DURECT CORP - Quarter Report: 2021 March (Form 10-Q)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2021
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-31615
DURECT CORPORATION
(Exact name of registrant as specified in its charter)
|
Delaware |
94-3297098 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
10260 Bubb Road
Cupertino, California 95014
(Address of principal executive offices, including zip code)
(408) 777-1417
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Trading Symbol |
|
Name of Each Exchange on Which Registered |
|
Common Stock $0.0001 par value per share
|
|
DRRX |
|
The NASDAQ Stock Market LLC (The Nasdaq Capital Market) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by a check mark whether the registrant a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 3, 2021, there were 227,406,182 shares of the registrant’s Common Stock outstanding.
INDEX
|
|
|
Page |
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. |
3 |
|
|
|
|
|
|
|
Condensed Balance Sheets as of March 31, 2021 and December 31, 2020 |
3 |
|
|
|
|
|
|
Condensed Statements of Comprehensive Loss for the three months ended March 31, 2021 and 2020 |
4 |
|
|
|
|
|
|
Condensed Statements of Stockholders’ Equity for the three months ended March 31, 2021 and 2020 |
5 |
|
|
|
|
|
|
Condensed Statements of Cash Flows for the three months ended March 31, 2021 and 2020 |
6 |
|
|
|
|
|
|
7 |
|
|
|
|
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
17 |
|
|
|
|
|
Item 3. |
35 |
|
|
|
|
|
|
Item 4. |
35 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. |
36 |
|
|
|
|
|
|
Item 1A. |
36 |
|
|
|
|
|
|
Item 2. |
60 |
|
|
|
|
|
|
Item 3. |
60 |
|
|
|
|
|
|
Item 4. |
60 |
|
|
|
|
|
|
Item 5. |
60 |
|
|
|
|
|
|
Item 6. |
60 |
|
|
|
|
|
|
61 |
||
2
PART I. FINANCIAL INFORMATION
|
Item 1. |
Financial Statements |
DURECT CORPORATION
CONDENSED BALANCE SHEETS
(in thousands)
|
|
|
March 31, 2021 |
|
|
December 31, 2020 |
|
||
|
|
|
(unaudited) |
|
|
(Note 1) |
|
||
|
A S S E T S |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
57,545 |
|
|
$ |
21,312 |
|
|
Cash held in escrow |
|
|
— |
|
|
|
14,979 |
|
|
Short-term investments |
|
|
39,538 |
|
|
|
19,421 |
|
|
Accounts receivable (net of allowances of $30 at March 31, 2021 and $72 at December 31, 2020) |
|
|
993 |
|
|
|
940 |
|
|
Inventories, net |
|
|
1,955 |
|
|
|
1,864 |
|
|
Prepaid expenses and other current assets |
|
|
4,780 |
|
|
|
4,545 |
|
|
Total current assets |
|
|
104,811 |
|
|
|
63,061 |
|
|
Property and equipment, net |
|
|
224 |
|
|
|
251 |
|
|
Operating lease right-of-use assets |
|
|
4,440 |
|
|
|
4,749 |
|
|
Goodwill |
|
|
6,169 |
|
|
|
6,169 |
|
|
Long-term investments |
|
|
— |
|
|
|
1,000 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
150 |
|
|
Other long-term assets |
|
|
261 |
|
|
|
261 |
|
|
Total assets |
|
$ |
116,055 |
|
|
$ |
75,641 |
|
|
L I A B I L I T I E S A N D S T O C K H O L D E R S’ E Q U I T Y |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
2,150 |
|
|
$ |
1,678 |
|
|
Accrued liabilities |
|
|
4,094 |
|
|
|
5,801 |
|
|
Contract research liabilities |
|
|
384 |
|
|
|
545 |
|
|
Deferred revenue, current portion |
|
|
373 |
|
|
|
— |
|
|
Term loan, current portion, net |
|
|
2,895 |
|
|
|
884 |
|
|
Operating lease liabilities, current portion |
|
|
1,808 |
|
|
|
1,795 |
|
|
Total current liabilities |
|
|
11,704 |
|
|
|
10,703 |
|
|
Deferred revenue, non-current portion |
|
|
812 |
|
|
|
812 |
|
|
Operating lease liabilities, non-current portion |
|
|
2,881 |
|
|
|
3,202 |
|
|
Term loan, non-current portion, net |
|
|
18,062 |
|
|
|
19,936 |
|
|
Other long-term liabilities |
|
|
873 |
|
|
|
873 |
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Common stock |
|
|
23 |
|
|
|
20 |
|
|
Additional paid-in capital |
|
|
581,632 |
|
|
|
529,884 |
|
|
Accumulated other comprehensive loss |
|
|
(14 |
) |
|
|
(5 |
) |
|
Accumulated deficit |
|
|
(499,918 |
) |
|
|
(489,784 |
) |
|
Stockholders’ equity |
|
|
81,723 |
|
|
|
40,115 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
116,055 |
|
|
$ |
75,641 |
|
The accompanying notes are an integral part of these condensed financial statements.
3
DURECT CORPORATION
CONDENSED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands, except per share amounts)
(unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Collaborative research and development and other revenue |
|
$ |
574 |
|
|
$ |
(30 |
) |
|
Product revenue, net |
|
|
1,638 |
|
|
|
1,625 |
|
|
Total revenues |
|
|
2,212 |
|
|
|
1,595 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Cost of product revenues |
|
|
352 |
|
|
|
396 |
|
|
Research and development |
|
|
7,975 |
|
|
|
7,587 |
|
|
Selling, general and administrative |
|
|
3,531 |
|
|
|
3,431 |
|
|
Total operating expenses |
|
|
11,858 |
|
|
|
11,414 |
|
|
Loss from operations |
|
|
(9,646 |
) |
|
|
(9,819 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest and other income |
|
|
37 |
|
|
|
258 |
|
|
Interest expense |
|
|
(525 |
) |
|
|
(592 |
) |
|
Net other expense |
|
|
(488 |
) |
|
|
(334 |
) |
|
Loss from continuing operations |
|
$ |
(10,134 |
) |
|
$ |
(10,153 |
) |
|
Income from discontinued operations |
|
|
— |
|
|
|
205 |
|
|
Net loss |
|
|
(10,134 |
) |
|
|
(9,948 |
) |
|
Net change in unrealized gain on available-for-sale securities, net of reclassification adjustments and taxes |
|
|
(9 |
) |
|
|
(15 |
) |
|
Total comprehensive loss |
|
$ |
(10,143 |
) |
|
$ |
(9,963 |
) |
|
Net Income (loss) per share |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
|
|
|
|
|
|
|
Loss from continuing operations |
|
$ |
(0.05 |
) |
|
$ |
(0.05 |
) |
|
Income from discontinued operations |
|
$ |
— |
|
|
$ |
0.00 |
|
|
Net loss per common share, basic and diluted |
|
$ |
(0.05 |
) |
|
$ |
(0.05 |
) |
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares used in computing net income (loss) per share, basic and diluted |
|
|
217,537 |
|
|
|
195,745 |
|
The accompanying notes are an integral part of these condensed financial statements.
4
DURECT CORPORATION
CONDENSED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except per share amounts)
(unaudited)
|
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated Other Comprehensive |
|
|
Accumulated |
|
|
Total Stockholders’ |
|
|||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
loss |
|
|
Deficit |
|
|
Equity |
|
||||||
|
Balance at December 31, 2020 |
|
|
203,533 |
|
|
$ |
20 |
|
|
$ |
529,884 |
|
|
$ |
(5 |
) |
|
$ |
(489,784 |
) |
|
$ |
40,115 |
|
|
Issuance of common stock upon exercise of stock options |
|
|
2,502 |
|
|
|
1 |
|
|
|
3,262 |
|
|
|
— |
|
|
|
— |
|
|
|
3,263 |
|
|
Issuance of common stock upon equity financings, net of issuance costs of $269 |
|
|
21,315 |
|
|
|
2 |
|
|
|
47,784 |
|
|
|
— |
|
|
|
— |
|
|
|
47,786 |
|
|
Stock-based compensation expense from stock options and ESPP shares |
|
|
— |
|
|
|
— |
|
|
|
702 |
|
|
|
— |
|
|
|
— |
|
|
|
702 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(10,134 |
) |
|
|
(10,134 |
) |
|
Unrealized loss on available-for-sale securities, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(9 |
) |
|
|
— |
|
|
|
(9 |
) |
|
Balance at March 31, 2021 |
|
|
227,350 |
|
|
$ |
23 |
|
|
$ |
581,632 |
|
|
$ |
(14 |
) |
|
$ |
(499,918 |
) |
|
$ |
81,723 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2019 |
|
|
195,257 |
|
|
$ |
19 |
|
|
$ |
512,046 |
|
|
$ |
(3 |
) |
|
$ |
(489,202 |
) |
|
$ |
22,860 |
|
|
Stock-based compensation expense from stock options and ESPP shares |
|
|
577 |
|
|
|
— |
|
|
|
761 |
|
|
|
— |
|
|
|
— |
|
|
|
761 |
|
|
Fully vested options issued to settle accrued liabilities |
|
|
— |
|
|
|
— |
|
|
|
416 |
|
|
|
— |
|
|
|
— |
|
|
|
416 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(9,948 |
) |
|
|
(9,948 |
) |
|
Unrealized loss on available-for-sale securities, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(15 |
) |
|
|
— |
|
|
|
(15 |
) |
|
Balance at March 31, 2020 |
|
|
195,834 |
|
|
$ |
19 |
|
|
$ |
513,223 |
|
|
$ |
(18 |
) |
|
$ |
(499,150 |
) |
|
$ |
14,074 |
|
The accompanying notes are an integral part of these condensed financial statements
5
DURECT CORPORATION
CONDENSED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(10,134 |
) |
|
$ |
(9,948 |
) |
|
Adjustments to reconcile net loss to net cash used in by operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
30 |
|
|
|
62 |
|
|
Stock-based compensation |
|
|
702 |
|
|
|
414 |
|
|
Amortization of debt issuance cost |
|
|
111 |
|
|
|
112 |
|
|
Net amortization on investments |
|
|
(44 |
) |
|
|
42 |
|
|
Changes in operating lease liabilities |
|
|
1 |
|
|
|
21 |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(53 |
) |
|
|
606 |
|
|
Inventories |
|
|
(91 |
) |
|
|
75 |
|
|
Prepaid expenses and other assets |
|
|
(235 |
) |
|
|
(1,343 |
) |
|
Accounts payable |
|
|
472 |
|
|
|
(29 |
) |
|
Accrued and other liabilities |
|
|
(1,680 |
) |
|
|
(2,800 |
) |
|
Contract research liabilities |
|
|
(161 |
) |
|
|
(537 |
) |
|
Deferred revenue |
|
|
373 |
|
|
|
465 |
|
|
Total adjustments |
|
|
(575 |
) |
|
|
(2,912 |
) |
|
Net cash used in by operating activities |
|
|
(10,709 |
) |
|
|
(12,860 |
) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(3 |
) |
|
|
(137 |
) |
|
Purchases of available-for-sale securities |
|
|
(29,698 |
) |
|
|
(4,545 |
) |
|
Proceeds from maturities of available-for-sale securities |
|
|
10,616 |
|
|
|
14,435 |
|
|
Net proceeds from sale of LACTEL product line |
|
|
14,979 |
|
|
|
— |
|
|
Net cash (used in) provided by investing activities |
|
|
(4,106 |
) |
|
|
9,753 |
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Payments on equipment financing obligations |
|
|
(1 |
) |
|
|
(1 |
) |
|
Net proceeds from issuances of common stock |
|
|
51,049 |
|
|
|
761 |
|
|
Net cash provided by financing activities |
|
|
51,048 |
|
|
|
760 |
|
|
Net increase (decrease) in cash, cash equivalents, and restricted cash |
|
|
36,233 |
|
|
|
(2,347 |
) |
|
Cash, cash equivalents, and restricted cash, beginning of the period (1) |
|
|
21,462 |
|
|
|
35,074 |
|
|
Cash, cash equivalents, and restricted cash, end of the period (1) |
|
$ |
57,695 |
|
|
$ |
32,727 |
|
|
|
|
|
|
|
|
|
|
|
|
(1) Includes restricted cash of $150,000 (in long term restricted investments) included in the condensed balance sheets at March 31, 2021 and December 31, 2020. |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed financial statements.
6
DURECT CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1. Summary of Significant Accounting Policies
Nature of Operations
DURECT Corporation (the Company) was incorporated in the state of Delaware on February 6, 1998. The Company is a biopharmaceutical company with research and development programs broadly falling into two categories: (i) new chemical entities derived from our Epigenetics Regulator Program, in which the Company attempts to discover and develop molecules which have not previously been approved and marketed as therapeutics, and (ii) Proprietary Pharmaceutical Programs, in which the Company applies its formulation expertise and technologies largely to active pharmaceutical ingredients whose safety and efficacy have previously been established but which the Company aims to improve in some manner through a new formulation. The Company has several products under development by itself and with third party collaborators. The Company also manufactures and sells osmotic pumps used in laboratory research, and manufactures certain excipients for certain clients for use as raw materials in their products. In addition, the Company conducts research and development of pharmaceutical products in collaboration with third party pharmaceutical and biotechnology companies.
Basis of Presentation
These financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (SEC), and therefore do not include all the information and footnotes necessary for a complete presentation of the Company’s results of operations, financial position and cash flows in conformity with U.S. generally accepted accounting principles (U.S. GAAP). The unaudited financial statements reflect all adjustments (consisting only of normal recurring adjustments) which are, in the opinion of management, necessary for a fair presentation of the financial position at March 31, 2021, the operating results and comprehensive loss, and stockholders’ equity for the three months ended March 31, 2021, and cash flows for the three months ended March 31, 2021 and 2020. The balance sheet as of December 31, 2020 has been derived from audited financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These financial statements and notes should be read in conjunction with the Company’s audited financial statements and notes thereto, included in the Company’s annual report on Form 10-K for the fiscal year ended December 31, 2020 filed with the SEC.
The results of operations for the interim periods presented are not necessarily indicative of results that may be expected for any other interim period or for the full fiscal year.
Discontinued Operations
In December 2020, the Company announced its decision to sell its LACTEL Absorbable Polymer (LACTEL) product line to Evonik, which was completed on December 31, 2020 and therefore the accompanying statement of operations for the three months ended March 31, 2020 has been recast to reflect the revenue and expenses related to the Company’s LACTEL product line as discontinued operations (see Note 8). The Company believes this format provides comparability with its previously filed financial statements.
Risks and Uncertainties
The pandemic caused by an outbreak of a new strain of coronavirus, COVID-19, has resulted, and is likely to continue to result, in significant national and global economic disruption and may adversely affect our business. COVID-19 initially also had a negative impact on orders for our ALZET product line as many ALZET customers reduced their activities during the pandemic. ALZET orders have recovered significantly in 2021, a trend that may or may not continue as the impact of the pandemic has proven to be difficult to predict. For DUR-928, the Company may experience delays in patient enrollment of the Phase 2b clinical trial in patients with alcohol-associated hepatitis (AH) or disruptions in supplies of DUR-928 or other items required for clinical trials. These delays and potential disruptions will increase the overall costs of development of DUR-928. The Company is actively monitoring the impact of COVID-19 and the possible effects on its financial condition, liquidity, operations, clinical trials, suppliers, industry and workforce. However, the full extent, consequences, and duration of the COVID-19 pandemic and the resulting impact on the Company cannot currently be predicted. The Company will continue to evaluate the impact that these events could have on the Company’s operations, financial position, and the results of operations and cash flows.
Liquidity and Need to Raise Additional Capital
As of March 31, 2021, the Company had an accumulated deficit of $499.9 million as well as negative cash flows from operating activities for the three months ended March 31, 2021, and expects to continue to incur negative cash flows from operating activities in the foreseeable future. The Company will continue to require substantial funds to continue research and development, including clinical trials of its product candidates. The Company believes its existing cash, cash equivalents and investments are sufficient to fund its operating cashflow requirements for a period greater than 12 months from the date of issuance of these condensed financial statements. Management’s plans in order to meet its operating cash flow requirements beyond the next 12 months from the date these
7
condensed financial statements are filed, may include seeking additional collaborative agreements for certain of the Company’s programs and achieving revenue from its collaboration and licensing agreements, as well as financing activities such as public offerings and private placements of its common stock, preferred stock offerings, issuances of debt and convertible debt instruments.
There are no assurances that such additional funding will be obtained or that the Company will succeed in its future operations. If the Company cannot successfully raise additional capital when needed and implement its strategic development plan, its liquidity, financial condition and business prospects will be materially and adversely affected.
Inventories
Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company may be required to expense previously capitalized inventory costs upon a change in management’s judgment due to new information that suggests that the inventory will not be saleable. If the Company is able to subsequently sell products made with raw materials that were previously written down, the Company will report an unusually high gross profit as there will be no associated cost of goods for these materials.
The Company’s inventories consist of the following (in thousands):
|
|
|
March 31, 2021 |
|
|
December 31, 2020 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Raw materials |
|
$ |
145 |
|
|
$ |
136 |
|
|
Work in process |
|
|
888 |
|
|
|
796 |
|
|
Finished goods |
|
|
922 |
|
|
|
932 |
|
|
Total inventories |
|
$ |
1,955 |
|
|
$ |
1,864 |
|
Revenue Recognition
The Company enters into license and collaboration agreements under which the Company may receive upfront license fees, research funding and contingent milestone payments and royalties.
Product Revenue, Net
The Company sells osmotic pumps used in laboratory research, and manufactures certain excipients for pharmaceutical clients for use as raw materials in their products.
Revenues from product sales are recognized when the customer obtains control of the Company’s product, which occurs at a point in time, typically upon shipment to the customer. The Company expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that the Company would have recognized is one year or less.
Trade Discounts and Allowances: The Company provides certain customers with discounts that are explicitly stated in the Company’s contracts and are recorded as a reduction of revenue in the period the related product revenue is recognized.
Product Returns: Consistent with industry practice, the Company generally offers customers a limited right of return for products that have been purchased from the Company. The Company estimates the amount of its product sales that may be returned by its customers and records this estimate as a reduction of revenue in the period the related product revenue is recognized. The Company currently estimates product return liabilities using its historical sales information. Consistent with historical experience, the Company continues to expect product returns to be minimal.
Collaborative Research and Development and Other Revenue
The Company enters into license agreements, under which it licenses certain rights to its product candidates to third parties. The terms of these arrangements typically include payment to the Company of one or more of the following: non-refundable, up-front license fees; reimbursement of development costs incurred by the Company under approved work plans; development, regulatory and commercial milestone payments; payments for manufacturing supply services the Company provides through its contract manufacturers; and royalties on net sales of licensed products. Each of these payments results in collaborative research and development revenues, except for revenues from royalties on net sales of licensed products, which are classified as other revenues.
In determining the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. As part of the accounting for these arrangements, the Company must develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the contract. The Company uses key assumptions to determine the standalone selling price, which may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical and regulatory success. The Company expects to recognize revenue for the variable consideration currently being constrained when it is probable that a significant revenue reversal will not occur.
8
Licenses of intellectual property: If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenues from non-refundable, up-front fees allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaborative research and development revenues and net income (loss) in the period of adjustment.
Milestone Payments: At the inception of each arrangement that includes development milestone payments, the Company evaluates whether the milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of the Company or the licensee, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. The transaction price is then allocated to each performance obligation on a relative stand-alone selling price basis, for which the Company recognizes revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of such development milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaborative research and development revenues and net income (loss) in the period of adjustment.
Manufacturing Supply Services: Arrangements that include a promise for future supply of drug product for either clinical development or commercial supply at the customer’s discretion are generally considered as options. The Company assesses if these options provide a material right to the customer and if so, they are accounted for as separate performance obligations. If the Company is entitled to additional payments when the customer exercises these options, any additional payments are recorded in collaborative research and development revenue when the customer obtains control of the goods, which is upon delivery.
Royalties and Earn-outs: For arrangements that include sales-based royalties or earn-outs, including milestone payments based on the level of sales, and the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized material royalty revenue resulting from the Company’s collaborative arrangements or material earn-out revenue from the Company’s patent purchase agreement with Indivior.
The Company receives payments from its customers based on development cost schedules established in each contract. Up-front payments are recorded as deferred revenue upon receipt or when due, and may require deferral of revenue recognition to a future period until the Company performs its obligations under these arrangements. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional. The Company does not assess whether a contract has a significant financing component if the expectation at contract inception is such that the period between payment by the customer and the transfer of the promised goods or services to the customer will be one year or less.
Total revenue by geographic region (based on the location of the customer) for the three months ended March 31, 2021 and 2020 are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
United States |
|
$ |
1,248 |
|
|
$ |
841 |
|
|
Japan |
|
|
480 |
|
|
|
274 |
|
|
Europe |
|
|
301 |
|
|
|
310 |
|
|
Other |
|
|
183 |
|
|
|
170 |
|
|
Total |
|
$ |
2,212 |
|
|
$ |
1,595 |
|
Prepaid and Accrued Contract Research Expenses
The Company incurs significant costs associated with third party consultants and organizations for pre-clinical studies, clinical trials, contract research and manufacturing, validation, testing, regulatory advice and other research and development-related services. The Company is required to estimate periodically the cost of services rendered but unbilled based on management’s estimates of project status. If these good faith estimates are inaccurate, actual expenses incurred could materially differ from these estimates.
Research and development expenses are primarily comprised of salaries and benefits associated with research and development personnel, overhead and facility costs, preclinical and non-clinical development costs, clinical trial and related clinical manufacturing costs, contract services, and other outside costs. Research and development costs are expensed as incurred. Research and development
9
costs paid to third parties under sponsored research agreements are recognized as the related services are performed. In addition, reimbursements of research and development expenses incurred by the Company’s partners are recorded as collaborative research and development revenue.
Comprehensive Loss
Components of other comprehensive loss are comprised entirely of unrealized gains and losses on the Company’s available-for-sale securities for all periods presented. Total comprehensive loss has been disclosed in the Company’s Statements of Comprehensive Loss.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding. Diluted net loss per share is computed using the weighted-average number of common shares outstanding and common stock equivalents (i.e., options to purchase common stock) outstanding during the period, if dilutive, using the treasury stock method for options.
The numerators and denominators in the calculation of basic and diluted net loss per share were as follows (in thousands except per share amounts):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Numerators: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(10,134 |
) |
|
$ |
(9,948 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted average shares used to compute basic net loss per share |
|
|
217,537 |
|
|
|
195,745 |
|
|
Dilutive common shares from stock options and ESPP |
|
|
— |
|
|
|
— |
|
|
Weighted average shares used to compute diluted net loss per share |
|
|
217,537 |
|
|
|
195,745 |
|
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.05 |
) |
|
$ |
(0.05 |
) |
|
Diluted |
|
$ |
(0.05 |
) |
|
$ |
(0.05 |
) |
Options to purchase approximately 4.8 million and 7.3 million shares of common stock were excluded from the denominator in the calculation of diluted net loss per share for the three months ended March 31, 2021 and March 31, 2020, respectively, as the effect would be anti-dilutive.
Recent Accounting Pronouncements
In June 2016, the FASB issued Accounting Standards Update No. 2016-13 (ASU 2016-13) “Financial Instruments - Credit Losses: Measurement of Credit Losses on Financial Instruments.” ASU 2016-13 requires measurement and recognition of expected credit losses for financial assets. This standard is effective for fiscal years beginning after December 15, 2022 for small reporting companies, including interim reporting periods within those years and must be adopted using a modified retrospective approach, with certain exceptions. Early adoption is permitted. The Company does not expect the adoption of this standard to have a material effect on its financial statements.
Note 2. Strategic Agreements
The collaborative research and development and other revenues associated with the Company’s collaborators or counterparties are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Collaborator/Counterparty |
|
|
|
|
|
|
|
|
|
Gilead (1) |
|
$ |
— |
|
|
$ |
(268 |
) |
|
Others (2) |
|
|
574 |
|
|
|
238 |
|
|
Total collaborative research and development and other revenue |
|
$ |
574 |
|
|
$ |
(30 |
) |
10
|
(1) |
Amounts related to recognition of a license upfront fee and milestone payment were zero and $(465,000) for the three months ended March 31, 2021 and 2020, respectively. The Company signed a license agreement with Gilead on July 19, 2019 and received a nonrefundable upfront license fee and a milestone payment totaling $35.0 million in 2019 which was being recognized as revenue as its obligation was being satisfied using the cost-to-cost input method. The Company recorded a net revenue reversal of $465,000 related to the upfront license fee and milestone payment in the three months ended March 31, 2020 due to a change in the Company’s estimated costs to complete its obligations under the license agreement, which was partially offset by $197,000 of reimbursable collaborative research and development services with Gilead earned during the quarter. In June 2020, the Company received notice that Gilead was terminating the License Agreement and a related R&D agreement between Gilead and the Company and as a result, the Company recognized all its remaining deferred revenue as there were no remaining substantive performance obligations to be provided to Gilead by the Company as of the date when the termination notice was received. |
|
(2) |
Includes: (a) amounts related to earn-out revenue from Indivior UK Limited (Indivior) with respect to PERSERIS net sales; (b) feasibility programs; (c) research and development activities funded by Santen Pharmaceutical Co. Ltd. (Santen); and (d) royalty revenue from OP Pharma with respect to Methydur net sales. Note that in January 2018, the Company was notified by Santen that due to a shift in priorities, Santen had elected to reallocate research and development resources and put the Company’s program on pause until further notice. While the main program is on pause, the parties are working together on a limited set of research and development activities funded by Santen. |
Patent Purchase Agreement with Indivior
On September 26, 2017, the Company entered into a Patent Purchase Agreement (the “Indivior Agreement”) with Indivior. Pursuant to the Indivior Agreement, the Company assigned to Indivior certain patent rights including granted patents extending through at least 2026. The Indivior Agreement contains customary representations, warranties and indemnities of the parties. Amounts recognized in the three months ended March 31, 2021 and 2020 related to earn-out revenues from PERSERIS have been immaterial and are included in collaborative research and development and other revenue.
