Odonate Therapeutics, Inc. - Annual Report: 2018 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2018
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 001-38318
Odonate Therapeutics, Inc
(Exact name of Registrant as specified in its charter)
|
Delaware |
|
|
|
82-2493065 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
|
|
(I.R.S. Employer Identification Number) |
4747 Executive Drive, Suite 510
San Diego, CA 92121
(858) 731-8180
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.01 per share |
|
Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to the filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☒ |
|
Non-accelerated filer |
☐ |
|
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.) Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Company as of June 29, 2018 was $215,063,401, based on the closing price on the Nasdaq Global Select Market reported for such date. Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 4, 2019, there were 26,752,669 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
|
|
|
|
|
Page |
|
|
|
|
1 |
|
|
|||
|
|
||||||
|
|
||||||
|
Item 1. |
|
|
2 |
|
|
|
|
Item 1A. |
|
|
34 |
|
|
|
|
Item 1B. |
|
|
54 |
|
|
|
|
Item 2. |
|
|
54 |
|
|
|
|
Item 3. |
|
|
54 |
|
|
|
|
Item 4. |
|
|
54 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 5. |
|
|
55 |
|
|
|
|
Item 6. |
|
|
55 |
|
|
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
56 |
|
|
|
Item 7A |
|
|
62 |
|
|
|
|
Item 8. |
|
|
62 |
|
|
|
|
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
62 |
|
|
|
Item 9A. |
|
|
62 |
|
|
|
|
Item 9B. |
|
|
63 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 10. |
|
|
64 |
|
|
|
|
Item 11. |
|
|
67 |
|
|
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
71 |
|
|
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
73 |
|
|
|
Item 14. |
|
|
75 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 15. |
|
|
76 |
|
|
|
|
Item 16. |
|
|
77 |
|
|
|
|
|
|
|
78 |
|
|
|
i
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the federal securities laws, which statements involve substantial risks and uncertainties. All statements, other than statements of historical facts included in this Annual Report on Form 10-K, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, financing needs, plans or intentions relating to acquisitions, business trends and other information referred to under “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. Forward-looking statements generally relate to future events or our future financial or operating performance. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms and similar expressions intended to identify forward-looking statements. Forward-looking statements are not historical facts and reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
There are a number of risks, uncertainties and other important factors that could cause our actual results to differ materially from the forward-looking statements contained in this Annual Report on Form 10-K. Such risks, uncertainties and other factors include, among others, the risks, uncertainties and factors disclosed under “Risk Factors” and the following risks, uncertainties and factors:
|
|
• |
our plans to develop and commercialize tesetaxel and any other product candidates; |
|
|
• |
our ongoing and planned clinical studies; |
|
|
• |
the timing of and our ability to obtain regulatory approvals for tesetaxel and any other product candidates; |
|
|
• |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
|
• |
our ability to identify additional products or product candidates with significant commercial potential that are consistent with our commercial objectives; |
|
|
• |
the rate and degree of market acceptance and clinical utility of tesetaxel and any other product candidates, if approved; |
|
|
• |
our commercialization, marketing and manufacturing capabilities and strategy; |
|
|
• |
significant competition in our industry; |
|
|
• |
our intellectual property position; |
|
|
• |
loss or retirement of key members of management; |
|
|
• |
failure to successfully execute our growth strategy, including any delays in our planned future growth; and |
|
|
• |
our failure to maintain effective internal controls. |
There may be other factors that may cause our actual results to differ materially from the forward-looking statements, including factors disclosed under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You should evaluate all forward-looking statements made in this Annual Report on Form 10-K in the context of these risks and uncertainties.
We caution you that the risks, uncertainties and factors referred to above may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. All forward-looking statements in this Annual Report on Form 10-K apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this Annual Report on Form 10-K. We undertake no obligation to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances.
1
In this Annual Report on Form 10-K, unless context requires otherwise, references to “we,” “us,” “our,” “Odonate” or the “Company” refer to: (i) Odonate Therapeutics, Inc. as of and following the completion of the Conversion described below; and (ii) Odonate Therapeutics, LLC before the completion of the Conversion. All shares of common stock and per share amounts for all periods presented in this Annual Report on Form 10-K have been adjusted retroactively, where applicable, to reflect the Conversion.
Company Overview
We are a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. Our initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several pharmacologic properties that make it unique among taxanes, including: oral administration with a low pill burden; a long (~8-day) terminal plasma half-life (“t1/2”) in humans, enabling the maintenance of adequate drug levels with relatively infrequent dosing; no history of hypersensitivity (allergic) reactions; and significant activity against chemotherapy-resistant tumors. In patients with metastatic breast cancer, tesetaxel was shown to have significant, single-agent antitumor activity in two multicenter, Phase 2 studies. We are conducting a multinational, multicenter, randomized, Phase 3 study in patients with locally advanced or metastatic breast cancer (“LA/MBC”), known as CONTESSA, and we expect to report top-line results from this study in 2020. Our goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
Breast Cancer and Its Treatment
Breast cancer is the second-most common cancer worldwide, with an estimated 2.1 million new cases diagnosed per year (World Health Organization). In Europe, an estimated 523,000 new cases are diagnosed and approximately 138,000 women will die of the disease each year, making it the leading cause of cancer death in women (World Health Organization). In the U.S., an estimated 269,000 new cases are diagnosed and approximately 41,000 women will die of the disease each year, making it the second-leading cause of cancer death in women (American Cancer Society). Estimated breast cancer incidence and deaths per year in Europe, the U.S. and worldwide are shown in the following table
Estimated Breast Cancer Incidence and Deaths per Year in Europe, the U.S. and Worldwide
|
|
|
(1) World Health Organization (2) American Cancer Society |
2
The prognosis for women with LA/MBC remains poor; the 5-year survival rate for metastatic disease is about 22% (American Cancer Society), making this an area of continued, high unmet medical need. Therefore, treatment that balances efficacy, tolerability and quality of life is preferred.
Clinical Benefit Is a Balance of Efficacy, Tolerability and Quality of Life
|
|
3
Breast cancer is a heterogeneous disease comprised of several molecular subtypes, which are commonly grouped into clinical subtypes based on receptor status (Perou et al., Nature 2000;406:747-752). Receptors that are assessed in standard clinical practice include the estrogen receptor (“ER”) and progesterone receptor (“PgR”), collectively the hormone receptors (“HR”), and human epidermal growth factor receptor 2 (“HER2”). Breast cancers generally are categorized by the presence or absence of these receptors. The most common type of breast cancer is HER2 negative and HR positive, accounting for approximately 64% of newly diagnosed cases (Howlader et al., Journal of the National Cancer Institute 2014;106(5):1-8). HER2 positive breast cancer and triple-negative breast cancer (“TNBC”), the latter of which lacks all three receptors, are less common, accounting for approximately 13% and 11% of breast cancers, respectively (Howlader et al., Journal of the National Cancer Institute 2014;106(5):1-8). Estimated U.S. breast cancer incidence by receptor status and MBC treatments by receptor status are shown in the following figure.
Estimated U.S. Breast Cancer Incidence by Receptor Status and MBC Treatments by Receptor Status
|
|
|
HER2=human epidermal growth factor receptor 2; HR=hormone receptor; MBC=metastatic breast cancer; TNBC=triple-negative breast cancer (1) Howlader et al., Journal of the National Cancer Institute 2014;106(5):1-8 (2) Caldeira et al., Oncology and Therapy 2016;4:189-197 |
Treatment of HER2 Negative, HR Positive LA/MBC
HER2 negative, HR positive disease, which represents the majority of all LA/MBC cases, remains an area of high unmet medical need. Over the past two decades, only modest survival benefits have been achieved in this patient population; hence, treatment goals emphasize controlling disease-related symptoms, minimizing toxicity and maximizing quality-of-life. Patients with HER2 negative, HR positive disease are typically treated with endocrine therapy (with or without a cyclin-dependent kinase [“CDK”] 4/6 inhibitor), chemotherapy or both.
Endocrine Therapy
Endocrine agents, which target certain hormone receptors inside and on the surface of tumor cells with the goal of slowing tumor growth, are preferred as initial treatment prior to chemotherapy for most patients with HER2 negative, HR positive MBC (National Comprehensive Cancer Network 2017). These agents, which typically are used sequentially, include aromatase inhibitors (e.g., anastrozole, exemestane and letrozole), selective estrogen receptor modulators (e.g., tamoxifen) and estrogen receptor downregulators (e.g., fulvestrant). As initial therapy in post-menopausal women with HER2 negative, ER positive disease, letrozole plus the recently approved CDK 4/6 inhibitor palbociclib resulted in median progression-free survival (“PFS”) of 24.8 months, compared to 14.5 months with letrozole alone (Finn et
4
al., New England Journal of Medicine 2016;375(20):1925-1936). As second-line endocrine therapy in women with HER2 negative, HR positive disease, fulvestrant plus palbociclib resulted in median PFS of 9.5 months, compared to 4.6 months with fulvestrant alone (Cristofanilli et al., The Lancet 2016;17(4):425-439). However, despite these recent advances in endocrine therapy, virtually all patients will eventually progress and require subsequent treatment with chemotherapy.
Chemotherapy
In HER2 negative, HR positive LA/MBC, chemotherapy generally is used following disease progression on endocrine therapy or in women for whom endocrine therapy is not indicated. While endocrine therapy is most often the first-line treatment for women with HER2 negative, HR positive LA/MBC, a significant percentage of women receive chemotherapy as their first treatment for advanced disease. In a recent analysis of a large patient record database (n=1,272-1,640 in Europe and n=2,225-2,760 in the U.S.), chemotherapy-only regimens were given as the first therapy following a diagnosis of advanced disease 33% to 35% of the time in Europe and 34% to 42% of the time in the U.S. (Caldeira et al., Oncology and Therapy 2016;4:189-197). This suggests that a significant percentage of women are not indicated for endocrine therapy in the advanced setting due to: (i) a short relapse-free interval while on adjuvant endocrine therapy (endocrine resistance); (ii) rapidly progressing disease/visceral crisis; or (iii) endocrine intolerance.
There are several approved chemotherapy agents for the treatment of HER2 negative LA/MBC. These include: paclitaxel, nab-paclitaxel and docetaxel (taxanes); capecitabine (a fluoropyrimidine); doxorubicin and epirubicin (anthracyclines); gemcitabine (a nucleoside inhibitor); ixabepilone (an epothilone approved in the U.S.); and eribulin (a non-taxane microtubule dynamics inhibitor). The taxanes and eribulin are approved as monotherapy; capecitabine is approved as both monotherapy and combination therapy (with docetaxel); gemcitabine is approved as combination therapy only (with paclitaxel); and ixabepilone is approved in the U.S. as both monotherapy and combination therapy (with capecitabine).
The choice and sequencing of chemotherapy regimens depend on a number of factors, including physician preference, previous therapies, pre-existing medical conditions, tumor burden and patient symptoms. As shown in the following chart, capecitabine, an oral chemotherapy and taxanes are the preferred first-line chemotherapy agents in HER2 negative, HR positive MBC.
Physician-reported Preferences for First-line Chemotherapy for Patients with HER2 Negative, HR Positive MBC(1)
|
|
|
Recent survey of 201 U.S. community-based oncologists (1) Lin et al., Cancer Medicine 2016;5(2):209-220 |
5
Taxanes are an established class of anticancer agents that are broadly used in various cancers, including breast cancer. Taxanes destroy cancer cells by preventing them from entering mitosis, a process of cell division, and thereby leading to apoptosis, or cell death. As shown in the figure below, taxanes are one of the most widely used classes of chemotherapy agents in both Europe and the U.S., with more than 2.8 million cycles administered in 2016 (Symphony Health Solutions 2016; IMS Health 2016).
>2.8 Million Cycles of Paclitaxel, Nab-paclitaxel and Docetaxel Administered in 2016 in Europe and the U.S.(1)
|
|
|
Recent survey of 201 U.S. community-based oncologists (1) Symphony Health Solutions 2016; IMS Health 2016 |
While paclitaxel and docetaxel, the first two taxanes approved for the treatment of breast cancer, possess robust antitumor activity, they have low oral bioavailability and low solubility. Therefore, these pharmaceutical agents must be delivered intravenously, typically at an infusion center, and also are formulated with solubilizing agents that are known to cause hypersensitivity reactions. Nab-paclitaxel, a different formulation of paclitaxel that also is approved for the treatment of breast cancer, has a greatly reduced risk of hypersensitivity reactions, but must still be delivered intravenously.
Therapies that must be given intravenously at an infusion center often are associated with(1):
|
• Fear of needles and complications associated with venous access; • Anxiety, including institutional-triggered side effects such as nausea and vomiting; • Heightened awareness of life-threatening disease presence; and • Disruption of daily activities. |
|
|
(1) Gornas et al., European Journal of Cancer Care 2010;19(1):131-136; Schott et al., BMC Cancer 2011;11:129 |
|
6
Tesetaxel: An Orally Administered Taxane with Improved Pharmacologic Properties
Tesetaxel, which we believe will qualify as a New Chemical Entity (“NCE”) if and when a New Drug Application (“NDA”) is submitted, retains the same taxane core as the approved taxanes, but includes the addition of two novel, nitrogen-containing functional groups. Tesetaxel is chemically designed to: (i) not be substantially effluxed by the P-glycoprotein (“P-gp”) pump, with the intent of retaining activity against chemotherapy-resistant tumor cells; (ii) have high oral bioavailability; (iii) have high solubility; and (iv) have a long (~8 day) t1/2 in humans. The table below includes the chemical and pharmacologic properties of paclitaxel, docetaxel and tesetaxel.
Chemical and Pharmacologic Properties of Paclitaxel, Docetaxel and Tesetaxel
|
|
|
(1) At therapeutic concentrations (2) The P-glycoprotein (P-gp) efflux pump mediates gastric absorption as well as chemotherapy resistance (3) Shanmugam et al., Drug Development and Industrial Pharmacy 2015;41(11):1864-1876 (4) McEntee et al., Veterinary and Comparative Oncology 2003;1(2):105-112 (5) At pH conditions similar to gastric fluid (6) Montaseri, Taxol: Solubility, Stability and Bioavailability 1997 (7) Bharate et al., Bioorganic & Medicinal Chemistry Letters 2015;25(7):1561-1567 (8) Tan et al., British Journal of Cancer 2014;110(11):2647-54 (9) Taxotere (docetaxel) prescribing label (10) Lang et al., 2012 American Society of Clinical Oncology (ASCO) Annual Meeting, Journal of Clinical Oncology 2012;20(15 supp):2555 |
7
Tesetaxel has a long (~8 day) t1/2 in humans (see figure below), which may allow adequate drug levels to be maintained with relatively infrequent dosing.
|
|
|
Cmax=maximum (plasma) concentration; GI50=the concentration of drug required to inhibit growth by 50% Q3/4W=once per week for 3 of 4 weeks; Q3W=once every 3 weeks (1) Shionoya et al., Cancer Science 2003;94(5):459-66 (2) Trock et al., Journal of the NCI 1997;89(13):917-31 (3) Tan et al., British Journal of Cancer 2014;110(11):2647-54 (4) Lang et al., 2012 American Society of Clinical Oncology (ASCO) Annual Meeting, Journal of Clinical Oncology 2012;20(15 supp):2555 |
We believe that tesetaxel’s unique properties may translate into significant benefits for patients. These may include:
|
|
• |
Oral administration with a low pill burden; |
|
|
• |
A long (~8-day) t1/2 in humans, enabling the maintenance of adequate drug levels with relatively infrequent dosing; |
|
|
• |
No history of hypersensitivity reactions; and |
|
|
• |
Significant activity against chemotherapy-resistant tumors. |
Nonclinical Studies
Tesetaxel has exhibited potent antitumor activity in both in vitro (in a test tube) and in vivo (in a live organism) nonclinical studies (Shionoya et al., Cancer Science 2003;94(5):459-466). Tesetaxel retains potent antitumor activity against chemotherapy-resistant tumor cells, including tumor cells over-expressing the P-gp efflux pump. A defense mechanism of tumor cells, this efflux pump functions to expel toxins, including many chemotherapy agents.
In Vitro Antitumor Activity
Tesetaxel has exhibited potent antitumor activity in in vitro nonclinical studies, with an overall GI50 (the concentration of drug required to inhibit growth by 50%) of less than 1 nM. GI50 is a commonly used nonclinical measurement of antitumor potency; lower GI50 numbers connote higher potency (1 nM is 1/1,000th of 1 µM). In human tumor cell lines, tesetaxel largely retained antitumor cytotoxic (cell-killing)
8
potency against taxane-resistant (P-gp positive) and other chemotherapy-resistant tumors, while paclitaxel and docetaxel lost considerable antitumor potency (Shionoya et al., Cancer Science 2003;94(5):459-466). Consistent with this finding, the uptake and accumulation of tesetaxel in both P-gp negative and P-gp positive tumor cells were greater than that of either paclitaxel or docetaxel. The relative loss of cytotoxic potency between P-gp negative and P-gp positive tumor cells and between non-drug-resistant and drug-resistant tumor cells for paclitaxel, docetaxel and tesetaxel is shown in the following table (low numbers connote high potency, and high numbers connote low potency).
Tesetaxel Retained Activity against Chemotherapy-resistant Tumors In Vitro(1)
|
|
|
(1) Shionoya et al., Cancer Science 2003;94(5):459-466 |
9
Tesetaxel exhibited significant growth-inhibitory effects (inhibition rate [“IR”] >94%) in a P-gp positive, human breast cancer (DU4475) mouse model that exhibited resistance to both paclitaxel and docetaxel (IR values of 26%-46%) (Shionoya et al., Cancer Science 2003;94(5):459-466). The relative in vivo antitumor activity for a control, paclitaxel, docetaxel and tesetaxel is shown in the following graph.
Antitumor Activity of Paclitaxel, Docetaxel and Tesetaxel in
P-gp Positive Breast Tumors in Mice(1)
|
|
|
(1) Shionoya et al., Cancer Science 2003;94(5):459-466 |
Clinical Studies
More than 500 patients have been treated with tesetaxel across 22 clinical studies. Tesetaxel was administered as monotherapy in 17 studies and in combination with other agents in 5 studies. Studies have been completed in LA/MBC, gastric cancer, colorectal cancer, non-small cell lung cancer and other cancers as first-line, second-line or salvage therapy.
Tesetaxel, administered both alone and in combination with capecitabine, has been generally well tolerated. In the 8 studies (927A-PRT001, 927A-PRT004, 927E-PRT003, 927E-PRT005, 927E-PRT007, 927A-PRT006, TOST107 and TOST107XT) for which final study data are available, a total of 187 patients were treated with tesetaxel at 27 mg/m2 once every three weeks as monotherapy (N=156) or in combination with capecitabine at 1,750-2,500 mg/m2 (N=31). In this population, the most common Grade ≥3 (severe or serious) treatment-related adverse event (“AE”) was neutropenia (low level of neutrophils, a type of white blood cell), (33%) febrile neutropenia (fever coinciding with neutropenia) occurred in 5% of patients. The most common non-hematologic Grade ≥3 treatment-related AEs were: dehydration (5%); diarrhea (5%); fatigue (5%); and anorexia (4%). Other non-hematologic Grade ≥ 3 treatment-related AEs include: nausea (3%); peripheral neuropathy (weakness, numbness and/or pain from damage to the nerves) (3%); and vomiting (2%). Treatment-related alopecia (hair loss) (any grade) occurred in 14% of patients overall, and Grade 2 treatment-related alopecia occurred in 3% of patients. There were no hypersensitivity reactions.
10
Tesetaxel’s adverse event profile across all completed studies is shown in the following table.
Adverse Event Profile of 187 Patients at Doses Consistent with CONTESSA
|
|
The results from a Phase 2 clinical study of tesetaxel monotherapy in MBC (TOB203) are summarized below.
Study TOB203: A Phase 2 Study of Tesetaxel as First-line Chemotherapy for MBC
In Study TOB203, 38 patients with HER2 negative, HR positive MBC received tesetaxel orally as a single agent once every three weeks at a starting dose of 27 mg/m2. Objective response rate (“ORR”) based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was the primary endpoint (Seidman et al., 2018 ASCO Annual Meeting).
11
Median age was 58 years (range: 36-80 years). Eighty-seven percent (87%) had visceral disease, 74% previously received at least one endocrine therapy, 68% previously received neoadjuvant or adjuvant chemotherapy and 53% previously received a taxane-containing regimen. The figures that follow show the study design and patient characteristics (Seidman et al., 2018 ASCO Annual Meeting).
|
|
|
|
In all 38 patients, the confirmed response rate was 45%. The confirmed response rate was consistent across subgroups. Forty-four percent (44%) of patients who did not previously receive a taxane-containing regimen achieved a confirmed response, compared to 45% of patients who previously received a taxane-containing regimen. Median duration of response was 10.9 months, and median progression-free survival was 5.4 months (Seidman et al., 2018 ASCO Annual Meeting).
Tumor change from baseline in target lesions by individual patient is shown in the following figure.
Tumor Change from Baseline in Target Lesions(1)
|
|
|
(1) Nadir change based on sum of the diameters |
12
Patient response is shown in the following figure.
Response
|
|
|
(1) Includes one patient who was not evaluated for response |
Patient response by prior taxane exposure is shown in the following figure.
Response by Prior Taxane Exposure
|
|
13
Tesetaxel was generally well tolerated. Neutropenia was the most common Grade ≥3 AE and occurred in 25% of the 24 patients who were not dose-escalated beyond the 27 mg/m2 starting dose (the dose selected for CONTESSA, our ongoing Phase 3 study); in these patients, febrile neutropenia occurred in one patient (4%) and Grade ≥3 neuropathy occurred in one patient (4%). There were no hypersensitivity reactions or drug-related deaths, and the rate of Grade 2 alopecia (significant hair loss) was 18%. A summary of Grade ≥3 AEs regardless of relationship is shown in the following table.
Grade ≥3 Adverse Events Regardless of Relationship
|
|
CONTESSA: A Multinational, Multicenter, Randomized, Phase 3 Study of Tesetaxel in MBC
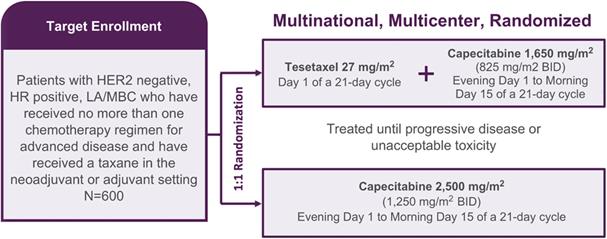
We are conducting a 600-patient, multinational, multicenter, randomized, Phase 3 study, known as CONTESSA, that is comparing tesetaxel dosed orally at 27 mg/m2 on the first day of a 21-day cycle plus a reduced dose of capecitabine (1,650 mg/m2/day on the first 14 days of a 21-day cycle) to the approved dose of capecitabine alone (2,500 mg/m2/day on the first 14 days of a 21-day cycle) in patients with HER2 negative, HR positive LA/MBC previously treated with a taxane in the neoadjuvant (prior to surgery) or adjuvant (immediately following surgery) setting. Where indicated, patients must have received endocrine therapy with or without a CDK 4/6 inhibitor. CONTESSA’s primary endpoint is PFS assessed by an Independent Radiologic Review Committee (“IRC”). CONTESSA’s secondary endpoints are overall survival (“OS”), ORR assessed by IRC and disease control rate assessed by IRC.
14
|
|
|
BID=twice per day; HER2=human epidermal growth factor receptor 2; HR=hormone receptor; LA/MBC=locally advanced or metastatic breast cancer |
In designing CONTESSA, we received non-binding advice from both the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”). We believe CONTESSA may serve as a single pivotal study sufficient for product registration, provided that the study demonstrates a statistically significant and clinically meaningful improvement in the primary endpoint, PFS, for tesetaxel plus a reduced dose of capecitabine as compared to the approved dose of capecitabine alone as well as an overall favorable benefit-risk profile for the tesetaxel plus a reduced dose of capecitabine regimen. Generally, a single pivotal study can be sufficient for FDA approval only when the study provides highly reliable and statistically strong evidence of an important clinical benefit and in which confirmation of the result in a second clinical trial would be practically or ethically impossible. There can be no assurance that the outcome of CONTESSA will be sufficient for the approval of tesetaxel by the FDA, EMA or other regulatory agencies or that tesetaxel will be approved at all.
Rationale for CONTESSA Study Design
CONTESSA is designed to evaluate whether tesetaxel plus a reduced dose of capecitabine results in improved PFS with manageable toxicity and favorable quality-of-life compared to the approved dose of capecitabine alone. Tesetaxel plus a reduced dose of capecitabine incorporates two agents with synergistic mechanisms of action and is an all-oral regimen that offers a significant pill-burden reduction compared to the approved dose of capecitabine alone.
