Philip Morris International Inc. - Quarter Report: 2022 March (Form 10-Q)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
| ☑ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the quarterly period ended March 31, 2022
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from to
Commission File Number 001-33708
Philip Morris International Inc. | ||||||||||||||
(Exact name of registrant as specified in its charter)
| Virginia | 13-3435103 | ||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | ||||
| 120 Park Avenue | New York | New York | 10017 | ||||||||
| (Address of principal executive offices) | (Zip Code) | ||||||||||
| Registrant’s telephone number, including area code | (917) | 663-2000 | ||||||
Former name, former address and former fiscal year, if changed since last report
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
| Common Stock, no par value | PM | New York Stock Exchange | ||||||||||||
| 2.375% Notes due 2022 | PM22B | New York Stock Exchange | ||||||||||||
| 2.500% Notes due 2022 | PM22 | New York Stock Exchange | ||||||||||||
| 2.500% Notes due 2022 | PM22C | New York Stock Exchange | ||||||||||||
| 2.625% Notes due 2023 | PM23 | New York Stock Exchange | ||||||||||||
| 2.125% Notes due 2023 | PM23B | New York Stock Exchange | ||||||||||||
| 3.600% Notes due 2023 | PM23A | New York Stock Exchange | ||||||||||||
| 2.875% Notes due 2024 | PM24 | New York Stock Exchange | ||||||||||||
| 2.875% Notes due 2024 | PM24C | New York Stock Exchange | ||||||||||||
| 0.625% Notes due 2024 | PM24B | New York Stock Exchange | ||||||||||||
| 3.250% Notes due 2024 | PM24A | New York Stock Exchange | ||||||||||||
| 2.750% Notes due 2025 | PM25 | New York Stock Exchange | ||||||||||||
| 3.375% Notes due 2025 | PM25A | New York Stock Exchange | ||||||||||||
| 2.750% Notes due 2026 | PM26A | New York Stock Exchange | ||||||||||||
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
| 2.875% Notes due 2026 | PM26 | New York Stock Exchange | ||||||||||||
| 0.125% Notes due 2026 | PM26B | New York Stock Exchange | ||||||||||||
| 3.125% Notes due 2027 | PM27 | New York Stock Exchange | ||||||||||||
| 3.125% Notes due 2028 | PM28 | New York Stock Exchange | ||||||||||||
| 2.875% Notes due 2029 | PM29 | New York Stock Exchange | ||||||||||||
| 3.375% Notes due 2029 | PM29A | New York Stock Exchange | ||||||||||||
| 0.800% Notes due 2031 | PM31 | New York Stock Exchange | ||||||||||||
| 3.125% Notes due 2033 | PM33 | New York Stock Exchange | ||||||||||||
| 2.000% Notes due 2036 | PM36 | New York Stock Exchange | ||||||||||||
| 1.875% Notes due 2037 | PM37A | New York Stock Exchange | ||||||||||||
| 6.375% Notes due 2038 | PM38 | New York Stock Exchange | ||||||||||||
| 1.450% Notes due 2039 | PM39 | New York Stock Exchange | ||||||||||||
| 4.375% Notes due 2041 | PM41 | New York Stock Exchange | ||||||||||||
| 4.500% Notes due 2042 | PM42 | New York Stock Exchange | ||||||||||||
| 3.875% Notes due 2042 | PM42A | New York Stock Exchange | ||||||||||||
| 4.125% Notes due 2043 | PM43 | New York Stock Exchange | ||||||||||||
| 4.875% Notes due 2043 | PM43A | New York Stock Exchange | ||||||||||||
| 4.250% Notes due 2044 | PM44 | New York Stock Exchange | ||||||||||||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer þ Accelerated filer ☐
Non-accelerated filer ☐ Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No þ
At April 22, 2022, there were 1,550,110,316 shares outstanding of the registrant’s common stock, no par value per share.
1
PHILIP MORRIS INTERNATIONAL INC.
TABLE OF CONTENTS
| Page No. | ||||||||
| PART I - | ||||||||
| Item 1. | ||||||||
| Condensed Consolidated Statements of Earnings for the | ||||||||
Three Months Ended March 31, 2022 and 2021 | ||||||||
| Condensed Consolidated Statements of Comprehensive Earnings for the | ||||||||
| Three Months Ended March 31, 2022 and 2021 | ||||||||
| Condensed Consolidated Balance Sheets at | ||||||||
March 31, 2022 and December 31, 2021 | ||||||||
| Condensed Consolidated Statements of Cash Flows for the | ||||||||
| Three Months Ended March 31, 2022 and 2021 | ||||||||
| Condensed Consolidated Statements of Stockholders’ (Deficit) Equity for the | ||||||||
| Three Months Ended March 31, 2022 and 2021 | ||||||||
| Item 2. | ||||||||
| Item 4. | ||||||||
| PART II - | ||||||||
| Item 1. | ||||||||
| Item 1A. | ||||||||
| Item 2. | ||||||||
| Item 6. | ||||||||
In this report, “PMI,” “we,” “us” and “our” refer to Philip Morris International Inc. and its subsidiaries.
Trademarks and service marks in this report are the registered property of, or licensed by, the subsidiaries of Philip Morris International Inc. and are italicized.
2
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements.
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Statements of Earnings
(in millions of dollars, except per share data)
(Unaudited)
| For the Three Months Ended March 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Revenues including excise taxes | $ | 19,341 | $ | 19,355 | |||||||
| Excise taxes on products | 11,595 | 11,770 | |||||||||
| Net revenues | 7,746 | 7,585 | |||||||||
| Cost of sales (Note 18) | 2,608 | 2,274 | |||||||||
| Gross profit | 5,138 | 5,311 | |||||||||
| Marketing, administration and research costs (Notes 16 & 18) | 1,802 | 1,849 | |||||||||
| Amortization of intangibles | 38 | 18 | |||||||||
| Operating income | 3,298 | 3,444 | |||||||||
| Interest expense, net | 154 | 167 | |||||||||
| Pension and other employee benefit costs (Note 3) | 4 | 28 | |||||||||
| Earnings before income taxes | 3,140 | 3,249 | |||||||||
| Provision for income taxes | 619 | 697 | |||||||||
| Equity investments and securities (income)/loss, net | 56 | (43) | |||||||||
| Net earnings | 2,465 | 2,595 | |||||||||
| Net earnings attributable to noncontrolling interests | 134 | 177 | |||||||||
| Net earnings attributable to PMI | $ | 2,331 | $ | 2,418 | |||||||
Per share data (Note 6): | |||||||||||
| Basic earnings per share | $ | 1.50 | $ | 1.55 | |||||||
| Diluted earnings per share | $ | 1.50 | $ | 1.55 | |||||||
See notes to condensed consolidated financial statements.
3
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Statements of Comprehensive Earnings
(in millions of dollars)
(Unaudited)
| For the Three Months Ended March 31, | ||||||||||||||
| 2022 | 2021 | |||||||||||||
| Net earnings | $ | 2,465 | $ | 2,595 | ||||||||||
| Other comprehensive earnings (losses), net of income taxes: | ||||||||||||||
Change in currency translation adjustments: | ||||||||||||||
Unrealized gains (losses), net of income taxes of $(31) in 2022 and $(85) in 2021 | (194) | 223 | ||||||||||||
Change in net loss and prior service cost: | ||||||||||||||
Amortization of net losses, prior service costs and net transition costs, net of income taxes of $(13) in 2022 and $(18) in 2021 | 55 | 81 | ||||||||||||
Change in fair value of derivatives accounted for as hedges: | ||||||||||||||
Gains (losses) recognized, net of income taxes of $(20) in 2022 and $(14) in 2021 | 110 | 76 | ||||||||||||
(Gains) losses transferred to earnings, net of income taxes of $2 in 2022 and $0 in 2021 | (9) | 21 | ||||||||||||
| Total other comprehensive earnings (losses) | (38) | 401 | ||||||||||||
Total comprehensive earnings | 2,427 | 2,996 | ||||||||||||
| Less comprehensive earnings attributable to: | ||||||||||||||
| Noncontrolling interests | 279 | 143 | ||||||||||||
| Comprehensive earnings attributable to PMI | $ | 2,148 | $ | 2,853 | ||||||||||
See notes to condensed consolidated financial statements.
4
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
(in millions of dollars)
(Unaudited)
| March 31, 2022 | December 31, 2021 | ||||||||||
| ASSETS | |||||||||||
| Cash and cash equivalents | $ | 4,622 | $ | 4,496 | |||||||
Trade receivables (less allowances of $32 in 2022 and $70 in 2021) | 3,650 | 3,123 | |||||||||
Other receivables (less allowances of $36 in 2022 and $36 in 2021) | 768 | 817 | |||||||||
Inventories: | |||||||||||
| Leaf tobacco | 1,691 | 1,642 | |||||||||
| Other raw materials | 2,297 | 1,652 | |||||||||
| Finished product | 4,696 | 5,426 | |||||||||
| 8,684 | 8,720 | ||||||||||
| Other current assets | 1,000 | 561 | |||||||||
Total current assets | 18,724 | 17,717 | |||||||||
Property, plant and equipment, at cost | 14,563 | 14,732 | |||||||||
| Less: accumulated depreciation | 8,559 | 8,564 | |||||||||
| 6,004 | 6,168 | ||||||||||
| Goodwill (Note 4) | 6,632 | 6,680 | |||||||||
| Other intangible assets, net (Note 4) | 2,786 | 2,818 | |||||||||
| Equity investments (Note 12) | 4,312 | 4,463 | |||||||||
| Deferred income taxes | 694 | 895 | |||||||||
Other assets (less allowances of $21 in 2022 and $21 in 2021) | 2,581 | 2,549 | |||||||||
| TOTAL ASSETS | $ | 41,733 | $ | 41,290 | |||||||
See notes to condensed consolidated financial statements.
Continued
5
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Balance Sheets (Continued)
(in millions of dollars, except share data)
(Unaudited)
| March 31, 2022 | December 31, 2021 | ||||||||||
| LIABILITIES | |||||||||||
| Short-term borrowings (Note 10) | $ | 2,441 | $ | 225 | |||||||
| Current portion of long-term debt (Note 10) | 2,897 | 2,798 | |||||||||
| Accounts payable | 3,203 | 3,331 | |||||||||
| Accrued liabilities: | |||||||||||
| Marketing and selling | 678 | 811 | |||||||||
| Taxes, except income taxes | 5,618 | 6,324 | |||||||||
| Employment costs | 864 | 1,146 | |||||||||
| Dividends payable | 1,958 | 1,958 | |||||||||
| Other | 1,854 | 1,637 | |||||||||
| Income taxes | 904 | 1,025 | |||||||||
| Total current liabilities | 20,417 | 19,255 | |||||||||
Long-term debt (Note 10) | 24,019 | 24,783 | |||||||||
| Deferred income taxes | 751 | 726 | |||||||||
| Employment costs | 2,880 | 2,968 | |||||||||
| Income taxes and other liabilities | 1,869 | 1,766 | |||||||||
| Total liabilities | 49,936 | 49,498 | |||||||||
Contingencies (Note 8) | |||||||||||
STOCKHOLDERS’ (DEFICIT) EQUITY | |||||||||||
Common stock, no par value (2,109,316,331 shares issued in 2022 and 2021) | — | — | |||||||||
| Additional paid-in capital | 2,118 | 2,225 | |||||||||
| Earnings reinvested in the business | 33,468 | 33,082 | |||||||||
| Accumulated other comprehensive losses | (9,760) | (9,577) | |||||||||
| 25,826 | 25,730 | ||||||||||
Less: cost of repurchased stock (559,231,389 and 559,146,338 shares in 2022 and 2021, respectively) | 35,924 | 35,836 | |||||||||
| Total PMI stockholders’ deficit | (10,098) | (10,106) | |||||||||
| Noncontrolling interests | 1,895 | 1,898 | |||||||||
| Total stockholders’ deficit | (8,203) | (8,208) | |||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | $ | 41,733 | $ | 41,290 | |||||||
See notes to condensed consolidated financial statements.
6
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(in millions of dollars)
(Unaudited)
| For the Three Months Ended March 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | |||||||||||
| Net earnings | $ | 2,465 | $ | 2,595 | |||||||
| Adjustments to reconcile net earnings to operating cash flows: | |||||||||||
| Depreciation and amortization | 253 | 245 | |||||||||
| Deferred income tax (benefit) provision | (1) | 33 | |||||||||
| Asset impairment and exit costs, net of cash paid (Note 16) | (28) | (36) | |||||||||
| Cash effects of changes, net of the effects from acquired companies: | |||||||||||
| Receivables, net | (553) | (427) | |||||||||
| Inventories | (232) | 305 | |||||||||
| Accounts payable | 14 | (67) | |||||||||
| Accrued liabilities and other current assets | (835) | (2,108) | |||||||||
| Income taxes | (93) | (91) | |||||||||
| Pension plan contributions | (34) | (30) | |||||||||
| Other | 162 | 16 | |||||||||
| Net cash provided by operating activities | 1,118 | 435 | |||||||||
| CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | |||||||||||
| Capital expenditures | (229) | (179) | |||||||||
| Equity investments | (20) | — | |||||||||
| Net investment hedges | 121 | 199 | |||||||||
| Other | (68) | 35 | |||||||||
| Net cash provided by (used in) investing activities | (196) | 55 | |||||||||
See notes to condensed consolidated financial statements.
Continued
7
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows (Continued)
(in millions of dollars)
(Unaudited)
| For the Three Months Ended March 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | |||||||||||
| Short-term borrowing activity by original maturity: | |||||||||||
| Net issuances (repayments) - maturities of 90 days or less | $ | 1,916 | $ | (49) | |||||||
| Issuances - maturities longer than 90 days | 305 | — | |||||||||
| Long-term debt repaid | (496) | (1,632) | |||||||||
| Repurchases of common stock | (209) | — | |||||||||
| Dividends paid | (1,952) | (1,879) | |||||||||
Payments to noncontrolling interests and Other (Note 17) | (265) | (89) | |||||||||
| Net cash used in financing activities | (701) | (3,649) | |||||||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (95) | (220) | |||||||||
Cash, cash equivalents and restricted cash (1): | |||||||||||
| Increase (Decrease) | 126 | (3,379) | |||||||||
| Balance at beginning of period | 4,500 | 7,285 | |||||||||
| Balance at end of period | $ | 4,626 | $ | 3,906 | |||||||
(1) The amounts for cash, cash equivalents and restricted cash shown above include restricted cash of $4 million and $4 million as of March 31, 2022 and 2021, respectively, and $4 million and $5 million as of December 31, 2021 and 2020, respectively, which were included in other current assets in the condensed consolidated balance sheets.
See notes to condensed consolidated financial statements.
8
Philip Morris International Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ (Deficit) Equity
For the Three Months Ended March 31, 2022 and 2021
(in millions of dollars, except per share amounts)
(Unaudited)
| PMI Stockholders’ (Deficit) Equity | |||||||||||||||||||||||||||||||||||||||||
| Common Stock | Additional Paid-in Capital | Earnings Reinvested in the Business | Accumulated Other Comprehensive Losses | Cost of Repurchased Stock | Noncontrolling Interests | Total | |||||||||||||||||||||||||||||||||||
| Balances, January 1, 2021 | $ | — | $ | 2,105 | $ | 31,638 | $ | (11,181) | $ | (35,129) | $ | 1,936 | $ | (10,631) | |||||||||||||||||||||||||||
| Net earnings | 2,418 | 177 | 2,595 | ||||||||||||||||||||||||||||||||||||||
| Other comprehensive earnings (losses), net of income taxes | 435 | (34) | 401 | ||||||||||||||||||||||||||||||||||||||
| Issuance of stock awards | (25) | 69 | 44 | ||||||||||||||||||||||||||||||||||||||
Dividends declared ($1.20 per share) | (1,878) | (1,878) | |||||||||||||||||||||||||||||||||||||||
| Payments to noncontrolling interests | (105) | (105) | |||||||||||||||||||||||||||||||||||||||
| Balances, March 31, 2021 | $ | — | $ | 2,080 | $ | 32,178 | $ | (10,746) | $ | (35,060) | $ | 1,974 | $ | (9,574) | |||||||||||||||||||||||||||
| Balances, January 1, 2022 | $ | — | $ | 2,225 | $ | 33,082 | $ | (9,577) | $ | (35,836) | $ | 1,898 | $ | (8,208) | |||||||||||||||||||||||||||
| Net earnings | 2,331 | 134 | 2,465 | ||||||||||||||||||||||||||||||||||||||
| Other comprehensive earnings (losses), net of income taxes | (12) | (26) | (38) | ||||||||||||||||||||||||||||||||||||||
| Issuance of stock awards | (77) | 111 | 34 | ||||||||||||||||||||||||||||||||||||||
Dividends declared ($1.25 per share) | (1,945) | (1,945) | |||||||||||||||||||||||||||||||||||||||
| Payments to noncontrolling interests | (101) | (101) | |||||||||||||||||||||||||||||||||||||||
| Common stock repurchased | (199) | (199) | |||||||||||||||||||||||||||||||||||||||
| Other (Note 17) | (30) | (171) | (10) | (211) | |||||||||||||||||||||||||||||||||||||
| Balances, March 31, 2022 | $ | — | $ | 2,118 | $ | 33,468 | $ | (9,760) | $ | (35,924) | $ | 1,895 | $ | (8,203) | |||||||||||||||||||||||||||
See notes to condensed consolidated financial statements.
9
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 1. Background and Basis of Presentation:
Background
Philip Morris International Inc. is a holding company incorporated in Virginia, U.S.A. (also referred to herein as the U.S., the United States or the United States of America), whose subsidiaries and affiliates and their licensees are primarily engaged in the manufacture and sale of cigarettes and reduced-risk products including heat-not-burn, vapor and oral nicotine products, in markets outside of the United States of America. Throughout these financial statements, the term "PMI" refers to Philip Morris International Inc. and its subsidiaries.
Reduced-risk products ("RRPs") is the term PMI uses to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing smoking. PMI has a range of RRPs in various stages of development, scientific assessment and commercialization.
"Platform 1" is the term PMI uses to refer to PMI’s reduced-risk product that uses a precisely controlled heating device into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol.
Basis of Presentation
The interim condensed consolidated financial statements of PMI are unaudited. These interim condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") and such principles are applied on a consistent basis. It is the opinion of PMI’s management that all adjustments necessary for a fair statement of the interim results presented have been reflected therein. All such adjustments were of a normal recurring nature. Net revenues and net earnings attributable to PMI for any interim period are not necessarily indicative of results that may be expected for the entire year.
In the third quarter of 2021, PMI acquired Fertin Pharma A/S, Vectura Group plc. and OtiTopic, Inc. On March 31, 2022, PMI launched a new Wellness and Healthcare business consolidating these entities, Vectura Fertin Pharma. The operating results of this new business is reported in an Other category. For further details, see Note 7. Segment Reporting and Note 17. Acquisitions.
Certain prior years' amounts have been reclassified to conform with the current year's presentation. During the first quarter of 2022, one of Fertin Pharma's product lines was moved from the Other category to the European Union segment (reduced-risk product category). For further details, see Note 4. Goodwill and Other Intangible Assets, net. The change did not have a material impact on PMI's consolidated financial position, results of operations or cash flows in any of the periods presented.
These statements should be read in conjunction with the audited consolidated financial statements and related notes, which appear in PMI’s Annual Report on Form 10-K for the year ended December 31, 2021.
10
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 2. Stock Plans:
In May 2017, PMI’s shareholders approved the Philip Morris International Inc. 2017 Performance Incentive Plan (the “2017 Plan”). Under the 2017 Plan, PMI may grant to eligible employees restricted shares and restricted share units, performance-based cash incentive awards and performance-based equity awards. Up to 25 million shares of PMI’s common stock may be issued under the 2017 Plan. At March 31, 2022, shares available for grant under the 2017 Plan were 12,644,751.
In May 2017, PMI’s shareholders also approved the Philip Morris International Inc. 2017 Stock Compensation Plan for Non-Employee Directors (the “2017 Non-Employee Directors Plan”). A non-employee director is defined as a member of the PMI Board of Directors who is not a full-time employee of PMI or of any corporation in which PMI owns, directly or indirectly, stock possessing at least 50% of the total combined voting power of all classes of stock entitled to vote in the election of directors in such corporation. Up to 1 million shares of PMI common stock may be awarded under the 2017 Non-Employee Directors Plan. At March 31, 2022, shares available for grant under the plan were 914,413.
Restricted share unit (RSU) awards
During the three months ended March 31, 2022 and 2021, shares granted to eligible employees, the weighted-average grant date fair value per share and the recorded compensation expense related to RSU awards were as follows:
| Number of Shares Granted | Weighted-Average Grant Date Fair Value Per RSU Award Granted | Compensation Expense Related to RSU Awards (in millions) | |||||||||||||||
| 2022 | 1,551,050 | $ 105.04 | $ | 39 | |||||||||||||
| 2021 | 1,972,770 | $ 81.86 | $ | 40 | |||||||||||||
As of March 31, 2022, PMI had $259 million of total unrecognized compensation cost related to non-vested RSU awards. The cost is recognized over the original restriction period of the awards, which is typically three years after the date of the award, or upon death, disability or reaching the age of 58.
During the three months ended March 31, 2022, 1,447,664 RSU awards vested. The grant date fair value of all the vested awards was approximately $112 million. The total fair value of RSU awards that vested during the three months ended March 31, 2022 was approximately $159 million.
Performance share unit (PSU) awards
During the three months ended March 31, 2022 and 2021, PMI granted PSU awards to certain executives. The PSU awards require the achievement of certain performance metrics, which are predetermined at the time of grant, typically over a three-year performance cycle. The performance metrics for such PSU's granted during the three months ended March 31, 2022 consisted of PMI's Total Shareholder Return ("TSR") relative to a predetermined peer group and on an absolute basis (40% weight), PMI’s currency-neutral compound annual adjusted diluted earnings per share growth rate (30% weight), and a Sustainability Index, which consists of two drivers:
•Product Sustainability (20% weight) measuring progress on PMI's efforts to maximize the benefits of smoke-free products, purposefully phase out cigarettes, seek net positive impact in wellness and healthcare, and reduce post-consumer waste; and
•Operational Sustainability (10% weight) measuring progress on PMI's efforts to tackle climate change, preserve nature, improve the quality of life of people in its supply chain, and foster an empowered, and inclusive workplace.
The performance metrics for such PSU's granted during the three months ended March 31, 2021 consisted of PMI's TSR relative to a predetermined peer group and on an absolute basis (40% weight), PMI’s currency-neutral compound annual adjusted diluted earnings per share growth rate (30% weight), and PMI’s performance against specific measures of PMI’s transformation, defined as net revenues from PMI's RRPs and any other non-combustible products as a percentage of PMI's total net revenues in the last year of the performance cycle (30% weight).
11
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The aggregate of the weighted performance factors for the three metrics in each such PSU award determines the percentage of PSUs that will vest at the end of the three-year performance cycle. The minimum percentage of such PSUs that can vest is zero, with a target percentage of 100 and a maximum percentage of 200. Each such vested PSU entitles the participant to one share of common stock. An aggregate weighted PSU performance factor of 100 will result in the targeted number of PSUs being vested. At the end of the performance cycle, participants are entitled to an amount equivalent to the accumulated dividends paid on common stock during the performance cycle for the number of shares earned.
During the three months ended March 31, 2022 and 2021, shares granted to eligible employee, the grant date fair value per share and the recorded compensation expense related to PSU awards were as follows:
| Number of Shares Granted | Grant Date Fair Value Subject to Other Performance Metrics | Grant Date Fair Value Subject to TSR Performance Metric | Compensation Expense Related to PSU Awards (in millions) | |||||||||||
| (Per Share) | (Per Share) | |||||||||||||
| 2022 | 451,790 | $ 105.07 | $ 143.94 | $ | 21 | |||||||||
| 2021 | 574,410 | $ 81.86 | $ 106.93 | $ | 12 | |||||||||
The grant date fair value of the PSU awards subject to the other performance metrics was determined by using the market price of PMI’s stock on the date of the grant. The grant date fair value of the PSU market based awards subject to the TSR performance metric was determined by using the Monte Carlo simulation model. The following assumptions were used to determine the grant date fair value of the PSU awards subject to the TSR performance metric:
| 2022 | 2021 | ||||||||||
Risk-free interest rate (a) | 1.6 | % | 0.2 | % | |||||||
Expected volatility (b) | 28.6 | % | 31.7 | % | |||||||
(a) Based on the U.S. Treasury yield curve.
(b) Determined using the observed historical volatility.
As of March 31, 2022, PMI had $76 million of total unrecognized compensation cost related to non-vested PSU awards. The cost is recognized over the performance cycle of the awards, or upon death, disability or reaching the age of 58.
During the three months ended March 31, 2022, 669,960 PSU awards vested. The grant date fair value of all the vested awards was approximately $54 million. The total fair value of PSU awards that vested during the three months ended March 31, 2022 was approximately $74 million.
Note 3. Benefit Plans:
Pension coverage for employees of PMI’s subsidiaries is provided, to the extent deemed appropriate, through separate plans, many of which are governed by local statutory requirements. In addition, PMI provides health care and other benefits to substantially all U.S. retired employees and certain non-U.S. retired employees. In general, health care benefits for non-U.S. retired employees are covered through local government plans.
Pension and other employee benefit costs per the condensed consolidated statements of earnings consisted of the following:
| For the Three Months Ended March 31, | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Net pension costs (income) | $ | (25) | $ | (1) | |||||||
| Net postemployment costs | 27 | 28 | |||||||||
| Net postretirement costs | 2 | 1 | |||||||||
| Total pension and other employee benefit costs | $ | 4 | $ | 28 | |||||||
12
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Pension Plans
Components of Net Periodic Benefit Cost
Net periodic pension cost consisted of the following:
Pension (1) | |||||||||||
| For the Three Months Ended March 31, | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Service cost | $ | 60 | $ | 75 | |||||||
| Interest cost | 20 | 13 | |||||||||
| Expected return on plan assets | (92) | (95) | |||||||||
| Amortization: | |||||||||||
| Net loss | 48 | 80 | |||||||||
| Prior service cost | (1) | 1 | |||||||||
| Net periodic pension cost | $ | 35 | $ | 74 | |||||||
(1) Primarily non-U.S. based defined benefit retirement plans.
Employer Contributions
PMI makes, and plans to make, contributions, to the extent that they are tax deductible and meet specific funding requirements of its funded pension plans. Employer contributions of $34 million were made to the pension plans during the three months ended March 31, 2022. Currently, PMI anticipates making additional contributions during the remainder of 2022 of approximately $73 million to its pension plans, based on current tax and benefit laws. However, this estimate is subject to change as a result of changes in tax and other benefit laws, as well as asset performance significantly above or below the assumed long-term rate of return on pension assets, or changes in interest and currency rates.
Note 4. Goodwill and Other Intangible Assets, net:
The movements in goodwill were as follows:
| (in millions) | European Union | Eastern Europe | Middle East & Africa | South & Southeast Asia | East Asia & Australia | Americas | Other | Total | ||||||||||||||||||
| Balances, December 31, 2021 | $ | 1,397 | $ | 295 | $ | 79 | $ | 2,828 | $ | 539 | $ | 611 | $ | 931 | $ | 6,680 | ||||||||||
| Changes due to: | ||||||||||||||||||||||||||
| Currency | (19) | (8) | 8 | (29) | (7) | 27 | (20) | (48) | ||||||||||||||||||
| Balances, March 31, 2022 | $ | 1,378 | $ | 287 | $ | 87 | $ | 2,799 | $ | 532 | $ | 638 | $ | 911 | $ | 6,632 | ||||||||||
At March 31, 2022, goodwill primarily reflects PMI’s business combinations in Colombia, Greece, Indonesia, Mexico, Pakistan, the Philippines and Serbia, as well as the preliminary purchase price allocation of Fertin Pharma A/S and Vectura Group plc., which were acquired in September 2021.
As discussed in Note 1. Background and Basis of Presentation, during the first quarter of 2022, one of Fertin Pharma's product lines was moved from the Other category to the European Union segment. As a result, the December 31, 2021 opening goodwill balance in the table above included a reclassification of $24 million from the Other category to the European Union segment.
13
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Details of other intangible assets were as follows:
| March 31, 2022 | December 31, 2021 | |||||||||||||||||||||||||
| (in millions) | Weighted-Average Remaining Useful Life | Gross Carrying Amount | Accumulated Amortization | Net | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||||||||||||
| Non-amortizable intangible assets | $ | 1,321 | $ | 1,321 | $ | 1,312 | $ | 1,312 | ||||||||||||||||||
| Amortizable intangible assets: | ||||||||||||||||||||||||||
| Trademarks | 11 years | 1,205 | $ | 656 | 549 | 1,201 | $ | 639 | 562 | |||||||||||||||||
| Developed technology, including patents | 12 years | 855 | 82 | 773 | 859 | 63 | 796 | |||||||||||||||||||
Other(1) | 11 years | 238 | 95 | 143 | 238 | 90 | 148 | |||||||||||||||||||
| Total other intangible assets | $ | 3,619 | $ | 833 | $ | 2,786 | $ | 3,610 | $ | 792 | $ | 2,818 | ||||||||||||||
(1) Primarily includes distribution networks and customer relationships.
Non-amortizable intangible assets substantially consist of trademarks from PMI’s acquisitions in Indonesia and Mexico, as well as the preliminary purchase price allocation associated with PMI's business combinations in 2021 (primarily in-process research and development). The increase since December 31, 2021 was due to currency movements of $9 million.
The change in the accumulated amortization from December 31, 2021, was mainly due to the 2022 amortization of $38 million, and currency movements of $3 million.
Amortization expense for each of the next five years is estimated to be $150 million or less, assuming no additional transactions occur that require the amortization of intangible assets.
Note 5. Financial Instruments:
Overview
PMI operates in markets outside of the United States of America, with manufacturing and sales facilities in various locations around the world. PMI utilizes certain financial instruments to manage foreign currency and interest rate exposures. Derivative financial instruments are used by PMI principally to reduce exposures to market risks resulting from fluctuations in foreign currency exchange and interest rates by creating offsetting exposures. PMI is not a party to leveraged derivatives and, by policy, does not use derivative financial instruments for speculative purposes. Substantially all of PMI's derivative financial instruments are subject to master netting arrangements, whereby the right to offset occurs in the event of default by a participating party. While these contracts contain the enforceable right to offset through close-out netting rights, PMI elects to present them on a gross basis in the consolidated balance sheets. Collateral associated with these arrangements is in the form of cash and is unrestricted. Financial instruments qualifying for hedge accounting must maintain a specified level of effectiveness between the hedging instrument and the item being hedged, both at inception and throughout the hedged period. PMI formally documents the nature and relationships between the hedging instruments and hedged items, as well as its risk-management objectives, strategies for undertaking the various hedge transactions and method of assessing hedge effectiveness. Additionally, for hedges of forecasted transactions, the significant characteristics and expected terms of the forecasted transaction must be specifically identified, and it must be probable that each forecasted transaction will occur. If it were deemed probable that the forecasted transaction would not occur, the gain or loss would be recognized in earnings.
PMI uses deliverable and non-deliverable forward foreign exchange contracts, foreign currency swaps and foreign currency options, collectively referred to as foreign exchange contracts ("foreign exchange contracts"), and interest rate contracts to
14
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
mitigate its exposure to changes in exchange and interest rates from third-party and intercompany actual and forecasted transactions. Both foreign exchange contracts and interest rate contracts are collectively referred to as derivative contracts ("derivative contracts"). The primary currencies to which PMI is exposed include the Euro, Indonesian rupiah, Japanese yen, Mexican peso, Philippine peso, Russian ruble and Swiss franc. At March 31, 2022, PMI had contracts with aggregate notional amounts of $25.8 billion of which $4.9 billion related to cash flow hedges, $7.9 billion related to hedges of net investments in foreign operations, $1.0 billion related to fair value hedges and $12.0 billion related to other derivatives that primarily offset currency exposures on intercompany financing.
