HCW Biologics Inc. - Quarter Report: 2023 September (Form 10-Q)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2023
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-40591
HCW Biologics Inc.
(Exact Name of Registrant as Specified in its Charter)
Delaware |
82-5024477 |
( State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
|
|
2929 N. Commerce Parkway Miramar, Florida |
33025 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (954) 842–2024
Securities registered pursuant to Section 12(b) of the Act:
Title of each class
|
Trading Symbol(s)
|
Name of each exchange
|
Common Stock, par value $0.0001 per share |
HCWB |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☒ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 13, 2023, the registrant had 35,974,570 shares of common stock, $0.0001 par value per share, outstanding.
Table of Contents
|
|
Page |
|
PART I. |
1 |
|
|
Item 1. |
1 |
|
|
|
Unaudited condensed interim financial statements as of and for the three and nine months ended September 30, 2022 and September 30, 2023: |
|
|
|
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
4 |
|
|
|
5 |
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
13 |
|
Item 3. |
26 |
|
|
Item 4. |
26 |
|
|
PART II. |
27 |
|
|
Item 1. |
27 |
|
|
Item 1A. |
27 |
|
|
Item 2. |
27 |
|
|
Item 3. |
27 |
|
|
Item 4. |
27 |
|
|
Item 5. |
27 |
|
|
Item 6. |
28 |
|
|
29 |
|
||
i
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
HCW Biologics Inc.
Condensed Balance Sheets
|
|
December 31, |
|
|
September 30, |
|
||
|
|
2022 |
|
|
2023 |
|
||
|
|
|
|
|
Unaudited |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
22,326,356 |
|
|
$ |
11,220,793 |
|
Short-term investments |
|
|
9,735,930 |
|
|
|
— |
|
Accounts receivable, net |
|
|
417,695 |
|
|
|
710,078 |
|
Prepaid expenses |
|
|
1,394,923 |
|
|
|
1,742,341 |
|
Other current assets |
|
|
196,015 |
|
|
|
174,881 |
|
Total current assets |
|
|
34,070,919 |
|
|
|
13,848,093 |
|
Investments |
|
|
1,599,751 |
|
|
|
1,599,751 |
|
Property, plant and equipment, net |
|
|
10,804,610 |
|
|
|
14,780,872 |
|
Deposit for interest reserve |
|
|
— |
|
|
|
5,250,000 |
|
Other assets |
|
|
333,875 |
|
|
|
137,626 |
|
Total assets |
|
$ |
46,809,155 |
|
|
$ |
35,616,342 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||
Liabilities |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
1,226,156 |
|
|
$ |
3,153,834 |
|
Accrued liabilities and other current liabilities |
|
|
1,730,325 |
|
|
|
2,262,839 |
|
Total current liabilities |
|
|
2,956,481 |
|
|
|
5,416,673 |
|
Debt, net |
|
|
6,409,893 |
|
|
|
6,332,736 |
|
Other liabilities |
|
|
14,275 |
|
|
|
— |
|
Total liabilities |
|
|
9,380,649 |
|
|
|
11,749,409 |
|
(Note 8) |
|
|
|
|
|
|
||
Stockholders’ equity: |
|
|
|
|
|
|
||
Common stock: |
|
|
|
|
|
|
||
Common, $0.0001 par value; 250,000,000 shares authorized |
|
|
3,588 |
|
|
|
3,593 |
|
Additional paid-in capital |
|
|
82,962,964 |
|
|
|
83,715,133 |
|
Accumulated deficit |
|
|
(45,538,046 |
) |
|
|
(59,851,793 |
) |
Total stockholders’ equity |
|
|
37,428,506 |
|
|
|
23,866,933 |
|
Total liabilities and stockholders’ equity |
|
$ |
46,809,155 |
|
|
$ |
35,616,342 |
|
See accompanying notes to the unaudited condensed interim financial statements.
1
HCW Biologics Inc.
Condensed Statements of Operations
(Unaudited)
|
|
Three Months Ended |
|
|
|
Nine Months Ended |
|
|
||||||||||
|
|
2022 |
|
|
2023 |
|
|
|
2022 |
|
|
2023 |
|
|
||||
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Revenues |
|
$ |
1,809,025 |
|
|
$ |
853,102 |
|
|
|
$ |
5,380,570 |
|
|
$ |
1,517,792 |
|
|
Cost of revenues |
|
|
(1,447,220 |
) |
|
|
(678,325 |
) |
|
|
|
(3,062,496 |
) |
|
|
(1,210,077 |
) |
|
Net revenues |
|
|
361,805 |
|
|
|
174,777 |
|
|
|
|
2,318,074 |
|
|
|
307,715 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
2,648,794 |
|
|
|
1,667,442 |
|
|
|
|
6,408,353 |
|
|
|
5,539,919 |
|
|
General and administrative |
|
|
1,732,666 |
|
|
|
3,585,215 |
|
|
|
|
5,321,262 |
|
|
|
9,716,765 |
|
|
Total operating expenses |
|
|
4,381,460 |
|
|
|
5,252,657 |
|
|
|
|
11,729,615 |
|
|
|
15,256,684 |
|
|
Loss from operations |
|
|
(4,019,655 |
) |
|
|
(5,077,880 |
) |
|
|
|
(9,411,541 |
) |
|
|
(14,948,969 |
) |
|
Interest expense |
|
|
(32,184 |
) |
|
|
(95,514 |
) |
|
|
|
(32,184 |
) |
|
|
(284,465 |
) |
|
Other (expense) income, net |
|
|
137,645 |
|
|
|
234,753 |
|
|
|
|
(38,237 |
) |
|
|
919,688 |
|
|
Net loss |
|
$ |
(3,914,194 |
) |
|
$ |
(4,938,641 |
) |
|
|
$ |
(9,481,962 |
) |
|
$ |
(14,313,746 |
) |
|
Net loss per share, basic and diluted |
|
$ |
(0.11 |
) |
|
$ |
(0.14 |
) |
|
|
$ |
(0.26 |
) |
|
$ |
(0.40 |
) |
|
Weighted average shares outstanding, basic and diluted |
|
|
35,835,135 |
|
|
|
35,926,921 |
|
|
|
|
35,809,216 |
|
|
|
35,907,123 |
|
|
See accompanying notes to the unaudited condensed interim financial statements.
2
HCW Biologics Inc.
Condensed Statements of Changes in Stockholders’ Equity
For the Nine Months Ended September 30, 2022 and September 30, 2023
(Unaudited)
|
|
Stockholders’ Equity |
|
|||||||||||||||||
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Total |
|
||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
Balance, December 31, 2021 |
|
|
35,768,264 |
|
|
$ |
3,577 |
|
|
$ |
81,827,006 |
|
|
$ |
(30,637,343 |
) |
|
$ |
51,193,240 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
11,225 |
|
|
|
1 |
|
|
|
2,272 |
|
|
|
— |
|
|
|
2,273 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
260,348 |
|
|
|
— |
|
|
|
260,348 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,057,207 |
) |
|
|
(2,057,207 |
) |
Balance, March 31, 2022 |
|
|
35,779,489 |
|
|
$ |
3,578 |
|
|
$ |
82,089,626 |
|
|
$ |
(32,694,550 |
) |
|
$ |
49,398,654 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
44,434 |
|
|
|
4 |
|
|
|
5,996 |
|
|
|
— |
|
|
|
6,000 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
271,335 |
|
|
|
|
|
|
271,335 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,510,561 |
) |
|
|
(3,510,561 |
) |
Balance, June 30, 2022 |
|
|
35,823,923 |
|
|
$ |
3,582 |
|
|
$ |
82,366,957 |
|
|
$ |
(36,205,111 |
) |
|
$ |
46,165,428 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
12,212 |
|
|
|
2 |
|
|
|
1,672 |
|
|
|
— |
|
|
|
1,674 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
302,320 |
|
|
|
— |
|
|
|
302,320 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,914,194 |
) |
|
|
(3,914,194 |
) |
Balance, September 30, 2022 |
|
|
35,836,135 |
|
|
$ |
3,584 |
|
|
$ |
82,670,949 |
|
|
$ |
(40,119,305 |
) |
|
$ |
42,555,228 |
|
|
|
Stockholders’ Equity |
|
|||||||||||||||||
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Total |
|
||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
Balance, December 31, 2022 |
|
|
35,876,440 |
|
|
$ |
3,588 |
|
|
$ |
82,962,964 |
|
|
$ |
(45,538,046 |
) |
|
$ |
37,428,506 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
10,195 |
|
|
|
1 |
|
|
|
1,900 |
|
|
|
— |
|
|
|
1,901 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
259,206 |
|
|
|
— |
|
|
|
259,206 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,070,686 |
) |
|
|
(5,070,686 |
) |
Balance, March 31, 2023 |
|
|
35,886,635 |
|
|
$ |
3,589 |
|
|
$ |
83,224,070 |
|
|
$ |
(50,608,732 |
) |
|
$ |
32,618,927 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
40,086 |
|
|
|
4 |
|
|
|
7,708 |
|
|
|
— |
|
|
|
7,712 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
263,423 |
|
|
|
— |
|
|
|
263,423 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,304,420 |
) |
|
|
(4,304,420 |
) |
Balance, June 30, 2023 |
|
|
35,926,721 |
|
|
|
3,593 |
|
|
|
83,495,201 |
|
|
|
(54,913,152 |
) |
|
|
28,585,642 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
600 |
|
|
|
— |
|
|
|
84 |
|
|
|
— |
|
|
|
84 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
219,848 |
|
|
|
— |
|
|
|
219,848 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,938,641 |
) |
|
|
(4,938,641 |
) |
Balance, September 30, 2023 |
|
|
35,927,321 |
|
|
$ |
3,593 |
|
|
$ |
83,715,133 |
|
|
$ |
(59,851,793 |
) |
|
$ |
23,866,933 |
|
See accompanying notes to the unaudited condensed interim financial statements.
