PFIZER INC - Annual Report: 2019 (Form 10-K)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION |
Washington, D.C. 20549 |
FORM 10-K
(Mark One) | |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 1-3619

PFIZER INC.
(Exact name of registrant as specified in its charter)
Delaware | 13-5315170 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
235 East 42nd Street, New York, New York 10017
(Address of principal executive offices) (zip code)
(212) 733-2323
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: | ||
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, $.05 par value | PFE | New York Stock Exchange |
0.000% Notes due 2020 | PFE20A | New York Stock Exchange |
0.250% Notes due 2022 | PFE22 | New York Stock Exchange |
1.000% Notes due 2027 | PFE27 | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files.) Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large Accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☐ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting stock held by non-affiliates of the registrant, computed by reference to the closing price as of the last business day of the registrant’s most recently completed second fiscal quarter, June 30, 2019, was approximately $241 billion. This excludes shares of common stock held by directors and executive officers at June 30, 2019. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, directly or indirectly, to direct or cause the direction of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant. The registrant has no non-voting common stock.
The number of shares outstanding of the registrant’s common stock as of February 25, 2020 was 5,547,639,005 shares of common stock, all of one class.
DOCUMENTS INCORPORATED BY REFERENCE | |
Portions of the 2019 Annual Report to Shareholders | Parts I, II and IV |
Portions of the Proxy Statement for the 2020 Annual Meeting of Shareholders | Part III |
TABLE OF CONTENTS |
Page | |
Pfizer Inc. | 2019 Form 10-K | i |
DEFINED TERMS |
Unless the context requires otherwise, references to “Pfizer,” “the Company,” “we,” “us” or “our” in this 2019 Form 10-K (defined below) refer to Pfizer Inc. and its subsidiaries. We also have used several other terms in this 2019 Form 10-K, most of which are explained or defined below.
2019 Financial Report | Exhibit 13 to this 2019 Form 10-K |
2019 Form 10-K | This Annual Report on Form 10-K for the fiscal year ended December 31, 2019 |
2020 Proxy Statement | Proxy Statement for the 2020 Annual Meeting of Shareholders |
ACA | U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act |
Alliance revenues | Revenues from alliance agreements under which we co-promote products discovered or developed by other companies or us |
Akcea | Akcea Therapeutics, Inc. |
Array | Array BioPharma Inc. |
Astellas | Astellas Pharma Inc., Astellas US LLC and Astellas Pharma US, Inc. |
Biopharma | Pfizer Biopharmaceuticals Group |
BMS | Bristol-Myers Squibb Company |
cGMPs | current Good Manufacturing Practices |
DEA | U.S. Drug Enforcement Agency |
Developed Markets | U.S., Western Europe, Japan, Canada, South Korea, Australia, Scandinavian countries, Finland and New Zealand |
EMA | European Medicines Agency |
Emerging Markets | Includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey |
EU | European Union |
Exchange Act | Securities Exchange Act of 1934, as amended |
FCPA | U.S. Foreign Corrupt Practices Act |
FDA | U.S. Food and Drug Administration |
FFDCA | U.S. Federal Food, Drug and Cosmetic Act |
GPD | Global Product Development organization |
GSK | GlaxoSmithKline plc |
Hospira | Hospira, Inc. |
Ionis | Ionis Pharmaceuticals, Inc. |
IPR&D | In-process Research and Development |
LIBOR | London Interbank Offered Rate |
LOE | Loss of Exclusivity |
MCO | Managed Care Organization |
Mylan | Mylan N.V. |
NMPA | National Medical Product Administration in China |
NYSE | New York Stock Exchange |
OTC | over-the-counter |
PBM | Pharmacy Benefit Manager |
PGS | Pfizer Global Supply |
Pfizer Inc. | 2019 Form 10-K | ii |
PMDA | Pharmaceuticals and Medical Device Agency in Japan |
QCE | quality consistency evaluation in China |
R&D | research and development |
SEC | U.S. Securities and Exchange Commission |
Teva | Teva Pharmaceuticals USA, Inc. |
U.K. | United Kingdom |
U.S. | United States |
VAI | Voluntary Action Indicated |
VBP | volume-based procurement in China |
WRDM | Worldwide Research, Development and Medical |
Pfizer Inc. | 2019 Form 10-K | iii |

 | |
 | |
 | Pfizer Biopharmaceuticals Group (Biopharma) (~$39.4 Billion 2019 Revenues) / Upjohn (~$10.2 Billion 2019 Revenues) / Consumer Healthcare |
 | Internal Medicine, Oncology, Hospital, Vaccines, Inflammation & Immunology and Rare Disease |
 | 20 Globally Recognized Brands and the Greenstone generics platform in Upjohn |
 | >125 Countries Where We Sell Our Products |
 | |
 | ~$8.7 Billion 2019 R&D Expense |
 | 42 Manufacturing Sites Worldwide Operated by PGS; 7 Manufacturing Sites Worldwide Operated by Upjohn |
 | |
Unless indicated otherwise, the information contained in this summary is as of December 31, 2019. This summary does not include information that will be incorporated by reference into Part III of this 2019 Form 10-K from our 2020 Proxy Statement.
* On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. On July 31, 2019, Pfizer’s Consumer Healthcare business, an over-the-counter medicines business, was combined with GSK’s consumer healthcare business to form a new consumer healthcare joint venture. For additional information, see the Item 1. Business—About Pfizer section in this 2019 Form 10-K.
** As of January 28, 2020
Pfizer Inc. | 2019 Form 10-K | iv |
PART I |
ITEM 1. | BUSINESS |
 ABOUT PFIZER
ABOUT PFIZER Pfizer Inc. is a research-based, global biopharmaceutical company. We apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development, manufacture and distribution of healthcare products, including innovative medicines and vaccines. We work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. Our revenues are derived from the sale of our products and, to a much lesser extent, from alliance agreements, under which we co-promote products discovered or developed by other companies or us. The majority of our revenues come from the manufacture and sale of biopharmaceutical products. The Company was incorporated under the laws of the State of Delaware on June 2, 1942.
We believe that our medicines provide significant value for both healthcare providers and patients, not only from the improved treatment of diseases but also from a reduction in other healthcare costs, such as emergency room or hospitalization costs, as well as improvements in health, wellness and productivity. We continue to actively engage in dialogues about the value of our medicines and how we can best work with patients, physicians and payers to prevent and treat disease and improve outcomes. We continue to work within the current legal and pricing structures, as well as continue to review our pricing arrangements and contracting methods with payers, to maximize patient access and minimize any adverse impact on our revenues. We remain firmly committed to fulfilling our Company’s purpose: Breakthroughs that change patients’ lives. By doing so, we expect to create value for the patients we serve and for our colleagues and shareholders.
With the formation of the GSK Consumer Healthcare joint venture and the pending combination of Upjohn with Mylan, which are further discussed below, Pfizer is transforming itself into a more focused, global leader in science-based innovative medicines.
We are committed to capitalizing on growth opportunities by advancing our own pipeline and maximizing the value of our in-line products, as well as through various forms of business development, which can include alliances, licenses, joint ventures, collaborations, equity- or debt-based investments, dispositions, mergers and acquisitions. We view our business development activity as an enabler of our strategies, and we seek to generate earnings growth and enhance shareholder value by pursuing a disciplined, strategic and financial approach to evaluating business development opportunities.
Our significant recent business development activities include:
• | License Agreement with Akcea Therapeutics, Inc.––In October 2019, we entered into a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases, with Akcea, a majority-owned affiliate of Ionis. The transaction closed in November 2019 and we made an upfront payment of $250 million to Akcea and Ionis. |
• | Formation of a New Consumer Healthcare Joint Venture—On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. The joint venture is a category leader in pain relief, respiratory and vitamins, minerals and supplements, and therapeutic oral health and is the largest global OTC consumer healthcare business. In exchange for contributing our Consumer Healthcare business to the joint venture, we received a 32% equity stake in the new company and GSK owns the remaining 68%. |
• | Acquisition of Array BioPharma Inc.—On July 30, 2019, we acquired Array, a commercial stage biopharmaceutical company focused on the discovery, development and commercialization of targeted small molecule medicines to treat cancer and other diseases of high unmet need, for $48 per share in cash. The total fair value of the consideration transferred for Array was approximately $11.2 billion ($10.9 billion, net of cash acquired). |
• | Agreement to Combine Upjohn with Mylan N.V.—On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. Under the terms of the agreement, which is structured as an all-stock, Reverse Morris Trust transaction, Upjohn is expected to be spun off or split off to Pfizer’s shareholders and, immediately thereafter, combined with Mylan. Pfizer shareholders would own 57% of the combined new company, and former Mylan shareholders would own 43%. The transaction is expected to be tax free to Pfizer and Pfizer shareholders. The transaction is anticipated to close in mid-2020, subject to Mylan shareholder approval and satisfaction of other customary closing conditions, including receipt of regulatory approvals. |
Pfizer Inc. | 2019 Form 10-K | 1 |
• | Acquisition of Therachon Holding AG—On July 1, 2019, we acquired all the remaining shares of Therachon Holding AG, a privately-held clinical-stage biotechnology company focused on rare diseases, with assets in development for the treatment of achondroplasia, a genetic condition and the most common form of short-limb dwarfism, for $340 million upfront, plus potential milestone payments of up to $470 million, contingent on the achievement of key milestones in the development and commercialization of the lead asset. |
For a further discussion of our strategy and our business development initiatives, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Business Development Initiatives and —Our Strategy sections and the Notes to Consolidated Financial Statements—Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements in our 2019 Financial Report.
Our businesses are heavily regulated in most of the countries in which we operate. In the U.S., the principal authority regulating our operations is the FDA. The FDA regulates the safety and efficacy of the products we offer and our research, quality, manufacturing processes, product promotion, advertising and product labeling. Similar regulations exist in most other countries, and in many countries the government also regulates our prices. In the EU, the EMA conducts the scientific evaluation, supervision and safety monitoring of our products, and employs a centralized procedure for approval of medicines for the EU and the European Economic Area countries. In China, the NMPA is the primary regulatory authority for approving and supervising medicines. In Japan, the PMDA is involved in a wide range of regulatory activities, including clinical studies, approvals, post-marketing reviews and pharmaceutical safety. Health authorities in many middle and lower income countries require marketing approval by a recognized regulatory authority (i.e., similar to the authority of the FDA or EMA) before they begin to conduct their application review process and/or issue their final approval. For additional information, see the Item 1. Business—Government Regulation and Price Constraints section in this 2019 Form 10-K.
Some amounts in this 2019 Form 10-K may not add due to rounding. All percentages have been calculated using unrounded amounts. All trademarks in this 2019 Form 10-K are the property of their respective owners.
Our website is located at www.pfizer.com. This 2019 Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, are, or will be, available (free of charge) on our website, in text format and, where applicable, in interactive data file format, as soon as reasonably practicable after we electronically file this material with, or furnish it to, the SEC.
Throughout this 2019 Form 10-K, we “incorporate by reference” certain information from other documents filed or to be filed with the SEC, including our 2020 Proxy Statement and our 2019 Financial Report, portions of which are filed as Exhibit 13 to this 2019 Form 10-K, and which also will be contained in Appendix A to our 2020 Proxy Statement. The SEC allows us to disclose important information by referring to it in that manner. Please refer to this information. Our 2019 Annual Report to Shareholders consists of our 2019 Financial Report and the Corporate and Shareholder Information attached to the 2020 Proxy Statement. Our 2019 Financial Report will be available on our website on or about February 27, 2020. Our 2020 Proxy Statement will be available on our website on or about March 13, 2020.
We may use our website as a means of disclosing material information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. These disclosures are included on our website in the “Investors” or “News” sections. Accordingly, investors should monitor these portions of our website, in addition to following Pfizer’s press releases, SEC filings, public conference calls and webcasts, as well as Pfizer’s social media channels (Pfizer’s Facebook, YouTube and LinkedIn pages and Twitter accounts (@Pfizer and @Pfizer_News)).
Information relating to corporate governance at Pfizer, including our Corporate Governance Principles; Director Qualification Standards; Pfizer Policies on Business Conduct (for all of our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer); Code of Business Conduct and Ethics for Members of the Board of Directors; information concerning our Directors; ways to communicate by e-mail with our Directors; Board Committees; Committee Charters; Charter of the Lead Independent Director; and transactions in Pfizer securities by Directors and Officers are available on our website. We will provide any of the foregoing information without charge upon written request to our Corporate Secretary, Pfizer Inc., 235 East 42nd Street, New York, NY 10017. We will disclose any future amendments to, or waivers from, provisions of the Pfizer Policies on Business Conduct affecting our Chief Executive Officer, Chief Financial Officer and Controller on our website as promptly as practicable, as may be required under applicable SEC and NYSE rules. Information relating to shareholder services, including the Computershare Investment Program, book-entry share ownership and direct deposit of dividends, is also available on our website.
The information contained on our website, our Facebook, YouTube and LinkedIn pages or our Twitter accounts is not incorporated by reference into this 2019 Form 10-K. Pfizer’s references to the URLs for websites are intended to be inactive textual references only.
Pfizer Inc. | 2019 Form 10-K | 2 |
At the beginning of our 2019 fiscal year, we began to manage our commercial operations through a new global structure consisting of three businesses—Pfizer Biopharmaceuticals Group (Biopharma), Upjohn and, through July 31, 2019, Consumer Healthcare, each led by a single manager. We have revised prior-period segment information in our 2019 Form 10-K to reflect the 2019 reorganization. Biopharma and Upjohn are the only reportable segments.
For additional information regarding the 2019 reorganization, as well as our Organizing for Growth initiative, see the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Strategy––Organizing for Growth section and the Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information in our 2019 Financial Report.
On July 31, 2019, Pfizer’s Consumer Healthcare business, an over-the-counter medicines business, was combined with GSK’s consumer healthcare business to form a new consumer healthcare joint venture in which we own a 32% equity stake. For additional information, see the Notes to Consolidated Financial Statements––Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation and Note 2C. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Equity-Method Investments and Assets and Liabilities Held for Sale in our 2019 Financial Report.
Some additional information about our Biopharma and Upjohn business segments follows: | |||
 | Pfizer Biopharmaceuticals Group |  | |
Biopharma is a science-based medicines business that includes six business units – Oncology, Inflammation & Immunology, Rare Disease, Hospital, Vaccines and Internal Medicine. The Hospital unit commercializes our global portfolio of sterile injectable and anti-infective medicines and includes Pfizer’s contract manufacturing operation, Pfizer CentreOne. At the beginning of our 2019 fiscal year, we also incorporated our biosimilar portfolio into the Oncology and Inflammation & Immunology business units and certain legacy established products into the Internal Medicine business unit. Each business unit is committed to delivering breakthroughs that change patients’ lives. | Upjohn is a global, primarily off-patent branded and generic medicines business, which includes a portfolio of 20 globally recognized solid oral dose brands, as well as a U.S.-based generics platform, Greenstone. | ||
Select products include: - Prevnar 13/Prevenar 13 - Ibrance - Eliquis - Xeljanz - Enbrel (outside the U.S. and Canada) - Chantix/Champix - Sutent - Xtandi - Vyndaqel/Vyndamax | Select products include: - Lyrica - Lipitor - Norvasc - Celebrex - Viagra - Certain generic medicines | ||
On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. For additional information, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Business Development Initiatives and —Our Strategy sections in our 2019 Financial Report.
For a further discussion of these operating segments, see the Pfizer Biopharmaceuticals Group (Biopharma) and Upjohn sections in this 2019 Form 10-K, the table captioned Revenues by Operating Segment and Geography in the Analysis of the Consolidated Statements of Income section and the Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information, including the tables therein captioned Selected Income Statement Information, Geographic Information and Significant Product Revenues, in our 2019 Financial Report, which are incorporated by reference.
Pfizer Inc. | 2019 Form 10-K | 3 |
PFIZER BIOPHARMACEUTICALS GROUP (BIOPHARMA)
The key therapeutic areas comprising our Biopharma business segment include:
Therapeutic Area | Description | Key Products |
Internal Medicine | Includes innovative brands from two therapeutic areas, Cardiovascular Metabolic and Pain, as well as regional brands. | Eliquis, Chantix/Champix and Premarin family |
Oncology | Includes innovative oncology brands of biologics, small molecules, immunotherapies, and biosimilars across a wide range of cancers. | Ibrance, Sutent, Xtandi, Xalkori, Inlyta and Braftovi + Mektovi |
Hospital | Includes our global portfolio of sterile injectable and anti-infective medicines, as well as Pfizer CentreOne, our contract manufacturing and active pharmaceutical ingredient sales operation. | Sulperazon, Medrol, Vfend and Zithromax |
Vaccines | Includes innovative vaccines brands across all ages—infants, adolescents and adults—in pneumococcal disease, Meningococcal disease and tick-borne encephalitis, with a pipeline focus on healthcare-acquired infections and maternal health. | Prevnar 13/Prevenar 13 (pediatric/adult), FSME-IMMUN, Nimenrix and Trumenba |
Inflammation and Immunology | Includes innovative brands and biosimilars for chronic immune and inflammatory diseases. | Xeljanz, Enbrel (outside the U.S. and Canada), Inflectra and Eucrisa |
Rare Disease | Includes innovative brands for a number of therapeutic areas with rare diseases, including amyloidosis, hemophilia, and endocrine diseases. | Vyndaqel/Vyndamax, BeneFIX, Genotropin and Refacto AF/Xyntha |
We recorded direct product and/or alliance revenues of more than $1 billion for each of six Biopharma products in 2019, seven Biopharma products in 2018 and six Biopharma products in 2017:
Biopharma $1 Billion+ Products | ||||
2019 | 2018 | 2017 | ||
Prevnar 13/Prevenar 13 | Prevnar 13/Prevenar 13 | Prevnar 13/Prevenar 13 | ||
Ibrance | Ibrance | Ibrance | ||
Eliquis* | Eliquis* | Eliquis* | ||
Xeljanz | Enbrel | Enbrel | ||
Enbrel | Xeljanz | Xeljanz | ||
Chantix/Champix | Chantix/Champix | Sutent | ||
Sutent | ||||
* Eliquis includes alliance revenues and direct sales in 2019, 2018 and 2017. | ||||
For a discussion of certain Biopharma products and additional information regarding collaboration and/or co-promotion agreements involving certain of these Biopharma products, see the Item 1A. Business—Collaboration and Co-Promotion Agreements and —Patents and Other Intellectual Property Rights sections of this 2019 Form 10-K; for additional information regarding the revenues of our Biopharma business, including revenues by geography and of significant Biopharma products, see the Analysis of the Consolidated Statements of Income—Revenues—Overview, —Revenues by Operating Segment and Geography and —Revenues—Selected Product Discussion sections and the Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information in our 2019 Financial Report; and for additional information on the key operational revenue drivers of our Biopharma business, see the Analysis of Operating Segment Information—Biopharma Operating Segment section in our 2019 Financial Report. For a discussion of the risks associated with our dependence on certain of our major products, see the Item 1A. Risk Factors—Dependence on Key In-Line Products section in this 2019 Form 10-K.
Pfizer Inc. | 2019 Form 10-K | 4 |
UPJOHN
Upjohn’s products are used to treat non-communicable diseases across a broad range of therapeutic areas, including:
• | Cardiovascular (Lipitor, Norvasc and Revatio); |
• | Pain and neurology (Lyrica and Celebrex); |
• | Psychiatry (Effexor, Zoloft and Xanax); |
• | Urology (Viagra); and |
• | Ophthalmology (Xalatan/Xalacom). |
We recorded direct product revenues of more than $1 billion for two Upjohn products in 2019, three Upjohn products in 2018, and three Upjohn products in 2017:
Upjohn $1 Billion+ Products | ||||
2019 | 2018 | 2017 | ||
Lyrica | Lyrica | Lyrica | ||
Lipitor | Lipitor | Lipitor | ||
Norvasc | Viagra | |||
For a discussion of certain Upjohn products and additional information regarding the revenues of our Upjohn business, including revenues by geography and of significant Upjohn products, see the Analysis of the Consolidated Statements of Income—Revenues—Overview, —Revenues by Operating Segment and Geography and —Revenues—Selected Product Discussion sections and the Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information in our 2019 Financial Report; and for additional information on the key operational revenue drivers of our Upjohn business, see the Analysis of Operating Segment Information—Upjohn Operating Segment section in our 2019 Financial Report. For a discussion of the risks associated with our dependence on certain of our major products, see the Item 1A. Risk Factors—Dependence on Key In-Line Products section in this 2019 Form 10-K.
COLLABORATION AND CO-PROMOTION AGREEMENTS
We are party to collaboration and/or co-promotion agreements relating to certain biopharmaceutical products, including, among others, Eliquis, Xtandi and Bavencio. Revenues from Eliquis (except in certain markets where we have direct sales), Xtandi and Bavencio are included in alliance revenues.
Eliquis has been jointly developed and is commercialized by Pfizer and BMS. Pfizer funds between 50% and 60% of all development costs depending on the study. Profits and losses are shared equally on a global basis, except in certain countries where Pfizer commercializes Eliquis and pays BMS compensation based on a percentage of net sales. We have full commercialization rights in certain smaller markets. BMS supplies the product to us at cost plus a percentage of the net sales to end-customers in these markets. Eliquis is part of the Novel Oral Anticoagulant market; the agents in this class were developed as alternative treatment options to warfarin in appropriate patients.
Xtandi is being developed and commercialized through a collaboration with Astellas. The two companies share equally in the gross profits (losses) related to U.S. net sales of Xtandi. Subject to certain exceptions, Pfizer and Astellas also share equally all Xtandi commercialization costs attributable to the U.S. market. In addition, Pfizer and Astellas share certain development and other collaboration expenses, and Pfizer receives tiered royalties as a percentage of international Xtandi net sales (recorded in Other (income)/deductions––net). Xtandi is an androgen receptor inhibitor that blocks multiple steps in the androgen receptor signaling pathway within tumor cells.
Bavencio (avelumab) is being developed and commercialized in collaboration with Merck KGaA. Both companies jointly fund the majority of development and commercialization costs, and split equally any profits related to net sales generated from selling any products containing avelumab from this collaboration. Bavencio is a human anti-programmed death ligand-1 (PD-L1) antibody.
Pfizer Inc. | 2019 Form 10-K | 5 |
RESEARCH AND DEVELOPMENT
Innovation is critical to the success of our Company, and drug discovery and development are time-consuming, expensive and unpredictable. Pfizer’s purpose is to deliver breakthroughs that change patients’ lives. R&D is at the heart of fulfilling Pfizer’s purpose as we work to translate advanced science and technologies into the therapies that matter most.
