OSI SYSTEMS INC - Annual Report: 2023 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) | |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2023 | |
OR | |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to | |
Commission File Number 000-23125

OSI SYSTEMS, INC.
(Exact name of registrant as specified in its charter)
Delaware | 33-0238801 |
12525 Chadron Avenue, Hawthorne, California | 90250 |
Registrant’s telephone number, including area code: (310) 978-0516
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading symbol(s) |
| Name of each exchange on which registered |
Common Stock, $0.001 par value | OSIS | The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes: ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes: ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes: ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes: ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ | Accelerated filer ☐ | Non-accelerated filer ☐ | Smaller reporting company ☐ |
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes: ☐ No ☒
The aggregate market value of the registrant’s voting and non-voting Common Stock held by non-affiliates computed by reference to the price at which the Common Stock was last sold on December 31, 2022, the last business day of the registrant’s most recently completed second fiscal quarter, was $1,258,906,407. For purposes of the foregoing calculation only, executive officers and directors of the registrant have been deemed to be affiliates of the registrant. The number of shares outstanding of the registrant’s Common Stock as of August 25, 2023 was 16,799,266.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive proxy statement relating to the 2023 annual meeting of stockholders are incorporated by reference into Part III. The proxy statement will be filed by the registrant with the Securities and Exchange Commission not later than 120 days after the end of the registrant’s fiscal year.
TABLE OF CONTENTS
PART I
Forward-Looking Statements
This report contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements relate to our current expectations, beliefs, and projections concerning matters that are not historical facts. Words such as “project,” “believe,” “anticipate,” “plan,” “expect,” “intend,” “may,” “should,” “will,” “would,” and similar words and expressions are intended to identify forward-looking statements. Forward-looking statements are not guarantees of future performance and involve uncertainties, risks, assumptions and contingencies, many of which are outside our control. Assumptions upon which our forward-looking statements are based could prove to be inaccurate, and actual results may differ materially from those expressed in or implied by such forward-looking statements. Important factors that could cause our actual results to differ materially from our expectations are disclosed in this report, including, without limitation, delays related to the award of domestic and international contracts; failure to secure the renewal of key customer contracts; delays in customer programs; delays in revenue recognition related to the timing of customer acceptance; the impact of potential information technology, cybersecurity or data security breaches; changes in domestic and foreign government spending, budgetary, procurement and trade policies adverse to our businesses; the impact of the Russia-Ukraine conflict, including the potential for broad economic disruption; global economic uncertainty; material delays and cancellations of orders or deliveries thereon, supply chain disruptions, plant closures, or other adverse impacts on our ability to execute business plans; unfavorable currency exchange rate fluctuations; effect of changes in tax legislation; market acceptance of our new and existing technologies, products and services; our ability to win new business and convert any orders received to sales within the fiscal year; contract and regulatory compliance matters, and actions, which if brought, could result in judgments, settlements, fines, injunctions, debarment or penalties; as well as other risks and uncertainties, including but not limited to those factors described in Part I, Item 1, “Business,” Part I, Item 1A, “Risk Factors” and Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as factors described elsewhere in this report and other documents filed by us from time to time with the Securities and Exchange Commission (“SEC”). All forward-looking statements contained in this report are qualified in their entirety by this section. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Investors should not place undue reliance on forward-looking statements as a prediction of actual results. We undertake no obligation other than as may be required under securities laws to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
ITEM 1. BUSINESS
General
OSI Systems, Inc., together with our subsidiaries, is a vertically integrated designer and manufacturer of specialized electronic systems and components for critical applications. We sell our products and provide related services in diversified markets, including homeland security, healthcare, defense and aerospace. Our company is incorporated in the State of Delaware and our principal office is located at 12525 Chadron Avenue, Hawthorne, California 90250.
We have three operating divisions: (a) Security, providing security and inspection systems and turnkey security screening solutions; (b) Healthcare, providing patient monitoring, cardiology and remote monitoring, and connected care systems and associated accessories; and (c) Optoelectronics and Manufacturing, providing specialized electronic components and electronic manufacturing services for our Security and Healthcare divisions, as well as to third parties for applications in the defense and aerospace markets, among others.
Industry Overview
We sell our security and inspection solutions and healthcare products primarily to end‑users, while we design and manufacture our optoelectronic devices and value‑added subsystems and provide electronics manufacturing services primarily for original equipment manufacturer (OEM) customers.
1
Security. A variety of technologies are currently used globally in security and inspection applications, including transmission and backscatter X-ray, and 3-D computed tomography, radiation detection, metal detection, millimeter wave imaging, explosive trace detection, and optical inspection. We believe that the market for security and inspection products will continue to be affected by the threat of terrorist incidents, drug and human trafficking, gun violence, and by new government mandates and appropriations for security and inspection products in the United States and internationally.
Security and inspection products are used at a wide range of facilities in addition to airports, such as border crossings, seaports, freight forwarding operations, correctional facilities, government and military installations, sports and concert venues and other locations where the interdiction of criminal activities is paramount. The U.S. Department of Homeland Security has undertaken numerous initiatives to prevent terrorists from entering the country, hijacking airplanes, and obtaining and transporting weapons of mass destruction and their components, to secure sensitive U.S. technologies, prevent human trafficking and to identify and screen cargo before it is loaded onto airplanes and ships. These initiatives, such as the Customs‑Trade Partnership Against Terrorism, the U.S. Transportation Security Administration’s Air Cargo Screening Mandate and the U.S. Customs and Border Protection Container Security Initiative, have resulted in increased demand for security and inspection products, as have similar programs undertaken by governments across the world.
Government sponsored initiatives in one nation often stimulate corresponding security programs by others in part because such initiatives frequently require that other nations bolster their security strategies, including acquiring or improving their security and inspection equipment and screening operations, in order to participate in international aviation and cross-border trade activities. The international market for non‑intrusive inspection equipment and related services, therefore, continues to expand as countries satisfy the requirements of these initiatives in order to maintain direct airline connections and ship goods internationally and as they themselves choose to procure and operate equipment to secure their own borders, transportation networks, facilities and other venues.
The U.S. Transportation Security Administration and other international air transportation security regulators around the world require the screening of passengers, carry-on bags and air cargo. Several of our screening system models have been approved by the U.S. Transportation Security Administration, as well as by various international regulatory bodies, for this purpose and are procured and used by government agencies, airlines, airports, freight forwarders, transportation companies and other businesses to fulfill their compliance requirements. These and other regulations promulgated by international organizations have resulted in an ongoing global demand for airline, cargo, port and border security and inspection technologies.
Healthcare. Healthcare has been, and we believe will continue to be, a growing economic sector throughout much of the world. Developing countries in Latin America and the Asia-Pacific region are expected to continue to build healthcare infrastructure to serve expanding middle class populations. In developed areas, especially the United States, Europe, and mature Asian countries, aging populations and extended life expectancy are projected to fuel growth in healthcare for the foreseeable future.
While we believe that the healthcare industry will continue to grow throughout much of the world, many factors are forcing healthcare providers to do more with less. These factors include stricter government requirements affecting staffing and accountability and uncertainty around potential U.S. healthcare legislation. The COVID-19 pandemic strained healthcare provider resources, placing increased focus on the advantages of remote monitoring and products which can be deployed flexibly, enabling hospitals to quickly reconfigure and adapt to unexpected changes. Our customers expect clinical value, economic value, and clinical decision support. Positioning our current healthcare products to demonstrate the competitive value in total cost of ownership is increasingly important in this environment. At the same time, the widespread introduction of mobile devices into the healthcare environment is creating an emerging demand for patient data acquisition and distribution. Our Healthcare division designs, manufactures and markets devices and software that respond to these factors, helping hospitals reduce costs, make better-informed clinical decisions, and more fully utilize resources.
We are a global manufacturer and distributor of patient monitoring, cardiology and remote monitoring, and connected care solutions for use in hospitals, medical clinics and physician offices. We design, manufacture and market patient monitoring solutions for critical, sub-acute and perioperative care areas of the hospital, wired and wireless networks and ambulatory blood pressure monitors, all aimed at providing caregivers with timely patient information. Our cardiology and remote monitoring systems include Holter recorders and analyzers, ambulatory blood pressure monitors, resting and stress electrocardiography (ECG) devices, and ECG management software systems and related software and services.
2
Optoelectronics and Manufacturing. We believe that continued advances in technology and reductions in the cost of key components of optoelectronic systems, including computer processing power and memory, have broadened the optoelectronics market by enabling the use of optoelectronic devices in a greater number of applications. In addition, we see a trend among OEMs to outsource the design and manufacture of optoelectronic devices as well as value-added subsystems to fully-integrated, independent manufacturers, like us, that may have greater specialization, broader expertise and more flexibility to respond to short cycle times and quicker market expectations.
Our optoelectronic devices are used in a wide variety of applications for diversified markets including aerospace and defense, avionics, medical imaging and diagnostics, biochemistry analysis, pharmaceutical, nanotechnology, telecommunications, construction and homeland security. Medical applications for our devices include diagnostic and imaging products, patient monitoring equipment, and glucose monitors. Aerospace and defense applications for our devices include satellite navigation sensors, laser guided munitions systems, range finders, weapons simulation systems, and other applications that require the conversion of optical signals into electrical signals. Homeland security applications for our devices include X-ray based and other detection systems. Our optoelectronic devices and value-added subsystems are also used in a wide variety of measurement control, monitoring and industrial applications and are key components in telecommunications technologies. We also offer electronics manufacturing services to broader markets, as well as to our optoelectronics customers and to our Security and Healthcare divisions. We offer full turnkey solutions as well as printed circuit board assembly, cable and harness assembly, liquid crystal displays and box build manufacturing services, in which we provide product design and development, supply chain management, and production manufacturing services. Additionally, our flexible circuit businesses offer design expertise, fabrication capabilities, and assembly of flexible and rigid circuit boards for applications in the industrial medical, military, and consumer markets.
Growth Strategy
We believe that one of our primary competitive strengths is our expertise in the cost effective design and manufacture of specialized electronic systems and components for critical applications. As a result, we will continue to leverage such expertise and capacity to gain price, performance and agility advantages over our competitors in the security, healthcare and optoelectronics fields, and to translate such advantages into profitable growth in those fields. At the same time, we continually seek to identify new markets in which our core expertise and capacity will provide us with competitive advantages. Key elements of our growth strategy include:
Capitalizing on Global Reach. We operate from multiple locations throughout the world. We view our international operations as providing an important strategic advantage over competitors. First, our international manufacturing facilities allow us to take advantage of competitive labor rates in order to lower our manufacturing costs. Second, our international offices strengthen our sales and marketing efforts and our ability to service and repair our systems by providing direct access to growing markets and to our existing international customer base. Third, our international manufacturing locations allow us to reduce delivery times to our global customer base. We intend to continue to enhance our international manufacturing and sales capabilities.
Capitalizing on Vertical Integration. Our vertical integration provides several advantages in each of our divisions. These advantages include reduced manufacturing and delivery times, lower costs due to our access to competitive international labor markets and direct sourcing of raw materials and sub components. We also believe that we offer significant added value to our customers by providing a full range of vertically-integrated services, including component design and customization, subsystem concept design and application engineering, product development and prototyping, efficient preproduction and short run manufacturing and competitive mass production capabilities. We believe that our vertical integration differentiates us from many of our competitors and provides value to our customers who can rely on us to be an integrated supplier.
Capitalizing on the Market for Security and Inspection Systems. The trend toward increased screening of goods entering and departing from ports and crossing borders has resulted, and may continue to result in, the growth in the market for cargo inspection systems and turnkey security screening services that are capable of inspecting shipping containers for contraband and assisting customs officials in the verification of shipping manifests. Package and cargo screening by freight forwarders, airlines and air cargo companies represents a growing sector, as regulations in the United States and Europe have continued to require screening of air cargo shipments. We plan to capitalize on opportunities to replace, service and upgrade existing security installations, and to offer turnkey security screening solutions in which we may construct, staff and/or operate on a long-term basis security screening checkpoints for our customers.
3
We expect that a market for software-as-a-service (SaaS) platforms that are capable of integrating the data that security inspection systems produce with related information derived from vehicle license plates, cargo container numbers, drivers’ licenses, government databases, and other sources will also continue to develop, mature and grow, particularly as customers shift their operating procedures to take advantage of secure, cloud-based, networking technologies. We are a leader in the development of these platforms, including the transmission of such data to operators that may be working within secure, remote screening facilities hundreds or thousands of miles away from the security checkpoint. Our software has been used by customs and tax authorities in the United States, Europe and Latin America to screen millions of containers and vehicles. We believe that government agencies and commercial customers will increasingly rely on such SaaS offerings to review and adjudicate screening decisions remotely, over secure networks, as well as to communicate with and monitor the performance of their employees working on the ground at distant ports, border crossings and other checkpoints.
Finally, we also intend to continue to develop new security and inspection products and technologies, including software, and to enhance our current product and service offerings through internal research and development and selective acquisitions.
Improving and Complementing Existing Medical Technologies. We develop and market patient monitoring systems, cardiology and remote monitoring products, and connected care systems and associated supplies and accessories. Our efforts to develop new products and improve our existing medical technologies are focused on the needs of healthcare organizations, caregivers, and their patients. Our efforts to improve existing medical technologies concentrate on providing products that are flexible and intuitive to use so that clinicians can deliver accurate, precise, reliable and cost-effective care.
Selectively Entering New Markets. We intend to continue to selectively enter new markets that complement our existing capabilities in the design, development and manufacture of specialized electronic systems and components for critical applications such as security inspection, patient monitoring and cardiology and remote monitoring. We believe that by manufacturing products that rely on our existing technological capabilities, we will leverage our integrated design and manufacturing infrastructure to build a larger presence in new markets that present attractive competitive dynamics. We intend to achieve this strategy through internal growth and through selective acquisitions.
Acquiring New Technologies and Companies. Our success depends in part on our ability to continually enhance and broaden our product offerings in response to changing technologies, customer demands and competitive pressures. We have developed expertise in our various lines of business and other areas through internal research and development efforts, as well as through selective acquisitions. We expect to continue to seek acquisition opportunities to broaden our technological expertise and capabilities, lower our manufacturing costs and facilitate our entry into new markets.
Products and Technology
We design, develop, manufacture and sell products ranging from security and inspection systems to patient monitoring and cardiology and remote monitoring systems to discrete optoelectronic devices and value-added subsystems.
Security and Inspection Systems. We design, manufacture and market security and inspection systems globally to end users primarily under the “Rapiscan” trade name. Our Security products are used to inspect baggage, parcels, cargo, people, vehicles and other objects for various contraband and prohibited items including weapons, explosives, drugs, and nuclear materials. These systems are also used for the safe, accurate and efficient verification of cargo manifests for the purpose of assessing duties and monitoring the export and import of controlled materials. Our Security products fall into the following categories: baggage and parcel inspection; cargo and vehicle inspection; hold (checked) baggage screening; people screening; radiation monitoring; explosive and narcotics trace detection; and optical inspection systems. We also offer turnkey security screening services, as well as related software integration platforms, operator training, and the staffing and operation of security screening checkpoints under the “S2” trade name. From time to time we form joint ventures to carry out our operations in certain geographies, including, for example, Albania.
In recent years, security and inspection products have increasingly been used at a wide range of facilities in addition to airports, such as border crossings, railways, seaports, cruise line terminals, sporting venues, freight forwarding operations, government and military installations and nuclear facilities. As a result of the use of security and inspection products at additional facilities, we have diversified our portfolio of security and inspection products and our sales channels.
4
Many of our security and inspection systems utilize dual-energy X-ray imaging technology, in combination with software enhanced imaging methods and algorithms to facilitate the detection of contraband materials and items such as explosives, weapons, narcotics, and bulk currency. Dual energy imaging allows some material properties to be identified. Additionally, dual-view X-ray imaging allows operators to view and examine objects from two directions simultaneously, thereby improving the operator’s ability to detect threats quickly and effectively. Some of our systems also use different types or combinations of X-ray imaging in addition to dual-energy, such as multi-view and computed tomography. Algorithms that process images and related data from these systems significantly enhance the overall probability of detection of a range of threat items and materials. Typical threat items include explosives and weapons.
Our inspection systems range in size from compact, handheld and table-top products to large systems comprising entire buildings in which trucks, shipping containers or pallets are inspected. Many of our inspection systems are also designed to be upgradeable to respond to new customer requirements as they emerge or change.
Our cargo and vehicle inspection applications, in which vehicles, cars, trucks, shipping containers, pallets and other large objects can be inspected, are designed in various configurations, including mobile, portal, gantry, and rail systems. Our customers use these products to verify the contents of cars, trucks, rail cars and cargo containers and to detect the presence of contraband, including narcotics, weapons, explosives, radioactive and nuclear materials and other smuggled items. Most of our cargo and vehicle inspection systems employ X‑ray imaging to inspect objects and present images to an inspector, including shapes, sizes, locations and relative densities of the contents. These systems utilize transmission imaging, backscatter imaging, or both technologies in combination. We also manufacture passive radiation monitoring devices for detecting nuclear materials utilizing their gamma and neutron signatures. Additionally, we have developed isotope‑specific identification algorithms. Many of these systems have been built to meet specific requirements of our government customers.
Our broad portfolio of non-intrusive inspection systems permits us to offer customers solutions that are tailored to their specific operational requirements, performance standards and budgets.
In many cases, we have designed our systems to meet the performance specifications of relevant regulators, including authorities located in the United States, United Kingdom and European Union. This is particularly the case with respect to systems used (or approved for use) to perform screening of airline passenger carry-on items, hold (checked) baggage and air cargo.
Our Security division also offers trace detection systems that are designed to detect trace amounts of explosives or narcotics and people screening products, such as walk-through metal detectors for use at security checkpoints at airports, government buildings, sports arenas and other venues.
Patient Monitoring and Cardiology and Remote Monitoring. Our Healthcare division designs, manufactures and markets products globally to end users primarily under the “Spacelabs” trade name.
Spacelabs products include patient monitors for use in perioperative, critical care and emergency care environments with neonatal, pediatric and adult patients. Our patient monitoring systems such as Xprezzon® and Qube® are supported by surveillance systems connected by wireless or hardwired networks, as well as standalone monitors that enable patient data to be transported physically from one monitor to another as the patient is moved. These systems enable hospital staff to access patient data where and when it is required. In addition, these products are designed to interact with hospital information systems.
Spacelabs SafeNSound™ assists hospitals in providing value-based care by streamlining workflows and improving communications. Features include comprehensive reporting tools, a communications dashboard for monitor technicians, and a device management system to admit patients to monitors/telemetry at the bedside. These tools help address top challenges facing hospitals today.
Spacelabs predictive analytics clinical decision support tools provide surveillance and deterioration alerting for patients in all levels of care in the hospital setting and includes FDA-cleared and regulated products featuring the Rothman Index, a proprietary patient condition score available through EMR-integrated, web-based, or mobile app interfaces.
5
For electrocardiograph monitoring or multiparameter monitoring of ambulatory patients, we offer a digital telemetry system. The system operates in government protected bands, which are not used for private land mobile radio, business radio services or broadcast analog or digital television. Spacelabs Intesys® Clinical Suite (ICS) provides a software suite allowing hospitals to leverage their infrastructure to capture data from the bedside, compact and telemetry monitors.
Our PathfinderSL® and Lifescreen™ Pro analysis tools provide clinicians the ability to save Holter analysis time and to do detailed analysis when needed inside or outside the hospital. Our Eclipse Pro Holter recorders provide up to 14 days of 3-channel recording or up to 72 hours of 12 lead with pacing. Our Eclipse Mini Ambulatory ECG Recorder provides up to 30 days of 3-channel ECG and when paired with Lifescreen™ Pro clinicians can analyze millions of heart beats within minutes.We are also a supplier of ambulatory blood pressure (ABP) monitors which are routinely used by physicians around the world and by contract research organizations. Many physicians are using ambulatory blood pressure monitoring to detect “white coat” hypertension, a condition in which people experience elevated blood pressure in the doctor’s office but not in their daily lives. Ambulatory blood pressure monitoring helps improve diagnostic accuracy and minimize the associated costs of treatment. Spacelabs OnTrak™ ambulatory blood pressure system has been validated for both pediatric and adult patient types and includes the capability to measure activity correlation with non-invasive blood pressure readings.
Our Sentinel® 11 Cardiology Information Management System is designed to provide an electronic, enterprise-wide scalable system for cardiology and remote monitoring. Sentinel integrates data from Spacelabs-branded products and third-party devices into a central enterprise-wide database system that can be accessed by care providers and medical facility administrators, thereby providing enhanced workflow and efficiencies. The system’s web-based solution enables the secure transfer of data from multiple remote sites. Sentinel supports mobile and remote working, taking ECG management to the point of care for flexible use of devices and capture of data.
In addition, the capital-intensive products that our Healthcare division sells have supplies and accessories associated with them that can represent annuity revenue opportunities. Additionally, our Healthcare division manufactures multivendor compatible accessories for use with third-party devices.
Optoelectronic Devices and Manufacturing Services. Optoelectronic devices designed, manufactured and sold through our Optoelectronics and Manufacturing division generally consist of both active and passive components. Active components sense light of varying wavelengths and convert the light detected into electrical signals, whereas passive components amplify, separate or reflect light. These products are manufactured in standard and customized configurations for specific applications and are offered either as components or as subsystems. Our optoelectronic products and services are provided primarily under the “OSI Optoelectronics,” “OSI LaserDiode,” “OSI Laserscan,” and “Advanced Photonix” trade names.
In addition to the manufacture of standard and OEM products, we also specialize in designing and manufacturing customized value-added subsystems for use in a wide range of products and equipment. An optoelectronic subsystem typically consists of one or more optoelectronic devices that are combined with other electronic components and packaging for use in an end product. The composition of a subsystem can range from a simple assembly of various optoelectronic devices that are incorporated into other subsystems (for example, a printed circuit board containing our optoelectronic devices) to complete end products (for example, pulse oximetry equipment).
We develop, manufacture and sell laser-based remote sensing devices that are used to detect and classify vehicles in toll and traffic management systems under the “OSI Laserscan” and “Autosense” trade names. We offer solid-state laser products for aerospace, defense, telecommunication and medical applications under the “OSI LaserDiode” trade name.
We also provide electronics design and manufacturing services in North America, the United Kingdom and in the Asia Pacific region with enhanced, Rohs compliant, printed circuit board and cable and harness assembly and box build manufacturing services utilizing automated surface mount technology lines. We offer electronics manufacturing services to OEM customers and end users for medical, automotive, defense, aerospace, industrial and consumer applications that do not utilize optoelectronic devices. We also manufacture LCD displays for medical, industrial and consumer electronics applications, and flex circuits for OEM customers from the prototype stage to mass production. Our electronics manufacturing services are provided primarily under the “OSI Electronics,” “APlus Products,” “Altaflex,” and “PFC Flexible Circuits” trade names.
6
Markets, Customers and Applications
Security and Inspection Products. Many security and inspection products were developed originally in response to civilian airline hijackings. Consequently, certain of our security and inspection products have been and continue to be sold for use at airports. Our security and inspection products are also used for security and customs purposes at locations in addition to airports, such as border crossings, shipping ports, sporting venues, military and other government installations, freight forwarding facilities, high-profile locations such as U.K. House of Parliament, Buckingham Palace, and the Vatican and for high-profile events such as the Olympic Games, FIFA World Cup, and other sporting events. We also provide turnkey security screening solutions, which can include the construction, staffing and long-term operation of security screening locations for our customers.
Our customers include, among many others, the U.S. Department of Homeland Security, U.S. Department of Defense, U.S. Department of State, U.S. Department of Commerce, and U.S. Department of Justice, as well as many premier international government agencies, including airports and other critical infrastructure agencies.
Our contracts with the U.S. Government are generally subject to termination for convenience at the election of the U.S.Government. For the fiscal year ended June 30, 2023, our Security division’s direct sales to the U.S. Government were approximately $235 million. Additionally, certain of our contracts with foreign governments also contain provisions allowing the government to terminate a contract for convenience. For further discussion, please refer to Item 1A. “Risk Factors.”
Patient Monitoring, Cardiology and Remote Monitoring, and Connected Care Systems. Our patient monitoring, cardiology and remote monitoring, and connected care systems are manufactured and distributed globally for use in critical care, emergency and perioperative areas within hospitals, as well as physicians’ offices, medical clinics and ambulatory surgery centers. We also provide wired and wireless networks, clinical information access solutions and ambulatory blood pressure monitors.
We sell products directly to end customers, as well as through integrated delivery networks and group purchasing organizations in the U.S., the NHS Supplies Organisation in the United Kingdom, UGAP in France, and to various government funded hospitals in the Middle East and several parts of Asia.
Optoelectronic Devices and Electronics Manufacturing Services. Our optoelectronic devices and the electronics we manufacture are used in a broad range of products by a variety of customers in the following market segments: defense, aerospace and avionics; analytical and medical imaging; healthcare; telecommunications; homeland security; toll and traffic management; and automotive.
Marketing, Sales and Service
We market and sell our security and inspection products and turnkey security screening solutions globally through a direct sales and marketing staff located in North America, South America, Europe, Middle East, Australia, and Asia, in addition to an expansive global network of independent distributors. This sales organization is supported by a service organization located in the same regions, as well as a global network of independent, authorized service providers.
We market and sell our healthcare products globally through a direct sales and marketing staff located in North America, South America, Europe and Asia, in addition to a global network of independent distributors. We also support these sales and customer service efforts by providing operator in service training, comprehensive interactive eLearning for all monitoring products, software updates and upgrades and service training for customer biomedical staff and distributors. We also provide IT specialists and clinical specialists to provide support both before and after product sale.
We market and sell our optoelectronic devices and value-added manufacturing services, through both our direct sales and marketing staff located in North America, Europe and Asia, and indirectly through a global network of independent sales representatives and distributors. Our sales staff is supported by an applications engineering group whose members are available to provide technical support, which includes designing applications, providing custom tooling and process integration and developing products that meet customer defined specifications.
7
We consider our maintenance service operations to be an important element of our business. After the expiration of our standard product warranty periods, we are often engaged by customers, either directly or through our network of authorized service providers, to provide maintenance services for our security and inspection products. In addition, we provide a variety of service and support options for our healthcare customers, including hospital on-site repair and maintenance service and telephone support, parts exchange programs for customers with the internal expertise to perform a portion of their own service needs and a depot repair center at our division headquarters. We believe that our international maintenance service capabilities allow us to be competitive in selling our security and inspection systems as well as our patient monitoring, cardiology and remote monitoring, and connected care systems.
Research and Development
Our security and inspection systems are primarily designed at our facilities in the United States and in the United Kingdom, Australia, Singapore, India, and Malaysia. These products include mechanical, electrical, analog and digital electronics, and software components and subsystems. In addition to product design, we provide civil works and system integration services to install and integrate our products with other systems, networks and facilities at the customer site. We support cooperative and government-funded research projects with universities and directly with government agencies themselves.
Our healthcare products are primarily designed at our facilities in the United States and in the United Kingdom with sustaining engineering efforts in India. These products include enterprise and embedded software, networking, connectivity, mechanical, electronic and software subsystems, most of which are designed by us. We are also currently involved, both in the United States and internationally, in research projects aimed at improving our medical systems and at expanding our current product lines.
We design and manufacture optoelectronic devices and we provide electronics manufacturing services primarily in our facilities in the United States and internationally in the United Kingdom, Canada, India, Indonesia, and Malaysia. We engineer and manufacture subsystems to solve the specific application needs of our OEM customers. In addition, we offer entire subsystem design and manufacturing solutions. We consider our engineering personnel to be an important extension of our core sales and marketing efforts.
In addition to close collaboration with our customers in the design and development of our current products, we maintain an active program for the development and introduction of new products, enhancements and improvements to our existing products, including the implementation of new applications of our technology. We seek to further enhance our research and development program and consider such program to be an important element of our business and operations.
Manufacturing and Materials
We currently manufacture our security and inspection systems domestically in California, Kentucky, Massachusetts, Tennessee, and Virginia, and internationally in Malaysia and the United Kingdom. We currently manufacture our patient monitoring and cardiology and remote monitoring systems in Washington state. We outsource manufacturing of certain of our supplies and accessories. We currently manufacture our optoelectronic devices and provide electronics manufacturing services domestically in California and New Jersey, and internationally in Canada, India, Indonesia, Malaysia, and the United Kingdom. Most of our high-volume, labor-intensive manufacturing activities are performed at our facilities in India, Indonesia and Malaysia. Our ability to manufacture products and provide follow-on service from offices located in these regions allows us to remain in close proximity to our customers, which is an important component of our global strategy.
Our global manufacturing organization has expertise in optoelectronic, microelectronic and integrated electronics for industrial and automation, medical, aerospace and defense industry applications. Our manufacturing includes silicon wafer processing and fabrication, optoelectronic device assembly and screening, thin and thick film microelectronic hybrid assemblies, surface mounted and thru-hole printed circuit board electronic assemblies, cable and harness assemblies, LCD and TFT displays, box-build manufacturing, and flex circuitry on a complete turnkey basis. To support our manufacturing operations, we outsource certain requirements, including sheet metal fabrication and plastic molding of components.
The principal raw materials and subcomponents used in producing our security and inspection systems consist of X-ray generators, linear accelerators, detectors, data acquisition and computer systems, conveyance systems, vehicles, and miscellaneous mechanical and electrical components. A large portion of the optoelectronic devices, subsystems and circuit card assemblies used in our inspection systems are manufactured in-house. A large proportion of our X-ray generators, linear accelerators, computers and conveyance systems used in our cargo and vehicle inspection systems are purchased from unaffiliated third-party providers.
8
The principal raw materials and subcomponents used in producing our healthcare products consist of printed circuit boards, housings, mechanical assemblies, pneumatic devices, touch screens, medical grade displays, cables, filters, textiles, fabric, gauges, fittings, tubing and packaging materials. We purchase finished medical devices, computers, peripheral accessories, and remote displays from unaffiliated third-party providers.
The principal raw materials and subcomponents used in producing our optoelectronic devices and electronic subsystems consist of silicon wafers, electronic components, light emitting diodes, scintillation crystals, passive optical components, printed circuit boards and packaging materials. The silicon-based optoelectronic devices manufactured by us are critical components in most of our products and subsystems. We purchase silicon wafers and other electronic components from unaffiliated third-party providers.
For cost, quality control, technological, and efficiency reasons, we purchase certain materials, parts, and components only from single vendors with whom we have ongoing relationships. We do, however, qualify alternative sources for many of our materials, parts, and components. We purchase most materials, parts, and components pursuant to purchase orders placed from time to time in the ordinary course of business. Since the initial onset of the COVID-19 pandemic, our divisions have experienced supply chain and labor availability challenges that have impacted the price and availability of parts, components, consumables, freight, shipping, and third-party services, adversely impacting our gross margin as well as delayed product deliveries, installations, maintenance and repair work, and technical support, among other work and services.
Information Technology and Cybersecurity Risk Management
We rely extensively on digital technology to conduct operations and engage with our customers and business partners. As the complexity of our engagements grows, so do the threats from cyber intrusion, ransomware, denial of service, phishing, account takeover, data manipulation and other cyber misconduct. To counter these threats, we have implemented an information security management system (ISMS) focused on data confidentiality, integrity, and availability. Our ISMS has been certified as ISO/IEC 27001 compliant and is re-evaluated annually by our external auditors. Similarly, we conduct external cyber penetration testing annually to assess and improve our security posture and reduce cybersecurity risk. No material information security breaches have occurred in the past three years. Through a combination of governance, risk, and compliance (GRC) resources, we also (i) proactively monitor IT controls to ensure compliance with legal and regulatory requirements, (ii) perform third-party risk management assessments, (iii) ensure essential business functions remain available during business disruptions, (iv) develop and update incident response plans to address potential weaknesses, and (v) maintain cyber incident management and reporting procedures. Our ISMS and GRC processes are designed to prioritize IT and cybersecurity risk areas, identify solutions that minimize such risks, pursue optimal outcomes, and maintain compliance with contractual obligations. We also maintain a global security operations center with real-time capability to investigate and trigger impact mitigation protocols. These capabilities allow us to reduce exposure should a security incident arise. For additional information regarding the risks associated with these matters, see Item 1A. “Risk Factors.”
Trademarks and Trade Names and Patents
Trademarks and Trade Names. We have used, registered and applied to register certain trademarks and service marks to distinguish our products, technologies and services from those of our competitors in the United States and in foreign countries. We monitor and, when necessary, enforce our trademark, service mark and trade name rights in the United States and abroad.
