dispute resolution through a formal arbitration process. The Company’s management may devote significant time and resources to this dispute resolution process, which may detract from time our management would otherwise devote to managing our operations and could have a material adverse effect on our business.
Our cash flows are likely to differ from our reported revenues
41
Our revenues will likely differ from our cash flows from revenue-generating activities. Upfront payments received upon execution of collaborative agreements are recorded as deferred revenue and generally recognized on a straight-line basis over the period of our continuing involvement with the third-party collaborator pursuant to the applicable agreement. The period of continuing involvement may also be revised on a prospective basis. As of March 31, 2018, we had $812,000 of deferred revenue which will be recognized in future periods and may cause our reported revenues to be greater than cash flows from our ongoing revenue-generating activities.
Our revenues also depend on milestone payments based on achievements by our third-party collaborators. Failure of such collaborators to attain such milestones would result in our not receiving additional revenues
In addition to payments based on our performance of research and development activities, our revenues also depend on the attainment of milestones set forth in our collaboration agreements. Such milestones are typically related to development activities, including regulatory milestones, or sales accomplishments. While our involvement is generally necessary to the achievement of development-based milestones, the performance of our third-party collaborators is also generally required to achieve those milestones. Under our third-party collaborative agreements, our third-party collaborators will take the lead in commercialization activities and we are typically not involved in the achievement of sales-based milestones. Therefore, we are even more dependent upon the performance of our third-party collaborators in achieving sales-based milestones. To the extent we and our third-party collaborators do not achieve such development-based milestones or our third-party collaborators do not achieve sales-based milestones, we will not receive the associated revenues, which could harm our financial condition and may cause us to defer or cut-back development activities or forego the exploitation of opportunities in certain geographic territories, any of which could have a material adverse effect on our business.
Our business strategy includes the entry into additional collaborative agreements. We may not be able to enter into additional collaborative agreements or may not be able to negotiate commercially acceptable terms for these agreements
Our current business strategy includes the entry into additional collaborative agreements for the development and commercialization of our product candidates, including, but not limited to DUR-928, ORADUR-Methylphenidate ER in markets not already licensed to Orient Pharma, including the United States and Europe, and others. The negotiation and consummation of these types of agreements typically involve simultaneous discussions with multiple potential collaborators and require significant time and resources from our officers, business development, legal, and research and development staff. In addition, in attracting the attention of pharmaceutical and biotechnology company collaborators, we compete with numerous other third parties with product opportunities as well as the collaborators’ own internal product opportunities. We may not be able to consummate additional collaborative agreements, or we may not be able to negotiate commercially acceptable terms for these agreements. If we do not consummate additional collaborative agreements, we may have to consume money more rapidly on our product development efforts, defer development activities or forego the exploitation of certain geographic territories, abandon development of certain product candidates or indications for certain product candidates, any of which could have a material adverse effect on our business.
We and our third-party collaborators may not be able to manufacture sufficient quantities of our pharmaceutical product candidates and components to support the non-clinical, clinical and commercial requirements of our collaborators and ourselves at an acceptable cost or in compliance with applicable government regulations, and we have limited manufacturing experience
We or our third-party collaborators to whom we have assigned such responsibility must manufacture our product candidates, and components (including active ingredients, excipients) in non-clinical, clinical and commercial quantities, either directly or through third parties, in compliance with regulatory requirements and at an acceptable cost. The manufacturing processes associated with our product candidates are complex. We and our third-party collaborators, where relevant, have not yet completed development of the manufacturing process for any product candidates or components, including DUR-928 ORADUR-Methylphenidate ER and POSIMIR. If we and our third-party collaborators, where relevant, fail to timely complete the development of the manufacturing process for our product candidates, we and our third-party collaborators, where relevant, will not be able to timely produce supplies for non-clinical, clinical trials and commercialization of our product candidates. We have also committed to manufacture and supply product candidates or components under a number of our collaborative agreements with third-party companies. We have limited experience manufacturing pharmaceutical products, and we may not be able to timely accomplish these tasks. If we and our third-party collaborators, where relevant, fail to develop manufacturing processes to permit us to manufacture a product candidate or component at an acceptable quality and cost, then we and our third-party collaborators may not be able to develop or commercialize that product candidate or we may be in breach of our supply obligations to our third-party collaborators.
Our manufacturing facility in Cupertino is a multi-disciplinary site that we have used to manufacture only research and clinical supplies of several of our product candidates, including POSIMIR and DUR-928. If we experience delays or technical difficulties in developing acceptable manufacturing processes or scaling up the manufacturing of our product candidates, it could result in delays or added cost in our development programs. We have not manufactured commercial quantities of any of our product candidates at our Cupertino facility. In the future, we intend to develop additional manufacturing capabilities for our product candidates and components to meet our demands and those of our third-party collaborators by contracting with third-party manufacturers and by potentially constructing additional manufacturing space at our facilities in California and Alabama. We have limited experience building and validating manufacturing facilities, and we may not be able to accomplish these tasks in a timely or cost effective manner.
42
If we and our third-party collaborators, where relevant, are unable to manufacture our product candidates or components in a timely manner or at an acceptable cost, quality or performance level, and are unable to attain and maintain compliance with applicable regulations, the non-clinical and clinical trials and the commercial sale of our product candidates and those of our third-party collaborators could be delayed or never occur. Additionally, we may need to alter our facility design or manufacturing processes, install additional equipment or do additional construction or testing in order to meet regulatory requirements, optimize the production process, increase efficiencies or production capacity or for other reasons, which may result in additional cost to us or delay production of product needed for the non-clinical trials, clinical trials, chemistry, manufacturing and controls (CMC) and commercial launch of our product candidates and those of our third-party collaborators.
If we or our third-party collaborators cannot manufacture our pharmaceutical product candidates or components in time to meet the clinical or commercial requirements of our collaborators or ourselves or at an acceptable cost, our operating results will be harmed.
Failure to comply with ongoing governmental regulations for our pharmaceutical product candidates could materially harm our business in the future
Developing, manufacturing, marketing or promoting a drug is subject to very strict controls. Furthermore, clearance or approval may entail ongoing requirements for post-marketing studies. The manufacture and marketing of drugs are subject to continuing FDA and foreign regulatory review and requirements that we update our regulatory filings. Later discovery of previously unknown problems with a product, manufacturer or facility, or our failure to update regulatory files, may result in restrictions, including withdrawal of the product from the market. Any of the following or other similar events, if they were to occur, could delay or preclude us from further developing, marketing or realizing full commercial use of our product candidates, which in turn would materially harm our business, financial condition and results of operations:
|
|
• |
failure to obtain or maintain requisite governmental approvals; |
|
|
• |
failure to meet GMP, GLP and/or other governmental requirements for drug development; |
|
|
• |
failure to obtain approvals for clinically intended uses of our pharmaceutical product candidates under development; or |
|
|
• |
FDA required product withdrawals or warnings arising from identification of serious and unanticipated adverse side effects in our product candidates. |
Manufacturers of drugs must comply with the applicable FDA good manufacturing practice regulations, which include production design controls, testing, quality control and quality assurance requirements as well as the corresponding maintenance of records and documentation. Compliance with current good manufacturing practices regulations is difficult and costly. Manufacturing facilities are subject to ongoing periodic inspection by the FDA and corresponding state and in some cases, foreign agencies, including unannounced inspections, and must be licensed before they can be used for the commercial manufacture of our development products. We and/or our present or future suppliers and distributors may be unable to comply with the applicable good manufacturing practice regulations and other FDA and/or foreign regulatory requirements. We have not been subject to a good manufacturing practice regulation inspection by the FDA relating to our product candidates. If we, our third-party collaborators or our respective suppliers do not achieve compliance for our product candidates we or they manufacture, the FDA or foreign equivalents may refuse or withdraw marketing clearance, put our or our partner’s clinical trial on hold, withdraw or reject an investigational new drug (IND) application or require product recall, which may cause interruptions or delays in the development, manufacture and sale of our product candidates.