The fair value of PMI’s derivative contracts included in the condensed consolidated balance sheets as of March 31, 2022 and December 31, 2021, were as follows:
| Derivative Assets | Derivative Liabilities | ||||||||||||||||||||||||||||||||||
| Fair Value | Fair Value | ||||||||||||||||||||||||||||||||||
| At | At | At | At | ||||||||||||||||||||||||||||||||
| (in millions) | Balance Sheet Classification | March 31, 2022 | December 31, 2021 | Balance Sheet Classification | March 31, 2022 | December 31, 2021 | |||||||||||||||||||||||||||||
| Derivative contracts designated as hedging instruments | Other current assets | $ | 291 | $ | 173 | Other accrued liabilities | $ | 46 | $ | 34 | |||||||||||||||||||||||||
| Other assets | 63 | 22 | Income taxes and other liabilities | 182 | 190 | ||||||||||||||||||||||||||||||
| Derivative contracts not designated as hedging instruments | Other current assets | 55 | 37 | Other accrued liabilities | 83 | 75 | |||||||||||||||||||||||||||||
| Other assets | — | — | Income taxes and other liabilities | 14 | — | ||||||||||||||||||||||||||||||
| Total gross amount derivatives contracts presented in the condensed consolidated balance sheets | $ | 409 | $ | 232 | $ | 325 | $ | 299 | |||||||||||||||||||||||||||
| Gross amounts not offset in the condensed consolidated balance sheets | |||||||||||||||||||||||||||||||||||
| Financial instruments | (199) | (126) | (199) | (126) | |||||||||||||||||||||||||||||||
| Cash collateral received/pledged | (200) | (93) | (87) | (151) | |||||||||||||||||||||||||||||||
| Net amount | $ | 10 | $ | 13 | $ | 39 | $ | 22 | |||||||||||||||||||||||||||
PMI assesses the fair value of its foreign exchange contracts and interest rate contracts using standard valuation models that use, as their basis, readily observable market inputs. The fair value of PMI’s foreign exchange forward contracts, foreign currency swaps and interest rate contracts is determined by using the prevailing foreign exchange spot rates and interest rate differentials, and the respective maturity dates of the instruments. The fair value of PMI’s currency options is determined by using a Black-Scholes methodology based on foreign exchange spot rates and interest rate differentials, currency volatilities and maturity dates. PMI’s derivative contracts have been classified within Level 2 at March 31, 2022 and December 31, 2021.
15
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
For the three months ended March 31, 2022 and 2021, PMI's derivative contracts impacted the condensed consolidated statements of earnings and comprehensive earnings as follows:
| (pre-tax, in millions) | For the Three Months Ended March 31, | ||||||||||||||||||||||||||||||||||||||||
| Amount of Gain/(Loss) Recognized in Other Comprehensive Earnings/(Losses) on Derivatives | Statement of Earnings Classification of Gain/(Loss) on Derivatives | Amount of Gain/(Loss) Reclassified from Other Comprehensive Earnings/(Losses) into Earnings | Amount of Gain/(Loss) Recognized in Earnings | ||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||
| Derivative contracts designated as hedging instruments: | |||||||||||||||||||||||||||||||||||||||||
| Cash flow hedges | $ | 130 | $ | 90 | |||||||||||||||||||||||||||||||||||||
| Net revenues | $ | 17 | $ | — | |||||||||||||||||||||||||||||||||||||
| Cost of sales | — | — | |||||||||||||||||||||||||||||||||||||||
| Marketing, administration and research costs | (3) | (18) | |||||||||||||||||||||||||||||||||||||||
| Interest expense, net | (3) | (3) | |||||||||||||||||||||||||||||||||||||||
| Fair value hedges | Interest expense, net | $ | (37) | $ | — | ||||||||||||||||||||||||||||||||||||
Net investment hedges (a) | 105 | 318 | Interest expense, net (b) | 33 | 42 | ||||||||||||||||||||||||||||||||||||
| Derivative contracts not designated as hedging instruments: | Interest expense, net | 8 | 11 | ||||||||||||||||||||||||||||||||||||||
Marketing, administration and research costs (c) | (1) | 287 | |||||||||||||||||||||||||||||||||||||||
| Total | $ | 235 | $ | 408 | $ | 11 | $ | (21) | $ | 3 | $ | 340 | |||||||||||||||||||||||||||||
(a) Amount of gains (losses) on hedges of net investments principally related to changes in exchange and interest rates between the Euro and U.S. dollar
(b) Represent the gains for amounts excluded from the effectiveness testing
(c) The gains (losses) from these contracts attributable to changes in foreign currency exchange rates substantially offset the (losses) and gains generated by the underlying intercompany and third-party loans being hedged
Cash Flow Hedges
PMI has entered into derivative contracts to hedge the foreign currency exchange and interest rate risks related to certain forecasted transactions. Gains and losses associated with qualifying cash flow hedge contracts are deferred as components of accumulated other comprehensive losses until the underlying hedged transactions are reported in PMI’s condensed consolidated statements of earnings. As of March 31, 2022, PMI has hedged forecasted transactions for periods not exceeding the next twenty-one months with the exception of one derivative contract that expires in May 2024. The impact of these hedges is primarily included in operating cash flows on PMI’s condensed consolidated statements of cash flows.
16
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Fair Value Hedges
PMI has entered into fixed-to-floating interest rate contracts, designated as fair value hedges to minimize exposure to changes in the fair value of fixed rate U.S. dollar-denominated debt that results from fluctuations in benchmark interest rates. For derivative contracts that are designated and qualify as fair value hedges the gain or loss on the derivative, as well as the offsetting gain or loss on the hedged items attributable to the hedged risk, is recognized in current earnings. The carrying amount of the debt hedged, which includes the cumulative adjustment for fair value gains/losses, as of March 31, 2022 was $957 million, and is recorded in long-term debt in the condensed consolidated balance sheets. The cumulative amount of fair value gains/(losses) included in the carrying amount of the debt hedged was $(36) million as of March 31, 2022.
Hedges of Net Investments in Foreign Operations
PMI designates derivative contracts and certain foreign currency denominated debt instruments as net investment hedges, primarily of its Euro net assets. For the three months ended March 31, 2022 and 2021, the amount of pre-tax gain/(loss) related to these debt instruments, that was reported as a component of accumulated other comprehensive losses within currency translation adjustments, was $66 million and $181 million, respectively. The premiums paid for, and settlements of, net investment hedges are included in investing cash flows on PMI’s condensed consolidated statements of cash flows.
Other Derivatives
PMI has entered into derivative contracts to hedge the foreign currency exchange and interest rate risks related to intercompany loans between certain subsidiaries, and third-party loans. While effective as economic hedges, no hedge accounting is applied for these contracts; therefore, the gains (losses) relating to these contracts are reported in PMI’s condensed consolidated statements of earnings.
Qualifying Hedging Activities Reported in Accumulated Other Comprehensive Losses
Derivative gains or losses reported in accumulated other comprehensive losses are a result of qualifying hedging activity. Transfers of these gains or losses to earnings are offset by the corresponding gains or losses on the underlying hedged item. Hedging activity affected accumulated other comprehensive losses, net of income taxes, as follows:
| (in millions) | For the Three Months Ended March 31, | |||||||
| 2022 | 2021 | |||||||
| Gain/(loss) as of January 1, | $ | 4 | $ | (85) | ||||
| Derivative (gains)/losses transferred to earnings | (9) | 21 | ||||||
| Change in fair value | 110 | 76 | ||||||
| Gain/(loss) as of March 31, | $ | 105 | $ | 12 | ||||
At March 31, 2022, PMI expects $76 million of derivative gains that are included in accumulated other comprehensive losses to be reclassified to the condensed consolidated statement of earnings within the next 12 months. These gains are expected to be substantially offset by the statement of earnings impact of the respective hedged transactions.
Contingent Features
PMI’s derivative instruments do not contain contingent features.
Credit Exposure and Credit Risk
PMI is exposed to credit loss in the event of non-performance by counterparties. While PMI does not anticipate non-performance, its risk is limited to the fair value of the financial instruments less any cash collateral received or pledged. PMI actively monitors its exposure to credit risk through the use of credit approvals and credit limits and by selecting and continuously monitoring a diverse group of major international banks and financial institutions as counterparties.
17
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Other Investments
A PMI investment, which is comprised primarily of money market funds, has been classified within Level 1 and had a fair value of $82 million at March 31, 2022. For the three months ended March 31, 2022 the unrealized pre-tax gains on these investments were immaterial.
Note 6. Earnings Per Share:
Basic and diluted earnings per share (“EPS”) were calculated using the following:
| (in millions) | For the Three Months Ended March 31, | |||||||
| 2022 | 2021 | |||||||
| Net earnings attributable to PMI | $ | 2,331 | $ | 2,418 | ||||
Less distributed and undistributed earnings attributable to share-based payment awards | 7 | 7 | ||||||
| Net earnings for basic and diluted EPS | $ | 2,324 | $ | 2,411 | ||||
| Weighted-average shares for basic EPS | 1,550 | 1,558 | ||||||
| Plus contingently issuable performance stock units (PSUs) | 2 | 2 | ||||||
| Weighted-average shares for diluted EPS | 1,552 | 1,560 | ||||||
Unvested share-based payment awards that contain non-forfeitable rights to dividends or dividend equivalents are participating securities and therefore are included in PMI’s earnings per share calculation pursuant to the two-class method.
For the 2022 and 2021 computations, there were no antidilutive stock awards.
Note 7. Segment Reporting:
PMI’s subsidiaries and affiliates are primarily engaged in the manufacture and sale of cigarettes and RRPs, including heat-not-burn, vapor and oral nicotine products, in markets outside of the United States of America. PMI's segments are generally organized by geographic region and managed by segment managers who are responsible for the operating and financial results of the regions inclusive of combustible and reduced-risk product categories sold in the region. PMI currently has six geographical segments: the European Union; Eastern Europe; Middle East & Africa; South & Southeast Asia; East Asia & Australia; and Americas; as well as an Other category. Other consists of the operating results of PMI's new Wellness and Healthcare business, Vectura Fertin Pharma. For further details on these acquisitions, see Note 17. Acquisitions. PMI records net revenues and operating income to its geographical segments based upon the geographic area in which the customer resides.
PMI’s chief operating decision maker evaluates geographical segment performance and allocates resources based on regional operating income, which includes results from all product categories sold in each region. Business operations in the Other category are managed and evaluated separately.
PMI disaggregates its net revenue from contracts with customers by both geographic location and product category for each of PMI's six geographical segments. For the new Wellness and Healthcare business discussed above, net revenues from contracts with customers are included in the Other category. PMI believes this best depicts how the nature, amount, timing and uncertainty of its revenue and cash flows are affected by economic factors.
18
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Segment data were as follows:
| (in millions) | For the Three Months Ended March 31, | |||||||
| 2022 | 2021 | |||||||
| Net revenues: | ||||||||
| European Union | $ | 3,012 | $ | 2,909 | ||||
| Eastern Europe | 726 | 796 | ||||||
| Middle East & Africa | 991 | 801 | ||||||
| South & Southeast Asia | 1,123 | 1,173 | ||||||
| East Asia & Australia | 1,404 | 1,472 | ||||||
| Americas | 424 | 434 | ||||||
| Other | 66 | — | ||||||
| Net revenues | $ | 7,746 | $ | 7,585 | ||||
| Operating income (loss): | ||||||||
| European Union | $ | 1,527 | $ | 1,490 | ||||
| Eastern Europe | 144 | 261 | ||||||
| Middle East & Africa | 521 | 335 | ||||||
| South & Southeast Asia | 445 | 529 | ||||||
| East Asia & Australia | 571 | 695 | ||||||
| Americas | 121 | 134 | ||||||
| Other | (31) | — | ||||||
| Operating income | $ | 3,298 | $ | 3,444 | ||||
Items affecting the comparability of results from operations were as follows:
•Asset impairment and exit costs - See Note 16. Asset Impairment and Exit Costs for details of the $48 million pre-tax charge and a breakdown of these costs by segment for the three months ended March 31, 2021.
•Charges related to the war in Ukraine - See Note 18. War in Ukraine for details of the $42 million pre-tax charges in the Eastern Europe segment for the three months ended March 31, 2022.
19
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
PMI's net revenues by product category were as follows:
| (in millions) | For the Three Months Ended March 31, | |||||||
| 2022 | 2021 | |||||||
| Net revenues: | ||||||||
| Combustible products: | ||||||||
| European Union | $ | 1,810 | $ | 1,950 | ||||
| Eastern Europe | 456 | 492 | ||||||
| Middle East & Africa | 929 | 780 | ||||||
| South & Southeast Asia | 1,118 | 1,171 | ||||||
| East Asia & Australia | 601 | 648 | ||||||
| Americas | 416 | 422 | ||||||
| Total combustible products | $ | 5,330 | $ | 5,463 | ||||
| Reduced-risk products: | ||||||||
| European Union | $ | 1,202 | $ | 959 | ||||
| Eastern Europe | 270 | 304 | ||||||
| Middle East & Africa | 62 | 21 | ||||||
| South & Southeast Asia | 5 | 2 | ||||||
| East Asia & Australia | 803 | 824 | ||||||
| Americas | 8 | 12 | ||||||
| Total reduced-risk products | $ | 2,350 | $ | 2,122 | ||||
| Other: | ||||||||
| Other | $ | 66 | $ | — | ||||
| Total PMI net revenues | $ | 7,746 | $ | 7,585 | ||||
Note: Sum of product categories or Regions might not foot to total PMI due to roundings.
Net revenues related to combustible products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of PMI's cigarettes and other tobacco products combined. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos, and do not include reduced-risk products.
Net revenues related to reduced-risk products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of PMI's heated tobacco units, heat-not-burn devices and related accessories, and other nicotine-containing products, which primarily include PMI's e-vapor and oral nicotine products.
Net revenues in the Other category primarily consist of operating revenues generated from the sale of inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of PMI's new Wellness and Healthcare business, Vectura Fertin Pharma.
20
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 8. Contingencies:
Tobacco-Related Litigation
Legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. Our indemnitees include distributors, licensees, and others that have been named as parties in certain cases and that we have agreed to defend, as well as to pay costs and some or all of judgments, if any, that may be entered against them. Pursuant to the terms of the Distribution Agreement between Altria Group, Inc. (“Altria”) and PMI, PMI will indemnify Altria and Philip Morris USA Inc. (“PM USA”), a U.S. tobacco subsidiary of Altria, for tobacco product claims based in substantial part on products manufactured by PMI or contract manufactured for PMI by PM USA, and PM USA will indemnify PMI for tobacco product claims based in substantial part on products manufactured by PM USA, excluding tobacco products contract manufactured for PMI.
It is possible that there could be adverse developments in pending cases against us and our subsidiaries. An unfavorable outcome or settlement of pending tobacco-related litigation could encourage the commencement of additional litigation.
Damages claimed in some of the tobacco-related litigation are significant and, in certain cases in Brazil, Canada and Nigeria, range into the billions of U.S. dollars. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. However, as discussed below, we have to date been largely successful in defending tobacco-related litigation.
We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. At the present time, except as stated otherwise in this Note 8. Contingencies, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
It is possible that our consolidated results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. Nevertheless, although litigation is subject to uncertainty, we and each of our subsidiaries named as a defendant believe, and each has been so advised by counsel handling the respective cases, that we have valid defenses to the litigation pending against us, as well as valid bases for appeal of adverse verdicts. All such cases are, and will continue to be, vigorously defended. However, we and our subsidiaries may enter into settlement discussions in particular cases if we believe it is in our best interests to do so.
CCAA Proceedings and Stay of Tobacco-Related Cases Pending in Canada
As a result of the Court of Appeal of Quebec’s decision in both the Létourneau and Blais cases described below, our subsidiary, Rothmans, Benson & Hedges Inc. (“RBH”), and the other defendants, JTI Macdonald Corp., and Imperial Tobacco Canada Limited, sought protection in the Ontario Superior Court of Justice under the Companies’ Creditors Arrangement Act (“CCAA”) on March 22, March 8, and March 12, 2019, respectively. CCAA is a Canadian federal law that permits a Canadian business to restructure its affairs while carrying on its business in the ordinary course. The initial CCAA order made by the Ontario Superior Court on March 22, 2019 authorizes RBH to pay all expenses incurred in carrying on its business in the ordinary course after the CCAA filing, including obligations to employees, vendors, and suppliers. As further described in Item 8, Note 20. Deconsolidation of RBH of PMI's Annual Report on Form 10-K for the year ended December 31, 2021, RBH's financial results have been deconsolidated from our consolidated financial statements since March 22, 2019. As part of the CCAA proceedings, there is currently a comprehensive stay up to and including September 30, 2022 of all tobacco-related litigation pending in Canada against RBH and the other defendants, including PMI and our indemnitees (PM USA and Altria), namely, the smoking and health class actions filed in various Canadian provinces and health care cost recovery actions. These proceedings are presented below under the caption “Stayed Litigation — Canada.” Ernst & Young Inc. has been appointed as monitor of RBH in the CCAA proceedings. In accordance with the CCAA process, as the parties work towards a plan of arrangement or compromise in a confidential mediation, it is anticipated that the court will set additional hearings and further extend the stay of proceedings. On April 17, 2019, the Ontario Superior Court ruled that RBH and the other defendants will not be allowed to file an application to the Supreme Court of Canada for leave to appeal the Court of Appeal’s decision in the Létourneau and the Blais cases so long as the comprehensive stay of all tobacco-related litigation in Canada remains in effect and that the time period to file the application would be extended by the stay period. While RBH believes that the findings of liability and damages in both Létourneau and the Blais cases were incorrect, the CCAA proceedings will provide a forum for
21
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
RBH to seek resolution through a plan of arrangement or compromise of all tobacco-related litigation pending in Canada. It is not possible to predict the resolution of the underlying legal proceedings or the length of the CCAA process.
Stayed Litigation — Canada
Smoking and Health Litigation — Canada
In the first class action pending in Canada, Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in November 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants. The plaintiffs, an anti-smoking organization and an individual smoker, sought compensatory and punitive damages for each member of the class who suffers allegedly from certain smoking-related diseases. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and found that the class members’ compensatory damages totaled approximately CAD 15.5 billion, including pre-judgment interest (approximately $12.2 billion). The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion, including pre-judgment interest (approximately $2.4 billion)). In addition, the trial court awarded CAD 90,000 (approximately $70,800) in punitive damages, allocating CAD 30,000 (approximately $23,600) to RBH. The trial court estimated the disease class at 99,957 members. RBH appealed to the Court of Appeal of Quebec. In October 2015, the Court of Appeal ordered RBH to furnish security totaling CAD 226 million (approximately $178 million) to cover both the Létourneau and Blais cases, which RBH has paid in installments through March 2017. The Court of Appeal ordered Imperial Tobacco Canada Ltd. to furnish security totaling CAD 758 million (approximately $596 million) in installments through June 2017. JTI Macdonald Corp. was not required to furnish security in accordance with plaintiffs’ motion. The Court of Appeal ordered that the security is payable upon a final judgment of the Court of Appeal affirming the trial court’s judgment or upon further order of the Court of Appeal.
On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the compensatory and punitive damages award while reducing the total amount of compensatory damages to approximately CAD 13.5 billion including interest (approximately $10.6 billion) due to the trial court’s error in the calculation of interest. The compensatory damages award is on a joint and several basis with an allocation of 20% to RBH (approximately CAD 2.7 billion, including pre-judgment interest (approximately $2.1 billion)). The Court of Appeal upheld the trial court’s findings that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking and by conspiring to prevent consumers from learning of the dangers of smoking. The Court of Appeal further held that the plaintiffs either need not prove, or had adequately proven, that these faults were a cause of the class members’ injuries. In accordance with the judgment, defendants were required to deposit their respective portions of the damages awarded in both the Létourneau case described below and the Blais case, approximately CAD 1.1 billion (approximately $865 million), into trust accounts within 60 days. RBH’s share of the deposit was approximately CAD 257 million (approximately $194 million). PMI recorded a pre-tax charge of $194 million in its consolidated results, representing $142 million net of tax, as tobacco litigation-related expense, in the first quarter of 2019. The charge reflects PMI’s assessment of the portion of the judgment that represents probable and estimable loss prior to the deconsolidation of RBH and corresponds to the trust account deposit required by the judgment.
In the second class action pending in Canada, Cecilia Létourneau v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI-Macdonald Corp., Quebec Superior Court, Canada, filed in September 1998, RBH and other Canadian cigarette manufacturers (Imperial Tobacco Canada Ltd. and JTI-Macdonald Corp.) are defendants. The plaintiff, an individual smoker, sought compensatory and punitive damages for each member of the class who is deemed addicted to smoking. The class was certified in 2005. The trial court issued its judgment on May 27, 2015. The trial court found RBH and two other Canadian manufacturers liable and awarded a total of CAD 131 million (approximately $103 million) in punitive damages, allocating CAD 46 million (approximately $36 million) to RBH. The trial court estimated the size of the addiction class at 918,000 members but declined to award compensatory damages to the addiction class because the evidence did not establish the claims with sufficient accuracy. The trial court found that a claims process to allocate the awarded punitive damages to individual class members would be too expensive and difficult to administer. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court’s findings of liability and the total amount of punitive damages awarded allocating CAD 57 million including interest (approximately $45 million) to RBH. See the Blais description above and Item 8, Note 20. Deconsolidation of RBH in PMI's Annual Report on Form 10-K for the year ended December 31, 2021 for further detail concerning the security order pertaining to both Létourneau and Blais cases and the impact of the decision on PMI’s financial statements.
22
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
RBH and PMI believe the findings of liability and damages in both Létourneau and the Blais cases were incorrect and in contravention of applicable law on several grounds including the following: (i) defendants had no obligation to warn class members who knew, or should have known, of the risks of smoking; (ii) defendants cannot be liable to class members who would have smoked regardless of what warnings were given; and (iii) defendants cannot be liable to all class members given the individual differences between class members.
In the third class action pending in Canada, Kunta v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Winnipeg, Canada, filed June 12, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic obstructive pulmonary disease (“COPD”), severe asthma, and mild reversible lung disease resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the fourth class action pending in Canada, Adams v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Saskatchewan, Canada, filed July 10, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, emphysema, heart disease, or cancer, as well as restitution of profits.
In the fifth class action pending in Canada, Semple v. Canadian Tobacco Manufacturers' Council, et al., The Supreme Court (trial court), Nova Scotia, Canada, filed June 18, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and COPD resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products.
In the sixth class action pending in Canada, Dorion v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Alberta, Canada, filed June 15, 2009, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic bronchitis and severe sinus infections resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. To date, we, our subsidiaries, and our indemnitees have not been properly served with the complaint.
In the seventh class action pending in Canada, McDermid v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and heart disease resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from heart disease allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed.
In the eighth class action pending in Canada, Bourassa v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada, filed June 25, 2010, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, the heir to a deceased smoker, alleges that the decedent was addicted to tobacco products and suffered from emphysema resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from chronic respiratory diseases allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed. In December 2014, plaintiff filed an amended statement of claim.
In the ninth class action pending in Canada, Suzanne Jacklin v. Canadian Tobacco Manufacturers' Council, et al., Ontario Superior Court of Justice, filed June 20, 2012, we, RBH, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised
23
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, heart disease, or cancer, as well as restitution of profits.
Health Care Cost Recovery Litigation — Canada
In the first health care cost recovery case pending in Canada, Her Majesty the Queen in Right of British Columbia v. Imperial Tobacco Limited, et al., Supreme Court, British Columbia, Vancouver Registry, Canada, filed January 24, 2001, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The plaintiff, the government of the province of British Columbia, brought a claim based upon legislation enacted by the province authorizing the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, resulting from a “tobacco related wrong.”
In the second health care cost recovery case filed in Canada, Her Majesty the Queen in Right of New Brunswick v. Rothmans Inc., et al., Court of Queen's Bench of New Brunswick, Trial Court, New Brunswick, Fredericton, Canada, filed March 13, 2008, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of New Brunswick based on legislation enacted in the province. This legislation is similar to the law introduced in British Columbia that authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the third health care cost recovery case filed in Canada, Her Majesty the Queen in Right of Ontario v. Rothmans Inc., et al., Ontario Superior Court of Justice, Toronto, Canada, filed September 29, 2009, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Ontario based on legislation enacted in the province. This legislation is similar to the laws introduced in British Columbia and New Brunswick that authorize the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fourth health care cost recovery case filed in Canada, Attorney General of Newfoundland and Labrador v. Rothmans Inc., et al., Supreme Court of Newfoundland and Labrador, St. Johns, Canada, filed February 8, 2011, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Newfoundland and Labrador based on legislation enacted in the province that is similar to the laws introduced in British Columbia, New Brunswick and Ontario. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the fifth health care cost recovery case filed in Canada, Attorney General of Quebec v. Imperial Tobacco Limited, et al., Superior Court of Quebec, Canada, filed June 8, 2012, we, RBH, our indemnitee (PM USA), and other members of the industry are defendants. The claim was filed by the government of the province of Quebec based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the sixth health care cost recovery case filed in Canada, Her Majesty in Right of Alberta v. Altria Group, Inc., et al., Supreme Court of Queen's Bench Alberta, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Alberta based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the seventh health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Manitoba v. Rothmans, Benson & Hedges, Inc., et al., The Queen's Bench, Winnipeg Judicial Centre, Canada, filed May 31, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Manitoba based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the eighth health care cost recovery case filed in Canada, The Government of Saskatchewan v. Rothmans, Benson & Hedges Inc., et al., Queen's Bench, Judicial Centre of Saskatchewan, Canada, filed June 8, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Saskatchewan based on legislation enacted in the province that is similar to the laws enacted in several other Canadian
24
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the ninth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Prince Edward Island v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Prince Edward Island (General Section), Canada, filed September 10, 2012, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Prince Edward Island based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
In the tenth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Nova Scotia v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Nova Scotia, Canada, filed January 2, 2015, we, RBH, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Nova Scotia based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a “tobacco related wrong.”
__________
The table below lists the number of tobacco-related cases pertaining to combustible products pending against us and/or our subsidiaries or indemnitees as of March 31, 2022, and March 31, 2021:¹
| Type of Case | Number of Cases Pending as of March 31, 2022 | Number of Cases Pending as of March 31, 2021 | ||||||||||||
| Individual Smoking and Health Cases | 41 | 43 | ||||||||||||
| Smoking and Health Class Actions | 9 | 9 | ||||||||||||
| Health Care Cost Recovery Actions | 17 | 17 | ||||||||||||
| Label-Related Class Actions | — | — | ||||||||||||
| Individual Label-Related Cases | 5 | 5 | ||||||||||||
| Public Civil Actions | 1 | 2 | ||||||||||||
Since 1995, when the first tobacco-related litigation was filed against a PMI entity, 524 Smoking and Health, Label-Related, Health Care Cost Recovery, and Public Civil Actions in which we and/or one of our subsidiaries and/or indemnitees were a defendant have been terminated in our favor. Fourteen cases have had decisions in favor of plaintiffs. Ten of these cases have subsequently reached final resolution in our favor and four remain on appeal.
______
¹ Includes cases pending in Canada.
25
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The table below lists the verdict and significant post-trial developments in the four pending cases where a verdict was returned in favor of the plaintiff:
| Date | Location of Court/Name of Plaintiff | Type of Case | Verdict | Post-Trial Developments | ||||||||||||||||||||||
| May 27, 2015 | Canada/Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais | Class Action | On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Blais class on liability and found the class members’ compensatory damages totaled approximately CAD 15.5 billion (approximately $12.2 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion including pre-judgment interest (approximately $2.4 billion)). The trial court awarded CAD 90,000 (approximately $70,800) in punitive damages, allocating CAD 30,000 (approximately $23,600) to our subsidiary. The trial court ordered defendants to pay CAD 1 billion (approximately $786 million) of the compensatory damage award, CAD 200 million (approximately $157 million) of which is our subsidiary’s portion, into a trust within 60 days. | In June 2015, RBH commenced the appellate process with the Court of Appeal of Quebec. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court's decision. (See “Stayed Litigation — Canada” for further detail.) | ||||||||||||||||||||||
26
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
| Date | Location of Court/Name of Plaintiff | Type of Case | Verdict | Post-Trial Developments | ||||||||||||||||||||||
| May 27, 2015 | Canada/Cecilia Létourneau | Class Action | On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Létourneau class on liability and awarded a total of CAD 131 million (approximately $103 million) in punitive damages, allocating CAD 46 million (approximately $36 million) to RBH. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days. The court did not order the payment of compensatory damages. | In June 2015, RBH commenced the appellate process with the Court of Appeal of Quebec. On March 1, 2019, the Court of Appeal issued a decision largely affirming the trial court's decision. (See “Stayed Litigation — Canada” for further detail.) | ||||||||||||||||||||||
| Date | Location of Court/Name of Plaintiff | Type of Case | Verdict | Post-Trial Developments | ||||||||||||||||||||||
| August 5, 2016 | Argentina/Hugo Lespada | Individual Action | On August 5, 2016, the Civil Court No. 14 - Mar del Plata, issued a verdict in favor of plaintiff, an individual smoker, and awarded him ARS 110,000 (approximately $963), plus interest, in compensatory and moral damages. The trial court found that our subsidiary failed to warn plaintiff of the risk of becoming addicted to cigarettes. | On August 23, 2016, our subsidiary filed its notice of appeal. On October 31, 2017, the Civil and Commercial Court of Appeals of Mar del Plata ruled that plaintiff's claim was barred by the statute of limitations and it reversed the trial court's decision. On May 17, 2021 plaintiff filed a federal extraordinary appeal. On November 1, 2021, the Supreme Court of the Province of Buenos Aires dismissed plaintiff's federal extraordinary appeal. On November 10, 2021, plaintiff filed a direct appeal before the Federal Supreme Court. | ||||||||||||||||||||||
27
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
| Date | Location of Court/Name of Plaintiff | Type of Case | Verdict | Post-Trial Developments | ||||||||||||||||||||||
| June 17, 2021 | Argentina/Claudia Milano | Individual Action | On June 17, 2021, the Civil Court No. 9 - Mar del Plata, issued a verdict in favor of plaintiff, an individual smoker, and awarded her smoking cessation treatments, ARS 150,000 (approximately $1,314), in compensatory and moral damages, and ARS 4,000,000 (approximately $35,028) in punitive damages, plus interest and costs. The trial court found that our subsidiary failed to warn plaintiff of the risk of becoming addicted to cigarettes. | On July 2, 2021, our subsidiary filed its notice of appeal. In addition, plaintiff filed an appeal challenging the dismissal of the claim for psychological damages. As required by local law, our subsidiary deposited the damages awarded, plus interest and costs, in total ARS 6,114,428 (approximately $53,544), into a court escrow account. Our subsidiary challenged the amount determined by the court. The Mar del Plata Court of Appeals granted our subsidiary's challenge to the escrow amount determined by the trial court. As a result, on December 16, 2021, ARS 893,428 (approximately $7,823) was returned to our subsidiary. If our subsidiary ultimately prevails on appeal, the remaining deposited amounts will be returned to our subsidiary. | ||||||||||||||||||||||
Pending claims related to tobacco products generally fall within the following categories:
Smoking and Health Litigation: These cases primarily allege personal injury and are brought by individual plaintiffs or on behalf of a class or purported class of individual plaintiffs. Plaintiffs' allegations of liability in these cases are based on various theories of recovery, including negligence, gross negligence, strict liability, fraud, misrepresentation, design defect, failure to warn, breach of express and implied warranties, violations of deceptive trade practice laws and consumer protection statutes. Plaintiffs in these cases seek various forms of relief, including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include licit activity, failure to state a claim, lack of defect, lack of proximate cause, assumption of the risk, contributory negligence, and statute of limitations.
As of March 31, 2022, there were a number of smoking and health cases pending against us, our subsidiaries or indemnitees, as follows:
•41 cases brought by individual plaintiffs in Argentina (30), Brazil (2), Canada (2), Chile (3), the Philippines (1), Turkey (1) and Scotland (1), as well as 1 case brought by an individual plaintiff in the United States District Court for the District of Oregon in May 2021 (See information regarding the provisions of the 2008 Share Distribution Agreement between PMI and Altria that provide for indemnities to PMI for certain liabilities concerning tobacco products under the caption "Tobacco-Related Litigation" described above), compared with 43 such cases on March 31, 2021; and
•9 cases brought on behalf of classes of individual plaintiffs, compared with 9 such cases on March 31, 2021.
The class actions pending in Canada are described above under the caption “Smoking and Health Litigation — Canada.”
Health Care Cost Recovery Litigation: These cases, brought by governmental and non-governmental plaintiffs, seek reimbursement of health care cost expenditures allegedly caused by tobacco products. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including unjust enrichment, negligence, negligent design, strict liability, breach of express and implied warranties, violation of a voluntary undertaking or special duty, fraud, negligent misrepresentation, conspiracy, public nuisance, defective product, failure to warn, sale of cigarettes to minors, and claims under statutes governing
28
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
competition and deceptive trade practices. Plaintiffs in these cases seek various forms of relief including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include lack of proximate cause, remoteness of injury, failure to state a claim, adequate remedy at law, “unclean hands” (namely, that plaintiffs cannot obtain equitable relief because they participated in, and benefited from, the sale of cigarettes), and statute of limitations.