Patents. We possess rights to a number of U.S. and foreign patents relating to various aspects of our security and inspection products, healthcare products and optoelectronic devices and subsystems. Our current patents will expire at various times between 2023 and 2041. While we continue to file new applications and pursue new patents, it remains possible that pending patent applications or other applications that may be filed may not result in issued patents. In addition, issued patents may not survive challenges to their validity or enforceability, or may be found to not be infringed by any third parties. Although we believe that our patents have value, our patents, or any additional patents that may be issued in the future, may not be able to provide meaningful protection from competition.
We believe that our trademarks and trade names and patents are important to our business. The loss of some of our trademarks or patents might have a negative impact on our financial results and operations. Nevertheless, with the exception of the loss of the, Rapiscan®, AS&E® or Spacelabs® trademarks, the impact of the loss of any single trademark or patent would not likely have a material adverse effect on our business.
9
Government Regulation of Medical Devices
The patient monitoring, cardiology and remote monitoring, and connected care systems we design, manufacture, and market are subject to regulation by numerous government agencies, principally the U.S. Food and Drug Administration (FDA), and by other federal, state, local and foreign authorities. These systems are also subject to various U.S. and foreign product performance and safety standards. Our medical device product candidates must undergo an extensive government regulatory clearance or approval process prior to sale in the United States and other countries, including submission demonstrating clinical safety and efficacy of intended use, as well as the continuing need for compliance with applicable laws and regulations.This may require significant interaction with regulatory agencies and the expenditure of substantial resources.
United States FDA. In the United States, the FDA has broad regulatory powers with respect to preclinical and clinical testing of new medical devices and the designing, manufacturing, labeling, storage, record keeping, marketing, advertising, promotion, distribution, post market monitoring and reporting and import and export of medical devices. Unless an exemption applies, federal law and FDA regulations require that all new or significantly modified medical devices introduced into the market be preceded either by a premarket notification clearance under section 510(k) of the Federal Food, Drug and Cosmetic Act (FDCA), or an approved premarket approval (PMA) application. Under the FDCA, medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness. Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to a set of regulations, referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation (QSR) facility registration and product listing, reporting of adverse events and malfunctions and truthful and non-misleading promotional materials. Some Class I devices, also called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below. Most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the General Controls, as well as Special Controls as deemed necessary by the FDA, which can include performance standards, guidelines and post market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process.
Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the product for which clearance has been sought is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA had not yet called for the submission of pre-market approval applications. After a 510(k) notice is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) notification. In that case, the applicant must correct the submission errors before resubmitting. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of, and clear or deny, a 510(k) notification within 90 days of receiving the 510(k) notification. The FDA may formally request additional information, which may toll or restart the 90 day deadline. As a practical matter, clearance often takes longer than 90 days and sometimes is not granted at all. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
To be substantially equivalent, the proposed device must have a substantially equivalent intended use and indications for use as the predicate device, and either have substantially equivalent technological characteristics to the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support the demonstration of substantial equivalence. Multiple interactions and/or the submission of additional information or documentation may be required to secure regulatory clearance.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination that a new submission is not required, the FDA may require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. In addition, in these circumstances, we may be subject to significant regulatory fines or penalties for failure to submit the requisite premarket notification or PMA submissions.
10
Class III devices include devices deemed by the FDA to pose the greatest risk such as life-supporting or life-sustaining devices, or implantable devices, in addition to those deemed not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the General Controls and Special Controls described above. Therefore, these devices are typically subject to the PMA application process, which is more costly and time consuming than the 510(k) process and requires substantial clinical data. To date, all of the patient monitoring and cardiology and remote monitoring systems we manufacture and sell in the United States have required only 510(k) pre-market notification clearance.
FDA clearance or approval, when granted, may entail limitations on the indicated uses for which a product may be marketed, and such product approvals, once granted, may be withdrawn if problems occur after initial marketing. Manufacturers of FDA-regulated products are subject to pervasive and continuing post-approval governmental regulation, including, but not limited to, the registration and listing regulation, which requires manufacturers to register all manufacturing facilities and list all medical devices placed into commercial distribution; Quality System (also known as Good Manufacturing Practices) Regulations, which requires manufacturers, including third-party manufacturers, to follow stringent design, risk management, validation, testing, production, control, supplier/contractor selection, complaint handling, documentation and other quality assurance procedures during the manufacturing process; product and promotional labeling regulations; advertising and promotion requirements; restrictions on sale, distribution or use of a device; PMA annual reporting requirements; the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; the Medical Device Reporting (MDR) regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to reoccur; medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and removals (“recalls”) if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; recall requirements, including a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death; an order of repair, replacement or refund; device tracking requirements; and post-approval study and post-market surveillance requirements. The FDA has also established a Unique Device Identification (“UDI”) system that requires manufacturers to mark certain medical devices distributed in the United States with unique device identifiers. Also, we must comply with cybersecurity requirements to assess cybersecurity and safety risks and design and develop our devices to ensure safe and effective performance in the face of cyber threats. It is also incumbent on us to monitor third-party software for new vulnerabilities and verify and validate any software updates or patches meant to address vulnerabilities.
Our facilities, records and manufacturing processes are subject to periodic scheduled and unscheduled inspections by the FDA. Failure to comply with the applicable United States medical device regulatory requirements could result in, among other things, warning letters, untitled letters, fines, injunctions, consent decrees, civil penalties, unanticipated expenditures, repairs, replacements, refunds, recalls or seizures of products, operating restrictions, total or partial suspension of production, the FDA’s refusal to issue certificates to foreign governments needed to export products for sale in other countries, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product clearances or approvals and criminal prosecution.
Coverage and Reimbursement. Government and private sector initiatives to limit the growth of healthcare costs, including price regulation and competitive pricing, coverage and payment policies, comparative effectiveness therapies, technology assessments and managed care arrangements, are continuing in many countries where we do business, including the United States, Europe and Asia. As a result of these changes, the marketplace has placed increased emphasis on the delivery of more cost-effective medical therapies. In addition, because there is generally no separate reimbursement from third-party payers to our customers for many of our products, the additional costs associated with the use of our products can impact the profit margin of our customers. Accordingly, these various initiatives have created increased price sensitivity over healthcare products generally and may impact demand for our products and technologies.
Healthcare cost containment efforts have also prompted domestic hospitals and other customers of medical devices to consolidate into larger purchasing groups to enhance purchasing power, and this trend is expected to continue. The medical device industry has also experienced some consolidation, partly in order to offer a broader range of products to large purchasers. As a result, transactions with customers are larger, more complex and tend to involve more long-term contracts than in the past. These larger customers, due to their enhanced purchasing power, may attempt to increase the pressure on product pricing.
11
Significant healthcare reforms have had an impact on medical device manufacturer and hospital revenues. The Patient Protection and Affordable Care Act as amended by the Health Care and Education and Reconciliation Act of 2010, collectively referred to as the Affordable Care Act, is a sweeping measure designed to expand access to affordable health insurance, control healthcare spending and improve healthcare quality. Many states have also adopted or are considering changes in healthcare policies, in part due to state budgetary pressures. Ongoing uncertainty regarding implementation of certain aspects of the Affordable Care Act makes it difficult to predict the impact the Affordable Care Act or state law proposals may have on our business. This has created uncertainty in the market, which could result in reduced demand for our products, additional pricing pressure, and increased demand for new and more flexible payment structures.
Other Healthcare Laws. In addition to FDA restrictions on marketing and promotion of drugs and devices, other federal and state laws restrict our business practices. These laws include, without limitation, data privacy and security laws, anti-kickback and false claims laws, and transparency laws regarding payments or other items of value provided to healthcare providers.
As a participant in the healthcare industry, we are subject to extensive regulations protecting the privacy and security of patient health information that we receive, including the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, which was enacted as part of the American Recovery and Reinvestment Act of 2009 (collectively, “HIPAA”). Among other things, these regulations impose extensive requirements for maintaining the privacy and security of individually identifiable health information, known as “protected health information.” The HIPAA privacy regulations do not preempt state laws and regulations relating to personal information that may also apply to us. Our failure to comply with these regulations could expose us to civil and criminal sanctions.
The HIPAA provisions also created federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A person or entity does not need to have actual knowledge of the statutes or specific intent to violate them in order to have committed a violation. Also, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payer, in addition to items and services reimbursed under Medicaid and other state programs.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, to induce or in return for the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease or order of items or services for which payment may be made, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Further, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government, or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. Government. Medical device manufacturers have been held liable under these laws if they are deemed to cause the submission of false or fraudulent claims by, for example, providing customers with inaccurate billing or coding information.
These laws impact the kinds of financial arrangements we may have with hospitals or other potential purchasers of our products. They particularly impact how we structure our sales offerings, including pricing, customer support, education and training programs, physician consulting, research grants and other service arrangements. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to material penalties, including potentially significant criminal and civil and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, and the curtailment or restructuring of our operations, any of which could materially and adversely affect our ability to operate our business and our results of operations.
12
Additionally, there has been a trend towards increased federal and state regulation of payments and other transfers of value provided to healthcare professionals or entities. The federal Physician Payment Sunshine Act requires that certain device manufacturers track and report to the government information regarding payments and other transfers of value to physicians, certain other clinical staff, and teaching hospitals, as well as ownership and investment interests held by physicians and their family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties for “knowing failures.” Certain states also mandate implementation of compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities.
We are subject to similar laws in foreign countries where we conduct business. For example, within the EU, the control of unlawful marketing activities is a matter of national law in each of the member states. The member states of the EU closely monitor perceived unlawful marketing activity by companies. We could face civil, criminal, and administrative sanctions if any member state determines that we have breached our obligations under its national laws. Industry associations also closely monitor the activities of member companies. If these organizations or authorities name us as having breached our obligations under their regulations, rules or standards, our reputation would suffer, and our business and financial condition could be adversely affected.
Other Foreign Healthcare Regulations
We are also subject to regulation in the foreign countries in which we manufacture, market, and/or import our products. For example, the commercialization of certain products, including medical devices, in the EU is regulated under a system that presently requires all such products sold in the EU to bear the CE marking—an international symbol of adherence to the medical device regulations and standards of the EU. Our manufacturing facilities in Hawthorne, California; Snoqualmie, Washington; Johor Bahru, Malaysia; Batam, Indonesia; and Hyderabad, India are all certified to the International Organization for Standardization’s ISO 13485 standard for quality management. Our Hawthorne, California and Snoqualmie, Washington facilities are also certified to the requirements of Annex II, section 3 of the Directive 93/42/EEC on Medical Devices, which allows them to self-certify that manufactured products can bear the CE marking. Further, the implementation of the Restriction of Hazardous Substance Directive (“ROHS”) requires that certain products, including medical devices, shipped into the EU eliminate targeted ROHS substances.
The International Medical Device Regulators Forum has implemented a global approach to auditing manufacturers of medical devices. This audit system, called the Medical Device Single Audit Program (“MDSAP”), provides for an annual audit of a medical device manufacturer by a certified body on behalf of various regulatory authorities. Current authorities participating in MDSAP include the Therapeutic Goods Administration of Australia, Brazil’s Agencia Nacional de Vigilancia Sanitaria, Health Canada, Japan’s Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency and the FDA. It is expected that more regulatory authorities will participate in MDSAP in the future.
We and other medical device manufacturers are being confronted with major changes in the EU’s decades-old regulatory framework governing market access to the EU. The EU’s Medical Devices Regulation (“EU MDR”) is replacing the EU’s Medical Device Directive (93/42/EEC) and the EU’s Directive on active implantable medical devices (90/385/EEC). The EU MDR imposes stricter requirements for the marketing and sale of medical devices, including in the area of clinical evaluation requirements, quality systems and post-market surveillance, than the medical device directives replaced by the EU MDR. The EU MDR became effective as of May 26, 2021.
Manufacturers of currently approved medical devices will have a transition time to meet the requirements of the EU MDR. The EU MDR differs in several important ways from the EU’s directives for medical devices and active implantable medical devices replaced thereby. The most significant changes in the regulations include:
| ● | The definition of medical devices covered under the EU MDR is significantly expanded to include devices that may not have a medical intended purpose, such as colored contact lenses. Also included in the scope of the regulation are devices designed for the purpose of “prediction and prognosis” of a disease or other health condition. |
| ● | Device manufacturers are being required to identify at least one person within their organization who is ultimately responsible for all aspects of compliance with the requirements of the new EU MDR. The organization must document the specific qualifications of this individual relative to the required tasks. |
13
| ● | The EU MDR requires rigorous post-market oversight of medical devices. |
| ● | The EU MDR allows the EU Commission or expert panels to publish “Common Specifications,” such as requirements for technical documentation, risk management, or clinical evaluation. |
| ● | Devices are to be reclassified according to risk, contact, duration, and invasiveness. |
| ● | Systematic clinical evaluation is being required for Class IIa and Class IIb medical devices. |
| ● | All approved devices must be recertified in accordance with the new EU MDR requirements. |
We have a team dedicated to updating and revising key systems, processes, and product technical documentation to meet the new EU MDR requirements.
Environmental Regulations
We are subject to various environmental laws, directives, and regulations pertaining to the use, storage, handling and disposal of hazardous substances used, and hazardous wastes generated, in the manufacture of our products. Such laws mandate the use of controls and practices designed to mitigate the impact of our operations on the environment, and under such laws we may be held liable for the costs associated with the remediation and removal of any unintended or previously unknown releases of hazardous substances on, beneath or from our property and associated operations, including the remediation of hazardous waste disposed off-site. Such laws may impose liability without regard to whether we knew of, or caused, the release of such hazardous substances. Any failure by us to comply with present or future regulations could subject us to the imposition of substantial fines, suspension of production, alteration of manufacturing processes or cessation of operations, any of which could have a material adverse effect on our business, financial condition and results of operations.
We believe that, except to an extent that would not have a material adverse effect on our business, financial condition or results of operations, we are currently in compliance with all environmental regulations in connection with our manufacturing operations, and that we have obtained all environmental permits necessary to conduct our business. The amount of hazardous substances used, and hazardous wastes generated, by us may increase in the future depending on changes in our operations. To ensure compliance and practice proper due diligence, we conduct appropriate environmental audits and investigations at our manufacturing facilities in North America, Asia Pacific, and Europe, and, to the extent practicable, on all new properties. Our manufacturing facilities conduct regular internal audits to ensure proper environmental permits and controls are in place to meet changes in operations. Third-party investigations address matters related to current and former occupants and operations, historical land use, and regulatory oversight and status of associated properties and operations (including surrounding properties). The purpose of these studies is to identify, as of the date of such report, potential areas of environmental concern related to past and present activities or from nearby operations. The scope and extent of each investigation is dependent upon the size, complexity and operation of the property and on recommendations by independent environmental consultants.
Competition
The markets in which we operate are highly competitive and characterized by evolving customer needs and rapid technological change. We compete with other manufacturers, some of which have significantly greater financial, technical and marketing resources than we have. In addition, some competitors may have the ability to respond quickly to new or emerging technologies, adapt more quickly to changes in customer requirements, have stronger customer relationships, have greater name recognition and devote greater resources to the development, promotion and sale of their products than we do. As a result, we may not be able to compete successfully against designers and manufacturers of specialized electronic systems and components or within the markets for security and inspection systems, patient monitoring, cardiology and remote monitoring, or optoelectronic devices. Future competitive pressures may materially and adversely affect our business, financial condition and results of operations.
14
In the security and inspection market, competition is based primarily on factors such as product performance specification standards, quality and reliability, government regulatory approvals and qualifications, the overall cost effectiveness of the system, prior customer relationships and reputation, technological capabilities of the products, price, local market presence, program execution capability, and breadth of sales and service organization. Competition results in price reductions and reduced margins and could result in loss of market share. Although our competitors offer products in competition with one or more of our products, we can supply a variety of system types and offer among the widest array of solutions available from a single supplier. This variety of technologies also permits us to offer unique hybrid systems to our customers that utilize two or more of these technologies, thereby optimizing flexibility, performance and cost to meet each customer’s unique application requirements.
In the patient monitoring, cardiology and remote monitoring, and connected care markets, competition is also based on a variety of factors including product performance, functionality, value and breadth of sales and service organization. Competition could result in price reductions, reduced margins and loss of our market share. We believe that our patient monitoring products are easier to use than the products of many of our competitors because we offer a consistent user interface throughout many of our product lines. We also believe that the capability of our monitoring systems to connect together, and to the hospital IT infrastructure, is a key competitive advantage. Further, while some of our competitors are also beginning to introduce portal technology, which allows remote access to data from the bedside monitor, central station or other point of care, we believe that our competing technologies bring valuable, instant access to labs, radiology and charting at the point of care.
In the markets in which we compete to provide optoelectronic devices and electronics manufacturing services, competition is based primarily on factors such as expertise in the design and development of optoelectronic devices, product quality, timeliness of delivery, price, technical support and the ability to provide fully integrated services from application development and design through production. Because we specialize in custom subsystems requiring a high degree of engineering expertise, we believe that we generally do not compete to any significant degree with any other large United States, European or Asian manufacturers of standard optoelectronic components. Competition in the extensive electronic manufacturing services market ranges from multinational corporations with sales in excess of several billion dollars, to large regional competitors and to small local assembly companies. In our experience, the OEM customers to whom we provide such services often prefer to engage companies that offer both local and lower-cost off-shore facilities. Along with a number of domestic competitors for these services, our high-volume, low-cost contract manufacturing locations in Southeast Asia compete with other manufacturers in the same region.
Backlog
We currently measure our backlog as quantifiable purchase orders or contracts that have been signed, for which revenues are expected to be recognized within the next five years. In instances where we are not able to estimate the value of a purchase order or contract, they are not included in backlog.
We ship most of our baggage and parcel inspection, people screening, trace detection, patient monitoring and cardiology and remote monitoring systems and optoelectronic devices and value-added subsystems within one to several months after receiving an order. However, such shipments may be delayed for a variety of reasons, including supply chain disruptions and any special design or requirements of the customer. In addition, large orders of security and inspection products typically require greater lead-times. Fulfillment of orders of our Rapiscan RTT hold (checked) baggage screening equipment generally requires longer lead times. Further, we provide turnkey screening services to certain customers for which we may recognize revenue over multi-year periods.
Certain of our cargo and vehicle inspection systems may require more than a year of lead-time. We have experienced some significant shipping delays associated with our cargo and vehicle inspection systems. Such delays can occur for many reasons, including: (i) additional time necessary to coordinate and conduct factory inspections with the customer before shipment; (ii) a customer’s need to engage in time-consuming site construction projects to accommodate the system, over which we may have no control or responsibility; (iii) additional fine tuning of such systems once they are installed; (iv) design or specification changes by the customer; (v) time needed to obtain export licenses and/or letters of credit; (vi) delays originating from other contractors on the project; and (vii) supply chain constraints. The COVID-19 pandemic exacerbated these challenges, and supply chain constraints that originated during the pandemic continue to affect our projects, even as international travel restrictions and other protective measures subside.
15
As of June 30, 2023, our consolidated backlog totaled approximately $1.8 billion, compared to $1.2 billion as of June 30, 2022. Approximately $0.8 billion of our backlog as of June 30, 2023 is not reasonably expected to be fulfilled in fiscal year 2024. Sales orders underlying our backlog are firm orders, although, from time to time we may agree to permit a customer to cancel an order, or an order may be cancelled for other reasons. Variations in the size of orders, product mix, or delivery requirements, among other factors, may result in substantial fluctuations in backlog from period to period. Backlog as of any particular date should not be relied upon as indicative of our revenues for any future period and should not be considered a meaningful indicator of our performance on an annual or quarterly basis.
Human Capital
The strength and talent of our workforce are critical to the success of our businesses, and we strive to attract, develop and retain personnel commensurate with the needs of our businesses. Our human capital management priorities are designed to support the execution of our business strategy and improve organizational effectiveness. We contribute to our employees’ financial, health, and social well-being through competitive compensation structures, including a robust employee stock purchase program and retirement benefits, as well as health and well-being programs focused on promoting the physical and mental health of our workforce. We also strive to create opportunities for career development and growth. We provide training and development programs to foster connections, leadership competency, and team and individual development, and we have a tuition reimbursement program to encourage ongoing education.
We understand the importance of a diverse workforce, and we are committed to upholding a culture of diversity, equity, and inclusion. We value the unique contributions of our employees, and we hold firm to the ideals of fairness, equal opportunity and mutual respect for all forms of diversity and differing abilities. We are committed to pay equity and protecting the rights of underrepresented groups within our organization, including women, racial and ethnic minorities, and members of the LGBTQ+ community. Our broader diversity strategies include focus at all levels of our organization, including with senior management and our Board of Directors. As of June 30, 2023, 46% of our global workforce was female and 52% of our U.S. workforce was ethnically diverse.
As of June 30, 2023, we employed 6,423 people, of whom 3,975 were employed in manufacturing, 543 were employed in engineering or research and development, 624 were employed in administration, 342 were employed in sales and marketing and 939 were employed in service capacities. Of the total employees, 2,014 were employed in the Americas, 3,527 were employed in Asia and 882 were employed in Europe.
Available Information
We are subject to the informational requirements of the Exchange Act. Therefore, we file periodic reports, proxy statements and other information with the SEC. The SEC maintains an internet website (http://www.sec.gov) that contains reports, proxy statements and other information that issuers are required to file electronically.
Our internet address is: http://www.osi-systems.com. The information found on, or otherwise accessible through, our website is not incorporated into, and does not form a part of this annual report on Form 10-K or any other report or document we file with or furnish to the SEC. We make available, free of charge through our internet website, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, and reports filed pursuant to Section 16 of the Exchange Act, as soon as reasonably practicable after electronically filing such material with, or furnishing it to, the SEC. Also available on our website free of charge are our Corporate Governance Guidelines, the Charters of our Nominating and Governance, Audit, Compensation and Benefits, Technology, and Risk Management Committees of our Board of Directors and our Code of Ethics and Conduct (which applies to all members of our Board of Directors and employees, including our principal executive officer, principal financial officer and principal accounting officer). A copy of this annual report on Form 10-K is available without charge upon written request addressed to: c/o Secretary, OSI Systems, Inc., 12525 Chadron Avenue, Hawthorne, CA 90250 or by calling telephone number (310) 978-0516.
16
ITEM 1A. RISK FACTORS
Set forth below and elsewhere in this report and in other documents we file with the SEC are descriptions of the risks and uncertainties that could materially and adversely affect our business, financial condition and results of operations and could make an investment in our securities speculative or risky. We encourage you to carefully consider all such risk factors when making investment decisions regarding our company. If any such risks, or any other risks that we do not currently consider to be material, or which are not known to us, materialize, our business, financial condition and operating results could be materially adversely affected.
Business and Industry Risks
If operators of, or algorithms installed on, our security and inspection systems fail to detect weapons, explosives or other devices or materials that are used to commit a terrorist act or other mass casualty event, we could be exposed to product and professional liability and related claims for which we may not have adequate insurance coverage.
Our business exposes us to potential product liability risks that are inherent in the development, manufacturing, sale and service of security and inspection systems, software and threat detection algorithms, as well as in the provision of training to our customers in the use and operation of such systems. Our customers use our security and inspection systems to help them detect items that could be used in performing terrorist acts, mass casualty events or other crimes. Some of our security and inspection systems require that an operator interpret an image of suspicious items within a bag, parcel, container, vehicle or other vessel. Others use algorithms to signal to the operator that further investigation is required. In addition, the training, reliability and competence of the customer’s operator are often crucial to the detection of suspicious items.
Security inspection systems that signal to the operator that further investigation is required are sometimes referred to in the security industry as “automatic” detection systems. Nevertheless, if such a system were to fail to signal to an operator when an explosive, weapon or other contraband was in fact present, resulting in significant loss of life or damage, we would be subject to risk of significant product liability claims. Security inspection by technological means is circumstance and application-specific. Our security and inspection systems offer significant capabilities, but also have performance limitations and cannot be designed to reveal or detect contraband under all circumstances, particularly if criminal actors successfully conceal such items. They can also malfunction or underperform, including if not properly maintained.
We also offer turnkey security screening solutions under which we perform some or all of the security screening tasks that have historically been performed by our customers. Such projects expose us to certain professional liability risks that are inherent in performing security inspection services for the purpose of detecting contraband items, including items that could be used in performing terrorist acts, mass casualty events or other crimes. If a contraband item were to pass through our operations and be used to perform a terrorist act, mass casualty event or other crime, we would be subject to risk of significant professional liability claims.
There are also many other factors beyond our control that could lead to liability claims should an act of terrorism, mass casualty event, or other crime occur. Past terrorism attacks in the U.S. and in other locations worldwide and the potential for future attacks have caused commercial insurance for such threats to become extremely difficult to obtain. In the event that we are found liable following an act of terrorism or other mass casualty event, the insurance we currently have in place would not fully cover the claims for damages. Further, if our security and inspection systems fail to, or are perceived to have failed to, help detect a threat, we could experience negative publicity and reputational harm, which could have a material adverse effect on our business, financial conditions and results of operations.
The loss of certain of our customers, including government agencies that can modify or terminate agreements more easily than other commercial customers with which we contract, the failure to continue to diversify our customer base or the non-renewal of certain material contracts could have a negative effect on our reputation and could have a material adverse effect on our business, financial condition and results of operations.
We sell many of our products to prominent, well-respected institutions, including agencies and departments of the U.S. Government, state and local governments, foreign governments, renowned hospitals and hospital networks, and large military defense and space industry contractors. Many of these larger customers spend considerable resources testing and evaluating our products and our design and manufacturing processes and services. Some of our smaller customers know this and rely on this as an indication of the quality and reliability of our products and services. As a result, part of our reputation and success depends on our ability to continue to sell to larger institutions that are known for demanding high standards of excellence. The loss or termination of a contract by such an institution, even if for reasons unrelated to the quality of our products or services, could therefore have a more wide-spread and potentially material adverse effect on our business, financial condition and results of operations.
17
Our acquisition and alliance activities could result in disruption of our ongoing business and other operational difficulties, unrecoverable costs, and other negative consequences, any of which could adversely impact our financial condition and results of operations.
We intend to continue to make investments in companies, products and technologies, either through acquisitions, investments or alliances. Acquisition and alliance activities often involve risks, including:
| ● | difficulty in assimilating the acquired operations and employees and realizing synergies expected to result from the acquisition; |
| ● | potential liabilities of, or claims against, an acquired company, some of which might not be known until after the acquisition; |
| ● | difficulty in managing product development activities with our alliance partners; |
| ● | difficulty in effectively coordinating sales and marketing efforts; |
| ● | difficulty in combining product offerings and product lines quickly and effectively; |
| ● | difficulty in retaining the key employees of the acquired operation; |
| ● | disruption of our ongoing business, including diversion of management time; |
| ● | inability to successfully integrate the acquired technologies and operations into our businesses and maintain uniform standards, controls, policies and procedures; |
| ● | unanticipated changes in market or industry practices that adversely impact our strategic and financial expectations regarding an acquired company or acquired assets and require us to write off or dispose of such acquired company or assets; |
| ● | lacking the experience necessary to enter into new product or technology markets successfully; and |
| ● | difficulty in integrating financial reporting systems and implementing controls, procedures and policies, including disclosure controls and procedures and internal control over financial reporting, appropriate for public companies of our size at companies that, prior to the acquisition, had lacked such controls, procedures and policies. |
Integrating acquired businesses has been and will continue to be complex, time consuming and expensive, and can negatively impact the effectiveness of our internal control over financial reporting. The use of debt to fund acquisitions or for other related purposes increases our interest expense and leverage. If we issue equity securities as consideration in an acquisition, current stockholders percentage ownership and earnings per share may be diluted. As a result of these and other risks, we cannot be certain that our previous or future acquisitions will be successful and will not materially adversely affect the conduct, operating results or financial condition of our business.
Substantial declines in crude oil prices or extended periods of low crude oil prices may adversely affect our business, financial condition, and results of operations.
Some of our international customers have procurement budgets that are strongly correlated with fluctuations in the price of crude oil. Historically, the market for crude oil has been volatile and unpredictable. Crude oil prices are subject to rapid and significant fluctuations in response to global events and relatively minor changes in supply and demand. While factors relating the price of crude oil to demand for our products and services are complex, a period of depressed crude oil prices may adversely affect our business, financial condition, and results of operations.
Unfavorable currency exchange rate fluctuations could adversely affect our financial results.
Our international sales and our operations in foreign countries expose us to risks associated with fluctuating currency values and exchange rates. Gains and losses on the conversion of accounts receivable, accounts payable and other monetary assets and liabilities to U.S. dollars may contribute to fluctuations in our results of operations. We also use forward contracts which are intended to mitigate the impact of certain foreign currency exposures. These forward contracts may not completely offset foreign currency gains and losses. In addition, since we conduct business in currencies other than the U.S. dollar but report our financial results in U.S. dollars, increases or decreases in the value of the U.S. dollar relative to other currencies could have a material adverse effect on our business, financial condition and results of operations.
18
U.S. budgeting process disruptions could reduce government spending, which could adversely impact our revenues, earnings, cash flows and financial condition.
Funding for U.S. federal Government activities takes place on an annual basis with the Government fiscal year beginning on October 1 and ending on September 30. In recent years, the budgeting process has often not been completed by October 1st, which has required temporary extensions of funding authority, known as a continuing resolution. Because the provision of appropriated funds is undertaken on an annual basis and subject to budgetary rules and requirements, there can be disruptions to federal funding of current and future procurements.
We face aggressive competition in each of our operating divisions. If we do not compete effectively, our business will be harmed.
We encounter aggressive competition from numerous competitors in each of our divisions. In the security and inspection and patient monitoring and cardiology systems markets, competition is based primarily on such factors as product performance, functionality and quality, prior customer relationships, technological capabilities of the product, price, certification by government authorities, past performance, local market presence and breadth of sales and service organization. In the optoelectronic devices and electronics manufacturing markets, competition is based primarily on factors such as expertise in the design and development of optoelectronic devices, product quality, timeliness of delivery, price, customer technical support and on the ability to provide fully-integrated services from application development and design through volume subsystem production. We may not be able to compete effectively with all of our competitors. To remain competitive, we must develop new products and enhance our existing products and services in a timely manner. We anticipate that we may have to downward adjust the prices of many of our products to stay competitive. In addition, new competitors may emerge and entire product lines or service offerings may be threatened by new technologies or market trends that reduce the value of these product lines or service offerings. Our failure to compete effectively could have a material adverse effect on our business, financial condition and results of operations.
Healthcare cost containment pressures and legislative or regulatory reforms may affect our ability to sell our products profitably.
Third-party payers globally are developing increasingly sophisticated methods of controlling healthcare costs which can limit the amount that healthcare providers may be willing to pay for medical devices. In the United States, hospital and other healthcare provider customers that purchase our products typically bill various third-party payers to cover all or a portion of the costs and fees associated with the procedures or tests in which our products are used and bill patients for any deductibles or copayments. Because there is often no separate reimbursement for our products, any decline in the amount payers are willing to reimburse our customers for the procedures and tests associated with our products could make it difficult for customers to continue using, or adopt, our products and create additional pricing pressure for us.
There have been, and we expect there will continue to be, legislative and regulatory proposals to change the healthcare system, and some could significantly affect the ways in which doctors, hospitals, healthcare systems and health insurance companies are compensated for the services they provide, which could have a material impact on our business. It is not clear at this time what changes may impact the ability of hospitals and hospital networks to purchase the patient monitoring, cardiology and remote monitoring, and connected care systems that we sell or if it will alter market-based incentives that hospitals and hospital networks currently face to continually improve, upgrade and expand their use of such equipment.
Efforts by governmental and third-party payers to reduce healthcare costs or the implementation of new legislative reforms imposing additional government controls could cause a reduction in sales or in the selling price of our products, which could adversely affect our business, financial condition and results of operations.
19
Consolidation in the healthcare industry could have a material and adverse effect on our revenues and results of operations.
The healthcare industry has been consolidating and organizations such as group purchasing organizations, independent delivery networks, and large single accounts, such as the United States Veterans Administration, continue to consolidate purchasing decisions for many of our healthcare provider customers. As a result, transactions with customers are larger, more complex and tend to involve more long-term contracts. The purchasing power of these larger customers has increased, and may continue to increase, causing downward pressure on product pricing. If we are not one of the providers selected by one of these organizations, we may be precluded from making sales to its members or participants. Even if we are one of the selected providers, we may be at a disadvantage relative to other selected providers that are able to offer volume discounts based on purchases of a broader range of products. Further, we may be required to commit to pricing that has a material adverse effect on our revenues and profit margins, business, financial condition and results of operations. We expect that market demand, governmental regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances, which may exert further downward pressure on the prices of our products and could materially and adversely impact our business, financial condition, and results of operations.
Technological advances and evolving industry and regulatory standards and certifications could reduce our future product sales, which could cause our revenues to grow more slowly or decline.
