MIMEDX GROUP, INC. - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ☑ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the fiscal year ended December 31, 2021 | |||||
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from __________to __________
Commission file number 001-35887
MIMEDX GROUP, INC.
(Exact name of registrant as specified in its charter)
| Florida | 26-2792552 | |||||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
1775 West Oak Commons Court, NE, Marietta, GA
(Address of principal executive offices)
30062
(Zip Code)
(770) 651-9100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||||||
Common Stock, par value $0.001 per share | MDXG | The Nasdaq Stock Market LLC | ||||||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§223.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☑ | Accelerated filer | ☐ | ||||||||
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ | ||||||||
| Emerging growth company | ☐ | ||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered accounting firm that prepared or its audit report ☑
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No þ
The aggregate market value of the registrant’s voting common equity held by non-affiliates of the registrant as of June 30, 2021 (the last business day of the registrant’s most recently completed second quarter) was approximately $1,381 million based upon the last sale price ($12.51) of the shares as reported on The Nasdaq Stock Market LLC on such date.
There were 112,359,601 shares of the registrant’s common stock, par value $0.001 per share, outstanding as of February 21, 2022.
Documents Incorporated By Reference
Portions of the proxy statement relating to the 2022 Annual Meeting of Shareholders, to be filed within 120 days after the end of the fiscal year to which this report relates, are incorporated by reference in Part III of this Report.
Table of Contents
| Item | Description | Page | ||||||
| Part I | ||||||||
| Item 1. | Business | |||||||
| Item 1A. | Risk Factors | |||||||
| Item 1B. | Unresolved Staff Comments | |||||||
| Item 2. | Properties | |||||||
| Item 3. | Legal Proceedings | |||||||
| Item 4. | Mine Safety Disclosures | |||||||
| Part II | ||||||||
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |||||||
| Item 6. | [Reserved] | |||||||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | |||||||
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | |||||||
| Item 8. | Financial Statements and Supplementary Data | F- 1 | ||||||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |||||||
| Item 9A. | Controls and Procedures | |||||||
| Item 9B. | Other Information | |||||||
| Item 9C. | Disclosures Regarding Foreign Jurisdictions that Prevent Inspections | |||||||
| Part III | ||||||||
| Item 10. | Directors, Executive Officers and Corporate Governance | |||||||
| Item 11. | Executive Compensation | |||||||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |||||||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | |||||||
| Item 14. | Principal Accounting Fees and Services | |||||||
| Part IV | ||||||||
| Item 15. | Exhibits, Financial Statement Schedules | |||||||
| Item 16. | Form 10-K Summary | |||||||
| Signatures | ||||||||
PART I
Explanatory Note and Important Cautionary Statement Regarding Forward-Looking Statements
As used herein, the terms “MiMedx,” “the Company,” “we,” “our” and “us” refer to MiMedx Group, Inc., a Florida corporation, and its consolidated subsidiaries as a combined entity, except where it is clear that the terms mean only MiMedx Group, Inc.
This Annual Report contains forward-looking statements. All statements relating to events or results that may occur in the future are forward-looking statements, including, without limitation, statements regarding the following:
•our strategic focus, by our current business priorities and our ability to implement these priorities, including as a result of our no longer being able to market our micronized products and certain other products;
•our expectations regarding the sufficiency of our liquidity and existing capital resources to implement our current business priorities;
•our expectations regarding our ability to fund our ongoing and future operating costs;
•our expectations regarding future income tax liability;
•the advantages of our products and development of new products;
•our expectations regarding the size of potential markets for our products and any growth in such markets;
•our expectations regarding the regulatory pathway for our products, including our existing and planned investigative new drug application and pre-market approval requirements; current plans, designs, expected timelines, and expectations for success of our clinical trials; and our expectations regarding timing and receipt of necessary regulatory approvals for certain of our products including Biological License Applications (“BLAs”);
•our expectations regarding ongoing regulatory obligations and oversight and the changing nature thereof impacting our products, research and clinical programs, and business, including those relating to patient privacy;
•our expectations regarding our ability to manufacture certain of our products in accordance with current Good Manufacturing Practices (“CGMP”) and in sufficient quantities to meet current and potential demand;
•our expectations regarding costs relating to compliance with regulatory requirements, including those arising from our clinical trials, pursuit of BLAs, and CGMP compliance;
•the likelihood, timing, and scope of possible regulatory approval and commercial launch of our late-stage product candidates and new indications for our products.
•our expectations regarding government and other third-party coverage and reimbursement for our products;
•our expectations regarding future revenue growth;
•our belief in the sufficiency of our intellectual property rights in our technology;
•our expectations regarding our ability to procure sufficient supplies of human tissue to manufacture and process our products;
•the outcome of pending litigation and investigations;
•our expectations regarding the ongoing and future effects arising from the investigation conducted by the Audit Committee (the “Audit Committee”) of our Board of Directors (the “Board”) concluded in May 2019 relating to allegations regarding certain sales and distribution practices at the Company and certain other matters (the “Investigation” or the “Audit Committee Investigation”), the restatement of our consolidated financial statements previously filed in our Annual Report for the year ended December 31, 2016, as well as selected unaudited condensed consolidated financial data as of and for the years ended December 31, 2015 (Restated) and 2014 (Restated), which reflected adjustments to our previously filed consolidated financial statements as of and for the years ended December 31, 2015 and 2014 (collectively, the “Restatement”), and related litigation;
4
•ongoing and future effects arising from the COVID-19 pandemic on our business, employees, suppliers and other third parties with whom we do business, and our responses intended to mitigate such effects;
•demographic and market trends;
•our expectations regarding research and development costs, including those arising from filing additional investigative new drug applications and pursuing new BLAs; and
•our ability to compete effectively.
Forward-looking statements generally can be identified by words such as “expect,” “will,” “change,” “intend,” “seek,” “target,” “future,” “plan,” “continue,” “potential,” “possible,” “could,” “estimate,” “may,” “anticipate,” “to be” and similar expressions. These statements are based on numerous assumptions and involve known and unknown risks, uncertainties and other factors that could significantly affect our operations and may cause our actual actions, results, financial condition, performance or achievements to differ materially from any future actions, results, financial condition, performance or achievements expressed or implied by any such forward-looking statements. Factors that may cause such a difference include, without limitation, those discussed under the heading “Risk Factors” in this Annual Report.
Unless required by law, the Company does not intend, and undertakes no obligation, to update or publicly release any revision to any forward-looking statements, whether as a result of the receipt of new information, the occurrence of subsequent events, a change in circumstances or otherwise. Each forward-looking statement contained in this Annual Report is specifically qualified in its entirety by the aforementioned factors. Readers are advised to carefully read this Annual Report in conjunction with the important disclaimers set forth above prior to reaching any conclusions or making any investment decisions and not to place undue reliance on forward-looking statements, which apply only as of the date of the filing of this Annual Report with the SEC.
Estimates and Projections
This discussion includes certain estimates, projections and other statistical data. These estimates and projections reflect management’s best estimates based upon currently available information and certain assumptions we believe to be reasonable. These estimates are inherently uncertain, subject to risks and uncertainties, many of which are not within our control, have not been reviewed by our independent auditors and may be revised as a result of management’s further review. In addition, these estimates and projections are not a comprehensive statement of our financial results, and our actual results may differ materially from these estimates and projections due to developments that may arise between now and the time the results are final. There can be no assurance that the estimates will be realized, and our results may vary significantly from the estimates, including as a result of unexpected issues in our business and operations. Accordingly, you should not place undue reliance on such information. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. See Item 1A — Risk Factors for further information.
5
Item 1. Business
Overview
MiMedx is a transformational placental biologics company, developing and distributing placental tissue allografts with patent-protected, proprietary processes for multiple sectors of healthcare. As a pioneer in placental biologics, we are focused on addressing unmet clinical needs in the areas of advanced wound care, surgical recovery applications, and musculoskeletal conditions. We derive our products from human placental tissues and process these tissues using our proprietary methods, including the PURION® process. We employ Current Good Tissue Practices (“CGTP”), Current Good Manufacturing Practices (“CGMP”), and terminal sterilization to produce our allografts. MiMedx provides products primarily for use in the wound care, burn, and surgical recovery sectors of healthcare. All of our products are regulated by the U.S. Food & Drug Administration (“FDA”).
At MiMedx, our vision is to advance regenerative science and innovative biologics that restore quality of life. Our mission is to improve people’s health and lives through innovation that makes healing possible. By advancing rigorous science and increasing access to evidence-based regenerative technologies, we elevate the standard of care. Our commitment to the highest quality standards maximizes our potential to reduce cost to the healthcare system and restore quality of life. Character, Customer Orientation, Innovation, Collaboration and Stewardship are our core values.
MiMedx is a leading supplier of human placental allografts, which are human tissues that are derived from one person (the donor) and used to produce products that treat another person (the recipient). MiMedx has supplied over two million allografts, through both direct and consignment shipments. Our primary platform technologies include EPIFIX®, AMNIOFIX®, EPICORD®, and AMNIOCORD®. AMNIOFIX and EPIFIX are our tissue allografts derived from the amnion and chorion layers of the human placental membrane. EPICORD and AMNIOCORD are tissue allografts derived from umbilical cord tissue.
Our EPIFIX and EPICORD products are marketed for external use, such as in advanced wound care applications, while our AMNIOFIX and AMNIOCORD products are positioned for use in surgical recovery applications, including lower extremity repair, plastic surgery, vascular surgery and multiple orthopedic repairs and reconstructions. We describe these in greater detail below under the heading “Our Product Portfolio.”
2017 FDA Guidance. The products we sell are regulated by the FDA. Generally, our products are regulated as Human Cells, Tissues and Cellular and Tissue – Based Products (“HCT/Ps”), which do not require pre-market clearance or approval by the FDA and are subject solely to Section 361 of the Public Health Service Act (“Section 361”) and related regulations. However, in November 2017 the FDA published a series of related guidances, including one entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue–Based Products: Minimal Manipulation and Homologous Use – Guidance for Industry and Food and Drug Administration Staff” (the “Guidance”). The Guidance established an updated framework for the FDA’s regulation of cellular and tissue-based products. Among other things, the Guidance clarified the FDA’s views about the criteria that differentiate those products subject to regulation solely under Section 361 (“Section 361 HCT/Ps”) from those cellular and tissue-based products considered to be drugs, devices, and/or biological products (“Section 351 HCT/Ps”) subject to licensure under Section 351 of the Public Health Service Act (“Section 351”) and related regulations.
Effect of Guidance on Our Products. Under the Guidance, we expect that the FDA will continue to regulate certain of our placental tissue products (EPIFIX, AMNIOFIX, EPICORD, AMNIOCORD and AMNIOBURN) as Section 361 HCT/Ps so long as the claims we make for them are consistent with the Section 361 framework. However, the FDA is now regulating certain of our other products, such as our micronized products (AMNIOFIX Injectable and EPIFIX Micronized, collectively “mdHACM” or “micronized dehydrated human amnion chorion membrane”) and our particulate product (AMNIOFILL), as Section 351 HCT/Ps and/or medical devices.
Enforcement Discretion. Under the Guidance, the FDA exercised enforcement discretion under limited conditions with respect to the Investigational New Drug (“IND”) application and pre-market approval requirements for certain HCT/Ps through May 31, 2021. We continued to market our micronized products (mdHACM) and our particulate product (AMNIOFILL) under this policy of enforcement discretion in the United States until May 31, 2021, while at the same time pursuing Biologics License Applications (“BLAs”) for certain of our micronized products in specific clinical applications. After May 31, 2021, we no longer sell our micronized and particulate products in the United States, and do not intend to sell such products in the United States until the FDA grants pre-market approval. As a result, we will only be able to market such products for indications that have been cleared or approved by the FDA. Similarly, we are engaged with the FDA regarding the classification of our umbilical cord products, EPICORD, EPICORD Expandable, and AMNIOCORD, which are tissue allografts derived from the structural, protective covering and extracellular matrix cushioning layers of the umbilical cord. If the FDA makes a final determination that our umbilical cord-derived products do not meet the requirements for regulation solely under Section 361, then the products will require additional pre-market clearance or approval. In 2021, revenues from US sales of our umbilical cord-derived products were $23.6 million. See discussion below – “Risk Factors” under the heading “Certain of our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act, which has resulted in removal of the applicable products from the market, made the introduction of
6
some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.”
Our History
Our current business began on February 8, 2008 when Alynx, Co., our predecessor company, acquired MiMedx, Inc., a development-stage medical device company, the assets of which included licenses to two development-stage medical device technology platforms which we do not currently market. On March 31, 2008, Alynx, Co. merged into MiMedx Group, Inc., a Florida corporation and wholly-owned subsidiary that had been formed for purposes of the merger, with MiMedx Group, Inc. as the surviving corporation in the merger. In January 2011, we acquired all of the outstanding equity interests of Surgical Biologics, LLC (n/k/a MiMedx Tissue Services, LLC).
Current Business Priorities and Strategy
As a pioneer in placental biologics, we are focused on addressing the needs of patients with acute and chronic non-healing wounds in the areas of advanced wound care and surgical recovery. We have a promising late-stage pipeline platform aimed at decreasing pain and improving function in patients with degenerative musculoskeletal conditions. There is significant unmet patient need due to an aging population, an increasing incidence of obesity and diabetes, and other contributing comorbidities that result in a higher susceptibility to non-healing across each of these therapeutic areas. An increasing number of patients require advanced treatment, which represents a significant cost burden on the healthcare system. By incorporating a strategy to advance the scientific and therapeutic potential of placental tissue and more rigorously establish the clinical and economic effectiveness of our products, we believe the Company can differentiate the value of our portfolio and address multiple areas of significant unmet clinical need. We have focused our priorities on initiatives across our Commercial, Operations and Research & Development organizations that position the Company to achieve its goal of sustainable double digit annual percentage growth in our business and advance our late-stage musculoskeletal pipeline.
We believe there are a number of large, underpenetrated market opportunities in the areas of advanced wound care and surgical recovery, including across multiple international markets. We anticipate receiving reimbursement approval in Japan in mid-2022, and plan to launch EPIFIX in Japan as the first amniotic tissue approved for wound treatment across a broad range of conditions. Domestically, we are expanding our addressable markets from the treatment of diabetic foot ulcers, venous leg ulcers, pressure ulcers and complex wounds into areas of surgical recovery where the use of our tissue products could help reduce complications across several specialties, including plastic surgery, general surgery, gynecology, urology, orthopedics, spinal surgery, lower extremity repair and sports medicine procedures. After studying the landscape of surgical procedures, we are targeting certain procedures based on potential complication rate, clinical relevance, economic factors and business priorities. We have a robust pipeline of organic products in development, and have a goal of improving the Company’s Product Vitality Index by launching two new organic products per year, beginning with AMNIOEFFECT™ and our Placental Collagen Matrix (“PCM”) product in 2022. We believe we have a sustainable competitive advantage with our customer focused ecosystem, consisting of a leading portfolio of products, proprietary technology, and best-in-class sales and support organization, together with our broad access and coverage, robust clinical support and medical education efforts, and record of proven outcomes focused on improving patient care.
The Company is also pursuing FDA approval for mdHACM as a platform technology to treat musculoskeletal degeneration across multiple indications, beginning with knee osteoarthritis (“KOA”). In late 2021, we reviewed the results of our Phase 2B KOA clinical trial, which did not meet its primary endpoints, but did yield significant outcomes from the “Pre-Interim Analysis Cohort” consisting of 190 patients. The 190 subjects enrolled prior to an interim analysis performed for sample size correction in July through August 2019 showed a statistically significant and clinically meaningful difference in favor of mdHACM in Western Ontario and McMaster Universities Arthritis Index (“WOMAC”) total scores, and in each of the pain and function subscales compared to the placebo. However, subjects enrolled after this interim analysis did not show separation from the placebo. Root-cause analysis determined that the potency of the investigational product faded as it aged, resulting in the study’s failure to meet its primary endpoints. Based on the data from the Pre-Interim Analysis Cohort in the Phase 2B trial, published retrospective data, extensive real-world clinical use, and ongoing scientific mechanism of action research, the Company expects to initiate a Phase 3 KOA program in 2022, with a BLA filing anticipated in late 2025, and will work closely with the FDA in advancing this program.
Our Product Portfolio
We sell our placenta-based allograft products under our own brands and, on a limited basis, through a private label or original equipment manufacturer (“OEM”). We maintain strict controls on quality at each step of the manufacturing process beginning at the time of procurement. Our Quality Management System is focused on compliance with the American Association of Tissue Banks’ (“AATB”) standards and the FDA’s CGTP, and we are implementing CGMP. We believe the application of CGMP will provide benefits throughout our entire product portfolio, and add to our competitive differentiation.
7
EPIFIX
EPIFIX is a semi-permeable, protective barrier allograft comprised of dehydrated human amnion/chorion membrane that may be used in the treatment of chronic wounds, including diabetic foot ulcers (“DFUs”), venous leg ulcers (“VLUs”), and pressure ulcers. EPIFIX is available in an assortment of sheet configurations and sizes to accommodate various wounds.
MiMedx also has a micronized version of this product that it no longer markets or sells in the United States. As further discussed below under the heading “Government Regulation - Recent FDA Guidance and Transition Policy for HCT/Ps,” the FDA clarified in its 2017 guidance that it regards micronized placental membrane products as subject to FDA licensure as biological products under Section 351.
AMNIOFIX
AMNIOFIX is a semi-permeable, protective barrier allograft comprised of dehydrated human amnion/chorion membrane that may be used in surgical recovery applications. AMNIOFIX is available in an assortment of sheet configurations and sizes for internal use, including in the areas of lower extremity repair, spine, orthopedic, sports medicine, gastrointestinal, urologic, and other general surgery applications.
mdHACM
mdHACM is a micronized form of AMNIOFIX, and supplied in powder form, reconstituted with 0.9% sterile saline for injection. This product is our lead BLA candidate. We completed three late-stage randomized controlled studies under open INDs, evaluating mdHACM in plantar fasciitis, Achilles tendonitis and knee osteoarthritis. While the trials did not meet their primary endpoints, we intend to initiate our Phase 3 clinical trial program for knee osteoarthritis in 2022. For further details, see “--Clinical Trials.”
AMNIOBURN
AMNIOBURN is a semi-permeable, protective barrier allograft comprised of dehydrated human amnion/chorion membrane that may be used in the treatment of partial-thickness and full-thickness burns.
EPICORD and AMNIOCORD
EPICORD and AMNIOCORD are dehydrated human umbilical cord allografts that may be used to provide a protective environment for the healing process and are used in the areas of advanced wound care and surgical recovery. These products are thicker than our amniotic membrane allografts and can be applied in deeper wounds or in areas where suturing the allograft in place may be advantageous.
EPICORD Expandable is an allograft derived from the umbilical cord, and can expand to twice its size, conforming to uneven surfaces and deep wounds. The thickness of the product allows for suturing as needed to keep the graft in place, and it provides healthcare professionals a new option to support the advanced wound care needs of their patients with larger, chronic, and hard-to-heal wounds.
AMNIOFILL
The Company ceased marketing and selling AMNIOFILL in the United States in May 2021, following the end of the FDA’s period of enforcement discretion. We have not yet initiated any clinical trials in furtherance of any regulatory approvals for this product.
OEM Products
We sell a selection of allografts on an OEM basis pursuant to an agreement under which we have granted a third party an exclusive license to some of our technology for use in dental applications.
We continue to research new opportunities for amniotic and other placental tissue, and we have additional offerings in various stages of conceptualization and development.
Placenta Donation Program
We partner with physicians and hospitals to recover donated placental tissue. Through our donor program, a mother who delivers a healthy baby via Caesarean section can donate her placenta and umbilical cord tissue in lieu of having it discarded as medical waste. After consent for donation is obtained, a blood sample from each donor is tested for communicable diseases, and
8
the donor is screened for risk factors in order to determine eligibility in compliance with federal regulations and AATB standards. We operate a licensed tissue bank that is registered as a tissue establishment with the FDA, and we are an accredited member of the AATB. All donor records and test results are reviewed by our Medical Director and staff prior to the release of the tissue for distribution. However, see discussion below, “Risk Factors” under the heading “The products we manufacture and process are derived from human tissue and therefore have the potential for disease transmission.”
We have developed a large, geographically diverse, network of hospitals that participate in our placenta donation program, and we employ a dedicated staff that work with these hospitals. We also utilize a third-party provider of placenta donations on an as- needed basis to mitigate business risk. We believe that we will be able to obtain an adequate supply of tissue to meet anticipated demand for the foreseeable future. However, see discussion below “Risk Factors” under the heading “Our products depend on the availability of tissue from human donors, and any disruption in supply could adversely affect our business.”
Processing (Manufacturing)
The Company has developed and patented a unique and proprietary technique (PURION) for processing allografts from the donated placental tissue. This technique specifically focuses on preserving the tissue’s natural growth factor content and regulatory proteins, and maintaining the structure and collagen matrix of the tissue. Our patented and proprietary processing method employs aseptic processing techniques in addition to terminal sterilization for increased product safety. Despite starting with similar placental tissues, all placental tissue products and processes are not the same – we believe that our proprietary tissue engineering process preserves more of the natural beneficial characteristics of the tissue than the processes used by many of our competitors.
The PURION process produces an allograft that retains the tissue’s inherent biological properties and regulatory proteins (including cytokines, chemokines, and growth factors) found in the placental tissue and produces an allograft that is safe and easy for healthcare providers to use. The allograft can be stored at room temperature and has a five-year shelf life. Each sheet allograft incorporates specialized visual embossments that assist the health care practitioner with allograft placement and orientation.
To ensure the safety of human tissue products, the FDA enforces CGTP manufacturing regulations. We believe that MiMedx has developed mature systems to comply with, and is in compliance with, these regulations. As an important part of the Company’s product safety compliance, MiMedx products are terminally sterilized to an internationally recognized industry standard in addition to having been processed via the PURION process.
Our facilities are subject to periodic unannounced inspections by regulatory authorities and may undergo compliance inspections conducted by the FDA and corresponding state and foreign agencies. We are registered with the FDA as a tissue establishment and are subject to the FDA’s CGTP quality program regulations, state regulations and regulations promulgated by various regulatory authorities outside the United States. The Company’s September 2018 FDA inspection for compliance with CGTP regulations resulted in no observations and a no action indicated (“NAI”) rating, which is the most favorable designation the FDA provides after an inspection. In December 2019, the FDA conducted CGMP inspections at our Marietta, Georgia, and Kennesaw, Georgia, processing facilities. The FDA issued a Form FDA 483 (“483”), which is a list of inspectional observations, at the conclusion of each inspection. Specifically, the FDA issued a 483 consisting of nine observations at our Marietta, Georgia processing facility, and a 483 consisting of 14 observations at our Kennesaw, Georgia processing facility. MiMedx timely responded to the FDA regarding each observation, providing substantive responses to all of the observations. The Company’s response included completed and planned actions to address each observation, and all of these remedial actions have been completed. The FDA classified its December 2019 inspection of our Kennesaw, Georgia facility as voluntary action indicated (“VAI”), which means objectionable conditions or practices were found in their December 2019 inspection but the agency is not taking or recommending any administrative or regulatory actions. The FDA also categorized its December 2019 inspection of our Marietta, Georgia facility as VAI. The Company believes it has significantly progressed its CGMP compliance and maintains a proactive dialogue with the FDA regarding its continued application of CGMP throughout its portfolio.
Intellectual Property
Our intellectual property includes owned and licensed patents, owned and licensed patent applications and patents pending, proprietary manufacturing processes and trade secrets, and trademarks associated with our technology. We believe that our patents, proprietary manufacturing processes, trade secrets, trademarks, and technology licensing rights provide us with important competitive advantages.
Patents and Patent Applications
Due to the substantial expertise and investment of time, effort and financial resources required to bring new regenerative biomaterial products and implants to the market, the importance of obtaining and maintaining patent protection for significant new technologies, products and processes cannot be underestimated. As of the date of the filing of this Annual Report, in addition to international patents and patent applications, we own 62 U.S. patents related to our amniotic tissue technology and products, and 32 additional patent applications covering aspects of this technology are pending at the United States Patent and Trademark Office. The vast majority of our domestic patents covering our core amniotic tissue technology and products will
9
not begin to expire until August 2027. See discussion below – “Risk Factors” under the heading “Risks Related to Our Intellectual Property.”
Market Overview
Domestic sales currently account for substantially all of our revenue, and we are actively pursuing international expansion, primarily targeting Japan and select countries in Europe, Asia Pacific, and the Middle East. In the United States, our primary areas of clinical use include advanced wound care and surgical recovery applications.
Wound Care
The broad wound care category includes traditional dressings such as bandages, gauzes and ointments, which are used to treat non-severe or non-chronic wounds, and advanced wound care products such as medical devices, advanced dressings, xenografts, biological products, and HCT/Ps, which are used as skin substitutes to treat severe wounds or chronic wounds that have not appropriately closed after four weeks of treatment with traditional or standard of care dressings.
In the United States, estimates indicate that in 2021, the prevalence of chronic wounds was 2% of the total U.S. population, or approximately 6.9 million people suffering from chronic wounds. Of these chronic wounds, approximately 58% or 3.9 million are categorized as chronic leg ulcers (which include DFUs and VLUs), with 43% treated with advanced wound care dressings such as skin substitutes (GlobalData: 2021 Wound Care Management- Tissue Engineered Skin Subs - US - 2015-2030). MiMedx is a leader in the cellular tissue products/skin substitute segment of the advanced wound care category and the amniotic tissue allograft sub-category. We expect these markets will continue to grow due to certain demographic trends, including an aging population, increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds. Furthermore, the increasing number of patients requiring advanced treatment represents a significant cost burden on the healthcare system. The overall cost of treating chronic wounds is rising sharply, and the current annual estimated cost in the United States exceeds $28 billion.
Traditional dressings such as bandages, gauzes and ointments, along with treatment of active infection and debridement, currently represent the “standard of care” for treating chronic wounds such as DFUs and VLUs. If, after four weeks of standard of care therapy, the wound has not responded appropriately or improved, clinical research has shown that advanced therapy such as a skin substitute can be beneficial as part of the patient’s treatment plan. However, often times advanced therapies are not employed due to current treatment guidelines, product access, or medical education around the clinical and economic benefits of advanced skin substitutes. We believe this represents a large opportunity for us to expand the market and drive initiatives resulting in market growth. According to data provided by BioMedGPS, MiMedx’s EPIFIX is the current product of choice for physicians choosing to use an amniotic skin substitute product as a barrier or cover. Our EPIFIX and EPICORD products can be stored at room temperature for up to five years compared to certain other skin substitutes currently on the market that require cryogenic freezer storage, have limited shelf life, and may not be human-derived. In addition, we market multiple sizes of EPIFIX and EPICORD sheets for use as protective barriers, which enables a healthcare provider to select an appropriate size graft based on the size of the wound to reduce product waste. Our EPICORD and EPICORD Expandable product lines also offer an alternative treatment option to address larger, deeper wounds in a cost-effective way earlier in the treatment algorithm.
Surgical Recovery
We are expanding beyond advanced wound care into areas of surgical recovery where the use of our tissue products could help reduce complications across several specialties, including plastic surgery, general surgery, gynecology, urology, orthopedics, spinal surgery, lower extremity repair and sports medicine procedures. Certain surgical procedures can have an increased likelihood of complications such as dehiscence, adhesions, and others that may affect both the recovery of the patient and the outcome of the surgery. The rate of complications can depend on a number of factors, including the complexity of the procedure and patient specific issues, such as obesity, diabetes or advanced age.
Surgical recovery applications focus on the use of tissue products to augment tissue, serve as a barrier membrane in procedures where scar tissue formation may be problematic or where a second surgery may be required, or aid in incisional closure with the goal of preventing or reducing procedural complications. Following a thorough review of surgical procedures and potential clinical applications across several specialties, we have identified those areas where we believe our tissue products could be incorporated. We are targeting certain procedures for use of our products based on unmet clinical need, potential procedural complication rate, clinical relevance, economic factors and overall business priorities. As in advanced wound care, we believe this market is expanding as a result of demographic trends, including an aging population, increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds.
International
The Company is actively pursuing international expansion, with an initial focus in Japan. 2021 estimates indicate that within a total Japanese population of approximately 126 million people, there are approximately 626,000 chronic leg ulcers, 100,000 of which are potential candidates for an advanced wound care product (GlobalData Tissue Engineered-Skin Sub Data Model
10
Wound Management Year). The Japanese population has the largest proportion of people 65 or older in the world, estimated to be approximately 36.2 million (28.8%) in 2020, increasing the potential need for healthcare products and services (Statistics Bureau of Japan, https://www.stat.go.jp/english/data/handbook/c0117.html). We believe these demographic trends, along with an increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds, present a significant unmet patient need and underpenetrated market opportunity.
MIMEDX received regulatory approval from the Japanese Ministry of Health, Labor and Welfare in June 2021 to market EPIFIX in Japan, as the first amniotic tissue approved for hard-to-heal chronic wounds, such as DFUs and VLUs, which do not respond to conventional therapy. We expect to secure reimbursement approval in mid- 2022, and are putting in place the necessary structure, medical education programs, and market development initiatives to operationalize our commercial strategy.
The Company is also evaluating opportunities for geographic expansion in the United Kingdom and certain other countries in Europe and the Middle East. Current efforts are focused on the collection of real-world evidence to support the development of patient treatment guidelines, health economic analysis, and product reimbursement in core markets.
Biologics License Application (BLA) Programs
In 2017 the FDA released guidance clarifying its views that certain cellular and tissue-based products, including certain products marketed by MiMedx, are considered drugs, devices, and/or biological products subject to Section 351 requirements under the federal Food, Drug and Cosmetic Act (the “FD&C Act”). In order to conform to this regulatory guidance, MiMedx is pursuing indications under the BLA pathway, although there can be no assurance that we will obtain a BLA and we may ultimately decide not to pursue a BLA for these products or indications. See Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.”
mdHACM is our lead BLA product candidate. We conducted three IND programs in the areas of plantar fasciitis (Phase 3 clinical trial conducted), Achilles tendonitis (Phase 3 clinical trial conducted) and knee osteoarthritis (Phase 2B clinical trial conducted). See Clinical Trials, below, for more information.
After oral non-habit forming pain medication fails to adequately relieve a patient’s joint, ligament or tendon pain, market available injections such as corticosteroids and hyaluronic acid are commonly used treatment options. However, a number of patients still do not get adequate relief from these injections, or do not want to use corticosteroids for a variety of reasons. Additionally, in light of the crisis with opioid abuse, non-surgical treatments and alternative approaches to musculoskeletal pain management are under consideration. Patients and physicians are searching for new products that are safe and effective for the management of chronic and degenerative musculoskeletal conditions.
Osteoarthritis (“OA”) is a disease characterized by progressive articular cartilage destruction, ultimately leading to disabling pain and joint dysfunction. The knee is the most commonly affected joint and knee OA represents the leading cause of disability in the adult population. Estimates indicate that approximately 17.5 million people suffered from symptomatic knee osteoarthritis in 2020 (GlobalData: 2020 Orthopedic Devices Knee Reconstruction - US - 2015-2030), and this number is expected to increase to 19 million people by 2025 (GlobalData: 2020 Orthopedic Devices - Knee Reconstruction - US - 2015-2030). According to the Arthritis Foundation, more than half of knee osteoarthritis sufferers are younger than 65 years old. Current treatment options include analgesics, non-steroidal anti-inflammatory drugs (“NSAIDs”), injectable corticosteroids, viscosupplements, platelet rich plasma, and other emerging therapies. Approximately 80% of symptomatic knee OA patients fail conservative therapy (GlobalData: 2020 Orthopedic Devices - Viscosupplementation - US - 2015-2030). When conservative and non-operative treatment options fail, patients often consider surgical intervention. According to estimates by Global Data’s United States Knee Reconstruction Model, approximately one million people required knee reconstruction surgery in 2020, with 2% needing bilateral knee replacement. Costs for knee replacement procedures can exceed $55,000, on average. We believe there is significant unmet need for a non-surgical treatment option to reduce pain and improve function in patients suffering from knee osteoarthritis. Current estimates of the potential addressable market for mdHACM are dependent on many factors, including the results of our clinical trial program, recommended place in the knee osteoarthritis treatment algorithm, anticipated dosing regimen, as well as the potential for our clinical trials to demonstrate disease modifying characteristics which could further amplify the market opportunity. However, mdHACM has not yet been approved by the FDA for any such use. See Item 1A - Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.”
Marketing and Sales
Our direct sales team includes field sales representatives and field sales management, who call on hospitals, wound care clinics, physician offices, and federal health care facilities such as the Department of Veterans Affairs (the “VA”) and Department of Defense (“DoD”) hospitals. Our direct sales force focuses on the advanced wound care and surgical recovery category through multiple sites of service. We also maintain a network of independent sales agents that focus on surgical recovery applications leveraging the complementary products in their portfolios, and provide access to certain customers, as well as sales coverage for areas where we do not have a full time sales representative.
11
We also sell our products through distributors. Distributors purchase products from us at wholesale prices and resell products to end users. See Note 15 to our consolidated audited financial statements included in Item 8 of this Annual Report, “Revenue.” As discussed above, we sell allografts for certain applications on an OEM basis pursuant to an agreement under which we grant a third party an exclusive license to some of our technology for use in certain fields.
Coverage and Reimbursement
With the exception of government accounts, most purchasers of our products include physicians, hospitals or ambulatory surgery centers (“ASCs”) that rely on reimbursement by third-party payers. Accordingly, our growth substantially depends on adequate levels of third-party reimbursement for our products from these payers. Third-party payers are sensitive to the cost of products and services and are increasingly seeking to implement cost containment measures to control, restrict access to, or influence the purchase of health care products and services. In the U.S., such payers include U.S. federal healthcare programs (e.g., Medicare and Medicaid), private insurance plans, managed care programs and workers’ compensation plans. Federal healthcare programs have prescribed coverage criteria and reimbursement rates for medical products, services and procedures. Similarly, private, third-party payers have their own coverage criteria and negotiate reimbursement amounts for medical products, services and procedures with providers. In addition, in the U.S., an increasing percentage of insured individuals are receiving their medical care through managed care programs (including managed federal healthcare programs) which monitor and may require pre- approval of the products and services that a member receives. Ultimately, however, each third-party payer determines whether and on what conditions they will provide coverage for our products, and such decisions often include each payer’s assessment of the science and efficacy of the applicable product.
A portion of our products are purchased by U.S. government accounts (e.g., the VA and the Public Health Service (including the Indian Health Service), which do not depend on reimbursement from third party payers. In order for us to be eligible to have our products purchased by such federal agencies and paid for by the Medicaid program, federal law requires us to participate in the VA Federal Supply Schedule (“FSS”) pricing program.
Medicare Coverage
The largest third-party payer in the United States is the Medicare program, which is a federally-funded program that provides healthcare coverage for senior citizens and certain disabled individuals. The Medicare program is administered by the Centers for Medicare and Medicaid Services (“CMS”), an agency within the U.S. Department of Health and Human Services (“HHS”). Medicare Administrative Contractors (“MACs”) are private insurance companies that serve as agents of CMS in the administration of the Medicare program and are responsible for making coverage decisions and paying claims for the designated Medicare jurisdiction. There are seven Part A/B MACs in the U.S., which cover 12 jurisdictions, each with its own geographical jurisdictions, and each MAC has its own standards and process for determining coverage and reimbursement for a procedure or product. Private payers often follow the lead of governmental payers in making coverage and reimbursement determinations. Therefore, achieving favorable Medicare coverage and reimbursement is usually a significant gating factor for successful coverage and reimbursement for a new product or clinical application by private payers.
The coverage and reimbursement framework for products under Medicare is determined in accordance with the Social Security Act and pursuant to regulations promulgated by CMS, as well as the agency’s coverage and reimbursement guidance. In some cases, CMS does not specify coverage, leaving each of the MACs to determine whether and on what conditions they will provide coverage for the product. Such decisions are based on each MAC’s assessments of the science and efficacy of the applicable product. As noted below under the heading “Research and Development,” we have devoted significant resources to clinical studies to provide data to the MACs, as well as other payers, in order to demonstrate the clinical efficacy and economic effectiveness of our tissue technologies. As of the date of this report, both EPIFIX and EPICORD allografts are eligible for coverage by all MACs. In January 2019, EPIFIX and EPICORD received separate CMS HCPCS Codes, Q4186 and Q4187, distinguishing each product in coverage and reimbursement policies.
For Medicare reimbursement purposes, our EPIFIX and EPICORD allografts are classified as “skin substitutes.” Current reimbursement methodology varies between the hospital outpatient department (“HOPD”) and ASC setting versus the physician office. Currently, skin substitutes are reimbursed under a “packaged” or “bundled” methodology along with the related application procedure under a two-tier payment system. In the HOPD and ASC setting, providers receive a single payment that reimburses them for the application of the product as well as the product itself. CMS classifies skin substitutes into low cost or high cost groups, based on a geometric mean unit cost and per day cost. For 2022, the geometric mean unit cost threshold applicable to both our EPIFIX and EPICORD allograft products was $48 per square centimeter, and the per day cost threshold was $949. The national HOPD average packaged (“bundled”) rate for our EPIFIX and EPICORD allograft products was $1,568 in 2018, was $1,549 in 2019, was $1,623 in 2020, was $1,715 in 2021, and is $1,749.26 in 2022. This “bundled” payment structure applies only to the HOPD and ASCs settings.
Currently, providers that administer EPIFIX or EPICORD allografts and other skin substitutes in the physician office setting are reimbursed based on the size of the graft, computed on a per square centimeter basis. The payment rate is calculated using the manufacturer’s reported average sales price (“ASP”) submitted quarterly to CMS. This payment methodology applies only to physician offices. The Medicare payment rates are updated quarterly based on this ASP information for many skin substitute
12
products but not all. EPIFIX and EPICORD are included on the Medicare national ASP Drug Pricing File. The published skin substitute Medicare payment rate established by statute is ASP plus 6%. Reimbursement for products not included on the Medicare national ASP Drug Pricing File are at the discretion of each MAC, which typically is invoice cost or wholesale acquisition cost (“WAC”) plus 6%.
Medicare payments for all items and services, including EPIFIX and EPICORD sheet products, since 2013 have been reduced by 2% under the sequestration required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012. Subsequent legislation extended the 2% reduction to 2030 (although the sequestration was suspended from May 1, 2020 through December 31, 2020 due to COVID-19). This 2% reduction in Medicare payments affects all parts of the Medicare program. The law allows for additional sequestration orders, potentially resulting in up to a 4% reduction in Medicare payments under a statutory PAYGO sequestration order. The Coronavirus Aid, Relief, and Economic Security (CARES) Act suspended the sequestration payment adjustment percentage of 2% applied to all Medicare Fee-for-Service (FFS) claims from May 1 through December 31, 2020. The Consolidated Appropriations Act, 2021, extended the suspension period to March 31, 2021. An Act to Prevent Across-the-Board Direct Spending Cuts, and for Other Purposes, signed into law on April 14, 2021, extended the suspension period to December 31, 2021.
Private Payers
We have devoted considerable resources to clinical trials to support coverage and reimbursement of our products. An increasing number of private payers reimburse for EPIFIX and EPICORD in the physician office, the HOPD and the ASC settings, and we have complete national commercial coverage for the use of EPIFIX in the treatment of DFUs. Coverage and reimbursement vary according to the patient’s health plan and related benefits. The majority of health plans currently provide coverage for EPIFIX and EPICORD for the treatment of DFUs, and many include treatment of VLUs. MiMedx has secured payer coverage for over 300 million covered lives, allowing a significant number of patients access to our products. Information contributing to the coverage determination included a third-party technical brief (by the Agency for Healthcare Research and Quality (“AHRQ”)) that evaluated a number of skin substitutes for treating chronic wounds, in which EPIFIX was noted to have the most Randomized Controlled Trials, a low risk of overall study bias, and statistically significant findings.
We have established and continue to grow a reimbursement support group to educate providers and patients with regard to accurate coverage and reimbursement information regarding our products, and plan to continue investing in clinical data supportive of coverage for our products in additional clinical areas of use. See discussion below – “Risk Factors” under the heading “Our revenues depend on adequate reimbursement from public and private insurers and health systems.”
Hospital Use
Products administered in the hospital inpatient setting are bundled when submitted as part of the hospital’s claim under a diagnosis-related group (“DRG”). In these cases, we continue to educate the hospital that our products are cost-effective, and have the potential to improve patient outcomes and reduce the length of stay. We are working to develop additional health economic data to support this effort. As noted above, the ability to sell products in a hospital is dependent upon demonstrating to the hospital the product’s efficacy and cost effectiveness.
Customer Concentration
For the years ended December 31, 2021, 2020, and 2019, our net sales to all U.S. government accounts comprised approximately 3%, 5% and 6% of our net sales, respectively. We have contracted with a third party as our indefinite delivery/ indefinite quantity channel partner into the VA and DoD markets. See discussion below – “Risk Factors” under the heading “A portion of our revenues and accounts receivable come from government accounts.”