We have a history of operating losses, expect to continue to have losses in the future and may never achieve or maintain profitability
We have incurred significant operating losses since our inception in 1998 and, as of March 31, 2019, had an accumulated deficit of approximately $475.7 million. We expect to continue to incur significant operating losses over the next several years as we continue to incur significant costs for research and development, clinical trials, manufacturing, sales, and general and administrative functions. Our ability to achieve profitability depends upon our ability, alone or with others, to successfully complete the development of our proposed product candidates, obtain the required regulatory clearances, and manufacture and market our proposed product candidates. Development of pharmaceutical product candidates is costly and requires significant investment. In addition, we may choose to license from third parties either additional drug delivery platform technology or rights to particular drugs or other appropriate technology and/or intellectual property rights for use in our product candidates. The license fees for these technologies or rights would increase the costs of our product candidates.
To date, we have not generated significant revenue from the commercial sale of our pharmaceutical product candidates and do not expect to do so in the near future. Our current revenues are from the ALZET product line, from the LACTEL product line, from certain excipient sales, from earn-out payments from Indivior related to sales of PERSERIS, and from payments under collaborative research and development agreements with third parties. We do not expect our product revenues to increase significantly in the near future, and we do not expect that collaborative research and development revenues will exceed our actual operating expenses. We do not anticipate meaningful revenues to derive from the commercialization and marketing of our product candidates in development in the near future, and therefore do not expect to generate sufficient revenues to cover expenses or achieve profitability in the near future.
43
We may develop our own sales force and commercial group to market future products but we have limited sales and marketing experience with respect to pharmaceuticals and may not be able to do so effectively
We have a small sales and marketing group focused on our ALZET and LACTEL product lines. We may choose to develop our own sales force and commercial group to market products that we may develop in the future. Developing a sales force and commercial group would require substantial expenditures and the hiring of qualified personnel. We have limited sales and marketing experience, and may not be able to effectively recruit, train or retain sales personnel. If we are not able to put in place an appropriate sales force and commercial group for our products in development, we may not be able to effectively launch these products. We may not be able to effectively sell our product candidates, if approved, and our failure to do so could limit or materially harm our business.
We and our third-party collaborators may not sell our product candidates effectively
We and our third-party collaborators compete with many other companies that currently have extensive and well-funded marketing and sales operations. Our marketing and sales efforts and those of our third-party collaborators may be unable to compete successfully against these other companies. We and our third-party collaborators, if relevant, may be unable to establish a sufficient sales and marketing organization on a timely basis, if at all. We and our third-party collaborators, if relevant, may be unable to engage qualified distributors. Even if engaged, these distributors may:
|
|
• |
fail to satisfy financial or contractual obligations to us; |
|
|
• |
fail to adequately market our product candidates; |
|
|
• |
cease operations with little or no notice to us; |
|
|
• |
offer, design, manufacture or promote competing product lines; |
|
|
• |
fail to maintain adequate inventory and thereby restrict use of our product candidates; or |
|
|
• |
build up inventory in excess of demand thereby limiting future purchases of our product candidates resulting in significant quarter-to-quarter variability in our sales. |
The failure of us or our third-party collaborators to effectively develop, gain regulatory approval for, sell, manufacture and market our product candidates will hurt our business, prospects, financial results and may impact our access to capital.
We rely heavily on third parties to support development, clinical testing and manufacturing of our product candidates
We rely on third-party contract research organizations, consultants, service providers and suppliers to provide critical services to support development, clinical testing, and manufacturing of our product candidates. For example, we currently depend on third-party vendors to manage and monitor our clinical trials. We rely on third-parties to manufacture or perform manufacturing steps relating to our product candidates or components. We anticipate that we will continue to rely on these and other third-party contractors to support development, clinical testing, and manufacturing of our product candidates. These third parties may not execute their responsibilities and tasks competently in compliance with applicable laws and regulations or in a timely fashion. Failure of these contractors to provide the required services in a competent or timely manner or on reasonable commercial terms could materially delay the development and approval of our development products, increase our expenses and materially harm our business, financial condition, results of operations and access to capital.
Key components of our product candidates are provided by limited numbers of suppliers, and supply shortages or loss of these suppliers could result in interruptions in supply or increased costs
Certain components and drug substances used in our and our collaborators’ product candidates, including DUR-928, POSIMIR, Methydur, and PERSERIS, are currently purchased from a single or a limited number of outside sources. In particular, Eastman Chemical is the sole supplier of our requirements of sucrose acetate isobutyrate, a necessary component of POSIMIR, ORADUR-Methylphenidate and certain other pharmaceutical product candidates we have under development. A third party manufacturer is our sole supplier for future clinical and commercial supplies of POSIMIR. Another third party manufacturer is our sole supplier for future non-clinical, clinical and commercial supplies of DUR-928. The reliance on a sole or limited number of suppliers could result in:
|
|
• |
delays associated with redesigning a pharmaceutical product candidate due to a failure to obtain a single source component; |
|
|
• |
delays associated with finding and contracting with a new supplier (if we can find one capable of replacing the old supplier and negotiate commercially reasonable terms) and then transferring the technology to the new supplier; |
|
|
• |
an inability to obtain an adequate supply of required product candidate, active pharmaceutical ingredient or excipients or other components; and |
|
|
• |
reduced control over pricing, quality and delivery time. |
We have entered into a commercial manufacturing and packaging agreement with a third party manufacturer for future supply of POSIMIR. This third party is our sole source for the drug product required for development and commercialization of this drug
44
candidate. There may be technical risks associated with establishing an alternative commercial manufacturer that could entail delays in supply, quality issues or delays in the possible regulatory approval of POSIMIR. Furthermore, we and our contract manufacturer may also need or choose to subcontract with additional third-party contractors to perform manufacturing steps of POSIMIR or supply required components for POSIMIR. Where third party contractors perform manufacturing services for us, we will be subject to the schedule, expertise and performance of third parties as well as incur significant additional costs. Failure of third parties to perform their obligations could adversely affect our operations, development timeline and financial results. If we proceed with the development of POSIMIR, we expect to put in place in the future second source supply arrangements, which may be costly and time consuming.
