MESA LABORATORIES INC /CO/ - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2022
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____ to ____
Commission File No: 0-11740
MESA LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
| Colorado | 84-0872291 |
| (State or other jurisdiction of | (I.R.S. Employer |
| Incorporation or organization) | Identification number) |
| 12100 West Sixth Avenue | |
| Lakewood, Colorado | 80228 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (303) 987-8000
Securities registered under Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||
| Common stock, no par value | MLAB | The Nasdaq Stock Market LLC |
Securities registered under Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (check one):
| Large accelerated filer ☒ | Accelerated filer ☐ | Non-accelerated filer ☐ | Smaller reporting company ☐ | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of voting stock held by non-affiliates of the registrant was $1,454 million based upon the closing market price and common shares outstanding as of September 30, 2021.
The number of outstanding shares of the Registrant’s common stock as of May 25, 2022 was 5,267,902.
This document (excluding exhibits) contains 80 pages.
DOCUMENTS INCORPORATED BY REFERENCE
Part III is incorporated by reference from the registrant’s definitive Proxy Statement for its 2022 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after the close of the registrant's fiscal year.
Forward-Looking Statements
In this annual report on Form 10-K, Mesa Laboratories, Inc., a Colorado corporation, together with its subsidiaries is collectively referred to as “we,” “us,” “our,” the “Company,” or "Mesa." Mesa was organized in 1982 as a Colorado corporation.
General
We are a multinational manufacturer, developer, and seller of life sciences tools and critical quality control products and services, many of which are sold into niche markets driven by regulatory requirements. We have manufacturing operations in the United States and Europe, and our products are marketed by our sales personnel in North America, Europe, and Asia Pacific, and by independent distributors in these areas as well as throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross margins.
We are headquartered in Lakewood, Colorado and our common stock is listed for trading on the Nasdaq Global Market (“Nasdaq”) under the symbol MLAB.
Our fiscal year ends on March 31. References in this Annual Report on Form 10-K (“annual report”) to a particular “fiscal year,” “year” or “year-end” mean our fiscal year, references to the first quarter of fiscal year 2022 refer to the period from April 1, 2021 through June 30, 2021, references to the second quarter of fiscal year 2022 refer to the period from July 1, 2021 through September 30, 2021, references to the third quarter of fiscal year 2022 refer to the period from October 1, 2021 through December 31, 2021, and references to the fourth fiscal quarter of fiscal year 2022 refer to the period from January 1, 2022 through March 31, 2022.
Strategy
We strive to create shareholder value and further our purpose of Protecting the Vulnerable® by growing our business both organically and through further strategic acquisitions, by improving our operating efficiency, and by continuing to hire, develop and retain top talent. As a business, we commit to our purpose of Protecting the Vulnerable® every day by taking a customer-focused approach to developing, building, and delivering our products. We serve a broad set of industries, in particular the pharmaceutical, healthcare services, and medical device verticals that require dependable life sciences tools and quality control and calibration solutions to ensure the safety and efficacy of the products they use.
Our revenues come from product sales, which include consumables and hardware, as well as services, which include discrete and ongoing calibration, testing, and maintenance services and contracts. We grow our revenues organically by expanding our customer base, increasing sales volumes, and implementing price increases, and inorganically through acquisitions. Our acquisition strategy is focused on businesses that complement our existing portfolio as well as those that expand our presence further into life sciences tools and critical quality control solutions for regulated applications in the pharmaceutical, healthcare and medical device industries.
We continue to focus on improving our operating efficiency through The Mesa Way, which is our customer-centric, lean based system for continuously improving and operating a set of high-margin, niche businesses. The Mesa Way is based on four pillars:
| – |
Measure what matters: We use “True North,” our customers’ perspective, to measure what matters most and to set high standards for performance. We manage to leading indicators whenever possible, which drives us to proactively avoid problems before they are apparent to our customers. |
| – |
Empower Teams: We move decision making as close to the customer as possible and provide the structure and real-time communication forum to align the whole organization towards surpassing customer expectations. |
| – |
Steadily Improve: We leverage a common and proven set of lean-based tools to identify the root cause of opportunities, prioritize our biggest opportunities, and enable change to be embraced and implemented quickly. |
| – |
Always Learn: We ensure that improvements are sustained, enabling us to raise performance expectations and repeat the cycle of improvement. Equally, this cycle strengthens the Mesa team by providing endless learning opportunities for our employees and helps us to become an employer of choice in our communities. |
Finally, we hire, develop, and retain top talent capable of taking on new challenges using a team approach to continuously improve our products, our services, and ourselves, resulting in long-term value creation for our shareholders.
Our Segments
As described in Note 14. "Segment Data" of the Notes to Consolidated Financial Statements contained within Item 8. Financial Statements and Supplementary Data of this annual report, following the acquisition (the "Agena Acquisition") of Agena Bioscience, Inc. ("Agena") on October 20, 2021, we changed our reporting segments to align with strategic changes in the way we manage our business units. We report our financial performance in four segments, or divisions: (1) Sterilization and Disinfection Control, (2) Biopharmaceutical Development, (3) Calibration Solutions, which represents a combination of the historical Instruments and Continuous Monitoring reporting segments, and (4) Clinical Genomics, a new reporting segment comprised entirely of Agena's operations. Non-reportable operating segments and unallocated corporate expenses are reported within Corporate and Other.
Sterilization and Disinfection Control
Our Sterilization and Disinfection Control division manufactures and sells biological, chemical, and cleaning indicators used to assess the effectiveness of sterilization processes, including steam, gas, hydrogen peroxide, ethylene oxide, radiation, and other processes in the pharmaceutical, medical device, hospital, and dental industries. The Sterilization and Disinfection Control division also provides related testing services, mainly to the dental industry.
Biological indicators consist of resistant spores of certain microorganisms that are applied on a convenient substrate, such as a small piece of filter paper. The spores are well characterized in terms of purity, population of spores, and resistance to sterilization. Our biological indicator products are manufactured by growing microbiological spores from raw materials, using the spores to form finished products, and testing the finished biological indicators using established quality control tests. Our dental sterilizer testing products are assembled into kits containing biological indicator spore strips. Our microbiological laboratory tests these kits when they are returned to us to determine the effectiveness of our customers' sterilization processes.
Our biological indicators are distinguished in the marketplace by their high level of quality, consistency, and flexibility. We offer a variety of different product formats, which allows our biological indicators to be used in many different types of processes, equipment, and environments. For example, our simple spore strips are used most often in small table-top steam sterilizers in dental offices, while our more complex self-contained biological indicators, either with or without process challenge devices ("PCDs"), may be used by medical device manufacturers to assure sterility in complex ethylene oxide sterilization processes. In either case, the population of spores contained on the carrier and the resistance of the spores to the sterilization process must be well characterized in order to accurately assess the effectiveness of sterilization. During manufacture, extensive quality control steps are used to ensure that the microorganism spores are well-characterized and their resistance is known following placement on the target carrier.
Chemical indicators use a chemical change (generally determined by color) to assess the exposure to sterilization conditions. Biological indicators and chemical indicators are often used together to monitor processes.
Cleaning indicators are used to assess the effectiveness of cleaning processes, including washer-disinfectors and ultrasonic cleaners in healthcare settings. Cleaning is the critical first step performed prior to disinfection and sterilization. Debris left on an instrument may interfere with microbial inactivation and can compromise disinfection or sterilization processes. Cleaning indicators complement sterilization and disinfection processes within central sterile supply departments, such as in hospitals. Our cleaning indicator products are manufactured by inoculating a test soil onto a stainless-steel coupon. The test soil is designed to mimic the challenge of removing blood and tissue from surgical instruments and evaluates the effectiveness of our customers' cleaning processes.
Our Bozeman, Montana and Munich, Germany locations manufacture our Sterilization and Disinfection Control division products, which include the EZTest®, ProSpore®, PCD®, Apex® and Simicon biological and cleaning indicators. Our Bozeman, Montana facility also provides sterility assurance testing services to dental offices in the United States and Canada. Sterilization and disinfection control products are disposable and are used on a routine basis, thus product sales are less sensitive to general economic conditions. We generate sales to end users through our direct sales personnel and independent distributors. Customers include hospitals, dental offices, contract sterilization providers and various industrial users involved in pharmaceutical and medical device manufacturing.
Our sterilization and disinfection control products are used in highly regulated industries and compete on the basis of quality, cost effectiveness, and suitability for intended use. We compete with various other sterilization and cleaning indicator providers, and additional products using new technologies that may be competitive with our products may be introduced.
Biopharmaceutical Development
Our Biopharmaceutical Development division develops, manufactures, and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Protein analysis and peptide synthesis solutions accelerate the discovery, development, and manufacture of biological therapies, among other applications. Customers include biopharmaceutical research, development, and manufacturing teams at biopharmaceutical companies and their contract research organization partners, as well as academic research and development laboratories. The Biopharmaceutical Development division sells two types of products: (1) protein analysis solutions, which are used to test for the existence or concentration of specific proteins in a fluid sample, and (2) peptide synthesis solutions, which automate the synthesis of peptides from amino acids; both are primarily used in biopharmaceutical research, discovery and development, and bioprocessing. Our Biopharmaceutical Development division develops and manufactures Gyrolab® xPand and Gyrolab xPlore® hardware and software, as well as Gyrolab Bioaffy® consumable microfluidic disks (“CDs”), and Gyrolab kits and Rexxip® buffers for protein analysis in Uppsala, Sweden, while PurePep® Chorus, Symphony® X, and Sonata® XT hardware and associated software for peptide synthesis are developed and manufactured in our Tucson, Arizona location. Products manufactured in Sweden are typically invoiced in U.S. Dollars or euros, whereas the costs to produce the products is incurred in Swedish Krona. As a result, the Biopharmaceutical Development segment is susceptible to changes in foreign currency. For a discussion of risks related to our non-U.S. operations and foreign currency exchange, refer to Item 1A. Risk Factors, “Foreign currency exchange rates may adversely affect our financial statements” and “Our international operations subject us to a wide range of risks.”
About one-third of our Biopharmaceutical Development revenues are from consumables used on a routine basis. Sales of these products are less sensitive to general economic conditions. Approximately 45% of revenues are from more discretionary hardware purchases that are more sensitive to general economic conditions. The remainder of sales are related to service and support agreements. We generate sales directly as well as through independent foreign distributors. Marketing activities include industry conferences, user meetings, educational webinars, and all forms of digital marketing, in addition to market sensing and capturing user requirements for new product roadmaps. In-person marketing largely resumed during the year ended March 31, 2022 as many COVID-19 restrictions gradually lifted.
The Biopharmaceutical Development division’s market success is primarily dependent upon creating innovative, high quality products that customers choose based on available features, cost-effectiveness, and performance. We believe we are one of the leading world-wide suppliers of protein analysis and peptide synthesis equipment to the biologics discovery and development market. We further believe that enhancements of our product offerings and new product development driven by our research and development team, the recognized quality of our products and support, and the ability to continue to bring novel, cutting edge products and solutions to the market will allow us to remain competitive in the growing markets we serve.
Protein Analysis
We develop, manufacture, and market protein analysis equipment, CDs, kits, and buffers that enable the detection and quantification of a target protein in a biological or bioprocess sample. Gyrolab technology is widely used across human and non-human applications, mainly for therapy development and bioprocess design. Customers, primarily pharmaceutical and biotech companies and their contract research organization partners developing protein-based therapies, use our CDs to deposit their samples for mixing with application specific reagents. The CDs and reagents are loaded into one of our instruments for processing and analysis. Our proprietary software interprets results and provides useful data analysis for decision-making. Our protein analysis products accelerate the development and processing of assays to obtain accurate results for pre-clinical and clinical studies as well as for upstream and downstream bioprocessing of biological therapies, thus meeting critical data and time requirements. Our analytical protein technologies provide superior data consistency and accuracy, and reduce labor and the attendant variability of more manual analysis methods.
Peptide Synthesizers
Our peptide synthesis solutions enable customers to automate the chemical synthesis of peptides used in the creation of peptide therapies, biomaterials, cosmetics, and general research. Our peptide synthesis products facilitate the ability to produce more complex and longer peptides with higher purity, and are designed to comply with related Food and Drug Administration ("FDA") and European Medicines Agency requirements. Customers of our peptide synthesizers include commercial and academic biopharmaceutical laboratories, as well as contract manufacturers of peptides.
Calibration Solutions
Our Calibration Solutions division develops, manufactures, and sells quality control and calibration products used to measure or calibrate temperature, pressure, pH, humidity, and other such parameters for health and safety purposes, primarily in hospital, medical device manufacturing, pharmaceutical manufacturing, and various laboratory and healthcare environments. Generally, our Calibration Solutions products are used for testing, quality control, safety, validation and regulatory compliance. As of March 31, 2022, our Lakewood, Colorado and Hanover, Germany facilities manufacture our Calibration Solutions products, which include continuous monitoring systems, dialysate meters and consumables, data loggers, gas flow calibration and air sampling equipment, and torque testing systems represented by the ViewPoint®, Point Six, CheckPoint®, AmegaView, FreshLoc®, DialyGuard®, DataTrace®, DryCal®, Torqo®, SureTorque®, IBP Medical, and BGI brands. During fiscal year 2022, we closed our Butler, New Jersey location, which previously manufactured our gas flow calibration and air sampling equipment products. These manufacturing operations have since moved to Lakewood, Colorado.
The majority of our Calibration Solutions products have relatively long lives and their purchase by customers is discretionary, so sales are more sensitive to general economic conditions. Service demand is driven by our customers’ quality control and regulatory environments, which require periodic repair and recalibration or certification of our instruments products. Most of our Calibration Solutions products are manufactured by assembling the products from purchased components and calibrating the final products prior to release, though certain continuous monitoring products are assembled and calibrated on site at customer locations. Our Calibration Solutions division's commercial efforts focus on offering quality products to our customers that will aid them in containing cost, improving the quality of their products and services, and meeting regulatory requirements. We generate sales through our direct sales personnel and independent distributors.
Continuous Monitoring
Our continuous monitoring products are used to monitor various environmental parameters such as temperature, humidity, and differential pressure to ensure that critical storage and processing conditions are maintained. Continuous monitoring systems are used in controlled environments such as refrigerators, freezers, warehouses, laboratory incubators, clean rooms, and a number of other settings. The continuous monitoring systems consist of wired or wireless sensors that are placed in controlled environments, hardware modules to receive the data, and various software programs to collect, store and process the data. Our systems are designed to operate continuously, providing data around the clock, 365 days per year. We provide recalibration of sensors through our dedicated service organization and SnapCalTM self-managed probe exchange program. Because of the advantages of our continuous monitoring systems, we have a solid market share in North America, but are not currently focused on international expansion.
A critical function of our systems is the ability to provide local alarms and notifications via e-mail, text, or telephone, if established environmental conditions are exceeded. Among the important competitive differentiators of our continuous monitoring systems are (1) their high degree of reliability and up-time; (2) a large variety of sensor types to meet the needs of most applications; (3) a skilled, distributed installation and service team; and (4) a full-featured and 21 CFR Part 11 (Electronic records; Electronic signatures) validated software program, providing extensive reporting and alarm capability. Key markets for our continuous monitoring systems are hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies, and laboratory environments, all located in North America.
Dialysate Meters and Calibration Consumables
Our medical meters are used to test various parameters of dialysis fluid (dialysate) and the proper calibration and operation of dialysis machines used in dialysis clinics. Each meter measures some combination of temperature, pressure, pH, conductivity and flow to ensure that the dialysate has the proper composition to promote the transfer of waste products from the blood to the dialysate. The meters provide a digital readout that the technician uses to verify that a dialysis machine is working within prescribed limits and delivering properly prepared dialysate. We manufacture two styles of medical meters: those designed for use by dialysis machine manufacturers and biomedical technicians, and those used primarily by dialysis clinicians. The meters for technicians are characterized by exceptional accuracy, stability and flexibility, and are used by the industry as the primary standard for the calibration of dialysis machines and water system testing. The meters designed for use by dialysis clinicians are known primarily for their ease of use and incorporate a previously patented, built-in syringe sampling system. These meters are used as the final quality control check on the dialysate just prior to starting a treatment. In addition to the dialysate meters, we market a line of standard consumable solutions for use in dialysis clinics for calibration of our meters. These standard solutions are regularly consumed by the dialysis clinics; thus, along with calibration services that we also provide, are less impacted by general economic conditions than dialysate meters sales. Customers that utilize these products include dialysis facilities, medical device manufacturers, and biomedical service companies. In addition to competition in the dialysis meter business, our products face regulatory and technological challenges. For a discussion of risks related to our regulatory and technological challenges, refer to Item 1A. Risk Factors "Changes in dialysis methods may decrease demand for our dialysis products and negatively impact our financial statements."
Data Loggers
Our data loggers are self-contained, wireless, high precision instruments used in critical manufacturing and quality control processes in the pharmaceutical, medical device, food, and tool industries. They are used to measure temperature, humidity and pressure inside a process or a product during manufacturing. In addition, data loggers can be used to validate the proper operation of laboratory or manufacturing equipment, either during installation or for annual re-certifications. The products consist of individual data loggers, a personal computer (“PC”) interface, software, and various accessories. Customers typically purchase a large number of data loggers along with a single PC interface and the software package. In practice, using the PC interface, the user programs the loggers to collect environmental data at a pre-determined interval, places the data loggers in the product or process, and then collects stored process data from the data logger either through the PC interface or wirelessly via a radio link. The user can then prepare tabular and graphical reports using the software. Unique aspects of our data loggers are their ability to operate at elevated temperatures and in explosive environments – important differentiating factors in the marketplace. Consequently, they are used by companies to control their most critical processes, such as sterilization. We face competition in data logger sales from several other companies, some of which have well-established commercial organizations, particularly in Europe.
Gas Flow Calibration and Air Sampling Equipment
We manufacture a variety of instruments and equipment for gas flow calibration and environmental air sampling. In the air sampling area, our technology is used primarily for the determination of particulate concentrations in air as a measure of urban or industrial air pollution, and for industrial hygiene assessments. The primary products include air samplers, particle separators and pumps. While both the public and private sector continue to focus on air quality and its impact on the environment and the health of populations, technological advances in real-time monitoring have made the traditional air sampling market more limited. In the environmental area, our particle samplers were some of the first on the market and they were recognized early-on as “reference samplers” by the U.S. Environmental Protection Agency. This product has a competitive advantage in the market because our particle separation cyclones hold the only “federal reference method” distinction for the measurement of PM2.5 in ambient air and are sold to most manufacturers of ambient particulate measurement instrumentation.
We also manufacture gas flow calibration instruments to support the use of our air sampling equipment, and for broader industrial applications. Our gas flow calibration instruments provide the precise standards required by laboratories and industry in the design, development, manufacture, installation and calibration of various gas flow meters and air sampling devices. Our flow calibrators are used by professionals in many industries, including (1) industrial hygienists and environmental technicians, (2) calibration and research laboratories, (3) manufacturers who design, develop and manufacture gas flow metering devices, and (4) industrial engineering and manufacturing companies that utilize gas flow metering devices. We see expanded opportunities in gas flow calibration as markets that heavily use and measure process gas are growing. There is competition in gas flow calibration; however, our products are distinguished against the competition by their unique dry piston technology and industry-leading accuracy and certifications.
Due to the relocation of gas flow calibration and air sampling equipment production from Butler, NJ to Lakewood, CO, sales of these products were lower during the year ended March 31, 2022; however, we expect a return to normal sales levels in future years.
Torque Testing Systems
Our automated torque testing systems are durable and reliable motorized cap torque analyzers that measure the amount of force required to open a container. The primary advantages of our torque instruments are their high accuracy and long-term consistency of measurement. Our motorized torque systems eliminate the errors associated with manual torque testing. With a motorized torque testing system, the force applied to a cap is precisely the same in each testing cycle, regardless of the strength of the machine's operator. Our torque systems provide information that helps the packaging operation track events and potential problems during the manufacturing process so corrections can be performed in a timely fashion. Industries utilizing these instruments include pharmaceutical and beverage and food processing companies. Given the niche nature of this product, there is a relatively low level of competition this product line; however, the growth of this line is limited by the growth of new manufacturing facilities and packaging regulations in pharmaceutical manufacturing.
Clinical Genomics
During the year ended March 31, 2022, we added a new reportable segment, Clinical Genomics, as a result of the Agena Acquisition discussed further in Note 4 to the financial statements included in this report, “Significant Transactions.” Our Clinical Genomics division develops, manufactures, and sells highly sensitive, low-cost, high-throughput genetic analysis tools used by labs to perform clinical genomic testing in several therapeutic areas.
Clinical Genomics’ MassARRAY® system couples mass spectrometry with end-point polymerase chain reaction ("PCR") methods, enabling highly multiplexed reactions under universal cycling conditions to provide accurate, sensitive, rapid genetic analysis. Using the MassARRAY® system and our proprietary consumables, including chips, panels, and chemical reagent solutions, customers can analyze DNA samples for a variety of high volume clinical testing applications such as inherited genetic disease testing, newborn screenings, pharmacogenetics, various oncology tests, infectious disease testing, and other highly-differentiated applications. The MassARRAY® system is differentiated in the market by its ability to target up to 50 specific DNA variants in a single PCR reaction and run up to 384 samples on one SpectroCHIP® array, up to eight times in a full workday, with the flexibility to process additional samples overnight. The system allows for the testing of hundreds of mutations, including SNPs, insertions, deletions, translocations, copy number variation, and methylation makers, all in a single, efficient workflow. Using time-of-flight mass spectrometry, genetic variants are distinguished by analysis of their individual mass, eliminating the need for fluorescence or labeling. The system's integrated software provides a user-friendly interface to generate reports that identify the targets and review spectra.
In addition to the MassARRAY® system and related consumable products, Clinical Genomics also sells services, including equipment maintenance contracts and custom laboratory services through which our scientists help customers develop specified assay designs. About 70% of our Clinical Genomics revenues to date are from consumables used on a routine basis. Sales of these products are less sensitive to general economic conditions. Approximately 20% of our Clinical Genomics revenues to date are from more discretionary hardware products that are more sensitive to general economic conditions. The remainder of Clinical Genomics revenues are related to service and support agreements.
Clinical Genomics sells its products and services primarily to clinical labs, including large specialty, reference, and pathology labs, as well as to a variety of academic, hospital, and government facilities. The majority of revenues are derived from customers in the United States and China. Our Clinical Genomics products are manufactured in San Diego, California, primarily by assembling purchased subcomponents designed to our specification into finished goods, and by processing and mixing reagents. Our Clinical Genomics products generate revenues primarily through direct sales, and also through independent distributors in certain regions.
Clinical Genomics products are manufactured under a quality system that complies with ISO 13485 for the design, development, manufacture, distribution, installation, and servicing of diagnostic products, reagents, and instruments for genetic analysis and life sciences.
Corporate and Other
Corporate and other consists of unallocated corporate expenses, the non-reportable operating segment Cold Chain Packaging division that ceased operations during the year ended March 31, 2020, and other business activities.
Other Matters Relating to our Business as a Whole
Acquisitions
Year Ended March 31, 2022 Acquisitions
On October 20, 2021, we completed the acquisition of 100% of the outstanding shares of Agena Bioscience, Inc. for adjusted cash consideration of $300.8 million. Agena is a leading clinical genomics tools company that develops, manufactures, markets, and supports proprietary instruments and related consumables and services that enable genetic analysis for a broad range of diagnostic and research applications. The acquisition of Agena moves our business toward the life sciences tools sector and expands our market opportunities, particularly in Asia.
Year Ended March 31, 2020 Acquisitions
On October 31, 2019, we completed the acquisition of 100% of the outstanding shares of Gyros Protein Technologies Holding AB ("GPT" or the "GPT acquisition") for adjusted cash consideration of $181.5 million. The acquisition expanded our presence into a new market - immunoassays and peptide synthesis solutions -that accelerate the development, and manufacture of biotherapeutic drugs. GPT systems include laboratory instruments, consumables, kits, and software that maximize laboratory productivity by miniaturizing and automating immunoassays at nanoliter scale.
On April 1, 2019, we completed the acquisition of 100% of the outstanding shares of IBP Medical GmbH, a company whose business manufactures medical meters used to test various parameters of dialysis fluid (dialysate), and the proper calibration and operation of dialysis machines.
Manufacturing and Materials
Most of the components, raw materials, and other supplies used in our product lines are available from a number of different suppliers. We generally maintain multiple sources of supply, but we are dependent on single sources for certain items, particularly in the Biopharmaceutical Development and Clinical Genomics divisions. We continue to emphasize reviewing our supply base and designs for single source or sole source suppliers that might affect our ability to supply critical product to our customers. We have experienced increased supply constraints for certain components used in our operations, particularly components used by the Calibration Solutions and Biopharmaceutical Development divisions, and to a lesser extent the Sterilization and Disinfection Control and Clinical Genomics divisions. We continue to work with our suppliers to understand the existing and potential future impacts to our supply chain, and we are making efforts to mitigate such impacts, including pre-ordering components in higher quantities than usual. See further discussion within Item 1A. Risk Factors,“We face numerous manufacturing and supply chain risks. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services could cause production interruptions, delays and inefficiencies.”
Major Customers
No individual customer represented more than 10% of our accounts receivable or revenues in any of the past three years.
Backlog
We define backlog as firm orders from customers for products and services where the order will be fulfilled within the next 12 months. Backlog as of March 31, 2022 and March 31, 2021 was approximately $21.3 million and $11.3 million, respectively. The increase in backlog is attributable to supply chain constraints causing order fulfillment delays, the addition of Clinical Genomics into our overall backlog, and delays resulting from moving production of our gas flow calibration and air sampling equipment from Butler, NJ to Lakewood, CO.
Research and Development
Research and development ("R&D") activities are primarily directed towards innovating new products and improving the quality and performance of our existing products or altering our current products to accommodate use of raw materials that are more readily available for purchase in our supply chain. Other R&D efforts also seek to develop or improve software that will be sold, leased, or marketed in the future, and improve manufacturing efficiencies.
Intellectual Property
We own numerous patents, trademarks, and other proprietary rights, each of which is important to the various facets of our business. Where appropriate, we seek patent protection for inventions and developments made by our personnel that are incorporated into our products or otherwise fall within our fields of interest. There can be no assurance, however, that any patent will provide adequate protection for the technology, system, product, service or process it covers. In addition, the process of obtaining and protecting patents can be long and expensive. We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our proprietary position. Our products and services are sold under various trade names, trademarks and brand names. We consider our trade names, trademarks and brand names to be valuable in the marketing of our products in each segment. We do not believe that the loss of any one patent or other proprietary right would have a material adverse effect on our overall business or on any of our reporting segments.
Regulatory Matters
Mesa's operations are global and are affected by complex state, federal and international laws relating to healthcare, environmental protection, antitrust, anti-corruption, marketing, fraud and abuse, import and export control, product safety and efficacy, employment, privacy, government contracts acquisition regulations, and other areas.
We are required to comply with certain ISO standards and United States Pharmacopeia standards in order to sell some of our products to certain customers. While our quality system and manufacturing processes are generally the same throughout the Calibration Solutions division, specific products are compliant under ISO 13485, ISO 17025, ISO 9001 and certain U.S. federal regulations. Our Uppsala, Sweden and Tucson, Arizona facilities, part of the Biopharmaceutical Development division, are ISO 9001:2015 certified. Clinical Genomics operates a quality management system which complies with the requirements of ISO 13485:2016 and EN ISO 13485:2016. We obtain third party certification to remain compliant with ISO standards.
Several products in the Sterilization and Disinfection Control, Calibration Solutions, and Clinical Genomics divisions are medical devices subject to the provisions of the Federal Food, Drug and Cosmetic Act, which requires any company proposing to market a medical device to notify the FDA of its intention at least 90 days before doing so. We have received permission from the FDA to market all of our products requiring such permission. Some of our facilities are subject to FDA regulations and inspections, which may be time-consuming and costly. This includes ongoing compliance with the FDA’s current Good Manufacturing Practices regulations that require, among other things, the systematic control of manufacture, packaging and storage of products intended for human use. Failure to comply with these practices renders the product adulterated and could subject us to an interruption of manufacturing and selling these products, and possible regulatory action by the FDA.
The manufacture and sale of medical devices is also regulated by some states. Although there is substantial overlap between state regulations and the regulations of the FDA, compliance with some state laws may require additional cost or effort; however, we do not anticipate that complying with state regulations will create any significant problems.
Foreign countries also have laws regulating medical devices sold in those countries, which require additional resources for compliance. The time required to obtain approval by the FDA and other foreign governmental agencies can be lengthy and the requirements may differ.
We are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal or sensitive data in the course of our business, including the EU General Data Protection Regulation which imposes strict requirements on how we collect, transmit, process and retain personal data.
Government Contracts
Although we transact business with various U.S. government agencies, no government contract or aggregate contracts are of such magnitude that a renegotiation of profits or termination of the contracts at the election of the government would have a material adverse effect on our financial results.
Human Capital Management
As a company, our vision is to Protect the Vulnerable® and we believe that our vision is achieved in large part through the strength of our workforce. Every day, our talented employees strive to implement lean based tools to find ways to continuously improve our products and services so that we may better serve our customers. We recruit top talent from all backgrounds using a combination of industry expert recruiters and recruiting tools. We support employees with compensation, benefits and development programs aimed at ensuring employees are productive and engaged.
Employees
As of March 31, 2022, we had 681 employees, of whom 298 are employed for manufacturing and quality assurance, 114 for research and development and engineering, 175 for sales and marketing, and 94 for administration. Our voluntary employee turnover was 12% during the year ended March 31, 2022. We believe that our turnover rate indicates that employees remain at Mesa because of the opportunities to grow and develop within the company.