Competition
Due to lower barriers of entry in the Section 361 HCT/P regulated market, competition in the placenta-based and allograft tissue field is intense and subject to new entrants and evolving market dynamics. Companies within the industry compete on the basis of price, ease of handling, logistics and efficacy. Another important factor is third-party reimbursement, which is difficult to obtain as it is a time-consuming and expensive process. We believe our success in obtaining third-party reimbursement, our strong position with group purchasing organizations, capabilities and investments to apply CGMP, and established clinical evidence for our products are competitive advantages.
In February 2020, the AHRQ published a technology assessment analyzing Skin Substitutes for Treating Chronic Wounds. AHRQ conducted a literature search yielding 164 studies and 81 Supplemental Evidence and Data for Systematic Reviews (“SEADs”) submissions. Only 22 randomized, controlled trials (“RCTs”) met the inclusion criteria to be reviewed in the AHRQ analysis, and out of the 22 RCTs MiMedx had six RCTs included in the final brief. Of the 22 studies reviewed, only 12 were assessed as low risk of bias, of which five were MiMedx RCTs. This important government assessment highlights our commitment to providing unbiased level 1 clinical evidence in advanced wound treatment. This dedication to elevating the standard of care is further underscored by the fact that the AHRQ points out in its assessment that MiMedx was the only entity
13
to provide two studies out of the 22 evaluated that performed a subgroup analysis of patients with diabetic foot ulcers that received adequate debridement. Both studies reported an increase in wounds healed with adequate debridement.
Advanced wound care therapies employ technologies to aid in wound healing in cases where the wound is chronic and healing progress has stalled or stopped. The primary competitive products in the skin substitutes category include, among others, placental-tissue allografts, tissue-engineered living skin equivalents, porcine-, bovine- and fish skin-derived xenografts and collagen matrix products. Xenografts, or tissue transplants from non-human species, serve mainly as an extracellular matrix and have to undergo aggressive processing to remove immunogenic animal products from the tissue. In addition, challenges with xenografts include limited clinical published data, and some products may require suturing or stapling to the wound bed, making handling more difficult. Furthermore, other skin substitutes currently on the market require cryogenic freezer storage and have limited shelf life.
Our main competitors in the skin substitute market include Integra LifeSciences Holdings Corporation, Organogenesis, Inc., and Smith & Nephew plc, which sell a variety of advanced wound care products, including skin substitutes and placental tissue allografts. In addition, the overall market is competitive, with a large number of other competitors that compete regionally and nationally.
See discussion below – “Risk Factors” under the heading “We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants.”
Government Regulation
The products manufactured and processed by the Company are derived from human tissue. As discussed below, our Section 361 HCT/Ps are tissue-based products that are regulated solely under Section 361 and do not require pre-market clearance or approval by the FDA. Our Section 351 HCT/Ps are also tissue products, but are regulated as biological products, and, in order to be lawfully marketed in the United States, require FDA pre-market approval. See discussion below – “Risk Factors” under the heading “Risks Related to Regulatory Approval of Our Products and Other Government Regulations.”
Tissue Products
In 1997, the FDA proposed a regulatory framework for cells and tissues. This framework was intended to provide adequate protection of public health while enabling the development of new therapies and products with limited regulatory burden. A key innovation in the system was that covered HCT/Ps would be regulated solely under Section 361 and would not be subject to pre-market clearance. The registration and listing rules were finalized in January 2001 in 21 CFR Part 1271. Additional rules regarding donor eligibility and good tissue practices were soon adopted. Together, these rules form a comprehensive system intended to encourage significant innovation.
The FDA requires each HCT/P establishment to register and establish that its product meets the requirements to qualify for regulation solely under Section 361. To be a Section 361 HCT/P, a cellular or tissue-based product generally must meet all four of the following criteria (fully set forth in 21 CFR Part 1271):
•it must be minimally manipulated;
•it must be intended for homologous use;
•its manufacture must not involve combination with another article, except for water, crystalloids or a sterilizing, preserving or storage agent; and
•it must not have a systemic effect and must not be dependent upon the metabolic activity of living cells for its primary function.
Certain amniotic and other birth tissues are considered cellular and tissue-based articles and are therefore eligible for regulation solely as a Section 361 HCT/P depending on whether the specific product at issue and the claims made for it are consistent with the criteria set forth above. HCT/Ps that do not meet these criteria are subject to more extensive regulation as drugs, medical devices, biological products or combination products.
Products Regulated Solely as Section 361 HCT/Ps
The FDA has specific regulations governing HCT/Ps, including some regulations specific to Section 361 HCT/Ps, which are set forth in 21 CFR Part 1271. All establishments that manufacture Section 361 HCT/Ps must register and list their HCT/Ps with the FDA’s Center for Biologics Evaluation and Research within five days after commencing operations. In addition, establishments are required to update their registration annually in December or within 30 days of certain changes and submit changes in HCT/P listing at the time of or within six months of such change.
The regulations in 21 CFR Part 1271 also require establishments to comply with donor screening, eligibility and testing requirements and CGTP to prevent the introduction, transmission and spread of communicable diseases. The CGTP govern, as may be applicable, the facilities, controls and methods used in the manufacture of all HCT/Ps, including processing, storage, recovery, labeling, packaging and distribution of Section 361 HCT/Ps. CGTP require us, among other things, to maintain a
14
quality program, train personnel, control and monitor environmental conditions as appropriate, control and validate processes, properly store, handle and test our products and raw materials, maintain our facilities and equipment, keep records and comply with standards regarding recovery, pre-distribution, distribution, tracking and labeling of our products and complaint handling. 21 CFR Part 1271 also mandates compliance with adverse reaction and CGTP deviation reporting and labeling requirements.
The FDA conducts periodic inspections of HCT/P manufacturing facilities, and contract manufacturers’ facilities, to assess compliance with CGTP. Such inspections can occur at any time, with or without written notice, at such frequency as determined by the FDA in its sole discretion. To determine compliance with the applicable provisions, the inspection may include, but is not limited to, an assessment of the establishment’s facilities, equipment, finished and unfinished materials, containers, processes, HCT/Ps, procedures, labeling, records, files, papers and controls required to be maintained under 21 CFR Part 1271. If the FDA were to find serious non-compliant manufacturing or processing practices during such an inspection, it could take regulatory actions that could adversely affect our business, results of operations, financial condition and cash flows. See Item 1A Risk Factors, “Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly, and our failure to comply could result in negative effects on our business, results of operations and financial condition.”
2017 FDA Guidance and Transition Policy for HCT/Ps
In November 2017, the FDA released four guidance documents that, collectively, the agency described as a “comprehensive policy framework” for applying existing laws and regulations governing regenerative medicine products, including HCT/Ps. One guidance document in particular, “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue – Based Products: Minimal Manipulation and Homologous Use – Guidance for Industry and Food and Drug Administration Staff,” offered important clarity.
The guidance documents confirmed that sheet forms of amniotic membrane generally are appropriately regulated as solely Section 361 HCT/Ps when intended for use as a barrier or covering. We continually evaluate our marketing materials for each of our products to align with FDA guidance.
Second, the guidance documents confirmed the FDA’s stance that all micronized amniotic membrane products are more than minimally manipulated, and therefore do not qualify as Section 361 HCT/Ps. However, the guidance documents also stated that the FDA intended to exercise enforcement discretion under limited conditions with respect to the IND application and pre-market approval requirements for certain HCT/Ps through November 2020, which was later extended through May 2021. This period of enforcement discretion was intended to give sponsors time to evaluate their products, have a dialogue with the agency and, if necessary, begin clinical trials and file the appropriate pre-market applications. The FDA’s approach was risk-based, and the guidance documents clarified that high-risk products and uses could be subject to immediate enforcement action.
This enforcement discretion applied across our industry, and during the period, the Company continued to market its products under this policy of enforcement discretion. After May 31, 2021, the Company no longer markets or sells its micronized and particulate products in the United States. We are pursuing the BLA pre-market approval process for certain uses of mdHACM. However, there is no assurance that the FDA will grant these approvals on a timely basis, or at all, or that we will not discontinue our pursuit of a BLA for certain products or indications. See “Clinical Trials” below for more information.
Products Regulated as Biologics – The BLA Pathway
The typical steps for obtaining FDA approval of a BLA to market a biological product in the United States include:
•Completion of preclinical laboratory tests, animal studies and formulations studies under the FDA’s Good Laboratory Practice regulations;
•Submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin and which must include independent Institutional Review Board approval at each clinical site before the trials may be initiated;
•Performance of adequate and well-controlled clinical trials in accordance with Good Clinical Practices to establish the safety and efficacy of the product and its dosage (as applicable) for each indication;
•Development of purity, potency and identity tests to demonstrate consistency and reliability of the manufacturing process through a chemistry, manufacturing and control program;
•Submission to the FDA of a BLA for marketing the product that includes, among other things, reports of the outcomes and full data sets of the clinical trials, and proposed labeling and packaging for the product;
•Satisfactory review of the contents of the BLA by the FDA, including the satisfactory resolution of any questions raised during the review;
•Satisfactory completion of an FDA Advisory Committee review, if applicable;
•Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with FDA’s CGMP regulations, to assure that the facilities, methods and controls are adequate to ensure the product’s identity, potency, quality and purity; and
•FDA approval of the BLA, including agreement on post-marketing commitments, if applicable.
Generally, clinical trials are conducted in three phases, though the phases may overlap or be combined. Phase 1 trials typically involve a small number of healthy volunteers and are designed to provide information about the product safety and to evaluate the pattern of drug distribution and metabolism within the body. Phase 2 trials are conducted in a larger but limited group of
15
patients afflicted with a particular disease or condition in order to determine preliminary efficacy, dosage tolerance and optimal dosing, and to identify possible adverse effects and safety risks. Dosage studies are typically designated as Phase 2A, and efficacy studies are designated as Phase 2B. Phase 3 clinical trials are generally large-scale, multi-center, comparative trials conducted with patients who have a particular disease or condition in order to provide statistically valid proof of efficacy, as well as safety and potency. In some cases, the FDA will require Phase 4, or post-marketing trials, to collect additional data after a product is on the market. All phases of clinical trials are subject to extensive record keeping, monitoring, auditing and reporting requirements.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that the Company has failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, such as issuing an FDA Form 483 notice of inspectional observations; sending a warning letter or untitled letter; issuing an order of retention, destruction, or cessation of marketing; imposing civil money penalties; suspending or delaying issuance of approvals; requiring product recalls; imposing a total or partial shutdown of production; withdrawing approvals or clearances already granted; pursuing product seizures, consent decrees or other injunctive relief; and criminal prosecution through the Department of Justice (“DOJ”).
Clinical Trials
Trial Overview
The Company recently completed three IND studies investigating the use of mdHACM to reduce pain and increase function in patients with plantar fasciitis, Achilles tendonitis, and knee osteoarthritis. As previously disclosed, the trials were developed and initially overseen by senior managers, many of whom are no longer with the Company. The Company has instituted several actions with respect to its ongoing and anticipated clinical trials to address the resources, capabilities and expertise needed for an effective dialogue with the FDA regarding our BLA progress. However, there can be no assurance that we will obtain BLA approval and we may ultimately decide not to pursue a BLA for certain products or indications. See Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.”
Plantar Fasciitis
In March 2015, we initiated a Phase 2B prospective, single-blinded, RCT investigating a single injection of 40 mg of mdHACM as compared to a single intra-plantar injection of saline (placebo control) in the treatment of patients with recalcitrant plantar fasciitis pain and foot dysfunction. This trial enrolled 145 patients at 15 study sites. In September 2017, we announced the trial had met its efficacy endpoints, and the three-month endpoint data were published in 2018.
In April 2017, we met with the FDA and informally discussed preliminary data from the Phase 2B study, our progress toward achieving CGMP compliance, and our proposed Phase 3 study design. Formal FDA feedback from this meeting was incorporated into our development plans. Based on this feedback and the Phase 2B interim data, in January 2018 we initiated a Phase 3 prospective, double-blinded, RCT to assess the safety and efficacy of a single 40 mg intra-plantar injection of mdHACM as compared to a single intra-plantar injection of saline (placebo control) to treat patients with recalcitrant plantar fasciitis pain. The trial plan was initially to enroll 164 patients, with an interim analysis to assess adequacy of this sample size built into the statistical plan. In July through August 2019, we conducted an interim analysis on subjects representing 50% of total enrollment that had reached the primary efficacy endpoint, to assess adequacy of the sample size to assess differences between the two treatment groups. This analysis indicated that a significant increase in sample size would be required to observe clinically and statistically significant improvement and separation between treatment and control groups. We determined that increasing the sample size to 276 patients would provide sufficient power to observe an efficacy result with statistical and clinical significance. We instituted these changes and amendments and completed enrollment of 277 subjects in September 2020. Following completion of the study, initial review of the trial data during the third quarter of 2021 revealed that the study did not meet its endpoints. The data from the study continue to be the subject of extended analyses, however, as previously disclosed, we do not expect to file a BLA or pursue further studies in this indication at this time.
Knee Osteoarthritis
In March 2018, the FDA granted mdHACM the Regenerative Medicine Advanced Therapy (“RMAT”) designation for use in the treatment of osteoarthritis of the knee. RMAT-designated products are eligible for increased and earlier interactions with the FDA, similar to those interactions available to fast-track and breakthrough-designated therapies. In addition, these products may be eligible for rolling review and accelerated approval. The meetings with sponsors of RMAT- designated products may include discussions of whether accelerated approval would be appropriate based on surrogate or intermediate endpoints reasonably likely to predict long-term clinical benefit or reliance upon data obtained from a meaningful number of sites.
In March 2018, we initiated a Phase 2B prospective, double-blinded RCT investigating a single intra-articular injection of 40 mg of mdHACM as compared to a single injection of saline (placebo control) in the treatment of pain and functional impairment in patients with osteoarthritis of the knee. This trial was planned to enroll 318 patients, with an interim analysis to assess adequacy of this sample size built into the statistical plan. This blinded interim analysis was performed in July through
16
August 2019 and revealed that while differences in the treatment groups were observed, the power to observe statistically and clinically significant results would be enhanced by increasing the sample size to 466 patients. Amendments to the protocol to allow this increase were subsequently approved. It should be noted that during the first half of 2020 in particular, study enrollment slowed considerably due to the ongoing COVID-19 pandemic , although this did begin to resolve in the third quarter of the year. Due to actual dropout rates observed in the study being lower than planned, in September 2020, we completed enrollment of 447 patients. We also amended the protocol to establish an open label extension to the trial and allow patients to receive a second injection of the active treatment at six months, nine months, or 12 months subsequent to their completion of study visits, if their pain has not resolved or responded, regardless of treatment arm. The study was still blinded to subjects, sites and MiMedx during this extension. The six months blinded efficacy visits in this study were completed during the second quarter of 2021, and analyses were completed during the third quarter of 2021. The final study visits are expected to occur (open label extension) during the second quarter of 2022.
As previously announced, the trial did not meet its primary endpoints, however it revealed that the 190 subjects enrolled prior to an interim analysis performed for sample size correction in July through August 2019 showed a statistically significant and clinically meaningful difference in favor of mdHACM in WOMAC total scores and both the pain and function subscales compared to the placebo. However, subjects enrolled after this interim analysis did not show separation from the placebo. Third-party biostatisticians validated the improvement in WOMAC Pain at three and six months, respectively (p=0.032 and p=0.009), WOMAC Function (p=0.046 and p=0.009), and WOMAC Total (p=0.038 and p=0.008) for the Pre-Interim Analysis Cohort of 190 patients. Our root-cause analysis has determined that the potency of the investigational product faded as it aged, resulting in the study’s failure to meet its primary endpoints. The Company’s proprietary biochemical and biological tests detected this reduced potency, related to the age of the investigational product used in the Phase 2B KOA study. Based on the clinically meaningful and statistically significant data from the Pre-Interim Analysis Cohort of 190 patients in the Phase 2B trial, published retrospective data, extensive real-world clinical use, and ongoing scientific mechanism of action research, the Company expects to initiate a Phase 3 KOA program in 2022, with a BLA filing anticipated in late 2025, and will work closely with the FDA in advancing these trials.
There can be no assurance, however, that our anticipated time frame for commencing the Phase 3 KOA program and submitting a BLA will be achieved or that we will receive FDA approval for mdHACM and be able to commercialize this product, or that such approval will not be delayed for a variety of reasons, including failure of the studies to achieve their endpoints; the impact of the COVID-19 pandemic on study enrollment and FDA operations; the potential that the results of the clinical studies do not merit further investment; and the work required to achieve commercial and manufacturing readiness. See discussion in Item 1A - “Risk Factors” under the heading “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time-consuming and may impede our ability to fully exploit our technologies.”
Achilles Tendonitis
In January 2018, we initiated a Phase 3 prospective, double-blinded RCT investigating a single intra-tendon injection of 40 mg of mdHACM as compared to a single injection of saline (placebo control) in the treatment of Achilles tendonitis. The planned trial enrollment was 158 patients, with an interim analysis to assess adequacy of the sample size built into the statistical plan. We analyzed data received from this sample size analysis, conducted on patients representing 50% of total enrollment that had reached the primary efficacy endpoint. This analysis indicated that a substantial increase in sample size would be required to observe clinically and statistically significant improvement and separation between treatment and control groups. With this in mind, we determined that the most reasonable approach was to continue the study to completion with the originally planned sample size, and analyze the final results to determine the adequacy of the measures employed and time points of observation to show meaningful clinical and statistical analyses. Enrollment for this study was completed and the last patient visit occurred in the first half of 2021. The data from this study are currently being prepared for analysis. We plan to review our options for this program after we have assessed the results of this study; however, as previously disclosed, we do not expect to file a BLA or pursue further studies in this indication at this time.
Prior to May 31, 2021, the date the FDA’s period of enforcement discretion ended, we filed appropriate investigational applications for AMNIOFILL and EPIFIX Micronized. Two INDs were approved for EPIFIX, one in chronic wounds, another in surgical incisions, and an investigational device exemption (“IDE”) was filed for AMNIOFILL. We have not yet initiated any clinical trials for AMNIOFILL or EPIFIX Micronized related to these applications, and have no immediate plans to advance these programs.
BLA Process
If study results support potential product approval and potential for commercialization, we intend to file BLAs as described above. The process of obtaining an approved BLA requires the expenditure of substantial time, effort and financial resources and may take years to complete. The fee for filing a BLA and the annual user fees payable with respect to any establishment that manufactures biologics and with respect to each approved product are substantial. While there can be no assurance that we will ultimately obtain regulatory approval for our micronized products, we have already completed substantial work towards our BLA program, including engineering our manufacturing processes to conform to CGMP requirements.
FDA Post–Market Regulation
17
Tissue processors regulated solely under Section 361 are still required to register as a tissue establishment with the FDA. As a registered tissue establishment, we are required to comply with regulations regarding labeling, record keeping, donor eligibility, screening and testing. We are also required to process the tissue in accordance with established CGTP, as well as report any deviations from core CGTP requirements or adverse reactions caused by a possible transmission of an infectious disease attributed to our tissue. Our facilities are also subject to periodic inspections to assess our compliance with the regulations.
Products covered by a BLA, New Drug Application, 510(k) clearance or a pre-market approval are subject to numerous additional regulatory requirements, which include, among others, compliance with CGMP (or, in the case of devices, with FDA’s Quality System Regulation), which imposes certain procedural, substantive and record keeping requirements, and labeling regulations to ensure the product’s identity, potency, quality, and purity. These products are also subject to the FDA’s general prohibition against promoting products for unapproved or “off-label” uses, and additional adverse reaction reporting.
As part of our BLA development effort, we are updating our manufacturing establishments into maintaining application of CGMP for production of our injectable and other applicable Section 351 products. The process includes development and enhancement of production processes, procedures, tests and assays, and it requires extensive validation work. It also involves the procurement and installation of new production and lab equipment. These efforts require human capital, expertise and resources. We have made significant improvements over the last two years. We have engaged industry experts to assess our state of compliance and to provide guidance on the additional activities needed to maintain CGMP. Significant improvements include a newly built, validated processing suite applying CGMP that is utilized for processing of Section 351 products. See discussion in Item 1A – “Risk Factors” under the heading “Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.”
Other Regulation Specific to Tissue Products
National Organ Transplant Act
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act (“NOTA”), which prohibits the transfer of certain human organs, including skin and related tissue, for valuable consideration, but permits the reimbursement of reasonable expenses associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. Our wholly-owned subsidiary, MiMedx Tissue Services, LLC, is registered with the FDA as an establishment that manufactures human cells, tissues and cellular and tissue- based products and is involved with the recovery and storage of donated human amniotic membrane. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery and storage of donated human tissue.
Tissue Bank Laws, Regulations, and Related Accreditation
As discussed above, we are required to register with the FDA as an establishment that manufactures human cells, tissues and cellular and tissue-based products. We are licensed, registered, or permitted as a tissue bank in California, New York, Delaware, Illinois, Oregon, and Maryland. Additionally, we received and actively maintain AATB accreditation. The AATB has issued operating standards for tissue banking. Compliance with these standards is required in order to become an AATB-accredited tissue establishment. AATB standards include specific requirements for recovery, screening, testing, labeling, processing, and storing of birth tissue. We believe we are compliant in all material respects with AATB standards and our state licensure requirements.
To the extent we sell our products outside of the United States, we also are subject to laws and regulations of foreign countries.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including CMS, other divisions of the HHS (e.g., the Office of Inspector General), the DOJ and individual United States Attorney offices within the DOJ, and state and local governments. These regulations include those described below. See also the discussion under “Risk Factors - We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause an adverse effect on our business, results of operations and financial condition.”
•The federal Anti-Kickback Statute, which is a criminal law that prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward referrals, purchases or orders, or arranging for or recommending the purchase, order or referral of any item or service for which payment may be made in whole or in part by a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. The Patient Protection and Affordable Care Act amended the intent requirement of the federal Anti-Kickback Statute, so that a person or entity no longer needs to have actual knowledge of this statute or
18
specific intent to violate it. A conviction for violation of the Anti-Kickback Statute results in criminal fines and requires mandatory exclusion from participation in federal health care programs. Although there are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common industry practices from prosecution, the exceptions and safe harbors are drawn narrowly, and arrangements may be subject to scrutiny or penalty if they do not fully satisfy all elements of an available exception or safe harbor.
•The federal False Claims Act (“FCA”) imposes significant civil liability on any person or entity that knowingly presents, or causes to be presented, a claim for payment to the U.S. government, including the Medicare and Medicaid programs, that is false or fraudulent. The FCA also allows a private individual or entity as a whistleblower to sue on behalf of the government to recover civil penalties and treble damages. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of between $11,181 and $22,363 per false claim or statement for penalties assessed after January 29, 2018, with respect to violations occurring after November 2, 2015. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. See also Item 3, “Legal Proceedings.”
•The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) fraud and abuse provisions prohibit executing a scheme to defraud any healthcare benefit program, willfully obstructing a criminal investigation of a health care offense, or making false statements or concealing a material fact relating to payment for healthcare benefits, items or services.
•While manufacturers of human cell and tissue products regulated solely under Section 361 are not subject to the federal Physician Payments Sunshine Act and its implementing regulations (together with the Act, the “Sunshine Act”), in the future, if we expand our product portfolio beyond those regulated solely under Section 361, this law will require us (with certain exceptions) to report information to CMS related to certain payments or other transfers of value we make to U.S.-licensed physicians and teaching hospitals, and for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists and certified nurse-midwives. If we receive a BLA approval, the Sunshine Act would also require us to report annually certain ownership and investment interests held by U.S.-licensed physicians and their immediate family members. Such information will subsequently be made publicly available by CMS on the Open Payments website. There is a risk that CMS or another government agency may take the position that our products are not human cell and tissue products regulated solely under Section 361, and thereby assert that we are currently subject to the Sunshine Act, which could subject us to civil penalties and the administrative burden of having to comply with the law.
•Federal conflicts of interest laws, the Standards of Ethical Conduct for Employees of the Executive Branch, and local site policies for each federal institution we call upon govern our interactions with federal employees at our various government accounts (e.g., DoD, VA, etc.) and impose a number of limitations on such interactions.
•There are state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of protected health information. Among other things, HITECH made HIPAA’s privacy and security standards directly applicable to “business associates,” independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Research and Development
Our research and development group has extensive experience in developing products for our target markets, and works to design products that are intended to improve patient outcomes, simplify techniques, shorten procedures, reduce hospitalization and rehabilitation times and, as a result, reduce costs. Our research and development group also works to establish scientific evidence in support of the use of our products. Clinical trials that demonstrate the safety, efficacy and cost effectiveness of our products are key to obtaining broader third-party reimbursement for our products. In addition to our internal staff, we contract with outside laboratories and physicians who aid us in our research and development process. See Part II, Item 7, below, for information regarding expenditures for research and development in each of the last three fiscal years.
19
Environmental Matters
Our tissue preservation activities generate a small amount of chemical and biomedical waste, consisting primarily of diluted alcohols and acids and human biological waste, including human tissue and body fluids removed during laboratory procedures. The biomedical waste generated by our tissue processing operations are placed in appropriately constructed and labeled containers and are segregated from other waste. We contract with third parties for transport, treatment, and disposal of our biomedical waste.
Human Capital
As of December 31, 2021, we had 811 full time employees. Generally, we consider our relationships with our employees to be good, and none of our employees are covered by a collective bargaining agreement. We conduct an annual survey of employees to monitor engagement levels and act on feedback received through this process.
We strive to promote diversity, inclusion and equal opportunity across the organization. In 2020, we formed an Inclusion and Diversity Council with the goal of supporting strategic initiatives and practices to foster an inclusive, diverse and equitable organization in order to better serve our customers and their patients. Women and minorities hold a third of the seats on our Board of Directors, including the Chair of the Board. As of December 31, 2021, 55% of our employees are women, and women comprised 56% and 57% of our new hires in 2021 and 2020, respectively. Additionally, as of December 31, 2021, approximately 22% of our workforce self-identifies as Black or African American, 7% as Hispanic or Latino, and 4% as other non-White (including American Indian, Alaskan Native, Asian, Native Hawaiian, or Other Pacific Islander).
We track turnover and retention for all employees. We also track time-to-hire and time-to-train for certain departments. In the last year, turnover has been elevated relative to historical trends. We have adopted specific measures and incentives to improve retention within the most affected organizational areas.
The health of our workforce is important to us, particularly that of our processing employees and other employees who, based on their specific job tasks and requirements, have not been able to work remotely during the ongoing COVID-19 pandemic. We employ approximately 77 highly-trained employees in our processing area. While we process donated tissue using aseptic techniques in a controlled environment, the manufacturing space is a confined space in which an employee with COVID-19 may spread the virus to other employees despite the use of personal protective equipment in all required areas at MiMedx. To date, we have been successful in mitigating these risks through a variety of measures, including screening employees for COVID-19 prior to entering our facilities at earlier stages of the pandemic, implementing a number of safety protocols, partnering with a testing facility to provide test kits and rapid results for employees that have symptoms or have a known risk of exposure, and supplying employees with appropriate personal protective equipment. However, there can be no assurance that we will continue to be successful. See Item 1A., Risk Factors, “The COVID-19 pandemic and governmental and societal responses thereto have adversely affected our business, results of operations and financial condition, and the continuation of the pandemic or the outbreak of other health epidemics could harm our business, results of operations, and financial condition.”
Available Information
We are required to file proxy statements, annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K with the SEC. The SEC maintains an internet site, www.sec.gov, where these reports are available free of charge. We also make these reports available free of charge on our website, www.mimedx.com, under the heading “Investors–SEC Filings.” In addition, our Audit Committee, Compensation Committee, Ethics and Compliance Committee, and Nominating and Corporate Governance Committee Charters as well as our Code of Business Conduct and Ethics, are on our website under the heading “Investors–Corporate Governance.” The reference to our website does not constitute incorporation by reference of any information contained on that site.
20
Item 1A. Risk Factors
An investment in our Common Stock involves a substantial risk of loss. Set forth below is a summary of the risks and uncertainties affecting our business that we currently believe to be material. We caution you to read the following risk factors, which have affected, and/or in the future could affect, our business, prospects, operating results, and financial condition. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also affect our business, prospects, operating results, and financial condition. Additional risks and uncertainties are described under other captions in this report and should also be considered by our stockholders. If any of these risks materialize, our business, financial condition or operating results could suffer. In this case, the trading price of our Common Stock could decline, and you may lose part or all of your investment.
Summary of Risk Factors Risks Related to Our Business and Industry •If we do not successfully execute our priorities, our business, operating results and financial condition could be adversely affected. •We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants. •Rapid technological change could cause our products to become obsolete. •Our products depend on the availability of tissue from human donors, and any disruption in supply could adversely affect our business. •The COVID-19 pandemic and governmental and societal responses thereto have adversely affected our business, and the continuation of the pandemic or the outbreak of other health epidemics could harm our business. •We depend on our senior leadership team and may not be able to retain or replace these employees or recruit additional qualified personnel. •A portion of our revenues and accounts receivable come from government accounts. •Our revenues depend on adequate reimbursement from public and private insurers and health systems. •Our revenue, results of operations and cash flows may suffer upon the loss of a GPO or IDN. •We contract with independent sales agents and distributors. •Disruption of our processing could adversely affect our business, financial condition and results of operations. •To be commercially successful, we must convince physicians, where appropriate, how and when our products are proper alternatives to existing treatments and that our products should be used in their procedures. •If we cannot successfully address quality issues that may arise with our products, our brand reputation could suffer, and our business, financial condition, and results of operations could be adversely impacted. •The formation of physician-owned distributorships (“PODs”) could result in increased pricing pressure on our products or harm our ability to sell our products to physicians who own or are affiliated with those distributorships. •We face the risk of product liability claims and may not be able to obtain or maintain adequate product liability insurance. •The products we manufacture and process are derived from human tissue and therefore have the potential for disease transmission. •We may implement a product recall or voluntary market withdrawal. •A cyberattack or significant disruptions of information technology systems could adversely affect our business. •We may expand or contract our business through acquisitions, divestitures, licenses, investments, and other commercial arrangements. •New lines of business or new products and services may subject us to additional risks. •Our international expansion and operations outside the U.S. expose us to risks. Risks Related to Regulatory Approval of Our Products and Other Government Regulations •Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future. •If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance. Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies. | ||
21
•If any of the BLAs are approved, we would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance. •Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies. •Our business is subject to extensive regulation by the FDA and other authorities, which is costly. •We may be subject to fines, penalties, injunctions and other sanctions if we are deemed to be promoting the use of our products for unapproved, or off-label, uses. •We and our sales representatives must comply with various federal and state anti-kickback, self-referral, false claims and similar laws. •Our results of operations may be adversely affected by current and potential future healthcare reforms. •We may fail to obtain or maintain foreign regulatory approvals to market our products in other countries. •Federal and state laws that protect the privacy and security of personal information may increase our costs and limit our ability to collect and use that information and subject us to liability if we are unable to fully comply with such laws. Risks Related to Our Intellectual Property •Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain and may be inadequate. •We may become subject to claims of infringement of the intellectual property rights of others. •We may be subject to damages resulting from claims that we, our employees, or our independent contractors have wrongfully used or disclosed alleged trade secrets, proprietary or confidential information of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors. Risks Related to our Past Audit Committee Investigation, Consolidated Financial Statements, Internal Controls and Related Matters •If we fail to maintain adequate internal control over financial reporting in the future, this could adversely affect our business, financial condition and operating results. •Negative publicity, including publicity relating to or arising from the Restatement, the Audit Committee Investigation, or related matters, has had and could continue to have an adverse effect on our business, results of operations and financial condition. •We are currently, in the past have been, and may in the future be, subject to substantial litigation and ongoing investigations that could cause us to incur significant legal expenses and result in harm to our business. Risks Related to the Securities Markets and Ownership of Our Common Stock •Our substantial indebtedness may adversely affect our financial health. •Our variable rate indebtedness under the Hayfin Loan Agreement subjects us to interest rate risk. •EW Healthcare Partners and its interests may conflict with those of our other shareholders. •Holders of shares of our Series B Preferred Stock have rights, preferences and privileges that are not held by, and are preferential to, the rights of, our common shareholders. •Our Series B Preferred Stock is convertible into shares of our Common Stock, and any such conversion may dilute the value of our Common Stock. •The price of our Common Stock has been, and will likely continue to be, volatile. •Securities analysts may elect not to report on our common stock or may issue negative reports that adversely affect the stock price. •Fluctuations in revenue or results of operations could cause additional volatility in our stock price. •We do not intend to pay cash dividends on our Common Stock. •Certain provisions of Florida law and anti-takeover provisions in our organizational documents may discourage or prevent a change of control. | ||
22
Risks Related to Our Business and Industry
If we do not successfully execute our priorities, our business, operating results and financial condition could be adversely affected.
Our priorities in our advanced wound care and surgical recovery business are to address large, underpenetrated market opportunities, domestically and internationally, including by launching new organic or inorganic products. We intend to implement and maintain rigorous CGMP standards throughout our entire supply chain and continue to advance the scientific body of evidence substantiating clinical efficacy, economic viability and the underlying mechanism of action for our PURION processed placental tissue platform through additional peer-reviewed publications, rigorous scientific research and clinical studies. We are also focused on pursuing FDA approval for mdHACM as a platform technology to treat musculoskeletal degeneration across multiple indications, beginning with initiating a Phase 3 KOA program.
We have sought and may continue to seek capital to implement our priorities. In developing our priorities, we evaluated many factors including, without limitation, those related to developments in our industry, customer demand, competition, regulatory developments, and general economic conditions. Actual conditions may be different from our assumptions, and we may not be able to successfully execute our priorities. If we do not successfully execute our priorities, or if actual results vary significantly from our assumptions, our business, operating results and financial condition could be adversely impacted.
We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants.
Our business is in a very competitive and evolving field. Competition from other tissue processors, medical device companies, and biotherapeutic companies, and from research and academic institutions, is intense, expected to increase and subject to rapid change and could be significantly affected by new product introductions. Established competitors and newer market entrants are investing in additional clinical research that may allow them to gain further clinician usage, adoption and payer coverage of their products. In addition, consolidation and cost containment measures in the healthcare industry may cause hospitals to consolidate their purchases with suppliers that have a broad portfolio of products. This would continue to give rise to demands for price concessions, which could have an adverse effect on our business, results of operations and financial condition. Further, competitors may introduce placental-based membrane products in the future at lower prices, adding new features or gaining additional reimbursement coverage, or utilize sales and marketing practices that negatively impact the industry. Further, they may copy our products outside the United States. The presence of this competition may lead to pricing pressure, which could have an adverse effect on our business, results of operations and financial condition.
Rapid technological change could cause our products to become obsolete and, if we do not enhance our product offerings through our research and development efforts, we may be unable to compete effectively.
The technologies underlying our products are subject to rapid and profound technological change. Competition intensifies as technical advances in each field are made and become more widely known. Others may develop services, products or processes with significant advantages over the products, services and processes that we offer or are seeking to develop. Any such occurrence could have an adverse effect on our business, results of operations and financial condition.
We plan to enhance and broaden our product offerings as part of a strategy that involves responding to changing customer demands and competitive pressure and technologies, among other factors. The success of any new product offering or enhancement to an existing product will depend on numerous factors, including our ability to:
•properly identify and anticipate physician and patient needs;
•acquire, through licensing, co-development or outright purchase, new technology developed outside of MiMedx;
•develop and introduce new products or product enhancements in a timely manner;
•adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties;
•demonstrate the safety and efficacy of new products; and
•obtain the necessary regulatory clearances or approvals for new products or product enhancements.
If we do not develop and, when necessary, obtain regulatory clearance or approval for new products or product enhancements in time to meet market demand, or if there is insufficient demand for these products or enhancements, our results of operations and financial condition will suffer. Our research and development efforts may require a substantial investment of time and resources, including additional capital, before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. In addition, even if we are able to successfully develop enhancements or new generations of our products, these enhancements or new generations of products may not produce sales in excess of the costs of development, or they may never receive required regulatory approval and they may be quickly rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
23
Our products depend on the availability of tissue from human donors, and any disruption in supply could adversely affect our business.
The success of our human tissue products depends upon, among other factors, the availability of tissue from human donors. Any failure to obtain tissue from our sources will interfere with our ability to effectively meet demand for our products incorporating human tissue. The availability of donated tissue could also be adversely impacted by regulatory changes, public opinion of the donor process and our own reputation in the industry. We may not be successful in our ability to scale tissue recovery efforts to meet the potential future demand of our pipeline. Obtaining adequate supplies of human tissue involves several risks, including limited control over availability (for example, access to hospital accounts and the number of consenting mothers), quality and delivery schedules. In addition, any interruption in the supply of any human tissue component could harm our ability to manufacture our products until a new source of supply, if any, could be found. We also utilize third-party providers of placental donations on an as-needed basis to mitigate risks but there can be no assurance that these third parties will be able to provide donated tissues at all times. We may be unable to find a sufficient alternative supply channel in a reasonable time period or on commercially reasonable terms, if at all, which would have an adverse effect on our business, results of operations and financial condition.
The COVID-19 pandemic and governmental and societal responses thereto have adversely affected our business, results of operations and financial condition, and the continuation of the pandemic or the outbreak of other health epidemics could harm our business, results of operations, and financial condition.
The COVID-19 pandemic and governmental and societal responses thereto have adversely affected our business, results of operations and financial condition, and will likely continue to do so. See Item 7, “Management’s Discussion and Analysis - Results of Operations.” The continuation or additional waves of the COVID-19 pandemic may continue to adversely affect our operations and increase our costs and expenses in numerous ways. For example:
–We source raw materials for our products from donated placentas from scheduled C-section births via a large, geographically-diverse network of donor hospitals. We may experience shortages of donated placentas if donors or our recovery specialists are excluded from hospitals, or if our donor recovery specialists contract COVID-19 and are required to quarantine. We experienced interruptions from a portion of our hospitals in certain geographic areas in the first half of 2020,in late 2021 and in early 2022. To date, we have been successful in mitigating this disruption to our supply by adding additional donor hospitals, increasing efforts at hospitals that did not impose access limits, and using third-party providers of donated placentas (where necessary and in accordance with MiMedx quality standards). However, there can be no assurance that our efforts to source raw materials for our products will continue to be successful, and we may experience shortages of raw materials, especially if the current pandemic, including further strains, or responses thereto intensify. Additionally, we may experience shortages of donated placentas if additional testing protocols are implemented for donated tissues based on guidance issued by the American Association of Tissue Banks, the FDA, or other standards, and are screened as ineligible.
–We process donated tissue using aseptic techniques in a controlled environment. However, the manufacturing space is a confined space area in which an infected employee may spread the virus to other employees despite the use of personal protective equipment required for all areas at MiMedx. To date, we have been successful in mitigating these risks through a variety of measures, including in the initial stages of the pandemic screening employees for COVID-19 prior to entering our facilities, implementing a number of safety protocols, partnering with a testing facility to provide test kits and rapid results for employees that have symptoms or have a known risk of exposure, and supplying appropriate employees with personal protective equipment. However, there can be no assurance that our efforts to prevent wide scale infections among our processing staff will continue to be successful, especially if the current pandemic or responses thereto intensify. If we experience widescale infections among our production staff, we may experience a shortage of finished goods.
–Our ability to sell our products was hampered by the pandemic. In many areas of the country, our sales force was excluded from hospitals and the offices of other health care providers. Additionally, many patients stayed away from hospitals and other medical facilities. This had an adverse effect on our revenues beginning late in the first quarter of 2020 and continuing into April 2020. By mid-May, access restrictions to hospitals and offices of healthcare providers had eased for our sales force, and significant numbers of patients began to return for treatment, including for elective procedures. This trend continued into the third and fourth quarters of 2020, where we saw net sales generally consistent with the comparable periods from 2019 on an “as-shipped” basis. In certain areas, local or regional surges of COVID-19 have continued, and future sales will depend on patients’ willingness and ability to visit healthcare providers for care, and our sales force’s access to healthcare providers. The timing, impact, and response to the pandemic has been uneven across the country. Subsequent waves may have a greater impact than did earlier waves,
24
depending on a myriad of factors, including, but not limited to, the availability and efficacy of vaccines, the emergence and severity of new variants of the virus, infection rates, mitigation efforts, and societal response. We are not able to estimate the future effect of COVID-19 on patient behavior and, consequently, future demand or the ability of providers to pay for our products.
–Similarly, our clinical researchers, clinical study coordinators, and their patients experienced restrictions in their access to hospitals and ability to access other healthcare providers, which slowed enrollment in our clinical trials. For example, from mid-March through mid-May 2020, many patients stayed away from hospitals and other medical facilities, which stalled enrollments in our clinical trials. We subsequently concluded enrollment in and completed our three IND trials, but plan to initiate new trials in 2022. If such access were to be restricted again, it might again impair or delay the initiation, approval and launch of future products or additional clinical trials. See “Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.”
If our leadership, employees, sales agents, suppliers, medical professionals, or users of our products are impacted by an epidemic, by illness, or through social distancing, quarantine or other precautionary measures taken in connection therewith, then our manufacturing operations, sales, demand for our products, and clinical trials may be adversely affected.
Disruptions to the health care system generally, such as if patients are unable or unwilling to visit health care providers, or if health care providers prioritize treatment of acute or communicable illnesses over wound care, have affected and may continue to adversely affect our revenues and results of operations.
