Phio Pharmaceuticals Corp. - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
A
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2021
Or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-36304
PHIO PHARMACEUTICALS CORP.
(Exact name of registrant as specified in its charter)
| Delaware | 45-3215903 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
257 Simarano Drive, Suite 101, Marlborough, Massachusetts 01752
(Address of principal executive offices and Zip Code)
(508) 767-3861
(Registrant’s telephone number, including area code)
|
Securities registered pursuant to Section 12(b) of the Act:
| ||
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value, $0.0001 per share | PHIO | The Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
The aggregate market value of the registrant’s common stock, $0.0001 par value per share (“Common Stock”), held by non-affiliates of the registrant, based on the closing sale price of the registrant’s Common Stock on June 30, 2021, was $30,488,446. Shares of Common Stock held by each officer and director and by each person who is known to own 10% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 11, 2022, the registrant had shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Definitive Proxy Statement to be filed for Phio Pharmaceuticals Corp.’s 2022 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
TABLE OF CONTENTS
PHIO PHARMACEUTICALS CORP.
ANNUAL REPORT ON FORM 10-K
For the Fiscal Year Ended December 31, 2021
| i |
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,” “expects,” “suggests,” “may,” “would,” “should,” “potential,” “designed to,” “will,” “ongoing,” “estimate,” “forecast,” “target,” “predict,” “could,” and similar references, although not all forward-looking statements contain these words. Forward-looking statements are neither historical facts nor assurances of future performance. These statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements as a result of a number of important factors, including, but not limited to:
| · | our business and operations may be materially and adversely affected by the coronavirus pandemic; | |
| · | we are dependent on the success of our INTASYL technology platform, and our product candidates based on this platform, which is unproven and may never lead to approved and marketable products; | |
| · | our product candidates are in an early stage of development and we may fail, experience significant delays, never advance to the clinic or not be successful in our efforts to identify or discover additional product candidates, which may materially and adversely impact our business; | |
| · | we are dependent on collaboration partners for the successful development of our adoptive cell therapy product candidates; | |
| · | topline data may not accurately reflect or may materially differ from the complete results of a study or clinical trial; | |
| · | a number of different factors could prevent us from advancing into clinical development, obtaining regulatory approval, and ultimately commercializing our product candidates on a timely basis, or at all; | |
| · | we are subject to significant competition and may not be able to compete successfully; | |
| · | we are dependent on the patents we own and the technologies we license, and if we fail to maintain our patents or lose the right to license such technologies, our ability to develop new products would be harmed; | |
| · | we will require substantial additional funds to complete our research and development activities; | |
| · | future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our stockholders or may otherwise adversely affect our business; and | |
| · | the price of our common stock has been and may continue to be volatile. |
The risks set forth above are not exhaustive and additional factors, including those identified in this Annual Report on Form 10-K under the heading “Risk Factors,” for reasons described elsewhere in this Annual Report on Form 10-K and in other filings Phio Pharmaceuticals Corp. periodically makes with the Securities and Exchange Commission, could adversely affect our business and financial performance. Therefore, you should not rely unduly on any of these forward-looking statements. Forward-looking statements contained in this Annual Report on Form 10-K speak as of the date hereof and Phio Pharmaceuticals Corp. does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this report.
| 1 |
PART I
Unless otherwise noted, (1) the term “Phio” refers to Phio Pharmaceuticals Corp. and our subsidiary, MirImmune, LLC and (2) the terms “Company,” “we,” “us” and “our” refer to the ongoing business operations of Phio and MirImmune, LLC, whether conducted through Phio or MirImmune, LLC.
| ITEM 1. | BUSINESS |
Overview
Phio Pharmaceuticals Corp. (“Phio,” “we,” “our” or the “Company”) is seeking to address the biggest challenges in immuno-oncology by creating new pathways to a cancer-free future for patients. We are developing therapeutics that leverage our INTASYL™ technology to target both tumor and immune cells by regulating genes to strengthen a patient’s immune system while weakening tumor defense mechanisms. With our INTASYL self-delivering RNAi technology, we aim to bring the benefits of RNA therapeutics into cancer care where other modalities may fall short.
Our Development Pipeline
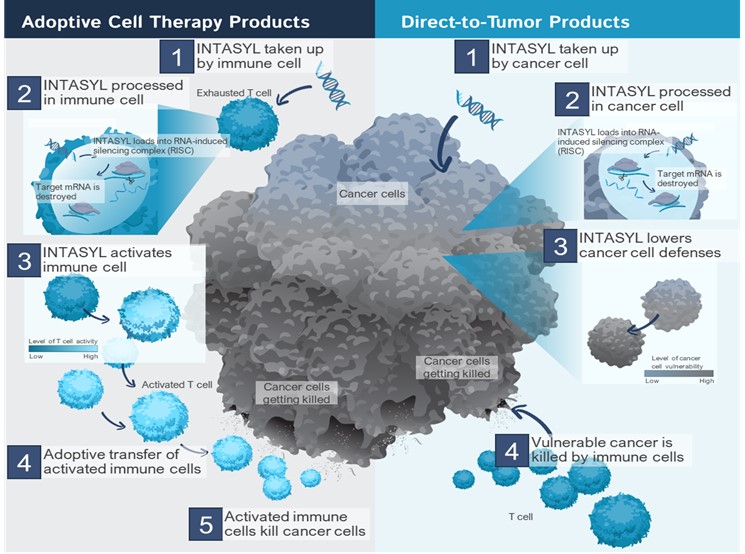
We are developing a pipeline of immuno-oncology therapies using our INTASYL technology that has the ability to attack cancers in multiple ways. Our INTASYL-based therapeutics are used to: (1) strengthen immune cells, for example those administered as part of adoptive cell therapy (“ACT”), and (2) directly modify cells in the tumor microenvironment (the “TME”) to weaken a tumor’s defense. These two strategies allow for multiple therapeutic applications of our INTASYL products.
| 2 |
In contrast to other RNA technologies and platforms, the self-delivering nature of our INTASYL platform makes it ideally suited for use with ACT treatments, as well as for direct therapeutic use. By using INTASYL technology during the manufacturing of ACT cell products we can improve the phenotype and function of these cells, potentially leading to better therapeutic outcomes. Multiple inhibitory mechanisms restrain immune cells from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence. Furthermore, the immunosuppressive TME can pose a formidable barrier to immune cell infiltration and function. By using INTASYL based drugs administered directly, we can also reprogram cells in the TME to help overcome these immunosuppressive mechanisms.
INTASYL Use To Improve Adoptive Cell Therapy Products
ACT consists of the administration of immune cells with antitumor properties to patients to fight cancer after growing the cells in a lab to large numbers. These cells can be derived from unmodified (i.e. naturally occurring) immune cells, immune cells isolated from resected tumors or genetically engineered immune cells that recognize tumor cells. These cells have several shortcomings that inhibit their full therapeutic potential in patients with solid tumors.
There are several types of ACT, including: a.) non-engineered cell therapy in which immune cells are grown from the patient’s tumor or blood, such as tumor infiltrating lymphocytes (“TILs”), or from donor blood or tissue such as natural killer (“NK”) cells, dendritic cells (“DC”) and macrophages, and b.) genetically engineered immune cells that are genetically modified to recognize specific tumor proteins and to remain in an activated state (such as T cell receptor technology (“TCRs”), chimeric antigen receptor (“CAR”) T cells, or CAR-NK cells).
Multiple inhibitory mechanisms restrain immune cells used in ACT from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence, and other barriers to immune cell infiltration and function mainly in solid tumors. When used in ACT, we believe our INTASYL compounds can improve immune cell function, differentiation and metabolism, in order to make these immune cells more effective without the need for additional complicated manufacturing steps and/or genetic engineering.
Our approach builds on well-established methodologies of ACT and involves the treatment of immune cells with our INTASYL compounds ex vivo while they are grown in the lab and before administering them to the patient. In contrast to other RNA technologies, our INTASYL compounds do not require a delivery vehicle to penetrate into the cells, therefore we are able to enhance the function of these cells by merely adding our INTASYL compounds during the expansion process and without the need for genetic engineering, complex delivery vehicles or formulations, or additional complex manufacturing steps, which in themselves may be detrimental to the cells. By adding INTASYL to the cell culture media used during the cell expansion, we can reduce or eliminate the expression of genes that make the immune cells less effective. For example, with our INTASYL compounds, we can reduce the expression of immunosuppressive proteins by the therapeutic immune cells, potentially enabling them to overcome tumor resistance mechanisms and thus improving their ability to destroy the tumor cells. In various types of immune cells tested to date, INTASYL treatment results in potent silencing with close to 100% transfection efficiency and while maintaining cell viability and cell growth rate. After expanding these cells and enhancing them with INTASYL ex vivo, they are returned to the patient for treatment.
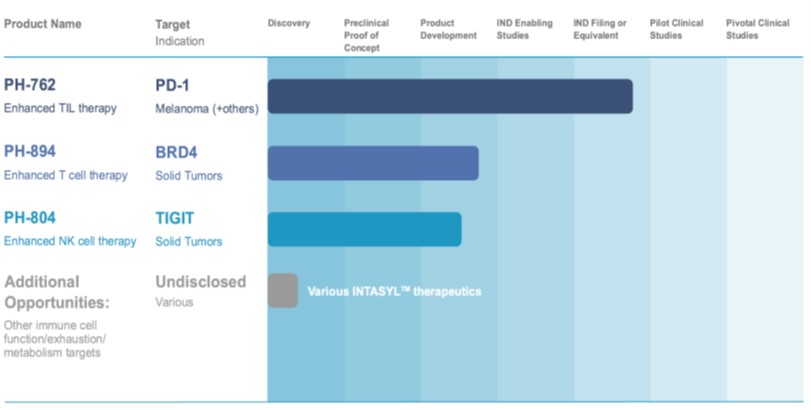
The table below sets forth the Company’s pipeline for its INTASYL product candidates for use in ACT:
| 3 |
Our lead product candidate, and our most advanced program being developed by the Company in ACT, is PH-762. PH-762 is an INTASYL compound that activates immune cells to better recognize and kill cancer cells by reducing the expression of the checkpoint protein PD-1, a clinically validated target for immunotherapy. Checkpoint proteins, such as PD-1, normally act as a type of “off switch” that prevent T cells, immune cells that protect the body from cancer cells and infections, from attacking certain cells in the body, such as cancer cells. The expression of PD-1 enables the cancer cell to evade the T cell. Reducing the expression of PD-1 can thereby reduce the ability of cancer cells to avoid T cell detection.
Data has shown that PH-762 silences PD-1 checkpoint expression in T cells, thereby removing the “off switch” and enabling T cells to overcome tumor resistance mechanisms, and thus improving their ability to destroy tumor cells. Preclinical studies show that PH-762 can silence the expression of PD-1 in target human T cells in a potent and durable manner and can increase their tumor cell-killing ability. Patient derived T cells treated with PH-762, in comparison to untreated T cells, were shown to have increased tumor killing potency against tumor cells of the same patient. As a result, we believe that PH-762 in ACT is well-positioned to enhance therapeutic responses in cancer.
In March 2021, the Company announced that it entered into a clinical development collaboration with AgonOx, Inc. (“AgonOx”), a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer, in which the companies will collaborate on the development of novel T cell-based therapies using PH-762 and AgonOx’s “double positive” TIL (“DP TIL”) technology. Per the terms of the clinical development agreement, AgonOx will receive financial support from Phio to conduct a clinical trial in ACT with their DP TIL technology and PH-762, and Phio will be entitled to certain future development milestones and sales-based royalty payments from AgonOx’s DP TIL technology. AgonOx has demonstrated that their DP TIL enriched cell populations have increased tumor killing activity when compared to TILs that were not enriched prior to expansion. Preclinical data from our research collaboration with AgonOx has shown that treating DP TILs with PH-762 increases the tumor killing activity of the DP TILs even further (a two-fold increase). As a result, we expect the use of PH-762 treated DP TILs to enhance therapeutic responses in cancer. Based on these data, our clinical development collaboration will focus on conducting a clinical study for PH-762 treated DP TILs. The Company expects to start the clinical trial evaluating the use of PH-762 and DP TILs in ACT in the second quarter of 2022.
PH-762 use in ACT is not limited to TILs, but can also be used on other forms of T cell-based cell therapy. We recently presented in vivo data showing that PH-762 significantly enhanced the antitumor efficacy of HER2-targeted CAR-T cells (“HER2CART”) in solid tumors. Compared to untreated HER2CART cells, HER2CART cells treated with PH-762 showed a statistically significant and durable inhibition of tumor growth. Analysis of the PH-762 treated HER2CART cells isolated from the tumors suggest that PH-762 enhances CAR-T function through multiple mechanisms including enhanced efficiency, degranulation and promotion of memory/stem populations. We believe that this data provides proof of concept for the application of PD-1 checkpoint silencing with INTASYL in CAR-T cells prior to ACT to enhance the therapeutic efficacy of CAR-T cell therapy in solid tumors.
Our second product candidate in development for use in ACT is PH-894. PH-894 is an INTASYL compound that silences the epigenetic protein BRD4, which is an intracellular regulator of gene expression that impacts cell differentiation, and hence, cell function. Like other epigenetic targets, BRD4 is a protein that has been shown to be difficult to target with current drug modalities. Since BRD4 is an intracellular protein, antibody therapies cannot be used and small molecule inhibitors tested to date typically lack the required specificity. As our INTASYL compounds can target intracellular proteins as well as extracellular proteins with a high level of specificity, we believe that PH-894 has significant potential. In collaboration with the Karolinska Institutet in Sweden, PH-894 has been shown to improve T cell function and persistence by differentiating T cells into a more active state (stem-cell like memory phenotype). We have demonstrated that the application of PH-894 is shown to silence BRD4 in human T cells during expansion for ACT, which has the potential to confer superior anti-tumor activity.
Our INTASYL compound PH-804 is also being developed for use in ACT. PH-804 targets the suppressive immune receptor TIGIT, which is a checkpoint protein present on immune cells, such as T cells and NK cells. Similar to PD-1, cancer cells can suppress the activity of these immune cells by activating TIGIT. This triggers an “off switch,” resulting in tumor immune evasion, which can be prevented by blocking or silencing TIGIT. PH-804 provides powerful dose-dependent silencing of TIGIT that can be seen in both T cells and NK cells and we have shown that PH-804 can silence the expression of TIGIT in these cells, overcoming their “off switch” and thereby becoming “weaponized” to kill cancer cells.
| 4 |
Direct Therapeutic Use of INTASYL Towards the Tumor Microenvironment
Cancer cells have evolved natural defenses that can suppress the immune system surrounding the tumor, in an area called the tumor microenvironment, which decreases the effectiveness of many traditional immunotherapies. Reprogramming different cell types in the TME, such as cancer cells and immune cells, may overcome these natural tumor defenses and decrease resistance to immunotherapy. An optimal treatment therapy should have the ability to address targets both inside and on the surface of tumor and immune cells, creating multiple ways to prevent tumors from evading immune detection. Our INTASYL compounds can target both intracellular and extracellular targets, and are also being developed for use as direct therapeutics to reprogram the TME, for example, by in situ transfection and activation of immune cells in the TME. Therefore, INTASYL-based drug therapy is a novel way of fighting cancer by reprogramming the cells in the TME to make cancer more responsive to a patient’s immune system and to other anti-cancer drugs.
The table below sets forth the Company’s pipeline for its direct-to-tumor INTASYL product candidates:
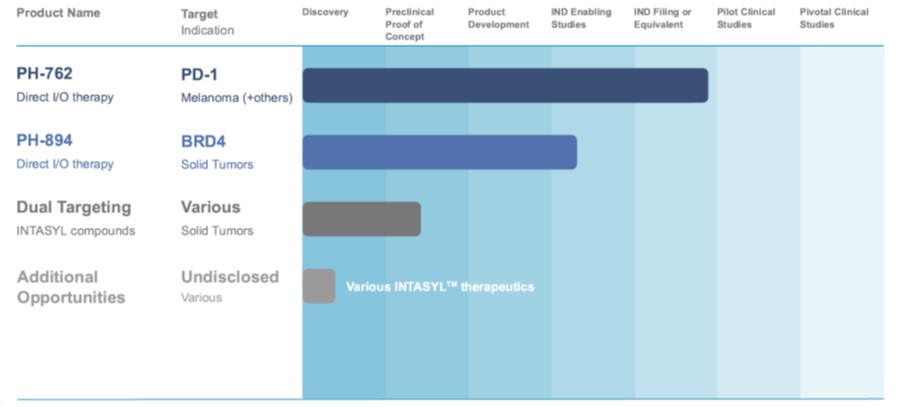
Our most advanced program being developed by the Company in our direct to tumor therapy programs, is PH-762. We have shown that we can reprogram the TME with PH-762 and achieve local activation of immune cells. Preclinical studies conducted by the Company showed that local administration of PH-762 through intratumoral injection resulted in potent anti-tumoral effects. Treated animals showed a complete and statistically significant inhibition of tumor growth, whereas placebo treated animals displayed exponential tumor growth. Recently announced in vivo data showed that intratumoral treatment with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Furthermore, on target efficacy was supported by modulation of immune cell populations toward anti-tumor phenotypes. Importantly, local administration of PH-762 resulted in activity against distal untreated tumors, indicative of a systemic anti-tumor response. The Company believes this data further supports the potential for PH-762 to provide a strong local immune checkpoint blockade without the dose immune-related adverse effects seen with systemic antibody therapy.
| 5 |
In January 2022, the Company was granted clinical trial authorization (CTA) by the French National Agency for the Safety of Medicines and Health Products to proceed with our first-in-human clinical trial for PH-762 to treat patients with melanoma at the Gustave Roussy Institute, one of the largest cancer centers in Europe. This first clinical trial with PH-762 will be a Phase 1b study to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of PH-762 in a neoadjuvant setting in subjects with advanced melanoma. Currently, there are no neoadjuvant treatment options approved for these patients. The clinical study will feature a dose escalation of PH-762 monotherapy and is designed to allow for a data driven evaluation of the recommended Phase 2 dose. The Company expects to start patient enrollment in the first quarter of 2022.
Our second direct to tumor product candidate is PH-894. In a study conducted in collaboration with the Karolinska Institutet, we demonstrated that PH-894 resulted in a strong, concentration dependent and durable silencing of BRD4 in T cells, and in various cancer cells. Data published with PH-894 in a hepatocellular carcinoma model showed potent and statistically significant anti-tumoral effects when administered locally. These data show that our PH-894 compound can reprogram T cells and other cells in the TME to provide enhanced immunotherapeutic activity. Recent in vivo data showed that local administration of PH-894 also resulted in a systemic anti-tumor response, similar to PH-762. PH-894 shows the power of our INTASYL compounds to modulate the expression of intracellular and/or commonly considered “undruggable” targets, a limitation for small molecule and antibody therapies. The Company currently expects to finalize investigational new drug (“IND”)-enabling studies for PH-894 in the second half of 2022.
We are also investigating the use of INTASYL to target multiple genes in a single formulation. New study data showed that PH-3861, a dual-targeting INTASYL towards PD-1 and BRD4, elicited complete cure of tumors in an in vivo hepatoma model and outperformed the efficacy of the small molecule and antibody control treatments toward the same targets. In addition, local INTASYL therapy was shown to induce a systemic anti-tumor response with clearance of untreated distal tumors. The animals which showed complete cure of their tumors were then rechallenged over two months after the original treatment of PH-3861 by re-implanting hepatoma cancer cells at a different location to the original tumor. All of the mice that were rechallenged with new tumors were cured again without requiring further treatment, while tumors grew steadily in the control group as expected. We believe that these data demonstrate that local administration of PH-3861 provides a durable and systemic anti-tumor immune response that can combat tumor growth.
Our INTASYL Platform
Our development efforts are based on our broadly patented INTASYL technology platform. Our INTASYL compounds do not require a delivery vehicle to penetrate into tissues and cells and are designed to “silence” or down-regulate the expression of a specific gene which is over-expressed in cancer.