Diversity and Inclusion
We are committed to diversity and inclusion (“D&I”), and we are always working to improve in this area. We train our managers annually on anti-discrimination and anti-harassment practices. We continue to evolve our talent acquisition process to focus on diversity for both external hires and succession planning. Our recruiting standards require that we consider candidates from two or more underrepresented categories for all director-level or higher positions, and our global cloud-based human capital management platform enables us to more accurately track employee representation and identify how we can better enhance our diversity around the world. Our executive officers have committed to help drive further D&I progress during our year ending March 31, 2023 and beyond. As of March 31, 2022, 50% of our board of directors are from under-represented categories.
Compensation and Benefits
Our compensation and benefits are competitive to market and create incentives to attract and retain employees. In determining merit increases, we evaluate individual performance—including measuring an individual's contribution to company goals and performing semi-annual performance reviews—to align financial incentives with individual contributions. Our compensation package includes market-competitive pay, cash bonuses, stock-based compensation to certain levels of employees, health care and retirement benefits, paid time off, and paid caregiver leave, among other benefits.
Communication and Engagement
We believe that our success depends in part on our employees understanding how their work contributes to our company purpose and strategy. To this end, we utilize a variety of channels to facilitate open and direct communication, including: (i) quarterly town hall meetings with our executive team; (ii) internally maintained websites; (iii) an anonymous whistleblower hotline that is advertised to our employees; and (iv) employee engagement surveys. We also measure employee net promoter scores, which is an employee ranking of how likely they are to recommend working at Mesa to a family member or friend. Our employee net promoter scores decreased during the year ended March 31, 2022. Based on the results of the net promoter score surveys, we are undertaking several initiatives to improve employee engagement, including implementing salary increases and leadership development programs. We will continue tracking and making efforts to improve the score going forward.
Available Information
We are subject to the reporting and other information requirements of the Securities Exchange Act of 1934, as amended (“Exchange Act”). We make available, free of charge, on or through our website at www.mesalabs.com under the link “Financials” on the Investor Relations section, our annual report on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, and other information. Information on our website is not incorporated into this annual report on Form 10-K and is not a part of this report. The Securities and Exchange Commission (“SEC”) also maintains a website at www.sec.gov containing reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Our code of ethics and Board of Directors committee charters and policies are also posted on the Investor Relations section of our website. The information on our website is not part of this or any other report Mesa files with, or furnishes to, the SEC.
In addition to the other information set forth in this Annual Report on Form 10-K and other documents we filed with the SEC, you should carefully consider the following factors, which could materially affect our business, financial condition or results of operations in future periods. The risks and uncertainties described below are those that we have identified as material, but these are not the only risks and uncertainties facing us. Our business is also subject to general risks and uncertainties that affect many other companies, such as market conditions, economic conditions, geopolitical events, changes in laws, regulations or accounting rules, fluctuations in interest rates, terrorism, wars or conflicts, major health concerns, natural disasters or other disruptions of expected business conditions. Additional risks and uncertainties not currently known to us or that we currently believe are immaterial also may impair our business, including our results of operations, liquidity and financial condition.
Business and Strategic Risks
Conditions in the global economy, the markets we serve, and the financial markets may adversely affect our business, financial statements, and access to capital markets.
Our business is sensitive to general economic conditions. Slow or disrupted global economic growth, volatility in the currency and credit markets, high levels of unemployment or underemployment, changes or anticipation of potential changes in government fiscal, tax, trade and monetary policies, changes in capital requirements for financial institutions, government deficit reduction and budget negotiation dynamics, sequestration, austerity measures, sovereign debt defaults, and other challenges that adversely affect the global economy could adversely affect us and our distributors, customers and suppliers, including having the effect of:
| ● |
reducing demand for our products and services, limiting the financing available to our customers and suppliers, increasing order cancellations and resulting in longer sales cycles and slower adoption of new technologies; |
| ● |
increasing the difficulty in collecting accounts receivable and the risk of excess and obsolete inventories; |
| ● |
increasing price competition in our served markets; |
| ● |
supply interruptions, which could disrupt our ability to produce our products; |
| ● |
increasing the risk of impairment of goodwill and other long-lived assets, and the risk that we may not be able to fully recover the value of other assets such as tax assets; |
| ● |
increasing the risk that counterparties to our contractual arrangements will become insolvent or otherwise unable to fulfill their contractual obligations, which could increase the risks identified above; and |
| ● |
adversely impacting market sizes and growth rates. |
If growth in the global economy or in any of the markets we serve slows for a significant period, if there is significant deterioration in the global economy or such markets or if improvements in the global economy do not benefit the markets we serve, our business and financial results could be adversely affected. We cannot predict the likelihood, duration or severity of any disruption in financial markets or any adverse economic conditions in the U.S. and other countries.
Our growth could suffer if the markets into which we sell our products and services decline, do not grow as anticipated or experience volatility.
Our growth depends in part on the growth of the markets which we serve, and visibility into our markets is limited (particularly for markets into which we sell through distribution). Any decline or lower than expected growth in our served markets could diminish demand for our products and services, which would adversely affect our financial statements. Certain of our businesses operate in industries that may experience periodic, cyclical downturns. In addition, in certain of our businesses’ demand depends on customers’ capital spending budgets as well as government funding policies, and matters of public policy and government budget dynamics as well as product and economic cycles can affect the spending decisions of these entities. Demand for our products and services is also sensitive to changes in customer order patterns, which may be affected by announced price changes, marketing or promotional programs, new product introductions, the timing of industry conferences, changes in distributor or customer inventory levels, or other factors. Any of these factors could adversely affect our growth and results of operations in any given period.
We face competition and if we are unable to compete effectively, we may experience decreased demand and decreased market share resulting in decreased revenues. Even if we compete effectively, we may be required to reduce prices for our products and services resulting in decreased profit margin.
The markets for our current and potential products are competitive. Because of the range of products and services we sell and the variety of markets we serve, we encounter a wide variety of competitors (refer to Item 1. Business for additional details), including several that possess both larger sales forces and greater capital resources.
In order to compete effectively, we must maintain longstanding relationships with major customers, continue to grow our business by establishing relationships with new customers, develop new products and services to maintain and expand our brand recognition and leadership position in various product and service categories, and penetrate new markets, including in developing countries and high growth markets. In addition, significant shifts in industry market share can occur in connection with product problems, safety alerts and publications about products, reflecting the competitive significance of product quality, product efficacy and quality systems in our industries. Our failure to compete effectively or pricing pressures resulting from competition may adversely impact our results of operations.
Changing industry trends may affect our results of operations.
Various changes within the industries we serve may limit future demand for our products and may include the following:
| ● |
changes in dialysis reimbursements that our customers may receive; |
|
| ● | mergers within other industries we serve, making us more dependent upon fewer, larger customers for our sales; | |
| ● | decreased product demand, driven by changes in our customers’ regulatory environments or standard industry practices; | |
| ● | price competition for key products. | |
| ● |
mergers within key industries we serve, concentrating our medical meter and solutions sales with a few, large customers; and |
| ● |
new competitor products that may result in customers discontinuing new orders or consumables orders. |
Our growth depends in part on the timely development, commercialization, and customer acceptance of new and enhanced products and services based on technological innovation.
Our growth depends on the acceptance of our products and services in the marketplace, the penetration achieved by the companies to which we sell, and our ability to introduce new and innovative products that meet the needs of the various markets we serve. We can offer no assurance that we will be able to continue to introduce new and enhanced products, that the products we introduce, or have introduced, will be widely accepted by the marketplace, or that our direct sales team or independent distributors will successfully penetrate our various markets. Our failure to introduce new and enhanced products or gain widespread acceptance of our products and services could adversely affect our financial results. If we fail to accurately predict future customer needs and preferences, fail to produce viable technologies, or to protect the intellectual property of such technologies, we may invest heavily in research and development of products and services that do not lead to significant revenues, which could adversely affect our profitability. Even if we successfully innovate and develop new and enhanced products and services, we may incur substantial costs in doing so, and our profitability may suffer. Competitors may also develop after-market services and parts for our products which attract customers and adversely affect our return on investment for new products. In addition, we face risks in connection with the retirement of old products and customer migration to new products.
If we are unable to continue to hire and retain personnel, then we will have trouble manufacturing and marketing our products.
Our success depends largely upon the continued service of our management and manufacturing employees and our ability to attract and retain manufacturing and management personnel, some of whom we are recruiting for in-person positions in competitive labor markets, particularly Bozeman, Montana. At times, we face significant competition in the hiring and retention of personnel in competitive markets where other employers may offer superior pay or benefits. Loss of key personnel or our inability to hire and retain personnel could materially adversely affect our manufacturing efforts and increase backlog. Further, if we have to pay manufacturing employees higher wages to attract and retain them, our gross margins and overall profitability may decline.
Adverse changes in our relationships with, or the financial condition, performance, purchasing patterns or inventory levels of, distributors and other channel partners could adversely affect our financial statements.
We sell a significant number of products to distributors and other channel partners that have valuable relationships with customers and end-users. Some of these distributors and other partners also sell our competitors’ products or compete with us directly, and if they favor competing products for any reason, they may fail to market our products effectively. Adverse changes in our relationships with these distributors and other partners, or adverse developments in their financial condition, performance or purchasing patterns, could adversely affect our business and financial statements.
The levels of inventory maintained by our distributors and other channel partners, and changes in those levels, can also negatively impact our results of operations in any given period. In addition, the consolidation of distributors could adversely impact our business and financial statements. We cannot directly control the actions of our distributors. Our distributors may not comply with export laws, or follow the terms of the distribution agreements which require compliance with export laws, which could have legal or financial implications for us.
Our international operations subject us to a wide range of risks.
Our operations and sales outside of the United States have increased as a result of our strategic acquisitions and the continued expansion of our commercial organization. Risks related to these increased foreign operations include:
| ● | fluctuations in foreign currency exchange rates, which may affect the costs incurred in international operations and could harm our results of operations and financial condition; | |
| ● |
interruption in the transportation of materials to us and finished goods to our customers; |
| ● |
differences in terms of sale, including longer payment terms than are typical in the United States; |
| ● |
local product preferences and product requirements; |
| ● |
trade protection measures, embargoes and import or export restrictions and requirements; |
| ● |
unexpected changes in laws or regulatory requirements, including changes in tax laws; |
| ● |
capital controls and limitations on ownership and on repatriation of earnings and cash; |
| ● |
changes in general economic and political conditions in countries where we operate, particularly as a result of ongoing economic instability within foreign jurisdictions; |
| ● |
difficulty in staffing and managing widespread operations; |
| ● |
differing labor or employment regulations; |
| ● |
difficulties in implementing restructuring actions on a timely or comprehensive basis; |
| ● |
differing protection of intellectual property; and |
| ● |
greater uncertainty, risk, expense and delay in commercializing products in certain foreign jurisdictions, including with respect to product and other regulatory approvals. |
International business risks have in the past and may in the future negatively affect our business and financial statements. A deterioration in diplomatic relations between the United States and any country where we conduct business could adversely affect our future operations and lead to a decline in profitability. Changes in U.S. policy regarding international trade, including import and export regulation and international trade agreements, could also negatively impact our business. Tariffs imposed by the U.S. on a broad range of imports or trade measures imposed by other countries could result in an increase in supply chain costs that we may not be able to offset or that could otherwise adversely impact our results of operations.
Our international operations are governed by the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws outside the U.S. Global enforcement of anti-corruption laws has increased in recent years, with more enforcement proceedings by U.S. and foreign governmental agencies and the imposition of significant fines and penalties. Our international operations, which often involve customer relationships with foreign governments, create the risk that there may be unauthorized payments or offers of payments made by employees, consultants, or distributors. Any alleged or actual violations of these laws may subject us to government investigations and significant criminal or civil sanctions and other liabilities, and negatively affect our reputation.
Uncertainties remain regarding the consequences of the UK ceasing to be a member state of the EU on January 31, 2020 (commonly referred to as “Brexit”), including the application of the terms of the trade and cooperation agreement with the EU, the impact of new or different laws and regulations as the UK determines which EU laws to replace or replicate, and trade and tax impacts as the UK negotiates its own tax and trade treaties with countries around the world. The impacts from Brexit could add time and expense to the conduct of our business, delay regulatory approval of products, adversely impact the manufacturing or movement of products, adversely impact customer demand, and otherwise adversely affect our business and financial statements both inside and outside the UK.
The COVID-19 pandemic has adversely impacted and continues to pose risks to our business.
Since December 2019, an outbreak of a novel strain of a virus named SARS-CoV-2, which causes COVID-19, spread to countries in which we or our customers and suppliers operate, including the United States and caused major disruption throughout the year. The COVID-19 pandemic continues to evolve, and to date, has led to the implementation of various responses, including government-imposed quarantines, extended business closures, travel restrictions and other public health safety measures, as well as reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries.
In response to COVID-19 and in accordance with direction from state and local government authorities, we restricted and may continue to restrict access to our facilities to our office-based employees and monitored and responded to certain external factors in instituting limited travel restrictions during fiscal year 2022. In addition, many of our customers and potential customers closed facilities or limited facility hours due to the spread of COVID-19. Such closures have resulted in, and may continue to result in, our inability to demonstrate and install some of our products, as well as lower demand for certain products. Any interruptions in the installation of ordered products could delay our ability to recognize revenues in a particular period. In addition, we must maintain sufficient production capacity in order to meet anticipated customer demand, which carries fixed costs that we may not be able to offset if installations cannot occur, which would adversely affect our operating margins.
We operate on a global basis with offices or operations in North America, Europe, and Asia, and global health crises, such as COVID-19, could result in a widespread economic downturn in the industries in which we and our customers operate. The extent to which outbreaks impacts our business and the businesses of our customers will depend on future developments, which remain highly uncertain and cannot be predicted with confidence, such as the continued geographic spread of the disease, the duration of outbreaks, and actions taken in the United States and elsewhere to contain outbreaks and treat the disease, such as vaccination rates and efficacy, social distancing and quarantines, business closures and business disruptions. Recent COVID-19 outbreaks and resulting lockdowns in China have adversely impacted revenues from our Clinical Genomics division during fiscal year 2022, and we expect continued adverse impacts on revenues from our Clinical Genomics division and, to a lesser extent, the rest of the company in fiscal year 2023. Some factors from the COVID-19 pandemic that could delay or otherwise adversely affect our operations and performance include:
| ● |
disruptions in our supply chains; |
| ● |
limitations on travel that could interrupt our ability to provide installation or maintenance services at customer sites and could impact our ability to effectively market our products; |
| ● |
interruption in global shipping affecting the transport of our products and other supplies; |
|
| ● | restrictions on business operations by local, state, or federal governments; |
| ● |
business disruptions or cybersecurity risks associates with a substantial portion of our workforce working from home for extended periods of time; |
| ● |
the impact of the valuation of our financial assets due to market volatility; |
| ● |
interruption or delays in the operations of the FDA and comparable foreign regulatory agencies, which may impact review, inspection, clearance, and approval timelines; |
|
| ● | delays in partner clinical trials due to government-imposed restrictions or lockdowns in China. |
The COVID-19 pandemic could also have the effect of heightening other risk factors described in this report.
Operational Risks
A significant disruption in, or breach in security of, our information technology systems or data could adversely affect our business, reputation and financial statements.
We rely on information technology systems, some of which are provided or managed by third-parties, to process, transmit and store electronic information (including sensitive data such as confidential business information and personally identifiable data relating to employees, customers, and other business partners), and to manage or support a variety of critical business processes and activities (such as receiving and fulfilling orders, billing, collecting and making payments, shipping products, providing services and support to customers and fulfilling contractual obligations). In addition, some products or software we sell to customers may connect to our systems for maintenance or other purposes, and we sell software as a service and cloud-based platforms. These systems, products and services (including those we acquire through business acquisitions) may be damaged, disrupted or shut down due to attacks by computer hackers, computer viruses, ransomware, human error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and in any such circumstances our system redundancy and other disaster recovery planning may be ineffective or inadequate. Attacks may also target hardware, software and information installed, stored or transmitted in our products after such products have been purchased and incorporated into third-party products, facilities or infrastructure. Security breaches of systems provided or enabled by us, regardless of whether the breach is attributable to a vulnerability in our products or services, could result in the misappropriation, destruction or unauthorized disclosure of confidential information or personal data belonging to us or to our employees, partners, customers, patients or suppliers. Our information technology systems have been subject to computer viruses, malicious codes, unauthorized access and other cyber-attacks and we expect the sophistication and frequency of such attacks to continue to increase. Unauthorized tampering, adulteration or interference with our products may also adversely affect product functionality and result in loss of data, risk to patient safety and product recalls or field actions.
Any attacks, breaches or other disruptions or damage could interrupt our operations or the operations of our customers and partners, delay production and shipments, result in theft of our and our customers’ intellectual property and trade secrets, damage customer, business partner, and employee relationships, and our reputation or result in defective products or services, legal claims and proceedings, liability and penalties under privacy laws and increased costs for security and remediation, each of which could adversely affect our business, reputation and financial statements.
Further, a significant number of our employees work remotely, which exposes us to greater cybersecurity risks. Any inability to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches can result in adverse regulatory consequences, business consequences and litigation.
We face numerous manufacturing and supply chain risks. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services could cause production interruptions, delays and inefficiencies.
We purchase materials, components and equipment from third parties for use in our manufacturing operations. Our results of operations could be adversely impacted if we are unable to adjust our purchases to reflect changes in customer demand and market fluctuations, including those caused by seasonality or cyclicality. Suppliers may extend lead times, limit supplies or increase prices. If we cannot purchase sufficient products at competitive prices and quality and on a timely enough basis to meet increasing demand, we may not be able to satisfy market demand, product shipments may be delayed, our costs may increase, or we may breach our contractual commitments and incur liabilities.
In addition, some of our businesses purchase certain required products from sole or limited source suppliers for reasons of quality assurance, regulatory requirements, cost effectiveness, availability or uniqueness of design. If these or other suppliers encounter financial, operating or other difficulties or if our relationship with them changes, we might not be able to quickly establish or qualify replacement sources of supply. The supply chains for our businesses were impacted in fiscal year 2022 and could also be disrupted in the future by supplier capacity constraints, supplier bankruptcy or exiting of the business for other reasons, decreased availability of key raw materials or commodities and external events such as natural disasters, pandemics or other public health problems, war, terrorist actions, governmental actions and legislative or regulatory changes. Any of these factors could result in production interruptions, delays, extended lead times and inefficiencies.
Because we cannot always immediately adapt our production capacity and related cost structures to changing market conditions, our manufacturing capacity may at times exceed or fall short of our production requirements. Any or all of these problems could result in the loss of customers, provide an opportunity for competing products to gain market acceptance, and otherwise adversely affect our financial condition.
Our financial results are subject to fluctuations in the cost and availability of components and commodities that we use in our operations.
As discussed in Item 1. Business—Manufacturing and Materials, our manufacturing and other operations employ a wide variety of components, and raw materials and other commodities, including metallic-based components, electronic components, chemicals, and plastics and other petroleum-based products. Prices for and availability of these components, and raw materials and other commodities have fluctuated significantly in the past, and more recently have increased. Any sustained interruption in the supply of these items could adversely affect our business. In addition, due to the highly competitive nature of the industries that we serve, the cost-containment efforts of our customers and the terms of certain contracts we are party to, if components and commodity prices rise, we may be unable to pass along cost increases through higher prices. If we are unable to fully recover higher costs through price increases or offset these increases through cost reductions, or if there is a time delay between the increase in costs and our ability to recover or offset these costs, our margins and profitability could decline, and our financial statements could be adversely affected.
We are dependent on our suppliers to deliver components, and a shortage of and increasing prices of components have and could continue to disrupt our production and delay order fulfillment.
Products we sell are made of components, including electronic components, glass, plastics, and packaging that we source globally from suppliers who, in turn, source components from their suppliers. If there is a shortage of a key component in our supply chain, and the component cannot be easily sourced from a different supplier, the shortage may disrupt our production which may, in turn, delay order fulfillment. We are currently experiencing long lead times, and in some cases, difficulty obtaining components from our existing suppliers, which has resulted in delayed order fulfillment to varying degrees in our divisions, especially the Calibration Solutions and Biopharmaceutical Development divisions, and to a lesser extent the Sterilization and Disinfection Control and Clinical Genomics divisions.
In addition, costs for components and transportation costs have increased, which may reduce our gross profit margins unless and until we are able to pass the cost increases along to our customers. There are several reasons for the supply chain disruptions to components that we rely on to manufacture our products, including: increased demand for other products that use the same components as those we purchase, manufacturing shut-downs during the past 18 months that reduced production of components, obsolescence of materials we have historically purchased, labor issues, and long lead times for raw materials used in the production of components. A continued shortage of components or other key materials that comprise the components could cause a significant disruption to our production schedule and have a substantial adverse effect on our financial condition or results of operations.
Significant developments or uncertainties stemming from the U.S. administration, including changes in U.S. trade policies, tariffs and the reaction of other countries thereto could have an adverse effect on our business.
Changes, potential changes or uncertainties in U.S. social, political, regulatory and economic conditions or laws and policies governing foreign trade, manufacturing, and development and investment in the territories and countries where we or our customers operate, or governing the health care system, can adversely affect our business and financial statements. For example, trade tensions between the United States and China remain high, and each country has continued to impose significant tariffs on a wide range of goods imported from the other country. China accounted for approximately 9% of our sales during the year ended March 31, 2022. These factors have adversely affected, and in the future could further adversely affect, our business and financial statements.
Macroeconomic pressures in the markets in which we operate may adversely affect our financial results.
Geopolitical issues around the world can impact macroeconomic conditions and could have a material adverse impact on our financial results. For example, the ultimate impact of the conflict in Ukraine on fuel prices, inflation, the global supply chain and other macroeconomic conditions is unknown and could materially adversely affect global economic growth, disrupting discretionary spending habits and generally decreasing demand for our products and services. While we do not purchase any of significant raw materials directly from Russia, it is a significant global producer of fuel, nickel, and copper. Disruptions in the markets for those inputs could negatively impact the macroeconomy. We cannot predict the extent or duration of sanctions in response to the conflict in Ukraine, nor can we predict the effects of legislative or other governmental actions or regulatory scrutiny of Russia and Belarus, Russia's other allies or other countries with which Russia has significant trade or financial ties, including China. The conflict in Ukraine may also exacerbate geopolitical tensions globally. While our sales to Russia have historically produced an immaterial amount of revenues and profitability compared to the overall company, we cannot predict the impact that the conflict may have on future financial results. For example, Russian customers for some of our product lines may choose a competitors' product and we may not be able to regain the business, including future sales to those Russian customers.
Violation of data privacy laws could adversely affect our business, reputation and financial statements.
If we are unable to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches, we may suffer adverse regulatory consequences, business consequences and litigation. As a multinational organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. The EU General Data Protection Regulation impose strict requirements in how we collect and process personal data, including, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. Data privacy laws in other jurisdictions, such as California and Colorado, also impose data privacy obligations. Government enforcement actions can be costly and interrupt the regular operation of our business, and data breaches or violations of data privacy laws can result in fines, reputational damage and civil lawsuits, any of which may adversely affect our business, reputation and financial statements. In addition, compliance with the varying data privacy regulations around the world may require significant expenditures and may require changes in our products or business models that increase competition or reduce revenues.
Changes to dialysis methods may decrease demand for our dialysis products and negatively impact our financial statements.
In July 2019, an executive order was signed by the President of the United States that is intended to change the way that kidney care is delivered to patients and reimbursed through government-sponsored medical programs. The executive order’s objectives included encouraging dialysis patients to receive treatments through in-home care rather than at a dialysis clinic and also reducing the number of people developing kidney failure. The extent of the impact of the executive order, as well as the timing of the impact on procedures and the market in general is currently unknown.
Currently, our Dialyguard product line accounts for approximately one-third of the revenues and gross margin associated with our Calibration Solutions division. The majority of the revenues in our Dialyguard business are associated with products that are used in dialysis clinics, while a smaller portion of our sales relate to in home care. Another recent development is dialysis machines that feature built-in dialysis calibration functionalities. Demand for our dialysis products may be adversely affected by these or other developments in the dialysis industry.
We may be unable to efficiently manage our growth as a larger and more geographically diverse organization.
Our strategic acquisitions and the continued organic expansion of our commercial sales operations have increased the scope and complexity of our business. As a result, we face challenges inherent in efficiently managing a more complex business with an increased number of employees over large geographic distances, including the need to implement appropriate systems, policies, benefits, and compliance programs. Our inability to manage successfully a substantially larger and geographically more diverse (including from a cultural perspective) organization could materially adversely affect our operating results and, as a result, the market price of our common stock.
If we suffer loss to our facilities, supply chains, distribution systems or information technology systems due to a catastrophic event, our operations could be seriously harmed.
Our facilities, supply chains, distribution systems and information technology systems are subject to catastrophic loss due to fire, flood, earthquake, hurricane, pandemics and epidemics and other public health crises, war, terrorism or other natural or man-made disasters. If any of these facilities, supply chains or systems were to experience a catastrophic loss, it could disrupt our operations, delay production and shipments, result in defective products or services, damage customer relationships and our reputation and result in legal exposure and large repair or replacement expenses. Our insurance coverage with respect to natural disasters is limited and is subject to deductible and coverage limits and may be unavailable or insufficient to protect us against such losses.
The health care industry and related industries that we serve have undergone, and are in the process of undergoing, significant changes in an effort to reduce costs, which could adversely affect our financial statements.
Participants in the health care industry and related industries have implemented, and are implementing, significant changes in an effort to reduce costs. Many of the end-users to whom our customers supply products rely on government funding of and reimbursement for health care products and services and research activities. The U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “PPACA”), health care austerity measures in other countries and other potential health care reform changes and government austerity measures have reduced and may further reduce the amount of government funding or reimbursement available to customers or end-users of our products and services and/or the volume of medical procedures using our products and services. Global economic uncertainty or deterioration can also adversely impact government funding and reimbursement.
These changes as well as other impacts from market demand, government regulations, third-party coverage and reimbursement policies and societal pressures have started changing the way healthcare is delivered, reimbursed and funded and may cause participants in the health care industry and related industries that we serve to purchase fewer of our products and services, reduce the prices they are willing to pay for our products or services, reduce the amounts of reimbursement and funding available for our products and services from governmental agencies or third-party payors, affect the acceptance rate of new technologies and products and increase our compliance and other costs. All of the factors described above could adversely affect our business and financial results.
Defects or quality issues associated with our products could adversely affect the results of our operations.
Manufacturing or design defects or “bugs” in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, standard use of, “off label” use of, or inadequate disclosure of risks relating to the use of products and services that we make or sell (including items that we source from third parties or that we provide to third parties who sell on our behalf) can lead to property damage, loss of profits or other liability. These events could lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us. Recalls, removals and product liability and similar claims (regardless of their validity or ultimate outcome) can result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services. Any of the above can result in the discontinuation of marketing of such products in one or more countries and give rise to claims for damages from persons who believe they have been injured as a result of product issues, including claims by individuals or groups seeking to represent a class.
The manufacture of many of our products is a highly exacting and complex process, and if we directly or indirectly encounter problems manufacturing products, our reputation, business and financial results could suffer.
The manufacture of many of our products is a highly exacting and complex process, due in part to strict regulatory requirements. Problems may arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials, natural disasters and environmental factors, and if not discovered before the product is released to market could result in recalls and product liability exposure. Because of the time required to approve and license certain regulated manufacturing facilities and other stringent regulations of the FDA and similar agencies regarding the manufacture of certain of our products, an alternative manufacturer may not be available on a timely basis to replace such production capacity. Any of these manufacturing problems could result in significant costs, liability and lost revenues, loss of market share as well as negative publicity and damage to our reputation that could reduce demand for our products.
Our failure to maintain appropriate environmental, social, and governance ("ESG") practices and disclosures could result in reputational harm, a loss of customer and investor confidence, and adverse business and financial results.
Governments, investors, customers, and employees are enhancing their focus on ESG practices and disclosures, and expectations in this area are rapidly evolving and increasing. While we monitor the various and evolving standards and associated reporting requirements, failure to adequately maintain appropriate ESG practices that meet diverse stakeholder expectations may result in the loss of business, reduced market valuation, an inability to attract customers, and an inability to attract and retain top talent.
Climate change, or legal or regulatory measures to address climate change, may negatively affect us.
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere could present risks to our operations. Physical risk resulting from acute changes (such as hurricane, tornado, wildfire or flooding) or chronic changes (such as droughts, heat waves or sea level changes) in climate patterns can adversely impact our facilities and operations and disrupt our supply chains and distribution systems. Concern over climate change can also result in new or additional legal or regulatory requirements designed to reduce greenhouse gas emissions and/or mitigate the effects of climate change on the environment (such as taxation of, or caps on the use of, carbon-based energy). Any such new or additional legal or regulatory requirements may increase the costs associated with, or disrupt, sourcing, manufacturing and distribution of our products, which may adversely affect our business and financial statements.
If we are unable to manufacture our products in sufficient quantities and in a timely manner, our operating results will be harmed, our ability to generate revenue could be diminished, and our gross margin may be negatively impacted.
Our revenues and other operating results will depend in large part on our ability to manufacture and assemble our products in sufficient quantities and in a timely manner. Any interruptions we experience in the manufacturing or shipping of our products - such as the delay that occurred when we moved our Butler, New Jersey facilities to Lakewood, Colorado resulting in changes to the way we manufacture certain products - could delay our ability to recognize revenues in a particular quarter. Manufacturing problems can and do arise, and as demand for our products increases, any such problems could have an increasingly significant impact on our operating results. We may not be able to quickly ship products and recognize anticipated revenues if we experience significant delays in the manufacturing process. In addition, we must maintain sufficient production capacity in order to meet anticipated customer demand, which carries fixed costs that we may not be able to offset if orders slow, which would adversely affect our operating margins. If we are unable to manufacture our products consistently, in sufficient quantities, and on a timely basis, our revenues, gross margins and our other operating results will be materially and adversely affected.