3
HCW Biologics Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
|
Nine Months Ended September 30, |
|
|||||
|
|
2022 |
|
|
2023 |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(9,481,962 |
) |
|
$ |
(14,313,746 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
456,696 |
|
|
|
860,634 |
|
Stock-based compensation |
|
|
834,003 |
|
|
|
742,477 |
|
Unrealized loss (gain) on investments, net |
|
|
253,550 |
|
|
|
(248,445 |
) |
Realized (gain) on investments |
|
|
— |
|
|
|
(15,625 |
) |
Reduction in the carrying amount of right-of-use asset |
|
|
1,463 |
|
|
|
(1,045 |
) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable |
|
|
(222,555 |
) |
|
|
(292,383 |
) |
Deposit for interest reserve |
|
|
— |
|
|
|
(5,250,000 |
) |
Prepaid expenses and other assets |
|
|
1,854,155 |
|
|
|
(251,008 |
) |
Accounts payable and other liabilities |
|
|
(202,979 |
) |
|
|
392,802 |
|
Operating lease liability |
|
|
(89,110 |
) |
|
|
(242,990 |
) |
Net cash used in operating activities |
|
|
(6,596,739 |
) |
|
|
(18,619,329 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
(10,206,441 |
) |
|
|
(2,486,950 |
) |
Proceeds for sale or maturities of short-term investments |
|
|
24,983,520 |
|
|
|
10,000,000 |
|
Net cash provided by investing activities |
|
|
14,777,079 |
|
|
|
7,513,050 |
|
Cash flows from financing activities: |
|
|
|
|
|
|
||
Proceeds from issuance of common stock |
|
|
9,947 |
|
|
|
9,697 |
|
Proceeds from issuance of debt, net |
|
|
6,448,166 |
|
|
|
— |
|
Offering costs |
|
|
(144,870 |
) |
|
|
— |
|
Debt repayment |
|
|
— |
|
|
|
(8,981 |
) |
Net cash provided by financing activities |
|
|
6,313,243 |
|
|
|
716 |
|
Net increase (decrease) in cash and cash equivalents |
|
|
14,493,583 |
|
|
|
(11,105,563 |
) |
Cash and cash equivalents at the beginning of the period |
|
|
11,730,677 |
|
|
|
22,326,356 |
|
Cash and cash equivalents at the end of the period |
|
$ |
26,224,260 |
|
|
$ |
11,220,793 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
||
Cash paid for interest |
|
$ |
— |
|
|
$ |
284,465 |
|
Noncash operating, investing and financing activities: |
|
|
|
|
|
|
||
Operating lease liabilities arising from obtaining right-of-use assets |
|
$ |
231,196 |
|
|
$ |
— |
|
Capital expenditures accrued, but not yet paid |
|
$ |
— |
|
|
$ |
2,095,724 |
|
See accompanying notes to the unaudited condensed interim financial statements.
4
HCW Biologics Inc.
Notes to Condensed Financial Statements
(Unaudited)
1. Organization and Summary of Significant Accounting Policies
Organization
HCW Biologics Inc. (the “Company”) is a clinical stage biopharmaceutical company focused on discovering and developing novel immunotherapies to lengthen healthspan by disrupting the link between chronic, low-grade inflammation and age-related diseases. The Company believes age-related low-grade chronic inflammation, or “inflammaging,” is a significant contributing factor to several chronic diseases and conditions, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and autoimmune diseases. The Company is located in Miramar, Florida and was incorporated in the state of Delaware in April 2018.
Liquidity
As of September 30, 2023, the Company had not generated any revenue from commercial product sales of its internally-developed immunotherapeutic products for the treatment of cancer and other age-related diseases. In the course of its development activities, the Company has sustained operating losses and expects to continue to incur operating losses for the foreseeable future. Since inception, substantially all the Company’s activities have consisted of research, development, establishing large-scale cGMP production for clinical trials, and raising capital. The Company's total revenues to date have been generated solely from the Wugen License and its manufacturing and supply arrangement with Wugen. In the three and nine months ended September 30, 2023, the Company recognized revenues from manufacturing and supply of materials for Wugen of $853,102 and $1.5 million, respectively.
As of September 30, 2023, the Company had cash and cash equivalents of $11.2 million and a deposit for interest reserve of $5.3 million. Since inception to September 30, 2023, the Company incurred cumulative net losses of $57.1 million. Management expects to incur additional losses in the future to conduct product research and development and recognizes the need to raise additional capital to fully implement its business plan. In November 2023, the Company expects to begin to draw down on its $26.3 million Development Line of Credit Agreement, the proceeds of which will be primarily used for the buildout of the Company's headquarters, including upgraded research laboratories and a new manufacturing facility. See Note 8. Commitments and Contingencies - Project Financing herein. The Company intends to raise capital through additional debt or equity financings. In addition, the Company intends to continue its efforts to enter into business development transactions. Business development efforts are focused on out-licensing rights to non-core assets or regional markets, third-party collaboration funding, cooperative agreements for clinical trials, and other transactions. However, if the Company is not able to obtain financing at adequate levels, it will need to reevaluate its operating plan and may be required to delay the development of some of its products.
Summary of Significant Accounting Policies
Basis of Presentation
Unaudited Interim Financial Information
The accompanying unaudited condensed interim financial statements as of September 30, 2023 and for the three and nine months ended September 30, 2022 and 2023 have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and pursuant to Article 10 of Regulation S-X of the Securities Act of 1933, as amended (the “Securities Act”). Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. These unaudited condensed interim financial statements include only normal and recurring adjustments that the Company believes are necessary to fairly state the Company’s financial position and the results of its operations and cash flows. The results for the three and nine months ended September 30, 2023 are not necessarily indicative of the results expected for the full fiscal year or any subsequent interim period. The condensed balance sheet at December 31, 2022 has been derived from the audited financial statements at that date but does not include all disclosures required by U.S. GAAP for complete financial statements. Because all of the disclosures required by U.S. GAAP for complete financial statements are not included herein, these unaudited condensed interim financial statements and the notes accompanying them should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2022 which appear in the Company’s Annual Report on Form 10-K (No. 001-40591) filed for the year ended December 31, 2022 with the Securities and Exchange Commission (the “SEC”) on March 28, 2023 and in other filings with the SEC.
5
Deposit for Interest Reserve
The Company has established an interest reserve account for the purpose of paying interest on outstanding debt under the Development Line of Credit Agreement which is further described in Note 8. Commitments and Contingencies - Project Financing herein. As of September 30, 2023, there was a balance of $5.3 million included in Deposit for interest reserve in noncurrent assets on the accompanying condensed balance sheet.
Revenue Recognition
The Company accounts for revenues in accordance with Accounting Standards Codification Topic 606, Revenue from Contracts with Customers (“Topic 606”). To determine revenue recognition for arrangements that fall within the scope of Topic 606, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the Company satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that it will collect the consideration it is entitled to in exchange for the goods or services transferred to the customer.
At contract inception, the Company assesses the goods or services promised within each contract, determines those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. To date, the Company's revenues have been generated solely from transactions with Wugen. The Wugen License includes licenses of intellectual property, cost reimbursements, upfront signing fees, milestone payments and royalties on future licensee’s product sales. In addition, the Company and Wugen have an agreement for supply of materials, from which the Company also recognizes revenues.
License Grants:
For out-licensing arrangements that include a grant of a license to the Company’s intellectual property, the Company considers whether the license grant is distinct from the other performance obligations included in the arrangement. For licenses that are distinct, the Company recognizes revenues from nonrefundable, upfront payments and other consideration allocated to the license when the license term has begun and the Company has provided all necessary information regarding the underlying intellectual property to the customer, which generally occurs at or near the inception of the arrangement.
Milestone and Contingent Payments:
At the inception of the arrangement and at each reporting date thereafter, the Company assesses whether it should include any milestone and contingent payments or other forms of variable consideration in the transaction price using the most likely amount method. If it is probable that a significant reversal of cumulative revenue would not occur upon resolution of the uncertainty, the associated milestone value is included in the transaction price. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of each such milestone and any related constraint and, if necessary, adjusts its estimate of the overall transaction price. Since milestone and contingent payments may become payable to the Company upon the initiation of a clinical study or filing for or receipt of regulatory approval, the Company reviews the relevant facts and circumstances to determine when the Company should update the transaction price, which may occur before the triggering event. When the Company updates the transaction price for milestone and contingent payments, the Company allocates the changes in the total transaction price to each performance obligation in the agreement on the same basis as the initial allocation. Any such adjustments are recorded on a cumulative catch-up basis in the period of adjustment, which may result in recognizing revenue for previously satisfied performance obligations in such period. The Company’s licensees will generally pay milestones payments subsequent to achievement of the triggering event.
Materials Supply:
The Company provides clinical and research grade materials so that licensees may develop products based on the licensed molecules. The Company plans to enter into commercialization supply agreements when licensees enter the commercial stage of their company. The amounts billed are recognized as revenue as the performance obligations are satisfied by the Company, once the Company determines that a contract exists.
On June 18, 2021, the Company entered into a master services agreement (“MSA”) for the supply of materials for clinical development of licensed products. Under the MSA, the Company enters into statements-of-work (“SOWs”) for transactions for the purchase of clinical and research grade materials which meet all requirements necessary to qualify as a contract under Topic 606. The
6
sale of clinical and research material supplied by the Company each represents a single performance obligation that is satisfied over time. The Company recognizes revenue using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company's progress toward completion. As part of the accounting for these arrangements, the Company must develop estimates and assumptions that require judgement to determine the progress towards completion. The Company reviews its estimate of the progress toward completion based on the best information available to recognize the cumulative progress toward completion as of the end of each reporting period, and makes revisions to such estimates, if facts and circumstances change during each reporting period.