While we have entered into contract manufacturing agreements with multiple vendors for DUR-928, we currently have a third party sole supplier for GMP supplies of DUR-928. This third party is our sole source for the drug product required for development and commercialization of this drug candidate. There can be no assurance that we will receive sufficient quantities of DUR-928 to commence and conduct the non-clinical trials, clinical trials and CMC activities we are planning, and delays in supply could delay development of DUR-928.
We have supply agreements in place for certain components of our pharmaceutical product candidates, but do not have in place long term supply agreements with respect to all of the components of any of our product candidates. Therefore the supply of a particular component could be terminated at any time without penalty to the supplier. In addition, we may not be able to procure required components or drugs from third-party suppliers at a quantity, quality and cost acceptable to us. Any interruption in the supply of single source components (including active pharmaceutical ingredients or excipients) or product candidates, could cause us to seek alternative sources of supply or manufacture these items internally if feasible. Furthermore, in some cases, we are relying on our third-party collaborators to procure supply of necessary components. If the supply of any components for our product candidates is interrupted, components from alternative suppliers may not be available in sufficient volumes or at acceptable quality levels within required timeframes, if at all, to meet our needs or those of our third-party collaborators. This could delay our ability to complete development and obtain approval for commercialization and marketing of our product candidates, causing us to lose sales, incur additional costs, delay new product introductions and could harm our reputation and make access to capital more difficult, expensive or impossible.
Some of our pharmaceutical product candidates contain controlled substances, the making, use, sale, importation, exportation and distribution of which are subject to regulation by state, federal and foreign law enforcement and other regulatory agencies
Some of our product candidates currently under development contain, and our products in the future may contain, controlled substances which are subject to state, federal and foreign laws and regulations regarding their manufacture, use, sale, importation and distribution. ORADUR-Methylphenidate ER and certain other product candidates we may develop contain active ingredients which are classified as controlled substances under the regulations of the U.S. Drug Enforcement Agency. For our product candidates containing controlled substances, we and our suppliers, manufacturers, contractors, customers and distributors are required to obtain and maintain applicable registrations from state, federal and foreign law enforcement and regulatory agencies and comply with state, federal and foreign laws and regulations regarding the manufacture, use, sale, importation and distribution of controlled substances. These regulations are extensive and include regulations governing manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, record keeping, reporting, handling, shipment and disposal. These regulations increase the personnel needs and the expense associated with development and commercialization of drug candidates including controlled substances. Failure to obtain and maintain required registrations or comply with any applicable regulations could delay or preclude us from developing and commercializing our product candidates containing controlled substances and subject us to enforcement action. In addition, because of their restrictive nature, these regulations could limit our commercialization of our product candidates containing controlled substances. In particular, among other things, there is a risk that these regulations may interfere with the supply of the drugs used in our clinical trials, and in the future, our ability to produce and distribute our products in the volume needed to meet commercial demand.
Write-offs related to the impairment of our goodwill, long-lived assets, inventories and other non-cash charges, as well as stock-based compensation expenses may adversely impact or delay our profitability
We may incur significant non-cash charges related to impairment write-downs of our long-lived assets, including goodwill. We are required to perform periodic impairment reviews of our goodwill at least annually. The carrying value of goodwill on our balance sheet was $6.4 million at March 31, 2019. To the extent these reviews conclude that the expected future cash flows generated from our business activities are not sufficient to recover the cost of our long-lived assets, we will be required to measure and record an impairment charge to write-down these assets to their realizable values. We completed our last review during the fourth quarter of 2018 and determined that goodwill was not impaired as of December 31, 2018. However, there can be no assurance that upon completion of subsequent reviews a material impairment charge will not be recorded. If future periodic reviews determine that our assets are impaired and a write-down is required, it will adversely impact or delay our profitability.
Inventories, in part, include certain excipients that are sold to customers and included in product candidates in development. These inventories are capitalized based on management’s judgment of probable sale prior to their expiration date which in turn is primarily based on management’s internal estimates. The valuation of inventory requires us to estimate the value of inventory that may become expired prior to use. We may be required to expense previously capitalized inventory costs upon a change in our
45
judgment, due to, among other potential factors, a denial or delay of approval of a product by the necessary regulatory bodies, changes in product development timelines, or other information that suggests that the inventory will not be saleable. In addition, these circumstances may cause us to record a liability related to minimum purchase agreements that we have in place for raw materials. For example, we recorded charges to cost of goods sold of approximately $926,000, of which approximately $426,000 related to the write-down of the cost basis of inventory and approximately $500,000 related to the prepaid inventory for the minimum purchase commitment for an excipient in the year ended December 31, 2016 as a result of a change in the forecasted demand for the excipients after Pain Therapeutics received a Complete Response Letter from the FDA on its resubmission of the NDA for REMOXY ER. In addition, during the year ended December 31, 2017, we recorded charges to cost of goods sold of approximately $2.0 million, of which approximately $503,000 related to the write-down of the cost basis of inventory on hand, $500,000 related to the prepaid inventory for the minimum purchase commitment for the excipient, and $1.0 million related to the recognition of our remaining minimum purchase commitment for the same excipient after we announced that PERSIST, the Phase 3 clinical trial for POSIMIR, did not meet its primary efficacy endpoint.
Global credit and financial market conditions could negatively impact the value of our current portfolio of cash equivalents, short-term investments or long-term investments and our ability to meet our financing objectives
Our cash and cash equivalents are maintained in highly liquid investments with remaining maturities of 90 days or less at the time of purchase. Our short-term investments consist primarily of readily marketable debt securities with original maturities of greater than 90 days from the date of purchase but remaining maturities of less than one year from the balance sheet date. Our long-term investments consist primarily of readily marketable debt securities with maturities in one year or beyond from the balance sheet date. While, as of the date of this filing, we are not aware of any downgrades, material losses, or other significant deterioration in the fair value of our cash equivalents, short-term investments or long-term investments since March 31, 2019, no assurance can be given that deterioration in conditions of the global credit and financial markets would not negatively impact our current portfolio of cash equivalents, short-term investments or long-term investments or our ability to meet our financing objectives.
We depend upon key personnel who may terminate their employment with us at any time, and we may need to hire additional qualified personnel
Our success will depend to a significant degree upon the continued services of key management, technical and scientific personnel. In addition, our success will depend on our ability to attract and retain other highly skilled personnel, particularly as we develop and expand our Epigenetic Regulator Program. Competition for qualified personnel is intense, and the process of hiring and integrating such qualified personnel is often lengthy. We may be unable to recruit such personnel on a timely basis, if at all. Our management and other employees may voluntarily terminate their employment with us at any time. The loss of the services of key personnel, or the inability to attract and retain additional qualified personnel, could result in delays to product development or approval, loss of sales and diversion of management resources as well as difficulties or inability to raise sufficient capital to fund the Company’s operations.
We may not successfully manage our company through varying business cycles
Our success will depend on properly sizing our company through growth and contraction cycles caused in part by changing business conditions, which places a significant strain on our management and on our administrative, operational and financial resources. To manage through such cycles, we must expand or contract our facilities, our operational, financial and management systems and our personnel. If we were unable to manage growth and contractions effectively our business would be harmed.