Acquisition Risks
Any inability to consummate acquisitions at our historical rate and at appropriate prices could negatively impact our growth rate and stock price.
Our ability to grow revenues, earnings and cash flows at or above our historic rates depends in part upon our ability to identify and successfully acquire and integrate businesses at appropriate prices and realize anticipated synergies. We may not be able to consummate acquisitions at rates similar to the past, which could adversely impact our growth rate and our stock price. Promising acquisitions are difficult to identify and complete for a number of reasons, including high valuations, competition among prospective buyers, the availability of affordable funding in the capital markets, and the need to satisfy applicable closing conditions. In addition, competition for acquisitions may result in higher purchase prices. Changes in accounting or regulatory requirements, or instability in the credit markets, or global crisis that prevents travelling or other activities necessary for acquisitions could also adversely impact our ability to consummate acquisitions.
Our acquisition of businesses could negatively impact our financial statements.
As an important part of our business strategy, we acquire businesses, some of which may be material. Please see Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations for additional details. These acquisitions involve a number of financial, accounting, managerial, operational, legal, compliance and other risks and challenges, including the following, any of which could adversely affect our business and our financial statements:
| ● |
any business, technology, service or product that we acquire could under-perform relative to our expectations and the price that we paid for it, or not perform in accordance with our anticipated timetable, or we could fail to make such business profitable; |
| ● |
we may incur or assume significant debt in connection with our acquisitions which could cause a deterioration of our credit rating, result in increased borrowing costs and interest expense and diminish our future access to the capital markets; |
| ● |
acquisitions could cause our results of operations to differ from our own or the investment community’s expectations in any given period, or over the long-term; |
| ● |
pre-closing and post-closing acquisition-related earnings charges could adversely impact our results of operations in any given period, and the impact may be substantially different from period to period; |
| ● |
acquisitions could create demands on our management, operational resources and financial and internal control systems that we are unable to effectively address, or for which we may incur additional costs; |
| ● |
we could experience difficulty in integrating personnel, operations, financial and other systems, and in retaining key employees and customers; |
| ● |
we may be unable to achieve cost savings or other synergies anticipated in connection with an acquisition; |
| ● |
we may assume by acquisition unknown liabilities, known contingent liabilities that become realized, known liabilities that prove greater than anticipated, internal control deficiencies or exposure to regulatory sanctions resulting from the acquired company’s activities. The realization of any of these liabilities or deficiencies may increase our expenses, adversely affect our financial position or cause us to fail to meet our public financial reporting obligations; |
| ● |
in connection with acquisitions, we often enter into post-closing financial arrangements such as purchase price adjustments, earn-out obligations and indemnification obligations, which may have unpredictable financial results; and |
| ● |
as a result of our acquisitions, we have recorded significant goodwill and intangible assets on our balance sheets. If we are not able to realize the value of these assets, we may be required to incur charges relating to the impairment of these assets, which could materially impact our financial results. |
If intangible assets and goodwill that we recorded in connection with our acquisitions become impaired, we may have to take significant charges against earnings.
In connection with the accounting for our completed acquisitions, we recorded a significant amount of intangible assets, including developed technology and customer relationships relating to the acquired product lines, and goodwill. Under accounting principles generally accepted in the United States (“GAAP”), we must assess, at least annually and potentially more frequently, whether the value of intangible assets and goodwill has been impaired. Intangible assets and goodwill will be assessed for impairment in the event of an impairment indicator. Any reduction or impairment of the value of intangible assets and goodwill will result in a charge against earnings, which could materially adversely affect our results of operations and shareholders’ equity in future periods.
The indemnification provisions of acquisition agreements by which we have acquired companies may not fully protect us and as a result we may face unexpected liabilities, or we may have acquisition agreements with no indemnification protection at all.
Certain of the acquisition agreements by which we have acquired companies require the former owners to indemnify us against certain liabilities related to the operation of the company before we acquired it. In most of these agreements, however, the liability of the former owners is limited, and certain former owners may be unable to meet their indemnification responsibilities. We cannot guarantee that these indemnification provisions will protect us fully or at all, and as a result we may face unexpected liabilities that could adversely impact our financial statements. In addition, we may enter into acquisition agreements that have no indemnification protection at all.
Future strategic transactions or acquisitions may require us to seek additional financing, which we may not be able to secure on favorable terms, if at all.
We actively evaluate various strategic transactions on an ongoing basis, and in order to complete such transactions, we may need to seek additional financing. We may not be able to secure such financing on favorable terms, or at all. In addition, future acquisitions may require the issuance or sale of additional equity or debt securities, which may result in additional dilution to our stockholders.
Legal, Regulatory, Compliance, and Reputational Risks
We are subject to lawsuits and regulatory proceedings.
We have been a defendant in a number of lawsuits, and in the future are subject to the possibility of a variety of litigation and regulatory proceedings, including claims for damages arising out of the use of products or services and claims relating to intellectual property matters, employment matters, tax matters, commercial disputes, product liability, marketing matters, insurance coverage, competition and sales and trading practices, environmental matters, product retirement, personal injury, and acquisition or divestiture-related matters, as well as regulatory investigations or enforcement. We may also become subject to lawsuits as a result of past or future acquisitions or as a result of liabilities retained from, or representations, warranties or indemnities provided in connection with, divested businesses. Any of these lawsuits may include claims for compensatory damages, punitive and consequential damages or injunctive relief. The defense of these lawsuits may divert our management’s attention, we may incur significant expenses in defending these lawsuits, and we may be required to pay damages or settlements or become subject to equitable remedies that could adversely affect our operations and financial results. Moreover, any insurance or indemnification rights that we may have may be insufficient or unavailable to protect us against such losses. In addition, developments in proceedings in any given period may require us to adjust the loss contingency estimates that we have recorded in our financial statements, record estimates for liabilities or assets previously not susceptible of reasonable estimates or pay cash settlements or judgments. Any of these developments could adversely affect our financial results in any given period. We cannot make assurances that our liabilities in connection with litigation and other legal regulatory proceedings will not exceed our estimates or adversely affect our financial results and business. Please see Note 13. “Commitments and Contingencies” of Notes to Consolidated Financial Statements contained in Item 8. Financial Statements and Supplementary Data for additional discussion.
Our reputation, ability to do business and prepare financial statements may be impaired by improper conduct by any of our employees, agents or business partners.
We cannot provide assurance that our internal controls and compliance systems will always protect us from acts committed by employees, agents or business partners of ours (or of businesses we acquire or partner with) that would violate U.S. and/or non-U.S. laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, export and import compliance, money laundering and data privacy.
If we do not or cannot adequately protect our intellectual property, if third parties infringe our intellectual property rights, or if we or our customers are alleged to infringe upon others’ intellectual property rights, we may suffer competitive injury or expend significant resources enforcing or defending our rights.
We own patents, trademarks, copyrights, trade secrets and other intellectual property and licenses to intellectual property owned by others, which in the aggregate are important to our business. The intellectual property rights that we obtain, however, may not be sufficiently broad or otherwise may not provide us a significant competitive advantage, and patents may not be issued for pending or future patent applications owned by or licensed to us. In addition, the steps that we and our licensors have taken to maintain and protect our intellectual property may not prevent it from being challenged, invalidated, circumvented or designed-around, particularly in countries where intellectual property rights are not highly developed or protected. In some circumstances, enforcement may not be available to us because an infringer has a dominant intellectual property position or for other business reasons, or countries may require compulsory licensing of our intellectual property. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements will adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights. In addition, we or our customers may be alleged to infringe upon the intellectual property of third parties. Our failure to obtain or maintain intellectual property rights that convey competitive advantage, adequately protect our intellectual property, detect or prevent circumvention or unauthorized use of such property, and the cost of enforcing our intellectual property rights or defending against any allegation of infringement, could adversely impact our competitive position and results of operations.
We are subject to extensive regulation.
The process of obtaining and maintaining required regulatory approvals is lengthy, expensive and uncertain. We can offer no assurance that delays will not occur in the future, which could have a significant adverse effect on our ability to introduce new products on a timely basis. Regulatory agencies periodically inspect our manufacturing facilities to ascertain compliance with “good manufacturing practices” and can subject approved products to additional testing and surveillance programs.
Failure to comply with applicable regulatory requirements can, among other things, result in fines, suspension of regulatory approvals, product recalls, operating restrictions and criminal penalties. If we fail to comply with regulatory requirements, it could have an adverse effect on our results of operations and financial condition. The regulations we are subject to have tended to become more stringent over time and may be inconsistent across jurisdictions. We, our representatives and the industries in which we operate may at times be under review and/or investigation by regulatory authorities. Compliance with applicable regulations may affect our returns on investment, require us to incur significant expenses or modify our business model or impair our flexibility in modifying product, marketing, pricing or other strategies for growing our business. Our products and operations are also often subject to the rules of industrial standards bodies such as the International Standards Organization, and failure to comply with these rules could result in withdrawal of certifications needed to sell our products and services and otherwise adversely impact our business and financial statements.
Certain of our products are medical devices and other products subject to regulation by the U.S. FDA, by other federal and state governmental agencies, by comparable agencies of other countries and regions. We cannot guarantee that we will be able to obtain regulatory clearance (such as 510(k) clearance) or approvals for our new products or modifications to (or additional indications or uses of) existing products within our anticipated timeframe or at all, and if we do obtain such clearance or approval, it may be time-consuming, costly and subject to restrictions. Our ability to obtain such regulatory clearances or approvals will depend on many factors and the process for obtaining such clearances or approvals could change over time and may require the withdrawal of products from the market until such clearances are obtained. The global regulatory environment has become increasingly stringent and unpredictable. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, and other countries have expanded, or plan to expand, their existing regulations. For example, the EU Medical Device Regulation (the “EU MDR”) imposes strict requirements for the marketing and sale of medical devices, including in the area of clinical evaluation requirements, quality systems and post-market surveillance. Failure to meet the requirements could adversely impact our business in the EU and other regions that tie their product registrations to the EU requirements.
Ensuring that our internal operations and business arrangements with third parties comply with applicable laws and regulations involves substantial costs. It is also possible that government authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law. Noncompliance with applicable laws and regulations can result in, among other things, fines, expenses, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, failure to receive 510(k) clearance of devices, withdrawal of marketing approvals, reputation damage, business disruption, loss of customers and disbarment from selling to certain federal agencies, criminal prosecutions and other adverse effects. Further, defending against any such actions can be costly and time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions brought against us, our business may be impaired.
Off-label marketing of our products could result in substantial penalties.
The FDA strictly regulates the promotional claims that may be made about approved or cleared products. In particular, any clearances we may receive only permit us to market our products for the uses indicated on the labeling cleared by the FDA. We may request additional label indications for our current products, and the FDA may deny those requests outright, require additional data to support any additional indications or impose limitations on the intended use of any cleared products as a condition of clearance. If the FDA determines that we have marketed our products for off-label use, we can be subject to fines, injunctions or other penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, substantial monetary penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, and/or the curtailment of our operations. Any of these events could significantly harm our business and financial results.
Certain modifications to our products may require new 510(k) clearances or other marketing authorizations and may require us to recall or cease marketing our products.
Once a medical device is permitted to be legally marketed in the United States pursuant to a 510(k) clearance, a manufacturer may be required to notify the FDA of certain modifications to the device. Manufacturers determine in the first instance whether a change to a product requires a new 510(k) clearance or premarket submission, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances are necessary. We have made modifications to our products in the past and have determined based on our review of the applicable FDA regulations and guidance that in certain instances new 510(k) clearances or other premarket submissions were not required. We may make similar modifications or add additional features in the future that we believe do not require a new 510(k) clearance. If the FDA disagrees with our determinations and requires us to submit new 510(k) notifications, we may be required to cease marketing or to recall the modified product until we obtain clearance, and we may be subject to significant regulatory fines or penalties.
Changes in governmental regulations may reduce demand for our products or services or increase our expenses.
We compete in markets in which we and our customers must comply with federal, state, and other jurisdictional regulations, such as regulations governing health and safety, food and drugs, privacy and electronic communications. We develop, configure and market our products and services to meet customer needs created by these regulations. These regulations are complex, change frequently, have tended to become more stringent over time and may be inconsistent across jurisdictions. Any significant change in any of these regulations (or in the interpretation or application thereof) could reduce demand for, increase our costs of producing or delay the introduction of new or modified products and services, or could restrict our existing activities, products and services. In addition, in certain of our international markets our growth depends in part upon the introduction of new regulations. In these markets, the delay or failure of governmental and other entities to adopt or enforce new regulations, the adoption of new regulations which our products and services are not positioned to address or the repeal of existing regulations, could adversely affect demand. In addition, regulatory deadlines may result in substantially different levels of demand for our products and services from period-to-period.
Product liability suits against us, product defects or unanticipated use or inadequate disclosure with respect to our products or services could adversely affect our business, reputation and our financial statements.
Manufacturing or design defects in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, or inadequate disclosure of risks relating to the use of products and services that we make or sell (including items that we source from third parties) can lead to personal injury, property damage or other liability. These events could lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us. Recalls, removals and product liability and similar claims (regardless of their validity or ultimate outcome) can result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services. Our product liability insurance may not adequately cover our costs arising from defects in our products or otherwise.
We are subject to export and import control laws and regulations that could impair our ability to compete in international markets or subject us to liability if we violate such laws and regulations.
We are subject to U.S. export controls and sanctions regulations that restrict the shipment or provision of certain products and services to certain countries, governments, and persons. While we take precautions to prevent our products and services from being exported in violation of these laws, we cannot guarantee that the precautions we take will prevent violations of export control and sanctions laws. If we are found to be in violation of U.S. sanctions or export control laws, it could result in substantial fines and penalties for us and for the individuals working for us. We may also be adversely affected through other penalties, reputational harm, loss of access to certain markets, or otherwise.
Complying with export control and sanctions regulations may be time-consuming and may result in the delay or loss of sales opportunities or impose other costs. Any change in export or import regulations, economic sanctions or related legislation, or change in the countries, governments, persons or technologies targeted by such regulations, could result in our decreased ability to export or sell certain products to existing or potential customers in affected jurisdictions. We are subject to laws and regulations governing government contracts.
We are subject to laws and regulations governing government contracts, and failure to address these laws and regulations or comply with government contracts could harm our business by leading to a reduction in revenues associated with these customers. We have agreements relating to the sale of our products to government entities and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government. We are also subject to investigation for compliance with the regulations governing government contracts. A failure to comply with these regulations could result in suspension of these contracts, criminal, civil and administrative penalties or debarment.
Financial and Tax Risks
We may be required to recognize additional impairment charges for our goodwill and other intangible assets.
As of March 31, 2022, the net carrying value of our goodwill and other intangible assets totaled $541.3 million. In accordance with generally accepted accounting principles, we periodically assess our assets to determine if they are impaired. Significant negative industry or economic trends, disruptions to our business, inability to effectively integrate acquired businesses, unexpected significant changes or planned changes in use of our assets, changes in the structure of our business, divestitures, market capitalization declines, or increases in associated discount rates may impair our goodwill and other intangible assets. Any charges relating to such impairments would adversely affect our financial statements in the periods recognized.
Foreign currency exchange rates may adversely affect our financial statements.
As a global company with substantial operations outside the U.S., sales and purchases in currencies other than the U.S. dollar expose us to fluctuations in foreign currencies relative to the U.S. dollar and may adversely affect our financial statements. Increased strength of the U.S. dollar increases the effective price of our products sold in U.S. dollars into other countries, which may require us to lower our prices or adversely affect sales to the extent we do not increase local currency prices. Decreased strength of the U.S. dollar could adversely affect the cost of materials, products and services we purchase overseas. Sales and expenses of our non-U.S. businesses are also translated into U.S. dollars for reporting purposes and the strengthening or weakening of the U.S. dollar could result in unfavorable translation effects. In addition, certain of our businesses may invoice customers in a currency other than the business’ functional currency, and movements in the invoiced currency relative to the functional currency could also result in unfavorable translation effects. We also face exchange rate risk from our investments in subsidiaries owned and operated in foreign countries. We do not enter into hedging arrangements to mitigate any foreign currency exposure.
Changes in accounting standards could affect our reported financial results.
New accounting standards or pronouncements that may become applicable to our Company from time to time, or changes in the interpretation of existing standards and pronouncements, could have a significant effect on our reported results of operations for the affected periods.
Changes in our tax rates or exposure to additional income tax liabilities or assessments could affect our profitability. In addition, audits by tax authorities could result in additional tax payments for prior periods.
We are subject to income taxes in the U.S. and in various non-U.S. jurisdictions. The amount of income taxes we pay is subject to ongoing audits by U.S. federal, state and local tax authorities and by non-U.S. tax authorities, such as those audits described elsewhere in this report. If audits result in payments or assessments different from our reserves, our future results may include unfavorable adjustments to our tax liabilities and our financial statements could be adversely affected. Any further significant changes to the tax system in the United States or in other jurisdictions (including changes in the taxation of international income as further described below) could adversely affect our financial statements.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report financial results or prevent fraud. If we identify a material weakness in our internal control over financial reporting, our ability to meet our reporting obligations and the trading price of our stock could be negatively affected.
Effective internal controls are necessary to provide reliable financial reports and to assist in the effective prevention of fraud. Any inability to provide reliable financial reports or prevent fraud could harm our business. We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the Sarbanes-Oxley Act of 2002 to report annually on our internal control over financial reporting. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. If we, or our independent registered public accounting firm, determine that our internal control over financial reporting is not effective, discover areas that need improvement in the future or discover a material weakness, these shortcomings could have an adverse effect on our business and financial results, and the price of our common stock could be negatively affected. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Accordingly, a material weakness increases the risk that the financial information we report contains material errors.
If we cannot conclude that we have effective internal control over our financial reporting, or if our independent registered public accounting firm is unable to provide an unqualified opinion regarding the effectiveness of our internal control over financial reporting, investors could lose confidence in the reliability of our financial statements, which could lead to a decline in our stock price. Failure to comply with reporting requirements could also subject us to sanctions and/or investigations by the SEC, The Nasdaq Stock Market or other regulatory authorities. We have previously implemented several significant ERP modules and have acquired businesses that were subsequently required to adopt our systems of internal controls. The implementation of these systems represents a change in our internal control over financial reporting. Although we continue to monitor and assess our internal controls environment as changes are made and new modules are implemented, and we have taken additional steps to modify and enhance the design and effectiveness of our internal control over financial reporting, there is a risk that deficiencies may occur that could aggregate to a material weakness. If we fail to remedy any deficiencies or maintain the adequacy of our internal controls, we could be subject to regulatory scrutiny, civil or criminal penalties or shareholder litigation. In addition, failure to maintain adequate internal controls could result in financial statements that do not accurately reflect our operating results or financial condition.
Our ability to use net operating losses and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and it is possible that certain transactions or a combination of certain transactions may result in material additional limitations on our ability to use our net operating loss and tax credit carryforwards.
Section 382 and 383 of the Internal Revenue Code of 1986, as amended, contain rules that limit the ability of a company that undergoes an ownership change, which is generally any change in ownership of more than 50% of its stock over a three-year period, to utilize its net operating loss and tax credit carryforwards and certain built-in losses recognized in years after the ownership change. These rules generally operate by focusing on ownership changes involving stockholders owning directly or indirectly 5% or more of the stock of a company and any change in ownership arising from a new issuance of stock by the company. Generally, if an ownership change occurs, the yearly taxable income limitation on the use of net operating loss and tax credit carryforwards and certain built-in losses is equal to the product of the applicable long-term, tax-exempt rate and the value of the company’s stock immediately before the ownership change. We may be unable to offset our taxable income with losses, or our tax liability with credits, before such losses and credits expire and therefore would incur larger federal income tax liability. We are in the process of conducting a new Section 382 study as a result of the acquisition of Agena, and while our most recent Section 382 analysis did not show any current exposure, future transactions or combinations of future transactions may result in a change in control under Section 382. Federal net operating losses generated after December 31, 2017 are not subject to expiration and generally may not be carried back to prior taxable years except that, under the Coronavirus Aid, Relief, and Economic Security Act, net operating losses generated in 2018, 2019 and 2020 may be carried back five taxable years. Additionally, for taxable years beginning after March 31, 2021, the deductibility of such deferral net operating losses is limited to 80% of our taxable income in any future taxable year.
Changes in tax law relating to multinational corporations could adversely affect our tax position.
The U.S. Congress, government agencies in non-U.S. jurisdictions where we and our affiliates do business, and the Organization for Economic Co-operation and Development (“OECD”) have recently focused on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where profits are claimed to be earned for tax purposes in low-tax jurisdictions, or payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. The OECD has released several components of its comprehensive plan to create an agreed set of international rules for addressing base erosion and profit shifting. As a result, the tax laws in the United States and other countries in which we do business could change on a prospective or retroactive basis, and any such changes could adversely affect our business and financial statements.
Our business is subject to sales tax in numerous states.
The application of indirect taxes, such as sales tax, is a complex and evolving issue. A company is required to collect and remit state sales tax from certain of its customers if that company is determined to have “nexus” in a particular state. The determination of nexus varies by state and often requires knowledge of each jurisdiction’s tax case law. The application and implementation of existing, new or future laws could change the states in which we are required to collect and remit sales taxes. If any jurisdiction determines that we have “nexus” in additional locations that we have not contemplated, it could have an adverse effect on our financial results.
If global credit market conditions deteriorate, our financial performance could be adversely affected.
The cost and availability of credit are subject to changes in the global economic environment. If conditions in major credit markets deteriorate, our ability to obtain debt financing or the terms associated with that debt financing may be negatively affected, which could affect our results of operations.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business or the ability to raise capital to repay our 1.375% convertible senior notes due August 15, 2025 (the “Notes”) at maturity or repurchase the notes in the event of a fundamental change, or if we borrow under our credit facility, swingline loan, and letters of credit (together referred to as the "Credit Facility") or if we incur more debt.
We incurred significant indebtedness in the amount of $172,500 in the form of the Notes which mature on August 15, 2025, unless earlier converted. We also have a revolving Credit Facility and could borrow additional amounts under that at any time, incurring more debt.
We currently expect to settle future conversions solely in shares of our common stock, which has the effect of including the shares of common stock issuable upon conversion of the Notes in our diluted earnings per share to the extent such shares are not anti-dilutive. We will reevaluate this policy from time to time in the event conversion notices are received from Note holders and if our stock price is above the strike price. Holders of the Notes also have the right to require us to repurchase all or a portion of their Notes upon the occurrence of a fundamental change (as defined in the applicable indenture governing the Notes) at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest. In addition, if the Notes have not previously been converted or repurchased due to a decline in share price, we will be required to repay the Notes in cash.
Our ability to make required cash payments in connection with conversions of the Notes, repurchase the Notes in the event of a fundamental change, or to repay or refinance the Notes at maturity will depend on market conditions and our future performance, which is subject to economic, financial, competitive, and other factors beyond our control.
In addition, our ability to repurchase or to pay cash upon conversion or at maturity of the Notes may be limited by law or regulatory authority. Our failure to repurchase Notes following a fundamental change as required by the applicable indenture would constitute a default under such indenture. A default under the indenture or agreements governing our future indebtedness could have a material adverse effect on our business, results of operations, and financial condition. If the payment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or to pay cash upon conversion or at maturity of the Notes.
Additional stock issuances could result in significant dilution to our stockholders.
We may issue additional equity securities to raise capital, make acquisitions, or for a variety of other purposes. Additional issuances of our stock may be made pursuant to the exercise or conversion of new or existing convertible debt securities, stock options, or other equity incentive awards. We rely on equity-based compensation as an important tool in recruiting and retaining employees. The amount of dilution due to equity-based compensation of our employees and other additional issuances could be substantial. In addition, in March 2022, we entered a sales agreement with Jefferies LLC ("Jefferies") to sell shares of our common stock, from time to time, with aggregate gross sales proceeds up to $150.0 million through an at-the-market equity offering program under which Jefferies will act as our sales agent. If we issue common stock or securities convertible into common stock for the above reasons, or any other reason, our common stockholders would experience additional dilution and, as a result, our stock price may decline.
Our stock price may be volatile, which may subject us to a securities class action litigation.
The trading price of our common stock price may be volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| ● |
general economic, industry and market conditions; |
| ● |
actions by institutional or other large stockholders; |
| ● |
the depth and liquidity of the market for our common stock; |
| ● |
volume and timing of orders for our products; |
| ● |
developments generally affecting medical device companies; |
| ● |
the announcement of new products or product enhancements by us or our competitors; |
| ● |
changes in earnings estimates or recommendations by securities analysts; |
| ● |
investor perceptions of us and our business, including changes in market valuations of medical device companies; and |
| ● |
our results of operations and financial performance. |
In addition, the stock market in general, and the Nasdaq Stock Market and the market for products and devices sold into the medical and healthcare industries in particular, have experienced substantial price and volume volatility that is often seemingly unrelated to the operating performance of particular companies. These broad market fluctuations may cause the trading price of our common stock to decline. In the past, securities class action litigation has often been brought against a company after a period of volatility in the market price of its common stock. We may become involved in this type of litigation in the future. Any securities litigation claims brought against us could result in substantial expense and the diversion of management’s attention from our business.
Item 1B. Unresolved Staff Comments
None.
As of March 31, 2022, we owned two facilities and both are material to our business: one in Lakewood, Colorado and the other in Bozeman, Montana. Both facilities are used for manufacturing, engineering, research and development, marketing, and administration. Two of our four segments use the properties: Sterilization and Disinfectant Control and Calibration Solutions. We have ten leased facilities which are individually immaterial.
For information regarding legal proceedings, refer to Note 13. “Commitments and Contingencies” in our Consolidated Financial Statements included in Item 8. Financial Statements and Supplementary Data.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded on the Nasdaq Global Market (“Nasdaq”) under the symbol “MLAB.”
While we have paid dividends to holders of our common stock on a quarterly basis since 2003, the declaration and payment of future dividends will depend on many factors, including, but not limited to, our earnings, financial condition, business development needs and regulatory considerations, and is at the sole discretion of our Board of Directors.
As of March 31, 2022, there were 66 holders of record of our common stock. This amount does not include “street name” holders or beneficial holders of our common stock, whose holders of record are banks, brokers and other financial institutions.
During the year ended March 31, 2022, we did not sell any equity securities that were not registered under the Securities Act of 1933, as amended.
On November 7, 2005, our Board of Directors adopted a share repurchase plan which allows for the repurchase of up to 300,000 of our common shares. This plan will continue until the maximum is reached or the plan is terminated by further action of the Board of Directors. We made no repurchases of our common stock during the years ended March 31, 2022, March 31, 2021, or March 31, 2020. As of March 31, 2022, 137,514 shares remained available to repurchase pursuant to the repurchase plan.
See Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters for information regarding securities authorized for issuance.
Set forth below is a line graph comparing, for the period March 31, 2016 through March 31, 2022, the cumulative total shareholder return on our common stock against the cumulative total return of (a) the S&P Composite Stock Index (b) the S&P Small Cap 600, and (c) a self-selected peer group, comprised of the following companies: Danaher Corp., Inc., Steris Corp., Fortive Corporation, Mettler Toledo International, Inc., Transcat Inc., Electro-Sensors Inc., Sotera Health, Hologic, Inc., NeoGenomics Laboratories, Inc., and Natus Medical Inc. The graph shows the value on March 31 of each year, assuming an original investment of $100 in each and reinvestment of cash dividends.
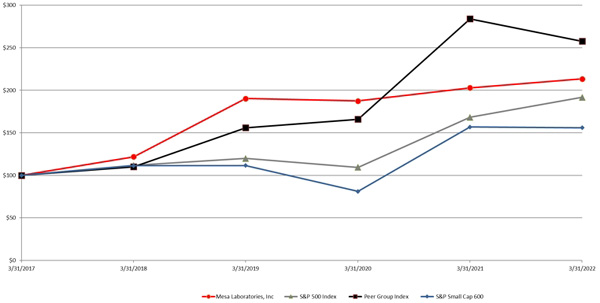
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
(Dollars in thousands, unless specified)
Overview
We are a multinational manufacturer, developer, and seller of life sciences tools and quality control products and services, many of which are sold into niche markets that are driven by regulatory requirements. We have manufacturing operations in the United States and Europe and our products are marketed by our sales personnel in North America, Europe, and Asia Pacific, and by independent distributors in these areas as well as throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross profit margins. As described in Note 14. "Segment Data" of the Notes to Consolidated Financial Statements contained within Item 8. Financial Statements and Supplementary Data of this annual report, during the third quarter of fiscal year 2022, following the acquisition of Agena, we changed our segment reporting to align with strategic changes in the way we manage our business units. As of March 31, 2022, we managed our operations in four reportable segments, or divisions: Sterilization and Disinfection Control, Biopharmaceutical Development, Calibration Solutions, and Clinical Genomics, which is comprised of the newly-acquired Agena. Each of our divisions are described further in "Results of Operations" below. Non-reportable operating segments (including our Cold Chain Packaging Division which ceased operations during the year ended March 31, 2020) and unallocated corporate expenses are reported within Corporate and Other.