The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to change. We do not yet know the full extent of delays or impacts on our business, our clinical trials, healthcare systems or the global economy as a whole, or how long such effects will endure. The effects of the COVID-19 pandemic or other health epidemics could have an adverse impact on our business, results of operations and financial condition.
We depend on our senior leadership team and may not be able to retain or replace these employees or recruit additional qualified personnel, which would harm our business, results of operations and financial condition.
Our business and success are materially dependent on attracting and retaining members of our senior leadership team to formulate and execute the Company’s business plans. Since June 2018, we have replaced a majority of our senior leadership team, and hired several new senior leaders.
Leadership changes can be inherently difficult to manage and may cause material disruption to our business or management team. Changes in senior management could also lead to an environment that presents additional challenges in recruiting and retaining employees, which could have an adverse effect on our business, results of operations and financial condition. We experienced difficulties in recruiting due to legal and business uncertainties resulting from the issues that were the subject of the Audit Committee Investigation.
Our future success will also depend, in part, upon our ability to attract and retain skilled personnel, including sales, managerial and technical personnel. There can be no assurance that we will be able to continue to find and attract additional qualified employees to support our expected growth or retain any such personnel.
A portion of our revenues and accounts receivable come from government accounts.
Some of our revenues are derived from sales, both direct and through a distributor, to the government. Any disruption of our products on the FSS, or of the use of Indefinite Delivery, Indefinite Quantity contracts (“IDIQ”), or any change in the way the government purchases products like ours or the price it is willing to pay for our products, could adversely affect our business, results of operations and financial condition.
Our revenues depend on adequate reimbursement from public and private insurers and health systems.
Our success depends on the extent to which our customers receive adequate reimbursement for the costs of our products and related treatments from third-party payers, including government healthcare programs, such as Medicare and Medicaid, as well as private insurers and health systems. Government and other third-party payers attempt to contain healthcare costs by limiting both coverage and the level of reimbursement of medical products, particularly new products. Therefore, significant
25
uncertainty may exist as to the reimbursement status of new healthcare products by third-party payers. Although EPIFIX and EPICORD have coverage with the majority of large payers, a significant number of public and private insurers and health systems currently do not cover or reimburse our other products.
If we are not successful in obtaining adequate coverage and reimbursement for our products from these third-party payers, it could have an adverse effect on market acceptance of our products. Inadequate reimbursement levels would likely also create downward price pressure on our products. Even if we do succeed in obtaining widespread coverage and reimbursement rates or policies for our products, future changes in coverage or reimbursement rates or policies could have a negative impact on our business, financial condition and results of operations.
Further, we have experienced some reluctance by payers to cover products for applications other than those for which we have published clinical efficacy data. Currently, there are four MACs that do not have a written medical policy in the form of a Local Coverage Determination (“LCD”) or a specific article for skin substitutes. In the absence of an LCD, MACs will reimburse based on medical necessity. If these three MACs created written medical policy criteria that limit providers to the use of products that have published clinical evidence for a specific wound type such as Diabetic Foot Ulcer or Venous Leg Ulcer only, we could experience a negative impact on revenue. Our future revenues could experience additional declines if other MACs or other payers further limit their coverage of our products to specific clinical uses. This decline would adversely affect our business, financial condition and results of operations.
Our revenue, results of operations and cash flows may suffer upon the loss of a Group Purchasing Order or Integrated Delivery Network.
As with many manufacturers in the healthcare space, the Company contracts with Group Purchasing Organizations (“GPOs”) and Integrated Delivery Networks (“IDNs”) to establish contracted pricing and terms and conditions for the members of GPOs and IDNs. Approximately three-quarters of our sales in the year ended December 31, 2021 came from customers that are members of our primary GPOs or IDNs.
Our agreements with GPOs and IDNs allow us to sell our products efficiently to large groups of customers. Our agreements with GPOs and IDNs typically provide their members with favorable ordering terms and conditions and access to favorable product pricing. These customers purchase our product through GPO and IDN arrangements in part because of the favorable pricing and terms and conditions. If our agreement with any GPO or IDN is terminated or expires without being extended, renewed or renegotiated, this could adversely affect our revenue, results of operations and cash flows.
We contract with and are dependent upon independent sales agents and distributors.
In 2021, approximately 20% of our sales were through our relationships with independent agents, and we also use a small number of distributors, primarily outside the United States, and may use more in the future. (Sales agents act directly on behalf of MiMedx to arrange sales, while distributors take title to product and may set their own prices.) See Note 15, “Revenue” to our consolidated audited consolidated financial statements included in Item 8, Consolidated Financial Statements and Supplementary Data.
If our relationships with our independent sales agents were terminated for any reason, it could materially and adversely affect our revenues and profits. Because the independent agent often controls the customer relationships within its territory, there is a risk that if our relationship with the agent ends, our relationship with the customer will be lost.
Because our agents and distributors are not employees, there is a risk we will be unable to ensure that our sales processes, compliance safeguards, and related policies will be adhered to despite our communication and training of agents and distributors regarding these requirements. Furthermore, if we fail to maintain relationships with our key independent agents, or fail to ensure that our independent agents adhere to our sales processes, compliance safeguards and related policies, there could be an adverse effect on our business, results of operations, and financial condition.
We may obtain the assistance of additional distributors and independent sales representatives to sell products in certain sales channels, particularly in territories and fields where agents are commonly used. Our success is partially dependent upon our ability to train, retain and motivate our independent sales agencies, distributors, and their representatives to appropriately and compliantly sell our products in certain territories or fields. They may not be successful in implementing our marketing plans or compliance safeguards. Some of our independent sales agencies and distributors do not sell our products exclusively and may offer similar products from other companies. Our independent sales agencies and distributors may terminate their contracts with us, may devote insufficient sales efforts to our products or may focus their sales efforts on other products that produce greater commissions for them, which could have an adverse effect on our business, results of operations and financial
26
condition. We also may not be able to find additional independent sales agencies and distributors who will agree to appropriately and compliantly market or distribute our products on commercially reasonable terms, if at all. If we are unable to establish new independent sales representative and distribution relationships or renew current sales agency and distribution agreements on commercially acceptable terms, our business, financial condition, and results of operations could be materially and adversely affected.
Disruption of our processing facilities could adversely affect our business, financial condition and results of operations.
Our business depends upon the continued operation of our processing facilities in Marietta, Georgia and Kennesaw, Georgia. Risks that could impact our ability to use these facilities include the occurrence of natural and other disasters, the outbreak of pandemics, and the need to comply with the requirements of directives from government agencies, including the FDA. See above, for example, “ - - The COVID-19 pandemic and governmental and societal responses thereto have adversely affected our business, results of operations and financial condition, and the continuation of COVID-19 or the outbreak of other health epidemics could harm our business, results of operations, and financial condition.”
Either of our two processing facilities can serve as a redundant processing facility for our Section 361 products in the event the other facility experiences a disaster event. For our 351 products, we have transitioned manufacturing to our Kennesaw, Georgia facility to comply with CGMP standards, and implemented these standards for upstream and downstream supply chain activities at our Marietta, Georgia facility. However, if our processing facilities were to become unavailable, this could have a material adverse effect on our business, financial condition and results of operations during the period of such unavailability.
To be commercially successful, we must educate physicians, where appropriate, how and when our products are proper alternatives to existing treatments and that our products should be used in their procedures.
We believe physicians will only use our products if they determine, based on their independent medical judgment and experience, clinical data, and published peer reviewed journal articles, that the use of our products in a particular procedure is a favorable alternative to other treatments. Physicians may be hesitant to change their existing medical treatment practices for the following reasons, among others:
•their lack of experience with advanced therapeutics, such as our placenta-based allografts;
•lack of evidence supporting additional patient benefits of advanced therapeutics, such as our placenta-based allografts, over conventional methods in certain therapeutic applications;
•perceived liability risks generally associated with the use of new products and procedures;
•limited availability of reimbursement from third-party payers;
•more favorable reimbursement for other market-available products; and
•the time that must be dedicated to physician training in the use of our products.
If we cannot successfully address quality issues that may arise with our products, our brand reputation could suffer, and our business, financial condition, and results of operations could be adversely impacted.
In the course of conducting our business, we must adequately address quality issues that may arise with our products, as well as defects in third-party components included in our products, as any quality issues or defects may negatively impact physician use of our products. Although we have established internal procedures to minimize risks that may arise from quality issues, we may not be able to eliminate or mitigate occurrences of these issues and associated liabilities. If the quality of our products does not meet the expectations of physicians or patients, then our brand reputation could suffer and our business could be adversely impacted. We must also ensure any promotional claims made for our products comport with government regulations.
The formation of physician-owned distributorships (“PODs”) could result in increased pricing pressure on our products or harm our ability to sell our products to physicians who own or are affiliated with those distributorships.
PODs are medical product distributors that are owned, directly or indirectly, by physicians. These physicians derive a proportion of their revenue from selling or arranging for the sale of medical products for use in procedures they perform on their own patients at hospitals that agree to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based directly or indirectly on those orders of medical products. The Office of Inspector General (“OIG”) of the Department of Health & Human Services has issued a Special Fraud Alert on PODs, indicating that they are inherently suspect under the federal Anti-Kickback Statute.
27
Our commercial strategy emphasizes selling directly to healthcare providers and, to a limited extent, through distributors. To our knowledge, we do not directly sell to or distribute any of our products through PODs. The number and strength of PODs in the industry may continue to grow as economic pressures increase throughout the industry and hospitals, insurers and physicians search for ways to reduce costs, and, in the case of the physicians, identify additional sources to increase their incomes. These companies and the physicians who own, or partially own, PODs may have significant market knowledge, access to and influence on the physicians who use our products and the hospitals that purchase our products, and we may not be able to compete effectively for business from physicians who own PODs.
We face the risk of product liability claims and may not be able to obtain or maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the manufacturing, processing and marketing of human tissue products. We may be subject to such claims if our products cause, or appear to have caused, an injury. Claims may be made by patients, healthcare providers or others selling our products. Product liability claims can be expensive to defend (regardless of merit), divert our management’s attention, result in substantial damage awards against us, harm our reputation, and generate adverse publicity, which could result in the withdrawal of, or reduced acceptance of, our products in the market.
Although we have product liability insurance that we believe is adequate, this insurance is subject to deductibles and coverage limitations, and we may not be able to maintain this insurance at an acceptable cost or on acceptable terms or be able to secure increased coverage (if needed), nor can we be sure that existing or future claims against us will be covered by our product liability insurance. Moreover, the existing coverage of our insurance or any rights of indemnification and contribution that we may have may not be sufficient to offset existing or future claims. If we are unable to maintain product liability insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect ourselves against potential product liability claims or we underestimate the amount of insurance we need, we could be exposed to significant liabilities, which may harm our business. A product liability claim or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could result in significant costs and significant harm to our business. Even if a claim is not successful, defending such claim would be time-consuming and expensive, may damage our reputation in the marketplace, and would likely divert our management’s attention.
The products we manufacture and process are derived from human tissue and therefore have the potential for disease transmission.
The utilization of human tissue creates the potential for transmission of communicable disease, including, without limitation, human immunodeficiency virus, viral hepatitis, syphilis and other viral, fungal or bacterial pathogens. We are required to comply with federal and state regulations intended to prevent communicable disease transmission.
We maintain strict quality controls designed in accordance with CGTP to ensure the safe procurement and processing of our tissue, including terminal sterilization of our products. These controls are intended to prevent the transmission of communicable disease. However, risks exist with any human tissue implantation. We are also implementing and maintaining CGMP systems to comply with the regulations that will apply to our Section 351 HCT/Ps, and believe this provides an added level of quality throughout our manufacturing process. However, negative publicity concerning disease transmission from other companies’ improperly processed donated tissue could have a negative impact on the demand for our products and adversely affect our business, financial condition and results of operations.
We may implement a product recall or voluntary market withdrawal, which could significantly increase our costs, damage our reputation, disrupt our business and adversely affect our business, results of operations and financial condition.
The processing and marketing of our tissue products involves an inherent risk that our tissue products or processes may not meet applicable quality standards and requirements. In the event that one or more of our products experiences a failure to meet such standards and requirements, we may voluntarily implement a recall or market withdrawal or may be required to do so by a regulatory authority.
A recall or market withdrawal of one of our products could be costly and may divert management resources. A recall or withdrawal of one of our products, or a similar product processed by another entity, also could impair sales of our products as a result of confusion concerning the scope of the recall or withdrawal, or as a result of the damage to our reputation for quality and safety.
A cyberattack or significant disruptions of our information technology systems could adversely affect our business, results of operation and financial condition.
28
A cyberattack, a disruption in availability, or the unauthorized alteration of systems or data could adversely affect our business, results of operations and financial condition. We rely on technology for day-to-day operations as well as positioning to enhance our stance in the market. We generate intellectual property that is central to the future success of the business and transmit large amounts of confidential information. Additionally, we collect, store and transmit confidential information of customers, patients, employees and third parties. We also have outsourced significant elements of our operations to third parties, including significant elements of our information technology infrastructure, and, as a result, we are managing many independent vendor relationships with third parties who may or could have access to our confidential information. The continually changing threat landscape of cybersecurity today makes our systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees, partners, and vendors, and from attacks by malicious third parties, including supply chain attacks originating at our third-party partners. Such attacks are of ever-increasing levels of sophistication. Attacks are made by individuals or groups that have varying levels of expertise, some of which are technologically advanced and well-funded including, without limitation, nation states, organized criminal groups and hacktivists organizations.
To ensure protection of our information, we have invested in cybersecurity and have implemented processes and procedural controls to maintain the confidentiality and integrity of such information. We measure these controls and their success through a cybersecurity framework that is based on industry standards. While we have invested in the protection of our data and technology, there can be no guarantees that our efforts will prevent all service interruptions or security breaches. Any such interruption or breach of our systems could adversely affect our business operations and result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal and reputational harm to our business, including legal claims and proceedings, liability under laws that protect the privacy of personal information, government enforcement actions and regulatory penalties, as well as remediation costs. We also maintain cyber liability insurance. However, this insurance may not be sufficient to cover the financial, legal or reputational losses that may result from an interruption or breach of our systems.
We may expand or contract our business through acquisitions, divestitures, licenses, investments, and other commercial arrangements with other companies or technologies, which may adversely affect our business, results of operations and financial condition.
We periodically evaluate opportunities to acquire companies or divest divisions, technologies, products, and rights through licenses, distribution agreements, investments, and outright acquisitions to grow our business. In connection with one or more of those transactions, we may, subject to the requirements and limitations set forth in our secured credit agreement (the “Hayfin Loan Agreement”) with Hayfin Services, LLP (“Hayfin”) an affiliate of Hayfin Capital Management LLP:
•issue additional equity securities that would dilute the value of equity currently held by our shareholders;
•divest or license existing products or technology;
•use cash that we may need in the future to operate our business;
•incur debt that could have terms unfavorable to us or that we might be unable to repay;
•structure the transaction in a manner that has unfavorable tax consequences, such as a stock purchase that does not permit a step-up in the tax basis for the assets acquired;
•be unable to realize the anticipated benefits, such as increased revenues, cost savings, or synergies from additional sales; and
•be unable to secure the services of key employees related to the transaction(s).
Any of these items could adversely affect our revenues, results of operations and financial condition. Business acquisitions also involve the risk of unknown liabilities associated with the acquired business, which could be material. Incurring unknown liabilities or the failure to realize the anticipated benefits of any transaction could adversely affect our business if we are unable to recover our initial investment. Inability to recover our investment, or any write off of such investment, associated goodwill or assets could have an adverse effect on our business, results of operations and financial condition.
New lines of business or new products and services may subject us to additional risks.
From time to time, we may implement or may acquire new lines of business or offer new products and services within existing lines of business. There are risks and uncertainties associated with these efforts, particularly in instances where the markets are not fully developed or are evolving. In developing and marketing new lines of business and new products and services, we may invest significant time and resources. External factors, such as regulatory compliance obligations, competitive alternatives, and shifting market preferences, may also impact the successful implementation of a new line of business or a new product or service. Failure to successfully manage these risks in the development and implementation of new lines of
29
business or new products or services could have an adverse effect on our business, results of operations and financial condition.
Our international expansion and operations outside the U.S. expose us to risks associated with international sales and operations.
We are pursuing further expansion outside the U.S., including in Japan. Managing a global organization is difficult, time consuming and expensive. Our ability to conduct international operations is affected by many of the same risks we face in our U.S. operations, as well as unique costs and difficulties of managing international operations. Risks inherent in international operations also include, among others, potential adverse tax consequences, greater difficulty in enforcing intellectual property rights, risks associated with the Foreign Corrupt Practices Act and local anti-bribery law compliance, and other international regulations. These regulations may limit our ability to market, sell, distribute or otherwise transfer our products to prohibited countries or persons. International regulations may also limit what promotional claims we may make for our products.
Compliance with these regulations and laws is costly, and failure to comply with applicable legal and regulatory obligations could adversely affect us in a variety of ways that include, without limitation, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities.
These risks may limit or disrupt our expansion, restrict the movement of funds or result in the deprivation of contractual rights or the taking of property by nationalization or expropriation without fair compensation. Operating outside of the U.S. also requires significant management attention and financial resources.
Risks Related to Regulatory Approval of Our Products and Other Government Regulations
Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.
The products we manufacture and process are derived from human tissue. Amniotic and other birth tissue have in the past generally been regulated as HCT/P and were therefore eligible to be subject to regulation solely under Section 361 (“Section 361 HCT/P”) depending on whether the specific product at issue and the claims made for it were consistent with the applicable criteria. HCT/Ps that do not meet these criteria are subject to more extensive regulation as drugs, medical devices, biological products, or combination products. These HCT/Ps must comply with both the FDA’s requirements for HCT/Ps and the requirements applicable to biologics, devices or drugs, including pre-market clearance or approval from the FDA. Obtaining FDA pre-market clearance or approval involves significant time and investment by the Company.
In November 2017, the FDA released a guidance document entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue - Based Products: Minimal Manipulation and Homologous Use - Guidance for Industry and Food and Drug Administration Staff.” The document confirmed the FDA’s stance that all micronized amniotic products require a biologics license to be lawfully marketed in the United States. It also confirmed that sheet forms of amniotic tissue are appropriately regulated as solely Section 361 HCT/Ps when manufactured in accordance with 21 CFR Part 1271 and intended for use as a barrier or covering. The final guidance also stated that the FDA intended to exercise enforcement discretion under limited conditions with respect to the IND application and pre-market approval requirements for certain HCT/Ps through November 2020, which was later extended through May 2021. The FDA’s approach was risk-based, and the Guidance clarified that high-risk products and uses could be subject to immediate enforcement action.
After May 31, 2021, the Company has not marketed or sold its micronized products in the United States, has requested the return of unused consignment inventory as of that date, and does not intend to sell such products in the United States until the FDA grants pre-market approval. Our sales of such products for all uses was $17.6 million, $31.8 million, and $42.4 million, respectively, in 2021, 2020, and 2019, primarily in the United States. However, we are pursuing the BLA pre-market approval process for certain of our micronized products, as more fully discussed under “Business - Government Regulation.” The loss of our ability to market and sell our micronized products has had an adverse impact on our revenues, business, financial condition and results of operations.
30
Also, the Company currently markets EPICORD and AMNIOCORD, tissue products derived from the protective covering and extracellular matrix cushioning layers of the human umbilical cord, as providing a protective environment or as a barrier. In warning letters to several companies marketing human umbilical cord derived products for a variety of uses, the FDA has stated that those products fail to meet one or more of the Section 361 criteria, including the minimal manipulation criterion, the dependence on the metabolic activity of living cells for their primary function criterion, and the homologous use criterion, as “the product is not intended to perform the same basic function or functions of umbilical cord in the recipient as in the donor, such as serving as a conduit.” We are engaged with the FDA regarding the classification of our umbilical cord-derived products. If the FDA makes a final determination that our umbilical cord products do not meet the requirements for regulation solely under Section 361, then pre-market clearance or approval will be required for those products. The loss of our ability to market and sell our umbilical cord derived products would have an adverse impact on our revenues, business, financial condition and results of operations. Included in net sales were sales of umbilical cord-derived products totaling $23.6 million, $16.1 million, $17.9 million, respectively, in 2021, 2020, and 2019, almost entirely in the United States.
Any future regulatory changes could also have adverse consequences for us and make it more difficult or expensive for us to conduct our business by requiring pre-market clearance or approval and compliance with additional post-market regulatory requirements with respect to those products. For example, the FDA may in the future impose conditions, such as labeling restrictions, and the requirement that a product be manufactured in compliance with CGMP. Although the Company is preparing for these requirements in connection with its pursuit of a BLA for certain of its products, earlier compliance with these conditions would require significant additional time and cost investments by the Company. Moreover, increased regulatory scrutiny within the industry in which we operate could lead to increased regulation of HCT/Ps, including Section 361 HCT/Ps, which could ultimately increase our costs and adversely impact our business, results of operations and financial condition. If the FDA approves the BLAs we seek, we will incur increased compliance costs on an ongoing basis. See “ - - If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.”
If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.
Products subject to the FDA’s BLA requirements must comply with a range of pre- and post-market provisions. Pre-market compliance includes the conduct of clinical trials in support of BLA approval, the development and submission of a BLA, and the production of product for use in the clinical trials that meets FDA’s quality expectations. We have been making enhancements in our fixed plant as well as incurring costs and reduced product yields from testing products to ensure quality, identity, purity, and potency. Post-approval requirements for BLA products include: compliance with CGMP, which will require us to comply with promotional and labeling requirements, which limit our ability to make claims about regulated products; submission of annual reports in appropriate circumstances; compliance with the FDA’s “Biological Product Deviation Reporting System,” when applicable; submission of adverse events; reporting and correcting product problems within established timeframes; recalling or stopping the manufacture of a product if a significant problem is detected; complying with the appropriate laws and regulations relevant to the biologics licensed and identifying any changes needed to help ensure product quality. In some instances, the FDA can also require that applicants conduct post-market studies or trials of the product. This additional compliance burden may increase costs, and failure to comply with such requirements may subject the Company to sanctions that would have an adverse impact on our business, results of operations and financial condition.
Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time consuming and may impede our ability to fully exploit our technologies.
The process of obtaining regulatory clearances or approvals to market a biological product or medical device from the FDA or similar regulatory authorities outside of the U.S. may be costly and time consuming, and such clearances or approvals may not be granted on a timely basis, or at all. We are pursuing approval of BLAs for certain of our micronized products, but have not yet submitted a BLA for review. Additionally, the FDA may take the position that some of the other products that we currently market require a BLA as well. Some of the future products and enhancements to our current products that we expect to develop or may acquire and market may require marketing clearance or approval from the FDA. However, clearance or approval may not be granted with respect to any of our products or enhancements and further FDA review may add delays that could adversely affect our ability to market such products or enhancements.
The process of obtaining an approved BLA, including clinical trial development and execution as well as manufacturing processes, requires the expenditure of substantial time, effort and financial resources and may take years to complete. The fee for filing a BLA and program fees payable with respect to any establishment that manufactures biologics are substantial. Additionally, there are significant costs associated with clinical trials that can be difficult to accurately estimate until a BLA is approved. Clinical trials may not be successful or may return results that do not support approval. Moreover, data obtained from clinical trials are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or
31
prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all, or we may decide not to pursue a BLA for certain products or indications, or need to conduct additional trials for a given indication. Additionally, the FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products. If we do receive approval, some types of changes to the approved product, such as adding new indications or doses, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval. Our revenues will be adversely affected if we fail to obtain BLA approvals on a timely basis or at all, or if the FDA limits the indications for use or requires other conditions that restrict the commercial application of our products.
Clinical trials will be necessary to support future BLA submissions and potential product approvals by the FDA. The clinical trial process is lengthy and expensive with uncertain outcomes, and often requires the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Delays or failures in our clinical trials could prevent us from commercializing any modified or new products and would adversely affect our business, operating results and prospects.
The results of early clinical trials are not necessarily predictive of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials. Our interpretation of data and results from our clinical trials does not ensure that we will achieve similar results in future clinical trials. In addition, clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their products performed satisfactorily in earlier clinical trials or retrospective studies have nonetheless failed to replicate results in later clinical trials. Products in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through nonclinical studies and earlier clinical trials and retrospective studies, and such failures can occur at any stage of clinical testing. Our clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and non-clinical testing in addition to those we have planned.
The initiation and completion of a trial may be prevented, delayed, or halted for numerous reasons, including, but not limited to, the following:
•regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol, or place a clinical study on hold;
•patients do not enroll in, or enroll at a lower rate than we expect or need, or do not complete a clinical study. For instance, in 2020, the time necessary to complete our studies was longer than expected as a result of access restrictions at hospitals and health care provider facilities as a result of the COVID-19 Pandemic;
•patients or investigators do not comply with study protocols;
•the FDA may require us to submit data on a greater number of patients than we originally anticipated and/or for a longer follow-up period or change the data collection requirements or data analysis applicable to our clinical trials;
•patients do not return for post-treatment follow-up at the expected rate;
•patients may experience serious or unexpected adverse side effects for a variety of reasons that may or may not be related to our product causing a clinical trial study to be put on hold;
•we may be unable to recruit a sufficient number of clinical trial sites;
•sites participating in an ongoing clinical study may withdraw, requiring us to engage new sites;
•third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule, or act in ways inconsistent with the investigator agreement, clinical study protocol, good clinical practices, or other regulatory requirements;
•third-party entities do not perform data collection and analysis in a timely or accurate manner;
•we may have to amend clinical trial protocols or conduct additional studies to reflect changes in regulatory requirements or guidance, which we may be required to submit to regulatory authorities for approval;
•the cost of clinical trials may be greater than we anticipate; and
•regulators or other reviewing bodies may fail to approve or subsequently find fault with our manufacturing processes or facilities, the supply of materials necessary to conduct clinical trials may be insufficient, inadequate or not available at an acceptable cost, or we may experience interruptions in supply.
Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of certification or regulatory approval of our product candidates.
Our ability to consistently and reliably manufacture our biologic products will be key to the marketing of any future Section 351 products. Also, our current manufacturing facilities may be inadequate to produce sufficient quantities if our planned BLA program is approved.
The manufacture of biologic products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, and the approval of BLAs require one to demonstrate the ability to manufacture pursuant to specified chemistry and manufacturing controls. Manufacturers of biologic products often encounter difficulties in production, particularly in scaling up initial production as would be the case at any new facility. These problems
32
can include difficulties with production costs and yields, quality control (including stability of the product candidate and quality assurance testing), shortages of qualified personnel, and compliance with strictly enforced federal, state and foreign regulations. If we were to encounter any of these difficulties, or otherwise fail to comply with our obligations under applicable regulations, then our ability to provide product candidates to patients in our clinical trials or commercially would be jeopardized, and any delay or interruption in the supply of product could delay the commercial launch of the product or impair our ability to meet demand for the product.
Our products can be manufactured only in a facility that has undergone a satisfactory inspection by the FDA and other relevant regulatory authorities. While we currently possess redundant manufacturing capacity, we may not be able to replace manufacturing capacity for our products quickly if we were unable to use our manufacturing facilities as a result of a fire, natural disaster (including an earthquake), equipment failure, or other difficulty, or if such facilities were deemed not in compliance with the regulatory requirements and such non-compliance could not be rapidly rectified. An inability or reduced capacity to manufacture our products could have a material adverse effect on our business, financial condition, and results of operations.
Our existing manufacturing facilities have been adequate for the products we currently sell, but may become inadequate for future products if our planned BLA for knee osteoarthritis is approved. Therefore, we have begun planning for additional manufacturing capacity. Failure to adequately expand capacity could delay commercialization of our current or future product candidates, depriving us of potential product revenue. Any manufacturing problem could be disruptive to our operations and result in lost sales.
Our business is subject to extensive regulation by the FDA and other authorities, which is costly, and our failure to comply could result in negative effects on our business, results of operations and financial condition.
As discussed above, the FDA has specific regulations governing our tissue-based products, or HCT/Ps. The FDA has broad post-market and regulatory and enforcement powers, even for Section 361 HCT/Ps. The FDA’s regulation of HCT/Ps includes requirements for registration and listing of products, donor screening and testing, processing and distribution, labeling, record keeping and adverse-reaction reporting, and inspection and enforcement.
HCT/Ps that are regulated as drugs, biological products or medical devices are subject to even more stringent regulation by the FDA. Even if pre-market clearance or approval is obtained, the approval or clearance may place substantial restrictions on the indications for which the product may be marketed or to whom it may be marketed, may require warnings to accompany the product or impose additional restrictions on the sale or use of the product. In addition, regulatory approval is subject to continuing compliance with regulatory standards, including the FDA’s quality system regulations.
If we fail to comply with the FDA regulations regarding our tissue products, the FDA could take enforcement action, including, without limitation, any of the following sanctions and the manufacture of our products or processing of our tissue could be delayed or terminated:
•untitled letters, warning letters, cease and desist orders, fines, injunctions, and civil penalties;
•recall or seizure of our products;
•operating restrictions, partial suspension or total shutdown of production;
•refusing our requests for clearance or approval of new products;
•withdrawing or suspending current applications for approval or approvals already granted;
•refusal to grant export approval for our products; and
•criminal prosecution.
The FDA’s regulation of HCT/Ps may continue to evolve. Complying with any such new regulatory requirements may entail significant time delays and expense, which could have an adverse effect on our business, results of operations and financial condition.
The AATB has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an accredited tissue bank. In addition, some states have their own tissue banking regulations.
In addition, procurement of certain human organs and tissue for transplantation is subject to the restrictions of the NOTA, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery and storage of donated human tissue. Although we have independent third party appraisals that confirm the reasonableness of the service fees we pay, if we were to be found to have violated NOTA’s prohibition on the sale or transfer
33
of human tissue for valuable consideration, we could potentially be subject to criminal enforcement sanctions, which could adversely affect our results of operations.
Finally, we and other manufacturers of skin substitutes are required to provide average ASP information to CMS on a quarterly basis. The Medicare payment rates are updated quarterly based on this ASP information. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, such manufacturer is subject to civil monetary penalties of up to $10,000 for each misrepresentation for each day in which the misrepresentation was applied, and potential False Claims Act liability. See “We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause an adverse effect on our business, results of operations and financial condition.”
We may be subject to fines, penalties, injunctions and other sanctions if we are deemed to be promoting the use of our products for unapproved, or off-label, uses.
As a general rule, FDA regulations require that the marketing of 361 HCT/Ps only be for appropriate homologous uses, and that the promotion of pre-approved biological products or devices only be for FDA-approved indications. Generally, unless the products are approved by the FDA for alternative uses, the FDA contends that we may not make claims about the safety or effectiveness of our products, or promote them as safe or effective for uses other than those specifically approved by the FDA. Such limitations present a risk that the FDA or other federal or state law enforcement authorities could determine that the nature and scope of our sales, marketing and support activities, though designed to comply with all FDA requirements, constitute the promotion of our products for an unapproved use in violation of the federal FD&C Act. We also face the risk that the FDA or other governmental authorities might pursue enforcement based on past activities that we have discontinued or changed, including sales activities, prior marketing materials, arrangements with institutions and doctors, educational and training programs and other activities.
Investigations concerning the promotion of unapproved product uses and related issues are typically expensive, disruptive and burdensome and generate negative publicity. If our promotional activities are found to be in violation of the law, we may face significant legal action, fines, penalties, and even criminal liability and may be required to substantially change our sales, promotion, grant and educational activities. There is also a possibility that we could be enjoined from selling some or all of our products for any unapproved use. In addition, as a result of an enforcement action against us or any of our executive officers, we could be excluded from participation in government healthcare programs such as Medicare and Medicaid.
However, under the Guidance, the FDA exercised enforcement discretion under limited conditions with respect to the investigative new drug application and pre-market approval requirements for certain HCT/Ps through May 31, 2021. We continued to market our micronized products (mdHACM) and our particulate product (AMNIOFILL) under this policy of enforcement discretion in the United States until May 31, 2021, while at the same time pursuing BLAs for certain of our micronized products in specific clinical applications. After May 31, 2021, we no longer sell our micronized and particulate products in the United States, and do not intend to sell such products in the United States until the FDA grants pre-market approval. We will ultimately only be able to market such products for indications that have been cleared or approved by the FDA.
Nevertheless, while we believe we are fully in compliance with the FDA's Guidance on HCT/Ps, there can be no assurance that we have correctly interpreted the FDA Guidance, or that we will not need to discontinue marketing a product and/or may be subject to fines, penalties, injunctions, and other sanctions if we are deemed to be promoting the use of our products for unapproved uses. Such regulatory penalties by the FDA could adversely affect our business and results of operations.
We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause an adverse effect on our business, results of operations and financial condition.
Our relationships with physicians, hospitals and other healthcare providers are subject to various federal and state healthcare fraud and abuse laws. Healthcare fraud and abuse laws are complex and, in some instances, even minor or inadvertent violations can give rise to liability. Possible sanctions for violation of the healthcare fraud and abuse laws include, without limitation, monetary fines, civil and criminal penalties, exclusion from participating in the federal and state healthcare programs, including, without limitation, Medicare, Medicaid, the VA health programs and TRICARE (the healthcare program administered by or on behalf of the U.S. Department of Defense for uniformed service members, including both those in active duty and retirees, as well as their dependents), and forfeiture of amounts collected in violation of such prohibitions. Many states have similar fraud and abuse laws, imposing substantial penalties for violations. A finding of a violation of one or more
34
of these laws, or even a government investigation or inquiry into the same, would likely result in a material adverse effect on the market price of our Common Stock, as well as on our business, results of operations, and financial condition.
The federal Anti-Kickback Statute is a criminal law that prohibits, among other things, any person from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward referrals, purchases or orders or arranging for or recommending the purchase, order or referral of any item or service for which payment may be made in whole or in part by a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. The Patient Protection and Affordable Care Act (the “PPACA”) amended the federal Anti-Kickback Statute to clarify the intent that is required to prove a violation. Under the federal Anti-Kickback Statute as amended, a person or entity need not have actual knowledge of this statute or specific intent to violate it. The PPACA also amended the federal Anti-Kickback Statute to provide that any claims for items or services resulting from a violation of the federal Anti-Kickback Statute are considered false or fraudulent for purposes of the federal FCA. A conviction for violation of the Anti-Kickback Statute results in criminal fines and requires mandatory exclusion from participation in federal health care programs. Although there are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common industry practices from prosecution, the exceptions and safe harbors are drawn narrowly, and arrangements may be subject to scrutiny or penalty if they do not fully satisfy all elements of an available exception or safe harbor. We have entered into consulting agreements, speaker agreements, research agreements and product development agreements with physicians, including some who may order or recommend our products or make decisions to use them. In addition, some of these physicians own our stock, which they purchased in arm’s-length transactions on terms identical to those offered to non-physicians, or received stock awards from us in the past as consideration for services performed by them. While we believe these transactions generally meet the requirements of applicable laws, including the federal Anti-Kickback Statute and analogous state laws, it is possible that our arrangements with physicians and other providers may be questioned by regulatory or enforcement authorities under such laws, which could lead us to redesign the arrangements and subject us to significant civil or criminal penalties. We have designed our policies and procedures to comply with the federal Anti-Kickback Statute, FCA, and industry best practices. In addition, we have conducted training sessions on these principles. If, however, regulatory or enforcement authorities were to view these arrangements as non-compliant with applicable laws, there would be risk of government investigations/inquiries or penalties. There is also risk that one or more of our employees or agents will disregard the rules we have established. Because our strategy relies on the involvement of physicians who consult with us on the design of our products, perform clinical research on our behalf or educate other health care professionals about the efficacy and uses of our products, we could be materially impacted if regulatory or enforcement agencies or courts interpret our financial relationships with physicians who refer or order our products to be in violation of applicable laws. This could harm our reputation and the reputations of the physicians we engage to provide services on our behalf. In addition, the cost of noncompliance with these laws could be substantial since we could be subject to monetary fines and civil or criminal penalties, and we could also be excluded from federally-funded healthcare programs, including Medicare, Medicaid, VA and TRICARE.
The FCA imposes civil liability on any person or entity that knowingly submits, or causes the submission of, a false or fraudulent claim to the U.S. government. Damages under the FCA can be significant and consist of the imposition of fines and penalties. The FCA also allows a private individual or entity to sue on behalf of the government to recover civil penalties and treble damages as a whistleblower. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of between $11,181 and $22,363 per false claim or statement for penalties assessed after January 29, 2018, with respect to violations occurring after November 2, 2015.
Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payers if they are deemed to “cause” the submission of false or fraudulent claims. The PPACA provides that claims tainted by a violation of the federal Anti-Kickback Statute are false for purposes of the FCA. The DOJ on behalf of the government has previously alleged that the marketing and promotional practices of pharmaceutical and medical device manufacturers, including the off-label promotion of products or the payment of prohibited kickbacks to doctors, violated the FCA, resulting in the submission of improper claims to federal and state healthcare programs such as Medicare and Medicaid. In certain cases, manufacturers have entered into criminal and civil settlements with the federal government under which they entered into plea agreements, paid substantial monetary amounts and entered into onerous corporate integrity agreements with the government that require, among other things, substantial reporting and remedial actions, as well as oversight and review by an outside entity, an Independent Review Organization (“IRO”), at substantial expense to the Company.
Under HIPAA criminal federal healthcare fraud statute, it is a crime to knowingly and willfully execute, or attempt to execute, a scheme or artifice to defraud any health care benefit program or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any health care benefit program, in connection with the delivery of or payment for health care benefits, items or services.
35
There are federal and state laws requiring detailed reporting of manufacturer interactions with and payments to healthcare providers, such as the federal Physician Payments Sunshine Act (“Sunshine Act”). The Sunshine Act requires, among others, “applicable manufacturers” of drugs, devices, biological products, and medical supplies reimbursed under Medicare, Medicaid or the Children’s Health Insurance Program to annually report to CMS information related to payments and other transfers of value provided to “covered recipients.” The term covered recipients includes U.S.-licensed physicians and teaching hospitals, and, for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives. While manufacturers of human cell and tissue products regulated solely under Section 361 are not subject to the Sunshine Act, in the future, if we receive a BLA, we will be subject to this law. There is the risk that CMS or another government agency may take the position that our products are not human cell and tissue products regulated solely under Section 361, and thereby assert that we are currently subject to the Sunshine Act, which could subject us to civil penalties and the administrative burden of having to comply with the law.
There are state law equivalents to the Anti-Kickback Statute and FCA. There are also so-called state “all-payer” anti-kickback laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, as well as when no insurer is involved (i.e. cash-pay patients).
The enforcement of all of these laws is uncertain and subject to rapid change. Federal or state regulatory or enforcement authorities may investigate or challenge our current or future activities under these laws. Any investigation or challenge could have a material adverse effect on our business, financial condition and results of operations. Any state or federal regulatory or enforcement review of us, regardless of the outcome, would be costly and time consuming. Additionally, we cannot predict the impact of any changes in these laws, whether these changes are retroactive or will have effect on a going-forward basis only.
Our results of operations may be adversely affected by current and potential future healthcare reforms.
In response to perceived increases in healthcare costs in recent years, there have been and continue to be proposals by the U.S. federal government, state governments, regulators and third-party payers to control these costs and, more generally, to reform the U.S. healthcare system. In the U.S., the PPACA was enacted in 2010 with a goal of reducing the cost of healthcare and substantially changing the way healthcare is financed by both government and private insurers.
In addition, other legislative changes have been proposed and adopted in the U.S. since the PPACA was enacted. The Budget Control Act of 2011 created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013. In January 2013, the American Taxpayer Relief Act was signed into law, which, among other things, further reduced Medicare payments to several provider types, including hospitals.
In addition to the ACA, the Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”) repealed the Sustainable Growth Rate formula used to calculate Medicare payment updates for physicians providing services to Medicare beneficiaries. In its place, MACRA introduced the Quality Payment Program (“QPP”), which is a value-based program that focuses on quality and outcomes as a metric for physician reimbursement. The Centers for Medicare and Medicaid Services released its final rules for the QPP in October 2016. The QPP, which impacts more than 600,000 physicians and other practice-based clinicians, represents a fundamental change in physician reimbursement, transitioning from a system that solely rewards volume of care to one that also rewards quality and value of care. The rule may have an impact on our revenue in the future. The program’s increased emphasis on quality and cost of care may encourage physicians to merge practices or seek direct employment with hospitals. In addition, the ACA encourages hospitals and physicians to work collaboratively through shared savings programs as well as other bundled payment initiatives. These shifts could lead to a consolidation of hospital providers into larger delivery networks with increased price negotiation strength resulting in downward pressure on our selling prices. Although we believe that we are well positioned to minimize any such impact on our business, our inability to address the consolidation trend could materially and adversely affect our business and results of operations.
There is uncertainty with respect to the impact the U.S. Administration, the executive order, and the attempted legislation may have, if any, and any changes will likely take time to unfold and could have an impact on coverage and reimbursement for healthcare items and services, including our products. We believe that substantial uncertainty remains regarding the net effect of the PPACA, or its repeal and potential replacement, on our business, including uncertainty over how benefit plans purchased on exchanges will cover our products, how the expansion or contraction of the Medicaid program will affect access to our products, the effect of risk-sharing payment models such as Accountable Care Organizations and other value-based purchasing programs on coverage for our product, and the effect of the general increase or decrease in federal oversight of healthcare payers. The taxes imposed and the expansion in government’s role in the U.S. healthcare industry under the
36
PPACA, if unchanged, may result in decreased revenues, lower reimbursements by payers for our products and reduced medical procedure volumes, all of which could have a material adverse effect on our business, results of operations and financial condition.