The Company recognized revenue related to sale of development supply materials to its licensee, Wugen, of $1.8 million and $5.4 million, respectively, for the three and nine months ended September 30, 2022; and $853,102 and $1.5 million, respectively, for three and nine months ended September 30, 2023.
Investments
The Company holds a minority interest in Wugen which is accounted for using the measurement alternative whereby the investment is recorded at cost less impairment, adjusted for observable price changes in orderly transactions for an identical or similar investment of the same investee. No impairment has been recognized on the minority interest in Wugen as of September 30, 2023. As of December 31, 2022 and September 30, 2023, the Company included $1.6 million for the investment in Wugen in Investments in the accompanying condensed balance sheets.
From time to time, the Company invests in bills and notes issued by the U.S. Treasury which are classified as trading securities. The Company reported a fair value of $9.7 million and nil for the fair value of investments in U.S. Treasury bills as of December 31, 2022 and September 30, 2023, respectively, included in Short-term investments in the accompanying condensed balance sheets.
Operating Leases
The Company determines if an arrangement is a lease at inception. Operating leases are included in Other assets, Accrued liabilities and other current liabilities, and Other liabilities on the accompanying condensed balance sheets. Operating lease Right of Use (“ROU”) assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. The Company has a lease agreement with lease and non-lease components, which are accounted for separately.
Net Loss Per Share
Basic loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during each period. Diluted loss per share of common stock includes the effect, if any, from the potential exercise of stock options and unvested shares of restricted stock, which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share due to the fact that when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
2. Accrued Liabilities and Other Current Liabilities
As of December 31, 2022, the Company had a balance of $1.7 million included in Accrued liabilities and other current liabilities in the accompanying condensed balance sheet, consisting primarily of $416,000 for legal expenses, $277,500 for clinical expenses, $524,000 for bonus expenses, $134,000 for salary and benefits, and $178,000 for a lease liability.
As of September 30, 2023, the Company had a balance of $2.3 million in Accrued liabilities and other current liabilities in the accompanying condensed balance sheet, consisting primarily of the following: $208,000 for retainage related to renovation of the Company's headquarters; $892,000 related to legal matters further described in Note 8. Commitments and Contingencies - Legal herein; $246,000 for clinical expenses; $365,000 related to performance-based bonuses; $81,000 for salary and benefits; $71,000 for a lease liability; $117,680 for the current portion of long-term debt; and $40,000 for legal fees related to patent prosecution as well as other legal expenses incurred in the ordinary course of business.
7
3. Debt, Net
On August 15, 2022, the Company entered into a loan and security agreement (the "2022 Loan Agreement") with Cogent Bank ("Cogent"), pursuant to which it received $6.5 million in gross proceeds to purchase a building that will become the Company's new headquarters. The Cogent loan is secured by a first priority lien on the building.
Under the terms of the 2022 Loan Agreement, the interest-only period is one year followed by 48 months of equal payments of principal and interest beginning on September 15, 2023 based on a 25-year amortization rate. The unamortized balance is due on August 15, 2027 (the “Cogent Maturity Date”), and bears interest at a fixed per annum rate equal to 5.75%. At the Cogent Maturity Date, a final payment of unamortized principal will be due. The Company has the option to prepay the outstanding balance of the loan prior to the Cogent Maturity Date without penalty. The Company is in compliance with all covenants under the 2022 Loan Agreement as of September 30, 2023.
As of December 31, 2022, the 2022 Loan Agreement consisted of $6.5 million which is included in Debt, net on the accompanying condensed balance sheet. As of September 30, 2023, it consisted of a current portion of $117,680 which is included in Accrued liabilities and other current liabilities and a noncurrent portion of $6.3 million which is included in Debt, net in the accompanying condensed balance sheet.
4. Preferred Stock
At December 31, 2022 and September 30, 2023, the Company had 10,000,000 shares of preferred stock authorized and no shares issued.
5. Net Loss Per Share
The following table summarizes the computation of the basic and diluted net loss per share:
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
|
||||||||||
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
||||
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net loss |
|
$ |
(3,914,194 |
) |
|
$ |
(4,938,641 |
) |
|
$ |
(9,481,962 |
) |
|
$ |
(14,313,746 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Weighted-average common shares outstanding |
|
|
35,835,135 |
|
|
|
35,926,921 |
|
|
|
35,809,216 |
|
|
|
35,907,123 |
|
|
Net loss per share, basic and diluted |
|
$ |
(0.11 |
) |
|
$ |
(0.14 |
) |
|
$ |
(0.26 |
) |
|
$ |
(0.40 |
) |
|
The following table summarizes the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because their inclusion would be anti-dilutive:
|
|
At September 30, |
|
|||||
|
|
2022 |
|
|
2023 |
|
||
Common stock options |
|
|
1,907,991 |
|
|
|
1,869,492 |
|
Potentially diluted securities |
|
|
1,907,991 |
|
|
|
1,869,492 |
|
8
6. Fair Value of Financial Instruments
The carrying amount of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, U.S. government-backed securities with maturity dates up to one year, accounts payable, accrued liabilities and other current liabilities, approximate fair value due to their short-term maturities. The balance of funds included in Deposit for interest reserve is held in a non-interest bearing account and its carrying value approximates its fair value.
Money market funds included in cash and cash equivalents and U.S. government-backed securities are measured at fair value based on quoted prices in active markets, which are considered Level 1 inputs. No transfers between levels occurred during the periods presented. The following table presents the Company’s assets which were measured at fair value at December 31, 2022 and September 30, 2023:
|
|
At December 31, 2022: |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
19,458,020 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
19,458,020 |
|
Treasury notes |
|
|
9,735,930 |
|
|
|
— |
|
|
|
— |
|
|
|
9,735,930 |
|
Total |
|
$ |
29,193,950 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
29,193,950 |
|
|
|
At September 30, 2023: |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds |
|
$ |
9,999,421 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
9,999,421 |
|
Total |
|
$ |
9,999,421 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
9,999,421 |
|
7. Income Taxes
The Company computes its quarterly income tax expense/(benefit) by using a forecasted annual effective tax rate and adjusts for any discrete items arising during the quarter. The Company did not have a provision for income taxes (current or deferred tax expense) as of December 31, 2022 and September 30, 2023. The Company will continue to maintain a 100% valuation allowance on total deferred tax assets. The Company believes it is more likely than not that the related deferred tax asset will not be realized. As a result, the Company’s effective tax rate will remain at 0.00% because no items either estimated or discrete items would impact the tax provision.
9
8. Commitments and Contingencies
Operating Leases
The Company has operating leases for approximately 12,250 square feet of space located in Miramar, Florida. The leases have a two-year term which commenced on March 1, 2022 and will terminate on February 29, 2024. Upon the commencement of the leases, the Company used its incremental borrowing rate of 6.0% to determine the amounts to recognize for a ROU asset and a lease liability. There are no obligations under finance leases.
The components of the lease expense for the three and nine months ended September 30, 2023 were as follows:
|
|
For the Three Months |
|
|
For the Nine Months Ended September 30, 2023 |
|
||
Operating lease cost |
|
$ |
42,413 |
|
|
$ |
127,238 |
|
Supplemental cash flow information related to lease for the nine months ended September 30, 2023 was as follows:
|
|
For the Nine Months Ended September 30, 2023 |
|
|
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
Operating cash flows |
|
$ |
127,229 |
|
Right-of-use assets obtained in exchange for lease obligations: |
|
|
|
|
Operating lease |
|
$ |
120,973 |
|
As of September 30, 2023, the supplemental balance sheet information related to leases was as follows:
|
|
As of September 30, 2023 |
|
|
Operating lease right-of-use assets |
|
$ |
69,624 |
|
Operating lease liabilities, current |
|
$ |
70,669 |
|
As of September 30, 2023, the remaining lease payments were as follows:
2023 |
|
$ |
42,831 |
|
2024 |
|
|
28,693 |
|
Total future minimum lease payments |
|
$ |
71,524 |
|
For the three months ended September 30, 2022 and 2023, rent expense recognized by the Company was $43,700 and $42,400, respectively, of which $22,200 and $22,200, respectively, is included in research and development in the accompanying condensed statements of operations.
For the nine months ended September 30, 2022 and 2023, rent expense recognized by the Company was $130,300 and $127,200, respectively, of which $57,800 and $66,600, respectively, is included in research and development in the accompanying condensed statements of operations.
10
Contractual Commitments
The Company entered into an agreement with a third-party global contract development and manufacturer of biologics for the manufacture of the Company’s proprietary molecules for use in clinical trials. At December 31, 2022 and September 30, 2023, future payment obligations under such agreements were $406,000 and $1.8 million, respectively. In addition, as of December 31, 2022, the Company committed to purchase upstream processing and fluid management equipment for $1.6 million, and it advanced $495,000 for this purchase as of September 30, 2023.
Project Financing
On April 21, 2023, the Company entered into a secured Development Line of Credit Agreement (such agreement as amended from time to time, the "2023 Loan Agreement") with Prime Capital Ventures, LLC ("Prime"), pursuant to which Prime will advance loans to the Company in a principal amount not to exceed $26.3 million with a scheduled maturity of April 20, 2028 (the "Prime Maturity Date"). The Company has the option to prepay the balance of the loan prior to the Prime Maturity date without penalty. The note issued pursuant to the 2023 Loan Agreement bears interest at a fixed rate equal to 7.00% per annum, due monthly in arrears on the first day of each month. The primary purpose of the loan is to provide the funding required to complete the buildout of the Company's new headquarters, including improved research laboratories and a vivarium to support the Company's preclinical research efforts, and a manufacturing facility to produce GMP material to support the Company's clinical development as well as produce material to support the clinical development of its licensee, Wugen.
