Oncology Institute, Inc. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the fiscal year ended December 31, 2022
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from _____ to _____
Commission file number 001-39248
The Oncology Institute, Inc.
(Exact name of registrant as specified in its charter)
Delaware | 84-3562323 | ||||||||||
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | ||||||||||
18000 Studebaker Rd, Suite 800 | |||||||||||
Cerritos, California 90703 | |||||||||||
(Address of Principal Executive Offices) | |||||||||||
(562) 735-3226
Registrant's telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
| Common Stock, $0.0001 par value per share | TOI | The Nasdaq Stock Market LLC | ||||||
| Warrants to purchase common stock | TOIIW | The Nasdaq Stock Market LLC | ||||||
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports); and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
1
| Large accelerated filer | ☐ | Accelerated filer | ☒ | ||||||||
Non-accelerated filer | ☐ | Smaller reporting company | ☒ | ||||||||
Emerging growth company | ☒ | ||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of voting stock held by non-affiliates of the Registrant, based on the closing price of $5.06 per shares of the Registrant’s common stock as reported by the Nasdaq Capital Market as of June 30, 2022, was approximately $125.7 million.
The registrant had outstanding 74,551,556 shares of common stock as of March 6, 2023.
DOCUMENTS INCORPORATED BY REFERENCE
Part III of this Form 10-K incorporates by reference information from the registrant’s proxy statement for the annual meeting of stockholders expected to be held on June 15, 2023.
2
Table of Contents
| Page | |||||
3
FORWARD-LOOKING STATEMENTS
In this Annual Report on Form 10-K, including "Business” in Item 1, “Risk Factors” in Item 1A, and "Management’s Discussion and Analysis of Financial Condition and Results of Operations" in Item 7, and the documents incorporated by reference herein, The Oncology Institute, Inc. ("TOI", "we", or "our") make forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements relate to expectations for future financial performance, business strategies or expectations for our business. These statements may be preceded by, followed by or include the words "may," "might," "will," "will likely result," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or similar expressions.
These forward-looking statements are based on information available to us as of the date they were made, and involve a number of risks and uncertainties which may cause them to turn out to be wrong. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date, and we do not undertake any obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward- looking statements. Some factors that could cause actual results to differ include:
•expectations and assumptions about growth rate and market opportunity of TOI;
•our public securities’ potential liquidity and trading;
•our ability to raise financing in the future;
•our success in retaining or recruiting, or changes required in, our officers, key employees or directors;
•the impact of the regulatory environment and complexities with compliance related to such environment;
•factors relating to the business, operations and financial performance of TOI, including:
◦the impact of the COVID-19 pandemic;
◦the ability of TOI to maintain an effective system of internal controls over financial reporting;
◦the ability of TOI to grow market share in its existing markets or any new markets it may enter;
◦the ability of TOI to respond to general economic conditions;
◦the ability of TOI to manage its growth effectively;
◦the ability of TOI to achieve and maintain profitability in the future;
◦the ability of TOI to attract new patients;
◦continued reimbursement from third-party payors; and
◦other factors detailed under the section titled “Risk Factors” within this 10-K.
4
PART I
Item 1. Business
Overview
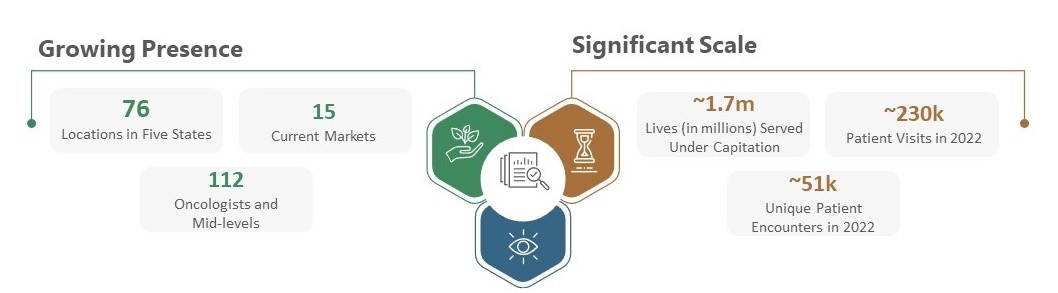
The Oncology Institute, Inc. ("TOI", the "Company", "we", or "our) is a leading value-based oncology company that manages community-based oncology practices that serve patients at 76 clinic locations across 15 markets and five states throughout the United States. Our community-based oncology practices are staffed with 112 oncologists and advanced practice providers. 62 of these clinics are staffed with 101 providers employed by our affiliated physician-owned professional corporations, referred to as the "TOI PCs", which provided care for more than 64,000 patients in 2022 and managed a population of approximately 1.7 million patients under value-based agreements as of December 31, 2022. We also provide management services to 14 clinic locations owned by independent oncology practices. Our mission is to heal and empower cancer patients through compassion, innovation, and state-of-the-art medical care.
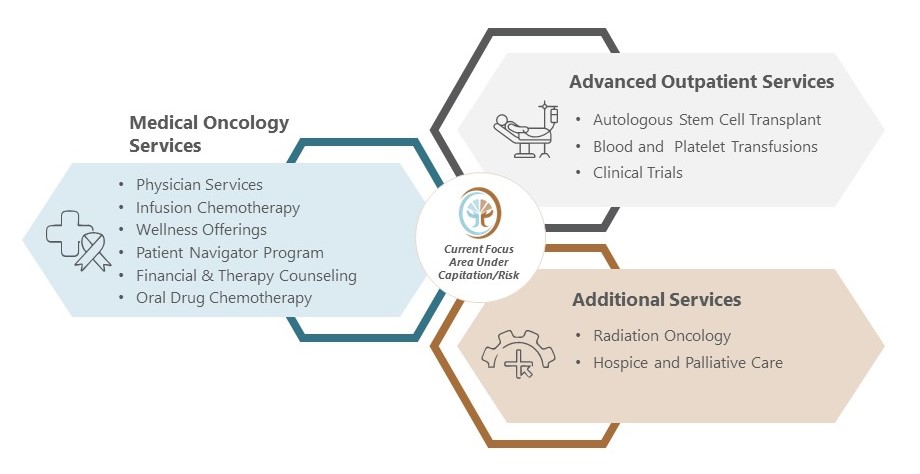
Our managed clinics provide a range of medical oncology services, including physician services, in-house infusion and dispensary, clinical trial services, radiation, innovative programs like outpatient blood product transfusions, along with 24/7 patient support. Through TOI Clinical Research, LLC ("TCR"), the clinical research arm of the TOI PCs, we also provide and manage clinical trial services and research for the benefit of cancer patients. Many of our services, such as managing clinical trials and palliative care programs, are traditionally accessed through academic and tertiary care settings, while the TOI PCs bring these services to patients in a community setting. As scientific research progresses and more treatment options become available, cancer care is shifting from acute care episodes to chronic disease management. With this shift, it is increasingly important for high-quality, high-value cancer care to be available in a local community setting to all patients in need.
5
As a value-based oncology company, we seek to deliver better quality care while managing costs for patients and payors that we serve. We define value-based care as care that focuses on providing optimal health outcomes and healthcare affordability and a value-based contract as any contract that removes the incentive to drive up cost of care or overutilization, while rewarding better clinical outcomes, cost and quality. We work to accomplish this goal by reducing wasteful, inefficient or futile care that drives up costs but does not improve outcomes. We believe payors and employers are aligned with the value-based model due to its enhanced access, improved outcomes, and lower costs. Patients under our affiliated providers’ care can benefit from evidence-based and personalized care plans, gain access to sub-specialized care in convenient community locations, and lower out-of-pocket costs. We believe our affiliated providers enjoy the stability and predictability of a large multi-state practice, are not incentivized or pressured to over-treat when it may be inconsistent with a patient’s goals of care and can focus on practicing outstanding evidence-based oncology care.
In contrast to value-based care, we believe much of traditional fee-for-service, or FFS, oncology care is plagued by misaligned incentives that drive up costs and often lower the quality of care. In FFS care, oncologists are reimbursed on a “cost-plus” basis for drugs which in turn are often responsible for the majority of a practice’s revenue. This "cost-plus" model may incentivize oncologists to prescribe the most expensive treatments even if lower cost alternatives that are still medically appropriate are available, as well as to continue to utilize chemotherapy in advanced cancer patients who may no longer benefit from such treatment. In these cases, patients and payors not only bear the burden of higher cost of care, but patients may also suffer negative health outcomes including higher rates of emergency room visits and hospitalizations for supportive care needs due to the side effects associated with chemotherapy.
In 2022, we generated more than 50% of our revenue from patients who are covered by value-based contracts. Historically, our value-based contracts have predominately taken the form of capitated contracts. Our capitated contracts remove incentives to drive up costs, and they also have incentives for meeting or exceeding certain quality metrics. In some capitated contracts we are penalized if we fail to meet certain quality metrics. In other capitated contracts, we receive bonuses/rewards if we meet or exceed certain quality metrics. Our value-based contracts could also take on other forms, such as sharing with payors in the cost savings generated for specific medical oncology costs (which we refer to as 'gain-sharing' contract), along with incentives to meet certain quality metrics. These contracts, despite their modifications on how reimbursement is structured, still meet the definition of value-based care. We and our affiliated providers have contractual relationships with payors serving a variety of patients, including Medicare Advantage ("MA"), Medicaid, and commercial patients. These payors include affiliates of Anthem, CareMore Health, Heritage Provider Network and Optum Care.
In 2023, we are continuing to evolve the way we structure our value-based arrangements, particularly in areas of the country where there are limits on medical group delegation, or an increased desire from groups to incentivize TOI for cost of care that extends beyond medical oncology and includes elements such as management of acute care utilization and/or management of non-employed oncologists. We believe that these new contracting methodologies are furthering our mission to provide access to world-class oncology care in an affordable manner to underserved populations.
We believe that our position in the market and focus on elevating the state of oncology care with a value-based care model positions our affiliated providers well for future growth. Our technology platform supports this growth and enables the TOI PCs to standardize and deliver consistent value-based care at scale. We believe that our model will support growth into new markets, allow us to continue service more patients across the United States.
Our website is www.theoncologyinstitute.com. The information contained on our website is not a part of this annual report.
Affiliated Physician Practices
Some states have laws that prohibit business entities with non-physician owners from practicing medicine, which are generally referred to as the corporate practice of medicine. States that have corporate practice of medicine laws require only physicians to practice medicine, exercise control over medical decisions or engage in certain arrangements with other physicians, such as fee-splitting. For example, under California's corporate practice of medicine doctrine, physicians and certain licensed professionals cannot be employed by non-professional corporations, except under limited exceptions which do not apply to us. Additionally, all clinical decisions and certain business or management decisions that result in control over a physician's practice of medicine must be made by a licensed physician and not by an unlicensed person or entity. California also prohibits professional fee-splitting arrangements, but management fees based on a percentage of gross revenue or similar arrangement that is commensurate with fair market value of services provided by the management company are generally permissible.
We have entered into a management services agreement with each of the TOI PCs, which are entirely physician owned. Under our management services agreements, we have agreed to serve, on an exclusive basis, as manager and administrator of each TOI PC’s non-medical functions and services related to healthcare services and items provided to patients by physicians and other licensed healthcare providers employed by or under contract with a TOI PC. The non-medical functions and services
6
we provide under the management services agreements include practice management services and non-clinical operational assistance for all TOI PC clinic locations, assistance with provider and payor contract negotiations and administration, billing and collection services, financial and accounting services, electronic medical records and practice management technology solutions, assistance in maintaining licensure, permits and other credentialing requirements for the TOI PCs, risk management services, non-clinical personnel services, provider recruitment services and other administrative services required for the day-to-day operations of the clinics and TOI PCs. Our management services agreements with the TOI PCs have 20-year terms, unless terminated upon mutual agreement of the parties or unilaterally by a party following a material breach or commencement of bankruptcy or liquidation events by the other party, or a governmental or judicial termination order against a party. Under the management services agreement, we receive a monthly management fee that is structured as direct reimbursement of all costs incurred plus a percentage of the TOI PC’s gross revenue, which is defined as the TOI PC’s total revenues payable for all healthcare services and items rendered by the TOI PC, adjusted for bad debt, discounts and payor contract adjustments. In accordance with relevant accounting guidance, each of the TOI PCs is determined to be a variable interest entity, or VIE, of the Company as the Company has the ability, through the management services agreement, to direct the activities (excluding clinical decisions) that most significantly affect the TOI PC’s economic performance.
Market Overview
Our business is focused on caring for adult and senior populations with medical oncology and related care needs, including members of MA plans run by private insurance companies on behalf of the Centers for Medicare and Medicaid Services, or CMS, as well as traditional FFS Medicare, Medicaid, other government healthcare programs and commercial insurance populations. One of our primary focuses is on value-based contracts in which we manage the medical oncology care for a population of patients for a pre-determined, population-based capitated payment. Many of the patients that we manage under value-based arrangements are referred to as “capitated” populations, however our affiliated providers also provide care to patients outside of these arrangements under traditional FFS arrangements as well as other types of value-based contract.
As of December 31, 2022, we were active in 15 markets in five states. Across these states, there were approximately 81 million commercial, Medicaid, and MA lives. This population provides us with a substantial opportunity to capture a portion of those lives in both our legacy, existing markets, as well as in our new expansion geographies.
Our Care Model
Since our founding over 15 years ago, we have built a solid track record around our care model for value-based oncology care. Our care model is focused on delivering personalized, evidenced-based care, consistently, and at scale. We seek to deliver better patient outcomes for lower costs, and to care for more of our payors’ patient populations.
Our care model is designed to remove physicians’ incentives to over-prescribe or prescribe high-cost chemotherapy that is of limited clinical utility to patients. We invest in nurse practitioners to help with advanced care planning and palliative care discussions with patients. We give patients the education and tools to make their own decisions about when the right time is to choose palliative care or hospice.
7

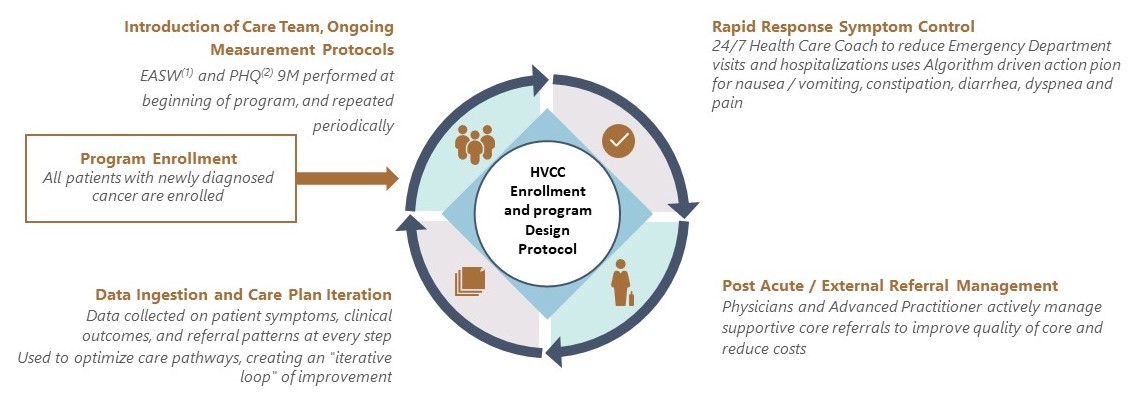
While the TOI PCs treat patients under both value-based and FFS contracts, our affiliated providers’ approach to care focuses on achieving the best outcomes at the lowest cost, regardless of the reimbursement methodology. We have developed a High Value Cancer Care, or HVCC, program, in which patients are able to access targeted care resources that augment and support their treatment. Our treatment regimens are based on algorithms established by the National Comprehensive Cancer Network (“NCCN”) and are evidence-based. NCCN is a not-for-profit alliance of 32 leading cancer centers devoted to patient care, research and education (not including TOI). NCCN focuses on improving cancer care through the input of clinical thought leaders at its member organizations. NCCN publishes guidelines developed from evidence- based medicine to ensure that all cancer patients receive preventative, diagnostic, treatment, and supportive services that are most likely to lead to optimal outcomes. The NCCN guidelines are widely recognized as the standard for clinical care in medical oncology, and the intent of the guidelines is to assist in clinical decision making. Our affiliated providers strive to ensure that clinical pathways in our electronic health records system, as well as recommendations on use of chemotherapy and supportive care medications are consistent with NCCN guidelines to ensure patients receive the best clinical care based on their individual disease and comorbidities. Moreover, the TOI PCs operate physician dispensaries that allow our affiliated providers to prescribe and dispense oral oncolytics and related medications to patients, alongside chemotherapy infusion and injections. This provides patients with holistic and convenient access to the most appropriate treatment pathways, all in a community setting. According to a study conducted by researchers at Stanford University on the TOI PCs’ patients in 2019 who were enrolled in our HVCC program, we saw improvements in several key metrics, including:

Overall, the study demonstrated greater than 25% lower median total healthcare costs from diagnosis to death. We are continuously improving and innovating our care model, using the clinical data from the HVCC program to develop evidence-based care and treatment protocols for all patients.
8
Our Value Proposition and Differentiated Care Model
Our managed clinics primarily serve adult and senior cancer patients in markets that have MA plans and primary care medical groups reimbursed on a capitated basis. Our affiliated providers provide these services primarily through employed providers who are responsible for patient care. We intend to leverage our long-established, strong relationships with payors to continue to build out our network and increase access to cancer patients in adjacent markets, while at the same time, decreasing oncology care costs for both patients and payors. Through the TOI PCs, we seek to provide high quality and lower cost care delivery through the following capabilities:
•recruiting process focused on selecting physicians that want to practice evidence-based medicine;
•technology-enabled care pathways ensuring adherence to evidence-based clinical protocols;
•strong clinical culture and physician oversight;
•care management to prevent unnecessary hospitalizations;
•care delivered in community clinics vs. hospital setting;
•clinically appropriate integration of palliative care and hospice aligned with patients’ goals of care;
•access to clinical trials providing cutting-edge treatment options at low or no cost to patients or payors; and
•appropriate provider training on clinical documentation to ensure proper risk adjustment and reimbursement for complex patients.
We strive to add value by consistently performing these activities effectively. The goal is a lower cost of care for the same or better clinical outcomes while providing a superior patient experience.
Patient Experience
We believe our patient-centric focus facilitates high levels of patient satisfaction and supports our care delivery model while strengthening payor relationships. We employ a continuous feedback mechanism to ensure superior patient experience and satisfaction among our affiliated physicians and advanced practice providers.
As of a recent patient survey in 2022, 88% of our oncologists and 90% of our locations were rated 4.0 or above as measured by our survey, which we distribute to patients via text or e-mail following their clinic visit.
Growth Strategy and Opportunities
To date, revenue has grown at a roughly 30% CAGR from 2016 to 2022, driven by robust growth in capitated lives under value-based contracts.
Our footprint as of December 31, 2022 spanned five states and is growing rapidly.
9
California | Arizona | Nevada | Florida | Texas | |||||||||||||||||||||||||
Markets | 7 | 1 | 1 | 5 | 1 | ||||||||||||||||||||||||
Managed and Affiliated Clinics(1) | 57 | 4 | 5 | 8 | 2 | ||||||||||||||||||||||||
Providers | 84 | 5 | 4 | 15 | 4 | ||||||||||||||||||||||||
(1) 62 clinics operated under the TOI PCs, whereby we receive a percentage of revenues under our MSAs and are consolidated; and 14 independent oncology practice locations that are under MSAs for limited management and administrative services but do not bear any direct operating costs.
We anticipate adding more TOI PC clinics and other managed practices in the future across our markets through acquisitions and through de novo clinic builds, and we are in constant discussion with payors and providers to enter new markets. We continually seek to evaluate our growth strategy and may continue to modify it in the future, and there can be no assurance that we will be able to successfully capitalize on growth strategies.
Our go-to-market strategy focuses on both payors and providers. This blend is important given the increasing penetration of non-traditional payors, such as Oscar and Bright HealthCare, and primary care risk models such as Agilon health and ChenMed LLC.
We believe that our existing payor relationships provide us leads on opportunities to enter new markets, and we often receive outreach from new management services organizations, health plans and risk bearing organizations. When evaluating a new market, we consider three primary factors:
1.the penetration and growth of Medicare Advantage and other value-based reimbursement models;
2.the presence of value-based primary care groups with whom we can partner to generate referrals and manage outcomes; and
3.how well oncology spend is currently managed in that market.
We believe that new markets we are focused on meet all of the above criteria and could provide us with significant opportunity to create value for patients, providers and payors.
We have multiple strategies we believe can achieve long term growth.
•Existing Market Contract Growth: Continue driving covered lives growth. Significant growth potential in existing markets can be achieved through expanding the scope of our services with existing partners and securing new contracts with new payors and independent practices. The addition of new de novo clinics and affiliated providers can drive additional growth. By continuing to build regional density in existing markets, we also have an opportunity to achieve efficiencies with increased scale.
•New Market Contract Growth: Our replicable operating model enables quick scaling in new markets. Oncology continues to be a key focus area for payors and providers, who are highly supportive of our entry into new markets. Our high priority markets have attractive market dynamics due to the high cost of oncology care in these geographies, the prevalence of risk-bearing organizations, and the presence of national payor partners who we collaborate with in existing markets. Our initial approach to value-based contracting in new markets is likely to be in the form of gain share contracts These new contracts, which will enable us to work with payors and risk-taking providers as they continue their shift to value-based care, are likely to produce lower revenue and profitability in the initial period as compared to full capitation. Once payors and risk-bearing providers in these new markets become comfortable with our ability to generate savings and better outcomes, we believe these contracts will shift to capitation.
•M&A Opportunities: Leveraging our existing pipeline and mergers and acquisition expertise can help us facilitate growth in both existing and new markets, allowing us to rapidly establish market presence. Once on-boarded, we can transition the affiliated practice to our value-based model, as well as expand and enhance the scope of services provided to patients by the affiliated practice, such as adding dispensary operations, managing clinical trials and access to our broad purchasing contracts. Independent oncologists continue to face multitude of challenges and our acquisition model offers a path for these oncologists to continue to practice in their community without the burdens of business building or administration, while at the same time working alongside a dynamic and growing organization at the forefront of value-based care. We look for acquisition targets where the practice is philosophically aligned with us in driving the shift to value-based care.
•Service Expansion: We can broaden scope and diversify service offerings, including ancillary and adjacent services focused on patient care and innovation and providing access to new oncology treatments being investigated in clinical trials that our affiliated practices manage. We have the potential to scale significantly faster with additional capital via
10
new oncologist on-boarding and training, further technology investments, investments in ancillaries, and strategic acquisitions. In Q4 2021, we acquired our first radiation oncology practice, adding a new service line to our business.
Contracting Overview
At a time when many FFS healthcare organizations have been struggling due to the decrease in service volumes, our value-based capitation payments have allowed us to maintain our level of member care and prioritize member safety by incentivizing the provision of care in the most appropriate setting.
We have focused our business on capitation arrangements and other types of value-based contracts, which we believe align provider incentives with both quality and efficiency of care. Under capitation arrangements, payors pay a fixed per member per month, or PMPM, amount for every plan member within a population assigned to us for oncology care.
Our affiliated providers are responsible for managing oncology care for this population based on a scope of medical services and drugs agreed upon by both parties. The PMPM rates for our capitation arrangements are determined based on our analysis of historical patient data and agreements with contractual partners. In new markets, this may require the TOI PCs to contract with both the health insurance company and their risk-bearing provider organization in order to service these members.
In addition to capitation-based arrangements, we continue to explore several other forms of value-based arrangements. Although many of these arrangements continue to be based on a FFS-based methodology, our affiliated providers are eligible to earn additional bonuses based on their ability to achieve oncology specific clinical and other quality of care based benchmarks. While these alternative value-based arrangements may not produce as much initial revenue on a PMPM basis as capitation, we believe this flexibility in contracting models will allow us to speed our expansion into new markets while preserving the value-based economics that are critical for our business’ growth and success.
Payor Relationships
Our ability to consistently attract patients across multiple geographic markets depends on our ability to contract with payors in each market. Depending on the market, payors can be delegated medical groups who are taking risk or insurance companies themselves. By opening clinics in locations where the TOI PCs currently manage the oncology care for a large number of insured Medicare, Commercial and Medicaid members, we believe we are creating net benefits for payors, as our affiliated providers are able to reduce unnecessary costs and improve patient care and experience. This also allows us to benefit from the value-based offerings already established by payors in the market, therefore not requiring us to single-handedly drive patient growth. Some of the biggest and most respected names in healthcare contract with the TOI PCs to provide oncology care to their members, including Anthem, CareMore Health, Heritage Provider Network and Optum Care. More than half of our revenue in 2022 was generated from value-based contracts where payors have made our affiliated providers their preferred or exclusive oncology group.
While our relationships with payors are very strong, we believe we have limited concentration risk as our largest customer by revenue in 2022 represented less than 20% of our revenue.
Provider and Clinic Capacity Growth
Our primary driver for growth in provider and clinic capacity is to create network adequacy to service members from payors with whom we have capitated or other value-based arrangements. For each market we currently operate in or are considering entering, we do a detailed assessment of the existing market landscape and determine the optimal approach to create the capacity we need given our payor relationships and pipeline of contracts. We can achieve capacity growth through multiple avenues, including practice acquisitions and de novo clinics. Practice acquisitions offer an opportunity to gain scale and market presence rapidly, while de novo clinics allow us to build out our network in a highly capital efficient manner. We believe both approaches can work in tandem to achieve optimal scale, network presence and speed to market. In addition, we have an active recruitment pipeline for providers to join our network and help us both manage patient load and grow the patient base.
We believe we have built a robust and data-driven approach to acquisitions, with a dedicated team to identify, assess and integrate physician practices into our network, and a strong pipeline of targets in both existing and new markets. We have invested in resources to continually add to our pipeline.
Clinic Structure, Staffing and Network Design
We have a standard clinic design and approach to staffing that has been refined over many years. Managed clinics typically range from 2,000 to 3,000 square feet with 3-4 providers (physicians and advanced practice providers) per clinic. We have flexibility around clinic size to allow us to establish smaller clinics and part time staffing in areas where needed to ensure the
11
TOI PCs can meet network adequacy under existing payor contracts. We group our managed clinics in a similar geographic area into pods, with multiple pods in each market. We have operations teams managing our markets and pods allowing us to drive performance and scale efficiently.
Competition
The U.S. healthcare industry is generally highly competitive. We compete with large and medium-sized local and national providers of cancer care services, such as health system affiliated practices, for, among other things, contracts with payors, recruitment of physicians and other medical and non-medical personnel and patients. The closest competitors are traditional oncology physician practices, such as American Oncology Network, LLC, Florida Cancer Specialists & Research Institute, LLC, U.S. Oncology Network, Inc., and OneOncology, Inc. These organizations are predominantly reimbursed via FFS contracts, which we believe can often lead to over utilization of treatments that may be medically appropriate but often results in higher costs. Secondary competitors may include specialty benefit managers. These include companies such as AIM Specialty Health, eviCore Healthcare, Magellan Health, New Century Health, and OncoHealth. These benefit managers seek to change provider behavior by reviewing and authorizing treatment requests. The benefit manager model can produce incremental improvement in utilization, but the benefit managers are often unable to achieve results comparable to managed healthcare practices like ours. Furthermore, the benefit manager model frequently results in an antagonistic relationship with physicians who are operating in a traditional FFS-based practice. We distinguish ourselves from other managed oncology practices and specialty benefit managers in our ability to align incentives across the care continuum, including physicians and payors in delivering high quality care at lower costs, and we believe there are currently no other value-based oncology management companies of meaningful scale in the U.S.
We believe the principal competitive factors for serving the healthcare market for Medicare beneficiaries include: patient experience, quality of care, health outcomes, total cost of care, brand identity and trust in that brand. We believe we compete favorably on all these factors.
Government Regulation
Regulatory Licensing, Accreditation and Certification
Many states, including California, require regulatory approval, including licensure, accreditation and certification before establishing certain types of clinics offering certain professional and ancillary services, including the services we offer. The operations of our managed clinics are subject to extensive federal, state and local regulation relating to, among other things, the adequacy of medical care, equipment, personnel, operating policies and procedures, dispensing of prescription drugs, fire prevention, rate-setting and compliance with building codes and environmental protection. Our ability to operate profitably will depend in part on the ability of our managed clinics and doctors to obtain and maintain all necessary licenses, accreditation and other approvals, and maintain updates to their enrollment in the Medicare and Medicaid programs, including the addition of new clinic locations, providers and other enrollment information. In addition, certain ancillary services such as the provision of diagnostic laboratory testing require additional state and federal licensure and regulatory oversight, including oversight by CMS, under Clinical Laboratory Improvement Amendments of 1988, which requires all clinical laboratories to meet certain quality assurance, quality control and personnel standards, and comparable state laboratory licensing authorities, including for example, the California Department of Public Health. Our dispensary operations must also comply with applicable laws. Sanctions for failure to comply with applicable state and federal licensing, accreditation, certification and other regulatory requirements include suspension, revocation or limitation of the applicable authorization, significant fines and penalties and/or an inability to receive reimbursement from government healthcare programs and other third-party payors.
State Corporate Practice of Medicine and Fee-Splitting Laws
Our arrangements with the TOI PCs are subject to various state laws, including California, commonly referred to as corporate practice of medicine and fee-splitting laws, which are intended to prevent unlicensed persons from interfering with or influencing the physician’s professional judgment, and prohibiting the sharing of professional service fees with non-professional or business interests. These laws vary from state to state and are subject to broad interpretation and enforcement by state regulators. A determination of non-compliance against us and/or the TOI PCs could lead to adverse judicial or administrative action, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, and/or restructuring of these arrangements.
Healthcare Fraud and Abuse Laws
We are subject to a number of federal and state healthcare regulatory laws that restrict certain business practices in the healthcare industry. These laws include, but are not limited to, federal and state anti- kickback, false claims, self-referral and other healthcare fraud and abuse laws.
12
The federal Anti-Kickback Statute, or AKS, prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. The AKS includes statutory exceptions and regulatory safe harbors that protect certain arrangements. The AKS safe harbors for value-based arrangements require, among other things, that the arrangement does not induce a person or entity to reduce or limit medically necessary items or services furnished to any patient. Failure to meet the requirements of the safe harbor, however, does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances, including the parties’ intent and the arrangement’s potential for abuse, and may be subject to greater scrutiny by enforcement agencies.
The Stark Law prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing designated health services, or DHS, from referring Medicare and Medicaid patients to such entities for the furnishing of DHS, unless an exception applies. The Stark Law also prohibits the entity from billing for any such prohibited referral. Unlike the AKS, the Stark Law is violated if the financial arrangement does not meet an applicable exception, regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral.
The Federal False Claims Act, or FCA, prohibits a person from knowingly presenting, or caused to be presented, a false or fraudulent request for payment from the federal government, or from making a false statement or using a false record to have a claim approved. The FCA further provides that a lawsuit thereunder may be initiated in the name of the United States by an individual, a “whistleblower,” who is an original source of the allegations. Moreover, the government may assert that a claim including items and services resulting from a violation of the AKS or the Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Penalties for a violation of the FCA include fines for each false claim, plus up to three times the amount of damages caused by each false claim.
Further, the Civil Monetary Penalties Statute authorizes the imposition of civil monetary penalties, assessments and exclusion against an individual or entity based on a variety of prohibited conduct, including, but not limited to offering remuneration to a federal health care program beneficiary that the individual or entity knows or should know is likely to influence the beneficiary to order or receive health care items or services from a particular provider.
Health Insurance Portability and Accountability Act of 1996 ("HIPAA") also established federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Several states in which we operate have also adopted similar fraud and abuse laws as described above. The scope of these laws and the interpretations of them vary from state to state and are enforced by state courts and regulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any payor, including patients and commercial insurers, not just those reimbursed by a federally funded healthcare program, including California's anti-kickback statutes and the Physician Ownership and Referral Act of 1993.
Violation of any of these laws or any other governmental regulations that apply may result in significant penalties, including, without limitation, administrative civil and criminal penalties, damages, disgorgement, fines, additional reporting requirements and compliance oversight obligations, contractual damages, the curtailment or restructuring of operations, exclusion from participation in governmental healthcare programs and/ or imprisonment.
Healthcare Reform
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system, many of which are intended to contain or reduce healthcare costs. By way of example, in the United States, the Affordable Care Act (“ACA”), substantially changed the way healthcare is financed by both governmental and private insurers. Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare. It is unclear how other
13
healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 and a 1% payment reduction from April 1, 2022 to June 30, 2022, unless additional Congressional action is taken. In addition, on January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
CMS, through the Centers for Medicare and Medicaid Innovation, or CMMI, has implemented or has announced plans to implement numerous demonstration models designed to test value-based reimbursement models, some of which are specifically focused on oncology services. For example, in 2016, CMS initiated the Oncology Care Model demonstration, which continues into 2022 and provides participating physician practices, including the TOI PCs that participate in this program, with performance-based financial incentives that aim to manage or reduce Medicare costs without negatively affecting the efficacy of care. In late 2019, CMS issued a request for information on the Oncology Care First model, a new voluntary model that, if implemented, would build on the Oncology Care Model. More recently, CMMI has announced plans to implement the Radiation Oncology Model, which would require radiotherapy providers in certain regions to participate in a prospective, episode-based payments model where payment is based on a patient’s diagnosis as opposed to the traditional volume-based FFS payment model. Although the Radiation Oncology Model was originally intended to begin on January 1, 2022, recent legislation delayed its implementation until January 1, 2023. There likely will continue to be regulatory proposals directed at containing or lowering the cost of healthcare. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue or attain growth, any of which could have a material impact on our business.
Further, healthcare providers and industry participants are also subject to a growing number of requirements intended to promote the interoperability and exchange of patient health information. For example, on April 5, 2021, healthcare providers and certain other entities became subject to information blocking restrictions pursuant to the Cures Act that prohibit practices that are likely to interfere with the access, exchange or use of electronic health information ("EHI"), except as required by law or specified by HHS as a reasonable and necessary activity.
Violations may result in penalties or other disincentives. It is unclear at this time what the costs of compliance with the new rules will be, and what additional risks there may be to our business.
Federal and State Insurance and Managed Care Laws
Regulation of downstream risk-sharing arrangements, including, but not limited to, global risk and other value-based arrangements, varies significantly from state to state. Some states require downstream entities and risk bearing organization ("RBOs") to obtain an insurance license, a certificate of authority, or an equivalent authorization, in order to participate in downstream risk-sharing arrangements with payors. In some states, statutes, regulations and/or formal guidance explicitly address whether and in what manner the state regulates the transfer of risk by a payor to a downstream entity. However, the majority of states do not explicitly address the issue, and in such states, regulators may nonetheless interpret statutes and regulations to regulate such activity. If downstream risk-sharing arrangements are not regulated directly in a particular state, the state regulatory agency may nonetheless require oversight by the licensed payor as the party to such a downstream risk-sharing arrangement. Such oversight is accomplished via contract and may include the imposition of reserve requirements, as well as reporting obligations. Further, state regulatory stances regarding downstream risk-sharing arrangements can change rapidly and codified provisions may not keep pace with evolving risk-sharing mechanisms and other new value-based reimbursement models. Certain of the states where we currently operate or may choose to operate in the future regulate the operations and financial condition of RBOs like the us and our affiliated providers. These regulations can include capital requirements, licensing or certification, governance controls and other similar matters. While these regulations have not had a material impact on our business to date, as we continue to expand, these rules may require additional resources and capitalization and add complexity to our business.
Privacy and Security
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of
14
the Federal Trade Commission ["FTC"] Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of the TOI PCs.
In addition, certain state laws, such as the California Consumer Privacy Act, or the CCPA and the California Privacy Rights Act of 2020, or the CPRA, govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other to complicate compliance efforts, and can result in investigations, proceedings, or actions that lead to significant civil and/or criminal penalties and restrictions on data processing.
Intellectual Property
At present, we own no material intellectual property.
Legal Proceedings
From time to time, we may be involved in various legal proceedings and subject to claims that arise in the ordinary course of business. Although the results of litigation and claims are inherently unpredictable and uncertain, we are not currently a party to any legal proceedings the outcome of which, if determined adversely to us, are believed to, either individually or taken together, have a material adverse effect on our business, operating results, cash flows or financial condition. Regardless of the outcome, litigation has the potential to have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Insurance
We maintain insurance, excess coverage, or reinsurance for property and general liability, professional liability, directors’ and officers’ liability, workers’ compensation, cybersecurity and other coverage in amounts and on terms deemed adequate by management, based on our actual claims experience and expectations for future claims. Future claims could, however, exceed our applicable insurance coverage.
Employees and Human Capital Resources
As of December 31, 2022, we and TOI PCs collectively had approximately 750 employees, including approximately 101 oncologists and advanced practice providers. We consider our relationship with our employees to be good. None of our employees are represented by a labor union or party to a collective bargaining agreement.
Our goal is to provide top quality oncology care to our patients, and we view our human capital-related initiatives as essential to continuing to reach that goal. Such initiatives include: (i) implementing a robust talent acquisition approach, including through competitive pay and benefits, (ii) implementing programs to promote diversity and foster a sense of connection and community throughout our company, (iii) offering an array of opportunities for learning and development opportunities, and (iv) conducting annual employee engagement surveys and developing action plans based on the survey outcomes.
Properties
Our principal executive offices are located in Cerritos, California where we occupy a suite under a lease that expires in 2026. We use this facility for administration, billing and collections, technology and development and professional services.
We intend to procure additional space as we add team members and expand geographically. We believe that our facilities are adequate to meet our needs for the immediate future, and that, should it be needed, suitable additional space will be available to accommodate any such expansion of our operations. As of December 31, 2022, we have leases for 62 clinics located in California, Arizona, Nevada, Florida and Texas. Generally, our leases are “net” leases, which require us to pay all of the cost of insurance, taxes, maintenance and utilities. We generally cannot cancel these leases at our option.
Availability of Information
We were originally incorporated in Delaware on November 19, 2019 as a special purpose acquisition company (f/k/a DFP Healthcare Acquisition Corp.). In November 2021, we consummated our business combination with TOI Parent, Inc. (the “Business Combination”). In connection with the closing of the Business Combination, TOI Parent, Inc. became our wholly owned subsidiary and we changed our name to The Oncology Institute, Inc. We file or furnish annual, quarterly and current
15
reports, proxy statements and other documents with the Securities and Exchange Commission (the “SEC”) under the Securities Exchange Act of 1934 (as amended, the "Exchange Act"). The SEC maintains an internet website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers, including us, that file electronically with the SEC.
We also make available free of charge through our website, https://investors.theoncologyinstitute.com/, electronic copies of certain documents that we file with the SEC, including our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information on our website or any other website is not incorporated by reference into, and does not constitute a part of, this Annual Report.
Item 1A. Risk Factors
We operate in a rapidly changing environment that involves a number of risks. Our operations and financial results are subject to various risks and uncertainties including those described below. You should consider carefully the risks and uncertainties described below, in addition to other information contained in this Annual Report on Form 10-K, including our consolidated financial statements and related notes, as well as our other public filings with the Securities and Exchange Commission. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely affect our business. If any of the following risks or others not specified below materialize, our business, financial condition and results of operations could be materially adversely affected. In that case, the trading price of our common stock could decline.
SUMMARY RISK FACTORS
The following is a summary of select risks and uncertainties that could materially adversely affect The Oncology Institute, Inc. ("TOI", "we", or "our") and its business, financial condition and results of operations. You should read this summary together with the full and complete discussion of risk factors contained below:
•Our growth strategy depends on our ability to build or acquire clinics to service our contracts and treat our patients.
•We have experienced, and may continue to experience, rapid growth and organizational change, which has placed, and may continue to place, significant demands on our management and our operational and financial resources.
•We have identified a material weakness in our internal control over financial reporting that, if not properly corrected, could materially adversely affect our operations and result in material misstatements in our financial statements.
•We have a history of net losses, we anticipate increasing expenses in the future, and we may not be able to achieve or maintain profitability.
•A pandemic, epidemic or outbreak of an infectious disease in the United States or worldwide, including the current COVID-19 pandemic, could adversely affect our business.
•Our services are concentrated in certain geographic areas and populations exposing us to unfavorable changes in local benefit costs, reimbursement rates, competition and economic conditions.
•If we are unable to attract new patients, our revenue growth will be adversely affected.
•We primarily depend on reimbursement from third-party payors, as well as payments by individuals, which could lead to delays, denials, or uncertainties in the reimbursement process.
•With many of our value-based agreements, our consolidating professional corporations ("TOI PCs") assume the risk that the cost of providing services will exceed our compensation. As oncology costs rise, if we do not accurately predict the cost to deliver care, some of the TOI PCs’ value-based agreements could become less profitable, or unprofitable.
•There are significant risks associated with estimating the amount of revenue that is recognized under TOI PCs’ risk agreements with health plans, and if our estimates of revenue are materially inaccurate, it could impact the timing and the amount of our revenue recognition or have a material adverse effect on our business, results of operations, financial condition and cash flows.
•A significant portion of our consolidated Patient Services revenue is derived from a limited number of health insurance, Independent Practice Associations, or IPAs and medical group companies. Those payors could take action to remove, exclude, delay, or otherwise prevent the inclusion of the TOI PCs in their provider networks.
•A significant portion of sales are from prescription drug sales reimbursed by a limited number of pharmacy benefit management companies with which TOI PCs contract. Those pharmacy benefit management companies could take action to remove, exclude, delay or otherwise prevent the inclusion of the TOI PCs in their provider networks.
16
•Reductions in Medicare reimbursement rates or changes in the rules governing the Medicare program could have a material adverse effect on our financial condition and results of operations.
•We cannot predict the effect that health care reform and other changes in government programs may have on our business, financial condition or results of operations.
•The transition from volume to value-based reimbursement models may have a material adverse effect on our operations.
•Changes in the payor mix of patients and potential decreases in reimbursement rates as a result of consolidation among our customers could adversely affect our revenues and results of operations.
•We face significant competition from other healthcare services providers. Our failure to adequately compete could adversely affect our business.
•Competition for physicians and clinical personnel, including nurses, shortages of qualified personnel or other factors could increase our labor costs and adversely affect our revenue, growth rate, profitability and cash flows.
•Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to execute our business strategies and growth plans.
•If we are unable to provide consistently high quality of care, our business will be adversely impacted.
•If certain of our suppliers do not meet our needs, if there are material price increases on supplies, if we are not reimbursed or adequately reimbursed for drugs we purchase or if we are unable to effectively access new technology or superior products, it could negatively impact our ability to effectively provide the services we offer and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
•We depend on our information technology systems, and those of our third-party vendors, contractors and consultants, and any failure or significant disruptions of these systems, security breaches or loss of data could materially adversely affect our business, financial condition and results of operations.
•We may be subject to legal proceedings and litigation, including intellectual property and privacy disputes, which are costly to defend and could materially harm our business and results of operations.
•Some jurisdictions preclude the TOI PCs from entering into non-compete agreements with our physicians, and other non-compete agreements and restrictive covenants applicable to certain physicians and other clinical employees may not be enforceable.
•Current and future acquisitions may use significant resources, may be unsuccessful, and could expose us to unforeseen liabilities.
•We conduct some clinical trials in contract with TOI Clinical Research, LLC ("TCR"). If we fail to perform our clinical trial services in accordance with contractual requirements, government regulations and ethical considerations, we could be subject to significant costs or liability and our reputation could be adversely affected.