15
CONTESSA: Tesetaxel Arm Offers Significant Pill Burden Reduction
|
|
|
(1) Xeloda (capecitabine) prescribing label (2) Illustration for patients with body surface area of 1.78-1.90m2 |
Our rationale for the CONTESSA study design includes the following points:
|
|
• |
Capecitabine is a preferred agent as a first- or second-line chemotherapy treatment for patients with HER2 negative, HR positive LA/MBC. Therefore, capecitabine, at the approved dose, is an appropriate control regimen for a registration-enabling Phase 3 study. |
|
|
• |
There is a high unmet medical need for combination chemotherapy regimens with improved benefit-risk profiles. |
|
|
• |
Combining the approved dose of capecitabine with currently available taxanes results in robust efficacy but with significant toxicity. |
|
|
• |
Nonclinical and clinical studies support investigating whether reducing the dose of capecitabine in combination with a taxane will reduce toxicity without a reduction in efficacy. |
|
|
• |
Single-agent tesetaxel has demonstrated antitumor activity in two Phase 2 studies in MBC. |
Capecitabine as an Appropriate Control Regimen
Capecitabine is a preferred agent as a first- or second-line chemotherapy treatment for patients with HER2 negative, HR positive LA/MBC, particularly those previously treated with a taxane in the neoadjuvant, adjuvant or advanced setting. Therefore, capecitabine, at the approved dose, is an appropriate control regimen for a registration-enabling Phase 3 study. The FDA- and EMA-approved capecitabine regimen is 2,500 mg/m2 on the first 14 days of a 21-day cycle.
Medical Need for Combination Chemotherapy Regimens with Improved Benefit-risk Profiles
To date, combination chemotherapy regimens generally have not demonstrated superior benefit-risk profiles as compared to single-agent, sequential chemotherapy. Specifically, while currently available combination regimens have been associated with increased PFS and, in the case of docetaxel-
16
capecitabine, increased OS, they also have been associated with significantly increased toxicity. As a result, single-agent, sequential chemotherapy remains the standard of care for many patients.
Nonetheless, there remains a large unmet need for chemotherapy regimens, including combination regimens, with improved benefit-risk profiles. In particular, newer combinations that preserve the response rates and PFS of currently available combination regimens, but are better tolerated and easier to take, are needed.
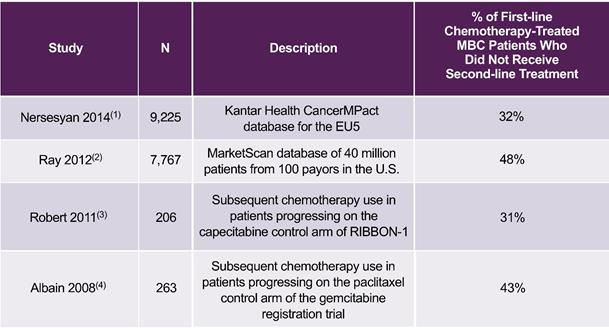
There is a need for improved combination chemotherapy regimens due to the fact that approximately a third or more of LA/MBC patients do not receive second-line chemotherapy after progressing on first-line chemotherapy, as shown in the following table. For these patients, who are not necessarily identifiable when first-line chemotherapy is initiated, there is no opportunity for a second chemotherapy treatment. Therefore, regimens that can significantly increase PFS with minimal increase in toxicity, including regimens that incorporate two therapeutic agents, would significantly benefit patients.
Percentage of LA/MBC Patients Who Did Not Receive Additional Chemotherapy after
Progressing on First-line Chemotherapy Treatment
|
|
|
EU5=European Union 5 (France, Germany, Italy, Spain and the United Kingdom) (1) Nersesyan et al., 2014 ISPOR 17th Annual European Congress (2) Ray et al., Journal of Clinical Oncology 2012;30(27):116 (3) Robert et al., Journal of Clinical Oncology 2011;29(10):1252-1260 (4) Albain et al., Journal of Clinical Oncology 2008;26(24):3950-3957 |
17
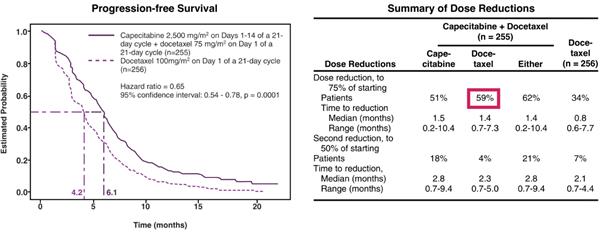
Combining the Approved Dose of Capecitabine with Docetaxel, an Approved Taxane
In a multicenter, randomized Phase 3 study in 511 patients receiving first-, second- or third-line chemotherapy that served as the basis for approval for capecitabine combined with docetaxel in the treatment of MBC, capecitabine at the approved dose of 2,500 mg/m2 (1,250 mg/m2 BID) on the first 14 days of a 21-day cycle combined with docetaxel resulted in superior time to disease progression (hazard ratio = 0.65, 95% confidence interval: 0.54-0.78, p=0.0001) and OS (hazard ratio = 0.78, 95% confidence interval: 0.63-0.95, p=0.01) as compared to docetaxel alone. However, there was significant drug-related toxicity on the combination regimen, resulting in a large percentage of dose reductions and discontinuations (59% of patients reduced their dose of docetaxel and 51% reduced their dose of capecitabine) (O'Shaughnessy et al., Journal of Clinical Oncology 2002;20(12):2812-2823). The results of this Phase 3 study are as follows.
Clinical Evidence of Increased Efficacy when Combining a Taxane with Capecitabine(1)
|
|
|
(1) O’Shaughnessy et al., Journal of Clinical Oncology 2002;20(12):2812-2823 |
18
Reducing the Dose of Capecitabine in Combination with a Taxane
Synergy when Combining a Taxane with Capecitabine in Nonclinical Studies
Nonclinical studies have shown synergy when combining a taxane with capecitabine. Taxanes up-regulate tumor levels of thymidine phosphorylase, the enzyme essential for the activation of capecitabine. These studies suggest the potential to reduce the dose of capecitabine without loss of efficacy. In two in vivo nonclinical studies of breast cancer, the combined administration of capecitabine and docetaxel resulted in antitumor efficacy significantly greater than the sum of the efficacy resulting from either agent administered as monotherapy (see the following figures). Furthermore, the synergy may be tumor-specific, as toxicity as measured by weight loss and effect on peripheral blood cells was minimal. These studies suggest the potential to reduce the dose of capecitabine without loss of efficacy.
In Vivo Synergy with Taxane-Capecitabine Combinations
|
|
Capecitabine at 1/2 MTD + Docetaxel at 1/8 MTD(1)(2) |
Capecitabine at 2/3 MTD + Docetaxel at 1/15 MTD(3)(4) |
|
|
|
|
|
|
MTD=maximum tolerated dose (1) Sawada et al., Clinical Cancer Research 1998;4:1013-1019 (2) Capecitabine dosed 5 times every 7 days; docetaxel dosed once every 7 days (3) Fujimoto-Ouchi et al., Clinical Cancer Research 2001;7(4):1079-1086 (4) Capecitabine dosed on Days 1-14 and 22-36; docetaxel dosed on Days 8 and 29 |
|
19
Clinical Studies Evaluating Lower Doses of Capecitabine Combined with a Taxane
Consistent with nonclinical findings of synergy between taxanes and capecitabine, clinical studies support investigating the combination of a taxane with a reduced dose of capecitabine such as 1,650 mg/m2/day on the first 14 days of a 21-day cycle, the dose of capecitabine chosen for combination with tesetaxel in CONTESSA. In a review of 18 first-line MBC studies of taxane plus capecitabine combinations shown in the following table, there was no apparent loss of efficacy when comparing capecitabine at 1,650 mg/m2/day to capecitabine at 2,000 mg/m2/day (on the first 14 days of a 21-day cycle). Among these studies, the capecitabine 1,650 mg/m2/day dose was the most studied dose less than 2,000 mg/m2/day (5/8 studies). According to Lortholary, the trend toward improved efficacy with lower doses of capecitabine may result from the significantly lower proportion of patients discontinuing study therapy prematurely because of toxicity, and highlights the importance of administering capecitabine using a schedule that optimizes dose intensity and tolerability (Lortholary et al, Breast Cancer Research and Treatment 2012;131:127-135).
Clinical Activity of Taxane and Capecitabine Regimen at Capecitabine Doses Ranging from 1,500 to 2,000 mg/m2/day
|
|
|
PFS=progression-free survival; ORR=objective response rate; OS=overall survival (1) Days 1-14 of a 21-day cycle (2) Bachelot et al., Oncology 2011;80(3-4):262-268; Campone et al., The Breast Journal 2013;19(3):240-249; Chitapanarux et al., Asia-Pacific Journal of Clinical Oncology 2012;8:76-82; Fan et al., Annals of Oncology 2013;24:1219-1225; Liao et al., Chemotherapy 2013;59:207-213; Michalaki et al., Anti-Cancer Drugs 2009;20(3):204-207; Michalaki et al., Anticancer Research 2010;30:3051-3054; Venturini et al., Cancer 2003;97(5):1174-1180; Wang et al., Cancer 2015;121:3412; Wardley et al., Journal of Clinical Oncology 2010;28(6):976-983 (3) Bisagni et al., Cancer Chemotherapy and Pharmacology 2013;71(4):1051-1057; Luck et al., Breast Cancer Research and Treatment 2015;149:141-149 (4) Hatschek et al., Breast Cancer Research and Treatment 2012;131(3):939-947; Lam et al., European Journal of Cancer 2014;50(18):3077-3088; Perez et al., Annals of Oncology 2010;21(2):269-274; Schwartzberg et al., Clinical Breast Cancer 2012;12(2):87-93; Tonyali et al., Journal of Cancer Research and Clinical Oncology 2013;139(6):981-986 (5) Silva et al., Clinical Breast Cancer 2008;8(2):162-167 |
In summary, we believe that the data support the investigation of tesetaxel at 27 mg/m2 on the first day of a 21-day cycle plus capecitabine at 1,650 mg/m2/day on the first 14 days of a 21-day cycle as a novel, all-oral regimen with a potentially favorable benefit-risk profile for the treatment of patients with HER2 negative, HR positive LA/MBC.
Competition
The biotechnology and pharmaceutical industries are extremely competitive. Our potential competitors in the field are many in number and include major and mid-sized pharmaceutical and biotechnology companies. Many of our potential competitors have significantly more financial, technical and other resources than we do, which may give them a competitive advantage. In addition, they may have substantially more experience in effecting strategic combinations, in-licensing technology, developing drugs, obtaining regulatory approvals and manufacturing and marketing products. We cannot give any assurances that we can compete effectively with these other biotechnology and pharmaceutical companies. Any products that we may develop or discover will compete in highly competitive markets. Our potential competitors in these markets may succeed in developing products that could render our product candidates obsolete or non-competitive.
20
Tesetaxel faces significant competition. Multiple chemotherapies are currently available to physicians and patients for the treatment of HER2 negative, HR positive LA/MBC. These include: paclitaxel, nab-paclitaxel and docetaxel (taxanes); capecitabine (a fluoropyrimidine); doxorubicin and epirubicin (anthracyclines); gemcitabine (a nucleoside inhibitor); ixabepilone (an epothilone that is approved in the U.S.); and eribulin (a non-taxane microtubule dynamics inhibitor). The taxanes and eribulin are approved as monotherapy; capecitabine is approved as both monotherapy and combination therapy (with docetaxel); gemcitabine is approved as combination therapy only (with paclitaxel); and ixabepilone is approved in the U.S. as both monotherapy and combination therapy (with capecitabine). In addition, there are novel chemotherapies in development, including new intravenous paclitaxel formulations, such as NantPharma’s Cynviloq and Sun Pharma’s Taclantis, and novel oral paclitaxel formulations, such as Athenex’s Oraxol and Daehwa Pharmaceutical’s DHP107. We believe that the extent to which tesetaxel is adopted by the marketplace, if it is approved, will depend on factors such as its safety and tolerability, efficacy, convenience, effect on quality-of-life and cost-effectiveness relative to other treatment alternatives.
Daiichi Sankyo License Agreement
In 2013, we licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Tesetaxel had previously been licensed to Genta Incorporated.
Under the Daiichi Sankyo license agreement, we currently hold exclusive rights to 15 issued patents covering tesetaxel. See “Business—Patents and Proprietary Rights.” We are obligated under the license agreement to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. We are required to make aggregate future milestone payments of up to $31.0 million, contingent on attainment of certain regulatory milestones, none of which have yet been achieved. Additionally, we will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel. To date, no payments have been made to Daiichi Sankyo under the license agreement. The license agreement and accompanying royalty obligation terminate on a country-by-country basis on the last-to-expire patent in each such country, which we expect will be between 2026 and 2031 in the U.S., 2025 and 2030 in European countries and 2025 and 2030 in Japan, depending on the availability and application of patent term extensions.
NCE Exclusivity
We believe that tesetaxel will qualify as an NCE if and when an NDA is submitted. If tesetaxel qualifies as an NCE, we believe that NCE regulatory exclusivity, combined with our intellectual property, assuming the availability of 5 years of patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Act, will provide exclusivity for tesetaxel in all major markets through at least 2031. Separate from patent protection, exclusivity refers to certain delays and prohibitions on approval of competitor drugs available under the statute that attach on approval of a drug.
Exclusivity in the U.S.
In the U.S., drugs approved by the FDA are eligible for regulatory exclusivity under the Federal Food, Drug, and Cosmetic Act (“FDCA”), which can delay the approval of generic competition by up to 7.5 years. Specifically, the FDCA provides a 5-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for an NCE. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all of the data required for approval. However, an application may be submitted after 4 years if it contains a certification of patent invalidity or
21
non-infringement. This certification will trigger an automatic 30-month stay in the approval of any generic competition, effectively extending the regulatory exclusivity period to 7.5 years.
NCE exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and adequate and well-controlled clinical studies necessary to demonstrate safety and effectiveness.
Exclusivity in Europe
In Europe, NCEs, sometimes referred to as new active substances, qualify for 8 years of data exclusivity upon marketing authorization and an additional two years of market exclusivity, for a total of 10 years of regulatory exclusivity. This exclusivity, if granted, prevents regulatory authorities in the EU from referring to the innovator’s data to assess a generic application for 8 years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, but the generic product may not be approved for two years. This 10-year period can be extended to a maximum of 11 years if, during the first 8 years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications that, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
However, even if a compound is considered to be an NCE and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full Marketing Authorization Application with a complete database of pharmaceutical tests, nonclinical studies and clinical studies and obtain marketing approval of its product.
Exclusivity in Japan
In Japan, an NCE is eligible for at least 8 years of regulatory exclusivity. Specifically, under the Pharmaceutical Affairs Law, the regulatory authority re-examines the safety and efficacy of drugs after drug approval. The data submitted to the regulatory authority is not available to generic drug companies during the re-examination period. This effectively makes the re-examination system a regulatory exclusivity system in Japan. The re-examination period is 10 years following approval for an orphan drug and 8 years for an NCE. Innovators may also benefit from an additional 4- to 10-month waiting period for generic pricing approval. There may be an additional 4 years of market protection granted if a new indication for a drug is registered in the first 8 years of the re-examination period.
Patents and Proprietary Rights
The proprietary nature of, and protection for, our product candidates, processes and know-how are important to our business. Our success depends in part on our ability to protect the proprietary nature of our product candidates, technology and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing on our proprietary rights. We seek and maintain patent protection in the U.S. and internationally for our product candidates and other technology. We endeavor to patent or in-license technology, inventions and improvements that we consider important to the development of our business. In addition to patent protection, we intend to use other means to protect our proprietary rights, including pursuing terms of marketing or data exclusivity, orphan drug status (if applicable) and similar rights that are available under regulatory provisions in certain territories, including the U.S., Europe and Japan. We also rely on trade secrets, know-how and continuing innovation to develop and maintain our competitive position.
The intellectual property portfolio protecting our tesetaxel program includes 9 U.S., 5 European and 7 Japanese patents, as well as one pending U.S. patent application and one pending international patent application. Of these, 5 U.S., 4 European and 6 Japanese patents are exclusively licensed to us by Daiichi Sankyo. The 21 issued patents consist of the following:
22
|
|
• |
Five (5) European patents, including: (i) two composition-of-matter patents expiring in 2020 and 2022; and (ii) two method-of-manufacture patents expiring in 2022 and 2025; and (iii) one patent with claims to manufacturing methods and intermediates expiring in 2031, without taking into account any potential patent term restoration. |
|
|
• |
Seven (7) Japanese patents, including: (i) three composition-of-matter patents expiring between 2020 and 2022; (ii) three method-of-manufacture patents expiring between 2022 and 2025; and (iii) one patent with both composition-of-matter and method-of-manufacture claims expiring in 2031, without taking into account any potential patent term restoration. |
Among these patents, one issued U.S. composition-of-matter patent (U.S. Patent No. 7,410,980) covers the crystal form of tesetaxel used in our clinical formulation and will expire in 2026. If tesetaxel is approved by the FDA, we will be entitled to request patent term restoration that could extend the protection of this patent until 2031. The exact duration of the extension depends on the time we spend in clinical studies as well as the time the FDA spends reviewing our NDA. See “Business—Government Regulation—U.S. Patent Term Restoration.”
Our success depends on an intellectual property portfolio that supports our future revenue streams. We are maintaining and building our patent portfolio through filing new patent applications and prosecuting existing applications. We cannot be certain that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology. Any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented, infringed or misappropriated, or such intellectual property and proprietary rights may not be sufficient to permit us to take advantage of current market trends or otherwise to provide competitive advantages. For more information, see “Risk Factors—Risks Relating to Intellectual Property.”
Manufacturing
We currently contract with third-party contract development and manufacturing organizations (“CDMOs”) for the manufacture of tesetaxel and intend to do so in the future. We do not own or operate manufacturing facilities and currently have no plans to build our own clinical- or commercial-scale manufacturing capabilities. Although we rely on CDMOs, we have personnel with extensive manufacturing experience to oversee these contract service providers.
We conduct our manufacturing activities under individual purchase orders with multiple CDMOs. To date, our third-party manufacturers have met our manufacturing requirements. We expect third-party manufacturers to be capable of providing sufficient quantities of tesetaxel to meet anticipated full-scale commercial demands. To meet our projected needs for commercial manufacturing, third parties with whom we currently work with might need to increase their scale of production, or we will need to secure alternate suppliers. We believe that there are alternate sources of supply that can satisfy our clinical and commercial requirements, although we cannot be certain that identifying and establishing relationships with such sources, if necessary, would not result in significant delay or material additional costs.
Sales and Marketing
In order to commercialize tesetaxel, if approved, or any other product candidates that we may develop, we must build marketing, sales and distribution capabilities or make arrangements with third parties to perform these services. The commercial infrastructure for oncology products typically consists of a sales
23
force that calls on oncologists, supported by sales management, medical liaisons, internal sales and marketing support and distribution support.
Additional capabilities important to the oncology marketplace include the management of key accounts such as managed care organizations, integrated delivery networks, group-purchasing organizations, specialty pharmacies and government accounts. To develop the appropriate commercial infrastructure, we will have to invest significant amounts of financial and management resources, some of which will be committed prior to any confirmation that any of our product candidates will be approved.
Where appropriate, we may elect in the future to utilize marketing partners, distributors or contract sales forces to assist in the commercialization of tesetaxel.
Government Regulation
Governmental authorities in the U.S., Europe, Japan and other countries where we may seek approval to commercialize tesetaxel extensively regulate the research, development, testing, manufacture, approval and marketing of pharmaceutical products. Our product candidates must be approved by these regulatory authorities before they may be legally marketed in the applicable jurisdictions. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Below is a general summary of applicable government regulations affecting our current and planned business activities in the U.S., Europe and Japan.
U.S. Government Regulation
In the U.S., the FDA regulates drugs under the FDCA and its implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development or approval process, or after approval, may subject an applicant to administrative or judicial sanctions, any of which could have a material adverse effect on us. These sanctions could include:
|
|
• |
refusal to approve pending applications; |
|
|
• |
withdrawal of an approval; |
|
|
• |
imposition of a clinical hold; |
|
|
• |
warning or untitled letters; |
|
|
• |
seizures or administrative detention of product; |
|
|
• |
total or partial suspension of production or distribution; or |
|
|
• |
injunctions, fines, restitution, disgorgement, refusal of government contracts or civil or criminal penalties. |
U.S. Drug Approval Process
The process required by the FDA before a pharmaceutical product may be marketed in the U.S. generally involves the following:
|
|
• |
completion of extensive nonclinical laboratory tests, in vivo nonclinical studies and formulation studies conducted according to Good Laboratory Practices and other applicable regulations; |
|
|
• |
submission to the FDA of an investigational new drug (“IND”) application, which must become effective before human clinical studies may begin; |
|
|
• |
performance of adequate and well-controlled human clinical studies according to Good Clinical Practices (“GCPs”) and other applicable regulations to establish the safety and efficacy of the product candidate for its intended use; |
24
|
|
• |
completion of an FDA pre-approval inspection of the manufacturing facility or facilities to assess compliance with current Good Manufacturing Practices (“cGMPs”) and conformance with the manufacturing-related elements of the application to assure consistent production of the product within required specifications; |
|
|
• |
potential FDA audit of the study sites that generated the data in support of the NDA; and |
|
|
• |
FDA review and approval of the NDA. |
Once a pharmaceutical candidate is identified for development, it enters the nonclinical testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the nonclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The IND will also include a protocol detailing the objectives of the clinical study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated.
All clinical studies must be conducted under the supervision of one or more qualified investigators in accordance with FDA requirements. An institutional review board (“IRB”) must review and approve the protocol and will monitor the study until completion. Clinical studies must be conducted under protocols detailing the objectives of the study, dosing procedures, research subject selection, inclusion and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol, and any material amendments to the protocol, must be submitted to the FDA as part of the IND, and sponsors must report to the FDA serious and unexpected adverse reactions in a timely manner. Sponsors also must make certain financial disclosures to the FDA regarding any financial relationships with study investigators.
Human clinical studies are typically conducted in three sequential phases that may overlap or be combined.
|
|
• |
Phase 1—The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. Initial human testing is often conducted in patients for product candidates intended to treat severe or life-threatening diseases, such as cancer, especially when the product candidate may be inherently too toxic to ethically administer to healthy volunteers. |
|
|
• |
Phase 2—Clinical studies are performed on a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
|
|
• |
Phase 3—Clinical studies are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide an adequate basis for product labeling. A pivotal study is a clinical study that is intended to meet regulatory authority requirements for the evaluation of a product candidate’s efficacy and safety such that it can be used to justify the approval of the product. |
Human clinical studies are inherently uncertain, and Phase 1, Phase 2 and Phase 3 studies may not be successfully completed or may not be completed at all. The FDA or the sponsor may suspend a clinical study at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study if the clinical study is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients.
The results of product development, nonclinical studies and clinical studies, along with descriptions of the manufacturing process, analytical tests and other control mechanisms, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the
25
product. Within 60 days following submission of the application, the FDA reviews the NDA to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to accept any NDA that it deems incomplete or not properly reviewable at the time of submission and may request additional information. Once the submission is accepted for filing, the FDA begins an in-depth, substantive review of the NDA, which includes an assessment of the nonclinical and clinical data, the product’s formulation and manufacturing and whether the product is safe and effective for the proposed intended use. The review timeline for NDAs for new molecular entities is 10 months from the date the application is accepted for filing for a standard review; and 6 months from the date the application is accepted for filing for a priority review.
FDA Expedited Review and Approval
The FDA has various programs, including fast-track designation, breakthrough therapy designation, accelerated approval and priority review, each of which are intended to facilitate and expedite the development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening conditions. Different qualifying criteria apply to each program, and each program offers different mechanisms to expedite the development or approval process, such as additional opportunities to meet with the FDA regarding the product candidate’s clinical development program, the opportunity for rolling review of a marketing application, approval based on a surrogate endpoint or, in the case of priority review, a shorter timeline for reviewing a marketing application. We may seek to take advantage of one or more of these expedited programs for tesetaxel or other product candidates in the future. However, even if a product candidate qualifies for one or more of these programs, the development or approval of the product candidate may not be shortened. The FDA may also later determine that a product candidate no longer meets the criteria for designation, and designation does not guarantee that the FDA will ultimately approve the product.
U.S. Patent Term Restoration
Depending on the timing, duration and specifics of FDA approval of the use of our product candidates, one of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to 5 years as compensation for patent term lost during product development and the FDA regulatory review process, provided the patent term restoration does not extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. In the future, if available, we intend to apply for an extension of patent term for one of our currently owned patents beyond its current expiration date; however, there can be no assurance that any such extension will be granted to us.