Under the 2023 Loan Agreement, the Company was required to fund an interest reserve bank account controlled by Prime in the amount of $5.3 million to fund the interest payments due thereunder. The balance of the reserve account is presented in Deposit for interest reserve in noncurrent assets on the accompanying condensed balance sheet. As of September 30, 2023, the Company had no outstanding borrowings under the 2023 Loan Agreement. On August 10, 2023, the Company obtained construction permits required to begin the buildout of its new headquarters. This satisfied the final condition precedent to accessing the $26.3 million line of credit. The Company will incur $1.8 million in debt issuance costs in connection to the Prime loan, which will be earned and payable upon the first draw down. In November 2023, the Company expects to begin to draw down funds available under the 2023 Loan Agreement.
Legal
From time to time, the Company is a party to or otherwise involved in legal proceedings, including suits, assessments, regulatory actions and investigations generally arising out of the normal course of business. In addition, the Company enters into agreements that may include indemnification provisions, pursuant to which the Company agrees to indemnify, hold harmless and defend the indemnified parties for losses suffered or incurred by the indemnified party. When the Company believes that the outcome of such a matter will result in a liability that is probable to be incurred and result in a potential loss, or range of loss, that can be reasonably estimated, the Company will accrue a liability and make the appropriate disclosure in the footnotes to the financial statements.
On December 23, 2022, Altor BioScience, LLC and NantCell, Inc. (“Altor/NantCell”) initiated an arbitration against Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, in California alleging breach of contract and fiduciary duty, among other claims. On that same date, Altor/NantCell filed a lawsuit against the Company in federal court alleging misappropriation of trade secrets, inducement of breach of contract and breach of fiduciary duty, among other claims against the Company. On January 31, 2023, the Company filed a motion to compel arbitration, a motion for the stay of the litigation, and a motion to dismiss the complaint (“motion to compel”). On April 18, 2023, the U.S. District Court for the Southern District of Florida (the “Court”) heard oral argument on the Company’s motion to compel and ordered the parties to provide supplemental briefing by April 28, 2023. Before the Court ruled on the Company’s motion to compel, on April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the Court approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Altor/NantCell’s proceeding against the Company is now proceeding in arbitration before JAMS.
11
Inflationary Cost Environment, Geopolitical Risks and Other Macroeconomic Factors
The operations have been affected by many headwinds, including inflationary pressures, rising interest rates, ongoing global supply chain disruptions resulting from increased geopolitical tensions such as the war in the Middle East, the conflict between Russia and Ukraine, Chinese aggression towards Taiwan, financial market volatility and currency movements. The Company has been impacted by inflation, and may continue to be so, when procuring materials required for the buildout of our new headquarters, the costs for recruiting and retaining employees and other employee-related costs. Management employs a number of strategies to effectively navigate these issues, including product redesign, alternate sourcing, and establishing contingencies in budgeting and timelines. Future developments in these and other areas present material uncertainty and risk with respect to the Company's clinical trials, IND-enabling activities, buildout of the new headquarters, as well as the Company's financial condition and results of operations. The extent and duration of such events and conditions, and resulting disruptions to our operations, are highly unpredictable.
9. Subsequent Events
Subsequent events have been evaluated through the date the financial statements were available to be issued. As of such date, there were no material subsequent events identified that required recognition or disclosure other than as disclosed in the footnotes herein.
12
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with (i) our unaudited condensed interim financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q and (ii) our audited financial statements and related notes and the discussion under the heading “Management's Discussion and Analysis of Financial Condition and Results of Operations” for the fiscal year ended December 31, 2022 included in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 28, 2023. Our historical results are not necessarily indicative of the results that may be expected for any period in the future. Unless the context requires otherwise, references in this Quarterly Report on Form 10-Q to the “Company,” “HCW Biologics,” “HCWB”, “we,” “us” and “our” refer to HCW Biologics Inc.
Forward-Looking Statements
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this quarterly report, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success of our clinical trials, plans and objectives of management for future operations, adequacy of our cash resources and working capital, future economic conditions or performance, and future results of anticipated products, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this Quarterly Report on Form 10-Q are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in this report in Part II, Item 1A -“Risk Factors,” in this Quarterly Report on Form 10-Q and in other filings we make with the SEC from time to time. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. These forward-looking statements speak only as of the date hereof. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Overview
HCW Biologics Inc. is a clinical-stage biopharmaceutical company focused on discovering and developing novel immunotherapies to lengthen healthspan by disrupting the link between chronic, low-grade inflammation and age-related diseases. We believe age-related, chronic, low-grade inflammation, or “inflammaging,” is a significant contributing factor to several diseases and conditions, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and autoimmune diseases.
The induction and retention of low-grade inflammation in an aging human body is mainly the result of the accumulation of non-proliferative but metabolically active senescent cells, which can also be caused by persistent activation of protein complexes, known as inflammasomes, in innate immune cells. These two elements share common mechanisms in promoting secretion of pro-inflammatory proteins and in many cases interact to drive senescence, and thus, inflammaging. Our novel approach is to reduce senescent cells and eliminate the pro-inflammatory factors they secrete systemically through multiple pathways. We believe our approach has the potential to fundamentally change the treatment of age-related diseases. Our lead product candidates address the two primary processes that promote chronic inflammation.
13
HCW9218. HCW9218 is our clinical stage lead product candidate which is administered by subcutaneous injection. This bi-functional immunotherapeutic has demonstrated the ability to reduce senescent cells as well as function as a senomorphic that eliminates senescence-associated pro-inflammatory factors. We have made progress in developing a deep understanding of the anti-cancer mechanism of action of HCW9218, especially in relation to how it complements immune checkpoint inhibitors and chemotherapies. In preclinical testing in different cold tumor models and data from correlative studies conducted in conjunction with our clinical trials, HCW9218 has demonstrated that it has a unique mechanism of action that we believe is the key to turning a ‘cold’ tumor into a ‘hot’ tumor, indicating the potential of the anti-cancer utility of HCW9218 in combination with standard-of-care anti-cancer therapies. The graphic below depicts the potential of HCW9218 in widely-used, FDA-approved anti-cancer therapies.
HCW9218 Platform to Boost Anti-Cancer Utility of Standard-of-Care Cancer Therapies
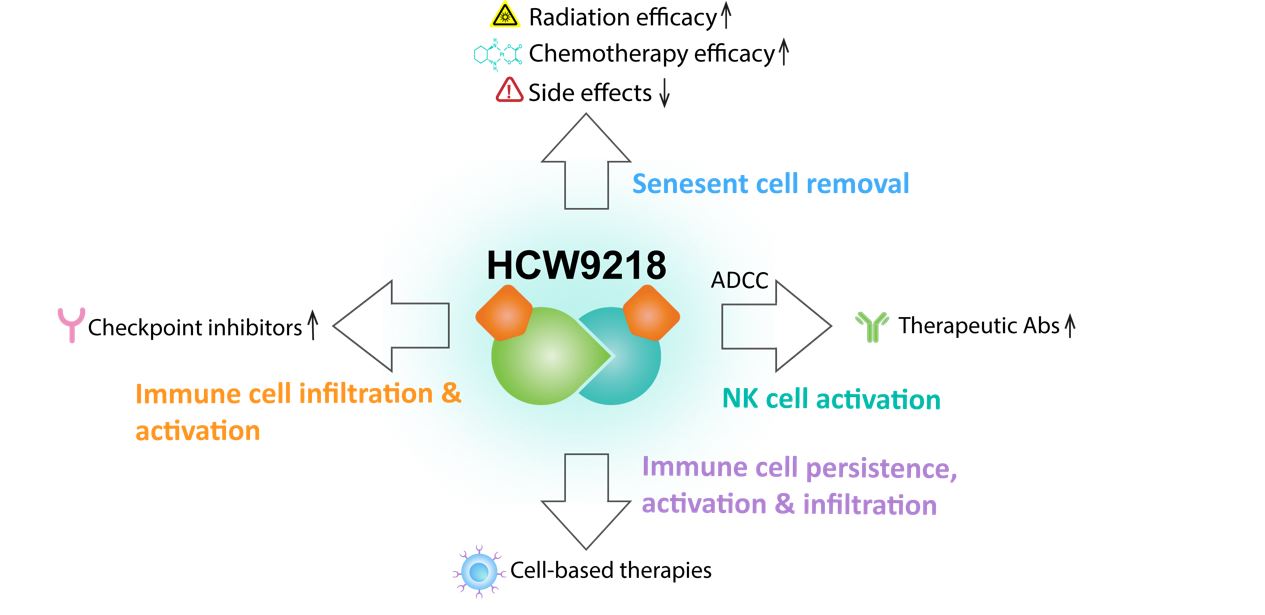
The Company plans to focus its clinical development for future Phase 2 clinical trials in two of the areas of utility depicted in the graphic above:
HCW9218 + chemotherapy. Chemotherapy is the current standard of care for treating most forms of cancer. Tumor cells can undergo senescence and secrete pro-inflammatory factors, or SASP factors, in response to chemotherapy, a process referred to as therapy-induced senescence (“TIS”). SASP factors promote TIS cancer cells to re-enter the growth cycle with stem-like characteristics which can result in disease relapse and metastasis. HCW9218 is designed to treat the impact of accumulated senescent cells and the SASP factors which they secrete by eliminating senescent cells (i.e., senescent cell reducing effect) and reducing SASP factors (i.e., senomorphic effect). One of the key SASP factors is TGF-β, well known for its immunosuppressive role in cancer progression. HCW9218 is designed with a TGF-β trap for TGF-β neutralization.