Agreement with Santen Pharmaceutical Co., Ltd.
On December 11, 2014, the Company and Santen Pharmaceutical Co., Ltd. (Santen) entered into a definitive agreement (the Santen Agreement). Pursuant to the Santen Agreement, the Company granted Santen an exclusive worldwide license to the Company’s proprietary SABER formulation platform and other intellectual property to develop and commercialize a sustained release product utilizing the Company’s SABER technology to deliver an ophthalmology drug. Santen controls and funds the development and commercialization program, and the parties established a joint management committee to oversee, review and coordinate the development activities of the parties under the Santen Agreement.
In connection with the Santen agreement, Santen agreed to pay the Company an upfront fee of $2.0 million in cash and to make contingent cash payments to the Company of up to $76.0 million upon the achievement of certain milestones, of which $13.0 million are development-based milestones and $63.0 million are commercialization-based milestones including milestones requiring the achievement of certain product sales targets (none of which has been achieved as of March 31, 2021). Santen will also pay for certain Company costs incurred in the development of the licensed product. If the product is commercialized, the Company would also receive a tiered royalty on annual net product sales ranging from single-digit to the low double digits, determined on a country-by-country basis. In January 2018, the Company was notified by Santen that due to a shift in near term priorities, Santen elected to reallocate research and development resources and put the Company’s program on pause until further notice. While the main program is on pause, the parties are working together on a limited set of research and development activities funded by Santen. As of March 31, 2021, the cumulative aggregate payments received by the Company under this agreement were $3.3 million.
Note 3. Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company’s valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company follows a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. These levels of inputs are the following:
|
|
• |
Level 1—Quoted prices in active markets for identical assets or liabilities. |
|
|
• |
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
|
|
• |
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
11
The Company’s financial instruments are valued using quoted prices in active markets or based upon other observable inputs. Money market funds are classified as Level 1 financial assets. Certificates of deposit, commercial paper, municipal bonds, corporate debt securities, and U.S. Government agency securities are classified as Level 2 financial assets. The fair value of the Level 2 assets is estimated using pricing models using current observable market information for similar securities. The Company’s Level 2 investments include U.S. government-backed securities and corporate securities that are valued based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications. The fair value of commercial paper is based upon the time to maturity and discounted using the three-month treasury bill rate. The average remaining maturity of the Company’s Level 2 investments as of March 31, 2021 is less than twelve months and these investments are rated by S&P and Moody’s at AAA or AA- for securities and A1, A2, P1 or P2 for commercial paper.
The following is a summary of available-for-sale securities as of March 31, 2021 and December 31, 2020 (in thousands):
|
|
|
March 31, 2021 |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Estimated Fair Value |
|
||||
|
Money market funds |
|
$ |
1,152 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,152 |
|
|
Certificates of deposit |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
Commercial paper |
|
|
86,094 |
|
|
|
— |
|
|
|
(10 |
) |
|
|
86,084 |
|
|
Municipal bonds |
|
|
4,259 |
|
|
|
— |
|
|
|
(3 |
) |
|
|
4,256 |
|
|
Corporate debt |
|
|
1,317 |
|
|
|
— |
|
|
|
(1 |
) |
|
|
1,316 |
|
|
|
|
$ |
92,972 |
|
|
$ |
— |
|
|
$ |
(14 |
) |
|
$ |
92,958 |
|
|
Reported as: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
53,272 |
|
|
$ |
— |
|
|
$ |
(2 |
) |
|
$ |
53,270 |
|
|
Short-term investments |
|
|
39,550 |
|
|
|
— |
|
|
|
(12 |
) |
|
|
39,538 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
|
|
$ |
92,972 |
|
|
$ |
— |
|
|
$ |
(14 |
) |
|
$ |
92,958 |
|
|
|
|
December 31, 2020 |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Unrealized Gain |
|
|
Unrealized Loss |
|
|
Estimated Fair Value |
|
||||
|
Money market funds |
|
$ |
522 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
522 |
|
|
Certificates of deposit |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
Commercial paper |
|
|
32,213 |
|
|
|
2 |
|
|
|
(2 |
) |
|
|
32,213 |
|
|
Municipal bonds |
|
|
6,310 |
|
|
|
— |
|
|
|
(5 |
) |
|
|
6,305 |
|
|
U.S. Government agencies |
|
|
1,000 |
|
|
|
— |
|
|
|
— |
|
|
|
1,000 |
|
|
|
|
$ |
40,195 |
|
|
$ |
2 |
|
|
$ |
(7 |
) |
|
$ |
40,190 |
|
|
Reported as: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
19,619 |
|
|
$ |
1 |
|
|
$ |
(1 |
) |
|
$ |
19,619 |
|
|
Short-term investments |
|
|
19,426 |
|
|
|
1 |
|
|
|
(6 |
) |
|
|
19,421 |
|
|
Long-term investments |
|
$ |
1,000 |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
1,000 |
|
|
Long-term restricted investments |
|
|
150 |
|
|
|
— |
|
|
|
— |
|
|
|
150 |
|
|
|
|
$ |
40,195 |
|
|
$ |
2 |
|
|
$ |
(7 |
) |
|
$ |
40,190 |
|
The following is a summary of the cost and estimated fair value of available-for-sale securities at March 31, 2021, by contractual maturity (in thousands):
|
|
|
March 31, 2021 |
|
|||||
|
|
|
Amortized Cost |
|
|
Estimated Fair Value |
|
||
|
Mature in one year or less |
|
|
91,670 |
|
|
|
91,656 |
|
|
Mature after one year through five years |
|
|
150 |
|
|
|
150 |
|
|
|
|
$ |
91,820 |
|
|
$ |
91,806 |
|
There were no securities that have had an unrealized loss for more than 12 months as of March 31, 2021.
12
As of March 31, 2021, unrealized losses on available-for-sale investments are not attributed to credit risk and are considered to be temporary. The Company believes that it is more-likely-than-not that investments in an unrealized loss position will be held until maturity or the recovery of the cost basis of the investment. To date, the Company has not recorded any impairment charges on marketable securities related to other-than-temporary declines in market value.
Note 4. Stock-Based Compensation
As of March 31, 2021, the Company has two stock-based compensation plans. The stock-based compensation cost that has been included in the statements of comprehensive loss is shown as below (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Cost of product revenues |
|
$ |
5 |
|
|
$ |
2 |
|
|
Research and development |
|
|
315 |
|
|
|
207 |
|
|
Selling, general and administrative |
|
|
382 |
|
|
|
178 |
|
|
Total stock-based compensation |
|
$ |
702 |
|
|
$ |
387 |
|
As of March 31, 2021 and 2020, $16,000 and $12,000 of stock-based compensation cost was capitalized in inventory on the Company’s balance sheets, respectively.
The Company uses the Black-Scholes option pricing model to value its stock options. The expected life computation is based on historical exercise patterns and post-vesting termination behavior. The Company considered its historical volatility in developing its estimate of expected volatility.
The Company used the following assumptions to estimate the fair value of stock options granted and shares purchased under its employee stock purchase plan for the three months ended March 31, 2021 and 2020:
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Stock Options |
|
|
|
|
|
|
|
|
|
Risk-free rate |
|
|
|
|
|
|
||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
Expected life of option (in years) |
|
|
|
|
|
|
||
|
Volatility |
|
|
|
|
|
|
||
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Employee Stock Purchase Plan |
|
|
|
|
|
|
|
|
|
Risk-free rate |
|
0.1% |
|
|
1.6% |
|
||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
Expected life of option (in years) |
|
|
|
|
|
|
||
|
Volatility |
|
78% |
|
|
103% |
|
||
Note 5. Term Loan
In July 2016, the Company entered into a $20.0 million secured single-draw term loan with Oxford Finance LLC (Oxford Finance). The Company and Oxford Finance entered into three subsequent amendments to the Loan Agreement in February 2018, November of 2018 and December 2019, for which the Company paid Oxford Finance loan modification fees of $100,000, $900,000 and $825,000 respectively. As amended, the Loan Agreement provides for interest only payments for the first 18 months, followed by consecutive monthly payments of principal and interest in arrears starting on December 1, 2021 and continuing through the maturity date of the term loan of May 1, 2024. The Loan Agreement provides for a floating interest rate (7.95% initially and 7.95% as of March 31, 2021) based on an index rate plus a spread. In addition, a payment equal to 10% of the principal amount of the term loan is due when the term loan becomes due or upon the prepayment of the facility. If the Company elects to prepay the loan, there is also a prepayment fee of between 0.75% and 2.5% of the principal amount of the term loan depending on the timing of prepayment. The $150,000 facility fee that was paid at the original closing, the loan modification fees and other debt offering/issuance costs have been recorded as debt discount on the Company’s balance sheet and together with the final $2.0 million payment are being amortized to interest expense using the effective interest method over the revised term of the loan.
The term loan is secured by substantially all of the assets of the Company, except that the collateral does not include any intellectual property (including licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The 2016 Loan Agreement contains customary representations, warranties and covenants by the Company, which covenants limit the
13
Company’s ability to convey, sell, lease, transfer, assign or otherwise dispose of certain assets of the Company; engage in any business other than the businesses currently engaged in by the Company or reasonably related thereto; liquidate or dissolve; make certain management changes; undergo certain change of control events; create, incur, assume, or be liable with respect to certain indebtedness; grant certain liens; pay dividends and make certain other restricted payments; make certain investments; and make payments on any subordinated debt.
The Loan Agreement also contains customary indemnification obligations and customary events of default, including, among other things, the Company’s failure to fulfill certain obligations of the Company under the Loan Agreement and the occurrence of a material adverse change which is defined as a material adverse change in the Company’s business, operations, or condition (financial or otherwise), a material impairment of the prospect of repayment of any portion of the loan, or a material impairment in the perfection or priority of lender’s lien in the collateral or in the value of such collateral. In the event of default by the Company under the Loan Agreement, the lender would be entitled to exercise its remedies thereunder, including the right to accelerate the debt, upon which the Company may be required to repay all amounts then outstanding under the Loan Agreement, which could harm the Company’s financial condition. The conditionally exercisable call option related to the event of default is considered to be an embedded derivative which is required to be bifurcated and accounted for as a separate financial instrument. In the periods presented, the value of the embedded derivative is not material, but could become material in future periods if an event of default became more probable than is currently estimated.
The fair value of the term loan approximates the carrying value. Future maturities and interest payments due under the term loan as of March 31, 2021, are as follows (in thousands):
|
Nine months ended December 31, 2021 |
|
$ |
2,543 |
|
|
2022 |
|
|
9,208 |
|
|
2023 |
|
|
8,563 |
|
|
2024 |
|
|
4,711 |
|
|
Total minimum payments |
|
|
25,025 |
|
|
Less amount representing interest |
|
|
(3,222 |
) |
|
Gross balance of term loan |
|
|
21,803 |
|
|
Less unamortized debt discount |
|
|
(846 |
) |
|
Carrying value of term loan, net |
|
|
20,957 |
|
|
Less term loan, current portion, net |
|
|
(2,895 |
) |
|
Term loan, non-current portion, net |
|
$ |
18,062 |
|
As of March 31, 2021, the Company was in compliance with all material covenants under the Loan Agreement and there had been no material adverse change.
Note 6. Commitments
Operating Leases
The Company has lease arrangements for its facilities in California and Alabama as follows.
|
Location |
|
Approximate Square Feet |
|
Operation |
|
Expiration |
|
Cupertino, CA |
|
|
|
Office, Laboratory and Manufacturing |
|
Lease expires 2024 (with an option to renew for an additional five years) |
|
|
|
|
|
|
|
|
|
Cupertino, CA |
|
|
|
Office and Laboratory |
|
Lease expires 2024 (with an option to renew for an additional five years)
|
|
Vacaville, CA |
|
|
|
Manufacturing |
|
Lease expires 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Under these leases, the Company is required to pay certain maintenance expenses in addition to monthly rent. Rent expense is recognized on a straight-line basis over the lease term for leases that have scheduled rental payment increases. The lease expense includes the amortization of the right-of-assets with the associated interest component estimated by applying the effective interest method. Rent expense under all operating leases was $479,000 and $484,000 for the three months ended March 31, 2021 and 2020, respectively.
14
Future minimum payments under these noncancelable leases are as follows (in thousands):
|
|
|
Operating Leases |
|
|
|
Nine months ended December 31, 2021 |
|
$ |
1,418 |
|
|
2022 |
|
|
1,991 |
|
|
2023 |
|
|
1,970 |
|
|
2024 |
|
|
275 |
|
|
|
|
$ |
5,654 |
|
Note 7. Stockholders’ Equity
In August 2018, the Company filed a shelf registration statement on Form S-3 with the SEC (the “2018 Registration Statement”) (File No. 333-226518), which upon being declared effective in October 2018, terminated the Company’s registration statement filed in November 2015 (File No. 333-207776) and allowed the Company to offer up to $175.0 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of shares of the Company’s common stock which the Company may sell, subject to certain limitations, pursuant to a sales agreement dated November 3, 2015 with Cantor Fitzgerald & Co. (the “2015 Sales Agreement”).
In February 2021, the Company completed an underwritten public offering of 20,364,582 shares of its common stock at a price of $2.2386 per share pursuant to an underwriting agreement with Cantor Fitzgerald & Co., raising total gross proceeds to the Company of approximately $45.6 million before deducting estimated offering expenses payable by the Company. Total stock issuance costs related to this financing were approximately $195,000. After deducting estimated offering expenses payable by the Company, the net proceeds to the Company were approximately $45.4 million.
During the three months ended March 31, 2021, the Company also raised net proceeds (net of commissions) of approximately $2.4 million from the sale of 950,009 shares of the Company’s common stock in the open market at a weighted average price of $2.60 per share pursuant to the October 2018 registration statement and the 2015 Sales Agreement. During the three months ended March 31, 2020, the Company did not issue any shares through its the 2015 Sales Agreement.
As of May 3, 2021, the Company had up to approximately $95.6 million of the Company’s securities available for sale under the 2018 Registration Statement, of which approximately $56.2 million of the Company’s common stock are available pursuant to the 2015 Sales Agreement.
Note 8.Discontinued Operations
On December 31, 2020, the Company completed the sale of its LACTEL Absorbable Polymers product line to Evonik. Under the terms of the Asset Purchase Agreement, Evonik paid DURECT approximately $15 million subject to certain adjustments, and also agreed to assume certain liabilities with respect to the transferred assets.
As a result of the sale of the LACTEL product line, the operating results from the Company’s LACTEL product line have been excluded from continuing operations and presented as discontinued operations in the accompanying Statement of Operations and Comprehensive Loss for the three months ended March 31, 2020. During the twelve months ended December 31, 2020, the Company recorded a gain on sale of the LACTEL product line of $12.8 million, upon the completion of sale to Evonik. The results of operations below include certain allocations that management believes fairly reflect the utilization of services provided to the LACTEL product line. The allocations do not include amounts related to general corporate administrative expenses or interest expense. Therefore, these results of operations do not necessarily reflect what the results of operations would have been had the LACTEL product line operated as a stand-alone entity.
15
The components of income from discontinued operations as reported in the Company’s statement of operations were as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|
|
|
|
2020 |
|
|
|
Product revenue, net |
|
$ |
1,180 |
|
|
Total revenues |
|
|
1,180 |
|
|
Operating expenses: |
|
|
|
|
|
Cost of product revenues |
|
|
836 |
|
|
Research and development |
|
|
130 |
|
|
Selling, general and administrative |
|
|
9 |
|
|
Total costs and expenses |
|
|
975 |
|
|
Net income from discontinued operations |
|
$ |
205 |
|
|
Net income per share |
|
|
|
|
|
Basic and diluted |
|
$ |
0.00 |
|
|
Weighted-average shares used in computing net income per share basic and diluted |
|
|
|
|
|
Basic and diluted |
|
|
195,745 |
|
The following table presents certain non-cash items related to discontinued operations, which are included in the Company’s statement of cash flows (in thousands):
|
|
|
Three months ended March 31, |
|
|
|
|
|
2020 |
|
|
|
Depreciation |
|
$ |
50 |
|
|
Stock-based compensation expense |
|
|
27 |
|
|
|
|
$ |
77 |
|
|
Gain on sale of the LACTEL product line |
|
$ |
— |
|
|
Non-cash items, net |
|
$ |
77 |
|
16
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three months ended March 31, 2021 should be read in conjunction with our annual report on Form 10-K for the year ended December 31, 2020 filed with the Securities and Exchange Commission and “Risk Factors” section included elsewhere in this Form 10-Q. This Form 10-Q contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. When used in this report, the words “believe,” “anticipate,” “intend,” “plan,” “estimate,” “expect,” “may,” “will,” “could,” “potentially” and similar expressions are forward-looking statements. Such forward-looking statements are based on current expectations and beliefs. Any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements as a result of various factors.
Forward-looking statements made in this report include, for example, statements about:
|
|
• |
the clinical trial plans and timelines for DUR-928; |
|
|
• |
potential uses and benefits of DUR-928 to treat alcohol-associated hepatitis (also called alcoholic hepatitis or AH), non-alcoholic steatohepatitis (NASH), or other conditions; |
|
|
• |
the results and timing of clinical trials, the ability to enroll patients in clinical trials in a timely and cost-effective manner; |
|
|
• |
the likelihood of future clinical trial results of DUR-928 being similar to results from previous trials, the possible commencement of future clinical trials, enrollment rates and timing of announcements of the findings from our clinical trials; |
|
|
• |
the potential earn-out payments we may receive from Indivior related to the commercialization of PERSERIS, and milestone and royalty payments we may receive from Santen or Orient Pharma; |
|
|
• |
the progress of our third-party collaborations, including estimated milestones; |
|
|
• |
our intention to seek, and ability to enter into and maintain strategic alliances and collaborations, including for the commercialization of POSIMIR; |
|
|
• |
POSIMIR’s commercial prospects, differentiating product attributes, potential for reimbursement and formulary access, the timing of post-marketing commitments and timing of potential commercial partnership and launch; |
|
|
• |
the potential benefits and uses of our products, product candidates and technologies, including POSIMIR, DUR-928 and our SABER, CLOUD and ORADUR technologies; |
|
|
• |
responsibilities of our third-party collaborators, including the responsibility to make cost reimbursement, milestone, royalty and other payments to us, and our expectations regarding our collaborators’ plans with respect to our product candidates and continued development of our product candidates; |
|
|
• |
our responsibilities to our third-party collaborators, including our responsibilities to conduct research and development, clinical trials and manufacture products or product candidates; |
|
|
• |
market opportunities for product candidates in our product development pipeline; |
|
|
• |
potential regulatory filings for or approval of DUR-928 or any of our or any third parties’ other product candidates; |
|
|
• |
the progress and results of our research and development programs and our evaluation of additional development programs; |
|
|
• |
requirements for us to purchase supplies and raw materials from third parties, and the ability of third parties to provide us with required supplies and raw materials; |
|
|
• |
conditions for obtaining regulatory approval of our product candidates; |
|
|
• |
submission and timing of applications for regulatory approval and timing of responses to our regulatory submissions; |
|
|
• |
the impact of FDA, EMA and other government regulation on our business; |
|
|
• |
our ability to obtain, assert and protect patents and other intellectual property rights, including intellectual property licensed to our collaborators, as well as avoiding the intellectual property rights of others; |
|
|
• |
products and companies that will compete with our products and the product candidates we develop and/or license to third-party collaborators; |
|
|
• |
the possibility we may commercialize our own products and build up our commercial, sales and marketing capabilities and other required infrastructure; |
|
|
• |
the possibility that we may develop additional manufacturing capabilities; |
|
|
• |
our employees, including the number of employees and the continued services of key management, technical and scientific personnel; |
|
|
• |
our future performance, including our anticipation that we will not derive meaningful revenues from our product candidates in development for at least the next twelve months, potential for future inventory write-offs and our expectations regarding our ability to achieve profitability; |
|
|
• |
sufficiency of our cash resources, anticipated capital requirements and capital expenditures, our ability to comply with covenants of our term loan, and our need or desire for additional financing, including potential sales under our shelf registration statement; |
|
|
• |
our expectations regarding research and development expenses, and selling, general and administrative expenses; |
17
|
|
• |
the composition of future revenues; and |
|
|
• |
accounting policies and estimates. |
Forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements as a result of various factors. For a more detailed discussion of such forward looking statements and the potential risks and uncertainties that may impact upon their accuracy, see the “Risk Factors” section and “Overview” section of this Management’s Discussion and Analysis of Financial Condition and Results of Operations. These forward-looking statements reflect our view only as of the date of this report. We undertake no obligations to update any forward-looking statements. You should also carefully consider the factors set forth in other reports or documents that we file from time to time with the Securities and Exchange Commission.
Overview
We are a biopharmaceutical company advancing novel and potentially lifesaving investigational therapies derived from our Epigenetic Regulator Program. DUR‑928, a new chemical entity in clinical development, is the lead candidate in our Epigenetic Regulator Program. An endogenous, orally bioavailable small molecule, DUR-928 has been shown in both in vitro and in vivo studies to play an important regulatory role in lipid metabolism, stress and inflammatory responses, and cell death and survival. Alcohol-associated hepatitis (AH) is a life-threatening acute liver condition with no approved therapeutics and a 28-day or 90-day historical mortality rate of 26% or 29%, respectively. After completing a Phase 2a trial where 100% of AH patients treated with DUR-928 survived the 28-day study period, we are now conducting a double-blind, placebo-controlled Phase 2b clinical trial called AHFIRM, in which we are evaluating DUR-928’s life saving potential compared to the current standard of care in patients with severe AH. If the AHFIRM trial is successful, it may support an NDA filing and we may decide to develop our own commercial, sales and marketing organization. DUR-928 is also being investigated in patients with nonalcoholic steatohepatitis (NASH). In March 2021, a peer-reviewed research paper describing the binding sites and proposed mechanism of action of DUR-928 was published in The Journal of Lipid Research. The publication shows that DUR-928 (referred to in the paper as 25HC3S) binds to and inhibits the activity of DNA methyltransferases (DNMTs) DNMT-1, 3a and 3b, epigenetic regulating enzymes that add methyl groups to DNA (a process called DNA methylation). As such, by inhibiting DNMT activity, DUR-928 inhibits DNA methylation, thereby regulating the expression of genes that modulate crucial cellular activities, including those associated with cell death, stress response, and lipid biosynthesis. These modulations may lead to improved cell survival, and reduced lipid accumulation and inflammation, as has been observed in various in vivo animal models and in results from DURECT’s completed clinical trials in AH and NASH.
In addition to our Epigenetic Regulator Program, we developed a novel and proprietary post-surgical pain product called POSIMIR that utilizes our innovative SABER platform technology to enable continuous sustained delivery of bupivacaine, a non-opioid local analgesic, over 3 days in adults. In February 2021, the U.S. Food and Drug Administration (FDA) approved POSIMIR for infiltration use in adults for administration into the subacromial space under direct arthroscopic visualization to produce post-surgical analgesia for up to 72 hours following arthroscopic subacromial decompression. The approval was based on positive data from a randomized, multicenter, assessor-blinded, placebo‑controlled clinical trial in patients undergoing arthroscopic subacromial decompression surgery with an intact rotator cuff. The primary outcome measures were mean pain intensity and total opioid rescue analgesia administered, both evaluated over the first 72 hours after surgery versus placebo. POSIMIR demonstrated a statistically significant improvement in both primary outcome measures: a 1.3 point, or 20%, reduction in mean pain intensity on a 0-10 point pain scale (p=0.01), and a 67% reduction in intravenous morphine-equivalent rescue opioid use, from a median of 12 mg in the placebo group to 4 mg in the POSIMIR group (p=0.01). We are in discussions with potential licensees regarding commercialization rights to POSIMIR, for which we hold worldwide rights.
NOTE: POSIMIR®, SABER®, CLOUDTM, ORADURTM and ALZET ® are trademarks of DURECT Corporation. Other trademarks referred to belong to their respective owners. Full prescribing information for POSIMIR, including BOXED WARNING and Medication Guide can be found at www.posimir.com. Full prescribing information for PERSERIS, including BOXED WARNING and Medication Guide can be found at www.perseris.com.
18
As a result of the assignment of certain patent rights, DURECT also receives single digit sales-based earn-out payments from U.S. net sales of Indivior’s PERSERIS (risperidone) drug for schizophrenia, which was launched commercially in the U.S. in February 2019. In addition, in September 2020, our licensee, Orient Pharma informed us that it had launched Methydur Sustained Release Capsules (Methydur) for the treatment of attention deficit hyperactivity disorder (ADHD) in Taiwan. We receive a single digit royalty on sales of Methydur by Orient Pharma. We also manufacture and sell ALZET osmotic pumps used in laboratory research and have several early-stage development programs with corporate collaborators on terms which typically call for our collaborator to fund all or a substantial portion of future development costs and then pay us milestone payments based on specific development or commercial achievements plus royalties on product sales.
Additional details of these programs and related strategic agreements are contained in our annual report on Form 10-K for the year ended December 31, 2020 and in Note 2 of the financial statements included in Item 1 above.
