HERBALIFE LTD. - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2021
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 1-32381
HERBALIFE NUTRITION LTD.
(Exact name of registrant as specified in its charter)
Cayman Islands |
98-0377871 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
P.O. Box 309GT
Ugland House, South Church Street
Grand Cayman, Cayman Islands
(Address of principal executive offices) (Zip Code)
(213) 745-0500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class: |
Trading Symbol(s): |
Name of each exchange on which registered: |
Common Shares, par value $0.0005 per share |
HLF |
New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☒ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
|
|
|
|
Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
There were 109,774,177 common shares outstanding as of February 16, 2022. The aggregate market value of the Registrant’s common shares held by non-affiliates was approximately $2,792 million as of June 30, 2021, based upon the last reported sales price on the New York Stock Exchange on that date of $52.73. For the purposes of this disclosure only, the registrant has assumed that its directors, executive officers, and the beneficial owners of 5% or more of the registrant’s outstanding common stock are the affiliates of the registrant.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission no later than 120 days after the end of the Registrant’s fiscal year ended December 31, 2021, are incorporated by reference in Part III of this Annual Report on Form 10-K.
1
TABLE OF CONTENTS
|
|
Page No. |
|
PART I |
|
|
|
|
Item 1. |
5 |
|
Item 1A. |
18 |
|
Item 1B. |
42 |
|
Item 2. |
42 |
|
Item 3. |
43 |
|
Item 4. |
43 |
|
|
|
|
|
PART II |
|
|
|
|
Item 5. |
44 |
|
Item 6. |
45 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
45 |
Item 7A. |
68 |
|
Item 8. |
70 |
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
70 |
Item 9A. |
70 |
|
Item 9B. |
71 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
71 |
|
|
|
|
PART III |
|
|
|
|
Item 10. |
72 |
|
Item 11. |
72 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
72 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
72 |
Item 14. |
72 |
|
|
|
|
|
PART IV |
|
|
|
|
Item 15. |
73 |
|
Item 16. |
127 |
2
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are “forward-looking statements” for purposes of federal and state securities laws, including any projections of earnings, revenue or other financial items; any statements of the plans, strategies and objectives of management, including for future operations, capital expenditures, or share repurchases; any statements concerning proposed new products, services, or developments; any statements regarding future economic conditions or performance; any statements of belief or expectation; and any statements of assumptions underlying any of the foregoing or other future events. Forward-looking statements may include, among other, the words “may,” “will,” “estimate,” “intend,” “continue,” “believe,” “expect,” “anticipate” or any other similar words.
Although we believe that the expectations reflected in any of our forward-looking statements are reasonable, actual results or outcomes could differ materially from those projected or assumed in any of our forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to change and to inherent risks and uncertainties, many of which are beyond our control. Additionally, many of these risks and uncertainties are, and may continue to be, amplified by the COVID-19 pandemic. Important factors that could cause our actual results, performance and achievements, or industry results to differ materially from estimates or projections contained in or implied by our forward-looking statements include the following:
3
Additional factors and uncertainties that could cause actual results or outcomes to differ materially from our forward-looking statements are set forth in this Annual Report on Form 10-K, including under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in our Consolidated Financial Statements and the related Notes. In addition, historical, current, and forward-looking sustainability-related statements may be based on standards for measuring progress that are still developing, internal controls and processes that continue to evolve, and assumptions that are subject to change in the future.
Forward-looking statements in this Annual Report on Form 10-K speak only as of the date hereof. We do not undertake any obligation to update or release any revisions to any forward-looking statement or to report any events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law.
The Company
“We,” “our,” “us,” “Company,” “Herbalife,” and “Herbalife Nutrition” refer to Herbalife Nutrition Ltd., a Cayman Islands exempted company incorporated with limited liability, and its subsidiaries. Herbalife Nutrition Ltd. is a holding company, with substantially all of its assets consisting of the capital stock of its direct and indirectly-owned subsidiaries.
4
PART I
Item 1. Business
GENERAL
Herbalife Nutrition is a global nutrition company that provides health and wellness products to consumers in 95 markets, which consists of countries and territories, through our direct-selling business model. Our products are primarily in the categories of weight management, sports nutrition, and targeted nutrition.
We use a direct-selling business model to distribute and market our nutrition products to and through a global network of independent members, or Members. Members include consumers who purchase products for their own personal use and distributors who wish to resell products or build a sales organization. We believe that direct selling is ideally suited for our business because the distribution and sales of our products with personalized support, coaching, and education provide a supportive and understanding community of like-minded people who prioritize health and nutrition.
In addition to the effectiveness of personalized selling through a direct-selling business model, we believe the primary drivers for our success throughout our 42-year operating history have been enhanced consumer awareness and demand for our products due to global trends such as the obesity epidemic, increasing interest in a fit and active lifestyle, living healthier, and the rise of entrepreneurship.
PRODUCT SALES
Our science-backed products help Members and their customers improve their overall health, enhance their wellness, and achieve their fitness and sport goals. As of December 31, 2021, we marketed and sold approximately 130 product types. Our products are often sold as part of a program and therefore our portfolio is comprised of a series of related products designed to simplify weight management, health and wellness, and overall nutrition for our Members and their customers. Our Formula 1 Nutritional Shake Mix, our best-selling product line, approximated 27% of our net sales for the year ended December 31, 2021.
The following table summarizes our products by product category:
|
|
Percentage of Net Sales |
|
|
|
|
||||
|
|
2021 |
|
2020 |
|
2019 |
|
Description |
|
Representative Products |
Weight Management |
|
58.1% |
|
59.8% |
|
61.8% |
|
Meal replacement, protein shakes, drink mixes, weight loss enhancers and healthy snacks |
|
Formula 1 Healthy Meal, Herbal Tea Concentrate, Protein Drink Mix, Personalized Protein Powder, Total Control®, Formula 2 Multivitamin Complex, Prolessa™ Duo, and Protein Bars |
Targeted Nutrition |
|
28.2% |
|
27.6% |
|
26.2% |
|
Functional beverages and dietary and nutritional supplements containing quality herbs, vitamins, minerals and other natural ingredients |
|
Herbal Aloe Concentrate, Active Fiber Complex, Niteworks®, and Herbalifeline® |
Energy, Sports, and Fitness |
|
9.5% |
|
7.9% |
|
7.2% |
|
Products that support a healthy active lifestyle |
|
Herbalife24® product line, N-R-G Tea, and Liftoff® energy drink |
Outer Nutrition |
|
1.9% |
|
2.0% |
|
2.0% |
|
Facial skin care, body care, and hair care |
|
Herbalife SKIN line and Herbal Aloe Bath and Body Care line |
Literature, Promotional, and Other |
|
2.3% |
|
2.7% |
|
2.8% |
|
Start-up kits, sales tools, and educational materials |
|
Herbalife Member Packs and BizWorks |
5
Product returns and buyback policies
We offer a customer satisfaction guarantee in substantially all markets where our products are sold. If for any reason a customer or preferred member is not satisfied with an Herbalife Nutrition product, they may return it or any unused portion of the product within 30 days from the time of receipt for a full refund or credit toward the exchange of another Herbalife Nutrition product.
In addition, in substantially all markets, we maintain a buyback program pursuant to which we will purchase back unsold products from a Member who decides to leave the business. Subject to certain terms and conditions that may vary by market, the buyback program generally permits a Member to return unopened products or sales materials in marketable condition purchased within the prior twelve-month period in exchange for a refund of the net price paid for the product and, in most markets, the cost of returning the products and materials to us.
Together, product returns and buybacks were approximately 0.1% of net sales for each of the years ended December 31, 2021, 2020, and 2019.
Product development
Our products are focused on nutrition and seek to help consumers achieve their goals in the areas of weight management; targeted nutrition (including everyday wellness and healthy aging); energy, sports, and fitness; and outer nutrition. We believe our focus on nutrition and botanical science and the combination of our internal efforts with the scientific expertise of outside resources, including our ingredient suppliers, major universities, and our Nutrition Advisory Board, have resulted in product differentiation that has given our Members and consumers increased confidence in our products.
We continue to invest in scientific and technical functions, including research and development associated with creating new or enhancing current product formulations and the advancement of personalized nutrition solutions; clinical studies of existing products or products in development; technical operations to improve current product formulations; quality assurance and quality control to establish the appropriate quality systems, controls, and standards; and rigorous ingredient and product testing to ensure compliance with regulatory requirements, as well as in the areas of regulatory and scientific affairs. Our personalized nutrition solutions include tools which aid in the development of optimal product packages specific to our customers’ individual nutritional needs, based on their expected wellness goals.
Our product development strategy is twofold: (1) to increase the value of existing customers by investing in products that address customers’ health, wellness and nutrition considerations, fill perceived gaps in our portfolios, add flavors, increase convenience by investing in snacks and bars, and expand afternoon and evening consumption with products like savory shakes or soups; and (2) to attract new customers by entering into new categories, offering more choices, increasing individualization, and expanding our current sports line. We have a keen focus on product innovation and aim to launch new products and variations on existing products on a regular basis. Once a particular market opportunity has been identified, our scientists, along with our operations, marketing, and sales teams, work closely with Member leadership to introduce new products and variations on existing products.
Our Nutrition Advisory Board and Dieticians Advisory Board are comprised of leading experts around the world in the fields of nutrition and health who educate our Members on the principles of nutrition, physical activity, diet, and healthy lifestyle. We rely on the scientific contributions from members of our Nutrition Advisory Board and our in-house scientific team to continually upgrade existing products or introduce new products as new scientific studies become available and are accepted by regulatory authorities around the world.
COMPETITION
The nutrition industry is highly competitive. Nutrition products are sold through a number of distribution channels, including direct selling, online retailers, specialty retailers, and the discounted channels of food, drug and mass merchandise. Our competitors include Conagra Brands, Hain Celestial, and Post. Additionally, we compete for the recruitment of Members from other network marketing organizations, including those that market nutrition products and other entrepreneurial opportunities. Our direct-selling competitors include Nu Skin, Tupperware, and USANA. Our ability to remain competitive depends on many factors, including having relevant products that meet consumer needs, a rewarding compensation plan, enhanced education and tools, innovation in our products and services, competitive pricing, a strong reputation, and a financially viable company.
We have differentiated ourselves from our competitors through our Members’ focus on the consultative sales process, which includes ongoing personal contact, coaching, behavior motivation, education, and the creation of supportive communities. For example, many Members have frequent contact with and provide support to their customers through a community-based approach to help them achieve nutrition goals. Some methods include Nutrition Clubs, Weight Loss Challenges, Wellness Evaluations, and Fit Camps.
6
For additional information regarding competition, see Part I, Item 1A, Risk Factors, of this Annual Report on Form 10-K.
OUR NETWORK MARKETING PROGRAM
General
Our products are sold and distributed through a global direct selling business model which individuals may join to become a Member of our network marketing program. We believe that the one-on-one personalized service inherent in the direct-selling business model is ideally suited to marketing and selling our nutrition products. Sales of nutrition products are reinforced by the ongoing personal contact, coaching, behavior motivation, education, and the creation of supportive communities. This frequent, personal contact can enhance consumers’ nutritional and health education as well as motivate healthy behavioral changes in consumers to begin and maintain an active lifestyle through wellness and weight management programs. In addition, our Members consume our products themselves, and, therefore, can provide first-hand testimonials of the use and effectiveness of our products and programs to their customers. The personalized experience of our Members has served as a very powerful sales tool for our products.
People become Herbalife Nutrition Members for a number of reasons. Many first start out as consumers of our products who want to lose weight or improve their nutrition, and are customers of our Members. Some later join Herbalife Nutrition and become Members themselves, which makes them eligible to purchase products directly from us, simply to receive a discounted price on products for them and their families. Some Members are interested in the entrepreneurial opportunity to earn compensation based on their own skills and hard work and join Herbalife Nutrition to earn part-time or full-time income.
Segmentation
In a number of markets, including the United States, Mexico, Brazil, Italy, Russia, and India, we have segmented our Member base into two categories: “preferred members” – who are consumers who wish to purchase product for their own household use, and “distributors” – who are Members who also wish to resell products or build a sales organization. This member segmentation provides a clear differentiation between those interested in retailing our products or building a sales organization, and those simply consuming our products as discount customers. This distinction allows us to more effectively communicate and market to each group, and provides us with better information regarding our Members within the context of their stated intent and goals. As of December 31, 2021, we had approximately 6.3 million Members, including 2.5 million preferred members and 2.3 million distributors in the markets where we have established these two categories and 0.3 million sales representatives and independent service providers in China.
The number of preferred members and distributors may change as a result of segmentation and/or conversion, and do not necessarily represent a change in the total number of Members. Any future change in the number of preferred members or distributors is not necessarily indicative of our future expected financial performance.
Our objective is sustainable growth in the sales of our products to our Members and their customers by increasing the productivity, retention and recruitment of our Member base through the structure of our network marketing program.
Our Members
We believe our Members are the most important differentiator as we go to market with our nutrition products, because of the one-on-one direct contact they have with their customers, along with the education, training and community support services that we believe help improve the nutrition habits of consumers.
We work closely with our entrepreneurial Members to improve the sustainability of their businesses and to reach consumers. We require our Members to fairly and honestly market both our products and the Herbalife Nutrition business opportunity. Our relationship with our Members is key to our continued success as they allow us direct access to the voice of consumers.
Many of our entrepreneurial Members identify and test new marketing efforts and programs developed by other Members and disseminate successful techniques to their sales organizations. For example, Members in Mexico developed businesses that became known as “Nutrition Clubs,” marketing techniques that improve the productivity and efficiency of our Members as well as the affordability of our weight loss products for their customers. Rather than buying several retail products, these businesses allow consumers to purchase and consume our products each day (a Member marketing technique we refer to as “daily consumption”), while continuing to benefit from the support and interaction with the Member as well as socializing with other customers in a designated location. Other programs to drive daily consumption, whether for weight management or for improved physical fitness, include Member-conducted weight loss contests, or Weight Loss Challenges, Member-led fitness programs, or Fit Camps, and Member-led Wellness Evaluations. We refer to successful Member marketing techniques that we disseminate throughout our Member network, such as Nutrition Clubs, Weight Loss Challenges, and Fit Camps, as Daily Methods of Operations, or DMOs.
7
We believe that personal and professional development is key to our Members’ success and, therefore, we and our sales leader Members – those that achieve certain levels within our Marketing Plan – have meetings and events to support this important objective. We and our Member leadership, which is comprised of sales leaders, conduct in-person and virtual training sessions on local, regional, and global levels attended by thousands of Members to provide updates on product education, sales and marketing training, and instruction on available tools. These events are opportunities to showcase and disseminate our Members’ evolving best marketing practices and DMOs from around the world and to introduce new or upgraded products. A variety of training and development tools are also available through online and mobile platforms.
On July 18, 2002, we entered into an agreement with our Members that provides that we will continue to distribute Herbalife Nutrition products exclusively to and through our Members and that, other than changes required by applicable law or necessary in our reasonable business judgment to account for specific local market or currency conditions to achieve a reasonable profit on operations, we will not make any material changes to certain aspects of our Marketing Plan that are adverse to our Members without the support of our Member leadership. Specifically, any such changes would require the approval of at least 51% of our Members then at the level of President’s Team earning at the production bonus level of 6% who vote, provided that at least 50% of those Members entitled to vote do in fact vote. We initiate these types of changes based on the assessment of what will be best for us and our Members and then submit such changes for the requisite vote. We believe that this agreement has strengthened our relationship with our existing Members, improved our ability to recruit new Members and generally increased the long-term stability of our business.
Member Compensation and Sales Leader Retention and Requalification
In addition to benefiting from discounted prices, Members interested in the entrepreneurial opportunity may earn profit from several sources. First, Members may earn profits by purchasing our products at wholesale prices, discounted depending on the Member’s level within our Marketing Plan, and reselling those products at prices they establish for themselves to generate retail profit. Second, Members who sponsor other Members and establish, maintain, coach, and train their own sales organizations may earn additional income based on the sales of their organization, which may include royalty overrides, production bonuses, and other cash bonuses. Members earning such compensation have generally attained the level of sales leader as described below. There are also many Members, which include distributors, who have not sponsored another Member. Members who have not sponsored another Member are generally considered discount buyers or small retailers. While a number of these Members have also attained the level of sales leader, they do not receive additional income as do Members who have sponsored other Members.
We assign point values, known as Volume Points, to each of our products to determine a Member’s level within the Marketing Plan. See Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Operating Results, of this Annual Report on Form 10-K for a further description of Volume Points. Typically, a Member accumulates Volume Points for a given sale at the time the Member pays for the product. However, since May 2017, a Member does not receive Volume Points for a transaction in the United States until that product is sold to a customer at a profit and it is documented in compliance with the consent order, or Consent Order, we entered into with the Federal Trade Commission, or the FTC, in 2016. The Member’s level within the Marketing Plan is used to determine the discount applied to their purchase of our products and whether they have qualified to become a sales leader. To become a sales leader, or qualify for a higher level within our Marketing Plan, Members must achieve specified Volume Point thresholds of product sales or earn certain amounts of royalty overrides during specified time periods and generally must re-qualify once each year. Qualification criteria vary somewhat by market. We have initial qualification methods of up to 12 months to encourage a more gradual qualification. We believe a gradual qualification approach is important to the success and retention of new sales leaders and benefits the business in the long term as it allows new Members to obtain product and customer experience as well as additional training and education on Herbalife Nutrition products, daily consumption based DMOs, and the business opportunity prior to becoming a sales leader.
The basis for calculating Marketing Plan payouts varies depending on product and market: for 2021, we utilized on a weighted-average basis approximately 90% of suggested retail price, to which we applied discounts of up to 50% for distributor allowances and payout rates of up to 15% for royalty overrides, up to 7% for production bonuses, and approximately 1% for a cash bonus known as the Mark Hughes bonus. We believe that the opportunity for Members to earn royalty overrides and production bonuses contributes significantly to our ability to retain our most active and productive Members.
Our Marketing Plan generally requires each sales leader to re-qualify for such status each year, prior to February, in order to maintain their 50% discount on products and be eligible to receive additional income. In February of each year, we demote from the rank of sales leader those Members who did not satisfy the re-qualification requirements during the preceding twelve months. The re-qualification requirement does not apply to new sales leaders (i.e. those who became sales leaders subsequent to the January re-qualification of the prior year).
8
As of December 31, 2021, prior to our February re-qualification process, approximately 793,000 of our Members have attained the level of sales leader, of which approximately 732,000 have attained this level in the 94 markets where we use our Marketing Plan and 61,000 independent service providers operating in our China business. See Business in China below for a description of our business in China.
The table below reflects sales leader retention rates by year and by region:
|
|
Sales Leader Retention Rate |
|
|||||||||
|
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
North America |
|
|
58.8 |
% |
|
|
70.8 |
% |
|
|
65.4 |
% |
Mexico |
|
|
68.6 |
% |
|
|
68.2 |
% |
|
|
66.6 |
% |
South and Central America |
|
|
70.2 |
% |
|
|
65.5 |
% |
|
|
60.7 |
% |
EMEA |
|
|
77.1 |
% |
|
|
72.7 |
% |
|
|
70.6 |
% |
Asia Pacific |
|
|
66.5 |
% |
|
|
63.5 |
% |
|
|
65.7 |
% |
Total sales leaders |
|
|
68.9 |
% |
|
|
67.9 |
% |
|
|
66.5 |
% |
For the latest twelve-month re-qualification period ending January 2022, approximately 68.9% of our sales leaders, excluding China, re-qualified, versus 67.9% for the twelve-month period ended January 2021. For each of these years, certain markets have utilized a lower re-qualification threshold, and these figures include the effect of the lower threshold. Excluding the impact of the lower re-qualification thresholds, the retention rates for 2022 and 2021 would have been 60.7% and 62.9%, respectively. Separately, with revised business requirements in place following the Consent Order, as described in Network Marketing Program below, we utilize a re-qualification equalization factor for U.S. Members to better align their re-qualification thresholds with Members in other markets, and retention results for each of the years presented include the effect of the equalization factor. We believe this factor preserves retention rate comparability across markets. Also, for each of the years presented, the retention results exclude certain markets for which, due to local operating conditions, sales leaders were not required to requalify; such exclusions are not material to our retention results.
We believe the sales leader retention rate of 68.9% for the year ended January 2022 is the result of efforts we have made to improve the sustainability of sales leaders’ businesses, such as encouraging Members to obtain experience retailing Herbalife Nutrition products before becoming a sales leader and providing them with advanced technology tools. The results of these efforts were partially offset by the effect of the significant increase in sales leaders during the second half of 2020, particularly in the North America region where a number of these first-time sales leaders did not requalify for the year ended January 2022 as reflected in the decline in the retention rate for that region. The decline for North America also contributed to the slight decline for the year in the global retention rate excluding the impact of lower re-qualification thresholds as described above. As our business operations evolve, including the segmentation of our Member base in certain markets and changes in sales leader re-qualification thresholds for other markets, management continues to evaluate the importance of sales leader retention rate information.
The table below reflects the number of sales leaders as of the end of February of the year indicated (subsequent to the annual re-qualification process) and by region:
|
|
Number of Sales Leaders |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
North America |
|
|
95,402 |
|
|
|
71,202 |
|
|
|
66,264 |
|
Mexico |
|
|
73,043 |
|
|
|
72,866 |
|
|
|
75,475 |
|
South and Central America |
|
|
58,316 |
|
|
|
61,535 |
|
|
|
64,929 |
|
EMEA |
|
|
158,153 |
|
|
|
130,438 |
|
|
|
121,297 |
|
Asia Pacific |
|
|
173,582 |
|
|
|
158,815 |
|
|
|
133,817 |
|
Total sales leaders |
|
|
558,496 |
|
|
|
494,856 |
|
|
|
461,782 |
|
China |
|
|
68,301 |
|
|
|
70,701 |
|
|
|
89,077 |
|
Worldwide total sales leaders |
|
|
626,797 |
|
|
|
565,557 |
|
|
|
550,859 |
|
The number of sales leaders as of December 31 will exceed the number immediately subsequent to the preceding re-qualification period because sales leaders qualify throughout the year but sales leaders who do not re-qualify are removed from the rank of sales leader the following February.
9
Business in China
Our business model in China includes unique features as compared to our traditional business model in order to ensure compliance with Chinese regulations. As a result, our business model in China differs from that used in other markets. Members in China are categorized differently than those in other markets. In China, we sell our products to and through independent service providers and sales representatives to customers and preferred customers, as well as through Company-operated retail platforms when necessary.
In China, while multi-level marketing is not permitted, direct selling is permitted. Chinese citizens who apply and become Members are referred to as sales representatives. These sales representatives are permitted to sell away from fixed retail locations in the provinces where we have direct selling licenses, including in the provinces of Jiangsu, Guangdong, Shandong, Zhejiang, Guizhou, Beijing, Fujian, Sichuan, Hubei, Shanxi, Shanghai, Jiangxi, Liaoning, Jilin, Henan, Chongqing, Hebei, Shaanxi, Tianjin, Heilongjiang, Hunan, Guangxi, Hainan, Anhui, Yunnan, Gansu, Ningxia, and Inner Mongolia. In Xinjiang province, where we do not have a direct selling license, we have a Company-operated retail store that can directly serve customers and preferred customers. With online orderings throughout China, there has been a declining demand in Company-operated retail stores.
Sales representatives receive scaled rebates based on the volume of products they purchase. Sales representatives who reach certain volume thresholds and meet certain performance criteria are eligible to apply to provide marketing, sales and support services. Once their application is accepted, they are referred to as independent service providers. Independent service providers are independent business entities that are eligible to receive compensation from Herbalife Nutrition for the marketing, sales and support services they provide so long as they satisfy certain conditions, including procuring the requisite business licenses, having a physical business location, and complying with all applicable Chinese laws and Herbalife Nutrition rules.
In China, our independent service providers are compensated for marketing, sales support, and other services, instead of the Member allowances and royalty overrides utilized in our global Marketing Plan. The service hours and related fees eligible to be earned by the independent service providers are based on a number of factors, including the sales generated through them and through others to whom they may provide marketing, sales support and other services, the quality of their service, and other factors. Total compensation available to our independent service providers in China can generally be comparable to the total compensation available to other sales leaders globally. The Company does this by performing an analysis in our worldwide system to estimate the potential compensation available to the service providers, which can generally be comparable to that of sales leaders in other countries. After adjusting such amounts for other factors and dividing by each service provider’s hourly rate, we then notify each independent service provider the maximum hours of work for which they are eligible to be compensated in the given month. In order for a service provider to be paid, the Company requires each service provider to invoice the Company for their services.
Member Technology
Many Members rely on the use of technology to support their goals and businesses. As part of our continued investment in technology to further support our Members and drive long-term growth, we have enhanced our product access and distribution network to support higher volumes of online or mobile orders, allowing Members and their customers to select home or business delivery options. We have also implemented information technology systems to support Members and their increasing demand to be more connected to Herbalife Nutrition, their business, and their consumers with tools such as HN MyClub, Engage, HNconnect, BizWorks, MyHerbalife, GoHerbalife, and Herbalife.com. Additionally, we continue to support a growing suite of point-of-sale tools to assist our Members with ordering, tracking, and customer relationship management. These tools allow our Members to manage their business and communicate with their customers more efficiently and effectively.
RESOURCES
We seek to provide the highest quality products to our Members and their customers through our “seed to feed” strategy, which includes significant investments in quality ingredients from traceable sources, scientific personnel, product testing, and increasing the amount of self-manufacturing of our top products.
Ingredients
Our seed to feed strategy is rooted in using quality ingredients from traceable sources. Our procurement process for many of our botanical products now stretches back to the farms and includes self-processing of teas and herbal ingredients into finished raw materials at our own facilities. Our Changsha, China facility provides high quality tea and herbal raw materials to all our manufacturing facilities as well as our third-party contract manufacturers around the world. We source the ingredients that we do not self-process from companies that we believe are well-established and reputable suppliers in their respective field. Our suppliers typically utilize similar quality processes, equipment, expertise, and traceability as we do with our own modern quality processes. As part of ensuring high quality ingredients, we also test our incoming raw materials for compliance to potency, identity, and adherence to strict specifications.
10
Manufacturing
The next key component of our seed to feed strategy involves the high-quality manufacturing of these ingredients into finished products, which are produced at both third-party manufacturers and our own manufacturing facilities. As part of our long-term strategy, we seek to expand and increase our self-manufacturing capabilities. Our manufacturing facilities, known as Herbalife Innovation and Manufacturing Facilities, or HIMs, include HIM Lake Forest, HIM Winston-Salem, HIM Suzhou, and HIM Nanjing. HIM Winston-Salem is currently our largest manufacturing facility at approximately 800,000 square feet. Together, our HIM manufacturing facilities produce approximately 60% of our inner nutrition products sold worldwide. Self-manufacturing also enables us greater control to reduce negative environmental impacts of our operations and supply chain. As described in the Sustainability section below, we are focused on developing science-based green-house gas emission and waste targets for our manufacturing facilities as part of our sustainability goals. We are also focused on reducing single-use plastics throughout our global distribution network and incorporating more sustainable content, such as post-consumer resin, into our packaging.
Finished products are analyzed for label claims and microbiological purity, thereby verifying product safety, meeting label claims, and assuring shelf life. For self-manufactured products, we conduct all of our testing in-house at our fully-equipped, modern quality control laboratories in the U.S. and China. We have two quality control laboratories in Southern California (including a Center of Excellence); Winston-Salem, North Carolina; Suzhou, China; Nanjing, China; and two laboratories in Changsha, China (including a Quality Center of Excellence). All HIM quality control labs contain modern analytical equipment and are backed by the expertise in testing and methods development of our scientists. In our U.S. HIM facilities, which manufacture products for the U.S. and most of our international markets, we operate and adhere to the regulations established by the U.S. Food and Drug Administration, or FDA, and strict Current Good Manufacturing Practice regulations, or CGMPs, for food, acidified foods, and dietary supplements.
We also work closely with our third-party manufacturers to ensure high quality products are produced and tested through a vigorous quality control process at approved contract manufacturer labs or third-party labs. For these products manufactured at other facilities, we combine four elements to ensure quality products: (1) the same selectivity and assurance in ingredients as noted above; (2) use of reputable, CGMP-compliant, quality- and sustainability-minded manufacturing partners; (3) supplier qualification through annual audit programs; and (4) significant product quality testing. During 2021, we purchased approximately 19% of our products from our top three third-party manufacturers.
Infrastructure
Our direct-selling business model enables us to grow our business with moderate investment in infrastructure and fixed costs. We incur no direct incremental cost to add a new Member in our existing markets, and our Member compensation varies directly with product sales. In addition, our Members also bear a portion of our consumer marketing expenses, and our sales leaders sponsor and coordinate Member recruiting and most meeting and training initiatives. Additionally, our infrastructure features scalable production and distribution of our products as a result of having our own manufacturing facilities and numerous third-party manufacturing relationships, as well as our global footprint of in-house and third-party distribution centers.
An important part of our seed to feed strategy is having an efficient infrastructure to deliver products to our Members and their customers. As the shift in consumption patterns continues to reflect an increasing daily consumption focus, one focus of this strategy is to provide more product access points closer to our Members and their customers. We have both Company-operated and outsourced distribution points ranging from our “hub” distribution centers in Los Angeles, Memphis, and Venray, Netherlands, to mid-size distribution centers in major countries, to small pickup locations spread throughout the world. We also expect to continue to improve our distribution channels relating to home delivery as we expect to see continued increased demands for our products being shipped to our Members in certain of our larger markets. In addition to these distribution points, we partner with certain retail locations to provide Member pickup points in areas which are not well serviced by our distribution points. We have also identified a number of methods and approaches that better support Members by providing access points closer to where they do business and by improving product delivery efficiency through our distribution channels. Specific methods vary by markets and consider local Member needs and available resources. In aggregate, we have over 1,600 distribution points and partner retail locations around the world. In addition to our distribution points, we contract third party-run drop-off locations where we can ship to and Members can pick up ordered products.
We leverage our technology infrastructure in order to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in technology, evolving industry and regulatory standards, emerging data security risks, and changing user patterns and preferences. We also continue to invest in our manufacturing and operational infrastructure to accelerate new products to market and accommodate planned business growth. We invest in business intelligence tools to enable better analysis of our business and to identify opportunities for growth. We will continue to build on these platforms to take advantage of the rapid development of technology around the globe to support a more robust Member and customer experience. In addition, we leverage an Oracle business suite platform, which was upgraded in 2017, to support our business operations, improve productivity and support our strategic initiatives. Our investment in technology infrastructure helps support our capacity to grow.
11
Intellectual property and branding
Marketing foods and supplement products on the basis of sound science means using ingredients in the exact composition and quantity as demonstrated to be effective in the relevant scientific literature. Use of these ingredients for their well-established purposes is by definition not novel, and for that reason, most food uses of these ingredients are not subject to patent protection. Notwithstanding the absence of patent protection, we do own proprietary formulations for substantially all of our weight management products and dietary and nutritional supplements. We take care in protecting the intellectual property rights of our proprietary formulas by restricting access to our formulas within the Company to those persons or departments that require access to them to perform their functions, and by requiring our finished goods suppliers and consultants to execute supply and non-disclosure agreements that contractually protect our intellectual property rights. Disclosure of these formulas, in redacted form, is also necessary to obtain product registrations in many countries. We also make efforts to protect certain unique formulations under patent law. We strive to protect all new product developments as the confidential trade secrets of the Company and its inventor employees.
We use the umbrella trademarks Herbalife®, Herbalife Nutrition®, and the Tri-Leaf design worldwide, and protect several other trademarks and trade names related to our products and operations, such as Niteworks® and Liftoff®. Our trademark registrations are issued through the United States Patent and Trademark Office, or USPTO, and comparable agencies in the foreign countries. We believe our trademarks and trade names contribute to our brand awareness.
To increase our brand awareness, we and our Members use a variety of tools and marketing channels. These can include anything from traditional media to social media and alliances with partners who can promote our goal of better living through nutrition. Herbalife Nutrition sponsorships of and partnerships with featured athletes, teams, and events promote brand awareness and the use of Herbalife Nutrition products. We continue to build brand awareness with a goal towards becoming the most trusted brand in nutrition. We also work to leverage the power of our Member base as a marketing and brand-building tool. We maintain a brand style guide and brand asset library so that our Members have access to the Herbalife Nutrition brand logo and marketing materials for use in their marketing efforts.
Sustainability
Our goals and objectives to nourish people and communities and to improve the planet are part of both our day-to-day activities and our long-term growth strategy. As a signatory of the United Nations Global Compact, or UNGC, we have aligned our sustainability initiatives outlined by the United Nations’ Sustainable Development Goals. Our current sustainability initiatives focus on issues including climate and emissions, packaging, and operational waste. For example, across our product packaging and operations in North America and Mexico, we have implemented projects that have reduced overall packaging materials and incorporated usage of recycled materials in the packaging of our flagship product, Formula 1 Healthy Meal Nutritional Shake. We are seeking opportunities across operations to reduce waste-prone materials such as single-use plastics. More information on these efforts is provided in the Manufacturing section above. For information relating to our culture, diversity, equity, and inclusion, please see the Human Capital section below.
REGULATION
General
In our United States and foreign markets, we are affected by extensive laws, governmental regulations, administrative determinations and guidance, court decisions and similar constraints that regulate the conduct of our business. Such laws, regulations and other constraints exist at the federal, state or local levels in the United States and at all levels of government in foreign jurisdictions, and include regulations pertaining to: (1) the formulation, manufacturing, packaging, labeling, distribution, importation, sale, and storage of our products; (2) product claims and advertising, including direct claims and advertising by us, as well as claims and advertising by Members, for which we may be held responsible; (3) our network marketing program; (4) transfer pricing and similar regulations that affect the level of U.S. and foreign taxable income and customs duties; (5) taxation of our Members (which in some instances may impose an obligation on us to collect the taxes and maintain appropriate records); (6) our international operations, such as import/export, currency exchange, repatriation and anti-bribery regulations; (7) antitrust issues; and (8) privacy and data protection. See Part I, Item 1A, Risk Factors, of this Annual Report on Form 10-K for additional information.
12
Products
In the United States, the formulation, manufacturing, packaging, holding, labeling, promotion, advertising, distribution, and sale of our products are subject to regulation by various federal governmental agencies, including: (1) the FDA; (2) the FTC; (3) the Consumer Product Safety Commission, or CPSC; (4) the United States Department of Agriculture, or USDA; (5) the Environmental Protection Agency, or EPA; (6) the United States Postal Service; (7) United States Customs and Border Protection; and (8) the Drug Enforcement Administration. Our activities also are regulated by various agencies of the states, localities and foreign countries in which our products are manufactured, distributed, or sold. The FDA, in particular, regulates the formulation, manufacture, and labeling of over-the-counter, or OTC, drugs, conventional foods, dietary supplements, and cosmetics such as those distributed by us. The majority of the products marketed by us in the United States are classified as conventional foods or dietary supplements under the Federal Food, Drug and Cosmetic Act, or FFDCA. Internationally, the majority of products marketed by us are classified as foods, health supplements, or food supplements.
FDA regulations govern the preparation, packaging, labeling, holding, and distribution of foods, OTC drugs, cosmetics, and dietary supplements. Among other obligations, they require us and our contract manufacturers to meet relevant CGMP regulations for the preparation, packaging, holding, and distribution of OTC drugs and dietary supplements. The FDA also requires identity testing of all incoming dietary ingredients used in dietary supplements, unless a company successfully petitions for an exemption from this testing requirement in accordance with the regulations. The CGMPs are designed to ensure that OTC drugs and dietary supplements are not adulterated with contaminants or impurities, and are labeled to accurately reflect the active ingredients and other ingredients in the products. We have implemented a comprehensive quality assurance program that is designed to maintain compliance with the CGMPs for products manufactured by us or on our behalf for distribution in the United States. As part of this program, we have regularly implemented enhancements, modifications and improvements to our manufacturing and corporate quality processes. We believe that we and our contract manufacturers are compliant with the FDA’s CGMPs and other applicable manufacturing regulations in the United States.
The U.S. Dietary Supplement Health and Education Act of 1994, or DSHEA, revised the provisions of FFDCA concerning the composition and labeling of dietary supplements. Under DSHEA, dietary supplement labeling may display structure/function claims that the manufacturer can substantiate, which are claims that the products affect the structure or function of the body, without prior FDA approval, but with notification to the FDA. They may not bear any claim that they can prevent, treat, cure, mitigate or diagnose disease (a drug claim). Apart from DSHEA, the agency permits companies to use FDA-approved full and qualified health claims for food and supplement products containing specific ingredients that meet stated requirements.
U.S. law also requires that all serious adverse events occurring within the United States involving dietary supplements or OTC drugs be reported to the FDA. We believe that we are in compliance with this law having implemented a worldwide procedure governing adverse event identification, investigation and reporting. As a result of reported adverse events, we may from time to time elect, or be required, to remove a product from a market, either temporarily or permanently.
Some of the products marketed by us are considered conventional foods and are currently labeled as such. Within the United States, this category of products is subject to the federal Nutrition, Labeling and Education Act, or NLEA, and regulations promulgated under the NLEA. The NLEA regulates health claims, ingredient labeling and nutrient content claims characterizing the level of a nutrient in the product. The ingredients in conventional foods must either be generally recognized as safe by experts for the purposes to which they are put in foods, or be approved as food additives under FDA regulations.
The federal Food Safety Modernization Act, or FSMA, is also applicable to some of our business. We follow a food safety plan and have implemented preventive measures required by the FSMA. Foreign suppliers of our raw materials are also subject to FSMA requirements, and we have implemented a verification program to comply with the FSMA. Dietary supplements manufactured in accordance with CGMPs and foods manufactured in accordance with the low acid food regulations are exempt.
In foreign markets, prior to commencing operations and prior to making or permitting sales of our products in the market, we may be required to obtain an approval, license or certification from the relevant country’s ministry of health or comparable agency. Prior to entering a new market in which a formal approval, license or certificate is required, we work with local authorities in order to obtain the requisite approvals. The approval process generally requires us to present each product and product ingredient to appropriate regulators and, in some instances, arrange for testing of products by local technicians for ingredient analysis. The approvals may be conditioned on reformulation of our products, or may be unavailable with respect to some products or some ingredients.
13
The FTC, which exercises jurisdiction over the advertising of all of our products in the United States, has in the past several years instituted enforcement actions against several dietary supplement and food companies and against manufacturers of weight loss products generally for false and misleading advertising of some of their products. In addition, the FTC has increased its scrutiny of the use of testimonials, which we also utilize, as well as the role of expert endorsers and product clinical studies. We cannot be sure that the FTC, or comparable foreign agencies, will not question our advertising or other operations in the future.
In Europe, where an EU Health Claim regulation is in effect, the European Food Safety Authority, or EFSA, issued opinions following its review of a number of proposed claims documents. ESFA’s opinions, which have been accepted by the European Commission, have limited the use of certain nutrition-specific claims made for foods and food supplements. Accordingly, we revised affected product labels to ensure regulatory compliance.
We are subject to a permanent injunction issued in October 1986 pursuant to the settlement of an action instituted by the California Attorney General, the State Health Director and the Santa Cruz County District Attorney. We consented to the entry of this injunction without in any way admitting the allegations of the complaint. The injunction prevents us from making specified claims in advertising of our products, but does not prevent us from continuing to make specified claims concerning our products, provided that we have a reasonable basis for making the claims. The injunction also prohibits certain recruiting-related investments from Members and mandates that payments to Members be premised on retail value (as defined); the injunction provides that we may establish a system to verify or document such compliance.
Network Marketing Program
Our network marketing program is subject to a number of federal and state regulations administered by the FTC and various state regulators as well as regulations in foreign markets administered by foreign regulators. Regulations applicable to network marketing organizations generally are directed at ensuring that product sales ultimately are made to consumers and that advancement within the organization is based on sales of the organization’s products rather than investments in the organization or other non-retail sales related criteria. When required by law, we obtain regulatory approval of our network marketing program or, when this approval is not required, the favorable opinion of local counsel as to regulatory compliance.
On July 15, 2016, we reached a settlement with the FTC and entered into a proposed Stipulation to Entry of Order for Permanent Injunction and Monetary Judgment, or the Consent Order, which resolved the FTC’s multi-year investigation of us. The Consent Order became effective on July 25, 2016, or the Effective Date, upon final approval by the U.S. District Court for the Central District of California. Pursuant to the Consent Order, we implemented and continue to enhance certain procedures in the U.S. and agreed to be subject to certain audits by an independent compliance auditor (Affiliated Monitors, Inc.) for a period of seven years. Among other requirements, the Consent Order requires us to categorize all existing and future Members in the U.S. as either “preferred members” – who are simply consumers who only wish to purchase product for their own household use — or “distributors” – who are Members who wish to resell some products or build a sales organization. We also agreed to compensate distributors on U.S. eligible sales within their downline organizations, which include purchases by preferred members, purchases by a distributor for his or her personal consumption within allowable limits and sales of product by a distributor to his or her customers. The Consent Order also requires distributors to meet certain conditions before opening Nutrition Clubs and/or entering into leases for their Herbalife Nutrition business in the United States.
The Consent Order also prohibits us from making expressly or by implication, any misrepresentation regarding certain lifestyles or amount or level of income, including full-time or part-time income that a participant can reasonably expect to earn in our network marketing program. The Consent Order also prohibits us and other persons who act in active concert with us from misrepresenting that participation in the network marketing program will result in a lavish lifestyle and from using images or descriptions to represent or imply that participation in the program is likely to result in a lavish lifestyle. In addition, the Consent Order prohibits specified misrepresentations in connection with marketing the program, including misrepresentations regarding any fact material to participation such as the cost to participate or the amount of income likely to be earned. The Consent Order also requires us to clearly and conspicuously disclose information related to our refund and buyback policy on certain company materials and websites.
The terms of the Consent Order do not change our going to market through direct selling by independent distributors, and compensating those distributors based upon the product they and their sales organization sell. We have implemented new and enhanced procedures required by the terms of the Consent Order and will continue to do so. We continue to monitor the impact of the Consent Order and our board of directors originally established the Implementation Oversight Committee in connection with monitoring compliance with the Consent Order, and more recently, our Audit Committee assumed oversight of continued compliance with the Consent Order. While we currently do not expect the Consent Order to have a long-term and material adverse impact on our business and our Member base, our business and our Member base, particularly in the U.S., have been in the past, and may in the future, be negatively impacted as we and they adjust to the changes. However, the terms of the Consent Order and the ongoing costs of compliance may adversely affect our business operations, our results of operations, and our financial condition. See Part I, Item 1A, Risk Factors, of this Annual Report on Form 10-K for a discussion of risks related to the settlement with the FTC.
14
On January 4, 2018, the FTC released its nonbinding Business Guidance Concerning Multi-Level Marketing, or MLM Guidance. The MLM Guidance explains, among other things, lawful and unlawful compensation structures, the treatment of personal consumption by participants in determining if an MLM’s compensation structure is unfair or deceptive, and how an MLM should approach representations to current and prospective participants. We believe our current business practices, which include new and enhanced procedures implemented in connection with the Consent Order, are in compliance with the MLM Guidance.
Additionally, the FTC has promulgated nonbinding Guides Concerning the Use of Endorsements and Testimonials in Advertising, or Guides, which explain how the FTC interprets Section 5 of the FTC Act’s prohibition on unfair or deceptive acts or practices. Consequently, the FTC could bring a Section 5 enforcement action based on practices that are inconsistent with the Guides. Under the Guides, advertisements that feature a consumer and convey his or her atypical experience with a product or service are required to clearly disclose the typical results that consumers can generally expect. The revised Guides also require advertisers to disclose connections between the advertiser and any endorsers that consumers might not expect, known as “material connections.” We have adapted our practices and rules regarding the practices of our Members to comply with the Guides and to comply with the Consent Order.
We also are subject to the risk of private party challenges to the legality of our network marketing program both in the United States and internationally. For example, in Webster v. Omnitrition International, Inc., 79 F.3d 776 (9th Cir. 1996), the network marketing program of Omnitrition International, Inc., or Omnitrition, was challenged in a class action by Omnitrition distributors who alleged that it was operating an illegal “pyramid scheme” in violation of federal and state laws. We believe that our network marketing program satisfies federal and other applicable state statutes and case law.
In some countries, regulations applicable to the activities of our Members also may affect our business because in some countries we are, or regulators may assert that we are, responsible for our Members’ conduct. In these countries, regulators may request or require that we take steps to ensure that our Members comply with local regulations. The types of regulated conduct include: (1) representations concerning our products; (2) income representations made by us and/or Members; (3) public media advertisements, which in foreign markets may require prior approval by regulators; (4) sales of products in markets in which the products have not been approved, licensed or certified for sale; and (5) classification by government agencies of our Members as employees of the Company.
In some markets, it is possible that improper product claims by Members could result in our products being reviewed by regulatory authorities and, as a result, being classified or placed into another category as to which stricter regulations are applicable. In addition, we might be required to make labeling changes.
We also are subject to regulations in various foreign markets pertaining to social security assessments and employment and severance pay requirements. As an example, in some markets, we are substantially restricted in the amount and types of rules and termination criteria that we can impose on Members without having to pay social security assessments on behalf of the Members and without incurring severance obligations to terminated Members. In some countries, we may be subject to these obligations in any event.
It is an ongoing part of our business to monitor and respond to regulatory and legal developments, including those that may affect our network marketing program. However, the regulatory requirements concerning network marketing programs do not include bright line rules and are inherently fact-based. An adverse judicial or regulatory determination with respect to our network marketing program could have a material adverse effect on our business, financial condition, and operating results and may also result in negative publicity, requirements to modify our network marketing program, or a negative impact on Member morale. In addition, adverse rulings by courts in any proceedings challenging the legality of network marketing systems, even in those not involving us directly, could have a material adverse effect on our operations.
Although questions regarding the legality of our network marketing program have come up in the past and may come up from time to time in the future, we believe, based in part upon guidance to the general public from the FTC, that our network marketing program is compliant with applicable law.
Income Tax, Transfer Pricing, and Other Taxes
In many countries, including the United States, we are subject to income tax, transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported as earned by our U.S. and local entities and are taxed accordingly. In addition, our operations are subject to regulations designed to ensure that appropriate levels of customs duties are assessed on the importation of our products.
15
Although we believe that we are in substantial compliance with all applicable tax rules, regulations, and restrictions, we are subject to the risk that governmental authorities could assert that additional taxes are owed based on findings of their audit. For example, we are currently subject to pending or proposed audits that are at various levels of review, assessment or appeal in a number of jurisdictions involving transfer pricing issues, income taxes, duties, value added taxes, withholding taxes and related interest and penalties in material amounts. In some circumstances, additional taxes, interest and penalties have been assessed, and we will be required to appeal or litigate to reverse the assessments. We have taken advice from our tax advisors and believe that there are substantial defenses to the allegations that additional taxes are owed, and we are vigorously defending against the imposition of additional proposed taxes. The ultimate resolution of these matters may take several years, and the outcome is uncertain.
In the event that the audits or assessments are concluded adversely, we may or may not be able to offset or mitigate the consolidated effect of foreign income tax assessments through the use of U.S. foreign tax credits. The laws and regulations governing U.S. foreign tax credits are complex and subject to periodic legislative amendment, and there are restrictions on the utilization of U.S. foreign tax credits. Therefore, we cannot be sure that we would in fact be able to take advantage of any foreign tax credits in the future.
Compliance Procedures
As indicated above, Herbalife Nutrition, our products and our network marketing program are subject, both directly and indirectly through Members’ conduct, to numerous federal, state and local regulations, in the United States and foreign markets. Beginning in 1985, we began to institute formal compliance measures by developing a system to identify specific complaints against Members and to remedy any violations of Herbalife Nutrition’s rules by Members through appropriate sanctions, including warnings, fines, suspensions and, when necessary, terminations. We prohibit Members from making therapeutic claims for our products or misrepresentations regarding participating in our network marketing program, including in our manuals, seminars, and other training programs and materials.
Our general policy is to reject Member applications from individuals who do not reside in one of our approved markets.
In order to comply with regulations that apply to both us and our Members, we research the applicable regulatory framework prior to entering any new market to identify necessary licenses and approvals and applicable limitations relating to our operations in that market and then work to bring our operations into compliance with the applicable limitations and to maintain such licenses. Typically, we conduct this research with the assistance of local legal counsel and other representatives. We also research laws applicable to Member operations and revise or alter our Member applications, rules, and other training materials and programs to provide Members with guidelines for operating their independent business, marketing and distributing our products and similar matters, as required by applicable regulations in each market. While we have rules and guidelines for our Members and monitor their market conduct, we are, however, unable to ensure that our Members will not distribute our products in countries where we have not commenced operations.
In addition, regulations in existing and new markets often are ambiguous and subject to considerable interpretive and enforcement discretion by the responsible regulators. Moreover, even when we believe that we and our Members are in compliance with all applicable regulations, new regulations are being added regularly and the interpretation of existing regulations is subject to change. Further, the content and impact of regulations to which we are subject may be influenced by public attention directed at us, our products, or our network marketing program, so that extensive adverse publicity about us, our products, or our network marketing program may increase the likelihood regulatory scrutiny or action.
HUMAN CAPITAL
At Herbalife Nutrition, our commitment to improving lives and our communities is at the core of everything we do. This commitment also informs how we value and treat our employees. We seek to provide a work environment where employees can grow and thrive while supporting our Members and their customers. We believe attracting, developing, and retaining a talented and diverse workforce are critical factors that contribute to the success and growth of our business.
Employees
We have operations globally, requiring investment to assess local labor market conditions and recruit and retain the appropriate human capital. Having a business presence in multiple domestic and international markets also requires us to monitor local labor and employment laws for which we often engage third-party advisors. We monitor the human capital needs of our departments and functions with particular focus on the areas where human capital resources are important to daily operations to ensure we can timely manufacture, distribute, and sell products to our Members. As of December 31, 2021, we had approximately 10,800 employees, of which approximately 3,100 were located in the United States.
16
Diversity, Equity, and Inclusion
We believe diversity is a strength and embrace a core vision that a diverse, equitable, and inclusive culture is imperative to enable us to better serve our Members, stakeholders, and communities. As such, we seek to promote a work environment where all people can thrive, and are committed to diversity, equity, and inclusion, or DE&I, at all levels, from our employees, management and executive leadership to our board of directors.
Our DE&I strategy is currently focused on creating opportunities to further recruit and support diverse talent at all levels, encouraging belonging, and embedding equity throughout our culture and operations. Current initiatives include the implementation of a global applicant tracking system to deepen our commitment to fair recruitment processes, offering unconscious bias trainings for all employees, and the expansion of existing employee networks to provide support to our employees. Additionally, in 2021 we set diversity goals and targets for women in leadership roles globally and for racial and ethnic minorities in leadership roles in the U.S.
Hiring, Development, Engagement, and Retention
We seek to attract and retain a talented workforce. To help foster an inclusive hiring process in the U.S., we use a tool that helps ensure that job descriptions do not unintentionally exclude potential applicants.
Because we believe the professional growth of our employees supports our retention efforts and helps establish a strong foundation for long-term success, we strive to create a learning culture, one in which development is an ongoing focus for all employees and managers. We also invest in our employees’ development through a variety of formal and informal programs. These programs are designed to help our employees grow professionally and strengthen their skills throughout their careers. Examples of these programs include the following:
Employee Compensation and Benefits
Our board of directors and its Compensation Committee establish our general compensation philosophy and oversee and approve the development, adoption, and implementation of compensation and benefits policies and programs, which are adapted to meet local country requirements as needed. We provide base pay that aligns with employee positions, skill levels, experience, contributions, and geographic location. In addition to base pay, we seek to reward employees with annual incentive awards, recognition programs, and equity awards for employees at certain job grades. Our benefit programs are designed to enhance employee well-being and assist employees in the event of illness, injury, or disability. To this end, we offer benefits that vary by country, but may include health insurance, retirement savings programs, and wellness incentives designed to promote a healthy and active lifestyle. We believe we offer our employees wages and benefits packages that are in line with respective local labor markets and laws.