Our business involves environmental risks and risks related to handling regulated substances
In connection with our research and development activities and our manufacture of materials and product candidates, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Our research and development involves the use, generation and disposal of hazardous materials, including but not limited to certain hazardous chemicals, solvents, agents and biohazardous materials. The extent of our use, generation and disposal of such substances has increased substantially since we started manufacturing and selling biodegradable polymers. Although we believe that our safety procedures for storing, handling and disposing of such materials comply with the standards prescribed by state and federal regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. We currently contract with third parties to dispose of these substances generated by us, and we rely on these third parties to properly dispose of these substances in compliance with applicable laws and regulations. If these third parties do not properly dispose of these substances in compliance with applicable laws and regulations, we may be subject to legal action by governmental agencies or private parties for improper disposal of these substances. The costs of defending such actions and the potential liability resulting from such actions are often very large. In the event we are subject to such legal action or we otherwise fail to comply with applicable laws and regulations governing the use, generation and disposal of hazardous materials and chemicals, we could be held liable for any damages that result, and any such liability could exceed our resources.
46
Cyber-attacks or other failures in telecommunications or information technology systems could result in information theft, data corruption and significant disruption of our business operations
We utilize information technology, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks and the confidentiality, availability and integrity of our data, and may cause a disruption in our operations, harm our reputation and increase our stock trading risk. There can be no assurance that we will be successful in preventing cyber-attacks or successfully mitigating their effects. Similarly, there can be no assurance that our third-party collaborators, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems. Any cyber-attack or destruction or loss of data could have a material adverse effect on our business and prospects. In addition, we may suffer reputational harm or face litigation or adverse regulatory action as a result of cyber-attacks or other data security breaches and may incur significant additional expense to implement further data protection measures.
Our corporate headquarters, certain manufacturing facilities and personnel are located in a geographical area that is seismically active and near wildfire zones.
Our corporate headquarters, certain manufacturing facilities and personnel are located in a geographical area that is known to be seismically active and prone to earthquakes, as well as wildfires. Should such a natural disaster occur, our ability to conduct our business could be severely restricted, and our business and assets, including the results of our research, development and manufacturing efforts, could be destroyed.
Risks Related to Our Intellectual Property
If we are unable to adequately protect, maintain or enforce our intellectual property rights or secure rights to third-party patents, we may lose valuable assets, experience reduced market share or incur costly litigation to protect our rights or our third-party collaborators may choose to terminate their agreements with us
Our ability to commercially exploit our products will depend significantly on our ability to obtain and maintain patents, maintain trade secret protection and operate without infringing the proprietary rights of others.
As of May 3, 2019, we owned or exclusively in-licensed over 55 unexpired issued U.S. patents and over 270 unexpired issued foreign patents (which include granted European patent rights that have been validated in various EU member states). In addition, we have over 30 pending U.S. patent applications and over 145 foreign applications pending in Europe, Australia, Japan, Canada and other countries.
The patent status of our most advanced drug candidates is as follows:
Our Epigenetic Regulator Program includes seven in-licensed patent families and one patent family solely owned by us. Three patent families each include granted patents providing protection until at least 2026, 2032, and 2034, respectively. The other patent families include pending patent applications, which if granted, could result in patents expiring in 2033, 2035, 2037, 2037 and 2037, respectively, plus any eligible patent term adjustments and extensions. Of the eight patent families covering DUR-928 and/or other molecules in the Epigenetic Regulator Program, two were only filed in the United States, and the other six have been filed or likely will be filed both in the U.S. and internationally. Since DUR-928 is an endogenous small molecule, patent claims directed to DUR-928 composition of matter may be more difficult to maintain or enforce in the United States under Myriad Genetics and other recent court decisions. One of the U.S. patents issued before Myriad Genetics, and four of the DUR-928 U.S. patents issued after Myriad Genetics. The granted claims in the U.S. include both composition of matter and method of treatment claims. There can be no assurance that the pending patent applications will be granted. Further, there can be no assurance that VCU will not attempt to terminate their license to us, which termination could result in the loss of our rights to these patent families.
In the United States, POSIMIR is covered by two patent families. One patent family includes granted patents expiring in at least 2025. Another patent family includes a pending patent application, which if granted, could result in a patent expiring in 2026, plus any eligible patent term adjustments and extensions. In Europe, POSIMIR is covered by five granted patents with three expiring in 2025 and two expiring in 2026, plus any eligible patent term extensions.
In the United States, our ORADUR methylphenidate patent portfolio includes four patent families. One patent family includes granted patents expiring in at least 2023. The other patent families include pending patent applications, which if granted, could result in patents expiring in 2026, 2028, and 2037, respectively, plus any eligible patent term adjustments and extensions. There can be no assurance that the pending patent applications will be granted.
The patent positions of pharmaceutical companies, including ours, are uncertain and involve complex legal and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, our patent applications or those that are licensed to us may not issue into patents, and any issued patents may not provide protection against competitive technologies or may be held invalid if challenged. Our competitors may also independently
47
develop products similar to ours or design around or otherwise circumvent patents issued to us or licensed by us. In addition, the laws of some foreign countries may not protect our proprietary rights to the same extent as U.S. law.
The patent laws of the United States have recently undergone changes through court decisions which may have significant impact on us and our industry. Decisions of the U.S. Supreme Court and other courts with respect to the standards of patentability, constitutionality of inter partes reviews, enforceability, availability of injunctive relief and damages may make it more difficult for us to procure, maintain, defend and enforce patents. In addition, the America Invents Act was signed into law in September 2011, which among other changes to the U.S. patent laws, changed patent priority from “first to invent” to “first to file,” implemented a post-grant opposition system for patents and provided a prior user defense to infringement. These judicial and legislative changes have introduced significant uncertainty in the patent law landscape and may potentially negatively impact our ability to procure, maintain and enforce patents to provide exclusivity for our products.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We require our employees, consultants, advisors and collaborators to execute appropriate confidentiality and assignment-of-inventions agreements with us. These agreements typically provide that all materials and confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances, and that all inventions arising out of the individual’s relationship with us will be our exclusive property. These agreements may be breached, and in some instances, we may not have an appropriate remedy available for breach of the agreements. Furthermore, our competitors may independently develop substantially equivalent proprietary information and techniques, reverse engineer our information and techniques, or otherwise gain access to our proprietary technology.
We may be unable to meaningfully protect our rights in trade secrets, technical know-how and other non-patented technology. We may have to resort to litigation or arbitration to protect our intellectual property rights, or to determine their scope, validity or enforceability. In addition, interference, derivation, post-grant oppositions, and similar proceedings may be necessary to determine rights to inventions in our patents and patent applications. Enforcing or defending our proprietary rights is expensive, could cause diversion of our resources and may be unsuccessful. Any failure to enforce or protect our rights could cause us to lose the ability to exclude others from using our technology to develop or sell competing products.