Recent Developments
The COVID-19 global pandemic poses a significant life-threatening and economic risk throughout the world and has had a negative impact on our business. Healthcare providers and hospitals have prioritized resources toward fighting the virus causing clinical trial activities to be deprioritized. As a result, clinical site initiations, patient enrollment and other activities that require visits to clinical sites, including data monitoring, have been and may continue to be delayed. COVID-19 initially also had a negative impact on orders for our ALZET product line as many ALZET customers reduced their activities during the pandemic. ALZET orders have recovered significantly in 2021, a trend that may or may not continue as the impact of the pandemic has proven to be difficult to predict. In addition, other than laboratory and manufacturing employees, many of our other employees continue to work from home, and, in keeping with social distancing requirements, we have limited the number of staff in our facilities. This partial disruption, although expected to be temporary, may impact our operations and overall business by delaying the progress of our research and development programs, including our ongoing and planned preclinical studies and clinical trials. The impact of COVID-19 is evolving rapidly and its future effects are uncertain. Given the uncertainty of the situation, the duration of the disruption and related financial impact cannot be reasonably estimated at this time. We will continue to evaluate the impact of the COVID-19 pandemic on our business and expect to reevaluate the timing of our anticipated preclinical and clinical objectives as we learn more and the impact of COVID-19 on our industry becomes clearer.
For additional information on the various risks posed by the COVID-19 pandemic, please read Item 1A. Risk Factors included in this report.
Epigenetic Regulator Program and New Chemical Entities
Epigenetic regulation involves biochemical modification of either DNA itself or proteins that are intimately associated with DNA. These modifications lead to changes in gene expression that facilitate downstream biological effects.
DURECT’s Epigenetic Regulator Program involves a multi-year collaborative effort with the Department of Internal Medicine at Virginia Commonwealth University (VCU), the VCU Medical Center and the McGuire VA Medical Center. The knowledge base supporting this program is a result of more than 30 years of lipid research by Shunlin Ren, M.D., Ph.D., Professor of Internal Medicine at the VCU Medical Center. The lead compound from this program, DUR-928 is an endogenous sulfated oxysterol and an epigenetic regulator. Epigenetic regulators are compounds that regulate patterns of gene expression without modifying the DNA sequence. In March 2021, DURECT announced the publication of a peer-reviewed research paper describing the proposed mechanism of action of DUR-928, in The Journal of Lipid Research. The publication shows that DUR-928 bound to and inhibited the activity of DNA methyltransferases (DNMTs), DNMT-1, 3a and 3b, enzymes that add methyl groups to DNA (a process called DNA methylation, one of the mechanisms of epigenetic regulation), in stressed cells. As such, by inhibiting DNMT activity, DUR-928 reduced DNA methylation in the promoter regions of more than 1,000 genes that were hypermethylated in stressed cells, thereby regulating the expression of these genes that are associated with crucial cellular activities, including cell death/survival, stress/inflammatory response, and lipid/energy homeostasis. These modulations may lead to improved cell survival, and reduced lipid accumulation and inflammation, as observed in various in vivo animal models and shown in results from DURECT’s completed clinical trials in alcohol-associated hepatitis (AH) and non-alcoholic steatohepatitis (NASH). Under a license with VCU, we hold the exclusive royalty-bearing worldwide right to develop and commercialize DUR-928 and related molecules discovered in the program.
19
The biological activity of DUR-928 has been demonstrated in over a dozen different animal disease models involving three animal species. Some of these models represent acute organ injuries (e.g., endotoxin shock, acute oxidative stress, ischemic-reperfusion injury in the kidney and brain) and several represent chronic metabolic disorders involving hepatic lipid accumulation and dysfunction (e.g., NASH and NAFLD).
Our major product research and development efforts for DUR-928 are in the following table:
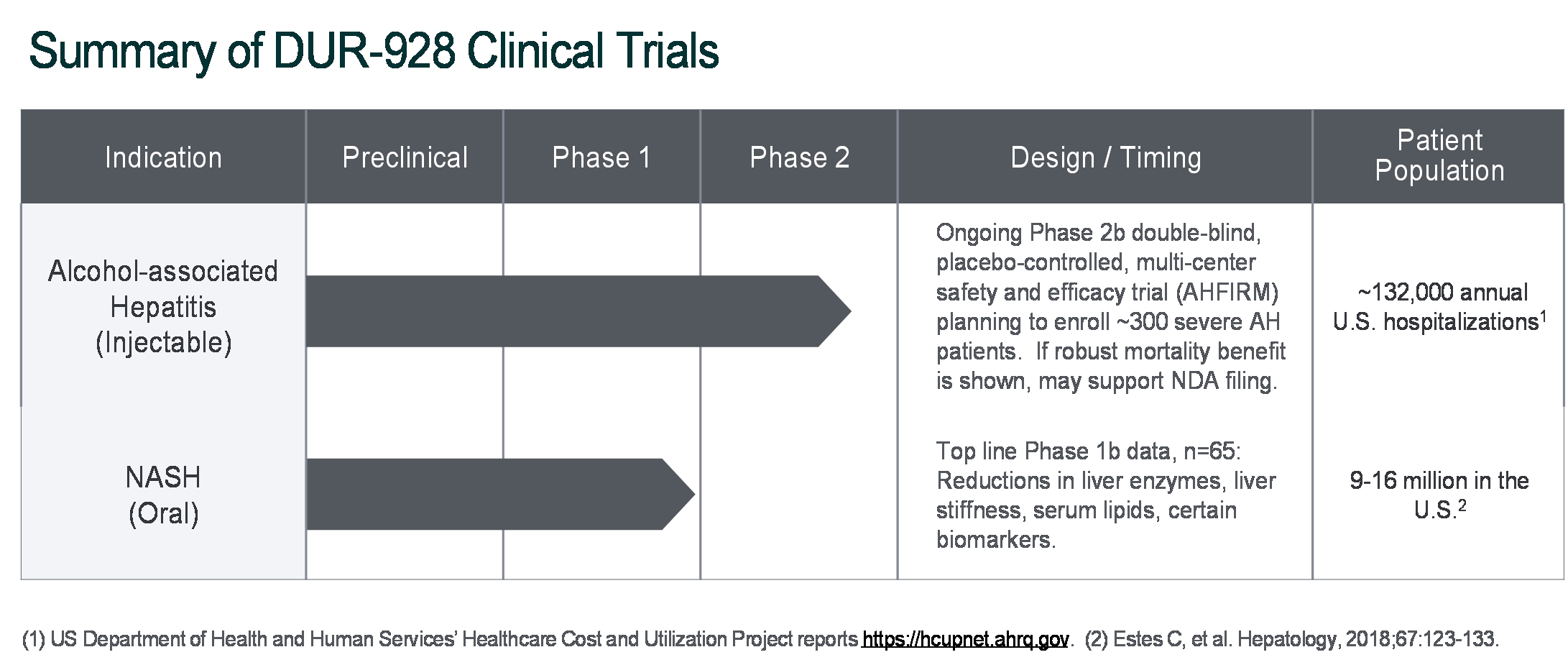
In pharmacokinetic (PK) and toxicology studies conducted in mice, hamsters, rats, rabbits, dogs, minipigs and monkeys, DUR-928 has been found to be tolerable and safe by all routes of administration tested to date. These results support the use of DUR-928 in completed, ongoing and planned human safety, PK, proof-of-concept, and efficacy trials. The chronic toxicity of DUR-928 was further assessed in a 6-month oral study in rats and in a 9-month oral study in dogs. These studies were completed successfully and support long duration human clinical trials of DUR-928.
Acute Organ Injury Program with Injectable DUR-928
Market Opportunity. Alcohol-associated hepatitis (also called alcoholic hepatitis or AH), an acute form of alcohol-associated liver disease (ALD), is associated with long-term heavy intake of alcohol and often occurs after a recent period of increased alcohol consumption. AH is typically characterized by recent onset jaundice and hepatic failure. An analysis of 77 studies published between 1971 and 2016, which included data from a total of 8,184 patients, showed the overall mortality from AH was 26% at 28 days and 29% at 90 days. According to the most recent data provided by the Agency for Healthcare Research and Quality (AHRQ), a part of the US Department of Health and Human Services (HHS), there were 131,950 hospitalizations for patients with AH in 2018. From a recent publication analyzing the mortality and costs associated with AH, the cost per patient is estimated at over $50,000 in the first year. ALD is one of the leading causes of liver transplants in the US, each of which cost over $875,000.
Clinical Program. In 2019, we completed a Phase 2a clinical trial evaluating intravenously infused DUR-928 in patients with moderate and severe AH. This was an open label, dose escalation (30, 90 and 150 mg), multi-center U.S. study, originally designed to be conducted in two sequential parts. Part A included patients with moderate AH and Part B included patients with severe AH. Severity of AH was determined by the Model of End-Stage Liver Disease (MELD) scores, a common scoring system to assess the severity and prognosis of AH patients; moderate was defined as MELD 11-20 and severe as MELD 21-30.
In this Phase 2a trial, dose escalation was permitted following review of safety and pharmacokinetic (PK) results of the prior dose level by a Dose Escalation Committee. The target number of patients for the study was 4 per dose group. Final enrollment included 19 patients with moderate and severe AH, who were administered DUR-928 intravenously at three different doses. Eight patients (four moderate and four severe) were dosed at 30mg, seven patients (three moderate and four severe) were dosed at 90mg and four patients (all severe) were dosed at 150mg. After being discharged on day two, one patient did not return for the scheduled day seven and day 28 follow-up visits; therefore Lille, bilirubin and MELD data reported below are based on 18 patients. The objectives of this study included assessment of safety, PK and pharmacodynamic (PD) signals, including liver biochemistry, biomarkers, and prognostic scores, including the Lille score, following DUR-928 treatment.
20
In November 2019, the results from this Phase 2a clinical trial of DUR-928 in alcoholic hepatitis (AH) were presented as a late-breaking oral presentation at The Liver Meeting®. The study results were also selected for inclusion in the ‘Best of The Liver Meeting’ summary slide presentation in the alcohol-related liver disease category.
All 19 patients treated with DUR-928 in this trial survived the 28-day follow-up period and there were no drug-related serious adverse events. Using an alternative measure of AH severity to MELD, Maddrey’s Discrimination Function (DF), 15 of the 19 patients had DF scores of 32 or greater, indicating that they had severe AH. Patients treated with DUR-928 had a statistically significant reduction from baseline in bilirubin at day 7 and 28 and MELD at day 28. Lille scores, described below, were also statistically significantly lower than those from a well-matched group of patients in a contemporary ongoing trial as well as several published historical controls. 74% of all DUR-928 treated patients and 67% of those with severe AH were discharged from the hospital within four days of receiving a single dose of DUR-928.
Lille
Lille scores are used in clinical practice to help determine the prognosis and response of AH patients after seven days of treatment. The lower the Lille score, the better the prognosis. Patients with a Lille score below 0.45 have a six-month survival rate of 85% compared to those with Lille scores above 0.45, who have only a 25% six-month survival rate. The chart below shows the Lille scores for individual AH patients treated with DUR-928 plotted as a function of their baseline MELD scores. In our study, the median Lille score for patients treated with DUR-928 was 0.10. The median Lille score among a cohort of 15 patients treated with standard of care at the University of Louisville (UL) was 0.41 (shown as historical control).
The chart below shows individual patient Lille scores plotted as a function of their baseline MELD scores.
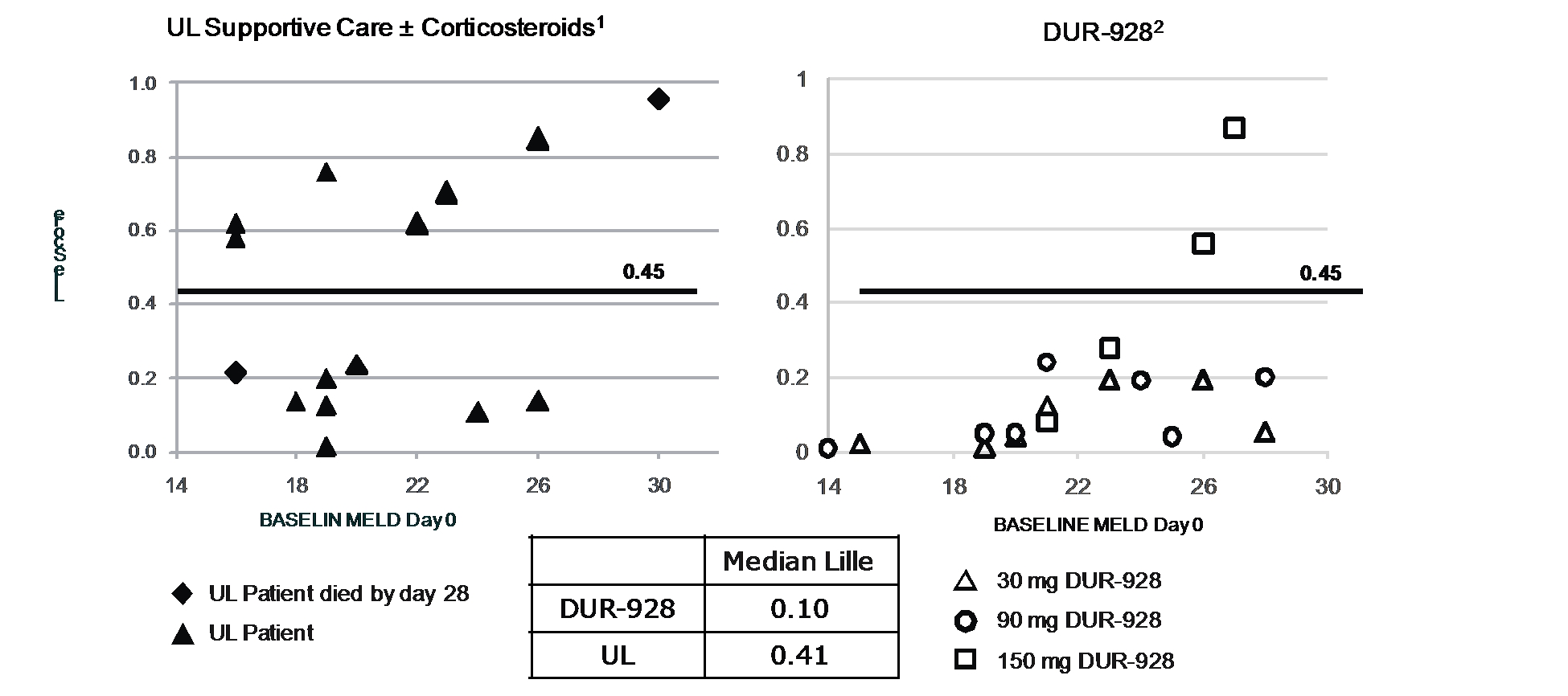
|
|
1) |
Our advisor, Dr. Craig McClain from the University of Louisville (UL), shared anonymized data from his study, in which 15 AH patients with initial MELD scores ranging from 15-30 received either supportive care alone (n=8 moderate AH patients) or supportive care with corticosteroids (n=7 severe AH patients). Two of the UL control patients died by day 28. |
|
|
2) |
One patient in the DUR-928 group did not return for the day 7 or 28 visit. All 19 patients, including this one, treated with DUR-928 in this trial survived the 28-day follow-up period. |
|
|
3) |
Lille scores in the DUR-928 group were significantly lower than that of the UL patients (p=0.01; Wilcoxon's Rank Sum Test). |
21
As shown below, 100% of patients in the 30 mg and 90 mg DUR-928 dosing groups were treatment responders based on their Lille scores. 89% of the overall DUR-928 patient population were treatment responders based on Lille. Patients with severe AH, as defined by Maddrey’s Discriminant Function (DF) ≥32 or MELD 21-30, and baseline serum bilirubin above 8 mg/dL, had similarly high response rates to DUR-928 treatment.
|
AH Patient Category |
|
n1 |
Responders (Lille<0.45) |
Lille Median (Quartile) |
|
All Patients2 30 or 90 mg DUR-9283 |
|
18 14 |
89% 100% |
0.10 (0.04, 0.20) 0.05 (0.04, 0.19) |
|
DF≥32 (SAH)2, 4 30 or 90 mg DUR-9283 |
|
15 11 |
87% 100% |
0.19 (0.05, 0.22) 0.12 (0.05, 0.19) |
|
MELD 21-302 30 or 90 mg DUR-9283 |
|
12 8 |
83% 100% |
0.19 (0.11, 0.25) 0.19 (0.10, 0.19) |
|
Baseline bilirubin >8mg/dl2 30 or 90 mg DUR-9283 |
|
11 8 |
82% 100% |
0.10 (0.05, 0.20) 0.10 (0.05, 0.19) |
|
|
1) |
One patient did not return for Day 7 and 28 visits; |
|
|
2) |
Including patients receiving 30, 90 and 150 mg of DUR-928; |
|
|
3) |
Excluding patients receiving 150 mg of DUR-928. |
|
|
4) |
Maddrey’s Discriminant Function (MDF or DF) is calculated using the patient’s prothrombin time and serum bilirubin level. DF was introduced in 1978 as a predictor of significant mortality risk for AH patients. A DF≥32 identified AH patients with a 30-day mortality rate of ≥50%. |
The Lille scores of patients treated with DUR-928 in this trial were also significantly lower than several selected published historical studies (Hepatology 2007, 45:1348-1354; Gut 2011, 60:255-260), in which patients had similar baseline bilirubin, albumin, creatinine, prothrombin time and DF scores, and were treated with standard of care with or without corticosteroids. Of course, due to the historical nature of these studies, such comparisons should be taken cautiously.
A sub-group analysis was conducted to compare severe AH patients in the 30 mg and 90 mg dosing groups (n=8) with well-matched severe AH patients (n=13) who received corticosteroids for 28 days in a contemporaneous study at the University of Louisville (UL). Patients shown below in the UL steroid group had a mean baseline MELD of 24.46 and mean baseline Maddrey’s DF score of 62.98. The 8 patients in the DUR-928 group had baseline mean MELD of 24.50 and mean baseline Maddrey’s DF score of 61.25. All patients treated with DUR-928 survived the 28-day follow up period, while 3 of the 13 patients (23%) in the UL steroid group died within the first 28 days.

The steroid group in the above graph includes the 7 severe AH patients treated with steroids from the UL group shown in the MELD vs Lille graph above plus an additional 6 severe AH patients subsequently treated in the UL study.
22
Bilirubin
Bilirubin is formed by the breakdown of red blood cells in the body. The level of total bilirubin in the blood is an indication of how the liver is functioning. High bilirubin levels are associated with liver dysfunction and disease. In this trial, patients treated with DUR-928 had a significant early reduction from baseline in bilirubin by day 7. Patients with more elevated bilirubin at baseline (serum bilirubin >8 mg/dL) had a median reduction from baseline of 25% by day 7 and 48% by day 28.
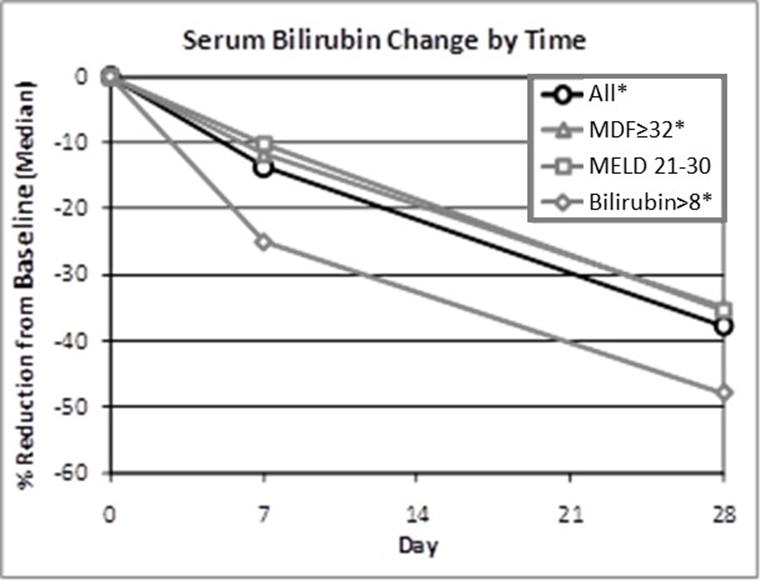
*p<0.05 compared to baseline (Wilcoxon's Signed Rank Test)
MELD is another common scoring system used to assess the severity and prognosis of AH patients. Patients with MELD scores of 11-20 are classified as having moderate AH and patients with MELD scores of 21-30 are classified as having severe AH. As with Lille scores, the lower the MELD score, the better the prognosis for the AH patient. In this study (shown in the chart below), the median reduction from baseline in MELD among all DUR-928 treated patients was >2 points and among those with baseline bilirubin levels >8 mg/dL was 5 points by day 28.
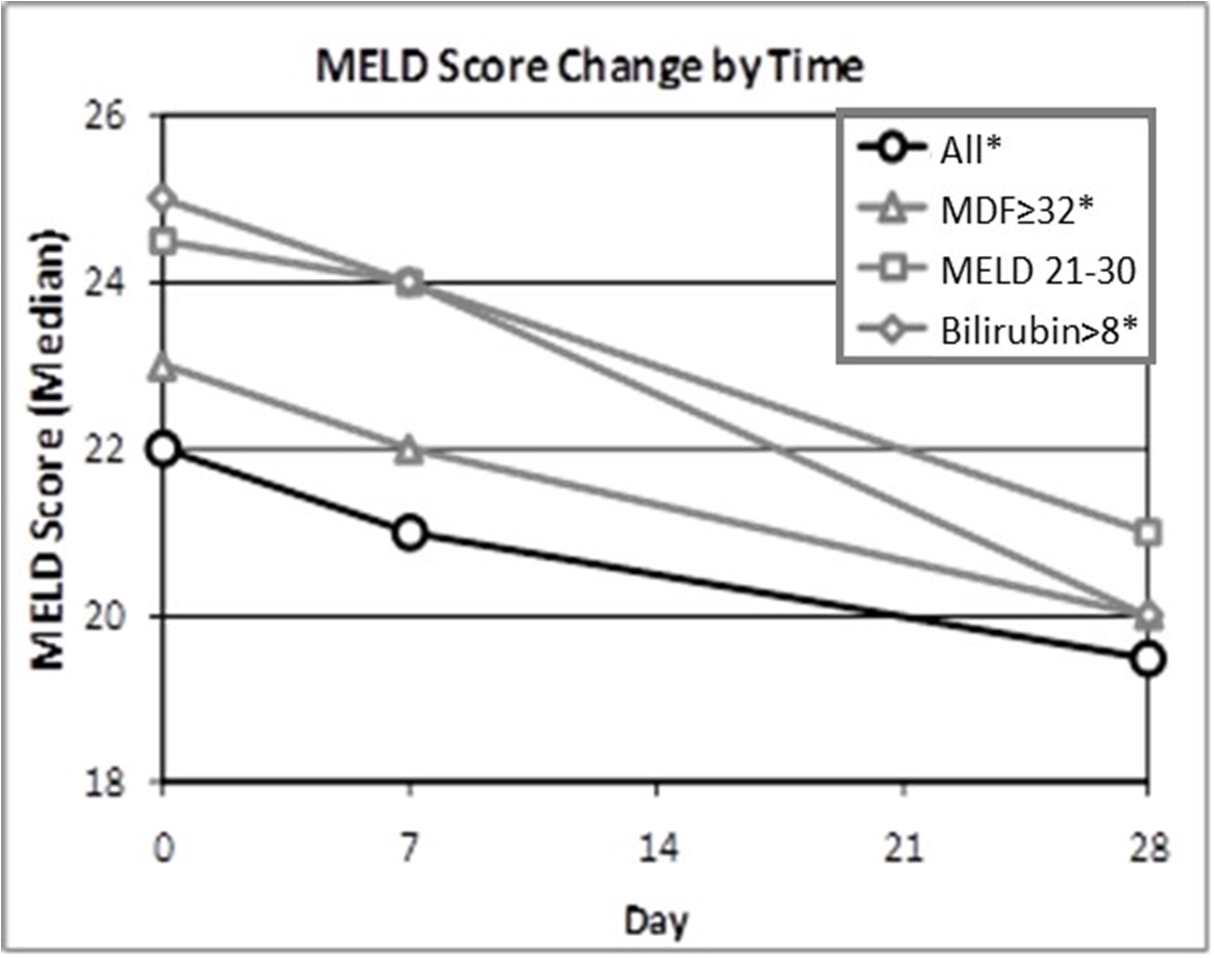
*p<0.05 compared to baseline (Wilcoxon's Signed Rank Test)
MELD is calculated based on (a) bilirubin, (b) serum creatinine (sCr), and (c) International Normalized Ratio (INR), which is a measure of prothrombin time.
23
Safety and Pharmacokinetics
In the Phase 2a study of DUR-928 in AH, DUR-928 was well tolerated at all doses tested. There were no drug-related serious adverse events and only three adverse events designated as possibly or probably related to DUR-928: one occurrence of moderate generalized pruritus, one mild rash and one grade two alkaline phosphatase elevation. There were no discontinuations, early withdrawals or termination of study drug or study participation due to adverse events. All patients treated with DUR-928 survived through the 28-day follow-up period. Drug exposures were dose proportional and were not affected by the severity of the disease.
In December 2020, we announced that the FDA had granted DUR-928 Fast Track Designation for the treatment of AH. The FDA grants Fast Track Designation to facilitate development and expedite the review of therapies with the potential to treat a serious condition where there is an unmet medical need. A therapeutic that receives Fast Track Designation may benefit from early and frequent communication with the agency in addition to a rolling submission of the marketing application, with the objective of getting important new therapies to patients more quickly.