Employee Safety, Healthy, and Well-Being
As a nutrition company, we believe the safety, health, and well-being of our employees is of the utmost importance. We endeavor to provide a safe and healthy work environment and encourage healthy, active lifestyles. Our efforts to provide a safe workplace are guided by various formal policies, trainings, and programs, which are designed to protect employees, contractors, and visitors from accidents, illnesses, and injuries, while operating in compliance with applicable regulations, including OSHA guidelines in the U.S. We also follow policies and programs regarding material health and safety risks, workplace violence prevention, and incident response and management. In the U.S., our manufacturing facilities in Winston-Salem and Lake Forest are ISO 45001 certified, an international standard for occupational health and safety management.
17
In addition, we believe in the importance of wellness and that being healthy and subscribing to an active lifestyle is an important part of being a team player. Our flagship wellness program in the U.S., “Wellness for Life,” offers employees a suite of activities to achieve overall wellness through improved fitness, nutrition, mental/emotional well-being, and financial literacy. The long-term plan is to expand this program to reach employees around the globe. We also have facilities and programs in place that allow employees to incorporate fitness into their daily schedule, such as gyms available at several facilities globally. By providing free access, we remove logistic obstacles that might get in the way of being physically active.
The COVID-19 pandemic has increased the resources required to keep our employees safe and healthy and we continue to make what we believe are the necessary investments to achieve this goal. In response to, and during various phases of, the pandemic, we have taken several actions, including supporting our employees to work from home when possible, offering mental wellness programs, and implementing the following safety measures at our U.S. facilities: required temperature screening, required face coverings, instituted social distancing protocols, enhanced robust cleaning and sanitation measures, and increased the filtration efficiency of existing ventilation systems. Earlier during the COVID-19 pandemic, we also offered incremental compensation to certain of our employees. Over the course of the pandemic, our senior management team has relied on cross-functional teams to monitor, review, and assess the evolving situation, and to recommend risk mitigation actions for the health and safety of our employees and, in the U.S., protocols to align with all federal, state, and local public health guidelines. We believe our proactive efforts have been successful in supporting our business growth despite the obstacles and challenges presented by the COVID-19 pandemic.
Our Members
We are dependent on our Members to sell and promote our products to their customers. We frequently interact and work directly with our sales leaders to explore ways to support our and our Members’ businesses, and their customers’ personal goals of living a healthier and more active lifestyle. See the Our Network Marketing Program – Member Compensation and Sales Leader Retention and Requalification section above for sales leader and requalification metrics and further discussion on our sales leaders.
Available Information
Our Internet website address is www.herbalife.com and our investor relations website is ir.herbalife.com. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, proxy statements, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practical after we file such material with, or furnish it to, the Securities and Exchange Commission, or SEC. The SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov. We also make available free of charge on our investor relations website at ir.herbalife.com our Principles of Corporate Governance, our Corporate Code of Business Conduct and Ethics, and the Charters of our Audit Committee, Nominating and Corporate Governance Committee, Compensation Committee, and ESG Committee of our board of directors. Unless expressly noted, the information on our website, including our investor relations website, or any other website is not incorporated by reference in this Annual Report on Form 10-K and should not be considered part of this Annual Report on Form 10-K or any other filing we make with the SEC.
Item 1A. Risk Factors
Please carefully consider the following discussion of significant factors, events, and uncertainties that make an investment decision regarding our securities risky. The factors, events, uncertainties, and consequences discussed in these risk factors could, in circumstances we may not be able to accurately predict, recognize, or control, have a material adverse effect on our business, reputation, prospects, financial condition, operating results, cash flows, liquidity, and share price. These risk factors do not identify all risks that we face. We could also be affected by factors, events, or uncertainties that are not presently known to us or that we currently do not consider to present material risks.
Additionally, the COVID-19 pandemic has amplified many of the other risks discussed below to which we are subject. We are unable to predict the duration and extent to which the pandemic and its related impacts will adversely impact our business, financial condition, and operating results as well as our share price. In addition, given the unpredictable, unprecedented, and fluid nature of the pandemic, it may also materially and adversely affect our business, financial condition, and operating results in ways that are not currently anticipated by or known to us or that we currently do not consider to present material risks.
18
Risk Factor Summary
This risk factor summary contains a high-level summary of certain of the principal factors, events and uncertainties that make an investment in our securities risky, including risks related to our business and industry, risks related to regulatory and legal matters, risks related to our international operations, risks related to our indebtedness and risks related to our common shares. The following summary is not complete and should be read together with the more detailed discussion of these and the other factors, events, and uncertainties set forth below before making an investment decision regarding our securities. The principal factors, events, and uncertainties that make an investment in our securities risky include the following:
Risks Related to Our Business and Industry
Risks Related to Regulatory and Legal Matters
19
Risks Related to Our International Operations
Risks Related to Our Indebtedness
Risks Related to Our Common Shares
20
Risks Related to Our Business and Industry
Our failure to establish and maintain Member and sales leader relationships could negatively impact sales of our products and materially harm our business, financial condition, and operating results.
We distribute our products exclusively to and through our independent Members, and we depend on them directly for substantially all of our sales. To increase our revenue, we must increase the number and productivity of our Members. Accordingly, our success depends in significant part on our relationships with our sales leaders and our ability to recruit, retain, and motivate a large base of Members, including through an attractive compensation plan, the quality of our reputation, the maintenance of an attractive product portfolio, the breadth and quality of our Member services, and other incentives. The loss of a significant number of Members, changes to our network marketing program, our inability to respond to Member demand or generate sufficient interest in our business opportunities, products, or services, decreases in Member engagement, loss of Member or consumer confidence, or any legal or regulatory impact to our Members’ ability to conduct their business could negatively impact sales of our products and our ability to attract and retain Members, each of which could have a material adverse effect on our business, financial condition, and operating results. In our efforts to attract and retain Members, we compete with other direct-selling organizations. In addition, our Member organization has a high turnover rate, which is common in the direct-selling industry, in part because our Members, including our sales leaders, may easily enter and exit our network marketing program without facing a significant investment or loss of capital. For example, the upfront financial cost to become a Member is low, we do not have time or exclusivity requirements, we do not charge for any required training, and, in substantially all jurisdictions, we maintain a buyback program.
We believe the COVID-19 pandemic could have an adverse impact on the pipeline of new Members and our Member turnover rate, and may impact our future net sales. See the COVID-19 Pandemic and Sales by Geographic Region sections in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of this Annual Report on Form 10-K for further discussion of the impacts of the COVID-19 pandemic on our business and results of operations. For additional information regarding sales leader retention rates, see Part I, Item 1, Business, of this Annual Report on Form 10-K.
Because we cannot exert the same level of influence or control over our Members as we could if they were our employees, our Members could fail to comply with applicable law or our rules and procedures, which could result in claims against us that could materially harm our business, financial condition, and operating results.
Our Members are independent contractors and, accordingly, we are not in a position to provide the same direction, motivation, and oversight as we could if Members were our employees. As a result, there can be no assurance that our Members will participate in our marketing strategies or plans, accept our introduction of new products, or comply with applicable legal requirements or our rules and procedures.
We are subject to extensive federal, state, local, and foreign laws, rules, and regulations that regulate our business, products, direct sales channel, and network marketing program. See the Regulation section of Part I, Item 1, Business, of this Annual Report on Form 10-K for additional information. While we have implemented policies and procedures designed to govern Member conduct and to protect the goodwill associated with Herbalife Nutrition, it can be difficult to enforce these policies and procedures because of our large number of Members and their status as independent contractors and because our policies and procedures differ by jurisdiction as a result of varying local legal requirements. In addition, although we train our Members and attempt to monitor our Members’ marketing materials, we cannot ensure that our Members will comply with applicable legal requirements or our policies and procedures or that such marketing materials or other Member practices comply with applicable laws, rules, and regulations. It is possible that a court could hold us liable for the actions of our Members, which could materially harm our business, financial condition, and operating results.
Adverse publicity associated with our Company or the direct-selling industry could materially harm our business, financial condition, and operating results.
Our reputation and the quality of our brand are critical to our business, and the size and success of our Member organization, our operating results, and our share price may be significantly affected by the public’s perception of Herbalife Nutrition and other direct-selling companies. This perception is dependent upon opinions concerning a number of factors, including:
21
Adverse publicity concerning any of the foregoing whether or not accurate or resulting in investigation, enforcement, or other legal or regulatory actions or the imposition of fines, penalties, or other sanctions, could negatively impact our reputation, our ability to attract, motivate, and retain Members, and our ability to generate revenue.
In addition, our Members’ and consumers’ perception of Herbalife Nutrition and our direct-selling business as well as similar companies can be significantly influenced by media attention, publicized scientific research or findings, product liability claims, and other publicity, whether or not it is legitimate. For example, as a result of the prevalence and marked increase in the use of blogs, social media platforms, and other forms of Internet-based communications, the opportunity for dissemination of information, both accurate and inaccurate, is seemingly limitless and readily available, and often does not provide any opportunity for correction or other redress.
Adverse publicity that associates use of our products or any similar products with adverse effects, questions the quality or benefits of any such products, or claims that any such products are ineffective, inappropriately labeled, or have inaccurate instructions as to their use, could lead to lawsuits or other legal or regulatory challenges and could materially and adversely impact our reputation, the demand for our products, and our business, financial condition, and operating results.
Adverse publicity relating to us has had, and could again have, a negative effect on our ability to attract, motivate, and retain Members, on consumer perception of Herbalife Nutrition, and on our share price. For example, the resulting adverse publicity from the 1986 permanent injunction entered in California caused a rapid, substantial loss of Members in the United States and a corresponding reduction in sales beginning in 1985. See also the risk factor titled “Our share price may be adversely affected by third parties who raise allegations about our Company.” We expect that adverse publicity will, from time to time, continue to negatively impact our business in particular markets and may adversely affect our share price.
Our failure to compete successfully could materially harm our business, financial condition, and operating results.
The business of developing and marketing weight management and other nutrition and personal care products is highly competitive and sensitive to the introduction of new products and weight management plans, including various prescription drugs, which may rapidly capture a significant share of the market. Our competitors include numerous manufacturers; distributors; marketers; online, specialty, mass, and other retailers; and physicians that actively compete for the business of consumers both in the United States and abroad. Some of our competitors have longer operating histories, significantly greater resources, better-developed and more innovative sales and distribution channels and platforms, greater name recognition, and larger established customer bases than we do. Our present and future competitors may be able to offer products at lower prices or better withstand reductions in prices or other adverse economic or market conditions than we can; develop products that are comparable or superior to those we offer; adapt more quickly or effectively to new technologies, changing regulatory requirements, evolving industry trends and standards, and customer requirements than we can; and/or devote greater resources to the development, promotion, and sale of their products than we do. We are also subject to significant competition for the recruitment of Members from other direct-selling organizations, including those that market weight management products, dietary and nutritional supplements, personal care products, and other types of products, as well as those organizations in which former employees or Members are involved. In addition, because the industry in which we operate is not particularly capital intensive or otherwise subject to high barriers to entry, it is relatively easy for new competitors to emerge that will compete with us, including for our Members and their customers. Accordingly, competition may intensify and we may not be able to compete effectively in our markets. If we are not able to retain our Members and their customers or otherwise compete successfully, our business, financial condition, and operating results would be materially adversely affected.
22
Our contractual obligation to sell our products only through our Member network and to refrain from changing certain aspects of our Marketing Plan may limit our growth.
We are contractually prohibited from expanding our business by selling Herbalife Nutrition products through other distribution channels that may be available to our competitors, such as over the Internet, through wholesale sales, by establishing retail stores, or through mail order systems. To the extent legally permitted, an agreement we entered into with our Members provides assurances that we will not sell Herbalife Nutrition products worldwide through any distribution channel other than our network of Members. Since this is an open-ended commitment, there can be no assurance that we will be able to take advantage of innovative new distribution channels that are developed in the future or appropriately respond to consumer preferences as they continue to evolve.
In addition, this agreement with our Members provides that we will not make any material changes adverse to our Members to certain aspects of our Marketing Plan that may negatively impact our Members without their approval as described in further detail below. For example, our agreement with our Members provides that we may increase, but not decrease, the discount percentages available to our Members for the purchase of products or the applicable royalty override percentages and production and other bonus percentages available to our Members at various qualification levels within our Member hierarchy. We may not modify the eligibility or qualification criteria for these discounts, royalty overrides, and production and other bonuses unless we do so in a manner to make eligibility and/or qualification easier than under the applicable criteria in effect as of the date of the agreement. Our agreement with our Members further provides that we may not vary the criteria for qualification for each Member tier within our Member hierarchy, unless we do so in such a way so as to make qualification easier.
We reserved the right to make changes to our Marketing Plan without the consent of our Members in the event that changes are required by applicable law or are necessary in our reasonable business judgment to account for specific local market or currency conditions to achieve a reasonable profit on operations. In addition, we may initiate other changes that are adverse to our Members based on an assessment of what will be best for the Company and its Members. Under the agreement with our Members, these other adverse changes would then be submitted to our Member leadership for a vote. The vote would require the approval of at least 51% of our Members then at the level of President’s Team earning at the production bonus level of 6% who vote, provided that at least 50% of those Members entitled to vote do in fact vote. While we believe this agreement has strengthened our relationship with our existing Members, improved our ability to recruit new Members, and generally increased the long-term stability of our business, there can be no assurance that our agreement with our Members will not restrict our ability to adapt our Marketing Plan or our business to the evolving requirements of the markets in which we operate. As a result, our growth may be limited.
Our failure to appropriately respond to changing consumer trends, preferences, and demand for new products and product enhancements could materially harm our Member relationships, Members’ customer relationships, and product sales or otherwise materially harm our business, financial condition, and operating results.
Our business is subject to rapidly changing consumer trends and preferences and product introductions, especially with respect to our nutrition products. Our continued success depends in part on our ability to anticipate and respond to these changes and introductions, and we may not respond or develop new products or product enhancements in a cost-effective, timely, or commercially appropriate manner, or at all, particularly while the COVID-19 pandemic persists. Current consumer trends and preferences have evolved and will continue to evolve as a result of, among other things, changes in consumer tastes; health, wellness, and nutrition considerations; competitive product and pricing pressures; changes in consumer preferences for certain sales channels; shifts in demographics; and concerns regarding the environmental and sustainability impact of the product manufacturing process.
The success of our response to changing consumer trends and preferences and product introductions, including any new product offerings and enhancements, depends on a number of factors, including our ability to:
23
Our failure to accurately predict changes in consumer demand and technological advancements could negatively impact consumer opinion of our products or our business, which in turn could harm our Member relationships and the Members’ relationships with their customers, and cause a loss of sales. In addition, if we do not introduce new products or make enhancements to meet the changing needs of our Members and their customers in a cost-effective, timely, and commercially appropriate manner, or if our competitors release new products or product enhancements before we do, some of our product offerings could be rendered obsolete, which could cause our market share to decline and negatively impact our business, financial condition, and operating results.
If we fail to further penetrate existing markets, the growth in sales of our products, along with our operating results, could be negatively impacted.
The success of our business is to a large extent contingent on our ability to further penetrate existing markets, which is subject to numerous factors, many of which are out of our control. Our ability to increase market penetration may be limited by the finite number of persons in a given country inclined to pursue a direct-selling business opportunity or consumers aware of, or willing to purchase, Herbalife Nutrition products. Moreover, our growth in existing markets will depend upon increased brand awareness and improved training and other activities that enhance Member retention in our markets. While we have recently experienced significant growth in certain of our foreign markets, we cannot assure you that such growth levels will continue in the immediate or long-term future. Furthermore, our efforts to support growth in such foreign markets could be hampered to the extent that our infrastructure in such markets is deficient when compared to our infrastructure in our more developed markets, such as the United States. For example, there can be no assurances that we will be able to successfully manage expansion of manufacturing operations and a growing and dynamic sales force in China. If we are unable to effectively scale our supply chain and manufacturing infrastructure to support future growth in China or other foreign markets, our operations in such markets may be adversely impacted. Therefore, we cannot assure you that our general efforts to increase our market penetration and Member retention in existing markets will be successful. If we are unable to further penetrate existing markets, our business, financial condition, and operating results could materially suffer.
Since one of our products constitutes a significant portion of our net sales, significant decreases in consumer demand for this product or our failure to produce a suitable replacement could materially harm our business, financial condition, and operating results.
Our Formula 1 Healthy Meal, which is our best-selling product line, approximated 27% of our net sales for the year ended December 31, 2021. If consumer demand for this product decreases significantly or we cease offering this product without a suitable replacement, or if the replacement product fails to gain market acceptance, our business, financial condition, and operating results could be materially harmed.
Our business could be materially and adversely affected by natural disasters, other catastrophic events, acts of war or terrorism, cybersecurity incidents, pandemics, and/or other acts by third parties.
We depend on the ability of our business to run smoothly, including the ability of Members to engage in their day-to-day selling and business building activities. In coordination with our suppliers, third-party manufacturers, and distributors, our ability to make and move our products reasonably unimpeded around the world is critical to our success. Any material disruption to our collective operations or supply, manufacturing, or distribution capabilities caused by unforeseen or catastrophic events, such as (i) natural disasters or severe weather conditions, including droughts, fires, floods, hurricanes, volcanic eruptions, and earthquakes; (ii) power loss or shortages; (iii) telecommunications or information technology infrastructure failures; (iv) acts or threats of war, terrorism, or other armed hostilities; (v) outbreaks of contagious diseases, epidemics, and pandemics; (vi) cybersecurity incidents, including intentional or inadvertent exposure of content perceived to be sensitive data; (vii) employee misconduct or error; and/or (viii) other actions by third parties and other similar disruptions, could materially adversely affect our ability to conduct business and our Members’ selling activities. For example, our operations in Central America were impacted in November 2020 when Hurricanes Eta and Iota made landfall in the region. The storms disrupted our supply chain transportation network and our ability to import product. In addition, our distribution center in Honduras experienced flooding, which damaged or destroyed product. Furthermore, our headquarters and one of our distribution facilities and manufacturing facilities are located in Southern California, an area susceptible to fires and earthquakes. Although the events in Central America did not have a material negative impact on our operations, we cannot make assurances that any future catastrophic events will not adversely affect our ability to operate our business or our financial condition and operating results. In addition, catastrophic events may result in significant cancellations or cessations of Member orders; contribute to a general decrease in local, regional, or global economic activity; directly impact our marketing, manufacturing, financial, or logistics functions; impair our ability to meet Member demands; harm our reputation; and expose us to significant liability, losses, and legal proceedings, any of which could materially and adversely affect our business, financial condition, and operating results.
24
In March 2020, the World Health Organization declared the COVID-19 outbreak a global pandemic. The COVID-19 pandemic has significantly impacted health and economic conditions globally, disrupted global supply chains, and has adversely affected the Company’s business and that of its Members in certain of the Company’s markets and may continue to impact those markets or others in the future. Government, agency, and other regulatory recommendations, guidelines, mandates, and actions to address public health concerns, including restrictions on movement, public gatherings, and travel and restrictions on, or in certain cases outright prohibitions of, companies’ ability to conduct normal business operations, have and may continue to adversely affect our business. Although we have been classified as an essential business in most jurisdictions where we operate, there is no guarantee that this classification will not change. We may also be forced to or voluntarily elect to limit or cease operations in one or more markets for other reasons, such as the health and safety of our employees or because of disruptions in the operation of our supply chain and sources of supply. For example, it is possible that closures of our manufacturing facilities or those of our third-party contract manufacturers or suppliers could impact our distribution centers and our ability to manufacture and deliver products to our Members. In general, our inventory of products continues to be adequate to meet demand, but we do expect our supply chain and our ability to source and/or manufacture products will be negatively impacted if the negative effects of the pandemic continue for a prolonged period of time or worsen. The pandemic has had an adverse impact on our distribution channels and Members’ product access in some markets, which may, and in some cases will, continue until conditions improve. Our third-party contract manufacturers and suppliers and our Members’ businesses are also subject to many of the same risks and uncertainties related to the COVID-19 pandemic, as well as other pandemic-related risks and uncertainties that may not directly impact our operations, any of which could adversely affect demand for our products. For example, limitations on public gatherings have restricted our Members’ ability to hold meetings with their existing customers and to attract new customers. Significant limitations on cash transactions could also have an adverse effect on sales of products in certain markets.
The COVID-19 pandemic has also adversely affected the economies and financial markets of many countries, at times causing a significant deceleration of or interruption to economic activity, which during various stages of the pandemic has reduced production, decreased demand for a broad variety of goods and services, diminished trade levels, and led to widespread corporate downsizing. We have also seen periods of significant disruption of and extreme volatility in the global capital markets, which could increase the cost of, or entirely restrict access to, capital. Further, while some countries have progressed in distributing COVID-19 vaccines to the general population, many countries have limited to no access to vaccines at this time. To the extent the global supply of vaccine remains limited or vaccination rates do not significantly increase, government restrictions in the countries with limited to no access or low vaccination rates may persist or increase and economic activity may remain at depressed levels in those countries or regions.
Despite the relaxation of pandemic-related constraints in certain markets, considerable uncertainty still surrounds the COVID-19 pandemic, its potential effects, and the extent and effectiveness of government responses to the pandemic. If the pandemic is not contained, or if new variants emerge or effective vaccines are not made available and utilized quickly enough, the adverse impacts of the COVID-19 pandemic could worsen, impacting all segments of the global economy, and result in a significant recession or worse. However, the unprecedented and sweeping nature of the COVID-19 pandemic makes it extremely difficult to predict how our business and operations will be affected in the long run. Further, the resumption of normal business operations after the disruptions caused by the COVID-19 pandemic may be delayed or constrained by the pandemic’s lingering effects on our Members, consumers, and third-party contract manufacturers and suppliers. Accordingly, our ability to conduct our business in the manner previously done or planned for the future could be materially and adversely affected, and any of the foregoing risks, or other cascading effects of the COVID-19 pandemic that are not currently foreseeable, could materially and adversely affect our business, financial condition, and operating results. See the COVID-19 Pandemic and Sales by Geographic Region sections in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of this Annual Report on Form 10-K for further discussion of the impacts of the COVID-19 pandemic on our business and operating results.
25
We depend on the integrity and reliability of our information technology infrastructure, and any related interruptions or inadequacies may have a material adverse effect on our business, financial condition, and operating results.
Our business, including our ability to provide products and services to and manage our Members, depends on the performance and availability of our information technology infrastructure, including our core transactional systems. The most important aspect of our information technology infrastructure is the system through which we record and track Member sales, Volume Points, royalty overrides, bonuses, and other incentives. The failure of our information systems to operate effectively, or a breach in security of these systems, could adversely impact the promptness and accuracy of our product distribution and transaction processing. While we continue to invest in our information technology infrastructure, there can be no assurance that there will not be any significant interruptions to such systems, that the systems will be adequate to meet all of our business needs, or that the systems will keep pace with continuing changes in technology, legal and regulatory standards. Our information technology infrastructure, as well as that of our Members and the other third parties with which we interact, may be damaged, disrupted, or breached or otherwise fail for a number of reasons, including power outages, computer and telecommunication failures, internal design, manual or usage errors, workplace violence or wrongdoing, or catastrophic events such as natural disasters, severe weather conditions, or acts of war or terrorism. In addition, numerous and evolving cybersecurity threats, including advanced and persistent cyberattacks, such as unauthorized attempts to access, disable, improperly modify, exfiltrate, or degrade our information technology infrastructure, or the introduction of computer viruses, malware, “phishing” emails, and other destructive software, and social engineering schemes, could compromise the confidentiality, availability, and integrity of our information technology infrastructure as well as those of the third parties with which we interact. These attacks may come from external sources, such as governments or hackers, or may originate internally from an employee or a third party with which we interact. We have been the target of, and may be the target of in the future, malicious cyberattacks, although to date none of these attacks have had a meaningful adverse impact on our business, financial condition, or operating results. The potential risk of cyberattacks may increase as we introduce new technology systems and services. Additionally, in response to the COVID-19 pandemic, many of our employees have been encouraged to work remotely, which may increase our exposure to significant systems interruptions, cybersecurity attacks, and otherwise compromise the integrity and reliability of our information technology infrastructure and our internal controls.
Any disruptions to, or failures or inadequacies of, our information technology infrastructure that we may encounter in the future may result in substantial interruptions to our operations, expose us to significant liability, and may damage our reputation and our relationships with, or cause us to lose, our Members, especially if the disruptions, failures, or inadequacies impair our ability to track sales and pay royalty overrides, bonuses, and other incentives, any of which would harm our business, financial condition, and operating results. Any such disruptions, failures, or inadequacies could also create compliance risks under the Consent Order and result in penalties, fines, or sanctions under any applicable laws or regulations. Furthermore, it may be expensive or difficult to correct or replace any aspect of our information technology infrastructure in a timely manner, if at all, and we may have little or no control over whether any malfunctioning information technology services supplied to us by third parties are appropriately corrected, if at all. We have encountered, and may encounter in the future, errors in our software and our enterprise network, and inadequacies in the software and services supplied by certain of our vendors, although to date none of these errors or inadequacies have had a meaningful adverse impact on our business, financial condition or operating results.
In addition, developments in technology are continuing to evolve and affecting all aspects of our business, including how we effectively manage our operations, interact with our Members, and commercialize opportunities that accompany the evolving digital and data driven economy. Therefore, one of our top priorities is to modernize our technology and data infrastructure by, among other things, creating more relevant and more personalized experiences wherever our systems interact with Members; and developing ways to create more powerful digital tools and capabilities for Members to enable them to grow their businesses. These initiatives to modernize our technology and data infrastructure are expected to be implemented over the course of many years and to require significant investments. If these initiatives are not successful, our ability to attract and retain Members, increase sales, and reduce costs may be negatively affected. Further, these initiatives may be subject to cost overruns and delays and may cause disruptions in our operations. These cost overruns and delays and disruptions could adversely impact our business, financial condition, and operating results.
Disruption of supply, shortage, or increases in the cost of ingredients, packaging materials, and other raw materials as well as climate change could materially harm our business, financial condition, and operating results.
We and our third-party contract manufacturers depend on third-party suppliers to supply us with the various ingredients, packaging materials, and other raw materials that we use in the manufacturing and distribution of our products. Our business could be materially harmed if we experience operational difficulties with our third-party suppliers, such as increases in costs, reductions in the availability of materials or production capacity, errors in complying with specifications or applicable law, insufficient quality control, and failures to meet production or shipment deadlines. If we fail to develop or maintain our relationships with our third-party suppliers or if such suppliers cease doing business with us or go out of business, we could face difficulties in finding or transitioning to alternative suppliers that meet our standards.
26
Many of the ingredients, packaging materials, and other raw materials we use are subject to fluctuations in availability and price due to a number of factors beyond our control, including crop size, ingredient, water, and land scarcity, market demand for raw materials, commodity market speculation, energy costs, currency fluctuations, supplier and logistics service capacities, import and export requirements, tariffs, and other government policies, and drought, excessive rain, temperature extremes, and other severe weather events. If we experience supply shortages, price increases, or supplier or regulatory impediments with respect to any of the materials we use in our products or packaging, we may need to seek alternative supplies or suppliers and may experience difficulties in finding replacements that are comparable in quality and price. For a discussion of the impacts of the COVID-19 pandemic on our supply chain see “If any of our manufacturing facilities or third-party manufacturers fail to reliably supply products to us at required levels of quality or fail to comply with applicable laws, our financial condition and operating results could be materially and adversely impacted” below.
Further, the risks related to our ability to adequately source the materials required to meet our needs may be exacerbated by the effects of climate change and the legal, regulatory, or market measures that may be implemented to address climate change. There is growing concern that carbon dioxide and other greenhouse gases in the atmosphere may have an adverse impact on global temperatures, weather patterns, and the frequency and severity of extreme weather and natural disasters. If climate change has a negative effect on agricultural productivity, we may be subject to decreased availability or less favorable pricing for certain raw materials that are necessary for our products, such as soybeans, wheat, tea leaves, and nuts. Severe weather conditions and natural disasters can reduce crop size and crop quality, which in turn could reduce our supplies of raw materials, lower recoveries of usable raw materials, increase the prices of our raw materials, increase our cost of storing and transporting our raw materials, or disrupt production schedules. The impacts of climate change may also cause unpredictable water availability or exacerbate water scarcity. In addition, the increasing concern over climate change and related sustainability matters may also result in more federal, state, local, and foreign legal and regulatory requirements relating to climate change, which may significantly increase our costs of operation and delivery.
If any of our manufacturing facilities or third-party manufacturers fail to reliably supply products to us at required levels of quality or fail to comply with applicable laws, our financial condition and operating results could be materially and adversely impacted.
We operate manufacturing facilities in the United States and around the world and also rely on third-party contract manufacturers to supply products to us. Any significant interruption of production at any of our manufacturing facilities or third-party contract manufacturers, or other interruption in our supply chain, may materially harm our business, financial condition, and operating results. For example, natural disasters, including droughts, earthquakes, fires, hurricanes, or floods, technical issues, work stoppages, or other unforeseen or catastrophic events, that result in significant interruption of production at any of our facilities or third-party contract manufacturers or suppliers could impede our ability to conduct business. While we have business continuity programs for our manufacturing facilities which contemplate and plan for such events, if we were to experience such an event resulting in the temporary, partial, or complete shutdown of one of these manufacturing facilities, we could be required to transfer manufacturing to a surviving facility and/or third-party contract manufacturers if permissible. When permissible, converting or transferring manufacturing could be expensive and time-consuming, result in delays in our production or shipping, reduce our net sales, damage our relationship with Members, and damage our reputation, any of which could harm our business, financial condition, and operating results. Additionally, we cannot assure you that our third-party contract manufacturers will continue to reliably supply products to us at the levels of quality, or the quantities, we require, and in compliance with applicable laws. Our product supply contracts generally have three-year terms. Except for force majeure events, such as natural disasters and other acts of God, and non-performance by Herbalife Nutrition, our contract manufacturers generally cannot unilaterally terminate these contracts. These contracts can generally be extended by us at the end of the relevant time-period and we have exercised this right in the past. Globally, we have over 50 contract manufacturers, with Fine Foods (Italy) being a major supplier for meal replacements, protein powders and nutritional supplements. Our contract manufacturers are also located in the United States, India, Brazil, South Korea, Taiwan, Germany, and the Netherlands. If any of our contract manufacturers were to become unable or unwilling to continue to provide us with products in required volumes, at suitable quality levels, or in a cost-effective manner, we would be required to identify and obtain replacement manufacturing sources. There is no assurance that we would be able to obtain acceptable alternative manufacturing sources on a cost-effective or timely basis, or at all. An extended interruption in the supply of our products, including any interruptions that may arise as a result of the COVID-19 pandemic, would result in the loss of sales, which could have a material adverse effect on our business, financial condition, or operating results.
27
In addition, our business depends in large part on our ability to maintain consumer confidence in the safety and quality of our products. We have rigorous product safety and quality standards, which we expect our manufacturing facilities as well as our contract manufacturers to meet. However, despite our commitment to product safety and quality, we or our contract manufacturers may not always meet these standards, particularly as we expand our manufacturing operations and product offerings. Further, our manufacturing operations are subject to numerous regulations, including food and drug, environmental, and labor regulations, which continue to expand and evolve and require substantial expenditures. If we or our contract manufacturers fail to comply with our product safety and quality standards or applicable law, or if our products are or become contaminated, damaged, adulterated, mislabeled, or misbranded, whether caused by us or someone in our supply chain or events outside of our or their control, we may be required to undertake costly remediation efforts, which may include product recalls, the destruction of inventory, temporarily facility closings, and supply chain interruption, and may become subject to negative publicity, regulatory fines, and product liability claims, which could materially harm our reputation, business, financial condition, and operating results. Further, any perceived degradation of product quality as a result of reliance on contract manufacturers may have an adverse effect on sales or result in increased product returns and buybacks.
As a result of the COVID-19 pandemic, our suppliers have experienced some delays in receiving and delivering certain ingredients and packaging components. While these delays have not materially impacted our supply levels, there is no guarantee that there will be sufficient global supply for us or our contract manufacturers to manufacture our products at sufficient levels to meet demand or at the pre-pandemic levels. Given the uncertainties surrounding COVID-19, including the duration and extent of the pandemic, and actions taken or to be taken by governmental authorities and the resulting impacts from those responses to us and our third-party suppliers and contract manufacturers, we cannot guarantee that we will have a sufficient and reliable supply of ingredients and other raw materials from our third-party suppliers or products from our contract manufacturers. In addition, if price changes within our supply chain lead to unexpected or significant increases in the costs of any ingredients, raw materials, or other products we source, we may be unwilling or unable to increase our product prices or unable to effectively hedge against price increases or find alternative suppliers at lower cost. We are actively monitoring the pandemic and economic conditions and their potential impact on our supply chain and operations. Additionally, while we are not presently aware of any current liquidity issues with our suppliers or contract manufacturers, we cannot assure you that they will not experience financial hardship as a result of the COVID-19 pandemic, economic conditions, or otherwise, which could impact their ability to meet our needs.
If we lose the services of members of our senior management team, our business, financial condition, and operating results could be materially harmed.
We depend on the continued services of our senior management team as it works closely with the senior Member leadership to create an environment of inspiration, motivation, and entrepreneurial business success. Although we have entered into employment agreements with certain members of our senior management team, and do not believe that any of them are planning to leave or retire in the near term, we cannot assure you that all members of our senior management team will remain with us. The loss or departure of any member of our senior management team, or our failure to adequately develop succession plans, could adversely impact our Member relations and operating results. Also, the loss of key personnel, including our regional and country managers, could negatively impact our ability to implement our business strategy. Further, to the extent we are required to replace members of senior management or key personnel, any significant leadership change or transition involves inherent risk and any failure to ensure a smooth transition could hinder our strategic planning and execution, adversely impact our Member relations, or cause our business to suffer. While we strive to mitigate any negative impact associated with changes to our senior management team or key personnel, there may be uncertainty among investors, employees, Members, and others concerning our future direction and performance. Any disruption in our operations or uncertainty could have a material adverse effect on our business, financial condition, and operating results.
Our continued success also depends on our ability to hire, develop, and retain qualified and diverse personnel with the requisite skills to meet our business needs. Identifying, recruiting, integrating, training, and retaining qualified personnel may require significant time, expense, and attention, and we may compete for such personnel with companies that have significant financial resources or recognized brands or that are able to offer more attractive or lucrative employment opportunities. If we are not able to hire, develop, and retain personnel, our business, financial, condition, and operating results may be adversely affected.
Our share price may be adversely affected by third parties who raise allegations about our Company.
Short sellers and others who raise allegations regarding our business activities, some of whom are positioned to profit if our share price declines, can negatively affect our share price. For example, in late 2012, a hedge fund manager publicly raised allegations regarding the legality of our network marketing program, our product safety, our accounting practices, and other matters, and announced that his fund had taken a significant short position regarding our common shares, leading to intense public scrutiny and significant share price volatility. Following this public announcement, our share price dropped significantly. Additionally, from time to time we are subject to various legal proceedings, including governmental and regulatory inquiries and inquiries from legislators, that may adversely affect our share price. Significant volatility of our share price may cause the value of a shareholder’s investment to decline rapidly.
28
ESG matters, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition, and operating results and may damage our reputation.
Companies across all industries are facing increasing scrutiny relating to their environmental, social, and governance practices. In particular, we expect many consumers will continue to put an increased priority on purchasing products that are sustainably and responsibly grown and made. Changing consumer preferences may result in increased demands regarding the source of origin of our ingredients, the recyclability of, and amount of recycled content contained in, our packaging containers, and other components of our products and supply chain and their respective environmental impact, including on sustainability. These demands could require additional transparency, due diligence, and reporting and could cause us to incur additional costs or to make changes to our operations to comply with such demands. We may also determine that certain changes are required in anticipation of further evolution of consumer preferences and demands. Increased focus and activism related to ESG may also result in investors reconsidering their investment decisions as a result of their assessment of a company’s ESG practices. Further, concern over climate change and other environmental sustainability matters, has and may in the future result in new or increased legal and regulatory requirements to reduce or mitigate impacts to the environment, including greenhouse gas emissions regulations, alternative energy policies, and sustainability initiatives, such as single use plastics. Increased regulatory requirements may be more aggressive than any sustainability measures we may be currently undertaking or may implement in the future and may cause disruptions in the supply and manufacture of our products or an increase in operating and compliance costs. If we fail to achieve any goals, targets, or objectives we may set with respect to ESG matters, if we do not meet or comply with new regulations or evolving consumer, investor, industry, or stakeholder expectations and standards, including those related to reporting, or if we are perceived to have not responded appropriately to the growing concern for ESG matters, we may face legal or regulatory actions, the imposition of fines, penalties, or other sanctions, adverse publicity, and decreased demand from consumers who may stop purchasing our products, or the price of our common shares could decline, any of which could materially harm our reputation or have a material adverse effect on our business, financial condition, or operating results.
Risks Related to Regulatory and Legal Matters
Our products are affected by extensive regulations and our failure or our Members’ failure to comply with any regulations could lead to significant penalties or claims, which could materially harm our financial condition and operating results.
The majority of our products are classified as foods, dietary supplements, and cosmetics. In both domestic and foreign markets, the formulation, manufacturing, packaging, labeling, distribution, advertising, importation, exportation, licensing, sale, and storage of our products are subject to extensive government regulation. This regulation takes the form of laws, governmental regulations, administrative determinations, court decisions, and other similar constraints and exists at the federal, state, and local levels in the United States and at all levels of government in foreign jurisdictions. There can be no assurance that we or our Members are, or will remain, in compliance with all of these regulations. Our failure or our Members’ failure to comply with applicable regulations could disrupt the manufacturing of our products, our marketing activity, our Members’ sale of our products, or lead to increased costs, legal or regulatory proceedings, the imposition of significant penalties, or harm our reputation, any of which could adversely impact our business, financial condition, and operating results. In addition, regulatory authorities periodically review legislative and regulatory policies and initiatives, and may promulgate new or revised, or adopt changes in the interpretation and enforcement of existing, regulations at any time. The adoption of new regulations or changes in the interpretations of existing regulations, such as those relating to genetically modified foods, may result in significant compliance costs or discontinuation of impacted product sales and may negatively impact the marketing of our products or require us to change or cease aspects of our business, any of which could result in significant loss of sales and harm our business, financial condition, and operating results.
For example, we are subject to the rules of the FDA, including for CGMPs. Any failure by us or our contract manufacturers to comply with the CGMPs could negatively impact our reputation and ability to sell our products even after the situation has been rectified and, in the case of our contract manufacturers, even though we are not directly liable under the CGMPs for their compliance. In complying with the dietary supplement CGMPs, we have experienced increases in production costs due to increases in required testing of raw ingredients, work in process, and finished products. In addition, regulators and other governmental authorities limit the types of claims that we and our Members can make about our products, including nutrition content claims, health claims, and therapeutic claims and otherwise regulate the marketing of our products. For example, the FTC’s Guides explain how the FTC interprets prohibitions on unfair or deceptive acts or practices. Consequently, the FTC could bring an enforcement action based on practices that are inconsistent with the Guides. The Consent Order entered into with the FTC in 2016 also includes restrictions regarding the marketing of our products. It is possible that our use, and that of our Members, of marketing materials, including testimonials about our products, may be significantly impacted by laws, rules, and regulations governing the marketing of our products and therefore might negatively impact our sales.
29
From time to time, we receive inquiries from regulators and third parties requesting information concerning our products. We fully cooperate with these inquiries including, when requested, by the submission of detailed technical documents addressing product composition, manufacturing, process control, quality assurance, and contaminant testing. We are confident in the safety of our products when used as directed. However, there can be no assurance that regulators, including in countries where we plan to commence or expand operations, will not take actions that may adversely affect our business and our sales, including preventing or delaying entry into markets or the introduction of new products or requiring the reformulation or the temporary or permanent withdrawal of certain of our existing products from their markets. Any such regulatory action, regardless of whether it results in a final determination adverse to us, could create negative publicity, with detrimental effects on the motivation and recruitment of Members and, consequently, on sales. For example, the Chinese government carried out a 100-day review, or the Review, in 2019 to investigate the unlawful promotion and sales of health products, which resulted in negative media attention to the health products industry and materially and adversely impacted our business in China in 2019 as Members significantly reduced activities and sales meetings during and following the Review. Additionally, in response to the COVID-19 pandemic, the FTC has increased its scrutiny of claims being made by companies and issued hundreds of warning letters to, and initiated enforcement actions against, companies making health claims related to the ability of their products to treat, cure, or prevent COVID-19 or business opportunity claims related to COVID-19.
Our network marketing program is subject to extensive regulation and scrutiny and any failure to comply, or alteration to our compensation practices in order to comply, with these regulations could materially harm our business, financial condition, and operating results.
Our network marketing program, like the compensation practices of other direct-selling organizations, is subject to a number of federal, state, and foreign regulations administered by the FTC and other federal, state, and foreign agencies. Regulations applicable to network marketing organizations generally are directed at preventing fraudulent or deceptive schemes, sometimes referred to as “pyramid” or “chain sales” schemes, by ensuring that product sales ultimately are made to consumers and that advancement within an organization is based on genuine demands and sales of the organization’s products rather than investments in the organization or other non-retail sales-related criteria. For example, in certain foreign countries, compensation to distributors in the direct-selling industry may be limited to a certain percentage of sales.
The regulatory requirements concerning network marketing programs do not include “bright line” rules and are inherently fact-based and, thus, we are subject to the risk that these regulations or the enforcement or interpretation of these regulations by regulators or courts can change. Regulatory authorities also periodically review legislative and regulatory policies and initiatives and may promulgate new or revised regulations. For example, in 2018, the FTC released its nonbinding Business Guidance Concerning Multi-Level Marketing, and in December 2021, India’s Ministry of Consumer Affairs, Food and Public Distribution, Government promulgated the Consumer Protection (Direct Selling) Rules, 2021 under the Consumer Protection Act, 2019. The adoption of new regulations, or changes in the interpretations or enforcement of existing regulations, may result in significant compliance costs or require us to change or cease aspects of our network marketing program. In addition, the ambiguity surrounding these regulations can also affect the public perception of the Company and our business model. For example, in the past, allegations regarding the legality of our network marketing program have been raised, which led to intense public scrutiny and significant share price volatility.
From time to time, we are a party to various regulatory proceedings related to compliance with regulations applicable to our network marketing program. We are also subject to the risk of private party challenges to the legality of our network marketing program, and similar programs of other companies have been successfully challenged in the past. Legal proceedings may cause us to incur significant expenses, including legal fees and costs for remediation efforts, and result in fines, penalties, sanctions, adverse judgments, or negative publicity, any of which could materially harm our business, financial condition, and operating results and impact our share price. For example, in one or more markets, our network marketing program could be found not to be in compliance, or a court could issue an adverse determination with respect to our network marketing program specifically or with respect to network marketing practices generally in proceedings not involving us, any of which may require us to alter our compensation practices under our network marketing program and adversely impact our ability to recruit and maintain Members or to obtain or maintain a license, permit, or similar certification. As previously disclosed, the Consent Order entered into with the FTC in 2016 and the 1986 permanent injunction entered in California required us to make changes to our network marketing program and our business operations. There can be no assurances that federal, state, or foreign regulators or courts will not require similar actions in the future. While we believe we are in compliance with regulations applicable to our network marketing program, including those enforced by the Consent Order and the permanent injunction in California, there is no assurance that any federal, state, or foreign courts or regulators or the independent compliance auditor under the Consent Order would agree. The failure of our network marketing program to comply with current or newly adopted laws, rules, and regulations, the Consent Order, or the California injunction, or any allegations or charges to that effect brought by federal, state, or foreign regulators, could have a material adverse impact our business in a particular market or in general and may adversely affect our share price.
30
We are subject to the Consent Order with the FTC, the effects of which, or any failure to comply therewith, could materially harm our business, financial condition, and operating results.
As previously disclosed, in July 2016, we entered into the Consent Order with the FTC. As part of the Consent Order, we agreed to make a payment of $200 million and to implement, and continue to enhance, certain procedures in the United States. We also agreed, among other things, to (i) be subject to certain audits by an independent compliance auditor, or the ICA, for a period of seven years; (ii) requirements regarding compliance certification and record creation and maintenance; (iii) a prohibition on misrepresentations and misleading claims made by us or our Members regarding our network marketing program, including the income potential of participants in our network marketing program and misleading depictions of lavish lifestyles; and (iv) restrictions on distributors’ ability to open Nutrition Clubs in the United States. The FTC and ICA have the right to inspect Company records and request additional compliance reports for purposes of conducting audits pursuant to the Consent Order. The terms of the Consent Order are described in greater detail in our Current Report on Form 8-K filed on July 15, 2016.
The Consent Order, including our compliance therewith and the procedures implemented as a result thereof, has impacted, and may continue to impact, our business operations, including our net sales and profitability. For example, the Consent Order includes a number of restrictions and requirements, including regarding the verification and receipting of sales, and therefore creates compliance risks and costs. As a result, we have implemented a number of enhanced procedures regarding, among other things, tracking retail sales and internal consumption by distributors. We have also instituted controls and procedures and developed technology solutions that we believe address our Consent Order requirements, including tools and software used by distributors to document their sales and more efficiently track and manage their customer base. However, there can be no assurances that some or all of these controls and procedures and technology solutions will continue to operate as expected. These controls and procedures and technology solutions have been, and may continue to be, costly. These extensive costs or any amounts in excess of our cost estimates could have a material adverse effect on our financial condition and operating results. In addition, any failure of these systems to operate as designed could cause us to fail to maintain the records required under, or otherwise violate terms of, the Consent Order.
Further, management and our board of directors have been, and may continue to be, required to focus a substantial amount of time on Consent Order compliance activities, which could divert their attention from running and growing our business. At any time, we may also be required to suspend or defer many or all of our current or anticipated business development, capital deployment, and other projects unrelated to compliance with the Consent Order to allow resources to be focused on our compliance efforts, which could cause us to fall short of any guidance or analyst or investor expectations. In addition, while we believe the Consent Order has set new standards within the direct-selling industry, our competitors are not required to comply with the Consent Order and may not be subject to similar actions, which could limit our ability to effectively compete for Members, consumers, and ultimately sales.
A number of our Members disagreed with our decision to enter into the Consent Order, whether because they disagreed with certain terms thereof, they believed it would negatively impact their personal business, or they would not have settled the investigation on any terms. Compliance with the Consent Order, however, requires the cooperation of our Members and, while we have updated our training programs and policies to address the Consent Order and expect our Members to cooperate, we do not have the same level of influence or control over our Members as we would if they were our employees. Failure by our Members to comply with the relevant aspects of the Consent Order could be a violation of the Consent Order and impact our ability to comply. In addition, the Consent Order provides that if the total eligible U.S. sales on which compensation may be paid falls below 80% of the Company’s total U.S. sales for a given year, compensation payable to distributors on eligible U.S. sales will be capped at 41.75% of the Net Rewardable Sales amount as defined in the Consent Order. Because our business is dependent on our Members, our business operations and net sales could be adversely affected if U.S. distributor compensation is restricted or if any meaningful number of Members are dissatisfied, choose to reduce activity levels, or leave our business altogether. Member dissatisfaction may also negatively impact the willingness of new Members to join Herbalife Nutrition as a distributor.
The Consent Order also creates additional third-party risks. The Consent Order does not prevent other third parties from bringing actions against us, whether in the form of other federal, state, or foreign regulatory proceedings or private litigation, any of which could lead to monetary settlements, fines, penalties, or injunctions. Although we neither admitted nor denied the allegations in the FTC’s complaint (except as to the Court having jurisdiction over the matter), third parties may use specific statements or other matters addressed in the Consent Order as the basis for their action. The Consent Order has caused, and any subsequent legal or regulatory claim may also lead to, negative publicity, whether because some view it as a condemnation of the Company or our direct-selling business model or because other third parties use it as justification to make unfounded and baseless assertions against us, our business model, or our Members. An increase in the number, severity or scope of third-party claims, actions or public assertions may result in substantial costs and harm to our reputation. The Consent Order may also impact third parties’ willingness to work with us as a company.
31
We believe we have complied with the Consent Order and we will continue to do so. However, the FTC or ICA may not agree now or in the future. In the event we are found to be in violation of the Consent Order, the FTC could take corrective actions such as initiating enforcement actions, seeking an injunction or other restrictive orders and imposing civil monetary penalties against us and our officers and directors. Further, the impact of the Consent Order on our business, including the effectiveness of the controls, procedures, and technology solutions implemented to comply therewith, and on our Member base could be significant. If our business or Member base is adversely impacted, it is uncertain as to whether, or how quickly, we would be able to restructure or rebuild, irrespective of market conditions. Our financial condition and operating results could be materially harmed if we fail to comply with the Consent Order, if costs related to compliance exceed our estimates, if it has a negative impact on net sales, or if it leads to further legal, regulatory, or compliance claims, proceedings, or investigations or litigation.
Our actual or perceived failure to comply with privacy and data protection laws, rules, and regulations could materially harm our business, financial condition, and operating results.
Our business requires the collection, transmission, and retention of large volumes of confidential and proprietary information, including personally identifiable information of our Members, customers, leads, vendors, and employees in various information technology systems that we maintain and in those maintained by third parties with which we interact. Anyone who is able to circumvent our security measures or those of our third-party service providers could misappropriate such confidential or proprietary information, including that of third parties such as our Members, cause interruption in our operations, damage our information technology infrastructure, damage our reputation, or otherwise damage our business. We may need to expend significant resources to protect against security breaches or to address problems caused by such breaches, and the potential risk of security breaches may increase as we introduce new technology systems and services. Any actual security breaches could result in legal and financial exposure, including litigation and other potential liability, and a loss of confidence in our security measures, which could have a material adverse effect on our business, financial condition, and operating results and our reputation as a brand, business partner, and employer. In addition, employee error or malfeasance or other errors in the storage, use, or transmission of any such information could result in disclosure to third parties. If this should occur, we could incur significant expenses addressing such problems. Since we collect and store Member, customer, and vendor information, including credit card banking information, these risks are heightened. In addition, our role as a credit card merchant may also put us at a greater risk of being targeted by hackers and requires us to comply with certain regulatory requirements. See also the risk factor titled “We depend on the integrity and reliability of our information technology infrastructure, and any related interruptions or inadequacies may have a material adverse effect on our business, financial condition, and operating results.”
In addition, the use and handling of certain types of information, including personally identifiable and financial information, is regulated by evolving and increasingly demanding laws, rules, and regulations, such as the European Union General Data Protection Regulation, which became effective in May 2018, the Brazil Law on General Data Protection, which became effective in September 2020, the California Consumer Privacy Act, or the CCPA, which became effective in January 2020, the European Union Payment Services Directive 2, which became effective in January 2021 and requires stronger customer authentication for online transactions in that region, and the China Personal Information Protection Law, which became effective in November 2021. These laws impose continuing, and at times new, responsibilities on our operations, including, among other things, the collection, deletion, disclosure, and maintenance of personally identifiable and financial information of our Members and their customers and could present technological challenges and negatively impact our sales. These privacy and data security laws, rules, and regulations are increasing in their complexity, enactment, and amendments. As such, compliance with these laws, rules, and regulations and potential and actual conflicts amongst them in the various jurisdictions in which we operate have resulted in greater compliance burden and risk and increased costs for us. If we fail to comply with these privacy and data security laws, rules, and regulations, we could be subject to significant litigation, monetary damages, and regulatory enforcement actions or fines in one or more jurisdictions, which could have a material adverse effect on our operating results.
32
We are subject to material product liability risks, which could increase our costs and materially harm our business, financial condition, and operating results.
Our ingestible products include vitamins, minerals, botanicals, and other ingredients and are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the United States. Our products could contain contaminated substances, and some of our products contain ingredients that do not have long histories of human consumption or use. Although we rely upon published and unpublished safety information, including clinical studies on ingredients used in our products, and conduct limited clinical studies on some key products, unknown adverse reactions resulting from human consumption or use of these ingredients could occur. We have been, and may again be, subjected to various product liability claims, including claims that the products contain contaminants, include inadequate instructions as to their uses, and include inadequate warnings concerning side effects and interactions with other substances. It is possible that widespread product liability claims could increase our costs and materially adversely affect our business, financial condition, and operating results. Even claims without merit could subject us to adverse publicity and require us to incur significant legal fees. Moreover, product liability claims may increase our costs through higher insurance premiums and deductibles, and may make it more difficult to secure adequate insurance coverage in the future. In addition, our product liability insurance may not cover all product liability claims, which may require us to pay substantial monetary damages. Finally, even if our insurance covers a claim, given the level of self-insured retentions that we have accepted under our current product liability insurance policies, which is $12.5 million, in certain cases we may be subject to the full amount of liability associated with any claims, which could be substantial.