As of March 31, 2022, there were 17 health care cost recovery cases pending against us, our subsidiaries or indemnitees in Brazil (1), Canada (10), Korea (1) and Nigeria (5), compared with 17 such cases on March 31, 2021.
The health care cost recovery actions pending in Canada are described above under the caption “Health Care Cost Recovery Litigation — Canada.”
In the health care cost recovery case in Brazil, The Attorney General of Brazil v. Souza Cruz Ltda., et al., Federal Trial Court, Porto Alegre, Rio Grande do Sul, Brazil, filed May 21, 2019, we, our subsidiaries, and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases in certain prior years, payment of anticipated costs of treating future alleged smoking-related diseases, and moral damages. Defendants filed answers to the complaint in May 2020.
In the first health care cost recovery case in Nigeria, The Attorney General of Lagos State v. British American Tobacco (Nigeria) Limited, et al., High Court of Lagos State, Lagos, Nigeria, filed March 13, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the second health care cost recovery case in Nigeria, The Attorney General of Kano State v. British American Tobacco (Nigeria) Limited, et al., High Court of Kano State, Kano, Nigeria, filed May 9, 2007, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of challenging the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the third health care cost recovery case in Nigeria, The Attorney General of Gombe State v. British American Tobacco (Nigeria) Limited, et al., High Court of Gombe State, Gombe, Nigeria, filed October 17, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In February 2011, the court ruled that the plaintiff had not complied with the procedural steps necessary to serve us. As a result of this ruling, plaintiff must re-serve its claim. We have not yet been re-served.
In the fourth health care cost recovery case in Nigeria, The Attorney General of Oyo State, et al., v. British American Tobacco (Nigeria) Limited, et al., High Court of Oyo State, Ibadan, Nigeria, filed May 25, 2007, we and other members of the industry are defendants. Plaintiffs seek reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We challenged service as improper. In June 2010, the court ruled that plaintiffs did not have leave to serve the writ of summons on the defendants and that they must re-serve the writ. We have not yet been re-served.
In the fifth health care cost recovery case in Nigeria, The Attorney General of Ogun State v. British American Tobacco (Nigeria) Limited, et al., High Court of Ogun State, Abeokuta, Nigeria, filed February 26, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In May 2010, the trial court rejected our objections to the court's jurisdiction. We have appealed. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the health care cost recovery case in Korea, the National Health Insurance Service v. KT&G, et. al., filed April 14, 2014, our subsidiary and other Korean manufacturers are defendants. Plaintiff alleges that defendants concealed the health hazards of smoking, marketed to youth, added ingredients to make their products more harmful and addictive, and misled consumers into believing that Lights cigarettes are safer than regular cigarettes. The National Health Insurance Service seeks to recover
29
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
damages allegedly incurred in treating 3,484 patients with small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer from 2003 to 2012. The trial court dismissed the case in its entirety on November 20, 2020. Plaintiff appealed.
Label-Related Cases: These cases, now brought only by individual plaintiffs, allege that the use of the descriptor “Lights” or other alleged misrepresentations or omissions of labeling information constitute fraudulent and misleading conduct. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including misrepresentation, deception, and breach of consumer protection laws. Plaintiffs seek various forms of relief including restitution, injunctive relief, and compensatory and other damages. Defenses raised include lack of causation, lack of reliance, assumption of the risk, and statute of limitations.
As of March 31, 2022, there were 5 label-related cases brought by individual plaintiffs in Italy (1) and Chile (4) pending against our subsidiaries, compared with 5 such cases on March 31, 2021.
Public Civil Actions: Claims have been filed either by an individual, or a public or private entity, seeking to protect collective or individual rights, such as the right to health, the right to information or the right to safety. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including product defect, concealment, and misrepresentation. Plaintiffs in these cases seek various forms of relief including injunctive relief such as banning cigarettes, descriptors, smoking in certain places and advertising, as well as implementing communication campaigns and reimbursement of medical expenses incurred by public or private institutions.
As of March 31, 2022, there was 1 public civil action pending against our subsidiary in Venezuela (1), compared with 2 such cases on March 31, 2021.
In a public civil action in Venezuela, Federation of Consumers and Users Associations (“FEVACU”), et al. v. National Assembly of Venezuela and the Venezuelan Ministry of Health, Constitutional Chamber of the Venezuelan Supreme Court, filed April 29, 2008, we were not named as a defendant, but the plaintiffs published a notice pursuant to court order, notifying all interested parties to appear in the case. In January 2009, our subsidiary appeared in the case in response to this notice. The plaintiffs purport to represent the right to health of the citizens of Venezuela and claim that the government failed to protect adequately its citizens' right to health. The claim asks the court to order the government to enact stricter regulations on the manufacture and sale of tobacco products. In addition, the plaintiffs ask the court to order companies involved in the tobacco industry to allocate a percentage of their “sales or benefits” to establish a fund to pay for the health care costs of treating smoking-related diseases. In October 2008, the court ruled that plaintiffs have standing to file the claim and that the claim meets the threshold admissibility requirements. In December 2012, the court admitted our subsidiary and BAT's subsidiary as interested third parties. In February 2013, our subsidiary answered the complaint.
Reduced-Risk Products
In Colombia, an individual filed a purported class action, Ana Ferrero Rebolledo v. Philip Morris Colombia S.A., et al., in April 2019 against our subsidiaries with the Civil Court of Bogota related to the marketing of our Platform 1 product. Plaintiff alleged that our subsidiaries advertise the product in contravention of law and in a manner that misleads consumers by portraying the product in a positive light, and further asserts that the Platform 1 vapor contains many toxic compounds, creates a high level of dependence, and has damaging second-hand effects. Plaintiff sought injunctive relief and damages on her behalf and on a behalf of two classes (class 1 - all Platform 1 consumers in Colombia who seek damages for the purchase price of the product and personal injuries related to the alleged addiction, and class 2 - all residents of the neighborhood where the advertising allegedly took place who seek damages for exposure to the alleged illegal advertising). Our subsidiaries answered the complaint in January 2020, and in February 2020, plaintiff filed an amended complaint. The amended complaint modifies the relief sought on behalf of the named plaintiff and on behalf of a single class (all consumers of Platform 1 products in Colombia who seek damages for the product purchase price and personal injuries related to the use of an allegedly harmful product). In June 2021, our subsidiaries answered the amended complaint.
30
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Other Litigation
The Department of Special Investigations of the government of Thailand ("DSI") conducted an investigation into alleged underpayment by our subsidiary, Philip Morris (Thailand) Limited ("PM Thailand"), of customs duties and excise taxes relating to imports from the Philippines covering the period 2003-2007. On January 18, 2016, the Public Prosecutor filed charges against our subsidiary and seven former and current employees in the Bangkok Criminal Court alleging that PM Thailand and the individual defendants jointly and with the intention to defraud the Thai government, under-declared import prices of cigarettes to avoid full payment of taxes and duties in connection with import entries of cigarettes from the Philippines during the period of July 2003 to June 2006. The government is seeking a fine of approximately THB 80.8 billion (approximately $2.4 billion). In May 2017, Thailand enacted a new customs act. The new act, which took effect in November 2017, substantially limits the amount of fines that Thailand could seek in these proceedings. PM Thailand believes that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and other Thai governmental agencies. Trial in the case began in November 2017 and concluded in September 2019. In November 2019, the trial court found our subsidiary guilty of under-declaration of the prices and imposed a fine of approximately THB 1.2 billion (approximately $35 million). The trial court dismissed all charges against the individual defendants. In December 2019, as required by the Thai law, our subsidiary paid the fine. This payment is included in other assets on the condensed consolidated balance sheets and negatively impacted net cash provided by operating activities in the condensed consolidated statements of cash flows in the period of payment. Our subsidiary filed an appeal of the trial court's decision. In addition, the Public Prosecutor filed an appeal of the trial court's decision challenging the dismissal of charges against the individual defendants and the amount of the fine imposed. If our subsidiary ultimately prevails on appeal, then Thailand will be required to return this payment to our subsidiary. The appellate court is scheduled to issue its decision on the appeals on June 1, 2022.
The DSI also conducted an investigation into alleged underpayment by PM Thailand of customs duties and excise taxes relating to imports from Indonesia covering the period 2000-2003. On January 26, 2017, the Public Prosecutor filed charges against PM Thailand and its former Thai employee in the Bangkok Criminal Court alleging that PM Thailand and its former employee jointly and with the intention to defraud the Thai government under-declared import prices of cigarettes to avoid full payment of taxes and duties in connection with import entries during the period from January 2002 to July 2003. The government is seeking a fine of approximately THB 19.8 billion (approximately $582 million). In May 2017, Thailand enacted a new customs act. The new act, which took effect in November 2017, substantially limits the amount of fines that Thailand could seek in these proceedings. PM Thailand believes that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law, and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and a Thai court. Trial in the case began in November 2018 and concluded in December 2019. In March 2020, the trial court found our subsidiary guilty of under-declaration of the prices and imposed a fine of approximately THB 130 million (approximately $4 million). The trial court dismissed all charges against the individual defendant. In April 2020, as required by Thai law, our subsidiary paid the fine. This payment is included in other assets on the condensed consolidated balance sheets and negatively impacted net cash provided by operating activities in the condensed consolidated statements of cash flows in the period of payment. Our subsidiary filed an appeal of the trial court's decision. In addition, the Public Prosecutor filed an appeal of the trial court's decision challenging the dismissal of charges against the individual defendant and the amount of the fine imposed. If our subsidiary ultimately prevails on appeal, then Thailand will be required to return this payment to our subsidiary. The appellate court is scheduled to issue its decision on the appeals on August 30, 2022.
The South Korean Board of Audit and Inspection (“BAI”) conducted an audit of certain Korean government agencies and the tobacco industry into whether inventory movements ahead of the January 1, 2015 increase of cigarette-related taxes by tobacco companies, including Philip Morris Korea Inc. ("PM Korea"), our South Korean subsidiary, were in compliance with South Korean tax laws. In November 2016, the tax authorities completed their audit and assessed allegedly underpaid taxes and penalties. In order to avoid nonpayment financial costs, PM Korea paid approximately KRW 272 billion (approximately $219 million), of which KRW 100 billion (approximately $80 million) was paid in 2016 and KRW 172 billion (approximately $138 million) was paid in the first quarter of 2017. These paid amounts are included in other assets in the condensed consolidated balance sheets and negatively impacted net cash provided by operating activities in the condensed consolidated statements of cash flows in the period of payment. PM Korea appealed the assessments. In January 2020, a trial court ruled that PM Korea did not underpay taxes in the amount of approximately KRW 218 billion (approximately $175 million). The tax authorities appealed this decision to the appellate court. In September 2020, the appellate court upheld the trial court's decision. The tax authorities have appealed to the Supreme Court of South Korea. In June 2020, another trial court ruled that PM Korea did not underpay approximately KRW 54 billion (approximately $43 million) of alleged underpayments. The government agencies appealed this decision. In January 2021, the appellate court upheld the trial court's decision. The government agencies appealed to the Supreme Court of South Korea. If the tax authorities and government agencies ultimately lose, then they would be required to return the paid amounts to PM Korea.
31
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The Saudi Arabia Customs General Authority issued its assessments requiring our distributors to pay additional customs duties in the amount of approximately 1.5 billion Saudi Riyal, or approximately $396 million, in relation to the fees paid by these distributors under their agreements with our subsidiary for exclusive rights to distribute our products in Saudi Arabia. In order to challenge these assessments, the distributors posted bank guarantees. To enable the distributors' challenge, our subsidiary agreed with the banks to bear a portion of the amount the authority may draw on the bank guarantees. In September and October 2020, respectively, the distributors lost their challenges of the assessments. Both distributors appealed, and in June 2021, the Customs Appeal Committee in Riyadh notified the distributors of its decisions to largely reject their appeals. On the basis of the above-mentioned decisions, in June 2021, PMI recorded a pre-tax charge of $246 million in relation to the period of 2014 through 2020 in line with existing and contemplated arrangements with the distributors. The estimated amounts for 2021 and 2022 are immaterial. In accordance with U.S. GAAP, the charge was recorded as a reduction in net revenues on the consolidated statements of earnings for the three months and six months ended June 30, 2021. Despite the unfavorable decisions, our subsidiary believes that customs duties paid in Saudi Arabia were in compliance with the applicable law and the WTO Customs Valuation Agreement.
A putative shareholder class action lawsuit, In re Philip Morris International Inc. Securities Litigation, is pending in the United States District Court for the Southern District of New York, purportedly on behalf of purchasers of Philip Morris International Inc. stock between July 26, 2016 and April 18, 2018. The lawsuit names Philip Morris International Inc. and certain officers and employees as defendants and includes allegations that the defendants made false and/or misleading statements and/or failed to disclose information about PMI’s business, operations, financial condition, and prospects, related to product sales of, and alleged irregularities in clinical studies of, PMI’s Platform 1 product. The lawsuit seeks various forms of relief, including damages. In November 2018, the court consolidated three putative shareholder class action lawsuits with similar allegations previously filed in the Southern District of New York (namely, City of Westland Police and Fire Retirement System v. Philip Morris International Inc., et al., Greater Pennsylvania Carpenters’ Pension Fund v. Philip Morris International Inc., et al., and Gilchrist v. Philip Morris International Inc., et al.) into these proceedings. A putative shareholder class action lawsuit, Rubenstahl v. Philip Morris International Inc., et al., that had been previously filed in December 2017 in the United States District Court for the District of New Jersey, was voluntarily dismissed by the plaintiff due to similar allegations in these proceedings. On February 4, 2020, the court granted defendants’ motion in its entirety, dismissing all but one of the plaintiffs’ claims with prejudice. The court noted that one of plaintiffs’ claims (allegations relating to four non-clinical studies of PMI’s Platform 1 product) did not state a viable claim but allowed plaintiffs to replead that claim by March 3, 2020. On February 18, 2020, the plaintiffs filed a motion for reconsideration of the court's February 4th decision; this motion was denied on September 21, 2020. On September 28, 2020, plaintiffs filed an amended complaint seeking to replead allegations relating to four non-clinical studies of PMI's Platform 1 product. On September 10, 2021, the court granted defendant's motion to dismiss plaintiffs' amended complaint in its entirety. Plaintiffs have filed an appeal with the U.S. Court of Appeal for the Second Circuit. We believe that this lawsuit is without merit and will continue to defend it vigorously.
In April 2020, affiliates of British American Tobacco plc (“BAT”) commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Altria Client Services LLC, et al., in the federal court in the Eastern District of Virginia, where PMI's subsidiary, Philip Morris Products S.A., as well as Altria Group, Inc.'s subsidiaries, are defendants. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in the United States. In April 2020, BAT affiliates filed a complaint against PMI, Philip Morris Products S.A., Altria Group, Inc., and its subsidiaries before the International Trade Commission ("ITC"). Plaintiffs seek an order to prevent the importation of Platform 1 products into the United States. The ITC evidentiary hearing closed on February 1, 2021. On May 14, 2021, the administrative law judge issued an Initial and Recommended Determination ("ID/RD") finding that the Platform 1 blade products infringe two of the three patents asserted by Plaintiffs, recommending that the ITC issue a Limited Exclusion order against infringing products, and recommending against a cease-and-desist, as well as recommending against a bond pending Presidential review of the ITC's Final Determination ("FD"). Defendants and Plaintiffs filed separate Petitions for Review with the ITC of the ID on May 28, 2021; on July 27, 2021, the ITC granted each of the petitions in part, deciding to review certain issues in the ID. Plaintiffs and Defendants also submitted brief statements of the public interest factors in issue to the ITC on June 15, 2021. On September 29, 2021, the ITC issued its FD finding a violation of section 337 of the U.S. Tariff Act and issued (a) a limited exclusion order against Philip Morris Products S.A., prohibiting, inter alia, the importation of Platform 1 product and infringing components; and (b) a cease-and-desist order against Altria Client Services, LLC and its affiliate prohibiting, inter alia, sales of imported Platform 1 products. The ITC predicated the orders on its finding that Platform 1 blade products infringe two patents owned by a BAT affiliate. The ITC also found that Platform 1 blade products do not infringe a third patent owned by a BAT affiliate. The ITC further held that there were insufficient concerns over public interest to prevent the issuance of remedial orders. Following the Presidential Review period, the orders became effective and Defendants filed a petition for review of the FD with the U.S. Court of Appeals for the Federal Circuit. Defendants also filed motions in the ITC and Federal Circuit for a stay of the orders
32
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
pending disposition of the appeal; the ITC denied the motion on January 20, 2022 and the Federal Circuit denied the motion on January 25, 2022. We estimate that an adverse ruling is probable due to our inability to import the products and components impacted by the ITC's FD with immaterial financial impact. In the Eastern District of Virginia case, the defendants also counterclaimed that BAT infringed their patents relating to certain e-vapor products, seeking damages for, and injunctive relief against, the commercialization of these products by BAT; defendants' claims against BAT are set for trial beginning the week of June 6, 2022. Upon petition of Philip Morris Products S.A., the Patent Trial and Appeal Board ("PTAB") of the United States Patent and Trademark Office has instituted review of certain claims pertaining to four of the six patents asserted by BAT affiliates in both proceedings. On January 11, 2022, PTAB issued its final decision on one of the two patents underlying the ITC's FD, invalidating all challenged claims of BAT's patent. On March 30, 2022, PTAB issued its final decision on the second of the two patents underlying the ITC's FD, finding the challenged claims patentable. The parties may appeal PTAB results to the U.S. Court of Appeals for the Federal Circuit.
In April 2020, BAT’s affiliate commenced patent infringement proceedings, Nicoventures Trading Limited v. PM GmbH, et al., against PMI’s German subsidiary, Philip Morris GmbH, and Philip Morris Products S.A., in the Regional Court in Munich, Germany. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in Germany. In June 2021, the court stayed the proceeding in respect of one of the two patents asserted by BAT’s Affiliate.
In September 2020, BAT’s affiliates commenced patent infringement and unfair competition proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Products S.A., et al., against Philip Morris Products S.A. and PMI’s Italian subsidiaries, Philip Morris Manufacturing & Technology Bologna S.p.A. and Philip Morris Italia S.r.l., in the Court of Milan, Italy. Plaintiffs seek damages, as well as injunctive relief against the manufacture in Italy of the Platform 1 blade heated tobacco units allegedly infringing the asserted patents and the commercialization of the Platform 1 blade products in Italy. As part of this proceeding, in October 2020, BAT’s affiliates filed a request based on one of the two asserted patents seeking preliminary injunctive relief against the manufacture and commercialization of the Platform 1 blade products in Italy.
In October 2020, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Japan, Limited, et al., against PMI’s Japanese subsidiary, Philip Morris Japan Limited, and a third-party distributor in the Tokyo District Court. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade products in Japan.
In November 2020, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Romania SRL, et al., against PMI’s Romanian subsidiaries, Philip Morris Romania S.R.L. and Philip Morris Trading S.R.L., and a third-party distributor in the Court of Law of Bucharest, Civil Registry. Plaintiffs seek damages and preliminary and permanent injunctive relief against the manufacture and commercialization of the Platform 1 blade products in Romania. In February 2021, the court dismissed plaintiffs’ request for a preliminary injunction. In April 2021, the appellate court denied plaintiffs' appeal, confirming the dismissal of plaintiffs' request for preliminary injunction. Plaintiffs' proceeding requesting damages and a permanent injunction remains pending before the Court of Law of Bucharest, Civil Registry. In an October 14, 2021 hearing, the court stayed the proceeding.
In March 2021, BAT’s affiliates commenced patent infringement proceedings, RAI Strategic Holdings, Inc., et al. v. Philip Morris Korea, Co., Ltd., against PM Korea in the Seoul Central District Court. Plaintiffs seek damages and injunctive relief against the commercialization of the Platform 1 blade heated tobacco units in South Korea.
In July, 2021, Philip Morris Products, S.A. filed a claim at the High Court of Justice of England and Wales against BAT affiliates Nicoventures Trading Limited and British American Tobacco (Investments) Limited seeking revocation of the UK parts of two BAT European patents. In January 2022, the court ordered that the trial of the action will be in September 2022, and the trial has subsequently been set to start on September 20, 2022. In March, the BAT affiliates stated that they would consent to revocation of one of the patents and filed an application with the court to bring a counterclaim against Philip Morris Products S.A. and Philip Morris Limited seeking from the court a declaration that the remaining BAT affiliate patent is infringed by Platform 1 induction products, as well as damages and injunctive relief against the commercialization of the Platform 1 induction products in the U.K.
Other patent challenges by both parties are pending in various jurisdictions.
We believe that the foregoing proceedings by the affiliates of BAT are without merit and will defend them vigorously.
We are also involved in additional litigation arising in the ordinary course of our business. While the outcomes of these proceedings are uncertain, management does not expect that the ultimate outcomes of other litigation, including any reasonably possible losses in excess of current accruals, will have a material adverse effect on our consolidated results of operations, cash flows or financial position.
33
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Third-Party Guarantees
On October 17, 2020, Medicago Inc., an equity method investee of Philip Morris Investments B.V. (“PMIBV”), a PMI subsidiary, entered into a contribution agreement with the Canadian government (the “Contribution Agreement”) whereby the Canadian government agreed to contribute up to CAD 173 million (approximately $131 million on the date of signing) to Medicago Inc., to support its on-going COVID-19 vaccine development and clinical trials, and for the construction of its Quebec City manufacturing facility (the “Project”). On March 31, 2022, the Contribution Agreement was amended (the “Contribution Agreement Amendment”) to reflect an additional contribution from the Canadian government up to CAD 27 million (approximately $22 million on the date of signing) to Medicago Inc. for the construction of its Quebec City manufacturing facility. PMIBV and the majority shareholder of Medicago Inc. are also parties to the Contribution Agreement and the Contribution Agreement Amendment as guarantors of Medicago Inc.’s obligations thereunder on a joint and several basis (“Co-Guarantors”). The Co-Guarantors agreed to repay amounts contributed by the Canadian government plus interest, if Medicago Inc. fails to do so, and could be responsible for the costs of other Medicago’s obligations (such as the achievement of specific milestones of the Project). The maximum amount of these obligations is currently non-estimable. As of March 31, 2022, PMI has determined that these guarantees did not have a material impact on its condensed consolidated financial statements.
In connection with the Contribution Agreement, PMIBV and the majority shareholder of Medicago Inc. entered into a guarantors’ agreement that apportions Co-Guarantors’ obligations and limits those of PMIBV to its share of holdings in Medicago Inc. During 2022, Medicago Inc. initiated additional rounds of equity funding in which PMIBV did not participate. As a result, PMIBV’s share of holdings in Medicago Inc. was reduced from approximately 23% as of December 31, 2021 to approximately 21% as of March 31, 2022. The guarantees are in effect through March 31, 2026.
Note 9. Income Taxes:
Income tax provisions for jurisdictions outside the United States of America, as well as state and local income tax provisions, were determined on a separate company basis, and the related assets and liabilities were recorded in PMI’s condensed consolidated balance sheets.
PMI’s effective tax rates for the three months ended March 31, 2022 and 2021 were 19.7% and 21.5%, respectively. The effective tax rate for the three months ended March 31, 2022, was favorably impacted by changes in earnings mix by taxing jurisdiction, as well as a decrease in deferred tax liabilities related to the fair value adjustment of equity securities held by PMI ($13 million). For further details, see Note 12. Related Parties - Equity Investments and Other. The effective tax rate for the three months ended March 31, 2021, was favorably impacted by the corporate income tax rate reduction in Indonesia (enacted in the second quarter of 2020) and the Philippines (enacted in the first quarter of 2021), as well as changes in earnings mix by taxing jurisdiction. PMI estimates that its full-year 2022 effective tax rate will be 21% to 22%, excluding discrete tax events. Changes in currency exchange rates, earnings mix by taxing jurisdiction or future regulatory developments may have an impact on the effective tax rates, which PMI monitors each quarter. Significant judgment is required in determining income tax provisions and in evaluating tax positions.
PMI is regularly examined by tax authorities around the world and is currently under examination in a number of jurisdictions. The U.S. federal statute of limitations remains open for the years 2017 and onward. Foreign and U.S. state jurisdictions have statutes of limitations generally ranging from 3 to 5 years. In October 2021, a subsidiary of PMI in Indonesia, PT Hanjaya Mandala Sampoerna Tbk ("HMS"), received a tax assessment in the amount of 3.8 trillion Indonesian rupiah (approximately $260 million) primarily relating to corporate income taxes on domestic and other intercompany transactions for the years 2017 to 2019. HMS paid the assessment in the fourth quarter of 2021 in order to avoid potential penalties and filed an objection letter with the tax office in January 2022. The amount paid was included in other assets in PMI’s condensed consolidated balance sheets at March 31, 2022 and December 31, 2021, and negatively impacted net cash provided by operating activities in the consolidated statements of cash flows in the period of payment.
It is reasonably possible that within the next 12 months certain tax examinations will close, which could result in a change in unrecognized tax benefits along with related interest and penalties. An estimate of any possible change cannot be made at this time.
34
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 10. Indebtedness:
Short-term Borrowings:
PMI's short-term borrowings, consisting of commercial paper and bank loans to certain PMI subsidiaries at March 31, 2022, and bank loans to certain PMI subsidiaries at December 31, 2021, had a carrying value of $2,441 million and $225 million, respectively. The fair values of PMI’s short-term borrowings, based on current market interest rates, approximate carrying value.
Long-term Debt:
At March 31, 2022 and December 31, 2021, PMI’s long-term debt consisted of the following:
| (in millions) | March 31, 2022 | December 31, 2021 | ||||||||||||
U.S. dollar notes, 0.875% to 6.375% (average interest rate 3.261%), due through 2044 | $ | 18,867 | $ | 19,397 | ||||||||||
| Foreign currency obligations: | ||||||||||||||
Euro notes, 0.125% to 3.125% (average interest rate 1.995%), due through 2039 | 7,561 | 7,687 | ||||||||||||
Swiss franc notes, 1.625%, due 2024 | 270 | 273 | ||||||||||||
Other (average interest rate 3.238%), due through 2029 (a) | 218 | 224 | ||||||||||||
| Carrying value of long-term debt | 26,916 | 27,581 | ||||||||||||
| Less current portion of long-term debt | 2,897 | 2,798 | ||||||||||||
| $ | 24,019 | $ | 24,783 | |||||||||||
(a) Includes mortgage debt in Switzerland as well as $68 million and $71 million in finance leases at March 31, 2022 and December 31, 2021, respectively.
35
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The fair value of PMI’s outstanding long-term debt, which is utilized solely for disclosure purposes, is determined using quotes and market interest rates currently available to PMI for issuances of debt with similar terms and remaining maturities. At March 31, 2022, the fair value of PMI's outstanding long-term debt, excluding the aforementioned finance leases, was as follows:
(in millions) | March 31, 2022 | ||||
| Level 1 | $ | 26,610 | |||
| Level 2 | 161 | ||||
For a description of the fair value hierarchy and the three levels of inputs used to measure fair values, see Item 8, Note 2. Summary of Significant Accounting Policies of PMI's Annual Report on Form 10-K for the year ended December 31, 2021.
Credit Facilities:
At March 31, 2022, PMI's total committed credit facilities were as follows:
(in billions)
Type | Committed Credit Facilities | ||||
364-day revolving credit, expiring January 31, 2023 | $ | 1.8 | |||
Multi-year revolving credit, expiring February 10, 2026 (1) | 2.0 | ||||
Multi-year revolving credit, expiring September 29, 2026 (2) | 2.5 | ||||
Total facilities | $ | 6.3 | |||
(1) On January 28, 2022, PMI entered into an agreement, effective February 10, 2022, to amend and extend the term of its $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(2) Includes pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets.
At March 31, 2022, there were no borrowings under these committed credit facilities, and the entire committed amounts were available for borrowing.
Note 11. Accumulated Other Comprehensive Losses:
PMI’s accumulated other comprehensive losses, net of taxes, consisted of the following:
| (Losses) Earnings | At | At | At | |||||||||||||||||
| (in millions) | March 31, 2022 | December 31, 2021 | March 31, 2021 | |||||||||||||||||
| Currency translation adjustments | $ | (7,039) | $ | (6,701) | $ | (6,586) | ||||||||||||||
| Pension and other benefits | (2,826) | (2,880) | (4,172) | |||||||||||||||||
| Derivatives accounted for as hedges | 105 | 4 | 12 | |||||||||||||||||
| Total accumulated other comprehensive losses | $ | (9,760) | $ | (9,577) | $ | (10,746) | ||||||||||||||
36
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Reclassifications from Other Comprehensive Earnings
The movements in accumulated other comprehensive losses and the related tax impact, for each of the components above, that are due to current period activity and reclassifications to the income statement, are shown on the condensed consolidated statements of comprehensive earnings for the three months ended March 31, 2022 and 2021. For additional information, see Note 3. Benefit Plans for disclosures related to PMI's pension and other benefits, Note 5. Financial Instruments for disclosures related to derivative financial instruments and Note 17. Acquisitions.
Note 12. Related Parties - Equity Investments and Other:
Equity Method Investments:
At March 31, 2022 and December 31, 2021, PMI had total equity method investments of $844 million and $879 million, respectively. Equity method investments are initially recorded at cost. Under the equity method of accounting, the investment is adjusted for PMI's proportionate share of earnings or losses, dividends, capital contributions, changes in ownership interests and movements in currency translation adjustments. The carrying value of our equity method investments at March 31, 2022 and December 31, 2021, exceeded our share of the investees' book value by $724 million and $764 million, respectively. The difference between the investment carrying value and the amount of underlying equity in net assets, excluding $684 million and $728 million attributable to goodwill as of March 31, 2022 and December 31, 2021, respectively, which consists primarily of definite-lived intangible assets is being amortized on a straight-line basis. At March 31, 2022 and December 31, 2021, PMI received year-to-date dividends from equity method investees of $8 million and $176 million, respectively.
PMI holds a 23% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis, PMI's distributor in Russia (Eastern Europe segment).
PMI holds a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) (“EITA”). PMI holds an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie (“STAEM”), an Algerian joint venture that is 51% owned by EITA and 49% by the Algerian state-owned enterprise Management et Développement des Actifs et des Ressources Holding ("MADAR Holding"), which manufactures and distributes under license some of PMI’s brands (Middle East & Africa segment).
The initial investments in Megapolis Distribution BV and EITA were recorded at cost and are included in equity investments on the condensed consolidated balance sheets.
Equity securities:
Following the deconsolidation of RBH on March 22, 2019, PMI recorded the continuing investment in RBH, PMI's wholly owned subsidiary in Canada, at fair value of $3,280 million at the date of deconsolidation, within equity investments. For further details, see Item 8, Note 20. Deconsolidation of RBH, in PMI's Annual Report on Form 10-K for the year ended December 31, 2021. Transactions between PMI and RBH are considered to be related party transactions from the date of deconsolidation and are included in the tables below.
The fair value of PMI’s other equity securities, which have been classified within Level 1, was $221 million at March 31, 2022. Unrealized pre-tax loss of $(60) million ($(47) million net of tax) on these equity securities was recorded in the condensed consolidated statement of earnings for the three months ended March 31, 2022.
Other related parties:
United Arab Emirates-based Trans-Emirates Trading and Investments (FZC) ("TTI") holds a 33% non-controlling interest in Philip Morris Misr LLC ("PMM"), an entity incorporated in Egypt which is consolidated in PMI’s financial statements in the Middle East & Africa segment. PMM sells, under license, PMI brands in Egypt through an exclusive distribution agreement with a local entity that is also controlled by TTI.
37
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Godfrey Phillips India Ltd ("GPI") is one of the non-controlling interest holders in IPM India, which is a 56.3% owned PMI consolidated subsidiary in the South & Southeast Asia segment. GPI also acts as contract manufacturer and distributor for IPM India. Amounts in the tables below include transactions between these related parties.
Financial activity with the above related parties:
PMI’s net revenues and expenses with the above related parties were as follows:
| For the Three Months Ended March 31, | ||||||||
| (in millions) | 2022 | 2021 | ||||||
| Net revenues: | ||||||||
| Megapolis Group | $ | 387 | $ | 483 | ||||
| Other | 291 | 280 | ||||||
Net revenues (a) | $ | 678 | $ | 763 | ||||
| Expenses: | ||||||||
| Other | $ | 12 | $ | 17 | ||||
| Expenses | $ | 12 | $ | 17 | ||||
(a) Net revenues exclude excise taxes and VAT billed to customers.