Our R&D Priorities and Strategy
Our R&D priorities include:
• | delivering a pipeline of highly differentiated medicines and vaccines where Pfizer has a unique opportunity to bring the most important new therapies to patients in need; |
• | advancing our capabilities that can position Pfizer for long-term R&D leadership; and |
• | advancing new models for partnerships with creativity, flexibility and urgency to deliver innovation to patients as quickly as possible. |
To that end, our R&D primarily focuses on:
• | Oncology; |
• | Inflammation and Immunology; |
• | Vaccines; |
• | Internal Medicine; |
• | Rare Diseases; and |
• | Hospital. |
While a significant portion of R&D is done internally, we continue to seek out promising chemical and biological lead molecules and innovative technologies developed by third parties to incorporate into our discovery and development processes or projects, as well as our product lines. We do so by entering into collaboration, alliance and license agreements with other companies, as well as leveraging acquisitions and equity- or debt-based investments. These agreements enable us to co-develop, license or acquire promising compounds, technologies and/or capabilities. We also enter into agreements pursuant to which a third party agrees to fund a portion of the development costs of one or more of our pipeline products in exchange for rights to receive potential milestone payments, revenue sharing payments, profit sharing payments and/or royalties. Collaboration, alliance, license and funding agreements and equity- or debt-based investments allow us to share risk and cost. They also enable us to access external scientific and technological expertise, as well as provide us the opportunity to advance our own products and in-licensed or acquired products.
For additional information, see the Notes to Consolidated Financial Statements—Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements in our 2019 Financial Report.
Our R&D Operations
We conduct R&D internally and also through contracts with third parties, through collaborations with universities and biotechnology companies and in cooperation with other pharmaceutical firms. In 2019, we continued to strengthen our global R&D organization and pursue strategies intended to improve innovation and overall productivity in R&D to achieve a sustainable pipeline that is positioned to deliver value in the near term and over time.
Our R&D spending is conducted through a number of matrix organizations:
• | Research Units within our WRDM organization are generally responsible for research and early-stage development assets for our Biopharma business (assets that have not yet achieved proof-of-concept). Our Research Units are organized by therapeutic area to enhance flexibility, cohesiveness and focus. Because of our structure, we are able to rapidly redeploy resources within a Research Unit between various projects as necessary because in many instances the workforce shares similar skills, expertise and/or focus. |
• | Our science-based and other platform-services organizations provide technical expertise and other services to the various R&D projects, and are organized into science-based functions (which are part of our WRDM organization), such as Pharmaceutical Sciences, Medicine Design, and non-science-based functions, such as Facilities, Digital and Finance. Within each of these functions, we are able to migrate resources among projects, candidates and/or targets in any therapeutic area and in most phases of development, allowing us to react quickly in response to evolving needs. In addition, the Worldwide Medical and Safety group, within WRDM, ensures that Pfizer provides all stakeholders––including patients, healthcare providers, pharmacists, payers and health authorities––with complete and up-to-date information on the risks and benefits associated with Pfizer products so that they can make appropriate decisions on how and when to use Pfizer’s medicines. |
• | Our R&D organization within Upjohn supports the off-patent branded and generic established medicines and helps to develop product enhancements, new indications and new market registrations for these medicines. |
Pfizer Inc. | 2019 Form 10-K | 6 |
• | Our Global Product Development (GPD) organization is a unified center for clinical development and regulatory activities that is generally responsible for the clinical development strategy and operational execution of clinical trials for both early-stage assets in the WRDM portfolio as well as late-stage assets in the Biopharma portfolio. |
We manage R&D operations on a total-company basis through our matrix organizations described above. Specifically, the Portfolio Strategy & Investment committee, comprised of senior executives, is accountable for aligning resources among all of our WRDM, GPD and Biopharma R&D projects and for seeking to ensure optimal capital allocation across the innovative R&D portfolio. We believe that this approach also serves to maximize accountability and flexibility. Our Upjohn R&D organization manages its resources separately from the WRDM and GPD organizations, with operational support from GPD for select clinical development regulatory activities and from WRDM for clinical supply operations and global pharmacovigilance processing.
Generally, we do not disaggregate total R&D expense by development phase or by therapeutic area since, as described above, we do not manage our R&D operations by development phase or by therapeutic area. Further, as we are able to adjust a significant portion of our spending quickly, we believe that any prior-period information about R&D expense by development phase or by therapeutic area would not necessarily be representative of future spending.
For additional information on our R&D operations and expenses, see the Costs and Expenses—Research and Development (R&D) Expenses section in our 2019 Financial Report.
Our R&D Pipeline and Competition
The discovery and development of safe, effective new products, as well as the development of additional uses for existing products, are necessary for the continued strength of our businesses. Drug candidates can fail at any stage of the process, and candidates may not receive regulatory approval even after many years of research and development. The process from discovery to development to regulatory approval can take more than ten years.
As of January 28, 2020, we had the following number of projects in various stages of R&D:

Development of a single compound is often pursued as part of multiple programs. While these drug candidates may or may not eventually receive regulatory approval, new drug candidates entering clinical development phases are the foundation for future products. In addition to discovering and developing new products, our R&D efforts seek to add value to our existing products by improving their effectiveness, enhancing ease of dosing and by discovering potential new indications for them.
Information concerning several of our drug candidates in development, as well as supplemental filings for existing products, is set forth in the Analysis of the Consolidated Statements of Income—Product Developments––Biopharmaceutical section in our 2019 Financial Report, which is incorporated by reference.
Our competitors also devote substantial funds and resources to R&D. We also compete against numerous small biotechnology companies in developing potential drug candidates. The extent to which our competitors are successful in their research could result in erosion of the sales of our existing products and potential sales of products in development, as well as unanticipated product obsolescence. In addition, several of our competitors operate without large R&D expenses and make a regular practice of challenging our product patents before their expiration. For additional information, see the Competition and Item 1A. Risk Factors—Competitive Products sections in this 2019 Form 10-K.
Pfizer Inc. | 2019 Form 10-K | 7 |
INTERNATIONAL OPERATIONS
We have significant operations outside the U.S. In 2019, operations in developed and emerging markets were managed through our business segments: Biopharma, Upjohn and, through July 31, 2019, Consumer Healthcare. Emerging markets are an important component of our strategy for global leadership, and our commercial structure recognizes that the demographics and rising economic power of the fastest-growing emerging markets are becoming more closely aligned with the profile found within developed markets. Urbanization and the rise of the middle class in emerging markets, particularly in Asia, provide growth opportunities for our medicines.
We sell our products in over 125 countries. Revenues from operations outside the U.S. of $27.9 billion accounted for 54% of our total revenues in 2019. Revenues exceeded $500 million in each of eleven countries outside the U.S. in 2019, 2018 and 2017. By total revenues, China and Japan are our two largest national markets outside the U.S. For a geographic breakdown of revenues, see the Analysis of the Consolidated Statements of Income—Revenues—Overview and —Revenues by Operating Segment and Geography sections and the table captioned Geographic Information in the Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information in our 2019 Financial Report.

Our international operations are subject, in varying degrees, to a number of risks inherent in carrying on business in other countries, including, among other things, currency fluctuations, capital and exchange control regulations and expropriation and other restrictive government actions. See the Item 1A. Risk Factors—International Operations section in this 2019 Form 10-K. Our international businesses are also subject to government-imposed constraints, including laws and regulations on pricing, reimbursement, and access to our products. See the Item 1. Business—Government Regulation and Price Constraints—Outside the United States section in this 2019 Form 10-K for a discussion of these matters.
Depending on the direction of change relative to the U.S. dollar, foreign currency values can increase or decrease the reported dollar value of our net assets and results of operations. While we cannot predict with certainty future changes in foreign exchange rates or the effect they will have on us, we attempt to mitigate their impact through operational means and by using various financial instruments, depending upon market conditions. For additional information, see the Notes to Consolidated Financial Statements—Note 7F. Financial Instruments: Derivative Financial Instruments and Hedging Activities in our 2019 Financial Report, which is incorporated by reference, as well as Item 7A. Quantitative and Qualitative Disclosures About Market Risk—Financial Risk Management section in this 2019 Form 10-K.
MARKETING
In our global biopharmaceutical businesses, we promote our products to healthcare providers and patients. Through our marketing organizations, we explain the approved uses, benefits and risks of our products to healthcare providers, such as doctors, nurse practitioners, physician assistants and pharmacists; MCOs that provide insurance coverage, such as hospitals, Integrated Delivery Systems, PBMs and health plans; and employers and government agencies who hire MCOs to provide health benefits to their employees. We also market directly to consumers in the U.S. through direct-to-consumer advertising that seeks to communicate the approved uses, benefits and risks of our products while motivating people to have meaningful conversations with their doctors. In addition, we sponsor general advertising to educate the public on disease awareness, prevention and wellness, important public health issues, and our patient assistance programs.
Our prescription pharmaceutical products are sold principally to wholesalers, but we also sell directly to retailers, hospitals, clinics, government agencies and pharmacies, and, in the case of our vaccines products in the U.S., we primarily sell directly to the U.S. Centers for Disease Control and Prevention, wholesalers, individual provider offices, retail pharmacies, and integrated delivery networks. We seek to gain access for our products on healthcare authority and PBM formularies, which are lists of approved medicines available to members of the PBMs. PBMs use various benefit designs, such as tiered co-pays for formulary
Pfizer Inc. | 2019 Form 10-K | 8 |
products, to drive utilization of products in preferred formulary positions. We may also work with payers on disease management programs that help to develop tools and materials to educate patients and physicians on key disease areas.
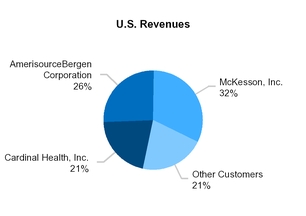
In 2019, our top three biopharmaceutical wholesalers accounted for approximately 37% of our total revenues (and approximately 79% of our total U.S. revenues).
% of 2019 Total Revenues and U.S. Revenues from
Major Biopharmaceutical Wholesalers and Other Customers

PATENTS AND OTHER INTELLECTUAL PROPERTY RIGHTS
Our products are sold around the world under brand-name, logo and certain product design trademarks that we consider, in the aggregate, to be of material importance to Pfizer. Trademark protection continues in some countries for as long as the mark is used and, in other countries, for as long as it is registered. Registrations generally are for fixed, but renewable, terms.
We own or license a number of U.S. and foreign patents. These patents cover pharmaceutical and other products and their uses, pharmaceutical formulations, product manufacturing processes and intermediate chemical compounds used in manufacturing.
Patents for individual products extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends upon the type of patent, the scope of its coverage and the availability of legal remedies in the country. Further, patent term extension may be available in many major countries to compensate for a regulatory delay in approval of the product. For additional information, see the Item 1. Business—Government Regulation and Price Constraints—Outside the United States—Intellectual Property section in this 2019 Form 10-K.
In various markets, a period of regulatory exclusivity may be provided to certain drugs upon approval. The scope and term of such exclusivity will vary but, in general, the period of regulatory exclusivity will run concurrently with the term of any existing patent rights associated with the drug at the time of approval.
In the aggregate, our patent and related rights are of material importance to our businesses in the U.S. and most other countries. Based on current product sales, and considering the vigorous competition with products sold by our competitors, the patent rights we consider most significant in relation to our business as a whole, together with the year in which the basic product patent expires (including, where applicable, grant of an additional six-month pediatric extension and/or the granted patent term extension in the U.S. and Japan and Supplementary Patent Certificate in Europe), are those for the medicines set forth in the table below. Unless otherwise indicated, the years set forth in the table below pertain to the basic product patent expiration for the respective products. Patent term extensions, supplementary protection certificates and pediatric exclusivity periods are not reflected in the expiration dates listed in the table below, unless they have been granted by the issuing authority. In some instances, there are later-expiring patents relating to our products directed to particular forms or compositions, to methods of manufacturing, or to use of the drug in the treatment of particular diseases or conditions. However, in some cases, such patents may not protect our drug from generic or, as applicable, biosimilar competition after the expiration of the basic patent.
Pfizer Inc. | 2019 Form 10-K | 9 |
Drug | U.S. Basic Product Patent Expiration Year | Major EU Basic Product Patent Expiration Year | Japan Basic Product Patent Expiration Year | |||
Lyrica | 2019(1) | 2014(2) | 2022(3) | |||
Chantix/Champix | 2020 | 2021 | 2022 | |||
Sutent | 2021 | 2022 | 2024 | |||
Ibrance | 2023 | 2028 | 2028 | |||
Vyndaqel/Vyndamax | 2024 | 2026 | 2026 | |||
Inlyta | 2025 | 2025 | 2025 | |||
Xeljanz | 2025 | 2028(4) | 2025 | |||
Prevnar 13/Prevenar 13 | 2026 | __(5) | 2029 | |||
Eliquis(6) | 2026 | 2026 | 2026 | |||
Xtandi(7) | 2027 | *(7) | *(7) | |||
Xalkori | 2029 | 2027 | 2028 | |||
Besponsa | 2030 | 2028 | 2028(8) | |||
Braftovi(9) | 2031 | *(9) | *(9) | |||
Mektovi(9) | 2031(10) | *(9) | *(9) | |||
Bavencio(11) | 2033 | 2032 | 2033 | |||
(1) | Lyrica lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. |
(2) | Lyrica regulatory exclusivity in the EU expired in July 2014. |
(3) | Lyrica is covered by a Japanese method-of-use patent which expires in 2022. The patent is currently subject to an invalidation action. |
(4) | Xeljanz EU expiry is provided by regulatory exclusivity. |
(5) | The EU patent that covers the combination of the 13 serotype conjugates of Prevenar 13 was revoked following an opposition and has now been withdrawn. There are other EU patents and pending applications covering the formulation, various aspects of the manufacturing process, and the combination of serotype conjugates of Prevenar 13 that remain in force. |
(6) | Eliquis was developed and is being commercialized in collaboration with BMS. |
(7) | Xtandi is being developed and commercialized in collaboration with Astellas, which has exclusive commercialization rights for Xtandi outside the U.S. Pfizer receives tiered royalties as a percentage of international Xtandi net sales. |
(8) | Besponsa Japan expiry is provided by regulatory exclusivity. |
(9) | Pfizer has exclusive rights to Braftovi and Mektovi in the U.S. The Pierre Fabre Group has exclusive rights to commercialize both products in Europe and Ono Pharmaceutical Co., Ltd. has exclusive rights to commercialize both products in Japan. Pfizer receives royalties from The Pierre Fabre Group and Ono Pharmaceutical Co., Ltd. on sales of Braftovi and Mektovi outside the U.S. |
(10) | The U.S. expiration date in the table for Mektovi is provided by a method-of-use patent. |
(11) | Bavencio is being developed and commercialized in collaboration with Merck KGaA. |
The loss, expiration or invalidation of intellectual property rights, patent litigation settlements with manufacturers and the expiration of co-promotion and licensing rights can have a significant adverse effect on our revenues. Many of our branded products have multiple patents that expire at varying dates, thereby strengthening our overall patent protection. However, once patent protection has expired or has been lost prior to the expiration date as a result of a legal challenge, we typically lose exclusivity on these products, and generic and biosimilar pharmaceutical manufacturers generally produce identical or highly similar products and sell them for a lower price. The date at which generic or biosimilar competition commences may be different from the date that the patent or regulatory exclusivity expires. However, when generic or biosimilar competition does commence, the resulting price competition can substantially decrease our revenues for the impacted products, often in a very short period of time. In some cases, however, we can continue to obtain commercial benefits from product manufacturing trade secrets; patents on uses for products; patents on processes and intermediates for the economical manufacture of the active ingredients; patents for special formulations of the product or delivery mechanisms; or conversion of the active ingredient to OTC products.
Also, if one of our patents is found to be invalid by judicial, court or administrative proceedings, such as inter partes review, post-grant review, re-examination or opposition proceedings, before the U.S. Patent and Trademark Office, the European Patent Office, or other foreign counterparts, generic or competitive products could be introduced into the market resulting in the erosion of sales of our existing products. For example, several of the patents in our pneumococcal vaccine portfolio were challenged in inter partes review and post-grant review proceedings in the U.S. For additional information, see the Item 1A. Risk Factors—Patent Protection section in this 2019 Form 10-K.
Companies have filed applications with the FDA seeking approval of product candidates that such companies claim either do not infringe our patents or our patents are invalid; these include candidates that would compete with, among other products, Eliquis, Ibrance and Xeljanz. We will continue to aggressively defend our patent rights whenever we deem appropriate. For additional
Pfizer Inc. | 2019 Form 10-K | 10 |
information, see the Notes to Consolidated Financial Statements—Note 16A1. Contingencies and Certain Commitments—Legal Proceedings––Patent Litigation in our 2019 Financial Report.
Recent Losses and Expected Losses of Product Exclusivity
Certain of our current products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and we expect certain products to face significantly increased generic competition over the next few years. For example, as a result of a patent litigation settlement, Teva launched a generic version of Viagra in the U.S. in December 2017. Lyrica lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. Also, the basic product patent for Chantix in the U.S. will expire in November 2020. See the table above for the basic product patent expiries of our most significant products.
We expect the impact of reduced revenues due to patent expiries will be significant in 2020, then moderating downward to a much lower level from 2021 through 2025. For additional information, see the Item 1A. Risk Factors—Dependence on Key In-Line Products section in this 2019 Form 10-K.
The following table provides information about certain products recently experiencing, or expected to experience in 2020, patent expirations or loss of regulatory exclusivity in the U.S., Europe or Japan. Our financial results in 2019 and our financial guidance for 2020 reflect the impact of the loss of exclusivity of various products discussed below:
(MILLIONS OF DOLLARS) | Product Revenues in Markets Impacted | |||||||||||||||
Products | Key Dates(a) | Markets Impacted | Year Ended December 31, | |||||||||||||
2019 | 2018 | 2017 | ||||||||||||||
Viagra(b) | June 2013 May 2014 December 2017 | Major European markets Japan U.S. | $ | 134 | $ | 274 | $ | 850 | ||||||||
Lyrica(c) | July 2014 June 2019 | Major European markets U.S. | 2,208 | 3,852 | 3,901 | |||||||||||
Pristiq(d) | March 2017 | U.S. | 42 | 71 | 133 | |||||||||||
Chantix(e) | November 2020 | U.S. | 899 | 838 | 742 | |||||||||||
(a) | Unless otherwise noted, “Key Dates” indicate patent-based expiration dates. |
(b) | As a result of a patent litigation settlement, Teva launched a generic version of Viagra in the U.S. in December 2017. |
(c) | Lyrica lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. |
(d) | As a result of a patent litigation settlement with several generic manufacturers, generic versions of Pristiq launched in the U.S. in March 2017. |
(e) | The basic product patent for Chantix in the U.S. will expire in November 2020, which includes the FDA’s grant of pediatric exclusivity that extended the period of market exclusivity in the U.S. for Chantix for an additional six months from May 2020. |
Biologic Products
Our biologic products, including BeneFIX, ReFacto, Xyntha, Bavencio, Prevnar 13/Prevenar 13 and Enbrel (we market Enbrel outside the U.S. and Canada), already face, or may face in the future, competition from biosimilars (also referred to as follow-on biologics). In the U.S., such biosimilars would reference our originator biologic products approved under the U.S. Public Health Service Act. Additionally, the FDA has approved a follow-on recombinant human growth hormone that referenced our biotechnology product, Genotropin, that was approved under the FFDCA.
Biosimilars are versions of biologic medicines that have been developed and proven to be highly similar to the original biologic in terms of safety and efficacy and that have no clinically meaningful differences in safety, purity or potency. Biosimilars have the potential to offer high-quality, lower-cost alternatives to biologic medicines. Abbreviated legal pathways for the approval of biosimilars exist in certain international markets and, since the passage of the ACA in 2010, a framework for such approval exists in the U.S. In Europe, the European Commission grants marketing authorizations for biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals.
As part of our business strategy, we are capitalizing on our expertise in biologics manufacturing, as well as our regulatory and commercial strengths, to develop and commercialize biosimilar medicines. Some of the biosimilars that we currently market include Inflectra, Nivestym, Retacrit, Zirabev, Ruxience and Trazimera in the U.S.; Inflectra, Retacrit, Nivestim and Trazimera in the EU; and Ixifi, Trazimera, Zirabev and Ruxience in Japan. See the Item 1A. Risk Factors—Biosimilars section in this 2019 Form 10-K.
Pfizer Inc. | 2019 Form 10-K | 11 |
We may face litigation with respect to the validity and/or scope of patents relating to our biologic products. Likewise, as we develop, manufacture and seek to launch biosimilars, patents may be asserted against us.
International
One of the main limitations on our operations in some countries outside the U.S. is the lack of effective intellectual property protection for our products. Under international and U.S. free trade agreements in recent years, we have seen some improvement in global protection of intellectual property rights. For additional information, see the Item 1. Business—Government Regulation and Price Constraints—Outside the United States—Intellectual Property section in this 2019 Form 10-K.
COMPETITION
Our businesses are conducted in intensely competitive and often highly regulated markets. Many of our prescription pharmaceutical products face competition in the form of branded or generic drugs or biosimilars that treat similar diseases or indications. The principal forms of competition include efficacy, safety, ease of use, and cost effectiveness. Though the means of competition vary among product categories and business groups, demonstrating the value of our products is a critical factor for success in all of our principal businesses.
Our competitors include other worldwide research-based biopharmaceutical companies, smaller research companies with more limited therapeutic focus and generic and biosimilar drug manufacturers. We compete with other companies that manufacture and sell products that treat diseases or indications similar to those treated by our major products.
This competition affects our core product business, which is focused on applying innovative science to discover and market products that satisfy unmet medical needs and provide therapeutic improvements. Our emphasis on innovation is underscored by our multi-billion-dollar investment in R&D, as well as our business development transactions, both designed to result in a strong product pipeline. Our investment in research does not stop with drug approval; we continue to invest in further demonstrating the value of our products for the conditions they treat, as well as potential new applications. We seek to protect the health and well-being of patients by striving to ensure that medically sound knowledge of the benefits and risks of our medicines is understood and communicated to patients, physicians, payers and global health authorities. We also seek to continually enhance the organizational effectiveness of all of our biopharmaceutical functions, including coordinating support for our efforts to accurately and ethically launch and promote our products to our customers.
Operating conditions have become more challenging under mounting global pressures of competition, industry regulation and cost containment. We continue to take measures to evaluate, adapt and improve our organization and business practices to better meet customer and public needs. We believe that we have taken an industry-leading role in evolving our approaches to U.S. direct-to-consumer advertising, interactions with, and payments to, healthcare professionals, and medical education grants. We also continue to sponsor programs to address patient affordability and access barriers, as we strive to advance fundamental health system change through support for better healthcare solutions.
Our vaccines business may face competition from the introduction of alternative vaccines. For example, Prevnar 13 may face competition in the form of competitor vaccines, including vaccines with additional serotypes or “next-generation” pneumococcal conjugate vaccines prior to or after the expiration of its patents, which may adversely affect our future results.