If we fail to protect our intellectual property, our ability to compete could be negatively affected, which could materially harm our financial condition and operating results.
Our success and the market for our products depend to a significant extent upon the goodwill associated with our trademark and tradenames and our ability to protect our proprietary rights in our innovative products and product enhancements. We own, or have licenses to use, the material trademark and trade name rights used in connection with the packaging, marketing, and distribution of our products in the markets where those products are sold. Therefore, trademark and trade name protection is important to our business. Although most of our trademarks are registered in the United States and in certain foreign countries in which we operate, we may not be successful in asserting trademark or trade name protection or obtaining new trademark registrations. We permit the limited use of our trademarks by our Members to assist them in marketing our products. It is possible that doing so may increase the risk of unauthorized use or misuse of our trademarks in markets where their registration status differs from that asserted by our Members, or they may be used in association with claims or products in a manner not permitted under applicable laws, rules, and regulations. Were these to occur, it is possible that this could diminish the value of these marks or otherwise impair our further use of these marks.
We attempt to protect our innovative products and product enhancements under a combination of copyright, trademark, and trade secret laws, confidentiality procedures, and contractual provisions. However, our products are generally not patented domestically or abroad, and the legal protections afforded by common law and contractual proprietary rights in our products provide only limited protection.
Monitoring infringement or misappropriation of intellectual property can be difficult and expensive, and we may not be able to detect every infringement or misappropriation of our proprietary rights or to prevent third parties from infringing upon or misappropriating our proprietary rights or from independently developing non-infringing products that are competitive with, equivalent to, or superior to our products. In addition, our actions to monitor our intellectual property rights may not prevent counterfeit reproductions of our products or products bearing confusingly similar trademarks from entering the markets in which we operate. Even if we do detect infringement or misappropriation of our proprietary rights, litigation to enforce these rights could cause us to divert financial and other resources away from our business operations and may result in the impairment or loss of all or portions of our proprietary rights. Further, the laws of some foreign countries do not protect our intellectual property to the same extent as do the laws of the United States. For example, there is limited protection of intellectual property available under Chinese law. Accordingly, we face an increased risk in China that unauthorized parties may attempt to copy or otherwise obtain or use our trademarks, copyrights, product formulations, or other intellectual property or sell counterfeit reproductions, including on popular e-commerce platforms. Further, because Chinese commercial law is relatively undeveloped, we may have limited legal recourse in the event we encounter significant difficulties with intellectual property theft or infringement. As a result, we cannot assure you that we will be able to adequately protect our intellectual property in any jurisdictions. The loss or infringement of our trademarks or tradenames or other proprietary rights could impair the goodwill associated with our brands and, with respect to the sale of counterfeit reproductions, could pose safety risks due to the lower quality of such products, divert sales from us, reduce the demand for our products, or damage our brand integrity. Any of the foregoing could materially harm our reputation, business, financial condition, and operating results.
33
If we infringe the intellectual property rights of others, our business, financial condition, and operating results could be materially harmed.
Third parties may claim that products or marks that we have independently developed or licensed, or which bear certain of our trademarks, infringe upon their intellectual property rights and there can be no assurance that one or more of our products or marks will not be found to infringe upon third-party intellectual property rights in the future and we may need to settle disputes on terms that are unfavorable to us, or we may be subject to an unfavorable judgment. Defending these and other intellectual property infringement claims can be time-consuming and costly and require the attention of management. The terms of any settlement or judgment may require us to pay substantial amounts to the other party or cease, or seek a license to continue, using products or marks found to be in violation of third-party intellectual property rights. A license may not be available on reasonable terms, or at all, and we may be required to develop alternative non-infringing products or marks or discontinue use of such products or marks. Any development efforts could require significant effort and expense. Any of the foregoing could have a material adverse effect on our business, financial condition, and operating results.
We may be held responsible for additional compensation, certain taxes, or assessments relating to the activities of our Members, which could materially harm our financial condition and operating results.
Our Members are subject to certain taxation, and in some instances, we are required to collect taxes from our Members, such as value-added tax, or VAT, and social contributions, and to maintain appropriate records. In addition, if local laws, rules, and regulations or their interpretation change to require us to treat our Members as employees, or if our Members are deemed by regulatory authorities to be our employees rather than independent contractors, in any such jurisdictions we may be held responsible for additional compensation, social security, or similar contributions, withholding, and related taxes, and workers’ compensation insurance, plus any related assessments and penalties, which could materially harm our financial condition and operating results. Our Members could face similar risks with respect to other Members in their sales organizations who may claim they are employees of that Member rather than independent contractors or independent business owners, which could impact their sales operations or lead them to cease their participation in our network marketing program. For example, California passed legislation, taking effect January 1, 2020, which seeks to expand the classification of employees. Other states may propose similar legislation or interpret existing laws, rules, and regulations to expand the classification of employees. Although the California legislation provides an exemption for direct sellers, there can be no assurance that other jurisdictions will provide such an exemption or that judicial or regulatory authorities will not assert interpretations that would mandate that we change our classification. See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a more specific discussion of contingencies related to the activities of our Members.
Risks Related to Our International Operations
A substantial portion of our business is conducted in foreign jurisdictions, exposing us to the risks associated with international operations.
Approximately 76% of our net sales for the year ended December 31, 2021 were generated outside the United States, exposing our business to risks associated with international operations. We have invested significant resources in our international operations and expect to continue to do so in the future. However, there are certain risks inherent in doing business in international markets, particularly in the direct-selling industry, which is regulated in many jurisdictions.
For example, a foreign government may impose trade restrictions or increased tariffs, require compliance with trade and economic sanctions laws, rules, or regulations, such as those administered by U.S. Customs and Border Protection and the U.S. Treasury Department’s Office of Foreign Assets Control, implement new or change existing trade policies, or otherwise limit or restrict our ability to import products in a cost-effective manner, or at all, any of which could negatively impact our operations. Additionally, we may be negatively impacted by conflicts with or disruptions caused or faced by our third-party importers, as well as conflicts between such importers and local governments or regulators.
Our operations in some jurisdictions also may be adversely affected by political, economic, legal, regulatory, and social conditions, or instability, as well as by economic and political tensions between governments. For example, tariffs enacted by the United States and other foreign governments, such as China or Mexico, that apply to our products or our ingredients may have an adverse impact on the costs and future sales of our products, particularly if we deem it necessary to increase product prices. In addition, our compliance with our code of conduct and anti-bribery laws, rules, and regulations may conflict with local customs and practices in certain of the jurisdictions in which we operate. See the risk factor titled “We are subject to the anti-bribery, laws, rules, and regulations of the United States and the other foreign jurisdictions in which we operate.”
34
Our ability to staff and manage our international operations could also be affected by laws and regulations related to immigration. For example, current and future tightening of U.S. immigration controls may adversely affect the residence status of non-U.S. employees in our U.S. locations or our ability to hire new non-U.S. employees in such locations and may adversely affect the ability of non-U.S. Members from entering the United States.
We are also exposed to risks associated with foreign currency fluctuations, foreign exchange controls, limitations on the repatriation of funds, and changes in currency policies or practices. For instance, purchases from suppliers are generally made in U.S. dollars while sales to Members are generally made in local currencies. Accordingly, any strengthening of the U.S. dollar versus a foreign currency could have a negative impact on us. Although we engage in transactions to protect against risks associated with foreign currency fluctuations, we cannot be certain any hedging activity will effectively reduce our exchange rate exposure. In addition, due to the possibility of government restrictions on transfers of cash out of a country and control of exchange rates, we may not be able to immediately repatriate cash at the official exchange rate. If this should occur, or if the official exchange rate devalues, it may have a material adverse effect on our business, assets, financial condition, liquidity, operating results, or cash flows. For example, currency restrictions enacted by the Venezuelan government continue to impact the ability of our subsidiary in Venezuela, or Herbalife Venezuela, to obtain U.S. dollars in exchange for Venezuelan Bolivars at the official foreign exchange rate and limit Herbalife Venezuela’s ability to import U.S. dollar denominated raw materials and finished goods, both of which have significantly negatively impacted our Venezuelan operations. We may be required to fundamentally change or cease operations in Venezuela or any other jurisdiction that may be similarly affected in the future. If these restrictions intensify or do not improve and impact our ability to control our Venezuelan operations, we may be required to deconsolidate Herbalife Venezuela for U.S. GAAP purposes and would be subject to the risk of further impairments.
Our overall success depends, in part, on our ability to anticipate and effectively manage these risks, and to coordinate the various legal and regulatory requirements of multiple jurisdictions that are constantly evolving and subject to change, and there can be no assurance that we will be able to do so without incurring unexpected or increased costs or at all. In certain regions, the degree of these risks may be higher due to more volatile economic, political, or social conditions; less developed and predictable legal and regulatory regimes; and increased potential for various types of adverse governmental action. As we continue to focus on expanding our existing international operations, these and other risks associated with international operations will likely increase, which could materially harm our business, financial condition, and operating results.
We are subject to the anti-bribery laws, rules, and regulations of the United States and the other foreign jurisdictions in which we operate.
We are subject to a variety of anti-bribery laws, rules, and regulations, including the U.S. Foreign Corrupt Practices Act, or the FCPA, the U.K. Bribery Act of 2010, and similar anti-bribery laws, rules, and regulations in the other foreign jurisdictions in which we operate. These regimes generally prohibit companies and their intermediaries from making improper payments for the purpose of obtaining or retaining business as well as require companies to maintain accurate books and records. There has been a substantial increase in anti-bribery law enforcement activity with more frequent and aggressive investigations and criminal and civil enforcement proceedings brought against companies and individuals by regulators, including the Department of Justice, or DOJ, and the SEC. Our policies mandate compliance with these anti-bribery laws, rules, and regulations, including the requirements to maintain accurate information and internal controls. We operate in many parts of the world that have experienced governmental corruption to some degree and in certain circumstances, strict compliance with anti-bribery laws, rules, and regulations may conflict with local customs and practices. Notwithstanding our compliance programs, which include annual training and certification requirements, there is no assurance that our internal policies and procedures will protect us from acts committed by our employees or agents. Additionally, we cannot predict the nature, scope, or effect of future anti-bribery requirements or the manner in which existing or new requirements might be administered or interpreted. Alleged or actual violations of any such existing or future laws, rules, or regulations, whether due to our own acts or inadvertence or to the acts or inadvertence of others, may result in criminal or civil sanctions, including fines, penalties, contract cancellations, or debarment, increased compliance costs, changes to our activities, and loss of reputation, any of which could have a material adverse effect on our business, financial condition, and operating results.
35
As previously disclosed, the SEC and the DOJ conducted investigations into our compliance with the FCPA in China. Also, as previously disclosed, we conducted our own review and implemented remedial and improvement measures based upon this review, including replacement of certain employees and enhancements of our policies and procedures in China. We cooperated with the SEC and the DOJ and have now reached separate resolutions with each of them. On August 28, 2020, the SEC accepted the Offer of Settlement and issued an administrative order finding that we violated the books and records and internal controls provisions of the FCPA. In addition, on August 28, 2020, we and the DOJ separately entered into a court-approved deferred prosecution agreement, or DPA, under which the DOJ deferred criminal prosecution of the Company for a period of three years related to a conspiracy to violate the books and records provisions of the FCPA. Among other things, we are required to undertake compliance self-reporting obligations for the three-year terms of the agreements with the SEC and the DOJ. If we remain in compliance with the DPA during its three-year term, the deferred charge against us will be dismissed with prejudice. In addition, we paid the SEC and the DOJ aggregate penalties, disgorgement, and prejudgment interest of approximately $123 million in September 2020. Any failure to comply with these agreements, or any resulting further government action, could result in a material and adverse impact to our business, financial condition, and operating results.
If we do not comply with transfer pricing, income tax, customs duties, VAT, and similar regulations, we may be subject to additional taxes, customs duties, interest, and penalties in material amounts, which could materially harm our financial condition and operating results.
As a multinational corporation operating in many countries, we are subject to transfer pricing, income tax, and other tax regulations designed to ensure that our intercompany transactions are consummated at prices that have not been manipulated to produce a desired tax result, that appropriate levels of income are reported as earned by our United States and local entities, and that we are taxed appropriately on such transactions. In addition, our operations are subject to regulations designed to ensure that appropriate levels of customs duties are assessed on the importation of our products.
If the United States Internal Revenue Service, or the IRS, or the taxing authorities of any other jurisdiction were to successfully challenge our transfer pricing practices or our positions regarding the payment of income taxes, customs duties, value added taxes, withholding taxes, and sales and use and other taxes, we could become subject to higher taxes and may increase product prices in certain jurisdictions accordingly. The imposition of new taxes, even pass-through taxes such as VAT could result in increased product prices in certain jurisdictions. Any increases in prices could adversely affect product demand and therefore could have a negative impact on our business, financial condition, and operating results. From time to time, we are a party to various regulatory proceedings related to compliance with applicable tax regulations, including audits, examinations, and investigations. We are currently subject to ongoing audits that are at various levels of review, assessment, or appeal in a number of jurisdictions involving issues of transfer pricing, income taxes, customs duties, value added taxes, withholding taxes, and sales and use and other taxes. In some circumstances, additional taxes, interest, and penalties have been assessed. We have reserved in our consolidated financial statements an amount that we believe represents the most likely outcome of the resolution of these audits, but if we are incorrect in our assessment, we may have to pay additional amounts, which could potentially be material. Ultimate resolution of these ongoing audits may take several years, and the outcome is uncertain. See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for further information on contingencies relating to tax matters.
In addition, any change in applicable tax laws, rules, treaties, or regulations, or their interpretation, could result in a higher effective tax rate on our worldwide earnings. For example, the Organisation for Economic Co-operation and Development, or OECD, has released guidance covering various international tax standards as part of its “base erosion and profit shifting,” or BEPS, initiative. The anticipated implementation of BEPS by non-U.S. jurisdictions in which we operate could result in changes to tax laws, rules, and regulations, including with respect to transfer pricing, that could materially increase our effective tax rate. On October 8, 2021, the OECD issued a statement announcing that 137 of its 140 members had agreed upon two groups of proposals for global tax reform, labeled “Pillar One” and “Pillar Two.” Pillar One is focused on providing a mechanism for taxing rights more closely aligned with market engagement (generally where people or consumers are located). Pillar Two is focused on establishing a global minimum tax rate and would apply when a country’s income tax rate is below a minimum tax rate of at least 15%. On December 20, 2021, the OECD published model rules consistent with the two Pillars announced in its October 2021 statement, and the model rules included the 15% global minimum tax rate previewed as part of Pillar Two in the OECD’s October 2021 statement. The framework’s implementation plan targets 2023 for full implementation of Pillar One and the end of 2022 for full implementation of Pillar Two, though details regarding the exact steps (and timing) of the framework’s implementation are unclear. This framework is wide ranging and could affect all multinational enterprises across all industries, including ours, by increasing our effective tax rate on earnings. No assurances can be given that future legislative, regulatory, or judicial developments will not result in an increase in the amount of taxes payable by us. If any such developments occur, our business, financial condition, and operating results could be materially and adversely affected.
36
Our business in China is subject to general, as well as industry-specific, economic, political, and legal developments and risks and requires that we utilize a modified version of the business model we use elsewhere in the world.
Our business and operations in China, which generated approximately 11% of our net sales for the year ended December 31, 2021, are subject to unique risks and uncertainties related to general economic, political, and legal developments. The Chinese government exercises significant control over the Chinese economy, including by controlling capital investments, allocating resources, setting monetary policy, controlling and monitoring foreign exchange rates, implementing and overseeing tax regulations, providing preferential treatment to certain industry segments or companies, and issuing necessary licenses to conduct business. Accordingly, any adverse change in the Chinese economy, the Chinese legal system, or Chinese governmental, economic, or other policies could have a material adverse effect on our business and operations in China and our prospects generally.
China has published regulations governing direct selling, prohibiting pyramid promotional schemes, governing food safety, and regulating e-commerce, and a number of related administrative methods and proclamations have been issued. To operate under these regulations, we created and introduced a modified business model specific to China based on our understanding of how Chinese regulators interpret and enforce these regulations, our own interpretation of applicable regulations and the enforcement thereof, and our understanding of the practices of other licensed direct-selling organizations in China.
In China, we sell our products to and through independent service providers and sales representatives, to preferred customers and other customers, as well as through Company-operated retail platforms when necessary. We also have a social e-commerce business in China, which enables our sales representatives who are also individual e-commerce promoters and independent service providers to promote our products and provide services to customers in China through virtual online stores. Our independent service providers must meet requirements to operate their own business under Chinese law, which prohibits fraudulent or misleading claims and engaging in any pyramid sales schemes, as well as our policies. In China, our independent service providers receive compensation for marketing, sales support, and other services instead of the Member allowances and royalty overrides utilized in our network marketing program outside China. The service hours and related fees eligible to be earned by the independent service providers are based on a number of factors, including the sales generated through them and through others to whom they may provide marketing, sales support and other services, the quality of their service, and other factors. Total compensation available to our independent service providers in China can generally be comparable to the total compensation available to other sales leaders globally. The Company does this by performing an analysis in our worldwide system to estimate the potential compensation available to the service providers, which can generally be comparable to that of sales leaders in other countries. After adjusting such amounts for other factors and dividing by each service provider’s hourly rate, we then notify each independent service provider the maximum hours of work for which they are eligible to be compensated in the given month. In order for a service provider to be paid, the Company requires each service provider to invoice the Company for their services and submit a timesheet of such services and, upon the Company’s request, service providers may be required to submit additional supporting documents for the Company’s further verification. These and other business model features in China are not common to the business model we employ elsewhere in the world, and we expect our business model in China will continue to incorporate some or all of these features, and any failure of this model or our business or our service providers to comply with Chinese law could materially and negatively impact our business, financial condition, and operating results.
Direct-selling regulations in China require us to apply for various approvals to conduct direct selling in China. The process for obtaining the necessary licenses to conduct direct selling is protracted and cumbersome and involves multiple layers of Chinese governmental authorities and numerous governmental employees at each layer. While direct-selling licenses are centrally issued, such licenses are generally valid only in the jurisdictions within which related approvals have been obtained, and such approvals are generally awarded on local and provincial bases. Accordingly, there can be no assurance that we will obtain additional, or maintain our existing, direct-selling licenses and approvals in China that are important to our business, which could materially and negatively impact our business, financial condition, and operating results. The approval process, like other aspects of our operations in China, is guided by distinct Chinese practices and customs, and is subject to applicable laws of China and the other jurisdictions in which we operate our business, including the United States, as well as our internal policies, such as our code of ethics. There is a risk that in attempting to comply with local customs and practices in China, including during the application process or otherwise, we will fail to comply with our policies, applicable requirements in China, or violate the laws of another jurisdiction, any of which could materially harm our business in China, prevent us from obtaining direct-selling licenses or other approvals, or result in adverse publicity or legal or regulatory proceedings. Furthermore, we rely on certain key personnel in China, including to assist us during the approval process and to maintain our licenses, and the loss of any such key personnel could delay or hinder our ability to obtain or maintain licenses or related approvals or otherwise negatively impact our operations in China.
37
Additionally, there continues to be uncertainty regarding the interpretation and enforcement of Chinese regulations. The regulatory environment in China continues to evolve, and officials at all levels of the Chinese, provincial, and local government exercise broad discretion in deciding how to interpret, apply, and enforce regulations as they deem appropriate. Regulators in China may modify existing, or introduce new, regulations or interpretations. There can be no guarantee that changes in regulations, or their interpretation or enforcement, will not negatively impact our business in China, create industry reputational risk, result in regulatory proceedings, or lead to fines or penalties against us or our independent service providers. If our business practices or those of our independent service providers are deemed to be in violation of applicable regulations, in particular with respect to the factors used in determining the services a service provider is eligible to perform and service fees they are eligible to earn and receive, we could be sanctioned and/or required to change our business model, either of which could have a significant adverse impact on our business in China. In addition, the Chinese government rigorously monitors markets, including the direct-selling market, in China and in the past has taken serious action against companies engaged in activities that the government regarded as in violation of applicable law, including shutting down their businesses and imposing substantial fines, such as the Review, which investigated unlawful promotion and sales within the health products industry. There is no guarantee the government will not revisit its focus on health products, expand its investigation to cover direct-selling business models, or otherwise launch into a new investigation or multiple investigations that may result in a material adverse effect to our business in China.
The United Kingdom’s exit from the European Union could adversely impact us.
On January 31, 2020, the U.K. formally exited the European Union. Although the British government reached a formal agreement with the European Union providing a framework for the U.K.’s relationship with the European Union, additional agreements and rules will need to be negotiated in the future and it remains unclear what long-term economic, financial, trade, and legal implications the exit of the U.K. from the European Union will have and how such exit could affect our business globally and in the region. The exit has disrupted and could continue to disrupt the movement of goods, services, and people between the U.K. and the European Union and other nations and could potentially undermine bilateral cooperation in key geographic areas. It has also led to legal uncertainty and could result in potentially divergent national laws and regulations as the U.K. determines which European Union laws to replace or replicate. In addition, Brexit may lead other European Union member countries to consider referendums regarding their European Union membership. Any of these events, along with any political, economic, and regulatory changes that may occur, could cause political and economic uncertainty in Europe and globally and materially harm our business, financial condition, and operating results.
Risks Related to Our Indebtedness
The terms and covenants in our existing indebtedness could limit our discretion with respect to certain business matters, which could harm our business, financial condition, and operating results.
Our senior secured credit facility, or the 2018 Credit Facility, and the indentures governing the senior notes due September 1, 2025, or the 2025 Notes, and the senior notes due June 1, 2029, or the 2029 Notes, have restrictive covenants that limit our and our subsidiaries’ ability to, among other things:
In addition, the 2018 Credit Facility requires us to meet certain financial ratios and financial conditions. These covenants could limit our ability to grow our business, take advantage of attractive business opportunities, successfully compete, obtain future financing, withstand future downturns in our business or the economy in general, or otherwise conduct necessary corporate activities.
Our ability to comply with these covenants may be affected by events beyond our control, including prevailing economic, financial and industry conditions. Failure to comply with these covenants could result in an event of default. Upon the occurrence of an event of default under any of our debt agreements, the lenders or noteholders, as applicable, could cause all outstanding amounts under such agreements to become due and payable, and it could trigger a cross-default with respect to other outstanding indebtedness under certain circumstances. The 2018 Credit Facility is secured by the equity interests of certain of our subsidiaries and substantially all of the assets of the domestic loan parties, and the lenders thereunder could proceed to foreclose on such assets if we are unable to repay or refinance any accelerated debt under the 2018 Credit Facility. Following an event of default, the lenders under our revolving credit facility would also have the right to terminate any commitments they have to provide further borrowings.
38
The required interest payments on our indebtedness under the 2018 Credit Facility or other agreements may be impacted by expected, and recently effective, reforms related to the London Interbank Offered Rate, or LIBOR. The variable interest rates applicable under the 2018 Credit Facility are linked to LIBOR as the benchmark rate for establishing such rates. Pursuant to national, international, and other regulatory guidance and reform proposals regarding LIBOR, certain LIBOR tenors were discontinued or otherwise became unavailable as benchmark rates at the end of 2021 and LIBOR is expected to be fully discontinued or become unavailable as a benchmark rate by June 2023. Although the 2018 Credit Facility includes mechanics to facilitate the adoption by us and our lenders of an alternative benchmark rate for use in place of LIBOR, no assurance can be made that such alternative benchmark rate will perform in a manner similar to LIBOR or result in interest rates that are at least as favorable to us as those that would have resulted had LIBOR remained in effect, which could result in an increase in our interest expense and other debt service obligations. In addition, the overall credit market may be disrupted as a result of the replacement of LIBOR or in the anticipation thereof, which could have an adverse impact on our ability to refinance, reprice, or amend our existing indebtedness or incur additional indebtedness on favorable terms or at all.
The conversion or maturity of our convertible notes may adversely affect our financial condition and operating results, and their conversion into common shares could have a dilutive effect that could cause our share price to go down.
We issued convertible senior notes due on March 15, 2024, or the 2024 Convertible Notes, in the aggregate principal amount of $550 million. Prior to December 15, 2023, under certain circumstances, holders of our 2024 Convertible Notes may convert their notes at their option. On and after December 15, 2023, holders may convert their 2024 Convertible Notes at any time.
The 2024 Convertible Notes may be settled, at our option, in cash or a combination of cash and common shares, so long as the principal amount of the 2024 Convertible Notes is settled in cash. If one or more holders elect to convert their 2024 Convertible Notes when conversion is permitted, we would be required to make cash payments to satisfy the principal amount due at conversion and could elect to make cash payments to satisfy our full conversion obligations, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their 2024 Convertible Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal amount of our 2024 Convertible Notes as a current rather than long-term liability, which could result in a material reduction of our net working capital. Payment of cash upon conversion of the 2024 Convertible Notes, or any adverse change in the accounting treatment of the 2024 Convertible Notes, may adversely affect our financial condition and operating results, each of which could in turn adversely impact the amount or timing of future potential share repurchases or the payment of dividends to our shareholders.
In addition, if a portion of the 2024 Convertible Notes are converted into common shares, our existing shareholders will experience immediate dilution of voting rights and our share price may decline. Furthermore, the perception that such dilution could occur may cause our share price to decline. Because the conversion rate of the 2024 Convertible Notes adjusts upward upon the occurrence of certain events, existing shareholders may experience further dilution if a portion of the 2024 Convertible Notes are converted into common shares and the currently effective adjusted conversion rate is further adjusted. For more information regarding the conversion features of our 2024 Convertible Notes, including the events that allow for early conversion and the current conversion rate, see Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K.
Risks Related to Our Common Shares
Holders of our common shares may face difficulties in protecting their interests because we are incorporated under Cayman Islands law.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, the Cayman Islands Companies Act (as revised), or the Companies Act, and the common law of the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly defined as and may be different from those under statutes or judicial precedent in existence in jurisdictions in the United States. In particular, the Cayman Islands has a less prescriptive body of corporate laws compared to the United States, and certain states, such as Delaware, may have more fulsome and judicially interpreted bodies of corporate law. Therefore, shareholders may have more difficulty in protecting their interests in the face of actions by our management or board of directors than would shareholders of a corporation incorporated in a jurisdiction in the United States.
For example, shareholders of Cayman Islands exempted companies such as Herbalife Nutrition Ltd. have no general rights under Cayman Islands law to inspect corporate records and accounts or to obtain copies of lists of shareholders. Our directors have discretion under our articles of association to determine whether, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
39
A shareholder may have a direct right of action against us where its individual rights have been, or are about to be, infringed. Our Cayman Islands counsel, Maples and Calder (Cayman) LLP, is not aware of any reported class action having been brought in a Cayman Islands court. Derivative actions have been brought in the Cayman Islands courts, and the Cayman Islands courts have confirmed the availability of such actions. In most cases, however, we would be the proper plaintiff where an action is brought to redress any loss or damage suffered by us, or based on a breach of duty owed to us, and a claim, for example, against our officers or directors, usually may not be brought by a shareholder. However, based on English authorities, which would likely be of persuasive authority and be applied by a court in the Cayman Islands, exceptions to the foregoing principle may apply where:
Provisions of our articles of association and Cayman Islands law may impede a takeover or make it more difficult for shareholders to change the direction or management of the Company, which could reduce shareholders’ opportunity to influence management of the Company.
Our articles of association contain certain provisions which could have an effect of discouraging a takeover or other transaction or preventing or making it more difficult for shareholders to change the direction or management of our Company. For example, our articles of association permit our board of directors to issue preference shares from time to time, with such rights and preferences as they consider appropriate. Our board of directors could authorize the issuance of preference shares with terms and conditions and under circumstances that could have an effect of discouraging a takeover or other transaction. In addition, our articles of association prohibit the ability of shareholders to act by written consent, limit the ability of shareholders to call special meetings of shareholders, and contain advance notice provisions. As a result, our shareholders may have less input into the management of our Company than they might otherwise have if these provisions were not included in our articles of association.
The Companies Act contains provisions to facilitate mergers and consolidations between Cayman Islands companies and non-Cayman Islands companies (provided that is facilitated by the laws of such other jurisdiction). These provisions, contained within Part XVI of the Companies Act, are broadly similar to the merger provisions provided for under Delaware law.
There are, however, important differences that could impede a takeover. For example, the threshold for approval of the merger plan by shareholders is higher. The threshold is a special resolution of the shareholders (being 66 ⅔% of those present in person or by proxy and voting) together with such other authorization, if any, as may be specified in the articles of association. Additionally, the consent of each holder of a fixed or floating security interest is required to be obtained unless the Grand Court of the Cayman Islands waives such requirement.
The Companies Act contains separate provisions that provide for the merger, reconstruction, and amalgamation of companies pursuant to court-approved arrangements. These are commonly referred to in the Cayman Islands as “schemes of arrangement.” The procedural and legal requirements necessary to consummate a scheme of arrangement are more rigorous and take longer to complete than the procedures typically required to consummate a merger in the United States. Under Cayman Islands law and practice, a scheme of arrangement in relation to a solvent Cayman Islands company must be approved at a shareholders’ meeting by a majority in number of each class of the company’s shareholders who are present and voting (either in person or by proxy) at such meeting. The shares voted in favor of the scheme of arrangement must also represent at least 75% of the value of each relevant class of the company’s shareholders present and voting at the meeting. The convening of these meetings and the terms of the arrangement must also be sanctioned by the Grand Court of the Cayman Islands. Although there is no requirement to seek the consent of the creditors of the parties involved in the scheme of arrangement, the Grand Court typically seeks to ensure that the creditors have consented to the transfer of their liabilities to the surviving entity or that the scheme of arrangement does not otherwise materially adversely affect creditors’ interests. Furthermore, the court will only approve a scheme of arrangement if it is satisfied that:
40
If the scheme of arrangement is approved, dissenting shareholders would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of U.S. corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
In addition, if an offer by a third party to purchase shares has been approved by the holders of at least 90% of the issued and outstanding shares (not including shares held by such third party) within four months of the third party making such offer, the third party may, during the two months following expiration of the four-month period, require the holders of the remaining shares to transfer their shares on the same terms on which the purchaser acquired the first 90% of the issued and outstanding shares. An objection can be made to the Grand Court of the Cayman Islands, but this is unlikely to succeed unless there is evidence of fraud, bad faith, collusion or inequitable treatment of the shareholders.
Further, transactions similar to a merger, reconstruction and/or an amalgamation may in some cases be achieved through means other than these statutory provisions, such as a share capital exchange, asset acquisition or control, or through contractual arrangements of an operating business.
There is uncertainty as to shareholders’ ability to enforce certain foreign civil liabilities in the Cayman Islands.
We are incorporated as an exempted company with limited liability under the laws of the Cayman Islands and a material portion of our assets are located outside of the United States.
Herbalife Nutrition Ltd. has been advised by its Cayman Islands legal counsel, Maples and Calder (Cayman) LLP, that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against Herbalife Nutrition Ltd. judgments of courts of the United States predicated upon the civil liability provisions of the securities laws of the United States or any state; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against Herbalife Nutrition Ltd. predicated upon the civil liability provisions of the securities laws of the United States or any state, so far as the liabilities imposed by those provisions are penal in nature. ln those circumstances, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign money judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud, or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). A Cayman Islands court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
Mail addressed to the Company and received at its registered office will be forwarded unopened to the forwarding address supplied by the Company. None of Herbalife Nutrition Ltd., its directors, officers, advisors or service providers (including the organization that provides registered office services in the Cayman Islands) will bear any responsibility for any delay caused in mail reaching the forwarding address.
U.S. Tax Reform may adversely impact certain U.S. shareholders of the Company.
If a U.S. shareholder owns 10% or more of our common shares, it may be subject to increased U.S. federal income taxation under the “controlled foreign corporation,” or CFC, rules. A non-U.S. corporation will be classified as a CFC for any particular taxable year, if U.S. persons (including individuals and entities) who own (directly, indirectly, or constructively) 10% or more of the voting power or value of shares, or 10% U.S. Shareholders, own, in the aggregate, more than 50% of the total combined voting power or value of the shares. In determining whether a shareholder is treated as a 10% U.S. Shareholder, the voting power of the shares and any special voting rights, such as to appoint directors, may also be taken into account. In addition, certain constructive ownership rules apply, which attribute share ownership among certain family members and certain entities and their owners. Such constructive ownership rules may also attribute share ownership to persons that are entitled to acquire shares pursuant to an option, such as the holders of our 2024 Convertible Notes.
41
As a result of certain changes to the CFC constructive ownership rules introduced by the Tax Cuts and Jobs Act of 2017, or U.S. Tax Reform, one or more of our non-U.S. corporate subsidiaries that were not previously classified as CFCs are now classified as CFCs, including on a retroactive basis. For 10% U.S. Shareholders, this may result in adverse tax consequences. Generally, 10% U.S. Shareholders of a CFC are required to include currently in gross income their respective shares of (i) the CFC’s “Subpart F income” (e.g. items of passive income and certain income resulting from inter-company sales and services), (ii) the CFC’s earnings (that have not been subject to tax under the Subpart F rules) to the extent the CFC holds certain U.S. property, and (iii) the CFC’s global intangible low-taxed income pursuant to the U.S. Tax Reform. Such 10% U.S. Shareholders are subject to current U.S. federal income tax with respect to the foregoing income items, even if the CFC has not made an actual distribution to such shareholders.
While we do not believe that Herbalife Nutrition Ltd. is classified as a CFC, such entity and one or more of our non-U.S. corporate subsidiaries not already classified as CFCs could become classified as CFCs either as a result of (i) additional changes to tax laws, rules, or regulations, including future pronouncements or other guidance from the IRS or (ii) an increase in the percentage ownership of our common shares by shareholders who hold, or in the future may hold, 10% or more of our common shares, whether as a result of future share acquisitions, the impact of any share repurchases we may undertake, or otherwise.
Shareholders who own, or contemplate owning, 10% or more of our shares (taking into account the impact of any share repurchases we may undertake and the constructive ownership rules) are urged to consult their tax advisors.
No assurances can be given that future legislative, administrative, or judicial developments will not result in an increase in the amount of U.S. taxes payable by an investor in our shares. If any such developments occur, such developments could have a material and adverse effect on an investment in our shares.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
As of December 31, 2021, we leased the majority of our physical properties. We currently lease approximately 95,000 square feet in downtown Los Angeles, California, including our corporate executive offices located in the LA Live complex with the lease term expiring in 2033. We also lease approximately 140,000 square feet, with the lease term expiring in 2033, and own approximately 189,000 square feet of general office space in Torrance, California, for our North America and South America regional headquarters, including some of our corporate support functions. Additionally, we lease distribution center facilities in Los Angeles, California and Memphis, Tennessee of approximately 255,000 square feet and 259,000 square feet, respectively. The Los Angeles and Memphis lease agreements have terms through 2031 and 2023, respectively. We also lease approximately 178,000 square feet of warehouse space for a distribution center in Hagerstown, Maryland, expiring in 2032. In Lake Forest, California, we lease warehouse, manufacturing plant, and office space of approximately 166,000 square feet expiring in 2029. In Venray, Netherlands, we lease our European centralized warehouse of approximately 344,000 square feet under an arrangement expiring in 2025. In Changsha, Hunan, China we are leasing our botanical extraction facility of approximately 154,000 square feet with the term expiring in 2022. In Suzhou, China we are leasing our manufacturing and warehouse facilities of approximately 81,000 square feet and 121,000 square feet, respectively, under leases expiring in 2022 and 2024, respectively. In Nanjing, China, we are leasing an additional manufacturing facility of approximately 372,000 square feet under a lease expiring in 2025. In Guadalajara, Mexico, we lease approximately 234,000 square feet of office space, the majority of which houses a Global Business Service Center that supports worldwide operations, under leases expiring in 2027. In Bangalore, India, we lease approximately 155,000 square feet of office space for our Global Business Service Center, which expires in 2026. We also lease office space for Global Business Service Centers in Querétaro, Mexico; Krakow, Poland; and Kuala Lumpur, Malaysia. In addition to the properties noted above, we also lease other warehouse, manufacturing, and office buildings in a majority of our other geographic areas of operation.
We own a manufacturing facility in Winston-Salem, North Carolina. The manufacturing facility contains approximately 800,000 square feet of manufacturing and office space. See Item 1, Business, for further discussion of the manufacturing facility purchased in Winston-Salem, North Carolina.
We believe that our existing facilities are adequate to meet our current requirements and that comparable space is readily available at each of these locations.
42
Item 3. Legal Proceedings
The information set forth under Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K is incorporated herein by reference.
Item 4. Mine Safety Disclosures
Not applicable.
43
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Information with Respect to our Common Shares
Our common shares are listed on the New York Stock Exchange, or NYSE, and trade under the symbol “HLF.” The market price of our common shares is subject to fluctuations in response to variations in our quarterly operating results, general trends in the market for our products, economic and currency exchange issues in the foreign markets in which we operate as well as other factors, many of which are not within our control. In addition, broad market fluctuations, as well as general economic, business and political conditions may adversely affect the market for our common shares, regardless of our actual or projected performance.
The closing price of our common shares on February 16, 2022, was $43.96. The approximate number of holders of record of our common shares as of February 16, 2022 was 488. This number of holders of record does not represent the actual number of beneficial owners of our common shares because shares are frequently held in “street name” by securities dealers and others for the benefit of individual owners who have the right to vote their shares.
Performance Graph
Set forth below is information comparing the cumulative total shareholder return and share price appreciation plus dividends on our common shares with the cumulative total return of the S&P 500 Index and a market-weighted index of publicly traded peers over the five-year period ended December 31, 2021. The graph assumes that $100 is invested in each of our common shares, the S&P 500 Index, and the index of publicly traded peers on December 31, 2016 and that all dividends were reinvested. The publicly traded companies in the peer group are Conagra Brands, Inc., The Hain Celestial Group, Inc., Nu Skin Enterprises, Inc., Post Holdings, Inc., Tupperware Brands Corporation, and USANA Health Sciences, Inc.
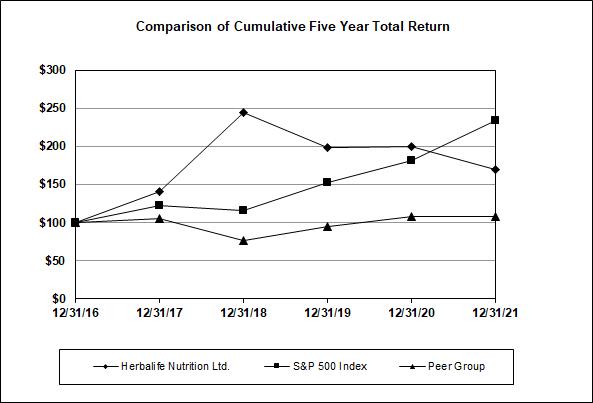
|
|
December 31, |
|
|||||||||||||||||||||
|
|
2016 |
|
|
2017 |
|
|
2018 |
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
||||||
Herbalife Nutrition Ltd. |
|
$ |
100.00 |
|
|
$ |
140.67 |
|
|
$ |
244.91 |
|
|
$ |
198.05 |
|
|
$ |
199.63 |
|
|
$ |
170.05 |
|
S&P 500 Index |
|
$ |
100.00 |
|
|
$ |
121.83 |
|
|
$ |
116.49 |
|
|
$ |
153.17 |
|
|
$ |
181.35 |
|
|
$ |
233.41 |
|
Peer Group |
|
$ |
100.00 |
|
|
$ |
106.02 |
|
|
$ |
76.74 |
|
|
$ |
94.88 |
|
|
$ |
108.05 |
|
|
$ |
108.41 |
|
44
Information with Respect to Dividends
We have not declared or paid cash dividends since 2014. The declaration of future dividends is subject to the discretion of our board of directors and will depend upon various factors, including our earnings, financial condition, Herbalife Nutrition Ltd.’s available distributable reserves under Cayman Islands law, restrictions imposed by the 2018 Credit Facility and the terms of any other indebtedness that may be outstanding, cash requirements, future prospects and other factors deemed relevant by our board of directors.
Information with Respect to Purchases of Equity Securities by the Issuer
On February 9, 2021, our board of directors authorized a new three-year $1.5 billion share repurchase program that will expire on February 9, 2024, which replaced our prior share repurchase authorization that was set to expire on October 30, 2023 and had approximately $7.9 million of remaining authorized capacity when it was replaced. This share repurchase program allows us, which includes an indirect wholly-owned subsidiary of Herbalife Nutrition Ltd., to repurchase our common shares at such times and prices as determined by management, as market conditions warrant, and to the extent Herbalife Nutrition Ltd.’s distributable reserves are available under Cayman Islands law. The 2018 Credit Facility permits us to repurchase our common shares as long as no default or event of default exists and other conditions, such as specified consolidated leverage ratios, are met. As of December 31, 2021, the remaining authorized capacity under our $1.5 billion share repurchase program was approximately $1.1 billion.
The following is a summary of our repurchases of common shares during the three months ended December 31, 2021. For further information on our share repurchases during the year ended December 31, 2021, see Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K.
|
|
Total Number of Shares Purchased |
|
|
Average Price Paid per Share |
|
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
|
|
Approximate Dollar Value of Shares That May Yet Be Purchased Under the Plans or Programs |
|
||||
October 1 — October 31 |
|
|
1,859,392 |
|
|
$ |
46.04 |
|
|
|
1,859,392 |
|
|
$ |
1,133,296,958 |
|
November 1 — November 30 |
|
|
380,000 |
|
|
$ |
42.07 |
|
|
|
380,000 |
|
|
$ |
1,117,311,565 |
|
December 1 — December 31 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
1,117,311,565 |
|
|
|
|
2,239,392 |
|
|
$ |
45.36 |
|
|
|
2,239,392 |
|
|
$ |
1,117,311,565 |
|
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with Part I, Item 1A, Risk Factors, and our consolidated financial statements and related notes, each included elsewhere in this Annual Report on Form 10-K.
This section of this Annual Report on Form 10-K generally discusses 2021 and 2020 items and year-over-year comparisons between 2021 and 2020. Discussions of 2019 items and year-over-year comparisons between 2020 and 2019 that are not included in this Annual Report on Form 10-K can be found in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of our Annual Report on Form 10-K for the year ended December 31, 2020, or the 2020 10-K.
Overview
We are a global nutrition company that sells weight management; targeted nutrition; energy, sports, and fitness; and outer nutrition products to and through independent members, or Members. In China, we sell our products to and through independent service providers and sales representatives to customers and preferred customers, as well as through Company-operated retail platforms when necessary. We refer to Members that distribute our products and achieve certain qualification requirements as “sales leaders.”
We provide high-quality, science-backed products to Members and their customers who seek a healthy lifestyle and we also offer a business opportunity to those Members who seek additional income. We believe enhanced consumer awareness and demand for our products due to global trends such as the obesity epidemic, increasing interest in a fit and active lifestyle, living healthier, and the rise of entrepreneurship, coupled with the effectiveness of personalized selling through a direct sales channel, have been the primary reasons for our continued success.
45
Our products are grouped in four principal categories: weight management; targeted nutrition; energy, sports, and fitness; and outer nutrition, along with literature, promotional, and other items. Our products are often sold through a series of related products and literature designed to simplify weight management and nutrition for consumers and maximize our Members’ cross-selling opportunities.
While we continue to monitor the current global financial environment and the impacts of the COVID-19 pandemic, we remain focused on the opportunities and challenges in retailing our products and enhancing the customer experience, sponsoring and retaining Members, improving Member productivity, further penetrating existing markets, globalizing successful Daily Methods of Operation, or DMOs, such as Nutrition Clubs, Fit Clubs, and Weight Loss Challenges, introducing new products and globalizing existing products, developing niche market segments and further investing in our infrastructure.
We sell our products in six geographic regions:
On July 15, 2016, we reached a settlement with the FTC and entered into the Consent Order, which resolved the FTC’s multi-year investigation of the Company. We continue to monitor the impact of the Consent Order and our Audit Committee assists our board of directors in overseeing continued compliance with the Consent Order. While we currently do not expect the settlement to have a long-term and materially adverse impact on our business and our Member base, our business and our Member base, particularly in the U.S., may be negatively impacted. The terms of the Consent Order do not change our going to market through direct selling by independent distributors, and compensating those distributors based upon the product they and their sales organization sell. See Part I, Item 1, Business, of this Annual Report on Form 10-K for further discussion about the Consent Order and Part I, Item 1A, Risk Factors, of this Annual Report on Form 10-K for a discussion of risks related to the Consent Order.
COVID-19 Pandemic
During March 2020, the World Health Organization declared the outbreak of coronavirus disease 2019, or COVID-19, as a pandemic. The outbreak and subsequent global spread of the virus has impacted the general public, companies and state, local and national governments and economies worldwide, as well as global financial markets. Public health organizations and international, federal, state and local governments have implemented measures to combat the spread of COVID-19, including restrictions on movement such as quarantines, “stay-at-home” orders and social distancing ordinances and restricting or prohibiting outright some or all forms of commercial and business activity. These measures, or others that may be implemented in the future, although temporary in nature, have continued intermittently for many markets.
Our business and operations have been affected by the pandemic in manners, in some cases adversely, and degrees that vary by market and we expect that the effects may extend through 2022 and possibly beyond. For the health and safety of our employees, our Members, and their customers, we implemented temporary access restrictions at many of our physical business locations and locations where Members conduct their business activities, some of which measures continue. Generally, we have been able to satisfy current levels of demand. While pandemic constraints have been lessened in most markets, including by the designation of our nutritional business as “essential” or other similar characterization, our operations have been and continue to be disrupted. The most significant impacts we have seen, depending on market, include:
46
We and our Members have responded to the pandemic and its impacts on our business and theirs by adapting operations and taking a number of proactive measures to mitigate those impacts. The most significant measures include:
We believe our cash on hand as of December 31, 2021 and as of the date of this filing, combined with cash flows from operating activities, is sufficient to meet our foreseeable needs for the next twelve months. We also have access to our revolving credit facility to supplement our cash-generating ability if necessary.
Although we believe that our responsive measures have been effective in limiting the adverse impact of the pandemic on most markets, the ongoing impact of the COVID-19 pandemic will affect our business, financial condition, and results of operations in future quarters, including their comparability to prior periods. Given the unpredictable, unprecedented, and fluid nature of the pandemic and its economic consequences, we are unable to predict the duration and extent to which the pandemic and its related impacts will impact our business, financial condition, and results of operations. A more detailed discussion of the pandemic’s impact on net sales for 2021 and its expected impact in future periods, as well as the impacts specific to each geographic region, are discussed further in the Sales by Geographic Region section below. See Part I, Item 1A, Risk Factors, of this Annual Report on Form 10-K for a further discussion of risks related to the COVID-19 pandemic.
Volume Points by Geographic Region
A key non-financial measure we focus on is Volume Points on a Royalty Basis, or Volume Points, which is essentially our weighted-average measure of product sales volume. Volume Points, which are unaffected by exchange rates or price changes, are used by management as a proxy for sales trends because in general, excluding the impact of price changes, an increase in Volume Points in a particular geographic region or country indicates an increase in our local currency net sales while a decrease in Volume Points in a particular geographic region or country indicates a decrease in our local currency net sales. The criteria we use to determine how and when we recognize Volume Points are not identical to our revenue recognition policies under U.S. GAAP. Unlike net sales, which are generally recognized when the product is delivered and when control passes to the Member, as discussed in greater detail in Note 2, Basis of Presentation, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K, we recognize Volume Points when a Member pays for the order, which is generally prior to the product being delivered. Further, the periods in which Volume Points are tracked can vary slightly from the fiscal periods for which we report our results under U.S. GAAP. Therefore, there can be timing differences between the product orders for which net sales are recognized and for which Volume Points are recognized within a given period. However, historically these timing differences generally have been immaterial in the context of using changes in Volume Points as a proxy to explain volume-driven changes in net sales.
47
The specific number of Volume Points assigned to a product, which is generally consistent across all markets, is based on a Volume Point to suggested retail price ratio for similar products. If a product is available in different quantities, the various sizes will have different Volume Point values. In general, once assigned, a Volume Point value is consistent in each region and country and does not change from year to year. We use Volume Points for Member qualification and recognition purposes, as well as a proxy for sales trends, and therefore we generally keep Volume Points for a similar or like product consistent on a global basis. However, because Volume Points are a function of value rather than product type or size, they are not a reliable measure for product mix. As an example, an increase in Volume Points in a specific country or region could mean a significant increase in sales of less expensive products or a marginal increase in sales of more expensive products.
|
|
Year Ended December 31, |
|
|||||||||||||||||||||
|
|
2021 |
|
|
2020 |
|
|
% Change |
|
|
2020 |
|
|
2019 |
|
|
% Change |
|
||||||
|
|
(Volume Points in millions) |
|
|||||||||||||||||||||
North America |
|
|
1,783.8 |
|
|
|
1,735.0 |
|
|
|
2.8 |
% |
|
|
1,735.0 |
|
|
|
1,317.0 |
|
|
|
31.7 |
% |
Mexico |
|
|
851.0 |
|
|
|
879.7 |
|
|
|
(3.3 |
)% |
|
|
879.7 |
|
|
|
882.8 |
|
|
|
(0.4 |
)% |
South and Central America |
|
|
497.8 |
|
|
|
535.2 |
|
|
|
(7.0 |
)% |
|
|
535.2 |
|
|
|
516.5 |
|
|
|
3.6 |
% |
EMEA |
|
|
1,629.3 |
|
|
|
1,562.5 |
|
|
|
4.3 |
% |
|
|
1,562.5 |
|
|
|
1,290.1 |
|
|
|
21.1 |
% |
Asia Pacific |
|
|
1,960.1 |
|
|
|
1,690.2 |
|
|
|
16.0 |
% |
|
|
1,690.2 |
|
|
|
1,565.0 |
|
|
|
8.0 |
% |
China |
|
|
375.8 |
|
|
|
523.8 |
|
|
|
(28.3 |
)% |
|
|
523.8 |
|
|
|
497.2 |
|
|
|
5.3 |
% |
Worldwide |
|
|
7,097.8 |
|
|
|
6,926.4 |
|
|
|
2.5 |
% |
|
|
6,926.4 |
|
|
|
6,068.6 |
|
|
|
14.1 |
% |
Volume Points increased 2.5% for 2021, including a mixed impact of COVID-19 pandemic conditions across our markets, after having increased 14.1% in 2020. The comparative 2021 and 2020 results by region discussed below are greatly impacted, we believe, by the significance and timing of pandemic conditions and our and our Members’ ability to respond to the conditions, which varied by region and by market within regions. Although pandemic conditions had adverse operational impacts across all markets, we believe during certain periods our Members in certain markets where we saw increased net sales and Volume Point growth for some periods were more focused on their business due to those conditions, particularly the North America region and certain EMEA markets during the second half of 2020 and first half of 2021.