Diseases are often related to the wrong protein being made, excessive amounts of a specific protein being made, or the correct protein being made but at the wrong location or time. Overall, RNA is involved in the synthesis, regulation and expression of proteins. RNA interference (“RNAi’) is a biological process in which specific RNA molecules inhibit gene expression or translation into proteins. RNAi offers a novel approach to drug development because RNAi compounds can be designed to silence any one of the thousands of human genes, many of which are “undruggable” by other modalities. The potential of RNAi as a powerful drug development platform has been shown by several RNAi based drugs becoming approved over the last few years.
The first design of RNAi compounds to be pursued for the development of human therapeutics were short, double-stranded RNAs that included limited modifications, known as small-interfering RNA (“siRNA”). Since the initial discovery of RNAi, drug delivery has been the primary challenge in developing RNAi-based therapeutics. One solution to the delivery problem involves encapsulation of siRNA into lipid-based formulations, such as liposomes, to improve cellular uptake. Another approach is to use chemical conjugations of a ligand, such as GalNAC, for cell specific delivery limited to hepatocytes. We have developed an alternative approach where delivery and drug-like properties are built directly into the RNAi compound itself, whereby the RNAi uptake is neither dependent on complex formulation nor limited to addressing a specific cell type. These novel compounds are termed self-delivering RNAi compounds, or INTASYL.
| 6 |
Our INTASYL compounds are hybrid oligonucleotide compounds that the Company believes combines the beneficial properties of both conventional RNAi and antisense technologies. In an attempt to combine the best properties of both technologies, INTASYL compounds have a single-stranded phosphorothioate region, a short duplex region, and contain a variety of nuclease-stabilizing and lipophilic chemical modifications. The combination of these features allows INTASYL compounds to achieve efficient spontaneous cellular uptake and potent, long-lasting intracellular activity.
The key to therapeutic success with RNAi lies in delivering intact RNAi compounds to the target tissue and the interior of the target cells. To accomplish this, our chemically synthesized INTASYL compounds are optimized for stability and efficacy and have unique properties that improve tissue and cell uptake.
We believe that our INTASYL platform uniquely positions the Company in the field of immuno-oncology for the following reasons:
| · | Targets multiple genes (i.e. multiple immunosuppression pathways) in a single therapeutic entity; | |
| · | Results in a sustained, or long-term, effect in vivo; | |
| · | Favorable clinical safety profile of INTASYL with local administration; | |
| · | Efficient uptake of INTASYL by target cells, obviating the need for facilitated delivery (e.g. mechanical or formulation which can be detrimental to the cells); and | |
| · | Readily manufactured under current good manufacturing practices. |
Intellectual Property
We protect our proprietary information by means of United States and foreign patents, trademarks and copyrights. In addition, we rely upon trade secret protection and contractual arrangements to protect certain of our proprietary information and products. We have pending patent applications that relate to potential drug targets, compounds we are developing to modulate those targets, methods of making or using those compounds and proprietary elements of our drug discovery platform.
Much of our technology and many of our processes depend upon the knowledge, experience and skills of key scientific and technical personnel. To protect our rights to our proprietary know-how and technology, we require all employees, as well as our consultants and advisors when feasible, to enter into confidentiality agreements that require disclosure and assignment to us of ideas, developments, discoveries and inventions made by these employees, consultants and advisors in the course of their service to us.
We have also obtained rights to various patents and patent applications under licenses with third parties, which require us to pay royalties, milestone payments, or both. The degree of patent protection for biotechnology products and processes, including ours, remains uncertain, both in the United States and in other important markets, because the scope of protection depends on decisions of patent offices, courts and lawmakers in these countries. There is no certainty that our existing patents or others, if obtained, will afford us substantial protection or commercial benefit. Similarly, there is no assurance that our pending patent applications or patent applications licensed from third parties will ultimately be granted as patents or that those patents that have been issued or are issued in the future will stand if they are challenged in court. We assess our license agreements on an ongoing basis and may from time to time terminate licenses to technology that we do not intend to employ in our technology platforms, or in our product discovery or development activities.
| 7 |
Patents and Patent Applications
We are actively seeking protection for our intellectual property and are prosecuting a number of patents and pending patent applications covering our compounds and technologies. A combined summary of these patents and patent applications is set forth below in the following table:
|
Pending Applications |
Issued Patents |
|||||||
| United States | 13 | 48 | ||||||
| Canada | 6 | 4 | ||||||
| Europe | 24 | 43 | ||||||
| Japan | 10 | 14 | ||||||
| Other Markets | 16 | 13 | ||||||
Our portfolio includes 122 issued patents, 85 of which cover our INTASYL platform. There are 17 patent families broadly covering both the composition and methods of use of our self-delivering platform technology and uses of our INTASYL compounds targeting immune checkpoint, cellular differentiation and metabolism targets for ex vivo cell-based cancer immunotherapies. These patents are scheduled to expire between 2029 and 2040. Furthermore, there are 69 patent applications, encompassing what we believe to be important new RNAi compounds and their use as therapeutics, chemical modifications of RNAi compounds that improve the compounds’ suitability for therapeutic uses (including delivery) and compounds directed to specific targets (i.e., that address specific disease states). The patents and any patents that may issue from these pending patent applications will, if issued, be set to expire between 2022 and 2040, not including any patent term extensions that may be afforded under the Federal Food, Drug, and Cosmetic Act (“FFDCA”) (and the equivalent provisions in foreign jurisdictions) for any delays incurred during the regulatory approval process relating to human drug products (or processes for making or using human drug products).
Key Intellectual Property License Agreements
As we develop our own proprietary compounds, we continue to evaluate our in-licensed portfolio as well as the field for new technologies that could be in-licensed to further enhance our intellectual property portfolio and unique intellectual property position.
Advirna LLC. On September 24, 2011, we entered into an agreement with Advirna, LLC (“Advirna”) pursuant to which Advirna assigned to us its existing patent and technology rights related to the INTASYL technology and we granted back to Advirna a license for use of the assigned patent and technology rights outside of human therapeutics and diagnostics. Under the terms of the agreement, in April 2012, the Company issued to Advirna shares of common stock equal to 5% of the Company’s fully-diluted shares outstanding at the time of issuance and paid a one-time milestone payment of $350,000 in 2014 upon the issuance of the first patent under the agreement. The Company also pays to Advirna an annual maintenance fee of $100,000 and is required to pay a low single-digit royalty on any license revenue received by the Company with respect to future licensing of the assigned Advirna patent and technology rights. To date, royalties owed to Advirna have been minimal.
Our rights under the Advirna agreement will expire upon the later of: (i) the expiration of the last-to-expire of the “patent rights” (as defined therein) or (ii) the abandonment of the last-to-be abandoned of such patents, unless earlier terminated in accordance with the provisions of the agreement. We may terminate the Advirna agreement at any time upon 90 days’ written notice to Advirna, and Advirna may terminate the agreement upon 90 days’ prior written notice in the event that we cease using commercially reasonable efforts to research, develop, license or otherwise commercialize the patent rights or “royalty-bearing products” (as defined therein), provided that we may refute such claim within such 90-day period by showing budgeted expenditures for the research, development, licensing or other commercialization consistent with other technologies of similar stage of development and commercial potential as the patent rights or royalty-bearing products. Further, either party at any time may provide to the other party written notice of a material breach of the agreement. If the other party fails to cure the identified breach within 90 days after the date of the notice, the aggrieved party may terminate the agreement by written notice to the party in breach.
| 8 |
Research and Development
Our research and development expense primarily consists of compensation and benefits for research and development personnel, facility-related expenses, supplies, external services, costs to acquire technology licenses, expenses associated with preclinical and clinical development activities and other operating costs.
Total research and development expense for the years ended December 31, 2021 and 2020 was $8,886,000 and $3,716,000, respectively.
Competition
The biotechnology and pharmaceutical industries, including the immuno-oncology field, are a constantly evolving landscape with rapidly advancing technologies and significant competition. There are a number of competitors in the immuno-oncology field including large and small pharmaceutical and biotechnology companies, academic institutions, government agencies and other private and public research organizations.
A variety of cell-based autologous and allogeneic approaches are being researched and developed for the treatment of cancer. We believe that competitors developing TIL-based and NK cell-based therapies in this field, our initial areas of focus in ACT, include, but are not limited to, Acepodia Inc., Achilles Therapeutics plc, AgonOx, Inc., Artiva Biotherapeutics, Inc., Caribou Biosciences, Inc., Century Therapeutics, Inc., Cytovia Therapeutics, Inc., Editas Medicine, Inc., Fate Therapeutics, Inc., Gamida Cell Ltd., Glycostem Therapeutics B.V., Instil Bio, Inc., Iovance Biotherapeutics, Inc., KSQ Therapeutics, Inc., Lyell Immunopharma, Inc., MiNK Therapeutics, Inc., Nkarta, Inc., ImmunityBio, Inc., NKGen Biotech, Inc., ONK Therapeutics Limited, Sanofi S.A., Shoreline Biosciences, Inc., Sorrento Therapeutics, Inc., SQZ Biotechnologies Company, Takeda Pharmaceutical Company Limited and Turnstone Biologics Corp. All of these companies are larger than us and have greater financial resources and human capital to develop competing products.
A number of companies have taken the direct therapeutic approach to modulating gene expression in the field of immuno-oncology and are conducting research and development. We believe that competitors in this field include, but are not limited to, Portage Biotech Inc., Cytovation ASA, Targovax ASA, Lytix Biopharma AS, Checkmate Pharmaceuticals, Inc., Idera Pharmaceuticals, Inc., SillaJen, Inc., Duet Therapeutics, a wholly owned subsidiary of Scopus Biopharma Inc., OncoSec Medical Incorporated and Philogen S.p.A.
Government Regulation
Review and Approval of Drugs in the United States
The United States and many other countries extensively regulate the preclinical and clinical testing, manufacturing, labeling, storage, record-keeping, advertising, promotion, export, marketing and distribution of drugs and biologic products. The U.S. Food and Drug Administration (“FDA”) regulates pharmaceutical and biologic products under the FFDCA, the Public Health Service Act and other federal statutes and regulations.
To obtain approval of our future product candidates from the FDA, we must, among other requirements, submit data supporting safety and efficacy for the intended indication as well as detailed information on the manufacture and composition of the product candidate. In most cases, this will require extensive laboratory tests and preclinical and clinical trials. The collection of these data, as well as the preparation of applications for review by the FDA involve significant time and expense. The FDA also may require post-marketing testing to monitor the safety and efficacy of approved products or place conditions on any approvals that could restrict the therapeutic claims and commercial applications of these products. Regulatory authorities may withdraw product approvals if we fail to comply with regulatory standards or if we encounter problems at any time following initial marketing of our products.
| 9 |
The first stage of the FDA approval process for a new biologic or drug involves completion of preclinical studies and the submission of the results of these studies to the FDA. These data, together with proposed clinical protocols, manufacturing information, analytical data and other information submitted to the FDA in an investigational new drug (“IND”) application, must become effective before human clinical trials may commence. Preclinical studies generally involve FDA regulated laboratory evaluation of product characteristics and animal studies to assess the efficacy and safety of the product candidate.
After the IND becomes effective, a company may commence human clinical trials. These are typically conducted in three sequential phases, but the phases may overlap. Phase 1 trials consist of testing the product candidate in a small number of patients or healthy volunteers, primarily for safety at one or more doses. Phase 2 trials, in addition to safety, evaluate the efficacy of the product candidate in a patient population somewhat larger than Phase 1 trials. Phase 3 trials typically involve additional testing for safety and clinical efficacy in an expanded population at multiple test sites. A company must submit to the FDA a clinical protocol, accompanied by the approval of the Institutional Review Board (“IRB”) at the institutions participating in the trials, prior to commencement of each clinical trial.
To obtain FDA marketing authorization, a company must submit to the FDA the results of the preclinical and clinical testing, together with, among other things, detailed information on the manufacture and composition of the product candidate, in the form of a new drug application (“NDA”), or, in the case of a biologic, a biologics license application (“BLA”).
The amount of time taken by the FDA for approval of an NDA or BLA will depend upon a number of factors, including whether the product candidate has received priority review, the quality of the submission and studies presented, the potential contribution that the compound will make in improving the treatment of the disease in question and the workload at the FDA.
The FDA may, in some cases, confer upon an investigational product the status of a fast track product. A fast track product is defined as a new drug or biologic intended for the treatment of a serious or life-threatening condition that demonstrates the potential to address unmet medical needs for this condition. The FDA can base approval of an NDA or BLA for a fast track product on an effect on a surrogate endpoint, or on another endpoint that is reasonably likely to predict clinical benefit. If a preliminary review of clinical data suggests that a fast track product may be effective, the FDA may initiate review of entire sections of a marketing application for a fast track product before the sponsor completes the application.
We anticipate that our products will be manufactured by our strategic partners, licensees or other third parties. Before approving an NDA or BLA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless the manufacturing facilities are in compliance with the FDA’s current good manufacturing practice regulations (“cGMP”), which are regulations that govern the manufacture, holding and distribution of a product. Manufacturers of biologics also must comply with the FDA’s general biological product standards. Our manufacturers also will be subject to regulation under the Occupational Safety and Health Act, the Nuclear Energy and Radiation Control Act, the Toxic Substance Control Act and the Resource Conservation and Recovery Act and other applicable environmental statutes. Following approval, the FDA periodically inspects drug and biologic manufacturing facilities to ensure continued compliance with the cGMP. Our manufacturers will have to continue to comply with those requirements. Failure to comply with these requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing or recall or seizure of product. Adverse patient experiences with the product must be reported to the FDA and could result in the imposition of marketing restrictions through labeling changes or market removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
The labeling, advertising, promotion, marketing and distribution of a drug or biologic product also must be in compliance with FDA and Federal Trade Commission requirements which include, among others, standards and regulations for off-label promotion, industry sponsored scientific and educational activities, promotional activities involving the internet, and direct-to-consumer advertising. We also will be subject to a variety of federal, state and local regulations relating to the use, handling, storage and disposal of hazardous materials, including chemicals and radioactive and biological materials. In addition, we will be subject to various laws and regulations governing laboratory practices and the experimental use of animals. In each of these areas, as above, the FDA has broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of product approvals, seize or recall products and deny or withdraw approvals.
We will also be subject to a variety of regulations governing clinical trials and sales of our products outside the United States. Whether or not FDA approval has been obtained, approval of a product candidate by the comparable regulatory authorities of foreign countries and regions must be obtained prior to the commencement of marketing the product in those countries. The approval process varies from one regulatory authority to another and the time may be longer or shorter than that required for FDA approval. In the European Union, Canada and Australia, regulatory requirements and approval processes are similar, in principle, to those in the United States.
| 10 |
Review and Approval of Drugs in the European Union Including France
In order to market any pharmaceutical product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions governing, among other things, research and development, testing, manufacturing, quality control, safety, efficacy, clinical trials, marketing authorization, packaging, storage, record keeping, reporting, export and import, advertising and other promotional practices involving pharmaceutical products, as well as commercial sales, distribution and post-approval monitoring and reporting of our products. Whether or not it obtains FDA approval for a pharmaceutical product, the company would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the pharmaceutical product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer and far more difficult than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
The United Kingdom (“UK”) formally left the European Union (“EU”) on January 31, 2020 and the transition period, during which EU laws continued to apply to the UK, expired on December 31, 2020. This means EU laws now only apply to the UK in respect of Northern Ireland as laid out in the Protocol on Ireland and Northern Ireland. Following the end of the transition period, the EU and the UK concluded a trade and cooperation agreement (“TCA”), which applied provisionally from January 1, 2021 and entered into force on May 1, 2021.
The TCA includes provisions affecting the life sciences sector (including on customs and tariffs) but areas for further discussion between the EU and the UK remain. In addition, there are some specific provisions concerning pharmaceuticals. These include the mutual recognition of Good Manufacturing Practice (“GMP”) and issued GMP documents. The TCA does not, however, contain wholesale mutual recognition of UK and EU pharmaceutical regulations and product standards.
Since January 1, 2021, the EU laws which have been transposed into UK law through secondary legislation continue to be applicable in the UK as “retained EU law”. As there is no general power to amend these regulations, the UK government has enacted the Medicines and Medical Devices Act 2021. The purpose of the act is to enable the existing regulatory frameworks in relation to human medicines, clinical trials of human medicines, veterinary medicines and medical devices to be updated. The powers under the act may only be exercised in relation to specified matters and must safeguard public health.
Specified provisions of the Medicines and Medical Devices Act 2021 entered into force on February 11, 2021. The remaining provisions came into effect within two months of February 11, 2021 or will otherwise come into effect as stipulated in subsequent statutory instruments. The Medicines and Medical Devices Act 2021 supplements the UK Medical Devices Regulations 2002 (UK Regulations), which are based on the EU Medical Devices Directive as amended to reflect the UK’s post-Brexit regulatory regime. Notably, the UK Regulations do not include any of the revisions that have been made by the EU Medical Devices Regulation (EU) 2017/745, which, since May 26, 2021, now applies in all EU Member States.
The UK’s Medicines and Healthcare products Regulatory Agency (“MHRA”) conducted a comprehensive consultation between September and November 2021 on proposals to develop a new UK regime for medical devices in the UK. The proposals include more closely aligning definitions for medical devices and in vitro medical devices with internationally recognized definitions and changing the classification of medical devices according to levels or risk. The proposals are intended to improve patient and public safety and increase the appeal of the UK market. The new regime is planned to come into force on July 1, 2023, which will align with the date from which the UK is due to stop accepting CE marked medical devices and require UKCA (UK Conformity Assessed) marking. It is envisaged that, in Northern Ireland, the amended regime could run in parallel with any existing or future EU rules in accordance with the Protocol on Ireland and Northern Ireland.
| 11 |
Drug Development Process
The conduct of clinical trials is currently governed by the EU Clinical Trials Directive 2001/20/EC (Clinical Trials Directive), and will be gradually replaced by the EU Clinical Trials Regulation (EU) No. 536/2014 (“CTR”). The CTR introduces a complete overhaul of the existing regulation of clinical trials for medicinal products in the EU. It entered into force on January 31, 2022.
Under the current regime, which will expire after a transition period of one or three years, respectively, as outlined below in more detail, before a clinical trial can be initiated, it must be approved in each EU Member State in which the clinical trial is to be conducted. The approval must be obtained from two separate entities: the National Competent Authority (“NCA”) and one or more Ethics Committees. The NCA of the EU Member States in which the clinical trial will be conducted must authorize the conduct of the trial, and the independent Ethics Committee must grant a positive opinion in relation to the conduct of the clinical trial in the relevant EU Member State before the commencement of the trial. Any substantial changes to the trial protocol or other information submitted with the clinical trial applications must be submitted to or approved by the relevant NCA and Ethics Committees. Under the current regime all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial must be reported to the NCA and to the Ethics Committees of the EU Member State where they occur.
A more unified procedure applies under the new CTR, which came into force on January 31, 2022. A sponsor is able to submit a single application for approval of a clinical trial through a centralized EU clinical trials portal. One national regulatory authority (the reporting EU Member State proposed by the applicant) takes the lead in validating and evaluating the application consult and coordinate with the other concerned Member States. If an application is rejected, it may be amended and resubmitted through the EU clinical trials portal. If an approval is issued, the sponsor may start the clinical trial in all concerned Member States. However, a concerned EU Member State may in limited circumstances declare an “opt-out” from an approval and prevent the clinical trial from being conducted in such Member State. The CTR also aims to streamline and simplify the rules on safety reporting, and introduces enhanced transparency requirements such as mandatory submission of a summary of the clinical trial results to the EU Database. While Member States will work in CTIS immediately after the system has gone live, the CTR provides for two transition periods for sponsors: For one year, until January 31, 2023, clinical trial sponsors can still choose whether to submit an initial clinical trial application in line with the current system (Clinical Trials Directive) or via CTIS. From January 31, 2023, submission of initial clinical trial applications via CTIS becomes mandatory, and by January 31, 2025, all ongoing trials approved under the current Clinical Trials Directive will be governed by the new Regulation and have to be transitioned to CTIS.
