Humacyte, Inc. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________
FORM 10-K
___________________________________
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the fiscal year ended December 31, 2022
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from to
Commission file number 001-39532
___________________________________
Humacyte, Inc.
(Exact name of registrant as specified in its charter)
___________________________________
Delaware | 85-1763759 | |||||||
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
2525 East North Carolina Highway 54 | ||||||||
| Durham, | NC | 27713 | ||||||
| (Address of principal executive offices) | (Zip code) | |||||||
(919) 313-9633
(Registrant's telephone number, including area code)
Securities registered pursuant to 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Common Stock, par value $0.0001 per share | HUMA | The Nasdaq Stock Market LLC | ||||||||||||
Redeemable Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 | HUMAW | The Nasdaq Stock Market LLC | ||||||||||||
Securities registered pursuant to 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports); and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | o | Accelerated filer | o | |||||||||||
Non-accelerated filer | x | Smaller reporting company | x | |||||||||||
Emerging growth company | x | |||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C.7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
As of June 30, 2022, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of common stock held by non-affiliates of the registrant was approximately $165.9 million (based on the closing price of the registrant’s common stock as reported on The Nasdaq Global Select Market on that date).
As of March 10, 2023, 103,329,133 shares of common stock, par value $0.0001, were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement relative to the 2023 Annual Meeting of Shareholders are incorporated by reference in Part III hereof.
Table of Contents
| Page No. | ||||||||
2
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements that involve substantial risks and uncertainties. “Forward-looking statements,” as that term is defined in the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”) are statements that are not historical facts and involve a number of risks and uncertainties. These statements include, without limitation, statements regarding the financial position, business strategy and the plans and objectives of management for future operations. These statements constitute projections, forecasts and forward-looking statements, and are not guarantees of performance. Such statements can be identified by the fact that they do not relate strictly to historical or current facts. When used therein, words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Such statements are based on the beliefs of, as well as assumptions made by and information currently available to, our management.
Forward-looking statements may include, for example, statements about:
•our plans and ability to execute product development, process development and preclinical development efforts successfully and on our anticipated timelines;
•our plans and ability to obtain marketing approval from the U.S. Food and Drug Administration (“FDA”) and other regulatory authorities, including the European Medicines Agency (“EMA”), for our bioengineered human acellular vessels (“HAVs”) and other product candidates;
•our ability to design, initiate and successfully complete clinical trials and other studies for our product candidates and our plans and expectations regarding our ongoing or planned clinical trials, including for our ongoing V005 Phase 2/3 clinical trial and V007 Phase 3 clinical trial;
•the outcome of our ongoing discussions with the FDA concerning the design of our clinical trials;
•our anticipated growth rate and market opportunities;
•the potential liquidity and trading of our securities;
•our ability to raise additional capital in the future;
•our ability to use our proprietary scientific technology platform to build a pipeline of additional product candidates;
•the characteristics and performance of our HAVs;
•our plans and ability to commercialize our HAVs and other product candidates, if approved by regulatory authorities;
•the expected size of the target populations for our product candidates;
•the anticipated benefits of our HAVs relative to existing alternatives;
•our assessment of the competitive landscape;
•the degree of market acceptance of HAVs, if approved, and the availability of third-party coverage and reimbursement;
•our ability to manufacture HAVs and other product candidates in sufficient quantities to satisfy our clinical trial and commercial needs;
•our expectations regarding our strategic partnership with Fresenius Medical Care Holdings, Inc. (“Fresenius Medical Care”) to sell, market and distribute our 6 millimeter HAV for certain specified indications and in specified markets;
•the performance of other third parties on which we rely, including our third-party manufacturers, our licensors, our suppliers and the organizations conducting our clinical trials;
•our ability to obtain and maintain intellectual property protection for our product candidates as well as our ability to operate our business without infringing, misappropriating or otherwise violating the intellectual property rights of others;
3
•our ability to maintain the confidentiality of our trade secrets, particularly with respect to our manufacturing process;
•our compliance with applicable laws and regulatory requirements, including FDA regulations, healthcare laws and regulations, and anti-corruption laws;
•our ability to attract, retain and motivate qualified personnel and to manage our growth effectively;
•our future financial performance and capital requirements;
•our ability to implement and maintain effective internal controls;
•the impact of the ongoing effects of the COVID-19 pandemic on our business, including our manufacturing efforts and clinical trials; and
•the impact of the overall global economy and increasing interest rates and inflation on our business.
We caution readers not to place undue reliance on any such forward-looking statements, which speak only as of the date they are made. Any forward-looking statement is based on information current as of the date of this Annual Report on Form 10-K and speaks only as of the date on which such statement is made. Actual events or results may differ materially from the results, plans, intentions or expectations anticipated in these forward-looking statements as a result of a variety of factors, many of which are beyond our control. More information on factors that could cause actual results to differ materially from those anticipated is included from time to time in our reports filed with the Securities and Exchange Commission (the “SEC”), including, but not limited to, those described in the sections of this Annual Report on Form 10-K titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” We disclaim any obligation, except as specifically required by law, to publicly update or revise any such statements to reflect any change in our expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
4
PART I
Item 1. Business
Business Overview
Executive Summary
Humacyte, Inc. is pioneering the development and manufacture of off-the-shelf, universally implantable, bioengineered human tissues, advanced tissue constructs and organ systems with the goal of improving the lives of patients and transforming the practice of medicine. We believe our regenerative medicine technology has the potential to overcome limitations in existing standards of care and address the lack of significant innovation in products that support tissue repair, reconstruction and replacement. We are leveraging our novel, scalable technology platform to develop proprietary, bioengineered, acellular human tissues for use in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas.
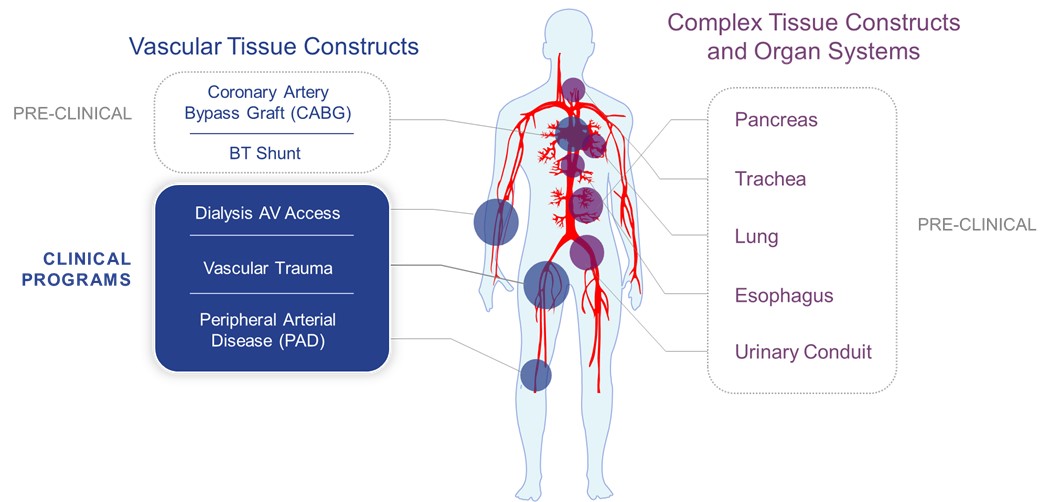
We are initially using our proprietary, scientific technology platform to engineer and manufacture Human Acellular VesselsTM, or HAVsTM. Our investigational HAVs are designed to be easily implanted into any patient without inducing a foreign body response or leading to immune rejection. We are developing a portfolio, or “cabinet”, of HAVs with varying diameters and lengths. The HAV cabinet would initially target the vascular repair, reconstruction and replacement market, including vascular trauma; arteriovenous (“AV”) access for hemodialysis; peripheral artery disease (“PAD”); and coronary artery bypass grafting (“CABG”). In addition, we are developing our HAVs for pediatric heart surgery and the delivery of cellular therapies, including pancreatic islet cell transplantation to treat Type 1 diabetes. We will continue to explore the application of our technology across a broad range of markets and indications including the development of urinary conduit, trachea, esophagus and other novel cell delivery systems.
We believe there is substantial clinical demand for safe and effective vascular conduits to replace and repair blood vessels throughout the body. Vascular injuries resulting from trauma are common in civilian and military populations, frequently resulting in the loss of either life or limb. Existing treatment options in the vascular repair, reconstruction and replacement market include the use of autologous vessels and synthetic grafts, which we believe suffer from significant limitations. For example, the use of autologous veins to repair traumatic vascular injuries can lead to significant morbidity associated with the surgical wounds created for vein harvest and prolonged times to restore blood flow to injured limbs, leading to an increased risk of amputation and infection. In addition, in many instances of vascular trauma, the patient may not have adequate vein available to make autologous graft repair feasible. Synthetic grafts are often contraindicated in the setting of vascular trauma due to higher infection risk that can lead to prolonged hospitalization and limb loss. Given the competitive advantages our HAVs are designed to have over existing vascular substitutes, we believe that HAVs have the potential to become the standard of care and lead to improved patient outcomes and lower healthcare costs.
As of December 31, 2022, our HAVs have been implanted in approximately 533 patients. We are currently conducting Phase 2 and Phase 3 trials of our 6 millimeter HAV across two therapeutic indications, vascular trauma and AV access for hemodialysis, as well as continuing long-term follow up of patients in our Phase 2 PAD studies. We were granted Fast Track designation by the FDA for our 6 millimeter HAV for use in AV access for hemodialysis in 2014. We also received the first Regenerative Medicine Advanced Therapy (“RMAT”) designation from the FDA, for the creation of vascular access for performing hemodialysis, in March 2017. In addition, in 2018 our HAV product candidate was assigned a priority designation by the Secretary of Defense under Public Law 115-92, enacted to expedite the FDA’s review of products that are intended to diagnose, treat or prevent serious or life-threatening conditions facing American military personnel. Upon completion of our Phase 3 trials, we intend to submit a Biologics License Application (“BLA”) to the FDA for an indication in vascular trauma and AV access for hemodialysis.
We have developed a novel paradigm for manufacturing human tissues that is intended to mimic key aspects of human physiology. We have an 83,000 square foot bioprocessing facility housing our modular manufacturing process with the ability to manufacture HAVs of different diameters and lengths at commercial scale. As we continue to expand production, we believe we will have the ability to take advantage of economies of scale to reduce costs of production. We believe our established, controlled manufacturing process demonstrates a significant competitive advantage in the regenerative medicine market.
Our technology is protected by our patent portfolio, which includes certain patents licensed from parties as well as intellectual property generated internally at Humacyte. Our patent portfolio is comprised of 18 families of patents, many of which generally relate to the scaffolds used to make our vessels, the composition of our vessels and systems and methods of manufacturing our vessels. For more information, see “— Intellectual Property” below.
5
We intend to continue to shape our commercial and distribution strategy by indication and pursue collaborations with partners in markets where such partners provide strategic opportunities in launching our product candidates and enabling access to specific patient populations.
Our world-class senior management team and board of directors will be instrumental in helping us achieve our goals. Our President and Chief Executive Officer, Laura Niklason M.D., PhD., who founded Legacy Humacyte (as defined below), is an internationally respected physician scientist and a world leader in regenerative medicine technologies. Dr. Niklason is also a member of three national academies — Inventors, Medicine and Engineering. Our current Chairman of the Board is Kathleen Sebelius, the former Secretary of the Department of Health and Human Services (“HHS”), and the former Governor of Kansas.
Merger
On August 26, 2021 (the “Closing Date”), Humacyte, Inc. (“Legacy Humacyte”), a Delaware corporation, and Alpha Healthcare Acquisition Corp. (“AHAC”), a Delaware corporation, consummated a merger pursuant to that certain Business Combination Agreement, dated as of February 17, 2021 (the “Merger Agreement”), by and among Legacy Humacyte, AHAC and Hunter Merger Sub (“Merger Sub”), a Delaware corporation and wholly owned subsidiary of AHAC. As contemplated by the Merger Agreement, Merger Sub merged with and into Legacy Humacyte, with Legacy Humacyte continuing as the surviving corporation and as a wholly owned subsidiary of AHAC (the “Merger”). On the Closing Date, AHAC changed its name to Humacyte, Inc. and Legacy Humacyte changed its name to Humacyte Global, Inc.
Unless the context indicates otherwise, references in this Annual Report on Form 10-K to the “Company,” “Humacyte,” “we,” “us,” “our” and similar terms refer to Humacyte, Inc. (formerly known as Alpha Healthcare Acquisition Corp.) and its consolidated subsidiaries (including Humacyte Global, Inc.) following the Merger. References to “AHAC” refer to Alpha Healthcare Acquisition Corp. prior to the Merger.
Our Approach
We have developed an approach that relies on two key complementary elements to address the significant market opportunity for the global treatment of patients in need of vascular replacement, repair and reconstruction, vascular access for dialysis and potential future indications including complex tissue and organ replacement and treatment of Type-1 diabetes:
•our proprietary scientific and engineering technology platform allows us to grow human tissues, which are ultimately decellularized and therefore expected to be non-immunogenic and universally implantable; and
•our novel, scalable manufacturing paradigm is designed to allow us to produce thousands of HAVs per year at the time of commercial launch, with the ability to expand manufacturing capacity and breadth to meet expected future global demand and the planned expansion of our pipeline of product candidates.
In the first employment of these platform and manufacturing approaches, we intend to develop a readily available “cabinet” of HAVs of varying diameters and lengths to address the significant unmet needs across multiple potential indications in vascular repair, reconstruction and replacement.
6
Illustration of our Proposed HAV “Cabinet” for Vascular Repair, Reconstruction, and Replacement

Our Proprietary Scientific Technology Platform
Our proprietary scientific technology platform uses primary human aortic vascular cells from a working cell stock, isolated from donor tissues and cryopreserved. The working cell stock is expanded using traditional cell culture techniques, and the cells are transferred onto a biocompatible, biodegradable polymer mesh within a flexible, single-use bioreactor bag. Over the course of weeks, the cells proliferate and build extracellular matrix while the polymer mesh degrades. The resulting bioengineered vessel is comprised of the aortic vascular cells and their deposited extracellular matrix. After completion of the culture period, we decellularize the bioengineered vessel using a proprietary combination of solutions. The resulting HAV retains the extracellular matrix constituents and, therefore, the biomechanical properties of the bioengineered vessel, but is cleansed of the cells and cellular components that could induce a foreign body response or immune rejection following implantation. Our functionally closed system allows for the HAV to be grown, decellularized and ultimately shipped within the same flexible bioreactor bag. Our HAVs are designed to be shipped to hospitals, trauma centers and outpatient surgical settings, where they can then be stored at refrigerated temperatures for immediate use by removing each HAV from its packaging.
7
The following image summarizes key information about our proprietary scientific technology platform:
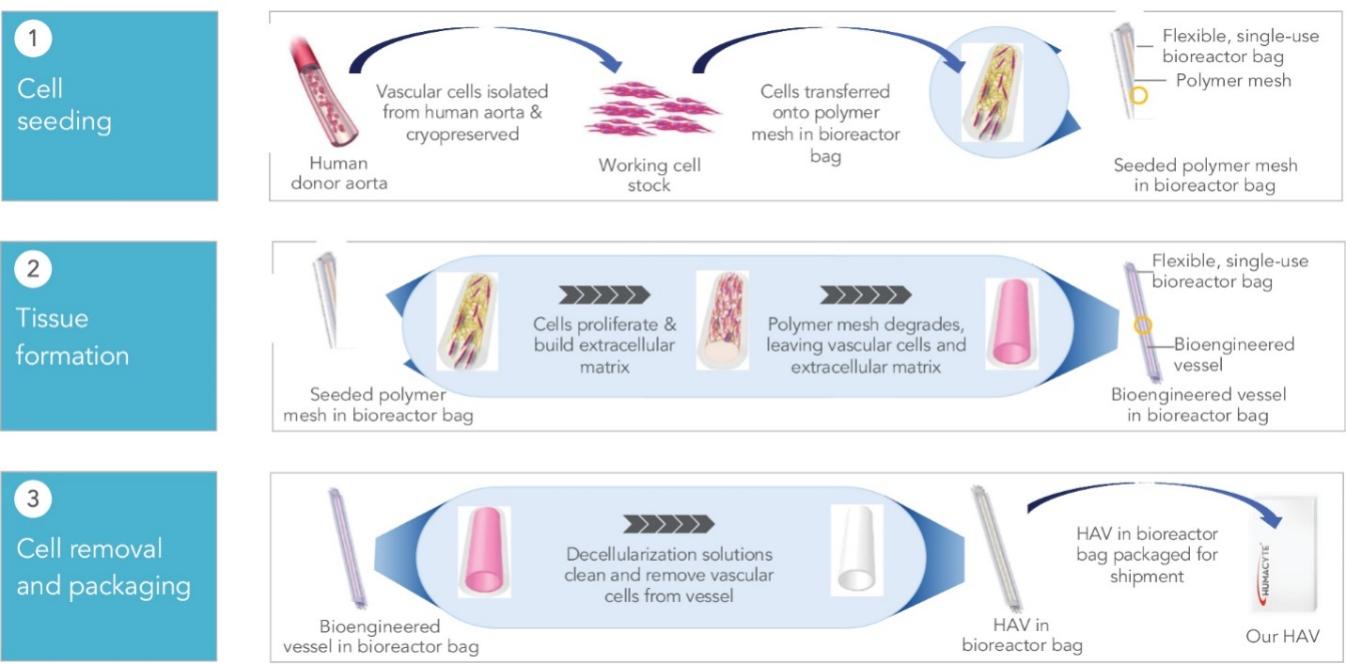
Our Novel Manufacturing Paradigm
We have developed a novel paradigm for manufacturing human tissues that is intended to mimic key aspects of human physiology. Our proprietary manufacturing process was designed with a modular approach allowing us to produce HAVs in smaller batches for clinical trials and scale out to larger batches for commercial manufacturing. The manufacturing system used to supply our clinical trials from 2016 to 2021, including our Phase 3 trials, utilized a single tray within one growth drawer holding ten HAVs per batch. In 2021 we commenced supplying our ongoing clinical trials with HAVs produced in our current, commercial-scale LUNA200TM system, which consists of 20 growth drawers per production unit for a total of 200 HAVs per batch. Each growth drawer is capable of producing ten 42cm HAVs, each of which is contained within an individual bioreactor bag. Inside a LUNA200, a tubing network connects all HAVs, allowing the entire system to share nutritive media. In this way, a single LUNA200 can produce up to 200 HAVs (42cm in length) per batch while maintaining the critical operating parameters, such as biomechanical pulsing, that affect growth.
A thorough comparability assessment was performed to evaluate HAV batches produced in the single drawer system and used in Phase 3 studies versus the 20-drawer LUNA200 system. The study assessed 22 separate comparisons on the identity, strength, quality, purity, and potency of the HAV product. In this study, we observed that HAVs produced in the LUNA200 system were comparable to HAVs produced in the single-drawer system. Additionally, a crossover study, called V011, was conducted in 30 subjects to evaluate HAVs that are manufactured on Humacyte’s commercial LUNA200 platform with the primary goal to evaluate the safety, efficacy and immunogenicity of the LUNA200-manufactured HAVs. In this trial we have observed comparable safety profile between HAV used in previous studies and the HAV manufactured in the LUNA200 commercial system. The results of the comparability assessment and the results from the V011 crossover study were submitted to the FDA. In 2021, the FDA authorized the use of HAVs produced in the commercial LUNA200 system to supply our ongoing clinical trials. We also plan to use the LUNA200 system for anticipated commercial launches of the HAV if it is approved.
8
Our current 83,000 square foot manufacturing facility has space to further expand manufacturing capacity as needed to over 40 LUNA200 systems. Currently, eight LUNA200 systems are installed and operational.
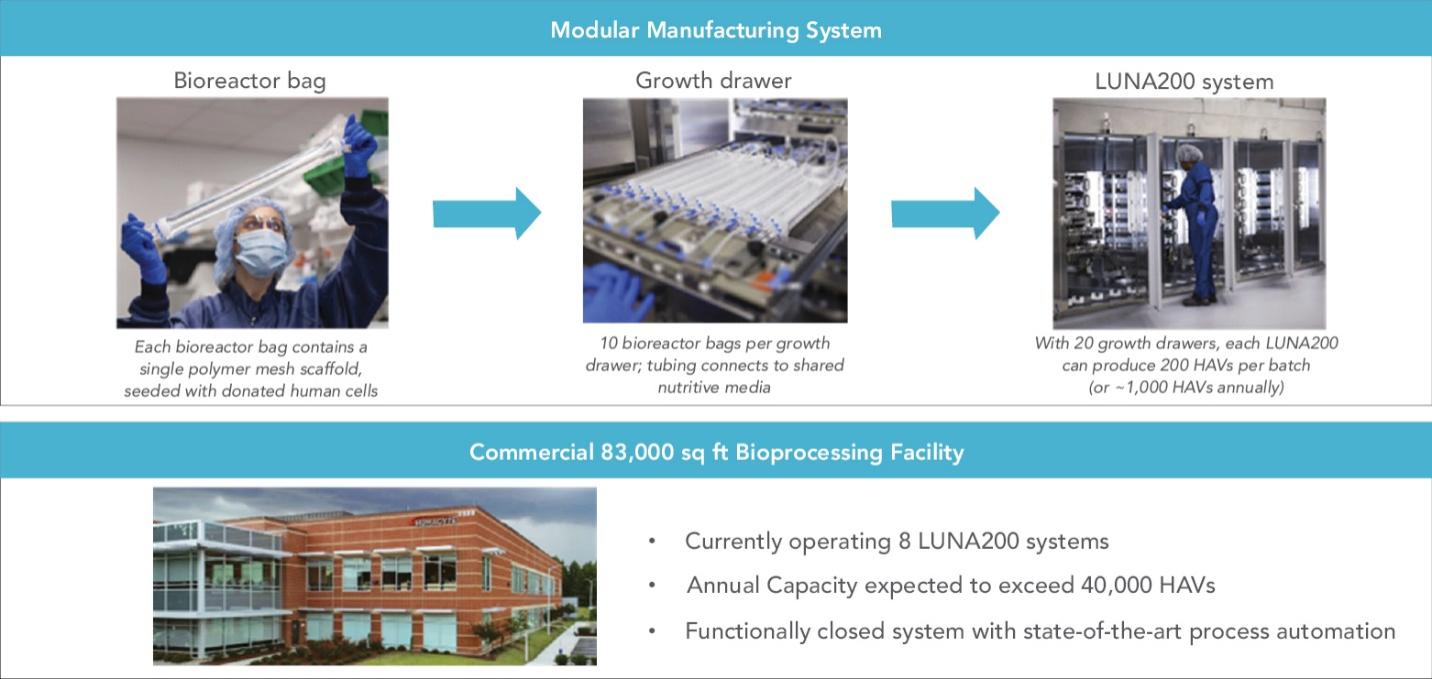
We believe that the LUNA200 can produce HAVs in diameter sizes from 3mm to 10mm and lengths from 10cm to 42cm, making the equipment suitable for the varied array of product candidates in our pipeline. We currently intend to introduce a 13cm-long HAV line extension after commercial launch of the 42cm HAV for surgeries that require shorter segments of HAV in the setting of vascular trauma and repair. Using our existing LUNA200 manufacturing equipment without modification, we believe we have the ability to generate 400 HAVs (13cm in length) or 200 HAVs (42cm in length) per manufactured batch. We have designed our manufacturing system to be functionally closed, to utilize single-use disposable materials with aseptic connections, and to be fully automated, which allows us to control and maximize HAV production.
Based on observations to date, the HAV has withstood maximal pressures that are comparable to those reported for native arteries. For example, the human aorta is reported to have rupture strengths around 1,400 mmHg, while human cerebral arteries rupture around 1,800 mmHg. We have observed HAVs withstanding maximal pressures of approximately 3,200 mmHg before rupturing, making their mechanical properties on par with native human blood vessels.
Our Market Opportunity
We are a biotechnology company with Phase 3 clinical trials in two indications and a strong pipeline for additional products and indications. Additionally, we have had significant interest from surgeons to use our HAV in life and limb saving surgeries as demonstrated by their requests to the FDA to use our HAV in multiple expanded access (compassionate use) cases where no alternative was available.
Our Initial Market Opportunity in Vascular Repair, Reconstruction and Replacement
We believe there is a significant market opportunity for our technology across a number of important clinical areas within vascular reconstruction and replacement including vascular trauma, AV access for hemodialysis, peripheral artery disease, and adult cardiac surgery. To treat these diseases and conditions, patients often require invasive cardiovascular surgery, which involves the use of alternative vascular synthetic materials or autologous vessels harvested from elsewhere in the body. For more information about our evaluation of market opportunity, see “Risk Factors — Risks Related to the Development and Commercialization of Our Product Candidates — The sizes of the market opportunities for our product candidates have not been established with precision and are estimates that management believes to be reasonable. If these market opportunities are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the relevant patient population, our revenue and ability to achieve profitability might be materially and adversely affected.”
9
Vascular Trauma: Arterial injuries resulting from vascular trauma are common in military and civilian populations, frequently resulting in the loss of life or limb. In military populations, as the rate of battlefield fatalities has been declining due to faster evacuations and more robust protection from body armor, the rate of survivable vascular injuries has been increasing. In civilian populations, trauma injuries are primarily caused by motor vehicle, workplace and sporting accidents, gun violence, mass casualty terrorist attacks, stabbings, blunt trauma, and iatrogenic injuries (injuries caused by medical treatment or examination). We estimate that central or peripheral vascular injuries in civilian patients account for approximately 150,000 of all injuries reported in global trauma patients. Furthermore, these injuries account for greater than 20% of all trauma-related deaths.
Civilian patients with central or peripheral vascular injuries are estimated to account for approximately 80,000 of all injuries reported in trauma patients in the United States, inclusive of urgent and iatrogenic vascular trauma injuries. However, these injuries account for greater than 20% of all trauma-related deaths.
We believe our HAVs will be a promising alternative that can address critical gaps in existing treatment options for acute vascular injuries due to trauma. We are developing our HAVs with the goal of providing an effective solution in all time-constrained surgical environments and in resource-limited, infection prone battlefield environments. The ability to provide immediately available, non-immunogenic, universally implantable human vessels that are less susceptible to infection represents a clinically significant advantage over existing treatment options.
Arteriovenous Access for Hemodialysis: An estimated $5 to $6 billion per year is spent on hospital admissions in hemodialysis patients with infection and access complications. In 2021, there were over 550,000 patients receiving hemodialysis in the United States. Annually, at least 160,000 existing or new dialysis patients require a new AV access in the U.S. and an additional 150,000 patients require a new AV access in Europe and Japan.
Hemodialysis patients are a chronically ill population, suffering an average of 1.8 hospital admissions, three visits to the emergency department, and four days hospitalized for infections each year. The two most common causes of hospital admissions in hemodialysis patients are infection and access complications. For hemodialysis patients, an infected access site can lead to sepsis, a life-threatening complication that is the most expensive cause for hospitalization in the United States and carries at least a 10% overall mortality rate.
We believe that our HAVs, when used as AV access for hemodialysis, can decrease infections and dialysis access failures, which would improve patient outcomes and lower the burden of dialysis costs on the healthcare system. We expect to file a BLA with the FDA, seeking approval for the use of HAV in AV access for hemodialysis, and to target our commercialization efforts particularly toward those patients who are at high risk of fistula failure or non-maturation, or for those patients at high risk of vascular access infection.
Peripheral Artery Disease (PAD): PAD is a cardiovascular disease of blood vessels located outside the brain and heart. PAD occurs when plaque builds up in arteries that carry blood to the head, organs, and limbs. PAD usually affects arteries
10
in the legs, but it can also affect arteries that carry blood from the heart to the head, arms, kidneys, and stomach. We believe our HAVs can be used as a bypass conduit in patients with PAD. Peripheral arterial bypass procedures are common with over 230,000 PAD related procedures reported annually in the U.S. Annual peripheral bypass procedures are over 200,000 per year in Europe, and approximately 220,000 per year in Asia.
While endovascular techniques have become more available over the past ten years to treat an array of vascular occlusions, depending on the nature and length of the blockage these types of treatment options have met with both mixed success and durability compared to conventional surgical bypass. Both angioplasty and stenting procedures provide near term success, however long-term durability has remained a question, as highlighted in the results of the recent BEST-CLI clinical trial published in the New England Journal of Medicine demonstrating that patients treated with surgical bypass had fewer major amputations and less need for repeat procedures than those treated with endovascular therapy.
Type I Diabetes: Type 1 diabetes, caused by auto-immune destruction of insulin-producing cells in the islets of the pancreas, is a devastating disease affecting more than 1.7 million people in the United States, and costing at least $10 billion to $14 billion annually. In the EU4 (France, Germany, Italy and Spain) and the UK, the number of patients suffering with Type 1 diabetes is estimated at approximately 1.5 million. Even with the newer insulin delivery technologies, less than one-third of patients achieve consistent target blood sugar levels.
Pancreas transplantation is limited due to the associated morbidity and cost of the whole pancreas organ transplantation procedure. As an alternative to pancreas transplantation, the “Edmonton Protocol” has been developed whereby insulin producing cells are transplanted into the portal vein in the liver. However, the majority of the injected cells are lost to inflammation and clotting, and only 16% of Type 1 diabetes patients who receive the Protocol are cured long term.
We believe our HAVs present a means to deliver a therapeutic number of pancreatic islets to patients with Type 1 diabetes. Pancreatic islets are embedded on the outer surface of our HAV and implanted as an AV graft, analogous to the outpatient procedure done for hemodialysis access. After implantation, the islets have the potential to sense blood glucose and then respond by secreting appropriate levels of insulin to maintain glucose levels in the blood. We have termed this new paradigm for pancreatic islet cell delivery the “Biovascular Pancreas (BVP).” Proof-of-concept studies in rodents and pigs have shown promise that the BVP can reduce glucose levels. Studies in non-human primates are planned to commence in 2023.
We believe that a reliable, low-risk, and easily implantable islet cell delivery method that could ensure the survival and functionality of a therapeutic number of islet cells in a human adult would be transformational for the treatment of Type 1 diabetes.
Coronary Artery Bypass Graft (“CABG”): CABG is a surgery used to treat a blockage or narrowing of one or more of the coronary arteries to restore the blood supply to the heart muscle. We believe our HAVs can replace existing vascular substitutes and improve patient outcomes, particularly in obese patients or those suffering from diabetes, in whom the risks of saphenous vein harvesting are more substantial. CABG procedures are common, with more than 200,000 CABG procedures reported annually in the U.S. and over 765,000 annual CABG procedures globally.
Typically, a CABG operation involves the use of both the patient’s own artery and vein. In patients who are obese, have diabetes, or who are very elderly, there are higher risks for vein harvest complications, including failure to heal the vein harvest incision, infection, and prolonged swelling of the operative leg. Furthermore, complications from the vein harvest incision site are more common than complications from the chest incision in CABG patients. It is estimated that approximately 20% of patients requiring bypass surgery have no suitable grafts available, with sources reporting as high as 45% of CABG patients are without suitable autologous vein.
Pediatric Heart Surgery: We are developing a smaller diameter HAV product for use in pediatric heart surgery as a Blalock Taussig (“BT”) shunt. The BT shunt is a surgical procedure that is used to increase pulmonary blood flow for the treatment of babies born with a complex congenital heart defect called Tetralogy of Fallot, a common type of “blue baby syndrome”. In 2021, there were 3.7 million babies born in the United States and approximately 1,500 to 2,000 of these babies were born with Tetralogy of Fallot. The BT shunt is a life-saving procedure for these babies, and we plan to submit an orphan drug application for use of our HAV as a BT shunt for infants born with cyanotic congenital heart defects. Although 3 – 4mm inner diameter expanded polytetrafluoroethylene (“ePTFE”) grafts are currently used as the most common BT shunt, they suffer from limitations that impact morbidity and mortality in these infants.
11
Our Clinical and Pre-Clinical Stage Product Pipeline
The following table highlights key information about our current product pipeline:
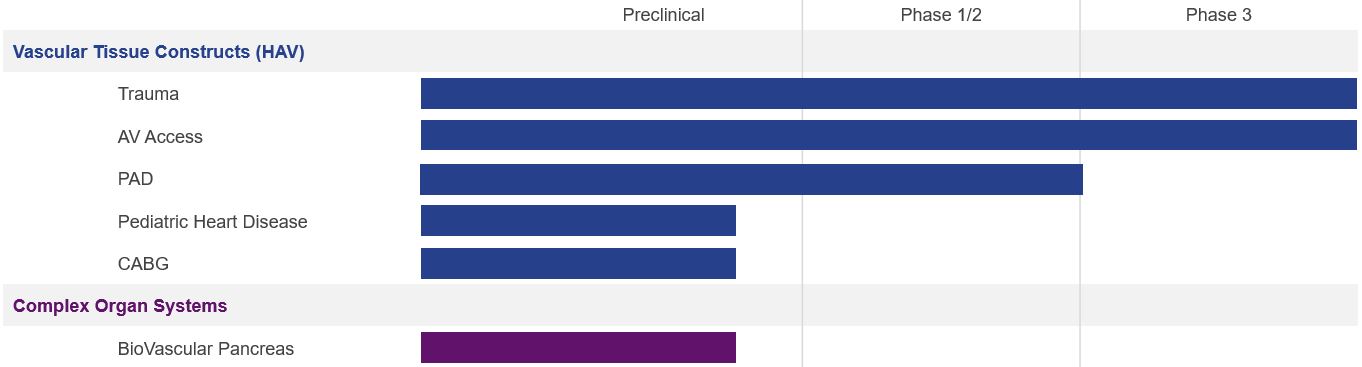
We began clinical evaluations of our HAVs in December 2012, with the enrollment of the first Phase 2 patient in our V001 hemodialysis access trial in Europe. Since then, we have completed one Phase 2 trial in the United States, and currently have seven trials either actively enrolling or in long-term follow-up. HAVs have been implanted in approximately 85 clinical centers in seven countries around the world, and by more than 100 practicing surgeons.
12
Overview of Clinical Trials Assessing the Safety and Efficacy of the HAV in Multiple Indications
| Clinical Trial Number | Indication | Begin Enrollment | Design/Phase | Number of Subjects | Status | Outcomes** | ||||||||||||||||||||||||||||||||
| Vascular Trauma | ||||||||||||||||||||||||||||||||||||||
| V005 | Vascular Trauma | 2018 | Phase 2/3 Single-arm Historical Comparator Unblinded | A total of 60 patients enrolled as of December 31, 2022. Primary analysis will be based on a total of 50 patients with injuries of extremities, 42 of which have been enrolled as of December 31, 2022 | Enrolling | Trial is currently enrolling | ||||||||||||||||||||||||||||||||
| Dialysis Access | ||||||||||||||||||||||||||||||||||||||
| V001 | Dialysis Access | 2012 | Phase 2 Single-arm | 40 | 10-year follow-up ongoing | 30-day PP: 95% 6-month SP: 100% 12-month SP: 97% 60-month SP: 58% Infection Rate/yr: 0% Number of Rejections: 0 | ||||||||||||||||||||||||||||||||
| V003 | Dialysis Access | 2013 | Phase 2 Single-arm | 20 | Complete 2-year follow-up | 30-day PP: 95% 6-month SP: 89% 12-month SP: 81% Infection Rate/yr: 4% (1 event) Number of Rejections: 0 | ||||||||||||||||||||||||||||||||
| V006 | Dialysis Access | 2016 | Phase 3 Prospective Randomized Blinded | 355 total; 177 received HAV 178 received ePTFE | 5-year follow-up ongoing | 30-day PP HAV: 93% 12-month SP HAV: 82% 24-month SP HAV: 67% 12-month SP ePTFE: 80% 24-month SP ePTFE: 74% Infection Rate HAV/yr: 0.93% Infection Rate ePTFE/yr: 4.5% Number of HAV Rejections: 0 | ||||||||||||||||||||||||||||||||
| V007 | Dialysis Access | 2017 | Phase 3 Prospective Randomized Blinded | Target 240 total; 230 enrolled (as of December 31, 2022) | Enrolling | Trial is currently enrolling | ||||||||||||||||||||||||||||||||
| V011 | Dialysis Access | 2019 | Phase 2 (LUNA200 manufacturing system) | 30 | 3-year follow-up ongoing | 30-day PP: 97% 30-day SP: 100% 12-month SP: 83% Infection Rate HAV/yr: 0% Number of HAV Rejections: 0 | ||||||||||||||||||||||||||||||||
| Peripheral Artery Disease | ||||||||||||||||||||||||||||||||||||||
| V002 | Peripheral Artery Disease | 2013 | Phase 2 Single-arm | 20 | 10-year follow-up ongoing | 30-day PP: 100% 6-month SP: 84% 12-month SP: 84% 72-month SP: 60% Infection Rate/yr: 0% Number of Rejections: 0 | ||||||||||||||||||||||||||||||||
| V004 | Peripheral Artery Disease | 2016 | Phase 2 Single-arm | 15 | 5-year follow-up ongoing | 30-day PP: 100% 6-month SP: 86% 12-month SP: 64% Infection Rate/yr: 0% Number of Rejections: 0 Number of Amputations: 0 | ||||||||||||||||||||||||||||||||
___________________________
** PP: Primary Patency, which is the interval of time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency, i.e. patent without interventions.
SP: Secondary Patency, which is the interval from the time of access placement until abandonment, i.e. patent with or without interventions.
13
As of December 31, 2022, approximately 533 patients worldwide have received our HAVs for the treatment of trauma, AV access for hemodialysis, PAD, and in expanded access cases resulting in approximately 1,080 subject-years of exposure to the HAV. Our cumulative HAV exposure is approximately 880 subject-years in the hemodialysis access population, 130 subject-years in the PAD population, and 70 subject-years in the arterial trauma population. The longest our HAV has been in a patient and used for dialysis is more than nine years and there have been more than 106,000 estimated dialysis sessions using our HAVs. Additionally, a total of 26 expanded access/compassionate use cases have been granted by the FDA, and another 18 patients with severe PAD have been treated with the HAV under an investigator IND at the Mayo Clinic. Lastly, ten patients suffering vascular injuries during the conflict in Ukraine have been treated with the HAV under a humanitarian program. Throughout all of these trials and other programs, we have observed that our HAVs functioned as intended and provided functional blood flow to affected limbs. We have also observed consistent durability with a strong tolerability profile. Furthermore, we have observed no evidence of clinically relevant immunologic reactions to our HAVs, supporting the potential use of our HAVs as off-the-shelf, universally implantable, bioengineered human tissues.
Overall, the HAV has functioned well and as intended, across eight different clinical trials in three clinical indications. The HAV has been implanted in approximately 533 patients, across approximately 85 clinical sites in seven countries, over more than nine years (as of December 31, 2022). Rates of primary and secondary patency were similar across trial designs and disease states, with 30-day primary patency ranging from 95% – 100%. Six-month secondary patency ranges from 84% – 100%, and 12-month secondary patency ranges from 81% – 97%, across multiple clinical trials, disease states, and patient age ranges and demographics.
We have observed zero instances of clinical rejection of the HAV in any clinical trial over the past nine years, suggesting that the HAV was not immunologically rejected after implantation.
Based on clinical trial results to date, we have observed that the HAVs were highly resistant to infection, with an infection rate averaging approximately 1.0% per patient-year in our AV access trials, and low infection rates currently in our trauma and PAD trials. Vascular graft infections are a potentially serious complication and can result in adverse outcomes such as sepsis, hospitalization, long-term antibiotic use, repeat procedures and even death.
HAVs Remodel with Host Cells After Implantation
Additionally, based on clinical samples obtained during our Phase 2 AV access trials and published in three peer reviewed journals, The Lancet in 2016, Science Translational Medicine in 2019, and in the Journal of Vascular Surgery in 2020, we observed that the HAV became populated with healthy, vascular cells from the patient. As described in these publications, over time the patient’s cells have been observed to transform the HAV into a multi-layered living tissue similar to native blood vessels. In these trials we have also observed ongoing cellular repair of HAV tissues that had been previously injured during cannulation with dialysis needles, which suggests that the recellularized HAV may be capable of self-healing. The image below shows an HAV that had been implanted in a hemodialysis patient for 44 weeks, that had developed alpha-actin positive vascular smooth muscle cells throughout the wall (red staining in the left-hand panel), and had developed a layer of CD31+ endothelial cells on the inner luminal surface of the HAV (line of red endothelial cells indicated in the right-hand panel).
Histological Images of HAV Repopulated with the Patient’s Own Vascular Cells
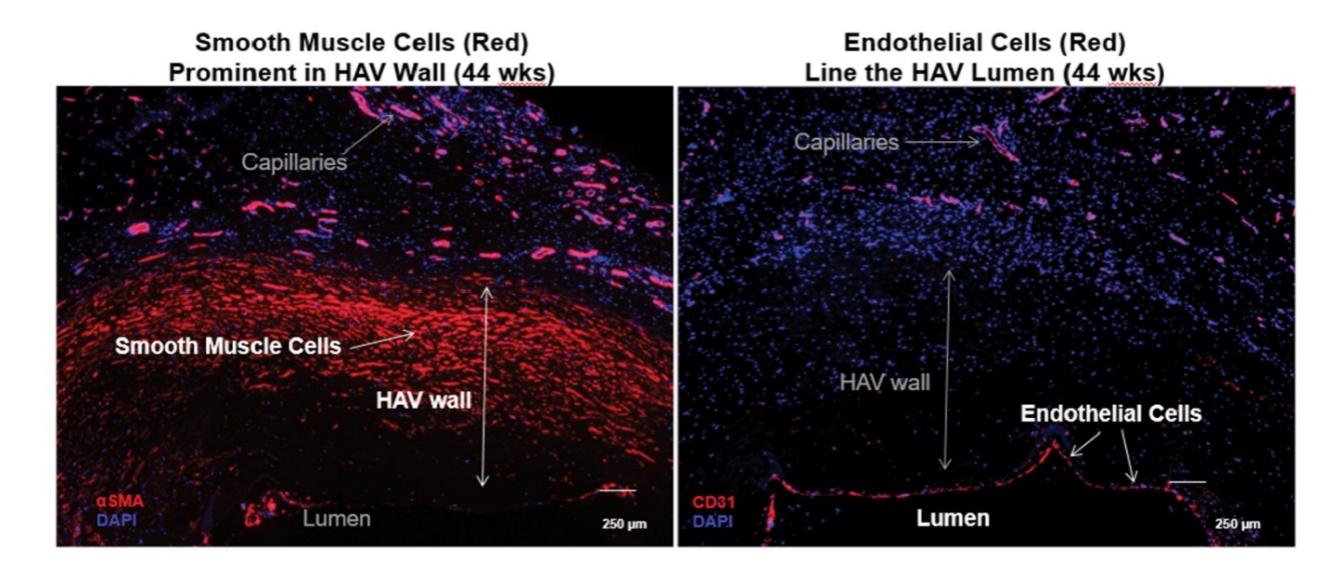
14
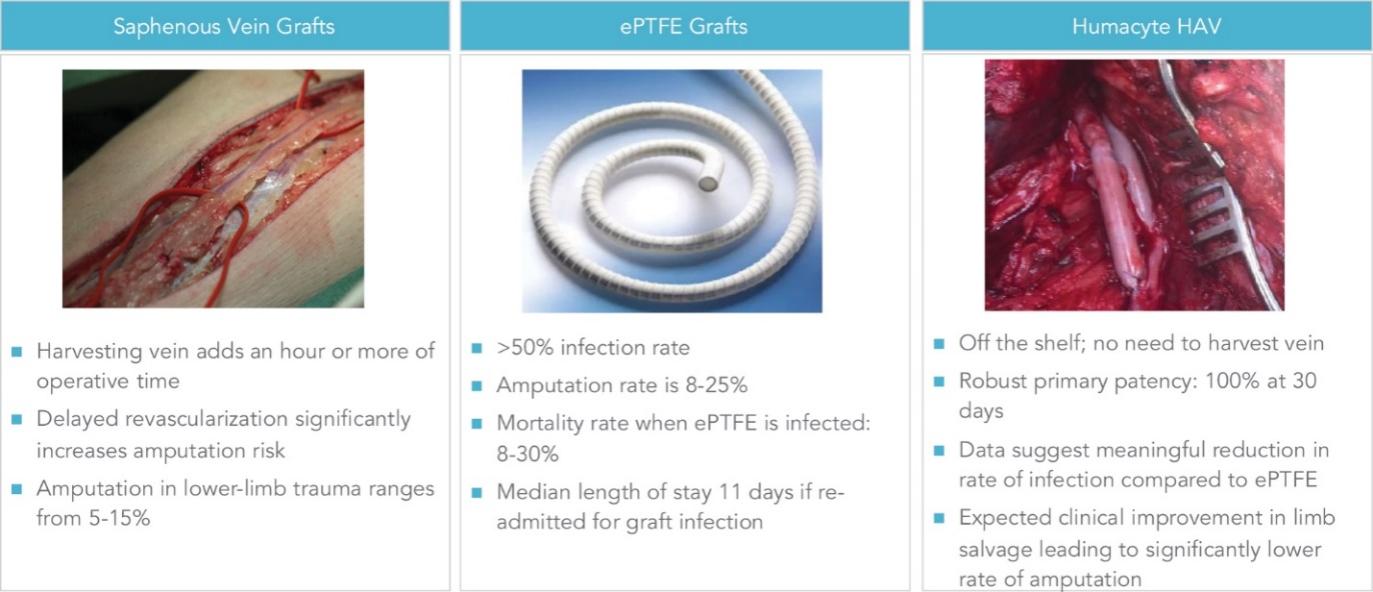
Existing Options for Surgical Treatment of Vascular Disease Are Not Sufficient
The table below contains a summary of the efficacy of autologous veins, ePTFE grafts, cryopreserved human cadaveric veins, and preserved bovine veins. For the treatment of vascular trauma, saphenous vein presents challenges in terms of time to procure the vein, and ePTFE grafts carry extremely high infection rates: 24% – 29% per patient year in the studies below. Similarly, autogenous fistulas and ePTFE grafts for dialysis access have low functional patencies at six and 12 months, and ePTFE is burdened with high rates of annual infection: 3% – 17% in the studies below. Both cryopreserved human cadaver vein, and preserved bovine veins, have low patency at 12 months, and also suffer from high rates of aneurysm formation. Lastly, for bypass of PAD, vein has acceptable patency but is not available for all subjects, while ePTFE carries lower patencies and higher infection risk, and bovine vein becomes aneurysmal at unacceptably high rates.
Published Studies in Vascular Surgery
We derived the data in the table below from data contained in certain published papers on vascular trauma, hemodialysis and PAD between 2002 and the present. These data are from different studies and thus are not directly comparable. In addition, many of these papers reported on additional endpoints that are not included in the table below.
| Clinical Indication | Type of Conduit | Year | Number of Patients | Published Secondary Patency Outcome | Infection (per patient-year) | Rejection Outcome | ||||||||||||||||||||||||||||||||
| Vascular Trauma | Saphenous Vein (autologous) | 2002 – 2012 | 24 | 12 months: ~78% function | 4% | N/A | ||||||||||||||||||||||||||||||||
| 2014 | 152 | 30 days: ~90% function | N/A | |||||||||||||||||||||||||||||||||||
| ePTFE (synthetic graft) | 2002 – 2012 | 25 | 12 months: ~50% function | 24% | N/A | |||||||||||||||||||||||||||||||||
| 2005 | 14 of 95 | 30 days: ~79% function | 29% | |||||||||||||||||||||||||||||||||||
| Hemodialysis Access | Fistula (autogenous) | Post-2005 | 2,800 | 12 months: 75% | 2% | N/A | ||||||||||||||||||||||||||||||||
| 2019 | 14892 | 6 months: 51% | N/A | |||||||||||||||||||||||||||||||||||
| 2017 | 6,439 | N/A | 4% | |||||||||||||||||||||||||||||||||||
| 2018 | 602 | 6 months: 61% | N/A | |||||||||||||||||||||||||||||||||||
| ePTFE (synthetic graft) | 2013 | 128 | 6 months: ~60% | N/A | N/A | |||||||||||||||||||||||||||||||||
| 2019 | > 400 | N/A | 3% – 17% | |||||||||||||||||||||||||||||||||||
| 2020 | > 3,000 | 12 months: 70% | 9% | |||||||||||||||||||||||||||||||||||
| Cryovein (cryopreserved human cadaver vein) | 2002 | 45 | 12 months: 80% | N/A | 100% | |||||||||||||||||||||||||||||||||
| 2004 | 49 | 12 months: ~65% Aneurysm rate: 18% | 0% | ~100% | ||||||||||||||||||||||||||||||||||
| Procol (bovine vein) | 2005 | 186 | 12 months: 66% Aneurysm rate: 3.2% | 5.3% | N/A | |||||||||||||||||||||||||||||||||
| Peripheral Artery Disease | Saphenous Vein (autologous) | 2008 | 60 | 12 months: ~86% | N/A | N/A | ||||||||||||||||||||||||||||||||
| ePTFE (synthetic graft) | 2008 | 61 | 12 months: ~80% | N/A | N/A | |||||||||||||||||||||||||||||||||
| 2013 | 101 | 12 months: 76% – 89% | ||||||||||||||||||||||||||||||||||||
| 2011 | 273 | 12 months: 81% | ||||||||||||||||||||||||||||||||||||
| 2013 | 496 | N/A | 3.8% | |||||||||||||||||||||||||||||||||||
| Procol (bovine vein) | 2008 | 7 | 6 months: 50% 12 months: 50% Aneurysm Rate: 29% | N/A | N/A | |||||||||||||||||||||||||||||||||
15
Proposed Indication #1: Use of HAV to Repair Vascular Trauma
Overview of Vascular Trauma
Arterial injuries resulting from vascular trauma are common in military and civilian populations, frequently resulting in the loss of life or limb. In military populations, as the rate of battlefield fatalities has been declining due to faster evacuations and more robust protection from body armor, the rate of survivable vascular injuries has been increasing. In civilian populations, trauma injuries are primarily caused by motor vehicle, workplace and sporting accidents, gun violence, mass casualty terrorist attacks, stabbings, blunt trauma and iatrogenic injuries (injuries caused by medical treatment or examination). Consequently, we believe there is an increasingly urgent unmet need for novel materials that are immediately available for permanent vascular repair for both civilian and military vascular trauma.
Options in Surgical Treatment of Vascular Trauma
Autologous vein is the preferred conduit for vascular repair. However, harvesting of autologous vein is not always feasible, due to damage to vein or lower limb, prior vein harvest, inadequate size of the vein or venous disease. Harvesting autologous vein is a serious operation that requires additional time and resources. Delaying the time from injury to operative intervention from less than one hour, to three hours or greater, more than doubles the risk of limb amputation. Limb amputation, in turn, almost triples the length of intensive care unit stay, nearly doubles the length of hospital stay, and is devastating to patient quality of life. Additionally, the morbidity associated with saphenous vein harvest includes surgical site infections, chronic pain, and limb swelling. Synthetic materials have been shown to be inferior to autologous vein in resistance to infection and durability and, therefore, are generally only used for vascular repair when autologous vein is not an option.
Our Solution for Vascular Trauma
We believe our HAVs will be a promising alternative that can address critical gaps in existing treatment options for acute vascular injuries due to trauma. We are developing our HAVs with the goal of providing an effective solution in all time-constrained surgical environments and in resource-limited, infection prone battlefield environments. The ability to create immediately available, non-immunogenic, universally implantable material that is less susceptible to infection represents a clinically significant advantage over existing options.
Humacyte has a strong working relationship with the Department of Defense (“DoD”) that has led to a partnership over the last decade to support their unmet need to reconstruct and repair vascular injuries through the development of our HAVs. As a result of this collaboration and partnership with the DoD, we anticipate Humacyte would supply HAVs for use in military hospitals to treat injured soldiers and veterans. The DoD assigned a priority designation to the HAV technology under Public Law 115-92. Under this law, FDA and DoD work together to expedite the development and review of critical technologies and therapies requested by DoD. Additionally, we have received an approximately $6.8 million grant from the DoD for the continued development of our HAVs for vascular reconstruction and repair.