U.S. Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval for various reasons, such as non-compliance with regulatory requirements or significant safety and performance problems with the product. Later discovery of previously unknown problems with a product may result in recalls or restrictions on the product or even complete withdrawal of the product from the market. Holders of an approved NDA are required to report certain adverse reactions to the FDA and maintain pharmacovigilance programs to proactively look for these adverse events. Manufacturers are also required to comply with restrictions on the advertising and promotion of their products, including restrictions on “off-label” promotion for uses outside those described in the approved product labeling.
In addition, after a product candidate has been approved, the FDA may require that certain additional post-approval requirements be satisfied, including the conduct of additional clinical studies. Certain changes to an approved product, such as adding new indications, making certain manufacturing changes or making certain additional labeling claims are subject to further FDA review and approval. Before a company can market products for additional indications, it must obtain approval from the FDA of a new NDA or NDA supplement, which generally requires that additional clinical studies be conducted. A company cannot be sure that any additional approval for new indications for any product candidate will be approved on a timely basis, or at all.
26
Changes to the manufacturing process for a given drug are strictly regulated and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements on us and any third-party manufacturers we use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance and be subject to periodic or for-cause inspection by the FDA and other regulatory authorities to ensure such compliance.
U.S. Reimbursement and Pricing
Significant uncertainty exists as to the coverage and reimbursement status of tesetaxel and any other products for which we may seek regulatory approval. Sales in the U.S. will depend in part on the availability of adequate financial coverage and reimbursement from third-party payors, which include government health programs such as Medicare, Medicaid, TRICARE and the Veterans Administration, as well as managed care organizations and private health insurers. Prices at which we or our customers seek reimbursement for our product candidates can be subject to challenge, reduction or denial by payors.
The process for determining whether a payor will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list or formulary, which might not include all of the FDA-approved products for a particular indication. Also, third-party payors may refuse to include a particular branded drug on their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available. Medicare Part D, Medicare’s outpatient prescription drug benefit, contains protections to ensure coverage and reimbursement for oral oncology products, and all Part D prescription drug plans are required to cover substantially all oral anti-cancer agents. However, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be available.
Private payors often rely on the lead of the governmental payors in rendering coverage and reimbursement determinations. Sales of our product candidates will therefore depend substantially on the extent to which the costs of our products will be paid by third-party payors. Achieving favorable coverage and reimbursement from the Centers for Medicare and Medicaid Services (“CMS”) and/or the Medicare Administrative Contractors is typically a significant gating issue for successful introduction of a new product.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for marketing, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, which would be in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider our product candidates to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development.
U.S. Healthcare Fraud and Abuse Laws and Compliance Requirements
We are subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales and marketing programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
27
|
|
recommendation of, an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example gifts, cash payments, donations, the furnishing of supplies or equipment, waivers of payment, ownership interests, and providing any item, service or compensation for something other than fair market value. Federal false claims and civil monetary penalties laws, including the federal civil False Claims Act, which prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we may not submit claims directly to payors, manufacturers can be held liable under these laws in a variety of ways. These include: providing inaccurate billing or coding information to customers or improperly promoting a product’s off-label use; violating the Anti-Kickback Statute; or misreporting pricing information to government programs. |
|
|
• |
Provisions of the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or services. |
|
|
• |
The federal Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act, which require manufacturers of certain drugs and biologics to track and report to CMS payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer. |
|
|
• |
Provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information. |
|
|
• |
Section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information. State law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state transparency reporting and compliance laws; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and which may not have the same effect, thus complicating compliance efforts. |
European Government Regulation
In Europe, tesetaxel and any future products we may seek to develop and commercialize may also be subject to extensive regulatory requirements. As in the U.S., medicinal products can only be marketed if a marketing authorization from the competent regulatory authorities has been obtained.
Similar to the U.S., the various phases of nonclinical and clinical research in Europe are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC (the “Directive”) has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical study can be initiated, it must be approved in each of the EU countries where the study is to be conducted by two distinct bodies: The National Competent Authority (“NCA”) and one or more Ethics Committees (“EC”). Under the current regime, all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical study have to be reported to the NCA and ECs of the Member State where they occurred.
In 2014, a new Clinical Trials Regulation 536/2014 (the “Regulation”), replacing the current Directive, was adopted. The new Regulation will become directly applicable in all EU Member States (without
28
national implementation) once the EU Portal and Database are fully functional. It is expected that the Regulation will apply in 2019. The new Regulation seeks to simplify and streamline the approval of clinical studies in the EU. For example, the sponsor shall submit a single application for approval of a clinical study via the EU Portal. As part of the application process, the sponsor shall propose a reporting Member State, which will coordinate the validation and evaluation of the application. The reporting Member State shall consult and coordinate with the other concerned Member States. If an application is rejected, it can be amended and resubmitted through the EU Portal. If an approval is issued, the sponsor can start the clinical study in all concerned Member States. However, a concerned Member State can, in limited circumstances, declare an “opt-out” from an approval. In such a case, the clinical study cannot be conducted in that Member State. The Regulation also aims to streamline and simplify the rules on safety reporting, and introduces enhanced transparency requirements such as mandatory submission of a summary of the clinical study results to the EU Database.
European Drug Approval Process
In the European Economic Area (“EEA”), which is comprised of the 28 Member States of the EU plus Norway, Iceland and Liechtenstein, medicinal products can only be commercialized after obtaining a Marketing Authorization (“MA”). There are two types of marketing authorizations:
|
|
• |
The Community MA, which is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use (“CHMP”), of the EMA and which is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of drugs, such as biotechnology medicinal drugs, orphan medicinal drugs and medicinal drugs containing a new active substance indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for drugs containing a new active substance not yet authorized in the EEA, or for drugs that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. Under the Centralized Procedure in the EU, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease (e.g., disabling or life-threatening diseases) to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days, excluding clock stops. |
|
|
• |
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for drugs not falling within the mandatory scope of the Centralized Procedure. Where a drug has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member States through the Mutual Recognition Procedure. If the drug has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure, an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State (“RMS”). Within 210 days after receipt of a valid application, the competent authority of the RMS prepares a draft assessment report, a draft summary of the drug characteristics (“SmPC”) and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SmPC, labeling or packaging proposed by the RMS, the drug is subsequently granted a national MA in all the Member States (i.e., in the RMS and the Member States Concerned). |
29
Under the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the drug on the basis of scientific criteria concerning its quality, safety and efficacy.
Patent Term Extension in Europe
Similar to the patent term extensions available in the U.S., European patent law offers the possibility to apply for a supplementary protection certificate (“SPC”) in order to compensate patent holders for the regulatory delays caused by marketing authorization procedures for medicinal products. SPCs extend the patent term for a period that is equal to the time that elapsed between the filing date of the patent application and the date of the first marketing authorization in the EU, minus 5 years. The overall term of an SPC may not exceed 5 years. An SPC may afford a maximum patent duration of 25 years or, when calculated from the date of first marketing approval, an effective patent exclusivity period of 15 years after first marketing authorization. Applications for SPCs must be filed and approved on a country-by-country basis.
Pharmaceutical Coverage, Reimbursement and Pricing in Europe
In Europe, similar political, economic and regulatory developments may affect our ability to profitably commercialize tesetaxel or any other products, if approved. European countries vary significantly in their approach to coverage, reimbursement and pricing assessments. Some countries allow drug products to be marketed only after a reimbursement price has been agreed to. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies, or so-called health technology assessments, in order to obtain reimbursement or pricing approval. The considerations in each country can also vary with respect to the value placed on unmet need, generic availability, dosing, administration, level of innovation and many other dynamics. In certain cases, these decisions are made sequentially and are often interdependent. Many also have multi-layered, decision-making bodies at the country, regional, local and even hospital level, with various responsibilities within the process.
For example, the United Kingdom typically bases reimbursement decision on a determination of cost effectiveness as defined by cost per quality-adjusted life year (“QALY”), as assessed by National Institute for Health and Care Excellence (“NICE”). Funding by the National Health Service is typically granted on an incremental cost-effectiveness ratio (“ICER”) of £30,000 or less per QALY. Unlike other countries, the United Kingdom does have a specific process for cancer therapies that do not gain NICE recommendation initially. Cancer therapies can enter into a conditional reimbursement agreement for no more than two years funded by the Cancer Drug Fund while clinical value continues to be assessed in order to inform the final guidance. At the end of this two-year period, recommendation may be granted for permanent reimbursement on fulfillment of the evidence commitment by the manufacturer.
Similarly, Germany also makes reimbursement decisions based on the determination of additional benefit. Unlike the ICER approach, an efficiency frontier of the total cost and total benefit of all available agents is employed as a cost-benefit methodology. Newly approved agents are compared in terms of cost-benefit ratio either against alternatives or within a specific indication.
France has its own system of therapeutic index to assess medicines for reimbursement and pricing. Reimbursement is determined through a Service Médical Rendu rating of clinical benefit (low, moderate, substantial), with the exception of hospital-only drugs that may be reimbursed at 100%. Pricing is negotiated based on an Amélioration du Service Médical Rendu rating of therapeutic improvement compared to available alternatives (negative (VI), no improvement (V), minor (IV), moderate (III), substantial (II), major (I)).
It is possible that we may be required to conduct cost-effectiveness studies of our product candidates relative to other available therapies in certain countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our financial results may suffer.
30
Japanese Government Regulation
Tesetaxel and any future products we may seek to develop and commercialize will also be subject to extensive regulatory requirements in Japan. As in the U.S., medicinal products can only be marketed if a marketing authorization from the competent regulatory authorities has been obtained. In Japan, the Ministry of Health, Labor and Welfare (“MHLW”) oversees the regulation and safety of pharmaceuticals, medical devices, cosmetics and food and is the organizational body responsible for approving or rejecting an NDA. Within MHLW, the Pharmaceuticals and Medical Devices Agency (“PMDA”) oversees regulatory affairs for drugs and medical devices and is the body responsible for regulatory review of NDAs.
Japanese Drug Approval Process
The drug approval process in Japan involves a series of activities, including nonclinical and clinical (Phase 1, 2 and 3) studies, bridging studies, submission of an NDA by the manufacturer and review of the NDA by the PMDA. MHLW and PMDA are the main regulatory authorities regulating clinical studies. To conduct a clinical study, a pharmaceutical company must register a protocol with MHLW. Prior to submitting a protocol to the MHLW, an applicant usually submits a Clinical Trial Notification to the PMDA. All documents must be translated into Japanese. The notification mainly consists of a description and product summary, nonclinical data, the clinical study protocol, analysis plan, SOPs, contact person and the names of participating research institution(s). Also, compliance with GCP often requires an IRB to review the clinical study protocol, provide written informed consent forms for participants and report adverse events.
In order to obtain marketing approval, an applicant must submit an NDA for drug marketing authorization to the PMDA for review. Once the PMDA has received the NDA, a team of reviewers evaluates the application data, including quality, pharmacology, pharmacokinetics, toxicology, clinical implications, biostatistics and GCP on-site inspection. During the review process, the reviewers exchange opinions with external experts (expert meetings) to discuss important problems. A general review conference attended by team members, external experts and representatives of the applicant is held after the expert meeting. After the review, the PMDA makes a recommendation and sends the application to the MHLW. MHLW then obtains a recommendation from the Pharmaceutical Affairs and Food Sanitation Council before making a decision regarding approval or rejection of the application.
When data from clinical studies performed in foreign countries are used for an NDA in Japan, the data are first checked to assure that it complies with legal requirements in Japan. Following the legal assessment, an evaluation to determine whether or not the drug is apt to be affected by ethnic factors (intrinsic or extrinsic factors) is conducted. When necessary, a bridging study in Japanese patients is performed, and, when it is concluded that the clinical study outcome in a foreign population can be extrapolated to the Japanese population, the foreign data can be accepted. It is mandatory to conduct pharmacokinetic studies in Japanese people.
Drug approval reviews are normally processed in the order in which the application forms are received. However, orphan drugs and other drugs considered to be especially important from a medical standpoint, such as new drugs to treat serious diseases, may be designated for priority review.
Products for priority review are given priority at each stage of the review process as much as possible. For example, for products designated for priority review at the development stage, it is possible to obtain priority interview advice on indications and other items concerning the designated product. The target review period for priority review is 9 months, compared to 12 months for standard reviews.
Patent-term Extension in Japan
The term of a patent that covers an approved drug may be extended for the shorter of 5 years or the period during which the patent could not be exploited due to obtaining regulatory approval. This period is calculated from the later of the patent registration date (grant date) or the clinical study start date to the
31
regulatory approval date. Unlike in the U.S., patent-term extension in Japan can be applied to more than one patent.
Pharmaceutical Coverage, Reimbursement and Pricing in Japan
In Japan, there is significant uncertainty as to the reimbursement and pricing status of tesetaxel and any other products for which we may seek regulatory approval. Japanese pricing of prescription pharmaceuticals is subject to tight government control. Drug prices are set both according to standardized formulas and through negotiations between government officials and applicant companies on a product-by-product basis. In those negotiations, the level of innovation, usefulness and marketability are important determinants of price. With few exceptions, the Japanese MHLW sets the reimbursement prices for all newly launched prescription drugs in Japan.
There are currently three main pricing methodologies, which are dependent on the number of similar products available. The Cost Calculation Method applies to first-to-market products and is based on manufacturer cost inputs as well as international reference markets. Comparison Method I applies to products with less than three non-generic competitors and allows for premiums based on innovation, usefulness and marketability among others, plus an adjustment to international reference markets. Comparison Method II is reserved for products with three or more non-generic competitors and anchors price to the lowest competitor in the basket, with only downward adjustments possible to the international reference markets. Over 80% of products in Japan are priced through Comparison Method I.
In order to control the proportion of the country’s total healthcare expenditure that is devoted to drugs, the government mandates revisions in the prices of all prescription drugs every two years. However, pricing reform introduced in 2016 may have an impact on the commercial landscape in future years. There are three main changes in discussion, including annual pricing reviews, the addition of a cost-effectiveness analysis, and the “huge sellers” provision to reduce budget risk for expensive drugs with significant budget impact. These are expected to go into effect in 2018, with additional proposals under consideration for later roll out, including annual reference pricing, indication expansion discounts and optimal use guidelines. These changes may impact the timeline for pricing negotiations as well as the result of these negotiations. The adoption of more restrictive pricing and reimbursement policies along with existing controls could limit our commercial revenue in the Japanese market.
Rest of the World Regulation
For other countries outside of the U.S., EU and Japan, such as Eastern Europe, Latin America, Asia and emerging markets, the requirements governing the conduct of clinical studies, drug licensing, pricing and reimbursement vary from country to country. In all cases, clinical studies must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Corporate Conversion
On December 6, 2017, we converted from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion with the Delaware Secretary of State, and we changed our name from “Odonate Therapeutics, LLC” to “Odonate Therapeutics, Inc.” (the “Conversion”). As a result of the Conversion, the membership interests of Odonate Therapeutics, LLC were converted into shares of common stock of Odonate Therapeutics, Inc. Prior to the Conversion, we formed a holding company named Odonate Holdings, LLC (“Odonate Holdings”) to own Odonate Therapeutics, LLC. Following the Conversion, Odonate Holdings distributed its interest in Odonate Therapeutics, Inc. to prior members of Odonate Therapeutics, LLC, but retained record title to 2,931,402 shares of common stock underlying outstanding incentive units previously granted to employees, officers, directors and consultants by Odonate Management Holdings, LLC and 154,285 shares of common stock beneficially owned by Tang Capital Partners, LP.
32
Implications of Being an Emerging Growth Company
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under this act, an emerging growth company may take advantage of certain exemptions from various reporting requirements for up to 5 years that are otherwise applicable to public companies. These exemptions include, among others:
|
|
• |
an exemption from the auditor attestation requirement on the effectiveness of our system of internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002, as amended; |
|
|
• |
reduced disclosure about executive compensation arrangements; |
|
|
• |
an exemption from the requirement to seek non-binding advisory votes on executive compensation and golden parachute arrangements; and |
|
|
• |
an exemption from compliance with any new requirements adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer. |
We have availed ourselves of certain of the exemptions in this Annual Report on Form 10-K, and we expect to continue to avail ourselves of certain of the exemptions available to emerging growth companies in future filings with the U.S. Securities and Exchange Commission (the “SEC”) as long as we are an emerging growth company. We will remain an emerging growth company until December 31, 2023 unless, prior to that time, we: (i) have more than $1.07 billion in annual gross revenue; (ii) have a market value for shares of our common stock held by non-affiliates of more than $700 million as of the last day of our second quarter of any year; or (iii) issue more than $1.0 billion of non-convertible debt over a three-year period.
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the U.S. Securities Act of 1933 for complying with new or revised accounting standards. An emerging growth company can therefore delay the adoption of certain new or revised accounting standards issued subsequent to the enactment of the JOBS Act until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Employees
As of December 31, 2018, we had 130 employees, of which 117 were full-time employees. None of our employees are represented by labor unions or covered by collective bargaining agreements.
Corporate and Other Information
We are a Delaware corporation that was initially formed as a Delaware limited liability company in March 2013. On December 6, 2017, in anticipation of our initial public offering, we converted into a Delaware corporation. We currently operate in one segment.
Our principal executive offices are located at 4747 Executive Drive, Suite 510, San Diego, California, 92121, and our telephone number is (858) 731-8180. Our corporate website address is www.odonate.com. Information contained on or accessible through our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is an inactive textual reference only.
We file electronically with the SEC our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.odonate.com,
33
under “Corporate Resources,” free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC. The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below before deciding whether to purchase shares of our common stock. In assessing these risks, you should also refer to the other information contained in this Annual Report on Form 10-K, including our audited financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our business, financial condition, results of operations, cash flow and prospects could be materially and adversely affected by any of these risks or uncertainties. In any such case, the trading price of shares of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business
We are substantially dependent on our ability to successfully develop and commercialize tesetaxel.
Since our inception, we have invested substantially all of our capital resources on the development of tesetaxel, which we initially are developing for the treatment of locally advanced or metastatic breast cancer (“LA/MBC”). We are conducting a multinational, multicenter, randomized, Phase 3 study of tesetaxel in patients with human epidermal growth factor receptor 2 (“HER2”) negative, hormone receptor (“HR”) positive LA/MBC who have received no more than one chemotherapy regimen for advanced disease and have received a taxane in the neoadjuvant (prior to surgery) or adjuvant (immediately following surgery) setting. If the results of this study, known as CONTESSA, are negative or inconclusive, we may be unable to obtain regulatory approval for tesetaxel. Further, if CONTESSA is discontinued early due to safety issues or efficacy futility, we may be unable to obtain regulatory approval for tesetaxel. Additionally, even if the results of CONTESSA are positive, we cannot assure you that the U.S. Food and Drug Administration (“FDA”), the European Medicines Agency (“EMA”) or any other regulatory authority will approve tesetaxel for marketing.
Our ability to generate revenue and our future success depends in large part on the success of CONTESSA, the approval of tesetaxel, the nature of any potential requirements for post-approval studies and the successful commercialization of tesetaxel, if approved. Delays in obtaining regulatory approval for tesetaxel would, among other consequences, require further development expenditures, delay the launch of tesetaxel and impact our ability to raise additional capital, all of which would have a material adverse effect on our business and financial condition.
The commercial adoption of tesetaxel and any other product candidates we develop will depend on the degree of their market acceptance.
Even with the requisite approvals from the FDA, the EMA and other regulatory authorities, the commercial adoption of tesetaxel and any other product candidates we develop will depend on the degree of their acceptance by physicians, patients, third-party payors and others in the medical community. The degree of market acceptance will depend on a number of factors, including:
|
|
• |
the safety and efficacy of the product as demonstrated in clinical studies; |
|
|
• |
the perception of physicians, patients, third-party payors and others in the medical community of the relative safety, efficacy, convenience, effect on quality-of-life and cost-effectiveness of the product, compared to those of other available treatments; |
34
|
|
• |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
|
|
• |
the strength of marketing and distribution support and timing of market introduction of competitive products; |
|
|
• |
the publicity concerning our products or competing products and treatments; |
|
|
• |
product liability litigation alleging injuries relating to our products or similar classes of drugs; |
|
|
• |
our ability to access third parties to manufacture or distribute our products on acceptable terms or at all; |
|
|
• |
any post-approval study requirements for our products and the results thereof; and |
|
|
• |
sufficient third-party insurance coverage and reimbursement. |
Even if a potential product such as tesetaxel displays a favorable efficacy and safety profile in nonclinical and clinical studies, market acceptance of the product is not fully known until after its commercial launch. Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our product candidates may require significant resources and may never be successful. If tesetaxel or other product candidates are approved but fail to achieve an adequate level of acceptance by physicians, patients, third-party payors and others in the medical community, we will not be able to generate sufficient revenue to become or remain profitable.
We have only limited assets and will need to raise additional capital before we can expect to generate revenue or become profitable.
As of December 31, 2018, we had no revenue, an accumulated deficit of $128.3 million and cash of $139.1 million. We believe that our existing cash as of December 31, 2018 will be sufficient to meet our anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (the “SEC”). However, to fund future operations to the point at which we are able to generate positive cash flow from sales of tesetaxel or other potential product candidates, we will need to raise significant additional capital. The amount and timing of future funding requirements will depend on many factors, including the timing and results of our ongoing development efforts, the potential expansion of our current development programs, potential new development programs and related general and administrative support. We anticipate that we will seek to fund our operations through public and private equity and debt financings or other sources, such as potential licenses or other collaboration agreements. We cannot assure you that anticipated additional financing will be available to us on favorable terms, or at all. Although we have been successful in obtaining financing through the issuance of our equity securities, we cannot assure you that we will be able to do so in the future. If we are unable to raise additional capital to fund our clinical development and commercialization of tesetaxel, if approved, and other business activities, we could be forced to abandon one or more programs and curtail or cease our operations.
We have never generated any revenue and may never be profitable.
We have no products approved for marketing, have never generated any revenue from product sales and have incurred losses in each year since our inception. Our ability to generate revenue and achieve profitability depends on our ability, alone or with marketing partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, tesetaxel. We expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase as we
35
continue to develop tesetaxel. Our ability to generate revenue from product sales depends heavily on our success in many areas, including but not limited to:
|
|
• |
successfully completing the development of tesetaxel; |
|
|
• |
obtaining regulatory approvals to market tesetaxel; |
|
|
• |
successfully managing third-party service providers involved in the manufacturing and development of tesetaxel; |
|
|
• |
successfully commercializing tesetaxel, either independently or with marketing partners; |
|
|
• |
obtaining market acceptance of tesetaxel, including garnering market share from existing and future treatment alternatives; |
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
|
|
• |
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how; and |
|
|
• |
attracting, hiring and retaining qualified personnel. |
If tesetaxel or any other product candidates we may develop are approved for marketing, we anticipate incurring significant commercialization costs. Our expenses could increase beyond our current expectations if we are required by the FDA, the EMA or other regulatory authorities to change our manufacturing processes or quality procedures or perform additional or unanticipated nonclinical, clinical or other studies. In cases where we are successful in obtaining regulatory approvals to market tesetaxel or other product candidates, our revenue will be dependent, in part, on the size of the markets in the territories for which we gain regulatory approval, the acceptance of the price of the product in those markets and the ability to obtain reimbursement at any price. If the number of our addressable patients is not as significant as we estimate, the indication approved by regulatory authorities is narrower than we expect or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of such products, even if approved. If we are not able to generate revenue from the sale of approved products, we may never become profitable.
We currently have no sales, marketing or distribution capabilities. If we elect to commercialize tesetaxel ourselves and we are unable to establish effective sales, marketing or distribution capabilities or if we are unable to enter into agreements with third parties to commercialize tesetaxel or other product candidates that we may develop, we may not be able to effectively generate product revenues.
We currently do not have sales, marketing or distribution capabilities. In order to commercialize tesetaxel, if approved, or any other product candidates that we may develop, we must build marketing, sales and distribution capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. If tesetaxel receives regulatory approval and we decide to commercialize tesetaxel ourselves, building the requisite sales, marketing or distribution capabilities will be expensive and time-consuming and will require significant attention of our leadership team to manage. Any failure or delay in the development of our sales, marketing or distribution capabilities would adversely affect the commercialization of any product. The competition for talented individuals experienced in selling and marketing pharmaceutical products is intense, and we cannot assure you that we can assemble an effective team. Additionally, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties on the commercialization of tesetaxel or any other product candidates that we may develop. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations.
36
We may be subject to additional risks related to operating in foreign countries either ourselves or through a third-party, including:
|
|
• |
differing regulatory requirements in foreign countries; |
|
|
• |
unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; |
|
|
• |
economic weakness, including inflation or political instability in particular foreign economies and markets; |
|
|
• |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
|
|
• |
foreign taxes, including withholding of payroll taxes; |
|
|
• |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
|
|
• |
difficulties staffing and managing foreign operations; |
|
|
• |
workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
|
|
• |
potential liability under the Foreign Corrupt Practices Act of 1977 or comparable foreign regulations; |
|
|
• |
challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.; |
|
|
• |
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
|
|
• |
business interruptions resulting from geopolitical actions, including war and terrorism. |
If we are not successful in commercializing tesetaxel or other product candidates, our future product revenue will suffer and we may incur significant additional losses.