Under both the current regime and the new CTR, national laws, regulations, and the applicable Good Clinical Practice and Good Laboratory Practice standards must also be respected during the conduct of the trials, including the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines on Good Clinical Practice (“GCP”) and the ethical principles that have their origin in the Declaration of Helsinki.
Marketing Authorization Procedures
In the EU and in Iceland, Norway and Liechtenstein (together, the European Economic Area or “EEA”), after completion of all required clinical testing, pharmaceutical products may only be placed on the market after obtaining a Marketing Authorization (“MA”). To obtain an MA of a drug under European Union regulatory systems, an applicant can submit a Marketing Authorization Application (“MAA”) through, amongst others, a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single MA by the European Commission (“EC”) that is valid for all EU Member States and, after respective national implementing decisions, in the three additional EEA Member States. The centralized procedure is compulsory for specific medicinal products, including for medicines developed by means of certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products (“ATMP”) and medicinal products with a new active substance indicated for the treatment of certain diseases (AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases).
For medicinal products containing a new active substance not yet authorized in the EEA before May 20, 2004 and indicated for the treatment of other diseases, medicinal products that constitute significant therapeutic, scientific or technical innovations or for which the grant of a MA through the centralized procedure would be in the interest of public health at EU level, an applicant may voluntarily submit an application for a marketing authorization through the centralized procedure.
| 12 |
Under the centralized procedure, the Committee for Medicinal Products for Human Use (“CHMP”), established at the European Medicines Agency (“EMA”), is responsible for conducting the initial assessment of a drug. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure, the timeframe for the evaluation of an MAA by the EMA’s CHMP is, in principle, 210 days from receipt of a valid MAA. However, this timeline excludes clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP, so the overall process typically takes a year or more, unless the application is eligible for an accelerated assessment. Accelerated assessment might be granted by the CHMP in exceptional cases when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. On request, the CHMP can reduce the time frame to 150 days if the applicant provides sufficient justification for an accelerated assessment. The CHMP will provide a positive opinion regarding the application only if it meets certain quality, safety and efficacy requirements. However, the EC has final authority for granting the MA within 67 days after receipt of the CHMP opinion.
The decentralized procedure permits companies to file identical MA applications for a medicinal product to the competent authorities in various EU Member States simultaneously if such medicinal product has not received marketing approval in any EU Member State before. This procedure is available for pharmaceutical products not falling within the mandatory scope of the centralized procedure.
The competent authority of a single EU Member State, known as the reference EU Member State, is appointed to review the application and provide an assessment report. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference EU Member State and concerned EU Member States. The reference EU Member State prepares a draft assessment report and drafts of the related materials within 120 days after receipt of a valid application. Subsequently each concerned EU Member State must decide whether to approve the assessment report and related materials. If an EU Member State cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the EC, whose decision is binding for all EU Member States.
All new MAAs must include a Risk Management Plan (“RMP”), describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. RMPs and Periodic Safety Update Reports (“PSURs”) are routinely available to third parties requesting access, subject to limited redactions.
Marketing Authorizations have an initial duration of five years. After these five years, the authorization may subsequently be renewed on the basis of a reevaluation of the risk-benefit balance. Once renewed, the MA is valid for an unlimited period unless the EC or the national competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with only one additional five-year renewal. Applications for renewal must be made to the EMA at least nine months before the five-year period expires.
Data and Market Exclusivity in the European Union
As in the United States, it may be possible to obtain a period of market and/or data exclusivity in the European Union that would have the effect of postponing the entry into the marketplace of a competitor’s generic, hybrid or biosimilar product (even if the pharmaceutical product has already received an MA) and prohibiting another applicant from relying on the MA holder’s pharmacological, toxicological and clinical data in support of another MA for the purposes of submitting an application, obtaining MA or placing the product on the market.
New medicinal products authorized in the European Union, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. The overall ten-year period of market exclusivity can be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
The data exclusivity period prevents generic or biosimilar applicants from relying on the preclinical and clinical trial data contained in the dossier of the reference medicinal product when applying for a generic or biosimilar marketing authorization in the European Union during a period of eight years from the date on which the reference product was first authorized in the European Union. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the European Union until 10 years have elapsed from the initial authorization of the reference product in the European Union.
| 13 |
Post-approval Regulation
Similar to the United States, both marketing authorization holders and manufacturers of pharmaceutical products are subject to comprehensive regulatory oversight by the EMA, the EC and/or the competent regulatory authorities of the EU Member States.
The holder of an EU marketing authorization for a pharmaceutical product must also comply with EU pharmacovigilance legislation and its related regulations and guidelines, which entail many requirements for conducting pharmacovigilance, or the assessment and monitoring of the safety of pharmaceutical products.
Failure by us or by any of our third-party partners, including suppliers, manufacturers and distributors to comply with EU laws and the EU Member State laws implementing Directive 2001/83/EC on pharmaceutical products for human use and other core legislation relating to pharmaceutical products, and other EU Member State laws that apply to the conduct of clinical trials, manufacturing approval, marketing authorization of pharmaceutical products and marketing of such products, both before and after grant of marketing authorization, manufacturing of pharmaceutical products, statutory health insurance, bribery and anti-corruption or with other applicable regulatory requirements may result in administrative, civil or criminal penalties. These penalties could include delays or refusal to authorize the conduct of clinical trials or to grant marketing authorization, product withdrawals and recalls, product seizures, suspension, withdrawal or variation of the marketing authorization, total or partial suspension of production, distribution, manufacturing or clinical trials, operating restrictions, injunctions, suspension of licenses, fines and criminal penalties.
Pricing and Reimbursement Environment
Even if a pharmaceutical product obtains a marketing authorization in the European Union, there can be no assurance that reimbursement for such product will be secured on a timely basis or at all. The EU Member States are free to restrict the range of pharmaceutical products for which their national health insurance systems provide reimbursement, and to control the prices and reimbursement levels of pharmaceutical products for human use. An EU Member State may approve a specific price or level of reimbursement for the pharmaceutical product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the pharmaceutical product on the market, including volume-based arrangements, caps and reference pricing mechanisms.
To obtain reimbursement or pricing approval in some countries, including the EU Member States, we may be required to conduct studies that compare the cost-effectiveness of our product candidates to other therapies that are considered the local standard of care. There can be no assurance that any country will allow favorable pricing, reimbursement and market access conditions for any of our products, or that we will be feasible to conduct additional cost-effectiveness studies, if required.
| 14 |
European Union Data Laws
The collection and use of personal health data and other personal information in the European Union is governed by the provisions of the General Data Protection Regulation (“GDPR”), which came into force in May 2018, and related implementing laws in individual EU Member States. In addition, following the United Kingdom’s formal departure from the European Union on January 31, 2020 and the end of the transition period on December 31, 2020, the United Kingdom has become a “third country” for the purposes of EU data protection law. A “third country” is a country other than the EU Member States and the three additional European Economic Area countries (Norway, Iceland and Liechtenstein) that have adopted a national law implementing the GDPR. However, the TCA includes a provision, whereby the transfer of personal data from the EU to the United Kingdom will not be considered as a transfer to a “third country” for a period of four months starting from the entry into force of the TCA. This period will be extended by two further months, unless the EU or the United Kingdom objects. Under the GDPR, personal data can only be transferred to third countries in compliance with specific conditions for cross-border data transfers. Appropriate safeguards are required to enable transfers of personal data from the EU Member States. This status has a number of significant practical consequences, in particular for international data transfers, competent supervisory authorities and enforcement of the GDPR. The GDPR increased responsibility and liability in relation to personal data that we process.
The GDPR imposes a number of strict obligations and restrictions on the ability to process (processing includes collection, analysis and transfer of) personal data, including health data from clinical trials and adverse event reporting. The GDPR also includes requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals prior to processing their personal data or personal health data, notification of data processing obligations to the national data protection authorities and the security and confidentiality of the personal data. The GDPR also prohibits the transfer of personal data to countries outside of the European Union that are not considered by the EU to provide an adequate level of data protection, except if the data controller meets very specific requirements. These countries include the United States, and following the end of the six month period as laid out in the TCA, it may include the United Kingdom if no adequacy decision is given prior to this. Following the Schrems II decision of the Court of Justice of the European Union on July 16, 2020, there is uncertainty as to the general permissibility of international data transfers under the GDPR. In light of the implications of this decision we may face difficulties regarding the transfer of personal data from the European Union to third countries. The European Data Protection Board has adopted draft recommendations for data controllers and processors who export personal data to third countries regarding supplementary measures to ensure compliance with the GDPR when transferring personal data outside of the EU. These recommendations were submitted to public consultation until December 21, 2020, however it is unclear when and in which form these recommendations will be published in final form.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EU Member States may result in significant monetary fines, other administrative penalties and a number of criminal offenses (punishable by uncapped fines) for organizations and in certain cases their directors and officers as well as civil liability claims from individuals whose personal data was processed. Data protection authorities from the different EU Member States may still implement certain variations, enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing personal data in the European Union. Guidance developed at both EU level and at the national level in individual EU Member States concerning implementation and compliance practices are often updated or otherwise revised.
There is, moreover, a growing trend towards required public disclosure of clinical trial data in the European Union which adds to the complexity of obligations relating to processing health data from clinical trials. Such public disclosure obligations are provided in the new EU Clinical Trials Regulation, EMA disclosure initiatives and voluntary commitments by industry. Failing to comply with these obligations could lead to government enforcement actions and significant penalties against us, harm to our reputation, and adversely impact our business and operating results. The uncertainty regarding the interplay between different regulatory frameworks, such as the Clinical Trials Regulation and the General Data Protection Regulation, further adds to the complexity that we face with regard to data protection regulation.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in the European Union, its Member States and other states of Europe that could significantly change the statutory provisions governing the testing, approval, manufacturing, marketing, coverage and reimbursement of pharmaceutical products. In addition to new legislation, pharmaceutical regulations and policies are often revised or interpreted by the EMA and national agencies in ways that may significantly affect our business and our products.
| 15 |
Environmental Compliance
Our research and development activities involve the controlled use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specific waste products. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of bio-hazardous materials. The cost of compliance with these laws and regulations could be significant and may adversely affect capital expenditures to the extent we are required to procure expensive capital equipment to meet regulatory requirements.
Human Capital Management
As of December 31, 2021, we had twelve full-time and no part-time employees at our facility in Marlborough, Massachusetts. None of our employees are represented by a labor union or covered by a collective bargaining agreement, nor have we experienced any work stoppages.
We expect to add additional employees in fiscal year 2022 to increase our expertise and resources available in our preclinical and clinical research and development. We continually evaluate our business needs and weigh the use of in-house expertise and capacity with outsourced expertise and capacity. The Company currently outsources substantial preclinical and clinical trial work to third party contract research organizations and drug manufacturing contractors.
Our ability to identify, attract, retain and integrate additional qualified key personnel is also critical to our success and the competition for skilled research, product development, regulatory and technical personnel is intense. To attract qualified applicants to the Company, we offer a total rewards package consisting of base salary and cash target bonus based on geography and size of company, a comprehensive benefit package and equity compensation for every employee. Bonus opportunity and equity compensation increase as a percentage of total compensation based on level of responsibility. Actual bonus payout is based on performance.
A large majority of Phio’s employees have obtained advanced degrees in their professions and we support our employees’ further development with individualized development plans, mentoring, coaching, group training, conference attendance and financial support including tuition reimbursement.
Corporate Information
We were incorporated in the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio Pharmaceuticals Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking immuno-oncology therapeutics. Our executive offices are located at 257 Simarano Drive, Suite 101, Marlborough, MA 01752, and our telephone number is (508) 767-3861.
The Company’s website address is http://www.phiopharma.com. We make available on our website, free of charge, copies of our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the Securities and Exchange Commission (the “SEC”). We also make available on our website the charters of our audit committee, compensation committee and nominating and corporate governance committee, as well as our corporate code of ethics and conduct.
You may read and copy any materials the Company files with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding Phio and other issuers that file electronically with the SEC. The SEC’s website address is http://www.sec.gov. The contents of these websites are not incorporated by reference into this report and should not be considered to be part of this report.
| 16 |
| ITEM 1A. | RISK FACTORS |
Risks Relating to Our Business and Industry
Our business and operations may be materially and adversely affected by the coronavirus pandemic.
Our business and operations may be materially and adversely affected by the ongoing coronavirus pandemic. From the first signs of the pandemic, we have taken proactive measures to protect the health and safety of our employees, such as working remotely and flexible scheduling, and our facilities have remained largely operational. The implementation of preventative and precautionary measures that we, companies we do business with and government authorities have taken to mitigate the spread of coronavirus have impacted, and may further impact or disrupt our business and operations. The effects of these measures and the extent of their impact will depend, in part, on the length and severity of the restrictions and the limitations on our ability to conduct our business in the ordinary course. These and future measures may negatively affect our business, results of operations, financial condition and cash flows.
As a result of the coronavirus pandemic, limited availability of certain services and supplies required for our preclinical programs significantly impacted our operations, causing delays to our clinical program timelines. The Company has undertaken efforts to mitigate potential future impacts by identifying and engaging alternative third-party service providers, however, if measures to overcome the pandemic continue or are insufficient, the availability of required services and supplies could be further delayed, which may in turn further slow or delay our preclinical and clinical activities. Additionally, the commencement of new clinical trials and the enrollment of patients in clinical trials have been affected by the coronavirus pandemic and while the steps to initiate our clinical trials are continuing and ongoing, the Company does not yet know the full extent of similar potential delays on our clinical trial activities.
We cannot predict the impact that the progression of the pandemic will have on future operations or financial results due to a number of factors including, but not limited to, the health and safety of our employees, the ability of the Company’s third-party providers to continue to operate, the availability of services and supplies for our programs, the ability to commence our clinical trials and the clinical sites to enroll patients, and the length of the coronavirus pandemic. The extent to which the coronavirus impacts our business, preclinical studies and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19, including mutations or variants, and the actions to contain the pandemic or treat its impact, among others. As a result, we may experience additional disruptions that could severely impact our business, preclinical studies and clinical trials, including delays in regulatory approvals to initiate any planned clinical trials, delays or difficulties in enrolling patients, interruptions or delays in preclinical studies due to limited operations at our facilities or the companies we do business with, and lack of availability or delays in supplies needed to conduct our preclinical and clinical activities.
The pandemic has impacted and may further impact the global economy and capital markets. Moreover, it has led to significant uncertainty and increased volatility in the capital markets. If these conditions in the capital markets continue it may reduce the Company’s ability to access capital and negatively affect our future liquidity. As a result, we may be compelled to take actions to preserve our cash flow.
The coronavirus pandemic continues to evolve and change rapidly. The ultimate impact of the coronavirus pandemic, or a similar public health emergency, is highly uncertain and subject to change. The Company does not yet know the full extent of potential delays or impacts on its business, financing activities, preclinical studies, clinical trial activities or the global economy as a whole. However, these effects could have a material impact on the Company’s liquidity, results of operations and financial condition.
| 17 |
We are dependent on the success of our INTASYL technology platform, and our product candidates based on this platform, which is unproven and may never lead to approved and marketable products.
Our research and development efforts have been focused on the development of product candidates based on our INTASYL technology platform. We have invested, and we expect to continue to invest, significant financial resources and efforts developing our product candidates. The ability to generate revenue is highly dependent on the successful development, regulatory approval and commercialization of our INTASYL product candidates by us or by collaborative partners, which may not occur for the foreseeable future, if ever, and is highly uncertain and depends on a number of factors, many of which are beyond our control. Therefore, it is difficult to accurately predict challenges we may face with our product candidates as they move through the discovery, preclinical and clinical development stages. We may spend large amounts of money trying to develop our INTASYL platform technology and may never succeed in doing so. In addition, our research methodology may be unsuccessful in identifying product candidates and results from preclinical and clinical studies may not predict the results that will be obtained in later phase trials of our product candidates or our product candidates may interact with patients in unforeseen or harmful ways that may make it impractical to manufacture, market or receive regulatory approval. If we are not successful in bringing an INTASYL product candidate to market, it could negatively impact our business and financial condition and we may not be able to identify and successfully implement an alternative product development strategy.
Our product candidates are in an early stage of development and we may fail, experience significant delays, never advance to the clinic or not be successful in our efforts to identify or discover additional product candidates, which may materially and adversely impact our business.
Our success depends heavily on the successful development of our product candidates, which may never occur. Our product candidates could be delayed, not advance into the clinic, or unexpectedly fail at any stage of development. Our ability to identify, develop and commercialize product candidates is dependent on extensive preclinical and other non-clinical tests in order to support an investigational new drug (“IND”) application in the United States, or the equivalent with regulatory authorities in other jurisdictions. These research programs to identify new product candidates require substantial financial and human resources, are difficult to design and can take many years.
We cannot be certain of the outcome of our research studies and the results from these studies may not predict the results that will be obtained in later stages of development and we may focus our efforts and resources on product candidates that may prove to be unsuccessful. There is no assurance that we will be able to successfully develop our product candidates, and we may forego opportunities with certain product candidates or for indications that later prove to have greater commercial potential. If we are not able to successfully develop our product candidates, we may be forced to abandon or delay our development efforts, which may materially and adversely affect our business, financial condition, and results of operations.
We are dependent on collaboration partners for the successful development of our adoptive cell therapy product candidates.
As we do not have direct access to the patient or donor cells used in cell therapy, we are not considered a cell therapy company and expect to depend on third-party collaborators to support the clinical development of our ACT product candidates. We have entered into a clinical collaboration development agreement with AgonOx, Inc. for the clinical development of our PH-762 product candidate in ACT and have entered into research agreements with our academic and industry collaborators, each of which is terminable by the relevant party at any time, subject to applicable notice periods. The success of our collaborations depends upon the efforts of our collaboration partners, and their performance in achieving the development activities to the extent they are responsible under our collaboration agreements. Each of our partners may not be successful in performing these activities, including completing the required preclinical studies and other information to be included in an IND application (or foreign equivalent), obtaining approval to initiate clinical trials, conducting the necessary clinical trials and arranging for the manufacturing or contract research organization (“CRO”) relationships and obtaining marketing authorization. Our partners work with other companies, potentially including some of our competitors, and their corporate objectives may not align with ours, they may change their strategic focus or pursue alternative technologies. If our collaborations are not successful or a partner terminates our collaboration agreement, our business, financial condition, results of operations could be materially and adversely affected.
| 18 |
Further, we may not be successful in negotiating agreements with these collaborators or with future collaborators for the development and commercialization of our ACT product candidates through collaborations such as joint development or licensing agreements. Our ability to successfully negotiate such agreements will depend on, among other things, potential partners’ evaluation of the superiority of our technology over competing technologies, the quality of preclinical data that we have generated, the perceived risks specific to developing our product candidates and our partners’ own strategic and corporate objectives. If we fail to negotiate these agreements, we may not be able commence clinical trials with our ACT product candidates or we may be required to obtain licenses from cell therapy companies and our business, financial condition, and results of operations could be materially and adversely affected.
If we experience delays or difficulties in identifying and enrolling patients in clinical trials, it may lead to delays in generating clinical data and the receipt of necessary regulatory approvals.
Clinical trials of a new drug candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease or condition the drug candidate is intended to treat and who meet other eligibility criteria. Rates of subject enrollment are affected by many factors, and delays in subject enrollment can result in increased costs and longer development times, which could materially and adversely impact our business and financial condition. We may experience slower than expected patient enrollment, including as a result of the coronavirus pandemic, in our current or future clinical trials. In addition, clinical trials for drug candidates that treat the same indications as our product candidates may result in subjects who would otherwise be eligible for our clinical trials instead enrolling in clinical trials for other drug candidates.