PMI’s balance sheet activity with the above related parties was as follows:
| (in millions) | At March 31, 2022 | At December 31, 2021 | |||||||||
| Receivables: | |||||||||||
| Megapolis Group | $ | 455 | $ | 319 | |||||||
| Other | 236 | 199 | |||||||||
| Receivables | $ | 691 | $ | 518 | |||||||
| Payables: | |||||||||||
| Other | $ | 24 | $ | 25 | |||||||
| Payables | $ | 24 | $ | 25 | |||||||
The activities with the above related parties are in the ordinary course of business, and are primarily for distribution, service fees, contract manufacturing and license agreements. PMI eliminated its respective share of all significant intercompany transactions with the equity method investees.
Note 13. Sale of Accounts Receivable:
To mitigate risk and enhance cash and liquidity management, PMI sells trade receivables to unaffiliated financial institutions. These arrangements allow PMI to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the condensed consolidated balance sheets. PMI sells trade receivables under two types of arrangements, servicing and non-servicing. For servicing arrangements, PMI continues to service the sold trade receivables on an administrative basis and does not act on behalf of the unaffiliated financial institutions. When applicable, a servicing liability is recorded for the estimated fair value of the servicing. The amounts associated with the servicing liability were not material as of March 31, 2022 and March 31, 2021. Under the non-servicing arrangements, PMI does not provide any administrative support or servicing after the trade receivables have been sold to the unaffiliated financial institutions.
Cumulative trade receivables sold, including excise taxes, for the three months ended March 31, 2022 and 2021, were $2.8 billion and $2.5 billion, respectively. PMI’s operating cash flows were positively impacted by the amount of the trade
38
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
receivables sold and derecognized from the condensed consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of March 31, 2022 and March 31, 2021, were $598 million, and $625 million, respectively. The net proceeds received are included in cash provided by operating activities in the condensed consolidated statements of cash flows. The difference between the carrying amount of the trade receivables sold and the sum of the cash received is recorded as a loss on sale of trade receivables within marketing, administration and research costs in the condensed consolidated statements of earnings. For the three months ended March 31, 2022 and 2021, the loss on sale of trade receivables was immaterial.
Note 14. Product Warranty:
PMI's heat-not-burn devices and e-vapor products are subject to standard product warranties generally for a period of 12 months from the date of purchase or such other periods as required by law. PMI generally provides in cost of sales for the estimated cost of warranty in the period the related revenue is recognized. PMI assesses the adequacy of its accrued product warranties and adjusts the amounts as necessary based on actual experience and changes in future estimates. Factors that affect product warranties may vary across markets but typically include device version mix, product failure rates, logistics and service delivery costs, and warranty policies. PMI accounts for its product warranties within other accrued liabilities. At March 31, 2022 and December 31, 2021, these amounts were as follows:
| (in millions) | As of and For the Three Months Ended March 31, 2022 | As of and For the Year Ended December 31, 2021 | |||||||||
| Balance at beginning of period | $ | 113 | $ | 137 | |||||||
| Changes due to: | |||||||||||
| Warranties issued | 46 | 154 | |||||||||
| Settlements | (26) | (177) | |||||||||
| Currency/Other | (3) | (1) | |||||||||
| Balance at end of period | $ | 130 | $ | 113 | |||||||
Note 15. Leases:
The components of PMI’s lease cost were as follows for the three months ended March 31, 2022 and 2021:
| For the Three Months Ended March 31, | ||||||||
| (in millions) | 2022 | 2021 | ||||||
| Operating lease cost | $ | 62 | $ | 60 | ||||
| Finance lease cost: | ||||||||
| Amortization of right-of-use assets | 16 | 8 | ||||||
Interest on lease liabilities | — | — | ||||||
| Short-term lease cost | 14 | 10 | ||||||
| Variable lease cost | 6 | 7 | ||||||
| Total lease cost | $ | 98 | $ | 85 | ||||
Note 16. Asset Impairment and Exit Costs:
For the three months ended March 31, 2022, PMI did not record any pre-tax charges for asset impairment and exit costs. For the three months ended March 31, 2021, PMI recorded total pre-tax asset impairment and exit costs of $48 million. These pre-tax charges for the three months ended March 31, 2021 were included in marketing, administration and research costs in the condensed consolidated statements of earnings.
39
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
South Korea
In the first quarter of 2021, PM Korea commenced the implementation of a new business operating model, which required the restructuring of its current distribution agreements. As a result, PMI recorded exit costs of $26 million in the three months ended March 31, 2021, related to contract terminations and restructuring with certain distributors.
Organizational Design Optimization
As part of PMI’s transformation to a smoke-free future, PMI sought to optimize its organizational design, which included the elimination, relocation and outsourcing of certain operations center and centralized activities. In January 2020, PMI commenced a multi-phase restructuring project in Switzerland. PMI initiated the employee consultation procedures, as required under Swiss law, for the impacted employees. The consultation procedures for the first two phases were completed in 2020 with the final phases initiated and completed in 2021. Additionally, since the commencement of this multi-phase restructuring project in 2020, PMI launched a voluntary separation program in Switzerland for certain eligible employees and announced the outsourcing of certain activities in Argentina, Indonesia, Poland and the United States. This multi-phase restructuring project was completed in the fourth quarter of 2021.
For the three months ended March 31, 2021, PMI recorded pre-tax charges of $22 million, related to the organizational design optimization. Since inception of this multi-phase restructuring project in January 2020 through December 31, 2021, approximately 1,020 positions in total were impacted, resulting in cumulative pre-tax charges of $308 million related to the organizational design optimization program. Of this cumulative pre-tax amount, $300 million related to separation program charges and $8 million related to asset impairment charges.
Asset Impairment and Exit Costs by Segment
PMI recorded the following pre-tax asset impairment and exit costs by segment:
| (in millions) | For the Three Months Ended March 31, | ||||
| 2021 | |||||
Separation programs: (1) | |||||
| European Union | $ | 9 | |||
| Eastern Europe | 2 | ||||
| Middle East & Africa | 2 | ||||
| South & Southeast Asia | 3 | ||||
| East Asia & Australia | 5 | ||||
| Americas | 1 | ||||
| Total separation programs | 22 | ||||
| Contract termination charges: | |||||
| East Asia & Australia | 26 | ||||
| Total contract termination charges | 26 | ||||
| Asset impairment and exit costs | $ | 48 | |||
(1) Organizational design optimization pre-tax charges in 2021 were allocated across all geographical segments.
40
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Movement in Exit Cost Liabilities
The movement in exit cost liabilities for the three months ended March 31, 2022 was as follows:
| (in millions) | |||||
| Liability balance, January 1, 2022 | $ | 142 | |||
| Charges, net | — | ||||
| Cash spent | (28) | ||||
| Currency/other | (1) | ||||
| Liability balance, March 31, 2022 | $ | 113 | |||
Future cash payments for exit costs incurred to date are anticipated to be substantially paid by the end of 2023, with approximately $72 million expected to be paid in the remainder of 2022.
Note 17. Acquisitions:
Purchase of Noncontrolling Interests
Turkey – In the first quarter of 2022, PMI acquired the remaining 25% stake of its holding in Philip Morris Tütün Mamulleri Sanayi ve Ticaret A.Ş. (formerly Philsa Philip Morris Sabancı Sigara ve Tütüncülük Sanayi ve Ticaret A.Ş.) and 24.75% stake in Philip Morris Pazarlama ve Satış A.Ş. (formerly Philip Morris SA, Philip Morris Sabancı Pazarlama ve Satış A.Ş.) from its Turkish partners, Sabanci Holding for a total acquisition price including transaction costs and remaining dividend entitlements of approximately $223 million. As a result of this acquisition, PMI now owns 100% of these Turkish subsidiaries. The purchase of the remaining stakes in these holdings resulted in a decrease to PMI's additional paid-in capital of $30 million and an increase to accumulated other comprehensive losses of $171 million primarily following the reclassification of accumulated currency translation losses from noncontrolling interests to PMI’s accumulated other comprehensive losses during the first quarter of 2022.
Business Combinations
AG Snus - On May 6, 2021, PMI acquired 100% of AG Snus Aktieselskab ("AG Snus"), a company based in Denmark, and its Swedish subsidiary Tobacco House of Sweden AB fully owned by AG Snus, which operates in the oral tobacco (i.e. snus) and modern oral (i.e. nicotine pouches) product categories. The purchase price was $28 million in cash, net of cash acquired, with additional contingent payments of up to $10 million, primarily relating to product development and performance targets over a less than two-year period. The operating results of AG Snus are included in the European Union segment, and were not material.
Fertin Pharma – On September 15, 2021, PMI acquired 100% of Fertin Pharma A/S (“Fertin Pharma”), a company based in Denmark. Fertin Pharma is a developer and manufacturer of pharmaceutical and well-being products based on oral and intra-oral delivery systems. The acquisition was funded with existing cash. The total consideration of $821 million (DKK 5.2 billion) included cash of $580 million and the payment of $241 million related to the settlement of Fertin Pharma’s indebtedness. The purchase price of $821 million was preliminarily allocated to cash ($24 million), current assets including receivables and inventories ($69 million), non-current assets including property, plant and equipment ($228 million), goodwill ($378 million), and other intangible assets ($245 million, which primarily consisted of customer relationships, developed technology, and in process research and development ("IPR&D")), partially offset by current liabilities ($44 million, which primarily consisted of accrued liabilities and accounts payable) and non-current liabilities ($79 million, primarily deferred income tax). Goodwill is primarily attributable to future growth opportunities provided by acquired R&D capabilities and any intangibles that did not qualify for separate recognition. The amortizable intangible assets are being amortized over their estimated useful lives of 8 to 19 years. During the first quarter of 2022, PMI did not record any measurement period adjustments to the preliminary purchase price allocation. The purchase price allocation is preliminary and continues to be subject to refinement. PMI is evaluating the deductibility of goodwill for income tax purposes.
41
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Vectura – During the third quarter and up to September 15, 2021, PMI acquired a controlling interest of 74.77% of the total issued shares in Vectura Group plc (“Vectura”), an inhaled therapeutics company based in the United Kingdom. The shares were acquired through a series of open market purchases and acceptances of the tender offer at a price of 165 pence per share. As a result of additional acceptances of the offer and the exercise of the right to acquire compulsorily the Vectura shares, in accordance with the applicable English law, PMI completed the acquisition of 100% of Vectura in the fourth quarter of 2021. The acquisition was funded with existing cash from a designated account operated solely for the purpose of funding this acquisition.
The total purchase price of $1,384 million (GBP 1.0 billion) for 100% of the Vectura shares was preliminarily allocated to cash ($136 million), current assets including receivables and inventories ($89 million), non-current assets including property, plant and equipment ($67 million), goodwill ($590 million), and other intangible assets ($719 million, which primarily consisted of developed technology, and IPR&D), partially offset by current liabilities ($100 million, primarily accrued liabilities), and non-current liabilities ($117 million, primarily deferred income tax). Goodwill is primarily attributable to future growth opportunities provided by acquired R&D capabilities and any intangibles that did not qualify for separate recognition. The amortizable intangible assets are being amortized over their estimated useful lives of 3 to 15 years. During the first quarter of 2022, PMI did not record any measurement period adjustments to the preliminary purchase price allocation. The purchase price allocation is preliminary and continues to be subject to refinement. PMI is evaluating the deductibility of goodwill for income tax purposes.
Pro forma results of operations for the above business combinations have not been presented as the aggregate impact is not material to PMI's consolidated statements of earnings.
Asset Acquisition
On August 9, 2021, PMI acquired 100% of OtiTopic, Inc., a U.S. respiratory drug development company with a late-stage dry powder inhalation aspirin treatment for acute myocardial infarction. The transaction price was $38 million in cash, plus transaction costs, with additional contingent payment of $13 million, primarily related to certain key milestones that PMI deemed probable. Additionally, PMI may owe up to $25 million in future additional contingent payments dependent upon the achievement of certain milestones. PMI accounted for this transaction as an asset acquisition since the IPR&D of the dry powder inhalation aspirin treatment represented substantially all of the fair value of the gross assets acquired. At the date of acquisition, PMI determined that the acquired IPR&D had no alternative future use. As a result, PMI recorded a charge of $51 million to research and development costs within marketing, administration and research costs in the third quarter of 2021.
As previously discussed in Note 1. Background and Basis of Presentation and Note 7. Segment Reporting, on March 31, 2022, PMI launched a new Wellness and Healthcare business, Vectura Fertin Pharma, which consolidates Fertin Pharma, Vectura and OtiTopic, Inc. This business is considered one operating segment with its operating results included in the Other category.
42
Philip Morris International Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 18. War in Ukraine:
In February 2022, the Russian Federation launched a military action against Ukraine. Since the onset of the war in Ukraine, PMI's main priority has been the safety and security of its more than 1,300 employees and their families in the country.
Ukraine
PMI temporarily suspended its operations in Ukraine, including the closing of its factory in Kharkiv at the end of February 2022. While the effects of the war are unpredictable and could trigger impairment reviews for long-lived assets, as of March 31, 2022, PMI is unable to estimate the information required to perform impairment analyses (i.e., forecast of revenues, manufacturing and commercial plans). PMI is not aware of any major damage to its production facilities, inventories or other assets in Ukraine. As a result, PMI has not recorded an impairment of long-lived assets. As of March 31, 2022, PMI’s Ukrainian operations had approximately $0.4 billion in total assets, excluding intercompany balances. These total assets included $105 million, $192 million and $44 million in receivables, inventories and property, plant and equipment, respectively.
As of March 31, 2022, PMI recorded in its condensed consolidated statements of earnings a pre-tax charge related to circumstances driven by the conflict of $27 million, primarily due to an inventory write down, additional allowance for receivables and the cost of PMI’s humanitarian efforts, which includes salary continuation for its employees.
Russia
PMI has suspended its planned investments in the Russian Federation including all new product launches and commercial, innovation, and manufacturing investments. PMI has also initiated plans to scale down its manufacturing operations in Russia amid ongoing supply chain disruptions and the evolving regulatory environment and is working on options to exit the Russian market in an orderly manner. As a result of PMI continuing operations within Russia as of March 31, 2022, it has not recorded an impairment of long-lived and other assets. PMI’s Russian operations as of March 31, 2022 had approximately $1.4 billion in total assets, excluding intercompany balances. These total assets included $464 million, $487 million and $357 million in receivables, inventories and property, plant and equipment, respectively.
As of March 31, 2022, PMI recorded in its condensed consolidated statements of earnings a pre-tax charge related to circumstances driven by the conflict of $15 million, which was primarily due to an inventory write down related to the commercial decisions noted above.
The pre-tax charges, for both PMI’s Ukrainian and Russian affiliates, of $26 million and $16 million were recorded in cost of sales and marketing, administration and research costs, respectively. PMI will continue to monitor the situation as it evolves and will determine if further charges are needed.
43
Item 2.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Description of Our Company
We are a leading international tobacco company working to deliver a smoke-free future and evolving our portfolio for the long-term to include products outside of the tobacco and nicotine sector. Our current product portfolio primarily consists of cigarettes and reduced-risk products, including heat-not-burn, vapor and oral nicotine products, which are sold in markets outside the United States. Since 2008, we have invested more than $9 billion to develop, scientifically substantiate and commercialize innovative smoke-free products for adults who would otherwise continue to smoke, with the goal of completely ending the sale of cigarettes. This includes the building of world-class scientific assessment capabilities, notably in the areas of pre-clinical systems toxicology, clinical and behavioral research, as well as post-market studies. The U.S. Food and Drug Administration ("FDA") has authorized the marketing of versions of our IQOS Platform 1 devices and consumables as Modified Risk Tobacco Products (MRTPs), finding that exposure modification orders for these products are appropriate to promote the public health. We describe the MRTP orders in more detail in the "Business Environment" section of this Item 2. With a strong foundation and significant expertise in life sciences, in February 2021, we announced our ambition to expand into wellness and healthcare areas and deliver innovative products and solutions that aim to address unmet consumer and patient needs.
We currently manage our business in six geographical segments and an Other category:
•European Union ("EU");
•Eastern Europe ("EE");
•Middle East & Africa ("ME&A"), which includes our international duty free business;
•South & Southeast Asia ("S&SA");
•East Asia & Australia ("EA&A");
•Americas ("AMCS"); and
•Other, which includes the operating results of our new Wellness and Healthcare business. In the third quarter of 2021, we acquired Fertin Pharma A/S, Vectura Group plc. (also known as Vectura Group Ltd.) and OtiTopic, Inc. On March 31, 2022, we launched a new Wellness and Healthcare business consolidating these entities, Vectura Fertin Pharma. The operating results of this new business is reported in this Other category. For further details, see Note 7. Segment Reporting and Note 17. Acquisitions.
Our cigarettes are sold in approximately 180 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands.
In addition to the manufacture and sale of cigarettes, we are engaged in the development and commercialization of reduced-risk products ("RRPs"). RRPs is the term we use to refer to products that present, are likely to present, or have the potential to present less risk of harm to smokers who switch to these products versus continuing smoking. IQOS is the leading brand in our smoke-free product portfolio. As of March 31, 2022, our smoke-free products were available for sale in 71 markets in key cities or nationwide.
During 2021, we laid the foundation for our long-term growth ambitions beyond nicotine in wellness and healthcare, including the milestone acquisitions of Vectura Group plc and Fertin Pharma A/S, as noted above, which provide essential capabilities for future product development.
We use the term net revenues to refer to our operating revenues from the sale of our products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. Our net revenues and operating income are affected by various factors, including the volume of products we sell, the price of our products, changes in currency exchange rates and the mix of products we sell. Mix is a term used to refer to the proportionate value of premium-price brands to mid-price or low-price brands in any given market (product mix). Mix can also refer to the proportion of shipment volume in more profitable markets versus shipment volume in less profitable markets (geographic mix).
44
Our cost of sales consists principally of: tobacco leaf, non-tobacco raw materials, labor and manufacturing costs; shipping and handling costs; and the cost of devices produced by third-party electronics manufacturing service providers. Estimated costs associated with device warranty programs are generally provided for in cost of sales in the period the related revenues are recognized.
Our marketing, administration and research costs include the costs of marketing and selling our products, other costs generally not related to the manufacture of our products (including general corporate expenses), and costs incurred to develop new products. The most significant components of our marketing, administration and research costs are marketing and sales expenses and general and administrative expenses.
Philip Morris International Inc. is a legal entity separate and distinct from its direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions that are otherwise compliant with law.
Executive Summary
The following executive summary provides the business update and significant highlights from the "Discussion and Analysis" that follows.
War in Ukraine
Since the onset of the war in Ukraine, our main priority has been the safety and security of our more than 1,300 employees and their families in the country. We have taken action to achieve three critical missions: (i) helping to evacuate more than 1,000 people from Ukraine and relocate over 2,700 others from conflict zones to locations in the country away from the heaviest fighting; (ii) providing critical aid to employees who cannot leave or who decide to remain in Ukraine; and (iii) providing those who have left the country with logistical, medical, financial, and other practical support in neighboring countries. We are continuing to pay salaries to all our Ukrainian employees and are also providing substantial in-kind support to them and their families. In addition, we have already contributed around $10 million in funds and donated essential items across the country, directly to humanitarian organizations and through our own employee-led initiative, Projects With a Heart.
On February 25, 2022, we announced the temporary suspension of our operations in Ukraine, including at our factory in Kharkiv. While activities in the east of Ukraine remain the most heavily impacted, we have seen some resumption of retail activities where safety allows, as we seek to provide product availability and service to adult consumers, using existing finished goods inventories on hand. We are also working on future supply from other production centers, although this may involve higher costs. We are applying increased security and safety measures for personnel.
In 2021, Ukraine accounted for around 2% of our total cigarette and heated tobacco unit shipment volume and under 2% of our total net revenues. As of March 31, 2022, our Ukrainian operations had approximately $0.4 billion in total assets, excluding intercompany balances.
On March 24, 2022, we announced concrete steps we had taken to suspend planned investments and scale down our manufacturing operations in Russia. This included:
•the discontinuation of a number of cigarette products offered in the market (representing approximately one-quarter of the company's domestic cigarette SKUs, including Marlboro and Parliament SKUs) and the reduction of our manufacturing activities accordingly;
•the suspension of our marketing activities in the country;
•the cancellation of all product launches planned for 2022 in the market, including the launch of its flagship heated tobacco product IQOS ILUMA, originally planned for March 2022; and
•the cancellation of our plans to manufacture TEREA heated tobacco units for IQOS ILUMA in Russia (with an eventual annualized capacity of more than 20 billion units) and the related ongoing investment of $150 million.
45
Further, we announced that our Board of Directors and senior executives are working on options to exit the Russian market in an orderly manner, in the context of an increasingly complex and rapidly changing regulatory and operating environment.
We employ more than 3,200 people in Russia and will continue to support our employees there, including paying their salaries, while continuing to fulfill our legal obligations. We will continue to make decisions with their safety and security as a priority.
In 2021, Russia made up almost 10% of total shipment volumes and around 6% of our total net revenues. As of March 31, 2022, our Russian operations had approximately $1.4 billion in total assets, excluding intercompany balances.
These developments above have and will continue to have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in impairment charges.
For further details, see Note 18. War in Ukraine to our condensed consolidated financial statements and the "Cautionary Factors That May Affect Future Results" section of this MD&A.
Consolidated Operating Results for the Three Months Ended March 31, 2022
•Net Revenues - Net revenues of $7.7 billion for the three months ended March 31, 2022 increased by $161 million, or 2.1%, from the comparable 2021 amount. The change in our net revenues from the comparable 2021 amount was driven by the following (variances not to scale with quarterly results):
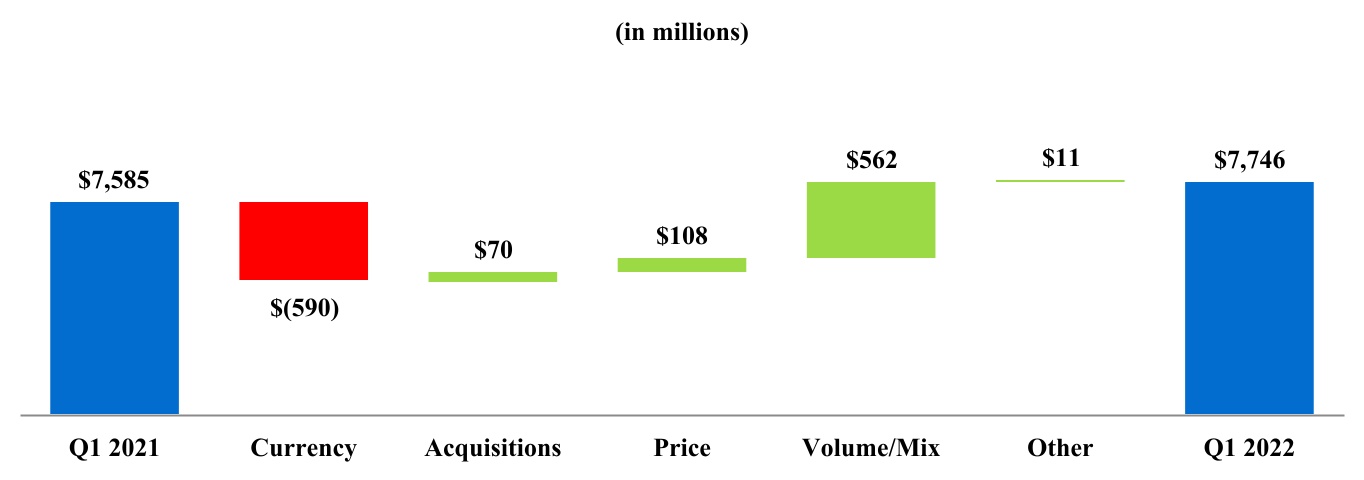
During the quarter, net revenues, excluding currency and acquisitions, increased by 9.0%, mainly reflecting: favorable volume/mix, primarily driven by higher heated tobacco unit volume (predominantly in the EU, particularly Germany, Italy and Poland), higher cigarette volume (mainly in Indonesia, PMI Duty Free and the Philippines, partly offset by Germany and Russia) and higher device volume (driven by the EU and Japan), partially offset by unfavorable cigarette mix (mainly in Japan); and a favorable pricing variance (notably driven by Germany, Russia and Turkey, partly offset by Indonesia and the Philippines).
During the quarter, Russia and Ukraine accounted for around 6% of PMI's total net revenues.
46
Net revenues by product category for the three months ended March 31, 2022 and 2021, are shown below:


•Diluted Earnings Per Share - The changes in our reported diluted earnings per share ("diluted EPS") for the three months ended March 31, 2022, from the comparable 2021 amounts, were as follows:
| Diluted EPS | % Change | |||||||
| For the three months ended March 31, 2021 | $ | 1.55 | ||||||
| 2021 Asset impairment and exit costs | 0.02 | |||||||
| 2021 Tax items | — | |||||||
| Subtotal of 2021 items | 0.02 | |||||||
2022 Charges related to the war in Ukraine | (0.03) | |||||||
| 2022 Fair value adjustment for equity security investments | (0.03) | |||||||
| 2022 Tax items | — | |||||||
| Subtotal of 2022 items | (0.06) | |||||||
| Currency | (0.23) | |||||||
| Interest | 0.01 | |||||||
| Change in tax rate | 0.03 | |||||||
| Operations | 0.18 | |||||||
| For the three months ended March 31, 2022 | $ | 1.50 | (3.2) | % | ||||
Asset impairment and exit costs – In the first quarter of 2021, we recorded pre-tax asset impairment and exit costs of $48 million, representing $38 million net of income tax and a diluted EPS charge of $0.02 per share, related to product distribution restructuring in South Korea and the organizational design optimization plan, primarily in Switzerland. The total pre-tax charge was included in marketing, administration and research costs on the condensed consolidated statements of earnings. For further details, see Note 16. Asset Impairment and Exit Costs.
Charges related to the war in Ukraine – In the first quarter of 2022, we recorded a pre-tax charge of $42 million, representing $39 million net of income tax and a diluted EPS charge of $0.03 per share, related to circumstances driven by the conflict, including inventory write downs, additional allowances for receivables and the cost of PMI’s humanitarian efforts. Of this total pre-tax charge, $26 million was recorded in cost of sales and $16 million was recorded in marketing, administration and research costs. For further details, see Note 18. War in Ukraine.
47
Fair value adjustment for equity security investments – In the first quarter of 2022, we recorded an unfavorable fair value adjustment for our equity security investments in India and Sri Lanka of $47 million after tax (or $0.03 per share decrease in diluted EPS). The fair value adjustment for our equity security investments was included in equity investments and securities (income)/loss, net ($60 million loss) and provision for income taxes ($13 million benefit) on the condensed consolidated statements of earnings for the three months ended March 31, 2022. For further details, see Note 12. Related Parties - Equity Investments and Other.
Income Taxes – The change in the tax rate that increased our diluted EPS by $0.03 per share in the table above was primarily due to changes in earnings mix by taxing jurisdiction.
Currency – The unfavorable impact of $0.23 per share during the reporting period primarily results from the fluctuations of the U.S. dollar, especially against the Euro, Japanese yen and Turkish lira. This unfavorable currency movement has impacted our profitability across our primary revenue markets and local currency cost bases.
Operations – The increase in diluted EPS of $0.18 from our operations in the table above was due primarily to the following segments:
•Middle East & Africa: Favorable pricing, favorable volume/mix and lower marketing, administration and research costs, partially offset by higher manufacturing costs; and
•European Union: Favorable volume/mix, partially offset by higher manufacturing costs;
partially offset by
•East Asia & Australia: Unfavorable volume/mix and higher manufacturing costs, partially offset by lower marketing, administration and research costs and favorable pricing;
•South & Southeast Asia: Unfavorable pricing, partially offset by favorable volume/mix;
•Eastern Europe: Unfavorable volume/mix and higher marketing, administration and research costs, partially offset by favorable pricing;
•Americas: Higher manufacturing costs and unfavorable volume/mix, partially offset by favorable pricing; and
•Other: Primarily reflecting the amortization of intangibles related to the acquisitions, investments in research and development, and expenses related to employee retention programs.
For further details, see the “Consolidated Operating Results” and “Operating Results by Business Segment” sections of the following “Discussion and Analysis.”
48
Discussion and Analysis
Consolidated Operating Results
See pages 82-92 for a discussion of our "Cautionary Factors That May Affect Future Results." Our net revenues and operating income by segment are shown in the table below:
| (in millions) | For the Three Months Ended March 31, | ||||||||||
| 2022 | 2021 | Change | |||||||||
| Net revenues: | |||||||||||
| European Union | $ | 3,012 | $ | 2,909 | 3.5 | % | |||||
| Eastern Europe | 726 | 796 | (8.8) | % | |||||||
| Middle East & Africa | 991 | 801 | 23.7 | % | |||||||
| South & Southeast Asia | 1,123 | 1,173 | (4.3) | % | |||||||
| East Asia & Australia | 1,404 | 1,472 | (4.6) | % | |||||||
| Americas | 424 | 434 | (2.3) | % | |||||||
| Other | 66 | — | — | % | |||||||
| Net revenues | $ | 7,746 | $ | 7,585 | 2.1 | % | |||||
| Operating income (loss): | |||||||||||
| European Union | $ | 1,527 | $ | 1,490 | 2.5 | % | |||||
| Eastern Europe | 144 | 261 | (44.8) | % | |||||||
| Middle East & Africa | 521 | 335 | 55.5 | % | |||||||
| South & Southeast Asia | 445 | 529 | (15.9) | % | |||||||
| East Asia & Australia | 571 | 695 | (17.8) | % | |||||||
| Americas | 121 | 134 | (9.7) | % | |||||||
| Other | (31) | — | — | % | |||||||
| Operating income | $ | 3,298 | $ | 3,444 | (4.2) | % | |||||
Items affecting the comparability of results from operations were as follows:
•Asset impairment and exit costs – See Note 16. Asset Impairment and Exit Costs for details of the $48 million pre-tax charge and a breakdown of these costs by segment for the three months ended March 31, 2021.
•Charges related to the war in Ukraine – See Note 18. War in Ukraine for details of the $42 million pre-tax charges in the Eastern Europe segment for the three months ended March 31, 2022.
49
Our net revenues by product category are shown in the table below:
| PMI Net Revenues by Product Category | |||||||||||
| (in millions) | For the Three Months Ended March 31, | ||||||||||
| 2022 | 2021 | Change | |||||||||
| Combustible Products | |||||||||||
| European Union | $ | 1,810 | $ | 1,950 | (7.2) | % | |||||
| Eastern Europe | 456 | 492 | (7.2) | % | |||||||
| Middle East & Africa | 929 | 780 | 19.1 | % | |||||||
| South & Southeast Asia | 1,118 | 1,171 | (4.6) | % | |||||||
| East Asia & Australia | 601 | 648 | (7.3) | % | |||||||
| Americas | 416 | 422 | (1.4) | % | |||||||
| Total Combustible Products | $ | 5,330 | $ | 5,463 | (2.4) | % | |||||
| Reduced-Risk Products | |||||||||||
| European Union | $ | 1,202 | $ | 959 | 25.4 | % | |||||
| Eastern Europe | 270 | 304 | (11.4) | % | |||||||
| Middle East & Africa | 62 | 21 | +100% | ||||||||
| South & Southeast Asia | 5 | 2 | +100% | ||||||||
| East Asia & Australia | 803 | 824 | (2.5) | % | |||||||
| Americas | 8 | 12 | (35.3) | % | |||||||
| Total Reduced-Risk Products | $ | 2,350 | $ | 2,122 | 10.7 | % | |||||
| Other | |||||||||||
| Other | 66 | — | — | % | |||||||
| Total PMI Net Revenues | $ | 7,746 | $ | 7,585 | 2.1 | % | |||||
Note: Sum of product categories or Regions might not foot to total PMI due to roundings.
Net revenues related to combustible products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of our cigarettes and other tobacco products combined. Other tobacco products primarily include roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos, and do not include reduced-risk products.
Net revenues related to reduced-risk products refer to the operating revenues generated from the sale of these products, including shipping and handling charges billed to customers, net of sales and promotion incentives, and excise taxes. These net revenue amounts consist of the sale of our heated tobacco units, heat-not-burn devices and related accessories, and other nicotine-containing products, which primarily include our e-vapor and oral nicotine products.
Net revenues in the Other category primarily consist of operating revenues generated from the sale of inhaled therapeutics, and oral and intra-oral delivery systems that are included in the operating results of our new Wellness and Healthcare business, Vectura Fertin Pharma.
PMI's heat-not-burn products include licensed KT&G heat-not-burn products.
References to "Cost/Other" in the Consolidated Financial Summary table of total PMI and the six geographical segments throughout this "Discussion and Analysis" reflects the currency-neutral variances of: cost of sales (excluding the volume/mix cost component); marketing, administration and research costs (including asset impairment and exit costs); and amortization of intangibles. “Cost/Other” also includes the currency-neutral net revenue variance, unrelated to volume/mix and price components, attributable to: fees for certain distribution rights billed to customers in certain markets in the ME&A Region.