Because a number of companies compete with us, many of which have greater resources than we do, and because we face rapid changes in science in our industry, we cannot be certain that our products will be accepted in the marketplace or capture market share.
Competition from other biotechnology and pharmaceutical companies is intense and is expected to increase. A number of companies are pursuing the development of pharmaceuticals in oncology, our area of focus. Many of these companies are very large, and have financial, technical, sales and distribution and other resources substantially greater than ours. The greater resources of these competitors may enable them to develop, obtain regulatory approval for or market competing products more quickly or effectively, making it extremely difficult for us to capture a share of the market for our products. Additionally, the biotechnology and pharmaceutical industries are subject to rapid changes in science, and our competitors may develop and market products with improved therapeutic profiles relative to our product candidates that would render our product candidates noncompetitive.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, reduce the commercial attractiveness of a prescribing label or result in significant negative consequences following regulatory approval, if approved.
Clinical studies of tesetaxel or other product candidates we may develop could reveal a high and unacceptable incidence and severity of undesirable side effects. Undesirable side effects could adversely affect patient enrollment in clinical studies, cause us or regulatory authorities to interrupt, delay or halt clinical studies or result in the delay, denial or withdrawal of regulatory approval by the FDA, the EMA or other regulatory authorities. For example, in 2007, tesetaxel was placed on clinical hold by the FDA while
37
in development by the original sponsor due to the occurrence of several fatalities in the setting of severe neutropenia (a low level of neutrophils, a type of white blood cell) in patients with advanced cancer. While this clinical hold was lifted in 2008, and tesetaxel has since been evaluated in multiple clinical studies in 300 patients without any interruption due to safety issues, we cannot assure you that safety-related interruptions in tesetaxel’s clinical development will not occur again in the future. Any such recurrence could potentially delay or prevent the ultimate approval of our product candidate. Undesirable or adverse side effects also could result in regulatory authorities mandating a more restrictive prescribing label for the product, which, in turn, could limit the market acceptance of the product even if approved for marketing and commercialization.
Drug-related side effects could result in potential product liability claims. We carry product liability insurance in the amount of $10.0 million in the aggregate. We believe our product liability insurance coverage is sufficient in light of our clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts or maintain coverage at all to protect us against losses due to liability. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations, business and financial condition. In addition, regardless of merit or eventual outcome, product liability claims may result in impairment of our business reputation, withdrawal of clinical study participants, costs due to related litigation, distraction of management’s attention from our primary business, initiation of investigations by regulators, substantial monetary awards to patients or other claimants, the inability to commercialize our product candidates and decreased demand for our product candidates, if approved for marketing.
Additionally, if one or more of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including but not limited to:
|
|
• |
the withdrawal of approvals by regulatory authorities; |
|
|
• |
the requirement of additional warnings on the prescribing label; |
|
|
• |
the requirement of a Risk Evaluation and Mitigation Strategy plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers and/or other elements to assure safe use; |
|
|
• |
litigation and the potential to be held liable for harm caused to patients; and |
|
|
• |
an adverse effect on our reputation. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate and could significantly harm our business, results of operations, financial condition and prospects.
We may not be successful in our efforts to identify, in-license or acquire, discover, develop or commercialize additional product candidates.
Although a substantial amount of our effort will focus on the development and potential commercialization of tesetaxel, we also may seek to identify, in-license or acquire, discover, develop and commercialize additional product candidates in the oncology field. We cannot assure you that our efforts to in-license or acquire additional product candidates will be successful. Even if we are successful in in-licensing or acquiring additional product candidates, their requisite development activities may require substantial resources, and we cannot assure you that these development activities will result in regulatory approvals.
38
We will need to increase the size and capabilities of our organization, and we may experience difficulties in managing our growth.
Odonate was formed in 2013 and had 130 employees as of December 31, 2018. As we advance the development of tesetaxel, we must continue to grow the size of the organization. Future growth will impose significant added responsibilities on members of management, including:
|
|
• |
identifying, recruiting, integrating, retaining and motivating additional employees; |
|
|
• |
effectively managing our development efforts, including the clinical development and FDA, EMA or other regulatory authority review processes for our product candidates; |
|
|
• |
effectively managing our third-party service providers involved in the development and manufacture of our product candidates; and |
|
|
• |
improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance and our ability to successfully develop and commercialize our product candidates will depend, in part, on our ability to effectively manage any future growth. Our management will have to dedicate a significant amount of its attention to managing these growth activities. In addition, we expect to incur additional costs in hiring, training and retaining such additional personnel.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully execute the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
Our future success depends on our ability to retain our key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our management and scientific teams. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we grant equity awards that vest over time. The value to employees of these equity awards that vest over time may be significantly affected by changes in the price of shares of our common stock that are beyond our control, and may at any time be insufficient to retain employees who receive more lucrative offers from other companies. Any of our employees could leave our employment at any time, with or without notice.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel or consultants will also be critical to our success. We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our discovery, development and commercialization strategies. The loss of the services of any of our executive officers, key employees or consultants could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy.
Replacing executive officers, key employees or consultants may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel or consultants on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel.
39
We may hire part-time employees or use consultants. As a result, certain of our employees, officers, directors or consultants may not devote all of their time to our business, and may from time to time serve as employees, officers, directors and consultants of other companies.
Our business and operations may be materially adversely affected in the event of computer system failures or security breaches.
Despite the implementation of security measures, our internal computer systems, and those of our third-party contract research organizations (“CROs”) and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyber-attacks, natural disasters, fire, terrorism, war and telecommunication and electrical failures. If such an event were to occur and interrupt our operations, it could result in a material disruption of our development programs. For example, the loss of data from ongoing or planned clinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, loss of trade secrets or inappropriate disclosure of confidential or proprietary information, including protected health information or personal data of employees or former employees, access to our clinical data or disruption of the manufacturing process, we could incur liability and the further development of our product candidates could be delayed. We may also be vulnerable to cyber-attacks by hackers or other malfeasance. This type of breach of our cybersecurity may compromise our confidential information or our financial information and adversely affect our business or result in legal proceedings.
Risks Related to Our Industry
Drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Clinical testing is expensive, can take many years to complete and its outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of nonclinical studies and early clinical studies of our product candidates may not be predictive of the results of later-stage clinical studies. Product candidates that have shown promising results in early-stage clinical studies may still suffer significant setbacks in subsequent clinical studies. For example, the safety or efficacy results generated to date in our clinical studies do not ensure that later clinical studies will demonstrate similar results. There is a high failure rate for pharmaceutical product candidates proceeding through clinical studies, and product candidates in later stages of clinical studies may fail to show the desired safety and efficacy, despite having progressed through nonclinical studies and initial clinical studies.
A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Moreover, nonclinical and clinical data often are susceptible to varying interpretations and analyses. We do not know whether any clinical studies we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain regulatory approval to market our product candidates.
Results from CONTESSA and any other clinical studies we may undertake may not be sufficient to obtain regulatory approvals to market our product candidates on a timely basis, if at all.
Pharmaceutical product candidates are subject to extensive government regulations related to development, clinical studies, manufacturing and commercialization. In order to sell any product that is under development, we must first receive regulatory approval. To obtain regulatory approval, we must conduct nonclinical and clinical studies that demonstrate that our product candidates are safe and effective. The process of obtaining FDA, EMA and other regulatory authority approvals is costly, time-consuming, uncertain and subject to unanticipated delays.
40
The FDA, EMA and other regulatory authorities have substantial discretion in the approval process and may not agree that we have demonstrated that our product candidates are safe and effective. If our product candidates are ultimately not found to be safe and effective, we would be unable to obtain regulatory approval to manufacture, market and sell them. We can provide no assurances that the FDA, EMA or other regulatory authorities will approve our product candidates or, if approved, what the scope of the approved indication might be.
CONTESSA and other clinical studies that we may undertake may be delayed or halted.
CONTESSA and any other clinical studies of our product candidates that we may conduct in the future may be delayed or halted for various reasons, including:
|
|
• |
insufficient financial resources; |
|
|
• |
insufficient supplies of drug product to treat the patients in the studies; |
|
|
• |
failure of patients to enroll in the studies at the rate we expect; |
|
|
• |
ineffectiveness of our product candidates; |
|
|
• |
patients experiencing unexpected side effects or other safety concerns being raised during treatment; |
|
|
• |
changes in governmental regulations or administrative actions; |
|
|
• |
failure to conduct studies in accordance with required clinical practices; |
|
|
• |
inspection of clinical study operations or study sites by the FDA or other regulatory authorities, resulting in a clinical hold; |
|
|
• |
political unrest at foreign clinical sites; |
|
|
• |
a shutdown of the U.S. government, including the FDA; or |
|
|
• |
natural disasters at any of our clinical sites. |
If studies are delayed or halted, we may incur significant additional expenses, and the potential approval of our product candidates may be delayed, which would have a material adverse effect on our business and financial condition.
Even if we obtain regulatory approval for tesetaxel or another product candidate, our products will remain subject to regulatory scrutiny.
If tesetaxel or other product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information.
Manufacturers and manufacturers’ facilities are required to comply with extensive FDA, EMA and other regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices (“cGMP”) regulations. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMPs and adherence to commitments made in any New Drug Application (“NDA”), Market Authorization Application (“MAA”) or other marketing application. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals we receive for our product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical studies,
41
which must comply with applicable Good Clinical Practice (“GCP”) regulations. We could also be asked to conduct post-marketing clinical studies to verify the safety and efficacy of our products in general or in specific patient subsets. If initial regulatory approval was obtained via the accelerated approval pathway, we could be required to conduct a successful post-marketing clinical study to confirm clinical benefit for our products. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of regulatory approval. We will be required to report certain adverse reactions and production problems, if any, to the FDA, EMA and other regulatory authorities. Any new legislation addressing drug safety or approval issues could result in delays in product development or commercialization, or increased costs to assure compliance. We will have to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have approval. The holder of an approved NDA or MAA must submit new or supplemental applications and obtain approval for certain changes to the approved product, product labeling or manufacturing process.
If a regulatory authority discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, such regulatory authority may impose restrictions on that product or us. If we fail to comply with applicable regulatory requirements, a regulatory or enforcement authority may, among other things:
|
|
• |
issue warning or untitled letters; |
|
|
• |
impose civil or criminal penalties; |
|
|
• |
suspend or withdraw regulatory approval; |
|
|
• |
suspend any of our ongoing clinical studies; |
|
|
• |
refuse to approve pending applications or supplements to approved applications submitted by us; |
|
|
• |
impose restrictions on our and our contract manufacturers’ operations, including closing manufacturers’ facilities; |
|
|
• |
seize or detain products; or |
|
|
• |
require a product recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, this would have a material adverse effect on our business and financial condition.
If we are unable to achieve and maintain coverage and adequate levels of reimbursement for tesetaxel and other product candidates, if approved, their commercial success may be severely hindered.
Successful sales of tesetaxel and any other product candidates that may receive regulatory approval depend on the availability of coverage and adequate reimbursement from third-party payors. Patients who are prescribed medications for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Coverage decisions may depend on clinical and economic standards that disfavor new products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Assuming we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate or may require co-
42
payments that patients find unacceptably high. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products.
The market for tesetaxel and any other product candidates that we attempt to commercialize will depend significantly on access to third-party payors’ drug formularies or lists of medications for which third-party payors provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical products. Also, third-party payors may refuse to include a particular branded drug in their formularies or otherwise restrict patient access through formulary controls or otherwise to a branded drug when a less costly generic equivalent or other alternative is available.
Third-party payors, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the U.S., no uniform policy requirement for coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
Third-party coverage and reimbursement for our product candidates for which we may receive regulatory approval may not be available or adequate in either the U.S. or international markets, which could have a material adverse effect on our business, results of operations, financial condition and prospects.
We will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy, security laws and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and physician sunshine laws and regulations. These laws will impact, among other things, our clinical development, proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The U.S. laws that will affect our ability to operate include:
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
|
|
• |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
43
|
|
• |
HIPAA, as amended by the federal Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
|
• |
the federal physician sunshine requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, (collectively, the “PPACA”), which require manufacturers of drugs, devices, biologics and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and |
|
|
• |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available thereunder, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare reform legislation has strengthened these laws. For example, the PPACA, among other things, amended the previous intent requirement of the federal Anti-Kickback Statute and criminal healthcare fraud statutes. Now, a person or entity does not have to have actual knowledge of the statutes or specific intent to violate them. The PPACA also provides that the federal government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of these laws or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if a person becomes subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Current and future legislation may increase the difficulty and cost of obtaining regulatory approval, and the subsequent commercialization, of our product candidates, if approved, and may affect the prices we may obtain.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain regulatory approval.
44
For example, in 2010, President Obama signed into law the PPACA, which contains provisions, among others, that may impact our potential product candidates, including:
|
|
• |
an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; |
|
|
• |
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
|
|
• |
expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance; |
|
|
• |
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices; |
|
|
• |
extension of manufacturers’ Medicaid rebate liability; |
|
|
• |
expansion of eligibility criteria for Medicaid programs; |
|
|
• |
new requirements to report financial arrangements with physicians and teaching hospitals; |
|
|
• |
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; |
|
|
• |
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
|
|
• |
the Independent Payment Advisory Board, which, if created, would have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs; and |
|
|
• |
a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending. |
Other legislative changes have been proposed and adopted since the PPACA was enacted. These changes included aggregate reductions of Medicare payments to providers of up to two percent per year. Additionally, the American Taxpayer Relief Act of 2012 reduced Medicare payments to certain providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. There have been judicial, Congressional and Executive Branch challenges to certain aspects of the PPACA, and we expect there will be additional challenges and/or amendments to the PPACA in the future. Further, it is possible that additional regulatory changes, as well as the repeal (in whole or in part) of the PPACA, could negatively affect insurance coverage and/or drug prices. These new laws also may result in additional reductions in Medicare and other healthcare funding as well as insurance coverage and payments.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the reimbursement received for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Moreover, there recently has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Individual states in the U.S. have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing,
45
including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. Additionally, legislation has been introduced to repeal the PPACA. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the regulatory approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent regulatory approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Governments outside the U.S. tend to impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly certain countries of the European Union (“EU”), the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of regulatory approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical study that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
Risks Relating to Our Reliance on Third Parties
We rely on third parties to conduct our nonclinical and clinical studies. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates, and our business could be substantially harmed.
We have agreements with third-party CROs to monitor and manage data for several nonclinical and clinical programs. We rely heavily on these third parties for execution of our nonclinical and clinical studies and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with cGCPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these cGCPs through periodic inspections of study sponsors, principal investigators and study sites. If we, the investigators, the sites or any of these CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical studies may be deemed unreliable and the regulatory authorities may require us to perform additional clinical studies before approving our marketing applications. We cannot assure you that, upon inspection, such regulatory authorities will determine that any of our clinical studies comply with the cGCP regulations. In addition, our clinical studies must be conducted with product produced under cGMP regulations. Failure to comply with these regulations may require us to repeat clinical studies, which would delay or compromise the regulatory approval process.
If our relationships with any of these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing nonclinical and clinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other
46
reasons, our clinical studies may be extended, delayed or terminated. As a result, we may not be able to obtain regulatory approval for, or successfully commercialize, our product candidates and may incur significant additional expenses. In addition, potential approval of our product candidates may be delayed, which would have a material adverse effect on our business, results of operations and financial condition.
If the third-party manufacturers on which we rely fail to produce our product candidates on a timely basis or comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the studies, regulatory submissions, required approvals or commercialization of our product candidates.
We contract with third-party contract development and manufacturing organizations (“CDMOs”) to manufacture our product candidate, and we would expect to rely on these CDMOs to produce commercial quantities of tesetaxel. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, which include difficulties with production costs and yields, quality control and assurance and shortages of qualified personnel, as well as compliance with strictly enforced governmental regulations, including cGMPs. The CDMOs we contract with may not perform as agreed or may terminate their agreements with us.
In addition to product approval, any facilities in which our product candidates are manufactured or tested for their ability to meet required specifications must be inspected and approved by regulatory authorities before a commercial product can be manufactured. Failure of such a facility to be approved could delay the approval of one or more of our product candidates.
Any of these factors could cause us to delay or suspend any clinical studies, regulatory submissions, required approvals or commercialization of one or more of our product candidates, entail higher costs and result in our being unable to effectively commercialize products.
Risks Relating to Intellectual Property
Our success in developing and marketing our product candidates depends significantly on our ability to obtain and maintain patent protection and operate without infringing on the rights of others.
We depend on patents and other intellectual property to prevent others from improperly benefiting from products or inventions that we developed or acquired. Our patents and patent applications cover our product candidates and inventions. The intellectual property portfolio protecting our tesetaxel program includes 9 U.S., 5 European and 7 Japanese patents, as well as one pending U.S. patent application and one pending international patent application. Of those, 5 U.S., 4 European and 6 Japanese patents are exclusively licensed to us by Daiichi Sankyo. Among the licensed patents, one issued U.S. patent (U.S. Patent No. 7,410,980) covers the crystal form of tesetaxel used in our clinical formulation and will expire in 2026. If tesetaxel is approved by the FDA, we will be entitled to request patent term restoration that could extend the protection of this patent until 2031. The exact duration of the extension depends on the time we spend in clinical studies as well as the time the FDA spends reviewing our NDA. See “Business—Government Regulation—U.S. Patent Term Restoration.” The licensed portfolio includes 4 other issued U.S. patents that cover compositions of matter and various methods useful for preparing tesetaxel, as well as European and Japanese counterparts of these U.S. patents. We also own 4 U.S. patents that cover additional methods useful for preparing tesetaxel and certain salt and crystal forms of tesetaxel, as well as one pending U.S. patent application and one pending international application related to tesetaxel. If the international patent application results in an issued U.S. patent, that patent would expire in 2038.
47
The patent position of pharmaceutical firms like ours is highly uncertain and involves complex legal and factual questions. We intend to continue to file patent applications because we believe it is appropriate to obtain patents covering our products and their manufacture and use. There can be no assurance, however, that any additional patents will issue, that the scope of any patent that has issued or may issue will be sufficient to protect our product candidates, or that any current or future issued patent will be held not invalid if subsequently challenged. Additionally, we may have to incur significant expense and expend management time defending or enforcing our patents. If we cannot obtain and maintain effective patent rights and/or regulatory exclusivity for our product candidates, we may not be able to compete effectively, and our business and results of operations would be harmed.
The scope and terms of our patents may be insufficient to protect our product candidates for an adequate amount of time.
In the U.S., the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.
Patents may be eligible for limited patent term extension in the U.S. under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. Similar patent extensions exist in the EU and Japan. The Hatch-Waxman Act permit a patent term extension of up to 5 years for a patent covering an approved product as compensation for patent term that elapsed during product development and the FDA regulatory review process, provided the extension does not extend the total patent term beyond 14 years from approval, and only one patent per approved product is extended. We may not receive an extension if we fail to apply within applicable deadlines or fail to apply prior to expiration of relevant patents. Moreover, the length of the extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner, impacting our revenue.
If the FDA or foreign regulatory authorities approve generic versions of any of our products that receive marketing approval or such authorities do not grant our products appropriate periods of exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
NDA applicants are required to list with the FDA each patent with claims covering the applicant’s product or method of using the product for which approval is sought. Upon approval of a drug, each of the patents listed in the application for the drug that cover the drug or an approved use of the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic or 505(b)(2) NDA applicants in support of approval of an Abbreviated New Drug Application (“ANDA”) or a 505(b)(2) NDA. An ANDA is a streamlined way to seek approval for marketing a drug product that has the same active ingredient in the same strength and dosage form as the listed drug and has been shown to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct or submit results of nonclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way can often be substituted by pharmacists under prescriptions written for the original listed drug.
Both ANDA and 505(b)(2) NDA applicants are required to make a certification to the FDA concerning any patents listed for the approved NDA product in the FDA’s Orange Book. Specifically, ANDA and 505(b)(2) NDA applicants must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the
48
new product. The ANDA applicant may also elect to submit a statement certifying that its proposed ANDA labeling does not contain (or carves out) any language regarding patented methods-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the submitted application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the proposed product will not infringe the already approved product’s listed patents, or that such patents are invalid or unenforceable, is called a Paragraph IV certification. If the applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) NDA has been received by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving a submitted application until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant or 505(b)(2) applicant.
In addition to the protections from competitors that may be afforded by patents, pharmaceutical products may also be eligible for regulatory exclusivity, such as the exclusivity that may be granted to New Chemical Entities (“NCEs”). While we believe that tesetaxel will qualify as an NCE if and when an NDA is submitted for tesetaxel, such determination is only made at, or after, the time of approval. Accordingly, we do not have any agreement with the FDA, EMA or other regulatory body that tesetaxel will in fact be regarded as an NCE, and there can be no assurance that it will be treated as such at the time of approval (if approval is granted).
Competition that our products may face from generic versions of our products could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
If we fail to comply with our obligations under our licenses, we may lose rights to critical patents that are important to the commercialization and revenue potential of tesetaxel.
We have licensed patent rights covering tesetaxel from Daiichi Sankyo. If, for any reason, our license agreement with Daiichi Sankyo is terminated or we otherwise lose those rights, it could adversely affect our business. Our license agreement with Daiichi Sankyo imposes, and any future collaboration agreements or license agreements we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement or other obligations on us. Failure to fulfill these obligations could pose a material risk to our patent protection for tesetaxel and any future product candidates.
If our product candidates infringe the rights of others, we could be subject to expensive litigation, become liable for substantial damages, be required to obtain licenses from others or be prohibited from selling our product candidates altogether.
Our competitors or others may have patent rights that they choose to assert against us or our licensors, licensees, suppliers, customers or potential marketing partners. Moreover, we may not know about patents or patent applications that our products would infringe. Because patent applications do not publish for at least 18 months, if at all, and can take many years to issue, there may be currently pending applications unknown to us that may later result in issued patents that our product candidates would infringe. In addition, if third parties file patent applications or obtain patents claiming inventions also claimed by us or our licensors in issued patents or pending applications, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office (“USPTO”) to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of our foreign patent applications.
49
If a third party claims that we infringe its proprietary rights, any of the following may occur:
|
|
• |
we may become involved in time-consuming and expensive litigation, even if the claim is without merit; |
|
|
• |
we may become liable for substantial damages for past infringement if a court decides that our science infringes a competitor’s patent; |
|
|
• |
a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross licenses to our patents; or |
|
|
• |
we may have to redesign our product candidates so that they do not infringe patent rights of others, which may not be possible or commercially feasible. |
Any of these events would have a material adverse effect on our business, results of operations and financial condition.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the U.S. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we or our licensors were the first to make the inventions claimed in our owned and licensed patents or pending applications, or that we or our licensor were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the U.S., prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while, outside the U.S., the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (“Leahy-Smith Act”), enacted on September 16, 2011, the U.S. has moved to a first-to-file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations and financial condition.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and provide new opportunities for third parties to challenge issued patents in the USPTO. We may be subject to the risk of third-party prior art submissions on pending applications or become a party to opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patents for tesetaxel. There is a lower standard of evidence necessary to invalidate a patent claim in a USPTO proceeding relative to the standard in U.S. district or federal court. This could lead third parties to challenge and successfully invalidate our patents that would not otherwise be invalidated if challenged through the court system.
In addition to patent protection, we will need to successfully preserve our trade secrets. If we are unable to maintain effective proprietary rights for tesetaxel or any future product candidates, we may not be able to compete effectively in our markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve information or know-how that is not covered by
50
patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary science and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, there remains the possibility that agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. For instance, the FDA has introduced its Transparency Initiative and is currently considering whether to publicly disclose additional information from drug sponsors; in such case, we cannot guarantee that our trade secrets will not be disclosed.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, science or information to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed, that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating our trade secrets.
Risks Related to Ownership of Shares of Our Common Stock
The price of shares of our common stock may be volatile, and you may lose all or part of your investment.