Topline data may not accurately reflect or may materially differ from the complete results of a study or clinical trial.
From time to time, we may publicly disclose topline or interim data from our preclinical and clinical studies based on a preliminary analysis of then-available data, of which the results, related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. Preliminary observations made in early stages of preclinical studies and clinical trials are not necessarily indicative of results that will be obtained when full data sets are analyzed or in subsequent preclinical and clinical studies and may not be replicated in subsequent studies. As a result, topline data may differ from future results from the same studies or different conclusions may qualify such results once additional data has been received and evaluated. Topline or interim data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data that we publicly disclose and should be viewed with caution until the complete data is available.
Further, the U.S. Food and Drug Administration (“FDA”), or equivalent foreign regulatory authority, may not accept the results of our preclinical or clinical studies and may require us to complete additional studies or impose stricter approval conditions than we expect, which could impact the value of a particular program, the approvability or commercialization of the particular product candidate or product and our Company in general. Because of these factors, it is difficult to predict the time and cost of the development of our product candidates. Any delay or failure in obtaining required approvals may prevent us from completing our preclinical or clinical studies and could have a material adverse effect on our ability to initiate or commercialize any drug candidate on a timely basis, or at all. Additionally, preclinical studies and clinical trials are lengthy and expensive and if our cash resources become limited we may not be able to commence, continue or complete our clinical trials. If the topline data we report differs from future analysis of results, or if others, including regulatory authorities, disagree with the conclusions reached, our business, financial condition, and results of operations could be materially and adversely affected.
We rely upon third-parties to conduct our clinical trials and other studies for our product candidates, and if they do not successfully fulfill their obligations, the development of our product candidates may be materially impacted.
We depend upon third-party CROs, medical institutions, clinical investigators, consultants and other third-parties to support and conduct our clinical trials and rely on these third-party CROs for the execution of certain of our preclinical studies and expect to continue to do so. Because we rely on these third-parties, we cannot necessarily control the timing, quality of work or amount of resources that our contract partners will devote to these activities. We and our CROs are responsible for ensuring that our clinical trials are conducted in accordance with applicable regulations and protocols. If we or our CROs fail to comply with these applicable regulations, the FDA, or equivalent foreign regulatory authority, may not accept these data and may require us to complete additional studies. which could result in significant additional costs and delays to us. Further, these third parties may face disruptions due to the coronavirus pandemic that may affect our ability to initiate and complete our clinical studies.
| 19 |
As we only control certain aspects of their activities, we cannot guarantee that these partners will fulfill their obligations to us under these arrangements. If these third-parties do not successfully carry out their responsibilities, as well as within a timely fashion, our clinical trials and preclinical studies may be delayed, unsuccessful or otherwise adversely affected. If we have to enter into alternative arrangements it may delay or adversely affect the development of our product candidates and our business operations. This could be difficult, costly or impossible, and our preclinical or clinical trials may need to be extended, delayed, terminated or repeated, and we may not be able to obtain regulatory approval in a timely fashion, or at all, for the applicable drug candidate, or to commercialize such drug candidate being tested in such trials.
France adopted the General Data Protection Regulation, a data privacy regulation, and as we are conducting a clinical study in France we are required to follow this law, which, if violated could subject us to significant fines.
The collection and use of personal health data and other personal information in the European Union is governed by the provisions of the European General Data Protection Regulation (EU) 2016/679 (“GDPR”), which came into force in May 2018 and related implementing laws in individual EU Member States.
The GDPR imposes a number of strict obligations and restrictions on the ability to process (processing includes collection, analysis and transfer of) personal data of individuals within the European Union and in the EEA, including health data from clinical trials and adverse event reporting. The GDPR also includes requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals prior to processing their personal data or personal health data, notification of data processing obligations to the national data protection authorities and the security and confidentiality of the personal data. EU Member States may also impose additional requirements in relation to health, genetic and biometric data through their national implementing legislation.
Under the GDPR, personal data can only be transferred within the EU Member States and the three additional European Economic Area countries (Norway, Iceland and Liechtenstein) that have adopted a national law implementing the GDPR. Appropriate safeguards are required to enable cross-border transfers of personal data from the EU and EEA Member States to a “third country” (a country outside the EU or EEA). This status has a number of significant practical consequences, in particular for international data transfers, competent supervisory authorities and enforcement of the GDPR.
In conclusion, the GDPR prohibits the transfer of personal data to countries outside of the European Union/EEA (including the United States) that are not considered by the European Commission to provide an adequate level of data protection, except if the data controller meets very specific requirements such as the use of standard contractual clauses (“SCCs”), issued by the European Commission. In this respect recent legal developments in Europe have created complexity and compliance uncertainty regarding certain transfers of personal data from the EU/EEA. For example, following the Schrems II decision of the Court of Justice of the European Union on July 16, 2020, in which the Court invalidated the Privacy Shield under which personal data could be transferred from the EU/EEA to United States entities who had self-certified under the Privacy Shield scheme, there is uncertainty as to the general permissibility of international data transfers under the GDPR. The Court did not invalidate the then current SCCs, but ruled that data exporters relying on these SCCs are required to verify, on a case-by-case basis, if the law of the third country ensures an adequate level of data protection that is essentially equivalent to that guaranteed in the EU/EEA. In light of the implications of this decision we may face difficulties regarding the transfer of personal data from the European Union/EEA to third countries. However, on June 4, 2021 the EU Commission issued a new set of SCCs for data transfers from controllers or processors in the EU/EEA to controllers or processors established outside the EU/EEA. These SCCs replace the old sets of SCCs that were adopted under the previous European Data Protection Directive 95/46. Since September 27, 2021, it is no longer possible to conclude contracts incorporating these previous versions of the SCCs. In addition, for contracts concluded before September 27, 2021, it is still possible to rely on the previous SCCs until the end of an additional 15 months transitional period (until December 27, 2022), provided that the processing operations which are the subject matter of the contract remain unchanged and reliance on previous SCCs ensures that the transfer is subject to appropriate safeguards. On November 11, 2021, the European Data Protection Board has adopted recommendations on such appropriate safeguards that supplement transfer mechanisms. These recommendations aim to assist data exporters with their duty to identify and implement appropriate supplementary measures where they are needed to ensure an essentially equivalent level of protection to the personal data they transfer to third countries.
| 20 |
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EU Member States may result in significant monetary fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater, other administrative penalties and a number of criminal offenses (punishable by uncapped fines) for organizations and in certain cases their directors and officers as well as civil liability claims from individuals whose personal data was processed. Data protection authorities from the different EU Member States may still implement certain variations, enforce the GDPR and national data protection laws differently, and introduce additional national regulations and guidelines, which adds to the complexity of processing personal data in the European Union. Guidance developed at both EU level and at the national level in individual EU Member States concerning implementation and compliance practices are often updated or otherwise revised. Ensuring compliance with GDPR is time-intensive and may increase the cost of doing business, and failure to comply with these laws may have a material impact on our operations and financial condition.
There is, moreover, a growing trend towards required public disclosure of clinical trial data in the European Union which adds to the complexity of obligations relating to processing health data from clinical trials. Such public disclosure obligations are provided in the new EU Clinical Trials Regulation, EMA disclosure initiatives and voluntary commitments by industry. Failing to comply with these obligations could lead to government enforcement actions and significant penalties against us, harm to our reputation, and adversely impact our business and operating results. The uncertainty regarding the interplay between different regulatory frameworks, such as the Clinical Trials Regulation and the GDPR, further adds to the complexity that we face with regard to data protection regulation.
On June 28, 2021 the European Commission adopted two adequacy decisions for the United Kingdom – one under the GDPR and the other for the Law Enforcement Directive. Personal data may now freely flow from the European Union to the United Kingdom since the United Kingdom is deemed to have an adequate data protection level. Additionally, following the UK's withdrawal from the European Union and the EEA, companies also have to comply with the UK’s data protection laws (including the UK GDPR, which is based on the EU GDPR), the latter regime having the ability to separately fine up to the greater of £17.5 million or 4% of global turnover. The adequacy decisions include a ‘sunset clause’ which entails that the decisions will automatically expire four years after their entry into force.
A number of different factors could prevent us from advancing into clinical development, obtaining regulatory approval, and ultimately commercializing our product candidates on a timely basis, or at all.
Before obtaining regulatory approval for the sale of any drug candidate, we must conduct extensive preclinical tests and successful clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Before human clinical trials may commence, we must submit to the FDA an IND application, or foreign equivalent. An IND application involves the completion of preclinical studies and the submission of the results, together with proposed clinical protocols, manufacturing information, analytical data and other data in the IND submission. The FDA may require us to complete additional preclinical studies or disagree with our clinical trial study design. Also, animal models may not exist for some of the disease areas we choose to develop our product candidates for. As a result, our clinical trials may be delayed or we may be required to incur more expense than we anticipated.
Clinical trials require the review and oversight of Institutional Review Boards (“IRB”), which approve and continually review clinical investigations and protect the rights and welfare of human subjects. Before our clinical trials can begin, we must also submit to the FDA a clinical protocol accompanied by the approval of the IRB at the institution(s) participating in the clinical trial. An inability or delay in obtaining IRB approval could prevent or delay the initiation and completion of our clinical trials, and the FDA may decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB review and approval.
Preclinical studies and clinical trials are lengthy and expensive, and their outcome is highly uncertain. Historical failure rates are high due to a number of factors, such as safety and efficacy of drug candidates. We, our collaborators, the FDA, or an IRB may suspend clinical trials of a drug candidate at any time for various reasons, including if we or they believe the subjects participating in such trials are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a drug candidate on subjects in a clinical trial could result in the FDA or other regulatory authorities suspending or terminating the clinical trial and refusing to approve a particular drug candidate for any or all indications of use.
| 21 |
We also are subject to numerous foreign regulatory requirements governing the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process includes all of the risks associated with the FDA approval described above, as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not assure approval by regulatory authorities outside of the United States.
An additional number of factors could affect the timing, cost or outcome of our drug development efforts, including the following:
| · | Delays in filing or acceptance of initial drug applications for our product candidates; |
| · | Difficulty in securing centers to conduct clinical trials; |
| · | Conditions imposed on us by the FDA or comparable foreign authorities regarding the scope or design of our clinical trials; |
| · | Problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies; |
| · | Difficulty in enrolling subjects in conformity with required protocols or projected timelines; |
| · | Third-party contractors failing to comply with regulatory requirements or to meet their contractual obligations to us in a timely manner; |
| · | Our drug candidates having unexpected and different chemical and pharmacological properties in humans than in laboratory testing and interacting with human biological systems in unforeseen, ineffective or harmful ways; |
| · | The need to suspend or terminate clinical trials if the participants are being exposed to unacceptable health risks; |
| · | Insufficient or inadequate supply or quality of our product candidates or other necessary materials necessary to conduct our clinical trials; |
| · | Effects of our product candidates not having the desired effects or including undesirable side effects or the product candidates having other unexpected characteristics; |
| · | The cost of our clinical trials being greater than we anticipate; |
| · | Negative or inconclusive results from our clinical trials or the clinical trials of others for similar product candidates or inability to generate statistically significant data confirming the efficacy of the product being tested; |
| · | Changes in the FDA’s requirements for testing during the course of that testing; |
| · | The impact from the ongoing coronavirus pandemic; |
| · | Reallocation of our limited financial and other resources to other clinical programs; and |
| · | Adverse results obtained by other companies developing similar drugs. |
A failure of any preclinical study or clinical trial can occur at any stage of testing. Any delay or failure in obtaining required approvals may prevent us from completing our preclinical or clinical studies and could have a material adverse effect on our ability to initiate or commercialize any drug candidate on a timely basis, or at all. Additionally, preclinical studies and clinical trials are lengthy and expensive and if our cash resources become limited we may not be able to commence, continue or complete our clinical trials, which could have a material impact on our business, financial condition, and results of operations.
| 22 |
We are subject to significant competition and may not be able to compete successfully.
The biotechnology and pharmaceutical industries have intense competition and contain a high degree of risk and there are many other companies actively engaged in the discovery, development and commercialization of products that may compete with our product candidates. We face a number of competitors that have substantially greater experience and greater research and development capabilities, staffing, financial, manufacturing, marketing, technical and other resources than us, and we may not be able to successfully compete with them. These companies include large and small pharmaceutical and biotechnology companies, academic institutions, government agencies and other private and public research organizations.
In addition, even if we are successful in developing our product candidates, in order to compete successfully we may need to be first to market or to demonstrate that our products are superior to therapies based on different technologies. Some of our competitors may develop and commercialize products that are introduced to market earlier than our product candidates or on a more cost-effective basis. A number of our competitors have already commenced clinical testing of product candidates and may be more advanced than we are in the process of developing products. If we are not first to market or are unable to demonstrate superiority, on a cost-effective basis or otherwise, any products for which we are able to obtain approval may not be successful.
Our competitors also compete with us in acquiring technologies complementary to our INTASYL technology. We may face competition with respect to product efficacy and safety, ease of use and adaptability to modes of administration, acceptance by physicians, timing and scope of regulatory approvals, reimbursement coverage, price and patent position, including dominant patent positions of others. If we are not able to successfully obtain regulatory approval or commercialize our product candidates, we may not be able to establish market share and generate revenues from our technology.
If we fail to attract, hire and retain qualified personnel, we may not be able to design, develop, market or sell our products or successfully manage our business.
We have a small management team and are particularly dependent on our core management team. Accordingly, our business prospects are dependent on the principal members of our executive team, the loss of whose services could make it difficult for us to manage our business successfully and achieve our business objectives. While we have entered into employment agreements with each of our executive officers, they could leave at any time, in addition to our other employees, who are all “at will” employees. Our ability to identify, attract, retain and integrate additional qualified key personnel is also critical to our success. Competition for skilled research, product development, regulatory and technical personnel is intense, and we may not be able to recruit and retain the personnel we need. The loss of the services of any key research, product development, regulatory and technical personnel, or our inability to hire new personnel with the requisite skills, could restrict our ability to develop our product candidates.
We are subject to potential liabilities from clinical testing and future product liability claims.
The use of our product candidates in clinical trials and, if any of our product candidates receive regulatory approval, the sale of our product candidates for commercial use expose us to the risk or product liability claims. Product liability claims may be brought against us by patients, healthcare providers, consumers or others who come into contact with our product candidates or approved products. We will seek to obtain clinical trial insurance for clinical trials that we conduct, as well as liability insurance for any products that we market. However, there is no assurance that we will be able to obtain insurance in the amounts we seek, or at all. We anticipate that licensees who develop our products will carry liability insurance covering the clinical testing of our product candidates and the marketing of those product candidates, if approved. There is no assurance, however, that any insurance maintained by us or our licensees will prove adequate in the event of a claim against us. If we cannot successfully defend against product liability claims, we could incur substantial liabilities. Even if claims asserted against us are unsuccessful, they may divert management’s attention from our operations and we may have to incur substantial costs to defend such claims. Any of these outcomes could materially impact our business and financial condition.
| 23 |
We rely upon third parties for the manufacture of the clinical supply for our product candidates.
We rely on third-party suppliers and manufacturers to provide us with the materials and services to manufacture our product candidates for certain preclinical studies and for our clinical trials, and we expect that we will continue to rely on third-party manufacturers for the supply of our product candidates in the future. We have limited in-house manufacturing capabilities and resources, and we do not own or lease manufacturing facilities or have our own supply source for the required materials to manufacture our compounds. Further, we have limited current good manufacturing practice (“cGMP”) manufacturing capabilities and limited experience in scale-up of clinical supply as our internal capabilities are limited to small-scale production of research material. Accordingly, we are dependent upon third-party suppliers and contract manufacturers to obtain supplies and manufacture our product candidates and we will need to either develop, contract for, or otherwise arrange for the necessary manufacturers for these supplies.
There are a limited number of manufacturers that make oligonucleotides and we currently contract with multiple manufacturers for the supply of our product candidates to reduce the risk of supply interruption or availability. However, there is no assurance that our supply of our product candidates will not be limited, interrupted, of satisfactory quality or be available at acceptable prices. For example, constraints on the supply chain and availability of resources due to the ongoing effort to address the coronavirus pandemic have resulted in delays and shortages at manufacturing facilities. While we have engaged with multiple manufacturers for the supply of our product candidates, there can be no assurance that our efforts will be successful. If for any reason we are unable to obtain the clinical supply of our product candidates from our current manufacturers, we would have to seek to contract with another major manufacturer. If we or any of these manufacturers is unable or unwilling to increase its manufacturing capacity or if we are unable to establish alternative arrangements on a timely basis or on acceptable terms, the development and commercialization of such an approved product may be delayed or there may be a shortage in supply. Any inability to manufacture our product candidates or future approved drugs in sufficient quantities when needed would seriously harm our business.
Approval of any of our product candidates will not occur unless the manufacturing facilities are in compliance with the FDA’s cGMP regulations, or a foreign equivalent’s regulations, in order to ensure that drug products are safe and that they consistently meet applicable requirements and specifications. These requirements are enforced by the FDA and other regulatory authorities through periodic inspections of the manufacturing facilities and can result in enforcement action, such as warning letters, fines and suspension of production if they are found to not be in compliance with the regulations. If our suppliers or manufacturers do not comply with the FDA or foreign regulations for our product candidates, we may experience delays in timing or supply, be forced to manufacture our product candidates ourselves or seek to enter contract with another supplier or manufacturer. If we are required to switch suppliers or manufacturers, we will be required to verify that the new supplier or manufacturer maintains facilities and processes in line with cGMP regulations, which may result in delays, additional expenses, and may have a material adverse effect on our ability to complete the development of our product candidates.
Risks Relating to Our Intellectual Property
We may be involved in litigation to protect our patents and intellectual property rights and our ability to protect our patents and intellectual property rights is uncertain and may subject us to potential liabilities.
We have filed patent applications, have pending patents that we have licensed and those that we own and expect to continue to file patent applications. We may also need to license patents and patent applications from research sponsored by us with third-parties. There is no assurance that these applications will result in any issued patents or that those patents would withstand possible legal challenges or protect our technologies from competition. The patent granting authorities have upheld stringent standards for the RNAi patents that have been prosecuted so far and, consequently, pending patents that we have licensed and those that we own may continue to experience long and difficult prosecution challenges and may ultimately issue with much narrower claims than those in the pending applications.
In addition, others may challenge the patents or patent applications that we currently license or may license in the future or that we own and, as a result, these patents could be narrowed, invalidated or rendered unenforceable, which would negatively affect our ability to exclude others from using the technologies described in these patents. There is no assurance that these patents or other pending applications or issued patents we license or that we own will withstand possible legal challenges. Moreover, the laws of some foreign countries may not protect our proprietary rights to the same extent as do the laws of the United States. Our efforts to enforce and maintain our intellectual property rights may not be successful and may result in substantial costs and diversion of management and key employee’s time. If we are unable to defend our licensed or owned intellectual property, it may have a materially and adverse impact on our business, results of operations and financial condition.
| 24 |
Third-parties may claim that we infringe their patents, which may result in substantial liabilities and prevent us from pursuing the development of our product candidates.
Because the field we operate in is relatively new, is constantly changing and patent applications are still being processed by government patent offices around the world, there is a great deal of uncertainty about which patents will issue, when, to whom and with what claims. Although we are not aware of any blocking patents or other proprietary rights, it is likely that there will be significant litigation and other proceedings, such as interference and opposition proceedings in various patent offices, relating to patent rights in the field we operate. Further, many patents in the fields we are pursuing have already been exclusively licensed to third-parties, including our competitors. It is possible that we may become a party to such proceedings.