•We are dependent on our relationships with the TOI PCs, which are affiliated professional entities that we do not own, to provide healthcare services, and our business would be harmed if those relationships were disrupted or if our arrangements with the TOI PCs become subject to legal challenges.
•Our managed clinics and the TOI PCs providing professional services at such clinics may become subject to medical liability claims, which could have a material adverse impact on our business.
•If there is a change in accounting standards by the Financial Accounting Standards Board or the interpretation thereof affecting consolidation of entities, it could have a material adverse effect on our consolidation of total revenues derived from the TOI PCs.
•Our managed clinics and the TOI PCs may be subject to third-party payor audits, which, if adversely determined against us or the TOI PCs, may have a material effect on our results of operations and financial condition.
•We are subject to extensive fraud, waste, and abuse laws that may give rise to federal and state audits, investigations, lawsuits and claims against us, the outcome of which may have a material adverse effect on our business.
•If any of our managed clinics or TOI PCs lose their regulatory licenses, permits and/or accreditation status, or become ineligible to receive reimbursement under Medicare or Medicaid or other third-party payors, there may be a material adverse effect on our business, financial conditions, cash flows or results of operations.
•If we or the TOI PCs fail to comply with applicable data interoperability and information blocking rules, our consolidated results of operations could be adversely affected.
•Actual or perceived failures to comply with applicable data protection, privacy and security, advertising and consumer protection laws, regulations, standards and other requirements could adversely affect our business, financial condition and results of operations.
•We and our TOI PCs are subject to federal, state and local laws and regulations that govern our business.
•We may not be able to utilize a portion of our net operating loss carry forwards (“NOLs”) to offset future taxable income for U.S. federal income tax purposes, which could adversely affect our net income and cash flows.
17
•Future changes to applicable tax laws and regulations and/or their interpretations may have an adverse effect on our business, financial condition and results of operations. Tax rules and regulations are subject to interpretation and require judgment by us that may be successfully challenged by the applicable taxation authorities upon audit, which could result in additional tax liabilities.
Risks Related to Our Business
Our growth strategy depends on our ability to build or acquire new TOI PC clinics to service our contracts and treat our patients.
Our business strategy is to grow rapidly by expanding our network of oncology care clinics and is significantly dependent our ability to open new TOI PC clinics in our existing markets, expand into new geographical locations through existing TOI PCs or affiliating with new professional entities that would become a TOI PC, recruit new patients and partner or contract with payors, existing medical practices or other healthcare providers to provide oncology care services. We seek growth opportunities both organically and through TOI PCs’ agreements with payors or other oncology care providers. Our ability to grow organically depends upon a number of factors, including our affiliated providers obtaining referrals for cancer patient care services, the TOI PCs entering into contracts with additional payors, identifying appropriate facilities, obtaining leases, completing internal build-outs of new facilities within proposed timelines and budgets and hiring care teams and other employees. We cannot guarantee that we will be successful in pursuing our growth strategy. If we fail to evaluate and execute new business opportunities properly, we may not achieve anticipated benefits and may incur increased costs.
Our growth strategy involves a number of risks and uncertainties, including that:
•the TOI PCs may not be able to successfully enter into contracts with local payors on terms favorable to us or at all. In addition, the TOI PCs compete for payor relationships with other healthcare organizations, some of whom may have greater resources than we do. This competition may intensify due to the ongoing consolidation in the healthcare industry, which may increase our costs to pursue such opportunities;
•through the TOI PCs, we may not be able to recruit or retain a sufficient number of new patients to execute our growth strategy, and we may incur substantial costs to recruit new patients and we may be unable to recruit a sufficient number of new patients to offset those costs;
•the TOI PCs may not be able to hire sufficient numbers of physicians and other staff and may fail to integrate our employees, particularly our medical personnel, into our care model;
•future value-based contracts may not be as favorable as current capitation contracts;
•when expanding our business into new states, we may be required to comply with laws and regulations that may differ from states in which we currently operate; and
•depending upon the nature of the local market, we may not be able to implement our business model in every local market that we enter, which could negatively impact our revenues and financial condition.
There can be no assurance that we will be able to successfully capitalize on growth opportunities, which may negatively impact our business model, revenues, results of operations and financial condition.
We have experienced, and may continue to experience, rapid growth and organizational change, which has placed, and may continue to place, significant demands on our management and our operational and financial resources.
Our organizational structure may become more complex as we improve our operational, financial and management controls, as well as our reporting systems and procedures. We may require significant capital expenditures and the allocation of valuable management resources to grow and change in these areas. We must effectively increase our headcount and continue to effectively train and manage our employees. We will be unable to manage our business effectively if we are unable to alleviate the strain on resources caused by growth in a timely and successful manner. If we fail to effectively manage our anticipated growth and change, the quality of our services may suffer, which could negatively affect our brand and reputation and harm our ability to attract and retain patients and employees.
In addition, as we expand our business, it is important that we continue to maintain a high level of patient service and satisfaction. As our patient base continues to grow, through the TOI PCs, we will need to expand our medical, patient services and other personnel, and our network of partners, to provide personalized patient service. If we are not able to continue to provide high quality medical care with high levels of patient satisfaction, our reputation, as well as our business, results of operations and financial condition could be adversely affected.
18
We have a history of net losses, we anticipate increasing expenses in the future, and we may not be able to achieve or maintain profitability.
We incurred a net loss of $10,927 in 2021, and a loss from operations in 2022. We expect our losses will continue as we expect to invest heavily in increasing our patient base, expanding our operations, and operating as a public company. These efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenue sufficiently to offset these higher expenses. To date, we have financed our operations principally from the sale of our equity, revenue from our patient services and the incurrence of indebtedness. We may not generate positive cash flow from operations or profitability in any given period, and our limited operating history may make it difficult for you to evaluate our current business and our future prospects.
We have encountered and will continue to encounter risks and difficulties frequently experienced by growing companies in rapidly changing industries, including increasing expenses as we continue to grow our business. We expect our operating expenses to increase significantly over the next several years as we continue to hire additional personnel, expand our operations and infrastructure, and continue to expand to reach more patients. In addition to the expected costs to grow our business, we also expect to incur additional legal, accounting and other expenses as a newly public company. These investments may be more costly than we expect, and if we do not achieve the benefits anticipated from these investments, or if the realization of these benefits is delayed, they may not result in increased revenue or growth in our business. If our growth rate were to decline significantly or become negative, it could adversely affect our financial condition and results of operations. If we are not able to achieve or maintain positive cash flow in the long term, we may require additional financing, which may not be available on favorable terms or at all and/or which would be dilutive to our stockholders. If we are unable to successfully address these risks and challenges as we encounter them, our business, results of operations and financial condition would be adversely affected. Our failure to achieve or maintain profitability could negatively impact the value of our Common Stock.
A pandemic, epidemic or outbreak of an infectious disease in the United States or worldwide, including the current COVID-19 pandemic, could adversely affect our business.
A pandemic, epidemic or outbreak of an infectious disease, including the current COVID-19 pandemic, that occurs in the United States or worldwide, may adversely affect our business.
Adverse market conditions resulting from the spread of COVID-19 could materially adversely affect our business and the value of our Common Stock. Preventative measures taken to alleviate any public health crises, such as “shelter-in-place” orders, quarantines, executive orders and similar government orders may result in largely remote operations at our headquarters, work stoppages among some vendors and suppliers, slowdowns and delays, travel restrictions and cancellation of events, among other effects, thereby significantly and negatively impacting our operations. Other disruptions or potential disruptions include restrictions on the ability of our personnel to travel; restrictions on our business development activities due to potential payors or other entities we and the TOI PCs engage with limiting their corresponding business development efforts; inability of our suppliers to manufacture goods and to deliver these to us on a timely basis, or at all; inventory shortages or obsolescence; delays in actions of regulatory bodies; diversion of or limitations on employee resources that would otherwise be focused on the operations of our business, including because of sickness of employees or their families or the desire of employees to avoid contact with groups of people; business adjustments or disruptions of certain third parties; and additional government requirements or other incremental mitigation efforts. The extent to which a pandemic impacts our business will depend on developments that are highly uncertain and cannot be predicted, including information that may emerge concerning the severity and spread of the pandemic and the actions to contain it or treat its impact, among others.
Because of our business model, the full impact of the COVID-19 pandemic may not be fully reflected in our results of operations and overall financial condition until future periods. It is not currently possible to reliably project the direct impact of the COVID-19 pandemic on our operating revenues and expenses. Key factors include the duration and extent of the outbreak in our service areas as well as societal and governmental responses. Patients may continue to be reluctant to seek necessary care given the risks of the COVID-19 pandemic. This could have the effect of deterring healthcare costs that we will need to incur to later periods and may also affect the health of patients who defer treatment, which may cause our costs to increase in the future. Further, as a result of the COVID-19 pandemic, we may experience slowed growth or a decline in new patient demand. We also may experience increased internal and third-party medical costs as the TOI PCs and our affiliated providers provide care for patients suffering from COVID-19. This increase in costs may be particularly significant given the number of patients who are under capitation agreements. Further, we may face increased competition due to changes to our competitors’ products and services, including modifications to their terms, conditions, and pricing that could materially adversely impact our business, results of operations, and overall financial condition in future periods.
19
The COVID-19 pandemic could also cause our third-party data center hosting facilities and cloud computing platform providers, which are critical to our infrastructure, to shut down their business, experience security incidents that impact our business, delay or disrupt performance or delivery of services, or experience interference with the supply chain of hardware required by their systems and services, any of which could materially adversely affect our business. Further, the COVID-19 pandemic has resulted in our employees and those of many of our vendors working from home and conducting work via the internet, and if the network and infrastructure of internet providers becomes overburdened by increased usage or is otherwise unreliable or unavailable, our employees’, and our customers’ and vendors’ employees’, access to the internet to conduct business could be negatively impacted. Limitations on access or disruptions to services or goods provided by or to some of our suppliers and vendors upon which our platform and business operations relies, could interrupt our ability to provide our platform, decrease the productivity of our workforce, and significantly harm our business operations, financial condition, and results of operations.
Our platform and the other systems or networks used in our business may experience an increase in attempted cyberattacks, targeted intrusion, ransomware, and phishing campaigns seeking to take advantage of shifts to employees working remotely using their household or personal internet networks and to leverage fears promulgated by the COVID-19 pandemic. The success of any of these unauthorized attempts could substantially impact our platform, the proprietary and other confidential data contained therein or otherwise stored or processed in our operations, and ultimately our business. Any actual or perceived security incident also may cause us to incur increased expenses to improve our security controls and to remediate security vulnerabilities.
To the extent the COVID-19 pandemic, or another pandemic, epidemic or outbreak of an infectious disease occurs in the United States or worldwide, adversely affects our business and financial results, it may also have the effect of heightening many of the other risks described in this “Risk Factors” section, including but not limited to those relating to cyberattacks and security vulnerabilities, interruptions or delays due to third-parties, or our ability to raise additional capital or generate sufficient cash flows necessary to fulfill our obligations under our existing indebtedness or to expand our operations.
Our services are concentrated in certain geographic areas and populations exposing us to unfavorable changes in local benefit costs, reimbursement rates, competition and economic conditions.
The TOI PCs’ membership remains concentrated in certain geographic areas in the United States. We have clinic locations in five states. As of December 31, 2022, the vast majority of the TOI PC members under capitation agreements were residents of California. In addition, during 2022, approximately 85% of our revenues were generated in California. Unfavorable changes in health care or other benefit costs or reimbursement rates or increased competition in the states in which we operate or any other geographic area where the TOI PCs’ membership becomes concentrated in the future could therefore have a disproportionately adverse effect on our operating results. Additionally, the geographic concentration of a significant portion of the TOI PCs’ membership may make them more vulnerable to events such as the COVID-19 pandemic.
If we are unable to attract new patients, our revenue growth will be adversely affected.
To increase our revenue, our business strategy is to expand the number of payor contracts entered into by the TOI PCs and clinic locations in our network. In order to support such growth, the TOI PCs must continue to win new contracts and retain or grow existing contracts with payors. We face competition from other oncology providers in the recruitment of potential patients. If the TOI PCs are unable to convince potential payors and patients of the benefits of our value-based system, or if potential or existing payors and patients prefer the care provider model of one of our competitors, we may not be able to effectively implement our growth strategy, which depends on our ability to grow organically and attract new patient referrals and payors for the TOI PCs. In addition, our growth strategy is dependent on payors electing to enter into capitation or other value-based arrangements and selecting the TOI PCs as their oncology provider. The TOI PCs’ inability to obtain new payor agreements and patient referrals and retain existing payors and patients, particularly those under capitation arrangements, would harm our ability to execute our growth strategy and may have a material adverse effect on our business operations and financial position.
We primarily depend on reimbursement by third-party payors, as well as payments by individuals, which could lead to delays and uncertainties in the reimbursement process.
The reimbursement process is complex and can involve lengthy delays. Although we recognize revenue when the TOI PCs and our affiliated providers provide services to patients, we may from time to time experience delays in receiving the associated capitation payments or, for patients on fee-for-service arrangements, the reimbursement for the service provided. In addition, third-party payors may disallow, in whole or in part, requests for reimbursement based on determinations that the patient is not eligible for coverage, certain amounts are not reimbursable under plan coverage or the services provided that were not medically necessary or additional supporting documentation is necessary. Retroactive adjustments may change amounts
20
realized from third-party payors. As described below, the TOI PCs are subject to audits by such payors, including governmental audits of our Medicare claims, and may be required to repay these payors if a finding is made that we were incorrectly reimbursed. Delays and uncertainties in the reimbursement process may adversely affect accounts receivable, increase the overall costs of collection and cause us to incur additional costs associated with raising capital. Third-party payors are also increasingly focused on controlling healthcare costs, and such efforts, including any revisions to reimbursement policies, may further complicate and delay the TOI PCs’ reimbursement claims.
In addition, certain of our patients are covered under health plans that require the patient to cover a portion of their own healthcare expenses through the payment of copayments or deductibles. The TOI PCs may not be able to collect the full amounts due with respect to these payments that are the patient’s financial responsibility, or in those instances where physicians provide services to uninsured individuals. To the extent permitted by law, amounts not covered by third-party payors are the obligations of individual patients for which the TOI PCs may not receive whole or partial payment. Any increase in cost shifting from third-party payors to individual patients, including as a result of high deductible plans for patients, increases our collection costs and reduces overall collections, which we may not be able to offset such additional costs with sufficient revenue.
In response to the COVID-19 pandemic, the Centers for Medicare and Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, made several changes in the manner in which Medicare will pay for telehealth visits, many of which relax previous requirements, including site requirements for both the providers and patients, telehealth modality requirements and others. The Consolidated Appropriations Act of 2023 extended many of the COVID-19 public health emergency provisions related to telehealth until December 31, 2024. State law applicable to telehealth, particularly licensure requirements, has also been relaxed in many jurisdictions as a result of the COVID-19 pandemic. It is unclear which, if any, of these changes will remain in place permanently and which will be rolled-back following the COVID-19 pandemic. If regulations change to restrict the TOI PCs’ or our affiliated providers ability to deliver care through telehealth modalities, our financial condition and results of operations may be adversely affected.
With many of our value-based agreements, the TOI PCs assume some or all of the risk that the cost of providing services will exceed compensation. If we do not accurately predict the cost to deliver care, some of the TOI PCs’ value-based agreements could become less profitable, or unprofitable.
Approximately 24% of our revenue for 2022 was derived from fixed fees paid by payors under capitation agreements with the TOI PCs. While there are variations specific to each agreement, the TOI PCs generally contract with payors to receive a fixed fee per month for professional services and assume the financial responsibility for the specified medical oncology and related expenses of our patients. This type of contract is referred to as a “capitation” contract. To the extent that patients require more care than is anticipated and/or the cost of care increases, aggregate fixed compensation amounts, or capitation payments, may be insufficient to cover the costs associated with treatment. If medical costs and expenses exceed estimates, except in very limited circumstances, the TOI PCs will not be able to increase the fee received under these risk agreements during their then-current terms and we could suffer losses with respect to such agreements.
Changes in our anticipated ratio of medical expense to revenue can significantly impact our financial results. Accordingly, the failure to adequately predict and control medical costs and expenses could have a material adverse effect on our business, results of operations, financial condition and cash flows. Additionally, the Medicare expenses of our patients may be outside of the TOI PCs control in the event that patients take certain actions that increase such expenses, such as unnecessary hospital visits.
Historically, the TOI PCs’ medical costs and expenses as a percentage of revenue have fluctuated. Factors that may cause medical expenses to exceed estimates include:
•the health status of patients;
•changes to oncology treatment guidelines which our affiliated providers follow;
•higher than expected utilization of new or existing healthcare services, drugs or technologies;
•an increase in the cost of healthcare services and supplies, whether as a result of inflation or otherwise;
•changes to mandated benefits or other changes in healthcare laws, regulations and practices;
•increased costs attributable to provider and support staff compensation or providers with which the TOI PCs contract to provide care to patients;
•changes in the demographics of our patients and medical trends;
21
•contractual or claims disputes with providers, hospitals or other service providers within and outside a health plan’s network; and
•the occurrence of catastrophes, major epidemics or acts of terrorism.
In addition, we are reliant on our customers under value-based contracts to provide us with data related to the population of patients for which we are at risk. This data, in particular, which relates to membership eligibility, is subject to frequent changes, omissions and errors which we cannot control. We work closely with our customers to reconcile this data, but we cannot be certain of the accuracy of this data. If we underestimate or do not correctly predict the cost of the oncology care the TOI PCs provide to patients, the TOI PCs might be underpaid for the care that must be provided to our patients, which could have a negative impact on our results of operations and financial condition.
There are significant risks associated with estimating the amount of revenue that is recognized under TOI PCs’ risk agreements with health plans, and if our estimates of revenue are materially inaccurate, it could impact the timing and the amount of our revenue recognition or have a material adverse effect on our business, results of operations, financial condition and cash flows.
There are significant risks associated with estimating the amount of revenues that is recognize under the TOI PCs’ risk agreements with health plans in a reporting period. The billing and collection process is complex due to ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage and other payor issues, such as ensuring appropriate documentation. Determining applicable primary and secondary coverage for our patients, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and Medicaid programs are also subject to estimating risk related to the amounts not paid by the primary government payor that will ultimately be collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor recoupments typically continue to occur for up to three years and longer after services are provided. If our estimates of revenues are materially inaccurate, it could impact the timing and the amount of our revenues recognition and have a material adverse impact on our business, results of operations, financial condition and cash flows.
A significant portion of our consolidated Patient Services revenue is derived from a limited number of health insurance, Independent Practice Associations ("IPAs"), and medical group companies. Those payors could take action to remove, exclude, delay, or otherwise prevent the inclusion of the TOI PCs in their provider networks.
Our operations are dependent on a concentrated number of payors with whom the TOI PCs contract to provide services to patients. We generally manage the TOI PCs’ payor contracts on a state by state basis, entering into a separate contract in each state with the local affiliate of the relevant payor such that no one local payor contract accounts for a majority of our collective revenue. Regal Medical Group accounted for a total of approximately 13% of the Patient Services revenue for the year ended December 31, 2022. No other non-government payor accounted for more than 10% of the Patient Services revenue in 2022. We believe that a majority of the TOI PCs’ revenues will continue to be derived from a limited number of key payors, which may terminate their contracts with the TOI PC or the individual TOI PC physicians credentialed by them upon the occurrence of certain events. The sudden loss of any of the TOI PCs’ payor partners, or the renegotiation of any of the TOI PCs’ payor contracts, could adversely affect our operating results. In the ordinary course of business we engage in active discussions and renegotiations with payors in respect of the services the TOI PCs provide and the terms of the TOI PCs’ payor agreements. As the payors’ businesses respond to market dynamics and financial pressures, and as payors make strategic business decisions in respect of the lines of business they pursue and programs in which they participate, certain of the payors may seek to renegotiate or terminate their agreements with the TOI PCs. These discussions could result in reductions to the fees and changes to the scope of services contemplated by the original payor contracts and consequently could negatively impact our revenues, business and prospects.
Because we rely on a limited number of payors for a significant portion of the TOI PCs’ revenues, we depend on the creditworthiness of these payors. The payors are subject to a number of risks including reductions in payment rates from governmental programs, higher than expected health care costs and lack of predictability of financial results when entering new lines of business, particularly with high-risk populations. If the financial condition of the TOI PCs’ payor partners declines, our financial results could be impacted. Should one or more of the TOI PCs’ significant payor partners declare bankruptcy, be declared insolvent or otherwise be restricted by state or federal laws or regulation from continuing in some or all of their operations, this could adversely affect our ongoing revenues, the collectability of our accounts receivable, our bad debt reserves and our net income.
Although the TOI PCs have long-term contracts with many payors, these contracts may be terminated before their term expires for various reasons, such as changes in the regulatory landscape and poor performance by the TOI PCs and our
22
affiliated providers, subject to certain conditions. Certain of the payor contracts are terminable immediately upon the occurrence of certain events. Certain of the payor contracts may be terminated immediately by the partner if the TOI PCs lose applicable licenses, go bankrupt, lose its liability insurance or receive an exclusion, suspension or debarment from state or federal government authorities. Additionally, if a payor were to lose applicable licenses, go bankrupt, lose liability insurance, become insolvent, file for bankruptcy or become subject to exclusion, suspension or debarment from state or federal government authorities, the TOI PC’s contract with such payor could in effect be terminated. In addition, certain of the payor contracts may be terminated immediately if a TOI PC becomes insolvent or file for bankruptcy. If any of the contracts with the TOI PCs’ payors is terminated, the TOI PCs may not be able to recover all fees due under the terminated contract, which may adversely affect our operating results.
A significant portion of sales are from prescription drug sales reimbursed by a limited number of pharmacy benefit management companies with which TOI PCs contract. Those pharmacy benefit management companies could take action to remove, exclude, delay or otherwise prevent the inclusion of the TOI PCs in their provider networks.
There is currently significant concentration in the U.S. healthcare industry, and in particular there are a limited number of pharmacy benefit managers, or PBMs, and a limited number of national pharmacy chains. CVS Caremark, OptumRx and Express Scripts together accounted for approximately 85% of our dispensary revenue in 2022. If the TOI PCs are unable to retain favorable contractual arrangements with PBMs, including any successor PBMs should there be further consolidation of PBMs, the negotiated rates provided by such PBMs may become less competitive, which could have an adverse impact on the TOI PCs’ ability to provide prescription drugs at the capitated rates negotiated with the payors with whom the TOI PCs contract to provide such drugs to patients. This could be exacerbated by further consolidation of PBMs or pharmacy chains. Specifically, PBMs have instituted Direct and Indirect Remuneration, or DIR, fees, which reduce the reimbursement for drugs dispensed by the TOI PCs. The impact of these fees in future is uncertain, and our ability to negotiate with PBMs on DIR fees is limited. In addition, PBMs could at any time change their contracting and/or credentialing requirements, the effect of which could prohibit the TOI PCs from billing for prescription drugs dispensed by the TOI PCs. If such changes, individually or in the aggregate, are material, they would have an adverse effect on our business, results of operations and financial condition.
Reductions in government reimbursement rates or changes in the rules governing government healthcare programs could have a material adverse effect on our financial condition and results of operations.
The TOI PCs receive a significant portion of revenue directly from Medicare, which accounted for approximately 16% of our Patient Services revenue in 2022. In addition, many private payors base their reimbursement rates on the published Medicare rates or, in the case of Medicare Advantage, are themselves reimbursed by Medicare for the services the TOI PCs provide. As a result, our results of operations are, in part, dependent on government funding levels for Medicare programs, particularly Medicare Advantage programs. Any changes that limit or reduce Medicare Advantage or general Medicare reimbursement levels, such as reductions in or limitations of reimbursement amounts or rates under programs, reductions in funding of programs, expansion of benefits without adequate funding, elimination of coverage for certain benefits, or elimination of coverage for certain individuals or treatments under programs, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
The Medicare program and its reimbursement rates and rules are subject to frequent change. These include statutory and regulatory changes, rate adjustments (including retroactive adjustments), administrative or executive orders and government funding restrictions, all of which may materially adversely affect the rates at which Medicare reimburses the TOI PCs for patient care services. Budget pressures often lead the federal government to reduce or place limits on reimbursement rates under Medicare. Implementation of these and other types of measures has in the past and could in the future result in substantial reductions in our revenue and operating margins.
In addition, CMS often changes the rules governing the Medicare program, including those governing reimbursement. Changes that could adversely affect our business include:
•administrative or legislative changes to rates or the bases of payment;
•limits on the services or types of providers for which Medicare will provide reimbursement;
•changes in methodology for patient assessment and/or determination of payment levels;
•the reduction or elimination of annual rate increases; or
•an increase in co-payments or deductibles payable by beneficiaries.
There is also uncertainty regarding both Medicare Advantage payment rates and beneficiary enrollment, which, if reduced, would reduce our overall revenues and net income, as well as future growth opportunities. For example, although the
23
Congressional Budget Office (“CBO”) predicted in 2010 that Medicare Advantage participation would drop substantially by 2020, the CBO has more recently predicted, without taking into account potential future reforms, that enrollment in Medicare Advantage (and other contracts covering Medicare Parts A and B) could reach 36 million by 2027. Although Medicare Advantage enrollment has increased significantly over the past decade, there can be no assurance that this trend will continue. Further, fluctuation in Medicare Advantage payment rates are evidenced by CMS’s annual announcement of the expected average change in revenue from the prior year: for 2021, CMS announced an average increase of 1.66%; and for 2022, 4.08%. Uncertainty over Medicare Advantage enrollment and payment rates present a continuing risk to our business.
According to the Kaiser Family Foundation, or KFF, Medicare Advantage enrollment continues to be highly concentrated among a few payors, both nationally and in local regions. In 2022, the KFF reported that two payors together accounted for nearly half of Medicare Advantage enrollment and seven firms accounted for nearly 85% of covered lives. Consolidation among Medicare Advantage plans in certain regions, or the Medicare program’s failure to attract additional plans to participate in the Medicare Advantage program, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Moreover, the Medicaid program and its reimbursement rates and policies are subject to frequent change. By way of example, Medi-Cal recently implemented a new policy regarding reimbursement for pharmacy services. Although the policy was not intended to change the manner in which physician-administered drugs billed under the medical benefit are reimbursed, certain Medi-Cal managed care plans nevertheless began to transition these claims to be payable as a pharmacy benefit and exclude coverage of prescription drugs formerly available through the medical benefit or direct their subcontractors or network providers to no longer bill for prescription drugs through their medical claims. The California Department of Health Care Services, or DHCS, later issued clarifying guidance which instructed Medi-Cal managed care plans to ensure all medically necessary prescription drugs administered in an outpatient office or clinic setting by a health care professional continue to be available through the medical benefit, even though some may be available as a pharmacy benefit. In addition, during the COVID-19 public health emergency, DHCS delayed the processing of Medi-Cal annual redeterminations and delayed discontinuances and negative actions for Medi-Cal and other state and county healthcare programs. DHCS has indicated that Medicaid redeterminations will resume on April 1, 2023, which may cause some of our patients to lose their coverage. As a result, the TOI PCs could experience a reduction in membership, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Any reductions in reimbursement rates or the scope of services, including pharmacy services, rendered by the TOI PCs being reimbursed could have a material, adverse effect on our financial condition and results of operations or even result in reimbursement rates that are insufficient to cover our operating expenses. Additionally, any delay or default by the government in making Medicare or Medicaid reimbursement payments to the TOI PCs or any reduction in patients eligible for such programs could materially and adversely affect our business, financial condition and results of operations.
We cannot predict the effect that health care reform and other changes in government programs may have on our business, financial condition or results of operations.
The impact of healthcare reform legislation and other changes in the healthcare industry and in healthcare spending is currently unknown, but may adversely affect our business, financial condition and results of operations. Our revenue is dependent on the healthcare industry and could be affected by changes in healthcare spending, reimbursement and policy. The healthcare industry is subject to changing political, regulatory and other influences. By way of example, the ACA, which was enacted in 2010, made major changes in how healthcare is delivered and reimbursed, and it increased access to health insurance benefits to the uninsured and underinsured populations of the United States.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of 2%, which began in 2013 and will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022. Under current legislation, the actual reduction in Medicare payments varies from 1% from April 1, 2022 to June 30, 2022, up to 3% in the final fiscal year of this sequester, unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments
24
to providers from three to five years. New laws may result in additional reductions in Medicare and other healthcare funding, which may materially adversely affect consumer demand and affordability for our products and services and, accordingly, the results of our financial operations. Additional changes that may affect our business include the expansion of new programs such as Medicare payment for performance initiatives for physicians under the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, which first affected physician payment in 2019. At this time, it is unclear how the introduction of the Medicare quality payment program will impact overall physician reimbursement. The Inflation Reduction Act of 2022, or IRA, signed into law on August 16, 2022, also contains a number of provisions designed to limit or reduce drug prices under the Medicare program, reduce beneficiary out-of-pocket spending under Medicare’s prescription drug benefit, and expand subsidies for individuals to obtain private health insurance under the ACA. While these provisions of the IRA do not apply directly to healthcare providers like the TOI PCs, we are continuing to evaluate the potential impact, if any, that the IRA may have on our business.
Such changes in the regulatory environment may also result in changes to our payer mix that may affect our operations and revenue. In addition, certain provisions of the ACA authorize voluntary demonstration projects, which include the development of bundling payments for acute, inpatient hospital services, physician services and post-acute services for episodes of hospital care. Further, the ACA may adversely affect payers by increasing medical costs generally, which could have an effect on the industry and potentially impact our business and revenue as payers seek to offset these increases by reducing costs in other areas.
Uncertainty regarding future amendments to the ACA as well as new legislative proposals to reform healthcare and government insurance programs, along with the trend toward managed healthcare in the United States, could result in reduced demand and prices for our services. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments and other third party payers will pay for healthcare products and services, which could adversely affect our business, financial condition and results of operations.
The transition from volume to value-based reimbursement models may have a material adverse effect on our operations.
Healthcare reform is causing some payors to transition from volume to value-based reimbursement models, which can include risk-sharing, bundled payment and other innovative approaches. While these models may provide us with opportunities to provide new or additional services and to participate in incentive- based payment arrangements, there can be no assurance that such new models and approaches will be profitable to us or the TOI PCs. Further, new models and approaches may require investment by us to develop technology or expertise to offer necessary and appropriate solutions or support to the TOI PCs, and we do not fully know the amount and timing for return of such investment at this time. In addition, some of these new models are being offered as pilot programs and there is no assurance that they will continue or be renewed. Many states in which these new value-based structures are being developed also lack regulatory guidance or a well-developed body of law for these new models and approaches, or may not have updated their laws or enacted legislation yet to reflect the new healthcare reform models. As a result, new and existing laws, regulations or guidance could have a material adverse effect on our operations and could subject us to the risk of restructuring or terminating our arrangements with the TOI PCs, as well as the risk of regulatory enforcement, penalties and sanctions, if state and federal enforcement agencies disagree with our interpretation of these laws.
CMS, through the Centers for Medicare and Medicaid Innovation, or the CMMI, has implemented or has announced plans to implement numerous demonstration models designed to test value-based reimbursement models, some of which are specifically focused on oncology services. For example, in 2016, CMS initiated the Oncology Care Model, or OCM demonstration, which continued through June 30, 2022 and provided participating physician practices with performance-based financial incentives that aim to manage or reduce Medicare costs without negatively affecting the efficacy of care. In June 2022, CMS issued a request for applications for the Enhancing Oncology Model, a new 5-year voluntary model that builds on the OCM demonstration. While the extent to which these models may impact our business is uncertain and will depend on future developments, such models may materially reduce Medicare reimbursement levels for our services or TOI PCs’ services and could have a material adverse effect on our results of operations and financial condition.
Changes in the payor mix of patients and potential decreases in reimbursement rates as a result of consolidation among plans could adversely affect our revenues and results of operations.
The amounts the TOI PCs receive for services provided to patients are determined by a number of factors, including the payor mix of patients and the reimbursement methodologies and rates utilized by our patients’ plans. Our Patient Services revenue consists of both capitation and fee-for-service agreements held by the TOI PCs. Reimbursement rates are generally higher for capitation agreements than they are under fee-for-service arrangements, and capitation agreements provide the TOI PCs with an opportunity to capture any additional surplus created by applying our care model. Under a capitation plan, the TOI PCs receive a fixed fee PMPM for services. Under a fee-for-service payor arrangement, the TOI PCs collect fees directly from
25
the payor as services are provided. Our Patient Services revenue accounted for approximately 66% of total revenue for the year ended December 31, 2022. A significant decrease in the number of capitation or FFS arrangements held by the TOI PCs could adversely affect our revenues and results of operation.
The healthcare industry has also experienced a trend of consolidation, resulting in fewer but larger payors that have significant bargaining power, given their market share. Payments from payors are the result of negotiated rates. These rates may decline based on renegotiations and larger payors have significant bargaining power to negotiate higher discounted fee arrangements with healthcare providers. As a result, payors increasingly are demanding discounted fee structures or the assumption by healthcare providers of all or a portion of the financial risk related to paying for care provided through capitation agreements.
We face significant competition from other healthcare services providers. Our failure to adequately compete could adversely affect our business.
We and the TOI PCs compete directly with national, regional and local providers of healthcare for patients and physicians. There are many other companies and individuals currently providing healthcare services, many of which have been in business longer and/or have substantially more resources. Other companies could enter the healthcare industry in the future and divert some or all of our business. If we expand to other geographies, we expect competition may change based on a number of factors, including the number of competing oncology care facilities in the local market and the types of services available at those facilities, our local and the TOI PCs reputation for quality care of patients, the commitment and expertise of the TOI PCs medical staff, our local service offerings and community programs, the cost of care in each locality, and the physical appearance, location, age and condition of our facilities. If we are unable to attract patients to our managed clinics, our revenue and profitability will be adversely affected. Some of our competitors may have greater recognition and be more established in their respective communities than we are, and may have greater financial and other resources than we have. Competing oncology care providers may also offer larger facilities or different programs or services than we do, which, combined with the foregoing factors, may result in our competitors being more attractive to our current patients, potential patients and referral sources. Furthermore, while we budget for routine capital expenditures at our managed clinics to keep them competitive in their respective markets, to the extent that competitive forces cause those expenditures to increase in the future, our financial condition may be negatively affected. In addition, our relationships with governmental and private third-party payors are not exclusive and our competitors have established or could seek to establish relationships with such payors to serve their covered patients. Additionally, as we expand into new geographies, we may encounter competitors with stronger relationships or recognition in the community in such new geography, which could give those competitors an advantage in obtaining new patients. Individual physicians, physician groups and companies in other healthcare industry segments, including those with which the TOI PCs have contracts, and some of which have greater financial, marketing and staffing resources, may become competitors in providing health care services, and this competition may have a material adverse effect on our business operations and financial position.
Competition for physicians and nurses, shortages of qualified personnel or other factors could increase our labor costs and adversely affect our revenue, profitability and cash flows.
Our operations are dependent on the efforts, abilities and experience of the TOI PCs’ physicians and clinical personnel. We compete with other healthcare providers, primarily hospitals and other oncology practices, in attracting physicians, nurses and medical staff to support our managed clinics, recruiting and retaining qualified management and support personnel responsible for the daily operations of each of our managed clinics and in the TOI PCs contracting with payors in each of our markets. In some markets, the lack of availability of clinical personnel has become a significant operating issue facing all healthcare providers. This shortage may require us and the TOI PCs to continue to enhance wages and benefits to recruit and retain qualified personnel or to contract for more expensive temporary personnel. We also depend on the available labor pool of semi-skilled and unskilled workers in each of the markets in which we operate.
If our labor costs increase, we may not be able to raise rates to offset these increased costs. Because a significant percentage of our revenue consists of fixed, prospective payments, our ability to pass along increased labor costs is limited. In particular, if labor costs rise at an annual rate greater than our net annual consumer price index basket update from Medicare, our results of operations and cash flows will likely be adversely affected. Any union activity at our managed clinics that may occur in the future could contribute to increased labor costs. Certain proposed changes in federal labor laws and the National Labor Relations Board’s modification of its election procedures could increase the likelihood of employee unionization attempts. Although none of our employees or the employees of the TOI PCs are currently represented by a collective bargaining agreement, to the extent a significant portion of our employee base unionizes, it is possible our labor costs could increase materially. Our failure to recruit and retain qualified management and medical personnel for the TOI PCs, or to control our collective labor costs, could have a material adverse effect on our business, prospects, results of operations and financial condition.
26
Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to execute our business strategies and growth plans.
To execute on our growth plan, we and the TOI PCs must attract and retain highly qualified personnel. Competition for highly qualified personnel is intense, especially for physicians and other medical professionals who are experienced in providing oncology care services. We and the TOI PCs have, from time to time, experienced, and we expect to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. Many of the companies and healthcare providers with which we compete for experienced personnel have greater resources than we have. If we and the TOI PCs hire employees from competitors or other companies or healthcare providers, their former employers may attempt to assert that these employees or we have breached certain legal obligations, resulting in a diversion of our time and resources.
As we become a more mature company, we may find our recruiting efforts more challenging. The incentives to attract, retain, and motivate employees provided by our stock options and other equity awards, or by other compensation arrangements, may not be as effective as in the past. As such, we may not be successful in continuing to attract and retain qualified personnel. Our recruiting efforts may also be limited by laws and regulations, such as restrictive immigration laws, and restrictions on travel or availability of visas (including during the ongoing COVID-19 pandemic). If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects could be harmed.
Certain of our management has limited experience in operating a public company.
Certain of our executive officers and certain directors have limited experience in the management of a publicly traded company. Our management team may not successfully or effectively manage its transition to a public company that is subject to significant regulatory oversight and reporting obligations under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which will result in less time being devoted to the management and growth of the company. It is possible that we will be required to expand our employee base and hire additional employees to support our operations as a public company, which will increase our operating costs in future periods.
If we are unable to provide consistently high quality of care, our business will be adversely impacted.
Our business is dependent upon the TOI PCs and our affiliated providers providing high-quality care to our patients. In particular, our ability to attract and retain patients and patient referrals dependent upon providing cost effective, quality patient care that meets or exceeds our patients’ and payors’ expectations. We depend on third parties for certain of our patient care needs. If we or the TOI PCs fail to provide service that meets our patients’ and payors’ expectations, we may have difficulty retaining or growing our patient base, which could adversely affect our business, financial condition and results of operations.
We expect the importance of high-quality patient experience to increase as we, through the TOI PCs, expand our business and pursue new lives served. Any failure to maintain high-quality patient experience, or a market perception that we do not maintain high-quality care, could harm the reputation of us and our affiliated providers and our ability to grow the number of lives served, and our business, results of operations, and financial condition. Additionally, as the number of lives served by the TOI PCs in our managed clinics grows, we will need to hire additional personnel to provide quality care at scale. If we and the TOI PCs are unable to provide such care, our business, results of operations, financial condition, and reputation could be harmed.
If certain of our suppliers do not meet our needs, if there are material price increases on supplies, if we are not reimbursed or adequately reimbursed for drugs purchased or if we are unable to effectively access new technology or superior products, it could negatively impact the ability of the TOI PCs to effectively provide the services we offer and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
The TOI PCs have significant drug suppliers that may be the sole or primary source of products critical to the services the TOI PCs provide, or to which we have committed obligations to make purchases, sometimes at particular prices. Approximately 76% of the TOI PCs’ total costs are related to drug purchases, including both oral and chemotherapy drugs, for the year ended December 31, 2022. If any of these suppliers do not meet the TOI PCs’ needs for the products they supply, including in the event of a product recall, shortage or dispute, and we are not able to find adequate alternative sources, if we experience material price increases from these suppliers that we are unable to mitigate, or if some of the drugs that the TOI PCs purchase are not reimbursed or not adequately reimbursed by commercial or government payors, it could have a material adverse impact on our business, results of operations, financial condition and cash flows. In addition, the technology related to the products critical to the services we provide is subject to new developments which may result in superior products. If we are not able to access superior products on a cost-effective basis or if suppliers are not able to fulfill our requirements for such products, we and the
27
TOI PCs could face patient attrition and other negative consequences which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We depend on our information technology systems, and those of our third-party vendors, contractors and consultants, and any failure or significant disruptions of these systems, security breaches or loss of data could materially adversely affect our business, financial condition and results of operations.
Our business is highly dependent on maintaining effective information systems as well as the integrity and timeliness of the data we use to serve our patients, support our care teams and operate our business. Because of the large amount of data that we collect and manage, it is possible that hardware failures or errors in our systems could result in data loss or corruption or cause the information that we collect to be incomplete or contain inaccuracies that our partners regard as significant. If our data were found to be inaccurate or unreliable due to fraud or other error, or if we, or any of the third-party service providers we engage, were to fail to maintain information systems and data integrity effectively, we could experience operational disruptions that may impact our patients and care teams and hinder our ability to provide services, establish appropriate pricing for services, retain and attract patients, manage our patient risk profiles, establish reserves, report financial results timely and accurately and maintain regulatory compliance, among other things.
Our information technology strategy and execution are critical to our continued success. We must continue to invest in long-term solutions that will enable us to anticipate patient needs and expectations, enhance the patient experience, act as a differentiator in the market and protect against cybersecurity risks and threats. We believe our success is dependent, in large part, on maintaining the effectiveness of existing technology systems and continuing to deliver and enhance technology systems that support our business processes in a cost-efficient and resource-efficient manner. Increasing regulatory and legislative changes will place additional demands on our information technology infrastructure that could have a direct impact on resources available for other projects tied to our strategic initiatives. In addition, recent trends toward greater patient engagement in health care require new and enhanced technologies, including more sophisticated applications for mobile devices. Connectivity among technologies is becoming increasingly important. We must also develop new systems to meet current market standards and keep pace with continuing changes in information processing technology, evolving industry and regulatory standards and patient needs. Failure to do so may present compliance challenges and impede our ability to deliver services in a competitive manner. Further, because system development projects are long-term in nature, they may be more costly than expected to complete and may not deliver the expected benefits upon completion.
Security incidents compromising the confidentiality, integrity, and availability of our confidential or personal information and our and our third-party service providers’ information technology systems could result from cyber-attacks, computer malware, viruses, social engineering (including spear phishing and ransomware attacks), credential stuffing, supply chain attacks, efforts by individuals or groups of hackers and sophisticated organizations, including state-sponsored organizations, errors or malfeasance of our personnel, and security vulnerabilities in the software or systems on which we and our third party service providers rely. As techniques used by cyber criminals change frequently, a disruption, cyberattack or other security breach of our information technology systems or infrastructure, or those of our third-party service providers, may go undetected for an extended period and could result in the theft, transfer, unauthorized access to, disclosure, modification, misuse, loss or destruction of our employee, representative, customer, vendor, consumer and/or other third-party data, including sensitive or confidential data, personal information and/or intellectual property. We and certain of our service providers are from time to time, subject to cyberattacks and security incidents, and we cannot guarantee that our security efforts will prevent breaches or breakdowns of our or our third-party service providers’ information technology systems. While we do not believe that we have experienced any significant system failure, accident or security breach to date, if we suffer a material loss or disclosure of health-related or other personal or confidential information as a result of a breach of our information technology systems, including those of our third-party service providers, we may suffer reputational, competitive and/or business harm, incur significant costs and be subject to government investigations, litigation, fines and/or damages, which could have a material adverse effect on our business, prospects, results of operations, financial condition and/or cash flows. Moreover, while we maintain cyber insurance that may help provide coverage for these types of incidents, we cannot assure you that our insurance will be adequate to cover costs and liabilities related to these incidents. Further, our failure to effectively invest in, implement improvements to and properly maintain the uninterrupted operation and data integrity of our information technology and other business systems could adversely affect our results of operations, financial position and cash flow.