We may fail to obtain or maintain foreign regulatory approvals to market our products in other countries.
We currently market our products in a small number of foreign countries, and intend to expand our international marketing, including in Japan. Foreign jurisdictions require separate regulatory approvals and compliance with numerous and varying regulatory requirements. The approval procedures vary among countries and may involve requirements for additional testing. Certain of our products require clearance or approval by the FDA. However, such clearance or approval does not ensure approval or certification by regulatory authorities in other countries or jurisdictions, and approval or certification by one foreign regulatory authority does not ensure approval or certification by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval or certification process may include all of the risks associated with obtaining FDA clearance or approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals or certifications and may not receive necessary approvals to commercialize our products in any foreign jurisdiction. Furthermore, many foreign jurisdictions operate under socialized medical care, and obtaining reimbursement for our products under that construct may also prove difficult. If we fail to receive necessary approvals, certifications, or reimbursements necessary to commercialize our products in foreign jurisdictions such as Japan on a timely basis, or at all, our business, results of operations and financial condition could be adversely affected. Further, governmental authorities outside the U.S. have become increasingly stringent in their regulation of medical devices, and our products may become subject to more rigorous regulation by non-U.S. governmental authorities in the future. U.S. or non-U.S. government regulations may be imposed in the future that may have a material adverse effect on our business and operations.
Federal and state laws that protect the privacy and security of personal information may increase our costs and limit our ability to collect and use that information and subject us to liability if we are unable to fully comply with such laws.
Numerous federal and state laws, rules and regulations govern the collection, dissemination, use, security and confidentiality of personal information, including protected health information and individually identifiable health information. These laws include:
•provisions of HIPAA that limit how covered entities and business associates may use and disclose protected health information, provide certain rights to individuals with respect to that information and impose certain security requirements;
•HITECH, which strengthened and expanded the HIPAA Privacy Rule and Security Rules, imposed data breach notification obligations, created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions;
•other federal and state laws restricting the use and protecting the privacy and security of personal information, including health information, many of which are not preempted by HIPAA;
•federal and state consumer protection laws; and
•federal and state laws regulating the conduct of research with human subjects.
The California Consumer Protection Act (“CCPA”), which became effective on January 1, 2020, is a privacy law that requires certain companies doing business in California to disclose information regarding the collection and use of a consumer’s personal data and to delete a consumer’s data upon request. The Act also permits the imposition of civil penalties and expands existing state security laws by providing a private right of action for consumers in certain circumstances where consumer data is subject to a breach. We are still evaluating whether and how this rule will impact our U.S. operations and/or limit the ways in which we can provide services or use personal data collected while providing services.
As part of our business operations, including our medical record keeping, third-party billing and reimbursement and research and development activities, we collect and maintain protected health information in paper and electronic format. Standards related to collecting and maintaining health information, whether implemented pursuant to HIPAA, HITECH, state laws, federal or state action or otherwise, could have a significant effect on the manner in which we handle personal information, including healthcare-related data, and communicate with payers, providers, patients, donors and others, and compliance with these standards could impose significant costs on us or limit our ability to offer services, thereby negatively impacting the business opportunities available to us.
37
If we are alleged to have not complied with existing or new laws, rules and regulations related to personal information, we could be subject to litigation and to sanctions that include monetary fines, civil or administrative penalties, civil damage awards or criminal penalties.
Risks Related to Our Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain and may be inadequate, which could have an adverse effect on our business, results of operations and financial condition.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology, including our licensed technology. These legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. The patent application process can be time consuming and expensive. Our pending patent applications might not result in issued patents. Competitors may be able to design around our patents or develop products that provide outcomes that are comparable or even superior to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with some of our officers, employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements.
The failure to obtain and maintain patents or protect our intellectual property rights could have an adverse effect on our business, results of operations, and financial condition. Whether a patent claim is valid is a complex matter of science, facts and law, and therefore we cannot be certain that, if challenged, our patent claims would be upheld. If any of those patent claims are invalidated, our competitive advantage may be reduced or eliminated.
In the event a competitor infringes upon our licensed patents, issued patents, pending patent applications or other intellectual property rights, enforcing those rights may be costly, uncertain, difficult and time consuming. Even if successful, litigation to enforce or defend our intellectual property rights could be expensive and time consuming and could divert our management’s attention. Further, bringing litigation to enforce our patents subjects us to the potential for counterclaims. Other companies or entities also have commenced, and may again commence, actions seeking to establish the invalidity of our patents and certain related claims. In the event that any of our patent claims are challenged, a court, the United States Patent and Trademark Office (“USPTO”), or the Patent Trial and Appeal Board (“PTAB”) of the USPTO may invalidate one or more challenged patent claims or determine that the patent is unenforceable, which could harm our competitive position. If the USPTO or the PTAB ultimately cancels or narrows the claim scope of any of our patents through these proceedings, it could prevent or hinder us from being able to enforce them against competitors. Such adverse decisions could negatively impact our business, results of operations, and financial condition.
In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in enforcing and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop infringement of our foreign patents, if obtained, or the misappropriation of our other intellectual property rights. For example, some foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, some countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in some countries may be inadequate.
We may become subject to claims of infringement of the intellectual property rights of others, which could prohibit us from developing our products, require us to obtain licenses from third parties or to develop non-infringing alternatives, and subject us to substantial monetary damages.
Third parties could assert that our products infringe their patents or other intellectual property rights. Whether a product infringes a patent claim or other intellectual property right involves a complex combination of legal and factual issues, the determination of which is often uncertain. Therefore, we cannot be certain that we have not infringed the intellectual property rights of others. Because patent applications may take years to issue, there also may be applications now pending of which we
38
are unaware that may later result in issued patent claims that our products or processes infringe. There also may be existing patents or pending patent applications of which we are unaware that our products or processes may inadvertently infringe.
Any infringement claim could cause us to incur significant costs, place significant strain on our financial resources, divert management’s attention from our business and harm our reputation. If the relevant patent claims at issue in such a dispute were upheld as valid and enforceable and we were found to infringe, we could be prohibited from selling any product that is found to infringe those claims unless we could obtain licenses to use the technology covered by the asserted patent claims or other intellectual property, or are able to design around the patent claim or claims at issue or other intellectual property. We may be unable to obtain such a license on terms acceptable to us, if at all, and we may not be able to redesign our products to avoid infringement. A court could also order us to pay compensatory damages for such infringement, plus prejudgment interest and could, in addition, treble the compensatory damages and award attorney fees. These damages could be substantial and could harm our reputation, business, financial condition and operating results. A court also could enter orders that temporarily, preliminarily or permanently enjoin us and our customers from making, using, or selling products, and could enter an order mandating that we undertake certain remedial measures. Depending on the nature of the relief ordered by the court, we could become liable for additional damages to third parties. Further, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our trade secrets or other confidential information could be compromised by inadvertent or court-ordered disclosure during this type of litigation.
We may be subject to damages resulting from claims that we, our employees, or our independent contractors have wrongfully used or disclosed alleged trade secrets, proprietary or confidential information of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Some of our employees were previously employed at other medical device, pharmaceutical or tissue companies. We may also hire additional employees who are currently employed at other medical device, pharmaceutical or tissue companies, including our competitors. Additionally, consultants or other independent agents with which we may contract may be or have been in a contractual arrangement with one or more of our competitors. Although no claims are currently pending, we may be subject to claims that we, our employees, or our independent contractors have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of these former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail to defend such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Any future litigation or the threat thereof may adversely affect our ability to hire additional direct sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business, financial condition and operating results.
Risks Related to Our Past Audit Committee Investigation, Consolidated Financial Statements, Internal Controls and Related Matters
If we fail to maintain adequate internal control over financial reporting in the future, this could adversely affect our business, financial condition and operating results.
We have in the past reported material weaknesses in our internal control over financial reporting which we have now remediated. If additional material weaknesses or deficiencies in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements might contain material misstatements and we could be required to restate our financial results. Moreover, because of the inherent limitations of any control system, material misstatements due to error or fraud may not be prevented or detected on a timely basis, or at all. If we are unable to provide reliable and timely financial reports in the future, our business and reputation may be further harmed. Failures in internal controls may also cause us to fail to meet reporting obligations, negatively affect investor confidence in our management and the accuracy of our financial statements and disclosures, or result in adverse publicity and concerns from investors, any of which could have a negative effect on the price of our Common Stock, subject us to regulatory investigations and penalties or shareholder litigation, and adversely impact our business, results of operations and financial condition.
Negative publicity, including publicity relating to or arising from the Restatement, the Audit Committee Investigation, or related matters, has had and could continue to have an adverse effect on our business, results of operations and financial condition.
We have been and could continue to be the subject of negative publicity focusing on the Restatement, the results of the Audit Committee Investigation, and related matters. As a result, our customers and others with whom we do business have voiced
39
concerns regarding our accounting and control environment and our ability to be a long-term provider to our customers. Further negative publicity could adversely affect our business, financial condition and results of operations.
We have incurred significant legal and accounting expenditures as a result of the Restatement and have become subject to a number of additional risks and uncertainties, including being a party to certain litigation relating to the Restatement. See Item 3. “Legal Proceedings” and Item 8. Financial Statements and Supplementary Data, Note 14, “Commitments and Contingencies” for additional information. As a result of the Restatement, we may continue to be at risk for further government investigations, shareholder litigation, and additional accounting and legal fees in connection therewith, as well as loss of investor confidence in us, and a negative impact on our stock price.
We are currently, in the past have been, and in the future may be, subject to substantial litigation and ongoing investigations that could cause us to incur significant legal expenses, divert management’s attention, and result in harm to our business.
We are exposed to potential liabilities and reputational risk associated with litigation, regulatory proceedings and government enforcement actions. We were party to a securities class action lawsuit subject to appeal alleging, among other things, violations of Section 10(b) of the Securities Exchange Act of 1934. See Item 3, “Legal Proceedings” and Item 8, Financial Statement and Supplementary Data, Note 14, “Commitments and Contingencies” for information regarding proceedings that we believe may be significant to the Company as of the date of the filing of this Annual Report. We may be subject to additional lawsuits, including class action or securities derivative lawsuits, and further government investigations as well as incur additional legal fees and may face negative impacts to our stock price and reputation. In addition, we are obligated to indemnify and advance expenses to certain individuals involved in certain of these proceedings.
Any adverse judgment in or settlement of any pending or any future litigation could result in significant payments, fines and penalties that could have a material adverse effect on our business, results of operations, financial condition and reputation. Such payments, damages or settlement costs, if any, related to these matters could be in excess of our insurance coverage. The amount of time that is required to resolve these lawsuits is unpredictable and any litigation or claims against us, even those without merit, may cause us to incur substantial costs, divert management’s attention from the day-to-day operation of our business, and materially harm our reputation.
Risks Related to the Securities Markets and Ownership of Our Common Stock
Our substantial indebtedness may adversely affect our financial health.
As of December 31, 2021, the Company had an aggregate of $50 million of borrowings outstanding under the Hayfin Loan Agreement. See Item 8, Financial Statements and Supplementary Data, Note 9, “Long-Term Debt.”
Our substantial outstanding debt may limit our ability to borrow additional funds or may adversely affect the terms on which such additional funds may be available. Additionally, a default under certain other indebtedness constitutes an event of default under the Hayfin Loan Agreement. Consequently, the effects of a default under other debt may be amplified by the lender exercising the remedies available to it in the Hayfin Loan Agreement for events of default, including foreclosure on the collateral securing our obligations and the declaration that all amounts outstanding under the Hayfin Loan Agreement are immediately due and payable. The limitations on our ability to access additional borrowing and the potential effects of a cross-default under the Hayfin Loan Agreement may limit our liquidity and have an adverse effect on our business, financial condition, and results of operations.
The restrictive covenants in the Hayfin Loan Agreement, and the Company’s obligation to make debt payments under the Hayfin Loan Agreement, limit our operating and financial flexibility and may adversely affect our business, results of operations and financial condition.
The Hayfin Loan Agreement, as amended, imposes operating and financial restrictions and covenants. For example, the Hayfin Loan Agreement, as amended, contains (a) covenants that impose certain reporting and/or performance obligations on the Company and its subsidiaries, including (i) a Minimum Consolidated Total Net Sales (as defined in the Hayfin Loan Agreement) of varying amounts from now until maturity at June 30, 2025, in each case tested quarterly; and (ii) Minimum Liquidity (as defined in the Hayfin Loan Agreement) of $20 million, an at-all-times covenant tested monthly and (b) certain negative covenants that generally limit, subject to various exceptions, the Company and its subsidiaries from taking certain actions, including, without limitation, incurring indebtedness, making investments, incurring liens, paying dividends and engaging in mergers and consolidations, sale and leaseback transactions and asset dispositions.
40
Our ability to comply with the financial covenants in the Hayfin Loan Agreement is in part dependent on our success in our overall strategies, including pursuing expansion beyond advanced wound care into areas of surgical recovery, introducing new products and seeking international growth. A breach of a financial covenant in the Hayfin Loan Agreement could result in an event of default that would trigger the lenders’ remedies, including the right to accelerate the entire principal balance of the loan under the Hayfin Loan Agreement. We currently have sufficient cash on hand to repay all amounts outstanding, however, there can be no assurances that we will be able to find alternative financing in case of such or other event of a default. Even if alternative financing were available should an event of a default occur under the Hayfin Loan Agreement, it might be on unfavorable terms, and the interest rate charged on any new borrowings could be substantially higher than the interest rate under the Hayfin Loan Agreement, thus adversely affecting our cash flows, liquidity, and results of operations. Acceleration of the repayment of the loan pursuant to the terms of the Hayfin Loan Agreement, in combination with the Company’s current commitments and contingent liabilities, could also cast doubt on the Company’s ability to continue as a going concern.
Our variable rate indebtedness under the Hayfin Loan Agreement subjects us to interest rate risk, which could result in higher expense in the event of increases in interest rates and adversely affect our business, financial condition, and results of operations.
Borrowings under the Hayfin Loan Agreement, as amended, bear interest at a per annum rate equal to London Interbank Offered Rate (“LIBOR”), subject to a “floor” of 1.5%, plus a margin of 6.75%. As a result, we are exposed to interest rate risk, which we do not hedge. If LIBOR rises, the interest rate on outstanding borrowings under the Hayfin Loan Agreement will increase. Therefore, an increase in LIBOR will increase our interest payment obligations under the Hayfin Loan Agreement and have a negative effect on our cash flows and liquidity, and could have a negative effect on our ability to make payments due under the Hayfin Loan Agreement.
EW Healthcare Partners and its interests may conflict with those of our other shareholders.
As of December 31, 2021, EW Healthcare Partners and their affiliates own 90% of the outstanding shares of our Series B Preferred Stock which upon conversion into shares of Common Stock, would result in an ownership interest of approximately 18.3% of our Common Stock (calculated on the basis described in Item 12, “ - - Security Ownership Of Certain Beneficial Owners And Management” below). Also, for as long as EW Healthcare Partners and its affiliates collectively hold at least (i) 10% of the outstanding shares of our Common Stock (calculated on an as converted basis), EW Healthcare Partners has the right to designate two directors to our Board and (ii) 5% (but less than 10%) of the outstanding shares of our outstanding Common Stock (calculated on an as converted basis), EW Healthcare Partners has the right to designate one individual to serve on our Board. Such individuals will initially be preferred directors and therefore not subject to election by the holders of Common Stock. EW Healthcare Partners designated Martin P. Sutter and William A. Hawkins, III, who continue to serve on our board as preferred directors. The interests of EW Healthcare Partners may conflict with those of our other shareholders, and EW Healthcare Partners may seek to influence, and may be able to influence, us through its director designation rights and its share ownership.
Holders of shares of our Series B Preferred Stock have rights, preferences and privileges that are not held by, and are preferential to, the rights of, our common shareholders.
Holders of shares of our Series B Preferred Stock were entitled to cumulative dividends at a rate of 4.0% per annum until June 30, 2021 and are entitled to 6.0% per annum thereafter, in each case compounding quarterly in arrears. The dividends are payable quarterly in whole or in part, in cash. However, the Company may, at its option, elect not to pay any such dividend in cash and instead to accrue the amount of such dividend. The payment of regular dividends in cash to the holders of Series B Preferred Stock could impact our liquidity and reduce the amount of cash available for working capital, capital expenditures, growth opportunities, acquisitions, and other general corporate purposes. If we elect to accrue the dividends in lieu of paying them in cash, holders of Common Stock could effectively be diluted because such accrual of dividends will increase the number of shares of Common Stock into which the Series B Preferred Stock would then be convertible. Our obligations to the holders of Series B Preferred Stock could also limit our ability to obtain additional equity or debt financing or increase our borrowing costs, which could have an adverse effect on our financial condition.
The Series B Preferred Stock ranks senior to our Common Stock with respect to dividends and distributions on liquidation, winding-up, and dissolution. Upon a liquidation, dissolution, or winding-up of the Company, holders of Series B Preferred Stock will be entitled to receive $1,000 per share of Series B Preferred Stock (subject to adjustment), plus any accrued and unpaid dividends. This amount will be payable prior to any distribution of our available assets to the holders of our Common Stock.
41
Holders of Series B Preferred Stock generally are entitled to vote together as a single class with the holders of the shares of Common Stock, on an as converted basis, on all matters submitted for a vote of holders of our Common Stock subject to certain limitations on their voting rights contained in the related Articles of Amendment to our Restated Articles of Incorporation. Additionally, certain matters will require the approval of the holders of a majority of the outstanding shares of Series B Preferred Stock, voting as a separate class, including the following actions:
•any changes to the rights, preferences, or privileges of the Series B Preferred Stock;
•amendments or restatements of any organizational document of the Company or its subsidiaries in a manner that materially, adversely, and disproportionately affects the rights, preferences, and privileges of the Series B Preferred Stock as compared to our Common Stock;
•the authorization or creation of any class or series of senior or parity equity securities;
•the declaration of any dividends or any other distributions, or the repurchase or redemption, of any equity securities of the Company ranking junior to or on parity with the Series B Preferred Stock (subject to certain exceptions);
•prior to January 2, 2023, the sale, transfer, or other disposition of any assets, business, or operations for $25 million or more (other than sales of inventory in the ordinary course of business), or the purchase or acquisition of any assets, business, or operations for $75 million or more;
•prior to January 2, 2023, the merger or consolidation of the Company unless either (x) the surviving company will have no class of equity securities ranking superior to or on parity with the Series B Preferred Stock or (y) the holders of shares of the Series B Preferred Stock will receive in connection therewith consideration per share of Series B Preferred Stock valued at 200% or more of the purchase price per share of $1,000;
•prior to January 2, 2023, commencing a voluntary case under any applicable bankruptcy, insolvency, or other similar law or consenting to the entry of an order for relief in an involuntary case under any such law, or effectuating any general assignment for the benefit of creditors; and
•prior to January 2, 2023, entering into any settlement agreement regarding the Company’s securities class action litigation.
The interests of our holders of Series B Preferred Stock and our Common Stock may conflict in certain circumstances, and these provisions may constrain the Company from taking certain actions that may be in the best interest of the holders of its Common Stock.
The conversion price of the Series B Preferred Stock is subject to anti-dilution adjustments in the event that the Company sells or issues Common Stock to any third-party investor at any time prior to July 2, 2022 at a price that is less than $3.85 per share of Common Stock (although such adjustments cannot result in a conversion price for the Series B Preferred Stock of less than $3.47). Additionally, as long as EW Healthcare Partners holds at least 10% of our outstanding Common Stock (calculated on an as converted basis), it has certain preemptive rights to participate in offerings of Common Stock to any person, subject to customary exceptions.
Furthermore, in the event that the Company undergoes a change of control (as defined), the holders of Series B Preferred Stock will have certain redemption rights, which, if exercised, could require us to repurchase all of the outstanding shares of Series B Preferred Stock for cash at the original purchase price of Series B Preferred Stock plus all accrued and unpaid dividends thereon. Any required repurchase of the outstanding Series B Preferred Stock could impact our liquidity and reduce the amount of cash available for working capital, capital expenditures, growth opportunities, acquisitions, and other general corporate purposes.
The preferential rights of the Series B Preferred Stock could also result in divergent interests between the holders of Series B Preferred Stock and our common shareholders.
See Item 8, Financial Statement and Supplementary Data, Note 11, “Equity” for more information regarding our Series B Preferred Stock.
42
Our Series B Preferred Stock is convertible into shares of our Common Stock, and any such conversion may dilute the value of our Common Stock.
Holders of shares of Series B Preferred Stock have the right, at their option, to convert each share of Series B Preferred Stock into shares of our Common Stock, except that no holder may convert its shares of Series B Preferred Stock into shares of Common Stock if such conversion would result in such holder and its affiliates holding more than 19.9% of the aggregate voting power of our Common Stock or beneficially owning in excess of 19.9% of our then-outstanding shares of Common Stock. Additionally, each share of Series B Preferred Stock (including any accrued and unpaid dividends) will automatically convert into shares of our Common Stock at any time after July 2, 2023, provided that our Common Stock has traded at 200% or more of the then conversion price (i) for 20 out of 30 consecutive trading days preceding, and (ii) as of the close of trading on the date immediately prior to conversion. The conversion of Series B Preferred Stock may significantly dilute our common shareholders and adversely affect both our net income per share of Common Stock and the market price of our Common Stock.
The price of our Common Stock has been, and will likely continue to be, volatile.
The market price of our Common Stock, like that of the securities of many other healthcare companies that are engaged in research, development, and commercialization, has fluctuated over a wide range, and it is likely that the price of our Common Stock will fluctuate in the future. The market price of our Common Stock could be impacted by a variety of factors, including:
•Fluctuations in stock market prices and trading volumes of similar companies or of the markets generally;
•Our ability to successfully launch, market and earn significant revenue from our products;
•Our ability to obtain additional financing to support our continuing operations;
•Disclosure of the details and results of our clinical trials and our regulatory applications and proceedings;
•Developments in and disclosure or publicity regarding existing or new litigation or contingent liabilities;
•Changes in government regulations or our failure to comply with any such regulations;
•Additions or departures of key personnel;
•Our investments in research and development or other corporate resources;
•Announcements of technological innovations or new commercial products by us or our competitors;
•Developments in the patents or other proprietary rights owned or licensed by us or our competitors;
•The timing of new product introductions;
•Actual or anticipated fluctuations in our operating results, including any restatements of previously reported results;
•Our ability to effectively and consistently manufacture our products and avoid costs associated with the recall of defective or potentially defective products;
•Our ability and the ability of our distribution partners to market and sell our products;
•Changes in reimbursement for our products or the price for our products to our customers;
•Removal of our products from the FSS, or changes in how government accounts purchase products such as ours or in the price for our products to government accounts;
•Activities of market participants and investors, including analysts and MiMedx shareholders;
•Material amounts of short-selling of our Common Stock; and
•The other risks detailed in this Item 1A.
Price volatility or a decrease in the market price of our Common Stock could have an adverse effect on our ability to raise capital, liquidity, business, financial condition and results of operations.
Securities analysts may elect not to report on our common stock or may issue negative reports that adversely affect the stock price.
We have conducted extensive investor relations outreach to the investment analysts community with the goal of attracting analyst coverage. However, at this time, only three securities analysts provide coverage on us, and we compensate one of those analyst’s firms. There can be no assurance that any other analysts will cover our stock or, if they do, that they will continue to report on our common stock or that additional analysts will initiate reporting on our common stock.
If we fail to attract the coverage or securities analysts, or if securities analysts discontinue covering our common stock, the lack of research coverage may adversely affect the actual and potential market price of our common stock. The trading market for our common stock may be affected in part by the research and reports that industry participants, industry analysts or financial analysts publish about our business. If one or more analysts elect to cover us and then downgrade the stock, the stock price would likely decline rapidly. If one or more of these analysts cease coverage of us, we could lose visibility in the market, which in turn could cause our stock price to decline.
43
Fluctuations in revenue or results of operations could cause additional volatility in our stock price.
Any unanticipated shortfall in our revenue in any fiscal quarter could have an adverse effect on our results of operations in that quarter. The effect on our net income of such a shortfall could be exacerbated by the relatively fixed nature of most of our costs, which primarily include personnel costs as well as facilities costs. These fluctuations could cause the trading price of our stock to be negatively affected. Our quarterly operating results have varied substantially in the past and may vary substantially in the future.
We do not intend to pay cash dividends on our Common Stock.
Holders of our Series B Preferred Stock are entitled to contractually-determined dividends before holders of our Common Stock. See above “Holders of shares of Series B Preferred Stock have rights, preferences and privileges that are not held by, and are preferential to, the rights of, our common shareholders.”
We have never declared or paid cash dividends on our Common Stock. We currently expect to use available funds and any future earnings to pay dividends on the Series B Preferred Stock; in the development, operation and expansion of our business; to repay debt; and, to the extent authorized by our Board, repurchasing our Common Stock. We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. As a result, capital appreciation, if any, of our Common Stock will be an investor’s only source of potential gain from our Common Stock for the foreseeable future.
Certain provisions of Florida law and anti-takeover provisions in our organizational documents may discourage or prevent a change of control, even if an acquisition would be beneficial to shareholders, which could affect our share price adversely and prevent attempts by shareholders to remove current management.
The Florida Business Corporation Act (the “FBCA”) includes several provisions applicable to the Company that may discourage potential acquirors. Such provisions include provisions that:
•allow directors to take other stakeholders into account in discharging their duties;
•a requirement that certain transactions with a shareholder of 10% or more ownership must be approved by the affirmative vote of two-thirds of the other shareholders unless approved by a majority of the disinterested directors or certain fair price requirements are met; and
•voting rights acquired by a shareholder at ownership levels at or above one-fifth, one-third and a majority of voting power are denied unless authorized by the Board prior to such acquisition or by a majority of the other shareholders (excluding interested shares (as defined in the FBCA)).
Additionally, our organizational documents contain provisions:
•authorizing the issuance of blank check preferred stock;
•restricting persons who may call shareholder meetings;
•permitting shareholders to remove directors only “for cause” and only by super-majority vote; and
•providing the Board with the exclusive right to fill vacancies and to fix the number of directors.
These provisions of Florida law and our articles of incorporation and bylaws could negatively affect our share price, prevent attempts by shareholders to remove current management, prohibit or delay mergers or other takeovers or changes of control of the Company and discourage attempts by other companies to acquire us, even if such a transaction would be beneficial to our shareholders.
44
Item 1B. Unresolved Staff Comments
There are no unresolved SEC Staff comments with respect to our SEC filings.
Item 2. Properties
Our corporate headquarters are located in Marietta, Georgia, where we lease office, laboratory, tissue processing and warehouse space. We also lease a facility in Kennesaw, Georgia, which primarily consists of laboratory, tissue processing and warehouse space, and additional warehouse space in Marietta, Georgia. All of our properties are used by our one business segment, which includes the design, manufacture and marketing of products and tissue processing services primarily for the wound care, burn, surgical recovery, and non-operative sports medicine sectors of healthcare.
The Company’s properties are suitable and adequate for current business operations. We are making investments to increase our manufacturing capacity, especially in the context of enhancements to facilitate the processing of products required to be manufactured under CGMP.
Item 3. Legal Proceedings
The description of our securities class action contained in Note 14, “Commitments and Contingencies,” to our financial statements included in Item 8 is incorporated herein by reference.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market for Common Stock
Our Common Stock trades on The Nasdaq Stock Market under the trading symbol “MDXG”.
Holders
Based upon information supplied from our transfer agent, there were approximately 869 shareholders of record of our Common Stock as of February 21, 2022.
Stock Performance Graph
The following graph compares the cumulative total stockholder return on our Common Stock with the cumulative total stockholder return of the Nasdaq Composite Index and the Nasdaq Biotechnology Index, assuming an investment of $100.00 on December 31, 2016, in each of our Common Stock, the stocks comprising the Nasdaq Composite Index, and the stocks comprising the Nasdaq Biotechnology Index.
45
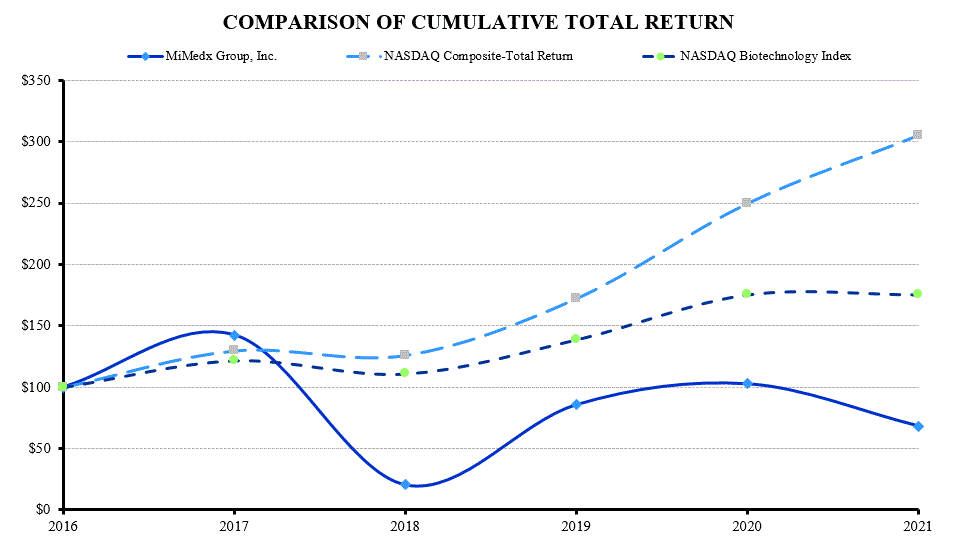
ASSUMES $100 INVESTED ON DEC. 31, 2016
ASSUMES DIVIDEND REINVESTMENT; NO DIVIDENDS ISSUED BY MIMEDX
FISCAL YEAR ENDED DEC. 31, 2021
ASSUMES DIVIDEND REINVESTMENT; NO DIVIDENDS ISSUED BY MIMEDX
FISCAL YEAR ENDED DEC. 31, 2021
Securities Authorized for Issuance Under Equity Compensation Plans
Information about securities authorized for issuance under our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report.
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
The following table sets forth information regarding the purchases of the Company’s equity securities made by or on behalf of the Company or any affiliated purchaser (as defined in Rule 10b-18 under the Exchange Act) during the three-month period ended December 31, 2021.
| Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs | ||||||||||
| October 1, 2021 - October 31, 2021 | — | $ | — | — | $ | — | ||||||||
| November 1, 2021 - November 30, 2021 | — | $ | — | — | $ | — | ||||||||
| December 1, 2021 - December 31, 2021 | — | $ | — | — | $ | — | ||||||||
| Total for the quarter | — | $ | — | — | $ | — | ||||||||
46
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
MIMEDX is a transformational placental biologics company, developing and distributing placental tissue allografts with patent-protected, proprietary processes for multiple sectors of healthcare. As a pioneer in placental biologics, we are focused on addressing unmet clinical needs in areas of advanced wound care, surgical recovery applications and musculoskeletal conditions. We derive our products from human placental tissues and process these tissues using our proprietary methods, including the PURION® process. We apply CGTP, CGMP, and terminal sterilization to produce our allografts. MIMEDX provides products primarily in the wound care, burn, and surgical recovery sectors of healthcare. All of our products are regulated by the FDA.
MIMEDX is a leading supplier of human placental allografts, which are human tissues that are derived from one person (the donor) and used to produce products that treat another person (the recipient). MIMEDX has supplied over two million allografts, through both direct and consignment shipments. Our platform technologies include tissue allografts derived from the amnion and chorion layers of the human placental membrane (EPIFIX and AMNIOFIX) and tissue allografts derived from human umbilical cord (EPICORD and AMNIOCORD).
EPIFIX and EPICORD products are marketed for external use, such as in advanced wound care applications, while our AMNIOFIX and AMNIOCORD products are positioned for use in surgical recovery applications, including lower extremity repair, plastic surgery, vascular surgery and multiple orthopedic repairs and reconstructions.
AMNIOFIX Injectable, or mdHACM is a micronized configuration of AMNIOFIX and is not currently marketed in the United States. mdHACM is our lead product candidate for our late-stage pipeline targeted at achieving FDA approval for specific clinical indications, including degenerative musculoskeletal conditions.
We have two classes of products: (1) Advanced Wound Care products, or Section 361 products, consisting of our tissue and cord sheet allograft products, and (2) Section 351 products, consisting of our micronized and particulate products, which, prior to May 31, 2021, the date of the FDA’s period of enforcement discretion ended, were used to treat a variety of clinical conditions, including both advanced wound care and musculoskeletal applications. Our Advanced Wound Care business includes two product categories, Tissue/Other and Cord products. We sell product through two distribution channels: (1) direct to customers (healthcare professionals and/or facilities); and (2) sales through distributors.
In November 2017, the FDA published a series of guidances that established an updated framework for the regulation of cellular and tissue-based products. These guidances clarified the FDA’s views about the criteria that differentiate those products subject to regulation under Section 361 of the Public Health Service Act from those considered to be drugs, devices, and/or biological products subject to licensure under Section 351 of the Public Health Service Act and related regulations. The FDA exercised enforcement discretion under limited conditions with respect to IND applications and pre-market approval requirements through May 31, 2021. The enforcement discretion period ended on May 31, 2021. We are not currently marketing our micronized and particulate products affected by the guidance in the United States.
This discussion, which presents our results for the fiscal years ended December 31, 2021 and December 31, 2020, should be read in conjunction with our Consolidated Financial Statements and the accompanying notes. Also please refer to Item 1 — Business and Item 1A — Risk Factors, which include detailed discussions of various items impacting our business, results of operations and financial condition. We intend for this discussion to provide the reader with information that will assist in understanding our financial statements, the changes in certain key items in those financial statements from period to period and the primary factors that accounted for those changes. We also discuss certain performance metrics that management uses to assess the Company's performance. Further information on the factors that can affect our operating results can be found in Part I under the caption “Explanatory Note and Important Cautionary Statement Regarding Forward-Looking Statements.”
Our Annual Report for the year ended December 31, 2020 includes a discussion and analysis of our financial condition and results of operations for the year ended December 31, 2019 in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Trends in Our Business
Analysis of our Phase 2B Knee Osteoarthritis clinical trial has identified a probable root cause of the failure for this study to meet its primary endpoints. We intend to use these findings to inform planned future clinical trials
47
In September 2021, we reported top-line data from the results of two late-stage musculoskeletal clinical trials of the Company’s mdHACM product, including a Phase 2B clinical trial for the treatment of Knee Osteoarthritis. Results from a topline analysis of the six-month efficacy data for the Phase 2B clinical trial for Knee Osteoarthritis revealed that the study did not meet its primary endpoints, but did reveal varied efficacy signals between patient cohorts evaluated pre- and post-blinded interim analysis performed in mid-2019.
A root-cause analysis of the Knee Osteoarthritis study indicated that the varied efficacy signals between the pre-interim analysis and post-interim analysis cohorts was the result of faded potency of the investigational product over time. We intend to incorporate these findings into the design of our Phase 3 program, which we plan to initiate in 2022.
We are expanding beyond advanced wound care and into areas of surgical recovery
Surgical recovery applications focus on the use of tissue products to augment tissue, serve as a barrier membrane, or aid in incisional closure with the goal of preventing or reducing procedural complications. Following a thorough review of surgical procedures and potential clinical applications across several specialties, we have identified those areas where we believe our tissue products could be incorporated. We are targeting certain procedures for use of our products based on unmet clinical need, potential procedural complication rate, clinical relevance, economic factors and overall business priorities. As in advanced wound care, we believe this market is expanding as a result of demographic trends, including an aging population, increasing incidence of obesity and diabetes and the associated higher susceptibility to non-healing chronic wounds.
We are actively pursuing growth strategies by expanding our geographic reach
We are actively pursuing international expansion, with an initial focus in Japan. We received regulatory approval by the Japanese Ministry of Health, Labor and Welfare in June 2021 to market EPIFIX in Japan. We expect to secure reimbursement approval in mid-2022, and are putting in place the necessary structure, medical education programs, and market development initiatives that will operationalize our commercial strategy. We are evaluating opportunities for geographic expansion in the United Kingdom, certain other areas of Europe and also the Middle East.
Impact of COVID-19 Pandemic
While the impact of the COVID-19 pandemic is still ongoing, the effects on our operations, such as access restrictions to hospitals and difficulties obtaining donor materials that we observed during the year ended December 31, 2020 did not materially affect our operations during the year ended December 31, 2021. We are continuously monitoring developments with respect to novel variants of the virus and government and societal responses to mitigate the continued spread of COVID-19, which could impact our operations.
We continue to exercise an abundance of caution with respect to the health and well being of our employees. We are providing employees with Personal Protective Equipment as needed, and advising all employees to receive a COVID-19 vaccine or booster as soon as reasonably possible. None of these efforts have materially affected the Company’s operations for the year ended December 31, 2021.
Components of and Key Factors Influencing Our Results of Operations
In assessing the performance of our business, we consider a variety of performance and financial measures. We believe the items discussed below provide insight into the factors that affect these key measures.
Net sales
Net sales is recognized based on the consideration we expect to receive from the sale. This consists of the gross selling price of the product, less any discounts, rebates, fees paid to GPOs, and returns.
We derive the majority of our revenue from selling our tissue and cord products in the United States. We are actively working to broaden our product portfolio in a number of clinical applications, while also seeking regulatory approval with the appropriate regulators to expand our geographic footprint.
We have two classes of products: (1) Advanced Wound Care products, or Section 361 products, consisting of our tissue and cord sheet allograft products, and (2) Section 351 products, consisting of our micronized and particulate products, which, prior to May 31, 2021, the date the FDA’s period of enforcement discretion ended, were used to treat a variety of patient needs, including both advanced wound care and musculoskeletal applications. Our Advanced Wound Care business includes two product categories, Tissue/Other and Cord products.
48
We have two distribution channels: (1) direct to customers and (2) sales through distributors.
Several factors affect our reported revenue in any period, including product, payer and geographic sales mix, operational effectiveness, pricing realization, marketing and promotional efforts, timing of orders and shipments, regulatory actions including healthcare reimbursement scenarios, competition, and business acquisitions that involve our customers or competitors.
Cost of goods sold and gross profit
Cost of goods sold includes product testing costs, quality assurance costs, personnel costs, manufacturing costs, raw materials and product costs, depreciation and facility costs associated with our manufacturing and warehouse facilities. Fluctuations in our cost of goods sold correspond with the fluctuations in these costs as well as in sales units driven by the changes in our sales force and sales territories, product portfolio offerings and the number of facilities that offer our products.
Gross profit is calculated as net sales less cost of goods sold. Our gross profit is affected by product and geographic sales mix, realized pricing of our products, the efficiency of our manufacturing operations and the costs of materials used to make our products. Regulatory actions, including with respect to reimbursement for our products, may require costly expenditures or result in pricing pressure, and may decrease our gross profit and gross margin.
Selling, general and administrative expense
Selling, general and administrative expense includes personnel costs, commissions, incentive compensation, customer support, administrative and labor costs, insurance, professional fees, depreciation and bad debt expense. We expect our selling, general and administrative expense to fluctuate based on revenue fluctuations, geographic changes, and any changes to the size of our headcount, particularly that of our sales and marketing forces.
Research and development expense
Research and development expense relates to our investments in clinical trials to expand our product pipeline and platforms, as well as investments in improvements to our manufacturing process and the enhancement of existing products. Our research and development costs also include expenses such as salaries and benefits related to our research department, consulting costs and advisory costs, and regulatory costs.
We expense research and development costs as incurred. Fluctuations in research and development expenses are potentially driven by the timing and cadence of our clinical trials.
Investigation, restatement and related expense
Investigation, restatement and related expense primarily relates to legal fees advanced to certain former officers and directors of the Company under certain indemnification agreements and the Company’s liability from legal proceedings taken against us, which arose from the findings of the Audit Committee Investigation. The timing and extent of these expenses depend on the stage and status of legal proceedings. Other activity includes amounts received from certain director and officer insurance providers.
Interest expense
We incur interest expense primarily through stated interest on our outstanding term loan. The interest on our term loan is tied to the three-month London Interbank Offered Rate (“LIBOR”), subject to a floor of 1.5%. Increases in LIBOR could cause our interest expense to increase. Other activity influencing interest expense relates to the amortization of deferred financing costs and original issue discount associated with credit facilities outstanding.