16
Our Current Phase 2/3 Trial for Vascular Trauma
Trial Design: Our ongoing V005 trial is a single-arm, multi-center, non-randomized clinical trial to evaluate the efficacy, safety and tolerability of our 6 millimeter HAV in replacement or reconstruction of vascular tissues in patients with life or limb-threatening vascular trauma. Since the V005 trial is a single-arm, non-randomized, open label study, we have the ability to track ongoing efficacy and safety. The primary efficacy endpoint will evaluate patency of the HAV at 30 days based on an assessment of 50 patients from the V005 trial who have vascular trauma of the extremity, excluding torso injuries and iatrogenic trauma patients. The Company plans to file a BLA with the FDA for an indication in vascular trauma approximately four months after completion of the V005 trial. The Company plans to seek accelerated approval of the HAV for urgent arterial repair following extremity vascular trauma when synthetic graft is contraindicated and when autologous vein is not feasible. Results from patients from V005 outside of the primary endpoint population, as well as results from patients treated in the Ukraine humanitarian program, will be included in the BLA as supportive data.
Status of Phase 3 Trial of HAV in Vascular Trauma (as of December 31, 2022)
| Clinical Trial Number | Indication | Begin Enrollment | Design/Phase | Number of Subjects | Status | Outcomes** | ||||||||||||||||||||||||||||||||
| V005 | Vascular Trauma | 2018 | Phase 2/3 Single-arm Historical Comparator Unblinded | A total of 60 patients enrolled as of December 31, 2022. Primary analysis will be based on a total of 50 patients with injuries of extremities, 42 of which have been enrolled as of December 31, 2022 | Enrolling | Trial is currently enrolling | ||||||||||||||||||||||||||||||||
___________________________
** PP: Primary Patency, which is the interval of time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency, i.e. patent without interventions.
SP: Secondary Patency, which is the interval from the time of access placement until abandonment, i.e. patent with or without interventions.
Current Trial Status: As of December 31, 2022, we had 17 clinical trial sites for the V005 trial in the United States and four in Israel. We are in the process of adding to the trial sites in Ukraine which are currently participating in the ongoing humanitarian program.
The range of trauma injuries in V005 has been broad, including penetrating trauma cases, blunt injury cases, and iatrogenic injuries. Mechanisms of injury have included motor vehicle accidents, gunshot wounds, industrial accidents, falls, and iatrogenic injuries from other interventional or surgical procedures. The HAVs have been placed throughout the body, including in the lower limbs, upper limb, and torso. The HAV has been used to repair the axillary artery, femoral artery, popliteal artery and vein, and the brachial artery in the V005 trial. Many of the injuries treated in the V005 trial, including industrial accidents, motor vehicle accidents, and some gunshot wounds, are contaminated injuries that are at elevated risk of graft infection. Nonetheless, as of December 31, 2022, there has been only one report of HAV infection in any V005 subject, despite a number of instances where the HAV has been implanted into contaminated surgical fields of acute vascular wounds. There have been several instances of local wound infections, but importantly only one of these instances has led to infection of the HAV material itself, which we believe represents a significant advantage of the HAV over ePTFE grafts, which have a higher propensity to become infected in contaminated or infected wound beds. There have been no reports of limb amputation that occurred as a result of HAV malfunction or loss of patency. The resistance to
17
infection we have observed in trials to date is particularly important in traumatic injuries which are often caused by infected material (i.e., knife, car accident, blast injury).
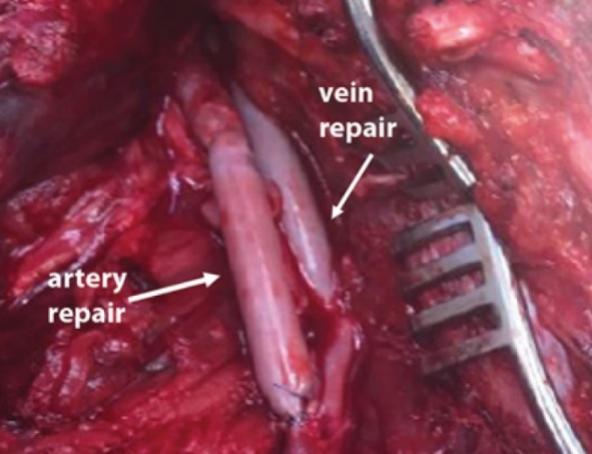
In the figure below, a photograph is shown of an HAV that was used to repair both an artery and a vein in the knee of a patient who suffered a gunshot wound. This patient was doing well at the 30-day follow-up visit with both repairs remaining patent and functional.
Intra-operative photograph of HAV repair of popliteal artery (left) and vein (right) in V005 subject.
In the second quarter of 2022, Humacyte launched a humanitarian initiative to provide its HAVs to hospitals in Ukraine for the treatment of wounded civilians and soldiers with vascular trauma injuries. Ukrainian surgeons presented patient outcomes from the use of the HAV to treat wartime vascular trauma at two vascular conferences in December 2022, the VI Congress of Vascular Surgeons, Phlebologists, and Angiologists of Ukraine in Kyiv, Ukraine, and the 11th Munich Vascular Conference (MAC) 2022. The surgeons described long-standing limitations in vascular tissue repair and replacement as well as the injuries that they have observed during the Russian-Ukrainian conflict. Surgeons have utilized the HAV to treat patients with wartime injuries including blast trauma, shrapnel injuries, and gunshot wounds. The surgeons observed that access to the HAV, a biologic conduit, has improved their ability to perform vascular reconstructions by eliminating the need to harvest a venous conduit. As of December 31, 2022, a total of ten vascular patients have been treated under this humanitarian program, and all patients are reported to have primary patency at 30 days and zero cases of infection despite the presence of contaminated wound beds.
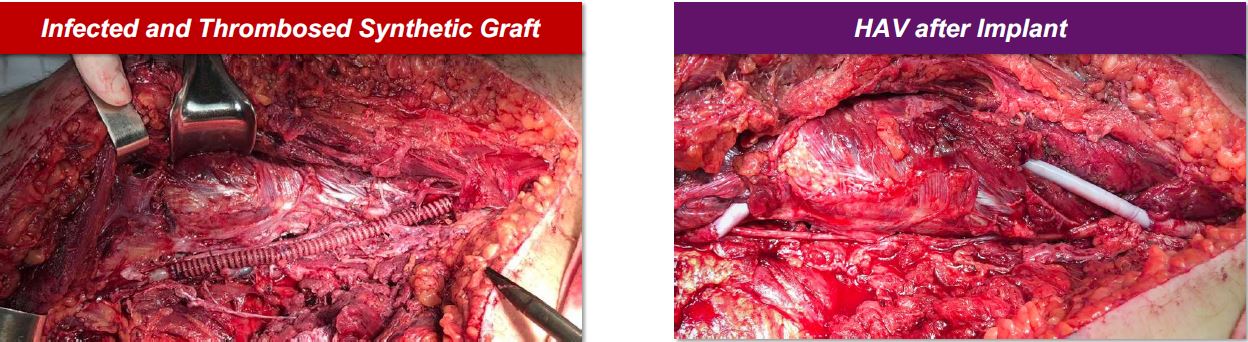
In the figure below, photographs are shown of the first patient treated under the humanitarian program in Ukraine. The patient was a 42-year-old male who suffered a gunshot wound in the leg which damaged his femoral artery. The patient was initially treated using synthetic graft which became infected, and the patient experienced critical right lower extremity ischemia. The HAV was implanted as a right superficial femoral artery reconstruction to achieve wound healing and limb salvage. After three months, the HAV was reported to have retained primary patency with no evidence of HAV infection.
Intra-operative photographs of attempted synthetic graft repair of femoral artery (left) and subsequent repair with HAV (right) in patient from Ukraine humanitarian program.
18
Proposed Indication #2: Use of the HAV for Arteriovenous Access for Hemodialysis
Overview of Hemodialysis and Existing Methods of Arteriovenous Access for Hemodialysis
End-stage renal disease (“ESRD”) develops when chronic kidney disease progresses to a point where either dialysis or a kidney transplant is required for the patient to survive. For hemodialysis to be conducted, a point of vascular access to the patient’s circulatory system must be created, termed vascular access, so that blood can be transported from the body to the dialyzer and then back to the body. The demand for vascular access conduits includes the need for both new hemodialysis patients who have progressed to ESRD requiring an initial access, and existing patients that require the replacement of their existing access. There are currently three traditional methods for obtaining vascular access for hemodialysis: an AV fistula, a synthetic graft, and a catheter. Each of these vascular access methods has substantial limitations, as outlined below:
Three Traditional Methods for Obtaining Vascular Access for Hemodialysis
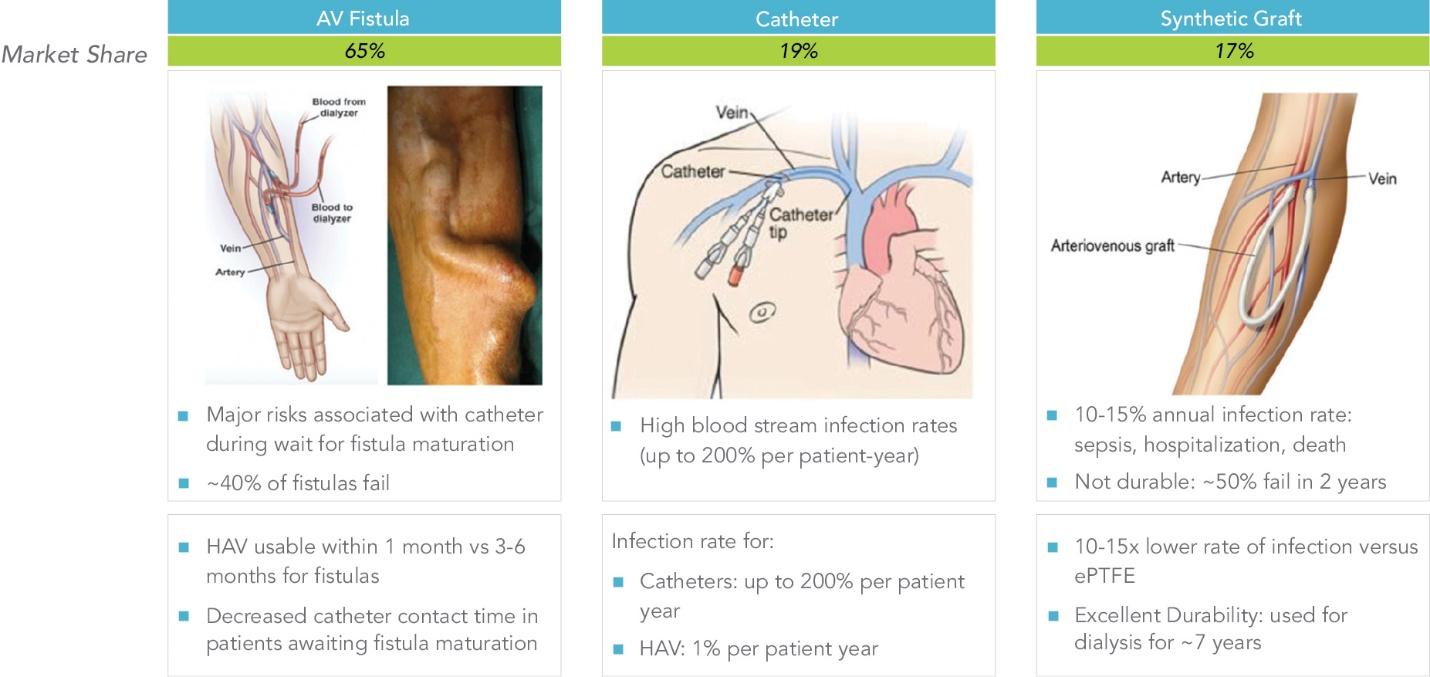
Fistula. An AV fistula is created by surgically connecting a vein to an artery, typically in the patient’s arm. Fistulae are often considered the preferred means of access for hemodialysis due to lower infection rates of approximately 0.5% – 1.5% per patient-year as well as long-term durability. However, many patients are not suitable candidates for fistula placement, due to small vessel anatomy, advanced age, obesity or other comorbidities. Approximately 40% of patients who undergo surgery for fistula creation will not gain any benefit from the surgery because the fistula lacks sufficient vein enlargement and increased blood flow, a process called fistula maturation, that is necessary for hemodialysis. Additionally, during the period in which the fistula is maturing, catheters are generally used to provide the patient access for dialysis. There is a high risk of infection and morbidity, and health care cost, associated with prolonged catheter dependence while waiting for the fistula to mature.
Catheters. A catheter, which is tunneled underneath the skin and placed directly into a large vein in the patient, is generally the least desirable access solution. Given the time necessary for fistulae to mature, the vast majority of patients in the United States begin hemodialysis using a catheter while awaiting fistula maturation. Catheters have rates of blood stream infections as high as 200% per patient-year, with high associated morbidity and health care costs.
Synthetic graft. A synthetic graft, typically made from ePTFE and sewn between an artery and vein in the patient’s arm, is generally used in patients who are not candidates for fistulae. The drawbacks of synthetic grafts include higher infection rates, which can be as high as 10% – 15% per patient-year, and gradual degradation of the non-healing ePTFE graft material caused by persistent needle punctures. A recent systematic meta-analysis measuring the functional patency of ePTFE grafts shows that, on average, only 70% of ePTFE dialysis access grafts remain functional one year after implantation.
Distribution of Hemodialysis Access Modes in Use in the United States
| Access Type | Fistulae | Catheters | Synthetic Grafts | |||||||||||||||||
Incident Patients: At Initiation of Hemodialysis | 16.7 | % | 80.3 | % | 3.0 | % | ||||||||||||||
Prevalent Patients: For Ongoing Hemodialysis | 64.5 | % | 18.9 | % | 16.6 | % | ||||||||||||||
19
Published Data in Hemodialysis Access
We derived the data in the table below from data contained in certain published papers on hemodialysis between 2002 and the present. These data are from different studies and thus are not directly comparable. In addition, many of these papers reported on additional endpoints that are not included in the table below.
| Clinical Indication | Type of Conduit | Year | Number of Patients | Published Secondary Patency Outcome | Infection (per patient-year) | Rejection Outcome | ||||||||||||||||||||||||||||||||
| Fistula (autogenous) | Post‑2005 | 2,800 | 12 months: 75% | 2% | N/A | |||||||||||||||||||||||||||||||||
| 2019 | 14,892 | 6 months: 51% | N/A | |||||||||||||||||||||||||||||||||||
| Hemodialysis Access | 2017 | 6,439 | N/A | 4% | ||||||||||||||||||||||||||||||||||
| 2018 | 602 | 6 months: 61% | N/A | |||||||||||||||||||||||||||||||||||
| ePTFE (synthetic graft) | 2013 | 128 | 6 months: ~60% | N/A | N/A | |||||||||||||||||||||||||||||||||
| 2019 | > 400 | N/A | 3% – 17% | |||||||||||||||||||||||||||||||||||
| 2020 | > 3,000 | 12 months: 70% | 9% | |||||||||||||||||||||||||||||||||||
| Cryovein (cryopreserved cadaver vein) | 2002 | 45 | 12 months: 80% | N/A | 100% | |||||||||||||||||||||||||||||||||
| 2004 | 49 | 12 months: ~65% Aneurysm rate: 18% | 0% | ~100% | ||||||||||||||||||||||||||||||||||
| Procol (bovine vein) | 2005 | 186 | 12 months: 66% Aneurysm rate: 3.2% | 5.3% | N/A | |||||||||||||||||||||||||||||||||
Overview of HAV Experience in Hemodialysis Access: A table listing our clinical trials of the HAV in hemodialysis access is included below. We have implanted the HAV into approximately 384 total patients for hemodialysis access, for a total of more than 880 patient-years of exposure, as of December 31, 2022. Throughout these trials, we have observed consistent and sustained high primary patency rates, ranging from 95% – 100% at 30 days. Secondary patency of the HAV at 6 months ranges from 84% – 100%. Consistently, we have observed zero instances of clinical rejection of any HAV in any hemodialysis access trial.
Implantation of HAV for Hemodialysis

We have also observed in multiple clinical trials that our HAVs had a low infection susceptibility during use for hemodialysis, with a rate lower than 1% per patient-year across all studies. The low infection susceptibility we observed in our trials of our HAVs may be a result of the HAV’s potential to become a living tissue as it becomes populated by cells from the patient’s body. Since living tissues are known to have resisted infection due to interactions with host white blood cells and immunological defense systems, it is possible that the repopulated HAV resists infection for the same reasons that native arteries and veins resist infections, as is observed with autogenous fistulas.
We have also observed early evidence of potential healing from the cells that repopulate the HAV after needle puncture for hemodialysis. In examining HAV explanted segments we have observed healed needle cannulation tracts with cells expressing smooth muscle markers. This self-healing indicates that the HAV may have repaired itself while being used as a hemodialysis access, which we believe is a distinct feature not present in synthetic materials, and, to our knowledge, has not been observed before for any other regenerative medicine product.
20
Our Current Phase 2 and Phase 3 Trials of the HAV in Hemodialysis Access
| Clinical Trial Number | Indication | Begin Enrollment | Design/Phase | Number of Subjects | Status | Outcomes** | ||||||||||||||||||||||||||||||||
| V001 | Dialysis Access | 2012 | Phase 2 Single-arm | 40 | 10‑year follow-up ongoing | 30‑day PP: 95% 6‑month SP: 100% 12‑month SP: 97% 60-month SP: 58% Infection Rate/yr: 0% Number of Rejections: 0 | ||||||||||||||||||||||||||||||||
| V003 | Dialysis Access | 2013 | Phase 2 Single-arm | 20 | Complete 2‑year follow-up | 30‑day PP: 95% 6‑month SP: 89% 12‑month SP: 81% Infection Rate/yr: 4% (1 event) Number of Rejections: 0 | ||||||||||||||||||||||||||||||||
| V006 | Dialysis Access | 2016 | Phase 3 Prospective Randomized Blinded | 355 total; 177 received HAV 178 received ePTFE | 5‑year follow-up ongoing | 30‑day PP HAV: 93% 12‑month SP HAV: 82% 24‑month SP HAV: 67% 12‑month SP ePTFE: 80% 24‑month SP ePTFE: 74% Infection Rate HAV/yr: 0.93% Infection Rate ePTFE/yr: 4.5% Number of HAV Rejections: 0 | ||||||||||||||||||||||||||||||||
| V007 | Dialysis Access | 2017 | Phase 3 Prospective Randomized Blinded | Target 240 total; 230 enrolled (as of December 31, 2022) | Enrolling | Trial is currently enrolling | ||||||||||||||||||||||||||||||||
| V011 | Dialysis Access | 2019 | Phase 2 (LUNA200 manufacturing system) | 30 | 3‑year follow-up ongoing | 30‑day PP: 97% 30‑day SP: 100% 12-month SP: 83% Infection Rate HAV/yr: 0% Number of HAV Rejections: 0 | ||||||||||||||||||||||||||||||||
___________________________
** PP: Primary Patency, which is the interval of time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency, i.e. patent without interventions.
SP: Secondary Patency, which is the interval from the time of access placement until abandonment, i.e. patent with or without interventions.
Long-Term Data from Early Phase 2 Trials in Hemodialysis: V001 and V003
Phase 2 Trial Design and Current Outcomes: We have completed or are in long-term follow-up on two open-label Phase 2 trials in 60 hemodialysis patients in the United States and Poland from December 2012 through May 2014, which we refer to as our V003 trial and V001 trial, respectively. Both the V001 and V003 studies were designed as single-arm trials to assess the safety and efficacy of the HAV for hemodialysis access, with assessments of patency at 6, 12, 18 and 24 months. In the 60 patients enrolled in these two studies, blood flow through all HAVs was appropriate for hemodialysis, averaging over 1,200 mL/minute. Secondary patency for the two combined trials was 97% at six months, 89% at 12-months, and 81% at 18-months. These results compare favorably to published reports of secondary patency for fistula of 51% – 61% at six months and 75% at 12 months. Long-term results from the V001 trial showing five-year secondary patency of 58% were published in the European Journal of Vascular and Endovascular Surgery companion journal EJVES Vascular Forum in February 2022, and patients from the V001 trial are currently in a 10-year follow-up period.
21
Images and long-term results from Phase 2 V001 trial of HAV in AV Access


Phase 3 V006 AV Access Study
Trial Design: Our V006 HUMANITY study is a prospective, multi-center, multinational, open-label, randomized, two-arm, comparative study. Eligible study subjects were randomized to receive either a HAV or a commercially available ePTFE graft and followed to 24 months post-implantation by routine study visits. After 24 months, subjects with a patent conduit are followed to five years post-implantation using a questionnaire at six-month intervals to ascertain patient and conduit status. The primary endpoint for the V006 HUMANITY trial was a non-inferiority analysis of secondary patency compared to ePTFE, to be assessed when all subjects are at least 18 months post-implantation. There were a total of 37 sites that participated in the study, enrolling a combined total of 355 subjects.
24-Month Results: The V006 study enrolled 355 subjects who were roughly equally matched in terms of demographics and co-morbidities. HAV subjects trended older (p=0.06) and had more prior strokes (p=0.02) than did ePTFE subjects.
22
Phase 3 V006 HUMANITY trial subject demographics
V006 Demographics (N=355) | ePTFE (n=178) | HAV (n=177) | p-value | |||||||||||||||||
| Age(years) | 59.9 | 62.6 | 0.06 | |||||||||||||||||
| Male (%) | 49.4% | 49.7% | NS | |||||||||||||||||
| Caucasian (%) | 65.2% | 69.5% | NS | |||||||||||||||||
| Black (%) | 27.5% | 24.9% | NS | |||||||||||||||||
| Hispanic (%) | 11.2% | 14.7% | NS | |||||||||||||||||
| Asian / Other (%) | 3.4% | 2.3% | NS | |||||||||||||||||
| Body Mass Index (BMI) | 29.2 | 28.9 | NS | |||||||||||||||||
| Hypertension (%) | 79.8% | 79.7% | NS | |||||||||||||||||
| Cardiac Disease (%) | 50.6% | 57.1% | NS | |||||||||||||||||
| Diabetes (%) | 29.2% | 32.8% | NS | |||||||||||||||||
| Prior Stroke (%) | 5.6% | 12.4% | 0.02 | |||||||||||||||||
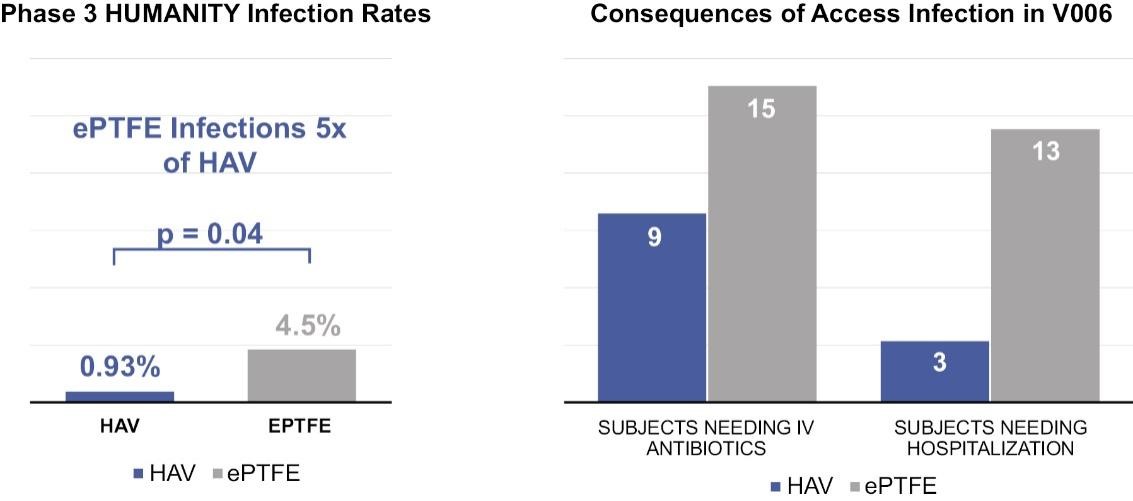
The secondary patency of the HAV was greater than that of ePTFE at six and 12 months but lower at 18 and 24 months, an outcome that had not been modelled in the V006 trial design. As per the pre-specified Cox Proportional Hazards test, the HAV did not achieve its primary efficacy endpoint regarding secondary patency. In terms of safety, the HAV had a statistically significant lower rate of conduit infections compared to ePTFE. Substantial differences in antibiotic use and need for hospitalization for infection were also noted in the V006 trial, all favoring the HAV. The safety advantage of the HAV over ePTFE may be clinically important as infection and sepsis are the second most common cause of death in dialysis patients.
Phase 3 V006 HUMANITY trial secondary patency results
| Secondary Patency | 6 months | 12 months | 18 months | 24 months | ||||||||||||||||||||||
HAV HUMANITY [Mean (95% CI)] | 92%(87 – 95%) | 82% (75 – 87%) | 73% (65 – 79%) | 67% (59 – 74%) | ||||||||||||||||||||||
| ePTFE HUMANITY [Mean (95% CI)] | 87% (81 – 85%) | 80% (73 – 85%) | 77% (70 – 83%) | 74% (67 – 81%) | ||||||||||||||||||||||
| Cox Proportional Hazards Model for Time to Loss of Secondary Patency | ||||||||||||||||||||||||||||||||
| Treatment Group (HAV vs ePTFE) | Hazard Ratio | Non-inferiority Margin Hazard | Non-inferiority Demonstrated (Yes/No) | |||||||||||||||||||||||||||||
| Estimate | 95% CI | |||||||||||||||||||||||||||||||
| 12 months | 0.869 | (0.528, 1.431) | 1.491 | Yes | ||||||||||||||||||||||||||||
| 24 months | 1.284 | (0.867, 1.903) | 1.488 | No | ||||||||||||||||||||||||||||
23
Phase 3 V006 HUMANITY trial rates of infection
The reported SAEs related to the HAV and ePTFE in the V006 trial, in this patient population, which typically has a high prevalence of existing medical conditions, are detailed in the table below.
SAEs Reported in V006 Phase 3 Clinical Study in AV Access
| Description of SAE | Number of SAEs (% of total subjects) | |||||||||||||
| HAV | ePTFE | |||||||||||||
| Number of subjects in V006 study | 177 | 178 | ||||||||||||
| General disorders and administration conditions: | ||||||||||||||
| Implant site extravasation | 0(0.0)% | 1(0.6)% | ||||||||||||
| Infections and infestations: | ||||||||||||||
| Vascular access site infection | 0(0.0)% | 5(2.8)% | ||||||||||||
| Injury, poisoning and procedural complications: | ||||||||||||||
| Anastomotic stenosis | 1(0.6)% | (0.0)% | ||||||||||||
| Vascular access site hematomas | 1(0.6)% | (0.0)% | ||||||||||||
| Vascular access site hemorrhage | 0(0.0)% | 3(1.7)% | ||||||||||||
| Vascular access site pain | 1(0.6)% | 0(0.0)% | ||||||||||||
| Vascular access site pseudoaneurysm | 10(5.6)% | 0(0.0)% | ||||||||||||
| Vascular access site rupture | 2(1.1)% | 0(0.0)% | ||||||||||||
| Vascular access site thrombosis | 41(23.2)% | 28(15.7)% | ||||||||||||
| Skin and subcutaneous tissue disorders: | ||||||||||||||
| Skin necrosis | 0(0.0)% | 1(0.6)% | ||||||||||||
| Vascular disorders: | ||||||||||||||
| Steal syndrome | 2(1.1)% | 2(1.1)% | ||||||||||||
| Subclavian vein occlusion | 0(0.0)% | 1(0.6)% | ||||||||||||
| Vascular stenosis | 34(19.2)% | 27(15.2)% | ||||||||||||
| Venous stenosis | 3(1.7)% | 9(0.0)% | ||||||||||||
24
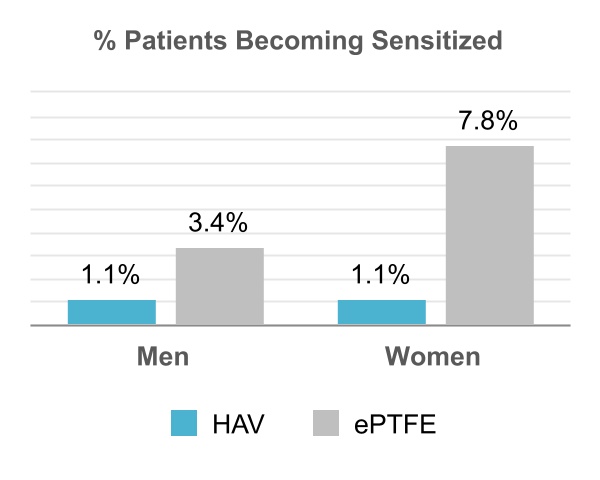
Through an Analysis of Panel Reactive Antibodies (“PRA”) in the V006 trial, we observed that subjects that received the ePTFE grafts were more likely to develop antibodies against human tissues, and to become more difficult to crossmatch for a future kidney transplant, than were patients who received the HAV. While the cause of this improvement in patient responses with the HAV is not clear, this may have been related to the lower number of severe infection events in HAV patients as compared to ePTFE patients in V006. The percentage of women becoming sensitized (i.e. cPRA values > 20%) was notably higher in the ePTFE group than the HAV group.
Phase 3 V006 HUMANITY trial % of patients developing antibodies against human tissues
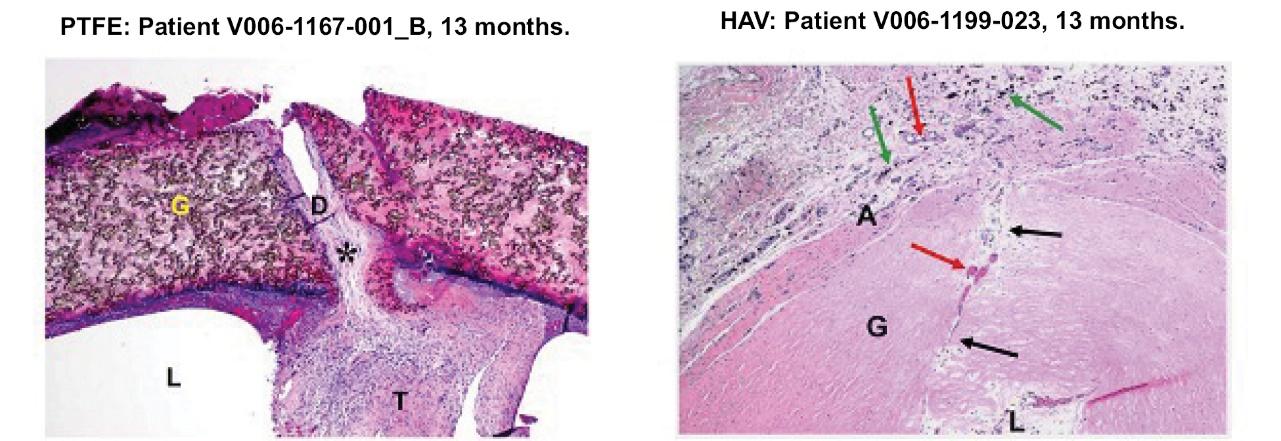
We also observed differences in the remodeling of the HAV and ePTFE implants in the V006 trial. Consistent with earlier observations from Phase 2 studies, microscopic examination of samples from HAV and ePTFE subjects suggest that the HAVs may have repopulated with host cells and microvasculature, while ePTFE grafts did not repopulate with cells and, in certain cases, fractured at sites of needle cannulation for hemodialysis (“G” is graft; “D” is defect’ “T” is thrombus (clot); “L” is lumen; and “A” is adventitia):
Phase 3 V006 HUMANITY trial microscopic examination of samples from ePTFE and HAV subjects
Comparison of flow rates within the HAV and ePTFE conduits revealed similarities in blood flow and diameter over the 24-month period of the trial. Bar graphs below display average blood flow rate, maximal blood flow rate, and mid-graft diameters, all of which were measured by periodic ultrasound examinations. Diameters of the HAV remained close to the nominal 6.0 millimeter diameter. Average blood flow rates exceeded 1.0 liters/minute, which is generally considered suitable for efficient hemodialysis.
25
Phase 3 V006 HUMANITY trial blood-flow rates and vessel diameters
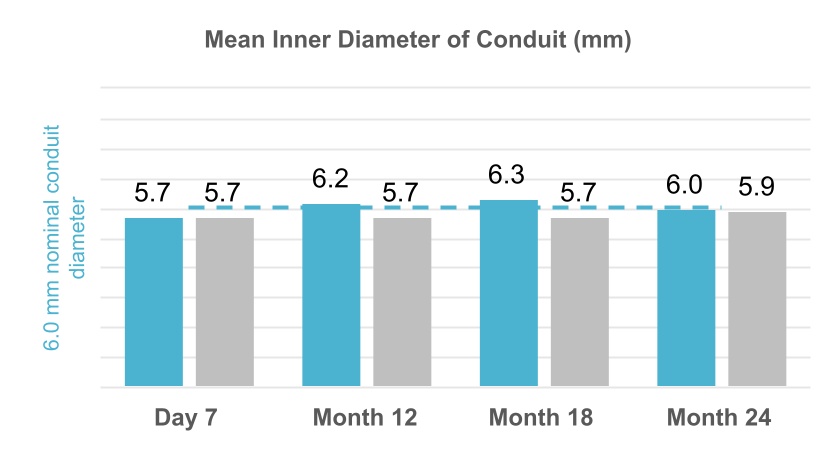
| HAV | ePTFE | ||||||||||||||||
Overall, although the primary efficacy endpoint concerning secondary patency was not met, the HAV performed in the V006 trial as was expected, based upon HAV performance in previous Phase 2 trials in hemodialysis and in other clinical applications. This outcome was due at least in part to unexpectedly high patency of the ePTFE grafts, particularly after 12 months. While the cause of this unexpectedly high patency is not clear, it is possible that study-mandated ultrasounds and examinations may have led to more aggressive vigilance with ePTFE grafts to maintain patency. In addition, the age and comorbidities of HAV subjects in V006 was somewhat worse than for ePTFE subjects.
In the V006 trial, the HAV displayed significantly fewer infections than did the ePTFE grafts. This was associated with fewer instances of immune sensitization in HAV subjects as compared to ePTFE subjects, which could translate to easier kidney transplantation at future times. Similar to prior studies, we observed that the HAV had good durability, blood flow rates and diameters similar to ePTFE grafts, and also host cell remodelling that was superior to that of ePTFE grafts.
Phase 3 V007 AV Access Study
Trial Design: We are currently enrolling a Phase 3 trial, called V007, in 240 patients with ESRD. V007 is a Phase 3, prospective, multi-center, open label, randomized, two-arm comparative study conducted in the United States. The V007 trial is designed to assess the usability of the HAV for dialysis at six and 12 months as a comparison to autogenous fistulas, which are known to exhibit a high rate of early maturation failure of approximately 40% at six months. Patients in the study are randomized to receive either the HAV for vascular access or an autogenous AV fistula. The objective of V007 is to compare the safety and efficacy of our 6 millimeter HAV to autogenous AV fistula for functional hemodialysis access.
Eligible study subjects in V007 are randomized to receive either an HAV or an autogenous fistula and followed to 24 months post-implantation by routine study visits. After 24 months, patients with functional accesses will be followed for up to five years. Efficacy endpoints include useability for dialysis at six and 12 months, as well as a comparison of secondary patency via a time-to-event analysis of all subjects at 12 months. Additional safety endpoints include the rate of dialysis access-related infections for HAV and fistula subjects.
Current Trial Status: As of December 31, 2022, there were 230 patients enrolled in the V007 trial, out of a target enrollment of 240 total. We currently expect, upon completion of the V007 trial, to file a BLA for the use of HAV in AV access for hemodialysis.
Proposed Indication #3: Peripheral Artery Disease
PAD involves partial or complete occlusion of blood vessels in the peripheral circulation and is a major cause of morbidity and mortality in the developed world. Patients with severe PAD undergo peripheral arterial bypass surgery where a conduit is implanted above and below the area of the arterial obstruction, to provide a “bypass” route for blood to flow around the blocked artery. The vast majority of these operations are performed in the lower limb. Other surgical alternatives include minimally invasive approaches such as stenting and angioplasties that are suitable for smaller atherosclerotic lesions and can delay — but oftentimes not prevent — the ultimate need for surgical revascularization.
26
Published Data in PAD
We derived the data in the table below from data contained in certain published papers on PAD between 2008 and the present. These data are from different studies and thus are not directly comparable. In addition, many of these papers reported on additional endpoints that are not included in the table below.
| Clinical Indication | Type of Conduit | Year | Number of Patients | Published Secondary Patency Outcome | Infection (per patient-year) | Rejection Outcome | ||||||||||||||||||||||||||||||||
| Peripheral Artery Disease | Saphenous Vein (autologous) | 2008 | 60 | 12 months: – 86% | N/A | N/A | ||||||||||||||||||||||||||||||||
| ePTFE | 2008 | 61 | 12 months: – 80% | |||||||||||||||||||||||||||||||||||
| (synthetic graft) | 2013 | 101 | 12 months: 76% – 89% | N/A | N/A | |||||||||||||||||||||||||||||||||
| 2011 | 273 | 12 months: 81% | ||||||||||||||||||||||||||||||||||||
| 2013 | 496 | N/A | 3.8% | |||||||||||||||||||||||||||||||||||
| 6 months: 50% | N/A | N/A | ||||||||||||||||||||||||||||||||||||
| Procol | 2008 | 7 | 12 months: 50% | |||||||||||||||||||||||||||||||||||
| (bovine vein) | Aneurysm Rate: 29% | |||||||||||||||||||||||||||||||||||||
We have observed strong patency rates and no reported cases of infection for the HAV in PAD in clinical studies to date. We are developing our 6 millimeter HAV for use as a bypass conduit for patients with PAD. We are conducting two Phase 2 trials to evaluate the safety and efficacy of our 6 millimeter HAV for use as a bypass conduit with PAD, which we refer to as our V002 and V004 trials. For both of these Phase 2 trials, the HAV is being implanted as a femoral popliteal bypass graft in patients with PAD.
Our Current Phase 2 Trials of the HAV in PAD
| Clinical Trial Number | Indication | Begin Enrollment | Design/ Phase | Number of Subjects | Status | Outcomes** | ||||||||||||||||||||||||||||||||
| V002 | Peripheral | 2013 | Phase 2 | 20 | 10-year | 30-day PP: 100% | ||||||||||||||||||||||||||||||||
| Artery Disease | Single-arm | follow-up | 6-month SP: 84% | |||||||||||||||||||||||||||||||||||
| ongoing | 12-month SP: 84% | |||||||||||||||||||||||||||||||||||||
| 72-month SP: 60% | ||||||||||||||||||||||||||||||||||||||
| Infection Rate/yr: 0% | ||||||||||||||||||||||||||||||||||||||
| Number of Rejections: 0 | ||||||||||||||||||||||||||||||||||||||
| V004 | Peripheral | 2016 | Phase 2 | 15 | 5-year | 30-day PP: 100% | ||||||||||||||||||||||||||||||||
| Artery Disease | Single-arm | follow-up | 6-month SP: 86% | |||||||||||||||||||||||||||||||||||
| ongoing | 12-month SP: 64% | |||||||||||||||||||||||||||||||||||||
| Infection Rate/yr: 0% | ||||||||||||||||||||||||||||||||||||||
| Number of Rejections: 0 | ||||||||||||||||||||||||||||||||||||||
| Number of Amputations: 0 | ||||||||||||||||||||||||||||||||||||||
___________________________
** PP: Primary Patency, which is the interval of time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency, i.e. patent without interventions.
SP: Secondary Patency, which is the interval from the time of access placement until abandonment, i.e. patent with or without interventions.
Trial Design: Both our V004 and V002 trials are prospective, open-label, single treatment arm, multi-center studies. We enrolled 20 patients in our V002 trial in Poland, and 15 patients in our V004 trial in the United States. Both trials have the primary objectives of evaluating the safety of the HAV as a femoral-to-popliteal bypass graft, and determining the primary, primary assisted, and secondary patency over 12 and 24 months.
27
Current Trial Status and Outcomes: V002 enrolled a total of 20 patients between the ages of 54 and 79 at three clinical sites. 24-month results of the V002 trial were published in 2020. After censoring for three deaths (none of which were determined to be related to the HAV or the implant procedure), we observed 24-month primary, primary assisted and secondary patency rates of 58%, 58%, and 74%, respectively. We observed through ultrasound data that the HAVs were mechanically stable during the follow-up period and did not develop aneurysmal dilatation in any patient. Overall, we also determined through the histological assessment of explanted specimens that there were normal vascular cells within the HAV and there was no infection or signs of immunological reaction to the graft.
There have been no HAV-related infections reported during the V002 trial as of December 31, 2022, and no amputations of the treated extremity. A sub-set of seven V002 subjects consented for long-term follow-up computerized tomography (“CT”) angiograms, which were obtained at 48 to 52 months after HAV implantation. In all cases, the HAV maintained normal architecture and function. A representative image is shown below, taken 50 months post-implantation. Proximal and distal anastomoses of HAV with recipient’s vasculature are noted, as is the scale bar on the right-hand side of each image. The image presents two views of the same subject, and shows uniform HAV diameter along the length of the implant.
A CT Angiogram from a V002 Subject at 51 months after HAV implantation

Patients in the V002 trial are currently in long-term follow-up out to ten years. In 2022, six-year results from V002 were published in Journal of Vascular Surgery – Vascular Science. The article, entitled “6-Year Outcomes of a Phase 2 Study of Human-Tissue Engineered Blood Vessels for Peripheral Arterial Bypass,” reported overall secondary patency rate of 60% at 72 months, including all patients originally enrolled, as estimated by Kaplan Meier analysis. There was no evidence of graft rejection or infection, and no patients underwent amputation of the affected limb out to six years.
Long-term results from V002 Phase 2 study in PAD
Result from V002 Phase 2 Trial in PAD (as of April 2021) | Pre-Op | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | 6 yr | Avg | ||||||||||||||||||||||||||||||||||||||||||
| Secondary Patency | — | 84% | 74% | 73% | 66% | 60% | 60% | — | ||||||||||||||||||||||||||||||||||||||||||
| Ankle-Brachial Index (median) | 0.64 | 0.90 | 0.96 | — | 1.07 | 0.98 | 0.94 (n=2) | 0.97 (post-op) | ||||||||||||||||||||||||||||||||||||||||||
| HAV Infection Rate | — | 0% | 0% | 0% | 0% | 0% | 0% | 0% | ||||||||||||||||||||||||||||||||||||||||||
28
The V004 trial enrolled 15 subjects in the United States, with the 12-month follow-up of the last enrolled patient occurring in December 2020. Patients in the V004 trial included Rutherford 4 and 5 subjects, with severe, debilitating limb ischemia. (Rutherford 4 and 5 patients are classified as patients with pain at rest due to limb ischemia (stage 4), and those patients suffering tissue loss in the limb as a result of ischemia (stage 5)). In addition, enrollment in V004 required that no autologous vein be available for bypass. Hence, the subjects enrolled in the V004 trial had severe and debilitating limb ischemia due to PAD and had no autologous vein that was suitable for lesion bypass and revascularization.
12-month results from V004 Phase 2 study in PAD
Result from V004 Trial (as of April 2021) | Pre-Op | 6 mos | 12 mos | |||||||||||||||||
| Secondary Patency | — | 86% | 64% | |||||||||||||||||
| Ankle-Brachial Index (median) | 0.51 | 0.85 | 0.90 | |||||||||||||||||
| Rate of Amputation | — | 0% | 0% | |||||||||||||||||
| VascuQol Quality of Life Assessment | 3.1 | 5.6 | 5.9 | |||||||||||||||||
In the V004 trial, HAV secondary patency was 86% at 6 months, and 64% at 12 months. While lower than patency values observed in the V002 trial, patients in the V004 trial had more severe PAD, which is associated with poorer arterial “run-off” and higher propensity for conduit occlusion. Assessment of Quality of Life by the validated VascuQol assessment demonstrated an increase in overall quality of life for V004 patients at 6 and 12 months. In addition, ankle-brachial index, a measurement of blood pressure in the operative limb, was increased at 6 and 12 months. There were no infections of the HAV reported in the V004 trial, despite the severity of the PAD and the often-associated tissue infection that can accompany this disease. There were zero reports of clinical HAV rejection. Lastly, there were zero reported amputations of any operative limb in the first 12 months of follow-up.
The SAEs reported for the HAV in our V002 and V004 Phase 2 clinical studies in PAD in 35 subjects, a patient population which typically has a high prevalence of existing medical conditions, are summarized in the table below.
SAEs Reported in V002 and V004 Phase 2 Clinical Studies in PAD
| Description of SAE | Number of SAEs (% of total subjects) | |||||||
Number of subjects in V002 and V004 studies | N=35 | |||||||
Arterial bypass thrombosis | 3(8)% | |||||||
Anastomotic stenosis | 1(3)% | |||||||
Graft Thrombosis | 2(6)% | |||||||
Vascular Graft Complication | 1(3)% | |||||||
Published literature reports of patients with Rutherford stage 4 and 5 PAD and no autologous vein available for revascularization show that outcomes can include amputation. For Rutherford 4,5 patients with no vein and no revascularization procedure, amputation rates at 6 months are reported at 31%. For stage 4,5 patients who do undergo saphenous vein revascularization, the amputation rate at one year is approximately 10%. The lack of amputation for stage 4,5 patients in the V004 trial at one year, none of whom had saphenous vein for revascularization, supports the use of the HAV in severe PAD.
Examples of the Use of Our 6 millimeter HAVs in Expanded Access Cases
The FDA has granted use of the HAV in 26 special expanded access cases through December 31, 2022. Each of these compassionate use cases was conducted under an individual, investigator-initiated IND with the FDA. Two cases are highlighted below.
70-year-old with Critical Limb Ischemia
The patient is a 70-year-old male with critical limb ischemia and no vein available to perform a bypass, as the vein was previously used for a CABG. He underwent a successful bypass with the HAV. Imaging at one year demonstrated a patent graft.
29
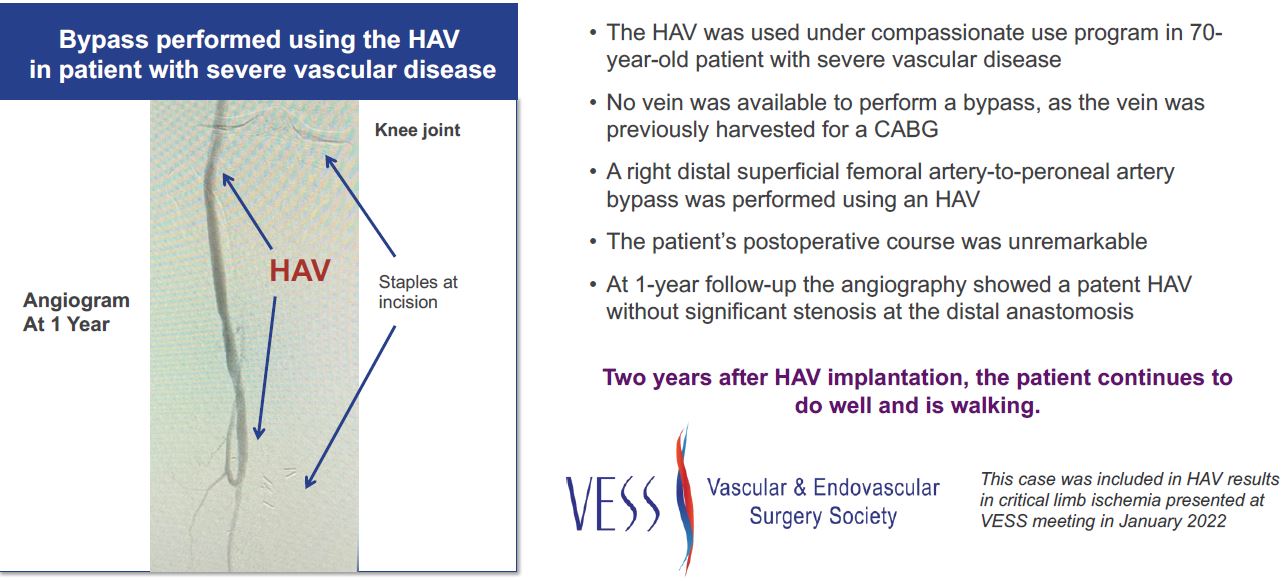
42-year-old with Infected Dacron Graft
An HAV was used in a 42-year-old female to replace an 8 mm Dacron iliac artery bypass graft that had become infected. The patient refused harvesting of the femoral vein for reconstruction and requested the HAV. The patient was seen at one, three, six, nine, and 12 months after HAV implantation. At all visits, the HAV appeared normal with unobstructed patency. Flow and velocities were normal. At three months, the patient was released to full activity. At six and 12 months, the graft was functioning well. At one-year imaging, the HAV was patent and appeared remarkably similar to the patient’s native blood vessels. The patient had no signs of infection in the HAV and continues to have no limitations or complications during normal activity or exercise.
Mayo Clinic Study in Severe PAD
The Mayo Clinic, Rochester, MN, is conducting a study in up to 25 patients with severe PAD under an investigator IND filed with the FDA. As of December 25, 2022, 18 patients have been implanted with the HAV under the study.
Preclinical Pipeline
Pancreatic Islet Transplantation for Type 1 Diabetes (“Biovascular Pancreas”)
The Biovascular Pancreas (“BVP”) is a modification of Humacyte’s HAV product, leveraging the HAV to deliver therapeutic cells within close proximity of the patient’s bloodstream. We believe that the HAV extracellular matrix material is both highly biocompatible, as evidenced by adaptive cellular repopulation after implantation, and also highly angiogenic, as evidenced by extensive formation of microvessels surrounding the HAV in vivo. These attributes mean that the HAV may serve as a suitable conduit for delivering large numbers of therapeutic cells to a patient.
Pancreatic islets, which sense blood glucose and respond by secreting insulin, are destroyed by an auto-immune attack in patients with Type I diabetes. The outer surface of our 42cm HAV has sufficient surface area to accommodate a monolayer of approximately 800,000 human pancreatic islets, which is approximately the number in an entire adult pancreas, and can reverse diabetes and restore glucose control.
30
We have performed mathematical modelling studies that predict, we believe, that a 42cm HAV could maintain viability of a therapeutic number of islets after implantation of the HAV into the arterial bloodstream, or after implantation as an AV conduit similar to that used for hemodialysis access. Bioreactor experiments have confirmed these mathematical conclusions. Furthermore, we have implanted rat-sized BVPs into the aortas of diabetic rats, and observed that the BVP could restore normal glucose levels in all treated animals, while control animals (“No Flow” in red in figure below) did not restore glucose control. Studies in large animal models are planned to commence in 2023 as the next step in the development of this product candidate.

Coronary Artery Bypass Graft (CABG)
Evaluation of 3- and 4mm diameter HAVs for coronary artery bypass is ongoing at Humacyte. Our initial pilot studies have included the use of our engineered vessels for CABG in canines, demonstrating functional patency and adequate blood flow for up to one month. To further evaluate the utility and durability of the HAV in a large animal model, we have initiated a preclinical study at Duke University to evaluate the use of our small diameter HAV for CABG in adult primates (baboons). The goal of this study is to assess patency and function for six to 12 months, as well as host responses and cellular remodeling. HAVs are followed by ultrasound imaging of the heart, and angiographic imaging of the conduits. In July 2022, preclinical data on use of the small-diameter HAV in CABG was presented at American Heart Association Basic Cardiovascular Sciences Scientific Sessions. Coronary bypass in a non-human primate model, with follow-up of six months, showed that the HAV maintained structural integrity and functioned well to conduct blood flow to the heart. In addition, the HAV was observed to have robust cell repopulation with vascular cells over time, becoming a living vascular tissue supplying the heart muscle. During 2023 we plan to commence IND-enabling preclinical studies in large animals to support potential advancement of the HAV into human clinical trials.