We may be subject to legal proceedings and litigation, including intellectual property and privacy disputes, which are costly to defend and could materially harm our business and results of operations.
We and the TOI PCs may be party to lawsuits and legal proceedings in the normal course of business. These matters are often expensive and disruptive to normal business operations. We may face allegations, lawsuits and regulatory inquiries, audits and investigations regarding data privacy, security, labor and employment, consumer protection and intellectual property
28
infringement, including claims related to privacy, patents, publicity, trademarks, copyrights and other rights. We may also face allegations or litigation related to our acquisitions, securities issuances or business practices, including public disclosures about our business. Litigation and regulatory proceedings may be protracted and expensive, and the results are difficult to predict. Certain of these matters may include speculative claims for substantial or indeterminate amounts of damages and include claims for injunctive relief. Additionally, our litigation costs could be significant. Adverse outcomes with respect to litigation or any of these legal proceedings may result in significant settlement costs or judgments, penalties and fines, or require us to modify our services or require us to stop serving certain patients or geographies, all of which could negatively impact our geographical expansion and revenue growth. The TOI PCs may also become subject to periodic audits, which would likely increase our regulatory compliance costs and may require us to change our business practices, which could negatively impact our revenue growth. Managing legal proceedings, litigation and audits, even if we achieve favorable outcomes, is time-consuming and diverts the attention of management and our affiliated providers from our business.
The results of regulatory proceedings, litigation, claims, and audits cannot be predicted with certainty, and determining reserves for pending litigation and other legal, regulatory and audit matters requires significant judgment. There can be no assurance that our expectations will prove correct, and even if these matters are resolved in our favor or without significant cash settlements, these matters, and the time and resources necessary to litigate or resolve them, could harm our reputation, business, financial condition, results of operations and the market price of our common stock.
Furthermore, our business exposes the TOI PCs and our affiliated providers to potential medical malpractice, professional negligence or other related actions or claims that are inherent in the provision of healthcare services. These claims, with or without merit, could cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management and our affiliated providers from our core business, harm our reputation and adversely affect the TOI PCs’ ability to attract and retain patients, any of which could have a material adverse effect on our business, financial condition and results of operations.
Although the TOI PCs and our affiliated providers maintain third-party professional liability insurance coverage, it is possible that claims against them may exceed the coverage limits of their insurance policies. Even if any professional liability loss is covered by an insurance policy, these policies typically have substantial deductibles for which the TOI PCs and our affiliated providers are responsible. Professional liability claims in excess of applicable insurance coverage could have a material adverse effect on our collective business, financial condition and results of operations. In addition, any professional liability claim brought against the TOI PCs or our affiliated providers, with or without merit, could result in an increase of their professional liability insurance premiums. Insurance coverage varies in cost and can be difficult to obtain, and we cannot guarantee that we will be able to obtain insurance coverage on behalf of the TOI PCs and our affiliated providers in the future on terms acceptable to us or at all. If costs of insurance and claims increase, then our collective earnings could decline.
Some jurisdictions preclude the TOI PCs from entering into non-compete agreements with physicians, and other non-compete agreements and restrictive covenants applicable to certain physicians and other clinical employees may not be enforceable.
The TOI PCs have employment contracts with physicians and other health professionals in many states. Some of these contracts include provisions preventing these physicians and other health professionals from competing with us both during and after the term of our contract with them. The law governing non- compete agreements and other forms of restrictive covenants varies from state to state. Some jurisdictions prohibit the TOI PCs from using non-competition covenants with our professional staff. Other states are reluctant to strictly enforce non-compete agreements and restrictive covenants applicable to physicians and other healthcare professionals. Additionally, the Federal Trade Commission recently proposed new rules which, if enacted, would ban non-compete agreements in employee contracts. There can be no assurance that the TOI PCs’ non-compete agreements related to physicians and other health professionals will be found enforceable if challenged in certain states. In such event, the TOI PCs would be unable to prevent physicians and other health professionals formerly employed by the TOI PCs from competing with us, potentially resulting in the loss of some of our patients.
Current and future acquisitions may use significant resources, may be unsuccessful, and could expose us to unforeseen liabilities.
As part of our growth strategy, we may pursue acquisitions of oncology and other physician practices and services. These acquisitions may involve significant cash expenditures, debt incurrence, additional operational losses and expenses, and compliance risks that could have a material adverse effect on our financial condition and results of operations. We may not be able to successfully integrate the acquired businesses into ours and the TOI PCs, and therefore, we may not be able to realize the intended benefits from an acquisition. These acquisitions could result in difficulties integrating acquired operations, technologies, and personnel into our business. Such difficulties may divert significant financial, operational, and managerial resources from our existing operations and make it more difficult to achieve our operating and strategic objectives. We and the
29
TOI PCs may fail to retain employees or patients acquired through these acquisitions, which may negatively impact the integration efforts. These acquisitions could also have a negative impact on our results of operations if it is subsequently determined that goodwill or other acquired intangible assets are impaired, thus resulting in an impairment charge in a future period.
In addition, these acquisitions involve risks that the acquired businesses will not perform in accordance with expectations; that we may become liable for unforeseen financial or business liabilities of the acquires businesses, including liabilities for failure to comply with applicable healthcare regulations; that the expected synergies associated with acquisitions will not be achieved; and that business judgments concerning the value, strengths and weaknesses of businesses acquired will prove incorrect, which could have a material adverse effect on our financial condition and results of operations.
If we are unable to protect the confidentiality of our trade secrets, know-how and other proprietary and internally developed information, the value of our technology could be adversely affected.
We may not be able to protect our trade secrets, know-how and other internally developed information adequately. Although we use reasonable efforts to protect this internally developed information and technology, our employees, consultants and other parties (including independent contractors and companies with which we conduct business) may unintentionally or willfully disclose our information or technology to competitors. Enforcing a claim that a third party illegally disclosed or obtained and is using any of our internally developed information or technology is difficult, expensive and time-consuming, and the outcome is unpredictable. We rely, in part, on non-disclosure, confidentiality and assignment-of-invention agreements with our employees, independent contractors, consultants and companies with which we conduct business to protect our internally developed information. These agreements may not be self-executing, or they may be breached and we may not have adequate remedies for such breach. Moreover, third parties may independently develop similar or equivalent proprietary information or otherwise gain access to our trade secrets, know- how and other internally developed information.
We conduct some clinical trials in contract with the TCR. If we fail to perform our clinical trial services in accordance with contractual requirements, government regulations and ethical considerations, we could be subject to significant costs or liability and our reputation could be adversely affected.
The TCR, contracts with biotechnology and pharmaceutical companies to perform services to assist them in bringing new drugs and biologics to market. TCR’s services include monitoring clinical trials, laboratory analysis, electronic data capture, patient recruitment, data analytics, technology solutions, and other related services. Such services are complex and subject to contractual requirements, government regulations, and ethical considerations. TCR’s services are subject to various regulatory requirements designed to ensure the quality and integrity of the clinical trial process. In the United States, clinical development services must be performed in compliance with applicable laws, rules and regulations enforced by the United States Food and Drug Administration, or FDA, including Good Clinical Practice, or GCP, requirements, which govern, among other things, the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials.
If TCR fails to perform services in accordance with these requirements, regulatory authorities may take action against TCR. Such actions may include injunctions or failure to grant marketing approval of products, imposition of clinical holds or delays, suspension or withdrawal of approvals, rejection of data collected in TCR’s studies, license revocation, product seizures or recalls, operational restrictions, civil or criminal penalties or prosecutions, damages, or fines. Additionally, there is a risk that actions by regulatory authorities, if they result in significant inspectional observations or other measures, could harm TCR’s reputation and cause customers not to award TCR future contracts or to cancel existing contracts. Clients may also bring claims against TCR for breach of TCR’s contractual obligations and patients in the clinical trials and patients taking drugs approved on the basis of those trials may bring personal injury claims against TCR. Any such action could have a material adverse effect on our results of operations, financial condition, and reputation.
Negative publicity regarding the managed healthcare industry generally could adversely affect our results of operations or business.
Negative publicity regarding the managed healthcare industry generally, or the MA program in particular, may result in increased regulation and legislative review of industry practices that further increase our costs of doing business and adversely affect our results of operations or business by:
•requiring us to change our products and services;
•increasing the regulatory, including compliance, burdens under which we operate, which, in turn, may negatively impact the manner in which the TOI PCs provide services and increase our costs of providing services;
30
•adversely affecting our ability to market the TOI PCs products or services through the imposition of further regulatory restrictions regarding the manner in which plans and providers market to MA enrollees; or
•adversely affecting our ability to attract and retain patients.
Our managed clinics may be negatively impacted by weather and other factors beyond our control.
Our results of operations may be adversely impacted by adverse conditions affecting our managed clinics, including severe weather events such as hurricanes and flooding, natural disasters such as earthquakes and forest fires, public health concerns such as contagious disease outbreaks, violence or threats of violence or other factors beyond our control that cause disruption of patient scheduling, displacement of our patients, employees and care teams, or force certain of our managed clinics to close temporarily. Our future operating results may be adversely affected by these and other factors that disrupt the operation of our managed clinics.
Risks Related to Our Regulatory Environment
We are dependent on our relationships with the TOI PCs, which are affiliated professional entities that we do not own, to provide healthcare services, and our business would be harmed if those relationships were disrupted or if our arrangements with the TOI PCs become subject to legal challenges.
Our contractual relationships with the TOI PCs may implicate certain state laws that generally prohibit non-professional entities from providing licensed medical services or exercising control over licensed physicians or other healthcare professionals (such activities generally referred to as the “corporate practice of medicine”) or engaging in certain practices such as fee-splitting with such licensed professionals. The interpretation and enforcement of these laws vary significantly from state to state. There can be no assurance that these laws will be interpreted in a manner consistent with our practices or that other laws or regulations will not be enacted in the future that could have a material and adverse effect on our business, financial condition and results of operations. Regulatory authorities, state boards of medicine, state attorneys general and other parties may assert that, despite the agreements through which we operate, we are engaged in the provision of medical services and/or that our arrangements with the TOI PCs constitute unlawful fee- splitting. If a jurisdiction’s prohibition on the corporate practice of medicine or fee-splitting is interpreted in a manner that is inconsistent with our practices, we would be required to restructure or terminate our arrangements with the TOI PCs to bring our activities into compliance with such laws. A determination of non-compliance, or the termination of or failure to successfully restructure these relationships could result in disciplinary action, penalties, damages, fines, and/or a loss of revenue, any of which could have a material and adverse effect on our business, financial condition and results of operations. State corporate practice and fee-splitting prohibitions also often impose penalties on healthcare professionals for aiding in the improper rendering of professional services, which could discourage physicians and other healthcare professionals from providing clinical services to members of the health plans with whom we contract.
Our managed clinics and the TOI PCs providing professional services at such clinics may become subject to medical liability claims, which could have a material adverse impact on our business.
Our business entails the risk of medical liability claims against us, the TOI PCs and their clinicians. Although we, the TOI PCs and their clinicians carry insurance covering medical malpractice claims in amounts that we believe are appropriate in light of the risks attendant to our business, successful medical liability claims could result in substantial damage awards that exceed the limits of our and our clinicians’ insurance coverage. In addition, professional liability insurance is expensive and insurance premiums may increase significantly in the future, particularly as we expand our services. As a result, adequate professional liability insurance may not be available to our clinicians, our affiliated practices or to us in the future at acceptable costs or at all.
Any claims made against us or the TOI PCs that are not fully covered by insurance could be costly to defend, result in substantial damage awards against us and divert the attention of our management and the TOI PCs from our operations, which could have a material adverse effect on our business, financial condition and results of operations. In addition, any claims may adversely affect our business or reputation.
If there is a change in accounting standards by the Financial Accounting Standards Board ("FASB") or the interpretation thereof affecting consolidation of entities, it could have a material adverse effect on our consolidation of total revenues derived from the TOI PCs.
Our financial statements are consolidated in accordance with applicable accounting standards and include the accounts of our subsidiaries and the TOI PCs, which we manage under long-term management services agreements but are not owned by us. Such consolidation for accounting and/or tax purposes does not, is not intended to, and should not be deemed to, imply or
31
provide us any control over the medical or clinical affairs of the TOI PCs. In the event a change in accounting standards promulgated by FASB or in interpretation of its standards, or if there is an adverse determination by a regulatory agency or a court, or a change in state or federal law relating to the ability to maintain present agreements or arrangements with the TOI PCs, we may not be permitted to continue to consolidate the total revenues of such practices.
Our managed clinics and the TOI PCs may be subject to third-party payor audits, which, if adversely determined against us or the TOI PCs, may have a material effect on our results of operations and financial condition.
As a result of the TOI PCs participation in the Medicare and Medicaid programs, our managed clinics and the TOI PCs are subject to various governmental inspections, reviews, audits and investigations to verify our compliance with these programs and applicable laws and regulations. Payors may also reserve the right to conduct audits. We also periodically conduct internal audits and reviews of our regulatory compliance. An adverse inspection, review, audit or investigation could result in:
•refunding amounts we have been paid pursuant to the Medicare or Medicaid programs or from payors;
•state or federal agencies imposing fines, penalties and other sanctions on us;
•temporary suspension of payment for new patients to the facility or agency;
•decertification or exclusion from participation in the Medicare or Medicaid programs or one or more payor networks;
•self-disclosure of violations to applicable regulatory authorities;
•damage to our reputation;
•the revocation of a facility’s or agency’s license; and
•loss of certain rights under, or termination of, our contracts with payors.
With respect to MA plans, the TOI PCs submit claims and encounter data to applicable MA plans that are used to establish the annual, average Medicare Risk Adjustment Factor, or RAF, scores attributable to each TOI PC’s MA population. These RAF scores determine, in part, the revenue to which the health plans and, in turn, the TOI PCs, are entitled for the provision of medical care to such population. The data submitted to CMS by each health plan is based, in part, on medical charts and diagnosis codes that the TOI PCs prepare and submit to the health plans. CMS audits MA plans for documentation to support RAF-related payments for enrollees chosen at random. The MA plans then ask providers to submit the underlying documentation for members that they serve. It is possible that claims associated with members with higher RAF scores could be subject to more scrutiny in a CMS or plan audit. There is a possibility that a MA plan may seek repayment from the TOI PCs should CMS make any payment adjustments to the MA plan as a result of its audits. CMS has indicated that payment adjustments will not be limited to RAF scores for the specific MA enrollees for which errors are found but may also be extrapolated to the entire MA plan subject to a particular CMS contract. Based on a recent final rule issued by CMS in January 2023, although 2011 to 2017 plan years are still subject to audit, overpayments to MA plans that are identified as a result of a Risk Adjustment Data Validation, or RADV, audit will only be subject to extrapolation for plan year 2018 and any subsequent plan year. In addition, CMS will not apply an adjustment factor, known as a Fee-For-Service, or FFS, Adjuster, in RADV audits to account for potential differences in diagnostic coding between the Medicare Advantage program and Medicare FFS program. We are continuing to assess the potential impact this final rule may have on our business and operations.
We have in the past and will likely in the future be required to refund amounts we have been paid and/or pay fines and penalties as a result of these inspections, reviews, audits and investigations. If adverse inspections, reviews, audits or investigations occur and any of the results noted above occur, it could have a material adverse effect on our business and operating results. Furthermore, the legal, document production and other costs associated with complying with these inspections, reviews, audits or investigations could be significant.
We are subject to extensive fraud, waste, and abuse laws that may give rise to federal and state audits, investigations, lawsuits and claims against us, the outcome of which may have a material adverse effect on our business, financial condition, cash flows, or results of operations.
The U.S. healthcare industry is heavily regulated and closely scrutinized by federal, state and local governments. Comprehensive statutes and regulations govern the manner in which we provide and bill for services and collect reimbursement from governmental programs and private payors, our contractual relationships and arrangements with healthcare providers and vendors, our marketing activities and other aspects of our operations. Of particular importance are:
•the federal Anti-Kickback Statute, or AKS, which prohibits the knowing and willful offer, payment, solicitation or receipt of any bribe, kickback, rebate or other remuneration for referring an individual, in return for ordering, leasing, purchasing or recommending or arranging for or to induce the referral of an individual or the ordering, purchasing or
32
leasing of items or services covered, in whole or in part, by any federal healthcare program, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•the federal physician self-referral law, the Stark Law, which, subject to limited exceptions, prohibits physicians from referring Medicare or Medicaid patients to an entity for the provision of certain designated health services, or DHS if the physician or a member of such physician’s immediate family has a direct or indirect financial relationship (including an ownership interest or a compensation arrangement) with the entity, and prohibits the entity from billing Medicare or Medicaid for such DHS;
•the FCA, which imposes civil and criminal liability on individuals or entities that knowingly submit false or fraudulent claims for payment to the government or knowingly make, or cause to be made, a false statement in order to have a false claim paid, including qui tam or whistleblower suits. There are many potential bases for liability under the FCA. The government has used the FCA to prosecute Medicare and other government healthcare program fraud such as coding errors, billing for services not provided, and providing care that is not medically necessary or that is substandard in quality. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS or Stark Law constitutes a false or fraudulent claim for purposes of the FCA;
•the Civil Monetary Penalties Law, which prohibits, among other things, an individual or entity from offering remuneration to a federal healthcare program beneficiary that the individual or entity knows or should know is likely to influence the beneficiary to order or receive healthcare items or services from a particular provider. We may also be subject to civil monetary penalties and other sanctions under the statute if we or the TOI PCs hire or contract with any individuals or entities that are or become excluded from government healthcare programs, for the provision of items or services for which payment may be made under such programs;
•the criminal healthcare fraud provisions of HIPAA and related rules that prohibit knowingly and willfully executing a scheme or artifice to defraud any healthcare benefit program or falsifying, concealing or covering up a material fact or making any material false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•reassignment of payment rules that prohibit certain types of billing and collection practices in connection with claims payable by the Medicare or Medicaid programs;
•similar state law provisions pertaining to anti-kickback, self-referral and false claims issues, some of which may apply to items or services reimbursed by any payor, including patients and commercial insurers;
•laws that regulate debt collection practices;
•a provision of the Social Security Act that imposes criminal penalties on healthcare providers who fail to disclose, or refund known overpayments;
•federal and state laws that prohibit providers from billing and receiving payment from Medicare and Medicaid for services unless the services are medically necessary, adequately and accurately documented, and billed using codes that accurately reflect the type and level of services rendered;
•federal and state laws and policies that require healthcare providers to maintain licensure, certification or accreditation to enroll and participate in the Medicare and Medicaid programs, to report certain changes in their operations to the agencies that administer these programs and, in some cases, to re-enroll in these programs when changes in direct or indirect ownership occur; and
•federal and state laws pertaining to the provision of services by nurse practitioners and physician assistants in certain settings, physician supervision of those services, and reimbursement requirements that depend on the types of services provided and documented and relationships between physician supervisors and nurse practitioners and physician assistants; and
•Medicare and Medicaid regulations, manual provisions, local coverage determinations, national coverage determinations and agency guidance imposing complex and extensive requirements upon healthcare providers.
The laws and regulations in these areas are complex, changing and often subject to varying interpretations. As a result, there is no guarantee that a government authority will find that we or the TOI PCs are in compliance with all such laws and regulations that apply to our business. Further, because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of the business activities undertaken by us or the TOI PCs could be subject to challenge under one or more of these laws, including, without limitation, our patient assistance programs that waive or reduce the patient’s obligation to pay copayments, coinsurance or deductible amounts owed for the services we
33
provide to them if they meet certain financial need criteria. If our or the TOI PCs’ operations are found to be in violation of any of such laws or any other governmental regulations that apply, we may be subject to significant penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations, integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and imprisonment. In addition, any action against us or the TOI PCs for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and result in adverse publicity, or otherwise experience a material adverse impact on our business, results of operations, financial condition, cash flows, reputation as a result.
If any of our managed clinics or TOI PCs lose their regulatory licenses, permits and/or accreditation status, or become ineligible to receive reimbursement under Medicare or Medicaid or other third-party Payors, there may be a material adverse effect on our business, financial condition, cash flows, or results of operations.
The operations of our managed clinics through the TOI PCs are subject to extensive federal, state and local regulation relating to, among other things, the adequacy of medical care, equipment, personnel, operating policies and procedures, dispensing of prescription drugs, fire prevention, rate-setting and compliance with building codes and environmental protection. Our managed clinics and TOI PCs are also subject to extensive laws and regulation relating to facility and professional licensure, conduct of operations, including financial relationships among healthcare providers, Medicare and Medicaid fraud and abuse and physician self-referrals, and maintaining updates to the TOI PCs’ enrollment in the Medicare and Medicaid programs, including addition of new clinic locations, providers and other enrollment information. Our managed clinics and TOI PCs are subject to periodic inspection by licensing authorities and accreditation organizations to assure their continued compliance with these various standards. There can be no assurance that these regulatory authorities will determine that all applicable requirements are fully met at any given time. Should any of our managed clinics or TOI PCs be found to be noncompliant with these requirements, we could be assessed fines and penalties, could be required to refund reimbursement amounts or could lose our licensure or Medicare and/or Medicaid certification or accreditation so that we or the TOI PCs are unable to receive reimbursement from such programs and possibly from other third-party payors, any of which could materially adversely affect our business, financial condition, cash flows or results of operations.
If we or the TOI PCs fail to comply with applicable data interoperability and information blocking rules, our consolidated results of operations could be adversely affected.
The 21st Century Cures Act (the “Cures Act”), which was passed and signed into law in December 2016, includes provisions related to data interoperability, information blocking and patient access. In March 2020, the HHS Office of the National Coordinator for Health Information Technology, or ONC, and CMS finalized and issued complementary rules that are intended to clarify provisions of the Cures Act regarding interoperability and information blocking, and include, among other things, requirements surrounding information blocking, changes to ONC’s health IT certification program and requirements that CMS- regulated payors make relevant claims/care data and provider directory information available through standardized patient access and provider directory application programming interfaces, or APIs, that connect to provider electronic health record systems, or EHRs. The companion rules will transform the way in which healthcare providers, health IT developers, health information exchanges/health information networks, or HIEs/HINs, and health plans share patient information, and create significant new requirements for healthcare industry participants. For example, the ONC rule, which went into effect on April 5, 2021, prohibits healthcare providers, health IT developers of certified health IT, and HIEs/HINs from engaging in practices that are likely to interfere with, prevent, materially discourage, or otherwise inhibit the access, exchange or use of electronic health information, or EHI, also known as “information blocking.” To further support access and exchange of EHI, the ONC rule identifies eight “reasonable and necessary activities” as exceptions to information blocking activities, as long as specific conditions are met. Any failure to comply with these rules could have a material adverse effect on our business, results of operations and financial condition.
Actual or perceived failures to comply with applicable data protection, privacy and security, advertising and consumer protection laws, regulations, standards and other requirements could adversely affect our business, financial condition and results of operations.
We and the TOI PCs collect, receive, generate, use, process, and store significant and increasing volumes of sensitive information, such as employee, individually identifiable health information and other personally identifiable information. We and the TOI PCs are subject to a variety of federal and state laws and regulations, as well as contractual obligations, relating to the collection, use, storage, retention, security, disclosure, transfer, return, destruction and other processing of personal information, including health- related information. Enforcement actions and consequences for noncompliance with such laws, directives and regulations are rising, and the regulatory framework for privacy, data protection and data transfers is complex and rapidly evolving and is likely to remain uncertain for the foreseeable future.
34
In the United States, numerous such federal and state laws and regulations, including data breach notification laws, health information privacy laws, and consumer protection laws and regulations, including those that govern the collection, use, disclosure, and protection of health-related and other personal information, could apply to our operations or the operations of the TOI PCs. For example, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder, which we refer to collectively as HIPAA, imposes privacy, security and breach notification obligations on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities. HIPAA requires covered entities, such as the TOI PCs, and business associates, such as us, to develop and maintain policies with respect to the protection of, use and disclosure of protected health information, or PHI, including the adoption of administrative, physical and technical safeguards to protect such information, and certain notification requirements in the event of a data breach.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, or PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. HIPAA also authorizes state Attorneys General to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Numerous other state and federal laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality, security and processing of personal information, including health-related information, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. In addition, these laws and regulations in many cases are more restrictive than, and may not be preempted by, HIPAA and may be subject to varying interpretations by courts and government agencies. Laws in all 50 states and other United States territories require businesses to provide notice to individuals whose personal information has been disclosed as a result of a data breach. Such laws are not always consistent, and compliance in the event of a widespread data breach is costly and may be challenging.
States are also constantly amending existing laws, requiring attention to frequently changing requirements, and we expect these changes to continue. For example, in June 2018, California enacted the California Consumer Privacy Act, or the CCPA, which became effective on January 1, 2020, and, among other things, requires covered companies to provide disclosures to California consumers, and affords such consumers certain data protection rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information that may increase data breach litigation. While the CCPA includes certain exceptions for health-related information, including PHI, it still may require us to modify our data practices and policies and to incur substantial costs and expenses in an effort to comply. Further, the California Privacy Rights Act, or CPRA, generally went into effect on January 1, 2023 and significantly amends the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. Additional compliance investment and potential business process changes may be required. Similar laws have passed in Virginia, Colorado, Connecticut and Utah, and have been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. The enactment of such laws could have potentially conflicting requirements that would make compliance challenging.
As required by certain laws, we publicly post documentation regarding our privacy practices concerning the collection, processing, use and disclosure of certain data. The publication of our privacy policy and other documentation that provide promises and assurances about privacy and security can subject us to potential state and federal action if they are found to be deceptive, unfair, or misrepresentative of our actual practices. In addition, although we endeavor to comply with our published policies and documentation, individuals could allege we have failed to do so, or we may at times actually fail to do so despite our efforts. Any failure by us, our third-party service providers or other parties with whom we do business to comply with this documentation or with laws or regulations applicable to our business could result in proceedings against us by governmental entities or others.
In addition, the Federal Trade Commission, or the FTC, expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Our failure to take any steps perceived by the FTC as
35
appropriate to protect consumers’ personal information may result in claims by the FTC that we have engaged in unfair or deceptive acts or practices in violation of Section 5(a) of the FTC Act. State consumer protection laws provide similar causes of action for unfair or deceptive practices for alleged privacy, data protection and data security violations.
In addition to government regulation, privacy advocates and industry groups may propose self- regulatory standards from time to time. These and other industry standards may legally or contractually apply to us, or we may elect to comply with such standards or to facilitate our customers’ compliance with such standards. We expect that there will continue to be new proposed laws and regulations concerning privacy, data protection, and information security, and we cannot yet determine the impact such future laws, regulations, and standards may have on our business. New laws, amendments to or re-interpretations of existing laws and regulations, industry standards, contractual and other obligations may require us to incur additional costs and restrict our business operations. Because the interpretation and application of laws, standards, contractual and other obligations relating to privacy and data protection are still uncertain and changing, it is possible that these laws, standards, contractual and other obligations may be interpreted and applied in a manner that is inconsistent with our data management practices, our privacy, data protection or data security policies or procedures or the features of our technology. If so, in addition to the possibility of fines, lawsuits, regulatory investigations, imprisonment of company officials and public censure, other claims and penalties, significant costs for remediation and damage to our reputation, we could be required to fundamentally change our business activities and practices or modify our technology, any of which could adversely affect our business. We may be unable to make such changes or modifications in a commercially reasonable manner, or at all, and our ability to develop new software or provide new services could be limited. Any inability to adequately address privacy, data protection or information security-related concerns, even if such concerns are unfounded, or to successfully negotiate privacy, data protection or information security- related contractual terms with customers, or to comply with applicable laws and regulations, or our policies relating to privacy, data protection, and information security, could result in additional cost and liability to us, harm our reputation and brand, and adversely affect our business, financial condition and results of operations.
We and our TOI PCs are subject to federal, state and local laws and regulations that govern our business. These include regulations of our employment practices, including minimum wage, living wage, and paid time-off requirements, permitting and licensing, employee health and safety and the storage, treatment and disposal of waste. Failure to comply with these laws and regulations, or changes to these laws and regulations that increase our expenses, could adversely impact our operations.
We and the TOI PCs are required to comply with all applicable federal, state and local laws and regulations related to the operation of our business. These regulations include regulations governing the TOI PCs’ dispensary services, the construction, the use of our managed clinics and the treatment of hazardous waste or drug products. Changes in regulations or new regulations could increase our costs, cause the TOI PCs to lose licenses or accreditations or otherwise harm our business or the business of the TOI PCs.
We and the TOI PCs are required to comply with all applicable federal, state and local laws and regulations relating to employment, including occupational safety and health requirements, wage and hour and other compensation requirements, employee benefits, providing leave and sick pay, employment insurance, proper classification of workers as employees or independent contractors, immigration and equal employment opportunity laws. These laws and regulations can vary significantly among jurisdictions and can be highly technical. Costs and expenses related to these requirements are a significant operating expense and may increase as a result of, among other things, changes in federal, state or local laws or regulations, or the interpretation thereof, requiring employers to provide specified benefits or rights to employees, increases in the minimum wage and local living wage ordinances, increases in the level of existing benefits or the lengthening of periods for which unemployment benefits are available. We may not be able to offset any increased costs and expenses. Furthermore, any failure to comply with these laws requirements, including even a seemingly minor infraction, can result in significant penalties which could harm our reputation and have a material adverse effect on our business.
We may not be able to utilize a portion of our NOLs to offset future taxable income for U.S. federal income tax purposes, which could adversely affect our net income and cash flows.
As of December 31, 2022, we had federal income tax NOLs of approximately $91,435 and state income tax NOLs of approximately $85,733 available to offset our future taxable income, if any, prior to consideration of annual limitations that may be imposed under Section 382 of the Code or otherwise. The federal NOLs will be carried forward indefinitely and the state NOLs begin expiring after 2040. Utilization of these NOLs depends on many factors, including our future income, which cannot be assured. Some of these NOLs could expire unused and be unavailable to offset our future income tax liabilities. In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, and corresponding provisions of state law, if a corporation undergoes an “ownership change” (very generally defined as a greater than 50% change, by value, in the corporation’s equity ownership by certain stockholders or groups of stockholders over a rolling three-year period), the corporation’s ability to use is pre-ownership change NOLs to offset its post-ownership change income may be limited. We are
36
in the process of completing an analysis to determine whether the Business Combination resulted in an ownership change to determine if there is a limitation on pre-ownership NOLs. Additionally, we may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If it is determined that an ownership change has occurred as a result of the Business Combination or we undergo an ownership change in the future, we may be prevented from fully utilizing our NOLs existing at the time of the ownership change prior to their expiration. The deferred tax asset associated with the Company’s federal and state net operating losses are fully offset by a valuation allowance. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. To the extent we are not able to offset future taxable income with our NOLs, our net income and cash flows may be adversely affected.
Future changes to applicable tax laws and regulations and/or their interpretation may have an adverse effect on our business, financial condition and results of operations. Tax rules and regulations are subject to interpretation and require judgment by us that may be successfully challenged by the applicable taxation authorities upon audit, which could result in additional tax liabilities.
Changes in tax laws or their interpretation could decrease the amount of revenues we receive, the value of any tax loss carry-forwards and tax credits recorded on our balance sheet and the amount of our cash flow, and adversely affect our business, financial condition or results of operations. In addition, other factors or events, including business combinations and investment transactions, changes in the valuation of our deferred tax assets and liabilities, adjustments to taxes upon finalization of various tax returns or as a result of deficiencies asserted by taxing authorities, increases in expenses not deductible for tax purposes, changes in available tax credits, other changes in the apportionment of our income, and changes in tax rates, could also increase our future effective tax rate.
In addition, our effective tax rate and tax liability are based on the application of current income tax laws, regulations and treaties. These laws, regulations and treaties are complex, and the manner which they apply to us and our diverse set of business arrangements is often open to interpretation, and can require us to take positions regarding the interpretation of applicable rules or the valuation of our assets that are subject to material uncertainty. Significant management judgment is required in determining our provision for taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. The proper tax treatment or characterization of many of the transactions we undertake, such as the transactions associated with our issuance of the Convertible Notes and DF Warrants, is often subject to significant uncertainty, and the resolution of any related issues could affect the withholding tax liabilities to which we are subject or the tax deductions that we are able to claim. The tax authorities could challenge our interpretation of laws, regulations and treaties or the positions that we have taken regarding the valuation of its assets, resulting in additional tax liability or adjustment to our income tax provision.
Our tax filings are subject to review or audit by various taxing authorities. As discussed above, we exercise significant judgment in determining our provision for taxes and, in the ordinary course of our business, there may be transactions and calculations where the proper tax treatment is uncertain. We may also be liable for taxes in connection with businesses we acquire. Our determinations are not binding on the IRS or any other taxing authorities, and accordingly the final determination in an audit or other proceeding may be materially different than the treatment reflected in our tax provisions, accruals and returns. An assessment of additional taxes because of an audit could have a material adverse effect on our business, financial condition, results of operations and cash flows.
New income, sales, use or other tax laws, statutes, rules, regulations or ordinances could be enacted at any time, or interpreted, changed, modified or applied adversely to us, any of which could adversely affect our business operations and financial performance. We are unable to predict what changes will occur and, if so, the ultimate impact on its business. To the extent that such changes have a negative impact on us, they may materially and adversely impact its business, financial condition, results of operations and cash flows.
Risks Related to Our Financial Condition
Goodwill and other intangible assets represent a significant portion of our total assets. Goodwill is tested for impairment at least annually, which could result in a material, non-cash write-down of goodwill and could have a material adverse effect on our results of operations and stockholders’ equity.
Goodwill represents the excess of cost over the fair market value of net assets acquired in business combinations. For example, if our market capitalization drops significantly below the amount of the carrying equity recorded on our balance sheet, it might indicate a decline in our fair value and would require us to further evaluate whether our goodwill has been impaired. If, as part of our annual review of goodwill, we are required to write down all or a significant part of our goodwill, our net earnings could be materially adversely affected, which could affect our flexibility to obtain additional financing. In addition, if our
37
assumptions used in preparing our valuations for purposes of impairment testing differ materially from actual future results, we may record impairment charges in the future and our financial results may be materially adversely affected. We had $21,418 and $26,626 of goodwill recorded on our Consolidated Balance Sheets at December 31, 2022 and 2021, respectively. Goodwill impairment charges of $9,944 and $0 were recorded during the years ended December 31, 2022 and 2021, respectively, based on management's evaluation of the value goodwill. It is not possible at this time, under current market conditions, to determine if there will be any future impairment charge, or if there is, whether such charges would be material.
We may need additional capital to fund its operations and finance its growth, and we may not be able to obtain it on acceptable terms, or at all, which may limit our ability to grow.
Our ability to maintain our operations and grow in existing and new markets may require additional capital, particularly if we were to accelerate its acquisition and expansion plans. Financing may not be available or may be available only on terms that are not favorable. If we are unable to obtain funds on acceptable terms, it may have to delay or abandon some or all of its growth strategies. Further, if additional funds are raised through the issuance of additional equity securities, the percentage ownership of our stockholders would be diluted. Any newly issued equity securities may have rights, preferences or privileges senior to those of the Common Stock.
Risks Related to Our Common Stock and Warrants
Our issuance of additional shares of Common Stock, Warrants or other convertible securities may dilute your ownership interest in us and could adversely affect our stock price.
From time to time in the future, we may issue additional shares of our Common Stock, Warrants or other securities convertible into Common Stock pursuant to a variety of transactions, including acquisitions. Additional shares of our Common Stock may also be issued upon exercise of outstanding stock options and Warrants. The issuance by us of additional shares of our Common Stock, Warrants or other securities convertible into our Common Stock would dilute your ownership interest in us and the sale of a significant amount of such shares in the public market could adversely affect prevailing market prices of our Common Stock and Warrants. Subject to the satisfaction of vesting conditions and the expiration of our lock-up, shares issuable upon exercise of options will be available for resale immediately in the public market without restriction.
In the future, we expect to obtain financing or to further increase our capital resources by issuing additional shares of our capital stock or offering debt or other equity securities, including senior or subordinated notes, debt securities convertible into equity, or shares of preferred stock. Issuing additional shares of our capital stock, other equity securities, or securities convertible into equity may dilute the economic and voting rights of our existing stockholders, reduce the market price of our Common Stock and Warrants, or both. Debt securities convertible into equity could be subject to adjustments in the conversion ratio pursuant to which certain events may increase the number of equity securities issuable upon conversion. Preferred stock, if issued, could have a preference with respect to liquidating distributions or a preference with respect to dividend payments that could limit our ability to pay dividends to the holders of our Common Stock. Our decision to issue securities in any future offering will depend on market conditions and other factors beyond our control, which may adversely affect the amount, timing or nature of our future offerings. As a result, holders of our Common Stock and Warrants bear the risk that our future offerings may reduce the market price of our Common Stock and Warrants and dilute their percentage ownership.
Future sales, or the perception of future sales, of our Common Stock and Warrants by us or our existing securityholders in the public market could cause the market price for our Common Stock and Warrants to decline.
The sale of substantial amounts of shares of our Common Stock or Warrants in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our Common Stock and Warrants. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
In addition, the shares of our Common Stock reserved for future issuance under the 2021 Plan will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements, lock-up provisions and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. The number of shares reserved for future issuance under the 2021 Incentive Plan is equal to the sum of (i) 7% of the aggregate number of shares of DFP Class A and DFP Class B Common Stock outstanding on a fully diluted basis as of the effective date of the 2021 Plan; (ii) up to 634,067 shares of Common Stock which are subject to options outstanding under the Prior Plan; (iii) an annual increase on January 1 of each calendar year (commencing January 1, 2022 and ending on and including January 1, 2031) equal to a number of shares of Common Stock equal to 4% of the aggregate shares of Common Stock outstanding on a fully diluted basis as of December 31 of the immediately preceding calendar year (or such lesser number of shares as is determined by the Board), subject to adjustment by the plan administrator in the event of certain changes in our corporate
38
structure, as described below, and (iv) up to 1,178,065 option holder earnout shares or stockholder earnout shares which may become available for issuance under the 2021 Plan. We have filed one or more registration statements on Form S-8 under the Securities Act to register shares of our Common Stock or securities convertible into or exchangeable for shares of our Common Stock issued pursuant to our equity incentive plans. Such Form S-8 registration statements automatically become effective upon filing. Accordingly, shares registered under such registration statements are available for sale in the open market.
Delaware law and provisions in our Charter and Bylaws could make a takeover proposal more difficult.
Our organizational documents are governed by Delaware law. Certain provisions of Delaware law and of our Charter and Bylaws could discourage, delay, defer or prevent a merger, tender offer, proxy contest or other change of control transaction that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares of Common Stock. These provisions include the ability of our Board to designate the terms of and issue new series of preference shares, which may make more difficult the removal of management and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
These anti-takeover provisions as well as certain provisions of Delaware law could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, stockholders of the Company may be limited in their ability to obtain a premium for their shares. If prospective takeovers are not consummated for any reason, we may experience negative reactions from the financial markets, including negative impacts on the price of our Common Stock and Warrants. These provisions could also discourage proxy contests and make it more difficult for stockholders of the Company to elect directors of their choosing and to cause us to take other corporate actions that stockholders of the Company desire. See “Description of Capital Stock.”
We are an “emerging growth company” and the reduced disclosure requirements applicable to emerging growth companies may make our Common Stock and Warrants less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. As an emerging growth company, we may follow reduced disclosure requirements and do not have to make all of the disclosures that public companies that are not emerging growth companies do. We will remain an emerging growth company until the earlier of (a) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (b) the last day of the fiscal year following the fifth anniversary of the date of the completion of the initial public offering of DFP; (c) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (d) the date on which we are deemed to be a large accelerated filer under the rules of the SEC, which means the market value of our Common Stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
•not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act;
•not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis);
•reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
•exemptions from the requirements of holding a nonbinding advisory vote of stockholders on executive compensation, stockholder approval of any golden parachute payments not previously approved and having to disclose the ratio of the compensation of our chief executive officer to the median compensation of our employees.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period for complying with new or revised accounting standards; and as a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We may choose to take advantage of some, but not all, of the available exemptions for emerging growth companies. We cannot predict whether investors will find our Common Stock or Warrants less attractive if we rely on these exemptions. If some investors find our Common Stock or Warrants less attractive as a result, there may be a less active trading market for our Common Stock and Warrants and our share and Warrant price may be more volatile.
39
Our certificate of incorporation and our bylaws provide that the Court of Chancery of the State of Delaware is the sole and exclusive forum for substantially all disputes between us and our stockholders, which limits our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our Charter and Bylaws provide that, unless we consent in writing to the selection of an alternative forum, the (a) Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (i) any derivative action, suit or proceeding brought on our behalf; (ii) any action, suit or proceeding asserting a breach of fiduciary duty owed by any current or former director, officer, stockholder or employee of the company to the company or its stockholders; (iii) any action, suit or proceeding asserting a claim against the Company arising under the DGCL, its certificate of incorporation or its bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware; (iv) any action, suit or proceeding as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (v) any action, suit or proceeding asserting a claim against the Company or any current or former director, officer or stockholder governed by the internal affairs doctrine, and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to (A) the personal jurisdiction of the state and federal courts within Delaware and (B) service of process on such stockholder’s counsel. The provision of the Charter described in the immediately preceding sentence does not apply to (i) suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction and (ii) any action arising under the Securities Act, as to which the federal district court for the United States of America shall have exclusive jurisdiction. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
Additionally, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. As noted above, our certificate of incorporation and our bylaws provide that the federal district courts of the United States shall have jurisdiction over any action arising under the Securities Act. Accordingly, there is uncertainty as to whether a court would enforce such provision. Our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
The market price of our Common Stock may be volatile or may decline regardless of our operating performance. You may lose some or all of your investment.
The market price of our Common Stock is likely to be volatile. The stock market recently has experienced extreme volatility. This volatility often has been unrelated or disproportionate to the operating performance of particular companies. You may not be able to resell your shares at an attractive price due to a number of factors such as those listed in this section and the following:
•the impact of the COVID-19 pandemic on our financial condition and the results of operations;
•our operating and financial performance and prospects;
•our quarterly or annual earnings or those of other companies in our industry compared to market expectations;
•conditions that impact demand for our products;
•future announcements concerning our business, our customers’ businesses or our competitors’ businesses;
•the public’s reaction to our press releases, other public announcements and filings with the SEC;
•the size of our public float;
•coverage by or changes in financial estimates by securities analysts or failure to meet their expectations;
•market and industry perception of our success, or lack thereof, in pursuing our growth strategy;
•strategic actions by us or our competitors, such as acquisitions or restructurings;
•changes in laws or regulations that adversely affect our industry or us;
•changes in accounting standards, policies, guidance, interpretations or principles;
•changes in senior management or key personnel;
•issuances, exchanges or sales, or expected issuances, exchanges or sales, of our capital stock;
•changes in our dividend policy;
•adverse resolution of new or pending litigation against us; and
40
•changes in general market, economic and political conditions in the United States and global economies or financial markets, including those resulting from natural disasters, terrorist attacks, acts of war and responses to such events.
These broad market and industry factors may materially reduce the market price of our Common Stock and Warrants, regardless of our operating performance. In addition, price volatility may be greater if the public float and trading volume of our Common Stock is low. As a result, you may suffer a loss on your investment.
In the past, following periods of market volatility, stockholders have instituted securities Class Action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
If securities analysts cease publishing research or reports about us, or if they issue unfavorable commentary about us or our industry or downgrade our Common Stock, the price of our Common Stock could decline.