Our generics and biosimilars businesses compete with branded products from competitors, as well as other generics and biosimilars manufacturers. Globally, Pfizer sells generic versions of Pfizer’s, as well as certain competitors’, solid oral dose and sterile injectable pharmaceutical products. We also sell biosimilars of certain inflammation & immunology and oncology biologic medicines globally. We seek to maximize the opportunity to establish a “first-to-market” or early market position for our generic injectable drugs and biosimilars, as a “first-to-market” position provides customers a lower-cost alternative immediately when available and also may provide us with potentially higher levels of sales and profitability until other generic or biosimilar competitors enter the market.
Managed Care Organizations
The evolution of managed care in the U.S. has been a major factor in the competitive makeup of the healthcare marketplace. Approximately 300 million people in the U.S. now have some form of health insurance coverage. Due to the expansion of health insurance coverage (see the Item 1. Business—Government Regulation and Price Constraints—In the United States section in this 2019 Form 10-K), the marketing of prescription drugs to both consumers and the entities that manage this expanded coverage in the U.S. continues to grow in importance.
The influence of MCOs has increased in recent years due to the growing number of patients receiving coverage through MCOs. At the same time, those organizations have been consolidating into fewer, even larger entities. This consolidation enhances both their ability to negotiate, as well as their importance to Pfizer.
The growth of MCOs has increased pressure on drug prices as well as revenues. One objective of MCOs is to contain and, where possible, reduce healthcare expenditures. MCOs typically negotiate prices with pharmaceutical providers by using
Pfizer Inc. | 2019 Form 10-K | 12 |
formularies (which are lists of approved medicines available to members of the MCOs), clinical protocols (requiring prior authorization for a branded product if a generic product is available or requiring the patient to first fail on one or more generic products before permitting access to a branded medicine), volume purchasing, long-term contracts and their ability to influence volume and market share of prescription drugs. In addition, by placing branded medicines on higher-tier status in their formularies (leading to higher patient co-pays) or non-preferred tier status, MCOs transfer a portion of the cost of the medicine to the patient, resulting in significant out-of-pocket expenses for the patient, especially for chronic treatments. This financial disincentive is a tool for MCOs to manage drug costs and channel patients to medicines preferred by the MCOs. MCOs also use additional measures such as new-to-market blocks, exclusion lists, indication-based pricing and “copay accumulator” programs to improve their cost containment efforts. We are closely monitoring these newer approaches and developing appropriate strategies to respond to them.
Due to their generally lower cost, generic medicines typically are placed in lowest cost tiers of MCO formularies. The breadth of the products covered by formularies can vary considerably from one MCO to another, and many formularies include alternative and competitive products for treatment of particular medical problems.
Exclusion of a product from a formulary or other MCO-implemented restrictions can significantly impact drug usage in the MCO patient population and beyond. Consequently, pharmaceutical companies compete to gain access to formularies for their products. Unique product features, such as greater efficacy, better patient ease of use, or fewer side effects, are generally beneficial to achieving access to formularies. However, lower overall cost of therapy is also an important factor. We have been generally, although not universally, successful in having our major products included on MCO formularies. However, increasingly our branded products are being placed on the higher tiers or in a non-preferred status.
MCOs also emphasize primary and preventive care, out-patient treatment and procedures performed at doctors’ offices and clinics as another way to manage costs. Hospitalization and surgery, typically the most expensive forms of treatment, are carefully managed. Since the use of certain drugs can reduce the need for hospitalization, professional therapy, or even surgery, such drugs can become favored first-line treatments for certain diseases.
The ACA has accelerated payment reform by distributing risk across MCOs and other stakeholders in care delivery with the intent of improving quality while reducing costs, which creates pressure on MCOs to tie reimbursement to defined outcomes. For additional information, see the Item 1. Business—Government Regulation and Price Constraints—In the United States—Healthcare Reform section in this 2019 Form 10-K.
Generic Products
One of the biggest competitive challenges that our branded products face is from generic pharmaceutical manufacturers. Upon the expiration or loss of patent protection for a product, especially a small molecule product, we can lose the major portion of revenues for that product in a very short period of time. Several competitors make a regular practice of challenging our product patents before their expiration. Generic competitors often operate without large R&D expenses, as well as without costs of conveying medical information about products to the medical community. In addition, the FDA approval process exempts generics from costly and time-consuming clinical trials to demonstrate their safety and efficacy, allowing generic manufacturers to rely on the safety and efficacy data of the innovator product. Generic competitors can market a competing version of our product after the expiration or loss of our patent and often charge much less. In China, for example, we are expected to face further intensified competition by certain generic manufacturers in 2020, which may result in price cuts and volume loss of some of our products.
In addition, our patent-protected products can face competition in the form of generic versions of competitors’ branded products that lose their market exclusivity.
As noted above, MCOs that focus primarily on the immediate cost of drugs often favor generics over brand-name drugs. Many governments also encourage the use of generics as alternatives to brand-name drugs in their healthcare programs, including Medicaid in the U.S. Laws in the U.S. generally allow, and in some cases require, pharmacists to substitute, for brand-name drugs, generic drugs that have been rated under government procedures to be chemically and therapeutically equivalent to brand-name drugs. In a small subset of states, prescribing physicians are able to expressly prevent such substitution. Favoring generics may reduce sales of our branded products.
RAW MATERIALS
Raw materials essential to our businesses are purchased worldwide in the ordinary course of business from numerous suppliers. In general, these materials are available from multiple sources. In 2019, we experienced periodic shortages of select materials due to constrained capacity or operational challenges with the associated suppliers. Supplier management activities are ongoing to work to ensure the necessary supply to meet our requirements for these materials. No significant impact to our operations is anticipated in 2020.
Pfizer Inc. | 2019 Form 10-K | 13 |
GOVERNMENT REGULATION AND PRICE CONSTRAINTS
Pharmaceutical companies are subject to extensive regulation by government authorities in the countries in which they do business. Certain laws and regulations that govern Pfizer’s business are discussed below.
General. Our business has been and will continue to be subject to numerous laws and regulations. Failure to comply with these laws and regulations, including those governing the manufacture and marketing of our products, could subject us to administrative and legal proceedings and actions by various governmental bodies. For additional information on these proceedings and actions, see the Notes to Consolidated Financial Statements—Note 16A. Contingencies and Certain Commitments—Legal Proceedings in our 2019 Financial Report. Criminal charges, substantial fines and/or civil penalties, warning letters and product recalls or seizures, delays in product approvals, as well as limitations on our ability to conduct business in applicable jurisdictions, could result from such proceedings and actions.
In the United States
Drug Regulation. In the U.S., biopharmaceutical products are subject to extensive pre- and post-market regulation by the FDA, including regulations that govern, among other things, the safety and efficacy of our medicines, clinical trials, advertising and promotion, manufacturing, labeling and record keeping. Our products are also subject to post-market surveillance under the FFDCA and its implementing regulations with respect to drugs, as well as the Public Health Service Act and its implementing regulations with respect to biologics.
Other U.S. federal agencies, including the DEA, also regulate certain of our products. Many of our activities also are subject to the jurisdiction of the SEC.
Biopharmaceutical companies seeking to market a product in the U.S. must first test the product to demonstrate that it is safe and effective for its intended use. If, after evaluation, the FDA determines the product is safe (i.e., its benefits outweigh its known risks) and effective, then the FDA will approve the product for marketing, issuing a New Drug Application or Biologics License Application, as appropriate. Companies seeking to market a generic prescription drug must scientifically demonstrate that the generic drug is bioequivalent to the innovator drug. The Abbreviated New Drug Application, or generic drug application, must show, among other things, that the generic drug is pharmaceutically equivalent to the brand, the manufacturer is capable of making the drug correctly, and the proposed label is the same as that of the innovator/brand drug’s label.
Even after a drug or biologic is approved for marketing, it may still be subject to postmarketing commitments or postmarketing requirements. Postmarketing commitments are studies or clinical trials that the drug or biologic sponsor has agreed to conduct, but are not required by law and/or regulation. Postmarketing requirements include studies and clinical trials that sponsors are required to conduct, by law and/or regulation, as a condition of approval. Postmarketing studies or clinical trials can be required in order to assess a known risk or demonstrate clinical benefit for drugs or biologics approved pursuant to accelerated approval. If a company fails to meet its postmarketing requirements, the FDA may assess a civil monetary penalty, issue a warning letter or deem the drug or biologic misbranded. Once a drug or biologic is approved, the FDA must be notified of any modifications to the product and the FDA may also require a manufacturer to submit additional studies or conduct clinical trials. In addition, we are also required to report adverse events and comply with cGMPs, as well as advertising and promotion regulations. Failure to comply with the FFDCA may subject us to administrative and/or judicial sanctions, including warning letters, product recalls, seizures, delays in product approvals, injunctions, fines, civil penalties and/or criminal prosecution.
Biosimilar Regulation. The ACA created a framework for the approval of biosimilars (also known as follow-on biologics) following the expiration of 12 years of exclusivity for the innovator biologic, with a potential six-month pediatric extension. Under the ACA, biosimilar applications may not be submitted until four years after the approval of the reference innovator biologic.
The FDA is responsible for implementation of the legislation and approval of new biosimilars. Through FDA approvals and the issuance of draft and final guidance, the FDA has addressed a number of issues related to the biosimilars approval pathway, such as the labeling expectations for biosimilars. For example, in 2019, the FDA issued final guidance regarding the standards for demonstrating interchangeability with a U.S.-licensed reference product. In addition, in 2017, the Biosimilar User Fee Act was reauthorized for a five-year period, which led to a significant increase in the FDA’s biosimilar user fee revenues, thereby providing the FDA with additional resources to process biosimilar applications. For example, since the enactment of the newly authorized fee structure, the FDA estimates its revenues from biosimilar user fees generally will exceed $40 million.
Sales and Marketing Laws and Regulations. The marketing practices of U.S. biopharmaceutical companies are generally subject to various federal and state healthcare laws that are intended, among other things, to prevent fraud and abuse in the healthcare industry and to protect the integrity of government healthcare programs. These laws include anti-kickback laws and false claims laws. Anti-kickback laws generally prohibit a biopharmaceutical company from soliciting, offering, receiving, or paying anything of value to generate business, including purchasing or prescribing of a particular product. False claims laws generally prohibit anyone from knowingly and willingly presenting, or causing to be presented, any claims for payment for goods (including drugs or biologics) or services to third-party payers (including Medicare and Medicaid) that are false or fraudulent and generally treat claims generated through kickbacks as false or fraudulent. Violations of fraud and abuse laws may be punishable by criminal or civil sanctions and/or exclusion from federal healthcare programs (including Medicare and Medicaid). The federal government
Pfizer Inc. | 2019 Form 10-K | 14 |
and various states also have enacted laws to regulate the sales and marketing practices of pharmaceutical companies. The laws and regulations generally limit financial interactions between manufacturers and healthcare providers, require disclosure to the federal or state government and the public of such interactions, and/or require the adoption of compliance standards or programs. Many of these laws and regulations contain ambiguous requirements or require administrative guidance for implementation. Individual states, acting through their attorneys general, have become active as well, seeking to regulate the marketing of prescription drugs under state consumer protection and false advertising laws. Given the lack of clarity in laws and their implementation, our activities could be subject to the penalties under the pertinent laws and regulations.
Pricing and Reimbursement. Pricing and reimbursement for our pharmaceutical products depends in part on government regulation. Pfizer must offer discounted pricing or rebates on purchases of pharmaceutical products under various federal and state healthcare programs, such as the Medicaid Drug Rebate Program, the “federal ceiling price” drug pricing program, the 340B drug pricing program and the Medicare Part D Program. Pfizer must also report specific prices to government agencies under healthcare programs, such as the Medicaid Drug Rebate Program and Medicare Part B. The calculations necessary to determine the prices reported are complex and the failure to report prices accurately may expose Pfizer to penalties. See the discussion regarding rebates in the Analysis of the Consolidated Statements of Income—Revenues—Overview section and the Notes to Consolidated Financial Statements—Note 1G. Basis of Presentation and Significant Accounting Policies: Revenues and Trade Accounts Receivable in our 2019 Financial Report, which are incorporated by reference.
Government and private third-party payers routinely seek to manage utilization and control the costs of our products. Efforts by government officials or legislators to implement measures to regulate prices or payment for pharmaceutical products, including proposed action on drug importation, could adversely affect our business if implemented. There continues to be considerable public and government scrutiny of pharmaceutical pricing, and measures to address the perceived high cost of pharmaceuticals are being considered by Congress, the Presidential Administration and select states. For example, recent legislation revised how manufacturers calculate the average manufacturer price on branded drugs with authorized generics under the Medicaid drug rebate program, which the Congressional Budget Office has estimated will reduce Medicaid costs by over $3 billion over the next decade. Proposals for even more far-reaching reform, such as immediately eliminating or phasing out private health insurance, are being proposed by some Democratic candidates for U.S. President. In particular, several states have enacted or are considering transparency laws that require prescription drug manufacturers to report to the state and make public price increases, and sometimes to provide a written justification for the increase. In addition to new state transparency laws and the introduction of several Federal pricing bills, we have also seen the Presidential Administration introduce proposals related to importation and express interest in international reference pricing in Medicare Part B. We expect to see continued focus in regulating pricing resulting in additional legislation and regulation that could adversely impact revenue. In addition, U.S. government action to reduce federal spending on entitlement programs including Medicare and Medicaid may affect payment for our products or services associated with the provision of our products. For additional information, see the Item 1A. Risk Factors—U.S. Entitlement Reform section in this 2019 Form 10-K. Also, the majority of states use preferred drug lists to restrict access to certain pharmaceutical products under Medicaid. Restrictions exist for some Pfizer products under certain state Medicaid programs. As another example, access to our products under the Medicaid managed care program is typically determined by the health plans with which state Medicaid agencies contract to provide services to Medicaid beneficiaries. States continue to explore options for controlling healthcare costs related to Medicaid and other state healthcare programs, including the implementation of supplemental rebate agreements under the Medicaid drug rebate program that are tied to patient outcomes. In addition, we expect that consolidation and integration among pharmacy chains and wholesalers, who collectively are the primary purchasers of our pharmaceutical products in the U.S., and PBMs will increase pricing pressures on pharmaceutical manufacturers, including us. For additional information, see the Item 1A. Risk Factors—Managed Care Trends section in this 2019 Form 10-K.
The potential for additional pricing and access pressures in the commercial sector continues to be significant. Many employers have adopted high deductible health plans, which can increase out-of-pocket costs for medicines. This is a trend that is likely to continue. Private third-party payers, such as health plans, increasingly challenge pharmaceutical product pricing, which could result in lower prices, lower reimbursement rates and a reduction in demand for our products. Pricing pressures for our products may occur as a result of highly competitive insurance markets. Healthcare provider purchasers, directly or through group purchasing organizations, are seeking enhanced discounts or implementing more rigorous bidding or purchasing review processes.
Overall, there is increasing pressure on U.S. providers to deliver healthcare at a lower cost and to ensure that those expenditures deliver demonstrated value in terms of health outcomes. Longer term, we are seeing a shift in focus away from fee-for-service payments towards outcomes-based payments and risk-sharing arrangements that reward providers for cost reductions and improved patient outcomes. These new payment models can, at times, lead to lower prices for, and restricted access to, new medicines. At the same time, these models can also promote utilization of drugs by encouraging physicians to screen and diagnose and consider drugs as a means of forestalling more costly medical interventions.
We believe medicines are the most efficient and effective use of healthcare dollars based on the value they deliver to the overall healthcare system. We work with law makers and advocate for solutions that effectively improve patient health outcomes, lower costs to the healthcare system, and ensure access to medicines within an efficient and affordable healthcare system. In addition, in response to the evolving U.S. and global healthcare spending landscape, we work with health authorities, health technology assessment and quality measurement bodies and major U.S. payers throughout the product-development process to better
Pfizer Inc. | 2019 Form 10-K | 15 |
understand how these entities value our compounds and products. Further, we seek to develop stronger internal capabilities focused on demonstrating the value of the medicines that we discover or develop, register and manufacture, by recognizing patterns of usage of our medicines and competitor medicines along with patterns of healthcare costs.
Healthcare Reform. There have been significant efforts at the federal and state levels to reform the healthcare system by enhancing access to healthcare, improving the delivery of healthcare and further rationalizing payment for healthcare. We face uncertainties due to federal legislative and administrative efforts to repeal, substantially modify or invalidate some or all of the provisions of the ACA. There is additional uncertainty given the ruling in December 2019 by the U.S. Circuit Court of Appeals for the Fifth Circuit in Texas v. Azar that the individual mandate, which is a significant provision of the ACA, is unconstitutional. The case has been remanded to a lower court to determine whether the individual mandate is inseparable from the entire ACA, in which case the ACA as a whole would be rendered unconstitutional. In the meantime, the remaining provisions of the law remain in effect. The revenues generated for Pfizer by the health insurance exchanges and Medicaid expansion under the ACA are not material, so the impact of full invalidation of the law is expected to be limited. However, any future replacement for the ACA may adversely affect our business and financial results, particularly if the legislation reduces incentives for employer-sponsored insurance coverage or dramatically increases industry taxes and fees. Any future healthcare reform efforts may adversely affect our business and financial results.
Anti-Corruption. The FCPA prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Data Privacy. Pfizer collects personal data as part of its regular business activities. The collection and use of this data is subject to privacy and data security laws and regulations, including oversight by various regulatory or other governmental bodies. For example, we are subject to the California Consumer Privacy Act (CCPA). The CCPA, which came into effect on January 1, 2020, imposes numerous obligations on us, including a duty to disclose the categories of personal data that we collect, sell, or share about California consumers, and gives those consumers rights regarding their personal data. Noncompliance with any of these laws could result in the imposition of fines, penalties, or orders to stop non-compliant activities, and could damage our reputation and harm our business.
Outside the United States
We encounter similar regulatory and legislative issues in most countries outside the U.S.
New Drug Approvals. In the EU, the approval of new drugs may be achieved using the Mutual Recognition Procedure, the Decentralized Procedure or the EU Centralized Procedure. These procedures apply in the EU member states, plus the European Economic Area countries, Norway, Iceland and Liechtenstein. The Centralized Procedure, managed by the EMA, results in one single authorization for the whole EU, which provides the most rapid and efficient means of gaining approval across the EU and is the one most commonly used for new products.
In China, the regulatory system historically presented numerous challenges for the pharmaceutical industry, as its requirements for drug development and registration were often inconsistent with U.S. or other international standards. In recent years, however, China has introduced reforms and draft reforms, which are discussed in more detail below, that attempt to address these challenges. Furthermore, in 2017, the China regulatory authority, the National Medical Products Administration (NMPA), became a member of the International Council for Harmonization (ICH), which has resulted in greater adoption of international technical guidelines and practices by the government. 2019 was another active year in this respect, with a number of reforms coming into effect, and more proposals and drafts being issued for consultation.
In Japan, the PMDA is the point of entry for businesses looking to sell drugs in the country. The PMDA, which is involved in a wide range of regulatory activities, including clinical studies, approvals, postmarketing reviews and pharmaceuticals safety, must approve an application before a new drug product may be marketed in Japan. The PMDA also offers consultations on clinical trials of new drugs and provides advice on product classifications and approvals.
Health authorities in many middle and lower income countries require marketing approval by a recognized regulatory authority (i.e., similar to the authority of the FDA or the EMA) before they begin to conduct their application review process and/or issue their final approval. Many authorities also require local clinical data in the country’s population in order to receive final marketing approval.
Pharmacovigilance. In the EU, the EMA’s Pharmacovigilance Risk Assessment Committee has the responsibility for reviewing and making recommendations on product safety issues for the EU authorities. EU regulators may require pharmaceutical companies to conduct post-authorization safety and efficacy studies at the time of approval, or at any time afterwards in light of scientific developments. There are also additional extensive requirements regarding adverse drug reaction reporting and additional monitoring of products. Outside developed markets such as the EU and Japan, pharmacovigilance requirements vary and are generally not as extensive, but there is a trend toward increasing regulation.
Pfizer Inc. | 2019 Form 10-K | 16 |
Pricing and Reimbursement. Certain governments, including the different EU member states, the U.K., China, Japan, Canada, South Korea and some other international markets, provide healthcare at low-to-zero direct cost to consumers at the point of care and have significant power as large single payers to regulate pharmaceutical prices or patient reimbursement levels to control costs for the government-sponsored healthcare system, particularly under recent global financing pressures. Governments may use a variety of cost-containment measures for our pharmaceutical products, including price cuts, mandatory rebates, health technology assessments, forced localization as a condition of market access, “international reference pricing” (i.e., the practice of a country linking its regulated medicine prices to those of other countries), quality consistency evaluation processes and volume-based procurement. In addition, the international patchwork of price regulation and differing economic conditions and incomplete value assessments across countries has led to varying access to quality medicines in many markets and some third-party trade in our products between countries.
In particular, international reference pricing adds to the regional impact of price cuts in individual countries and hinders patient access and innovation. Price variations, exacerbated by international reference pricing systems, also have resulted from exchange rate fluctuations. The downward pricing pressure resulting from this dynamic can be expected to continue as a result of reforms to international reference pricing policies and measures targeting pharmaceuticals in some European countries.
In addition, several important multilateral organizations, such as the United Nations, including the World Health Organization (WHO), and the Organization for Economic Cooperation and Development, are increasing scrutiny of international pharmaceutical pricing through issuing reports and policy recommendations. In 2019, the WHO continued exerting pressure on pharmaceutical pricing practices by supporting strategies to reduce medicine prices, including calling for greater transparency around the cost of research and development and production of medicines, as well as disclosure of net prices.
In Japan, the pricing environment for innovative medicines further deteriorated in 2019 with the introduction of a health technology assessment (HTA) system to inform price adjustments of healthcare technologies after launch. Expansion of this system for reimbursement decisions, as seen in other HTA markets, remains a risk. While significant challenges remain, the 2020 Drug Pricing Reform Package, unlike the last reform package in 2018, is not expected to fundamentally change the access landscape. Furthermore, the eligibility criteria for the Price Maintenance Premium, a key policy that protects against price erosion for certain products, is expected to be somewhat enhanced while expedited regulatory pathways are codified in law.
In Canada, the Patented Medicine Prices Review Board (PMPRB) released draft guidelines to implement new pricing regulations in November 2019, which will go into force in July 2020. These regulations drop the U.S. from the reference basket of countries used to determine price and add economic factors for setting ceiling prices for new medicines. An initial analysis of the potential impact of these proposed changes to the PMPRB regulations estimated an approximately $26 billion reduction in industry revenues over the next decade.
China Pricing Pressures. In China, healthcare is largely driven by a public payer system, with public medical insurance as the largest single payer for pharmaceuticals, and pricing pressures have increased in recent years. Government officials have consistently emphasized the importance of improved health outcomes, the need for healthcare reform and decreased drug prices as key indicators of progress towards reform. While the government provides basic health insurance for the vast majority of Chinese citizens, that insurance is not adequate to cover many innovative medicines, and alternative funding sources for innovative medicines remain suboptimal.