In January 2021, we announced the dosing of the first patient in our Phase 2b study in subjects with AH to evaluate saFety and effIcacy of DUR-928 treatMent (AHFIRM). AHFIRM is a randomized, double-blind, placebo-controlled, international, multi-center Phase 2b study to evaluate the safety and efficacy of DUR-928 in approximately 300 patients with severe AH. The study is comprised of three arms targeting approximately 100 patients each: (1) Placebo plus standard of care (SOC, which may include the use of methylprednisolone, a corticosteroid, at the discretion of the treating physician); (2) DUR-928 (30 mg); and (3) DUR-928 (90 mg). All patients in the trial receive supportive care. Patients receive an intravenous (IV) dose of DUR-928 or placebo (sterile water) on day 1 and a second IV dose on day 4 if they are still hospitalized. The primary outcome measure will be the 90-day survival rate for patients treated with DUR-928 compared to those treated with placebo plus SOC. Secondary endpoints include 28-day survival, the rate of adverse events, Lille and MELD (prognostic scores), and time in the intensive care unit. We are targeting approximately 50 to 60 clinical trial sites in the United States, U.K., E.U. and Australia.
Phase 1 trials of DUR-928 administered through injection have supported the development of DUR-928 in AH. The initial Phase 1 trial in healthy subjects was a single-site, randomized, double-blinded, placebo-controlled, single-ascending-dose study that evaluated the safety, tolerability and PK of intramuscular (IM) injected DUR-928. The 24-subject study (16 healthy volunteers on the drug and 8 on placebo) of four escalating dose levels resulted in dose proportional systemic exposure of DUR-928. DUR-928 was well-tolerated at all dose levels, with no serious treatment-related adverse events reported. We also conducted a multiple-dose study involving 10 healthy subjects, in which participants received IM-injected DUR-928 for 5 consecutive days (8 subjects on the drug, 2 on placebo) using the next to highest dose from the single dose study. No serious treatment related adverse events were reported, no subjects withdrew from the study, no accumulation in plasma concentrations were observed with repeat dosing, and the pain scores and injection site reactions were minimal. We also conducted a single-ascending dose intravenous (IV) infusion study with 16 healthy subjects and observed no treatment-related serious adverse events. The systemic exposure following IV infusion was dose proportional.
A Phase 1 drug-drug interaction study conducted in healthy subjects demonstrated that neither orally administered nor intravenously injected DUR-928 at doses tested affected the safety and PK of midazolam, a drug metabolized by CYP3A4, which is one of the important enzymes associated with clinically relevant drug-drug interactions.
We have also conducted a Phase 1b study with injected DUR-928 in patients with impaired kidney function (stage 3 and 4 chronic kidney disease (CKD)) and matched control subjects (MCS), matched by age, body mass and gender with normal kidney function. This study was a single-site, open-label, single-ascending-dose study in two successive cohorts (first a low dose of 30 mg and then a high dose of 120 mg) evaluating safety and PK of intramuscular injected DUR-928. The low dose cohort consisted of 6 patients with CKD and 3 MCS; the high dose cohort consisted of 5 CKD patients and 3 MCS. In this trial, DUR-928 was well tolerated among all subjects and the PK parameters between the kidney function impaired patients and the MCS were comparable.
Chronic Liver Disease Program with Orally Administered DUR-928
Market Opportunity. Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in both children and adults. It is estimated that NAFLD affects approximately 30% to 40% of adults and 10% of children in the United States. Non-alcoholic steatohepatitis (NASH), a more severe and progressive form of NAFLD, is one of the most common chronic liver diseases worldwide, with an estimated prevalence of 3-5% globally. No drug is currently approved for treatment of NAFLD or NASH. In addition to these liver diseases, there are a number of orphan liver diseases for which we may seek to develop DUR-928.
Clinical Program. In 2020, we completed a Phase 1b randomized, multi-center, and open-label clinical study in the United States to evaluate safety, PK and signals of biological activity of DUR-928 in NASH patients with stage 1-3 fibrosis. DUR-928 (at doses of 50 mg QD, 150 mg QD and 300 mg BID) was administered orally for 28 days with 20 patients or more per dose group for a total of 65 patients in the trial as shown below. Key endpoints included safety and PK, clinical chemistry and biomarkers (e.g., ALT, AST, GGT, triglycerides, Non-HDL-C, CK-18s, inflammatory cytokines) as well as liver fat content and liver stiffness by imaging (e.g., MRI-PDFF and FibroScan®).
24
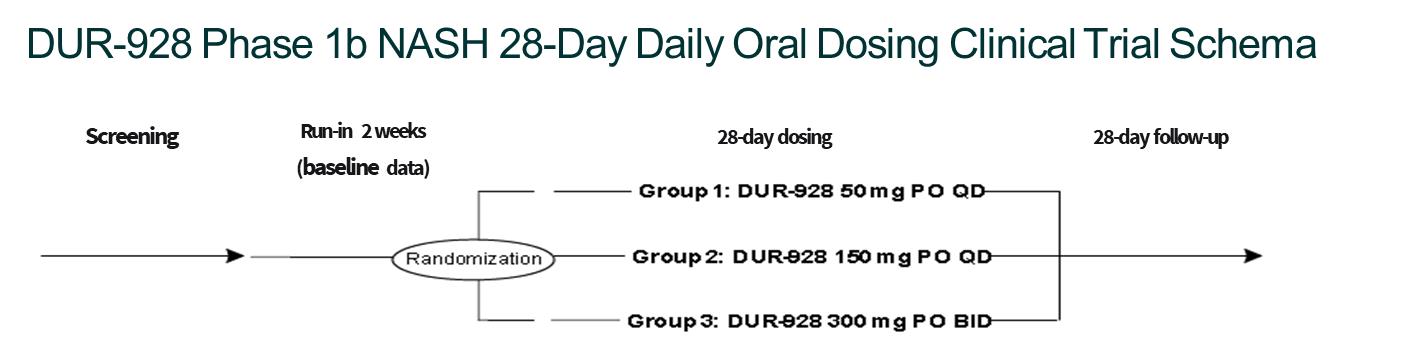
In May 2020, we reported positive topline results from this Phase 1b clinical study of orally administered DUR-928 in NASH patients. In November 2020, we presented additional safety data and efficacy signals at the The AASLD Liver Meeting Digital Experience™ (TLMdX).
A total of 65 patients completed the study, in which DUR-928 was orally administered daily for 4 weeks at 50 mg (n=23), 150 mg (n=21), or 600 mg (300 mg BID (n=21)) and followed up for an additional 4 weeks. Both the 50 mg and 600 mg dose groups showed a statistically significant median reduction at day 28 from baseline of serum alanine aminotransferase (ALT) levels at -16% and -17%, respectively. The 600 mg dose group also showed statistically significant median reductions at day 28 from baseline of serum aspartate aminotransferase (AST) (-18%) and gamma-glutamyl transferase (GGT) (-8%), and the 50 mg dose group had a statistically significant reduction at day 28 from baseline in liver stiffness as measured by Fibroscan (-10%).
Patients in the 50 mg or 150 mg dose groups also had statistically significant median reduction at day 28 from baseline of serum triglycerides (-13% in the 50 mg group) or LDL-C (-11% in the 150mg group). Patients with elevated baseline triglycerides (≥200 mg/dL; n=16) across all dose groups had a median reduction at day 28 from baseline of -24% (p <0.01).
At day 28, 43% of patients in all three dose groups showed ≥ 10% liver fat reduction from baseline as measured by magnetic resonance imaging - proton density fat fraction (MRI-PDFF). In this subgroup, there was a significant reduction from baseline in median liver fat content (-18%, -19%, and -23%, in the 50 mg, 150 mg and 600 mg groups respectively). The reduction of liver fat content was accompanied by a significant median reduction from baseline of serum ALT (-21%, -19%, and -32%, in the 50 mg, 150 mg and 600 mg groups respectively), as well as both CK-18, M30 and M65 in the 50 mg and 600 mg groups. We may report additional efficacy signals of this trial at a future medical conference.
DUR-928 was well tolerated at all three doses evaluated. There were no serious adverse events reported during the study, and no discontinuations, early withdrawals or termination of study drug or study participation due to adverse events. PK parameters after repeat dosing were comparable to those after a single dose (from a prior study), indicating no accumulation of the drug after repeat dosing. We will present additional data from this trial at an upcoming medical conference and we are working with a number of disease experts to determine next steps for DUR-928 in NASH.
25
Topline Data Summary (Day 28 vs Baseline)
|
Median at Day 28 * p < 0.05 ** p < 0.01 *** p < 0.001 |
All Subjects |
Patients with ≥ 10% Reduction in MRI-PDFF |
|||||
|
50 mg QD (n=21-23) |
150 mg QD (n=20-21) |
300 mg BID (n=20-21) |
50 mg QD (n=9) |
150 mg QD (n=8) |
300 mg BID (n=9) |
||
|
Liver Enzymes |
ALT |
-16%* |
-10% |
-17%*** |
-21%** |
-19%* |
-32%*** |
|
AST |
-14% |
-9% |
-18%** |
-24%** |
-21% |
-39%*** |
|
|
GGT |
-6% |
-1% |
-8%* |
-13%*** |
-16%* |
-14% |
|
|
Imaging |
MRI-PDFF |
-7% |
-7% |
-4% |
-18%*** |
-19%*** |
-23%*** |
|
FibroScan |
-10%** |
-9% |
-1% |
-7% |
-9%** |
-9% |
|
|
Serum Lipids |
LDL-C |
-6% |
-11%* |
-7% |
-7% |
-11% |
-8%* |
|
Non-HDL-C |
-8% |
-5% |
-1% |
-10% |
-8%* |
-12%* |
|
|
Triglycerides |
-13%* |
-3% |
-2% |
-9% |
0% |
-8% |
|
|
Biomarkers |
CK-18, M30 |
-14.6% |
-8.6% |
-16.1% |
-22.8%*** |
-3.8% |
-42.1%* |
|
CK-18, M65 |
-18.1% |
-9.9% |
-35.0% |
-28.1%*** |
-8.7% |
-55.8%* |
|
ALT (alanine aminotransferase); AST (aspartate aminotransferase); GGT (gamma-glutamyl transferase); MRI-PDFF (Magnetic Resonance Imaging - Proton Density Fat Fraction) is a non-invasive measure of the proportion of liver tissue which is composed of fat; FibroScan is a specialized ultrasound machine that measures the stiffness of liver tissue. LDL-C ( Low-Density Lipoprotein – Cholesterol); Non-HDL-C (Total cholesterol excluding High-Density Lipoprotein-Cholesterol); QD (once a day); BID (twice a day); CK-18 (cytokeritin 18)
We have completed multiple Phase I trials in healthy subjects with orally administered DUR-928. These included single-ascending-dose and multiple-ascending-dose studies as well as a food effect study. In all of these studies DUR-928 was well-tolerated at all dose levels, with no serious treatment-related adverse events reported. Dose related increases in plasma concentrations were observed and no accumulation in plasma concentrations or food effects were observed with repeat dosing.
We also conducted a Phase 1b trial in cirrhotic and non-cirrhotic NASH patients and matched control subjects (MCS) (matched by age, body mass index and gender with normal liver function) utilizing orally administered DUR-928. This was an open-label, single-ascending-dose safety and PK study conducted in Australia in two successive dose cohorts (first a low dose of 50 mg and then a high dose of 200 mg). Both cohorts consisted of 10 NASH patients and 6 MCS. Data from this study was presented at the International Liver Congress™ 2017 organized by the European Association for the Study of the Liver (EASL) in Amsterdam in April 2017. All patients and MCS in this study tolerated DUR-928 well. One patient (with a prior history of arrhythmia and an ongoing viral infection) in the high dose cohort experienced a serious adverse event (shortness of breath), which occurred without unusual biochemical changes and resolved without intervention but was considered possibly treatment related by the physician due to its temporal association with dosing. In both low and high dose cohorts, the PK parameters were comparable between the NASH patients and the MCS. In addition, the systemic exposure following the low and high doses of DUR-928 was dose dependent.
26
While this study was not designed to assess efficacy, we observed statistically significant reductions from baseline of several biomarkers after both doses of DUR-928. A single oral dose of DUR-928 significantly reduced the levels of both full-length (M65) and cleaved (M30) cytokeratin-18 (CK-18), bilirubin, hsCRP, and IL-18 in these subjects. The mean decrease of full-length CK-18 (a generalized cell death marker) at the measured time point of greatest effect (12 hours after dosing) was 33% in the low dose cohort and 41% in the high dose cohort. The mean decrease of cleaved CK-18 (a cell apoptosis marker) at the measured time point of greatest effect (12 hours after dosing) was 37% in the low dose cohort and 47% in the high dose cohort. The mean reduction of total bilirubin (a liver function marker) at the measured time point of greatest effect (12 hours after dosing) was 27% in the low dose cohort and 31% in the high dose cohort. The mean decrease of high sensitivity C-Reactive Protein (hsCRP) (a marker of inflammation) at the measured time point of greatest effect (24 hours after dosing) was 8% in the low dose cohort and 13% in the high dose cohort. The mean decrease of IL-18 (an inflammatory mediator) at the measured time point of greatest effect (8 hours after dosing) was 4% in the low dose cohort and 8% in the high dose cohort.
Collectively, the biological signals observed in NASH patients plus results from our animal and cell culture studies suggest potential therapeutic activity of DUR-928 for patients with liver diseases. However, additional studies are required to evaluate the safety and efficacy of DUR-928, and there is no assurance that these biomarker, clinical chemistry and liver imaging effects will be associated with clinically relevant benefits, or that DUR-928 will demonstrate safety or efficacy in treating liver diseases in our ongoing or future trials.
We have also conducted a Phase 1b safety and PK study in subjects with moderate and severe hepatic function impairment (Child-Pugh Class B (n=10) and C (n=7) and matched control subjects (n=10)) following a single oral dose of DUR-928 to support potential inclusion of patients with advanced fibrosis (f4) and cirrhosis in future clinical trials. DUR-928 was well tolerated by these subjects with no drug-related serious adverse events reported in this trial. We plan to report results of this trial at a future medical conference.
27
Approved and Commercial Pharmaceutical Products

POSIMIR® (bupivacaine solution)
POSIMIR (bupivacaine solution) for infiltration use is a novel and proprietary product that combines the strength of 660 mg of bupivacaine base with the innovative SABER® platform technology, enabling continuous sustained delivery of a non-opioid local analgesic over 3 days in adults, which we believe coincides with the time period of the greatest need for post-surgical pain control in most patients. POSIMIR contains more bupivacaine than any other approved single-dose sustained-release bupivacaine product. At the end of surgery, POSIMIR is administered into the subacromial space under direct arthroscopic visualization, where it continuously releases bupivacaine for 72 hours or more. We are in discussions with potential licensees regarding commercialization rights to POSIMIR, for which we hold worldwide rights.
In February 2021, the U.S. Food and Drug Administration (FDA) approved POSIMIR for infiltration use in adults for administration into the subacromial space under direct arthroscopic visualization to produce post-surgical analgesia for up to 72 hours following arthroscopic subacromial decompression. The approval was based on positive data from a randomized, multicenter, assessor-blinded, placebo‑controlled clinical trial in patients undergoing arthroscopic subacromial decompression surgery with an intact rotator cuff. The primary outcome measures were mean pain intensity and total opioid rescue analgesia administered, both evaluated over the first 72 hours after surgery versus placebo. POSIMIR demonstrated a statistically significant improvement in both primary outcome measures: a 1.3 point, or 20%, reduction in mean pain intensity on a 0-10 point pain scale (p=0.01), and a 67% reduction in I.V. morphine-equivalent rescue opioid use, from a median of 12 mg in the placebo group to 4 mg in the POSIMIR group (p=0.01). In connection with this approval, we or our licensee, will be required to conduct two postmarketing non-clinical studies. Full Prescribing Information, including the Boxed Warning, is available at www.POSIMIR.com.
Market Opportunity. Arthroscopic Subacromial Decompression (ASD) Shoulder Surgery
Subacromial decompression is a type of shoulder surgery used to treat impingement syndrome, a common repetitive-use injury that causes pain when the arm is raised over the head. The procedure is typically performed arthroscopically, meaning that several small incisions are made in the skin and muscle of the shoulder through which a camera lens (arthroscope) and surgical instruments are inserted during surgery. Arthroscopic subacromial decompression is generally considered outpatient surgery, and most patients go home within a few hours of surgery. The recovery period may extend from weeks to months, but the most intense pain typically occurs during the first 3 days after surgery and is often managed with oral opioids. There are over 600,000 surgeries involving arthroscopic subacromial decompression performed each year in the U.S.
While ASD is the first approved indication for POSIMIR, there may be opportunities to expand the indication and addressable patient population based on existing data or data generated from additional POSIMIR trials. According to data published by the Center for Disease Control and Prevention, there are approximately 72 million ambulatory and inpatient surgical procedures performed annually in the U.S. Insufficient postoperative pain control remains a significant problem, with studies indicating that roughly 65% of patients experience moderate-to-extreme pain after surgery. The current standard of care for post-surgical pain
28
includes a variety of opiate and non-opiate analgesics and muscle relaxants. While systemic opioids can effectively reduce post-surgical pain, they commonly cause side effects including drowsiness, constipation, nausea and vomiting, and cognitive impairment, and have the potential for addiction and abuse. Post-surgical pain also can be treated effectively with local anesthetics; however, their usefulness often is limited by their short duration of action.
PERSERIS™(risperidone)
In September 2017, we entered into an agreement with Indivior, under which we assigned to Indivior certain patents that may provide further intellectual property protection for PERSERIS, Indivior’s extended-release injectable suspension for the treatment of schizophrenia in adults. In consideration for such assignment, Indivior has made non-refundable upfront and milestone payments to DURECT totaling $17.5 million. Additionally, under the terms of the agreement with Indivior, DURECT receives quarterly earn-out payments that are based on a single digit percentage of U.S. net sales of PERSERIS into 2026. Indivior commercially launched PERSERIS in the U.S. in February 2019.
ORADUR-ADHD Program
In collaboration with Orient Pharma (OP), we developed a drug candidate based on our ORADUR technology for the treatment of ADHD. This drug candidate is intended to provide once-a-day dosing. In August 2009, we entered into a development and license agreement, as amended, with OP, a diversified multinational pharmaceutical, healthcare and consumer products company with headquarters in Taiwan, under which we granted to OP development and commercialization rights in certain defined Asian and South Pacific countries to ORADUR-Methylphenidate ER (Methydur). We retain rights to North America, Europe and all other countries not specifically licensed to OP.
OP has informed us that they launched Methydur commercially in Taiwan in September 2020. Methydur is indicated for the treatment of ADHD and is available in three strengths (22 mg, 33 mg and 44 mg) in Taiwan. They may seek a partner in other countries within their territory, including China and may pursue regulatory approvals in selected other countries in Southeast Asia where OP has commercialization rights and a commercialization presence. We receive a single digit royalty on sales of Methydur by OP and retain rights to this product in markets not specifically licensed to OP.
Drug Delivery Technologies and Programs
Our drug delivery technologies are designed to deliver the right drug to the right place, in the right amount and at the right time to treat a variety of chronic, acute and episodic diseases and conditions. We aim to improve therapy for a given disease or patient population by controlling the rate and duration of drug administration. In addition, if advantageous for the therapy, our technologies can target the delivery of the drug to its intended site of action.
Our technologies are suitable for providing long-term drug therapy because they can often store highly concentrated, stabilized drugs in a small volume and protect the drug from degradation by the body. This, in combination with the ability to continuously deliver desired doses of a drug, can extend the therapeutic value of a wide variety of drugs, including, in some cases, those which would otherwise be ineffective, too unstable, too potent or cause adverse side effects. In some cases, delivering the drug directly to the intended site of action can improve efficacy while minimizing unwanted side effects elsewhere in the body, which often limit the long-term use of many drugs. Our technologies may thus provide better therapy for chronic diseases or conditions, or for certain acute conditions where longer drug dosing is required or advantageous, by replacing multiple injection therapy or oral dosing, improving drug efficacy, reducing side effects and ensuring dosing compliance. Our technology may thereby improve patients’ quality of life by eliminating more repetitive treatments, reducing dependence on caregivers and allowing patients to lead more independent lives.
We currently have several active drug delivery technology platforms:
The SABER and CLOUD Bioerodible Injectable Depot Systems
Our bioerodible injectable depot systems include our SABER and CLOUD platform technologies. SABER uses a high viscosity base component, such as sucrose acetate isobutyrate (SAIB), to provide controlled release of a drug. When the high viscosity SAIB is formulated with drug, biocompatible excipients and other additives, the resulting formulation is easily injectable with standard syringes and needles. After injection of a SABER formulation, the excipients diffuse away over time, leaving a viscous depot which provides controlled sustained release of drug. CLOUD is a class of bioerodible injectable depot technology which generally does not contain SAIB but includes various other release rate modifying excipients and/or bioerodible polymers to achieve the delivery of drugs for periods of days to months from a single injection.
The SABER technology is the basis of POSIMIR (described above). The SABER technology is also utilized in our ophthalmic program with Santen Pharmaceutical Co., Ltd. (Santen), as well as in feasibility programs.
The SABER technology is also the basis for SucroMate™ Equine, an injectable animal health drug utilizing our SABER technology to deliver the peptide deslorelin. This was the first FDA approved SABER injectable product when it was launched in 2011 by CreoSalus, Inc.
29
Sustained Release Gel Cap Technology
We believe that our ORADUR sustained release technology can transform short-acting oral capsule dosage forms into sustained release oral products. Products based on our ORADUR technology can take the form of an easy to swallow capsule that uses a high-viscosity base component such as sucrose acetate isobutyrate (SAIB) to provide controlled release of active ingredients for an extended period of time. Oral dosage forms based on the ORADUR gel-cap may also have the added benefit of being less prone to abuse (e.g., by crushing and then snorting, smoking, injecting or extracting by mixing with alcohol or water). These properties have the potential to make ORADUR-based products an attractive option for pharmaceutical companies that seek to develop abuse-deterrent oral products.
The ORADUR technology is the basis of our ORADUR-Methylphenidate ER program (described above).
Product Revenues
We also currently generate product revenue from the sale of two product lines:
|
|
• |
ALZET® osmotic pumps which are used for animal research; and |
|
|
• |
certain key excipients that are included in Methydur and one excipient that is included in a marketed animal health product. |
In December 2020, we completed the sale of our LACTEL Absorbable Polymers product line to Evonik Corporation. Under the terms of the Asset Purchase Agreement, Evonik paid us approximately $15 million subject to certain adjustments, and also agreed to assume certain liabilities with respect to the transferred assets and assembled workforce. LACTEL product line is reflected as a discontinued operation in our statements of operations and comprehensive loss and balance sheets. Our financial information discussed in the Management’s Discussion and Analysis of Financial Condition and Results of Operations excludes the impact of LACTEL product line.
Because we consider our core business to be developing and commercializing pharmaceuticals, we do not intend to significantly increase our investments in or efforts to sell or market any of our existing product lines. However, we expect that we will continue to make efforts to increase our revenues related to collaborative research and development by entering into additional research and development agreements with third-party collaborators to develop product candidates based on our drug delivery technologies.
Operating Results
Since our inception in 1998, we have had a history of operating losses. At March 31, 2021, we had an accumulated deficit of $499.9 million. Our net loss was $10.1 million for the three months ended March 31, 2021 compared with $9.9 million for the corresponding period in 2020. Our losses have resulted primarily from costs incurred to research and develop our product candidates and, to a lesser extent, from selling, general and administrative costs associated with our operations and product sales. We expect our research and development expenses in the near future to increase compared to the first quarter of 2021 as we experience higher research and development expenses related to DUR-928. We expect selling, general and administrative expenses excluding the effects of stock-based compensation in the near future to be comparable to the first quarter of 2021. We do not anticipate meaningful revenues from our products in development, should they be approved, for at least the next twelve months. Therefore, we expect to incur continuing losses and negative cash flows from operations for the foreseeable future. Depending on whether we enter into additional collaborative agreements in the near term and the extent to which we earn milestone revenues, we may be required to raise additional capital through a variety of sources.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. The most significant estimates and assumptions relate to revenue recognition, prepaid and accrued contract research expenses, and stock-based compensation. Actual amounts could differ significantly from these estimates. There have been no material changes to our other critical accounting policies and estimates as compared to the disclosures in our annual report on Form 10-K for the year ended December 31, 2020.
Results of Operation
Three months ended March 31, 2021 and 2020
Collaborative research and development and other revenue
We recognize revenues from collaborative research and development activities and service contracts. Collaborative research and development revenue primarily represents reimbursement of qualified expenses related to collaborative agreements with various third parties to research, develop and commercialize potential products using our drug delivery technologies, and revenue from the recognition of upfront fees and milestone payments in connection with our collaborative agreements.
30
We expect our collaborative research and development and other revenue to fluctuate in future periods depending on the success of our efforts to enter into potential new collaborations, our existing third-party collaborators’ commitment to and progress in the research and development programs, and any royalty or earn-out revenue recognized from collaborators or counterparties.
The collaborative research and development and other revenues associated with the Company’s collaborators or counterparties were $574,000 for the three months ended March 31, 2021, compared to $(30,000) for the corresponding period in 2020. These revenues were primarily related to revenue recognized associated with the Company’s license and feasibility agreements.