We believe North America’s Volume Point increase for 2021 reflects both the continuing success and expansion of our Distributors as well as the enhanced motivation and focus of our Members due to pandemic conditions seen through the first half of 2021. The significant decline in the North America 2021 growth rate compared to the 2020 growth rate reflects in part, we believe, the comparison to a 2020 year period that included record levels of sales in the second half of the year, and fewer new distributors and preferred customers joining our business in 2021. We believe Mexico’s Volume Point decreases for 2021, continuing modest declines seen in recent years, is due to ongoing difficult economic conditions in the market, exacerbated by the adverse impact of intermittent pandemic-related constraints. The South and Central America region saw a decrease in Volume Points for 2021, after a small increase for 2020. The current year was impacted by continuing intermittent adverse impacts of the pandemic seen across most markets in the region. The volume decline was greatest in Brazil, our largest market in the region, as it saw adverse pandemic effects as well as longer-term negative momentum. EMEA has seen Volume Point growth in recent years, a result we believe of customer-oriented efforts including Member training, brand awareness, and product line expansion, as well as Member success in leveraging online approaches and new Member recruitment, plus, during the second half of 2020 and first half of 2021, enhanced motivation and focus of our Members due to pandemic conditions. The significant decline in the EMEA 2021 growth rate compared to the 2020 growth rate reflects in part, we believe, the comparison to a 2020 year period that included record levels of sales in the second half of 2020, and fewer new distributors and preferred customers joining our business in 2021. Though results were mixed by market, the Asia Pacific region saw a Volume Point increase for 2021, led by the India market and continuing favorable long-term trends seen in the region that are a result, we believe, of a customer-focused business, daily consumption DMOs including Nutrition Clubs, and ongoing product line expansion. China saw a significant Volume Point decrease for 2021, versus a modest increase for 2020. Results for 2021 reflect, we believe, an adverse near-term impact of changes we are making that we believe will ultimately strengthen the consistency and sustainability of our business in China, as well as the continuing impact on sales and training meetings of pandemic conditions and residual effects of the Chinese government's 100-day review of the health product industry, or the Review, which concluded in April 2019. Also notable is that the China growth rate for 2020 was favorably impacted by a weakened 2019 year period due to disruption from the Review.
Across most markets, we expect COVID-19 pandemic conditions to continue to impact Volume Point results; however, we are unable to predict the duration or magnitude of these effects. Results and more regional or country-specific impacts of the COVID-19 pandemic are discussed further below in the applicable sections of Sales by Geographic Region.
48
Presentation
“Net sales” represent product sales to our Members, net of “distributor allowances,” and inclusive of any shipping and handling revenues, as described further below.
Our Members purchase product from us at a suggested retail price, less discounts referred to as “distributor allowance.” Each Member’s level of discount is determined by qualification based on their volume of purchases. In cases where a Member has qualified for less than the maximum discount, the remaining discount, which we also refer to as a wholesale commission, is received by their sponsoring Members. Distributor allowances may also vary by country depending upon regulatory restrictions that limit or otherwise restrict distributor allowances. We also offer reduced distributor allowances with respect to certain products worldwide.
For U.S. GAAP purposes, shipping and handling services relating to product sales are recognized as fulfillment activities on our performance obligation to transfer products and are therefore recorded within net sales as part of product sales and are not considered as separate revenues.
In certain geographic markets, we have introduced segmentation of our Member base into two categories: “preferred members” – who are simply consumers who wish to purchase product for their own household use, and “distributors” – who are Members who also wish to resell products or build a sales organization. Additionally, in certain markets we are simplifying our pricing by eliminating certain shipping and handling charges and recovering those costs within suggested retail price.
Our international operations have provided and will continue to provide a significant portion of our total net sales. As a result, total net sales will continue to be affected by fluctuations in the U.S. dollar against foreign currencies. In order to provide a framework for assessing how our underlying businesses performed excluding the effect of foreign currency fluctuations, in addition to comparing the percent change in net sales from one period to another in U.S. dollars, we also compare the percent change in net sales from one period to another period using “net sales in local currency.” Net sales in local currency is not a U.S. GAAP financial measure. Net sales in local currency removes from net sales in U.S. dollars the impact of changes in exchange rates between the U.S. dollar and the local currencies of our foreign subsidiaries, by translating the current period net sales into U.S. dollars using the same foreign currency exchange rates that were used to translate the net sales for the previous comparable period. We believe presenting net sales in local currency is useful to investors because it allows a meaningful comparison of net sales of our foreign operations from period to period. However, net sales in local currency measures should not be considered in isolation or as an alternative to net sales in U.S. dollar measures that reflect current period exchange rates, or to other financial measures calculated and presented in accordance with U.S. GAAP.
Additionally, the impact of foreign currency fluctuations in Venezuela and the price increases we implement as a result of the highly inflationary economy in that market can each, when considered in isolation, can have a disproportionately large impact to our consolidated results despite the offsetting nature of these drivers and that net sales in Venezuela, which represent less than 1% of our consolidated net sales, are not material to our consolidated results. Therefore, in certain instances, we believe it is helpful to provide additional information with respect to these factors as reported and excluding the impact of Venezuela to illustrate the disproportionate nature of Venezuela’s individual pricing and foreign exchange impact to our consolidated results. However, excluding the impact of Venezuela from these measures is not in accordance with U.S. GAAP and should not be considered in isolation or as an alternative to the presentation and discussion thereof calculated in accordance with U.S. GAAP.
Our “gross profit” consists of net sales less “cost of sales,” which represents our manufacturing costs, the price we pay to our raw material suppliers and manufacturers of our products as well as shipping and handling costs including duties, tariffs, and similar expenses.
While certain Members may profit from their activities by reselling our products for amounts greater than the prices they pay us, Members that develop, retain, and manage other Members may earn additional compensation for those activities, which we refer to as “Royalty overrides.” Royalty overrides are a significant operating expense and consist of:
Royalty overrides are compensation to Members for the development, retention and improved productivity of their sales organizations and are paid to several levels of Members on each sale. Royalty overrides are compensation for services rendered to us and, as such, are recorded as an operating expense.
49
In China, our independent service providers are compensated for marketing, sales support, and other services instead of the distributor allowances and royalty overrides utilized in our global Marketing Plan. Service fees to China independent service providers are included in selling, general, and administrative expenses.
Because of local country regulatory constraints, we may be required to modify our Member incentive plans as described above. We also pay reduced royalty overrides with respect to certain products worldwide. Consequently, the total Royalty override percentage may vary over time.
Our “contribution margins” consist of net sales less cost of sales and Royalty overrides.
“Selling, general, and administrative expenses” represent our operating expenses, which include labor and benefits, service fees to China independent service providers, sales events, professional fees, travel and entertainment, Member promotions, occupancy costs, communication costs, bank fees, depreciation and amortization, foreign exchange gains and losses, and other miscellaneous operating expenses.
Our “other operating income” consists of government grant income related to China and the finalization of insurance recoveries in connection with the flooding at one of our warehouses in Mexico during September 2017.
Our “other expense (income), net” consists of non-operating income and expenses such as gains or losses on extinguishment of debt and gains or losses due to subsequent changes in the fair value of the non-transferable contractual contingent value right, or CVR, provided for each share tendered in the October 2017 modified Dutch auction tender offer. See Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for further information on the CVR.
Most of our sales to Members outside the United States are made in the respective local currencies. In preparing our financial statements, we translate revenues into U.S. dollars using average exchange rates. Additionally, the majority of our purchases from our suppliers generally are made in U.S. dollars. Consequently, a strengthening of the U.S. dollar versus a foreign currency can have a negative impact on our reported sales and contribution margins and can generate foreign currency losses on intercompany transactions. Foreign currency exchange rates can fluctuate significantly. From time to time, we enter into foreign currency derivatives to partially mitigate our foreign currency exchange risk as discussed in further detail in Part II, Item 7A, Quantitative and Qualitative Disclosures about Market Risk, of this Annual Report on Form 10-K.
Results of Operations
Our results of operations for the periods below are not necessarily indicative of results of operations for future periods, which depend upon numerous factors, including our ability to sponsor Members and retain sales leaders, further penetrate existing markets, introduce new products and programs that will help our Members increase their retail efforts and develop niche market segments.
The following table sets forth selected results of our operations expressed as a percentage of net sales for the periods indicated:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
Operations: |
|
|
|
|
|
|
|
|
|
|||
Net sales |
|
|
100.0 |
% |
|
|
100.0 |
% |
|
|
100.0 |
% |
Cost of sales |
|
|
21.4 |
|
|
|
20.8 |
|
|
|
19.6 |
|
Gross profit |
|
|
78.6 |
|
|
|
79.2 |
|
|
|
80.4 |
|
Royalty overrides(1) |
|
|
31.6 |
|
|
|
30.5 |
|
|
|
29.7 |
|
Selling, general, and administrative expenses(1) |
|
|
34.6 |
|
|
|
37.4 |
|
|
|
39.8 |
|
Other operating income |
|
|
(0.3 |
) |
|
|
(0.3 |
) |
|
|
(0.8 |
) |
Operating income |
|
|
12.7 |
|
|
|
11.6 |
|
|
|
11.7 |
|
Interest expense |
|
|
2.7 |
|
|
|
2.5 |
|
|
|
3.1 |
|
Interest income |
|
|
0.1 |
|
|
|
0.2 |
|
|
|
0.4 |
|
Other expense (income), net |
|
|
0.4 |
|
|
|
— |
|
|
|
(0.3 |
) |
Income before income taxes |
|
|
9.7 |
|
|
|
9.3 |
|
|
|
9.3 |
|
Income taxes |
|
|
2.0 |
|
|
|
2.6 |
|
|
|
2.9 |
|
Net income |
|
|
7.7 |
% |
|
|
6.7 |
% |
|
|
6.4 |
% |
(1) Service fees to our independent service providers in China are included in selling, general, and administrative expenses while Member compensation for all other countries is included in Royalty overrides.
50
Changes in net sales are directly associated with the retailing of our products, recruitment of new Members, and retention of sales leaders. Our strategies involve providing quality products, improved DMOs, including daily consumption approaches such as Nutrition Clubs, easier access to product, systemized training and education of Members on our products and methods, leveraging technology to make it easier for our Members to do business, and continued promotion and branding of Herbalife Nutrition products.
Management’s role, in-country and at the region and corporate level, is to provide Members with a competitive, broad, and innovative product line, offer leading-edge business tools and technology services, and encourage strong teamwork and Member leadership to make doing business with Herbalife Nutrition simple. We have provided to our Members enhanced technology tools for ordering, business performance, and customer retailing to make it easier for them to do business with us and to optimize their customers’ experiences. Management uses the Marketing Plan, which reflects the rules for our global network marketing organization that specify the qualification requirements and general compensation structure for Members, coupled with educational and motivational programs and promotions to encourage Members to increase retailing, retention, and recruiting, which in turn affect net sales. Such programs include sales events such as Extravaganzas, Leadership Development Weekends and World Team Schools where large groups of Members network with other Members, learn retailing, retention, and recruiting techniques from our leading Members, and become more familiar with how to market and sell our products and business opportunities. Accordingly, management believes that these development and motivation programs increase the productivity of the sales leader network. The expenses for such programs are included in selling, general, and administrative expenses. We also use event and non-event product promotions to motivate Members to increase retailing, retention, and recruiting activities. These promotions have prizes ranging from qualifying for events to product prizes and vacations. A program that we have seen success with in many markets is the Member Activation Program, under which new Members, who order a modest number of Volume Points in each of their first three months, earn a prize. Our objective is to improve the quality of sales leaders by encouraging new Members to begin acquiring retail customers before attempting to qualify for sales leader status. Additionally, in a number of markets we have segmented our Member base into “preferred members” and “distributors” for more targeted and efficient communication and promotions for these two differently motivated types of Members. In certain other markets that have not been segmented, we have begun using Member data to similarly categorize Members for communication and promotion efforts.
DMOs are being generated in many of our markets and are globalized where applicable through the combined efforts of Members and country, regional and corporate management. While we support a number of different DMOs, one of the most popular DMOs is the daily consumption DMO. Under our traditional DMO, a Member typically sells to its customers on a somewhat infrequent basis (e.g., monthly) which provides fewer opportunities for interaction with their customers. Under a daily consumption DMO, a Member interacts with its customers on a more frequent basis, including such activities as weekly weigh-ins, which enables the Member to better educate and advise customers about nutrition and the proper use of the products and helps promote daily usage as well, thereby helping the Member grow his or her business. Specific examples of globalized DMOs include the Nutrition Club concept in Mexico, the Healthy Breakfast concept in Russia, and the Weight Loss Challenge in the United States. Management’s strategy is to review the applicability of expanding successful country initiatives throughout a region, and where appropriate, support the globalization of these initiatives.
As discussed further by market in the Sales by Geographic Region below, the Company has responded to COVID-19 pandemic conditions by adapting how it communicates with, services, and transacts with our Members and our Members have similarly adapted their DMOs and other activities. These responsive actions have varied by region and by market due to the differing market- and regional-specific impacts of the pandemic and the conditions and challenges unique to a particular market or region independent of the impacts of the pandemic. The factors described above help Members increase their business, which in turn helps drive Volume Point growth in our business, and thus, net sales growth. The discussion below of net sales details some of the specific drivers of changes in our business and causes of sales fluctuations during the year ended December 31, 2021 as compared to the same period in 2020, as well as the unique growth or contraction factors specific to certain geographic regions or significant markets within a region during these periods. Net sales fluctuations, both Company-wide and within a particular geographic region or market, are primarily the result of changes in volume, changes in prices, or changes in foreign currency translation rates. The discussion of changes in net sales quantifies the impact of those drivers that are quantifiable such as changes in foreign currency translation rates, and cites the estimated impact of any significant price changes. The remaining drivers, which management believes are the primary drivers of changes in volume, are typically qualitative factors whose impact cannot be quantified. We use Volume Points as an indication for changes in sales volume.
We expect the impact of the COVID-19 pandemic to impact our results of operations in future quarters and their comparability to prior periods, both on a consolidated basis and at the regional level. However, given the unpredictable, unprecedented, and fluid nature of the pandemic and its economic consequences, we are unable to predict the extent to which the pandemic and its related impacts will adversely impact our business, financial condition, and results of operations, including the impact it may have on our regions and individual markets. See the Sales by Geographic Region below for a more detailed discussion of the pandemic’s impact on net sales for the year for each geographic region and individual market.
51
Financial Results for the Year Ended December 31, 2021 Compared to the Year Ended December 31, 2020
Net sales were $5,802.8 million for the year ended December 31, 2021. Net sales increased $261.0 million, or 4.7% ($260.8 million, or 4.7% excluding Venezuela), for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 3.3% (2.9% excluding Venezuela) for the year ended December 31, 2021 as compared to the same period in 2020. The 4.7% increase in net sales for the year ended December 31, 2021 was primarily driven by a 2.9% favorable impact of price increases (2.5% favorable impact excluding Venezuela), an increase in sales volume, as indicated by a 2.5% increase in Volume Points, and a 1.4% favorable impact of fluctuations in foreign currency rates (1.9% favorable impact excluding Venezuela), partially offset by a 2.6% unfavorable impact of country sales mix.
Net income was $447.2 million, or $4.13 per diluted share, for the year ended December 31, 2021. Net income increased $74.6 million, or 20.0%, for the year ended December 31, 2021 as compared to the same period in 2020. The increase in net income for the year ended December 31, 2021 was mainly due to $62.9 million lower selling, general, and administrative expenses primarily driven by $103.9 million in lower service fees for China independent service providers due to lower sales in China and $83.1 million of expenses relating to the SEC and DOJ investigations relating to the FCPA matter in China in 2020 (See Selling General, and Administrative Expenses below and Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K), $30.2 million lower income taxes, and $28.7 million higher contribution margin driven by higher net sales, partially offset by a $24.6 million loss on extinguishment of our 2026 Notes (See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K) and $24.5 million higher net interest expense.
Net income for the year ended December 31, 2021 included a $24.6 million pre-tax unfavorable impact ($19.1 million post-tax) of loss on extinguishment of our 2026 Notes; a $23.7 million unfavorable impact of non-cash interest expense related to the 2024 Convertible Notes (See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K); a $13.8 million pre-tax unfavorable impact ($11.2 million post-tax) from expenses related to the COVID-19 pandemic, and such expenses are expected to continue in future periods; a $12.9 million pre-tax unfavorable impact ($11.5 million post-tax) of transformation initiative expenses, primarily relating to professional fees; a $12.5 million pre-tax unfavorable impact ($9.6 million post-tax) of expenses relating to the class action lawsuit titled Rodgers, et al. v Herbalife Ltd., et al. (See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K); a $7.4 million pre-tax favorable impact ($5.6 million post-tax) of net benefit on non-income tax items; and a $1.7 million pre-tax unfavorable impact ($1.3 million post-tax) of debt issuance costs related to the amendment of our 2018 Credit Facility.
Net income for the year ended December 31, 2020 included a $85.9 million unfavorable impact ($85.4 million post-tax) from expenses related to regulatory inquiries and a legal accrual, which includes $83.1 million of expenses relating to the SEC and DOJ investigations relating to the FCPA matter in China; a $21.8 million unfavorable impact of non-cash interest expense related to the 2024 Convertible Notes; a $21.2 million pre-tax unfavorable impact ($19.2 million post-tax) from expenses related to the COVID-19 pandemic; and a $0.5 million pre-tax unfavorable impact ($0.4 million post-tax) of debt issuance costs related to the amendment of our 2018 Credit Facility.
Reporting Segment Results
We aggregate our operating segments, excluding China, into a reporting segment, or the Primary Reporting Segment. The Primary Reporting Segment includes the North America, Mexico, South and Central America, EMEA, and Asia Pacific regions. China has been identified as a separate reporting segment as it does not meet the criteria for aggregation. See Note 10, Segment Information, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for further discussion of our reporting segments. See below for discussions of net sales and contribution margin by our reporting segments.
Net Sales by Reporting Segment
The Primary Reporting Segment reported net sales of $5,173.3 million for the year ended December 31, 2021, representing an increase of $441.1 million, or 9.3% ($441.0 million, or 9.3% excluding Venezuela), as compared to the same period in 2020. In local currency, net sales increased 8.5% (8.0% excluding Venezuela) for the year ended December 31, 2021 as compared to the same period in 2020. The 9.3% increase in net sales for the year ended December 31, 2021 was primarily due to an increase in sales volume, as indicated by a 5.0% increase in Volume Points, a 3.4% favorable impact of price increases (2.9% favorable impact excluding Venezuela), and a 0.8% favorable impact of fluctuations in foreign currency exchange rates (1.3% favorable impact excluding Venezuela).
52
For a discussion of China’s net sales for the year ended December 31, 2021 as compared to the same period in 2020, see the China section of Sales by Geographic Region below.
Contribution Margin by Reporting Segment
As discussed above under “Presentation,” contribution margin consists of net sales less cost of sales and Royalty overrides.
The Primary Reporting Segment reported contribution margin of $2,175.6 million, or 42.1% of net sales, for the year ended December 31, 2021, representing an increase of $192.0 million, or 9.7% ($191.4 million, or 9.6% excluding Venezuela), as compared to the same period in 2020. The 9.7% increase in contribution margin for the year ended December 31, 2021 was primarily the result of a 5.5% favorable impact of price increases (4.7% favorable impact excluding Venezuela) and a 5.0% favorable impact of volume increases.
China reported contribution margin of $554.2 million for the year ended December 31, 2021, representing a decrease of $163.3 million, or 22.8%, as compared to the same period in 2020. The 22.8% decrease in contribution margin for the year ended December 31, 2021 was primarily the result of a 28.3% unfavorable impact of volume decreases, partially offset by a 5.5% favorable impact of fluctuations in foreign currency exchange rates.
Sales by Geographic Region
Net sales by geographic region were as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
% Change |
|
|||
|
|
(Dollars in millions) |
|
|||||||||
North America |
|
$ |
1,428.9 |
|
|
$ |
1,372.9 |
|
|
|
4.1 |
% |
Mexico |
|
|
463.7 |
|
|
|
436.9 |
|
|
|
6.1 |
% |
South and Central America |
|
|
359.2 |
|
|
|
366.4 |
|
|
|
(2.0 |
)% |
EMEA |
|
|
1,335.4 |
|
|
|
1,208.3 |
|
|
|
10.5 |
% |
Asia Pacific |
|
|
1,586.1 |
|
|
|
1,347.7 |
|
|
|
17.7 |
% |
China |
|
|
629.5 |
|
|
|
809.6 |
|
|
|
(22.2 |
)% |
Worldwide |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
|
4.7 |
% |
North America
The North America region reported net sales of $1,428.9 million for the year ended December 31, 2021. Net sales increased $56.0 million, or 4.1%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 3.9% for the year ended December 31, 2021 as compared to the same period in 2020. The 4.1% increase in net sales for the year ended December 31, 2021 was primarily due to an increase in sales volume, as indicated by a 2.8% increase in Volume Points, and a 1.3% favorable impact of price increases.
Net sales in the U.S. were $1,386.7 million for the year ended December 31, 2021. Net sales increased $52.2 million, or 3.9%, for the year ended December 31, 2021 as compared to the same period in 2020.
The region has seen growth in recent years, supported by product line expansion and deployment of enhanced technology tools and social media to support our distributors’ businesses and optimize their customers’ experiences with Herbalife Nutrition. The number of active Nutrition Clubs in the region has continued to grow and the Nutrition Club DMO is a focus area for training and technological support of our Members. Our communications, promotions, and other operations in the region are targeted to our distributors, or their preferred members or retail customers as appropriate. Our promotional program is designed to encourage consistency and sustainability in our Members’ businesses. Additionally, we believe that pandemic conditions may have been a contributing factor in the motivation and focus of our Members during the second half of 2020 and the first half of 2021, contributing to year-over-year sales volume increases for the first half of 2021. The second half of 2021 saw a sales volume decline. We believe that contributing factors to the second half decline, besides comparison to the second half of 2020 that saw a record level of sales, include fewer new distributors and preferred customers joining our business in 2021. Higher sales in the Energy, Sports, and Fitness product category contributed to sales volume for the year.
53
In response to the pandemic conditions, product distribution to our Members was temporarily altered during 2020 to allow online and phone-in orders only. Currently, two of our three major U.S. distribution centers are shipping product only, with no in-person pick-ups permitted, and our sales centers are for pick-up only, with no orders taken on-site; however, our Members’ ability to obtain product has not materially decreased. Our access points, including distribution centers and sales centers, generally allow in-person pick-up orders, with exceptions as local conditions warrant; however, our access points generally continue to not allow in-person orders. Members’ Nutrition Clubs, which represent a major DMO for the region, are operating in some areas as pick-up points for product only, and returning to traditional on-site consumption approach as local conditions allow. Nutrition Club sales volume increased for 2021 versus 2020, including the impact of home deliveries from Nutrition Clubs to their customers, an approach that has seen increased use as a response to the pandemic. Our Member training and promotion events, such as our Success Training Seminars and our Leadership Development Weekends, have shifted to include “virtual” online approaches, with in-person events reinstituted during the fourth quarter of 2021 and continuing on a case-by-case basis as conditions allow. Promotional activities aimed at our Members continue, though prizes that have involved travel to events have shifted in some cases to cash and other awards. Certain modified practices by us and our Members may prove to be lasting improvements, such as events and trainings that are offered virtually as well as in-person and expanded use of social media channels.
Mexico
The Mexico region reported net sales of $463.7 million for the year ended December 31, 2021. Net sales increased $26.8 million, or 6.1%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 0.7% for the year ended December 31, 2021 as compared to the same period in 2020. The 6.1% increase in net sales for the year ended December 31, 2021 was primarily due to a 5.4% favorable impact of fluctuations in foreign currency exchange rates and a 3.9% favorable impact of price increases, partially offset by a decrease in sales volume, as indicated by a 3.3% decrease in Volume Points.
Mexico has faced ongoing difficult economic conditions, as well as the adverse impact of intermittent pandemic-related constraints, particularly on Nutrition Club operations which are important to the market. Although nearly all product access points in Mexico, both Company-operated and third party, have remained open, in some areas Nutrition Clubs are operating under restrictions such as for product pick-up only. We have seen some decline in new Members during 2021 attributable in part, we believe, to Members adapting to Member segmentation introduced in March 2021 and to pandemic conditions constraining Members’ ability to hold in-person meetings with customers.
South and Central America
The South and Central America region reported net sales of $359.2 million for the year ended December 31, 2021. Net sales decreased $7.2 million, or 2.0% ($7.3 million, or 2.0% excluding Venezuela), for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 8.9% (2.5% excluding Venezuela) for the year ended December 31, 2021 as compared to the same period in 2020. The 2.0% decrease in net sales for the year ended December 31, 2021 was due to a 10.9% unfavorable impact of fluctuations in foreign currency exchange rates (4.5% unfavorable impact excluding Venezuela) and a decrease in sales volume, as indicated by a 7.0% decrease in Volume Points, partially offset by an 11.2% favorable impact of price increases (5.0% favorable impact excluding Venezuela) and a 3.5% favorable impact of sales mix.
Most markets in the region saw volume declines for 2021, particularly in the second half of the year, due in part, we believe, to the intermittent operating constraints and adverse macroeconomic impacts of the COVID-19 pandemic. Sales volume declines were greatest in Brazil, our largest market in the region; sales volume increases were led by Chile.
Pandemic impacts have varied by market across the region, but have at times included product shipping delays and suspension of product access points and Members’ Nutrition Clubs, requiring reliance on shipping product to Members’ and customers’ homes. More broadly, markets across the region are focused on building more sustainable business for our Members through daily product consumption and retailing. Efforts within the region include leveraging social media, utilizing cash prize promotions, and using the weight management challenge DMO. The region has introduced Member segmentation in most markets, adding preferred customer program options for our Members, and is leveraging this segmentation in marketing and promotions.
54
Net sales in Brazil were $64.6 million for the year ended December 31, 2021. Net sales decreased $19.6 million, or 23.3%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales decreased 18.3% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had an unfavorable impact of $4.1 million on net sales for the year ended December 31, 2021. Sales volumes declined in the market for the year versus the prior year. COVID-19 pandemic conditions and their macroeconomic impact continue in the market and have constrained our business and that of our Members, particularly the important Nutrition Club DMO. Although Members’ Nutrition Clubs are currently operating, we have seen declines in both the number of Clubs and their customer traffic. Home delivery is operating and other product access points are open for pick-up. More broadly, the market has seen some years of negative momentum, though we continue to support our Members with innovative measures, such as the Preferred Member program, and work with Member leadership to identify best practices for replication within the market.
Net sales in Peru were $59.6 million for the year ended December 31, 2021. Net sales decreased $2.1 million, or 3.4%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 7.1% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had an unfavorable impact of $6.4 million on net sales for the year ended December 31, 2021. Sales volume increased slightly for the year, attributable in part to comparison to a 2020 year period weakened by the severe initial stages of pandemic conditions in the market. Sales volume for the second half of 2021 declined year-over-year. The market continues to experience intermittent disruptions due to COVID-19 pandemic conditions, and we have adapted our business with steps including taking orders by Internet and phone and shipping product to Member homes. Members’ Nutrition Clubs were also modified for home delivery only or are open for partial operation. The market has seen Members’ leveraging social media and using the weight management challenge DMO.
EMEA
The EMEA region reported net sales of $1,335.4 million for the year ended December 31, 2021. Net sales increased $127.1 million, or 10.5%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 8.1% for the year ended December 31, 2021 as compared to the same period in 2020. The 10.5% increase in net sales for the year ended December 31, 2021 was primarily due to an increase in sales volume, as indicated by a 4.3% increase in Volume Points, a 3.3% favorable impact of price increases, and a 2.4% favorable impact of fluctuations in foreign currency exchange rates.
The Volume Point growth that has been seen across the EMEA region for a number of years reflects, we believe, efforts to enhance the quality and activity of sales leaders including Member training, brand awareness, and product line expansion, as well as enhanced technology tools for ordering, business performance, and customer retailing. Also, we believe that pandemic conditions may have been a contributing factor in the motivation and focus of our Members in certain markets of the region during the second half of 2020 and the first half of 2021. The sales volume increase for the full year of 2021 included a year-over-year sales volume decline for the second half of the year after year-over-year growth for the first half. Nearly all markets in the region saw second-half year-over-year sales volume trend performance below that of the first half, including second half declines after first half growth for Spain, Italy, and a number of other markets. Year-over-year declines for the second half include, we believe, the effect of comparison to the second half of 2020 that saw record levels of sales and fewer new distributors and preferred customers joining our business in 2021. Significant sources of growth for the full year included Italy, Spain, and Turkey; declines were most significant for South Africa.
Due to COVID-19 pandemic conditions, our sales centers and other product access points in certain markets within the region have at times been closed or open for limited operations only, leaving shipping for home delivery as the primary distribution channel while those conditions persist. Members are turning further to social media to carry out their sales and oversight activities. Although we and our Members have begun to reinstitute face-to-face approaches as conditions allow, we believe these adaptations have been successful in limiting the adverse impact of the pandemic.
Net sales in Spain were $187.0 million for the year ended December 31, 2021. Net sales increased $15.0 million, or 8.7%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 4.3% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $7.7 million on net sales for the year ended December 31, 2021. In recent years, Spain has generally seen sales volume increases as it benefited from programs of promotions and sponsorships that have raised brand awareness through healthy active lifestyle and contributed to broad-based success across Member sales organizations in the market. After the first quarter of 2020 saw a small sales volume decline due to pandemic disruption, subsequent quarters saw volume increases as our Members continued to adapt to pandemic conditions, such as leveraging online tools for meetings, trainings, and selling activities. The second half of 2021 saw a sales volume decrease year-over-year as the second half of 2020 reflected significant strength and fewer new distributors and preferred customers joined our business in 2021. In response to pandemic conditions, we have temporarily shifted our Member support operations to primarily online activities, as well as continuing normal home delivery.
55
Net sales in Italy were $164.1 million for the year ended December 31, 2021. Net sales increased $22.2 million, or 15.7%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 10.8% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $6.8 million on net sales for the year ended December 31, 2021. Sales volume increased for the year versus the prior year. After weakened performance in our business and pandemic conditions in the country contributed to a sales volume decline for the first quarter of 2020, we believe adaptation by Members to pandemic conditions, such as online communication with Members and home delivery, as well as heightened demand for our products and business opportunity, have been contributing factors to sales volume increases. The second half of 2021 saw a sales volume decrease year-over-year as the second half of 2020 reflected significant strength and fewer new distributors and preferred customers joined our business in 2021.
Net sales in Russia were $144.1 million for the year ended December 31, 2021. Net sales decreased $3.1 million, or 2.1%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales remained flat for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had an unfavorable impact of $3.1 million on net sales for the year ended December 31, 2021. Russia saw a slight sales volume decline for 2021 versus the prior year. Members’ Nutrition Clubs, a key DMO for the market, are operating primarily online due to pandemic conditions, an approach that Members are taking time to adapt to, as supported by Herbalife Nutrition with new products, training, and promotion for all levels of Membership. Our sales centers are open for product pick-up, although we continue to support home delivery for the market. Product access expansion, such as same-day express delivery in many areas has enabled growth in smaller cities. During the third quarter of 2020, we introduced Member segmentation to the market by adding a preferred customer program option, which has been a significant source of new Members. Russia had a 4% price increase in September 2021 and a 5% price increase in September 2020.
Asia Pacific
The Asia Pacific region, which excludes China, reported net sales of $1,586.1 million for the year ended December 31, 2021. Net sales increased $238.4 million, or 17.7%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 16.1% for the year ended December 31, 2021 as compared to the same period in 2020. The 17.7% increase in net sales for the year ended December 31, 2021 was primarily due to an increase in sales volume, as indicated by a 16.0% increase in Volume Points, a 3.3% favorable impact of price increases, and a 1.6% favorable impact of fluctuations in foreign currency exchange rates, partially offset by a 3.6% unfavorable impact of sales mix.
Volume Point and net sales increases in recent years for most markets in the region are a result, we believe, of a customer-focused business, daily consumption DMOs including Nutrition Clubs, and ongoing product line expansion. COVID-19 pandemic conditions, such as closed sales centers and operating constraints on Members’ Nutrition Clubs, have had an intermittent adverse impact on results. The volume results for 2021 were mixed across the markets of the region. Volume increases for the year versus the prior year were led by India and Vietnam; volume declines were greatest for Indonesia.
Net sales in India were $519.1 million for the year ended December 31, 2021. Net sales increased $170.0 million, or 48.7%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 48.4% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $1.0 million on net sales for the year ended December 31, 2021. Sales volumes have increased in India in recent years as we continued to expand our product line and make it easier for our Members to do business, such as by adding product access points and payment methods, and introducing a customer-direct shipping capability for our Members. Additionally, we believe adaption by our Members to pandemic-related operating constraints, such as greater use of online marketing and training tools and online Nutrition Club operation, has broadened their geographic reach enabling them to expand their businesses.
Pandemic-related operating constraints continue to be intermittent in the market, as conditions evolve by area and Indian states institute constraints as warranted. We are increasing our manufacturing capacity to continue to meet demand. We continue to take Member orders and payments online. Company locations are now open for the taking of orders and payments and pick-up of product, though home delivery volumes continue to exceed pre-pandemic levels and home delivery is becoming an important distribution channel for the market. Disruption to our collections and expenditures of cash have eased, though most product ordering and payment are now online for Member convenience.
Regulatory restrictions on direct selling in India, including registration requirements for our distributors, have reduced the number of new distributors. However, we have seen a significant increase in new Preferred Members, which do not have similar registration requirements, as distributors encourage the Preferred Membership pathway for their customers.
56
Net sales in Vietnam were $272.6 million for the year ended December 31, 2021. Net sales increased $60.0 million, or 28.2%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 26.6% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $3.4 million on net sales for the year ended December 31, 2021. Vietnam continues to see sales volume growth as sales leadership continues to focus on sustainable, consumption-oriented business practices and the number of product access points are increased. Our sales centers continue to be closed for in-person product orders or pick-up due to COVID-19, and other pandemic-related operating constraints, such as the closure of some Members’ Nutrition Clubs for in-person customer servicing, continue to be intermittent. These constraints contributed, we believe, to a year-over-year decline in volume for the fourth quarter of 2021. We and our Members have adapted to constraints by moving events, trainings, and product ordering online, as well as providing home delivery. Further changes to direct-selling regulations in the market are expected to receive government approval in early 2022. We continue to assess and monitor these proposed regulations.
Net sales in Indonesia were $163.2 million for the year ended December 31, 2021. Net sales decreased $18.0 million, or 9.9%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales decreased 11.3% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $2.4 million on net sales for the year ended December 31, 2021. The sales volume decline for the year versus the prior year is attributable in part, we believe, to ongoing pandemic conditions. These conditions include intermittent constraints on the operating hours and capacity of our product access points. Our sales centers have continued to operate via online ordering, home delivery, and pick-up, which were already established methods for the market. Many Members’ Nutrition Clubs, the major DMO for the market, have experienced pandemic-related constraints on their activities, including limitations on operating hours and capacity. Additionally, we have applied to comply with certain applicable licensing and filing requirements, subject to review by the government; we continue to monitor this review.
Net sales in South Korea were $136.7 million for the year ended December 31, 2021. Net sales increased $7.7 million, or 6.0%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales increased 2.8% for the year ended December 31, 2021 as compared to the same period in 2020. The fluctuation of foreign currency exchange rates had a favorable impact of $4.1 million on net sales for the year ended December 31, 2021. Sales volumes increased for the year versus the prior year as pandemic conditions in the market eased somewhat and social interactions such as visits to Members’ Nutrition Clubs increased, and as our Members continue to adapt to virtual approaches for their businesses. However, pandemic conditions continue to be intermittent with potential ongoing adverse impact on sales volumes, including suspension of our training facilities, safety guidelines for Nutrition Clubs operations, and restrictions on gatherings.
China
The China region reported net sales of $629.5 million for the year ended December 31, 2021. Net sales decreased $180.1 million, or 22.2%, for the year ended December 31, 2021 as compared to the same period in 2020. In local currency, net sales decreased 27.5% for the year ended December 31, 2021 as compared to the same period in 2020. The 22.2% decrease in net sales for the year ended December 31, 2021 was primarily due to a decrease in sales volume, as indicated by a 28.3% decrease in Volume Points, partially offset by a 5.3% favorable impact of fluctuations in foreign currency exchange rates.
The volume decline for the year was attributable, we believe, to several factors including changes we are making that we believe ultimately strengthen the consistency and sustainability of our business in China. In December 2020 we increased the requirements for our sales representatives in China to be eligible to apply to become independent service providers, with further modification during the third quarter of 2021. We believe these changes will ultimately strengthen our business by improving the quality of our independent service providers, but as our Members acclimate to these new requirements we have seen declines in the number of new independent service providers and net sales. Also, the frequency and attendance of our and our Members’ in-person training and sales meetings, which are important to the business as they are a central channel for attracting and retaining customers, providing personal and professional development for our Members, and promoting our products, were below the levels of prior years due to continuing intermittent effects of the COVID-19 pandemic and, earlier during 2020, due to residual effects of the Chinese government’s 100-day review of the health product industry, which concluded in April 2019.
Focus areas for China include enhancing our digital capabilities and offerings, such as improving the integration of our technological tools to make it easier for our Members to do business, and supporting our Members’ establishment of daily consumption-oriented Nutrition Clubs. We have expanded our product line for the China market and continue to conduct sales promotions in the region.
57
Sales by Product Category
Net sales by product category were as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
% Change |
|
|||
|
|
(Dollars in millions) |
|
|||||||||
Weight Management |
|
$ |
3,370.4 |
|
|
$ |
3,312.8 |
|
|
|
1.7 |
% |
Targeted Nutrition |
|
|
1,636.6 |
|
|
|
1,527.4 |
|
|
|
7.1 |
% |
Energy, Sports, and Fitness |
|
|
551.8 |
|
|
|
437.4 |
|
|
|
26.2 |
% |
Outer Nutrition |
|
|
107.8 |
|
|
|
111.3 |
|
|
|
(3.1 |
)% |
Literature, Promotional, and Other(1) |
|
|
136.2 |
|
|
|
152.9 |
|
|
|
(10.9 |
)% |
Total |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
|
4.7 |
% |
(1) Product buybacks and returns in all product categories are included in the Literature, Promotional, and Other category.
Net sales for the majority of product categories increased for the year ended December 31, 2021 as compared to the same period in 2020. The trends and business factors described in the above discussions of the individual geographic regions apply generally to all product categories. The Energy, Sports, and Fitness category saw a significant increase for the year ended December 31, 2021 as compared to the same period in 2020 based on strength in the North America region.
Gross Profit
Gross profit was $4,563.5 million and $4,391.2 million for the years ended December 31, 2021 and 2020, respectively. Gross profit as a percentage of net sales was 78.6% and 79.2% for the years ended December 31, 2021 and 2020, respectively, or an unfavorable net decrease of 59 basis points.
The decrease in gross profit as a percentage of net sales for the year ended December 31, 2021 as compared to the same period in 2020 included unfavorable changes in country mix of 52 basis points, unfavorable cost changes related to self-manufacturing and sourcing of 34 basis points primarily related to increased freight costs, the unfavorable impact of foreign currency fluctuations of 20 basis points (unfavorable impact of 11 basis points excluding Venezuela), and the unfavorable impact of higher inventory write-downs of 17 basis points, partially offset by the favorable impact of retail price increases of 61 basis points (favorable impact of 52 basis points excluding Venezuela). There was no net impact of foreign currency fluctuations and retail price increases in Venezuela for the year ended December 31, 2021 as compared to the same period in 2020.
We expect our gross margin to be negatively impacted in 2022 primarily due to increased costs related to raw materials, manufacturing labor costs, and freight costs.
Generally, gross profit as a percentage of net sales may vary from period to period due to the impact of foreign currency fluctuations, changes in country mix as volume changes among countries with varying margins, retail price increases, cost changes related to self-manufacturing and sourcing, and inventory write-downs.
Royalty Overrides
Royalty overrides were $1,833.7 million and $1,690.1 million for the years ended December 31, 2021 and 2020, respectively. Royalty overrides as a percentage of net sales were 31.6% and 30.5% for the years ended December 31, 2021 and 2020, respectively.
The increase in royalty overrides as a percentage of net sales for the year ended December 31, 2021 as compared to the same period in 2020 was primarily due to lower net sales in China as a proportion of our total worldwide net sales. Service fees to our independent service providers in China are included in selling, general, and administrative expenses while Member compensation for all other countries is included in Royalty overrides.
Generally, Royalty overrides as a percentage of net sales may vary from period to period due to changes in the mix of products and countries because full royalty overrides are not paid on certain products and in certain countries.
58
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses were $2,012.1 million and $2,075.0 million for the years ended December 31, 2021 and 2020, respectively. Selling, general, and administrative expenses as a percentage of net sales were 34.6% and 37.4% for the years ended December 31, 2021 and 2020, respectively.
The decrease in selling, general, and administrative expenses for the year ended December 31, 2021 as compared to the same period in 2020 was driven by $103.9 million in lower service fees for China independent service providers due to lower sales in China and $83.1 million of expenses relating to the SEC and DOJ investigations relating to the FCPA matter in China in 2020 (See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K), partially offset by $62.7 million in higher labor and benefits costs, $30.6 million in higher professional fees, $19.5 million in higher Member event and promotion costs, and $12.5 million of expenses relating to the class action lawsuit titled Rodgers, et al. v Herbalife Ltd., et al. (See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K).
Other Operating Income
The $16.4 million of other operating income for the year ended December 31, 2021 consisted of $16.4 million of government grant income for China (See Note 2, Basis of Presentation, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K).
The $14.5 million of other operating income for the year ended December 31, 2020 consisted of $14.5 million of government grant income for China.
Interest Expense, Net
Interest expense, net is as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Interest expense |
|
$ |
153.1 |
|
|
$ |
133.0 |
|
Interest income |
|
|
(4.4 |
) |
|
|
(8.8 |
) |
Interest expense, net |
|
$ |
148.7 |
|
|
$ |
124.2 |
|
The increase in interest expense, net for the year ended December 31, 2021 as compared to the same period in 2020 was primarily due to an increase in our overall weighted-average borrowings, partially offset by a decrease in our overall weighted-average interest rate.
Other Expense (Income), Net
The $24.6 million of other expense, net for the year ended December 31, 2021 consisted of a loss on the extinguishment of the 2026 Notes (See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K).
We did not recognize any other expense (income), net for the year ended December 31, 2020.
Income Taxes
Income taxes were $113.6 million and $143.8 million for the years ended December 31, 2021 and 2020, respectively. The effective income tax rate was 20.3% and 27.8% for the years ended December 31, 2021 and 2020, respectively. The decrease in the effective tax rate for the year ended December 31, 2021 as compared to the same period in 2020 was primarily due to changes in the geographic mix of our income and an increase in net tax benefits from discrete events.
59
Liquidity and Capital Resources
We have historically met our short- and long-term working capital and capital expenditure requirements, including funding for expansion of operations, through net cash flows provided by operating activities. Variations in sales of our products directly affect the availability of funds. There are no material contractual restrictions on our ability to transfer and remit funds among our international affiliated companies. However, there are foreign currency restrictions in certain countries which could reduce our ability to timely obtain U.S. dollars. Even with these restrictions and the impacts of the COVID-19 pandemic, we believe we will have sufficient resources, including cash flow from operating activities and access to capital markets, to meet debt service obligations in a timely manner and be able to continue to meet our objectives.
Historically, our debt has not resulted from the need to fund our normal operations, but instead has resulted primarily from our share repurchase programs. Since inception in 2007, total share repurchases amounted to approximately $6.4 billion. While a significant net sales decline could potentially affect the availability of funds, many of our largest expenses are variable in nature, which we believe protects our funding in all but a dramatic net sales downturn. Our $601.5 million cash and cash equivalents as of December 31, 2021 and our senior secured credit facility, in addition to cash flow from operations, can be used to support general corporate purposes, including any future share repurchases, dividends, and strategic investment opportunities.
We have a cash pooling arrangement with a financial institution for cash management purposes. This cash pooling arrangement allows certain of our participating subsidiaries to withdraw cash from this financial institution based upon our aggregate cash deposits held by subsidiaries who participate in the cash pooling arrangement. We did not owe any amounts to this financial institution under the pooling arrangement as of December 31, 2021 and 2020.
For the year ended December 31, 2021, we generated $460.3 million of operating cash flow as compared to $628.6 million for the same period in 2020. The decrease in our operating cash flow was the result of $258.5 million of unfavorable changes in operating assets and liabilities, partially offset by $90.2 million of higher net income excluding non-cash and reconciling items disclosed within our consolidated statement of cash flows. The $258.5 million change in operating assets and liabilities was primarily the result of unfavorable changes in inventories, prepaid expenses and other current assets, royalty overrides, and other current liabilities, partially offset by a favorable change in receivables. The unfavorable change in other current liabilities included unfavorable changes in accrued compensation, accrued interest, accrued member events and promotions, and settlement of Mexico VAT assessments, partially offset by a favorable impact in 2021 due to the prior-year settlement of the SEC and DOJ investigations relating to the FCPA matter in China. The $90.2 million of higher net income excluding non-cash and reconciling items was primarily driven by higher contribution margin driven by higher net sales (See Financial Results for the Year Ended December 31, 2021 Compared to the Year Ended December 31, 2020 above for further discussion) and lower selling, general, and administrative expenses (See Selling, General, and Administrative Expenses above for further discussion).
Capital expenditures, including accrued capital expenditures, for the years ended December 31, 2021 and 2020 were $159.1 million and $116.8 million, respectively. The majority of these expenditures represented investments in management information systems, including initiatives to develop web-based Member tools, as well as expansion and enhancement of our manufacturing and distribution facilities. We expect to continue our investments in these areas and expect to incur total capital expenditures of approximately $175 million to $225 million for the full year of 2022.
In March 2021, we hosted our annual global Herbalife Honors event virtually where sales leaders from around the world met, shared best practices, and conducted leadership training, and our management awarded Members $81.1 million of Mark Hughes bonus payments related to their 2020 performance. In March 2020, our management awarded Members $71.3 million of Mark Hughes bonus payments related to their 2019 performance.
In the fourth quarter of 2021, we initiated a global transformation program to optimize global processes for future growth, or the Transformation Program. The Transformation Program involves the investment in certain new technologies and the realignment of infrastructure and the locations of certain functions to better support distributors and customers. For the first phase of the Transformation Program, we currently expect total pre-tax expenses in the range of $25 million to $30 million through 2023, of which $12.9 million was recognized in selling, general, and administrative expenses within our consolidated statement of income during the year ended December 31, 2021. In addition, we expect a total of $11 million to $14 million of related capital expenditures through 2023, primarily relating to technology, to support both the first and second phase of the Transformation Program. In addition to better aligning our internal resources to better effect regional and market level strategies, the first phase of the Transformation Program is expected to deliver annual incremental savings in the range of $10 million to $15 million, with some savings beginning in 2022 and full-year savings expected to be realized in 2024. We are still assessing the scope, timing, and execution plan of the second phase of the Transformation Program.
60
Senior Secured Credit Facility
On August 16, 2018, we entered into a $1.25 billion senior secured credit facility, or the 2018 Credit Facility, consisting of a $250.0 million term loan A, or the 2018 Term Loan A, a $750.0 million term loan B, or the 2018 Term Loan B, and a $250.0 million revolving credit facility, or the 2018 Revolving Credit Facility, with a syndicate of financial institutions as lenders. The 2018 Term Loan B matures upon the earlier of: (i) August 18, 2025, or (ii) December 15, 2023 if the outstanding principal on the 2024 Convertible Notes, as defined below, exceeds $350.0 million and we exceed certain leverage ratios as of that date. All obligations under the 2018 Credit Facility are unconditionally guaranteed by certain direct and indirect wholly-owned subsidiaries of Herbalife Nutrition Ltd. and secured by the equity interests of certain of Herbalife Nutrition Ltd.’s subsidiaries and substantially all of the assets of the domestic loan parties. Also on August 16, 2018, we issued $400.0 million aggregate principal amount of senior unsecured notes, or the 2026 Notes as described below, and used the proceeds from the 2018 Credit Facility and the 2026 Notes to repay in full the $1,178.1 million outstanding under our prior senior secured credit facility.
On December 12, 2019, we amended the 2018 Credit Facility which, among other things, reduced the interest rate for borrowings under the 2018 Term Loan B. We incurred approximately $1.2 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC Topic 470, Debt, or ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. The debt issuance costs were recognized in interest expense within our consolidated statement of income during the fourth quarter of 2019.
On March 19, 2020, we amended the 2018 Credit Facility which, among other things, extended the maturity of both the 2018 Term Loan A and 2018 Revolving Credit Facility to the earlier of: (i) March 19, 2025 or (ii) September 15, 2023 if the outstanding principal on the 2024 Convertible Notes, as defined below, exceeds $350.0 million and we exceed certain leverage ratios as of that date; increased borrowings under the 2018 Term Loan A from $234.4 million to a total of $264.8 million; increased the total available borrowing capacity under 2018 Revolving Credit Facility from $250.0 million to $282.5 million; and reduced the interest rate for borrowings under both the 2018 Term Loan A and 2018 Revolving Credit Facility. We incurred approximately $1.6 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. Of the $1.6 million of debt issuance costs, approximately $1.1 million was recorded on our consolidated balance sheet and is being amortized over the life of the 2018 Credit Facility using the effective-interest method, and approximately $0.5 million was recognized in interest expense within our consolidated statement of income during the first quarter of 2020.
On February 10, 2021, we amended the 2018 Credit Facility which, among other things, reduced the interest rate for borrowings under the 2018 Term Loan B. We incurred approximately $1.1 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. The debt issuance costs were recognized in interest expense within our consolidated statement of income during the first quarter of 2021.
On July 30, 2021, we amended the 2018 Credit Facility which, among other things, increased borrowings under the 2018 Term Loan A from $245.0 million to a total of $286.2 million; increased the total available borrowing capacity under the 2018 Revolving Credit Facility from $282.5 million to $330.0 million; reduced the interest rate for borrowings under the 2018 Term Loan A and 2018 Revolving Credit Facility; and amended the commitment fee on the undrawn portion of the 2018 Revolving Credit Facility. As a result of the amendment, the applicable margin for the 2018 Term Loan A and 2018 Revolving Credit Facility may also be subject to certain premiums or discounts tied to criteria determined by certain sustainability targets. We incurred approximately $1.4 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. Of the $1.4 million of debt issuance costs, approximately $0.8 million was recorded on our consolidated balance sheet and is being amortized over the life of the 2018 Credit Facility using the effective-interest method, and approximately $0.6 million was recognized in interest expense within our consolidated statement of income during the third quarter of 2021.
The 2018 Credit Facility requires us to comply with a leverage ratio. The 2018 Credit Facility also contains affirmative and negative covenants customary for financings of this type, including, among other things, limitations or prohibitions on repurchasing common shares, declaring and paying dividends and other distributions, redeeming and repurchasing certain other indebtedness, loans and investments, additional indebtedness, liens, mergers, asset sales and transactions with affiliates. In addition, the 2018 Credit Facility contains customary events of default. As of December 31, 2021 and 2020, we were in compliance with our debt covenants under the 2018 Credit Facility.
61
The 2018 Term Loan A and 2018 Term Loan B are payable in consecutive quarterly installments which began on December 31, 2018. Interest is due at least quarterly on amounts outstanding under the 2018 Credit Facility. In addition, beginning in 2020, we may be required to make mandatory prepayments towards the 2018 Term Loan B based on our consolidated leverage ratio and annual excess cash flows as defined under the terms of the 2018 Credit Facility. We are also permitted to make voluntary prepayments. Amounts outstanding under the 2018 Term Loan A and 2018 Term Loan B may be voluntarily prepaid without premium or penalty, subject to customary breakage fees in connection with the prepayment of a eurocurrency loan. These prepayments, if any, will be applied against remaining quarterly installments owed under the 2018 Term Loan A and 2018 Term Loan B in order of maturity with the remaining principal due upon maturity, unless directed otherwise by us. Based on the 2021 consolidated leverage ratio and excess cash flow calculation, both as defined under the terms of the 2018 Credit Facility, we will not be required to make a mandatory prepayment in 2022 toward the 2018 Term Loan B.