The trading market for our Common Stock depends, in part, on the research and reports that third-party securities analysts publish about us and the industries in which we operate. We may be unable or slow to attract research coverage, and if one or more analysts cease coverage of us, the price and trading volume of our securities would likely be negatively impacted. If any of the analysts that may cover us change their recommendation regarding our Common Stock adversely, or provide more favorable relative recommendations about our competitors, the price of our Common Stock would likely decline. If any analyst that may cover us ceases covering us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the price or trading volume of our Common Stock to decline. Moreover, if one or more of the analysts who cover us downgrades our Common Stock, or if our reporting results do not meet their expectations, the market price of our Common Stock could decline.
The obligations associated with being a public company involve significant expenses and require significant resources and management attention, which may divert from our business operations.
We are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal control over financial reporting. As a result, we will incur increased legal, accounting and other expenses that Legacy TOI did not previously incur. Our entire management team and many of our other employees will need to devote substantial time to compliance and may not effectively or efficiently manage our transition into a public company.
In addition, the need to establish the corporate infrastructure demanded of a public company may also divert management’s attention from implementing our business strategy, which could prevent us from improving our business, results of operations and financial condition. We have made, and will continue to make, changes to our internal control over financial reporting, including IT controls, and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to satisfy our obligations as a public company. If we do not continue to develop and implement the right processes and tools to manage our changing enterprise and maintain our culture, our ability to compete successfully and achieve our business objectives could be impaired, which could negatively impact our business, financial condition and results of operations. In addition, we cannot predict or estimate the amount of additional costs we may incur to comply with these requirements. We anticipate that these costs will materially increase our general and administrative expenses.
These rules and regulations result in our incurring legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on our board of directors, on our board committees or as executive officers.
We do not intend to pay dividends on our Common Stock for the foreseeable future.
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and future earnings, if any, to fund the development and growth of the business, and therefore, do not anticipate declaring or paying any cash dividends on Common Stock in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our business prospects, results of operations, financial condition, cash requirements and availability, debt repayment obligations, capital expenditure needs, contractual restrictions, covenants in the agreements governing current and future indebtedness, industry trends, the provisions of Delaware law
41
affecting the payment of dividends and distributions to stockholders and any other factors or considerations the board of directors deems relevant.
We have identified a material weakness in our internal controls over financial reporting. We may not be able to timely and effectively implement controls and procedures required by Section 404 of the Sarbanes-Oxley Act that are applicable to us.
As a public company, we are required to comply with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley Act, which require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of internal control over financial reporting. To comply with the requirements of being a public company, we are required to provide attestation on internal controls, and we may need to undertake various actions, such as implementing additional internal controls and procedures and hiring additional accounting or internal audit staff. The standards required for a public company under Section 404 of the Sarbanes-Oxley Act are significantly more stringent than those that were required of TOI as a privately held company. Management may not be able to effectively and timely implement controls and procedures that adequately respond to the increased regulatory compliance and reporting requirements that became applicable to us after the Business Combination. If we are not able to implement the additional requirements of Section 404 in a timely manner or with adequate compliance, we may not be able to assess whether our internal controls over financial reporting are effective, which may subject us to adverse regulatory consequences and could harm investor confidence and the market price of our Common Stock. Further, as an emerging growth company, our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 until the date we are no longer an emerging growth company. At such time, our independent registered public accounting firm may issue a report that is adverse in the event that it is not satisfied with the level at which our controls are documented, designed or operating.
We have identified a material weakness in our internal control over financial reporting over complex accounting transactions. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
We have implemented a remediation plan to remediate the material weakness identified but can give no assurance that the measures we take will prevent any future material weaknesses or deficiencies in our internal control over financial reporting. The material weakness will not be considered remediated until management completes the design and implementation of processes and controls in our remediation plan and management has concluded, through testing, that these controls are effective.
Maintaining effective internal controls over financial reporting is necessary for us to produce reliable financial reports and helping prevent financial fraud. If we are unable to maintain adequate internal controls over financial reporting, our business and operating results could be harmed.
Our warrants may have an adverse effect on the market price of our Common Stock.
Simultaneously with the closing of its IPO, DFP Healthcare Acquisitions Corp., issued in a private placement an aggregate of 4,333,333 private placement warrants, each exercisable to purchase one share of Common Stock at $11.50 per share. As of December 31, 2022, there were 3,177,542 private placement warrants outstanding. To the extent such warrants are exercised, additional shares of our Common Stock will be issued, which will result in dilution to our stockholders and increase the number of shares of Common Stock eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of our Common Stock.
Risks Related to Our Indebtedness
The Facility Agreement and the associated restrictive covenants thereunder could adversely affect our financial condition and will restrict our ability to raise capital.
On August 9, 2022, we entered into the Facility Agreement with Deerfield Partners and certain of its affiliates. The Facility Agreement contains various covenants, including a requirement to retain $40,000 in unrestricted cash and cash equivalents, and maintain a minimum revenue of $50,000, $75,000, and $100,000 for each fiscal quarter ending during the fiscal year 2023, 2024, and 2025, respectively. In addition, the Facility Agreement restricts our and the guarantors’ ability to, among other things, (i) merge, consolidate, dissolve or liquidate into or convey, transfer, lease or dispose of all or substantially all of its assets (other than into another Loan Party or if the Company determines in good faith in the best interest of a subsidiary and not materially disadvantageous), (ii) create or incur any lien on our assets beyond those outstanding on the date
42
of the Facility Agreement and certain other permitted liens, (iii) dispose of any assets or property or issue, transfer, or provide a controlling, management, or other interest in certain securities of the Company or its guarantors, (iv) incur any indebtedness not to exceed $1,000 or as otherwise permitted, (v) make any investments other than as otherwise permitted, (vi) amend our organizational documents or any material agreements in a manner that would reasonably be expected to be materially adverse to the rights of the lenders or (vii) change our reporting practices or fiscal year, in each case, subject to exceptions set forth in the Facility Agreement. Furthermore, under the Facility Agreement, we are required to, among other things, (i) remain a reporting company and maintain the listing of our common shares on an eligible market, (ii) provide the lenders with information regarding any event of default or the occurrence of any material adverse event and (iii) publicly disclose material, nonpublic information that is provided to the lenders without their prior written consent. Subject to customary exceptions and exclusions, our obligations under the Facility Agreement are guaranteed by a perfected, first-priority security interest in substantially all of our personal property, including our intellectual property and the equity ownership interests directly and indirectly held by us in our wholly-owned subsidiaries. Compliance with such covenants and our indebtedness will result in the following, which could materially and adversely affect our business, financial condition and results of operations:
•require us to dedicate a substantial portion of cash and cash equivalents to the payment of interest on, and principal of, the indebtedness, which will reduce the amounts available to fund working capital, capital expenditures, product development efforts and other general corporate purposes;
•oblige us to comply with negative covenants restricting our activities, including limitations on dispositions, mergers or acquisitions, encumbering our intellectual property, incurring indebtedness or liens, paying dividends, making investments and engaging in certain other business transactions;
•limit our flexibility in planning for, or reacting to, changes in our business and our industry;
•place us at a competitive disadvantage compared to our competitors who have less debt or competitors with comparable debt at more favorable interest rates; and
•limit our ability to borrow additional amounts for working capital, capital expenditures, research and development efforts, acquisitions, debt service requirements, execution of our business strategy and other purposes and otherwise restrict our financing options.
Furthermore, because the interests of the lenders may potentially differ from ours and from those of our stockholders, we may be unable to engage in transactions or other activities that may be beneficial to our stockholders. The covenants under the Facility Agreement could materially and adversely affect our business, financial condition and results of operations.
Upon the occurrence of a Major Transaction, as defined under the Senior Secured Convertible Note, the holders of the convertible notes may elect to require us to redeem all or any portion of the notes for an amount equal to the principal amount thereof (in addition to accrued and unpaid interest, a make-whole amount and an exit fee, as applicable). There can be no assurance that we will have sufficient capital to redeem such notes upon the occurrence of a Major Transaction, under the Senior Secured Convertible Note.
Servicing our indebtedness requires a significant amount of cash. Our ability to repay the principal of, to pay interest on or to refinance our indebtedness depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our indebtedness. If we are unable to generate cash flow, we may be required to adopt one or more alternatives, such as restructuring debt or obtaining additional financing on terms that may be unfavorable to us or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at the time we seek to refinance such indebtedness. Our inability to satisfy our debt obligations could materially and adversely affect our financial position and results of operations.
A failure to comply with the conditions of the Facility Agreement or the Senior Secured Convertible Note could result in an event of default. An event of default under the Facility Agreement includes, among other things, a failure to pay any amount due under the Facility Agreement or to issue common stock when required upon conversion of the Senior Secured Convertible Note as well as the occurrence of a criminal proceeding pursuant to which the remedy sought includes forfeiture of a material portion of property. If we fail to comply with any of the covenants under our indebtedness and are unable to obtain a waiver or amendment, the lenders may, among other things, accelerate our outstanding indebtedness and exercise rights with respect to collateral securing our outstanding indebtedness, each of which could have an adverse effect on our business, financial condition and results of operations.
Any of these events could materially and adversely affect our business, financial condition and results of operations.
43
The terms of the Senior Secured Convertible Note may have a negative impact on our business and the value of our securities and may result in substantial dilution to our other equity securityholders.
The Senior Secured Convertible Note provides for certain terms which may have a negative impact on our business. Obligations under such agreement mature on August 9, 2027 and carry the possibility of the issuance of Convertible Note Warrants upon prepayment.
The obligations under the Senior Secured Convertible Note are secured and the lenders thereunder will have a claim against the assets and equity interests securing the related debt obligations that will have priority to claims of the Company’s equity securityholders generally. Additionally, the Convertible Note is guaranteed by certain of our subsidiaries, effectively providing for claims against such subsidiaries which are structurally senior to our other equity securityholders generally.
The Senior Secured Convertible Note is convertible into common stock, subject to certain terms and conditions, which may result in dilution to our other equity securityholders.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our principal executive offices are located in Cerritos, California where we occupy a suite under a lease that expires in 2026. We use this facility for administration, billing and collections, technology and development and professional services.
We intend to procure additional space as we add team members and expand geographically. We believe that our facilities are adequate to meet our needs for the immediate future, and that, should it be needed, suitable additional space will be available to accommodate any such expansion of our operations. As of December 31, 2022, we have leases for 62 clinics located in California, Arizona, Nevada, Florida, and Texas. Generally, our leases are “net” leases, which require us to pay all of the cost of insurance, taxes, maintenance and utilities. We generally cannot cancel these leases at our option.
Item 3. Legal Proceedings
From time to time, we may be involved in various legal proceedings and subject to claims that arise in the ordinary course of business. Although the results of litigation and claims are inherently unpredictable and uncertain, we are not currently a party to any legal proceedings the outcome of which, if determined adversely to us, are believed to, either individually or taken together, have a material adverse effect on our business, operating results, cash flows or financial condition.
Item 4. Mine Safety Disclosures
Not applicable.
44
Table of Contents
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Stock Price Information
Our common stock trades on the Nasdaq under the symbol “TOI.” Our publicly traded warrants trade on Nasdaq under the symbol “TOIIW.”
Holders
As of March 8, 2023, we had approximately 89 holders of record of our common stock.
Dividends
We have never declared or paid any cash dividends on our Common Stock or any other securities. Subject to applicable law and the rights and preferences of any holders of any outstanding series of preferred stock, under our third amended and restated certificate of incorporation, holders of our common stock will be entitled to the payment of dividends when, as and if declared by our board in accordance with applicable law.
Recent Sales of Unregistered Securities
None
Equity Compensation Plan Information
See Item 12 - “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Item 6. [ Reserved ]
45
Table of Contents
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis provides information that management believes is relevant to an assessment and understanding of the consolidated results of operations and financial condition of The Oncology Institute, Inc. ("TOI") along with its consolidating subsidiaries (the "Company"). The discussion should be read together with the historical audited annual financial statements for the years ended December 31, 2022 and 2021, and the related notes that are included elsewhere in this Annual Report. The information in this discussion contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 (as amended, "Securities Act"), as amended, and Section 21E of the Securities and Exchange Act of 1934 (as amended, the "Exchange Act"). Such statements are based upon current expectations, as well as management's beliefs and assumptions and involve a high degree of risk and uncertainty. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Statements that include the words "believes," "anticipates," "plans," "expects." "intends," and similar expressions that convey uncertainty of future events or outcomes are forward-looking statements. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. All forward-looking statements in this document are based on information available to us as of the filing date of this Annual Report on Form 10-K and we assume no obligation to update any forward-looking statements or the reasons why our actual results may differ. All dollar values are expressed in thousands, unless otherwise noted.
Overview
The Company is a leading value-based oncology company that manages community-based oncology practices that serve patients at 76 clinic locations across 15 markets and five states throughout the United States. Our community-based oncology practices are staffed with 112 oncologists and advanced practice providers. 62 of these clinics are staffed with 101 providers employed by our affiliated physician-owned professional corporations, referred to as the "TOI PCs", which provided care for more than 64,000 patients in 2022 and managed a population of approximately 1.7 million patients under value-based agreements as of December 31, 2022. The Company also provides management services to 14 clinic locations owned by independent oncology practices. The Company's mission is to heal and empower cancer patients through compassion, innovation, and state-of-the-art medical care.
Operationally, the Company’s medical centers provide a complete suite of medical oncology services including: physician services, in-house infusion and pharmacy, clinical trials, radiation, educational seminars, support groups, counseling, and 24/7 patient assistance. Many of our services, such as managing clinical trials and palliative care programs, are traditionally accessed through academic and tertiary care settings, while the TOI PCs bring these services to patients in a community setting. As scientific research progresses and more treatment options become available, cancer care is shifting from acute care episodes to chronic disease management. With this shift, it is increasingly important for high-quality, high-value cancer care to be available in a local community setting to all patients in need.
As a value-based oncology company, the Company seeks to deliver both better quality care and lower cost of care. The Company works to accomplish this goal by reducing wasteful, inefficient or counterproductive care that drives up costs but does not improve outcomes. The Company believes payors and employers are aligned with the value-based model due to its enhanced access, improved outcomes, and lower costs. Patients under the Company's affiliated providers’ care can benefit from evidence-based and personalized care plans, gain access to sub-specialized care in convenient community locations, and lower out-of-pocket costs. The Company believes its affiliated providers enjoy the stability and predictability of a large multi-state practice, are not incentivized or pressured to overtreat when it may be inconsistent with a patient’s goals of care, and can focus on practicing outstanding evidence-based medicine, rather than business building.
2022 Highlights
•Completed a $110 million strategic investment from Deerfield Management Company, L.P. through secured senior convertible notes on August 9, 2022
•Ended the fiscal year 2022 with $132 million in cash, cash equivalents, and investments
•Increased market count to 15 at year-end from 10 at the prior year end, including new markets in California, Florida and Texas
•Remediated two of the previously disclosed material weaknesses surrounding controls over review of revenue and segregation of duties within the financial close and reporting process. For the remaining one, Management has developed and continues to execute a remediation plan to address the previously disclosed material weakness around treatment of complex accounting transactions
•Received Agency for Healthcare Research and Quality's ("AHRQ") certification as an accredited Patient Safety Organization
•Generated over $1.7 million in savings to patients through the Company's dispensary co-pay assist program
46
Table of Contents
•Added 3 new gain share contracts in Florida
•Grew Capitated Membership by over 100 thousand lives
•Completed 6 practice acquisitions
The Business Combination
On June 28, 2021, DFP Healthcare Acquisition Corp. ("DFPH"), Orion Merger Sub I, Inc. ("First Merger Sub") and Orion Merger Sub II, LLC ("Second Merger Sub") entered into an agreement and plan of merger ("Merger Agreement") with TOI Parent, Inc. ("TOI Parent") (collectively, the "Business Combination"). In connection with the Business Combination, DFPH entered into subscription agreements with certain investors (the “PIPE Investors”), whereby it issued 17.5 million shares of common stock at $10.00 per share and 100,000 shares of preferred stock at $1,000.00 per share (“PIPE Shares”) for an aggregate investment of $275,000 (“PIPE Investment”), which closed simultaneously with the consummation of the Business Combination.
The Business Combination closed on November 12, 2021 ("Closing Date"). On the Closing Date, (i) First Merger Sub merged with and into TOI Parent, with TOI Parent being the surviving corporation and (ii) immediately following, TOI Parent merged with and into Second Merger Sub ("Legacy TOI"), with Second Merger Sub being the surviving entity and a wholly owned subsidiary of DFPH. DFPH was renamed “The Oncology Institute, Inc.” and TOI Common Stock and Public Warrants continued to be listed on Nasdaq under the ticker symbols “TOI” and “TOIIW,” respectively.
The total merger consideration on the Closing Date was $762,052, consisting of 51.3 million shares of common stock, valued at $10.00 per share (aggregate $595,468, inclusive of shares of DFPH common stock issuable per restricted stock units and the exercise of Legacy TOI stock options), and $166,584 in cash. Legacy TOI also issued 12.5 million shares of common stock pursuant to the terms of an earnout (“Earnout Shares”). The earnout shares are allocable to both Legacy TOI stockholders and Legacy TOI option holders. On the Closing Date, shares of DFPH common stock that were not otherwise redeemed as part of the DFPH public stockholder vote and the PIPE Shares automatically converted into shares of TOI stock on a one-for-one basis.
The Business Combination was accounted for as a reverse recapitalization in accordance with U.S. generally accepted accounting principles (“U.S. GAAP"). Under this method of accounting, DFPH was treated as the “acquired” company for accounting purposes and the Business Combination was treated as the equivalent of Legacy TOI issuing stock for the net assets of DFPH, accompanied by a recapitalization. The net assets of DFPH are stated at historical cost, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination were those of Legacy TOI.
Components of Results of Operations
Revenue
The Company receives payments from the following sources for services rendered: (i) commercial insurers; (ii) pharmacy benefit managers (“PBMs”), (iii) the federal government under the Medicare program administered by the Centers for Medicare and Medicaid Services (“CMS”); (iv) state governments under Medicaid and other programs; (v) other third-party payors and managed care organizations (e.g., risk bearing organizations and independent practice associations (“IPAs”)); and (vi) individual patients and clients.
Revenue primarily consists of capitation revenue, fee-for-service (“FFS”) revenue, dispensary revenue, and clinical trials revenue. Capitation and FFS revenue comprise the revenues within the Company’s patient services segment and are presented together in the results of operations. The following paragraphs provide a summary of the principal forms of our billing arrangements and how revenue is recognized for each type of revenue.
Capitation
Capitation revenues consist primarily of fees for medical services provided by the TOI PCs to the Company's patients under a capitated arrangement with various managed care organizations. Capitation revenue is paid monthly based on the number of enrollees by the contracted managed care organization (per member per month or “PMPM”). Capitation contracts generally have a legal term of one year or longer. Payments in capitation contracts are variable since they primarily include PMPM fees associated with unspecified membership that fluctuates throughout the term of the contract; however, based on our experience, our total underlying membership generally increases over time as penetration of MA products grows. Certain contracts include terms for a capitation deduction where the cost of out-of-network referrals of members are deducted from the future payment. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time.
47
Table of Contents
Fee-for-service revenue
FFS revenue represents revenue earned under contracts in which we bill and collect for medical services rendered by the TOI PCs’ employed physicians. The terms for FFS contracts are short in duration and only last for the period over which services are rendered (typically, one day). FFS revenue consists of fees for medical services provided to patients. As specialist providers, our FFS revenue is dependent on referrals from other physicians, such as primary care physicians. The Company's affiliated providers build trusted, professional relationships with these physicians and their associated medical groups, which can lead to recurring FFS volume; however, this volume is subject to numerous factors the Company cannot control and can fluctuate over time. The Company also receives FFS revenue for capitated patients that receive medical services which are excluded from the Company's capitation contracts. Under the FFS arrangements, third-party payors and patients are billed for patient care services provided by the TOI PCs. Payments for services provided are generally less than billed charges. The Company records revenue net of an allowance for contractual adjustments, which represents the net revenue expected to be collected from third-party payors (including managed care, commercial, and governmental payors such as Medicare and Medicaid), and patients. These expected collections are based on fees and negotiated payment rates in the case of third-party payors, the specific benefits provided for under each patient’s healthcare plan, mandated payment rates in the case of Medicare and Medicaid programs, and historical cash collections (net of recoveries). The recognition of net revenue (gross charges less contractual allowances) from such services is dependent on certain factors, such as the proper completion of medical charts following a patient visit, the forwarding of such charts to our billing center for medical coding and entering into the Company's billing system, and the verification of each patient’s submission or representation at the time services are rendered as to the payor(s) responsible for payment of such services. Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into the Company's billing systems as well as an estimate of the revenue associated with medical services.
Dispensary
Oral prescription drugs prescribed by doctors to their patients are sold directly through the TOI PCs’ dispensaries. Revenue for the prescriptions is based on fee schedules set by various PBMs and other third-party payors. The fee schedule is often subject to direct and indirect remuneration (“DIR”) fees, which are based primarily on pre-established metrics. DIR fees may be assessed in the periods after payments are received against future payments. The Company recognizes revenue, deducted by estimated DIR fees, at the time the patient takes possession of the oral drug.
Clinical trials & other revenue
The TOI PCs also enter into contracts to perform clinical research trials. The terms for clinical trial contracts last many months as the clinical research is performed. Each contract represents a single, integrated set of research activities that are satisfied over time as the output of results from the trial is captured for the trial sponsor to review. Under the clinical trial contracts, the TOI PCs receive a fixed payment for administrative, set-up, and close-down fees; a fixed amount for each patient site visit; and certain expense reimbursements. The Company recognizes revenue for these arrangements on the fees earned to date based on the state of the trial, as established under contract with the customer.
Operating Expenses
Direct costs - patient services
Direct costs - patient services primarily includes chemotherapy drug costs, clinician salaries and benefits, and medical supplies. Clinicians include oncologists, advanced practice providers such as physician assistants and nurse practitioners, and registered nurses employed by the TOI PCs.
Direct costs - dispensary
Direct costs - dispensary primarily includes the cost of oral medications dispensed in the TOI PCs’ clinic locations.
Direct costs - clinical trials & other
Direct costs - clinical trials & other primarily includes costs related to clinical trial contracts and medical supplies.
Selling, general and administrative expense
Selling, general and administrative expenses include employee-related expenses, including both clinic and field support staff as well as central administrative and corporate staff. These expenses include salaries and related costs and share-based compensation for our executives and physicians. The Company's selling, general and administrative expenses also includes
48
Table of Contents
occupancy costs, technology infrastructure, operations, clinical and quality support, finance, legal, human resources, and business development. Following the consummation of the Business Combination, general and administrative expenses have increased, and the Company expects continued increases over time, due to the additional legal, accounting, insurance, investor relations and other costs that the Company incurs as a public company, as well as other costs associated with continuing to grow the business. While the Company expects its selling, general and administrative expenses to increase in absolute dollars in the foreseeable future. such expenses are on average expected to decrease as a percentage of revenue over the long term.
Results of Operations
The following table sets forth our Consolidated Statements of Operations data expressed as a percentage of total revenues for the periods indicated. The Company’s management is not aware of material events or uncertainties that would cause the financial information below to not be indicative of future operating results or results of future financial condition. The results of operations for the year ended December 31, 2022 and as of December 31, 2022 reflect the adoption of ASU 2016-02, Leases (“Topic 842”). See Notes 2 and 10 of the notes to consolidated financial statements for more information. Financial data for the years ended December 31, 2021 and as of December 31, 2021 does not reflect the adoption of Topic 842.
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Revenue | ||||||||||||||
| Patient services | 66.1 | % | 61.2 | % | ||||||||||
| Dispensary | 31.4 | % | 35.7 | % | ||||||||||
| Clinical trials & other | 2.5 | % | 3.1 | % | ||||||||||
| Total operating revenue | 100.0 | % | 100.0 | % | ||||||||||
| Operating expenses | ||||||||||||||
| Direct costs – patient services | 53.4 | % | 49.0 | % | ||||||||||
| Direct costs – dispensary | 25.8 | % | 30.6 | % | ||||||||||
| Direct costs – clinical trials & other | 0.2 | % | 0.3 | % | ||||||||||
| Goodwill impairment charges | 3.9 | % | — | % | ||||||||||
| Selling, general and administrative expense | 47.4 | % | 41.1 | % | ||||||||||
| Depreciation and amortization | 1.7 | % | 1.6 | % | ||||||||||
| Total operating expenses | 132.4 | % | 122.6 | % | ||||||||||
| Loss from operations | (32.4) | % | (22.6) | % | ||||||||||
| Other non-operating expense (income) | ||||||||||||||
| Interest expense, net | 1.6 | % | 0.2 | % | ||||||||||
| Change in fair value of derivative warrant liabilities | (0.7) | % | (1.8) | % | ||||||||||
| Change in fair value of earnout liabilities | (23.5) | % | (12.3) | % | ||||||||||
| Change in fair value of conversion option derivative liabilities | (9.6) | % | — | % | ||||||||||
| Gain on loan forgiveness | (0.1) | % | (2.4) | % | ||||||||||
| Other, net | (0.1) | % | (0.5) | % | ||||||||||
| Total other non-operating income | (32.4) | % | (16.8) | % | ||||||||||
| Loss before provision for income taxes | — | % | (5.8) | % | ||||||||||
| Income tax benefit | 0.1 | % | 0.3 | % | ||||||||||
| Net income (loss) | 0.1 | % | (5.5) | % | ||||||||||
49
Table of Contents
Comparison of the Years Ended December 31, 2022 and 2021
Revenue
| Year Ended December 31, | Change | |||||||||||||||||||||||||
| (dollars in thousands) | 2022 | 2021 | $ | % | ||||||||||||||||||||||
| Patient services | $ | 166,785 | $ | 124,074 | $ | 42,711 | 34.4 | % | ||||||||||||||||||
| Dispensary | 79,343 | 72,550 | 6,793 | 9.4 | % | |||||||||||||||||||||
| Clinical trials & other | 6,355 | 6,379 | (24) | (0.4) | % | |||||||||||||||||||||
| Total operating revenue | $ | 252,483 | $ | 203,003 | $ | 49,480 | 24.4 | % | ||||||||||||||||||
Patient services
The increase in patient services revenue was primarily due to a 28.9% increase in FFS revenue as a result of practice acquisitions and an overall increase in clinic count as well as a 5.2% increase in capitation revenue due to new capitation contracts entered into during 2022 and in the latter half of 2021.
Dispensary
The increase in dispensary revenue was primarily due to a 7.5% increase in the average revenue per fill and a 1.7% increase in the number of fills.
Clinical trials & other
For the year ended December 31, 2022, the decrease in clinical trials and other revenue was primarily due to a decrease in other revenue compared to the prior year.
Operating Expenses
| Year Ended December 31, | Change | |||||||||||||||||||||||||
| (dollars in thousands) | 2022 | 2021 | $ | % | ||||||||||||||||||||||
| Direct costs – patient services | $134,761 | $ | 99,401 | $ | 35,360 | 35.6 | % | |||||||||||||||||||
| Direct costs – dispensary | 65,111 | 62,102 | 3,009 | 4.8 | % | |||||||||||||||||||||
| Direct costs – clinical trials & other | 518 | 652 | (134) | (20.6) | % | |||||||||||||||||||||
| Goodwill impairment charges | 9,944 | — | 9,944 | N/A | ||||||||||||||||||||||
| Selling, general and administrative expense | 119,689 | 83,365 | 36,324 | 43.6 | % | |||||||||||||||||||||
| Depreciation and amortization | 4,411 | 3,341 | 1,070 | 32.0 | % | |||||||||||||||||||||
| Total operating expenses | $334,434 | $ | 248,861 | $ | 85,573 | 34.4 | % | |||||||||||||||||||
Patient services cost
The increase in patient services cost was primarily due to a 21.5% increase in intravenous drug costs, primarily driven by the Company's patient mix and volume, as well as the rising rate of inflation during 2022. In addition, clinical payroll costs increased 12.4% from the prior year due to the growth in clinic count.
Dispensary cost
The increase in dispensary cost was primarily due to a 3.1% increase in the average cost of the prescriptions filled, and a 1.7% increase in the number of prescriptions filled.
50
Table of Contents
Goodwill impairment charges
During the year ended December 31, 2022, impairment charges of $9,944 and $0 were recorded related to goodwill and intangible assets, respectively. See Note 2 and Note 18 in Item. 8 Financial Statements and Supplementary Data for additional detail.
Selling, general and administrative expense
The increase in selling, general and administrative expense was primarily driven by a 16.5% increase in salaries and benefits due to the growth in the Company's management and corporate team, as well as a 6.2% increase in office expenses, 5.5% increase in insurance, and 5.5% increase in professional fees primarily related to the continued growth of our business. In addition, share-based compensation expense increased 3.8%, and contingent consideration from acquisitions resulted in the increase to selling, general, and administrative expense.
Other Non-Operating Expenses (Income)
| Year Ended December 31, | Change | |||||||||||||||||||||||||
| (dollars in thousands) | 2022 | 2021 | $ | % | ||||||||||||||||||||||
| Interest expense, net | $ | 4,082 | $ | 320 | $ | 3,762 | 1,175.6 | % | ||||||||||||||||||
| Change in fair value of derivative warrant liabilities | (1,843) | (3,686) | 1,843 | (50.0) | % | |||||||||||||||||||||
| Change in fair value of earnout liabilities | (59,215) | (24,891) | (34,324) | 137.9 | % | |||||||||||||||||||||
| Change in fair value of conversion option derivative liabilities | (24,200) | — | (24,200) | N/A | ||||||||||||||||||||||
| Gain on loan forgiveness | (183) | (4,957) | 4,774 | (96.3) | % | |||||||||||||||||||||
| Other, net | (501) | (1,046) | 545 | (52.1) | % | |||||||||||||||||||||
| Total other non-operating expense (income) | $ | (81,860) | $ | (34,260) | $ | (47,600) | 138.9 | % | ||||||||||||||||||
Interest expense
The increase in interest expense was primarily the result of interest and amortization related to the Senior Secured Convertible Notes issued during the year ended December 31, 2022.
Change in fair value of liabilities
The increase in non-operating (income) expense was primarily due to gains of $59,215 and $24,200, respectively, as a result of decreases in the fair value of earnout liabilities and conversion option derivative liabilities, which were created as part of the Business Combination and the issuance of the Senior Secured Convertible Note, respectively.
Gain on loan forgiveness
During the year ended December 31, 2022, gain on loan forgiveness of $183 was a result of a CARES Act loan that was acquired as part of a physician practice acquisition and subsequently forgiven. During the year ended December 31, 2021, gain on loan forgiveness of $4,957 was a result of forgiveness of all CARES Act loans, including those obtained through physician practice acquisition.
Other, net
The change in other, net was primarily due to Provider Relief Funding received under the CARES Act during the year ended December 31, 2021 that did not occur in 2022.
Key Business Metrics
In addition to our financial information, the Company's management reviews a number of operating and financial metrics, including the following key metrics, to evaluate our business, measure our performance, identify trends affecting our business, formulate business plans, and make strategic decisions.
51
Table of Contents
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
Clinics (1) | 76 | 67 | ||||||||||||
| Markets | 15 | 10 | ||||||||||||
| Lives under value-based contracts (millions) | 1.7 | 1.6 | ||||||||||||
| Net income (loss) | $ | 152 | $ | (10,927) | ||||||||||
Adjusted EBITDA (in thousands) (2) | $ | (23,542) | $ | (5,377) | ||||||||||
(1) Includes independent oncology practices to which we provide limited management services, but do not bear the operating costs.
(2) Adjusted EBITDA is a "non-GAAP" financial measure with the meaning of Item 10 of Regulation S-K promulgated by the SEC. The Company defines adjusted EBITDA as net income (loss) excluding:
•Depreciation and amortization,
•Interest expense, net,
•Income tax expense,
•Non-cash addbacks,
•Share-based compensation,
•Goodwill impairment charges,
•Changes in fair value of liabilities,
•Unrealized (gains) losses on investments
•Practice acquisition-related costs,
•Practice acquisition deferred purchase price,
•Consulting and legal fees,
•Public company transaction costs, and
•Other specific charges.
The Company includes adjusted EBITDA because it is an important measure upon which our management uses to assess the results of operations, to evaluate factors and trends affecting the business, and to plan and forecast future periods.
Management believes that this measure provides an additional way of viewing aspects of the Company's operations that, when viewed with the GAAP results, provides a more complete understanding of the Company's results of operations and the factors and trends affecting the business. However, non-GAAP financial measures should be considered a supplement to, and not as a substitute for, or superior to, the corresponding measures calculated in accordance with U.S. GAAP. Non-GAAP financial measures used by management may differ from the non-GAAP measures used by other companies, including the Company's competitors. Management encourages investors and others to review the Company's financial information in its entirety, not to rely on any single financial measure.
52
Table of Contents
The following tables provide a reconciliation of net income (loss), the most closely comparable GAAP financial measure, to Adjusted EBITDA:
| Year Ended December 31, | Change | ||||||||||||||||||||||
| (dollars in thousands) | 2022 | 2021 | $ | % | |||||||||||||||||||
| Net income (loss) | $ | 152 | $ | (10,927) | $ | 11,079 | (101.4) | % | |||||||||||||||
| Depreciation and amortization | 4,411 | 3,341 | 1,070 | 32.0 | % | ||||||||||||||||||
| Interest expense, net | 4,082 | 320 | 3,762 | 1,175.6 | % | ||||||||||||||||||
| Income tax benefit | (243) | (671) | 428 | (63.8) | % | ||||||||||||||||||
Non-cash addbacks(1) | 1,208 | (5,115) | 6,323 | (123.6) | % | ||||||||||||||||||
| Share-based compensation | 27,683 | 24,535 | 3,148 | 12.8 | % | ||||||||||||||||||
| Goodwill impairment charges | 9,944 | — | 9,944 | N/A | |||||||||||||||||||
| Change in fair value of liabilities | (85,258) | (28,577) | (56,681) | 198.3 | % | ||||||||||||||||||
| Unrealized (gains) losses on investments | (640) | — | (640) | N/A | |||||||||||||||||||
Practice acquisition-related costs(2) | 790 | 476 | 314 | 66.0 | % | ||||||||||||||||||
Post-combination compensation expense(3) | 2,243 | — | 2,243 | N/A | |||||||||||||||||||
Consulting and legal fees(4) | 3,797 | 1,826 | 1,971 | 107.9 | % | ||||||||||||||||||
Other, net(5) | 5,030 | 1,692 | 3,338 | 197.3 | % | ||||||||||||||||||
Transaction costs(6) | 3,259 | 7,723 | (4,464) | (57.8) | % | ||||||||||||||||||
| Adjusted EBITDA | $ | (23,542) | $ | (5,377) | $ | (18,165) | 337.8 | % | |||||||||||||||
(1) During the year ended December 31, 2022, non-cash addbacks were primarily comprised of non-cash rent of $711, net bad debt write-offs of $476, and other miscellaneous charges of $22. During the year ended December 31, 2021, non-cash addbacks were primarily comprised of a $4,957 gain on loan forgiveness and $417 of net bad debt recoveries, partially offset by deferred rent of $109 and other miscellaneous charges of $150.
(2) Practice acquisition-related costs were comprised of consulting and legal fees incurred to perform due diligence, execute, and integrate acquisitions of various oncology practices.
(3) Deferred consideration payments for practice acquisitions that are contingent upon the seller’s future employment at the Company.
(4) Consulting and legal fees were comprised of a subset of the Company’s total consulting and legal fees during the years ended December 31, 2022 and 2021, and related to certain advisory projects, software implementations, and legal fees for debt financing and predecessor litigation matters.
(5) Other, net is comprised of severance expenses resulting from cost rationalization programs of $248 and $127, as well as temporary labor of $1,830 and $1,182, recruiting expenses to build out corporate infrastructure of $2,835 and $1,275, and other miscellaneous expense of $117 and $131 during the years ended December 31, 2022 and 2021, respectively. During the years ended December 31, 2022 and 2021 such expenses were partially offset by $0 and $1,023, respectively, of stimulus funds received under the CARES Act.
(6) Transaction costs incurred related to the issuance of the Senior Secured Convertible Note such as legal, audit, administrative, and registration fees during the year ended December 31, 2022, and related to the Business Combination during the year ended December 31, 2021.
Liquidity and Capital Resources
General
To date, the Company has financed its operations principally through debt facilities, issuances of equity securities and payments received from various payors. As of December 31, 2022, the Company had $14,010 of cash and cash equivalents, none of which are restricted cash, as well as $59,796 of current marketable securities and $58,354 of noncurrent marketable securities.
The Company expects to incur operating losses and generate negative cash flows from operations for the foreseeable future due to the investments management intends to continue to make in expanding operations and sales and marketing and due to
53
Table of Contents
additional general and administrative expenses management expects to incur in connection with operating as a public company. As a result, the Company may require additional capital resources to execute strategic initiatives to grow the business.
Management believes that the cash on hand and investments in marketable securities will be sufficient to fund the Company's operating and capital needs for at least the next 12 months. Management's assessment of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties. The Company's actual results could vary because of, and its future capital requirements will depend on, many factors, including our growth rate, the timing and extent of spending to open or acquire new clinics and expand into new markets and the expansion of sales and marketing activities. The Company may in the future enter into arrangements to acquire or invest in complementary businesses, services and technologies, including intellectual property rights. The Company has based this estimate on assumptions that may prove to be wrong, and the Company could use its available capital resources sooner than management currently expects. The Company may be required to seek additional equity or debt financing. In the event that additional financing is required from outside sources, the Company may not be able to raise it on terms acceptable to management or at all. If unable to raise additional capital when desired, or if the Company cannot expand operations or otherwise capitalize on business opportunities because the Company's lack of sufficient capital, the Company's business, results of operations, and financial condition would be adversely affected.
Cash Flows
The following table presents a summary of the Company's consolidated cash flows from operating, investing, and financing activities for the periods indicated.
| Year Ended December 31, | Change | ||||||||||||||||||||||
| (dollars in thousands) | 2022 | 2021 | $ | % | |||||||||||||||||||
| Net cash, cash equivalents, and restricted cash used in operating activities | $ | (61,756) | $ | (32,680) | $ | (29,076) | 89.0 | % | |||||||||||||||
| Net cash, cash equivalents, and restricted cash used in investing activities | (131,614) | (12,154) | (119,460) | 982.9 | % | ||||||||||||||||||
| Net cash, cash equivalents, and restricted cash provided by financing activities | 92,206 | 154,010 | (61,804) | (40.1) | % | ||||||||||||||||||
| Net (decrease) increase in cash, cash equivalents, and restricted cash | $ | (101,164) | $ | 109,176 | $ | (210,340) | (192.7) | % | |||||||||||||||
| Cash, cash equivalents, and restricted cash at beginning of period | 115,174 | 5,998 | 109,176 | 1,820.2 | % | ||||||||||||||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 14,010 | $ | 115,174 | $ | (101,164) | (87.8) | % | |||||||||||||||
Operating Activities
Significant changes impacting net cash, cash equivalents, and restricted cash used in operating activities for the year ended December 31, 2022 as compared to the year ended December 31, 2021 were as follows:
•Net income increased $11,079, primarily as a result of a decrease in the fair value of liabilities of $85,258 for the year ended December 31, 2022 as compared to a decrease in fair value of liabilities of $28,577 during the year ended December 31, 2021;
•Cash used by accounts receivable increased $18,090 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 due to the growth in the Company's business;
•Cash used by accounts payable, accrued expenses and income taxes payable increased $2,535 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 primarily due to an increase in vendor payables due to the growth in the Company's business; and
•Cash used by purchasing inventory decreased $110 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 as a result of an increase in inventory acquired through practice acquisition.
•Cash provided by prepaid and other current assets increased $13,373 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 primarily due to the financing of the Company's directors and officers insurance policy that occurred in 2021.
54
Table of Contents
Investing Activities
Net cash used in investing activities increased $119,460 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 due to purchases of marketable securities of $117,508 that did not occur in the prior year and an increase in cash used for purchases of property and equipment of $2,682 for new clinic builds and clinic remodels, offset by a decrease in cash used for purchases of practice acquisitions and intangibles of $730.
Financing Activities
Net cash provided by financing activities decreased $61,804 for the year ended December 31, 2022 as compared to the year ended December 31, 2021 primarily due to cash received in connection with the Business Combination and the issuance of $20,000 of Legacy Preferred Stock during the year ended December 31, 2021, that did not occur in 2022 offset primarily by $110,000 of proceeds from the issuance of the Senior Secured Convertible Note during year ended December 31, 2022.
Material Cash Requirements
The Company's material cash requirements for the following five years consist of principal and interest due on the convertible note, operating leases and other miscellaneous administrative expenses. Additionally, the Company is subject to certain outside claims and litigation arising out of the ordinary course of business, however, no such litigation requires future cash expenditure as of December 31, 2022.
| Material Cash Requirements Due by the Year Ended December 31, | ||||||||||||||||||||||||||||||||
| (dollars in thousands) | 2023 | 2024-2025 | 2026-2027 | Thereafter | Total | |||||||||||||||||||||||||||
Convertible note1 | $ | 10,666 | $ | 21,624 | $ | 127,648 | $ | — | $ | 159,938 | ||||||||||||||||||||||
Operating leases | 6,637 | 11,778 | 8,711 | 4,600 | 31,726 | |||||||||||||||||||||||||||
| Deferred acquisition and contingent consideration | 2,584 | 525 | — | — | 3,109 | |||||||||||||||||||||||||||
Other2 | 3,131 | 119 | 68 | — | 3,318 | |||||||||||||||||||||||||||
| Total material cash requirements | $ | 23,018 | $ | 34,046 | $ | 136,427 | $ | 4,600 | $ | 198,091 | ||||||||||||||||||||||
(1) Includes principal and interest payments due.
(2) Other is comprised of finance leases and directors and officers insurance premiums.
JOBS Act
The Company qualifies as an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and has elected to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies, but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of our financial statements with another public company that is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Critical Accounting Policies
The Company prepares its financial statements in accordance with U.S. GAAP, which requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates under different assumptions or conditions.
Variable Interest Entities
The Company consolidates entities for which it has a variable interest and is determined to be the primary beneficiary. The Company holds variable interests in the TOI PCs, comprised of The Oncology Institute CA, a Professional Corporation (“TOI
55
Table of Contents
CA”) and The Oncology Institute FL, LLC (“TOI FL”) and The Oncology Institute TX, a Professional Association ("TOI TX"), all of which the Company cannot legally own due to jurisdictional laws governing the corporate practice of medicine. The TOI PCs employ physicians and other clinicians in order to provide professional services to patients of our managed clinics, and under substantially similar MSAs, we serve as the exclusive manager and administrator of the TOI PCs’ non-medical functions and services. The TOI PCs are considered variable interest entities (“VIEs”) as they do not have sufficient equity to finance their activities without additional financial support from the Company. An enterprise having a controlling financial interest in a VIE must consolidate the VIE if it has both power and benefits — that is, it has (1) the power to direct the activities of a VIE that most significantly impacts the VIE’s economic performance (power), and (2) the obligation to absorb the losses of the VIE that potentially could be significant to the VIE or the right to receive benefits from the VIE that potentially could be significant to the VIE (benefits). The Company has the power to control all financial activities of the TOI PCs, the rights to receive substantially all benefits from the VIEs, and consequently consolidates the TOI PCs. Revenues, expenses, and income from the TOI PCs are included in the consolidated amounts as presented on the Consolidated Statements of Operations.