49
Results of Operations for 2021 Compared to 2020
| Year Ended December 31, | |||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| 2021 | 2020 | $ Change | % Change | ||||||||||||||||||||
| Net sales | $ | 258,615 | $ | 248,234 | $ | 10,381 | 4.2 | % | |||||||||||||||
| Gross profit | 215,332 | 208,904 | 6,428 | 3.1 | % | ||||||||||||||||||
| Selling, general and administrative | 198,359 | 181,022 | 17,337 | 9.6 | % | ||||||||||||||||||
| Research and development | 17,344 | 11,715 | 5,629 | 48.0 | % | ||||||||||||||||||
| Investigation, restatement and related | 3,791 | 59,465 | (55,674) | (93.6) | % | ||||||||||||||||||
| Amortization of intangible assets | 820 | 1,073 | (253) | (23.6) | % | ||||||||||||||||||
| Impairment of intangible assets | 53 | 1,027 | (974) | (94.8) | % | ||||||||||||||||||
| Loss on extinguishment of debt | — | (8,201) | 8,201 | — | |||||||||||||||||||
| Interest expense, net | (4,980) | (7,941) | 2,961 | (37.3) | % | ||||||||||||||||||
| Other expense, net | (23) | (3) | (20) | — | |||||||||||||||||||
| Income tax provision (expense) benefit | (247) | 12,259 | (12,506) | — | |||||||||||||||||||
| Net loss | $ | (10,285) | $ | (49,284) | $ | 38,999 | (79.1) | % | |||||||||||||||
Net Sales
We recorded net sales for the year ended December 31, 2021 of $258.6 million, an increase of $10.4 million or 4.2% over 2020 net sales of $248.2 million. Net sales for 2021 and 2020 include collections on the Remaining Contracts of $1.0 million and $7.8 million, respectively. Refer to Item 8, Note 2, “Significant Accounting Policies,” of the consolidated financial statements for additional details regarding the Remaining Contracts.
Adjusted Net Sales, which excludes cash collected on the Remaining Contracts, were $257.6 million in 2021, an increase of $17.1 million or 7.1%, compared to $240.5 million in 2020. Adjusted Net Sales in these periods included net sales of Section 351 products of $17.6 million and $31.8 million in 2021 and 2020, respectively. Adjusted Net Sales is a Non-GAAP measure intended to remove cash collections from the Remaining Contracts, which are not a reflection of recurring revenue. We expect that collections on the Remaining Contracts will be negligible in 2022 and beyond. Refer to the section “Non-GAAP Financial Measures” below for more information.
Sales of our Advanced Wound Care products, which excludes the Section 351 Products, increased $31.4 million or 15.0%, year-over-year. This increase was primarily the result of an increase in sales volume due to lessening of restrictions implemented at the onset of the COVID-19 pandemic, including access to hospitals and travel restrictions. The increase also reflects the initial results of our commercial focus on areas of surgical recovery. We also saw growth in our EPIFIX sheet portfolio and the positive impact of sales of our EPICORD Expandable product launched in September 2020.
Refer to Item 8, Note 15, “Revenue”, for a disaggregation of our sales by product.
Gross Margin
Gross margin in 2021 was 83.3%, compared to 84.2% in 2020. The decrease in gross margin was driven primarily by write-downs of discontinued product recorded during 2021. The write-downs related to our Section 351 Products, which we no longer market in the United States after May 31, 2021, the date the FDA’s period of enforcement discretion ended, and certain Advanced Wound Care product lines which we no longer intend to market. We do not currently anticipate significant write-downs of our inventory to recur in 2022.
Selling, General and Administrative Expense
Selling, general and administrative (“SG&A”) expense increased $17.3 million, or 9.6%, to $198.4 million for 2021, compared to $181.0 million for 2020.
The increase in SG&A expense was driven by:
•the restoration of full-salary levels, which were restricted for a portion of 2020 as part of our response to the COVID-19 pandemic, and merit increases;
50
•incremental costs associated with the expansion of our sales force;
•higher travel costs during 2021 compared to 2020, as travel was restricted at the onset of the COVID-19 pandemic in 2020;
•a proxy contest during the second quarter of 2021, totaling $3.9 million of expenses; and
•increases in sales commissions, resulting from higher sales volumes.
Research and Development Expense
Our research and development expense increased $5.6 million, or 48.0%, to $17.3 million in 2021, compared to $11.7 million in the prior year.
The increase was driven by higher personnel costs due to headcount increases to support investments in our clinical trials and the restoration of full salary levels and merit increases, which were restricted for a portion of 2020. We also incurred higher consulting fees in 2021, primarily to assist in the evaluation of the results of our clinical trials.
Investigation, Restatement and Related Expense
Investigation, restatement, and related expenses decreased $55.7 million, or 93.6% to $3.8 million for 2021 compared to $59.5 million for 2020. The decrease was the result of:
•lower fees advanced under indemnification agreements with certain former members of management during 2021 compared to 2020;
•recoveries from certain director and officer insurance policies relating to previously-recognized legal expenses in 2021;
•negotiated reductions in previously-recognized legal expenses in 2021; and
•year-over-year reductions in costs related to the restatement of our prior period financial information.
The funds received from insurance providers and reductions in legal expenses were reflected as reductions to expense in the periods in which those transactions occurred.
The restatement of our prior period financial information concluded in 2020 and we will not incur any expenses related to the restatement moving forward.
We remain subject to indemnification agreements with certain former officers and directors of the Company (other than our former Chief Executive Officer and our former Chief Operating Officer) for whom legal proceedings are still ongoing.
Amortization of Intangible Assets
Amortization expense related to intangible assets decreased $0.3 million to $0.8 million in 2021, compared to $1.1 million in 2020. The decrease was the result of intangible assets impaired in 2020.
Impairment of Intangible Assets
Impairment of intangible assets of $0.1 million was recorded in 2021 related to the impairment of a supplier relationship acquired as part of the acquisition of Surgical Biologics, LLC (“SB”) in 2011.
Impairment of intangible assets of $1.0 million was recorded in 2020 related to the impairment of customer relationships acquired as part of the SB acquisition.
51
Loss on Extinguishment of Debt
Loss on extinguishment of debt of $8.2 million was recorded in 2020 resulting from the repayment and termination of a previous term loan agreement.
Interest Expense, Net
Interest expense, net decreased by $2.9 million to $5.0 million during 2021 from $7.9 million during 2020. The decrease was the result of less principal outstanding, a lower stated interest rate, and lower amortization of deferred financing costs and original issue discount under the Hayfin Loan Agreement, as defined and described in the Liquidity and Capital Resources section below, compared to our previous term loan agreement which was outstanding for the first half of 2020.
Other Expense, Net
Other expense was negligible in both periods.
Income Tax Provision (Expense) Benefit
The effective tax rate for 2021 was (2.5)% on pre-tax book loss of $10.0 million, primarily reflecting a current tax expense associated with state income taxes and adjustment to federal income tax refund receivable.
Liquidity and Capital Resources
We require capital for our operating activities, including costs associated with the sale of product through direct and indirect sales channels, the conduct of clinical trials and other research and development activities, compliance costs, costs to sell and market our products, regulatory fees, and legal and consulting fees in connection with ongoing litigation and other matters. We generally fund our operating capital requirements through our operating activities and cash reserves. We expect to use capital in the near and medium term to commence late-stage clinical trials for certain of our products, invest in the international expansion of our business and the broadening of our product portfolio, and invest in certain capital projects.
As of December 31, 2021, we had $87.1 million of cash and cash equivalents.
Our net working capital at December 31, 2021 was $106.2 million, an increase of $4.7 million from $101.5 million at December 31, 2020. Our current ratio (current assets divided by current liabilities) was 3.5 to 1 as of December 31, 2021 and 2.7 to 1 as of December 31, 2020.
The Company is currently paying its obligations in the ordinary course of business. We believe that our anticipated cash from operating activities and existing cash and cash equivalents will enable us to meet our operational liquidity needs for the twelve months following the filing date of this Annual Report.
We expect to incur additional costs in connection with the commencement of two late-stage clinical trials. This includes development of protocols, site selection, patient recruitment, start-up costs, ongoing monitoring, and the costs advanced to sites for carrying out such trials. These efforts also require human capital, expertise and resources.
Contractual Obligations
Contractual obligations associated with ongoing business activities are expected to result in cash payments in future periods. The table below summarizes the amounts and estimated timing of these future cash payments as of December 31, 2021 (in thousands):
| Less than | |||||||||||||||||||||||||||||
| Contractual Obligations | Total | 1 year | 1-3 years | 3-5 years | Thereafter | ||||||||||||||||||||||||
| Hayfin Term Loan Principal | $ | 50,000 | $ | — | $ | — | $ | 50,000 | $ | — | |||||||||||||||||||
| Hayfin Term Loan Interest | 14,632 | 4,182 | 8,376 | 2,074 | — | ||||||||||||||||||||||||
| Operating lease obligations | 5,886 | 1,566 | 3,174 | 779 | 367 | ||||||||||||||||||||||||
| Finance lease obligations | 170 | 55 | 110 | 5 | — | ||||||||||||||||||||||||
| Meeting space commitments | 701 | 383 | 318 | — | — | ||||||||||||||||||||||||
| Total | $ | 71,389 | $ | 6,186 | $ | 11,978 | $ | 52,858 | $ | 367 | |||||||||||||||||||
52
We have not declared or paid any cash dividends on our Series B Convertible Preferred Stock since their issuance. Dividends in arrears as of December 31, 2021 were $7.2 million. These were convertible into 27,850,916 shares as of December 31, 2021. Assuming we do not declare or pay a cash dividend, the holders do not exercise their option to convert, and the other conversion or redemption features are not triggered, we would accrue $6.6 million of dividends in 2022, $14.4 million in aggregate in 1-3 years, and $16.3 million in aggregate in 3-5 years. Refer to Item 8, Note 11, “Equity” for more detailed discussion regarding the rights and preferences of our Series B Convertible Preferred Stock.
Term Loan
The Hayfin Loan Agreement was funded on July 2, 2020 and provided us with a senior secured term loan in an aggregate amount of $50 million (the “Term Loan”). The Term Loan matures on June 30, 2025 (the “Maturity Date”). On February 28, 2022 (the “Amendment Date”), we executed an Amendment to the Hayfin Loan Agreement (the “Amendment”).
No principal payments are due on the Term Loan until the Maturity Date.
Interest is payable on the Term Loan for principal outstanding quarterly through the Maturity Date. The interest rate applicable to any borrowings under the Term Loan is equal to LIBOR (subject to a floor of 1.5%) plus a margin of 6.75%. If LIBOR is unavailable, the loan will carry interest at the greatest of the Prime Rate, the Federal Funds Rate plus 0.5% per annum, and 2.5% plus the Margin.
An additional 3.0% margin would be applied to the interest rate upon the occurrence of an Event of Default as defined in the Hayfin Loan Agreement. At issuance, and as of December 31, 2021, the Term Loan carried an interest rate of 8.3%.
Prior to the Amendment Date, the Hayfin Loan Agreement contained financial covenants requiring the Company, on a consolidated basis, to maintain the following:
•Maximum Total Net Leverage Ratio, required to be calculated on a quarterly basis, of 4.0x; and
•Minimum Liquidity, as defined in the Hayfin Term Loan Agreement, of $10 million, an at-all-times financial covenant, tested monthly.
We were in compliance with all debt covenants as of December 31, 2021.
The Amendment changed these financial covenants and requires the Company, on a consolidated basis, to maintain the following beginning on the Amendment Date and continuing through the Maturity Date:
•Minimum Consolidated Total Net Sales (as defined in the Amendment) of varying amounts, required to be calculated on a quarterly basis,
•Minimum Liquidity of $20 million, an at-all-times financial covenant, tested monthly.
The Hayfin Loan Agreement, as amended, also specifies that any prepayment of the loan, voluntary or mandatory, as defined in the Hayfin Loan Agreement, will subject us to a prepayment premium applicable as of the date of the prepayment calculated as follows:
•On or before July 2, 2023: 2% of the principal balance repaid.
•After July 2, 2023, but on or before July 2, 2024: 1% of the principal balance repaid.
•After July 2, 2024: no premium.
The Hayfin Loan Agreement also includes certain negative covenants and events of default customary for facilities of this type, and upon the occurrence of such events of default, subject to customary cure rights, all outstanding loans under the Hayfin Loan Agreement may be accelerated or the lender’s commitments terminated. The mandatory prepayments are also required in the event of a change in control, incurring other indebtedness, certain proceeds from disposal of assets and insured casualty event.
From January 1, 2021, we are required to prepay the outstanding loans based on a percentage of Excess Cash Flow, as defined in the Hayfin Loan Agreement, if Excess Cash Flow is generated, with the percentage determined based on the Total Net Leverage thresholds. To date, we have not been required to make any such prepayments.
A breach of a financial covenant in the Hayfin Loan Agreement, if uncured or unable to be cured, would likely result in an event of default that could trigger the lender’s remedies, including acceleration of the entire principal balance of the loan as well
53
as any applicable prepayment premiums. Future compliance with the financial covenants, as amended, requires continuing growth in net sales consistent with the Company’s business strategy and plans. Our business is subject to inherent uncertainties that could impact the Company’s net sales growth, including, but not limited to, the regulatory pathway of our cord-derived product.
While we currently have sufficient cash to repay all such amounts in an event of default, we may require alternative financing to cover other obligations. Even if alternative financing were available in an event of default under the Hayfin Loan Agreement, it might be on unfavorable terms, and the interest rate charged on any new borrowings may be substantially higher than the interest rate under the Hayfin Loan Agreement, thus adversely affecting our future cash flows, liquidity, and results of operations.
Series B Preferred Stock
The Company has 100,000 shares of Series B Preferred Stock outstanding as of December 31, 2021.
The Series B Preferred Stock paid a 4.0% cumulative dividend per annum prior to June 30, 2021, and pays a 6.0% cumulative dividend per annum thereafter. Dividends are declared at the sole discretion of our board of directors. Dividends, if declared, are paid in cash at the end of each quarter based on dividend amounts that accumulate beginning on the last payment date through the day prior to the end of each quarter. In lieu of paying a dividend in cash, we may elect to accrue the dividend owed to shareholders. Dividend balances accumulate at the prevailing dividend rate for each dividend period for which they are outstanding.
Each share of Series B Preferred Stock, including any accrued and unpaid dividends, is convertible into our common stock at any time at the option of the holder at a conversion price of $3.85 per common share, or 259.74 common shares for each Series B Preferred Share prior to any accrued and unpaid dividends. The Series B Preferred Stock, including any accrued and unpaid dividends, automatically converts into common stock at any time after July 2, 2023, provided that the common stock has traded at $7.70 or higher (i) for 20 out of 30 consecutive trading days and (ii) on such date of conversion.
If we undergo a change of control, we will have the option to repurchase some or all of the then-outstanding shares of Series B Preferred Stock for cash in an amount equal to the liquidation preference and any accumulated and unpaid dividends, subject to the rights of the holders of the Series B Preferred Stock in connection with such change in control. If we do not exercise such repurchase right, holders of the Series B Preferred Stock will have the option to (1) require us to repurchase any or all of our then-outstanding shares of Series B Preferred Stock for cash in an amount equal to the liquidation preference or (2) convert the Series B Preferred Stock, including accrued and unpaid dividends into common stock and receive its pro rata consideration thereunder.
Liquidity Considerations
Our net sales increased 4% in 2021 compared to 2020. This increase was due primarily to increases in sales volume due to lessening of access restrictions imposed by hospitals and travel restrictions implemented at the onset of the COVID-19 pandemic. However, our sales were negatively impacted by our inability to market our Section 351 products in the United States after May 31, 2021. Sales of our Section 351 products were $17.6 million and $31.8 million in 2021 and 2020, respectively. In addition, there is a possibility that the FDA may rule that our cord-derived products do not meet the requirements to be regulated solely under the authority of Section 361 of the Public Health Service Act, in which case we might need to cease marketing such products in the United States until FDA approval or clearance is secured. Sales of our cord products were $23.6 million and $16.1 million in 2021 and 2020, respectively.
See Item 1A - Risk Factors - “Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.”
Further, our liquidity will be impacted by expected and unexpected costs, investments in clinical trials to support BLAs, and contingent liabilities:
•Advancement of our clinical trials and BLA pipeline will involve substantial cost. Products subject to the FDA’s BLA requirements must comply with a range of pre- and post-market provisions. Pre-market compliance includes the conduct of clinical trials in support of BLA approval, the development and submission of a BLA, and the production of product for use in the clinical trials that meets the FDA’s quality expectations. See Item 1A - Risk Factors - “Obtaining and maintaining the necessary regulatory approvals for certain of our products will be expensive and time
54
consuming and may impede our ability to fully exploit our technologies,” and “If any of the BLAs are approved, the Company would be subject to additional regulation which will increase costs and could result in adverse sanctions for non-compliance.”
•The international expansion of our business will require investment through the costs to achieve necessary regulatory approvals and reimbursement schemes, establishing a physical presence through office and warehouse space, identifying and hiring employees, and other costs to establish ongoing operations. Whether we pursue such opportunities will depend on a myriad of factors and the amount and timing of these costs are uncertain at this time.
•We are exposed to potential liabilities and reputational risk associated with litigation, regulatory proceedings, and government enforcement actions. The amounts, if any, for which we may be liable resulting from such proceedings are highly uncertain. See Item 3, “Legal Proceedings” and Item 8, Note 14, “Commitments and Contingencies” and Item 1A, “Risk Factors” - “We are currently, and may in the future be, subject to substantial litigation and ongoing investigations that could cause us to incur significant legal expenses and result in harm to our business.
•The application of CGMP requires investment in our manufacturing establishments for production for our micronized products. The transition process includes development and enhancement of production processes, procedures, test and assays, and it requires extensive validation work. It can also involve the procurement and installation of new production or lab equipment. These efforts require human capital, expertise and resources. See Item 1A. – “Risk Factors” under the heading “Certain of our products no longer qualify for regulation as human cells, tissues and cellular and tissue-based products solely under Section 361 of the Public Health Service Act (“Section 361”), which has resulted in removal of the applicable products from the market, made the introduction of some new tissue products more expensive, significantly delayed the expansion of our tissue product offerings and subjected us to additional post-market regulatory requirements. Additional regulatory requirements may be imposed in the future.”
Moreover, the COVID-19 pandemic may affect our operations in 2022 and beyond. More specifically:
•Our results of operations may be adversely affected if our customers restrict access to hospitals and our ability to access other healthcare providers, particularly for elective procedures.
•Our manufacturing operations, sales and demand for our products, and clinical trials may be adversely affected if our leadership, employees, sales agents, suppliers, medical professionals, or users of our products are impacted by illness or through actions taken to stop or slow the spread of the COVID-19 pandemic.
•Our results of operations may be adversely affected if we experience shortages of donated placentas because donors or our recovery specialists are excluded from hospitals, or because additional testing protocols are implemented for donated tissues based on guidance issued by the AATB, FDA, or other standards and are screened as ineligible.
•Because our sales are not evenly spread across the United States, to the extent that areas most impacted by COVID- are those where we have more of our sales, the pandemic will have a greater adverse impact on our results from operations.
•While vaccines have been approved by the FDA, the continued efficacy of the vaccine against current and future variants, as well as the general willingness to accept the vaccine and any recommended “boosters”, could influence the magnitude of the impact of the COVID-19 pandemic and any of the factors noted above.
The ultimate impact of the COVID-19 pandemic is highly uncertain. The duration and magnitude of these impacts on our business is uncertain.
Expectations for 2022 Operating Results
We expect net sales of our Advanced Wound Care products, which were $240 million in 2021, to grow 11% to 14% in 2022. We expect gross margin for 2022 to be slightly lower than 2021. We anticipate beginning the Phase 3 Knee Osteoarthritis clinical trial program in 2022, and expect the cost to be approximately $30 million, representing $15 million per trial for two trials incurred over the next three years. We expect research and development expense to increase over 2021 as we plan and begin to execute new clinical trials and execute other product development initiatives. However, the amount and timing of these expenses are dependent on many factors.
Discussion of Cash Flows
Operating Activities
55
During the year ended December 31, 2021, net cash used in operating activities decreased $28.3 million to $2.0 million compared to $30.3 million for the year ended December 31, 2020. The decrease in cash used was primarily attributable to year-over-year reductions in amounts paid related to the Audit Committee Investigation, the Restatement, and related expenses, particularly those incurred with respect to the Restatement and the indemnification of certain former officers and directors of the Company. In addition, we received $9.2 million in income tax refunds during 2021. These effects were offset by year-over-year increases in SG&A and research and development expense.
Investing Activities
During the year ended December 31, 2021, net cash used in investing activities was $3.4 million, a decrease of $1.2 million, compared to $4.6 million for the year ended December 31, 2020. The primary reason for the decrease was a $1.0 million decrease in capital expenditures, year-over-year. The remaining variance was the result of a year-over-year decrease in paid for patent application costs as well as collections on a note receivable in 2021.
Financing Activities
During the year ended December 31, 2021, net cash used in financing activities was approximately $3.4 million compared to cash provided from financing activities of $61.6 million for the year ended December 31, 2020. Activity in 2020 was driven by the issuance of our Series B Convertible Preferred Stock, for which we received proceeds of $92.5 million, net of stock issuance costs. In addition, we received net proceeds on the borrowing of our Term Loan of $46.3 million, net of deferred financing costs and original issue discount. These proceeds were used to repay the outstanding principal and prepayment premium on a previous term loan of $73.4 million. We did not have a similar financing transaction in 2021.
The remaining variance was driven by year-over-year increases in the cash paid for shares repurchased for tax withholding ($2.4 million), offset by increases in proceeds from option exercises ($1.0 million).
Non-GAAP Financial Measures
In addition to our GAAP results, we provide the following Non-GAAP measures: Adjusted Net Sales, Earnings Before Interest, Taxes, Depreciation and Amortization (“EBITDA”), and Adjusted EBITDA. We believe that the presentation of these measures provides important supplemental information to management and investors regarding our performance. These measurements are not, and should not be used as, a substitute for GAAP measures. Company management uses these Non-GAAP measures as aids in monitoring our on-going financial performance from quarter-to-quarter and year-to-year on a regular basis and for benchmarking against comparable companies.
Adjusted Net Sales
We provide Adjusted Net Sales to provide a normalized view of revenue by removing effects related to our Transition Adjustment in revenue recognition practices. Specifically, we recognized a one-time Transition Adjustment in 2019 to reflect the change in our pattern of revenue recognition from a “cash receipts” to an “as-shipped” basis. Since the third quarter of 2019, we have recognized revenue from cash collections related to the Remaining Contracts, or transactions which occurred prior to the Transition but for which we had not previously recognized revenue. Refer to Item 8, Note 2, “Significant Accounting Policies,” of the consolidated financial statements for additional details regarding the Transition Adjustment and the Remaining Contracts.
Adjusted Net Sales provides comparative assessments of our revenue and assists in evaluating our sales performance. Adjusted Net Sales consists of GAAP net sales less the effects of the Transition. For 2019, this includes the Transition Adjustment and cash received from the Remaining Contracts. For 2020 and 2021, this reflects cash received from the Remaining Contracts. A reconciliation of GAAP net sales to Adjusted Net Sales is provided in the table below (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net sales | $ | 258,615 | $ | 248,234 | $ | 299,255 | |||||||||||
| Effect of change in revenue recognition | (1,038) | (7,767) | (29,604) | ||||||||||||||
| Adjusted net sales | $ | 257,577 | $ | 240,467 | $ | 269,651 | |||||||||||
56
EBITDA and Adjusted EBITDA
We provide EBITDA and Adjusted EBITDA to facilitate comparisons to results of other companies. We use EBITDA as a measure of our operating performance, planning, and budgeting purposes as it eliminates the effects of financing and investing activities. EBITDA is widely used by investors and analysts to measure operating performance and evaluate enterprise value.
EBITDA consists of GAAP net loss excluding: (i) depreciation, (ii) amortization of intangibles, (iii) interest expense, net, (iv) loss on extinguishment of debt, and (v) income tax provision.
Adjusted EBITDA is intended to provide an enduring, normalized view of EBITDA and our broader business operations that we expect to experience on an ongoing basis by removing from EBITDA certain items which may be irregular, non-recurring, or non-cash items not excluded when calculating EBITDA. This enables us to identify underlying trends in our business that could otherwise be masked by such items.
Adjusted EBITDA consists of GAAP net loss excluding: (i) depreciation, (ii) amortization of intangibles, (iii) interest expense, net, (iv) loss on extinguishment of debt, (v) income tax provision, (vi) costs incurred in connection with Audit Committee Investigation and Restatement, (vii) the effect of the change in revenue recognition on net income, (viii) share-based compensation, and (ix) impairment of intangible assets.
A reconciliation of GAAP net loss to EBITDA and Adjusted EBITDA appears in the table below (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net loss | $ | (10,285) | $ | (49,284) | $ | (25,580) | |||||||||||
| Non-GAAP Adjustments: | |||||||||||||||||
| Depreciation expense | 4,363 | 5,782 | 6,546 | ||||||||||||||
| Amortization of intangible assets | 820 | 1,073 | 1,039 | ||||||||||||||
| Interest expense, net | 4,980 | 7,941 | 4,708 | ||||||||||||||
| Loss on extinguishment of debt | — | 8,201 | — | ||||||||||||||
| Income tax provision expense (benefit) | 247 | (12,259) | (5) | ||||||||||||||
| EBITDA | $ | 125 | $ | (38,546) | $ | (13,292) | |||||||||||
| Additional Non-GAAP Adjustments: | |||||||||||||||||
| Costs incurred in connection with Audit Committee Investigation and Restatement | 3,791 | 59,465 | 66,504 | ||||||||||||||
| Effect of change in revenue recognition | (864) | (6,680) | (24,450) | ||||||||||||||
| Share-based compensation | 14,757 | 15,357 | 12,064 | ||||||||||||||
| Impairment of intangible assets | 53 | 1,027 | 1,258 | ||||||||||||||
| Adjusted EBITDA | $ | 17,862 | $ | 30,623 | $ | 42,084 | |||||||||||
Critical Accounting Estimates
This Management’s Discussion and Analysis of Financial Condition and Results of Operations discusses our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of these financial statements requires that we make judgments and estimates which may affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. We derive these judgments and estimates on historical experience and other relevant factors which we believe to be reasonable. Actual results may differ from these estimates.
57
Net Sales
Description
We record estimates for returns and allowances as a reduction to net sales based on our expectation for such returns.
Judgments and Uncertainties
We sell our products to individual customer and independent distributors (collectively referred to as “customers”). Customers obtain and use products either through ship and bill sales or consignment arrangements. We recognize revenue as performance obligations are fulfilled, which generally occurs upon the shipment of product to customers for ship and bill sales or upon implantation for consignment sales. We recognize revenue based on consideration we expect to receive from the sale. This consists of the gross selling price of the product, less any discounts, rebates, fees paid to GPOs, and an expectation for sales returns.
We maintain a return policy that allows our customers to return product for any reason within 30 days of sale, and to return product that is damaged or non-conforming, ordered in error, or due to recall at any time.
We derive an expectation for product returns based on historical return patterns and other factors, including shifts in our regulatory environment and product recalls. Determinations involving other factors are based on our estimates for product at customer sites that are eligible for return.
Additions or reversals to our return allowance, as determined necessary, are accounted for prospectively and recorded as a decrease or increase to net sales, respectively. Actual returns are recorded against the recorded accrual.
Sensitivity of Estimate to Change
We have accrued $0.8 million for sales returns as of December 31, 2021. Changes in return patterns or unforeseen changes in regulations or identified product recalls could cause returns significantly in excess of this estimate.
Contingencies
Description
We record contingent liabilities related to legal and other proceedings at such point in time when loss is probable and reasonably estimable.
Judgments and Uncertainties
We evaluate the probability of loss and the range of potential losses based on salient details about a case. These evaluations consider evidence derived from discussions with counsel and include the merits and jurisdiction of the proceeding, the nature and the number of other similar current and past proceedings, damages sought by the counterparty, settlement offers we have extended to the counterparty and other factors. From this information, we make a judgmental determination of whether loss from a case is probable and whether a reasonable estimate of loss can be derived. In situations where a reasonable estimate is a range of estimates, we record the most likely amount in the range or, if no single amount is more likely than any of the others, we record the minimum amount of the range.
Sensitivity of Estimate to Change
We have accrued $1.0 million as of December 31, 2021 for potential losses relating to legal proceedings discussed in Item 8, Note 14, “Commitments and Contingencies.” The outcome of court judgments could lead to a change in our evaluation of probability of loss or our estimate for such loss. In addition, court judgments may result from matters for which we had previously assessed loss as being not probable or which result in losses which materially depart from our estimate, both favorably or unfavorably.
We believe that our estimates applied are based on reasonable assumptions, but are inherently uncertain. Actual results may differ from the assumptions and judgments used to derive our accrual.
Income Taxes
Description
58
We record a valuation allowance to offset our net deferred tax asset to the extent that realization is not likely.
Judgments and Uncertainties
Deferred income taxes arise from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements. Transactions which result in lower taxable income in the future give rise to deferred tax assets.
We evaluate our ability to recover deferred tax assets based on projected future taxable income, scheduled reversals of deferred tax liabilities, tax planning strategies, and our recent operating results. Judgment is required to determine whether the totality of this evidence suggests that we can recover our deferred tax assets in the future.
Sensitivity of Estimate to Change
As of December 31, 2021, we had valuation allowances recorded of $41.1 million, fully offsetting our net deferred tax asset. This determination may change due to changes in tax law, a revision to our expectation regarding taxable income in the future, taxable income generated in a period in which we had not previously anticipated taxable income, a change in scheduled reversals of deferred tax liabilities, and other changes.
Historically, exclusive of changes in tax law such as that enacted under the Coronavirus Aid, Relief and Economic Security Act, we have not reversed our valuation allowance.
If the weight of available evidence suggests that some or all of this amount is more likely than not to be realized, we will derecognize the valuation allowance as an income tax benefit to the extent that the underlying deferred tax asset is more likely than not to be realized.
Recently Adopted Accounting Pronouncements
See Note 2, “Significant Accounting Policies,” in the Consolidated Financial Statements for recently adopted accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Based on our lack of market risk sensitive instruments outstanding at December 31, 2021, we have determined that we had no material market risk exposure as of such date.
59
Item 8. Financial Statements and Supplementary Data
| Index to Financial Statements | |||||
Report of Deloitte & Touch LLP, Independent Registered Public Accounting Firm (PCAOB ID: 34) | F- 2 | ||||
Report of BDO USA, LLP, Independent Registered Public Accounting Firm (PCAOB ID: 243) | |||||
Consolidated Balance Sheets – As of December 31, 2021 and 2020 | F- 6 | ||||
Consolidated Statements of Operations – For the years ended December 31, 2021, 2020 and 2019 | F- 7 | ||||
Consolidated Statements of Stockholders’ Equity (Deficit) – For the years ended December 31, 2021, 2020 and 2019 | F- 8 | ||||
Consolidated Statements of Cash Flows – For the years ended December 31, 2021, 2020 and 2019 | F- 9 | ||||
F- 10 | |||||
| Schedule II - Valuation and Qualifying Accounts | F- 41 | ||||
F- 1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of MiMedx Group, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of MiMedx Group, Inc. and subsidiaries (the "Company") as of December 31, 2021, the related consolidated statements of operations, stockholders' equity (deficit), and cash flows, for the year then ended, and the related notes and the schedule listed in the Index at Item 8 (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 28, 2022, expressed an unqualified opinion on the Company's internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Net Sales - Timing of Revenue Recognition — Refer to Note 2 to the Financial Statements
Critical Audit Matter Description
The Company sells its products primarily to individual customers and independent distributors (collectively referred to as “customers”). Customers obtain and use products either through ship and bill sales or consignment arrangements. Under ship and bill arrangements, the Company retains possession of the product until the customer submits an order and the product ordered is shipped to the customer. Under consignment arrangements, the customer takes possession of the product, but the Company retains title until the implantation, or application of the Company’s product to the end user. The Company recognizes revenue as performance obligations are fulfilled, which generally occurs upon the shipment of product to the customers for ship and bill orders or upon implantation for consignment sales.
We identified the timing of revenue recognition for ship and bill and consignment sales at or near year-end as a critical audit matter because of the judgments involved in evaluating that the performance obligations are fulfilled. This required extensive audit effort due to the volume of transactions and a high degree of auditor judgment when performing audit procedures and evaluating the results of those procedures.
How the Critical Audit Matter Was Addressed in the Audit
F-2
Our audit procedures related to the timing of revenue recognition transactions included the following, among others:
•We created data visualizations using a detail of all revenue transactions and evaluated trends in the transactional revenue data with emphasis on activity at or near period end.
•We evaluated and tested corollary relationships between revenue and related accounts.
•We evaluated the appropriateness and consistency of the methods and assumptions utilized by management to estimate consignment revenue.
•We tested a sample of consignment revenue transactions manually accrued as of year-end and evaluated whether the transactions were recorded in the correct period.
•We tested a sample of ship and bill revenue transactions close to period end by agreeing the amounts recognized to source documents and evaluating whether the transaction was recorded in the correct period.
•We tested a sample of credits issued after year-end by agreeing to documents supporting the authorization for the issuance of the credit and to evaluate if the credit was issued in the correct period.
/s/ Deloitte & Touche LLP
Atlanta, Georgia
February 28, 2022
Atlanta, Georgia
February 28, 2022
We have served as the Company's auditor since 2021.
F-3
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
MiMedx Group, Inc.
Marietta, Georgia
MiMedx Group, Inc.
Marietta, Georgia
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of MiMedx Group, Inc. (the “Company”) as of December 31, 2020, the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2020, and the related notes and schedule (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2020, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the audit of the consolidated financial statements for the year ended December 31, 2020 that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Evaluation of the audit evidence for revenue recognition
The Company recorded consolidated net sales of $248.2 million for the year ended December 31, 2020. As more fully described in Note 2 to the consolidated financial statements, during 2018 and into part of 2019, the Company’s control environment was such that it created uncertainty surrounding all of its customer arrangements. The control environment allowed for the existence of extra-contractual or undocumented terms or arrangements initiated by or agreed to by the Company and former members of Company management at the outset of the transactions (side agreements). Concessions were also agreed to subsequent to the initial sale (e.g. sales above established customer credit limits, extended and unusually long payment terms, return or exchange rights, and contingent payment obligations). Beginning October 1, 2019, for all new customer arrangements, the Company determined adequate measures were in place to understand the terms of its contracts with customers. As such, the Company concluded that the Step 1 Criteria (identify the contracts with a customer) for revenue recognition would be met prior to shipment of product to the customer or implantation of the products on consignment.
We identified the evaluation of the sufficiency of audit evidence over revenue recognition as a critical audit matter. Evaluating the sufficiency of audit evidence required especially challenging auditor judgment to determine that extracontractual arrangements or side agreements did not exist at the onset of the transaction and that fictitious customer purchase orders were not entered into the system by sales personnel.
F-4
The primary procedures we performed to address this critical audit matter included:
•Testing the design and operating effectiveness of internal controls over the Company’s revenue processes, including controls over management’s review of the Step 1 Criteria.
•Testing the existence of revenue by selecting a sample of revenue transactions and comparing the amounts recorded for consistency with the underlying documentation, including the customer contract, purchase order, sales invoice, third party shipping documents, support documenting the implantation date (for consignment revenue), authorized pricing tables and customer payment support.
•Obtaining the monthly sales returns information recorded during 2020 to determine whether any unauthorized side agreements existed.
•Obtaining the January and February 2021 sales returns information to determine the completeness of the sales returns and associated credit memos.
•Performing data analytics over revenue transactions (excluding consignment and cash basis revenue) during the year ensuring a match of the sales order, sales invoice, shipping documents and payment support and investigating any items that did not agree.
/s/ BDO USA, LLP
We served as the Company's auditor from 2019 to 2020.
Atlanta, Georgia
March 8, 2021
F-5
MIMEDX GROUP, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except share data)
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | 87,083 | $ | 95,812 | |||||||
| Accounts receivable, net | 40,353 | 35,423 | |||||||||
| Inventory | 11,389 | 10,361 | |||||||||
| Prepaid expenses | 6,146 | 5,605 | |||||||||
| Income tax receivable | 743 | 10,045 | |||||||||
| Other current assets | 2,809 | 3,371 | |||||||||
| Total current assets | 148,523 | 160,617 | |||||||||
| Property and equipment, net | 9,165 | 11,437 | |||||||||
| Right of use asset | 4,696 | 3,623 | |||||||||
| Goodwill | 19,976 | 19,976 | |||||||||
| Intangible assets, net | 5,383 | 6,004 | |||||||||
| Other assets | 186 | 375 | |||||||||
| Total assets | $ | 187,929 | $ | 202,032 | |||||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK, AND STOCKHOLDERS’ EQUITY (DEFICIT) | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 7,385 | $ | 8,765 | |||||||
| Accrued compensation | 23,595 | 18,467 | |||||||||
| Accrued expenses | 9,812 | 30,460 | |||||||||
| Other current liabilities | 1,565 | 1,470 | |||||||||
| Total current liabilities | 42,357 | 59,162 | |||||||||
| Long term debt, net | 48,127 | 47,697 | |||||||||
| Other liabilities | 4,869 | 3,755 | |||||||||
| Total liabilities | $ | 95,353 | $ | 110,614 | |||||||
| Commitments and contingencies (Note 14) | |||||||||||
Convertible preferred stock Series B; $.001 par value; 100,000 shares authorized, issued and outstanding at December 31, 2021 and December 31, 2020 | $ | 92,494 | $ | 91,568 | |||||||
| Stockholders’ equity (deficit): | |||||||||||
Preferred stock Series A; $.001 par value; 5,000,000 shares authorized; 0 issued and outstanding at December 31, 2021 and 0 issued and outstanding at December 31, 2020 | $ | — | $ | — | |||||||
Common stock; $.001 par value; 187,500,000 shares authorized, 112,703,926 issued, and 111,925,216 outstanding at December 31, 2021 and 110,930,243 outstanding at December 31, 2020 | 113 | 113 | |||||||||
| Additional paid-in capital | 165,695 | 158,610 | |||||||||
Treasury stock at cost; 778,710 shares at December 31, 2021 and 1,773,683 shares at December 31, 2020 | (4,017) | (7,449) | |||||||||
| Accumulated deficit | (161,709) | (151,424) | |||||||||
| Total stockholders’ equity (deficit) | 82 | (150) | |||||||||
| Total liabilities, convertible preferred stock, and stockholders’ equity (deficit) | $ | 187,929 | $ | 202,032 | |||||||
See notes to the consolidated financial statements.
F-6
MIMEDX GROUP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net sales | $ | 258,615 | $ | 248,234 | $ | 299,255 | |||||||||||
| Cost of sales | 43,283 | 39,330 | 43,081 | ||||||||||||||
| Gross profit | 215,332 | 208,904 | 256,174 | ||||||||||||||
| Operating expenses: | |||||||||||||||||
| Selling, general and administrative | 198,359 | 181,022 | 198,205 | ||||||||||||||
| Research and development | 17,344 | 11,715 | 11,140 | ||||||||||||||
| Investigation, restatement and related | 3,791 | 59,465 | 66,504 | ||||||||||||||
| Amortization of intangible assets | 820 | 1,073 | 1,039 | ||||||||||||||
| Impairment of intangible assets | 53 | 1,027 | 446 | ||||||||||||||
| Operating loss | (5,035) | (45,398) | (21,160) | ||||||||||||||
| Other (expense) income | |||||||||||||||||
| Loss on extinguishment of debt | — | (8,201) | — | ||||||||||||||
| Interest expense, net | (4,980) | (7,941) | (4,708) | ||||||||||||||
| Other (expense) income, net | (23) | (3) | 283 | ||||||||||||||
| Loss before income tax provision | (10,038) | (61,543) | (25,585) | ||||||||||||||
| Income tax provision (expense) benefit | (247) | 12,259 | 5 | ||||||||||||||
| Net loss | $ | (10,285) | $ | (49,284) | $ | (25,580) | |||||||||||
| Net loss available to common stockholders (Note 10) | $ | (16,421) | $ | (83,328) | $ | (25,580) | |||||||||||
| Net loss per common share - basic | $ | (0.15) | $ | (0.77) | $ | (0.24) | |||||||||||
| Net loss per common share - diluted | $ | (0.15) | $ | (0.77) | $ | (0.24) | |||||||||||
| Weighted average common shares outstanding - basic | 110,353,406 | 108,257,112 | 106,946,384 | ||||||||||||||
| Weighted average common shares outstanding - diluted | 110,353,406 | 108,257,112 | 106,946,384 | ||||||||||||||
See notes to the consolidated financial statements.