During the year ended December 31, 2021, we borrowed an aggregate amount of $671.2 million under the 2018 Credit Facility, which includes $630.0 million of borrowings under the 2018 Revolving Credit Facility, and repaid a total amount of $561.3 million on amounts outstanding under the 2018 Credit Facility, which includes $480.0 million of repayments on amounts outstanding under the 2018 Revolving Credit Facility and a $60.0 million prepayment on amounts outstanding under the 2018 Term Loan B. During the year ended December 31, 2020, we borrowed an aggregate amount of $30.4 million under the 2018 Credit Facility and repaid a total amount of $20.7 million on amounts outstanding under the 2018 Credit Facility. As of December 31, 2021 and 2020, the U.S. dollar amount outstanding under the 2018 Credit Facility was $1,094.6 million and $984.7 million, respectively. Of the $1,094.6 million outstanding under the 2018 Credit Facility as of December 31, 2021, $279.0 million was outstanding under the 2018 Term Loan A, $665.6 million was outstanding under the 2018 Term Loan B, and $150.0 million was outstanding under the 2018 Revolving Credit Facility. Of the $984.7 million outstanding under the 2018 Credit Facility as of December 31, 2020, $251.6 million was outstanding under the 2018 Term Loan A and $733.1 million was outstanding under the 2018 Term Loan B. There were no borrowings outstanding under the 2018 Revolving Credit Facility as of December 31, 2020. There were no outstanding foreign currency borrowings under the 2018 Credit Facility as of December 31, 2021 and 2020. As of December 31, 2021 and 2020, the weighted-average interest rate for borrowings under the 2018 Credit Facility was 2.62% and 3.39%, respectively.
See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on the 2018 Credit Facility.
Convertible Senior Notes due 2019
In February 2014, we issued $1.15 billion aggregate principal amount of convertible senior notes due 2019, or the 2019 Convertible Notes. The 2019 Convertible Notes were senior unsecured obligations which ranked effectively subordinate to any of our existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2019 Convertible Notes paid interest at a rate of 2.00% per annum payable semiannually in arrears on February 15 and August 15 of each year, beginning on August 15, 2014. Unless earlier repurchased or converted, the 2019 Convertible Notes matured on August 15, 2019. The primary purpose of the issuance of the 2019 Convertible Notes was for share repurchase purposes.
In March 2018, we issued $550.0 million aggregate principal of new convertible senior notes due 2024 as described below, and subsequently used the proceeds, along with cash on hand, to repurchase $475.0 million of our existing 2019 Convertible Notes from a limited number of holders in privately negotiated transactions for an aggregate purchase price of $583.5 million, which included $1.0 million of accrued interest.
In August 2019, we repaid a total amount of $675.0 million to repay in full amounts outstanding on the 2019 Convertible Notes upon maturity, as well as $6.7 million of accrued interest. See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our 2019 Convertible Notes.
Convertible Senior Notes due 2024
In March 2018, we issued $550.0 million aggregate principal amount of convertible senior notes due 2024, or the 2024 Convertible Notes. The 2024 Convertible Notes are senior unsecured obligations which rank effectively subordinate to any of our existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2024 Convertible Notes pay interest at a rate of 2.625% per annum payable semiannually in arrears on March 15 and September 15 of each year, beginning on September 15, 2018. Unless redeemed, repurchased or converted in accordance with their terms prior to such date, the 2024 Convertible Notes mature on March 15, 2024. The primary purpose of the issuance of the 2024 Convertible Notes was to repurchase a portion of the 2019 Convertible Notes.
62
In December 2021, the Company made an irrevocable election under the indenture governing the 2024 Convertible Notes to require the principal portion of the 2024 Convertible Notes to be settled in cash and any excess in shares or cash. See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our 2024 Convertible Notes.
Senior Notes due 2025
In May 2020, we issued $600.0 million aggregate principal amount of senior notes due 2025, or the 2025 Notes. The 2025 Notes are senior unsecured obligations which rank effectively subordinate to any of our existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2025 Notes pay interest at a rate of 7.875% per annum payable semiannually in arrears on March 1 and September 1 of each year, beginning on March 1, 2021. The 2025 Notes mature on September 1, 2025, unless redeemed or repurchased in accordance with their terms prior to such date. The primary purpose of the issuance of the 2025 Notes was for general corporate purposes, including share repurchases and other capital investment projects. See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our 2025 Notes.
Senior Notes due 2026
In August 2018, we issued $400.0 million aggregate principal amount of senior notes due 2026, or the 2026 Notes. The 2026 Notes were senior unsecured obligations which ranked effectively subordinate to any of our existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2026 Notes paid interest at a rate of 7.250% per annum payable semiannually in arrears on February 15 and August 15 of each year, beginning on February 15, 2019. The 2026 Notes were to mature on August 15, 2026, unless redeemed or repurchased in accordance with their terms prior to such date. The primary purpose of the issuance of the 2026 Notes was to refinance a portion of our prior senior secured credit facility.
In May 2021, we issued $600.0 million aggregate principal of new senior notes due 2029 as described below, and subsequently used a portion of the proceeds to redeem all $400.0 million of our existing 2026 Notes for an aggregate purchase price of $428.5 million, which included $7.7 million of accrued interest. See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our 2026 Notes.
Senior Notes due 2029
In May 2021, we issued $600.0 million aggregate principal amount of senior notes due 2029, or the 2029 Notes. The 2029 Notes are senior unsecured obligations which rank effectively subordinate to any of our existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2029 Notes pay interest at a rate of 4.875% per annum payable semiannually in arrears on June 1 and December 1 of each year, beginning on December 1, 2021. The 2029 Notes mature on June 1, 2029, unless redeemed or repurchased in accordance with their terms prior to such date. The primary purpose of the issuance of the 2029 Notes was to repurchase the 2026 Notes as well as for general corporate purposes, which may include shares repurchases and other capital investment projects. See Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our 2029 Notes.
Contractual Obligations
Our inventory purchase commitments are generally short-term in nature and have ordinary commercial terms. We did not have any material long-term inventory purchase commitments as of December 31, 2021. Our leases generally consist of long-term operating leases, which are payable monthly and relate to our office space, warehouses, distribution centers, manufacturing centers, and equipment.
For a further discussion on our debt and operating lease commitments as of December 31, 2021, see the sections above as well as Note 4, Leases, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K and Note 5, Long-Term Debt, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K.
63
Cash and Cash Equivalents
The majority of our foreign subsidiaries designate their local currencies as their functional currencies. As of December 31, 2021, the total amount of our foreign subsidiary cash and cash equivalents was $329.3 million, of which $14.1 million was held in U.S. dollars. As of December 31, 2021, the total amount of cash and cash equivalents held by Herbalife Nutrition Ltd. and its U.S. entities, inclusive of U.S. territories, was $272.2 million.
For earnings not considered to be indefinitely reinvested, deferred taxes have been provided. For earnings considered to be indefinitely reinvested, deferred taxes have not been provided. Should we make a determination to remit the cash and cash equivalents from our foreign subsidiaries that are considered indefinitely reinvested to Herbalife Nutrition Ltd. for the purpose of repatriation of undistributed earnings, we would need to accrue and pay taxes. As of December 31, 2021, Herbalife Nutrition Ltd. had approximately $2.8 billion of permanently reinvested unremitted earnings relating to its operating subsidiaries. As of December 31, 2021, we do not have any plans to repatriate these unremitted earnings to Herbalife Nutrition Ltd.; therefore, we do not have any liquidity concerns relating to these unremitted earnings and related cash and cash equivalents. See Note 12, Income Taxes, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for additional discussion on our unremitted earnings.
Off-Balance Sheet Arrangements
As of December 31, 2021 and 2020, we had no material off-balance sheet arrangements.
Dividends
We have not declared or paid cash dividends since 2014. The declaration of future dividends is subject to the discretion of our board of directors and will depend upon various factors, including our earnings, financial condition, Herbalife Nutrition Ltd.’s available distributable reserves under Cayman Islands law, restrictions imposed by the 2018 Credit Facility and the terms of any other indebtedness that may be outstanding, cash requirements, future prospects, and other factors deemed relevant by our board of directors.
Share Repurchases
On February 9, 2021, our board of directors authorized a new three-year $1.5 billion share repurchase program that will expire on February 9, 2024, which replaced our prior share repurchase authorization that was set to expire on October 30, 2023 and had approximately $7.9 million of remaining authorized capacity when it was replaced. This share repurchase program allows us, which includes an indirect wholly-owned subsidiary of Herbalife Nutrition Ltd., to repurchase our common shares at such times and prices as determined by management, as market conditions warrant, and to the extent Herbalife Nutrition Ltd.’s distributable reserves are available under Cayman Islands law. The 2018 Credit Facility permits us to repurchase our common shares as long as no default or event of default exists and other conditions, such as specified consolidated leverage ratios, are met. As of December 31, 2021, the remaining authorized capacity under our $1.5 billion share repurchase program was approximately $1.1 billion.
In conjunction with the issuance of the 2019 Convertible Notes during February 2014, we paid approximately $685.8 million to enter into prepaid forward share repurchase transactions, or the Forward Transactions, with certain financial institutions, or the Forward Counterparties, pursuant to which we purchased approximately 19.9 million common shares, at an average cost of $34.51 per share, for settlement on or around the August 15, 2019 maturity date for the 2019 Convertible Notes, subject to the ability of each Forward Counterparty to elect to settle all or a portion of its Forward Transactions early. The shares are treated as retired shares for basic and diluted EPS purposes. See Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on the Forward Transactions.
In January 2021, we repurchased from Mr. Carl C. Icahn and certain of his affiliates an aggregate of approximately 12.5 million common shares of ours at an aggregate cost of approximately $600.0 million, or $48.05 per share, and subsequently retired these shares. In addition, during the year ended December 31, 2021, we repurchased approximately 7.9 million of our common shares through open-market purchases at an aggregate cost of approximately $382.7 million, or an average cost of $48.17 per share, and subsequently retired these shares. In total, during the year ended December 31, 2021, we repurchased approximately 20.4 million of our common shares at an aggregate cost of approximately $982.7 million, or an average cost of $48.10 per share. In August 2020, we completed our modified Dutch auction tender offer and then subsequently paid cash to repurchase and retire a total of approximately 15.4 million of our common shares at an aggregate cost of approximately $750.0 million, or $48.75 per share. In addition, during the year ended December 31, 2020, we repurchased approximately 3.0 million of our common shares through open-market purchases at an aggregate cost of approximately $142.1 million, or an average cost of $47.40 per share, and subsequently retired these shares. In total, during the year ended December 31, 2020, we repurchased approximately 18.4 million of our common shares at an aggregate cost of approximately $892.1 million, or an average cost of $48.53 per share.
64
As of both December 31, 2021 and 2020, we held approximately 10.0 million of treasury shares for U.S. GAAP purposes. These treasury shares increased our shareholders’ deficit and are reflected at cost within our accompanying consolidated balance sheets. Although these shares are owned by an indirect wholly-owned subsidiary of ours and remain legally outstanding, they are reflected as treasury shares under U.S. GAAP and therefore reduce the number of common shares outstanding within our consolidated financial statements and the weighted-average number of common shares outstanding used in calculating earnings per share. The common shares of Herbalife Nutrition Ltd. held by the indirect wholly-owned subsidiary, however, remain outstanding on the books and records of our transfer agent and therefore still carry voting and other share rights related to ownership of our common shares, which may be exercised. So long as it is consistent with applicable laws, such shares will be voted by such subsidiary in the same manner, and to the maximum extent possible in the same proportion, as all other votes cast with respect to any matter properly submitted to a vote of Herbalife Nutrition Ltd.’s shareholders.
See Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on our share repurchases.
In connection with our October 2017 modified Dutch auction tender offer, we incurred $1.6 million in transaction costs and also provided a non-transferable CVR for each share tendered, allowing participants in the tender offer to receive a contingent cash payment in the event Herbalife Nutrition is acquired in a going-private transaction (as defined in the CVR Agreement) within two years of the commencement of the tender offer. The initial fair value of the CVR was $7.3 million, which was recorded as a liability in the fourth quarter of 2017 with a corresponding decrease to shareholders’ equity. In determining the initial fair value of the CVR, we used a lattice model, which included inputs such as the underlying stock price, strike price, time to expiration, and dividend yield. Subsequent changes in the fair value of the CVR liability, using a similar valuation approach as the initial fair value determination, were recognized within our consolidated balance sheets with corresponding gains or losses being recognized in other expense (income), net within our consolidated statements of income during each reporting period until the CVR expired in August 2019 or was terminated due to a going-private transaction, which was also incorporated in the valuation of the CVR; this going-private probability input was considered to be a Level 3 input in the fair value hierarchy and any increase or decrease in this input could have significantly impacted the fair value of the CVR as of the reporting date. The CVR expired without value on August 21, 2019, the two-year anniversary of August 21, 2017, the date we commenced the related modified Dutch auction tender offer.
During the year ended December 31, 2019, we recognized a $15.7 million gain in other expense (income), net within our consolidated statement of income due to the change in the fair value of the CVR, which was driven by its expiration during August 2019.
See Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion on the CVR.
Capped Call Transactions
In February 2014, in connection with the issuance of the 2019 Convertible Notes, we paid approximately $123.8 million to enter into capped call transactions with respect to our common shares, or the Capped Call Transactions, with certain financial institutions. The Capped Call Transactions were expected generally to reduce the potential dilution upon conversion of the 2019 Convertible Notes in the event that the market price of the common shares was greater than the strike price of the Capped Call Transactions, initially set at $43.14 per common share, with such reduction of potential dilution subject to a cap based on the cap price initially set at $60.39 per common share.
During March 2018, in connection with our repurchase of a portion of the 2019 Convertible Notes, we entered into partial settlement agreements with the option counterparties to the Capped Call Transactions to terminate a portion of the Capped Call Transactions, in each case, in a notional amount corresponding to the aggregate principal amount of the 2019 Convertible Notes that were repurchased.
On August 15, 2019, the 2019 Convertible Notes matured and the remaining Capped Call Transactions expired unexercised. The expiration of the Capped Call Transactions did not have an impact on our consolidated financial statements. See Note 8, Shareholders’ Deficit, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion of the Capped Call Transactions.
Working Capital and Operating Activities
As of December 31, 2021 and 2020, we had working capital of $351.4 million and $648.5 million, respectively, or a decrease of $297.1 million. The decrease was primarily due to a decrease in cash and cash equivalents, partially offset by increases in inventories and prepaid expenses and other current assets and a decrease in other current liabilities.
65
We expect that cash and funds provided from operations, available borrowings under the 2018 Credit Facility, and access to capital markets will provide sufficient working capital to operate our business, to make expected capital expenditures, and to meet foreseeable liquidity requirements for the next twelve months and thereafter.
The majority of our purchases from suppliers are generally made in U.S. dollars, while sales to our Members generally are made in local currencies. Consequently, strengthening of the U.S. dollar versus a foreign currency can have a negative impact on net sales and contribution margins and can generate transaction gains or losses on intercompany transactions. For discussion of our foreign exchange contracts and other hedging arrangements, see Part II, Item 7A, Quantitative and Qualitative Disclosures about Market Risk, of this Annual Report on Form 10-K.
Contingencies
See Note 7, Contingencies, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for information on our contingencies as of December 31, 2021.
Critical Accounting Policies
U.S. GAAP requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the year. We regularly evaluate our estimates and assumptions related to revenue recognition, allowance for product returns, inventory, goodwill and purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, tax contingencies, and other loss contingencies. We base our estimates and assumptions on current facts, historical experience and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of revenue, costs and expenses. Actual results could differ from those estimates. We consider the following policies to be most critical in understanding the judgments that are involved in preparing the financial statements and the uncertainties that could impact our operating results, financial condition and cash flows.
We are a nutrition company that sells a wide range of weight management; targeted nutrition; energy, sports, and fitness; and outer nutrition products. Our products are manufactured by us in our Changsha, Hunan, China extraction facility; Suzhou, China facility; Nanjing, China facility; Lake Forest, California facility; and Winston-Salem, North Carolina facility; and by third-party providers, and then are sold to Members who consume and sell Herbalife Nutrition products to retail consumers or other Members. As of December 31, 2021, we sold products in 95 markets throughout the world and we are organized and managed by geographic region. We aggregate our operating segments into one reporting segment, except China, as management believes that our operating segments have similar operating characteristics and similar long-term operating performance. In making this determination, management believes that the operating segments are similar in the nature of the products sold, the product acquisition process, the types of customers to whom products are sold, the methods used to distribute the products, the nature of the regulatory environment, and their economic characteristics.
We generally recognize revenue upon delivery when control passes to the Member. Product sales are recognized net of product returns, and discounts referred to as “distributor allowances.” We generally receive the net sales price in cash or through credit card payments at the point of sale. Royalty overrides are generally recorded when revenue is recognized. See Note 2, Basis of Presentation, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion of distributor compensation in the U.S.
Allowances for product returns, primarily in connection with our buyback program, are provided at the time the sale is recorded. This accrual is based upon historical return rates for each country and the relevant return pattern, which reflects anticipated returns to be received over a period of up to 12 months following the original sale. Historically, product returns and buybacks have not been significant. Product returns and buybacks were approximately 0.1% of net sales for both of the years ended December 31, 2021 and 2020.
We adjust our inventories to lower of cost and net realizable value. Additionally we adjust the carrying value of our inventory based on assumptions regarding future demand for our products and market conditions. If future demand and market conditions are less favorable than management’s assumptions, additional inventory write-downs could be required. Likewise, favorable future demand and market conditions could positively impact future operating results if previously written down inventories are sold. We have obsolete and slow moving inventories which have been adjusted downward $31.4 million and $23.0 million to present them at their lower of cost and net realizable value in our consolidated balance sheets as of December 31, 2021 and 2020, respectively.
Goodwill and marketing-related intangible assets not subject to amortization are tested annually for impairment, and are tested for impairment more frequently if events and circumstances indicate that the asset might be impaired.
66
Under the quantitative method for impairment testing of goodwill, which is done at the reporting unit level, we primarily use an income approach in order to determine the fair value of a reporting unit and compare it to its carrying amount. The determination of the fair value of the reporting units requires us to make significant estimates and assumptions. These estimates and assumptions include estimates of future revenues and expense growth rates, capital expenditures and the depreciation and amortization related to these capital expenditures, discount rates, and other inputs. Due to the inherent uncertainty involved in making these estimates, actual future results could differ. Changes in assumptions regarding future results or other underlying assumptions could have a significant impact on the fair value of the reporting unit. If the carrying amount of a reporting unit exceeds its fair value, an impairment loss is recognized for any excess of the carrying amount of the reporting unit over its fair value. During fiscal year 2021, we performed a quantitative assessment and determined that the fair value of each reporting unit was significantly greater than its respective carrying value.
Under the quantitative method for impairment testing of our marketing-related intangible assets, we use a discounted cash flow model, or the income approach, under the relief-from-royalty method to determine the fair value of our marketing-related intangible assets in order to confirm there is no impairment required. An impairment loss is recognized to the extent that the carrying amount of the assets exceeds their fair value. During fiscal year 2021, we performed a quantitative assessment of our marketing-related intangible assets and determined that the fair value of the assets was significantly greater than their carrying value.
As of December 31, 2021 and 2020, we had goodwill of approximately $95.4 million and $100.5 million, respectively. The decrease in goodwill during the year ended December 31, 2021 was due to foreign currency translation adjustments. As of both December 31, 2021 and 2020, we had marketing-related intangible assets of approximately $310.0 million. No goodwill or marketing-related intangibles impairment was recorded during the years ended December 31, 2021 and 2020. See Note 2, Basis of Presentation, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a further discussion.
Contingencies are accounted for in accordance with FASB ASC Topic 450, Contingencies, or ASC 450. ASC 450 requires that we record an estimated loss from a loss contingency when information available prior to issuance of our financial statements indicates that it is probable that an asset has been impaired or a liability has been incurred at the date of the financial statements and the amount of the loss can be reasonably estimated. We also disclose material contingencies when we believe a loss is not probable but reasonably possible as required by ASC 450. Accounting for contingencies such as legal and non-income tax matters requires us to use judgment related to both the likelihood of a loss and the estimate of the amount or range of loss. Many of these legal and tax contingencies can take years to be resolved. Generally, as the time period increases over which the uncertainties are resolved, the likelihood of changes to the estimate of the ultimate outcome increases.
As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate prior to the completion and filing of tax returns for such periods. These estimates involve complex issues and require us to make judgments about the likely application of the tax law to our situation, as well as with respect to other matters, such as anticipating the positions that we will take on tax returns prior to us actually preparing the returns and the outcomes of disputes with tax authorities. The ultimate resolution of these issues may take extended periods of time due to examinations by tax authorities and statutes of limitations. In addition, changes in our business, including acquisitions, changes in our international corporate structure, changes in the geographic location of business functions or assets, changes in the geographic mix and amount of income, as well as changes in our agreements with tax authorities, valuation allowances, applicable accounting rules, applicable tax laws and regulations, rulings and interpretations thereof, developments in tax audit and other matters, and variations in the estimated and actual level of annual pre-tax income can affect the overall effective income tax rate.
We evaluate the realizability of our deferred tax assets by assessing the valuation allowance and by adjusting the amount of such allowance, if necessary. Although realization is not assured, we believe it is more likely than not that the net carrying value will be realized. The amount of the carryforwards that is considered realizable, however, could change if estimates of future taxable income are adjusted. The ability to forecast income over multiple years at a jurisdictional level is subject to uncertainty especially when our assessment of valuation allowances factor in longer term income forecasts. The impact of increasing or decreasing the valuation allowance could be material to our consolidated financial statements. See Note 12, Income Taxes, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for additional information on our net deferred tax assets and valuation allowances.
We account for uncertain tax positions in accordance with FASB ASC Topic 740, Income Taxes, or ASC 740, which provides guidance on the determination of how tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, we must recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution.
67
We have made an accounting policy election to account for global intangible low-taxed income as a period cost if and when incurred.
We account for foreign currency transactions in accordance with FASB ASC Topic 830, Foreign Currency Matters. In a majority of the countries where we operate, the functional currency is the local currency. Our foreign subsidiaries’ asset and liability accounts are translated for consolidated financial reporting purposes into U.S. dollar amounts at period-end exchange rates. Revenue and expense accounts are translated at the average rates during the year. Our foreign currency translation adjustments are included in accumulated other comprehensive loss on our accompanying consolidated balance sheets. Foreign currency transaction gains and losses and foreign currency remeasurements are generally included in selling, general, and administrative expenses in the accompanying consolidated statements of income.
New Accounting Pronouncements
See discussion under Note 2, Basis of Presentation, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for information on new accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risks, which arise during the normal course of business from changes in interest rates and foreign currency exchange rates. On a selected basis, we use derivative financial instruments to manage or hedge certain of these risks. All hedging transactions are authorized and executed pursuant to written guidelines and procedures.
We apply FASB ASC Topic 815, Derivatives and Hedging, or ASC 815, which established accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. All derivatives, whether designated in hedging relationships or not, are required to be recorded on the balance sheet at fair value. If the derivative is designated as a fair-value hedge, the changes in the fair value of the derivative and the underlying hedged item are recognized concurrently in earnings. If the derivative is designated as a cash flow hedge, changes in the fair value of the derivative are recorded in other comprehensive income (loss) and are recognized in the consolidated statements of income when the hedged item affects earnings. ASC 815 defines the requirements for designation and documentation of hedging relationships as well as ongoing effectiveness assessments in order to use hedge accounting. For a derivative that does not qualify as a hedge, changes in fair value are recognized concurrently in earnings.
A discussion of our primary market risk exposures and derivatives is presented below.
Foreign Exchange Risk
We transact business globally and are subject to risks associated with changes in foreign exchange rates. Our objective is to minimize the impact to earnings and cash flow associated with foreign exchange rate fluctuations. We enter into foreign exchange derivatives in the ordinary course of business primarily to reduce exposure to currency fluctuations attributable to intercompany transactions, translation of local currency earnings, inventory purchases subject to foreign currency exposure, and to partially mitigate the impact of foreign currency rate fluctuations. Due to volatility in foreign exchange markets, our current strategy, in general, is to hedge some of the significant exposures on a short-term basis. We will continue to monitor the foreign exchange markets and evaluate our hedging strategy accordingly. With the exception of our foreign currency forward contracts relating to forecasted inventory purchases and intercompany management fees discussed below, all of our foreign exchange contracts are designated as freestanding derivatives for which hedge accounting does not apply. The changes in the fair value of the derivatives not qualifying as cash flow hedges are included in selling, general, and administrative expenses within our consolidated statements of income.
The foreign currency forward contracts and option contracts designated as freestanding derivatives are primarily used to hedge foreign currency-denominated intercompany transactions and to partially mitigate the impact of foreign currency fluctuations. The fair value of foreign exchange derivative contracts is based on third-party quotes. Our foreign currency derivative contracts are generally executed on a monthly basis.
68
We also purchase foreign currency forward contracts in order to hedge forecasted inventory transactions and intercompany management fees that are designated as cash flow hedges and are subject to foreign currency exposures. We applied the hedge accounting rules as required by ASC 815 for these hedges. These contracts allow us to buy and sell certain currencies at specified contract rates. As of December 31, 2021 and 2020, the aggregate notional amounts of these contracts outstanding were approximately $54.5 million and $56.4 million, respectively. As of December 31, 2021, the outstanding contracts were expected to mature over the next thirteen months. Our derivative financial instruments are recorded on the consolidated balance sheets at fair value based on quoted market rates. For the forecasted inventory transactions, the forward contracts are used to hedge forecasted inventory transactions over specific months. Changes in the fair value of these forward contracts designated as cash flow hedges, excluding forward points, are recorded as a component of accumulated other comprehensive loss within shareholders’ deficit, and are recognized in cost of sales within our consolidated statement of income during the period which approximates the time the hedged inventory is sold. We also hedge forecasted intercompany management fees over specific months. Changes in the fair value of these forward contracts designated as cash flow hedges, excluding forward points, are recorded as a component of accumulated other comprehensive loss within shareholders’ deficit, and are recognized in selling, general, and administrative expenses within our consolidated statement of income during the period when the hedged item and underlying transaction affect earnings. As of December 31, 2021, we recorded assets at fair value of $0.3 million and liabilities at fair value of $1.7 million relating to all outstanding foreign currency contracts designated as cash flow hedges. As of December 31, 2020, we recorded liabilities at fair value of $3.9 million relating to all outstanding foreign currency contracts designated as cash flow hedges. These hedges remained effective as of December 31, 2021 and 2020.
As of both December 31, 2021 and 2020, the majority of our outstanding foreign currency forward contracts had maturity dates of less than twelve months with the majority of freestanding derivatives expiring within one month.
See Note 11, Derivative Instruments and Hedging Activities, to the Consolidated Financial Statements included in Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K for a description of foreign currency forward contracts that were outstanding as of December 31, 2021 and 2020, which discussion is incorporated herein by reference.
The majority of our foreign subsidiaries designate their local currencies as their functional currencies. See Liquidity and Capital Resources — Cash and Cash Equivalents in Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, of this Annual Report on Form 10-K for further discussion of our foreign subsidiary cash and cash equivalents.
Interest Rate Risk
As of December 31, 2021, the aggregate annual maturities of the 2018 Credit Facility were expected to be $29.0 million for 2022, $29.0 million for 2023, $36.1 million for 2024, and $1,000.5 million for 2025. As of December 31, 2021, the fair values of the 2018 Term Loan A, 2018 Term Loan B, and 2018 Revolving Credit Facility were approximately $278.0 million, $663.1 million, and $150.0 million, respectively, and the carrying values were $278.1 million, $660.5 million, and $150.0 million, respectively. As of December 31, 2020, the fair values of the 2018 Term Loan A and 2018 Term Loan B were approximately $251.9 million and $734.0 million, respectively, and the carrying values were $250.5 million and $726.0 million, respectively. There were no outstanding borrowings on the 2018 Revolving Credit Facility as of December 31, 2020. The 2018 Credit Facility bears variable interest rates, and as of December 31, 2021 and 2020, the weighted-average interest rate for borrowings under the 2018 Credit Facility was 2.62% and 3.39%, respectively.
During the first quarter of 2020, we entered into various interest rate swap agreements with effective dates ranging between February 2020 and March 2020. These agreements collectively provide for us to pay interest at a weighted-average fixed rate of 0.98% on aggregate notional amounts of $100.0 million under the 2018 Credit Facility until their respective expiration dates ranging between February 2022 and March 2023, while receiving interest based on LIBOR on the same notional amounts for the same periods. At inception, these swap agreements were designated as cash flow hedges against the variability in certain LIBOR-based borrowings under the 2018 Credit Facility, effectively fixing the interest rate on such notional amounts at a weighted-average effective rate of, depending on our total leverage ratio, between 2.73% and 3.23%. The fair values of the interest rate swap agreements are based on third-party bank quotes, and as of December 31, 2021 and 2020, we recorded liabilities at fair value of $0.1 million and $1.0 million, respectively, relating to these interest rate swap agreements.
69
Our exposure to interest rate volatility risk related to our 2018 Credit Facility is partially mitigated by our interest rate swaps. If interest rates were to increase or decrease by 1% for the year and our borrowing amounts on our 2018 Credit Facility and related interest rate swaps remained constant, our annual interest expense could increase by approximately $9.9 million or decrease by approximately $1.0 million, respectively. The variable interest rates payable under our 2018 Credit Facility are linked to LIBOR as the benchmark for establishing such rates. Recent national, international and other regulatory guidance and reform proposals regarding LIBOR are requiring certain LIBOR tenors to be discontinued or become unavailable by the end of 2021 and LIBOR to be fully discontinued or become unavailable as a benchmark rate by June 2023. Our 2018 Credit Facility includes mechanics to facilitate the adoption by us and our lenders of an alternative benchmark rate for use in place of LIBOR which may result in interest rates that are higher or lower than those that would have resulted had LIBOR remained in effect.
As of December 31, 2021, the fair value of the liability component of the 2024 Convertible Notes was approximately $547.4 million, and the carrying value was $486.0 million. As of December 31, 2020, the fair value of the liability component of the 2024 Convertible Notes was approximately $541.8 million and the carrying value was $460.6 million. The 2024 Convertible Notes pay interest at a fixed rate of 2.625% per annum payable semiannually in arrears on March 15 and September 15 of each year, beginning on September 15, 2018. Unless redeemed, repurchased or converted in accordance with their terms prior to such date, the 2024 Convertible Notes mature on March 15, 2024.
As of December 31, 2021, the fair value of the 2025 Notes was approximately $639.7 million and the carrying value was $594.2 million. As of December 31, 2020, the fair value of the 2025 Notes was approximately $656.3 million and the carrying value was $592.9 million. The 2025 Notes pay interest at a fixed rate of 7.875% per annum payable semiannually in arrears on March 1 and September 1 of each year, beginning on March 1, 2021. The 2025 Notes mature on September 1, 2025, unless redeemed or repurchased in accordance with their terms prior to such date. The 2025 Notes are recorded at their carrying value and their fair value is used only for disclosure purposes, so an increase or decrease in interest rates would not have any impact to our consolidated financial statements; however, if interest rates were to increase or decrease by 1%, their fair value could decrease by approximately $4.1 million or increase by approximately $4.2 million, respectively.
As of December 31, 2021, the fair value of the 2029 Notes was approximately $588.9 million and the carrying value was $592.8 million. The 2029 Notes pay interest at a fixed rate of 4.875% per annum payable semiannually in arrears on June 1 and December 1 of each year, beginning on December 1, 2021. The 2029 Notes mature on June 1, 2029, unless redeemed or repurchased in accordance with their terms prior to such date. The 2029 Notes are recorded at their carrying value and their fair value is used only for disclosure purposes, so an increase or decrease in interest rates would not have any impact to our consolidated financial statements; however, if interest rates were to increase or decrease by 1%, their fair value could decrease by approximately $33.8 million or increase by approximately $28.6 million, respectively.
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements and notes thereto and the report of PricewaterhouseCoopers LLP, independent registered public accounting firm, are set forth in the Index to Financial Statements under Part IV, Item 15, Exhibits, Financial Statement Schedules, of this Annual Report on Form 10-K, and are incorporated herein by reference.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on an evaluation of the Company’s disclosure controls and procedures as of December 31, 2021 conducted by the Company’s management, with the participation of the Chief Executive Officer and Chief Financial Officer, the Chief Executive Officer and Chief Financial Officer have concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2021.
70
Management’s Report on Internal Control over Financial Reporting
The SEC, as directed by Section 404 of the Sarbanes-Oxley Act of 2002, adopted rules which require the Company to include in this Annual Report on Form 10-K, an assessment by management of the effectiveness of the Company’s internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. In addition, the Company’s independent auditors must attest to and report on the effectiveness of the Company’s internal control over financial reporting.
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of the effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
The Company’s management carried out an evaluation, under the supervision and with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2021 based on the framework in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based upon this evaluation, under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2021.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2021 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report incorporated by reference in Part II, Item 8, Financial Statements and Supplementary Data, of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act that occurred during the fourth quarter ended December 31, 2021 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
71
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required under this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2021.
Item 11. Executive Compensation
The information required under this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2021.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required under this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2021.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required under this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2021.
Item 14. Principal Accountant Fees and Services
The information required under this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2021.
72
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as part of this Annual Report on Form 10-K, or incorporated herein by reference:
|
Page No. |
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES |
|
Report of Independent Registered Public Accounting Firm (PCAOB ID: 238) |
77 |
Consolidated Balance Sheets as of December 31, 2021 and 2020 |
79 |
Consolidated Statements of Income for the years ended December 31, 2021, 2020, and 2019 |
80 |
81 |
|
82 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2021, 2020, and 2019 |
83 |
84 |
73
EXHIBIT INDEX
74
10.26# |
|
|
(o) |
|
10.27# |
|
|
(p) |
|
10.28# |
|
|
(p) |
|
10.29 |
|
|
(q) |
|
10.30 |
|
|
(s) |
|
10.31# |
|
Retention Agreement, effective as of April 6, 2020, by and between Mark Schissel and the Company |
|
(aa) |
10.32 |
|
|
(v) |
|
10.33 |
|
|
(v) |
|
10.34 |
|
|
(w) |
|
10.35 |
|
|
(z) |
|
21.1 |
|
|
* |
|
23.1 |
|
Consent of PricewaterhouseCoopers LLP — Independent Registered Public Accounting Firm |
|
* |
31.1 |
|
|
* |
|
31.2 |
|
|
* |
|
32.1 |
|
|
** |
|
32.2 |
|
|
** |
|
101.INS |
|
Inline XBRL Instance Document – The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
* |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
* |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
* |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
* |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
* |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
* |
104 |
|
Cover Page Interactive Data File – The cover page from the Company’s Annual Report on Form 10-K for the year ended December 31, 2021 is formatted in Inline XBRL (included as Exhibit 101) |
|
* |
* Filed herewith.
** Furnished herewith.
# Management contract or compensatory plan or arrangement.
(a) Previously filed on October 1, 2004 as an Exhibit to the Company’s registration statement on Form S-1 (File No. 333-119485) and is incorporated herein by reference.
(b) Previously filed on December 2, 2004 as an Exhibit to Amendment No. 4 to the Company’s registration statement on Form S-1 (File No. 333-119485) and is incorporated herein by reference.
(c) Previously filed on December 14, 2004 as an Exhibit to Amendment No. 5 to the Company’s registration statement on Form S-1 (File No. 333-119485) and is incorporated herein by reference.
75
(d) Previously filed on May 5, 2015 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and is incorporated herein by reference.
(e) Previously filed on August 5, 2015 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and is incorporated herein by reference.
(f) Previously filed on May 5, 2016 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and is incorporated herein by reference.
(g) Previously filed on July 15, 2016 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(h) Previously filed on February 23, 2017 as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2016 and is incorporated herein by reference.
(i) Previously filed on August 1, 2017 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2017 and is incorporated herein by reference.
(j) Previously filed on February 22, 2018 as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2017 and is incorporated herein by reference.
(k) Previously filed on March 29, 2018 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(l) Previously filed on May 3, 2018 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 and is incorporated herein by reference.
(m) Previously filed on August 22, 2018 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(n) Previously filed on February 19, 2019 as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 and is incorporated herein by reference.
(o) Previously filed on August 1, 2019 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2019 and is incorporated herein by reference.
(p) Previously filed on October 29, 2019 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 and is incorporated herein by reference.
(q) Previously filed on December 12, 2019 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(r) Previously filed on February 18, 2020 as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019.
(s) Previously filed on March 19, 2020 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(t) Previously filed on May 7, 2020 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2020 and is incorporated herein by reference.
(u) Previously filed on May 29, 2020 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(v) Previously filed on November 5, 2020 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2020 and is incorporated herein by reference.
(w) Previously filed on February 11, 2021 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(x) Previously filed on May 4, 2021 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2021 and is incorporated herein by reference.
(y) Previously filed on May 20, 2021 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(z) Previously filed on July 30, 2021 as an Exhibit to the Company’s Current Report on Form 8-K and is incorporated herein by reference.
(aa) Previously filed on November 2, 2021 as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2021 and is incorporated herein by reference.
76
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Herbalife Nutrition Ltd.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Herbalife Nutrition Ltd. and its subsidiaries (the “Company”) as of December 31, 2021 and 2020, and the related consolidated statements of income, of comprehensive income, of changes in shareholders’ deficit, and of cash flows for each of the three years in the period ended December 31, 2021, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2021 based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021 based on criteria established in Internal Control – Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
77
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Loss Contingencies
As described in Note 7 to the consolidated financial statements, the Company is from time to time engaged in routine litigation. As disclosed by management, an estimated loss from a loss contingency is recorded when information available prior to issuance of the Company’s financial statements indicates that it is probable that an asset has been impaired or a liability has been incurred at the date of the financial statements and the amount of the loss can be reasonably estimated. Management also discloses material contingencies when they believe a loss is not probable but reasonably possible. Management regularly reviews all pending litigation matters in which it is involved and establishes reserves for these litigation matters when a probable loss estimate can be made. Accounting for contingencies such as legal and non-income tax matters requires management to use judgment related to both the likelihood of a loss and the estimate of the amount or range of loss.
The principal considerations for our determination that performing procedures relating to loss contingencies is a critical audit matter are the significant judgment by management when assessing the likelihood of a loss being incurred and when determining whether a reasonable estimate of the loss or range of loss for each matter can be made, which in turn led to a high degree of auditor judgment and effort in evaluating management’s assessment of loss contingencies associated with legal and non-income tax matters. In addition, the audit effort involved the use of professionals with specialized skill and knowledge to assist in performing procedures and evaluating the audit evidence obtained from these procedures.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s evaluation of loss contingencies associated with legal and non-income tax matters, including controls over determining whether a loss is probable and whether the amount of loss can be reasonably estimated, as well as financial statement disclosures. These procedures also included, among others, obtaining and evaluating the letters of audit inquiry from the Company’s external legal counsel, evaluating the reasonableness of management’s assessment regarding whether an unfavorable outcome is reasonably possible or probable and reasonably estimable, and evaluating the sufficiency of the Company’s contingency disclosures. Professionals with specialized skill and knowledge were used to assist in the evaluation of the completeness and measurement of certain contingencies, evaluation of whether the positions taken by management are reasonable and assessing the audit evidence obtained.
/s/ PricewaterhouseCoopers LLP
Los Angeles, California
February 23, 2022
We have served as the Company’s auditor since 2013.
78
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions, except share and par value amounts) |
|
|||||
ASSETS |
|
|||||||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
601.5 |
|
|
$ |
1,045.4 |
|
Receivables, net of allowance for doubtful accounts |
|
|
66.9 |
|
|
|
83.3 |
|
Inventories |
|
|
575.7 |
|
|
|
501.4 |
|
Prepaid expenses and other current assets |
|
|
187.7 |
|
|
|
145.7 |
|
Total current assets |
|
|
1,431.8 |
|
|
|
1,775.8 |
|
Property, plant, and equipment, at cost, net of accumulated depreciation and amortization |
|
|
442.1 |
|
|
|
390.2 |
|
Operating lease right-of-use assets |
|
|
220.0 |
|
|
|
222.8 |
|
Marketing-related intangibles and other intangible assets, net |
|
|
317.3 |
|
|
|
313.3 |
|
Goodwill |
|
|
95.4 |
|
|
|
100.5 |
|
Other assets |
|
|
313.2 |
|
|
|
273.5 |
|
Total assets |
|
$ |
2,819.8 |
|
|
$ |
3,076.1 |
|
LIABILITIES AND SHAREHOLDERS’ DEFICIT |
|
|||||||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
92.0 |
|
|
$ |
88.7 |
|
Royalty overrides |
|
|
363.2 |
|
|
|
358.2 |
|
Current portion of long-term debt |
|
|
29.4 |
|
|
|
22.9 |
|
Other current liabilities |
|
|
595.8 |
|
|
|
657.5 |
|
Total current liabilities |
|
|
1,080.4 |
|
|
|
1,127.3 |
|
Long-term debt, net of current portion |
|
|
2,733.2 |
|
|
|
2,405.5 |
|
Non-current operating lease liabilities |
|
|
201.2 |
|
|
|
206.7 |
|
Other non-current liabilities |
|
|
196.5 |
|
|
|
192.7 |
|
Total liabilities |
|
|
4,211.3 |
|
|
|
3,932.2 |
|
|
|
|
|
|
|
|||
Shareholders’ deficit: |
|
|
|
|
|
|
||
Common shares, $0.0005 par value; 2.0 billion shares authorized; 100.8 million (2021) and 120.1 million (2020) shares outstanding |
|
|
0.1 |
|
|
|
0.1 |
|
Paid-in capital in excess of par value |
|
|
318.1 |
|
|
|
342.3 |
|
Accumulated other comprehensive loss |
|
|
(211.8 |
) |
|
|
(182.2 |
) |
Accumulated deficit |
|
|
(1,169.0 |
) |
|
|
(687.4 |
) |
Treasury stock, at cost, 10.0 million (2021) and 10.0 million (2020) shares |
|
|
(328.9 |
) |
|
|
(328.9 |
) |
Total shareholders’ deficit |
|
|
(1,391.5 |
) |
|
|
(856.1 |
) |
Total liabilities and shareholders’ deficit |
|
$ |
2,819.8 |
|
|
$ |
3,076.1 |
|
See the accompanying notes to consolidated financial statements.
79
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions, except per share amounts) |
|
|||||||||
Net sales |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
$ |
4,877.1 |
|
Cost of sales |
|
|
1,239.3 |
|
|
|
1,150.6 |
|
|
|
958.0 |
|
Gross profit |
|
|
4,563.5 |
|
|
|
4,391.2 |
|
|
|
3,919.1 |
|
Royalty overrides |
|
|
1,833.7 |
|
|
|
1,690.1 |
|
|
|
1,448.2 |
|
Selling, general, and administrative expenses |
|
|
2,012.1 |
|
|
|
2,075.0 |
|
|
|
1,940.3 |
|
Other operating income |
|
|
(16.4 |
) |
|
|
(14.5 |
) |
|
|
(37.5 |
) |
Operating income |
|
|
734.1 |
|
|
|
640.6 |
|
|
|
568.1 |
|
Interest expense |
|
|
153.1 |
|
|
|
133.0 |
|
|
|
153.0 |
|
Interest income |
|
|
4.4 |
|
|
|
8.8 |
|
|
|
20.6 |
|
Other expense (income), net |
|
|
24.6 |
|
|
|
— |
|
|
|
(15.7 |
) |
Income before income taxes |
|
|
560.8 |
|
|
|
516.4 |
|
|
|
451.4 |
|
Income taxes |
|
|
113.6 |
|
|
|
143.8 |
|
|
|
140.4 |
|
Net income |
|
$ |
447.2 |
|
|
$ |
372.6 |
|
|
$ |
311.0 |
|
Earnings per share: |
|
|
|
|
|
|
|
|
|
|||
Basic |
|
$ |
4.22 |
|
|
$ |
2.83 |
|
|
$ |
2.26 |
|
Diluted |
|
$ |
4.13 |
|
|
$ |
2.77 |
|
|
$ |
2.20 |
|
Weighted-average shares outstanding: |
|
|
|
|
|
|
|
|
|
|||
Basic |
|
|
105.9 |
|
|
|
131.5 |
|
|
|
137.4 |
|
Diluted |
|
|
108.3 |
|
|
|
134.5 |
|
|
|
141.6 |
|
See the accompanying notes to consolidated financial statements.
80
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Net income |
|
$ |
447.2 |
|
|
$ |
372.6 |
|
|
$ |
311.0 |
|
Other comprehensive (loss) income: |
|
|
|
|
|
|
|
|
|
|||
Foreign currency translation adjustment, net of income taxes of $0.2 (2021), $(2.0) (2020), and $0.1 (2019) |
|
|
(33.2 |
) |
|
|
33.2 |
|
|
|
— |
|
Unrealized gain (loss) on derivatives, net of income taxes of $— (2021), $(0.4) (2020), and $— (2019) |
|
|
3.6 |
|
|
|
(2.9 |
) |
|
|
(2.7 |
) |
Total other comprehensive (loss) income |
|
|
(29.6 |
) |
|
|
30.3 |
|
|
|
(2.7 |
) |
Total comprehensive income |
|
$ |
417.6 |
|
|
$ |
402.9 |
|
|
$ |
308.3 |
|
See the accompanying notes to consolidated financial statements.
81
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
|
|
Common Shares |
|
|
Treasury Stock |
|
|
Paid-in Capital in Excess of Par Value |
|
|
Accumulated Other Comprehensive Loss |
|
|
Accumulated Deficit |
|
|
Total Shareholders’ Deficit |
|
||||||
|
|
(in millions) |
|
|||||||||||||||||||||
Balance as of December 31, 2018 |
|
$ |
0.1 |
|
|
$ |
(328.9 |
) |
|
$ |
341.5 |
|
|
$ |
(209.8 |
) |
|
$ |
(526.3 |
) |
|
$ |
(723.4 |
) |
Issuance of 1.0 common shares from exercise of stock options, SARs, restricted stock units, employee stock purchase plan, and other |
|
|
— |
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
|
|
3.2 |
|
|||
Additional capital from share-based compensation |
|
|
|
|
|
|
|
|
38.6 |
|
|
|
|
|
|
|
|
|
38.6 |
|
||||
Repurchases of 0.4 common shares |
|
|
— |
|
|
|
|
|
|
(16.7 |
) |
|
|
|
|
|
|
|
|
(16.7 |
) |
|||
Forward Counterparties’ delivery of 6.0 common shares to the Company |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|||
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
311.0 |
|
|
|
311.0 |
|
||||
Foreign currency translation adjustment, net of income taxes of $0.1 |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||||
Unrealized loss on derivatives, net of income taxes of $— |
|
|
|
|
|
|
|
|
|
|
|
(2.7 |
) |
|
|
|
|
|
(2.7 |
) |
||||
Balance as of December 31, 2019 |
|
|
0.1 |
|
|
|
(328.9 |
) |
|
|
366.6 |
|
|
|
(212.5 |
) |
|
|
(215.3 |
) |
|
|
(390.0 |
) |
Issuance of 1.7 common shares from exercise of stock options, SARs, restricted stock units, employee stock purchase plan, and other |
|
|
— |
|
|
|
|
|
|
3.5 |
|
|
|
|
|
|
|
|
|
3.5 |
|
|||
Additional capital from share-based compensation |
|
|
|
|
|
|
|
|
51.0 |
|
|
|
|
|
|
|
|
|
51.0 |
|
||||
Repurchases of 19.0 common shares |
|
|
— |
|
|
|
|
|
|
(78.8 |
) |
|
|
|
|
|
(844.7 |
) |
|
|
(923.5 |
) |
||
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
372.6 |
|
|
|
372.6 |
|
||||
Foreign currency translation adjustment, net of income taxes of $(2.0) |
|
|
|
|
|
|
|
|
|
|
|
33.2 |
|
|
|
|
|
|
33.2 |
|
||||
Unrealized loss on derivatives, net of income taxes of $(0.4) |
|
|
|
|
|
|
|
|
|
|
|
(2.9 |
) |
|
|
|
|
|
(2.9 |
) |
||||
Balance as of December 31, 2020 |
|
|
0.1 |
|
|
|
(328.9 |
) |
|
|
342.3 |
|
|
|
(182.2 |
) |
|
|
(687.4 |
) |
|
|
(856.1 |
) |
Issuance of 1.7 common shares from exercise of stock options, SARs, restricted stock units, employee stock purchase plan, and other |
|
|
— |
|
|
|
|
|
|
4.2 |
|
|
|
|
|
|
|
|
|
4.2 |
|
|||
Additional capital from share-based compensation |
|
|
|
|
|
|
|
|
54.1 |
|
|
|
|
|
|
|
|
|
54.1 |
|
||||
Repurchases of 21.0 common shares |
|
|
— |
|
|
|
|
|
|
(82.5 |
) |
|
|
|
|
|
(928.8 |
) |
|
|
(1,011.3 |
) |
||
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
447.2 |
|
|
|
447.2 |
|
||||
Foreign currency translation adjustment, net of income taxes of $0.2 |
|
|
|
|
|
|
|
|
|
|
|
(33.2 |
) |
|
|
|
|
|
(33.2 |
) |
||||
Unrealized gain on derivatives, net of income taxes of $— |
|
|
|
|
|
|
|
|
|
|
|
3.6 |
|
|
|
|
|
|
3.6 |
|
||||
Balance as of December 31, 2021 |
|
$ |
0.1 |
|
|
$ |
(328.9 |
) |
|
$ |
318.1 |
|
|
$ |
(211.8 |
) |
|
$ |
(1,169.0 |
) |
|
$ |
(1,391.5 |
) |
See the accompanying notes to consolidated financial statements.
82
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|||
Net income |
|
$ |
447.2 |
|
|
$ |
372.6 |
|
|
$ |
311.0 |
|
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization |
|
|
107.6 |
|
|
|
100.3 |
|
|
|
97.7 |
|
Share-based compensation expenses |
|
|
54.1 |
|
|
|
51.0 |
|
|
|
38.6 |
|
Non-cash interest expense |
|
|
30.1 |
|
|
|
26.7 |
|
|
|
43.7 |
|
Deferred income taxes |
|
|
(33.3 |
) |
|
|
2.0 |
|
|
|
15.4 |
|
Inventory write-downs |
|
|
28.8 |
|
|
|
20.6 |
|
|
|
19.1 |
|
Foreign exchange transaction loss |
|
|
14.3 |
|
|
|
9.9 |
|
|
|
2.1 |
|
Loss on extinguishment of debt |
|
|
24.6 |
|
|
|
— |
|
|
|
— |
|
Other |
|
|
5.2 |
|
|
|
5.3 |
|
|
|
(7.9 |
) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|||
Receivables |
|
|
9.6 |
|
|
|
(5.8 |
) |
|
|
(14.4 |
) |
Inventories |
|
|
(129.1 |
) |
|
|
(76.6 |
) |
|
|
(68.6 |
) |
Prepaid expenses and other current assets |
|
|
(49.3 |
) |
|
|
(11.9 |
) |
|
|
28.3 |
|
Accounts payable |
|
|
6.9 |
|
|
|
5.5 |
|
|
|
0.1 |
|
Royalty overrides |
|
|
17.8 |
|
|
|
61.2 |
|
|
|
11.5 |
|
Other current liabilities |
|
|
(68.8 |
) |
|
|
77.6 |
|
|
|
(5.5 |
) |
Other |
|
|
(5.4 |
) |
|
|
(9.8 |
) |
|
|
(13.6 |
) |
Net cash provided by operating activities |
|
|
460.3 |
|
|
|
628.6 |
|
|
|
457.5 |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|||
Purchases of property, plant, and equipment |
|
|
(151.4 |
) |
|
|
(112.0 |
) |
|
|
(106.1 |
) |
Other |
|
|
(5.0 |
) |
|
|
(11.2 |
) |
|
|
(1.9 |
) |
Net cash used in investing activities |
|
|
(156.4 |
) |
|
|
(123.2 |
) |
|
|
(108.0 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|||
Borrowings from senior secured credit facility, net of discount |
|
|
671.1 |
|
|
|
31.5 |
|
|
|
— |
|
Principal payments on senior secured credit facility and other debt |
|
|
(563.5 |
) |
|
|
(24.5 |
) |
|
|
(24.5 |
) |
Repayment of convertible senior notes |
|
|
— |
|
|
|
— |
|
|
|
(675.0 |
) |
Proceeds from senior notes |
|
|
600.0 |
|
|
|
600.0 |
|
|
|
— |
|
Repayment of senior notes |
|
|
(420.7 |
) |
|
|
— |
|
|
|
— |
|
Debt issuance costs |
|
|
(8.4 |
) |
|
|
(7.9 |
) |
|
|
— |
|
Share repurchases |
|
|
(1,011.3 |
) |
|
|
(923.5 |
) |
|
|
(16.7 |
) |
Other |
|
|
4.2 |
|
|
|
3.5 |
|
|
|
3.2 |
|
Net cash used in financing activities |
|
|
(728.6 |
) |
|
|
(320.9 |
) |
|
|
(713.0 |
) |
Effect of exchange rate changes on cash, cash equivalents, and restricted cash |
|
|
(18.9 |
) |
|
|
22.0 |
|
|
|
(4.0 |
) |
Net change in cash, cash equivalents, and restricted cash |
|
|
(443.6 |
) |
|
|
206.5 |
|
|
|
(367.5 |
) |
Cash, cash equivalents, and restricted cash, beginning of period |
|
|
1,054.0 |
|
|
|
847.5 |
|
|
|
1,215.0 |
|
Cash, cash equivalents, and restricted cash, end of period |
|
$ |
610.4 |
|
|
$ |
1,054.0 |
|
|
$ |
847.5 |
|
Cash paid during the year: |
|
|
|
|
|
|
|
|
|
|||
Interest paid |
|
$ |
143.5 |
|
|
$ |
78.9 |
|
|
$ |
114.3 |
|
Income taxes paid |
|
$ |
156.3 |
|
|
$ |
138.2 |
|
|
$ |
147.9 |
|
See the accompanying notes to consolidated financial statements.