Before (left) and after (right) implantation of HAV CABG in baboon
31
Pediatric Heart Surgery: Modified Blalock-Taussig-Thomas (mBTT) Shunt
Tetrology of Fallot is a relatively common congenital heart defect, that is often treated using a modified Blalock-Taussig-Thomas (“mBTT”). To support a potential future IND filing with the FDA, we have evaluated the use of our HAV as an mBTT shunt for up to six months in juvenile primates at the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio.
BT Shunt Implant Schematic
In November 2021, the results for this preclinical study were presented at the American Heart Association’s Scientific Sessions 2021 meeting. In this study, five non-immunosuppressed juvenile primates were surgically implanted with the 3.5mm diameter HAVs as mBTT shunts. The 3.5mm HAVs were implanted into primates as mBTT shunts using standard surgical techniques, and the animals were studied for three to six months. Each of the HAVs remained parent during the study and exhibited repopulation with vascular cells. Two of the primates showed a stronger xenogeneic to the human HAV material.
The 3.5mm diameter HAV has smaller product dimensions but is manufactured using a similar process as Humacyte's 6mm HAV system currently being evaluated in clinical trials in vascular trauma, AV access for hemodialysis, and PAD. We believe that the production of the functional 3.5mm HAV is indicative of the potentially broad application of our proprietary bioengineered tissue platform and manufacturing processes.
Imaging of 3.5mm HAV mBTT shunt in juvenile primate followed for 6 months
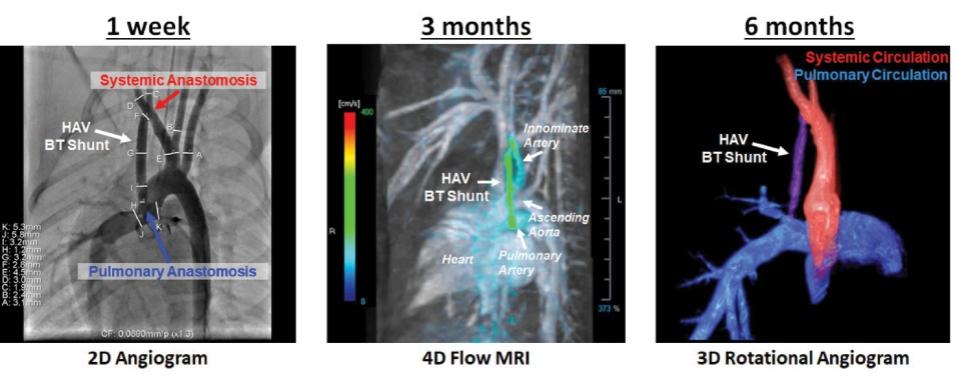
Engineered Trachea for Treatment of Severe Airway Injuries
Each year in the United States, approximately 4,000 operations are performed to repair or reconstruct the trachea or mainstem bronchi. But unlike most other connective tissues in the body — such as blood vessel, bone, skin and tendon — there currently are no replacements for tracheal tissue that are in widespread clinical use. For long tracheal or bronchial defects, some sort of tracheal replacement is often needed, yet none exists currently. The lack of a functional tracheal conduit commits patients to, sometimes, slow suffocation.
32
We have modified the HAV production process to enable the embedding of a biocompatible medical-grade stent within the wall of the engineered vessel. Combining a non-degradable stent with the degradable polymer scaffold used for HAV production results in a composite scaffold that can be seeded with smooth muscle cells and grown in culture. After decellularization, the engineered trachea consists of the extracellular matrix contained in the HAV, along with an embedded stent that prevents the collapse of the engineered airway with inspiration or neck movements.
Summary of Process to Generate Engineered Tracheas

In models where engineered tracheas were implanted into rats and non-human primates, we have observed that the implants repopulate with cells from the host, including cuboidal respiratory epithelium that lines the native airway progressively from two to eight weeks after implantation. We have further observed that the engineered tracheas can function out to two months. Future studies in large animal models are planned.
Photograph (A) of Implantation of Engineered Trachea into Non-Human Primate Airway; Microscope Imaging of Cells Repopulating the Trachea after 2 and 8 weeks (B, C)
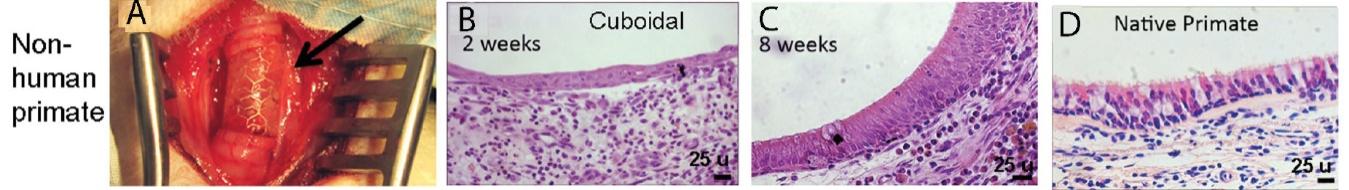
Engineered Whole Lung Organs
End-stage lung disease is the fourth leading cause of death in the U.S., and lung transplantation remains severely limited by donor organ shortages. Dr. Niklason’s laboratory at Yale University has pioneered the development of using decellularized native lungs, combined with targeted recellularization of the lung scaffolds within biomimetic bioreactors, to produce whole lungs that are capable of exchanging gas. Gas exchange for several hours has been observed in studies in rodents. Efforts to scale-up the technology to human-sized organs are ongoing.
Structure of Lung, Scaffold for Lung Engineering, and Implanted Engineered Lung
33
Manufacturing
We have developed a novel paradigm for manufacturing human tissues that mimics key aspects of human physiology. Recognizing that commercial scale production capacity of bioengineered tissue has been non-existent, we prioritized the development of a scalable, reproduceable, commercial biomanufacturing process. At our 83,000 square foot manufacturing facility in Durham, North Carolina, we have industrialized this concept and created a scalable modular manufacturing process that enables us to engineer our HAVs in commercial quantities in a system designed for cGMP compliance.
Our proprietary manufacturing process was designed with a modular approach allowing us to produce HAVs in smaller batches for clinical trials and scale out to larger batches for commercial manufacturing. The system used to produce HAVs for use in our clinical trials from 2016 to 2021, including Phase 3 trials, utilized a single tray within one growth drawer holding ten HAVs per batch. These batches were manufactured at a contract manufacturer. The current, commercial-scale LUNA200 system utilizes 20 growth drawers holding ten HAVs each for a total of 200 HAVs per batch. Since 2021 this system has been utilized to produce clinical product for use in our ongoing Phase 3 trials, and is planned for use to supply our anticipated commercial launches upon approval.
Our manufacturing process utilizes our LUNA200 system, consisting of 20 “growth drawers.” Each growth drawer is capable of producing ten 42cm HAVs and each HAV remains contained within an individual bioreactor bag. Inside a LUNA200, a closed tubing network connects all 20 growth drawers as well as the ten bioreactor bags in each drawer, allowing the entire system to share cells and nutritive media. In this way, a single LUNA200 can produce up to 200 HAVs per batch while maintaining the critical operating parameters that direct growth, creating a gross capacity of approximately 900 HAVs per system annually.
A thorough comparability assessment was performed to evaluate HAV batches produced in the single-drawer system versus batches produced in the 20-drawer LUNA200 system. The study assessed 22 separate comparisons on the identity, strength, quality, purity, and potency of the HAV product. In this study, we observed that HAVs produced in the LUNA200 system were comparable to HAVs produced in the single-drawer system. Additionally, a crossover study, called V011, enrolled 30 subjects to evaluate HAVs that were manufactured on Humacyte’s commercial LUNA200 platform with the primary goal to evaluate the safety, efficacy and immunogenicity of the LUNA200-manufactured HAVs. In this trial we observed comparable safety profile between HAV used in previous studies and the HAV manufactured in the LUNA200 commercial system. The results of the comparability assessment and from the V011 crossover study were submitted to the FDA. In 2021, the FDA authorized the use of HAVs produced in the commercial LUNA200 system to supply our ongoing clinical trials. We plan to also use the LUNA200 system for anticipated commercial launches of the HAV if it is approved.
We have designed the LUNA200 to have the ability to produce HAVs in diameter sizes from 3mm to 10mm and lengths from 10cm to 42cm, making the equipment suitable for the varied array of product candidates in our pipeline. We intend to introduce a 13cm HAV line extension after commercial launch of the 42cm HAV. Using our existing LUNA manufacturing equipment, we can generate 400 13cm HAVs per batch. Our modular manufacturing platform can be scaled without impacting the operating parameters that support the HAV growth process. We have designed our manufacturing system to be functionally closed, to utilize single-use disposable materials with aseptic connections, and to be fully automated.
Modular Manufacturing Platform Allows for Production of Multiple Product Lengths Using the Same Equipment

34
We currently have eight LUNA200 systems installed, commissioned and qualified in our manufacturing facility, creating an annual gross HAV capacity of approximately 7,200 HAVs. Our manufacturing facility contains space to increase capacity in future years to approximately 40 LUNA200 systems in total. As we continue to expand production, we believe that we will have the ability to take advantage of economies of scale and reduce production costs. The initiation and pace of the expansion of vessel capacity will be determined based on our assessment of market opportunity.
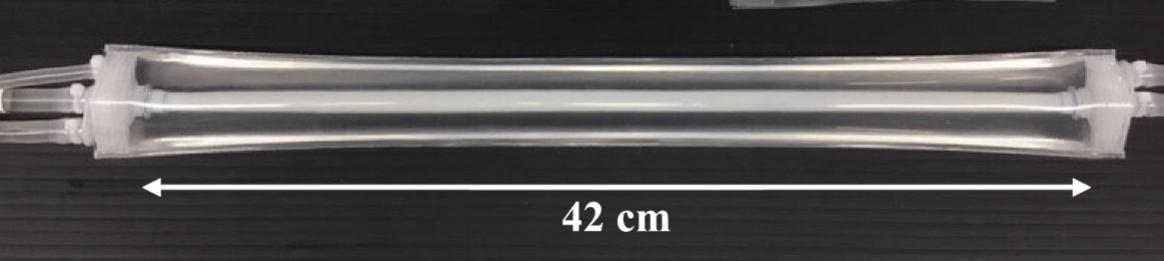
We initiate HAV production using primary human aortic vascular cells from a working cell stock (“WCS”) that is isolated from FDA-compliant donor tissues and cryopreserved. The WCS vials are stored at two separate qualified facilities to mitigate the risk of single site storage. We qualify all new WCSs for use in HAV manufacturing utilizing biochemical and gene expression assays. Each qualified primary isolation can produce approximately 500,000 to one million HAVs.
The WCS expanded using traditional cell culture techniques, and the cells are transferred onto a biocompatible, biodegradable polymer mesh within a flexible, single-use bioreactor bag. Cells inoculated onto this tubular mesh are cultured utilizing a proprietary culture medium and subjected to cyclic mechanical stretch for a period of approximately eight weeks. During this period, the cells proliferate and build extracellular matrix while the polymer mesh degrades. The resulting bioengineered vessel is comprised of the aortic vascular cells and their deposited extracellular matrix. After completion of the culture period, we decellularize the bioengineered vessel using a proprietary combination of salts, enzymes and detergents, followed by numerous washes in excipient grade neutral pH buffered saline. The resulting HAV retains the human extracellular matrix constituents and, therefore, the biomechanical properties of the bioengineered vessel, but cells and cellular components, which could induce a foreign body response or immune rejection following implantation, are removed. After decellularization, our HAVs are packaged for distribution inside the same flexible bioreactor bag in which they were produced, with sterile phosphate buffered saline as the excipient. Once the package is delivered to the operating room, the HAV is removed from the bioreactor bag by the surgical staff.
Suppliers
We source critical components and necessary raw materials from vendors that have been approved and qualified through our vendor management program. SeraCare, which was subsequently acquired by LGC Clinical Diagnostics, Inc. (“SeraCare”), is the current single source supplier of human plasma used in our manufacturing process and Confluent Medical Technologies, Inc. (“Confluent”) is the current single source supplier of the polymer mesh we use. We source custom, Humacyte-designed, pre-sterilized (gamma irradiated) assemblies and single-use tubing sets through multiple approved vendors. We source bioprocess solutions, including culture media and decellularization buffers, from a division of Thermo Fisher Scientific, which has a second production site to provide redundant media/buffer production capacity. We are in the process of developing redundant vendors for all critical materials and we manage all vendor changes through a robust change control process.
Supply Agreement with SeraCare
In January 2014, we entered into a supply agreement with SeraCare for the supply of human plasma, which was amended in October 2018. We refer to the supply agreement, as amended, as the SeraCare Agreement. Under the SeraCare Agreement, we agreed to purchase at least a substantial majority of our human plasma requirements from SeraCare. In the event SeraCare is unable to fulfill our requirements, and subject to certain conditions, we may engage another plasma supplier during the period in which SeraCare is unable to fulfill our requirements. The SeraCare Agreement is subject to annual price modifications in the case of significant changes in SeraCare’s cost of raw materials, with any modification to be determined at least three months prior to the end of the relevant year. The initial term of the SeraCare Agreement expires on October 12, 2023, but automatically extends for subsequent one-year periods unless terminated by either party at least 18 months prior to the end of the initial term. Either party may terminate the SeraCare Agreement for uncured material breach or for the insolvency of the other party at any time. In addition, either party may terminate the SeraCare Agreement without cause upon 12 months’ written notice. We may also terminate the agreement in the event of certain supply interruptions. Each party also agreed to indemnify the other against certain third-party claims up to a specified cap.
35
Supply Agreement with Confluent
In August 2015, we entered into an agreement for the supply of polymer mesh, which we refer to as the mesh supply agreement, with Biomedical Structures LLC. Biomedical Structures’ rights and obligations under the mesh supply agreement were subsequently assigned to Confluent in connection with Confluent’s acquisition of Biomedical Structures in 2016. In 2020, the agreement was amended to align with the growth expected with the transition to commercial distribution following FDA approval. Pursuant to the mesh supply agreement, the price of polymer mesh we purchase from Confluent is subject to potential adjustment if Confluent’s cost of raw materials increases above a specified threshold pursuant to good faith negotiations from both parties, which negotiation Confluent may not request more than once in a 12-month period. The 2020 amendment also provided volume driven discounts. Confluent is obligated to partner with Humacyte in order to establish redundant facilities for the manufacture of the polymer mesh at established contractual volume thresholds. The amended mesh supply agreement has a term of three years, which can be automatically extended for subsequent one-year periods and will continue to do so unless either party provides notice of non-renewal at least 120 days prior to the end of the then-current term or otherwise terminates in accordance with the agreement. We and Confluent are each also permitted to terminate the mesh supply agreement for convenience, however Confluent must provide us with at least 365 days written notice and we are obligated to provide 180 days’ notice, prior to such a termination. In addition, each party is permitted to terminate the mesh supply agreement for an uncured material breach by the other party following failure to remedy the breach during a sixty-day cure period. Both parties have agreed to indemnify one another for certain third-party claims.
Distribution
Commercialization Strategy Within United States and for Earlier-Stage Pipeline Programs
For our vascular repair and replacement applications of our technology, including renal replacement therapy for dialysis access, the treatment of PAD, and the treatment of vascular trauma, we have retained the right to commercialize our HAV within the United States, and expect to commercialize the HAV through a combination of our own direct sales and marketing team combined with our partnership with Fresenius Medical Care described below. We plan to own end-to-end commercialization while pursuing collaborations with appropriate strategic partners who have established distribution channels for supplying customer care centers.
Our first expected market launch, in the treatment of vascular trauma, is a highly concentrated market of approximately 200 Level I Trauma Centers that may be reached with a small field sales forces of no more than 20 representatives. Many of the major trauma centers already have familiarity with our HAV product candidate through their participation in our clinical trials. Our sales effort will include dual targeting of surgeons to create pull-through demand and hospital administration (trauma center Value Analysis Committees) to assure adoption and uptake of the HAV in vascular trauma.
We expect that the large market potential of earlier-stage applications of our technology platform such as CABG and biovascular pancreas for diabetes will provide additional collaboration opportunities, and we expect explore strategic partnerships for these product candidates as preclinical and clinical results providing additional proof of concept are generated.
Distribution Agreement with Fresenius Medical Care
We entered into a distribution agreement with Fresenius Medical Care in June 2018 which, as amended as of February 16, 2021, granted Fresenius Medical Care and its affiliates exclusive rights to develop outside the United States and European Union (the “EU”) and commercialize outside of the United States our 6 millimeter x 42cm HAV and all improvements thereto, and modifications and derivatives thereof (including any changes to the length, diameter or configuration of the foregoing), for use in vascular creation, repair, replacement or construction, including renal replacement therapy for dialysis access, the treatment of peripheral artery disease, and the treatment of vascular trauma, but excluding coronary artery bypass graft, pediatric heart surgery, or adhering pancreatic islet cells onto the outer surface of the distribution product for use in diabetic patients. Within the United States, Fresenius Medical Care will collaborate with Humacyte in its commercialization of the product in the field, including adoption of the distribution product as a standard of care in patients for which such use is supported by clinical results and health economic analyses.
We are responsible for developing and seeking regulatory approval for the distribution product in the field in the United States. For countries outside the United States, the parties agreed to use commercially reasonable efforts to satisfy certain agreed minimum market entry criteria for the distribution product in the field in such country. For the EU, once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory
36
approval for the distribution product in the field in the applicable country, we agreed to use commercially reasonable efforts to obtain such regulatory approval (other than pricing approval), and Fresenius Medical Care agreed to use commercially reasonable efforts to obtain the corresponding pricing approval. For the rest of the world (i.e., outside the United States and the EU), once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory and pricing approval for the distribution product in the field in the applicable country, Fresenius Medical Care agreed to use commercially reasonable efforts to obtain such approvals, and we agreed to use commercially reasonable efforts to support Fresenius Medical Care in its efforts.
Under the distribution agreement, we grant an exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by us during the term to commercialize the distribution product in the field outside the United States, subject to our retained rights to carry out our obligations under the distribution agreement. We also grant a non-exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by us during the term to develop the distribution product in accordance with the terms of the distribution agreement. In addition, we grant to Fresenius Medical Care, among other things, a perpetual, irrevocable, non-exclusive sublicensable license under the patents and know-how that primarily relate to the distribution product or its manufacture and that were created, conceived or developed solely or jointly by or on behalf of Fresenius Medical Care in the performance of its activities under the distribution agreement.
The distribution agreement provides that we will own all know-how and patents that primarily relate to the distribution product or its manufacture that are created, conceived or developed by or on behalf of either party in the performance of activities under the distribution agreement. Ownership of all other know-how, patents, materials and other intellectual property created, conceived or developed during the performance of activities under the distribution agreement will be determined in accordance with U.S. patent laws for determining inventorship.
We are obligated to make payments to Fresenius Medical Care based on a share of aggregate net sales by or on behalf of us of the distribution product in the United States in the field. Such revenue-share payments will be a percentage of net sales in the low double digits, without regard to the calendar year in which such net sales are attributable, until such time that we have paid to Fresenius Medical Care a certain total amount, at which time the revenue-share will decrease to a percentage of net sales in the mid-single digits. The amounts that Fresenius Medical Care will be obligated to pay us under the distribution agreement for sales of the distribution product in the field outside of the United States will vary. Fresenius Medical Care agreed to pay us initially, on a country-by-country basis for sales outside of the United States, the amount equal to the average cost of manufacturing our distribution product plus a fixed dollar amount per unit. Following a specified period, on a country-by-country basis outside of the United States, Fresenius Medical Care will pay us a fixed percentage of net sales for each unit sold in such country, such that the Company will receive more than half of such net sales.
The distribution agreement will generally continue on a country-by-country basis until the later of the tenth anniversary of the launch date of the distribution product in the relevant country or (b) the expiration of the last-to-expire valid claim of specified patents in such country. Each party is permitted to terminate the distribution agreement for insolvency of, or, under certain circumstances, including various cure periods, material breach by the other party. Subject to a cure period, Fresenius Medical Care may also terminate the distribution agreement in its entirety or on a country-by-country basis (i) for certain withdrawals of regulatory approval or (ii) for termination or expiration of any of our in-licenses that is necessary for the exercise of Fresenius Medical Care’s rights, or the satisfaction of its obligations, under the distribution agreement. In addition, Fresenius Medical Care may terminate the distribution agreement for convenience on a country-by-country basis upon not less than 12 months’ written notice to us, although Fresenius Medical Care is not permitted to give such notice prior to the end of the second year following launch of the distribution product in such country. Each party is required to indemnify one another for certain third-party claims.
Third-Party Reimbursement
We anticipate that coverage and reimbursement by the Centers for Medicare and Medicaid Services (“CMS”) and private payors will be essential for most patients and health care providers to afford our treatments, particularly in the applications of renal replacement therapy for dialysis access and the treatment of PAD. Accordingly, sales of our products will depend substantially, both domestically and abroad, on reimbursement by government authorities, private health coverage insurers and other third-party payors. Our strategy around HAV reimbursement focuses on achieving alignment and agreement from CMS on coding and payment pathways; both are critical to influencing and achieving optimal reimbursement payment from private payor sources. Therefore, Humacyte continues to develop a comprehensive reimbursement strategy including CMS, private payors, and other key stakeholders to ensure a clear and sustainable reimbursement path for all HAV product opportunities.
37
We are pursuing a dual regulatory and legislative reimbursement strategy to ensure separate Medicare payment for the HAV at an appropriate price. The regulatory strategy includes (1) engaging CMS political and career staff directly on coverage, payment, and coding followed by (2) submission of formal applications in these areas once FDA approval is obtained. Currently, no RMAT tissue engineered product has established coverage and reimbursement by CMS, and it is difficult to predict what CMS will decide with respect to coverage and reimbursement for fundamentally novel products. See “Risk Factors — Risks Related to the Development and Commercialization of Our Product Candidates” for further information. Even if we receive marketing approval for our HAVs, there is uncertainty with respect to third-party coverage and reimbursement of our HAVs. They may also be subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, any of which could harm our business, prospects, operating results and financial condition.
Containment of healthcare costs has been a priority of federal, state, and foreign governments, and the prices of drug products have been a focus of this effort. Governments have shown significant interest in implementing cost-containment programs. This interest has resulted in significant proposed and enacted reform measures affecting healthcare reimbursement and drug pricing, including the enactment in August 2022 of significant changes to potential Medicare drug product reimbursement through government negotiation of certain drug prices, as well as manufacturer discount and inflation rebate obligations under the Inflation Reduction Act (the “IRA”).
Intellectual Property
We strive to protect and enhance the proprietary technology, inventions and improvements that are commercially important to the development of our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also rely on trade secrets relating to our proprietary technology platform and on know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen and maintain our proprietary position that may be important for the development of our business. We additionally may rely on regulatory protection afforded through data exclusivity, market exclusivity and patent term extensions where available.
Our success will depend significantly on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, preserve the confidentiality of our trade secrets, and operate without infringing the valid and enforceable patents and proprietary rights of third parties.
As of December 31, 2022, our patent estate is comprised of 18 families of patents. Of these families, 14 are solely owned by Humacyte, one is jointly owned by Humacyte and Global Life Sciences Solutions USA LLC, one is jointly owned by Humacyte and Yale University, one is exclusively licensed to Humacyte from Duke University and one is exclusively licensed to Humacyte from Yale University. For more information regarding these license agreements, see “— License Agreement with Duke University” and “— License Agreements with Yale University.”
Our 18 families of patents are comprised of:
(i)eight issued U.S. patents, 74 foreign patents in Austria, Australia, Belgium, Canada, China, Cyprus, Denmark, France, Germany, Greece, Hong Kong, Hungary, Ireland, Italy, Japan, Netherlands, Portugal,Spain, Sweden, Switzerland, Turkey, and the UK, nine pending U.S. non-provisional patent applications, one pending PCT patent application and seven pending foreign applications in Australia, Canada, China, Europe and Japan, which are solely owned by us,
(ii)three issued U.S. patents, 19 issued foreign patents in Australia, Austria, Belgium, Canada, Denmark, France, Germany, Ireland, Italy, Japan, Netherlands, Spain, Sweden, Switzerland, Turkey, and the UK, one pending U.S. non-provisional patent application, and two pending foreign patent applications in Europe and Canada, which we co-own, and
(iii)one issued U.S. patents, seven issued foreign patents in Canada, France, Germany, Italy, Spain, Sweden, and the UK, one pending U.S. non-provisional patent application, and six pending foreign patent applications in Australia, Canada, Europe, Japan, China, and Hong Kong, which we exclusively license.
Many of these patents and patent applications generally relate to the scaffolds used to make our vessels, the composition of our vessels, and systems and methods of manufacturing our vessels. Excluding any patent term adjustment or patent term extension, the U.S. patent relating to the scaffold used to make our vessels expires in 2032, the U.S. patents relating to the composition of our vessels expire in 2032 and the U.S. patents relating to the systems and methods of manufacturing our vessels expires in 2032. The U.S. patent relating to the entangler machinery used to make tubular
38
scaffolds expires in 2035. Included in our patent portfolio are nine pending, Humacyte-owned non-provisional applications relating to the manufacturing of engineered tissues at commercial scale, as well as other technologies and product candidates. If these non-provisional applications are allowed, such additional patents issuing therefrom would be expected to expire around 2043.
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position for our product candidates will depend on our success in obtaining effective patent claims and enforcing those claims if granted. However, our owned and licensed pending patent applications, and any patent applications that we may in the future file or license from third parties, may not result in the issuance of patents. For more information, see “Risk Factors — Risks Related to Our Intellectual Property.”
We have also registered trademarks for use in connection with our products. These include registrations for HUMACYL™ in the United States, Europe, Australia, Canada, China, and Israel; HUMAGRAFT™ in Australia, China, Europe, and Israel; HUMAPASS™ in Europe, Australia, and Israel; and HUMACYTE, in the United States, Europe, Australia, Canada, and Israel. We may pursue additional registrations for future products in markets of interest.
In addition to the above, we have established expertise and development capabilities focused in the areas of preclinical research and development, manufacturing process scale-up, cGMP manufacturing, quality control, quality assurance, compliance, regulatory affairs and clinical trial design and execution. We believe that our focus and expertise will help us develop and expand technology-based applications leveraging our proprietary intellectual property.
Finally, we rely, in some circumstances, on trade secrets to protect our technology. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems.
In addition to the intellectual property that we have developed internally, we license rights to certain intellectual property that is material to our business prospects. We have summarized our material license agreements below.
License Agreement with Duke University
In March 2006, we entered into a license agreement with Duke University (“Duke”), which was subsequently amended in 2011, 2014, 2015, 2018, 2019 and January 2022. We refer to the license agreement, as amended, as the Duke License Agreement. Under the Duke License Agreement, Duke granted us a worldwide, exclusive, sublicensable license to certain patents related to decellularized tissue engineering, which we refer to as the patent rights, as well as a non-exclusive license to use and practice certain know-how related to the patent rights. The relevant licensed patent on decellularization of tissue expired in 2021. We have agreed to use commercially reasonable efforts to develop, register, market and sell products utilizing the patent rights, which we refer to as the licensed products. Any services provided to a third party utilizing licensed products are referred to as licensed services. We have also agreed to meet certain benchmarks in our development efforts, including as to development events, clinical trials, regulatory submissions and marketing approval, within specified timeframes. Under the Duke License Agreement, Duke retains the right to use the patent rights for its own educational and research purposes, and to provide the patent rights to other non-profit, governmental or higher-learning institutions for non-commercial purposes without paying royalties or other fees.
In connection with our entry into the Duke License Agreement, we granted equity consideration to Duke in the form of 52,693 shares of our post-Merger common stock. Under the Duke License Agreement, we have also agreed to pay Duke: a low single-digit percentage royalty on eligible sales of licensed products and licensed services, plus a low double-digit percentage of any sublicensing revenue; an annual minimum royalty beginning in 2012, which increases in the calendar year immediately following the first commercial sale of licensed products or licensed services (whichever occurs first); and an additional amount in license fees, as certain scientific milestones are met.
The Duke License Agreement remains effective until the latter of (i) the last of the patent rights expires or (ii) four years after our first commercial sale, unless earlier terminated. Either party may terminate the agreement for fraud, willful misconduct or illegal conduct, or uncured material breach. Duke may terminate the agreement if we become insolvent. Duke may also terminate the license, convert the license into a non-exclusive license or seek assignment of any sublicense if we fail to reach diligence milestones within the applicable time period. If we abandon any claim, patent or patent application, our rights under the license with respect to such patent rights will be terminated in the territory in which we abandon such rights. We may terminate the Duke License Agreement unilaterally upon three months’ prior notice to Duke. We agree to indemnify Duke against certain third-party claims.
39
License Agreements with Yale University
Large Diameter HAV
In August 2019, we entered into a license agreement with Yale University (“Yale”) that granted us a worldwide license to the patents jointly owned with us related to tubular prostheses which are large diameter versions of our vessels, which may or may not contain a stent. The license granted under the agreement is exclusive in the field of engineered urinary conduits, engineered tracheae/airways and engineered esophagi, except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. We have agreed to use reasonable commercial efforts to develop and commercialize the licensed patents and any licensed products and methods, and to use reasonable efforts to make the licensed products available to patients in low and low-middle income countries. We are also obligated to provide Yale periodically an updated and revised copy of our plan, which must indicate progress of our development and commercialization. We may also sublicense our rights without Yale’s prior written consent, but such sublicense is subject to certain conditions.
In connection with our entry into the Yale License Agreement, we paid Yale an upfront cash fee of less than $0.1 million. We have also agreed to pay to Yale: an annual maintenance fee, increasing between the first anniversary of the agreement until the fifth anniversary up to a maximum of less than $0.1 million per year; milestone payments upon achievement of certain regulatory and commercial milestones of $0.2 million and $0.6 million for this license; a low single-digit percentage royalty on worldwide net sales, subject to reductions for third-party license fees; and a low double-digit percentage of sublicensing income.
If we or any of our future sublicensees bring a patent challenge against Yale or assist another party in bringing a patent challenge against Yale, the license fees described above will be subject to certain increases and penalties.
The agreement expires on a country-by-country basis on the date on which the last of the patents in such country expires, lapses or is declared invalid. Issued patents and additional patents issuing from this licensed portfolio will expire no earlier than 2032, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Issued U.S. patent No. 10,172,707 will expire no earlier than 2035. Issued patents and additional patents issuing from this licensed portfolio will expire no earlier than 2032, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Issued U.S. patent No. 10,172,707 will expire no earlier than 2035. Yale may terminate the agreement if we fail to (i) provide written diligence reports, (ii) provide a commercially reasonable diligence plan, (iii) implement the plan in accordance with the obligations under the agreement, or (iv) reach certain research and development milestones within the scheduled timeframe set forth in the agreement; however, any such termination right would be limited in scope to the country or countries to which such failure relates. Yale may also terminate for our non-payment, uncured material breach, failure to obtain adequate insurance, bringing or assisting in bringing of a patent challenge against Yale, abandonment of the research and development of our product or insolvency. We may terminate the license agreement (i) on 90 days’ prior written notice to Yale, provided we are not in breach of the license agreement and have made all required payments to Yale thereunder and (ii) on written notice to Yale following an uncured material breach. Under certain circumstances, Yale may, at its option, convert the exclusive license to a non-exclusive license if we decline to initiate certain infringement or interference proceedings with respect to the licensed patents. We have agreed to indemnify Yale against certain third-party claims.
Small Diameter HAV Coating
In February 2014, we entered into a license agreement with Yale that granted us a worldwide license to the patents related to coatings for small-diameter vessels to inhibit clotting. The license granted under the agreement is exclusive in the field of engineered vascular tissues and tissues and extracellular matrix-based implants used for vascular repair, reconstruction and replacement (provided that all uses are vascular tissues within the range of 1-12mm in diameter), except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. We have agreed to use reasonable commercial efforts to develop and commercialize the licensed patents and any licensed products and methods, and to use reasonable efforts to make the licensed products available to patients in low and low-middle income countries. We are also obligated to provide Yale periodically an updated and revised copy of our plan, which must indicate progress of our development and commercialization. We may also sublicense our rights without Yale’s prior written consent, but such sublicense is subject to certain conditions.
In connection with our entry into the Yale License Agreement, we paid Yale an upfront cash fee of less than $0.1 million. We have also agreed to pay to Yale: an annual maintenance fee, increasing between the first anniversary of the agreement until the fifth anniversary up to a maximum of less than $0.1 million per year; milestone payments upon
40
achievement of certain regulatory and commercial milestones of $0.2 million and $0.6 million for this license; a low single-digit percentage royalty on worldwide net sales, subject to reductions for third-party license fees; and a low double-digit percentage of sublicensing income.
If we or any of our future sublicensees bring a patent challenge against Yale or assist another party in bringing a patent challenge against Yale, the license fees described above will be subject to certain increases and penalties.
The agreement expires on a country-by-country basis on the date on which the last of the patents in such country expires, lapses or is declared invalid. Issued patents and additional patents issuing from this licensed portfolio will expire no earlier than 2034, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Issued patents and additional patents issuing from this licensed portfolio will expire no earlier than 2034, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Yale may terminate the agreement if we fail to (i) provide written diligence reports, (ii) provide a commercially reasonable diligence plan, (iii) implement the plan in accordance with the obligations under the agreement, or (iv) reach certain research and development milestones within the scheduled timeframe set forth in the agreement; however, any such termination right would be limited in scope to the country or countries to which such failure relates. Yale may also terminate for our non-payment, uncured material breach, failure to obtain adequate insurance, bringing or assisting in bringing of a patent challenge against Yale, abandonment of the research and development of our product or insolvency. We may terminate the license agreement (i) on 90 days’ prior written notice to Yale, provided we are not in breach of the license agreement and have made all required payments to Yale thereunder and (ii) on written notice to Yale following an uncured material breach. Our rights under the license agreement will also terminate automatically with respect to a patent application or patent within the licensed patents in a specified country if, upon receipt of written notice from Yale, we do not agree to pay the patent filing, prosecution and maintenance fees incurred by Yale for such patent applications or patents in the specified country. Under certain circumstances, Yale may, at its option, convert the exclusive license to a non-exclusive license if we decline to initiate certain infringement or interference proceedings with respect to the licensed patents. We have agreed to indemnify Yale against certain third-party claims.
On December 21, 2022, we provided notice to Yale that we were terminating the license effective March 21, 2023 as we do not intend to use the technology underlying the patents.
Biovascular Pancreas
In August 2019, we entered into a license agreement with Yale that granted us a worldwide license to its patents related to a biovascular pancreas. The license granted under the agreement is exclusive in the field of acellular vascular tissues that deliver pancreatic islet cells to patients, except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. We have agreed to use reasonable commercial efforts to develop and commercialize the licensed patents and any licensed products and methods, and to use reasonable efforts to make the licensed products available to patients in low and low-middle income countries. We are also obligated to provide Yale periodically an updated and revised copy of our plan, which must indicate progress of our development and commercialization. We may also sublicense our rights without Yale’s prior written consent, but such sublicense is subject to certain conditions.
In connection with our entry into the Yale License Agreement, we paid Yale an upfront cash fee of less than $0.1 million. We have also agreed to pay to Yale: an annual maintenance fee, increasing between the first anniversary of the agreement until the fifth anniversary up to a maximum of less than $0.1 million per year; milestone payments upon achievement of certain regulatory and commercial milestones of $0.1 million and $0.2 million for this license; a low single-digit percentage royalty on worldwide net sales, subject to reductions for third-party license fees; and a low double-digit percentage of sublicensing income.
If we or any future sublicensees bring a patent challenge against Yale or assist another party in bringing a patent challenge against Yale, the license fees described above will be subject to certain increases and penalties.
The agreement expires on a country-by-country basis on the date on which the last of the patents in such country expires, lapses or is declared invalid. Patents issuing from this licensed portfolio will expire no earlier than 2039, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Patents issuing from this licensed portfolio will expire no earlier than 2039, and the term of each patent may be extended by patent term adjustment, patent term extension, or foreign equivalents thereof. Yale may terminate the agreement if we fail to (i) provide written diligence reports, (ii) provide a commercially reasonable diligence plan, (iii) implement the plan in accordance with the obligations under the agreement, or (iv) reach certain research and development milestones within the scheduled timeframe set forth in the agreement; however, any such termination right would be limited in scope to the
41
country or countries to which such failure relates. Yale may also terminate for our non-payment, uncured material breach, failure to obtain adequate insurance, bringing or assisting in bringing of a patent challenge against Yale, abandonment of the research and development of our product or insolvency. We may terminate the license agreement (i) on 90 days’ prior written notice to Yale, provided we are not in breach of the license agreement and have made all required payments to Yale thereunder and on written notice to Yale following an uncured material breach. Our rights under the license agreement will also terminate automatically with respect to a patent application or patent within the licensed patents in a specified country if, upon receipt of written notice from Yale, we do not agree to pay the patent filing, prosecution and maintenance fees incurred by Yale for such patent applications or patents in the specified country. Under certain circumstances, Yale may, at its option, convert the exclusive license to a non-exclusive license if we decline to initiate certain infringement or interference proceedings with respect to the licensed patents. We have agreed to indemnify Yale against certain third-party claims.
Competition
Despite the magnitude and critical nature of the diseases and conditions we are targeting, no significant advances in the open surgical market have been made in the last 35 years, and current treatment and products used in vascular repair, reconstruction and replacement suffer from various drawbacks. The large majority of vascular repair, reconstruction and replacement procedures rely on either harvesting autologous veins or using synthetic grafts. However, each method presents significant limitations as discussed below:
Autologous Veins
The harvest of autologous veins is a serious operation that can result in numerous complications, including infection, chronic pain, and limb swelling that severely impact the patient’s quality of life. In addition, this procedure can often result in long recovery times, increased hospital stays, and increased risk of hospital readmission. In order to obtain an autologous vein, such as a saphenous vein, for use in a surgical procedure, a second operation must be performed on the patient to harvest the vein. The harvesting process must be completed before the bypass procedure occurs and can take significant time to complete, which increases costs related to the additional operative time and staff required to perform the operation. Even if successful, the patient’s recovery time could increase as the patient must recover from two surgical procedures instead of one, further increasing morbidity and cost. Additionally, a significant percentage of patients are not suitable for vein harvesting either due to vein or limb damage, limited vein supply from prior harvest, venous disease or the surgeon’s desire to preserve the vein for future coronary or other bypass procedures. In acute trauma, the time to restore blood flow to injured limbs is delayed when a vein must be harvested from the patient, which puts the limbs at greater risk of reduced function or amputation. For patients suffering from vascular trauma, some types of injury preclude the harvesting of autologous saphenous vein due to concomitant injuries of one or both legs. Furthermore, time is required to prepare the vein harvest site and to remove the vein from the leg, which adds to ischemia time and can increase the risk of tissue and limb loss. Rates of traumatic limb loss are strongly tied to ischemia time, and therefore rapid revascularization using an off-the-shelf HAV conduit may decrease ischemia time and lead to better outcomes.
The use of autologous vein for creating an AV fistula for use in hemodialysis is often limited by vein size and location. The vast majority of veins must go through a process of enlargement, known as maturation, prior to use for hemodialysis. For approximately 40% of patients receiving fistulae, the vein does not mature sufficiently to allow for hemodialysis even after six months. Even in patients having adequate veins for fistula creation, the fistula often becomes large, tortuous and disfiguring and can be at risk for sometimes fatal rupture.
Synthetic Grafts
Use of synthetic materials, such as ePTFE and Dacron, while widely available, have known complications, such as continuous chronic risk of infection and clotting inside the graft. Risk of infection is significantly increased in acute battlefield and civilian injuries, as well as in contaminated wounds. The body recognizes any synthetic materials as foreign and, therefore, can mount a host foreign body response following implantation. Synthetic materials also have been shown to be inferior to autologous vein in resisting infection, and generally only are used for vascular repair when autologous vein is not an option.
In hemodialysis access, persistent puncture presents an ongoing risk of graft infection. The annual risk of infection of ePTFE grafts in hemodialysis patients can be as high as 10% – 15% per patient-year. Furthermore, gradual degradation of the non-healing ePTFE graft material caused by persistent needle punctures can eventually lead to graft failure. In traumatic vascular injury, ePTFE grafts are generally contraindicated, due to the high rates of contamination of the wound that can lead to synthetic graft infection and failure.
42
Two lesser used products, cryopreserved human blood vessels, known as allografts, and animal-derived vessels, known as xenografts, also involve significant limitations.
Cryopreserved Blood Vessels
To eliminate the need for harvesting autologous vein, some surgeons use allogeneic vessels that have been previously harvested from cadavers and cryogenically preserved. These allogeneic vessels are stored at -80 degrees Celsius and must be thawed prior to use, which can take up to 60 minutes. The supply of cryopreserved vessels is limited by the number of cadaveric donors available, and the vessels are often non-uniform in size. In addition, because the vessels contain human cells from a donor, they can generate an immune rejection response that can lead to aneurismal degradation or catastrophic failure. Furthermore, development of antibodies to the implanted cryopreserved human vessel frequently has a detrimental impact on the ability of the patient to receive a transplant in the future. Cryopreserved blood vessels are only rarely used in the treatment of vascular trauma, due to the time required for procurement and thawing, and the high rates of rejection response.
Animal-Derived Vessels
Xenogeneic tissues, including cow, pig or sheep-derived vessels, are used less frequently in vascular surgery, in part due to the risk of thrombosis and structural deterioration over time. The limited clinical data that are available for existing xenografts in vascular reconstruction indicates lower patency rates and higher incidence of complications when compared to autologous vein. Xenografts are all chemically treated in efforts to minimize rejection to animal components, and therefore do not respond like living tissue. Some of these products require rinsing to remove toxic chemicals used for storage.
Our Solution
We believe our HAVs combine the off-the-shelf availability of synthetic grafts with the regenerative capabilities of autologous vessels. We believe these and other attributes have the potential to address unmet clinical needs in a range of disease states, including atherosclerosis, end-stage kidney disease, coronary artery disease, vascular trauma, pediatric congenital heart disease, airway disease, and others. We believe that the HAV’s multiple key characteristics will drive rapid clinical adoption amongst surgeons and the broader healthcare community:
•Off-the-Shelf: Our “cabinet” of HAVs of varying diameters and lengths is designed to be stored on-site at facilities such as hospitals, trauma centers and outpatient surgical centers.
•Immediately Available: When needed, our HAVs are available for immediate use by opening and removing the HAV from its original flexible bioreactor bag. Since our HAV does not need flushing, harvesting or thawing, as is common with other vascular substitute alternatives, we believe hospitals will be able to use our HAVs for vascular surgery more quickly with smaller surgical teams, reduced logistics and decreased overall cost.
•No Surgical Harvesting: The use of our HAVs does not subject patients to the serious operation of harvesting an autologous vein, which can result in greater procedure and recovery time, potential scarring and disfigurement, increased costs, and numerous potential health complications.
•Non-Immunogenic and No Foreign Body Response: Given their acellular nature, our HAVs have the potential to be universally implantable and durable across patients. Because our HAVs are derived from human tissue (but cleansed of all cells and cellular components), we believe (and have observed in clinical trials to date) that they do not generate the foreign body response associated with the use of synthetic grafts, or the immune response associated with cryopreserved vessels.
•Low Infection Susceptibility: In clinical trials to date, we have observed reduced rates of infection in our HAVs as compared to synthetic materials. As a result, we believe our HAVs may be used in complicated and potentially contaminated wounds with fewer patient complications following the initial procedure.
•Uniform and Predictable Size, Structure and Quality: Harvested veins vary in size, structure and quality by donor. We manufacture our HAVs to precise specifications under controlled quality standards, which will allow surgeons the flexibility to quickly and easily select an HAV in the appropriate size and shape for each indication.
•Regenerative Potential: Our HAVs repopulate with the patient’s own vascular cells, creating a living vascular tissue with the associated long-term benefits of self-healing and infection resistance.
43
If approved, we expect our HAVs would compete with the use of a patient’s own blood vessels, as well as a variety of marketed products, such as conventional synthetic grafts, xenografts, and allografts, as well as developing technologies. We expect the key competitive factors affecting the commercial success of our HAVs to likely be efficacy, safety, convenience, pricing and reimbursement.
Other Commercial Entities
There are several conventional synthetic grafts made of ePTFE or Dacron presently on the market from companies such as Bard Peripheral Vascular, Inc., W.L. Gore & Associates, Inc., Terumo Medical Systems, and Atrium (Maquet Getinge Group) that are used for both AV access for hemodialysis and vascular repair. Xenograft and allograft products are also available, but not widely used. Xenografts, such as Artegraft® and Procol®, are processed animal-derived vessels, while allografts are processed allogeneic cellular vessels, such as CryoVein® and AngioGRAFT®.
There are also a number of companies of which we are aware that have preclinical and early clinical-stage research programs underway to develop products that could potentially compete with our HAVs, including NovaHep AB, Xeltis AG, Hancock Jaffe, and Vascudyne Inc. We may face competition from these and other emerging technologies such as bioabsorbable polymetric implants and electrospun or 3D printed tubular conduits.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are more effective, safer, have fewer or less severe side effects, are more convenient or are less expensive than the products that we develop. Our competitors also may obtain FDA or other marketing approval for their products more rapidly than we may obtain the same approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Government Regulation
Overview
The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements on the research, development, testing, manufacture, quality control, safety, effectiveness, packaging, labeling, storage, record keeping, marketing, advertising and promotion, import/export, and distribution of our vessels.
In the United States, the FDA regulates pharmaceutical drugs, medical devices and biologic products under the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service Act (“PHSA”), FDA implementing regulations, and other laws. Our vessels are subject to regulation by the FDA as biologics. Biologics require the submission of a BLA and approval by the FDA before being marketed in the United States. None of our vessels have been approved by the FDA for marketing in the United States, and we currently have no BLAs pending. If we fail to comply with applicable FDA or other requirements at any time during the product development process, clinical testing, and the approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any FDA enforcement action could have a material adverse effect on us.
Marketing Approval — Biological Products in the United States
Before a biologic is approved in the United States, an applicant must submit a BLA that includes sufficient evidence to establish the safety, purity, and potency of the product candidate for its intended indications, including from the results of preclinical studies and clinical trials. A BLA must also contain extensive information about manufacturing and product quality control testing, and the applicant must pass an FDA preapproval inspection of the manufacturing facility or facilities at which the biologic product is produced and distributed from to assess compliance with current good manufacturing practices, or cGMPs.
The steps for obtaining FDA approval of a BLA to market a biologic product in the United States generally include:
•Completion of extensive preclinical laboratory tests and preclinical animal studies performed in accordance with the FDA’s current good laboratory practice (“GLP”) regulations;
•Submission to the FDA of an Investigational New Drug application (“IND”), which must become effective before human clinical trials in the United States may begin;
44
•Approval of the protocol and related documentation by an Institutional Review Board (“IRB”) or ethics committee representing each clinical site before each clinical trial may be initiated;
•Performance of adequate and well-controlled human clinical trials according to the FDA’s regulations commonly referred to as GCPs and any additional requirements for the protection of human research subjects and their health information, to establish the safety and efficacy of the product candidate for each proposed indication;
•Submission to the FDA of a BLA;
•Satisfactory completion of an FDA inspection of the manufacturing facility or facilities and distribution site at which the product is produced: to assess compliance with cGMP regulations; to assure that the facilities, production methods, testing and controls are adequate; and, if applicable, to assure compliance with cGTP requirements for human cellular and tissue-derived products;
•Potential FDA audit of the nonclinical study and clinical trial sites that generated the data in support of the BLA;
•Review of the product candidate by an FDA advisory committee, if applicable;
•Payment of user fees for FDA review of the BLA (unless a fee waiver applies); and
•FDA review and approval, or licensure, of the BLA prior to any commercial marketing, sale or shipment of the product.
U.S. Biological Products Development Process
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our vessels will be granted on a timely basis, if at all.
Once a product candidate is identified for development, that biologic candidate enters the preclinical testing stage. Preclinical studies include laboratory evaluations of product chemistry, toxicity, formulation and stability, as well as animal studies to evaluate the product’s potential safety and activity. The results of the preclinical studies, together with manufacturing information, analytical data, and at least one protocol for clinical study, are submitted to the FDA as part of an IND. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. This is known as a “clinical hold.” In such a case, the IND sponsor must resolve all of the FDA’s concerns to the agency’s satisfaction before the clinical trial can begin. Submission of an IND may result in the FDA not allowing the clinical trials to commence or not allowing the clinical trials to commence on the terms originally specified in the IND. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development, and the FDA must grant permission, either explicitly or implicitly by not objecting, before each clinical trial can begin. Even after a clinical trial has begun, the FDA can issue a clinical hold at any time if it concludes that certain conditions exist, such as patients may be exposed to an unreasonable and significant risk of illness or injury.
Clinical trials involve the use of the product candidate in human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be used. Each protocol must be submitted to the FDA as part of the IND. An independent IRB for each medical center proposing to conduct a clinical trial must also review and approve a plan for any clinical trial before it can begin at that center and the IRB must monitor the clinical trial until it is completed. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. Some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a data safety monitoring board or committee. This group provides authorization as to whether or not a trial may move forward at designated check points based on access that only the group maintains to available data from the study. Clinical testing also must satisfy extensive GCP requirements, including the requirements for informed consent. Information about clinical trials must be submitted within specific timeframes to the National Institutes of Health (“NIH”) for public dissemination on its ClinicalTrials.gov website.
For purposes of BLA submission and approval, clinical trials are typically conducted in three sequential phases, which may overlap or be combined. For Humacyte’s development of product candidates, Phase 1 and Phase 2 trials have heretofore been combined into a single trial design.
45
•Phase 1. The biological product is initially introduced into human subjects and tested for safety. These initial trials to evaluate the potential toxicity and pharmacological activity of the investigational product (including pharmacokinetics, if applicable), and, if possible, gain early evidence on effectiveness.
•Phase 2. The biological product is evaluated in a limited patient population to identify potential adverse events and safety risks, to evaluate preliminarily the efficacy of the product candidate for specific targeted indications in patients with the disease or condition under trial, and, when applicable, to evaluate dosage tolerance and appropriate dosage.
•Phase 3. The biological product is administered to an expanded patient population, often large numbers of patients of several hundred to several thousand and generally at geographically dispersed clinical trial sites. These trials are designed to generate enough data to statistically evaluate clinical effectiveness and safety as well as to establish the overall benefit-risk relationship of the investigational new biological product, and to provide an adequate basis for product approval. FDA typically requires at least two Phase 3 trials to support approval, but in some cases may approve an application on the basis of one trial.
In some cases, the FDA may condition approval of a BLA on the sponsor’s agreement to conduct additional clinical trials to further assess the biologic’s safety and effectiveness after BLA approval. Such post-approval clinical trials are typically referred to as Phase 4 clinical trials.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the progress of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators detailing serious and unexpected adverse events, any findings from other studies that suggest a significant risk to human patients, tests in laboratory animals or in vitro testing that suggest a significant risk for human patients, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information.
The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk, including risks inferred from other trials on other products. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the biological product has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal trials and must also develop additional information about the characteristics of the biologic and finalize a process for manufacturing the biologic in commercial quantities in accordance with cGMP and, when applicable, GTP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
BLA Review Process
The results of preclinical studies and of the clinical trials, together with other detailed information, including extensive manufacturing information, information on the composition of the biologic, and proposed labeling, are submitted to the FDA in the form of a BLA requesting approval to market the biologic in the United States for one or more specified indications. The FDA reviews a BLA to determine, among other things, whether a biologic is safe and effective for its intended use.