Segment Reporting
The Company presents the financial statements by segment in accordance with the relevant accounting literature to provide investors with transparency into how the chief operating decision maker (“CODM”) manages the business. The Company's CODM is our Chief Executive Officer. The CODM reviews financial information and allocates resources across three operating segments: dispensary, patient care, and clinical trials & other.
Revenue Recognition
The Company recognizes consolidated revenue based upon the principle of the transfer of control of our goods and services to customers in an amount that reflects the consideration it expects to be entitled. This principle is achieved through applying the following five-step approach:
1.Identification of the contract, or contracts, with a customer.
2.Identification of the performance obligations in the contract.
3.Determination of the transaction price.
4.Allocation of the transaction price to the performance obligations in the contract.
5.Recognition of revenue when, or as, the entity satisfies a performance obligation.
Consolidated revenue primarily consists of capitation revenue, fee-for-service (FFS) revenue, dispensary revenue, and clinical trials revenue. Revenue is recognized in the period in which services are rendered or the period in which the TOI PCs are obligated to provide services. The form of billing and related risk of collection for such services may vary by type of revenue and the payor. The following paragraphs provide a summary of the principal forms of billing arrangements and how revenue is recognized for each.
Capitation
Capitation contracts have a single performance obligation that is a stand ready obligation to perform specified healthcare services to the population of enrolled members and constitutes a series for the provision of managed healthcare services for the term of the contract, which is deemed to be one month since the mix of patient-customers can and do change month over month. The transaction price for capitation contracts is variable as it primarily includes PMPM fees associated with unspecified membership that fluctuates throughout the term of the contract. Further, we adjust the transaction price for capitation deductions based on historical experience. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time. If subsequent information resolves uncertainties related to the transaction price, adjustments will be recognized in the period they are resolved. When payment has been received but services have not yet been rendered, the payment is recognized as a contract liability.
Fee For Service
FFS revenue consists of fees for medical services actually provided to patients. These medical services are distinct since the patient can benefit from the medical services on their own. Each service constitutes a single performance obligation for which the patient accepts and receives the benefit of the medical services as they are performed.
56
Table of Contents
The transaction price from FFS arrangements is variable in nature because fees are based on patient encounters, credits due to patients, and reimbursement of provider costs, all of which can vary from period to period. The Company estimates the transaction price using the most likely methodology and amounts are only included in the net transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any uncertainty is resolved. As a practical expedient, the Company adopted a portfolio approach to determine the transaction price for the medical services provided under FFS arrangements. Under this approach, the Company bifurcated the types of services provided and grouped health plans with similar fees and negotiated payment rates.
At these levels, portfolios share the characteristics conducive to ensuring that the results do not materially differ from the standard applied to individual patient contracts related to each medical service provided.
Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into our billing systems as well as an estimate of the revenue associated with medical services. When the performance obligation is not satisfied, the billing is recognized as a contract liability.
Dispensary
Dispensed prescriptions that are filled and delivered to the patient are considered a distinct performance obligation. The transaction price for the prescriptions is based on fee schedules set by PBMs and other third-party payors. The fee schedule is often subject to DIR fees, which are based primarily on pre-established metrics. DIR fees may be assessed in periods after payments are received against future payments. The Company estimates DIR fees to arrive at the transaction price for prescriptions. Revenue is recognized based on the transaction at the time the patient takes possession of the oral drug.
Clinical Research & Other
Clinical research contracts represent a single, integrated set of research activities and thus are a single performance obligation. The performance obligation is satisfied over time as the output is captured in data and documentation that is available for the customer to consume over the course of arrangement and furthers progress of the clinical trial. The Company has elected to recognize revenue for clinical trials using the ‘as-invoiced’ practical expedient. The customer is invoiced periodically based on the progress of the trial such that each invoice captures the revenue earned to date based on the state of the trial as established under contract with the customer.
Leases
On January 1, 2022, the Company adopted ASU 2016-02, Leases, with various amendments issued in 2018 and 2019 (collectively, “ASC 842”) using the modified retrospective approach, for leases that existed on January 1, 2022. ASC 842 requires lessees to recognize assets and liabilities for most leases. The Company evaluates whether an arrangement is or contains a lease at contract inception. A lease exists when a contract conveys to the customer the right to control the use of an identified asset for a period of time in exchange for consideration. Upon lease commencement, the date on which a lessor makes the underlying asset available to the Company for use, the Company classifies the lease as either an operating or finance lease. The Company applied certain practical expedients permitted under the transition guidance, including the package of practical expedients, which permits the Company not to reassess its prior conclusions related to lease identification, lease classification, and initial direct costs capitalization. The Company solely acts as a lessee and its leases primarily consist of operating leases for its real estate in the states in which the Company operates. The Company has other operating or financing leases for various clinical and non-clinical equipment.
Generally, upon the commencement of a lease, the Company will record a right-of-use (“ROU”) asset and lease liability. An ROU asset represents the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Lease liabilities are measured at the present value of the remaining, fixed lease payments at lease commencement. The Company uses its incremental borrowing rate, based on the information available at the later of adoption, inception, or modification in determining the present value of lease payments. ROU assets are measured at an amount equal to the initial lease liability, plus any prepaid lease payments (less any incentives received) and initial direct costs, at the lease commencement date. The Company has elected to account for lease and non-lease components as a single lease component for all underlying classes of assets. As a result, the fixed payments that would otherwise be allocable to the non-lease components are account for as lease payments and included in the measurement of the Company’s right-of-use asset and lease liability.
Lease arrangements with an initial term of 12 months or less are considered short-term leases and are not recorded on the balance sheet. The short-term lease payments are recognized as an expense on a straight-line basis over the lease term. The
57
Table of Contents
lease term includes any period covered by renewal options available that the Company is reasonably certain to exercise and any options to terminate the lease that the Company is not reasonably certain to exercise.
Direct Costs of Sales
Direct cost of sales primarily consists of wages paid to clinical personnel and other health professionals, oral and IV drug costs, and other medical supplies used to provide patient care. Costs for clinical personnel wages are expensed as incurred and costs for inventory and medical supplies are expensed when used, generally by applying the specific identification method.
Goodwill and Intangible Assets
The Company accounts for goodwill and intangible assets under Accounting Standards Codification Topic No. 350, Goodwill and Other (“ASC 350”). Goodwill represents the excess of the fair value of the consideration conveyed in acquisition over the fair value of net assets acquired.
Goodwill is not amortized but is required to be evaluated for impairment at the same time every year. The Company performs annual testing of impairment for goodwill in the fourth quarter of each year. When impairment indicators are identified, the Company compares the reporting unit’s fair value to its carrying amount, including goodwill. An impairment loss is recognized as the difference, if any, between the reporting unit’s carrying amount and its fair value to the extent the difference does not exceed the total amount of goodwill allocated to the reporting unit.
Under ASC 350, finite-lived intangible assets are stated at acquisition-date fair value. Intangible assets are amortized using the straight-line method.
Finite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When circumstances indicate that recoverability may be impaired, the Company assesses its ability to recover the carrying value of the asset group from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than the carrying value of such asset, an impairment loss is recognized for the difference between estimated fair value and carrying value. Fair value is determined based on appropriate valuation techniques.
Recent Accounting Pronouncements
For information regarding recent accounting pronouncements, refer to Note 2 of our consolidated financial statements included in this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of exposure due to potential changes in inflation or interest rates. We do not hold financial instruments for trading purposes.
Interest Rate Risk
We held cash and cash equivalents of $14,010, current marketable securities of $59,796, and noncurrent marketable securities of $58,354 as of December 31, 2022, consisting of bank deposits and Treasury bills. Such interest-earning instruments carry a degree of interest rate risk. The goals of our investment policy are liquidity and capital preservation. We believe that we do not have any material exposure to changes in the fair value of these assets as a results of changes in interest rates due to the short-term nature of our cash and cash equivalents.
Inflation Risk
Recently, inflation has increased throughout the U.S. economy. Inflation can adversely affect us by increasing the costs of drugs, clinical trials and research, administration and other costs of doing business. We may experience increases in the prices of labor and other costs of doing business. In an inflationary environment, cost increases may outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If this happens, we may need to raise additional capital to fund our operations, which may not be available in sufficient amounts or on reasonable terms, if at all, sooner than expected.
Impairment Risk
Impairment risk refers to the risk that the Company will write down a material amount of its goodwill or intangible assets. This risk is assessed at least annually in the fourth quarter each year when the Company performs its impairment testing. To the
58
Table of Contents
extent that, among other factors, (i) there is underperformance in one or more reporting units (ii) a potential recession further disrupts the economic environment or (iii) interest rates continue to rise in response to persistent inflation, the fair value of one or more of the reporting units could fall below their carrying value, resulting in a goodwill or intangible impairment charge.
For the year ended December 31, 2022, the Company recognized an impairment charge of $9,944 to write-down the carrying value of goodwill related to patient services in excess of the fair value.
59
Table of Contents
Item 8. Financial Statements and Supplementary Data
Table of Contents
| Page Number(s) | |||||
Report of Independent Registered Public Accounting Firm (BDO USA, LLP; Costa Mesa, California; PCAOB ID#243) | |||||
60
Table of Contents
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
The Oncology Institute, Inc.
Cerritos, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of The Oncology Institute, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations, convertible preferred stock and changes in stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Method Related to Leases
As discussed in Notes 2 and 10 to the consolidated financial statements, the Company changed its method of accounting for leases due to the adoption of Accounting Standards Codification 842, Leases effective January 1, 2022 under the modified retrospective approach.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company’s auditor since 2019.
Costa Mesa, California
March 16, 2023
61
Table of Contents
THE ONCOLOGY INSTITUTE, INC. CONSOLIDATED BALANCE SHEETS (US Dollars in thousands, except share data) | |||||||||||
| December 31, 2022 | December 31, 2021 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
Cash and cash equivalents (includes restricted cash of $0 and $875 as of December 31, 2022 and December 31, 2021) | $ | 14,010 | $ | 115,174 | |||||||
| Marketable securities | 59,796 | — | |||||||||
| Accounts receivable | 39,816 | 20,007 | |||||||||
| Other receivables | 617 | 1,237 | |||||||||
| Inventories, net | 9,261 | 6,438 | |||||||||
| Prepaid expenses | 6,918 | 11,200 | |||||||||
| Total current assets | 130,418 | 154,056 | |||||||||
| Non-current investments | 58,354 | — | |||||||||
| Property and equipment, net | 8,547 | 4,192 | |||||||||
| Operating right of use assets | 24,494 | — | |||||||||
| Intangible assets, net | 17,957 | 18,245 | |||||||||
| Goodwill | 21,418 | 26,626 | |||||||||
| Other assets | 477 | 320 | |||||||||
| Total assets | $ | 261,665 | $ | 203,439 | |||||||
| Liabilities and stockholders’ equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 9,372 | $ | 15,559 | |||||||
| Current portion of operating lease liabilities | 5,498 | — | |||||||||
| Current portion of long-term debt | — | 183 | |||||||||
| Income taxes payable | 255 | 132 | |||||||||
| Accrued expenses and other current liabilities | 14,595 | 13,924 | |||||||||
| Total current liabilities | 29,720 | 29,798 | |||||||||
| Operating lease liabilities | 22,060 | — | |||||||||
| Derivative warrant liabilities | 350 | 2,193 | |||||||||
| Derivative earnout liabilities | 803 | 60,018 | |||||||||
| Conversion option derivative liabilities | 3,960 | — | |||||||||
| Long-term debt, net of unamortized debt issuance costs | 80,621 | — | |||||||||
| Other non-current liabilities | 868 | 6,900 | |||||||||
| Deferred income taxes liability | 108 | 371 | |||||||||
| Total liabilities | 138,490 | 99,280 | |||||||||
| Commitments and contingencies (Note 15) | — | — | |||||||||
| Stockholders’ equity: | |||||||||||
Common Stock, $0.0001 par value, authorized 500,000,000 shares; 73,265,621 and 73,249,042 shares issued and outstanding at December 31, 2022 and December 31, 2021 | 7 | 7 | |||||||||
Series A Convertible Preferred Stock, $0.0001 par value, authorized 10,000,000 shares; 165,045 and 163,510 shares issued and outstanding at December 31, 2022 and December 31, 2021 | — | — | |||||||||
| Additional paid-in capital | 186,250 | 167,386 | |||||||||
| Accumulated deficit | (63,082) | (63,234) | |||||||||
| Total stockholders’ equity | 123,175 | 104,159 | |||||||||
| Total liabilities and stockholders’ equity | $ | 261,665 | $ | 203,439 | |||||||
62
Table of Contents
Note: The Company’s consolidated balance sheets include the assets and liabilities of its consolidated variable interest entities (“VIEs”). The consolidated balance sheets include total assets that can be used only to settle obligations of the Company’s consolidated VIEs totaling $70,994 and $42,332 as of December 31, 2022 and December 31, 2021, respectively, and total liabilities of the Company’s consolidated VIEs for which creditors do not have recourse to the general credit of the Company totaling $154,572 and $79,579 as of December 31, 2022 and December 31, 2021, respectively. See Note 17 for further details.
See accompanying notes to the consolidated financial statements.
63
Table of Contents
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(US Dollars in thousands, except share data)
| Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Revenue | |||||||||||
| Patient services | $ | 166,785 | $ | 124,074 | |||||||
| Dispensary | 79,343 | 72,550 | |||||||||
| Clinical trials & other | 6,355 | 6,379 | |||||||||
| Total operating revenue | 252,483 | 203,003 | |||||||||
| Operating expenses | |||||||||||
| Direct costs – patient services | 134,761 | 99,401 | |||||||||
| Direct costs – dispensary | 65,111 | 62,102 | |||||||||
| Direct costs – clinical trials & other | 518 | 652 | |||||||||
| Goodwill impairment charges | 9,944 | — | |||||||||
| Selling, general and administrative expense | 119,689 | 83,365 | |||||||||
| Depreciation and amortization | 4,411 | 3,341 | |||||||||
| Total operating expenses | 334,434 | 248,861 | |||||||||
| Loss from operations | (81,951) | (45,858) | |||||||||
| Other non-operating expense (income) | |||||||||||
| Interest expense, net | 4,082 | 320 | |||||||||
| Change in fair value of derivative warrant liabilities | (1,843) | (3,686) | |||||||||
| Change in fair value of earnout liabilities | (59,215) | (24,891) | |||||||||
| Change in fair value of conversion option derivative liabilities | (24,200) | — | |||||||||
| Gain on loan forgiveness | (183) | (4,957) | |||||||||
| Other, net | (501) | (1,046) | |||||||||
| Total other non-operating income | (81,860) | (34,260) | |||||||||
| Loss before provision for income taxes | (91) | (11,598) | |||||||||
| Income tax benefit | 243 | 671 | |||||||||
| Net income (loss) | $ | 152 | $ | (10,927) | |||||||
| Net income (loss) per share attributable to common stockholders: | |||||||||||
| Net income (loss) attributable to common stockholders, basic | $ | 68 | $ | (10,628) | |||||||
| Weighted-average number of shares outstanding, basic | 72,793,497 | 66,230,606 | |||||||||
| Net income (loss) per share attributable to common stockholders, basic | $ | — | $ | (0.16) | |||||||
| Net loss attributable to common stockholders, diluted | $ | (16,980) | $ | (10,628) | |||||||
| Weighted-average number of shares outstanding, diluted | 80,605,600 | 66,230,606 | |||||||||
| Net loss per share attributable to common stockholders, diluted | $ | (0.21) | $ | (0.16) | |||||||
See accompanying notes to the consolidated financial statements.
64
Table of Contents
THE ONCOLOGY INSTITUTE, INC.
CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND CHANGES IN STOCKHOLDERS’ EQUITY
(US Dollars in thousands, except share and per share data)
| Common Stock | Series A Convertible Preferred Stock | |||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Additional paid in capital | Retained Earnings/ (Accumulated Deficit) | Total Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||
| Balance at December, 31, 2020 | 59,160,192 | $ | 6 | — | $ | — | $ | 80,402 | $ | (52,307) | $ | 28,101 | ||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | (10,927) | (10,927) | |||||||||||||||||||||||||||||||||||||
| Common stock issued in connection with the Closing of the Business Combination (refer to Note 1) | 14,088,850 | 1 | — | — | 46,098 | — | 46,099 | |||||||||||||||||||||||||||||||||||||
| Preferred stock issued in connection with the Closing of the Business Combination (refer to Note 1) | — | — | 163,510 | — | 16,351 | — | 16,351 | |||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | 24,535 | — | 24,535 | |||||||||||||||||||||||||||||||||||||
| Balance at December, 31, 2021 | 73,249,042 | $ | 7 | 163,510 | $ | — | $ | 167,386 | $ | (63,234) | $ | 104,159 | ||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | 152 | 152 | |||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of RSUs | 696,690 | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of options | 973,389 | — | — | — | 858 | — | 858 | |||||||||||||||||||||||||||||||||||||
| Exchange of common stock for preferred stock | (153,500) | — | 1,535 | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Repurchase and retirement of common stock from related party | (1,500,000) | — | — | — | (9,000) | — | (9,000) | |||||||||||||||||||||||||||||||||||||
| Net settlement of taxes for equity awards | — | — | — | — | (413) | — | (413) | |||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — | — | — | 27,419 | — | 27,419 | |||||||||||||||||||||||||||||||||||||
| Balance at December, 31, 2022 | 73,265,621 | $ | 7 | 165,045 | $ | — | $ | 186,250 | $ | (63,082) | $ | 123,175 | ||||||||||||||||||||||||||||||||
See accompanying notes to the consolidated financial statements.
65
Table of Contents
| THE ONCOLOGY INSTITUTE, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (US Dollars in thousands) | |||||||||||
| Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Cash flows from operating activities: | |||||||||||
| Net income (loss) | $ | 152 | $ | (10,927) | |||||||
| Adjustments to reconcile net income (loss) to cash, cash equivalents, and restricted cash used in operating activities: | |||||||||||
| Depreciation and amortization | 4,411 | 3,341 | |||||||||
| Amortization of debt issuance costs | 2,444 | 53 | |||||||||
| Goodwill impairment charges | 9,944 | — | |||||||||
| Share-based compensation | 27,683 | 24,535 | |||||||||
| Decrease in fair value of liability classified warrants | (1,843) | (3,686) | |||||||||
| Decrease in fair value of liability classified earnouts | (59,215) | (24,891) | |||||||||
| Decrease in fair value of liability classified conversion option derivatives | (24,200) | — | |||||||||
| Unrealized (gain) loss on investments | 378 | — | |||||||||
| Accretion of discount on investment securities | (1,020) | — | |||||||||
| Deferred taxes | (263) | (1,242) | |||||||||
| Gain on loan forgiveness | (183) | (4,957) | |||||||||
| Bad debt expense (recovery) | 476 | (417) | |||||||||
| Loss on disposal of property and equipment | 21 | — | |||||||||
| Changes in operating assets and liabilities, net of business combinations: | |||||||||||
| Accounts receivable | (20,285) | (2,195) | |||||||||
| Inventories | (1,732) | (1,842) | |||||||||
| Other receivables | 620 | (792) | |||||||||
| Prepaid expenses | 4,282 | (9,091) | |||||||||
| Operating lease right-of-use assets | 5,404 | — | |||||||||
| Other assets | (157) | (198) | |||||||||
| Accrued expenses and other current liabilities | 2,349 | (3,084) | |||||||||
| Income taxes payable | 123 | (1,012) | |||||||||
| Accounts payable | (6,187) | 2,916 | |||||||||
| Current and long-term operating lease liabilities | (3,801) | — | |||||||||
| Other non-current liabilities | (1,157) | 809 | |||||||||
| Net cash, cash equivalents, and restricted cash used in operating activities | (61,756) | (32,680) | |||||||||
| Cash flows from investing activities: | |||||||||||
| Purchases of property and equipment | (5,529) | (2,847) | |||||||||
| Purchases of intangible asset in practice acquisitions | — | (200) | |||||||||
| Cash paid for practice acquisitions, net | (8,577) | (9,107) | |||||||||
| Purchases of marketable securities/investments | (117,508) | — | |||||||||
| Net cash, cash equivalents, and restricted cash used in investing activities | (131,614) | (12,154) | |||||||||
| Cash flows from financing activities: | |||||||||||
| Proceeds from recapitalization transaction | — | 333,946 | |||||||||
| Transaction costs | — | (33,145) | |||||||||
| Payments as a result of recapitalization transaction | — | (167,510) | |||||||||
| Proceeds from issuance of long-term debt | 110,000 | ||||||||||
| Transactions costs related to issuance of long-term debt | (3,663) | — | |||||||||
| Proceeds from financing of insurance payments | — | 8,429 | |||||||||
| Payments made for financing of insurance payments | (5,009) | (409) | |||||||||
| Payment of deferred consideration liability for acquisition | (509) | (50) | |||||||||
| Principal payments on long-term debt | — | (7,219) | |||||||||
| Principal payments on financing leases | (58) | (32) | |||||||||
| Common stock repurchase from related party | (9,000) | — | |||||||||
| Common stock issued for options exercised | 858 | — | |||||||||
| Taxes for common stock net settled | (413) | — | |||||||||
| Issuance of Legacy TOI preferred stock | — | 20,000 | |||||||||
| Net cash, cash equivalents, and restricted cash provided by financing activities | 92,206 | 154,010 | |||||||||
66
Table of Contents
| THE ONCOLOGY INSTITUTE, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (US Dollars in thousands) | |||||||||||
| Year Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net (decrease) increase in cash, cash equivalents, and restricted cash | (101,164) | 109,176 | |||||||||
| Cash, cash equivalents, and restricted cash at beginning of period | 115,174 | 5,998 | |||||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 14,010 | $ | 115,174 | |||||||
| Supplemental disclosure of cash flow information: | |||||||||||
| Cash paid for: | |||||||||||
| Income taxes | $ | 150 | $ | 1,727 | |||||||
| Interest | $ | 224 | $ | 275 | |||||||
| Supplemental disclosure of noncash investing and financing activities: | |||||||||||
| Reclassification of public warrant derivative liability related to the recapitalization transaction into equity | $ | — | $ | 10,580 | |||||||
| Fair value of assets acquired as part of acquisition (except for cash and goodwill) | $ | — | $ | 1,175 | |||||||
| Deferred consideration as part of practice acquisitions | $ | — | $ | 4,468 | |||||||
| Discount on senior secured convertible note | $ | 28,160 | $ | — | |||||||
See accompanying notes to the consolidated financial statements.
67
Table of Contents
THE ONCOLOGY INSTITUTE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2022 and December 31, 2021 and for the years ended December 31, 2022 and 2021
(US Dollars in thousands, except share data)
Note 1. Description of the Business
Overview of the Business
The Oncology Institute, Inc. (“TOI”) is the successor entity to DFP Healthcare Acquisitions Corp. ("DFPH"). DFPH is a Delaware corporation originally formed in 2019 as a publicly-traded special purpose acquisition company for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization, or similar business combination ("Business Combination"). TOI was originally founded in 2007 and is a community oncology practice that operates value-based oncology services platforms. TOI has three wholly-owned subsidiaries, TOI Parent, Inc. ("TOI Parent"), The Oncology Institute of Hope and Innovation Patient Safety Organization, LLC, and TOI Management, LLC (“TOI Management”). Additionally, TOI Management holds master services agreements with affiliated physician-owned professional entities ("TOI PCs") that confer controlling financial interest over the professional entities and their wholly-owned subsidiaries (TOI PCs, together with TOI, the “Company”).
On November 12, 2021 ("Closing Date"), the Business Combination closed following a series of mergers, which resulted in DFPH emerging as the parent of the combined entity Orion Merger Sub II, LLC and TOI Parent (together, "Legacy TOI"). DFPH was renamed “The Oncology Institute, Inc.” and common stock and "Public Warrants" continued to be listed on Nasdaq under the ticker symbols “TOI” and “TOIIW,” respectively (See Note 16).
Operationally, the Company’s medical centers provide a complete suite of medical oncology services including: physician services, in-house infusion and pharmacy, clinical trials, radiation, educational seminars, support groups, counseling, and 24/7 patient assistance. TOI’s mission is to heal and empower cancer patients through compassion, innovation and state-of-the-art medical care. The Company brings comprehensive, integrated cancer care into the community setting, including clinical trials, palliative care programs, stem cell transplants, and other care delivery models traditionally associated with non-community-based academic and tertiary care settings. In addition, the Company, through it consolidating subsidiary TOI Clinical Research, LLC ("TCR"), performs cancer clinical trials through a network of cancer care specialists. TCR conducts clinical trials for a broad range of pharmaceutical and medical device companies from around the world.
The Company has 101 oncologists and mid-level professionals across 62 clinic locations located within five states: California, Florida, Arizona, Nevada, and Texas. The Oncology Institute CA, a Professional Corporation ("TOI CA"), one of the TOI PCs, is comprised of the clinic locations in California, Nevada, and Arizona. The Company has contractual relationships with multiple payors, serving Medicare, including Medicare Advantage, Medi-Cal, and commercial patients.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States ("U.S. GAAP").
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of TOI, its subsidiaries, all of which are controlled by TOI through majority voting control, and variable interest entities (“VIE”) for which TOI (through TOI Management) is the primary beneficiary. The Company consolidates entities in which it has a controlling financial interest based on either the variable interest entity or voting interest model. All significant intercompany balances and transactions have been eliminated in consolidation.
68
Table of Contents
Variable Interest Entities
The Company consolidates entities for which it has a variable interest and is determined to be the primary beneficiary. Noncontrolling interests in less-than-wholly-owned consolidated subsidiaries of the Company are presented as a component of total equity to distinguish between the interests of the Company and the interests of the noncontrolling owners. Revenues, expenses, and net income from these subsidiaries are included in the consolidated amounts as presented on the Consolidated Statements of Operations.
The Company holds variable interests in TOI PCs, which it cannot legally own, as a result of entering into master services agreements ("MSAs"). As of December 31, 2022, TOI held variable interest in TOI CA, The Oncology Institute FL, LLC, a Professional Corporation ("TOI FL"), and The Oncology Institute TX, a Professional Association ("TOI TX"), all of which are VIEs. The Company is the primary beneficiary of the TOI PCs, and thus, consolidates the TOI PCs in its financial statements. As discussed in Note 17, the stockholders of the Company's consolidating VIEs own a minority of the issued and outstanding common shares of the Company.
Business Combinations
The Company accounts for all transactions that represent business combinations using the acquisition method of accounting under Accounting Standards Codification Topic No. 805, Business Combinations (“ASC 805”). The company first assesses whether an acquisition constitutes a business combination or asset acquisition by applying the screening test and analyzing whether the acquired entity has substantive inputs, processes, and the ability to produce outputs. Upon concluding an acquisition is a business combination, per ASC 805, the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquired entity are recognized and measured at their fair values on the date an entity obtains control of the acquiree. Such fair values that are not finalized for reporting periods following the acquisition date are estimated and recorded as provisional amounts. Adjustments to these provisional amounts during the measurement period (defined as the date through which all information required to identify and measure the consideration transferred, the assets acquired, the liabilities assumed, and the noncontrolling interests obtained, limited to one year from the acquisition date) are recorded when identified. Goodwill is determined as the excess of the fair value of the consideration exchanged in the acquisition over the fair value of the net assets acquired.
The DFPH-Legacy TOI Business Combination was accounted for as a reverse recapitalization. Under this method of accounting, DFPH was treated as the “acquired” company for accounting purposes and the Business Combination was treated as the equivalent of Legacy TOI issuing stock for the net assets of DFPH, accompanied by a recapitalization. The net assets of DFPH are stated at historical cost, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination were those of TOI Parent.
Segment Reporting
The Company presents the financial statements by segment in accordance with Accounting Standard Codification Topic No. 280, Segment Reporting (“ASC 280”) to provide investors with transparency into how the chief operating decision maker (“CODM”) manages the business. The Company determined the CODM is its Chief Executive Officer. The CODM reviews financial information and allocates resources across three operating segments: patient services, dispensary, and clinical trials & other. Each of the operating segments is also a reporting segment as described further in Note 20.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates under different assumptions or conditions. Significant items subject to such estimates and assumptions include judgements related to revenue recognition, estimated accounts receivable, useful lives and recoverability of long-lived and intangible assets, recoverability of goodwill, fair values of acquired identifiable assets and assumed liabilities in business combinations, fair value of intangible assets and goodwill, fair value of share-based compensation, fair value of liability classified instruments, and judgements related to deferred income taxes.
Net Income (Loss) Per Share
Basic and diluted net income (loss) per share attributable to common stockholders is presented in conformity with the two-class method required for participating securities such as our preferred stock and convertible note. Basic and diluted net income
69
Table of Contents
(loss) per share has been retrospectively adjusted for all periods presented prior to the Business Combination. The retroactive adjustment is based on the same number of weighted average shares outstanding in each historical period.
Under the two-class method, basic and diluted net income (loss) per share attributable to common stockholders is computed by dividing the basic and diluted net income (loss) attributable to common stockholders by the basic and diluted weighted-average number of shares of common stock outstanding during the period. Diluted net income per share attributable to common stockholders adjusts basic net income per share for the potentially dilutive impact of stock options, restricted stock units, Medical RSUs (defined in Note 14), earnout shares (defined in Note 14), public warrants, private placement warrants, and Senior Secured Convertible Notes (defined in Note 11).
The treasury stock method is used to calculate the potentially dilutive effect of stock options, RSUs, public warrants, and private placement warrants. The if-converted method is used to calculate the potentially dilutive effect of the Senior Secured Notes. In both methods, diluted net income (loss) attributable to common stockholders and diluted weighted-average shares outstanding are adjusted to account for the impact of the assumed issuance of potential common shares that are dilutive, subject to dilution sequencing rules. The earnout shares are contingently issuable; therefore, the earnout shares are excluded from basic and diluted net income (loss) per share until the market conditions have been met (see more detail on the earnout shares in Note 14). The Medical RSUs (defined in Note 14) are also contingently issuable; therefore, they are excluded from basic net income (loss) per share until the performance and service conditions have been met (see more detail in Note 14). Further, the number of contingently issuable Medical RSUs included in diluted net income (loss) per share is based on the number of shares, if any, that would be issuable if the end of the reporting period were the end of the contingency period and if the result would be dilutive. For the periods presented, the public and private placement warrants are out of the money; therefore, the public and private placement warrants are antidilutive and excluded from diluted net income per share.
Revenue Recognition
The Company follows the accounting requirements of Accounting Standard Codification Topic No. 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services. This principle is achieved through applying the following five-step approach:
1.Identification of the contract, or contracts, with a customer.
2.Identification of the performance obligations in the contract.
3.Determination of the transaction price.
4.Allocation of the transaction price to the performance obligations in the contract.
5.Recognition of revenue when, or as, an entity satisfies a performance obligation.
The Company receives payments from the following sources for services rendered: (i) commercial insurers; (ii) the federal government under the Medicare program administered by the Centers for Medicare and Medicaid Services (“CMS”); (iii) state governments under the Medicaid and other programs; (iv) other third-party payors (e.g., hospitals and independent practice associations (“IPAs”)); and (v) individual patients and clients.
Revenue primarily consists of capitation revenue, fee-for-service (“FFS”) revenue, dispensary revenue, and clinical trials revenue. Revenue is recognized in the period in which services are rendered or the period in which the Company is obligated to provide services. The form of billing and related risk of collection for such services may vary by type of revenue and the payor. The following paragraphs provide a summary of the principal forms of the Company’s billing arrangements and how revenue is recognized for each.
Capitation
Capitation revenues of the Company consist primarily of fees for medical services provided to patients by the Company under a capitated arrangement with various managed care organizations. Capitation revenue is paid monthly to the Company based on the number of enrollees assigned to the Company by the contracted managed care organization (per member, per month; or “PMPM”). Capitation contracts generally have a legal term of one year or longer. Capitation contracts have a single performance obligation that is a stand ready obligation to perform healthcare services to the population of enrolled members and constitutes a series for the provision of managed healthcare services for the term of the contract, which is deemed to be one month since the mix of patient-customers can and do change month over month. The transaction price for capitation contracts is variable as it primarily includes PMPM fees associated with unspecified membership that fluctuates throughout the contract. The Company generally estimates the transaction price using the most likely methodology and amounts are only included in the transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any
70
Table of Contents
uncertainty is resolved. Certain contracts include terms for a capitation deduction where the cost of out-of-network referrals of members by the Company are deducted from the future payment. The deductions vary depending on the payor and are often not known until a future period. As such, the Company adjusts the transaction price for capitation deductions based on historic experience such that the capitation revenue is recognized to the extent that it is not probable a significant reversal of revenue will occur in the future. Revenue is recognized in the month services are rendered on the basis of the transaction price established at that time. If subsequent information resolves uncertainties related to the transaction price, adjustments will be recognized in the period they are resolved. When payment has been received but services have not yet been rendered, the payment is recognized as a contract liability.
Fee-for-Service Revenue
FFS revenue represents revenue earned under contracts in which the Company bills and collects for medical services rendered by the Company’s employed physicians. The terms for FFS contracts are short in duration and only last for the period over which services are rendered (typically, one day). FFS revenue consists of fees for medical services provided to patients. These medical services are capable of being distinct since the patient can benefit from the medical services on their own. Each service constitutes a single performance obligation for which the patient accepts and receives the benefit of the medical services as they are performed.
Under the FFS arrangements, the Company bills third-party payors and patients for patient care services provided. Payments for services provided are generally less than billed charges. The Company records revenue net of an allowance for contractual adjustments, which represents the net revenue expected to be collected from third-party payors (including managed care, commercial, and governmental payors such as Medicare and Medicaid), and patients. These expected collections are based on fees and negotiated payment rates in the case of third-party payors, the specific benefits provided for under each patient’s healthcare plans, mandated payment rates in the case of Medicare and Medicaid programs, and historical cash collections (net of recoveries).
The transaction price from FFS arrangements is variable in nature because fees are based on patient encounters, credits due to patients, and reimbursement of provider costs, all of which can vary from period to period. The Company estimates the transaction price using the most likely methodology and amounts are only included in the net transaction price to the extent that it is probable that a significant reversal of cumulative revenue will not occur once any uncertainty is resolved. As a practical expedient, the Company uses a portfolio approach to determine the transaction price for the medical services provided under FFS arrangements. Under this approach, the Company bifurcates the types of services provided and grouped health plans with similar fees and negotiated payment rates. At these levels, portfolios share the characteristics conducive to ensuring that the results do not materially differ from the standard applied to individual patient contracts related to each medical service provided.
The recognition of net revenue (gross charges less contractual allowances) from such services is dependent on such factors as proper completion of medical charts following a patient visit, the forwarding of such charts to the Company’s billing center for medical coding and entering into the Company’s billing system, and the verification of each patient’s submission or representation at the time services are rendered as to the payor(s) responsible for payment of such services. Revenue is recorded on the date the services are rendered based on the information known at the time of entering of such information into the Company’s billing systems as well as an estimate of the revenue associated with medical services. When the performance obligation is not satisfied, the billing is recognized as a contract liability.
Dispensary
The Company sells oral prescription drugs directly through its dispensaries. Each prescription filled and delivered to the customer is a distinct performance obligation. The transaction price for the prescriptions is based on fee schedules set by various pharmacy benefit managers (“PBMs”) and other third party payors. The fee schedule is often subject to direct and indirect remuneration (“DIR”) fees, which are based primarily on pre-established metrics. DIR fees may be assessed in periods after payments are received against future payments. The Company estimates DIR fees to arrive at the transaction price for prescriptions. The Company recognizes revenue based on the transaction at the time the customer takes possession of the oral drug.
Clinical Trials & Other Revenue
The Company enters into contracts to perform clinical research trials. The terms for clinical trial contracts last many months as the clinical research is performed. Each contract represents a single, integrated set of research activities and thus is a single performance obligation. The performance obligation is satisfied over time as the output is captured in data and documentation that is available for the customer to consume over the course of arrangement and furthers progress of the clinical
71
Table of Contents
trial. Under the clinical trial contracts, the Company receives a fixed payment for administrative, set-up, and close-down fees; a fixed amount for each patient site visit; and certain expense reimbursements. Under ASC 606, the Company has elected to recognize revenue for these arrangements using the ‘as-invoiced’ practical expedient. The Company invoices the customer periodically based on the progress of the trial such that each invoice captures the revenue earned to date based on the state of the trial as established between the Company and the customer.
Direct Costs of Sales
Direct cost of sales primarily consists of wages paid to clinical personnel and other health professionals, oral and IV drug costs, and other medical supplies used to provide patient care. The Company’s costs for clinical personnel wages are expensed as incurred and the Company’s costs for inventory and medical supplies are expensed when used, generally by applying the specific identification method.
Cash, Restricted Cash, and Cash Equivalents
Cash primarily consists of deposits with banking institutions. The carrying value of the Company’s cash approximates fair value due to the short-term maturity of these instruments (less than three months). Pursuant to a covenant arising from a corporate credit card program, the Company holds cash on deposit with a banking institution that is subject to legal restrictions on withdrawal. The Company considers all highly liquid investments that are both readily convertible into cash and mature within 90 days from the date of purchase to be cash equivalents. As of December 31, 2022, the Company's cash equivalents consist of U.S. Treasury bills with a maturity date less than 90 days from the date of purchase.
Accounts Receivable
The Company accounts for accounts receivable under Accounting Standard Codification Topic No. 310, Receivables (“ASC 310”). Accounts receivable includes capitation receivables, FFS reimbursement for patient care, dispensary receivables and contract receivables. Accounts receivable are recorded and stated at the amount expected to be collected determined by each payor.
For third-party payors including Medicare, Medicaid, managed care providers, and commercial payors, the collectable amount is based on the estimated contractual reimbursement percentage, which is based on current contract prices or historical paid claims data by payor. For self-pay accounts receivable, which includes patients who are uninsured and the patient responsibility portion for patients with insurance, the collectable amount is determined using estimates of historical collection experience without regard to aging category. These estimates are adjusted for estimated conversions of patient responsibility portions, expected recoveries, and any anticipated changes in trends.
Accounts receivable can be impacted by the effectiveness of the Company’s collection efforts. Additionally, significant changes in payor mix, business office operations, economic conditions, or trends in federal and state governmental healthcare coverage could affect the collectable amount of accounts receivable. The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends, and changes in customer payment patterns to evaluate the adequacy of these reserves. The Company also regularly analyzes the ultimate collectability of accounts receivable after certain stages of the collection cycle using a look-back analysis to determine the amount of receivables subsequently collected, and adjustments are recorded when necessary.
The Company continuously monitors its collections of receivables and its policy is to write off receivables when they are determined to be uncollectible. As of December 31, 2022 and 2021, the Company does not have an allowance for doubtful accounts.
Inventories, net
The Company accounts for inventory under Accounting Standard Codification Topic No. 330, Inventory (“ASC 330”). Inventories consist of intravenous chemotherapy drugs and oral prescription drugs. Inventories are stated at the lower of cost, determined using the weighted average cost method of inventory valuation, or net realizable value. Net realizable value is determined using the selling price, less costs to sell.
The Company receives purchase discounts on products purchased. Contractual arrangements with vendors, including manufacturers and wholesalers, normally provide for the Company to receive purchase discounts from established list prices in one, or a combination, of the following forms: (i) a direct discount at the time of purchase or (ii) a discount for the prompt payment of invoices. Additionally, in other circumstances, the Company may receive rebates when products are purchased indirectly from a manufacturer (e.g., through a wholesaler). These rebates are recognized when intravenous chemotherapy drugs
72
Table of Contents
and oral prescription drugs are dispensed and are generally calculated by manufacturers within 30 days after the end of each completed quarter. The Company also receives additional rebate under its wholesaler contracts if it exceeds contractually defined annual purchase volumes. Purchase rebates are recorded as reductions to cost of services.
Property and Equipment, net
The Company accounts for property and equipment under Accounting Standard Codification Topic No. 360, Property, Plant, and Equipment (“ASC 360”). As required under ASC 360, the Company states property and equipment at cost, net of accumulated depreciation. Property and equipment is depreciated using the straight-line method over the estimated useful lives of the related assets, as described further in Note 8. Maintenance and repairs are charged to expense as incurred. Significant renewals and improvements are capitalized. At the time of retirement or other disposition of property and equipment, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is reflected in the Consolidated Statements of Operations.
When events or changes in circumstances indicate that the carrying amount of long-lived assets, including property and equipment, or other long-lived assets, may not be recoverable, an evaluation of the recoverability of currently recorded costs is performed. When an evaluation is performed, the estimated value of undiscounted future net cash flows associated with the asset groups is compared to the asset groups’ carrying value to determine if a write-down to fair value is required. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset group exceeds the fair value of the assets. There were no impairment adjustments recorded for long-lived assets during the years ended December 31, 2022 and 2021.
Accounts Payable, Accrued Expenses, and Other Current Liabilities
Accounts payable primarily consists of unpaid invoices related to routine operating expenses. Accrued expenses and other current liabilities primarily consist of accruals made for payroll expenses, deferred capitation, and FFS revenue.
Leases
Effective January 1, 2022, the Company accounts for its leasing arrangements in accordance with Accounting Standards Codification, Topic No. 842, Leases ("ASC 842"), which requires lessees to recognize assets and liabilities for most leases. The Company evaluates whether an arrangement is or contains a lease at contract inception. A lease exists when a contract conveys to the customer the right to control the use of an identified asset for a period of time in exchange for consideration. Upon lease commencement, the date on which a lessor makes the underlying asset available to the Company for use, the Company classifies the lease as either an operating or finance lease. The Company applied certain practical expedients permitted under the transition guidance, including the package of practical expedients, which permits the Company not to reassess its prior conclusions related to lease identification, lease classification, and initial direct costs capitalization. The Company solely acts as a lessee and its leases primarily consist of operating leases for its real estate in the states in which the Company operates. The Company has other operating and financing leases for various clinical and non-clinical equipment.
Generally, upon the commencement of a lease, the Company will record a right-of-use (“ROU”) asset and lease liability. An ROU asset represents the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Lease liabilities are measured at the present value of the remaining, fixed lease payments at lease commencement. The Company uses its incremental borrowing rate, based on the information available at the later of adoption, inception, or modification in determining the present value of lease payments. ROU assets are measured at an amount equal to the initial lease liability, plus any prepaid lease payments (less any incentives received) and initial direct costs, at the lease commencement date. The Company has elected to account for lease and non-lease components as a single lease component for all underlying classes of assets. As a result, the fixed payments that would otherwise be allocable to the non-lease components are accounted for as lease payments and included in the measurement of the Company’s right-of-use asset and lease liability.
Lease arrangements with an initial term of 12 months or less are considered short-term leases and are not recorded on the balance sheet. The operating lease payments are recognized as an expense on a straight-line basis over the lease term. The lease term includes any period covered by renewal options available that the Company is reasonably certain to exercise and any options to terminate the lease that the Company is not reasonably certain to exercise.
The Company displays ROU assets, current lease liabilities, and long term lease liabilities arising from operating leases as separate line items on the consolidated balance sheet. The Company includes ROU assets, current lease liabilities, and long term lease liabilities arising from finance leases within property and equipment, net; accrued expenses and other current liabilities; and other non-current liabilities.