HCW9218 + immune checkpoint inhibitors. HCW9218 addresses a key challenge for immune checkpoint inhibitors: Exhausted T cells. Preclinical studies have shown that HCW9218 stimulates and expands progenitor exhausted stem-like and transitory CD8+ T cells in the tumor draining lymph nodes followed by trafficking of these cells into the tumors. This opens a pathway for enhancing the anti-tumor activity of immune checkpoint inhibitors. HCW9218 also substantially lowers the TGF-β activity in the tumor microenvironment to lessen immunosuppression which the Company believes will also enhance the effectiveness of immune checkpoint inhibitors.
14
Outside of the Company's main focus are two other standard-of-care cancer treatments shown above, antibody- and cell-based therapies. Our preclinical studies have demonstrated HCW9218 may have the potential to boost the effectiveness of these two anti-cancer therapies. HCW9218 activates natural killer (“NK”) cells to increase their antibody-dependent cytotoxicity against cancer cells. As a result, combination therapy of HCW9218 and a tumor-specific antibody was more efficacious than either therapy alone in a mouse melanoma model. HCW9218 treatment also can activate and induce adoptively transferred tumor-specific CD8+ T cells to traffic into tumors and carry out their effector functions in reducing tumor growth. Our business development activities include a search for partners who are focused on these areas for out-license arrangements and collaborations.
HCW9302. HCW9302 is our preclinical lead product candidate administered by subcutaneous injection. This immunotherapeutic is designed to reduce senescence by suppressing the activity of inflammasome-bearing cells and the inflammatory factors they secrete through activation and expansion of regulatory T cells (“Treg”) cells. This molecule is a single-chain, IL-2-based fusion protein. Preclinical studies in mouse models have demonstrated the ability of HCW9302 to expand and activate Treg cells and reduce inflammation-related diseases, supporting the potential of HCW9302 to treat a wide variety of autoimmune and pro-inflammatory diseases, such as atherosclerosis. We are in the process of completing IND-enabling studies and intend to prepare and submit an Investigational New Drug (“IND”) application to the FDA for permission to conduct a clinical trial in an autoimmune indication in the fourth quarter of 2023.
Recent Developments
15
Trends and Uncertainties
Inflationary Cost Environment, Geopolitical Risks and Other Macroeconomic Factors
The Company's operations have been affected by many headwinds, including inflationary pressures, rising interest rates, ongoing global supply chain disruptions resulting from increased geopolitical tensions such as the war in the Middle East, the conflict between Russia and Ukraine, Chinese aggression towards Taiwan, financial market volatility and currency movements. The Company has been impacted by inflation, and may continue to be so, especially when procuring materials required for the buildout of our new headquarters, the costs for recruiting and retaining employees and other employee-related costs. Management employs a number of strategies to effectively navigate these issues, including product redesign, alternate sourcing, and establishing contingencies in budgeting and timelines. Future developments in these and other areas, such as the Company's ongoing legal proceedings, present material uncertainty and risk with respect to the Company's clinical trials, IND-enabling activities, buildout of the new headquarters, as well as the Company's financial condition and results of operations. The extent and duration of such events and conditions, and resulting disruptions to our operations, are highly unpredictable.
Components of our Results of Operation
Revenues
We have no products approved for commercial sale and have not generated any revenue from commercial product sales of internally-developed immunotherapeutic products for the treatment of cancer and other age-related diseases. The principal source of our revenues to date have been generated from our Wugen License and Master Services Agreement (the “MSA”) with Wugen. See Note 1 to our condensed financial statements included elsewhere in this Quarterly Report for these definitions and more information.
We derive revenue from a license agreement granting rights to Wugen to further develop and commercialize products based on two of our internally-developed molecules. Consideration under our contract included a nonrefundable upfront payment, development, regulatory and commercial milestones, and royalties based on net sales of approved products. Additionally, HCW Biologics retained manufacturing rights and has agreed to provide Wugen with clinical and research grade materials for clinical development and commercialization of licensed products under separate agreements. We assessed which activities in the Wugen License should be considered distinct performance obligations that should be accounted for separately. We develop assumptions that require judgement to determine whether the license to our intellectual property is distinct from the research and development services or participation in activities under the Wugen License.
16
Performance obligations relating to the granting a license and delivery of licensed product and R&D know-how were satisfied when transferred upon the execution of the Wugen License on December 24, 2020. The Company recognized revenue for the related consideration at a point in time. The revenue recognized from a transaction to supply clinical and research grade materials entered into under the MSA and covered by a statement-of-work (“SOW”), represents one performance obligation that is satisfied over time. The Company recognizes revenue generated for supply of material for clinical development using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company’s progress toward completion.
Operating Expenses
Our operating expenses are reported as research and development expenses and general and administrative expenses.
Research and Development
Our research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
We expense research and development costs as they are incurred. Costs for contract manufacturing are recognized based on an evaluation of the progress to completion of specific tasks using information provided to us by our vendors. Payments for these activities are based on the terms of the agreement, and the pattern of payments for goods and services will change depending on the material. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses and expensed as the related goods are delivered or the services are performed.
We expect research and development expenses to increase substantially for the foreseeable future as we continue the development of our product candidates. We cannot reasonably determine the nature, timing, and costs of the efforts that will be necessary to complete the development of, and obtain regulatory approval for, any of our product candidates. Product candidates in later stages of development generally have higher development costs than those in earlier stages. See “Risk Factors -- Risks Related to the Development and Clinical Testing of Our Product Candidates,” in our Annual Report for the year ended December 31, 2022 filed with the SEC on March 28, 2023 for a discussion of some of the risks and uncertainties associated with the development and commercialization of our product candidates. Any changes in the outcome of any of these risks and uncertainties with respect to the development of our product candidates in preclinical and clinical development could mean a significant change in the costs and timing associated with the development of these product candidates. For example, if the FDA or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect or if we experience significant delays in enrollment in any of our planned clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development of that product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of employee-related expenses, including salaries, related benefits, and stock-based compensation expense for employees in the executive, legal, finance and accounting, human resources, and other administrative functions. General and administrative expenses also include third-party costs such as insurance costs, fees for professional services, such as legal, auditing and tax services, facilities administrative costs, and other expenses.
17
During the period ended December 31, 2022, Altor/NantCell, a former employer of Dr. Hing C. Wong, our Founder and Chief Executive Officer, initiated legal proceedings against Dr. Wong and the Company. On April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the U.S. District Court for the Southern District of Florida (the “Court”) with jurisdiction over lawsuit against the Company approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Altor/NantCell’s proceeding against the Company is now proceeding in arbitration before JAMS. In connection with claims brought against Dr. Wong, Altor/NantCell has advancement obligations to him for claims brought against him. Thus, legal expenses incurred by him in connection with his arbitration will be advanced by Altor/NantCell; however, under certain circumstances, the Company may be required to advance his legal fees. The Company incurred legal expenses on its own behalf in the period ended September 30, 2023, and we expect to continue to incur material costs and expenses in connection with defending the Company in the foregoing legal matters through the end of 2023 and into 2024.
We expect general and administrative expenses incurred in the normal course of business for other purposes, such as costs for recruitment and retention of personnel, service fees for consultants, advisors and accountants, as well as costs to comply with government regulations, corporate governance, internal control over financial reporting, insurance and other requirements for a public company, to continue to increase for the foreseeable future as we scale our operations.
Interest Expense and Other (Expense) Income, Net
Interest expense reflects the interest paid for loans. Other (expense) income, net consists of interest earned on our cash, cash equivalents, unrealized and realized gains and losses related to our investments in U.S. government-backed securities, income related to non-operating activities, and miscellaneous non-operating expenses.
Income related to non-operating activities includes rent earned under a short-term, market rate lease, which the Company entered into with the former owner of the building purchased by the Company on August 15, 2022. The lease provided the former owner with the right to occupy offices that comprise approximately 15,000 square feet of the building for a period of one year, ending August 14, 2023, which the Company agreed to extend to September 30, 2023. During the three and nine months ended September 30, 2023, the Company reported rental income of $60,003 and $178,910, respectively, which is included within Other (expense) income, net in the interim condensed statement of operations.
18
Results of Operations
|
|
Three Months Ended |
|
|
Nine Months Ended |
|
|
||||||||||
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
||||
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Revenues |
|
$ |
1,809,025 |
|
|
$ |
853,102 |
|
|
$ |
5,380,570 |
|
|
$ |
1,517,792 |
|
|
Cost of revenues |
|
|
(1,447,220 |
) |
|
|
(678,325 |
) |
|
|
(3,062,496 |
) |
|
|
(1,210,077 |
) |
|
Net revenues |
|
|
361,805 |
|
|
|
174,777 |
|
|
|
2,318,074 |
|
|
|
307,715 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
2,648,794 |
|
|
|
1,667,442 |
|
|
|
6,408,353 |
|
|
|
5,539,919 |
|
|
General and administrative |
|
|
1,732,666 |
|
|
|
3,585,215 |
|
|
|
5,321,262 |
|
|
|
9,716,765 |
|
|
Total operating expenses |
|
|
4,381,460 |
|
|
|
5,252,657 |
|
|
|
11,729,615 |
|
|
|
15,256,684 |
|
|
Loss from operations |
|
|
(4,019,655 |
) |
|
|
(5,077,880 |
) |
|
|
(9,411,541 |
) |
|
|
(14,948,969 |
) |
|
Interest expense |
|
|
(32,184 |
) |
|
|
(95,514 |
) |
|
|
(32,184 |
) |
|
|
(284,465 |
) |
|
Other (expense) income, net |
|
|
137,645 |
|
|
|
234,753 |
|
|
|
(38,237 |
) |
|
|
919,688 |
|
|
Net loss |
|
$ |
(3,914,194 |
) |
|
$ |
(4,938,641 |
) |
|
$ |
(9,481,962 |
) |
|
$ |
(14,313,746 |
) |
|
Comparison of the Three Months ended September 30, 2022 and September 30, 2023
Revenues
The Company recognized $1.8 million and $853,102 of revenues for the three months ended September 30, 2022 and 2023, respectively. All revenues were generated under the development supply agreement with our licensee, Wugen. Revenue was recognized for all transactions made under SOWs pursuant to our existing MSA, since a contract existed for these transactions and all of the other conditions for revenue recognition were met under Topic 606. For those transactions for which revenue was not recognized because one or more of the criteria for revenue recognition had not been met, the Company records deferred revenue. There were no deferred revenues as of September 30, 2022 or September 30, 2023.