Corporate Strategy
We strive to create shareholder value and further our purpose of Protecting the Vulnerable® by growing our business both organically and through acquisitions, by improving our operating efficiency, and by continuing to hire, develop and retain top talent. As a business, we commit to our purpose of Protecting the Vulnerable® every day by taking a customer-focused approach to developing, building, and delivering our products. We serve a broad set of industries, in particular the pharmaceutical, healthcare services, and medical device verticals, that require dependable quality control and calibration solutions to ensure the safety and efficacy of the products they use. By delivering the highest quality products possible, we are committed to protecting people, the environment, and end products.
Organic Revenues Growth
Organic revenues growth is primarily driven by the expansion of our customer base, increases in sales volumes, and price increases. Our ability to increase organic revenues is affected by general economic conditions, both domestic and international, customer capital spending trends, competition, and the introduction of new products. We typically evaluate costs and pricing annually. Our policy is to price our products competitively and, where possible, we pass along cost increases to our customers in order to maintain our margins.
Gross profit is affected by many factors including our product mix, manufacturing efficiencies, foreign currency rates, and price competition. Historically, as we have integrated our acquisitions and taken advantage of manufacturing efficiencies, our gross profit percentages for some products have improved. There are, however, differences in gross profit percentages between product lines, and ultimately the mix of sales and prices will continue to impact our overall gross profit.
Inorganic Revenues Growth - Acquisitions
During fiscal year 2022, we completed the acquisition of Agena for an aggregate purchase price of $300,793, net of cash acquired, subject to customary purchase price adjustments. Agena is a leading clinical genomics tools company that develops, manufactures, and sells highly sensitive, low-cost, high-throughput genetic analysis tools used by clinical labs to perform genomic clinical testing in several therapeutic areas, such as newborn screenings, pharmacogenetics and oncology. The acquisition of Agena accelerates Mesa's strategic trajectory towards higher growth applications within the regulated segments of the life sciences tools market.
Over the past decade, we have consummated a number of acquisitions as a part of our growth strategy. The acquisitions of these businesses have allowed us to expand our product offerings, globalize our company, and increase the scale at which we operate, which in turn affords us the ability to improve our operating efficiency, extend our customer base, and further the pursuit of our purpose: Protecting the Vulnerable®.
Improving Our Operating Efficiency
We maximize value in both our existing businesses and those we acquire by implementing efficiencies in our manufacturing, commercial, engineering, and administrative operations. We achieve efficiencies using the four pillars that make up The Mesa Way, which is our customer-centric, lean-based system for continuously improving and operating a set of high-margin, niche businesses. The Mesa Way is focused on: Measuring what matters using our customers' perspective and setting high standards for performance; Empowering teams to improve operationally and exceed customer expectations; Steadily improving using lean-based tools designed to help us identify the root cause of opportunities and prioritize the biggest opportunities; and Always learn so that performance continuously improves. As we integrate Agena into our business, we will focus on applying The Mesa Way to its operations which we hope will improve efficiency in some areas of Agena’s business.
Hire, Develop, and Retain Top Talent
At the center of our organization are talented people who are capable of taking on new challenges using a team approach. It is our exceptionally talented workforce that works together and uses our lean-based tool set to find ways to continuously improve our products, our services, and ourselves, resulting in long-term value creation for our shareholders.
COVID-19 and Business Update
The COVID-19 pandemic began to broadly impact our business late in fiscal year 2020, and its impacts continued to affect our business in various ways throughout fiscal year 2021 and, to a lesser extent, into fiscal years 2022 and 2023. We continue to monitor the impacts of COVID-19, including the current spread of certain variants of the virus, and we have taken and will continue to take steps to identify and mitigate the adverse impact on, and risks to, our business (including but not limited to our employees, customers, vendors, manufacturing capabilities and capacity, and supply and distribution channels) posed by the spread of COVID-19 and the government responses thereto.
COVID-19 has caused or exacerbated broad market phenomena such as supply chain disruptions, inflation, and wage pressure to which we are susceptible. Throughout fiscal year 2022, we experienced increased supply constraints for certain components used in our operations, particularly components used by the Calibration Solutions and Biopharmaceutical Development divisions, and to a lesser extent the Sterilization and Disinfection Control and Clinical Genomics divisions. We continue to work with our suppliers to understand the existing and potential future impacts to our supply chain and are taking actions in an effort to mitigate such impacts, including pre-ordering components in higher quantities than usual, which has resulted in increased raw materials balances on our balance sheet as of March 31, 2022. The impact of supply chain disruptions is discussed in more detail in our "Results of Operations" and Item 1A. Risk Factors. Even after the COVID-19 pandemic has largely subsided as a public health matter, we may experience material adverse impacts to our business as a result of the pandemic's adverse impact on the global economy, in-person collaboration and sales efforts, and our customers’ changed purchasing behaviors and confidence.
The COVID-19 pandemic and related public health recommendations and mandated precautions to mitigate the spread of the virus, including regulations to close or limit the operating hours of our laboratory and facilities of our customers, and to prevent non-essential personnel from going on-site to customer locations to service or market our products, have negatively affected our operations. While many recommendations and precautions that affected us in fiscal year 2021 have been rescinded in the United States, some restrictions were reimposed for portions of the year ended March 31, 2022 as COVID-19 variants spread widely. Our operations in Europe and Asia have been most impacted because regulations and restrictions have tended to be more widespread in those areas. In contrast to the negative impacts experienced by our other divisions, our Clinical Genomics division produces a consumable reagent that can be used with its proprietary MassARRAY® instruments to accurately identify the presence of the COVID-19 virus and identify the variant from a biological sample. As a result, the Clinical Genomics division has benefited to some extent from outbreaks and resulting increased testing efforts. However, as in our other divisions, regulatory restrictions, particularly in Asia have negatively impacted commercial execution, limiting sales of Clinical Genomics products to new customers. We expect regulatory restrictions in Asia to continue into fiscal year 2023 which may negatively impact our Clinical Genomics division, and to a lesser extent, the rest of the company.
Our revenues are generated from product sales, which include consumables and hardware; as well as services, which include discrete and ongoing calibration, testing, and maintenance services. Revenues increase as a result of organic or inorganic revenues growth. Inorganic revenues growth is driven by acquisitions. Sales of our hardware products have historically been more sensitive to general economic conditions than sales of our consumables. Even as the broad healthcare industry has returned to more normal operations resulting in increased sales levels in most of our divisions, outbreaks and increasing numbers of COVID-19 cases in many areas, especially Asia, have and may continue to result in the reinstatement of strict regulations, which we expect will result in lower sales levels. However, as vaccine distribution progresses, we are hopeful that any reinstatements of strict regulations will be less frequent and shorter in duration.
We are working on several research and development projects that, if completed, may result in enhanced or new products for both existing customers and new markets. We are hopeful that we will have enhanced or new products and services available for sale in the coming fiscal year.
As discussed in Note 15. "Subsequent Events" within Item 8. Financial Statements and Supplementary Data, we entered into an Open Market Sale AgreementSM with Jefferies LLC as sales agent subsequent to the year ended March 31, 2022.
Revenues for our reportable segments increased 38% for the year ended March 31, 2022. Revenues growth was primarily attributable to the acquisition of Agena; however, organic revenues growth was 13%. Gross profit as a percentage of revenues decreased 6 percentage points for the year ended March 31, 2022, primarily as a result of a $7,462 charge recorded as we amortized the fair value of inventory step up recorded as part of purchase accounting. Results by reportable segment are as follows:
| Revenues |
Organic Revenues Growth |
Gross Profit as a % of Revenues |
||||||||||||||||||||||
| Year Ended March 31, 2022 |
Year Ended March 31, 2021 |
Year Ended March 31, 2022 |
Year Ended March 31, 2021 |
Year Ended March 31, 2022 |
Year Ended March 31, 2021 |
|||||||||||||||||||
| Sterilization and Disinfection Control |
$ | 59,044 | $ | 53,119 | 11 | % | 7 | % | 74 | % | 75 | % | ||||||||||||
| Biopharmaceutical Development |
45,579 | 33,892 | 34 | % | 19 | % | 63 | % | 62 | % | ||||||||||||||
| Calibration Solutions |
46,872 | 46,926 | - | % | (9 | %) | 53 | % | 56 | % | ||||||||||||||
| Clinical Genomics |
32,840 | - | N/A | N/A | 36 | % | N/A | |||||||||||||||||
| Reportable segments |
$ | 184,335 | $ | 133,937 | 13 | % | 1 | % | 59 | % | 65 | % | ||||||||||||
Results of Operations
Our results of operations and year-over-year changes are discussed in the following section. The tables and discussion below should be read in conjunction with the accompanying Consolidated Financial Statements and the notes thereto appearing in Item 8. Financial Statements and Supplementary Data (in thousands, except percent data).
Refer to Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended March 31, 2021, filed on June 1, 2021, for a comparison of results of operations for the years ended March 31, 2021 and March 31, 2020.
Our condensed consolidated results of operations are as follows:
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | 184,335 | $ | 133,937 | $ | 117,687 | 38 | % | 14 | % | ||||||||||
| Gross profit |
109,090 | 87,014 | 65,362 | 25 | % | 33 | % | |||||||||||||
| Operating expenses |
104,388 | 74,656 | 57,439 | 40 | % | 30 | % | |||||||||||||
| Operating income |
4,702 | 12,358 | 7,923 | (62 | %) | 56 | % | |||||||||||||
| Net income |
$ | 1,871 | $ | 3,274 | $ | 1,778 | (43 | %) | 84 | % | ||||||||||
Reportable Segments
Sterilization and Disinfection Control
Our Sterilization and Disinfection Control division manufactures and sells biological, cleaning, and chemical indicators which are used to assess the effectiveness of sterilization and disinfection processes in the pharmaceutical, medical device, dental, and hospital industries. The division also provides testing and laboratory services, mainly to the dental industry. Sterilization and disinfection control products are disposable and are used on a routine basis.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | 59,044 | $ | 53,119 | $ | 49,660 | 11 | % | 7 | % | ||||||||||
| Gross profit |
43,720 | 39,870 | 35,797 | 10 | % | 11 | % | |||||||||||||
| Gross profit as a % of revenues |
74 | % | 75 | % | 72 | % | (1 | %) | 3 | % | ||||||||||
Sterilization and Disinfection Control revenues increased 11% as a result of organic revenues growth, which was achieved through modest price increases, effective efforts by our sales team to market and sell certain products to a larger customer base, and volume increases with existing customers particularly in the biopharmaceutical markets.
Sterilization and Disinfection Control's gross profit percentage decreased one percentage point during the year ended March 31, 2022 primarily due to higher labor costs as a result of strong competition for employees in the labor market, and higher freight costs as a result of the global supply chain disruptions.
Biopharmaceutical Development
Our Biopharmaceutical Development division develops, manufactures, and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Immunoassays and peptide synthesis solutions accelerate the discovery, development, and manufacture of biotherapeutic drugs.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | 45,579 | $ | 33,892 | $ | 13,851 | 34 | % | 145 | % | ||||||||||
| Gross profit |
28,605 | 21,035 | 382 | 36 | % | 5,407 | % | |||||||||||||
| Gross profit as a % of revenues |
63 | % | 62 | % | 3 | % | 1 | % | 59 | % | ||||||||||
The results of the Biopharmaceutical Development division were consolidated into our results beginning on November 1, 2019, the first day following our acquisition of Gyros Protein Technologies Holding AB. Biopharmaceutical Development's revenues increased 34% as a result of resumed in-person sales efforts and a marketing strategy focused on packaging our hardware and software products in a format that is more appealing to our customers. These efforts resulted in increased hardware sales and to a lesser extent increased consumables sales, particularly in the cell and gene therapy market.
Biopharmaceutical Development's gross profit percentage increased one percentage point during the year ended March 31, 2022 as a result of a favorable mix shift towards immunoassay products, as well as production efficiencies resulting from increased revenues, partially offset by higher labor costs.
Substantially all of this division's sales are invoiced in either euros or U.S. dollars ("USD"); however, the majority of the costs in this division are recorded in Swedish Krona ("SEK") and translated to USD for reporting purposes. During periods in which the USD is weaker against the SEK, such as in the first and second quarters of fiscal year 2022, our USD reported costs are inflated and gross profit percentage is lower. In periods in which the USD strengthens against the SEK, such as in the third and fourth quarters of fiscal year 2022, our USD reported costs are lower and gross profit percentage is higher.
Calibration Solutions
This new reportable segment is comprised of the historical Instruments and Continuous Monitoring segments. The Calibration Solutions division designs, manufactures, and markets quality control and calibration products used to measure or calibrate temperature, pressure, pH, humidity, and other such parameters for health and safety purposes, primarily in hospital, medical device manufacturing, pharmaceutical, and laboratory environments.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | 46,872 | $ | 46,926 | $ | 51,713 | - | % | (9 | %) | ||||||||||
| Gross profit |
24,989 | 26,112 | 28,765 | (4 | %) | (9 | %) | |||||||||||||
| Gross profit as a % of revenues |
53 | % | 56 | % | 56 | % | (3 | %) | - | % | ||||||||||
Calibration Solutions' revenues were flat for the year ended March 31, 2022, primarily as a result of supply and labor constraints limiting our ability to manufacture ordered quantities of certain products, partially offset by slightly higher service revenues. As COVID-19 related restrictions were gradually lifted, our service technicians were able to go to client sites to complete service requests and hardware installations. Despite fulfillment delays for many customer orders, demand for the division's products increased during fiscal year 2022, and to date, we have been able to retain the substantial majority of our customers and orders.
Calibration Solutions' gross profit percentage decreased three percentage points during the year ended March 31, 2022. The decrease in gross profit percentage resulted primarily from increased freight on purchased components, higher labor costs as a result of strong competition for employees in the labor market, and costs associated with outsourcing certain calibration functions while we completed the move and manufacturing set up of certain product sets from our Butler, New Jersey facility to Lakewood, Colorado. While we no longer are outsourcing our calibration functions, supply chain disruptions and higher labor costs are expected to continue through fiscal year 2023.
Clinical Genomics
This is a new reportable segment comprised of the recently acquired Agena. The Clinical Genomics division develops, manufactures, and sells highly sensitive, low-cost, high-throughput genetic analysis tools used by clinical labs to perform genomic clinical testing in several therapeutic areas, such as newborn screenings, pharmacogenetics and oncology.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | 32,840 | $ | - | $ | - | - | % | N/A | |||||||||||
| Gross profit |
11,941 | - | - | - | % | N/A | ||||||||||||||
| Gross profit as a % of revenues |
36 | % | N/A | N/A | N/A | N/A | ||||||||||||||
Revenues in the Clinical Genomics division represent revenues from October 20, 2021 until March 31, 2022. Of the revenues reported, $2,871 represents revenues from COVID-19-related sales, of which the substantial majority are consumables. We expect sales in fiscal year 2023 will be negatively impacted by continued restrictions and lockdowns imposed in China as a result of the COVID-19 pandemic, which have limited our sales efforts beginning in late fiscal year 2022.
Clinical Genomics' gross profit was $11,941 for the period from October 20, 2021 until March 31, 2022. Gross profit includes a $7,462 charge related to amortization of an inventory step-up to fair value recorded in purchase accounting. Excluding the step-up amortization, gross profit for the period ended March 31, 2022 would have been $19,403, and gross profit as a percentage of revenues would have been 59%. Gross profit also includes $2,538 of amortization of intellectual property from the Agena Acquisition. Going forward, we expect gross profit as a percentage of revenues to range from the high 50s to the low 60s, including an annual impact of $5,675 of non-cash amortization of intellectual property.
Corporate and Other
Corporate and Other primarily consists of results from our Cold Chain Packaging division, which was dissolved during the year ended March 31, 2020 and is no longer considered a reportable segment, as well as unallocated corporate expenses.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Revenues |
$ | - | $ | - | $ | 2,463 | N/A | (100% | ) | |||||||||||
| Gross (loss) profit |
(165 | ) | (3 | ) | 418 | 5400 | % | (101% | ) | |||||||||||
| Gross profit as a % of revenues |
N/A | N/A | 17 | % | N/A | N/A | ||||||||||||||
Operating Expenses
Operating expenses for the year ended March 31, 2022 increased 40% in total compared to the year ended March 31, 2021.
Selling
Selling expense is driven primarily by labor costs, including salaries and commissions; accordingly, it may vary with sales levels.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Selling expense |
$ | 28,310 | $ | 18,480 | 12,910 | 53 | % | 43 | % | |||||||||||
| As a percentage of revenues |
15 | % | 14 | % | 11 | % | 1 | % | 3 | % | ||||||||||
Selling expense increased 53% for the year ended March 31, 2022 primarily as a result of the acquisition of Agena. Excluding the impact of Agena, selling expense increased 15% for the year ended March 31, 2022, as we executed on our previously-announced plan to invest in sales and marketing resources in order to increase organic revenues growth. We hired several sales employees throughout fiscal year 2022, resulting in higher labor-related costs and higher commission expense resulting from increased revenues. Further, travel-related costs increased as we resumed various in-person sales events as restrictions on gatherings lifted compared to fiscal year 2021. Including the acquisition of Agena and its sales force, we expect total selling expense will approximate 16% to 18% of revenues for fiscal year 2023.
General and Administrative
Labor costs, non-cash stock-based compensation, and amortization of intangible assets drive the substantial majority of general and administrative expense.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| General and administrative expense |
$ | 60,311 | $ | 45,788 | $ | 38,174 | 32 | % | 20 | % | ||||||||||
| As a percentage of revenues |
33 | % | 34 | % | 32 | % | (1 | %) | 2 | % | ||||||||||
General and administrative expenses increased 32% for the year ended March 31, 2022, primarily as a result of the acquisition of Agena. Excluding the impact of Agena, general and administrative expenses increased 15% for the year ended March 31, 2022 as a result of acquisition and integration costs, higher annual bonus accruals based on our financial results for the year ended March 31, 2022, and increased stock-based compensation expense as we expanded the number of participants in our stock-based compensation programs.
Research and Development
Research and development expense is predominantly comprised of labor costs and third-party consultants.
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Research and development expense |
$ | 15,767 | $ | 10,388 | $ | 6,355 | 52 | % | 63 | % | ||||||||||
| As a percentage of revenues |
9 | % | 8 | % | 5 | % | 1 | % | 3 | % | ||||||||||
Research and development expenses for the year ended March 31, 2022 increased 52% primarily as a result of expenses attributable to Agena. Excluding the impact of Agena, research and development costs for the year ended March 31, 2022 increased 12% primarily as a result of higher personnel and third-party contractor expenditures supporting our continued incremental investments in enhancing existing products as well as the development of new products and features. We expect research and development expenses will approximate 9% to 12% of revenues for fiscal year 2023.
Nonoperating Expense
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Nonoperating expense |
$ | 1,128 | 10,055 | 4,061 | (89 | %) | 148 | % | ||||||||||||
Nonoperating expense for the year ended March 31, 2022 is composed primarily of interest expense and amortization of the debt discount associated with our 1.375% convertible senior notes issued in August 2019, gains and losses on foreign currency transactions, and interest income earned on cash and cash equivalents.
During the year ended March 31, 2022, we incurred significant realized and unrealized foreign currency gains as a result of the USD strengthening significantly, particularly against the Swedish Krona.
Interest expense and amortization of debt discount was lower for the year ended March 31, 2022 compared to the year ended March 31, 2021 due to our adoption of ASU 2020-06, which resulted in a $4,090 reduction in non-cash interest expense related to the Notes. See Note 1. "Description of Business and Summary of Significant Accounting Policies" within Item 8. Financial Statements and Supplementary Data.
Income Taxes
| Year Ended March 31, |
Percentage Change |
|||||||||||||||||||
| 2022 |
2021 |
2020 |
2022 vs. 2021 |
2021 vs. 2020 |
||||||||||||||||
| Income tax expense (benefit) |
$ | 1,703 | $ | (971 | ) | $ | 2,084 | (275 | %) | (147 | %) | |||||||||
| Effective tax rate |
48 | % | (42 | %) | 54 | % | 90 | % | (96 | %) | ||||||||||
Our income tax rate varies based upon many factors, but in general we anticipate that on a go-forward basis, our effective tax rate will be approximately 26%, plus or minus the impact of excess tax benefits and deficiencies associated with share-based payment awards to employees; (please see Note 12. “Income Taxes” within Item 8. Financial Statements and Supplementary Data). Our effective tax rate increased during the year ended March 31, 2022 due to the limitations imposed by Section 162(m), and higher federal and state income taxes, partially offset by tax benefits, notably the exercise of stock options. The excess tax benefits and deficiencies associated with share-based payment awards to our employees have caused and, in the future, may cause large fluctuations in our realized effective tax rate based on timing, volume, and nature of stock options exercised under our share-based payment program.
Net Income
Net income for the year ended March 31, 2022 varied with the changes in revenues, gross profit, and operating expenses (including, respectively, $21,806, $11,391, and $7,462 of non-cash amortization of intangible assets acquired in a business combination, stock-based compensation expense, and amortization of inventory step up). Prior to the adoption of ASU 2020-06 on April 1, 2021, we were required to recognize non-cash interest expense related to the amortization of debt discounts and issuance costs. Subsequent to the adoption, we recognize non-cash interest expense related to amortization of debt issuance costs only, resulting in higher net income subsequent to the adoption of ASU 2020-06.
Non-GAAP reconciliation
Adjusted operating income (which excludes the non-cash impact of amortization of intangible assets acquired in a business combination, stock-based compensation and impairment of goodwill and long-lived assets) is used by management as a supplemental performance measure, in order to compare current financial performance to historical performance, assess the ability of our assets to generate cash, and evaluate potential acquisitions.
Adjusted operating income should not be considered an alternative to, or more meaningful than, net income, operating income, cash flow from operating activities or any other measure of financial performance presented in accordance with GAAP as measures of operating performance or liquidity.
The following table sets forth our reconciliation of adjusted operating income, a non-GAAP measure:
| Year Ended March 31, |
||||||||||||
| 2022 |
2021 |
2020 |
||||||||||
| Operating income |
$ | 4,702 | $ | 12,358 | $ | 7,923 | ||||||
| Amortization of intangible assets acquired in a business combination |
21,806 | 14,513 | 10,637 | |||||||||
| Stock-based compensation |
11,391 | 9,268 | 5,525 | |||||||||
| Impairment loss on goodwill and long-lived assets |
- | - | 276 | |||||||||
| Adjusted Operating Income |
$ | 37,899 | $ | 36,139 | $ | 24,361 | ||||||
Liquidity and Capital Resources
Our sources of liquidity include cash generated from operations, cash and cash equivalents on hand, cash available from our revolving credit facility, swingline loan, and letters of credit (together referred to as the "Credit Facility"), working capital and potential additional equity and debt offerings. We continue to believe that we have the liquidity required to continue operations even if volatility in the economic environment reoccurs. We believe that cash and cash equivalents on hand and cash generated from operations, as well as the remainder of the unused capacity under our Credit Facility, and potential funds from our Open Market Sale AgreementSM, will be sufficient to meet our short-term and long-term needs.
Our more significant uses of resources have historically included acquisitions, long-term capital expenditures, payment of debt and interest obligations, and quarterly dividends to shareholders. Working capital is the amount by which current assets exceed current liabilities. We had working capital of $76,263 and $271,166 on March 31, 2022 and 2021, respectively. We also had $49,346 and $263,865 of cash and cash equivalents as of March 31, 2022 and 2021, respectively. We consider all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents.
On October 20, 2021 we completed the acquisition of Agena for an adjusted purchase price, net of cash acquired, of $300,793.
On March 5, 2021, we entered into a four-year senior secured credit agreement that includes 1) a revolving credit facility in an aggregate principal amount of up to $75,000, 2) a swingline loan in an aggregate principal amount not exceeding $5,000, and 3) letters of credit in an aggregate stated amount not exceeding $2,500 at any time. The Credit Facility also provides for an incremental term loan or an increase in revolving commitments in an aggregate principal amount of at a minimum $25,000 and at a maximum $75,000, subject to the satisfaction of certain conditions and lender considerations. During the third quarter of fiscal year 2022, we borrowed $70,000 under the line of credit to fund the acquisition of Agena and repaid $21,000 during the third and fourth quarters of fiscal year 2022 using cash on hand and cash generated from operations. As of March 31, 2022, we had $26,000 remaining to draw on the Credit Facility.
As of March 31, 2022, we have $172,500 aggregate principal of senior convertible notes outstanding. The Notes bear interest at a rate of 1.375% payable semi-annually in arrears on February 15 and August 15 of each year. The Notes can be converted prior to maturity if certain conditions are met; no such conditions were met during the year ended March 31, 2022. We currently expect to settle future conversions of the Notes entirely in shares of our common stock and will reevaluate this policy from time to time in the event that conversion conditions are met, and conversion notices are received from holders of the Notes. We were in compliance with all debt agreements on March 31, 2022 and for all prior years presented and have met all debt payment obligations. Refer to Note 8. "Indebtedness" within Item 8. Financial Statements and Supplementary Data for more details on these transactions.
In April 2022 we entered into an Open Market Sale AgreementSM, pursuant to which we may issue and sell, from time to time, shares of our common stock with an aggregate value of up to $150 million.
Future material acquisitions may require that we obtain additional capital, assume additional third-party debt or incur other long-term obligations. We believe that we have the ability to issue more equity or debt in the future in order to finance our acquisition and investment activities; however additional equity or debt financing, or other transactions, may not be available on acceptable terms, if at all.
We may from time to time repurchase or otherwise retire our debt. These actions may include retirements or refinancing of outstanding debt, privately negotiated transactions, or otherwise. The amount of debt that may be retired, if any, could be material and would be decided at the sole discretion of our Board of Directors and will depend on market conditions, our cash position and other considerations.
Dividends
We have paid regular quarterly dividends since 2003. We declared and paid dividends of $0.16 per share each quarter of the years ended March 31, 2022, 2021, and 2020.
In April 2022, our Board of Directors declared a quarterly cash dividend of $0.16 per share of common stock, payable on June 15, 2022, to shareholders of record at the close of business on May 31, 2022
Cash Flows
Our cash flows from operating, investing, and financing activities were as follows:
| Year Ended March 31, |
||||||||||||
| 2022 |
2021 |
2020 |
||||||||||
| Net cash provided by operating activities |
$ | 39,223 | $ | 37,073 | $ | 26,988 | ||||||
| Net cash (used in) investing activities |
(305,225 | ) | (1,992 | ) | (185,585 | ) | ||||||
| Net cash provided by financing activities |
52,576 | 146,228 | 231,277 | |||||||||
Cash flows from operating activities for the year ended March 31, 2022 provided $39,223. The $2,150 increase in cash flows from operating activities primarily resulted from non-cash adjustments to net income, particularly increased depreciation and amortization as a result of higher intangibles balances resulting from the Agena Acquisition and amortization of the inventory step-up associated with the Agena Acquisition. Further, cash provided by operating assets and liabilities decreased by $12,444 for the year ended March 31, 2022 compared to the year ended March 31, 2021, primarily as a result of the impact of timing on our working capital accounts. Cash used in investing activities was higher during the year ended March 31, 2022 compared to the year ended March 31, 2021, due to cash expended on the Agena Acquisition, and to a lesser extent purchases of property, plant, and equipment, primarily to support the renovations of our Lakewood, Colorado facility. Cash provided by financing activities primarily resulted from a $70,000 draw on our Credit Facility, net of $21,000 repaid during the year. The draw on our Credit Facility was used to fund a portion of the purchase price of the Agena Acquisition. Our equity raise completed during the year ended March 31, 2021 provided $145,935.
Critical Accounting Policies and Estimates
Our Consolidated Financial Statements are prepared in accordance with accounting principles generally accepted in the United States, which require management to make estimates, judgments, and assumptions that affect the amounts reported in our Consolidated Financial Statements and accompanying notes. We believe that the following are the more critical judgment areas in the application of accounting policies that currently affect our financial condition and results of operations. Management has discussed the development, selection, and disclosure of critical accounting policies and estimates with the Audit Committee of our Board of Directors. While our estimates and assumptions are based on our knowledge of current events and circumstances and actions we may take in the future, actual results may ultimately differ from these estimates and assumptions. For a discussion of our significant accounting policies, see Note 1. “Description of Business and Summary of Significant Accounting Policies” in Item 8. Financial Statements and Supplementary Data.
Revenue Recognition
Our revenues come from product sales, which include consumables and hardware; as well as services, which include discrete and ongoing calibration, testing, and maintenance services and contracts. Revenues are recognized when we satisfy our performance obligations under the terms of a contract, which occurs when control of the promised products or services transfers to our customers. We recognize as revenue the amount of consideration we expect to receive in exchange for transferring products or services to our customers (the transaction price). For all revenue arrangements, prices are fixed at the time of purchase and no price protections or variables are offered. The significant majority of our revenues and related receivables are generated from contracts with customers that are 12 months or less in duration. We generally recognize revenues as follows:
Product sales: Our performance obligations related to product sales generally consist of the promise to sell tangible goods to distributors or end users. Control of these goods is typically transferred upon shipment, at which time our performance obligation is satisfied and revenue is recognized. For products requiring Mesa's personnel to complete installation, control transfers to the customer and revenue is recognized when our technicians have completed the installation at the customer’s location. Purchase orders typically provide evidence of an arrangement for product sales. Products sold include an assurance-type warranty which is accounted for as part of accrued warranty expense.
Services: We generate service revenues from discrete or contracted calibration, testing, and maintenance services performed on our hardware products. Performance obligations arise when discrete services are contracted in advance and performed at a future time, often at the time of the customer’s choosing. In such cases, our performance obligation is satisfied and revenue is recognized upon completion of the specified work. Alternately, performance obligations arising from ongoing service contracts are satisfied by completing any service that is contractually required during the contract period, if requested by the customer, or simply by the passage of time if no services are requested. For ongoing service contracts, revenue is recognized on a straight-line basis over the life of the contract in a faithful depiction of our obligation to provide services over the contract period. Evidence of a service arrangement may be in the form of a formal contract or a purchase order.