73
Table of Contents
For years 2021 and prior, the lease agreements are evaluated to determine whether they are capital or operating leases in accordance with Accounting Standards Codification, Topic No. 840, Leases (“ASC 840”). When any one of the four test criteria in ASC 840 is met, the lease then qualifies as a capital lease. Capital leases are capitalized at the lower of the net present value of the total amount payable under the leasing agreement (excluding finance charges) or the fair market value of the leased asset. Capital lease assets are depreciated on a straight-line basis, over a period consistent with the Company’s normal depreciation policy for tangible fixed assets. The Company allocates each lease payment between a reduction of the lease obligation and interest expense using the effective interest method. Rent expense for operating leases, which may include free rent or fixed escalation amounts in addition to minimum lease payments, is recognized on a straight-line basis over the duration of the lease term. The Company reports the current and long-term portions of capital lease obligations within and , respectively, on the consolidated balance sheets.
Goodwill
The Company accounts for goodwill under Accounting Standards Codification Topic No. 350, Intangibles - Goodwill and Other (“ASC 350”). Goodwill represents the excess of the fair value of the consideration conveyed in and acquisition over the fair value of net assets acquired.
Goodwill is not amortized but is required to be evaluated for impairment at the same time every year. The Company performs its annual testing of impairment for goodwill in the fourth quarter of each year. When impairment indicators are identified, the Company compares the reporting unit’s fair value to its carrying amount, including goodwill. An impairment loss is recognized as the difference, if any, between the reporting unit’s carrying amount and its fair value to the extent the difference does not exceed the total amount of goodwill allocated to the reporting unit.
During the year ended December 31, 2022, the Company's share price experienced significant declines. The Company performed a qualitative analysis of impairment over goodwill and noted the following indicators of potential impairment: (i) underperformance in one or more reporting units, (ii) the continued threat of a national recession, and (iii) interest rates rising in response to persistent inflation. Based on these indicators, the Company proceeded to perform a quantitative analysis of potential impairment of goodwill by comparing the fair value of goodwill at each reporting unit to the carrying value. The quantitative assessment considers fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company’s quantitative analysis considered and evaluated each of the three traditional approaches to value: the income approach, the market approach, and the asset approach. The Company primarily relied on the discounted cash flow method within the income approach and the guideline public company method to value the reporting units. Impairment charges are based on both historic and future expected business results that no longer support the carrying value of the reporting unit. The quantitative assessment determined that goodwill was impaired. As a result, the Company recorded an impairment charge of $9,944 to goodwill.
For the year ended December 31, 2021, the Company performed a qualitative analysis and determined that there were no indicators of impairment. Therefore, no goodwill impairment charge was recorded.
Intangible Assets
Under ASC 350, finite-lived intangible assets are stated at acquisition-date fair value. Intangible assets are amortized using the straight-line method.
Finite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When circumstances indicate that recoverability may be impaired, the Company assesses its ability to recover the carrying value of the asset group from the expected future pre-tax cash flows (undiscounted and without interest charges) of the related operations. If these cash flows are less than the carrying value of such asset, an impairment loss is recognized for the difference between estimated fair value and carrying value. Fair value is determined based on appropriate valuation techniques.
For the years ended December 31, 2022 and 2021, the Company performed a qualitative analysis and determined that there were no indicators of impairment. Therefore, no impairment charge of its finite-lived intangible assets was recorded.
Investments in Marketable Securities
The Company's investments in marketable securities are classified as available-for-sale and are carried at fair value. The Company accounts for its investment securities available for sale using the fair value election pursuant to ASC 825, Financial Instruments ("ASC 825"), where changes in fair value are recorded in unrealized gains (losses), net on the Company's Consolidated Statements of Operations. The Company determines the appropriate classification of these investments at the time
74
Table of Contents
of purchase and reevaluates such designation at each balance sheet date. The Company’s marketable securities are classified as current assets if the maturity date is less than one year from the date of purchase and they may be readily liquidated.
Interest income, realized gains and losses on sales of securities, and other-than-temporary declines in the fair value of marketable securities, if any, are included as a component of other income (expense), net in the Consolidated Statements of Operations. The cost of securities sold is based on the First In, First Out method.
At each reporting period, the Company evaluates available-for-sale marketable securities, to the extent the fair value option is not elected, for any credit-related impairment when the fair value of the investment is less than its amortized cost. If the Company determines that the decline in fair value is below the carrying value and this decline is other-than-temporary, credit-related impairment is recognized in the Consolidated Statements of Operations in accordance with ASC 320, Debt Securities. As of December 31, 2022, there were no available-for-sale instruments for which the fair value option was not elected.
Debt
The Company accounts for debt net of debt issuance costs and debt discount. Debt issuance costs and debt discount are capitalized, netted against the related debt for presentation purposes, and amortized to interest expense over the terms of the related debt using the effective interest method.
The Company accounts for bifurcated, debt-classified embedded features separately as derivative liabilities pursuant to Accounting Standards Codification Topic No. 815, Derivatives and Hedging ("ASC 815"). Bifurcated, debt-classified embedded features are recorded at fair value on the Company's balance sheet with subsequent changes in fair value recorded in the Consolidated Statement of Operations each reporting period.
Public Warrants and Private Placement Warrants
Upon completion of the Business Combination, the Company assumed public and private placement warrants that were issued by DFPH in connection with its initial public offering (declared effective by the Securities and Exchange Commission on March 10, 2020) whereby holders of the public and private placement warrants are entitled to acquire common stock of the Company.
Prior to the Business Combination, the public warrants were accounted for as liabilities per Accounting Standards Codification Subtopic No. 815-40 Contracts on an Entity's Own Equity ("ASC 815-40"). Following the Business Combination, the shares of common stock underlying the public warrants are not redeemable and the Company has one single class of voting stock; therefore, the public warrants are not precluded from being considered indexed to the Company’s common stock which allows the public warrants to meet the criteria for equity classification per ASC 815-40. Warrants classified as equity are recorded at their issuance cost and are not subject to remeasurement at each subsequent balance sheet date.
Prior to the Business Combination, the private placement warrants were accounted for as liabilities per ASC 815-40. The private placement warrants are not considered indexed to the Company’s stock per ASC 815-40 and are therefore recorded as liabilities, given the settlement of the private placement warrants is dependent, in part, on who holds the warrants at the time of the settlement. Warrants classified as liabilities are recorded at their estimated fair value on the Closing Date and are revalued at each subsequent balance sheet date, with fair value changes recognized in other non-operating expense (income) in the accompanying Consolidated Statements of Operations. The Company estimates the value of these warrants using a Binomial Lattice valuation model in a risk-neutral framework.
Earnout Liability
As part of the Business Combination, DFPH issued to eligible Legacy TOI stockholders and Legacy TOI employees the contingent right to receive up to 12.5 million additional shares of common stock (“Legacy TOI Earnout Shares”), in two tranches of 5.0 million and 7.5 million, respectively, upon the Company common stock achieving a price per share of $12.50 during the two-year period following the Closing or a price per share of $15.00 during the three-year period following the Closing, in each case, as its last reported sales price per share for any 20 trading days within any 30 consecutive trading day period within the applicable period ("Earnout Terms"); provided, that (i) if one or both of the share price triggers has not been achieved prior to the end of the three-year period following the Closing, (ii) the Company enters into a definitive agreement that would result in a change of control and (iii) the price per share of the Company’s common stock in such transaction is equal to or greater than one or both of the share price triggers, then at the Closing of such transaction, the Company shall issue the applicable portion of the Legacy TOI Earnout Shares as if such share price trigger had been achieved.
In addition, certain DFPH common stockholders deposited 575,000 shares of DFPH common stock in an escrow account that will vest and be released to such holders in two tranches of 50%, each (“DFPH Earnout Shares”), upon the Company
75
Table of Contents
common stock achieving the Earnout Terms as described above; provided, that (i) if one or both of the share price triggers has not been achieved prior to the end of the three-year period following the closing, (ii) the Company enters into a definitive agreement that would result in a change of control and (iii) the price per share of common stock in such transaction is equal to or greater than one or both of the share price triggers, then at the closing of such transaction, the Company shall issue the applicable portion of the DFPH Earnout Shares as if such share price trigger had been achieved. To the extent any DFPH Earnout Shares remain unvested at the expiration of the three-year period following the closing, such DFPH Earnout Shares shall be forfeited and cancelled without any consideration.
Collectively, the Legacy TOI Earnout Shares and DFPH Earnout Shares constitute the “Earnout Shares”, the “Earnout”, and the “Earnout Liability”.
The Company determined that Earnout Shares issuable to Legacy TOI stockholders and DFPH stockholders fail to meet equity classification criteria under ASC 815-40 and therefore, represents a liability that meets the definition of a derivative and recognized it on the balance sheet at its fair value upon the Closing Date. The right to Earnout Shares issuable to Legacy TOI stockholders and DFPH stockholders are remeasured at fair value using a Monte Carlo simulation model each period through earnings. See Note 7 for further discussion.
Earnout Shares issuable to Legacy TOI employees is considered a share-based compensation award under Accounting Standards Codification Topic No. 718, Stock Based Compensation (“ASC 718”) due to the requirement that Legacy TOI employees must remain employed by the Company in order to not forfeit such unvested Earnout Shares. Such Earnout Shares are accounted for within equity over the service period. See Note 14 for further discussion.
Income Taxes
The Company accounts for income taxes under the asset and liability method under Accounting Standards Codification Topic No. 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest related to unrecognized tax benefits in interest expense and penalties in selling, general, and administrative expenses.
Retirement Plans
The Company provides a qualified 401(K) plan to all eligible employees which is administered through the John Hancock Life Insurance Company (U.S.A.). Employees are eligible to participate in the plan on the first day of the month subsequent to completing two months of service. Eligible employees may, subject to statutory limitations, contribute a portion of their salary to the plan through payroll deduction. In 2022 and 2021, the Company provided a matching contribution of 100% of the elective deferral that does not exceed 4% of compensation. Participants are always fully vested in their own contributions and the Company’s matching contributions vest immediately. The Company expensed to selling, general and administrative expenses $1,108 and $787 in matching contributions related to the 401(K) plan during the years ended December 31, 2022 and December 31, 2021, respectively.
Share-Based Compensation Plan
The Company accounts for share-based compensation under Accounting Standards Codification Topic No. 718, Compensation - Stock Compensation ("ASC 718"). As required under ASC 718, the Company accounts for employee and nonemployee share-based compensation as an expense in the consolidated financial statements. Equity-classified awards are measured at the grant date fair value of the award. Liability-classified awards are remeasured at fair value each reporting end date. For stock options, the Company estimates grant date fair value using the Black-Scholes-Merton option-pricing model. For restricted stock units (“RSU”), the fair value is based on the Company’s share price on the grant date. Liability-classified awards are settled in a variable number of the Company’s common stock on the vesting date based on a fixed monetary value. The Company accounts for forfeitures as incurred.
Excess tax benefits of awards related to stock option exercises are recognized as an income tax benefit in the Consolidated Statements of Operations and reflected in operating activities in the Consolidated Statement of Cash Flows.
76
Table of Contents
Commitments and Contingencies
The Company accounts for contingent liabilities under Accounting Standards Codification Subtopic No. 450-20, Contingencies (“ASC 450-20”). As required by ASC 450-20, liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred.
Fair Value Measurements
The Company accounts for fair value measurements under Accounting Standards Codification Topic No. 820, Fair Value Measurements (“ASC 820”). The Company uses valuation approaches that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible. The Company determines fair value based on assumptions that market participants would use in pricing an asset or liability in the principal or most advantageous market. When considering market participant assumptions in fair value measurements, the following fair value hierarchy distinguishes between observable and unobservable inputs, which are categorized in one of the following levels (see Note 7 for further discussion):
Level 1inputs: Unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement date.
Level 2inputs: Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the asset or liability.
Level 3inputs: Unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at measurement date.
The Company's fair value measurement methodology for cash and cash equivalents, accounts receivable, other receivables, and accounts payable approximates fair value because of the short maturity and high liquidity of these instruments. Fair value measurement of investment securities available for sale is based upon quoted prices from active markets, if available (Level 1). If quoted prices are not available, fair values are measured using independent pricing models or other model-based valuation methodologies. Level 2 investment securities include US Treasuries purchased in the secondary market that use pricing inputs other than quoted prices in active markets and fair value is determined using pricing models or other valuation methodologies such as broker price indications, which are based on quoted prices for identical or similar notes, which are Level 2 input measures. Fair value measurements used for the goodwill and intangible assets are based on the discounted cash flow method within the income approach and guideline public company method to value the reporting units, which is considered to be a Level 3 fair value measurement. The unobservable inputs utilized in determining the fair value of goodwill based on the income approach primarily include estimated future cash flows, discounted at a rate that approximates the cost of capital of a market participant. Inputs used to calculate the fair value based on the market approach include the revenue and EBITDA multiples based on guidelines for similar publicly traded companies and recent transactions. Fair value measurements of derivative warrants and earnout liabilities are based on Binomial Lattice and Monte-Carlo Simulation Models, respectively, which are considered to be Level 3 fair value measurements. The primary unobservable input utilized in determining the fair value of the derivative warrants and earnouts is the expected volatility of the common stock. Fair value measurements of the convertible note warrant and conversion option derivative liabilities are based on the Black-Derman-Toy model implemented in the Binomial Lattice and Black-Scholes Models, which are considered to be Level 3 fair value measurements. The primary unobservable input utilized in determining the fair value of the convertible note warrant and conversion option derivative liabilities is the expected volatility of the common stock.
Emerging Growth Company
Pursuant to the Business Combination, the Company qualifies as an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933 ("Securities Act"), as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and has elected to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies, but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth
77
Table of Contents
company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company, nor an emerging growth company which has opted out of using the extended transition period, difficult or impossible because of the potential differences in accounting standards used.
Recently Adopted Accounting Standards
Leases
On January 1, 2022, the Company adopted Accounting Standards Update 2016-02, Leases, with various amendments issued in 2018 and 2019 (collectively, ASC 842) using the modified retrospective approach, for leases that existed on January 1, 2022. Due to the Company's modified retrospective adoption, prior periods are still presented in accordance with Accounting Standards Codification, Topic No. 840, Leases (“ASC 840”), with capital leases being capitalized at the lower of the net present value of the total amount payable under the leasing agreement (excluding finance charges) or the fair market value of the leased asset and rent for operating leases being expensed on a straight-line basis over the duration of the lease term.
As a result of the Company's adoption of ASC 842, the Company recorded an initial adjustment to the January 1, 2022 balance sheet of $17,832 to operating ROU assets, $4,056 to current portion of operating lease liabilities, $15,104 to long term operating lease liabilities, $43 to property and equipment, net; $19 to other current liabilities; and $21 to other non-current liabilities. The impact of ASC 842 was not material to the Consolidated Statements of Operations.
Other
In May 2021, the FASB issued Accounting Standards Update 2021-04, Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options (“ASU 2021-04”). The guidance in ASU 2021-04 requires the issuer to treat a modification of an equity-classified written call option that does not cause the option to become liability-classified as an exchange of the original option for a new option. This guidance applies whether the modification is structured as an amendment to the terms and conditions of the option or as termination of the original option and issuance of a new option. The Company adopted ASU 2021-04 as of January 1, 2022. The adoption of this standard did not have an impact on the Company’s Consolidated Statements of Operations or Cash Flows and did not result in a cumulative catch-up adjustment to the opening balance of retained earnings.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes (“ASU 2019-12”), which amends ASC 740, Income Taxes. This new standard is intended to simplify accounting for income taxes by removing certain exceptions to the general principles in ASC 740 and amending existing guidance to improve consistent application of ASC 740. The guidance in the new standard has various elements, some of which are applied on a prospective basis and others on a retrospective basis with earlier application permitted. The Company adopted ASU 2019-12 as of December 31, 2022. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements and related disclosures.
Recently Issued Accounting Standards
In June 2016, the FASB issued Accounting Standards Update 2016-13, Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which changes the way entities recognize impairment of many financial assets by requiring immediate recognition of estimated credit losses expected to occur over their remaining life, instead of when incurred. In November 2018, the FASB issued Accounting Standard Update 2018-19, Codification Improvements to Topic 326, Financial Instruments — Credit Losses (“ASU 2018-19”), which amends Subtopic 326-20 (created by ASU 2016-13) to explicitly state that operating lease receivables are not in the scope of Subtopic 326-20. Additionally, in April 2019, the FASB issued Accounting Standard Update 2019-04, Codification Improvements to Topic 326, Financial Instruments — Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments (“ASU 2019-04”); in May 2019, the FASB issued Accounting Standards Update 2019-05, Financial Instruments — Credit Losses (Topic 326): Targeted Transition Relief (“ASU 2019-05”); and in November 2019, the FASB issued Accounting Standards Update 2019-10, Financial Instruments — Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842): Effective Dates and ASU 2019-11, Codification Improvements to Topic 326, Financial Instruments — Credit Losses (“ASU 2019-10”), to provide further clarifications on certain aspects of ASU 2016-13 and to extend the nonpublic entity effective date of ASU 2016-13. The changes (as amended) are effective for the Company for annual and interim periods in fiscal years beginning January 1, 2023. ASU 2016-13 will not have a material effect on its consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and
78
Table of Contents
Contracts in an Entity’s Own Equity ("ASU 2020-06"), which simplifies accounting for convertible instruments by removing major separation models required under current U.S. GAAP. ASU 2020-06 also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings per share calculation in certain areas. The new standard is effective for the Company beginning January 1, 2024. The Company is currently evaluating the effect of ASU 2020-06 on the Company’s consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-08, Business Combinations: Accounting for Contract Assets and Contract Liabilities from Contracts with Customers ("ASU 2021-08"). Under ASU 2021-08, an acquirer must recognize, and measure contract assets and contract liabilities acquired in a business combination in accordance with ASC 606, Revenue from Contract with Customers (“ASC 606”). The guidance is effective for interim and annual periods beginning after December 15, 2023, with early adoption permitted. The Company will adopt ASU 2021-08 on January 1, 2024 on a prospective basis. The Company is currently evaluating the effect of ASU 2021-08 on the Company’s consolidated financial statements and related disclosures.
Note 3. Significant Risks and Uncertainties Including Business and Credit Concentrations
Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, accounts receivable, and investment securities.
Cash accounts in a financial institution may, at times, exceed the Federal Deposit Insurance Corporation coverage of $250 per account ownership category. The Company has not experienced losses on these accounts, and management believes the Company is not exposed to significant risks on such accounts.
The Company’s accounts receivable has implicit collection risk. The Company grants credit without collateral to their patients, most of whom are local residents and are insured under third-party payor agreements. The Company believes this risk is partially mitigated by the Company’s establishment of long-term agreements and relationships with third-party payors that provide the Company with insight into historic collectability and improve the collections process.
The Company's investment securities portfolio is managed by a third party vendor to provide a relatively stable source of investment income from excess liquidity while satisfactorily managing risk, including credit risk, reinvestment risk, liquidity risk, and interest rate risk.
Revenue Concentration Risk
The concentration of net revenue on a percentage basis for major payors for the years ended December 31, 2022 and 2021 are as follows:
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Percentage of Net Revenue: | ||||||||||||||
| Payor A | 13 | % | 17 | % | ||||||||||
| Payor B | 16 | % | 14 | % | ||||||||||
The concentration of gross receivables on a percentage basis for major payors at December 31, 2022 and December 31, 2021 are as follows:
| December 31, 2022 | December 31, 2021 | ||||||||||
| Percentage of Gross Receivables: | |||||||||||
| Payor B | 13 | % | 19 | % | |||||||
| Payor C | 10 | % | 14 | % | |||||||
All of the Company’s revenue is generated from customers located in the United States.
79
Table of Contents
Vendor Concentration Risk
The concentration of cost of sales on a percentage basis for major vendors for the years ended December 31, 2022 and 2021 are as follows:
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Percentage of Cost of Sales: | ||||||||||||||
| Vendor A | 76 | % | 50 | % | ||||||||||
| Vendor B | 21 | % | 48 | % | ||||||||||
The concentration of gross payables on a percentage basis for major payors at December 31, 2022 and December 31, 2021 are as follows:
| December 31, 2022 | December 31, 2021 | ||||||||||
| Percentage of Gross Payables: | |||||||||||
| Vendor A | 66 | % | 39 | % | |||||||
| Vendor B | N/A | 47 | % | ||||||||
COVID-19 Pandemic
In January 2020, the Secretary of the U.S. Department of Health and Human Services (“HHS”) declared a national public health emergency due to a novel strain of coronavirus (“COVID-19”). In March 2020, the World Health Organization declared the outbreak of COVID-19, a disease caused by this coronavirus, a pandemic. The resulting measures to contain the spread and impact of COVID-19 and other developments related to COVID-19 have affected the Company’s results of operations during 2021 and 2022. Where applicable, the impact resulting from the COVID-19 pandemic has been considered, including updated assessments of the recoverability of assets and evaluation of potential credit losses. As a result of the COVID-19 pandemic, federal and state governments passed legislation, promulgated regulations, and took other administrative actions intended to assist healthcare providers in providing care to COVID-19 and other patients during the public health emergency. Sources of relief include the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”), which was enacted on March 27, 2020, the Paycheck Protection Program and Health Care Enhancement Act (the “PPPHCE Act”), which was enacted on April 24, 2020, and the Consolidated Appropriations Act, 2021 (the “CAA”), which was enacted on December 27, 2020. In total, the CARES Act, PPPHCE Act and the CAA authorized $178,000,000 in funding to be distributed to hospitals and other healthcare providers through the Public Health and Social Services Emergency Fund (the “PHSSEF”). In addition, the CARES Act provided for an expansion of the Medicare Accelerated and Advance Payment Program whereby inpatient acute care hospitals and other eligible providers were able to request accelerated payment of up to 100% of their Medicare payment amount for a six-month period to be repaid through withholding of future Medicare fee-for-service payments. Various other state and local programs also exist to provide relief, either independently or through distribution of monies received via the CARES Act. During the year ended December 31, 2021, the Company was a beneficiary of these stimulus measures. The Company’s accounting policies for the recognition of these stimulus monies is as follows.
The Company directly received $4,993 in Paycheck Protection Program (“PPP”) loans under the CARES Act and indirectly received an additional $332 in PPP loans through acquisitions (see Note 16). PPP loans may be eligible for forgiveness if the funds were used for eligible payroll costs, payments on business mortgage interest payments, rent, or utilities during either the 8- or 24-week period after disbursement. The Company elected to account for the loans as current debt until such loans were forgiven. Forgiveness for $4,957 of the PPP loans was received during the year ended December 31, 2021. As of December 31, 2022, the balance of all PPP loans has been forgiven. As such, the Company recognized the loan principal balance and accrued interest as a gain on loan forgiveness in the Consolidated Statement of Operations.
The Company received $2,727 from CMS under the Accelerated and Advance Payment Program which is an advance on future Medicare payments and will be recouped from future payments due to the Company by Medicare after 120 days. Effective October 1, 2020, the program was amended such that providers are required to repay accelerated payments beginning one year after the payment was issued. After such one-year period, Medicare payments owed to providers will be recouped against Medicare payments according to the repayment terms. As of December 31, 2021, the Medicare accelerated payments are reflected within accrued expenses and other current liabilities in the consolidated balance sheets. As of December 31, 2022, the Company repaid all advances received from CMS under the Accelerated and Advance Payment Program.
80
Table of Contents
The Company received funding from United States Department of HHS as part of the Provider Relief Funding under the CARES Act. Provider Relief Funding is paid in the form of a grant and does not require repayment if used to cover lost revenue, as defined, attributable to COVID-19 and healthcare-related expenses, as defined, including qualifying direct labor, paid or purchased to prevent, prepare for, and respond to COVID-19. Under International Accounting Standard No. 20, Accounting for Government Grants (“IAS 20”), grants are recognized when an entity has reasonable assurance that 1) it will comply with the relevant conditions and 2) the grant will be received. The Company recognized $1,023 in other income related to HHS funding during the year ended December 31, 2021 by applying IAS 20 by analogy.
Note 4. Accounts Receivable
The Company’s accounts receivable consists primarily of amounts due from third-party payors and patients. See Note 2 for a summary of the Company’s policies relating to accounts receivable.
Accounts Receivable as of December 31, 2022 and December 31, 2021 consist of the following:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Oral drug accounts receivable | $ | 4,165 | $ | 2,097 | |||||||
| Capitated accounts receivable | 1,623 | 665 | |||||||||
| FFS accounts receivable | 26,313 | 12,530 | |||||||||
| Clinical trials accounts receivable | 2,443 | 1,823 | |||||||||
| Other trade receivables | 5,272 | 2,892 | |||||||||
| Total | $ | 39,816 | $ | 20,007 | |||||||
During the years ended December 31, 2022 and 2021, the Company had net bad debt recoveries of $169, and bad debt recoveries of $465, respectively, and bad debt expense of $307 and $48, respectively. Bad debt write-offs were a result of accounts receivable on completed contracts that were deemed uncollectible during the period due to delayed collection efforts.
Note 5. Revenue
Management recognizes revenue in accordance with ASC 606 on the basis of its satisfaction of outstanding performance obligations. Management typically fulfills its performance obligations over time, either over the course of a single treatment (FFS), a month (capitation), or a number of months (clinical research). Management also has revenue that is satisfied at a point in time (dispensary). See Note 2 for summary of the Company’s policies and significant assumptions related to revenue recognition.
Disaggregation of Revenue
The Company categorizes revenue based on various factors such as the nature of contracts, payors, order to billing arrangements, and cash flows received by the Company, as follows:
| (in thousands) | Year Ended December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Patient services | ||||||||||||||
| Capitated revenue | $ | 61,341 | $ | 54,285 | ||||||||||
| FFS revenue | 105,444 | 69,789 | ||||||||||||
| Subtotal | 166,785 | 124,074 | ||||||||||||
| Dispensary revenue | 79,343 | 72,550 | ||||||||||||
| Clinical research trials and other revenue | 6,355 | 6,379 | ||||||||||||
| Total | $ | 252,483 | $ | 203,003 | ||||||||||
Refer to Note 20 for Segment Reporting for disaggregation of revenue by reporting segment.
Contract Asset and Liabilities
Under ASC 606, contract assets represent rights to payment for performance contingent on something other than the passage of time and accounts receivable are rights to payment for performance without contingencies. The Company does not
81
Table of Contents
have any contract assets as of December 31, 2022 and December 31, 2021. Refer to Note 4 for accounts receivable as of December 31, 2022 and December 31, 2021.
Contract liabilities represent cash that has been received for contracts, but for which performance is still unsatisfied. As of December 31, 2022 and December 31, 2021, contract liabilities amounted to $1,139 and $220, respectively. Contract liabilities are included within other current liabilities and presented in Note 9 along with refund liabilities.
Remaining Unsatisfied Performance Obligations
The accounting terms for the Company’s patient services and dispensary contracts do not extend past a year in duration. Additionally, the Company applies the ‘as invoiced’ practical expedient to its clinical research contracts.
Note 6. Inventories
The Company purchases intravenous chemotherapy drugs and oral prescription drugs from various suppliers. See Note 2 for a summary of the Company’s policies relating to intravenous chemotherapy and oral prescription drugs inventory.
The Company’s inventories as of December 31, 2022 and December 31, 2021 were as follows:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Oral drug inventory | $ | 2,130 | $ | 1,484 | |||||||
| IV drug inventory | 7,131 | 4,954 | |||||||||
| Total | $ | 9,261 | $ | 6,438 | |||||||
Note 7. Marketable Securities and Fair Value Measurements
Marketable Securities
The Company accounts for its investment securities available for sale using the fair value election pursuant to ASC 825, where changes in fair value are recorded in Other non-operating expense (income) on the Company's Consolidated Statements of Operations. The Company’s investments in cash equivalents and marketable securities at December 31, 2022 is as follows:
| December 31, 2022 | |||||||||||||||||||||||
| (in thousands) | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
| U.S. Treasury Bills | $ | 2,573 | $ | — | $ | — | $ | 2,573 | |||||||||||||||
| Marketable securities: | |||||||||||||||||||||||
| Short-term U.S. Treasuries | 59,876 | 6 | (86) | 59,796 | |||||||||||||||||||
| Long-term U.S. Treasuries | 58,652 | — | (298) | 58,354 | |||||||||||||||||||
| Total available for sale securities | $ | 121,101 | $ | 6 | $ | (384) | $ | 120,723 | |||||||||||||||
The contractual maturities of the Company's investments in cash equivalents and marketable securities as of December 31, 2022 is as follows:
| (in thousands) | Due in One Year or less | Due After One Year through Five Years | Due After Five Years | Total | |||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
| U.S. Treasury Bills | $ | 2,573 | $ | — | $ | — | $ | 2,573 | |||||||||||||||
| Marketable securities: | |||||||||||||||||||||||
| Short-term U.S. Treasuries | 59,796 | — | — | 59,796 | |||||||||||||||||||
| Long-term U.S. Treasuries | 10,523 | 47,831 | — | 58,354 | |||||||||||||||||||
| Total available for sale securities | $ | 72,892 | $ | 47,831 | $ | — | $ | 120,723 | |||||||||||||||
82
Table of Contents
The Company recorded a net unrealized loss of $378 for the year ended December 31, 2022. At December 31, 2022, eight securities were in an unrealized loss position. The decline in fair value of our securities since acquisition was attributable to a combination of changes in interest rates and general volatility in the credit market conditions in response to the economic uncertainty caused by the global pandemic, rising inflation, and conflict between Russia and Ukraine. We do not currently intend to sell any of the securities in an unrealized loss position and further believe, it is more likely than not, that we will not be required to sell these securities before their anticipated recovery. There were no investment securities held as of December 31, 2021.
Accrued interest receivable on cash equivalents and marketable securities was $274 and $0, respectively, at December 31, 2022 and December 31, 2021, and is included within in the Consolidated Balance Sheets.
Fair Value Measurements
The following tables present the carrying amounts of the Company’s financial instruments at December 31, 2022 and December 31, 2021:
| December 31, 2022 | |||||||||||||||||||||||
| (in thousands) | Total | Level 1 | Level 2 | Level 3 | |||||||||||||||||||
| Financial assets: | |||||||||||||||||||||||
| Treasury bills | $ | 2,573 | $ | — | $ | 2,573 | — | ||||||||||||||||
| Marketable securities | 59,796 | — | 59,796 | — | |||||||||||||||||||
| Non-current investments | 58,354 | — | 58,354 | — | |||||||||||||||||||
| Goodwill | 21,418 | — | — | 21,418 | |||||||||||||||||||
| Financial liabilities: | |||||||||||||||||||||||
| Derivative warrant liabilities | $ | 350 | — | — | $ | 350 | |||||||||||||||||
| Earnout liabilities | 803 | — | — | 803 | |||||||||||||||||||
| Conversion option derivative liabilities | 3,960 | — | — | 3,960 | |||||||||||||||||||
| December 31, 2021 | |||||||||||||||||||||||
| (in thousands) | Total | Level 1 | Level 2 | Level 3 | |||||||||||||||||||
| Financial liabilities: | |||||||||||||||||||||||
| Derivative warrant liabilities | 2,193 | — | — | 2,193 | |||||||||||||||||||
| Earnout liabilities | 60,018 | — | — | 60,018 | |||||||||||||||||||
The carrying amounts of cash, accounts receivable, other receivables, and accounts payable approximate fair value because of the short maturity and high liquidity of these instruments.
The Company measures its investments (including cash equivalents, marketable securities, and non-current investments) at fair value on a recurring basis and classifies those instruments within Level 2 of the fair value hierarchy. Investment securities, including U.S. Treasury Bills purchased in the secondary market and U.S. Treasury bonds, are classified within Level 2 of the fair value hierarchy because pricing inputs are other than quoted prices in active markets, which are either directly or indirectly observable as of the reporting date, and fair value is determined using models or other valuation methodologies. The Company measures its derivative warrant, earnout, convertible note warrant derivative liability, optional redemption derivative liability and conversion option derivative liability on a recurring basis and classifies those instruments within Level 3 of the fair value hierarchy because unobservable inputs are used to measure fair value. See Note 2 for a summary of the Company’s policies relating to fair value measurements, and Note 11 for more detail on the convertible note warrant, optional redemption, and conversion option derivative liabilities.
The Company measures goodwill at fair value on a nonrecurring basis and classifies goodwill within Level 3 of the fair value hierarchy. Due to significant declines in the Company's share price during the year ended December 31, 2022, the Company performed qualitative and quantitative analysis of impairment over goodwill and determined goodwill was impaired. As a result, the Company recorded an impairment charge of $9,944. Goodwill was valued using an equally weighted income approach and market approach. The unobservable inputs utilized in determining the fair value of the goodwill, which is categorized as a Level 3 instrument, are the discount rate of 25.0% and various revenue growth rates utilized in the financial forecast of future cash flows. See Note 2 for further detail on the impairment evaluation and Note 18 for goodwill.
83
Table of Contents
The following table presents information about the Company’s Level 3 liabilities that are measured at fair value on a recurring basis at December 31, 2022:
| (in thousands) | Private Warrant Liability | Earnout Liability | Conversion Option Derivative Liability | ||||||||||||||
| Balance at December 31, 2020 | $ | — | $ | — | $ | — | |||||||||||
| Private placement warrant liability acquired as part of the Business Combination | 5,879 | — | — | ||||||||||||||
| Earnout liability acquired as part of the Business Combination | — | 84,909 | — | ||||||||||||||
| Decrease in fair value included in other expense | (3,686) | (24,891) | — | ||||||||||||||
| Balance at December 31, 2021 | $ | 2,193 | $ | 60,018 | $ | — | |||||||||||
| Conversion option derivative liability acquired (See Note 11 for detail) | — | — | 28,160 | ||||||||||||||
| Decrease in fair value included in other expense | (1,843) | (59,215) | (24,200) | ||||||||||||||
| Balance at December 31, 2022 | $ | 350 | $ | 803 | $ | 3,960 | |||||||||||
The derivative warrant and earnout liabilities were valued using a Binomial Lattice and Monte-Carlo Simulation Model, respectively, which are considered to be Level 3 fair value measurements. The convertible note warrant and conversion option derivative liabilities were valued using the Binomial Lattice and Black-Scholes Models, which are considered to be Level 3 fair value measurements. The primary unobservable input utilized in determining the fair value of our Level 3 liabilities is the expected volatility of the common stock. A summary of the inputs used in the valuations is as follows:
| December 31, 2022 | |||||||||||||||||||||||||||||
| Derivative Warrant Liability | First Tranche Earnout | Second Tranche Earnout | Convertible Note Warrant Derivative Liability | Conversion Option Derivative Liability | |||||||||||||||||||||||||
| Unit price | $ | 1.65 | $ | 1.65 | $ | 1.65 | $ | 1.65 | $ | 1.65 | |||||||||||||||||||
| Term (in years) | 3.87 | 1.54 | 1.55 | 4.61 | 4.61 | ||||||||||||||||||||||||
| Volatility | 71.80 | % | 70.00 | % | 70.00 | % | 40.00 | % | 40.00 | % | |||||||||||||||||||
| Risk-free rate | 4.08 | % | 4.45 | % | 4.45 | % | 3.99 | % | 3.99 | % | |||||||||||||||||||
| Dividend yield | — | — | — | — | — | ||||||||||||||||||||||||
| Cost of equity | — | 13.60 | % | 13.60 | % | — | — | ||||||||||||||||||||||
| December 31, 2021 | |||||||||||||||||
| Derivative Warrant Liability | First Tranche Earnout | Second Tranche Earnout | |||||||||||||||
| Unit price | $ | 9.75 | $ | 9.75 | $ | 9.75 | |||||||||||
| Term (in years) | 4.87 | 1.87 | 2.87 | ||||||||||||||
| Volatility | 12.80 | % | 35.00 | % | 35.00 | % | |||||||||||
| Risk-free rate | 1.24 | % | 0.94 | % | 0.94 | % | |||||||||||
| Dividend yield | — | — | — | ||||||||||||||
| Cost of equity | — | 11.14 | % | 11.14 | % | ||||||||||||
On August 9, 2022, the Company issued a senior secured convertible note that contains embedded warrant, optional redemption, and conversion option features. Due to the economic disincentive to redeem and the make whole amount that would be required to be paid, it is highly unlikely that the optional redemption would occur, reducing the value during the period to a qualitatively immaterial amount. See Note 11 for additional detail. A summary of the inputs used in the initial measurement of the convertible note warrant and conversion option derivative liabilities is as follows:
84
Table of Contents
| August 9, 2022 | |||||||||||
| (Initial Measurement) | |||||||||||
| Convertible Note Warrant Derivative Liability | Conversion Option Derivative Liability | ||||||||||
| Unit price | $ | 6.63 | $ | 6.63 | |||||||
| Term (in years) | 5.00 | 5.00 | |||||||||
| Volatility | 42.5 | % | 42.5 | % | |||||||
| Risk-free rate | 3.0 | % | 3.0 | % | |||||||
| Dividend yield | — | — | |||||||||
| Cost of equity | — | — | |||||||||
There were no transfers between fair value measurement levels during the years ended December 31, 2022 and 2021.
Uncertainty of Fair Value Measurement from Use of Significant Unobservable Inputs
The inputs to estimate the fair value of the Company’s derivative warrant, earnout, convertible note warrant, and conversion option derivative liabilities were the market price of the Company’s common stock, their remaining expected term, the volatility of the Company’s common stock price and the risk-free interest rate over the expected term. Significant changes in any of those inputs in isolation can result in a significant change in the fair value measurement.
Generally, an increase in the market price of the Company’s shares of common stock, an increase in the volatility of the Company’s shares of common stock, and an increase in the remaining term of the derivative liabilities would each result in a directionally similar change in the estimated fair value of the Company’s derivative liabilities. Such changes would increase the associated liability while decreases in these assumptions would decrease the associated liability. An increase in the risk-free interest rate would result in a decrease in the estimated fair value measurement and thus a decrease in the associated liability. The Company has not, and does not plan to, declare dividends on its common stock and, as such, there is no change in the estimated fair value of the derivative warrant liabilities due to the dividend assumption.
Note 8. Property and Equipment, Net
The Company accounts for property and equipment at historical cost less accumulated depreciation. See Note 2 for a summary of the Company’s policies relating to property and equipment.
Property and equipment, net, consist of the following:
| (in thousands) | Useful lives | December 31, 2022 | December 31, 2021 | ||||||||||||||
| Computers and software | 60 months | $ | 2,139 | $ | 961 | ||||||||||||
| Office furniture | 84 months | 606 | 343 | ||||||||||||||
| Leasehold improvements | Shorter of lease term or estimated useful life | 6,655 | 3,387 | ||||||||||||||
| Medical equipment | 60 months | 1,138 | 805 | ||||||||||||||
| Construction in progress | 1,144 | 518 | |||||||||||||||
| Finance lease ROU assets | Shorter of lease term or estimated useful life | 371 | 162 | ||||||||||||||
| Less: accumulated depreciation | (3,506) | (1,984) | |||||||||||||||
| Total property and equipment, net | $ | 8,547 | $ | 4,192 | |||||||||||||
Depreciation expense for the years ended December 31, 2022 and 2021 was $1,526 and $826, respectively.
85
Table of Contents
Note 9. Accrued Expenses and Other Current and Non-Current Liabilities
Accrued expenses and other current liabilities as of December 31, 2022 and December 31, 2021 consist of the following:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Compensation, including bonuses, fringe benefits, and payroll taxes | $ | 5,310 | $ | 3,325 | |||||||
| Contract liabilities | 1,139 | 262 | |||||||||
| Directors and officers insurance premiums | 3,010 | 5,009 | |||||||||
| Deferred acquisition and contingent consideration (see Note 16) | 802 | 2,359 | |||||||||
| Accrued interest | 1,100 | — | |||||||||
| Other liabilities | 3,234 | 2,969 | |||||||||
| Total accrued expenses and other current liabilities | $ | 14,595 | $ | 13,924 | |||||||
Contract liabilities as of December 31, 2022 and December 31, 2021 consist of cumulative adjustments made to capitated revenue recognized in prior periods.
Pursuant to the Business Combination, the Company has agreed to indemnify members of the Board and certain officers if they are named or threatened to be named as a party to any proceeding by reason of the fact that they acted in such capacity. The Company entered into a financing arrangement to pay premiums for directors’ and officers’ (“D&O”) insurance coverage to protect against such losses on November 12, 2021. As of December 31, 2022, the remaining D&O principal balance was $3,010, all of which is due to be paid before December 31, 2023 and classified as a current liability.
Note 10. Leases
The Company leases clinics, office buildings, and certain equipment under noncancellable financing and operating lease agreements that expire at various dates through November 2031. See Note 2 for a summary of the Company’s policies relating to leases, and the Company's adoption of Accounting Standards Update 2016-02, Leases (ASC 842), as of January 1, 2022.
The initial terms of operating leases range from 0 to 10 years and certain leases provide for free rent periods, periodic rent increases, and renewal options. Monthly payments for these leases range from $0 to $37. All lease agreements generally require the Company to pay maintenance, repairs, property taxes, and insurance costs, which are generally variable amounts based on actual costs incurred during each applicable period.
The Company has determined that periods covered by options to extend the Company's leases are excluded from the lease terms as it is not reasonably certain the Company will exercise such options. Operating lease expenses, including expenses related to short-term leases, were $6,364, $4,281, and $3,680, respectively, for the years ended December 31, 2022, 2021, and 2020.
Lease Expense
The components of lease expense were as follows for the year ended December 31, 2022:
| (in thousands) | Year Ended December 31, 2022 | ||||
| Operating lease costs: | $ | 6,002 | |||
| Finance lease costs: | |||||
| Amortization of ROU asset | $ | 62 | |||
| Interest expense | $ | 8 | |||
| Other lease costs: | |||||
| Short-term lease costs | $ | 362 | |||
| Variable lease costs | $ | 967 | |||
| Total lease costs | $ | 7,401 | |||
Operating and other lease costs are presented as part of selling, general, and administrative expenses. The components of finance lease costs appear in depreciation and amortization and interest expense.
86
Table of Contents
Maturity of Lease Liabilities
The aggregate future lease payments for the Company's leases in years subsequent to December 31, 2022 are as follows:
| (in thousands) | Operating Leases | Finance Leases | ||||||
| 2023 | $ | 6,637 | $ | 84 | ||||
| 2024 | 6,202 | 77 | ||||||
| 2025 | 5,576 | 42 | ||||||
| 2026 | 4,834 | 39 | ||||||
| 2027 | 3,877 | 29 | ||||||
| Thereafter | 4,600 | — | ||||||
| Total future lease payment | $ | 31,726 | $ | 271 | ||||
| Less: amount representing interest | (4,168) | (30) | ||||||
| Present value of future lease payment (lease liability) | $ | 27,558 | $ | 241 | ||||
| Reported as: | ||||||||
| Lease liabilities, current | $ | 5,498 | $ | 72 | ||||
| Lease liabilities, noncurrent | 22,060 | 169 | ||||||
| Total lease liabilities | $ | 27,558 | $ | 241 | ||||
Lease Term and Discount Rate
The following table provides the weighted average remaining lease terms and weighted average discount rates for the Company's leases as of December 31, 2022:
| December 31, 2022 | |||||
| Weighted-average remaining lease term (in years) | |||||
| Operating | 5.32 | ||||
| Finance | 3.75 | ||||
| Weighted-average discount rate | |||||
| Operating | 4.94 | % | |||
| Finance | 6.02 | % | |||
Supplemental Cash Flow Information
The following table provides certain cash flow and supplemental noncash information related to the Company's lease liabilities for the year ended December 31, 2022.
| (in thousands) | Year Ended December 31, 2022 | ||||
| Supplemental cash flow information | |||||
| Cash paid for amounts included in the measurement of lease liabilities: | |||||
| Operating cash payment from operating leases | $ | 5,342 | |||
| Financing cash payments for finance leases | 73 | ||||
| Lease liabilities arising from obtaining right-of-use assets: | |||||
| Operating leases | $ | 30,800 | |||
| Finance leases | 203 | ||||
During the year ended December 31, 2022, ROU assets of $11,668 were obtained in exchange for lease obligations.