The market price of shares of our common stock could fluctuate significantly, and you may not be able to resell your shares near, at or above the price that you purchased them. Those fluctuations could be based on various factors in addition to those otherwise described in this Annual Report on Form 10-K, including those described under “Risk Factors—Risks Related to Our Business,” “Risk Factors—Risks Related to Our Industry,” “Risk Factors—Risks Related to Our Reliance on Third Parties” and “Risk Factors—Risks Related to Intellectual Property,” and the following:
|
|
• |
unfavorable developments relating to the regulatory status of our product candidates, such as the FDA refusing to accept for filing our NDA or issuing a complete response letter, or a delay in the regulatory review process; |
|
|
• |
adverse actions taken by regulatory authorities with respect to our clinical studies, manufacturing supply chain or future sales and marketing activities; |
|
|
• |
unfavorable results from our clinical studies; |
|
|
• |
delays in the initiation or completion of our clinical studies; |
|
|
• |
adverse changes to our relationships with third-party service providers; |
|
|
• |
manufacture, supply or distribution shortages; |
|
|
• |
departures of our management; |
|
|
• |
a change in competitive landscape that is unfavorable to our product candidates; |
|
|
• |
actual or threatened intellectual property litigation that involves our product candidates; |
|
|
• |
adverse developments concerning the pharmaceutical industry in general; |
|
|
• |
higher-than-expected expenses related to our development programs or overall corporate operations; |
|
|
• |
financial results that are not in line with analyst expectations; |
51
|
|
• |
press reports or other negative publicity, whether or not true, about our business; |
|
|
• |
release or expiry of lock-up or other transfer restrictions on our outstanding shares of common stock; |
|
|
• |
sales or perceived potential sales of additional shares of common stock; |
|
|
• |
sales of shares of our common stock by us, our executive officers and directors or our stockholders in the future; |
|
|
• |
fluctuations in the stock prices of pharmaceutical and biotechnology stocks; and |
|
|
• |
general economic and market conditions and overall fluctuations in the U.S. equity markets. |
Any of these factors may result in large and sudden changes in the volume and trading price of shares of our common stock. In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of management, result in negative press reports and, if adversely determined, have a material adverse effect on our results of operations and financial condition.
In addition, the Nasdaq Global Select Market, in general, and the stocks of small pharmaceutical and biotechnology companies, in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of shares of our common stock, regardless of our actual operating performance. Further, a decline in the financial markets and related factors beyond our control may cause the market price of shares of our common stock to decline rapidly and unexpectedly.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC.
Disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Future sales of shares of our common stock, or the perception that such sales may occur, could depress the market price of shares of our common stock.
We will need additional capital in the future to continue our planned operations. Sales of a substantial number of shares of our common stock in the public market, or the perception that such sales may occur, could depress the market price of shares of our common stock. All of our outstanding shares are freely tradable except for any shares held or acquired by persons who may be deemed to be our affiliates. Shares of our common stock held by our affiliates continue to be subject to the volume and other restrictions of Rule 144 under the U.S. Securities Act of 1933 (the “Securities Act”). Sales of a substantial
52
number of such shares, or the perception that such sales may occur, could cause our market price to fall or make it more difficult for you to sell your shares of common stock at a time and price that you deem appropriate.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of shares of our common stock for return on your investment.
We intend to retain most, if not all, of our available funds and earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in shares of our common stock as a source for any future dividend income.
Our board of directors (“Board”) has significant discretion as to whether to distribute dividends and in what amounts. Even if our Board decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our Board.
Our directors, executive officers and principal stockholders have substantial control over the Company, which could limit your ability to influence the outcome of key transactions, including a change of control.
Our current directors, officers and stockholders who own greater than 5% of our outstanding shares of common stock, together with their affiliates, beneficially own, in the aggregate, approximately 72% of our outstanding shares of common stock, based on the number of shares outstanding as of February 4, 2019. As a result, these current directors, officers and stockholders, if they act, will be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers, acquisitions or other extraordinary transactions. In addition, our current directors, officers and stockholders, acting together, would have the ability to control the management and affairs of our company. They may also have interests that differ from yours and may vote in a way with which you disagree and that may be adverse to your interests. This concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their shares of common stock as part of a sale of our company and could affect the market price of shares of our common stock.
We are a controlled company within the meaning of the Nasdaq rules, and, as a result, we qualify for and rely on exemptions from certain corporate governance requirements.
We are considered a "controlled company" for the purposes of Nasdaq rules and corporate governance standards because Tang Capital Partners, LP, together with its affiliates, controls more than 50% of our voting power. As a controlled company, we are exempt from certain Nasdaq corporate governance requirements, including requirements that our Board have a majority of independent directors and that we either establish Compensation and Nominating and Corporate Governance Committees comprised entirely of independent directors, or otherwise ensure that the compensation of our executive officers and nominees for directors are determined or recommended to the Board by the independent members of the Board. While we have established Compensation and Nominating and Corporate Governance Committees comprised entirely of independent directors, our Board does not currently have a majority of independent directors. Accordingly, our stockholders do not have the same protections as stockholders of companies that are subject to all of the Nasdaq corporate governance requirements.
53
We are an emerging growth company, as defined in the Securities Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make shares of our common stock less attractive to investors.
We are an emerging growth company, as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we may take advantage of certain exemptions from various reporting requirements that are otherwise applicable to public companies, including, among others, an exemption from the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended; reduced disclosure about executive compensation arrangements in our registration statements, periodic reports and proxy statements; and an exemption from the requirement to seek non-binding advisory votes on executive compensation and golden parachute arrangements. As a result, our stockholders may not have access to certain information that they may deem important. We will remain an emerging growth company until December 31, 2023 unless, prior to that time, we: (i) have more than $1.07 billion in annual gross revenue; (ii) have a market value for shares of our common stock held by non-affiliates of more than $700 million as of the last day of our second quarter of any year; or (iii) issue more than $1.0 billion of non-convertible debt over a three-year period.
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. An emerging growth company can therefore delay the adoption of certain new or revised accounting standards issued subsequent to the enactment of the JOBS Act until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We cannot predict if investors will find shares of our common stock less attractive because we may rely on these exemptions. If some investors find shares of our common stock less attractive as a result, there may be a less active trading market for shares of our common stock and our stock price may be more volatile.
Item 1B. Unresolved Staff Comments
Not applicable.
Our principal executive offices are located at 4747 Executive Drive, Suite 510, San Diego, California 92121 (the “San Diego Lease”). We also maintain offices at 3 East 28th Street, New York, New York 10016 (the “New York Lease”). We lease approximately 8,365 square feet of office space in San Diego and approximately 5,575 square feet of office space in New York. The San Diego Lease expires on December 31, 2019. The New York Lease expires on February 28, 2025, and we have an option to extend the New York Lease for an additional three years at the end of the initial term.
We are not currently a party to any material legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
54
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Shares of our common stock began trading on the Nasdaq Global Select Market on December 7, 2017 under the symbol “ODT.” Prior to that time, there was no public market for shares of our common stock.
Holders of Record
As of February 4, 2019, we had 4 holders of record of shares of our common stock. Certain shares of common stock are held in “street” name, and, accordingly, the number of beneficial owners of such shares of common stock is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid any cash dividends on shares of our common stock and do not currently intend to do so for the foreseeable future. Any determination to pay dividends to holders of shares of our common stock will be at the discretion of our Board and will depend on many factors, including our financial condition, results of operations, projections, liquidity, earnings, legal requirements, restrictions in the agreements governing any indebtedness we may enter into and other factors that our Board deems relevant.
Use of Proceeds from Initial Public Offering
On December 11, 2017, we closed our initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters exercised their option to purchase 441,073 additional shares of common stock in the Company’s IPO. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs. The offer and sale of the shares of common stock in the IPO were registered pursuant to a registration statement on Form S-1 (File No. 333-221533), which the SEC declared effective on December 6, 2017.
There has been no material change in the use of proceeds from our IPO as described in our final prospectus filed with the SEC pursuant to Rule 424(b)(4) on December 8, 2017.
Item 6. Selected Financial Data
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
55
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our audited financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, include forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” set forth in this Annual Report on Form 10-K for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. Our initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several pharmacologic properties that make it unique among taxanes, including: oral administration with a low pill burden; a long (~8-day) terminal plasma half-life in humans, enabling the maintenance of adequate drug levels with relatively infrequent dosing; no history of hypersensitivity (allergic) reactions; and significant activity against chemotherapy-resistant tumors. In patients with metastatic breast cancer, tesetaxel was shown to have significant, single-agent antitumor activity in two multicenter, Phase 2 studies. We are conducting a multinational, multicenter, randomized, Phase 3 study in patients with locally advanced or metastatic breast cancer, known as CONTESSA, and we expect to report top-line results from this study in 2020. Our goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
Components of Our Results of Operations
Research and Development Expense
Research and development expense consists primarily of costs associated with the development of our product candidates and includes: (i) salaries, benefits, travel, equity-based compensation expense and facility-related expense for personnel engaged in research and development functions; (ii) expense incurred under agreements with contract research organizations (“CROs”), investigative sites and consultants that conduct our nonclinical and clinical studies; (iii) manufacturing development and scale-up expense and the cost of acquiring and manufacturing clinical study materials and commercial materials, including manufacturing registration and validation batches; (iv) payments to consultants engaged in the development of our product candidates, including equity-based compensation expense, travel and other expense; and (v) costs related to compliance with quality and regulatory requirements.
Research and development expense is charged to operations as incurred when the expenditures relate to our research and development efforts and have no alternative future uses. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
All of our research and development expense incurred to date has been incurred in connection with tesetaxel. We expect our research and development expense to increase for the foreseeable future as we advance tesetaxel through clinical development, including the conduct of our ongoing Phase 3 study, CONTESSA.
General and Administrative Expense
General and administrative expense consists primarily of salaries, benefits, travel, equity-based compensation expense and facility-related expense for personnel in finance and administrative functions.
56
General and administrative expense also includes professional fees for legal, patent, consulting, accounting and audit services and other related costs. We expect our general and administrative expense to remain consistent in the future as we continue to support our research and development of tesetaxel.
Results of Operations
The following table summarizes our results of operations for each of the periods below (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Research and development expense |
|
$ |
79,948 |
|
|
$ |
27,902 |
|
|
General and administrative expense |
|
$ |
10,816 |
|
|
$ |
4,842 |
|
|
Interest income |
|
$ |
1,804 |
|
|
$ |
- |
|
Research and Development Expense
The following table summarizes our research and development expense for each of the periods below (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Clinical development costs |
|
$ |
52,489 |
|
|
$ |
18,129 |
|
|
Personnel and related costs |
|
|
21,177 |
|
|
|
6,957 |
|
|
Equity-based compensation expense |
|
|
5,659 |
|
|
|
2,633 |
|
|
Other research and development costs |
|
|
623 |
|
|
|
183 |
|
|
Total research and development expense |
|
$ |
79,948 |
|
|
$ |
27,902 |
|
Research and development expense was $79.9 million and $27.9 million for the years ended December 31, 2018 and 2017, respectively. The increase of $52.0 million was primarily due to increased activities in connection with our tesetaxel clinical development program, resulting in increased clinical development costs of $34.4 million, increased personnel and related costs of $14.2 million and increased equity-based compensation expense of $3.0 million.
General and Administrative Expense
The following table summarizes our general and administrative expense for each of the periods below (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
General and administrative costs |
|
$ |
9,754 |
|
|
$ |
4,291 |
|
|
Equity-based compensation expense |
|
|
1,062 |
|
|
|
551 |
|
|
Total general and administrative expense |
|
$ |
10,816 |
|
|
$ |
4,842 |
|
General and administrative expense was $10.8 million and $4.8 million for the years ended December 31, 2018 and 2017, respectively. The increase of $6.0 million was due to increased administrative support costs in connection with our tesetaxel clinical development program, including increased personnel, professional and other general administrative costs of $5.5 million and increased equity-based compensation expense of $0.5 million.
57
Interest income was $1.8 million for the year ended December 31, 2018 and consists of income generated from cash held in savings accounts. No interest income was generated for the year ended December 31, 2017.
Liquidity and Capital Resources
As of December 31, 2018 and 2017, we had cash in the amount of $139.1 million and $198.1 million, respectively. We believe that our existing cash as of December 31, 2018 will be sufficient to meet our anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission.
We have incurred losses in each year since our inception. Our net loss was $89.0 million and $32.7 million for the years ended December 31, 2018 and 2017, respectively. As of December 31, 2018 and 2017, we had an accumulated deficit of $128.3 million and $39.3 million, respectively. Substantially all of our operating losses resulted from expenses incurred in connection with advancing tesetaxel through development activities and general and administrative costs associated with our operations.
To date, we have funded our operations through the sale of equity securities. Since our inception, we have raised $238.4 million in net proceeds from the sale of equity securities.
The following table summarizes our net cash flow activity for each of the periods below (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(67,094 |
) |
|
$ |
(25,858 |
) |
|
Investing activities |
|
|
(1,907 |
) |
|
|
(83 |
) |
|
Financing activities |
|
|
10,197 |
|
|
|
221,447 |
|
|
Net (decrease) increase in cash and restricted cash |
|
$ |
(58,804 |
) |
|
$ |
195,506 |
|
Net cash used in operating activities was $67.1 million and $25.9 million for the years ended December 31, 2018 and 2017, respectively. Net cash used in operating activities was the result of our net loss and change in working capital, partially offset by equity-based compensation expense, depreciation and amortization expense and non-cash contributions for expenses by an affiliate of a significant stockholder.
Net cash used in investing activities was $1.9 million and $0.1 million for the years ended December 31, 2018 and 2017, respectively. Net cash used in investing activities was the result of purchases of property and equipment.
Net cash provided by financing activities was $10.2 million and $221.4 million for the years ended December 31, 2018 and 2017, respectively. For the year ended December 31, 2018, net cash provided by financing activities was the result of net proceeds of $9.8 million from the exercise of the underwriters’ option to purchase additional shares of common stock in our initial public offering (“IPO”) and $0.3 million in proceeds from the issuance of stock under our employee stock plans. For the year ended December 31, 2017, net cash provided by financing activities was the result of our sale of shares of common stock in our IPO for net proceeds of $137.5 million and, prior to our IPO, our sale of shares of common stock in private financings for net proceeds of $84.0 million.
Until such time as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic partnerships and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the
58
terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic partnerships or licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, associated intellectual property, our other technologies, future revenue streams or research programs or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our product candidate even if we would otherwise prefer to develop and market such product candidate ourselves.
Contractual Obligations and Commitments
We enter into contracts in the normal course of business with CROs, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancelable contracts.
In 2013, we licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Under the Daiichi Sankyo license agreement, we are obligated to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. We are required to make aggregate future milestone payments of up to $31.0 million, contingent on attainment of certain regulatory milestones. Additionally, we will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel.
Off-Balance Sheet Arrangements
During the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under the rules of the SEC.
Jumpstart Our Business Startups Act
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under this act, an emerging growth company can delay the adoption of new or revised accounting standards issued subsequent to the enactment of the JOBS Act until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. However, we intend to rely on other exemptions provided by the JOBS Act, including without limitation, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended. We will remain an emerging growth company until December 31, 2023 unless, prior to that time, we: (i) have more than $1.07 billion in annual gross revenue; (ii) have a market value for our common stock held by non-affiliates of more than $700 million as of the last day of our second quarter of any year; or (iii) issue more than $1.0 billion of non-convertible debt over a three-year period.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our audited financial statements, which have been prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses during the reporting periods. These items are monitored and analyzed by us for
59
changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our audited financial statements elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies related to accrued expenses and equity-based compensation are most critical to understanding and evaluating our reported financial results.
Accrued Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued expenses include costs associated with conducting our development and regulatory activities, including fees paid to third-party professional consultants and service providers, and costs to develop and manufacture clinical study materials.
We base our accrued expenses on our estimates of the services received and efforts expended pursuant to our contractual arrangements. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our service providers will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepayment accordingly.
Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differs from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. To date, there have been no material differences from our estimates to the amount actually incurred.
Equity-based Compensation Expense
Equity-based compensation expense represents the grant-date fair value of employee awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. We account for awards to non-employees using the fair value approach. Non-employee awards are subject to periodic revaluation over their vesting terms.
Following the Conversion, we adopted the Odonate Therapeutics, Inc. 2017 Stock Option Plan (the “2017 Plan”) in order to grant stock options to our employees, officers, directors and consultants. Recipients of stock options are eligible to purchase shares of our common stock at an exercise price equal to the fair market value of such stock on the date of grant. The maximum term of options granted under the 2017 Plan is 10 years. The options generally vest over a 4-year period from either the date of grant or the commencement of service.
60
Following the Conversion, we adopted the Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan (the “ESPP”) in order to provide a means for eligible employees to accumulate shares of our common stock over time through regular payroll deductions. Under the ESPP, eligible employees may purchase shares of our common stock twice per month at a price equal to 85% of the closing price of our common stock on the date of each purchase. Eligible employees purchasing shares under the ESPP are subject to an annual cap equal to the lesser of $25,000 or 10% of the employee’s annual cash compensation. Shares purchased under the ESPP cannot be sold for a period of one year following the purchase date (or such shorter period of time if the participating employee’s employment terminates before this one-year anniversary).
Prior to the Conversion, we, through Odonate Management Holdings, LLC (“Management Holdings”), issued an aggregate of 2,931,402 incentive units under the Odonate Management Holdings Equity Incentive Plan (the “Management Plan”). The incentive units were issued to certain of our directors, officers, employees and consultants in consideration for bona fide services provided to us. Pursuant to the Management Plan, the incentive units are considered “profits interests” within the meaning of U.S. federal and state tax rules. Incentive units do not entitle their holders to receive distributions if we were to be liquidated immediately after the grant. Instead, the incentive unitholders are entitled to receive an allocation of a portion of our profits arising after the date of the grant and, subject to vesting conditions, distributions made out of a portion of our profits arising after the grant date of the incentive units. Accordingly, the financial benefits of incentive units to the awardee, and the costs to the issuing company, are substantially similar to a stock option grant. The incentive units generally vest over a 4-year period from either the date of grant or the commencement of service. Following the Conversion, we have not granted, and will no longer grant, incentive units under the Management Plan.
Following the Conversion, the outstanding incentive units of Management Holdings remain outstanding, but represent an indirect interest in shares of our common stock on vesting of the awards. As part of the Conversion, the shares of common stock underlying the outstanding incentive units were issued to a newly formed entity, Odonate Holdings, LLC (“Odonate Holdings”). While Odonate Holdings holds the shares of common stock underlying outstanding incentive units, it has no other operations. In addition, Odonate Holdings granted us a proxy directing us to vote all shares of common stock underlying outstanding incentive units held by Odonate Holdings in the same proportion as the votes cast by all other stockholders, which is sometimes called “mirrored voting.” In the event that any incentive units in Management Holdings are forfeited, the shares of common stock underlying such forfeited incentive units will be transferred from Odonate Holdings to us and cancelled.
As of December 31, 2018 and 2017, there were 2,096,758 and 2,931,402 incentive units outstanding, respectively, all of which are incentive units in Odonate Holdings and represent an indirect right, subject to vesting, in shares of our common stock. As of December 31, 2018 and 2017, we had 3,265,162 and 183,699 stock options outstanding, respectively. The grant-date fair value of equity awards was estimated using a Black-Scholes option-pricing model. The estimated volatility was based on the historical equity volatility of comparable companies.
Prior to our IPO, there was no public market for shares of our common stock and incentive units at the time of grant. As such, the fair value of the shares of common stock and incentive units were estimated on each grant date by our management. In order to determine the fair value of shares of our common stock and incentive units, our management considered, among other things, recently available independent valuations and valuations derived from the sale of our equity securities to third parties in recent equity financings. These independent valuations were performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation.
Following the closing of our IPO, we determine the fair value of shares of our common stock based on the closing price of shares of our common stock as reported on the Nasdaq Global Select Market.
61
Recent Accounting Pronouncements
See Note 2 to the audited financial statements included in Item 8 of this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
Item 8. Financial Statements and Supplementary Data
Our financial statements, together with the report of our independent registered public accounting firm, appear in this Annual Report on Form 10-K beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Management’s Evaluation of our Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Annual Report on Form 10-K, the effectiveness of our disclosure controls and procedures. Based on that evaluation of our disclosure controls and procedures as of December 31, 2018, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date are effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the U.S. Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive officer and our principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the U.S.
As of December 31, 2018, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (“2013 Framework”). Based on this assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2018.
62
This Annual Report on Form 10-K does not include an attestation report on internal control over financial reporting issued by our independent registered accounting firm. Our auditors will not be required to formally opine on the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002 until we are no longer an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our most recent quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
63
Item 10. Directors, Executive Officers and Corporate Governance
Directors and Executive Officers
The following table sets forth certain information regarding members of our Board of Directors (the “Board”) and our executive officers as of the date of this Annual Report on Form 10-K. There are no family relationships among any of our directors or executive officers.
|
Name |
|
Age |
|
Position |
|
Kevin Tang |
|
51 |
|
Chairman and Chief Executive Officer |
|
Jeff Vacirca, M.D. |
|
50 |
|
Vice Chairman |
|
Aaron Davis(1)(2)(3) |
|
40 |
|
Director |
|
Craig Johnson(1)(2)(3) |
|
57 |
|
Director |
|
Laura Johnson Douglass(1) |
|
54 |
|
Director |
|
Robert Rosen |
|
63 |
|
Director |
|
George Tidmarsh, M.D., Ph.D. |
|
59 |
|
Director |
|
Joseph O’Connell, M.D. |
|
64 |
|
Chief Medical Officer |
|
John Lemkey |
|
38 |
|
Chief Operating Officer |
|
Michael Hearne |
|
56 |
|
Chief Financial Officer |
|
(1) |
Member of the Audit Committee |
|
(2) |
Member of the Compensation Committee |
|
(3) |
Member of the Nominating and Corporate Governance Committee |
Each of our directors stands for election at each annual meeting of stockholders and, if elected, will serve from the time of election and qualification until the next annual meeting. Each director’s term continues until the election and qualification of his or her successor, or his or her earlier death, resignation or removal. Each of our executive officers was appointed to serve until such officer’s resignation or removal.
The following is a biographical summary of the experience of our directors and executive officers:
Kevin Tang
Mr. Tang has served as our Chairman and Chief Executive Officer since the Company’s inception in 2013. Mr. Tang also serves as President of Tang Capital Management, LLC, a life sciences-focused investment company he founded in 2002 and an affiliate of the Company. Since 2014, Mr. Tang has served as a director and Chairman of La Jolla Pharmaceutical Company. Since 2009, Mr. Tang has served as a director of Heron Therapeutics, Inc. and, since 2012, has served as Chairman. From 2009 through its acquisition by Endo Pharmaceuticals, Inc. in 2010, he served as a director of Penwest Pharmaceuticals Co. In 2006, Mr. Tang co-founded Ardea Biosciences, Inc. and served as a director from inception through its acquisition by AstraZeneca PLC in 2012. From 2001 to 2008, he was a director of Trimeris, Inc. From 1993 to 2001, Mr. Tang held various positions at Deutsche Banc Alex Brown, Inc., an investment banking firm, most recently serving as Managing Director and head of the firm’s Life Sciences research group. Mr. Tang received a B.S. degree from Duke University. The Board has concluded that Mr. Tang should serve as a director based on his experience forming and building biotechnology companies, serving as a director of numerous biotechnology companies and serving as a manager of funds specializing in the area of life sciences.
Jeff Vacirca, M.D.
Dr. Vacirca has served as our Vice Chairman since July 2016, as a director since January 2017 and, from March 2017 to September 2017, as our Chief Medical Officer. Since 2008, Dr. Vacirca has served as Chief Executive Officer and Managing Partner/Director of Clinical Research at New York Cancer Specialists. Since 2014, he has served on the Scientific Advisory Board of Caris Life Sciences.
64
Dr. Vacirca currently serves as the President of Community Oncology Alliance, a non-profit organization dedicated to advocating for community oncology practices and patients with cancer. Dr. Vacirca received a B.A. degree in human biology from the University at Albany and an M.D. degree from St. George’s University. The Board has concluded that Dr. Vacirca should serve as a director based on his substantial business experience and clinical expertise in oncology.
Aaron Davis
Mr. Davis has served as a director since December 2016. Mr. Davis has been the Chief Executive Officer of Boxer Capital, the healthcare arm of Tavistock Group, since 2012. Mr. Davis co-founded Boxer Capital in 2005 and, prior to being appointed Chief Executive Officer, served as Portfolio Manager. Since 2016, Mr. Davis has served as a director of CiVi Biopharma, Inc. From 2006 to 2008, he served as a director of Kalypsys, Inc. From 2000 to 2004, Mr. Davis worked in the Global Healthcare Investment Banking and Private Equity Group at UBS Warburg, LLC. Mr. Davis received an M.A. degree in biotechnology from Columbia University and a B.B.A. degree in finance from Emory University. The Board has concluded that Mr. Davis should serve as a director based on his experience serving as a director of biotechnology companies and as a manager of funds specializing in the area of life sciences.