50
Our shipment volume by segment for cigarettes and heated tobacco units is shown in the table below:
| PMI Shipment Volume (Million Units) | |||||||||||
| For the Three Months Ended March 31, | |||||||||||
| 2022 | 2021 | Change | |||||||||
| Cigarettes | |||||||||||
| European Union | 36,444 | 36,769 | (0.9) | % | |||||||
| Eastern Europe | 18,514 | 19,966 | (7.3) | % | |||||||
| Middle East & Africa | 29,471 | 27,642 | 6.6 | % | |||||||
| South & Southeast Asia | 37,461 | 34,888 | 7.4 | % | |||||||
| East Asia & Australia | 11,553 | 11,362 | 1.7 | % | |||||||
| Americas | 14,795 | 14,885 | (0.6) | % | |||||||
| Total Cigarettes | 148,238 | 145,512 | 1.9 | % | |||||||
| Heated Tobacco Units | |||||||||||
| European Union | 8,566 | 6,426 | 33.3 | % | |||||||
| Eastern Europe | 5,866 | 5,635 | 4.1 | % | |||||||
| Middle East & Africa | 897 | 396 | +100% | ||||||||
| South & Southeast Asia | 94 | 33 | +100% | ||||||||
| East Asia & Australia | 9,288 | 9,139 | 1.6 | % | |||||||
| Americas | 108 | 105 | 2.9 | % | |||||||
| Total Heated Tobacco Units | 24,819 | 21,734 | 14.2 | % | |||||||
| Cigarettes and Heated Tobacco Units | |||||||||||
| European Union | 45,010 | 43,195 | 4.2 | % | |||||||
| Eastern Europe | 24,380 | 25,601 | (4.8) | % | |||||||
| Middle East & Africa | 30,368 | 28,038 | 8.3 | % | |||||||
| South & Southeast Asia | 37,555 | 34,921 | 7.5 | % | |||||||
| East Asia & Australia | 20,841 | 20,501 | 1.7 | % | |||||||
| Americas | 14,903 | 14,990 | (0.6) | % | |||||||
| Total Cigarettes and Heated Tobacco Units | 173,057 | 167,246 | 3.5 | % | |||||||
Heated tobacco units ("HTU") is the term we use to refer to heated tobacco consumables, which include our HEETS, HEETS Creations, HEETS Dimensions, HEETS Marlboro and HEETS FROM MARLBORO (defined collectively as HEETS), Marlboro Dimensions, Marlboro HeatSticks, Parliament HeatSticks and TEREA, as well as the KT&G-licensed brands, Fiit and Miix (outside of South Korea).
Market share for HTUs is defined as the total sales volume for HTUs as a percentage of the total estimated sales volume for cigarettes and HTUs.
References to total industry, total market, our shipment volume and our market share performance reflect cigarettes and heated tobacco units, unless otherwise stated.
As of the first quarter of 2022, total industry volume, PMI in-market sales volume and PMI market share for the following geographies include the cigarillo category in Japan: the total international market, East Asia & Australia Region, and Japanese domestic market.
References to total international market, defined as worldwide cigarette and heated tobacco unit volume excluding the United States, total industry, total market and market shares throughout this "Discussion and Analysis" are our estimates for tax-paid products based on the latest available data from a number of internal and external sources and may, in defined instances, exclude the People's Republic of China and/or our duty free business.
51
2021 and 2022 estimates for total industry volume and market share in certain geographies reflect limitations on the availability and accuracy of industry data during pandemic-related restrictions.
First-quarter 2022 estimates for total industry volume and market share in the Philippines reflect limitations on the availability and accuracy of industry data.
In-market sales ("IMS") is defined as sales to the retail channel, depending on the market and distribution model.
North Africa is defined as Algeria, Egypt, Libya, Morocco and Tunisia.
The Gulf Cooperation Council ("GCC") is defined as Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and the United Arab Emirates (UAE).
Southeast Europe is defined as Albania, Bosnia & Herzegovina, Kosovo, Montenegro, North Macedonia and Serbia.
From time to time, PMI’s shipment volumes are subject to the impact of distributor inventory movements, and estimated total industry/market volumes are subject to the impact of inventory movements in various trade channels that include estimated trade inventory movements of PMI’s competitors arising from market-specific factors that significantly distort reported volume disclosures. Such factors may include changes to the manufacturing supply chain, shipment methods, consumer demand, timing of excise tax increases or other influences that may affect the timing of sales to customers. In such instances, in addition to reviewing PMI shipment volumes and certain estimated total industry/market volumes on a reported basis, management reviews these measures on an adjusted basis that excludes the impact of distributor and/or estimated trade inventory movements. Management also believes that disclosing PMI shipment volumes and estimated total industry/market volumes in such circumstances on a basis that excludes the impact of distributor and/or estimated trade inventory movements improves the comparability of performance and trends for these measures over different reporting periods.
52
Key market data regarding total market size, our shipments and market share are shown in the tables below:
| For the Three Months Ended March 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PMI Shipments (billion units) | PMI Market Share (%)(2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Market | Total Market (billion units) | Total | Cigarette | Heated Tobacco Unit | Total | Heated Tobacco Unit | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||||||||||||
| Total (1) | 563.0 | 556.6 | 173.1 | 167.2 | 148.2 | 145.5 | 24.8 | 21.7 | 26.7 | 26.0 | 3.5 | 2.9 | ||||||||||||||||||||||||||||||||||||||||||||
| European Union | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| France | 7.8 | 8.2 | 3.5 | 3.8 | 3.5 | 3.7 | 0.1 | 0.1 | 45.0 | 43.6 | 0.7 | 0.6 | ||||||||||||||||||||||||||||||||||||||||||||
| Germany | 16.1 | 17.4 | 6.8 | 7.1 | 5.9 | 6.5 | 0.9 | 0.6 | 42.3 | 40.8 | 5.9 | 3.6 | ||||||||||||||||||||||||||||||||||||||||||||
| Italy | 16.8 | 15.9 | 9.7 | 9.6 | 7.1 | 7.5 | 2.6 | 2.2 | 54.2 | 52.8 | 14.8 | 11.3 | ||||||||||||||||||||||||||||||||||||||||||||
| Poland | 12.8 | 10.8 | 4.8 | 4.0 | 3.8 | 3.4 | 1.0 | 0.6 | 37.3 | 36.7 | 7.6 | 5.4 | ||||||||||||||||||||||||||||||||||||||||||||
| Spain | 10.0 | 9.6 | 3.3 | 2.7 | 3.2 | 2.6 | 0.2 | 0.1 | 30.4 | 31.1 | 1.5 | 1.2 | ||||||||||||||||||||||||||||||||||||||||||||
| Eastern Europe | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Russia | n/a | 49.1 | 14.1 | 15.7 | 10.8 | 12.1 | 3.4 | 3.6 | n/a | 31.0 | n/a | 7.7 | ||||||||||||||||||||||||||||||||||||||||||||
| Middle East & Africa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Saudi Arabia | 5.2 | 5.5 | 2.1 | 2.2 | 2.1 | 2.2 | — | — | 41.1 | 42.3 | 0.8 | 0.8 | ||||||||||||||||||||||||||||||||||||||||||||
| Turkey | 23.6 | 25.3 | 11.0 | 11.0 | 11.0 | 11.0 | — | — | 46.9 | 43.4 | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| South & Southeast Asia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indonesia | 75.1 | 71.0 | 20.9 | 19.9 | 20.9 | 19.9 | — | — | 27.8 | 28.0 | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Philippines | 15.6 | 13.1 | 9.7 | 8.2 | 9.6 | 8.1 | — | — | 62.0 | 62.4 | 0.3 | 0.2 | ||||||||||||||||||||||||||||||||||||||||||||
| East Asia & Australia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Australia | 2.2 | 2.4 | 0.8 | 0.8 | 0.8 | 0.8 | — | — | 33.9 | 32.7 | — | — | ||||||||||||||||||||||||||||||||||||||||||||
Japan (3) | 34.4 | 35.6 | 14.2 | 13.8 | 6.1 | 5.9 | 8.1 | 7.9 | 37.2 | 36.1 | 22.9 | 21.5 | ||||||||||||||||||||||||||||||||||||||||||||
| South Korea | 16.8 | 16.8 | 3.3 | 3.4 | 2.2 | 2.2 | 1.1 | 1.1 | 19.6 | 20.1 | 6.5 | 6.8 | ||||||||||||||||||||||||||||||||||||||||||||
| Americas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Argentina | 7.7 | 7.7 | 4.9 | 5.3 | 4.9 | 5.3 | — | — | 63.5 | 68.2 | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Mexico | 6.5 | 6.8 | 4.1 | 4.0 | 4.0 | 4.0 | — | — | 62.5 | 59.6 | 0.4 | 0.3 | ||||||||||||||||||||||||||||||||||||||||||||
| (1) Total market and market share estimates exclude Russia & Ukraine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (2) Market share estimates are calculated using IMS data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (3) Total market and market share estimates include cigarillos | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Consolidated Operating Results for the Three Months Ended March 31, 2022
The following discussion compares our consolidated operating results for the three months ended March 31, 2022, with the three months ended March 31, 2021.
During the first quarter, our total shipment volume increased by 3.5%, driven by:
•the EU, reflecting higher heated tobacco unit shipment volume across the Region (primarily in Germany, Italy and Poland), partly offset by lower cigarette shipment volume (mainly in Germany and Italy, partially offset by Poland and Spain);
•Middle East & Africa, mainly reflecting higher cigarette shipment volume, primarily in Egypt and PMI Duty Free, partly offset by Algeria;
53
•South & Southeast Asia, primarily reflecting higher cigarette shipment volume, mainly in Indonesia and the Philippines; and
•East Asia & Australia, reflecting higher cigarette and heated tobacco unit shipment volume, primarily in Japan;
partly offset by
•Eastern Europe, reflecting lower cigarette shipment volume, primarily in Russia, partly offset by higher heated tobacco unit shipment volume, mainly in Ukraine and Southeast Europe, partially offset by Russia; and
•Americas, primarily reflecting lower cigarette shipment volume, mainly in Argentina, partly offset by Brazil.
Excluding Russia and Ukraine, our total shipment volume increased by 4.9%, reflecting an increase of 4.5% in the Eastern Europe Region.
First-Quarter Impact of Inventory Movements
Excluding the net favorable impact of estimated distributor inventory movements of approximately 1.7 billion units, our total in-market sales increased by 2.5%, driven by a 16.7% increase in heated tobacco units and a 0.4% increase in cigarettes.
The net favorable impact of approximately 1.7 billion units reflected:
•A net favorable impact of 2.1 billion cigarettes, mainly driven by Japan, PMI Duty Free and Spain; partly offset by
•A net unfavorable impact of 0.5 billion heated tobacco units, primarily due to Russia.
Excluding Russia and Ukraine, our total in-market sales increased by 3.8%.
Our total heated tobacco unit in-market sales volume in the quarter was 24.8 billion units, or 19.5 billion units excluding Russia and Ukraine, representing growth of 16.7% and 19.5%, respectively.
Our cigarette shipment volume by brand and heated tobacco units shipment volume are shown in the table below:
| PMI Shipment Volume by Brand (Million Units) | |||||||||||
| First-Quarter | |||||||||||
| 2022 | 2021 | Change | |||||||||
| Cigarettes | |||||||||||
| Marlboro | 57,264 | 53,682 | 6.7 | % | |||||||
| L&M | 20,198 | 20,367 | (0.8) | % | |||||||
| Chesterfield | 15,606 | 12,758 | 22.3 | % | |||||||
| Philip Morris | 9,753 | 10,184 | (4.2) | % | |||||||
| Sampoerna A | 9,718 | 8,698 | 11.7 | % | |||||||
| Parliament | 9,152 | 8,957 | 2.2 | % | |||||||
| Dji Sam Soe | 5,772 | 5,704 | 1.2 | % | |||||||
| Lark | 3,452 | 3,899 | (11.5) | % | |||||||
| Others | 17,323 | 21,263 | (18.5) | % | |||||||
| Total Cigarettes | 148,238 | 145,512 | 1.9 | % | |||||||
| Heated Tobacco Units | 24,819 | 21,734 | 14.2 | % | |||||||
| Total Cigarettes and Heated Tobacco Units | 173,057 | 167,246 | 3.5 | % | |||||||
Note: Lark includes Lark Harmony; Philip Morris includes Philip Morris/Dubliss; and Sampoerna A includes Sampoerna.
54
The increase in our heated tobacco unit shipment volume was mainly driven by the EU (notably Germany, Italy and Poland), Japan and the Middle East & Africa (notably Egypt). Excluding Russia and Ukraine, our heated tobacco unit shipment volume increased by 18.4%.
Our cigarette shipment volume of the following brands increased:
•Marlboro, mainly driven by PMI Duty Free, the Philippines, Russia, Spain and Turkey, partly offset by Algeria and Italy;
•Chesterfield, primarily driven by the Philippines and Russia;
•Sampoerna A in Indonesia, primarily driven by premium A Mild;
•Parliament, mainly driven by Turkey, partly offset by Saudi Arabia; and
•Dji Sam Soe in Indonesia, primarily driven by Dji Sam Soe Magnum Mild.
Our cigarette shipment volume of the following brands decreased:
•L&M, primarily due to Germany and Turkey, partly offset by Egypt and the GCC;
•Philip Morris, mainly due to Russia, partly offset by Japan;
•Lark, primarily due to Japan and Turkey; and
•"Others," mainly due to: mid-price Fortune (Philippines), Sampoerna Hijau (Indonesia) and Sampoerna U (Indonesia); and low-price Bond Street (primarily due to Russia).
Excluding Russia and Ukraine, our cigarette shipment volume increased by: 5.1% for Marlboro, 0.1% for L&M, 10.8% for Chesterfield, 13.3% for Philip Morris and 4.9% for Parliament.
First-Quarter International Share of Market
Our total international market share (excluding China and the United States, as well as Russia and Ukraine), defined as our cigarette and heated tobacco unit sales volume as a percentage of total industry cigarette and heated tobacco unit sales volume, increased by 0.7 points to 26.7%, reflecting:
•Market share for heated tobacco units of 3.5%, up by 0.6 points; and
•Market share for cigarettes of 23.3%, up by 0.2 points.
Excluding China and the United States, as well as Russia and Ukraine, our total international cigarette sales volume as a percentage of total industry cigarette sales volume increased by 0.4 points to 24.4%, mainly reflecting higher cigarette market share and/or a favorable geographic mix impact, notably in Indonesia, PMI Duty Free and the Philippines, partly offset by Algeria and Germany.
| Financial Summary | |||||||||||||||||||||||||||||||||||||||||
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 7,746 | $ | 7,585 | 2.1 | % | 9.0 | % | $ | 161 | $ | (590) | $ | 70 | $ | 108 | $ | 562 | $ | 11 | |||||||||||||||||||||
Cost of Sales (1) | (2,608) | (2,274) | (14.7) | % | (18.3) | % | (334) | 125 | (42) | — | (298) | (119) | |||||||||||||||||||||||||||||
Marketing, Administration and Research Costs (2) | (1,802) | (1,849) | 2.5 | % | 1.7 | % | 47 | 50 | (35) | — | — | 32 | |||||||||||||||||||||||||||||
| Amortization of Intangibles | (38) | (18) | -(100)% | (5.6) | % | (20) | — | (19) | — | — | (1) | ||||||||||||||||||||||||||||||
| Operating Income | $ | 3,298 | $ | 3,444 | (4.2) | % | 8.6 | % | $ | (146) | $ | (415) | $ | (26) | $ | 108 | $ | 264 | $ | (77) | |||||||||||||||||||||
(1) Cost/Other variance includes charges in 2022 of $26 million related to the war in Ukraine. For more details, see Note 18. War in Ukraine.
55
(2) Cost/Other variance includes charges in 2022 of $16 million related to the war in Ukraine and charges in 2021 of $48 million related to asset impairment and exit costs. For more details, see Note 16. Asset Impairment and Exit Costs and Note 18. War in Ukraine.
During the quarter, net revenues, excluding currency and acquisitions, increased by 9.0%, mainly reflecting: favorable volume/mix, primarily driven by higher heated tobacco unit volume (predominantly in the EU, particularly Germany, Italy and Poland), higher cigarette volume (mainly in Indonesia, PMI Duty Free and the Philippines, partly offset by Germany and Russia) and higher device volume (driven by the EU and Japan), partially offset by unfavorable cigarette mix (mainly in Japan); and a favorable pricing variance (notably driven by Germany, Russia and Turkey, partly offset by Indonesia and the Philippines).
During the quarter, Russia and Ukraine accounted for around 6% of PMI's total net revenues.
The unfavorable currency in net revenues was due primarily to the Euro, Japanese yen, Russian ruble and Turkish lira.
Net revenues include $2.3 billion in 2022 and $2.1 billion in 2021 related to the sale of RRPs. IQOS devices accounted for approximately 5% of RRP net revenues in the first quarter of 2022, reflecting higher year-over year device volumes as supply constraints ease and ILUMA's positive performance.
Operating income, excluding currency and acquisitions, increased by 8.6%, primarily reflecting: favorable volume/mix, mainly driven by higher heated tobacco unit volume and higher cigarette volume (each mainly reflecting the same geographies as for net revenues noted above), partly offset by unfavorable cigarette mix (primarily in the EU and Japan) and device mix (predominantly in Japan); and a favorable pricing variance; partly offset by higher manufacturing costs (primarily due to higher logistics costs and other inflationary impacts, partially offset by productivity).
Interest expense, net, of $154 million decreased by $13 million (7.8)%.
Our effective tax rate decreased by 1.8 percentage points to 19.7%. We estimate that our full-year 2022 effective tax rate will be 21% to 22%, excluding discrete tax events. For further details, see Note 9. Income Taxes.
Net earnings attributable to PMI of $2.3 billion decreased by $87 million or 3.6%. This decrease was due primarily to lower operating income as discussed above, partially offset by a lower effective tax rate. Diluted and basic EPS of $1.50 decreased by 3.2%. Excluding an unfavorable currency impact of $0.23, diluted EPS increased by 11.6%.
Operating Results by Business Segment
Business Environment
Taxes, Legislation, Regulation and Other Matters Regarding the Manufacture, Marketing, Sale and Use of Tobacco Products
The tobacco industry and our company face a number of challenges that may adversely affect our business, volume, results of operations, cash flows and financial position. These challenges, which are discussed below and in “Cautionary Factors That May Affect Future Results,” include:
•regulatory restrictions on our products, including restrictions on the packaging, marketing, and sale of tobacco or other nicotine-containing products that could reduce our competitiveness, eliminate our ability to communicate with adult consumers, or even ban certain of our products;
•fiscal challenges, such as excessive excise tax increases and discriminatory tax structures;
•illicit trade in cigarettes and other tobacco and nicotine-containing products, including counterfeit, contraband and so-called “illicit whites”
•intense competition, including from non-tax paid volume by certain local manufacturers;
•pending and threatened litigation as discussed in Note 8. Contingencies; and
•governmental investigations.
Regulatory Restrictions: The tobacco industry operates in a highly regulated environment. The well-known risks of smoking have led regulators to impose significant restrictions and high excise taxes on cigarettes.
56
Much of the regulation that shapes the business environment in which we operate is driven by the Framework Convention on Tobacco Control (the “FCTC”) of the World Health Organization (the “WHO”), which entered into force in 2005. The FCTC has as its main objective to establish a global agenda for tobacco regulation, with the purpose of reducing tobacco use. To date, 182 countries and the European Union are Parties to the FCTC. The treaty requires Parties to have in place various tobacco control measures and recommends others. The FCTC governing body, the Conference of the Parties (“CoP”), has also adopted non-binding guidelines and policy recommendations related to certain articles of the FCTC that go beyond the text of the treaty. In October 2018, the CoP recognized the need for more scientific assessment and improved reporting to define policy on heated tobacco products. Similar to its previous policy recommendations on e-cigarettes, the CoP invited countries to regulate, restrict or prohibit heated tobacco products, as appropriate under their national laws.
Prior to CoP 9 that took place in November 2021, the WHO and the WHO FCTC Secretariat published two reports on novel and emerging tobacco products. The reports were noted by CoP 9 and related substantive discussions and decisions were deferred to CoP 10, currently scheduled for 2023. It is not possible to predict whether or to what extent measures recommended by the WHO's reports will be implemented as the reports are not binding to the WHO Member States.
We believe that when better alternatives to cigarettes exist, the discussion should not be whether these alternatives should be made available to the more than one billion people who smoke today, but how fast, and within what regulatory framework to maximize their adoption while minimizing unintended use. Therefore, we advocate for regulatory frameworks that recognize a significant difference on a risk continuum between combustible tobacco on the one hand and non-combustible tobacco and other nicotine-containing products on the other. Regulation should include measures that will accelerate switching to non-combustible products, for example, by allowing adult consumers who would not otherwise quit to receive truthful and non-misleading information about such products to enable them to make informed decisions and by applying uniform product standards to enable manufacturers to demonstrate the safety of these products as well as the absence of combustion. Regulation should also include specific rules for ingredients, labeling and consumer communication, and should ensure that the public is informed about the health risks of all combustible and non-combustible tobacco and nicotine-containing products. Importantly, regulation must include measures designed to prevent initiation by youth and non-smokers. We support mandated health warnings, minimum age laws, restrictions on advertising, and public place smoking restrictions. We also support regulatory measures that help reduce illicit trade.
Certain measures are discussed in more detail below and in the Reduced-Risk Products (RRPs) section.
Fiscal Challenges: Excessive and disruptive excise, sales and other tax increases and discriminatory tax structures are expected to continue to have an adverse impact on our profitability, due to lower consumption and consumer down-trading to non-premium, discount, other low-price or low-taxed combustible tobacco products such as fine cut tobacco and illicit cigarettes. In addition, in certain jurisdictions, some of our combustible products are subject to tax structures that discriminate against premium-price products and manufactured cigarettes. We believe that such tax policies undermine public health by encouraging consumers to turn to illicit trade, and ultimately undercut government revenue objectives, disrupt the competitive environment, and encourage criminal activity. Other jurisdictions have imposed, or are seeking to impose, levies or other taxes specifically on tobacco companies, such as taxes on revenues and/or profits.
World Customs Organization Developments: In 2020, the World Customs Organization (the “WCO”) amended the harmonized system nomenclature to introduce dedicated custom codes for novel tobacco and nicotine products, including heated tobacco products, e-cigarettes and other nicotine-containing products. The amendments became effective as of January 1, 2022. These amendments require WCO member states to transfer products from customs codes in the current nomenclature to the new one. These amendments are not expected to significantly impact current customs duty rates.
EU Tobacco Products Directive: In April 2014, the EU adopted a significantly revised EU Tobacco Products Directive (the “TPD”), which entered into force in May 2016. All member states have adopted laws transposing the TPD. The TPD sets forth a comprehensive set of regulatory requirements for tobacco products, including:
•health warnings covering 65% of the front and back panels of cigarette packs, with an option for member states to further standardize tobacco packaging, including the introduction of plain packaging;
•a ban on characterizing flavors in some tobacco products, with a transition period for menthol that expired in May 2020;
•security features and tracking and tracing measures that became effective in May 2019; and
57
•a framework for the regulation of novel tobacco products and e-cigarettes, including requirements for health warnings and information leaflets, a prohibition on product packaging text related to reduced risk, and the introduction of notification requirements or authorization procedures in advance of commercialization.
In May 2021, the European Commission published its first report on the application of the TPD. The report identifies significant progress made due to the implementation of the TPD and where there is still room for improvement. Most notably, it finds that the EU legislation has enhanced tobacco control, contributed to protecting the health of EU citizens by providing Member States with strong rules to address the use of tobacco products in the EU. The TPD reportedly achieved the 2% reduction target of the impact assessment with decreased smoking prevalence among youth. The report also concludes that there is scope for improvement in certain areas, such as enforcement at national level, assessment of ingredients, and a better consideration for novel and emerging products.
In November 2021, the European Commission published the implementation roadmap to Europe's Beating Cancer Plan (the "Plan"). According to the Plan, a revision of the TPD is planned for 2024.
EU Tobacco Excise Directive ("TED"): The EU Commission is preparing a legislative proposal for the revision of the 2011 EU Tobacco Excise Directive that may include definitions and tax treatment for novel tobacco and nicotine-containing products, including heated tobacco products and e-cigarettes. The proposal is expected to be published in the course of 2022 and adopted by the EU Council in 2023. Any final amendments to TED require unanimous agreement by all EU member states, followed by transposition of TED into national legislation. The potential effective date for any changes to TED, after the transposition period, is early 2024.
Plain Packaging and Other Packaging Restrictions: Plain packaging legislation bans the use of branding, logos and colors on packaging other than the brand name and variant that may be printed only in specified locations and in a uniform font. To date, plain packaging laws have been adopted in certain markets in all of our operating segments, including the key markets of Australia, France, Saudi Arabia and Turkey. Some countries, such as Canada, Denmark and Israel adopted plain packaging regulations that apply to all tobacco products, including RRPs. Other countries are also considering plain packaging legislation.
Some countries have adopted, or are considering adopting, packaging restrictions that could have an impact similar to plain packaging. Examples of such restrictions include standardizing the shape and size of packages, prohibiting certain colors or the use of certain descriptive phrases on packaging, and requiring very large graphic health warnings that leave little space for branding.
Restrictions and Bans on the Use of Ingredients: The WHO and others in the public health community have recommended restrictions or total bans on the use of some or all ingredients in tobacco products, including menthol. Broad restrictions and ingredient bans would require us to reformulate our American blend tobacco products and could reduce our ability to differentiate these products in the market in the long term. In many countries, menthol bans would eliminate the entire category of mentholated tobacco products. The European Union banned cigarettes and roll-your-own tobacco products with characterizing flavors. Other tobacco products, including heated tobacco products, are currently exempted from this characterizing flavor ban. However, the European Union Commission has issued a draft delegated directive that would end this exemption. While the timing and final form of the directive is not yet known, if adopted it would likely ban the use of characterizing flavors from heated tobacco products in the European Union, impacting a significant proportion of our RRP products currently sold in the European Union. Other countries may follow the EU’s approach toward tobacco product ingredients. Turkey banned menthol as of May 2020. Broader ingredient bans have been adopted by Brazil and Canada.
Bans on Display of Tobacco Products at Retail: In a number of our markets, including, but not limited to, Australia and Russia, governments have banned the display of tobacco products at the point of sale. Other countries are considering similar bans.
Bans and Restrictions on Advertising, Marketing, Promotions and Sponsorships: For many years, the FCTC has called for, and countries have imposed, partial or total bans on tobacco advertising, marketing, promotions and sponsorships, including bans and restrictions on advertising on radio and television, in print and on the Internet. The FCTC's non-binding guidelines recommend that governments prohibit all forms of communication with adult smokers.
Restrictions on Product Design: Some members of the public health community are calling for the further standardization of tobacco products by requiring, for example, that cigarettes have a certain minimum diameter, which would amount to a ban on slim cigarettes, or requiring the use of standardized filter and cigarette paper designs. In addition, at its meeting in November 2016, the CoP adopted non-binding guidelines recommending that countries regulate product design features that increase the attractiveness of tobacco products, such as the diameter of cigarettes and the use of flavor capsules.
58
Restrictions on Public Smoking and Use of Nicotine-Containing Products in Public: The pace and scope of restrictions on the use of our products have increased significantly in most of our markets. Many countries around the world have adopted, or are likely to adopt, regulations that restrict or ban smoking and use of nicotine-containing products in public and/or work places, restaurants, bars and nightclubs. Some public health groups have called for, and some countries, regional governments and municipalities have adopted or proposed, bans on smoking in outdoor places, as well as bans on smoking in cars (typically, when minors are present) and private homes.
Other Regulatory Issues: Some regulators are considering, or in some cases have adopted, regulatory measures designed to reduce the supply of tobacco products. These include regulations intended to reduce the number of retailers selling tobacco products by, for example, reducing the overall number of tobacco retail licenses available or banning the sale of tobacco products within specified distances of certain public facilities. In addition, South Africa banned the sale of tobacco products, e-cigarettes, and electronic devices that heat tobacco for several months during the COVID-19 pandemic. The ban, which was lifted on August 17, 2020, resulted in a significant increase of illicit trade of tobacco products.
In a limited number of markets, most notably Japan, we are dependent on governmental approvals that may limit our pricing flexibility.
The EU Single-Use Plastics Directive, which will require tobacco manufacturers and importers to cover the costs of public collection systems for tobacco product filters, under Extended Producer Responsibility ("EPR") schemes, entered into force on July 2, 2019. To date, some member states transposed the Directive into national legislation. We expect remaining member states to transpose the EU Single-Use Plastics Directive into national legislation including EPR schemes by January 2023. While we cannot predict the impact of this initiative on our business at this time, we are monitoring developments in this area.
Illicit Trade: Illicit tobacco trade creates a cheap and unregulated supply of tobacco products, undermines efforts to reduce smoking prevalence, especially among youth, damages legitimate businesses and intellectual property rights, stimulates organized crime, increases corruption and reduces government tax revenue. We generally estimate that, excluding China and the U.S., illicit trade may account for as much as 12% of global cigarette consumption; this includes counterfeit, contraband and the persistent problem of “illicit whites,” which are cigarettes legally produced in one jurisdiction for the sole purpose of being exported and illegally sold in another jurisdiction where they have no legitimate market. Currently, we estimate that illicit trade in the European Union accounted for approximately 8% of total cigarette consumption in 2021.
A number of jurisdictions are considering actions to prevent illicit trade. In November 2012, the FCTC adopted the Protocol to Eliminate Illicit Trade in Tobacco Products (the “Protocol”), which includes supply chain control measures, such as licensing of manufacturers and distributors, enforcement of these control measures in free trade zones, controls on duty free and Internet channels and the implementation of tracking and tracing technologies. To date, 64 Parties, including the European Union, have ratified it. The Protocol came into force in September 2018. Parties must start implementing its provisions in their national legislation. In November 2021, the second Meeting of the Parties to the Protocol decided, among others, to focus on the implementation of a framework for global information sharing to combat illicit tobacco trade and enable the parties to exchange products' tracking and tracing information in a secure manner. We welcome this decision and expect that other Parties will ratify the Protocol.
We devote substantial resources to help prevent illicit trade in combustible tobacco products and RRPs. For example, we engage with governments, our business partners and other stakeholders to implement effective measures to combat illicit trade and, in some instances, pursue legal remedies to protect our intellectual property rights.
The tracking and tracing regulations for cigarettes and roll-your-own products manufactured or destined for the EU became effective on May 20, 2019. The effective date for other tobacco-containing products, including some of our RRPs such as heated tobacco units, is May 20, 2024. While we expect that this regulation will increase our operating expenses, we do not expect this increase to be significant.
In 2009, our Colombian subsidiaries entered into an Investment and Cooperation Agreement with the national and regional governments of Colombia to promote investment in, and cooperation on, anti-contraband and anti-counterfeit efforts. The agreement provides $200 million in funding over a 20-year period to address issues such as combating illegal cigarette trade and increasing the quality and quantity of locally-grown tobacco.
In May 2016, PMI launched PMI IMPACT, a global initiative that supports third-party projects dedicated to fighting illegal trade and related crimes such as corruption, organized criminal networks and money laundering. The centerpiece of PMI IMPACT is a council of external independent experts in the fields of law, anti-corruption and law enforcement. The experts are
59
responsible for evaluating and approving funding proposals for PMI IMPACT grants. PMI has pledged $100 million to fund projects within PMI IMPACT over three funding rounds.
Reduced-Risk Products (RRPs)
Our Approach to RRPs: We recognize that smoking cigarettes causes serious diseases and that the best way to avoid the harms of smoking is never to start or to quit. Nevertheless, it is predicted that by 2025, the number of smokers will remain largely unchanged from the current estimate of 1.1 billion, despite the considerable efforts to discourage smoking.
Cigarettes burn tobacco, which produces smoke. As a result of the combustion process, the smoker inhales various toxic substances. In contrast, RRPs do not burn tobacco and therefore contain significantly lower levels of harmful and potentially harmful constituents ("HPHCs") than found in cigarette smoke.
For adult smokers who would otherwise continue to smoke, we believe that RRPs, while not risk-free, offer a much better consumer choice. Accordingly, our key strategic priorities are to: (i) to develop and commercialize products that present less risk of harm to adult smokers who switch to those products versus continued smoking; and (ii) educate and convince current adult smokers who would otherwise continue to smoke to switch to those products.