Lease Modifications
During the year ended December 31, 2022, the Company expanded its lease space and extended its lease term for two clinics and two corporate offices in California. These expansions and extensions constitute lease modifications that qualify as a change of accounting for the original leases and not separate contracts. Accordingly, in the year ended December 31, 2022, the
87
Table of Contents
Company recognized the difference of $2,186 as an increase to the operating lease liability; $2,052, net of lease incentives, as an increase to operating lease right-of-use asset, and $39 as a net increase to rent expense.
Operating Lease Contractual Commitments
As of December 31, 2021, future minimum lease payments under noncancellable operating leases (with initial or remaining lease terms in excess of one year) and future minimum capital lease payments were:
| (in thousands) | Capital leases | Operating leases | |||||||||
| Year ending December 31: | |||||||||||
| 2022 | $ | 37 | $ | 4,263 | |||||||
| 2023 | 37 | 3,946 | |||||||||
| 2024 | 29 | 3,291 | |||||||||
| 2025 | — | 2,718 | |||||||||
| 2026 | — | 1,954 | |||||||||
| Thereafter | — | 1,044 | |||||||||
| Total minimum lease payments | $ | 103 | $ | 17,216 | |||||||
Less: amount representing interest (6% interest rate) | (7) | ||||||||||
| Present value of net minimum capital lease payments | $ | 96 | |||||||||
| Less current installments of obligations under capital leases | (33) | ||||||||||
| Obligations under capital leases, excluding current installments | $ | 63 | |||||||||
Note 11. Debt
Senior Secured Convertible Note
On August 9, 2022, TOI entered into a Facility Agreement (the “Facility Agreement”) with certain lenders (“Lenders”) and Deerfield Partners L.P. (“Agent”), pursuant to which, TOI borrowed cash loans from the Lenders in the amount of $110,000, in exchange for which, TOI issued to each Lender a secured convertible promissory note (“Senior Secured Convertible Note”), which is payable to such Lenders in an amount equal to the unpaid principal amount of loans held by such Lender.
The Senior Secured Convertible Note will mature on August 9, 2027 (the “Maturity Date”) and shall bear interest at the rate of 4.00% per annum from August 9, 2022, on the outstanding principal amount, any overdue interest and any other amounts and obligations. The interest shall be paid in cash quarterly in arrears commencing on October 1, 2022. In case of any prepayment, repayment or redemption of the Senior Secured Convertible Note, the Company shall pay any accrued and unpaid interest on the principal, along with a make whole amount and an exit fee.
The Facility Agreement requires the Company to meet certain operational and reporting requirements, including, but not limited to, customary regulatory, financial reporting, and disclosure requirements. Additionally, limitations are placed on the Company's ability to merge with other companies and enter into other debt arrangements and permitted investments are limited to amounts specified in the Facility Agreement. The Facility Agreement also provides certain restrictions on dividend payments and other equity transactions and requires the Company to make prepayments under specified circumstances. Financial covenants in the Facility Agreement require the Company to maintain a minimum unrestricted cash and Cash Equivalent balance of $40,000 and a minimum net quarterly revenues of $50,000 during fiscal year 2023; $75,000 during fiscal year 2024; and $100,000 during fiscal year 2025. Cash Equivalents as defined by the Facility Agreement means (a) any readily-marketable securities (i) issued by, or directly, unconditionally and fully guaranteed or insured by the United States federal government or (ii) issued by any agency of the United States federal government the obligations of which are fully backed by the full faith and credit of the United States federal government, (b) any readily-marketable direct obligations issued by any other agency of the United States federal government, any state of the United States or any political subdivision of any such state or any public instrumentality thereof, in each case having a rating of at least “A-1” from S&P or at least “P-1” from Moody’s, (c) any commercial paper rated at least “A-1” by S&P or “P-1” by Moody’s and issued by any Person organized under the laws of any state of the United States, (d) any United States dollar-denominated time deposit, insured certificate of deposit, overnight bank deposit or bankers’ acceptance issued or accepted by any commercial bank that (A) is organized under the laws of the United States, any state thereof or the District of Columbia, (B) is “adequately capitalized” (as defined in the regulations of its primary federal banking regulators) and (C) has Tier 1 capital (as defined in such regulations) in excess of $250,000 and (e) shares of any United States money market fund that (i) has substantially all of its assets invested continuously in the types of investments
88
Table of Contents
referred to in clause (a), (b), (c) and/or (d) above with maturities as set forth in the proviso below, (ii) has net assets in excess of $500,000 and (iii) has obtained from either S&P or Moody’s the highest rating obtainable for money market funds in the United States; provided, however, that the maturities of all obligations specified in any of clause (a), (b), (c) and (d) above shall not exceed one year. Additionally, the Registration Rights Agreement requires the Company to have an effective registration statement and calls for payment should the registration statement cease to remain effective. The Company was in compliance with the covenants of the Facility Agreement as of December 31, 2022.
Conversion Options
The Senior Secured Convertible Note contains several embedded conversion options (the “Conversion Options”) that grant the holders of the Senior Secured Convertible Note the ability to convert the Senior Secured Convertible Note at any time on or after date of issuance of the note. The Conversion Options are convertible into shares of the Company’s common stock (such converted shares, “Conversion Shares”) and, in certain circumstances, a combination of cash and shares of the Company’s common stock, or a combination of cash, other assets and securities or other property of any Company successor entity. The Conversion Shares or settlement amounts shall be computed on the basis of predefined formulae, with a set conversion price of $8.567 as one of the inputs and a conversion cap of 14,663,019 shares. The if-converted value did not exceed the principal amount as of December 31, 2022. No Conversion Shares were issued as of December 31, 2022.
The Company evaluated the Conversion Options of the Senior Secured Convertible Note under ASC 815 and concluded that they require bifurcation from the host contract as a separate unit of account. The Conversion Options do not meet the criteria to be classified in stockholders’ equity and hence, are accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings.
The Conversion Options contain certain limits on exercise if, after giving effect to the exercise, the Lender would beneficially own a number of shares of common stock of the Company in excess of those permissible under the terms of the Senior Secured Convertible Note. The number of shares to be issued against these notes and conversion price are each subject to adjustments provided under the terms of Senior Secured Convertible Note.
The holder shall receive dividends on the Senior Secured Convertible Note and distributions of any kind made to the holders of common stock, other than dividends of, or distributions in, shares, to the same extent as if the holder had converted the Senior Secured Convertible Note into such shares and had held such shares on the record date for such dividends and distributions any limitations on conversion options.
Optional Redemption
The Facility Agreement also provides the Company the right to redeem the outstanding principal amount of each note (“Optional Redemption”) for the principal amount, plus undiscounted interest. The Company shall not affect any Optional Redemption under this Senior Secured Convertible Note unless along with this, the Company effects an optional redemption under all other notes in accordance with the terms thereof, on a pro rata basis, based upon the respective applicable original principal amount of each of the notes outstanding as of the date the notice for Optional Redemption is delivered to the holders.
The Company evaluated the Optional Redemption feature of the Senior Secured Convertible Note under ASC 815 and concluded that it requires bifurcation from the host contract as a separate unit of account. The Optional Redemption feature does not meet the criteria to be classified in stockholders’ equity and hence, is accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings. The fair value of the Optional Redemption feature is de minimis.
If the principal redemption amount specified in an Optional Redemption notice is less than the entire principal amount then outstanding, the principal amount specified in each conversion notice shall be applied (i) first, to reduce, on a dollar-for-dollar basis, the principal amount of the note in excess of the principal redemption amount until such excess principal amount is reduced to zero and (ii) to reduce, on a dollar-for-dollar basis, the principal redemption amount until all of such principal redemption amount shall have been converted.
Convertible Note Warrants
The Facility Agreement also provides for the issuance of warrants (the “Convertible Note Warrants”) on each date any principal amount of any Senior Secured Convertible Note is paid, repaid, redeemed, or prepaid at any time prior to the Maturity Date. Convertible Note Warrants are exercisable from their original issue date to August 9, 2027, for purchase of an aggregate amount of Conversion Shares into which such principal amount of Senior Secured Convertible Note was convertible into, immediately prior to such payment, at an exercise price of $8.567. The holder of Convertible Note Warrants may pay the
89
Table of Contents
exercise price in cash or exercise the warrant on cashless basis or through a reduction of an amount of principal outstanding under any Senior Secured Convertible Note held by such holder. In the event that the Convertible Note Warrant has not been exercised in full as of the last business day during its term, the holder shall be deemed to have exercised the purchase rights represented by the Convertible Note Warrant in full as a cashless exercise, in which event the Company shall issue number of shares to the holder computed on the basis of a predefined formula.
The Company evaluated the Convertible Note Warrants of the Senior Secured Convertible Note under ASC 815 and concluded that they require bifurcation from the host contract as a separate unit of account. The Convertible Note Warrants do not meet the criteria to be classified in stockholders’ equity and hence, are accounted for as a derivative liability remeasured at fair value at each balance sheet date with changes in fair value reported in earnings.
The Convertible Note Warrant holder shall be entitled to receive any dividend or distribution made by the Company to the holders of common stock to the same extent as if the holder had exercised the Convertible Note Warrants in full in a cash exercise.
The number of shares to be issued against these warrants and exercise price are each subject to adjustments provided under the terms of Convertible Note Warrants. The Convertible Note Warrants contain certain limits on exercise if, after giving effect to the exercise, the Lender would beneficially own a number of shares of common stock of the Company in excess of those permissible under the terms of the Convertible Note Warrants. Further, the Convertible Note Warrants can be fully or partially settled in cash in certain cases in accordance with the terms of issuance such as when shares issuable upon exercise of the warrants exceed a predefined number, upon occurrence of predefined event of default and upon occurrence of predefined events that will bring a fundamental change in the Company such as merger, consolidation, business combination, recapitalization, reorganization, reclassification or other similar event.
As of December 31, 2022, there are no Convertible Note Warrants outstanding.
Allocation of Proceeds
The Company has allocated total issuance proceeds of $110,000 among the Senior Secured Convertible Note and Convertible Note Warrants based on fair value. Upon issuance of the Convertible Note Warrants, the Company recorded Convertible Note Warrants, Optional Redemption, and Conversion Options of $0, $0 and $28,160, which were recorded as a debt discount to the Senior Secured Convertible Note of $110,000. The Company will amortize the debt discount over a period of 5 years (of which 4.61 years remain).
The total issuance costs of $4,924 was allocated among the Senior Secured Convertible Note, Convertible Note Warrants, Optional Redemption, and Conversion Options, by allocating costs of $0, $0, and $1,261 to the Convertible Note Warrants, Optional Redemption, and Conversion Options with the residual cost of $3,663 being allocated to the Senior Secured Convertible Note (in addition to the debt discount). The Company immediately expensed issuance costs allocated to Warrants, Optional Redemption, and Conversion Options at inception and will amortize the costs allocated to the Senior Secured Convertible Note over a period of 5 years (of which 4.61 years remain).
Amounts Outstanding and Recognized during the Periods Presented
The Senior Secured Convertible Note as of December 31, 2022 consists of the following:
| December 31, 2022 | |||||
| Senior Secured Convertible Note, due August 9, 2027 | $ | 110,000 | |||
| Less: Unamortized debt issuance costs | 3,454 | ||||
| Less: Unamortized debt discount | 25,925 | ||||
| Long-term debt, net of unamortized debt discount and issuance costs | $ | 80,621 | |||
The amortization of the debt issuance costs was charged to interest expense for all periods presented. For the year ended December 31, 2022, the effective yield was 13.38%. The amount of debt issuance costs included in interest expense for the year ended December 31, 2022 was $2,444. The Company had interest expense of $1,772 on the Credit Agreement term loan for the year ended December 31, 2022, including $1,100 of accrued interest as of December 31, 2022.
On August 9, 2022, the Company also entered into the Guarantee and Security Agreement (“Guarantee Agreement”) with the Agent for the purpose of providing a guarantee of all the obligations under the Facility Agreement (refer to Note 15. Commitments and Contingencies for detail).
90
Table of Contents
Debt Maturities
The following table summarizes the stated debt maturity related to the Senior Secured Convertible Note as of December 31, 2022:
| (in thousands) | ||||||||
| 2023 | $ | — | ||||||
| 2024 | — | |||||||
| 2025 | — | |||||||
| 2026 | — | |||||||
| 2027 | 110,000 | |||||||
| Total debt | $ | 110,000 | ||||||
PPP Loan
The Company recorded a PPP loan as a result of the acquisition of the practice of Leo E. Orr, MD on November 12, 2021 with Pacific Western Bank in the amount of $183, with interest bearing at 1%. The maturity date of the loan is October 24, 2026. The application for the PPP funds required an entity to, in good faith, certify that the current economic uncertainty made the loan request necessary to support the ongoing operations of the entity. This certification further required the entity to take into account its current business activity and its ability to access other sources of liquidity sufficient to support ongoing operations in a manner that is not significantly detrimental to the business. The receipt of these funds, and the forgiveness of the loan attendant to these funds, is dependent on the entity having initially qualified for the loan and qualifying for the forgiveness of such loan based on its future adherence to the forgiveness criteria. During the year ended December 31, 2022, the Company received notice of forgiveness of its PPP loan and accordingly has recognized the loan principal balance and accrued interest as a gain on loan forgiveness in the Consolidated Statement of Operations.
Note 12. Income Taxes
The components of the provision (benefit) for income taxes consists of:
| (in thousands) | Current | Deferred | Total | ||||||||||||||
| Year ended December 31, 2022: | |||||||||||||||||
| U.S. federal | $ | — | $ | (135) | $ | (135) | |||||||||||
| State and local | 20 | (128) | (108) | ||||||||||||||
| $ | 20 | $ | (263) | $ | (243) | ||||||||||||
| (in thousands) | Current | Deferred | Total | ||||||||||||||
| Year ended December 31, 2021: | |||||||||||||||||
| U.S. federal | $ | (180) | $ | (904) | $ | (1,084) | |||||||||||
| State and local | 751 | (338) | 413 | ||||||||||||||
| $ | 571 | $ | (1,242) | $ | (671) | ||||||||||||
The Company’s income tax expense differs from the amount that would have resulted from applying the federal statutory rate of 21% to pretax income from operations because of the effect of the following items:
| (in thousands) | Year Ended December 31, | ||||||||||
| 2022 | 2021 | ||||||||||
| Income tax at federal statutory rate | $ | (19) | $ | (2,436) | |||||||
| State tax, net federal benefit | (101) | (241) | |||||||||
| Meals and entertainment | 14 | 11 | |||||||||
| Transaction costs | 684 | 349 | |||||||||
| Fines and penalties | — | 28 | |||||||||
| Stock based compensation | 1,411 | (122) | |||||||||
| Warrant expense | (387) | (774) | |||||||||
91
Table of Contents
| (in thousands) | Year Ended December 31, | ||||||||||
| 2022 | 2021 | ||||||||||
| Earnout expense | (12,435) | (5,227) | |||||||||
| PPP loan forgiveness | — | (1,058) | |||||||||
| 162(m) Analysis | — | 1,717 | |||||||||
| 162(m) Deferred haircut | 1,433 | — | |||||||||
| 163(l) Interest expense limitation | 885 | — | |||||||||
| DFP derivative expense | (5,082) | — | |||||||||
| Goodwill impairment | 569 | — | |||||||||
| Prior year deferred true-ups | (2,100) | — | |||||||||
| Other state items | 24 | — | |||||||||
| Change in valuation allowance | 14,856 | 6,941 | |||||||||
| Other | 5 | 141 | |||||||||
| Income tax (benefit) expense | $ | (243) | $ | (671) | |||||||
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at December 31, 2022 and 2021 are presented below.
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Deferred tax assets: | |||||||||||
| Deferred rent | $ | — | $ | 173 | |||||||
| Accrued Expenses | 1,293 | 606 | |||||||||
| Net operating loss carryforwards | 26,357 | 12,686 | |||||||||
| Impaired assets | 1,313 | 1,751 | |||||||||
| Deferred revenue | 334 | 77 | |||||||||
| Stock based compensation | 3,497 | 1,088 | |||||||||
| Interest expense limitation | 21 | — | |||||||||
| Charitable contributions | 1 | — | |||||||||
| Tenant improvement allowance | (43) | — | |||||||||
| ROU Lease liability | 7,913 | — | |||||||||
| Financing lease liability | 177 | — | |||||||||
| Unrealized gain/loss | 112 | — | |||||||||
| Intangibles | 2,530 | — | |||||||||
| Total gross deferred tax assets | 43,505 | 16,381 | |||||||||
| Valuation allowance | (34,915) | (14,719) | |||||||||
| Net deferred tax assets | $ | 8,590 | $ | 1,662 | |||||||
| Deferred tax liabilities: | |||||||||||
| Property, plant, and equipment | $ | (1,507) | $ | (706) | |||||||
| Intangibles | — | (1,327) | |||||||||
| ROU Asset | (7,013) | 0 | |||||||||
| Financial lease asset | (176) | 0 | |||||||||
| IRC 174 expenditures | (2) | 0 | |||||||||
| Total gross deferred liabilities | $ | (8,698) | $ | (2,033) | |||||||
| Net deferred tax liabilities | $ | (108) | $ | (371) | |||||||
The valuation allowance for deferred tax assets as of December 31, 2022 and 2021, was $34,915 and $14,719, respectively. The net change in the total valuation allowance was an increase of $20,196 in 2022 and an increase of $9,268 in 2021.
The valuation allowance at December 31, 2022 was primarily related to net operating loss carryforwards of TOI, Inc., TOI CA, and TOI FL that, in the judgment of management, are not more likely than not to be realized. TOI Inc., TOI CA, TOI FL,
92
Table of Contents
and TOI TX will elect to file a consolidated 2022 federal return and state income tax return. Accordingly, net operating losses of TOI CA, TOI FL, and TOI TX can offset taxable income of TOI Parent for federal and state tax purposes. Deferred tax assets and deferred tax liabilities have been separately determined for all groups, as has the valuation allowance assessment for each. The table above reflects the combined deferred tax assets, deferred tax liabilities, and valuation allowance for TOI Inc., TOI CA, TOI FL, and TOI TX. Of the $34,915 total valuation allowance, $24,967 is attributable to the Federal Group, $9,928 is attributable to TOI CA, $18 is attributable to TOI FL, and $2 to TOI TX.
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities (including the effect of available carry back and carryforward periods), projected future taxable income, and tax-planning strategies in making this assessment. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will realize the benefits of these deductible differences, net of the existing valuation allowances at December 31, 2022. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carryforward period are reduced.
At December 31, 2022, the Company has net operating loss carryforwards for Federal income tax purposes of $91,435, with $73,071 attributable to the Practice and $18,364 attributable to TOI Parent, which are available to offset future Federal taxable income of the Practice and Parent indefinitely. The Company has net operating loss carryforwards for state income tax purposes of $85,733, of which $68,617 is attributable to the Practice and will begin to expire after 2040, and $17,116 is attributable to Parent and will begin to expire after 2041.
Pursuant to Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, and corresponding provisions of state law, if a corporation undergoes an “ownership change” (very generally defined as a greater than 50% change, by value, in the corporation’s equity ownership by certain stockholders or groups of stockholders over a rolling three-year period), the corporation’s ability to use its pre-ownership change NOLs to offset its post-ownership change income may be limited. We are in the process of completing an analysis to determine whether there was a change in ownership during the year ended December 31, 2022 in order to determine if there is a limitation on pre-ownership NOLs. Additionally, we may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If it is determined that an ownership change has occurred as a result of the Business Combination or we undergo an ownership change in the future, we may be prevented from fully utilizing our NOLs existing at the time of the ownership change prior to their expiration.
The deferred tax asset associated with the Company’s federal and state net operating losses are fully offset by a valuation allowance. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. A summary of the changes in the amount of unrecognized tax benefits (excluding interest and penalties) for 2022 and 2021 is as follows:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Beginning balance of unrecognized tax benefits | $ | 99 | $ | 1,903 | |||||||
| Additions based on tax positions related to the current year | — | — | |||||||||
| Reductions based on tax positions of prior years | — | (1,804) | |||||||||
| Reductions due to lapse of applicable statute of limitation | — | — | |||||||||
| Settlements | — | — | |||||||||
| Ending balance of unrecognized tax benefits | $ | 99 | $ | 99 | |||||||
The Company does not anticipate a significant change in the amount of its unrecognized tax within the next 12 months. The Company recognizes interest and penalties related to unrecognized tax benefits in income tax expense. Due to the Company’s NOL position, no interest or penalties have been recognized with respect to unrecognized tax benefits, as such amounts are considered immaterial. The Company includes unrecognized tax benefits within other non-current liabilities on its consolidated balance sheet.
The Company is subject to taxation in the U.S., California, Arizona, and Florida. As of December 31, 2022, the statute of limitations remains open for tax year 2018 through the current year.
93
Table of Contents
Note 13. Stockholders' Equity
The Consolidated Statements of Convertible Preferred Stock and Changes in Stockholders’ Equity has been retroactively adjusted for all periods presented to reflect the Business Combination and reverse recapitalization described in Note 1. The balances as of December 31, 2022 from the consolidated financial statements of Legacy TOI as of that date, share activity (Legacy TOI preferred stock, Legacy TOI common stock, and additional paid-in capital) and per share amounts were retroactively adjusted, where applicable, using the Common Stock Exchange Ratio (as defined below).
Common Stock
Upon the Closing Date of the Business Combination on November 12, 2021, pursuant to the terms of the Amended and Restated Certificate of Incorporation, the Company authorized 500,000,000 shares of common stock with a par value of $0.0001. As of December 31, 2022 and December 31, 2021, there were 73,265,621 and 73,249,042 shares, respectively, of common stock outstanding.
In connection with the Closing Date, all previously issued and outstanding shares of Legacy TOI preferred stock were converted into Legacy TOI common stock and received (i) shares of Company common stock pursuant to a 591:1 ratio of Company common shares to Legacy TOI common shares (the "Common Stock Exchange Ratio") and ii) cash. The Company has retroactively adjusted shares issued and outstanding prior to November 11, 2021 to give effect to the Common Stock Exchange Ratio to determine the number of shares of common stock into which they were converted.
Voting
The holders of the Company’s common stock are entitled to one vote for each share of common stock held at all meetings of stockholders (and written actions in lieu of meetings), and there is no cumulative voting.
Dividends
Common stockholders are entitled to receive dividends whenever funds are legally available and when declared by the board of directors. No dividends have been declared as of December 31, 2022.
Preferred Stock
Upon the Closing Date of the Business Combination, pursuant to the terms of the Amended and Restated Certificate of Incorporation, the Company authorized 10,000,000 shares of Series A Common Equivalent Preferred Stock (“preferred stock”) with a par value and liquidation preference of $0.0001 per share. The Company’s board of directors has the authority, without further action by the stockholders to issue such shares of preferred stock in one or more series, to establish, from time to time the number of shares to be included in each such series, and to fix the dividend, voting, and other rights, preferences, and privileges of the shares. Immediately following the Closing Date and as of December 31, 2021, there were 163,510 shares of preferred stock outstanding. As of December 31, 2022, there were 165,045 shares of preferred stock outstanding.
Conversion
Each share of preferred stock is convertible, at any time on the part of the holder except with respect to the Beneficial Ownership Limitation (defined below), into 100 shares of common stock.
Blocker/Beneficial Ownership Limitation
The preferred stock is subject to a beneficial ownership limitation such that the preferred stock may not, at any time, be convertible into more than 4.9% of the total number of shares of common stock outstanding (“Beneficial Ownership Limitation”).
Voting
The holders of preferred stock do not have voting rights in the Company.
Dividends
The holders of preferred stock are entitled to receive dividends whenever funds are legally available and when declared by the board of directors on an as-converted basis. No dividends have been declared as of December 31, 2022.
94
Table of Contents
Assumed Public Warrants and Private Placement Warrants
Following the consummation of the Business Combination, holders of the public warrants and private placement warrants are entitled to acquire common stock of the Company. The warrants became exercisable 30 days from the completion of the Business Combination, on December 12, 2021, and will expire five years after the completion of the Business Combination or earlier upon redemption or liquidation. As of December 31, 2022, there are 5,749,986 public warrants outstanding and 3,177,542 private placement warrants outstanding.
Each warrant entitles the holder to purchase one share of common stock for $11.50 per share. Private warrants held by the initial purchaser or certain permitted transferees may be exercised on a cashless basis.
If the reported last sale price of the common stock equals or exceeds $18.00 per share for any 20 trading days within a 30-trading day period ending three business days before the Company sends the notice of redemption to the warrant holders, the Company may redeem all the public warrants at a price of $0.01 per warrant upon not less than 30 days’ prior written notice.
If the Company calls the public warrants for redemption, management will have the option to require all holders that wish to exercise the public warrants to do so on a cashless basis. The Company will not be required to net cash settle the warrants.
The private warrants are exercisable on a cashless basis and are non-redeemable so long as they are held by the initial purchasers or their permitted transferees. If the private warrants are held by someone other than the initial purchasers of their permitted transferees, the private warrants will be redeemable by the Company and exercisable by such holders on the same basis as the public warrants.
Share Repurchase Program
On May 10, 2022, the Company's Board consented to the adoption and approval of the Share Repurchase Program, authorizing up to $20,000 to be spent on the repurchase of the Company's common stock, expiring on December 31, 2022. The Company repurchased and immediately retired 1,500,000 shares of its common stock for $9,000 from a related party (see Note 21) during the year ended December 31, 2022.
Note 14. Share-Based Compensation
Non-Qualified Stock Option Plan
On January 2, 2019, the Company issued and adopted the 2019 Non-Qualified Stock Option Plan (the “2019 Plan”) to incentivize directors, consultants, advisors, and other key employees of the Company and its subsidiaries to continue their association by providing opportunities to participate in the ownership and further growth of the Company. The 2019 Plan provides for the grant of options (the “Stock Options”) to acquire common shares of the Company.
Stock Options are exercised from the pool of shares designated by the appropriate Committee of the Board of Directors. The grant-date fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton option-pricing model. The grant date fair value of the service vesting and the performance vesting options is recognized as an expense over the requisite service period or vesting period and upon the achievement of the performance condition deemed probable of being achieved, respectively. The exercise price of each Stock Option shall be determined by the Committee and may not be less than the fair market value of the common stock on the date of grant. Stock Options have 10-year terms, after which they expire and are no longer exercisable.
The total number of shares of common stock for which Stock Options may be granted under the 2019 Plan shall not exceed 13,640. The 2019 Plan was amended on November 6, 2020, pursuant to which the total number of common shares for which Stock Options may be granted under the 2019 Plan shall not exceed 15,640.
Stock Options become vested upon fulfillment of either service vesting conditions, performance vesting conditions, or both, as determined by the award agreement entered into by the Company and optionee. The service vesting requirement states that: (i) 25% of the service vesting options shall vest on the first anniversary of the grant date and (ii) the remaining 75% shall vest on an equal monthly-basis, so long as the optionee has remained continuously employed by the Company from the date of the award through the fourth anniversary of the grant date. The performance vesting requirement states that Stock Options shall vest upon sale of the Company only if the optionee has been continuously employed by the Company or its subsidiaries from the grant date through the date of such sale of the Company. For the awards vesting based on service conditions only and that have a graded vesting schedule, the Company recognizes compensation expense for vested awards in earnings, net of actual forfeitures in the period they occur, on a straight-line basis over the requisite service period.
95
Table of Contents
Conversion of the Stock Options
In conjunction with the Business Combination, the Company amended and fully restated the 2019 Plan through the establishment of the 2021 Incentive Plan (“2021 Plan”). Pursuant to the 2021 Plan, each remaining legacy Stock Option from the 2019 Plan that was outstanding immediately prior to the Business Combination, whether vested or unvested, was converted into an option to purchase a number of shares of common stock (each such option, an “Exchanged Option”) equal to the product (rounded down to the nearest whole number) of (i) the number of shares of Legacy TOI stockholders subject to such Stock Option immediately prior to the Business Combination, and (ii) an exercise price per share equal to (A) the exercise price per share of such Stock Option immediately prior to the consummation of the Business Combination, divided by (B) the Common Stock Exchange Ratio ("Stock Option Exchange Ratio"). Following the Business Combination, each Exchanged Option that was previously subject to time vesting only, will continue to be governed by the same terms and conditions (including vesting and exercisability terms) as were applicable to the corresponding former old Stock Option immediately prior to the consummation of the Business Combination. Each Exchanged Option that was previously subject to performance vesting, will no longer be subject to the sale of the Company, and was modified to include service requirements only, under which, the Exchange Options will vest on a monthly-basis, so long as the optionee has remained continuously employed by the Company from the date of the Business Combination through the third anniversary of the Closing Date. The Company treated the Exchanged Options that were previously subject to performance conditions as a new award granted at the Closing Date. The Exchanged Options that were previously subject to service vesting only were not modified as a result of the Business Combination. All stock option activity was retroactively restated to reflect the Exchanged Options.
As of the Closing Date, the 11,850 Stock Options outstanding under the 2019 Plan were converted into 6,925,219 Exchanged Options after effect of the Common Stock Exchange Ratio. This effect of the Common Stock Exchange Ratio has been retroactively adjusted throughout the Company's consolidated financial statements.
As of December 31, 2022, the total number of shares of common stock remaining available for future awards (e.g., non-qualified stock options, incentive stock options, restricted stock units, restricted stock awards) under the 2021 Plan is 8,808,435.
The weighted average assumptions used in the Black-Scholes-Merton option-pricing model for the units granted during the years ended December 31, 2022 and 2021 Stock Options are provided in the following table:
| December 31, 2022 | December 31, 2021 | ||||||||||
| Valuation assumptions: | |||||||||||
| Expected dividend yield | —% | —% | |||||||||
| Expected volatility | 35.0% to 60.0% | 35.00% to 40.20% | |||||||||
| Risk-free interest rate | 2.33% to 3.87% | 0.76% to 1.30% | |||||||||
| Expected term (years) | 5.75 to 6.65 | 7.00 | |||||||||
The Company used the simplified method to calculate the expected term of stock option grants because sufficient historical exercise data was not available to provide a reasonable basis for the expected term. Under the simplified method, the expected term is estimated to be the mid-point between the vesting date and the contractual term of the option.
Stock option activity during the years ended December 31, 2022 and 2021 is as follows:
| Stock options | Number of shares | Weighted average exercise price | Weighted average remaining contractual term | Aggregate intrinsic value (in thousands) | ||||||||||||||||||||||
| Balance at January 1, 2022 | 6,921,180 | $ | 0.88 | |||||||||||||||||||||||
| Granted | 2,940,064 | 4.67 | ||||||||||||||||||||||||
Exercised | (973,389) | 0.90 | ||||||||||||||||||||||||
| Forfeited | (836,505) | 2.35 | ||||||||||||||||||||||||
| Expired | (1,876) | 0.97 | ||||||||||||||||||||||||
Balance at December 31, 2022 | 8,049,474 | $ | 2.14 | 7.64 | $ | 4,081 | ||||||||||||||||||||
Vested Options Exercisable at December 31, 2022 | 2,860,085 | $ | 1.34 | 6.90 | $ | 2,061 | ||||||||||||||||||||
96
Table of Contents
| Stock options | Number of shares | Weighted average exercise price | Weighted average remaining contractual term | Aggregate intrinsic value (in thousands) | ||||||||||||||||||||||
| Balance at January 1, 2021 | 8,683,952 | $ | 0.85 | |||||||||||||||||||||||
| Granted | 1,182,218 | 1.08 | ||||||||||||||||||||||||
| Exercised | (2,175,986) | 0.87 | ||||||||||||||||||||||||
| Forfeited | (769,004) | 0.87 | ||||||||||||||||||||||||
| Expired | — | — | ||||||||||||||||||||||||
Balance at December 31, 2021 | 6,921,180 | $ | 0.88 | 8.92 | $ | 61,379 | ||||||||||||||||||||
Vested Options Exercisable at December 31, 2021 | 1,821,909 | $ | 0.87 | 7.78 | $ | 16,185 | ||||||||||||||||||||
Total share-based compensation expense during the years ended December 31, 2022 and 2021 was $11,602 and $1,775, excluding costs associated with rolled over units and new units issued or replaced in connection with the Business Combination, respectively. In addition, pursuant to the Business Combination, the Company accelerated and settled in cash 3,724 Legacy TOI Stock Options in a total cash amount of $20,597. The Company recognized compensation expense in the amount of $19,953 related to the share-based compensation units that are subject to performance vesting conditions immediately prior to the Business Combination.
In June 2021, the Company and certain participants in the Plan entered into agreements to amend the terms of the Stock Options previously issued to the participants during the first two quarters of 2021. The amendment primarily related to updating the exercise price, vesting conditions, and the number of Stock Options. The modification to the Stock Options resulted in immaterial incremental share-based compensation expense recorded in the Company's statement of operations.
At December 31, 2022 there was $22,521 of total unrecognized compensation cost related to unvested service Stock Options granted under the 2021 Plan and 2019 Plan that are expected to vest. That cost is expected to be recognized over a weighted average period of 2.67 and 2.98 years for the years ended December 31, 2022 and 2021, respectively. During the year ended December 31, 2022, the Company received $858 in cash and $2,799 in tax benefit from the stock options exercised. The total fair value of common shares vested during the years ended December 31, 2022 and 2021 was $2,951 and $1,349, respectively.
Restricted Stock Awards (“RSAs”) and Restricted Stock Units (“RSUs”)
Agajanian Holdings (“Holdings”), a holder of Series A Preferred Stock of Legacy TOI, entered into arrangements with physicians employed by the TOI PCs to issue RSAs which represent Series A Preferred Stock of Legacy TOI. The Legacy TOI RSAs only have performance vesting requirements linked to the sale of the Company so long as the grantee remains continuously and actively employed by the Company’s subsidiaries through the vesting date.
Conversion of the RSAs
Each of the Legacy TOI RSAs, from the Plan that was outstanding immediately prior to the Business Combination, whether vested or unvested, was converted into an RSU equal to the product (rounded down to the nearest whole number) of (i) the number of shares of RSAs immediately prior to the Business Combination, (ii) conversion rate of 1:10 of the Series A Preferred Stock of Legacy TOI, and (iii) the Common Stock Exchange Ratio. Following the Business Combination, each RSU is no longer subject to the sale of the Company event in order to vest, but was modified to include service requirements only. The service vesting requirement states that: (i) 16.67% of the RSUs shall vest on the sixth month anniversary of the Closing Date, and (ii) the remaining 83.33% shall vest on an equal quarterly-basis, so long as the grantee has remained continuously employed by the Company from the date of the award through the third anniversary of the grant date. The Company treated the RSUs that were previously subject to performance conditions as a new award granted at the Closing Date. All RSAs activity was retroactively restated to reflect the RSUs.
As of the Closing Date, the 2,210 RSAs outstanding under the Plan were converted into 1,291,492 RSUs upon the completion of the Business Combination after effect of the Common Stock Exchange Ratio. This effect of the Common Stock Exchange Ratio has been retroactively adjusted throughout our consolidated financial statements.
The weighted-average grant date fair values of the RSUs granted during the year ended December 31, 2022 and 2021 were determined to be $5.74 and $10.98, respectively, based on the fair value of the Company’s common shares at the grant date.
97
Table of Contents
A summary of the activity for the RSUs and RSAs for the years ended December 31, 2022 and 2021, respectively, are shown in the following tables:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2022 | 2021 | |||||||||||||||||||||||||
| Number of Shares | Weighted Average Grant Date Fair Value | Number of Shares | Weighted Average Grant Date Fair Value | |||||||||||||||||||||||
| Unvested at beginning of year | 1,291,492 | $ | 10.98 | 1,390,839 | 10.98 | |||||||||||||||||||||
| Granted | 2,163,135 | 5.74 | — | — | ||||||||||||||||||||||
| Vested | (760,973) | 9.31 | — | — | ||||||||||||||||||||||
| Forfeited | (587,114) | 7.21 | (99,347) | 10.98 | ||||||||||||||||||||||
| Unvested at end of year | 2,106,540 | $ | 7.25 | 1,291,492 | $ | 10.98 | ||||||||||||||||||||
The total share-based compensation expense during the year ended December 31, 2022 was $8,284 related to the RSUs. The total share-based compensation expense during the period between the Closing Date and December 31, 2021 was $640 related to the RSUs.
As of December 31, 2022, there was $15,281 of unrecognized compensation expense related to the RSUs and RSAs that are expected to vest. That cost is expected to be recognized over a weighted average period of 2.40 years as of December 31, 2022. As of December 31, 2022, 64,331 RSU shares were net settled to cover the required withholding tax upon vesting.
RSUs granted to Medical Employees and Nonemployees
In 2022, the Company entered into arrangements with certain medical directors and supervisors of advanced practice providers employed by or engaged as independent contractors of TOI to issue RSUs of the Company (“Medical RSUs”). Vesting on each annual Medical RSU award is dependent on the participant performing a specified minimum number of service hours during the calendar year (“one-Year Term”) and further contingent upon the participant’s continued service to, or employment by, the Company through the grant date. The Company’s regular grant date for these Medical RSU awards is in the first quarter of the calendar year following the one-Year Term.
The number of Medical RSUs granted to each such participant is determined by dividing a fixed monetary value by the trailing five-day closing price per share of the Common Stock preceding the grant date. Due to the calculation, some Medical RSU awards are liability-classified whereas other Medical RSU awards have a fixed number of shares and are equity-classified.
In the fourth quarter of 2022, the Company amended the terms of Medical RSUs previously issued to approximately 21 participants during the first quarter of 2022. The amendment primarily updated the vesting period and conditions. The original terms of the Medical RSU awards were deemed improbable of vesting at the modification date whereas the amended Medical RSU awards were deemed probable of vesting at the modification date, and thus are a Type III modification under ASC 718. The modification to the Medical RSUs resulted in $187 incremental share-based compensation expense before forfeitures, $(11) after accounting for forfeitures related to participants who did not perform the minimum number of service hours specified, recorded in the Company's statement of operations.
The total fair value of the liability-classified Medical RSU awards granted in 2022 and outstanding as of December 31, 2022 was approximately $264, which represents the fixed monetary value of the awards. The weighted-average grant-date fair value, based on the Company’s share price on the modification date, was $3.56 for equity-classified Medical RSUs granted during 2022 and outstanding as of December 31, 2022
A summary of the activity for the equity-classified Medical RSUs for the year ended December 31, 2022 is shown in the following table:
| Number of Shares | |||||
| Balance at January 1, 2022 | — | ||||
| Granted | 208,881 | ||||
| Vested | — | ||||
| Forfeited | (61,411) | ||||
| Balance at December 31, 2022 | 147,470 | ||||
98
Table of Contents
Total compensation costs for Medical RSUs were $618 for the year-ended December 31, 2022. As of December 31, 2022, there was $278 of unrecognized compensation expense related to these Medical RSUs that are expected to vest. That cost is expected to be recognized over a weighted average period of 0.25 years as of December 31, 2022. As of December 31, 2022, none of the Medical RSUs have vested.
Earnout Shares granted to Employees
As described in Note 2, the Company issued Earnout Shares to Legacy TOI option holders and Legacy RSU holders (“Option-holders Earnout” and “RSU-holders Earnout”, respectively, together “Employees Earnout Shares”).
The Option-holders Earnout vests upon the Company common stock achieving the price per share as provided above, so long as the optionee has remained continuously employed by the Company at that date. The RSU-holders Earnout vests upon (a) the Company common stock achieving the price per share as provided above, and (b) the underlying RSU vested, so long as the optionee has remained continuously employed by the Company at that date.
The grant date fair value of the First Earnout Tranche and Second Earnout Tranche as of Closing Date was determined to be $8.35 and $6.76, respectively. The assumptions used in the Monte-Carlo Simulation model for the Earnout Shares granted on the Closing Date are provided in the following table:
| November 12, 2021 | |||||
| Valuation assumptions | |||||
| Expected dividend yield | — | % | |||
| Expected volatility | 35.00 | % | |||
| Risk-free interest rate | 0.85 | % | |||
A summary of the activity for the Employees Earnout Shares for the years ended December 31, 2022 and 2021 is shown in the following tables:
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Outstanding at beginning of year | 1,602,435 | — | ||||||||||||
| Granted | — | 1,603,322 | ||||||||||||
| Forfeited | (184,803) | (887) | ||||||||||||
| Outstanding at end of year | 1,417,632 | 1,602,435 | ||||||||||||
The total share-based compensation expense during the year ended December 31, 2022 was $7,911, related to the Employees Earnout Shares, respectively. The total share-based compensation expense during the period between the Closing Date and December 31, 2021 was $2,167.
As of December 31, 2022, there was $552 of unrecognized compensation expense related to the Employees Earnout Shares, that are expected to vest. That cost is expected to be recognized over a weighted average period of 0.05 years as of December 31, 2022. As of December 31, 2022, none of the Employee Earnout Shares have vested.
Note 15. Commitments and Contingencies
The Company evaluates contingencies based upon available evidence. In addition, allowances for losses are provided each year for disputed items which have continuing significance. The Company believes that allowances for losses have been provided to the extent necessary, and that its assessment of contingencies is reasonable. Due to the inherent uncertainties and subjectivity involved in accounting for contingencies, there is at least a reasonable possibility that recorded estimates will change by a material amount in the near term. To the extent that the resolution of contingencies results in amounts which vary from management’s estimates, future operating results will be charged or credited. The principal commitments and contingencies are described below.
Legal Matters
The Company is subject to certain outside claims and litigation arising in the ordinary course of business. In the opinion of Management, the outcome of such matters will not have a material effect on the Company’s consolidated financial statements. Loss contingencies entail uncertainty and a possibility of loss to an entity. If the loss is probable and the amount of loss can be
99
Table of Contents
reasonably estimated, the loss should be accrued according to Accounting Standards Codification No. 450-20, Disclosure of Certain Loss Contingencies. As of December 31, 2021, the Company settled and accrued for a loss contingency for a legal matter related to an employee lawsuit for $350, which was then paid in 2022.
Indemnities
The Company’s Articles of Incorporation and bylaws require it, among other things, to indemnify the director or officer against specified expenses and liabilities, such as attorneys’ fees, judgments, fines, and settlements, paid by the individual in connection with any action, suit, or proceeding arising out of the individual’s status or service as its director or officer, other than liabilities arising from willful misconduct or conduct that is knowingly fraudulent or deliberately dishonest, and to advance expenses incurred by the individual in connection with any proceeding against the individual with respect to which the individual may be entitled to indemnification by the Company. The Company also indemnifies its lessor in connection with its facility lease for certain claims arising from the use of the facilities. These indemnities do not provide for any limitation of the maximum potential future payments it could be obligated to make. Historically, the Company has not incurred any payments for these obligations and, therefore, no liabilities have been recorded for these indemnities in the accompanying consolidated balance sheets.
The Health Insurance Portability and Accountability Act
The Health Insurance Portability and Accountability Act (“HIPAA”) assures health insurance portability, reduces healthcare fraud and abuse, guarantees security and privacy of health information, and enforces standards for health information. Organizations are required to be in compliance with HIPAA provisions. The Health Information Technology for Economic and Clinical Health Act (“HITECH”) imposes notification requirements in the event of certain security breaches relating to protected health information. Organizations are subject to significant fines and penalties if found not to be compliant with the provisions outlined in the regulations. The Company believes it is in compliance with these laws.