F-7
MIMEDX GROUP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share data)
| Common Stock | Additional Paid-in | Treasury Stock | Accumulated | ||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Capital | Shares | Amount | Deficit | Total | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2018 | 112,703,926 | $ | 113 | $ | 164,744 | 3,605,263 | $ | (38,642) | $ | (76,560) | $ | 49,655 | |||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | 11,689 | — | — | — | 11,689 | ||||||||||||||||||||||||||||||||||
| Exercise of stock options | — | — | (1,343) | (150,000) | 1,451 | — | 108 | ||||||||||||||||||||||||||||||||||
| Issuance of restricted stock | — | — | (37,798) | (3,084,875) | 37,798 | — | — | ||||||||||||||||||||||||||||||||||
| Restricted stock shares canceled/forfeited | — | — | 9,939 | 1,084,971 | (9,939) | — | — | ||||||||||||||||||||||||||||||||||
| Shares repurchased for tax withholding | — | — | — | 429,918 | (1,474) | — | (1,474) | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (25,580) | (25,580) | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2019 | 112,703,926 | $ | 113 | $ | 147,231 | 1,885,277 | $ | (10,806) | $ | (102,140) | $ | 34,398 | |||||||||||||||||||||||||||||
| Issuance of Series B Convertible Preferred Stock | — | — | 32,954 | — | — | — | 32,954 | ||||||||||||||||||||||||||||||||||
| Deemed dividends | — | — | (32,028) | — | — | — | (32,028) | ||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | 15,733 | — | — | — | 15,733 | ||||||||||||||||||||||||||||||||||
| Exercise of stock options | — | — | (3,180) | (359,328) | 3,591 | — | 411 | ||||||||||||||||||||||||||||||||||
| Issuance of restricted stock | — | — | (5,463) | (613,146) | 5,463 | — | — | ||||||||||||||||||||||||||||||||||
| Restricted stock shares canceled/forfeited | — | — | 3,363 | 425,388 | (3,363) | — | — | ||||||||||||||||||||||||||||||||||
| Shares repurchased for tax withholding | — | — | — | 435,492 | (2,334) | — | (2,334) | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (49,284) | (49,284) | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2020 | 112,703,926 | $ | 113 | $ | 158,610 | 1,773,683 | $ | (7,449) | $ | (151,424) | $ | (150) | |||||||||||||||||||||||||||||
| Deemed dividends | — | — | (926) | — | — | — | (926) | ||||||||||||||||||||||||||||||||||
| Shares repurchased for tax withholding | — | — | — | 469,239 | (4,751) | — | (4,751) | ||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | 14,757 | — | — | 14,757 | |||||||||||||||||||||||||||||||||||
| Exercise of stock options | — | — | (1,199) | (487,361) | 2,636 | — | 1,437 | ||||||||||||||||||||||||||||||||||
| Issuance of restricted stock | — | — | (4,053) | (810,405) | 4,053 | — | — | ||||||||||||||||||||||||||||||||||
| Restricted stock shares canceled/forfeited | — | — | 515 | 73,056 | (515) | — | — | ||||||||||||||||||||||||||||||||||
| Other | — | — | (2,009) | (239,502) | 2,009 | — | — | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (10,285) | (10,285) | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | 112,703,926 | $ | 113 | $ | 165,695 | 778,710 | $ | (4,017) | $ | (161,709) | $ | 82 | |||||||||||||||||||||||||||||
See notes to the consolidated financial statements.
F-8
MIMEDX GROUP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Cash flows from operating activities: | |||||||||||||||||
| Net loss | $ | (10,285) | $ | (49,284) | $ | (25,580) | |||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||||||
| Share-based compensation | 14,757 | 15,357 | 12,064 | ||||||||||||||
| Depreciation | 4,363 | 5,782 | 6,546 | ||||||||||||||
| Amortization of deferred financing costs and debt discount | 1,055 | 2,276 | 1,431 | ||||||||||||||
| Non cash lease expenses | 989 | 983 | 947 | ||||||||||||||
| Amortization of intangible assets | 820 | 1,073 | 1,039 | ||||||||||||||
| Loss on fixed asset disposal | 262 | 1 | 318 | ||||||||||||||
| Accretion of asset retirement obligation | 81 | 10 | — | ||||||||||||||
| Impairment of intangible assets | 53 | 1,027 | 1,258 | ||||||||||||||
| Loss on extinguishment of debt | — | 8,201 | — | ||||||||||||||
| Effect of change in revenue recognition | — | — | (17,382) | ||||||||||||||
| Increase (decrease) in cash resulting from changes in: | |||||||||||||||||
| Accounts receivable | (4,930) | (3,096) | (10,938) | ||||||||||||||
| Inventory | (1,028) | (1,257) | 6,882 | ||||||||||||||
| Prepaid expenses | (542) | 1,064 | 4 | ||||||||||||||
| Other assets | 675 | (119) | (5,770) | ||||||||||||||
| Accounts payable | (326) | 177 | (6,171) | ||||||||||||||
| Accrued compensation | 5,128 | (2,459) | (1,722) | ||||||||||||||
| Accrued expenses | (21,197) | 1,746 | (57) | ||||||||||||||
| Income taxes | 9,302 | (10,027) | 436 | ||||||||||||||
| Other liabilities | (1,159) | (1,718) | (2,717) | ||||||||||||||
| Net cash flows used in operating activities | (1,982) | (30,263) | (39,412) | ||||||||||||||
| Cash flows from investing activities: | |||||||||||||||||
| Purchases of property and equipment | (3,218) | (4,228) | (1,752) | ||||||||||||||
| Patent application costs | (252) | (327) | (466) | ||||||||||||||
| Principal payments from note receivable | 75 | — | 2,722 | ||||||||||||||
| Net cash flows (used in) provided by investing activities | (3,395) | (4,555) | 504 | ||||||||||||||
| Cash flows from financing activities: | |||||||||||||||||
| Stock repurchased for tax withholdings on vesting of restricted stock | (4,751) | (2,334) | (1,474) | ||||||||||||||
| Proceeds from exercise of stock options | 1,437 | 411 | 108 | ||||||||||||||
| Payments under finance lease obligations | (38) | — | — | ||||||||||||||
| Proceeds from sale of Series B convertible preferred stock | — | 100,000 | — | ||||||||||||||
| Stock issuance costs | — | (7,470) | — | ||||||||||||||
| Proceeds from term loans | — | 59,500 | 72,750 | ||||||||||||||
| Deferred financing costs | — | (3,235) | (6,650) | ||||||||||||||
| Repayment of term loans | — | (83,872) | (1,875) | ||||||||||||||
| Prepayment premium on early repayment of term loan | — | (1,439) | — | ||||||||||||||
| Net cash flows (used in) provided by financing activities | (3,352) | 61,561 | 62,859 | ||||||||||||||
| Net change in cash | (8,729) | 26,743 | 23,951 | ||||||||||||||
| Cash and cash equivalents, beginning of year | 95,812 | 69,069 | 45,118 | ||||||||||||||
| Cash and cash equivalents, end of year | $ | 87,083 | $ | 95,812 | $ | 69,069 | |||||||||||
F-9
MIMEDX GROUP, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1.Nature of Business
MiMedx Group, Inc. (together with its subsidiaries, except where the context otherwise requires, “MIMEDX,” or the “Company”) is a transformational placental biologics company, developing and distributing placental tissue allografts with patent-protected, proprietary processes for multiple sectors of healthcare. As a pioneer in placental biologics, the Company is focused on addressing unmet clinical needs in the areas of advanced wound care, surgical recovery applications, and musculoskeletal conditions. We derive our products from human placental tissues and process these tissues using our proprietary methods, including the PURION® process. The Company applies Current Good Tissue Practices, Current Good Manufacturing Practices, and terminal sterilization to produce its allografts. MIMEDX provides products primarily in the wound care, burn, and surgical recovery sectors of healthcare. All of its products are regulated by the U.S. Food & Drug Administration (“FDA”).
The Company’s business model is focused primarily on the United States of America but the Company is pursuing opportunities for international expansion.
Effect of the COVID-19 Pandemic
On March 11, 2020, the World Health Organization designated the outbreak of a novel strain of coronavirus as a global pandemic. The COVID-19 pandemic and associated governmental and societal responses have affected the Company’s business, results of operations and financial condition in the past and could continue to have an adverse impact on the Company’s business, results of operations, and financial condition in the future.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) was signed into law. The CARES Act included provisions relating to refundable payroll tax credits, deferment of the employer portion of certain payroll taxes, loans, and grants to certain businesses, net operating loss carryback periods, alternative minimum tax credit refunds, modifications to the net interest deduction limitations and technical corrections to tax depreciation methods for qualified improvement property. Certain of these provisions were extended or expanded as a result of the Consolidated Appropriations Act, 2021, which was signed into law on December 27, 2020. As a result of these laws, the Company recorded a federal tax benefit of $11.3 million due to the release of a previously-recorded valuation allowance in 2020. Of this amount, the Company received $9.2 million and $1.2 million during the years ended December 31, 2021 and 2020, respectively. The remaining $0.9 million is recorded as part of income tax receivable on the consolidated balance sheet as of December 31, 2021.
In addition, the CARES Act provided an employee retention credit (“ERC”), which was a refundable tax credit against certain payroll taxes. Upon determination that the Company had complied with all of the conditions required to receive the credit, the Company qualified and filed to claim the ERC. The Company reflected the ERC as a reduction to the respective captions on the consolidated statements of operations associated with the employees to which the payroll tax benefit related. For the year ended December 31, 2021, the Company recorded a $1.6 million reduction to selling, general and administrative expense. As of December 31, 2021, the Company recorded $1.6 million as other current assets in the consolidated balance sheet.
Enforcement Discretion
In November 2017, the FDA published a series of guidances that established an updated framework for the regulation of cellular and tissue-based products. These guidances clarified the FDA’s views about the criteria that differentiate those products subject to regulation under Section 361 of the Public Health Service Act from those considered to be drugs, devices, and/or biological products subject to licensure under Section 351 of the Public Health Service Act and related regulations. The Company identified its micronized and particulate products (collectively, the “Section 351 Products”) as being subject to regulation under Section 351, requiring pre-market approval from the FDA for a specified indication with demonstrated clinical efficacy.
The FDA exercised enforcement discretion with respect to Investigational New Drug (“IND”) applications and pre-market approval requirements through May 31, 2021. As of May 31, 2021, the Company stopped marketing its Section 351 Products in the United States and is precluded from marketing such products until a Biologics License Application (“BLA”) is granted. If and when the FDA approves a BLA, the Company expects to be allowed to market its Section 351 Products in the United States, but only for specific indications as permitted by the FDA. Sales of the Company’s Section 351 Products were $17.6 million and $31.8 million for the years ended December 31, 2021 and 2020, respectively. Sales of Section 351 Products for the year ended December 31, 2021 reflects the sale of such products in the United States through May 31, 2021.
F-10
The Company currently markets EPICORD® and AMNIOCORD® tissue products derived from human umbilical cord as providing a protective environment or as a barrier. If the FDA were to determine that EPICORD and AMNIOCORD do not meet the requirements for regulation solely under Section 361, then pre-market clearance or approval would be required. The loss of the Company’s ability to market and sell its umbilical cord-derived products would have an adverse effect on the Company’s revenue, business, financial condition, and results of operations. Net sales of the Company’s umbilical cord-derived products were $23.6 million and $16.1 million for the years ended December 31, 2021 and 2020, respectively. The Company’s cord inventory was $1.9 million as of December 31, 2021.
Out-of-Period Adjustment
During the year ended December 31, 2021, the Company identified certain Restricted Stock Unit and Performance Stock Unit awards that were not appropriately reflected in the Company’s balance of common stock outstanding beginning in 2019. The effects of these errors caused misstatements in the Company’s balance of treasury stock, additional paid-in capital, and common stock outstanding on each of the Company’s reported consolidated balance sheets and consolidated statements of stockholders’ equity (deficit) for interim and annual periods beginning with those statements as of and for the year ended December 31, 2019. The identified errors did not affect total stockholders’ equity (deficit) or earnings per share in any period.
The Company recorded an out-of-period adjustment during the year ended December 31, 2021, which resulted in a decrease of $2.0 million to the balance of additional paid-in capital for the year ended December 31, 2021 and an increase of $2.0 million to the balance of treasury stock.
The Company concluded the effect of the misstatement was not material, qualitatively or quantitatively, to any interim or annual period. These amounts are reflected as part of other in the consolidated statement of stockholders’ equity (deficit) for the year ended December 31, 2021.
2. Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of MiMedx Group, Inc. and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated upon consolidation.
Use of Estimates
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). Generally accepted accounting principles require management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported consolidated statements of operations during the reporting period. Actual results could differ from those estimates. Significant estimates include estimated useful lives and potential impairment of property and equipment, goodwill and intangible assets, estimates of loss for contingent liabilities, estimate of allowance for doubtful accounts, management’s assessment of the Company’s ability to continue as a going concern, estimate of fair value of share-based payments, estimates of returns and allowances, and valuation of deferred tax assets.
Segment Reporting
Accounting Standards Codification (“ASC”) 280, “Segment Reporting” requires the use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s chief operating decision maker organizes segments within the Company for which separate discrete financial information is available regarding resource allocation and assessing performance. The Company has determined it operates as one operating segment.
Cash and Cash Equivalents
Cash and cash equivalents include cash held at various banks. The Company considers all highly-liquid investments purchased with an original maturity of three months or less at the date of purchase and money market mutual funds to be cash equivalents.
F-11
Market Concentrations and Credit Risk
The Company places its cash and cash equivalents on deposit with U.S.-based financial institutions. The U.S. Federal Deposit Insurance Corporation (“FDIC”) provides insurance coverage for deposits up to $250,000 for substantially all depository accounts. As of December 31, 2021 and 2020, the Company had cash and cash equivalents of approximately $86.4 million and $95.1 million, respectively, in excess of the insured amounts in four depository institutions.
Accounts Receivable
Accounts receivable represent amounts due from customers for which revenue has been recognized. Generally, the Company does not require collateral or any other security to support its receivables.
Bad debt expense and the allowance for doubtful accounts are based on historical trends and current expectations for credit losses. The Company’s policy to reserve for potential bad debts is based on the aging of the individual receivables as well as customer-specific qualitative factors, such as bankruptcy proceedings. The Company manages credit risk by routinely performing credit checks on customers prior to sales. The individual receivables are written-off after all reasonable efforts to collect the funds have been made. Actual write-offs may differ from the amounts reserved.
Inventory
Inventory is valued at the lower of cost or net realizable value. Costs of inventory sold are recognized using the first–in, first-out (“FIFO”) method. Inventory is tracked through raw material, work-in-process, and finished goods stages as the product progresses through various production steps and stocking locations. Labor and overhead costs are absorbed through the various production processes up to when the work order closes. Historical yields and normal capacities are utilized in the calculation of production overhead rates. Write-downs are utilized to account for slow-moving inventory as well as inventory no longer needed due to diminished demand or regulatory action.
Property and Equipment
Property and equipment are recorded at cost and depreciated on a straight-line method over their estimated useful lives, principally to seven years. Leasehold improvements are depreciated on a straight-line method over the shorter of the estimated useful lives and the remaining lease term.
Asset Retirement Obligations
The Company records obligations associated with the legal requirement to retire long-lived assets at the sooner of the imposition of the legal requirement and when an estimate for the cost of retirement can reasonably be made. The Company reviews legal obligations associated with the retirement of long-lived assets that result from contractual obligations or the acquisition, construction, development and/or normal use of the assets. If it is determined that a legal obligation exists, regardless of whether the obligation is conditional on a future event, the fair value of the liability for an asset retirement obligation is recognized in the period in which it is incurred, if a reasonable estimate of fair value can be made. The fair value is calculated as the estimate of the expected cash outflow to satisfy the legal obligation discounted to present value using the Company’s incremental borrowing rate. At such point in time, an asset and liability are recorded for the amount of the expected liability. The asset amount is depreciated, straight-line, over the life of the underlying asset, while the liability is accreted to the amount of the expected outflow through selling, general and administrative expense using the effective interest method. Subsequent revisions to estimates for future cash flows related to the asset retirement obligations are recorded as equal increases or decreases to the retirement asset and liability.
Impairment of Long-lived Assets
The Company evaluates the recoverability of its long-lived assets (property, equipment, right of use, and intangible assets with finite lives) whenever adverse events or changes in business climate indicate that the expected undiscounted future cash flows from the related assets may be less than their carrying amounts. When a situation arises which results in a conclusion that it is more likely than not that an asset is not recoverable, the Company estimates cash flows expected to be derived from the continuing use and eventual disposition of the asset. If the sum of those cash flows, not discounted to present value, does not exceed the net book value of the asset, the Company estimates the fair value of the asset. Impairment loss is recorded to the extent that the net book value exceeds the fair value of the asset.
F-12
Impairment reviews are based on an estimated future cash flow approach that requires significant judgment with respect to future revenue and expense growth rates, selection of appropriate discount rate, asset groupings, and other assumptions and estimates. The Company uses estimates that are consistent with its business plans and a market participant view of the assets being evaluated. Actual results may differ from these estimates.
The Company recorded impairment losses on amortizable intangible assets of $0.1 million, $1.0 million, and $0.5 million in in 2021, 2020, and 2019, respectively. The Company recorded no impairment losses with respect to any other classes of long-lived assets in those periods.
Goodwill and Indefinite-lived Intangible Assets
Goodwill represents the excess of purchase price over the fair value of net assets of acquired businesses. The Company assesses goodwill for impairment at least annually on October 1, or more frequently whenever events or substantive changes in circumstances indicate that it is more likely than not that goodwill is impaired. In performing the goodwill impairment test, the Company assesses qualitative factors to determine the existence of impairment. If the qualitative factors indicate that it is more likely than not that the carrying value of the reporting unit exceeds its fair value, the Company proceeds to a quantitative test to measure the existence and amount of goodwill impairment. The Company may also choose to bypass the qualitative assessment and proceed directly to the quantitative analysis.
The Company has one reporting unit.
In performing the quantitative test, impairment loss is recorded to the extent that the carrying value of the reporting unit exceeds its assessed fair value, not to exceed goodwill allocated to that reporting unit. No impairment is recognized if fair value is determined to exceed carrying value. The Company determines the fair value utilizing the income and market approaches. Under the income approach, the fair value of the reporting unit is the present value of its future cash flows. These future cash flows are derived from expectations of revenue, expenses, tax deductions, working capital flows, capital expenditures, and other projected sources and uses of cash. Value indications are developed by discounting expected cash flows to their present value at a risk-adjusted weighted average cost of capital using the capitalization of market-comparable companies. The weighted average cost of capital is rooted in the risk-free rate of a U.S. Treasury with a similar maturity to the time period evaluated, credit risk specific to the Company, relevant equity risk premia, the Company’s incremental borrowing rate, and the prevailing marginal income tax rate. Under the market approach, the Company uses its market capitalization, which is calculated by taking the Company’s share price multiplied by the number of outstanding common shares plus the number of common shares to which the holders of the Company’s Convertible preferred stock Series B would be entitled to upon conversion.
Acquired indefinite-lived intangible assets are tested for impairment annually on October 1 or whenever events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable. The Company’s impairment reviews are based on an estimated future cash flow approach that requires significant judgment with respect to future revenue and expense growth estimates. The Company uses estimates consistent with business plans and a market participant view of the assets being evaluated. Actual results may differ from the estimates used in these analyses.
For the goodwill impairment test performed on October 1, 2021, the Company performed a qualitative assessment for its reporting unit, concluding that it was not more likely than not that the carrying value of the reporting unit exceeded its fair value. Therefore, the Company did not perform a quantitative assessment and no goodwill impairment was recognized related to this test.
There were no recorded impairment losses related to goodwill in 2021, 2020, or 2019. The Company recorded impairment losses related to our indefinite-lived intangible assets of $0, $0, and $0.8 million related to the abandonment of patents in process during 2021, 2020, and 2019, respectively.
Patent Costs
The Company incurs certain legal and related costs in connection with patent applications. The Company capitalizes such costs to be amortized over the expected life of the patent to the extent that an economic benefit is anticipated from the resulting patent or an alternative future use is available to the Company. The Company capitalized $0.3 million, $0.3 million, and $0.5 million of patent costs for the years ended December 31, 2021, 2020, and 2019, respectively.
Leases
The Company determines if a contract is, or contains, a lease at inception. Leases provide the Company with the right to control an underlying asset for a contractual term, subject to certain renewal and other rights, in exchange for a series of stipulated cash
F-13
flows. Right of use (“ROU”) assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the Company's obligation to make lease payments arising from the lease.
Lease assets and liabilities are recognized at the lease commencement date based on the estimated present value of lease payments over the lease term. The Company calculates the present value of lease payments by discounting the lease payments using the Company’s incremental borrowing rate for a collateralized or secured borrowing over a term equivalent to that of the lease. Lease payments that vary according to an index or rate are measured using the index or rate at lease inception. The lease term and applicable payments include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Options to renew or terminate a lease are included in the lease term to the extent that such provisions are reasonably certain to be exercised. This determination is reassessed as new information arises and is accounted for prospectively. As an accounting policy election, the Company does not capitalize leases having initial terms of 12 months or fewer. The Company has made an accounting policy election not to separate lease components from non-lease components in the event that the agreement contains both.
Operating lease right of use assets and the related liabilities are included in right of use asset, other current liabilities, and other liabilities, respectively, in the consolidated balance sheets. Lease expense associated with operating leases is recognized, straight-line, over the lease term. The Company does not recognize interest expense as part of operating lease liabilities.
Finance lease right of use assets and the related liabilities are included in property and equipment, net, other current liabilities, and other liabilities, respectively, in the consolidated balance sheets. Finance lease right of use assets are amortized, straight-line, over the lease term as depreciation expense. Interest expense is recognized using the effective interest method on finance lease liabilities as part of interest expense, net.
Treasury Stock
Shares repurchased by the Company are recorded as treasury stock at the cost to acquire such shares. Subsequent issuances of shares held in treasury are assumed to be released on a FIFO basis.
Contingencies
The Company is or has been subject to various patent challenges, product liability claims, government investigations, former employee matters, and other legal proceedings, see Note 14, “Commitments and Contingencies.” Legal fees and other expenses related to litigation are expensed as incurred and included in selling, general and administrative expenses in the consolidated statements of operations. The Company records an accrual for resolution costs and other contingencies in the consolidated financial statements when the Company determines that a loss is both probable and reasonably estimable. Subsequent revisions to the Company’s accrual are made as new information emerges and are accounted for prospectively. The Company discloses all ongoing legal matters for which a loss is reasonably possible, regardless of whether an estimate can be reasonably determined.
Due to the fact that legal proceedings and other contingencies are inherently unpredictable, the Company’s estimates of the probability and amount of any such liabilities involve significant judgment regarding future events. The actual costs of resolving a claim may be substantially different from the amount of reserve the Company recorded. The Company records a receivable from its insurance carriers only when the resolution of any dispute has been reached and realization of the amounts equal to the potential claim for recovery is considered probable. Any recovery of an amount in excess of the related recorded contingent loss will be recognized only when all contingencies relating to recovery have been resolved.
Revenue Recognition
The Company sells its products primarily to individual customers and independent distributors (collectively referred to as “customers”). Customers obtain and use products either through ship and bill sales or consignment arrangements. Under ship and bill arrangements, the Company retains possession of the product until the customer submits an order. Upon approval of the sales order, the Company ships product to the customer and invoices them for the product sold. Under consignment arrangements, the customer takes possession of the product, but the Company retains title until the implantation, or application of the Company’s product to the end user.
Subsequent to the Transition (as defined below) and including all of the years ended December 31, 2021 and 2020, the Company recognizes revenue as performance obligations are fulfilled, which generally occurs upon the shipment of product to the customers for ship and bill orders or upon implantation for consignment sales.
Revenue is recognized based on the consideration the Company expects to receive from the sale. This consists of the gross selling price of the product, less any discounts, rebates, fees paid to Group Purchasing Organizations (“GPOs”), and returns
F-14
(collectively, “deductions” or “sales deductions”). Gross selling price is a standard set by the Company for all customers unless a contract governing the sale provides for a specified price. Sales deductions are specified in individual contracts with customers. The Company estimates the total sales deductions which a specific customer will achieve over the relevant term and applies the reduction to sales as they are made throughout the period.
Sales deductions owed to customers and other parties are accrued and recorded in accrued expenses on the consolidated balance sheets.
The Company acts as the principal in all of its customer arrangements and records revenue on a gross basis. Shipping is considered immaterial in the context of the overall customer arrangement, and damages or loss of goods in transit are rare. Therefore, shipping is not deemed a separately recognized performance obligation and the Company has elected to treat shipping costs as activities to fulfill the promise to transfer the product. The Company maintains a returns policy that allows its customers to return product that is damaged or non-conforming, ordered in error, or due to a recall. The estimate of the provision for returns is based upon historical experience with actual returns. The Company’s payment terms for customers are typically 30 to 60 days from receipt of title of the goods.
Previous Revenue Recognition Policy and Transition
During the first three quarters of 2019, the Company’s control environment was such that it created uncertainty surrounding all of its customer arrangements, which required consideration related to the proper revenue recognition under the applicable literature. The control environment allowed for the existence of extra-contractual or undocumented terms or arrangements initiated by or agreed to by the Company and former members of Company management at the outset of the transactions (side agreements). Concessions were also agreed to subsequent to the initial sale (e.g. sales above established customer credit limits extended and unusually long payment terms, return or exchange rights, and contingent payment obligations) that precluded the Company from recognizing revenue at the time that product was shipped to a customer.
Because of the prevalence of these arrangements, the Company’s sales arrangements did not qualify as contracts under ASC 606, Revenue from Contracts with Customers, until consideration was collected from customers. This determination precluded the recognition of revenue at the time of shipment. Instead, recognition of revenue was deferred until: (1) the customer returned the product prior to payment; or (2) the Company received payment from the customer. Cost of sales associated with product shipped was deferred until collection was received.
The Company implemented changes and remediated weaknesses, which gave rise to the above conclusion beginning in mid-2018. Management concluded that these efforts had been sufficiently implemented such that customers were aware of the Company’s sales policies and procedures and that a contract existed prior to the transfer of title or the implantation of product for ship-and-bill and consignment sales, respectively, by the third quarter of 2019. Accordingly, the Company changed its pattern of revenue recognition effective October 1, 2019 to the policy described under the section titled “Current Policy” above.
The Company also reassessed whether the revenue recognition criteria had been met for all shipments of products where payment had not been received as of September 30, 2019. While the measures summarized above provided significant evidence necessary to understand the terms of the Company’s contractual arrangements with its customers, certain of these customers continued to exhibit behaviors that resulted in extended periods until cash collection. Such delays in collection suggested that uncertainty regarding extra-contractual arrangements may continue, particularly as it relates to payment terms. As a result, the Company concluded the following for any existing arrangements, which remained unpaid at September 30, 2019:
•For customer arrangements where collection was considered probable within 90 days from the date of original shipment or implantation of the products, the Company concluded the revenue recognition criteria were met (the “Transition Adjustment”).
•For the remaining customer arrangements (the “Remaining Contracts”), the Company concluded that, due to the uncertainty that extra-contractual arrangements may continue, the revenue recognition criteria would not be satisfied until the Company received payment from the customer. At that point, the Company determined that an accounting contract would exist and the performance obligations of the Company to deliver product and the customer to pay for the product would be satisfied. The Company continued to reassess the Remaining Contracts for settlement of the revenue recognition criteria prior to payment, concluding that the revenue recognition criteria continued to not be met due to the same circumstances described above.
The effect of the Transition Adjustment and cash collections on the Remaining Contracts on net sales and cost of sales for each of the years ended December 31, 2021, 2020, and 2019 were as follows (amounts in thousands):
F-15
| Year Ended December 31, | |||||||||||||||||
| Net sales | 2021 | 2020 | 2019 | ||||||||||||||
| Transition Adjustment | $ | — | $ | — | $ | 21,385 | |||||||||||
| Collections on Remaining Contracts | 1,038 | 7,767 | 8,219 | ||||||||||||||
| Net sales | 1,038 | 7,767 | 29,604 | ||||||||||||||
| Cost of sales | |||||||||||||||||
| Transition Adjustment | — | — | 2,565 | ||||||||||||||
| Collections on Remaining Contracts | 145 | 1,087 | 1,151 | ||||||||||||||
| Write-off of cost of sales deemed uncollectible | 29 | — | 1,438 | ||||||||||||||
| Cost of sales | 174 | 1,087 | 5,154 | ||||||||||||||
| Gross profit | $ | 864 | $ | 6,680 | $ | 24,450 | |||||||||||
Group Purchasing Organization Fees
The Company sells to Group Purchasing Organization (“GPO”) members who transact directly with the Company at GPO-agreed pricing. GPOs are funded by administrative fees that are paid by the Company. These fees are set as a percentage of the purchase volume, which is typically 3% of sales made to the GPO members. Fees paid to GPOs are presented as a reduction to net sales.
Cost of Sales
Cost of sales includes all costs directly related to bringing the Company’s products to their final selling destination. Amounts include direct and indirect costs to manufacture products including raw materials, personnel costs and direct overhead expenses necessary to convert collected tissues into finished goods, product testing costs, quality assurance costs, facility costs associated with the Company’s manufacturing and warehouse facilities, including depreciation, freight charges, costs to operate equipment and other shipping and handling costs for products shipped to customers.
The Company obtains raw material in the form of human placenta donations from participating mothers who give birth via scheduled Caesarean section.
Subsequent to the Transition Adjustment, the Company deferred cost of sales related to the Remaining Contracts. Deferred cost of sales were $0 and $0.2 million as of December 31, 2021 and 2020, respectively. These amounts were recorded within other current assets on the consolidated balance sheet.
Research and Development Costs
Research and development costs consist of direct and indirect costs associated with the development of the Company’s technologies. These costs are expensed as incurred.
Advertising expense
Advertising expense consists primarily of print media promotional materials. Advertising costs are expensed as incurred. Advertising expense for each of the years ended December 31, 2021, 2020, and 2019 amounted to $0.1 million.
Income Taxes
Income tax provision (expense) benefit, deferred tax assets and liabilities, and liabilities for unrecognized tax benefits reflect management’s best assessment of estimated current and future taxes to be paid. The Company is subject to income taxes in the United States and numerous states.
Deferred income taxes arise from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements, which will result in taxable or deductible amounts in the future. The Company recognizes deferred tax assets to the extent that it believes these assets are more likely than not to be realized. If the Company determines that it would be able to realize its deferred tax assets in the future in excess of their net recorded amount, the Company would make an adjustment to the deferred tax asset valuation allowance.
F-16
In evaluating the Company’s ability to recover its deferred tax assets within the jurisdiction from which they arise, management considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax-planning strategies, results of recent operations, and changes in tax laws. In projecting future taxable income, the Company begins with historical results and incorporates assumptions about the amount of future state and federal pretax operating income adjusted for items that do not have tax consequences. The assumptions about future taxable income require significant judgment and are consistent with the plans and estimates the Company uses to manage the underlying businesses. In evaluating the objective evidence that historical results provide, management considers three years of cumulative income (loss). The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the tax provision (benefit) in the period that includes the enactment date.
The calculation of income tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations both for U.S. federal income tax purposes and across numerous state jurisdictions. Accounting Standards Codification Topic 740, Income Taxes, states that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, on the basis of the technical merits. The Company (1) records unrecognized tax benefits as liabilities in accordance with ASC 740 included within other liabilities on the consolidated balance sheets, and (2) adjusts these liabilities when management’s judgment changes as a result of the evaluation of new information not previously available. Because of the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from management’s current estimate of the unrecognized tax benefit liabilities. These differences will be reflected as increases or decreases to the deferred tax asset or income tax expense in the period in which new information is available.
The Company records uncertain tax positions in accordance with ASC 740 on the basis of a two-step process whereby (1) it determines whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position, and (2) for those tax positions that meet the more-likely-than-not recognition threshold, it recognizes the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
The Company recognizes interest and penalties related to unrecognized tax benefits within the income tax expense line in the consolidated statements of operations. Accrued interest and penalties, if any, are included within the related deferred tax liability line in the consolidated balance sheets and recorded as a component of income tax expense.
Share-based Compensation
The Company grants share-based awards to employees and members of the Company’s Board of Directors (the “Board”). Awards to employees and the Board are generally made annually. Grants are issued outside of the annual cadence for certain new hires, promotions, and other events.
The amount of expense to be recognized is determined by the fair value of the award using inputs available as of the grant date. The fair value of restricted common stock is the value of common stock on the grant date. The fair value of stock option grants is estimated using the Black-Scholes option pricing model. Use of the valuation model requires management to make certain assumptions with respect to selected model inputs.
For awards with service-based vesting conditions only, the Company recognizes share-based compensation expense on a straight-line basis through the vesting date of the last tranche of the award. For awards with service- and performance-based vesting conditions, the Company recognizes stock-based compensation expense using the graded-vesting method, treating each tranche as if it were a separately-granted award and recognizing expense through the vesting date of each individual tranche. In each scenario, the Company recognizes share-based compensation expense based upon the probability that the award will ultimately vest. The Company recognizes the cumulative effect of changes in the probability outcomes in the period in which the changes occur.
F-17
Basic and Diluted Net Loss per Common Share
Basic net loss per common share is calculated as net loss available to common stockholders divided by weighted average common shares outstanding for the applicable period. Net loss available to common stockholders is calculated by adjusting net loss for periodic preferred accrued or deemed dividends. These amounts include (i) dividends accumulated on the Company’s Series B Convertible Preferred Stock during the period, (ii) periodic amortization of the beneficial conversion feature, and (iii) periodic accretion of the increasing-rate dividend feature.
This amount is divided by the weighted average common shares outstanding during the period. Weighted average common shares outstanding is calculated as shares of the Company outstanding adjusted for the portion of the period for which they are outstanding. Unvested restricted stock awards are excluded from the calculation of weighted average common shares outstanding until they have vested.
Diluted net loss per common share adjusts basic net loss per common share for convertible securities, options, restricted stock unit awards, and other share-based payment awards which have yet to vest, to the extent such adjustments reduce basic net loss per common share.
The Company uses the if-converted method to calculate the dilutive effect of the Series B Convertible Preferred Stock, and other convertible securities, to the extent they are outstanding. The if-converted method assumes that convertible securities are converted at the later of the issuance date or the beginning of the period. If the hypothetical conversion of convertible securities, and the consequential avoidance of any deemed or accumulated preferred dividends, would decrease basic net loss per common share, these effects are incorporated in the calculation of diluted net loss per common share, adjusted for the proportion of the period the securities were outstanding.
The Company uses the treasury stock method to calculate the dilutive effect of outstanding options, restricted stock awards, and other share-based payments. The treasury stock method assumes that the proceeds from exercise are used to repurchase common shares at the weighted average market price during the period, increasing the denominator for the net effect of shares issued upon exercise less hypothetical shares repurchased.
If the dilutive effects noted above would cause diluted net loss per common share to exceed basic net loss per common share, such effects are not incorporated into the calculation, as they are deemed antidilutive. For all periods with a net loss available to common stockholders, any adjustment for potential common shares would be naturally anti-dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted net loss per common share are the same for periods with a net loss.
Fair Value of Financial Instruments and Fair Value Measurements
The respective carrying value of certain on-balance sheet financial instruments approximated their fair values due to the short-term nature and type of these instruments. These financial instruments include cash and cash equivalents, accounts receivable, notes receivable, and certain other financial assets and liabilities.
The Company measures certain non-financial assets at fair value on a non-recurring basis. These non-recurring valuations include evaluating assets such as long-lived assets, and non-amortizing intangible assets for impairment, allocating value to assets in an acquired asset group, and accounting for business combinations. The Company uses the fair value measurement framework to value these assets and reports these fair values in the periods in which they are recorded or written down.
Fair value financial instruments are recorded in accordance with the fair value measurement framework. The fair value measurement framework includes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair values in their broad levels. These levels from highest to lowest priority are as follows:
•Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities;
•Level 2: Quoted prices in active markets for similar assets or liabilities or observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
•Level 3: Unobservable inputs or valuation techniques that are used when little or no market data is available.
The determination of fair value and the assessment of a measurement’s placement within the hierarchy require judgment. Level 3 valuations often involve a higher degree of judgment and complexity. Level 3 valuations may require the use of various valuation methodologies which incorporate unobservable inputs, management estimates, and assumptions. Management’s
F-18
assumptions could vary depending on the asset or liability valued and the valuation method used. Such assumptions could include: estimates of prices, earnings, costs, actions of market participants, market factors, or the weighting of various valuation methods. The Company may also engage external advisors to assist it in determining fair value, as appropriate.
Although the Company believes that the recorded fair value of its financial instruments is appropriate, these fair values may not be indicative of net realizable value or reflective of future fair values.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-06, “Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity,” which simplifies and clarifies certain calculation and presentation matters related to convertible equity and debt instruments. Specifically, this ASU simplifies the accounting for such instruments by removing requirements to separately account for conversion features as a derivative under ASC Topic 815 and removing the requirement to account for beneficial conversion features on such instruments. Accounting Standards Update 2020-06 also provides clearer guidance surrounding disclosure of such instruments and provides specific guidance for how such instruments are to be incorporated in the calculation of Diluted EPS. The guidance under ASU 2020-06 is effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020. The Company adopted this standard on January 1, 2021 on a modified retrospective basis. There was no impact upon adoption.
Recently Issued Accounting Pronouncements Not Yet Adopted
In March 2020, the FASB issued ASU 2020-04, “Reference Rate Reform (Topic 848)”, which provides temporary, optional expedients and exceptions to accounting guidance for certain contract modifications and hedging arrangements to ease financial reporting burdens as a result of market transitions from the London Interbank Offered Rate (“LIBOR”) to alternative reference rates. The guidance is available for prospective application upon its issuance and can generally be applied to contract modifications and hedging relationships entered into beginning March 12, 2020 through December 31, 2022. As of December 31, 2021, the Company has long-term debt outstanding which carries an interest rate tied to LIBOR, the agreement for which contemplates an interest rate alternative in the event that LIBOR is unavailable. The Company is evaluating the possibility of adoption and the related impact on its financial statements. If adopted, the Company does not expect the provisions of this ASU to have a material impact on its consolidated financial statements.
In November 2021, the FASB issued ASU 2021-10, “Government Assistance (Topic 832)”, which provides disclosure requirements regarding government grants and contributions. The ASU requires disclosure of the nature of transactions and related accounting policies used to account for transactions, the effect, including amounts, of government assistance on individual line items on the financial statements, and significant terms and conditions of the transactions, including commitments and contingencies. This ASU is effective for fiscal years beginning after December 15, 2021. The Company does not expect the provisions of this ASU to have a material impact on its consolidated financial statements.
All other ASUs issued and not yet effective as of December 31, 2021, and through the date of this report, were assessed and determined to be either not applicable or are expected to have minimal impact on the Company’s current or future financial position or results of operations.
3. Accounts Receivable, Net
Accounts receivable, net, consists of the following (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Accounts receivable, gross | $ | 41,540 | $ | 36,160 | |||||||
| Allowance for doubtful accounts | (1,187) | (737) | |||||||||
| Accounts receivable, net | $ | 40,353 | $ | 35,423 | |||||||
Bad debt expense for the years ended December 31, 2021, 2020, and 2019 was $0.8 million, $0.7 million, and $0, respectively.
F-19
4. Inventory
Inventory consists of the following (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Raw materials | $ | 364 | $ | 314 | |||||||
| Work in process | 6,112 | 4,316 | |||||||||
| Finished goods | 4,913 | 5,731 | |||||||||
| Inventory | $ | 11,389 | $ | 10,361 | |||||||
Write-downs recorded against the inventory balance as of December 31, 2020, which were presented separately in previously-issued financial statements, have been reclassified as reductions to raw materials, work in process, and finished goods.
As a result of the conclusion of the FDA’s period of enforcement discretion on May 31, 2021, the Company wrote down $1.0 million of its Section 351 product inventory and $0.7 million related to discontinued product during the year ended December 31, 2021.
Consignment inventory, included as a component of finished goods in the table above, was $2.6 million and $3.2 million as of December 31, 2021 and 2020, respectively.
5. Property and Equipment, Net
Property and equipment consist of the following (in thousands):
.
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Leasehold improvements | $ | 9,052 | $ | 6,010 | |||||||
| Laboratory and clean room equipment | 16,567 | 15,524 | |||||||||
| Furniture and office equipment | 14,975 | 15,295 | |||||||||
| Construction in progress | 397 | 3,321 | |||||||||
| Asset retirement cost | 863 | 785 | |||||||||
| Finance lease right of use assets | 189 | — | |||||||||
| Property and equipment, gross | 42,043 | 40,935 | |||||||||
| Less accumulated depreciation and amortization | (32,878) | (29,498) | |||||||||
| Property and equipment, net of accumulated depreciation | $ | 9,165 | $ | 11,437 | |||||||
Depreciation expense for each of the years ended December 31, 2021, 2020, and 2019 was recorded in certain captions of the consolidated statements of operations for those periods in the amounts shown in the table below (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Cost of sales | $ | 1,787 | $ | 2,022 | $ | 1,965 | |||||||||||
| Selling, general and administrative expenses | 2,278 | 3,416 | 4,223 | ||||||||||||||
| Research and development expenses | 298 | 344 | 358 | ||||||||||||||
| Total | $ | 4,363 | $ | 5,782 | $ | 6,546 | |||||||||||
F-20
6. Leases
The Company has leases for corporate offices, manufacturing facilities, vehicles, and certain equipment. Such leases do not require any contingent rental payments, impose any financial restrictions, or contain any residual value guarantees.
The Company subleases one of its leased industrial warehouse spaces. The sublease income from the facility offsets the lease expense associated with the facility. Sublease income for the facility was $0.1 million, $0.1 million, and $0 for the years ended December 31, 2021, 2020, and 2019, respectively, and is presented as a reduction to selling, general, and administrative expense on the consolidated statements of operations in those periods.