The FDA has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the FDA’s threshold determination that the application is sufficiently complete to permit substantive review. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe and potent, or effective, for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with cGMPs (and, where applicable, GTPs) to assure and preserve the product’s identity, safety, strength, quality, potency, and purity, and biological product standards. The FDA may refer applications for novel biological products or biological
46
products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes outside clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an application, the FDA will, among other things, inspect the facility or the facilities at which the biologic product is manufactured and distributed, and will not approve the product unless cGMP compliance is satisfactory. The FDA may also inspect the sites at which the clinical trials were conducted to assess their compliance, and may refuse to approve the biologic if compliance with GCP requirements is found to be unsatisfactory. For a human cellular or tissue product the FDA also may refuse to approve the product if the manufacturer is not in compliance with GTP requirements, in addition to cGMPs.
The FDA also has authority to require a Risk Evaluation and Mitigation Strategy (“REMS”) from manufacturers to ensure that the benefits of a biological product outweigh its risks. A sponsor may also voluntarily propose a REMS as part of the BLA submission. The need for a REMS is determined as part of the review of the BLA. Based on statutory standards, elements of a REMS may include “dear doctor letters,” a medication guide, more elaborate targeted educational programs, and in some cases restrictions on distribution and/or use. These elements are negotiated as part of the BLA approval, and in some cases may delay the approval date. Once adopted, REMS are subject to periodic assessment and modification.
The testing and approval processes require substantial time, effort and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all. Even if we believe a clinical trial has demonstrated safety and efficacy of one of our vessels for the treatment of a disease, the results may not be satisfactory to the FDA. Preclinical and clinical data may be interpreted by the FDA in different ways, which could delay, limit or prevent regulatory approval. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our vessels. The FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products.
Biologics may be marketed only for the FDA approved indications and in accordance with the provisions of the approved labeling. Further, if there are any modifications to the biologic, including changes in indications, labeling, or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new BLA or BLA supplement, which may require developing additional data or conducting additional preclinical studies and clinical trials. As with new BLAs, the review process is often significantly extended by FDA requests for additional information or clarification.
The Biologics Price Competition and Innovation Act (“BPCIA”), amended the PHSA to authorize the FDA to approve similar versions of innovative biologics, commonly known as biosimilars. A competitor seeking approval of a biosimilar must file an application to establish its product as highly similar to an approved innovator biologic, among other requirements. The BPCIA, however, bars the FDA from approving biosimilar applications for 12 years after an innovator biological product receives initial marketing approval. This bar does not apply to submission or approval of full BLAs. Because FDA has determined that our HAVs are regulated as biologics and require a BLA for marketing, we believe that our lead product will be entitled to 12 years of exclusivity upon approval. Nevertheless, the BPCIA is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty.
Expedited Development and Review Programs
The FDA offers various programs, including Fast Track designation, Breakthrough Therapy Designation, accelerated approval, priority review and RMAT designation, that are intended to expedite the process for the development and FDA review of biological products that are intended for the treatment of serious or life-threatening diseases or conditions. To be eligible for Fast Track designation, biological product candidates must be intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a biological product candidate may request the FDA to designate the biologic as a Fast Track product at any time during the clinical development of the product. The sponsor of a Fast Track product has opportunities for more frequent interactions with the applicable FDA review team during product development and, once a BLA is submitted, the product candidate may be eligible for priority review. A Fast Track product may also be eligible for rolling review, where the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the BLA, the FDA agrees to accept sections of the BLA and
47
determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the BLA.
A biological product candidate may be eligible for Breakthrough Therapy Designation if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product candidate, alone or in combination with one or more other drugs or biologics, may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough Therapy Designation provides all the features of Fast Track designation in addition to intensive guidance on an efficient development program beginning as early as Phase 1, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced review staff in a cross-disciplinary review, where appropriate.
Any marketing application for a biological product submitted to the FDA for approval, including a product candidate with a Fast Track designation and/or Breakthrough Therapy Designation, may be eligible for other types of FDA programs intended to expedite the FDA review and approval process, such as priority review and accelerated approval. Any product candidate is eligible for priority review if it is designed to treat a serious or life-threatening disease or condition, and if approved, would provide a significant improvement in safety or effectiveness compared to available alternatives for such disease or condition. The FDA will attempt to direct additional resources to the evaluation of an application for a biological product candidate designated for priority review in an effort to facilitate the review. Under priority review, the FDA’s goal is to review an application within six months of the 60-day filing date, compared to ten months for a standard review.
Additionally, FDA may grant accelerated approval to a product candidate intended to treat a serious or life-threatening disease or condition upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. Products receiving accelerated approval may be subject to expedited withdrawal procedures if the sponsor fails to conduct the required post-marketing studies or if such studies fail to verify the predicted clinical benefit. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
In 2017, the FDA established a new RMAT designation as part of its implementation of the 21st Century Cures Act. The RMAT designation program is intended to fulfill the 21st Century Cures Act requirement that the FDA facilitate an efficient development program for, and expedite review of, any biological product that meets the following criteria: (i) the biological product qualifies as an RMAT, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (ii) the biological product is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and preliminary clinical evidence indicates that the biological product has the potential to address unmet medical needs for such a disease or condition. RMAT designation provides all the benefits of Breakthrough Therapy Designation, including more frequent meetings with the FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Product candidates granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of clinical trial sites, including through expansion of trials to additional sites.
Fast Track designation, Breakthrough Therapy Designation, priority review, accelerated approval, and RMAT designation do not change the standards for approval but may expedite the development or approval process. Even if a product candidate qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Additionally, on December 12, 2017, Public Law No. 115-92 amended the FDCA to, among other things, allow the DoD to request, and FDA to provide assistance to expedite development and the FDA’s review of products to diagnose, prevent, treat or mitigate a specific and life-threatening risk to the U.S. military. Similar to the designations described above that FDA may grant, a priority designation by the DoD does not change the standards for approval but may expedite the development or approval process.
48
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a biological product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation must be requested before submitting a BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
Orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA, to market the same biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity, or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity.
A designated orphan drug many not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or, as noted above, if a second applicant demonstrates that its product is clinically superior to the approved product with orphan exclusivity or the manufacturer of the approved product is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Other U.S. Regulatory Requirements
For biologics that are human cells, tissues, and cellular and tissue-based products (“HTC/Ps”), manufacturers must also comply with the FDA’s HCT/P regulations at 21 C.F.R. Part 1271. These regulations impose a variety of specialized requirements as follows:
HCT/P registration and listing. Every establishment that manufactures an HCT/P must register with the FDA and provide a list of every HCT/P that the establishment manufactures. The definition of manufacture is broad and includes any and all steps in the recovery, processing, storage, labeling, packaging or distribution of any human cell or tissue and the screening or testing of the cell or tissue donor.
Donor eligibility. HCT/P manufacturers must maintain procedures for testing, screening and determining the eligibility of donors of cells and tissues used in HCT/Ps. An HCT/P may not be transferred or implanted into an individual until the donor has been determined to be eligible under these procedures. These procedures must involve, among other things, testing donors for certain communicable diseases and the use of quarantines for HCT/Ps that have not yet been shown to meet the eligibility requirements. Manufacturers must keep detailed records regarding donor eligibility determinations.
Current Good Tissue Practices. HCT/Ps must be recovered, processed, stored, labeled, packaged and distributed in a manner that is consistent with the FDA’s cGTP regulations. Cells and tissues must also be screened and tested according to these regulations. The goal of cGTPs is to prevent the introduction, transmission or spread of communicable diseases. The FDA’s cGTPs regulations require companies to establish a comprehensive quality program and to comply with rules related to personnel, facilities and equipment used to manufacture HCT/Ps, as well as rules on how these HCT/Ps are processed, labeled and stored. Companies must also keep detailed manufacturing records and product complaint files.
Adverse Reaction Reports. Manufacturers of nonreproductive HCT/Ps must investigate and report to the FDA certain adverse reactions.
Inspections. Establishments that manufacture HCT/Ps must allow the FDA to inspect the establishment and company records.
49
Post-Approval Requirements
Any biologics manufactured or distributed by us or our collaborators pursuant to FDA approvals would be subject to continuing post-approval regulation by the FDA, including recordkeeping requirements and reporting of adverse experiences associated with the product, as well as any post-marketing surveillance requested by the FDA as a condition to BLA approval. Manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, require us to recall our product from distribution or withdraw approval of the BLA for that product.
The FDA closely regulates the post-approval marketing and promotion of biologics to healthcare professionals, including standards and regulations for direct-to-consumer advertising, false or misleading claims, off-label promotion, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, and potential civil and criminal penalties. Physicians may prescribe legally available biologics for uses that are not described in the product’s labelling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
U.S. Healthcare Reform
Political, economic and regulatory influences are subjecting the healthcare industry in the United States to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals to significantly change the healthcare system. For example, the Patient Protection and Affordable Care Act (the “ACA”) was enacted to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. In December 2017, portions of the ACA dealing with the individual mandate insurance requirement were effectively repealed by the Tax Cuts and Jobs Act of 2017.
U.S. Third-Party Payor Coverage and Reimbursement
Although none of our vessels have been commercialized for any indication, if they are approved for marketing, commercial success of our vessels will depend, in part, upon the availability of coverage and reimbursement from third-party payors at the federal, state and private levels. Government payor programs, including Medicare and Medicaid, private health care insurance companies and managed-care plans have attempted to control costs by limiting coverage and the amount of reimbursement for particular procedures or treatments. The U.S. Congress and state legislatures from time to time propose and adopt initiatives aimed at cost-containment. Ongoing federal and state government initiatives directed at lowering the total cost of health care will likely continue to focus on health care reform and on the reform of the Medicare and Medicaid payment systems. Examples of how limits on coverage and reimbursement in the United States may cause reduced payments for products in the future include: changing Medicare reimbursement methodologies; fluctuating decisions on which drugs to include in formularies; allowing the federal government to negotiate drug prices for federal healthcare programs; revising drug rebate calculations under the Medicaid program; and reforming drug importation laws.
Some third-party payors also require pre-approval of coverage for new or innovative devices or therapies before they will reimburse health care providers who use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, the announcement or adoption of these proposals could have a material adverse effect on our ability to obtain adequate prices for our vessels and operate profitably. Significant cost containment pressure and downward pricing pressures exist in the U.S. and around the world, which may negatively affect reimbursement at any time.
50
Other Healthcare Laws and Regulations
We are also subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we conduct our business. The laws that may affect our ability to operate include but are not limited to:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
•federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
•the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (collectively, “HIPAA”), which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information;
•the federal Physician Payments Sunshine Act, which requires drug and device companies to annually report to CMS all payments and transfers of value provided to physicians and teaching hospitals for posting on a public website; and
•state law equivalents of many of the above federal laws, including anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers.
If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, the exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and impact our financial results.
International Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our future products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
EU Requirements Applicable to Medicinal Products
In the EU, medicinal products are subject to extensive pre-and post-market regulation by regulatory authorities at both the EU and national levels.
Clinical Trials
Clinical trials of medicinal products in the EU must be conducted in accordance with EU (previously, Directive 2001/20/EC applied; as of January 31, 2022, Regulation EU No 536/2014 applies) and national regulations and the International Conference on Harmonization (“ICH”) guidelines on GCP.
Prior to commencing a clinical trial, the sponsor must obtain a clinical trial authorization from the competent authority, and a positive opinion from an independent ethics committee of the relevant EU Member State in which the clinical trial will be carried out. Any substantial changes to the trial protocol or other information submitted with the clinical trial applications must be notified to or approved by the relevant competent authorities and ethics committees.
51
The sponsor of a clinical trial must register the clinical trial in advance, and certain information related to the clinical trial will be made public as part of the registration. The results of the clinical trial must be submitted to the competent authorities and, with the exception of non-pediatric Phase 1 trials, will be made public at the latest within 12 months after the end of the trial.
During the development of a medicinal product, the European Medicines Agency (“EMA”) and national medicines regulators within the EU provide the opportunity for dialogue and guidance on the development program. At the EMA level, this is usually done in the form of scientific advice, which is given by the Scientific Advice Working Party of the Committee for Medicinal Products for Human Use (“CHMP”). Advice is not legally binding with regard to any future marketing authorization application of the product concerned. To date, we have not initiated any scientific advice procedures with the EMA, but we have obtained confirmation from the EMA that our HAVs would be eligible for the EMA’s scientific advice procedures.
Marketing Authorizations
After completion of the required clinical testing, we must obtain a marketing authorization before we may place a medicinal product on the market in the EU. There are various application procedures available, depending on the type of product involved.
All application procedures require an application in the common technical document format, which includes the submission of detailed information about the manufacturing and quality of the product, and non-clinical and clinical trial information. There is an increasing trend in the EU towards greater transparency and, while the manufacturing or quality information is currently generally protected as confidential information, the EMA and national regulatory authorities are now liable to disclose much of the non-clinical and clinical information in marketing authorization dossiers, including the full clinical study reports, in response to freedom of information requests after the marketing authorization has been granted.
The centralized procedure gives rise to marketing authorizations that are valid throughout the EU. Applicants file marketing authorization applications with the EMA, where they are reviewed by a relevant scientific committee, in most cases the CHMP (although other specialist committees may also be involved; for example, the Committee for Advanced Therapies will also be involved in the review of advanced therapy medicinal products (“ATMP”), and HAVs could potentially be classified as an ATMP). The EMA forwards CHMP opinions to the European Commission, which uses them as the basis for deciding whether to grant a marketing authorization. The centralized procedure is compulsory for medicinal products that (1) are derived from biotechnology processes, (2) contain a new active substance (not yet approved on 20 November 2005) indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders, viral diseases or autoimmune diseases and other immune dysfunctions, (3) are orphan medicinal products or (4) are advanced therapy medicinal products. For medicines that do not fall within these categories, an applicant may voluntarily submit an application for a centralized marketing authorization to the EMA, as long as the CHMP agrees that (i) the medicine concerned contains a new active substance (not yet approved on November 20, 2005), (ii) the medicine is a significant therapeutic, scientific, or technical innovation, or if its authorization under the centralized procedure would be in the interest of public health.
For those medicinal products for which the centralized procedure is not available, the applicant must submit marketing authorization applications to the national medicines regulators through one of three procedures: (1) a national procedure, which results in a marketing authorization in a single EU member state; (2) the decentralized procedure, in which applications are submitted simultaneously in two or more EU member states; and (3) the mutual recognition procedure, in which the EU member states are required to grant an authorization recognizing an existing authorization in another EU member state, unless they identify a serious risk to public health.
Data Exclusivity
Marketing authorization applications for generic medicinal products do not need to include the results of preclinical and clinical trials, but instead can refer to the data included in the marketing authorization of a reference product for which regulatory data exclusivity has expired. If a marketing authorization is granted for a medicinal product containing a new active substance or to a different marketing authorization holder that has carried out a complete set of pre-clinical tests and clinical trials, that product benefits from eight years of data exclusivity, during which generic marketing authorization applications referring to the data of that product may not be accepted by the regulatory authorities, and a further two years of market exclusivity, during which such generic products may not be placed on the market. The two-year period may be extended to three years if during the first eight years a new therapeutic indication with significant clinical benefit over existing therapies is approved.
52
There is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product, for example, because of differences in raw materials or manufacturing processes. For such products, while a full set of pre-clinical tests and trials are not necessary, the results of appropriate preclinical or clinical trials must be provided, and guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product.
Pediatric Development
In the EU, companies developing a new medicinal product must agree to a Pediatric Investigation Plan (“PIP”) with the EMA and must conduct pediatric clinical trials in accordance with that PIP. The marketing authorization application for the product must ordinarily include the results of pediatric clinical trials conducted in accordance with the PIP. It is possible to obtain a deferral, in which case the pediatric clinical trials must be completed at a later date, or a complete waiver from the obligation to conduct pediatric clinical trials (e.g., because the relevant disease or condition occurs only in adults).
Post-Approval Controls
The holder of a marketing authorization is subject to various post-approval controls, such as obligations to maintain a pharmacovigilance system and report adverse reactions, and requirements relating to promotional activities, including a prohibition on the promotion of prescription medicines to the general public. Manufacturers/importers and distributors of medicinal products must obtain authorizations from the competent national authorities and are subject to periodic inspections for compliance with cGMPs and current good distribution practices (“cGDPs”), respectively. The regulatory authorities may also impose specific obligations as a condition of the marketing authorization, such as additional safety monitoring or the conduct of additional clinical trials or post-authorization safety studies.
EU Requirements Applicable to Medical Devices
Under the previous medical devices directive, Directive 93/42/EEC, our HAVs were not classified as medical devices in the EU because, with limited exceptions, products incorporating or derived from tissues or cells of human origin are expressly excluded from the scope of the EU medical devices rules under Directive 93/42. However, as of May 26, 2021, Regulation (EU) 2017/745 applies, and this will bring us within the scope of the EU medical device rules products containing or derived from tissues or cells of human origin that are non-viable or are rendered non-viable.
Medical devices are generally governed by Regulation (EU) 2017/745 on Medical Devices that directly applies in all EU Member States and harmonizes the conditions for placing medical devices on the EU market. This Regulation, however, does not regulate certain important marketing aspects, such as pricing and reimbursement, which remain governed by national law. Additionally, certain areas, such as advertising, may be governed by additional national requirements.
A medical device may be placed on the market within the EU if it conforms to certain “general product safety requirements” or “GSPRs.” These are general in nature and broad in scope. A fundamental GSPR, for example, is that a device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users or other persons.
The manufacturer is obliged to demonstrate that the device conforms to the relevant GSPRs through a conformity assessment procedure. Once the appropriate conformity assessment procedure for a medical device has been completed, the manufacturer must draw up a written declaration of conformity and affix the CE mark to the device. The device can then be marketed throughout the EU.
The nature of the conformity assessment depends upon the classification of the device. The classification rules are mainly based on three criteria: the length of time the device is in contact with the body, the degree of invasiveness, and the extent to which the device affects the anatomy. As a general rule, Class I (low risk) devices are those that do not enter or interact with the body; Class IIa and IIb (medium risk) devices are invasive or implantable or interact with the body; and Class III (high risk) devices are those that affect the vital organs.
Conformity assessment procedures for all but the lowest risk classification of device involve a notified body, which are non-governmental, private entities licensed to provide independent certification of certain classes of medical device. EU regulatory bodies are not involved in the premarket approval of medical devices, with only very limited exceptions (such as medical devices that incorporate a medicinal product as an ancillary substance, in which case these regulatory bodies
53
review the medicinal product). The onus of ensuring a device is safe enough to be placed on the market is ultimately the responsibility of the manufacturer and the notified body.
As part of the conformity assessment procedure, the manufacture will need to conduct a clinical evaluation of the device. This clinical evaluation may consist of an analysis of the scientific literature relating to similar devices, new clinical investigations of the device, or a combination of the two. For Class III and implantable devices, the conduct of clinical investigations is mandatory (with limited exceptions). If a manufacturer wishes to conduct a clinical investigation in the EU, the manufacturer must notify the competent national regulatory authorities in advance and obtain ethics committee approval of the study.
EU Requirements Applicable to Human Cells and Tissues
EU rules, notably Directive 2004/23/EC and other implementing directives, govern the donation, procurement, testing and storage of human cells and tissues intended for human application, whether or not they are medicinal products. These rules also cover the donation, testing, processing, preservation, storage and distribution of human cell and tissues that are not medicinal products. Establishments that conduct such activities must be licensed and are subject to inspection by regulatory authorities. Such establishments must implement appropriate quality systems and maintain appropriate records to ensure that cells and tissues can be traced from the donor to the recipient and vice versa. There are also requirements to report SAEs and reactions linked to the quality and safety of cells and tissues. More detailed rules may exist at the national level.
In addition to regulations in Europe and the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial distribution of our future products.
Facilities
Our corporate headquarters, manufacturing, and research and development facilities are located in Durham, North Carolina where we lease approximately 83,000 square feet of space. This space includes approximately 55,000 square feet for production and distribution operations including manufacturing, bioprocessing, quality control, mechanical space and inventory. The remainder of the facility consists of offices, laboratories, and common spaces.
Employees and Human Capital Management
As of December 31, 2022, we had 164 employees that were all full-time. None of our employees are represented by a collective bargaining agreement, and we have never experienced any work stoppage. We believe we have good relations with our employees.
Financing Arrangements
In March 2021, Legacy Humacyte entered into a Loan Agreement with Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P., which provides a term loan facility of up to $50.0 million with a maturity date of March 1, 2025. We became a co-borrower under the Loan Agreement in connection with the Merger. The obligations of Humacyte and Legacy Humacyte under the Loan Agreement are secured by substantially all of their assets, except for their intellectual property. In connection with the Loan Agreement, the lenders were each granted a warrant to purchase Legacy Humacyte common stock, with an exercise price of $2.699 per share, subject to customary adjustments. In connection with the closing of the Merger, these warrants were exchanged for warrants to purchase 287,704 shares of our common stock at an exercise price of $10.28 per share. On October 13, 2021, we borrowed an additional $10.0 million under the Loan Agreement, and in connection with that borrowing, issued Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P. warrants to purchase an aggregate of 123,302 shares of our common stock at an exercise price of $10.28 per share. The Loan Agreement contains certain customary covenants, including, but not limited to, those relating to additional indebtedness, liens, asset divestitures, and affiliate transactions. We may use the proceeds of borrowings under the Loan Agreement as working capital and to fund our general business requirements.
As of February 28, 2023, we had borrowed $30.0 million of principal under the Loan Agreement.
Additional Information
We were incorporated in Delaware on July 1, 2020, under the name Alpha Healthcare Acquisition Corp. (“AHAC”), in order to effectuate a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses or entities. AHAC completed its initial public offering on September 22, 2020.
54
On August 26, 2021, AHAC and Legacy Humacyte consummated the transactions contemplated by the Merger Agreement. In connection with the closing of the Merger, we changed our name to Humacyte, Inc.
Our principal executive office is located at 2525 East North Carolina Highway 54, Durham, North Carolina 27713, and our telephone number is (919) 313-9633.
Our website address is www.humacyte.com and our investor relations website is located at https://investors.humacyte.com. The information posted on our website is not incorporated into this Annual Report on Form 10-K. The U.S. Securities and Exchange Commission (“SEC”) maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at http://www.sec.gov. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) are also available free of charge on our investor relations website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
We provide notifications of news or announcements regarding our financial performance, including SEC filings, investor events, and press releases, as part of our investor relations website. The contents of these websites are not intended to be incorporated by reference into this report or in any other report or document we file.
Item 1A. Risk Factors
Our operations and financial results are subject to a high degree of risk. These risks include, but are not limited to, those described below, each of which may have a material and adverse effect on our business, prospects, operating results, financial condition and the trading price of our common stock. You should carefully consider the risks described below, together with all of the other information included in this Annual Report on Form 10-K. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business. In that event, the trading price of our common stock could decline and you could lose all or part of your investment.
Summary of Risk Factors
The following is a summary of the principal risks to which our business, operations and financial performance are subject. Each of these risks is more fully described in the individual risk factors immediately following this summary.
•We have never generated product revenue and have incurred significant losses to date. We expect to continue to incur losses for the foreseeable future and may never generate product revenue or be profitable. We will need to raise additional capital to finance our operations, which we may not be able to do on acceptable terms or at all.
•If our clinical trials fail to demonstrate safety and efficacy to the satisfaction of the FDA or similar regulatory authorities outside the United States or do not otherwise produce favorable results, we may incur significant additional costs or experience significant delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
•Our near-term prospects are dependent on the success of our 6 millimeter HAV, and if we are unable to successfully develop and commercialize it, our business, operating results and financial condition will be materially harmed.
•We may experience delays or difficulties in the enrollment of patients in our clinical trials, which may delay or prevent additional clinical trials and our receipt of necessary marketing approvals.
•Lack of experience by investigators and surgeons with our HAVs can lead to incorrect implantation or follow-up procedures which could harm the results of our clinical trials and market acceptance of our HAVs, if approved.
•We may not be successful in our efforts to use our proprietary scientific technology platform to build a pipeline of additional product candidates.
•Even if our HAVs receive marketing approval in the future for one or more of our product candidates, they may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
55
•The sizes of the market opportunities for our product candidates have not been established with precision and are estimates that management believes to be reasonable. If these market opportunities are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the relevant patient population, our revenue and ability to achieve profitability might be materially and adversely affected.
•Our distribution agreement with Fresenius Medical Care imposes obligations on us that may restrict our ability to operate our business in ways we believe to be in our long-term best interest.
•If we receive approval for a product candidate that is not subject to our distribution agreement with Fresenius Medical Care, and we are unable to establish our own marketing, sales and distribution capabilities or are unable to enter into agreements with third parties do so, we may not be able to generate product revenue and will have to alter our development and commercialization plans.
•The ongoing effects of the COVID-19 pandemic may continue to adversely impact our business, including our manufacturing efforts and clinical trials.
•The manufacture of our product candidates is complex, we have not manufactured commercial product, and we may encounter difficulties in production. If we or any third-party manufacturer encounter such difficulties, our ability to supply our product candidates for clinical trials or, if approved, for commercial sale could be delayed or halted entirely.
•The terms of our existing indebtedness may limit our ability to incur future debt.
•We rely on third parties to conduct and support our clinical trials, and those third parties may not perform satisfactorily, including by failing to adhere to regulatory requirements or our stated protocols or to meet deadlines for the completion of such trials.
•We rely on third-party suppliers, including sole source suppliers, to provide certain components for our product candidates. Any failure by a third-party supplier to supply these components for manufacture may delay or impair our ability to complete our clinical trials and to commercialize our product candidates.
•We intend to rely on our strategic, global partnership with Fresenius Medical Care to undertake, or assist with, the marketing, sale and distribution of certain of our product candidates in certain markets if we receive marketing approval from relevant regulatory authorities. Disruption of this arrangement could materially adversely affect our business, prospects, operating results and financial condition.
•Our ability to successfully commercialize our products may be impaired if we are unable to obtain and maintain effective intellectual property rights for our proprietary scientific technology platform and product candidates.
•We may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
Risks Related to the Development and Commercialization of Our Product Candidates
If our clinical trials fail to demonstrate safety and efficacy to the satisfaction of the FDA or similar regulatory authorities outside the United States or do not otherwise produce favorable results, we may incur significant additional costs or experience significant delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates. If we experience significant delays or significant additional costs, our business will be materially harmed.
Before obtaining marketing approval for any of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive and time-consuming, and its outcomes are uncertain. We believe the novelty of our research and development efforts, which are focused on the development of bioengineered human, acellular, tissue-based vessels for use across a wide spectrum of applications in vascular surgery, augments this uncertainty. The scientific discoveries that form the basis for our efforts to develop our product candidates are relatively new, and the scientific evidence to support the feasibility of developing product candidates based on these discoveries is both preliminary and limited. At this time, no products based on HAVs have been approved in the United States or in Europe. The clinical trial requirements of the FDA and other regulatory agencies and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to
56
the type, complexity, novelty and intended use and market of the potential product, and we may not succeed in obtaining marketing approval even if we view our clinical trials as successful. Data obtained from preclinical and clinical activities, and manufacturing comparability studies, are also subject to varying interpretations, which may delay, limit or prevent marketing approval. In such circumstances, we could experience significant delays, or be prevented from, developing or commercializing our HAVs, and our business, prospects, operating results and financial condition could be materially harmed.
Our V006 trial did not meet its primary endpoint, and if we fail to achieve the primary endpoint of our other ongoing or future clinical trials, or if safety issues arise, or the results from our clinical trials are otherwise inadequate to support regulatory approval of our product candidates, we may incur significant additional costs or experience significant delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates. Even if our clinical trials achieve their primary endpoints, the FDA may still determine that such trials do not adequately establish the safety and effectiveness of our products. In such a circumstance, the FDA may require that we design and conduct new, additional clinical trials to demonstrate safety and effectiveness, or may determine not to approve our products at all.
Even if we receive FDA approval for our HAVs, we may face a number of difficulties if the results of our clinical trials are unfavorable, inconclusive, or only modestly favorable or if there are safety concerns, such as adverse events (“AEs”) or serious adverse events (“SAEs”), which could include clotting, mechanical failure, immunological rejection or infection, that could outweigh potential benefits associated with such product candidates. This could result in:
•obtaining approval for indications or patient populations that are not as broad as intended or desired;
•obtaining approval with, or later becoming subject to, labelling that includes significant use or distribution restrictions or significant safety warnings;
•being subject to a REMS or equivalent requirement from a comparable foreign regulatory agency, to ensure that the benefits of a biological product outweigh its risks or to change the way the product is used;
•being required to perform additional clinical trials to support approval or comparability or being subject to additional post-marketing testing requirements;
•having regulatory authorities withdraw their approval of the product;
•being sued; or
•suffering damage to our reputation.
Any of these events could cause us to incur significant additional costs, significant delays and prevent us from achieving or maintaining market acceptance of or commercializing one or more of our product candidates.
If we experience failures or delays in our preclinical and clinical programs, we would be prevented from developing and commercializing our product candidates in a timely matter, if at all.
A number of factors impact the timing of our preclinical and clinical programs and the development and commercialization of our product candidates. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. Events that prevent successful or timely completion of the development of our product candidates beyond unfavorable or inconclusive clinical trial results include, among others, the following:
•delays in the testing, validation, manufacturing or delivery of our product candidates to the clinical sites;
•delays in reaching — or inability to reach — agreement with the FDA or other regulatory agencies on trial design;
•delays in reaching agreement on acceptable terms with prospective clinical research organizations (“CROs”) and clinical trial sites;
•delays in obtaining required IRB approval at each clinical trial site;
•delays in recruiting suitable patients in sufficient volume to participate in our clinical trials and in having those patients complete participation in our clinical trials or return for follow-up, including delays related to the ongoing effects of the COVID-19 pandemic;
57
•the occurrence of SAEs associated with any of our product candidates that are viewed to outweigh their potential benefits;
•imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical trial sites;
•failure by CROs, other third parties or us to adhere to clinical trial requirements;
•failure to perform in accordance with the FDA’s good clinical practices (“GCP”) or current good tissue practices (“cGTP”), or applicable regulatory guidelines in other countries;
•clinical trial sites dropping out of, or being removed from, a trial; or
•changes in regulatory requirements and guidance that require amending or submitting new clinical protocols or data.
Any inability to successfully complete development of our product candidates would likely result in significant additional costs to us, create delays in filing a BLA for regulatory approval of our product candidates and impair our ability to generate revenue. Clinical trial delays could also allow our competitors to bring products to market before we do, which could materially impair our ability to successfully commercialize our product candidates and may harm our business and prospects.
Our progress in early stage clinical trials may not be indicative of long-term efficacy in late stage clinical trials, and our progress in trials for one product candidate may not be indicative of progress in trials for another product candidate.
The product candidates in our pipeline are at various stages of development. Trial designs and results from previous studies are not necessarily predictive of our future clinical trial designs or results, and initial results of ongoing trials may not be confirmed upon full analysis of the complete trial data. A number of companies in the biotechnology industry have suffered significant setbacks in late-stage clinical trials even after achieving promising results in earlier stage clinical trials, and we may experience similar setbacks. Favorable results in clinical trials for one of our product candidates also do not necessarily indicate that we will obtain positive results in clinical trials related to other product candidates. The novelty of our proprietary scientific technology platform adds another layer of risk that early-stage clinical trials may not be indicative of long-term efficacy in our late-stage clinical trials. If we are unable to demonstrate favorable results in future clinical trials for our various product candidates, we expect that our business, prospects, operating results and financial condition will be materially adversely affected.
Additionally, several of our past, planned and ongoing clinical trials utilize an “open-label” trial design. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate. Some open-label clinical trials test only the investigational product candidate without a comparator. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. Open-label clinical trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The results from an open-label trial may not be predictive of future clinical trial results with any of our product candidates when studied in an environment with an active control.
Interim, “topline,” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary or topline data from our clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, although we may not have received or had the opportunity to fully and carefully evaluate all data at the time such preliminary or topline results are released. As a result, the topline or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, topline data should be viewed with caution until the final data are
58
available. From time to time, we may also disclose interim data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available, or as patients from our clinical trials continue other treatments for their disease. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects.
If the interim, topline, or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition. In addition, the information we choose to publicly disclose regarding a particular clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If SAEs occur at an unacceptable rate or other unacceptable side effects are identified in our HAVs we may need to delay, abandon or limit development and marketing of our product candidates.
Our HAVs may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining marketing approval. The reported SAEs related to the HAV for hemodialysis access, a patient population which typically has a high prevalence of existing medical conditions, are detailed in the table below which summarizes results from our V006 HUMANITY Phase 3 study in which subjects were randomized to receive either a HAV or a commercially available expanded polytetrafluoroethylene (“ePTFE”) graft.
SAEs Reported in V006 Phase 3 Clinical Study in AV Access
| Number of SAEs (% of total subjects) | ||||||||||||||
| Description of SAE | HAV | ePTFE | ||||||||||||
| Number of subjects in V006 study | 177 | 178 | ||||||||||||
| General disorders and administration conditions: | ||||||||||||||
| Implant site extravasation | 0(0.0)% | 1(0.6)% | ||||||||||||
| Infections and infestations | ||||||||||||||
| Vascular access site infection | 0(0.0)% | 5(2.8)% | ||||||||||||
| Injury, poisoning and procedural complications: | ||||||||||||||
| Anastomotic stenosis | 1(0.6)% | (0.0)% | ||||||||||||
| Description of SAE | Number of SAEs (% of total subjects) | |||||||||||||
| HAV | ePTFE | |||||||||||||
| Vascular access site hematomas | 1(0.6)% | (0.0)% | ||||||||||||
| Vascular access site hemorrhage | 0(0.0)% | 3(1.7)% | ||||||||||||
| Vascular access site pain | 1(0.6)% | 0(0.0)% | ||||||||||||
| Vascular access site pseudoaneurysm | 10(5.6)% | 0(0.0)% | ||||||||||||
| Vascular access site rupture | 2(1.1)% | 0(0.0)% | ||||||||||||
| Vascular access site thrombosis | 41(23.2)% | 28(15.7)% | ||||||||||||
| Skin and subcutaneous tissue disorders: | ||||||||||||||
| Skin necrosis | 0(0.0)% | 1(0.6)% | ||||||||||||
| Vascular disorders: | ||||||||||||||
| Steal syndrome | 2(1.1)% | 2(1.1)% | ||||||||||||
| Subclavian vein occlusion | 0(0.0)% | 1(0.6)% | ||||||||||||
| Vascular stenosis | 34(19.2)% | 27(15.2)% | ||||||||||||
| Venous stenosis | 3(1.7)% | 9(0.0)% | ||||||||||||
59
In our V002 and V004 Phase 2 clinical studies in PAD in 35 subjects, another patient population which typically has a high prevalence of existing medical conditions, the SAEs reported for the HAV are detailed in the table below.
SAEs Reported in V002 and V004 Phase 2 Clinical Studies in PAD
| Description of SAE | Number of SAEs (% of total subjects) | |||||||
Number of subjects in V002 and V004 studies | N=35 | |||||||
Arterial bypass thrombosis | 3(8)% | |||||||
Anastomotic stenosis | 1(3)% | |||||||
Graft thrombosis | 2(6)% | |||||||
Vascular graft complication | 1(3)% | |||||||
If our HAVs are associated with undesirable side effects in clinical trials or have negative characteristics that are unexpected, we may need to perform additional clinical trials, abandon their development or limit development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Even if one of our product candidates is approved, the FDA and other regulatory authorities may take action to withdraw it from the market if serious safety concerns emerge. Any of these events could cause us to delay, abandon or limit the development and, if approved, marketing of our product candidates.
We may experience delays or difficulties in the enrollment of patients in our clinical trials, which may delay or prevent additional clinical trials and our receipt of necessary marketing approvals.
We are currently enrolling patients in several clinical trials, including in our V005 trial, which is a Phase 2/3 clinical trial of our 6 millimeter HAV in traumatic vascular repair and our V007 trial, which is a Phase 3 clinical trial comparing the safety and efficacy of our 6 millimeter HAV to AV fistula for hemodialysis access. Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends in part on the rate at which we can recruit patients to participate in such trials. Additionally, the COVID-19 pandemic has had and may continue to have a sustained impact on our ability to recruit and follow up with patients. We may not be able to initiate or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA and other regulatory authorities, and as such our product candidates could be delayed or otherwise adversely affected. Patient enrollment and trial completion is affected by many factors including the:
•size of the patient population and process for identifying subjects;
•availability of clinical trial research resources at clinical sites due to ongoing effects of the COVID-19 pandemic;
•design of the trial protocol;
•inclusion and exclusion criteria;
•safety profile to date of the product candidate under study;
•perceived risks and benefits of the product candidate under study;
•availability of competing therapies and clinical trials;
•severity of the disease under investigation;
•degree of progression of the subject’s disease at the time of enrollment;
•proximity and availability of clinical trial sites for prospective subjects;
•the ongoing impact of the COVID-19 pandemic or future pandemics or similar events on patients’ willingness and ability to participate in clinical trials or on study site policies;
•ability to obtain and maintain subject consent;
•risk that enrolled subjects will drop out before completion of the trial;
60
•patient referral practices of physicians; and
•ability to monitor subjects adequately during and after treatment.
If we have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay, limit or terminate ongoing or planned clinical trials, any of which would have an adverse effect on our business, financial condition, results of operations and prospects.
Lack of experience by investigators and surgeons with our HAVs can lead to incorrect implantation or follow-up procedures which could harm the results of our clinical trials and market acceptance of our HAVs, if approved.
Our HAVs are currently in various stages of preclinical and clinical testing and have not been widely used. We do not have the personnel capacity to directly conduct or manage all of the clinical trials that are necessary for the development of our HAVs. Therefore, we rely, and will continue to rely, on third parties to assist us in managing, monitoring and conducting our clinical trials. Some of the investigators in our clinical trials have not been, and, if our HAVs receive marketing approval, surgeons may not be, previously exposed to the implantation and follow-up procedures related to their use. As a result, our HAVs may be, and have been in the past, incorrectly implanted and follow-up procedures may be performed incorrectly, resulting in violations of our trial protocols, increased interventions or failure of the HAV. Our efforts to educate investigators, surgeons and interventionalists regarding the proper techniques for use of our HAVs both during clinical trials and following potential commercialization may be costly, prove unsuccessful and could materially harm our ability to continue the clinical trials or marketing of our HAVs. Regulatory authorities may also seek to impose restrictive labeling or proactive communication obligations on any marketing approval granted for use of our HAVs as a result, which could reduce market acceptance of any of our HAVs that receive marketing approval.
Our near-term prospects are dependent on the success of our 6 millimeter HAV, and if we are unable to successfully develop and commercialize it, our business, operating results and financial condition will be materially harmed.
We currently have no products approved for sale and, while we are developing a number of product candidates, we have invested and continue to invest a substantial portion of our efforts and financial resources in the development of our 6 millimeter HAV. None of our remaining product geometries and modifications have advanced beyond preclinical development. As a result, in the near term we are dependent on the success of our 6 millimeter HAV, and if we are unable to successfully develop, obtain marketing approval for, and commercialize it, our business, along with our operating results and financial condition, will be materially harmed. Even if we succeed with the development of our 6 millimeter HAV, our ability to generate product revenue and become profitable from our 6 millimeter HAV depends on our assumptions regarding the relevant market opportunity and the degree of market acceptance for our products, once approved, for which our estimates may prove inaccurate, and market acceptance in any approved indication, which may never occur.
We may not be successful in our efforts to use our proprietary scientific technology platform to build a pipeline of additional product candidates.
A key element of our strategy is to use our proprietary scientific technology platform to expand our pipeline of HAVs and to progress other product candidates into and through clinical development. We may not be able to identify or develop future product candidates that are safe and effective. Even if we are successful in building our pipeline, the potential product candidates that we identify may not be suitable for clinical development, including if they have harmful side effects or other characteristics that render them unlikely to receive marketing approval or achieve market acceptance. Research programs to identify new product candidates require substantial technical, financial and human resources, and we may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. If we do not successfully develop and commercialize additional product candidates based upon our technology, we may have difficulty generating product revenue in the future, which could result in significant harm to our business, prospects, operating results and financial condition and adversely affect our stock price.
Even if our HAVs receive marketing approval in the future for one or more of our product candidates, they may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
Even with the requisite approvals from the FDA in the United States, the European Commission in the EU and other regulatory authorities internationally, the commercial success of our HAVs will depend, in part, on the acceptance of physicians, patients and health care payors, as medically necessary, cost-effective and safe. Any product that we commercialize may not gain acceptance by physicians, patients, health care payors and others in the medical community
61
due to ethical, social, medical and legal concerns. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable.
The degree of market acceptance of any of our product candidates that receives marketing approval will depend on a number of factors, including:
•the efficacy and potential advantages of our product candidates compared with alternative products or methods, including convenience and ease of administration;
•the prices we charge for our products, if approved;
•the availability of third-party coverage and adequate reimbursement;
•the willingness of the target patient population to try new products and methods and of physicians to use these products and methods;
•the quality of our relationships with patient advocacy groups;
•the strength of marketing and distribution support;
•the availability of the product and our ability to meet market demand;
•the prevalence and severity of any side effects; and
•any restrictions on the use of our products, if approved.
The sizes of the market opportunities for our product candidates have not been established with precision and are estimates that management believes to be reasonable. If these market opportunities are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the relevant patient population, our revenue and ability to achieve profitability might be materially and adversely affected.
Our estimates of the market opportunity for certain of our product candidates are based on a number of internal and third-party estimates. While we believe our assumptions and the data underlying these estimates are reasonable, they may be inaccurate or based on imprecise data. In addition, the assumptions and conditions underlying the estimates may change at any time. For example, the number of patients who ultimately use our product candidates, if approved by regulatory authorities, and our total market opportunities for such product candidates, will depend on, among other things, pricing and reimbursement, market acceptance of those product candidates and patient access, and may be lower than we estimate. Additionally, any approval we receive for our product candidates may be based on a narrower definition of the relevant patient population than we have estimated. Either of these circumstances could materially harm our business, financial condition, results of operations and prospects.
We face and will continue to face substantial competition, which may result in others discovering, developing or commercializing competing products before or more successfully than we do, which may adversely affect our ability to successfully market or commercialize our HAVs.
The development and commercialization of new biological products is highly competitive and subject to rapid change and technological advancements. If approved, we expect our HAVs would compete with the use of a patient’s own blood vessels, as well as a variety of marketed products, such as conventional synthetic grafts, xenografts, and allografts, as well as developing technologies.
We expect to face competition with respect to any additional product candidates that we may seek to develop or commercialize in the future from a variety of sources, including major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies, hospital product-focused companies, as well as public and private universities and research organizations.
Many of our existing or potential competitors, either alone or with their strategic partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing approvals and marketing and commercializing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also
62
compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than the products that we develop. Our competitors also may obtain FDA or other marketing approval for their products more rapidly than we may obtain the same approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
We plan to seek marketing approval for our HAVs in the United States as a biologic and in the EU as a medicinal product. In both the United States and the EU, our competitors may try to market vascular conduits similar to our product candidates as medical devices. Such competitive products could have comparable characteristics and could function similarly in the body (and could even be protein-based like our product candidates). Companies may be able to obtain marketing approval for such products on the basis of less data than the data required for a BLA and marketing similar products as devices could permit our competitors to circumvent regulatory exclusivity for biologics in the United States and medicinal products in the EU.
Our distribution agreement with Fresenius Medical Care imposes obligations on us that may restrict our ability to operate our business in ways we believe to be in our long-term best interest.
We expect to rely on our strategic, global relationship with Fresenius Medical Care for the development and commercialization of certain of our product candidates. As discussed in more detail in the section of this Annual Report on Form 10-K titled “Business — Distribution — Distribution Agreement with Fresenius Medical Care,” Fresenius Medical Care will have the exclusive right to develop outside of the United States and EU and commercialize outside of the United States, among other things, our 6 millimeter x 42 centimeter HAV and all improvements thereto, and modifications and derivatives thereof (including any changes to the length, diameter, or configuration of the foregoing), which we refer to as the distribution product, for use in vascular creation, repair, replacement or construction (including renal replacement therapy for dialysis access, the treatment of vascular trauma, and the treatment of PAD, but excluding coronary artery bypass graft, pediatric heart surgery, or adhering pancreatic islet cells onto the outer surface of the distribution product for use in diabetic patients). We refer to these indications wherein Fresenius Medical Care has rights to develop and commercialize Humacyte’s products as the field. The distribution agreement also imposes a number of restrictions on our business. For instance, outside the United States, the distribution agreement restricts our ability to engage a distributor for the distribution product outside the field or for HAV products other than the distribution product: we have granted Fresenius Medical Care (i) an exclusive right of first negotiation for exclusive distribution rights outside the United States for the distribution product for use outside the field, and (ii) an exclusive right of first negotiation for exclusive distribution rights outside the United States for our other HAV products, if any, subject, in each case, to certain conditions. These and other obligations may restrict our ability to operate our business in ways we believe are in our long-term best interest, which could harm our business and our prospects.
If we receive approval for a product candidate that is not subject to our distribution agreement with Fresenius Medical Care, and we are unable to establish our own marketing, sales and distribution capabilities or are unable to enter into agreements with third parties do so, we may not be able to generate product revenue and will have to alter our development and commercialization plans.
We currently have limited internal marketing, sales or distribution capabilities, and our management team has limited experience commercializing products following marketing approval. If one of our product candidates that is not subject to the distribution agreement with Fresenius Medical Care receives marketing approval, we will be required either to develop these capabilities internally or to make arrangements with third parties for the marketing, sales and distribution of the relevant product candidate. The establishment and development of our own marketing, sales and distribution functions will be expensive and time-consuming and may delay any product launch, and we may ultimately be unable to successfully develop the product candidate. In addition, or in the alternative, we could seek one or more partners to handle some or all of the marketing, sales and distribution activities associated with any such product candidate. However, we may face significant competition in seeking appropriate strategic partners, and the negotiation process is time consuming and complex. Therefore, we may not be able to enter into arrangements with third parties to do so on favorable terms or at all. In the event we are unable to develop our own marketing, sales and distribution functions or collaborate with a third-party organization for this purpose, we may not be able to successfully commercialize a product candidate that is not subject to the distribution agreement with Fresenius Medical Care, which would adversely affect our ability to generate revenue. Further, whether we commercialize any such product candidate on our own or rely on a third party to do so, our ability to generate revenue will be dependent on the effectiveness of the organization performing these functions.
63
Even if we receive marketing approval for our HAVs, there is uncertainty with respect to third-party coverage and reimbursement of our HAVs. They may also be subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, any of which could harm our business, prospects, operating results and financial condition.
There is uncertainty around third-party coverage and reimbursement of newly approved regenerative medicine type products, even those with the RMAT designation from FDA, such as our 6 millimeter HAV for AV access for performing hemodialysis, which received the RMAT designation in 2017. In the United States, third-party payors, including government payors such as the Medicare and Medicaid programs, play an important role in determining the extent to which medical products and biologics will be covered and reimbursed. The Medicare and Medicaid programs increasingly are used as models for how private payors and government payors develop their coverage and reimbursement policies. Currently, no RMAT tissue engineered product has established coverage and reimbursement by the CMS. Even if our HAVs receive approval from regulatory authorities, it is difficult to predict what CMS or any comparable foreign regulatory agency will decide with respect to coverage and reimbursement for novel products such as ours, as there is no body of established practices and precedents for these types of products.
The healthcare industry is acutely focused on cost containment, both in the United States and elsewhere. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives could also cause us to decrease any price we might establish for products, which could result in lower than anticipated product revenue. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including our costs related to research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the product and the clinical setting in which it is used. If the prices for our products, if any, decrease or if governmental and other third-party payors do not provide adequate coverage or reimbursement, our business, prospects, operating results and financial condition will suffer, perhaps materially.
On August 16, 2022, President Biden signed the IRA into law, which sets forth meaningful changes to drug product reimbursement by Medicare. Among other actions, the IRA permits HHS to engage in price-capped negotiation to set the price of certain drugs and biologics reimbursed under Medicare Part B and Part D. The IRA contains statutory exclusions to the negotiation program, including for certain orphan designated drugs for which the only approved indication (or indications) is for the orphan disease or condition. Should our product candidates be approved and covered by Medicare Part B or Part D, and fail to fall within a statutory exclusion, such as that for an orphan drug, those products could, after a period of time, be selected for negotiation and become subject to prices representing a significant discount from average prices to wholesalers and direct purchasers. The IRA also establishes a rebate obligation for drug manufacturers that increase prices of Medicare Part B and Part D covered drugs at a rate greater than the rate of inflation. The inflation rebates may require us to pay rebates if we increased the cost of a covered Medicare Part B or Part D approved product faster than the rate of inflation. In addition, the law eliminates the “donut hole” under Medicare Part D beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and requiring manufacturers to subsidize, through a newly established manufacturer discount program, 10% of Part D enrollees’ prescription costs for brand drugs below the out-of-pocket maximum and 20% once the out-of-pocket maximum has been reached. Our cost-sharing responsibility for any approved product covered by Medicare Part D could be significantly greater under the newly designed Part D benefit structure compared to the pre-IRA benefit design. Additionally, manufacturers that fail to comply with certain provisions of the IRA may be subject to penalties, including civil monetary penalties. The IRA is anticipated to have significant effects on the pharmaceutical industry and may reduce the prices we can charge and reimbursement we can receive for our products, among other effects.
Any reduction in reimbursement from Medicare resulting from the IRA or other legislative or policy changes, or from other government programs may result in a similar reduction in payments from private payers. These healthcare reforms and the implementation of any future cost containment measures or other reforms may prevent us from being able to generate sufficient revenue, attain and/or maintain profitability or commercialize our drug candidates. We cannot be sure whether additional legislative changes will be enacted, or the effect of forthcoming guidance implementing the IRA, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on our product candidates or the marketing approvals of our product candidates, if any, may be.
In some countries, particularly in Europe, the pricing of our product may be subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a
64
clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products, if approved, is unavailable or more limited in scope or amount than we anticipate, or if pricing is set at even lower levels than we anticipate, our business could be harmed, possibly materially.
Product liability lawsuits against us could cause us to incur substantial liabilities that may not be covered by our limited product liability insurance and may limit development, approval and commercialization of our HAVs and any other product candidates that we develop in the future.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk, if and when we commercially sell our HAVs and any other product candidates that we may develop. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
•decreased demand for any product candidates or products that we develop or sell, leading to loss of revenue;
•injury to our reputation and significant negative media attention;
•withdrawal, or slower enrollment, of clinical trial participants;
•significant costs to defend the related litigation and reduced resources of our management to pursue our business strategy;
•substantial monetary awards to trial participants or patients; and
•inability to further develop or commercialize our product candidates.
We currently hold limited product liability insurance coverage, and it may not be adequate to cover all liabilities that we may incur. We also may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
The ongoing effects of the COVID-19 pandemic may continue to adversely impact our business, including our manufacturing efforts and clinical trials.
The ongoing effects of the COVID-19 pandemic has impacted our business and we expect it to continue to do so. We have experienced delays in the ongoing enrollment of our clinical trials as a result of COVID-19. If there is a resurgence of COVID-19 in the United States and elsewhere, we may experience disruptions that could severely impact our business and clinical trials, including:
•further delays or difficulties in enrolling patients in our clinical trials;
•delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
•delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials, including interruption in global shipping that may affect the transport of clinical trial materials;
•shortages of clinical trial site and hospital and clinic staff supporting the conduct of our clinical trials;
•risk that participants enrolled in our clinical trials will acquire COVID-19 while the clinical trial is ongoing, or will withdraw from the clinical trial due to concerns over COVID-19, which could impact the results of the clinical trial, including by increasing the number of observed adverse events, or reducing the statistical power of the clinical trials;
•delays in necessary interactions with regulators, ethics committees and other important agencies and contractors due to limitations in employee resources;
•changes to the clinical endpoints, statistical analysis plan, or enrollment plans for ongoing clinical trials due to limitations in patients, resources, or sites, including due to COVID-19; and
•unanticipated deaths of clinical trial patients due to COVID-19 or due to lack of healthcare resources and follow-up as a consequence of COVID-19.