The markets for our products are characterized by rapidly changing technology, changing customer needs, evolving industry or regulatory standards and certifications and frequent new product introductions and enhancements. The emergence of new industry or regulatory standards and certification requirements in related fields may adversely affect the demand for our products. This could happen, for example, if new standards and technologies emerge that were incompatible with customer deployments of our applications. In addition, any products or processes that we currently offer or plan to develop may become obsolete or uneconomical before we recover all or any of the expenses incurred in connection with their development. We cannot provide assurance that we will succeed in developing and marketing product enhancements or new products that respond to technological change, new industry standards, changed customer requirements or competitive products on a timely and cost‑effective basis. Additionally, even if we are able to develop new products and product enhancements to meet any such standards, we cannot provide assurance that they will be profitable or that they will achieve market acceptance.
We develop certain of our security inspection technologies to meet the certification requirements of various government regulatory agencies worldwide, including the U.S. Transportation Security Administration and the European Civil Aviation Conference among others. Such standards change as threat and risk assessments evolve and as new technology becomes available within the industry, which enables regulators to demand performance improvements. We may not ultimately be able to develop technologies, or develop in a timely way solutions that are ultimately able to meet the new standards.
Certain of our U.S. Government contracts are dependent upon our employees obtaining and maintaining required security clearances, as well as our ability to obtain security clearances for the facilities in which we perform sensitive government work.
Certain of our U.S. Government contracts require our employees to maintain various levels of security clearances, and we are required to maintain certain facility security clearances. If we cannot maintain or obtain the required security clearances for our facilities and our employees, or obtain these clearances in a timely manner, we may be unable to perform certain U.S. Government contracts. Further, loss of a facility clearance, or an employee’s failure to obtain or maintain a security clearance, could result in a U.S. Government customer terminating an existing contract or choosing not to renew a contract. Lack of required clearances could also impede our ability to bid on or win new U.S. Government contracts. This could damage our reputation and adversely affect our business, financial condition and results of operations.
We could be subject to changes in our tax rates, the adoption of new U.S. or international tax legislation, or exposure to additional tax liabilities.
We are subject to taxes in the U.S. and numerous foreign jurisdictions. Tax rates in various jurisdictions may be subject to significant change due to economic and political conditions or otherwise. Our effective tax rates could be affected by changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, or adoption of new tax legislation or changes in tax laws or their interpretation.
20
We are also subject to the examination of our tax returns and other tax matters by the U.S. Internal Revenue Service and other tax authorities and governmental bodies. We regularly assess the likelihood of an adverse outcome resulting from these examinations to determine the adequacy of our provision for taxes. There can be no assurance as to the outcome of these examinations. If our effective tax rates were to increase, or if the ultimate determination of our taxes owed is for an amount in excess of amounts previously accrued, our financial condition and operating results could be materially adversely affected.
The conflict between Russia and Ukraine and the related implications may negatively impact our operations.
In February 2022, Russia invaded Ukraine. As a result, the U.S. and certain other countries have imposed sanctions on Russia and could impose further sanctions that could damage or disrupt international commerce and the global economy. It is not possible to predict the broader or longer-term consequences of this conflict or the sanctions imposed to date, which could include further sanctions, embargoes, regional instability, geopolitical shifts and adverse effects on macroeconomic conditions, security conditions, currency exchange rates and financial markets. Such geopolitical instability and uncertainty could have a negative impact on our ability to sell to, ship products to, collect payments from, and support customers in certain regions based on trade restrictions, embargoes and export control law restrictions, and logistics restrictions including closures of air space, and could increase the costs, risks and adverse impacts from supply chain and logistics challenges.
As a result of the conflict between Russia and Ukraine, there is also an increased likelihood of cyberattacks or cybersecurity incidents that could either directly or indirectly impact our operations. Any attempts by cyber attackers to disrupt our information systems or the information systems of our vendors, if successful, could harm our business, result in the misappropriation of funds, be expensive to remedy, and damage our reputation or brand. Insurance may not be sufficient to cover significant expenses and losses related to such cyberattacks and cybersecurity incidents.
The potential effects of the conflict between Russia and Ukraine also could impact many of the other risk factors described herein. Given the evolving nature of this conflict, the related sanctions, potential governmental actions and economic impact, such potential impacts remain uncertain. We have certain research and development activities within Ukraine for our Healthcare division which have been somewhat impacted and while we expect the impacts of conflict between Russia and Ukraine to continue to have an effect on our business, financial condition and results of operations, we are unable to predict the extent or nature of these impacts at this time.
Operational Risks
As a U.S. Government contractor, we are subject to extensive Federal procurement rules and regulations as well as contractual obligations that are unique to doing business with the U.S. Government. Non-compliance with any such rules, regulations or contractual obligations could negatively affect current programs, potential awards and our ability to do business with the U.S. Government in the future.
U.S. Government contractors must comply with extensive procurement regulations and other requirements including, but not limited to, those appearing in the Federal Acquisition Regulation (FAR) and its supplements, as well as specific procurement rules and contractual conditions imposed by various U.S. Government agencies. In addition, U.S. Government contracts typically contain provisions and are subject to laws and regulations that provide government agencies rights not typically found in commercial contracts, including the ability to: (i) terminate, reduce the value of, or otherwise modify existing contracts; (ii) suspend or prohibit us from doing business with the government or a specific government agency; and (iii) claim rights in technologies and systems invented, developed or produced by us at the government’s expense.
U.S. Government agencies and the agencies of many other governments with which we contract can terminate their contracts with us for convenience, and in that event, we generally may recover only our incurred costs and expenses on the work completed prior to termination. If an agency terminates a contract with us for default, we may be denied any recovery and may be liable for excess costs incurred by the agency in procuring undelivered items from an alternative source. Decisions by an agency to terminate one of our contracts for default could negatively affect our ability to win future awards not only from such agency, but also from other government agencies and commercial customers, many of whom evaluate past performance, or are required to review past performance information, when making their procurement decisions.
21
U.S. Government agencies may also initiate civil False Claims Act litigation against us based on allegations related to our performance of contracts for the U.S. Government, or to our compliance with procurement regulations and other legal requirements to which such contracts are subject, or both. Such litigation can be expensive to defend and if found liable can result in treble damages and significant civil penalties. The U.S. Government may also initiate administrative proceedings that, if resulting in an adverse finding against us or any of our subsidiaries as to our present responsibility to be a U.S. Government contractor or subcontractor, could result in our company or our subsidiaries being suspended for a period of time from eligibility for award of new government contracts or task orders or in a loss of export privileges and, if satisfying the requisite level of seriousness, in our debarment from contracting with the U.S. Government for a specified term as well as being subject to other remedies available to the U.S. Government. The occurrence of any of the foregoing events could result in a material adverse effect on our business, financial condition and results of operations.
Due to the competitive process to obtain contracts and the likelihood of protests, we may be unable to achieve or sustain revenue growth and profitability.
A significant portion of our business is generally awarded through a competitive bidding process, which involves substantial costs, including cost and time to prepare bids and proposals for contracts that may not be awarded to us, may be split among competitors or that may be awarded but for which we do not receive meaningful task orders. Following contract award, we may encounter significant expense, delay, contract modifications or even contract loss as a result of our competitors protesting the award of contracts to us in competitive bidding. Any resulting loss or delay of start-up and funding of work under protested contract awards may adversely affect our revenues and profitability. In addition, multi-award contracts require that we make sustained post-award efforts to obtain task orders under the contract. As a result, we may not be able to obtain these task orders or recognize revenues under these multi-award contracts. Our failure to compete effectively in this procurement environment would adversely affect our revenues and profitability.
Our revenues are dependent on orders of security and inspection systems, turnkey security screening solutions and patient monitoring and cardiology and remote monitoring systems, which may have lengthy and unpredictable sales cycles.
Sales of security and inspection systems and turnkey security screening solutions often depend upon the decision of governmental agencies to upgrade or expand existing airports, border crossing inspection sites, seaport inspection sites, military facilities and other security installations. In the case of turnkey security screening solutions, the commencement of screening operations may be dependent on the approval, by a government agency, of the protocols and procedures that our personnel are to follow during the performance of their activities. In addition, turnkey screening solutions projects, in contrast to the sale and installation of security inspection equipment, also require that we hire and manage large numbers of local personnel in jurisdictions where we may not have previously operated. Sales outside of the United States of our patient monitoring and cardiology and remote monitoring systems depend in significant part on the decision of governmental agencies to build new medical facilities or to expand or update existing medical facilities. Accordingly, a significant portion of our sales of security and inspection systems, turnkey security screening solutions and our patient monitoring and cardiology and remote monitoring systems is often subject to delays associated with the lengthy approval processes. During these approval periods, we expend significant financial and management resources in anticipation of future revenues that may not occur. If we fail to receive such revenues after expending such resources, such failure could have a material adverse effect on our business, financial condition and results of operations.
If we do not introduce new products in a timely manner, our products could become obsolete and our operating results would suffer.
We sell many of our products in industries characterized by rapid technological changes, frequent new product and service introductions and evolving industry standards and customer needs. Without the timely introduction of new products and enhancements, our products could become technologically obsolete over time, in which case our revenue and operating results would suffer. The success of our new product offerings will depend upon several factors, including our ability to: (i) accurately anticipate customer needs; (ii) innovate and develop new technologies and applications; (iii) successfully commercialize new technologies in a timely manner; (iv) price our products competitively and manufacture and deliver our products in sufficient volumes and on time; and (v) differentiate our offerings from our competitors’ offerings. Some of our products are used by our customers to develop, test and manufacture their products. We therefore must anticipate industry trends and develop products in advance of the commercialization of our customers’ products. In developing any new product, we may be required to make a substantial investment before we can determine the commercial viability of the new product. If we fail to accurately foresee our customers’ needs and future activities, we may invest heavily in research and development of products that do not lead to significant revenues.
22
Interruptions in our ability to purchase raw materials and subcomponents may adversely affect our profitability.
We purchase raw materials and certain subcomponents from third parties. We generally do not have guaranteed long-term supply arrangements with our suppliers. In addition, for certain raw materials and subcomponents that we use, there are a limited number of potential suppliers that we have qualified or that we are currently able to qualify. Consequently, some of the key raw materials and subcomponents that we use are currently available to us only from a single vendor. The reliance on a single qualified vendor could result in delays in delivering products or increases in the cost of manufacturing the affected products. Any material interruption in our ability to purchase necessary raw materials or subcomponents or a significant increase in price of raw materials or subcomponents could adversely affect our ability to fulfill customer orders and therefore could ultimately have a material adverse effect on our business, financial condition and results of operations.
We contract with third parties that may be unable to fulfill contracts on time.
We contract with third-party vendors to service our equipment in the field. We have made such arrangements because sometimes it is more efficient to outsource these activities than it is for our own employees to service our equipment. In addition, some of these vendors maintain stocks of spare parts that are more efficiently accessed in conjunction with a service agreement than would be the case if we were to maintain such spare parts independently. Any material interruption in the ability of our vendors to fulfill such service contracts could adversely affect our ability to fulfill customer orders and therefore could ultimately have a material adverse effect on our business, financial condition and results of operations.
Additionally, purchasers of our security and inspection systems and turnkey security screening solutions sometimes require the construction of the facilities that will house our systems and/or operations. We engage qualified construction firms to perform this work. However, if such firms experience delays, if they perform sub-standard work or if we fail to properly monitor the quality of their work or the timeliness of their progress, we may not be able to complete our construction projects on time. In any such circumstance, we could face the imposition of delay penalties and breach of contract claims by our customer. Any material delay caused by our construction firm subcontractors could therefore ultimately have a material adverse effect on our business, financial condition and results of operations.
We accumulate excess inventory from time to time.
Because of long lead times and specialized product designs, in certain cases we purchase components and manufacture products in anticipation of customer orders based on customer forecasts. For a variety of reasons, such as decreased end-user demand for our products or other factors, our customers might not purchase all the products that we have manufactured or for which we have purchased components. To the extent that we are unsuccessful in recouping our material and manufacturing costs, this could have a material adverse effect on our business, financial condition and results of operations. In addition, because of the complex customer acceptance criteria associated with some of our products, on some occasions, products the title of which has passed to our customers are still included in our inventory until revenue recognition criteria are met. As a result, inventory levels are elevated from time to time.
Economic, political, legal, operational and other risks associated with international sales and operations could adversely affect our financial performance.
Our businesses are subject to risks associated with doing business internationally. We anticipate that revenues from international operations will continue to represent a substantial portion of our total revenue. In addition, many of our manufacturing facilities, and therefore employees, suppliers, real property, capital equipment, cash and other assets are located outside the United States. Accordingly, our future results could be harmed by a variety of factors, including without limitation:
| ● | changes in foreign currency exchange rates; |
| ● | changes in a country’s or region’s political or economic conditions, particularly in developing or emerging markets; |
| ● | political and economic instability, including the possibility of civil unrest, terrorism, mass violence or armed conflict; |
| ● | longer payment cycles of foreign customers and difficulty of collecting receivables in foreign jurisdictions; |
| ● | imposition of domestic and international taxes, export controls, tariffs, embargoes, sanctions, trade disputes, and other trade restrictions; |
| ● | difficulty in staffing and managing widespread operations; |
| ● | difficulty in managing distributors and sales agents and their compliance with applicable laws; |
| ● | changes in a foreign government’s budget, leadership and national priorities; |
| ● | increased legal risks arising from differing legal systems; and |
| ● | compliance with export control and anticorruption legislation, including but not limited to, the Foreign Corrupt Practices Act and UK Bribery Act and International Traffic in Arms Regulations. |
23
There are inherent risks associated with operations in Mexico.
We are currently in the process of fulfilling agreements to provide cargo and vehicle inspection systems and related services to government customers in Mexico. These agreements are significant to our business, financial condition and results of operations. The following are certain risks to operating in Mexico that could adversely impact our operations and have a material adverse effect on our business, financial condition and results of operations: (i) ability of key suppliers and subcontractors to fulfill obligations on a timely basis; (ii) cooperation of various departments of the Mexican government in issuing permits, and inspecting our operations on a timely basis; (iii) receipt of payments in a timely manner; (iv) significant penalties in the event of our late delivery or non-performance; (v) termination or change in scope of program at the election of the Mexican government; (vi) regional political and economic instability; (vii) high rate of crime in Mexico where we conduct operations; and (viii) change in the value of the Mexican peso.
Our operations are vulnerable to interruption or loss due to natural disasters, epidemics or pandemics such as COVID-19, terrorist acts and other events beyond our control, which could adversely impact our operations.
Although we perform manufacturing in multiple locations, we generally do not have redundant manufacturing capabilities in place for any particular product or component. As a result, we depend on our current facilities for the continued operation of our business. A natural disaster, epidemic or pandemic, terrorist act, act of war, civil unrest, or other natural or manmade disaster affecting any of our facilities could significantly disrupt our operations, or delay or prevent product manufacturing and shipment for the time required to repair, rebuild, or replace our manufacturing facilities. This delay could be lengthy and we could incur significant expenses to repair or replace the facilities. Any similar natural or manmade disaster that affects a key supplier or customer could lead to a similar disruption in our business.
As an example, the COVID-19 pandemic resulted in governments around the world implementing stringent measures to help combat the spread of the virus, including quarantines, “shelter in place” and “stay at home” orders, travel restrictions, business curtailments, school closures, and other measures, which has led to a global economic slowdown and impacted the financial markets of many countries. In particular, the COVID-19 pandemic significantly reduced airline passenger traffic, which reduced demand for certain of our security screening products and services. To slow and limit the transmission of COVID-19, governments across the world imposed significant air travel restrictions. These restrictions reduced demand for security screening products and related services at airport checkpoints globally. The global supply chain has also been disrupted. Staffing or personnel shortages due to pandemic-related shelter-in-place orders and quarantines have impacted and may continue to impact us and our suppliers. There have been widespread shortages in certain product categories. If the supply chain for materials used in our production process continues to be adversely impacted by COVID-19 or otherwise, our business, financial condition, and results of operations may be materially and adversely impacted.
Any recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products that leads to corrective actions, could have a material adverse impact on us.
Although we believe that existing data continue to support the efficacy and safety of our patient monitoring, cardiology, and connected care products, in the future, longer term study outcomes could demonstrate conflicting clinical effectiveness, a reduction of effectiveness, no clinical effectiveness or longer-term safety issues. This type of differing data could have a detrimental effect on the market penetration and usage of our medical device products. As a result, our sales may decline or expected growth would be negatively impacted. This could negatively impact our operating condition and financial results.
More generally, all medical devices can experience performance problems that require review and possible corrective action by us or a component supplier. We cannot provide assurance that there will not be component failures, manufacturing errors, noncompliance with quality system requirements or good manufacturing practices, design defects, software defects or labeling inadequacies in any device that could result in an unsafe condition or injury to the patient. The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or if a product poses an unacceptable risk to health. Manufacturers may also, under their own initiative, stop shipment or recall a product if any material deficiency is found or withdraw a product to improve device performance or for other reasons. A government mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, noncompliance with good manufacturing practices or quality system requirements, design or labeling defects or other deficiencies and issues. Similar regulatory agencies in other countries have similar authority to recall products because of material deficiencies or defects in design or manufacture that could endanger health. A recall involving our products could be particularly harmful to our business, financial and operating results. In addition, under the FDA’s medical device reporting regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA or a foreign governmental authority could take enforcement action for failing to report the recalls when they were conducted.
24
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA or applicable foreign regulatory authority may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties, civil penalties or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales as well as face material adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future.
Any adverse event involving our products, whether in the United States or abroad, could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall, orders of repair, replacement or refund or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital and may harm our reputation and financial results.
We rely on third parties and our own systems for interaction with our customers and suppliers and employees, and a failure of a key information technology system, process or site or any other failure or interruption in the services provided by these third parties or our own systems could have a material adverse impact on our ability to conduct business.
We rely extensively on our information technology systems and systems and services provided by third parties to interact with our employees and our customers and suppliers. These interactions include, but are not limited to, ordering and managing materials from suppliers, converting materials to finished products, shipping product to customers, processing transactions, summarizing and reporting results of operations, transmitting data used by our service personnel and by and among our wide-spread personnel and facilities, complying with regulatory, legal and tax requirements, and other processes necessary to manage our business. We do not control our third-party service providers and we do not maintain redundant systems for some of such services, increasing our vulnerability to problems with such services. If the systems on which we rely are damaged or cease to function properly due to any number of causes, ranging from failures of our third-party service providers to catastrophic events, to power outages, to security breaches, we may suffer interruptions in our ability to manage operations which may adversely impact our business, results of operations and/or financial condition.
We could suffer a loss of revenue and increased costs, exposure to significant liability, reputational harm, and other serious negative consequences if we sustain cyber-attacks or other data security breaches that disrupt our operations or result in the dissemination of proprietary or confidential information about us or our customers, suppliers, or other third parties; our products and services may be subject to potential cyber-attacks or other information technology vulnerabilities.
We manage and store proprietary, sensitive and confidential data related to our business operations. We may be subject to cyber-attacks and breaches of the information technology systems we use for these purposes. Experienced programmers and hackers may be able to penetrate our network security and misappropriate or compromise our confidential information or that of third parties, create system disruptions, or cause shutdowns. Hackers may also be able to develop and deploy viruses, worms, malware, ransomware and other malicious software programs that attack our systems or otherwise exploit security vulnerabilities in our systems or products. In addition, sophisticated hardware and operating system software and applications that we produce or procure from third parties may contain defects in design or manufacturing, including “bugs” and other problems that could unexpectedly interfere with the operation of our systems or products. Cyber-threats vary in technique, are persistent, frequently change, and increasingly are more sophisticated, targeted, and difficult to detect or prevent.
We expend significant capital and resources to protect against the threat of security breaches, including cyber-attacks, viruses, worms, malware, ransomware and other malicious software programs. Substantial additional expenditures may be required before or after a cyber-attack to mitigate or alleviate problems caused by the unauthorized access, theft of data stored within our information systems, or the introduction of computer malware or ransomware to our environment. Our remediation efforts may not be successful, and there could be interruptions, delays, or cessation of service due to cyber-attacks or other data security breaches.
We often identify attempts to gain unauthorized access to our systems. Given the rapidly evolving nature and proliferation of cyber threats, there can be no assurance that our employee training, operational, and other technical security measures or other controls will detect, prevent or remediate security or data breaches in a timely manner or otherwise prevent unauthorized access, damage, or interruption of our systems and operations. We are likely to face attempted cyber-attacks in the future. Accordingly, we may be vulnerable to losses associated with the improper functioning, security breach, or unavailability of our information systems as well as any systems used in acquired operations.
25
In addition, breaches of our security measures and the unapproved use or disclosure of proprietary information or sensitive or confidential data about us or our suppliers, customers or other third parties could expose us or any such affected third party to a risk of loss or misuse of this information, result in litigation and potential liability for us, damage our brand and reputation or otherwise harm our business, even if we were not responsible for the breach. Furthermore, we are exposed to additional risks because we rely in certain capacities on third-party software, data management, and cloud service providers with possible security problems and security vulnerabilities beyond our control. Media or other reports of perceived security vulnerabilities to our systems or those of our third-party suppliers, even if no breach has been attempted or occurred, could adversely impact our brand and reputation and materially impact our business.
Our products and services may also be at risk of cyber-attacks and security breaches. While we design and build security measures into our products and services, once installed and implemented at customer sites those measures may not prevent all cybersecurity attacks targeted against their networks and datacenters, such as the unauthorized access, capture, or alteration of information; the exposure or exploitation of potential security vulnerabilities; distributed denial of service attacks; the installation of malware or ransomware; acts of vandalism; computer viruses; or misplaced data or data loss.
A significant actual or perceived (whether or not valid) theft, loss, fraudulent use or misuse of customer, employee, or other personally identifiable data, whether by us, our partners and vendors, or other third parties, or as a result of employee error or malfeasance or otherwise, non-compliance with applicable industry standards or our contractual or other legal obligations regarding such data, or a violation of our privacy and information security policies with respect to such data, could result in costs, fines, litigation, or regulatory actions against us. Such an event could additionally result in unfavorable publicity and therefore materially and adversely affect the market’s perception of the security and reliability of our products and services and our credibility and reputation with our customers.
Given increasing cyber security threats, there can be no assurance that we will not experience business interruptions, data loss, ransom, misappropriation, or corruption or theft or misuse of proprietary information or related litigation and investigation, any of which could have a material adverse effect on our financial condition and results of operations and harm our business reputation.
Delays, costs, and disruptions that result from upgrading, integrating and maintaining the security of our information and technology networks and systems could materially adversely affect us.
We are dependent on information technology networks and systems, including Internet and Internet-based or “cloud” computing services, to collect, process, transmit, and store electronic information. We are currently modernizing and upgrading our information technology systems while simultaneously integrating systems from our various acquisitions, including making changes to legacy systems, and replacing some legacy systems with new and advanced functionality. While upgrading and implementing change to any one of our systems could present challenges, the age of our systems and architecture may present unique challenges that we have not previously encountered as we undertake these efforts. There are inherent costs and risks associated with integrating, replacing and changing these systems and implementing new systems, including potential disruption of our sales and operations, potential disruption of our internal control structure, substantial capital expenditures, additional administration and operating expenses, demands on management time, securing our systems along with dependent processes from cybersecurity threats, and other risks and costs of delays or difficulties in transitioning to new systems or of integrating new systems into our current systems. The implementation of or delay in implementing new information technology systems may also cause disruptions in our business operations and impede our ability to comply with constantly evolving laws, regulations and industry standards addressing information and technology networks, privacy and data security, any of which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Our inability to successfully manage the implementation of a company-wide enterprise resource planning (“ERP”) system could adversely affect our operating results.
We are in the process of implementing a new company-wide ERP system. This process has been and continues to be complex and time-consuming and we expect to incur additional capital outlays and expenses. This ERP system will modernize and replace many of our existing operating and financial systems, which is a major undertaking from a financial management and personnel perspective. Should the new ERP system not be implemented successfully throughout all our business units, be significantly delayed or over-budget or if the system does not perform in a satisfactory manner, it could be disruptive and adversely affect our operations, including our potential ability to report accurate, timely and consistent financial results, our ability to purchase supplies, components and raw materials from suppliers, and our ability to timely deliver products and services to customers and/or collect receivables from them. If the new ERP system is not successfully and fully implemented, it could negatively affect our financial reporting, inventory management, future sales, profitability and financial condition.
26
Our credit facility contains provisions that could restrict our ability to finance our future operations or engage in other business activities that may be in our interest.
Our credit facility contains a number of significant covenants that, among other things, limit our ability to: (i) dispose of assets; (ii) incur certain additional indebtedness; (iii) repay certain indebtedness; (iv) create liens on assets; (v) pay dividends on our Common Stock; (vi) make certain investments, loans and advances; (vii) repurchase or redeem capital stock; (viii) make certain capital expenditures; (ix) engage in acquisitions, mergers or consolidations; and (x) engage in certain transactions with subsidiaries and affiliates.
These covenants could limit our ability to plan for or react to market conditions, finance our operations, engage in strategic acquisitions or disposals or meet our capital needs or could otherwise restrict our activities or business plans. Our ability to comply with these covenants may be affected by events beyond our control. In addition, our credit facility also requires us to maintain compliance with certain financial ratios. Our inability to comply with the required financial ratios or covenants could result in an event of default under our credit facility. A default, if not cured or waived, may permit acceleration of our indebtedness. In addition, our lenders could terminate their commitments to make further extensions of credit under our credit facility. If our indebtedness is accelerated, we cannot be certain that we will have sufficient funds to pay the accelerated indebtedness or that we will have the ability to refinance accelerated indebtedness on terms favorable to us or at all. If we are not able to refinance existing indebtedness on acceptable terms, our ability to finance our operations, engage in strategic acquisitions, and otherwise meet our capital needs would be significantly impaired.
Legal and Regulatory Risks
The Support Anti-terrorism by Fostering Effective Technologies Act of 2002 (SAFETY Act) may not shield us against legal claims we may face following an act of terrorism.
The SAFETY Act provides important legal liability protections for providers of qualified anti-terrorism products and services. Under the SAFETY Act, providers, such as our Security division, may apply to the U.S. Department of Homeland Security for coverage of their products and services. If granted coverage, such providers receive certain legal protections against product liability, professional liability and certain other claims that could arise following an act of terrorism. We have applied to the U.S. Department of Homeland Security for many of the products and services offered by our Security division, but we do not enjoy coverage under the SAFETY Act (or the highest level of coverage) for every product line, model number and service offering that our Security division provides. In addition, the terms of the SAFETY Act coverage decisions awarded to us by the U.S. Department of Homeland Security restrict coverage to specific model numbers, software, and options within our product lines, sales to specific customers, and impose various other limitations, and contain conditions and requirements that we may not (or may not be able to) continue to satisfy in the future. Delays by the U.S. Department of Homeland Security in granting coverage (or extensions of coverage) and in our ability to meet the evolving standards of the SAFETY Act application process has and may in the future continue to result in coverage limitations for our products and services.
If we fail to maintain SAFETY Act protections for each of our product models, options, offerings, software and services, or fail to apply in a timely way for coverage for new products, models, and services as we acquire or introduce them, or if the U.S. Department of Homeland Security limits the scope of any coverage previously awarded to us, denies us coverage or continued coverage for a particular product, product line, model, option, offering, software feature, or service, or delays in making decisions about whether to grant us coverage, we may become exposed to legal claims that the SAFETY Act was otherwise designed to prevent. Moreover, the SAFETY Act was not designed to shield providers of qualified anti-terrorism products and services from all types of claims that may arise from acts of terrorism, including from many types of claims lodged in courts outside of the United States or acts of terrorism that occur outside of the United States, which exposes us to legal claims and litigation defense costs despite the SAFETY Act awards we have received.
Our patient monitoring, cardiology and remote monitoring, and connected care systems could give rise to product liability claims and product recall events that could materially and adversely affect our financial condition and results of operations.
The development, manufacturing and sale of medical devices expose us to significant risk of product liability claims, product recalls and, sometimes, product failure claims. We face an inherent business risk of financial exposure to product liability claims if the use of our medical devices results in personal injury or death. Substantial product liability litigation currently exists within the medical device industry. Some of our patient monitoring, cardiology and remote monitoring, and connected care products may become subject to product liability claims and/or product recalls. Future product liability claims and/or product recall costs may exceed the limits of our insurance coverages, or such insurance may not continue to be available to us on commercially reasonable terms, or at all. In addition, a significant product liability claim or product recall could significantly damage our reputation for producing safe, reliable and effective products, making it more difficult for us to market and sell our products in the future. Consequently, a product liability claim, product recall or other claim could have a material adverse effect on our business, financial condition and results of operations.
27
Our global operations expose us to legal compliance risks related to certain anti-bribery and anti-corruption laws.
We are required to comply with the U.S. Foreign Corrupt Practices Act, which prohibits United States companies from engaging in bribery or making other prohibited payments to foreign officials for the purpose of obtaining or retaining business. It also requires us to maintain specific record-keeping standards and adequate internal accounting controls. In addition, we are subject to similar requirements in other countries. Bribery, corruption, and trade laws and regulations, and the enforcement thereof, are increasing in frequency, complexity and severity on a global basis. Although we have internal policies and procedures with the intention of assuring compliance with these laws and regulations, our employees, distributors, resellers and contractors involved in our international sales may take actions in violations of such policies. If our internal controls and compliance program do not adequately prevent or deter our employees, distributors, resellers, contractors and/or other third parties with which we do business from violating anti-bribery, anti-corruption or similar laws and regulations, we may incur severe fines, penalties and reputational damage.
We are subject to import and export controls that could subject us to liability or impair our ability to compete in international markets.
Due to the international scope of our operations, we are subject to a complex system of import- and export-related laws and regulations, including U.S. export control and customs regulations and customs regulations of other countries. These regulations are complex and vary among the legal jurisdictions in which we operate. Any alleged or actual failure to comply with such regulations may subject us to government scrutiny, investigation, and civil and criminal penalties, and may limit our ability to import or export our products or to provide services outside the United States. Depending on severity, any of these penalties could have a material impact on our business, financial condition and results of operations.
Our business is subject to complex and evolving U.S. and international laws and regulation regarding privacy and data protection. If we fail to meet our compliance obligations under applicable privacy and data protection regulations, even if such compliance by us is inadvertent, or if we are unable to comply with changes to such requirements, we might be subject to fines, legal disputes, or other liabilities that could have a material adverse effect on our financial condition and results of operations.
Regulatory authorities around the world are considering legislative and regulatory proposals concerning data protection, and the interpretation and application of data protection laws in the U.S., the EU, and elsewhere are often uncertain and in flux. These laws may be interpreted and applied in a manner that is inconsistent with our data practices. If our data practices are found to be in conflict with privacy and data protection laws or regulations, we could face fines or orders requiring that we change our data practices, which could have an adverse effect on our business, financial condition and results of operations.
We must comply with extensive federal and state requirements regarding the use, retention, security, and re-disclosure of patient healthcare information. HIPAA and the regulations that have been issued under it contain substantial restrictions and complex requirements with respect to the use and disclosure of certain individually identifiable health information, referred to as “protected health information”. The HIPAA Privacy Rule prohibits a covered entity or a business associate from using or disclosing protected health information unless the use or disclosure is validly authorized by the individual or is specifically required or permitted under the HIPAA Privacy Rule and only if certain complex requirements are met. The HIPAA Security Rule establishes administrative, organizational, physical, and technical safeguards to protect the privacy, integrity, and availability of electronic protected health information maintained or transmitted by covered entities and business associates. The HIPAA Breach Notification Rule requires that covered entities and business associates, under certain circumstances, notify patients when there has been an improper use or disclosure of protected health information. Any failure or perceived failure of our Company or our products to meet HIPAA standards and related regulatory requirements could expose us to certain notification, penalty, and enforcement risks, damage our reputation, and adversely affect demand for our products and force us to expend significant capital and other resources to address the privacy and security requirements of HIPAA.
In addition, there are other federal laws that include specific privacy and security obligations, above and beyond HIPAA, for certain types of health information and impose additional sanctions and penalties. All 50 states, the District of Columbia, Guam, Puerto Rico, and the Virgin Islands have enacted legislation requiring notice to individuals of security breaches involving protected health information, which is not uniformly defined among the breach notification laws. Organizations must review each state’s definitions, mandates, and notification requirements and timelines to appropriately prepare and notify affected individuals and government agencies, including the attorney general, in compliance with such state laws. Further, most states have enacted patient confidentiality laws that protect against the disclosure of confidential medical information, and many states have adopted or are considering adopting further legislation in this area. These state laws may be more stringent than HIPAA requirements. California passed the California Consumer Privacy Act, which imposes significant changes in data privacy regulation, and New York has passed the Stop Hacks and Improve Electronic Data Security Act, which expands the state’s existing privacy laws. GDPR, a regulation implemented on May 25, 2018 in the EU on data protection and privacy for all individuals in the EU and the EEA, applies to all enterprises, regardless of location, that are doing business in the EU or that collect and analyze data tied to EU and EEA residents. GDPR creates a range of compliance obligations, including stringent technical and security controls surrounding the storage, use, and disclosure of personal information, and significantly increases financial penalties for noncompliance.