The collaborative research and development and other revenues associated with our collaborators or counterparties are as follows (in thousands):
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
Collaborator/Counterparty |
|
|
|
|
|
|
|
|
|
Gilead (1) |
|
$ |
— |
|
|
$ |
(268 |
) |
|
Others (2) |
|
|
574 |
|
|
|
238 |
|
|
Total collaborative research and development and other revenue |
|
$ |
574 |
|
|
$ |
(30 |
) |
|
(1) |
Amounts related to recognition of an upfront license fee and milestone payment were zero for the three months ended March 31, 2021 compared to $(465,000) for the corresponding period in 2020. We signed a license agreement with Gilead on July 19, 2019 and received a nonrefundable upfront license fee and a milestone payment totaling $35.0 million in 2019 which was being recognized as revenue as its obligation was being satisfied using the cost-to-cost input method. We recorded a net revenue reversal of $465,000 related to the upfront license fee and milestone payment in the three months ended March 31, 2020 due to a change in our estimated costs to complete our obligations under the license agreement, which was partially offset by $197,000 of reimbursable collaborative research and development services with Gilead earned during the quarter. In June 2020, we received notice that Gilead was terminating the License Agreement and a related R&D agreement between Gilead and us and as a result, we recognized all our remaining deferred revenue as there were no remaining substantive performance obligations to be provided to Gilead by us as of the date when the termination notice was received. |
|
(2) |
Includes: (a) amounts related to earn-out revenue from Indivior UK Limited (Indivior) with respect to PERSERIS net sales; (b) feasibility programs; (c) research and development activities funded by Santen Pharmaceutical Co. Ltd. (Santen); and (d) royalty revenue from OP Pharma with respect to Methydur net sales. Note that in January 2018, we were notified by Santen that due to a shift in priorities, Santen had elected to reallocate research and development resources and put our program on pause until further notice. While the main program is on pause, the parties are working together on a limited set of research and development activities funded by Santen. |
Product revenue
In December 2020, we completed the sale of our LACTEL Absorbable Polymers product line to Evonik Corporation. Revenue from LACTEL product line, related cost of product revenues, associated research and development and selling, general and administrative has been reclassified to discontinued operations the three months ended March 31, 2020.
A portion of our revenues is derived from product sales, which include our ALZET mini pump product line, and certain excipients that are included in Methydur and in a currently marketed animal health product. Net product revenues were $1.6 million in each of the three months ended March 31, 2021 and 2020. Product revenues from our ALZET mini pump product line in the three months ended March 31, 2021 were comparable to the corresponding period in 2020 despite of the impact of COVID-19.
COVID-19 may have an adverse impact on the economies and financial markets of many countries, resulting in a severe and prolonged global economic downturn that could affect demand for our ALZET product line. We expect that our product revenues may fluctuate compared to historic levels as a result of the COVID-19 pandemic, as some customers may be limiting or reducing their operations or requesting changes to payment terms.
Cost of product revenues
Cost of product revenues was $352,000 for the three months ended March 31, 2021 compared with $396,000 for the corresponding period in 2020. Cost of product revenues in the three months ended March 31, 2021 decreased primarily due to lower manufacturing costs for the units sold from our ALZET product line compared to the corresponding period in 2020. Stock-based compensation expense recognized related to cost of product revenues was $5,000 for the three months ended March 31, 2021 compared to $2,000 for the corresponding period in 2020.
We had 10 manufacturing employees as of March 31, 2021 compared with 9 as of March 31, 2020. We expect the number of employees involved in manufacturing will remain comparable in the near future.
31
Research and development
Research and development expenses are primarily comprised of salaries, benefits, stock-based compensation and other compensation cost associated with research and development personnel, overhead and facility costs, preclinical and non-clinical development costs, clinical trial and related clinical manufacturing costs, contract services, and other outside costs.
Research and development expenses were $8.0 million for the three months ended March 31, 2021 compared to $7.6 million for the corresponding period in 2020. The increase in the three months ended March 31, 2021 was primarily attributable to higher research and development costs associated with DUR-928, the depot injectable programs and other research programs, partially offset by lower research and development costs associated with the Gilead program and POSIMIR, compared to the corresponding period in 2020, as more fully discussed below. Stock-based compensation expense recognized related to research and development personnel was $315,000 for the three months ended March 31, 2021compared to $207,000 for the corresponding periods in 2020. As of March 31, 2021, we had 46 research and development employees compared with 43 as of March 31, 2020. We expect research and development expenses in the near future to increase compared to the first quarter of 2021 as we expect to incur higher research and development expenses for DUR-928.
|
|
|
Three months ended March 31, |
|
|||||
|
|
|
2021 |
|
|
2020 |
|
||
|
DUR-928 |
|
$ |
5,516 |
|
|
$ |
4,991 |
|
|
POSIMIR |
|
|
1,674 |
|
|
|
1,691 |
|
|
Depot injectable programs |
|
|
262 |
|
|
|
177 |
|
|
Gilead |
|
|
65 |
|
|
|
447 |
|
|
Others |
|
|
458 |
|
|
|
281 |
|
|
Total research and development expenses |
|
$ |
7,975 |
|
|
$ |
7,587 |
|
DUR-928
Our research and development expenses for DUR-928 were $5.5 million the three months ended March 31, 2021 compared to $5.0 million for the corresponding period in 2020. The increase in the three months ended March 31, 2021 was primarily due to higher contract manufacturing expenses and higher employee related costs for this drug candidate compared with the corresponding period in 2020.
We continue to assess the impact of the COVID-19 outbreak on our business, including our DUR-928 Phase 2b trial in alcohol-associated hepatitis, but COVID-19 may affect our ability to initiate and/or complete recruitment and data analysis for our clinical trials, including DUR-928 trials, in our planned timeframe.
POSIMIR
Our research and development expenses for POSIMIR, consisting primarily of consulting expenses and employee related costs, were $1.7 million in each of the three months ended March 31, 2021 and 2020.
It is possible that the COVID-19 pandemic will adversely impact our efforts to out-license POSIMIR commercial rights and the timing of potential commercialization of POSIMIR.
Depot injectable programs
Our research and development expenses for depot injectable programs were $262,000 in the three months ended March 31, 2021 compared to $177,000 for the corresponding period in 2020. The increase in the three months ended March 31, 2021 was primarily due to higher employee-related costs and higher outside expenses for these programs compared with the corresponding period in 2020.
Gilead Program
Our research and development expenses for the Gilead program were $65,000 in the three months ended March 31, 2021 compared to $447,000 for the corresponding period in 2020. The decrease in the three months ended March 31, 2021 was primarily due to lower employee-related costs and outside costs devoted to the program compared with the corresponding period in 2020. In June 2020, Gilead provided notice that it was terminating the Gilead Agreement and a related R&D agreement between us.
Other DURECT research programs
Our research and development expenses for all other programs were $458,000 in the three months ended March 31, 2021 compared to $281,000 for the corresponding period in 2020. The increase in the three months ended March 31, 2021 was primarily due to higher employee-related costs associated with these programs compared with the corresponding period in 2020.
We cannot reasonably estimate the timing and costs of our research and development programs due to the risks and uncertainties associated with developing pharmaceuticals as outlined in the “Risk Factors” section of this report. The duration of development of our research and development programs may span as many as ten years or more, and estimation of completion dates or costs to
32
complete would be highly speculative and subjective due to the numerous risks and uncertainties associated with developing pharmaceutical products, including significant and changing government regulation, the uncertainties of future preclinical and clinical study results, uncertainties related to the COVID-19 pandemic, the uncertainties with our collaborators’ commitment to and progress in the programs and the uncertainties associated with process development and manufacturing as well as sales and marketing. In addition, with respect to our development programs subject to third-party collaborations, the timing and expenditures to complete the programs are subject to the control of our collaborators. Therefore, we cannot reasonably estimate the timing and costs of the efforts necessary to complete the research and development programs. For additional information regarding these risks and uncertainties, see “Risk Factors” above.
Selling, general and administrative. Selling, general and administrative expenses are primarily comprised of salaries, benefits, stock-based compensation and other compensation costs associated with finance, legal, business development, sales and marketing and other administrative personnel, overhead and facility costs, and other general and administrative costs.
Selling, general and administrative expenses were $3.5 million in the three months ended March 31, 2021 compared to $3.4 million for the corresponding period in 2020. Selling, general and administrative expenses increased in the three months ended March 31, 2021 primarily due to higher employee related costs for selling, general and administrative personnel. Stock-based compensation expense recognized related to selling, general and administrative personnel was $382,000 for the three months ended March 31, 2021 compared to $178,000 for the corresponding periods in 2020.
We had 25 selling, general and administrative employees as of March 31, 2021 and March 31, 2020. We expect selling, general and administrative expenses in the near future to be comparable to the first quarter of 2021.
Other income (expense). Interest and other income was $37,000 for the three months ended March 31, 2021 compared to $258,000 for the corresponding period in 2020. The decrease in interest and other income in the three months ended March 31, 2021 was primarily due to lower interest rates associated with our cash and investments compared with the corresponding period in 2020.
Interest and other expense was $525,000 for the three months ended March 31, 2021 compared to $592,000 for the corresponding period in 2020. The decrease in interest and other expense in the three months ended March 31, 2021 was primarily due to lower interest rates associated with the term loan with Oxford Finance compared with the corresponding period in 2020.
Liquidity and Capital Resources
We had cash, cash equivalents and investments totaling $97.2 million at March 31, 2021 compared to cash, cash equivalents, cash held in escrow and investments of $56.9 million at December 31, 2020. These balances include $150,000 of interest-bearing marketable securities classified as restricted investments on our balance sheets as of March 31, 2021 and December 31, 2020. The increase in cash, cash equivalents and investments during the three months ended March 31, 2021 was primarily due to cash received from the February 2021 equity financings and from the exercise of stock options, cash received from the December 2020 sale of the LACTEL product line as well as payments received from collaboration partners and customers, partially offset by cash used in ongoing operating activities and interest payments.
We used $10.7 million of cash in operating activities in the three months ended March 31, 2021 compared to $12.9 million received for the corresponding period in 2020. The cash used for operations was primarily to fund operations as well as our working capital requirements, partially offset by the changes in account receivable, prepaid expenses and other assets, and accrued and other liabilities.
We used $4.1 million of cash in investing activities for the three months ended March 31, 2021 compared to $9.8 million provided by investing activities for the corresponding period in 2020. The decrease in cash provided by investing activities was primarily due to an increase in net purchases of available-for-sale securities for the three months ended March 31, 2021 compared to the corresponding period in 2020, partially offset by cash received from the sale of the LACTEL product line.
We received $51.0 million of cash from financing activities for three months ended March 31, 2021 compared to $760,000 for the corresponding period in 2020. The increase in cash received from financing activities was primarily due to higher net proceeds received from our February 2021 underwritten public offering of our common stock and from the exercise of stock options in the three months ended March 31, 2021 compared with the corresponding period in 2020.
We anticipate that cash used in operating activities in the near future will increase compared to the first quarter of 2021 as we experience higher research and development expenses related to DUR-928.
In August 2018, we filed a shelf registration statement on Form S-3 with the SEC (the “2018 Registration Statement”) (File No. 333-226518), which upon being declared effective in October 2018, terminated our registration statement filed in November 2015 (File No. 333-207776) and allowed us to offer up to $175.0 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of our common stock which we may sell, subject to certain limitations, pursuant to a sales agreement dated November 3, 2015 with Cantor Fitzgerald & Co. (the “2015 Sales Agreement”).
In February 2021, we completed an underwritten public offering of 20,364,582 shares of our common stock at a price of $2.2386 per share pursuant to an underwriting agreement with Cantor Fitzgerald & Co., raising total gross proceeds of approximately
33
$45.6 million before deducting estimated offering expenses. Total stock issuance costs related to this financing were approximately $195,000, resulting in net proceeds of approximately $45.4 million. From January 1, 2021 to March 31, 2021, we also raised net proceeds (net of commissions) of approximately $2.4 million from the sale of 950,009 shares of our common stock in the open market at a weighted average price of $2.60 per share pursuant to the October 2018 registration statement and 2015 Sales Agreement. As of May 3, 2021, we had up to approximately $56.2 million of common stock available for sale under the Controlled Equity Offering program and approximately $39.3 million of common stock available for sale under our shelf registration statement.
Any material sales in the public market of our common stock, under the 2015 Sales Agreement or otherwise under the 2018 Registration Statement, could adversely affect prevailing market prices for our common stock.
During the three months ended March 31, 2021, there were no significant changes in our commercial commitments and contractual obligations as compared with the information presented in our Annual Report on Form 10-K for the year ended December 31, 2020.
The COVID-19 pandemic is impacting our business in several ways. COVID-19 initially also had a negative impact on orders for our ALZET product line as many ALZET customers reduced their activities during the pandemic. ALZET orders have recovered significantly in 2021, a trend that may or may not continue as the impact of the pandemic has proven to be difficult to predict. For DUR-928, the Company may experience delays in patient enrollment of the Phase 2b clinical trial in patients with alcohol-associated hepatitis (AH) or disruptions in supplies of DUR-928 or other items required for clinical trials. These delays and potential disruptions will increase the overall costs of development of DUR-928. The Company is actively monitoring the impact of COVID-19 and the possible effects on its financial condition, liquidity, operations, clinical trials, suppliers, industry and workforce. However, the full extent, consequences, and duration of the COVID-19 pandemic and the resulting impact on the Company cannot currently be predicted. The Company will continue to evaluate the impact that these events could have on the Company’s operations, financial position, and the results of operations and cash flows. Additional volatility in capital markets and/or clinical trial delays resulting from the impacts of COVID-19 may also limit our ability to raise capital on acceptable terms, if at all.
We believe that our existing cash, cash equivalents and investments and anticipated revenues will be sufficient to fund our planned operations, existing debt and contractual commitments and planned capital expenditures through at least the next 12 months from the date these interim condensed financial statements are filed. We may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. Additionally, we do not expect to generate significant revenues from our pharmaceutical product candidates currently under development for at least the next twelve months, if at all. Depending on whether we enter into additional collaborative agreements in the near term and the extent to which we earn milestone revenues, we may be required to raise additional capital through a variety of sources, including:
|
|
• |
the public equity markets; |
|
|
• |
private equity financings; |
|
|
• |
collaborative arrangements; and/or |
|
|
• |
public or private debt. |
There can be no assurance that we will enter into additional collaborative agreements, will earn milestone revenues or that additional capital will be available on favorable terms, if at all. If adequate funds are not available, we may be required to significantly reduce or refocus our operations or to obtain funds through arrangements that may require us to relinquish rights to certain of our products, technologies or potential markets, any of which could have a material adverse effect on our business, financial condition and results of operations. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in ownership dilution to our existing stockholders (assuming convertible debt securities were converted into shares).
Our cash and investments policy emphasizes liquidity and preservation of principal over other portfolio considerations. We select investments that maximize interest income to the extent possible given these two constraints. We satisfy liquidity requirements by investing excess cash in securities with different maturities to match projected cash needs and limit concentration of credit risk by diversifying our investments among a variety of high credit-quality issuers.
We are experiencing certain operational and other challenges as a result of the COVID-19 global pandemic, which could delay or halt our research and development programs or clinical programs. See Recent Developments and Item 1A - Risk Factors for further discussion of the current and expected impact on our business and programs.
Off-Balance Sheet Arrangements
As of March 31, 2021, we did not have any off-balance sheet arrangements, as defined under SEC Regulation S-K Item 303(a)(4)(ii).
34
Available information
Our corporate website address is www.durect.com. We use the investor relations page of our website for purposes of compliance with Regulation FD and as a routine channel for distribution of important information, including news releases, analyst presentations, financial information and corporate governance practices. Our filings with the SEC are posted on our website and available free of charge as soon as reasonably practical after they are electronically filed with, or furnished to, the SEC. The SEC's website, www.sec.gov, contains reports, proxy statements and other information regarding issuers that file electronically with the SEC. The content on any website referred to in this Quarterly Report on Form 10-Q is not incorporated by reference in this Form 10-Q unless expressly noted. Further, the Company’s references to website URLs are intended to be inactive textual references only.
|
Item 3. |
Quantitative and Qualitative Disclosures about Market Risk |
During the three months ended March 31, 2021, there have been no significant changes in market risks as disclosed in our Annual Report on Form 10-K for the year ended December 31, 2020.
|
Item 4. |
Controls and Procedures |
Evaluation of Disclosure Controls and Procedures: The Company’s principal executive and principal financial officers reviewed and evaluated the Company’s disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) as of the end of the period covered by this Form 10-Q. Based on that evaluation, the Company’s principal executive and principal financial officers concluded that the Company’s disclosure controls and procedures are effective at ensuring that information required to be disclosed by the Company in reports that the Company files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to management, including the Company’s principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting: There were no significant changes in the Company’s internal control over financial reporting (as defined in Exchange Act Rule 13a-15(f)) during the Company’s most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
35
PART II—OTHER INFORMATION
|
Item 1. |
Legal Proceedings |
We are not a party to any material legal proceedings.
|
Item 1A. |
Risk Factors. |
In addition to the other information in this Form 10-Q, a number of factors may affect our business and prospects. These factors include but are not limited to the following, which you should consider carefully in evaluating our business and prospects. If any of the following risks actually occur, our business, financial condition, results of operations and growth prospects may be materially and adversely affected.
Those risk factors below denoted with a “*” are newly added or have been materially updated from our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or the SEC, on March 5, 2021.
Summary
|
• |
We are dependent on the success of DUR-928 and we cannot be certain that it will ever receive regulatory approval, or if approved, for what indication or be successfully commercialized and the path to regulatory approval is uncertain |
|
• |
Fast Track Designation for DUR-928 in AH may not actually lead to faster FDA review or an approval |
|
• |
Indications of activity from completed DUR-928 clinical trials may not predict safety or efficacy in future trials |
|
• |
Open-label trials of DUR-928 in NASH and AH have inherent limitations |
|
• |
Ongoing and planned clinical trials for DUR-928 may be delayed and may not demonstrate efficacy or safety |
|
• |
Clinical trial delays could increase expenses, delay clinical data and delay or prevent approvals |
|
• |
The COVID-19 outbreak has and will adversely impact our business, including posing challenges to conducting clinical trials |
|
• |
We are in discussion with potential commercial licensees for POSIMIR and may be unable to enter an agreement |
|
• |
The approved indication for POSIMIR may limit its market, and we or a commercial licensee may not be able to expand the label to include additional indications |
|
• |
In connection with the approval of POSIMIR, we are required to conduct two postmarketing non-clinical studies. If the results from either of these studies are unfavorable, the FDA may require additional studies, or may take actions that are adverse to the regulatory status, commercial prospects and market acceptability of POSIMIR |
|
• |
We have a significant amount of debt. Compliance with repayment obligations and other covenants may be difficult; failure to fulfill our obligations may cause the repayment obligations to accelerate |
|
• |
We will require and may have difficulty raising needed capital in the future |
|
• |
For certain of our product candidates, we depend on third-party collaborators, and we have limited or no control over their development, regulatory strategy or potential commercialization |
|
• |
Cancellation of collaborations may adversely affect potential economic benefits |
|
• |
Our revenues may decrease and losses may increase due to the sale of the LACTEL product line in 2020 |
|
• |
Our business strategy includes entering into additional collaborative agreements to support development, clinical trials, manufacturing and commercialization of product candidates. We may not be able to successfully negotiate or enter into acceptable collaboration agreements |
|
• |
We and our third-party collaborators may not be able to manufacture sufficient quantities of our product candidates and components at an acceptable cost or in compliance with applicable government regulations, and we have limited manufacturing experience |
|
• |
Failure to comply with governmental regulations could materially harm our business |
|
• |
We have a history of operating losses, expect to continue to have losses and may never achieve profitability; and we may not successfully manage our company through varying business cycles |
|
• |
We may develop our own sales force and commercial group to market future products but we have limited sales and marketing experience and may not be able to do so effectively |
|
• |
Key product candidate components are provided by a limited number of suppliers, and supply shortages or loss of these suppliers could result in interruptions in supply or increased costs |
|
• |
Write-offs related to impairment of goodwill, long-lived assets, inventories and other non-cash charges may adversely impact profitability and cause cash flows to differ from reported revenues |
|
• |
Global credit and financial market conditions could negatively impact the value of our investments |
|
• |
We depend upon key personnel who may terminate their employment with us at any time, and we may not be able to attract and retain sufficient qualified personnel |
|
• |
Cyber-attacks or other failures in telecommunications or information technology systems could result in information theft, data corruption and significant disruption of our business operations |
36
|
• |
Our corporate headquarters, certain manufacturing facilities and personnel are located in a seismically active area near wildfire zones; our business also involves environmental risks and risks related to handling regulated substances |
|
• |
As a non-accelerated filer, we are not required to comply with auditor attestation requirements of the Sarbanes-Oxley Act and, consequently, investors may find our common stock less attractive |
|
• |
If we are unable to protect, maintain or enforce our IP rights or secure rights to third-party IP, we may lose valuable assets, lose market share or incur costly litigation or our third-party collaborators may choose to terminate their agreements with us, which may depend on our IP |
|
• |
We may be sued by third parties claiming that our product candidates infringe on their IP rights, particularly because there is substantial uncertainty about the validity and breadth of medical patents |
|
• |
Competitive products or technologies could impair our ability to maintain or grow our business |
|
• |
Our relationships with customers and third-party payers are subject to anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings |
|
• |
We could be exposed to significant product liability claims and we are subject to healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages and reputational harm |
|
• |
Healthcare reform measures could hinder or prevent our product candidates’ commercial success |
|
• |
Market acceptance of our product candidates is uncertain, and failure to achieve market acceptance or adequate reimbursement from third-party payers will harm our future revenues and profitability |
|
• |
Inability to train physicians to use our products may prevent market acceptance of our products |
|
• |
Risks related to actions on trade by the U.S. and foreign governments, new accounting pronouncements and legislative actions could adversely affect the Company's results of operations and financial condition |
|
• |
Our operating history makes evaluating our stock difficult, the price of our stock may be volatile |
|
• |
Investors may experience substantial dilution of their investment |
|
• |
Our ability to use net operating losses and other tax attributes is uncertain and may be limited |
|
• |
We have broad discretion over the use of our cash and investments, which may not always yield a favorable return |
|
• |
Our certificate of incorporation, bylaws and Delaware law could discourage an acquisition of us |
|
• |
Having Delaware as the exclusive forum for substantially all disputes between us and our stockholders could limit our stockholders’ ability to obtain a favorable judicial forum for disputes |
37
Risks Related To Our Business
We are dependent on the success of DUR-928 and we cannot be certain that it will receive regulatory approval or be commercialized
Our business depends substantially on the successful development of DUR-928, which has completed a Phase 1b clinical trial in NASH and a Phase 2a clinical trial in AH, and is currently recruiting patients for a Phase 2b clinical trial (AHFIRM) in patients with severe AH. Ongoing and future clinical trials will need to establish clinically and statistically significant proof of efficacy, and/or sufficient evidence of safety to support filing for regulatory approval and/or additional clinical trials and ultimately regulatory approval. DUR-928 will require additional development, including more clinical trials as well as further preclinical studies, including those designed to evaluate its dosage, dosing regimen, toxicology, carcinogenicity, pharmacokinetics and other non-clinical parameters, as well as regulatory clearances before it can be commercialized. Positive results obtained during early development do not necessarily mean later development will succeed or that regulatory clearances will be obtained. Our drug development efforts may not lead to commercial drugs, for several reasons such as if DUR-928 fails to be shown to be safe and effective or if we do not have adequate financial or other resources to advance DUR-928 through the pre-clinical and clinical development and approval processes. We consider DUR-928 to be our lead and most important asset. If DUR-928 fails to demonstrate safety or efficacy at any time or during any phase of development, we would experience potentially significant delays in, or be required to abandon, development of DUR-928, any of which would materially harm our business. Even if the Phase 2b AHFIRM trial successfully demonstrates a survival benefit over placebo, (1) additional clinical trial(s) may be required to support an NDA filing and ultimately to support approval by FDA and/or other regulatory bodies; and (2) accelerated regulatory pathways (such as an FDA priority review designation) may not be available.
We do not anticipate that DUR-928 will be eligible to receive regulatory approval from the FDA or comparable foreign authorities and begin commercialization for a number of years, if ever. Even if we ultimately receive regulatory approval for DUR-928, we or our potential future partners, if any, may be unable to commercialize it successfully for a variety of reasons. These include, for example, the availability of alternative, potentially superior or less expensive treatments or vaccines, lack of cost-effectiveness, the lack of favorable access and/or commercial pricing, the cost or technical challenges of manufacturing the product on a commercial scale and competition with other drugs or vaccines. The success of DUR-928 may also be limited by the prevalence and severity of any adverse side effects, including mortality. If we fail to commercialize DUR-928, we may be unable to generate sufficient revenues to attain or maintain profitability, and our financial condition and stock price may decline.
Fast Track designation of DUR-928 by the FDA may not actually lead to a faster development or regulatory review or approval
The FDA grants Fast Track designation to therapies that are considered capable of addressing unmet medical needs and possess the potential to treat serious or life-threatening disease conditions in order to facilitate its development and expedite the review procedure. Even though DUR-928 has received Fast Track designation for the treatment of AH, we may not experience a faster development process, review or approval compared to conventional FDA procedures, or receive FDA approval at all, in that indication or any other. A Fast Track designation does not change the standards for approval. The FDA may also withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program.
Indications of activity from completed Phase 1 and 2 clinical trials of DUR-928 may not predict safety or therapeutic efficacy in future trials
Although Phase 1 and Phase 2 clinical trials of DUR-928 have shown positive initial data in AH patients, including reductions in bilirubin and MELD scores from baseline and promising Lille scores, and demonstrated in NASH patients that DUR-928 can lead to the reduction from baseline in liver enzymes, liver stiffness and serum lipids as well as certain biomarkers, such initial results, indications of activity and biomarker changes may ultimately not be correlated with treatment or improvement in the associated disease, and there is a risk that DUR-928 may not demonstrate therapeutic efficacy in larger controlled trials, despite encouraging initial data and improvements in biomarker levels in smaller, early trials. The failure of DUR-928 to show efficacy in one indication may negatively affect its perceived value in other indications, and the emergence of safety signals in ongoing or future clinical trials would significantly harm our business.