65
The extent to which ongoing effects of the COVID-19 pandemic impacts our business and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence.
Risks Related to Manufacturing Our Product Candidates
The manufacture of our product candidates is complex, we have not manufactured commercial product, and we may encounter difficulties in production. If we or any third-party manufacturer encounter such difficulties, our ability to supply our product candidates for clinical trials or, if approved, for commercial sale could be delayed or halted entirely.
The process of manufacturing our HAVs is complex, highly regulated and subject to multiple risks. The manufacture of biologics such as our HAVs has been, and continues to be, susceptible to product loss due to contamination, equipment failure, temporary power outages, improper installation or operation of equipment, damage to facilities, vendor or operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes has resulted, and could in the future result, in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination, which would harm our business, operating results and financial condition as well as our reputation. We depend on cell banks in our manufacturing process, and the loss of our master cell banks would result in significant disruptions to that process.
We currently manufacture the 6 millimeter HAVs for our clinical trials at our manufacturing facility in Durham, North Carolina, where we have created a scalable modular manufacturing process, which we refer to as the LUNA200 system, that we believe will enable us to manufacture our HAVs, if approved, in commercial quantities in compliance with current good manufacturing practices (“cGMPs”). Our efforts to scale out our manufacturing operations may not succeed. Scaling out a biologic manufacturing process is a difficult task, as there are risks including, among others, cost overruns, process reproducibility, stability issues, lot consistency and timely availability of raw materials. Prior to the establishment of our internal manufacturing facility, we employed a contract manufacturer who produced our HAVs using a smaller-production system known as the AURA system. We have limited years of experience manufacturing our HAVs in-house with the LUNA200 system, and no experience manufacturing the volume that we anticipate will be required to supply all of our clinical trials or to achieve planned levels of commercial sales following marketing approval, if received. Additionally, our manufacturing process has evolved over time and we may not have the experience, resources, or facility capacity to handle adoption of future changes or expansion of capacity. The forecasts of demand we plan to use to determine order quantities and lead times for components from outside suppliers may be incorrect, and we may be unable to obtain such components when needed and at a reasonable cost. We also may experience interruptions in the supply of the raw materials required to manufacture our product candidates, or increased costs due to supply chain disruptions or inflation in the cost of goods, services or other operating inputs. Likewise, supply chain interruptions could affect the transport of clinical trial materials, such as our HAVs and other supplies used in our clinical trials, which would negatively impact our ability to conduct our clinical trials. In addition, we may not be able to develop and implement efficient manufacturing capabilities and processes to manufacture our HAVs in sufficient volumes that also satisfy the legal, regulatory, quality, price, durability, engineering, design and production standards required to commercialize our HAVs successfully.
If we are unable to produce sufficient quantities of our HAVs for our clinical trial needs or commercialization due to production system limitations, we may need to make additional changes to our manufacturing processes and procedures. Such changes to our manufacturing platform could trigger the need to conduct additional bridging studies between our prior clinical supply and that of any new manufacturing processes and procedures. Should we experience delays or be unable to produce sufficient quantities of our HAVs utilizing our current or a modified version of our manufacturing system, we expect that our development and commercialization efforts would be impaired as a result, which would likely materially adversely affect our business, prospects, operating results and financial condition.
Manufacturing facilities are subject to significant government regulations and approvals, which are often costly and could result in adverse consequences to our business if we fail to comply with the regulations or maintain the approvals.
Our manufacturing facility is subject to ongoing regulation and periodic inspection by the FDA and other regulatory authorities to ensure compliance with cGMPs. Failure to follow and document adherence to such regulations or other regulatory requirements may (i) lead to significant delays in the availability of product for our clinical trials, (ii) result in the termination of or a hold being placed on one or more of our clinical trials, or (iii) delay or prevent filing or approval of marketing applications for our HAVs.
To monitor compliance with applicable regulations, the FDA routinely conducts inspections of facilities and may identify potential deficiencies. For example, the FDA issues what are referred to as “Form 483s” that set forth observations
66
and concerns that are identified during its inspections. Failure to satisfactorily address the concerns or potential deficiencies identified in a Form 483 could result in the issuance of a warning letter, which is a notice of the issues that the FDA believes to be significant regulatory violations requiring prompt corrective actions. Failure to respond adequately to a warning letter, or to otherwise fail to comply with applicable regulatory requirements could result in enforcement, remedial or punitive actions by the FDA or other regulatory authorities.
Risks Related to Our Reliance on Third Parties
We rely on third parties to conduct and support our clinical trials, and those third parties may not perform satisfactorily, including by failing to adhere to regulatory requirements or our stated protocols or to meet deadlines for the completion of such trials.
We do not independently conduct clinical trials for our product candidates and instead rely on third parties, such as CROs, clinical data management organizations, medical institutions and clinical investigators, to perform various functions, including implanting our HAVs and monitoring patients. The FDA and other regulatory authorities require us and these third parties to comply with GCP and, where applicable, cGTPs for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of patients in clinical trials are protected; ultimately, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and trial protocol. Failure by us or these third parties to do so could require us to enroll additional trial subjects beyond those we anticipate, could require us to modify our protocol, which may cause us to lose previously established Special Protocol Assessment (“SPA”) agreements with the FDA or similar agreements with other regulatory authorities concerning whether the design and size of our clinical trial adequately addresses scientific and regulatory requirements to support marketing approval, or could materially harm our ability to complete our clinical trials, including as a result of the need to remove trial sites and participants from the trial. We have in the past and may in the future need to terminate trial sites due to failure to conduct a trial in accordance with its protocol, applicable regulations, and generally accepted research standards.
The performance of the sites for our clinical trials may also be adversely affected by various other issues, including the lack of familiarity with the properties of our HAVs, intervention rates, insufficient training of personnel, variances in medical infrastructure, lack of familiarity with conducting clinical trials in accordance with international regulatory standards, communication difficulties or changes in local regulations. If these third parties do not successfully conduct our clinical trials in accordance with regulatory requirements or our stated protocols, carry out their contractual duties, or meet expected deadlines, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and may not be able to, or may be delayed in our efforts to, successfully commercialize our products if approved by regulatory authorities.
We rely on third-party suppliers, including sole source suppliers, to provide certain components for our product candidates. Any failure by a third-party supplier to supply these components for manufacture may delay or impair our ability to complete our clinical trials and to commercialize our product candidates.
We currently rely, and expect to continue to rely, on third parties for the supply of certain components necessary for our product candidates, such as donor tissue, other biologically derived substances, the PGA polymer mesh and the bioreactor bags in which our HAVs are grown. Our suppliers for certain of these materials, including SeraCare for the supply of human plasma and Confluent for the supply of polymer mesh, are sole source suppliers. Failure of one or more of our suppliers, including these sole source suppliers, to deliver components necessary for the production of our HAVs in a timely and sufficient manner, whether due to shortages of such materials, difficulties in scaling up supply to satisfy our clinical trial and commercial needs, contamination, recall, the COVID-19 pandemic or otherwise, or to source or manufacture such components in accordance with cGMPs and cGTPs, as applicable, could delay our ability to complete our clinical trials, obtain marketing approval and commercialize our product candidates. Establishing additional or replacement suppliers for these components could take a substantial amount of time and it may be difficult to establish replacement suppliers who meet regulatory requirements. In addition, as part of the FDA’s approval of our product candidates, the FDA must review and approve the individual components of our production process, which includes raw materials, the manufacturing processes and facilities of our suppliers. Some of our current suppliers have not undergone this process nor have they had any components included in any product approved by the FDA. If our suppliers fail to comply with applicable regulations, and if we do not qualify alternate suppliers, the clinical development, marketing approval or commercialization of our product candidates could be delayed, thereby increasing our costs to complete clinical development and to obtain marketing approval and depriving us of potential product revenue.
67
We intend to rely on our strategic, global relationship with Fresenius Medical Care to undertake, or assist with, the development and commercialization of certain of our product candidates if we receive marketing approval from relevant regulatory authorities. Disruption of this arrangement could materially adversely affect our business, prospects, operating results and financial condition.
Under the distribution agreement, Fresenius Medical Care has the exclusive right to sell and distribute the distribution product in the field outside of the United States. In addition, under the terms of the distribution agreement, Fresenius Medical Care will collaborate with Humacyte in its commercialization of the distribution product in the field in the United States, including adoption of the distribution product as a standard of care in patients for which such use is supported by clinical results and health economic analyses. As a result of our arrangement with Fresenius Medical Care, we expect to be reliant on Fresenius Medical Care to undertake or assist with the development and commercialization, as well as, in some cases, obtaining and maintaining regulatory approval, of the distribution product in the field and for Fresenius Medical Care to do so in a manner consistent with applicable law and regulatory requirements outside of the United States. If Fresenius Medical Care otherwise fails to undertake or assist with the development or commercialization, or obtaining or maintaining regulatory approvals, of the distribution product in accordance with the terms of the distribution agreement, our business, prospects, operating results and financial condition would be adversely affected, perhaps materially.
Fresenius Medical Care also maintains certain discretionary termination rights on a country-by-country basis with respect to any country outside of the United States under the distribution agreement, as discussed in more detail in the section of this Annual Report on Form 10-K titled “Business — Distribution — Distribution Agreement with Fresenius Medical Care.” If the distribution agreement is terminated, we may not be able to secure an alternative distributor in the applicable country on a timely basis or at all, in which case our ability to generate revenues from the distribution product in such country would be harmed.
In addition, if Fresenius Medical Care fails to undertake or assist with the development or commercialization, or obtaining or maintaining regulatory approval, as applicable, of the distribution product in a manner consistent with applicable law and regulatory requirements, patient access to, and demand for, the distribution product could be reduced, our reputation could be damaged, and, under certain circumstances, we could be exposed to potential liability. Furthermore, while Fresenius Medical Care has certain commercialization diligence obligations, Fresenius Medical Care is not restricted from offering its own products and services or the products and services of other companies that compete with the distribution product, and may not undertake or assist with the development or commercialization of the distribution product effectively.
Risks Related to Our Financial Position and Need for Additional Funding
We have never generated product revenue and have incurred significant losses to date. We expect to continue to incur losses for the foreseeable future and may never generate product revenue or be profitable.
Since inception, we have generated no product revenue, and prior to receipt of marketing approval from regulatory authorities, we will be unable to do so. We incurred net losses of $12.0 million and $26.5 million for the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022 and 2021, we had an accumulated deficit of $426.5 million and $414.6 million, respectively. Up to the date of the consummation of the Merger, we financed our operations primarily through the sale of equity securities and convertible debt and, to a lesser extent, through grants from governmental agencies. We have devoted substantially all of our financial resources and efforts to research and development, including preclinical studies and clinical trials and development of manufacturing technology, and we anticipate that our expenses will continue to increase over the next several years as we continue these activities. Our V005 and V007 trials are currently enrolling, and we currently intend to submit a BLA to the FDA relating to vascular trauma and a subsequent BLA filing related to AV access for hemodialysis. We also intend to continue scaling out our manufacturing facility to satisfy potential demand if the FDA approves our BLA, advancing preclinical and clinical development of additional clinical applications for our HAVs and funding our operations. Accordingly, we expect to continue to incur substantial operating losses for the foreseeable future, which may fluctuate significantly from quarter-to-quarter and year-to-year.
To become and remain profitable, we must succeed in obtaining marketing approval for our HAVs in the United States, in commercializing our HAVs, and in developing and commercializing additional product candidates that generate significant revenue. We may never succeed in these activities and, even if we do, may never generate revenue that is sufficient to achieve profitability.
Even if we do achieve profitability, we may not be able to sustain or increase profitability. Our failure to become and remain profitable would depress the value of our company and could impair our ability to maintain our research and
68
development efforts, expand our business, diversify our product offerings or even continue our operations. A decline in the value of Humacyte could also cause you to lose all or part of your investment in our securities.
Our ability to use our net operating loss and tax credit carryforwards to offset future taxable income may be subject to certain limitations.
As of December 31, 2022, we had net operating loss carryforwards for federal and state tax purposes of approximately $322.4 million and $323.9 million, respectively, which begin to expire in 2025. In addition, we had tax credit carryforwards for federal and state tax purposes of approximately $18.1 million, as of December 31, 2022, which begin to expire in 2025 and will expire completely in 2042. The future utilization of net operating loss and tax credit carryforwards may be limited due to changes in ownership. In general, if we experience a greater than 50% aggregate change in ownership of certain significant stockholders or groups over a three-year period (which constitutes an ownership change under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”)), utilization of our pre-change net operating loss carryforwards is subject to an annual limitation under Section 382 of the Code (and similar state laws). The annual limitation generally is determined by multiplying the value of our stock at the time of such ownership change (subject to certain adjustments) by the applicable long-term tax-exempt rate. Such limitations may result in expiration of a portion of the pre-change net operating loss carryforwards before utilization and may be substantial. In the past we may have experienced, and in the future may experience, ownership changes as a result of subsequent shifts in our stock ownership. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us.
We expect to need to raise additional funding, which may not be available on acceptable terms, or at all, and any failure to obtain capital when needed may force us to delay, limit or terminate our product development or commercialization efforts.
We expect to incur significant expenses in connection with our ongoing activities as we seek to (i) scale out our manufacturing facility to satisfy potential demand if our HAVs receive marketing approval in the United States, (ii) continue our preclinical and clinical development efforts, including the ongoing clinical trials, and (iii) obtain marketing approval for our 6 millimeter HAV, and, if marketing approval is obtained, to commercialize our HAVs for one or more approved indications. We will need additional funding in connection with these activities. Our future capital requirements will depend on many factors, including:
•the progress and results of our clinical trials and interpretation of those results by the FDA and other regulatory authorities;
•the cost, timing and outcome of regulatory review of our product candidates, particularly for approval of our HAVs in the United States;
•the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our additional product candidates;
•the cost and timing of our future commercialization activities, including product manufacturing, marketing and distribution for our HAVs if approved by the FDA, and any other product candidate for which we receive marketing approval in the future;
•the amount and timing of revenues, if any, that we receive from commercial sales of any product candidates for which we receive marketing approval; and
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims.
Adequate capital may not be available to us when needed or on acceptable terms. If we are unable to raise capital, we could be forced to delay, reduce, suspend or cease our research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition. As of December 31, 2022 and 2021, we had $151.9 million and $225.5 million, respectively, in cash and cash equivalents and short-term investments. Based upon our current operating plan, we believe that our cash and cash equivalents and short-term investments will be sufficient to fund our operations, including clinical trial expenses and capital expenditure requirements, for at least 12 months from the date of this Annual Report on Form 10-K.
69
Pursuant to the terms of our outstanding indebtedness, we may be limited in our ability to incur future debt.
In March 2021, Humacyte Global, Inc., or Legacy Humacyte, entered into a Loan and Security Agreement (as amended, the “Loan Agreement”) with Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P., which provides a term loan facility of up to $50.0 million with a maturity date of March 1, 2025. We became a co-borrower under the Loan Agreement in connection with the Merger. The obligations of Humacyte and Legacy Humacyte under the Loan Agreement are secured by substantially all of their assets, except for their intellectual property.
Pursuant to the terms of the Loan Agreement, we are limited in our ability to incur additional indebtedness. In addition, a failure to comply with the covenants under the Loan Agreement could result in an event of default and an acceleration of amounts due. If an event of default occurs that is not waived by the lenders, and the lenders accelerate any amounts due, we may not be able to make accelerated payments, and the lenders could seek to enforce their security interests in the collateral securing such indebtedness, which could have a material adverse effect on our business and results of operations. Our payment obligations under the Loan Agreement reduce cash available to fund working capital, capital expenditures, research and development and other corporate purposes, and limit our ability to obtain additional financing for working capital, capital expenditures, expansion plans and other investments, which may in turn limit our ability to implement our business strategy, heighten our vulnerability to downturns in our business, the industry, or in the general economy, limit our flexibility in planning for, or reacting to, changes in our business and the industry and prevent us from taking advantage of business opportunities as they arise. If market rates increase, we will have to pay additional interest on this indebtedness, which would further reduce cash available for our other business needs.
We cannot assure you that our business will generate sufficient cash flow from operations or that future financing will be available to us in amounts sufficient to enable us to make required and timely payments on our indebtedness, or to fund our operations.
To date, we have not requested or obtained marketing approval for, or commercialized, any of our product candidates, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are a development-stage company. Our operations to date, with respect to the development of our product candidates, have been limited to organizing and staffing our company, business planning, raising capital, identifying markets for our product candidates, undertaking preclinical studies and clinical trials of our product candidates for various potential indications and establishing research and development, manufacturing and distributing collaborations. We have not yet demonstrated the ability to obtain marketing approval for a product, to manufacture an approved product at commercial scale or to successfully commercialize an approved product. Consequently, any predictions you make about our financial prospects may not be as accurate as they could be if we had received marketing approval and begun commercializing a product.
Risks Related to Government Regulation
We may not obtain marketing approval from the FDA for any of our product candidates even if we successfully complete our clinical trials, which failure would materially harm our business, prospects, operating results and financial condition.
Prior to commercialization, biologics, like our HAVs, require the submission of a BLA to, and approval of the BLA by, the FDA. A BLA must be supported by extensive preclinical and clinical data, as well as extensive information regarding chemistry, manufacturing and controls (“CMC”), sufficient to demonstrate the safety, purity, potency and effectiveness of the applicable product candidate to the satisfaction of the FDA. We have never submitted a BLA for approval or otherwise obtained FDA approval for any of our product candidates.
The BLA approval process is expensive and uncertain, it may take several years to complete, and we may not be successful in obtaining such approval. The FDA has substantial discretion in the approval process and decisions made by the FDA can be unpredictable. The FDA may use its discretion at any time to withdraw statements or representations, including written statements, that it has made or may make to us regarding our product candidates, clinical trials or the BLA process. The number and types of preclinical studies and clinical trials that will be required for BLA approval varies depending on the product candidate, the disease or the condition that the product candidate is designed to target and the regulations applicable to any particular product candidate. The FDA could delay, limit or deny approval of our product candidates for many reasons, including because it:
•may not deem the product candidate to be adequately safe or effective;
70
•may not find the data from preclinical studies, clinical trials or CMC data sufficient to support approval;
•may not approve the manufacturing processes or facilities associated with the product candidate;
•may conclude that the long-term integrity of the product candidate for which approval is being sought has not been sufficiently demonstrated;
•may change approval policies or adopt new regulations; or
•may not accept a submission due to, among other reasons, the content or formatting of the submission.
In some cases, the FDA may agree to an SPA for a clinical trial, when it determines that the trial is adequately designed to provide necessary data to support a license application. Even in such cases, however, the FDA may subsequently abandon the SPA if a substantial scientific issue essential to determining the safety or effectiveness of the product candidate has been identified after the testing has begun. In addition, if a company alters the protocol for a trial, the SPA may no longer apply. Further, the results of pivotal clinical trials are always subject to thorough FDA review. Even highly significant clinical trial results are no guarantee of approval.
We currently intend to submit a BLA to the FDA relating to vascular trauma and a BLA for AV access in hemodialysis, based on the results and trial design of our V005 and V007 trials, respectively. The FDA may decline to approve our 6 millimeter HAV on the basis of these or other trial results, or for other reasons.
Even if we obtain and maintain approval for our HAVs from the FDA, we may never obtain approval for our HAVs outside of the United States, where the regulatory process is also complex and subject to significant uncertainty. Failure to do so would limit our market opportunities and adversely affect our business.
Even if we receive FDA approval to market any biologic in the United States, we must comply with the numerous and varying regulatory and compliance related requirements of other countries, including the submission of extensive preclinical and clinical data, manufacturing and quality information regarding the process and facility, scientific data characterizing the relevant product candidate and other supporting data in order to establish safety and effectiveness. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods, including obtaining reimbursement and pricing approval in select markets. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The marketing approval process in other countries may include all of the risks associated with FDA approval as well as additional, presently unanticipated, risks. Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may negatively impact the regulatory process in others, including the risk that our product candidates may not be approved for all indications requested and that such approval may be subject to limitations on the indicated uses for which the product candidate may be marketed.
Even if we seek “rolling review” or priority review, the review time for BLAs for our product candidates may be longer and more expensive than for other products because of the novelty and complexity of our product candidates, which would delay our ability to begin commercialization and earn product revenues.
The marketing approval process for novel product candidates such as ours may take longer to complete and be more expensive than the process for other, better known or extensively studied pharmaceutical or other product candidates. We may be eligible for a “rolling review” of a BLA, which means we may submit completed modules of a BLA rather than waiting until every module of the BLA is completed before submitting the full BLA for FDA review. Such “rolling review” is common for indications that are part of one of FDA’s expedited programs, such as our 6 millimeter HAV, which has received Fast Track and RMAT designations for AV access in hemodialysis. The FDA may also designate one or more of our product candidates for priority review after we submit a BLA. Under priority review, the FDA’s goal is to review an application within six months of the 60-day filing date, compared to ten months for a standard review. Even if we are able to utilize a “rolling review” and/or the FDA designates one or more of our product candidates for priority review, it may not lead to a shorter review period. The FDA could also decide to consult an advisory committee as part of our BLA review process, which often leads to a longer review time. We are not permitted to commercialize our product candidates in the United States until they have been approved by the FDA, and if we experience a lengthier review period than expected, our ability to generate product revenues would be materially harmed.
71
We may in the future seek orphan drug designation for the use of our HAVs to treat congenital pediatric heart defects. We may be unable to obtain such designation or to maintain the benefits associated with orphan drug designation, including market exclusivity, which may cause our revenue, if any, to be reduced.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 in the United States, or a patient population of 200,000 or more in the United States when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a BLA. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. After the FDA grants orphan drug designation, the generic identity of the drug or biologic and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. Even if one of our product candidates receives orphan exclusivity, the FDA can still approve other drugs that have a different active ingredient for use in treating the same indication or disease, as well as the same drug or biologic for a different indication or disease. The FDA can also approve the same drug or biologic for the same indication or disease if the subsequent drug or biologic demonstrates clinical superiority. Furthermore, the FDA can waive orphan exclusivity if we are unable to manufacture sufficient supply of our product.
Inadequate funding for the FDA and other government agencies, including from government shut downs, global health concerns or other disruptions to these agencies’ operations, could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, policy changes, and the effects of the COVID-19 pandemic. Average review times at the FDA have fluctuated in recent years as a result.
Disruptions at the FDA and other agencies may also slow the time necessary for new product candidates to be reviewed and/or approved by necessary government agencies, which could adversely affect our business. In addition, government funding of other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
We may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials and the review process. In addition, disruptions at the FDA and other agencies may also slow the time necessary for new product candidates to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Even if we receive marketing approval for a product candidate, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to significant penalties if we fail to comply with applicable regulatory requirements.
If we obtain marketing approval for any of our product candidates, the approved product will be subject to ongoing regulatory requirements from the FDA and, if applicable, non-U.S. regulatory authorities. Any marketing approval that we receive for our product candidates may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing follow-up trials to monitor the safety and efficacy of the product. The FDA could also approve our product candidates with a REMS, which could include significant restrictions on distribution and/or use of our products. In addition, if the FDA and non-U.S. regulatory authorities approve any of our product candidates, we will be subject to extensive and ongoing regulatory requirements by the FDA and other regulatory authorities with regard to the manufacturing, labelling, packaging, AE reporting, storage, advertising, distribution, promotion and recordkeeping for our products. If we, our product candidates or the manufacturing facilities
72
for our product candidates fail to comply with regulatory requirements of the FDA and, if relevant, other non-U.S. regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including the following:
•issuance of warning letters or untitled letters by regulatory authorities asserting that we are in violation of the law;
•imposition of injunctions or significant civil monetary penalties or pursuit by regulatory authorities of civil or criminal prosecutions and fines against us or our responsible officers;
•suspension or withdrawal of marketing approval;
•suspension of any ongoing clinical trials or refusal by regulatory authorities to approve pending marketing applications or supplements to approved applications;
•seizure of products or refusal to allow us to enter into supply contracts, including government contracts, or to import or export products;
•voluntary or mandatory product recalls and publicity requirements; and
•restrictions on operations, including marketing efforts, or restrictions that mandate costly new manufacturing requirements.
Any of these events could reduce market acceptance of any or our product candidates that had received marketing approval, substantially reduce our revenue, increase the costs of operating our business, and cause us significant reputational damage, among other consequences. If we ultimately receive approval for any product candidates in jurisdictions outside the U.S., we expect to be subject to similar ongoing regulatory oversight by the relevant foreign regulatory authorities.
Our products may be subject to product recalls that could harm our reputation and could materially and adversely affect our business, financial condition, operating results, cash flows and prospects. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, if approved. In particular, while the FDA permits the dissemination of truthful and non-misleading information about an approved product, the FDA restricts our ability to promote a product for uses that are not approved by the FDA. The misuse or off-label use of our product may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory authorities if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business. We may also face risks in other non-U.S. jurisdictions from product recalls and advertising/promotion rules.
We could also face product liability suits or regulatory delays due to defects in our products, which could be expensive and time-consuming and result in substantial damages payable by us and increases in our insurance rates.
Designation of our product candidates for expedited programs, such as Fast Track designation, Breakthrough Therapy Designation, or RMAT designation, or accelerated approval by the FDA, or priority designation by the Department of Defense, may not lead to a faster development or regulatory review or approval process, and even if granted, will not increase the likelihood that our product candidates will receive marketing approval.
In 2014, the FDA granted Fast Track designation for our 6 millimeter HAV for use in the creation of AV access for hemodialysis, and, in 2017, the FDA granted RMAT designation for our 6 millimeter HAV for the creation of vascular access for performing hemodialysis. We have submitted a request for RMAT designation of the HAV for vascular trauma but there is no guarantee that the FDA will grant this request. And we have not received designations pursuant to any of the FDA’s expedited programs for peripheral artery disease or our other indications, although we may in the future seek such designations if such product candidates meet the criteria for that designation. As a result, even if we submit a BLA for trauma, our Fast Track and RMAT designations, and their attendant benefits, may not apply to this requested indication unless the FDA grants our request for RMAT designation. In addition, even with one or more of these designations, we may not experience a faster development process, or faster review or approval, for our product candidates compared to product candidates that are not part of the expedited programs. Further, the FDA may withdraw a designation if it believes that the designation is no longer supported by data from our clinical development program. In addition, a product candidate may no longer demonstrate a potential to address unmet medical need if, for example, a new product is approved that addresses the same need, which could lead to loss of a designation. The loss of a designation under an expedited program, including a Fast Track designation, Breakthrough Therapy Designation, or RMAT designation, could significantly increase the costs of development and length of time required before we could seek marketing approval of such a product candidate.
73
We may seek accelerated approval for our HAV relating to vascular trauma. A product candidate may be eligible for accelerated approval if it treats a serious or life-threatening condition, generally provides a meaningful advantage over available therapies, and demonstrates an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. As a condition of accelerated approval, the FDA may require that a sponsor of a product receiving accelerated approval perform adequate and well-controlled confirmatory clinical trials post-approval. These confirmatory trials must be completed with due diligence. In addition, the FDA currently requires the pre-approval of promotional materials as a condition for accelerated approval, which could adversely impact the timing of the commercial launch of the product. Even if we do receive accelerated approval, we may not experience a faster development or regulatory review or approval process, and receiving accelerated approval does not provide assurance of ultimate full FDA approval. Accelerated approval may also be withdrawn if, among other things, a confirmatory trial required to verify the predicted clinical benefit of the product fails to verify such benefit or if such trial is not conducted with due diligence.
In addition, in 2018, our HAV product candidate was assigned a priority designation by the Secretary of Defense under Public Law 115-92. Similar to the designations described above that FDA may grant, a priority designation by the Department of Defense does not change the standards for approval but may expedite the development or approval process.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success.
Our industry is highly regulated, and changes in or revisions to laws and regulations that make gaining coverage of and adequate reimbursement for our product candidates more difficult or subject to different criteria and standards may adversely impact our business, prospects, operating results and financial condition. In the United States, there have been and we expect there will continue to be a number of legislative, regulatory and other changes to the healthcare system to contain or reduce healthcare costs that may adversely affect our ability to set a price we believe is fair for our product candidates, our ability to generate revenues and achieve or maintain profitability, and the availability of capital.
Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. For example, ACA, enacted in 2010 and amended by the Health Care and Education Reconciliation Act, contains a number of provisions that were intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies for fraud and abuse, add transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry, and impose additional health policy reforms. The Bipartisan Budget Act of 2018, among other things, amended the ACA to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole,” in part by requiring greater discounts from manufacturers. Various members of Congress have expressed a desire to repeal all or portions of the ACA, and in December 2017, portions of the ACA dealing with the individual mandate insurance requirement were effectively repealed by the Tax Cuts and Jobs Act of 2017. On February 10, 2021, the Biden administration withdrew the federal government’s support for overturning the ACA. Further, on January 28, 2021, President Biden issued an executive order to initiate a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which ran until August 15, 2021. The executive order also instructs certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. In June 2021, the United States Supreme Court held that the individual plaintiffs and states lacked standing to challenge the constitutionality of the ACA.
Additionally, in December 2018, CMS published a final rule permitting further collections and payments to and from certain ACA qualified health plans and health insurance issuers under the ACA risk adjustment program in response to the outcome of federal district court litigation regarding the method CMS uses to determine this risk adjustment. Since then, the ACA risk adjustment program payment parameters have been updated annually. In addition, CMS published a final rule that gave states greater flexibility, starting in 2020, in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces.
At this time, it remains unclear whether there will be further changes made to the ACA. The ACA, as currently enacted or as amended in the future, may adversely affect our business and operating results, and we do not know how future federal or state legislative or administrative changes relating to healthcare reform will affect our business. Other legislative changes that have been adopted since enactment of the ACA could also affect potential pricing and utilization of our product candidates.
74
In addition, the Secretary of Health and Human Services, various members of Congress and CMS have made statements and issued proposals regarding containment of drug prices through various means, including enabling CMS to negotiate U.S. drug pricing to align with foreign drug pricing, pricing transparency measures, reform of drug rebate programs, and conditioning coverage and reimbursement of certain drugs upon the prior failure or inadequacy of less expensive therapies, sometimes referred to as “step therapy.” Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies. At the federal level, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. In addition, at the state level, individual states have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Beginning in fiscal year 2018, CMS altered the reimbursement formula on specified covered outpatient drugs (“SCODs”). A SCOD drug product may also be a covered outpatient drug under the 340B program, which allows 340B-participating hospitals to purchase the drug product at the 340B discounted rate and, when prescribing it to a Medicare patient, be reimbursed at the Medicare rate. Under the prior Medicare reimbursement rate, this created a significant, positive gap for 340B-participating health care facilities. CMS’s change in the Medicare reimbursement rate for SCODs significantly impacted, or eliminated, the positive gap for 340B-participating health care facilities. The District Court for the District of Columbia invalidated the formula change, but the U.S. Court of Appeals for the District of Columbia Circuit reversed the district court’s decision and found that the changes were within the Secretary’s authority. The case is currently under review by the U.S. Supreme Court, and a decision is expected by summer of 2022. It is unlikely the Medicare rate litigation will impact 340B pricing for our approved products in the future, but it possible it could affect covered hospitals who might purchase our products.
The FDA also released a final rule on September 24, 2020, which went into effect on November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Additionally, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The 2020 rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. Pursuant to a court order, HHS subsequently delayed the effective date for aspects of the rule, including those relating to pharmacy benefit managers, until 2023. The rule was then effectively delayed until January 1, 2026, as part of the Infrastructure Investment and Jobs Act, which was signed into law on November 15, 2021. In addition, on November 19, 2021, the House of Representatives passed a version of the Build Back Better Act that includes a provision prohibiting the implementation, administration, or enforcement of the rule. Although a number of these, and other proposed measures may require authorization through additional legislation to become effective, and the Biden administration may reverse or otherwise change these measures, Congress has indicated that it will continue to seek new legislative measures to control drug costs.
The ultimate content, timing, or effect of any healthcare reform legislation or executive order or the impact that the resulting changes may have on us is uncertain, but we expect there will continue to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, prospects, operating results and financial condition could be adversely affected.
Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business even though we do not and will not control referrals of healthcare services. We could also be subject to patient privacy regulation by both the U.S. Government and the states in which we conduct our business. Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements. The regulations that may affect our ability to operate include, without limitation:
•the federal Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either
75
the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs, such as the Medicare and Medicaid programs, even if the person does not have actual knowledge of the statute or specific intent to violate it;
•the federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false claims, or knowingly using false statements, to obtain payment from the U.S. Government;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
•the anti-inducement law, which prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of items or services reimbursable by a federal or state governmental program;
•the federal transparency requirements under the ACA, including the provision commonly referred to as the Physician Payments Sunshine Act and its implementing regulations, which require applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the State Children’s Health Insurance Program to report annually to CMS information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members. Effective January 1, 2022, these reporting obligations were extended to include transfers of value made to certain non-physician providers such as physician assistants and nurse practitioners;
•federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•HIPAA, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; and
•state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participating in federal health care programs and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment, exclusion, or restructuring of our operations could adversely affect our ability to operate our business, prospects, operating results and financial condition. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy and fraud and abuse laws may prove costly.
Our business and operations, including our development programs, could be materially disrupted in the event of system failures, security breaches, violations of data protection laws or data loss or damage by us or third parties on which we rely, including our CROs or other contractors or consultants.
Our internal computer systems (including our LUNA200 manufacturing system) and those of third parties on which we rely, including our CROs and other contractors and consultants, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. These risks may be compounded as our information technology hardware ages. If such an event were to occur and cause interruptions in our operations, it could have a material adverse effect on our business operations, including a material disruption of our development program. Unauthorized disclosure of sensitive or confidential patient or employee data, including personally identifiable information, whether through breach of computer systems, systems failure, employee negligence, fraud or misappropriation, or otherwise, or unauthorized access to or through our information systems and networks, whether by our
76
employees or third parties, could result in negative publicity, legal liability and damage to our reputation. Unauthorized disclosure of personally identifiable information could also expose us to sanctions for violations of data privacy laws and regulations around the world. To the extent that any disruption or security breach resulted in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed. For example, the loss of or damage to clinical trial data, such as from completed or ongoing clinical trials, for any of our product candidates would likely result in delays in our marketing approval efforts and significantly increased costs in an effort to recover or reproduce the data.
We have previously been, and expect to remain, the target of cyber-attacks. During late 2020 and early 2021, a professional services firm providing services to the Company was the target of a cyber-attack. The Company believes that it was not materially impacted by the attack. As we become more dependent on information technologies to conduct our operations, cyber incidents, including deliberate attacks, such as ransomware attacks, and attempts to gain unauthorized access to computer systems (including our LUNA200 manufacturing system) and networks, may increase in frequency and sophistication. These incidents pose a risk to the security of our systems and networks, the confidentiality and the availability and integrity of our data and these risks apply both to us, and to third parties on whose systems we rely for the conduct of our business. While the effect of these incidents has not historically been material to our results of operations, financial condition or prospects, cyber threats are persistent and constantly evolving. Such threats have increased in frequency, scope and potential impact in recent years, which increase the difficulty of detecting and successfully defending against them. As cyber threats continue to evolve, we may be required to incur additional expenses in order to enhance our protective measures or to remediate any information security vulnerability. There can be no assurance that we or our third-party providers will be successful in preventing cyber-attacks or successfully mitigating their effects. Similarly, there can be no assurance that our collaborators, CROs, third-party logistics providers, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems. Any cyber-attack or destruction or loss of data could have a material adverse effect on our business and prospects. In addition, we may suffer reputational harm or face litigation or adverse regulatory action as a result of cyber-attacks or destruction or loss of data and may incur significant additional expense to implement further data protection measures. It is also possible that unauthorized access to data may be obtained through inadequate use of security controls by our suppliers or other vendors. In 2021, a remote code execution vulnerability in Apache Log4j was identified as affecting large amounts of systems worldwide. We were not impacted by the Log4j vulnerability, however we cannot provide assurance that these and other attacks will not have an impact in the future.
Although we have general liability insurance coverage, our insurance may not cover all claims, continue to be available on reasonable terms or be sufficient in amount to cover one or more large claims. Additionally, the insurer may disclaim coverage as to any claim. The successful assertion of one or more large claims against us that exceed or are not covered by our insurance coverage or changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could have a material adverse effect on our business, prospects, operating results and financial condition.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines and penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials and produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. In the event of contamination or injury resulting from our use or production of hazardous materials, we could be held liable for any resulting damages even if we contract with a third party for their disposal, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties resulting from contamination or injury from our use or production of hazardous materials. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use or production of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous materials.
In addition, we may be required to incur substantial costs to comply with future environmental, health and safety laws and regulations. Compliance with such laws and regulations may divert resources away from our research, development and manufacturing efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
77
Failure to comply with health and data protection laws and regulations could lead to government enforcement actions (which could include civil or criminal penalties), private litigation and adverse publicity and could negatively affect our operating results and business.
We and any potential collaborators may be subject to federal, state and foreign data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state data breach notification laws, state privacy and health information privacy laws and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA. Depending on the facts and circumstances, we could be subject to civil or criminal penalties if we obtain, use, or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
International data protection laws, including Regulation 2016/679, known as the General Data Protection Regulation (“GDPR”), may also apply to health-related and other personal information obtained outside of the United States. The GDPR will increase our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional mechanisms to ensure compliance with the new EU (which also includes the European Economic Area, or “EEA”) data protection rules. Further, the United Kingdom’s vote in favor of exiting the EU, often referred to as Brexit, has created more uncertainty with regard to data protection regulation in the United Kingdom (the “UK”). The UK retained the GDPR in UK law, which sits alongside the amended version of the Data Protection Act 2018. The EU adopted an adequacy decision so data can be transferred from the EU to the UK. Additionally, there are no new requirements for transfer from the UK to the EU. However, going forward, the EU and UK’s data protection rules could diverge and data transfers may not be possible and/or new arrangements may need to be put in place. In particular, it is unclear to what extent the UK regime will begin diverging from the GDPR and how data transfers to and from the UK will be regulated.
In addition, California recently enacted the California Consumer Privacy Act (“CCPA”), which creates new individual privacy rights for California consumers (as defined in the law) and places increased privacy and security obligations on entities handling personal data of consumers or households. The CCPA became effective on January 1, 2020, but the California Consumer Rights Act (“CPRA”) was recently enacted to strengthen elements of the CCPA effective January 1, 2023. In addition, there are a number of other states that have considered similar privacy proposals, with states like Virginia and Colorado enacting their own privacy laws (also scheduled to come into effect in January 1, 2023 and July 1, 2023, respectively). These privacy laws may impact our business activities and exemplify the vulnerability of our business to the evolving regulatory environment related to personal data.
Compliance with U.S. and international data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with U.S. and international data protection laws and regulations could result in government enforcement actions (which could include civil or criminal penalties), private litigation and adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects about whom we or our potential collaborators obtain information, as well as the providers who share this information with us, may contractually limit our ability to use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
We or the third parties upon which we depend may be adversely affected by natural disasters, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Natural disasters such as hurricanes could severely disrupt our operations and have a material adverse effect on our business, prospects, operating results and financial condition. In addition, flooding, lightning strikes, meteor strikes, and polar vortices could affect our building operations. If a natural disaster, power outage or other unforeseen event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as our in-house manufacturing facility, or that otherwise significantly disrupted our operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently may prove inadequate in the event of a natural disaster or similar event. We may incur substantial expenses as a result of any natural disaster, which could have a material adverse effect on our business.
78
We are subject to anti-corruption and a variety of other laws governing our international operations. If we fail to comply with these laws, we could be subject to, among other things, civil or criminal penalties, other sanctions and remedial measures, and reputational damage, which could adversely affect our business, prospects, operating results and financial condition.
Our operations are subject to anti-corruption laws, including the U.S. Foreign Corrupt Practices Act (“FCPA”), the U.K. Bribery Act and other anti-corruption laws. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and partners from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We are conducting certain of our trials at a number of trial sites around the world. Certain of these jurisdictions pose a risk of potential FCPA violations, and we have relationships with third parties, including government-affiliated hospitals and universities, whose actions could potentially subject us to liability under the FCPA or local anti-corruption laws.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the U.S. Department of Commerce’s Bureau of Industry and Security, the U.S. Department of the Treasury’s Office of Foreign Assets Control, and various non-U.S. government entities, including applicable economic sanctions on countries and persons, customs requirements, currency exchange regulations and transfer pricing regulations.
If we fail to comply with applicable anti-corruption laws and other legal requirements, we may become subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, including the loss of export or import privileges and debarment, and face substantial legal expenses. Likewise, even an investigation by U.S. or foreign authorities of potential violations of such laws could damage our reputation. In either case, our business, prospects, operating results and financial condition could be adversely affected. Under certain circumstances, we could also be held liable for the activities of our employees, contractors, and partners that violate anti-corruption laws, even if we do not explicitly authorize or have actual knowledge of such activities. Even allegations of such violations could potentially damage our reputation and harm our business.
Risks Related to Our Intellectual Property
Our ability to successfully commercialize our products may be impaired if we are unable to obtain and maintain effective intellectual property rights for our proprietary scientific technology platform and product candidates.
Our success depends in large part on our and our licensors’ ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary scientific technology platform and products. We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and product candidates that we and/or our licensors view as important to our business. This process is expensive and time-consuming, and we and our licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we and/or our licensors will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents or enforce the patents, covering technology or products that we license from third parties. Our existing patents and any future patents and the existing and any future licenses to third-party patents we obtain may not be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies.
The patent position of biotechnology companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unresolved. In recent years, patent rights have been the subject of significant litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors’ patent rights are highly uncertain. Additionally, changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our owned or licensed patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. We, or our licensors, may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, we may miss potential opportunities to strengthen our patent position. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or
79
held unenforceable, which could limit our ability to stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of future product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage.
The patent protection we obtain for our product candidates may not be sufficient enough to provide us with any competitive advantage or our owned or licensed patents may be challenged.
In some instances, agreements through which we license patent rights may not give us control over patent prosecution or maintenance, so that we may not be able to control which claims or arguments are presented, how claims are amended, and may not be able to secure, maintain, or successfully enforce necessary or desirable patent protection from those patent rights. We have not had and do not have primary control over patent prosecution and maintenance for certain of the patents and patent applications we license and therefore cannot guarantee that these patents and applications will be prosecuted or maintained in a manner consistent with the best interests of our business. We cannot be certain that patent prosecution and maintenance activities by our licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents. Moreover, some of our in-licensed patents and patent applications are, and our future owned and licensed patents may be, co-owned with third parties. If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in any future patents or patent applications, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of our patents in order to enforce such patents against third parties, and such cooperation may not be provided to us.
It is possible that defects of form in the preparation or filing of our owned or licensed patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments or extensions. If we or our partners, collaborators, licensees, or licensors, whether current or future, fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our partners, collaborators, licensees, or licensors, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form, preparation, prosecution, or enforcement of our owned or licensed patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
Pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent.
However, prior to March 16, 2013, in the United States, the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Similarly, we cannot be certain that parties from whom we do or may license or purchase patent rights were the first to make relevant claimed inventions, or were the first to file for patent protection for them. If third parties have filed prior patent applications on inventions claimed in our owned or licensed patents or applications that were filed on or before March 15, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of such owned or licensed patent applications. If third parties have filed such prior applications after March 15, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our owned or licensed invention was derived from theirs.
Moreover, because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, our owned and licensed patents or pending patent applications may be challenged in the courts or patent offices in the United States and abroad. There is no assurance that all of the potentially relevant prior art relating to our owned or licensed patents and patent applications has been found. If such prior art exists, it may be used to invalidate a patent, or may prevent a patent from issuing from a pending patent application. For example, such patent filings may be subject to a third-party submission of prior art to the U.S. Patent and Trademark Office (“USPTO”), or to other patent offices around the world. Alternately or additionally, we may become involved in post-grant review procedures, oppositions, derivation
80
proceedings, ex parte reexaminations, inter partes review, supplemental examinations, or interference proceedings or challenges in district court, in the United States or in various foreign patent offices, including both national and regional, challenging patents or patent applications in which we have rights, including patents on which we rely to protect our business. An adverse determination in any such challenges may result in loss of the patent or in patent or patent application claims being narrowed, invalidated or held unenforceable, in whole or in part, or in denial of the patent application or loss or reduction in the scope of one or more claims of the patent or patent application, any of which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. In addition, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
Pending and future patent applications may not result in patents being issued that protect our business, in whole or in part, or which effectively prevent others from commercializing competitive products.
Competitors may also be able to design around our owned or licensed patents. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our owned or licensed patents or narrow the scope of our patent protection. In addition, the laws of foreign countries may not protect our rights to the same extent or in the same manner as the laws of the United States. For example, patent laws in various jurisdictions, including significant commercial markets such as Europe, restrict the patentability of methods of treatment of the human body more than United States law does. If these developments were to occur, they could have a material adverse effect on our ability to generate revenue.
Issued patents that we have or may obtain or license may not provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend or assert our owned or licensed patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our owned or licensed patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe, misappropriate or violate our owned or licensed patents or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly and refuse to stop the other party from using the technology at issue on the grounds that our owned or licensed patents do not cover such technology. The standards that courts use to interpret patents are not always applied predictably or uniformly and can change, particularly as new technologies develop. As a result, we do not know how much protection, if any, will be given to our owned or licensed patents if we attempt to enforce them and they are challenged in court. An adverse result in any litigation proceeding could put one or more of our owned or licensed patents at risk of being invalidated or interpreted narrowly.
Inequitable conduct is frequently raised as a defense during intellectual property litigation. It is believed that all parties involved in the prosecution of our patent applications have complied with their duties of disclosure in the course of prosecuting our patent applications; however, it is possible that legal claims to the contrary could be asserted if we were engaged in intellectual property litigation, and the results of any such legal claims are uncertain due to the inherent uncertainty of litigation. If a court determines that any party involved in the prosecution of our owned or licensed patents failed to comply with its duty of candor, the subject patent could be held to be unenforceable.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. Intellectual property litigation or other legal proceedings may cause us to incur significant expenses and may also absorb significant management time. Uncertainties resulting from our participation in patent litigation or other proceedings could have a material adverse effect on our business.
81
Third parties may initiate legal proceedings alleging that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could harm our business, prospects, operating results and financial condition.
Third parties may assert infringement, misappropriation or other claims against us, or other parties we have agreed to indemnify, based on existing third-party patents or patents that may be granted in the future as well as other intellectual property rights. There may be existing third-party patents or patent applications covering aspects of our technology. Furthermore, because patent applications are published sometime after filing, and because applications can take several years to issue, there may be additional currently pending third-party patent applications that are unknown to us, which may later result in issued patents. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. We may not have sufficient resources to bring these actions to a successful conclusion. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of shares of our common stock.
Because of the inevitable uncertainty in intellectual property litigation, we could lose a patent infringement or other action asserted against us regardless of our perception of the merits of the case. If we are found to infringe upon, misappropriate or otherwise violate a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and commercializing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the implicated technology or product. In addition, in any such proceeding or litigation, we could be found liable for monetary damages, which could be significant, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. A finding of infringement, misappropriation or that we otherwise violated intellectual property rights could prevent us from commercializing our product candidates or force us to cease some or all of our business operations.
If we fail to comply with our obligations in our intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are a party to intellectual property license agreements with third parties. For example, we have licenses with each of Duke University and Yale University for patents associated with our proprietary technology, and may enter into additional license agreements in the future. Our existing license agreements impose, and we expect that our future license agreements will impose, various diligence, royalty payment, milestone payment, insurance and other obligations on us. If we fail to comply with these obligations or other obligations in our license agreements, our licensors may have the right to terminate these agreements, in which event we may not be able to develop and market any product or use any platform technology that is covered by these agreements. If our license agreements terminate, or we experience a reduction or elimination of licensed rights under these agreements, we may have to negotiate new or reinstated licenses with less favorable terms or we may not have sufficient intellectual property rights to operate our business. The occurrence of such events could materially harm our business.
Further, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. Accordingly, disputes may arise between us and our licensor, our licensor and its licensors, regarding intellectual property subject to a license agreement, including those relating to:
•the scope of rights, if any, granted under the license agreement and other interpretation-related issues;
•whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the license agreement;
•whether our licensor or its licensor had the right to grant the license agreement;
•whether third parties are entitled to compensation or equitable relief, such as an injunction, for our use of the intellectual property without their authorization;
•our right to sublicense patent and other rights to third parties under collaborative development relationships;
•whether we are complying with our obligations with respect to the use of the licensed technology in relation to our development and commercialization of product candidates;
82
•our involvement in the prosecution of the licensed patents and our licensors’ overall patent enforcement strategy;
•the allocation of ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and by us and our partners; and
•the amounts of royalties, milestones or other payments due under the license agreement.
The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement. If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, or are insufficient to provide us the necessary rights to use the intellectual property, we may be unable to successfully develop and commercialize the affected product candidates. If we or any such licensors fail to adequately protect this intellectual property, our ability to commercialize our products could suffer. Any disputes with our licensors or any termination of the licenses on which we depend could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may not be successful in obtaining necessary intellectual property rights to product candidates for our development pipeline through acquisitions and in-licenses.
Although we intend to develop product candidates through our own internal research, we may need to obtain additional licenses from others to advance our research or allow commercialization of our product candidates. However, we may be unable to acquire or in-license intellectual property rights relating to, or necessary for, any such product candidates from third parties on commercially reasonable terms or at all. In that event, we may be unable to develop or commercialize such product candidates. We may also be unable to identify additional, future product candidates that we believe are an appropriate strategic fit for our company and intellectual property relating to, or necessary for, such product candidates.
The in-licensing and acquisition of third-party intellectual property is a competitive area, and a number of more established companies are also pursuing strategies to in-license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. Furthermore, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. In addition, we expect that competition for the in-licensing or acquisition of third-party intellectual property rights for product candidates that are attractive to us may increase in the future, which may mean fewer suitable opportunities for us as well as higher acquisition or licensing costs. We may be unable to in-license or acquire the third-party intellectual property rights for product candidates on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to suitable product candidates, our business, financial condition, results of operations and prospects for growth could suffer.
We may be unable to protect the confidentiality of our trade secrets, particularly in light of our reliance on third parties, which increases the possibility that such trade secrets will be disclosed or misappropriated, thus harming our business and competitive position.
In addition to our patented technology and products, we rely upon trade secrets, including unpatented know-how, technology and other proprietary information to develop and maintain our competitive position, particularly with respect to our manufacturing process. We seek to protect our trade secrets, in part, through confidentiality agreements with our employees, collaborators and consultants. We seek to have agreements with our employees and selected consultants that obligate them to assign any inventions created during their tenure with us. However, we may not obtain these agreements in all circumstances and the assignment of intellectual property under such agreements may not be self-executing. If the employees, collaborators or consultants that are parties to these agreements breach or violate their respective terms, we may not have adequate remedies for any such breach or violation. Our trade secrets could also be misappropriated by our competitors. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, time-consuming and potentially distracting, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent such a party from using that technology or information to compete with us. If our trade secrets are disclosed to or misappropriated or independently developed by a third party, it would harm our ability to protect our rights and could materially harm our business and competitive position.
83
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We may employ individuals or engage consultants that previously worked with other organizations, including our competitors or potential competitors. Although we seek to ensure that such persons do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or they, or both, have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third party. Litigation may be necessary to defend against these claims. If we fail in defending any such claims or settling those claims, we may lose valuable intellectual property rights or personnel in addition to paying monetary damages or a settlement. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Patent terms may be inadequate to protect our competitive position on our HAVs or our other product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our HAVs are obtained, once the patent life has expired, we may face competition, including from other competing technologies. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, defending and enforcing patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in countries outside the United States, or from selling or importing products made using our inventions in and into or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement rights are not as strong as those in the United States. These products may compete with our products, to the extent approved, and our owned or licensed patents or other intellectual property rights may not be effective or sufficient to prevent them from doing so.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our owned or licensed patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our owned or licensed patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded to us, if any, may not be commercially meaningful.