Collectability is reasonably assured through our customer review process, and payment is typically due within 60 days or less. Upon adoption of Accounting Standards Codification ("ASC") 606, we elected the practical expedient to expense commission costs as incurred. The substantial majority of our contracts have original durations of one year or less, and we have elected not to disclose the expected timing or allocated transaction prices of future performance obligations. Additionally, we have elected the practical expedient to not assess whether a significant financing component exists when the period between when we perform our performance obligation and when the customer remits payment is one year or less. None of our contracts contained a financing component as of March 31, 2022 or March 31, 2021.
Contracts with customers may contain multiple performance obligations. For such arrangements, the transaction price is allocated to each performance obligation based on the estimated relative standalone selling prices of the promised products or services underlying each performance obligation. Standalone selling prices are based on the price at which the performance obligation is sold separately. If the standalone selling price is not observable through past transactions, we estimate the standalone selling price considering available information such as market conditions and internally approved pricing guidelines. Discounts may be approved at the time of purchase and are included within a contract’s fixed transaction price. Discounts are typically allocated to the performance obligations included in the contract based on the standalone values of such obligations.
Inventories
Inventories are stated at the lower of cost or net realizable value using a weighted average costing methodology. Work in progress and finished goods inventory acquired in an acquisition are recorded at fair market value. Our work in process and finished goods inventories include the costs of raw materials, labor and overhead, which are estimated based on trailing twelve months of expense and standard labor hours for each product. We evaluate labor and overhead costs annually unless specific circumstances necessitate a mid-year evaluation for specific items.
We monitor inventory costs relative to selling prices and perform physical cycle count procedures on inventories throughout the year to determine if a lower of cost or net realizable value reserve is necessary. We estimate and maintain an inventory reserve as needed for such matters as excess or obsolete inventory, shrinkage, and scrap. This reserve may fluctuate as our assumptions change due to new information, discrete events, or changes in our business, such as entering new markets or discontinuing a specific product; however, once inventory is written down, a new cost basis is established that is not subsequently written back up in future periods.
Purchase Accounting for Acquisitions
We account for all business combinations in which we obtain control over another entity using the acquisition method of accounting, which requires most assets (both tangible and intangible) and liabilities (including contingent consideration) to be recognized at fair value at the date of acquisition. The excess of the purchase price over the fair value of assets less liabilities is recognized as goodwill. We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses. These types of analyses require us to make and monitor assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, discount rates and cash flow. Certain adjustments to the assessed fair values of acquired assets or liabilities made subsequent to the acquisition date but within the measurement period are recorded as adjustments to goodwill. Any adjustments subsequent to the measurement period are recorded within earnings. We expense all costs as incurred related to an acquisition in selling, general, and administrative expenses.
Results of operations of the acquired company are included in our Consolidated Financial Statements from the date of the acquisition forward. If actual results are not consistent with our assumptions and estimates, or if our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future.
Acquired Intangible Assets
Our business acquisitions typically result in the recognition of goodwill and other intangible assets, which affect the amount of future period amortization expense and possible impairment charges we may incur.
Intangible assets with a definite life are amortized over their useful lives using the straight-line method and the amortization expense is recorded within cost of products or selling, general and administrative expense in the Consolidated Statements of Income. Intangible assets and their related useful lives are reviewed at least annually to determine if any adverse conditions exist that would indicate the carrying value of these assets may not be recoverable. More frequent impairment assessments are conducted if certain conditions exist, including a change in the competitive landscape, any internal decisions to pursue new or different technology strategies, a loss of a significant customer, or a significant change in the marketplace, including changes in the prices paid for our products or changes in the size of the market for our products. If impairment indicators are present, we determine whether the underlying intangible asset is recoverable through estimated future undiscounted cash flows. The fair value measurement for asset impairment is based on Level 3 inputs. If the asset is not found to be recoverable, it is written down to the estimated fair value of the asset based on the sum of the future cash flows expected to result from the use and disposition of the asset. If the estimate of an intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset is amortized prospectively over the revised remaining useful life. We continue to believe that our definite lived intangible assets are recoverable as of March 31, 2022.
We test goodwill for impairment on an annual basis and between annual tests if events and circumstances indicate it is more likely than not that the fair value of a reporting unit is less than its carrying value. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to: current economic and market conditions, including a decline in market capitalization; a significant adverse change in legal factors; business climate or operational performance of the business; and an adverse action or assessment by a regulator. Goodwill is tested for impairment during the fourth quarter of each year, or more frequently as warranted by events or changes in circumstances mentioned above. Our impairment tests for other indefinite lived intangible assets are similar to the tests performed for goodwill but are conducted at the individual asset level. We accounted for the economic uncertainty caused by the COVID-19 pandemic when conducting our impairment analyses of goodwill and other indefinite lived intangible assets during the fourth quarter of our year ended March 31, 2022.
Our impairment tests begin with the optional qualitative assessment to determine whether it is more likely than not that the carrying value of a goodwill reporting unit or other intangible asset exceeds its fair value, as permitted by the accounting guidance. If, after this qualitative assessment, we determine it is more likely than not that the fair value is greater than the carrying amount, then no further quantitative testing is necessary. A quantitative assessment is performed if the qualitative assessment results in a more likely than not determination or if a qualitative assessment is not performed. The quantitative assessment considers whether the carrying amount of a reporting unit or indefinite lived intangible asset exceeds its fair value, in which case an impairment charge is recorded to the extent carrying value exceeds fair value. Fair value is determined using an income approach, which relies heavily on Level 3 inputs. Our qualitative assessments over each of our reportable segments and our other indefinite lived intangible assets during the year ended March 31, 2022 concluded that no impairment exists as of March 31, 2022.
Debt Accounting
As of March 31, 2022, our long-term debt balance is related to our 1.375% convertible senior notes due 2025, which were issued in August 2019 and are carried at their principal amount less unamortized debt discount. We account for our convertible notes as liabilities. For a discussion on the change in accounting for convertible debt, refer to Note 1. "Description of Business and Summary of Significant Accounting Policies" in Item 8. Financial Statements and Supplementary Data. Debt discount is amortized to interest expense in our Consolidated Statements of Income over the term of the convertible notes using the effective interest rate method.
Stock-based Compensation
We recognize compensation expense for equity awards over the vesting period based on the award’s fair value. We use the Black-Scholes valuation model to determine the fair value of our stock options. The Black-Scholes model requires assumptions to be made regarding our stock price volatility, the expected life of the award, and expected dividend rates. The volatility assumption and the expected life assumptions are based on our historical data. The compensation expense of performance share awards is based in part on the estimated probability of achieving levels of performance associated with particular levels of payout for performance shares. We determine the probability of achievement of future levels of performance by comparing the relevant performance level with our internal estimates of future performance. Those estimates are based on a number of assumptions, and different assumptions may have resulted in different conclusions regarding the probability of achieving future levels of performance relevant to the payout levels for the awards. Had we arrived at different assumptions of stock price volatility or expected lives of our options, or different assumptions regarding the probability of our achieving future levels of performance with respect to performance share awards, our stock-based compensation expense and results of operations could have been different.
Income Taxes
Our provision for income taxes requires the use of estimates in determining the timing and amounts of deductible and taxable items including impacts on effective tax rates, deferred tax items and valuation allowances based on management’s interpretation and application of complex tax laws and accounting guidance. We establish reserves for uncertain tax positions for material, known tax exposures relating to deductions, transactions and other matters involving some uncertainty as to the measurement and recognition of the item. While we believe that our reserves are adequate, issues raised by a tax authority may be finally resolved at an amount different than the related reserve and could materially increase or decrease our income tax provision in the current and/or future periods.
Recent Accounting Standards and Pronouncements
For a discussion of the new accounting standards impacting the Company, refer to Note 1. “Description of Business and Summary of Significant Accounting Policies” in Item 8. Financial Statements and Supplementary Data.
Contractual Obligations, Commitments and Off-Balance Sheet Arrangements
Off-Balance Sheet Arrangements
As of March 31, 2022, we have no obligations or interests which qualify as off-balance sheet arrangements.
Contractual Obligations
We are party to many contractual obligations that involve commitments to make payments to third parties in the ordinary course of business. For a description of our contractual obligations and other commercial commitments as of March 31, 2021, see our Annual Report on Form 10-K for the fiscal year ended March 31, 2021, filed with the Securities and Exchange Commission on June 1, 2021. As a result of the Agena Acquisition in the third quarter of fiscal year 2022, we assumed certain contractual obligations, including an additional $9,884 of payments under existing lease agreements, and $4,373 of open purchase orders as of March 31, 2022.
On a consolidated basis, at March 31, 2022, we had contractual obligations for open purchase orders of approximately $19,025 for routine purchases of supplies and inventory, of which the substantial majority are payable in less than one year. Open purchase orders continue to increase as we take proactive steps to mitigate risks in supply by increasing our orders of certain critical raw materials.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We have no derivative instruments and minimal exposure to commodity market risks.
Foreign Currency Exchange Rates
We face exchange rate risk from transactions with customers in countries outside the United States and from intercompany transactions between affiliates. Transactional exchange rate risk arises from the purchase and sale of goods and services in currencies other than our functional currency or the functional currency of the applicable subsidiary. We also face translational exchange rate risk related to the translation of financial statements of our foreign operations into U.S. dollars, our functional currency. Costs incurred and sales recorded by subsidiaries operating outside of the United States are translated into U.S. dollars using exchange rates effective during the respective period. As a result, we are exposed to movements in the exchange rates of various currencies against the U.S. dollar. Currency exposures have increased as a result of the GPT Acquisition, which incurs a substantial portion of its expenses in Swedish Krona, while most revenue contracts for GPT are in U.S. Dollars and euros. Therefore, when the Swedish Krona strengthens or weakens against the U.S. dollar, operating profits are decreased or increased, respectively. The effect of a change in currency exchange rates on our international subsidiaries' assets and liabilities is reflected in the accumulated other comprehensive income component of stockholders’ equity.
To the extent material, we have discussed the impact of the change in foreign currency within Item 7. "Results of Operations." A hypothetical 10 percent reduction in currency exchange rates compared to the U.S. dollar (U.S. dollar weakening) would result in an estimated $170 after tax reduction in net earnings over a one-year period. Actual changes in market prices or rates may differ from hypothetical changes.
Interest Rates
Beginning during our year ended March 31, 2020, we held investments in money market funds. As a result, we are exposed to potential loss from market risks that may occur as a result of changes in interest rates, credit quality of the issuer, or other factors.
During our year ended March 31, 2021, we entered into the Credit Facility. Based on the Company’s variable-rate debt outstanding as of March 31, 2022, we estimate that a 1 percentage point increase in interest rates would have increased interest expense by $193 for the year ended March 31, 2022.
Item 8. Financial Statements and Supplementary Data
[REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM]
Stockholders and Board of Directors
Mesa Laboratories, Inc.
Lakewood, Colorado
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Mesa Laboratories, Inc. (the “Company”) as of March 31, 2022 and 2021, the related consolidated statements of income, comprehensive (loss) income, stockholders' equity, and cash flows for each of the years in the three-year period ended March 31, 2022, and the related notes (collectively referred to as the “financial statements”). We also have audited the Company's internal control over financial reporting as of March 31, 2022, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO framework”).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the three-year period ended March 31, 2022, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2022, based on criteria established in the COSO framework.
Basis for Opinions
The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s financial statements and an opinion on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Report on Internal Control over Financial Reporting, management has excluded an acquired entity from its assessment of internal control over financial reporting as of March 31, 2022 because it was acquired by the Company in a purchase business combination during the year ended March 31, 2022. We have also excluded this entity from our audit of internal control over financial reporting. The acquired entity represents approximately 32% and 18% of assets (exclusive of intangible assets and goodwill) and revenues, respectively, for the year ended March 31, 2022.
Definition and Limitations of Internal Control over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matters or on the accounts or disclosures to which they relate.
Business Combination – Refer to Notes 1 and 4
Critical Audit Matter Description
As disclosed in Note 4 to the consolidated financial statements, the Company completed an acquisition of Agena Bioscience, Inc. for total cash consideration of approximately $300.8 million, net of cash acquired, on October 20, 2021. The Company accounted for the transaction as a business combination using the acquisition method of accounting. Accordingly, the assets acquired and liabilities assumed were recognized at their respective acquisition date fair values.
We identified the allocation of the purchase price related to the Agena Bioscience, Inc. acquisition as a critical audit matter. The principal considerations for our determination include the inherent judgment involved in selecting market-based assumptions used in the estimated cash flow projections, including forecasts of future revenue growth rates, customer attrition rates, royalty rates and discount rates.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures performed to address this critical audit matter included the following, among others:
| ● |
We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over business combinations |
| ● |
Tested management’s process for estimating the fair value of intangible assets. This included evaluating, with the assistance of our fair value specialists, the appropriateness of the valuation methods, testing the completeness and accuracy of data provided by management, and evaluating the reasonableness of key assumptions with respect to the expected future net discounted cash flows including the future revenue growth rates, customer attrition rates, royalty rates, and discount rates. |
|
|
● |
Evaluated the reasonableness of the expected future net discounted cash flows including the future revenue growth rates, the customer attrition rates, the royalty rates, and the discount rates involved considering the past performance of the acquired business and the Company, as well as economic and industry forecasts, and considering whether they were consistent with evidence obtained in other areas of the audit. Additionally, evaluated the reconciliation of the weighted average cost of capital to the internal rate of return for reasonableness and consistency. |
| ● |
We performed sensitivity analyses of the significant assumptions around the future revenue growth rate, the customer attrition rate, the royalty rates, and discount rates within the valuation models. | |
| ● | We evaluated the Company’s disclosures related to the business combinations. |
Income Taxes – Refer to Notes 1 and 12
Critical Audit Matter Description
The Company’s income tax expense includes U.S., state, local and international income taxes. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting basis and the tax basis of existing assets and liabilities. The tax rate used to determine the deferred tax assets and liabilities is based on the enacted tax rate for the year and the manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
We identified management’s calculation of the provision for income taxes as a critical audit matter because of the significant judgments and estimates management makes to determine these amounts. Performing audit procedures to evaluate the reasonableness of management’s interpretation of tax law in various foreign jurisdictions, and its estimate of the associated provisions and tax charges required a high degree of auditor judgment and increased effort.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures performed to address this critical audit matter included the following, among others:
| ● |
We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over income tax balances and disclosures, including the provision for income taxes |
| ● |
We assessed the Company’s income tax provision by: |
| ● |
Testing the provision for income taxes, including the effective tax rate reconciliation, permanent and temporary differences and uncertain tax positions, by evaluating communications with tax advisors, and testing the underlying data for completeness and accuracy. |
| ● |
Utilizing personnel with specialized knowledge and skill in domestic and international tax to assist in (i) evaluating management’s application of domestic and foreign tax laws and (ii) evaluating the calculation of the deferred tax attributes. |
| ● |
Evaluating the significant assumptions used by management in establishing and measuring tax-related assets and liabilities, including the application of recent tax laws and regulations. |
| ● |
Evaluating the Company’s disclosures related to the provision for income taxes. |
/s/ Plante & Moran, PLLC
We have served as the Company’s auditor since 1986.
Denver, Colorado
May 31, 2022
Mesa Laboratories, Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
| March 31, | March 31, | |||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 49,346 | $ | 263,865 | ||||
| Accounts receivable, less allowances of $ and $, respectively | 41,224 | 23,787 | ||||||
| Inventories, net | 24,606 | 11,178 | ||||||
| Prepaid expenses and other | 9,142 | 4,919 | ||||||
| Total current assets | 124,318 | 303,749 | ||||||
| Property, plant and equipment, net | 28,620 | 21,998 | ||||||
| Deferred tax asset | 1,318 | 616 | ||||||
| Other assets | 11,830 | 2,530 | ||||||
| Customer relationships, net | 176,688 | 93,548 | ||||||
| Intellectual property, net | 53,273 | 12,606 | ||||||
| Other intangibles, net | 20,156 | 5,587 | ||||||
| Goodwill | 291,166 | 160,841 | ||||||
| Total assets | $ | 707,369 | $ | 601,475 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 7,897 | $ | 4,473 | ||||
| Accrued payroll and benefits | 14,717 | 9,388 | ||||||
| Unearned revenues | 13,830 | 8,777 | ||||||
| Other accrued expenses | 11,611 | 9,945 | ||||||
| Total current liabilities | 48,055 | 32,583 | ||||||
| Deferred tax liability | 39,224 | 16,275 | ||||||
| Other long-term liabilities | 7,924 | 715 | ||||||
| Credit facility | 49,000 | - | ||||||
| Convertible senior notes, net of discounts and debt issuance costs | 169,365 | 145,675 | ||||||
| Total liabilities | 313,568 | 195,248 | ||||||
| Stockholders’ equity | ||||||||
| Common stock, par value; authorized shares; issued and outstanding, and shares, respectively | 313,460 | 317,652 | ||||||
| Retained earnings | 76,675 | 72,459 | ||||||
| Accumulated other comprehensive income | 3,666 | 16,116 | ||||||
| Total stockholders’ equity | 393,801 | 406,227 | ||||||
| Total liabilities and stockholders’ equity | $ | 707,369 | $ | 601,475 | ||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Income
(In thousands, except per share data)
| Year Ended March 31, |
||||||||||||
| 2022 |
2021 |
2020 |
||||||||||
| Revenues |
||||||||||||
| Product |
$ | 149,422 | $ | 107,028 | $ | 93,401 | ||||||
| Service |
34,913 | 26,909 | 24,286 | |||||||||
| Total revenues |
184,335 | 133,937 | 117,687 | |||||||||
| Cost of revenues |
||||||||||||
| Cost of products |
54,747 | 33,120 | 40,445 | |||||||||
| Cost of services |
20,498 | 13,803 | 11,880 | |||||||||
| Total cost of revenues |
75,245 | 46,923 | 52,325 | |||||||||
| Gross profit |
109,090 | 87,014 | 65,362 | |||||||||
| Operating expenses |
||||||||||||
| Selling |
28,310 | 18,480 | 12,910 | |||||||||
| General and administrative |
60,311 | 45,788 | 38,174 | |||||||||
| Research and development |
15,767 | 10,388 | 6,355 | |||||||||
| Total operating expenses |
104,388 | 74,656 | 57,439 | |||||||||
| Operating income |
4,702 | 12,358 | 7,923 | |||||||||
| Nonoperating (income) expenses |
||||||||||||
| Interest expense and amortization of debt discount |
3,885 | 8,024 | 5,504 | |||||||||
| Other (income) expense, net |
(2,757 | ) | 2,031 | (1,443 | ) | |||||||
| Total nonoperating expense |
1,128 | 10,055 | 4,061 | |||||||||
| Earnings before income taxes |
3,574 | 2,303 | 3,862 | |||||||||
| Income tax expense (benefit) |
1,703 | (971 | ) | 2,084 | ||||||||
| Net income |
$ | 1,871 | $ | 3,274 | $ | 1,778 | ||||||
| Earnings per share |
||||||||||||
| Basic |
$ | 0.36 | $ | 0.66 | $ | 0.42 | ||||||
| Diluted |
$ | 0.35 | $ | 0.64 | $ | 0.41 | ||||||
| Weighted-average common shares outstanding |
||||||||||||
| Basic |
5,212 | 4,975 | 4,200 | |||||||||
| Diluted |
5,335 | 5,124 | 4,371 | |||||||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Comprehensive (Loss) Income
(In thousands except per share data)
| Year Ended March 31, |
||||||||||||
| 2022 |
2021 |
2020 |
||||||||||
| Net income |
$ | 1,871 | $ | 3,274 | $ | 1,778 | ||||||
| Other comprehensive (loss) income |
||||||||||||
| Foreign currency translation adjustments |
(12,450 | ) | 26,485 | (8,367 | ) | |||||||
| Comprehensive (loss) income |
$ | (10,579 | ) | $ | 29,759 | $ | (6,589 | ) | ||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
| Common Stock | ||||||||||||||||||||
| Number of Shares | Amount | Retained Earnings | AOCI* | Total | ||||||||||||||||
| March 31, 2019 | 3,890,138 | $ | 39,823 | $ | 73,303 | $ | (1,815 | ) | $ | 111,311 | ||||||||||
| Proceeds from issuance of common stock, net of issuance costs of $ | 431,250 | 84,995 | - | - | 84,995 | |||||||||||||||
| Proceeds from conversion feature of convertible senior notes, due 2025, net of allocated costs and deferred taxes of $ | - | 22,735 | - | - | 22,735 | |||||||||||||||
| Exercise of stock options and vesting of restricted stock units, net of shares withheld for taxes | 65,752 | 4,945 | - | - | 4,945 | |||||||||||||||
| Dividends paid, $0.64 per share | - | - | (2,722 | ) | - | (2,722 | ) | |||||||||||||
| Stock-based compensation expense | - | 5,525 | - | - | 5,525 | |||||||||||||||
| Currency translation recognized in earnings from the exit of Cold Chain Packaging Division | - | - | - | (187 | ) | (187 | ) | |||||||||||||
| Foreign currency translation | - | - | - | (8,367 | ) | (8,367 | ) | |||||||||||||
| Net income | - | - | 1,778 | - | 1,778 | |||||||||||||||
| March 31, 2020 | 4,387,140 | 158,023 | 72,359 | (10,369 | ) | |||||||||||||||
| Proceeds from the issuance of common stock, net of issuance costs of $ | 690,000 | 145,935 | - | - | 145,935 | |||||||||||||||
| Exercise of stock options and vesting of restricted stock units, net of shares withheld for taxes | 63,428 | 4,426 | - | - | 4,426 | |||||||||||||||
| Dividends paid, $ per share | - | - | (3,165 | ) | - | (3,165 | ) | |||||||||||||
| Stock-based compensation expense | - | 9,268 | - | - | 9,268 | |||||||||||||||
| Foreign currency translation | - | - | - | 26,485 | 26,485 | |||||||||||||||
| Adoption of accounting standards, net | - | - | (9 | ) | - | (9 | ) | |||||||||||||
| Net income | - | - | 3,274 | - | 3,274 | |||||||||||||||
| March 31, 2021 | 5,140,568 | 317,652 | 72,459 | 16,116 | ||||||||||||||||
| Exercise of stock options and vesting of restricted stock units, net of shares withheld for taxes | 125,059 | 7,152 | - | - | 7,152 | |||||||||||||||
| Dividends paid, $ per share | - | - | (3,339 | ) | - | (3,339 | ) | |||||||||||||
| Stock-based compensation expense | - | 11,391 | - | - | 11,391 | |||||||||||||||
| Foreign currency translation | - | - | - | (12,450 | ) | (12,450 | ) | |||||||||||||
| Cumulative adjustment due to adoption of ASU 2020-06 | - | (22,735 | ) | 5,684 | - | (17,051 | ) | |||||||||||||
| Net income | - | - | 1,871 | - | 1,871 | |||||||||||||||
| March 31, 2022 | 5,265,627 | $ | 313,460 | $ | 76,675 | $ | 3,666 | $ | 393,801 | |||||||||||
*Accumulated Other Comprehensive Income (Loss).
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended March 31, |
||||||||||||
| 2022 |
2021 |
2020 |
||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net income |
$ | 1,871 | $ | 3,274 | $ | 1,778 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
25,068 | 17,660 | 12,990 | |||||||||
| Stock-based compensation |
11,391 | 9,268 | 5,525 | |||||||||
| Non-cash interest and debt amortization |
1,029 | 5,397 | 3,314 | |||||||||
| Amortization of step-up in inventory basis |
7,462 | (436 | ) | 8,502 | ||||||||
| Deferred taxes |
128 | (3,503 | ) | (1,971 | ) | |||||||
| Other |
(534 | ) | 161 | (13 | ) | |||||||
| Cash provided by changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable, net |
(6,752 | ) | (647 | ) | (1,665 | ) | ||||||
| Inventories |
(1,045 | ) | 929 | 414 | ||||||||
| Prepaid expenses and other assets |
(3,606 | ) | 2,878 | (432 | ) | |||||||
| Accounts payable |
1,370 | 967 | (61 | ) | ||||||||
| Accrued liabilities and taxes payable |
255 | (317 | ) | (2,147 | ) | |||||||
| Unearned revenues |
2,586 | 1,442 | 754 | |||||||||
| Net cash provided by operating activities |
39,223 | 37,073 | 26,988 | |||||||||
| Cash flows from investing activities: |
||||||||||||
| Acquisitions, net of cash acquired |
(300,793 | ) | - | (184,102 | ) | |||||||
| Purchases of property, plant and equipment |
(4,432 | ) | (1,992 | ) | (1,498 | ) | ||||||
| Proceeds from the sale of assets |
- | - | 15 | |||||||||
| Net cash (used in) investing activities |
(305,225 | ) | (1,992 | ) | (185,585 | ) | ||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from the issuance of debt |
70,000 | - | - | |||||||||
| Payments of debt |
(21,000 | ) | - | (23,000 | ) | |||||||
| Dividends |
(3,339 | ) | (3,165 | ) | (2,722 | ) | ||||||
| Proceeds from the exercise of stock options |
7,152 | 4,426 | 4,945 | |||||||||
| Payments of contingent consideration |
(237 | ) | (304 | ) | (11 | ) | ||||||
| Proceeds from the issuance of common stock, net |
- | 145,935 | 84,995 | |||||||||
| Proceeds from the issuance of convertible senior notes, net |
- | - | 172,500 | |||||||||
| Payment of debt issuance costs |
- | (664 | ) | (5,430 | ) | |||||||
| Net cash provided by financing activities |
52,576 | 146,228 | 231,277 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(1,093 | ) | 1,176 | (1,485 | ) | |||||||
| Net (decrease) increase in cash and cash equivalents |
(214,519 | ) | 182,485 | 71,195 | ||||||||
| Cash and cash equivalents at beginning of period |
263,865 | 81,380 | 10,185 | |||||||||
| Cash and cash equivalents at end of period |
$ | 49,346 | $ | 263,865 | $ | 81,380 | ||||||
| Cash paid for: |
||||||||||||
| Income taxes paid |
$ | 3,048 | $ | 1,367 | $ | 2,634 | ||||||
| Interest paid |
$ | 2,762 | $ | 2,372 | $ | 1,627 |
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Notes to Consolidated Financial Statements
(dollar and share amounts in thousands, unless otherwise specified)
Note 1. Description of Business and Summary of Significant Accounting Policies
Description of Business
In this Annual Report on Form 10-K, Mesa Laboratories, Inc., a Colorado corporation, together with its subsidiaries is collectively referred to as “we,” “us,” “our,” the “Company,” or "Mesa."
We are a multinational manufacturer, developer, and seller of life sciences tools and critical quality control products and services, many of which are sold into niche markets driven by regulatory requirements. We have manufacturing operations in the United States and Europe, and our products are marketed by our sales personnel in North America, Europe, and Asia Pacific, and by independent distributors in these areas as well as throughout the rest of the world. We prefer markets in which we can establish a strong presence and achieve high gross margins.
As described in Note 14. "Segment Data," following the acquisition of Agena Bioscience, Inc. on October 20, 2021, we changed our financial reporting segments to align with strategic shifts in the way we manage our business units. As of March 31, 2022, we managed our operations in four reportable segments, or divisions:
| ● | Sterilization and Disinfection Control - manufactures and sells biological, cleaning, and chemical indicators which are used to assess the effectiveness of sterilization and disinfection processes in the hospital, dental, medical device, and pharmaceutical industries. The division also provides testing and laboratory services, mainly to the dental industry. |
| ● | Biopharmaceutical Development - develops, manufactures, and sells automated systems for protein analysis (immunoassays) and peptide synthesis solutions. Immunoassays and peptide synthesis solutions accelerate the discovery, development, and manufacture of biotherapeutic drugs. Customers include biopharmaceutical research, development, and manufacturing teams at biopharmaceutical companies and academic research and development laboratories. |
| ● | Calibration Solutions - develops, manufactures, and sells quality control and calibration products used to measure or calibrate temperature, pressure, pH, humidity, and other such parameters for health and safety purposes, primarily in hospital, medical device manufacturing, pharmaceutical manufacturing, and various laboratory environments. This division represents a combination of the historical Instruments and Continuous Monitoring reportable segments. |
| ● | Clinical Genomics - develops, manufactures, and sells highly sensitive, low-cost, high-throughput genetic analysis tools used by labs to perform clinical genomic testing in several therapeutic areas such as newborn screenings, pharmacogenetics, and oncology. This division is a new reportable segment comprised entirely of Agena’s operations. For more information on Mesa’s acquisition of Agena, see Note 4. “Significant Transactions.” |
Non-reportable operating segments (including our Cold Chain Packaging division which ceased operations during the year ended March 31, 2020) and unallocated corporate expenses are reported within Corporate and Other.
Principles of Consolidation and Basis of Presentation
Our Consolidated Financial Statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and include our accounts and wholly owned subsidiaries after elimination of all intercompany accounts and transactions. Agena results are consolidated with Mesa's financial statements beginning October 20, 2021, the day of the acquisition. Prior period results have not been recast and are therefore not comparable with the year ending March 31, 2022, except all prior year segment data presented has been reclassified to conform to current year presentation, as described in Note 14. "Segment Data." Our change in financial reporting segments has not resulted in any change to previously reported consolidated amounts.
Prior Period Reclassification
Certain amounts presented in Note 2. "Revenue Recognition" in prior periods of fiscal year 2022 have been reclassified out of revenues from consumables and into revenues from hardware and services. These reclassifications have not resulted in any change to consolidated financial statements for the year ended March 31, 2022.