We recognize that this transformation from cigarettes to RRPs will take time and that the speed of transformation will depend in part upon factors beyond our control, such as the willingness of governments, regulators and other policy groups to embrace RRPs as a desired alternative to continued cigarette smoking. For as long as a significant number of adult smokers continues to smoke, responsible leadership of the category is critical. We aim to maintain our competitive position in the cigarette market through selective investment. As a leading international cigarette manufacturer, we will continue to accelerate this transformation by using our regulatory and commercial expertise and extensive commercial and distribution infrastructure as an effective platform for the commercialization of our RRPs and communication with adult smokers and trade partners about the benefits of switching to our RRPs.
While seeking to remain competitive in the cigarette market, we are judiciously reallocating resources from cigarettes to RRPs and are streamlining our cigarette portfolio.
We have a range of RRPs in various stages of development, scientific assessment and commercialization. We conduct rigorous scientific assessments of our RRP platforms to substantiate that they reduce exposure to HPHCs and, ultimately, that these products present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to them versus continued smoking. We draw upon a team of expert scientists and engineers from a broad spectrum of scientific disciplines and our extensive learnings of adult consumer preferences to develop and assess our RRPs. Our efforts are guided by the following key objectives:
•to develop RRPs that adult smokers who would otherwise continue to smoke find to be satisfying alternatives to smoking;
•for those adult smokers, our goal is to offer RRPs with a scientifically substantiated risk-reduction profile that approaches as closely as possible that associated with smoking cessation;
•to substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on scientific evidence of the highest standard that is made available for scrutiny and review by external independent scientists and relevant regulatory bodies; and
•to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs, including the communication of scientifically substantiated information to enable adult smokers to make better consumer choices.
Our RRP Platforms: Our product development is based on the elimination of combustion via tobacco heating and other innovative systems, which we believe are the most promising path to providing a better consumer choice for those who would otherwise continue to smoke. We recognize that no single product will appeal to all adult smokers. Therefore, we are developing a portfolio of products intended to appeal to a variety of distinct adult consumer preferences.
Five PMI-developed or improved RRP platforms are in various stages of development and commercialization readiness:
Platform 1 uses a precisely controlled heating device incorporating our IQOS HeatControl technology, into which a specially designed and proprietary tobacco unit is inserted and heated to generate an aerosol. We have conducted a series of
60
clinical studies for this platform, the results of which were included in our submission to the U.S. Food and Drug Administration (“FDA”) described below. We completed a 6+6-month exposure response study and shared the results with the FDA in April 2020. The study showed that for the group that switched to our Platform 1 product, the eight clinical risk endpoints that were tested as co-primary endpoints in the first six-month term moved in the same direction as observed for smoking cessation after 12 months of use of this product. In addition, we completed an 18-month combined chronic toxicity and carcinogenicity study in mice, which was on-going at the time of our FDA submission. We shared the results with the FDA in August 2018. In addition to the original version of Platform 1 which relies on a heating technology using a blade, a new version of Platform 1 is now available using induction. All studies referenced above were conducted with the blade version of Platform 1. We believe that there is full comparability between the versions, therefore the data from these studies remain valid.
Platform 2 used a pressed carbon heat source which, when ignited, generates a nicotine-containing aerosol by heating tobacco. As a result of consumer testing feedback, the design of our current Platform 2 technology has been discontinued. We are assessing alternative designs for this consumer segment.
Platform 3 provides an aerosol of nicotine salt. We have explored two routes for this platform, one with electronics and one without, and conducted nicotine pharmacokinetic studies with both versions. The results of our pharmacokinetic study related to the version without electronics indicate this product's potential as an acceptable alternative to continued cigarette smoking in terms of product satisfaction. In February 2020, we completed a one-month product use and adaptation study in adult smokers for the product variant without electronics. The results of the study indicated that while during the study period, the adult smokers did not fully switch from smoking cigarettes to this Platform 3 product, on average, they used this product on a daily basis and significantly reduced their daily consumption of cigarettes. We are working on product modifications to enable switching by those adult smokers who are looking for better alternatives to cigarettes.
Platform 4 covers e-vapor products, which are battery-powered devices that produce an aerosol by vaporizing a nicotine-containing liquid solution. In 2020, our e-vapor products comprised devices with the "coil and wick" technology as well as our e-vapor mesh technology designed to ensure the consistency and quality of the generated aerosol compared to the products with the “coil and wick” technology. Recently, we discontinued the commercialization of devices with the “coil and wick” technology. We conducted a nicotine pharmacokinetic study with respect to products with our e-vapor mesh technology in 2017. The results of this study indicate that these products are an effective means of nicotine delivery while being a satisfying alternative for e-cigarette users. In March 2019, a six-month pre-clinical study in mice evaluating the impact of e-cigarette vapor on the risks of pulmonary and cardiovascular disease compared to cigarette smoke was completed; this study did not pertain to a specific product. The study demonstrated that e-cigarette vapors induce significantly lower biological responses associated with cardiovascular and pulmonary diseases compared with cigarette smoke. Recently, we designed a new consumable for our e-vapor mesh technology to deliver real tobacco taste satisfaction in an E-Vapor product liquid-using patented technology, where flavors and nicotine are extracted directly from the tobacco leaves and captured in a liquid solution, without having to add flavoring ingredients.
Platform 5 covers Modern Oral Nicotine Pouches, which consist of white pre-portioned pouches containing nicotine derived from tobacco. Users place a pouch between the upper lip and gum and leave it there while the nicotine and taste are being released. At the end of the use, the user can dispose of the pouch. Nicotine pouches are inherently smoke-free as they are consumed orally, and no combustion process occurs during use. Our nicotine pouches do not contain tobacco. Instead, they contain primarily nicotine, flavors, and a cellulose substrate. The nicotine used in the pouches is of pharmaceutical-grade like the nicotine used in medicinal products, such as gums and inhalers, while the flavors are approved for use in food in accordance with the product quality standards for nicotine pouches developed by the Swedish Institute for Standards. In 2021, PMI acquired AG Snus as well as Fertin Pharma, two companies manufacturing and/or marketing nicotine pouches.
We aim to expand our brand portfolio and market positions with additional RRPs. In addition, we are continuing to use our expertise, technology and capabilities to explore new growth opportunities beyond our current business, including products that do not contain nicotine or tobacco.
After we receive the results of our scientific studies, including those mentioned above, in accordance with standard scientific practices, we share the conclusions in scientific forums and submit them for inclusion in peer-reviewed publications.
Commercialization of RRPs: We are building a new product category and tailor our commercialization strategy to the characteristics of each specific market. We focus our commercialization efforts on consumer retail experience, guided consumer trials and customer care, and increasingly, digital communication programs and e-commerce. In order to accelerate switching to our Platform 1 products, our initial market introductions typically entail one-to-one consumer engagement (in person or by digital means) and device discounts. These initial commercialization efforts require substantial investment, which we believe
61
will moderate over time and further benefit from the increased use of digital engagement capabilities. During the COVID-19 pandemic, we accelerated our investments in, and pivot to, digital consumer engagement.
As of March 31, 2022, PMI's smoke-free products are available for sale in 71 markets in key cities or nationwide.
In 2014, we introduced our Platform 1 product in pilot city launches in Nagoya, Japan, and in Milan, Italy. Since then, we have continuously expanded our commercialization activities.
We believe that only a very small percentage of adult smokers who convert to our Platform 1 product switch back to cigarettes.
We have integrated the production of our heated tobacco units into a number of our existing manufacturing facilities, are progressing with our plans to build manufacturing capacity for our other RRP platforms, and continue to optimize our manufacturing infrastructure.
An adequate supply chain for our RRP portfolio, including the supply of electronic devices, is important to our business. We work with four electronics manufacturing service providers for the supply of our Platform 1 and Platform 4 devices, and a small number of other providers for other products in our RRP portfolio and related accessories. Due to the COVID-19 pandemic, the operations of our two main electronic manufacturing service providers were temporarily suspended at different times. Even though these suspensions did not materially affect our operations, if one or more of these service providers were significantly constrained at the same time, the supply of the devices could be disrupted. Although we work closely with these service providers on monitoring their production capability and financial health, we cannot guarantee that they will remain capable of meeting their commitments, particularly during the COVID-19 pandemic and the armed conflict between Russia and Ukraine; if they will not, the commercialization of our RRPs could be adversely affected. The production of our RRP portfolio requires various metals, and we believe that there is an adequate supply of such metals in the world markets to satisfy our current and anticipated production requirements. However, some components and materials necessary for the production of our RRPs, including those for the electronic devices, are obtained from single or limited sources, and can be subject to industry-wide shortages and price fluctuations. While we were successful in maintaining adequate supply of such components and materials so far, we may not be able to secure such supply going forward, particularly during the COVID-19 pandemic; this could negatively impact the commercialization of our RRPs.
In addition, we are also exposed to a world-wide shortage of semiconductors, which continues to put constraints on our device supplies for RRPs. We believe, however, that the overall impact of this shortage remains manageable, and we have adjusted our device assortments to limit the effect on consumer availability of our RRPs.
We discuss product warranties in more detail in Note 14. Product Warranty. The significance of warranty claims is dependent on a number of factors, including device version mix, product failure rates, logistics and service delivery costs, and warranty policies, and may increase with the number of devices sold.
Product quality may affect consumer acceptance of our RRPs.
Our near-term planned commercialization efforts for the other PMI-developed RRP platforms are as follows:
•We started commercializing an improved version of our IQOS MESH product in Canada, Croatia, the Czech Republic, Finland, Italy, Ukraine and New Zealand under the IQOS VEEV or VEEV brand names. We currently plan to launch this product in additional markets.
•Following the consumer test conducted in 2020, and the results of the product use and adaptation study described above, we are incorporating our learnings into our plans to improve our Platform 3 product.
•We launched a Platform 5 product in Sweden in January 2022, that is a reformulated version of the already commercialized nicotine pouches bearing the Shiro brand by our newly acquired affiliate AG Snus.
Due to the COVID-19 pandemic and the armed conflict between Russia and Ukraine, certain of these commercialization efforts could be delayed.
RRP Regulation and Taxation: RRPs contain nicotine and are not risk-free. As we describe in more detail above, we support science-based regulation and taxation of RRPs, and believe that regulation and taxation should differentiate between cigarettes and products that present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to
62
these products versus continued smoking and should recognize a continuum of risk for tobacco and other nicotine-containing products. Regulation, as well as industry practices, should reflect the fact that youth should not consume nicotine in any form.
Some governments have banned or are seeking to ban or severely restrict emerging tobacco and nicotine-containing products such as our RRPs and communication of truthful and non-misleading information about such products.
These regulations might foreclose or unreasonably restrict adult consumer access even to products that might be shown to be a better consumer choice than continuing to smoke. During the COVID-19 pandemic, some governments have been and may continue to be temporarily unable to focus on the development of science-based regulatory frameworks for the development and commercialization of RRPs or on the enforcement or implementation of regulations that are significant to our business.
We oppose blanket bans and unreasonable restrictions of products that have the potential to present less risk of harm compared to continued smoking. By contrast, we support regulation that sets clear standards for all RRP categories and propels innovation to benefit adult smokers who would otherwise continue to smoke.
In the United States, an established regulatory framework for assessing “Modified Risk Tobacco Products” and “New Tobacco Products” exists under the jurisdiction of the FDA. We submitted to the FDA a Modified Risk Tobacco Product Application (“MRTPA”) for our Platform 1 product in December 2016, and a Premarket Tobacco Product Application (“PMTA”) for our Platform 1 product in March 2017.
On April 30, 2019, the FDA determined that a version of our Platform 1 product, namely, IQOS 2.4 and three related consumables, is appropriate for the protection of public health and authorized it for sale in the United States. The FDA’s decision followed its comprehensive assessment of our PMTA. On December 7, 2020, the FDA reached the same determination for the IQOS 3 device and authorized that version of our Platform 1 product for sale in the United States.
On July 7, 2020, the FDA determined that the available scientific evidence demonstrates that the issuance of an exposure modification order would be appropriate for the promotion of public health and authorized the marketing of a version of our Platform 1 product, namely IQOS 2.4 and three related consumables, as a "modified risk tobacco product." The FDA authorized the marketing of this product in the U.S. with the following information:
"AVAILABLE EVIDENCE TO DATE:
•the IQOS system heats tobacco but does not burn it.
•this significantly reduces the production of harmful and potentially harmful chemicals.
•scientific studies have shown that switching completely from conventional cigarettes to the IQOS system significantly reduces your body’s exposure to harmful or potentially harmful chemicals."
We must request and receive authorization from the FDA in order to continue marketing this product with the same modified exposure information after the present order expires in four years from the date of the orders.
On March 18, 2021, we submitted to the FDA a supplemental MRTPA ("sMRTPA") for IQOS 3 requesting authorization to market this version of the device as a Modified Risk Tobacco Product with reduced exposure information like IQOS 2.4. In June 2021, the FDA formally accepted and filed our sMRTPA for substantive scientific review and, already in May 2021, the FDA opened the period for the public to provide comments on our application. The public comment period, which was initially scheduled to be closed on August 2, 2021, was extended on July 20, 2021 to provide time for the public to review application materials that were not previously posted by FDA. The FDA closed the comment period for IQOS 3's sMRTPA on December 10, 2021. On March 11, 2022, the FDA authorized our sMRTPA for IQOS 3 by issuing a Modified Risk Granted Order – Exposure Modification.
There are two types of MRTP orders the FDA may issue: a “risk modification” order or an “exposure modification” order. We had requested both types of orders for IQOS 2.4 and an initial selection of 3 consumables' variants. After review, the FDA determined that the evidence did not support issuing a "risk modification" order at this time but that it did support issuing an "exposure modification" order for the product. This determination included a finding that issuance of the exposure modification order is expected to benefit the health of the population as a whole. We requested (and received) an exposure modification order for IQOS 3.
We look forward to working with the FDA to provide any additional information they may require in order to market this product with reduced risk claims.
63
The FDA’s PMTA and MRTP orders do not mean that the agency “approved” our Platform 1 product. These authorizations are subject to strict marketing, reporting and other requirements, and are not a guarantee that the product will remain authorized, particularly if there is a significant uptake in youth or non-smoker initiation. The FDA will monitor the marketing of the product.
Some states and municipalities in the U.S. have introduced severe restrictions for the sale of certain e-cigarettes and tobacco products, including those authorized by the FDA. We believe that such restrictions on FDA-authorized products will not advance public health and will unreasonably limit adult consumer access to products that are shown to be a better alternative to continued smoking.
In March 2020, we requested a clarification from the FDA regarding the applicability of its new health warning requirements to our heated tobacco units sold in the United States. In June 2021, the FDA responded to our letter and requested additional information regarding the applicability of the cigarette health warnings rule to the IQOS System and HeatSticks. Philip Morris Products S.A. is committed to providing adult consumers of tobacco products with complete, accurate and non-misleading information regarding the health risks associated with the use of the IQOS System and HeatSticks. We shared our views with the FDA on the applicability of new health warnings to our products in our submission on December 2, 2021.
In the U.S., tobacco and nicotine-containing products that were not commercially marketed as of February 15, 2007, are subject to review and authorization by the FDA. Manufacturers of all non-authorized products currently on the market were required to file a PMTA with the FDA by September 9, 2020. The FDA announced on September 9, 2020 that it will prioritize enforcement against any tobacco and nicotine-containing product sold without a PMTA. On October 5, 2021, FDA published its final PMTA rule in the Federal Register, which is effective November 4, 2021. All future applications will have to comply with the requirements in the PMTA rule, which is substantially similar to the version of the final PMTA rule which was posted on Advanced Federal Register on January 19, 2021.
FDA actions may influence the regulatory approach of other governments.
On September 29, 2021, the International Trade Commission ("ITC") issued its Final Determination ("FD"), Limited Exclusion Order ("LEO") and Cease and Desist Order ("CDO"). The ITC upheld the finding of infringement in the ID and found a subsequent violation. The ITC issued a LEO prohibiting the importation of infringing tobacco heating articles and components thereof and cease and desist orders against Philip Morris USA, Inc. and Altria Client Services, LLC, which went into effect at the end of the 60-day Presidential review period on November 28, 2021. We have appealed the patent issues. Furthermore, lawsuits based on the same patent families have been repeatedly and universally rejected in European courts and the European Patent Office. The decision has no bearing outside the United States.
On March 15, 2022, the Consolidated Appropriations Act, 2022 amended the definition of “tobacco product” contained in the Federal Food, Drug and Cosmetic Act ("FD&C Act") to “any product made or derived from tobacco, or containing nicotine from any source, that is intended for human consumption.” Under this definition, products containing synthetic nicotine, e.g., e-cigarettes, are subject to the FDA’s tobacco regulatory authority. According to this new provision, effective April 14, 2022, manufacturers of tobacco products containing nicotine not derived from tobacco will be required to submit a PMTA to the FDA and obtain authorization from the Agency to market their product under the FD&C Act or they will be subject to FDA enforcement actions. This new provision does not have any impact on tobacco products developed by PMI and intended for commercialization in the U.S.
Until recently, there were no countries with specific product standards for heat-not-burn products. Currently, national standards setting minimum quality and safety requirements for such products have been adopted in several countries with technical heat-not-burn specifications and/or methods for demonstrating the absence of combustion. They are mandatory in Egypt, Jordan, Saudi Arabia, Tunisia, the UAE, Uzbekistan and Bahrain, and voluntary in Armenia, Costa Rica, Indonesia, Kazakhstan, Kyrgyzstan, Russia, Tajikistan, Vietnam, the U.K. and Ukraine. In Japan, a voluntary standard sets minimum safety requirements for tobacco heating devices. We expect other governments to consider similar product standards and encourage making them mandatory.
All EU member states have transposed the EU Tobacco Products Directive, including the provisions on novel tobacco products, such as heated tobacco units, and e-cigarettes. Most of the EU member states require a notification submitted six months before the intended placing on the market of such products, while some require pre-market authorizations for the introduction of such products. To date, we have filed a comprehensive dossier summarizing our scientific assessment of our Platform 1 product in over 20 member states.
64
In addition, in Italy, in April 2018, we submitted an application for HEETS, used with the IQOS device, requesting regulatory recognition of the reduction of toxic substances and potential risk reduction resulting from switching to this product compared to continued cigarette smoking. In January 2019, our application was not granted primarily on the grounds of insufficient data and questions of methodology. Due to the constraints of the review process, we had been unable to supplement the application with all the data we subsequently filed with the FDA and to address methodological questions during the review. We plan to submit a new application where we will clarify the concerns raised by the decision and further strengthen our application by submitting additional evidence that became available since we submitted our first application, consistent with our FDA filings. We are confident that our evidence supports our application.
On October 31, 2019, our Australian subsidiary, Philip Morris Limited (“PML”), submitted an application to the Scheduling Committee of the Therapeutic Goods Administration of Australia (“TGA”) seeking to exempt heated tobacco products from being prohibited in Australia. In August 2020, the TGA issued its decision denying the application and stating that it did not present compelling evidence to establish a public health benefit from greater access to nicotine in heated tobacco products.
To date, several governmental agencies have published their scientific findings that analyze the harm-reduction potential of certain RRPs versus continuing smoking, including:
In December 2017, at the request of the U.K. Department of Health and Public Health England, the U.K. Committee on Toxicity published its assessment of the risk of heat-not-burn products relative to cigarette smoking. This assessment included analysis of scientific data for two heat-not-burn products, one of which was our Platform 1 product. The assessment concluded that, while still harmful to health, compared with the known risks from cigarettes, heat-not-burn products are probably less harmful. Subsequently, in February 2018, Public Health England published a report stating that the available evidence suggests that heat-not-burn products may be considerably less harmful than cigarettes and more harmful than e-cigarettes.
In May 2018, the German Federal Institute for Risk Assessment (“BfR”) published a study on the Platform 1 aerosol relative to cigarette smoke using the Health Canada Intense Smoking Regimen. BfR found reductions in selected HPHCs in a range of 80-99%. This publication indicates that significant reductions in the levels of selected toxicants are likely to reduce toxicant exposure, which BfR stated might be regarded as a discrete benefit compared to combustible cigarettes.
In May 2018, the Dutch National Institute for Public Health and Environment (“RIVM”) published a factsheet on novel tobacco products that heat rather than burn tobacco, focusing on our Platform 1 product. RIVM analyzed the aerosol generated by our Platform 1 product and concluded that the use of this product, while still harmful to health, is probably less harmful than continued smoking.
In June 2018, the Korean Food and Drug Administration (“KFDA”) issued a statement on products that heat rather than burn tobacco. The KFDA tested three heat-not-burn products, one of which was our Platform 1 product. The KFDA confirmed that the levels of the nine HPHCs tested in the aerosol of these products were on average approximately 90% lower compared to those measured in the cigarette smoke of the top five cigarette brands in South Korea. However, the KFDA stated that it could not establish that the tested heat-not-burn products are less harmful than cigarettes. In October 2018, our Korean subsidiary filed a request with a local court seeking information underlying KFDA’s analysis, conclusions and public statements. In May 2020, the court ordered KFDA to produce certain records.
In August 2018, the Science & Technology Committee of the U.K. House of Commons published a report of its inquiry into e-cigarettes and heat-not-burn products. The report concluded that e-cigarettes are significantly less harmful to health than smoking tobacco. The report also observed that for those smokers who do not accept e-cigarettes, heat-not-burn products may offer a public health benefit despite their relative risk. The report called for a risk-proportionate regulatory environment for both e-cigarettes and heat-not-burn products and noted that e-cigarettes should remain the least taxed, cigarettes the most taxed, with heat-not-burn products falling between the two. The U.K. Committee on Advertising Practice announced the removal of a prohibition of health claims in the advertising of e-cigarettes in the U.K. effective November 2018.
In November 2018, the Eurasian Economic Commission (regulatory body of the Eurasian Union consisting of Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) published the results of its commissioned study on novel nicotine-containing products, including our Platform 1 product. The study confirms significantly lower levels of HPHCs in the aerosol generated by this product compared to cigarette smoke.
In January 2019, scientific media published the results of the study of the China National Tobacco Quality Supervision and Test Centre (“CNTQST”) comparing the aerosol generated by our Platform 1 product with cigarette smoke. The CNTQST found that the former contained fewer, and lower levels of, harmful constituents than the latter and concluded that the lower
65
temperature of heating tobacco in our Platform 1 product contributed to the difference. The CNTQST stated that the reduction in emissions of harmful constituents cannot be interpreted as a harm/risk reduction for smokers in the same proportion.
In 2020, the Superior Health Council of Belgium (“SHC”) published results of its inquiry into heat-not-burn products. The SHC concluded that heat-not-burn products, while not safe, have a more favorable toxicity profile than cigarettes. However, in light of the uncertainty of such products’ short and long-term impacts, the toxic effects of the dual use with cigarettes, and the existence of approved smoking cessation tools, the SHC recommended that current regulations for cigarettes should apply to heat-not-burn products.
The foregoing scientific findings of government agencies may not be indicative of the measures that the relevant government authorities could take in regulating our products.
We make our scientific findings publicly available for scrutiny and peer review through several channels, including our websites. From time to time, adult consumers, competitors, members of the scientific community, and others inquire into our scientific methodologies, challenge our scientific conclusions or request further study of certain aspects of our RRPs and their health effects. We are committed to a robust and open scientific debate and believe that such debate should be based on accurate and reliable scientific information. We seek to provide accurate and reliable scientific information about our RRPs; nonetheless, we may not be able to prevent third-party dissemination of false, misleading or unsubstantiated information about these products. The dissemination of scientifically unsubstantiated information or studies with a strong confirmation bias by third parties may cause confusion among adult smokers and affect their decision to switch to better alternatives to continued smoking, such as our RRPs.
To date, we have been largely successful in demonstrating to regulators that our heated tobacco units are not cigarettes due to the absence of combustion, and as such they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Although we believe that this is sensible from the public health perspective, we cannot guarantee that regulators will continue this approach.
There can be no assurance that we will succeed in our efforts to replace cigarettes with RRPs or that regulation will allow us to commercialize RRPs in all markets, to communicate about our RRPs, including making scientifically substantiated risk-reduction claims, or to treat RRPs differently from cigarettes.
Legal Challenges to RRPs: We face various administrative and legal challenges related to certain RRP activities, including allegations concerning product classification, advertising restrictions, corporate communications, product coach activities, scientific substantiation, product liability, and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize RRPs and to communicate publicly. The outcomes of these matters may affect our RRP commercialization and public communication activities and performance in one or more markets.
Our RRP Business Development Initiatives: In December 2013, we established a strategic framework with Altria Group, Inc. (“Altria”) setting out terms on how the parties would collaborate to develop and commercialize e-vapor products and commercialize two of our RRPs in the U.S. In late 2018, Altria announced that it will participate in the e-vapor category only through another e-vapor company in which Altria acquired a minority interest. In September 2019, Altria's subsidiary, Philip Morris USA Inc. (“PM USA”), began commercialization of a version of our Platform 1 product in the U.S. Under the agreement, PM USA must achieve certain milestones to maintain its exclusive distribution right and additional milestones to extend the agreement after the initial 5-year term. PMI and Altria are currently discussing these milestones, the contractual obligations, and the impact of the United States International Trade Commission (“ITC”) Orders (For more details please refer to Note 8. Contingencies).
In January 2020, we announced an agreement with KT&G, a leading tobacco and nicotine company in South Korea, for the commercialization of KT&G’s smoke-free products outside of South Korea on an exclusive basis. For more information, see Acquisitions and Other Business Arrangements below.
Other Developments: In September 2017, we announced our support of the Foundation for a Smoke-Free World. In September 2020, our pledge agreement with the Foundation was amended. We contributed $45 million in 2020, $40 million in 2021, and expect to contribute $35 million annually from 2022 through 2029, as specified in the amended pledge agreement. To date, we contributed a total of $249.5 million. The Foundation is an independent body and is governed by its independent Board of Directors. The Foundation’s role, as set out in its corporate charter, includes funding research in the field of tobacco harm
66
reduction, encouraging measures that reduce the harm caused by smoking, and assessing the effect of reduced cigarette consumption on the industry value chain.
Governmental Investigations
From time to time, we are subject to governmental investigations on a range of matters, including tax, customs, antitrust, advertising, and labor practices. We describe certain matters pending in Russia, South Korea and Thailand in Note 8. Contingencies.
In November 2010, a World Trade Organization ("WTO") panel issued its decision in a dispute relating to facts that arose from August 2006, between the Philippines and Thailand, concerning a series of Thai customs and tax measures affecting cigarettes imported by PM Thailand into Thailand (see Note 8. Contingencies for additional information). The WTO panel decision, which was upheld by the WTO Appellate Body, concluded that Thailand had no basis to find that PM Thailand's declared customs values and taxes paid were too low, as alleged by the Department of Special Investigations of the government of Thailand ("DSI") in 2009. The decision also created obligations for Thailand to revise its laws, regulations, or practices affecting the customs valuation and tax treatment of future cigarette imports. Thailand agreed in September 2011 to fully comply with the decision by October 2012. The Philippines asserts that to date Thailand has not fully complied with the WTO panel decision and commenced challenges at the WTO Appellate Body. The WTO Appellate Body is not operational, and the appeals by Thailand are suspended indefinitely. In December 2020, the Philippines and Thailand agreed to pursue facilitator-assisted discussions aimed at progressing and resolving outstanding issues. It is not possible to predict any future developments in these proceedings or the outcome of these discussions.
The Public Prosecutor’s office of Rome, Italy, notified our Italian subsidiary, Philip Morris Italia S.r.l. (“PM Italia”), as well as three former or current employees and a former external consultant of PM Italia in July 2020 and March 2020, respectively, that it concluded a preliminary investigation against them for alleged contravention of anti-corruption laws and related disruption of trade freedom. The Public Prosecutor alleges that the individuals involved promised certain personal favors to government officials from January to July of 2018 in exchange for favorable treatment for PM Italia, and that PM Italia lacked appropriate organizational controls to prevent the alleged actions by the individuals. At the first trial hearing held on September 22, 2021, BAT filed a civil claim against PM Italia claiming vicarious liability for any wrongdoing of its former or current employees. BAT claims EUR 50 million in damages. The court admitted the claim as a matter of course and issued summons for PM Italia to appear as civil party in the case. The next trial hearing is scheduled for June 1, 2022. PM Italia believes the charges brought against it by the Public Prosecutor are without merit and will defend them vigorously.
Vectura Fertin Pharma
Subsequent to the end of the first quarter, on April 5, 2022, the European Commission issued a proposal for a regulation on fluorinated greenhouse gases. If accepted, the proposal also establishes quantitative limits on usage of certain propellant gases which are components of certain products sold by our Vectura Fertin Pharma business. This may impact the related products development and market strategies, may have an adverse impact on the value of technology and goodwill acquired in PMI’s 2021 purchase of Vectura Group plc, and may result in impairment charges.
Asset Impairment and Exit Costs
We discuss asset impairment and exit costs in Note 16. Asset Impairment and Exit Costs to our condensed consolidated financial statements.
U.S. GAAP Treatment of Turkey as a Highly Inflationary Economy
Following the categorization of Turkey by the International Practices Task Force of the Center for Audit Quality as a country with a three-year cumulative inflation rate greater than 100%, the country is considered highly inflationary in accordance with U.S. GAAP. Consequently, PMI has begun to account for the operations of its Turkish affiliates as highly inflationary, and treat the U.S. dollar as the functional currency of the affiliates, effective April 1, 2022.
67
Acquisitions and Other Business Arrangements
We discuss our acquisitions in Note 17. Acquisitions to our condensed consolidated financial statements.
Global Collaboration Agreement with KT&G
In January 2020, PMI announced a global collaboration agreement with the leading tobacco and nicotine company in South Korea, KT&G, to commercialize KT&G’s smoke-free products outside of the country. The agreement will run for an initial period of three years. The two companies plan for global collaboration with the intention to actively expand to cover many markets, based on commercial success. The agreement allows PMI to distribute current KT&G smoke-free products, and their evolutions, on an exclusive basis, and does not restrict PMI from distributing its own or third-party products. KT&G’s smoke-free product brand portfolio includes heat-not-burn tobacco products (e.g., LIL Mini and LIL Plus), hybrid technologies that combine heat-not-burn tobacco and e-vapor technologies (e.g., LIL HYBRID), and e-vapor products (e.g., LIL Vapor). PMI will be responsible for the commercialization of smoke-free products supplied under the agreement.
Products sold under the agreement are subject to careful assessment to ensure they meet the regulatory requirements in the markets where they are launched, as well as our standards of quality and scientific substantiation to confirm the absence of combustion and significant reductions of emissions of harmful chemicals compared to cigarettes. PMI and KT&G will seek any necessary regulatory approvals that may be required on a market-by-market basis. There are no current plans to commercialize KT&G products in the United States.
Since the third quarter of 2020, we have launched commercial initiatives for licensed KT&G products in select markets.
Equity Investments
We discuss our equity investments in Note 12. Related Parties - Equity Investments and Other to our condensed consolidated financial statements.
Trade Policy
We are subject to various trade restrictions imposed by the U.S. and countries in which we do business (“Trade Sanctions”), including the trade and economic sanctions administered by the U.S. Department of the Treasury's Office of Foreign Assets Control and the U.S. Department of State. It is our policy to comply fully with these Trade Sanctions.
Tobacco products are agricultural products under U.S. law and are not technological or strategic in nature. From time to time we make sales in countries subject to Trade Sanctions, either where such sanctions do not apply to our business or pursuant to exemptions or licenses. On March 11, 2022, the U.S. introduced a ban on export, re-export, sale, or supply, directly or indirectly, from the U.S., or by a U.S. person, wherever located, of certain tobacco and manufactured tobacco substitutes to Russia and Belarus.
From time to time, a subsidiary sells products to distributors that, in turn, sell those products to duty free customers that supply U.N. peacekeeping forces around the world, including those in the U.N. peacekeeping mission located in Abyei, a special administrative territory in Sudan. We do not believe that these sales, which are not subject to Trade Sanctions, and are de minimis in volume and value, present a material risk to our shareholders, our reputation or the value of our shares. We have no employees, operations or assets in Sudan.
We do not sell products in Iran, North Korea and Syria. From time to time, we explore opportunities to sell our products in one or more of these countries, as permitted by law.
We sell cigarettes in Cuba under a distribution agreement. These sales are permitted by U.S. law under a License Exception for Agricultural Commodities, issued by the United States Department of Commerce (Bureau of Industry and Security), granted to our distributor.
Certain states within the U.S. have enacted legislation permitting or requiring state pension funds to divest or abstain from future investment in stocks of companies that do business with certain countries that are sanctioned by the U.S. Because we do business in certain of these countries, these state pension funds may have divested of our stock or may not invest in our stock. We do not believe such legislation has had a material effect on the price of our shares.