If a claim should be brought against us and we are found to infringe the rights of others, we may be required to pay substantial damages, be forced to stop the development of product candidates affected by the claim, and/or establish licenses or similar arrangements. Furthermore, any such licenses may not be available when needed, on commercially reasonable terms or at all. Whether an infringement claim is successful or not, the cost of these proceedings may be significant and divert the attention of management and other key employees. As a result, we cannot be certain that our patents or those we license will not be challenged by others, which could have a material adverse effect on our business, results of operations and financial condition.
We are dependent on the patents we own and the technologies we license, and if we fail to maintain our patents or lose the right to license such technologies, our ability to develop new products would be harmed.
Our success depends upon our ability to obtain and maintain intellectual property protection for our product candidates. Any patents issued to us or our licensors may not provide us with any competitive advantages, and there is no assurance that the patents of others will not have an adverse effect on our ability to do business or to continue to develop our product candidates freely. Pending patents that we have licensed and those that we own may continue to experience long and difficult prosecution challenges and may ultimately issue with much narrower claims than those in the pending applications. Because of the extensive time required for development, testing, and regulatory review of a potential product, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thus reducing any advantage provided by the patent. Further, even if our rights are valid, enforceable and broad in scope, competitors may develop products based on technology that is not covered by our licenses or patents or patent applications that we own. If we are unable to derive value from our licensed or owned intellectual property, it may have a materially and adverse impact on our business, results of operations and financial condition.
Third parties may hold or seek to obtain additional patents that could make it more difficult or impossible for us to develop products based on our technologies without obtaining a license to such patents, which licenses may not be available on attractive terms, or at all. If there is any dispute or issue of non-performance between us and the respective licensing partner regarding the rights or obligations under the license agreements, the ability to develop and commercialize the affected product candidate may be adversely affected. Moreover, if any of our existing licenses are terminated, the development of the product candidates contemplated by the licenses could be delayed or terminated and we may not be able to negotiate additional licenses on acceptable terms, if at all, which would have a material adverse effect on our business. To the extent that we are required and are able to obtain multiple licenses from third parties to develop or commercialize a product candidate, the aggregate licensing fees and milestones and royalty payments made to these parties may materially reduce our economic returns or even cause us to abandon development or commercialization of a product candidate.
| 25 |
Risks Relating to Our Financial Condition
We have a history of net losses, and we expect to continue to incur net losses for the foreseeable future and may not achieve or maintain profitability.
We have generated significant losses to date, have not generated any product revenue and may not generate product revenue in the foreseeable future, or ever. We expect to incur significant operating losses as we advance our product candidates through drug development and the regulatory process. Our ability to achieve profitability, if ever, will depend on, among other things, us or our collaborators, obtaining regulatory approvals and successfully commercializing our drug candidates. Even if we are able to successfully commercialize our drug candidates, we may not be able to achieve or sustain profitability, which could have a material adverse effect on our business, financial condition and results of operations.
We will require substantial additional funds to complete our research and development activities.
We have used substantial funds to develop our product candidates and will need to raise additional substantial funds to continue our drug development efforts and support our operations. Our future capital requirements and the period for which our existing resources are able to support our operations may vary significantly from what we expect. We anticipate that we will need to raise substantial amounts of money to fund a variety of future activities integral to the development of our business, which may include but is not limited to the following:
| · | To conduct research and development to successfully develop our product candidates; |
| · | To obtain regulatory approval for our products; |
| · | To file and prosecute patent applications and to defend and assess patents to protect our technologies; |
| · | To retain qualified employees, particularly in light of intense competition for qualified personnel; |
| · | To manufacture products ourselves or through third parties; |
| · | To market our products, either through building our own sales and distribution capabilities or relying on third parties; and |
| · | To acquire new technologies, licenses or products. |
In the future, we will be dependent on obtaining funding from third parties, such as proceeds from the issuance of debt, sale of equity or strategic opportunities, in order to maintain our operations. We cannot assure you that additional financing will be available to us on acceptable terms, or at all. If we cannot, or are limited in the ability to, issue equity, incur debt or enter into strategic collaborations, we may be unable to fund the discovery and development of our product candidates, address gaps in our product offerings or improve our technology. If we fail to obtain additional funding when needed, we may ultimately be unable to continue to develop and potentially commercialize our product candidates, and we may be forced to scale back or terminate our operations or seek to merge with or be acquired by another company.
| 26 |
Future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our stockholders or may otherwise adversely affect our business.
If we raise funds through the issuance of debt or equity, any debt securities or preferred stock issued will have rights, preferences and privileges senior to those of holders of our common stock in the event of a liquidation. In such event, there is a possibility that once all senior claims are settled, there may be no assets remaining to pay out to the holders of common stock. The terms of debt securities may also impose restrictions on our operations, which may include limiting our ability to incur additional indebtedness, to pay dividends on or repurchase our capital stock, or to make certain acquisitions or investments. In addition, we may be subject to covenants requiring us to satisfy certain financial tests and ratios, and our ability to satisfy such covenants may be affected by events outside of our control. If we raise funds through the issuance of additional equity, whether through private placements or public offerings, such an issuance would dilute current stockholders’ ownership in us, perhaps substantially. The issuance of a significant amount of shares of common stock could cause the market price of our common stock to decline or become highly volatile.
We expect to continue to incur significant research and development expenses, which may make it difficult for us to attain profitability, and may lead to uncertainty as to our ability to continue as a going concern.
We expend substantial funds to develop our technologies, and additional substantial funds will be required for further research and development, including preclinical testing and clinical trials of any product candidates, and to manufacture and market any products that are approved for commercial sale. Because the successful development of our products is uncertain, we are unable to precisely estimate the actual funds we will require to develop and potentially commercialize them. In addition, we may not be able to generate enough revenue, even if we are able to commercialize any of our product candidates, to become profitable.
Based on our current operating plans and liquidity, we believe that our existing cash at December 31, 2021 will be sufficient to fund our currently planned operations for at least the next 12 months from the date of release of the associated financial statements. However, if we are unable to achieve or sustain profitability or to secure additional financing, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our common stockholders losing their entire investment. There is no guarantee that we will become profitable or secure additional financing. Changes in our operating plans, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, increased expenses, potential acquisitions or other events will all affect our ability to continue as a going concern.
Our ability to utilize net operating loss carryforwards and other tax benefits may be limited.
We have historically incurred net losses and may never achieve or sustain profitability. Under the Internal Revenue Code of 1986, as amended (the “Code”), a corporation is generally allowed a deduction for net operating losses carried forward from a prior taxable year. Under that provision, we can carryforward our net operating losses to offset our future taxable income, if any, until such net operating losses are used or expire. As a result of the Tax Cuts and Jobs Act of 2017 legislation, net operating losses incurred in tax years beginning after December 31, 2017 may be carried forward indefinitely and can offset up to 80% of future taxable income. Under the Coronavirus Aid, Relief, and Economic Security Act of 2020, net operating losses arising in years beginning 2018 through 2020 may be carried back five years and the 80% net operating loss deduction limit is temporarily lifted for net operating loss carryforwards to years beginning before January 1, 2021. These net operating loss carryforwards could expire unused before offsetting potential future income tax liabilities.
Additionally, an ownership change, as defined by Section 382 of the Code, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50% over a three-year period. If the Company has experienced a change of control, as defined by Section 382 of the Code, at any time since inception, utilization of the Company’s net operating loss carryforwards would be subject to an annual limitation. Any limitation may result in expiration of a portion of the net operating loss carryforwards before utilization. During 2021, the Company completed an assessment of the available net operating loss carryforwards under Section 382 and determined that the Company underwent multiple ownership changes during the period from 2012 to 2021. As a result, our net operating losses are subject to substantial annual limitations under Section 382 due to these ownership changes. The Company has adjusted its net operating loss carryforwards to address the impact of the 382 ownership change. We may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If an ownership change occurs and our ability to use our net operating loss carryforwards is materially limited, it would harm our future operating results by effectively increasing our future tax obligations.
| 27 |
Risks Relating to Our Securities
The price of our common stock has been and may continue to be volatile.
Our stock price has historically fluctuated widely and is likely to continue to be volatile. Because we are at an early stage of development and in the absence of product revenue as a measure of operating performance, we anticipate that the market price for our common stock may be influenced by, but not limited to, such factors as:
| · | Announcements regarding the initiation or completion, and the results of preclinical studies and clinical trials of our product candidates; |
| · | Announcements regarding clinical trial results or development announcements concerning our competitors product candidates; |
| · | Regulatory or legal developments in the United States and other countries; |
| · | The recruitment or departure of key personnel; |
| · | The issuance of competitive patents or disallowance or loss of our patent rights; |
| · | Our ability to raise additional capital and the terms on which additional capital is raised; |
| · | To acquire new technologies, licenses or products; |
| · | Natural disasters and calamities, including the coronavirus pandemic; and |
| · | General economic, industry and market conditions. |
The stock markets, in general, and the markets for drug delivery and pharmaceutical company stocks, in particular, have experienced extreme volatility, that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock and could result in the loss of all or part of your investment. In addition, the limited trading volume of our stock may contribute to its volatility. Moreover, our stock has recently traded below $1.00 for an extended period of time. If we are unable to trade above $1.00 for a certain period of time (which may require a reverse split of our outstanding common stock), The Nasdaq Stock Market may delist our common stock. Delisting our common stock from Nasdaq would adversely affect our trading volume and would likely negatively impact our trading price.
Our Board of Directors has the authority to issue shares of “blank check” preferred stock and the terms of the preferred stock may reduce the value of our common stock.
We are authorized to issue up to 10,000,000 shares of preferred stock in one or more series. Our Board of Directors may determine the terms of future preferred stock offerings without further action by our stockholders. The issuance of our preferred stock could affect the rights of existing stockholders or reduce the value of our outstanding preferred stock or common stock. In particular, rights granted to holders of certain series of preferred stock may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights and restrictions on our ability to merge with or sell our assets to a third party.
| 28 |
We may acquire other businesses or form joint ventures that may be unsuccessful and could dilute your ownership interest in the Company.
As part of our business strategy, we may pursue future acquisitions of other complementary businesses and technology licensing arrangements. We also may pursue strategic alliances. We have limited experience with respect to acquiring other companies and with respect to the formation of collaborations, strategic alliances and joint ventures. We may not be able to integrate such acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. We also could experience adverse effects on our reported results of operations from acquisition related charges, amortization of acquired technology and other intangibles and impairment charges relating to write-offs of goodwill and other intangible assets from time to time following the acquisition. Integration of an acquired company requires management resources that otherwise would be available for ongoing development of our existing business. We may not realize the anticipated benefits of any acquisition, technology license or strategic alliance. There is no assurance that we will be successful in developing such assets, and a failure to successfully develop such assets could diminish our prospects.
To finance future acquisitions, we may choose to issue shares of our common stock or preferred stock as consideration, which would dilute current stockholders’ ownership interest in us. Alternatively, it may be necessary for us to raise additional funds through public or private financings. Additional funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders. Any future acquisitions by us also could result in large and immediate write-offs, the incurrence of contingent liabilities or amortization of expenses related to acquired intangible assets, any of which could harm our operating results.
Provisions of our certificate of incorporation and bylaws and Delaware law might discourage, delay or prevent a change of control of the Company or changes in our management and, as a result, depress the trading price of our common stock.
Our certificate of incorporation and bylaws contain provisions that could discourage, delay or prevent a change of control of the Company or changes in our management that the stockholders of the Company may deem advantageous. These provisions:
| · | Authorize the issuance of “blank check” preferred stock that our Board of Directors could issue to increase the number of outstanding shares and to discourage a takeover attempt; |
| · | Prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; |
| · | Provide that the Board of Directors is expressly authorized to adopt, alter or repeal our bylaws; and |
| · | Establish advance notice requirements for nominations for election to our Board of Directors or for proposing matters that can be acted upon by stockholders at stockholder meetings. |
Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our Board of Directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management team by making it more difficult for stockholders to replace members of our Board of Directors, which is responsible for appointing the members of our management.
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
| 29 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
On December 17, 2013, we entered into a lease (the “Lease”), as subsequently amended on January 22, 2019, with 257 Simarano Drive, LLC, Brighton Properties, LLC, Robert Stubblebine 1, LLC and Robert Stubblebine 2, LLC to lease office and laboratory space in the building known as the “Main Building” located at 257 Simarano Drive, Marlborough, Massachusetts, covering 7,581 square feet. The premises are used by the Company for office and laboratory space. The term of the Lease commenced on April 1, 2014 and expires on March 31, 2024, for a total of a ten year lease term. The base rent for the premises is $124,865 per annum, payable on a monthly basis. Each year thereafter, the base rent shall increase by approximately 3% over the base rent from the prior year. With six months’ advance notice, either party had the option to terminate the lease on March 31, 2021, paying the non-terminating party six months’ rent as a penalty or on March 31, 2022, paying the non-terminating party three months’ rent as a penalty. The option to terminate the Lease early was not exercised by either party and has expired.
We believe that our facilities are suitable for our current needs.
| ITEM 3. | LEGAL PROCEEDINGS |
From time to time, we may become a party to various legal proceedings and complaints arising in the ordinary course of business. There are none deemed to be material at this time.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| 30 |
PART II.
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is listed on The Nasdaq Capital Market under the symbol “PHIO.”
Holders
At March 11, 2022, there were approximately 17 holders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of individual stockholders represented by these holders of record.
Dividends
We have never paid any cash dividends and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We expect to retain future earnings, if any, for use in our development activities and the operation of our business. The payment of any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, our results of operations, financial condition, cash requirements, prospects and other factors that our board of directors may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
See Item 12 to this Annual Report on Form 10-K for additional information about the securities authorized for issuance under our equity compensation plans.
Recent Sales of Unregistered Sales of Securities
No sales or issues of unregistered securities occurred that have not previously been disclosed in a Quarterly Report on Form 10-Q or in a Current Report on Form 8-K.
Purchases of Equity Securities by the Issuer and Affiliated Purchases
We did not repurchase any shares of our common stock during the years ended December 31, 2021 or 2020.
| ITEM 6. | RESERVED |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the notes to those consolidated financial statements included in Item 8 of this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve significant risks and uncertainties. As a result of many factors, such as those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K, our actual results may differ materially from those anticipated in these forward-looking statements. Please refer to the discussion under the heading “Forward-Looking Statements” above.
| 31 |
Overview
Phio Pharmaceuticals Corp. (“Phio,” “we,” “our” or the “Company”) is seeking to address the biggest challenges in immuno-oncology by creating new pathways to a cancer-free future for patients. We are developing therapeutics that leverage our INTASYL™ technology to target both tumor and immune cells by regulating genes to strengthen a patient’s immune system while weakening tumor defense mechanisms. With our INTASYL self-delivering RNAi technology, we aim to bring the benefits of RNA therapeutics into cancer care where other modalities may fall short.
We are developing a pipeline of immuno-oncology therapies using our INTASYL technology that has the ability to attack cancers in multiple ways. Our INTASYL-based therapeutics are used to: (1) strengthen immune cells, for example those administered as part of adoptive cell therapy (“ACT”), and (2) directly modify cells in the tumor microenvironment (the “TME”) to weaken a tumor’s defense. These two strategies allow for multiple therapeutic applications of our INTASYL products.
In contrast to other RNA technologies and platforms, the self-delivering nature of our INTASYL platform makes it ideally suited for use with ACT treatments, as well as for direct therapeutic use. By using INTASYL technology during the manufacturing of ACT cell products we can improve the phenotype and function of these cells, potentially leading to better therapeutic outcomes. Multiple inhibitory mechanisms restrain immune cells from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence. Furthermore, the immunosuppressive TME can pose a formidable barrier to immune cell infiltration and function. By using INTASYL based drugs administered directly, we can also reprogram cells in the TME to help overcome these immunosuppressive mechanisms.
INTASYL Use To Improve Adoptive Cell Therapy Products
ACT consists of the administration of immune cells with antitumor properties to patients to fight cancer after growing the cells in a lab to large numbers. These cells can be derived from unmodified (i.e. naturally occurring) immune cells, immune cells isolated from resected tumors or genetically engineered immune cells that recognize tumor cells. These cells have several shortcomings that inhibit their full therapeutic potential in patients with solid tumors.
There are several types of ACT, including: a.) non-engineered cell therapy in which immune cells are grown from the patient’s tumor or blood, such as tumor infiltrating lymphocytes (“TILs”), or from donor blood or tissue such as natural killer (“NK”) cells, dendritic cells (“DC”) and macrophages, and b.) genetically engineered immune cells that are genetically modified to recognize specific tumor proteins and to remain in an activated state (such as T cell receptor technology (“TCRs”), chimeric antigen receptor (“CAR”) T cells, or CAR-NK cells).
Multiple inhibitory mechanisms restrain immune cells used in ACT from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence, and other barriers to immune cell infiltration and function mainly in solid tumors. When used in ACT, we believe our INTASYL compounds can improve immune cell function, differentiation and metabolism, in order to make these immune cells more effective without the need for additional complicated manufacturing steps and/or genetic engineering.
Our approach builds on well-established methodologies of ACT and involves the treatment of immune cells with our INTASYL compounds ex vivo while they are grown in the lab and before administering them to the patient. In contrast to other RNA technologies, our INTASYL compounds do not require a delivery vehicle to penetrate into the cells, therefore we are able to enhance the function of these cells by merely adding our INTASYL compounds during the expansion process and without the need for genetic engineering, complex delivery vehicles or formulations, or additional complex manufacturing steps, which in themselves may be detrimental to the cells. By adding INTASYL to the cell culture media used during the cell expansion, we can reduce or eliminate the expression of genes that make the immune cells less effective.
Our lead product candidate, and our most advanced program being developed by the Company in ACT, is PH-762. PH-762 is an INTASYL compound that activates immune cells to better recognize and kill cancer cells by reducing the expression of the checkpoint protein PD-1, a clinically validated target for immunotherapy. Checkpoint proteins, such as PD-1, normally act as a type of “off switch” that prevent T cells, immune cells that protect the body from cancer cells and infections, from attacking certain cells in the body, such as cancer cells. The expression of PD-1 enables the cancer cell to evade the T cell. Reducing the expression of PD-1 can thereby reduce the ability of cancer cells to avoid T cell detection.
| 32 |
Data has shown that PH-762 silences PD-1 checkpoint expression in T cells, thereby removing the “off switch” and enabling T cells to overcome tumor resistance mechanisms, and thus improving their ability to destroy tumor cells. Preclinical studies show that PH-762 can silence the expression of PD-1 in target human T cells in a potent and durable manner and can increase their tumor cell-killing ability. Patient derived T cells treated with PH-762, in comparison to untreated T cells, were shown to have increased tumor killing potency against tumor cells of the same patient. As a result, we believe that PH-762 in ACT is well-positioned to enhance therapeutic responses in cancer.
In March 2021, the Company announced that it entered into a clinical development collaboration with AgonOx, Inc. (“AgonOx”), a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer, in which the companies will collaborate on the development of novel T cell-based therapies using PH-762 and AgonOx’s “double positive” TIL (“DP TIL”) technology. Per the terms of the clinical development agreement, AgonOx will receive financial support from Phio to conduct a clinical trial in ACT with their DP TIL technology and PH-762, and Phio will be entitled to certain future development milestones and sales-based royalty payments from AgonOx’s DP TIL technology. AgonOx has demonstrated that their DP TIL enriched cell populations have increased tumor killing activity when compared to TILs that were not enriched prior to expansion. Preclinical data from our research collaboration with AgonOx has shown that treating DP TILs with PH-762 increases the tumor killing activity of the DP TILs even further (a two-fold increase). As a result, we expect the use of PH-762 treated DP TILs to enhance therapeutic responses in cancer. Based on this data, our clinical development collaboration will focus on conducting a clinical study for PH-762 treated DP TILs. As a result of impacts from the coronavirus pandemic, the availability of certain materials for the clinical trial are delayed. Based upon current information, the Company expects to start the clinical trial evaluating the use of PH-762 and DP TILs in ACT in the second quarter of 2022.