Our collaboration agreements may depend on our intellectual property
We are party to collaborative agreements with Orient Pharma and Santen among others. Our third-party collaborators have entered into these agreements based on the exclusivity that our intellectual property rights confer on the products being developed. The loss or diminution of our intellectual property rights could result in a decision by our third-party collaborators to terminate their agreements with us. In addition, these agreements are generally complex and contain provisions that could give rise to legal disputes, including potential disputes concerning ownership of intellectual property and data under collaborations. Such disputes can lead to lengthy, expensive litigation or arbitration requiring us to devote management time and resources to such dispute which we would otherwise spend on our business. To the extent that our agreements call for future royalties to be paid conditional on our having patents covering the royalty-bearing subject matter, the decision by the Supreme Court in the case of MedImmune v. Genentech and other case law could encourage our licensees to challenge the validity of our patents and thereby seek to avoid future royalty obligations without losing the benefit of their license. Should they be successful in such a challenge, our ability to collect future royalties could be substantially diminished.
We may be sued by third parties claiming that our product candidates infringe on their intellectual property rights, particularly because there is substantial uncertainty about the validity and breadth of medical patents
We or our collaborators may be exposed to future litigation by third parties based on claims that our product candidates or activities infringe the intellectual property rights of others or that we or our collaborators have misappropriated the trade secrets of others. This risk is exacerbated by the fact that the validity and breadth of claims covered in medical technology patents and the breadth and scope of trade secret protection involve complex legal and factual questions for which important legal principles are unresolved. Any litigation or claims against us or our collaborators, whether or not valid, could result in substantial costs, could place a significant strain on our financial resources and could harm our reputation. We also may not have sufficient funds to litigate against parties with substantially greater resources. In addition, pursuant to our collaborative agreements, we have provided our collaborators with the right, under specified circumstances, to defend against any claims of infringement of the third party intellectual property rights, and such collaborators may not defend against such claims adequately or in the manner that we would do ourselves. Intellectual property litigation or claims could force us or our collaborators to do one or more of the following, any of which could harm our business or financial results:
|
|
• |
cease selling, incorporating or using any of our product candidates that incorporate the challenged intellectual property, which would adversely affect our revenue; |
|
|
• |
obtain a license from the holder of the infringed intellectual property right, which license may be costly or may not be available on reasonable terms, if at all; or |
|
|
• |
redesign our product candidates, which would be costly and time-consuming. |
48
The markets for our pharmaceutical product candidates and for our ALZET and LACTEL product lines are rapidly changing and competitive, and new products or technologies developed by others could impair our ability to maintain or grow our business and remain competitive
The pharmaceutical industry is subject to rapid and substantial technological change. Developments by others may render our product candidates under development or technologies noncompetitive or obsolete, or we may be unable to keep pace with technological developments or other market factors. Technological competition in the industry from pharmaceutical and biotechnology companies, universities, governmental entities and others diversifying into the field is intense and is expected to increase.
We may face competition from other companies in numerous industries including pharmaceuticals, biotechnology, medical devices and drug delivery. Competition for DUR-928, if approved, will depend on the specific indications for which DUR-928 is approved. Intercept, Gilead, Shire, Conatus Pharmaceuticals, Galectin Therapeutics, Genfit, Pfizer, Roche, Bristol Myers Squibb, Novartis, Terns Pharmaceuticals, Galmed Pharmaceuticals, Enanta Pharmaceuticals, Novo Nordisk, Takeda, Vital Therapies, Allergan, Akarna Therapeutics, Inventiva Pharma, Genkyotex, VBL Therapeutics, NGM Biopharmaceuticals, Gemphire Therapeutics, Albireo Pharma, CymaBay Therapeutics, Madrigal Pharmaceuticals, Viking Therapeutics, CohBar, FALK Pharma, Acorda, and others have development plans for products to treat NAFLD/NASH, PSC or other liver diseases. AbbVie, Ischemix, Thrasos Therapeutics, AM-Pharma, Complexa, Quark Pharmaceuticals and others have development plans for products to treat acute kidney injury. Bristol Myers Squibb, Novartis, Eli Lilly, Almirall, LEO Pharma, Pfizer, Janssen, AbbVie, Boerhinger-Ingelheim, Amgen, Sandoz, Astra-Zeneca, Valeant, Takeda, Merck, Idera Pharmaceuticals and others have development plans for products to treat psoriasis.
POSIMIR, if approved, will compete with currently marketed oral opioids, transdermal opioids, local anesthetic patches, implantable and external infusion pumps which can be used for infusion of opioids and local anesthetics. Products of these types are marketed by Pacira, Purdue Pharma, AbbVie, Janssen, Actavis, Medtronic, Endo, AstraZeneca, Pernix Therapeutics, Tricumed, Halyard Health, Cumberland Pharmaceuticals, Acorda Therapeutics, Mallinckrodt, Inspirion Delivery Technologies, Mylan, Shire, Johnson & Johnson, Eli Lilly, Pfizer, Novartis, Egalet, Teva Pharmaceuticals, Collegium Pharmaceutical and others. PERSERIS competes with currently marketed or approved products by Johnson & Johnson, Eli Lilly, Otsuka, Alkermes, Merck, Allergan, Novartis, and others. Our ORADUR-ADHD product candidates, if approved, will compete with currently marketed or approved products by Shire, Johnson & Johnson, UCB, Novartis, Noven, Eli Lilly, Pfizer and others.
Numerous companies are applying significant resources and expertise to the problems of drug delivery and several of these are focusing or may focus on delivery of drugs to the intended site of action, including Pacira, Heron Therapeutics, Alkermes, Immune Pharmaceuticals, Innocoll, Nektar, Kimberly-Clark, Acorda Therapeutics, Flamel, Alexza, Mallinckrodt, Hospira, Pfizer, Cumberland Pharmaceuticals, Egalet, Acura, Elite Pharmaceuticals, Phosphagenics, Intellipharmaceutics, Collegium Pharmaceutical, Charleston Laboratories, Daiichi Sankyo and others. Some of these competitors may be addressing the same therapeutic areas or indications as we are. Our current and potential competitors may succeed in obtaining patent protection or commercializing products before us. Many of these entities have significantly greater research and development capabilities than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us. Acquisitions of, or investments in, competing pharmaceutical or biotechnology companies by large corporations could increase such competitors’ financial, marketing, manufacturing and other resources.
Competition for our ALZET product line primarily consists of customers choosing to utilize delivery methods for their research projects other than an osmotic pump. Competition for our LACTEL product line comes from companies including Evonik, Corbion, FUJIFILM Wako Pure Chemical Corporation, PCAS and others. Many of these entities have significantly greater research and development capabilities than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us. We may in the future face competition for our ALZET and LACTEL product lines from other companies including low cost foreign competitors.
We are engaged in the development of novel therapeutic technologies. Our resources are limited and we may experience technical challenges inherent in such novel technologies. Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competitive products. Some of these products may have an entirely different approach or means of accomplishing similar therapeutic effects than our product candidates. Our competitors may develop products that are safer, more effective or less costly than our product candidates and, therefore, present a serious competitive threat to our product offerings.
The widespread acceptance of therapies that are alternatives to ours may limit market acceptance of our product candidates even if commercialized. Chronic and post-operative pain are currently being treated by oral medication, transdermal drug delivery systems, such as drug patches, injectable products and implantable drug delivery devices which will be competitive with our product candidates. These treatments are widely accepted in the medical community and have a long history of use. The established use of these competitive products may limit the potential for our product candidates to receive widespread acceptance if commercialized.