Management Estimates
The preparation of our Consolidated Financial Statements in conformity with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our Consolidated Financial Statements and accompanying notes. Actual results could differ from our estimates under different assumptions or conditions.
Summary of Significant Accounting Policies
Foreign Currency
Exchange rate adjustments resulting from foreign currency transactions are recognized in net earnings, whereas effects resulting from the translation of financial statements are reflected as a component of accumulated other comprehensive income within stockholders’ equity. Assets and liabilities of subsidiaries operating outside the United States with a functional currency other than the U.S. dollar are translated into U.S. dollars at period end exchange rates, and revenue and expense accounts are translated at weighted average period rates.
Fair Value of Financial Instruments
Fair value is the price we would receive to sell an asset or pay to transfer a liability (exit price) in an orderly transaction between market participants. We determine fair value based on the following input hierarchy:
Level 1: Quoted prices for identical assets or liabilities in active markets.
Level 2: Observable inputs other than prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated with observable market data.
Level 3: Unobservable inputs supported by little or no market activity. Pricing models, discounted cash flow methodologies, and other similar techniques involving significant management judgment or estimation typically require unobservable inputs.
Revenue Recognition
Our revenues come from product sales, which include consumables and hardware; as well as services, which include discrete and ongoing calibration, testing, and maintenance services and contracts. Revenues are recognized when we satisfy our performance obligations under the terms of a contract, which occurs when control of the promised products or services transfers to our customers. We recognize as revenue the amount of consideration we expect to receive in exchange for transferring products or services to our customers (the transaction price). For all revenue contracts, prices are fixed at the time of purchase and no price protections or variables are offered. The significant majority of our revenues and related receivables are generated from contracts with customers that are 12 months or less in duration. We generally recognize revenues as follows:
Product sales: Our performance obligations related to product sales generally consist of the promise to sell tangible goods and integrated software to distributors or end users. Control of these goods is typically transferred upon shipment, at which time our performance obligation is satisfied and revenue is recognized. For products requiring Mesa's personnel to complete installation, control transfers to the customer and revenue is recognized when our technicians have completed the installation at the customer’s location. Purchase orders typically provide evidence of an arrangement for product sales. Products sold include an assurance-type warranty which is accounted for as part of accrued warranty expense.
Services: We generate service revenues from discrete or contracted calibration, testing, and maintenance services performed on our hardware products. Performance obligations arise when discrete services are contracted in advance and performed at a future time, often at the time of the customer’s choosing. In such cases, our performance obligation is satisfied and revenue is recognized upon completion of the specified work. Alternately, performance obligations arising from ongoing service contracts are satisfied by completing any service that is contractually required during the contract period, if requested by the customer, or simply by the passage of time if no services are requested. For ongoing service contracts, revenue is recognized on a straight-line basis over the life of the contract in a faithful depiction of our obligation to provide services over the contract period. Evidence of a service arrangement may be in the form of a formal contract or a purchase order.
Collectability is reasonably assured through our customer review process, and payment is typically due within 60 days or less. Upon adoption of Accounting Standards Codification 606, we elected the practical expedient to expense commission costs as incurred. The substantial majority of our contracts have original durations of one year or less, and we have elected not to disclose the expected timing or allocated transaction prices of future performance obligations. Additionally, we have elected the practical expedient to not assess whether a significant financing component exists when the period between when we perform our performance obligation and when the customer remits payment is one year or less. None of our contracts contained a financing component as of March 31, 2022 or March 31, 2021.
Contracts with customers may contain multiple performance obligations. For such arrangements, the transaction price is allocated to each performance obligation based on the estimated relative standalone selling prices of the promised products or services underlying each performance obligation. Standalone selling prices are based on the price at which the performance obligation is sold separately. If the standalone selling price is not observable through past transactions, we estimate the standalone selling price considering available information such as market conditions and internally approved pricing guidelines. Discounts may be approved at the time of purchase and are included within a contract’s fixed transaction price. Discounts are typically allocated to the performance obligations included in the contract based on the standalone values of such obligations.
Shipping and handling
Payments made by customers to us for shipping and handling costs are included in revenues on the Consolidated Statements of Income, and our expenses are included in cost of revenues. Our performance obligation with respect to shipping and handling consists of a promise to secure such services from a third party on behalf of our customers. Shipping and handling for inventory and materials we purchase is included as a component of inventory on the Consolidated Balance Sheets, and expensed to cost of revenues when products are sold
.
Unearned Revenues
Certain of our products may be sold with associated time-based service contracts whereby we provide repairs, technical support, parts, and various analytical or maintenance services. In the event these contracts are paid in advance by the customer, the associated amounts are recorded as an unearned revenue liability and recognized as revenue ratably over the term of the service period, generally one year.
Accrued Warranty Expense
We typically provide assurance-type limited product warranties on our products and, accordingly, accrue for estimates of related warranty expenses.
Cash and Cash Equivalents
We classify any highly liquid investments with maturities of three months or less at the date of purchase as cash equivalents. All cash equivalents are carried at cost, approximating fair value.
Accounts Receivable and Allowance for Doubtful Accounts
All trade accounts receivable are reported at net realizable value on the accompanying Consolidated Balance Sheets, adjusted for any write-offs and net of allowances for doubtful accounts. Allowances for doubtful accounts represent our best estimate and current expectation of future credit losses from trade accounts. We estimate credit losses based on historical information, current and expected future economic and market conditions, and reviews of the current status of customers’ trade accounts receivable. Customers are pooled based on shared specific risk factors such as historical credit loss patterns. In circumstances in which we become aware of a specific customer’s inability to meet its financial obligations, a specific reserve is recorded against amounts due to reduce the recognized receivable to the amount reasonably expected to be collected. We do not believe our trade accounts receivable represent significant concentrations of credit risk due to our diversified portfolio of individual customers and geographical areas. Differences may arise between estimated and actual losses, which could materially affect the provision for credit losses and, therefore, net earnings. We recorded $304, $100, and $1 of expense associated with doubtful accounts for the years ended March 31, 2022, 2021, and 2020, respectively.
Inventories
Inventories are stated at the lower of cost or net realizable value using a weighted average costing methodology. Inventories acquired in an acquisition are recorded at fair market value. Our work in process and finished goods inventories include the costs of raw materials, labor and overhead, which are estimated based on trailing twelve months of expense and standard labor hours for each product. We evaluate labor and overhead costs annually unless specific circumstances necessitate a mid-year evaluation for specific items.
We monitor inventory costs relative to selling prices and perform physical cycle count procedures on inventories throughout the year to determine if a lower of cost or net realizable value reserve is necessary. We estimate and maintain an inventory reserve as needed for such matters as excess or obsolete inventory, shrinkage, and scrap. This reserve may fluctuate as our assumptions change due to new information, discrete events, or changes in our business, such as entering new markets or discontinuing a specific product; however, once inventory is written down, a new cost basis is established that is not subsequently written back up in future periods.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, except for assets acquired in acquisitions, which are recorded at fair value. Expenditures for major renewals and improvements that extend the life of the asset are capitalized, while expenditures for minor replacements, maintenance, and repairs are expensed as incurred. Depreciation is calculated using the straight-line method over the assets’ estimated useful lives. Upon asset retirement or disposal, accounts are relieved of cost and accumulated depreciation, and any related gain or loss is reflected in our results of operations. For certain business consolidation activities, accelerated depreciation may be required for the revised remaining useful lives of assets designated to be abandoned. At least annually, we evaluate and adjust as necessary the estimated lives of property, plant and equipment. Any changes in estimated useful lives are recorded prospectively. Estimated useful lives of significant classes of depreciable assets are as follows:
| Category | Useful Lives |
| Buildings / Building improvements | |
| Office equipment | |
| Manufacturing equipment | |
| Computer equipment | |
| Leasehold Improvements | Lesser of the economic life or the remaining term in the respective lease |
Land is not depreciated and construction in progress is not depreciated until placed in service, at which time it is assigned a useful life consistent with the nature of the asset.
Leases
Under ASC 842, we determine whether contractual arrangements contain a lease at the inception of the arrangement. If a lease is identified in an arrangement, we recognize a right-of-use asset ("ROU") and liability on our Consolidated Balance Sheets and determine whether the lease should be classified as a finance or operating lease. We do not have any finance leases. We do not recognize assets or liabilities for leases with terms of less than 12 months, and our short-term leases are not material.
A contract is a lease or contains one when (1) the contract contains an explicitly or implicitly identified asset and (2) the customer obtains substantially all of the economic benefits from the use of that underlying asset and directs how and for what purpose the asset is used during the term of the contract in exchange for consideration. Operating lease assets and liabilities are recognized at the lease commencement date. Operating lease liabilities represent the present value of lease payments not yet paid. Operating lease assets represent our right to use an underlying asset and are based upon the operating lease liabilities adjusted for prepayments. Adjustments would also be made for accrued lease payments, initial direct costs, lease incentives, and impairment of operating lease assets, none of which are present in any of our current lease contracts. When readily determinable, the discount rate used to calculate the lease liability is the rate implicit in the lease, otherwise we use our incremental borrowing rate based on the information available at lease commencement. When we acquire a business, we retain the acquiree's classification of its leases. We evaluate the ROU assets and liabilities in accordance with ASC 842.
Our leases typically contain rent escalations over the lease term. We recognize expense for these leases on a straight-line basis over the lease term. Lease expense is recorded in cost of products, selling, general and administrative, or research and development on our Consolidated Statements of Income, depending on the nature of use of the underlying asset. Many of our leases include one or more renewal or termination options exercisable at our discretion, which are included in the determination of the lease term if we are reasonably certain to exercise the option. We have also entered into lease agreements that have variable payments related to certain indexes. Variable lease payments are recognized in the period in which those payments are incurred. All non-lease components are readily identifiable in our lease contract. We account for non-lease components separately from the lease component to which it is related.
Acquired Intangible Assets
Our goodwill and other intangible assets result from acquisitions of existing businesses. Upon acquisition, we record the fair values of identifiable indefinite and definite lived intangible assets using, among other sources of relevant information, independent appraisals, or actuarial or other valuations. Intangible assets affect the amount of future amortization expense and possible impairment charges we may incur.
Goodwill and indefinite lived intangible assets (certain tradenames we intend to renew and continue using indefinitely) are not subject to amortization and are tested for impairment qualitatively, and if necessary, quantitatively, at least annually during the fourth quarter of our fiscal year, or when events or changes in circumstances indicate it may be more likely than not that carrying value exceeds fair value. We perform impairment tests of goodwill at the reporting unit level and tests for other indefinite lived intangible assets at the asset level.
Intangible assets deemed to have definite lives are amortized on a straight-line basis over their useful lives, generally ranging from to years (See Note 6. “Goodwill and Intangible Assets”). We determine the useful lives of finite intangible assets based on the specific facts and circumstances related to each asset, and we evaluate the appropriateness of assigned useful lives at least annually. Factors we consider when determining useful lives include the contractual term of any agreement related to the asset, the historical performance of the asset, our long-term strategy for using the asset, any laws or other local regulations which could impact the useful life of the asset, and economic factors such as competition or specific market conditions. Definite-lived intangible assets are tested for impairment only if events or changes in circumstances indicate that the carrying amount of a long-lived asset or asset group might not be recoverable.
The fair value measurement used in testing intangible asset impairment is typically based on discounted cash flow projection models, using Level 3 inputs. See “Fair Value of Financial Instruments” for a description of input levels. Significant assumptions include, among others, the weighted average cost of capital, net sales growth, and terminal growth rates. In certain cases, management uses other market information when available to estimate fair value. Impairment charges represent the excess carrying amount over estimated fair value. We do not believe our goodwill and other intangible assets are impaired as of March 31, 2022.
Research & Development Costs
We conduct research and development activities for the purpose of developing new products and enhancing the functionality, effectiveness, reliability, and accuracy of existing products. Research and development expense is predominantly comprised of labor costs and third-party consultants, but we may from time to time, purchase in-process research and development with the intention of developing a saleable product. Research and development costs are expensed as incurred.
Convertible Debt
We issue shares in the form of stock options and full-value awards as part of employee compensation pursuant to the Mesa Laboratories, Inc. 2014 Equity Plan (the "2014 Equity Plan") and Mesa Laboratories, Inc. 2021 Equity Incentive Plan (the "2021 Equity Plan" or together, "the Equity Plans").
Stock options and service-based stock awards generally vest equally over a to year term and stock options generally expire after to years. Awards granted to non-employee directors generally vest year from the grant date. We recognize stock-based compensation expense based on the fair value of stock awards at the grant date and recognize the expense over the related service period using a straight line vesting expense schedule. The 2021 Equity plan includes retiree provisions, which result in the acceleration of stock-based compensation for expense for retiree-eligible participants. Compensation expense related to employees eligible to retire and retain full rights to the awards is recognized over the calculated service period required to earn the award according to the plan provisions.
The fair value of each granted stock option is estimated on the grant date using the Black-Scholes option valuation model. The assumptions used to calculate the fair value of granted options reflect market conditions and our historical experience. We estimate forfeitures using a dynamic forfeiture model based on historical data when determining the amount of stock-based compensation costs to recognize each period.
Restricted stock units ("RSUs") issued by us are equivalent to nonvested shares under the applicable accounting guidance. The fair value of RSUs is based on the closing price of Mesa's common stock on the award date, less the present value of expected dividends not received during the vesting period.
Expense for performance-based RSUs ("PSUs") is recognized when it is probable the performance goal will be achieved. Performance goals are determined by the Board of Directors and may include measures such as revenues growth and profitability targets. Compensation expense on stock awards subject to performance conditions is recognized over the longer of the estimated performance goal attainment period or time vesting period. As of each reporting period, we estimate the number of PSUs expected to vest based on our current estimate of performance compared to the target metrics in the award documents, and if necessary, a cumulative-effect adjustment is recorded.
We allocate stock-based compensation expense to cost of revenues, selling, research and development, and general and administrative expense in the Consolidated Statements of Income.
Earnings Per Share
Basic earnings per share (“EPS”) is computed by dividing net income by the weighted-average number of common shares outstanding during the reporting period. Diluted earnings per share (“diluted EPS”) is computed similarly to basic earnings per share, except it includes the effects of potential common shares related to stock options, restricted stock units, performance share units, and convertible debt in periods in which such effects are dilutive. Potentially dilutive securities are excluded from the calculation of diluted EPS in the event they are subject to performance conditions that have not yet been achieved. See Note 10. “Earnings per Share” for EPS calculations for the years ended March 31, 2022, 2021 and 2020.
Income Taxes
Income tax expense includes U.S., state, local and international income taxes, plus a provision for U.S. taxes on undistributed earnings of foreign subsidiaries and other prescribed foreign entities not deemed to be indefinitely reinvested. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting basis and the tax basis of existing assets and liabilities. The tax rate used to determine the deferred tax assets and liabilities is based on the enacted tax rate for the year and the manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
We are involved in various tax matters, some of which have uncertain outcomes. We establish reserves to remove some or all of the tax benefits related to our tax positions at the time we determine one of the following conditions exists: (1) the tax position is not “more likely than not” to be sustained, (2) the tax position is “more likely than not” to be sustained, but for a lesser amount, or (3) the tax position is “more likely than not” to be sustained, but not in the financial period in which the tax position was originally taken. For purposes of evaluating whether a tax position is uncertain, (1) we presume the tax position will be examined by the relevant taxing authority that has full knowledge of all relevant information; (2) the technical merits of a tax position are derived from authorities such as legislation and statutes, legislative intent, regulations, rulings and case law and their applicability to the facts and circumstances of the tax position; and (3) each tax position is evaluated without consideration of the possibility of offset or aggregation with other tax positions taken. A number of years may elapse before a particular uncertain tax position is audited and finally resolved or when a tax assessment is raised. The number of years subject to tax assessments varies depending on the tax jurisdiction. A tax benefit that has been previously reserved because of a failure to meet the “more likely than not” recognition threshold would be recognized in income tax expense in the first period when the uncertainty disappears under any of the following conditions: (1) the tax position is “more likely than not” to be sustained, (2) the tax position, amount, and/or timing is ultimately settled through negotiation or litigation, or (3) the statute of limitations for the tax position has expired (See Note 12. “Income Taxes”).
Acquisition Related Contingent Consideration Liabilities
Acquisition related contingent consideration liabilities consist of estimated amounts due under various acquisition agreements and are typically based on either revenues growth or specified profitability growth metrics. At each reporting period, we evaluate the expected future payments and the associated discount rate to determine the fair value of the contingent consideration, and we record any necessary adjustments in other expense, net on the Consolidated Statements of Income. As of March 31, 2022, there are no outstanding contingent consideration liabilities.
Legal Contingencies
We are party to various claims and legal proceedings that arise in the normal course of business. We record an accrual for legal contingencies when we determine it is probable we have incurred a liability and can reasonably estimate the amount of the loss (See Note 13. “Commitments and Contingencies”).
Purchase Accounting for Acquisitions
We account for all business combinations in which we obtain control over another entity using the acquisition method of accounting, which requires most assets (both tangible and intangible) and liabilities (including contingent consideration) to be recognized at fair value at the date of acquisition. The excess of the purchase price over the fair value of assets less liabilities is recognized as goodwill. We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses. These types of analyses require us to make and monitor assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, discount rates and cash flow. Certain adjustments to the assessed fair values of acquired assets or liabilities made subsequent to the acquisition date but within the measurement period are recorded as adjustments to goodwill. Any adjustments subsequent to the measurement period are recorded within earnings. We expense all acquisition costs as incurred related to an acquisition in selling, general, and administrative expenses.
Results of operations of the acquired company are included in our Consolidated Financial Statements from the date of the acquisition forward. If actual results are not consistent with our assumptions and estimates, or if our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future. For the years ended March 31, 2022, 2021 and 2020, our acquisitions of businesses (net of cash acquired) totaled $300,793, $0, and $184,102 respectively.
Business Consolidation Costs
We estimate our liabilities for business closure activities by gathering detailed estimates of costs and, if applicable, asset sale proceeds, for each business consolidation initiative. For a typical business consolidation initiative, we estimate costs of employee severance, impairment of property and equipment and other assets including estimating net realizable value, if necessary, accelerated depreciation, termination payments for contracts and leases, and any other qualifying costs related to the exit plan. Such charges represent our best estimates; however, they require assumptions about plans that may change over time. The estimated costs are grouped by specific projects within the overall exit plan and are monitored at each reporting period, and any subsequent change to the original estimate is recorded in current earnings.
Risks and Uncertainties
The preparation of financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities at the reporting date and revenues and expenses during the reporting periods. These estimates represent management's judgement about the outcome of future events. The current global business environment continues to be impacted directly and indirectly by the effects of the novel coronavirus ("COVID-19"), the conflict in Ukraine, and other factors. It is not possible to accurately predict the future impact of such events and circumstances. However, we have reviewed the estimates used in preparing the financial statements and have identified the following factors that have a reasonable possibility of being materially affected in the near term:
| ● | Estimates regarding the future financial performance of the business used in the impairment tests for goodwill and long-lived assets acquired in a business combination; however, our impairment test conducted during the quarter ended March 31, 2022 concluded that goodwill is impaired; |
| ● | Estimates regarding the recoverability of deferred tax assets and estimates regarding cash needs and associated indefinite reinvestment assertions; |
| ● | Estimates regarding recoverability for customer receivables; |
| ● | Estimates of the net realizable value of inventory. |
Recently Issued Accounting Pronouncements
We have reviewed all recently issued accounting pronouncements and have concluded that they are either not applicable to us or are not expected to have a significant impact on our consolidated financial statements.
Recently Adopted Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update No. 2020-06, Debt with Conversion and Other Options and Derivatives and Hedging Accounting for Convertible Instruments and Contracts in an Entity's Own Equity ("ASU 2020-06"), which simplifies the accounting for certain financial instruments with characteristics of both liabilities and equity, such as the Notes due 2025. ASU 2020-06 also enhances transparency and improves disclosures for convertible instruments and earnings per share guidance. It is effective for annual reporting periods beginning after December 15, 2021, including interim periods within those fiscal years. Early adoption is permitted at the beginning of any fiscal year after December 15, 2020. The update permits the use of either the modified retrospective or full retrospective method of transition.
We early adopted ASU 2020-06 effective April 1, 2021 on a modified retrospective basis, and our adoption of this standard had a material effect on our consolidated financial statements. Upon adoption, we derecognized the $22,735 equity conversion feature, net of taxes, that was recorded to common stock, and we derecognized the deferred tax liability of $5,747. We recorded an increase of $22,799 in aggregate to the Notes balance as a result of the reversal of the separation of the debt and equity components of the convertible debt. The net effect of these adjustments, which represents $5,683 of historical non-cash interest expense, net of taxes, was recorded as an increase in the balance of beginning retained earnings as of April 1, 2021. The adoption of this standard has significantly decreased the amount of non-cash interest expense recognized in our Consolidated Statement of Income as a result of eliminating the discount associated with the equity component. Our statements of cash flows reflect the lower non-cash interest expense in effect after the adoption of ASU 2020-06.
In each period in which the Notes have been outstanding, we have always intended to settle the Notes in shares of common stock rather than in cash, and therefore, we have applied the if-converted method to calculate the potentially dilutive impact of the Notes on earnings per share. In each reporting period, we have determined that the Notes were antidilutive. Due to decreases in non-cash interest expense that will result from the adoption of ASU 2020-06, it is likely the Notes will have a dilutive effect in future periods, which would decrease our diluted earnings per share.
On October 28, 2021, the FASB issued Accounting Standard Update No. 2021-08 ("ASU 2021-08"), Accounting for Contract Assets and Contract Liabilities from Contracts with Customers, which amends ASC 805 to require acquiring entities to apply ASC 606 to recognize and measure contract assets and contract liabilities acquired in a business combination. Prior to adoption, an acquirer generally recognized such items at fair value on acquisition date.
We early adopted ASU 2021-08 upon its issuance effective October 28, 2021 and applied the amendments retrospectively to the Agena Acquisition. As a result of adopting ASU 2021-08, we recognized Agena's deferred revenue at its recorded book value rather than at fair value, after determining that Agena's application of ASC 606 was appropriate and the underlying accounting for deferred revenue included no material errors.
Note 2. Revenue Recognition
We develop, manufacture, market, sell, and maintain life sciences tools and quality control instruments and related software, consumables, and services.
Sales of hardware and software, such as instruments used for molecular and genetic analysis, protein synthesizers, medical meters, wireless sensor systems, and data loggers, are generally driven by our acquisition of new customers, growth of existing customers, or customers replacing existing equipment. Hardware sales may be offered with accompanying software licenses, which in some cases are required for the hardware to function. We also offer discrete and ongoing service and maintenance contracts on our instruments.
Consumables are typically used on a one-time basis and require frequent replacement in our customers' operating cycles. Some of our consumables, such as biological indicator test strips, are used on a standalone basis. Others, including reagents used for molecular and genetic analysis and solutions used for protein synthesis and instrument calibrations, are critical to the ongoing use of our instruments.
Revenues from our new Clinical Genomics segment are derived from our recently acquired Agena business (See Note 4. "Significant Transactions"). These revenues consist of sales of instruments and consumables used in molecular and genetic analysis, as well as sales of discrete and contracted instrument maintenance agreements.
We evaluate our revenues internally based on operating segment, the timing of revenue generation, and the nature of goods and services provided. Typically, discrete revenues are recognized at shipping point or upon completion of a service, while contracted revenues are recognized over time based on the performance obligation period in the applicable contract. The significant majority of our revenues and related receivables are generated from contracts with customers that are 12 months or less in duration.
The following tables present disaggregated revenues for the years ended March 31, 2022, 2021 and 2020:
| Year Ended March 31, 2022 | ||||||||||||||||||||||||
| Sterilization and Disinfection Control | Biopharmaceutical Development | Calibration Solutions | Clinical Genomics (1) | Corporate and Other | Total | |||||||||||||||||||
| Discrete Revenues | ||||||||||||||||||||||||
| Consumables | $ | 50,311 | $ | 15,551 | $ | 3,675 | $ | 22,271 | $ | - | $ | 91,808 | ||||||||||||
| Hardware and Software | 700 | 21,651 | 28,537 | 6,726 | - | 57,614 | ||||||||||||||||||
| Services | 2,225 | 3,864 | 11,212 | 1,796 | - | 19,097 | ||||||||||||||||||
| Contracted Revenues | ||||||||||||||||||||||||
| Services | 5,808 | 4,513 | 3,448 | 2,047 | - | 15,816 | ||||||||||||||||||
| Total Revenues | $ | 59,044 | $ | 45,579 | $ | 46,872 | $ | 32,840 | $ | - | $ | 184,335 | ||||||||||||
| Year Ended March 31, 2021 | ||||||||||||||||||||||||
| Sterilization and Disinfection Control | Biopharmaceutical Development | Calibration Solutions | Clinical Genomics (1) | Corporate and Other | Total | |||||||||||||||||||
| Discrete Revenues | ||||||||||||||||||||||||
| Consumables | $ | 45,869 | $ | 13,942 | $ | 3,198 | $ | - | $ | - | $ | 63,009 | ||||||||||||
| Hardware and Software | 505 | 13,545 | 29,969 | - | - | 44,019 | ||||||||||||||||||
| Services | 1,848 | 2,928 | 10,850 | - | - | 15,626 | ||||||||||||||||||
| Contracted Revenues | ||||||||||||||||||||||||
| Services | 4,897 | 3,477 | 2,909 | - | - | 11,283 | ||||||||||||||||||
| Total Revenues | $ | 53,119 | $ | 33,892 | $ | 46,926 | $ | - | $ | - | $ | 133,937 | ||||||||||||
| Year Ended March 31, 2020 | ||||||||||||||||||||||||
| Sterilization and Disinfection Control | Biopharmaceutical Development (2) | Calibration Solutions | Clinical Genomics (1) | Corporate and Other | Total | |||||||||||||||||||
| Discrete Revenues | ||||||||||||||||||||||||
| Consumables | $ | 42,654 | $ | 4,981 | $ | 3,240 | $ | - | $ | 2,436 | $ | 53,311 | ||||||||||||
| Hardware and Software | 551 | 6,015 | 33,524 | - | - | 40,090 | ||||||||||||||||||
| Services | 1,592 | 1,761 | 11,556 | - | 27 | 14,936 | ||||||||||||||||||
| Contracted Revenues | ||||||||||||||||||||||||
| Services | 4,863 | 1,094 | 3,393 | - | - | 9,350 | ||||||||||||||||||
| Total Revenues | $ | 49,660 | $ | 13,851 | $ | 51,713 | $ | - | $ | 2,463 | $ | 117,687 | ||||||||||||
(1) Revenues in the Clinical Genomics division represent transactions subsequent to the Agena Acquisition on October 20, 2021.
(2) Revenues in the Biopharmaceutical Development division represent transactions subsequent to the acquisition of Gyros Protein Technologies Holding AB on October 31, 2019.
Contract Balances
Our contracts have varying payment terms and conditions. Some customers prepay for products and services, resulting in either unearned revenues or customer deposits, called contract liabilities, which are included within unearned revenues, other accrued expenses, and other long-term liabilities in the accompanying Consolidated Balance Sheets. Contract assets would exist when sales are recorded (for example, the control of the goods or services has been transferred to the customer), but customer payment is contingent on a future event besides the passage of time (such as satisfaction of additional performance obligations). We do have any contract assets. Unbilled receivables, which are not classified as contract assets, represent arrangements in which sales have been recorded prior to billing and our right to payment is unconditional.
A summary of contract liabilities is as follows:
| Contract liabilities as of March 31, 2021 | $ | 8,994 | ||
| Prior year liabilities recognized in revenues during the year ended March 31, 2022 | (5,791 | ) | ||
| Contract liabilities added during the year ended March 31, 2022, net of revenues recognized | 11,866 | |||
| Contract liabilities balance as of March 31, 2022 | $ | 15,069 |
Contract liabilities primarily relate to service contracts with original expected durations of 12 months or less and will be recognized to revenue as time passes. Contract liabilities of added during the year ended March 31, 2022 are attributable to the acquisition of Agena. See Note 4. "Significant Transactions."
Note 3. Fair Value Measurements
Our financial instruments consist primarily of cash and cash equivalents, trade accounts receivable, obligations under trade accounts payable, and debt. Due to their short-term nature, the carrying values for cash and cash equivalents, trade accounts receivable, and trade accounts payable approximate fair value. Cash equivalents on our Consolidated Balance Sheets consisted of $0 held in a money market account as of March 31, 2022, compared to $230,822 held in a money market account as of March 31, 2021. We used the money market funds for the Agena Acquisition, see Note 4. "Significant Transactions." We measure our cash equivalents at fair value using quoted market prices in an active market, and we classify them within Level 1 of the fair value hierarchy.
Historically, the financial instruments that subject us to the highest concentration of credit risk are cash and cash equivalents and accounts receivable. It is our policy to invest in highly liquid cash equivalent financial instruments with high credit ratings and to maintain low single issuer exposure (except U.S. treasuries). Concentration of credit risk with respect to accounts receivable is limited to customers to which we make significant sales. To manage credit risk, we consider the creditworthiness of new and existing customers, and we regularly review outstanding balances and payment histories. We may require pre-payments from customers under certain circumstances and may limit future purchases until payments are made on past due amounts. We reserve an allowance for potential write-offs of accounts receivable, but we have not written off any significant accounts to date.