PH-762 use in ACT is not limited to TILs, but can also be used on other forms of T cell-based cell therapy. We recently presented in vivo data showing that PH-762 significantly enhanced the antitumor efficacy of HER2-targeted CAR-T cells (“HER2CART”) in solid tumors. Compared to untreated HER2CART cells, HER2CART cells treated with PH-762 showed a statistically significant and durable inhibition of tumor growth. Analysis of the PH-762 treated HER2CART cells isolated from the tumors suggest that PH-762 enhances CAR-T function through multiple mechanisms including enhanced efficiency, degranulation and promotion of memory/stem populations. We believe that this data provides proof of concept for the application of PD-1 checkpoint silencing with INTASYL in CAR-T cells prior to ACT to enhance the therapeutic efficacy of CAR-T cell therapy in solid tumors.
Our second product candidate in development for use in ACT is PH-894. PH-894 is an INTASYL compound that silences the epigenetic protein BRD4, which is an intracellular regulator of gene expression that impacts cell differentiation, and hence, cell function. Like other epigenetic targets, BRD4 is a protein that has been shown to be difficult to target with current drug modalities. Since BRD4 is an intracellular protein, antibody therapies cannot be used and small molecule inhibitors tested to date typically lack the required specificity. As our INTASYL compounds can target intracellular proteins as well as extracellular proteins with a high level of specificity, we believe that PH-894 has significant potential. In collaboration with the Karolinska Institutet in Sweden, PH-894 has been shown to improve T cell function and persistence by differentiating T cells into a more active state (stem-cell like memory phenotype). We have demonstrated that the application of PH-894 is shown to silence BRD4 in human T cells during expansion for ACT, which has the potential to confer superior anti-tumor activity.
Our INTASYL compound PH-804 is also being developed for use in ACT. PH-804 targets the suppressive immune receptor TIGIT, which is a checkpoint protein present on immune cells, such as T cells and NK cells. Similar to PD-1, cancer cells can suppress the activity of these immune cells by activating TIGIT. This triggers an “off switch,” resulting in tumor immune evasion, which can be prevented by blocking or silencing TIGIT. PH-804 provides powerful dose-dependent silencing of TIGIT that can be seen in both T cells and NK cells and we have shown that PH-804 can silence the expression of TIGIT in these cells, overcoming their “off switch” and thereby becoming “weaponized” to kill cancer cells.
Direct Therapeutic Use of INTASYL Towards the Tumor Microenvironment
Cancer cells have evolved natural defenses that can suppress the immune system surrounding the tumor, in an area called the tumor microenvironment, which decreases the effectiveness of many traditional immunotherapies. Reprogramming different cell types in the TME, such as cancer cells and immune cells, may overcome these natural tumor defenses and decrease resistance to immunotherapy. An optimal treatment therapy should have the ability to address targets both inside and on the surface of tumor and immune cells, creating multiple ways to prevent tumors from evading immune detection. Our INTASYL compounds can target both intracellular and extracellular targets, and are also being developed for use as direct therapeutics to reprogram the TME, for example, by in situ transfection and activation of immune cells in the TME. Therefore, INTASYL-based drug therapy is a novel way of fighting cancer by reprogramming the cells in the TME to make cancer more responsive to a patient’s immune system and to other anti-cancer drugs.
| 33 |
Our most advanced program being developed by the Company in our direct to tumor therapy programs, is PH-762. We have shown that we can reprogram the TME with PH-762 and achieve local activation of immune cells. Preclinical studies conducted by the Company showed that local administration of PH-762 through intratumoral injection resulted in potent anti-tumoral effects. Treated animals showed a complete and statistically significant inhibition of tumor growth, whereas placebo treated animals displayed exponential tumor growth. Recently announced in vivo data showed that intratumoral treatment with PH-762 inhibits tumor growth in a dose dependent fashion in PD-1 responsive and refractory models. Furthermore, on target efficacy was supported by modulation of immune cell populations toward anti-tumor phenotypes. Importantly, local administration of PH-762 resulted in activity against distal untreated tumors, indicative of a systemic anti-tumor response. The Company believes this data further supports the potential for PH-762 to provide a strong local immune checkpoint blockade without the dose immune-related adverse effects seen with systemic antibody therapy.
In January 2022, the Company was granted clinical trial authorization (CTA) by the French National Agency for the Safety of Medicines and Health Products to proceed with our first-in-human clinical trial for PH-762 to treat patients with melanoma at the Gustave Roussy Institute, one of the largest cancer centers in Europe. This first clinical trial with PH-762 will be a Phase 1b study to evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of PH-762 in a neoadjuvant setting in subjects with advanced melanoma. Currently, there are no neoadjuvant treatment options approved for these patients. The clinical study will feature a dose escalation of PH-762 monotherapy and is designed to allow for a data driven evaluation of the recommended Phase 2 dose. The Company expects to start patient enrollment in the first quarter of 2022.
Our second direct to tumor product candidate is PH-894. In a study conducted in collaboration with the Karolinska Institutet, we demonstrated that PH-894 resulted in a strong, concentration dependent and durable silencing of BRD4 in T cells, and in various cancer cells. Data published with PH-894 in a hepatocellular carcinoma model showed potent and statistically significant anti-tumoral effects when administered locally. These data show that our PH-894 compound can reprogram T cells and other cells in the TME to provide enhanced immunotherapeutic activity. Recent in vivo data showed that local administration of PH-894 also resulted in a systemic anti-tumor response, similar to PH-762. PH-894 shows the power of our INTASYL compounds to modulate the expression of intracellular and/or commonly considered “undruggable” targets, a limitation for small molecule and antibody therapies. The Company currently expects to finalize IND-enabling studies for PH-894 in the second half of 2022.
We are also investigating the use of INTASYL to target multiple genes in a single formulation. New study data showed that PH-3861, a dual-targeting INTASYL towards PD-1 and BRD4, elicited complete cure of tumors in an in vivo hepatoma model and outperformed the efficacy of the small molecule and antibody control treatments toward the same targets. In addition, local INTASYL therapy was shown to induce a systemic anti-tumor response with clearance of untreated distal tumors. The animals which showed complete cure of their tumors were then rechallenged over two months after the original treatment of PH-3861 by re-implanting hepatoma cancer cells at a different location to the original tumor. All of the mice that were rechallenged with new tumors were cured again without requiring further treatment, while tumors grew steadily in the control group as expected. We believe that these data demonstrate that local administration of PH-3861 provides a durable and systemic anti-tumor immune response that can combat tumor growth.
Impact of COVID-19 on our Business
While measures to contain and prevent the spread of coronavirus and its variants may be modified or extended, we expect that our activities, including our internal research and development functions, will continue to remain largely operational, though we have experienced and may continue to experience delays in our clinical activities. We have implemented safety measures following the guidance provided by the World Health Organization (the “WHO”), the Centers for Disease Control (the “CDC”) and governmental authorities, such as working remotely and flexible scheduling. We expect to continue following these safety measures and may take further actions as we require, as government authorities require or recommend, or as we determine to be in the best interests of our employees.
| 34 |
Current and future pandemic-related restrictions may further impact our operations and may slow or diminish our research and development activities.
As a result of the coronavirus pandemic, certain of our third-party suppliers and service providers on which we rely have seen impacts to their operations. The Company has undertaken efforts to mitigate potential future impacts by identifying and engaging alternative third-party service providers and suppliers, and because of that, the Company has been able to limit the impact of delays from our third-party service providers to our program’s anticipated timelines. However, the continued impacts to our third-party service providers, including, for example, limited availability of certain services and supplies, began to significantly affect our operations in the second quarter of 2021, resulting in delays to certain of our clinical program timelines. Further, while the steps required for us to initiate our clinical trials with PH-762 are ongoing, the commencement of new clinical trials and the enrollment and participation of patients in clinical trials have been impacted as a result of the coronavirus pandemic, and the Company does not yet know the full extent of similar potential delays or impacts related to its planned clinical activities. If measures to overcome the pandemic are insufficient, the availability of supplies and services that we purchase and rely on could be further reduced or delayed, which may in turn further slow or delay our preclinical and clinical activities.
In May 2020, the Company applied for and received a loan of $231,252 under the Paycheck Protection Program (the “PPP”) as part of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”). In February 2021, the Small Business Administration (the “SBA”) approved the Company’s application for full loan forgiveness, and the full amount of the PPP loan was remitted to the lender for forgiveness. We believe this loan helped to mitigate the financial impact to us of the coronavirus pandemic on our financial condition.
While we believe that the coronavirus pandemic has not had a significant impact on our financial condition to date, the extent to which the pandemic impacts our results will depend on future developments, which are highly uncertain and cannot be predicted. There may be developments outside of our control that require us to adjust our operating plans, including new information which may emerge concerning the actions to contain the coronavirus or treat its impact, among others. We do not yet know the full extent of potential delays or impacts on our business or our preclinical and clinical trial activities, and, therefore, given the nature of the situation, we cannot reasonably estimate the impact of the coronavirus on our financial condition, results of operations or cash flows in the future.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions and could have a material impact on our reported results. While our significant accounting policies are more fully described in the notes to our consolidated financial statements included elsewhere in this Annual Report, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our financial statements.
Research and Development Expenses
Research and development expenses are charged to expense as incurred. Payments made by the Company in advance for research and development services not yet provided and/or for materials not yet received are recorded as prepaid expenses and expensed when the service has been performed or when the goods have been received. Accrued liabilities are recorded with respect to services provided and/or materials that it has received for which vendors have not yet billed the Company. The financial terms of these contracts are subject to negotiation, vary from provider to provider and may result in uneven payment flows. There may be instances in which payments made to our vendors exceed the level of services provided and result in a prepayment of the expense. In other instances, payment depends on factors such as the successful completion of milestones.
| 35 |
We are required to estimate our accrued research and development expenses, of which a significant portion relate to third party providers the Company has contracted with to perform various research activities on our behalf for the continued development of our product candidates. This process includes reviewing open contracts and purchase orders, estimating the service performed and the associated cost incurred for research and development services not yet billed or otherwise notified of actual cost. Accrued liabilities for the services provided by contract research organizations are recorded during the period incurred based on such estimates and assumptions as expected cost, passage of time, the level of effort to be expended in each period, the achievement of milestones and other information available to us. Estimates of our research and development accruals are assessed on a quarterly basis based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and facts and circumstances known to us at that time, and adjusted accordingly.
Actual results may differ from these estimates and could have a material impact on the Company’s reported results. The Company’s historical accrual estimates have not been materially different from its actual costs. Due to the nature of estimates, we cannot provide assurance that we will not make changes to our estimates in the future as we become aware of additional information about the conduct of our research activities.
Stock-based Compensation
The Company follows the provisions of the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 718, “Compensation – Stock Compensation” (“ASC 718”), which requires the measurement and recognition of compensation expense for all stock-based payment awards. The fair value of restricted stock units is based upon our closing stock price at the grant date. We use the Black-Scholes option-pricing model to estimate the fair value of stock options at the grant date. The Black-Scholes valuation model requires the input of valuation assumptions to calculate the value of stock options, including expected volatility, expected term, risk-free interest rate and expected dividends. Stock-based compensation expense is recognized over the requisite service period, which generally represents the vesting period, and commences at the date of grant based on the fair value of the award.
Stock-based compensation expense recognized in the financial statements is based on awards that are ultimately expected to vest. Accordingly, we are also required to estimate forfeitures at the time of grant and to revise those estimates in subsequent periods if actual forfeitures differ from estimates. We use historical data to estimate pre-vesting award forfeitures and record stock-based compensation expense only for those awards that are expected to vest. Our forfeiture rate estimates are based on an analysis of our actual forfeiture experience, employee turnover behavior, and other factors. The impact of any adjustments to our forfeiture rates is recorded as a cumulative adjustment in the period of adjustment. To the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates are revised.
Derivative Financial Instruments
During the normal course of business we may issue warrants to vendors as consideration to perform services. We may also issue warrants as part of a debt or equity financing. Warrants and other derivative financial instruments are accounted for either as equity or as an asset or liability, depending on the characteristics of each derivative financial instrument. Financial instruments that do not meet the definition of a derivative are classified as equity and measured at fair value and recorded as additional paid in capital in stockholders’ equity at the date of issuance. No further adjustments to their valuation are made. Financial instruments that meet the definition of a derivative are classified as an asset or liability are measured at fair value on the issuance date and are revalued on each subsequent balance sheet date. The changes in the fair value are recognized as current period income or loss.
Leases
At the inception of a contract, the Company determines whether the contract is or contains a lease based on all relevant facts and circumstances. For contracts that contain a lease, the Company identifies the lease and non-lease components, determines the consideration in the contract and recognizes the classification of the lease as operating or financing. For leases with a term greater than one year, the Company recognizes a liability to make lease payments and an asset representing the right to use the underlying asset during the lease term at the commencement date of the lease.
Lease liabilities and the corresponding right of use assets are recorded based on the present value of lease payments to be made over the lease term. The discount rate used to calculate the present value is the rate implicit in the lease, or if not readily determinable, the Company’s incremental borrowing rate. The Company’s incremental borrowing rate is the rate of interest that the Company would have to pay to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right of use asset may be required for items such as initial direct costs or incentives received. Lease payments, including scheduled increases, on operating leases are recognized on a straight-line basis over the expected term of the lease. Lease payments on financing leases are recognized using the effective interest method.
| 36 |
Financial Operations Overview
Revenues
To date, we have primarily generated revenues through government grants. We have not generated any commercial product revenue.
In the future, we may generate revenue from a combination of government grants, research and development agreements, license fees and other upfront payments, milestone payments, product sales and royalties in connection with future strategic collaborators and partners. We expect that any revenue we generate will fluctuate from period to period as a result of the timing of the achievement of any preclinical, clinical or commercial milestones and the timing and amount of payments received relating to those milestones and the extent to which any of our product candidates are approved and successfully commercialized by us or strategic collaborators and partners. If the Company or any future partner fails to develop product candidates in a timely manner or obtain regulatory approval for them, then our ability to generate future revenue and our results of operations and financial position would be adversely affected.
Research and Development Expenses
Research and development expenses relate to compensation and benefits for research and development personnel, facility-related expenses, supplies, external services, costs to acquire technology licenses, research activities under our research collaborations, expenses associated with preclinical and clinical development activities and other operating costs. Our research and development programs are focused on the development of immuno-oncology therapeutics based on our INTASYL therapeutic platform. Since we commenced operations, research and development has composed a significant portion of our total operating expenses and is expected to compose the majority of our spending for the foreseeable future.
General and Administrative Expenses
General and administrative expenses relate to compensation and benefits for general and administrative personnel, facility-related expenses, professional fees for legal, audit, tax and consulting services, as well as other general corporate expenses.
Other Income, net
Other income consists primarily of interest income and expense and various income or expense items of a non-recurring nature.
Results of Operations
The following data summarizes our results of operations for the periods indicated, in thousands:
| Years Ended December 31, | Dollar | |||||||||||
| 2021 | 2020 | Change | ||||||||||
| Operating expenses | $ | 13,511 | $ | 8,793 | $ | 4,718 | ||||||
| Operating loss | (13,511 | ) | (8,793 | ) | (4,718 | ) | ||||||
| Net loss | $ | (13,287 | ) | $ | (8,794 | ) | $ | (4,493 | ) | |||
| 37 |
Comparison of the Years Ended December 31, 2021 and 2020
Operating Expenses
The following table summarizes our total operating expenses, for the periods indicated, in thousands:
| Years Ended December 31, | Dollar | |||||||||||
| 2021 | 2020 | Change | ||||||||||
| Research and development | $ | 8,886 | $ | 3,716 | $ | 5,170 | ||||||
| General and administrative | 4,625 | 5,077 | (452 | ) | ||||||||
| Total operating expenses | $ | 13,511 | $ | 8,793 | $ | 4,718 | ||||||
Research and Development Expenses
Research and development expenses for the year ended December 31, 2021 increased 139% compared with the year ended December 31, 2020. The increase in research and development expenses was primarily due to manufacturing costs for the Company’s PH-762 and PH-894 INTASYL compounds, fees for the required preclinical studies in support of the Company’s clinical trials for PH-762 and CRO and consulting related costs to support the initiation of the Company’s clinical trials as compared to the same period in the prior year.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2021 decreased 9% compared with the year ended December 31, 2020. The decrease in general and administrative expenses was primarily due to a decrease in patent and legal fees partially offset by increases in the use of an outside consultant to support business development activities and corporate insurance premiums.
Other Income
Other income for the year ended December 31, 2021 increased by $225,000 as compared with the year ended December 31, 2020, primarily due to the full forgiveness of the Company’s PPP loan by the SBA in the first quarter of 2021.
Liquidity and Capital Resources
Historically, the Company’s primary source of funding has been through the sale of its securities. In the future, we will be dependent on obtaining funding from third parties, such as proceeds from the issuance of debt, sale of equity or strategic opportunities, in order to maintain our operations. We have reported recurring losses from operations since inception and expect that we will continue to have negative cash flows from our operations for the foreseeable future. At December 31, 2021, we had cash of $24,057,000 as compared with $14,244,000 at December 31, 2020.
In August 2019, the Company entered into a purchase agreement (the “Purchase Agreement”) with Lincoln Park Capital, LLC (“LPC”), pursuant to which the Company has the right to sell to LPC up to $10,000,000 in shares of the Company’s common stock, subject to certain limitations and conditions set forth in the agreement. The Company is initially limited to the issuance of 19.99% of the Company’s shares outstanding on the date of the Purchase Agreement unless stockholder approval is obtained to issue more than such amount or the average price of all sales under the Purchase Agreement exceeds certain amounts set forth in the agreement. The Purchase Agreement expires in May 2022. To date, no shares of common stock have been sold to LPC under the Purchase Agreement.
In January 2021, the Company sold 4,420,863 shares of Company common stock at a purchase price per share of $3.07, pre-funded warrants to purchase an aggregate of 140,065 shares of Company common stock at a purchase price per pre-funded warrant share of $3.069, and warrants to purchase an aggregate of 3,420,696 shares of the Company’s common stock with an exercise price of $3.00 per warrant share in a private placement transaction. Net proceeds to the Company were $12,669,000 after deducting placement agent fees and offering expenses.
In February 2021, the Company sold 2,246,784 shares of Company common stock at a purchase price of $3.42 per share in a registered direct offering under the Company’s Form S-3 “shelf” registration statement. Net proceeds to the Company were $6,908,000 after deducting placement agent fees and offering expenses.
| 38 |
We believe that our existing cash at December 31, 2021 should be sufficient to fund operations for at least the next 12 months from the date of the release of the associated financial statements.
The following table summarizes our cash flows for the periods indicated, in thousands:
| Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Net cash used in operating activities | $ | (11,858 | ) | $ | (8,802 | ) | ||
| Net cash used in investing activities | (51 | ) | (19 | ) | ||||
| Net cash provided by financing activities | 21,722 | 16,131 | ||||||
| Net increase in cash and restricted cash | $ | 9,813 | $ | 7,310 | ||||
Net Cash Flow from Operating Activities
Net cash used in operating activities was $11,858,000 for the year ended December 31, 2021, as compared with $8,802,000 for the year ended December 31, 2020. The increase in cash used in operating activities was primarily due to increases in net loss and changes in operating assets and liabilities as a result of increased spending primarily related to the Company’s manufacturing activities and preclinical studies in support of the clinical trials for PH-762.
Net Cash Flow from Investing Activities
Net cash used in investing activities was $51,000 for the year ended December 31, 2021, as compared with $19,000 for the year ended December 31, 2020. The increase in cash used in investing activities was primarily related to laboratory and computer equipment purchases.
Net Cash Flow from Financing Activities
Net cash provided by financing activities was $21,722,000 for the year ended December 31, 2021, as compared with $16,131,000 for the year ended December 31, 2020. The increase in cash provided by financing activities was primarily due to the net proceeds received by the Company from capital raising activities and warrant exercises.