49
Our relationships with customers and third-party payers will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings
Healthcare providers, physicians and third-party payers will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payers and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we would market, sell and distribute our products. As a pharmaceutical company, even though we do not and may not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payers, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. These regulations include:
|
|
• |
the Federal Healthcare Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid, and which will constrain our marketing practices and the marketing practices of our licensees, educational programs, pricing policies, and relationships with healthcare providers or other entities; |
|
|
• |
the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of “designated health services” with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies; |
|
|
• |
federal false claims laws that prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other government reimbursement programs that are false or fraudulent, and which may expose entities that provide coding and billing advice to customers to potential criminal and civil penalties, including through civil whistleblower or qui tam actions, and including as a result of claims presented in violation of the Federal Healthcare Anti-Kickback Statute, the Stark Law or other healthcare-related laws, including laws enforced by the FDA; |
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services, and which as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
|
• |
federal physician sunshine requirements under the Affordable Care Act, which requires manufacturers of drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; |
|
|
• |
the Federal Food, Drug, and Cosmetic Act, which, among other things, strictly regulates drug product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; and |
|
|
• |
state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non- governmental third-party payers, including private insurers, state laws requiring pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and which may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state and foreign laws governing the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws such as HIPAA, thus complicating compliance efforts. |
50
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success.
In the United States and some non-U.S. jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post‑approval activities, affect our ability to profitably sell any product candidates for which we obtain marketing and otherwise affect our future revenue and profitability and the future revenue and profitability of our collaborators or potential collaborators.
For example, in March 2010, the Affordable Care Act was enacted in the United States to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The law has continued the downward pressure on the pricing of medical items and services, especially under the Medicare program, and increased the industry’s regulatory burdens and operating costs. Among the provisions of the Affordable Care Act of importance to us are the following:
|
|
• |
imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” and biologic agents; |
|
|
• |
imposes an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States; |
|
|
• |
increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%; |
|
|
• |
requires collection of rebates for drugs paid by Medicaid managed care organizations; |
|
|
• |
addresses new methodologies by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and for drugs that are line extension products; |
|
|
• |
requires manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
|
|
• |
extends manufacturers’ Medicaid rebate liability to individuals enrolled in Medicaid managed care organizations; |
|
|
• |
mandates a further shift in the burden of Medicaid payments to the states; |
|
|
• |
expands the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
|
|
• |
establishes a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; |
|
|
• |
establishes a new Patient Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research; and |
|
|
• |
establishes an independent payment advisory board that will submit recommendations to Congress to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. The new Presidential Administration and U.S. Congress will likely continue to seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the Affordable Care Act. It is uncertain the extent to which any such changes may impact our business or financial condition. Since January 2017, President Trump has signed two Executive Orders designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the Affordable Care Act have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”.
51
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. These changes include the Budget Control Act of 2011, which, among other things, resulted in reductions to Medicare payments to providers of 2% per fiscal year and will remain in effect through 2025; the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years; and the Medicare Access and CHIP Reauthorization Act of 2015, which, among other things, ended the use of the sustainable growth rate formula and provides for a 0.5% update to physician payment rates for each calendar year through 2019, after which there will be a 0% annual update each year through 2025. More recently, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. For example, the current administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. The Department of Health and Human Services (HHS) has already started the process of soliciting feedback on some of these measures and is immediately implementing others under its existing authority. In addition, the marketing, pricing and sale of the Company’s products are subject to regulation, investigations and legal actions including under the Medicaid Drug Rebate Program under the Affordable Care Act, which has increased the statutory minimum rebates a manufacturer must pay under the program as well as a new methodology by which rebates are owed calculated for drugs that are inhaled, infused, instilled, implanted or injected. We are also subject to federal and state false claims acts, as well as federal and state antitrust and consumer protection laws. Increased scrutiny of health care industry business practices in recent years by government agencies and state attorneys general in the U.S., and any resulting investigations and prosecutions, carry risk of significant civil and criminal penalties including, but not limited to, debarment from participation in such government healthcare programs.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product and medical device pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, in October 2017, California passed a new law, to become effective in January 2019, which will require transparency from biopharmaceutical companies regarding price increases for prescription drugs. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and medical devices to purchase and which suppliers will be included in their prescription drug and other healthcare programs.
We expect that the Affordable Care Act, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we receive for any approved or cleared product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to new requirements or policies, or if we are not able to maintain regulatory compliance, our products and product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, or be able to enter attractive collaboration agreements, which would adversely affect our business.
We could be exposed to significant product liability claims which could be time consuming and costly to defend, divert management attention and adversely impact our ability to obtain and maintain insurance coverage
The testing, clinical development, manufacture, marketing and sale of our product candidates involve an inherent risk that product liability claims will be asserted against us. Although we are insured against such risks up to an annual aggregate limit in connection with clinical trials and commercial sales of our product candidates, our present product liability insurance may be inadequate and may not fully cover the costs of any claim or any ultimate damages we might be required to pay. Product liability claims or other claims related to our product candidates, regardless of their outcome, could require us to spend significant time and money in litigation or to pay significant damages. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. In addition, product liability coverage may cease to be available in sufficient amounts or at an acceptable cost. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our product candidates. A product liability claim could also significantly harm our reputation and delay market acceptance of our product candidates.
Acceptance of our pharmaceutical product candidates in the marketplace is uncertain, and failure to achieve market acceptance will delay our ability to generate or grow revenues
Our future financial performance will depend upon the successful introduction and customer acceptance of our product candidates in research and development, including DUR-928, and POSIMIR, if approved, and including Indivior’s PERSERIS. Even
52
if approved for marketing, our product candidates may not achieve market acceptance. The degree of market acceptance will depend upon a number of factors, including:
|
|
• |
the receipt of regulatory clearance of marketing claims for the uses that we are developing; |
|
|
• |
the establishment and demonstration in the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapeutic products; and |
|
|
• |
pricing and reimbursement policies of government and third-party payors such as insurance companies, health maintenance organizations, hospital formularies and other health plan administrators. |
In addition, market adoption of POSIMIR and other products in development may depend on what is included in the approved product label, including which clinical data, warnings and precautions is included, and there can be no assurance as to what the final product labels will contain. Physicians, patients, payers or the medical community in general may be unwilling to accept, utilize or recommend any of our products. If we are unable to obtain regulatory approval, commercialize and market our future products when planned and achieve market acceptance, we will not achieve anticipated revenues.
If users of our products are unable to obtain adequate reimbursement from third-party payers, or if new restrictive legislation is adopted, market acceptance of our products may be limited and we may not achieve anticipated revenues
The continuing efforts of government and insurance companies, health maintenance organizations and other payers of healthcare costs to contain or reduce costs of health care may affect our future revenues and profitability, and the future revenues and profitability of our potential customers, suppliers and third-party collaborators and the availability of capital. For example, in certain foreign markets, pricing or profitability of prescription pharmaceuticals is subject to government control. In the United States, recent federal and state government initiatives have been directed at lowering the total cost of health care, and the U.S. Congress and state legislatures will likely continue to focus on health care reform, the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid systems. While we cannot predict whether any such legislative or regulatory proposals will be adopted, the announcement or adoption of such proposals could materially harm our business, financial condition and results of operations.