Supplemental balance sheet information related to the Company’s leases, including the financial statement caption in which the amounts are presented, is as follows (amounts in thousands, except lease term and discount rate):
| Operating Leases | Finance Leases | ||||||||||||||||||||||
| December 31, | December 31, | ||||||||||||||||||||||
| 2021 | 2020 | 2021 | 2020 | ||||||||||||||||||||
| Assets | |||||||||||||||||||||||
| $ | 4,696 | $ | 3,623 | $ | — | $ | — | ||||||||||||||||
| Property and equipment, net | — | — | 145 | — | |||||||||||||||||||
| Total assets | $ | 4,696 | $ | 3,623 | $ | 145 | $ | — | |||||||||||||||
| Liabilities | |||||||||||||||||||||||
| $ | 1,203 | $ | 1,176 | $ | 45 | $ | — | ||||||||||||||||
| 3,812 | 2,960 | 106 | — | ||||||||||||||||||||
| Total liabilities | $ | 5,015 | $ | 4,136 | $ | 151 | $ | — | |||||||||||||||
| Weighted-average remaining lease term (years) | 4.0 | 4.4 | 3.1 | ||||||||||||||||||||
| Weighted-average discount rate | 8.4 | % | 10.0 | % | 8.3 | % | |||||||||||||||||
Information related to lease costs are as follows (amounts in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Operating lease cost | $ | 1,327 | $ | 1,392 | $ | 1,469 | |||||||||||
| Depreciation of finance lease ROU assets | 43 | — | — | ||||||||||||||
| Interest expense on finance lease liabilities | 13 | — | — | ||||||||||||||
Maturities of lease liabilities are as follows (amounts in thousands):
| Year Ending December 31, | Operating Leases | Finance Leases | Total | ||||||||||||||
| 2022 | $ | 1,566 | $ | 55 | $ | 1,621 | |||||||||||
| 2023 | 1,605 | 55 | 1,660 | ||||||||||||||
| 2024 | 1,569 | 55 | 1,624 | ||||||||||||||
| 2025 | 441 | 5 | 446 | ||||||||||||||
| 2026 | 338 | — | 338 | ||||||||||||||
| Thereafter | 367 | — | 367 | ||||||||||||||
| Total lease payments | 5,886 | 170 | 6,056 | ||||||||||||||
| Less: imputed interest | (871) | (19) | (890) | ||||||||||||||
| Lease liability | $ | 5,015 | $ | 151 | $ | 5,166 | |||||||||||
Certain lease agreements require the Company to return designated areas of leased space to its original condition upon termination of the lease agreement, for which the Company records an asset retirement obligation and a corresponding capital
F-21
asset in an amount equal to the estimated fair value of the obligation. In subsequent periods, the asset retirement obligation is accreted for the change in its present value and the capitalized asset is depreciated, both over the term of the associated lease agreement. Asset retirement obligations of $1.0 million and $0.8 million as of December 31, 2021 and 2020, respectively, are included in other liabilities in the consolidated balance sheets.
7. Goodwill and Intangible Assets
Goodwill
For the impairment test performed October 1, 2021, the Company performed a qualitative assessment to determine the existence of impairment. The qualitative assessment concluded that it was not more likely than not that goodwill was impaired. The Company did not proceed to the quantitative assessment, and no impairment was recorded for the year ended December 31, 2021.
For the impairment tests performed on September 30, 2020 and October 1, 2020, the Company performed a quantitative analysis to determine the existence and extent of impairment. The quantitative analysis concluded that the fair value of the Company’s reporting unit exceeded its carrying value. As a result of these assessments, the Company concluded that there was no impairment. Accordingly, no impairment was recorded for the year ended December 31, 2020.
The following table indicates the changes in the carrying amount of goodwill for 2021 and 2020 (in thousands):
| Balance as of January 1, 2020 | $ | 19,976 | |||
| Activity | — | ||||
| Balance as of December 31, 2020 | 19,976 | ||||
| Activity | — | ||||
| Balance as of December 31, 2021 | $ | 19,976 | |||
Intangible Assets
Intangible assets are summarized as follows (in thousands):
| December 31, 2021 | December 31, 2020 | ||||||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | ||||||||||||||||||
| Amortized intangible assets | |||||||||||||||||||||||
| Patents and know-how | $ | 9,578 | $ | (6,408) | $ | 3,170 | $ | 9,510 | $ | (5,730) | $ | 3,780 | |||||||||||
| Licenses | — | — | — | 1,414 | (1,334) | 80 | |||||||||||||||||
| Customer and supplier relationships | — | — | — | 241 | (172) | 69 | |||||||||||||||||
| Non-compete agreements | — | — | — | 120 | (98) | 22 | |||||||||||||||||
| Total amortized intangible assets | $ | 9,578 | $ | (6,408) | $ | 3,170 | $ | 11,285 | $ | (7,334) | $ | 3,951 | |||||||||||
| Unamortized intangible assets | |||||||||||||||||||||||
| Tradenames and trademarks | $ | 1,008 | $ | 1,008 | $ | 1,008 | $ | 1,008 | |||||||||||||||
| Patents in process | 1,205 | 1,205 | 1,045 | 1,045 | |||||||||||||||||||
| Total intangible assets | $ | 11,791 | $ | 5,383 | $ | 13,338 | $ | 6,004 | |||||||||||||||
Amortization expense and impairment expense for the years ended December 31, 2021, 2020, and 2019, is summarized in the table below (amounts in thousands):
F-22
| Year ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Amortization of intangible assets | $ | 820 | $ | 1,073 | $ | 1,039 | |||||||||||
| Impairment of intangible assets | 53 | 1,027 | 1,258 | ||||||||||||||
Impairment of intangible assets in 2021 related to supplier relationship assets that were determined to be unrecoverable due to attrition. Impairment of intangible assets in 2020 related to customer relationship assets that were determined to be unrecoverable due to lower than expected margins. Impairment of intangible assets in 2019 were related to the abandonment of patents in process and customer relationship assets.
Expected future amortization of intangible assets as of December 31, 2021, is as follows (in thousands):
| Estimated | |||||
| Amortization | |||||
| Year Ending December 31, | Expense | ||||
| 2022 | $ | 679 | |||
| 2023 | 679 | ||||
| 2024 | 679 | ||||
| 2025 | 284 | ||||
| 2026 | 129 | ||||
| Thereafter | 720 | ||||
| Total amortization expense | $ | 3,170 | |||
8. Accrued Expenses
Accrued expenses consist of the following (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Legal and settlement costs | $ | 2,806 | $ | 24,797 | |||||||
| External commissions | 2,630 | 2,141 | |||||||||
| Estimated returns | 788 | 688 | |||||||||
| Accrued clinical trials | 694 | 651 | |||||||||
| Accrued rebates | 1,343 | 886 | |||||||||
| Accrued GPO fees | 559 | 554 | |||||||||
| Other | 992 | 743 | |||||||||
| Total | $ | 9,812 | $ | 30,460 | |||||||
The Company’s accrual for settlement costs, which was presented separately in previously-issued financial statements, is included as part of legal and settlement costs in the table above. Accrued settlement costs were $10.0 million as of December 31, 2020.
9. Long Term Debt
Hayfin Loan Agreement
On June 30, 2020, the Company entered into a Loan Agreement with, among others, Hayfin Services, LLP, (“Hayfin”) an affiliate of Hayfin Capital Management LLP (the “Hayfin Loan Agreement”), which was funded on July 2, 2020 (the “Closing Date”) and provided the Company with a senior secured term loan in an aggregate amount of $50.0 million (the “Term Loan”). The Term Loan matures on June 30, 2025 (the “Maturity Date”). Interest is payable quarterly on the Term Loan for the principal balance outstanding through the Maturity Date. No principal payments are due and payable until the Maturity Date.
The Hayfin Loan Agreement also provided the Company with an option to draw on an additional delayed draw term loan (the “DD TL”, collectively with the Term Loan, the “Credit Facilities”) in the form of a committed but undrawn $25.0 million
F-23
facility until June 30, 2021. The Company did not exercise the option. On February 28, 2022 (the “Amendment Date”), the Company executed an Amendment to the Hayfin Loan Agreement (the “Amendment”).
The interest rate applicable to the Term Loan is equal to LIBOR (subject to a floor of 1.5%) plus a margin (the “Margin”), as determined below. If LIBOR is unavailable, the loan will carry interest at the greatest of the Prime Rate, the Federal Funds Rate plus 0.5% per annum, and 2.5%, plus the Margin.
Prior to the Amendment Date, the Margin on the Term Loan was calculated based on the Company’s Total Net Leverage Ratio (as defined in the Hayfin Loan Agreement) for the quarter, as follows:
•6.75% per annum if the Total Net Leverage Ratio is greater than 2.0x,
•6.5% per annum if the Total Net Leverage Ratio is less than 2.0x but greater than or equal to 1.0x, or
•6.0% per annum if the Total Net Leverage Ratio is less than 1.0x.
After the Amendment Date, the Margin is fixed at 6.75% through the Maturity Date.
An additional 3.0% margin is applied to the interest rate in the event of default as defined by the Hayfin Loan Agreement. Both at issuance and as of December 31, 2021, the Term Loan carried an interest rate of 8.3%.
The Term Loan contained financial covenants requiring the Company, on a consolidated basis, to maintain the following through the Amendment Date:
•Maximum Total Net Leverage Ratio of 4.0x for the remaining life of the loan, required to be calculated on a quarterly basis,
•Minimum Liquidity (as defined in the Hayfin Loan Agreement) of $10 million, an at-all-times financial covenant, tested monthly.
The Company is in compliance with all debt covenants as of December 31, 2021.
The Amendment changed these financial covenants and requires the Company, on a consolidated basis, to maintain the following beginning on the Amendment Date:
•Minimum Consolidated Total Net Sales (as defined in the Amendment) of varying amounts, required to be calculated on a quarterly basis,
•Minimum Liquidity of $20 million, an at-all-times financial covenant, tested monthly.
The Hayfin Loan Agreement, as amended, also specifies that any prepayment of the loan, voluntary or mandatory, will subject the Company to a prepayment premium applicable as of the date of the prepayment:
•On or before July 2, 2023: 2% of the principal balance repaid.
•After July 2, 2023, but on or before July 2, 2024: 1% of the principal balance repaid.
•After July 2, 2024: no premium.
The Hayfin Loan Agreement also includes events of default customary for facilities of this type, and upon the occurrence of such events of default, subject to customary cure rights, the Term Loan may be accelerated or the lenders’ commitments terminated. The mandatory prepayments are also required in the event of a change in control (as defined in the Hayfin Loan Agreement), incurring other indebtedness, certain proceeds from disposal of assets and an insured casualty event.
Beginning with the fiscal year ending December 31, 2021, the Company is required to prepay the outstanding loans based on the percentage of Excess Cash Flow (as defined in the Hayfin Loan Agreement), if Excess Cash Flow is generated, with the percentage determined based on the Total Net Leverage thresholds. The Company is not required to make any payments under this provision as of December 31, 2021.
Hayfin maintains a first-priority security interest in substantially all of the Company’s assets.
F-24
A breach of a financial covenant in the Hayfin Loan Agreement, if uncured or unable to be cured, would likely result in an event of default that could trigger the lender’s remedies, including acceleration of the entire principal balance of the loan as well as any applicable prepayment premiums. Future compliance with the financial covenants, as amended, requires continuing growth in net sales consistent with the Company’s business strategy and plans. Our business is subject to inherent uncertainties that could impact the Company’s net sales growth, including, but not limited to, the regulatory pathway of the Company’s cord-derived products. While we currently have sufficient cash to repay all such amounts in an event of default, we may require alternative financing to cover other obligations. Even if alternative financing were available in an event of default under the Hayfin Loan Agreement, it might be on unfavorable terms, and the interest rate charged on any new borrowings may be substantially higher than the interest rate under the Hayfin Loan Agreement, thus adversely affecting our future cash flows, liquidity, and results of operations.
Original issue discount and deferred financing costs were allocated between the sale of the Series B Convertible Preferred Stock (which occurred simultaneously with the Hayfin Term Loan, collectively the “Financing Transactions”) and the Term Loan on the basis of the relative fair values of the transactions. The costs allocated to the Term Loan were further allocated between the Term Loan and the DD TL on the basis of the maximum potential principal outstanding between the Credit Facilities. The allocation of the deferred financing costs and original issue discount between Term Loan and the DD TL on July 2, 2020 was as follows (amounts in thousands):
| July 2, 2020 | |||||||||||||||||
| Term Loan | DD TL | Total | |||||||||||||||
| Long term debt | Other current assets | ||||||||||||||||
| Original issue discount | $ | 333 | $ | 167 | $ | 500 | |||||||||||
| Deferred financing costs | 2,169 | 1,084 | 3,253 | ||||||||||||||
Deferred financing costs and original issue discount allocated to the Term Loan are amortized using the effective interest method through the Maturity Date. The amortization of such amounts is presented as part of interest expense, net on the consolidated statement of operations for the years ended December 31, 2021 and 2020 .
Deferred financing costs and original issue discount associated with the DD TL were amortized using the straight-line method through the expiration of the DD TL commitment term on June 30, 2021. Amortization of these amounts are presented as part of interest expense, net on the consolidated statements of operations. Unamortized deferred financing costs and original issue discount associated with the DD TL are presented as other current assets on the consolidated balance sheet as of December 31, 2020.
The balances of the Term Loan as of December 31, 2021 and 2020 were as follows (amounts in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Outstanding principal | $ | 50,000 | $ | 50,000 | |||||||
| Deferred financing costs | (1,624) | (1,996) | |||||||||
| Original issue discount | (249) | (307) | |||||||||
| Long term debt, net | $ | 48,127 | $ | 47,697 | |||||||
Components of interest expense related to the Term Loan, included in interest expense, net in the consolidated statements of operations, was as follows (amounts in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Stated interest | $ | 4,182 | $ | 2,085 | |||||||
| Amortization of deferred financing costs | 372 | 173 | |||||||||
| Accretion of original issue discount | 58 | 26 | |||||||||
| Interest expense | $ | 4,612 | $ | 2,284 | |||||||
F-25
Interest expense related to the DD TL, included in interest expense, net in the consolidated statements of operations, was as follows (amounts in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Commitment fee | $ | 126 | $ | 128 | |||||||
| Amortization of deferred financing costs | 542 | 542 | |||||||||
| Accretion of original issue discount | 83 | 83 | |||||||||
| Interest expense | $ | 751 | $ | 753 | |||||||
Scheduled principal payments on the Term Loan as of December 31, 2021 are as follows:
| Year ending December 31, | Principal | |||||||
| 2022 | $ | — | ||||||
| 2023 | — | |||||||
| 2024 | — | |||||||
| 2025 | 50,000 | |||||||
| 2026 | — | |||||||
| Thereafter | — | |||||||
| Outstanding principal | $ | 50,000 | ||||||
The DD TL was not funded as of December 31, 2021. Consequently, no principal payments are owed.
As of December 31, 2021, the fair value of the Term Loan was $50.7 million. This valuation was calculated based on a series of Level 2 and Level 3 inputs, including a discount rate based on the credit risk spread of debt instruments of similar risk character in reference to U.S. Treasury instruments with similar maturities, with an incremental risk premium for risk factors specific to the Company. The remaining cash flows associated with the Term Loan were discounted to December 31, 2021 using this discount rate to derive the fair value.
BT Term Loan
On June 10, 2019, the Company entered into a loan agreement (the “BT Loan Agreement”) with Blue Torch Finance LLC (“Blue Torch”), as administrative agent and collateral agent, to borrow funds with a face value of $75.0 million (the “BT Term Loan”), of which the full amount was borrowed and funded. The proceeds from the BT Term Loan were used (i) for working capital and general corporate purposes and (ii) to pay transaction fees, costs and expenses incurred in connection with the BT Term Loan and the related transactions. The BT Term Loan would have matured on June 20, 2022 and was repayable in quarterly installments of $0.9 million, with the balance due on June 20, 2022. Blue Torch maintained a first-priority security interest in substantially all the Company’s assets. The BT Term Loan was issued net of the original issue discount of $2.3 million. The Company incurred $6.7 million of deferred financing costs.
On April 22, 2020, the Company amended the BT Loan Agreement with Blue Torch. The amendment provided for an increase in the maximum Total Leverage Ratio, which was a quarterly test, for the remainder of 2020, and also provided for a reduction in the minimum Liquidity requirement from April 2020 through November 2020. In connection with the amendment, the Company agreed to pay a one-time fee of approximately $0.7 million, added to the principal balance, and a 1 percentage point increase in the interest rate to LIBOR plus 9%.
On July 2, 2020, a portion of the proceeds from the Financing Transactions was used to repay the outstanding balance of principal, accrued but unpaid interest, and prepayment premium under the BT Loan Agreement. In connection with the repayment of the BT Term Loan, the Company terminated the BT Loan Agreement. The Company has no continuing obligations related to the BT Term Loan as of December 31, 2021.
The Company recorded a loss on extinguishment of debt of $8.2 million during the year ended December 31, 2020. The composition of the loss on extinguishment of debt was as follows (amounts in thousands):
F-26
| July 2, 2020 | |||||
| Unamortized deferred financing costs | $ | 4,528 | |||
| Unamortized original issue discount | 1,538 | ||||
| Unamortized amendment fee | 671 | ||||
| Prepayment premium | 1,439 | ||||
| Other fees | 25 | ||||
| Loss on extinguishment of debt | $ | 8,201 | |||
Interest expense related to the BT Term Loan, included in interest (expense) income, net in the consolidated statements of operations was as follows (amounts in thousands):
| Year ended December 31, | |||||||||||
| 2020 | 2019 | ||||||||||
| Interest on principal balance | $ | 3,773 | $ | 4,331 | |||||||
| Accretion of original issue discount | 354 | 360 | |||||||||
| Accretion of amendment fee | 53 | — | |||||||||
| Amortization of deferred financing costs | 1,051 | 1,071 | |||||||||
| Total BT Term Loan interest expense | $ | 5,231 | $ | 5,762 | |||||||
Paycheck Protection Program Loan
The Company applied for and, on April 24, 2020, received proceeds of $10.0 million in the form of a loan under the Paycheck Protection Program (the “PPP Loan”). On May 11, 2020, the Company repaid the PPP Loan in full. There are no continuing obligations under the PPP Loan as of December 31, 2021.
10. Basic and Diluted Net Loss Per Common Share
Net loss per common share is calculated using two methods: basic and diluted.
Basic Net Loss Per Common Share
The following table provides a reconciliation of net loss to net loss available to common shareholders and calculation of basic net loss per common share for each of the years ended December 31, 2021, 2020, and 2019 (amounts in thousands, except share and per-share amounts):
| Year ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net loss | $ | (10,285) | $ | (49,284) | $ | (25,580) | |||||||||||
| Adjustments to reconcile to net loss available to common stockholders: | |||||||||||||||||
| Accumulated dividend on convertible preferred stock Series B | 5,210 | 2,016 | — | ||||||||||||||
| Amortization of beneficial conversion feature | — | 31,110 | — | ||||||||||||||
| Accretion of increasing-rate dividend feature | 926 | 918 | — | ||||||||||||||
| Total adjustments | 6,136 | 34,044 | — | ||||||||||||||
| Net loss available to common stockholders | $ | (16,421) | $ | (83,328) | $ | (25,580) | |||||||||||
| Weighted average common shares outstanding | 110,353,406 | 108,257,112 | 106,946,384 | ||||||||||||||
| Basic net loss per common share | $ | (0.15) | $ | (0.77) | $ | (0.24) | |||||||||||
Diluted Net Loss Per Common Share
The following table sets forth the computation of basic and diluted net loss per common share (in thousands, except share and per-share amounts):
F-27
| Year ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net loss available to common stockholders | $ | (16,421) | $ | (83,328) | $ | (25,580) | |||||||||||
| Dividends on convertible preferred stock Series B | 6,136 | 34,044 | — | ||||||||||||||
| Numerator - net loss available to common stockholders adjusted for hypothetical conversion of Series B Convertible Preferred Stock (a) | $ | (16,421) | $ | (83,328) | $ | (25,580) | |||||||||||
| Denominator - weighted average common shares outstanding adjusted for potential common shares (b) | 110,353,406 | 108,257,112 | 106,946,384 | ||||||||||||||
| Diluted net loss per common share | $ | (0.15) | $ | (0.77) | $ | (0.24) | |||||||||||
(a)Diluted net loss per common share is not adjusted for dividends on the Series B convertible preferred stock in 2021 or 2020 because the effect of a hypothetical conversion was determined to be anti-dilutive.
(b)Weighted average common shares outstanding for the calculation of diluted net loss per common share does not include the following adjustments for potential common shares below because their effects were determined to be anti-dilutive for the periods presented:
| Year ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Convertible preferred stock Series B | 26,497,570 | 12,987,013 | — | ||||||||||||||
| Restricted stock awards | 1,121,019 | 1,299,770 | 1,157,563 | ||||||||||||||
| Outstanding stock options | 771,409 | 752,499 | 978,243 | ||||||||||||||
| Restricted stock unit awards | 1,393,910 | 616,141 | — | ||||||||||||||
| Performance stock unit awards | 17,928 | 31,621 | — | ||||||||||||||
| Potential common shares | 29,801,836 | 15,687,044 | 2,135,806 | ||||||||||||||
11. Equity
Convertible Preferred Stock Series B
The Company’s Convertible preferred stock Series B (the “Series B Preferred Stock”) are convertible, cumulative securities which rank senior to the Company’s Series A Junior Participating Preferred Stock and the Company’s common stock. The Series B Preferred Stock accumulated a 4.0% cumulative dividend per annum through June 30, 2021, and accumulates a 6.0% cumulative dividend per annum thereafter. Dividends are declared at the sole discretion of the Board. Dividends are paid at the end of each quarter based on dividend amounts that accumulate beginning on the last payment date through the day prior to the end of each quarter. In lieu of paying a dividend, the Company may elect to accrue the dividend owed to holders of the Series B Preferred Stock. Accrued dividend balances accumulate dividends at the prevailing dividend rate for each dividend period for which they are outstanding.
Each share of Series B Preferred Stock is convertible into Company’s common stock at any time at the option of the Holder. Shares are converted based on the liquidation preference of $1,000 per share (the “Liquidation Preference”) plus any accrued or accumulated dividends through the date of the conversion at a conversion price of $3.85 per common share. The Series B Preferred Stock, including any accumulated and unpaid dividends, automatically converts into common stock at any time after July 2, 2023, provided that the common stock has traded at $7.70 per common share or more (i) for 20 out of 30 consecutive trading days and (ii) on such date of conversion.
The holders of the Series B Preferred Stock, voting as a class, are entitled to appoint two members to the board of directors. The Holders vote are entitled to vote on all matters on an as-converted basis as a single class with the common stock assuming a conversion price of $5.25 per share; provided that the votes represented by the Series B Preferred Stock cannot exceed 19.9% of the total voting stock of the Company.
Holders of the Series B Preferred Stock are also entitled to the Liquidation Preference and all accumulated and unpaid dividends in the event of a liquidation, dissolution, or winding-up of the Company.
If the Company undergoes a change of control (as defined), the Company will have the option to repurchase some or all of the then-outstanding shares of Series B Preferred Stock for cash in an amount equal to the liquidation preference plus any accumulated and unpaid dividends, subject to the rights of the holders in connection with such change in control. If the
F-28
Company does not exercise such repurchase right, the Holders will have the option to (1) require the Company to repurchase any or all of its then-outstanding shares of Series B Preferred Stock for cash in an amount equal to the liquidation preference or (2) convert the Series B Preferred Stock, including accumulated and unpaid dividends into common stock and receive their pro rata consideration thereunder.
The Company evaluated its Series B Preferred Stock and determined that it was considered an equity host under ASC 815, Derivatives and Hedging. As a result of the Company’s conclusion that the Series B Preferred Stock represented an equity host, the conversion feature of all Series B Preferred Stock was considered to be clearly and closely related to the associated Series B Preferred Stock host instrument. Accordingly, the conversion feature of all Series B Preferred Stock was not considered an embedded derivative that required bifurcation. At the time of the issuance of the Series B Preferred Stock, the Company’s common stock, into which the Company’s Series B Preferred Stock is convertible, had an estimated fair value exceeding the effective conversion price of the Series B Preferred Stock, giving rise to a beneficial conversion feature in the amount of $31.1 million. This amount was immediately recognized as a deemed dividend on the commitment date since there is no stated redemption date and the Series B Preferred Stock is immediately convertible.
The Series B Preferred Stock instrument contains an increasing-rate cumulative dividend feature. The Company determined the present value of the difference between the (1) dividends that will be payable in the period preceding commencement of the perpetual dividend and (2) the perpetual dividend amount for a corresponding number of periods in order to ascribe a fair value to this feature. These amounts were discounted to present value using a market rate for dividend yield as of the date on which the Series B Preferred Stock was issued. The Company calculated the amount of the increasing-rate dividend feature as $1.8 million. This amount is amortized as a deemed dividend to preferred shareholders using the effective interest method through June 30, 2021. During each of the years ended December 31, 2021 and 2020, the Company recognized $0.9 million of deemed dividends related to the amortization of the increasing-rate dividend feature.
The below table illustrates changes in the Company’s balance of the Series B Preferred Stock for the years ended December 31, 2021 and 2020 (in thousands, except per share amounts):
| Convertible preferred stock Series B | |||||||||||
| Shares | Amount | ||||||||||
Balance at December 31, 2019 | — | $ | — | ||||||||
| Issuance of Series B Preferred Stock | 100,000 | 59,540 | |||||||||
| Deemed dividends | — | 32,028 | |||||||||
Balance at December 31, 2020 | 100,000 | $ | 91,568 | ||||||||
| Deemed dividends | — | 926 | |||||||||
Balance at December 31, 2021 | 100,000 | $ | 92,494 | ||||||||
The Company has not declared or paid any dividends on the Series B Preferred Stock since issuance. Dividends in arrears as of December 31, 2021 were $7.2 million. As this amount has not been declared, the Company has not recorded this amount on its consolidated balance sheet as of December 31, 2021.
As of December 31, 2021, based on accumulated dividends as of that date, the Series B Preferred Stock was convertible into an aggregate of 27,850,916 shares of the Company’s common stock.
Stock Incentive Plans
The Company has two share-based compensation plans which provide for the granting of equity awards, including qualified incentive and non-qualified stock options and restricted stock awards: the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan Amended and Restated through October 2, 2020 (the “2016 Plan”), which was approved by shareholders on May 18, 2016, and the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (the “Prior Incentive Plan”). During the years ended December 31, 2021, 2020, and 2019 the Company used only the 2016 Plan to make grants.
The 2016 Plan permits the grant of equity awards to the Company’s employees, directors, consultants and advisors for up to 8,400,000 shares of the Company’s common stock plus (i) the number of shares of the Company’s common stock that remain available for issuance under the Prior Incentive Plan, and (ii) the number of shares that are represented by outstanding awards that later become available because of the expiration or forfeiture of the award without the issuance of the underlying shares. The awards are subject to a vesting schedule as set forth in each individual agreement.
F-29
Stock Options
Option awards are generally granted with an exercise price equal to the market price of the Company’s stock at the date of grant. Option awards generally vest based on three years of continuous service and have 10-year contractual terms. Certain option and restricted stock awards provide for accelerated vesting if there is a change in control or upon death or disability.
A summary of stock option activity for the year ended December 31, 2021, and changes during the year then ended are presented below:
| Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | ||||||||||||||||||||
| Outstanding at January 1, 2021 | 2,025,683 | $ | 4.62 | ||||||||||||||||||||
| Granted | — | — | |||||||||||||||||||||
| Exercised | (529,171) | 3.43 | |||||||||||||||||||||
| Unvested options forfeited | — | — | |||||||||||||||||||||
| Vested options expired | (51,667) | 1.23 | |||||||||||||||||||||
| Outstanding at December 31, 2021 | 1,444,845 | 5.18 | 1.51 | 1,960,006 | |||||||||||||||||||
| Exercisable at December 31, 2021 | 1,444,845 | $ | 5.18 | 1.51 | $ | 1,960,006 | |||||||||||||||||
The intrinsic values of the options exercised during the years ended December 31, 2021, 2020 and 2019 were $3.3 million, $1.9 million, and $0.6 million, respectively. Cash received from option exercise under all share-based payment arrangements for the years ended December 31, 2021, 2020 and 2019 was $1.4 million, $0.4 million, and $0.1 million, respectively. The actual tax benefit for the tax deductions from option exercise of the share-based payment arrangements totaled $2.0 million, $1.6 million, and $0.2 million, respectively, for the years ended December 31, 2021, 2020 and 2019. The Company has a policy of using its available repurchased treasury stock to satisfy option exercises.
The fair value of options vested during the years ended December 31, 2021, 2020 and 2019 were $0, $0, and $1.4 million, respectively. There were no options granted during the years ended December 31, 2021, 2020 and 2019 and there was no unrecognized compensation expense at December 31, 2021.
On June 13, 2019, our Board of Directors (prior to the election or appointment of any of the Company’s current non-executive Board members), in its capacity as Administrator of the Prior Incentive Plan, extended the contractual life of 612,000 fully vested share options held by 7 members of the Board and 278,916 fully vested share options held by a former employee. As a result of that modification, the Company recognized incremental share-based compensation expense of $0.4 million during the year ended December 31, 2019.
The incremental fair value of the modified options was estimated on the modification date using the Black-Scholes option-pricing model that uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. Expected volatilities were the blend of the Company’s historical stock price volatility as well as that of market comparable publicly traded peer companies and other factors estimated over the expected term of the options. The term of the modified options was the remaining time until the end of the contractual maturity of ten years. The risk-free rate was based on the U.S. Treasury yield curve in effect at the time of modification for the period of the expected term.
| 2019 Option Modification | |||||
| Expected volatility | 65% - 95% | ||||
| Expected life (in years) | 0.28 - 5.12 | ||||
| Expected dividend yield | 0 | ||||
| Risk-free interest rate | 1.56% - 2.02% | ||||
Restricted Stock Awards
F-30
The Company has issued several classes of restricted stock awards to employees: restricted stock (“RSAs”), restricted stock unit awards (“RSUs”), and performance stock unit awards (“PSUs”). The following is summary information for restricted stock awards for the year ended December 31, 2021. Restricted stock and RSUs vest over a - to three-year period in equal annual increments and require continuous service. Performance stock unit awards vest based on the achievement of specific performance targets subject to agreements with employees and require continuous service through the specified event.
As of December 31, 2021, there was approximately $23.9 million of total unrecognized stock-based compensation related to unvested restricted stock awards. That expense is expected to be recognized over a weighted-average period of 2.01 years, which approximates the remaining vesting period of these grants. All RSAs noted below as unvested are considered issued and outstanding at December 31, 2021, while unvested RSUs and PSUs are not considered issued and outstanding as of December 31, 2021.
| RSA | RSU | PSU | ||||||||||||||||||||||||
| Number of Shares | Weighted-Average Grant Date Fair Value | Number of Shares | Weighted-Average Grant Date Fair Value | Number of Shares | Weighted-Average Grant Date Fair Value | |||||||||||||||||||||
| Unvested at January 1, 2021 | 2,175,859 | $ | 4.78 | 2,325,273 | $ | 5.90 | 35,212 | $ | 7.10 | |||||||||||||||||
| Granted | — | — | 3,020,935 | 10.07 | — | — | ||||||||||||||||||||
| Vested | (1,225,606) | 5.24 | (775,193) | 5.89 | (35,212) | 7.10 | ||||||||||||||||||||
| Forfeited | (73,056) | 3.34 | (342,096) | 8.84 | — | — | ||||||||||||||||||||
| Unvested at December 31, 2021 | 877,197 | $ | 4.26 | 4,228,919 | $ | 8.64 | — | $ | — | |||||||||||||||||
The total fair value of restricted stock awards vested during the years ended December 31, 2021, 2020 and 2019, was $20.1 million, $10.1 million, and $5.2 million, respectively.
During the year ended December 31, 2019, the Company granted a fixed-dollar value RSU award to the members of its Board in the amount of $1.6 million. The RSU awards vested at the date of the 2019 Annual Meeting and were settled in common stock with the number of shares of common stock based on the closing price of the Company’s share price on August 5, 2020, a date thirty days after the Company became current on its SEC filings. Upon this event, these awards were modified from a fixed dollar-amount of awards to be settled in a variable number of shares to a fixed number of shares based on the closing price of the Company’s common stock on August 5, 2020. This event constituted a modification of the awards from liability-based awards to equity-based awards. This event did not change the total amount of expense recognized. Prior to August 5, 2020, the Company recorded $1.3 million of expense, of which $0.9 million and $0.4 million were recognized during the years ended December 31, 2020 and 2019, respectively. The Company reclassified $1.3 million of recorded liability to additional paid-in capital to reflect this modification on August 5, 2020. Subsequent to the modification, $0.3 million of expense was recognized as additional paid-in capital during the year ended December 31, 2020.
For the years ended December 31, 2021, 2020, and 2019 the Company recognized share-based compensation as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Cost of sales | $ | 813 | $ | 520 | $ | 477 | |||||||||||
| Research and development | 836 | 288 | 265 | ||||||||||||||
| Selling, general and administrative | 13,108 | 14,549 | 11,322 | ||||||||||||||
| Total share-based compensation | 14,757 | 15,357 | 12,064 | ||||||||||||||
| Income tax benefit, before consideration of valuation allowance | (3,649) | (3,792) | (3,081) | ||||||||||||||
| Total share-based compensation, net of tax benefit | $ | 11,108 | $ | 11,565 | $ | 8,983 | |||||||||||
Treasury Stock
Repurchases of shares of Common Stock in connection with the satisfaction of employee tax withholding obligations upon vesting of restricted stock and exercise of stock options for the years ended December 31, 2021, 2020, and 2019 were 469,239, 435,492, and 429,918, respectively, for an aggregate purchase price of $4.8 million, $2.3 million, and $1.5 million, respectively.
F-31
During 2020 and 2021, certain stock option holders elected to return restricted shares to the Company as consideration to exercise stock options. In total, 41,810 and 148,972 shares were returned to the Company during the year ended December 31, 2021 and 2020, respectively, for an aggregate fair value of $0.4 million and $0.9 million, respectively.
12. Income Taxes
On March 27, 2020, the U.S. government enacted the CARES Act which, among other changes, eliminated the taxable income limit for certain net operating losses (“NOL”), allowed businesses to carry back NOLs arising in 2018, 2019, and 2020 to the five prior years, and provided a payment delay of employer payroll taxes during 2020 after the date of enactment. These provisions allowed the Company to carry back federal tax losses related to 2018 and 2019. The Company recorded net tax receivable totaling $11.3 million in 2020 related to these provisions, of which $1.2 million had been collected as of December 31, 2020, and another $9.2 million was collected during the year ended December 31, 2021. The remaining $0.9 million is reflected in income tax receivable on the consolidated balance sheet as of December 31, 2021.
The Company has deferred payment on $2.2 million in employer taxes, $1.1 million of which was paid in January 2022 and the remainder is due December 2022. The $2.2 million is included as part of accrued compensation on the consolidated balance sheet as of December 31, 2021.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
Significant components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Deferred Tax Assets: | |||||||||||
| Net operating loss | $ | 23,333 | $ | 17,010 | |||||||
| Research and development and other tax credits | 6,297 | 5,920 | |||||||||
| Share-based compensation | 4,220 | 3,259 | |||||||||
| Interest limitation carryforward | 3,970 | 2,992 | |||||||||
| Accrued expenses | 3,385 | 2,918 | |||||||||
| Accrued settlement costs | 235 | 2,464 | |||||||||
| Bad debts | 601 | 2,138 | |||||||||
| Lease obligation | 1,277 | 1,021 | |||||||||
| Sales return and allowances | 195 | 170 | |||||||||
| Other | 1,115 | 1,075 | |||||||||
| Deferred Tax Liabilities: | |||||||||||
| Prepaid expenses | (1,337) | (1,170) | |||||||||
| Property and equipment | (705) | (1,073) | |||||||||
| Right of use asset | (1,197) | (895) | |||||||||
| Intangible assets | (263) | (160) | |||||||||
| Deferred costs of goods sold | — | (43) | |||||||||
| Net Deferred Tax Assets | 41,126 | 35,626 | |||||||||
| Less: Valuation allowance | (41,126) | (35,626) | |||||||||
| Net Deferred Tax Assets after Valuation Allowance | $ | — | $ | — | |||||||
F-32
The reconciliation of the federal statutory income tax rate of 21% to the effective rate is as follows:
| Year ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Federal statutory rate | 21.00 | % | 21.00 | % | 21.00 | % | |||||||||||
| State taxes, net of federal benefit | 4.53 | % | (0.20) | % | (1.36) | % | |||||||||||
| Nondeductible compensation | (13.77) | % | (0.89) | % | (1.49) | % | |||||||||||
| Meals and entertainment | (1.13) | % | (0.50) | % | (2.04) | % | |||||||||||
| Share-based compensation | 23.31 | % | (1.24) | % | (5.05) | % | |||||||||||
| Employee retention credit | 3.37 | % | — | % | — | % | |||||||||||
| Tax credits | 2.01 | % | 0.32 | % | 0.45 | % | |||||||||||
| Uncertain tax positions | 0.02 | % | 0.24 | % | 1.22 | % | |||||||||||
| NOL carryback rate differential | — | % | 10.99 | % | — | % | |||||||||||
| Other | 10.90 | % | (1.66) | % | 0.12 | % | |||||||||||
| Valuation allowance | (52.70) | % | (8.14) | % | (12.83) | % | |||||||||||
| Effective tax rate | (2.46) | % | 19.92 | % | 0.02 | % | |||||||||||
The tax benefit associated with the change in the valuation allowance had a significant impact on the Company’s effective tax rate for the year ended December 31, 2021. Additionally, the effective tax rate was affected by other permanent differences, such as share based compensation, executive compensation limitations and employee retention credit benefit.
The tax benefit associated with the carryback of federal net operating losses under the CARES Act had a significant impact on the Company’s effective tax rate for the year ended December 31, 2020. Additionally, the effective tax rate was affected by other permanent differences, as well as the change in the valuation allowance.
Current and deferred income tax (benefit) expense is as follows (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Current: | |||||||||||||||||
| Federal | $ | 91 | $ | (12,418) | $ | (53) | |||||||||||
| State | 156 | 159 | 48 | ||||||||||||||
| Total current | 247 | (12,259) | (5) | ||||||||||||||
| Deferred: | |||||||||||||||||
| Federal | — | — | — | ||||||||||||||
| State | — | — | — | ||||||||||||||
| Total deferred | — | — | — | ||||||||||||||
| Total expense (benefit) | $ | 247 | $ | (12,259) | $ | (5) | |||||||||||
Certain items of income and expense are not reported in tax returns and financial statements in the same year. The tax effect of such temporary differences is reported as deferred income taxes. The measurement of deferred tax assets is reduced, if necessary, by the amount of any tax benefit that, based on available evidence, is not expected to be realized. The Company establishes a valuation allowance for deferred tax assets for which realization is not likely. As of each reporting date, management considers new evidence, both positive and negative, that could affect its view of the future realization of deferred tax assets.
A valuation allowance of $41.1 million and $35.6 million was recorded against the deferred tax asset balance as of December 31, 2021 and 2020, respectively. The Company maintains a full valuation allowance because it is not more likely than not the
F-33
deferred tax assets will be utilized based on all available positive and negative evidence. In the event that the weight of the evidence changes in the future, any reduction in the valuation allowance would result in an income tax benefit.
At December 31, 2021 and 2020, the Company had income tax net operating loss (“NOL”) carryforwards for federal and state purposes of $84.2 million and $104.2 million and $62.7 million and $68.5 million, respectively. A portion of the Company’s NOLs and tax credits are subject to annual limitations due to ownership change limitations provided by Internal Revenue Code Section 382. If not utilized, the federal and state tax NOL carryforwards will expire between 2027 and 2037. As of December 31, 2021, the Company has recorded a deferred tax asset for both federal and state NOL carryforwards of approximately $17.7 million and $5.6 million, respectively. As of December 31, 2020, the Company has recorded a deferred tax asset for federal and state NOL carryforwards of $13.2 million and approximately $4.0 million, respectively.
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits (in thousands) included in other liabilities in the consolidated balance sheets:
| 2021 | 2020 | 2019 | |||||||||||||||
| Unrecognized tax benefits - January 1 | $ | 477 | $ | 627 | $ | 938 | |||||||||||
| Gross increases - tax positions in current period | 20 | — | 56 | ||||||||||||||
| Decreases in prior year positions | (28) | (150) | (367) | ||||||||||||||
| Unrecognized tax benefits - December 31 | $ | 469 | $ | 477 | $ | 627 | |||||||||||
Included in the balance of unrecognized tax benefits as of both December 31, 2021 and 2020 were $0.5 million of tax benefits that, if recognized, would affect the effective tax rate.
The Company recognizes accrued interest related to unrecognized tax benefits and penalties as income tax expense. Related to the unrecognized tax benefits noted above, the Company accrued $0 of interest during 2021. The Company accrued $0.1 million of interest during 2019 and, in total, as of December 31, 2019 had recognized $0.1 million of interest. The Company accrued $0.1 million of interest during 2018, and, in total, as of December 31, 2018 had recognized $0.1 million of interest.
The Company is subject to taxation in the U.S. and various state jurisdictions. As of December 31, 2021, the Company’s tax returns for 2018 through 2020 generally remain open for exam by taxing jurisdictions. Additional prior years may be open to the extent attributes are being carried forward to an open tax year.