83
HERBALIFE NUTRITION LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization
Herbalife Nutrition Ltd., a Cayman Islands exempted company with limited liability, was incorporated on April 4, 2002. Herbalife Nutrition Ltd. (and together with its subsidiaries, the “Company,” “Herbalife,” or “Herbalife Nutrition”) is a global nutrition company that sells weight management; targeted nutrition; energy, sports, and fitness; and outer nutrition products to and through a network of independent members, or Members. In China, the Company sells its products to and through independent service providers and sales representatives to customers and preferred customers, as well as through Company-operated retail platforms when necessary. The Company sells its products in six geographic regions: North America; Mexico; South and Central America; EMEA, which consists of Europe, the Middle East, and Africa; Asia Pacific (excluding China); and China.
2. Basis of Presentation
The Company’s consolidated financial statements refer to Herbalife Nutrition Ltd. and its subsidiaries.
Recently Adopted Pronouncements
In August 2018, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, No. 2018-14, Compensation — Retirement Benefits — Defined Benefit Plans — General (Subtopic 715-20): Disclosure Framework — Changes to the Disclosure Requirements for Defined Benefit Plans. This ASU removes disclosures that are no longer considered cost beneficial, clarifies the specific requirements of disclosures, and adds disclosure requirements identified as relevant. The amendments in this update are effective for reporting periods beginning after December 15, 2020, with early adoption permitted. The adoption of this guidance during the first quarter of 2021 did not have a material impact on the Company’s consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This ASU simplifies the accounting for income taxes by eliminating some exceptions to the general approach in the FASB Accounting Standards Codification, or ASC, Topic 740, Income Taxes, and clarifies certain aspects of the existing guidance to promote more consistent application, among other things. The amendments in this update are effective for reporting periods beginning after December 15, 2020, with early adoption permitted. The adoption of this guidance during the first quarter of 2021 did not have a material impact on the Company’s consolidated financial statements.
New Accounting Pronouncements
In August 2020, the FASB issued ASU No. 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, or ASU 2020-06. This ASU simplifies the accounting for convertible instruments by eliminating certain accounting models, resulting in fewer embedded conversion features being separately recognized from the host contract, and also amends the guidance for derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. Additionally, the amendments in this ASU affect the diluted EPS calculation for convertible instruments. It will require that the effect of potential share settlement be included in the diluted EPS calculation when a convertible instrument may be settled in cash or shares; the if-converted method as opposed to the treasury stock method would be required to calculate diluted EPS for these types of convertible instruments. The amendments in this update are effective for reporting periods beginning after December 15, 2021, with early adoption permitted. The Company plans to adopt this guidance during the first quarter of 2022 using the modified retrospective method and recognize a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. The Company expects the adoption of this guidance to increase long-term debt by approximately $59.1 million, reduce paid-in capital in excess of par value by approximately $136.7 million, and decrease accumulated deficit by approximately $77.6 million within its consolidated balance sheet. Future non-cash interest expense related to convertible instruments will be lower as a result of adoption of this guidance and net income per share will be computed using the if-converted method for convertible instruments.
In December 2021, the Company made an irrevocable election under the indenture governing the convertible senior notes due 2024, or the 2024 Convertible Notes, to require the principal portion of the 2024 Convertible Notes to be settled in cash and any excess in shares or cash. Following the irrevocable election, only the amounts expected to be settled in excess of the principal will be considered in diluted earnings per share under the if-converted method pursuant to ASU 2020-06. This irrevocable election under the indenture had no impact to the Company’s consolidated financial statements as of and for the year ended December 31, 2021.
84
In November 2021, the FASB issued ASU No. 2021-10, Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance. This ASU increases the transparency of government assistance including the disclosure of: (1) the types of assistance, (2) an entity’s accounting for the assistance, and (3) the effect of the assistance on an entity’s financial statements. The amendments in this update are effective for reporting periods beginning after December 15, 2021, with early adoption permitted. The adoption of this guidance will not have a material impact on the Company’s consolidated financial statements.
Significant Accounting Policies
Consolidation Policy
The consolidated financial statements include the accounts of Herbalife Nutrition Ltd. and its subsidiaries. All significant intercompany transactions and accounts have been eliminated.
Foreign Currency Translation and Transactions
In the majority of the countries that the Company operates, the functional currency is the local currency. The Company’s foreign subsidiaries’ asset and liability accounts are translated for consolidated financial reporting purposes into U.S. dollar amounts at year-end exchange rates. Revenue and expense accounts are translated at the average rates during the year. Foreign exchange translation adjustments are included in accumulated other comprehensive loss on the accompanying consolidated balance sheets. Foreign currency transaction gains and losses, which include the cost of foreign currency derivative contracts and the related settlement gains and losses but excluding certain foreign currency derivatives designated as cash flow hedges as discussed in Note 11, Derivative Instruments and Hedging Activities, are included in selling, general, and administrative expenses within the accompanying consolidated statements of income. The Company recorded net foreign currency transaction losses of $6.2 million, $14.8 million, and $2.0 million for the years ended December 31, 2021, 2020, and 2019, respectively.
Forward Exchange Contracts, Option Contracts, and Interest Rate Swaps
The Company enters into foreign currency derivatives, primarily comprised of foreign currency forward contracts and option contracts, in managing its foreign exchange risk on sales to Members, inventory purchases denominated in foreign currencies, and intercompany transactions and loans. The Company also enters into interest rate swaps in managing its interest rate risk on its variable rate senior secured credit facility. The Company does not use the contracts for trading purposes.
In accordance with FASB ASC Topic 815, Derivatives and Hedging, or ASC 815, the Company designates certain of its derivative instruments as cash flow hedges and formally documents its hedge relationships, including identification of the hedging instruments and the hedged items, as well as its risk management objectives and strategies for undertaking the hedge transaction, at the time the derivative contract is executed. The Company assesses the effectiveness of the hedge both at inception and on an ongoing basis and determines whether the hedge is highly or perfectly effective in offsetting changes in cash flows of the hedged item. The Company records changes in the estimated fair value in accumulated other comprehensive loss and subsequently reclassifies the related amount of accumulated other comprehensive loss to earnings when the hedged item and underlying transaction impacts earnings. If it is determined that a derivative has ceased to be a highly effective hedge, the Company will discontinue hedge accounting for such transaction. For derivatives that are not designated as hedges, all changes in estimated fair value are recognized in the consolidated statements of income.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity of three months or less to be cash equivalents. Cash and cash equivalents are comprised primarily of domestic and foreign bank accounts and money market funds. These cash and cash equivalents are valued based on Level 1 inputs, which consist of quoted prices in active markets. To reduce its credit risk, the Company monitors the credit standing of the financial institutions that hold the Company’s cash and cash equivalents.
The Company has a cash pooling arrangement with a financial institution for cash management purposes. This cash pooling arrangement allows certain of the Company’s participating subsidiaries to withdraw cash from this financial institution based upon the Company’s aggregate cash deposits held by subsidiaries who participate in the cash pooling arrangement. To the extent any participating location on an individual basis is in an overdraft position, these overdrafts will be recorded as liabilities and reflected as financing activities in the Company’s consolidated balance sheets and consolidated statements of cash flows, respectively. The Company did not owe any amounts to this financial institution as of December 31, 2021 and 2020.
85
Accounts Receivable
Accounts receivable consist principally of receivables from credit card companies, arising from the sale of products to the Company’s Members, and receivables from importers, who are utilized in a limited number of countries to sell products to Members. The Company believes the concentration of its collection risk related to its credit card receivables is reduced due to geographic dispersion. Credit card receivables were $53.0 million and $65.2 million as of December 31, 2021 and 2020, respectively. Substantially all credit card receivables were current as of December 31, 2021 and 2020. For the Company’s receivables from its importers, the Company performs ongoing credit evaluations of its importers and maintains an allowance for potential credit losses. The Company considers customer credit-worthiness, past and current transaction history with the customer, contractual terms, current economic industry trends, and changes in customer payment terms when determining whether collectability is reasonably assured and whether to record allowances for its receivables. If the financial condition of the Company’s customers deteriorates and adversely affects their ability to make payments, additional allowances will be recorded. The Company believes that it provides adequate allowances for receivables from its Members and importers which are not material to its consolidated financial statements. The Company recorded bad-debt expense related to allowances for the Company’s receivables of $0.1 million, $1.7 million, and $3.0 million during the years ended December 31, 2021, 2020, and 2019, respectively. As of December 31, 2021 and 2020, the Company’s allowance for doubtful accounts was $2.5 million and $3.3 million, respectively. As of December 31, 2021 and 2020, the majority of the Company’s total outstanding accounts receivable were current.
Fair Value of Financial Instruments
The Company applies the provisions of FASB authoritative guidance as it applies to its financial and non-financial assets and liabilities. The FASB authoritative guidance clarifies the definition of fair value, prescribes methods for measuring fair value, establishes a fair value hierarchy based on the inputs used to measure fair value, and expands disclosures about fair value measurements.
The Company has estimated the fair value of its financial instruments using the following methods and assumptions:
Inventories
Inventories are stated at lower of cost (primarily on the first-in, first-out basis) and net realizable value.
Debt Issuance Costs
Debt issuance costs represent fees and expenses related to the borrowing of the Company’s long-term debt and are generally amortized over the term of the related debt using the effective interest method. Debt issuance costs, except for those related to the Company’s revolving credit facility, are recorded as a reduction to debt (contra-liability) within the Company’s consolidated balance sheets. Total amortization expense related to debt issuance costs were $6.0 million, $4.6 million, and $5.3 million for the years ended December 31, 2021, 2020, and 2019, respectively. As of December 31, 2021 and 2020, the Company’s remaining unamortized debt issuance costs were $23.9 million and $25.4 million, respectively.
86
Long-Lived Assets
As of December 31, 2021 and 2020, the Company’s net property, plant, and equipment consisted of the following:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Property, plant, and equipment, at cost: |
|
|
|
|
|
|
||
Land and buildings |
|
$ |
51.2 |
|
|
$ |
51.1 |
|
Furniture and fixtures |
|
|
27.9 |
|
|
|
26.1 |
|
Equipment |
|
|
1,127.1 |
|
|
|
1,023.7 |
|
Building and leasehold improvements |
|
|
254.5 |
|
|
|
222.8 |
|
Total property, plant, and equipment, at cost |
|
|
1,460.7 |
|
|
|
1,323.7 |
|
Less: accumulated depreciation and amortization |
|
|
(1,018.6 |
) |
|
|
(933.5 |
) |
Property, plant, and equipment, at cost, net of accumulated depreciation and amortization |
|
$ |
442.1 |
|
|
$ |
390.2 |
|
Depreciation of furniture, fixtures, and equipment (including computer hardware and software) is computed on a straight-line basis over the estimated useful lives of the related assets, which range from to ten years. The Company capitalizes eligible costs to acquire or develop internal-use software that are incurred subsequent to the preliminary project stage. Computer hardware and software, the majority of which is comprised of capitalized internal-use software costs, were $199.3 million and $188.7 million as of December 31, 2021 and 2020, respectively, net of accumulated depreciation. Leasehold improvements are amortized on a straight-line basis over the life of the related asset or the term of the lease, whichever is shorter. Buildings are depreciated over 40 years. Building improvements are generally depreciated over to fifteen years. Land is not depreciated. Depreciation and amortization expenses recorded to selling, general, and administrative expenses totaled $89.2 million, $80.9 million, and $78.8 million, for the years ended December 31, 2021, 2020, and 2019, respectively.
Long-lived assets are reviewed for impairment based on undiscounted cash flows whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Measurement of an impairment loss is based on the estimated fair value of the asset.
Goodwill and marketing-related intangible assets with indefinite lives are evaluated on an annual basis for impairment or more frequently if events or changes in circumstances indicate that the asset might be impaired. For goodwill, the Company performed a quantitative assessment during the fourth quarter of 2021, in which it used a discounted cash flow approach to estimate the fair value of a reporting unit, and determined that the fair value of each reporting unit was greater than its respective carrying value. If the fair value of the reporting unit was less than the carrying value, then a goodwill impairment amount would be recorded for the difference. For the marketing-related intangible assets, the Company performed a quantitative assessment during the fourth quarter of 2021, in which it used a discounted cash flow model under the relief-from-royalty method in order to determine the fair value, and determined that the fair value of the assets was greater than their carrying value. If the fair value of the assets was less than the carrying value, then an impairment amount would be recorded for the difference. During the years ended December 31, 2021, 2020, and 2019, there were no additions to or impairments of marketing-related intangible assets. As of both December 31, 2021 and 2020, the marketing-related intangible asset balance was $310.0 million and consisted of the Company’s trademark, trade name, and marketing franchise. During the years ended December 31, 2021 and 2019, there were no additions to or impairments of goodwill. During the year ended December 31, 2020, goodwill increased by $9.0 million, of which $7.0 million was due to an immaterial acquisition and $2.0 million was due to foreign currency translation adjustments. During the year ended December 31, 2020, there was no impairment of goodwill. As of December 31, 2021 and 2020, the goodwill balance was $95.4 million and $100.5 million, respectively. The decrease in goodwill during the year ended December 31, 2021 was due to foreign currency translation adjustments. The cash paid for the immaterial acquisition during 2020 is reflected as other cash flows from investing activities within the Company’s consolidated statements of cash flows.
87
Restricted Cash
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the Company’s consolidated balance sheets that sum to the total of the same such amounts shown in the Company’s consolidated statements of cash flows:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Cash and cash equivalents |
|
$ |
601.5 |
|
|
$ |
1,045.4 |
|
Restricted cash included in Prepaid expenses and other current assets |
|
|
2.6 |
|
|
|
2.5 |
|
Restricted cash included in Other assets |
|
|
6.3 |
|
|
|
6.1 |
|
Total cash, cash equivalents, and restricted cash shown in the statement of cash flows |
|
$ |
610.4 |
|
|
$ |
1,054.0 |
|
The majority of the Company’s consolidated restricted cash is held by certain of its foreign entities and consists of cash deposits that are required due to the business operating requirements in those jurisdictions.
Income Taxes
Income tax expense includes income taxes payable for the current year and the change in deferred income tax assets and liabilities for the future tax consequences of events that have been recognized in the Company’s financial statements or income tax returns. A valuation allowance is recognized to reduce the carrying value of deferred income tax assets if it is believed to be more likely than not that a component of the deferred income tax assets will not be realized.
The Company accounts for uncertainty in income taxes in accordance with FASB authoritative guidance which clarifies the accounting and reporting for uncertainties in income taxes recognized in an enterprise’s financial statements. This guidance prescribes a comprehensive model for the financial statement recognition, measurement, presentation and disclosure of uncertain tax positions taken or expected to be taken in income tax returns.
The Company has made an accounting policy election to account for global intangible low-taxed income as a period cost if and when incurred.
Royalty Overrides
Certain Members may earn commissions called royalty overrides, which include production bonuses, based on retail sales volume. Royalty overrides are based on the retail sales volume of certain other Members who are sponsored directly or indirectly by the Member. Royalty overrides are recorded when the products are delivered and revenue is recognized. The royalty overrides are compensation to Members for services rendered including the development, retention and the improved productivity of their sales organizations. As such royalty overrides are classified as an operating expense. Non-U.S. royalty override checks that have aged, for a variety of reasons, beyond a certainty of being paid, are taken back into income. Management has estimated this period of certainty to be three years worldwide.
Distributor Compensation – U.S.
In the U.S., distributor compensation, including Royalty overrides, is capped if the Company does not meet an annual requirement as described in the consent order discussed in more detail in Note 7, Contingencies. On a periodic basis, the Company evaluates if this requirement will be achieved by year-end to determine if a cap on distributor compensation will be required, and then determines the appropriate amount of distributor compensation expense, which may vary in each reporting period. The Company determined that the cap to distributor compensation will not be applicable for the year ended December 31, 2021 as the annual requirement was met.
Comprehensive Income
Comprehensive income consists of net income, foreign currency translation adjustments, and unrealized gains or losses on derivatives. See Note 8, Shareholders’ Deficit, for the description and detail of the components of accumulated other comprehensive loss.
88
Operating Leases
The Company leases most of its physical properties under operating leases. The Company recognizes rent expense on a straight-line basis for its operating leases. Certain lease agreements generally include rent holidays and tenant improvement allowances. Prior to January 1, 2019, the Company recognized rent holiday periods on a straight-line basis over the lease term beginning when the Company had the right to the leased space; the Company also recorded tenant improvement allowances and rent holidays as deferred rent liabilities and amortized the deferred rent over the terms of the lease to rent expense. Prior to January 1, 2019, the Company did not recognize its operating leases on its balance sheet. Beginning January 1, 2019, the Company recognizes a right of use asset and lease liability within its consolidated balance sheets for operating leases with terms greater than twelve months. The initial measurement of the lease liability is measured at the present value of lease payments not yet paid discounted generally using the Company’s incremental borrowing rate at the commencement date. Leases with an initial term of twelve months or less are not recorded on the Company’s consolidated balance sheets, and the Company does not separate nonlease components from lease components.
Research and Development
The Company’s research and development is performed by in-house staff and outside consultants. For all periods presented, research and development costs were expensed as incurred and were not material.
Other Operating Income
To encourage local investment and operations, governments in various China provinces conduct grant programs. The Company applied for and received several such grants in China. Government grants are recorded into income when a legal right to the grant exists, there is a reasonable assurance that the grant proceeds will be received, and the substantive conditions under which the grants were provided have been met. Generally, these substantive conditions are the Company maintaining operations and paying certain taxes in the relevant province and obtaining government approval by completing an annual application process. The Company believes the continuing obligation with respect to the funds is a general requirement that they are used only for its business in China. The Company recognized government grant income related to its regional headquarters and distribution centers within China of approximately $16.4 million, $14.5 million, and $31.5 million during the years ended December 31, 2021, 2020, and 2019, respectively, in other operating income within its consolidated statements of income. The Company intends to continue applying for government grants in China when programs are available; however, there is no assurance that the Company will receive grants in future periods.
During the year ended December 31, 2019, the Company also recognized $6.0 million in other operating income within its consolidated statement of income related to the finalization of insurance recoveries in connection with the flooding at one of its warehouses in Mexico during September 2017, which damaged certain of the Company’s inventory stored within the warehouse. See Note 7, Contingencies, to the Consolidated Financial Statements included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2018 for further discussion.
Other Expense (Income), Net
During the year ended December 31, 2021, the Company recognized a $24.6 million loss on the extinguishment of the 2026 Notes (See Note 5, Long-Term Debt) in other expense (income), net within its consolidated statements of income. During the year ended December 31, 2020, the Company did not recognize any other expense (income), net. During the year ended December 31, 2019, the Company recognized a gain of $15.7 million on the revaluation of the non-transferable contractual contingent value right, or CVR, provided for each share tendered in the October 2017 modified Dutch auction tender offer (See Note 8, Shareholders’ Deficit) in other expense (income), net within its consolidated statements of income.
Professional Fees
The Company expenses professional fees, including legal fees, as incurred. These professional fees are included in selling, general, and administrative expenses within the Company’s consolidated statements of income.
Advertising
Advertising costs, including Company sponsorships, are expensed as incurred and amounted to approximately $47.3 million, $39.0 million, and $41.4 million for the years ended December 31, 2021, 2020, and 2019, respectively. These expenses are included in selling, general, and administrative expenses within the Company’s consolidated statements of income.
89
Earnings Per Share
Basic earnings per share represents net income divided by the weighted-average number of common shares outstanding for the period. Diluted earnings per share represents net income divided by the weighted-average number of common shares outstanding, inclusive of the effect of dilutive securities, such as outstanding stock appreciation rights, or SARs, stock units, and convertible notes.
The following are the common share amounts used to compute the basic and diluted earnings per share for each period:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Weighted-average shares used in basic computations |
|
|
105.9 |
|
|
|
131.5 |
|
|
|
137.4 |
|
Dilutive effect of exercise of equity grants outstanding |
|
|
2.4 |
|
|
|
3.0 |
|
|
|
3.5 |
|
Dilutive effect of 2019 Convertible Notes |
|
|
— |
|
|
|
— |
|
|
|
0.7 |
|
Weighted-average shares used in diluted computations |
|
|
108.3 |
|
|
|
134.5 |
|
|
|
141.6 |
|
There were an aggregate of 1.0 million, 0.8 million, and 0.8 million of equity grants, consisting of SARs and stock units that were outstanding during the years ended December 31, 2021, 2020, and 2019, respectively, but were not included in the computation of diluted earnings per share because their effect would be anti-dilutive or the performance condition of the award had not been satisfied.
Since the Company was required to settle the principal amount of its 2019 Convertible Notes in cash and settle the conversion feature for the amount above the conversion price in common shares, or the conversion spread, the Company used the treasury stock method for calculating any potential dilutive effect of the conversion spread on diluted earnings per share, if applicable. The conversion spread would have had a dilutive impact on diluted earnings per share when the average market price of the Company’s common shares for a given period exceeded the conversion price of the 2019 Convertible Notes. The dilutive impact for the year ended December 31, 2019 is disclosed in the table above. The initial conversion rate and conversion price for the 2019 Convertible Notes are described further in Note 5, Long-Term Debt.
For the 2024 Convertible Notes, the Company is required to settle the principal amount in cash and has the option to settle the conversion feature for the amount above the conversion price, or the conversion spread, in common shares or cash. The Company uses the treasury stock method for calculating any potential dilutive effect of the conversion spread on diluted earnings per share, if applicable. The conversion spread will have a dilutive impact on diluted earnings per share when the average market price of the Company’s common shares for a given period exceeds the conversion price of the 2024 Convertible Notes. For the years ended December 31, 2021, 2020, and 2019, the 2024 Convertible Notes have been excluded from the computation of diluted earnings per share, as the effect would be anti-dilutive since the conversion price of the 2024 Convertible Notes exceeded the average market price of the Company’s common shares for the years ended December 31, 2021, 2020, and 2019. The initial conversion rate and conversion price for the 2024 Convertible Notes are described further in Note 5, Long-Term Debt.
The capped call transactions executed in connection with the issuance of the 2019 Convertible Notes, or the Capped Call Transactions, were excluded from the calculation of diluted earnings per share because their impact is always anti-dilutive. Additionally, the prepaid forward share repurchase transactions executed in connection with the issuance of the 2019 Convertible Notes, or the Forward Transactions, were treated as retired shares for basic and diluted EPS purposes, in each case for the periods the transactions were in effect. On August 15, 2019, the remaining Capped Call Transactions expired unexercised and all shares were retired under the Forward Transactions. See Note 8, Shareholders’ Deficit, for additional discussion regarding the Capped Call Transactions and Forward Transactions.
See Note 8, Shareholders’ Deficit, for a discussion of how common shares repurchased by the Company’s indirect wholly-owned subsidiary are treated under U.S. GAAP.
90
Revenue Recognition
The Company’s net sales consist of product sales. In general, the Company’s performance obligation is to transfer its products to its Members. The Company generally recognizes revenue when product is delivered to its Members. For the majority of China independent service providers and for third-party importers utilized in certain other countries where sales historically have not been material, the Company recognizes revenue based on the Company’s estimate of when the service provider or third-party importer sells the products because the Company is deemed to be the principal party of these product sales due to the additional selling and operating requirements relating to pricing of products, conducting business with physical locations, and other selling and marketing activities required of the service providers and third-party importers. Beginning January 1, 2022, the Company will begin recognizing revenue for certain China independent service providers upon delivery as such Members will have pricing discretion and increased fulfillment responsibilities and accordingly have been determined to be the Company’s customers for accounting purposes.
The Company’s Members, excluding its China independent service providers, may receive distributor allowances, which are comprised of discounts, rebates, and wholesale commission payments from the Company. Distributor allowances resulting from the Company’s sales of its products to its Members are recorded against net sales because the distributor allowances represent discounts from the suggested retail price.
The Company compensates its sales leader Members with royalty overrides for services rendered relating to the development, retention, and management of their sales organizations. Royalty overrides are payable based on achieved sales volume. Royalty overrides are classified as an operating expense reflecting the services provided to the Company. The Company compensates its China independent service providers and third-party importers utilized in certain other countries for providing marketing, selling, and customer support services. As the Company is the principal party for the majority of product sales as described above, the service fees payable to China independent service providers and the compensation received by third-party importers for the services they provide, which represents the discount provided to them, are recorded in selling, general, and administrative expenses within the Company’s consolidated statements of income. For those certain China independent service providers who are deemed to be the Company’s customers for accounting purposes, a portion of the service fees payable to these Members will be classified as a reduction of net sales as opposed to the entire service fee being recognized within selling, general, and administrative expenses.
The Company recognizes revenue when it delivers products to its United States Members; distributor allowances, inclusive of discounts and wholesale commissions, are recorded as a reduction to net sales; and royalty overrides are classified as an operating expense.
Shipping and handling services relating to product sales are recognized as fulfillment activities on the Company’s performance obligation to transfer products and are therefore recorded within net sales as part of product sales and are not considered as separate revenues. Shipping and handling costs paid by the Company are included in cost of sales.
The Company presents sales taxes collected from customers on a net basis.
The Company generally receives the net sales price in cash or through credit card payments at the point of sale.
The Company records advance sales deposits when payment is received but revenue has not yet been recognized. In the majority of the Company’s markets, advance sales deposits are generally recorded to income when the product is delivered to its Members. Additionally, advance sales deposits also include deferred revenues due to the timing of revenue recognition for products sold through China independent service providers. The estimated deferral period for advance sales deposits is generally within one week. During the year ended December 31, 2021, the Company recognized substantially all of the revenues that were included within advance sales deposits as of December 31, 2020 and any remaining such balance was not material as of December 31, 2021. Advance sales deposits are included in other current liabilities on the Company’s consolidated balance sheets. See Note 15, Detail of Certain Balance Sheet Accounts, for further information.
In general, if a Member returns product to the Company on a timely basis, they may obtain replacement product from the Company for such returned products. In addition, in general the Company maintains a buyback program pursuant to which it will repurchase products sold to a Member who has decided to leave the business. Allowances for product returns, primarily in connection with the Company’s buyback program, are provided at the time the sale is recorded. This accrual is based upon historical return rates for each country and the relevant return pattern, which reflects anticipated returns to be received over a period of up to 12 months following the original sale. Allowances for product returns were $3.4 million and $3.7 million as of December 31, 2021 and 2020, respectively.
91
The Company’s products are grouped in five principal categories: weight management; targeted nutrition; energy, sports, and fitness; outer nutrition; and literature and promotional items. However, the effect of economic factors on the nature, amount, timing, and uncertainty of revenue recognition and cash flows are similar among all five product categories. The Company defines its operating segments through six geographic regions. The effect of economic factors on the nature, amount, timing, and uncertainty of revenue recognition and cash flows are similar among the geographic regions within the Company’s Primary Reporting Segment. See Note 10, Segment Information, for further information on the Company’s reportable segments and the Company’s presentation of disaggregated revenue by reportable segment.
Non-Cash Investing and Financing Activities
During the years ended December 31, 2021, 2020, and 2019, the Company recorded $24.6 million, $18.0 million, and $14.1 million, respectively, of non-cash capital expenditures.
During the years ended December 31, 2021 and 2020, the Company did not record any non-cash borrowings. During the year ended December 31, 2019, the Company recorded $5.9 million of non-cash borrowings that were used to finance software maintenance. Additionally, see Note 8, Shareholders’ Deficit, for information on the Company’s non-cash financing activities related to the CVR, as well as share repurchases for which payment was made subsequent to year end.
Share-Based Payments
The Company accounts for share-based compensation in accordance with FASB authoritative guidance which requires the measurement of share-based compensation expense for all share-based payment awards made to employees. The Company measures share-based compensation cost at the grant date, based on the fair value of the award. The Company recognizes share-based compensation expense for service condition awards on a straight-line basis over the employee’s requisite service period. The Company recognizes share-based compensation expense for performance condition awards over the vesting term using the graded vesting method.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions. Such estimates and assumptions affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors, including the current economic environment, which the Company believes to be reasonable under the circumstances. The Company adjusts such estimates and assumptions when facts and circumstances dictate. Illiquid credit markets, volatile equity, and foreign currency have combined to increase the uncertainty inherent in such estimates and assumptions. As future events and their effects cannot be determined with precision, actual results could differ from these estimates. Changes in estimates resulting from continuing changes in the economic environment will be reflected in the financial statements in future periods.
COVID-19 Pandemic
During March 2020, the World Health Organization characterized the outbreak of coronavirus disease 2019, or COVID-19, as a pandemic. In response to the spread of COVID-19, certain government agencies and the Company itself have mandated various measures and recommended others, in each to protect the public and the Company’s employees, which have disrupted certain areas of the Company’s business including, but not limited to, distribution and selling activities. Despite the pandemic having a negative impact in certain of the Company’s markets, the Company’s consolidated net sales was higher for the year ended December 31, 2021 as compared to the same period in 2020. The ultimate extent and magnitude of the impact of COVID-19 is not known and could have a material adverse impact to the Company’s business and future financial condition and results of operations. Management has been and continues to actively monitor the impact of COVID-19 generally and on the Company.
The Company’s consolidated financial statements presented herein reflect the latest estimates and assumptions made by management that affect the reported amounts of assets and liabilities and related disclosures as of the date of the consolidated financial statements and reported amounts of revenue and expenses during the reporting periods presented. The Company believes it has used reasonable estimates and assumptions to assess the fair values of its goodwill, marketing-related intangible assets, and long-lived assets; assessment of the annual effective tax rate; valuation of deferred income taxes; and the allowance for doubtful accounts. After reviewing historical and forward-looking information, the Company determined there were no impairments required relating to its goodwill, marketing-related intangible assets, and long-lived assets during the year ended December 31, 2021.
92
3. Inventories
The following are the major classes of inventory:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Raw materials |
|
$ |
81.8 |
|
|
$ |
80.1 |
|
Work in process |
|
|
8.6 |
|
|
|
7.9 |
|
Finished goods |
|
|
485.3 |
|
|
|
413.4 |
|
Total |
|
$ |
575.7 |
|
|
$ |
501.4 |
|
4. Leases
Generally, the Company leases certain office space, warehouses, distribution centers, manufacturing centers, and equipment. A contract is or contains a lease if the contract conveys the right to control the use of identified property, plant, or equipment (an identified asset) for a period of time in exchange for consideration. The Company also rents or subleases certain real estate to third parties. Sublease income was not material for the years ended December 31, 2021, 2020, and 2019.
In general, the Company’s leases include one or more options to renew, with renewal terms that generally vary from one to ten years. The exercise of lease renewal options is generally at the Company’s sole discretion. Certain leases also include options to purchase the leased property. The depreciable life of assets and leasehold improvements are limited by the expected lease term, unless there is a transfer of title or purchase option reasonably certain of exercise.
The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants.
Leases with an initial term of twelve months or less are not recorded on the Company’s consolidated balance sheets, and the Company does not separate nonlease components from lease components. The Company’s lease assets and liabilities recognized within its consolidated balance sheets were as follows:
|
|
December 31, |
|
|
|
|||||
|
|
2021 |
|
|
2020 |
|
|
Balance Sheet Location |
||
|
|
(in millions) |
|
|
|
|||||
ASSETS: |
|
|
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
$ |
220.0 |
|
|
$ |
222.8 |
|
|
|
Finance lease right-of-use assets |
|
|
1.1 |
|
|
|
0.5 |
|
|
|
Total lease assets |
|
$ |
221.1 |
|
|
$ |
223.3 |
|
|
|
LIABILITIES: |
|
|
|
|
|
|
|
|
||
Current: |
|
|
|
|
|
|
|
|
||
Operating lease liabilities |
|
$ |
42.8 |
|
|
$ |
35.5 |
|
|
|
Finance lease liabilities |
|
|
0.4 |
|
|
|
0.2 |
|
|
|
Non-current: |
|
|
|
|
|
|
|
|
||
Operating lease liabilities |
|
|
201.2 |
|
|
|
206.7 |
|
|
|
Finance lease liabilities |
|
|
0.7 |
|
|
|
0.3 |
|
|
|
Total lease liabilities |
|
$ |
245.1 |
|
|
$ |
242.7 |
|
|
|
(1) Finance lease assets are recorded net of accumulated amortization of $1.9 million and $1.7 million as of December 31, 2021 and 2020, respectively.
93
Lease cost is recognized on a straight-line basis over the lease term. The components of lease cost are as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Operating lease cost(1)(2) |
|
$ |
67.1 |
|
|
$ |
63.8 |
|
|
$ |
65.7 |
|
Finance lease cost |
|
|
|
|
|
|
|
|
|
|||
Amortization of right-of-use assets |
|
|
0.3 |
|
|
|
0.4 |
|
|
|
0.4 |
|
Interest on lease liabilities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Net lease cost |
|
$ |
67.4 |
|
|
$ |
64.2 |
|
|
$ |
66.1 |
|
(1)
(2)
As of December 31, 2021, annual scheduled lease payments were as follows:
|
|
Operating Leases(1) |
|
|
Finance Leases |
|
||
|
|
(in millions) |
|
|||||
2022 |
|
$ |
53.1 |
|
|
$ |
0.4 |
|
2023 |
|
|
40.9 |
|
|
|
0.4 |
|
2024 |
|
|
37.2 |
|
|
|
0.3 |
|
2025 |
|
|
29.2 |
|
|
|
— |
|
2026 |
|
|
23.2 |
|
|
|
— |
|
Thereafter |
|
|
117.2 |
|
|
|
— |
|
Total lease payments |
|
|
300.8 |
|
|
|
1.1 |
|
Less: imputed interest |
|
|
56.8 |
|
|
|
— |
|
Present value of lease liabilities |
|
$ |
244.0 |
|
|
$ |
1.1 |
|
(1)
In general, for the majority of the Company’s material leases, the renewal options are not included in the calculation of its right-of-use assets and lease liabilities, as the Company does not believe that it is reasonably certain that these renewal options will be exercised. Periodically, the Company assesses its leases to determine whether it is reasonably certain that these renewal options will be exercised.
The majority of the Company’s leases are for real estate and in general, the individual lease contracts do not provide information about the rate implicit in the lease. Because the Company is not able to determine the rate implicit in its leases, it instead generally uses its incremental borrowing rate to determine the present value of lease liabilities. In determining its incremental borrowing rate, the Company reviewed the terms of its leases, its senior secured credit facility, swap rates, and other factors. The weighted-average remaining lease term and weighted-average discount rate used to calculate the present value of lease liabilities are as follows:
|
|
December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
Weighted-average remaining lease term: |
|
|
|
|
|
|
|
|
|
|||
Operating leases |
|
7.8 years |
|
|
8.3 years |
|
|
8.3 years |
|
|||
Finance leases |
|
3.0 years |
|
|
3.1 years |
|
|
3.2 years |
|
|||
Weighted-average discount rate: |
|
|
|
|
|
|
|
|
|
|||
Operating leases |
|
|
4.8 |
% |
|
|
5.5 |
% |
|
|
5.6 |
% |
Finance leases |
|
|
3.6 |
% |
|
|
5.1 |
% |
|
|
5.4 |
% |
94
Supplemental cash flow information related to leases is as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
|
|
|||
Operating cash flows for operating leases |
|
$ |
50.2 |
|
|
$ |
50.3 |
|
|
$ |
45.9 |
|
Operating cash flows for finance leases |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Financing cash flows for finance leases |
|
|
0.3 |
|
|
|
0.5 |
|
|
|
0.4 |
|
Right-of-use assets obtained in exchange for new lease liabilities: |
|
|
|
|
|
|
|
|
|
|||
Operating leases |
|
|
46.0 |
|
|
|
74.2 |
|
|
|
55.2 |
|
Finance leases |
|
|
1.0 |
|
|
|
0.1 |
|
|
|
0.6 |
|
5. Long-Term Debt
Long-term debt consists of the following:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Borrowings under senior secured credit facility, carrying value |
|
$ |
1,088.6 |
|
|
$ |
976.5 |
|
2.625% convertible senior notes due , carrying value of liability component |
|
|
486.0 |
|
|
|
460.6 |
|
7.875% senior notes due , carrying value |
|
|
594.2 |
|
|
|
592.9 |
|
7.250% senior notes due , carrying value |
|
|
— |
|
|
|
395.9 |
|
4.875% senior notes due , carrying value |
|
|
592.8 |
|
|
|
— |
|
Other |
|
|
1.0 |
|
|
|
2.5 |
|
Total |
|
|
2,762.6 |
|
|
|
2,428.4 |
|
Less: current portion |
|
|
29.4 |
|
|
|
22.9 |
|
Long-term portion |
|
$ |
2,733.2 |
|
|
$ |
2,405.5 |
|
Senior Secured Credit Facility
On August 16, 2018, the Company entered into a $1.25 billion senior secured credit facility, or the 2018 Credit Facility, consisting of a $250.0 million term loan A, or the 2018 Term Loan A, a $750.0 million term loan B, or the 2018 Term Loan B, and a $250.0 million revolving credit facility, or the 2018 Revolving Credit Facility, with a syndicate of financial institutions as lenders. The 2018 Term Loan B matures upon the earlier of: (i) August 18, 2025; or (ii) December 15, 2023 if the outstanding principal on the 2024 Convertible Notes, as defined below, exceeds $350.0 million and the Company exceeds certain leverage ratios as of that date. All obligations under the 2018 Credit Facility are unconditionally guaranteed by certain direct and indirect wholly-owned subsidiaries of Herbalife Nutrition Ltd. and secured by the equity interests of certain of Herbalife Nutrition Ltd.’s subsidiaries and substantially all of the assets of the domestic loan parties. Also on August 16, 2018, the Company issued $400.0 million aggregate principal amount of senior unsecured notes, or the 2026 Notes as described below, and used the proceeds from the 2018 Credit Facility and the 2026 Notes to repay in full the $1,178.1 million outstanding under the Company’s prior senior secured credit facility.
The 2018 Term Loan B was issued to the lenders at a 0.25% discount, or $1.9 million. The Company incurred approximately $11.7 million of debt issuance costs in connection with the 2018 Credit Facility. The discount and debt issuance costs are recorded on the Company’s consolidated balance sheet and are being amortized over the life of the 2018 Credit Facility using the effective-interest method.
On December 12, 2019, the Company amended the 2018 Credit Facility which, among other things, reduced the interest rate for borrowings under the 2018 Term Loan B from either the eurocurrency rate plus a margin of 3.25% or the base rate plus a margin of 2.25% to either the eurocurrency rate plus a margin of 2.75% or the base rate plus a margin of 1.75%. The Company incurred approximately $1.2 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. The debt issuance costs were recognized in interest expense within the Company’s consolidated statement of income during the fourth quarter of 2019.
95
On March 19, 2020, the Company amended the 2018 Credit Facility which, among other things, extended the maturity of both the 2018 Term Loan A and 2018 Revolving Credit Facility to the earlier of: (i) March 19, 2025; or (ii) September 15, 2023 if the outstanding principal on the 2024 Convertible Notes, as defined below, exceeds $350.0 million and the Company exceeds certain leverage ratios as of that date; increased borrowings under the 2018 Term Loan A from $234.4 million to a total of $264.8 million; increased the total available borrowing capacity under 2018 Revolving Credit Facility from $250.0 million to $282.5 million; and reduced the interest rate for borrowings under both the 2018 Term Loan A and 2018 Revolving Credit Facility from either the eurocurrency rate plus a margin of 3.00% or the base rate plus a margin of 2.00% to either the eurocurrency rate plus a margin of 2.50% or the base rate plus a margin of 1.50%. The Company incurred approximately $1.6 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. Of the $1.6 million of debt issuance costs, approximately $1.1 million was recorded on the Company’s consolidated balance sheet and is being amortized over the life of the 2018 Credit Facility using the effective-interest method, and approximately $0.5 million was recognized in interest expense within the Company’s consolidated statement of income during the first quarter of 2020.
On February 10, 2021, the Company amended the 2018 Credit Facility which, among other things, reduced the interest rate for borrowings under the 2018 Term Loan B from either the eurocurrency rate plus a margin of 2.75% or the base rate plus a margin of 1.75% to either the eurocurrency rate plus a margin of 2.50% or the base rate plus a margin of 1.50%. The Company incurred approximately $1.1 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. The debt issuance costs were recognized in interest expense within the Company’s consolidated statement of income during the first quarter of 2021.
On July 30, 2021, the Company amended the 2018 Credit Facility which, among other things, increased borrowings under the 2018 Term Loan A from $245.0 million to a total of $286.2 million; increased the total available borrowing capacity under the 2018 Revolving Credit Facility from $282.5 million to $330.0 million; reduced the interest rate for borrowings under the 2018 Term Loan A and 2018 Revolving Credit Facility from either the eurocurrency rate plus a margin of 2.50% or the base rate plus a margin of 1.50% to, depending on the Company’s total leverage ratio, either the eurocurrency rate plus a margin of between 1.75% and 2.25% or the base rate plus a margin of between 0.75% and 1.25%; and amended the commitment fee on the undrawn portion of the 2018 Revolving Credit Facility from 0.35% per annum to, depending on the Company’s total leverage ratio, between 0.25% to 0.35% per annum. As a result of the amendment, the applicable margin for the 2018 Term Loan A and 2018 Revolving Credit Facility may also be subject to certain premiums or discounts tied to criteria determined by certain sustainability targets. The Company incurred approximately $1.4 million of debt issuance costs in connection with the amendment. For accounting purposes, pursuant to ASC 470, this transaction was accounted for as a modification of the 2018 Credit Facility. Of the $1.4 million of debt issuance costs, approximately $0.8 million was recorded on the Company’s consolidated balance sheet and is being amortized over the life of the 2018 Credit Facility using the effective-interest method, and approximately $0.6 million was recognized in interest expense within the Company’s consolidated statement of income during the third quarter of 2021.
Under the 2018 Credit Facility, borrowings under both the 2018 Term Loan A and 2018 Revolving Credit Facility bear interest at, depending on the Company’s total leverage ratio, either the eurocurrency rate plus a margin of between 1.75% and 2.25% or the base rate plus a margin of between 0.75% and 1.25%. The applicable margin may also be subject to certain premiums or discounts tied to criteria determined by certain sustainability targets. Borrowings under the 2018 Term Loan B bear interest at either the eurocurrency rate plus a margin of 2.50% or the base rate plus a margin of 1.50%. The eurocurrency rate is based on adjusted LIBOR and is subject to a floor of 0.00%. The base rate represents the highest of the Federal Funds Rate plus 0.50%, one-month adjusted LIBOR plus 1.00%, and the prime rate quoted by The Wall Street Journal, and is subject to a floor of 1.00%. The Company is required to pay a commitment fee on the 2018 Revolving Credit Facility of, depending on the Company’s total leverage ratio, between 0.25% to 0.35% per annum on the undrawn portion of the 2018 Revolving Credit Facility. Interest is due at least quarterly on amounts outstanding under the 2018 Credit Facility.
The 2018 Credit Facility requires the Company to comply with a leverage ratio. The 2018 Credit Facility also contains affirmative and negative covenants customary for financings of this type, including, among other things, limitations or prohibitions on repurchasing common shares, declaring and paying dividends and other distributions, redeeming and repurchasing certain other indebtedness, loans and investments, additional indebtedness, liens, mergers, asset sales and transactions with affiliates. In addition, the 2018 Credit Facility contains customary events of default. As of December 31, 2021 and 2020, the Company was in compliance with its debt covenants under the 2018 Credit Facility.
96
The 2018 Term Loan A and 2018 Term Loan B are payable in consecutive quarterly installments which began on December 31, 2018. In addition, beginning in 2020, the Company may be required to make mandatory prepayments towards the 2018 Term Loan B based on the Company’s consolidated leverage ratio and annual excess cash flows as defined under the terms of the 2018 Credit Facility. The Company is also permitted to make voluntary prepayments. Amounts outstanding under the 2018 Term Loan A and 2018 Term Loan B may be voluntarily prepaid without premium or penalty, subject to customary breakage fees in connection with the prepayment of a eurocurrency loan. These prepayments, if any, will be applied against remaining quarterly installments owed under the 2018 Term Loan A and 2018 Term Loan B in order of maturity with the remaining principal due upon maturity, unless directed otherwise by the Company. Based on the 2021 consolidated leverage ratio and excess cash flow calculation, both as defined under the terms of the 2018 Credit Facility, the Company will not be required to make a mandatory prepayment in 2022 toward the 2018 Term Loan B.
As of December 31, 2021 and 2020, the weighted-average interest rate for borrowings under the 2018 Credit Facility was 2.62% and 3.39%, respectively.
During the year ended December 31, 2021, the Company borrowed an aggregate amount of $671.2 million under the 2018 Credit Facility, which includes $630.0 million of borrowings under the 2018 Revolving Credit Facility, and repaid a total amount of $561.3 million on amounts outstanding under the 2018 Credit Facility, which includes $480.0 million of repayments on amounts outstanding under the 2018 Revolving Credit Facility and a $60.0 million prepayment on amounts outstanding under the 2018 Term Loan B. During the year ended December 31, 2020, the Company borrowed an aggregate amount of $30.4 million under the 2018 Credit Facility and repaid a total amount of $20.7 million on amounts outstanding under the 2018 Credit Facility. During the year ended December 31, 2019, the Company did not make any borrowings under the 2018 Credit Facility and repaid a total amount of $20.0 million on amounts outstanding under the 2018 Credit Facility. As of December 31, 2021 and 2020, the U.S. dollar amount outstanding under the 2018 Credit Facility was $1,094.6 million and $984.7 million, respectively. Of the $1,094.6 million outstanding under the 2018 Credit Facility as of December 31, 2021, $279.0 million was outstanding under the 2018 Term Loan A, $665.6 million was outstanding under the 2018 Term Loan B, and $150.0 million was outstanding under the 2018 Revolving Credit Facility. Of the $984.7 million outstanding under the 2018 Credit Facility as of December 31, 2020, $251.6 million was outstanding under the 2018 Term Loan A and $733.1 million was outstanding under the 2018 Term Loan B. There were no borrowings outstanding under the 2018 Revolving Credit Facility as of December 31, 2020. There were no outstanding foreign currency borrowings under the 2018 Credit Facility as of December 31, 2021 and 2020.
During the year ended December 31, 2021, the Company recognized $33.9 million of interest expense relating to the 2018 Credit Facility, which included $0.4 million relating to non-cash interest expense relating to the debt discount and $2.3 million relating to amortization of debt issuance costs. During the year ended December 31, 2020, the Company recognized $37.2 million of interest expense relating to the 2018 Credit Facility, which included $0.3 million relating to non-cash interest expense relating to the debt discount and $1.8 million relating to amortization of debt issuance costs. During the year ended December 31, 2019, the Company recognized $58.9 million of interest expense relating to the 2018 Credit Facility, which included $0.2 million relating to non-cash interest expense relating to the debt discount and $1.7 million relating to amortization of debt issuance costs.
The fair value of the outstanding borrowings on the 2018 Term Loan A is determined by utilizing over-the-counter market quotes for similar instruments, which are considered Level 2 inputs as described in Note 13, Fair Value Measurements. As of December 31, 2021 and 2020, the carrying value of the 2018 Term Loan A was $278.1 million and $250.5 million, respectively, and the fair value was approximately $278.0 million and $251.9 million, respectively. The fair value of the outstanding borrowings under the 2018 Term Loan B are determined by utilizing over-the-counter market quotes, which are considered Level 2 inputs as described in Note 13, Fair Value Measurements. As of December 31, 2021 and 2020, the carrying amount of the 2018 Term Loan B was $660.5 million and $726.0 million, respectively, and the fair value was approximately $663.1 million and $734.0 million, respectively. The fair value of the outstanding borrowings on the 2018 Revolving Credit Facility approximated its carrying value of $150.0 million as of December 31, 2021 due to its variable interest rate which reprices frequently and represents floating market rates.
97
Convertible Senior Notes due 2019
In February 2014, the Company initially issued $1 billion aggregate principal amount of convertible senior notes, or the 2019 Convertible Notes, in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. The Company granted an option to the initial purchasers to purchase up to an additional $150.0 million aggregate principal amount of 2019 Convertible Notes which was subsequently exercised in full in February 2014, resulting in a total issuance of $1.15 billion aggregate principal amount of 2019 Convertible Notes. The 2019 Convertible Notes were senior unsecured obligations which ranked effectively subordinate to any of the Company’s existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2019 Convertible Notes paid interest at a rate of 2.00% per annum payable semiannually in arrears on February 15 and August 15 of each year, beginning on August 15, 2014. Unless earlier repurchased or converted, the 2019 Convertible Notes matured on August 15, 2019. The Company could not redeem the 2019 Convertible Notes prior to their stated maturity date. Upon conversion, the 2019 Convertible Notes were to be settled in cash and, if applicable, the Company’s common shares, based on the applicable conversion rate at such time. The 2019 Convertible Notes had an initial conversion rate of 23.1816 common shares per $1,000 principal amount of the 2019 Convertible Notes, or an initial conversion price of approximately $43.14 per common share.
The Company incurred approximately $26.6 million of issuance costs during the first quarter of 2014 relating to the issuance of the 2019 Convertible Notes. Of the $26.6 million issuance costs incurred, $21.5 million and $5.1 million were recorded as debt issuance costs and additional paid-in capital, respectively, in proportion to the allocation of the proceeds of the 2019 Convertible Notes. The $21.5 million of debt issuance costs recorded on the Company’s consolidated balance sheet were amortized over the contractual term of the 2019 Convertible Notes using the effective-interest method.
In February 2014, the $1.15 billion aggregate principal amount of the 2019 Convertible Notes were initially allocated between long-term debt, or liability component, and additional paid-in capital, or equity component, within the Company’s consolidated balance sheet at $930.9 million and $219.1 million, respectively. The liability component was measured using the nonconvertible debt interest rate. The carrying amount of the equity component representing the conversion option was determined by deducting the fair value of the liability component from the face value of the 2019 Convertible Notes as a whole. Since the Company was required to settle these 2019 Convertible Notes at face value at or prior to maturity, this liability component was accreted up to its face value resulting in additional non-cash interest expense being recognized within the Company’s consolidated statements of income while the 2019 Convertible Notes remained outstanding. The effective interest rate on the 2019 Convertible Notes was approximately 6.2% per annum. The equity component was not to be remeasured as long as it continued to meet the conditions for equity classification.
In March 2018, the Company issued $550.0 million aggregate principal amount of new convertible senior notes due 2024, or 2024 Convertible Notes as described below, and subsequently used the proceeds, along with cash on hand, to repurchase $475.0 million of its existing 2019 Convertible Notes from a limited number of holders in privately negotiated transactions for an aggregate purchase price of $583.5 million, which included $1.0 million of accrued interest. For accounting purposes, pursuant to ASC 470, these transactions were accounted for as an extinguishment of 2019 Convertible Notes and an issuance of new 2024 Convertible Notes. The Company allocated the purchase price between the fair value of the liability component and the equity component of the 2019 Convertible Notes at $459.4 million and $123.0 million, respectively. As a result, the Company recognized $446.4 million as a reduction to long-term debt representing the carrying value of the liability component and $123.0 million as a reduction to additional paid-in capital representing the equity component of the repurchased 2019 Convertible Notes. The $13.1 million difference between the fair value and carrying value of the liability component of the repurchased 2019 Convertible Notes was recognized as a loss on extinguishment of debt as a result of the transaction and was recorded in other expense (income), net within the Company’s consolidated statement of income during the first quarter of 2018. The accounting impact of the 2024 Convertible Notes is described in further detail below.