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended September 30, 2022 and September 30, 2023:
|
|
Three Months Ended |
|
|
|
|
|
|
|
|||||||
|
|
2022 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Salaries, benefits and related expenses |
|
$ |
693,774 |
|
|
$ |
692,607 |
|
|
$ |
(1,167 |
) |
|
NM |
|
|
Manufacturing and materials |
|
|
751,945 |
|
|
|
206,150 |
|
|
|
(545,795 |
) |
|
|
(73 |
)% |
Preclinical expenses |
|
|
729,172 |
|
|
|
306,344 |
|
|
|
(422,828 |
) |
|
|
(58 |
)% |
Clinical trials |
|
|
292,276 |
|
|
|
343,821 |
|
|
|
51,545 |
|
|
|
18 |
% |
Other expenses |
|
|
181,627 |
|
|
|
118,520 |
|
|
|
(63,107 |
) |
|
|
(35 |
)% |
Total research and development expenses |
|
$ |
2,648,794 |
|
|
$ |
1,667,442 |
|
|
$ |
(981,352 |
) |
|
|
(37 |
)% |
NM - Not meaningful
Research and development expenses decreased by $981,352, or 37%, from $2.6 million for the three months ended September 30, 2022 to $1.7 million for the three months ended September 30, 2023. This decrease was primarily due to a decline in expenses for manufacturing and preclinical activities, and allocation for depreciation expense, partially offset by an increase in clinical trials expenses.
19
Manufacturing and materials expense decreased by $545,795, or 73%, from $751,945 for the three months ended September 30, 2022 to $206,150 for the three months ended September 30, 2023. In the three months ended September 30, 2022, expenses were primarily attributable to the initiation of a 1000L GMP manufacturing run for HCW9218. As of September 30, 2023, the Company anticipated that adequate supply of clinical development material for the Company's two lead molecules, HCW9218 and HCW9302, had been put in place for clinical development activities planned for the next 20-24 months. In the three months ended September 30, 2023, costs were incurred primarily for master cell bank characterization for HCW9101H, a high-producing cell line of a key component of the manufacturing process for the Company's proprietary molecules including those licensed to Wugen, as well as ancillary activities such as shipping, insurance and storage.
Expenses associated with preclinical activities decreased by $422,828, or 58%, from $729,172 for the three months ended September 30, 2022 to $306,344 for the three months ended September 30, 2023. In the three months ended September 30, 2022, expenses were attributable primarily to the cost of toxicology studies and experimental materials related to IND-enabling activities required to prepare our IND for clinical trials to evaluate HCW9302 in an autoimmune indication. In the three months ended September 30, 2023, costs were incurred primarily for additional studies required for submission of the HCW9302 IND.
Expenses associated with clinical activities increased by $51,545, or 18%, from $292,276 for the three months ended September 30, 2022 to $343,821 for the three months ended September 30, 2023. The increase was primarily due to a $122,838 increase in patient fees and clinical trial fees, partially offset by a $84,152 decrease in clinical site start-up costs. In the three months ended September 30, 2022, the Company had one ongoing Phase 1 clinical trial to evaluate HCW9218 in solid tumors, sponsored by the University of Minnesota (“UMN”), which initiated in May 2022. In the three months ended September 30, 2023, there were two ongoing clinical studies: The UMN study and a Company-sponsored Phase 1b clinical trial to evaluate HCW9218 in chemo-resistant/chemo-refractory pancreatic cancer, which initiated in October 2022.
In the three months ended September 30, 2023, the UMN trial completed dose escalation and began enrollment in the cohort expansion at the highest planned dose level. A human data readout was presented at the 2024 SITC conference, showing clinical safety and tumor response endpoints and results of correlative studies for 15 patients with heavily pretreated advanced solid tumors. These results supported the anti-cancer utility of HCW9218 and provided the rationale for future Phase 2 clinical trials using HCW9218 in combination with standard-of-care cancer treatments. There has been one dose limiting toxicity event among the patients participating in the study, which did not disrupt this patient's continued participation in the study. The Company expects the UMN study to be complete in the fourth quarter of 2023. Clinical expenses for the three months ended September 30, 2023 also include expenses related to a Company-sponsored, multi-center Phase 1b/2 clinical trial to evaluate HCW9218 in advanced pancreatic cancer. Patient recruitment continues to progress at the five clinical sites, led by the National Cancer Institute. The trial completed three dose escalation cohorts and has begun a fourth, with no dose-limiting toxicity to date. We expect to complete the Phase 1b portion of the pancreatic cancer study in late 2023 or early 2024, followed by a human data readout of clinical data expected in the first half of 2024.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the three months ended September 30, 2022 and September 30, 2023:
|
|
Three Months Ended |
|
|
|
|
|
|
|
|||||||
|
|
2022 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Salaries, benefits and related expenses |
|
$ |
779,713 |
|
|
$ |
775,956 |
|
|
$ |
(3,757 |
) |
|
NM |
|
|
Professional services |
|
|
352,166 |
|
|
|
2,283,094 |
|
|
|
1,930,928 |
|
|
|
548 |
% |
Facilities and office expenses |
|
|
85,661 |
|
|
|
175,694 |
|
|
|
90,033 |
|
|
|
105 |
% |
Depreciation |
|
|
31,939 |
|
|
|
63,083 |
|
|
|
31,144 |
|
|
|
98 |
% |
Rent and occupancy expense |
|
|
31,217 |
|
|
|
36,372 |
|
|
|
5,155 |
|
|
|
17 |
% |
Other expenses |
|
|
451,970 |
|
|
|
251,016 |
|
|
|
(200,954 |
) |
|
|
(44 |
)% |
Total general and administrative expenses |
|
$ |
1,732,666 |
|
|
$ |
3,585,215 |
|
|
$ |
1,852,549 |
|
|
|
107 |
% |
NM - Not meaningful
20
General and administrative expenses increased by $1.9 million, or 107%, from $1.7 million for the three months ended September 30, 2022 to $3.6 million for the three months ended September 30, 2023. The increase was primarily due to an increase in professional fees, which includes legal fees associated with the Altor/NantCell matter.
Salaries, benefits and related expenses did not have a meaningful net change. The components of the change consisted of a $55,547 increase in salaries and wages, a $21,606 increase in employee benefits, partially offset by a $80,580 decrease in expenses arising from stock-based compensation.
Professional services increased by $1.9 million, or 548%, from $352,166 for the three months ended September 30, 2022 to $2.3 million for the three months ended September 30, 2023. Professional services include corporate legal services, expenses related to the Altor/NantCell matter, legal fees associated with patent prosecution, and other professional services, such as auditing and tax advisory fees. The $1.9 million increase consists of a $2.0 million increase in legal fees associated with the Altor/NantCell matter and a $18,196 increase in fees associated with other professional services, such as audit fees and tax advisory services, partially offset by a $58,479 decrease in legal fees associated with patent prosecution.
Facilities and office expenses increased by $90,033, or 105%, from $85,661 for the three months ended September 30, 2022 to $175,694 for the three months ended September 30, 2023. The increase was primarily due to a $77,598 increase in IT-related expenses and a $10,291 increase in facility-related expenses.
Depreciation allocation to general and administrative expenses increased by $31,144, or 98%, from $31,939 for the three months ended September 30, 2022 to $63,083 for the three months ended September 30, 2023. The increase in the depreciation allocation to general and administrative expenses is related to the Company's acquisition of a property for its new headquarters in the third quarter of 2022.
Other expenses decreased by $200,954, or 44%, from $451,970 for the three months ended September 30, 2022 to $251,016 for the three months ended September 30, 2023. The decrease consists primarily of a $151,576 decrease in costs for filing fees, as the shelf registration filing in August 2022 did not recur during the three months ended September 30, 2023, and a $85,510 decrease in insurance premiums, partially offset by a $33,572 increase in taxes.
Comparison of the Nine Months ended September 30, 2022 and September 30, 2023
Revenues
The Company recognized $5.4 million and $1.5 million of revenues for the nine months ended September 30, 2022 and 2023, respectively. All revenues were generated under the development supply agreement with Wugen. The decline in revenue is attributed to a change in Wugen's clinical development plan, a delay in the ramp up of its manufacturing process, as well as transactions for which not all of the elements for revenue recognition were met. The revenue recognized in the nine months ended September 30, 2022 reflects recognition of revenues that had previously been classified as deferred revenue. Revenue may be recognized for all transactions made under the MSA for which the Company entered SOWs, since both of these elements must be in place for a contract to exist and to fulfill all of the other conditions required for revenue recognition under Topic 606. For those transactions for which revenues were not recognized because one or more of the criteria for revenue recognition had not been met under Topic 606, the Company records deferred revenue. There were no deferred revenues as of September 30, 2022 and September 30, 2023.