Open-label trials of DUR-928 in NASH and AH have inherent limitations
The most recently completed NASH and AH trials of DUR-928 are open-label trials with no control groups. Open label trials have inherent risk of bias given that the patients and physicians know that they received active study drug, which can lead to placebo effects. Trials without control groups have an inherent risk in that the comparisons used to determine the study drug’s effect and side effect profile are based on comparisons with baseline (pre-treatment) levels (for blood chemistry and biomarker endpoints) and/or with historical controls, which may not have been conducted under similar enough conditions to make accurate comparisons and/or draw accurate conclusions from those comparisons. Any initial data collected from these open-label trials also cannot be meaningfully analyzed or relied upon until after the completion of the trials due to the limited number of patients involved, open-label nature and lack of control groups. Additionally, larger controlled clinical trials will be required to evaluate the safety and efficacy of DUR-928 to treat any indication, including AH and NASH. There can be no assurance that ongoing or future studies will demonstrate the safety or efficacy of DUR-928 in a statistically significant or clinically meaningful manner.
38
*Ongoing and planned clinical trials for DUR-928 in AH may be delayed and may not demonstrate efficacy or safety in the indications tested
The Phase 2b AHFIRM trial of DUR-928 in patients with AH is subject to potential delays resulting from COVID-19 as well as timing of entering contracts with clinical sites and contract research organizations, obtaining institutional review board approvals and delays in other activities that need to be put in place prior to clinical trial initiation at each clinical trial site. Given uncertainty of COVID-19-related impacts on clinical trial sites in the U.S., U.K., E.U. and Australia, the timing of availability of top-line data from this trial cannot be predicted with certainty. There can be no assurance that the trial will enroll as anticipated if at all, and delays in enrollment could add to the costs and expenses of this trial and harm our business. There can also be no assurance that biological activity demonstrated in previous animal disease models or earlier clinical trials of DUR-928 will also be seen in ongoing trials or future clinical trials, or that any clinically relevant biological activity will be observed, or that enrollment rates will be favorable or that these additional trials will not identify safety issues. Failure of the AHFIRM trial to achieve desired results in its anticipated timeframe would negatively impact our business and ability to raise additional capital.
The COVID-19 outbreak has impacted and will adversely impact our business
The global COVID-19 pandemic has disrupted our operations and delayed our clinical trials. In particular, the COVID-19 pandemic delayed the initiation of our AHFIRM Phase 2b clinical trial to evaluate the safety and efficacy of DUR-928 in severe alcohol-associated hepatitis (AH) patients, and it may delay the pace of enrollment in this trial and other clinical trials. As a result of the COVID-19 pandemic, there are also supply shortages of components needed for commercialization supplies of POSIMIR, which has delayed and may further delay availability of commercial supplies of POSIMIR and may adversely affect the timing for the commercial launch of POSIMIR, and there has been reduced demand for our ALZET products, which are used in scientific and pre-clinical research. In addition, COVID-19 may have an adverse impact on the economies and financial markets of many countries, resulting in a severe and prolonged global economic downturn that could continue to affect demand for our ALZET product lines and POSIMIR and impact our operating results. We also need to raise additional capital to provide sufficient funding to continue our product development efforts, including clinical trials. COVID-19 initially had an adverse impact on the capital markets and could again, which would make it more difficult for companies such as ours to access capital. The extent to which the pandemic impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, and the actions that may be required to contain the COVID-19 virus or treat its impact. As a result of the COVID-19 pandemic, we may continue to experience disruptions that could severely impact our business, preclinical studies and clinical trials including:
|
|
• |
|
delays or difficulties in enrolling patients in our clinical trials; |
|
|
• |
|
delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; |
|
|
• |
|
diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
|
|
• |
|
interruption of key clinical trial activities, such as clinical trial site data monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others or interruption of clinical trial subject visits and study procedures, which may impact the integrity of subject data and clinical study endpoints, the ability to collect, ship and analyze biological samples from clinical trial patients due to concerns about potential contamination of samples and/or exposure of clinical staff to patients with the COVID-19; |
|
|
• |
|
interruption or delays in the operations of the FDA or other regulatory authorities, which may impact review and approval timelines; |
|
|
• |
|
disruption or delays in manufacturing of clinical and commercial supplies due to issues experienced by our contract manufacturing organizations and/or shortages and delays in obtaining raw materials and supplies required in the manufacturing processes; |
|
|
• |
|
interruption of, or delays in receiving, supplies of our product candidates from our contract manufacturing organizations due to staffing shortages, production slowdowns or stoppages, prioritization of pandemic-related activities over ours and disruptions in delivery systems; |
|
|
• |
|
interruptions in preclinical studies due to restricted or limited operations at laboratory facilities; |
|
|
• |
|
limitations on employee resources that would otherwise be focused on the conduct of our preclinical studies and clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with groups of people; and |
|
|
• |
|
material delays and complications with respect to our research and development programs. |
39
We may not successfully manage our company through varying business cycles, including the COVID-19 pandemic
Our success will depend on properly sizing our company through growth and contraction cycles caused in part by changing business conditions, which places a significant strain on our management and on our administrative, operational and financial resources. For example, in connection with the COVID-19 pandemic, we asked most of our personnel, including all of our administrative employees, to work remotely, restricted on-site staff to only those personnel who must perform activities that must be completed on-site, implemented social distancing on-site, and closed certain of our offices temporarily. Our increased reliance on personnel working from home may negatively impact productivity, or disrupt, delay, or otherwise adversely impact our business. In addition, this could increase our cyber security risk, create data accessibility concerns, and make us more susceptible to communication disruptions, any of which could adversely impact our business operations or delay necessary interactions with the FDA, manufacturing sites, research or clinical trial sites. To manage through such cycles, we may expand or contract our facilities, our operational, financial and management systems and our personnel. If we were unable to manage growth and contractions effectively our business would be harmed.
*The path to regulatory approval of DUR-928 is uncertain
DUR-928 is in clinical development for the potential treatment of AH; NASH is also being explored. In AH and NASH, there are no currently approved drugs. Accordingly, as the treatment landscape changes, we will have to interact with the FDA and other regulatory agencies regarding important aspects of the clinical development program, potentially including the size of clinical trials, the specific primary and secondary endpoints for the clinical trials, inclusion and exclusion criteria, stopping rules, duration of follow up, size of the safety databases, statistical analysis plans and other matters. This uncertainty may make it difficult to predict the timing or expense required to obtain regulatory approval for DUR-928. We also may need to revise our clinical development plans after trials have commenced or been completed, which could add to the time and expense associated with the clinical development of DUR-928. If we are unable to reach agreement with the FDA or other regulatory agencies regarding clinical development plans for DUR-928, we may curtail or limit our development activities for this product candidate.
*We are in discussions with potential commercial licensees for POSIMIR and may be unable to enter an agreement
We are in discussions to license POSIMIR to a third party for commercialization. There can be no assurance that we will be able to enter into an agreement with a third party for the commercialization of POSIMIR at all, or that any agreement we enter into will result in material payments to us. If we are not able to enter into such an agreement we may not commercially launch POSIMIR, or if such agreement does not result in material payments to us, our financial prospects may be harmed.
*The approved indication for POSIMIR may limit its market, and we or a commercial licensee may not be able to expand the label to include additional indications
The FDA approval for POSIMIR is limited to use in arthroscopic subacromial decompression (ASD) surgery and contains a Boxed Warning about risks of intravenous administration. We or a commercialization partner cannot currently promote POSIMIR for use in other indications, physicians may elect not to use POSIMIR in surgeries involving ASD in combination with other procedures, and the Boxed Warning may deter physicians from using it, which may limit the market opportunity for POSIMIR. While we intend to seek approval from FDA for POSIMIR in additional indications, there is no assurance that we or a commercialization partner will be able to do so, in which case sales of POSIMIR may be limited.
In connection with the approval of POSIMIR, we are required to conduct two postmarketing non-clinical studies. If the results from either of these studies are unfavorable, the FDA may require additional studies, or may take actions that are adverse to the regulatory status, commercial prospects and market acceptability of POSIMIR
In connection with the approval of POSIMIR, we are required to conduct two postmarketing non-clinical studies to determine the ultimate impact of the drug product and fate of the vehicle if it is inadvertently administered into the intravascular space and to assess the potential serious risk of POSIMIR-induced Local Anesthetic Systemic Toxicity (LAST) following inadvertent intravenous route of administration and the ability of lipid infusions to treat the clinical manifestations of LAST. We will need to reach agreement with the FDA on the appropriate protocols for these non-clinical studies, complete them and submit final reports to the FDA by September 2023.
The FDA may require us to update POSIMIR’s labeling with the results from these studies, including any positive or negative results, which could have adverse impact on POSIMIR’s commercial prospects and market acceptability. We may be unable to complete our postmarketing studies in accordance with the required timeline, if at all. For example, it may not be feasible to assess the risk of LAST following inadvertent intravenous route of administration and the ability of lipid infusions to treat the clinical manifestations of LAST. If the FDA determines the study cannot be completed as designed, but the study objectives remain important, the FDA may require one or more new postmarketing studies with new commitments and schedules. The FDA may also amend the labeling or take other actions that are adverse to POSIMIR’s regulatory status. If there are delays in completing the postmarketing studies, the FDA may post information about the delayed status on its website. Any of the above FDA actions could adversely affect us or POSIMIR’s commercial prospects, or have other collateral effects, such as making it more difficult for us to find a third-party licensee to commercialize POSIMIR. In POSIMIR clinical studies, no inadvertent intravascular injections were observed.
40
Delays or difficulties in the enrollment of subjects in clinical trials may increase our overall development expenses and delay clinical trial data and receipt of necessary regulatory approvals
Successful and timely completion of clinical trials will require that we enroll a sufficient number of subjects and/or patients. Enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, our ability to recruit clinical sites and the ability of clinical sites to successfully recruit subjects to participate in clinical trials. Initiation of and enrollment in many clinical trials is being adversely affected by COVID-19, which has caused many institutions to stop enrolling patients, has created a large number of clinical trial proposals for potential clinical trial sites to review and consider, and has caused many individuals to avoid contact with hospitals or other healthcare providers. Additionally, some of the patients in our clinical trials, including AH patients, are hospitalized and concerns about exposure to COVID-19 limit clinical trial staff’s access to patients, the frequency of interactions between patients and staff, the ability to obtain blood draws and other biological sample collection, and may limit the ability to ship samples to outside laboratories for analysis. In areas heavily impacted by COVID-19, there may be limited hospital staff available for clinical trial activities due to staff becoming infected or due to deprioritization of clinical trial activities. Trials may be subject to delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. We may not be able to initiate or continue clinical trials for DUR-928 if we are unable to sign sufficient clinical sites, locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States or if we are unable to collect and analyze biological samples required for trial endpoints. It is possible that the inclusion and exclusion criteria for patients to be included in these trials or COVID-19-related issues may make the trials more difficult to conduct or may significantly extend the time required for enrollment and the cost of these trials.
We cannot predict how successful we will be at enrolling patients in our clinical trials. Enrollment is affected by many factors including:
|
|
• |
the eligibility criteria for the trial in question; |
|
|
• |
the prevalence and incidence of the conditions being studied in the clinical trials; |
|
|
• |
COVID-19-related challenges with patient access, hospital prioritization, clinical trial staff availability, ability to collect, ship and analyze patients’ biological samples, availability of personal protective equipment (PPE), swabs, reagents and other materials and supplies; |
|
|
• |
the perceived risks and benefits of our product candidates; |
|
|
• |
clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs or therapeutic biologics that may be approved for the indications we are investigating; |
|
|
• |
the efforts to facilitate timely enrollment in clinical trials; |
|
|
• |
competition for clinical sites and patients from other clinical trials; |
|
|
• |
the willingness of potential clinical trial patients to provide informed consent to participate in the trial; |
|
|
• |
the patient referral practices of physicians; |
|
|
• |
the ability to monitor patients adequately during and after treatment; and |
|
|
• |
the proximity and availability of clinical trial sites for prospective patients. |
Our inability to sign up sufficient clinical trial sites and/or enroll a sufficient number of patients for clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether. Enrollment delays in these clinical trials may result in increased development costs for our drug candidates or delays in regulatory filings and progression, which would cause the value of our company to decline and limit our ability to obtain additional financing.
The FDA or other regulatory agencies may require more information or clinical studies for all of our product candidates, and our product candidates may never be approved
The failure to adequately demonstrate the safety and effectiveness of a pharmaceutical product candidate under development to the satisfaction of FDA and other regulatory agencies will result in delays to the regulatory approval or non-approvability of our product candidates, and could materially harm our business. Clinical trials may not demonstrate the sufficient levels of safety and efficacy necessary to obtain the requisite regulatory approvals for our product candidates, or may require such significant numbers of patients or additional costs to make it impractical to satisfy the regulatory agency’s requirements, and thus our product candidates may not be approved for marketing. During the review process, the FDA or other regulatory agencies may request more information regarding the safety of our product candidates, as the FDA did in their Complete Response Letter for POSIMIR, and answering such questions could require significant additional work and expense, and take a significant amount of time, resulting in a material delay of approval or the failure to obtain approval or lead the company to abandon the development of that product candidate. During the review process, the FDA, or other regulatory agencies, may also request more information regarding the chemistry, manufacturing or
41
controls related to our product candidates, and answering such questions could require significant additional work and expense, and take a significant amount of time, resulting in a material delay of approval or the failure to obtain approval or abandonment of the product candidate. Additionally, even if our product candidates receive FDA or other regulatory agency approval, the regulatory agency may require that we conduct additional clinical or non-clinical studies after such approval, place limitations on the use of our products in applicable labels, require marketing under a REMS program, include commercially unattractive language in the approved product label, delay approval to market our products or limit the indicated use of our products, which may harm our business and results of operations.
We currently have a significant amount of debt. Compliance with repayment obligations and other covenants may be difficult, and failure by us to fulfill our obligations under the applicable loan agreements may cause the repayment obligations to accelerate
In July 2016, we entered into a Loan and Security Agreement (the Loan Agreement) with Oxford Finance LLC (Oxford Finance), pursuant to which Oxford Finance provided a $20 million secured single-draw term loan to us with an initial maturity date of August 1, 2020. The term loan was fully drawn at close and the proceeds may be used for working capital and general business requirements. The term loan repayment schedule provided initially for interest only payments for the first 18 months, followed by consecutive monthly payments of principal and interest in arrears starting on March 1, 2018 and continuing through the maturity date of August 1, 2020. Following three amendments, we make interest only payments under the amended Loan Agreement until December 1, 2021 and the final maturity date of the loan is May 1, 2024. The Loan Agreement provides for a floating interest rate (7.95% initially and 7.95% as of March 31, 2021) based on an index rate plus a spread and an additional payment equal to 10% of the principal amount of the term loan, which is due when the term loan becomes due or upon the prepayment of the facility. If we elect to prepay the loan, there is also a prepayment fee between 0.75% and 2.5% of the principal amount of the term loan depending on the timing of prepayment. Our debt repayment obligations under the Loan Agreement, as amended, may prove a burden to the Company as they become due, particularly following the expiration of the interest-only period.
The Loan Agreement contains customary events of default, including, among other things, our failure to fulfill certain of our obligations under the Loan Agreement and the occurrence of a material adverse change in our business, operations or condition (financial or otherwise), a material impairment of the prospect of repayment of any portion of the loan, the failure to deliver an unqualified audit report and board approved financial projections within time periods set forth in the Loan Agreement, or a material impairment in the perfection or priority of lender’s lien in the collateral or in the value of such collateral. In the event of default by us under the Loan Agreement, the lender would be entitled to exercise its remedies thereunder, including the right to accelerate the debt, upon which we may be required to repay all amounts then outstanding under the Loan Agreement, which could harm our business, operations and financial condition.
In addition, the term loan is secured by substantially all of our assets, except that the collateral does not include any equity interests in the Company, any intellectual property (including all licensing, collaboration and similar agreements relating thereto), and certain other excluded assets. The Loan Agreement contains customary representations, warranties and covenants by us, which covenants limit our ability to convey, sell, lease, transfer, assign or otherwise dispose of certain of our assets; engage in any business other than the businesses currently engaged in by us or reasonably related thereto; liquidate or dissolve; make certain management changes; undergo certain change of control events; create, incur, assume, or be liable with respect to certain indebtedness; grant certain liens; pay dividends and make certain other restricted payments; make certain investments; make payments on any subordinated debt; and enter into transactions with any of our affiliates outside of the ordinary course of business or permit our subsidiaries to do the same. Complying with these covenants may make it more difficult for us to successfully execute our business strategy.
We will require and may have difficulty raising needed capital in the future
Our business currently does not generate sufficient revenues to meet our capital requirements and we do not expect that it will do so in the near future. We have expended and will continue to expend substantial funds to complete the research, development and clinical testing of our product candidates. We will require additional funds for these purposes, to establish additional clinical- and commercial-scale manufacturing arrangements and facilities, and to provide for the marketing and distribution of our product candidates. Additional funds may not be available on acceptable terms, if at all, and such availability will depend on a number of factors, some of which are outside of our control, including general capital markets conditions and investors’ view of our prospects and valuation. If adequate funds are unavailable from operations or additional sources of financing, we may have to delay, reduce the scope of or eliminate one or more of our research or development programs which would materially harm our business, financial condition and results of operations.
We believe that our cash, cash equivalents and investments and anticipated revenues will be adequate to satisfy our capital needs for at least the next 12 months from the date the financial statements are filed. However, our independent auditors may not agree with this assessment, and our actual capital requirements will depend on many factors, including:
42
|
|
• |
continued progress and cost of our research and development programs; |
|
|
• |
progress with preclinical studies and clinical trials; |
|
|
• |
the time and costs involved in obtaining regulatory clearance; |
|
|
• |
costs involved in establishing manufacturing capabilities for pre-clinical, non-clinical, clinical and commercial quantities of our product candidates; |
|
|
• |
success in entering into collaboration agreements and achieving milestones under such agreements; |
|
|
• |
the continuation of our collaborative agreements that provide financial funding for our activities; |
|
|
• |
regulatory actions with respect to our and our collaborators’ product candidates; |
|
|
• |
costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; |
|
|
• |
costs of developing sales, marketing and distribution channels and our ability and that of our collaborators to sell our products, products we have a financial interest in and eventually, product candidates; |
|
|
• |
competing technological and market developments; |
|
|
• |
market acceptance of our products, products we have a financial interest in and, eventually, product candidates; |
|
|
• |
any failure to comply with the covenants in our debt instruments that results in acceleration of repayment obligations; |
|
|
• |
impacts of the COVID-19 crisis; |
|
|
• |
costs for recruiting and retaining employees and consultants; and |
|
|
• |
unexpected legal, accounting and other costs and liabilities related to our business. |
We may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. We may seek to raise additional funds through equity or debt financings, convertible debt financings, collaborative arrangements with corporate collaborators or other sources, which may be dilutive to existing stockholders and may cause the price of our common stock to decline. In addition, in the event that additional funds are obtained through arrangements with collaborators or other sources, we may have to relinquish rights to some of our technologies or pharmaceutical product candidates that we would otherwise seek to develop or commercialize ourselves. If adequate funds are not available, we may be required to significantly reduce or refocus our product development efforts, resulting in delays in generating future product revenue.
We do not control the commercialization of PERSERIS or Methydur
We rely on Indivior for the commercialization of PERSERIS. There can be no assurance that PERSERIS will obtain meaningful sales. If Indivior does not successfully commercialize PERSERIS, the earn-out payments we receive under our agreement with them will be limited. We rely on Orient Pharma for the commercialization of Methydur in the territories licensed to Orient Pharma. If Orient Pharma does not successfully commercialize Methydur throughout their territory, the royalty payments we receive under our agreement with them may be limited. The sales of both of these products may be negatively impacted by the COVID-19 pandemic.
Development of our pharmaceutical product candidates is not complete, and we cannot be certain that our product candidates will be able to be commercialized
To be profitable, we or our third-party collaborators must successfully research, develop, obtain regulatory approval for, manufacture, introduce, market and distribute our pharmaceutical product candidates under development. For each product candidate that we or our third-party collaborators intend to commercialize, we must successfully meet a number of critical developmental milestones for each disease or medical condition targeted, including:
|
|
• |
demonstrating through clinical trials that each product candidate is safe and effective in patients for the intended indication at an achievable dose and that the product candidate’s benefits outweigh its risks; |
|
|
• |
with respect to each product candidate based on a new chemical entity, determining appropriate indication(s); |
|
|
• |
with respect to our proprietary pharmaceutical programs based on our drug delivery technologies, selecting and developing a drug delivery technology to deliver the proper dose of drug over the desired period of time; |
|
|
• |
determining the appropriate route of administration and drug dosage for each product candidate in each indication; |
|
|
• |
developing product candidates that will be tolerated, safe and effective; |
43
|
|
• |
demonstrating each product candidate will be chemically and physically stable for commercially reasonable time periods; and |
|
|
• |
completing the manufacturing development and scale-up to permit manufacture of the product candidate in commercial quantities and at acceptable cost. |
The time frame necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we may not successfully complete these milestones for any of our product candidates in development. Except for marketing authorization for POSIMIR in the U.S. for post-surgical pain reduction for up to 72 hours following arthroscopic subacromial decompression (ASD), PERSERIS by Indivior in the U.S. and for Orient Pharma’s approval of Methydur in Taiwan, development is incomplete for all product candidates in our development programs, including DUR-928. We may not be able to finalize the design or formulation of any of our product candidates. In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not been previously approved for use in pharmaceutical products, which may require us or our collaborators to perform additional studies and may delay clinical testing and regulatory approval of our product candidates. Even after we complete the design of a product candidate, the product candidate must still complete required clinical trials and additional safety testing in animals before approval for commercialization. We are continuing testing and development of our product candidates and may explore possible design or formulation changes to address issues of safety, manufacturing efficiency, stability and performance. We or our collaborators may not be able to complete development of any product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. If we or our third-party collaborators are unable to complete development of DUR-928 or other product candidates, we will not be able to earn revenue from them, which would materially harm our business.
For certain of our product candidates, we depend to a large extent on third-party collaborators, and we have limited or no control over the development, sales, distribution and disclosure for those product candidates
Our performance for certain of our product candidates depends to a large extent on the ability of our third-party collaborators to successfully develop and obtain regulatory approvals. We have entered into agreements with Indivior, Santen and Orient Pharma under which we granted such third parties the right to develop, apply for regulatory approval for, market, promote or distribute certain product candidates, subject to payments to us in the form of product royalties, earn-out and other payments. We have limited or no control over the expertise or resources that any collaborator may devote to the development, clinical trial strategy, regulatory approval, marketing or sale of these product candidates, or the timing of their activities. Any of our present or future collaborators may not perform their obligations as expected. These collaborators may breach or terminate their agreement with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Enforcing any of these agreements in the event of a breach by the other party could require the expenditure of significant resources and consume a significant amount of management time and attention. Our collaborators may also conduct their activities in a manner that is different from the manner we would recommend or would have chosen, had we been developing such product candidates ourselves. Further, our collaborators may elect not to develop or commercialize product candidates arising out of our collaborative arrangements or not devote sufficient resources to the development, clinical trials, regulatory approval, manufacture, marketing or sale of these product candidates. If any of these events occur, we may not recognize revenue from the commercialization of our product candidates based on such collaborations. In addition, these third parties may have similar or competitive products to the ones which are the subject of their collaborations with us, or relationships with our competitors, which may reduce their interest in developing or selling our product candidates. We may not be able to control public disclosures made by some of our third-party collaborators, which could negatively impact our stock price.
Cancellation of collaborations regarding our product candidates may adversely affect potential economic benefits
Third-party collaboration agreements typically allow the third party to terminate the agreement (or a specific program within an agreement) at will by providing notice. Termination can result from failure of the collaboration to achieve anticipated milestones, for changes in strategy of the other party or for other reasons. In these cases, the product rights revert to us or certain rights of the partner to use our proprietary technology are terminated. If there have been payments under such agreements that are being recognized over time, termination of such agreements (or programs) can lead to a near-term increase in our reported revenues resulting from the immediate recognition of the balance of such payments. Termination deprives us of potential future economic benefits under such agreements, and may make it more difficult or impossible to enter into agreements with other third parties for use of the assets and/or technologies that were subject to the terminated agreement. For example, termination of our agreements with Santen or Orient Pharma could have negative effects on the Company.
A significant component of our revenues resulted from collaboration agreements with other companies that have terminated
Our revenues have been based to a significant extent on collaborative arrangements with third parties, pursuant to which we receive payments based on our performance of research and development activities set forth in these agreements. For example, approximately 58% of our total revenues were derived from our collaboration agreement with Gilead in 2019. In June 2020, Gilead notified us that they were terminating this collaboration. In addition, we have seen periodic fluctuations in revenues associated with our other collaboration agreements, which reflect the current development stage of the product candidates subject to those agreements, and our collaborator’s needs for our services. Long-term growth of our collaboration revenues requires us to enter into new collaboration agreements, and there can be no assurance that we will do so. Even if we enter into new collaboration agreements, we
44
may not be able to fulfill our obligations or attain milestones set forth in any specific agreement, which could cause our revenues and/or cash flows to fluctuate or be less than anticipated and may expose us to liability for contractual breach. In addition, these agreements may require us to devote significant time and resources to communicating with and managing our relationships with such collaborators and resolving possible issues of contractual interpretation which may detract from time our management would otherwise devote to managing our operations. Such agreements are generally complex and contain provisions that could give rise to legal disputes, including potential disputes concerning ownership of intellectual property under collaborations. Such disputes can delay or prevent the development of potential new product candidates, or can lead to lengthy, expensive litigation or arbitration. In general, our collaboration agreements, including our agreements with Orient Pharma with respect to Methydur and Santen with respect to an investigational ophthalmic product, may be terminated by the other party at will or upon specified conditions including, for example, if we fail to satisfy specified performance milestones or if we breach the terms of the agreement. Acquisitions of our collaborators or strategic changes or re-organizations or re-prioritizations of our collaborators can lead to turnover of program staff, a review of development programs and strategies by the acquirer, and other events that can disrupt a program, resulting in program delays or discontinuations.