Off-Balance Sheet Arrangements
In connection with certain license agreements, we are required to indemnify the licensor for certain damages arising in connection with the intellectual property rights licensed under the agreement. In addition, we are a party to a number of agreements entered into in the ordinary course of business that contain typical provisions that obligate us to indemnify the other parties to such agreements upon the occurrence of certain events. These indemnification obligations are considered off-balance sheet arrangements in accordance with ASC Topic 460, “Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others.” To date, we have not encountered material costs as a result of such obligations and have not accrued any liabilities related to such obligations in our financial statements. See Note 9 to our consolidated financial statements for further discussion of these indemnification agreements.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
As a smaller reporting company, we are not required to provide this information.
| 39 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| 40 |
Report of Independent Registered Public Accounting Firm
Stockholders and Board of Directors
Phio Pharmaceuticals Corp.
Marlborough, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Phio Pharmaceuticals Corp. (the “Company”) and subsidiary as of December 31, 2021 and 2020, the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Accounting for warrants issued as part of equity offering
As described in Note 10 to the consolidated financial statements, the Company completed a private placement offering in January 2021 that included the issuance of common stock and warrants to purchase common stock. The warrants were evaluated for proper classification on the consolidated balance sheet and it was determined that the warrants issued in this equity offering should be classified within stockholders’ equity.
We identified the accounting for warrants issued as part of the equity offering in January 2021 as a critical audit matter. Our principal considerations included the existence of accounting complexities related to certain provisions of the warrant agreements, including provisions of cash settlement and derivative elements. Auditing these elements required especially challenging auditor judgement and significant audit effort as well as the need for specialized knowledge and skill assessing these elements of the agreement.
The primary procedures we performed to address this critical audit matter included:
| · | Reading the agreements related to the warrants issued along with management’s technical accounting memo to understand the facts and circumstances within the warrant agreements and other assumptions impacting the determination of warrant classification. |
| · | Utilizing personnel with specialized knowledge and skill in debt and equity accounting to evaluate the appropriateness of management’s interpretation on how to apply the relevant accounting guidance for the classification of the warrants issued, including the evaluation of derivative characteristics and the terms associated with the Company’s control that could require cash settlement of the warrants. |
/s/ BDO USA, LLP
We have served as the Company’s auditor since 2011.
Boston, Massachusetts
March 22, 2022
| F-1 |
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share data)
| December 31, 2021 | December 31, 2020 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 24,057 | $ | 14,244 | ||||
| Restricted cash | 50 | 50 | ||||||
| Prepaid expenses and other current assets | 620 | 870 | ||||||
| Total current assets | 24,727 | 15,164 | ||||||
| Right of use asset, net | 283 | 400 | ||||||
| Property and equipment, net | 133 | 157 | ||||||
| Other assets | 27 | 18 | ||||||
| Total assets | $ | 25,170 | $ | 15,739 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 283 | $ | 728 | ||||
| Accrued expenses | 2,660 | 1,352 | ||||||
| Lease liability | 125 | 116 | ||||||
| Total current liabilities | 3,068 | 2,196 | ||||||
| Lease liability, net of current portion | 170 | 295 | ||||||
| Long-term debt | – | 231 | ||||||
| Total liabilities | 3,238 | 2,722 | ||||||
| Commitments and contingencies (Footnote 9) | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $ par value, shares authorized | – | – | ||||||
| Common stock, $ par value, shares authorized; and shares issued and outstanding at December 31, 2021 and December 31, 2020, respectively | 1 | 1 | ||||||
| Additional paid-in capital | 138,831 | 116,629 | ||||||
| Accumulated deficit | (116,900 | ) | (103,613 | ) | ||||
| Total stockholders’ equity | 21,932 | 13,017 | ||||||
| Total liabilities and stockholders’ equity | $ | 25,170 | $ | 15,739 | ||||
See accompanying notes to consolidated financial statements.
| F-2 |
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share data)
| Twelve Months Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 8,886 | $ | 3,716 | ||||
| General and administrative | 4,625 | 5,077 | ||||||
| Total operating expenses | 13,511 | 8,793 | ||||||
| Operating loss | (13,511 | ) | (8,793 | ) | ||||
| Other income (expense) | ||||||||
| Gain on extinguishment of debt | 233 | – | ||||||
| Interest (expense) income, net | (9 | ) | (1 | ) | ||||
| Total other income (expense) | 224 | (1 | ) | |||||
| Loss before income taxes | (13,287 | ) | (8,794 | ) | ||||
| Provision for income taxes | – | – | ||||||
| Net loss | $ | (13,287 | ) | $ | (8,794 | ) | ||
| Net loss per share: | ||||||||
| Basic and diluted | $ | (1.04 | ) | $ | (1.92 | ) | ||
| Weighted average number of common shares outstanding | ||||||||
| Basic and diluted | 12,830,809 | 4,587,346 | ||||||
See accompanying notes to consolidated financial statements.
| F-3 |
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(Amounts in thousands, except share data)
| Common Stock | Additional Paid-in | Accumulated | ||||||||||||||||||
| Shares | Amount | Capital | Deficit | Total | ||||||||||||||||
| Balance at December 31, 2019 | 669,433 | $ | 1 | $ | 100,566 | $ | (94,819 | ) | $ | 5,748 | ||||||||||
| Cash in lieu of fractional shares for 1:55 reverse stock split | (1,364 | ) | (15 | ) | (15 | ) | ||||||||||||||
| Issuance of common stock under employee stock purchase plan | 153 | 1 | 1 | |||||||||||||||||
| Issuance of common stock and warrants in connection with registered direct and private placement offerings, net of offering costs | 1,910,120 | 4,994 | 4,994 | |||||||||||||||||
| Issuance of common stock, pre-funded warrants and warrants in connection with underwritten public offering, net of offering costs | 993,633 | 7,093 | 7,093 | |||||||||||||||||
| Issuance of common stock upon the exercise of warrants | 2,205,663 | 3,856 | 3,856 | |||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | 3,335 | (2 | ) | (2 | ) | |||||||||||||||
| Stock-based compensation expense | – | 136 | 136 | |||||||||||||||||
| Net loss | – | (8,794 | ) | (8,794 | ) | |||||||||||||||
| Balance at December 31, 2020 | 5,780,973 | 1 | 116,629 | (103,613 | ) | 13,017 | ||||||||||||||
| Issuance of common stock, pre-funded warrants and warrants in connection with private placement, net of offering costs | 4,420,863 | 12,669 | 12,669 | |||||||||||||||||
| Issuance of common stock in registered direct offering, net of offering costs | 2,246,784 | 6,908 | 6,908 | |||||||||||||||||
| Issuance of common stock upon the exercise of warrants | 1,083,321 | 2,146 | 2,146 | |||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | 3,055 | (1 | ) | (1 | ) | |||||||||||||||
| Stock-based compensation expense | – | 480 | 480 | |||||||||||||||||
| Net loss | – | (13,287 | ) | (13,287 | ) | |||||||||||||||
| Balance at December 31, 2021 | 13,534,996 | $ | 1 | $ | 138,831 | $ | (116,900 | ) | $ | 21,932 | ||||||||||
See accompanying notes to consolidated financial statements.
| F-4 |
PHIO PHARMACEUTICALS CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
Twelve Months Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (13,287 | ) | $ | (8,794 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 75 | 72 | ||||||
| Non-cash lease expense | 117 | 111 | ||||||
| Non-cash stock-based compensation | 480 | 136 | ||||||
| Forgiveness of debt | (233 | ) | – | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other assets | 241 | (554 | ) | |||||
| Accounts payable | (445 | ) | (81 | ) | ||||
| Accrued expenses and other liabilities | 1,310 | 415 | ||||||
| Lease liability | (116 | ) | (107 | ) | ||||
| Net cash used in operating activities | (11,858 | ) | (8,802 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Cash paid for purchase of property and equipment | (51 | ) | (19 | ) | ||||
| Net cash used in investing activities | (51 | ) | (19 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Net proceeds from the issuance of common stock and warrants | 19,577 | 12,088 | ||||||
| Net proceeds from the exercise of warrants | 2,146 | 3,856 | ||||||
| Proceeds from debt | – | 231 | ||||||
| Cash paid in lieu of fractional shares for 1:55 reverse stock split | – | (15 | ) | |||||
| Payments of taxes for net share settled restricted stock unit issuances | (1 | ) | (2 | ) | ||||
| Payments of capital lease obligations less than one year | – | (27 | ) | |||||
| Net cash provided by financing activities | 21,722 | 16,131 | ||||||
| Net increase in cash and restricted cash | 9,813 | 7,310 | ||||||
| Cash and restricted cash at the beginning of period | 14,294 | 6,984 | ||||||
| Cash and restricted cash at the end of period | $ | 24,107 | $ | 14,294 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Interest paid | $ | 8 | $ | 6 | ||||
See accompanying notes to consolidated financial statements.
| F-5 |
PHIO PHARMACEUTICALS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Operations
Phio Pharmaceuticals Corp. (“Phio,” “we,” “our” or the “Company”) is seeking to address the biggest challenges in immuno-oncology by creating new pathways to a cancer-free future for patients. We are developing therapeutics that leverage our INTASYL™ technology to target both tumor and immune cells by regulating genes to strengthen a patient’s immune system while weakening tumor defense mechanisms. With our INTASYL self-delivering RNAi technology, we aim to bring the benefits of RNA therapeutics into cancer care where other modalities may fall short.
Our operations are being conducted in accordance with guidance provided by the World Health Organization, the Center for Disease Control and governmental authorities, including the implementation of safety measures such as working remotely and flexible scheduling. We expect to continue following these safety measures and may take further actions as we require, as government authorities require or recommend, or as we determine to be in the best interests of our employees.
As a result of the coronavirus pandemic, certain of our third-party providers on which we rely have seen impacts to their operations. The Company had and continues to undertake efforts to mitigate potential future impacts by identifying and engaging alternative third-party providers to limit the impact of potential delays on our program’s anticipated timelines. However, the continued impacts to these third-party providers, including, for example, limited availability of certain services and supplies, began significantly affecting our operations in the second quarter of 2021, which resulted in delays to the start of our clinical trials. If measures to overcome the pandemic are insufficient, it could further reduce or delay the availability of supplies and services that we purchase and rely on, which may in turn further slow or delay our preclinical and clinical activities.
We believe that the coronavirus pandemic has not had a significant impact on our financial condition to date; however a variety of factors such as those described above, may further impact our operations and slow or diminish our research and development activities, which in turn may impact our financial condition in the future. The extent to which the coronavirus pandemic impacts our results will depend on future developments, which are highly uncertain and cannot be predicted.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). Additionally, certain prior year amounts have been reclassified for consistency with the current year presentation. The Company made an adjustment to the consolidated statements of operations to reflect patent costs within general and administrative operating expenses in the consolidated statements of operations. The reclassification increased general and administrative operating expenses and reduced research and development operating expenses by $715,000 for the year ended December 31, 2020. This reclassification had no effect on total operating expenses, net loss, net loss per share and had no impact on the Company’s consolidated balance sheets, statement of stockholders’ equity and statement of cash flows for the prior year period.
Principles of Consolidation
The consolidated financial statements include the accounts of Phio and its wholly-owned subsidiary, MirImmune, LLC. All material intercompany accounts have been eliminated in consolidation.
| F-6 |
Uses of Estimates in Preparation of Financial Statements
The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The areas subject to significant estimates and judgement include, among others, those related to the fair value of equity awards, accruals for research and development expenses, useful lives of property and equipment, income taxes, and our valuation allowance on our deferred tax assets. On an ongoing basis we evaluate our estimates and base our estimates on historical experience and other relevant assumptions that we believe are reasonable under the circumstances, including as a result of new information that may emerge concerning the coronavirus pandemic. We have made estimates of the impact of the coronavirus pandemic within our financial statements and there may be changes to those estimates in future periods. Actual results could differ materially from these estimates.
Restricted Cash
Restricted cash consists of certificates of deposit held by financial institutions as collateral for the Company’s corporate credit cards. The following table provides a reconciliation of cash and restricted cash reported within the balance sheet that sum to the total of the same such amounts shown in the statement of cash flows (in thousands):
| Schedule of Cash | ||||||||
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash | $ | 24,057 | $ | 14,244 | ||||
| Restricted cash | 50 | 50 | ||||||
| Cash and restricted cash shown in the statement of cash flows | $ | 24,107 | $ | 14,294 | ||||
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash. The Company maintains cash balances in several accounts with a financial institution that management believes is creditworthy, which at times are in excess of federally insured limits. These accounts are insured by the Federal Deposit Insurance Corporation (the “FDIC”) for up to $250,000 per institution.
Property and Equipment
Property and equipment are stated at cost and depreciated using the straight-line method based on the estimated useful lives of the related assets. The Company provides for depreciation over the assets’ estimated useful lives as follows:
| Schedule of estimated useful lives | |
| Computer equipment | 3 years |
| Machinery & equipment | 5 years |
| Furniture & fixtures | 5 years |
| Leasehold improvements | 5 years |
Impairment of Long-Lived Assets
The Company reviews long-lived assets for impairment whenever an event occurs or change in circumstances that the related carrying amounts may not be recoverable. An impairment loss would be recognized based on the difference between the carrying value of the asset and its estimated fair value, which would be determined based on either discounted future cash flows or other appropriate fair value methods. The Company believes no impairment existed as of December 31, 2021 or 2020.
Fair Value of Financial Instruments
The carrying amounts reported in the balance sheet for restricted cash, accounts payable and accrued expenses approximate their fair values due to their short-term nature.
The Company follows the provisions of the FASB ASC Topic 820, “Fair Value Measurements and Disclosures,” for the Company’s financial assets and liabilities that are re-measured and reported at fair value each reporting period and are re-measured and reported at fair value at least annual using a fair value hierarchy that is broken down into three levels. Level inputs are defined as follows:
Level 1 – quoted prices in active markets for identical assets or liabilities.
Level 2 – other significant observable inputs for the assets or liabilities through corroboration with market data at the measurement date.
Level 3 – significant unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities at the measurement date.
For the years ended December 31, 2021 and 2020, the Company categorized its restricted cash of $50,000 as Level 2 hierarchy. The assets classified as Level 2 have initially been valued at the applicable transaction price and subsequently valued, at the end of each reporting period, using other market observable data. Observable market data points include quoted prices, interest rates, reportable trades and other industry and economic events.
| F-7 |
Leases
At the inception of a contract, the Company determines whether the contract is or contains a lease based on all relevant facts and circumstances. For contracts that contain a lease, the Company identifies the lease and non-lease components, determines the consideration in the contract and recognizes the classification of the lease as operating or financing. For leases with a term greater than one year, the Company recognizes a liability to make lease payments and an asset representing the right to use the underlying asset during the lease term at the commencement date of the lease.
Lease liabilities and the corresponding right of use assets are recorded based on the present value of lease payments to be made over the lease term. The discount rate used to calculate the present value is the rate implicit in the lease, or if not readily determinable, the Company’s incremental borrowing rate. The Company’s incremental borrowing rate is the rate of interest that the Company would have to pay to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right of use asset may be required for items such as initial direct costs or incentives received. Lease payments on operating leases, including scheduled increases, are recognized on a straight-line basis over the expected term of the lease. Lease payments on financing leases are recognized using the effective interest method.
Debt
On March 27, 2020, the United States enacted the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) in response to the coronavirus pandemic. The CARES Act is an emergency economic stimulus package passed in response to the coronavirus outbreak that includes, but is not limited to, provisions providing aid to small businesses in the form of loans and grants and numerous tax provisions including, certain payroll tax benefits, changes to the net operating loss rules and changes to the business interest expense deduction rules. In May 2020, the Company received loan proceeds pursuant to the Paycheck Protection Program (the “PPP”) offered under the CARES Act, and the loan was subsequently forgiven in February 2021. Outside of the PPP, the Company has not utilized the other CARES Act loan programs and tax provisions, such as certain payroll tax benefits.
The Company followed the guidance under the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 470, “Debt” (“ASC 470”) in assessing the accounting for the PPP loan proceeds. Per ASC 470, the Company recorded a liability on the balance sheet for the full amount of the PPP loan proceeds received and accrued interest over the term of the loan. Upon loan forgiveness, the Company recognized the extinguishment of the liability in the consolidated statement of operations as a gain on extinguishment of debt.
Derivative Financial Instruments
Financial instruments that meet the definition of a derivative are classified as an asset or liability and measured at fair value on the issuance date and are revalued on each subsequent balance sheet date. The changes in fair value are recognized as current period income or loss. Financial instruments that do not meet the definition of a derivative are classified as equity and measured at fair value and recorded as additional paid-in capital in stockholders’ equity at the date of issuance. No further adjustments to their valuation are made.
Research and Development Expenses
Research and development expenses relate to compensation and benefits for research and development personnel, facility-related expenses, supplies, external services, costs to acquire technology licenses, research activities under our research collaborations, expenses associated with preclinical and clinical development activities and other operating costs. Research and development expenses are charged to expense as incurred. Payments made by the Company in advance for research and development services not yet provided and/or for materials not yet received are recorded as prepaid expenses and expensed when the service has been performed or when the goods have been received.
Accrued liabilities are recorded related to those expenses for which vendors have not yet billed the Company with respect to services provided and/or materials that it has received. Accrued liabilities for the services provided by contract research organizations are recorded during the period incurred based on such estimates and assumptions as expected cost, passage of time, the achievement of milestones and other information available to us and are assessed on a quarterly basis. Actual results may differ from these estimates and could have a material impact on the Company’s reported results. The Company’s historical accrual estimates have not been materially different from its actual costs.
| F-8 |
Collaborative Arrangements
The Company follows the provisions of the FASB ASC Topic 808, “Collaborative Arrangements,” (“Topic 808”) when collaboration agreements involve joint operating activities in which both parties are active participants and that are also both exposed to significant risks and rewards. The Company also considers the guidance in the FASB ASC Topic 606, “Revenue from Contracts with Customers,” (“Topic 606”) in determining the appropriate treatment for activities between the Company and its collaborative partners that are more reflective of a vendor-customer relationship and therefore, within the scope of Topic 606. Under Topic 808, the Company determines an appropriate recognition method, either by analogy to appropriate accounting literature or by applying a reasonable accounting policy election. Generally, the classification of transactions under the collaborative arrangements is determined based on the nature and contractual terms of the arrangement along with the nature of the operations of the participants. Payments or reimbursements that are the result of a collaborative relationship instead of a customer relationship, such as co-development activities, are recorded as increases or decreases to research and development expense.
Patents and Patent Application Costs
Although the Company believes that its patents and underlying technology have continuing value, the amount of future benefits to be derived from the patents is uncertain. Patent costs are, therefore, expensed as general and administrative costs as incurred.
The Company follows the provisions of the FASB ASC Topic 718, “Compensation — Stock Compensation” (“ASC 718”), which requires the measurement and recognition of compensation expense for all stock-based payment awards. The fair value of restricted stock units is based upon the Company’s closing stock price at the grant date. The Company uses the Black-Scholes option-pricing model to estimate the fair value of stock options at the grant date. The Black-Scholes valuation model requires the input of valuation assumptions to calculate the value of stock options, including expected volatility, expected term, risk-free interest rate and expected dividends. Stock-based compensation expense is recognized over the requisite service period, which generally represents the vesting period, and commences at the date of grant based on the fair value of the award.
Stock-based compensation expense recognized in the financial statements is based on awards that are ultimately expected to vest. Accordingly, we are also required to estimate forfeitures at the time of grant and to revise those estimates in subsequent periods if actual forfeitures differ from estimates. We use historical data to estimate pre-vesting award forfeitures and record stock-based compensation expense only for those awards that are expected to vest. Our forfeiture rate estimates are based on an analysis of our actual forfeiture experience, employee turnover behavior, and other factors. The impact of any adjustments to our forfeiture rates is recorded as a cumulative adjustment in the period of adjustment. To the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates are revised.