28
We are facing an increasingly complex international regulatory environment which is constantly changing and if we fail to comply with international regulatory requirements, or are unable to comply with changes to such requirements, our financial performance may be harmed.
Our international operations and sales subject us to an international regulatory environment which is becoming increasingly complex and is constantly changing due to factors beyond our control. Risks associated with our international operations and sales include, without limitation, those arising from the following factors:
| ● | differing legal and court systems and changes to such systems; |
| ● | differing labor laws and changes in those laws; |
| ● | differing tax laws and changes in those laws; |
| ● | differing environmental laws and changes in those laws; |
| ● | differing laws governing our distributors and sales agents and changes in those laws; |
| ● | differing protection of intellectual property and changes in that protection; and |
| ● | differing import and export requirements and changes to those requirements. |
If we fail to comply with applicable international regulatory requirements, even if such non-compliance by us is inadvertent, or if we are unable to comply with changes to such requirements, our financial performance may be harmed.
Substantial government regulation in the United States and abroad may restrict our ability to sell our patient monitoring, cardiology and remote monitoring, and connected care systems, and failure to comply with such laws and regulations may have a material adverse impact on our business.
The FDA and comparable regulatory authorities in foreign countries extensively and rigorously regulate our patient monitoring, cardiology and remote monitoring, and connected care systems, including the research and development, design, testing, clinical trials, manufacturing, clearance or approval, safety and efficacy, labeling, advertising, promotion, pricing, recordkeeping, reporting, import and export, post-approval studies and sale and distribution of these products. In the United States, before we can market a new medical device, or a new use of, new claim for, or significant modification to, an existing product, we must first receive clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act as discussed under Part I, Item 1, “Business - Regulation of Medical Devices.” Some modifications made to products cleared through a 510(k) may require a new 510(k). The FDA can delay, limit or deny clearance or approval of a device for many reasons.
Our future products may not obtain FDA clearance on a timely basis, or at all. Further, the FDA makes periodic inspections of medical device manufacturers and in connection with such inspections issues observations when the FDA believes the manufacturer has failed to comply with applicable regulations. If FDA observations are not addressed to the FDA’s satisfaction, the FDA may issue a warning letter or proceed directly to other forms of enforcement action, which could include the shutdown of our production facilities, adverse publicity, and civil and criminal penalties. The expense and costs of any corrective actions that we may take, which may include product recalls, correction and removal of products from customer sites and/or changes to our product manufacturing and quality systems, could adversely impact our financial results. Issuance of a warning letter may also lead customers to delay purchasing decisions or cancel orders.
Our patient monitoring, cardiology and remote monitoring, and connected care systems must also comply with the laws and regulations of foreign countries in which we develop, manufacture and market such products. In general, the extent and complexity of medical device regulation is increasing worldwide. This trend is likely to continue, and the cost and time required to obtain marketing clearance in any given country may increase as a result. Our products may not obtain any necessary foreign clearances on a timely basis, or at all.
29
Once any of our patient monitoring, cardiology and remote monitoring, or connected care systems is cleared for sale, regulatory authorities may still limit the use of such product, prevent its sale or manufacture or require a recall or withdrawal of such product from the marketplace. Following initial clearance from regulatory authorities, we continue to be subject to extensive regulatory requirements. Government authorities can withdraw marketing clearance or impose sanctions due to our failure to comply with regulatory standards or due to the occurrence of unforeseen problems following initial clearance. Ongoing regulatory requirements are wide-ranging and govern, among other things: (i) annual inspections to retain a CE mark for sale of products in the EU; (ii) product manufacturing; (iii) patient health data protection and medical device security; (iv) supplier substitution; (v) product changes; (vi) process modifications; (vii) medical device reporting; and (viii) product sales and distribution.
Legislative or regulatory reforms such as the new EU Medical Devices Regulation may make it more difficult and costly for us to obtain certification, regulatory clearance, or approval of any future products and to manufacture, market, and distribute our products after certification, clearance, or approval is obtained.
Following its entry into application on May 26, 2021, the EU MDR introduced substantial changes to the obligations with which medical device manufacturers must comply in the EEA. High risk medical devices are subject to additional scrutiny during the conformity assessment procedure. Unlike directives such as the EU Medical Devices Directive, which must be implemented into the national laws of EEA countries, the EU MDR is directly applicable, without the need for adoption by EEA country laws implementing them, in all EEA countries and intended to eliminate current differences in regulation of medical devices among EEA countries. The EU MDR, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices to ensure a high level of safety and health while supporting innovation.
The EU MDR imposes a number of new requirements on manufacturers of medical devices and imposes increased compliance obligations for us to access the EEA market. Our failure to comply with applicable foreign regulatory requirements, including those administered by authorities of the EEA countries, could result in enforcement actions against us and impair our ability to market products in the EEA in the future. Any changes to the membership of the EU, such as the departure of the United Kingdom under Brexit, may impact the regulatory requirements for impacted countries and impair our business operations and our ability to market products in such countries. For further discussion of the EU MDR, see Part I, Item 1, “Business - Regulation of Medical Devices.”
We may be subject to fines, penalties, injunctions, or other enforcement actions if we are determined to be promoting the use of our products for unapproved or “off label” uses, resulting in damage to our reputation and business.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by the FDA known as “off label” use. If the FDA determines that our promotional materials or training constitutes promotion of an off label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of warning letters, untitled letters, fines, penalties, consent decrees, injunctions, or seizures, which could have an adverse impact on our reputation and financial results. We could also be subject to enforcement action under other federal or state laws, including the False Claims Act.
Our failure to comply with federal, state, and foreign laws and regulations relating to our healthcare business could have a material and adverse effect on our business.
Although we do not provide healthcare services, submit claims for third-party reimbursement or receive payments directly from Medicare, Medicaid or other third-party payers for our products, we are subject to healthcare fraud and abuse regulation and enforcement by federal and state governments. Healthcare fraud and abuse and health information privacy and security laws potentially applicable to our operations are discussed in Part I, Item 1, “Business – Regulation of Medical Devices.”
The risk of our being found in violation of these laws and regulations is increased because many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amended the intent requirement of the federal Anti Kickback Statute and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them to have committed a violation. In addition, the Affordable Care Act provided that the government may assert that a claim including items or services resulting from a violation of the federal Anti Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
30
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Any action against us for violation of these laws could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could impair our ability to operate our business, financial condition and our financial results.
General Risks
Significant inflation and increasing interest rates could materially and adversely affect our business and financial results.
The current inflation rate could materially and adversely affect us by increasing our operating costs, including our materials, freight, and labor costs, which are already under pressure due to supply chain constraints. In a highly inflationary environment, we may be unable to raise the sales prices of our products to match the rate of inflation or our increasing operating costs, which could reduce our profit margins and have a material and adverse effect on our financial performance. Further, pressures from inflation could negatively impact the willingness and ability of our customers to purchase our products in the same volumes as have been purchased in the past or are currently being purchased.
As interest rates rise to address inflation or otherwise, such increases will impact the base rates applicable in our credit arrangements and will result in borrowed funds becoming more expensive to us over time. These financing pressures also can have a negative impact on customers’ willingness to purchase our products in the same volumes as previously purchased. We also use forward contracts which are intended to mitigate the impact of certain foreign currency exposures. These forward contracts may not completely offset foreign currency gains and losses.
Our insurance coverage may be inadequate to cover all significant risk exposures.
We maintain insurance for certain risks, and we believe our insurance coverage is consistent with general practices within our industry. However, the amount of our insurance coverage may not cover all claims or liabilities and we may be forced to bear substantial costs. Consistent with market conditions in the insurance industry, premiums and deductibles for some of our insurance policies have been increasing and may continue to increase in the future. In some instances, some types of insurance may become available only for reduced amounts of coverage, if at all. In addition, there can be no assurance that our insurers would not challenge coverage for certain claims. If we were to incur a significant liability for which we were not fully insured or that our insurers disputed, it could have a material adverse effect on our business, financial condition and results of operations.
We are involved in various litigation matters, which could have a material adverse effect on our business, financial condition or operating results.
Litigation can be lengthy, expensive and disruptive to our operations, and can divert our management’s attention away from the running of our business. Claims arising out of actual or alleged violations of law could be asserted against us by individuals, either individually or through class actions, or by governmental entities in investigations and proceedings. If we are unsuccessful in our defense in litigation matters, or any other legal proceeding, we may be forced to pay damages or fines, some of which may be in excess of our insurance coverage, and/or change our business practices, any of which could have a material adverse effect on our business, financial condition and results of operations. For more information about our litigation matters, see “Legal Proceedings” and Note 11 to the consolidated financial statements.
31
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
As of June 30, 2023, we owned the following principal facilities:
| Approximate | |||
Square | ||||
Location |
| Description of Facility |
| Footage |
Billerica, Massachusetts |
| Manufacturing, engineering, sales and marketing and service for our Security division |
| 186,200 |
Snoqualmie, Washington |
| Headquarters and administrative, manufacturing, engineering, sales, marketing and service for our Healthcare division |
| 177,000 |
Batam, Indonesia |
| Manufacturing for our Optoelectronics and Manufacturing division |
| 93,500 |
Stoke on Trent, United Kingdom |
| Manufacturing, engineering, sales, marketing and service for our Security division |
| 90,000 |
Surrey, United Kingdom |
| Manufacturing, engineering, sales, marketing and service for our Security division |
| 59,000 |
As of June 30, 2023, we leased the following principal facilities:
| Approximate |
| ||||
Location |
| Description of Facility |
| Square Footage |
| Expiration |
Hawthorne, California | Corporate headquarters and administrative, manufacturing, engineering, sales and marketing and service for our Optoelectronics and Manufacturing division | 88,000 | 2028 | |||
Johor Bahru, Malaysia(1) |
| Manufacturing, engineering, sales and service for our Security division |
| 167,600 |
| 2024 ~ 2025 |
Johor Bahru, Malaysia(1) |
| Manufacturing, engineering, sales and service for our Optoelectronics and Manufacturing division |
| 110,100 |
| 2024 ~ 2025 |
Torrance, California |
| Manufacturing, engineering, sales and marketing and service for our Security division |
| 91,900 |
| 2027 |
Batam, Indonesia (1) |
| Manufacturing for our Optoelectronics and Manufacturing division |
| 105,400 |
| 2023 ~ 2028 |
Andover, Massachusetts |
| Manufacturing, engineering, sales and marketing and service for our Security division |
| 64,200 |
| 2027 |
| (1) | This is comprised of multiple leases at the same or nearby facilities. |
32
We believe that our facilities are in adequate condition to support our current operations but expect to expand as necessary to support our anticipated future growth. We currently anticipate that we will be able to renew the leases that are scheduled to expire in the next few years on terms that are substantially the same as those currently in effect. However, even if we were not able to renew one or more of the leases, we believe that suitable substitute space is available to relocate any of the facilities. Accordingly, we do not believe that our failure to renew any of the leases that are scheduled to expire in the next few years will have a material adverse effect on our operations.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are subject to legal proceedings, claims, and litigation arising in the ordinary course of our business or otherwise. More information regarding legal proceedings in which we are involved can be found under Note 11, “Commitments and Contingencies” of the Notes to the Consolidated Financial Statements in Item 8, which is incorporated by reference into this Item 3.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable
33
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Stock Market and Other Information
Our Common Stock is traded on The Nasdaq Global Select Market under the symbol “OSIS.”
As of August 21, 2023, there were approximately 94 holders of record of our Common Stock. This number does not include beneficial owners holding shares through nominees or in “street” name.
Dividends
We have not paid any dividends since the consummation of our initial public offering in 1997, and we have no intention of paying dividends for the foreseeable future.
Unregistered Sales of Equity Securities
We did not sell any unregistered shares of Common Stock during the fiscal year ended June 30, 2023.
Issuer Purchases of Equity Securities
We did not repurchase any shares of Common Stock during the quarter ended June 30, 2023.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides information concerning our equity compensation plans as of June 30, 2023.
|
|
| Number of securities |
| ||||
remaining available for |
| |||||||
Number of securities to | Weighted‑average | future issuance under |
| |||||
be issued upon exercise | exercise price of | equity compensation |
| |||||
of outstanding options, | outstanding options, | plans (excluding securities |
| |||||
Plan category | warrants and rights | warrants and rights | reflected in column (a)) |
| ||||
| (a) |
| (b) |
| (c) | |||
Equity compensation plans approved by security holders |
| 83,677 |
| $ | 87.09 |
| 764,333 | (1)(2) |
Equity compensation plans not approved by security holders |
| — | N/A |
| — | |||
Total |
| 83,677 |
| $ | 87.09 |
| 764,333 | |
| (1) | These shares are available for future issuance under our Amended and Restated 2012 Incentive Award Plan (the “OSI Plan”), which was approved by our shareholders on December 10, 2020. |
| (2) | Awards of restricted stock units or other awards that convey the full value of the shares subject to the award are counted as 1.87 shares for every one award granted. |
Performance Graph
The graph below compares the cumulative total stockholder return for the period beginning on the market close on the last trading day before the beginning of our fifth preceding fiscal year through and including the end of our last completed fiscal year with (a) The Nasdaq Composite Index and (b) a peer group of publicly traded issuer(s) with which we have generally competed.
The peer group includes the following companies: Conmed Corp, Leidos Holdings Inc. and Smiths Group Plc.
34
The graph assumes that $100.00 was invested on June 30, 2018 in (a) our Common Stock, (b) The Nasdaq Composite Index, and (c) the companies comprising the peer group described above (weighted according to the issuer’s stock market capitalization at the beginning of each period for which a return is indicated). The graph assumes that all dividends were reinvested. Historical stock price performance is not necessarily indicative of future stock price performance.
This performance graph shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or incorporated by reference into any Company filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
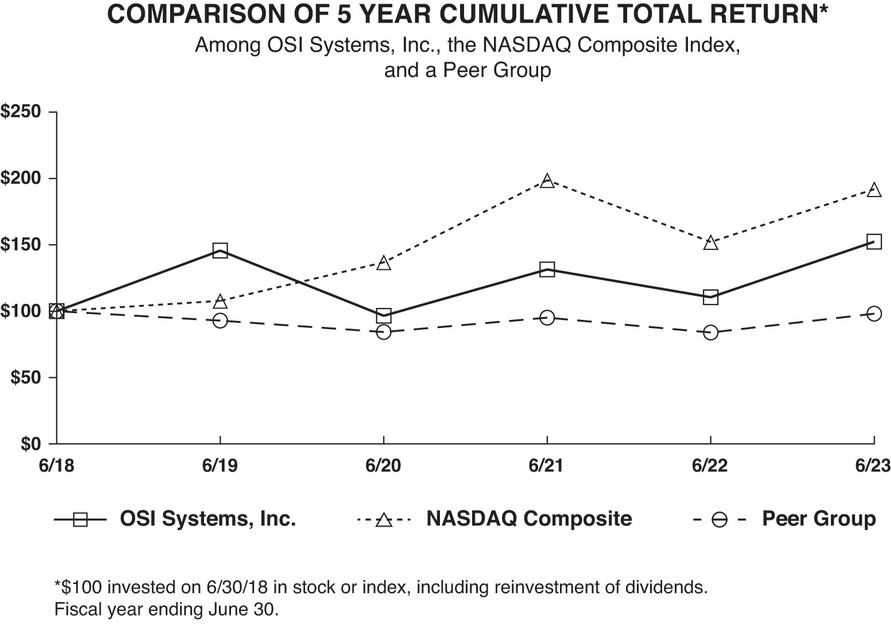
The following table provides the same information in tabular form as of June 30:
| 2018 |
| 2019 |
| 2020 |
| 2021 |
| 2022 |
| 2023 | |
OSI Systems, Inc. |
| 100.00 |
| 145.65 |
| 96.52 |
| 131.44 |
| 110.49 |
| 152.37 |
The Nasdaq Composite Index |
| 100.00 |
| 107.78 |
| 136.82 |
| 198.71 |
| 152.16 |
| 191.93 |
Peer Group |
| 100.00 |
| 92.88 |
| 84.28 |
| 95.11 |
| 83.91 |
| 98.10 |
ITEM 6. [RESERVED]
35
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management’s discussion and analysis of financial condition and results of operations (“MD&A”) is intended to help the reader understand our results of operations and financial condition. MD&A is provided as a supplement to, and should be read in conjunction with, our financial statements and the accompanying notes. This MD&A contains forward-looking statements and the matters discussed in these forward-looking statements are subject to risks, uncertainties, and other factors that could cause actual results to differ materially from those projected or implied in the forward-looking statements. Please see “Risk Factors” and “Forward-Looking Statements” for a discussion of the uncertainties, risks and assumptions associated with these statements.
Overview
We are a vertically integrated designer and manufacturer of specialized electronic systems and components for critical applications. We sell our products and provide related services in diversified markets, including homeland security, healthcare, defense and aerospace. We have three operating divisions, each of which is a reportable segment: (a) Security, providing security and inspection systems and turnkey security screening solutions; (b) Healthcare, providing patient monitoring, cardiology and remote monitoring, and connected care systems and associated accessories; and (c) Optoelectronics and Manufacturing, providing specialized electronic components and electronic manufacturing services for our Security and Healthcare divisions, as well as to third parties for applications in the defense and aerospace markets, among others.
Security Division. Through our Security division, we provide security screening products, software, and services globally, as well as turnkey security screening solutions. These products and services are used to inspect baggage, parcels, cargo, people, vehicles and other objects for weapons, explosives, drugs, radioactive and nuclear materials and other contraband. Revenues from our Security division accounted for 59% of our total consolidated revenues for fiscal 2023.
As a result of terrorist attacks and smuggling operations against the U.S. and in other locations worldwide, security and inspection products have increasingly been used at a wide range of facilities in addition to airports, such as border crossings, seaports, freight forwarding operations, sporting venues, government and military installations, railways, and nuclear facilities. We believe that our wide-ranging product portfolio together with our ability to provide turnkey screening solutions position us to pursue security and inspection opportunities as they arise throughout the world.
Currently, the U.S. Government is discussing various options to address the U.S. Government’s overall fiscal challenges and we cannot predict the outcome of these efforts. While we believe that national security spending will continue to be a priority, U.S. government budget deficits and the national debt have created increasing pressure to examine and reduce spending across many federal agencies. Additionally, there continues to be volatility in international markets that has impacted international security spending. We believe that the diversified product portfolio and international customer mix of our Security division position us well to withstand the impact of these uncertainties and even benefit from specific initiatives within various governments. However, future budgetary reductions may be implemented as both the U.S. Government and other international government customers manage fiscal challenges including those stemming from government spending that occurred during the COVID-19 pandemic; such reductions could have a material, adverse effect on our business, financial condition and results of operations.
Healthcare Division. Through our Healthcare division, we design, manufacture, market and service patient monitoring, cardiology and remote monitoring, and connected care systems globally for sale primarily to hospitals and medical centers. Our products monitor patients in critical, emergency and perioperative care areas of the hospital and provide information, through wired and wireless networks, to physicians and nurses who may be at the patient’s bedside, in another area of the hospital or even outside the hospital. Revenues from our Healthcare division accounted for 15% of our total consolidated revenues for fiscal 2023.
36
The healthcare markets in which we operate are highly competitive. We believe that our customers choose among competing products on the basis of product performance, functionality, price, value and service. Although there has been an increase in demand for patient monitoring products due to the COVID-19 pandemic, there is continued uncertainty regarding the U.S. federal government budget and the Affordable Care Act, either of which may impact hospital spending, third-party payer reimbursement and fees to be levied on certain medical device revenues, any of which could adversely affect our business and results of operations. In addition, hospital capital spending appears to have been impacted by strategic uncertainties surrounding the Affordable Care Act and economic pressures. We also believe that global economic uncertainty has caused some hospitals and healthcare providers to delay purchases of our products and services. During this period of uncertainty, sales of our healthcare products may be negatively impacted. A prolonged delay could have a material adverse effect on our business, financial condition and results of operations.
Optoelectronics and Manufacturing Division. Through our Optoelectronics and Manufacturing division, we design, manufacture and market optoelectronic devices and flex circuits and provide electronics manufacturing services globally for use in a broad range of applications, including aerospace and defense electronics, security and inspection systems, medical imaging and diagnostics, telecommunications, office automation, computer peripherals, industrial automation, and consumer products. We also provide our optoelectronic devices and electronics manufacturing services to OEM customers, and our own Security and Healthcare divisions. Revenues from external customers in our Optoelectronics and Manufacturing division accounted for 26% of our total consolidated revenues for fiscal 2023.
Consolidated Results
Discussion and analysis of our financial condition and results of operations for fiscal 2021 has been omitted from this Annual Report on Form 10-K, and is available in Item 7 of Part II, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K for the year ended June 30, 2022.
Fiscal 2023 Compared with Fiscal 2022. We reported consolidated sales of $1,278.4 million in fiscal 2023, an 8.0% increase compared to the prior year. Our income from operations increased to $135.3 million in fiscal 2023 or 11.1% growth from the prior year driven primarily by increased sales and a reduction in operating expenses of $7.4 million.
Acquisitions. We acquired four businesses during fiscal 2023 and two businesses during fiscal 2022, as described in Note 2 to the Consolidated Financial Statements. None of such acquisitions was considered material.
Trends and Uncertainties
The following is a discussion of certain trends and uncertainties that we believe have influenced, and may continue to influence, our results of operations.
Global Economic Considerations. Our products and services are sold in numerous countries worldwide, with a large percentage of our sales generated outside the United States. Therefore, we are exposed to and impacted by global macroeconomic factors, U.S. and foreign government policies and foreign exchange fluctuations. There is uncertainty surrounding macroeconomic factors in the U.S. and globally characterized by the supply chain environment, inflationary pressure, rising interest rates, and labor shortages. These global macroeconomic factors, coupled with the U.S. political climate and political unrest internationally, have created uncertainty and impacted demand for certain of our products and services. Also, the continued conflict between Russia and Ukraine and the sanctions imposed in response to this conflict have increased global economic and political uncertainty. While the impact of these factors remains uncertain, we will continue to evaluate the extent to which these factors will impact our business, financial condition or results of operations. We do not know how long this uncertainty will continue. These factors could have a material negative effect on our business, results of operations and financial condition.
37
Global Trade. The current domestic and international political environment, including in relation to recent and further potential changes by the U.S. and other countries in policies on global trade and tariffs, have resulted in uncertainty surrounding the future state of the global economy and global trade. This uncertainty is exacerbated by sanctions imposed by the U.S. government against certain businesses and individuals in select countries. Continued or increased uncertainty regarding global trade due to these or other factors may require us to modify our current business practices and could have a material adverse effect on our business, results of operations and financial condition.
Healthcare Considerations. As described below, our Healthcare division experienced some increased demand for its patient monitoring products as a result of the COVID-19 pandemic during the earlier stages of the pandemic. Increased healthcare capital purchases made in prior periods may result in fewer capital purchases in subsequent periods.
Government Policies. Our results of operations and cash flows could be materially affected by changes in U.S. or foreign government legislative, regulatory or enforcement policies.
Changes in Costs and Supply Chain Disruptions. Our costs are subject to fluctuations, particularly due to changes in raw material, component, and logistics costs. Our manufacturing and supply chain operations, including freight and shipping activities, have been and may continue to be impacted by increased vendor costs as well as the current global supply chain challenges. Specifically, we are impacted by the global shortage of electronic components and other materials needed for production and freight availability. We expect continued disruptions in obtaining material and freight availability as the world economies react to and recover from supply chain shortages. If we are unable to mitigate the impact of increased costs through pricing or other actions, there could be a negative impact on our business, results of operations, and financial condition.
Russia’s Invasion of Ukraine. The invasion of Ukraine by Russia and the sanctions imposed in response to this conflict have increased global economic and political uncertainty. This has the potential to indirectly disrupt our supply chain and access to certain resources. While we have not experienced significant adverse impacts to date and will continue to monitor for any impacts and seek to mitigate disruption that may arise, we have certain research and development activities within Ukraine for our Healthcare division which have been somewhat impacted. The conflict also has increased the threat of malicious cyber activity from nation states and other actors.
Currency Exchange Rates. On a year-over-year basis, currency exchange rates negatively impacted reported sales by approximately 1.0% for the year ended June 30, 2023 compared to the year ended June 30, 2022, primarily due to the strengthening of the U.S. dollar against other foreign currencies in fiscal 2023. Any further strengthening of the U.S. dollar against foreign currencies would adversely impact our sales for the remainder of the year, and any weakening of the U.S. dollar against foreign currencies would positively impact our sales for the remainder of the year.
Coronavirus Pandemic. The coronavirus disease 2019 (“COVID-19”) pandemic dramatically impacted the global health and economic environment, with millions of confirmed cases, business slowdowns and shutdowns, and market volatility. The COVID-19 pandemic caused, and may continue to cause, significant economic disruptions and impacted, and may continue to impact, our operations and the operations of our suppliers, logistics providers and customers as a result of supply chain disruptions and delays, as well as labor challenges. During the early stages of the pandemic, our Healthcare division experienced increased demand for certain products as a result of COVID-19. In our Security division, throughout the pandemic, receipt of certain orders was delayed, most notably with respect to our aviation and cargo products, and our revenues were adversely impacted as a result of the pandemic.
Significant International Security Contracts. During fiscal year 2023, our Security division was awarded two significant international contracts valued in aggregate greater than $700 million with expected revenues to be recognized over multiple years.
Critical Accounting Policies and Estimates
The following discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”). Our preparation of these consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. As a result, actual results may differ from such estimates. Our senior management has reviewed these critical accounting policies and estimates and related disclosures with the Audit Committee of our Board of Directors. The following summarizes our critical accounting policies and estimates used in preparing our consolidated financial statements:
38
Revenue Recognition. We recognize revenue when performance obligations under the terms of the contracts with our customers are satisfied. Our performance obligations are broadly categorized as product sales, service revenue, and project-specific contract revenue. Revenue from sales of products is recognized upon shipment or delivery when control of the product transfers to the customer, depending on the terms of each sale, and when collection is probable. Revenue from services includes installation and implementation of products and turnkey security screening services and after-market services. Generally, revenue from services is recognized over time as the services are performed. Sales agreements with customers can be project specific, cover a period of time, and can be renewable periodically. The contracts may contain terms and conditions with respect to payment, delivery, installation, services, warranty and other rights. Contracts with customers may include the sale of products and services.
In certain instances, contracts with customers can contain multiple performance obligations such as civil works to prepare a site for equipment installation, training of customer personnel to operate equipment, and after-market service of equipment. We generally assign multiple elements in a contract into separate performance obligations if those elements are distinct, both individually and in the context of the contract. If multiple promises comprise a series of distinct services which are substantially the same and have the same pattern of transfer, they are combined and accounted for as a single performance obligation.
Inventory. Inventories are stated at the lower of cost or net realizable value. We write down inventory for slow-moving and obsolete inventory based on historical usage, orders on hand, assessments of future demands, and market conditions, among other items. If these factors are less favorable than those projected, additional inventory write-downs may be required.
Income Taxes. Our annual tax rate is based on our income, statutory tax rates and tax planning opportunities available to us in the various jurisdictions in which we operate. Tax laws are complex and subject to different interpretations by the taxpayer and respective governmental taxing authorities. Significant judgment is required in determining our tax expense and in evaluating our tax positions including evaluating uncertainties. We review our tax positions quarterly and adjust the balances as new information becomes available.
Deferred income tax assets represent amounts available to reduce income taxes payable on taxable income in future years. Such assets arise because of temporary differences between the financial reporting and tax bases of assets and liabilities, as well as from net operating loss and tax credit carryforwards. We evaluate the recoverability of these future tax deductions by assessing the adequacy of future expected taxable income from all sources, including reversal of taxable temporary differences, forecasted operating earnings and available tax planning strategies. These sources of income inherently rely on estimates. To provide insight, we use our historical experience and our short and long-range business forecasts. We believe it is more likely than not that a portion of the deferred income tax assets may expire unused and therefore have established a valuation allowance against them. Although realization is not assured for the remaining deferred income tax assets, we believe it is more likely than not that the deferred tax assets will be fully recoverable within the applicable statutory expiration periods. However, deferred tax assets could be reduced in the near term if our estimates of taxable income are significantly reduced or available tax planning strategies are no longer viable.
Business Combinations. In connection with the acquisition of a business, we record the fair value of purchase consideration for the tangible and intangible assets acquired, and liabilities assumed based on their estimated fair values. The excess of the fair value of purchase consideration over the fair values of these identifiable assets and liabilities is recorded as goodwill. Such valuations require management to make significant estimates and assumptions, especially with respect to intangible assets. Significant estimates in valuing certain intangible assets include, but are not limited to, future expected cash flows from acquired customers, acquired technology, trade names, useful lives and discount rates. Our estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates. During the measurement period, which is until we have all the necessary information about the facts and circumstances that existed as of the acquisition date up to one year from the acquisition date, we may record adjustments to the provisional amounts initially recorded for the assets acquired and liabilities assumed, with the corresponding offset to goodwill. Upon the conclusion of the measurement period, any subsequent adjustments are recorded to earnings.
Legal and Other Contingencies. We are subject to various claims and legal proceedings. We review the status of each significant legal dispute to which we are a party and assess our potential financial exposure, if any. If the potential financial exposure from any claim or legal proceeding is considered probable and the amount can be reasonably estimated, we record a liability and an expense for the estimated loss. Significant judgment is required in both the determination of probability and the determination as to whether an exposure is reasonably estimable. Because of uncertainties related to these matters, accruals are based only on the best information available at the time. As additional information becomes available, we reassess the potential liability related to our pending claims and litigation and revise our estimates accordingly. Such revisions in the estimates of the potential liabilities could have a material impact on our results of operations and financial position.
39
Net Revenues
The table below and the discussion that follows are based upon the way we analyze our business. See Note 14 to the consolidated financial statements for additional information about business segments.
Fiscal | Fiscal | |||||||||||||||||||
| Fiscal |
| % of |
| Fiscal |
| % of |
| Fiscal | % of | 2021-2022 |
| 2022-2023 | |||||||
| 2021 |
| Net Revenues |
| 2022 |
| Net Revenues |
| 2023 |
| Net Revenues |
| % Change |
| % Change | |||||
(Dollars in millions) | ||||||||||||||||||||
Security | $ | 633.3 | 55 | % | $ | 663.2 | 56 | % | $ | 760.3 | 59 | % | 5 | % | 15 | % | ||||
Healthcare |
| 212.3 | 19 | % | 205.7 |
| 17 | % | 190.5 | 15 | % | (3) | % | (7) | % | |||||
Optoelectronics / Manufacturing |
| 301.3 | 26 | % | 314.3 |
| 27 | % | 327.6 | 26 | % | 4 | % | 4 | % | |||||
Total Net Revenues | $ | 1,146.9 | $ | 1,183.2 | $ | 1,278.4 | 3 | % | 8 | % | ||||||||||
Fiscal 2023 Compared with Fiscal 2022. Revenues for the Security division during the fiscal year ended June 30, 2023 increased on a year-over-year basis due to an increase in product and service revenues of approximately $66.1 million and $31.0 million, respectively. The increase in both product and service revenue was primarily driven by increased sales of cargo and vehicle inspection systems.
Revenues for the Healthcare division during the fiscal year ended June 30, 2023 decreased year-over-year due to a reduction in patient monitoring and cardiology sales of $12.2 million and $2.9 million, respectively.
Revenues for the Optoelectronics and Manufacturing division during the fiscal year ended June 30, 2023 increased year-over-year as a result of increases in revenue in our optoelectronics and contract manufacturing businesses of approximately $9.2 million and $4.1 million, respectively.