In 2019, China’s government negotiated with companies to add approximately 90 innovative drugs (mainly oncology medicines) to the National Reimbursement Drug List. This builds on 60 drugs already added through negotiation in 2017 and 2018. Prices for drugs have been reduced dramatically through this government-led process. While these negotiations have included a path to access for companies, market access is not assured. In addition, significant questions about the processes and negotiations for provincial tendering remain, as well as the need for multi-layered negotiations across provincial, municipal and hospital levels.
In the off-patent space, in 2013, China began to implement a quality consistency evaluation (QCE) process in order to improve the quality of domestically-manufactured generic drugs, primarily by requiring such drugs to pass a test to assess their bioequivalence to a qualified reference drug (typically the originator drug). In 2018, numerous local generics were officially deemed bioequivalent under QCE. A pilot project for centralized volume-based procurement (VBP) was then initiated including 25 molecules of drugs covering 11 major Chinese cities. Under this procurement model, a tender process has been established where a certain portion of included molecule volumes are guaranteed to tender winners. The program is intended to contain healthcare costs by driving utilization of generics that have passed QCE, which has resulted in dramatic price cuts for off-patent medicines.
Upjohn and most off-patent originators were not successful in the first bidding process under this pilot, which was finalized in December 2018 and implemented in March 2019, and most contracts went to local generic companies. The first bidding process resulted in significant price cuts by the successful bidders, with some bidders reducing the price of their products by as much as 96 percent, as companies attempted to secure volumes on the Chinese pharmaceutical market. The drugs that lost the bidding were also requested to reduce their selling price up to 30 percent based on the price difference with the successful bidder. China’s government began nationwide expansion of the VBP pilot in December 2019. The expanded model, which is being implemented nationwide, applies to certain drugs that are purchased for public hospitals as well as some military and private medical institutions. As in the first bidding process, our Upjohn business unit and most originator brands were not successful in
Pfizer Inc. | 2019 Form 10-K | 17 |
the bidding process for this nationwide expansion, and those contracts mostly went to local Chinese generic companies. The QCE-qualified generic makers of atorvastatin and amlodipine bid aggressively, lowering prices even further from the March 2019 tender. Our Upjohn business unit continues to take steps to mitigate the revenue impact of these initiatives but anticipates that they will continue to affect our Upjohn business in China in the future. We expect to utilize our presence in the retail channel, private hospitals and tendering capabilities to mitigate some of these pricing pressures. In addition, we believe that our geographic expansion to under-penetrated and lower-tiered cities and counties and additional focus on non-tendered products will increase sales volumes in greater China and partially mitigate pressures from QCE.
In late 2019, China announced another round of expansion of the national VBP program, which covers 33 new molecules, including Biopharma’s Zithromax tablets and Diflucan tablets and no Upjohn products. Biopharma was not successful in the bidding process for this expansion.
Furthermore, the Chinese government has discussed moving toward efforts to unify the reimbursement price between QCE-approved generic medicines and the applicable original medicines. The government currently plans to implement this universal reimbursement price initiative within the next two to three years. If this policy is implemented, the new reimbursement level for Upjohn’s products will likely be lower than the current reimbursement level, placing additional pressures on price and/or patient copays. There remains uncertainty as to whether, when and how this policy may be officially implemented. The Chinese government could also enact other policies that may increase pricing pressures or have the effect of reducing the volume of sales available to Upjohn’s products. This potential policy, and any other policies like it that could increase pricing and copay pressures on Upjohn’s drug products in China, could have an adverse effect on our business, financial condition and results of operations. The government has indicated that additional post-LOE drugs could be subjected to QCE qualification in future rounds, which could also be tied to volume-based procurement. The scope of future QCE products and timing of any program expansion is currently unknown, making it difficult to determine the impact on Pfizer’s business and financial condition. We will continue to monitor the market for developments.
EU Regulatory Changes. The EU adopted a new Clinical Trials Regulation in May 2014, but its implementation has been delayed by the need for the EU authorities to establish new technical systems. This regulation is aimed at simplifying and harmonizing the administrative processes and governance of clinical trials in the EU and will require increased public posting of clinical trial results. It is currently not anticipated to be fully implemented until the first half of 2022 at the earliest.
Brexit. In June 2016, the U.K. electorate voted in a referendum to leave the EU, which is commonly referred to as “Brexit”. The U.K. left the EU on January 31, 2020 with status quo arrangements through a transition period scheduled to end on December 31, 2020. The consequences of the U.K. leaving the EU and the terms of the future trading relationship continue to be highly uncertain, which may pose certain implications to our research, commercial and general business operations in the U.K. and the EU, including the approval and supply of our products. However, both the U.K. and the EU have issued detailed guidance for the industry on how medicines, medical devices and clinical trials will be separately regulated in their respective territories. Pfizer has substantially completed its preparations for Brexit, having made the changes necessary to meet relevant regulatory requirements in the EU and the U.K., through the transition period and afterwards, especially in the regulatory, research, manufacturing and supply chain areas. Between 2018 and 2021, we expect to spend up to approximately $60 million in one-time costs to make these adaptations. For additional information on Brexit, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—The Global Economic Environment section in our 2019 Financial Report.
China Regulatory Changes. In an effort to encourage drug innovation and reduce backlogs for existing applications for drug approval, in recent years, the NMPA has unveiled numerous reform initiatives for China’s drug approval system and engaged in significant efforts to build its capabilities. The NMPA divides drugs into new drugs and generics, with the definition for new drugs changed from “China New” to “Global New.” This means that drugs previously approved in other markets (such as the U.S. or Europe) are not considered new drugs under China’s regulatory regime. This change in definition creates more opportunities for China’s domestic drug manufacturers than for multinational firms, because multinational firms have historically had significant competitive advantage in successfully achieving regulatory approvals for drugs first approved outside of China. Revisions in 2019 made clear, however, that regulatory approval from the FDA or the EMA would no longer be required for approval of imported drugs, though a notable exception persists for imported vaccines, which still require prior approval from a reference regulatory agency such as the FDA. In 2019, China published a revision to its Drug Administration Law and introduced a “marketing authorization holder” system, which grants the NMPA more authority over regulating manufacturers and provides manufacturers more flexibility in contract manufacturing arrangements and manufacturing site transfers.
While challenges remain, a number of other policy changes are streamlining and accelerating approvals of domestic and imported drugs in China. These reforms, along with China’s June 2018 elevation to the ICH Management Committee, are expected to pave the way for integration of Chinese regulations with global practices. These changes include introducing more streamlined processes for maintaining renewal of product registrations, reduction in importing testing requirements, and establishing an expedited registration pathway for drugs to treat rare diseases and serious, life-threatening illnesses with no effective treatment. Though certain details on implementation are unclear (e.g., evolving list of qualified rare diseases and no guidance on what qualifies as serious, life threatening), the NMPA aims to build expedited pathways for certain categories of products similar to the U.S. and European regulatory systems. Additionally, the NMPA published changes to China’s registration requirements that align more with international practices, including a 60-day review timeline for clinical trial authorizations and
Pfizer Inc. | 2019 Form 10-K | 18 |
guidance for acceptance of foreign clinical data and the utilization of real world data in drug development and regulatory decision making.
Although a number of regulatory changes better support China’s inclusion in simultaneous global drug development, unique regulatory requirements continue to pose challenges for multinational companies, including China’s Human Genetic Resources process for exporting clinical trial samples (which adds months to starting a clinical trial in China); mismatched China Pharmacopoeia and manufacturing data requirements that require standards exceeding acceptable practices in the U.S., EU, and Japan; and unpredictable and inconsistent clinical trial inspection practices.
Healthcare Provider Transparency and Disclosures. A number of countries have implemented laws requiring (or their industry associations have recommended) disclosure of transfers of value made by pharmaceutical companies to healthcare providers. For example, the European Federation of Pharmaceutical Industries and Associations’ disclosure code requires all members, including Pfizer, to disclose transfers of value to healthcare professionals and healthcare organizations.
Intellectual Property. The World Trade Organization Agreement on Trade Related Aspects of Intellectual Property Rights (WTO-TRIPS) required participant countries to amend their intellectual property laws to provide patent protection for pharmaceutical products by 2005, with an extension until 2033 for least-developed countries. While we still face patent grant, enforcement and other intellectual property challenges around the world, some countries have made improvements. We include stronger patent protection among the factors we consider for continued business expansion in other participant countries.
While the global intellectual property environment has generally improved following WTO-TRIPS and bilateral/multilateral trade agreements, our future business growth depends on further progress in intellectual property protection. In emerging market countries in particular, governments have used intellectual property policies as a tool to force innovators to accept less than fair value for medicines, as well as to protect their local pharmaceutical industries. Considerable political and economic pressure exists to weaken current intellectual property protection and resist implementation of any further protection, which has led to policies such as more restrictive standards for obtaining patents and more difficult procedures for patenting biopharmaceutical inventions, restrictions on patenting certain types of inventions (e.g., new medical treatment methods), revocation of patents, laws or regulations that promote or provide broad discretion to issue a compulsory license, weak intellectual property enforcement and failure to implement effective regulatory data protection. Our industry advocacy efforts focus on seeking a more balanced business environment for foreign manufacturers, as well as on underscoring the importance of strong intellectual property systems for local innovative industries and helping improve patients’ access to innovative medicines. In developed countries as well, including the EU, we are facing an increasingly challenging intellectual property environment.
As part of the Canada/EU Comprehensive Economic & Trade Agreement (CETA), Canada now provides sui generis protection, commonly referred to as patent term restoration, for patent term extensions for basic patents; however, the extension is capped at two years, whereas the international norm is five years. In addition, the implementing regulations may create obstacles for patentees applying for patent term restoration via a Certificate of Supplementary Protection (CSP), and Canada’s proposed drug pricing reforms may negatively impact the benefit of a CSP. Furthermore, the United States-Mexico-Canada Agreement (USMCA) will, when implemented, require Canada and Mexico to make certain improvements to their current intellectual property regimes, including the establishment of patent term adjustment for unreasonable delays in the grant of patents.
In China, the intellectual property environment has improved in recent years, although effective enforcement and adequate legal remedies remain areas of concern. The government has taken steps to protect intellectual property rights in conformity with World Trade Organization provisions, although China remained on the U.S. Trade Representative’s Priority Watch List for 2019 due to ongoing enforcement challenges and China’s failure to make certain structural reforms. Further, the standards for patentability in China remain more restrictive than in other major markets, including the U.S., Europe and Japan. Also, while a framework exists for protecting patents for 20 years, enforcement mechanisms are often lacking or inconsistent. For example, the absence of effective patent linkage mechanisms and preliminary injunctions, impractical evidentiary burdens, and heightened sufficiency standards have been used to invalidate patents at the enforcement stage. In 2019, the regulatory authority granted marketing approval to generic products while the reference product in each case are still subject to patent protection, and there is no effective legal means to resolve patent disputes prior to the marketing of those infringing drugs. The U.S. and China recently signed an initial agreement in which China has committed to address some patent-related concerns, and both governments have indicated that they will continue bilateral discussions on implementation of these commitments and other intellectual property issues in 2020.
In Brazil and other Latin American countries, the role of health regulatory authorities in reviewing patents (e.g., National Health Surveillance Agency in Brazil), restrictive patentability rules, ambiguity regarding the term of certain patents and backlogs at patent agencies may limit our ability to protect our products through patents. The lack of regulatory data protection and difficulties in protecting certain types of inventions, such as new medical uses of drug products, may limit the commercial lifespan of some pharmaceutical products. Additionally, an increased threat of issuance of compulsory licenses for biopharmaceutical products exists, which adds to business uncertainty.
In India, we have seen some progress in terms of expediting patent approval processes to reduce pendency rates and implementing training programs to enhance enforcement. Despite these positive steps, gaps remain in terms of addressing longstanding intellectual property concerns. For example, policies favoring compulsory licensing of patents, the tendency of the
Pfizer Inc. | 2019 Form 10-K | 19 |
Indian Patent Office to revoke pharmaceutical patents in opposition proceedings (both pre- and post-grant), and restrictive standards for patentability of pharmaceutical products have made it difficult to safeguard many of our inventions and our investments in innovation. These policies heighten the risk of additional patent challenges targeting innovative pharmaceutical products, especially in areas perceived as being important to the public health of the population. Challenges against Pfizer patents in India are ongoing.
Data Privacy. Outside of the U.S., many countries where we conduct business, including the EU, have privacy and data security laws and regulations concerning the collection and use of personal data, and we must comply with these laws and regulations as well. One applicable law is the EU’s General Data Protection Regulation (GDPR). The GDPR imposes detailed obligations on companies that collect, use, or otherwise process personal data and penalties for noncompliance may include fines of up to 4 percent of the company’s global annual revenue. Additionally, the legislative and regulatory framework for privacy and data protection issues worldwide is rapidly evolving as countries continue to adopt privacy and data security laws. Any inability to comply with applicable laws, regulations, policies, industry standards or other legal obligations regarding data protection or privacy could result in additional costs and liability to Pfizer as well as reputational harm and may adversely affect our business.
ENVIRONMENTAL MATTERS
Most of our operations are affected by national, state and/or local environmental laws. We have made, and intend to continue to make, the expenditures necessary for compliance with applicable laws. We also are cleaning up environmental contamination from past industrial activity at certain sites. See the Notes to Consolidated Financial Statements—Note 16A3. Contingencies and Certain Commitments—Legal Proceedings––Commercial and Other Matters in our 2019 Financial Report. As a result, we incurred capital and operational expenditures in 2019 for environmental compliance purposes and for the clean-up of certain past industrial activity as follows:
• | environment-related capital expenditures— $31 million; and |
• | other environment-related expenses— $136 million. |
While capital expenditures or operating costs for environmental compliance cannot be predicted with certainty, we do not currently anticipate they will have a material effect on our capital expenditures or competitive position.
Climate change presents risks to our operations, including the potential for additional regulatory requirements and associated costs, and the potential for more frequent and severe weather events and water availability challenges that may impact our facilities and those of our suppliers. For example, in 2017, our manufacturing and commercial operations in Puerto Rico were impacted by hurricanes as our three manufacturing sites in Puerto Rico sustained damage and became inoperable due to issues impacting Puerto Rico overall. All three sites resumed operations, and remediation activities were completed in 2018. We cannot provide assurance that physical risks to our facilities and supply chain due to climate change will not occur in the future; however, we have a program for reviewing our vulnerability to potential weather-related risks and other natural disasters and we update our assessments periodically. To date, we have concluded that, because of our facility locations, our existing distribution networks and our controls, we do not anticipate that these risks will have a material impact on Pfizer in the near term.
TAX MATTERS
The discussion of tax-related matters in the Notes to Consolidated Financial Statements—Note 5. Tax Matters in our 2019 Financial Report is incorporated by reference.
EMPLOYEES
In our innovation-intensive business, our employees are vital to our success. We generally believe we have good relationships with our employees. As of December 31, 2019, we employed approximately 88,300 people in our operations throughout the world.
DISCLOSURE PURSUANT TO SECTION 219 OF THE IRAN THREAT REDUCTION AND SYRIA HUMAN RIGHTS ACT OF 2012
Section 219 of the Iran Threat Reduction and Syria Human Rights Act of 2012 (ITRSHRA) requires disclosure by public companies of certain transactions involving the Government of Iran, as well as entities and individuals designated under Executive Order 13382 and Executive Order 13224.
As a global biopharmaceutical company, we conduct business in multiple jurisdictions throughout the world. During 2019, our activities included supplying medicine and medical products (Pfizer products) for patient and consumer use in Iran. We ship Pfizer products to Iran, and conduct related activities, in accordance with licenses issued by the U.S. Department of the Treasury’s Office of Foreign Assets Control and other U.S. and non-U.S. governmental entities, and in line with our corporate policies. We will continue our global activities to improve the health and well-being of patients and consumers in a manner consistent with applicable laws and our corporate policies. To our knowledge, none of our activities during 2019 are required to be disclosed pursuant to ITRSHRA.
Pfizer Inc. | 2019 Form 10-K | 20 |
ITEM 1A. | RISK FACTORS |
The statements in this Section describe the major risks to our business and should be considered carefully. In addition, these statements constitute our cautionary statements under the Private Securities Litigation Reform Act of 1995.
Our disclosure and analysis in this 2019 Form 10-K and in our 2019 Annual Report to Shareholders contain forward-looking statements. From time to time, we also provide forward-looking statements in other materials we release to the public, as well as oral forward-looking statements. Such forward-looking statements involve substantial risks and uncertainties. We have tried, wherever possible, to identify such statements by using words such as “will,” “may,” “could,” “likely,” “ongoing,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “assume,” “target,” “forecast,” “guidance,” “goal,” “objective,” “aim,” “seek” and other words and terms of similar meaning or by using future dates in connection with any discussion of, among other things, our anticipated operating and financial performance, business plans and prospects, expectations for our product pipeline, in-line products and product candidates, including anticipated regulatory submissions, data read-outs, study starts, approvals, revenue contribution, growth, performance, timing of exclusivity and potential benefits, strategic reviews, capital allocation objectives, plans for and prospects of our acquisitions and other business-development activities, benefits anticipated from the reorganization of our commercial operations in 2019, sales efforts, expenses, interest rates, foreign exchange rates, the outcome of contingencies, such as legal proceedings, government regulation, our ability to successfully capitalize on growth opportunities or prospects, manufacturing and product supply and plans relating to share repurchases and dividends. In particular, these include statements relating to future actions, including, among others, the expected timing, benefits, charges and/or costs in connection with our agreement to combine Upjohn with Mylan to create a new global pharmaceutical company, Viatris, set forth in the Item 1. Business––About Pfizer and Item 1A. Risk Factors––Pending Combination of Upjohn with Mylan sections in this 2019 Form 10-K and the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Business Development Initiatives and ––Our Strategy sections and the Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies––Basis of Presentation in our 2019 Financial Report; the expected impact of patent expiries on our business set forth in the Item 1. Business––Patents and Other Intellectual Property Rights section in this 2019 Form 10-K and in the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Operating Environment––Industry-Specific Challenges––Intellectual Property Rights and Collaboration/Licensing Rights section in our 2019 Financial Report; the expected competition from certain generic manufacturers in China in the Item 1. Business––Competition––Generic Products and Item 1A. Risk Factors––Generic Competition sections in this 2019 Form 10-K; the anticipated costs related to our preparations for Brexit set forth in the Item 1. Business––Government Regulation and Price Constraints––Outside the United States––Brexit section in this 2019 Form 10-K and the Overview of Our Performance, Operating Environment, Strategy and Outlook––The Global Economic Environment section in our 2019 Financial Report; the availability of raw materials for 2020 set forth in Item 1. Business––Raw Materials in this 2019 Form 10-K; the expected pricing pressures on our products in the U.S. and internationally and the anticipated impact to our business set forth in the Item 1. Business––Government Regulation and Price Constraints and Item 1A. Risk Factors––Pricing and Reimbursement sections in this 2019 Form 10-K; the anticipated impact of climate change on Pfizer set forth in Item 1. Business––Environmental Matters in this 2019 Form 10-K; the expected demerger of the GSK Consumer Healthcare joint venture set forth in the Item 1A. Risk Factors––Consumer Healthcare Joint Venture with GSK section in this 2019 Form 10-K; the benefits expected from the reorganization of our commercial operations in 2019 and our expectations regarding growth set forth in the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Strategy––Organizing for Growth section in our 2019 Financial Report; our anticipated liquidity position set forth in the Overview of Our Performance, Operating Environment, Strategy and Outlook—The Global Economic Environment and the Analysis of Financial Condition, Liquidity and Capital Resources sections in our 2019 Financial Report; the anticipated costs and savings from certain of our initiatives, including Transforming to a More Focused Company initiative, set forth in the Overview of Our Performance, Operating Environment, Strategy and Outlook—Transforming to a More Focused Company and Costs and Expenses—Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives sections and the Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives in our 2019 Financial Report; our plans for increasing investment in the U.S. set forth in the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Strategy––Capital Allocation and Expense Management––Increasing Investment in the U.S. section in our 2019 Financial Report; the financial guidance set forth in the Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Financial Guidance for 2020 section in our 2019 Financial Report; the expected impact of the Advisory Committee on Immunization Practices recommendation for Prevnar 13 for adults 65 and older on Prevnar 13’s revenues set forth in the Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion––Prevnar 13/Prevenar 13 (Biopharma) section in our 2019 Financial Report; the expected impact of updates to the prescribing information for Xeljanz on its growth set forth in the Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion––Xeljanz (Biopharma) section in our 2019 Financial Report; the benefits expected from our business development transactions; the planned capital spending set forth in the Analysis of Financial Condition, Liquidity and Capital Resources—Selected Measures of Liquidity and Capital Resources—Contractual Obligations section in our 2019 Financial Report; the expected payments to our unfunded U.S. supplemental (non-qualified) pension plans, postretirement plans and deferred compensation plans and expected funding obligations set forth in the Analysis of Financial Condition, Liquidity and Capital Resources—Selected Measures of Liquidity and Capital Resources—Contractual Obligations section; and the voluntary contribution we expect to make during 2020 for the U.S. qualified plans set forth in the Notes to Consolidated Financial Statements—Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans in our 2019 Financial Report.
Pfizer Inc. | 2019 Form 10-K | 21 |
We cannot guarantee that any forward-looking statement will be realized. Achievement of anticipated results is subject to substantial risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. You should bear this in mind as you consider forward-looking statements, and you are cautioned not to put undue reliance on forward-looking statements.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects. Also note that we provide the following cautionary discussion of risks, uncertainties and possibly inaccurate assumptions relevant to our businesses. These are factors that, individually or in the aggregate, may cause our actual results to differ materially from expected, projected or historical results. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
RISKS RELATED TO OUR BUSINESS, INDUSTRY AND OPERATIONS:
MANAGED CARE TRENDS
Private third-party payers, such as health plans, and other managed care entities, such as PBMs, continue to take action to manage the utilization of drugs and control the cost of drugs. Consolidation among MCOs has increased the negotiating power of MCOs and other private third-party payers. Private third-party payers, as well as governments, increasingly employ formularies to control costs by taking into account discounts in connection with decisions about formulary inclusion or favorable formulary placement. Failure to obtain or maintain timely or adequate pricing or favorable formulary placement for our products, or failure to obtain such formulary placement at favorable pricing, could adversely impact revenue. Private third-party payers often implement formularies with copayment tiers to encourage utilization of certain drugs and have also been raising co-payments required from beneficiaries, particularly for branded pharmaceuticals and biotechnology products. Private third-party payers are also implementing new initiatives like so-called “copay accumulators” (policies that provide that the value of copay assistance does not count as out-of-pocket costs that are applied toward deductibles) that can shift more of the cost burden to manufacturers and patients. This cost shifting has increased consumer interest and input in medication choices, as they pay for a larger portion of their prescription costs and may cause consumers to favor lower cost generic alternatives to branded pharmaceuticals. Third-party payers also use additional measures such as new-to-market blocks, exclusion lists, indication-based pricing, and value-based pricing/contracting to improve their cost containment efforts, and are also increasingly imposing utilization management tools, such as clinical protocols, requiring prior authorization for a branded product if a generic product is available or requiring the patient to first fail on one or more generic products before permitting access to a branded medicine. As the U.S. private third-party payer market consolidates further and as more drugs become available in generic form, biopharmaceutical companies may face greater pricing pressure from private third-party payers, who will continue to drive more of their patients to use lower cost generic alternatives.