Craig Johnson
Mr. Johnson has served as a director since July 2017. Mr. Johnson also has been a director of Heron Therapeutics, Inc. since 2014, La Jolla Pharmaceutical Company since 2013 and Mirati Therapeutics, Inc. since 2013. Mr. Johnson also served as a director of Ardea Biosciences, Inc. from 2008 until its acquisition by AstraZeneca PLC in 2012, Adamis Pharmaceuticals Corporation from 2011 to 2014 and GenomeDx Biosciences, Inc. from 2015 to 2018. From 2004 through its acquisition by Raptor Pharmaceuticals Corp. in 2009, he served as Vice President and Chief Financial Officer of TorreyPines Therapeutics, Inc. and, from 2009 to 2010, as Vice President of a wholly-owned subsidiary of Raptor Pharmaceutical Corp. From 1994 to 2004, Mr. Johnson held various positions at MitoKor, Inc., most recently serving as Chief Financial Officer and Senior Vice President of Operations. Earlier in his career, Mr. Johnson practiced as a Certified Public Accountant with Price Waterhouse. Mr. Johnson received a B.B.A. degree in accounting from the University of Michigan-Dearborn. The Board has concluded that Mr. Johnson should serve as a director based on his experience serving as a director of biotechnology companies and his expertise in financial management.
Laura Johnson Douglass
Ms. Douglass has served as a director since December 2018. Ms. Douglass is the President and Chief Executive Officer of Next Generation Clinical Research Consulting, Inc., a contract research organization servicing the pharmaceutical industry that she founded in 1999. Additionally, Ms. Douglass is the President and Chief Executive Officer of Eufaeria Biosciences, Inc., a biotechnology company that she founded in 2016. Since 2013, she has served as a director of La Jolla Pharmaceutical Company. Ms. Douglass is also a founder and director of SB Bancorp, Inc. and Settlers Bank, Inc. Ms. Douglass received a nursing degree from The University of the State of New York-Albany. The Board has concluded that Ms. Douglass should serve as a director based on her substantial operating experience and expertise in clinical study management.
Robert Rosen
Mr. Rosen has served as a director since July 2017. From 2013 to February 2019, Mr. Rosen served as President and as a director of Heron Therapeutics, Inc. and, from 2012 to 2013, served as Senior Vice President and Chief Commercial Officer of Heron Therapeutics, Inc. Since 2014, he has served as a director of La Jolla Pharmaceutical Company. From 2014 to 2015, Mr. Rosen served as a director of Conkwest, Inc. (now NantKwest, Inc.). In 2012, he served as Managing Partner of Scotia Nordic LLC, a life sciences advisory firm. From 2011 to 2012, Mr. Rosen served as Senior Vice President of Global Commercial Operations at Dendreon Corporation. From 2005 to 2011, he served as Global Head of Oncology at Bayer HealthCare Pharmaceuticals. From 2002 to 2005, Mr. Rosen was Vice President of
65
the Oncology Business Unit at Sanofi-Synthèlabo Inc. Mr. Rosen received a B.S. degree in pharmacy from Northeastern University. The Board has concluded that Mr. Rosen should serve as a director based on his leadership experience in the biotechnology and pharmaceutical industries and expertise in commercializing pharmaceutical products.
George Tidmarsh, M.D., Ph.D.
Dr. Tidmarsh has served as a director since December 2016. Since 2012, Dr. Tidmarsh has served as President, Chief Executive Officer and a director of La Jolla Pharmaceutical Company. In 2005, Dr. Tidmarsh founded Horizon Pharma, Inc., where he served as President and Chief Executive Officer until 2008 and as a director and consultant until 2011. In 2001, he founded Threshold Pharmaceuticals, Inc., where he served as President until 2005. From 1996 to 2000, Dr. Tidmarsh held various senior positions at Coulter Pharmaceutical, Inc., most recently serving as Chief Medical Officer. Earlier in his career, Dr. Tidmarsh held various scientific and clinical positions at Sequus Pharmaceuticals, Inc., Gilead Sciences, Inc. and SyStemix, Inc. Dr. Tidmarsh received a Ph.D. degree and an M.D. degree from the Stanford University School of Medicine and a B.S. degree in microbiology from Stanford University. The Board has concluded that Dr. Tidmarsh should serve as a director based on his experience forming, building and leading biotechnology companies.
Joseph O’Connell, M.D.
Dr. O’Connell has served as our Chief Medical Officer since September 2017. From 2015 to 2017, Dr. O’Connell served as Vice President, Medical and Scientific Affairs, Hematology and Oncology at inVentiv Health Clinical, LLC. From 2007 to 2015, Dr. O’Connell held various positions at Pfizer Inc., most recently serving as Senior Director and Asset Global Clinical Lead for Oncology. Prior to 2007, he practiced adult medical oncology for more than 15 years, most recently as a Medical Oncologist at the Yale Cancer Center. Dr. O’Connell received a B.S. degree in biology from Fordham University and an M.D. degree from the State University of New York.
John Lemkey
Mr. Lemkey has served as our Chief Operating Officer since November 2018. Mr. Lemkey previously served as our Chief Financial Officer from the Company’s inception in 2013 to November 2018. Since 2012, Mr. Lemkey has also served as Chief Operating Officer of Tang Capital Management, LLC, a life sciences-focused investment company and an affiliate of the Company, which Mr. Lemkey joined in 2006. From 2003 to 2006, Mr. Lemkey was a Senior Auditor at Ernst & Young LLP. Mr. Lemkey received a B.S. degree in accounting from the University of Southern California and is a Certified Public Accountant (inactive) in the state of California.
Michael Hearne
Mr. Hearne has served as our Chief Financial Officer since November 2018. Mr. Hearne previously served as our Vice President of Finance and Accounting from 2015 to November 2018. Since 2015, Mr. Hearne has also served as Chief Financial Officer of Tang Capital Management, LLC, a life sciences-focused investment company and an affiliate of the Company. From 2014 to 2015, Mr. Hearne served as a Partner at Weaver & Tidwell, LLP. From 2008 to 2014, Mr. Hearne had his own financial consulting business. From 2000 to 2008, Mr. Hearne served as a Partner at Rothstein Kass & Company, P.C. Mr. Hearne started his public accounting career at Coopers & Lybrand in 1988. Mr. Hearne received a B.S. degree in accounting and a Masters of Accountancy, Taxation from Brigham Young University and is a Certified Public Accountant (inactive) in the state of California.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers and persons who beneficially own more than 10% of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities and to furnish us with copies of all Section 16(a) forms they file.
66
To our knowledge, based solely on our review of Forms 3, 4 and 5 furnished to us or written representations that no Form 5 was required, during the year ended December 31, 2018, our directors, executive officers and persons who beneficially own more than 10% of a registered class of our equity securities met all applicable filing requirements under Section 16(a) of the Exchange Act in a timely manner.
Code of Conduct
We have adopted a Code of Conduct that applies to all of our directors, officers and employees, including our principal executive officer and principal financial and accounting officer. Our Code of Conduct is posted on our website located at www.odonate.com. We intend to disclose any material future amendments to, or waivers of, provisions of the Code of Conduct on our website within 4 business days following the date of the amendment or waiver.
Audit Committee and Audit Committee Financial Expert
We have a separately-designated standing audit committee (“Audit Committee”) established in accordance with Section 3(a)(58)(A) of the Exchange Act. The Audit Committee is comprised of Messrs. Davis and Johnson and Ms. Douglass, each of whom qualify as an independent director, as defined under applicable Nasdaq qualification standards. Additionally, Mr. Johnson qualifies as an “audit committee financial expert” as that term is defined in the rules and regulations established by the SEC.
Item 11. Executive Compensation
Summary Compensation Table
The following table summarizes information for the years ended December 31, 2018 and 2017 concerning the compensation paid or awarded to our principal executive officer and the two other most highly compensated executive officers as of December 31, 2018 (the “Named Executive Officers” or “NEOs”):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Option |
|
|
Incentive Plan |
|
|
All Other |
|
|
|
|
|
|
||||||
|
Name and Principal Position |
|
Year |
|
Salary |
|
|
Awards(1) |
|
|
Compensation(2) |
|
|
Compensation(3) |
|
|
Total |
|
||||||||||
|
Kevin Tang(4) |
|
2018 |
|
$ |
|
1 |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
1 |
|
|
Chairman and Chief Executive Officer |
|
2017 |
|
$ |
|
1 |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
1 |
|
|
Joseph O’Connell, M.D.(5) |
|
2018 |
|
$ |
|
353,300 |
|
|
$ |
|
944,898 |
|
|
$ |
|
113,056 |
|
|
$ |
|
35,578 |
|
|
$ |
|
1,446,832 |
|
|
Chief Medical Officer |
|
2017 |
|
$ |
|
108,712 |
|
|
$ |
|
2,590,030 |
|
|
$ |
|
60,000 |
|
|
$ |
|
6,861 |
|
|
$ |
|
2,765,603 |
|
|
John Lemkey(6) |
|
2018 |
|
$ |
|
200,000 |
|
|
$ |
|
577,995 |
|
|
$ |
|
64,000 |
|
|
$ |
|
7,920 |
|
|
$ |
|
849,915 |
|
|
Chief Operating Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
The amounts reported in this column reflect the grant-date fair values of option awards granted to each NEO, calculated in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718, Compensation-Stock Compensation. For a discussion of the assumptions used to calculate the value of our option awards, see Note 6 to our audited financial statements included in Item 8 of this Annual Report on Form 10-K. |
|
(2) |
The amounts reported in this column reflect each NEO’s performance-based cash bonus paid. |
|
(3) |
The amounts reported in this column reflect the Company’s annual and matching contributions to each NEO’s 401(k) plan account, life insurance premiums paid by the Company and tax gross-up payments related to expense reimbursements. |
|
(4) |
Mr. Tang has elected to receive an annual salary of $1.00 and to not receive any bonuses, option awards or other compensation. |
|
(5) |
The grant-date fair value of option awards granted to Mr. O’Connell in 2018 includes $182,803 related to his January 2, 2018 grant for services rendered in 2017 and $755,352 related to his December 31, 2018 grant for services rendered in 2018. The grant-date fair value of option awards granted to Mr. O’Connell in 2017 relates to his new hire grant. |
|
(6) |
Mr. Lemkey became an NEO in 2018. |
67
Outstanding Option Awards as of December 31, 2018
The following table presents information regarding the outstanding option awards held by each of the NEOs as of December 31, 2018:
|
|
|
|
|
|
|
Option Awards |
|||||||||||||
|
|
|
|
|
|
|
Number |
|
|
Number |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
of Securities |
|
|
of Securities |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
Underlying |
|
|
Underlying |
|
|
Option |
|
|
Option |
||||
|
|
|
|
|
Vesting |
|
Unexercised |
|
|
Unexercised |
|
|
Award |
|
|
Award |
||||
|
|
|
Award |
|
Commencement |
|
Option Awards (#) |
|
|
Option Awards (#) |
|
|
Exercise |
|
|
Expiration |
||||
|
Name |
|
Type |
|
Date |
|
Exercisable |
|
|
Unexercisable(1) |
|
|
Price ($) |
|
|
Date(2) |
||||
|
Kevin Tang(3) |
|
- |
|
- |
|
|
- |
|
|
|
- |
|
|
$ |
|
- |
|
|
- |
|
Joseph O’Connell, M.D. |
|
Incentive Unit |
|
9/9/2017 |
|
|
60,369 |
|
|
|
132,813 |
|
|
$ |
|
14.89 |
|
|
- |
|
|
|
Stock Option |
|
1/2/2018 |
|
|
- |
|
|
|
11,265 |
|
|
$ |
|
24.73 |
|
|
1/2/2028 |
|
|
|
Stock Option |
|
4/13/2018 |
|
|
- |
|
|
|
15 |
|
|
$ |
|
21.65 |
|
|
4/13/2028 |
|
|
|
Stock Option |
|
4/30/2018 |
|
|
- |
|
|
|
17 |
|
|
$ |
|
21.08 |
|
|
4/30/2028 |
|
|
|
Stock Option |
|
5/15/2018 |
|
|
- |
|
|
|
15 |
|
|
$ |
|
23.17 |
|
|
5/15/2028 |
|
|
|
Stock Option |
|
5/31/2018 |
|
|
- |
|
|
|
14 |
|
|
$ |
|
23.99 |
|
|
5/31/2028 |
|
|
|
Stock Option |
|
6/15/2018 |
|
|
- |
|
|
|
13 |
|
|
$ |
|
26.52 |
|
|
6/15/2028 |
|
|
|
Stock Option |
|
6/29/2018 |
|
|
- |
|
|
|
16 |
|
|
$ |
|
22.08 |
|
|
6/29/2028 |
|
|
|
Stock Option |
|
7/13/2018 |
|
|
- |
|
|
|
15 |
|
|
$ |
|
23.95 |
|
|
7/13/2028 |
|
|
|
Stock Option |
|
7/31/2018 |
|
|
- |
|
|
|
17 |
|
|
$ |
|
19.77 |
|
|
7/31/2028 |
|
|
|
Stock Option |
|
8/15/2018 |
|
|
- |
|
|
|
19 |
|
|
$ |
|
18.74 |
|
|
8/15/2028 |
|
|
|
Stock Option |
|
8/31/2018 |
|
|
- |
|
|
|
18 |
|
|
$ |
|
19.19 |
|
|
8/31/2028 |
|
|
|
Stock Option |
|
9/14/2018 |
|
|
- |
|
|
|
18 |
|
|
$ |
|
19.13 |
|
|
9/14/2028 |
|
|
|
Stock Option |
|
9/28/2018 |
|
|
- |
|
|
|
18 |
|
|
$ |
|
19.41 |
|
|
9/28/2028 |
|
|
|
Stock Option |
|
10/15/2018 |
|
|
- |
|
|
|
64 |
|
|
$ |
|
16.09 |
|
|
10/15/2028 |
|
|
|
Stock Option |
|
10/31/2018 |
|
|
- |
|
|
|
72 |
|
|
$ |
|
14.50 |
|
|
10/31/2028 |
|
|
|
Stock Option |
|
11/15/2018 |
|
|
- |
|
|
|
63 |
|
|
$ |
|
16.52 |
|
|
11/15/2028 |
|
|
|
Stock Option |
|
11/30/2018 |
|
|
- |
|
|
|
66 |
|
|
$ |
|
15.76 |
|
|
11/30/2028 |
|
|
|
Stock Option |
|
12/14/2018 |
|
|
- |
|
|
|
68 |
|
|
$ |
|
15.12 |
|
|
12/14/2028 |
|
|
|
Stock Option |
|
12/31/2018 |
|
|
- |
|
|
|
80,074 |
|
|
$ |
|
14.08 |
|
|
12/31/2028 |
|
John Lemkey |
|
Stock Option |
|
4/13/2018 |
|
|
- |
|
|
|
45 |
|
|
$ |
|
21.65 |
|
|
4/13/2028 |
|
|
|
Stock Option |
|
4/30/2018 |
|
|
- |
|
|
|
46 |
|
|
$ |
|
21.08 |
|
|
4/30/2028 |
|
|
|
Stock Option |
|
5/15/2018 |
|
|
- |
|
|
|
43 |
|
|
$ |
|
23.17 |
|
|
5/15/2028 |
|
|
|
Stock Option |
|
5/31/2018 |
|
|
- |
|
|
|
40 |
|
|
$ |
|
23.99 |
|
|
5/31/2028 |
|
|
|
Stock Option |
|
6/15/2018 |
|
|
- |
|
|
|
37 |
|
|
$ |
|
26.52 |
|
|
6/15/2028 |
|
|
|
Stock Option |
|
6/29/2018 |
|
|
- |
|
|
|
45 |
|
|
$ |
|
22.08 |
|
|
6/29/2028 |
|
|
|
Stock Option |
|
7/13/2018 |
|
|
- |
|
|
|
41 |
|
|
$ |
|
23.95 |
|
|
7/13/2028 |
|
|
|
Stock Option |
|
7/31/2018 |
|
|
- |
|
|
|
49 |
|
|
$ |
|
19.77 |
|
|
7/31/2028 |
|
|
|
Stock Option |
|
8/15/2018 |
|
|
- |
|
|
|
53 |
|
|
$ |
|
18.74 |
|
|
8/15/2028 |
|
|
|
Stock Option |
|
8/31/2018 |
|
|
- |
|
|
|
51 |
|
|
$ |
|
19.19 |
|
|
8/31/2028 |
|
|
|
Stock Option |
|
9/14/2018 |
|
|
- |
|
|
|
51 |
|
|
$ |
|
19.13 |
|
|
9/14/2028 |
|
|
|
Stock Option |
|
9/28/2018 |
|
|
- |
|
|
|
51 |
|
|
$ |
|
19.41 |
|
|
9/28/2028 |
|
|
|
Stock Option |
|
10/15/2018 |
|
|
- |
|
|
|
61 |
|
|
$ |
|
16.09 |
|
|
10/15/2028 |
|
|
|
Stock Option |
|
10/31/2018 |
|
|
- |
|
|
|
67 |
|
|
$ |
|
14.50 |
|
|
10/31/2028 |
|
|
|
Stock Option |
|
11/15/2018 |
|
|
- |
|
|
|
59 |
|
|
$ |
|
16.52 |
|
|
11/15/2028 |
|
|
|
Stock Option |
|
11/30/2018 |
|
|
- |
|
|
|
63 |
|
|
$ |
|
15.76 |
|
|
11/30/2028 |
|
|
|
Stock Option |
|
12/14/2018 |
|
|
- |
|
|
|
64 |
|
|
$ |
|
15.12 |
|
|
12/14/2028 |
|
|
|
Stock Option |
|
12/31/2018 |
|
|
- |
|
|
|
60,070 |
|
|
$ |
|
14.08 |
|
|
12/31/2028 |
68
|
(2) |
The incentive units do not expire. |
|
(3) |
Mr. Tang has elected to receive an annual salary of $1.00 and to not receive any bonuses, option awards or other compensation. Accordingly, there were no option awards held by Mr. Tang as of December 31, 2018. |
Outstanding option awards held by each of the NEOs as of December 31, 2018 are comprised of incentive units issued under the Odonate Management Holdings Equity Incentive Plan (the “Management Plan”) and stock options issued under the Odonate Therapeutics, Inc. 2017 Stock Option Plan (the “2017 Plan”). The Management Plan and 2017 Plan were adopted in order to allow for directors, officers, employees and consultants of Odonate (the “Management Plan Participants” and the “2017 Plan Participants”) to share in the performance of the Company. The incentive units issued under the Management Plan were issued to Odonate Management Holdings, LLC, which issued incentive units to the Management Plan Participants on the same terms and conditions. The incentive units generally vest over a 4-year period from either the date of grant or the commencement of service and are subject to continued service requirements. Generally, on termination of services, unvested incentive units are forfeited. Following the initial public offering, the vested incentive units may be exercised by the Management Plan Participants, with the value received by the Management Plan Participants in the form of cash or shares of common stock equal to the fair market value on the date of exercise less the exercise price of the incentive unit. Following the Conversion, as defined in Note 1 to our audited financial statements included in Item 8 of this Annual Report on Form 10-K, the Company has not granted, and will no longer grant, incentive units. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to the fair market value of such stock on the date of grant. The maximum term of options granted under the 2017 Plan is 10 years. The options generally vest over a 4-year period from either the date of grant or the commencement of service.
Compensation Arrangements with Named Executive Officers
All compensation arrangements with NEOs constitute “at-will” employment, meaning the NEO can terminate employment at any time, for any reason or for no reason. Similarly, the Company is free to terminate an NEO at any time, for any reason or for no reason. NEOs receive an annual base salary and are eligible to earn an annual performance-based cash bonus. In determining the annual performance-based cash bonus for each NEO, the NEO’s annual base salary is multiplied by his or her target bonus percentage, and the resulting amount is then multiplied by the corporate performance percentage approved by the Board, which is dependent on the achievement of corporate performance goals. NEOs are also eligible to receive an equity interest in the Company in the form of option awards. Option awards generally vest over a 4-year period from either the date of grant or the commencement of service and are subject to continued service requirements. Option awards are subject to accelerated vesting in the event of an NEO’s termination of employment by reason of death or disability or by the Company without cause within 24 months following the occurrence of a change in control. Compensation arrangements with NEOs do not provide for severance benefits.
Defined Contribution Plan
We adopted a defined contribution 401(k) plan available to eligible employees, including NEOs. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under U.S. federal tax regulations. We make a mandatory annual contribution of 3% of the eligible employees’ compensation to the 401(k) plan. In addition, we make matching contributions of up to 6% of the eligible employees’ compensation to the 401(k) plan.
69
We determined the ratio of the annual total compensation of our Chief Executive Officer to the annual total compensation of our median employee (the “CEO Pay Ratio”) in accordance with Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of Regulation S-K.
For the year ended December 31, 2018:
|
|
• |
the annual total compensation of Kevin Tang, our Chief Executive Officer, was $1.00; |
|
|
• |
the annual total compensation of our median employee was $195,621; and |
|
|
• |
the CEO Pay Ratio was 0.000005:1. |
We identified our median employee as of December 31, 2018 based on a consistently applied compensation measure defined as the sum of: (1) annualized base salary paid in 2018; (2) the grant-date fair value of option awards granted in 2018; and (3) annualized performance-based cash bonus paid in 2018. We then calculated the annual total compensation of the median employee using the same methodology we used for our NEOs in the Summary Compensation Table above.
Director Compensation
We adopted a director compensation plan whereby we pay an annual retainer of $50,000 per year to each non-employee director, with an additional $25,000 annual fee for service as the vice chairman of the Board, $15,000 annual fee for service as chairperson of the Audit Committee and $10,000 annual fee for service as chairperson of the Compensation Committee. Such cash fees are generally paid quarterly in arrears. We also may make periodic equity grants to such directors, but we have no current commitments to do so. We reimburse our directors for their reasonable out-of-pocket expenses incurred in performing services, including expenses for attending board and committee meetings.
The following table shows the compensation earned in 2018 by the directors who served on the Board during the year ended December 31, 2018:
|
|
|
Fees Earned or |
|
|
Option |
|
|
|
|
|
|
||||
|
Name |
|
Paid in Cash |
|
|
Awards(1) |
|
|
Total |
|
||||||
|
Kevin Tang(2) |
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
Jeff Vacirca, M.D. |
|
$ |
|
75,000 |
|
|
$ |
|
315,569 |
|
|
$ |
|
390,569 |
|
|
Aaron Davis(2) |
|
$ |
|
- |
|
|
$ |
|
- |
|
|
$ |
|
- |
|
|
Craig Johnson |
|
$ |
|
75,000 |
|
|
$ |
|
315,569 |
|
|
$ |
|
390,569 |
|
|
Laura Johnson Douglass(3) |
|
$ |
|
3,973 |
|
|
$ |
|
468,112 |
|
|
$ |
|
472,085 |
|
|
Robert Rosen |
|
$ |
|
50,000 |
|
|
$ |
|
315,569 |
|
|
$ |
|
365,569 |
|
|
George Tidmarsh, M.D., Ph.D. |
|
$ |
|
50,000 |
|
|
$ |
|
315,569 |
|
|
$ |
|
365,569 |
|
|
(1) |
The amounts reported in this column reflect the grant-date fair values of stock options granted to the directors calculated in accordance with FASB ASC Topic 718, Compensation-Stock Compensation. For a discussion of the assumptions used to calculate the value of our stock options, see Note 6 to our audited financial statements included in Item 8 of this Annual Report on Form 10-K. The aggregate number of option awards outstanding for Dr. Vacirca, Mr. Johnson, Ms. Douglass, Mr. Rosen and Dr. Tidmarsh as of December 31, 2018 was 657,732, 103,530, 47,500, 103,530 and 271,822, respectively. There were no option awards held by Messrs. Tang and Davis as of December 31, 2018. |
|
(2) |
Messrs. Tang and Davis have waived all compensation for their services on the Board. |
|
(3) |
Fees earned or paid in cash were pro-rated based on the portion of the year such director served on the Board. |
70
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
The following table presents information regarding beneficial ownership of our equity interests as of February 4, 2019 by:
|
|
• |
each stockholder or group of stockholders known by us to be the beneficial owner of more than 5% of our outstanding equity interests; |
|
|
• |
our Named Executive Officers (“NEOs”); |
|
|
• |
each of our directors; and |
|
|
• |
all of our executive officers and directors as a group. |
The percentage ownership information shown in the column titled “Percentage of Shares Beneficially Owned” in the table below is based on 26,752,669 shares of common stock outstanding as of February 4, 2019.