Many countries have compulsory licensing laws under which a patent owner may be compelled under specified circumstances to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In those countries, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license, which could adversely affect our business, financial condition, results of operations, and prospects.
84
Some of our internal intellectual property and most of our in-licensed intellectual property has been generated under U.S. Government grants and contracts that trigger certain obligations and U.S. Government rights and thus is subject to federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies. Compliance with such regulations may limit our exclusive rights and limit our ability to contract with non-U.S. manufacturers.
Some of our internal intellectual property and most of our in-licensed intellectual property has been generated under U.S. Government grants and contracts that trigger certain obligations and U.S. Government rights under federal statutes and regulations, including the Bayh-Dole Act of 1980 and the Federal Technology Transfer Act of 1986. For example, the U.S. Government has a non-exclusive, non-transferable, irrevocable worldwide license to inventions conceived or first actually reduced to practice in the performance of a U.S. Government agreement. In addition, the U.S. Government has certain “march-in” rights to require us to grant exclusive, partially exclusive, or non-exclusive licenses to such inventions for the benefit of a third party if the U.S. Government determines that: (i) action is necessary to alleviate health or safety needs not reasonably met by us, our assignees, our licensees, or, in some cases, our licensors, (ii) action is necessary due to noncompliance with a U.S.-based manufacturing requirement applicable to exclusive licenses, (iii) action is necessary to meet requirements for public use specified by federal regulations and such requirements are not reasonably satisfied by us, our assignees, our licensees, and, in some cases, our licensors, and (iv) with respect to inventions made under funding agreements, adequate steps have not been taken to achieve practical application of the invention. The U.S. Government also has the right to take title to these inventions if we, or the applicable licensor, fails to disclose, elect title to, file or prosecute a patent application for, or defend or obtain a patent covering such inventions within time limits specified in particular funding agreements. The U.S. Government also has varying rights to use and disclose information, including copyrighted works, generated or delivered under a U.S. Government agreement depending on the terms of the agreement and the nature of the information. Intellectual property generated under a U.S. Government agreement is also subject to certain reporting requirements, compliance with which may require us or the applicable licensor to expend substantial resources. In addition, when inventions that are conceived or first actually reduced to practice under a U.S. Government funding agreement are exclusively licensed, products embodying or produced through the use of such inventions must be manufactured substantially in the United States. This U.S.-based manufacturing requirement may limit our ability to contract with non-U.S. companies to produce a covered product, although this requirement can be waived in certain circumstances. To the extent that any of our licensors’ current or future intellectual property is generated in the performance of U.S. Government grants or contracts, these requirements may apply to such intellectual property.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our marks of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to make products that are similar to any product candidates we may develop or utilize similar technology but that are not covered by the claims of the patents that we own or license or may own or license in the future;
•we, or our current or future licensors might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or license or may own or license in the future;
•we, or our current or future licensors might not have been the first to file patent applications covering certain of our or their inventions;
85
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights;
•it is possible that our and our licensors’ pending owned or licensed patent applications or those that we may own or license in the future will not lead to issued patents;
•issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may harm our business; and
•we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
Should any of these events occur, they could harm our business, financial condition, results of operations, and prospects.
Risks Related to Business Matters and Our Ability to Manage Growth
Our future success depends on our ability to retain our key employees, consultants and advisors and to attract, retain and motivate qualified personnel.
We are highly dependent on the research and development, clinical, regulatory, financial, commercial, and manufacturing expertise of the principal members of our management, scientific and clinical teams. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, losing or replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain marketing approval of and commercialize our product candidates. Competition to hire from this limited pool is intense. We also experience competition for the hiring of scientific and clinical personnel from public and private universities and research institutions. In addition, we rely on consultants and advisors, including scientific, commercial and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may have commitments under employment, consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
We expect to increase the size of our workforce in the future, and we may encounter difficulties in managing this growth, which could harm our operations.
As of December 31, 2022, we had 164 employees. As we move forward in our efforts to commercialize our HAVs, if approved, we expect to continue to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of development, regulatory affairs, manufacturing and quality and compliance and support functions. Due to our limited financial resources, we may not be able to effectively manage the expansion of our operations, maintain competitive compensation packages, or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage this growth effectively could delay the execution of our business plans or harm our operations.
Risks Related to Ownership of Our Securities
The price of our common stock may be volatile.
The price of our common stock may fluctuate due to a variety of factors, including:
•actual or anticipated fluctuations in our quarterly and annual results and those of other public companies in our industry;
86
•mergers and strategic alliances in the industry in which we operate;
•market prices and conditions in the industry in which we operate;
•changes in government regulation;
•potential or actual military conflicts or acts of terrorism;
•announcements concerning Humacyte or our competitors; and
•the general state of the securities markets.
These market and industry factors may materially reduce the market price of our common stock, regardless of our operating performance.
Reports published by analysts, including projections in those reports that differ from our actual results, could adversely affect the price and trading volume of our common stock.
We expect that securities research analysts will establish and publish their own periodic projections for the business of Humacyte. These projections may vary widely and may not accurately predict the results we actually achieve. Our stock price may decline if our actual results do not match the projections of these securities research analysts. Similarly, if one or more of the analysts who write reports on Humacyte downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price could decline. If one or more of these analysts ceases coverage of Humacyte or fails to publish reports on Humacyte regularly, our stock price or trading volume could decline.
We may issue additional shares of common stock or other equity securities without stockholder approval, which would dilute your ownership interests and may depress the market price of our common stock.
As of December 31, 2022, we had warrants outstanding to purchase up to an aggregate of 5,588,506 shares of our common stock and options outstanding to purchase up to an aggregate of 7,203,874 shares of our common stock. Under the Humacyte, Inc. 2021 Long-Term Incentive Plan (the “2021 Plan”) and the Humacyte, Inc. 2021 Employee Stock Purchase Plan (the “ESPP”), as of December 31, 2022 we also have the ability to issue 6,700,888 shares and 1,030,033 shares, respectively. In addition, the aggregate number of shares under the 2021 Plan and the ESPP will automatically increase on January 1 of each year commencing January 1, 2022, in an amount equal to 5% and 1%, respectively, of the number of shares of our capital stock outstanding on December 31 of the preceding year, unless our board of directors (the “Board”) acts prior to January 1 of a given year to provide that the increase for such year will be a lesser number. At the end of 2021 and 2022, our Board elected not to increase the number of shares under the 2021 Plan and the ESPP. We may also issue additional shares of common stock or other equity securities of equal or senior rank in the future in connection with, among other things, future acquisitions or repayment of outstanding indebtedness, without stockholder approval, in a number of circumstances.
Our issuance of additional shares of common stock or other equity securities of equal or senior rank would have the following effects:
•our existing stockholders’ proportionate ownership interest in Humacyte will decrease;
•the amount of cash available per share, including for payment of dividends in the future, may decrease;
•the relative voting strength of each previously outstanding share of common stock may be diminished; and
•the market price of shares of our common stock may decline.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gains and you may never receive a return on your investment.
We may retain future earnings, if any, for future operations, expansion and debt repayment and have no current plans to pay any cash dividends for the foreseeable future. Any decision to declare and pay dividends as a public company in the future will be made at the discretion of the Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that the Board may deem relevant. In addition, our ability to pay dividends may be limited by covenants of any existing and future outstanding indebtedness we
87
or our subsidiaries incur. As a result, you may not receive any return on an investment in our securities unless you sell your securities for a price greater than that which you paid for it.
The Public Warrants may not be in the money in the future, and they may expire worthless, and the terms of the Public Warrants may be amended in a manner adverse to a holder if holders of at least 50% of the then outstanding Public Warrants approve of such amendment.
In connection with the Merger, the Company assumed 5,000,000 publicly-traded warrants (“Public Warrants”) and 177,500 private placement warrants issued to AHAC Sponsor LLC (the “Sponsor”), Oppenheimer & Co. Inc. and Northland Securities, Inc, in connection with AHAC’s initial public offering (“Private Placement Warrants” and, together with the Public Warrants, the “Warrants”). The Warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and our predecessor AHAC. The warrant agreement provides that the terms of the Warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision or correct any mistake, but requires the approval by the holders of at least 50% of the then-outstanding Public Warrants to make any change that adversely affects the interests of the registered holders of Public Warrants. Accordingly, we may amend the terms of the Public Warrants in a manner adverse to a holder if holders of at least 50% of the then-outstanding Public Warrants approve of such amendment and, solely with respect to any amendment to the terms of the Private Placement Warrants or any provision of the warrant agreement with respect to the Private Placement Warrants, holders of at least 50% of the number of the then outstanding Private Placement Warrants. Although our ability to amend the terms of the Public Warrants with the consent of at least 50% of the then-outstanding Public Warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the Warrants, convert the Warrants into cash, shorten the exercise period or decrease the number of shares of common stock purchasable upon exercise of a Warrant.
We may redeem your unexpired Public Warrants prior to their exercise at a time that is disadvantageous to you, thereby making your Public Warrants worth less than they would be if you held and exercised them at a later time.
We have the ability to redeem outstanding Public Warrants prior to their expiration, at a price of $0.01 per Warrant, provided that the last reported sales price of our common stock equals or exceeds $18.00 per share (as adjusted for share subdivisions, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to the date we send the notice of redemption to the holders thereof. If and when the Public Warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding Public Warrants could force you to: (i) exercise your Public Warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so; (ii) sell your Public Warrants at the then-current market price when you might otherwise wish to hold your Public Warrants; or (iii) accept the nominal redemption price which, at the time the outstanding Public Warrants are called for redemption, is likely to be substantially less than the market value of your Public Warrants.
The value received upon exercise of the Public Warrants (i) may be less than the value the holders would have received if they had exercised their Public Warrants at a later time where the underlying share price is higher and (ii) may not compensate the holders for the value of the Public Warrants.
The Private Placement Warrants are not subject to the same risk of redemption as the Public Warrants as the Private Placement Warrants are not redeemable so long as they are held by the Sponsor, the underwriters of AHAC’s initial public offering or their permitted transferees. If the Private Placement Warrants are held by holders other than the Sponsor, the underwriters or their permitted transferees, the Private Placement Warrants will be redeemable by us.
We have derivative securities that are accounted for as liabilities and the changes in value of such derivative securities could have a material effect on our financial results.
Included on the Company’s consolidated balance sheets as of December 31, 2022 are derivative liabilities related to the Contingent Consideration and the Private Placement Warrants. Accounting Standards Codification 815, Derivatives and Hedging (“ASC 815”), provides for the remeasurement of the fair value of such derivatives at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value being recognized in earnings in the statement of operations. As a result of the recurring fair value measurement, our financial statements and results of operations may fluctuate quarterly, based on factors which are outside of our control. Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on the Contingent Consideration and the Private Placement Warrants each reporting period and that the amount of such gains or losses could be material.
88
Prior to the Merger, on April 12, 2021, the Acting Director of the Division of Corporation Finance and Acting Chief Accountant of the SEC together issued a statement regarding the accounting and reporting considerations for warrants issued by special purpose acquisition companies entitled “Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”)” (the “SEC Statement”). Specifically, the SEC Statement focused on certain settlement terms and provisions related to certain tender offers following a business combination, which terms are similar to those contained in the Public Warrants. As a result of the SEC Statement, prior to the Merger, AHAC reevaluated the accounting treatment of the Public Warrants and determined to classify the Public Warrants as derivative liabilities measured at fair value, with changes in fair value each period reported in earnings. As a result, included on AHAC’s balance sheet as of December 31, 2020 are derivative liabilities related to embedded features contained within the Public Warrants.
In connection with its Amended Annual Report on Form 10-K/A for the year ended December 31, 2020, AHAC reached a determination to restate certain previously issued financial statements and related disclosures for the periods disclosed in order to correct the accounting treatment for the Warrants following the publication of the SEC Statement. As a result, prior to the Merger, AHAC incurred unanticipated costs for accounting and legal fees in connection with or related to the restatement, and we may become subject to additional risks and uncertainties related to the restatement.
AHAC restated certain previously issued financial statements and related disclosures for the periods disclosed, and as of September 30, 2021, our management concluded that the conditions causing the material weakness that led to these restatements did not exist. However, in the future, we may determine that we have additional material weaknesses. Our failure to remediate any material weaknesses or failure to identify and address any material weaknesses or control deficiencies could result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis, which could cause investors to lose confidence in our reported financial information, which may result in volatility in and a decline in the market price of our common stock.
Our business could be adversely impacted by inflation.
Increases in inflation may have an adverse effect on our business. Current and future inflationary effects may be driven by, among other things, supply chain disruptions and governmental stimulus or fiscal policies. Continuing increases in inflation could impact the overall demand for our products, our costs for labor, material and services, and the margins we are able to realize on our products, all of which could have an adverse impact on our business, financial position, results of operations and cash flows. Inflation may also result in higher interest rates, which in turn would result in higher interest expense related to our variable rate indebtedness and any borrowings we undertake to refinance existing fixed rate indebtedness.
We may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
We may be forced to write-down or write-off assets, restructure our operations, or incur impairment or other charges that could result in losses. Even though these charges may be non-cash items and may not have an immediate impact on our liquidity, the fact that we may report charges of this nature could contribute to negative market perceptions about our securities. In addition, charges of this nature may cause us to be unable to obtain future financing on favorable terms or at all. Accordingly, a stockholder could suffer a reduction in the value of their shares.
The obligations associated with being a public company involve significant expenses and will require significant resources and management attention, which may divert from our business operations.
As a public company, we are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act. The Exchange Act requires the filing of annual, quarterly and current reports with respect to a public company’s business and financial condition. The Sarbanes-Oxley Act requires, among other things, that a public company establish and maintain effective internal control over financial reporting. As a result, we will incur significant legal, accounting and other expenses that we did not incur as a private company. Our entire management team and many of its other employees will need to devote substantial time to compliance, and may not effectively or efficiently manage our transition into a public company.
These rules and regulations will result in our incurring substantial legal and financial compliance costs and will make some activities more time-consuming and costly. For example, these rules and regulations have made it more difficult and more expensive for Humacyte to obtain director and officer liability insurance, and it has accepted reduced coverage. As a
89
result, it may be difficult for us to attract and retain qualified people to serve on the Board or committees of the Board or as executive officers.
We are an “emerging growth company” and a “smaller reporting company” within the meaning of the rules adopted by the SEC, and if we take advantage of certain exemptions from disclosure requirements available to emerging growth companies and smaller reporting companies, this could make our securities less attractive to investors and may make it more difficult to compare our performance with other public companies.
We are an emerging growth company as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, our stockholders may not have access to certain information they may deem important. We could be an emerging growth company for up to five years from the closing of AHAC’s initial public offering, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 before that time, in which case we would no longer be an emerging growth company as of the following December 31. We cannot predict whether investors will find our securities less attractive because we will rely on these exemptions. If some investors find our securities less attractive as a result of our reliance on these exemptions, the trading prices of our securities may be lower than they otherwise would be, there may be a less active trading market for our securities and the trading prices of our securities may be more volatile.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies, but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Additionally, we are a “smaller reporting company” as defined under the Exchange Act. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company for so long as (1) the market value of our common stock held by non-affiliates is less than $250 million as of the last business day of the second fiscal quarter, or (2) our annual revenues in our most recent fiscal year completed before the last business day of our second fiscal quarter are less than $100 million and the market value of our common stock held by non-affiliates is less than $700 million as of the last business day of the second fiscal quarter. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports. Any failure to implement new or improved controls necessary to maintain effective internal control over financial reporting, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations.
In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or our independent registered public accounting firm, may identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Ineffective internal control over financial reporting could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock. In connection with its
90
Amended Annual Report on Form 10-K/A for the year ended December 31, 2020, AHAC reached a determination to restate certain previously issued financial statements and related disclosures for the periods disclosed in order to correct the accounting treatment for the Warrants following the publication of the SEC Statement.
Our assessment is that, after the Merger, we have a sufficiently staffed and technically experienced finance and accounting team to address the financial reporting requirements of a public company. Because the conditions causing the material weakness no longer existed, and are not expected to exist in the foreseeable future, we determined the material weakness did not exist in internal control over financial reporting as of September 30, 2021. Prior to the Merger, AHAC’s management concluded that its disclosure controls and procedures were not effective as of December 31, 2020, and that its internal control over financial reporting was not effective as of December 31, 2020, as a result of a material weakness in controls related to the accounting for the Warrants. As a result, AHAC incurred unanticipated costs for accounting and legal fees in connection with or related to the restatement, and we may become subject to additional risks and uncertainties related to the restatement, such as a negative impact on investor confidence in the accuracy of our financial disclosures, and may face reputational risks for our business. Effective as of the closing of the Merger, our management is responsible for internal control over financial reporting and the former management of AHAC no longer participates in financial reporting.
As long as we are an emerging growth company under the JOBS Act or a non-accelerated filer and a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act. An independent assessment of the effectiveness of our internal control over financial reporting could detect deficiencies that our management’s assessment might not. Undetected material weaknesses in our internal control over financial reporting could lead to financial statement restatements and require us to incur the expense of remediation.
Anti-takeover provisions in our Second Amended and Restated Certificate of Incorporation and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult, and may prevent attempts by our stockholders to replace or remove our current management.
Our Second Amended and Restated Certificate of Incorporation (the “Charter”) contains provisions that may delay or prevent an acquisition of the company or change in our management. These provisions may make it more difficult for stockholders to replace or remove members of the Board. Because the Board is responsible for appointing the members of the management team, these provisions could in turn frustrate or prevent any attempt by our stockholders to replace or remove our current management. In addition, these provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. Among other things, these provisions include:
•the limitation of the liability of, and the indemnification of, our directors and officers;
•provisions that permit only (i) the chairperson of the Board, (ii) our chief executive officer or (iii) a majority of our Board to call special meetings of stockholders and therefore do not permit our stockholders to call stockholder meetings;
•a prohibition on actions by our stockholders by written consent; and
•the ability of the Board to issue preferred stock without stockholder approval.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), which prohibits a person who owns 15% or more of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired 15% or more of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. This could discourage, delay or prevent a third party from acquiring or merging with us, whether or not it is desired by, or beneficial to, our stockholders. This could also have the effect of discouraging others from making tender offers for our common stock, including transactions that may be in our stockholders’ best interests. Finally, these provisions establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. These provisions would apply even if the offer may be considered beneficial by some stockholders.
Our Charter provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware and the federal district courts of the United States of America are the exclusive forums for
91
substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our Charter provides that the Court of Chancery of the State of Delaware will be the exclusive forum for the following types of actions or proceedings under Delaware statutory or common law:
•any derivative action or proceeding brought on our behalf;
•any action asserting a breach of fiduciary duty;
•any action asserting a claim against us arising under the DGCL, our Charter or our amended and restated bylaws (the “Bylaws”);
•any action or proceeding asserting a claim as to which the DGCL confers jurisdiction upon the Court of Chancery of the State of Delaware; and
•any action asserting a claim against us that is governed by the internal affairs doctrine or otherwise related to our internal affairs.
This exclusive forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, other employees or stockholders, which may discourage lawsuits with respect to such claims. We cannot be certain that a court will decide that this provision is either applicable or enforceable, and if a court were to find the choice of forum provision contained in our Charter to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition.
This exclusive forum provision will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. In addition, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall, to the fullest extent permitted by law, be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act or the rules and regulations promulgated thereunder.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate headquarters, manufacturing, and research and development facilities are located in Durham, North Carolina where we lease approximately 83,000 square feet of space. This space includes approximately 55,000 square feet for production and distribution operations including manufacturing, bioprocessing, quality control, mechanical space and inventory. The remainder of the facility consists of offices, laboratories, and common spaces.
Item 3. Legal Proceedings
The Company currently is not aware of any legal proceedings or claims that management believes will have, individually or in the aggregate, a material adverse effect on the Company’s business, financial condition, results of operations, or cash flows.
Item 4. Mine Safety Disclosures.
None.
92
Part II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is currently listed on The Nasdaq Global Select Market under the symbol “HUMA.” Prior to the consummation of the Merger, our common stock was listed on The Nasdaq Global Select Market under the symbol “AHAC.” As of March 10, 2023, there were 201 holders of record of our common stock.
Dividend Policy
We have never declared or paid any dividends on shares of our common stock. We anticipate that we will retain all of our future earnings, if any, for use in the operation and expansion of our business and do not anticipate paying cash dividends in the foreseeable future. We are currently not permitted to pay cash dividends under the Loan Agreement with Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P. Any decision to declare and pay dividends in the future will be made at the sole discretion of our Board will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our board of directors may deem relevant.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our accompanying consolidated financial statements and the related notes contained in Part II, Item 8 of this Annual Report on Form 10-K. Unless the context indicates otherwise, references in this Annual Report on Form 10-K to the “Company,” “Humacyte,” “we,” “us,” “our” and similar terms refer to Humacyte, Inc. (formerly known as Alpha Healthcare Acquisition Corp.) and its consolidated subsidiaries (including Humacyte Global, Inc.) following the Merger (defined below); references to “Legacy Humacyte” refer to Humacyte, Inc. prior to the Merger; and references to “AHAC” refer to Alpha Healthcare Acquisition Corp. prior to the Merger.
Cautionary Statement Regarding Forward-Looking Statements
In addition to historical information, some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, future financial performance, expense levels and liquidity sources, includes forward-looking statements that involve risks and uncertainties. You should read the sections of this Annual Report on Form 10-K titled “Forward-Looking Statements” and “Risk Factors” for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are pioneering the development and manufacture of off-the-shelf, universally implantable, bioengineered human tissues, advanced tissue constructs and organ systems with the goal of improving the lives of patients and transforming the practice of medicine. We believe our regenerative medicine technology has the potential to overcome limitations in existing standards of care and address the lack of significant innovation in products that support tissue repair, reconstruction and replacement. We are leveraging our novel, scalable technology platform to develop proprietary product candidates for use in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas.
We are initially using our proprietary, scientific technology platform to engineer and manufacture HAVs. Our investigational HAVs are designed to be easily implanted into any patient without inducing a foreign body response or leading to immune rejection. We are developing a portfolio, or “cabinet”, of HAVs with varying diameters and lengths. The HAV cabinet would initially target the vascular repair, reconstruction and replacement market, including use in vascular trauma; AV access for hemodialysis; PAD; and CABG. In addition, we are developing our HAVs for pediatric heart surgery and the delivery of cellular therapies, including pancreatic islet cell transplantation to treat Type 1 diabetes (our biovascular pancreas). We will continue to explore the application of our technology across a broad range of markets and indications, including the development of urinary conduit, trachea, esophagus and other novel cell delivery systems.
93
We believe there is substantial clinical demand for safe and effective vascular conduits to replace and repair blood vessels throughout the body. Vascular injuries resulting from trauma are common in civilian and military populations, frequently resulting in the loss of either life or limb. Existing treatment options in the vascular repair, reconstruction and replacement market include the use of autologous vessels and synthetic grafts, which we believe suffer from significant limitations. For example, the use of autologous veins to repair traumatic vascular injuries can lead to significant morbidity associated with the surgical wounds created for vein harvest and prolonged times to restore blood flow to injured limbs leading to an increased risk of amputation and infection. Synthetic grafts are often contraindicated in the setting of vascular trauma due to higher infection risk that can lead to prolonged hospitalization and limb loss. Given the competitive advantages our HAVs are designed to have over existing vascular substitutes, we believe that HAVs have the potential to become the standard of care and lead to improved patient outcomes and lower healthcare costs.
We are currently conducting Phase 2 and Phase 3 trials of our 6 millimeter HAV across two therapeutic indications, vascular trauma and AV access for hemodialysis, as well as continuing long-term follow up of patients in our Phase 2 PAD studies. We were granted Fast Track designation by the FDA for our 6 millimeter HAV for use in AV access for hemodialysis in 2014. We also received the first RMAT designation from the FDA, for the creation of vascular access for performing hemodialysis, in March 2017. In addition, in 2018 our HAV product candidate was assigned a priority designation by the Secretary of Defense under Public Law 115-92, enacted to expedite the FDA’s review of products that are intended to diagnose, treat or prevent serious or life-threatening conditions facing American military personnel. Upon completion of our Phase 3 trials, we intend to submit a BLA to the FDA for an indication in vascular trauma.
We have generated no product revenue and incurred operating losses and negative cash flows from operations in each year since our inception in 2004. As of December 31, 2022 and 2021, we had an accumulated deficit of $426.5 million and $414.6 million, respectively, and working capital of $134.6 million and $218.3 million, respectively. Our operating losses were approximately $84.6 million and $81.2 million for the years ended December 31, 2022 and 2021, respectively. Net cash flows used in operating activities were $71.1 million and $81.2 million during the years ended December 31, 2022 and 2021, respectively. Substantially all of our operating losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to incur substantial operating losses and negative cash flows from operations for the foreseeable future as we advance our product candidates.
As of December 31, 2022, we had cash and cash equivalents and short-term investments of $151.9 million. We believe our cash and cash equivalents and short-term investments on hand will be sufficient to fund operations, including clinical trial expenses and capital expenditure requirements for at least the next 12 months from the date of this Annual Report on Form 10-K. See Note 1 — Organization and Description of Business in the notes to our accompanying consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K for additional information regarding this assessment.
Our need for additional capital will depend in part on the scope and costs of our development and commercial manufacturing activities. To date, we have not generated any revenue from the sale of commercialized products. Our ability to generate product revenue will depend on the successful development and eventual commercialization of one or more of our product candidates. Until such time, if ever, we expect to finance our operations through the use of existing cash and cash equivalents and short-term investments, the sale of equity or debt, borrowings under credit facilities, or through potential collaborations, other strategic transactions or government and other grants. Adequate capital may not be available to us when needed or on acceptable terms. If we are unable to raise capital, we could be forced to delay, reduce, suspend or cease our research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition. See “Risk Factors” for additional information.
We expect to continue to incur significant expenses and to increase operating losses for at least the next several years. We anticipate that our expenses will increase substantially as we seek to:
•obtain marketing approval for our 6 millimeter HAV for vascular repair, reconstruction and replacement, including for vascular trauma and AV access for hemodialysis;
•commercialize the HAV via U.S. market launches in vascular trauma and hemodialysis AV access;
•scale out our manufacturing facility to the extent required to satisfy potential demand following any receipt of marketing approval;
•continue our preclinical and clinical development efforts;
94
•maintain, expand and protect our intellectual property portfolio;
•add operational, financial and management information systems and personnel to support, among other things, our product development and commercialization efforts and operations; and
•continue operating as a public company, which includes higher costs associated with hiring additional personnel, director and officer insurance premiums, audit and legal fees, expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC and The Nasdaq Stock Market LLC (“Nasdaq”).
Merger
On August 26, 2021 (the “Closing Date”), Legacy Humacyte and AHAC consummated a merger pursuant to that certain Business Combination Agreement, dated as of February 17, 2021 (the “Merger Agreement”), by and among Legacy Humacyte, AHAC and Hunter Merger Sub, Inc. (“Merger Sub”), a Delaware corporation and wholly owned subsidiary of AHAC. As contemplated by the Merger Agreement, Merger Sub merged with and into Legacy Humacyte, with Legacy Humacyte continuing as the surviving corporation and as a wholly owned subsidiary of AHAC (the “Merger”). On the Closing Date, AHAC changed its name to Humacyte, Inc. and Legacy Humacyte changed its name to Humacyte Global, Inc. Operations prior to the Merger included in this Annual Report on Form 10-K are those of Legacy Humacyte.
Pursuant to the terms of the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (1) each outstanding share of common stock of Legacy Humacyte (“Legacy Humacyte common stock”) was cancelled and converted into the right to receive approximately 0.26260 shares of the Company’s common stock, par value $0.0001 per share (“Common Stock”), and (2) each outstanding share of preferred stock of Legacy Humacyte (“Legacy Humacyte preferred stock”) was cancelled and converted into the aggregate number of shares of Common Stock that would be issued upon conversion of the shares of Legacy Humacyte preferred stock based on the applicable conversion ratio immediately prior to the Effective Time, multiplied by approximately 0.26260, resulting in the issuance of a total of 75,656,935 shares of Common Stock. Prior holders of shares of Legacy Humacyte common stock and Legacy Humacyte preferred stock also received the contingent right to receive certain Contingent Earnout Shares (as defined below), for each share owned by each such Legacy Humacyte stockholder that was outstanding immediately prior to the closing of the Merger (the “Closing”). In addition, certain investors purchased an aggregate of 17,500,000 shares of Common Stock (such investors, the “PIPE Investors”) in a private placement that closed concurrently with the Closing for an aggregate purchase price of $175 million (the “PIPE Financing”). Additionally, at the Closing, 2,500,000 shares of AHAC’s Class B common stock (“Founder Shares”) automatically converted into shares of Common Stock on a one-for-one basis.
Following the Closing Date, former holders of Legacy Humacyte common stock and Legacy Humacyte preferred stock may receive up to 15,000,000 additional shares of Common Stock (“Contingent Earnout Shares”) in the aggregate in two equal tranches if the volume-weighted average closing sale price of our Common Stock is greater than or equal to $15.00 and $20.00, respectively, for any 20 trading days within any 30 consecutive trading day period.
Unless otherwise noted, the Company has retroactively adjusted all common and preferred share and related price information to give effect to the exchange ratio established in the Merger Agreement.
Impact of COVID-19
The COVID-19 outbreak and government measures taken in response have had a significant impact, both direct and indirect, on our business, as supply chains have been disrupted and enrollment in clinical trials has been delayed. To date, the COVID-19 pandemic has not resulted in material financial impacts or impairment losses in the carrying values of our assets and we are not aware of any specific related event or circumstance that would require us to revise the estimates reflected in our financial statements. The extent to which the ongoing effects of the COVID-19 pandemic will directly or indirectly impact our business, results of operations and financial condition, including current and future clinical trials and research and development costs and timelines, will depend on future developments that are highly uncertain, including as a result of new information that may emerge concerning COVID-19, the emergence of new virus variants, and the duration and intensity of the related economic impact of the COVID-19 pandemic.
95
Components of Results of Operations
Revenue
To date, we have not generated revenue from the sale of any products. All of our revenue has been derived from government and other grants. Since inception we have been awarded grants from the California Institute of Regenerative Medicine (“CIRM”), NIH, and the DoD, to support our development, production scaling and clinical trials of our product candidates. We may generate revenue in the future from government and other grants, payments from future license or collaboration agreements and, if any of our product candidates receive marketing approval, from product sales. We expect that any revenue we generate will fluctuate from quarter to quarter. If we fail to complete the development of, or obtain marketing approval for, our product candidates in a timely manner, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely affected.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, developing our manufacturing process and activities related to regulatory filings for our product candidates. We recognize research and development expenses as they are incurred. Our research and development expenses consist primarily of:
•salaries and related overhead expenses for personnel in research and development functions, including stock-based compensation and benefits;
•fees paid to consultants and CROs, including in connection with our clinical trials, and other related clinical trial fees, such as for investigator grants, patient screening, laboratory work and statistical compilation and analysis;
•allocation of facility lease and maintenance costs;
•depreciation of leasehold improvements, laboratory equipment and computers;
•costs related to purchasing raw materials and producing our product candidates for clinical trials;
•costs related to compliance with regulatory requirements;
•costs related to our manufacturing development and expanded-capabilities initiatives; and
•license fees related to in-licensed technologies.
The majority of our research and development resources are currently focused on our Phase 2 and 3 clinical trials for our 6 millimeter HAV and other work needed to obtain marketing approval for our 6 millimeter HAV for use for vascular repair, reconstruction and replacement, including vascular trauma and AV access in hemodialysis in the United States. We have incurred and expect to continue to incur significant expenses in connection with these and our other clinical development efforts, including expenses related to regulatory filings, trial enrollment and conduct, data analysis, patient follow up and study report generation for our Phase 2 and Phase 3 clinical trials. We do not allocate all of our costs by each research and development program for which we are developing our cabinet of HAVs, as a significant amount of our development activities broadly support multiple programs that use our technology platform. We plan to further increase our research and development expenses for the foreseeable future as we continue the development of our proprietary scientific technology platform and our novel manufacturing paradigm.
The successful development of our preclinical and clinical product candidates is highly uncertain. At this time, we cannot estimate with any reasonable certainty the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our preclinical or clinical product candidates or the period, if any, in which material net cash inflows from these product candidates may commence. This is due to the numerous risks and uncertainties associated with the development of our product candidates, including:
•the scope, rate of progress, expense and results of our preclinical development activities, our ongoing clinical trials and any additional clinical trials that we may conduct, and other research and development activities;
•successful patient enrollment in and the initiation and completion of clinical trials;
96
•the timing, receipt and terms of any marketing approvals from applicable regulatory authorities including the FDA and non-U.S. regulators;
•the extent of any required post-marketing approval commitments to applicable regulatory authorities;
•development of clinical and commercial manufacturing capabilities or making arrangements with third-party manufacturers in order to ensure that it or its third-party manufacturers are able to successfully manufacture our product;
•obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights;
•significant and changing government regulations;
•launching commercial sales of our product candidates, if approved, whether alone or in collaboration with others;
•the degree of market acceptance of any product candidates that obtain marketing approval; and
•maintaining a continued acceptable safety profile following approval, if any, of our product candidates.
A change in the outcome of any of these variables could lead to significant changes in the costs and timing associated with the development of our product candidates. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate being required to conduct in order to complete the clinical development of any of our product candidates, or if we experience significant delays in the enrollment or the conduct of any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for employees in executive, finance, human resources, commercialization, and administrative support functions, which also include stock-based compensation expenses and benefits for such employees. Other significant general and administrative expenses include facilities costs, professional fees for accounting and legal services and expenses associated with obtaining and maintaining patents.
We expect our general and administrative expenses will continue to increase for the foreseeable future to support our expanded infrastructure and increased costs of operating as a public company and as we prepare for our anticipated commercial launch of the HAV. These increases are expected to include increased employee-related expenses, increased sales and marketing expenses, and increased director and officer insurance premiums, audit and legal fees, and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC, as well as Nasdaq rules.
Other Income (Expense), Net
Total other income (expense), net consists of (i) the change in fair value of the Contingent Earnout Liability that was accounted for as a liability as of the date of the Merger, and is remeasured to fair value at each reporting period, resulting in a non-cash gain or loss, (ii) interest income earned on our cash and cash equivalents and short-term investments, (iii) interest expense incurred on our Loan Agreement, finance leases, and our PPP loan during the periods each were outstanding, (iv) a change in fair value of private placement common stock warrant liabilities related to the Private Placement Warrants, which we assumed in connection with the Merger, and which are subject to remeasurement to fair value at each balance sheet date resulting in a non-cash gain or loss, (v) a non-cash gain on PPP loan forgiveness during the year ended December 31, 2021, and (vi) during the year ended December 31, 2021, legal, accounting, and underwriting fees and other costs directly related to the consummation of the Merger that were associated with the aforementioned warrant liabilities.
97
Results of Operations
Comparison of the Years Ended December 31, 2022 and 2021
| Year Ended December 31, | Change | ||||||||||||||||||||||
| ($ in thousands) | 2022 | 2021 | $ | % | |||||||||||||||||||
| Grant revenue | $ | 1,565 | $ | 1,263 | $ | 302 | 24 | % | |||||||||||||||
Operating expenses: | |||||||||||||||||||||||
Research and development | 63,260 | 61,341 | 1,919 | 3 | % | ||||||||||||||||||
General and administrative | 22,883 | 21,130 | 1,753 | 8 | % | ||||||||||||||||||
Total operating expenses | 86,143 | 82,471 | 3,672 | 4 | % | ||||||||||||||||||
Loss from operations | (84,578) | (81,208) | (3,370) | 4 | % | ||||||||||||||||||
| Other income (expense), net: | |||||||||||||||||||||||
| Interest income | 2,629 | 16 | 2,613 | * | |||||||||||||||||||
| Change in fair value of Contingent Earnout Liability | 75,767 | 55,772 | 19,995 | 36 | % | ||||||||||||||||||
| Gain on PPP loan forgiveness | — | 3,284 | (3,284) | (100) | % | ||||||||||||||||||
| Interest expense | (6,200) | (4,348) | (1,852) | 43 | % | ||||||||||||||||||
| Other income, net | 417 | 7 | 410 | * | |||||||||||||||||||
Total other income, net | 72,613 | 54,731 | 17,882 | 33 | % | ||||||||||||||||||
Net loss | $ | (11,965) | $ | (26,477) | $ | 14,512 | (55) | % | |||||||||||||||
* Not meaningful
Grant Revenue
For the years ended December 31, 2022 and 2021, revenue was approximately $1.6 million and $1.3 million, respectively, and related to the reimbursement of qualifying expenses incurred in connection with our grant from DoD. The increase in revenue of $0.3 million, or 24%, relates to the timing of reimbursement of certain allowable costs related to our grant from DoD, which totaled approximately $6.8 million over the life of the grant before this program ended in November 2022.
Research and Development Expenses
The following table discloses the breakdown of research and development expenses:
| Year Ended December 31, | Change | ||||||||||||||||||||||
| ($ in thousands) | 2022 | 2021 | $ | % | |||||||||||||||||||
External services | $ | 15,583 | $ | 15,786 | $ | (203) | (1) | % | |||||||||||||||
Materials and supplies | 11,210 | 10,901 | 309 | 3 | % | ||||||||||||||||||
Payroll and personnel expenses | 23,678 | 23,227 | 451 | 2 | % | ||||||||||||||||||
Other research and development expenses | 12,789 | 11,427 | 1,362 | 12 | % | ||||||||||||||||||
| $ | 63,260 | $ | 61,341 | $ | 1,919 | 3 | % | ||||||||||||||||
Research and development expenses increased from $61.3 million for the year ended December 31, 2021 to $63.3 million for the year ended December 31, 2022. The increase of $1.9 million, or 3%, was primarily driven by expenses incurred to support our expanded research and development initiatives, including a $1.4 million increase in other research and development expenses driven by expanded preclinical studies of our earlier-stage pipeline programs combined with smaller increases in supplies and personnel.
General and Administrative Expenses
General and administrative expenses were $22.9 million and $21.1 million for the years ended December 31, 2022 and 2021, respectively. The increase in general and administrative expenses during this period of $1.8 million, or 8%, was
98
primarily driven by expenses associated with the transition to public company status and company growth, including (i) a $1.2 million increase in salaries and benefits and recruiting costs primarily due to higher headcount, including the initial members of the planned commercial launch team, (ii) a $1.1 million increase in external services, (iii) a $0.6 million increase in insurance costs, (iv) a $0.6 million increase in other general and administrative expenses, including software costs, travel and expenses related to our board of directors, partially offset by a $1.8 million decrease in non-cash stock compensation expense primarily due to higher costs in 2021 resulting from restructuring of the management team to accommodate the transition to being a public company.
Total Other Income, net
Total other income, net was $72.6 million and $54.7 million for the years ended December 31, 2022 and 2021, respectively. The increase of $17.9 million in income primarily resulted from (i) a $20.0 million increase in the non-cash gain related to the remeasurement of the Contingent Earnout Liability during the year ended December 31, 2022 compared to the year ended December 31, 2021, (ii) a $2.6 million increase in interest income, and (iii) a $0.4 million increase in the non-cash gain related to the remeasurement of our private placement warrant liability, partially offset by (i) a $3.3 million gain on PPP loan forgiveness we recognized during the year ended December 31, 2021 and (ii) a $1.9 million increase in interest expense primarily related to our loan facility with Silicon Valley Bank, which commenced in March 2021.
Liquidity and Capital Resources
Sources of Liquidity
To date, we have financed our operations primarily through the sale of equity securities and convertible debt, proceeds from the Merger and related PIPE Financing, borrowings under loan facilities and, to a lesser extent, through grants from governmental and other agencies. Since our inception, we have incurred significant operating losses and negative cash flows. As of December 31, 2022 and 2021, we had an accumulated deficit of $426.5 million and $414.6 million, respectively.
As of December 31, 2022 and 2021, we had cash and cash equivalents and short-term investments of $151.9 million and $225.5 million, respectively. We believe our cash and cash equivalents and short-term investments will be sufficient to fund operations, including clinical trial expenses and capital expenditure requirements for at least 12 months from the date of this Annual Report on Form 10-K. See Note 1 to our accompanying consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K for additional information on our assessment. We believe that our longer-term working capital, planned research and development, capital expenditures and other general corporate funding requirements will be satisfied through the sale of equity, debt, borrowings under credit facilities or through potential collaborations with other companies, other strategic transactions or government or other grants. Our liquidity plans are subject to a number of risks and uncertainties, including those described in the sections of this Annual Report on Form 10-K titled “Forward-Looking Statements” and “Risk Factors.”
As of December 31, 2022 and 2021, we had working capital of $134.6 million and $218.3 million, respectively. As of December 31, 2022, we had $30.0 million outstanding principal and $20.0 million of contingent borrowing capacity under our Loan Agreement (as defined below). We do not currently have any committed external source of funds beyond the Loan Agreement.
Material Cash Requirements
Our known material cash requirements include: (1) the purchase of supplies and services that are primarily for research and development; (2) debt repayments (for additional information, see below and Note 7 to our consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K); (3) employee wages, benefits, and incentives; and (4) financing and operating lease payments (for additional information see below and Note 8 to our accompanying consolidated financial statements in Part II, Item 8 of this Annual Report on Form 10-K). We have also entered into contracts with CROs primarily for clinical trials. These contracts generally provide for termination upon limited notice, and therefore we believe that our non-cancellable obligations under these agreements are not material. Moreover, we may be subject to additional material cash requirements that are contingent upon the occurrence of certain events, for example, legal contingencies, uncertain tax positions, and other matters.
As of December 31, 2022, we had non-cancellable purchase commitments of $32.0 million for supplies and services that are primarily for research and development. We have existing license agreements with Duke University and Yale University and have a distribution agreement with Fresenius Medical Care. The amount and timing of any potential milestone payments, license fee payments, royalties and other payments that we may be required to make under these
99
agreements are unknown or uncertain at December 31, 2022. For additional information regarding these agreements and the nature of payments that could become due thereunder, see the sections in this Annual Report on Form 10-K titled “Business — Distribution” and “Business — Intellectual Property.”
Debt
In March 2021, we entered into the Loan Agreement with Silicon Valley Bank and SVB Innovation Credit Fund VIII, L.P., as amended in June and September 2021, which provides a term loan facility of up to $50.0 million, with a maturity date of March 1, 2025. The initial term loan tranche of $20.0 million was funded upon the closing of the Loan Agreement, and on October 13, 2021, we borrowed an additional $10.0 million under the Loan Agreement. The additional $20.0 million becomes accessible in two tranches of $10.0 million each contingent on the achievement of certain business and clinical development milestones, although we currently do not expect to make any additional borrowings under the Loan Agreement. As a result of the additional borrowing in October 2021, the commencement of repayment of principal was deferred until no earlier than July 2023 and potentially later if the remaining tranches are drawn. As of December 31, 2022, principal of $30.0 million was outstanding under the Loan Agreement and we were in compliance with all covenants in all material respects. Assuming no additional borrowings under the Loan Agreement, we expect to make interest payments of approximately $5.1 million under the Loan Agreement from January 1, 2023 through March 1, 2025, approximately $3.4 million of which we expect to pay during the year ended December 31, 2023.
Our obligations under the Loan Agreement are secured by substantially all of our assets, except for our intellectual property. The Loan Agreement contains certain customary covenants, including, but not limited to, those relating to additional indebtedness, liens, asset divestitures, and affiliate transactions. We may use the proceeds of borrowings under the Loan Agreement as working capital and to fund our general business requirements.
Borrowings under the Loan Agreement bear interest at a rate of 7.5% or the sum of the Wall Street Journal Prime Rate plus 4.25%, whichever is greater (11.75% as of December 31, 2022). In addition, the lenders were granted warrants to purchase common stock. Interest-only payments on the principal amount outstanding are due monthly beginning in the first month after the loan is dispersed. We are required to repay principal beginning on July 1, 2023, unless we draw the remaining two loan tranches, in which case repayment of the outstanding principal amount will begin no later than April 1, 2024. Additionally, we are obligated to pay to the lenders a final payment fee of $1.5 million upon the maturity of the loan.
Our contractual obligations under the Loan Agreement as of December 31, 2022, include $8.6 million in cash payments related to principal within one year and $21.4 million within one to three years.
In April 2020, we received loan proceeds in the amount of approximately $3.3 million under the PPP established under the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”). The loan and accrued interest were forgivable after a 24-week period as long as we used the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintained our payroll levels. On May 25, 2021, the Small Business Administration approved the forgiveness of the outstanding amount of the PPP loan and we recognized a gain from loan extinguishment in the amount of $3.3 million during the year ended December 31, 2021.
Leases
Our finance lease relates to our headquarters facility containing our manufacturing, research and development and general and administrative functions, which was substantially completed in June 2018 and leased through May 2033, and our operating lease relates to the land lease associated with our headquarters. See Note 8 to our accompanying consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K for further information regarding our leases. Our future contractual obligations under our lease agreements as of December 31, 2022 are as follows:
| ($ in thousands) | Total | Less than 1 year | 1 – 3 years | 3 – 5 years | More than 5 years | ||||||||||||||||||||||||
Finance leases | $ | 29,133 | $ | 3,965 | $ | 8,232 | $ | 6,910 | $ | 10,026 | |||||||||||||||||||
Operating leases | 994 | 105 | 210 | 210 | 469 | ||||||||||||||||||||||||
ATM Facility
On September 1, 2022, we entered into an agreement for the sale from time to time up to $80.0 million of shares of Common Stock pursuant to a sales agreement (the “ATM Facility”). As of December 31, 2022, we have not conducted any sales of Common Stock under the ATM Facility.
100
Future Funding Requirements
We expect to incur significant expenses in connection with our ongoing activities as we seek to (i) continue clinical development of our 6 millimeter HAV for use in vascular trauma and hemodialysis AV access and submit biologics license applications for FDA approval, (ii) if marketing approval is obtained, to launch and commercialize our HAVs for hemodialysis AV access and vascular repair in the U.S. market, including subsequent launches in key international markets, (iii) advance our pipeline in major markets, including PAD Phase 3 trials and continue preclinical development and advance to planned clinical studies in CABG and biovascular pancreas for diabetes, and (iv) scale out our manufacturing facility as required to satisfy potential demand if our HAVs receive marketing approval. We will need additional funding in connection with these activities.
Our future funding requirements, both short-term and long-term, will depend on many factors, including:
•the progress and results of our clinical trials and interpretation of those results by the FDA and other regulatory authorities;
•the cost, timing and outcome of regulatory review of our product candidates, particularly for marketing approval of our HAVs in the United States;
•the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our additional product candidates;
•the cost and timing of our future commercialization activities, including product manufacturing, marketing and distribution for our HAVs if approved by the FDA, and any other product candidate for which we receive marketing approval in the future;
•the amount and timing of revenues, if any, that we receive from commercial sales of any product candidates for which we receive marketing approval;
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and
•the costs of operating as a public company, including hiring additional personnel as well as increased director and officer insurance premiums, audit and legal fees, and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC and Nasdaq.
Until such time, if ever, as we are able to successfully develop and commercialize one or more of our product candidates, we expect to continue financing our operations through the sale of equity, debt, borrowings under credit facilities or through potential collaborations with other companies, other strategic transactions or government or other grants. Adequate capital may not be available to us when needed or on acceptable terms. We do not currently have any committed external source of funds beyond the Loan Agreement. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures. Debt financing would also result in fixed payment obligations. If we are unable to raise capital, we could be forced to delay, reduce, suspend or cease our research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition.
Our principal use of cash in recent periods has been primarily to fund our operations, including the clinical and preclinical development of our product candidates. Our future capital requirements, both short-term and long-term, will depend on many factors, including the progress and results of our clinical trials and preclinical development, timing and extent of spending to support development efforts, cost and timing of future commercialization activities, and the amount and timing of revenues, if any, that we receive from commercial sales. See “Risk Factors” for additional risks associated with our substantial capital requirements.
101
Cash Flows
The following table shows a summary of our cash flows for each of the periods shown below:
| Year Ended December 31, | |||||||||||
| ($ in thousands) | 2022 | 2021 | |||||||||
| Net loss | $ | (11,965) | $ | (26,477) | |||||||
Non-cash adjustments to reconcile net loss to net cash used in operating activities(1): | (60,193) | (39,695) | |||||||||
| Payment of liabilities assumed in Merger | — | (14,461) | |||||||||
| Changes in operating assets and liabilities: | 1,029 | (557) | |||||||||
| Net cash used in operating activities | (71,129) | (81,190) | |||||||||
| Net cash provided by (used in) investing activities | 4,845 | (8,220) | |||||||||
| Net cash (used in) provided by financing activities | (1,446) | 266,983 | |||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (67,730) | $ | 177,573 | |||||||
| Cash and cash equivalents at the beginning of the period | $ | 217,502 | $ | 39,929 | |||||||
| Cash and cash equivalents at the end of the period | $ | 149,772 | $ | 217,502 | |||||||
___________________________
(1) Includes depreciation, amortization related to our leases and our debt discount, stock-based compensation expense, the change in fair value of our Contingent Earnout Liability and our common stock warrant liabilities, and in 2021 includes a gain on PPP loan forgiveness.
Cash Flow from Operating Activities
The decrease in net cash used in operating activities from 2021 to 2022 was primarily due to $14.5 million in payments of liabilities acquired in the Merger during the year ended December 31, 2021, partially offset by increased spending on pre-clinical, clinical and pre-commercial activities as well as payroll and personnel expenses.
Cash Flow from Investing Activities
The increase in net cash provided by investing activities from 2021 to 2022 was primarily due to a net cash inflow of $5.9 million related to investments in certificates of deposit classified as short-term investments on our consolidated balance sheets.
Cash Flow from Financing Activities
The decrease in net cash provided by financing activities for the year ended 2022 was primarily due to $242.4 million of proceeds received in August 2021 in connection with the Merger, including proceeds from the trust account that we obtained in connection with the closing of the Merger, as well as from the PIPE Financing, along with $29.7 million of net proceeds in connection with draws under our loan facility with Silicon Valley Bank in March 2021.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in SEC rules and regulations.
102
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues, and expenses, and disclosure of contingent liabilities. We base our estimates and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances. We evaluate our estimates and assumptions on an ongoing basis. Although we believe that our estimates, assumptions, and judgments are reasonable, they are based upon information presently available. Actual results may differ significantly from these estimates based on different assumptions, judgments, or conditions.
An accounting estimate or assumption is considered critical if both (a) the nature of the estimate or assumption involves a significant level of estimation uncertainty, and (b) the impact within a reasonable range of outcomes of the estimate and assumption is material to our financial condition. Our critical accounting policies are summarized below.
Common Stock Warrants
Under the Merger, we assumed 5,000,000 publicly-traded warrants (“Public Warrants”) and 177,500 private placement warrants issued to AHAC in connection with AHAC’s initial public offering (“Private Placement Warrants” and, together with the Public Warrants, the “Common Stock Warrants”). We account for the Common Stock Warrants in accordance with the guidance contained in ASC Topic 480, Distinguishing Liabilities from Equity and ASC Topic 815, Derivatives and Hedging (“ASC 815”).
We account for the Private Placement Warrants in accordance with the guidance contained in ASC 815, under which the warrants do not meet the criteria for equity treatment and must be recorded as liabilities. As the Private Placement Warrants meet the definition of a derivative under ASC 815, we recorded these warrants as liabilities on the consolidated balance sheet at fair value, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date. The fair value of the warrants was estimated using a Monte Carlo simulation value model utilizing assumptions including our current common stock price, expected volatility, risk-free rate, expected term and expected dividend yield. The fair value of the Private Placement Warrants is based on significant unobservable inputs, which represent Level 3 fair value measurements within the fair value hierarchy (see “Fair Value of Financial Instruments” accounting policy described in Note 2 to our accompanying consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K). Determining the fair value of the Private Placement Warrants involves certain assumptions requiring significant judgment and actual results can differ from assumed and estimated amounts.