GENERIC COMPETITION
Competition from manufacturers of generic drugs is a major challenge for our branded products around the world, and the loss or expiration of intellectual property rights can have a significant adverse effect on our revenues. In addition, our patented products may face generic competition before patent exclusivity expires, including upon the “at-risk” launch (despite pending patent infringement litigation against the generic product) by a manufacturer of a generic version of one of our patented products. Generic competition could lead to our loss of a major portion of revenues for that product in a very short period of time. A number of our products have experienced significant generic competition over the last few years. For example, Lyrica (a product in our Upjohn business) lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. Also, the basic product patent for Chantix in the U.S. will expire in November 2020. In China, we are expected to face further intensified competition by certain generic manufacturers, which may result in price cuts and volume loss of some of our products.
Also, generic manufacturers have filed applications with the FDA seeking approval of product candidates that such companies claim do not infringe our patents or that our patents are not valid; these include candidates that would compete with, among other products, Eliquis, Ibrance and Xeljanz. Our licensing and collaboration partners also face challenges by generic drug manufacturers to patents covering products for which we have licenses or co-promotion rights. In addition, our patent-protected products may face competition in the form of generic versions of competitors’ branded products that lose their market exclusivity.
Pfizer Inc. | 2019 Form 10-K | 22 |
COMPETITIVE PRODUCTS
We cannot predict with accuracy the timing or impact of the introduction of competitive products, including new product entrants, in-line branded products, generic products, private label products, biosimilars and product candidates that treat diseases and conditions similar to those treated by our in-line drugs and drug candidates. The introduction of competitive products can result in erosion of the sales of our existing products and potential sales of products in development, as well as unanticipated product obsolescence. Competitive product launches have occurred in recent years, and certain potentially competitive products are in various stages of development. Some of these have been filed for approval with the FDA and with regulatory authorities in other countries.
We also produce generic and biosimilar pharmaceutical products that compete with products from competitors, including other generic and biosimilar manufacturers. The ability to launch a generic or biosimilar pharmaceutical product at or before the anticipated formation of the generic or biosimilar marketplace is important to that product’s profitability. With increasing competition in the generic or biosimilar product markets, our success will depend on our ability to bring new products to market quickly. The FDA, along with other regulatory agencies around the world, has been experiencing a backlog of generic drug applications, which may result in delayed approvals of new generic products over the next few years. Also, we may face access challenges for our biosimilar products where our product may not receive appropriate coverage/reimbursement access or remains in a disadvantaged position relative to the innovator product. For example, Inflectra has experienced access challenges among commercial payers. In September 2017, Pfizer filed suit in the U.S. District Court for the Eastern District of Pennsylvania against Johnson & Johnson (J&J) alleging that J&J’s exclusionary contracts and other anticompetitive practices concerning Remicade® (infliximab) violate federal antitrust laws.
DEPENDENCE ON KEY IN-LINE PRODUCTS
We recorded direct product and/or alliance revenues of more than $1 billion for each of eight biopharmaceutical products in 2019: Prevnar 13/Prevenar 13, Ibrance, Eliquis, Lyrica, Xeljanz, Lipitor, Enbrel and Chantix/Champix. Those products accounted for 49% of our total revenues in 2019. If these products or any of our other major products were to become subject to problems such as loss of patent protection (if applicable), changes in prescription growth rates, material product liability litigation, unexpected side effects, regulatory proceedings, publicity affecting doctor or patient confidence, pressure from existing competitive products, changes in labeling, pricing and access pressures, supply shortages or, if a new, more effective treatment should be introduced, the adverse impact on our revenues could be significant. A number of our products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and patents covering a number of our best-selling medicines are, or have been, the subject of pending legal challenges. For example, as a result of a patent litigation settlement, Teva Pharmaceuticals USA, Inc. launched a generic version of Viagra (a product in our Upjohn business) in the U.S. in December 2017. In addition, Lyrica (a product in our Upjohn business) lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. Also, the basic product patent for Chantix in the U.S. will expire in November 2020. In addition, our revenues could be significantly impacted by the timing and rate of commercial acceptance of key new products. For additional information, see the Item 1. Business––Patents and Other Intellectual Property Rights section in this 2019 Form 10-K. Further, our Alliance revenues will be adversely affected by the termination or expiration of collaboration and co-promotion agreements that we have entered into and that we may enter into from time to time.
RESEARCH AND DEVELOPMENT INVESTMENT
The discovery and development of safe, effective new products, as well as the development of additional uses for existing products, are necessary for the continued strength of our businesses. Our product lines must be replenished over time in order to offset revenue losses when products lose their market exclusivity, as well as to provide for earnings growth. Our growth potential depends in large part on our ability to identify and develop new products or new indications for existing products that address unmet medical needs and receive reimbursement from payers, either through internal R&D or through collaborations, acquisitions, joint ventures or licensing or other arrangements with third parties. However, balancing current growth, investment for future growth and the delivery of shareholder return remains a major challenge. The average costs of product development continue to rise, as do the regulatory requirements in many therapeutic areas, which may affect the number of candidates funded as well as the sustainability of the R&D portfolio. Our ongoing investments in new product introductions and in R&D for new products and existing product extensions could exceed corresponding sales growth.
Additionally, our R&D investment plans and resources may not be correctly matched between science and markets, and failure to invest in the right technology platforms, therapeutic segments, product classes, geographic markets and/or in-licensing and out-licensing opportunities could adversely impact the productivity of our pipeline. Further, even if the areas with the greatest market attractiveness are identified, the scientific approach may not succeed for any given program despite the significant investment required for R&D, and the commercial potential of the product may not be as competitive as expected because of the highly dynamic market environment and the hurdles in terms of access and reimbursement.
We continue to strengthen our global R&D organization and pursue strategies intended to improve innovation and overall productivity in R&D to achieve a sustainable pipeline that is positioned to deliver value in the near-term and over time. These strategies may not deliver the desired result, which could affect growth and profitability in the future.
Pfizer Inc. | 2019 Form 10-K | 23 |
BIOSIMILARS
Abbreviated legal pathways for the approval of biosimilars exist in many international markets and, since the passage of the ACA, a framework for such approval exists in the U.S. If competitors are able to obtain marketing approval for biosimilars referencing our biologic products, our biologic products may become subject to competition from these biosimilars, with attendant competitive pressure, and price reductions could follow. For example, Enbrel faces ongoing biosimilar competition in most European markets. The loss of patent rights, due to patent expiration or litigation, could trigger competition.
We are developing and commercializing biosimilar medicines. Risks related to our commercialization of biosimilars include the potential for steeper than anticipated price erosion due to increased competitive intensity, coupled with intellectual property challenges that may preclude timely commercialization of our potential biosimilar products. There is also a risk of lower uptake for biosimilars due to various factors that may vary for different biosimilars (e.g., anti-competitive practices, physician reluctance to prescribe biosimilars for existing patients taking the originator product, or misaligned financial incentives). See also the Competitive Products risk factor above.
RESEARCH STUDIES
Decisions about research studies made early in the development process of a drug or vaccine candidate can have a substantial impact on the marketing strategy and payer reimbursement possibilities if it receives regulatory approval. For example, a wider range of studies can lead to approval for a broader set of indications that may impact the marketing and payer reimbursement process. However, each additional indication and its reimbursement potential must be balanced against the time and resources required to demonstrate benefit, the increased complexity of development and manufacturing and the potential delays to approval of the lead indication. We try to plan clinical trials prudently and to reasonably anticipate and address challenges, but there is no guarantee that an optimal balance between trial conduct, speed and desired outcome will be achieved each time. The degree to which such potential challenges are foreseen and adequately addressed could affect our future results.
INTERNATIONAL OPERATIONS
Our international operations could be affected by currency fluctuations, capital and exchange controls, economic conditions, expropriation and other restrictive government actions, changes in intellectual property legal protections and remedies, trade regulations and procedures and actions affecting approval, production, pricing, and marketing of, reimbursement for and access to our products, as well as by political unrest, unstable governments and legal systems and inter-governmental disputes. Any of these changes could adversely affect our business.
Many emerging markets have experienced growth rates in excess of developed markets, leading to an increased contribution to the industry’s global performance. As a result, we have been employing strategies to grow in emerging markets. However, our strategies in emerging markets may not be successful and these countries may not continue to sustain these growth rates. For example, even though China is growing faster than most emerging markets, we face certain challenges in China due to government imposed pricing controls affecting certain Pfizer medicines. In addition, some emerging market countries may be particularly vulnerable to periods of financial or political instability or significant currency fluctuations or may have limited resources for healthcare spending. Even though we constantly monitor the evolving emerging markets for any unanticipated risk to Pfizer, certain financial or political events in such markets can adversely affect our results.
SPECIALTY PHARMACEUTICALS
Specialty pharmaceuticals are medicines that treat rare or life-threatening conditions that typically have smaller patient populations. The growing availability and use of innovative specialty pharmaceuticals, combined with their relative higher cost as compared to other types of pharmaceutical products, has generated payer interest in developing cost-containment strategies targeted to this sector. The impact of payers’ efforts to control access to and pricing of specialty pharmaceuticals is increasing. A number of factors create a more challenging paradigm for Pfizer given our growing specialty business portfolio such as formulary restrictions and increasing use of utilization management tools such as step edits, which can lead to higher negotiated rebates or discounts to health plans and PBMs in the U.S., as well as the increasing use of health technology assessments and government pressures in markets around the world.
PRODUCT MANUFACTURING, SALES AND MARKETING RISKS
Difficulties or delays in product manufacturing, sales or marketing could affect future results through regulatory actions, shut-downs, work stoppages or strikes, approval delays, withdrawals, recalls, penalties, supply disruptions, shortages or stock-outs, reputational harm, product liability or unanticipated costs. Examples of such difficulties or delays include, but are not limited to, the inability to increase production capacity commensurate with demand; the failure to predict market demand for, or to gain market acceptance of, approved products; the possibility that the supply of component materials is delayed or unavailable and that the quality of such materials are substandard and not detected; the possibility that we may fail to maintain appropriate quality standards throughout our internal and external supply network and/or comply with cGMPs and other applicable regulations such as serialization (which allows for track and trace of products in the supply chain to enhance patient safety);
Pfizer Inc. | 2019 Form 10-K | 24 |
risks to supply chain continuity and commercial operations as a result of natural (including hurricanes, earthquakes and floods) or man-made disasters (including arson or terrorist attacks) at our facilities or at a supplier or vendor, including those that may be related to climate change; failure to maintain the integrity of our supply chains against economic adulteration, product diversion, product theft, counterfeit goods and cyberattacks. As an example, we have been experiencing production issues with Genotropin that will decrease revenue from that product.
Regulatory agencies periodically inspect our drug manufacturing facilities to evaluate compliance with cGMP or other applicable requirements. Failure to comply with these requirements may subject us to possible legal or regulatory actions, such as warning letters, suspension of manufacturing, seizure of product, injunctions, debarment, recall of a product, delays or denials of product approvals, import bans or denials of import certifications, any of which could have a material adverse effect on our business, financial condition and results of operations. In February 2017, for example, we received a warning letter from the FDA communicating the FDA’s view that certain violations of cGMP regulations exist at Hospira’s manufacturing facility in McPherson, Kansas. We undertook corrective actions to address the concerns raised by the FDA. In January 2018, the FDA upgraded the status of Pfizer’s McPherson manufacturing facility to VAI based on an October 2017 inspection. The change to VAI status lifted the compliance hold that the FDA placed on approval of pending applications. In June 2018, the FDA informed us that it had completed an evaluation of corrective actions and closed out the February 2017 warning letter issued to our McPherson manufacturing facility after determining that we had addressed the violations contained in the warning letter. In July-August 2018, the FDA conducted a follow-up inspection of our McPherson facility and issued an inspection report noting several findings. Pfizer responded to the FDA’s findings, and is in the process of implementing a corrective and preventive action plan to address the FDA’s concerns. On the basis of the July-August 2018 FDA inspection, the FDA changed the inspection classification of the McPherson site to Official Action Indicated (OAI). Future FDA inspections and regulatory activities will further assess the adequacy and sustainability of these corrections implemented at the site. Communication with the FDA on the status of the McPherson site is ongoing. As a result of the current OAI classification, the FDA may refuse to grant premarket approval of applications and/or the FDA may refuse to grant export certificates related to products manufactured at our McPherson site until the site status is upgraded, which upgrade would be based on a re-inspection by the FDA. We have been experiencing shortages of products from the legacy Hospira portfolio, among others, largely driven by capacity constraints, technical issues, supplier quality concerns or unanticipated increases in demand. We have made considerable progress in remediating issues at legacy Hospira facilities manufacturing sterile injectables and have substantially improved supply from most of these sites. Continuing product shortage interruption at these manufacturing facilities could negatively impact our financial results.
In addition, in September 2017, Meridian Medical Technologies, Inc., a subsidiary of Pfizer Inc., received a warning letter from the FDA asserting the FDA’s view that certain violations of cGMP and Quality System Regulations exist at Meridian’s manufacturing sites in St. Louis, Missouri and classifying the site as OAI. Meridian responded to the warning letter and committed to making improvements across the sites. We have made considerable progress addressing the concerns raised by the FDA, and communication with the FDA is ongoing. Future FDA inspections and regulatory activities will further assess the adequacy and sustainability of these corrections implemented at the site. As a result of the OAI classification, the FDA may refuse to grant premarket approval of applications and/or the FDA may refuse to grant export certificates related to products manufactured at our St. Louis sites.
COLLABORATIONS AND OTHER RELATIONSHIPS WITH THIRD PARTIES
We depend on third-party collaborators, service providers, and others in the research, development, manufacturing and commercialization of our products and product candidates and also enter into joint ventures and other business development transactions in connection with our business. To achieve expected longer term benefits, we may make substantial upfront payments in such transactions, which may negatively impact our reported earnings. We rely heavily on these parties for multiple aspects of our drug development, manufacturing and commercialization activities, but we do not control many aspects of those activities. We also outsource certain services to other parties, including transaction processing, accounting, information technology, manufacturing, clinical trial recruitment and execution, clinical lab services, non-clinical research, safety services, integrated facilities management and other areas. Failure by one or more of these third parties to complete activities on schedule or in accordance with our expectations; failure by one or more of these parties to meet their contractual or other obligations to Pfizer; failure of one or more of these parties to comply with applicable laws or regulations; or any disruption in the relationships between Pfizer and one or more of these third parties, could delay or prevent the development, approval, manufacturing or commercialization of our products and product candidates, could expose us to suboptimal quality of service delivery or deliverables, could result in repercussions such as missed deadlines or other timeliness issues, erroneous data and supply disruptions, and could also result in non-compliance with legal or regulatory requirements or industry standards or reputational harm, all with potential negative implications for our product pipeline and business.
BIOPHARMACEUTICAL WHOLESALERS
In 2019, our largest biopharmaceutical wholesaler accounted for approximately 16% of our total revenues (and approximately 32% of our total U.S. revenues), and our top three biopharmaceutical wholesalers accounted for approximately 37% of our total revenues (and approximately 79% of our total U.S. revenues). If one of our significant biopharmaceutical wholesalers should encounter financial or other difficulties, such wholesaler might decrease the amount of business that it does with us, and we might be unable to collect all the amounts that the wholesaler owes us on a timely basis or at all, which could negatively impact
Pfizer Inc. | 2019 Form 10-K | 25 |
our results of operations. In addition, we expect that consolidation and integration of pharmacy chains and wholesalers will increase competitive and pricing pressures on pharmaceutical manufacturers, including us.
BUSINESS DEVELOPMENT ACTIVITIES
We expect to continue to enhance our in-line products and product pipeline through various forms of business development, which can include alliances, licenses, joint ventures, collaborations, equity- or debt-based investments, dispositions, divestments, mergers and acquisitions. However, these enhancement plans are subject to the availability and cost of appropriate opportunities, competition from other pharmaceutical companies that are seeking similar opportunities and our ability to successfully identify, structure and execute transactions, including the ability to satisfy the conditions to closing of announced transactions in the anticipated timeframes or at all, and successfully integrate acquisitions. Pursuing these opportunities may require us to obtain additional equity or debt financing, and could result in increased leverage and/or a downgrade of our credit ratings. Where we acquire debt or equity securities as all or part of the consideration for business development activities, such as in connection with our contribution agreement entered into with Allogene Therapeutics, Inc., the value of those securities will fluctuate, and may depreciate in value. We may not control the company in which we acquire securities, such as in connection with a divestiture or collaborative arrangement, and as a result, we will have limited ability to determine its management, operational decisions and policies. Further, while we seek to mitigate risks and liabilities of such transactions through, among other things, due diligence, there may be risks and liabilities that such due diligence efforts fail to discover, that are not disclosed to us, or that we inadequately assess. Legal proceedings or regulatory issues often arise as a result of activities that occurred at acquired companies, their partners and other third parties. In 2016, for example, we paid $784.6 million to resolve allegations related to Wyeth’s reporting of prices to the government with respect to Protonix for activities that occurred prior to our acquisition of Wyeth. For these and other reasons, we may not realize the anticipated benefits of such transactions, and expected synergies and accretion may not be realized within the expected timeframes, or at all.
COUNTERFEIT PRODUCTS
A counterfeit medicine is one that has been deliberately and fraudulently mislabeled as to its identity and source. A counterfeit Pfizer medicine, therefore, is one manufactured by someone other than Pfizer, but which appears to be the same as an authentic Pfizer medicine. The prevalence of counterfeit medicines is a significant and growing industry-wide issue due to a variety of factors, including, but not limited to, the following: the widespread use of the Internet, which has greatly facilitated the ease by which counterfeit medicines can be advertised, purchased and delivered to individual patients; the availability of sophisticated technology that makes it easier for counterfeiters to make counterfeit medicines; the growing involvement in the medicine supply chain of under-regulated wholesalers and repackagers; the lack of adequate inspection at certain international postal facilities as counterfeit medicines are increasingly delivered direct to customers in small parcel packages; the tendency to misuse and abuse medicines; and the relatively modest risk of penalties faced by counterfeiters compared to the large profits that can be earned by them from the sale of counterfeit medicines. Further, laws against pharmaceutical counterfeiting vary greatly from country to country, and the enforcement of existing law varies greatly from jurisdiction to jurisdiction. For example, in some countries, pharmaceutical counterfeiting is not a crime; in others, it may result in only minimal sanctions. In addition, those involved in the distribution of counterfeit medicines use complex transport routes in order to evade customs controls by disguising the true source of their products.
Pfizer’s global reputation makes its medicines prime targets for counterfeiting organizations. Counterfeit medicines continue to pose a significant risk to patient health and safety because of the conditions under which they are manufactured—often in unregulated, unlicensed, uninspected and unsanitary sites—as well as the lack of regulation of their contents. Counterfeiters have been recently evolving to counterfeit life sustaining medications such as oncology medicines. This shift significantly increases the risk to patients who, for instance, unsuspectingly purchase counterfeit oncology medications from illicit online “pharmacies” operated by criminal counterfeiting organizations. Failure to mitigate this new threat posed by counterfeit biopharma medicines could adversely impact our business, by, among other things, causing the loss of patient confidence in the Pfizer name and in the integrity of our medicines, potentially resulting in lost sales, product recalls, and an increased threat of litigation.
We have an enterprise-wide strategy to counteract the threats associated with counterfeit medicines, and focused on educating patients and health care providers to reduce demand through awareness; increasing engagement and education of global law enforcement, customs and regulatory agencies about the growing prevalence of counterfeit life sustaining medicines; enhancing online identification and disruption efforts in partnership with pharmaceutical associations to optimize resources and impact; educating legislators about the risk to the security of the international drug supply chain by illicit manufacturing and distribution networks operated by transnational criminal organizations; supporting efforts by law enforcement authorities to prosecute counterfeiters; assessing new and existing technologies to seek to make it more difficult for counterfeiters to copy our products and easier for patients and healthcare providers to distinguish authentic from counterfeit medicines; and using data analytics and risk assessment tools to better target the factors that give rise to the counterfeiting problem in the first place. However, our efforts and the efforts of others may not be entirely successful, and the presence of counterfeit medicines may continue to increase.
Pfizer Inc. | 2019 Form 10-K | 26 |
RISKS RELATED TO GOVERNMENT REGULATION AND LEGAL PROCEEDINGS:
PRICING AND REIMBURSEMENT
U.S. and international governmental regulations that mandate price controls and limitations on patient access to our products or establish prices paid by government entities or programs for our products impact our business, and our future results could be adversely affected by changes in such regulations or policies.
In the U.S., many of our products are subject to increasing pricing pressures. Pharmaceutical product pricing is subject to enhanced government and public scrutiny and calls for reform. Some states have implemented, and other states are considering, pharmaceutical price controls or patient access constraints under the Medicaid program, and some states are considering price-control regimes that would apply to broader segments of their populations that are not Medicaid-eligible. There have also been recent state legislative efforts to address drug costs, which generally have focused on increasing transparency around drug costs or limiting drug prices. Efforts by government officials or legislators to implement measures to regulate prices or payment for pharmaceutical products, including legislation on drug importation, could adversely affect our business if implemented. See the discussion regarding pricing and reimbursement in the Item 1. Business—Government Regulation and Price Constraints—In the United States—Pricing and Reimbursement section in this 2019 Form 10-K.
We encounter similar regulatory and legislative issues in most other countries. In certain international markets, such as the different EU member states, the U.K., China, Japan, Canada and South Korea, governments have significant power as large single payers to regulate prices, access criteria (e.g., through public or private health technology assessments), or other means of cost control, particularly under recent global financing pressures. As a result, we expect that pressures on the pricing component of operating results will continue. For example, China, in 2013, began to implement a QCE process, under which numerous local generics have officially been deemed bioequivalents of a qualified reference drug. China’s government subsequently initiated a pilot project for centralized VBP in 2018, which included 25 molecules of drugs and covered 11 major Chinese cities. Under this procurement model, a tender process was established whereby a certain portion of included molecule volumes were guaranteed to tender winners. This tender process was intended to contain healthcare costs by driving utilization of generics and bioequivalents that had passed QCE, and has resulted in dramatic price cuts for off-patent medicines. China’s government began nationwide expansion of the VBP pilot in December 2019. See the discussion regarding these government initiatives in China in the Item 1. Business—Government Regulation and Price Constraints—Outside the United States—China Pricing Pressures section in this 2019 Form 10-K. We anticipate that these initiatives will continue to increase pricing pressures on our drug products in China in the future.
The adoption of restrictive price controls in new jurisdictions or more restrictive ones in existing jurisdictions or the failure to obtain or maintain timely or adequate pricing could also adversely impact revenue. In our vaccines business, we participate in a tender process in many countries for participation in national immunization programs. Failure to secure participation in national immunization programs or to obtain acceptable pricing in the tender process could adversely affect our business.