Beneficial ownership is determined in accordance with the rules of the U.S. Securities and Exchange Commission (the “SEC”) and, thus, represents voting or investment power with respect to our securities as of February 4, 2019. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting and sole investment power with respect to all equity interests beneficially owned, subject to community property laws where applicable. Unless otherwise indicated, the address of each individual listed in this table is 4747 Executive Drive, Suite 510, San Diego, California 92121.
|
|
|
Number of Shares |
|
|
Percentage of Shares |
||||
|
Name and Address of Beneficial Owner |
|
Beneficially Owned |
|
|
Beneficially Owned |
||||
|
Greater than 5% Holders |
|
|
|
|
|
|
|
|
|
|
Tang Capital Partners, LP(1) |
|
|
14,046,979 |
|
|
|
52.5 |
|
% |
|
Affiliates of Boxer Capital(2) |
|
|
3,624,518 |
|
|
|
13.5 |
|
% |
|
Franklin Resources, Inc.(3) |
|
|
1,601,213 |
|
|
|
6.0 |
|
% |
|
Named Executive Officers and Directors |
|
|
|
|
|
|
|
|
|
|
Kevin Tang(1) |
|
|
14,046,979 |
|
|
|
52.5 |
|
% |
|
Aaron Davis(2) |
|
|
645,756 |
|
|
|
2.4 |
|
% |
|
Jeff Vacirca, M.D.(4) |
|
|
428,965 |
|
|
|
1.6 |
|
% |
|
George Tidmarsh, M.D., Ph.D.(5) |
|
|
146,139 |
|
|
* |
|
% |
|
|
Joseph O'Connell, M.D.(6) |
|
|
76,696 |
|
|
* |
|
% |
|
|
Craig Johnson(7) |
|
|
45,221 |
|
|
* |
|
% |
|
|
Robert Rosen(8) |
|
|
45,221 |
|
|
* |
|
% |
|
|
John Lemkey(9) |
|
|
1,099 |
|
|
* |
|
% |
|
|
Laura Johnson Douglass |
|
|
- |
|
|
|
- |
|
% |
|
All executive officers and directors as a group (10 persons)(10) |
|
|
15,436,872 |
|
|
|
57.7 |
|
% |
|
* |
Indicates ownership of less than one percent. |
|
(1) |
These securities are beneficially owned by Tang Capital Partners, LP ("TCP"). TCP shares voting and dispositive power over such shares of common stock with Tang Capital Management, LLC (“TCM”) and Kevin Tang. TCM, as the general partner of TCP, and Kevin Tang, as the manager of TCM, may be deemed to beneficially own the shares of the common stock owned by TCP. Mr. Tang is the Chairman and Chief Executive Officer of the Company. Additionally, these securities include a total of 1,255,226 shares of common stock that are held of record by Odonate Holdings, LLC (“Odonate Holdings”). Odonate Holdings has granted a proxy to TCP giving TCP the authority to vote 154,285 shares of common stock. Odonate Holdings has also granted a proxy to the Company giving the Company the authority to vote 2,096,758 shares of common stock in the same proportion as the votes cast by all other stockholders of the Company. Of the 2,096,758 shares of common stock, TCP controls the voting of 1,100,941 shares of common stock based on its proportional ownership in the Company. The address of the foregoing entities is 4747 Executive Drive, Suite 510, San Diego, California 92121. |
71
|
common stock held by Boxer Capital. The address for the Affiliates of Boxer Capital is 11682 El Camino Real, Suite 320, San Diego, California 92130. |
|
(3) |
Based on the Schedule 13G filed with the SEC on January 28, 2019 by Franklin Resources, Inc. (“FRI”). These securities are beneficially owned by one or more open‑ or closed‑end investment companies or other managed accounts that are investment management clients of investment managers that are direct and indirect subsidiaries (each, an “Investment Management Subsidiary” and, collectively, the “Investment Management Subsidiaries”) of FRI. When an investment management contract (including a sub‑advisory agreement) delegates to an Investment Management Subsidiary investment discretion or voting power over the securities held in the investment advisory accounts that are subject to that agreement, FRI treats the Investment Management Subsidiary as having sole investment discretion or voting authority, as the case may be, unless the agreement specifies otherwise. Accordingly, Franklin Advisers, Inc. and Fiduciary Trust Company International report sole investment discretion and voting authority over 1,591,113 and 10,100 shares of common stock, respectively, and may be deemed to be the beneficial owners of these securities. The principal address of the foregoing entities is One Franklin Parkway, San Mateo, California 94403. |
|
(4) |
Includes 415,456 shares of common stock underlying incentive units and 12,500 shares of common stock underlying stock options exercisable by Dr. Vacirca within 60 days of February 4, 2019. |
|
(5) |
Includes 133,639 shares of common stock underlying incentive units and 12,500 shares of common stock underlying stock options exercisable by Dr. Tidmarsh within 60 days of February 4, 2019. |
|
(6) |
Includes 72,443 shares of common stock underlying incentive units and 3,521 shares of common stock underlying stock options exercisable by Dr. O’Connell within 60 days of February 4, 2019. |
|
(7) |
Includes 32,721 shares of common stock underlying incentive units and 12,500 shares of common stock underlying stock options exercisable by Mr. Johnson within 60 days of February 4, 2019. |
|
(8) |
Includes 32,721 shares of common stock underlying incentive units and 12,500 shares of common stock underlying stock options exercisable by Mr. Rosen within 60 days of February 4, 2019. |
|
(9) |
Mr. Lemkey is the Chief Operation Officer of the Company, as well as the Chief Operating Officer of TCM. Mr. Lemkey has a pecuniary interest in a portion of the shares beneficially held by TCP. |
|
(10) |
Includes the shares of common stock underlying incentive units and shares of common stock underlying stock options exercisable within 60 days of February 4, 2019 referred to in footnotes (4), (5), (6), (7) and (8). |
Equity Plan Compensation Information
The following table provides certain information regarding our equity compensation plans in effect as of December 31, 2018:
|
|
|
|
|
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
Securities Remaining |
|
||
|
|
|
Securities to Be |
|
|
Weighted-average |
|
|
Available for Future |
|
||||
|
|
|
Issued upon Exercise |
|
|
Exercise Price |
|
|
Issuance under Equity |
|
||||
|
|
|
of Outstanding |
|
|
of Outstanding |
|
|
Compensation Plans, |
|
||||
|
|
|
Options, Warrants |
|
|
Options, Warrants |
|
|
excluding Securities |
|
||||
|
|
|
and Rights |
|
|
and Rights |
|
|
Reflected in Column (1) |
|
||||
|
Plan Category |
|
(1) |
|
|
(2) |
|
|
(3) |
|
||||
|
Equity compensation plans approved by security holders(1) |
|
|
5,361,920 |
|
|
$ |
|
12.63 |
|
|
|
2,014,058 |
|
|
Equity compensation plans not approved by security holders |
|
|
- |
|
|
$ |
|
- |
|
|
|
- |
|
|
Total |
|
|
5,361,920 |
|
|
$ |
|
12.63 |
|
|
|
2,014,058 |
|
|
(1) |
Includes the Odonate Therapeutics, Inc. 2017 Stock Option Plan, Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan and Odonate Management Holdings Equity Incentive Plan. |
72
Item 13. Certain Relationships and Related Transactions, and Director Independence
We have described below each transaction or series of similar transactions since January 1, 2017, or any currently proposed transaction, to which we were or are a party in which:
|
|
• |
the amount involved exceeded or exceeds $120,000; and |
|
|
• |
any of our directors or executive officers, any beneficial owner of more than 5% of any class of our voting securities or any member of their immediate family had or will have a direct or indirect material interest. |
Beneficial ownership is determined in accordance with the rules of the U.S. Securities and Exchange Commission and generally includes voting or investment power with respect to such securities.
Related Party Transactions
The following table sets forth a summary of the sale of our securities to related parties since January 1, 2017. For a description of beneficial ownership, see Item 12 of this Annual Report on Form 10-K.
|
|
|
Shares of |
|
|
Total Purchase |
|
|||
|
Purchaser |
|
Common Stock |
|
|
Price |
|
|||
|
Tang Capital Partners, LP(1) |
|
|
5,255,434 |
|
|
$ |
|
68,519,009 |
|
|
Boxer Capital, LLC(2) |
|
|
1,431,440 |
|
|
$ |
|
19,101,091 |
|
|
Franklin Resources, Inc.(3) |
|
|
1,370,427 |
|
|
$ |
|
28,000,007 |
|
|
(1) |
These securities were purchased by Tang Capital Partners, LP ("TCP"). TCP beneficially owns more than 5% of our outstanding equity interests. TCP shares voting and dispositive power over such shares of common stock with Tang Capital Management, LLC (“TCM”) and Kevin Tang. TCM, as the general partner of TCP, and Mr. Tang, as the manager of TCM, may be deemed to beneficially own the shares of the common stock owned by TCP. Mr. Tang is the Chairman and Chief Executive Officer of the Company. Prior to the initial public offering (“IPO”), certain of these shares of common stock were acquired by Tang Capital Partners II, LP (“TCPII”) and TCPIL BL, LLC (“TCPIL”), which are affiliates of TCP. In connection with the IPO, TCPII and TCPIL transferred their shares of common stock to TCP. |
|
(2) |
Aaron Davis is the Chief Executive Officer of Boxer Capital, LLC (“Boxer Capital”) and is also a director of the Company. Boxer Capital beneficially owns more than 5% of our outstanding equity interests. Boxer Asset Management Inc. (“Boxer Management”) is the majority owner of Boxer Capital. Joe Lewis, a natural person, is the sole indirect beneficial owner of and controls Boxer Management. Mr. Davis is not deemed to be the beneficial owner of the shares of common stock held by Boxer Capital. |
|
(3) |
These securities were purchased by one or more open‑ or closed‑end investment companies or other managed accounts that are investment management clients of investment managers that are direct and indirect subsidiaries (each, an “Investment Management Subsidiary” and, collectively, the “Investment Management Subsidiaries”) of Franklin Resources, Inc. (“FRI”). FRI beneficially owns more than 5% of our outstanding equity interests. When an investment management contract (including a sub‑advisory agreement) delegates to an Investment Management Subsidiary investment discretion or voting power over the securities held in the investment advisory accounts that are subject to that agreement, FRI treats the Investment Management Subsidiary as having sole investment discretion or voting authority, as the case may be, unless the agreement specifies otherwise. Accordingly, the Investment Management Subsidiaries reporting sole investment discretion and voting authority over shares of common stock may be deemed to be the beneficial owners of these securities. Prior to the IPO, certain of these shares of common stock were acquired by funds managed by Franklin Advisers, Inc. (“FAV”), including Franklin Strategic Series–Franklin Biotechnology Discovery Fund and Franklin Templeton Investment Funds–Franklin Biotechnology Discovery Fund. FAV is an indirect wholly owned subsidiary of FRI. |
In March 2017, we raised $10.0 million through the sale of 2,593,024 shares of common stock at a price of $3.86 per share of common stock (the “March 2017 Financing”). In the March 2017 Financing, we sold a total of 1,949,658 shares of common stock to entities affiliated with Tang Capital Partners, LP (“TCP”), which is beneficially owned by Mr. Tang, our Chairman and Chief Executive Officer, and 544,816 shares of common stock to Boxer Capital. Mr. Davis is the Chief Executive Officer of Boxer Capital and is also a director of the Company.
In September 2017, we raised $74.0 million through the sale of 4,968,132 shares of common stock at a price of $14.90 per share of common stock (the “September 2017 Financing”). In the September 2017 Financing, we sold a total of 2,014,110 shares of common stock to entities affiliated with TCP, which is beneficially owned by Mr. Tang, our Chairman and Chief Executive Officer, and 469,958 shares of
73
common stock to Boxer Capital. Mr. Davis is the Chief Executive Officer of Boxer Capital and is also a director of the Company. We also sold 537,094 shares of common stock to Franklin Resources, Inc. (“FRI”).
In December 2017, we closed our initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. In January 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs. In the IPO, TCP, which is beneficially owned by Mr. Tang, our Chairman and Chief Executive Officer, purchased 1,291,666 shares of common stock through the underwriters at the IPO price for an aggregate purchase price of $31.0 million. Boxer Capital, where Mr. Davis, one of our directors, is the Chief Executive Officer, purchased 416,666 shares of common stock through the underwriters at the IPO price for an aggregate purchase price of $10.0 million. FRI purchased 833,333 shares of common stock through the underwriters at the IPO price for an aggregate purchase price of $20.0 million.
During the years ended December 31, 2018 and 2017, we received certain services and other benefits from an affiliate (the “Affiliate”) of our Chairman and Chief Executive Officer. We were not charged any fees for these services and other benefits, which included personnel costs for research and development and administrative functions, rent and facility costs and other direct expenses. We recorded expense, and corresponding increases to additional paid-in capital, of $0.1 million and $1.8 million for the years ended December 31, 2018 and 2017, respectively, for services and other benefits provided without charge to us. See Note 9 to our audited financial statements included in Item 8 of this Annual Report on Form 10-K. In 2017, for his services as interim Chief Medical Officer, our Vice Chairman was paid cash compensation of $201,383.
Director Independence
Our Board has reviewed the independence of all directors in light of each director’s (or any family member’s, if applicable) affiliations with the Company and members of management, as well as significant holdings of the Company’s securities. The Board uses the definition of independence from the Nasdaq Stock Market listing standards to assess independence of our directors, (the “Nasdaq Independence Rules”). The Nasdaq Independence Rules have objective tests and a subjective test for determining who is an “independent director.” The subjective test states that an independent director must be a person who lacks a relationship that, in the opinion of the Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Board has not established categorical standards or guidelines to make these subjective determinations but considers all relevant facts and circumstances. After considering the foregoing factors, our Board has determined that Messrs. Davis and Johnson and Ms. Douglass qualify as “independent directors,” as defined by Nasdaq Independence Rules. Mr. Tang and Dr. Vacirca are deemed to not be independent under Nasdaq Independence Rules by virtue of their current and past employment with the Company, respectively. Dr. Tidmarsh and Mr. Rosen are deemed to not be independent due to Mr. Tang’s prior and current service on the compensation committees of La Jolla Pharmaceutical Company and Heron Therapeutics, Inc., respectively.
The members of our Audit Committee must satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act (“Rule 10A-3”). In order to be considered independent for purposes of Rule 10A-3, no member of the Audit Committee may, other than in his or her capacity as a member of the Audit Committee, the Board or any other committee of the Board: (i) accept, directly or indirectly, any consulting, advisory or other compensatory fee from us or any of our subsidiaries; or (ii) directly, or indirectly through one or more intermediaries, control, be controlled by or be under common control with us or any of our subsidiaries.
We are considered a “controlled company” within the meaning of Rule 5615(c)(1) of the Nasdaq Independence Rules because Tang Capital Partners, LP, together with its affiliates, beneficially owns
74
52.5% of our outstanding common stock. As a controlled company, we are not required to maintain a majority of independent directors.
Item 14. Principal Accountant Fees and Services
The following table represents aggregate fees billed to us by Squar Milner LLP, our principal accountant, for each of the periods below:
|
|
|
Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
2018 |
|
|
2017 |
|
||||
|
Audit Fees(1) |
|
$ |
|
109,383 |
|
|
$ |
|
203,342 |
|
|
Audit-related Fees |
|
|
|
- |
|
|
|
|
- |
|
|
Tax Fees |
|
|
|
- |
|
|
|
|
- |
|
|
All Other Fees |
|
|
|
- |
|
|
|
|
- |
|
|
Total |
|
$ |
|
109,383 |
|
|
$ |
|
203,342 |
|
|
(1) |
“Audit Fees” consists of fees for professional services provided in connection with: (i) the audit of our annual financial statements; (ii) the reviews of our quarterly financial statements; and (iii) the consents and other services related to U.S. Securities and Exchange Commission filings. |
Our Audit Committee approves in advance all services provided by our independent registered public accounting firm. All engagements of our independent registered public accounting firm for 2018 and 2017 were approved by the Audit Committee.
75
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Financial Statements
The response to this portion of Item 15 is set forth under Item 8 hereof.
(a)(2) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not required or is shown in the financial statements or the notes thereto.
(a)(3) Exhibits
76
|
|
Description |
|
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
10.1+ |
|
|
|
|
|
|
|
10.2+ |
|
|
|
|
|
|
|
10.3+ |
|
|
|
|
|
|
|
10.4+ |
|
|
|
|
|
|
|
10.5† |
|
|
|
|
|
|
|
10.6+ |
|
|
|
|
|
|
|
24.1 |
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
31.2* |
|
|
|
|
|
|
|
32.1# |
|
|
|
|
|
|
|
101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema |
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase |
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase |
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase |
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase |
|
* |
Filed herewith |
|
† |
Confidential treatment has been granted with respect to certain portions (indicated by asterisks) of this exhibit. Omitted portions have been filed separately with the SEC. |
|
+ |
Indicates a management contract or compensatory plan or arrangement |
|
# |
Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act |
None.
77
Pursuant to the requirements of Section 13 of 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
ODONATE THERAPEUTICS, INC. |
||
|
|
|
|
|
|
Date: February 22, 2019 |
By: |
|
/s/ Kevin Tang |
|
|
|
|
Kevin Tang |
|
|
|
|
Chairman and Chief Executive Officer |
78
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below hereby constitutes and appoints Kevin Tang, John Lemkey and Michael Hearne, and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, to sign in any and all capacities (including, without limitation, the capacities listed below), with respect to this Annual Report on Form 10-K, and any and all amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the U.S. Securities and Exchange Commission (the “SEC”), and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done to enable Odonate Therapeutics, Inc. to comply with the provisions of the Securities Exchange Act of 1934 (the “Exchange Act”) and all the requirements of the SEC, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Exchange Act, this report has been signed by the following persons in the capacities set forth opposite their names and on the dates indicated below.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Kevin Tang Kevin Tang |
|
Chairman and Chief Executive Officer (principal executive officer) |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Michael Hearne Michael Hearne |
|
Chief Financial Officer (principal financial and accounting officer) |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Jeff Vacirca, M.D. Jeff Vacirca, M.D. |
|
Director, Vice Chairman |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Aaron Davis Aaron Davis |
|
Director |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Craig Johnson Craig Johnson |
|
Director |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Laura Johnson Douglass Laura Johnson Douglass |
|
Director |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ Robert Rosen Robert Rosen |
|
Director |
|
February 22, 2019 |
|
|
|
|
|
|
|
/s/ George Tidmarsh George Tidmarsh, M.D., Ph.D. |
|
Director |
|
February 22, 2019 |
79
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
Odonate Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Odonate Therapeutics, Inc. (the Company) as of December 31, 2018 and 2017, the related statements of operations, stockholders’ equity and cash flows for the years then ended, and the related notes to the financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Squar Milner LLP
We have served as the Company's auditor since 2017.
San Diego, California
February 22, 2019
F-2
(in thousands, except par value and share amounts)
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2018 |
|
|
2017 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
139,050 |
|
|
$ |
198,105 |
|
|
Prepaid expenses |
|
|
750 |
|
|
|
4,841 |
|
|
Total current assets |
|
|
139,800 |
|
|
|
202,946 |
|
|
Property and equipment, net |
|
|
1,899 |
|
|
|
165 |
|
|
Restricted cash |
|
|
251 |
|
|
|
- |
|
|
Other |
|
|
723 |
|
|
|
383 |
|
|
Total assets |
|
$ |
142,673 |
|
|
$ |
203,494 |
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
10,777 |
|
|
$ |
4,302 |
|
|
Accrued expenses |
|
|
7,365 |
|
|
|
3,210 |
|
|
Deferred rent, current portion |
|
|
66 |
|
|
|
- |
|
|
Total current liabilities |
|
|
18,208 |
|
|
|
7,512 |
|
|
Deferred rent, less current portion |
|
|
461 |
|
|
|
- |
|
|
Total liabilities |
|
|
18,669 |
|
|
|
7,512 |
|
|
Commitments and contingencies (Note 4) |
|
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
|
Common stock, $0.01 par value—100,000,000 shares authorized; 26,747,438 and 26,890,356 shares issued and outstanding at December 31, 2018 and 2017, respectively |
|
|
244 |
|
|
|
240 |
|
|
Additional paid-in capital |
|
|
252,012 |
|
|
|
235,034 |
|
|
Accumulated deficit |
|
|
(128,252 |
) |
|
|
(39,292 |
) |
|
Total stockholders' equity |
|
|
124,004 |
|
|
|
195,982 |
|
|
Total liabilities and stockholders' equity |
|
$ |
142,673 |
|
|
$ |
203,494 |
|
See accompanying notes.
F-3
Statements of Operations
(in thousands, except share and per share amounts)
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
79,948 |
|
|
$ |
27,902 |
|
|
General and administrative |
|
|
10,816 |
|
|
|
4,842 |
|
|
Total operating expenses |
|
|
90,764 |
|
|
|
32,744 |
|
|
Loss from operations |
|
|
(90,764 |
) |
|
|
(32,744 |
) |
|
Interest income |
|
|
1,804 |
|
|
|
- |
|
|
Net loss |
|
$ |
(88,960 |
) |
|
$ |
(32,744 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(3.64 |
) |
|
$ |
(2.31 |
) |
|
Weighted-average shares outstanding: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
24,462,293 |
|
|
|
14,169,464 |
|
See accompanying notes.
F-4
Statements of Stockholders’ Equity
(in thousands, except share amounts)
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Total Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
|
Balance at December 31, 2016 |
|
|
12,082,514 |
|
|
$ |
102 |
|
|
$ |
8,729 |
|
|
$ |
(6,548 |
) |
|
$ |
2,283 |
|
|
Issuance of common stock, net of issuance costs |
|
|
13,811,156 |
|
|
|
138 |
|
|
|
221,309 |
|
|
|
- |
|
|
|
221,447 |
|
|
Issuance of common stock underlying incentive units |
|
|
996,686 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Non-cash contributions for expenses |
|
|
- |
|
|
|
- |
|
|
|
1,812 |
|
|
|
- |
|
|
|
1,812 |
|
|
Equity-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
3,184 |
|
|
|
- |
|
|
|
3,184 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(32,744 |
) |
|
|
(32,744 |
) |
|
Balance at December 31, 2017 |
|
|
26,890,356 |
|
|
$ |
240 |
|
|
$ |
235,034 |
|
|
$ |
(39,292 |
) |
|
$ |
195,982 |
|
|
Issuance of common stock, net of issuance costs |
|
|
441,073 |
|
|
|
4 |
|
|
|
9,844 |
|
|
|
- |
|
|
|
9,848 |
|
|
Issuance of common stock under employee stock plans |
|
|
21,624 |
|
|
|
- |
|
|
|
349 |
|
|
|
- |
|
|
|
349 |
|
|
Forfeiture of common stock underlying incentive units |
|
|
(605,615 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Non-cash contributions for expenses |
|
|
- |
|
|
|
- |
|
|
|
64 |
|
|
|
- |
|
|
|
64 |
|
|
Equity-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
6,721 |
|
|
|
- |
|
|
|
6,721 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(88,960 |
) |
|
|
(88,960 |
) |
|
Balance at December 31, 2018 |
|
|
26,747,438 |
|
|
$ |
244 |
|
|
$ |
252,012 |
|
|
$ |
(128,252 |
) |
|
$ |
124,004 |
|
See accompanying notes.
F-5
(in thousands)
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(88,960 |
) |
|
$ |
(32,744 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Equity-based compensation expense |
|
|
6,721 |
|
|
|
3,184 |
|
|
Depreciation and amortization |
|
|
350 |
|
|
|
20 |
|
|
Non-cash contributions for expenses |
|
|
64 |
|
|
|
1,812 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Prepaid expenses and other assets |
|
|
3,751 |
|
|
|
(4,956 |
) |
|
Accounts payable |
|
|
6,392 |
|
|
|
3,642 |
|
|
Accrued expenses |
|
|
4,155 |
|
|
|
3,184 |
|
|
Deferred rent |
|
|
433 |
|
|
|
- |
|
|
Net cash used in operating activities |
|
|
(67,094 |
) |
|
|
(25,858 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(1,907 |
) |
|
|
(83 |
) |
|
Net cash used in investing activities |
|
|
(1,907 |
) |
|
|
(83 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net of issuance costs |
|
|
9,848 |
|
|
|
221,447 |
|
|
Proceeds from issuance of common stock under employee stock plans |
|
|
349 |
|
|
|
- |
|
|
Net cash provided by financing activities |
|
|
10,197 |
|
|
|
221,447 |
|
|
Net (decrease) increase in cash and restricted cash |
|
|
(58,804 |
) |
|
|
195,506 |
|
|
Cash and restricted cash, beginning of period |
|
|
198,105 |
|
|
|
2,599 |
|
|
Cash and restricted cash, end of period |
|
$ |
139,301 |
|
|
$ |
198,105 |
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
Issuance costs included in accounts payable and accrued expenses |
|
$ |
- |
|
|
$ |
571 |
|
|
Property and equipment purchases included in accounts payable |
|
$ |
83 |
|
|
$ |
88 |
|
See accompanying notes.