The successful commercialization of our product candidates will depend in part on the extent to which appropriate reimbursement levels for the cost of our product candidates and related treatment are obtained by governmental authorities, private health insurers and other organizations, such as HMOs. Third-party payers often limit payments or reimbursement for medical products and services. Also, the trend toward managed health care in the United States and the concurrent growth of organizations such as HMOs, which could control or significantly influence the purchase of health care services and products, as well as legislative proposals to reform health care or reduce government insurance programs, may limit reimbursement or payment for our products. The cost containment measures that health care payers and providers are instituting and the effect of any health care reform could materially harm our ability to operate profitably and access capital.
If we or our third-party collaborators are unable to train physicians to use our pharmaceutical product candidates to treat patients’ diseases or medical conditions, we may incur delays in market acceptance of our products
Broad use of our certain of our product candidates, such as POSIMIR, will require extensive training of numerous physicians on the proper and safe use of our product candidates. The time required to begin and complete training of physicians could delay introduction of our products and adversely affect market acceptance of our products. We or third parties selling our product candidates may be unable to rapidly train physicians in numbers sufficient to generate adequate demand for our product candidates. Any delay in training would materially delay the demand for our product candidates and harm our business and financial results. In addition, we may expend significant funds towards such training before any orders are placed for our products, which would increase our expenses and harm our financial results.
Potential new accounting pronouncements and legislative actions are likely to impact our future financial position or results of operations
Future changes in financial accounting standards may cause adverse, unexpected fluctuations in the timing of the recognition of revenues or expenses and may affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with frequency and may occur in the future and we may make changes in our accounting policies in the future. Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new SEC regulations, PCAOB pronouncements and NASDAQ rules, are creating uncertainty for companies such as ours and insurance, accounting and auditing costs are high as a result of this uncertainty and other factors. Compliance with evolving corporate governance and public disclosure standards may result in increased general and administrative expenses and a diversion of management time and attention from value-creating activities to compliance activities.
Risks related to actions on trade by the U.S. and foreign governments could adversely affect the Company's results of operations and financial condition
The U.S. government has indicated its intent to adopt a new approach to trade policy and in some cases to renegotiate, or potentially terminate, certain existing bilateral or multilateral trade agreements. It has also initiated or is considering the imposition
53
of tariffs on certain foreign products. Changes in U.S. trade policy have resulted in, and could continue to result in, one or more U.S. trading partners adopting responsive trade policy making it more difficult or costly for us to export our products to those countries. These measures could also result in increased costs for goods imported into the United States. This in turn could require us to increase prices to our customers which may reduce demand, or, if we are unable to increase prices, result in lowering our margin on products sold.
There is also a concern that the imposition of additional tariffs by the United States could result in the adoption of tariffs by other countries. A potential resulting trade war could have a significant adverse effect on world trade and the world economy. We cannot predict future trade policy or the terms of any renegotiated trade agreements and their impact on our business. The adoption and expansion of trade restrictions, the occurrence of a trade war, or other governmental action related to tariffs or trade agreements or policies has the potential to adversely impact demand for our products, our costs, our customers, our suppliers, and the U.S. economy, which in turn could adversely impact our business, financial condition and results of operations.
Risks Related To Our Common Stock
Our stock price does not currently meet the minimum bid price for continued listing on Nasdaq. Our ability to continue operations or to publicly or privately sell equity securities and the liquidity of our common stock could be adversely affected if we are delisted from Nasdaq
On December 24, 2018, we received written notification from Nasdaq informing us that because the closing bid price of our common stock was below $1.00 for 30 consecutive trading days, our shares no longer complied with the minimum closing bid price requirement for continued listing on the Nasdaq Global Market under Nasdaq Marketplace Rule 5450(a)(1).
The Company has until June 24, 2019 to regain compliance with Nasdaq’s listing requirements by having the closing bid price of our common stock listed on Nasdaq be at least $1.00 for at least 10 consecutive trading days. If the Company does not regain compliance within this time period, we may transfer our common stock listing to The Nasdaq Capital Market, provided that the Company (i) meets the applicable market value of publicly held shares requirement for continued listing and all other applicable requirements for initial listing on The Nasdaq Capital Market (except for the closing bid price requirement) and (ii) notifies Nasdaq of its intent to cure this deficiency. Following a transfer to The Nasdaq Capital Market, the Company would be afforded the remainder of an additional 180 calendar day grace period in order to regain compliance with the minimum closing bid price requirement of $1.00 per share under The Nasdaq Capital Market, unless it does not appear to NASDAQ that it would be possible for the Company to cure the deficiency.
If compliance is not demonstrated within the applicable compliance period, Nasdaq will notify the Company that its securities will be subject to delisting. The Company may appeal Nasdaq’s determination to delist its securities to a Hearings Panel. During any appeal process, shares of the Company’s common stock would continue to trade on the Nasdaq Global Market or Nasdaq Capital Market, as applicable.
There can be no assurance that we will maintain or regain compliance with the requirements for listing our common stock on the Nasdaq Global Market or if we were not in compliance, that our common stock would be eligible for transfer to the Nasdaq Capital Market and remain in compliance with the requirements for listing on that market. Delisting from Nasdaq would constitute an event of default under our loan facility with Oxford, entitling Oxford to accelerate our obligations under such facility, among other actions. Under such circumstances, we could be required to renegotiate the repayment terms of our loan facility, on terms which would not be favorable to the Company as our current terms, or we could be required to take other actions, such as discontinuing some or all of our operations, selling assets, or other action. Delisting could also adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly affect the ability of investors to trade our securities and would negatively affect the value and liquidity of our common stock. Delisting could also have other negative results, including the potential loss of confidence by employees, the loss of institutional investor interest and fewer business development opportunities.
Our operating history makes evaluating our stock difficult
Our quarterly and annual results of operations have historically fluctuated and we expect will continue to fluctuate for the foreseeable future. We believe that period-to-period comparisons of our operating results should not be relied upon as predictive of future performance. Our prospects must be considered in light of the risks, expenses and difficulties encountered by companies with no approved pharmaceutical products, particularly companies in new and rapidly evolving markets such as pharmaceuticals, drug delivery and biotechnology. To address these risks, we must, among other things, obtain regulatory approval for and commercialize our product candidates, which may not occur. We may not be successful in addressing these risks and difficulties. We may require additional funds to complete the development of our product candidates and to fund operating losses to be incurred in the next several years.