If we do not enter into new collaboration agreements, our anticipated revenues and/or cash flows will be reduced relative to periods of increased R&D revenues, such as occurred in 2020.
*Our cash flows are likely to differ from our reported revenues
Our revenues will likely differ from our cash flows from revenue-generating activities. Upfront payments received upon execution of collaborative agreements are recorded as deferred revenue and generally recognized over the period of our performance obligations with the third-party collaborator pursuant to the applicable agreement. The period of performance obligations may also be revised on a prospective basis. As of March 31, 2021, we had $1.2 million of deferred revenue which will be recognized in future periods and may cause our reported revenues to be greater than cash flows from our ongoing revenue-generating activities. Assumptions related to revenue recognition of deferred revenue are reviewed in each accounting period and changes are recorded in the current period. In certain circumstances, changes in assumptions related to the timing and amount of work required to complete a performance obligation tied to deferred revenue can result in negative revenue for an accounting period or the accelerated recognition of non-cash revenue.
Our revenues may decrease and our losses may increase compared to prior years as a result of the sale of the LACTEL® polymer product line
In December 2020 we completed the sale of our LACTEL polymer product line to Evonik for approximately $15 million subject to certain adjustments. Sales of LACTEL polymers contributed to our product revenues and earnings, and the loss of these revenues and earnings will decrease our future period product related revenues and operating results.
Our business strategy includes the entry into additional collaborative agreements. We may not be able to enter into additional collaborative agreements or may not be able to negotiate commercially acceptable terms for these agreements
Our current business strategy includes the entry into additional collaborative agreements for the development and commercialization of our product candidates, including, but not limited to POSIMIR, DUR-928 and others. The negotiation and consummation of these types of agreements typically involve simultaneous discussions with multiple potential collaborators and require significant time and resources from our officers, business development, legal, and research and development staff. In addition, in attracting the attention of pharmaceutical and biotechnology company collaborators, we compete with numerous other third parties with product opportunities as well as the collaborators’ own internal product opportunities. We may not be able to consummate additional collaborative agreements, or we may not be able to negotiate commercially acceptable terms for these agreements. If we do not consummate additional collaborative agreements, we may have to consume money more rapidly on our product development efforts, defer development activities or forgo the exploitation of certain opportunities, abandon development of certain product candidates or indications for certain product candidates, any of which could have a material adverse effect on our business.
We and our third-party collaborators may not be able to manufacture sufficient quantities of our pharmaceutical product candidates and components to support the non-clinical, clinical and commercial requirements of our collaborators and ourselves at an acceptable cost or in compliance with applicable government regulations, and we have limited manufacturing experience
We or our third-party collaborators to whom we have assigned such responsibility must manufacture our products, product candidates and components (including active ingredients and excipients) in non-clinical (e.g., toxicology), clinical and commercial quantities, either directly or through third parties, in compliance with regulatory requirements and at an acceptable cost. The manufacturing processes associated with our products and product candidates are complex. We and our third-party collaborators, where relevant, have not yet completed development of the manufacturing process for any of our product candidates or components that are in pre-clinical or clinical development, including DUR-928. If we and our third-party collaborators, where relevant, fail to timely complete the development of the manufacturing process for our product candidates, we and our third-party collaborators, where relevant, will not be able to timely produce supplies for non-clinical, clinical trials and commercialization of our product candidates. We have also committed to manufacture and supply product candidates or components under a number of our collaborative agreements with third-party companies. We have limited experience manufacturing pharmaceutical products, and we may not be able
45
to timely accomplish these tasks. If we and our third-party collaborators, where relevant, fail to develop manufacturing processes to permit us to manufacture a product candidate or component at an acceptable quality and cost, then we and our third-party collaborators may not be able to develop or commercialize that product candidate or we may be in breach of our supply obligations to our third-party collaborators.
Our manufacturing facility in Cupertino is a multi-disciplinary site that we have used to manufacture only research and clinical trial supplies of several of our product candidates, including DUR-928 and POSIMIR. If we experience delays or technical difficulties in developing acceptable manufacturing processes or scaling up the manufacturing of our product candidates, it could result in delays or added cost in our development programs. We have not manufactured commercial quantities of any of our product candidates at our Cupertino facility. In the future, we may develop additional manufacturing capabilities for our product candidates and components to meet our demands and those of our third-party collaborators by contracting with third-party manufacturers and/or by potentially constructing additional manufacturing space at our facilities in California or elsewhere. We have limited experience building and validating manufacturing facilities, and we may not be able to accomplish these tasks in a timely or cost-effective manner.
If we and our third-party collaborators, where relevant, are unable to manufacture our products, product candidates or components in a timely manner or at an acceptable cost, quality or performance level, and are unable to attain and maintain compliance with applicable regulations, the non-clinical and clinical trials and the commercial sale of our products and product candidates and those of our third-party collaborators could be delayed or never occur. Additionally, we may need to alter our facility design or manufacturing processes, install additional equipment or do additional construction or testing in order to meet regulatory requirements, optimize the production process, increase efficiencies or production capacity or for other reasons, which may result in additional cost to us or delay production needed for the non-clinical trials, clinical trials, chemistry, manufacturing and controls (CMC) and commercial launch of our products, product candidates and those of our third-party collaborators.
If we or our third-party collaborators cannot manufacture our products, product candidates or components in time to meet the clinical or commercial requirements of our collaborators or ourselves or at an acceptable cost, our operating results will be harmed.
Failure to comply with ongoing governmental regulations for our pharmaceutical product candidates could materially harm our business in the future
Developing, manufacturing, marketing or promoting a drug is subject to very strict regulations and controls. Furthermore, clearance or approval may entail ongoing requirements for post-marketing studies. The manufacture and marketing of drugs are subject to continuing FDA and foreign regulatory review and requirements that we update our regulatory filings. Later discovery of previously unknown problems with a product, manufacturer or facility, or our failure to update regulatory files, may result in restrictions, including withdrawal of the product from the market. Any of the following or other similar events, if they were to occur, could delay or preclude us from further developing, marketing or realizing full commercial use of our products or product candidates, which in turn would materially harm our business, financial condition and results of operations:
|
|
• |
failure to obtain or maintain requisite governmental approvals; |
|
|
• |
failure to meet GMP, GLP and/or other governmental requirements for drug development; |
|
|
• |
failure to obtain approvals for clinically intended uses of our pharmaceutical product candidates under development; or |
|
|
• |
FDA required product withdrawals or warnings arising from identification of serious adverse side effects in our products and product candidates. |
Manufacturers of drugs must comply with the applicable FDA good manufacturing practice (GMP) regulations, which include production design controls, testing, quality control and quality assurance requirements as well as the corresponding maintenance of records and documentation. Compliance with current GMP regulations is difficult and costly. Manufacturing facilities are subject to ongoing periodic inspection by the FDA and corresponding state and in some cases, foreign agencies, including unannounced inspections, and must be licensed before they can be used for the commercial manufacture of our products and product candidates. We and/or our present or future suppliers and distributors may be unable to comply with the applicable GMP regulations and other FDA and/or foreign regulatory requirements. If we, our third-party collaborators or our respective suppliers do not achieve compliance for our products or product candidates we or they manufacture, the FDA or foreign equivalents may refuse or withdraw marketing clearance, put our or our partner’s clinical trial on hold, withdraw or reject an investigational new drug (IND) application or require product recall, which may cause interruptions or delays in the development, manufacture and sale of our products and product candidates.
We have a history of operating losses, expect to continue to have losses in the future and may never achieve or maintain profitability
We have incurred significant operating losses since our inception in 1998 and, as of March 31, 2021, had an accumulated deficit of approximately $499.9 million. We expect to continue to incur significant operating losses over the next several years as we continue to incur significant costs for research and development, clinical trials, manufacturing, sales, and general and administrative functions. Our ability to achieve profitability depends upon our ability, alone or with others, to successfully complete the development of our proposed product candidates, obtain the required regulatory clearances, manufacture and market our proposed product candidates and successfully commercialize our approved products. Development of pharmaceutical product candidates is costly and requires significant investment. In addition, we may choose to license from third parties either additional drug delivery platform
46
technology or rights to particular drugs or other appropriate technology and/or intellectual property rights for use in our products and product candidates. The license fees for these technologies or rights would increase the costs of our products and product candidates.
To date, we have not generated significant revenue from the commercial sale of our products or product candidates and do not expect to do so in the near future. Our current revenues are from the ALZET product line, from certain excipient sales, from earn-out payments from Indivior related to sales of PERSERIS, from royalty payments from Orient Pharma related to sales of Methydur in Taiwan, and from payments under collaborative research and development agreements with third parties. We do not expect our product revenues to increase significantly in the near future, and we do not expect that collaborative research and development revenues will exceed our actual operating expenses in the near future. We do not anticipate meaningful revenues to derive from the commercialization and marketing of our product candidates in development in the near future, and therefore do not expect to generate sufficient revenues to cover expenses or achieve profitability in the near future.
We may develop our own sales force and commercial group to market future products but we have limited sales and marketing experience with respect to pharmaceuticals and may not be able to do so effectively
We have a small sales and marketing group focused on our ALZET product line. We may choose to develop our own sales force and commercial group to market products that we have developed or may develop in the future, including POSIMIR and/or DUR-928, if approved. Developing a sales force and commercial group would require substantial expenditures and the hiring of qualified personnel. We have limited sales and marketing experience, and may not be able to effectively recruit, train or retain sales and marketing personnel. If we are not able to put in place an appropriate sales force and commercial group for our products in development, we may not be able to effectively launch these products. We may not be able to effectively sell our product candidates, if approved, and our failure to do so could limit or materially harm our business.
We and our third-party collaborators may not sell our product candidates effectively
We and our third-party collaborators compete with many other companies that currently have extensive and well-funded marketing and sales operations. Our marketing and sales efforts and those of our third-party collaborators may be unable to compete successfully against these other companies. We and our third-party collaborators, if relevant, may be unable to establish a sufficient sales and marketing organization on a timely basis, if at all. We and our third-party collaborators, if relevant, may be unable to engage qualified distributors. Even if engaged, these collaborators and distributors may:
|
|
• |
fail to adequately market our product candidates; |
|
|
• |
fail to satisfy financial or contractual obligations to us; |
|
|
• |
cease operations with little or no notice to us; |
|
|
• |
offer, design, manufacture or promote competing product lines; |
|
|
• |
fail to maintain adequate inventory and thereby restrict use of our product candidates; or |
|
|
• |
build up inventory in excess of demand thereby limiting future purchases of our product candidates resulting in significant quarter-to-quarter variability in our sales. |
The failure of us or our third-party collaborators to effectively develop, gain regulatory approval for, sell, manufacture and market our product candidates will hurt our business, prospects, financial results and may impact our access to capital.
We rely heavily on third parties to support development, clinical testing and manufacturing of our product candidates
We rely on third-party contract research organizations, consultants, service providers and suppliers to provide critical services to support development, clinical testing, and manufacturing of our product candidates. For example, we currently depend on third-party vendors to manage and monitor most of our clinical trials. We rely on third-parties to manufacture or perform manufacturing steps relating to our product candidates and components. We anticipate that we will continue to rely on these and other third-party contractors to support development, clinical testing, and manufacturing of our product candidates. These third parties may not execute their responsibilities and tasks competently in compliance with applicable laws and regulations or in a timely or cost-effective fashion. Failure of these contractors to provide the required services in a competent or timely manner or on reasonable commercial terms could materially delay the development and approval of our product candidates, increase our expenses and materially harm our business, financial condition, results of operations and access to capital.
Key components of our products and product candidates are provided by a limited number of suppliers, and supply shortages or loss of these suppliers could result in interruptions in supply or increased costs
Certain components and drug substances used in our and our collaborators’ products and product candidates, including DUR-928, POSIMIR, Methydur and PERSERIS, are currently purchased from a single or a limited number of outside sources. In particular, Eastman Chemical is the sole supplier of our requirements of sucrose acetate isobutyrate, a necessary component of POSIMIR and certain pharmaceutical product candidates we have under development. A third-party manufacturer is our sole supplier for future commercial supplies of POSIMIR. Another third- party manufacturer is our sole supplier for future non-clinical, clinical and commercial supplies of DUR-928. The reliance on a sole or limited number of suppliers could result in:
47
|
|
• |
an inability to obtain an adequate supply of required product candidate, active pharmaceutical ingredient or excipients or other components; |
|
|
• |
delays associated with finding and contracting with a new supplier (if we can find one capable of replacing the old supplier and negotiate commercially reasonable terms) and then transferring the technology required to perform the services to the new supplier; |
|
|
• |
delays associated with redesigning a pharmaceutical product candidate due to a failure to obtain a single source component; and |
|
|
• |
reduced control over pricing, quality and delivery time. |
While we have entered into contract manufacturing agreements with multiple vendors for DUR-928, we currently have a third-party sole supplier for GMP supplies of DUR-928. This third party is our sole source for the drug product required for development and commercialization of this drug candidate. There can be no assurance that we will receive sufficient quantities of DUR-928 to commence and conduct the non-clinical trials, clinical trials and CMC activities we are planning, and delays in supply could delay development of DUR-928. In addition, certain of our third-party manufacturers and suppliers may be experiencing delays as a result of the COVID-19 pandemic or have otherwise encountered delays in providing their services. As a result, we may not be able to manufacture our product candidates for our clinical trials and conduct other research and development operations and maintain current clinical and pre-clinical timelines. In addition, if additional third parties in our supply chain are adversely impacted by restrictions resulting from the pandemic, including staffing shortages, raw material shortages, production slowdowns and disruptions in delivery systems, our supply chain may be disrupted in other ways, further limiting our ability to manufacture our product candidates for our clinical trials and conduct our research and development operations.
We have entered into a commercial manufacturing and packaging agreement with a third-party manufacturer for supply of POSIMIR. This third party is our sole source for the drug product required for commercialization of this drug candidate. There may be technical risks associated with establishing an alternative commercial manufacturer that could entail delays in supply or quality issues related to POSIMIR. Furthermore, we and our contract manufacturer may also need or choose to subcontract with additional third-party contractors to perform manufacturing steps of POSIMIR or supply required components for POSIMIR. Where third party contractors perform manufacturing services for us, we will be subject to the schedule, expertise and performance of third parties as well as incur significant additional costs. Failure of third parties to perform their obligations could adversely affect our operations, development timeline and financial results. If we or a commercialization partner proceed with the commercialization of POSIMIR, we expect to put in place in the future second source supply arrangements, which may be costly and time consuming.
We have supply agreements in place for certain components of our pharmaceutical product candidates, but do not have in place long term supply agreements with respect to all of the components of any of our products or product candidates. Therefore, the supply of a particular component could be terminated at any time without penalty to the supplier. In addition, we may not be able to procure required components or drugs from third-party suppliers at a quantity, quality, cost and timing acceptable to us. In addition, certain of our suppliers may be experiencing delays as a result of the COVID-19 pandemic or have otherwise encountered delays in providing their services. Any interruption in the supply of single source components (including active pharmaceutical ingredients, excipients, or components like vials, stoppers, filters and the like), products or product candidates, could cause us to seek alternative sources of supply or manufacture these items internally if feasible. Furthermore, in some cases, we are relying on our third-party collaborators to procure supply of necessary components. If the supply of any components for our products or product candidates is interrupted, components from alternative suppliers may not be available in sufficient volumes or at acceptable quality levels within required timeframes, if at all, to meet our needs or those of our third-party collaborators. This could delay our ability to obtain commercial product supplies or complete development and obtain approval for commercialization and marketing of our product candidates, causing us to lose sales, incur additional costs, delay new product introductions and could harm our reputation and make access to capital more difficult, expensive or impossible. The spread of COVID-19 has and is likely to continue to affect the manufacturing and shipment of goods globally. Many countries have imposed or are imposing certain restrictions on the movement of people and goods and may continue to lift and reimpose such restrictions as needed. Any delay in production or delivery of the components and drug substances used in our products or product candidates due to an extended closure of our suppliers’ plants as a result of efforts to limit the spread of COVID-19 could adversely impact our business and hinder our growth.
48
Write-offs related to the impairment of our goodwill, long-lived assets, inventories and other non-cash charges, as well as stock-based compensation expenses may adversely impact or delay our profitability
We may incur significant non-cash charges related to impairment write-downs of our long-lived assets, including goodwill. We are required to perform periodic impairment reviews of our goodwill at least annually. The carrying value of goodwill on our balance sheet was $6.2 million at March 31, 2021. To the extent these reviews conclude that the expected future cash flows generated from our business activities are not sufficient to recover the cost of our long-lived assets, we will be required to measure and record an impairment charge to write-down these assets to their realizable values. We completed our last review during the fourth quarter of 2020 and determined that goodwill was not impaired as of December 31, 2020. However, there can be no assurance that upon completion of subsequent reviews a material impairment charge will not be recorded. If future periodic reviews determine that our assets are impaired and a write-down is required, it will adversely impact or delay our profitability.
Inventories, in part, include certain excipients that are sold to customers and included in products and product candidates in development. These inventories are capitalized based on management’s judgment of probable sale prior to their expiration date which in turn is primarily based on management’s internal estimates. The valuation of inventory requires us to estimate the value of inventory that may become expired prior to use. We may be required to expense previously capitalized inventory costs upon a change in our judgment, due to, among other potential factors, a denial or delay of approval of a product by the necessary regulatory bodies, changes in product development timelines, or other information that suggests that the inventory will not be saleable.
Global credit and financial market conditions could negatively impact the value of our current portfolio of cash equivalents, short-term investments or long-term investments and our ability to meet our financing objectives
Our cash and cash equivalents are maintained in highly liquid investments with remaining maturities of 90 days or less at the time of purchase. Our short-term investments consist primarily of readily marketable debt securities with original maturities of greater than 90 days from the date of purchase but remaining maturities of less than one year from the balance sheet date. Our long-term investments consist primarily of readily marketable debt securities with maturities in one year or beyond from the balance sheet date. While, as of the date of this filing, we are not aware of any downgrades, material losses, or other significant deterioration in the fair value of our cash equivalents, short-term investments or long-term investments since March 31, 2021, no assurance can be given that deterioration in conditions of the global credit and financial markets would not negatively impact our current portfolio of cash equivalents, short-term investments or long-term investments or our ability to meet our financing objectives.
We depend upon key personnel who may terminate their employment with us at any time, and we may need to hire additional qualified personnel
Our success will depend to a significant degree upon the continued services of key management, technical and scientific personnel. In addition, our success will depend on our ability to attract and retain other highly skilled personnel, particularly as we develop and expand our Epigenetic Regulator Program. Competition for qualified personnel is intense, and the process of hiring and integrating such qualified personnel is often lengthy. We may be unable to recruit such personnel on a timely basis, if at all. Our management and other employees may voluntarily terminate their employment with us at any time. The loss of the services of key personnel, or the inability to attract and retain additional qualified personnel, could result in delays to product development or approval, loss of sales and diversion of management resources as well as difficulties or inability to raise sufficient capital to fund the Company’s operations.
49
*Our business involves environmental risks and risks related to handling regulated substances
In connection with our research and development activities and our manufacture of materials, products and product candidates, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Our research and development involves the use, generation and disposal of hazardous materials, including but not limited to certain hazardous chemicals, solvents, agents and biohazardous materials. The extent of our use, generation and disposal of such substances increased substantially when we started manufacturing and selling biodegradable polymers. Although we no longer manufacture and sell biodegradable polymers, we still handle and dispose of certain materials, biological specimens and wastes, and although we believe that our safety procedures for storing, handling and disposing of such materials comply with the standards prescribed by state and federal regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. We currently contract with third parties to dispose of these substances generated by us, and we rely on these third parties to properly dispose of these substances in compliance with applicable laws and regulations. If these third parties do not properly dispose of these substances in compliance with applicable laws and regulations, we may be subject to legal action by governmental agencies or private parties for improper disposal of these substances. The costs of defending such actions and the potential liability resulting from such actions are often very large. In the event we are subject to such legal action or we otherwise fail to comply with applicable laws and regulations governing the use, generation and disposal of hazardous materials and chemicals, we could be held liable for any damages that result, and any such liability could exceed our resources.
Cyber-attacks or other failures in telecommunications or information technology systems could result in information theft, data corruption and significant disruption of our business operations
We utilize information technology, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks and the confidentiality, availability and integrity of our data, and may cause a disruption in our operations, harm our reputation, cause us to pay to retrieve our data if it becomes infected or otherwise subject to ransomware and increase our stock trading risk. There can be no assurance that we will be successful in preventing cyber-attacks or successfully mitigating their effects. Similarly, there can be no assurance that our third-party collaborators, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems. Any cyber-attack or destruction or loss of data could have a material adverse effect on our business and prospects. In addition, we may suffer reputational harm or face litigation or adverse regulatory action as a result of cyber-attacks or other data security breaches and may incur significant additional expense to implement further data protection measures.
Our corporate headquarters, certain manufacturing facilities and personnel are located in a geographical area that is seismically active and near wildfire zones
Our corporate headquarters, certain manufacturing facilities and personnel are located in a geographical area that is known to be seismically active and prone to earthquakes, as well as wildfires and related power outages or power shortages. Should such a natural disaster occur or power outage or power shortage, our ability to conduct our business could be severely restricted, and our business and assets, including the results of our research, development and manufacturing efforts, could be harmed or destroyed.
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act and, consequently, some investors may find our common stock less attractive
We are a non-accelerated filer as defined by Rule 12b-2 of the Exchange Act, and as such, are not required to provide an auditor attestation of management’s assessment of internal control over financial reporting, which is generally required for SEC reporting companies under Section 404(b) of the Sarbanes-Oxley Act. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. Because we are not required to have our auditors provide an attestation of our management’s assessment of internal control over financial reporting, a material weakness in internal control may remain undetected for a longer period. In addition, we cannot predict if investors will find our common stock less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and the trading price for our common stock may be negatively affected.
50
Risks Related to Our Intellectual Property
If we are unable to adequately protect, maintain or enforce our intellectual property rights or secure rights to third-party patents, we may lose valuable assets, experience reduced market share or incur costly litigation to protect our rights or our third-party collaborators may choose to terminate their agreements with us
Our ability to commercially exploit our products will depend significantly on our ability to obtain and maintain patents, maintain trade secret protection and operate without infringing the proprietary rights of others.
As of May 3, 2021, we owned or exclusively in-licensed over 35 unexpired issued U.S. patents and over 140 unexpired issued foreign patents (which include granted European patent rights that have been validated in various EU member states). In addition, we have over 55 pending U.S. patent applications and over 140 foreign applications pending in Europe, Australia, Japan, Canada and other countries.
There can be no assurance that the pending patent applications will be granted. Further, there can be no assurance that VCU will not attempt to terminate their license to us, which termination could result in the loss of our rights to these patent families
The patent positions of pharmaceutical companies, including ours, are uncertain and involve complex legal and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, our patent applications or those that are licensed to us may not issue into patents, and any issued patents may not provide protection against competitive technologies or may be held invalid if challenged. Our competitors may also independently develop products similar to ours or design around or otherwise circumvent patents issued to us or licensed by us. In addition, the laws of some foreign countries may not protect our proprietary rights to the same extent as U.S. law, if at all.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We require our employees, consultants, advisors and collaborators to execute appropriate confidentiality and assignment-of-inventions agreements with us. These agreements typically provide that all materials and confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances, and that all inventions arising out of the individual’s relationship with us will be our exclusive property. These agreements may be breached, and in some instances, we may not have an appropriate remedy available for breach of the agreements. Furthermore, our competitors may independently develop substantially equivalent proprietary information and techniques, reverse engineer our information and techniques, or otherwise gain access to our proprietary technology.
We may be unable to meaningfully protect our rights in trade secrets, technical know-how and other non-patented technology. We may have to resort to litigation or arbitration to protect our intellectual property rights, or to determine their scope, validity or enforceability. In addition, interference, derivation, post-grant oppositions, and similar proceedings may be necessary to determine rights to inventions in our patents and patent applications. Enforcing or defending our proprietary rights is expensive, could cause diversion of our resources and may be unsuccessful. Any failure to enforce or protect our rights could cause us to lose the ability to exclude others from using our technology to develop or sell competing products.
Our collaboration agreements may depend on our intellectual property
We are party to collaborative agreements with Orient Pharma and Santen among others. Our third-party collaborators have entered into these agreements based on the exclusivity that our intellectual property rights confer on the products being developed. The loss or diminution of our intellectual property rights could result in a decision by our third-party collaborators to terminate their agreements with us. In addition, these agreements are generally complex and contain provisions that could give rise to legal disputes, including potential disputes concerning ownership of intellectual property and data under collaborations. Such disputes can lead to lengthy, expensive litigation or arbitration requiring us to devote management time and resources to such dispute which we would otherwise spend on our business.