Gross Profit
Fiscal | % of | Fiscal | % of | Fiscal | % of | |||||||||||
| 2021 |
| Net Revenues |
| 2022 |
| Net Revenues |
| 2023 |
| Net Revenues | |||||
(Dollars in millions) | ||||||||||||||||
Gross profit | $ | 419.9 | 36.6 | % | $ | 424.4 |
| 35.9 | % | $ | 430.5 | 33.7 | % | |||
Fiscal 2023 Compared with Fiscal 2022. Gross profit is impacted by sales volume and changes in overall manufacturing-related costs, such as raw materials and component costs, warranty expense, provision for inventory, freight, and logistics. Gross profit increased approximately $6.1 million in fiscal 2023 as compared to the prior year on an 8% increase in sales. The gross margin declined from 35.9% to 33.7% driven by the mix of sales and increased costs. Our cost of goods sold increased year-over-year primarily as a result of the increase in revenues and higher raw material costs. Gross profit as a percentage of net revenues during the fiscal year ended June 30, 2023 decreased on a year-over-year basis due to (i) a reduction in the Security division gross margin due to a decrease in margin from product sales driven by a less favorable product mix and increased component costs, (ii) a reduction in sales in the Healthcare division, which carries the highest gross margin of our three divisions, and (iii) an increase in sales in the Optoelectronics and Manufacturing division, which carries the lowest gross margin of our three divisions.
Operating Expenses
Fiscal | Fiscal | |||||||||||||||||||
Fiscal |
| % of |
| Fiscal |
| % of |
| Fiscal | % of | 2021-2022 | 2022-2023 | |||||||||
| 2021 | Net Revenues | 2022 | Net Revenues | 2023 |
| Net Revenues | % Change | % Change | |||||||||||
(Dollars in millions) | ||||||||||||||||||||
Selling, general and administrative | $ | 240.7 | 21.0 | % | $ | 235.6 | 19.9 | % | $ | 228.3 | 17.9 | % | (2) | % | (3) | % | ||||
Research and development |
| 53.7 | 4.7 | % |
| 59.6 | 5.0 | % | 59.4 | 4.6 | % | 11 | % | (0) | % | |||||
Impairment, restructuring and other charges |
| 10.1 | 0.9 | % |
| 7.5 | 0.6 | % | 7.6 | 0.6 | % | (25) | % | 1 | % | |||||
Total operating expenses | $ | 304.5 | 26.5 | % | $ | 302.7 | 25.6 | % | $ | 295.3 | 23.1 | % | (1) | % | (2) | % | ||||
40
Selling, General and Administrative
Our significant selling, general and administrative (“SG&A”) expenses include employee compensation, sales commissions, travel, professional services, marketing expenses, and depreciation and amortization expense.
Fiscal 2023 Compared with Fiscal 2022. SG&A expense for the fiscal year ended June 30, 2023 was $7.3 million lower than such expenses in the same prior-year period primarily due to a $5 million reduction in compensation and external commission expenses and a $1 million reduction in marketing expense, a reduction in the fair value of certain contingent liabilities, partially offset by $2 million lower bad debt recoveries and increased travel and meeting expenses compared to the same prior-year period.
Research and Development
Our Security and Healthcare divisions have historically invested substantial amounts in research and development (“R&D”). We intend to continue this trend in future years, although specific programs may or may not continue to be funded and funding levels may fluctuate. R&D expenses included research related to new product development and product enhancement expenditures.
Fiscal 2023 Compared with Fiscal 2022. R&D expense during the fiscal year ended June 30, 2023 was comparable to the prior fiscal year.
Impairment, Restructuring and Other Charges
Impairment, restructuring and other charges generally consist of charges relating to reductions in our workforce, facilities consolidation, impairment of assets, costs related to acquisition activity, legal charges and other non-recurring charges. We have undertaken certain restructuring activities in an effort to align our global capacity and infrastructure with demand by our customers and fully integrate acquisitions, thereby improving our operational efficiency. Our efforts have helped enhance our ability to improve operating margins, retain and expand existing relationships with customers and attract new business. We may utilize similar measures in the future to realign our operations to further increase our operating efficiencies. The effect of these efforts may materially affect our future operating results.
Fiscal 2023 Compared with Fiscal 2022. During the fiscal year ended June 30, 2023, impairment, restructuring and other charges were $7.6 million and consisted of $3.9 million for legal charges, net of insurance reimbursements, $1.7 million for employee terminations, $1.5 million for other facility closure costs for operational efficiency activities, and $0.4 million in acquisition related costs. During the fiscal year ended June 30, 2022, impairment, restructuring and other charges were $7.5 million and consisted of $5.1 million for legal charges primarily related to class action litigation and government investigations, net of insurance reimbursements, $1.1 million in charges for employee terminations, $0.3 million in acquisition related costs, and $1.0 million in impairment charges.
Other Income
Fiscal 2023 Compared with Fiscal 2022. During the fiscal year ended June 30, 2023, there was no other income. For the fiscal year ended June 30, 2022, other income was $27.4 million, driven by the gain on sale of property and equipment primarily from the sale of corporate owned real estate.
Interest and Other Expense, Net
Fiscal | Fiscal | Fiscal | |||||||
| 2021 |
| 2022 |
| 2023 | ||||
(Dollars in millions) | |||||||||
Interest and other expense, net | $ | 16.7 | $ | 9.0 | $ | 20.0 | |||
Fiscal 2023 Compared with Fiscal 2022. For the fiscal year ended June 30, 2023, interest and other expense, net was $20.0 million as compared to $9.0 million in the comparable prior-year period. This increase was driven by higher average interest rates and higher average levels of borrowing under our credit facility during the year ended June 30, 2023 in comparison with the interest rates and levels of borrowing during the same period in the prior year. The 1.25% convertible notes that were previously outstanding during the year ended June 30, 2022 were retired in September 2022 using borrowings from our credit facility which carries a higher interest rate than the convertible notes.
41
Provision for Income Taxes
| Fiscal |
| Fiscal |
| Fiscal | ||||
2021 | 2022 | 2023 | |||||||
(Dollars in millions) | |||||||||
Provision for income taxes | $ | 24.6 | $ | 24.8 | $ | 23.5 | |||
The effective tax rate for a particular period varies depending on a number of factors including (i) the mix of income earned in various tax jurisdictions, each of which applies a unique range of income tax rates and income tax credits, (ii) changes in previously established valuation allowances for deferred tax assets (changes are based upon our current analysis of the likelihood that these deferred tax assets will be realized), (iii) the level of non-deductible expenses, (iv) certain tax elections, (v) tax holidays granted to certain of our international subsidiaries, (vi) return to provision adjustments and (vii) changes in tax legislation.
Fiscal 2023 Compared with Fiscal 2022. For the fiscal years ended June 30, 2023 and 2022, we recognized a provision for income taxes of $23.5 million and $24.8 million, respectively. The effective tax rate for the fiscal years ended June 30, 2023 and 2022 was 20.4% and 17.7%, respectively. During the fiscal years ended June 30, 2023 and 2022, we recognized a net discrete tax benefit of $2.8 million and $7.0 million, respectively, primarily related to equity-based compensation under ASU 2016-09, adjustments to prior year estimates, and changes in uncertain tax positions.
Liquidity and Capital Resources
Our principal sources of liquidity are our cash and cash equivalents, cash generated from operations and our credit facility. Cash and cash equivalents totaled $76.8 million at June 30, 2023, compared to $64.2 million at June 30, 2022. During fiscal 2023, we generated $94.8 million of cash flow from operations. These proceeds and $5.9 million of net bank borrowings and long-term debt were used for the following: $15.8 million invested in capital expenditures, $7.1 million for the acquisition of four businesses and $46.7 million for share repurchases and taxes paid related to the net share settlement of equity awards. If we continue to net settle equity awards, we will use additional cash to pay our tax withholding obligations in connection with such settlements. We currently anticipate that our available funds, credit facilities and cash flow from operations will be sufficient to meet our operational cash needs for the next 12 months and foreseeable future. In addition, we anticipate that cash generated from operations, without repatriating earnings from our non-U.S. subsidiaries, and our credit facilities will be sufficient to satisfy our obligations in the U.S.
We have a $750 million credit facility that is comprised of a $600 million revolving credit facility, which includes a $300 million sub-facility for letters of credit, and a $150 million term loan. As of June 30, 2023, there was $215.0 million outstanding under our revolving credit facility, $143.1 million outstanding under the term loan, and $48.5 million of outstanding letters of credit. As of June 30, 2023, the total amount available under these credit facilities was $336.5 million. See Note 8 to the consolidated financial statements for further discussion.
Cash Provided by Operating Activities. Cash flows from operating activities can fluctuate significantly from period to period, as net income, adjusted for non-cash items, and working capital fluctuations impact cash flows. During fiscal 2023, we generated cash from operations of $94.8 million compared to $63.8 million in the prior fiscal year. This increase was driven by lower increases in inventory, increased accounts payable and other changes in net working capital.
Cash Used in Investing Activities. Net cash used in investing activities was $40.5 million during fiscal 2023 as compared to $12.7 million used during the prior year. During fiscal 2022, we received proceeds of $32 million from the sale of corporate owned real estate thereby reducing the amount of net cash used in investing activities in such year. During fiscal 2023, we used cash of $7.1 million for the acquisition of businesses as compared to $14.1 million in the prior fiscal year. Net capital expenditures in fiscal 2023 were $15.8 million compared to $14.9 million in the prior fiscal year. Expenditures for intangible and other assets in fiscal 2023 were $16.4 million compared to $15.6 million in the prior fiscal year. In addition, purchases of certificates of deposit in fiscal 2023 were $5.3 million compared to $2.2 million in the same prior-year period.
Cash Used in Financing Activities. Net cash used in financing activities was $37.2 million during fiscal 2023, compared to $64.0 million during the prior fiscal year. The changes in cash flows from financing activities primarily relate to (i) net repayments on bank lines of credit and the term loan of $5.9 million in fiscal 2023 compared to $64.3 million in the prior fiscal year; and (ii) $46.7 million used for share repurchases and taxes paid related to the net share settlement of equity awards in fiscal 2023 compared to $131.0 million in the prior fiscal year.
42
Material Cash Requirements
Our material cash requirements include the following contractual and other obligations.
Borrowings. Outstanding lines of credit and current and long-term debt totaled $359.6 million at June 30, 2023, an increase of $6.2 million from $353.4 million at June 30, 2022. As of June 30, 2023, we were in compliance with all financial covenants under our various borrowing agreements. See Note 8 to the consolidated financial statements for further discussion. We anticipate that cash generated from our operations, in addition to existing cash borrowing arrangements and future access to capital markets should be sufficient to meet our cash requirements for at least the next 12 months. However, our future capital requirements will depend on many factors, including future business acquisitions, capital expenditures, litigation, stock repurchases and levels of research and development spending, among other factors. The adequacy of available funds will depend on many factors, including the success of our businesses in generating cash, continued compliance with financial covenants contained in our credit facility and the health of capital markets in general, among other factors.
Leases. We have lease arrangements for certain facilities and equipment under various operating lease agreements. As of June 30, 2023, we had lease payment obligations of $33.5 million, with $10.8 million payable within the next 12 months.
Cash Held by Foreign Subsidiaries
Our cash and cash equivalents totaled $76.8 million at June 30, 2023. Of this amount, approximately 97% was held by our foreign subsidiaries and subject to repatriation tax considerations. These foreign funds were held primarily by our subsidiaries in the United Kingdom, Qatar, Singapore, India, Malaysia and Canada, and to a lesser extent in Indonesia, Australia, Germany and Mexico among other countries. We intend to permanently reinvest certain earnings from foreign operations, and we currently do not anticipate that we will need this cash in foreign countries to fund our U.S. operations. In the event we repatriate cash from certain foreign operations and if taxes have not previously been withheld on the related earnings, we would provide for withholding taxes at the time we change our intention with regard to the reinvestment of those earnings.
Stock Repurchase Program
In September 2022, our Board of Directors increased to 2,000,000 shares the maximum number of shares authorized under the stock repurchase program. This program does not expire unless our Board of Directors acts to terminate the program. During fiscal 2023, we repurchased 400,230 shares. As of June 30, 2023, 1,721,870 shares remained available for repurchase.
The timing and actual numbers of shares purchased depends on a variety of factors, including stock price, general business and market conditions and other investment opportunities. Repurchases may be made from time to time under the program through open-market purchases or privately-negotiated transactions at our discretion. Upon repurchase, the shares are restored to the status of authorized but unissued shares, and we record them as a reduction in the number of shares of Common Stock issued and outstanding in our consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market Risk
We are exposed to certain market risks, which are inherent in our financial instruments and arise from transactions entered into in the normal course of business. We may enter into derivative financial instrument transactions in order to manage or reduce market risk in connection with specific foreign currency denominated transactions. We do not enter into derivative financial instrument transactions for speculative purposes.
We are subject to interest rate risk on our borrowings under our bank lines of credit. Consequently, our interest expense fluctuates with changes in the general level of these interest rates as we borrow under the credit facility.
Importance of International Markets
International markets provide us with significant growth opportunities. Our financial results in future periods could, however, be adversely affected by periodic economic downturns in different regions of the world, changes in trade policies or tariffs, civil or military conflict and other political instability. We monitor economic and currency conditions around the world to evaluate whether there may be any significant effect on our international sales in the future.
43
Foreign Currency
Our international operations are subject to certain opportunities and risks, including from foreign currency fluctuations and governmental actions. We conduct business in more than 30 countries. We closely monitor our operations in each country in which we do business and seek to adopt appropriate strategies that are responsive to changing economic and political environments, and to fluctuations in foreign currencies. Weaknesses in the currencies of some of the countries in which we do business are often offset by strengths in other currencies. Foreign currency financial statements are translated into U.S. dollars at period-end rates, except that revenues, costs and expenses are translated at average rates during the reporting period. We include gains and losses resulting from foreign currency transactions in income, while we exclude those resulting from translation of financial statements from income and include them as a component of accumulated other comprehensive loss. Transaction gains and losses, which were included in our consolidated statement of operations, amounted to a net gain (loss) of approximately $(1.3) million, $0.6 million, and $2.0 million for the fiscal years ended June 30, 2021, 2022 and 2023, respectively. A 10% appreciation of the U.S. dollar relative to the local currency exchange rates would have resulted in a net increase in our operating income of approximately $13.5 million in fiscal 2023. Conversely, a 10% depreciation of the U.S. dollar relative to the local currency exchange rates would have resulted in a net decrease in our operating income of approximately $13.5 million in fiscal 2023.
Inflation
Heightened levels of inflation continue to present risk for us. We have experienced impacts to our materials and manufacturing costs and labor rates, and suppliers have signaled inflation-related cost pressures, which could flow through to our costs and pricing. If inflation remains at current levels for an extended period, or increases, and we are unable to successfully mitigate the impact, our costs could increase, resulting in pressure on our profits and margins. In addition, inflation and the increases in the cost of borrowing from rising interest rates could constrain the overall purchasing power of our customers for our products and services. Rising interest rates also will increase our borrowing costs. We remain committed to our ongoing efforts to increase the efficiency of our operations and improve the cost competitiveness of our products and services, which may, in part, offset cost increases from inflation.
Interest Rate Risk
The principal maturity and estimated value of our long-term debt exposure for each of the fiscal years set forth below as of June 30, 2023 were as follows (dollars in thousands):
Maturity |
| ||||||||||||||||||||||||
2029 and |
| ||||||||||||||||||||||||
| 2024 |
| 2025 |
| 2026 |
| 2027 |
| 2028 |
| Thereafter |
| Total |
| Fair Value |
| |||||||||
Term loan | $ | 7,500 | $ | 7,500 | $ | 7,500 | $ | 120,625 | $ | — | $ | — | $ | 143,125 | $ | 143,125 | |||||||||
Average interest rate | 6.20 | % | 6.20 | % | 6.20 | % | 6.20 | % | — | % | — | % | 6.20 | % | 6.20 | % | |||||||||
Finance lease obligations | $ | 576 | $ | 492 | $ | 301 | $ | 73 | $ | — | $ | — | $ | 1,442 | $ | 1,442 | |||||||||
Average interest rate of finance lease obligations |
| 3.5 | % |
| 3.5 | % |
| 3.5 | % |
| 3.5 | % |
| — | % |
| — | % |
| 3.5 | % |
| 3.5 | % | |
At June 30, 2023, we had $215.0 million of borrowings under our revolving credit facility and $143.1 million of term loan outstanding.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
We make reference here to the Index to Consolidated Financial Statements that appears on page F-1 of this report. The Report of Independent Registered Public Accounting Firm from Grant Thornton LLP, the Consolidated Financial Statements, the Notes to Consolidated Financial Statements, and Supplementary Data—Unaudited Quarterly Results listed in the Index to Consolidated Financial Statements, which appear beginning on page F-2 of this report, are incorporated by reference into this Item 8.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
44
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of June 30, 2023, the end of the period covered by this report, our management, including our Chief Executive Officer and our Chief Financial Officer, reviewed and evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a15(e) or 15d15(e) of the Exchange Act). Based upon management’s review and evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this Annual Report on Form 10-K, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified by the SEC and is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rule 13a-15(f) or 15d-15(f) of the Exchange Act) for the Company. Under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework and criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013. Based on that evaluation, management concluded that our internal control over financial reporting was effective as of June 30, 2023.
The effectiveness of the Company’s internal control over financial reporting as of June 30, 2023 has been audited by Grant Thornton LLP, an independent registered public accounting firm, as stated in its report, which is included in Item 8 of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the fourth quarter of fiscal 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud within the Company have been detected.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
45
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by Item 10 is incorporated by reference from our definitive proxy statement for our annual stockholders’ meeting, presently scheduled to be held in December 2023.
ITEM 11. EXECUTIVE COMPENSATION
The information required by Item 11 is incorporated by reference from our definitive proxy statement for our annual stockholders’ meeting, presently scheduled to be held in December 2023.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 12 is incorporated by reference from our definitive proxy statement for our annual stockholders’ meeting, presently scheduled to be held in December 2023.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 is incorporated by reference from our definitive proxy statement for our annual stockholders’ meeting, presently scheduled to be held in December 2023.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by Item 14 is incorporated by reference from our definitive proxy statement for our annual stockholders’ meeting, presently scheduled to be held in December 2023.
46
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | The following documents are filed as part of this report: |
| 1. | Financial Statements. Please see the accompanying Index to Consolidated Financial Statements, which appears on page F-1 of the report. The Report of Independent Registered Public Accounting Firm, the Consolidated Financial Statements and the Notes to Consolidated Financial Statements listed in the Index to Consolidated Financial Statements, which appear beginning on page F-2 of this report, are incorporated by reference into Item 8 above. |
| 2. | Financial Statement Schedules. |
Supplementary Data—Unaudited Quarterly Results
No other financial statement schedules are presented as the required information is either not applicable or included in the Consolidated Financial Statements or Notes thereto.
| 3. | Exhibits. Reference is made to item 15(b) below. |
| (b) | Exhibits. The exhibits listed on the accompanying Exhibit Index immediately preceding the signature page are filed as part of, or are incorporated by reference into, this report. |
| (c) | Financial Statement Schedules. Reference is made to Item 15(a)(2) above. |
ITEM 16. FORM 10-K SUMMARY
None.
47
OSI SYSTEMS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | |
Report of Independent Registered Public Accounting Firm (Grant Thornton LLP, Los Angeles, CA, PCAOB ID: 248) | F-2 | |
Report of Independent Registered Public Accounting Firm (Grant Thornton LLP, Los Angeles, CA) | F-4 | |
Report of Independent Registered Public Accounting Firm (Moss Adams LLP, Los Angeles, CA, PCAOB ID: 659) | F-5 | |
F-6 | ||
F-7 | ||
F-8 | ||
F-9 | ||
F-10 | ||
F-11 | ||
F-41 |
F-1
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
OSI Systems, Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheet of OSI Systems, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of June 30, 2023, the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for the year ended June 30, 2023, and the related notes and financial statement schedules included under Item 15(a) (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2023, and the results of its operations and its cash flows for the year ended June 30, 2023, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of June 30, 2023, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”), and our report dated August 29, 2023 expressed an unqualified opinion.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical audit matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Determination of standalone selling price - Security Segment Product Revenue
As described in Note 1 to the consolidated financial statements, the Company’s revenue contracts in the security segment may include multiple performance obligations, which are accounted for separately when they are distinct. The Company derives revenues in the security segment mainly from sales of products, installation, project management and training services. The Company allocates the transaction price to the distinct performance obligations on a relative stand-alone selling price basis and recognizes revenue when control is transferred. Product revenues are recognized at the point in time when product has been delivered.
Auditing the Company’s product revenue stand-alone selling price in the security segment was complex due to the subjectivity of the assumptions that were used in developing the stand-alone selling price of distinct performance obligations. Evaluating the appropriateness of these assumptions requires extensive audit effort due to the complexity of these contracts and a high degree of auditor judgment when performing audit procedures and evaluating the results of those procedures.
F-2
We obtained an understanding, evaluated design and tested the operating effectiveness of internal controls related to the determination of the stand-alone selling prices related to the security segment.
To test management’s determination of stand-alone selling price for each performance obligation, we performed procedures to evaluate the methodology applied. We evaluated the Company’s analysis of stand-alone selling price, including inspecting a sample of executed contracts. For the sample selected we evaluated the contracts to determine the appropriateness of the method used and the underlying data including cost details and margin percentages to estimate the stand-alone selling price.
/s/ Grant Thornton LLP |
| |
We have served as the Company’s auditor since 2023. | ||
Los Angeles, California | ||
August 29, 2023 |
F-3
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
OSI Systems, Inc.
Opinion on internal control over financial reporting
We have audited the internal control over financial reporting of OSI Systems, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of June 30, 2023, based on criteria established in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2023, based on criteria established in the 2013 Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated financial statements of the Company as of and for the year ended June 30, 2023, and our report dated August 29, 2023 expressed an unqualified opinion on those financial statements.
Basis for opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and limitations of internal control over financial reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Grant Thornton LLP |
| |
We have served as the Company’s auditor since 2023. | ||
Los Angeles, California | ||
August 29, 2023 |
F-4
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of
OSI Systems, Inc.
Opinions on the Financial Statements
We have audited the accompanying consolidated balance sheet of OSI Systems, Inc. and subsidiaries (the “Company”) as of June 30, 2022, the related consolidated statements of operations, comprehensive income, stockholders’ equity and cash flows for each of the two years in the period ended June 30, 2022, and the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of June 30, 2022, and the consolidated results of its operations and its cash flows for each of the two years in the period ended June 30, 2022, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 1 to the consolidated financial statements, the Company changed its method of accounting for its convertible notes as of July 1, 2021 due to the adoption of Accounting Standards Update No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
/s/ Moss Adams LLP |
| |
August 19, 2022 | ||
We served as the Company’s auditor from 2006 to 2023. |
F-5
OSI SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(amounts in thousands, except share amounts and par value)
June 30, | ||||||
| 2022 |
| 2023 | |||
ASSETS | ||||||
CURRENT ASSETS: | ||||||
Cash and cash equivalents | $ | 64,202 | $ | 76,750 | ||
Accounts receivable, net |
| 307,973 |
| 380,845 | ||
Inventories |
| 333,907 |
| 338,008 | ||
Prepaid expenses and other current assets |
| 40,062 |
| 44,300 | ||
Total current assets |
| 746,144 |
| 839,903 | ||
Property and equipment, net |
| 109,684 |
| 108,933 | ||
Goodwill |
| 336,357 |
| 349,505 | ||
Intangible assets, net |
| 138,370 |
| 140,857 | ||
Other assets |
| 112,595 |
| 116,488 | ||
Total assets | $ | 1,443,150 | $ | 1,555,686 | ||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||
CURRENT LIABILITIES: | ||||||
Bank lines of credit | $ | 60,000 | $ | 215,000 | ||
Current portion of long-term debt |
| 244,575 |
| 8,076 | ||
Accounts payable |
| 125,204 |
| 139,011 | ||
Accrued payroll and related expenses |
| 46,379 |
| 51,243 | ||
Advances from customers |
| 19,917 |
| 21,250 | ||
Other accrued expenses and current liabilities |
| 117,879 |
| 137,114 | ||
Total current liabilities |
| 613,954 |
| 571,694 | ||
Long-term debt, net |
| 48,668 |
| 136,491 | ||
Deferred income taxes |
| 11,112 |
| 6,571 | ||
Other long-term liabilities |
| 130,992 |
| 114,765 | ||
Total liabilities |
| 804,726 |
| 829,521 | ||
Commitments and contingencies (Note 11) | ||||||
Stockholders’ Equity: | ||||||
Preferred stock, $0.001 par value— 10,000,000 shares authorized; no shares issued or outstanding |
|
| ||||
Common stock, $0.001 par value—100,000,000 shares authorized; and , 16,870,050 and 16,755,772 shares at June 30, 2022 and 2023, respectively |
| 17 |
| 9,835 | ||
Retained earnings |
| 663,869 |
| 735,957 | ||
Accumulated other comprehensive loss |
| (25,462) |
| (19,627) | ||
Total stockholders’ equity |
| 638,424 |
| 726,165 | ||
Total liabilities and stockholders’ equity | $ | 1,443,150 | $ | 1,555,686 | ||
See accompanying notes to Consolidated Financial Statements.
F-6
OSI SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(amounts in thousands, except per share data)
Year Ended June 30, | |||||||||
| 2021 |
| 2022 |
| 2023 | ||||
Net revenues: |
| ||||||||
Products | $ | 872,809 | $ | 897,259 | $ | 958,827 | |||
Services |
| 274,093 |
| 285,977 |
| 319,600 | |||
Total net revenues |
| 1,146,902 |
| 1,183,236 |
| 1,278,427 | |||
Cost of goods sold: | |||||||||
Products |
| 586,935 |
| 608,990 |
| 676,772 | |||
Services |
| 140,049 |
| 149,819 |
| 171,145 | |||
Total cost of goods sold |
| 726,984 |
| 758,809 |
| 847,917 | |||
Gross profit |
| 419,918 |
| 424,427 |
| 430,510 | |||
Operating expenses: | |||||||||
Selling, general and administrative |
| 240,747 |
| 235,553 |
| 228,313 | |||
Research and development |
| 53,696 |
| 59,583 |
| 59,352 | |||
Impairment, restructuring and other charges, net |
| 10,104 |
| 7,542 |
| 7,566 | |||
Total operating expenses |
| 304,547 |
| 302,678 |
| 295,231 | |||
Income from operations |
| 115,371 |
| 121,749 |
| 135,279 | |||
Interest and other expense, net |
| (16,731) |
| (8,962) |
| (20,041) | |||
Other income | — | 27,373 | — | ||||||
Income before income taxes |
| 98,640 |
| 140,160 |
| 115,238 | |||
Provision for income taxes |
| (24,591) |
| (24,813) |
| (23,460) | |||
Net income | $ | 74,049 | $ | 115,347 | $ | 91,778 | |||
Earnings per share: | |||||||||
Basic | $ | 4.12 | $ | 6.57 | $ | 5.45 | |||
Diluted | $ | 4.03 | $ | 6.45 | $ | 5.34 | |||
Shares used in per share calculation: | |||||||||
Basic |
| 17,968 |
| 17,551 |
| 16,828 | |||
Diluted |
| 18,388 |
| 17,870 |
| 17,190 | |||
See accompanying notes to Consolidated Financial Statements.
F-7
OSI SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(amounts in thousands)
Year Ended June 30, | |||||||||
| 2021 |
| 2022 |
| 2023 | ||||
Net income | $ | 74,049 | $ | 115,347 | $ | 91,778 | |||
Other comprehensive income (loss): | |||||||||
Foreign currency translation adjustment, net of tax |
| 10,186 |
| (10,202) |
| 267 | |||
Other, net of tax | 262 | (514) | 5,568 | ||||||
Other comprehensive income (loss) | 10,448 | (10,716) | 5,835 | ||||||
Comprehensive income | $ | 84,497 | $ | 104,631 | $ | 97,613 | |||
See accompanying notes to Consolidated Financial Statements.
F-8
OSI SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(amounts in thousands, except share data)
Accumulated | ||||||||||||||
Common Stock | Other | |||||||||||||
Number of | Retained | Comprehensive | ||||||||||||
| Shares |
| Amount |
| Earnings |
| Loss |
| Total | |||||
Balance-July 1, 2020 |
| 18,011,982 | $ | 122,553 | $ | 474,793 | $ | (25,194) | $ | 572,152 | ||||
Exercise of stock options | 88,657 |
| 1,302 |
| — |
| — |
| 1,302 | |||||
Vesting of restricted stock/RSUs | 313,892 |
| — | — | — | — | ||||||||
Shares issued under employee stock purchase program | 68,180 |
| 4,215 |
| — |
| — |
| 4,215 | |||||
Stock-based compensation | — |
| 26,771 |
| — |
| — |
| 26,771 | |||||
Repurchase of common stock | (452,005) | (37,468) | — | — | (37,468) | |||||||||
Taxes paid related to net share settlement of equity awards | (176,596) |
| (11,649) |
| — |
| — |
| (11,649) | |||||
Net income | — | — | 74,049 | — | 74,049 | |||||||||
Other comprehensive income |
| — |
| — |
| — |
| 10,448 |
| 10,448 | ||||
Balance-June 30, 2021 |
| 17,854,110 | $ | 105,724 | $ | 548,842 | $ | (14,746) | $ | 639,820 | ||||
Exercise of stock options | 166,629 |
| 460 |
| — |
| — |
| 460 | |||||
Vesting of RSUs | 337,442 |
| — | — | — | — | ||||||||
Shares issued under employee stock purchase program | 60,065 |
| 4,297 |
| — |
| — |
| 4,297 | |||||
Stock-based compensation | — |
| 28,072 |
| — |
| — |
| 28,072 | |||||
Repurchase of common stock | (1,294,594) |
| (92,351) |
| (19,276) |
| — |
| (111,627) | |||||
Taxes paid related to net share settlement of equity awards | (253,602) |
| (19,422) |
| — |
| — |
| (19,422) | |||||
Adoption of ASU 2020-06 for convertible notes | — | (26,763) | 18,956 | — | (7,807) | |||||||||
Net income | — | — | 115,347 | — | 115,347 | |||||||||
Other comprehensive loss | — |
| — |
| — |
| (10,716) |
| (10,716) | |||||
Balance-June 30, 2022 |
| 16,870,050 | $ | 17 | $ | 663,869 | $ | (25,462) | $ | 638,424 | ||||
Exercise of stock options | 47,354 | 3,666 | — | — | 3,666 | |||||||||
Vesting of RSUs | 313,862 | — | — | — | — | |||||||||
Shares issued under employee stock purchase program | 59,255 | 4,041 | — | — | 4,041 | |||||||||
Stock-based compensation | — | 29,124 | — | — | 29,124 | |||||||||
Repurchase of common stock | (400,230) | (17,067) | (17,682) | — | (34,749) | |||||||||
Taxes paid related to net share settlement of equity awards | (134,519) | (9,946) | (2,008) | — | (11,954) | |||||||||
Net income | — | — | 91,778 | — | 91,778 | |||||||||
Other comprehensive income | — | — | — | 5,835 | 5,835 | |||||||||
Balance-June 30, 2023 |
| 16,755,772 | $ | 9,835 | $ | 735,957 | $ | (19,627) | $ | 726,165 | ||||
See accompanying notes to Consolidated Financial Statements.