Research and Development Expenses
The following table summarizes our research and development expenses for the nine months ended September 30, 2022 and September 30, 2023:
|
|
Nine Months Ended |
|
|
|
|
|
|
|
|||||||
|
|
2022 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Salaries, benefits and related expenses |
|
$ |
2,268,755 |
|
|
$ |
2,195,265 |
|
|
$ |
(73,490 |
) |
|
|
(3 |
)% |
Manufacturing and materials |
|
|
1,273,902 |
|
|
|
623,785 |
|
|
|
(650,117 |
) |
|
|
(51 |
)% |
Preclinical expenses |
|
|
1,841,809 |
|
|
|
1,367,725 |
|
|
|
(474,084 |
) |
|
|
(26 |
)% |
Clinical trials |
|
|
486,992 |
|
|
|
788,116 |
|
|
|
301,124 |
|
|
|
62 |
% |
Other expenses |
|
|
536,895 |
|
|
|
565,028 |
|
|
|
28,133 |
|
|
|
5 |
% |
Total research and development expenses |
|
$ |
6,408,353 |
|
|
$ |
5,539,919 |
|
|
$ |
(868,434 |
) |
|
|
(14 |
)% |
21
Research and development expenses decreased by $868,434, or 14%, from $6.4 million for the nine months ended September 30, 2022 to $5.5 million for the nine months ended September 30, 2023. This decrease was primarily due to a decrease in manufacturing and preclinical expenses, partially offset by an increase in clinical trials expenses.
Salaries, benefits, and related expenses decreased by $73,490, or 3%, from $2.3 million for the nine months ended September 30, 2022 to $2.2 million for the nine months ended September 30, 2023. This decrease was primarily attributable to an allocation of labor costs for manufacturing of clinical materials for Wugen and the impact of the reimbursement of expenses provided for under the Wugen License.
Manufacturing and materials expense decreased by $650,117, or 51%, from $1.3 million for the nine months ended September 30, 2022 to $623,785 for the nine months ended September 30, 2023. In the nine months ended September 30, 2022, costs were primarily attributable to HCW9302 technology transfer and development process closeout through finalization of reports and the project initiation and production costs for a 1000L GMP manufacturing run for HCW9218. In the nine months ended September 30, 2023, costs were incurred primarily for production activities associated with the master cell bank characterization for HCW9101H; a 200L cGMP manufacturing run of HCW9302; and ancillary activities such as shipping, insurance and storage.
Expenses associated with preclinical activities decreased by $474,084, or 26%, from $1.8 million for the nine months ended September 30, 2022 to $1.4 million for the nine months ended September 30, 2023. In the nine months ended September 30, 2022, expenses were related primarily to the cost of toxicology studies and experimental materials used for IND-enabling activities required to prepare our IND for clinical trials to evaluate HCW9302 in an autoimmune indication. In the nine months ended September 30, 2023, costs were incurred to complete the toxicology study and for additional studies required for submission of the HCW9302 IND.
Expenses associated with clinical activities increased by $301,124, or 62%, from $486,992 for the nine months ended September 30, 2022 to $788,116 for the nine months ended September 30, 2023. In the nine months ended September 30, 2022, the UMN study was the only ongoing clinical trial. In the nine months ended September 30, 2023, in addition to the UMN study, there was an ongoing Company-sponsored Phase 1b clinical trial to evaluate HCW9218 in chemo-refractory/chemo-resistant pancreatic cancer.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the nine months ended September 30, 2022 and September 30, 2023:
|
|
Nine Months Ended |
|
|
|
|
|
|
|
|||||||
|
|
2022 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Salaries, benefits and related expenses |
|
$ |
2,237,841 |
|
|
$ |
2,408,622 |
|
|
$ |
170,781 |
|
|
|
8 |
% |
Professional services |
|
|
1,098,530 |
|
|
|
5,618,898 |
|
|
|
4,520,368 |
|
|
|
411 |
% |
Facilities and office expenses |
|
|
299,595 |
|
|
|
439,373 |
|
|
|
139,778 |
|
|
|
47 |
% |
Depreciation |
|
|
84,484 |
|
|
|
188,454 |
|
|
|
103,970 |
|
|
|
123 |
% |
Rent expense |
|
|
96,493 |
|
|
|
118,296 |
|
|
|
21,803 |
|
|
|
23 |
% |
Other expenses |
|
|
1,504,319 |
|
|
|
943,122 |
|
|
|
(561,197 |
) |
|
|
(37 |
)% |
Total general and administrative expenses |
|
$ |
5,321,262 |
|
|
$ |
9,716,765 |
|
|
$ |
4,395,503 |
|
|
|
83 |
% |
General and administrative expenses increased by $4.4 million, or 83%, from $5.3 million for the nine months ended September 30, 2022 to $9.7 million for the nine months ended September 30, 2023. The increase was primarily due to a $4.5 million increase in professional fees, consisting of a $4.3 million increase in legal fees associated with the Altor/NantCell matter, a $224,201 increase in legal fees associated with patent prosecution, and a $92,921 increase for other professional services such as audit fees and tax advisory fees.
Salaries, benefits and related expenses increased by $170,781, or 8%, from $2.2 million for the nine months ended September 30, 2022 to $2.4 million for the nine months ended September 30, 2023. The increase is primarily attributable to a $188,224 increase in salaries and wages, a $40,887 increase in expenses for health insurance premium for employee benefits, and a $20,165 increase in payroll tax expense, partially offset by a $94,041 decrease in stock-based compensation expense.
22
Professional services increased by $4.5 million, or 411%, from $1.1 million for the nine months ended September 30, 2022 to $5.6 million for the nine months ended September 30, 2023. The $4.5 million increase consists of a $4.3 million increase in legal fees associated with the Altor/NantCell matter, a $224,201 increase for legal fees associated with patent prosecution, and a $92,921 increase in fees associated with other professional services such as audit fees and tax advisory services, partially offset by a $117,492 decrease in fees for consulting and advisory services.
Facilities and office expenses increased by $139,778, or 47%, from $299,595 for the nine months ended September 30, 2022 to $439,373 for the nine months ended September 30, 2023. This increase was primarily due to a $84,353 increase in IT-related expenses and a $45,222 increase in facilities expenses.
Depreciation allocation to general and administrative expenses increased by $103,970, or 123%, from $84,484 for the nine months ended September 30, 2022 to $188,454 for the nine months ended September 30, 2023. The increase in the depreciation allocation to general and administrative expenses reflects the Company's acquisition of a property for its new headquarters in the third quarter of 2022.
Other expenses decreased by $561,197, or 37%, from $1.5 million for the nine months ended September 30, 2022 to $943,122 for the nine months ended September 30, 2023. The decrease is primarily due to a $527,306 decrease in the insurance premiums and a $133,639 decrease in costs for filing, as the shelf registration filing in August 2022 did not recur during the nine months ended September 30, 2023, partially offset by a $115,124 increase in taxes.
Liquidity and Capital Resources
Sources of Liquidity
As of September 30, 2023, our principal source of liquidity was $11.2 million in cash and cash equivalents and a $5.3 million deposit for interest reserve. Since inception, our principal sources of liquidity have been $49.2 million in net proceeds from our initial public offering, a $6.5 million bank loan for the acquisition of a property the Company will use as its new headquarters; and a $26.3 million loan to finance to renovate our new headquarters. The Company intends to raise capital through additional debt or equity financings. In addition, we intend to continue our efforts to enter into business development transactions. Business development efforts are focused on out-licensing rights to non-core assets or regional markets, third-party collaboration funding, cooperative agreements for clinical trials, and other transactions. However, if we are not able to obtain financing at adequate levels, we will need to reevaluate its operating plan and may be required to delay the development of some of its products.
On August 15, 2022, we purchased a 36,000 square foot building located in Miramar, Florida for approximately $10.1 million, including transaction costs. A portion of the acquisition cost was funded with a $6.5 million five-year loan obtained from Cogent Bank (the “2022 Loan Agreement”) and is secured by the building. As of September 30, 2023, the Company owed $6.5 million on the 2022 Loan Agreement and was in compliance with all covenants thereunder.
On April 21, 2023, the Company entered into the 2023 Loan Agreement with Prime Capital Ventures, LLC (“Prime”), pursuant to which Prime will advance loans to the Company in a principal amount not to exceed $26.3 million pursuant to a loan agreement (the “2023 Loan Agreement”) with a scheduled maturity of April 20, 2028 (the “Prime Maturity Date”). In November 2023, the Company expects to begin to draw down funds under the 2023 Loan Agreement. We expect to draw on the loan over the next ten months, up to the maximum loan amount of $26.3 million. Some of the proceeds of the loan will be used to recoup $4.4 million in funds advanced from the Company's operating capital for the building project prior to drawing funds available under the 2023 Loan Agreement.
The Company has the option to terminate the 2023 Loan Agreement prior to the Prime Maturity Date. The note issued pursuant to the 2023 Loan Agreement bears interest of 7.00% per annum, due monthly in arrears on the first day of each month. The primary use for borrowings under the 2023 Loan Agreement is to obtain the funding required to complete the buildout of the Company's new headquarters, including improved research laboratories, a vivarium, and a manufacturing facility.
Under the 2023 Loan Agreement, the Company was required to fund a reserve bank account controlled by Prime in the amount of $5.3 million, with the purpose to fund the interest due on outstanding amounts under the 2023 Loan Agreement. The balance of the reserve account is presented in Deposit for interest reserve in noncurrent assets on the accompanying condensed balance sheet. As of September 30, 2023, the Company had no borrowings under the 2023 Loan Agreement. The Company incurred $1.3 million in debt issuance costs in connection with a lender's fee, which is payable in equal installments from the first four draws from the 2023 Loan Agreement. We intend to repay the 2022 Loan Agreement in six equal installments from the first six draws of the 2023 Loan Agreement. We will not incur any prepayment penalties as a result of prepayment of the 2022 Loan Agreement.