13. Supplemental Disclosure of Cash Flow and Non-Cash Investing and Financing Activities
Selected cash payments, receipts, and noncash activities are as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Cash paid for interest | $ | 4,327 | $ | 7,456 | $ | 4,331 | |||||||||||
| Income taxes paid | 169 | 208 | 308 | ||||||||||||||
| Cash paid for operating leases | 1,522 | 1,569 | 1,650 | ||||||||||||||
| Non-cash activities: | |||||||||||||||||
| Purchases of equipment included in accounts payable | 8 | 1,062 | 1,184 | ||||||||||||||
| Deferred financing costs | — | 53 | 6,650 | ||||||||||||||
| Note receivable for sale of property and equipment | 75 | — | — | ||||||||||||||
| Deemed dividends of Series B Convertible Preferred Stock | 926 | 32,028 | — | ||||||||||||||
| Amendment fee on previous term loan | — | 722 | — | ||||||||||||||
| Lease right of use asset and liability | 2,251 | 1,169 | — | ||||||||||||||
| Fair value of non-cash consideration received for option exercise | 380 | 922 | — | ||||||||||||||
14. Commitments and Contingencies
Contractual Commitments
F-34
The Company has commitments for meeting space. These commitments expire over 3 years following December 31, 2021, and generally contain renewal options.
The estimated meeting space commitments are as follows (in thousands):
| Years Ended December 31, | |||||
| 2022 | $ | 383 | |||
| 2023 | 204 | ||||
| 2024 | 114 | ||||
| $ | 701 | ||||
Litigation and Regulatory Matters
In the ordinary course of business, the Company and its subsidiaries may be a party to pending and threatened legal, regulatory, and governmental actions and proceedings (including those described below). In view of the inherent difficulty of predicting the outcome of such matters, particularly where the plaintiffs or claimants seek very large or indeterminate damages or where the matters present novel legal theories or involve a large number of parties, the Company generally cannot predict what the eventual outcome of the pending matters will be, what the timing of the ultimate resolution of these matters will be, or what the eventual recovery, loss, fines or penalties related to each pending matter may be.
In accordance with applicable accounting guidance, the Company accrues a liability when those matters present loss contingencies that are both probable and estimable. The Company's financial statements at December 31, 2021 reflect the Company's current best estimate of probable losses associated with these matters, including costs to comply with various settlement agreements, where applicable. As of December 31, 2021, the Company had accrued $1.0 million related to the matters described below. Of this amount, the Company is indemnified for $0.6 million from its insurance providers.
The Company paid $6.7 million to settle legal proceedings during 2021. In addition, $1.1 million was paid on the Company’s behalf through an insurance provider during 2021 relating directly to settlement matters. In addition, during 2021, the Company received funds from certain director and officer insurance policies for previously-incurred legal expenses under the Company’s indemnification agreements. These funds were recognized as a reduction to investigation, restatement and related expense on the consolidated statement of operations.
The Company paid $7.4 million to settle legal proceedings during 2020. In addition, $3.5 million was paid on the Company’s behalf through an insurance provider during 2020.
The actual costs of resolving these matters may be in excess of the amounts reserved.
Securities Class Action
On January 16, 2019, the United States District Court for the Northern District of Georgia entered an order consolidating two purported securities class actions (MacPhee v. MiMedx Group, Inc., et al. filed February 23, 2018 and Kline v. MiMedx Group, Inc., et al. filed February 26, 2018). The order also appointed Carpenters Pension Fund of Illinois (“CPFI”) as lead plaintiff. On May 1, 2019, CPFI filed a consolidated amended complaint, naming as defendants the Company, Michael J. Senken, Parker H. “Pete” Petit, William C. Taylor, Christopher M. Cashman and Cherry Bekaert & Holland LLP. The amended complaint (the “Securities Class Action Complaint”) alleged violations of Section 10(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 10b-5 promulgated thereunder, and Section 20(a) of the Exchange Act. It asserted a class period of March 7, 2013 through June 29, 2018. Following the filing of motions to dismiss by the various defendants, CPFI was granted leave to file an amended complaint. CPFI filed its amended complaint against the Company, Michael J. Senken, Parker H. Petit, William C. Taylor, and Cherry Bekaert & Holland (Christopher Cashman was dropped as a defendant) on March 30, 2020; defendants filed motions to dismiss on May 29, 2020. On March 25, 2021, the Court granted defendants’ respective motions to dismiss, finding that CPFI lacked standing to bring the underlying claims and also could not establish loss causation because it sold all of its shares in MIMEDX prior to any corrective disclosures, and dismissed the case. On April 22, 2021, CPFI filed a motion for reconsideration of the dismissal and for leave to amend to add a new plaintiff to attempt to cure the standing and loss causation issues. The Company opposed CPFI’s motions and the hearing on the same was held on September 24, 2021.
On January 28, 2022, the Court denied CPFI’s motion to reconsider and motion to substitute class representative. On February 25, 2022, CPFI filed a Notice of Appeal in the 11th Circuit Court of Appeals.
F-35
Investigations
On February 8, 2021, the Company received a subpoena issued by the Department of Defense Office of Inspector General seeking records regarding the sales of the Company’s micronized and other products to federal medical facilities and federal contracting offices, including those operated by the Department of Veterans Affairs or the Department of Defense. The subpoena also seeks information regarding the Company’s communications with the FDA regarding its products. The Company understands that the Office of the United States Attorney for the Western District of Washington Civil Division is overseeing the investigation, which is being conducted principally by agents employed by the Department of the Army Criminal Investigation Command. The Company is cooperating with the government’s investigation and at this time the Company is unable to predict the outcome of the investigation, including whether the investigation will result in any action or proceeding against the Company.
Former Employee Litigation and Related Matters
On January 12, 2021, the Company filed suit in the Circuit Court of the Eleventh Judicial District in and for Miami-Dade County, Florida (MiMedx Group, Inc. v. Petit, et. al.) against its former CEO, Parker H. “Pete” Petit, and its former COO, William C. Taylor, seeking a determination of its rights and obligations under indemnification agreements with Petit and Taylor following a federal jury’s guilty verdict against Petit for securities fraud and Taylor for conspiracy to commit securities fraud. The Company is seeking a declaratory judgment that it is not obligated to indemnify or advance expenses to Petit and Taylor in connection with certain cases to which Petit and Taylor are parties and also seeking to recoup amounts previously paid on behalf of Petit and Taylor in connection with such cases. On April 22, 2021, Petit and Taylor filed an answer and asserted counterclaims against the Company alleging breach of their indemnification agreements, breach of the covenant of good faith and fair dealing with respect to their indemnification agreements, and seeking a declaration that the Company remains obligated to indemnify and advance fees in connection with certain cases. Petit and Taylor simultaneously filed a motion seeking to compel the Company to advance and reinstate its payments of Petit and Taylor’s legal expenses. The Company opposed Petit and Taylor’s motion and a hearing was set for June 23, 2021. At the joint request of the parties, the hearing was cancelled to allow the parties to attend a mediation to attempt a resolution of this matter; such mediation was held on August 11, 2021. Negotiations are ongoing.
Defamation Claims
On June 4, 2018, Sparrow Fund Management, LP (“Sparrow”) filed a complaint against the Company and Petit, including claims for defamation and civil conspiracy in the United States District Court for the Southern District of New York (Sparrow Fund Management, L.P. v. MiMedx Group, Inc., et. al.). The complaint seeks monetary damages and injunctive relief and alleges the defendants commenced a campaign to publicly discredit Sparrow by falsely claiming it was a short seller who engaged in illegal and criminal behavior by spreading false information in an attempt to manipulate the price of our common stock. The Company has settled this matter.
Other Matters
Under the Florida Business Corporation Act and agreements with its current and former officers and directors, the Company is obligated to indemnify its current and former officers and directors who are made party to a proceeding, including a proceeding brought by or in the right of the corporation, with certain exceptions, and to advance expenses to defend such matters. The Company has already borne substantial costs to satisfy these indemnification and expense advance obligations and may continue to do so in the future.
In addition to the matters described above, the Company is a party to a variety of other legal matters that arise in the ordinary course of the Company’s business, none of which is deemed to be individually material at this time.
Previously-Settled Matters
The matters discussed below have been settled with the counterparty and their resolution has been disclosed in previously-issued financial statements. There are no contingent or continuing obligations associated with these matters.
Shareholder Derivative Suits
On December 6, 2018, the United States District Court for the Northern District of Georgia entered an order consolidating three shareholder derivative actions (Evans v. Petit, et al. filed September 25, 2018, Georgalas v. Petit, et al. filed September 27, 2018, and Roloson v. Petit, et al. filed October 22, 2018) that had been filed in the Northern District of Georgia. On January 22, 2019, plaintiffs filed a verified consolidated shareholder derivative complaint. The consolidated action sets forth claims of breach of fiduciary duty, corporate waste and unjust enrichment against certain former officers, and certain current and former
F-36
directors, of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Larry W. Papasan, Luis A. Aguilar, Bruce L. Hack, Charles E. Koob, Neil S. Yeston and Christopher M. Cashman. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to stay on February 18, 2019, pending the completion of the investigation by the Company’s Special Litigation Committee. The Special Litigation Committee completed its investigation relating to this action and filed an executive summary of its findings with the Court on July 1, 2019. The parties (together with parties from the Hialeah derivative lawsuit, the Nix and Demaio derivative lawsuit, and the Murphy derivative lawsuit, each described below) held a mediation on February 11, 2020. Following continued discussions, on May 1, 2020, the parties notified the Court that plaintiffs and the Company had reached an agreement in principle to settle this consolidated derivative action, which settlement also encompasses all claims asserted in the Hialeah derivative lawsuit, the Nix and Demaio derivative lawsuit, and the Murphy derivative lawsuit. The hearing on final approval was held on December 21, 2020 and the Court entered an Order granting final approval of the settlement the same day.
On October 29, 2018, the City of Hialeah Employees Retirement System (“Hialeah”) filed a shareholder derivative complaint in the Circuit Court for the Second Judicial Circuit in and for Leon County, Florida (the “Florida Court”). The complaint alleges claims for breaches of fiduciary duty and unjust enrichment against certain former officers, and certain current and former directors, of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Joseph G. Bleser, J. Terry Dewberry, Charles R. Evans, Bruce L. Hack, Charles E. Koob, Larry W. Papasan, and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company moved to stay the action on February 7, 2019, to allow the prior-filed consolidated derivative action in the Northern District of Georgia to be resolved first and to allow the Company’s Special Litigation Committee time to complete its investigation. The Company also filed a motion to dismiss on April 8, 2019. As discussed above, the plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia and is a party to the agreement settling that consolidated derivative action. In accordance with the terms of the settlement, Hialeah filed a motion for leave to dismiss its derivative action with prejudice on January 4, 2021.
On May 15, 2019, two individuals purporting to be shareholders of the Company filed a shareholder derivative complaint in the Superior Court for Cobb County, Georgia. (Nix and Demaio v. Evans, et al.) The complaint alleges claims for breaches of fiduciary duty, corporate waste and unjust enrichment against certain current and former directors and officers of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Chris Cashman, Lou Roselli, Mark Diaz, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Court ordered this matter stayed pending the resolution of the consolidated derivative suit pending in the Northern District of Georgia. As discussed above, the plaintiffs participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia and are a party to the agreement settling that consolidated derivative action. In accordance with the terms of the settlement, plaintiffs filed a notice of settlement and voluntary dismissal with prejudice on January 13, 2021.
On August 12, 2019, John Murphy filed a shareholder derivative complaint in the United States District Court for the Southern District of Florida (Murphy v. Petit, et al.). The complaint alleged claims for breaches of fiduciary duty and unjust enrichment against certain former officers, and certain current and former directors, of the Company: Parker H. Petit, William C. Taylor, Michael J. Senken, John E. Cranston, Alexandra O. Haden, Charles R. Evans, Luis A. Aguilar, Joseph G. Bleser, J. Terry Dewberry, Bruce L. Hack, Charles E. Koob, Larry W. Papasan and Neil S. Yeston. The allegations generally involve claims that the defendants breached their fiduciary duties by causing or allowing the Company to misrepresent its financial statements as a result of improper revenue recognition. The Company filed a motion to transfer this action to the Northern District of Georgia. Prior to resolution of that motion, the plaintiff voluntarily dismissed this action without prejudice. As discussed above, the plaintiff participated in the mediation that took place in connection with the prior-filed consolidated derivative action in the Northern District of Georgia and is a party to the agreement settling that consolidated derivative action. Pursuant to the terms of the settlement, this action is deemed dismissed with prejudice.
Qui Tam Matters
On January 19, 2017, a former employee of the Company filed a qui tam False Claims Act complaint in the United States District Court for the District of South Carolina (United States of America, ex rel. Jon Vitale v. MiMedx Group, Inc.) alleging that the Company’s donations to the patient assistance program, Patient Access Network Foundation, violated the Anti-Kickback Statute and resulted in submission of false claims to the government. The government declined to intervene and the complaint was unsealed on August 10, 2018. The Company filed a motion to dismiss on October 1, 2018. The Company’s
F-37
motion to dismiss was granted in part and denied in part on May 15, 2019. The parties have reached an agreement to resolve this matter.
On January 20, 2017, two former employees of the Company, filed a qui tam False Claims Act complaint in the United States District Court for the District of Minnesota (Kruchoski et. al. v. MiMedx Group, Inc.). An amended complaint was filed on January 27, 2017. The operative complaint alleges that the Company failed to provide truthful, complete and accurate information about the pricing offered to commercial customers in connection with the Company’s Federal Supply Schedule contract. On May 7, 2019, the Department of Justice (“DOJ”) declined to intervene, and the case was unsealed. In April 2020, without admitting the allegations, the Company agreed to pay $6.5 million to the DOJ to resolve this matter. This amount was paid during the year ended December 31, 2020.
Former Employee Matters
In December 2019, MiMedx received notice of a complaint filed in July 2018 with the Occupational Safety and Health Administration (“OSHA”) section of the Department of Labor (“DOL”) by Thomas Tierney, a former Regional Sales Director, against MiMedx and the referenced individuals, Tierney v. MiMedx Group, Inc., Parker Petit, William Taylor, Christopher Cashman, Thornton Kuntz, Jr. and Alexandra Haden, DOL No. 4-5070-18-243. Mr. Tierney alleged that he was terminated from MiMedx in retaliation for reporting concerns about revenue recognition practices, compliance issues, and the corporate culture, in violation of the anti-retaliation provisions of the Sarbanes-Oxley Act. The parties settled this matter and OSHA dismissed the complaint on May 20, 2020.
On January 21, 2019, a former employee filed a complaint in the Fifth Judicial Circuit, Richland County, South Carolina (Jon Michael Vitale v. MiMedx Group, Inc. et. al.) against the Company alleging retaliation, defamation and unjust enrichment and seeking monetary damages. The former employee claims he was retaliated against after raising concerns related to insurance fraud and later defamed by comments concerning the indictments of three South Carolina VA employees. On February 19, 2019, the case was removed to the U.S. District Court for the District of South Carolina. The Company filed a motion to dismiss on April 8, 2019, which was denied by the Court. This matter is resolved.
Intellectual Property Litigation
The NuTech Action
On March 2, 2015, the Company filed a patent infringement lawsuit against NuTech Medical, Inc. (“NuTech”) and DCI Donor Services, Inc. (“DCI”) in the United States District Court for the Northern District of Alabama (MiMedx Group, Inc. v. NuTech Medical, Inc. et. al.). The Company has alleged that NuTech and DCI infringed and continue to infringe on the Company’s patents through the manufacture, use, sale and/or offering of their tissue graft product. The Company has also asserted that NuTech knowingly and willfully made false and misleading representations about its products to customers and prospective customers. The Company is seeking permanent injunctive relief and unspecified damages. The case was stayed pending the restatement of the Company’s financial statements. Since the Company has completed its restatement, the case resumed. The parties have reached a settlement in the matter and the case was dismissed with prejudice.
The Osiris Action
On February 20, 2019, Osiris Therapeutics, Inc. (“Osiris”) refiled its trade secret and breach of contract action against the Company (which had been dismissed in a different forum) in the United States District Court for the Northern District of Georgia (Osiris Therapeutics, Inc. v. MiMedx Group, Inc.). The parties have reached a settlement in the matter and the case was dismissed with prejudice on October 26, 2020.
15. Revenue
Disaggregation of Revenue by Product
MIMEDX has two classes of products: (1) Advanced Wound Care, or Section 361, products, consisting of its sheet allograft products, and (2) Section 351 products, consisting of the Company’s micronized and particulate products. Advanced Wound Care is further disaggregated between the Company’s Tissue/Other and Cord products.
F-38
Below is a summary of net sales by each class of product (in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Advanced Wound Care | |||||||||||
| Tissue/Other | $ | 216,418 | $ | 192,566 | |||||||
| Cord | 23,599 | 16,073 | |||||||||
| Advanced Wound Care | 240,017 | 208,639 | |||||||||
| Section 351 | 17,610 | 31,828 | |||||||||
| Other | 988 | 7,767 | |||||||||
Total | $ | 258,615 | $ | 248,234 | |||||||
Due to the disconnection between the performance obligations related to sales and the recognition of revenue on such sales, it is not practical for the Company to allocate these amounts to specific product lines related to the Remaining Contracts (included in “Other” in the table above) as well as revenue recognized during the year ended December 31, 2019.
Disaggregation of Revenue by Customer
Prior to May 31, 2021, the conclusion of the FDA’s enforcement discretion period, the Company evaluated its revenue on the basis of its two primary distribution channels: (1) direct to customers (healthcare professionals and/or facilities) (“Direct Customers”); and (2) sales through distributors (“Distributors”).
Below is a summary of net sales by each customer type (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
Direct Customers | $ | 250,009 | $ | 240,690 | $ | 288,800 | |||||||||||
Distributors | 8,606 | 7,544 | 10,455 | ||||||||||||||
Total | $ | 258,615 | $ | 248,234 | $ | 299,255 | |||||||||||
The Company did not have significant foreign operations or a single external customer from which 10% or more of revenues were derived during the years ended December 31, 2021, 2020, and 2019.
16. 401(k) Plan
The Company has a 401(k) plan (the “401(k) Plan”) covering all employees who have completed one month of service. Under the 401(k) Plan, participants could defer up to 90% of their eligible wages to a maximum of $19,500 per year (annual limit for 2021). Employees age 50 or over in 2021 could make additional pre-tax contributions up to $6,500. In 2021, the Company matched 50% of employee contributions up to 8% of the employee’s eligible compensation. In 2020 and 2019, the Company matched 50% of employee contributions up to 5% of the employee’s eligible compensation. The matching contribution for the years ended December 31, 2021, 2020, and 2019 was $2.7 million, $1.5 million, and $1.5 million, respectively.
17. Related Party Transactions
The Company has employed Thomas Koob as its Chief Scientific Officer (a non-executive officer) since 2006. Thomas Koob is the brother of a former director, Charles Koob. Subsequent to the Company’s employment of Thomas Koob, Charles Koob was appointed as a director of the Company in March 2008. Charles Koob's term as a Director expired at the 2020 Annual Meeting held on November 20, 2020. In 2019, the Company paid Thomas Koob a salary of $0.2 million and provided equity, incentive compensation and other compensation of $0.2 million. In 2020, the Company paid Thomas Koob an annual salary of $0.2 million and provided equity, incentive compensation and other compensation of $0.3 million.
The Company employs Simon Ryan, the brother-in-law of the Company’s former General Counsel, Alexandra O. Haden, as a sales representative. In 2019, the Company paid Mr. Ryan total compensation of $0.2 million, consisting of a salary of $0.1 million and sales commissions, equity and other compensation of $0.1 million. Ms. Haden resigned from her position as General Counsel and Secretary of the Company, effective August 12, 2019, to accept another position.
F-39
18. Restructuring
Set forth below are disclosures relating to restructuring initiatives that resulted in material expenses or cash expenditures during the year ended December 31, 2019, and resulted in material restructuring liabilities at December 31, 2019. Employee retention and certain other employee benefit-related costs related to the Company’s restructuring are expensed ratably over an agreed-upon service period. One-time employee separation and related employee benefit costs are generally expensed as incurred.
In December 2018, the Company announced a reduction of the Company’s workforce by approximately 240 full-time employees, or 24% of its total workforce, of which approximately half were sales personnel as part of the plans to implement a broad-based organizational realignment, cost reduction and efficiency program to better ensure the Company’s cost structure was appropriate given its revenue expectations.
As a result of the December 2018 broad-based organizational realignment, cost reduction and efficiency program, the Company incurred pre-tax charges of $8.5 million during the years ended December 31, 2019. The charges related to employee retention and other one-time employee separation benefit-related costs. These charges are included in the cost of sales, research and development, and selling, general and administrative expenses in the consolidated statements of operations.
The Company’s restructuring program concluded in 2020. All obligations related to the Company’s restructuring program have been settled as of December 31, 2020.
Changes to this liability during the years ended December 31, 2020 and 2019 were as follows (in thousands):
| Liability balance as of December 31, 2018 | $ | 5,607 | |||
| Expenses | 8,543 | ||||
| Cash distributions | (10,589) | ||||
| Liability balance as of December 31, 2019 | 3,561 | ||||
| Expenses | — | ||||
| Cash distributions | (3,561) | ||||
| Liability balance as of December 31, 2020 | $ | — | |||
19. Subsequent Events
On February 28, 2022, the Company executed an Amendment to the Hayfin Loan Agreement (the “Amendment”). Material provisions of the Amendment are detailed in Note 9, Long-Term Debt.
F-40
Schedule II Valuation and Qualifying Accounts
| MIMEDX GROUP, INC. AND SUBSIDIARIES SCHEDULE II VALUATION AND QUALIFYING ACCOUNTS | ||||||||||||||||||||||||||
Years ended December 31, 2021, 2020 and 2019 (in thousands) | ||||||||||||||||||||||||||
| Balance at Beginning of Year | Additions charged to Expense or Revenue | Deductions and write-offs | Balance at End of Year | |||||||||||||||||||||||
For the year ended December 31, 2021 | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 737 | $ | 791 | $ | (341) | $ | 1,187 | ||||||||||||||||||
| Allowance for product returns | $ | 2,321 | $ | 2,508 | $ | (2,280) | $ | 2,549 | ||||||||||||||||||
For the year ended December 31, 2020 | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | — | $ | 719 | $ | 18 | $ | 737 | ||||||||||||||||||
| Allowance for product returns | $ | 4,115 | $ | 705 | $ | (2,499) | $ | 2,321 | ||||||||||||||||||
For the year ended December 31, 2019 | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
| Allowance for product returns | $ | 8,510 | $ | — | $ | (4,395) | $ | 4,115 | ||||||||||||||||||
F-41
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of MiMedx Group, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of MiMedx Group, Inc. and subsidiaries (the “Company”) as of December 31, 2021, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2021, of the Company and our report dated February 28, 2022, expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touch LLP
Atlanta, Georgia
February 28, 2022
79
Evaluation of Disclosure Controls and Procedures
Management maintains a set of disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to management, including our CEO and CFO, to allow for timely decisions regarding required disclosure.
An evaluation of the effectiveness of the design and operation of our disclosure controls and procedures was performed under the supervision and with the participation of our management, including our CEO and CFO. As a result of this evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of December 31, 2021.
Management's Report on Internal Control Over Financial Reporting
Management, including our CEO and CFO, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act and based upon the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the "COSO framework"). The Company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with United States Generally Accepted Accounting Principles (“GAAP”).
An effective internal control system, no matter how well designed, has inherent limitations, including the possibility of human error or overriding of controls, and therefore can provide only reasonable assurance with respect to reliable financial reporting. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may demonstrate.
Under the supervision and with the participation of our management, including our CEO and CFO, we have conducted an evaluation of the effectiveness of our internal control over financial reporting based on the COSO framework. Based on evaluation under these criteria, management determined that we did maintain effective internal control over financial reporting as of December 31, 2021.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that a reasonable possibility exists that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
The Company previously disclosed material weaknesses in internal control over financial reporting as of December 31, 2020 in Item 9A of our Annual Report in Form 10-K for the year ended December 31, 2020 related to certain control activities for which we did not have proper segregation of duties, were not sufficiently evidenced, or included assumptions which were not evaluated for completeness, accuracy or application of GAAP as part of the control.
Our independent registered public accounting firm, Deloitte & Touche LLP, has audited the effectiveness of our internal control over financial reporting as of December 31, 2021, as stated in their report which appears on page 79 of this Form 10-K.
Remediated Material Weaknesses
Remediation of the previously identified material weaknesses and strengthening our internal control environment were priorities for us throughout 2021. We implemented and tested the design and operating effectiveness of new and existing controls related to the previously identified material weaknesses, as follows:
•The Company enhanced its financial close process by introducing additional layers of independent reviews by appropriately qualified individuals and improving the precision and timeliness of reviews applied to various financial result analyses, including revenue recognition, recording of inventory and accrued expenses. Additionally, the Company enhanced the level of evidence of review required to be maintained to evidence the operation of controls.
•The Company enhanced its sales order review process to ensure compliance with Company sales policies by requiring retention of appropriate evidence of customer arrangements and establishing a quarterly review of key revenue metrics by finance and accounting personnel.
•The Company enhanced the operation of controls to address the accuracy and completeness of information used in the performance of controls, including retention of evidence of review and assessment of significant judgements to ensure
80
proper application of GAAP specific to accounting for revenue, inventory, goodwill impairment and the provision for income taxes.
•Management enhanced the controls that validate the completeness and accuracy of data utilized in financial forecasting and periodic goodwill analyses, employing the use of checklists and assessing the appropriateness of significant estimates.
Management has deemed the newly implemented or enhanced controls described above to be operating effectively as of December 31, 2021, and has determined them to have appropriately remediated the previously identified material weaknesses.
Changes in Internal Control Over Financial Reporting
Other than the changes described above in “Remediated Material Weaknesses,” there were no changes during the quarter ended December 31, 2021 in our internal control over financial reporting (as such term is defined in the Exchange Act) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Item 1.01 Entry into a Material Definitive Agreement
Amendment to Hayfin Loan Agreement
On February 28, 2022, the Company entered into the Amendment. Refer to Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources for details.
Item 9C. Disclosures Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information required by this Item will be contained in our definitive proxy statement relating to our 2022 Annual Meeting of Shareholders under the captions “Executive Officers,” “Election of Directors” and “Delinquent Section 16(a) Reports,” or similar captions which are incorporated herein by reference.
Item 11. Executive Compensation
Information required by this Item will be contained in our definitive proxy statement relating to our 2022 Annual Meeting of Shareholders under the caption “Executive Compensation Discussion and Analysis,” “Summary Compensation Table (2021, 2020 and 2019,” “Grants of Plan Based Awards for 2021,” “Outstanding Equity Awards on December 31, 2021,” “2021 Options Exercised and Stock Vested Table,” “2021 Potential Payments Upon Termination or Change in Control,” “2021 Director Compensation,” “Compensation Committee Report” and “Compensation Committee Interlocks and Insider Participation” or similar captions which are incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by this Item will be contained in our definitive proxy statement relating to our 2022 Annual Meeting of Shareholders under the captions “Security Ownership of Certain Beneficial Owners and Management,” and “Equity Compensation Plan Information,” or similar captions which are incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by this Item will be contained in our definitive proxy statement relating to our 2022 Annual Meeting of Shareholders under the captions “Policies and Procedures for Approval of Related Party Transactions,” “Related Party Transactions,” and "Director Independence" or similar captions which are incorporated herein by reference.
81
Item 14. Principal Accounting Fees and Services
Information required by this Item will be contained in our definitive proxy statement relating to our 2022 Annual Meeting of Shareholders under the captions “Audit Matters,” or a similar caption which is incorporated herein by reference.
82
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)Documents filed as part of this report:
(i)Financial Statements
(ii)Financial Statement Schedule:
The following Financial Statement Schedule is filed as part of this Report:
Schedule II Valuation and Qualifying Accounts for the years ended December 31, 2021, 2020 and 2019
(iii)Exhibits
See Item 15(b) below. Each management contract or compensation plan has been identified with an asterisk.
(b)Exhibits
Notes
| * | Indicates a management contract or compensatory plan or arrangement | ||||
| # | Filed herewith | ||||
| ## | Certain exhibits and schedules have been omitted pursuant to Item 601(b)(10) of Regulation S-K, but a copy will be furnished supplementally to the Securities and Exchange Commission upon request. | ||||
| Exhibit Number | Description | ||||||||||
| 3.1 | Restated Articles of Incorporation, adopted March 4, 2021, effective March 5, 2021 (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 10-K filed March 8, 2021). | ||||||||||
| 3.2 | Articles of Amendment to Restated Articles of Incorporation, effective June 3, 2021 (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed June 10, 2021). | ||||||||||
| 3.3 | Articles of Amendment to Restated Articles of Incorporation, effective June 3, 2021 (incorporated by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K filed June 10, 2021). | ||||||||||
| 3.4 | Bylaws of MiMedx Group, Inc., as amended and restated as of April 19, 2021 (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-K filed on April 21, 2021). | ||||||||||
| 3.5 | Amendment No. 1 to the Company’s Bylaws effective May 27, 2021 (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K Filed June 3, 2021). | ||||||||||
| 4.1 | The description of the Registrant’s Securities Registered Pursuant to Section 12 of the Securities Exchange Act of 1934, incorporated by reference to Registration Statement on Form 8-A filed November 2, 2020. | ||||||||||
| 10.1## | Loan Agreement dated as of June 30, 2020 by and among MiMedx Group, Inc., certain subsidiaries of MiMedx Group, Inc. parties thereto, the Lenders from time to time party hereto, Hayfin Services LLP, as administrative agent for the Lenders and as collateral agent for the Secured Parties, incorporated by reference to Exhibit 10.36 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.2## | Securities Purchase Agreement, dated as of June 30, 2020, by and between MiMedx Group, Inc., Falcon Fund 2 Holding Company, L.P. and certain other investors, incorporated by reference to Exhibit 10.38 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.3 | Registration Rights Agreement dated as of July 2, 2020, by and between MiMedx Group, Inc. and Falcon Fund 2 Holding Company, L.P., incorporated by reference to Exhibit 10.39 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.4 | Lease effective May 1, 2013 between Hub Properties of GA, LLC and MiMedx Group, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q filed on May 10, 2013). | ||||||||||
| 10.5 | First Amendment to Lease dated March 7, 2017 between CPVF II West Oak LLC (as successor in interest to HUB Properties of GA, LLC) and MiMedx Group, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on March 13, 2017). | ||||||||||
83
| Exhibit Number | Description | ||||||||||
| 10.6# | Third Amendment to Lease made as of November 30, 2021 for real property and improvements located at 1775 West Oak Commons Court, Marietta, Georgia between RE Fields, LLC, successor in interest to HUB Properties GA, LLC, and CPVF II West Oak LLC, and MiMedx Group, Inc., dated January 25, 2013, as amended March 7, 2017. | ||||||||||
| 10.7* | MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan, as amended and restated effective February 25, 2014 (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K filed on March 3, 2014). | ||||||||||
| 10.8* | Form of Incentive Stock Option Agreement under the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (incorporated by reference to Exhibit 10.4 to the Registrant’s Form 10-K filed on March 4, 2014). | ||||||||||
| 10.9* | Form of Nonqualified Stock Option Agreement under the MiMedx Group, Inc. Assumed 2006 Stock Incentive Plan (incorporated by reference to Exhibit 10.5 to the Registrant’s Form 10-K filed on March 4, 2014). | ||||||||||
| 10.10* | Form of Restricted Stock Agreement for Non-Employee Directors under the MiMedx Group, Inc. 2006 Assumed Stock Incentive Plan (incorporated by reference to Exhibit 10.66 to the Registrant’s Form 10-Q filed on August 8, 2013). | ||||||||||
| 10.11* | Form of Restricted Stock Agreement under the MiMedx Group, Inc. 2006 Assumed Stock Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Form 10-K filed on March 4, 2014). | ||||||||||
| 10.12* | 2016 Equity and Cash Incentive Plan, as amended and restated through October 2, 2020, incorporated by reference to Exhibit 4.6 to Registration Statement on Form S-8 filed December 17, 2020. | ||||||||||
| 10.13* | Form of Incentive Stock Option Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.2 to the Registrant’s Form 10-Q filed on August 2, 2016). | ||||||||||
| 10.14* | Form of Restricted Stock Agreement under the MiMedx Group, Inc 2016 Equity and Cash Incentive Plan (for shares not registered under the Securities Act of 1933) (incorporated by reference to Exhibit 10.9 to the Registrant’s Form 8-K filed on May 30, 2019). | ||||||||||
| 10.15* | Form of Restricted Stock Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Form 10-Q filed on August 2, 2016). | ||||||||||
| 10.16* | Form of Restricted Stock Agreement for Non-Employee Directors under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.11 to the Registrant’s Form 8-K filed on May 30, 2019). | ||||||||||
| 10.17* | Form of Nonqualified Stock Option Agreement under the MiMedx Group, Inc. 2016 Equity and Cash Incentive Plan (incorporated by reference to Exhibit 10.4 to the Registrant’s Form 10-Q filed on August 2, 2016). | ||||||||||
| 10.18* | Form of Director Restricted Stock Unit Award Agreement (incorporated by reference to Exhibit 10.16 to the Registrant’s Annual Report on Form 10-K filed March 17, 2020). | ||||||||||
| 10.19* | Form of Employee (Time-Vested) Restricted Stock Unit Award Agreement, incorporated by reference to Exhibit 10.33 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.20* | Form of Employee (Performance-Vested, uncertain number of shares) Restricted Stock Unit Award Agreement, incorporated by reference to Exhibit 10.34 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.21* | Form of Employee (Performance-Vested, certain number of shares) Restricted Stock Unit Award Agreement, incorporated by reference to Exhibit 10.35 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.22* | Form of Non-Employee Restricted Stock Award Agreement (vest into retirement), incorporated by reference to Exhibit 10.4 to Quarterly Report on Form 10-Q filed August 4, 2020. | ||||||||||
| 10.23* | Form of Employee (Time-Vested) Restricted Stock Unit Award Agreement, incorporated by reference to Exhibit 10.25 to the Annual Report on form 10-K file on March 8, 2021. | ||||||||||
| 10.24* | Letter Agreement dated April 10, 2019 between MiMedx Group, Inc. and Timothy R. Wright (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on May 9, 2019). | ||||||||||
| 10.25* | Employment Offer Letter between the Company and Peter M. Carlson, as amended and restated on June 30, 2021, incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed August 3, 2021. | ||||||||||
| 10.26* | Employment Offer Letter between the Company and William F. Hulse IV as of November 4, 2019, incorporated by reference to Exhibit 10.30 to Annual Report on Form 10-K filed July 6, 2020. | ||||||||||
| 10.27* | Employment Offer Letter between the Company and Rohit Kashyap dated as of July 23, 2020, incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed August 4, 2020. | ||||||||||
| 10.28* | Employment Offer Letter between the Company and Robert B. Stein effective August 1, 2020, incorporated by reference to Exhibit 10.3 to Quarterly Report on Form 10-Q filed August 4, 2020. | ||||||||||
| 10.29* | Form of Key Employee Retention and Restrictive Covenant Agreement, incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed December 21, 2020. | ||||||||||
| 10.30* | 2020 Management Incentive Plan, incorporated by reference to Exhibit 10.35 to the Annual Report on form 10-K file on March 8, 2021. | ||||||||||
| 10.31* | Management Incentive Plan, incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed December 21, 2020. | ||||||||||
84
| Exhibit Number | Description | ||||||||||
| 10.32* | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.65 to the Registrant’s Form 8-K filed July 15, 2008). | ||||||||||
| 10.33* | Form of Director Restricted Stock Unit Award Agreement (Type I - Initial Grant, Full Amount), incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed August 3, 2021. | ||||||||||
| 10.34* | Form of Director Restricted Stock Unit Award Agreement (Type II - Initial Grant, Pro Rata Amount), incorporated by reference to Exhibit 10.3 to Quarterly Report on Form 10-Q filed August 3, 2021. | ||||||||||
| 10.35* | Form of Director Restricted Stock Unit Award Agreement (Type III - Annual Grant), incorporated by reference to Exhibit 10.4 to Quarterly Report on Form 10-Q filed August 3, 2021. | ||||||||||
| 10.36 | Technology License Agreement dated January 29, 2007 between MiMedx, Inc., Shriner's Hospitals for Children and University of South Florida Research Foundation (incorporated by reference to Exhibit 10.32 to the Registrant’s Form 8-K filed on February 8, 2008). | ||||||||||
| 10.37 | Cooperation Agreement dated as of May 29, 2019 among MiMedx Group, Inc., M. Kathleen Behrens Wilsey, K. Todd Newton, Richard J. Barry, Prescience Partners, LP, Prescience Point Special Opportunity LP, Prescience Capital LLC, Prescience Investment Group, LLC d/b/a Prescience Point Capital Management LLC and Eiad Asbahi (incorporated by reference to Exhibit 10.32 to the Registrant’s Form 8-K filed on May 30, 2019). | ||||||||||
| 10.38# ## | Amendment No. 1 to Loan Agreement dated as of February 28, 2022, which amends that certain Loan Agreement dated as of June 30, 2020 by and among MiMedx Group, Inc., certain subsidiaries of MiMedx Group, Inc. parties thereto, the Lenders from time to time party hereto, Hayfin Services LLP, as administrative agent for the Lenders and as collateral agent for the Secured Parties. | ||||||||||
| 16.1 | Letter from BDO USA, LLP dated March 30, 2021, incorporated by reference to Exhibit 16.1 to the Registrant’s Current Report on Form 8-K filed March 30, 2021. | ||||||||||
| 21.1# | |||||||||||
| 23.1# | |||||||||||
| 23.2# | |||||||||||
| 24.1# | Power of Attorney (included on the signature page to this Report). | ||||||||||
| 31.1# | |||||||||||
| 31.2# | |||||||||||
| 32.1# | |||||||||||
| 32.2# | |||||||||||
| 101.INS# | XBRL Instance Document | ||||||||||
| 101.SCH# | XBRL Taxonomy Extension Schema Document | ||||||||||
101.CAL# | XBRL Taxonomy Extension Calculation Linkbase Document | ||||||||||
| 101.DEF# | XBRL Taxonomy Extension Definition Linkbase Document | ||||||||||
| 101.LAB# | XBRL Taxonomy Extension Label Linkbase Document | ||||||||||
| 101.PRE# | XBRL Taxonomy Extension Presentation Linkbase Document | ||||||||||
Item 16. Form 10-K Summary
Not applicable.
85
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MIMEDX GROUP, INC. | ||||||||
| February 28, 2022 | By: | /s/ Peter M. Carlson | ||||||
| Peter M. Carlson | ||||||||
| Chief Financial Officer and Principal Financial Officer | ||||||||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints William F. Hulse IV and Sajid N. Ajmeri and each of them acting individually, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her in any and all capacities, to sign any and all amendments to this Annual Report for the year ended December 31, 2021, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming our signatures as they may be signed by our said attorney to any and all amendments to said Annual Report.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
86
| Signature / Name | Title | Date | ||||||||||||
| /s/ Timothy R. Wright | Chief Executive Officer and Director | February 28, 2022 | ||||||||||||
| Timothy R. Wright | (Principal Executive Officer) | |||||||||||||
| /s/ Peter M. Carlson | Chief Financial Officer | February 28, 2022 | ||||||||||||
| Peter M. Carlson | (Principal Financial Officer) | |||||||||||||
| /s/ William L. Phelan | Senior Vice President and Chief Accounting Officer | February 28, 2022 | ||||||||||||
| William L. Phelan | (Principal Accounting Officer) | |||||||||||||
| /s/ M. Kathleen Behrens | Chair of the Board (Director) | February 28, 2022 | ||||||||||||
| M. Kathleen Behrens | ||||||||||||||
| /s/ James L. Bierman | Director | February 28, 2022 | ||||||||||||
| James L. Bierman | ||||||||||||||
| /s/ Michael J. Giuliani | Director | February 28, 2022 | ||||||||||||
| Michael J. Giuliani | ||||||||||||||
| /s/ William A. Hawkins III | Director | February 28, 2022 | ||||||||||||
| William A. Hawkins III | ||||||||||||||
| /s/ Cato T. Laurencin | Director | February 28, 2022 | ||||||||||||
| Cato T. Laurencin | ||||||||||||||
| /s/ K. Todd Newton | Director | February 28, 2022 | ||||||||||||
| K. Todd Newton | ||||||||||||||
| /s/ Martin P. Sutter | Director | February 28, 2022 | ||||||||||||
| Martin P. Sutter | ||||||||||||||
| /s/ Phyllis I. Gardner | Director | February 28, 2022 | ||||||||||||
| Phyllis I. Gardner | ||||||||||||||
87
Similar companies
See also STRYKER CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also BECTON DICKINSON & CO - Annual report 2022 (10-K 2022-09-30) Annual report 2023 (10-Q 2023-06-30)
See also 3M CO - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also BOSTON SCIENTIFIC CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-03-31)
See also RESMED INC - Annual report 2023 (10-K 2023-06-30) Annual report 2023 (10-Q 2023-09-30)