F-6
1. Business
Odonate Therapeutics, Inc. (“Odonate” or the “Company”) is a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. The Company’s initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several pharmacologic properties that make it unique among taxanes, including: oral administration with a low pill burden; a long (~8-day) terminal plasma half-life in humans, enabling the maintenance of adequate drug levels with relatively infrequent dosing; no history of hypersensitivity (allergic) reactions; and significant activity against chemotherapy-resistant tumors. In patients with metastatic breast cancer, tesetaxel was shown to have significant, single-agent antitumor activity in two multicenter, Phase 2 studies. The Company is conducting a multinational, multicenter, randomized, Phase 3 study in patients with locally advanced or metastatic breast cancer, known as CONTESSA. The Company’s goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
On December 6, 2017, the Company converted from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion with the Delaware Secretary of State, and the Company changed its name from “Odonate Therapeutics, LLC” to “Odonate Therapeutics, Inc.” (the “Conversion”). As a result of the Conversion, the membership interests of Odonate Therapeutics, LLC were converted into shares of Odonate Therapeutics, Inc. Prior to the Conversion, the Company formed a holding company named Odonate Holdings, LLC (“Odonate Holdings”) to own Odonate Therapeutics, LLC. Following the Conversion, Odonate Holdings distributed its interest in Odonate Therapeutics, Inc. to prior members of Odonate Therapeutics, LLC, but retained record title to 2,931,402 shares of common stock underlying outstanding incentive units previously granted to employees, officers, directors and consultants by Odonate Management Holdings, LLC and 154,285 shares of common stock beneficially owned by Tang Capital Partners, LP. All shares of common stock and per share amounts for all periods presented in the accompanying financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect the Conversion.
On December 11, 2017, the Company closed its initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters exercised their option to purchase 441,073 additional shares of common stock in the Company’s IPO. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs.
As of December 31, 2018, the Company had $139.1 million in cash. The Company has incurred operating losses and negative cash flows from operations since inception. Management believes the Company’s existing cash as of December 31, 2018 will be sufficient to meet the Company’s anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (the “SEC”).
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s financial statements are prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”). The preparation of the Company’s financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in the Company’s financial statements and accompanying notes. The most significant estimates in the Company’s financial statements relate to accrued expenses and equity-based compensation expense. These estimates and assumptions are based on current facts, historical experience and various other factors believed to be
F-7
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s future results of operations will be affected.
Common Stock Split
In November 2017, the Company completed a 2-for-1 forward split of the Company’s outstanding shares of common stock. The accompanying financial statements and notes to the financial statements give retroactive effect to the forward split for all periods presented.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment.
Fair Value Measurements
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or non-recurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs such as quoted prices in active markets.
Level 2: Inputs, other than the quoted prices in active markets that are observable either directly or indirectly.
Level 3: Unobservable inputs for which there are little or no market data, which require the reporting entity to develop its own assumptions.
The carrying amounts of the Company’s cash, prepaid expenses, accounts payable and accrued expenses are considered to be representative of their respective fair values because of the short-term nature of those instruments. As of December 31, 2018 and 2017, the Company had no financial assets or liabilities measured at fair value on a recurring basis.
Cash and Restricted Cash
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. The Company maintains its cash in checking and savings accounts. Income generated from cash held in savings accounts is recorded as interest income. As of December 31, 2018 and 2017, the Company held no cash equivalents. Cash is classified as restricted cash when it is reserved for a specific purpose and, therefore, is not available for immediate or general business use.
F-8
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash. The Company maintains deposits at federally insured financial institutions in excess of federally insured limits. The Company has not experienced any losses in such accounts, and management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
Property and Equipment
Property and equipment consists of office equipment, software, furniture and fixtures and leasehold improvements. Office equipment, software and furniture and fixtures are stated at cost and depreciated on a straight-line basis over the estimated useful life of the related assets, which generally ranges from three to five years. Leasehold improvements are amortized over the lesser of the estimated useful life or the remaining term of the lease.
Deferred Rent
Rent expense is recorded on a straight-line basis over the term of the lease. The difference between rent expense and amounts paid under the lease agreement is recorded as deferred rent. Tenant improvement allowances and other lease incentives are recorded as deferred rent and amortized on a straight-line basis over the term of the lease as reductions to rent expense.
Research and Development Expense
Research and development expense consists primarily of costs associated with the development of the Company’s product candidates and includes: (i) salaries, benefits, travel, equity-based compensation expense and facility-related expense for personnel engaged in research and development functions; (ii) expense incurred under agreements with contract research organizations (“CROs”), investigative sites and consultants that conduct the Company’s nonclinical and clinical studies; (iii) manufacturing development and scale-up expense and the cost of acquiring and manufacturing clinical study materials and commercial materials, including manufacturing registration and validation batches; (iv) payments to consultants engaged in the development of the Company’s product candidates, including equity-based compensation expense, travel and other expense; and (v) costs related to compliance with quality and regulatory requirements.
Research and development expense is charged to operations as incurred when the expenditures relate to the Company’s research and development efforts and have no alternative future uses. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
Patent Costs
Costs related to filing and pursuing patent applications are recorded as general and administrative expense and are expensed as incurred, since recoverability of such expenditures is uncertain.
Equity-based Compensation Expense
The Company issues stock options and had historically issued incentive units, considered “profits interests” within the meaning of U.S. federal and state tax rules, to employees, consultants and certain directors. Equity-based compensation expense represents the cost of the grant-date fair value of the awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. The Company accounts for awards to non-employees using the fair value approach. Non-employee awards are subject to periodic revaluation over their vesting terms.
F-9
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Income taxes are accounted for using the asset and liability method. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period of enactment. A valuation allowance against deferred tax assets is recorded if, based on the weight of all available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the financial statements. The Company’s policy is to record interest and penalties, if any, related to uncertain tax positions as a component of income tax expense.
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources. There have been no items qualifying as other comprehensive loss, and, therefore, for all periods presented, the Company’s comprehensive loss was the same as its reported net loss.
Net Loss per Share
Basic net loss per share is calculated by dividing net loss by the weighted average common shares outstanding during the period, without consideration of common stock equivalents. Basic net loss per share excludes 2,096,758 and 2,931,402 outstanding shares of common stock held by Odonate Holdings as of December 31, 2018 and 2017, respectively, to be used to settle incentive units previously issued under the Odonate Management Holdings Equity Incentive Plan (the “Management Plan”). These shares of common stock are subject to transfer to the Company and cancellation until such incentive units are vested and exercised and, as such, are considered common stock equivalents. Therefore, the shares of common stock held by Odonate Holdings are excluded from the basic net loss per share calculation until the incentive units are exercised.
Diluted net loss per share is calculated by adjusting weighted average common stock outstanding for the dilutive effect of common stock equivalents outstanding for the period. Common stock equivalents, which consist of shares of common stock underlying incentive units and vested stock options, were excluded from the calculation of diluted net loss per stock because they were anti-dilutive.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (“ASU 2016-02”). This guidance requires lessees to recognize operating leases with a term greater than one year on the balance sheet as a right-of-use asset and corresponding lease liability. ASU 2016-02 is effective for annual and interim reporting periods beginning after December 15, 2018, and early adoption is permitted. Although ASU 2016-02 is required to be adopted at the earliest period presented using a modified retrospective approach, the FASB issued ASU No. 2018-11, Leases (Topic 842): Targeted Improvements (“ASU 2018-11”), which allows for an alternative transition method of adoption by recognizing a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. The Company plans to adopt ASU 2016-02 on January 1, 2019 utilizing the alternative transition method allowed for under ASU 2018-11. Although the Company is in the process of evaluating the impact the adoption of ASU 2016-02 will have on its financial statements, the Company expects the most significant changes will be the recognition of right-of-use assets and lease liabilities on the Company’s balance sheet for its San Diego Lease and New York Lease (see Note 4).
F-10
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Property and equipment consisted of the following (in thousands):
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2018 |
|
|
2017 |
|
||
|
Leasehold improvements |
|
$ |
1,113 |
|
|
$ |
- |
|
|
Computer equipment |
|
|
504 |
|
|
|
143 |
|
|
Furniture and fixtures |
|
|
432 |
|
|
|
- |
|
|
Software |
|
|
126 |
|
|
|
42 |
|
|
Total gross property and equipment |
|
|
2,175 |
|
|
|
185 |
|
|
Less accumulated depreciation |
|
|
(276 |
) |
|
|
(20 |
) |
|
Property and equipment, net |
|
$ |
1,899 |
|
|
$ |
165 |
|
Depreciation expense was $0.3 million and $20,000 for the years ended December 31, 2018 and 2017, respectively.
Accrued expenses consisted of the following (in thousands):
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2018 |
|
|
2017 |
|
||
|
Accrued clinical development costs |
|
$ |
5,732 |
|
|
$ |
3,033 |
|
|
Accrued compensation and related expenses |
|
|
1,619 |
|
|
|
169 |
|
|
Other accrued expenses |
|
|
14 |
|
|
|
8 |
|
|
Total accrued expenses |
|
$ |
7,365 |
|
|
$ |
3,210 |
|
4. Commitments and Contingencies
Commitments
In March 2018, the Company entered into an agreement to lease office space in San Diego, California (the “San Diego Lease”) with aggregate payments of approximately $0.8 million over the term of the lease. The San Diego Lease expires on December 31, 2019.
In February 2018, the Company entered into an agreement to lease office space in New York, New York (the “New York Lease”) with aggregate payments of approximately $2.8 million over the 7-year term of the lease. The Company has an option to extend the New York Lease for an additional three years at the end of the initial term. Further, the Company provided a standby letter of credit of $0.3 million in lieu of a security deposit during the term of the lease, subject to a reduction 3.5 years after the lease commencement. As of December 31, 2018, $0.3 million was pledged as collateral for the letter of credit and recorded as restricted cash.
Future minimum lease payments under the New York Lease as of December 31, 2018 are as follows (in thousands):
|
2019 |
|
$ |
367 |
|
|
2020 |
|
|
376 |
|
|
2021 |
|
|
385 |
|
|
2022 |
|
|
399 |
|
|
2023 |
|
|
427 |
|
|
Thereafter |
|
|
806 |
|
|
Total future minimum lease payments |
|
$ |
2,760 |
|
Rent expense under leases was $0.7 million for the year ended December 31, 2018. No rent expense was recorded for the year ended December 31, 2017.
F-11
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
The Company enters into contracts in the normal course of business with third-party contract research organizations, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancellable contracts and not considered contractual obligations and commitments.
Contingencies
From time to time, the Company may become subject to claims and litigation arising in the ordinary course of business. The Company is not a party to any material legal proceedings, nor is it aware of any pending or threatened litigation.
5. Stockholders’ Equity
Common Stock Sales
On December 11, 2017, the Company closed its IPO of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters exercised their option to purchase 441,073 additional shares of common stock in the Company’s IPO. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs.
In September 2017, the Company raised $74.0 million in cash (net of $15,000 of issuance costs) through the sale of 4,968,132 shares of common stock at a price of $14.90 per share to new and certain existing investors.
In March 2017, the Company raised $10.0 million in cash (net of $14,000 of issuance costs) through the sale of 2,593,024 shares of common stock at a price of $3.86 per share to certain existing investors.
Non-cash Contributions for Expenses
Non-cash contributions for expenses represent certain services and other benefits received by the Company from an affiliate without charge to the Company (see Note 9). These services and other benefits are recorded as expense with corresponding increases to additional paid-in capital. The Company recorded expense, and corresponding increases to additional paid-in capital, of $0.1 million and $1.8 million for the years ended December 31, 2018 and 2017, respectively, for services and other benefits provided without charge to the Company.
6. Equity Incentive Plans
2017 Stock Option Plan
Following the Conversion, the Company adopted the Odonate Therapeutics, Inc. 2017 Stock Option Plan (the “2017 Plan”) in order to grant stock options to employees, officers, directors and consultants of the Company. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to the fair market value of such stock on the date of grant. The maximum term of options granted under the 2017 Plan is 10 years. The options generally vest over a 4-year period from either the date of grant or the commencement of service.
A total of 4,800,000 shares of common stock have been reserved for issuance under the 2017 Plan. As of December 31, 2018, 1,534,838 shares of common stock remained available for future grants under the 2017 Plan.
2017 Employee Stock Purchase Plan
Following the Conversion, the Company adopted the Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan (the “ESPP”) in order to provide a means for eligible employees to accumulate
F-12
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
shares of the Company’s common stock over time through regular payroll deductions. Under the ESPP, eligible employees may purchase shares of the Company’s common stock twice per month at a price equal to 85% of the closing price of shares of the Company’s common stock on the date of each purchase. Eligible employees purchasing shares under the ESPP are subject to an annual cap equal to the lesser of $25,000 or 10% of the employee’s annual cash compensation. Shares purchased under the ESPP cannot be sold for a period of one year following the purchase date (or such shorter period of time if the participating employee’s employment terminates before this one-year anniversary).
A total of 500,000 shares of common stock have been reserved for issuance under the ESPP. As of December 31, 2018, 479,220 shares of common stock remained available for future grants under the ESPP.
Management Plan
In August 2016, the Company adopted the Management Plan in order to allow for directors, officers, employees and consultants of Odonate (the “Management Plan Participants”) to share in the performance of the Company. The incentive units issued under the Management Plan were issued to Odonate Management Holdings, LLC, which issued incentive units to the Management Plan Participants on the same terms and conditions. The incentive units generally vest over a 4-year period from either the date of grant or the commencement of service and are subject to continued service requirements. Generally, on termination of services, unvested incentive units are forfeited. Following the IPO, the vested incentive units may be exercised by the Management Plan Participants, with the value received by the Management Plan Participants in the form of cash or shares of common stock equal to the fair market value on the date of exercise less the exercise price of the incentive unit. Following the Conversion, the Company has not granted, and will no longer grant, incentive units.
Prior to the Conversion, the Company issued an aggregate of 2,931,402 incentive units under the Management Plan. As of December 31, 2018, 2,096,758 outstanding shares of common stock were held by Odonate Holdings to be used to settle incentive units previously issued under the Management Plan.
Equity Awards
The activity related to equity awards, which are comprised of stock options and incentive units, during the year ended December 31, 2018 is summarized as follows:
|
|
|
Equity Awards |
|
|
Weighted- average Exercise Price per Share |
|
|
Weighted- average Remaining Contractual Term(1) (years) |
|
|
Aggregate Intrinsic Value(2) (millions) |
|
||||
|
Outstanding at December 31, 2017 |
|
|
3,115,101 |
|
|
$ |
5.50 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
3,352,787 |
|
|
$ |
18.05 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(239,712 |
) |
|
$ |
0.82 |
|
|
|
|
|
|
|
|
|
|
Cancelled/forfeited |
|
|
(866,256 |
) |
|
$ |
11.23 |
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2018 |
|
|
5,361,920 |
|
|
$ |
12.63 |
|
|
|
9.7 |
|
|
$ |
20.7 |
|
|
Exercisable at December 31, 2018 |
|
|
1,002,800 |
|
|
$ |
4.02 |
|
|
|
9.0 |
|
|
$ |
10.6 |
|
|
(1) |
Represents the weighted-average remaining contractual term of stock options. The incentive units have no expiration. |
|
(2) |
Aggregate intrinsic value represents the product of the number of equity awards outstanding or equity awards exercisable multiplied by the difference between the Company’s closing stock price per share on the last trading day of the period, which was $14.08 as of December 31, 2018, and the exercise price. |
The total intrinsic value of equity awards exercised during the years ended December 31, 2018 was $4.2 million. No equity awards were exercised during the year ended December 31, 2017. The total fair value of equity awards vested during the years ended December 31, 2018 and 2017 was $7.5 million and $0.3 million, respectively.
F-13
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Equity-based Compensation Expense
For the years ended December 31, 2018 and 2017, the weighted average grant-date fair value per equity award was $11.92 and $17.47, respectively. The Company estimated the fair value of each equity award on the grant date using the Black-Scholes option-pricing model with the following assumptions:
|
|
|
Year Ended |
||
|
|
|
December 31, |
||
|
|
|
2018 |
|
2017 |
|
Expected volatility |
|
70% - 75% |
|
73% - 78% |
|
Expected life |
|
6 years |
|
6 years |
|
Risk-free interest rate |
|
2.3% - 3.0% |
|
2.0% - 2.3% |
|
Expected dividend yield |
|
0% |
|
0% |
Expected Volatility. Due to the Company’s lack of Company-specific historical or implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar public companies in the life sciences industry. The Company selected the peer group based on comparable characteristics, including development stage, product pipeline and enterprise value. The Company computed historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the equity-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own share price becomes available.
Expected Life. The expected life represents the period that the equity awards are expected to be outstanding. It is based on the “simplified method” for developing the estimate of the expected life. Under this approach, the expected life is presumed to be the midpoint between the average vesting date and the end of the contractual term.
Risk-free Interest Rate. The Company bases the risk-free interest rate assumption on U.S. Treasury constant maturities with maturities similar to those of the expected term of the equity award being valued.
Expected Dividend Yield. The Company bases the expected dividend yield assumption on the fact that it has never paid dividends and does not expect to pay dividends in the foreseeable future.
In addition to assumptions used in the Black-Scholes option-pricing model, the Company estimates a forfeiture rate to calculate the equity-based compensation expense for equity awards. The forfeiture rate is based on an analysis of actual and estimated forfeitures.
Under the ESPP, eligible employees may purchase shares of the Company’s common stock twice per month at a price equal to 85% of the closing price of shares of the Company’s common stock on the date of each purchase. The benefit received by the employees, which is equal to a 15% discount on the shares of the Company’s common stock purchased, is recognized as equity-based compensation expense on the date of each purchase.
The classification of equity-based compensation expense is summarized as follows (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Equity-based compensation expense: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
5,659 |
|
|
$ |
2,633 |
|
|
General and administrative |
|
|
1,062 |
|
|
|
551 |
|
|
Total equity-based compensation expense |
|
$ |
6,721 |
|
|
$ |
3,184 |
|
As of December 31, 2018, total unrecognized compensation cost related to unvested equity awards was $37.7 million, which will be recognized over a weighted average period of 3.2 years. As of
F-14
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
December 31, 2018, there was no unrecognized equity-based compensation expense related to shares of common stock issued under the ESPP.
7. Income Taxes
Prior to the Conversion, the Company operated as a limited liability company (“LLC”). An LLC combines corporate protection from personal liability for business debts along with the pass-through tax structure of a partnership or sole proprietorship. Business income is passed through the business to the LLC members, who report their share of profits or losses on their respective income tax returns. Net operating losses incurred by the Company through the date of the Conversion are passed through to the LLC members and are not able to be carried forward to the Company.
From the Conversion through December 31, 2018, the Company did not record a provision for income taxes due to a full valuation allowance against its deferred tax assets.
In December 2017, the Tax Cuts and Jobs Act (the “2017 Tax Act”) was enacted. The 2017 Tax Act included a number of changes to existing U.S. tax laws that impacted the Company, most notably a reduction of the U.S. corporate income tax rate from 34% to 21% for tax years beginning after December 31, 2017. The 2017 Tax Act also provided for a one-time transition tax on certain foreign earnings, the acceleration of depreciation for certain assets placed into service after September 27, 2017 and other prospective changes beginning in 2018, including repeal of the domestic manufacturing deduction, acceleration of tax revenue recognition, capitalization of research and development expenditures, additional limitations on executive compensation and limitations on the deductibility of interest.
The Company recognized the income tax effects of the 2017 Tax Act in its 2017 financial statements in accordance with ASC Topic 740, Income Taxes, during the reporting period in which the 2017 Tax Act was signed into law. As such, the Company’s financial results reflect the income tax effects of the 2017 Tax Act for which the accounting under ASC Topic 740 is complete.
The difference between the provision for income taxes and income taxes computed using the U.S. federal income effective tax rate is as follows (in thousands):
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Federal statutory rate |
|
$ |
(18,682 |
) |
|
$ |
(1,296 |
) |
|
State taxes, net of federal benefit |
|
|
(6,213 |
) |
|
|
(222 |
) |
|
Research and development credits |
|
|
(2,725 |
) |
|
|
(214 |
) |
|
Change in valuation allowance |
|
|
26,295 |
|
|
|
907 |
|
|
Equity-based compensation expense |
|
|
1,227 |
|
|
|
116 |
|
|
Impact of the 2017 Tax Act |
|
|
- |
|
|
|
709 |
|
|
Other permanent differences |
|
|
98 |
|
|
|
- |
|
|
Income tax provision |
|
$ |
- |
|
|
$ |
- |
|
F-15
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Deferred income tax assets arising from differences between accounting for financial statement purposes and tax purposes, less valuation allowance are as follows (in thousands):
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2018 |
|
|
2017 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
23,450 |
|
|
$ |
945 |
|
|
Capitalized research and development |
|
|
3,594 |
|
|
|
306 |
|
|
Equity-based compensation expense |
|
|
714 |
|
|
|
- |
|
|
Depreciation and amortization |
|
|
574 |
|
|
|
786 |
|
|
Total gross deferred tax assets |
|
|
28,332 |
|
|
|
2,037 |
|
|
Less valuation allowance |
|
|
(28,332 |
) |
|
|
(2,037 |
) |
|
Total deferred tax assets |
|
$ |
- |
|
|
$ |
- |
|
As of December 31, 2018 and 2017, the Company established a full valuation allowance against its federal and state deferred tax assets due to the uncertainty surrounding the realization of such assets.
As of December 31, 2018 and 2017, the Company had federal net operating loss carryforwards of $83.3 million and $3.4 million, respectively, and state net operating loss carryforwards of $85.4 million and $3.4 million, respectively. The federal and state net operating loss carryforwards begin to expire in 2037, unless utilized. As of December 31, 2018 and 2017, the Company also had federal research and development credit carryforwards of $3.2 million and $0.2 million, respectively, and state research development credit carryforwards of $0.4 million and $0.1 million, respectively. The federal research and development credit carryforwards will begin to expire in 2037, unless utilized. The state research and development credit carryforwards will carry forward indefinitely, unless utilized.
Pursuant to Section 382 and 383 of the Internal Revenue Code (“IRC”), utilization of the Company’s federal net operating loss carryforwards and research and development credit carryforwards may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating loss carryforwards and research and development credit carryforwards prior to utilization. The Company has not completed an IRC Section 382 and 383 analysis regarding the limitation of net operating loss carryforwards and research and development credit carryforwards.
As of December 31, 2018 and 2017, the Company had no unrecognized tax benefits. The Company does not anticipate there will be a significant change in unrecognized tax benefits within the next 12 months.
The Company had no accrual for interest or penalties on the balance sheets as of December 31, 2018 and 2017 and has not recognized interest or penalties in the statements of operations for the years ended December 31, 2018 and 2017.
The Company is subject to taxation in the U.S. and various state jurisdictions. The Company’s tax returns for the tax years 2015 through 2017 are open and are subject to examination by federal and state taxing authorities. If such examinations result in adjustments to the income or expense amounts, the amounts allocated to the LLC members could be adjusted accordingly. The Company is not currently undergoing a tax audit in any federal or state jurisdiction.
8. License Agreement
In 2013, the Company licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Under the Daiichi Sankyo license agreement, the Company is obligated to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. The Company is required to make aggregate future milestone payments of up to
F-16
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
$31.0 million, contingent on attainment of certain regulatory milestones. Additionally, the Company will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel.
9. Related Party Transactions
Commencing in 2016, the Company received certain services and other benefits from an affiliate (the “Affiliate”) of the Chairman and Chief Executive Officer of the Company. The Company was not charged any fees for these services and other benefits, which included personnel costs for research and development and administrative functions, rent and facility costs and other direct expenses. The Company recorded expense, and corresponding increases to additional paid-in capital, of $0.1 million and $1.8 million for the years ended December 31, 2018 and 2017 respectively, for services and other benefits provided without charge to the Company. Personnel costs were based on actual costs incurred by the Affiliate, which were allocated based on the estimated percentage of time employees spent on Odonate on an employee-by-employee basis. Rent and facility costs were based on actual costs incurred by the Affiliate and allocated based on the Company’s use of shared space using headcount. Other direct expenses paid by the Affiliate were specifically identifiable to the Company and were allocated directly to the Company. The Chairman and Chief Executive Officer of the Company has elected to receive an annual salary of $1.00 and to not receive any bonuses, equity or other compensation.
Management believes that the method used to allocate costs is a fair and reasonable reflection of the utilization of the services provided to, or the benefit received by, the Company during the periods presented. The allocations may not, however, reflect the costs that the Company would have incurred if the Company had not received these services. Actual costs would depend on a number of factors, including strategic decisions in the areas of hiring, facility location and whether to outsource certain functions.
10. 401(k) Plan
During 2016, the Company adopted a defined contribution 401(k) plan available to eligible employees. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under U.S. federal tax regulations. The Company makes a mandatory annual contribution of 3% of the eligible employees’ compensation to the 401(k) plan. In addition, the Company makes matching contributions of up to 6% of the eligible employees’ compensation to the 401(k) plan. For the years ended December 31, 2018 and 2017, the Company incurred costs of $1.0 million and $0.2 million, respectively, related to the 401(k) plan.
F-17
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-09-29)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S