We may be sued by third parties claiming that our product candidates infringe on their intellectual property rights, particularly because there is substantial uncertainty about the validity and breadth of medical patents
We or our collaborators may be exposed to future litigation by third parties based on claims that our product candidates or activities infringe the intellectual property rights of others or that we or our collaborators have misappropriated the trade secrets of others. This risk is exacerbated by the fact that the validity and breadth of claims covered in medical technology, pharmaceutical and biotechnology patents and the breadth and scope of trade secret protection involve complex legal and factual questions for which important legal principles are unresolved. Any litigation or claims against us or our collaborators, whether or not valid, could result in substantial costs, could place a significant strain on our financial resources and could harm our reputation. We also may not have sufficient funds to litigate against parties with substantially greater resources. In addition, pursuant to our collaborative agreements, we have provided our collaborators with the right, under specified circumstances, to defend against any claims of infringement of the third-party intellectual property rights, and such collaborators may not defend against such claims adequately or in the manner that we would do ourselves. Intellectual property litigation or claims could force us or our collaborators to do one or more of the following, any of which could harm our business or financial results:
51
|
|
• |
cease selling, incorporating or using any of our products or product candidates that incorporate the challenged intellectual property, which would adversely affect our revenue; |
|
|
• |
obtain a license from the holder of the infringed intellectual property right, which license may be costly or may not be available on reasonable terms, if at all; or |
|
|
• |
redesign our products or product candidates, which would be costly and time-consuming. |
Risks Related To Our Industry
The markets for our pharmaceutical products, product candidates and for our ALZET product line are rapidly changing and competitive, and new products or technologies developed by others could impair our ability to maintain or grow our business and remain competitive
The pharmaceutical industry is subject to rapid and substantial technological change. Developments by others may render our products, product candidates under development or technologies noncompetitive or obsolete, or we may be unable to keep pace with technological developments or other market factors. Technological competition in the industry from pharmaceutical and biotechnology companies, universities, governmental entities and others diversifying into the field is intense and is expected to increase.
We may face competition from other companies in numerous industries including pharmaceuticals, biotechnology, medical devices and drug delivery. Competition for DUR-928, if approved, will depend on the specific indication(s) for which DUR-928 is approved. 89Bio, AbbVie, Afimmune, Akero Therapeutics, Ascletis, AstraZeneca, Axcella Health, BMS, Cirius Therapeutics, CytoDyn, Dr. Falk Pharma, Eli Lilly, Enanta, ENYO Pharma, Galectin, Galmed, Genentech, Genfit, Gilead, Hanmi, HighTide Biopharma, Intercept, Inventiva Pharma, Ionis Pharmaceuticals, Inc., Isotechnika, Kowa, LifeMax, Lipidio, Lipocine, Madrigal, MediciNova, MedImmune, Mitsubishi Tanabe, NGM Biopharmaceuticals, Nimbus, NorthSea Therapeutics, Novartis, Novo Nordisk, Pfizer, PharmaKing, Poxel, Promethera Biosciences, Seal Rock Therapeutics, Terns Pharmaceutical, Thera Technologies, Viking, and others have development plans for products to treat NAFLD/NASH, AH or other liver diseases.
POSIMIR, if commercially launched, will compete with currently marketed oral opioids, transdermal opioids, local anesthetic patches, implantable and external infusion pumps which can be used for infusion of opioids and local anesthetics. Products of these types are marketed by Pacira, Innocoll, Purdue Pharma, AbbVie, Janssen, Actavis, Medtronic, Endo, AstraZeneca, Pernix Therapeutics, Tricumed, Halyard Health, Cumberland Pharmaceuticals, Acorda Therapeutics, Mallinckrodt, Inspirion Delivery Technologies, Mylan, Shire, Johnson & Johnson, Eli Lilly, Pfizer, Novartis, Zyla Life Sciences, Teva Pharmaceuticals, Collegium Pharmaceutical and others. Additional competition for POSIMIR may come from Heron Therapeutics if their product candidate, HTX-011, is approved. PERSERIS competes with currently marketed or approved products by Johnson & Johnson, Eli Lilly, Otsuka, Alkermes, Merck, Allergan, Novartis, and others. Orient Pharma’s Methydur competes with multiple ADHD products in Taiwan.
Numerous companies are applying significant resources and expertise to the problems of drug delivery and several of these are focusing or may focus on delivery of drugs to the intended site of action, including Pacira, Heron Therapeutics, Alkermes, Immune Pharmaceuticals, Innocoll, Nektar, Acorda Therapeutics, Flamel, Alexza, Mallinckrodt, Pfizer, Cumberland Pharmaceuticals, Zyla Life Sciences, Acura, Elite Pharmaceuticals, Phosphagenics, Intellipharmaceutics, Collegium Pharmaceutical, Charleston Laboratories, Daiichi Sankyo and others. Some of these competitors may be addressing the same therapeutic areas or indications as we are. Our current and potential competitors may succeed in obtaining patent protection or commercializing products before us. Many of these entities have significantly greater research and development capabilities than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us. Acquisitions of, or investments in, competing pharmaceutical or biotechnology companies by large corporations could increase such competitors’ financial, marketing, manufacturing and other resources.
Competition for our ALZET product line primarily consists of customers choosing to utilize delivery methods for their research projects other than an osmotic pump. We may also face competition for our ALZET product line from other companies including low cost foreign competitors.
We are engaged in the development of novel therapeutic technologies. Our resources are limited and we may experience technical challenges inherent in such novel technologies. Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competitive products. Some of these products may have an entirely different approach or means of accomplishing similar therapeutic effects than our product candidates. Our competitors may develop products that are safer, more effective or less costly than our product candidates and, therefore, present a serious competitive threat to our product candidates and product offerings.
52
The widespread acceptance of therapies that are alternatives to ours may limit market acceptance of our products and product candidates if commercialized. Post-operative pain is currently being treated by oral medication, transdermal drug delivery systems, such as drug patches, long-acting and short-acting injectable products and implantable drug delivery devices which will be competitive with our products and product candidates. Many of these treatments are widely accepted in the medical community and have a long history of use. The established use of these competitive products may limit the potential for our products and product candidates to receive widespread acceptance if and when commercialized.
Our relationships with customers and third-party payers are subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings
Healthcare providers, physicians and third-party payers will play a primary role in the recommendation and prescription of POSIMIR and any additional product candidates for which we obtain marketing approval. Our future arrangements with third-party payers and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we may market, sell and distribute our products. As a pharmaceutical company, even though we do not and may not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payers, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. These regulations include:
|
|
• |
the Federal Healthcare Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid, and which will constrain our marketing practices and the marketing practices of our licensees, educational programs, pricing policies, and relationships with healthcare providers or other entities; |
|
|
• |
the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of “designated health services” with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies; |
|
|
• |
federal false claims laws that prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other government reimbursement programs that are false or fraudulent, and which may expose entities that provide coding and billing advice to customers to potential criminal and civil penalties, including through civil whistleblower or qui tam actions, and including as a result of claims presented in violation of the Federal Healthcare Anti-Kickback Statute, the Stark Law or other healthcare-related laws, including laws enforced by the FDA; |
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services, and which as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
|
• |
federal physician sunshine requirements under the Affordable Care Act, which requires manufacturers of drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; |
|
|
• |
the Federal Food, Drug, and Cosmetic Act, which, among other things, strictly regulates drug product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; and |
|
|
• |
state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non- governmental third-party payers, including private insurers, state laws requiring pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and which may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state and foreign laws governing the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws such as HIPAA, thus complicating compliance efforts. |
53
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success
In the United States and some non-U.S. jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities, affect our ability to profitably sell any product candidates for which we obtain marketing and otherwise affect our future revenue and profitability and the future revenue and profitability of our collaborators or potential collaborators.
For example, in March 2010, the Affordable Care Act was enacted in the United States to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The law appears likely to continue the downward pressure on the pricing of medical items and services, especially under the Medicare program, and increased the industry’s regulatory burdens and operating costs.
The previous U.S. presidential administration signed two Executive Orders designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the Affordable Care Act have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” As legislative and regulatory developments are ongoing, we cannot predict the ultimate content, timing or effect of healthcare reform legislation or the impact of potential legislation on our business.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. These changes include reductions to Medicare payments to providers of up to 2% per fiscal year that will remain in effect through 2027; the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. More recently, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. For example, the marketing, pricing and sale of the Company’s products are subject to regulation, investigations and legal actions including under the Medicaid Drug Rebate Program under the Affordable Care Act, which has increased the statutory minimum rebates a manufacturer must pay under the program as well as a new methodology by which rebates are owed for drugs that are inhaled, infused, instilled, implanted or injected. We are also subject to federal and state false claims acts, as well as federal and state antitrust and consumer protection laws. Increased scrutiny of health care industry business practices in recent years by government agencies and state attorneys general in the U.S., and any resulting investigations and prosecutions, carry risk of significant civil and criminal penalties including, but not limited to, debarment from participation in such government healthcare programs.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product and medical device pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, in October 2017, California passed a new law, effective in January 2019, which requires transparency from biopharmaceutical companies regarding price increases for prescription drugs. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and medical devices to purchase and which suppliers will be included in their prescription drug and other healthcare programs.
54
We expect that the Affordable Care Act, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we receive for any approved or cleared product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to new requirements or policies, or if we are not able to maintain regulatory compliance, our products and product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, or be able to enter attractive collaboration agreements, which would adversely affect our business.
We could be exposed to significant product liability claims which could be time consuming and costly to defend, divert management attention and adversely impact our ability to obtain and maintain insurance coverage
The testing, clinical development, manufacture, marketing and sale of our products and product candidates involve an inherent risk that product liability claims will be asserted against us. Although we are insured against such risks up to an annual aggregate limit in connection with clinical trials and commercial sales of our product candidates, our present product liability insurance may be inadequate and may not fully cover the costs of any claim or any ultimate damages we might be required to pay. Product liability claims or other claims related to our product candidates, regardless of their outcome, could require us to spend significant time and money in litigation or to pay significant damages. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. In addition, product liability coverage may cease to be available in sufficient amounts or at an acceptable cost. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products or product candidates if and when approved. A product liability claim could also significantly harm our reputation and delay market acceptance of our products and product candidates.
Acceptance of our products and product candidates in the marketplace is uncertain, and failure to achieve market acceptance will delay our ability to generate or grow revenues
Our future financial performance will depend upon the successful introduction and customer acceptance of our products and product candidates in research and development, including DUR-928, and our FDA approved product, POSIMIR, if commercially launched, and including Indivior’s PERSERIS and Orient Pharma’s Methydur. Even if approved for marketing, these products and product candidates may not achieve market acceptance. The degree of market acceptance will depend upon a number of factors, including:
|
|
• |
the receipt of regulatory clearance of marketing claims for the uses that we are developing; |
|
|
• |
the approved product labeling; |
|
|
• |
pricing, reimbursement and formulary access; |
|
|
• |
the establishment and demonstration in the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapies; and |
|
|
• |
pricing and reimbursement policies of government and third-party payors such as insurance companies, health maintenance organizations, hospital formularies and other health plan administrators. |
Physicians, patients, payers or the medical community in general may be unwilling to accept, utilize or recommend any of our products. If we are unable to obtain regulatory approval, commercialize and market our current and future products when planned and achieve market acceptance, we will not achieve meaningful revenues.
If users of our products are unable to obtain adequate reimbursement from third-party payers, or if new restrictive legislation is adopted, market acceptance of our products may be limited and we may not achieve meaningful revenues or profitability
The continuing efforts of government and insurance companies, health maintenance organizations and other payers of healthcare costs to contain or reduce costs of health care may affect our future revenues and profitability, and the future revenues and profitability of our potential customers, suppliers and third-party collaborators and the availability of capital. For example, in certain foreign markets, pricing or profitability of prescription pharmaceuticals is subject to government control. In the United States, recent federal and state government initiatives have been directed at lowering the total cost of health care, and the U.S. Congress and state legislatures will likely continue to focus on health care reform, the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid systems. While we cannot predict whether any such legislative or regulatory proposals will be adopted, the announcement or adoption of such proposals could materially harm our business, financial condition and results of operations.
The successful commercialization of our current and future products will depend in part on the extent to which appropriate reimbursement levels for the cost of our products and related treatment are obtained from governmental authorities, private health insurers and other organizations, such as HMOs. Third-party payers often limit payments or reimbursement for medical products and services. Also, the trend toward managed health care in the United States and the concurrent growth of organizations such as HMOs, which could control or significantly influence the purchase of health care services and products, as well as legislative proposals to reform health care or reduce government insurance programs, may limit reimbursement or payment for our products. The cost containment measures that health care payers and providers are instituting and the effect of any health care reform could materially harm our ability to operate profitably and access capital.
55
If we or our third-party collaborators are unable to train physicians to use our products and product candidates to treat patients’ diseases or medical conditions, we may incur delays in market acceptance of our products
Broad use of certain of our products, such as POSIMIR, will require extensive training of numerous physicians on their proper and safe use. The time required to begin and complete training of physicians could delay introduction of our products and adversely affect market acceptance of our products. We or third parties selling our products may be unable to rapidly train physicians in numbers sufficient to generate adequate demand for our products. Any delay in training would materially delay the demand for our products and harm our business and financial results. In addition, we may expend significant funds towards such training before any orders are placed for our products, which would increase our expenses and harm our financial results.
Potential new accounting pronouncements and legislative actions are likely to impact our future financial position or results of operations
Future changes in financial accounting standards may cause adverse, unexpected fluctuations in the timing of the recognition of revenues or expenses and may affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with frequency and may occur in the future and we may make changes in our accounting policies in the future. Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new SEC regulations, PCAOB pronouncements and NASDAQ rules, are creating uncertainty for companies such as ours and insurance, accounting and auditing costs are high as a result of this uncertainty and other factors. Compliance with evolving corporate governance and public disclosure standards may result in increased general and administrative expenses and a diversion of management time and attention from value-creating activities to compliance activities.
Risks related to actions on trade by the U.S. and foreign governments could adversely affect the Company's results of operations and financial condition
The U.S. government under the previous administration indicated its intent to adopt a new approach to trade policy and in some cases to renegotiate, or potentially terminate, certain existing bilateral or multilateral trade agreements. It also initiated the imposition of tariffs on certain foreign products. Changes in U.S. trade policy have resulted in, and could continue to result in, one or more U.S. trading partners adopting responsive trade policy making it more difficult or costly for us to export our products to those countries. These measures could also result in increased costs for goods imported into the United States. This in turn could require us to increase prices to our customers which may reduce demand, or, if we are unable to increase prices, result in lowering our margin on products sold.
There is also a concern that the imposition of additional tariffs by the United States could result in the adoption of tariffs by other countries. A potential resulting trade war could have a significant adverse effect on world trade and the world economy. We cannot predict future trade policy or the terms of any renegotiated trade agreements and their impact on our business. The adoption and expansion of trade restrictions, the occurrence of a trade war, or other governmental action related to tariffs or trade agreements or policies has the potential to adversely impact demand for our products, our costs, our customers, our suppliers, and the U.S. economy, which in turn could adversely impact our business, financial condition and results of operations.
Risks Related To Our Common Stock
Our operating history makes evaluating our stock difficult
Our quarterly and annual results of operations have historically fluctuated and we expect will continue to fluctuate for the foreseeable future. We believe that period-to-period comparisons of our operating results should not be relied upon as predictive of future performance. Our prospects must be considered in light of the risks, expenses and difficulties encountered by companies with a limited number of approved pharmaceutical products, particularly companies in new and rapidly evolving markets such as pharmaceuticals and biotechnology. To address these risks, we must, among other things, obtain regulatory approval for and commercialize our product candidates, which may not occur. We may not be successful in addressing these risks and difficulties. We may require additional funds to complete the development of our product candidates and to fund operating losses to be incurred in the next several years.
56
*Investors may experience substantial dilution of their investment
In order to raise capital and for other purposes, we may in the future offer and issue additional shares of our common stock or other securities convertible into or exchangeable for our common stock, and the price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share at which investors in our common stock bought their shares. In August 2018, we filed a shelf registration statement on Form S-3 with the SEC that allows us to offer up to $175 million of securities from time to time in one or more public offerings, inclusive of up to $75.0 million of additional shares of common stock which the Company may sell, subject to certain limitations, under the 2015 Sales Agreement through Cantor Fitzgerald, acting as agent. Through several financings between 2019 and March 31, 2021, and through our 2015 Sales Agreement with Cantor Fitzgerald during this period, we have raised an aggregate of $79.4 million, such that we may sell an additional $95.6 million under our existing shelf registration statement. We may also file in the future a new shelf registration statement on Form S-3 for the potential sale of additional securities. Any additional sales in the public market of our common stock, under our 2015 Sales Agreement with Cantor Fitzgerald, in other offerings under the shelf registration statement or otherwise, could adversely affect prevailing market prices for our common stock. In addition, as of March 31, 2021, 25,328,732 shares of our common stock were issuable upon exercise of stock options outstanding under our stock option plans at a weighted average exercise price of $1.39 per share, 7,043,094 additional shares of common stock were reserved for potential future issuance under our stock option plan, and an aggregate of 497,270 shares of common stock were reserved for potential future issuance under our 2000 Employee Stock Purchase Plan. Investors will incur dilution from the sale of any additional shares or upon the issuance of any shares pursuant to such plans, or upon exercise of any outstanding options.
Our stock price has in the past and may in the future not meet the minimum bid price for continued listing on Nasdaq. Our ability to continue operations or to publicly or privately sell equity securities and the liquidity of our common stock could be adversely affected if we are delisted from Nasdaq
In several instances in the past, including as recently as December 2018, we received written notification from Nasdaq informing us that because the closing bid price of our common stock was below $1.00 for 30 consecutive trading days, our shares no longer complied with the minimum closing bid price requirement for continued listing on Nasdaq under Nasdaq Marketplace Rules. Each time, we were given a period of 180 days from the date of the notification and in one case an extra 180-day period to regain compliance with Nasdaq’s listing requirements by having the closing bid price of our common stock listed on Nasdaq be at least $1.00 for at least 10 consecutive trading days.
While we have regained compliance within the applicable time periods in the past, if our shares again no longer comply with the minimum closing bid price requirement for continued listing on the Nasdaq Capital Market under Nasdaq Marketplace Rule 5550(a)(2) and we do not regain compliance within the applicable 180-day time period, Nasdaq will notify us that our securities will be subject to delisting. One strategy to regain compliance in such circumstances would be to implement a reverse stock split. The Company may appeal Nasdaq’s determination to delist its securities to a Hearings Panel. During any appeal process, shares of our common stock would continue to trade on the Nasdaq Capital Market.
There can be no assurance that we will maintain compliance with the requirements for listing our common stock on the Nasdaq Capital Market. Delisting from Nasdaq would constitute an event of default under our loan facility with Oxford, entitling Oxford to accelerate our obligations under such facility, among other actions. Under such circumstances, we could be required to renegotiate the repayment terms of our loan facility, on terms which would not be as favorable to the Company as our current terms, or we could be required to take other actions, such as discontinuing some or all of our operations, selling assets, or other action. Delisting could also adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly affect the ability of investors to trade our securities and would negatively affect the value and liquidity of our common stock. Delisting could also have other negative results, including the potential loss of confidence by employees, the loss of institutional investor interest and fewer business development opportunities.
Our ability to use net operating losses and certain other tax attributes is uncertain and may be limited
Our ability to use our federal and state net operating losses to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income before the expiration dates of the net operating losses, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use any or all of our net operating losses. In addition, utilization of net operating losses to offset potential future taxable income and related income taxes that would otherwise be due is subject to annual limitations under the “ownership change” provisions of Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (Internal Revenue Code) and similar state provisions, which may result in the expiration of net operating losses before future utilization. In general, under the Code, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating losses and other pre-change tax attributes (such as research and development credit carryforwards) to offset its post-change taxable income or taxes may be limited. Our equity offerings and other changes in our stock ownership, some of which are outside of our control, may have resulted or could in the future result in an ownership change. If an ownership change limitation were to apply, utilization of our domestic net operating losses and tax credit carryforwards could be limited in future periods and a portion of the carryforwards could expire before being available to reduce future income tax liabilities.
57
*The price of our common stock may be volatile
The stock markets in general, and the markets for pharmaceutical stocks in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock.
Price declines in our common stock could result from general market and economic conditions and a variety of other factors, including:
|
|
• |
adverse results (including adverse events or failure to demonstrate safety or efficacy) or delays in our clinical and non-clinical trials of DUR-928 or other product candidates; |
|
|
• |
announcements of FDA non-approval of our product candidates, approvals with commercially limiting labels, or delays in the FDA or other foreign regulatory agency review process; |
|
|
• |
adverse actions taken by regulatory agencies or law enforcement agencies with respect to our product candidates, clinical trials, manufacturing processes, accounting practices or sales and marketing activities, or those of our third-party collaborators; |
|
|
• |
announcements of technological innovations, patents, product approvals or new products by our competitors; |
|
|
• |
failure of third-party collaborators to continue development or commercialization of the respective product candidates they are developing or commercializing; |
|
|
• |
failure or delays to enter an agreement with a third party to commercialize POSIMIR, or entering into such an agreement on terms deemed to be unfavorable relative to market expectations |
|
|
• |
failure by us or our commercial licensee to successfully launch POSIMIR within a reasonable time and/or to successfully commercialize POSIMIR |
|
|
• |
regulatory, judicial and patent developments in the United States and foreign countries; |
|
|
• |
any lawsuit or arbitration involving us or our product candidates including intellectual property infringement or product liability suits; |
|
|
• |
announcements concerning our competitors, or the biotechnology or pharmaceutical industries in general; |
|
|
• |
developments concerning our strategic alliances or acquisitions or termination of such alliances; |
|
|
• |
actual or anticipated variations in our operating results; |
|
|
• |
changes in recommendations by securities analysts, misstatements or mischaracterizations in analyst reports or dropping or lack of analyst coverage; |
|
|
• |
negative press coverage or online misinformation about the Company or its partners or their respective products or personnel; |
|
|
• |
deviations in our operating results from the estimates of analysts; |
|
|
• |
sales of our common stock by our executive officers or directors or sales of substantial amounts of common stock by us or others; |
|
|
• |
potential failure to meet continuing listing standards from The Nasdaq Capital Market; |
|
|
• |
loss or disruption of facilities due to natural disasters; |
|
|
• |
acceleration of our debt obligations due to a determination by our lender that a material adverse change has occurred; |
|
|
• |
changes in accounting principles; or |
|
|
• |
loss of any of our key scientific or management personnel. |
The market price of our common stock may fluctuate significantly in response to factors which are beyond our control. The stock market in general has recently experienced extreme price and volume fluctuations. For example, the outbreak of the COVID-19 coronavirus, oil price volatility and other factors have caused broad stock market and industry fluctuations. In addition, the market prices of securities of technology and pharmaceutical companies have also been extremely volatile, and have experienced fluctuations that often have been unrelated or disproportionate to the operating performance of these companies. These broad market fluctuations could result in extreme fluctuations in the price of our common stock, which could cause a decline in the value of our common stock.
58
In the past, following periods of volatility in the market price of a particular company’s securities, litigation has often been brought against that company. If litigation of this type is brought against us, it could be extremely expensive, particularly if we were to lose the lawsuit and have to pay damages, and divert management’s attention and our company’s resources.
We have broad discretion over the use of our cash and investments, and their investment may not always yield a favorable return
Our management has broad discretion over how our cash and investments are used and may from time to time invest in ways with which our stockholders may not agree and that do not yield favorable returns.
Our certificate of incorporation, our bylaws and Delaware law contain provisions that could discourage another company from acquiring us
Provisions of Delaware law, our certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions include:
|
|
• |
authorizing the issuance of “blank check” preferred stock without any need for action by stockholders; |
|
|
• |
providing for a classified board of directors with staggered terms; |
|
|
• |
requiring supermajority stockholder voting to effect certain amendments to our certificate of incorporation and bylaws; |
|
|
• |
eliminating the ability of stockholders to call special meetings of stockholders; |
|
|
• |
prohibiting stockholder action by written consent; and |
|
|
• |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings. |
Our bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees
Our bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on behalf of the Company, any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company, any action asserting a claim arising pursuant to any provision of the General Corporation Law of Delaware or our Certificate of Incorporation or bylaws or any action asserting a claim governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees.
Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
59
|
Item 2. |
Unregistered Sales of Equity Securities and Use of Proceeds |
None
|
Item 3. |
Defaults Upon Senior Securities |
None
|
Item 4. |
Mine Safety Disclosures |
Not applicable
|
Item 5. |
Other Information |
None
|
Item 6. |
Exhibits |
|
Exhibit Number |
|
Exhibit Name |
|
|
|
|
|
|
|
|
|
|
|
|
31.1* |
|
||
|
|
|
|
|
|
31.2* |
|
||
|
|
|
|
|
|
32.1** |
|
||
|
|
|
|
|
|
32.2** |
|
||
|
|
|
|
|
|
101.INS* |
|
INLINE XBRL Instance Document - The instance document does not appear in the interactive data file because its XBRL tags are embedded within the inline XBRL document. |
|
|
|
|
|
|
|
101.SCH* |
|
INLINE XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
101.CAL* |
|
INLINE XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
101.DEF* |
|
INLINE XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
101.LAB* |
|
INLINE XBRL Taxonomy Extension Labels Linkbase Document |
|
|
|
|
|
|
|
101.PRE* |
|
INLINE XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL with applicable taxonomy extension information contained in Exhibits 101.) |
|
|
|
|
|
|
|
* |
Filed herewith. |
|
** |
Furnished, not filed. |
60
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
DURECT CORPORATION |
||
|
|
|
|
|
|
|
By: |
|
/S/ JAMES E. BROWN |
|
|
|
|
James E. Brown Chief Executive Officer |
|
|
|
|
|
|
Date: May 5, 2021 |
|
|
|
|
|
|
|
|
|
|
By: |
|
/S/ MICHAEL H. ARENBERG |
|
|
|
|
Michael H. Arenberg Chief Financial Officer and Principal Accounting Officer |
|
|
|
|
|
|
Date: May 5, 2021 |
|
|
|
61
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-29)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