68
PMI is also subject to various Trade Sanctions imposed by the EU and other jurisdictions ("Trade Sanctions"). We comply fully with these Trade Sanctions.
On June 24, 2021, the EU introduced sanctions regarding the Republic of Belarus ("Belarus") aimed at specific sectors of the Belarus economy, including the tobacco sector. Subsequently, seven non-EU countries (Norway, Iceland, Liechtenstein, North Macedonia, Bosnia and Herzegovina, Montenegro, and Albania) announced that they “aligned themselves” with the majority of the EU sanctions. Switzerland and the UK have also imposed sanctions similar in scope to the EU sanctions.
On August 9, 2021, the U.S. imposed blocking sanctions on certain Belarusian individuals and entities pursuant to an Executive Order, which expanded the bases for the imposition of sanctions, including, among others, by authorizing the imposition by OFAC of blocking sanctions on persons operating in the tobacco sector of the Belarus economy. In 2021, the U.S., the EU, the U.K., Switzerland and several other jurisdictions supplemented their respective sanctions lists by including additional Belarusian sanctions targets.
Following the start of the conflict in Ukraine on February 24, 2022, the U.S., the EU, the UK, Switzerland, Canada, Australia, New Zealand, Singapore, South Korea, Japan and other countries introduced extensive economic sanctions and export controls against Russia. While the introduced sanctions slightly vary from jurisdiction to jurisdiction, they are largely aligned. The restrictions are primarily targeted at the Russian financial, banking, oil, military, aviation and marine sectors. Among sanctions targets are Russian political figures and military personnel, certain oligarchs and journalists, and companies operating in the above-mentioned sectors. Export to Russia of certain luxury goods, and goods and technology which might contribute to Russia’s technological enhancement was banned. Seven non-EU countries (Norway, Iceland, Liechtenstein, North Macedonia, Bosnia and Herzegovina, Montenegro, and Albania) announced that they “aligned themselves” with the majority of the EU sanctions. Ukraine imposed its own sanctions against Russia, and certain targeted Russian entities and individuals.
Russia introduced certain countermeasures aimed at reducing the effect of Western sanctions. Countermeasures include the ban on export of certain goods from Russia, including tobacco-related production equipment, restrictions on lending to foreign borrowers and transactions with securities and real estate involving companies from “hostile” countries (i.e., those which introduced sanctions regarding Russia).
PMI complies with all applicable laws and regulations, including sanctions, in the markets where it operates. We have taken appropriate actions in response to the latest sanctions to ensure full compliance with the relevant restrictions.
Operating Results – Three Months Ended March 31, 2022
The following discussion compares operating results within each of our geographical segments and Other category for the three months ended March 31, 2022, with the three months ended March 31, 2021.
Unless otherwise stated, references to total industry, total market, our shipment volume and our market share performance reflect cigarettes and heated tobacco units. Estimates for total industry volume and market share in certain geographies reflect limitations on the availability and accuracy of industry data during pandemic-related restrictions.
European Union:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 3,012 | $ | 2,909 | 3.5 | % | 10.5 | % | $ | 103 | $ | (206) | $ | 4 | $ | 5 | $ | 300 | $ | — | |||||||||||||||||||||
| Operating Income | $ | 1,527 | $ | 1,490 | 2.5 | % | 12.3 | % | $ | 37 | $ | (145) | $ | (2) | $ | 5 | $ | 208 | $ | (29) | |||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, increased by 10.5%, reflecting: favorable volume/mix, mainly driven by higher heated tobacco unit volume (notably in Germany, Italy and Poland) and higher device volume (notably in Italy), partly offset by lower cigarette volume (mainly in Germany, partly offset by Spain). Pricing variance was slightly
69
favorable, primarily reflecting higher combustible pricing (driven by Germany), largely offset by lower pricing for devices (notably in Italy).
Operating income, excluding currency and acquisitions, increased by 12.3%, primarily reflecting: favorable volume/mix, mainly driven by higher heated tobacco unit volume (primarily reflecting the same geographies as for net revenues noted above), partly offset by lower cigarette volume (mainly in Germany); partially offset by higher manufacturing costs (primarily due to inflationary impacts).
European Union - Total Market, PMI Shipment Volume and Market Share Commentaries
Total market and market share performance are shown in the table below:
| European Union Key Data | First-Quarter | ||||||||||
| Change | |||||||||||
| 2022 | 2021 | % / pp | |||||||||
| Total Market (billion units) | 109.6 | 106.4 | 3.0 | % | |||||||
| PMI Market Share | |||||||||||
| Marlboro | 16.2 | % | 17.0 | % | (0.8) | ||||||
| L&M | 5.5 | % | 5.8 | % | (0.3) | ||||||
| Chesterfield | 5.6 | % | 5.5 | % | 0.1 | ||||||
| Philip Morris | 2.2 | % | 2.2 | % | — | ||||||
| Heated Tobacco Units | 7.6 | % | 5.6 | % | 2.0 | ||||||
| Others | 3.0 | % | 3.2 | % | (0.2) | ||||||
| Total European Union | 40.1 | % | 39.3 | % | 0.8 | ||||||
In the quarter, the estimated total market in the EU increased by 3.0% to 109.6 billion units, primarily driven by:
•Italy, up by 5.3%, mainly reflecting the impact on adult smoker average daily consumption of the easing of pandemic-related measures; and
•Poland, up by 18.7%, primarily reflecting a lower estimated prevalence of illicit trade and higher border sales due to the easing of pandemic-related measures;
partly offset by
•Germany, down by 7.5%, primarily reflecting the impact of excise tax-driven price increases and higher cross-border (non-domestic) purchases due to the easing of pandemic-related measures.
70

Our total shipment volume increased by 4.2% to 45.0 billion units, mainly driven by:
•Poland, up by 20.7%, mainly reflecting the higher total market, as well as a higher market share driven by heated tobacco units; and
•Spain, up by 23.1%, or by 1.7% excluding the net favorable impact of estimated distributor inventory movements, reflecting a higher total market, partly offset by a lower market share due to cigarettes.
Excluding the net unfavorable impact of estimated distributor inventory movements (notably in Italy, partly offset by Spain), our total in-market sales volume increased by 5.2%.
Eastern Europe:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 726 | $ | 796 | (8.8) | % | 0.3 | % | $ | (70) | $ | (72) | $ | — | $ | 36 | $ | (34) | $ | — | |||||||||||||||||||||
| Operating Income | $ | 144 | $ | 261 | (44.8) | % | (21.8) | % | $ | (117) | $ | (60) | $ | — | $ | 36 | $ | (26) | $ | (67) | |||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, increased by 0.3%, reflecting: a favorable pricing variance, mainly driven by higher combustible pricing (primarily in Russia); largely offset by unfavorable volume/mix, mainly due to unfavorable cigarette volume (mainly in Russia).
During the quarter, Russia and Ukraine accounted for around 65% of PMI's total net revenues in the Region.
Operating income, excluding currency and acquisitions, decreased by 21.8%, notably reflecting pre-tax charges in the first quarter of 2022 related to the war in Ukraine ($42 million), as well as unfavorable volume/mix, mainly due to unfavorable cigarette volume/mix (primarily in Russia); and higher marketing, administration and research costs; partly offset by a favorable pricing variance.
71
Eastern Europe - Total Market, PMI Shipment Volume and Market Share Commentaries
Given the company's intention to exit the Russian market and the impact of the war in Ukraine on business operations in the country, PMI is providing a total market estimate for the Eastern Europe Region excluding Russia and Ukraine.
In the quarter, the estimated total market in Eastern Europe, excluding Russia and Ukraine, increased, mainly driven by:
•Kazakhstan, up by 6.2%, primarily reflecting a favorable comparison versus the prior year period due to the temporary supply chain impact of the introduction, in January 2021, of certain mandatory requirements related to the sale of tobacco products; and
•Southeast Europe, up by 10.0%, mainly reflecting increased in-bound travel and the impact on adult smoker average daily consumption of the easing of pandemic-related measures.
The company's reported shipment volume, presented below, includes Russia and Ukraine.

Our total shipment volume decreased by 4.8% to 24.4 billion units, primarily due to:
•Russia, down by 9.9%.
During the quarter, Russia and Ukraine accounted for around 73% of PMI's total shipment volume in the Region. Excluding Russia and Ukraine, total shipment volume increased by 4.5%.
72
Middle East & Africa:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 991 | $ | 801 | 23.7 | % | 42.3 | % | $ | 190 | $ | (149) | $ | — | $ | 163 | $ | 165 | $ | 11 | |||||||||||||||||||||
| Operating Income | $ | 521 | $ | 335 | 55.5 | % | 89.9 | % | $ | 186 | $ | (115) | $ | — | $ | 163 | $ | 125 | $ | 13 | |||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, increased by 42.3%, primarily reflecting: favorable volume/mix, mainly driven by higher cigarette volume (primarily in Kuwait and PMI Duty Free, partly offset by Algeria) and higher heated tobacco unit volume (notably in Egypt); and a favorable pricing variance, driven by combustible pricing (mainly in Turkey).
Operating income, excluding currency and acquisitions, increased by 89.9%, mainly reflecting: a favorable pricing variance; favorable volume/mix, mainly driven by the same factors and geographies as for net revenues noted above; and lower marketing, administration and research costs; partly offset by higher manufacturing costs (primarily due to inflationary impacts).
Middle East & Africa - Total Market, PMI Shipment Volume and Market Share Commentaries
In the quarter, the estimated total market in the Middle East & Africa decreased, mainly due to:
•Algeria, down by 37.5%, or by 8.2% excluding the net unfavorable impact of estimated trade inventory movements, primarily reflecting industry supply chain disruptions; and
•Turkey, down by 6.8%, mainly reflecting a higher estimated prevalence of illicit trade, partly offset by the impact on adult smoker average daily consumption of the easing of pandemic-related measures, coupled with increased in-bound tourism;
partly offset by
•International Duty Free, up by 40.2%, reflecting the impact of reduced government travel restrictions and increased passenger traffic in certain geographies.
73

Our total shipment volume increased by 8.3% to 30.4 billion units, notably driven by:
•PMI Duty Free, up by +100%, or by 50.8% excluding the net favorable impact of estimated distributor inventory movements, reflecting the higher total market and a higher market share; and
•Egypt, up by 10.7%, primarily reflecting a higher market share, driven by cigarettes and heated tobacco units;
partly offset by
•Algeria, down by 27.5%, mainly reflecting the lower total market, partly offset by a higher market share driven by cigarettes.
Excluding the net favorable impact of estimated distributor inventory movements, our total in-market sales volume increased by 4.3%.
South & Southeast Asia:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 1,123 | $ | 1,173 | (4.3) | % | (0.5) | % | $ | (50) | $ | (44) | $ | — | $ | (145) | $ | 139 | $ | — | |||||||||||||||||||||
| Operating Income | $ | 445 | $ | 529 | (15.9) | % | (11.2) | % | $ | (84) | $ | (25) | $ | — | $ | (145) | $ | 87 | $ | (1) | |||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, decreased by 0.5%, reflecting: an unfavorable pricing variance, due to combustible pricing (mainly in Indonesia and the Philippines); largely offset by favorable volume/mix, primarily driven by higher cigarette volume (mainly in Indonesia and the Philippines) and favorable cigarette mix (primarily in Indonesia, partly offset by the Philippines).
Operating income, excluding currency and acquisitions, decreased by 11.2%, primarily reflecting: an unfavorable pricing variance; partly offset by favorable volume/mix, mainly driven by higher cigarette volume (primarily in Indonesia and the Philippines).
74
South & Southeast Asia - Total Market, PMI Shipment Volume and Market Share Commentaries
In the quarter, the estimated total market in South & Southeast Asia increased, mainly due to:
•India, up by 7.2%, primarily reflecting a favorable comparison versus the prior year period, during which pandemic-related restrictions impacted the movement of certain products, including tobacco;
•Indonesia, up by 5.8%, mainly reflecting impact on adult smoker consumption of the easing of pandemic-related measures, which drove growth in the tax-advantaged 'below tier one' segment; and
•the Philippines, up by 19.2%, primarily reflecting the net favorable impact of estimated trade inventory movements related to first-quarter 2022 price increases;
partly offset by
•Bangladesh, down by 12.9%, mainly reflecting the impact of pandemic-related restrictions on mobility during February 2022; and
•Thailand, down by 13.3%, primarily reflecting the impact of excise tax-driven price increases.

Our total shipment volume increased by 7.5% to 37.6 billion units, mainly driven by:
•Indonesia, up by 5.2%, primarily reflecting the higher total market, partly offset by a lower market share (mainly due to adult smoker down-trading to the 'below tier one' segment as a result of significantly lower retail prices, partly offset by share growth for our premium and hand-rolled portfolio); and
•the Philippines, up by 18.4%, primarily reflecting the higher total market.
75
East Asia & Australia:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ 1,404 | $ 1,472 | (4.6) | % | 2.6 | % | $ | (68) | $ | (106) | $ | — | $ | 30 | $ | 8 | $ | — | |||||||||||||||||||||||
| Operating Income | $ 571 | $ 695 | (17.8) | % | (8.1) | % | $ | (124) | $ | (68) | $ | — | $ | 30 | $ | (121) | $ | 35 | |||||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, increased by 2.6%, reflecting: a favorable pricing variance, primarily driven by higher combustible pricing (predominantly in Japan). Volume/mix was slightly favorable, mainly reflecting higher device and heated tobacco unit volume in Japan, largely offset by unfavorable cigarette mix (primarily in Japan) and unfavorable device mix in Japan.
Operating income, excluding currency and acquisitions, decreased by 8.1%, mainly reflecting: unfavorable volume/mix, primarily due to unfavorable mix for cigarettes and devices in Japan; and higher manufacturing costs (mainly due to higher logistics costs); partly offset by lower marketing, administration and research costs (notably driven by a favorable comparison related to 2021 asset impairment and exit costs of $31 million); and a favorable pricing variance.
East Asia & Australia - Total Market, PMI Shipment Volume and Market Share Commentaries
In the quarter, the estimated total market in East Asia & Australia, excluding China, decreased, mainly due to:
•Japan, down by 3.2%, primarily reflecting the impact of the October 2021 excise tax-driven price increase.

Our total shipment volume increased by 1.7% to 20.8 billion units, mainly driven by:
•Japan, up by 3.2%. Excluding the net favorable impact of estimated distributor inventory movements, total in-market sales volume was essentially stable.
Excluding the net favorable impact of estimated distributor inventory movements, our total in-market sales volume decreased by 1.0%.
76
Americas:
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 424 | $ | 434 | (2.3) | % | 0.7 | % | $ | (10) | $ | (13) | $ | — | $ | 19 | $ | (16) | $ | — | |||||||||||||||||||||
| Operating Income | $ | 121 | $ | 134 | (9.7) | % | (8.2) | % | $ | (13) | $ | (2) | $ | — | $ | 19 | $ | (9) | $ | (21) | |||||||||||||||||||||
During the quarter, net revenues, excluding currency and acquisitions, increased by 0.7%, reflecting: a favorable pricing variance, driven by combustible products (mainly in Argentina, Brazil and Mexico); partly offset by unfavorable volume/mix, mainly due to lower cigarette volume (primarily in Argentina) and unfavorable cigarette mix (primarily in Brazil).
Operating income, excluding currency and acquisitions, decreased by 8.2%, mainly reflecting: higher manufacturing costs (primarily due to inflationary impacts); and unfavorable volume/mix (mainly due to the same factors and geographies as for net revenues noted above); partly offset by a favorable pricing variance.
Americas - Total Market, PMI Shipment Volume and Market Share Commentaries
In the quarter, the estimated total market in Americas, excluding the U.S., decreased, primarily due to:
•Canada, down by 14.3%, notably reflecting the impact of price increases and out-switching from cigarettes to e-vapor products; and
•Mexico, down by 3.8%. Excluding the net unfavorable impact of estimated trade inventory movements, the total market increased by 1.4%, mainly driven by the impact on adult smoker average daily consumption of the easing of pandemic-related measures, coupled with the impact of increased in-bound tourism;
partly offset by
•Brazil, up by 4.1%, primarily reflecting a lower estimated prevalence of illicit trade.
77

Our total shipment volume decreased by 0.6% to 14.9 billion units, mainly due to:
•Argentina, down by 7.1%, primarily reflecting a lower market share due to adult smoker down-trading to ultra-low-price brands produced by local manufacturers;
partly offset by
•Brazil, up by 5.4%, mainly reflecting the higher total market and a higher market share driven by Chesterfield.
Other:
In the third quarter of 2021, we acquired Fertin Pharma A/S, Vectura Group plc. and OtiTopic, Inc. On March 31, 2022, we launched a new Wellness and Healthcare business consolidating these entities, Vectura Fertin Pharma. The operating results of this new business are reported in the Other category. The business operations of our Wellness and Healthcare business are managed and evaluated separately from the geographical segments.
| Financial Summary - Quarters Ended March 31, | Change Fav./(Unfav.) | Variance Fav./(Unfav.) | |||||||||||||||||||||||||||||||||||||||
| 2022 | 2021 | Total | Excl. Curr. & Acquis. | Total | Cur- rency | Acqui-sitions | Price | Vol/ Mix | Cost/ Other | ||||||||||||||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||||||||||||||||||||
| Net Revenues | $ | 66 | $ | — | — | % | — | % | $ | 66 | $ | — | $ | 66 | $ | — | $ | — | $ | — | |||||||||||||||||||||
| Operating Income / (Loss) | $ | (31) | $ | — | — | % | — | % | $ | (31) | $ | — | $ | (24) | $ | — | $ | — | $ | (7) | |||||||||||||||||||||
During the quarter, we recorded net revenues of $66 million, as well as an operating loss of $31 million, primarily reflecting the amortization of intangibles related to the acquisitions, investments in research and development, and expenses related to employee retention programs.
78
Financial Review
Cash Flow Highlights



| For the Three Months Ended March 31, | ||||||||
| (in millions) | 2022 | 2021 | ||||||
| Net cash provided by operating activities | $ | 1,118 | $ | 435 | ||||
| Net cash provided by (used in) investing activities | (196) | 55 | ||||||
| Net cash used in financing activities | (701) | (3,649) | ||||||
Net Cash Provided by Operating Activities
During the first quarter of 2022, net cash provided by operating activities increased by $0.7 billion compared with the first quarter of 2021. Excluding unfavorable currency movements of $0.4 billion, net cash provided by operating activities increased by $1.1 billion, due primarily to higher currency-neutral net earnings and lower working capital requirements of $0.7 billion.
The lower working capital requirements in the first quarter of 2022 as compared with the first quarter of 2021 were primarily due to the timing of excise tax-paid inventory movements and excise tax payments, including the 2021 settlement of excise tax liabilities driven by the COVID-19 pandemic-related build-up of inventory.
Net Cash Provided by (Used in) Investing Activities
During the first quarter of 2022, net cash used in investing activities was $196 million as compared to $55 million of net cash provided by investing activities in the first quarter of 2021. This change was due primarily to unfavorable movements of $78 million in cash collateral exchanged with financial institutions to secure derivatives designated as net investment hedges of Euro assets principally related to changes in exchange rates between the Euro and the U.S. dollar, higher capital expenditures and other investments. For further detail on our derivatives designated as net investment hedges and other investments, see Note 5. Financial Instruments.
Capital expenditures of $229 million during the first quarter of 2022 increased slightly as compared with the first quarter of 2021. The 2022 capital expenditures were primarily related to our ongoing investments in RRPs. We expect total capital expenditures in 2022 to be approximately $1.0 billion.
79
Net Cash Used in Financing Activities
During the first quarter of 2022, net cash used in financing activities decreased by $2.9 billion compared to the first quarter of 2021. The lower cash usage primarily reflected proceeds from short-term borrowings in 2022 (primarily commercial paper of $2.2 billion) and lower long-term debt repayments ($0.5 billion in 2022 compared with 1.6 billion in 2021), partially offset by share repurchases in the first quarter of 2022 under our share repurchase program and the purchase of the remaining stakes in our Turkish affiliates in the first quarter of 2022. For further details on the purchase of the remaining stakes in our Turkish affiliates, see Note 17. Acquisitions.
Debt and Liquidity
We define cash and cash equivalents as short-term, highly liquid investments, readily convertible to known amounts of cash that mature within a maximum of three months and have an insignificant risk of change in value due to interest rate or credit risk changes. As a policy, we do not hold any investments in structured or equity-linked products. Our cash and cash equivalents are predominantly held with institutions that have investment-grade long-term credit rating. As part of our cash management strategy and in order to manage counterparty exposure, we also enter into reverse repurchase agreements. Such agreements are collateralized with government or corporate securities held by a custodial bank and, at maturity, cash is paid back to PMI, and the collateral is returned to the bank. For the three months ended March 31, 2022 and the full-year 2021, the activities for such reverse repurchase agreements were not material.
We utilize long-term and short-term debt financing, including a commercial paper program that is regularly used to finance ongoing liquidity requirements, as part of our overall cash management strategy. Our ability to access the capital and credit markets as well as overall dynamics of these markets may impact borrowing costs. We expect that the combination of our long-term and short-term debt financing, the commercial paper program and the committed credit facilities, coupled with our operating cash flows, will enable us to meet our liquidity requirements.
In August 2021, we published a business transformation-linked financing framework (“Framework”), which integrates the company’s smoke-free transformation into its financing strategy. The Framework outlines the guidelines that we will follow in issuing business transformation-linked financing instruments in the debt capital and loan markets, which may include public notes offerings, private placements, loans, and other relevant financing instruments.
Credit Ratings – The cost and terms of our financing arrangements as well as our access to commercial paper markets may be affected by applicable credit ratings. At March 31, 2022, our credit ratings and outlook by major credit rating agencies were as follows:
| Short-term | Long-term | Outlook | ||||||||||||||||||
| Moody’s | P-1 | A2 | Stable | |||||||||||||||||
| Standard & Poor’s | A-1 | A | Stable | |||||||||||||||||
| Fitch | F1 | A | Stable | |||||||||||||||||
80
Credit Facilities
At March 31, 2022, our committed credit facilities were as follows:
| (in billions) | ||||||||
| Type | Committed Credit Facilities | |||||||
364-day revolving credit, expiring January 31, 2023 | $ | 1.8 | ||||||
Multi-year revolving credit, expiring February 10, 2026 (1) | 2.0 | |||||||
Multi-year revolving credit, expiring September 29, 2026 (2) | 2.5 | |||||||
Total facilities | $ | 6.3 | ||||||
(1) On January 28, 2022, we entered into an agreement, effective February 10, 2022, to amend and extend the term of our $2.0 billion multi-year revolving credit facility, for an additional year covering the period February 11, 2026 to February 10, 2027, in the amount of $1.9 billion.
(2) Includes business transformation-linked pricing adjustments that may result in the reduction or increase in both the interest rate and commitment fee under the credit agreement if PMI achieves, or fails to achieve, certain specified targets based on its business transformation goals.
At March 31, 2022, there were no borrowings under the committed credit facilities, and the entire committed amounts were available for borrowing. Subject to market conditions, we currently expect to request an extension of the terms of our $2.5 billion multi-year revolving credit facility for an additional one-year period, in accordance with and subject to the terms and conditions of the relevant revolving credit facility agreement.
All banks participating in our committed credit facilities have an investment-grade long-term credit rating from the credit rating agencies. We continuously monitor the credit quality of our banking group, and at this time we are not aware of any potential non-performing credit provider.
These facilities do not include any credit rating triggers, material adverse change clauses or any provisions that could require us to post collateral. We expect to continue to meet our covenants.
In addition to the committed credit facilities discussed above, certain of our subsidiaries maintain short-term credit arrangements to meet their respective working capital needs. These credit arrangements, which amounted to approximately $2.3 billion at March 31, 2022 and December 31, 2021, are for the sole use of our subsidiaries. Borrowings under these arrangements and other bank loans amounted to $208 million at March 31, 2022, and $225 million at December 31, 2021.
Commercial Paper Program – We continue to have access to liquidity in the commercial paper market through programs in place in the U.S. and in Europe having an aggregate issuance capacity of $8.0 billion. At March 31, 2022, we had $2.2 billion of commercial paper outstanding. At December 31, 2021, we had no commercial paper outstanding. The average commercial paper balance outstanding during the first three months of 2022 was $3.7 billion. The average commercial paper balance outstanding during 2021 was $1.1 billion.
Sale of Accounts Receivable – To mitigate credit risk and enhance cash and liquidity management, we sell trade receivables to unaffiliated financial institutions. These arrangements allow us to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the condensed consolidated balance sheets. We sell trade receivables under two types of arrangements, servicing and nonservicing.
Our operating cash flows were positively impacted by the amount of the trade receivables sold and derecognized from the condensed consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of March 31, 2022, and March 31, 2021 were $598 million and $625 million, respectively. The net proceeds received are included in cash provided by operating activities in the condensed consolidated statements of cash flows.
For further details, see Note 13. Sale of Accounts Receivable to our condensed consolidated financial statements.
Debt – Our total debt was $29.4 billion at March 31, 2022 and $27.8 billion at December 31, 2021.
81
On February 11, 2020, we filed a shelf registration statement with the U.S. Securities and Exchange Commission, under which we may from time to time sell debt securities and/or warrants to purchase debt securities over a three-year period.
Guarantees – At March 31, 2022, we have guarantees of our own performance, which are primarily related to excise taxes on the shipment of our products. There is no liability in the condensed consolidated financial statements associated with these guarantees. These guarantees have not had, and are not expected to have, a significant impact on PMI’s liquidity. In October 2020, we guaranteed an obligation for an equity method investee. For further details, see Note 8. Contingencies to our condensed consolidated financial statements.
Equity and Dividends
We discuss our stock awards as of March 31, 2022 in Note 2. Stock Plans to our condensed consolidated financial statements.
On June 11, 2021, our Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period. On July 22, 2021, we began repurchasing shares under this new share repurchase program. From July 22, 2021 through March 31, 2022, we repurchased 10.5 million shares of our common stock at a cost of approximately $1.0 billion. During the first quarter of 2022, we repurchased 2.0 million shares of our common stock at a cost of $199 million.
Dividends paid in the first quarter of 2022 were $2.0 billion. During the third quarter of 2021, our Board of Directors approved a 4.2% increase in the quarterly dividend to $1.25 per common share. As a result, the present annualized dividend rate is $5.00 per common share.
Market Risk
Counterparty Risk - We predominantly work with financial institutions with strong short- and long-term credit ratings as assigned by Standard & Poor’s and Moody’s. These banks are also part of a defined group of relationship banks. Non-investment grade institutions are only used in certain emerging markets to the extent required by local business needs. We have a conservative approach when it comes to choosing financial counterparties and financial instruments. As such, we do not invest or hold investments in any structured or equity-linked products. The majority of our cash and cash equivalents is currently invested with maturities of less than 30 days.
We continuously monitor and assess the credit worthiness of all our counterparties.
Derivative Financial Instruments - We operate in markets outside of the United States of America, with manufacturing and sales facilities in various locations throughout the world. Consequently, we use certain financial instruments to manage our foreign currency and interest rate exposure. We use derivative financial instruments principally to reduce our exposure to market risks resulting from fluctuations in foreign exchange and interest rates by creating offsetting exposures. We are not a party to leveraged derivatives and, by policy, do not use derivative financial instruments for speculative purposes.
See Note 5. Financial Instruments to our condensed consolidated financial statements for further details on our derivative financial instruments and the related collateral arrangements.
Contingencies
See Note 8. Contingencies to our condensed consolidated financial statements for a discussion of contingencies.
Cautionary Factors That May Affect Future Results
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "aspires," "estimates," "intends," "projects," "aims," "goals," "targets," "forecasts" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
82
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Our RRPs constitute a new product category in its early stages that is less predictable than our mature cigarette business. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in the "Business Environment" section. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Overall Business Risks
We may be unsuccessful in our attempts to introduce reduced-risk products, and regulators may not permit the commercialization of these products or the communication of scientifically substantiated information and claims.
Our key strategic priorities are to: (i) develop and commercialize products that present less risk of harm to adult smokers who switch to those products versus continued smoking; and (ii) convince and educate current adult smokers who would otherwise continue to smoke to switch to those RRPs. For our efforts to be successful, we must:
| • | develop RRPs that adult smokers find acceptable alternatives to smoking; | ||||
| • | conduct rigorous scientific studies to substantiate that they reduce exposure to harmful and potentially harmful constituents in smoke and, ultimately, that these products present, are likely to present, or have the potential to present less risk of harm to adult smokers who switch to them versus continued smoking; and | ||||
| • | effectively advocate for a timely development of science-based regulatory frameworks for the development and commercialization of RRPs, including communication of scientifically substantiated information to enable adult smokers to make better consumer choices. | ||||
We might not succeed in our efforts. If we do not succeed, but others do, or if heat-not-burn products are inequitably regulated compared to other RRP categories without regard to the totality of the scientific evidence available for such products, we may be at a competitive disadvantage. In addition, actions of some market entrants, such as the inappropriate marketing of e-vapor products to youth, as well as alleged health consequences associated with the use of certain e-vapor products, may unfavorably impact public opinion and/or mischaracterize all e-vapor products or other RRPs to consumers, regulators and policy makers without regard to the totality of scientific evidence available for specific products. This may impede our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs. We cannot predict whether regulators will permit the sale and/or marketing of RRPs with scientifically substantiated information and claims. Such restrictions could limit the success of our RRPs.
The WHO study group on tobacco product regulation ("TobReg") published their eighth report on the scientific basis of tobacco product regulation in May 2021. The report is based on a review of scientific evidence related to novel and emerging nicotine and tobacco products, such as electronic nicotine delivery systems ("ENDS"), electronic non-nicotine delivery systems ("ENNDS") and heated tobacco products ("HTPs") on a number of scientific topics. The report concludes by making a number of policy recommendations on HTPs and ENDS that, if implemented, could restrict both the availability of these products, and the access to accurate information about them. In August 2021, the WHO FCTC Secretariat published two reports to the ninth session of the Conference of the Parties ("CoP") of the FCTC, which are not materially different from the WHO study group report.
Prior to CoP 9 that took place in November 2021, the WHO and the WHO FCTC Secretariat published two reports on novel and emerging tobacco products. The reports were noted by CoP 9 and related substantive discussions and decisions were deferred to CoP 10, currently scheduled for 2023. It is not possible to predict whether or to what extent measures recommended by the WHO's reports will be implemented as the reports are not binding to the WHO Member States.
Additionally, any claims, regardless of merit, challenging our research and clinical data available to date, may impact the development of science-based regulatory frameworks for the commercialization of the RRP category and the commercialization of the RRP category in general.
83
Our RRPs and commercial activities for these products are designed for, and directed toward, current adult smokers and users of nicotine-containing products, and not for non-smokers or youth. We put significant effort in place to restrict access of our products to non-smokers or youth. Nevertheless, technological, operational, regulatory and/or commercial setbacks might prevent us from delivering necessary infrastructure required to fulfill our commitment of having 100% of our RRP device portfolio equipped with “Age Verification”-technology and device activation features by 2023.
If nonetheless there is a significant usage of our products or competitive products among youth or non-smokers, even in situations over which we have no control, our credibility may suffer, and our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs may be significantly impacted.
Moreover, the FDA’s premarket tobacco product and modified risk tobacco product authorizations of two versions of our Platform 1 product are subject to strict marketing, reporting and other requirements. Although we have received these products' authorizations from the FDA, there is no guarantee that the product will remain authorized for sale in the U.S., particularly if there is a significant uptake in youth or non-smoker initiation.
The financial and business performance of our reduced-risk products is less predictable than our cigarette business.
Our RRPs are novel products in a new category, and the pace at which adult smokers adopt them may vary, depending on the competitive, regulatory, fiscal and cultural environment, and other factors in a specific market. There may be periods of accelerated growth and periods of slower growth for these products, the timing and drivers of which may be more difficult for us to predict versus our mature cigarette business. The impact of this lower predictability on our projected results for a specific period may be significant, particularly during the early stages of this new product category, during the COVID-19 pandemic as a result of unpredictability due to shortage of key components in our supply chain or due to geopolitical or macroeconomic events that negatively impact RRP availability or adoption.
We may be unsuccessful in our efforts to differentiate reduced-risk products and cigarettes with respect to taxation.
To date, we have been largely successful in demonstrating to regulators that our RRPs are not cigarettes due to the absence of combustion, and as such they are generally taxed either as a separate category or as other tobacco products, which typically yields more favorable tax rates than cigarettes. Nevertheless, we are unable to predict whether regulators will be issuing new regulations where RRP will be equally taxed in line with other tobacco products such as ordinary cigarettes. However, if we cease to be successful in these efforts, RRP unit margins may be materially adversely affected.
Consumption of tax-paid cigarettes continues to decline in many of our markets.