On August 15, 2019, the 2019 Convertible Notes matured and the Company repaid the $675.0 million outstanding principal in cash, as well as $6.7 million of accrued interest.
During the year ended December 31, 2019, the Company recognized $27.0 million of interest expense relating to the 2019 Convertible Notes, which included $17.0 million relating to non-cash interest expense relating to the debt discount and $1.7 million relating to amortization of debt issuance costs.
98
In conjunction with the issuance of the 2019 Convertible Notes, during February 2014, the Company paid approximately $685.8 million to enter into prepaid forward share repurchase transactions, or the Forward Transactions, with certain financial institutions, and paid approximately $123.8 million to enter into capped call transactions with respect to its common shares, or the Capped Call Transactions, with certain financial institutions. Subsequently, in conjunction with the repurchase of a portion of the 2019 Convertible Notes, during March 2018, the Company entered into agreements with the option counterparties to the Capped Call Transactions to terminate a portion of such existing transactions. See Note 8, Shareholders’ Deficit, for additional discussion on the Forward Transactions and Capped Call Transactions entered into in conjunction with the issuance of these 2019 Convertible Notes.
Convertible Senior Notes due 2024
In March 2018, the Company issued $550.0 million aggregate principal amount of convertible senior notes, or the 2024 Convertible Notes, in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. The 2024 Convertible Notes are senior unsecured obligations which rank effectively subordinate to any of the Company’s existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2024 Convertible Notes pay interest at a rate of 2.625% per annum payable semiannually in arrears on March 15 and September 15 of each year, beginning on September 15, 2018. Unless redeemed, repurchased or converted in accordance with their terms prior to such date, the 2024 Convertible Notes mature on March 15, 2024. Holders of the 2024 Convertible Notes may convert their notes at their option under the following circumstances: (i) during any calendar quarter commencing after the calendar quarter ending June 30, 2018, if the last reported sale price of the Company’s common shares for at least 20 trading days (whether or not consecutive) in a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter exceeds 130% of the conversion price for the 2024 Convertible Notes on each applicable trading day; (ii) during the five business-day period immediately after any five consecutive trading day period, or the measurement period, in which the trading price per $1,000 principal amount of 2024 Convertible Notes for each trading day of that measurement period was less than 98% of the product of the last reported sale price of the Company’s common shares and the conversion rate for the 2024 Convertible Notes for each such day; (iii) if the Company calls the 2024 Convertible Notes for redemption; or (iv) upon the occurrence of specified corporate events. On and after December 15, 2023, holders may convert their 2024 Convertible Notes at any time, regardless of the foregoing circumstances. In December 2021, the Company made an irrevocable election under the indenture governing the 2024 Convertible Notes to require the principal portion of the 2024 Convertible Notes to be settled in cash and any excess in shares or cash. Upon conversion, the 2024 Convertible Notes will be settled in cash and, if applicable, the Company’s common shares, based on the applicable conversion rate at such time. The 2024 Convertible Notes had an initial conversion rate of 16.0056 common shares per $1,000 principal amount of the 2024 Convertible Notes, or an initial conversion price of approximately $62.48 per common share. The conversion rate is subject to adjustment upon the occurrence of certain events and was 16.0467 common shares per $1,000 principal amount of the 2024 Convertible Notes, or a conversion price of approximately $62.32 per common share, as of December 31, 2021.
The Company incurred approximately $12.9 million of issuance costs during the first quarter of 2018 relating to the issuance of the 2024 Convertible Notes. Of the $12.9 million issuance costs incurred, $9.6 million and $3.3 million were recorded as debt issuance costs and additional paid-in capital, respectively, in proportion to the allocation of the proceeds of the 2024 Convertible Notes. The $9.6 million of debt issuance costs, which was recorded as an additional debt discount on the Company’s consolidated balance sheet, are being amortized over the contractual term of the 2024 Convertible Notes using the effective-interest method.
In March 2018, the $550.0 million aggregate principal amount of the 2024 Convertible Notes were initially allocated between long-term debt, or liability component, and additional paid-in-capital, or equity component, within the Company’s consolidated balance sheet at $410.1 million and $139.9 million, respectively. The liability component was measured using the nonconvertible debt interest rate. The carrying amount of the equity component representing the conversion option was determined by deducting the fair value of the liability component from the face value of the 2024 Convertible Notes as a whole. Since the Company must still settle these 2024 Convertible Notes at face value at or prior to maturity, this liability component will be accreted up to its face value resulting in additional non-cash interest expense being recognized within the Company’s consolidated statements of income while the 2024 Convertible Notes remain outstanding. The effective interest rate on the 2024 Convertible Notes is approximately 8.4% per annum. The equity component is not remeasured as long as it continues to meet the conditions for equity classification.
As of December 31, 2021, the outstanding principal on the 2024 Convertible Notes was $550.0 million, the unamortized debt discount and debt issuance costs were $64.0 million, and the carrying amount of the liability component was $486.0 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. As of December 31, 2020, the outstanding principal on the 2024 Convertible Notes was $550.0 million, the unamortized debt discount and debt issuance costs were $89.4 million, and the carrying amount of the liability component was $460.6 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. The fair value of the liability component relating to the 2024 Convertible Notes was approximately $547.4 million and $541.8 million as of December 31, 2021 and 2020, respectively.
99
During the years ended December 31, 2021, 2020, and 2019, the Company recognized $39.8 million, $37.7 million, and $35.8 million, respectively, of interest expense relating to the 2024 Convertible Notes, which included $23.7 million, $21.8 million, and $20.0 million, respectively, relating to non-cash interest expense relating to the debt discount and $1.6 million, $1.5 million, and $1.4 million, respectively, relating to amortization of debt issuance costs.
Senior Notes due 2025
In May 2020, the Company issued $600.0 million aggregate principal amount of senior notes, or the 2025 Notes, in a private offering in the United States to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended, and outside the United States pursuant to Regulation S under the Securities Act of 1933, as amended. The 2025 Notes are senior unsecured obligations which rank effectively subordinate to any of the Company’s existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2025 Notes pay interest at a rate of 7.875% per annum payable semiannually in arrears on March 1 and September 1 of each year, beginning on March 1, 2021. The 2025 Notes mature on September 1, 2025.
At any time prior to September 1, 2022, the Company may redeem all or part of the 2025 Notes at a redemption price equal to 100% of their principal amount, plus a “make whole” premium as of the redemption date, and accrued and unpaid interest to the redemption date. In addition, at any time prior to September 1, 2022, the Company may redeem up to 40% of the aggregate principal amount of the 2025 Notes with the proceeds of one or more equity offerings, at a redemption price equal to 107.875%, plus accrued and unpaid interest. Furthermore, at any time on or after September 1, 2022, the Company may redeem all or part of the 2025 Notes at the following redemption prices, expressed as percentages of principal amount, plus accrued and unpaid interest thereon to the redemption date, if redeemed during the twelve-month period beginning on September 1 of the years indicated below:
|
|
Percentage |
|
|
2022 |
|
|
103.938 |
% |
2023 |
|
|
101.969 |
% |
2024 and thereafter |
|
|
100.000 |
% |
The 2025 Notes contain customary negative covenants, including, among other things, limitations or prohibitions on restricted payments, incurrence of additional indebtedness, liens, mergers, asset sales and transactions with affiliates. In addition, the 2025 Notes contain customary events of default.
The Company incurred approximately $7.9 million of issuance costs during the second quarter of 2020 relating to the issuance of the 2025 Notes. The $7.9 million of debt issuance costs, which was recorded as a debt discount on the Company’s consolidated balance sheet, are being amortized over the contractual term of the 2025 Notes using the effective-interest method.
As of December 31, 2021, the outstanding principal on the 2025 Notes was $600.0 million, the unamortized debt issuance costs were $5.8 million, and the carrying amount was $594.2 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. As of December 31, 2020, the outstanding principal on the 2025 Notes was $600.0 million, the unamortized debt issuance costs were $7.1 million, and the carrying amount was $592.9 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. The fair value of the 2025 Notes was approximately $639.7 million and $656.3 million as of December 31, 2021 and 2020, respectively, and was determined by utilizing over-the-counter market quotes and yield curves, which are considered Level 2 inputs as defined in Note 13, Fair Value Measurements.
During the years ended December 31, 2021 and 2020, the Company recognized $48.6 million and $28.5 million, respectively, of interest expense relating to the 2025 Notes, which included $1.3 million and $0.7 million, respectively, relating to amortization of debt issuance costs.
Senior Notes due 2026
In August 2018, the Company issued $400.0 million aggregate principal amount of senior notes, or the 2026 Notes, in a private offering in the United States to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended, and outside the United States pursuant to Regulation S under the Securities Act of 1933, as amended. The 2026 Notes were senior unsecured obligations which ranked effectively subordinate to any of the Company’s existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2026 Notes paid interest at a rate of 7.250% per annum payable semiannually in arrears on February 15 and August 15 of each year, beginning on February 15, 2019. The 2026 Notes were to mature on August 15, 2026.
100
The Company incurred approximately $5.4 million of issuance costs during the third quarter of 2018 relating to the issuance of the 2026 Notes. The $5.4 million of debt issuance costs, which was recorded as a debt discount on the Company’s consolidated balance sheet, were being amortized over the contractual term of the 2026 Notes using the effective-interest method.
In May 2021, the Company issued $600.0 million aggregate principal of new senior notes due 2029, or the 2029 Notes as described below, and subsequently used a portion of the proceeds to redeem all $400.0 million of its existing 2026 Notes for an aggregate purchase price of $428.5 million, which included $7.7 million of accrued interest. For accounting purposes, pursuant to ASC 470, these transactions were accounted for as an extinguishment of the 2026 Notes. The Company recognized a loss on extinguishment of $24.6 million as a result, which was recorded in other expense (income), net within the Company’s consolidated statement of income during the second quarter of 2021.
As of December 31, 2020, the outstanding principal on the 2026 Notes was $400.0 million, the unamortized debt issuance costs were $4.1 million, and the carrying amount was $395.9 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. The fair value of the 2026 Notes was approximately $425.0 million as of December 31, 2020 and was determined by utilizing over-the-counter market quotes and yield curves, which are considered Level 2 inputs as defined in Note 13, Fair Value Measurements.
During the years ended December 31, 2021, 2020, and 2019, the Company recognized $11.5 million, $29.6 million, and $29.5 million, respectively, of interest expense relating to the 2026 Notes, which included $0.2 million, $0.6 million, and $0.5 million, respectively, relating to amortization of debt issuance costs.
Senior Notes due 2029
In May 2021, the Company issued $600.0 million aggregate principal amount of senior notes, or the 2029 Notes, in a private offering in the United States to qualified institutional buyers, pursuant to Rule 144A under the Securities Act of 1933, as amended, and outside the United States pursuant to Regulation S under the Securities Act of 1933, as amended. The 2029 Notes are senior unsecured obligations which rank effectively subordinate to any of the Company’s existing and future secured indebtedness, including amounts outstanding under the 2018 Credit Facility, to the extent of the value of the assets securing such indebtedness. The 2029 Notes pay interest at a rate of 4.875% per annum payable semiannually in arrears on June 1 and December 1 of each year, beginning on December 1, 2021. The 2029 Notes mature on June 1, 2029.
At any time prior to June 1, 2024, the Company may redeem all or part of the 2029 Notes at a redemption price equal to 100% of their principal amount, plus a “make whole” premium as of the redemption date, and accrued and unpaid interest to the redemption date. In addition, at any time prior to June 1, 2024, the Company may redeem up to 40% of the aggregate principal amount of the 2029 Notes with the proceeds of one or more equity offerings, at a redemption price equal to 104.875%, plus accrued and unpaid interest. Furthermore, at any time on or after June 1, 2024, the Company may redeem all or part of the 2029 Notes at the following redemption prices, expressed as percentages of principal amount, plus accrued and unpaid interest thereon to the redemption date, if redeemed during the twelve-month period beginning on June 1 of the years indicated below:
|
|
Percentage |
|
|
2024 |
|
|
102.438 |
% |
2025 |
|
|
101.219 |
% |
2026 and thereafter |
|
|
100.000 |
% |
The 2029 Notes contain customary negative covenants, including, among other things, limitations or prohibitions on restricted payments, incurrence of additional indebtedness, liens, mergers, asset sales and transactions with affiliates. In addition, the 2029 Notes contain customary events of default.
The Company incurred approximately $7.7 million of issuance costs during the second quarter of 2021 relating to the issuance of the 2029 Notes. The $7.7 million of debt issuance costs, which was recorded as a debt discount on the Company’s consolidated balance sheet, are being amortized over the contractual term of the 2029 Notes using the effective-interest method.
As of December 31, 2021, the outstanding principal on the 2029 Notes was $600.0 million, the unamortized debt issuance costs were $7.2 million, and the carrying amount was $592.8 million, which was recorded to long-term debt within the Company’s consolidated balance sheet. The fair value of the 2029 Notes was approximately $588.9 million as of December 31, 2021 and was determined by utilizing over-the-counter market quotes and yield curves, which are considered Level 2 inputs as defined in Note 13, Fair Value Measurements.
101
During the year ended December 31, 2021, the Company recognized $18.4 million of interest expense relating to the 2029 Notes, which included $0.5 million relating to amortization of debt issuance costs.
Valuation of 2024 Convertible Notes – Level 2 and Level 3 Inputs
In order to determine the initial value of the 2024 Convertible Notes, the Company determined the fair value of the liability component of the 2024 Convertible Notes using two valuation methods. The Company reviewed market data that was available for publicly traded, senior, unsecured nonconvertible corporate bonds issued by companies with similar credit ratings. Assumptions used in the estimate represent what market participants would use in pricing the liability component, including market yields and credit standing to develop the straight debt yield estimate. The Company also used a lattice model, which included inputs such as stock price, the Convertible Note trading price, volatility and dividend yield to estimate the straight debt yield. The Company combined the results of the two valuation methods to determine the fair value of the liability component of the 2024 Convertible Notes. Most of these inputs are primarily considered Level 2 and Level 3 inputs. The Company used similar valuation approaches to determine the subsequent fair value of the liability component only for disclosure purposes, which includes using a lattice model and (1) reviewing market data relating to its 2025 Notes and 2029 Notes and comparable yield curves to determine its straight debt yield estimate, or (2) reviewing market data relating to publicly traded, senior, unsecured nonconvertible corporate bonds issued by companies with similar credit ratings in order to determine its straight debt yield estimate.
Total Debt
The Company’s total interest expense was $153.1 million, $133.0 million, and $153.0 million, for the years ended December 31, 2021, 2020, and 2019, respectively, which was recognized within its consolidated statements of income.
As of December 31, 2021, annual scheduled principal payments of debt were as follows:
|
|
Principal Payments |
|
|
|
|
(in millions) |
|
|
2022 |
|
$ |
29.4 |
|
2023 |
|
|
29.4 |
|
2024 |
|
|
586.4 |
|
2025 |
|
|
1,600.6 |
|
2026 |
|
|
— |
|
Thereafter |
|
|
600.0 |
|
Total |
|
$ |
2,845.8 |
|
Certain vendors and government agencies may require letters of credit or similar guaranteeing arrangements to be issued or executed. As of December 31, 2021, the Company had $20.7 million of issued but undrawn letters of credit or similar arrangements.
6. Employee Compensation Plans
In the United States, the Company maintains a profit sharing plan pursuant to Sections 401(a) and (k) of the Internal Revenue Code of 1986, as amended, or the Code. The plan is available to substantially all employees who meet the length of service requirements. The Company’s contribution expense relating to this profit sharing plan was $9.4 million, $6.6 million, and $5.2 million during the years ended December 31, 2021, 2020, and 2019, respectively.
The Company has employees in international countries that are covered by various deferred compensation plans. These plans are administered based upon the legal requirements in the countries in which they are established. The Company’s compensation expenses relating to these plans were $9.8 million, $8.8 million, and $7.6 million for the years ended December 31, 2021, 2020, and 2019, respectively.
The Company has non-qualified deferred compensation plans for select groups of management: the Herbalife Management Deferred Compensation Plan and the Herbalife Senior Executive Deferred Compensation Plan. The matching contribution was 3.5% of a participant’s annual base salary in excess of the Qualified Plan annual compensation limit and the amount by which deferrals reduce 401(k)-eligible pay below the IRS limit.
102
Each participant in either of the non-qualified deferred compensation plans discussed above has, at all times, a fully vested and non-forfeitable interest in each year’s contribution, including interest credited thereto, and in any Company matching contributions, if applicable. In connection with a participant’s election to defer an annual deferral amount, the participant may also elect to receive a short-term payout, equal to the annual deferral amount plus interest. Such amount is payable in five or more years from the first day of the year in which the annual deferral amount is actually deferred.
The total for the two non-qualified deferred compensation plans, excluding participant contributions, was an expense of $8.6 million, $9.5 million, and $9.4 million for the years ended December 31, 2021, 2020, and 2019, respectively. The total long-term deferred compensation liability under the two deferred compensation plans was $80.5 million and $72.3 million as of December 31, 2021 and 2020, respectively, and is included in other non-current liabilities within the Company’s consolidated balance sheets.
The deferred compensation plans are unfunded and their benefits are paid from the general assets of the Company, except that the Company has contributed to a “rabbi trust” whose assets will be used to pay the benefits if the Company remains solvent, but can be reached by the Company’s creditors if the Company becomes insolvent. The value of the assets in the “rabbi trust” was $48.2 million and $43.8 million as of December 31, 2021 and 2020, respectively, and is included in other assets within the Company’s consolidated balance sheets.
7. Contingencies
The Company is from time to time engaged in routine litigation. The Company regularly reviews all pending litigation matters in which it is involved and establishes reserves deemed appropriate by management for these litigation matters when a probable loss estimate can be made.
The matters described in this Note may take several years to resolve. While the Company believes it has meritorious defenses, it cannot be sure of their ultimate resolution. Although the Company may reserve amounts for certain matters that the Company believes represent the most likely outcome of the resolution of these related disputes, if the Company is incorrect in its assessment, the Company may have to record additional expenses, when it becomes probable that an increased potential liability is warranted.
Tax Matters
The Mexican Tax Administration Service commenced audits of the Company’s Mexican subsidiaries for the period from January to September 2007 and on May 10, 2013, the Company received an assessment related to that period. This assessment is subject to interest and inflationary adjustments. On July 11, 2013, the Company filed an administrative appeal disputing the assessment. On September 22, 2014, the Mexican Tax Administration Service denied the Company’s administrative appeal. The Company commenced litigation in the Tax Court of Mexico in November 2014 to dispute the assertions made by the Mexican Tax Administration Service in the case. On January 16, 2018, the Tax Court of Mexico issued a verdict upholding the assessment issued by the Mexican Tax Administration Service. On April 16, 2018, the Company filed an appeal of this verdict, and in July 2019, the Circuit Court issued a written verdict upholding the assessment and the judgment of the Tax Court of Mexico. On August 12, 2019, the Company filed an appeal with the Supreme Court of Mexico. On October 16, 2019, the Supreme Court of Mexico refused to hear the Company’s appeal. On October 21, 2019, the Company filed a petition with the Supreme Court of Mexico, asking them to reconsider their previous decision. On April 29, 2020, the Supreme Court of Mexico declined the Company’s second petition and the adverse verdicts of the lower courts became final. The Company previously recognized a loss of $19.0 million in selling, general, and administrative expenses within the Company’s consolidated statement of income during the year ended December 31, 2019. The Company paid the assessment during the second quarter of 2021 and the case is now closed.
The Mexican Tax Administration Service has delayed processing VAT refunds for companies operating in Mexico and the Company believes that the process for its Mexico subsidiary to receive VAT refunds may be delayed. As of December 31, 2021, the Company had $24.1 million of Mexico VAT-related assets, of which $8.6 million was recognized in prepaid expenses and other current assets and $15.5 million was recognized in other assets within its consolidated balance sheet. This amount relates to VAT payments made over various periods and the Company believes these amounts are recoverable by refund or they may be applied against certain future tax liabilities. Effective January 1, 2019, a tax reform law changed the rules concerning possible use of VAT assets, specifically providing that, for VAT balances generated after December 31, 2018, those balances could not be offset against taxes other than VAT obligations currently due. The Company has not recognized any losses related to these VAT-related assets as the Company does not believe a loss is probable.
103
The Company has received tax assessments for multiple years from the Federal Revenue Office of Brazil related to withholding/contributions based on payments to the Company’s Members. The aggregate combined amount of all these assessments is equivalent to approximately $9.9 million, translated at the December 31, 2021 spot rate. The Company is currently litigating these assessments. The Company has not accrued a loss for the majority of the assessments because the Company does not believe a loss is probable. The Company is currently unable to reasonably estimate the amount of the loss that may result from an unfavorable outcome if additional assessments for other periods were to be issued.
The Company is under examination in several Brazilian states related to ICMS and ICMS-ST taxation. Some of these examinations have resulted in assessments for underpaid tax that the Company has appealed. The State of São Paulo has audited the Company for the 2013 and 2014 tax years. During July 2016, for the State of São Paulo, the Company received an assessment in the aggregate amount of approximately $28.8 million, translated at the December 31, 2021 spot rate, relating to various ICMS issues for its 2013 tax year. In August 2016, the Company filed a first-level administrative appeal which was denied in February 2017. The Company filed a further appeal on March 9, 2017. On March 20, 2018, the Court held a hearing and a verdict was issued in June 2019, remanding the case back to the first-level administrative court. During August 2017, for the State of São Paulo, the Company received an assessment in the aggregate amount of approximately $10.7 million, translated at the December 31, 2021 spot rate, relating to various ICMS issues for its 2014 tax year. In September 2017, the Company filed a first-level administrative appeal for the 2014 tax year. The first-level administrative appeal was denied. The Company filed an appeal at the second-level administrative court in December 2018 and a verdict was issued in April 2019, remanding the case back to the first-level administrative court. During September 2018, for the State of Rio de Janeiro, the Company received an assessment in the aggregate amount of approximately $6.3 million, translated at the December 31, 2021 spot rate, relating to various ICMS-ST issues for its 2016 and 2017 tax years. On November 8, 2018, the Company filed a first-level administrative appeal, which was subsequently denied. On April 5, 2019, the Company appealed this tax assessment to the Administrative Council of Tax Appeals (second-level administrative appeal). The Company has also received other ICMS tax assessments in Brazil. During the fourth quarter of 2015, the Company filed appeals with state judicial courts against three of the assessments. The Company had issued surety bonds in the aggregate amount of $6.1 million, translated at the December 31, 2021 spot rate, to guarantee payment of some of the tax assessments as required while the Company pursues the appeals. In addition, the Company has received several ICMS tax assessments in the aggregate amount of $5.2 million, translated at the December 31, 2021 spot rate, from several other Brazilian states where surety bonds have not been issued. Litigation in all these cases is currently ongoing. The Company has not recognized a loss relating to any of these cases, assessments, and matters as the Company does not believe a loss is probable.
The Company has received various tax assessments in multiple jurisdictions in India for multiple years from the Indian VAT and Service Tax authorities in an amount equivalent to approximately $14.1 million, translated at the December 31, 2021 spot rate. These assessments are for underpaid VAT and the ability to claim input Service Tax credits. The Company is litigating these cases at the tax administrative level and the tax tribunal levels as it believes it has meritorious defenses. The Company has not recognized a loss as it does not believe a loss is probable. In addition, the Indian income tax authorities audited the Company’s fiscal years ended March 31, 2017 and 2018 and the Company has received assessments of approximately $13.3 million and $13.8 million for those respective years, translated at the December 31, 2021 spot rate. These assessments are subject to interest and penalties adjustments. The Company is currently litigating these cases. The Company currently believes that it is more likely than not that it will be successful in supporting its positions relating to these assessments. Accordingly, the Company has not accrued any amounts relating to these matters. In addition, the Indian income tax authorities are auditing the Company’s fiscal years ended March 31, 2013 and 2020 and it is uncertain whether additional assessments will be received.
104
The Korea Customs Service audited the importation activities of Herbalife Korea for the period January 2011 through May 2013. The total assessment for the audit period is $29.8 million, translated at the December 31, 2021 spot rate. The Company has paid the assessment and has recognized these payments in other assets within its consolidated balance sheet as of December 31, 2021. The Company lodged a first-level administrative appeal, which was denied on October 21, 2016. On January 31, 2017, the Company filed a further appeal to the National Tax Tribunal of Korea. In November 2018, the Company received an unfavorable decision from the National Tax Tribunal of Korea. In February 2019, the Company submitted an appeal to the Seoul Administrative Court. On February 17, 2021, the Seoul Administrative Court issued a verdict in favor of the Company. On March 10, 2021, the Korea Customs Service filed an appeal to the High Court against the verdict and the appeal is currently pending. The Korea Customs Service audited the importation activities of Herbalife Korea for the period May 2013 through December 2013. The total assessment for the audit period is $9.7 million, translated at the December 31, 2021 spot rate. The Company has paid the assessment and has recognized this payment in other assets within its consolidated balance sheet as of December 31, 2021. The Korea Customs Service audited the importation activities of Herbalife Korea for the period January 2014 through December 2014. The total assessment for the audit period is $15.1 million, translated at the December 31, 2021 spot rate. The Company paid the assessment in September 2020 and has recognized this payment in other assets within its consolidated balance sheet as of December 31, 2021. The Company is currently litigating both of these assessments at the Seoul Administrative Court. The Korea Customs Service audited the importation activities of Herbalife Korea for the period January 2015 through December 2017. The total assessment for the audit period is $12.3 million, translated at the December 31, 2021 spot rate. The Company has paid the assessment and has recognized this payment in other assets within its consolidated balance sheet as of December 31, 2021. The Company is currently litigating this assessment at the Seoul Administrative Court. The Company disagrees with the assertions made in all of these assessments, as well as the calculation methodology used in the assessments. The Company has not recognized a loss as the Company does not believe a loss is probable.
During the course of 2016, the Company received various questions from the Greek Social Security Agency and on December 29, 2016, the Greek Social Security Agency issued an assessment with respect to Social Security Contributions on Member earnings for the 2006 year. For Social Security issues, the statute of limitations is open for 2012 and later years in Greece. Despite the assessment amount being immaterial, the Company could receive similar assessments covering other years. The Company continues to litigate the assessment. The Company has not recognized a loss as it does not believe a loss is probable. The Company is currently unable to reasonably estimate the amount of the loss that may result from an unfavorable outcome if additional assessments for other periods were to be issued.
During March 2018, the Chinese Customs Service began an audit of the Company’s Chinese importations initially covering the periods 2015 through 2017 and had subsequently expanded its audit. The Company responded to the initial questions from the Customs Service. The Custom cases are now closed and resulted in immaterial settlement amounts that were previously fully accrued for.
U.S. Federal Trade Commission Consent Order
On July 15, 2016, the Company and the Federal Trade Commission, or the FTC, entered into a proposed Stipulation to Entry of Order for Permanent Injunction and Monetary Judgment, or the Consent Order. The Consent Order was lodged with the U.S. District Court for the Central District of California on July 15, 2016 and became effective on July 25, 2016, or the Effective Date. The Consent Order resolved the FTC’s multi-year investigation of the Company.
Pursuant to the Consent Order, under which the Company neither admitted nor denied the FTC’s allegations (except as to the Court having jurisdiction over the matter), the Company made, through its wholly-owned subsidiary Herbalife International of America, Inc., a $200 million payment to the FTC. Additionally, the Company implemented and continues to enhance certain existing procedures in the U.S. Among other requirements, the Consent Order requires the Company to categorize all existing and future Members in the U.S. as either “preferred members” – who are simply consumers who only wish to purchase products for their own household use, or “distributors” – who are Members who wish to resell some products or build a sales organization. The Company also agreed to compensate distributors on eligible U.S. sales within their downline organization, which include purchases by preferred members, purchases by a distributor for his or her personal consumption within allowable limits and sales of product by a distributor to his or her customers. The Consent Order also imposes restrictions on a distributor’s ability to open Nutrition Clubs in the United States. The Consent Order subjects the Company to certain audits by an independent compliance auditor for a period of seven years; imposes requirements on the Company regarding compliance certification and record creation and maintenance; and prohibits the Company, its affiliates and its distributors from making misrepresentations and misleading claims regarding, among other things, income and lavish lifestyles. The FTC and the independent compliance auditor have the right to inspect Company records and request additional compliance reports for purposes of conducting audits pursuant to the Consent Order. In September 2016, the Company and the FTC mutually selected Affiliated Monitors, Inc. to serve as the independent compliance auditor. The Company continues to monitor the impact of the Consent Order and, while the Company currently does not expect the settlement to have a long-term and materially adverse impact on its business and its Member base, the Company’s business and its Member base, particularly in the United States, may be negatively impacted. If the Company is unable to comply with the Consent Order then this could result in a material and adverse impact to the Company’s results of operations and financial condition.
105
Other Matters
As a marketer of foods, dietary and nutritional supplements, and other products that are ingested by consumers or applied to their bodies, the Company has been and is currently subjected to various product liability claims. The effects of these claims to date have not been material to the Company. The Company currently maintains product liability insurance with an annual deductible of $12.5 million.
As previously disclosed, the SEC and the Department of Justice, or DOJ, conducted investigations into the Company’s compliance with the Foreign Corrupt Practices Act, or FCPA, in China. Also, as previously disclosed, the Company conducted its own review and implemented remedial and improvement measures based upon this review, including replacement of certain employees and enhancements of Company policies and procedures in China. The Company cooperated with the SEC and the DOJ and has now reached separate resolutions with each of them.
On August 28, 2020, the SEC accepted the Offer of Settlement and issued an administrative order finding that the Company violated the books and records and internal controls provisions of the FCPA. In addition, on August 28, 2020, the Company and the DOJ separately entered into a court-approved deferred prosecution agreement, or DPA, under which the DOJ deferred criminal prosecution of the Company for a period of three years related to a conspiracy to violate the books and records provisions of the FCPA. Among other things, the Company is required to undertake compliance self-reporting obligations for the three-year terms of the agreements with the SEC and the DOJ. If the Company remains in compliance with the DPA during its three-year term, the deferred charge against the Company will be dismissed with prejudice. In addition, the Company paid the SEC and the DOJ aggregate penalties, disgorgement and prejudgment interest of approximately $123 million in September 2020, of which $83 million and $40 million were recognized in selling, general, and administrative expenses within the Company’s consolidated statements of income for the years ended December 31, 2020 and 2019, respectively, related to this matter. Any failure to comply with these agreements, or any resulting further government action, could result in a material and adverse impact to the Company’s business, financial condition, and operating results.
On September 18, 2017, the Company and certain of its subsidiaries and Members were named as defendants in a purported class action lawsuit, titled Rodgers, et al. v Herbalife Ltd., et al. and filed in the U.S. District Court for the Southern District of Florida, which alleges violations of Florida’s Deceptive and Unfair Trade Practices statute and federal Racketeer Influenced and Corrupt Organizations statutes, unjust enrichment, and negligent misrepresentation. On August 23, 2018, the U.S. District Court for the Southern District of Florida issued an order transferring the action to the U.S. District Court for the Central District of California as to four of the putative class plaintiffs and ordering the remaining four plaintiffs to arbitration, thereby terminating the Company defendants from the Florida action. The plaintiffs seek damages in an unspecified amount. While the Company continues to believe the lawsuit is without merit, and without admitting liability or wrongdoing, the Company and the plaintiffs have agreed on the principal terms of a settlement and continue to negotiate a stipulation of settlement. Under the principal terms of the settlement, the Company would pay $12.5 million into a fund to be distributed to qualified claimants. As of December 31, 2021, this amount has been adequately reserved for within the Company's consolidated financial statements. Any settlement would be subject to the preliminary and final approval of the U.S. District Court for the Central District of California.
8. Shareholders’ Deficit
The Company had 100.8 million, 120.1 million, and 137.4 million common shares outstanding as of December 31, 2021, 2020, and 2019, respectively. In December 2004, the Company authorized 7.5 million preference shares at $0.002 par value. The 7.5 million authorized preference shares remained unissued as of December 31, 2021. Preference shares may be issued from time to time in one or more series, each of such series to have such voting powers (full or limited or without voting powers), designations, preferences and relative, participating, optional or other special rights and qualifications, limitations or restrictions as determined by the Company’s board of directors.
Dividends
The Company has not declared or paid cash dividends since 2014. The declaration of future dividends is subject to the discretion of the Company’s board of directors and will depend upon various factors, including its earnings, financial condition, Herbalife Nutrition Ltd.’s available distributable reserves under Cayman Islands law, restrictions imposed by the 2018 Credit Facility and the terms of any other indebtedness that may be outstanding, cash requirements, future prospects and other factors deemed relevant by its board of directors.
106
Share Repurchases
On February 9, 2021, the Company’s board of directors authorized a new three-year $1.5 billion share repurchase program that will expire on February 9, 2024, which replaced the Company’s prior share repurchase authorization that was set to expire on October 30, 2023 and had approximately $7.9 million of remaining authorized capacity when it was replaced. This share repurchase program allows the Company, which includes an indirect wholly-owned subsidiary of Herbalife Nutrition Ltd., to repurchase the Company’s common shares at such times and prices as determined by management, as market conditions warrant, and to the extent Herbalife Nutrition Ltd.’s distributable reserves are available under Cayman Islands law. The 2018 Credit Facility permits the Company to repurchase its common shares as long as no default or event of default exists and other conditions, such as specified consolidated leverage ratios, are met. As of December 31, 2021, the remaining authorized capacity under the Company’s $1.5 billion share repurchase program was approximately $1.1 billion.
In conjunction with the issuance of the 2019 Convertible Notes during February 2014, the Company paid approximately $685.8 million to enter into Forward Transactions with certain financial institutions, or the Forward Counterparties, pursuant to which the Company purchased approximately 19.9 million common shares, at an average cost of $34.51 per share, for settlement on or around the August 15, 2019 maturity date for the 2019 Convertible Notes, subject to the ability of each Forward Counterparty to elect to settle all or a portion of its Forward Transactions early. The Forward Transactions were generally expected to facilitate privately negotiated derivative transactions between the Forward Counterparties and holders of the 2019 Convertible Notes, including swaps, relating to the common shares by which holders of the 2019 Convertible Notes establish short positions relating to the common shares and otherwise hedge their investments in the 2019 Convertible Notes concurrently with, or shortly after, the pricing of the 2019 Convertible Notes. The approximate 19.9 million common shares effectively repurchased through the Forward Transactions were treated as retired shares for basic and diluted EPS purposes. During the year ended December 31, 2019, the Forward Counterparties delivered approximately 6.0 million to the Company, which were subsequently retired by the Company. As of December 31, 2019, the Forward Counterparties had delivered all of the approximate 19.9 million common shares effectively repurchased through the Forward Transactions and no shares remained legally outstanding.
As a result of the Forward Transactions, the Company’s total shareholders’ equity within its consolidated balance sheet was reduced by approximately $685.8 million during the first quarter of 2014, with amounts of $653.9 million and $31.9 million being allocated between retained earnings and additional paid-in capital, respectively, within total shareholders’ equity. Also, upon executing the Forward Transactions, the Company recorded, at fair value, $35.8 million in non-cash issuance costs to other assets and a corresponding amount to additional paid-in capital within its consolidated balance sheet. These non-cash issuance costs were amortized to interest expense over the contractual term of the Forward Transactions. During the year ended December 31, 2019, the Company recognized $1.2 million of non-cash interest expense relating to amortization of these non-cash issuance costs within its consolidated statements of income.
During January 2021, the Company repurchased from Mr. Carl C. Icahn and certain of his affiliates an aggregate of approximately 12.5 million common shares of the Company at an aggregate cost of approximately $600.0 million, or $48.05 per share, and subsequently retired these shares. In addition, during the year ended December 31, 2021, the Company repurchased approximately 7.9 million of its common shares through open-market purchases at an aggregate cost of approximately $382.7 million, or an average cost of $48.17 per share, and subsequently retired these shares. In total, during the year ended December 31, 2021, the Company repurchased approximately 20.4 million of its common shares at an aggregate cost of approximately $982.7 million, or an average cost of $48.10 per share. In August 2020, the Company completed its modified Dutch auction tender offer and then subsequently paid cash to repurchase and retire a total of approximately 15.4 million of its common shares at an aggregate cost of approximately $750.0 million, or $48.75 per share. In addition, during the year ended December 31, 2020, the Company repurchased approximately 3.0 million of its common shares through open-market purchases at an aggregate cost of approximately $142.1 million, or an average cost of $47.40 per share, and subsequently retired these shares. In total, during the year ended December 31, 2020, the Company repurchased approximately 18.4 million of its common shares at an aggregate cost of approximately $892.1 million, or an average cost of $48.53 per share.
As of both December 31, 2021 and 2020, the Company held approximately 10.0 million of treasury shares for U.S. GAAP purposes. These treasury shares increased the Company’s shareholders’ deficit and are reflected at cost within the Company’s accompanying consolidated balance sheets. Although these shares are owned by an indirect wholly-owned subsidiary of the Company and remain legally outstanding, they are reflected as treasury shares under U.S. GAAP and therefore reduce the number of common shares outstanding within the Company’s consolidated financial statements and the weighted-average number of common shares outstanding used in calculating earnings per share. The common shares of Herbalife Nutrition Ltd. held by the indirect wholly-owned subsidiary, however, remain outstanding on the books and records of the Company’s transfer agent and therefore still carry voting and other share rights related to ownership of the Company’s common shares, which may be exercised. So long as it is consistent with applicable laws, such shares will be voted by such subsidiary in the same manner, and to the maximum extent possible in the same proportion, as all other votes cast with respect to any matter properly submitted to a vote of Herbalife Nutrition Ltd.’s shareholders.
107
In connection with the Company’s October 2017 modified Dutch auction tender offer, the Company incurred $1.6 million in transaction costs and also provided a non-transferable contractual CVR for each share tendered, allowing participants in the tender offer to receive a contingent cash payment in the event Herbalife Nutrition was acquired in a going-private transaction (as defined in the CVR Agreement) within two years of the commencement of the tender offer. The initial fair value of the CVR was $7.3 million, which was recorded as a liability in the fourth quarter of 2017 with a corresponding decrease to shareholders’ equity. In determining the initial fair value of the CVR, the Company used a lattice model, which included inputs such as the underlying stock price, strike price, time to expiration, and dividend yield. Subsequent changes in the fair value of the CVR liability, using a similar valuation approach as the initial fair value determination, were recognized within the Company’s consolidated balance sheets with corresponding gains or losses being recognized in other expense (income), net within the Company’s consolidated statements of income during each reporting period until the CVR expired in or was terminated due to a going-private transaction, which was also incorporated in the valuation of the CVR; this going-private probability input was considered to be a Level 3 input in the fair value hierarchy and any increase or decrease in this input could have significantly impacted the fair value of the CVR as of the reporting date. The CVR expired without value on August 21, 2019, the two-year anniversary of August 21, 2017, the date the Company commenced the related modified Dutch auction tender offer.
During the year ended December 31, 2019, the Company recognized a $15.7 million gain in other expense (income), net within its consolidated statement of income due to the change in the fair value of the CVR, which was driven by its expiration during August 2019.
The number of shares issued upon vesting or exercise for certain restricted stock units and SARs granted pursuant to the Company’s share-based compensation plans is net of the statutory withholding requirements that the Company pays on behalf of its employees. Although shares withheld are not issued, they are treated as common share repurchases in the Company’s consolidated financial statements, as they reduce the number of shares that would have been issued upon vesting. These shares do not count against the authorized capacity under the Company’s share repurchase program described above. During the years ended December 31, 2021, 2020, and 2019, the Company withheld shares on its vested restricted stock units and exercised SARs relating to its share-based compensation plans.
The Company reflects the aggregate purchase price of its common shares repurchased as an increase to shareholders’ deficit. The Company generally allocated the purchase price of the repurchased shares to accumulated deficit, common shares, and additional paid-in capital, with the exception of treasury shares, which are recorded separately on the Company’s consolidated balance sheets.
For the years ended December 31, 2021, 2020, and 2019, the Company’s share repurchases, inclusive of transaction costs, were $982.7 million, $893.9 million, and zero, respectively, under the Company’s share repurchase programs, which include open-market purchases and the tender offers described above, and $28.6 million, $29.6 million, and $16.7 million, respectively, due to shares withheld for tax purposes related to the Company’s share-based compensation plans. For the years ended December 31, 2021, 2020, and 2019, the Company’s total share repurchases, including shares withheld for tax purposes, were $1,011.3 million, $923.5 million, and $16.7 million, respectively, and have been recorded as an increase to shareholders’ deficit within the Company’s consolidated balance sheets.
Capped Call Transactions
In February 2014, in connection with the issuance of the 2019 Convertible Notes, the Company paid approximately $123.8 million to enter into Capped Call Transactions with certain financial institutions. The Capped Call Transactions were expected generally to reduce the potential dilution upon conversion of the 2019 Convertible Notes in the event that the market price of the common shares was greater than the strike price of the Capped Call Transactions, initially set at $43.14 per common share, with such reduction of potential dilution subject to a cap based on the cap price initially set at $60.39 per common share. The strike price and cap price were subject to certain adjustments under the terms of the Capped Call Transactions. Therefore, as a result of executing the Capped Call Transactions, the Company in effect was only exposed to potential net dilution once the market price of its common shares exceeded the adjusted cap price. As a result of the Capped Call Transactions, the Company’s additional paid-in capital within shareholders’ deficit on its consolidated balance sheet was reduced by $123.8 million during the first quarter of 2014.
During March 2018, in connection with the Company’s repurchase of a portion of the 2019 Convertible Notes, the Company entered into partial settlement agreements with the option counterparties to the Capped Call Transactions to terminate a portion of such existing transactions, in each case, in a notional amount corresponding to the aggregate principal amount of 2019 Convertible Notes that were repurchased. As a result of terminating a portion of the Capped Call Transactions, which were in a favorable position, the Company received $55.9 million in cash and recognized an offsetting increase to additional paid-in capital during 2018.
On August 15, 2019, the 2019 Convertible Notes matured and the remaining Capped Call Transactions expired unexercised. The expiration of the Capped Call Transactions did not have an impact on the Company’s consolidated financial statements
108
Accumulated Other Comprehensive Loss
The following table summarizes changes in accumulated other comprehensive loss by component during the years ended December 31, 2021, 2020, and 2019:
|
|
Changes in Accumulated Other Comprehensive Loss by Component |
|
|||||||||
|
|
Foreign Currency Translation Adjustments |
|
|
Unrealized Gain (Loss) on Derivatives |
|
|
Total |
|
|||
|
|
(in millions) |
|
|||||||||
Balance as of December 31, 2018 |
|
$ |
(211.6 |
) |
|
$ |
1.8 |
|
|
$ |
(209.8 |
) |
Other comprehensive loss before reclassifications, net of tax |
|
|
— |
|
|
|
(1.9 |
) |
|
|
(1.9 |
) |
Amounts reclassified from accumulated other comprehensive loss to income, net of tax(1) |
|
|
— |
|
|
|
(0.8 |
) |
|
|
(0.8 |
) |
Total other comprehensive loss, net of reclassifications |
|
|
— |
|
|
|
(2.7 |
) |
|
|
(2.7 |
) |
Balance as of December 31, 2019 |
|
|
(211.6 |
) |
|
|
(0.9 |
) |
|
|
(212.5 |
) |
Other comprehensive income before reclassifications, net of tax |
|
|
33.2 |
|
|
|
1.7 |
|
|
|
34.9 |
|
Amounts reclassified from accumulated other comprehensive loss to income, net of tax(1) |
|
|
— |
|
|
|
(4.6 |
) |
|
|
(4.6 |
) |
Total other comprehensive income (loss), net of reclassifications |
|
|
33.2 |
|
|
|
(2.9 |
) |
|
|
30.3 |
|
Balance as of December 31, 2020 |
|
|
(178.4 |
) |
|
|
(3.8 |
) |
|
|
(182.2 |
) |
Other comprehensive (loss) income before reclassifications, net of tax |
|
|
(33.2 |
) |
|
|
0.2 |
|
|
|
(33.0 |
) |
Amounts reclassified from accumulated other comprehensive loss to income, net of tax(1) |
|
|
— |
|
|
|
3.4 |
|
|
|
3.4 |
|
Total other comprehensive (loss) income, net of reclassifications |
|
|
(33.2 |
) |
|
|
3.6 |
|
|
|
(29.6 |
) |
Balance as of December 31, 2021 |
|
$ |
(211.6 |
) |
|
$ |
(0.2 |
) |
|
$ |
(211.8 |
) |
(1)
Other comprehensive income (loss) before reclassifications was net of tax expense of $0.2 million for foreign currency translation adjustments for the year ended December 31, 2021.
Other comprehensive income (loss) before reclassifications was net of tax benefit of $2.0 million and $0.6 million for foreign currency translation adjustments and unrealized gain (loss) on derivatives, respectively, for the year ended December 31, 2020. Amounts reclassified from accumulated other comprehensive loss to income was net of tax expense of $0.2 million for unrealized gain (loss) on derivatives for the year ended December 31, 2020.
Other comprehensive income (loss) before reclassifications was net of tax expense of $0.1 million for foreign currency translation adjustments for the year ended December 31, 2019.
109
9. Share-Based Compensation
The Company has the following share-based compensation plans: the Amended and Restated Herbalife Ltd. 2005 Stock Incentive Plan, or the 2005 Stock Incentive Plan, and the Amended and Restated Herbalife Ltd. 2014 Stock Incentive Plan, or the 2014 Stock Incentive Plan. Additionally, the Company has the Amended and Restated Non-Management Directors Compensation Plan, or the Non-Management Directors Plan, the purpose of which is to facilitate equity ownership in the Company by its directors through equity awards under the 2005 Stock Incentive Plan, and for grants made on and after April 30, 2014, under the 2014 Stock Incentive Plan. The 2014 Stock Incentive Plan replaced the 2005 Stock Incentive Plan and after the adoption thereof, no additional awards were made under the 2005 Stock Incentive Plan. The terms of the 2014 Stock Incentive Plan are substantially similar to the terms of the 2005 Stock Incentive Plan. The 2014 Stock Incentive Plan authorizes the issuance of 24.8 million common shares pursuant to awards granted under the plan, plus any shares that remained available for issuance under the 2005 Stock Incentive Plan as of April 29, 2014. As of December 31, 2021, an aggregate of approximately 7.1 million common shares remain available for future issuance under the 2014 Stock Incentive Plan.
The Company’s share-based compensation plans generally provide for grants of stock options, SARs, and stock unit awards, which are collectively referred to herein as awards. Certain SARs generally vest annually over a three-year period. The contractual term of stock options and SARs is generally ten years. Certain stock unit awards under the 2014 Stock Incentive Plan vest annually over a three-year period. Certain stock unit awards subject to service and performance conditions vest after the passage of a performance period as determined by the compensation committee of the Company’s board of directors. Stock unit awards granted to directors generally vest over a one-year period.
Awards can be subject to the following: market and service conditions, or market condition awards; performance and service conditions, or performance condition awards; market, service and performance conditions, or market and performance condition awards; or be subject only to continued service with the Company, or service condition awards. All awards granted by the Company are market condition awards, performance condition awards, or service condition awards. Unless otherwise determined at the time of grant, upon vesting, each stock unit award represents the right to receive one common share. For stock unit awards, the Company issues new shares, net of shares withheld for tax purposes, when vested. For SARs, the Company issues new shares based on the intrinsic value when exercised, net of shares withheld for tax purposes. The Company’s stock compensation awards outstanding as of December 31, 2021 included SARs and stock unit awards.
The SARs with performance conditions generally vest 20% in the first succeeding year, 20% in the second succeeding year, and 60% in the third succeeding year, subject to achievement of certain sales leader retention metrics. The fair value of these SARs was determined on the date of grant using the Black-Scholes-Merton option pricing model. The compensation expense for these grants is recognized over the vesting term using the graded vesting method. The Company did not grant any SARs with performance conditions during the years ended December 31, 2021, 2020, and 2019.
The fair value of SARs with service conditions was determined on the date of grant using the Black-Scholes-Merton option pricing model. The compensation expense for these grants is recognized over the vesting term using the straight-line method. The Company did not grant any SARs with service conditions during the years ended December 31, 2021, 2020, and 2019.
During the years ended December 31, 2021, 2020, and 2019, the Company granted performance stock unit awards to certain executives, which will vest on December 31, 2023, , and , respectively, subject to their continued employment through that date and the achievement of certain performance conditions. Generally, performance conditions include targets for local currency net sales, adjusted earnings before interest and taxes, and/or adjusted earnings per share. These performance stock unit awards can vest at between 0% and 200% of the target award based on the achievement of the performance conditions.
During the years ended December 31, 2021, 2020, and 2019, the Company granted stock unit awards with service conditions to directors and certain employees, which generally vest annually over a one-year and three-year period, respectively.
Share-based compensation expense is included in selling, general, and administrative expenses within the Company’s consolidated statements of income. The Company’s policy is to estimate the number of forfeitures expected to occur. Share-based compensation expense relating to service condition awards amounted to $43.6 million, $38.3 million, and $31.8 million for the years ended December 31, 2021, 2020, and 2019, respectively. Share-based compensation expense relating to performance condition awards amounted to $10.5 million, $12.7 million, and $6.8 million for the years ended December 31, 2021, 2020, and 2019, respectively. The related income tax benefits recognized in earnings for all awards amounted to $10.5 million, $9.6 million, and $8.3 million for the years ended December 31, 2021, 2020, and 2019, respectively. Excess tax benefits on share-based compensation arrangements totaled $3.5 million, $3.1 million, and $5.8 million for the years ended December 31, 2021, 2020, and 2019, respectively.
110
As of December 31, 2021, the total unrecognized compensation cost related to non-vested service condition stock awards was $58.2 million and the related weighted-average period over which it is expected to be recognized is approximately 1.4 years. As of December 31, 2021, the total unrecognized compensation cost related to non-vested performance condition awards was $12.9 million and the related weighted-average period over which it is expected to be recognized is approximately 1.3 years.
Stock unit awards are valued at the market value on the date of grant. The fair value of service condition SARs and performance condition SARs are estimated on the date of grant using the Black-Scholes-Merton option-pricing model. The fair value of SARs with market conditions or with market and performance conditions are estimated on the date of grant using the Monte Carlo lattice model. The Company calculates the expected term of its SARs based on historical data. All groups of employees have been determined to have similar historical exercise patterns for valuation purposes. The expected volatility of the SARs is based upon the historical volatility of the Company’s common shares and is also validated against the volatility rates of a peer group of companies. The risk-free interest rate is based on the implied yield on a U.S. Treasury zero-coupon issue with a remaining term equal to the expected term of the SARs. The expected dividend yield assumption is based on the Company’s historical and expected amount of dividend payouts.
There were no SARs granted during the years ended December 31, 2021, 2020, and 2019.
The following table summarizes the activities for all SARs under the Company’s share-based compensation plans for the year ended December 31, 2021:
|
|
Number of Awards |
|
|
Weighted-Average Exercise Price Per Award |
|
|
Weighted-Average Remaining Contractual Term |
|
Aggregate Intrinsic Value(1) |
|
|||
|
|
(in thousands) |
|
|
|
|
|
|
|
(in millions) |
|
|||
Outstanding as of December 31, 2020(2)(3) |
|
|
3,737 |
|
|
$ |
27.23 |
|
|
4.6 years |
|
$ |
77.8 |
|
Granted |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
Exercised(4) |
|
|
(1,092 |
) |
|
$ |
27.60 |
|
|
|
|
|
|
|
Forfeited |
|
|
(23 |
) |
|
$ |
25.03 |
|
|
|
|
|
|
|
Outstanding as of December 31, 2021(3) |
|
|
2,622 |
|
|
$ |
27.10 |
|
|
3.8 years |
|
$ |
36.3 |
|
Exercisable as of December 31, 2021(5) |
|
|
2,622 |
|
|
$ |
27.10 |
|
|
3.8 years |
|
$ |
36.3 |
|
Vested and expected to vest as of December 31, 2021 |
|
|
2,622 |
|
|
$ |
27.10 |
|
|
3.8 years |
|
$ |
36.3 |
|
(1) The intrinsic value is the amount by which the current market value of the underlying stock exceeds the exercise price of the stock awards.