The Public Warrants are considered to be “indexed to the Company’s own stock” and as we have a single class of common stock, a qualifying cash tender offer of more than 50% of the Company’s common stock will always result in a change-in-control and would not preclude permanent equity classification of the Public Warrants. Based on this evaluation, we concluded that the Public Warrants meet the criteria to be classified within stockholders’ equity.
Contingent Earnout Liability
In connection with the Reverse Recapitalization, Legacy Humacyte equity holders are entitled to receive as additional merger consideration of up to 15,000,000 shares of our common stock in the aggregate, in two equal tranches of 7,500,000 shares of common stock per tranche, for no consideration upon the occurrence of certain triggering events, including a change of control event that is not solely indexed to the common stock. In accordance with ASC 815-40, as the Contingent Earnout Shares were not indexed to the common stock, they were accounted for as a liability at the Reverse Recapitalization date and subsequently remeasured at each reporting date with changes in fair value recorded as a component of other income (expense), net in the consolidated statements of operations and comprehensive loss.
The estimated fair value of the Contingent Earnout Shares was determined using a Monte Carlo simulation valuation model using a distribution of potential outcomes on a monthly basis over a 10-year period prioritizing the most reliable information available. The assumptions utilized in the calculation were based on the achievement of certain stock price milestones, including our current common stock price, expected volatility, risk-free rate, expected term and expected dividend yield. See Note 9 to our accompanying consolidated financial statements contained in Part II, Item 8 of this Annual Report on Form 10-K for further information regarding the assumptions used in the valuation at December 31, 2022 and 2021.
103
The Contingent Earnout Shares are categorized as a Level 3 fair value measurement (see “Fair Value of Financial Instruments” accounting policy described in Note 2 to our financial statements contained elsewhere in this Annual Report on Form 10-K) because we estimated projections over a ten-year period utilizing unobservable inputs. Contingent earnout payments involve certain assumptions requiring significant judgment and actual results can differ from assumed and estimated amounts.
Stock-Based Compensation
We measure and recognize compensation expense for all options based on the estimated fair value of the award on the grant date. We use the Black-Scholes option-pricing model to estimate the fair value of option awards. The fair value is recognized as expense on a straight-line basis over the requisite service period. We account for forfeitures as they occur.
The determination of the grant date fair value of options using an option pricing model is affected principally by our estimated fair value of shares of our common stock and requires management to make a number of other assumptions, including the expected term of the option, the volatility of the underlying shares, the risk-free interest rate and expected dividends. The assumptions used in our Black-Scholes option-pricing model represent management’s good faith estimates at the time of measurement. These estimates are complex, involve a number of variables, uncertainties and assumptions and the application of management’s judgment, as they are inherently subjective. If any assumptions change, our stock-based compensation expense could be materially different in the future.
These assumptions are estimated as follows:
•Fair Value of Common Stock. Subsequent to the Merger, the fair value of our Common Stock has been determined based on the closing price of the shares on Nasdaq. Prior to the Merger, as our common stock was not publicly traded, the fair value of the shares of our common stock underlying the options was determined by our board of directors with input from management, after considering independent third-party valuation reports. See “—Fair Value of Common Stock” and “—Common Stock Valuation Methodology.”
•Expected Term. The expected term represents the period that stock options are expected to be outstanding. We calculated the expected term using the simplified method for options, which is available where there is insufficient historical data about exercise patterns and post-vesting employment termination behavior. The simplified method is based on the vesting period and the contractual term for each grant, or for each vesting-tranche for awards with graded vesting. The mid-point between the vesting date and the maximum contractual expiration date is used as the expected term under this method. For awards with multiple vesting-tranches, the times from grant until the mid-points for each of the tranches may be averaged to provide an overall expected term.
•Expected Volatility. The expected volatility was determined based on a blended approach using the historical share volatility of our Common Stock and that of several publicly traded peer companies over a period of time equal to the expected term of the options, as we have limited trading history. For purposes of identifying these peer companies, we considered the industry, stage of development, size and financial leverage of potential comparable companies.
•Risk-Free Interest Rate. The risk-free interest rate was based on the yields of U.S. Treasury zero-coupon securities with maturities similar in duration to the expected term of the options.
•Expected Dividend Yield. We have not paid dividends on our Common Stock nor do we expect to pay dividends in the foreseeable future. Accordingly, we have estimated the dividend yield to be zero.
Fair Value of Common Stock Post-Merger
Following the closing of the Merger, the fair value of our Common Stock is determined based on the closing price of our Common Stock on Nasdaq on the date stock options or other awards are granted under the 2021 Plan.
Fair Value of Common Stock Pre-Merger
Historically, for all periods prior to the Merger, the fair values of the shares of common stock underlying our options were determined on each grant date by our board of directors with input from management. In order to determine the fair value, our board of directors considered, among other things, contemporaneous valuations of our common stock and preferred stock prepared by an unrelated third-party valuation firm in accordance with the guidance provided by the American Institute of Certified Public Accountants 2013 Practice Aid, Valuation of Privately-Held-Company Equity
104
Securities Issued as Compensation (the “Practice Aid”). Given the absence of a public trading market of our capital stock, the assumptions used to determine the estimated fair value of our common stock were based on a number of objective and subjective factors, including:
•our stage of development and business strategy;
•the prices, rights, preferences and privileges of our redeemable convertible preferred stock relative to our common stock;
•our business, financial condition and results of operations, including related industry trends affecting our operations;
•the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company, given prevailing market conditions;
•the lack of marketability of our common stock;
•the market performance of comparable publicly traded companies; and
•U.S. and global economic and capital market conditions and outlook.
Common Stock Valuation Methodology
The Practice Aid identifies various available methods for allocating enterprise value across classes and series of capital stock to determine the estimated fair value of common stock at each valuation date. In accordance with the Practice Aid, we considered the following methods:
•Option Pricing Method. Under the option pricing method (“OPM”), shares are valued by creating a series of call options with exercise prices based on the liquidation preferences and conversion terms of each equity class. The estimated fair values of the preferred and common stock are inferred by analyzing these options.
•Probability-Weighted Expected Return Method. The probability-weighted expected return method (“PWERM”) is a scenario-based analysis that estimates value per share based on the probability-weighted present value of expected future investment returns, assuming various outcomes, as well as the economic and control rights of each share class.
Based on our early stage of development, we determined that the PWERM method, incorporating the OPM as one of several scenarios, was the most appropriate method for allocating our enterprise value to determine the estimated fair value of Legacy Humacyte common stock for valuations performed as of November 13, 2020 and October 23, 2019, which resulted in common stock valuations of $10.28 and $8.48 per share, respectively. In January, February, March and June 2021, stock options were granted at fair market value with an exercise price of $10.28, consistent with the fair market value determined two months earlier in November 2020. The $10.28 exercise price is greater than the public trading price of the AHAC Class A common stock as of the date of grant and also greater than the share price reflected in the $800 million equity value agreed upon in connection with the Merger. We also utilized the PWERM method for our valuation as of June 25, 2018, which resulted in a common stock valuation of $8.48 per share. In determining the estimated fair value of Legacy Humacyte common stock, our board of directors also considered the fact that our stockholders could not freely trade our common stock in the public markets. Accordingly, we applied discounts to reflect the lack of marketability of our common stock based on the weighted-average expected time to liquidity. The estimated fair value of our common stock at each grant date reflected a non-marketability discount partially based on the anticipated likelihood and timing of a future liquidity event.
Income Taxes
Income taxes are computed using the asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements. In estimating future tax consequences, we consider all expected future events other than enactment of changes in tax laws or rates. A valuation allowance is recorded to reduce net deferred tax assets to their realizable values if management does not believe it is more likely than not that the net deferred tax assets will be realized. As of December 31, 2022 and 2021, we have recorded a full valuation allowance against our net deferred tax assets.
105
We recognize the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. Assessing an uncertain tax position begins with the initial determination of the sustainability of the position and is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed. Additionally, we must accrue interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws. We have not identified any uncertain tax positions for the years ended December 31, 2022 and 2021.
We have analyzed our filing positions in all significant Federal and state jurisdictions where we are required to file income tax returns, as well as open tax years in these jurisdictions. As of December 31, 2022 and 2021, we have determined that no uncertain tax positions would have a material impact on our financial statements. We are no longer subject to U.S. Federal, state, and local tax examinations by tax authorities for years before 2019 although carry-forward attributes that were generated prior to 2019 may still be adjusted upon examination by the taxing authorities if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
As of December 31, 2022 and 2021, we had not recorded any amounts for unrecognized tax benefits. Our policy is to recognize interest and penalties related to uncertain tax positions in the provision for income taxes, if any. As of December 31, 2022 and 2021, we had no accrued interest or penalties related to uncertain tax positions, and no amounts had been recognized in our statements of operations and comprehensive loss.
Emerging Growth Company and Smaller Reporting Company Status
We are an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012 (the “JOBS Act”), and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies until it is no longer an emerging growth company. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards. We expect to use the extended transition period and, therefore, while we are an emerging growth company we will not be subject to new or revised accounting standards at the same time that they become applicable to other public companies that are not emerging growth companies, unless we choose to early adopt a new or revised accounting standard. This may make it difficult or impossible to compare our financial results with the financial results of another public company because of the potential differences in accounting standards used.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K under the Exchange Act (“Regulation S-K”). Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company if (1) the market value of Common Stock held by non-affiliates is less than $250 million as of the last business day of the second fiscal quarter, or (2) our annual revenues in our most recent fiscal year completed before the last business day of its second fiscal quarter are less than $100 million and the market value of Common Stock held by non-affiliates is less than $700 million as of the last business day of the second fiscal quarter.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We qualify as a smaller reporting company, as defined by Item 10 of Regulation S-K and, thus, are not required to provide the information required by this Item.
106
Item 8. Financial Statements and Supplementary Data.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
HUMACYTE, INC.
| Page | |||||
Report of Independent Registered Public Accounting Firm (PCAOB ID 238) | |||||
107
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Humacyte, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Humacyte, Inc. and its subsidiary (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations and comprehensive loss, of changes in redeemable convertible preferred stock and stockholders’ equity (deficit) and of cash flows for the years then ended, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Raleigh, North Carolina
March 24, 2023
We have served as the Company’s auditor since 2013.
108
HUMACYTE, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands except for share and per share amounts)
As of December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| ASSETS | |||||||||||
| Current assets | |||||||||||
Cash and cash equivalents | $ | 149,772 | $ | 217,502 | |||||||
| Short-term investments | 2,107 | 8,000 | |||||||||
Accounts receivable | 31 | 176 | |||||||||
Prepaid expenses and other current assets | 2,298 | 3,662 | |||||||||
Total current assets | 154,208 | 229,340 | |||||||||
Finance lease right-of-use assets, net | 19,373 | 21,432 | |||||||||
Operating lease right-of-use assets, net | 682 | 727 | |||||||||
Property and equipment, net | 30,039 | 35,034 | |||||||||
Total assets | $ | 204,302 | $ | 286,533 | |||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities | |||||||||||
Accounts payable | $ | 1,595 | $ | 2,094 | |||||||
Accrued expenses | 7,108 | 6,757 | |||||||||
| SVB loan payable, current portion | 8,571 | — | |||||||||
Finance lease obligation, current portion | 2,256 | 1,981 | |||||||||
Operating lease obligation, current portion | 50 | 45 | |||||||||
Deferred payroll tax | — | 173 | |||||||||
Total current liabilities | 19,580 | 11,050 | |||||||||
| Contingent Earnout Liability | 27,893 | 103,660 | |||||||||
| SVB loan payable, net of current portion | 20,336 | 27,361 | |||||||||
Finance lease obligation, net of current portion | 18,853 | 21,109 | |||||||||
Operating lease obligation, net of current portion | 632 | 682 | |||||||||
| Common stock warrant liabilities | 80 | 497 | |||||||||
Total liabilities | 87,374 | 164,359 | |||||||||
| Commitments and contingencies (Note 13) | |||||||||||
| Stockholders’ equity | |||||||||||
Preferred stock, $0.0001 par value; 20,000,000 shares designated as of December 31, 2022 and 2021; 0 shares issued and outstanding as of December 31, 2022 and 2021 | — | — | |||||||||
Common stock, $0.0001 par value; 250,000,000 shares authorized as of December 31, 2022 and 2021; 103,229,013 and 103,003,646 shares issued and outstanding as of December 31, 2022 and 2021, respectively | 10 | 10 | |||||||||
Additional paid-in capital | 543,456 | 536,737 | |||||||||
Accumulated deficit | (426,538) | (414,573) | |||||||||
Total stockholders’ equity | 116,928 | 122,174 | |||||||||
Total liabilities and stockholders’ equity | $ | 204,302 | $ | 286,533 | |||||||
The accompanying notes are an integral part of these financial statements.
109
HUMACYTE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands except for share and per share amounts)
Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
Grant revenue | $ | 1,565 | $ | 1,263 | |||||||
| Operating expenses: | |||||||||||
| Research and development | 63,260 | 61,341 | |||||||||
General and administrative | 22,883 | 21,130 | |||||||||
Total operating expenses | 86,143 | 82,471 | |||||||||
Loss from operations | (84,578) | (81,208) | |||||||||
| Other income (expense), net | |||||||||||
Interest income | 2,629 | 16 | |||||||||
| Change in fair value of Contingent Earnout Liability | 75,767 | 55,772 | |||||||||
| Change in fair value of common stock warrant liabilities | 417 | 56 | |||||||||
| Gain on PPP loan forgiveness | — | 3,284 | |||||||||
Interest expense | (6,200) | (4,348) | |||||||||
| Transaction costs expensed | — | (49) | |||||||||
Total other income, net | 72,613 | 54,731 | |||||||||
Net loss and comprehensive loss | $ | (11,965) | $ | (26,477) | |||||||
Net loss per share attributable to common stockholders, basic and diluted | $ | (0.12) | $ | (0.66) | |||||||
Weighted-average shares outstanding used in computing net loss per share attributable to common stockholders, basic and diluted | 103,051,366 | 39,970,398 | |||||||||
The accompanying notes are an integral part of these financial statements.
110
HUMACYTE, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands except for share amounts)
| Redeemable Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders’ (Deficit) Equity | ||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||
Balance as of December 31, 2020 | 69,613,562 | 420,989 | 5,822,396 | $ | 1 | $ | 37,778 | $ | (388,096) | $ | (350,317) | |||||||||||||||||||||||||||||||||
| Issuance of warrants in conjunction with debt | — | — | — | — | 3,275 | — | 3,275 | |||||||||||||||||||||||||||||||||||||
| Conversion of redeemable convertible preferred stock into common stock in connection with the Merger and related PIPE financing | (69,613,562) | (420,989) | 69,613,562 | 7 | 420,982 | — | 420,989 | |||||||||||||||||||||||||||||||||||||
| The Merger and related PIPE financing, net of transaction costs and acquired liabilities | — | — | 27,346,449 | 2 | 209,478 | — | 209,480 | |||||||||||||||||||||||||||||||||||||
| Public warrants assumed upon the Merger, net of transaction costs | — | — | — | — | 13,912 | — | 13,912 | |||||||||||||||||||||||||||||||||||||
| Contingent Earnout Liability recognized upon closing of the reverse recapitalization | — | — | — | — | (159,432) | — | (159,432) | |||||||||||||||||||||||||||||||||||||
| Proceeds from the exercise of stock options | — | — | 221,239 | — | 598 | — | 598 | |||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 10,146 | — | 10,146 | |||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (26,477) | (26,477) | |||||||||||||||||||||||||||||||||||||
Balance as of December 31, 2021 | — | — | 103,003,646 | $ | 10 | $ | 536,737 | $ | (414,573) | $ | 122,174 | |||||||||||||||||||||||||||||||||
| Proceeds from the exercise of stock options | — | — | 225,367 | — | 535 | — | 535 | |||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 6,184 | — | 6,184 | |||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (11,965) | (11,965) | |||||||||||||||||||||||||||||||||||||
Balance as of December 31, 2022 | — | — | 103,229,013 | $ | 10 | $ | 543,456 | $ | (426,538) | $ | 116,928 | |||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
111
HUMACYTE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Cash flows from operating activities | |||||||||||
| Net loss | $ | (11,965) | $ | (26,477) | |||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation expense | 6,151 | 6,181 | |||||||||
| Stock-based compensation expense | 6,184 | 10,146 | |||||||||
| Change in fair value of Contingent Earnout Liability | (75,767) | (55,772) | |||||||||
| Change in fair value of common stock warrant liabilities | (417) | (56) | |||||||||
| Loss on disposal of property and equipment | 6 | — | |||||||||
| Amortization expense | 2,059 | 2,060 | |||||||||
| Non-cash operating lease costs | 45 | 42 | |||||||||
| Amortization of SVB debt discount | 1,546 | 977 | |||||||||
| Accrued interest on PPP loan obligation | — | 11 | |||||||||
| Gain on PPP loan forgiveness | — | (3,284) | |||||||||
| Payment of liabilities assumed in Merger | — | (14,461) | |||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable | 145 | (63) | |||||||||
| Prepaid expenses and other current assets | 1,364 | (2,174) | |||||||||
| Accounts payable | (509) | (197) | |||||||||
| Accrued expenses | 247 | 2,035 | |||||||||
| Operating lease obligation | (45) | (42) | |||||||||
| Deferred payroll taxes | (173) | (116) | |||||||||
| Net cash used in operating activities | (71,129) | (81,190) | |||||||||
| Cash flows from investing activities | |||||||||||
| Purchase of short-term investments (certificates of deposit) | (10,107) | (8,000) | |||||||||
| Proceeds from maturity of short-term investments (certificates of deposit) | 16,000 | — | |||||||||
| Purchase of property and equipment | (1,048) | (220) | |||||||||
| Net cash provided by (used in) investing activities | 4,845 | (8,220) | |||||||||
| Cash flows from financing activities | |||||||||||
| Proceeds from the exercise of stock options | 535 | 598 | |||||||||
| Payment of finance lease principal | (1,981) | (1,729) | |||||||||
| Proceeds from Merger and PIPE financing, net of offering costs paid | — | 242,400 | |||||||||
| Payment of transaction costs related to Merger | — | (3,945) | |||||||||
| Proceeds from SVB loan | — | 29,659 | |||||||||
| Net cash (used in) provided by financing activities | (1,446) | 266,983 | |||||||||
| Net (decrease) increase in cash and cash equivalents | (67,730) | 177,573 | |||||||||
| Cash and cash equivalents at the beginning of the period | 217,502 | 39,929 | |||||||||
| Cash and cash equivalents at the end of the period | 149,772 | 217,502 | |||||||||
| Supplemental disclosure | |||||||||||
| Cash paid for interest on SVB loan | $ | 2,668 | $ | 1,123 | |||||||
| Supplemental disclosure of noncash activities: | |||||||||||
| Purchase of property and equipment in accounts payable and accrued expenses | $ | 135 | $ | 21 | |||||||
| Issuance of warrants in conjunction with debt | $ | — | $ | 3,275 | |||||||
| Unpaid liabilities assumed in connection with Merger | $ | — | $ | 130 | |||||||
| Conversion of redeemable convertible preferred stock into common stock in connection with the reverse capitalization | $ | — | $ | 420,989 | |||||||
| Contingent Earnout Liability recognized upon the closing of the reverse recapitalization | $ | — | $ | 159,432 | |||||||
The accompanying notes are an integral part of these financial statements.
112
1. Organization and Description of Business
Organization
Humacyte, Inc. and subsidiary (the “Company”), is pioneering the development and manufacture of off-the-shelf, universally implantable, bioengineered human tissues, advanced tissue constructs and organ systems designed to improve the lives of patients and transform the practice of medicine. The Company is leveraging its regenerative medicine technology platform to develop proprietary product candidates for use in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas.
On August 26, 2021 (the “Closing Date”), Alpha Healthcare Acquisition Corp. (“AHAC”) consummated a merger pursuant to a Business Combination Agreement, dated as of February 17, 2021 (the “Merger Agreement”), by and among Humacyte, Inc., a Delaware Corporation (“Legacy Humacyte”), AHAC and Hunter Merger Sub, Inc. (“Merger Sub”), a Delaware corporation and wholly owned subsidiary of AHAC. As contemplated by the Merger Agreement, Merger Sub merged with and into Legacy Humacyte, with Legacy Humacyte continuing as the surviving corporation and as a wholly-owned subsidiary of AHAC (such transactions, the “Merger,” and, collectively with the other transactions described in the Merger Agreement, the “Reverse Recapitalization”). On the Closing Date, AHAC changed its name to Humacyte, Inc. (“New Humacyte”) and Legacy Humacyte changed its name to Humacyte Global, Inc. The Merger is accounted for as a reverse recapitalization in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), and under this method of accounting, AHAC is treated as the acquired company for financial reporting purposes and Legacy Humacyte is treated as the acquirer. Operations prior to the Merger are those of Legacy Humacyte.
Refer to Note 3 — Reverse Recapitalization for further details of the Merger.
Liquidity and Going Concern
Since its inception in 2004, the Company has generated no product revenue and has incurred operating losses and negative cash flows from operations in each year. To date, the Company has financed its operations primarily through the sale of equity securities and convertible debt, proceeds from the Reverse Recapitalization, borrowings under loan facilities and, to a lesser extent, through governmental and other grants. At December 31, 2022 and December 31, 2021, the Company had an accumulated deficit of $426.5 million and $414.6 million, respectively. The Company’s operating losses were $84.6 million and $81.2 million for the years ended December 31, 2022 and 2021, respectively. Net cash flows used in operating activities were $71.1 million and $81.2 million during the years ended December 31, 2022 and 2021, respectively. Substantially all of the Company’s operating losses resulted from costs incurred in connection with the Company’s research and development programs and from general and administrative costs associated with the Company’s operations. The Company expects to incur substantial operating losses and negative cash flows from operations for the foreseeable future as the Company advances its product candidates.
As of December 31, 2022, the Company had cash and cash equivalents and short-term investments of $151.9 million. The Company believes its combined cash and cash equivalents and short-term investments on hand will be sufficient to fund operations, including clinical trial expenses and capital expenditure requirements, for at least 12 months from the issuance date of these financial statements.
Impact of COVID-19
The COVID-19 outbreak and government measures taken in response have had a significant impact, both direct and indirect, on the Company’s business, as supply chains have been disrupted and enrollment in clinical trials has been delayed. To date, there have been no material financial impacts or impairment losses in the carrying values of the Company’s assets as a result of the pandemic and the Company is not aware of any specific related event or circumstance that would require it to revise the estimates reflected in these financial statements. The extent to which the ongoing effects of the COVID-19 pandemic will directly or indirectly impact the Company’s business, results of operations and financial condition, including current and future clinical trials and research and development costs and timelines, will depend on future developments that are highly uncertain, including as a result of new information that may emerge concerning COVID-19, the emergence of new virus variants, and the duration and intensity of the related economic impact of the pandemic.
113
2. Summary of Significant Accounting Policies
Basis of Presentation
The Company has prepared the accompanying financial statements in conformity with U.S. GAAP. The Company’s consolidated financial statements reflect the operations of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Unless otherwise noted, the Company has retroactively adjusted all common and preferred share and related share price information to give effect to the exchange ratio established in the Merger Agreement. Operations prior to the Merger are those of Legacy Humacyte.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates in the financial statements include stock-based compensation costs, right-of-use assets, accruals for research and development activities, contingent earnout liability, fair value of common stock warrants, redeemable convertible preferred stock and income taxes. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors and adjusts those estimates and assumptions when facts and circumstances dictate. Actual results could differ from those estimates.
Segments
The Company operates and manages its business as one reportable and operating segment. The Company is developing proprietary, bioengineered, acellular human tissues, advanced tissue constructs and organ systems that are designed to be used in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas. The Company’s chief executive officer, who is the chief operating decision maker, reviews financial information on an aggregate basis for purposes of evaluating financial performance and allocating resources.
Comprehensive Loss
Comprehensive loss includes net loss as well as other changes in stockholders’ equity that result from transactions and economic events other than those with stockholders. There was no difference between net loss and comprehensive loss for the years ended December 31, 2022 and 2021.
Cash and Cash Equivalents
The Company considers all short-term, highly liquid investments, including certificates of deposit (“CDs”) purchased with an original maturity of three months or less at the date of purchase, to be cash equivalents. Cash deposits are held with financial institutions with investment-grade ratings in the United States of America, or U.S. Cash deposits typically exceed federally insured limits. As of December 31, 2022 and 2021, cash and cash equivalents consisted of cash on deposit with banks denominated in U.S. dollars, investments in money market funds, and CDs maturing within three months of their purchase date.
Short-term Investments
The Company classifies its certificates of deposit as cash and cash equivalents or short-term investments and reassesses the appropriateness of the classification of its investments at the end of each reporting period. Certificates of deposit held for investment with an original maturity greater than three months are carried at amortized cost and reported as short-term investments on the consolidated balance sheets. The type of certificates of deposit that the Company invests in are not considered debt securities under Financial Accounting Standards Board ("FASB") Accounting Standards Codification (“ASC”) 320, Investments - Debt Securities.
As of December 31, 2022 and 2021, the Company had approximately $10.1 million and $10.0 million, respectively, in CDs. These cash deposits are deposited at a bank that is a member of the Certificate of Deposit Account Registry Service
114
(“CDARS”), in which large deposits are divided into smaller amounts and placed with other Federal Deposit Insurance Corporation (“FDIC”) insured banks which are also members of the CDARS network. Those members issue CDs in amounts under $250,000, so that the entire deposit balance is eligible for FDIC insurance. As of December 31, 2022, the Company classified $8.0 million of its CDs as cash and cash equivalents and $2.1 million of its CDs as short-term investments on its consolidated balance sheets. As of December 31, 2021, the Company classified $2.0 million of its CDs as cash and cash equivalents and $8.0 million of its CDs as short-term investments on its consolidated balance sheets.
Revenue Recognition
The Company’s revenues generally consist of grant revenues, including revenues generated under government and other awarded grants.
Grant Revenue
The Company generates revenue primarily from government and other awarded grants that reimburse the Company for certain allowable costs related to research and development efforts.
In August 2017, the Department of Defense (“DoD”) granted the Company a cash award for work to support human tissue engineered blood vessels for vascular reconstruction in the injured warfighter. The final amount awarded to the Company totaled $6.8 million and the program ended in November 2022. Based on the terms of the research project award agreement associated with the DoD grant, allowable costs were reimbursed to the Company based on the percentage of completion of project milestones in accordance with milestone payment schedules set forth in the agreement. During the years ended December 31, 2022 and 2021, the Company recognized revenue of $1.6 million and $1.3 million, respectively, for reimbursement of certain allowable costs related to this grant. Revenue related to the DoD grant is included in grant revenue in the Company’s consolidated statements of operations and comprehensive loss. As of December 31, 2022 and 2021, there was $31 thousand and $176 thousand, respectively, of accounts receivable related to the DoD grant included in the Company’s consolidated balance sheets.
The Company has determined that the grant is not within the scope of ASC 606 as it does not meet the definition of a contract with a customer. The Company has concluded that the grant meets the definition of a contribution and is a nonexchange transaction and has applied the contribution accounting model in Subtopic 958-605, Not-for-Profit-Entities-Revenue Recognition by analogy.
The Company recognizes funding received from grants as revenue, rather than as a reduction of research and development expenses, because the Company is the principal in conducting the research and development activities and these grants are central to the Company’s ongoing operations. The Company recognizes revenue only after the qualifying expenses related to the grants have been incurred and it is reasonably assured that the expenses will be reimbursed and the revenue will be collectible. The related costs incurred are included in research and development expense in the Company’s consolidated statements of operations and comprehensive loss.
Revenue from Customers
Under ASC 606, revenue is recognized when a customer obtains control of promised goods or services and is recognized in an amount that reflects the consideration that an entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. ASC 606 also impacts certain other areas, such as the accounting for costs to obtain or fulfill a contract.
In addition, ASC 606 requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers.
For contracts where the period between when the Company transfers a promised good or service to the customer and when the customer pays is one year or less, the Company has elected the practical expedient to not adjust the promised amount of consideration for the effects of a significant financing component.
115
Concentration of Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and short-term investments consisting of CDs. Total cash balances exceeded insured balances by the FDIC as of December 31, 2022 and 2021. The Company has cash equivalents that are invested in highly rated money market funds that are invested only in obligations of the U.S. government and its agencies.
During the years ended December 31, 2022 and 2021, 100% of the Company’s total revenue relates to the award it received from the DoD in August 2017. As of December 31, 2022 and 2021, 100% of the Company’s accounts receivable relates to the DoD grant.
Net Loss per Share Attributable to Common Stockholders
The Company follows the two-class method to compute basic and diluted net loss per share attributable to common stockholders when shares meet the definition of participating securities. The two-class method determines net loss per common share for each class of common and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires income available to common stockholders for the period to be allocated between common and participating securities based upon their respective rights to share in the earnings as if all income for the period had been distributed. During periods of loss, there is no allocation required under the two-class method since the redeemable convertible preferred stock did not have a contractual obligation to share in the Company’s losses.
Basic net loss per share attributable to common stockholders is computed by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period without consideration of potentially dilutive common stock. Diluted net loss per share attributable to common stockholders reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the Company unless inclusion of such shares would be anti-dilutive. As the Company has incurred losses for the years ended December 31, 2022 and 2021, basic and diluted net loss per share is the same for each period.
The potential shares of common stock that were excluded from the computation of diluted net loss per share for each period because including them would have had an antidilutive effect were as follows:
Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Exercise of options under stock plan | 7,203,874 | 6,711,192 | |||||||||
| Warrants to purchase common stock | 5,588,506 | 5,588,506 | |||||||||
The 15,000,000 Contingent Earnout Shares, as defined in Note 3, are excluded from the anti-dilutive table for all periods presented, as such shares are contingently issuable until the share price of the Company exceeds specified thresholds that have not yet been achieved, or upon the occurrence of a change in control.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants at the measurement date. ASC 820, Fair Value Measurement and Disclosures, establishes a hierarchy whereby inputs to valuation techniques used in measuring fair value are prioritized, or the fair value hierarchy. There are three levels to the fair value hierarchy based on reliability of inputs, as follows:
•Level 1 — Observable inputs that reflect unadjusted quoted prices for identical assets or liabilities in active markets.
116
•Level 2 — Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 — Unobservable inputs in which little or no market data exists, therefore requiring the Company to develop its own assumptions.
The Company evaluates assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level at which to classify them for each reporting period, utilizing valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible. The determination requires significant judgments to be made by the Company.
Property and Equipment, Net
Property and equipment, net are recorded at cost less accumulated depreciation. Expenditures for major additions and improvements are capitalized and minor replacements, maintenance, and repairs are charged to expense as incurred. When property and equipment are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the balance sheet accounts and any resulting gain or loss is included in the results of operations for the respective period. Depreciation is calculated using the straight-line method over the estimated useful lives of the related assets. The estimated useful lives for significant asset categories are as follows:
| Property and equipment | Estimated Useful Lives (Years) | |||||||
| Scientific equipment | 5 – 7 | |||||||
| Computer equipment | 5 | |||||||
| Software | 3 | |||||||
| Furniture and fixtures | 5 – 7 | |||||||
| Leasehold improvements | Lesser of useful life or life of lease | |||||||
| Construction in progress | N/A | |||||||
Impairment of Long-Lived Assets
The Company reviews the carrying value of property and equipment for indicators of possible impairment whenever events and circumstances indicate that the carrying value of an asset or asset group may not be recoverable from the estimated future net undiscounted cash flows expected to result from its use and eventual disposition. In cases where estimated future net undiscounted cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of the asset or asset group. The factors considered by management in performing this assessment include current operating results, trends and prospects, the manner in which the property is used, and the effects of obsolescence, demand, competition, and other economic factors. Based on this assessment, during the years ended December 31, 2022 and 2021, respectively, the Company concluded there were no such events or changes in circumstances requiring review of the carrying amount of the Company’s long-lived assets and there was no impairment during the years ended December 31, 2022 and 2021.
Income Taxes
Income taxes are computed using the asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s financial statements. In estimating future tax consequences, the Company considers all expected future events other than enactment of changes in tax laws or rates. A valuation allowance is recorded, if necessary, to reduce net deferred tax assets to their realizable values if management does not believe it is more likely than not that the net deferred tax assets will be realized. As of December 31, 2022 and 2021, the Company has recorded a full valuation allowance against its deferred tax assets.
The Company applies the accounting guidance for uncertainties in income taxes, which prescribes a recognition threshold and measurement process for recording uncertain tax positions taken, or expected to be taken, in a tax return in the financial statements. Additionally, the guidance also prescribes the treatment for derecognition, classification,
117
accounting in interim periods and disclosure requirements for uncertain tax positions. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes.
The Company recognizes the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. Assessing an uncertain tax position begins with the initial determination of the sustainability of the position and is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed. Additionally, the Company must accrue interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws.
The Company has analyzed its filing positions in all significant Federal and state jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. As of December 31, 2022 and 2021, the Company has determined that no uncertain tax positions would have a material impact on the financials statements of the Company. The Company is no longer subject to Federal, state, and local tax examinations by tax authorities for years before 2019 although carry-forward attributes that were generated prior to 2019 may still be adjusted upon examination by the taxing authorities if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
As of December 31, 2022 and 2021, the Company had not recorded any amounts for unrecognized tax benefits. The Company’s policy is to recognize interest and penalties related to uncertain tax positions in the provision for income taxes. As of December 31, 2022 and 2021, the Company had no accrued interest or penalties related to uncertain tax positions, and no amounts had been recognized in the Company’s statements of operations and comprehensive loss.
Intellectual Property
The Company seeks to protect its intellectual property by filing patent applications in the United States and abroad related to novel technologies and product candidates that it views as important to its business. The patent positions of biotechnology companies generally, including the Company’s patent positions, is highly uncertain and involves complex legal and factual questions for which legal principles remain unresolved. Patent costs have been expensed as incurred as general and administrative expense.
Research and Development
The Company expenses research and development costs as operating expenses as incurred. Research and development expenses consist primarily of:
•salaries and related overhead expenses for personnel in research and development functions, including stock-based compensation and benefits;
•fees paid to consultants and CROs, including in connection with clinical trials, and other related clinical trial fees, such as for investigator grants, patient screening, laboratory work and statistical compilation and analysis; allocation of facility lease and maintenance costs;
•depreciation of leasehold improvements, laboratory equipment and computers;
•costs related to purchasing raw materials for and producing product candidates for clinical trials;
•costs related to compliance with regulatory requirements;
•costs related to the manufacturing scale-out initiative; and
•license fees related to in-licensed technologies.
118
Accrued Research and Development
The Company has entered into various agreements with CROs and a CMO, which conduct preclinical studies and clinical trials and contract manufacturing activities. The Company’s research and development accruals are estimated based on the level of services performed, progress of the studies, including the phase or completion of events, and contracted costs. The estimated costs of research and development provided, but not yet invoiced, are included in accrued expenses on the balance sheet. If the actual timing of the performance of services or the level of effort varies from the original estimates, the Company will adjust the accrual accordingly. Payments made under these arrangements in advance of the performance of the related services are recorded as prepaid expenses and other current assets until the services are rendered. The Company terminated its agreement with its CMO on March 6, 2020. The remaining HAV inventory at the CMO was fully depleted during the year ended December 31, 2021.
Stock-Based Compensation
The Company accounts for stock-based compensation for employees and non-employees measured at grant date, based on the fair value of the award. The Company measures the fair value of awards granted using the Black-Scholes option pricing model and recognizes the expense over the requisite service period using the straight-line method. Option valuation models, including the Black-Scholes option-pricing model, require the input of highly subjective assumptions, and changes in the assumptions used can materially affect the grant-date fair value of an award. These assumptions include the risk-free rate of interest, expected dividend yield, expected volatility, the expected term of the award, and the fair value of the underlying common stock on the date of grant. Forfeitures are accounted for as they occur.
Common Stock Warrants
In connection with the Merger, the Company assumed 5,000,000 publicly-traded warrants (“Public Warrants”) and 177,500 private placement warrants issued to AHAC Sponsor LLC (the “Sponsor”), Oppenheimer & Co. Inc. and Northland Securities, Inc, in connection with AHAC’s initial public offering (“Private Placement Warrants” and, together with the Public Warrants, the “Common Stock Warrants”). The Common Stock Warrants entitle the holder to purchase one share of the Company’s Common stock, par value $0.0001 (“Common Stock”), at an exercise price of $11.50 per share. The Public Warrants are publicly traded and are exercisable for cash unless certain conditions occur, such as the failure to have an effective registration statement related to the shares issuable upon exercise or redemption by the Company under certain conditions, at which time the warrants may be eligible for a cashless exercise. The Private Placement Warrants are non-redeemable for cash so long as they are held by the initial purchasers or their permitted transferees. If the Private Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Private Placement Warrants are redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
The Company evaluated the Common Stock Warrants to determine the appropriate financial statement classification upon the consummation of the Merger. The Common Stock Warrants are not mandatorily redeemable and are considered to be freestanding instruments as they are separately exercisable into common shares. As such, the Common Stock Warrants were not classified as liabilities under FASB ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”). The Company then evaluated the Common Stock Warrants under FASB ASC Topic 815, Derivatives and Hedging.
The agreement governing the Common Stock Warrants includes a provision (“Replacement of Securities Upon Reorganization”), the application of which could result in a different settlement value for the Private Placement Warrants depending on their holder. Because the holder of an instrument is not an input into the pricing of a fixed-for-fixed option on the Company’s ordinary shares, the Private Placement Warrants are not considered to be “indexed to the Company’s own stock” and therefore are not classified in stockholders’ equity. As the Private Placement Warrants meet the definition of a derivative, the Company recorded these warrants as liabilities on the consolidated balance sheet at fair value, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date.
The Public Warrants are considered to be “indexed to the Company’s own stock”. The agreement provides that in the event of a tender or exchange offer made to and accepted by holders of more than 50% of the outstanding shares of the Company’s common shares, all holders of the Common Stock Warrants (both the Public Warrants and the Private Placement Warrants) would be entitled to receive cash for all of their Common Stock Warrants. As the Company has a single class of common stock, a qualifying cash tender offer of more than 50% of the Company’s common stock will
119
always result in a change-in-control and would not preclude permanent equity classification of the Public Warrants. Based on this evaluation, the Company concluded that the Public Warrants meet the criteria to be classified within stockholders’ equity.
Contingent Earnout Liability
In connection with the Reverse Recapitalization and pursuant to the Merger Agreement, Legacy Humacyte equity holders are entitled to receive as additional merger consideration of up to 15,000,000 shares of the Company’s Common Stock (the “Contingent Earnout Shares”), comprised of two separate tranches of 7,500,000 shares per tranche, for no consideration upon the occurrence of certain triggering events, including a change of control event that is not solely indexed to the common stock. In accordance with ASC 815-40, as the earnout shares were not indexed to the common stock, they were accounted for as a liability at the Reverse Recapitalization date and subsequently remeasured at each reporting date with changes in fair value recorded as a component of other income (expense), net in the consolidated statements of operations and comprehensive loss.
The estimated fair value of the Contingent Earnout Shares was determined using a Monte Carlo simulation using a distribution of potential outcomes on a monthly basis over a 10-year period prioritizing the most reliable information available. The assumptions utilized in the calculation were based on the achievement of certain stock price milestones, including the current Company common stock price, expected volatility, risk-free rate, expected term and expected dividend yield.
The Contingent Earnout Shares are categorized as a Level 3 fair value measurement (see “Fair Value of Financial Instruments” accounting policy described above) because the Company estimated projections over a 10-year period utilizing unobservable inputs. Contingent earnout payments involve certain assumptions requiring significant judgment and actual results can differ from assumed and estimated amounts.
Leases
The Company accounts for its leases under ASC 842, Leases. The Company determines if an arrangement is or contains a lease and the classification of that lease at inception of a contract. The Company’s operating lease assets are included in “operating lease right-of-use assets, net”, and the current and non-current portions of the operating lease liabilities are included in “operating lease obligation, current portion”, and “operating lease obligation, net of current portion”, respectively, on the balance sheets. The Company’s finance lease assets are included in “finance lease right-of-use assets, net”, and the current and non-current portions of the finance lease liabilities are included in “finance lease obligation, current portion”, and “finance lease obligation, net of current portion”, respectively, on the consolidated balance sheets.
Under this guidance, arrangements meeting the definition of a lease are classified as operating or finance leases, and are recorded on the balance sheet as both a right-of-use asset and lease liability, calculated by discounting fixed lease payments over the lease term at the rate implicit in the lease or the Company’s incremental borrowing rate. Lease right-of-use assets and lease obligations are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. Operating lease right-of-use assets are adjusted for (i) payments made at or before the commencement date, (ii) initial direct costs incurred, and (iii) tenant incentives under the lease. As the implicit rate for the operating leases were not determinable, the Company used an incremental borrowing rate based on the information available at the respective lease commencement dates in determining the present value of future payments. The incremental borrowing rate represents the interest rate the Company would expect to incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease. The Company determined the incremental borrowing rate by considering various factors, such as its credit rating, interest rates of similar debt instruments of entities with comparable credit ratings, the lease term and the currency in which the lease was denominated. The Company considers a lease term to be the noncancelable period that it has the right to use the underlying asset, including any periods where it is reasonably certain the Company will exercise any option to extend the contract.
Lease expenses for minimum lease payments for operating leases are recognized on a straight-line basis over the lease term. Amortization expense of the right-of-use asset for finance leases is recognized on a straight-line basis over the lease term and interest expense for finance leases is recognized based on the incremental borrowing rate. Lease liabilities are increased by interest and reduced by payments each period, and the right of use asset is amortized over the lease term.
120
In calculating the right-of-use assets and lease liabilities, the Company has elected to combine lease and non-lease components for all asset classes. The Company excludes short-term leases, if any, having initial terms of 12 months or less from the new guidance as an accounting policy election, and recognizes rent expense on a straight-line basis over the lease term.
Other Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, successful discovery and development of its product candidates, the success of clinical trials and other studies for its product candidates, including for its ongoing V005 Phase 2/3 clinical trial and V007 Phase 3 clinical trial, the regulatory approval and commercialization of its HAVs and other product candidates, the expected size of the target populations for the Company’s product candidates, the degree of market acceptance of the HAVs, if approved, the availability of third-party coverage and reimbursement, development by competitors of new technological innovations, the ability to manufacture HAVs and other product candidates in sufficient quantities, expectations regarding the Company’s strategic partnerships, dependence on third parties, key personnel and the ability to attract and retain qualified employees, protection of proprietary technology and confidentiality of trade secrets, compliance with governmental regulations, the impact of the COVID-19 pandemic, the Company’s implementation and maintenance of effective internal controls, and the ability to secure additional capital to fund operations and commercial success of its product candidates.
Product candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel, and infrastructure and extensive compliance-reporting capabilities. Even if the Company’s commercialization efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales, and the Company may depend on certain strategic relationships to distribute its products, including the Company’s strategic partnership with Fresenius Medical Care Holdings, Inc., (“Fresenius Medical Care”) to sell, market and distribute its 6 millimeter HAV for certain specified indications outside the United States.
Recently Adopted Accounting Pronouncements
In May 2021, the FASB issued ASU No. 2021-04, “Earnings Per Share (Topic 260), Debt-Modifications and Extinguishments (Subtopic 470-50), Compensation-Stock Compensation (Topic 718), and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options” (“ASU 2021-04”). The FASB issued this update to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity classified written call options (for example, warrants) that remain equity classified after modification or exchange. ASU 2021-04 is effective for all entities for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. An entity should apply the amendments prospectively to modifications or exchanges occurring after the effective date of the amendments. The Company adopted ASU 2021-04 as of January 1, 2022. The adoption of this ASU had no impact on the Company’s consolidated financial statements and disclosures.
In November 2021, the FASB issued ASU No. 2021-10, “Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance” (“ASU 2021-10”) to improve financial reporting by requiring disclosures that increase the transparency of transactions with a government accounted for by applying a grant or contribution model by analogy (for example, guidance on contributions for not-for-profit-entities in ASC 958-605). For transactions within the scope, ASU 2021-10 requires the disclosure of (i) the types of transactions, (ii) an entity’s accounting for those transactions, and (iii) the effect of those transactions on an entity’s financial statements. ASU 2021-10 is effective for all entities within their scope for financial statements issued for annual periods beginning after December 15, 2021. The Company adopted ASU 2021-10 effective January 1, 2022 and elected to apply the amendments prospectively to all transactions within the scope of the amendment that are reflected in the financial statements at the date of adoption. The adoption did not have a material impact to the Company’s consolidated financial statements or disclosures. See the above section titled “Grant Revenue” for disclosure related to the Company’s government grants.
121
3. Reverse Recapitalization
On August 26, 2021, Merger Sub, a wholly-owned subsidiary of AHAC, merged with Legacy Humacyte, with Legacy Humacyte surviving as a wholly-owned subsidiary of AHAC. At the effective time of the Merger:
•each outstanding share of Legacy Humacyte common stock was converted into approximately 0.26260 shares of Common Stock;
•each outstanding share of preferred stock of Legacy Humacyte was cancelled and converted into the aggregate number of shares of Common Stock that would be issued upon conversion of the shares of Legacy Humacyte preferred stock based on the applicable conversion ratio immediately prior to the effective time, multiplied by approximately 0.26260; and
•each outstanding option or warrant to purchase Legacy Humacyte common stock was converted into an option or warrant, as applicable, to purchase a number of shares of Common Stock equal to the number of shares of Legacy Humacyte common stock subject to such option or warrant multiplied by approximately 0.26260, at an exercise price per share equal to the current exercise price per share for such option or warrant divided by approximately 0.26260;
in each case, rounded down to the nearest whole share.
In addition, upon the closing of the Merger (the “Closing”), 2,500,000 Class B shares of AHAC (the “Founder Shares”) automatically converted into shares of Common Stock, on a one-for-one basis.
Former holders of the Legacy Humacyte common stock and Legacy Humacyte preferred stock are eligible to receive up to an aggregate of 15,000,000 additional shares of Common Stock (the “Contingent Earnout Shares”) in the aggregate, comprised of two equal tranches of 7,500,000 shares per tranche if the volume-weighted average closing sale price of the Common Stock is greater than or equal to $15.00 and $20.00, respectively, for any 20 trading days within any 30 consecutive trading day period. At the Closing on August 26, 2021, the Company recorded a liability (“Contingent Earnout Liability”) of $159.4 million, based on the estimated fair value of the 15,000,000 Contingent Earnout Shares with a corresponding reduction of additional paid-in capital in the equity section of the Company’s consolidated balance sheet.
Concurrently with the execution of the Merger Agreement, AHAC entered into subscription agreements (the “Subscription Agreements”) with certain investors (the “PIPE Investors”). Pursuant to the Subscription Agreements, the PIPE Investors purchased an aggregate of 17,500,000 shares of Common Stock (the “PIPE Shares”) in a private placement at a price of $10.00 per share for an aggregate purchase price of $175 million (the “PIPE Financing”). The PIPE Financing was consummated in connection with the Closing.
The number of shares of Common Stock outstanding immediately following the consummation of the Merger was:
| Shares | ||||||||
| Common stock of AHAC, outstanding prior to Merger | 10,355,000 | |||||||
| Less redemption of AHAC shares | (3,008,551) | |||||||
| Common stock of AHAC | 7,346,449 | |||||||
| AHAC Founder Shares | 2,500,000 | |||||||
| New Humacyte shares issued to PIPE Investors | 17,500,000 | |||||||
| Issuance of common stock upon reverse recapitalization and PIPE Financing | 27,346,449 | |||||||
| New Humacyte shares issued in Merger to Legacy Humacyte stockholders | 75,656,935 | (1) | ||||||
| Total shares of Common Stock immediately after Merger | 103,003,384 | |||||||
___________________________
(1)Includes 69,613,562 shares of Common Stock issued upon conversion of Legacy Humacyte’s redeemable convertible preferred stock.
122
The Merger was accounted for as a reverse recapitalization in accordance with U.S. GAAP. Under this method of accounting, AHAC was treated as the acquired company for financial reporting purposes and Legacy Humacyte was treated as the acquirer. This determination was primarily based on the fact that subsequent to the Merger, the Legacy Humacyte stockholders held a majority of the voting rights of the combined company, Legacy Humacyte comprised all of the ongoing operations of the combined company, Legacy Humacyte comprised a majority of the governing body of the combined company, and Legacy Humacyte’s senior management comprised all of the senior management of the combined company. Accordingly, for accounting purposes, the Merger was treated as the equivalent of Legacy Humacyte issuing shares for the net assets of AHAC, accompanied by a recapitalization. The net assets of AHAC were stated at historical costs. No goodwill or other intangible assets were recorded. Operations prior to the Merger are those of Legacy Humacyte.
In connection with the Merger, the Company received $242.4 million in proceeds from the Merger and related PIPE Financing. The Company incurred $3.9 million of transaction costs, consisting of banking, legal, and other professional fees, of which $3.9 million was recorded as a reduction of proceeds to additional paid-in capital, and less than $0.1 million related to the Private Placement Warrants, which are classified as liabilities in the consolidated balance sheets, was expensed in the consolidated statements of operations and comprehensive loss. All transaction costs were paid as of December 31, 2021. Legacy Humacyte assumed $15.2 million of liabilities, including PIPE Financing fees and legal fees, and $0.1 million of assets from AHAC. Of the $15.2 million of liabilities assumed from AHAC, $0.1 million was included in accrued expenses as of December 31, 2021, and there were no unpaid liabilities as of December 31, 2022.
4. Fair Value Measurements
The Company’s assets and liabilities that were measured at fair value on a recurring basis were as follows:
Fair Value Measured as of December 31, 2022 | ||||||||||||||||||||||||||
| ($ in thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||
| Cash equivalents (money market funds) | $ | 141,159 | $ | — | $ | — | $ | 141,159 | ||||||||||||||||||
| Cash equivalents (certificates of deposit) | — | 8,000 | — | 8,000 | ||||||||||||||||||||||
| Short-term investments (certificates of deposit) | — | 2,107 | — | 2,107 | ||||||||||||||||||||||
| Total financial assets | $ | 141,159 | $ | 10,107 | $ | — | $ | 151,266 | ||||||||||||||||||
| Liabilities: | ||||||||||||||||||||||||||
| Contingent Earnout Liability | $ | — | $ | — | $ | 27,893 | $ | 27,893 | ||||||||||||||||||
| Private Placement Warrants liability | — | — | 80 | 80 | ||||||||||||||||||||||
| Total financial liabilities | $ | — | $ | — | $ | 27,973 | $ | 27,973 | ||||||||||||||||||
Fair Value Measured as of December 31, 2021 | ||||||||||||||||||||||||||
| ($ in thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||
| Cash equivalents (money market funds) | $ | 208,821 | $ | — | $ | — | $ | 208,821 | ||||||||||||||||||
| Cash equivalents (certificates of deposit) | — | 2,000 | — | 2,000 | ||||||||||||||||||||||
| Short-term investments (certificates of deposit) | — | 8,000 | — | 8,000 | ||||||||||||||||||||||
| Total financial assets | $ | 208,821 | $ | 10,000 | $ | — | $ | 218,821 | ||||||||||||||||||
| Liabilities: | ||||||||||||||||||||||||||
| Contingent Earnout Liability | $ | — | $ | — | $ | 103,660 | $ | 103,660 | ||||||||||||||||||
| Private Placement Warrants liability | — | — | 497 | 497 | ||||||||||||||||||||||
| Total financial liabilities | $ | — | $ | — | $ | 104,157 | $ | 104,157 | ||||||||||||||||||
123
The following tables present a summary of the changes in the fair value of the Company’s Level 3 financial instruments:
| Contingent Earnout Liability | ||||||||||||||
| Year Ended December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
| Fair value as of beginning of period | $ | (103,660) | $ | — | ||||||||||
| Contingent Earnout Liability recognized upon the closing of the reverse recapitalization | — | (159,432) | ||||||||||||
| Change in fair value included in other income, net | 75,767 | 55,772 | ||||||||||||
| Fair value as of end of period | $ | (27,893) | $ | (103,660) | ||||||||||
| Private Placement Warrants | ||||||||||||||
| Year Ended December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
| Fair value as of beginning of period | $ | (497) | $ | — | ||||||||||
| Private Placement Warrants liability acquired as part of the Merger | — | (553) | ||||||||||||
| Change in fair value included in other income, net | 417 | 56 | ||||||||||||
| Fair value as of end of period | $ | (80) | $ | (497) | ||||||||||
The fair value of the Contingent Earnout Liability and Private Placement Warrants (as defined in Note 9 — Stockholders’ Equity (Deficit)) liability are based on significant unobservable inputs, which represent Level 3 measurements within the fair value hierarchy.