F-9
OSI SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(amounts in thousands)
Year Ended June 30, | |||||||||
| 2021 |
| 2022 |
| 2023 | ||||
CASH FLOWS FROM OPERATING ACTIVITIES | |||||||||
Net income | $ | 74,049 | $ | 115,347 | $ | 91,778 | |||
Adjustments to reconcile net income to net cash provided by operating activities, net of effects from acquisitions: | |||||||||
Depreciation and amortization |
| 43,855 |
| 38,679 |
| 38,513 | |||
Stock-based compensation |
| 26,771 |
| 28,072 |
| 29,124 | |||
Provision for (recovery of) losses on accounts receivable | 9,823 | (5,978) | (3,899) | ||||||
Deferred income taxes | 432 | 3,520 | (3,978) | ||||||
Amortization of debt discount and issuance costs |
| 9,756 |
| 1,343 |
| 196 | |||
Impairment charges | 552 | 1,006 | — | ||||||
Gain on sale of property and equipment | — | (27,373) | — | ||||||
Other |
| (109) |
| (1,326) |
| 250 | |||
Changes in operating assets and liabilities—net of business acquisitions: | |||||||||
Accounts receivable |
| (28,955) |
| (13,710) |
| (66,088) | |||
Inventories |
| (47,768) |
| (44,662) |
| (115) | |||
Prepaid expenses and other assets |
| (34,430) |
| 22,323 |
| (5,422) | |||
Accounts payable |
| 55,601 |
| (15,055) |
| 10,756 | |||
Accrued payroll and related expenses | 10,486 | (1,998) | 4,716 | ||||||
Advances from customers |
| 9,796 |
| (18,423) |
| 1,356 | |||
Other |
| 9,207 |
| (17,957) |
| (2,375) | |||
Net cash provided by operating activities |
| 139,066 |
| 63,808 |
| 94,812 | |||
CASH FLOWS FROM INVESTING ACTIVITIES | |||||||||
Acquisition of property and equipment |
| (16,896) |
| (14,921) |
| (15,811) | |||
Proceeds from sale of property and equipment | 1,136 | 34,132 | 347 | ||||||
Purchases of certificates of deposit | (4,892) | (2,243) | (5,280) | ||||||
Proceeds from maturities of certificates of deposit | 2,710 | 56 | 3,827 | ||||||
Acquisition of businesses, net of cash acquired |
| (3,000) |
| (14,132) |
| (7,101) | |||
Payments for intangible and other assets |
| (13,751) |
| (15,566) |
| (16,443) | |||
Net cash used in investing activities |
| (34,693) |
| (12,674) |
| (40,461) | |||
CASH FLOWS FROM FINANCING ACTIVITIES | |||||||||
Net borrowings (repayments) on bank lines of credit |
| (59,000) |
| 60,000 |
| 155,000 | |||
Proceeds from long-term debt |
| 739 |
| 50,388 |
| 100,766 | |||
Payments on long-term debt |
| (1,057) |
| (46,074) |
| (249,842) | |||
Proceeds from exercise of stock options and employee stock purchase plan |
| 5,517 |
| 4,796 |
| 7,707 | |||
Payment of contingent consideration | (1,007) | (2,061) | (4,103) | ||||||
Repurchase of common stock |
| (37,468) |
| (111,627) |
| (34,749) | |||
Taxes paid related to net share settlement of equity awards |
| (11,649) |
| (19,430) |
| (11,954) | |||
Net cash used in financing activities |
| (103,925) |
| (64,008) |
| (37,175) | |||
Effect of exchange rate changes on cash |
| 4,063 |
| (3,537) |
| (4,628) | |||
Net increase (decrease) in cash and cash equivalents |
| 4,511 |
| (16,411) |
| 12,548 | |||
Cash and cash equivalents—beginning of year |
| 76,102 |
| 80,613 |
| 64,202 | |||
Cash and cash equivalents—end of year | $ | 80,613 | $ | 64,202 | $ | 76,750 | |||
Supplemental disclosure of cash flow information: | |||||||||
Interest | $ | 5,979 | $ | 6,979 | $ | 20,277 | |||
Income taxes | $ | 12,778 | $ | 16,658 | $ | 19,439 | |||
See accompanying notes to Consolidated Financial Statements.
F-10
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE YEARS ENDED JUNE 30, 2023
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business—OSI Systems, Inc., together with our subsidiaries, is a vertically integrated designer and manufacturer of specialized electronic systems and components for critical applications. We sell our products and provide related services in diversified markets, including homeland security, healthcare, defense and aerospace.
We have three reporting segments: (i) Security, providing security and inspection systems and turnkey security screening solutions; (ii) Healthcare, providing patient monitoring, cardiology and remote monitoring, and connected care systems and associated accessories; and (iii) Optoelectronics and Manufacturing, providing specialized electronic components and electronic manufacturing services for our Security and Healthcare divisions, as well as third parties for applications in the defense and aerospace markets, among others.
Through our Security segment, we provide security screening products and related services globally. These products and services are used to inspect baggage, parcels, cargo, people, vehicles and other objects for weapons, explosives, drugs, radioactive and nuclear materials and other contraband. In addition to these products, we also provide site design, installation, training and technical support services to our customers. We also provide turnkey security screening solutions, which can include the construction, staffing and long-term operation of security screening checkpoints for our customers.
Through our Healthcare segment, we design, manufacture, market and service patient monitoring, cardiology and remote monitoring, and connected care systems and associated accessories globally. These products are used by care providers in critical care, emergency and perioperative areas within the hospital and provide information, through wired and wireless networks, to physicians and nurses who may be at the patient’s bedside, in another area of the hospital or even outside the hospital.
Through our Optoelectronics and Manufacturing segment, we design, manufacture and market optoelectronic devices and flex circuits and provide electronics manufacturing services globally for use in a broad range of applications, including aerospace and defense electronics, security and inspection systems, medical imaging and diagnostics, telecommunications, office automation, computer peripherals, industrial automation and consumer products. This division provides products and services to OEM customers and to our own Security and Healthcare divisions.
Consolidation—The consolidated financial statements include the accounts of OSI Systems, Inc. and our wholly-owned and majority-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation. Investments in joint ventures over which we have significant influence but do not have voting control are accounted for using the equity method. Investments over which we do not have significant influence or control are not material and are carried at cost as there is no readily determinable fair value for the equity interests.
Use of Estimates—The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of sales,costs of sales and expenses during the reporting period. The most significant of these estimates and assumptions for our company relate to contract revenue, fair values of assets acquired and liabilities assumed in business combinations, values for inventories reported at lower of cost or net realizable value, stock-based compensation expense, income taxes, accrued warranty costs, contingent consideration, allowance for doubtful accounts, and the recoverability, useful lives and valuation of recorded amounts of long-lived assets, identifiable intangible assets and goodwill. Changes in estimates are reflected in the periods during which they become known. Due to the inherent uncertainty involved in making estimates, our actual amounts reported in future periods could differ materially from these estimates.
Cash and Cash Equivalents—We consider all highly liquid investments with maturities of three months or less as of the acquisition date to be cash equivalents.
F-11
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Our cash and cash equivalents totaled $76.8 million at June 30, 2023. Of this amount, approximately 97% was held by our foreign subsidiaries and subject to repatriation tax considerations. These foreign funds were held primarily by our subsidiaries in the United Kingdom, Qatar, Singapore, India, Malaysia and Canada, and to a lesser extent in Indonesia, Australia, Germany and Mexico among other countries. We have cash holdings in financial institutions that exceed insured limits for such financial institutions; however, we mitigate this risk by utilizing international financial institutions of high credit quality.
Accounts Receivable—We monitor collections and payments from our customers, and we maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We determine the allowance based on known troubled accounts, historical experience, current economic trends that might impact the level of credit losses in the future and other available information. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances could be required.
Inventories—Inventories are generally stated at the lower of cost or net realizable value. We write down inventory for slow-moving and obsolete inventory based on historical usage, orders on hand, assessments of future demands, market conditions among other items. If these factors are less favorable than those projected, additional inventory write-downs may be required.
Property and Equipment—Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization are charged while assets are used in service and are computed using the straight-line method over the estimated useful lives of the assets taking into consideration any estimated salvage value. Amortization of leasehold improvements is calculated on the straight-line method over the shorter of the useful life of the asset or the lease term. Right-of-use assets from finance leases are included in property and equipment. Amortization of property and equipment under finance leases is included with depreciation expense. In the event that property and equipment are idle, as a result of excess capacity or the early termination, non-renewal or reduction in scope of a turnkey screening operation, such assets are assessed for impairment on a periodic basis or if any indicators of impairment exist.
Goodwill and Other Intangible Assets and Valuation of Long-Lived Assets—Goodwill represents the excess purchase price over the estimated fair value of the assets acquired and liabilities assumed in a business combination. Goodwill is allocated to our reporting units based on the nature of the product line of the acquired business. The carrying value of goodwill is not amortized but is annually tested for impairment as of the end of the second quarter and more frequently if there is an indicator of impairment. We assess qualitative factors of each of our three reporting units to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill. The assessments conducted as of December 31, 2022 indicated that it is not more likely than not that the fair values of our three reporting units are less than their carrying amounts, including goodwill. There were no qualitative factors which would trigger impairment testing between measurement dates. Thus, we have determined that there is no goodwill impairment for any of the three reporting units.
We evaluate long-lived assets with finite lives for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Impairment is considered to exist if the total estimated future cash flows on an undiscounted basis are less than the carrying amount of the assets. If impairment does exist, we measure the impairment loss and record it based on the discounted estimate of future cash flows. In estimating future cash flows, we group assets at the lowest level for which there are identifiable cash flows that are largely independent of the cash flows from other asset groups. Our estimate of future cash flows is based upon, among other things, certain assumptions about expected future operating performance, growth rates and other factors.
Income Taxes—Deferred income taxes are provided for temporary differences between the financial statement and income tax basis of our assets and liabilities, based on enacted tax rates. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred income tax assets will not be realized. Income tax accounting standards prescribe a two-step process for the financial statement measurement and recognition of a tax position taken or expected to be taken in a tax return. The first step involves the determination of whether it is more likely than not (greater than 50 percent likelihood) that a tax position will be sustained upon examination, based on the technical merits of the position. The second step requires that any tax position that meets the more likely than not recognition threshold be measured and recognized in the financial statements at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. See Note 10 for additional information.
F-12
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Fair Value of Financial Instruments—Our financial instruments consist primarily of cash and cash equivalents, insurance company contracts, accounts receivable, accounts payable, debt instruments, an interest rate swap contract and foreign currency forward contracts. The carrying values of financial instruments, other than long-term debt instruments and the interest rate swap contract, are representative of their fair values due to their short-term maturities. The carrying values of our long-term debt instruments are considered to approximate their fair values because the interest rates of these instruments are variable or comparable to current rates for financing available to us. The fair values of our foreign currency forward contracts were not significant as of June 30, 2023.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The “Level 1” category includes assets and liabilities at quoted prices in active markets for identical assets and liabilities. The “Level 2” category includes assets and liabilities from observable inputs other than quoted market prices. The “Level 3” category includes assets and liabilities for which valuation techniques are unobservable and significant to the fair value measurement. Our contingent payment obligations related to acquisitions, which are further discussed in Note 11 to the consolidated financial statements, are in the “Level 3” category for valuation purposes.
The fair values of our financial assets and liabilities as of June 30, 2022 and 2023 are categorized as follows (in thousands):
| June 30, 2022 |
| June 30, 2023 | |||||||||||||||||||||
| Level 1 |
| Level 2 |
| Level 3 |
| Total |
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |||||||||
Assets—Insurance company contracts | $ | — | $ | 40,284 | $ | — | $ | 40,284 | $ | — | $ | 47,181 | $ | — | $ | 47,181 | ||||||||
Assets—Interest rate swap contract | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 5,369 | $ | — | $ | 5,369 | ||||||||
Liabilities—Convertible debt | $ | — | $ | 242,302 | $ | — | $ | 242,302 | $ | — | $ | — | $ | — | $ | — | ||||||||
Liabilities—Contingent consideration | $ | — | $ | — | $ | 28,212 | $ | 28,212 | $ | — | $ | — | $ | 21,181 | $ | 21,181 | ||||||||
Derivative Instruments and Hedging Activity—Our use of derivatives consists of foreign currency forward contracts and an interest rate swap contract. The foreign currency forward contracts are utilized to partially mitigate certain balance sheet exposures or used as a net investment hedge to protect against potential changes resulting from short-term foreign currency fluctuations. These contracts have original maturities of up to three months. We also manage our risk to changes in interest rates using derivative instruments. We use fixed interest rate swaps to effectively convert a portion of the variable interest rate payments to fixed interest rate payments. We do not use hedging instruments for speculative purposes.
The net investment hedge has been designated as a hedge instrument and accounted for under Accounting Standards Codification (“ASC”) 815 Derivatives and Hedging. Hedge effectiveness is assessed using the spot method, consistent with guidance in ASC 815 whereby the change in fair value of the forward contract is recorded in the same manner as the related currency translation adjustments, within other comprehensive income, as the hedging instrument is expected to be fully effective unless the amount hedged exceeds the net investment in the foreign operation, or the foreign operation is liquidated. We settled the net investment hedge during fiscal 2021, and the amount recorded in other comprehensive loss was not significant. There were no net investment hedges outstanding as of June 30, 2023.
The net gains or losses from our foreign currency forward contracts, which are not designated as hedge instruments, are reported in the consolidated statements of operations, and the amounts reported for the years ending June 30, 2021, 2022 and 2023 were not significant. The fair value of our foreign currency forward contracts is estimated using a standard valuation model and market-based observable inputs over the contractual term. Unrealized gains are recognized as assets and unrealized losses are recognized as liabilities. As of June 30, 2022 and 2023, we held foreign currency forward contracts with notional amounts totaling $22.9 million and $21.6 million, respectively. Unrealized gains and losses from our foreign currency forward contracts as of June 30, 2022 and 2023 were not significant.
F-13
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
The interest rate swap agreement was entered into to improve the predictability of cash flows from interest payments related to our variable, Secured Overnight Financing Rate (“SOFR”) based debt. The interest rate swap matures in December 2026. The interest rate swap is considered an effective cash flow hedge, and as a result, the net gains or losses on such instrument are reported as a component of other comprehensive income in the consolidated financial statements and are reclassified as net income when the underlying hedged interest expense impacts earnings. A qualitative and quantitative assessment over the hedge effectiveness is performed on a quarterly basis, unless facts and circumstances indicate that the hedge may no longer be highly effective.
As of June 30, 2022 and 2023, the notional amount of the derivative instruments designated as an interest rate swap hedge was $0 and $175 million, respectively. The fair value of the interest rate swap contract as of June 30, 2023 was $5.4 million and is recorded in Other assets within the consolidated balance sheet.
The effect of the cash flow hedges on other comprehensive income (loss) and earnings for the periods presented was as follows:
Fiscal Year Ended June 30, | ||||||
| 2022 |
| 2023 | |||
Total interest and other expense, net presented in the condensed consolidated statements of operations in which the effects of cash flow hedges are recorded | $ | — | $ | (20,041) | ||
Gain recognized in other comprehensive income |
| — |
| 3,892 | ||
Amount reclassified from accumulated other comprehensive income (loss) to interest expense, net |
| — |
| 1,343 | ||
Revenue Recognition
We recognize revenue under Accounting Standards Codification Topic 606, Revenue from Contracts with Customers (“ASC 606”), which superseded all prior revenue recognition methods and industry-specific guidance. The core principle of ASC 606 is that an entity should recognize revenue to depict the transfer of control for promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In applying the revenue recognition principles, an entity is required to identify the contract(s) with a customer, identify the performance obligations, determine the transaction price, allocate the transaction price to the performance obligations and recognize revenue as the performance obligations are satisfied (i.e., either over time or at a point in time). ASC 606 further requires that companies disclose sufficient information to enable readers of financial statements to understand the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers.
Product Sales. We recognize revenue from sales of products upon shipment or delivery when control of the product transfers to the customer, depending on the terms of each sale, and when collection is probable. In the circumstance where terms of a product sale include subjective customer acceptance criteria, revenue is deferred until we have achieved the customer acceptance criteria unless such acceptance criteria are perfunctory or inconsequential. We generally offer customers payment terms of less than one year. In cases when payment terms extend beyond one year, we consider whether the contract has a significant financing component.
Service Revenue. Revenue from services includes installation and implementation of products and turnkey security screening services and after-market services. Generally, revenue from services is recognized over time as the services are performed. Revenues from out of warranty service maintenance contracts are recognized ratably over the respective terms of such contracts. Deferred revenue for such services arises from payments received from customers for services not yet performed.
Contract Revenue. Sales agreements with customers can be project specific, cover a period of time, and can be renewable periodically. The contracts may contain terms and conditions with respect to payment, delivery, installation, services, warranty and other rights. In certain instances, we consider an accepted customer order, governed by a master sales agreement, to be the contract with the customer when legal rights and obligations exist. Contracts with customers may include the sale of products and services, as discussed in the paragraphs above. In certain instances, contracts can contain multiple performance obligations as discussed in the paragraph below. According to the terms of a sale contract, we may receive consideration from a customer prior to transferring goods to the customer, and we record these prepayments as an advance receipt. We also record deferred revenue, typically related to service contacts, when consideration is received before the services have been performed. We recognize contract liabilities and deferred revenue as net sales after all revenue recognition criteria are met.
F-14
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
When determining revenue recognition for contracts, we make judgments based on our understanding of the obligations in each contract. We determine whether or not customer acceptance criteria are perfunctory or inconsequential. The determination of whether or not customer acceptance terms are perfunctory or inconsequential impacts the amount and timing of revenue recognition. Judgments also include estimates of warranty reserves, which are established based on historical experience and knowledge of the product under warranty.
Multiple Performance Obligations. Certain agreements with customers include the sale of capital equipment involving multiple elements that may include civil works to prepare a site for the installation of equipment, manufacture and delivery of equipment, installation and integration of equipment, training of customer personnel to operate the equipment and after-market service of the equipment. We generally assign multiple elements in a contract into separate performance obligations if those elements are distinct, both individually and in the context of the contract. If multiple promises comprise a series of distinct services which are substantially the same and have the same pattern of transfer, they are combined and accounted for as a single performance obligation.
In cases where obligations in a contract are distinct and thus require separation into multiple performance obligations, revenue recognition guidance requires that contract consideration be allocated to each distinct performance obligation based on its relative standalone selling price. The value allocated to each performance obligation is then recognized as revenue when the revenue recognition criteria for each distinct obligation or bundle of obligations has been met.
The standalone selling price for each performance obligation is an amount that depicts the amount of consideration to which the entity expects to be entitled in exchange for transferring the good or service. When there is only one performance obligation associated with a contract, the entire sale value is attributed to that obligation. When a contract contains multiple performance obligations, the standalone selling price is first estimated using the observable price, which is generally a list price net of applicable discount, or the price used to sell the good or service in similar circumstances. In circumstances when a selling price is not directly observable, we will estimate the standalone selling price using information available to us including our market assessment and/or expected cost plus margin.
The timetable for fulfilment of each of the distinct performance obligations can range from completion in a short amount of time and entirely within a single reporting period to completion over several reporting periods. The timing of revenue recognition for each performance obligation may be dependent upon several milestones, including physical delivery of equipment, completion of factory acceptance test, completion of site acceptance test, installation and connectivity of equipment, certification of training of personnel and, in the case of after-market service deliverables, the passage of time (typically evenly over the post-warranty period of the service deliverable).
We often provide a guarantee to support our performance under multiple performance obligations. In the event that customers are permitted to terminate such arrangements, the underlying contract typically requires payment for deliverables and reimbursement of costs incurred through the date of termination.
We disaggregate revenue by reporting segment (Security, Optoelectronics and Manufacturing, and Healthcare) to depict the nature of revenue in a manner consistent with our business operations and to be consistent with other communications and public filings. Refer to Note 14 for additional details of revenues by reporting segment.
Contract Assets and Liabilities. We enter into contracts to sell products and provide services, and we recognize contract assets and liabilities that arise from these transactions. We recognize revenue and corresponding accounts receivable according to ASC 606. When we recognize revenue in advance of the point in time at which contracts give us the right to invoice a customer, we record this as unbilled revenue, which is included in accounts receivable, net, on the consolidated balance sheet. We may also receive consideration, per the terms of a contract, from customers prior to transferring goods to the customer. We record customer deposits as contract liabilities. Additionally, we may receive payments, most typically under service and warranty contracts, at the onset of the contract and before services have been performed. In such instances, we record a deferred revenue liability. We recognize these contract liabilities as sales after all revenue recognition criteria are met.
F-15
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Practical Expedients. In cases where we are responsible for shipping after the customer has obtained control of the goods, we have elected to treat the shipping activities as fulfillment activities rather than as a separate performance obligation. Additionally, we have elected to capitalize the cost to obtain a contract only if the period of amortization would be longer than one year. We only give consideration to whether a customer agreement has a financing component if the period of time between transfer of goods and services and customer payment is greater than one year.
Freight—We record shipping and handling fees that we charge to our customers as revenue and related costs as cost of goods sold.
Research and Development Costs—Research and development costs are those costs related to the development of a new product, process or service, or significant improvement to an existing product, process or service. Such costs are charged to operations as incurred.
Stock-Based Compensation—Stock-based compensation cost is measured at the grant date based on the estimated fair value of the award and is recognized as expense over the employee’s requisite service period for all stock-based awards granted or modified. Certain restricted stock unit awards vest based on the achievement of pre-established performance criteria. The fair value of performance-based awards is estimated at the date of grant based upon the probability that the specified performance criteria will be met, adjusted for estimated forfeitures. Each quarter we update our assessment of the probability that the specified performance criteria will be achieved and adjust the estimate of the expenses of the performance-based awards if necessary. We amortize the fair value of performance-based awards over the requisite service period for each separately vesting tranche of the award. See Note 9 to the consolidated financial statements.
Impairment, Restructuring and Other Charges—We account for certain charges related to restructuring activities, litigation, acquisition-related costs and other non-routine charges as Impairment, restructuring and other charges in the consolidated financial statements. See Note 7 for additional information about these charges.
Credit Risk and Concentration— Financial instruments that are potentially subject to concentrations of credit risk consist primarily of cash, cash equivalents, marketable securities and accounts receivable. We restrict investments in cash equivalents to financial institutions with high credit standing. Credit risk on accounts receivable is minimized as a result of the large and diverse nature of our company’s worldwide customer base. As of June 30, 2022 and 2023, no customer accounted for greater than 10% of accounts receivable. In fiscal years 2021 and 2022, no customer accounted for greater than 10% of revenues. In fiscal year 2023, one customer accounted for 11% of revenues. We perform ongoing credit evaluations of our customers’ financial condition and maintain allowances for potential credit losses.
Our cash and cash equivalents totaled $64.2 million and $76.8 million at June 30, 2022 and 2023, respectively. Of these amounts, approximately 78% and 97% was held by our foreign subsidiaries at June 30, 2022 and 2023, respectively.
For cost, quality control, technological, and efficiency reasons, we purchase certain materials, parts, and components only from single vendors with whom we have ongoing relationships. We do, however, qualify second sources for many of our materials, parts, and components. While management believes that relying on key vendors improves the efficiency and reliability of business operations, relying on any one vendor for a significant aspect of business can have a significant negative impact on revenue and profitability if that vendor fails to perform at acceptable service levels for any reason, including financial difficulties of the vendor.
F-16
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Foreign Currency Translation and Transactions— We transact business in various foreign currencies. In countries where the functional currency of the underlying operations has been determined to be the local country’s currency, revenues and expenses of operations outside the United States are translated into United States dollars using average exchange rates while assets and liabilities of operations outside the United States are translated into United States dollars using period-end exchange rates. The effects of foreign currency translation adjustments are included in stockholders’ equity as a component of accumulated other comprehensive income (loss) in the accompanying consolidated balance sheets. We also have subsidiaries where the United States dollar has been designated as the functional currency based on individual facts and circumstances. Remeasurement of non-United States dollar monetary assets and liabilities are translated using period-end exchange rates and associated gains and losses are recognized in the consolidated statements of operations. Non-monetary assets and liabilities are translated using historical exchange rates. Transaction gains and losses, which were included in our consolidated statement of operations, amounted to a net gain (loss) of approximately $(1.3) million, $0.6 million and $2.0 million for the fiscal years ended June 30, 2021, 2022 and 2023, respectively.
Business Combinations—Under ASC 805, the acquisition method of accounting requires us to record assets acquired and liabilities assumed from an acquisition at their estimated fair values at the date of acquisition. Any excess of the total estimated purchase price over the estimated fair value of the net assets acquired should be recorded as goodwill. Such valuations require management to make significant estimates and assumptions, especially with respect to intangible assets. Significant estimates in valuing certain intangible assets include, but are not limited to, future expected cash flows from acquired customers, acquired technology, trade names, useful lives and discount rates. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates. During the measurement period, which is until we have all the necessary information about the facts and circumstances that existed as of the acquisition date up to one year from the acquisition date, as additional information that existed at the acquisition date becomes available for preliminary estimates, we may record adjustments to the provisional amounts initially recorded for assets acquired and liabilities assumed. Upon the conclusion of the measurement period, any subsequent adjustments are included in earnings.
Earnings per Share—We compute basic earnings per share by dividing net income available to common stockholders by the weighted average number of common shares outstanding during the period. We compute diluted earnings per share by dividing net income available to common stockholders by the sum of the weighted average number of common shares and dilutive potential common shares outstanding during the period. Potential common shares consist of the shares issuable upon the exercise of stock options and restricted stock unit awards under the treasury stock method. In periods where a net loss is reported, basic and diluted net loss per share are the same since the effect of potential common shares is antidilutive and therefore excluded. There was no dilutive effect of the senior convertible notes (See Note 8) for the fiscal years ended June 30, 2021, 2022 and 2023.
The following table sets forth the computation of basic and diluted earnings per share (in thousands, except per share amounts):
| 2021 |
| 2022 |
| 2023 | ||||
Net income available to common stockholders | $ | 74,049 | $ | 115,347 | $ | 91,778 | |||
Weighted average shares outstanding—basic |
| 17,968 |
| 17,551 |
| 16,828 | |||
Dilutive effect of equity awards |
| 420 |
| 319 |
| 362 | |||
Weighted average shares outstanding—diluted |
| 18,388 |
| 17,870 |
| 17,190 | |||
Basic earnings per share | $ | 4.12 | $ | 6.57 | $ | 5.45 | |||
Diluted earnings per share | $ | 4.03 | $ | 6.45 | $ | 5.34 | |||
Weighted average shares excluded from diluted earnings per share due to their anti-dilutive effect | 47 | 47 | 49 | ||||||
F-17
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Warranty Provision—We offer our customers warranties on many of the products that we sell. These warranties typically provide for repairs and maintenance of the products if problems arise during a specified time period after original shipment. Concurrent with the sale of products, we record a provision for estimated warranty expenses with a corresponding increase in cost of goods sold. We periodically adjust this provision based on historical experience and anticipated expenses. We charge actual expenses of repairs under warranty, including parts and labor, to this provision when incurred. The current obligation for warranty provision is included in other accrued expenses and current liabilities and the noncurrent portion is included in other long-term liabilities in the consolidated balance sheets, whose activity for each of the three fiscal years ended June 30, 2023 is summarized in the following table (in thousands):
Warranty provision as of June 30, 2020 |
| $ | 20,825 |
Warranty claims provided for/assumed in acquisition |
| 5,419 | |
Settlements made |
| (6,508) | |
Warranty provision as of June 30, 2021 | $ | 19,736 | |
Warranty claims provided for/assumed in acquisition |
| 3,474 | |
Settlements made |
| (9,863) | |
Warranty provision as of June 30, 2022 | $ | 13,347 | |
Warranty claims provided for/assumed in acquisition |
| 4,193 | |
Settlements made |
| (6,391) | |
Warranty provision as of June 30, 2023 | $ | 11,149 |
Leases—Right-of-use (“ROU”) assets represent our right to use an underlying asset during the reasonably certain lease terms, and lease liabilities represent our obligation to make lease payments arising from the leases. We recognize ROU lease assets and lease liabilities at lease commencement on our consolidated balance sheet based on the present value of lease payments over the lease term using a discount rate determined based on our incremental borrowing rate since the rate implicit in each lease is not readily determinable. We elected the package of practical expedients, which permits us to not reassess (1) whether any expired or existing contracts are or contain leases, (2) the lease classification of any expired or existing leases, and (3) any initial direct costs for any existing leases as of the effective date. We elected the practical expedient to account for each separate lease component of a contract and its associated non-lease components as a single lease component. We also elected the practical expedient, which allows us to use hindsight in determining the lease term. We do not record a ROU asset and corresponding lease liability for leases with an initial term of one year or less (“short-term leases”). The terms in our leases may include options to extend or terminate the lease. We recognize ROU assets and liabilities when it is reasonably certain that we will exercise those options. Judgment is required in our assessment as to whether renewal or termination options are reasonably certain to be exercised and factors such as contractual terms compared to current market rates and the importance of the facility and location to our operations, among others, are considered. Lease payments are made in accordance with the lease terms, and lease expense, including short-term lease expense, is recognized on a straight-line basis over the lease term.
We lease facilities and certain equipment under various operating lease agreements. The majority of our lease arrangements are comprised of fixed payments while certain of our other leases provide for periodic rent increases. Our leases may contain escalation clauses and renewal options. Most of the leases require us to pay for certain other costs such as common area maintenance and property taxes. Rent expense for leases with periodic rent increases or escalation clauses is recognized on a straight-line basis over the minimum lease term. The lease agreements do not contain any material residual value guarantees or material restrictive covenants. We also have finance leases for fleet vehicles that are not material to the consolidated financial statements.
Subsequent Events— In accordance with ASC 855, our management evaluated material events after the balance sheet date through the date of the filing of this report with the SEC, and there are no disclosable subsequent events.
Recent Accounting Guidance
From time to time, new accounting pronouncements are issued by the FASB and other regulatory bodies that are adopted as of the specified effective dates. Unless otherwise discussed, management believes that the impact of recently issued standards, which are not yet effective, will not have a material impact on our Consolidated Financial Statements upon adoption.
F-18
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
2. BUSINESS COMBINATIONS
Fiscal Year 2023 Business Acquisitions
In April 2023, we (through our Optoelectronics and Manufacturing division) acquired a privately held provider of engineering and contract manufacturing solutions for approximately $2.5 million plus up to $2.5 million in potential contingent consideration. The acquisition was financed with cash on hand.
In February 2023, we (through our Healthcare division) acquired a privately held provider of software and solutions for approximately $2.1 million plus up to $5.0 million in potential contingent consideration. The acquisition was financed with cash on hand.
Through our Security division, we acquired (i) in December 2022 certain assets of a provider of baggage and parcel inspection systems for approximately $1.6 million and (ii) in August 2022 a privately held provider of training software and solutions for approximately $1.9 million plus an immaterial amount of potential contingent consideration. These acquisitions were financed with cash on hand. The goodwill recognized for each of the fiscal year 2023 business acquisitions is not deductible for income tax purposes.
Fiscal Year 2022 Business Acquisitions
In February 2022, we (through our Security division) acquired a privately held provider of intelligent inspection, sensory, and recognition solutions for approximately $14.0 million, plus up to $25.0 million in potential contingent consideration. The acquisition was financed with cash on hand and borrowings under our revolving bank line of credit. The goodwill recognized for this business is not deductible for income tax purposes.
In February 2022, we (through our Security division) acquired a privately held sales and services company for approximately $1.1 million, plus an immaterial amount of potential contingent consideration. The acquisition was financed with cash on hand. The goodwill recognized for this transaction is deductible for income tax purposes.
Fiscal Year 2021 Business Acquisition
In fiscal 2021, we (through our Healthcare division) acquired a privately-held software development company for $3.0 million, plus up to $12.0 million in potential contingent consideration. This acquisition was financed with available cash on hand. The goodwill recognized for this business is deductible for income tax purposes.
These business acquisitions in fiscal 2021, 2022 and 2023, individually and in the aggregate, were not material to our consolidated financial statements. Accordingly, pro-forma historical results of operations and other disclosures related to these businesses have not been presented.
F-19
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
3. BALANCE SHEET DETAILS
The following tables provide details of selected balance sheet accounts (in thousands):
June 30, | ||||||
Accounts receivable, net |
| 2022 |
| 2023 | ||
Accounts receivable | $ | 326,849 |
| $ | 395,218 | |
Less allowance for doubtful accounts |
| (18,876) |
| (14,373) | ||
Total | $ | 307,973 | $ | 380,845 | ||
June 30, | ||||||
Inventories |
| 2022 |
| 2023 | ||
Raw materials | $ | 213,290 |
| $ | 233,217 | |
Work-in-process |
| 46,873 |
| 56,329 | ||
Finished goods |
| 73,744 |
| 48,462 | ||
Total | $ | 333,907 | $ | 338,008 | ||
Estimated | ||||||||
Useful | June 30, | |||||||
Property and equipment, net |
| Lives |
| 2022 | 2023 | |||
Land | N/A | $ | 15,028 |
| $ | 15,691 | ||
Buildings, civil works and improvements | 5-40 years |
| 47,309 |
| 49,166 | |||
Leasehold improvements | 1-13 years |
| 11,599 |
| 13,553 | |||
Equipment and tooling | 3-10 years |
| 128,425 |
| 135,703 | |||
Furniture and fixtures | 3-10 years |
| 3,592 |
| 3,632 | |||
Computer equipment | 3-5 years |
| 21,208 |
| 24,119 | |||
Computer software | 3-10 years |
| 25,153 |
| 26,981 | |||
Computer software implementation in process | N/A | 9,422 | 9,705 | |||||
Construction in process | N/A |
| 5,283 |
| 4,108 | |||
Total |
| 267,019 |
| 282,658 | ||||
Less accumulated depreciation and amortization |
| (157,335) |
| (173,725) | ||||
Property and equipment, net | $ | 109,684 | $ | 108,933 | ||||
During fiscal 2021, 2022 and 2023, depreciation expense was approximately $22.4 million, $21.0 million and $19.5 million, respectively.