This decline is due to multiple factors, including increased taxes and pricing, governmental actions, the diminishing social acceptance of smoking and health concerns, competition, continuing economic and geopolitical uncertainty, and the continuing prevalence of illicit products. These factors and their potential consequences are discussed more fully below and in the Business Environment section. A continuous decline in the consumption of cigarettes could have a material adverse effect on our revenue and profitability.
Cigarettes are subject to substantial taxes. Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions. These tax increases may disproportionately affect our profitability and make us less competitive versus certain of our competitors.
Tax regimes, including excise taxes, sales taxes and import duties, can disproportionately affect the retail price of cigarettes versus other combustible tobacco products, or disproportionately affect the relative retail price of our cigarette brands versus cigarette brands manufactured by certain of our competitors. Because our portfolio is weighted toward the premium-price cigarette category, tax regimes based on sales price can place us at a competitive disadvantage in certain markets. Furthermore, our volume and profitability may be adversely affected in these markets.
In addition, increases in cigarette taxes are expected to continue to have an adverse impact on our sales of cigarettes, due to resulting lower consumption levels, a shift in sales from manufactured cigarettes to other combustible tobacco products and from the premium-price to the mid-price or low-price cigarette categories, where we may be under-represented, from local sales to legal cross-border purchases of lower price products, or to illicit products such as contraband, counterfeit and "illicit whites."
84
Our business faces significant governmental action aimed at increasing regulatory requirements with the goal of reducing or preventing the use of tobacco products.
Governmental actions, combined with the diminishing social acceptance of smoking and private actions to restrict smoking, have resulted in reduced industry volumes for our products in many of our markets, and we expect that such factors will continue to reduce consumption levels and will increase down-trading and the risk of counterfeiting, contraband, "illicit whites" and legal cross-border purchases. Significant regulatory developments will continue to take place over the next few years in most of our markets, driven principally by the World Health Organization's Framework Convention on Tobacco Control (the "FCTC"). Since it came into force in 2005, the FCTC has led to increased efforts by tobacco control advocates and public health organizations to promote increasingly restrictive regulatory measures on the marketing and sale of tobacco products to adult smokers. Regulatory initiatives that have been proposed, introduced or enacted by governmental authorities in various jurisdictions include:
| • | restrictions on or licensing of outlets permitted to sell cigarettes; | ||||
| • | the levying of substantial and increasing tax and duty charges; | ||||
| • | restrictions or bans on advertising, marketing and sponsorship; | ||||
| • | the display of larger health warnings, graphic health warnings and other labeling requirements; | ||||
| • | restrictions on packaging design, including the use of colors, and mandating plain packaging; | ||||
| • | restrictions on packaging and cigarette formats and dimensions; | ||||
| • | restrictions or bans on the display of tobacco product packaging at the point of sale and restrictions or bans on vending machines; | ||||
| • | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents; | ||||
| • | disclosure, restrictions, or bans of tobacco product ingredients, including bans on the flavors of certain tobacco products; | ||||
| • | increased restrictions on smoking and use of tobacco and nicotine-containing products in public and work places and, in some instances, in private places and outdoors; | ||||
| • | restrictions or prohibitions of novel tobacco or nicotine-containing products; | ||||
| • | elimination of duty free sales and duty free allowances for travelers; | ||||
| • | encouraging litigation against tobacco companies; and | ||||
| • | excluding tobacco companies from transparent public dialogue regarding public health and other policy matters. | ||||
Our financial results could be materially affected by regulatory initiatives resulting in a significant decrease in demand for our brands. More specifically, requirements that lead to a commoditization of tobacco products or impede adult consumers' ability to convert to our RRPs, as well as any significant increase in the cost of complying with new regulatory requirements could have a material adverse effect on our financial results.
Changes in the earnings mix and changes in tax laws may result in significant variability in our effective tax rates. Our ability to receive payments from foreign subsidiaries or to repatriate royalties and dividends could be restricted by local country currency exchange controls and other regulations.
We are subject to income tax laws in the United States and numerous foreign jurisdictions. The new administration resulting from the 2020 U.S. presidential and congressional elections could lead to changes in the U.S. tax system, including significant increases in the U.S. corporate income tax rate and the minimum tax rate on certain earnings of foreign subsidiaries. If ultimately enacted into law, such changes could have a material adverse impact on our effective tax rate thereby reducing our net earnings. Further changes in the tax laws of foreign jurisdictions could arise as a result of the base erosion and profit shifting project undertaken by the Organisation for Economic Co-operation and Development, which recommended changes to numerous long-standing tax principles. If implemented, such changes, as well as changes in taxing jurisdictions’ administrative interpretations, decisions, policies, or positions, could also have a material adverse impact on our effective tax rate thereby reducing our net earnings. In future periods, our ability to recover deferred tax assets could be subject to additional uncertainty
85
as a result of such developments. Furthermore, changes in the earnings mix or applicable foreign tax laws may result in significant variability in our effective tax rates.
As a result of Russia’s invasion of Ukraine, certain taxing jurisdictions, including the U.S., have proposed punitive tax legislation applicable to companies doing business in Russia. While we are working on options to exit the Russian market in an orderly manner, these punitive tax law changes, if enacted, could also have a material adverse impact on our effective tax rate thereby reducing our net earnings. Further, in the event we are able to successfully exit the Russian market, the sale of our Russian assets may result in U.S. tax liabilities that are material to our financial results.
Because we are a U.S. holding company, our most significant source of funds is distributions from our non-U.S. subsidiaries. Certain countries in which we operate have adopted or could institute currency exchange controls and other regulations that limit or prohibit our local subsidiaries' ability to convert local currency into U.S. dollars or to make payments outside the country. This could subject us to the risks of local currency devaluation and business disruption.
Risks Related to the Impact of the War in Ukraine on our Business
Our business, results of operations, cash flows and financial position may be adversely impacted by the continuation and consequences of the war in Ukraine.
As previously disclosed in this Form 10-Q, in 2021, Russia accounted for around 10% of our total cigarette and heated tobacco unit shipment volume, and around 6% of our total net revenues. Ukraine accounted for around 2% of our total cigarette and heated tobacco unit shipment volume, and under 2% of our total net revenues. We also produce finished goods in Ukraine for export and manufacture products in Russia. As a result of Russia’s invasion of Ukraine, we have suspended planned investments in Russia, including all new product launches and commercial, innovation, and manufacturing investments. We have also initiated plans to scale down our Russian manufacturing operations and are working on options to exit the Russian market in an orderly manner. In Ukraine, we have temporarily reduced operations, including closing our factory in the country.
The short and long-term implications of the Russian invasion of Ukraine for our operations in those countries are impossible to predict at this time. There can be no assurance that our exit from the Russian market will be successful, orderly, or represent a fair value for our Russian assets and operations. The likelihood of retaliatory action by the Russian government against companies, including us, as a result of actions and statements made in response to the Russian invasion, including the possibility of legal action against us or our employees or nationalization of foreign businesses or assets, including intangible assets such as trademarks, is impossible to predict. Further, if we are able to divest our Russian assets in an orderly manner, the nature of our divestiture may adversely impact the value we receive for those assets. In Ukraine, there is no way to know when and to what extent we will be able to normalize our operations or to what extent our workforce, facilities, inventory, and other assets will remain intact. These developments have and will continue to have a material adverse impact on our business, results of operations, cash flows and financial position, and may result in impairment charges.
The conflict also elevates the likelihood of supply chain disruptions, both in the region and globally, and may inhibit our ability to timely source materials and services needed to make and sell our products. For example, we source certain finished goods, production materials and components from both Russia and Ukraine, including printed materials and filters, and the invasion has, and may continue to, disrupt the availability of and impact our supply chain for these materials. These disruptions, to the extent we are unable to find alternative sources or otherwise address these supply constraints, may impact the availability and cost of our products in other markets, which would adversely impact our business, results of operations, cash flows and financial position, and may result in impairment charges.
The broader consequences of the invasion are also impossible to predict, but could include reputational consequences, further sanctions, financial or currency restrictions, punitive tax law changes, embargoes, regional instability, and geopolitical shifts as well as adverse effects on macroeconomic conditions, security conditions, currency exchange rates, and financial markets. Given the nature of our business and global operations, such geo-political instability and uncertainty could increase the costs of our materials and operations; reduce demand for our products; have a negative impact on our supply chains, manufacturing capabilities, or distribution capabilities; increase our exposure to currency fluctuations; constrain our liquidity or our ability to access capital markets; create staffing or operations difficulties; or subject us to increased cyber-attacks. While we will continue to monitor this fluid situation and develop contingency plans as necessary to address any disruptions to our business operations as they develop, the extent of the conflict’s effect on our business and results of operations as well as the global economy, cannot be predicted.
The conflict may also have the effect of heightening many other risks disclosed in this Form 10-Q, any of which could adversely affect our business, results of operations, cash flows or financial position. Such risks include, but are not limited to, the achievement of our strategic priorities, including achievement of our RRP growth targets; the availability of third-party
86
manufacturing resources; the availability of attractive acquisition and strategic business opportunities and our ability to fully realize the benefits of these transactions; our ability to attract, motivate, and retain the best global talent; and our loss of revenue from counterfeiting and similar illicit activities.
Risks Related to Sourcing of Materials, Products and Services
Use of third-party resources may negatively impact quality and availability of our products and services, and we may be required to replace third-party contract manufacturers or service providers with our own resources.
We increasingly rely on third-party resources and their subcontractors/suppliers to manufacture some of our products and product parts (particularly, the electronic devices and accessories), and to provide services, including to support our finance, commercialization and information technology processes. While many of these arrangements improve efficiencies and decrease our operating costs, they also diminish our direct control. Such diminished control may have a material adverse effect on the quality and availability of products or services, our supply chain, and the speed and flexibility in our response to changing market conditions and adult consumer preferences, all of which may place us at a competitive disadvantage. In addition, we may be unable to renew these agreements on satisfactory terms for numerous reasons, including government regulations, and our costs may increase significantly if we must replace such third parties with our own resources.
Government mandated prices, production control programs, shifts in crops driven by economic conditions and the impact of climate change may increase the cost or reduce the quality of the tobacco and other agricultural products used to manufacture our products.
As with other agricultural commodities, the price of tobacco leaf and cloves can be influenced by imbalances in supply and demand and the impacts of natural disasters and pandemics such as COVID-19. Furthermore, crop quality may be influenced by variations in weather patterns, including those caused by climate change. Tobacco production in certain countries is subject to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for agricultural products could cause farmers to produce less tobacco or cloves. Any significant change in tobacco leaf and clove prices, quality and quantity could affect our profitability and our business.
Risks Related to our International Operations
Because we have operations in numerous countries, our results may be adversely impacted by economic, regulatory and political developments, natural disasters, pandemics or conflicts.
Some of the countries in which we operate face the threat of civil unrest and can be subject to regime changes. In others, nationalization, terrorism, conflict and the threats of war or acts of war may have a significant impact on the business environment. Natural disasters, pandemics, economic, political, regulatory, acts of war or threats of war, or other developments could disrupt our supply chain, manufacturing capabilities or distribution capabilities, and our business continuity plans and other safeguards might not always be effective to fully mitigate their impact. In addition, such developments could increase costs of our materials and operations and lead to loss of property or equipment that are critical to our business in certain markets and difficulty in staffing and managing our operations, all of which could have a material adverse effect on our operations, volumes, revenue, net earnings and profitability. We discuss risks associated with the invasion of Ukraine above and with the COVID-19 pandemic below.
In certain markets, we are dependent on governmental approvals of various actions such as price changes, and failure to obtain such approvals could impair growth of our profitability.
In addition, despite our high ethical standards and rigorous controls and compliance procedures aimed at preventing and detecting unlawful conduct, given the breadth and scope of our international operations, we may not be able to detect all potential improper or unlawful conduct by our employees and partners. Such improper or unlawful conduct (actual or alleged) could lead to litigation and regulatory action, cause damage to our reputation and that of our brands, and result in substantial costs.
Our reported results could be adversely affected by unfavorable currency exchange rates, and currency fluctuations could impair our competitiveness.
We conduct our business primarily in local currency and, for purposes of financial reporting, the local currency results are translated into U.S. dollars based on average exchange rates prevailing during a reporting period. Foreign currencies may fluctuate significantly against the U.S. dollar reducing our net revenues, operating income and EPS. Our primary local currency
87
cost bases may be different from our primary currency revenue markets, and U.S. dollar fluctuations against various currencies may have disproportionate negative impact on net revenues as compared to our gross profit and operating income margins.
Risks Related to Legal Challenges and Investigations
Litigation related to tobacco use and exposure to environmental tobacco smoke could substantially reduce our profitability and could severely impair our liquidity.
There is litigation related to tobacco products pending in certain jurisdictions in which we operate. Damages claimed in some tobacco-related litigation are significant and, in certain cases in Brazil, Canada, and Nigeria, range into the billions of U.S. dollars. We anticipate that new cases will continue to be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our consolidated results of operations, cash flows or financial position could be materially adversely affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. We face various administrative and legal challenges related to certain RRP activities, including allegations concerning product classification, advertising restrictions, corporate communications, product coach activities, scientific substantiation, product liability, and unfair competition. While we design our programs to comply with relevant regulations, we expect these or similar challenges to continue as we expand our efforts to commercialize RRPs and to communicate publicly. The outcomes of these matters may affect our RRP commercialization and public communication activities and performance in one or more markets. Also see Note 8. Contingencies to our condensed consolidated financial statements for a discussion of pending litigation.
From time to time, we are subject to governmental investigations on a range of matters.
Investigations include allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within certain markets, allegations of underpayment of income taxes, customs duties and/or excise taxes, allegations of false and misleading usage of descriptors, allegations of unlawful advertising, and allegations of unlawful labor practices. We cannot predict the outcome of those investigations or whether additional investigations may be commenced, and it is possible that our business could be materially adversely affected by an unfavorable outcome of pending or future investigations. See Note 8. Contingencies—Other Litigation and “Management's Discussion and Analysis of Financial Condition and Results of Operations—Operating Results by Business Segment—Business Environment—Governmental Investigations” for a description of certain governmental investigations to which we are subject.
We may be unable to adequately protect our intellectual property rights, and disputes relating to intellectual property rights could harm our business.
Our intellectual property rights are valuable assets, and their protection is important to our business. If the steps we take to protect our intellectual property rights globally, including through applying for, prosecuting, maintaining and enforcing, where relevant, a combination of trademark, design, copyright, patent, trade secrets and other intellectual property rights, are inadequate, or if others infringe or misappropriate our intellectual property rights, notwithstanding legal protection, our business could be adversely impacted. Moreover, failing to manage our existing and/or future intellectual property may place us at a competitive disadvantage. Intellectual property rights of third parties may limit our ability to develop, manufacture and/or commercialize our products in one or more markets. Competitors or other third parties may claim that we infringe their intellectual property rights. Any such claims, regardless of merit, could divert management’s attention, be costly, disruptive, time-consuming and unpredictable and expose us to significant litigation costs and damages, and may impede our ability to develop, manufacture and/or commercialize new RRPs and improve our products, and thus have a material adverse effect on our revenue and our profitability. In addition, if, as a result, we are unable to manufacture or sell our RRPs or improve their quality in one or more markets, our ability to convert adult smokers to our RRPs in such markets would be adversely affected. See Note 8. Contingencies— Other Litigation to our condensed consolidated financial statements for a description of certain intellectual property proceedings.
Risks Related to our Competitive Environment
We face intense competition, and our failure to compete effectively could have a material adverse effect on our profitability and results of operations.
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, R&D, innovation, packaging, customer service, marketing, advertising and retail price and, increasingly, adult smoker willingness to convert to our RRPs. The competitive environment and our competitive
88
position can be significantly influenced by weak economic conditions, erosion of consumer confidence, competitors' introduction of lower-price products or innovative products, higher tobacco product taxes, higher absolute prices and larger gaps between retail price categories, and product regulation that diminishes the ability to differentiate tobacco products and restricts adult consumer access to truthful and non-misleading information about our RRPs. Competitors in our industry include three large international tobacco companies, new market entrants, particularly with respect to innovative products, several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, the PRC, Taiwan, Thailand and Vietnam. Some competitors have different profit, volume and regulatory objectives, and some international competitors are susceptible to changes in different currency exchange rates. Certain new market entrants in the non-combustible product category may alienate consumers from innovative products through inappropriate marketing campaigns, messaging and inferior product satisfaction, while not relying on scientific substantiation based on appropriate R&D protocols and standards. The growing use of digital media could increase the speed and extent of the dissemination of inaccurate and misleading information about our RRPs, all of which could have a mutual adverse effect on our profitability and results of operations.
We may be unable to anticipate changes in adult consumer preferences.
Our business is subject to changes in adult consumer preferences, which may be influenced by local economic conditions, accessibility to our products and availability of accurate information related to our products.
To be successful, we must:
| • | promote brand equity successfully; | ||||
| • | anticipate and respond to new adult consumer trends; | ||||
| • | ensure that our products meet our quality standards; | ||||
| • | develop new products and markets and broaden brand portfolios; | ||||
| • | improve productivity; | ||||
| • | educate and convince adult smokers to convert to our RRPs; | ||||
| • | ensure effective adult consumer engagement, including communication about product characteristics and usage of RRPs; | ||||
| • | provide excellent customer care; | ||||
| • | ensure adequate production capacity to meet demand for our products; and | ||||
| • | be able to protect or enhance margins through price increases. | ||||
In periods of economic uncertainty, adult consumers may tend to purchase lower-price brands, and the volume of our premium-price and mid-price brands and our profitability could be materially adversely impacted as a result. Such down-trading trends may be reinforced by regulation that limits branding, communication and product differentiation.
Our ability to grow profitability may be limited by our inability to introduce new products, enter new markets or improve our margins through higher pricing and improvements in our brand and geographic mix.
Our profit growth may be materially adversely impacted if we are unable to introduce new products or enter new markets successfully, to raise prices or to improve the proportion of our sales of higher margin products and in higher margin geographies.
We may be unable to expand our brand portfolio through successful acquisitions or the development of strategic business relationships, and the intended benefits from our investments may not materialize.
One element of our growth strategy is to expand our brand portfolio and market positions through selective acquisitions and the development of strategic business relationships. Acquisition and strategic business development opportunities are limited and present risks of failing to achieve efficient and effective integration, strategic objectives and/or anticipated revenue improvements and cost savings. There is no assurance that we will be able to acquire attractive businesses or enter into strategic business relationships on favorable terms ahead of our competitors, or that such acquisitions or strategic business development relationships will be accretive to earnings or improve our competitive position. In addition, we may not have a controlling position in certain strategic investments or relationships, which could impact the extent to which the intended financial growth and other benefits from these investments or relationships may ultimately materialize.
89
Our ability to achieve our strategic goals may be impaired if we fail to attract, motivate and retain the best global talent and effectively align our organizational design with the goals of our transformation.
To be successful, we must continue transforming our culture and ways of working, align our talent and organizational design with our increasingly complex business needs, and innovate and transform to a consumer-centric business. We compete for talent, including in areas that are new to us, such as digital, information technology, life sciences, with companies in the consumer products, technology, pharmaceutical and other sectors that enjoy greater societal acceptance. As a result, we may be unable to attract, motivate and retain the best global talent with the right degree of diversity, experience and skills to achieve our strategic goals.
Risks Related to the Impact of COVID-19 on our Business
Our business, results of operations, cash flows and financial position may be adversely impacted during the continuation of the COVID-19 pandemic.
The ongoing COVID-19 pandemic has created significant societal and economic disruption, and resulted in closures of stores, factories and offices, and restrictions on manufacturing, distribution and travel, all of which have and will continue to adversely impact our business, results of operations, cash flows and financial position. Our business continuity plans and other safeguards may not be effective to mitigate the impact of the pandemic.
An adequate supply chain for our cigarettes and RRP portfolio, including the supply of electronic devices, is important to our business. We work with four electronics manufacturing service providers for the supply of our Platform 1 and Platform 4 devices, and a small number of other providers for other products in our RRP portfolio and related accessories. Due to the COVID-19 pandemic, the operations of our two main electronic manufacturing service providers were temporarily suspended at different times. Even though these suspensions did not materially affect our operations, if one or more of these service providers were significantly constrained at the same time, the supply of the devices could be disrupted. Although we work closely with these service providers on monitoring their production capability and financial health, we cannot guarantee that they will remain capable of meeting their commitments, particularly during the ongoing COVID-19 pandemic; if they will not, the commercialization of our RRPs could be adversely affected. The production of our RRP portfolio requires various metals, and we believe that there is an adequate supply of such metals in the world markets to satisfy our current and anticipated production requirements. However, some components and materials necessary for the production of our RRPs, including those for the electronic devices, are obtained from single or limited sources, and can be subject to industry-wide shortages and price fluctuations. While we were successful in maintaining adequate supply of such components and materials so far, we may not be able to secure such supply going forward, particularly during the COVID-19 pandemic; this could negatively impact the commercialization of our RRPs.
Significant risks to our business during the ongoing COVID-19 pandemic also include our diminished ability to convert adult smokers to our RRPs, significant volume declines in our duty-free business and certain other key markets, disruptions or delays in our manufacturing and supply chain, including delays and increased costs in the shipment of parts to manufacture our products or for the products themselves, increased currency volatility, and delays in certain cost saving, transformation and restructuring initiatives. Our business could also be adversely impacted if key personnel or a significant number of employees or business partners become unavailable due to the COVID-19 outbreak. The significant adverse impact of COVID-19 on the economic or political conditions in markets in which we operate could result in changes to the preferences of our adult consumers and lower demand for our products, particularly for our mid-price or premium-price brands.
Continuation of the pandemic could disrupt our access to the credit markets or increase our borrowing costs. Governments may temporarily be unable to focus on the development of science-based regulatory frameworks for the development and commercialization of RRPs or on the enforcement or implementation of regulations that are significant to our business. In addition, messaging about the potential negative impacts of the use of our products on COVID-19 risks may lead to increasingly restrictive regulatory measures on the sale and use of our products, negatively impact demand for our products and the willingness of adult consumers to switch to our RRPs, and adversely impact our efforts to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs. All of the aforementioned impacts of the ongoing COVID-19 pandemic could have a material adverse effect on our business, operations, results of operations, revenues, cash flow and profitability.
The impact of these risks also depends on factors beyond our knowledge or control, including the duration and severity of the COVID-19 pandemic in general and specifically in the jurisdictions in which we operate, its recurrence in our key markets, actions taken to contain its spread and to mitigate its public health effects, and the ultimate economic consequences thereof.
90
Risks Related to Illicit Trade
We lose revenues as a result of counterfeiting, contraband, cross-border purchases, "illicit whites," non-tax-paid volume produced by local manufacturers, and counterfeiting of our Platform 1 device and heated tobacco units.
Large quantities of counterfeit cigarettes are sold in the international market. We believe that Marlboro is the most heavily counterfeited international cigarette brand, although we cannot quantify the revenues we lose as a result of this activity. In addition, our revenues are reduced by contraband, legal cross-border purchases, "illicit whites" and non-tax-paid volume produced by local manufacturers. Our revenues and consumer satisfaction with our Platform 1 device and heated tobacco units may be adversely affected by counterfeit products that do not meet our product quality standards and scientific validation procedures.
Risks Related to Cybersecurity and Data Governance
The failure of our information systems and systems owned and operated by our business partners to function as intended, or their penetration with the intent to corrupt them or to extract data from them, or our and our business partners failure to adhere to strict data governance and cybersecurity protocols, and to comply with privacy laws and regulations, could result in business disruption, loss of reputation, litigation and regulatory action, and loss of revenue, assets or personal or other confidential data.
We as well as our business partners use information systems to help manage business processes, collect and interpret data and communicate internally and externally with employees, suppliers, consumers, customers and others. Some of these information systems are managed by third-party service providers. Our business continuity plans and other safeguards may not be effective to mitigate the impact of service interruptions, connectivity outages, and other failures of these systems to function as intended, or to mitigate the penetration of these systems or systems owned and operated by our business partners by parties intent on extracting or corrupting information or otherwise disrupting business processes. These events could place us at a competitive disadvantage, result in a loss of revenue, assets, including our intellectual property, personal or other sensitive data, result in litigation and regulatory action, impact our operations, cause damage to our reputation and that of our brands and result in significant remediation and other costs. Failure to protect personal data, respect the rights of data subjects, and adhere to strict data governance and cybersecurity protocols could subject us to substantial fines and other legal challenges under regulations such as the EU General Data Protection Regulation. As we are increasingly relying on digital platforms in our business, and as privacy, data and cyber laws in the jurisdictions in which we do business are introduced or become more stringent, the magnitude of these risks is likely to increase.
Risks Related to the Acquisitions of OtiTopic, Inc. ("OtiTopic"), Fertin Pharma A/G ("Fertin Pharma") and Vectura Group Ltd. ("Vectura")
As previously disclosed in this Form 10-Q, we have acquired Fertin Pharma and Vectura, and have since launched a new Wellness and Healthcare business consolidating these entities, Vectura Fertin Pharma (with the Fertin Pharma acquisition and the Vectura acquisition being collectively referred to in these Risk Factors as the “Acquisitions”).
We may be unable to successfully integrate and realize the expected benefits from the Acquisitions.
The successful integration of the acquired businesses and their operations into those of our own and our ability to realize the benefits of the Acquisitions, are subject to a number of risks and uncertainties, many of which are not in our control. The risks and uncertainties relating to integrating the businesses acquired include, among other things: (i) the challenge of integrating complex organizations, systems, operating procedures, industry specific compliance programs, technology, networks and other assets of the businesses that we acquire, and the costs related to such integration efforts; (ii) the possibility that we are unable to gain access to differentiated proprietary technology and pharmaceutical development expertise as anticipated by these Acquisitions, and thus fail to realize our desired entry into additional smoke-free, wellness, therapeutic and healthcare platforms; (iii) the challenge of integrating the cultures and business practices of each of Fertin Pharma and Vectura to our culture and business practices, which if not managed correctly, could lead to difficulties in retaining key management and other key employees; and (iv) the challenge of achieving a successful integration as a result of our affiliation to our combustible product portfolio. In addition, even if we are able to successfully integrate, the anticipated benefits of the Acquisitions may not be realized fully, or at all, or may take longer to realize than expected. Furthermore, the success of the Acquisition also depends on the success of the research and development efforts of Vectura Fertin Pharma, including the ability to obtain regulatory approval for new products, and the ability to commercialize or license these new products developed by them. Moreover, our affiliation to its combustible product portfolio may stand in the way of introducing and growing new product categories, and
91
may prevent us in being successful in developing a long-term sustainable ecosystem of products in the wellness, therapeutic and healthcare categories.
The businesses that we acquire in the Acquisitions may have liabilities that are not known to us.
The businesses that we have acquired in the Acquisitions may have liabilities that we were unable to identify, or were unable to discover, in the course of performing our due diligence investigations during the Acquisitions thereof. We cannot assure you that the indemnification available to us under the respective acquisition agreements that we have negotiated, will be sufficient in amount, scope or duration to fully offset the possible liabilities associated with the respective business or property that we will assume upon consummation of each acquisition. Any such liabilities, individually or in the aggregate, could have a material adverse effect on our business, financial condition and results of operations.
Accounting adjustments related to the Acquisitions could adversely affect our financial results.
We have accounted for the completion of the Acquisitions using the acquisition method of accounting. Differences between preliminary estimates and the final acquisition accounting may occur, and these differences could have a material impact on the consolidated financial statements and our future results of operations and financial position in combination with the businesses acquired. Furthermore, given the nature of the assets being acquired in the Acquisitions, we may not be able to avoid future impairments of those assets, which may also have a material impact on our future results of operation and financial position.
PMI and Vectura Fertin Pharma may be subject to uncertainties that could adversely affect our respective businesses, and adversely affect the financial results of our combined businesses.
Our success following these Acquisitions will depend in part upon our ability, and the ability of Vectura Fertin Pharma, to maintain business relationships. Uncertainty about the effect of the Acquisitions on customers, suppliers, employees and other constituencies of each of Fertin Pharma and Vectura, may have a material adverse effect on us and/or the businesses that we have acquired with the proposed Acquisitions. Customers, suppliers and others who do business with Vectura Fertin Pharma may delay or defer business decisions, decide to terminate, modify or renegotiate their relationships, or take other actions as a result of the Acquisitions, which could negatively affect the revenues, earnings and cash flows of our company or the businesses that we have acquired with these Acquisitions. If we are unable to maintain the business and operational relationships of Vectura Fertin Pharma, our financial position, results of operations or cash flows upon combining with these companies could be adversely affected.
92
Item 4. Controls and Procedures.
PMI carried out an evaluation, with the participation of PMI’s management, including PMI’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of PMI’s disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based upon that evaluation, PMI’s Chief Executive Officer and Chief Financial Officer concluded that PMI’s disclosure controls and procedures are effective. There have been no changes in PMI’s internal control over financial reporting during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, PMI’s internal control over financial reporting.
93
Part II - OTHER INFORMATION
Item 1.Legal Proceedings.
See Note 8. Contingencies of the Notes to the Condensed Consolidated Financial Statements included in Part I – Item 1 of this report for a discussion of legal proceedings pending against Philip Morris International Inc. and its subsidiaries.
Item 1A. Risk Factors.
Information regarding Risk Factors appears in “MD&A – Cautionary Factors That May Affect Future Results,” in Part I – Item 2 of this Form 10-Q and in Part I – Item 1A. Risk Factors of our Annual Report on Form 10-K for the year ended December 31, 2021.
94
Item 2.Unregistered Sales of Equity Securities and Use of Proceeds.
Our share repurchase activity for each of the three months in the quarter ended March 31, 2022, was as follows:
| Period | Total Number of Shares Repurchased | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Approximate Dollar Value of Shares that May Yet be Purchased Under the Plans or Programs | ||||||||||||||||||||||
| January 1, 2022 – January 31, 2022 (1) | 377,393 | $ | 97.65 | 8,892,022 | $ | 6,178,541,816 | ||||||||||||||||||||
| February 1, 2022 – February 28, 2022 (1) | 668,911 | $ | 107.16 | 9,560,933 | $ | 6,106,858,356 | ||||||||||||||||||||
| March 1, 2022 – March 31, 2022 (1) | 920,426 | $ | 97.79 | 10,481,359 | $ | 6,016,847,275 | ||||||||||||||||||||
| Pursuant to Publicly Announced Plans or Programs | 1,966,730 | $ | 100.95 | |||||||||||||||||||||||
| January 1, 2022 – January 31, 2022 (2) | 14,393 | $ | 96.86 | |||||||||||||||||||||||
| February 1, 2022 – February 28, 2022 (2) | 218,668 | $ | 110.52 | |||||||||||||||||||||||
| March 1, 2022 – March 31, 2022 (2) | 2,884 | $ | 100.55 | |||||||||||||||||||||||
| For the Quarter Ended March 31, 2022 | 2,202,675 | $ | 101.88 | |||||||||||||||||||||||
(1)On June 11, 2021, our Board of Directors authorized a new share repurchase program of up to $7 billion, with target spending of $5 billion to $7 billion over a three-year period that commenced in July 2021. These share repurchases have been made pursuant to the $7 billion program.
(2)Shares repurchased represent shares tendered to us by employees who vested in restricted and performance share unit awards and used shares to pay all, or a portion of, the related taxes.
95
Item 6.Exhibits.
| 10.1 | Amendment and Extension Agreement, effective February 1, 2022, among PMI, the lenders named therein and Citibank Europe PLC, UK Branch (legal successor to Citibank International Limited), as administrative agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 1, 2022). | |||||||
| 10.2 | ||||||||
| 10.3 | ||||||||
| 10.4 | ||||||||
| 10.5 | ||||||||
| 10.6 | ||||||||
| 10.7 | ||||||||
| 10.8 | ||||||||
| 10.9 | ||||||||
| 10.10 | ||||||||
| 10.11 | ||||||||
| 10.12 | ||||||||
| 31.1 | ||||||||
| 31.2 | ||||||||
| 32.1 | ||||||||
| 32.2 | ||||||||
| 101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |||||||
| 101.SCH | XBRL Taxonomy Extension Schema. | |||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase. | |||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase. | |||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase. | |||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase. | |||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |||||||
96
Signature
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| PHILIP MORRIS INTERNATIONAL INC. | ||
| /s/ EMMANUEL BABEAU | ||
| Emmanuel Babeau | ||
| Chief Financial Officer | ||
| April 28, 2022 | ||
97
Similar companies
See also British American Tobacco p.l.c.See also ALTRIA GROUP, INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also SWEDISH MATCH CORP
See also RLX Technology Inc.
See also VECTOR GROUP LTD - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