In determining the fair value of the Contingent Earnout Liability, the Company used the Monte Carlo simulation value model using a distribution of potential outcomes on a monthly basis over a 10-year period prioritizing the most reliable information available. The assumptions utilized in the calculation were based on the achievement of certain stock price milestones, including the current Common Stock price, expected volatility, risk-free rate, expected term and expected dividend yield (see Note 9 — Stockholders’ Equity (Deficit)). Contingent earnout payments involve certain assumptions requiring significant judgment and actual results can differ from assumed and estimated amounts.
In determining the fair value of the Private Placement Warrants liability, the Company used the Monte Carlo simulation valuation model to estimate the fair value utilizing assumptions including the current Company stock price, expected volatility, risk-free rate, expected term and expected dividend yield (see Note 9 — Stockholders’ Equity (Deficit)).
The Company’s money market funds are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices. Certificates of deposit are carried at amortized cost in the Company’s consolidated balance sheets, which approximates their fair value based on Level 2 inputs. The carrying values of other receivables, accounts payable and accrued expenses as of December 31, 2022 and 2021 approximated their fair values due to the short-term nature of these items.
124
5. Property and Equipment, Net
Property and equipment, net consist of the following:
As of December 31, | ||||||||||||||
($ in thousands) | 2022 | 2021 | ||||||||||||
Scientific equipment(1) | $ | 27,821 | $ | 27,641 | ||||||||||
Computer equipment | 167 | 155 | ||||||||||||
Software | 209 | 335 | ||||||||||||
Furniture and fixtures | 988 | 988 | ||||||||||||
Leasehold improvements | 26,355 | 26,355 | ||||||||||||
| Construction in progress | 680 | — | ||||||||||||
| 56,220 | 55,474 | |||||||||||||
Accumulated depreciation | (26,181) | (20,440) | ||||||||||||
Property and equipment, net | $ | 30,039 | $ | 35,034 | ||||||||||
___________________________
(1)As of December 31, 2021, includes $3.6 million related to scientific equipment not depreciated until being placed into service during the third quarter of 2022.
Depreciation expense totaled $6.2 million for each of the years ended December 31, 2022 and 2021. All long-lived assets are maintained in the United States.
6. Accrued Expenses
Accrued expenses consisted of the following:
As of December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
Accrued external research, development and manufacturing costs | $ | 2,437 | $ | 2,520 | ||||||||||
Accrued employee compensation and benefits | 4,227 | 3,943 | ||||||||||||
Accrued professional fees | 444 | 294 | ||||||||||||
Total | $ | 7,108 | $ | 6,757 | ||||||||||
7. Debt
On March 30, 2021, the Company entered into a term loan agreement with Silicon Valley Bank (“SVB”) and SVB Innovation Credit Fund VIII, L.P., as amended in June 2021 and September 2021 (the “Loan Agreement”), which provides a term loan facility of up to $50.0 million with a maturity date of March 1, 2025, or the Loan Agreement. The Company’s obligations under the Loan Agreement are secured by substantially all of its assets except for its intellectual property. The Loan Agreement contains certain customary covenants, including, but not limited to, those relating to additional indebtedness, liens, asset divestitures, and affiliate transactions. If a minimum liquidity amount is not maintained, 50% of the outstanding principal and interest will become cash collateralized. As of December 31, 2022, the Company was in compliance with all covenants. The Company may use the proceeds of borrowings under the Loan Agreement as working capital and to fund its general business requirements.
The Loan Agreement provides that the term loans will be distributed in tranches. The initial term loan tranche of $20.0 million was drawn on March 31, 2021, and on October 13, 2021, the Company borrowed an additional $10.0 million under the Loan Agreement. Borrowings under the Loan Agreement are accounted for net of issuance costs which are being accreted to interest expense over the term of the loan using the effective interest method. As of December 31, 2022, two subsequent $10.0 million term loan tranches will be eligible to be drawn at the request of the Company during specified draw periods prior to May 15, 2023, the first tranche subject to submission by the Company of its first Biologics License
125
Application (“BLA”) to the FDA for its HAV prior to March 31, 2023, and the second tranche subject to the first approval from the FDA of any BLA for the HAV prior to March 31, 2023 and the Company having borrowed the first remaining tranche.
Borrowings bear interest at the greater of 7.5% or the Wall Street Journal Prime Rate plus 4.25% (11.75% as of December 31, 2022). Interest only payments on the principal amount outstanding are due monthly beginning in the first month after the loan is dispersed. Repayment of principal may begin as soon as July 1, 2023 under the level of borrowing outstanding at December 31, 2022, and no later than April 1, 2024 if the remaining two loan tranches are drawn. The term loans may only be prepaid in full, and such prepayment requires 30 days’ advance notice and was subject initially to a prepayment fee of 3.00% (that was decreased to 2.00% after March 30, 2022 (with a further decrease to 1.00% after March 30, 2023). The Company is not obligated to pay a prepayment fee if the Company makes a prepayment after March 30, 2024.
In connection with the Loan Agreement, the Company granted warrants to the lenders to purchase shares of Common Stock at an exercise price of $10.28 per share, of which 287,704 warrants were immediately exercisable. The warrants are classified within stockholders’ equity as the settlement of the warrants is indexed to the Common Stock. The Company recognized the fair value of the warrants immediately exercisable within stockholders’ equity using a Black-Scholes valuation model at issuance.
At issuance, the Company initially determined that the funding of an additional tranche was not probable, and therefore no value was ascribed to the remaining 123,302 warrants that were only exercisable upon the funding of the first additional tranche. As a result of the Company’s additional $10.0 million borrowings under the Loan Agreement on October 13, 2021, the warrants to purchase the additional 123,302 shares of Common Stock became exercisable at an exercise price of $10.28 per share and the value of the warrants was recorded as of that date. The additional warrants are classified within stockholders’ equity using a Black-Scholes valuation model, as the settlement of the warrants is indexed to the Common Stock.
As of December 31, 2022, the fair value of warrants ($3.3 million), a 5% final payment fee ($1.5 million) and debt issuance costs ($0.3 million) are being accreted to interest expense over the term of the loan using the effective interest method.
SVB loan payable and net discount or premium balances are as follows:
| ($ in thousands) | December 31, 2022 | |||||||
Principal amount of SVB loan payable | $ | 30,000 | ||||||
Final payment amount of SVB loan payable | 1,500 | |||||||
Net premium associated with accretion of final payment and other debt issuance costs | (2,593) | |||||||
SVB loan payable, current and noncurrent | 28,907 | |||||||
Less SVB loan payable, current portion | (8,571) | |||||||
SVB loan payable, noncurrent portion | $ | 20,336 | ||||||
Future minimum payments of principal on the Company’s outstanding variable rate borrowings as of December 31, 2022 are as follows:
Year ending December 31: | ($ in thousands) | |||||||
| 2023 | $ | 8,571 | ||||||
| 2024 | 17,143 | |||||||
| 2025 | 4,286 | |||||||
Total future payments | $ | 30,000 | ||||||
126
On April 30, 2020, the Company received loan proceeds in the amount of approximately $3.3 million under the Paycheck Protection Program (“PPP”). The loan and accrued interest were forgivable after a 24-week period as long as the Company used the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintained its payroll levels. On May 25, 2021, the PPP loan was forgiven and the Company recognized a gain from loan extinguishment in the amount of $3.3 million during the year ended December 31, 2021.
8. Leases
The Company’s finance lease relates to its headquarters, which was substantially completed in June 2018 and leased through May 2033, and its operating lease relates to the land lease associated with its headquarters.
At December 31, 2022 and 2021, the Company had finance lease liabilities of $21.1 million and $23.1 million, respectively, and right-of-use assets of $19.4 million and $21.4 million, respectively, and operating lease liabilities of $0.7 million and $0.7 million, respectively, and right-of-use assets of $0.7 million and $0.7 million, respectively, all of which were included in the consolidated balance sheets.
The Company’s leases do not require any contingent rental payments, impose any financial restrictions, or contain any residual value guarantees. Certain of the Company’s leases include renewal options and escalation clauses; renewal options have been included in the calculation of the lease liabilities and right of use assets as the Company is reasonably certain to exercise the options due to the specialized nature of the leased building. Variable expenses generally represent the Company’s share of the landlord’s operating expenses. The Company does not act as a lessor in any lease arrangements.
The following summarizes quantitative information about the Company’s leases:
| Year Ended December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
Finance lease cost | ||||||||||||||
| Amortization of right-of-use assets | $ | 2,059 | $ | 2,060 | ||||||||||
Interest on lease liabilities | 1,887 | 2,044 | ||||||||||||
Total finance lease cost | 3,946 | 4,104 | ||||||||||||
Operating lease cost | 105 | 105 | ||||||||||||
Total lease cost | $ | 4,051 | $ | 4,209 | ||||||||||
Year Ended December 31, 2022 | Year Ended December 31, 2021 | |||||||||||||||||||||||||
| ($ in thousands) | Finance Leases | Operating Leases | Finance Leases | Operating Leases | ||||||||||||||||||||||
| Operating cash flows from leases | $ | (1,887) | $ | (105) | $ | (2,044) | $ | (105) | ||||||||||||||||||
| Financing cash flows from leases | $ | (1,981) | $ | — | $ | (1,729) | $ | — | ||||||||||||||||||
| Weighted-average remaining lease term | 4.57 | 5.24 | 5.04 | 5.74 | ||||||||||||||||||||||
| Weighted-average discount rate | 8.50 | % | 8.50 | % | 8.50 | % | 8.50 | % | ||||||||||||||||||
127
As of December 31, 2022, the maturities of the Company’s lease liabilities were as follows:
($ in thousands) | Finance Leases | Operating Leases | ||||||||||||
| 2023 | $ | 3,965 | $ | 105 | ||||||||||
| 2024 | 4,065 | 105 | ||||||||||||
| 2025 | 4,167 | 105 | ||||||||||||
| 2026 | 4,237 | 105 | ||||||||||||
2027 | 2,673 | 105 | ||||||||||||
Thereafter | 10,026 | 469 | ||||||||||||
Total | 29,133 | 994 | ||||||||||||
Less: present value discount | (8,024) | (312) | ||||||||||||
Lease liabilities | $ | 21,109 | $ | 682 | ||||||||||
9. Stockholders’ Equity (Deficit)
Redeemable Convertible Preferred Stock
Immediately prior to the Merger, Legacy Humacyte had outstanding series A redeemable convertible preferred stock, series B redeemable convertible preferred stock, series C redeemable convertible preferred stock and series D redeemable convertible preferred stock, which are collectively referred to as “redeemable convertible preferred stock.”
In connection with the Merger, all previously issued and outstanding redeemable convertible preferred stock was converted into an equivalent number of shares of Common Stock of the Company on a one-for-one basis, then multiplied by the exchange ratio pursuant to the Merger Agreement and the amounts were reclassified as additional paid-in capital.
Common Stock
On August 26, 2021, the Merger and related PIPE Financing was consummated and the Company issued 27,346,449 shares of Common Stock for proceeds of $242.4 million. The Company incurred $3.9 million of transaction costs, consisting of banking, legal, and other professional fees. Legacy Humacyte assumed $15.2 million of liabilities, including PIPE Financing fees and legal fees, and $0.1 million of assets from AHAC. Immediately following the Merger, there were 103,003,384 shares of Common Stock outstanding with a par value of $0.0001 per share.
As of December 31, 2022, the Company’s Second Amended and Restated Certificate of Incorporation authorized the Company to issue 250,000,000 shares of Common Stock. The number of authorized shares of Common Stock may be increased or decreased (but not below the number of shares then outstanding or reserved for issuance) by the affirmative vote of the holders of a majority of the capital stock of the Company entitled to vote and may require a separate class vote of the Common Stock.
The holders of Common Stock are entitled to receive dividends from time to time as may be declared by the Company’s board of directors. Through December 31, 2022, no dividends have been declared.
The holders of Common Stock are entitled to one vote for each share held with respect to all matters voted on by the common stockholders of the Company.
In the event of a reorganization of the Company, after payment to the preferred stockholders of their liquidation preferences, holders of Common Stock are entitled to share ratably in all remaining assets of the Company.
128
As of December 31, 2022 and 2021, the Company had reserved Common Stock for future issuances as follows:
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Common stock reserved for Contingent Earnout Shares | 15,000,000 | 15,000,000 | |||||||||
Exercise of options under stock plans | 7,203,874 | 6,711,192 | |||||||||
Issuance of options under stock plans | 6,700,888 | 7,418,937 | |||||||||
| Shares available for grant under ESPP | 1,030,033 | 1,030,033 | |||||||||
Warrants to purchase Common Stock | 5,588,506 | 5,588,506 | |||||||||
| 35,523,301 | 35,748,668 | ||||||||||
Preferred Stock
The Company’s Second Amended and Restated Certificate of Incorporation provides the Company’s board of directors with the authority to issue preferred stock, par value $0.0001 per share, in one more series and to establish from time to time the number of shares to be included in each such series, by adopting a resolution and filing a certification of designations. Voting powers, designations, powers, preferences and relative, participating, optional, special and other rights shall be stated and expressed in such resolutions. There were 20,000,000 shares designated as preferred stock and none were outstanding as of December 31, 2022 and 2021.
Warrants
The Company had the following common stock warrants outstanding as of December 31, 2022 and 2021:
| Common Stock Warrants Outstanding | |||||
Legacy Humacyte Common Stock Warrants | 411,006 | ||||
Private Placement Warrants | 177,500 | ||||
| Public Warrants | 5,000,000 | ||||
Total Common Stock Warrants | 5,588,506 | ||||
See Note 7 — Debt for a discussion of common stock warrants issued in conjunction with the Company’s Loan Agreement in 2021 (such warrants, “Legacy Humacyte Common Stock Warrants”). There were no issuances, exercises or expirations of warrants during the year ended December 31, 2022. During the year ended December 31, 2021, there were 32,961 warrants exercised that were issued in conjunction with a long-term debt agreement repaid in a prior reporting period. There were no expirations of warrants during the year ended December 31, 2021.
Private Placement Warrants
The Private Placement Warrants were initially recognized as a liability on the Closing Date, at a fair value of $0.6 million, and the liability was remeasured to an estimated fair value of $0.5 million as of December 31, 2021. See Note 4 — Fair Value Measurements for a summary of the change in the fair value of the Private Placement Warrants during the years ended December 31, 2022 and 2021. The remeasurement of the Private Placement Warrant liability to a fair value of $0.1 million as of December 31, 2022 resulted in a non-cash gain of $0.4 million for the year ended December 31, 2022, compared to a non-cash gain of $0.1 million for the year ended December 31, 2021. The remeasurement of the Private Placement Warrant liability is classified within Change in fair value of common stock warrant liabilities in the consolidated statements of operations and comprehensive loss.
129
The Private Placement Warrants were valued using the following assumptions under the Monte Carlo simulation value model:
As of December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
Market price of public stock | $ | 2.11 | $ | 7.25 | |||||||
Exercise price | $ | 11.50 | $ | 11.50 | |||||||
Expected term (years) | 3.65 | 4.65 | |||||||||
Expected share price volatility | 78.3 | % | 61.0 | % | |||||||
Risk-free interest rate | 4.14 | % | 1.21 | % | |||||||
Estimated dividend yield | 0 | % | 0 | % | |||||||
Public Warrants
The Public Warrants may only be exercised for a whole number of shares and will expire five years after the completion of the Merger. The Public Warrants became exercisable 30 days after the completion of the Merger.
The Public Warrants were initially recognized as equity on the Closing Date at a fair value of $2.80 per share. There were no exercises of the Public Warrants during the year ended December 31, 2022.
Contingent Earnout Liability
Following the Closing, former holders of Legacy Humacyte common and preferred shares may receive up to 15,000,000 additional shares of Common Stock in the aggregate, in two equal tranches of 7,500,000 shares of Common Stock per tranche. The first and second tranches are issuable if the closing volume weighted average price (“VWAP”) per share of Common Stock quoted on Nasdaq (or the exchange on which the shares of Common Stock are then listed) is greater or equal to $15.00 and $20.00, respectively, over any 20 trading days within any 30 consecutive trading day period.
Upon the Closing, the contingent obligation to issue Contingent Earnout Shares was accounted for as a liability because the triggering events that determine the number of Contingent Earnout Shares required to be issued include events that are not solely indexed to the Common Stock. The estimated fair value of the total Contingent Earnout Shares at the Closing on August 26, 2021 was $159.4 million based on a Monte Carlo simulation valuation model using a distribution of potential outcomes on a monthly basis over a 10-year period using the most reliable information available. The estimated fair value of the total Contingent Earnout Shares at December 31, 2021 was $103.7 million.
See Note 4 — Fair Value Measurements for a summary of the change in the fair value of the Contingent Earnout Liability during the years ended December 31, 2022 and 2021. The remeasurement of the Contingent Earnout Liability to a fair value of $27.9 million as of December 31, 2022, resulted in a non-cash gain of $75.8 million for the year ended December 31, 2022, compared to a non-cash gain of $55.8 million for the year ended December 31, 2021. The remeasurement of the Contingent Earnout Liability is classified within Change in fair value of Contingent Earnout Liability in the consolidated statements of operations and comprehensive loss.
Assumptions used in the valuations are described below:
As of December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Current stock price | $ | 2.11 | $ | 7.25 | |||||||
| Expected share price volatility | 89.0 | % | 85.8 | % | |||||||
| Risk-free interest rate | 3.88 | % | 1.52 | % | |||||||
| Estimated dividend yield | 0 | % | 0 | % | |||||||
| Expected term (years) | 10.00 | 10.00 | |||||||||
130
10. Stock-based Compensation
At Closing, the 2021 Long-Term Incentive Plan, (the “2021 Plan”), and the 2021 Employee Stock Purchase Plan, (the “ESPP”), became effective. As of December 31, 2022, 6,700,888 and 1,030,033 shares of Common Stock were available under the 2021 Plan and ESPP, respectively. The 2021 Plan and ESPP provide that on January 1 of each year commencing January 1, 2022, the 2021 Plan and the ESPP reserve will automatically increase in an amount equal to the lesser of (a) 5% and 1%, respectively, of the number of shares of the Company’s Common Stock outstanding on December 31 of the preceding year and (b) a number of shares of Common Stock determined by the Company’s board of directors. In both December 2021 and 2022, the Company’s board of directors determined that there would be no automatic increase in the number of shares reserved under the 2021 Plan or the ESPP on either January 1, 2022 or January 1, 2023.
Under the 2021 Plan, the Company can grant non-statutory stock options, (“NSOs”), incentive stock options, ISOs, stock appreciation rights, restricted stock, restricted stock units, unrestricted stock, performance awards and other forms of awards. Under the ESPP, when and if implemented, eligible employees will be permitted to purchase shares of the Company’s Common Stock at the lower of 85% of the closing trading price per share of the Company’s Common Stock on the first day of the offering or 85% of the closing trading price per share on the exercise date, which will occur on the last day of each offering.
Prior to the Closing, Legacy Humacyte had two equity incentive plans, the 2015 Omnibus Incentive Plan, as amended, (the “2015 Plan”), and the 2005 Stock Option Plan (the “2005 Plan”). As a result of the Merger, no further awards may be granted under either the 2015 plan or the 2005 Plan. All awards previously granted and outstanding as of the effective date of the Merger were adjusted to reflect the impact of the Merger as set forth in the Merger Agreement, but otherwise remain in effect pursuant to their original terms. The shares underlying any award granted under the 2021 Plan or the 2015 Plan that are forfeited, cancelled or reacquired by the Company prior to vesting, that expire or that are paid out in cash rather than shares will become available for grant and issuance under the 2021 Plan. As of December 31, 2022, 3,632,237 and 484,562 shares of Common Stock remain reserved for outstanding options issued under the 2015 Plan and 2005 Plan, respectively.
The Company’s stock option plans allow for the grant of awards that the Company believes aid in aligning the interests of award recipients with those of its stockholders. The Company’s board of directors or compensation committee determines the specific terms of equity incentive grants, including the exercise price per share and vesting period for option awards. Option awards are granted with an exercise price equal to the fair market value of the Company’s Common Stock at the date of grant.
The Company has granted options that include either a service-based or performance-based vesting condition, or both, and a 10-year contractual term. The service-based vesting condition for the plans is generally satisfied over 36 to 48 months from the date of grant. The performance-based vesting conditions are satisfied upon the attainment of certain product development milestones. The Company recognizes stock-based compensation expense based on the grant date fair value of the awards measured using the Black-Scholes option pricing model. Compensation expense related to awards with service-based vesting conditions is recognized on a straight-line basis over the requisite service period.
Compensation expense related to awards with performance-based vesting conditions is recognized over the requisite service period using the accelerated attribution method to the extent achievement of the performance-based condition is probable. The Company does not recognize compensation expense related to awards with performance-based vesting conditions until it is probable that the performance-based vesting condition will be achieved. Forfeitures are accounted for as they occur.
Option awards under the Company’s option plans generally provide for accelerated vesting of the unvested portions of any option award in the event of an involuntary termination, as such term is defined in the relevant stock option agreement, of a grantee’s employment during the period that commences 30 days prior to the effective date of a corporate transaction and that ends 12 months following the effective date of such transaction. Additionally, the Company’s board of directors may, in its sole discretion, accelerate the vesting of any unvested stock options in the event of a corporate transaction.
131
The Company estimated the fair value of the stock options on the date of grant using the following assumptions in the Black-Scholes option-pricing model:
Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
Estimated dividend yield | 0 | % | 0 | % | |||||||
| Expected share price volatility (weighted average and range, if applicable) | 88.8% (87.4% to 100.0%) | 91.4% (90.7% to 93.1%) | |||||||||
| Risk-free interest rate (weighted average and range, if applicable) | 3.50% (1.89% to 3.69%) | 0.78% (0.62% to 1.32%) | |||||||||
| Expected term of options (in years) (weighted average and range, if applicable) | 6.25 | 6.05 (6.00 to 6.25) | |||||||||
•Fair Value of Common Stock. Prior to the Merger, as the Company’s common stock was not publicly traded, the fair value of the shares of its common stock underlying the options was determined by the Company’s board of directors with input from management, after considering independent third-party valuation reports. Subsequent to the Merger, the fair value of the Common Stock has been determined based on the closing price of the shares on Nasdaq.
•Expected Term. The expected term represents the period that stock options are expected to be outstanding. The Company calculated the expected term using the simplified method for options, which is available where there is insufficient historical data about exercise patterns and post-vesting employment termination behavior. The simplified method is based on the vesting period and the contractual term for each grant, or for each vesting-tranche for awards with graded vesting. The mid-point between the vesting date and the maximum contractual expiration date is used as the expected term under this method. For awards with multiple vesting-tranches, the times from grant until the mid-points for each of the tranches may be averaged to provide an overall expected term.
•Expected Volatility. The expected volatility was determined based on a blended approach using the historical share volatility of the Company’s Common Stock and that of several publicly traded peer companies over a period of time equal to the expected term of the options, as the Company has a limited trading history. For purposes of identifying these peer companies, the Company considered the industry, stage of development, size and financial leverage of potential comparable companies.
•Risk-Free Interest Rate. The risk-free interest rate was based on the yields of U.S. Treasury zero-coupon securities with maturities similar in duration to the expected term of the options.
•Expected Dividend Yield. The Company has not paid dividends on its Common Stock nor does it expect to pay dividends in the foreseeable future. Accordingly, the Company has estimated the dividend yield to be zero.
At December 31, 2022, there were 6,700,888 options remaining available for grant under the 2021 Plan. The Company has sufficient authorized and unissued shares to issue Common Stock in satisfaction of any awards available for grant under the 2021 Plan.
The following table shows a summary of stock-based compensation expense included in the consolidated statements of operations and comprehensive loss for the years ended December 31, 2022 and 2021:
Year Ended December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
Research and development | $ | 1,034 | $ | 3,220 | ||||||||||
General and administrative | 5,150 | 6,926 | ||||||||||||
Total | $ | 6,184 | $ | 10,146 | ||||||||||
132
A summary of option activity under the Company’s stock option plans during the year ended December 31, 2022 is presented below:
| Number of Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (years) | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
Options outstanding at December 31, 2021 | 6,711,192 | $ | 7.48 | 5.3 | $ | 8,276 | |||||||||||||||||
| Granted | 2,791,029 | 3.54 | |||||||||||||||||||||
| Exercised | (225,367) | 2.37 | |||||||||||||||||||||
| Forfeited | (2,072,980) | 8.21 | |||||||||||||||||||||
Options outstanding at December 31, 2022 | 7,203,874 | $ | 5.90 | 7.5 | $ | 429 | |||||||||||||||||
Vested and exercisable, December 31, 2022 | 3,375,289 | $ | 6.46 | 5.3 | $ | 429 | |||||||||||||||||
Vested and expected to vest, December 31, 2022 | 7,203,874 | $ | 5.90 | 7.5 | $ | 429 | |||||||||||||||||
The weighted-average grant-date fair value per share of options granted during the years ended December 31, 2022 and 2021 was $2.70 and $7.63, respectively. The total intrinsic value of options exercised during the years ended December 31, 2022 and 2021 was $0.3 million and $1.4 million, respectively. As of December 31, 2022, unrecognized stock-based compensation cost for options was $13.3 million and is expected to be recognized over a weighted-average period of 2.4 years.
11. Income Taxes
The Company did not record any income tax expense or benefit during the years ended December 31, 2022 and 2021. The Company has a net operating loss and has provided a valuation allowance against net deferred tax assets due to uncertainties regarding the Company’s ability to realize these assets. All losses before income taxes arose in the U.S.
133
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and deferred tax liabilities, including valuation allowances, are as follows:
As of December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
| Deferred tax assets: | ||||||||||||||
| Net operating loss | $ | 67,879 | $ | 58,646 | ||||||||||
| Capitalized research and development | 43,818 | 36,830 | ||||||||||||
| Research credits | 18,054 | 16,765 | ||||||||||||
| Stock-based compensation | 3,047 | 1,323 | ||||||||||||
| Right of use lease liability | 144 | 153 | ||||||||||||
| Accrued expenses | 65 | 92 | ||||||||||||
| Other | 1 | 1 | ||||||||||||
| Total deferred tax asset | 133,008 | 113,810 | ||||||||||||
| Less: valuation allowance | (131,151) | (111,575) | ||||||||||||
| Total net deferred tax asset | 1,857 | 2,235 | ||||||||||||
| Deferred tax liabilities: | ||||||||||||||
| Basis difference in fixed assets | (1,713) | (2,082) | ||||||||||||
| Right of use lease assets | (144) | (153) | ||||||||||||
| Total deferred tax liability | (1,857) | (2,235) | ||||||||||||
| Total net deferred tax asset/(liability) | $ | — | $ | — | ||||||||||
A valuation allowance is provided for deferred tax assets where the recoverability of the assets is uncertain. The determination to provide a valuation allowance is dependent upon the assessment of whether it is more likely than not that sufficient future taxable income will be generated to utilize the deferred tax assets. Based on the weight of the available evidence, which includes the Company’s historical operating losses, lack of taxable income and the accumulated deficit, the Company provided a full valuation allowance against the deferred tax assets resulting from the tax loss and credits carried forward as of December 31, 2022 and December 31, 2021.
On November 18, 2021, North Carolina enacted the 2021 Appropriations Act, which included a gradual corporate income tax rate decrease from the current 2.5% to 0% by 2030. The Company is in a cumulative loss position and does not have significant deferred tax liabilities that can be utilized as a source of taxable income in the future. Therefore, the Company has reduced its North Carolina deferred tax assets, including the net operating losses, to zero, as no benefit is expected to be realized from these deferred tax assets prior to 2030 when there would be no income tax in North Carolina. The reduction in the value of the deferred tax assets resulted in $6.9 million of tax expense in 2021, which was offset fully by the reduction in the corresponding valuation allowance. If the Company becomes profitable prior to 2030, the Company will recognize an income tax benefit related to the portion of its North Carolina deferred tax assets utilized.
134
The reasons for the difference between the actual income tax benefit for the years ended December 31, 2022 and 2021, and the amount computed by applying the statutory Federal income tax rate to losses before income taxes are as follows:
| December 31, | ||||||||||||||||||||||||||
| 2022 | 2021 | |||||||||||||||||||||||||
| ($ in thousands) | Amount | Rate | Amount | Rate | ||||||||||||||||||||||
| Income tax benefit at statutory rate | $ | (2,512) | 21.0 | % | $ | (5,560) | 21.0 | % | ||||||||||||||||||
| State income taxes, net of federal benefit | (1,767) | 14.8 | % | (1,706) | 6.4 | % | ||||||||||||||||||||
| Tax credits | (2,325) | 19.4 | % | (2,662) | 10.1 | % | ||||||||||||||||||||
| Other nondeductible expenses | (15,735) | 131.5 | % | (11,991) | 45.3 | % | ||||||||||||||||||||
| Deferred rate changes | 1,698 | (14.2) | % | 8,981 | (33.9) | % | ||||||||||||||||||||
Deferred tax true-up(1) | 1,065 | (8.9) | % | 3,120 | (11.8) | % | ||||||||||||||||||||
| Change in valuation allowance | 19,576 | (163.6) | % | 9,818 | (37.1) | % | ||||||||||||||||||||
| Provision for income taxes | $ | — | 0.0 | % | $ | — | 0.0 | % | ||||||||||||||||||
___________________________
(1)The deferred tax true-up for 2022 and 2021 primarily relates to executive compensation subject to IRC Section 162(m) limitations and the 2021 research and development and net operating loss carryforwards were adjusted due to application of the employee retention credit determined in 2022.
As of December 31, 2022 the Company had approximately $322.4 million and $323.9 million of Federal and state net operating losses, respectively. Of this amount, $161.1 million of Federal net operating losses are subject to an 80% limitation on taxable income, do not expire and will carry forward indefinitely, while the remaining amount begins to expire in 2025. Some of these state net operating losses included in these amounts follow the Federal Tax Cuts and Jobs Act and are carried over indefinitely. The Company’s state net operating losses began to expire in 2020 and will expire completely in 2042. The state operating loss carryforwards are inclusive of North Carolina net operating losses, which are recorded at a zero benefit.
As of December 31, 2022 and 2021, the Company had Federal and state research tax credit carryforwards of $18.1 million and $15.7 million, respectively. These credit carryforwards will begin to expire in 2025 and will expire completely in 2042.
Net operating loss carryforwards and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service, or IRS, and may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders or groups over a three-year period in excess of 50% as defined under Sections 382 and 383 in the Internal Revenue Code, which could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has not determined whether there have been any cumulative ownership changes or the impact on the utilization of the loss carryforwards if such changes have occurred. A section 382 study will be performed at a time when forthcoming profitability is reasonably anticipated.
12. Retirement Plan
The Company maintains two defined contribution employee retirement plans, or 401(k) plans, for all employees upon their date of hire. The 401(k) plans are intended to qualify as tax-qualified plans under Section 401(k) of the Internal Revenue Code of 1986, as amended. The plans permit employees to contribute, on a pre-tax basis, a portion of their salary up to the Federally mandated limits. The Company matches an employee’s contribution up to 4% of the employee’s compensation. Contributions to the plans by the Company totaled $0.7 million and $0.6 million for the years ended December 31, 2022 and 2021, respectively.
135
13. Commitments and Contingencies
Patent License Agreements
Duke University
In March 2006, the Company entered into a license agreement with Duke University (“Duke”), which was subsequently amended in 2011, 2014, 2015, 2018, 2019 and January 2022. Under this license agreement, Duke granted the Company a worldwide, exclusive, sublicensable license to certain patents related to decellularized tissue engineering, referred to as the patent rights, as well as a non-exclusive license to use and practice certain know-how related to the patent rights. The relevant licensed patent on decellularization of tissue expired in 2021. The Company has agreed to use commercially reasonable efforts to develop, register, market and sell products utilizing the patent rights, referred to as the licensed products. Any services provided to a third party utilizing licensed products are referred to as licensed services. The Company has also agreed to meet certain benchmarks in its development efforts, including as to development events, clinical trials, regulatory submissions and marketing approval, within specified timeframes. Under the license agreement, Duke retains the right to use the patent rights for its own educational and research purposes, and to provide the patent rights to other non-profit, governmental or higher-learning institutions for non-commercial purposes without paying royalties or other fees.
In connection with the Company’s entry into the license agreement, the Company granted equity consideration to Duke in the form of 52,693 shares of the Company’s common stock. Under the license agreement, the Company also agreed to pay Duke:
•a low single-digit percentage royalty on eligible sales of licensed products and licensed services, plus a low double-digit percentage of any sublicensing revenue;
•an annual minimum royalty beginning in 2012, which increases in the calendar year immediately following the first commercial sale of licensed products or licensed services (whichever occurs first); and
•an additional amount in license fees, as certain milestones are met.
The license agreement remains effective until the later of (i) the last of the patent rights expires or (ii) four years after the Company’s first commercial sale, unless terminated earlier. Either party may terminate the agreement for fraud, willful misconduct or illegal conduct, or uncured material breach. Duke may terminate the agreement if the Company becomes insolvent. Duke may also terminate the license, convert the license into a non-exclusive license or seek assignment of any sublicense if the Company fails to reach diligence milestones within the applicable time period. If the Company abandons any claim, patent or patent application, its rights under the license with respect to such patent rights will be terminated in the territory in which the Company abandons such rights. The Company may terminate the license agreement unilaterally upon three months’ prior notice to Duke. The Company agrees to indemnify Duke against certain third-party claims. Payments to Duke under the license agreement were immaterial during the periods presented.
Yale University
In February 2014, the Company entered into a license agreement with Yale University (“Yale”) that granted the Company a worldwide license to the patents related to coatings for small-diameter vessels to inhibit clotting. The license granted under the agreement is exclusive in the field of engineered vascular tissues and tissues and extracellular matrix-based implants used for vascular repair, reconstruction and replacement (provided that all uses are vascular tissues within the range of 1 – 12mm in diameter), except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. The Company has agreed to pay to Yale an annual maintenance fee, increasing between the first and fourth anniversaries of the agreement up to a maximum of less than $0.1 million per year for this license. In December 2022, in accordance with the terms of the agreement, the Company provided Yale with 90 days written notice of termination, effective March 21, 2023.
In August 2019, the Company entered into a license agreement with Yale that granted the Company a worldwide license to the patents related to Bioartificial Vascular Pancreas (“BVP”). The license granted under the agreement is exclusive in the field of engineered vascular tissues that deliver pancreatic islet cells to patients, except that it is subject to
136
Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. The Company has agreed to pay to Yale an annual maintenance fee, increasing between the first and fourth anniversaries of the agreement up to a maximum of less than $0.1 million per year for this license.
In August 2019, the Company entered into a license agreement with Yale that granted the Company a worldwide license to the patents related to tubular prostheses. The license granted under the agreement is exclusive in the field of engineered urinary conduits, engineered tracheas/airways, and engineered esophagi, except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. The Company has agreed to pay to Yale an annual maintenance fee, increasing between the first and fourth anniversaries of the agreement up to a maximum of less than $0.1 million per year for this license.
The Company has agreed to use reasonable commercial efforts to develop and commercialize the licensed patents and any licensed products and methods, and to use reasonable efforts to make the licensed products available to patients in low and low-middle income countries. The Company is also obligated to provide Yale periodically an updated and revised copy of its plan for each license, which must indicate progress of its development and commercialization. The Company may also sublicense the Company’s rights without Yale’s prior written consent, but such sublicense is subject to certain conditions.
In connection with its entry into the license agreement, the Company paid Yale upfront cash fees. The Company has also agreed to pay Yale:
•annual maintenance fees, increasing between the first anniversary of the agreement until the fifth anniversary for the coating (see above) and BVP licenses and until the fourth anniversary for the tubular prostheses license up to a maximum of less than $0.1 million per year;
•milestone payments upon achievement of certain regulatory and commercial milestones of $0.2 million and $0.6 million, respectively;
•a low single-digit percentage royalty on worldwide net sales, subject to reductions for third-party license fees; and
•a low double-digit percentage of sublicensing income.
If the Company or any of its future sublicensees bring a patent challenge against Yale or assists another party in bringing a patent challenge against Yale, the license fees described above will be subject to certain increases and penalties.
The agreements expire on a country-by-country basis on the date on which the last of the patents in such country expires, lapses or is declared invalid. Yale may terminate the agreements if the Company fails to (i) provide written diligence reports, (ii) provide commercially reasonable diligence plans, (iii) implement the plans in accordance with the obligations under the agreements, or (iv) reach certain research and development milestones within the scheduled timeframe set forth in the agreements; however, any such termination right would be limited in scope to the country to which such failure relates. Yale may also terminate for the Company’s non-payment, uncured material breach, failure to obtain adequate insurance, bringing or assisting in bringing of a patent challenge against Yale, abandonment of the research and development of the Company’s products or insolvency. The Company may terminate the license agreements (i) on 90 days’ prior written notice to Yale, provided the Company is not in breach of the license agreements and has made all required payments to Yale thereunder and (ii) on written notice to Yale following an uncured material breach. With respect to the license agreements related to small-diameter vessels and BVP, the Company’s rights under the license agreements will also terminate automatically with respect to a patent application or patent within the licensed patents in a specified country if, upon receipt of written notice from Yale, the Company does not agree to pay the patent filing, prosecution and maintenance fees incurred by Yale for such patent applications or patents in the specified country. Under certain circumstances, Yale may, at its option, convert the exclusive licenses to non-exclusive licenses if the Company declines to initiate certain infringement or interference proceedings with respect to the licensed patents. The Company has agreed to indemnify Yale against certain third-party claims. Payments to Yale under the license agreement were immaterial during the periods presented.
137
Legal Matters
The Company currently is not aware of any legal proceedings or claims that management believes will have, individually or in the aggregate, a material adverse effect on the Company’s business, financial condition, results of operations, or cash flows.
Indemnification
To the extent permitted under Delaware law, the Company has agreed to indemnify its directors and officers for certain events or occurrences while the director or officer is, or was serving, at the Company’s request in such capacity. The indemnification period covers all pertinent events and occurrences during the director’s or officer’s service. The maximum potential amount of future payments the Company could be required to make under these indemnification arrangements is not specified in such arrangements; however, the Company has director and officer insurance coverage that is intended to reduce its exposure and enable the Company to recover a portion of any potential future amounts the Company could be required to make. To date, the Company has not incurred any costs as a result of such obligations and has not accrued any liabilities related to such obligations in the consolidated financial statements.
14. Related Party Transactions
Fresenius Medical Care investments and distribution agreement
In June 2018, the Company completed a $150 million financing transaction pursuant to which Fresenius Medical Care purchased shares of series D redeemable convertible preferred stock that at the Closing of the Merger converted into 15,812,735 shares of the Company’s common stock. In August 2021, Fresenius Medical Care invested $25 million as part of the PIPE Financing and received an additional 2.5 million shares of the Company’s common stock.
In addition, the Company entered into a distribution agreement with Fresenius Medical Care in June 2018 which, as amended as of February 16, 2021, granted Fresenius Medical Care and its affiliates exclusive rights to develop outside the United States and EU and commercialize outside of the United States the Company’s 6 millimeter x 42 centimeter HAV and all improvements thereto, and modifications and derivatives thereof (including any changes to the length, diameter or configuration of the foregoing), for use in vascular creation, repair, replacement or construction, including renal replacement therapy for dialysis access, the treatment of peripheral artery disease, and the treatment of vascular trauma, but excluding coronary artery bypass graft, pediatric heart surgery, or adhering pancreatic islet cells onto the outer surface of the distribution product for use in diabetic patients. Within the United States, Fresenius Medical Care will collaborate with the Company in its commercialization of the product in the field, including adoption of the distribution product as a standard of care in patients for which such use is supported by clinical results and health economic analyses.
The Company is responsible for developing and seeking regulatory approval for the distribution product in the field in the United States. For countries outside the United States, the parties agreed to use commercially reasonable efforts to satisfy certain agreed minimum market entry criteria for the distribution product in the field in such country. For the EU, once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory approval for the distribution product in the field in the applicable country, the Company agreed to use commercially reasonable efforts to obtain such regulatory approval (other than pricing approval), and Fresenius Medical Care agreed to use commercially reasonable efforts to obtain the corresponding pricing approval. For the rest of the world (i.e., outside the United States and the EU), once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory and pricing approval for the distribution product in the field in the applicable country, Fresenius Medical Care agreed to use commercially reasonable efforts to obtain such approvals, and the Company agreed to use commercially reasonable efforts to support Fresenius Medical Care in its efforts.
Under the distribution agreement, the Company grants an exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by the Company during the term to commercialize the distribution product in the field outside the United States, subject to the Company’s retained rights to carry out its obligations under the distribution agreement. The Company also grants a non-exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by the Company during the term to develop the distribution product in accordance with the terms of the distribution agreement. In addition, the Company grants to Fresenius Medical Care, among other things, a perpetual, irrevocable, non-exclusive sublicensable license under the patents and know-how that primarily relate to the distribution product or its manufacture and that were created, conceived or
138
developed solely or jointly by or on behalf of Fresenius Medical Care in the performance of its activities under the distribution agreement.
The distribution agreement provides that the Company will own all know-how and patents that primarily relate to the distribution product or its manufacture that are created, conceived or developed by or on behalf of either party in the performance of activities under the distribution agreement. Ownership of all other know-how, patents, materials and other intellectual property created, conceived or developed during the performance of activities under the distribution agreement will be determined in accordance with U.S. patent laws for determining inventorship.
The Company is obligated to make payments to Fresenius Medical Care based on a share of aggregate net sales by or on behalf of the Company of the distribution product in the United States in the field. Such revenue-share payments will be a percentage of net sales in the low double digits, without regard to the calendar year in which such net sales are attributable, until such time that the Company has paid to Fresenius Medical Care a certain total amount, at which time the revenue-share will decrease to a percentage of net sales in the mid-single digits. The amounts that Fresenius Medical Care will be obligated to pay the Company under the distribution agreement for sales of the distribution product in the field outside of the United States will vary. Fresenius Medical Care agreed to pay the Company initially, on a country-by-country basis for sales outside of the United States, the amount equal to the average cost of manufacturing the Company’s distribution product plus a fixed dollar amount per unit. Following a specified period, on a country-by-country basis outside of the United States, Fresenius Medical Care will pay the Company a fixed percentage of net sales for each unit sold in such country, such that the Company will receive more than half of such net sales.
The distribution agreement will generally continue on a country-by-country basis until the later of (a) the tenth anniversary of the launch date of the distribution product in the relevant country or (b) the expiration of the last-to-expire valid claim of specified patents in such country. Each party is permitted to terminate the distribution agreement for insolvency of, or, under certain circumstances, including various cure periods, material breach by the other party. Subject to a cure period, Fresenius Medical Care may also terminate the distribution agreement in its entirety or on a country-by-country basis (i) for certain withdrawals of regulatory approval or (ii) for termination or expiration of any of our in-licenses that is necessary for the exercise of Fresenius Medical Care’s rights, or the satisfaction of its obligations, under the distribution agreement. In addition, Fresenius Medical Care may terminate the distribution agreement for convenience on a country-by-country basis upon not less than 12 months’ written notice to the Company, although Fresenius Medical Care is not permitted to give such notice prior to the end of the second year following launch of the distribution product in such country. Each party is required to indemnify one another for certain third-party claims.
Arrangements with Yale University
Dr. Niklason serves as an Adjunct Professor in Anesthesia at Yale University. As of December 31, 2022 and 2021, the Company was a party to license agreements with Yale University, as described in Note 13 — Commitments and Contingencies above.
The following table shows a summary of related party expenses included in the statements of operations and comprehensive loss for the years ended December 31, 2022 and 2021:
Year Ended December 31, | ||||||||||||||
| ($ in thousands) | 2022 | 2021 | ||||||||||||
| License expenses | 100 | 85 | ||||||||||||
| Other | 19 | 91 | ||||||||||||
| Total | $ | 119 | $ | 176 | ||||||||||
There was $50 thousand of license expenses payable to Yale University included in accounts payable on the Company’s consolidated balance sheets, as of December 31, 2022.
139
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is (i) recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
As of December 31, 2022, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2022.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statements for external purposes in accordance with U.S. GAAP. A control system, no matter how well designed and operated, can only provide reasonable, not absolute, assurance that the objectives of the control system are met. Because of these inherent limitations, management does not expect that our internal controls over financial reporting will prevent all errors and all fraud. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with our policies and procedures may deteriorate. Our management, under the supervision of and with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the results of its evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2022.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting as we are an “emerging growth company” as defined in the JOBS Act. For as long as we remain an “emerging growth company,” we are exempt from the auditor attestation requirement in the assessment of the effectiveness of our internal control over financial reporting.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
140
Part III
Item 10. Directors, Executive Officers and Corporate Governance.
Incorporated by reference from the information in our Proxy Statement for our 2023 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
Item 11. Executive Compensation.
Incorporated by reference from the information in our Proxy Statement for our 2023 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Incorporated by reference from the information in our Proxy Statement for our 2023 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Incorporated by reference from the information in our Proxy Statement for our 2023 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
Item 14. Principal Accountant Fees and Services
Incorporated by reference from the information in our Proxy Statement for our 2023 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
141
Part IV
Item 15. Exhibits and Financial Statement Schedules.
(a) The following financial statements are included in this Annual Report on Form 10-K:
(1) List of Financial Statements:
The financial statements required by this item are listed in Item 8, “Financial Statements and Supplementary Data” herein.
(2) List of Financial Statement Schedules:
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or notes thereto.
(3) List of Exhibits:
| Exhibit No. | Description | |||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
| 4.3* | ||||||||
| 4.4 | ||||||||
| 4.5 | ||||||||
| 4.6 | ||||||||
| 10.1 | ||||||||
| 10.2 | ||||||||
| 10.2.1 | ||||||||
| 10.2.2 | ||||||||
10.3˄ | ||||||||
10.3.1˄ | ||||||||
142
| Exhibit No. | Description | |||||||
10.3.2˄ | ||||||||
10.4˄ | ||||||||
| 10.5˄ | ||||||||
| 10.6˄ | ||||||||
| 10.7˄ | ||||||||
| 10.7.1˄ | ||||||||
| 10.7.2˄ | ||||||||
| 10.7.3˄ | ||||||||
| 10.7.4˄ | ||||||||
| 10.7.5˄ | ||||||||
| 10.7.6^ | ||||||||
| 10.8˄ | ||||||||
| 10.8.1˄ | ||||||||
| 10.8.2˄ * | ||||||||
| 10.9˄ * | ||||||||
| 10.10+* | ||||||||
| 10.11+! | ||||||||
| 10.12+! | ||||||||
| 10.13+ | ||||||||
| 10.13.1+ | ||||||||
| 10.14+ | ||||||||
143
| Exhibit No. | Description | |||||||
| 10.15+ | ||||||||
| 10.16+ | ||||||||
| 10.16.1+ | ||||||||
| 10.16.2+ | ||||||||
| 10.16.3+ | ||||||||
| 10.16.4+ | ||||||||
| 10.16.5+ | ||||||||
| 10.17+ | ||||||||
| 10.17.1+ | ||||||||
| 10.17.2+ | ||||||||
| 10.17.3+ | ||||||||
| 10.17.4+ | ||||||||
| 10.18+ | ||||||||
| 10.19 | ||||||||
| 10.19.1 | ||||||||
| 10.19.2 | ||||||||
| 10.19.3 | ||||||||
| 10.19.4 | ||||||||
| 21.1 | ||||||||
| 23.1! | ||||||||
| 31.1! | ||||||||
| 31.2! | ||||||||
144
| Exhibit No. | Description | |||||||
| 32.1! | ||||||||
| 32.2! | ||||||||
| 101! | The following materials from Humacyte, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2022, formatted in Inline XBRL (Inline eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2022 and 2021, (ii) Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2022 and 2021, (iii) Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit) for the years ended December 31, 2022 and 2021, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021, and (v) Notes to Consolidated Financial Statements. | |||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | |||||||
___________________________
! Filed herewith.
* Annexes, schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The registrant agrees to furnish supplementally a copy of any omitted attachment to the Securities and Exchange Commission on a confidential basis upon request.
^ Certain confidential information contained in this exhibit, market by brackets, has been omitted because the information (i) is not material and (ii) is the type of information the company both customarily and actually treats as private or confidential.
+ Management contract or compensatory plan or arrangement.
Item 16. Form 10-K Summary.
None.
145
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act of 1934, as amended, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| HUMACYTE, INC. | ||||||||
| By: | /s/ Laura E. Niklason | |||||||
| Name: Laura E. Niklason, M.D., Ph.D. | ||||||||
| Title: President and Chief Executive Officer | ||||||||
Date: March 24, 2023
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Annual Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Name | Position | Date | ||||||||||||
| /s/ Laura E. Niklason | President, Chief Executive Officer and Director (Principal Executive Officer) | March 24, 2023 | ||||||||||||
| Laura E. Niklason, M.D., Ph.D. | ||||||||||||||
| /s/ Dale A. Sander | Chief Financial Officer, Chief Corporate Development Officer and Treasurer (Principal Financial and Accounting Officer) | March 24, 2023 | ||||||||||||
| Dale A. Sander | ||||||||||||||
| Director | March 24, 2023 | |||||||||||||
| Kathleen Sebelius | ||||||||||||||
| Director | March 24, 2023 | |||||||||||||
| Gordon M. Binder | ||||||||||||||
| Director | March 24, 2023 | |||||||||||||
| Emery N. Brown, M.D., Ph.D. | ||||||||||||||
| /s/ Michael T. Constantino | Director | March 24, 2023 | ||||||||||||
| Michael T. Constantino | ||||||||||||||
| /s/ Brady W. Dougan | Director | March 24, 2023 | ||||||||||||
| Brady W. Dougan | ||||||||||||||
| /s/ C. Bruce Green | Director | March 24, 2023 | ||||||||||||
| C. Bruce Green, M.D. | ||||||||||||||
| /s/ Todd M. Pope | Director | March 24, 2023 | ||||||||||||
| Todd M. Pope | ||||||||||||||
| /s/ Diane Seimetz | Director | March 24, 2023 | ||||||||||||
| Diane Seimetz, Ph.D. | ||||||||||||||
| /s/ Rajiv Shukla | Director | March 24, 2023 | ||||||||||||
| Rajiv Shukla | ||||||||||||||
| /s/ Max Wallace | Director | March 24, 2023 | ||||||||||||
| Max Wallace, J.D. | ||||||||||||||
| /s/ Susan Windham-Bannister | Director | March 24, 2023 | ||||||||||||
| Susan Windham-Bannister, Ph.D. | ||||||||||||||
146
Similar companies
See also AMGEN INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also GILEAD SCIENCES, INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Moderna, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also BioNTech SE
See also BIOGEN INC. - Annual report 2023 (10-K 2023-12-31) Annual report 2025 (10-Q 2025-03-31)