(2)
(3)
(4)
(5)
The total intrinsic value of service condition SARs exercised during the years ended December 31, 2021, 2020, and 2019 was $19.1 million, $28.0 million, and $33.7 million, respectively. The total intrinsic value of performance condition SARs exercised during the years ended December 31, 2021, 2020, and 2019 was $6.5 million, $28.8 million, and $0.1 million, respectively. The total intrinsic value of market condition SARs exercised during the years ended December 31, 2021, 2020, and 2019 was $1.1 million, zero, and zero, respectively.
The following table summarizes the activities for all stock units under the Company’s share-based compensation plans for the year ended December 31, 2021:
|
|
Number of Shares |
|
|
Weighted-Average Grant Date Fair Value Per Share |
|
||
|
|
(in thousands) |
|
|
|
|
||
Outstanding and nonvested as of December 31, 2020(1) |
|
|
3,068 |
|
|
$ |
44.01 |
|
Granted(2) |
|
|
1,537 |
|
|
$ |
48.32 |
|
Vested(3) |
|
|
(941 |
) |
|
$ |
45.19 |
|
Forfeited(4) |
|
|
(264 |
) |
|
$ |
48.80 |
|
Outstanding and nonvested as of December 31, 2021(1) |
|
|
3,400 |
|
|
$ |
45.26 |
|
Expected to vest as of December 31, 2021(5) |
|
|
2,955 |
|
|
$ |
45.07 |
|
111
(1)
(2)
(3)
(4)
(5)
The total vesting date fair value of stock units which vested during the years ended December 31, 2021, 2020, and 2019 was $43.2 million, $23.0 million, and $13.0 million, respectively.
Employee Stock Purchase Plan
During 2007, the Company adopted a qualified employee stock purchase plan, or ESPP, which was implemented during the first quarter of 2008. In connection with the adoption of the ESPP, the Company has reserved for issuance a total of 4.0 million common shares. As of December 31, 2021, approximately 3.0 million common shares remain available for future issuance. Under the terms of the ESPP, rights to purchase common shares may be granted to eligible qualified employees subject to certain restrictions. The ESPP enables the Company’s eligible employees, through payroll withholdings, to purchase a limited number of common shares at 85% of the fair market value of a common share at the purchase date. Purchases are made on a quarterly basis.
10. Segment Information
The Company is a nutrition company that sells a wide range of weight management; targeted nutrition; energy, sports, and fitness; and outer nutrition products. The Company’s products are manufactured by the Company in its Changsha, Hunan, China extraction facility; Suzhou, China facility; Nanjing, China facility; Lake Forest, California facility; and Winston-Salem, North Carolina facility, as well as by third-party providers, and then are sold to Members who consume and sell Herbalife Nutrition products to retail consumers or other Members. Revenues reflect sales of products by the Company to its Members and are categorized based on geographic location.
As of December 31, 2021, the Company sold products in 95 markets throughout the world and was organized and managed by six geographic regions: North America, Mexico, South and Central America, EMEA, Asia Pacific, and China. The Company defines its operating segments as those geographical operations. The Company aggregates its operating segments, excluding China, into a reporting segment, or the Primary Reporting Segment, as management believes that the Company’s operating segments have similar operating characteristics and similar long-term operating performance. In making this determination, management believes that the operating segments are similar in the nature of the products sold, the product acquisition process, the types of customers to whom products are sold, the methods used to distribute the products, the nature of the regulatory environment, and their economic characteristics. China has been identified as a separate reporting segment as it does not meet the criteria for aggregation. The Company reviews its net sales and contribution margin by operating segment, and reviews its assets and capital expenditures on a consolidated basis and not by operating segment. Therefore, net sales and contribution margin are presented by reportable segment and assets and capital expenditures by segment are not presented.
112
Operating information for the two reportable segments, sales by product line, and sales by geographic area are as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Net sales: |
|
|
|
|
|
|
|
|
|
|||
Primary Reporting Segment |
|
$ |
5,173.3 |
|
|
$ |
4,732.2 |
|
|
$ |
4,125.1 |
|
China |
|
|
629.5 |
|
|
|
809.6 |
|
|
|
752.0 |
|
Total net sales |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
$ |
4,877.1 |
|
Contribution margin(1): |
|
|
|
|
|
|
|
|
|
|||
Primary Reporting Segment |
|
$ |
2,175.6 |
|
|
$ |
1,983.6 |
|
|
$ |
1,793.6 |
|
China(2) |
|
|
554.2 |
|
|
|
717.5 |
|
|
|
677.3 |
|
Total contribution margin |
|
$ |
2,729.8 |
|
|
$ |
2,701.1 |
|
|
$ |
2,470.9 |
|
Selling, general, and administrative expenses(2) |
|
|
2,012.1 |
|
|
|
2,075.0 |
|
|
|
1,940.3 |
|
Other operating income |
|
|
(16.4 |
) |
|
|
(14.5 |
) |
|
|
(37.5 |
) |
Interest expense |
|
|
153.1 |
|
|
|
133.0 |
|
|
|
153.0 |
|
Interest income |
|
|
4.4 |
|
|
|
8.8 |
|
|
|
20.6 |
|
Other expense (income), net |
|
|
24.6 |
|
|
|
— |
|
|
|
(15.7 |
) |
Income before income taxes |
|
|
560.8 |
|
|
|
516.4 |
|
|
|
451.4 |
|
Income taxes |
|
|
113.6 |
|
|
|
143.8 |
|
|
|
140.4 |
|
Net income |
|
$ |
447.2 |
|
|
$ |
372.6 |
|
|
$ |
311.0 |
|
Net sales by product line: |
|
|
|
|
|
|
|
|
|
|||
Weight Management |
|
$ |
3,370.4 |
|
|
$ |
3,312.8 |
|
|
$ |
3,012.5 |
|
Targeted Nutrition |
|
|
1,636.6 |
|
|
|
1,527.4 |
|
|
|
1,278.5 |
|
Energy, Sports, and Fitness |
|
|
551.8 |
|
|
|
437.4 |
|
|
|
352.0 |
|
Outer Nutrition |
|
|
107.8 |
|
|
|
111.3 |
|
|
|
97.3 |
|
Literature, Promotional, and Other(3) |
|
|
136.2 |
|
|
|
152.9 |
|
|
|
136.8 |
|
Total net sales |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
$ |
4,877.1 |
|
Net sales by geographic area: |
|
|
|
|
|
|
|
|
|
|||
United States |
|
$ |
1,386.7 |
|
|
$ |
1,334.5 |
|
|
$ |
1,002.6 |
|
China |
|
|
629.5 |
|
|
|
809.6 |
|
|
|
752.0 |
|
India |
|
|
519.1 |
|
|
|
349.1 |
|
|
|
321.3 |
|
Mexico |
|
|
463.7 |
|
|
|
436.9 |
|
|
|
473.6 |
|
Others |
|
|
2,803.8 |
|
|
|
2,611.7 |
|
|
|
2,327.6 |
|
Total net sales |
|
$ |
5,802.8 |
|
|
$ |
5,541.8 |
|
|
$ |
4,877.1 |
|
(1) Contribution margin consists of net sales less cost of sales and Royalty overrides. For the China segment, contribution margin does not include service fees to China independent service providers.
(2) Service fees to China independent service providers totaling $350.1 million, $454.0 million, and $418.9 million for the years ended December 31, 2021, 2020, and 2019, respectively, are included in selling, general, and administrative expenses.
(3) Product buybacks and returns in all product categories are included in the Literature, Promotional, and Other category.
As of December 31, 2021 and 2020, goodwill allocated to the Company’s reporting units included in the Company’s Primary Reporting Segment was $92.1 million and $97.2 million, respectively, and goodwill allocated to the China segment was $3.3 million and $3.3 million, respectively.
113
The following table sets forth property, plant, and equipment and deferred tax assets by geographic area:
|
|
December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Property, plant, and equipment, net: |
|
|
|
|
|
|
|
|
|
|||
United States |
|
$ |
348.3 |
|
|
$ |
303.2 |
|
|
$ |
292.4 |
|
Foreign |
|
|
93.8 |
|
|
|
87.0 |
|
|
|
79.1 |
|
Total property, plant, and equipment, net |
|
$ |
442.1 |
|
|
$ |
390.2 |
|
|
$ |
371.5 |
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
|||
United States |
|
$ |
142.6 |
|
|
$ |
123.8 |
|
|
$ |
114.5 |
|
Foreign |
|
|
78.0 |
|
|
|
76.6 |
|
|
|
68.3 |
|
Total deferred tax assets |
|
$ |
220.6 |
|
|
$ |
200.4 |
|
|
$ |
182.8 |
|
11. Derivative Instruments and Hedging Activities
Interest Rate Risk Management
The Company engages in an interest rate hedging strategy for which the hedged transactions are forecasted interest payments on the Company’s 2018 Credit Facility, which are based on variable rates.
During the first quarter of 2020, the Company entered into various interest rate swap agreements with effective dates ranging between February 2020 and March 2020. These agreements collectively provide for the Company to pay interest at a weighted-average fixed rate of 0.98% on aggregate notional amounts of $100.0 million under the 2018 Credit Facility until their respective expiration dates ranging between February 2022 and March 2023, while receiving interest based on LIBOR on the same notional amounts for the same periods. At inception, these swap agreements were designated as cash flow hedges against the variability in certain LIBOR-based borrowings under the 2018 Credit Facility, effectively fixing the interest rate on such notional amounts at a weighted-average effective rate of, depending on the Company’s total leverage ratio, between 2.73% and 3.23%. These hedge relationships qualified as effective under FASB ASC Topic 815, Derivatives and Hedging, or ASC 815, and consequently all changes in the fair value of these interest rate swaps are recorded as a component of accumulated other comprehensive loss within shareholders’ deficit, and are recognized in interest expense within the Company’s consolidated statement of income during the period when the hedged item and underlying transaction affect earnings. The fair values of the interest rate swap agreements are based on third-party bank quotes, and as of December 31, 2021 and 2020, the Company recorded liabilities at fair value of $0.1 million and $1.0 million, respectively, relating to these interest rate swap agreements.
Foreign Currency Instruments
The Company designates certain foreign currency derivatives, primarily comprised of foreign currency forward contracts and option contracts, as freestanding derivatives for which hedge accounting does not apply. The changes in the fair market value of these freestanding derivatives are included in selling, general, and administrative expenses within the Company’s consolidated statements of income. The Company primarily uses freestanding foreign currency derivatives to hedge foreign currency-denominated intercompany transactions and to partially mitigate the impact of foreign currency fluctuations. The fair value of the freestanding foreign currency derivatives is based on third-party quotes. The Company’s foreign currency derivative contracts are generally executed on a monthly basis.
The Company designates as cash flow hedges those foreign currency forward contracts it enters into to hedge forecasted inventory purchases and intercompany management fees that are subject to foreign currency exposures. Forward contracts are used to hedge forecasted inventory purchases over specific months. Changes in the fair value of these forward contracts designated as cash flow hedges, excluding forward points, are recorded as a component of accumulated other comprehensive loss within shareholders’ deficit, and are recognized in cost of sales within the Company’s consolidated statement of income during the period which approximates the time the hedged inventory is sold. The Company also hedges forecasted intercompany management fees over specific months. These contracts allow the Company to sell Euros in exchange for U.S. dollars at specified contract rates. Changes in the fair value of these forward contracts designated as cash flow hedges, excluding forward points, are recorded as a component of accumulated other comprehensive loss within shareholders’ deficit, and are recognized in selling, general, and administrative expenses within the Company’s consolidated statement of income during the period when the hedged item and underlying transaction affect earnings. The Company has elected to record changes in the fair value of amounts excluded from the assessment of effectiveness currently in earnings.
114
As of December 31, 2021 and 2020, the aggregate notional amounts of all foreign currency contracts outstanding designated as cash flow hedges were approximately $54.5 million and $56.4 million, respectively. As of December 31, 2021, these outstanding contracts were expected to mature over the next months. The Company’s derivative financial instruments are recorded on the consolidated balance sheets at fair value based on third-party quotes. As of December 31, 2021, the Company recorded assets at fair value of $0.3 million and liabilities at fair value of $1.7 million relating to all outstanding foreign currency contracts designated as cash flow hedges. As of December 31, 2020, the Company recorded liabilities at fair value of $3.9 million relating to all outstanding foreign currency contracts designated as cash flow hedges. The Company assesses hedge effectiveness at least quarterly and the hedges remained effective as of December 31, 2021 and 2020.
As of both December 31, 2021 and 2020, the majority of the Company’s outstanding foreign currency forward contracts had maturity dates of less than months with the majority of freestanding derivatives expiring within month.
115
The tables below provide information about the details of all foreign currency forward contracts that were outstanding as of December 31, 2021 and 2020:
|
|
Weighted-Average Contract Rate |
|
|
Notional Amount |
|
|
Fair Value Gain (Loss) |
|
|||
|
|
(in millions, except weighted-average contract rate) |
|
|||||||||
As of December 31, 2021 |
|
|
|
|
|
|
|
|
|
|||
Buy Brazilian real sell U.S. dollar |
|
|
5.77 |
|
|
$ |
6.8 |
|
|
$ |
0.1 |
|
Buy British pound sell Euro |
|
|
0.85 |
|
|
|
3.3 |
|
|
|
— |
|
Buy Chinese yuan sell Euro |
|
|
7.73 |
|
|
|
50.5 |
|
|
|
1.9 |
|
Buy Chinese yuan sell U.S. dollar |
|
|
6.63 |
|
|
|
103.2 |
|
|
|
3.7 |
|
Buy Colombian peso sell U.S. dollar |
|
|
4,009.53 |
|
|
|
1.1 |
|
|
|
— |
|
Buy Danish krone sell U.S. dollar |
|
|
6.59 |
|
|
|
0.9 |
|
|
|
— |
|
Buy Euro sell British pound |
|
|
0.85 |
|
|
|
8.9 |
|
|
|
(0.1 |
) |
Buy Euro sell Canadian dollar |
|
|
1.45 |
|
|
|
1.0 |
|
|
|
— |
|
Buy Euro sell Chilean peso |
|
|
987.89 |
|
|
|
1.1 |
|
|
|
— |
|
Buy Euro sell Chinese yuan |
|
|
7.26 |
|
|
|
1.8 |
|
|
|
— |
|
Buy Euro sell Hong Kong dollar |
|
|
8.81 |
|
|
|
4.0 |
|
|
|
— |
|
Buy Euro sell Indian rupee |
|
|
85.76 |
|
|
|
3.2 |
|
|
|
— |
|
Buy Euro sell Indonesian rupiah |
|
|
16,154.00 |
|
|
|
11.2 |
|
|
|
0.1 |
|
Buy Euro sell Japanese yen |
|
|
128.73 |
|
|
|
1.3 |
|
|
|
— |
|
Buy Euro sell Kazakhstani tenge |
|
|
501.00 |
|
|
|
1.5 |
|
|
|
— |
|
Buy Euro sell Malaysian ringgit |
|
|
4.77 |
|
|
|
21.3 |
|
|
|
(0.2 |
) |
Buy Euro sell Mexican peso |
|
|
24.84 |
|
|
|
47.4 |
|
|
|
(1.7 |
) |
Buy Euro sell Peruvian nuevo sol |
|
|
4.56 |
|
|
|
2.5 |
|
|
|
— |
|
Buy Euro sell Philippine peso |
|
|
56.41 |
|
|
|
2.3 |
|
|
|
0.1 |
|
Buy Euro sell Russian ruble |
|
|
84.24 |
|
|
|
10.5 |
|
|
|
0.2 |
|
Buy Euro sell South African rand |
|
|
18.03 |
|
|
|
1.6 |
|
|
|
— |
|
Buy Euro sell Taiwan dollar |
|
|
31.28 |
|
|
|
1.8 |
|
|
|
— |
|
Buy Euro sell Thai baht |
|
|
38.04 |
|
|
|
2.4 |
|
|
|
— |
|
Buy Euro sell Turkish lira |
|
|
15.54 |
|
|
|
1.5 |
|
|
|
— |
|
Buy Euro sell U.S. dollar |
|
|
1.14 |
|
|
|
19.3 |
|
|
|
— |
|
Buy Euro sell Ukrainian hryvnia |
|
|
31.46 |
|
|
|
1.5 |
|
|
|
— |
|
Buy Euro sell Vietnamese dong |
|
|
25,979.00 |
|
|
|
33.1 |
|
|
|
— |
|
Buy Indian rupee sell Euro |
|
|
84.85 |
|
|
|
3.2 |
|
|
|
— |
|
Buy Indonesian rupiah sell U.S. dollar |
|
|
14,379.57 |
|
|
|
6.9 |
|
|
|
— |
|
Buy Kazakhstani tenge sell Euro |
|
|
493.00 |
|
|
|
1.1 |
|
|
|
— |
|
Buy Norwegian krone sell U.S. dollar |
|
|
8.96 |
|
|
|
2.1 |
|
|
|
— |
|
Buy Polish zloty sell U.S. dollar |
|
|
4.13 |
|
|
|
0.9 |
|
|
|
— |
|
Buy South African rand sell U.S. dollar |
|
|
16.04 |
|
|
|
2.6 |
|
|
|
— |
|
Buy Swedish krona sell U.S. dollar |
|
|
9.15 |
|
|
|
2.0 |
|
|
|
— |
|
Buy Taiwan dollar sell U.S. dollar |
|
|
27.61 |
|
|
|
8.0 |
|
|
|
— |
|
Buy Thai baht sell Euro |
|
|
38.02 |
|
|
|
0.9 |
|
|
|
— |
|
Buy U.S. dollar sell Brazilian real |
|
|
5.79 |
|
|
|
18.8 |
|
|
|
(0.4 |
) |
Buy U.S. dollar sell Chinese yuan |
|
|
6.51 |
|
|
|
45.2 |
|
|
|
(0.9 |
) |
Buy U.S. dollar sell Colombian peso |
|
|
4,015.75 |
|
|
|
2.1 |
|
|
|
— |
|
Buy U.S. dollar sell Euro |
|
|
1.13 |
|
|
|
169.2 |
|
|
|
(0.8 |
) |
Buy U.S. dollar sell Indian rupee |
|
|
75.36 |
|
|
|
3.0 |
|
|
|
— |
|
Buy U.S. dollar sell Korean won |
|
|
1,179.42 |
|
|
|
4.0 |
|
|
|
— |
|
Buy U.S. dollar sell Mexican peso |
|
|
22.43 |
|
|
|
10.6 |
|
|
|
(0.2 |
) |
Buy U.S. dollar sell Philippine peso |
|
|
50.27 |
|
|
|
6.0 |
|
|
|
0.2 |
|
Buy U.S. dollar sell South African rand |
|
|
15.95 |
|
|
|
3.0 |
|
|
|
— |
|
Buy U.S. dollar sell Thai baht |
|
|
33.71 |
|
|
|
5.9 |
|
|
|
(0.1 |
) |
Buy Ukrainian hryvnia sell Euro |
|
|
31.62 |
|
|
|
1.2 |
|
|
|
— |
|
Buy Vietnamese dong sell Euro |
|
|
25,865.00 |
|
|
|
3.3 |
|
|
|
— |
|
Total forward contracts |
|
|
|
|
$ |
645.0 |
|
|
$ |
1.9 |
|
|
116
|
|
Weighted-Average Contract Rate |
|
|
Notional Amount |
|
|
Fair Value Gain (Loss) |
|
|||
|
|
(in millions, except weighted-average contract rate) |
|
|||||||||
As of December 31, 2020 |
|
|
|
|
|
|
|
|
|
|||
Buy British pound sell Euro |
|
|
0.92 |
|
|
$ |
3.4 |
|
|
$ |
0.1 |
|
Buy British pound sell U.S. dollar |
|
|
1.34 |
|
|
|
1.5 |
|
|
|
— |
|
Buy Chinese yuan sell Euro |
|
|
8.14 |
|
|
|
52.1 |
|
|
|
(0.1 |
) |
Buy Chinese yuan sell U.S. dollar |
|
|
7.13 |
|
|
|
99.3 |
|
|
|
8.5 |
|
Buy Colombian peso sell U.S. dollar |
|
|
3,422.09 |
|
|
|
1.1 |
|
|
|
— |
|
Buy Danish krone sell U.S. dollar |
|
|
6.10 |
|
|
|
1.0 |
|
|
|
— |
|
Buy Euro sell Australian dollar |
|
|
1.61 |
|
|
|
1.4 |
|
|
|
— |
|
Buy Euro sell British pound |
|
|
0.91 |
|
|
|
2.6 |
|
|
|
— |
|
Buy Euro sell Canadian dollar |
|
|
1.56 |
|
|
|
1.1 |
|
|
|
— |
|
Buy Euro sell Chilean peso |
|
|
896.37 |
|
|
|
2.6 |
|
|
|
(0.1 |
) |
Buy Euro sell Hong Kong dollar |
|
|
9.49 |
|
|
|
4.4 |
|
|
|
— |
|
Buy Euro sell Indian rupee |
|
|
90.29 |
|
|
|
4.9 |
|
|
|
(0.1 |
) |
Buy Euro sell Indonesian rupiah |
|
|
17,189.96 |
|
|
|
4.1 |
|
|
|
— |
|
Buy Euro sell Israeli shekel |
|
|
3.98 |
|
|
|
0.9 |
|
|
|
— |
|
Buy Euro sell Kazakhstani tenge |
|
|
520.25 |
|
|
|
3.1 |
|
|
|
— |
|
Buy Euro sell Malaysian ringgit |
|
|
4.98 |
|
|
|
1.2 |
|
|
|
— |
|
Buy Euro sell Mexican peso |
|
|
26.22 |
|
|
|
55.7 |
|
|
|
(3.4 |
) |
Buy Euro sell Peruvian nuevo sol |
|
|
4.41 |
|
|
|
1.6 |
|
|
|
— |
|
Buy Euro sell Philippine peso |
|
|
59.13 |
|
|
|
1.2 |
|
|
|
— |
|
Buy Euro sell Russian ruble |
|
|
92.13 |
|
|
|
7.7 |
|
|
|
(0.1 |
) |
Buy Euro sell South African rand |
|
|
18.00 |
|
|
|
5.4 |
|
|
|
— |
|
Buy Euro sell Taiwan dollar |
|
|
34.30 |
|
|
|
1.2 |
|
|
|
— |
|
Buy Euro sell Turkish lira |
|
|
9.32 |
|
|
|
2.9 |
|
|
|
(0.1 |
) |
Buy Euro sell Vietnamese dong |
|
|
27,872.81 |
|
|
|
17.5 |
|
|
|
0.2 |
|
Buy Indonesian rupiah sell U.S. dollar |
|
|
14,115.17 |
|
|
|
7.1 |
|
|
|
0.1 |
|
Buy Kazakhstani tenge sell Euro |
|
|
517.65 |
|
|
|
2.4 |
|
|
|
— |
|
Buy Korean won sell U.S. dollar |
|
|
1,114.85 |
|
|
|
10.0 |
|
|
|
0.2 |
|
Buy Mexican peso sell U.S. dollar |
|
|
19.97 |
|
|
|
35.1 |
|
|
|
— |
|
Buy Norwegian krone sell U.S. dollar |
|
|
8.66 |
|
|
|
2.2 |
|
|
|
— |
|
Buy Russian ruble sell Euro |
|
|
90.11 |
|
|
|
1.2 |
|
|
|
— |
|
Buy Swedish krona sell U.S. dollar |
|
|
8.25 |
|
|
|
2.7 |
|
|
|
— |
|
Buy Taiwan dollar sell U.S. dollar |
|
|
27.67 |
|
|
|
15.2 |
|
|
|
— |
|
Buy U.S. dollar sell Australian dollar |
|
|
0.76 |
|
|
|
2.0 |
|
|
|
— |
|
Buy U.S. dollar sell Chinese yuan |
|
|
6.75 |
|
|
|
47.1 |
|
|
|
(1.3 |
) |
Buy U.S. dollar sell Colombian peso |
|
|
3,525.72 |
|
|
|
4.2 |
|
|
|
(0.1 |
) |
Buy U.S. dollar sell Euro |
|
|
1.20 |
|
|
|
24.9 |
|
|
|
(0.4 |
) |
Buy U.S. dollar sell Korean won |
|
|
1,107.10 |
|
|
|
16.3 |
|
|
|
(0.3 |
) |
Buy U.S. dollar sell Mexican peso |
|
|
21.94 |
|
|
|
11.3 |
|
|
|
(0.6 |
) |
Buy U.S. dollar sell Philippine peso |
|
|
48.51 |
|
|
|
8.2 |
|
|
|
(0.1 |
) |
Buy U.S. dollar sell Thai baht |
|
|
31.08 |
|
|
|
3.2 |
|
|
|
(0.1 |
) |
Total forward contracts |
|
|
|
|
$ |
471.0 |
|
|
$ |
2.3 |
|
|
The following tables summarize the derivative activity during the years ended December 31, 2021, 2020, and 2019 relating to all the Company’s derivatives.
117
Gains and Losses on Derivative Instruments
The following table summarizes gains (losses) relating to derivative instruments recorded in other comprehensive loss during the years ended December 31, 2021, 2020, and 2019:
|
|
Amount of Gain (Loss) Recognized in Other Comprehensive (Loss) Income |
|
|||||||||
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Derivatives designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to inventory and intercompany management fee hedges |
|
$ |
0.1 |
|
|
$ |
2.3 |
|
|
$ |
(1.9 |
) |
Interest rate swaps |
|
|
|
|
|
(1.6 |
) |
|
|
— |
|
|
As of December 31, 2021, the estimated amount of existing net losses related to cash flow hedges recorded in accumulated other comprehensive loss that are expected to be reclassified into earnings over the next twelve months was $0.7 million.
The effect of cash flow hedging relationships on the Company’s consolidated statements of income for the years ended December 31, 2021, 2020, and 2019 was as follows:
|
|
Location and Amount of (Loss) Gain Recognized in Income on Cash Flow Hedging Relationships |
|
|||||||||
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|||||||||
|
|
Cost of sales |
|
|
Selling, general, and administrative expenses |
|
|
Interest expense |
|
|||
|
|
(in millions) |
|
|||||||||
Total amounts presented in the consolidated statements of income |
|
$ |
1,239.3 |
|
|
$ |
2,012.1 |
|
|
$ |
153.1 |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to inventory hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
(2.4 |
) |
|
|
— |
|
|
|
— |
|
Amount of loss excluded from assessment of effectiveness recognized in income |
|
|
(3.6 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to intercompany management fee hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
(0.2 |
) |
|
|
— |
|
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
0.1 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate swaps: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
— |
|
|
|
(0.9 |
) |
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
118
|
|
Location and Amount of Gain (Loss) Recognized in Income on Cash Flow Hedging Relationships |
|
|||||||||
|
|
Year Ended December 31, |
|
|||||||||
|
|
2020 |
|
|||||||||
|
|
Cost of sales |
|
|
Selling, general, and administrative expenses |
|
|
Interest expense |
|
|||
|
|
(in millions) |
|
|||||||||
Total amounts presented in the consolidated statements of income |
|
$ |
1,150.6 |
|
|
$ |
2,075.0 |
|
|
$ |
133.0 |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to inventory hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of gain reclassified from accumulated other comprehensive loss to income |
|
|
5.1 |
|
|
|
— |
|
|
|
— |
|
Amount of loss excluded from assessment of effectiveness recognized in income |
|
|
(3.3 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to intercompany management fee hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
(0.2 |
) |
|
|
— |
|
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
0.1 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate swaps: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
— |
|
|
|
(0.5 |
) |
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
119
|
|
Location and Amount of (Loss) Gain Recognized in Income on Cash Flow Hedging Relationships |
|
|||||||||
|
|
Year Ended December 31, |
|
|||||||||
|
|
2019 |
|
|||||||||
|
|
Cost of sales |
|
|
Selling, general, and administrative expenses |
|
|
Interest expense |
|
|||
|
|
(in millions) |
|
|||||||||
Total amounts presented in the consolidated statements of income |
|
$ |
958.0 |
|
|
$ |
1,940.3 |
|
|
$ |
153.0 |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to inventory hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of loss reclassified from accumulated other comprehensive loss to income |
|
|
(0.2 |
) |
|
|
— |
|
|
|
— |
|
Amount of loss excluded from assessment of effectiveness recognized in income |
|
|
(3.3 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts relating to intercompany management fee hedges: |
|
|
|
|
|
|
|
|
|
|||
Amount of gain reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
1.0 |
|
|
|
— |
|
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
0.2 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate swaps: |
|
|
|
|
|
|
|
|
|
|||
Amount of gain reclassified from accumulated other comprehensive loss to income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Amount of gain excluded from assessment of effectiveness recognized in income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
The following table summarizes gains (losses) recorded to income relating to derivative instruments not designated as hedging instruments during the December 31, 2021, 2020, and 2019:
|
|
Amount of Gain Recognized in Income |
|
|
|
|||||||||
|
|
Year Ended December 31, |
|
|
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|
Location of Gain Recognized in Income |
|||
|
|
(in millions) |
|
|
|
|||||||||
Derivatives not designated as hedging instruments: |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts |
|
$ |
5.9 |
|
|
$ |
2.5 |
|
|
$ |
1.0 |
|
|
Selling, general, and administrative expenses |
The Company reports its derivatives at fair value as either assets or liabilities within its consolidated balance sheets. See Note 13, Fair Value Measurements, for information on derivative fair values and their consolidated balance sheet locations as of December 31, 2021 and 2020.
12. Income Taxes
The components of income before income taxes were as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Domestic |
|
$ |
53.4 |
|
|
$ |
152.5 |
|
|
$ |
48.6 |
|
Foreign |
|
|
507.4 |
|
|
|
363.9 |
|
|
|
402.8 |
|
Total |
|
$ |
560.8 |
|
|
$ |
516.4 |
|
|
$ |
451.4 |
|
120
Income taxes were as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Current: |
|
|
|
|
|
|
|
|
|
|||
Foreign |
|
$ |
121.8 |
|
|
$ |
122.0 |
|
|
$ |
100.6 |
|
Federal |
|
|
20.1 |
|
|
|
13.7 |
|
|
|
22.1 |
|
State |
|
|
5.0 |
|
|
|
6.1 |
|
|
|
2.3 |
|
|
|
|
146.9 |
|
|
|
141.8 |
|
|
|
125.0 |
|
Deferred: |
|
|
|
|
|
|
|
|
|
|||
Foreign |
|
|
(17.0 |
) |
|
|
(2.7 |
) |
|
|
12.8 |
|
Federal |
|
|
(16.0 |
) |
|
|
3.9 |
|
|
|
1.3 |
|
State |
|
|
(0.3 |
) |
|
|
0.8 |
|
|
|
1.3 |
|
|
|
|
(33.3 |
) |
|
|
2.0 |
|
|
|
15.4 |
|
|
|
$ |
113.6 |
|
|
$ |
143.8 |
|
|
$ |
140.4 |
|
The significant categories of temporary differences that gave rise to deferred tax assets and liabilities were as follows:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Deferred income tax assets: |
|
|
|
|
|
|
||
Accruals not currently deductible |
|
$ |
94.9 |
|
|
$ |
98.5 |
|
Tax loss and credit carryforwards of certain foreign subsidiaries |
|
|
122.7 |
|
|
|
128.3 |
|
Domestic tax credit carryforwards |
|
|
204.5 |
|
|
|
208.7 |
|
Deferred compensation plan |
|
|
40.0 |
|
|
|
39.6 |
|
Deferred interest expense |
|
|
115.5 |
|
|
|
67.4 |
|
Inventory reserve |
|
|
9.3 |
|
|
|
5.5 |
|
Operating lease liabilities |
|
|
39.1 |
|
|
|
36.6 |
|
Other |
|
|
6.5 |
|
|
|
6.6 |
|
Gross deferred income tax assets |
|
|
632.5 |
|
|
|
591.2 |
|
Less: valuation allowance |
|
|
(411.9 |
) |
|
|
(390.8 |
) |
Total deferred income tax assets |
|
$ |
220.6 |
|
|
$ |
200.4 |
|
Deferred income tax liabilities: |
|
|
|
|
|
|
||
Intangible assets |
|
$ |
72.4 |
|
|
$ |
71.2 |
|
Unremitted foreign earnings |
|
|
20.9 |
|
|
|
14.9 |
|
Operating lease assets |
|
|
34.3 |
|
|
|
32.9 |
|
Other |
|
|
5.6 |
|
|
|
24.7 |
|
Total deferred income tax liabilities |
|
|
133.2 |
|
|
|
143.7 |
|
Total net deferred tax assets |
|
$ |
87.4 |
|
|
$ |
56.7 |
|
Tax loss and credit carryforwards of certain foreign subsidiaries for 2021 and 2020 were $122.7 million and $128.3 million, respectively. If unused, tax loss and credit carryforwards of certain foreign subsidiaries of $102.7 million will expire between 2022 and 2038 and $20.0 million can be carried forward indefinitely. U.S. foreign tax credit carryforwards for 2021 and 2020 were $195.1 million and $202.1 million, respectively, which are included in Domestic tax credit carryforwards in the table above. If unused, U.S. foreign tax credit carryforwards will expire between 2023 and 2031. U.S. research and development tax credit carryforwards for 2021 and 2020 were $12.0 million and $9.0 million, respectively. If unused, U.S. research and development tax credit carryforwards begin expiring in 2035. The deferred interest expense can be carried forward indefinitely.
121
The Company recognizes valuation allowances on deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. As of December 31, 2021 and 2020, the Company held valuation allowances against net deferred tax assets of certain subsidiaries, primarily related to tax loss carryforwards and U.S. foreign tax credits, in the amount of $411.9 million and $390.8 million, respectively. The change in the Company’s valuation allowance during 2021 of $21.1 million was primarily attributable to foreign deferred interest expense and tax loss carryforwards. The change in the Company’s valuation allowance during 2020 of $60.5 million was primarily attributable to foreign deferred interest expense and tax loss carryforwards. The change in the Company’s valuation allowance during 2019 of $11.2 million was primarily related to foreign deferred interest expense and tax loss carryforwards.
As of December 31, 2021, Herbalife Nutrition Ltd. had approximately $2.8 billion of permanently reinvested unremitted earnings relating to its operating subsidiaries. Since Herbalife Nutrition Ltd.’s unremitted earnings have been permanently reinvested, deferred taxes were not provided on these unremitted earnings. Further, it is not practicable to determine the amount of unrecognized deferred taxes with respect to these unremitted earnings. If the Company were to remit these unremitted earnings, it would be subject to income tax on these remittances. Deferred taxes have been accrued for earnings that are not considered indefinitely reinvested. The deferred tax liabilities on the unremitted foreign earnings as of December 31, 2021 and 2020 were $20.9 million and $22.3 million, respectively.
The applicable statutory income tax rate in the Cayman Islands was zero for Herbalife Nutrition Ltd. for the years being reported. For purposes of the reconciliation between the provision for income taxes at the statutory rate and the provision for income taxes at the effective tax rate, a notional 21% tax rate is applied for the years ended December 31, 2021, 2020, and 2019 as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Tax expense at United States statutory rate |
|
$ |
117.8 |
|
|
$ |
108.4 |
|
|
$ |
94.8 |
|
Increase (decrease) in tax resulting from: |
|
|
|
|
|
|
|
|
|
|||
Differences between U.S. and foreign tax rates on foreign income, including withholding taxes |
|
|
(15.1 |
) |
|
|
(19.5 |
) |
|
|
50.9 |
|
U.S. tax (benefit) on foreign income, net of foreign tax credits |
|
|
(21.9 |
) |
|
|
(20.5 |
) |
|
|
(10.1 |
) |
Increase in valuation allowances |
|
|
21.1 |
|
|
|
60.6 |
|
|
|
11.4 |
|
State taxes, net of federal benefit |
|
|
3.0 |
|
|
|
5.2 |
|
|
|
3.1 |
|
Unrecognized tax benefits |
|
|
9.3 |
|
|
|
3.9 |
|
|
|
(6.9 |
) |
Excess tax benefits on equity awards |
|
|
(3.5 |
) |
|
|
(3.1 |
) |
|
|
(5.8 |
) |
Other |
|
|
2.9 |
|
|
|
8.8 |
|
|
|
3.0 |
|
Total |
|
$ |
113.6 |
|
|
$ |
143.8 |
|
|
$ |
140.4 |
|
As of December 31, 2021, the total amount of unrecognized tax benefits, including related interest and penalties was $72.5 million. If the total amount of unrecognized tax benefits was recognized, $48.1 million of unrecognized tax benefits, $15.1 million of interest, and $3.3 million of penalties would impact the effective tax rate. As of December 31, 2020, the total amount of unrecognized tax benefits, including related interest and penalties was $65.9 million. If the total amount of unrecognized tax benefits was recognized, $46.9 million of unrecognized tax benefits, $11.4 million of interest, and $1.8 million of penalties would impact the effective tax rate.
The Company accounts for the interest and penalties generated by tax contingencies as a component of income tax expense. During the year ended December 31, 2021, the Company recorded an increase in interest and penalty expense related to uncertain tax positions of $4.1 million and $1.5 million, respectively. During the year ended December 31, 2020, the Company recorded an increase in interest expense related to uncertain tax positions of $2.4 million and a decrease in penalty expense related to uncertain tax positions of $0.1 million. During the year ended December 31, 2019, the Company recorded a decrease in interest expense related to uncertain tax positions of $0.7 million and an increase in penalty expense related to uncertain tax positions of $0.2 million. As of December 31, 2021, the total amount of interest and penalties related to unrecognized tax benefits recognized in the consolidated balance sheet was $15.1 million and $3.3 million, respectively. As of December 31, 2020, the total amount of interest and penalties related to unrecognized tax benefits recognized in the consolidated balance sheet was $11.4 million and $1.8 million, respectively. As of December 31, 2019, the total amount of interest and penalties related to unrecognized tax benefits recognized in the consolidated balance sheet was $9.1 million and $1.9 million, respectively.
122
The following changes occurred in the amount of unrecognized tax benefits during the years ended December 31, 2021, 2020, and 2019:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2021 |
|
|
2020 |
|
|
2019 |
|
|||
|
|
(in millions) |
|
|||||||||
Beginning balance of unrecognized tax benefits |
|
$ |
52.7 |
|
|
$ |
48.9 |
|
|
$ |
53.5 |
|
Additions for current year tax positions |
|
|
9.2 |
|
|
|
9.7 |
|
|
|
8.4 |
|
Additions for prior year tax positions |
|
|
5.1 |
|
|
|
1.3 |
|
|
|
6.1 |
|
Reductions for prior year tax positions |
|
|
(2.3 |
) |
|
|
(0.6 |
) |
|
|
(15.4 |
) |
Reductions for audit settlements |
|
|
(5.2 |
) |
|
|
(4.7 |
) |
|
|
(0.1 |
) |
Reductions for the expiration of statutes of limitations |
|
|
(4.1 |
) |
|
|
(2.1 |
) |
|
|
(3.6 |
) |
Changes due to foreign currency translation adjustments |
|
|
(1.3 |
) |
|
|
0.2 |
|
|
|
— |
|
Ending balance of unrecognized tax benefits (excluding interest and penalties) |
|
|
54.1 |
|
|
|
52.7 |
|
|
|
48.9 |
|
Interest and penalties associated with unrecognized tax benefits |
|
|
18.4 |
|
|
|
13.2 |
|
|
|
11.0 |
|
Ending balance of unrecognized tax benefits (including interest and penalties) |
|
$ |
72.5 |
|
|
$ |
65.9 |
|
|
$ |
59.9 |
|
The amount of income taxes the Company pays is subject to ongoing audits by taxing jurisdictions around the world. The Company’s estimate of the potential outcome of any uncertain tax position is subject to management’s assessment of relevant risks, facts, and circumstances existing at that time. The Company believes that it has adequately provided for these matters. However, the Company’s future results may include favorable or unfavorable adjustments to its estimates in the period the audits are resolved, which may impact the Company’s effective tax rate. As of December 31, 2021, the Company’s tax filings are generally subject to examination in major tax jurisdictions for years ending on or after December 31, 2012.
The Company believes that it is reasonably possible that the amount of unrecognized tax benefits could decrease by up to approximately $6.4 million within the next twelve months. Of this possible decrease, $5.5 million would be due to the expiration of statute of limitations in various jurisdictions. The remaining possible decrease of $0.9 million would be due to settlement of audits or resolution of administrative or judicial proceedings.
13. Fair Value Measurements
The Company applies the provisions of FASB ASC Topic 820, Fair Value Measurements and Disclosures, or ASC 820, for its financial and non-financial assets and liabilities. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC 820 establishes a fair value hierarchy, which prioritizes the inputs used in measuring fair value into three broad levels as follows:
Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date.
Level 2 inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability, and inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 3 inputs are unobservable inputs for the asset or liability.
The Company measures certain assets and liabilities at fair value as discussed throughout the notes to its consolidated financial statements. Foreign exchange currency contracts and interest rate swaps are valued using standard calculations and models. Foreign exchange currency contracts are valued primarily based on inputs such as observable forward rates, spot rates, and foreign currency exchange rates at the reporting period ended date. Interest rate swaps are valued primarily based on inputs such as LIBOR and swap yield curves at the reporting period ended date.
123
The Company’s derivative assets and liabilities are measured at fair value and consisted of Level 2 inputs and their amounts are shown below at their gross values as of December 31, 2021 and 2020:
|
|
Significant Other Observable Inputs (Level 2) Fair Value as of December 31, |
|
|
Significant Other Observable Inputs (Level 2) Fair Value as of December 31, |
|
|
Balance Sheet Location |
||
|
|
(in millions) |
|
|
|
|||||
ASSETS: |
|
|
|
|
|
|
|
|
||
Derivatives designated as hedging instruments: |
|
|
|
|
|
|
|
|
||
Foreign exchange currency contracts relating to inventory and intercompany management fee hedges |
|
$ |
0.3 |
|
|
$ |
— |
|
|
Prepaid expenses and other current assets |
Derivatives not designated as hedging instruments: |
|
|
|
|
|
|
|
|
||
Foreign exchange currency contracts |
|
|
6.6 |
|
|
|
9.4 |
|
|
Prepaid expenses and other current assets |
|
|
$ |
6.9 |
|
|
$ |
9.4 |
|
|
|
LIABILITIES: |
|
|
|
|
|
|
|
|
||
Derivatives designated as hedging instruments: |
|
|
|
|
|
|
|
|
||
Foreign exchange currency contracts relating to inventory and intercompany management fee hedges |
|
$ |
1.7 |
|
|
$ |
3.9 |
|
|
Other current liabilities |
Interest rate swaps |
|
|
0.1 |
|
|
|
1.0 |
|
|
Other current liabilities |
Derivatives not designated as hedging instruments: |
|
|
|
|
|
|
|
|
||
Foreign exchange currency contracts |
|
|
3.4 |
|
|
|
3.2 |
|
|
Other current liabilities |
|
|
$ |
5.2 |
|
|
$ |
8.1 |
|
|
|
The Company’s deferred compensation plan assets consist of Company-owned life insurance policies. As these policies are recorded at their cash surrender value, they are not required to be included in the fair value table above. See Note 6, Employee Compensation Plans, for a further description of its deferred compensation plan assets.
The following tables summarize the offsetting of the fair values of the Company’s derivative assets and derivative liabilities for presentation in the Company’s consolidated balance sheets as of December 31, 2021 and 2020:
|
|
Offsetting of Derivative Assets |
|
|||||||||
|
|
Gross Amounts of Recognized Assets |
|
|
Gross Amounts Offset in the Balance Sheet |
|
|
Net Amounts of Assets Presented in the Balance Sheet |
|
|||
|
|
(in millions) |
|
|||||||||
December 31, 2021 |
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts |
|
$ |
6.9 |
|
|
$ |
(2.2 |
) |
|
$ |
4.7 |
|
Total |
|
$ |
6.9 |
|
|
$ |
(2.2 |
) |
|
$ |
4.7 |
|
December 31, 2020 |
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts |
|
$ |
9.4 |
|
|
$ |
(1.8 |
) |
|
$ |
7.6 |
|
Total |
|
$ |
9.4 |
|
|
$ |
(1.8 |
) |
|
$ |
7.6 |
|
124
|
|
Offsetting of Derivative Liabilities |
|
|||||||||
|
|
Gross Amounts of Recognized Liabilities |
|
|
Gross Amounts Offset in the Balance Sheet |
|
|
Net Amounts of Liabilities Presented in the Balance Sheet |
|
|||
|
|
(in millions) |
|
|||||||||
December 31, 2021 |
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts |
|
$ |
5.1 |
|
|
$ |
(2.2 |
) |
|
$ |
2.9 |
|
Interest rate swaps |
|
|
0.1 |
|
|
|
— |
|
|
|
0.1 |
|
Total |
|
$ |
5.2 |
|
|
$ |
(2.2 |
) |
|
$ |
3.0 |
|
December 31, 2020 |
|
|
|
|
|
|
|
|
|
|||
Foreign exchange currency contracts |
|
$ |
7.1 |
|
|
$ |
(1.8 |
) |
|
$ |
5.3 |
|
Interest rate swaps |
|
|
1.0 |
|
|
|
— |
|
|
|
1.0 |
|
Total |
|
$ |
8.1 |
|
|
$ |
(1.8 |
) |
|
$ |
6.3 |
|
The Company offsets all of its derivative assets and derivative liabilities in its consolidated balance sheets to the extent it maintains master netting arrangements with related financial institutions. As of December 31, 2021 and 2020, all of the Company’s derivatives were subject to master netting arrangements and no collateralization was required for the Company’s derivative assets and derivative liabilities.
14. Transformation Program
In the fourth quarter of 2021, the Company initiated a global transformation program to optimize global processes for future growth, or the Transformation Program. The Transformation Program involves the investment in certain new technologies and the realignment of infrastructure and the locations of certain functions to better support distributors and customers. For the first phase of the Transformation Program, the Company currently expects total pre-tax expenses in the range of $25 million to $30 million through 2023, of which $12.9 million was recognized in selling, general, and administrative expenses within its consolidated statement of income during the year ended December 31, 2021. The Company expects to complete the first phase of the Transformation Program in 2023. The Company is still assessing the scope, timing, and execution plan of the second phase of the Transformation Program, and accordingly cannot estimate the amounts to be incurred for the program in totality.
Costs related to the Transformation Program for the year ended December 31, 2021 were as follows:
|
|
Year Ended December 31, |
|
|
|
|
2021 |
|
|
|
|
(in millions) |
|
|
Professional fees |
|
$ |
9.7 |
|
Retention and separation |
|
|
3.0 |
|
Other |
|
|
0.2 |
|
Total |
|
$ |
12.9 |
|
Changes in the liabilities related to the Transformation Program, which were recognized in other current liabilities within the Company’s consolidated balance sheets, were as follows:
|
|
Professional Fees |
|
|
Retention and Separation |
|
|
Other |
|
|
Total |
|
||||
|
|
(in millions) |
|
|||||||||||||
Expenses |
|
$ |
9.7 |
|
|
$ |
3.0 |
|
|
$ |
0.2 |
|
|
$ |
12.9 |
|
Cash payments |
|
|
(7.7 |
) |
|
|
(0.2 |
) |
|
|
(0.2 |
) |
|
|
(8.1 |
) |
Non-cash items and other |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Balance as of December 31, 2021 |
|
$ |
2.0 |
|
|
$ |
2.8 |
|
|
$ |
— |
|
|
$ |
4.8 |
|
15. Detail of Certain Balance Sheet Accounts
Other Assets
The Other assets on the Company’s accompanying consolidated balance sheets included deferred compensation plan assets of $48.2 million and $43.8 million and deferred tax assets of $118.0 million and $96.0 million as of December 31, 2021 and 2020, respectively.
125
Other Current Liabilities
Other current liabilities consisted of the following:
|
|
December 31, |
|
|||||
|
|
2021 |
|
|
2020 |
|
||
|
|
(in millions) |
|
|||||
Accrued compensation |
|
$ |
171.9 |
|
|
$ |
163.8 |
|
Accrued service fees to China independent service providers |
|
|
48.5 |
|
|
|
63.3 |
|
Accrued advertising, events, and promotion expenses |
|
|
55.9 |
|
|
|
67.6 |
|
Current operating lease liabilities |
|
|
42.8 |
|
|
|
35.5 |
|
Advance sales deposits |
|
|
63.0 |
|
|
|
68.5 |
|
Income taxes payable |
|
|
13.7 |
|
|
|
23.0 |
|
Other accrued liabilities |
|
|
200.0 |
|
|
|
235.8 |
|
Total |
|
$ |
595.8 |
|
|
$ |
657.5 |
|
Other Non-Current Liabilities
The Other non-current liabilities on the Company’s accompanying consolidated balance sheets included deferred compensation plan liabilities of $80.5 million and $72.3 million and deferred income tax liabilities of $30.6 million and $39.3 million as of December 31, 2021 and 2020, respectively. See Note 6, Employee Compensation Plans, for a further description of the Company’s deferred compensation plan assets and liabilities.
126
Item 16. Form 10-K Summary
None.
127
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
HERBALIFE NUTRITION LTD. |
|
|
|
|
|
By: |
/s/ ALEXANDER AMEZQUITA |
|
|
Alexander Amezquita Chief Financial Officer |
Dated: February 23, 2022
128
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ JOHN O. AGWUNOBI |
|
Chairman of the Board and Chief Executive Officer (Principal Executive Officer and Director) |
|
February 23, 2022 |
John O. Agwunobi |
|
|
||
|
|
|
|
|
/s/ ALEXANDER AMEZQUITA |
|
Chief Financial Officer (Principal Financial Officer) |
|
February 23, 2022 |
Alexander Amezquita |
|
|
||
|
|
|
|
|
/s/ JEHANGIR IRANI |
|
Senior Vice President, Principal Accounting Officer (Principal Accounting Officer) |
|
February 23, 2022 |
Jehangir “Bobby” Irani |
|
|
||
|
|
|
|
|
/s/ RICHARD H. CARMONA |
|
Director |
|
February 23, 2022 |
Richard H. Carmona |
|
|
||
|
|
|
|
|
/s/ KEVIN M. JONES |
|
Director |
|
February 23, 2022 |
Kevin M. Jones |
|
|
||
|
|
|
|
|
/s/ ALAN W. LEFEVRE |
|
Director |
|
February 23, 2022 |
Alan W. LeFevre |
|
|
||
|
|
|
|
|
/s/ SOPHIE L’HÉLIAS |
|
Director |
|
February 23, 2022 |
Sophie L’Hélias |
|
|
||
|
|
|
|
|
/s/ JUAN MIGUEL MENDOZA |
|
Director |
|
February 23, 2022 |
Juan Miguel Mendoza |
|
|
||
|
|
|
|
|
/s/ DONAL MULLIGAN |
|
Director |
|
February 23, 2022 |
Donal Mulligan |
|
|
||
|
|
|
|
|
/s/ MARIA OTERO |
|
Director |
|
February 23, 2022 |
Maria Otero |
|
|
||
|
|
|
|
|
/s/ JOHN TARTOL |
|
Director |
|
February 23, 2022 |
John Tartol |
|
|
129
Similar companies
See also MCKESSON CORP - Annual report 2023 (10-K 2023-03-31) Annual report 2023 (10-Q 2023-09-30)See also Cencora, Inc. - Annual report 2023 (10-K 2023-09-30) Annual report 2023 (10-Q 2023-06-30)
See also CARDINAL HEALTH INC - Annual report 2023 (10-K 2023-06-30) Annual report 2023 (10-Q 2023-09-30)
See also NU SKIN ENTERPRISES, INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PetIQ, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