U.S. HEALTHCARE REFORM
The U.S. healthcare industry is highly regulated and subject to frequent and substantial changes. For example, the ACA was enacted by Congress in March 2010 and established a major expansion of healthcare coverage, financed in part by a number of new rebates, discounts, and taxes that had a significant effect on our expenses and profitability. See the discussion in the Item 1. Business—Government Regulation and Price Constraints—In the United States section in this 2019 Form 10-K. We face uncertainties due to federal legislative and administrative efforts to repeal, substantially modify or invalidate some or all of the provisions of the ACA. There is additional uncertainty given the ruling in December 2019 by the U.S. Circuit Court of Appeals for the Fifth Circuit in Texas v. Azar that the individual mandate, which is a significant provision of the ACA, is unconstitutional. The case has been remanded to a lower court to determine whether the individual mandate is inseparable from the entire ACA, in which case the ACA as a whole would be rendered unconstitutional. In the meantime, the remaining provisions of the law remain in effect. The revenues generated for Pfizer by the health insurance exchanges and Medicaid expansion under the ACA are not material, so the impact of full invalidation of the law is expected to be limited. However, any future replacement of the ACA may adversely affect our business and financial results, particularly if the legislation reduces incentives for employer-sponsored insurance coverage or dramatically increases industry taxes and fees. Any future healthcare reform efforts may adversely affect our business and financial results.
Other U.S. federal or state legislative or regulatory action and/or policy efforts could adversely affect our business, including, among others, general budget control actions, changes in patent laws, the importation of prescription drugs from outside the U.S. at prices that are regulated by governments of various foreign countries (which is among the U.S. Presidential Administration’s policy proposals), revisions to reimbursement of biopharmaceuticals under government programs (such as the implementation of international reference pricing for Medicare Part B drugs, or changes to protected class criteria for Part D drugs), restrictions on U.S. direct-to-consumer advertising, limitations on interactions with healthcare professionals, or the use of comparative effectiveness methodologies that could be implemented in a manner that focuses primarily on cost differences and minimizes the therapeutic differences among pharmaceutical products and restricts access to innovative medicines.
Pfizer Inc. | 2019 Form 10-K | 27 |
U.S. ENTITLEMENT REFORM
In the U.S., government action to reduce federal spending on entitlement programs including Medicare and Medicaid may affect payment for our products or services provided using our products. The Congressional Budget Office routinely releases options for reducing federal spending, and the December 2018 release includes proposals to cap federal Medicaid payments to the states, and to require manufacturers to pay a minimum rebate on drugs covered under Medicare Part D for low-income beneficiaries. Significant Medicare reductions could also result if, for example, Congress proceeds with certain proposals to convert the Medicare fee-for-service program into a premium support program, or Congress chooses to implement the recommendations made annually by the Medicare Payment Advisory Commission, which are primarily intended to extend the fiscal solvency of the Medicare program. These and any other significant spending reductions or cost controls affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented could have an adverse impact on our results of operations.
SUBSTANTIAL REGULATION
We are subject to extensive, complex, costly and evolving regulation by federal and state governmental authorities in the U.S., principally by the FDA and the DEA, and foreign regulatory authorities. Failure to comply with all applicable regulatory requirements may subject us to operating restrictions and criminal prosecution, monetary penalties and other disciplinary actions, including, sanctions, warning letters, product seizures, recalls, fines, injunctions, suspension, revocation of approvals, corporate integrity or deferred prosecution agreements or exclusion from future participation in government healthcare programs, as well as reputational harm.
DEVELOPMENT, REGULATORY APPROVAL AND MARKETING OF PRODUCTS
Innovation is critical to the success of our Company, and drug discovery and development are time-consuming, expensive and unpredictable. The outcome of the lengthy and complex process of identifying new compounds and developing new products is inherently uncertain and involves a high degree of risk and cost. The process from early discovery to design and adequate implementation of clinical trials to regulatory approval can take many years. Drug candidates can and do fail at any stage of the process, including as the result of unfavorable pre-clinical and clinical trial results, or unfavorable new clinical data and further analyses of existing clinical data, including results that may not support further clinical development of the applicable product candidate or indication. We may not be able to meet anticipated pre-clinical or clinical endpoints, commencement and/or completion dates for our pre-clinical or clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates. Similarly, we may not be able to successfully address all of the comments received from regulatory authorities such as the FDA and the EMA, or obtain approval from regulators. Regulatory approval of drug or biologic products depends on myriad factors, including a regulator making a determination as to whether a product’s benefits outweigh its known risks and a determination of the product’s efficacy. Additionally, clinical trial data are subject to differing interpretations and assessments by regulatory authorities. Even after a drug or biologic is approved, it could be adversely affected by regulatory decisions impacting labeling, manufacturing processes, safety and/or other matters. We may not be able to receive or maintain favorable recommendations by technical or advisory committees, such as the Advisory Committee on Immunization Practices that may impact the use of our vaccines. Further, claims and concerns that may arise regarding the safety and efficacy of in-line products and product candidates can result in a negative impact on product sales, product recalls or withdrawals, and/or consumer fraud, product liability and other litigation and claims. Increasing regulatory scrutiny of drug safety and efficacy, with regulatory authorities increasingly focused on product safety and the risk/benefit profile of products as they relate to already-approved products, has resulted in a more challenging, expensive and lengthy regulatory approval process due to requests for, among other things, additional or more extensive clinical trials prior to granting approval or increased post-approval requirements. For these and other reasons discussed in Item 1A. Risk Factors, we may not obtain the approvals we expect within the timeframe we anticipate, or at all.
POST-APPROVAL DATA
As a condition to granting marketing approval of a product, the FDA may require a company to conduct additional clinical trials. The results generated in these Phase 4 trials could result in the loss of marketing approval, changes in product labeling, and/or new or increased concerns about the side effects or efficacy of a product. Regulatory agencies in countries outside the U.S. often have similar authority and may impose comparable requirements. For example, in July and December 2019, the FDA updated the U.S. prescribing information for Xeljanz to include three additional boxed warnings as well as changes to the indication and dosing for ulcerative colitis. In January 2020, the EMA revised the summary of product characteristics (SmPC) for Xeljanz to include new warnings and recommendations for use of Xeljanz due to an increased risk of venous thromboembolism and, due to an increased risk of infections, revised warnings in patients older than 65 years of age. These updates were based on the FDA’s and EMA’s review of data from the ongoing post-marketing requirement rheumatoid arthritis study A3921133. Postmarketing studies, whether conducted by us or by others and whether mandated by regulatory agencies or voluntary, and other emerging data about marketed products, such as adverse event reports, may also adversely affect the availability or commercial potential of our products. Further, the discovery of significant problems with a product similar to one of our products could implicate the entire class of products; and this, in turn, could have an adverse effect on the availability or commercial viability of our product(s) as well as other products in the class.
Pfizer Inc. | 2019 Form 10-K | 28 |
INTERACTIONS WITH HEALTHCARE PROFESSIONALS AND GOVERNMENT OFFICIALS
Risks and uncertainties apply if we provide, offer, or promise something of value to a healthcare professional, other healthcare provider and/or government official. Requirements or industry standards in the U.S. and certain jurisdictions abroad that require pharmaceutical manufacturers to track and disclose financial interactions with healthcare professionals and healthcare providers increase government and public scrutiny of such financial interactions. If an interaction is found to be improper, government enforcement actions and penalties could result. These risks may increase as both U.S. and foreign enforcement agencies adopt or increase enforcement efforts in respect of existing and new laws and regulations governing product promotion, marketing, anti-bribery and kickbacks, industry regulations, and codes of conduct.
CHANGES IN LAWS AND ACCOUNTING STANDARDS
Our future results could be adversely affected by changes in interpretations of existing laws and regulations, or changes in laws and regulations, including, among others, changes in accounting standards, taxation requirements (including tax rate changes, new tax laws, changes to existing tax laws and revised tax law and regulatory clarifications and/or interpretations, including changes affecting the taxation by the U.S. of income earned outside the U.S. that may result from pending and possible future proposals, including further clarifications and/or interpretations of or changes to the U.S. Tax Cuts and Jobs Act of 2017), competition laws, privacy laws and environmental laws in the U.S. and other countries. For additional information, see the Provision/(Benefit) for Taxes on Income—Changes in Tax Laws and New Accounting Standards sections, and the Notes to Consolidated Financial Statements—Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2019 in our 2019 Financial Report.
LEGAL PROCEEDINGS
We and certain of our subsidiaries are involved in various legal proceedings, including patent litigation, such as claims that our patents are invalid and/or do not cover the product of the generic drug manufacturer or where one or more third parties seeks damages and/or injunctive relief to compensate for alleged infringement of its patents by our commercial or other activities, product liability and other product-related litigation, including personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, commercial, environmental, government investigations, employment, tax litigation and other legal proceedings, including various means for resolving asbestos litigation, that arise from time to time in the ordinary course of our business. Litigation is inherently unpredictable, and excessive verdicts do occur. Although we believe that our claims and defenses in matters in which we are a defendant are substantial, we could in the future incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations in the period in which the amounts are accrued and/or our cash flows in the period in which the amounts are paid.
Claims against our patents include challenges to the coverage and/or validity of our patents on various products or processes. Although we believe we have substantial defenses to these challenges with respect to all of our material patents, there can be no assurance as to the outcome of these matters, and a loss in any of these cases could result in a loss of patent protection for the product at issue, which could lead to a significant loss of sales of that product and could materially affect future results of operations.
Like other pharmaceutical companies, we are subject to extensive regulation by government agencies in the U.S., other developed markets and multiple emerging markets in which we operate. Criminal charges, substantial fines and/or civil penalties, limitations on our ability to conduct business in applicable jurisdictions, corporate integrity or deferred prosecution agreements, as well as reputational harm and increased public interest in the matter could result from government investigations in the U.S. and other jurisdictions in which we do business. In addition, in a qui tam lawsuit in which the government declines to intervene, the relator may still pursue a suit for the recovery of civil damages and penalties on behalf of the government.
Our activities relating to the sale and marketing and the pricing of our products are subject to extensive regulation under the FFDCA, the Medicaid Drug Rebate Program, the FCPA and other federal and state statutes, including those discussed elsewhere in this 2019 Form 10-K, as well as anti-kickback and false claims laws, and similar laws in international jurisdictions. Like many companies in our industry, we have from time to time received inquiries and subpoenas and other types of information demands from government authorities, and been subject to claims and other actions related to our business activities brought by governmental authorities, as well as by consumers and private payers. In some instances, we have incurred significant expense, civil payments, fines and other adverse consequences as a result of these claims, actions and inquiries. For example, these claims, actions and inquiries may relate to alleged failures to accurately interpret or identify or prevent non-compliance with the laws and regulations associated with the dissemination of product information (approved and unapproved), potentially resulting in government enforcement and damage to our reputation. This risk may be heightened by digital marketing, including social media, mobile applications and blogger outreach.
In connection with the resolution of a U.S. government investigation concerning independent copay assistance organizations that provide financial assistance to Medicare patients, in May 2018, we entered into a Corporate Integrity Agreement (CIA) with the Office of the Inspector General of the U.S. Department of Health and Human Services, which is effective for a period of five
Pfizer Inc. | 2019 Form 10-K | 29 |
years. In the CIA, we agreed to implement and/or maintain certain compliance program elements to promote compliance with federal healthcare program requirements. Breaches of the CIA could result in severe sanctions against us.
For additional information, including information regarding certain legal proceedings in which we are involved in, see the Notes to Consolidated Financial Statements—Note 16A. Contingencies and Certain Commitments—Legal Proceedings in our 2019 Financial Report.
ENVIRONMENTAL CLAIMS AND PROCEEDINGS
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business relating to environmental claims and proceedings. Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. While we have accrued for worldwide environmental liabilities, there is no guarantee that additional costs will not be incurred beyond the amounts accrued. If we fail to properly manage the safety of our facilities and the environmental risks associated therewith or if we are required to increase our accruals for contingencies for environmental claims and proceedings in the future, it could potentially have an adverse effect on our results of operations.
RISKS RELATED TO INTELLECTUAL PROPERTY:
PATENT PROTECTION
Our long-term success largely depends on our ability to market technologically competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret and domain name protection laws, as well as confidentiality and license agreements, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from launching generic or biosimilar versions of our branded products, using our proprietary technologies or from marketing products that are very similar or identical to ours. Our currently pending or future patent applications may not result in issued patents, or be granted on a timely basis. Similarly, any term extensions that we seek may not be granted on a timely basis, if at all. In addition, our issued patents may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area. The scope of our patent claims also may vary between countries, as individual countries have distinct patent laws. We may be subject to challenges by third parties regarding our intellectual property, including, among others, claims regarding validity, enforceability, scope and effective term.
Our ability to enforce our patents also depends on the laws of individual countries and each country’s practice with respect to enforcement of intellectual property rights, and the extent to which certain sovereigns may seek to engage in policies or practices that may weaken its intellectual property framework (e.g., laws or regulations that promote or provide broad discretion to issue a compulsory license). In countries that provide some form of regulatory exclusivity, mechanisms exist permitting some form of challenge to our patents by competitors or generic drug marketers prior to or immediately following the expiration of such regulatory exclusivity, and generic companies are increasingly employing aggressive strategies, such as “at risk” launches that challenge our patent rights. Most of the suits involve claims by generic drug manufacturers that patents covering our products, processes or dosage forms are invalid and/or do not cover the product of the generic drug manufacturer. Independent actions have been filed alleging that our assertions of, or attempts to enforce, patent rights with respect to certain products constitute unfair competition and/or violations of antitrust laws. Such claims may also be brought as counterclaims to actions we bring to enforce our patents. We are also party to other patent damages suits in various jurisdictions pursuant to which generic drug manufacturers, payers, governments or other parties are seeking damages from us for alleged delay of generic entry. We also are often involved in other proceedings, such as inter partes review, post-grant review, re-examination or opposition proceedings, before the U.S. Patent and Trademark Office, the European Patent Office, or other foreign counterparts relating to our intellectual property or the intellectual property rights of others. Also, if one of our patents is found to be invalid in such proceedings, generic or competitive products could be introduced into the market resulting in the erosion of sales of our existing products. For example, several of the patents in our pneumococcal vaccine portfolio were challenged in inter partes review and post-grant review proceedings in the U.S. In October 2017, the Patent Trial and Appeal Board (PTAB) refused to initiate proceedings as to two patents. In June 2018, the PTAB ruled on another patent, holding that one claim was valid and that all other claims were invalid. The party challenging that patent has appealed the decision. In November 2019, the Federal Circuit vacated the PTAB’s ruling and requested that the PTAB redecide the challenge. In March and June 2019, an additional patent was found invalid in separate proceedings by the PTAB. We have appealed. Challenges to other patents remain pending in jurisdictions outside the U.S. The invalidation of all of these patents in our pneumococcal portfolio could potentially allow a competitor pneumococcal vaccine into the marketplace. Further, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, including because such agreements expire or are terminated, our operating results and financial condition could be materially adversely affected.
Likewise, in the U.S. and other countries, we currently hold issued trademark registrations and have trademark applications pending, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the trademark. As our products mature, our reliance on our trademarks and trade dress to differentiate us from our competitors increases and as a result, if we are unable to prevent third parties from adopting, registering or using trademarks
Pfizer Inc. | 2019 Form 10-K | 30 |
and trade dress that infringe, dilute or otherwise violate our trademark rights, our business could be materially adversely affected. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants, other advisors and other third parties to execute proprietary information and confidentiality agreements upon the commencement of their employment, engagement or other relationship. Despite these efforts and precautions, we may be unable to prevent a third party from copying or otherwise obtaining and using our trade secrets or our other intellectual property without authorization, and legal remedies in some countries may not adequately compensate us for the damages caused by such unauthorized use. Further, others may independently and lawfully develop substantially similar or identical products that circumvent our intellectual property by means of alternative designs or processes or otherwise.
THIRD PARTY INTELLECTUAL PROPERTY CLAIMS
A properly functioning intellectual property regime is essential to our business model. We are committed to respecting the valid intellectual property rights of other companies, but the patent granting process is imperfect. Accordingly, the pursuit of valid business opportunities may require us to challenge intellectual property rights held by other companies that we believe were improperly granted. Such challenges may include negotiation and litigation, which may not always be successful.
Part of our business depends upon successfully identifying generic pharmaceutical product and biosimilar opportunities and launching products to take advantage of those opportunities, which may involve litigation, associated costs and time delays, and may ultimately not be successful. These opportunities may arise in situations where patent protection of equivalent branded products has expired, where patents have been declared invalid, or where products do not infringe the patents of others, and in some circumstances we may take action, such as litigation, asserting that our products do not infringe patents of existing products or that those patents are invalid or unenforceable in order to achieve a “first-to-market” or early market position for our products.
Third parties may claim that our products infringe one or more patents owned or controlled by the third party. Claims of intellectual property infringement can be costly and time-consuming to resolve, may delay or prevent product launches, and may result in significant damages. We are involved in patent-related disputes with third parties over our attempts to market generic pharmaceutical products and biosimilars. Once we have final regulatory approval of the related generic pharmaceuticals products or biosimilars, we may decide to commercially market these products even though associated legal proceedings (including any appeals) have not been resolved (i.e., “at-risk” launch). If one of our marketed products is found to infringe valid patent rights of a third party, such third party may be awarded significant damages, or we may be prevented from further sales of that product. Such damages may be enhanced as much as three-fold in the event that we or one of our subsidiaries, like Hospira, is found to have willfully infringed valid patent rights of a third party. Any of these adverse consequences could have a material adverse effect on our profitability and financial condition.
RISK RELATED TO TECHNOLOGY:
INFORMATION TECHNOLOGY AND SECURITY
Significant disruptions of information technology systems or breaches of information security could adversely affect our businesses. We rely to a large extent upon sophisticated information technology systems to operate our businesses. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property), and we deploy and operate an array of technical and procedural controls to maintain the confidentiality and integrity of such confidential information. We also have outsourced significant elements of our operations to third parties, including significant elements of our information technology infrastructure and, as a result, we are managing many independent vendor relationships with third parties who may or could have access to our confidential information. The size and complexity of our information technology and information security systems, and those of our third-party vendors with whom we contract (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from attacks by malicious third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage) and expertise, including organized criminal groups, “hacktivists,” nation states and others. As a global pharmaceutical company, our systems are subject to frequent attacks. Due to the nature of some of these attacks, there is a risk that they may remain undetected for a period of time. While we have invested in the protection of data and information technology, our efforts may not prevent service interruptions or security breaches. Any such interruption or breach of our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us. We maintain cyber liability insurance; however, this insurance may not be sufficient to cover the financial, legal, business or reputational losses that may result from an interruption or breach of our systems.
Pfizer Inc. | 2019 Form 10-K | 31 |
RISKS RELATED TO OUR STRATEGIC TRANSACTIONS:
STRATEGIC ACQUISITIONS
The success of any of our strategic acquisitions will depend, in large part, on our ability to realize anticipated benefits from combining these businesses with Pfizer. We, for example, may fail to achieve cost savings anticipated with certain of these acquisitions, or such cost savings within the expected time frame. Similarly, the accretive impact anticipated from certain of these acquisitions may not be realized or may be delayed. Integration of these businesses may result in the loss of key employees, the disruption of ongoing business, including third-party relationships, or inconsistencies in standards, controls, procedures and policies. We also may fail to generate the revenue growth for the acquired business that we expected at the time of entering into the transaction. Expected revenue from acquired products and product candidates also may be constrained by developments outside of our control. Unsuccessful clinical trials, regulatory hurdles and commercialization challenges may adversely impact revenue and income contribution from products and product candidates, including those acquired in these acquisitions. Hospira, for example, has experienced manufacturing disruptions and substantial regulatory scrutiny due to quality issues. Manufacturing problems, as well as any corrective actions and their operational implementation, could adversely impact the revenue we generate from products acquired from Hospira and result in substantial unanticipated costs. For additional information, see the Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Business Development Initiatives section in our 2019 Financial Report.
PENDING COMBINATION OF UPJOHN WITH MYLAN
Pfizer, Mylan and Upjohn may be unable to satisfy the conditions or obtain the approvals required to complete the combination of Upjohn with Mylan (the Combination), and regulatory agencies may delay or impose conditions on approval of the Combination, which may diminish the anticipated benefits of the Combination.
The consummation of the Combination is subject to numerous conditions, including the receipt by Pfizer of an Internal Revenue Service ruling and an opinion of its tax counsel to the effect that, among other things, certain transactions related to the Combination and certain related transactions will constitute a tax-free “reorganization” within the meaning of Section 368(a)(1)(D) of the Internal Revenue Code, the approval of the Combination by Mylan shareholders, and other customary conditions, certain of which are dependent upon the actions of third parties. As a result of such conditions, Pfizer cannot make any assurances that the Combination will be consummated on the terms or timeline currently contemplated, or at all.
Completion of the Combination is also conditioned upon the receipt of certain required government consents and approvals, including certain approvals required from regulatory agencies. While Pfizer, Mylan and Upjohn intend to pursue vigorously all required governmental approvals, the requirement to receive these approvals prior to the consummation of the Combination could delay the completion of the Combination, possibly for a significant period of time. Any delay in the completion of the Combination could diminish the anticipated benefits of the Combination or result in additional transaction costs, loss of revenue or other effects associated with uncertainty about the Combination, including delaying Pfizer’s ability to capitalize on its strategy of becoming a more focused, innovative company as well as Upjohn’s ability to optimize the execution of its growth strategies.
Pfizer may be subject to shareholder lawsuit, or other actions filed in connection with or in opposition to the Combination or any related transactions. Such litigation could have an adverse effect on the business, financial condition and results of operations of Pfizer and could prevent or delay the consummation of the Combination.
Pfizer has expended and will continue to expend significant management time and resources and has incurred and will continue to incur significant expenses due to legal, advisory, printing and financial services fees related to the Combination, including costs required to obtain the required government consents or defend or settle actions noted above. We expect to incur costs of approximately $500 million in connection with fully separating Upjohn, inclusive of $145 million incurred in 2019. Such charges will include costs and expenses related to separation of legal entities and anticipated transaction costs. Many of these expenses must be paid regardless of whether the Combination is consummated, and even if the expected benefits of the Combination are not achieved. Additionally, the completion of the Combination, including for example, obtaining regulatory approvals, will require significant time and attention from Pfizer management and may divert attention from the day-to-day operations of our business.
Even if the Combination is completed as anticipated, Pfizer may not realize some or all of the expected benefits. Furthermore, Upjohn may experience operational challenges in integrating the Upjohn and Mylan businesses, which may also diminish the anticipated benefits of the Combination.