Investors may experience substantial dilution of their investment
In order to raise capital and for other purposes, we may in the future offer and issue additional shares of our common stock or other securities convertible into or exchangeable for our common stock, and the price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or
54
lower than the price per share at which investors in our common stock bought their shares. In August 2018, we filed a shelf registration statement on Form S-3 with the SEC that allows us to offer up to $175 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of additional shares of common stock which the Company may sell, subject to certain limitations, under the 2015 Sales Agreement through Cantor Fitzgerald, acting as agent. As of May 3, 2019, we raised net proceeds (net of commissions) of approximately $184,000 from the sale of 242,750 shares of the Company’s common stock in the open market at a weighted average price of $0.78 per share pursuant to the October 2018 registration statement. Any additional sales in the public market of our common stock, under our Controlled Equity Offering program with Cantor Fitzgerald, in other offerings under the shelf registration statement or otherwise, could adversely affect prevailing market prices for our common stock. In addition, as of March 31, 2019, 32,411,700 shares of our common stock were issuable upon exercise of stock options outstanding under our stock option plans at a weighted average exercise price of $1.43 per share and 7,163,848 additional shares of common stock reserved for potential future issuance under our stock option plan, and an aggregate of 361,185 shares of common stock reserved for potential future issuance under our 2000 Employee Stock Purchase Plan. Investors will incur dilution from the sale of any additional shares or upon the issuance of any shares pursuant to such plans, or upon exercise of any outstanding options.
Our ability to use net operating losses and certain other tax attributes is uncertain and may be limited
Our ability to use our federal and state net operating losses to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income before the expiration dates of the net operating losses, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our net operating losses. In addition, utilization of net operating losses to offset potential future taxable income and related income taxes that would otherwise be due is subject to annual limitations under the “ownership change” provisions of Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (Internal Revenue Code) and similar state provisions, which may result in the expiration of net operating losses before future utilization. In general, under the Code, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating losses and other pre-change tax attributes (such as research and development credit carryforwards) to offset its post-change taxable income or taxes may be limited. Our equity offerings and other changes in our stock ownership, some of which are outside of our control, may have resulted or could in the future result in an ownership change. If an ownership change limitation were to apply, utilization of our domestic net operating losses and tax credit carryforwards could be limited in future periods and a portion of the carryforwards could expire before being available to reduce future income tax liabilities.
The price of our common stock may be volatile
The stock markets in general, and the markets for pharmaceutical stocks in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock.
Price declines in our common stock could result from general market and economic conditions and a variety of other factors, including:
|
|
• |
adverse results (including adverse events or failure to demonstrate safety or efficacy) or delays in our clinical and non-clinical trials of DUR-928 or other product candidates; |
|
|
• |
announcements of FDA non-approval of our product candidates, or delays in the FDA or other foreign regulatory agency review process; |
|
|
• |
adverse actions taken by regulatory agencies or law enforcement agencies with respect to our product candidates, clinical trials, manufacturing processes or sales and marketing activities, or those of our third-party collaborators; |
|
|
• |
announcements of technological innovations, patents, product approvals or new products by our competitors; |
|
|
• |
failure of third-party collaborators to continue development of the respective product candidates they are developing; |
|
|
• |
regulatory, judicial and patent developments in the United States and foreign countries; |
|
|
• |
any lawsuit involving us or our product candidates including intellectual property infringement or product liability suits; |
|
|
• |
announcements concerning our competitors, or the biotechnology or pharmaceutical industries in general; |
|
|
• |
developments concerning our strategic alliances or acquisitions; |
|
|
• |
actual or anticipated variations in our operating results; |
|
|
• |
changes in recommendations by securities analysts or lack of analyst coverage; |
|
|
• |
negative press coverage or online misinformation about the Company or its partners or their respective products or personnel; |
55
|
|
• |
sales of our common stock by our executive officers or directors or sales of substantial amounts of common stock by us or others; |
|
|
• |
potential failure to meet continuing listing standards from The Nasdaq Global Market; |
|
|
• |
loss or disruption of facilities due to natural disasters; |
|
|
• |
acceleration of our debt obligations due to a determination by our lender that a material adverse change has occurred; |
|
|
• |
an unfavorable vote in an FDA advisory committee meeting; |
|
|
• |
changes in accounting principles; or |
|
|
• |
loss of any of our key scientific or management personnel. |
The market price of our common stock may fluctuate significantly in response to factors which are beyond our control. The stock market in general has recently experienced extreme price and volume fluctuations. In addition, the market prices of securities of technology and pharmaceutical companies have also been extremely volatile, and have experienced fluctuations that often have been unrelated or disproportionate to the operating performance of these companies. These broad market fluctuations could result in extreme fluctuations in the price of our common stock, which could cause a decline in the value of our common stock.
In the past, following periods of volatility in the market price of a particular company’s securities, litigation has often been brought against that company. If litigation of this type is brought against us, it could be extremely expensive and divert management’s attention and our company’s resources.
We have broad discretion over the use of our cash and investments, and their investment may not always yield a favorable return
Our management has broad discretion over how our cash and investments are used and may from time to time invest in ways with which our stockholders may not agree and that do not yield favorable returns.
Executive officers, directors and principal stockholders have substantial control over us, which could delay or prevent a change in our corporate control favored by our other stockholders
Our directors, executive officers and principal stockholders, together with their affiliates, have substantial control over us. The interests of these stockholders may differ from the interests of other stockholders. As a result, these stockholders, if acting together, could have the ability to exercise control over all corporate actions requiring stockholder approval irrespective of how our other stockholders may vote, including:
|
|
• |
the election of directors; |
|
|
• |
the amendment of charter documents; |
|
|
• |
the approval of certain mergers and other significant corporate transactions, including a sale of substantially all of our assets; or |
|
|
• |
the defeat of any non-negotiated takeover attempt that might otherwise benefit the public stockholders. |
Our certificate of incorporation, our bylaws and Delaware law contain provisions that could discourage another company from acquiring us
Provisions of Delaware law, our certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions include:
|
|
• |
authorizing the issuance of “blank check” preferred stock without any need for action by stockholders; |
|
|
• |
providing for a classified board of directors with staggered terms; |
|
|
• |
requiring supermajority stockholder voting to effect certain amendments to our certificate of incorporation and bylaws; |
|
|
• |
eliminating the ability of stockholders to call special meetings of stockholders; |
|
|
• |
prohibiting stockholder action by written consent; and |
|
|
• |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings. |
56
Our bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees
Our bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on behalf of the Company, any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company, any action asserting a claim arising pursuant to any provision of the General Corporation Law of Delaware or our Certificate of Incorporation or bylaws or any action asserting a claim governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees.
Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
57
None
None
Not applicable
None
|
Exhibit Number |
|
Exhibit Name |
|
|
|
|
|
|
|
31.1* |
|
||
|
|
|
|
|
|
31.2* |
|
Rule 13a-14(a) Section 302 Certification of Michael H. Arenberg. |
|
|
|
|
|
|
|
32.1** |
|
||
|
|
|
|
|
|
32.2** |
|
||
|
|
|
|
|
|
101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Labels Linkbase Document |
|
|
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
* Filed herewith.
58
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
DURECT CORPORATION |
||
|
|
|
|
|
|
|
By: |
|
/S/ JAMES E. BROWN |
|
|
|
|
James E. Brown Chief Executive Officer |
|
|
|
|
|
|
Date: May 7, 2019 |
|
|
|
|
|
|
|
|
|
|
By: |
|
/S/ MICHAEL H. ARENBERG |
|
|
|
|
Michael H. Arenberg Chief Financial Officer and Principal Accounting Officer |
|
|
|
|
|
|
Date: May 7, 2019 |
|
|
|
59
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-29)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