Regulatory Matters
Laws and regulations governing the Medicare program and healthcare generally, are complex and subject to interpretation. The Company believes that it is in compliance with all applicable laws and regulations and is not aware of any pending or threatened investigations involving allegations of potential wrongdoing. While no regulatory inquiries have been made, compliance with such laws and regulations can be subject to future government review and interpretation as well as significant regulatory action including fines, penalties, and exclusion from the Medicare and Medi-Cal programs.
Many of the Company’s payor and provider contracts are complex in nature and may be subject to differing interpretations regarding amounts due for the provision of medical services. Such differing interpretations may not come to light until a substantial period of time has passed following contract implementation. Liabilities for claims disputes are recorded when the loss is probable and can be estimated. Any adjustments to reserves are reflected in current operations. The Company does not have any reserves for regulatory matters as of December 31, 2022 and December 31, 2021.
Liability Insurance
The Company believes that its insurance coverage is appropriate based upon the Company’s claims experience and the nature and risks of the Company’s business. In addition to the known incidents that have resulted in the assertion of claims, the Company cannot be certain that its insurance coverage will be adequate to cover liabilities, arising out of claims asserted against the Company or the Company’s affiliated professional organizations, in the future where the outcomes of such claims are unfavorable.
The Company believes that the ultimate resolution of all pending claims, including liabilities in excess of the Company’s insurance coverage, will not have a material adverse effect on the Company’s financial position, results of operations or cash flows; however, there can be no assurance that future claims will not have such a material adverse effect on the Company’s business. Contracted physicians are required to obtain their own insurance coverage.
Guarantees
The Company, along with certain of the Company's subsidiaries from time to time party to the Facility Agreement (“Guarantors”), has pledged a first priority perfected lien on substantially all of their respective personal and real property, as collateral security for the payment of outstanding obligations, under the Facility Agreement.
100
Table of Contents
Note 16. Business Combinations
During the year ended December 31, 2021, the Company merged with DFPH with the intent to raise capital and gain access to the public markets. Additionally, the Company closed on five business combinations and one asset acquisition, consistent with the intent to strategically grow its existing markets and expand into new markets. During the year ended December 31, 2022, the Company closed on five business combinations and one asset acquisition.
DFPH-Legacy TOI Merger
On June 28, 2021, DFPH, Orion Merger Sub I, Inc. ("First Merger Sub"), and Orion Merger Sub II, LLC ("Second Merger Sub") entered into an agreement and plan of merger ("Merger Agreement") with Legacy TOI to affect the Business Combination. In connection with the Business Combination, DFPH entered into subscription agreements with certain investors (the “PIPE Investors”), whereby it issued 17.5 million shares of common stock at $10.00 per share and 100,000 shares of preferred stock (collectively, the “PIPE Shares”) for an aggregate investment of $275,000 (“PIPE Investment”), which closed simultaneously with the consummation of the Business Combination.
On the Closing Date, (i) First Merger Sub merged with and into Legacy TOI, with Legacy TOI being the surviving corporation and (ii) immediately following, Legacy TOI merged with and into Second Merger Sub, with Second Merger Sub being the surviving entity and a wholly owned subsidiary of DFPH.
The total merger consideration on the Closing Date was $762,052, consisting of $595,468 in share consideration (consisting of 51.3 million shares of DFPH common stock issued to Legacy TOI at $10.00 per share as well as shares of DFPH common stock issuable in respect of restricted stock units and the exercise of Legacy TOI stock options), and $166,584 in cash. Gross proceeds from the transaction were $333,946. Of that, $167,510 was cash consideration to Legacy TOI equity holders. Legacy TOI also issued 12.5 million shares of common stock pursuant to the terms of an earnout (“Earnout Shares”). The earnout shares are allocable to both Legacy TOI stockholders and Legacy TOI option holders. In connection with the Business Combination, the Company incurred $39,914 of equity issuance costs, consisting of advisory, legal, deferred underwriting, share registration, and other professional fees, of which $6,769 was ascribed to the earnout liability and expensed with the remainder being netted against additional paid-in capital.
On the Closing Date, shares of DFPH common stock that were not otherwise redeemed as part of the DFPH public stockholder vote were automatically converted into shares of TOI common stock on a one-for-one basis. Further, PIPE Shares as well as DFPH common stock that was not otherwise forfeited or subject to earnout automatically converted into TOI common stock on a one-for-one basis. Additionally, holders of DFPH forfeited 555,791 Private Placement Warrants.
All periods prior to the Closing Date reflect the balances and activity of Legacy TOI. The consolidated balances as of December 31, 2020 from the audited consolidated financial statements of Legacy TOI as of that date, share activity (convertible redeemable preferred stock and common stock) and per share amounts in these Consolidated Statements of Convertible Preferred Stock and Changes in Stockholders' Equity were retroactively adjusted, where applicable, using the recapitalization exchange ratio of 591:1. All previously issued and outstanding shares of Legacy TOI preferred stock classified as mezzanine equity were converted into Legacy TOI common stock and was retroactively adjusted and reclassified to permanent equity as a result of the reverse recapitalization. As a result of the Business Combination, $142,557 of additional paid-in capital was recognized.
Practice Acquisitions
For the acquisition of various clinical practices, the Company applied the acquisition method of accounting, where the total purchase price was allocated, or preliminarily allocated, to the tangible and intangible assets acquired and liabilities assumed, based on their fair values as of the acquisition dates.
Raiker Practice Acquisition
On February 12, 2021 ("Raiker Acquisition Date"), the Company entered into an asset purchase agreement and master services agreement ("Raiker MSA") with Anil N Raiker, M.D., P.L.C., d/b/a Pinellas Cancer Center (the "Raiker Practice" or "PCC") and Anil Raiker, M.D., an individual. Pursuant to the asset purchase agreement, the Company purchased from PCC certain non-clinical assets, properties, and rights. Pursuant to the Raiker MSA, TOI Management established an ongoing management services agreement which grants TOI Management the right to control the non-clinical and management operations of the Raiker Practice. Anil Raiker, M.D. continued to own all of the issued and outstanding equity interests of the Raiker Practice.
101
Table of Contents
Pursuant to the Raiker MSA, and as further described in Note 17, TOI Management became the Raiker Practice's primary beneficiary and thus consolidated the Raiker Practice and its subsidiaries. The consolidation of the Raiker Practice (the "Raiker Practice Acquisition") at the Raiker Acquisition Date constituted a business combination in accordance with ASC 805.
The total consideration for the Acquisition was $1,710, comprised of a cash payment of $892 and deferred consideration of $818. The deferred cash consideration is to be paid in two equal installments on the first and second anniversary of the Raiker Acquisition Date (February 12, 2022 and 2023, respectively). On February 12, 2022, the Company transferred the first installment of deferred consideration of $409. Considering the Company's incremental borrowing rate, the present value of the deferred cash consideration is not materially different than its stated value.
Subsequent to the Acquisition, the Company filed an amendment to the articles of incorporation of PCC to legally change the name to The Oncology Institute FL, LLC. The change was solely nominal, and the legal form, tax attributes, and books and records of PCC all remained.
Grant Practice Acquisition
On November 12, 2021 ("Grant Acquisition Date"), the Company acquired certain non-clinical assets of Ellsworth Grant, M.D., A Medical Corporation (the “Grant Practice”) from Ellsworth Grant, M.D. (“Dr. Grant”). Further, TOI CA acquired certain clinical assets of the Grant Practice from Dr. Grant. Intangible assets of $450 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years The Company transferred cash consideration of $849 and contingent consideration of $200 to Dr. Grant for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the Grant Acquisition Date (November 12, 2022 and 2023, respectively), pending Dr. Grant's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Grant. As of December 31, 2022, the first installment of the contingent cash consideration was paid to Dr. Grant.
The Grant Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Orr Practice Acquisition
On November 12, 2021 ("Orr Acquisition Date"), the Company acquired certain non-clinical assets of Leo E. Orr, M.D., Inc. (the “Orr Practice”) from Leo E. Orr, M.D. (“Dr. Orr”). Further, TOI CA acquired certain clinical assets of the Orr Practice from Dr. Orr. Intangible assets of $150 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years. The Company transferred cash consideration of $816 and contingent consideration of $200 to Dr. Orr for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the Orr Acquisition Date (November 12, 2022 and 2023, respectively), pending Dr. Orr's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Orr. As of December 31, 2022, the first installment of the contingent cash consideration was paid to Dr. Orr.
The Orr Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Dave Practice Acquisition
On November 19, 2021 ("Dave Acquisition Date"), the Company acquired certain non-clinical assets of Sulaba Dave M.D., d.b.a. Radiation Oncology Associates (the “Dave Practice”) from Sulaba Dave M.D. (the “Dr. Dave”). Further, TOI CA acquired certain clinical assets of the Dave Practice from Dr. Dave. Intangible assets of $77 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 2 years. The Company transferred cash consideration of $2,000 and contingent consideration of $750 to Dr. Dave for the purchase. The contingent cash consideration is to be paid in three equal installments on the six, twelfth, and eighteen month anniversaries of the Dave Acquisition Date (May 19, 2022, November 19, 2022, and May 19, 2023, respectively), pending Dr. Dave's continued employment and certain patient metrics being met at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Dave. As of December 31, 2022, the first two installments of the contingent cash consideration was paid to Dr. Dave.
The Dave Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Yang Practice Acquisition
On December 9, 2021 ("Yang Acquisition Date"), the Company, acquired certain non-clinical assets of Global Oncology, Inc. (the “Yang Practice”) from Dr. Honghao Yang M.D. (“Dr. Yang”). Further, TOI CA acquired certain clinical assets of the Practice from Dr. Yang. Intangible assets of $68 were recognized pursuant to the acquisition in the form of clinical contracts
102
Table of Contents
with a weighted average amortization period of 5 years. The Company transferred cash consideration of $4,615 and contingent consideration of $2,500 to Dr. Yang for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the Yang Acquisition Date (December 9, 2022 and 2023, respectively), pending Dr. Yang's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Yang. As of December 31, 2022, the first installment of the contingent cash consideration was paid to Dr. Yang.
The Yang Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Perkins Practice Acquisition
On April 30, 2022 ("Perkins Acquisition Date"), the Company acquired certain non-clinical assets of California Oncology of the Central Valley Medical Group, Inc., (the “Perkins Practice”) from Christopher Perkins, M.D. (“Dr. Perkins”). Further, TOI CA acquired certain clinical assets of the Perkins Practice from Dr. Perkins. In conjunction with the acquisition, the Company also entered into a Professional Service Agreement with Oncology Associates of Fresno Medical Group, Inc. Intangible assets were recognized pursuant to the acquisition in the form of trade names of $2,480 and clinical contracts of $70, with weighted average amortization periods of 10 years and 5 years respectively. The Company transferred cash consideration of $8,920 and contingent consideration of $2,000 to Dr. Perkins for the purchase. The contingent cash consideration was to be paid in two equal installments on the first and second anniversary of the transaction closing date (April 29, 2023 and 2024, respectively), pending Dr. Perkins' continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Perkins. The contingent consideration is accounted for as post-combination compensation expense to Dr. Perkins. As of December 31, 2022, Dr. Perkins is no longer employed by TOI and therefore, no contingent consideration will be paid thereby reducing compensation expense in subsequent periods.
The Perkins Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Parikh Practice Acquisition
On July 22, 2022 ("Parikh Acquisition Date"), the Company acquired certain non-clinical assets of Nutan K. Parikh, M.D., LTD., (the “Parikh Practice”) from Nutan K. Parikh, M.D. (“Dr. Parikh”). Further, TOI CA acquired certain clinical assets of the Parikh Practice from Dr. Parikh. Intangible assets of $20 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 3 years. The Company transferred cash consideration of $1,908 and contingent consideration of $400 to Dr. Parikh for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the transaction closing date (July 22, 2023 and 2024, respectively), pending Dr. Parikh's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Parikh.
The Parikh Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Barreras Practice Acquisition
On August 30, 2022 ("Barreras Acquisition Date"), the Company acquired certain non-clinical assets of Broward Oncology Associates, P.A., (the “Barreras Practice”) from Luis Barreras, M.D. (“Dr. Barreras”). Further, TOI FL acquired certain clinical assets of the Barreras Practice from Dr. Barreras. Intangible assets of $3 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years. The Company transferred cash consideration of $929 and contingent consideration of $250 to Dr. Barreras for the purchase. The contingent cash consideration is to be paid in two equal installments on the first and second anniversary of the transaction closing date (August 30, 2023 and 2024, respectively), pending Dr. Barreras's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Barreras.
The Barreras Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
De La Rosa Costa Practice Acquisition
On October 7, 2022 ("De La Rosa Costa Acquisition Date"), the Company acquired certain non-clinical assets of Pedro De La Rosa Costa, M.D. PA, (the “De La Rosa Costa Practice”) from Pedro U De La Rosa Costa, M.D. (“Dr. De La Rosa Costa”). Further, TOI FL acquired certain clinical assets of the De La Rosa Costa Practice from Dr. De La Rosa Costa. The Company transferred cash consideration of $25 to Dr. De La Rosa Costa for the purchase.
The De La Rosa Costa Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
103
Table of Contents
Hashimi Practice Acquisition
On November 21, 2022 ("Hashimi Acquisition Date"), the Company acquired certain non-clinical assets of Intercommunity Oncology of Chino Hills, A.P.C., Inc., (the “Hashimi Practice”) from Labib Hashimi, M.D. (“Dr. Hashimi”). Further, TOI CA acquired certain clinical assets of the Hashimi Practice from Dr. Hashimi. Intangible assets of $24 were recognized pursuant to the acquisition in the form of clinical contracts with a weighted average amortization period of 5 years. The Company transferred cash consideration of $445 and contingent consideration of $150 to Dr. Hashimi for the purchase. The contingent cash consideration is to be paid in three equal installments on the first, second, and third anniversary of the transaction closing date (November 21, 2023, 2024, and 2025, respectively), pending Dr. Hashimi's continued employment at that time. The contingent consideration is accounted for as post-combination compensation expense to Dr. Hashimi.
The Hashimi Practice Acquisition was determined to constitute a business combination in accordance with ASC 805.
Summary of Consideration Transferred
Goodwill is calculated as the excess of the consideration transferred over the net assets recognized and represents the estimated future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Such assets include synergies we expect to achieve, such as the use of our existing infrastructure to support the added membership, and future economic benefits arising from the assembled workforce. The purchase consideration for the acquisitions has been allocated under the acquisition method of accounting to the estimated fair market value of the net assets acquired including a residual amount of tax deductible goodwill as noted in the provisional fair value table below.
Acquisition costs amounted to $790 and $476 for the years ended December 31, 2022 and 2021 respectively, and were recorded as “General and administrative expenses” in the accompanying Consolidated Statements of Operations.
The following table summarizes the provisional fair values assigned to identifiable assets acquired and liabilities assumed.
| Acquisition | |||||||||||||||||||||||||||||||||||
| (in thousands) | Raiker | Grant | Orr | Dave | Yang | Perkins | Parikh | Barreras | De La Rosa Costa | Hashimi | Total | ||||||||||||||||||||||||
| Consideration: | |||||||||||||||||||||||||||||||||||
| Cash | $ | 892 | $ | 849 | $ | 816 | $ | 2,000 | $ | 4,615 | $ | 8,920 | $ | 1,908 | $ | 929 | $ | 25 | $ | 445 | $ | 21,399 | |||||||||||||
| Deferred | 818 | — | — | — | — | — | — | — | — | — | 818 | ||||||||||||||||||||||||
| Fair value of total consideration transferred | $ | 1,710 | $ | 849 | $ | 816 | $ | 2,000 | $ | 4,615 | $ | 8,920 | $ | 1,908 | $ | 929 | $ | 25 | $ | 445 | $ | 22,217 | |||||||||||||
| Estimated fair value of identifiable assets acquired and liabilities assumed: | |||||||||||||||||||||||||||||||||||
| Cash | $ | 65 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 65 | |||||||||||||
| Accounts receivable | 398 | — | 183 | — | — | — | — | — | — | — | 581 | ||||||||||||||||||||||||
| Inventory | 62 | 49 | 16 | — | 115 | 408 | 307 | 279 | — | 95 | 1,331 | ||||||||||||||||||||||||
| Property and equipment, net | — | — | 13 | 35 | 19 | 123 | 15 | 23 | — | 5 | 233 | ||||||||||||||||||||||||
| Operating right of use assets | — | — | — | — | — | 447 | 1,118 | 83 | 6 | 88 | 1,742 | ||||||||||||||||||||||||
| Clinical contracts and noncompetes | — | 450 | 150 | 77 | 68 | 70 | 20 | 3 | — | 24 | 862 | ||||||||||||||||||||||||
| Trade names | — | — | — | — | — | 2,480 | — | — | — | — | 2,480 | ||||||||||||||||||||||||
| Goodwill | 1,454 | 350 | 637 | 1,895 | 4,413 | 5,851 | 1,566 | 624 | 25 | 321 | 17,136 | ||||||||||||||||||||||||
| Total assets acquired | 1,979 | 849 | 999 | 2,007 | 4,615 | 9,379 | 3,026 | 1,012 | 31 | 533 | 24,430 | ||||||||||||||||||||||||
| Accounts payable | 120 | — | — | — | — | — | — | — | — | — | 120 | ||||||||||||||||||||||||
| Current portion of operating lease liabilities | — | — | — | — | — | 135 | 169 | 60 | 6 | 26 | 396 | ||||||||||||||||||||||||
| Accrued liabilities | — | — | — | 7 | — | 12 | — | — | — | — | 19 | ||||||||||||||||||||||||
| Current portion of long term debt | 149 | — | 183 | — | — | — | — | — | — | — | 332 | ||||||||||||||||||||||||
| Operating lease liabilities | — | — | — | — | — | 312 | 949 | 23 | — | 62 | 1,346 | ||||||||||||||||||||||||
| Total liabilities assumed | 269 | — | 183 | 7 | — | 459 | 1,118 | 83 | 6 | 88 | 2,213 | ||||||||||||||||||||||||
| Net assets acquired | $ | 1,710 | $ | 849 | $ | 816 | $ | 2,000 | $ | 4,615 | $ | 8,920 | $ | 1,908 | $ | 929 | $ | 25 | $ | 445 | $ | 22,217 | |||||||||||||
The establishment of the allocation to goodwill requires the extensive use of accounting estimates and management judgement. The fair values assigned to the assets acquired are based on estimates and assumptions from data that is readily available.
104
Table of Contents
Summary of Unaudited Supplemental Pro Forma Information
The Company recognized $12,981 cumulative revenue and $5 cumulative net loss in its Consolidated Statement of Operations for the year ended December 31, 2022, from the clinical practices acquired during the year ended December 31, 2022.
The pro forma results presented below include the effects of the Acquisitions which occurred during the year ended December 31, 2022, as if they had occurred on January 1, 2021. The pro forma results for the year ended December 31, 2022 and 2021 include the additional amortization resulting from the adjustments to the value of intangible assets resulting from purchase accounting. The pro forma results do not include any anticipated synergies or other expected benefits of the acquisitions. The pro forma information does not purport to be indicative of what the Company's results of operations would have been if the acquisitions had in fact occurred at the beginning of the period presented and is not intended to be a projection of the Company's future results of operations. Transaction expenses are included within the pro forma results.
| (in thousands) | Year Ended December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Revenue | $ | 266,230 | $ | 234,053 | ||||||||||
| Net income (loss) | $ | 2,140 | $ | (6,030) | ||||||||||
Mendez Asset Acquisition
On May 1, 2021, TOI Management, through PCC, entered into a purchase agreement to acquire certain clinical assets from Oncology Association, P.A. ("OA") from Pedro Mendez, M.D. Management determined the acquisition of OA is an asset acquisition. The Company paid $500, consisting of cash consideration of $200 and deferred cash consideration of $300, in exchange for intangible assets in the form of payor contracts. The entire $500 was assigned to the payor contract intangible asset class with a weighted average amortization period of 10 years. The deferred cash consideration is to be paid in three equal installments on the first, second, and third anniversaries of the Mendez Asset Acquisition Date (May 1, 2022, May 1, 2023, and May 1, 2024, respectively). On May 1, 2022, the Company transferred the first installment of deferred consideration of $100. Considering the Company's incremental borrowing rate, the present value of the deferred cash consideration is not materially different than its stated value.
Sapra Asset Acquisition
On July 1, 2022 ("Sapra Acquisition Date"), the Company acquired certain clinical assets of Ranjan K. Sapra, M.D. (the “Sapra Practice”) from Ranjan K. Sapra, M.D. (“Dr. Sapra”). The Company transferred cash consideration of $1 to Dr. Sapra for the purchase, which was assigned to property and equipment.
Note 17. Variable Interest Entities
The Company prepares its consolidated financial statements in accordance with Accounting Standards Codification Topic No. 810, Consolidations (“ASC 810”), which provides for the consolidation of VIEs of which an entity is the primary beneficiary.
Pursuant to the MSAs established with the TOI PCs, TOI Management is entitled to receive a management fee, which represents a variable interest in and the right to receive the benefits of the TOI PCs. Through the terms of the MSAs, TOI Management receives the right to direct the most significant activities of the TOI PCs. Therefore, the TOI PCs are variable interest entities and TOI Management is the primary beneficiary that consolidates the TOI PCs, and their subsidiaries.
The consolidated financial statements include the accounts of TOI and its subsidiaries and VIEs. All inter-company profits, transactions, and balances have been eliminated upon consolidation.
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and restricted cash | $ | 1,070 | $ | 1,618 | |||||||
| Accounts receivable | 39,817 | 20,007 | |||||||||
| Other receivables | 220 | 935 | |||||||||
| Inventories, net | 9,262 | 6,438 | |||||||||
105
Table of Contents
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Prepaid expenses | 841 | 781 | |||||||||
| Total current assets | 51,210 | 29,779 | |||||||||
| Property and equipment, net | 168 | — | |||||||||
| Other assets | 441 | 276 | |||||||||
| Intangible assets, net | 3,343 | 1,181 | |||||||||
| Goodwill | 15,832 | 11,096 | |||||||||
| Total assets | $ | 70,994 | $ | 42,332 | |||||||
| Liabilities | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 8,296 | $ | 14,204 | |||||||
| Income taxes payable | 132 | 132 | |||||||||
| Accrued expenses and other current liabilities | 5,129 | 5,539 | |||||||||
| Current portion of long-term debt | — | 183 | |||||||||
| Amounts due to affiliates | 140,218 | 56,312 | |||||||||
| Total current liabilities | 153,775 | 76,370 | |||||||||
| Other non-current liabilities | 739 | 3,203 | |||||||||
| Deferred income taxes liability | 58 | 6 | |||||||||
| Total liabilities | $ | 154,572 | $ | 79,579 | |||||||
Single physician holders, who are officers of the Company, retain equity ownership in TOI CA, TOI FL and TOI TX, which represents nominal noncontrolling interests. The noncontrolling interests do not participate in the profit or loss of TOI CA, TOI FL, or TOI TX, however.
Note 18. Goodwill and Intangible Assets
The Company accounts for goodwill at acquisition-date fair value and other intangible assets at acquisition-date fair value less accumulated amortization. See Note 2 for a summary of the Company’s policies relating to goodwill and intangible assets, as well as a discussion of the goodwill impairment charges recorded for the year ended December 31, 2022.
Intangible Assets
As of December 31, 2022, the Company’s intangible assets, net consists of the following:
| (in thousands) | Weighted average amortization period | Gross carrying amount | Accumulated amortization | Net carrying amount | |||||||||||||||||||
| Intangible assets | |||||||||||||||||||||||
| Amortizing intangible assets: | |||||||||||||||||||||||
| Payor contracts | 10 years | $ | 19,400 | $ | (8,038) | $ | 11,362 | ||||||||||||||||
| Trade names | 10 years | 6,650 | (1,941) | 4,709 | |||||||||||||||||||
| Clinical contracts and noncompetes | 8 years | 3,025 | (1,139) | 1,886 | |||||||||||||||||||
| Total intangible assets | $ | 29,075 | $ | (11,118) | $ | 17,957 | |||||||||||||||||
106
Table of Contents
As of December 31, 2021, the Company’s intangible assets, net consists of the following:
| (in thousands) | Weighted average amortization period | Gross carrying amount | Accumulated amortization | Net carrying amount | |||||||||||||||||||
| Intangible assets | |||||||||||||||||||||||
| Amortizing intangible assets: | |||||||||||||||||||||||
| Payor contracts | 10 years | $ | 19,400 | $ | (6,152) | $ | 13,248 | ||||||||||||||||
| Trade names | 10 years | 4,170 | (1,350) | 2,820 | |||||||||||||||||||
| Clinical contracts and noncompetes | 10 years | 2,909 | (732) | 2,177 | |||||||||||||||||||
| Total intangible assets | $ | 26,479 | $ | (8,234) | $ | 18,245 | |||||||||||||||||
The estimated aggregate amortization expense for each of the five succeeding fiscal years as of December 31, 2022 is as follows:
| (in thousands) | Amount | ||||
| Year ending December 31: | |||||
| 2023 | $ | 2,913 | |||
| 2024 | 2,913 | ||||
| 2025 | 2,909 | ||||
| 2026 | 2,884 | ||||
| 2027 | 2,757 | ||||
| Thereafter | 3,581 | ||||
| Total | $ | 17,957 | |||
The aggregate amortization expense during the year ended December 31, 2022 and 2021 were $2,885 and $2,515, respectively.
Goodwill
The Company evaluates goodwill at the reporting unit level, which, for the Company, is at the level of the reportable segments of patient services, dispensary, and clinical trials & other. The goodwill allocated to each of the reporting units as of December 31, 2022 and December 31, 2021 is as follows:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Patient services | $ | 16,235 | $ | 21,443 | |||||||
| Dispensary | 4,551 | 4,551 | |||||||||
| Clinical trials & other | 632 | 632 | |||||||||
| Total goodwill | $ | 21,418 | $ | 26,626 | |||||||
The changes in the carrying amounts of goodwill for the year ended December 31, 2022 and for the year ended December 31, 2021 are as follows:
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Balance as of January 1: | $ | 26,626 | $ | 14,227 | |||||||
| Goodwill acquired | 4,736 | 12,399 | |||||||||
| Goodwill impairment charges (see Note 2) | (9,944) | — | |||||||||
| Goodwill, net as of December 31 | $ | 21,418 | $ | 26,626 | |||||||
107
Table of Contents
Note 19. Net Income (Loss) Per Share
The following table sets forth the computation of the Company's basic net income (loss) per share to common stockholders for the years ended December 31, 2022 and 2021.
| (in thousands, except share data) | Year Ended December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Net income (loss) attributable to TOI | $ | 152 | $ | (10,927) | ||||||||||
| Less: Deemed dividend | 64 | — | ||||||||||||
| Net income (loss) attributable to TOI available for distribution | 88 | (10,927) | ||||||||||||
| Net income (loss) attributable to participating securities, basic | 20 | (299) | ||||||||||||
| Net income (loss) attributable to common stockholders, basic | $ | 68 | $ | (10,628) | ||||||||||
| Weighted average common shares outstanding, basic | 72,793,497 | 66,230,606 | ||||||||||||
| Net income (loss) per share attributable to common stockholders, basic | $ | — | $ | (0.16) | ||||||||||
The following table sets forth the computation of the Company's diluted net loss per share to common stockholders for the years ended December 31, 2022 and 2021.
| (in thousands, except share data) | Year Ended December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Net income (loss) attributable to TOI | $ | 152 | $ | (10,927) | ||||||||||
| Less: Deemed dividend | 64 | — | ||||||||||||
Less: Change in fair value of convertible option derivative liabilities(1) | 20,656 | — | ||||||||||||
| Net loss attributable to TOI available for distribution | (20,568) | (10,927) | ||||||||||||
| Net loss attributable to participating securities, diluted | (3,588) | (299) | ||||||||||||
| Net loss attributable to common stockholders, diluted | $ | (16,980) | $ | (10,628) | ||||||||||
| Weighted average common shares outstanding, basic | 72,793,497 | 66,230,606 | ||||||||||||
| Dilutive effect of stock options | 2,572,570 | — | ||||||||||||
| Dilutive effect of RSUs | 77,717 | — | ||||||||||||
| Dilutive effect of Medical RSUs | 61,007 | — | ||||||||||||
| Dilutive effect of convertible note | 5,100,809 | — | ||||||||||||
| Weighted average shares outstanding, diluted | 80,605,600 | 66,230,606 | ||||||||||||
| Net loss per share attributable to common stockholders, diluted | $ | (0.21) | $ | (0.16) | ||||||||||
(1) Inclusive of interest expense and amortization of debt issuance cost and debt discount related to the Senior Secured Convertible Note.
The following potentially dilutive outstanding securities were excluded from the computation of diluted net loss per share because their effect would have been anti-dilutive for the periods presented:
| Year Ended December 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Stock options | 4,461,592 | 6,921,180 | ||||||||||||
| RSUs | 1,677,516 | 1,291,492 | ||||||||||||
| Medical RSUs | 301,396 | — | ||||||||||||
| Earnout Shares | 1,417,632 | 1,602,435 | ||||||||||||
| Public Warrants | 5,749,986 | 5,749,986 | ||||||||||||
| Private Warrants | 3,177,542 | 3,177,542 | ||||||||||||
108
Table of Contents
Note 20. Segment Information
The Company operates its business and reports its results through three operating and reportable segments: dispensary, patient services, and clinical trials & other in accordance with ASC 280. See Note 2 for a summary of the Company’s policy on segment information.
Summarized financial information for the Company’s segments is shown in the following tables:
| (in thousands) | Year Ended December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Revenue | ||||||||||||||
| Patient services | $ | 166,785 | $ | 124,074 | ||||||||||
| Dispensary | 79,343 | 72,550 | ||||||||||||
| Clinical trials & other | 6,355 | 6,379 | ||||||||||||
| Consolidated revenue | 252,483 | 203,003 | ||||||||||||
| Direct costs | ||||||||||||||
| Patient services | 134,761 | 99,401 | ||||||||||||
| Dispensary | 65,111 | 62,102 | ||||||||||||
| Clinical trials & other | 518 | 652 | ||||||||||||
| Total segment direct costs | 200,390 | 162,155 | ||||||||||||
| Depreciation expense | ||||||||||||||
| Patient services | 1,202 | 659 | ||||||||||||
| Dispensary | 4 | 1 | ||||||||||||
| Clinical trials & other | 1 | 123 | ||||||||||||
| Total segment depreciation expense | 1,207 | 783 | ||||||||||||
| Amortization of intangible assets | ||||||||||||||
| Patient services | 2,675 | 2,305 | ||||||||||||
| Dispensary | — | — | ||||||||||||
| Clinical trials & other | 211 | 211 | ||||||||||||
| Total segment amortization | 2,886 | 2,516 | ||||||||||||
| Operating income | ||||||||||||||
| Patient services | 28,147 | 21,709 | ||||||||||||
| Dispensary | 14,228 | 10,447 | ||||||||||||
| Clinical trials & other | 5,625 | 5,393 | ||||||||||||
| Total segment operating income | 48,000 | 37,549 | ||||||||||||
| Goodwill impairment charges | ||||||||||||||
| Patient services | 9,944 | — | ||||||||||||
| Dispensary | — | — | ||||||||||||
| Clinical trials & other | — | — | ||||||||||||
| Total impairment charges | 9,944 | — | ||||||||||||
| Selling, general and administrative expense | 119,689 | 83,365 | ||||||||||||
| Non-segment depreciation and amortization | 318 | 42 | ||||||||||||
| Total consolidated operating loss | $ | (81,951) | $ | (45,858) | ||||||||||
109
Table of Contents
| (in thousands) | December 31, 2022 | December 31, 2021 | |||||||||
| Assets | |||||||||||
| Patient services | $ | 64,869 | $ | 44,223 | |||||||
| Dispensary | 7,194 | 4,277 | |||||||||
| Clinical trials & other | 11,496 | 14,504 | |||||||||
| Non-segment assets | 178,106 | 140,435 | |||||||||
| Total assets | $ | 261,665 | $ | 203,439 | |||||||
Note 21. Related Party Transactions
Related party transactions include payments for consulting services provided to the Company, clinical trials, board fees, and share repurchases. Related party payments for the years ended December 31, 2022 and 2021 were as follows:
| (in thousands) | Year Ended December 31, | ||||||||||||||||
| Type | 2022 | 2021 | |||||||||||||||
| American Institute of Research | Consulting | $ | 100 | $ | 152 | ||||||||||||
| Karen M Johnson | Board Fees | 56 | — | ||||||||||||||
| Richard Barasch | Board Fees | 12 | — | ||||||||||||||
| Anne M. McGeorge | Board Fees | 44 | — | ||||||||||||||
| Mohit Kaushal | Board Fees | 57 | — | ||||||||||||||
| Ravi Sarin | Board Fees | 57 | — | ||||||||||||||
| Maeve O'Meara Duke | Board Fees | 57 | — | ||||||||||||||
| Havencrest Capital Management, LLC | Management Fees | — | 166 | ||||||||||||||
| M33 Growth LLC | Management Fees | — | 353 | ||||||||||||||
Richy Agajanian MD(1) | Share Repurchase | 8,745 | — | ||||||||||||||
| Richy Agajanian MD | Clinical Trials | 22 | 21 | ||||||||||||||
| Veeral Desai | Board Fees | — | 52 | ||||||||||||||
| Total | $ | 9,150 | $ | 744 | |||||||||||||
(1) Net of strike price.
110
Table of Contents
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures are designed to ensure that the information relating to our Company, including our consolidated subsidiaries, that are required to be disclosed in our Securities and Exchange Commission ("SEC") reports, is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow for timely decisions regarding required disclosure. We conducted an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2022. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2022, our disclosure controls and procedures were not effective due to the material weakness in internal control over financial reporting described below. Notwithstanding the identified material weakness, management, including our Chief Executive Officer and Chief Financial Officer, who serve as our principal executive officer and principal financial officer, respectively, believes the consolidated financial statements included in this Annual Report on Form 10-K fairly represent, in all material respects, our financial condition, results of operations and cash flows as of and for the periods presented in accordance with U.S. Generally Accepted Accounting Principles.
Management's Annual Report on Internal Controls Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Management has evaluated the effectiveness of our internal controls over financial reporting based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result of this assessment, management has concluded that, as of December 31, 2022, our internal controls over financial reporting was not effective due to the material weakness in internal controls over financial reporting described below.
Material Weakness in Internal Controls Over Financial Reporting
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
As of December 31, 2022, we have identified deficiencies in our control environment. These deficiencies include a material weakness related to internal controls over review of complex accounting transactions. We previously disclosed this material weakness in our Annual Report on Form 10-K for the year ended December 31, 2021 as well as in our Quarterly Reports on Form 10-Q for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022.
During 2022, our management continued to execute against the remediation plan under the oversight of the Audit Committee. This involved hiring and training additional qualified personnel, performed detailed risk assessments in key process areas to identify risks of material misstatement, further documented and implemented control procedures to address the identified risks of material misstatements, and implemented monitoring activities over such control procedures.
Remediation of Previously Reported Material Weaknesses in Internal Control over Financial Reporting
As previously reported in our Annual Report on Form 10-K for the year ended December 31, 2021, material weaknesses in our internal controls existed relating to: (i) segregation of duties in the financial closing and reporting process and (ii) internal control over reviews of revenue process. During the year ended December 31, 2022, we remediated these material weaknesses as follows:
•Added resources with technical expertise in designing and testing internal controls
•We hired outside experts to assist with the preparation and review of the financial statement close process
•We re-designed controls and processes to ensure proper written documentation existed and
•The design of controls were reviewed and updated to ensure that there was a preparer of the control and a separate reviewer of the control
111
Table of Contents
During the quarter ended December 31, 2022, we completed the testing of the controls described above and remediated the material weaknesses.
Attestation Report of the Independent Registered Public Accounting Firm
As an emerging growth company, we are not required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm in this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
Except for the remediation of the material weaknesses noted above, there were no changes in the Company’s internal control over financial reporting that occurred during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Limitations on Effectiveness of Disclosure Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management, including the Chief Executive Officer and Chief Financial Officer, recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdiction that Prevent Inspections.
Not applicable.
112
Table of Contents
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2023 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2022.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2023 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2022.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2023 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2022.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2023 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2022.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated herein by reference to our definitive proxy statement for our 2023 Annual Meeting of Stockholders, or Proxy Statement, to be filed with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2022.
113
Table of Contents
PART IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this Report:
(1) Financial Statements: All financial statement schedules are filed as part of this report under Item 8 - Financial Statements and Supplementary Data. See Index to Consolidated Financial Statements on Page 60 of this Annual Report.
(2) Financial Statement Schedules: No financial statement schedules are included in this Annual Report as such schedules are not required or the information that would be included in such schedules is not material or is otherwise furnished.
(3) Exhibits: See Index to Exhibits below.
| Incorporated by Reference | Filed or Furnished Herewith | |||||||||||||||||||||||||||||||||||||
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date | |||||||||||||||||||||||||||||||||
| 2.1 | S-4/A | 333-258152 | 2.1 | October 20, 2021 | ||||||||||||||||||||||||||||||||||
| 3.1 | 8-K | 001-39248 | 3.1 | November 18, 2021 | ||||||||||||||||||||||||||||||||||
| 3.2 | 8-K | 001-39248 | 3.2 | November 18, 2021 | ||||||||||||||||||||||||||||||||||
| 3.3 | 8-K/A | 001-39248 | 3.3 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 4.1 | 8-K | 001-39248 | 4.1 | March 13, 2020 | ||||||||||||||||||||||||||||||||||
| 4.2 | 8-K/A | 001-39248 | 4.2 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 4.3 | 8-K | 001-39248 | 4.1 | August 10, 2022 | ||||||||||||||||||||||||||||||||||
| 4.4 | 8-K | 001-39248 | 4.2 | August 10, 2022 | ||||||||||||||||||||||||||||||||||
| 10.1 | S-4/A | 333-258152 | 10.1 | October 1, 2021 | ||||||||||||||||||||||||||||||||||
| 10.2 | S-4/A | 333-258152 | 10.2 | October 1, 2021 | ||||||||||||||||||||||||||||||||||
| 10.3 | 8-K/A | 001-39248 | 10.1 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 10.4* | 8-K/A | 001-39248 | 10.2 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 10.5* | 8-K/A | 001-39248 | 10.3 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 10.6 | 8-K/A | 001-39248 | 10.5 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 10.7 | 8-K/A | 001-39248 | 10.6 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
| 10.8* | 8-K/A | 001-39248 | 10.7 | November 22, 2021 | ||||||||||||||||||||||||||||||||||
114
Table of Contents
| Incorporated by Reference | Filed or Furnished Herewith | |||||||||||||||||||||||||||||||||||||
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date | |||||||||||||||||||||||||||||||||
| 10.9* | 8-K | 001-39248 | 10.1 | March 7, 2022 | ||||||||||||||||||||||||||||||||||
| 10.10* | 8-K | 001-39248 | 10.2 | March 7, 2022 | ||||||||||||||||||||||||||||||||||
| 10.11* | 10-Q | 001-39248 | 10.1 | August 9, 2022 | ||||||||||||||||||||||||||||||||||
| 10.12* | 10-Q | 001-39248 | 10.2 | August 9, 2022 | ||||||||||||||||||||||||||||||||||
| 10.13 | 8-K | 001-39248 | 10.1 | August 10, 2022 | ||||||||||||||||||||||||||||||||||
| 10.14 | 8-K | 001-39248 | 10.2 | August 10, 2022 | ||||||||||||||||||||||||||||||||||
| 10.15 | 8-K | 001-39248 | 10.3 | August 10, 2022 | ||||||||||||||||||||||||||||||||||
| 21.1 | S-1 | 333-261740 | 21.1 | December 17, 2021 | ||||||||||||||||||||||||||||||||||
| 23.1 | X | |||||||||||||||||||||||||||||||||||||
| 31.1 | X | |||||||||||||||||||||||||||||||||||||
| 31.2 | X | |||||||||||||||||||||||||||||||||||||
| 32.1 | X | |||||||||||||||||||||||||||||||||||||
| 32.2 | X | |||||||||||||||||||||||||||||||||||||
| 101 | Interactive Data File — the following financial statements from The Oncology Institute's Annual Report on Form 10-K formatted in inline XBRL (Extensible Business Reporting Language) includes: (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Convertible Preferred Stock and Changes in Stockholders’ Equity), (iv) the Consolidated Statements of Cash Flows, and (v) Notes to the Consolidated Financial Statements. | |||||||||||||||||||||||||||||||||||||
101.INS | XBRL Instance Document | |||||||||||||||||||||||||||||||||||||
101.SCH | XBRL Taxonomy Extension Schema Document | |||||||||||||||||||||||||||||||||||||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||||||||||||||||||||||||||||||
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |||||||||||||||||||||||||||||||||||||
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |||||||||||||||||||||||||||||||||||||
| 104 | Cover Page Interactive Data File - (formatted as Inline XBRL and contained in Exhibit 101) | |||||||||||||||||||||||||||||||||||||
115
Table of Contents
* Management contract or compensatory plan or arrangement
† Certain of the exhibits and schedules to this Exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Registrant agrees to furnish a copy of all omitted exhibits and schedules to the SEC upon its request.
Item 16. Form 10-K Summary
None
116
Table of Contents
Signatures
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned hereunto duly authorized, on this the day of March 16, 2023.
THE ONCOLOGY INSTITUTE, INC. | |||||
| By: | /s/ Mihir Shah | ||||
| Mihir Shah | |||||
Chief Financial Officer (Duly Authorized Officer) | |||||
SIGNATURES AND POWER OF ATTORNEY
We, the undersigned officers and directors of The Oncology Institute, Inc., hereby severally constitute and appoint Brad Hively and Mihir Shah, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for him and in his name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature | Title | Date | ||||||||||||
| /s/ Brad Hively | ||||||||||||||
| Brad Hively | Chief Executive Officer and Director (Principal Executive Officer) | March 16, 2023 | ||||||||||||
| /s/ Mihir Shah | ||||||||||||||
| Mihir Shah | Chief Financial Officer (Principal Financial and Accounting Officer) | March 16, 2023 | ||||||||||||
| /s/ Richard Barasch | ||||||||||||||
| Richard Barasch | Director | March 16, 2023 | ||||||||||||
| /s/ Karen Johnson | ||||||||||||||
| Karen Johnson | Director | March 16, 2023 | ||||||||||||
| /s/ Mohit Kaushal | ||||||||||||||
| Mohit Kaushal | Director | March 16, 2023 | ||||||||||||
| /s/ Gabriel Ling | ||||||||||||||
| Gabriel Ling | Director | March 16, 2023 | ||||||||||||
| /s/ Anne McGeorge | ||||||||||||||
| Anne McGeorge | Director | March 16, 2023 | ||||||||||||
| /s/ Maeve O'Meara | ||||||||||||||
| Maeve O’Meara | Director | March 16, 2023 | ||||||||||||
117
Table of Contents
| Signature | Title | Date | ||||||||||||
| /s/ Mark Pacala | ||||||||||||||
| Mark Pacala | Director | March 16, 2023 | ||||||||||||
| /s/ Ravi Sarin | ||||||||||||||
| Ravi Sarin | Director | March 16, 2023 | ||||||||||||
118
Similar companies
See also Teladoc Health, Inc. - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)See also 1Life Healthcare Inc - Annual report 2022 (10-K 2022-12-31) Annual report 2022 (10-Q 2022-09-30)
See also Cano Health, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Hims & Hers Health, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Airsculpt Technologies, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