We have outstanding $172,500 aggregate principal of 1.375% convertible senior notes due August 15, 2025. We estimate the fair value of the Notes based on the last actively traded price or observable market input preceding the end of the reporting period. The estimated fair value and carrying value of the Notes were as follows:
| March 31, 2022 | March 31, 2021 | |||||||||||||||
| Carrying Value | Fair Value (Level 2) | Carrying Value | Fair Value (Level 2) | |||||||||||||
| Notes | $ | 169,365 | $ | 185,438 | $ | 145,675 | $ | 188,780 | ||||||||
The carrying value of the Notes increased as a result of the adoption of ASU 2020-06, discussed further in Note 1. "Description of Business and Summary of Significant Accounting Policies" and Note 8. "Indebtedness."
Assets recognized or disclosed at fair value in the Consolidated Financial Statements on a nonrecurring basis include items such as property and equipment, operating lease assets, goodwill, and other intangible assets, including those that were part of the Agena Acquisition. These assets are measured at fair value if determined to be impaired. Preliminary fair values assigned to assets acquired and liabilities assumed in the Agena Acquisition, except deferred revenues, were measured using Level 3 inputs, as discussed further in Note 4. "Significant Transactions." There were no transfers between the levels of the fair value hierarchy during the fiscal years ended March 31, 2022 and March 31, 2021.
Note 4. Significant Transactions
Acquisitions
Acquisition of Agena Bioscience, Inc.
On October 20, 2021, we completed the acquisition of Agena Bioscience, Inc., which aligned with our overall acquisition strategy, moved our business towards the life sciences tools sector, and expanded our market opportunities, particularly in Asia. Agena is a leading clinical genomics tools company that develops, manufactures, markets, and supports proprietary instruments and related consumables and services that enable genetic analysis for a broad range of diagnostic and research applications. Using Agena's MassARRAY® instruments and chemical reagent solutions, customers can analyze DNA samples for a variety of high volume clinical testing applications, such as inherited genetic disease testing, pharmacogenetics, various oncology tests, infectious disease testing, and other highly-differentiated applications. Agena sells its products primarily to clinical labs, including large specialty, reference and pathology labs, as well as a variety of academic, hospital, and government facilities. Agena’s products are marketed directly to laboratories as well as to in vitro diagnostic development partners globally. Agena's products are differentiated in the market because they combine the throughput and analytical capabilities of mass spectrometry with the flexibility, ease-of-use and cost advantages of PCR methods.
We funded the acquisition and transactions relating thereto with cash on hand and borrowings under the Credit Facility. See Note 8. "Indebtedness" for additional details regarding the Credit Facility. At the completion of the Agena Acquisition on October 20, 2021, each Agena common share issued and outstanding was converted into the right to receive $5.96 per share in cash, subject to adjustment, without interest. We paid $300,793, net of cash acquired, but inclusive of working capital adjustments, to complete the Agena Acquisition. Of the cash consideration we paid, approximately $267,000 represented cash consideration to holders of Agena’s preferred and common stock, approximately $2,000 represented cash consideration paid for the settlement of Agena’s warrants, and approximately $31,800 represented cash consideration for the settlement of Agena's vested stock options as of the closing date.
Preliminary Allocation of Purchase Price
We accounted for the Agena Acquisition as a business combination using the acquisition method of accounting. Under the acquisition method of accounting, the acquiree's identifiable assets acquired and liabilities assumed are recorded at their acquisition date fair values, except contract assets and liabilities recorded at book value in accordance with ASU 2021-08, and are consolidated with those of Mesa. Significant judgments and estimates are required when performing valuations. The relief from royalty method was used to value our trade names and developed technology, while the multi-period excess earnings method, a form of the income approach, was used to value our customer relationships. These methods involve the use of significant estimates and assumptions depending on the underlying asset being valued, but may include internal rate of return, revenue growth rates, customer attrition rate, and royalty rates, all of which are considered Level 3 inputs. We obtained the information used to prepare the preliminary valuation during due diligence and from other sources. These estimates were based on assumptions that we believe to be reasonable; however, actual results may differ from these estimates. Some of these estimates, especially customer attrition and internal rate of return are highly sensitive and a small change in estimate could materially change the calculated value of intangibles.
During the quarter ended March 31, 2022, we continued refining the valuation of net assets acquired in the Agena Acquisition. The significant purchase price allocation changes during quarter ended March 31, 2022 included: a net decrease of $4,300 in the value of intangible assets; an increase of $1,400 in the value of the inventory step-up; and a decrease of $1,144 in the value of property, plant and equipment, net. We also made adjustments to deferred tax assets and deferred tax liabilities primarily due to the tax effect of these changes to the purchase price allocation. In addition to changes to valuation of intangible assets, we reassessed our estimate of the remaining useful lives of intangible assets and property, plant and equipment acquired. The net effect of the changes to the expected remaining useful life and the intangible asset valuation was a cumulative net increase to amortization expense amounting to $1,932, of which $472 of expense was recorded to cost of revenues and $1,460 was recorded in general and administrative costs during the quarter ended March 31, 2022.
The following table summarizes the allocation of the preliminary purchase price as of October 20, 2021:
| Life (in years) | Amount | |||||||
| Cash and cash equivalents | $ | 7,544 | ||||||
| Accounts receivable (a) | 11,100 | |||||||
| Other current assets (b) | 25,480 | |||||||
| Total current assets | 44,124 | |||||||
| Property, plant and equipment/noncurrent assets | 15,832 | |||||||
| Deferred tax asset | 811 | |||||||
| Intangible assets: | ||||||||
| Goodwill (c) | N/A | 135,880 | ||||||
| Customer relationships (d) | 12 | 103,800 | ||||||
| Intellectual property (d) | 8 | 45,400 | ||||||
| Tradenames (d) | 12 | 15,700 | ||||||
| Total Assets acquired | $ | 361,547 | ||||||
| Accounts payable | 2,174 | |||||||
| Unearned revenues | 2,713 | |||||||
| Other current liabilities | 12,295 | |||||||
| Total current liabilities | 17,182 | |||||||
| Deferred tax liability | 27,765 | |||||||
| Other noncurrent liabilities | 8,263 | |||||||
| Total liabilities assumed | $ | 53,210 | ||||||
| Total purchase price, net of cash acquired | $ | |||||||
(a) Trade receivables, which is expected to be collected.
(b) Includes $7,462 of inventory step-up, which was amortized entirely within fiscal year 2022. Our evaluation of the valuation of inventory was complete as of March 31, 2022.
(c) Acquired goodwill of $135,880, all of which is allocated to the Clinical Genomics reportable segment, represents the value expected to arise from the value of expanded market opportunities, expected synergies, and assembled workforce, none of which qualify as amortizable intangible assets. The goodwill acquired is not deductible for income tax purposes.
(d) Customer relationships, intellectual property, and tradenames are currently expected to be amortized on a straight line basis over a weighted average 10.9 year period. The identified intangible assets will be amortized on a straight line basis over their useful lives, which approximates the pattern over which the assets' economic benefits are expected to be consumed over time. Amortization expense for customer relationships and tradenames will be amortized to general and administrative expenses; amortization expense for intellectual property will be recorded to cost of revenues. During the period from October 20, 2021 until March 31, 2022, $4,454 of amortization expense was recorded to general and administrative costs and $2,538 of amortization expense was recorded to cost of revenues in the Clinical Genomics Division, including the cumulative effect catch up. Our valuation of intangible assets is considered to be complete as of March 31, 2022. Going forward, we expect to record amortization expense of $2,490 and $1,419 to general and administrative costs and costs of revenues, respectively, each quarter.
This preliminary purchase price allocation is subject to revision as more detailed analyses are completed with respect to prepaid taxes, tax accruals, and deferred tax positions. If additional information about the fair value of assets acquired and liabilities assumed becomes available, we may further revise the preliminary purchase price allocation as soon as is practical, but will not do so more than one year from the acquisition date. Only items identified as of the acquisition date are considered for subsequent adjustment. Any such revisions or changes may be material.
Acquisition-related costs, such as legal and advisory fees of $1,244 for the year ended March 31, 2022, are not included as a component of consideration transferred, but are expensed in the periods in which the costs are incurred and are reflected on the Consolidated Statements of Income in general and administrative expenses.
Unaudited Pro Forma Information
Agena's operations contributed $32,840 to revenues and ($7,779) of net loss to our consolidated results during fiscal year 2022, including the inventory-step up amounting to $7,462 that was fully amortized in fiscal year 2022 and $1,949 of additional intangible assets amortization related to the application of purchase accounting. We included the operating results of Agena in our Consolidated Statements of Income beginning on October 20, 2021, the acquisition date. The following pro forma financial information presents the combined results of operations of Mesa and Agena as if the acquisition had occurred on April 1, 2020 after giving effect to certain pro forma adjustments. The pro forma adjustments reflected only include those adjustments that are directly attributable to the Agena Acquisition, are factually supportable and have a recurring impact; they do not reflect any adjustments for anticipated expense savings resulting from the acquisition and are not necessarily indicative of the operating results that would have actually occurred had the transaction been consummated on April 1, 2020 or of future results.
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Pro forma total revenues (1) | $ | $ | ||||||
| Pro forma net income (2) | 6,193 | (3,879 | ) | |||||
(1) Net revenues were adjusted to include net revenues of Agena.
(2) Pro forma adjustments to net earnings attributable to Mesa include the following:
| ● | Excludes acquisition-related transaction costs incurred in the year ended March 31, 2022. |
| ● | Excludes interest expense attributable to Agena external debt that was paid off as part of the acquisition. |
| ● | Amortization expense of $15,636 for the years ended March 31, 2022 and 2021, respectively, based on the fair value of amortizable intangible assets acquired. |
| ● | $7,462 was excluded from the year ended March 31, 2022 based on the step up value of inventory which would have been fully amortized within the first six months of the acquisition. Additional charge to cost of revenues of was included in the year ended March 31, 2021 based on the step up value of inventory. |
| ● | Additional stock based compensation expense representing expense for performance share units awarded to certain key Agena employees. |
| ● | Income tax effect of applicable adjustments made at a blended federal and state statutory rate (approximately 26%). |
GPT Acquisition
On October 31, 2019, we completed the acquisition of 100% of the outstanding shares of GPT, which comprises our Biopharmaceutical Development segment. The acquisition of GPT expanded our presence into a new market, immunoassays and peptide synthesis solutions that accelerate the discovery, development, and manufacture of biotherapeutic drugs. GPT systems include laboratory instruments, consumables, kits, and software that maximize laboratory productivity by miniaturizing and automating immunoassays at the nanoliter scale. GPT's protein detection is used most frequently by pharmaceutical and biotech companies that are developing protein-based drugs. This division also provides instruments, consumables, and software for the chemical synthesis of peptides from amino acids which are used in the discovery of new peptide-based drug therapies. After adjustments, we paid cash consideration of $181,547 to the sellers in the transaction. The acquisition was considered a stock purchase for tax purposes.
IBP Acquisition
On April 1, 2019, we completed a business combination whereby we acquired all of the common stock of IBP Medical GmbH, a company whose business manufactures medical meters used to test various parameters of dialysis fluid (dialysate) and the proper calibration and operation of dialysis machines.
Restructuring
Butler, New Jersey
We completed the previously announced closure of our Butler, New Jersey facility during the year ended March 31, 2022. The facility was primarily used in the production of our gas flow calibration and air sampling equipment, which is part of our Calibration Solutions division. Our manufacturing facility in Lakewood, Colorado is currently undergoing renovations that will allow it to better accommodate the production of the gas flow calibration and air sampling equipment. Consolidating the production of these products is expected to reduce facilities costs and streamline our use of lean manufacturing tools under central management to further encourage production efficiencies. As a result of the facility consolidation, we incurred $77 of severance costs during the year ended March 31, 2022, which were recorded to cost of revenues, selling, and general and administrative expense on the Consolidated Statement of Income. As of March 31, 2022, there were no outstanding accrued costs, and we do not expect to incur any material expenses related to the Butler, New Jersey facility closure in future periods.
Note 5. Leases
We have operating leases for buildings and office equipment. The following table presents the lease balances within the Consolidated Balance Sheets related to our operating leases:
| Lease Assets and Liabilities | Balance Sheet Location | March 31, 2022 | March 31, 2021 | ||||||
| Operating lease ROU asset | Other assets | $ | 10,201 | $ | 1,801 | ||||
| Current operating lease liabilities | Other accrued expenses | 2,768 | 1,023 | ||||||
| Noncurrent operating lease liabilities | Other long-term liabilities | 7,436 | 677 | ||||||
Operating lease right of use assets and liabilities increased significantly during the year ended March 31, 2022 due to the Agena Acquisition. See Note 4. "Significant Transactions" for details. We accounted for the property leases acquired as part of our acquisition of Agena by measuring the lease liability at the present value of the remaining lease payments as if the acquired lease were a new lease for Mesa. These properties are used for office, laboratory, and manufacturing space.
The components of lease costs, the weighted average remaining lease term and the weighted average discount rate were as follows:
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating lease expense | $ | 1,973 | $ | 1,130 | ||||
| Variable lease expense | 419 | 272 | ||||||
| Total lease expense | $ | 2,392 | $ | 1,402 | ||||
| Weighted average remaining lease term in years | 4.3 | 1.8 | ||||||
| Weighted average discount rate | 1.7 | % | 3.3 | % | ||||
The weighted average discount rate on operating leases declined significantly as a result of the new leases acquired in the Agena Acquisition. These new lease ROU assets and liabilities were calculated using lower discount rates than leases commenced prior to fiscal year 2022.
Supplemental cash flow information related to leases was as follows:
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash paid for amounts included in the measurements of lease liabilities | $ | 1,896 | $ | 1,192 | ||||
| Operating lease assets obtained in exchange for operating lease obligations | 10,577 | 558 | ||||||
Maturities of lease liabilities are as follows as for the years ending March 31:
| 2023 | $ | 2,905 | ||
| 2024 | 2,195 | |||
| 2025 | 1,999 | |||
| 2026 | 1,954 | |||
| 2027 | 1,490 | |||
| Future value of lease liabilities | 10,543 | |||
| Less: imputed interest | 339 | |||
| Present value of lease liabilities | $ | 10,204 |
Note 6. Goodwill and Intangible Assets
Goodwill arises from the excess purchase price of acquired businesses over the fair value of acquired tangible and intangible assets, less assumed liabilities.
The change in the carrying amount of goodwill was as follows:
| Sterilization and Disinfection Control | Biopharmaceutical Development | Calibration Solutions | Clinical Genomics | Total | ||||||||||||||||
| March 31, 2020 | $ | 29,594 | 74,716 | $ | 37,226 | $ | - | $ | 141,536 | |||||||||||
| Effect of foreign currency translation | 559 | 10,715 | 63 | - | 11,337 | |||||||||||||||
| Goodwill related to GPT acquisition | - | 7,968 | - | - | 7,968 | |||||||||||||||
| March 31, 2021 | $ | 30,153 | $ | 93,399 | $ | 37,289 | $ | - | $ | 160,841 | ||||||||||
| Effect of foreign currency translation | (403 | ) | (5,134 | ) | (52 | ) | 34 | (5,555 | ) | |||||||||||
| Goodwill related to Agena acquisition | - | - | - | 135,880 | 135,880 | |||||||||||||||
| March 31, 2022 | $ | 29,750 | $ | 88,265 | $ | 37,237 | $ | 135,914 | $ | 291,166 | ||||||||||
Other intangible assets were as follows:
| March 31, 2022 | March 31, 2021 | |||||||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | |||||||||||||||||||
| Customer relationships | $ | 244,157 | $ | (67,469 | ) | $ | 176,688 | $ | 145,754 | $ | (52,206 | ) | $ | 93,548 | ||||||||||
| Intellectual property | 65,893 | (12,620 | ) | 53,273 | 21,201 | (8,595 | ) | 12,606 | ||||||||||||||||
| Other Intangibles | 25,350 | (5,194 | ) | 20,156 | 9,911 | (4,324 | ) | 5,587 | ||||||||||||||||
| Total | $ | 335,400 | $ | (85,283 | ) | $ | 250,117 | $ | 176,866 | $ | (65,125 | ) | $ | 111,741 | ||||||||||
The increase in the goodwill and intangible assets balance from March 31, 2021 to March 31, 2022 is related to the Agena Acquisition, partially offset by changes in foreign currency rates. See Note 4. "Significant Transactions" for more information.
The range of useful lives and weighted-average remaining useful lives of amortizable intangible assets as of March 31, 2022 were as follows:
| Est. Useful | Weighted Avg. | ||||||||
| Life | Remaining Life | ||||||||
| Description | (Years) | (Years) | |||||||
| Customer Relationships | 5 - 15 | 8.2 | |||||||
| Intellectual Property | 5 - 15 | 7.4 | |||||||
| Other Intangibles | 5 - 15 | 11.4 | |||||||
The following is estimated amortization expense for the years ending March 31:
| 2023 | 29,745 | |||
| 2024 | 29,229 | |||
| 2025 | 27,645 | |||
| 2026 | 26,873 | |||
| 2027 | 26,364 |
Amortization expense of intangibles acquired in a business combination for the years ended March 31, 2022, 2021 and 2020 was $21,806, $14,513, and $10,637 respectively.
Note 7. Supplemental Balance Sheets Information
Accrued payroll and benefits consisted of the following:
| March 31, 2022 | March 31, 2021 | |||||||
| Bonus payable | $ | 7,468 | $ | 3,504 | ||||
| Wages and paid-time-off payable | 3,677 | 3,562 | ||||||
| Payroll related taxes | 2,069 | 2,043 | ||||||
| Other benefits payable | 1,503 | 279 | ||||||
| Total accrued payroll and benefits | $ | 14,717 | $ | 9,388 | ||||
Other accrued expenses consisted of the following:
| March 31, 2022 | March 31, 2021 | |||||||
| Accrued business taxes | $ | 4,967 | $ | 4,749 | ||||
| Current operating lease liabilities | 2,768 | 1,023 | ||||||
| Customer deposits | 751 | 514 | ||||||
| Income taxes payable | 928 | 1,648 | ||||||
| Other | 2,197 | 2,011 | ||||||
| Total other accrued expenses | $ | 11,611 | $ | 9,945 | ||||
Property, plant and equipment consisted of the following:
| March 31, 2022 | March 31, 2021 | |||||||
| Land | $ | 889 | $ | 889 | ||||
| Buildings | 21,537 | 18,857 | ||||||
| Manufacturing equipment | 17,336 | 12,163 | ||||||
| Computer equipment | 4,519 | 4,350 | ||||||
| Construction in progress | 487 | 985 | ||||||
| Other | 1,578 | 1,084 | ||||||
| Gross total | 46,346 | 38,328 | ||||||
| Accumulated depreciation | (17,726 | ) | (16,330 | ) | ||||
| Property, plant and equipment, net | $ | 28,620 | $ | 21,998 | ||||
Depreciation expense for the years ended March 31, 2022, 2021 and 2020 was $3,262, $2,959, and $2,234, respectively.
Inventories consisted of the following:
| March 31, 2022 | March 31, 2021 | |||||||
| Raw materials | $ | 14,172 | $ | 5,755 | ||||
| Work in process | 4,419 | 426 | ||||||
| Finished goods | 6,015 | 4,997 | ||||||
| Inventories, net | $ | 24,606 | $ | 11,178 | ||||
As of March 31, 2022, $11,802 of total inventory on hand is attributable to the new Clinical Genomics division.
Note 8. Indebtedness
Credit Facility
On March 5, 2021, we entered into a -year senior secured credit agreement that includes 1) a revolving credit facility in an aggregate principal amount of up to $75,000, 2) a swingline loan in an aggregate principal amount not exceeding $5,000, and 3) letters of credit in an aggregate stated amount not exceeding $2,500 at any time. The agreement also provides for an incremental term loan or an increase in revolving commitments in an aggregate principal amount of at a minimum $25,000 and at a maximum $75,000, subject to the satisfaction of certain conditions and lender considerations.
Amounts borrowed under the Credit Facility bear interest at either a base rate or a Eurodollar rate, plus an applicable spread. The weighted average interest rate on borrowing under our line of credit during the year ended March 31, 2022 was 1.5%. We are obligated to pay quarterly unused commitment fees of between 0.15% and 0.35% of the Credit Facility’s aggregate principal amount, based on our leverage ratio. Since the Credit Facility's inception, the rate applied to our unused commitment fees has been 0.15%. We incurred unused commitment fees of $78 for the year ended March 31, 2022, and the balance of unamortized customary lender fees was $484 and $650 as of March 31, 2022 and March 31, 2021, respectively. On our Consolidated Balance Sheets, the short term portion of unamortized fees is recorded within prepaid expenses and other, and the long term portion is recorded in other assets. The fees are being expensed on a straight line basis over the life of the agreement.
The financial covenants in the Credit Facility include a maximum leverage ratio of 5.50 to 1.00 for the first four testing dates on which the line of credit is outstanding; 5.0 to 1.0 on each of the fifth, sixth, seventh, and eighth testing dates; and 4.5 to 1.0 on each testing date following the eighth testing date, except that we may have a leverage ratio of 5.75 to 1.0 for a period of four consecutive quarters following a permitted acquisition. The Credit Facility also stipulates a minimum fixed charge coverage ratio of 1.25 to 1.0. Other covenants include restrictions on our ability to incur debt, grant liens, make fundamental changes, engage in certain transactions with affiliates, or conduct asset sales. As of March 31, 2022, we were in compliance with all required covenants.
On October 18, 2021, we borrowed $70,000 under the Credit Facility to provide a portion of the cash needed to complete the Agena Acquisition as further discussed in Note 4. "Significant Transactions." Subsequent to the Agena Acquisition, we repaid $21,000 against our outstanding balance during the year ended March 31, 2022. As of March 31, 2022, the outstanding balance under our Credit Facility was
Convertible Notes
On August 12, 2019, we issued an aggregate principal amount of $172,500 of convertible senior notes. The Notes mature on August 15, 2025, unless earlier repurchased or converted, and bear interest at a rate of 1.375% payable semi-annually in arrears on February 15 and August 15 each year beginning on February 15, 2020. The Notes are initially convertible at a conversion rate of 3.5273 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of approximately $283.50 per share of common stock. Noteholders may convert their Notes at their option only in the following circumstances:
| (i) | during any calendar quarter commencing after the calendar quarter ended on December 31, 2019 (and only during such calendar quarter), if the last reported sale price per share of our common stock exceeds 130% of the conversion price for each of at least 20 trading days during the 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter; |
| (ii) | during the five consecutive business days immediately after any 10 consecutive trading day period (such 10 consecutive trading day period, the “measurement period”) in which the trading price per $1,000 principal amount of Notes for each trading day of the measurement period was less than 98% of the product of the last reported sale price per share of our common stock on such trading day and the conversion rate on such trading day; |
| (iii) | upon the occurrence of certain corporate events or distributions on our common stock, including certain distributions, the occurrence of a fundamental change (as defined in the indenture governing the Notes) or a transaction resulting in the Company’s common stock converting into other securities or property or assets; and |
| (iv) | at any time from, and including, April 15, 2025 until the close of business on the second scheduled trading day immediately before the maturity date. |
Upon conversion, we will pay or deliver, as the case may be, cash, shares of our common stock, or a combination of cash and shares of our common stock, at our election. Our current intent is to settle conversions entirely in shares of common stock. We will reevaluate this policy from time to time as we receive conversion notices from note holders. The circumstances necessary for conversion were not met during the year ended March 31, 2022. As of March 31, 2022, the Notes are classified as a long-term liability on our Consolidated Balance Sheets as the circumstances necessary for conversion were not satisfied as of the end of the period. The if-converted value of the Notes did not exceed the principal balance as of March 31, 2022.
Debt issuance costs related to the Notes are comprised of discounts and commissions payable to the initial purchasers of $5,175 and third party offering costs of $255. The debt issuance costs are being amortized to interest expense using the effective interest method over the six-year contractual term of the Notes.
Due to our adoption of ASU 2020-06 on April 1, 2021, we no longer bifurcate the Notes into a liability and an equity component in our Consolidated Balance Sheets (see Note 1. "Description of Business and Summary of Significant Accounting Policies"). The Notes are accounted for entirely as a liability, and the issuance costs of the Notes are accounted for wholly as debt issuance costs. The equity conversion feature that was recorded to common stock, as well as the unamortized debt discount and amortization expense attributable to equity, have been derecognized.
The net carrying amount of the Notes was as follows:
| March 31, 2022 | March 31, 2021 | |||||||
| Principal outstanding | $ | 172,500 | $ | 172,500 | ||||
| Unamortized debt discount attributable to equity | - | (23,497 | ) | |||||
| Unamortized debt issuance costs | (3,135 | ) | (3,328 | ) | ||||
| Net carrying value | $ | 169,365 | $ | 145,675 | ||||
We recognized interest expense on the Notes as follows:
| Year Ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Coupon interest expense at 1.375% | $ | 2,372 | $ | 2,372 | ||||
| Amortization of debt discounts and issuance costs | 890 | 5,397 | ||||||
| Total | $ | 3,262 | $ | 7,769 | ||||
The effective interest rate of the liability component of the note is approximately 1.9%. Prior to the adoption of ASU 2020-06, the effective interest rate was approximately 5.5%.
Note 9. Stock Transactions and Stock-Based Compensation
(dollars and shares in thousands, except per share values)
In November 2005, our Board of Directors approved a program to repurchase up to 300,000 shares of our outstanding common stock. Under the program, shares of common stock may be purchased from time to time in the open market at prevailing prices or in negotiated transactions off the market. Shares of common stock repurchased will be cancelled and repurchases of shares of common stock will be funded through existing cash reserves. There were no repurchases of our shares of common stock under this plan during the years ended March 31, 2022, 2021 and 2020. As of March 31, 2022, we have purchased 162 shares under this plan.
Under applicable law, Colorado corporations are not permitted to retain treasury stock. The price paid for repurchased shares is allocated between common stock and retained earnings based on management’s estimate of the original sales price of the underlying shares.
Public Offerings of Common Stock
On June 12, 2020, we completed the sale and issuance of a total of 600 shares of our common stock, and on June 19, 2020, our underwriters exercised in full their option to purchase an additional 90 shares of our common stock. The offering price to the public was $225.00 per share. The total proceeds we received from the offering, net of underwriting discounts and commissions and other offering expenses we paid, was $145,935.
On August 12, 2019, we completed the sale and issuance of a total of 431 shares of our common stock, which includes our underwriters' exercise in full of an option to purchase up to 56 additional shares. The offering price to the public was $210.00 per share. The total proceeds we received from the offering, net of underwriting discounts and commissions and other offering expenses we paid, was $84,995.
Stock-Based Compensation
During fiscal year 2022, our shareholders approved the Mesa Laboratories, Inc. 2021 Equity Incentive Plan (the "2021 Equity Plan"), which authorizes the issuance of 330 shares of common stock to eligible participants. The 2021 Equity Plan is administered by the Compensation Committee of the Board of Directors, which has the authority to grant equity awards, or to delegate its authority under the plan to make grants (subject to certain legal and regulatory restrictions), including the authority to determine the individuals to whom awards will be granted, the type of awards and when the awards are to be granted, the number of shares to be covered by each award, the vesting schedule, and all other terms and conditions of the awards. 203 shares were available for future grants as of March 31, 2022. Our 2021 Equity Plan includes retiree provisions, which result in the acceleration of stock-based compensation expense for retiree-eligible participants.
Pursuant to the Mesa Laboratories, Inc. 2014 Equity Plan and the 2021 Equity Plan (together referred to as "the 2014 and 2021 Equity Plans"), we grant stock options, RSUs and PSUs to employees and non-employee directors. For purposes of counting the shares remaining available under the 2014 Equity Plan, each share issuable pursuant to outstanding full value awards, such as RSUs and PSUs, counts as five shares issued, whereas each share underlying a stock option counts as one share issued. For purposes of counting the shares remaining under the 2021 Equity Plan, each share underlying a stock option or a full value award counts as one share used. We issue new shares of common stock upon the exercise of stock options and the vesting of RSUs and PSUs.
Under the 2014 Plan, 1,100 shares of common stock have been authorized and reserved for eligible participants, all of which have been issued as of March 31, 2022. Shares issued pursuant to awards granted prior to the 2014 Equity Plan were issued subject to previous stock plans, and 3 vested awards are still outstanding under previous plans.
Stock-based compensation expense recognized in the Consolidated Financial Statements was as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Stock-based compensation expense | $ | 11,391 | $ | 9,268 | $ | 5,525 | ||||||
| Amount of income tax (benefit) recognized in earnings | ) | ) | ) | |||||||||
| Stock-based compensation expense, net of tax | $ | 7,336 | $ | 7,452 | $ | 3,949 | ||||||
Stock Options
The weighted average assumptions utilized in the Black-Scholes option-pricing model to estimate the fair value of stock option awards granted each year were as follows:
| 2022 | 2021 | 2020 | ||||||||||
| Risk-free interest rate | 0.46 | % | 0.27 | % | 1.80 | % | ||||||
| Expected life (years) | 3.52 | 3.86 | 4.33 | |||||||||
| Expected dividend yield | 0.06 | % | 0.10 | % | 0.13 | % | ||||||
| Volatility | 38.82 | % | 38.83 | % | 36.52 | % | ||||||
| Weighted-average Black-Scholes fair value per share at date of grant | $ | 76.02 | $ | 67.66 | $ | 66.02 | ||||||
The expected life of options represents the estimated period of time until exercise and is based on historical experience of similar awards, giving consideration to the contractual terms, vesting schedules, and expectations of future employee behavior. The substantial majority of options granted during the years ended March 31, 2022 and March 31, 2021 vest equally on the first, second, and third anniversary of the grant date. Expected stock price volatility is based on the historical volatility of our own stock price over the period of time commensurate with the expected life of the award. The risk-free rate is based on the United States Treasury yield curve in effect at the time of grant for the estimated life of the stock option. The dividend yield assumption is based on our anticipated cash dividend payouts. The amounts shown above for the estimated fair value per option granted are before the estimated effect of forfeitures, which reduces the amount of expense recorded in our Consolidated Statements of Income. We base forfeiture rates on company-specific historical experience of similar awards for similar subsets of our employee population.