F-20
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
4. GOODWILL AND INTANGIBLE ASSETS
The changes in the carrying amount of goodwill by segment for fiscal 2022 and 2023 are as follows (in thousands):
Optoelectronics | ||||||||||||
and | ||||||||||||
Security | Healthcare | Manufacturing | ||||||||||
| Division |
| Division |
| Division |
| Consolidated | |||||
Balance as of June 30, 2021 | $ | 206,426 | $ | 43,584 | $ | 70,294 | $ | 320,304 | ||||
Goodwill acquired or adjusted during the period |
| 19,436 |
| — |
| — |
| 19,436 | ||||
Foreign currency translation adjustment |
| (307) |
| (397) |
| (2,679) |
| (3,383) | ||||
Balance as of June 30, 2022 | $ | 225,555 | $ | 43,187 | $ | 67,615 | $ | 336,357 | ||||
Goodwill acquired or adjusted during the period |
| 5,021 |
| 5,161 |
| 2,574 |
| 12,756 | ||||
Foreign currency translation adjustment |
| 86 |
| 107 |
| 199 |
| 392 | ||||
Balance as of June 30, 2023 | $ | 230,662 | $ | 48,455 | $ | 70,388 | $ | 349,505 | ||||
Intangible assets consisted of the following (dollar amounts in thousands):
June 30, 2022 | June 30, 2023 | |||||||||||||||||||
Weighted | Gross | Gross | ||||||||||||||||||
Average | Carrying | Accumulated | Intangibles | Carrying | Accumulated | Intangibles | ||||||||||||||
| Lives |
| Value |
| Amortization |
| Net |
| Value |
| Amortization |
| Net | |||||||
Amortizable assets: | ||||||||||||||||||||
Software development costs |
| 7-8 years | $ | 64,096 | $ | (18,934) | $ | 45,162 | $ | 77,844 | $ | (20,285) | $ | 57,559 | ||||||
Patents |
| 19 years |
| 8,541 |
| (2,987) |
| 5,554 |
| 8,636 |
| (3,404) |
| 5,232 | ||||||
Developed technology |
| 10 years |
| 66,901 |
| (31,071) |
| 35,830 |
| 68,274 |
| (38,353) |
| 29,921 | ||||||
Customer relationships |
| 7-8 years |
| 53,736 |
| (32,785) |
| 20,951 |
| 55,780 |
| (39,101) |
| 16,679 | ||||||
Total amortizable assets |
| 193,274 |
| (85,777) |
| 107,497 |
| 210,534 |
| (101,143) |
| 109,391 | ||||||||
Non-amortizable assets: | ||||||||||||||||||||
In-process R&D | 533 | — | 533 | 533 | — | 533 | ||||||||||||||
Trademarks |
| 30,340 |
| — |
| 30,340 |
| 30,933 |
| — |
| 30,933 | ||||||||
Total intangible assets | $ | 224,147 | $ | (85,777) | $ | 138,370 | $ | 242,000 | $ | (101,143) | $ | 140,857 | ||||||||
Amortization expense related to intangible assets was $21.5 million, $17.7 million and $19.0 million for fiscal 2021, 2022 and 2023, respectively.
At June 30, 2023, the estimated future amortization expense was as follows (in thousands):
2024 |
| $ | 18,758 |
2025 |
| 16,042 | |
2026 |
| 13,112 | |
2027 |
| 9,054 | |
2028 | 6,544 | ||
Thereafter |
| 45,881 | |
Total | $ | 109,391 |
F-21
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Software development costs for software products incurred before establishing technological feasibility are charged to operations. Software development costs incurred after establishing technological feasibility are capitalized on a product-by-product basis until the product is available for general release to customers at which time amortization begins. Annual amortization, charged to cost of goods sold, is the amount computed using the ratio that current revenues for a product bear to the total current and anticipated future revenues for that product. In the event that future revenues are not estimable, such costs are amortized on a straight-line basis over the remaining estimated economic life of the product. Amortizable assets that have not yet begun to be amortized are included in “Thereafter” in the table above. During fiscal 2021, 2022 and 2023, we capitalized software development costs in the amounts of $12.9 million, $15.2 million and $16.2 million, respectively.
5. CONTRACT ASSETS AND LIABILITIES
The table below shows the balance of contract assets and liabilities as of June 30, 2022 and 2023, including the change between the periods. There were no substantial non-current contract assets for the periods presented.
Contract Assets (dollar amounts in thousands)
June 30, | June 30, |
| ||||||||||
| 2022 |
| 2023 |
| Change |
| % Change |
| ||||
Unbilled revenue (included in accounts receivable, net) | $ | 43,287 | $ | 86,818 | $ | 43,531 |
| 101 | % | |||
Contract Liabilities (dollar amounts in thousands)
June 30, | June 30, |
| ||||||||||
| 2022 |
| 2023 |
| Change |
| % Change | |||||
Advances from customers | $ | 19,917 | $ | 21,250 | $ | 1,333 |
| 7 | % | |||
Deferred revenue—current |
| 31,396 |
| 43,861 |
| 12,465 |
| 40 | % | |||
Deferred revenue—long-term |
| 20,476 |
| 22,200 |
| 1,724 |
| 8 | % | |||
Contract Assets. Contract assets increased approximately $43.5 million as a result of unbilled revenue primarily from the timing and nature of milestones met in contracts for a number of customers in our Security Division, both within the United States and internationally, where we met the revenue recognition criteria under ASC 606 in advance of the time when contracts give us the right to invoice customers.
Remaining Performance Obligations. Remaining performance obligations related to ASC 606 represent the portion of the transaction price allocated to performance obligations under an original contract with a term greater than one year which are fully or partially unsatisfied at the end of the period. As of June 30, 2023, the aggregate portion of the transaction price allocated to remaining performance obligations was approximately $1,011.2 million. We expect to recognize revenue on approximately 55% of the remaining performance obligations over the next 12 months, and the remainder is expected to be recognized thereafter. During the fiscal year ended June 30, 2023, we recognized revenue of $50.9 million from contract liabilities existing as of July 1, 2022.
Practical Expedients. In cases where we are responsible for shipping after the customer has obtained control of the goods, we have elected to treat the shipping activities as fulfillment activities rather than as a separate performance obligation. Additionally, we have elected to capitalize the cost to obtain a contract only if the period of amortization would be longer than one year. We only give consideration to whether a customer agreement has a financing component if the period of time between transfer of goods and services and customer payment is greater than one year.
F-22
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
6. LEASES
The components of operating lease expense for the fiscal years ended June 30, 2022 and 2023 were as follows (in thousands):
| Fiscal Year Ended June 30, | |||||
2022 |
| 2023 | ||||
Operating lease cost | $ | 10,390 | $ | 11,364 | ||
Variable lease cost |
| 856 |
| 1,323 | ||
Short-term lease cost |
| 1,061 |
| 923 | ||
$ | 12,307 | $ | 13,610 | |||
Sale-leaseback Transaction. In March 2022, we completed a sale-leaseback transaction for our manufacturing facilities and corporate headquarters in Hawthorne, California (the “Hawthorne Property”). We sold the Hawthorne Property for $32 million and recognized a gain on sale of $27.4 million which is included in Other income on the statement of operations for the fiscal year ended June 30, 2022. We also entered into a lease agreement for the Hawthorne Property expiring in March 2028, with two 5-year renewal options.
Supplemental balance sheet assets and liabilities related to operating leases were as follows (dollar amounts in thousands):
| Balance Sheet Category |
| June 30, 2022 |
| June 30, 2023 |
| ||||
Operating lease ROU assets, net |
| $ | 39,461 | $ | 32,618 | |||||
Operating lease liabilities, current portion |
| $ | 9,700 | $ | 9,787 | |||||
Operating lease liabilities, long-term |
|
| 30,363 |
| 23,733 | |||||
Total operating lease liabilities | $ | 40,063 | $ | 33,520 | ||||||
Weighted average remaining lease term |
|
|
|
| 4.2 years | |||||
Weighted average discount rate |
|
|
|
| 3.7 | % | ||||
Supplemental cash flow information related to operating leases for the year ended June 30, 2023 was as follows (in thousands):
Fiscal Year Ended June 30, | ||||||
| 2022 |
| 2023 | |||
Cash paid for operating lease liabilities | $ | 10,046 | $ | 11,418 | ||
ROU assets obtained in exchange for new lease obligations |
| 27,402 |
| 14,574 | ||
Maturities of operating lease liabilities at June 30, 2023 were as follows (in thousands):
| June 30, 2023 | ||
Less than one year | $ | 10,813 | |
1 – 2 years |
| 8,596 | |
2 – 3 years |
| 7,032 | |
3 – 4 years |
| 6,101 | |
4 – 5 years |
| 1,748 | |
Thereafter |
| 1,966 | |
| 36,256 | ||
Less: Imputed interest |
| (2,736) | |
Total lease liabilities | $ | 33,520 | |
F-23
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
7. IMPAIRMENT, RESTRUCTURING AND OTHER CHARGES
We endeavor to align our global capacity and infrastructure with demand by our customers as well as fully integrate acquisitions and thereby improve operational efficiency.
During the fiscal year ended June 30, 2023, we recognized $7.6 million in impairment, restructuring and other charges, which included $3.9 million in legal charges primarily related to class action litigation and government investigations, $1.7 million for employee terminations, $1.5 million for other facility closure costs for operational efficiency activities, and $0.4 million in acquisition related costs.
During the fiscal year ended June 30, 2022, we recognized $7.5 million in impairment, restructuring and other charges, which included $5.1 million in legal charges primarily related to class action litigation and government investigations, $1.1 million for employee terminations, $1.0 million for impairment of software assets, $0.3 million in acquisition related costs, and a net benefit for facility closures activity of a nominal amount.
During the fiscal year ended June 30, 2021, we incurred $7.2 million for exit activities associated with an expired turnkey contract in Mexico. Such exit costs include $2.8 million for employee terminations, $1.1 million for facility closure and other exit costs, direct transaction costs of $2.7 million, and $0.6 million for ROU asset impairment for a leased facility. We also incurred costs of $1.6 million for other employee terminations and $0.5 million for other facility closure costs for operational efficiency activities, $0.3 million for acquisition-related activities, and $0.5 million for certain legal charges, net of insurance reimbursements.
The following tables summarize impairment, restructuring and other charges for the periods set forth below (in thousands):
Fiscal 2021 | |||||||||||||||
Optoelectronics | |||||||||||||||
and | |||||||||||||||
Security | Healthcare | Manufacturing | |||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Total | ||||||
Impairment charges | $ | 552 | $ | — | $ | — | $ | — | $ | 552 | |||||
Acquisition-related costs | 249 | 27 | — | — | 276 | ||||||||||
Employee termination costs |
| 4,130 |
| — |
| 315 |
| — |
| 4,445 | |||||
Mexico transaction costs |
| 2,691 |
| — |
| — |
| — |
| 2,691 | |||||
Facility closures/consolidation |
| 1,675 |
| — |
| — |
| — |
| 1,675 | |||||
Legal costs, net |
| — | — | — | 465 | 465 | |||||||||
Total expensed | $ | 9,297 | $ | 27 | $ | 315 | $ | 465 | $ | 10,104 | |||||
Fiscal 2022 | |||||||||||||||
Optoelectronics | |||||||||||||||
and | |||||||||||||||
Security | Healthcare | Manufacturing | |||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Total | ||||||
Impairment charges | $ | — | $ | — | $ | — | $ | 1,006 | $ | 1,006 | |||||
Acquisition-related costs | 232 | — | — | 56 | 288 | ||||||||||
Employee termination costs |
| 1,077 |
| — |
| 100 |
| — |
| 1,177 | |||||
Facility closures/consolidation |
| (33) |
| — |
| — |
| — |
| (33) | |||||
Legal costs, net |
| — |
| — |
| — |
| 5,104 |
| 5,104 | |||||
Total expensed | $ | 1,276 | $ | — | $ | 100 | $ | 6,166 | $ | 7,542 | |||||
F-24
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Fiscal 2023 | |||||||||||||||
Optoelectronics | |||||||||||||||
and | |||||||||||||||
Security | Healthcare | Manufacturing | |||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Total | ||||||
Acquisition-related costs | $ | 23 | $ | 225 | $ | 7 | $ | 127 | $ | 382 | |||||
Employee termination costs | 849 | 355 | 532 | — | 1,736 | ||||||||||
Facility closures/consolidation | 35 | — | 1,504 | — | 1,539 | ||||||||||
Legal costs, net | 808 | 2,497 | 464 | 140 | 3,909 | ||||||||||
Total expensed | $ | 1,715 | $ | 3,077 | $ | 2,507 | $ | 267 | $ | 7,566 | |||||
The accrued liability for restructuring and other charges is included in other accrued expenses and current liabilities in the consolidated balance sheet. The changes in the accrued liability for restructuring and other charges for fiscal 2022 and 2023 were as follows (in thousands):
Facility | |||||||||||||||
Acquisition- | Employee | Closure / | Legal | ||||||||||||
Related | Termination | Consolidation | Costs and | ||||||||||||
| Costs |
| Costs |
| Cost |
| Settlements |
| Total | ||||||
Balance as of June 30, 2021 | $ | — | $ | 250 | $ | 386 | $ | 2,772 | $ | 3,408 | |||||
Restructuring and other charges (benefit), net | 288 | 1,177 | (33) | 6,110 | 7,542 | ||||||||||
Payments, adjustments and reimbursements, net | (288) | (1,246) | (330) | (7,102) | (8,966) | ||||||||||
Balance as of June 30, 2022 | $ | — | $ | 181 | $ | 23 | $ | 1,780 | $ | 1,984 | |||||
Restructuring and other charges (benefit), net |
| 383 |
| 1,736 |
| 1,539 |
| 3,909 |
| 7,567 | |||||
Payments, adjustments and reimbursements, net |
| (376) |
| (1,810) |
| 47 |
| (5,033) |
| (7,172) | |||||
Balance as of June 30, 2023 | $ | 7 | $ | 107 | $ | 1,609 | $ | 656 | $ | 2,379 | |||||
8. BORROWINGS
Revolving Credit Facility
In December 2021, we entered into an amendment to the senior secured credit facility that increased the aggregate amount available to borrow from $535 million to $750 million. The amended facility matures in December 2026 and is comprised of a $600 million revolving credit facility and a $150 million delayed draw term loan. The revolving credit facility includes a $300 million sub-limit for letters of credit. Under certain circumstances and subject to certain conditions, we can increase the revolving credit facility by $250 million plus such amount as would not cause our consolidated secured net leverage ratio to exceed a specified level. Borrowings under the amended facility bore interest at SOFR plus a margin of 1.0% as of June 30, 2023 (which margin can range from 1.0% to 1.75% based on our consolidated net leverage ratio as defined in the credit facility). Letters of credit reduce the amount available to borrow under the credit facility by their face value amount. The unused portion of the facility bore a commitment fee of 0.10% as of June 30, 2023 (which fee can range from 0.10% to 0.25% based on our consolidated net leverage ratio as defined in the credit facility). Our borrowings under the credit agreement are guaranteed by certain of our U.S.-based subsidiaries and are secured by substantially all of our assets and substantially all the assets of certain of our subsidiaries. The credit facility contains various representations and warranties, affirmative, negative and financial covenants and events of default. As of June 30, 2023, there were $215.0 million of borrowings outstanding under the revolving credit facility, $48.5 million outstanding under the letters of credit sub-facility, and $143.1 million outstanding under the term loan. As of June 30, 2023, the amount available to borrow under the revolving credit facility was $336.5 million. Loan amounts under the revolving credit facility may be borrowed, repaid and re-borrowed during the term. The principal amount of each loan is due and payable in full on the maturity date. We have the right to repay each loan in whole or in part from time to time without penalty. It is our practice to routinely borrow and repay several times per year under the revolving facility and therefore, borrowings under the revolving credit facility are included in current liabilities. As of June 30, 2023, we were in compliance with all financial covenants under this credit facility. In September 2022, we entered into an interest rate swap in order to mitigate the interest rate risk on a portion of the interest payments expected to be made on the borrowings outstanding under the revolving credit facility and term loan. Refer to Note 1 for details.
F-25
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
1.25% Convertible Senior Notes Due 2022
In February 2017, we issued $287.5 million of the Notes in a private offering. The Notes were governed by an indenture dated February 22, 2017. The maturity date for the payment of principal was September 1, 2022. The Notes bore interest at the rate of 1.25% and were payable in cash semiannually in arrears on each March 1 and September 1. On September 1, 2022, we repurchased and cancelled the then-remaining $242.3 million balance of the Notes utilizing proceeds from the senior secured credit facility.
Issuance costs of $7.7 million were allocated between debt ($6.5 million) and equity ($1.2 million) components with the portion allocated to the debt presented as an offset against long-term debt in the consolidated balance sheet and was being amortized as interest expense over the life of the Notes using the effective interest method. Total interest expense recognized for the year ended June 30, 2021 related to the Notes was $13.4 million, which consisted of $3.6 million of contractual interest expense, $8.6 million of debt discount amortization, and $1.2 million of amortization of debt issuance costs. Total interest expense for the year ended June 30, 2022 related to the Notes was $4.7 million, which consisted of $3.5 million of contractual interest expense and $1.2 million of amortization of debt issuance costs. Total interest expense for the year ended June 30, 2023 related to the Notes was $0.7 million, which consisted of $0.5 million of contractual interest expense and $0.2 million of amortization of debt issuance costs.
Other Borrowings
Several of our foreign subsidiaries maintain bank lines-of-credit, denominated in local currencies and U.S. dollars, primarily for the issuance of letters-of-credit. As of June 30, 2023, $51.7 million was outstanding under these letter-of-credit facilities. As of June 30, 2023, the total amount available under these credit facilities was $24.5 million.
Long-term debt consisted of the following (in thousands):
| June 30, | |||||
2022 |
| 2023 | ||||
1.25% convertible notes due September 1, 2022: | ||||||
Principal amount | $ | 242,302 | $ | — | ||
Unamortized debt issuance costs | (196) | — | ||||
242,106 | — | |||||
Term loan | 50,000 | 143,125 | ||||
Other long-term debt |
| 1,137 |
| 1,442 | ||
| 293,243 |
| 144,567 | |||
Less current portion of long-term debt |
| (244,575) |
| (8,076) | ||
Long-term portion of debt | $ | 48,668 | $ | 136,491 | ||
Fiscal year principal payments of long-term debt as of June 30, 2023 are as follows (in thousands):
2024 |
| $ | 8,076 |
2025 |
| 7,992 | |
2026 |
| 7,801 | |
2027 |
| 120,698 | |
2028 and thereafter |
| — | |
Total | $ | 144,567 |
F-26
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
9. STOCKHOLDERS’ EQUITY
Stock-based Compensation
As of June 30, 2023, we maintained the OSI Plan as a stock-based employee compensation plan.
We recorded stock-based compensation expense in the consolidated statements of operations as follows (in thousands):
| 2021 |
| 2022 |
| 2023 | ||||
Cost of goods sold | $ | 760 | $ | 812 | $ | 911 | |||
Selling, general and administrative |
| 25,457 |
| 26,749 |
| 27,716 | |||
Research and development |
| 554 |
| 511 |
| 497 | |||
Stock-based compensation expense | $ | 26,771 | $ | 28,072 | $ | 29,124 | |||
As of June 30, 2023, total unrecognized compensation cost related to stock-based compensation grants under the OSI Plan were estimated at $0.7 million for stock options and $13.9 million for restricted stock units (“RSUs”). We expect to recognize these costs over a weighted-average period of 2.0 years with respect to the stock options and 2.0 years for grants of RSUs.
OSI Plan
Awards are granted in the form of incentive options, nonqualified options, restricted stock awards, stock appreciation rights, RSUs, performance shares and stock bonuses, amongst other forms of equity, to qualified employees, directors and consultants.
Under the OSI Plan, the exercise price of nonqualified options and incentive stock options may not be less than the fair market value of our Common Stock on the date of grant. The exercise price of nonqualified options and incentive stock options granted to individuals who own more than 10% of our may not be less than 110% of the of our Common Stock on the date of grant. Stock options granted under the OSI Plan typically vest over three years based on continued service. Restricted stock and RSUs typically vest over to four years based on continued service. Certain restricted stock awards granted to senior management vest based on the achievement of pre-established performance criteria.
Stock Option Fair Value Estimation Assumptions. We estimate the fair value of our stock options at the date of grant using the Black-Scholes option-pricing valuation model. Our valuation model is affected by our stock price as well as weighted average assumptions for a number of subjective variables described below.
Expected Dividend. Expected dividend is based on historical patterns and our anticipated dividend payments over the expected holding period.
Risk-Free Interest Rate. The risk-free interest rate for stock options is based on U.S. Treasuries for a maturity matching the expected holding period.
Expected Volatility. Expected volatility is based on implied volatility and/or our historical share price volatility matching the expected holding period. No single method of estimating volatility is proper under all circumstances and to the extent that a company can derive implied volatility based on the trading of its financial instruments on a public market, it may be appropriate to use both implied and historical volatility in its assumptions. We have certain financial instruments that are publicly traded from which we can derive the implied volatility. Therefore, we use implied and historical volatility for valuing our stock options. We believe that implied and historical volatility is a better indicator of expected volatility because it is generally reflective of both historical volatility and expectations of how future volatility will differ from historical volatility.
Expected Holding Period. We use historical stock option exercise data to estimate the expected holding period.
F-27
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Changes in assumptions can materially impact the estimated fair value of stock options. The weighted average assumptions used in the valuation model are presented in the table below.
| 2021 |
| 2022 |
| 2023 |
| |
Expected dividend |
| — | — | — | |||
Risk-free interest rate |
| 0.4 | % | 1.2 | % | 3.9 | % |
Expected volatility |
| 26.0 | % | 31.0 | % | 31.0 | % |
Expected holding period (in years) |
| 4.5 | 4.5 | 4.5 |
The following summarizes stock option activity for fiscal years 2021, 2022 and 2023:
Weighted- | ||||||||||
Average | Weighted-Average | Aggregate | ||||||||
Number of | Exercise | Remaining Contractual | Intrinsic Value | |||||||
| Options |
| Price |
| Term |
| (in thousands) | |||
Outstanding at June 30, 2020 |
| 326,304 |
| $ | 44.41 | |||||
Granted |
| 22,171 |
| 82.17 | ||||||
Exercised |
| (88,657) |
| 35.19 | ||||||
Expired or forfeited |
| (4,598) |
| 80.46 | ||||||
Outstanding at June 30, 2021 |
| 255,220 |
| $ | 50.24 | |||||
Granted |
| 22,954 |
| 96.38 | ||||||
Exercised |
| (166,629) |
| 35.09 | ||||||
Expired or forfeited |
| (900) |
| 73.99 | ||||||
Outstanding at June 30, 2022 |
| 110,645 |
| $ | 82.43 |
| ||||
Granted |
| 23,351 | 87.90 | |||||||
Exercised |
| (47,354) | 77.42 | |||||||
Expired or forfeited |
| (2,965) | 74.06 | |||||||
Outstanding at June 30, 2023 |
| 83,677 | $ | 87.09 | 7.1 years | $ | 2,572 | |||
Exercisable at June 30, 2023 | 38,977 | $ | 83.79 |
| 5.1 years | $ | 1,327 | |||
The per-share weighted-average grant-date fair value of stock options granted under the OSI Plan was $18.37, $26.72 and $28.46 for fiscal 2021, 2022 and 2023, respectively. The total intrinsic value of options exercised during fiscal 2023 was $1.5 million.
Restricted Stock Units—A summary of RSU activity for the periods indicated was as follows:
Weighted- | |||||
Average | |||||
| Shares |
| Fair Value | ||
Nonvested at June 30, 2020 |
| 423,590 | $ | 88.68 | |
Granted |
| 339,311 |
| 80.40 | |
Vested |
| (313,892) |
| 86.12 | |
Forfeited |
| (13,084) |
| 85.78 | |
Nonvested at June 30, 2021 |
| 435,925 | $ | 84.16 | |
Granted |
| 334,435 |
| 90.31 | |
Vested |
| (337,442) |
| 82.66 | |
Forfeited |
| (5,471) |
| 83.66 | |
Nonvested at June 30, 2022 |
| 427,447 | $ | 90.17 | |
Granted |
| 357,475 | 87.90 | ||
Vested |
| (313,862) | 96.36 | ||
Forfeited |
| (15,545) | 88.42 | ||
Nonvested at June 30, 2023 |
| 455,515 | $ | 85.15 | |
F-28
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
The per-share weighted average grant-date fair value of RSUs granted under the OSI Plan was $80.40, $90.31, and $87.90 for fiscal 2021, 2022 and 2023, respectively. The total fair value of shares vested during fiscal 2021, 2022 and 2023 was $27.0 million, $27.9 million, and $27.9 million, respectively.
In December 2020, our shareholders authorized an increase of 1.65 million shares for the OSI Plan resulting in a maximum pool of 7.1 million shares. As of June 30, 2023, there were approximately 0.7 million shares available for grant under the OSI Plan. Under the terms of the OSI Plan, RSUs and restricted stock granted from the pool of shares available for grant reduce the pool by 1.87 shares for each award granted. RSUs and restricted stock forfeited and returned to the pool of shares available for grant increase the pool by 1.87 shares for each award forfeited.
We granted 136,242, 96,620, and 110,811 performance-based awards during fiscal 2021, 2022 and 2023, respectively. These performance-based RSU awards are contingent on the achievement of certain performance metrics. The payout related to these awards can range from zero to 376% of the original number of shares or units awarded. Compensation cost associated with these performance based RSUs are recognized based on the estimated number of shares that we ultimately expect will vest. If the estimated number of shares to vest is revised in the future, then stock-based compensation expense will be adjusted accordingly.
Employee Stock Purchase Plan
We have an employee stock purchase plan under which eligible employees may purchase a limited number of shares of Common Stock at a discount of up to 15% of the market value of such stock at pre-determined, plan-defined dates. During the years ended June 30, 2021, 2022 and 2023, employees purchased 63,499, 60,708, and 60,465 shares, respectively. As of June 30, 2023, there were 416,762 shares of our Common Stock available for issuance under the plan.
Stock Repurchase Program
In September 2022, our Board of Directors increased the stock repurchase authorization to a total of two million shares of Common Stock. This program does not expire unless our Board of Directors acts to terminate the program. The timing and actual numbers of shares purchased depends on a variety of factors, including stock price, general business and market conditions and other investment opportunities. Repurchases may be made from time to time under the program through open-market purchases or privately-negotiated transactions at our discretion. Upon repurchase, the shares are restored to the status of authorized but unissued shares and we record them in our consolidated financial statements as a reduction in the number of shares of Common Stock issued and outstanding.
During fiscal 2021, 2022 and 2023, we repurchased 452,005 shares, 1,294,594 shares and 400,230 shares, respectively, of common stock under our then current programs. As of June 30, 2023, there were 1,721,870 shares remaining available for repurchase under the authorized repurchase program.
Dividends
We have not paid any dividends since the consummation of our initial public offering in 1997 and we do not currently intend to pay any dividends in the foreseeable future. Our Board of Directors will determine the payment of future dividends, if any. Certain of our current bank credit facilities restrict the payment of dividends and future borrowings may contain similar restrictions.
F-29
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
10. INCOME TAXES
The following is a geographical breakdown of income before the provision for income taxes (in thousands):
| 2021 |
| 2022 |
| 2023 | ||||
Pre-tax income: | |||||||||
United States | $ | 34,323 | $ | 51,295 | $ | 7,114 | |||
Foreign |
| 64,317 |
| 88,865 |
| 108,124 | |||
Total pre-tax income | $ | 98,640 | $ | 140,160 | $ | 115,238 | |||
Our provision (benefit) for income taxes consists of the following (in thousands):
| 2021 |
| 2022 |
| 2023 | ||||
Current: | |||||||||
Federal | $ | 4,407 | $ | 6,216 | $ | 6,860 | |||
State |
| 1,190 |
| 1,964 |
| 861 | |||
Foreign |
| 18,562 |
| 13,113 |
| 19,717 | |||
Total current provision |
| 24,159 |
| 21,293 |
| 27,438 | |||
Deferred: | |||||||||
Federal | $ | 679 | $ | 3,915 | $ | (2,547) | |||
State |
| 464 |
| 133 |
| (678) | |||
Foreign |
| (711) |
| (528) |
| (753) | |||
Total deferred provision (benefit) |
| 432 |
| 3,520 |
| (3,978) | |||
Total provision | $ | 24,591 | $ | 24,813 | $ | 23,460 | |||
As of June 30, 2022 and 2023, our liability for uncertain tax positions was $8.2 million and $12.0 million, respectively. The amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate was $12.0 million as of June 30, 2023.
We recognize potential interest and penalties related to income tax matters in income tax expense. As of June 30, 2023, we have accrued $0.4 million for interest and penalties. Our uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. These include fiscal years after 2019 for federal purposes, fiscal years after 2018 for state purposes and fiscal years after 2015 for various foreign jurisdictions. Facts and circumstances could arise that could cause us to reduce the liability for unrecognized tax benefits, including, but not limited to, settlement of income tax positions or expiration of statutes of limitation. Since the ultimate resolution of uncertain tax positions depends on many factors and assumptions, we are not able to estimate the range of potential changes in the liability for unrecognized tax benefits or the timing of such changes.
A summary of activity of unrecognized tax benefits for fiscal 2022 and 2023 is as follows (in thousands).
Balance at June 30, 2021 |
| $ | 19,677 |
Additions on tax positions for the current year |
| 3,084 | |
Additions on tax positions from prior years |
| 1,479 | |
Reduction in tax positions from prior year |
| (10,663) | |
Balance at June 30, 2022 | $ | 13,577 | |
Additions on tax positions for the current year |
| 3,225 | |
Additions on tax positions from prior years |
| 2,582 | |
Reduction in tax positions from prior year |
| (4,406) | |
Balance at June 30, 2023 | $ | 14,978 |
F-30
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Deferred income tax assets (liabilities) consisted of the following (in thousands):
June 30, | ||||||
| 2022 |
| 2023 | |||
Deferred income tax assets: | ||||||
Tax credit carryforwards | $ | 13,130 | $ | 5,934 | ||
Net operating loss carryforwards |
| 6,494 |
| 3,678 | ||
Customer advances |
| 2,848 |
| 3,443 | ||
Allowance for doubtful accounts |
| 4,471 |
| 2,994 | ||
Inventory reserve |
| 11,636 |
| 11,026 | ||
Inventory capitalization |
| 406 |
| 481 | ||
Accrued liabilities |
| 3,241 |
| 2,956 | ||
Operating lease liabilities | 8,714 | 8,043 | ||||
Stock and deferred compensation |
| 10,601 |
| 12,224 | ||
Other assets |
| 1,446 |
| 1,164 | ||
Total deferred income tax assets |
| 62,987 |
| 51,943 | ||
Valuation allowance |
| (12,301) |
| (8,433) | ||
Net deferred income tax assets |
| 50,686 |
| 43,510 | ||
Deferred income tax liabilities: | ||||||
Depreciation |
| (7,604) |
| (5,860) | ||
Amortization of intangible assets |
| (31,518) |
| (21,617) | ||
Withholding tax on unrepatriated foreign earnings | (6,851) | (6,851) | ||||
Operating lease ROU assets | (8,480) | (7,931) | ||||
State transition tax | (1,754) | (1,754) | ||||
Other liabilities |
| (1,750) |
| (1,824) | ||
Total deferred income tax liabilities |
| (57,957) |
| (45,837) | ||
Net deferred income tax liability | $ | (7,271) | $ | (2,327) | ||
The components of the net deferred income tax liability are classified in the consolidated balance sheets as follows (in thousands):
June 30, | ||||||
| 2022 |
| 2023 | |||
Long term deferred income tax asset, included in other assets | $ | 3,841 | $ | 4,244 | ||
Long term deferred income tax liability |
| (11,112) |
| (6,571) | ||
Net deferred income tax liability | $ | (7,271) | $ | (2,327) | ||
The components of current taxes receivable and payable and prepaid taxes are classified in the consolidated balance sheets as follows (in thousands):
| June 30, | |||||
2022 |
| 2023 | ||||
Current taxes receivable and prepaid taxes, included in prepaid expenses and other current assets | $ | 7,843 | $ | 7,216 | ||
Current taxes payable, included in other accrued expenses and current liabilities |
| (7,722) |
| (13,692) | ||
Net tax receivable (payable) | $ | 121 | $ | (6,476) | ||
F-31
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
As of June 30, 2023, we had federal, state and foreign net operating losses carryforwards of approximately $1.1 million, $22.6 million and $7.9 million, respectively. Our net operating loss carryforwards will begin to expire in the tax year ending June 30, 2028. As of June 30, 2023, we had federal and state tax credit carryforwards of approximately $0.5 million and $8.1 million, respectively. Our credit carryforwards will begin to expire in the tax year ending June 30, 2026.