Even if the Combination is completed, the anticipated operational, financial, strategic and other benefits of the Combination may not be achieved. There are many factors that could impact the anticipated benefits from the Combination, including, among others, strategic adjustments required to reflect the nature of our business following the Combination, any negative reaction to the Combination by our customers and business partners, and increased risks resulting from Pfizer becoming a company that is more focused on innovative medicines. In addition, Pfizer has agreed to provide certain transition services to the combined company, generally for an initial period of 24 months following the completion of the Combination (with certain possibilities for extension). These obligations under the transition agreements may result in additional expenses and may divert
Pfizer Inc. | 2019 Form 10-K | 32 |
Pfizer’s focus and resources that would otherwise be invested into maintaining or growing Pfizer’s business. An inability to realize the full extent of the anticipated benefits of the Combination, as well as any delays encountered in the process, could have an adverse effect on the revenues, level of expenses and operating results of our business.
Furthermore, the Combination is a complex, costly and time-consuming process. Even if Upjohn and Mylan successfully integrate, Pfizer, Upjohn and Mylan cannot predict with certainty if or when the anticipated synergies, growth opportunities and benefits resulting from the Combination will occur, or the extent to which they actually will be achieved. For example, the benefits from the Combination may be offset by costs incurred in integrating the companies or by required capital expenditures related to the combined businesses. In addition, the quantification of synergies expected to result from the Combination is based on significant estimates and assumptions that are subjective in nature and inherently uncertain. Realization of any benefits and synergies could be affected by a number of factors beyond Pfizer’s, Mylan’s, Upjohn’s or the combined company’s control, including, without limitation, general economic conditions, increased operating costs, regulatory developments and the other risks described in these risk factors. The amount of synergies actually realized in the Combination, if any, and the time periods in which any such synergies are realized, could differ materially from the synergies anticipated to be realized, regardless of whether the two business operations are combined successfully. If the integration is unsuccessful or if the combined company is unable to realize the anticipated synergies and other benefits of the Combination, there could be a material adverse effect on the combined company’s share price, business, financial condition and results of operations.
CONSUMER HEALTHCARE JOINT VENTURE WITH GSK
On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. Following the integration of the combined business, GSK intends to separate the joint venture as an independent company via a demerger of its equity interest to its shareholders and a listing of the combined business on the U.K. equity market. In February 2020, GSK announced the initiation of a two-year program to prepare for the separation of GSK into two companies, including a standalone Consumer Healthcare company. Until the fifth anniversary of the closing of the transaction, GSK will have the sole right to decide whether and when to initiate a separation and listing, and may also sell all or part of its stake in the joint venture in a contemporaneous initial public offering. Should a separation and listing occur during the first five years after closing, Pfizer has the option to participate through the distribution of some or all of its equity interest in the joint venture to its shareholders. Following a separation or listing, and subject to customary lock-up or similar restrictions, Pfizer will also have the ability to sell its equity interest in the joint venture through the capital markets. After the fifth anniversary of the closing of the transaction, both GSK and Pfizer will have the right to decide whether and when to initiate a separation and public listing of the joint venture. The planned separation and public listing transactions may not be initiated or completed within the expected time periods or at all, and both the timing and success of any separation and public listing transaction, as well as the value generated for Pfizer or its shareholders in any such transaction, will be subject to prevailing market conditions and other factors at the time of such transaction. Although Pfizer is entitled to participate in any separation and listing transaction initiated by GSK prior to the fifth anniversary of the closing, it is not required to do so, and any future distribution or sale of Pfizer’s equity stake in the joint venture will similarly be subject to prevailing market conditions and other factors at the time of such transaction. Pfizer’s ability to complete any such future distribution or sale may also be impacted by the size of Pfizer’s retained equity stake at the time. The uncertainty relating to the separation and public listing transactions, their implementation, their timing and their yet to be determined effects on the joint venture’s business may subject us and the joint venture to risks and uncertainties that may adversely affect our business and financial results.
Moreover, although we have certain consent, board representation and other governance rights with respect to the joint venture, Pfizer is a minority owner of the joint venture. As a result, Pfizer does not have control over the joint venture, its management or its policies and we may have business interests, strategies and goals that differ in certain respects from those of GSK or the joint venture.
In addition, the joint venture will be subject to the risks associated with the joint venture’s consumer healthcare business, and the business, financial condition and results of operations of the joint venture may be affected by factors that are different from or in addition to those that previously affected the business, financial condition and results of operations of Pfizer’s historical consumer healthcare business. Many of these factors are outside of our and the joint venture’s control, and could materially impact the business, financial condition and results of operations of the joint venture.
The success of the transaction will also depend, in part, on the joint venture’s ability to realize the anticipated benefits and cost synergies from the transaction. These anticipated benefits and cost savings may not be realized or may not be realized within the expected time period. The joint venture’s integration of Pfizer’s and GSK’s historic consumer healthcare businesses may result in material unanticipated problems, costs, expenses, liabilities, competitive responses, and loss of customer and other business relationships. Any material unanticipated issues arising from the integration process could negatively impact our stock price and our or the joint venture’s future business and financial results.
Pfizer Inc. | 2019 Form 10-K | 33 |
OTHER RISKS:
THE GLOBAL ECONOMIC ENVIRONMENT
Like all businesses of our size, we are exposed to both global and industry-specific economic conditions. Governments, corporations, and insurance companies, which provide insurance benefits to patients, have implemented increases in cost-sharing and restrictions on access to medicines, potentially causing patients to switch to generic or biosimilar products, delay treatments, skip doses or use less effective treatments. As discussed above, government financing pressures can lead to negative pricing pressure in various markets where governments take an active role in setting prices, access criteria (e.g., through public or private health technology assessments), or other means of cost control.
The global economic environment has not had, nor do we anticipate that it will have, a material impact on our liquidity or capital resources. Due to our significant operating cash flows, financial assets, access to capital markets and available lines of credit and revolving credit agreements, we continue to believe that we have, and will maintain, the ability to meet our liquidity needs for the foreseeable future. We monitor our liquidity position continuously in the face of evolving economic conditions, but there can be no guarantee that changes in global financial markets and global economic conditions will not affect our liquidity or capital resources or impact our ability to obtain financing in the future.
We continue to monitor credit, capital restrictions and economic situations in volatile regions and markets, especially where the ability to obtain U.S. dollars for local currency is unpredictable and challenging. We cannot predict the likelihood of future changes in these economic conditions, or what impact they may have on our results of operations, financial condition or business.
In addition, given that a significant portion of our business is conducted in the EU, including the U.K., the formal change in the relationship between the U.K. and the EU caused by Brexit may pose certain implications for our research, commercial and general business operations in the U.K. and the EU, including the approval and supply of our products. Details on how Brexit will be finally executed and the impact on the remaining EU countries will dictate how and whether the broader EU will be impacted and what the resulting impact on our business may be. For additional information, see the Overview of Our Performance, Operating Environment, Strategy and Outlook––The Global Economic Environment section in our 2019 Financial Report.
Public health epidemics or outbreaks could adversely impact our business. In December 2019, a novel strain of coronavirus (COVID-19) emerged in Wuhan, Hubei Province, China. While initially the outbreak was largely concentrated in China and caused significant disruptions to its economy, it has now spread to several other countries and infections have been reported globally. The extent to which the coronavirus impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information which may emerge concerning the severity of the coronavirus and the actions to contain the coronavirus or treat its impact, among others. In particular, the continued spread of the coronavirus globally could adversely impact our operations, including among others, our manufacturing and supply chain, sales and marketing and clinical trial operations and could have an adverse impact on our business and our financial results.
We also continue to monitor the global trade environment and potential trade conflicts and impediments. If trade restrictions or tariffs reduce global economic activity, or if other factors lead to a general economic downturn, potential impacts could include declining sales; increased costs; volatility in foreign exchange rates; a decline in the value of our financial assets and pension plan investments; required increases of our pension funding obligations; increased government cost control efforts; delays or failures in the performance of customers, suppliers, and other third parties on whom we may depend for the performance of our business; and the risk that our allowance for doubtful accounts may not be adequate.
FOREIGN EXCHANGE AND INTEREST RATE RISK
Significant portions of our revenues, costs and expenses, as well as our substantial international net assets, are exposed to changes in foreign exchange rates. 54% of our total 2019 revenues were derived from international operations, including 21% from Europe and 24% from China, Japan and the rest of Asia. As we operate in multiple foreign currencies, including the euro, the Chinese renminbi, the Japanese yen, the Canadian dollar, the U.K. pound and approximately 100 other currencies, changes in those currencies relative to the U.S. dollar will impact our revenues and expenses. If the U.S. dollar were to weaken against another currency, assuming all other variables remained constant, our revenues would increase, having a positive impact on earnings, and our overall expenses would increase, having a negative impact on earnings. Conversely, if the U.S. dollar were to strengthen against another currency, assuming all other variables remained constant, our revenues would decrease, having a negative impact on earnings, and our overall expenses would decrease, having a positive impact on earnings. Therefore, significant changes in foreign exchange rates can impact our results and our financial guidance.
The impact of possible currency devaluations in countries experiencing high inflation rates or significant exchange fluctuations, including Venezuela and Argentina, can impact our results and financial guidance. For additional information about our exposure to foreign currency risk, see the Item 7A. Quantitative and Qualitative Disclosures About Market Risk—Foreign Exchange Risk section in this 2019 Form 10-K and the Overview of Our Performance, Operating Environment, Strategy and
Pfizer Inc. | 2019 Form 10-K | 34 |
Outlook––Our Financial Guidance for 2020 and Analysis of Financial Condition, Liquidity and Capital Resources sections in our 2019 Financial Report.
In addition, our interest-bearing investments and borrowings, and our pension benefit obligations, net, and our postretirement benefit obligations, net, are subject to risk from changes in interest rates and foreign exchange rates. These risks related to interest-bearing investments and borrowings and the measures we have taken to help contain them are discussed in the Item 7A. Quantitative and Qualitative Disclosures About Market Risk—Financial Risk Management section in this 2019 Form 10-K. For additional details, see the Significant Accounting Policies and Application of Critical Accounting Estimates and Assumptions––Benefit Plans section and the Notes to Consolidated Financial Statements—Note 7F. Financial Instruments: Derivative Financial Instruments and Hedging Activities and —Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans in our 2019 Financial Report, which are incorporated by reference.
From time to time, we issue variable rate debt based on LIBOR, or undertake interest rate swaps that contain a variable element based on LIBOR. The U.K. Financial Conduct Authority announced in July 2017 that it will no longer compel banks to submit rates that are currently used to calculate LIBOR after 2021. Various governing parties, including government agencies, are working on a benchmark transition plan for LIBOR (and other interbank offered rates globally). We are monitoring their progress, and we will likely amend contracts to accommodate any replacement rate where it is not already provided. As a result, our interest expense could increase and our available cash flow for general corporate requirements may be adversely affected. Additionally, uncertainty as to the nature of a potential discontinuance, modification, alternative reference rates or other reforms may materially adversely affect the trading market for securities linked to such benchmarks. For additional information, see the Analysis of Financial Condition, Liquidity and Capital Resources—Selected Measures of Liquidity and Capital Resources—LIBOR section in our 2019 Financial Report.
Notwithstanding our efforts to foresee and mitigate the effects of changes in external fiscal circumstances, we cannot predict with certainty changes in currency and interest rates, inflation or other related factors affecting our businesses.
MARKET FLUCTUATIONS IN OUR EQUITY INVESTMENTS
In 2018, we adopted a new accounting standard whereby certain equity investments are measured at fair value with changes in fair value now recognized in net income. We expect the adoption of this new accounting standard may increase the volatility of our income in future periods due to changes in the fair value of certain equity investments. For additional information, see the Notes to Consolidated Financial Statements—Note 4. Other (Income)/Deductions—Net in our 2019 Financial Report and the Item 7A. Quantitative and Qualitative Disclosures About Market Risk—Financial Risk Management section in this 2019 Form 10-K.
Our pension benefit obligations and postretirement benefit obligations, net of our plan assets, are subject to volatility from changes in fair value of equity investments and other investment risk. For additional information, see the Significant Accounting Policies and Application of Critical Accounting Estimates and Assumptions—Benefit Plans section and the Notes to Consolidated Financial Statements—Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans in our 2019 Financial Report.
COST AND EXPENSE CONTROL/UNUSUAL EVENTS/FAILURE TO REALIZE THE ANTICIPATED BENEFITS OF STRATEGIC INITIATIVES AND ACQUISITIONS
Growth in costs and expenses, changes in product, segment and geographic mix and the impact of acquisitions, divestitures, restructurings, internal reorganizations, product withdrawals, recalls and other unusual events that could result from evolving business strategies, evaluation of asset realization and organizational restructuring could adversely affect future results. Such risks and uncertainties include, in particular, our ability to realize the projected benefits of (i) our cost-reduction and productivity initiatives; (ii) the reorganization of our commercial operations in 2019; (iii) any other corporate strategic initiatives; and (iv) any acquisitions, divestitures or other initiatives, such as our agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, which is anticipated to close in mid-2020, our acquisition of Array and the formation of the new consumer healthcare joint venture with GSK.
INTANGIBLE ASSETS, GOODWILL AND EQUITY-METHOD INVESTMENTS
Our consolidated balance sheet contains significant amounts of intangible assets, including goodwill. For IPR&D assets, the risk of failure is significant, and there can be no certainty that these assets ultimately will yield successful products. The nature of the biopharmaceutical business is high-risk and requires that we invest in a large number of projects in an effort to achieve a successful portfolio of approved products. Our ability to realize value on these significant investments is often contingent upon, among other things, regulatory approvals and market acceptance. As such, we expect that many of these IPR&D assets will become impaired and be written off at some time in the future. If the associated R&D effort is abandoned, the related IPR&D assets will likely be written-off, and we will record an impairment charge. For goodwill, all reporting units can confront events and circumstances that can lead to a goodwill impairment charge (such as, among other things, unanticipated competition, an adverse action or assessment by a regulator, a significant adverse change in legal matters or in the business climate and/or a failure to replace the contributions of products that lose exclusivity). Any such charge may be significant. Our other intangible
Pfizer Inc. | 2019 Form 10-K | 35 |
assets, including developed technology rights and brands, face similar risks for impairment and charges related to such assets may be significant as well. For additional details, see the Significant Accounting Policies and Application of Critical Accounting Estimates and Assumptions section in our 2019 Financial Report.
We also regularly review our equity-method investments for impairment. An impairment charge may result from the occurrence of unexpected adverse events or management decisions that impact our estimates of expected cash flows to be generated from these investments. We may recognize impairment charges as a result of a weak economic environment, events related to particular customers or asset types, challenging market conditions or decisions by management.
TERRORIST ACTIVITY
Our future results could be adversely affected by changes in business, political and economic conditions, including the cost and availability of insurance, due to the threat of terrorist activity in the U.S. and other parts of the world and related U.S. military action overseas.
Pfizer Inc. | 2019 Form 10-K | 36 |
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
ITEM 2. | PROPERTIES |
As of December 31, 2019, we had 453 owned and leased properties, amounting to approximately 47 million square feet.
In 2019, we reduced the number of properties in our portfolio by 45 sites and 6 million square feet, which reflects the divestment of properties in connection with the formation of the GSK Consumer Healthcare joint venture and the addition of properties in connection with the acquisition of Array.
Pfizer continues to own and lease space around the world for sales and marketing, customer service, regulatory compliance, R&D, manufacturing and distribution, and administrative support functions. In many locations, business lines and operations are co-located to achieve synergy and operational efficiencies.
Pfizer’s corporate headquarters are in New York City and Pfizer’s properties extend internationally to approximately 90 countries.
In April 2018, we entered an agreement to lease space at the Spiral, an office building in the Hudson Yards neighborhood of New York City. We will relocate our global headquarters to this property with occupancy expected beginning in 2022. In July 2018, we completed the sale of our current headquarters in New York City. We remain in a lease-back arrangement with the buyer while we complete our relocation. We continue to advance our global workplace strategy to provide workplaces that enable collaboration and foster innovation.
We have numerous facilities across the world to support our R&D organizations, with a heavy concentration in North America. In 2019, we operationalized the new R&D facilities in St. Louis, Missouri and Andover, Massachusetts. We also purchased an R&D property in Durham, North Carolina in 2019 and expect to renovate and fit out the space over the next several years.
Our PGS division is headquartered in various locations, with leadership teams primarily in New York City, New York and in Peapack, New Jersey. As of December 31, 2019, PGS had responsibility for 42 plants around the world, which manufacture products for our commercial divisions. Locations with major manufacturing facilities include Belgium, China, Germany, India, Ireland, Italy, Japan, Singapore and the U.S. Our PGS division’s plant network strategy is expected to result in the exit of two of these sites over the next several years. PGS also operates multiple distribution facilities around the world. In 2019, seven manufacturing plants transferred from PGS’s responsibility to Upjohn’s responsibility, and an additional two plants are expected to be fully migrated from PGS’s responsibility to Upjohn’s responsibility over the next several years.
In general, we believe that our properties are well-maintained, adequate and suitable for their current requirements and for our operations in the foreseeable future. See the Notes to Consolidated Financial Statements—Note 9. Property, Plant and Equipment in our 2019 Financial Report, which provides amounts invested in land, buildings and equipment and which is incorporated by reference.
ITEM 3. | LEGAL PROCEEDINGS |
Certain legal proceedings in which we are involved are discussed in the Notes to Consolidated Financial Statements—Note 16A. Contingencies and Certain Commitments—Legal Proceedings in our 2019 Financial Report, which is incorporated by reference.
ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
Pfizer Inc. | 2019 Form 10-K | 37 |
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The executive officers of the Company are set forth in this table. Each holds the office or offices indicated until his or her successor is chosen and qualified at the regular meeting of the Board of Directors to be held on the date of the 2020 Annual Meeting of Shareholders, or until his or her earlier death, resignation or removal. Each of the executive officers is a member of the Pfizer Executive Leadership Team.
Name | Age | Position | ||
Albert Bourla | 58 | Chairman of the Board since January 2020 and Chief Executive Officer since January 2019. Chief Operating Officer from January 2018 until December 2018; Group President, Pfizer Innovative Health from June 2016 until December 2017; Group President, Global Innovative Pharma Business (responsible for Vaccines, Oncology and Consumer Healthcare since 2014) from February 2016 until June 2016. President and General Manager of Established Products Business Unit from December 2010 until December 2013. Our Director since February 2018. Board member of Pharmaceutical Research and Manufacturers of America (PhRMA). Board member of the Pfizer Foundation, which promotes access to quality healthcare. Member of the Board of Directors of the Partnership for New York City and Catalyst, a global non-profit organization accelerating progress for the advancement of women into leadership. | ||
Frank A. D’Amelio | 62 | Chief Financial Officer, Executive Vice President, Business Operations and Global Supply since November 2018. Executive Vice President, Business Operations and Chief Financial Officer from December 2010 until October 2018. Senior Vice President and Chief Financial Officer from September 2007 until December 2010. Director of Zoetis Inc. and Humana Inc. and Chair of the Humana Inc. Board of Directors’ Audit Committee. Director of the Independent College Fund of New Jersey. | ||
Mikael Dolsten | 61 | Chief Scientific Officer, President, Worldwide Research, Development and Medical since January 2019. President of Worldwide Research and Development from December 2010 until December 2018. Senior Vice President; President of Worldwide Research and Development from May 2010 until December 2010. Senior Vice President; President of Pfizer BioTherapeutics Research & Development Group from October 2009 until May 2010. He was Senior Vice President of Wyeth and President, Wyeth Research from June 2008 until October 2009. Director of Karyopharm Therapeutics Inc. Chairman of the Translational Advisory Board of Apple Tree Partners from 2016 to 2017. | ||
Lidia Fonseca | 51 | Chief Digital and Technology Officer, Executive Vice President since January 2019. Chief Information Officer and Senior Vice President of Quest Diagnostics Incorporated from 2014 to 2018. Senior Vice President of Laboratory Corporation of America Holdings from 2008 until March 2013. Director of Tegna, Inc. | ||
Angela Hwang | 54 | Group President, Pfizer Biopharmaceuticals Group since January 2019. Group President, Pfizer Essential Health from January 2018 until December 2018. Global President, Pfizer Inflammation and Immunology from January 2016 until December 2017. Regional Head, U.S. Vaccines from January 2014 until December 2015. Vice President, Emerging Markets for the Primary Care business from September 2011 until December 2013. Vice President, U.S. Brands business within Essential Health from October 2009 until August 2011. | ||
Rady A. Johnson | 58 | Chief Compliance, Quality and Risk Officer, Executive Vice President since January 2019. Executive Vice President, Chief Compliance and Risk Officer from December 2013 until December 2018. Senior Vice President and Associate General Counsel from October 2006 until December 2013. | ||
Douglas M. Lankler | 54 | General Counsel, Executive Vice President since December 2013. Corporate Secretary from January 2014 until February 2014. Executive Vice President, Chief Compliance and Risk Officer from February 2011 until December 2013. Executive Vice President, Chief Compliance Officer from December 2010 until February 2011. Senior Vice President and Chief Compliance Officer from January 2010 until December 2010. Senior Vice President, Deputy General Counsel and Chief Compliance Officer from August 2009 until January 2010. | ||
Pfizer Inc. | 2019 Form 10-K | 38 |
Name | Age | Position | ||
A. Rod MacKenzie | 60 | Chief Development Officer, Executive Vice President since June 2016. Senior Vice President, Chief Development Officer from March 2016 until June 2016. Group Senior Vice President and Head, Pharma Therapeutics Research and Development from 2010 until March 2016. Dr. MacKenzie represents Pfizer as a member of the Board of Directors of ViiV Healthcare Limited, TransCelerate Biopharma Inc. and the National Health Council. | ||
Dawn Rogers | 55 | Chief Human Resources Officer, Executive Vice President since January 2019. Executive Vice President, Worldwide Human Resources from June 2018 until December 2018. Senior Vice President, Human Resources for the Chief Operating Officer from November 2017 until May 2018. Senior Vice President of Human Resources for Pfizer Essential Health, Global Product Development, and the Legal and Compliance Divisions from 2016 until November 2017. Senior Vice President of Human Resources for the Global Innovative Pharma Business from 2013 until 2016. Senior Vice President of Human Resources for the Primary Care Business Unit from 2011 until 2013. Senior Vice President of Human Resources for Worldwide Research and Development from 2008 until 2011. | ||
Sally Susman | 58 | Chief Corporate Affairs Officer, Executive Vice President since January 2019. Executive Vice President, Corporate Affairs (formerly Policy, External Affairs and Communications) from December 2010 until December 2018. Senior Vice President, Policy, External Affairs and Communications from December 2009 until December 2010. Director of WPP plc. | ||
John D. Young | 55 | Chief Business Officer, Group President since January 2019. Group President, Pfizer Innovative Health from January 2018 until December 2018. Group President, Pfizer Essential Health from June 2016 until December 2017; Group President, Global Established Pharma Business from January 2014 until June 2016. President and General Manager, Pfizer Primary Care from June 2012 until December 2013. Primary Care Business Unit’s Regional President for Europe and Canada from 2009 until June 2012. Director of Johnson Controls International plc. Mr. Young represents Pfizer as a member of the Board of Directors of the GSK Consumer Healthcare joint venture. | ||
Pfizer Inc. | 2019 Form 10-K | 39 |
PART II |
ITEM 5. | MARKET FOR THE COMPANY’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
The principal market for our common stock is the NYSE. Our common stock currently trades on the NYSE under the symbol “PFE”. As of February 25, 2020, there were 142,524 holders of record of our common stock. Additional information required by this item is incorporated by reference from the Selected Quarterly Financial Data (Unaudited) and Peer Group Performance Graph sections in our 2019 Financial Report.