Stock option activity under the 2021 Equity Plan and legacy plans as of March 31, 2022, and changes for the year then ended are presented below:
| Stock Options | ||||||||||||||||
| Shares Subject to Options | Weighted- Average Exercise Price per Share | Weighted-Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding as of March 31, 2021 | 253 | $ | 129.55 | 2.7 | $ | 28,856 | ||||||||||
| Awards granted | 37 | 268.81 | ||||||||||||||
| Awards forfeited or expired | (4 | ) | 191.52 | |||||||||||||
| Awards exercised or distributed | (84 | ) | 96.68 | |||||||||||||
| Outstanding as of March 31, 2022 | 202 | $ | 167.14 | 2.9 | $ | 18,261 | ||||||||||
| Exercisable as of March, 31, 2022 | 100 | $ | 128.32 | 1.9 | $ | 12,636 | ||||||||||
| Exercisable and expected to vest, March 31, 2022 | 199 | $ | 174.79 | 3.0 | $ | 18,357 | ||||||||||
The total intrinsic value of stock options exercised during the years ended March 31, 2022, 2021 and 2020 was $15,209, $9,559, and $9,574, respectively. Unrecognized stock-based compensation expense for stock options as of March 31, 2022 was $3,915 and is expected to be recognized over a weighted average period of 1.8 years. The total fair value of options vested was $2,856, $2,005, and $1,912 during the years ended March 31, 2022, 2021 and 2020, respectively. The weighted-average grant price of awards granted during the years ended March 31, 2021 and 2020 was $226.72 and $206.35, respectively.
Time-Based Restricted Stock Units (RSUs)
RSU activity under the 2014 and 2021 Equity Plans was as follows (shares and dollars in thousands, except per-share data):
| Time-Based Restricted Stock Units | ||||||||||||||||
| Number of Shares | Weighted- Average Grant Date Fair Value per Share | Weighted- average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Nonvested at March 31, 2021 | 37 | $ | 206.56 | 1.1 | $ | 8,948 | ||||||||||
| Awards granted | 37 | 274.55 | ||||||||||||||
| Awards forfeited or expired | (3 | ) | 250.09 | |||||||||||||
| Awards distributed | (20 | ) | 208.52 | |||||||||||||
| Nonvested as of March 31, 2022 | 51 | $ | 252.86 | 1.0 | $ | 13,019 | ||||||||||
There were 48 time-based RSUs with a weighted average grant date fair value per share of $251.94 that are expected to vest as of March 31, 2022. For the years ended March 31, 2021 and 2020, the weighted average fair value per RSU granted was $231.61 and $213.31, respectively. Unrecognized stock-based compensation expense for RSUs that we have determined are probable of vesting was $7,942 as of March 31, 2022. The total fair value of RSUs vested was $5,320, $1,819, $959 during the years ended March 31, 2022, 2021 and 2020.
Performance-Based Restricted Stock Units (PSUs)
PSU activity under the 2014 and 2021 Equity Plans was as follows:
| Performance-Based Restricted Stock Units | ||||||||||||||||
| Number of Shares | Weighted- Average Grant Date Fair Value per Share | Weighted- average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Nonvested at March 31, 2021 at target | 20 | $ | 207.88 | 0.8 | $ | 4,884 | ||||||||||
| Awards granted | 48 | 302.15 | ||||||||||||||
| Performance adjustment | 16 | |||||||||||||||
| Awards distributed | (29 | ) | 197.81 | |||||||||||||
| Nonvested as of March 31, 2022 at target | 55 | $ | 288.45 | 4.3 | $ | 14,093 | ||||||||||
| Expected to vest | 53 | $ | 283.88 | 2.8 | 13,531 | |||||||||||
(A) During the quarter ended June 30, 2021, the fiscal year 2019 PSUs vested and were paid at 280% of target, based on actual performance results and completion of service conditions. In addition, the PSUs granted to employees of Gyros Protein Technologies Holding AB vested at 60% of target, following a modification of the performance targets by the Compensation Committee of the Board of Directors during fiscal year 2021.
There were no PSUs granted during the year ended March 31, 2021. For the year ended March 31, 2020, the average fair value per PSU granted was $215.47. Unrecognized stock-based compensation expense for PSUs that we have determined probable of vesting was $11,651 as of March 31, 2022 and is expected to be recognized over a weighted average period of 2.8 years. No PSUs were distributed during the years ended March 31, 2021 and 2020.
During the third quarter of fiscal year 2022, we awarded 7 PSUs to key employees of Agena that are subject to both service and performance conditions ("Agena PSUs"). The Agena PSUs had a grant date fair value of $305.79 per share and vest based on continued service, completion of certain compliance requirements, and achievement of specific financial performance targets for the period from October 20, 2021 through March 31, 2023. The quantity of shares that will be issued upon vesting will range from 50% to if financial performance is less than 50% of targets, then no shares will vest. Based on actual and projected performance through the year ended March 31, 2022, we decreased our estimate of Agena PSUs expected to vest from 8 to 4 shares, resulting in a release of $295 of expense recorded to selling and administrative expense during the year ended March 31, 2022.
On October 28, 2021, the Compensation Committee of the Board of Directors granted a special long-term equity award consisting of performance stock units covering a target of 40 shares (“PSUs”) that is subject to both performance and service conditions to our Chief Executive Officer. The performance period of the award is the -year period from April 1, 2021 through March 31, 2024 and the service period commences on October 28, 2021 and ends on October 27, 2024, October 27, 2025, and October 27, 2026, on which dates eligible PSUs will vest and be distributed. The performance metrics are cumulative GAAP revenues over the performance period and cumulative adjusted operating income over the performance period. The quantity of shares that will be issued upon vesting will range from 0 to 40; if financial performance targets are not met, then no shares will vest.
During the year ended March 31, 2022, the Compensation Committee of the Board of Directors modified a time-based restricted stock award granted to our Chief Executive Officer during fiscal year 2017, distributing 3 remaining outstanding shares effective June 8, 2021. The original award required vesting of 1 award on each of March 20, 2022, 2023, and 2024. As a result of the modification, we recognized the previously unrecognized compensation cost of $351 during the year ended March 31, 2022.
Performance-based RSUs vest upon completion of the service period described in the award agreement and based on achievement of the financial targets described in the award agreements. We recognize the expense relating to the performance-based RSUs based on the probable outcome of achievement of the financial targets on a straight-line basis over the service period. During fiscal year 2020, we awarded 8 PSUs (the "FY 20 PSUs") that are subject to both service and performance conditions to eligible employees. The FY 20 PSUs had a grant date fair value of $202.00 per share and vest based on our achievement of specific performance criteria for the -year period from April 1, 2019 through March 31, 2022 and on a pro-rata basis after 12 months of continued service through June 15, 2022. The quantity of shares that will be issued upon vesting will range from 0% to 200% of the targeted number of shares; if the defined minimum targets are not met, then no shares will vest. Based on actual performance through the year ended March 31, 2022, we increased our estimate of FY 20 PSUs expected to vest from 6 to 9 shares, resulting in a cumulative effect true up of $650 recorded during the year ended March 31 2022. We expect to record $129 of expense related to the FY 20 PSUs in the first quarter of fiscal year 2023.
Note 10. Earnings Per Share
(dollars and shares in thousands, except per share values)
Basic earnings per share is computed by dividing net income by the weighted-average number of common shares outstanding during the reporting period. Diluted earnings per share is computed similarly to basic earnings per share, except that it includes the potential dilution that could occur if dilutive securities were exercised. Potentially dilutive securities include common shares related to stock options and RSUs (collectively “stock awards”) and convertible debt. Stock awards are excluded from the calculation of diluted EPS in the event that they are subject to performance conditions that have not yet been achieved or are antidilutive. Diluted EPS considers the impact of potentially dilutive securities except in periods in which there is a loss because the inclusion of the potential common shares would have an antidilutive effect.
The impact of the assumed conversion of the Notes calculated under the if-converted method was anti-dilutive, and as such shares underlying the Notes were excluded from the diluted EPS calculation for the years ended March 31, 2022.
The following table presents a reconciliation of the denominators used in the computation of basic and diluted earnings per share:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Net income available for shareholders | $ | 1,871 | $ | 3,274 | $ | 1,778 | ||||||
| Weighted average outstanding shares of common stock | 5,212 | 4,975 | 4,200 | |||||||||
| Dilutive effect of stock options | 100 | 125 | 159 | |||||||||
| Dilutive effect of RSUs | 20 | 10 | 12 | |||||||||
| Dilutive effect of PSUs | 3 | 14 | - | |||||||||
| Fully diluted shares | 5,335 | 5,124 | 4,371 | |||||||||
| Basic earnings per share | $ | 0.36 | $ | 0.66 | $ | 0.42 | ||||||
| Diluted earnings per share | $ | 0.35 | $ | 0.64 | $ | 0.41 | ||||||
The following stock awards were excluded from the calculation of diluted EPS:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Assumed conversion of convertible debt | 608 | 608 | 387 | |||||||||
| Stock awards that were anti-dilutive | 40 | 44 | 24 | |||||||||
| Stock awards subject to performance conditions | 26 | 14 | 18 | |||||||||
| Total stock awards excluded from diluted EPS | 674 | 666 | 429 | |||||||||
Note 11. Employee Benefit Plans
We adopted the Mesa Laboratories, Inc. 401(K) Retirement Plan effective January 1, 2000. Under this plan, we match 100% of the first 4% of pay contributed by each eligible employee, and contributions vest immediately. Participation is voluntary, and employees are eligible on the first day of the month following their start date. This plan also became effective for Agena employees upon completion of the Agena Acquisition on October 20, 2021.
Prior to the year ended March 31, 2022, certain employees of our Biopharmaceutical Development division were subject to the terms of a 401(K) plan in effect when we originally acquired the businesses comprising the division. Under the pre-existing plan, we matched 100% of the first 6% of pay contributed by each eligible employee, and contributions vested over three years. In July 2022, all employees under the pre-existing plan became subject to the terms of the Mesa Laboratories, Inc. 401(K) Retirement Plan.
During the years ended March 31, 2022, 2021 and 2020, respectively, we contributed $1,185, $935, and $661 to Mesa Laboratories, Inc. 401(K) retirement plans on behalf of employees.
Note 12. Income Taxes
Earnings before income taxes are as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Domestic | $ | 4,579 | $ | 6,297 | $ | 16,059 | ||||||
| Foreign | (1,005 | ) | (3,994 | ) | (12,197 | ) | ||||||
| Total earnings before income taxes | $ | 3,574 | $ | 2,303 | $ | 3,862 | ||||||
The components of our provision for income taxes are as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Current tax provision | ||||||||||||
| U.S. Federal | $ | (83 | ) | $ | 1,500 | $ | 2,348 | |||||
| U.S. State | 286 | 628 | 814 | |||||||||
| Foreign | 1,372 | 404 | 993 | |||||||||
| Total current tax expense | 1,575 | 2,532 | 4,155 | |||||||||
| Deferred tax provision: | ||||||||||||
| U.S. Federal | 1,707 | (2,410 | ) | 60 | ||||||||
| U.S. State | 337 | (619 | ) | 599 | ||||||||
| Foreign | (1,916 | ) | (474 | ) | (2,730 | ) | ||||||
| Total deferred tax expense | 128 | (3,503 | ) | (2,071 | ) | |||||||
| Total income tax expense (benefit) | $ | 1,703 | $ | (971 | ) | $ | 2,084 | |||||
The components of net deferred tax assets and liabilities are as follows:
| March 31, 2022 | March 31, 2021 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss | $ | 11,274 | $ | 8,990 | ||||
| Credits | 5,321 | 169 | ||||||
| Stock compensation deductible differences | 2,137 | 2,099 | ||||||
| Inventories | 1,316 | 838 | ||||||
| Allowances and reserves | 1,977 | 1,471 | ||||||
| Accrued employee-related expenses | 296 | 209 | ||||||
| Debt related | 91 | -- | ||||||
| Other | 7 | 25 | ||||||
| Total deferred tax assets | 22,419 | 13,801 | ||||||
| Deferred tax liabilities: | ||||||||
| Goodwill and intangible assets | (56,145 | ) | (23,029 | ) | ||||
| Property, plant and equipment | (3,284 | ) | (1,275 | ) | ||||
| Debt | -- | (4,723 | ) | |||||
| Currency translation adjustment | (185 | ) | -- | |||||
| Other | (3 | ) | (29 | ) | ||||
| Total deferred tax liabilities | (59,617 | ) | (29,056 | ) | ||||
| Valuation allowance | (708 | ) | (404 | ) | ||||
| Net deferred tax (liability) | $ | (37,906 | ) | $ | (15,659 | ) | ||
A reconciliation of our income tax provision and the amounts computed by applying statutory rates to income before income taxes is as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Federal income taxes at statutory rates | $ | 751 | $ | 483 | $ | 811 | ||||||
| State income taxes, net of federal benefit | 628 | (221 | ) | 1,122 | ||||||||
| Tax benefit of stock option exercises | (4,055 | ) | (1,816 | ) | (1,576 | ) | ||||||
| Foreign-derived intangible income deduction | -- | (999 | ) | -- | ||||||||
| Research and development credit | (495 | ) | (165 | ) | (191 | ) | ||||||
| Interest reserve adjustment | 668 | -- | -- | |||||||||
| Limitation for 162(m) | 4,039 | 1,113 | 1,112 | |||||||||
| Foreign rate differential | 152 | 810 | 657 | |||||||||
| Other | 15 | (176 | ) | 149 | ||||||||
| Total income tax expense (benefit) | $ | 1,703 | $ | (971 | ) | $ | 2,084 | |||||
We or one of our subsidiaries files income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. Our federal tax returns for all years after state tax returns after and foreign tax returns after 2017 are subject to future examination by tax authorities for all our tax jurisdictions. Although the outcome of tax audits, if any, is always uncertain, we believe that we have adequately accrued for all amounts of tax, including interest and penalties and any adjustments that may result. The tax year ended December 31, 2018 for Gyros US, Inc., and its subsidiary (together "Gyros U.S."), which we acquired as part of the GPT Acquisition, is under examination by the IRS. Additionally, the tax year ended March 31, 2019 for Mesa Laboratories, Inc. is under review by the IRS. We expect the examinations for these tax years to be completed during the year ending March 31, 2023.
We recognize interest and penalties related to unrecognized tax benefits in other expense and general and administrative expense, respectively. Accrued interest and penalties related to unrecognized tax benefits were $0, $0 and $19 as of March 31, 2022, 2021 and 2020, respectively.
A reconciliation of the changes in the balance of unrecognized tax benefit amounts is as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Beginning balance | $ | 64 | $ | 653 | $ | 1,361 | ||||||
| Increase (decreases) related to prior period tax positions | (629 | ) | (1,027 | ) | ||||||||
| Increases related to current period tax positions | 40 | 319 | ||||||||||
| Ending balance | $ | 1,329 | $ | 64 | $ | 653 | ||||||
During the year ended March 31, 2022, we recorded an income tax expense of approximately $1,179 related to our reserve associated with the acquired Agena Federal and California Research and Development credits, which increased the effective tax rate by 33.0%. The remaining amount of tax benefits that, if recognized, would affect the effective tax rate was $1,329 as of March 31, 2022, excluding interest and penalties. We expect that the remaining amount of unrecognized tax benefits will change in the next 12 months; however, we do not expect the change to have a significant impact on our consolidated statements of income or consolidated balance sheets. At this time, we expect resolution of the uncertain tax position within 12 months.
As of March 31, 2022, and March 31, 2021, undistributed earnings of our foreign subsidiaries amounted to $11,580 and $9,951, respectively. Those earnings are considered indefinitely reinvested and, accordingly, no U.S. federal and state income taxes have been provided thereon. Upon distribution of those earnings in the form of dividends or otherwise, we would be subject to both U.S. income taxes (subject to an adjustment for foreign tax credits) and withholding taxes payable to the various foreign countries. Determination of the amount of unrecognized deferred U.S. income tax liability is not practicable because of the complexities associated with its hypothetical calculation; however, unrecognized foreign tax credits would be available to reduce a portion of the U.S. tax liability. Furthermore, as a result of the Tax Cuts and Jobs Act, a significant portion of the distribution may not be subject to current U.S. income taxes, resulting in no foreign tax credits.
As of March 31, 2022, we had of gross net operating losses for foreign tax purposes. The foreign net operating losses do not expire. Furthermore, Gyros U.S. had gross net operating losses of $7,870 and $3,941, for federal and state tax purposes, respectively, of which the federal net operating losses do not expire, while the state net operating losses began to expire in the 2022 tax year. Agena Bioscience had domestic gross net operating losses of $11,667 and $6,744, for federal and state tax purposes, respectively, of which the federal net operating losses do not expire, and the state net operating losses begin to expire in the 2034 tax year. In addition, we had $16 of foreign tax credit carryovers which will expire in the tax year 2029. Gyros U.S. also had $212 and $105, for federal and state purposes, respectively, of Research and Development credit carryforward which will begin to expire in the 2030 tax year for federal purposes and begin to expire in the 2037 tax year for state purposes. Agena Bioscience had $3,718 and $3,244, for federal and state tax purposes, respectively, of Research and Development credit carryforward, which will begin to expire in the 2034 tax year for federal purposes, and do not expire for state purposes.
Note 13. Commitments and Contingencies
We are party to various legal proceedings arising in the ordinary course of business. As of March 31, 2022, we are not party to any legal proceeding that management believes could have a material adverse effect on our consolidated financial position, results of operations, or cash flows.
Companies are required to collect and remit sales tax from certain customers if the company is determined to have nexus in a particular state. The determination of nexus varies by state and often requires technical knowledge of each jurisdiction's tax case law. During the year ended March 31, 2021, we determined that certain subsidiaries of GPT had established nexus in various jurisdictions during prior periods without properly collecting and remitting sales tax, and in certain cases had collected sales tax and not remitted it. The estimated accrued liability for this matter is included in other accrued expenses on the Consolidated Balance Sheets. The balance was $2,080 and $2,714 as of March 31, 2022 and 2021, respectively. Approximately $1,899 of the liability is considered a preacquisition contingency and was included in purchase accounting. The amount ultimately remitted may differ from our estimates, which could materially impact the financial statements. We reevaluate the estimated liability each reporting period. We expect to resolve the liability during the fiscal year ending March 31, 2023.
Note 14. Segment Data
Segment information is prepared on the same basis that our CEO, who is our Chief Operating Decision Maker, uses to manage the segments, evaluate financial results, and make key operating decisions. The acquisition of Agena discussed in Note 4. "Significant Transactions," expanded our presence further into the life sciences tools market and provided an impetus for the creation of our new Clinical Genomics reportable segment. This strategic shift in our business also resulted in a change to the way we manage other business units, and as a result, our historical Instruments and Continuous Monitoring reportable segments have been combined to create Calibration Solutions. Prior year amounts have been recast to conform to current year presentation. Our change in financial reporting segments has not resulted in any change to previously reported consolidated amounts.
We have four reportable segments organized primarily by product type: Sterilization and Disinfection Control, Biopharmaceutical Development, Calibration Solutions, and Clinical Genomics. When determining our reportable segments, we aggregated operating segments based on their similar economic and operating characteristics. We evaluate the performance of our operating segments based on revenues, organic revenues growth, and gross profit. The accounting policies of the operating segments are the same as those described in Note 1. "Description of Business and Summary of Significant Accounting Policies."
The following tables set forth our segment information:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| Revenues (a): | ||||||||||||
| Sterilization and Disinfection Control | $ | 59,044 | $ | 53,119 | $ | 49,660 | ||||||
| Biopharmaceutical Development | 45,579 | 33,892 | 13,851 | |||||||||
| Calibration Solutions | 46,872 | 46,926 | 51,713 | |||||||||
| Clinical Genomics | 32,840 | - | - | |||||||||
| Reportable segment revenues | 184,335 | 133,937 | 115,224 | |||||||||
| Corporate and Other (b) | - | - | 2,463 | |||||||||
| Total revenues | $ | 184,335 | $ | 133,937 | $ | 117,687 | ||||||
| Gross profit: | ||||||||||||
| Sterilization and Disinfection Control | $ | 43,720 | $ | 39,870 | $ | 35,797 | ||||||
| Biopharmaceutical Development | 28,605 | 21,035 | 382 | |||||||||
| Calibration Solutions | 24,989 | 26,112 | 28,765 | |||||||||
| Clinical Genomics | 11,941 | - | - | |||||||||
| Reportable segment gross profit | 109,255 | 87,017 | 64,944 | |||||||||
| Corporate and Other (b) | (165 | ) | (3 | ) | 418 | |||||||
| Gross profit | $ | 109,090 | $ | 87,014 | $ | 65,362 | ||||||
| Reconciling items: | ||||||||||||
| Operating expenses | 104,388 | 74,656 | 57,439 | |||||||||
| Operating income | 4,702 | 12,358 | 7,923 | |||||||||
| Nonoperating expense | 1,128 | 10,055 | 4,061 | |||||||||
| Earnings before income taxes | $ | 3,574 | $ | 2,303 | $ | 3,862 | ||||||
| (a) | Intersegment revenues are not significant and are eliminated to arrive at consolidated totals. |
| (b) | Non-reportable operating segments (including our Cold Chain Packaging Division which ceased operations during the year ended March 31, 2020) and unallocated corporate expenses are reported within Corporate and Other. |
The following table sets forth net inventories by reportable segment. Our chief operating decision maker is not provided with any other segment asset information.
| March 31, | March 31, | |||||||
| 2022 | 2021 | |||||||
| Sterilization and Disinfection Control | $ | 2,176 | $ | 2,333 | ||||
| Biopharmaceutical Development | 4,495 | 4,162 | ||||||
| Calibration Solutions | 6,133 | 4,683 | ||||||
| Clinical Genomics | 11,802 | - | ||||||
| Reportable segment inventory | 24,606 | 11,178 | ||||||
| Corporate and Other | - | - | ||||||
| Total inventories, net | $ | 24,606 | $ | 11,178 | ||||
The following table sets forth a summary of long-lived assets by geographic area. Long-lived assets exclude goodwill and intangible assets acquired in a business combination and deferred tax assets.
| As of March 31, | ||||||||
| 2022 | 2021 | |||||||
| United States | $ | 36,475 | $ | 21,443 | ||||
| Foreign | 3,975 | 3,085 | ||||||
| Total | $ | 40,450 | $ | 24,528 | ||||
Revenues from external customers are attributed to individual countries based upon locations to which the product is shipped or exported, as follows:
| Year Ended March 31, | ||||||||||||
| 2022 | 2021 | 2020 | ||||||||||
| United States | $ | 99,068 | $ | 71,387 | $ | 66,344 | ||||||
| Foreign | 85,267 | 62,550 | 51,343 | |||||||||
| Total revenues | $ | 184,335 | $ | 133,937 | $ | 117,687 | ||||||
No customer accounts for 10% or more of our revenues. No foreign country exceeds 10% of total revenues.
Note 15. Subsequent Events
On April 5, 2022, we entered into an Open Market Sale AgreementSM with Jefferies LLC as sales agent, pursuant to which we may issue and sell, from time to time, through Jefferies, shares of our common stock with an aggregate value of up to $150 million.
In April 2022, we announced a corporate restructuring that, among other things, resulted in the elimination of the Senior Vice President of Commercial Operations role. As a result, we are formally aligning each of our business units under general managers who will oversee sales, customer service, research and development, as well as financial operations of the business unit for which they are responsible. We incurred of general and administrative expenses associated with the corporate restructuring in the fourth quarter of fiscal year 2022. These changes, among others, are expected to result in a total of $195 of severance in the first quarter of fiscal year 2023.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) that are designed to reasonably ensure that information required to be disclosed by us in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Our management evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of March 31, 2022. Based on that evaluation, our management concluded that our disclosure controls and procedures were effective as of March 31, 2022.
Management's Annual Report on Internal Control Over Financial Reporting
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles in the United States. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness for future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management evaluated the effectiveness of our internal control over financial reporting as of March 31, 2022, using the framework in “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013. Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of March 31, 2022.
Our independent auditor, Plante & Moran, PLLC, a registered public accounting firm, is appointed by the Audit Committee of our Board of Directors, subject to ratification by our shareholders. Plante & Moran, PLLC has issued an unqualified opinion on the effectiveness of our internal controls over financial reporting as of March 31, 2022, which appears in Item 8. Financial Statements and Supplementary Data of this Annual Report on Form 10-K.
Changes in internal control over financial reporting
The Agena Acquisition was completed on October 20, 2021. The financial results of Agena are included in our consolidated financial statements as of March 31, 2022 and for the year then ended. The Agena business represented $32,840 of revenues and ($7,779) of net loss, respectively, for the year ended March 31, 2022. As this acquisition occurred in the third quarter of fiscal year 2022, the scope of our assessment of our internal control over financial reporting does not include Agena. This exclusion is in accordance with the Securities and Exchange Commission’s general guidance that an assessment of a recently acquired business may be omitted from our scope in the year of acquisition.
During the second quarter of fiscal year 2022, we implemented a new human resources information and payroll system, which is considered to be a key system as part of our internal controls over financial reporting. We continued to integrate the software with our processes, systems, and controls in the fourth quarter of fiscal year 2022. There were no other changes during the quarter ended March 31, 2022 in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
Item 10. Directors, Executive Officers and Corporate Governance
Incorporated by reference from the definitive Proxy Statement for our 2022 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2022.
Item 11. Executive Compensation
Incorporated by reference from the definitive Proxy Statement for our 2022 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2022.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Securities Authorized for Issuance Under Equity Compensation Plans
The following table presents information regarding options and rights outstanding under our equity compensation plans as of March 31, 2022. All options reflected are options to purchase common stock.
| (a) Number of Securities to be Issued upon Exercising of Outstanding Options and Rights (1) |
(b) Weighted-Average Exercise Price of Outstanding Options and Rights (1) |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (2) |
||||||||||
| Equity Compensation Plan Approved by Security Holders |
305,854 | $ | 167.14 | 202,627 | ||||||||
| Equity Compensation Plans Not Approved by Security Holders |
None |
N/A | None |
|||||||||
| Total |
305,854 | $ | 167.14 | 202,627 | ||||||||
| 1. |
Includes shares issuable in connection with awards with performance conditions, which will be issued based on achievement of performance criteria associated with the awards, with the number of shares issuable dependent on our level of performance. We have accounted for the shares based on the current achievement as of March 31, 2022. The weighted average exercise price in column (b) includes the weighted average exercise price of options only. |
| 2. |
Includes 202,627 shares remaining available under the 2021 Equity Plan. Each share underlying a full value award such as restricted stock or performance shares count as one share used against the total number of securities authorized under the plan. |
Additional information for this item is incorporated by reference from the definitive Proxy Statement for our 2021 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2022.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Incorporated by reference from the definitive Proxy Statement for our 2022 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2022.
Item 14. Principal Accountant Fees and Services
Plante & Moran, Denver, Colorado, PCAOB ID 166 is the Company's independent registered public accounting firm.
Incorporated by reference from the definitive Proxy Statement for our 2022 Annual Meeting of Stockholders or an amendment to this report to be filed no later than 120 days after March 31, 2022.
Item 15. Exhibits and Financial Statement Schedules
| a) |
Consolidated Financial Statements |
The Consolidated Financial Statements of the Registrant listed on the accompanying index (please see Item 8. Financial Statements and Supplementary Data) are filed as part of this Annual Report.
All financial statement schedules have been omitted either because they are not applicable or required, or the information that would be required to be included is disclosed in the notes to the Consolidated Financial Statements.
| b) |
Exhibits |
* Indicates a management contract or compensatory plan, contract or arrangement.
α Mesa Laboratories, Inc. has entered into an Executive Employment Agreement with each of Gary M. Owens, John V. Sakys, and Brian Archbold.
+ Filed electronically herewith.
None.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MESA LABORATORIES, INC. | |||
| Registrant | |||
|
|
|
|
|
|
|
|
|
|
| Date: May 31, 2022 |
By: |
/s/ Gary M. Owens |
|
|
|
|
Gary M. Owens |
|
|
|
|
Chief Executive Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name |
Title |
Date |
|
| /s/John J. Sullivan, Ph.D. |
Chairman of the Board of Directors |
May 31, 2022 | |
| John J. Sullivan |
|||
| /s/Gary M. Owens |
Chief Executive Officer, President, |
May 31, 2022 | |
| Gary M. Owens |
and Director |
||
| /s/John V. Sakys |
Chief Financial and Chief Accounting Officer, and Treasurer |
May 31, 2022 | |
| John V. Sakys |
|
||
| /s/John B. Schmieder |
Director |
May 31, 2022 | |
| John B Schmieder |
|||
| /s/Jennifer S. Alltoft |
Director |
May 31, 2022 | |
| Jennifer S. Alltoft |
|||
| /s/Evan Guillemin |
Director |
May 31, 2022 | |
| Evan Guillemin |
|||
| /s/ Shannon Hall | Director |
May 31, 2022 | |
| Shannon Hall |
|||
| /s/ Shiraz Ladiwala | Director | May 31, 2022 | |
| Shiraz Ladiwala |
Similar companies
See also DANAHER CORP /DE/ - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-29)See also ROPER TECHNOLOGIES INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Keysight Technologies, Inc. - Annual report 2022 (10-K 2022-10-31) Annual report 2024 (10-Q 2024-07-31)
See also AMETEK INC/ - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Fortive Corp - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-27)