23
We believe that our cash and cash equivalents and short-term investments as of September 30, 2023 will be sufficient to meet our capital requirements and fund our operations for at least the next 12 months. We have based our projections of operation expenses requirements on assumptions, including our existing commitments and contingencies, that may prove to be incorrect, and we may use all of our available capital sooner than we expect. Because of the numerous risks and uncertainties associated with the clinical development and commercialization of immunotherapeutics, we are unable to estimate the exact amount of capital requirements to pursue these activities. Our funding requirements will depend on many factors, including, but not limited to:
A change in the outcome of any of these or other factors with respect to the clinical development and commercialization of our product candidates could significantly change the costs and timing associated with the development of that product candidate.
Further, our operating plan may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development expenditures.
Comparison of the Cash Flows for the Nine Months Ended September 30, 2022 and September 30, 2023
The following table summarizes our cash flows for the nine months ended September 30, 2022 and September 30, 2023:
|
|
Nine Months Ended |
|
|||||
|
|
2022 |
|
|
2023 |
|
||
Cash used in operating activities |
|
$ |
(6,596,739 |
) |
|
$ |
(18,619,329 |
) |
Cash provided by investing activities |
|
|
14,777,079 |
|
|
|
7,513,050 |
|
Cash provided by financing activities |
|
|
6,313,243 |
|
|
|
716 |
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
14,493,583 |
|
|
$ |
(11,105,563 |
) |
Operating Activities
Cash used in operating activities for the nine months ended September 30, 2022 consisted primarily of a net loss of $9.5 million, a $222,555 cash decrease arising from an increase accounts receivable, and a $202,979 cash decrease arising from a decrease in accounts payable and other current liabilities. These uses were partially offset by $1.9 million of cash provided by a decrease in prepaid expenses and other assets and noncash adjustments, consisting of $834,003 for stock-based compensation expense and $456,696 for depreciation and amortization expense.
24
Cash used in operating activities for the nine months ended September 30, 2023 consisted primarily of a net loss of $14.3 million, as well as a deposit $5.3 million used to establish an interest reserve for future interest payments, as required under the terms of the 2023 Loan Agreement. In addition, other uses of cash include a $292,383 increase in accounts receivable and a $251,008 increase in prepaid expenses and other current assets, partially offset by cash provided by a $392,802 net increase in accounts payable and other current liabilities. Further offset to the use of cash resulted from net noncash adjustments of $1.1 million, consisting primarily of $860,634 of cash provided by an adjustment for depreciation and amortization, $742,477 of cash provided by an adjustment for stock-based compensation, reduced by $248,445 of cash used for an adjustment for unrealized gains on investments.
Investing Activities
Cash provided by investing activities for the nine months ended September 30, 2022 consisted of $25.0 million of cash provided when short-term investments reached maturity, partially offset by $10.2 million of cash used to purchase property, plant and equipment.
Cash used by investing activities for the nine months ended September 30, 2023, consisted of $10.0 million of cash provided when short-term investments reached maturity, partially offset by $2.5 million of cash used to purchase equipment.
Financing Activities
Cash provided by financing activities for the nine months ended September 30, 2022 resulted primarily from obtaining the Cogent Loan, in the amount of $6.5 million, to provide purchase financing to acquire our new headquarters building.
Cash provided by financing activities for the nine months ended September 30, 2023 resulted from issuance of common stock upon exercise of vested employee stock options, partially offset by a principal repayment, as required under the 2022 Loan Agreement.
Critical Accounting Policies, Significant Judgements and Use of Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our unaudited condensed interim financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgements and estimates.
Revenue Recognition
We recognize revenue under the guidance of Topic 606. To determine the appropriate amount of revenue to be recognized for arrangements determined to be within the scope of Topic 606, we perform the following five steps: (i) identification of the contract(s) with the customer, (ii) identification of the promised goods or services in the contract and determination of whether the promised goods or services are performance obligations, (iii) measurement of the transaction price, (iv) allocation of the transaction price to the performance obligations, and (v) recognition of revenue when (or as) we satisfy each performance obligation. We only apply the five-step model to contracts when it is probable that we will collect the consideration we are entitled to in exchange for the goods or services we transfer to our customer. See Note 1 to our condensed interim financial statements appearing elsewhere in this Quarterly Report on Form 10-Q for more information.
Other than the above, there have been no material changes to our critical accounting policies and estimates from those described under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations— Critical Accounting Policies, Significant Judgements and Use of Estimates” in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the SEC on March 28, 2023.
Recent Accounting Pronouncements
See Note 1 to our unaudited condensed interim financial statements appearing elsewhere in this Quarterly Report for more information about recent accounting pronouncements.
25
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As of September 30, 2023, we had cash and cash equivalents of $11.2 million and a deposit for interest reserve of $5.3 million. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. We are exposed to market risk related to the marketability of our Wugen common stock reported within Investments in the accompanying condensed balance sheet. Until such time as these shares become publicly traded, we will have limited access to liquidity for these securities.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act, is recorded, communicated to our management to allow timely decisions regarding required disclosure, summarized and reported within the time periods specified in the SEC’s rules and forms. Any disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objective and management necessarily applies its judgement in evaluating the cost-benefit relationship of possible controls and procedures.
Under the supervision and with the participation of our management, including the Chief Executive Officer, or CEO, and Chief Financial Officer, or CFO, we conducted an evaluation of the effectiveness of our disclosure controls and procedures, as such term is defined under Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of September 30, 2023. Based on that evaluation, the CEO and CFO have concluded that, as of such date, our disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the quarter ended September 30, 2023, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
26
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
On December 23, 2022, Altor BioScience, LLC and NantCell, Inc. initiated an arbitration against Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, in California alleging breach of contract and fiduciary duty, among other claims. On that same date, Altor/NantCell filed a lawsuit against the Company in the U.S. District Court for the Southern District of Florida (the “Court”) alleging misappropriation of trade secrets, inducement of breach of contract and breach of fiduciary duty, among other claims against the Company. On January 31, 2023, the Company filed a motion to compel arbitration, a motion for the stay of the litigation, and a motion to dismiss the complaint (“motion to compel”). On April 18, 2023, the Court heard oral argument on the Company’s motion to compel and ordered the parties to provide supplemental briefing by April 28, 2023. Before the Court ruled on the Company’s motion to compel, on April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the Court approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Altor/NantCell’s proceeding against the Company is now proceeding in arbitration before JAMS. Although adverse decisions (or settlements) may occur in the lawsuit described above, it is not possible to reasonably estimate the possible loss or range of loss, if any, associated therewith at this time. As such, no accrual for these matters has been recorded within the financial statements.
Item 1A. Risk Factors.
There have been no material changes to the risk factors previously disclosed by us in our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on March 28, 2023. The risk factors included in the Form 10-K continue to apply to us and describe risks and uncertainties that could cause actual results to differ materially from the results expressed or implied by the forward-looking statements contained in this Quarterly Report on Form 10-Q. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business, financial condition and results of operations.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Unregistered Sales of Equity Securities
None.
Issuer Repurchases of Equity Securities
None.
Use of Proceeds
There has been no material change in the planned use of proceeds from our IPO from that described in the final prospectus filed by us with the SEC on July 21, 2021.
Item 3. Defaults Upon Senior Securities.
Not Applicable.
Item 4. Mine Safety Disclosures.
Not Applicable.
Item 5. Other Information.
Insider Adoption or Termination of Trading Agreements
During the three months ended September 30, 2023, none of our directors or officers informed us of the adoption, amendment, or termination of a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading agreement” as those terms are defined in Regulation S-K, Item 408.
27
Item 6. Exhibits.
The exhibits filed or furnished as part of this Quarterly Report on Form 10-Q are set forth on the Exhibit Index, which Exhibit Index is incorporated herein by reference.
Exhibit Number
|
|
Incorporated by Reference
|
Filed
|
||
Description
|
Form
|
Date
|
Number
|
||
|
|
|
|
|
|
31.1 |
|
|
|
X |
|
|
|
|
|
|
|
31.2 |
|
|
|
X |
|
|
|
|
|
|
|
32.1* |
|
|
|
X |
|
|
|
|
|
|
|
32.2* |
|
|
|
X |
|
|
|
|
|
|
|
101 |
The following materials from the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) the Condensed Balance Sheets as of December 31, 2022 and September 30, 2023 (unaudited); (ii) the Condensed Statements of Operations for the three and nine months ended September 30, 2022 (unaudited) and September 30, 2023 (unaudited); (iv) the Condensed Statements of Stockholders’ Equity for the nine months ended September 30, 2022 (unaudited) and September 30, 2023 (unaudited); (v) the Condensed Statements of Cash Flows for the nine months ended September 30, 2022 (unaudited) and September 30, 2023 (unaudited); and (vi) the notes to the Condensed Financial Statements (unaudited). |
|
|
|
X |
|
|
|
|
|
|
104 |
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
X |
|
|
* |
This certificate is deemed not filed for purpose of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities act or the Exchange Act. |
|
|
28
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
HCW Biologics Inc. |
|
|
|
|
|
Date: November 14, 2023 |
|
By: |
/s/ Hing C. Wong |
|
|
|
Hing C. Wong |
|
|
|
Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
|
|
|
|
Date: November 14, 2023 |
|
By: |
/s/ Rebecca Byam |
|
|
|
Rebecca Byam |
|
|
|
Chief Financial Officer |
|
|
|
(Principal Financial and Accounting Officer) |
29
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-29)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