The following table provides certain information with respect to our purchases of shares of the Company’s common stock during the fourth fiscal quarter of 2019:
Issuer Purchases of Equity Securities(a)
Period | Total Number of Shares Purchased(b) | Average Price Paid per Share(b) | Total Number of Shares Purchased as Part of Publicly Announced Plan | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plan(a) | |||||||||
September 30, 2019 through October 27, 2019 | 32,848 | $ | 36.06 | — | $ | 5,292,881,709 | |||||||
October 28, 2019 through November 30, 2019 | 13,399 | $ | 37.50 | — | $ | 5,292,881,709 | |||||||
December 1, 2019 through December 31, 2019 | 67,767 | $ | 38.86 | — | $ | 5,292,881,709 | |||||||
Total | 114,014 | $ | 37.89 | — | |||||||||
(a) | For additional information, see the Notes to Consolidated Financial Statements––Note 12. Equity in our 2019 Financial Report, which is incorporated by reference. |
(b) | These columns represent (i) 108,367 shares of common stock surrendered to the Company to satisfy tax withholding obligations in connection with the vesting of awards under our long-term incentive programs and (ii) the open market purchase by the trustee of 5,647 shares of common stock in connection with the reinvestment of dividends paid on common stock held in trust for employees who were granted performance share awards and who deferred receipt of such awards. |
Pfizer Inc. | 2019 Form 10-K | 40 |
ITEM 6. | SELECTED FINANCIAL DATA |
Information required by this item is incorporated by reference from the discussion under the heading Financial Summary in our 2019 Financial Report.
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Information required by this item is incorporated by reference from the discussion under the heading Financial Review in our 2019 Financial Report.
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Financial Risk Management
The objective of our financial risk management program is to minimize the impact of foreign exchange rate movements and interest rate movements on our earnings. We manage these financial exposures through operational means and through the use of third-party instruments. These practices may change as economic conditions change.
Foreign Exchange Risk
We operate globally and, as such, we are subject to foreign exchange risk in our commercial operations, as well as in our financial assets (investments) and liabilities (borrowings). Our net investments in foreign subsidiaries are also subject to currency risk.
On the commercial side, a significant portion of our revenues and earnings is exposed to changes in foreign exchange rates. See the Overview of Our Performance, Operating Environment, Strategy and Outlook—The Global Economic Environment section in our 2019 Financial Report for the key currencies in which we operate. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenues in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. Where foreign exchange risk cannot be mitigated via operational means, we may use foreign currency forward-exchange contracts and/or foreign currency swaps to manage that risk.
With respect to our financial assets and liabilities, our primary foreign exchange exposure arises predominantly from short-term and long-term intercompany receivables and payables, and, to a lesser extent, from short-term and long-term investments and debt, where the assets and/or liabilities are denominated in currencies other than the functional currency of the business entity.
We also hedge some forecasted intercompany sales denominated in euro, Japanese yen, Chinese renminbi, U.K. pound, Canadian dollar, and Australian dollar to protect against longer-term movements.
In addition, under certain market conditions, we may seek to protect against possible declines in the reported net investments of our foreign business entities. In these cases, we may use foreign currency swaps, foreign currency forward-exchange contracts and/or foreign currency debt.
For details about these and other financial instruments, including fair valuation methodologies, see the Notes to Consolidated Financial Statements—Note 7A. Financial Instruments: Fair Value Measurements in our 2019 Financial Report.
The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to foreign exchange rate changes. In this sensitivity analysis, holding all other assumptions constant and assuming that a change in one currency’s rate relative to the U.S. dollar would not have any effect on another currency’s rates relative to the U.S. dollar, if the dollar were to appreciate against all other currencies by 10%, as of December 31, 2019, the expected adverse impact on our net income would not be significant.
Interest Rate Risk
We are subject to interest rate risk on our investments and on our borrowings. We manage interest rate risk in the aggregate, while focusing on Pfizer’s immediate and intermediate liquidity needs.
With respect to our investments, we strive to maintain a predominantly floating-rate basis position, but our strategy may change based on prevailing market conditions. Our floating-rate assets are subject to the risk that short-term interest rates may fall and, as a result, the investments would generate less interest income. Fixed-rate investments provide a known amount of interest income regardless of a change in interest rates. We sometimes use interest rate swaps in our financial investment portfolio.
Pfizer Inc. | 2019 Form 10-K | 41 |
We borrow primarily on a long-term, fixed-rate basis. From time to time, depending on market conditions, we will change the profile of our outstanding debt by entering into derivative financial instruments like interest rate swaps.
For details about these and other financial instruments, including fair valuation methodologies, see the Notes to Consolidated Financial Statements—Note 7A. Financial Instruments: Fair Value Measurements in our 2019 Financial Report.
The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to interest rate changes. In this sensitivity analysis, holding all other assumptions constant and assuming a parallel shift in the interest rate curve for all maturities and for all instruments, if there were a one hundred basis point increase in interest rates as of December 31, 2019, the expected adverse impact on our net income would not be significant.
Equity Price Risk
We hold equity securities with readily determinable fair values in life science companies as a result of certain business development transactions. While we are holding such securities, we are subject to equity price risk, and this may increase the volatility of our income in future periods due to changes in the fair value of equity investments. From time to time, we will sell such equity securities based on our business considerations, which may include limiting our price risk.
Our equity securities with readily determinable fair values are analyzed at year-end to determine their sensitivity to equity price rate changes. In this sensitivity analysis, the expected adverse impact on our net income would not be significant.
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Information required by this item is incorporated by reference from the Report of Independent Registered Public Accounting Firm in our 2019 Financial Report and from the consolidated financial statements, related notes and supplementary data in our 2019 Financial Report.
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls
As of the end of the period covered by this 2019 Form 10-K, we carried out an evaluation, under the supervision and with the participation of our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures are effective in alerting them in a timely manner to material information required to be disclosed in our periodic reports filed with the SEC.
Internal Control over Financial Reporting
Management’s report on the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act), and the related report of our independent registered public accounting firm, are included in our 2019 Financial Report under the headings Management’s Report on Internal Control Over Financial Reporting and Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting, respectively, and are incorporated by reference.
Changes in Internal Controls
During our most recent fiscal quarter, there has not been any change in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. | OTHER INFORMATION |
Not applicable.
Pfizer Inc. | 2019 Form 10-K | 42 |
PART III |
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information about our Directors is incorporated by reference from the discussion under the heading Item 1—Election of Directors in our 2020 Proxy Statement. Information about the Pfizer Policies on Business Conduct governing our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer, and the Code of Business Conduct and Ethics for Members of the Board of Directors, is incorporated by reference from the discussions under the headings Governance—Pfizer Policies on Business Conduct and —Code of Conduct for Directors in our 2020 Proxy Statement. Information regarding the procedures by which our shareholders may recommend nominees to our Board of Directors is incorporated by reference from the discussion under the headings Item 1—Election of Directors—Criteria for Board Membership and Submitting Proxy Proposals and Director Nominations for the 2021 Annual Meeting in our 2020 Proxy Statement. Information about our Audit Committee, including the members of the Committee, and our Audit Committee financial experts, is incorporated by reference from the discussion under the heading Governance—Board Information—Board and Committee Information—Board Committees—The Audit Committee in our 2020 Proxy Statement. The balance of the information required by this item is contained in the discussion entitled Information about Our Executive Officers in Part I of this 2019 Form 10-K.
ITEM 11. | EXECUTIVE COMPENSATION |
Information about Director and executive compensation is incorporated by reference from the discussion under the headings Non-Employee Director Compensation; Executive Compensation; and Governance—Board Information—Board and Committee Information—Board Committees—The Compensation Committee—Compensation Committee Interlocks and Insider Participation in our 2020 Proxy Statement.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information required by this item is incorporated by reference from the discussion under the headings Executive Compensation—Compensation Tables—Equity Compensation Plan Information and Securities Ownership in our 2020 Proxy Statement.
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information about certain relationships and transactions with related parties is incorporated by reference from the discussion under the headings Related Person Transactions and Indemnification—Transactions with Related Persons in our 2020 Proxy Statement. Information about director independence is incorporated by reference from the discussion under the heading Governance—Other Governance Practices and Policies—Director Independence in our 2020 Proxy Statement.
ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
Information about the fees for professional services rendered by our independent registered public accounting firm in 2019 and 2018 is incorporated by reference from the discussion under the heading Item 2—Ratification of Selection of Independent Registered Public Accounting Firm—Audit and Non-Audit Fees in our 2020 Proxy Statement. Our Audit Committee’s policy on pre-approval of audit and permissible non-audit services of our independent registered public accounting firm is incorporated by reference from the discussion under the heading Item 2—Ratification of Selection of Independent Registered Public Accounting Firm—Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm in our 2020 Proxy Statement.
Pfizer Inc. | 2019 Form 10-K | 43 |
PART IV |
ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
15(a)(1) Financial Statements. The following consolidated financial statements, related notes, report of independent registered public accounting firm and supplementary data from our 2019 Financial Report are incorporated by reference into Item 8 of Part II of this 2019 Form 10-K:
• | Report of Independent Registered Public Accounting Firm on the Consolidated Financial Statements |
• | Consolidated Statements of Income |
• | Consolidated Statements of Comprehensive Income |
• | Consolidated Balance Sheets |
• | Consolidated Statements of Equity |
• | Consolidated Statements of Cash Flows |
• | Notes to Consolidated Financial Statements |
• | Selected Quarterly Financial Data (Unaudited) |
15(a)(2) Financial Statement Schedules. Schedules are omitted because they are not required or because the information is provided elsewhere in the financial statements. The financial statements of unconsolidated subsidiaries are omitted because, considered in the aggregate, they would not constitute a significant subsidiary.
15(a)(3) Exhibits. These exhibits are available upon request. Requests should be directed to our Corporate Secretary, Pfizer Inc., 235 East 42nd Street, New York, New York 10017. The exhibit numbers preceded by an asterisk (*) indicate exhibits filed with this 2019 Form 10-K. All other exhibit numbers indicate exhibits filed by incorporation by reference. Exhibit numbers 10.1 through 10.38 are management contracts or compensatory plans or arrangements.
Stock and Asset Purchase Agreement, dated December 19, 2018, by and among Pfizer Inc., GlaxoSmithKline plc and GlaxoSmithKline Consumer Healthcare Holdings Limited is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the Stock and Asset Purchase Agreement.) | ||
Business Combination Agreement, dated July 29, 2019, by and among Pfizer Inc., Upjohn Inc., Utah Acquisition Sub Inc., Mylan N.V., Mylan I B.V. and Mylan II B.V. is incorporated by reference from our Current Report on Form 8-K filed on July 29, 2019 (File No. 001-03619). (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the Business Combination Agreement.) | ||
Separation and Distribution Agreement, dated as of July 29, 2019, by and between Pfizer Inc. and Upjohn Inc. is incorporated by reference from our Current Report on Form 8-K filed on July 29, 2019 (File No. 001-03619). (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the Separation and Distribution Agreement.) | ||
*2.4 | Amendment No. 1 to the Separation and Distribution Agreement, dated as of February 18, 2020, by and between Pfizer Inc. and Upjohn Inc. | |
Our Restated Certificate of Incorporation dated April 12, 2004, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended March 28, 2004 (File No. 001-03619). | ||
Amendment dated May 1, 2006 to Restated Certificate of Incorporation dated April 12, 2004, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 2, 2006 (File No. 001-03619). | ||
Our By-laws, as amended December 18, 2017, are incorporated by reference from our Current Report on Form 8-K filed on December 21, 2017 (File No. 001-03619). | ||
Indenture, dated as of January 30, 2001, between us and The Chase Manhattan Bank, is incorporated by reference from our Current Report on Form 8-K filed on January 30, 2001 (File No. 001-03619). | ||
First Supplemental Indenture, dated as of March 24, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 28, 2009 (File No. 001-03619). | ||
Pfizer Inc. | 2019 Form 10-K | 44 |
Second Supplemental Indenture, dated as of June 2, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2009 (File No. 001-03619). | ||
Third Supplemental Indenture, dated as of June 3, 2013, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2013 (File No. 001-03619). | ||
Fourth Supplemental Indenture, dated as of May 15, 2014, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on May 15, 2014 (File No. 001-03619). | ||
Fifth Supplemental Indenture, dated as of October 5, 2015, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on October 6, 2015 (File No. 001-03619). | ||
Sixth Supplemental Indenture, dated as of June 3, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on June 3, 2016 (File No. 001-03619). | ||
Seventh Supplemental Indenture, dated as of November 21, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on November 21, 2016 (File No. 001-03619). | ||
Eighth Supplemental Indenture, dated as of March 17, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (successor to the Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 17, 2017 (File No. 001-03619). | ||
Ninth Supplemental Indenture, dated as of March 6, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent and calculation agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 6, 2017 (File No. 001-03619). | ||
Tenth Supplemental Indenture, dated as of December 19, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on December 19, 2017 (File No. 001-03619). | ||
Indenture, dated as of April 10, 1992, between Wyeth (formerly American Home Products Corporation) and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3 (File No. 33-57339), filed on January 18, 1995. | ||
Supplemental Indenture, dated as of October 13, 1992, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3 (File No. 33-57339), filed on January 18, 1995. | ||
Fifth Supplemental Indenture, dated as of December 16, 2003, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s 2003 Annual Report on Form 10-K (File No. 001-01225). | ||
Sixth Supplemental Indenture, dated as of November 14, 2005, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on November 15, 2005 (File No. 001-01225). | ||
Seventh Supplemental Indenture, dated as of March 27, 2007, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on March 28, 2007 (File No. 001-01225). | ||
Eighth Supplemental Indenture, dated as of October 30, 2009, between Wyeth, us and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, formerly The Chase Manhattan Bank), as trustee, to Indenture dated as of April 10, 1992 (as amended on October 13, 1992), is incorporated by reference from our Current Report on Form 8-K filed on November 3, 2009 (File No. 001-03619). | ||
Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018 (File No. 001-03619). | ||
Pfizer Inc. | 2019 Form 10-K | 45 |
First Supplemental Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018 (File No. 001-03619). | ||
Second Supplemental Indenture, dated as of March 11, 2019, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on March 11, 2019 (File No. 001-03619). | ||
*4.21 | Description of Pfizer’s Securities. | |
4.22 | Except as set forth in Exhibits 4.1-21 above, the instruments defining the rights of holders of long-term debt securities of the Company and its subsidiaries have been omitted.1 | |
2001 Stock and Incentive Plan is incorporated by reference from our Proxy Statement for the 2001 Annual Meeting of Shareholders (File No. 001-03619). | ||
Pfizer Inc. 2004 Stock Plan, as Amended and Restated is incorporated by reference from our 2011 Annual Report on Form 10-K (File No. 001-03619). | ||
Pfizer Inc. 2014 Stock Plan is incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Shareholders (File No. 001-03619). | ||
Form of Acknowledgment and Consent and Summary of Key Terms for Stock Option Grants, RSUs and TSRUs is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619). | ||
Form of Executive Grant Letter is incorporated by reference from our 2015 Annual Report on Form 10-K (File No. 001-03619). | ||
Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 1 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). | ||
Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2016 (File No. 001-03619). | ||
Amendment No. 1 to the Pfizer Supplemental Savings Plan (Amended and Restated as of January 1, 2016), is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2017 (File No. 001-03619). | ||
Amendment No. 2 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 3 to the Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 30, 2018 (File No. 001-03619). | ||
Amendment No. 4 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 5 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 6 to the Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 30, 2019 (File No. 001-03619). | ||
Amendment No. 7 to the Pfizer Supplemental Savings Plan. | ||
Pfizer Inc. Global Performance Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2017 (File No. 001-03619). | ||
Executive Annual Incentive Plan is incorporated by reference from our 2012 Annual Report on Form 10-K (File No. 001-03619). | ||
Amended and Restated Deferred Compensation Plan is incorporated by reference from our 2012 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment to Amended and Restated Deferred Compensation Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 2 to Amended and Restated Deferred Compensation Plan, dated April 27, 2016, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 3, 2016 (File No. 001-03619). | ||
Wyeth 2005 (409A) Deferred Compensation Plan (frozen as of January 2012), together with all material Amendments, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619). | ||
1 We agree to furnish to the Securities and Exchange Commission, upon request, a copy of each instrument with respect to issuances of long-term debt of the Company and its subsidiaries. | ||
Pfizer Inc. | 2019 Form 10-K | 46 |
Amended and Restated Wyeth Supplemental Employee Savings Plan (effective as of January 1, 2005 and frozen as of January 2012), together with all material Amendments is incorporated by reference from our 2011 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment to Amended and Restated Wyeth Supplemental Employee Savings Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619). | ||
The form of Indemnification Agreement with each of our non-employee Directors is incorporated by reference from our 1996 Annual Report on Form 10-K (File No. 001-03619). | ||
The form of Indemnification Agreement with each of the Named Executive Officers identified in our Proxy Statement for the 2019 Annual Meeting of Shareholders is incorporated by reference from our 1997 Annual Report on Form 10-K (File No. 001-03619). | ||
Letter to Frank A. D’Amelio regarding replacement pension benefit dated August 22, 2007 is incorporated by reference from our Current Report on Form 8-K filed on August 22, 2007 (File No. 001-03619). | ||
Pfizer Inc. Executive Severance Plan is incorporated by referenced from our Current Report on Form 8-K filed on February 20, 2009 (File No. 001-03619). | ||
Amendment No. 1 to the Pfizer Inc. Executive Severance Plan is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). | ||
Amendment No. 2 to the Pfizer Inc. Executive Severance Plan. | ||
Annual Retainer Unit Award Plan (for Non-Employee Directors) (frozen as of March 1, 2006) as amended, is incorporated by reference from our 2008 Annual Report on Form 10-K (File No. 001-03619). | ||
Nonfunded Deferred Compensation and Unit Award Plan for Non-Employee Directors, as amended, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 28, 2014 (File No. 001-03619). | ||
Form of Special Award Letter Agreement is incorporated by reference from our Current Report on Form 8-K filed on October 28, 2009 (File No. 001-03619). | ||
Offer Letter to G. Mikael Dolsten, dated April 6, 2009, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2011 (File No. 001-03619). | ||
Form of Special Performance-Based Incentive Award Letter is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619). | ||
Form of Special Performance-Based Incentive Grant Letter is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619). | ||
Pfizer Inc. 2019 Stock Plan is incorporated by reference from our Proxy Statement for the 2019 Annual Meeting of Shareholders (File No. 001-03619). | ||
Time Sharing Agreement, dated December 17, 2018, by and between Pfizer Inc. and Ian C. Read is incorporated by reference from our 2018 Annual Report on Form 10-K (File No. 001-03619). | ||
Consulting Agreement, dated December 13, 2019, between Ian C. Read and Pfizer Inc. is incorporated by reference from our Current Report on Form 8-K filed on December 19, 2019 (File No. 001-03619). | ||
*13 | Portions of the 2019 Financial Report, which, except for those sections incorporated by reference, are furnished solely for the information of the SEC and are not to be deemed “filed.” | |
*21 | Subsidiaries of the Company. | |
*23 | Consent of Independent Registered Public Accounting Firm. | |
*24 | Power of Attorney (included as part of signature page). | |
*31.1 | Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
*31.2 | Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
*32.1 | Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
*32.2 | Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
Exhibit 101: | ||
*101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |
*101.SCH | Inline XBRL Taxonomy Extension Schema | |
*101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase | |
Pfizer Inc. | 2019 Form 10-K | 47 |
*101.LAB | Inline XBRL Taxonomy Extension Label Linkbase | |
*101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase | |
*101.DEF | Inline XBRL Taxonomy Extension Definition Document | |
104 | Cover Page Interactive Data File - the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |
ITEM 16. | FORM 10-K SUMMARY |
A Form 10-K summary is provided at the beginning of this 2019 Form 10-K, with hyperlinked cross-references. This allows users to easily locate the corresponding items in this 2019 Form 10-K, where the disclosure is fully presented. The summary does not include certain Part III information that is incorporated by reference from our 2020 Proxy Statement.
Pfizer Inc. | 2019 Form 10-K | 48 |
SIGNATURES
Under the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, this report was signed on behalf of the Registrant by the authorized person named below.
Pfizer Inc. | |||
Dated: February 27, 2020 | By: | /S/ MARGARET M. MADDEN | |
Margaret M. Madden Senior Vice President and Corporate Secretary Chief Governance Counsel | |||
We, the undersigned directors and officers of Pfizer Inc., hereby severally constitute Douglas M. Lankler and Margaret M. Madden, and each of them singly, our true and lawful attorneys with full power to them and each of them to sign for us, in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K filed with the Securities and Exchange Commission.
Under the requirements of the Securities Exchange Act of 1934, this report was signed by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
Signature | Title | Date | ||
/S/ ALBERT BOURLA Albert Bourla | Chairman and Chief Executive Officer (Principal Executive Officer) | February 25, 2020 | ||
/S/ FRANK A. D’AMELIO Frank A. D’Amelio | Chief Financial Officer, Executive Vice President, Business Operations and Global Supply (Principal Financial Officer) | February 25, 2020 | ||
/S/ LORETTA V. CANGIALOSI Loretta V. Cangialosi | Senior Vice President—Controller (Principal Accounting Officer) | February 25, 2020 | ||
/S/ RONALD E. BLAYLOCK Ronald E. Blaylock | Director | February 25, 2020 | ||
/S/ W. DON CORNWELL W. Don Cornwell | Director | February 25, 2020 | ||
/S/ JOSEPH J. ECHEVARRIA Joseph J. Echevarria | Director | February 25, 2020 | ||
/S/ SCOTT GOTTLIEB Scott Gottlieb | Director | February 25, 2020 | ||
/S/ HELEN H. HOBBS Helen H. Hobbs | Director | February 25, 2020 | ||
Signature | Title | Date | ||
/S/ JAMES M. KILTS James M. Kilts | Director | February 25, 2020 | ||
/S/ DAN R. LITTMAN Dan R. Littman | Director | February 25, 2020 | ||
/S/ SHANTANU NARAYEN Shantanu Narayen | Director | February 25, 2020 | ||
/S/ SUZANNE NORA JOHNSON Suzanne Nora Johnson | Director | February 25, 2020 | ||
/S/ JAMES C. SMITH James C. Smith | Director | February 25, 2020 | ||
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
See also Merck & Co., Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-03-31)