We have established valuation allowances that relate to the net operating losses of certain subsidiaries, capital losses, and tax credits. During the year ended June 30, 2023, we recorded a net aggregated decrease of $3.9 million to these valuation allowances. We review the adequacy of individual valuation allowances and release such allowances when it is determined that it is more likely than not that the related benefits will be realized.
We recognized all excess tax benefits and tax deficiencies as income tax expense or benefit in the current year. An income tax benefit of approximately $2.0 million and $0.6 million was recognized in fiscal 2022 and 2023, respectively.
The consolidated effective income tax rate differs from the federal statutory income tax rate due primarily to the following:
June 30, |
| ||||||
| 2021 |
| 2022 |
| 2023 |
| |
Provision for income taxes at federal statutory rate | 21.0 | % | 21.0 | % | 21.0 | % | |
Research and development tax credits | (1.7) | (1.3) | (1.5) | ||||
Foreign income subject to tax at other than federal statutory rate | 0.6 | 0.2 | 0.2 | ||||
Stock compensation | (0.9) | (1.2) | (0.4) | ||||
Officers’ compensation | 5.8 | 4.3 | 5.5 | ||||
Change in valuation allowance | (5.9) | (4.0) | (0.5) | ||||
Unrecognized tax expense (benefit) | 4.2 | (1.4) | 0.3 | ||||
Tax on foreign currency gains and losses | (0.2) | — | (0.6) | ||||
State tax expense | 1.2 | 1.0 | 0.3 | ||||
U.S. tax on foreign earnings | (1.8) | 0.9 | 1.4 | ||||
Changes in prior year estimates | — | (0.6) | (1.1) | ||||
Global intangible low-taxed income, net of foreign tax credits | 0.5 | 0.3 | 0.8 | ||||
Foreign Derived Intangible Income Benefit | (1.3) | (1.3) | (1.8) | ||||
Non-taxable earnings from acquisitions | (0.4) | (0.6) | (2.1) | ||||
Patent box benefit | — | (0.3) | (1.9) | ||||
Withholding tax on foreign earnings | 3.4 | — | — | ||||
Other | 0.4 | 0.7 | 0.8 | ||||
Effective income tax rate | 24.9 | % | 17.7 | % | 20.4 | % | |
The provision for income taxes consists of provisions for federal, state, and foreign income taxes. We operate in an international environment with significant operations in various locations outside the U.S. Accordingly, the consolidated income tax rate is a composite rate reflecting the earnings in the various locations and the applicable rates.
F-32
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
11. COMMITMENTS AND CONTINGENCIES
Acquisition-Related Contingent Obligations—Under the terms and conditions of the purchase agreements associated with certain acquisitions, we may be obligated to make additional payments based on the achievement of certain sales or profitability milestones through the acquired operations. For agreements that contain contingent consideration caps, the remaining maximum amount of such potential future payments is $55.3 million as of June 30, 2023.
We account for such contingent payments for acquisitions which occurred through the end of fiscal year 2009 as additions to the purchase price of the acquired business. We made contingent payments relating to such acquisitions of $1.0 million, $1.9 million and $3.4 million, respectively, during the fiscal years ended June 30, 2021, 2022 and 2023, respectively.
For acquisitions completed after fiscal 2009, pursuant to ASC 805, the estimated fair value of these obligations is recorded as a liability at the time of the acquisition with subsequent revisions recorded in Selling, general and administrative expense in the consolidated financial statements. The estimated fair value measurements of contingent earnout obligations are primarily based on unobservable inputs, which may include projected revenues, gross margins, operating income and the estimated probability of achieving the earnouts.
These projections and probabilities are used to estimate future contingent earnout payments, which are discounted back to present value to compute contingent earnout liabilities. The following table provides a roll-forward from June 30, 2022 to June 30, 2023 of the contingent consideration liability, which is included in other accrued expenses and current liabilities, and other long-term liabilities in our consolidated balance sheets (in thousands):
Beginning fair value, June 30, 2022 |
| $ | 28,212 |
Addition of contingent earnout obligations | 5,506 | ||
Foreign currency translation adjustment | 27 | ||
Changes in fair value for contingent earnout obligations |
| (11,901) | |
Payments on contingent earnout obligations |
| (663) | |
Ending fair value, June 30, 2023 | $ | 21,181 |
Advances from Customers—We receive advances from customers associated with certain contracts. These advances are paid in cash by customers, and we account for these as liabilities until our contractual obligations are complete.
Environmental Contingencies—We are subject to various environmental laws. We often conduct environmental investigations at our manufacturing facilities in North America, Asia-Pacific, and Europe, and, to the extent practicable, on all new properties in order to identify, as of the date of such investigation, potential areas of environmental concern related to past and present activities or from nearby operations.
We have not accrued for loss contingencies relating to environmental matters because we believe that, although unfavorable outcomes are possible, they are not considered by our management to be probable and reasonably estimable. If one or more of these environmental matters are resolved in a manner adverse to us, the impact on our business, financial condition, results of operations and cash flow could be material.
Indemnifications and Certain Employment-Related Contingencies—In the normal course of business, we have agreed to indemnify certain parties with respect to certain matters. We have agreed to hold certain parties harmless against losses arising from a breach of representations, warranties or covenants, or intellectual property infringement or other claims made by third parties. These agreements may limit the time within which an indemnification claim can be made and the amount of the claim. In addition, we have entered into indemnification agreements with our directors and certain of our officers. It is not possible to determine the maximum potential amount under these indemnification agreements due to the limited history of prior indemnification claims and the unique facts and circumstances involved in each particular agreement. We have not recorded any liability for costs related to contingent indemnification obligations as of June 30, 2023.
F-33
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
On December 31, 2017, we and Deepak Chopra, our Chief Executive Officer, entered into an amendment to Mr. Chopra’s employment agreement that, among other things, provides for a $13.5 million bonus payment to Mr. Chopra on or within 45 days of January 1, 2024 contingent upon Mr. Chopra’s continued employment with us through that date, subject to accelerated payout terms in the event of Mr. Chopra’s death or disability. The bonus is recorded in the financial statements over the remaining term of the employment agreement and is included in accrued payroll and related expenses at June 30, 2023 and in other long-term liabilities at June 30, 2022.
Legal Proceedings— In February 2023, we received a subpoena from the U.S. Department of Justice (“DoJ”) relating to a former employee of an OSI Systems subsidiary. The DoJ is currently prosecuting the former employee for embezzlement and other conduct occurring before he was hired by our subsidiary and while he was employed by another company in the United States and Mexico. The subpoena requests documents and records relating to, among other things, the former employee and the Company’s business dealings in Mexico since 2020. We are working in coordination with the DoJ to identify and produce the relevant documents and records.
We are involved in various other claims and legal proceedings arising in the ordinary course of business. In our opinion after consultation with legal counsel, the ultimate disposition of such proceedings is not likely to have a material adverse effect on our business, financial condition, results of operations or cash flows. We have not accrued for loss contingencies relating to any non-ordinary course matters because we believe that, although unfavorable outcomes in the proceedings are possible, they are not considered by management to be probable and reasonably estimable. If one or more of these matters are resolved in a manner adverse to our company, the impact on our business, financial condition, results of operations and cash flows could be material.
12. RELATED-PARTY TRANSACTIONS
In 1994, we, together with an unrelated company, formed ECIL-Rapiscan Security Products Limited, a joint venture organized under the laws of India. We own a 36% interest in the joint venture, our Chairman and Chief Executive Officer owns a 10.5% interest, and one of our Executive Vice Presidents owns a 4.5% ownership interest. Our initial investment in the joint venture was approximately $0.1 million. For each of the years ended June 30, 2021, 2022 and 2023 our equity earnings in the joint venture were less than $0.1 million. We, our Chairman and Chief Executive Officer and our Executive Vice President collectively control less than 50% of the board of directors voting power in the joint venture. As a result, we account for the investment under the equity method of accounting. The joint venture was formed for the purpose of the manufacture, assembly, service and testing of security and inspection systems and other products. Some of our subsidiaries are suppliers to the joint venture partner, which in turn manufactures and sells the resulting products. Sales to the joint venture partner for fiscal 2021, 2022 and 2023 were approximately $2.4 million, $2.3 million and $6.9 million, respectively. Receivables from the joint venture were $0.6 million and $1.9 million as of June 30, 2022 and 2023, respectively.
F-34
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
13. EMPLOYEE BENEFIT PLANS
Employee Retirement Savings Plans
We have various qualified employee retirement savings plans. Participants can contribute certain amounts to the plans and we match a certain portion of employee contributions. We contributed approximately $6.7 million, $6.9 million and $7.2 million to the plans for the fiscal years ended June 30, 2021, 2022 and 2023, respectively.
Deferred Compensation Plan
We have a deferred compensation plan, which meets the requirements for deferred compensation under Section 409A of the Internal Revenue Code. The plan provides that selected employees are eligible to defer up to 80% of their salaries and up to 100% of their bonuses. We may also make employer contributions to participant accounts in certain circumstances. The benefits under this plan are unsecured. Participants are generally eligible to receive payment of their vested benefit at the end of their elected deferral period or after termination of their employment for any reason or at a later date to comply with the restrictions of Section 409A. Discretionary company contributions and the related earnings are subject to a vesting schedule dependent upon years of service to us and, also, vest completely upon the participant’s disability or death while employed by us or immediately prior to a change of control. We made contributions of $0.5 million, $0.5 million and $0.6 million for fiscal year 2021, 2022 and 2023, respectively. As of June 30, 2023, we held assets of $36.8 million and liabilities of $33.6 million related to this plan. Assets related to this plan are included in other assets and liabilities related to this plan are included in other long-term liabilities in the consolidated balance sheets. The plan liabilities include accrued employer contributions not yet funded to the plan.
Employee Pension Plans
We sponsor a number of qualified and nonqualified pension plans for our employees at certain locations. In accordance with accounting standards for employee pension and postretirement benefits, we fully recognize the overfunded or underfunded status of each of our defined benefit plans as an asset or liability in the consolidated balance sheets. The asset or liability equals the difference between the fair value of the plans’ assets and their benefit obligations. The liabilities associated with underfunded plans are classified as noncurrent, except to the extent the fair value of the plans’ assets is less than the plans’ estimated benefit payments over the next . We measure our pension and postretirement benefit plans’ assets and benefit obligations as of June 30.
F-35
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
The following provides a reconciliation of the changes in the plans’ benefit obligations and fair value of assets for fiscal years 2022 and 2023, and a statement of the funded status as of June 30, 2022 and 2023 (in thousands):
| 2022 |
| 2023 | |||
Change in Benefit Obligation | ||||||
Benefit obligation at beginning of year | $ | 18,434 | $ | 18,464 | ||
Translation adjustment |
| (708) |
| 149 | ||
Interest costs |
| 464 |
| 624 | ||
Amendment | 1,345 | — | ||||
Actuarial gain | (900) | (847) | ||||
Benefits paid |
| (171) |
| (162) | ||
Benefit obligation at end of year |
| 18,464 |
| 18,228 | ||
Change in Plan Assets | ||||||
Fair value of plan assets at beginning of year |
| 7,010 |
| 5,977 | ||
Translation adjustment |
| (860) |
| 243 | ||
Actual return on plan assets |
| (47) |
| 413 | ||
Benefits paid |
| (126) |
| (120) | ||
Fair value of plan assets at end of year |
| 5,977 |
| 6,513 | ||
Funded status and net amount recognized | $ | (12,487) | $ | (11,715) | ||
Amount recognized in consolidated balance sheets consists of: | ||||||
Net benefit asset (included in other current assets) | $ | 2,275 | $ | 3,264 | ||
Current portion of net pension liability (included in other current liabilities) | (180) | (6,189) | ||||
Net long term pension liability (included in other long-term liabilities) |
| (14,582) |
| (8,790) | ||
Accumulated other comprehensive income |
| 4,609 |
| 2,365 | ||
One of our defined benefit pension plans is considered a nonqualified plan, therefore we have funded a separate rabbi trust which comprises insurance company contracts with fair values of $11.9 million and $13.6 million as of June 30, 2022 and 2023, respectively. These amounts are not included in the fair value of plan assets in the table above.
The following table provides the net periodic benefit costs for the fiscal years ended June 30, (in thousands):
| 2021 |
| 2022 |
| 2023 | ||||
Net Periodic Benefit Costs | |||||||||
$ | 477 | $ | 464 | $ | 624 | ||||
Service costs | — | — | — | ||||||
| (242) |
| (279) |
| (340) | ||||
| 668 |
| 1,115 |
| 1,330 | ||||
| 75 |
| 41 |
| 83 | ||||
$ | 978 | $ | 1,341 | $ | 1,697 | ||||
Plan Assumptions
| 2022 |
| 2023 |
| |
Weighted average assumptions at year-end: | |||||
Discount rate |
| 3.0 | % | 5.3 | % |
Expected return on plan assets |
| 4.2 | % | 5.8 | % |
Rate of compensation increase |
| — | % | — | % |
The long-term return on assets has been derived from the weighted average of assumed returns on each of the major asset categories. The weighted average is based on the actual proportion of each major asset class held, rather than a benchmark portfolio of assets. The expected returns for each major asset class have been derived from a combination of both historical market returns and current market data as well as the views of a range of investment managers. There is no assumed rate of compensation increase as most of the plan participants are retirees or no longer employed by OSI.
F-36
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Plan Assets and Investment Policy
Fiscal year ended | Fiscal year ended |
| |||||||
June 30, 2022 | June 30, 2023 |
| |||||||
Proportion of | Expected Rate | Proportion of | Expected Rate |
| |||||
| Fair Value |
| of Return |
| Fair Value |
| of Return |
| |
Equity securities | 85 | % | 4.9 | % | 86 | % | 6.6 | % | |
Debt securities |
| 14 | % | 0.8 | % | 13 | % | 0.8 | % |
Cash |
| 1 | % | 0.4 | % | 1 | % | 0.4 | % |
Combined |
| 100 | % | 4.2 | % | 100 | % | 5.8 | % |
The defined benefit plans’ assets are invested in a range of pooled investment funds that provide access to a diverse range of asset classes. The investment objective is to maximize the investment return over the long term without exposing the fund to an unnecessary level of risk. Within this objective, it is recognized that benefits will be secured by the purchase of annuities at the time of employee retirement.
The benchmark is to hold assets in both equity and debt securities. The proportion in each investment class is not mandated and is allowed to fluctuate with market movements. The equity holdings are maintained in balanced funds under the control of investment managers.
Day-to-day equities selection decisions are delegated to investment managers, although these are monitored against performance and risk targets. Due to the nature of the pooled funds, there are no significant holdings in any single company (greater than 5% of the total assets). The investment strategy is reviewed on a regular basis, based on the results of third-party liability studies.
Projected Benefit Payments
The following table reflects estimated benefits payments, based upon the same assumptions used to measure the benefit obligation and net pension cost, as of June 30, 2023 (in thousands):
| Pension Benefits | ||
July 1, 2023 to June 30, 2024 | $ | 6,189 | |
July 1, 2024 to June 30, 2025 | 1,525 | ||
July 1, 2025 to June 30, 2026 | 2,284 | ||
July 1, 2026 to June 30, 2027 | 2,301 | ||
July 1, 2027 to June 30, 2028 | 2,317 | ||
July 1, 2028 to June 30, 2033 | 3,537 | ||
Company Contribution
There were no company contributions for the fiscal year ended June 30, 2023.
F-37
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
14. SEGMENT INFORMATION
We have determined that we operate in three identifiable industry segments: (a) security and inspection systems (Security division), (b) medical monitoring systems (Healthcare division) and (c) optoelectronic devices and manufacturing (Optoelectronics and Manufacturing division). We also have a corporate segment (Corporate) that includes executive compensation and certain other general and administrative expenses; expenses related to stock issuances and legal, audit and other professional service fees not allocated to industry segments. Both the Security and Healthcare divisions comprise primarily end-product businesses whereas the Optoelectronics and Manufacturing division primarily supplies components and subsystems to external OEM customers, as well as to the Security and Healthcare divisions. Sales between divisions are at transfer prices that approximate market values. All other accounting policies of the segments are the same as described in Note 1, Summary of Significant Accounting Policies.
The following tables present our results of operations and identifiable assets by industry segment (in thousands):
Fiscal 2021 | ||||||||||||||||||
Optoelectronics | ||||||||||||||||||
and | ||||||||||||||||||
Security | Healthcare | Manufacturing | ||||||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Eliminations |
| Consolidated | |||||||
Revenues: |
|
|
|
|
|
| ||||||||||||
External customer revenue | $ | 633,340 | $ | 212,315 | $ | 301,247 | $ | — | $ | — | $ | 1,146,902 | ||||||
Revenue between product segments |
| — |
| — |
| 48,640 |
| — |
| (48,640) |
| — | ||||||
Total revenues | $ | 633,340 | $ | 212,315 | $ | 349,887 | $ | — |
| (48,640) | $ | 1,146,902 | ||||||
Income (loss) from operations | $ | 85,515 | $ | 31,563 | $ | 38,465 | $ | (39,769) | $ | (403) | $ | 115,371 | ||||||
Segments assets | $ | 798,192 | $ | 220,411 | $ | 282,039 | $ | 121,293 | $ | (37,568) | $ | 1,384,367 | ||||||
Capital expenditures | $ | 3,290 | $ | 2,144 | $ | 6,714 | $ | 3,612 | $ | — | $ | 15,760 | ||||||
Depreciation and amortization | $ | 26,572 | $ | 5,364 | $ | 9,325 | $ | 2,594 | $ | — | $ | 43,855 | ||||||
Fiscal 2022 | ||||||||||||||||||
Optoelectronics | ||||||||||||||||||
and | ||||||||||||||||||
Security | Healthcare | Manufacturing | ||||||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Eliminations |
| Consolidated | |||||||
Revenues: |
|
|
|
|
|
| ||||||||||||
External customer revenue | $ | 663,159 | $ | 205,658 | $ | 314,419 | $ | — | $ | — | $ | 1,183,236 | ||||||
Revenue between product segments |
| — |
| — |
| 52,242 |
| — |
| (52,242) |
| — | ||||||
Total revenues | $ | 663,159 | $ | 205,658 | $ | 366,661 | $ | — |
| (52,242) | $ | 1,183,236 | ||||||
Income (loss) from operations | $ | 98,784 | $ | 24,696 | $ | 45,030 | $ | (46,950) | $ | 189 | $ | 121,749 | ||||||
Segments assets | $ | 839,769 | $ | 231,423 | $ | 301,483 | $ | 104,834 | $ | (34,359) | $ | 1,443,150 | ||||||
Capital expenditures | $ | 5,513 | $ | 2,295 | $ | 4,533 | $ | 2,580 | $ | — | $ | 14,921 | ||||||
Depreciation and amortization | $ | 22,970 | $ | 5,915 | $ | 8,098 | $ | 1,696 | $ | — | $ | 38,679 | ||||||
F-38
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Fiscal 2023 | ||||||||||||||||||
Optoelectronics | ||||||||||||||||||
and | ||||||||||||||||||
Security | Healthcare | Manufacturing | ||||||||||||||||
| Division |
| Division |
| Division |
| Corporate |
| Eliminations |
| Consolidated | |||||||
Revenues: |
|
|
|
|
|
| ||||||||||||
External customer revenue | $ | 760,291 | $ | 190,488 | $ | 327,648 | $ | — | $ | — | $ | 1,278,427 | ||||||
Revenue between product segments |
| — |
| — |
| 59,783 |
| — |
| (59,783) |
| — | ||||||
Total revenues | $ | 760,291 | $ | 190,488 | $ | 387,431 | $ | — | $ | (59,783) | $ | 1,278,427 | ||||||
Income (loss) from operations | $ | 115,023 | $ | 11,365 | $ | 46,680 | $ | (39,075) | $ | 1,286 | $ | 135,279 | ||||||
Segments assets | $ | 948,126 | $ | 245,856 | $ | 310,930 | $ | 94,678 | $ | (43,904) | $ | 1,555,686 | ||||||
Capital expenditures | $ | 3,689 | $ | 2,726 | $ | 7,390 | $ | 1,968 | $ | 38 | $ | 15,811 | ||||||
Depreciation and amortization | $ | 23,504 | $ | 5,757 | $ | 7,582 | $ | 1,670 | $ | — | $ | 38,513 | ||||||
The following tables present the revenues and identifiable assets by geographical area (in thousands):
Fiscal 2021 | |||||||||||||||
External | Intersegment | Total | Long-lived | Long-lived | |||||||||||
| revenues |
| revenues |
| Consolidated |
| tangible assets |
| assets | ||||||
Geographic region: |
|
|
|
|
| ||||||||||
United States | $ | 589,579 | $ | 17,498 | $ | 607,077 | $ | 126,100 | $ | 493,423 | |||||
Mexico |
| 10,583 |
| — |
| 10,583 |
| 2,379 |
| 2,379 | |||||
Other Americas |
| 66,732 |
| — |
| 66,732 |
| 8,055 |
| 29,960 | |||||
Total Americas |
| 666,894 |
| 17,498 |
| 684,392 |
| 136,534 | 525,762 | ||||||
United Kingdom |
| 221,423 |
| 874 |
| 222,297 |
| 25,183 | 80,348 | ||||||
Other Europe, Middle East and Africa |
| 29,879 |
| — |
| 29,879 |
| 8,389 | 8,389 | ||||||
Total EMEA |
| 251,302 |
| 874 |
| 252,176 |
| 33,572 | 88,737 | ||||||
Asia-Pacific |
| 228,706 |
| 30,268 |
| 258,974 |
| 29,346 | 32,865 | ||||||
Eliminations |
| — |
| (48,640) |
| (48,640) |
| — |
| — | |||||
Total | $ | 1,146,902 | $ | — | $ | 1,146,902 | $ | 199,452 | $ | 647,364 | |||||
Fiscal 2022 | |||||||||||||||
External | Intersegment | Total | Long-lived | Long-lived | |||||||||||
| revenues |
| revenues |
| Consolidated |
| tangible assets |
| assets | ||||||
Geographic region: |
|
|
|
|
| ||||||||||
United States | $ | 569,601 | $ | 16,322 | $ | 585,923 | $ | 117,622 | $ | 514,489 | |||||
Mexico |
| 8,109 |
| — |
| 8,109 |
| 261 |
| 261 | |||||
Other Americas |
| 47,737 |
| — |
| 47,737 |
| 8,091 |
| 27,676 | |||||
Total Americas |
| 625,447 |
| 16,322 |
| 641,769 |
| 125,974 | 542,426 | ||||||
United Kingdom |
| 276,658 |
| 2,887 |
| 279,545 |
| 27,749 | 80,758 | ||||||
Other Europe, Middle East and Africa |
| 52,952 |
| — |
| 52,952 |
| 4,837 | 6,776 | ||||||
Total EMEA |
| 329,610 |
| 2,887 |
| 332,497 |
| 32,586 | 87,534 | ||||||
Asia-Pacific |
| 228,179 |
| 33,002 |
| 261,181 |
| 20,589 | 23,916 | ||||||
Eliminations |
| — |
| (52,211) |
| (52,211) |
| — |
| — | |||||
Total | $ | 1,183,236 | $ | — | $ | 1,183,236 | $ | 179,149 | $ | 653,876 | |||||
F-39
OSI SYSTEMS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
FOR THE THREE YEARS ENDED JUNE 30, 2023
Fiscal 2023 | |||||||||||||||
External | Intersegment | Total | Long-lived | Long-lived | |||||||||||
| revenues |
| revenues |
| Consolidated |
| tangible assets |
| Assets | ||||||
Geographic region: |
|
|
|
|
| ||||||||||
United States | $ | 653,127 | $ | 17,461 | $ | 670,588 | $ | 126,388 | $ | 534,417 | |||||
Mexico |
| 23,467 |
| — |
| 23,467 |
| 609 |
| 609 | |||||
Other Americas |
| 63,416 |
| — |
| 63,416 |
| 8,050 |
| 25,844 | |||||
Total Americas |
| 740,010 |
| 17,461 |
| 757,471 |
| 135,047 | 560,870 | ||||||
United Kingdom |
| 280,268 |
| 5,835 |
| 286,103 |
| 27,952 | 87,289 | ||||||
Other Europe, Middle East and Africa |
| 44,498 |
| — |
| 44,498 |
| 4,233 | 6,198 | ||||||
Total EMEA |
| 324,766 |
| 5,835 |
| 330,601 |
| 32,185 | 93,487 | ||||||
Asia-Pacific |
| 213,651 |
| 36,487 |
| 250,138 |
| 21,478 | 24,715 | ||||||
Eliminations |
| — |
| (59,783) |
| (59,783) |
| — |
| — | |||||
Total | $ | 1,278,427 | $ | — | $ | 1,278,427 | $ | 188,710 | $ | 679,072 | |||||
Pursuant to ASC 280 Segment Reporting, external revenues are attributed to individual countries based upon the location of our selling entity.
* * * * * *
F-40
SUPPLEMENTARY DATA
UNAUDITED QUARTERLY RESULTS
The following tables present unaudited quarterly financial information for the four quarters in the fiscal years ended June 30, 2022 and 2023 (in thousands, except per share data):
Quarter Ended | ||||||||||||
September 30, | December 31, | March 31, | June 30, | |||||||||
| 2021 |
| 2021 |
| 2022 |
| 2022 | |||||
(Unaudited) | ||||||||||||
Net revenues | $ | 279,257 | $ | 276,681 | $ | 290,477 | $ | 336,821 | ||||
Costs of goods sold |
| 179,927 |
| 176,908 |
| 187,619 |
| 214,355 | ||||
Gross profit |
| 99,330 |
| 99,773 |
| 102,858 |
| 122,466 | ||||
Operating expenses: |
|
|
|
| ||||||||
Selling, general and administrative |
| 57,323 |
| 54,879 |
| 57,813 |
| 65,538 | ||||
Research and development |
| 14,817 |
| 14,977 |
| 15,150 |
| 14,639 | ||||
Impairment, restructuring and other charges, net |
| 2,510 |
| 831 |
| 1,469 |
| 2,732 | ||||
Total operating expenses |
| 74,650 |
| 70,687 |
| 74,432 |
| 82,909 | ||||
Income from operations |
| 24,680 |
| 29,086 |
| 28,426 |
| 39,557 | ||||
Interest and other expense, net |
| (2,016) |
| (2,217) |
| (2,301) |
| (2,428) | ||||
Other income | — | — | 27,373 | — | ||||||||
Income before income taxes |
| 22,664 |
| 26,869 |
| 53,498 |
| 37,129 | ||||
Provision for income taxes |
| (3,612) |
| (7,072) |
| (10,763) |
| (3,366) | ||||
Net income | $ | 19,052 | $ | 19,797 | $ | 42,735 | $ | 33,763 | ||||
Basic earnings per common share | $ | 1.06 | $ | 1.11 | $ | 2.45 | $ | 1.99 | ||||
Diluted earnings per common share | $ | 1.04 | $ | 1.09 | $ | 2.41 | $ | 1.94 | ||||
Quarter Ended | ||||||||||||
September 30, | December 31, | March 31, | June 30, | |||||||||
| 2022 |
| 2022 |
| 2023 |
| 2023 | |||||
(Unaudited) | ||||||||||||
Net revenues | $ | 268,071 | $ | 295,597 | $ | 302,889 | $ | 411,870 | ||||
Costs of goods sold |
| 180,574 |
| 199,390 |
| 199,103 |
| 268,850 | ||||
Gross profit |
| 87,497 |
| 96,207 |
| 103,786 |
| 143,020 | ||||
Operating expenses: |
|
|
|
| ||||||||
Selling, general and administrative |
| 53,438 |
| 54,003 |
| 53,707 |
| 67,165 | ||||
Research and development |
| 14,540 |
| 14,456 |
| 14,852 |
| 15,504 | ||||
Impairment, restructuring and other charges, net |
| 1,219 |
| 2,257 |
| 890 |
| 3,200 | ||||
Total operating expenses |
| 69,197 |
| 70,716 |
| 69,449 |
| 85,869 | ||||
Income from operations |
| 18,300 |
| 25,491 |
| 34,337 |
| 57,151 | ||||
Interest and other expense, net |
| (3,432) |
| (5,180) |
| (5,727) | (5,702) | |||||
Other income | — | — | — | — | ||||||||
Income before income taxes |
| 14,868 |
| 20,311 |
| 28,610 |
| 51,449 | ||||
Provision for income taxes |
| (3,633) |
| (3,957) |
| (6,802) |
| (9,068) | ||||
Net income | $ | 11,235 | $ | 16,354 | $ | 21,808 | $ | 42,381 | ||||
Basic earnings per common share | $ | 0.66 | $ | 0.97 | $ | 1.30 | $ | 2.53 | ||||
Diluted earnings per common share | $ | 0.65 | $ | 0.96 | $ | 1.27 | $ | 2.46 | ||||
F-41
INDEX TO EXHIBITS
No. |
| EXHIBIT DESCRIPTION |
|---|---|---|
(vi) the notes to the consolidated financial statements, tagged in summary and detail | ||
104 | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101) |
*Filed herewith
† | Denotes a management contract or compensatory plan or arrangement. |
| (1) | Previously filed with our Current Report on Form 8-K filed on March 8, 2010. |
| (2) | Previously filed with our Quarterly Report on Form 10-Q filed on May 2, 2014. |
| (3) | Previously filed with our Current Report on Form 8-K filed on October 10, 2008. |
| (4) | Previously filed with our Quarterly Report on Form 10-Q filed on October 24, 2014. |
| (5) | Previously filed with our Annual Report on Form 10-K filed on August 27, 2010. |
| (6) | Previously filed with our Current Report on Form 8-K filed on April 6, 2012. |
| (7) | Previously filed with our Current Report on Form 8-K filed on January 5, 2018. |
| (8) | Previously filed with our Proxy Statement on Schedule 14A filed on October 21, 2020. |
| (9) | Previously filed with our Registration Statement on Form S-8 filed on August 16, 2013. |
| (10) | Previously filed with our Current Report on Form 8-K filed on May 23, 2016. |
| (11) | Previously filed with our Quarterly Report on Form 10-Q filed on January 28, 2016. |
| (12) | Previously filed with our Quarterly Report on Form 10-Q filed on October 30, 2015. |
| (13) | Previously filed with our Current Report on Form 8-K filed on February 22, 2017. |
| (14) | Previously filed with our Proxy Statement on Schedule 14A filed on October 21, 2016. |
| (15) | Previously filed with our Quarterly Report on Form 10-Q filed on May 2, 2019. |
| (16) | Previously filed with our Annual Report on Form 10-K filed on August 21, 2020. |
| (17) | Previously filed with our Quarterly Report on Form 10-Q filed on October 29, 2021. |
| (18) | Previously filed with our Annual Report on Form 10-K filed on August 19, 2022. |
| (19) | Previously filed with our Quarterly Report on Form 10-Q filed on January 27, 2023. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
OSI SYSTEMS, INC. | |||
| |||
Date: August 29, 2023 | By: | /s/ ALAN EDRICK Alan Edrick, | |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below does hereby constitute and appoint Deepak Chopra, Alan Edrick and Victor Sze, and each of them singly, our true and lawful attorneys with full power to them, and each of them singly, to sign for us and in our names in the capacities indicated below, the Form 10-K filed herewith and any and all amendments to said Form 10-K, and generally to do all such things in our names and in our capacities as officers and directors to enable OSI Systems, Inc. to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all requirements of the Securities and Exchange Commission in connection therewith, hereby ratifying and confirming our signatures as they may be signed by our said attorneys, or any of them, to said Form 10-K and any and all amendments thereto.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Name |
| Title |
| Date |
/s/ DEEPAK CHOPRA Deepak Chopra | Chairman of the Board, | August 29, 2023 | ||
/s/ ALAN EDRICK Alan Edrick | Executive Vice President and Chief | August 29, 2023 | ||
/s/ WILLIAM F. BALLHAUS, JR. William F. Ballhaus, Jr. | Director | August 29, 2023 | ||
/s/ GERALD CHIZEVER Gerald Chizever | Director | August 29, 2023 | ||
/s/ JAMES B. HAWKINS James B. Hawkins | Director | August 29, 2023 | ||
/s/ MEYER LUSKIN Meyer Luskin | Director | August 29, 2023 | ||
/s/ KELLI BERNARD Kelli Bernard | Director | August 29, 2023 |
II-2
Similar companies
See also TAIWAN SEMICONDUCTOR MANUFACTURING CO LTDSee also NVIDIA CORP - Annual report 2023 (10-K 2023-01-29) Annual report 2023 (10-Q 2023-07-30)
See also Broadcom Inc. - Annual report 2022 (10-K 2022-10-30) Annual report 2023 (10-Q 2023-07-30)
See also TEXAS INSTRUMENTS INC - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-03-31)
See also Ascent Solar Technologies, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