Income Taxes
The Company recognizes assets or liabilities for the deferred tax consequences of temporary differences between the tax basis of assets or liabilities and their reported amounts in the financial statements in accordance with the FASB ASC Topic 740, “Accounting for Income Taxes” (“ASC 740”). These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled. Those temporary differences referred to as deferred tax assets and liabilities are determined at the end of each period using the tax rate expected to be in effect when taxes are actually paid or recovered. Valuation allowances are established if, based on the weight of available evidence, it is more likely than not that all or a portion of a deferred tax asset will not be realized. The provision for income taxes, if any, represents the tax payable for the period and the change in deferred income tax assets and liabilities during the period.
Immaterial Revision
In 2021, the Company completed an Internal Revenue Code Section 382 analysis of its historical net operating loss carry-forward amount. As a result, the prior year net operating loss carry-forward was determined to be limited. See Note 13 Income Taxes, for further details.
| F-9 |
Comprehensive Loss
The Company’s comprehensive loss is equal to its net loss for all periods presented.
Basic net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding. Diluted net loss per share is computed by dividing the Company’s net loss by the weighted average number of common shares outstanding and the impact of all dilutive potential common shares outstanding, except where such dilutive potential common shares would be anti-dilutive. Dilutive potential common shares primarily consist of warrants, restricted stock units and stock options.
3. Liquidity and Going Concern
The Company has reported recurring losses from operations since its inception and expects to continue to have negative cash flows from operations for the foreseeable future. Historically, the Company’s primary source of funding has been from sales of its securities. The Company’s ability to continue to fund its operations is dependent on obtaining funding from third parties, such as proceeds from the issuance of debt, sale of equity, or strategic opportunities, in order to maintain its operations. This is dependent on a number of factors, including the market demand or liquidity of the Company’s common stock. There is no guarantee that debt, additional equity or other funding will be available to us on acceptable terms, or at all. If we fail to obtain additional funding when needed, we would be forced to scale back or terminate our operations or seek to merge with or to be acquired by another company.
While we believe that the coronavirus pandemic has not had a significant impact on our financial condition and results of operations at this time, the potential economic impact brought by, and the duration of, the coronavirus pandemic is difficult to assess or predict. There may be developments outside of our control that require us to adjust our operating plans and given the nature of the situation, we cannot reasonably estimate the impact of the coronavirus pandemic on our financial conditions, results of operations or cash flows in the future.
The Company believes that its existing cash should be sufficient to fund operations for at least the next 12 months from the date of the release of these financial statements.
4. Recent Accounting Pronouncements
In December 2019, the FASB issued Accounting Standards Update (“ASU”) 2019-12, “Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes” (“ASU 2019-12”). The amendments in the update simplify the accounting for income taxes by eliminating the exceptions related to the incremental approach for intraperiod tax allocation, the recognition of a deferred tax liability for equity method investments, not recognizing a deferred tax liability for a foreign subsidiary and the general methodology for calculating income taxes in an interim period. The amendments also clarify and simplify other aspects of the accounting for income taxes. The amendments in ASU 2019-12 are effective for public entities for fiscal years, and the interim periods within those fiscal years, beginning after December 20, 2020. The Company adopted ASU 2019-12 on January 1, 2021. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, “Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815 – 40)” (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts in an entity’s own equity. ASU 2020-06 is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. For convertible instruments, the accounting models for instruments issued with beneficial conversion features or cash conversion features are removed. For contracts in an entity’s own equity, ASU 2020-06 simplifies the settlement assessment by removing the requirements to (1) consider whether the contract would be settled in registered shares, (2) consider whether collateral is required to be posted, and (3) assess shareholder rights. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, and interim periods within those fiscal years. The Company early adopted ASU 2020-06 on January 1, 2021. The adoption of this standard did not have an impact on the Company’s consolidated financial statements.
| F-10 |
In May 2021, the FASB issued ASU 2021-04, “Earnings per Share (Topic 260), Debt – Modifications and Extinguishments (Subtopic 470-50), Compensation – Stock Compensation (Topic 718), and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40)” (“ASU 2021-04”). The amendments in the updates are intended to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options that remain equity classified after modification or exchange. The amendments in ASU 2021-04 are effective for all entities for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. An entity should apply the amendments prospectively to modifications or exchanges occurring on or after the effective date of the amendments. Early adoption is permitted for all entities, including within an interim period. The Company does not expect the adoption of ASU 2021-04 to have a material impact on its consolidated financial statements.
5. Property and Equipment
The following table summarizes the Company’s major classes of property and equipment:
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Computer equipment | $ | 108 | $ | 195 | ||||
| Machinery & equipment | 1,035 | 992 | ||||||
| Furniture & fixtures | 49 | 46 | ||||||
| Leasehold improvements | 46 | 46 | ||||||
| Total gross fixed assets | 1,238 | 1,279 | ||||||
| Less: accumulated depreciation and amortization | (1,105 | ) | (1,122 | ) | ||||
| Property and equipment, net | $ | 133 | $ | 157 | ||||
Depreciation and amortization expense for the years ended December 31, 2021 and 2020 was $75,000 and $72,000, respectively.
6. Accrued Expenses
Accrued expenses consist of the following, in thousands:
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Compensation and benefits | $ | 572 | $ | 618 | ||||
| Professional fees | 102 | 81 | ||||||
| Research and development costs | 1,986 | 549 | ||||||
| Other | 104 | |||||||
| Total accrued expenses and other current liabilities | $ | 2,660 | $ | 1,352 | ||||
7. Leases
In January 2019, the Company amended the lease for its corporate headquarters and primary research facility in Marlborough, Massachusetts. The lease is for a total of 7,581 square feet of office and laboratory space and will expire on March 31, 2024. The lease contains an option to terminate after two or three years by providing advance written notice of termination pursuant to the terms of the agreement. The exercise of this option was not determined to be reasonably certain and thus was not included in the lease liability on the Company’s balance sheet. The Company did not exercise its option to terminate in either the second or third year of the lease, and the option to terminate has expired. Additionally, the lease agreement did not contain information to determine the borrowing rate implicit in the lease. As such, the Company calculated its incremental borrowing rate based on what the Company would have to pay to borrow on a collateralized basis over the lease term for an amount equal to the remaining lease payments, taking into consideration such assumptions as, but not limited to, the U.S. treasury yield rate and borrowing rates from a creditworthy financial institution using the above lease factors.
| F-11 |
The lease for our corporate headquarters represents substantially all of our significant lease obligations. The amounts reported in the consolidated balance sheets for operating leases in which the Company is the lessee and other supplemental balance sheet information is set forth as follows, in thousands, except the lease term (number of years) and discount rate:
| December 31, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Right of use asset | $ | 283 | $ | 400 | ||||
| Liabilities | ||||||||
| Lease liability, current | 125 | 116 | ||||||
| Lease liability, non-current | 170 | 295 | ||||||
| Total lease liability | $ | 295 | $ | 411 | ||||
| Lease Term and Discount Rate | ||||||||
| Weighted average remaining lease term | 2.25 | 3.25 | ||||||
| Weighted average discount rate | 4.70% | 4.70% | ||||||
Operating lease costs included in operating expense were $132,000 and $132,000 for the years ended December 31, 2021 and 2020, respectively. Short-term lease costs were not material for the years ended December 31, 2021 and 2020.
Cash paid for the amounts included in the measurement of the operating lease liability on the Company’s consolidated balance sheets and included within changes in the lease liability in the operating activities of our consolidated statement of cash flows was $131,600 and $127,800 for the years ended December 31, 2021 and 2020, respectively.
Future lease payments for our non-cancellable operating leases and a reconciliation to the carrying amount of the operating lease liability presented in the consolidated balance sheet as of December 31, 2021 is as follows, in thousands:
| 2022 | $ | 135 | ||
| 2023 | 140 | |||
| 2024 | 35 | |||
| Total lease payments | 310 | |||
| Less: Imputed interest | (15 | ) | ||
| Total operating lease liabilities (includes current portion) | $ | 295 |
8. Debt
In May 2020, the Company received loan proceeds in the amount of $231,252 from Bank of America, N.A., as lender, pursuant to the PPP under the CARES Act. The PPP loan had a maturity date of May 11, 2022, interest at a rate of 1% per year and monthly principal and interest payments that were deferred to the date that the Small Business Administration (the “SBA”) remitted the borrower’s loan forgiveness amount to the lender. When applying for the PPP loan, the Company carefully assessed the requirements for application under the program and believed that the loan was necessary to support its operations. The loans under the PPP may be forgiven if used for eligible purposes, including payroll, benefits, rent and utilities.
The Company followed the guidance under ASC 470 in assessing the accounting for the PPP loan proceeds. Per ASC 470, the Company recorded a liability on the balance sheet for the full amount of PPP loan proceeds received and accrued interest over the term of the loan. The Company believed it used the loan proceeds for eligible purposes and applied for full loan forgiveness. In February 2021, the SBA approved the Company’s application for full loan forgiveness, and the full amount of the PPP loan was remitted to the lender for forgiveness. Upon loan forgiveness, the Company recognized a gain on the extinguishment of debt of $233,000 for the loan proceeds received and interest accrued in the consolidated statements of operations for the year ended December 31, 2021.
| F-12 |
9. Commitments and Contingencies
License Commitments
The Company acquires assets under development and enters into research and development arrangements with third parties that often require milestone and royalty payments based on the progress of the asset through development stages. Milestone payments may be required, for example, upon approval of the product for marketing by a regulatory agency. In certain agreements, the Company is required to make royalty payments based upon a percentage of the sales of the products licensed pursuant to such agreements. Because of the contingent nature of these payments, they are not included in the table of contractual obligations shown below. During the years ended December 31, 2021 and 2020, the Company did not trigger any milestone payments.
The Company’s contractual license obligations that will require future cash payments as of December 31, 2021 are as follows, in thousands:
| Year Ending December 31, | ||||
| 2022 | $ | 100 | ||
| 2023 | 100 | |||
| 2024 | 100 | |||
| 2025 | 100 | |||
| 2026 | 100 | |||
| Thereafter | 300 | |||
| Total | $ | 800 | ||
The Company applies the disclosure provisions of the FASB ASC Topic 460, “Guarantor’s Accounting and Disclosure Requirements for Guarantees, Including Indirect Guarantees of Indebtedness of Others” (“ASC 460”), to its agreements that contain guarantee or indemnification clauses. The Company provides: (i) indemnifications of varying scope and size to certain investors and other parties for certain losses suffered or incurred by the indemnified party in connection with various types of third-party claims; and (ii) indemnifications of varying scope and size to officers and directors against third-party claims arising from the services they provide to us. These indemnifications give rise only to the disclosure provisions of ASC 460. To date, the Company has not incurred costs as a result of these obligations and does not expect to incur material costs in the future. Accordingly, the Company has not accrued any liabilities in its consolidated financial statements related to these indemnifications.
Litigation
From time to time, the Company is party to legal proceedings. There are none deemed to be material at this time. Accordingly, the Company has not accrued any liabilities in its consolidated financial statements related to proceedings.
10. Stockholders’ Equity
January 2021 Private Placement — On January 25, 2021, the Company completed a private placement of shares of the Company’s common stock at a purchase price per share of $, pre-funded warrants to purchase an aggregate of shares of the Company’s common stock (the “January 2021 Pre-Funded Warrants”) at a purchase price per pre-funded warrant share of $ and warrants to purchase an aggregate of shares of the Company’s common stock with an exercise price of $3.00 per warrant share (the “January 2021 Warrants”) (the “Private Placement”). In connection with the Private Placement, the Company issued warrants to the placement agent, H.C. Wainwright & Co., LLC (“HCW”), to purchase a total of shares of the Company’s common stock at an exercise price of $3.8375 (the “January 2021 Placement Agent Warrants”). Net proceeds to the Company from the Private Placement were $12,669,000 after deducting placement agent fees and offering expenses.
February 2021 Registered Direct Offering — On February 17, 2021, the Company completed a registered direct offering of shares of the Company’s common stock at a purchase price of $per share (the “Offering”). In connection with the Offering, the Company issued warrants to the placement agent, HCW, to purchase a total of shares of the Company’s common stock at an exercise price of $4.275 (the “February 2021 Placement Agent Warrants”). Net proceeds to the Company from the Offering were $6,908,000 after deducting placement agent fees and offering expenses.
| F-13 |
February 2020 Registered Direct Offering and Private Placement — On February 6, 2020, the Company completed a registered direct offering (the “February 2020 Registered Offering”) of shares of the Company’s common stock at a purchase price of $per share and in a concurrent private placement, sold warrants to purchase an aggregate of shares of the Company’s common stock at a purchase price of $per underlying warrant share and with an exercise price of $8.71 per share (the “February 2020 Registered Direct Warrants”). In connection with the February 2020 Registered Offering, the Company also issued warrants to purchase a total of shares of the Company’s common stock with an exercise price of $11.0375 per share (the “February 2020 Placement Agent Warrants”) to the placement agent, HCW. Net proceeds to the Company from the February 2020 Registered Offering were $1,467,000 after deducting placement agent fees and offering expenses paid by the Company.
February 2020 Underwritten Public Offering — On February 13, 2020, the Company completed an underwritten public offering of shares of the Company’s common stock at a purchase price per share of $, pre-funded warrants (the “2020 Pre-Funded Warrants”) to purchase an aggregate of shares of the Company’s common stock at a purchase price per pre-funded warrant share of $ and warrants (the “February 2020 Warrants”) to purchase an aggregate of shares of the Company’s common stock with an exercise price of $4.00 per warrant share (the “February 2020 Underwritten Offering”). The 2020 Pre-Funded Warrants were immediately exercisable at an exercise price per share of $0.001 and each share of Company common stock or 2020 Pre-Funded Warrant, as applicable, was sold with a February 2020 Warrant. In connection with the February 2020 Underwritten Offering, the Company issued warrants to purchase up to shares of Company common stock, immediately exercisable at an exercise price of $5.00 per share (the “February 2020 Underwriter Warrants”) to HCW, as underwriter.
In connection with the February 2020 Underwritten Offering, the Company also granted to the underwriter, HCW, a 30-day option to purchase up to an additional shares of the Company’s common stock at a purchase price of $ per such share and/or warrants to purchase up to shares of the Company’s common stock at a purchase price of $per such warrant. Such warrants have the same terms as the February 2020 Warrants. On February 12, 2020, HCW exercised its option to purchase warrants to purchase an aggregate of shares of the Company’s common stock.
Net proceeds from the February 2020 Underwritten Offering were $7,093,000 after deducting underwriting discounts and commissions and offering expenses paid by the Company.
April 2020 Registered Direct Offering and Private Placement — On April 2, 2020, the Company completed a registered direct offering (the “April 2020 Offering”) of shares of the Company’s common stock at a purchase price of $2.21 per share and in a concurrent private placement, sold warrants to purchase an aggregate of shares of the Company’s common stock at a purchase price of $ per underlying warrant share and with an exercise price of $per share (the “April 2020 Warrants”). In connection with the April 2020 Offering, the Company also issued warrants to purchase a total of shares of the Company’s common stock with an exercise price of $2.9188 per share (the “April 2020 Placement Agent Warrants”) to the placement agent, HCW. Net proceeds to the Company from the April 2020 Offering were $3,527,000 after deducting placement agent fees and offering expenses paid by the Company.
Warrants
The Company first assesses the warrants it issues under the FASB ASC Topic 480, “Distinguishing Liabilities from Equity” (“ASC 480”) to determine whether they are within the scope of ASC 480. As there were no instances outside of the Company’s control that could require cash settlement of the warrants issued in the Private Placement and Offering, as well as warrants issued in the Company’s prior financing transactions, the Company’s outstanding warrants are outside the scope of ASC 480.
The Company then applies and follows the applicable accounting guidance in ASC 815. Financial instruments are accounted for as either derivative liabilities or as equity instruments depending on the specific terms of the agreement. The warrants issued in the Private Placement and the Offering do not meet the definition of a derivative instrument as they are indexed to the Company’s common stock and classified within stockholders’ equity, as are the warrants issued in the Company’s prior financing transactions. Based on this determination, the Company’s warrants are classified within stockholders’ equity.
| F-14 |
The following table summarizes the Company’s outstanding equity-classified warrants at December 31, 2021:
| Exercise | Expiration | Balance December 31, | Warrants | Warrants | Warrants | Balance December 30, | ||||||||||||||||||||
| Description | Price | Date | 2020 | Issued | Exercised | Expired | 2021 | |||||||||||||||||||
| December 2016 Warrants | $ | 495.00 | 12/21/2021 | 23,233 | (23,233 | ) | – | |||||||||||||||||||
| April 2018 Warrants | $ | 173.25 | 5/31/2023 | 20,599 | 20,599 | |||||||||||||||||||||
| April 2018 Placement Agent Warrants | $ | 223.00 | 4/9/2023 | 1,373 | 1,373 | |||||||||||||||||||||
| October 2018 Warrants | $ | 10.45 | 10/3/2025 | 389,610 | 389,610 | |||||||||||||||||||||
| October 2018 Underwriter Warrants | $ | 13.06 | 10/1/2023 | 29,220 | 29,220 | |||||||||||||||||||||
| November 2019 Placement Agent Warrants | $ | 6.875 | 11/18/2024 | 13,636 | 13,636 | |||||||||||||||||||||
| February 2020 Registered Direct Warrants | $ | 8.71 | 8/6/2025 | 197,056 | 197,056 | |||||||||||||||||||||
| February 2020 Placement Agent Warrants | $ | 11.0375 | 2/4/2025 | 14,779 | 14,779 | |||||||||||||||||||||
| February 2020 Warrants | $ | 4.00 | 2/13/2025 | 1,326,500 | 1,326,500 | |||||||||||||||||||||
| February 2020 Underwriter Warrants | $ | 5.00 | 2/11/2025 | 150,000 | 150,000 | |||||||||||||||||||||
| April 2020 Warrants | $ | 2.21 | 10/2/2025 | 1,284,798 | (856,532 | ) | 428,266 | |||||||||||||||||||
| April 2020 Placement Agent Warrants | $ | 2.9188 | 3/31/2025 | 128,480 | (86,724 | ) | 41,756 | |||||||||||||||||||
| January 2021 Pre-Funded Warrants | $ | 0.001 | No expiration | 140,065 | (140,065 | ) | – | |||||||||||||||||||
| January 2021 Warrants | $ | 3.00 | 7/27/2026 | 3,420,696 | 3,420,696 | |||||||||||||||||||||
| January 2021 Placement Agent Warrants | $ | 3.8375 | 7/27/2026 | 342,070 | 342,070 | |||||||||||||||||||||
| February 2021 Placement Agent Warrants | $ | 4.275 | 2/12/2026 | 168,509 | 168,509 | |||||||||||||||||||||
| 3,579,284 | 4,071,340 | (1,083,321 | ) | (23,233 | ) | 6,544,070 | ||||||||||||||||||||
| F-15 |
The Company received net proceeds of $2,146,000 and $3,856,000 from the exercise of warrants during the years ended December 31, 2021 and 2020, respectively.
Of the warrants exercised during the year ended December 31, 2020, of the Company’s April 2020 Warrants were exercised via a cashless exercise transaction and as a result a total of shares of common stock were issued. There were no such cashless exercises of warrants during the year ended December 31, 2021.
The following table sets forth the potential common shares excluded from the calculation of net loss per share because their inclusion would be anti-dilutive:
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Options to purchase common stock | 2,499 | 2,570 | ||||||
| Unvested restricted stock units | 367,101 | 9,699 | ||||||
| Warrants to purchase common stock | 6,544,070 | 3,579,284 | ||||||
| Total | 6,913,670 | 3,591,553 | ||||||
Stock Plans
The Company’s approved equity plans include the Phio Pharmaceuticals Corp. 2020 Long Term Incentive Plan (the “2020 Plan”) and the Phio Pharmaceuticals Corp. 2012 Long Term Incentive Plan (the “2012 Plan”). These plans are administered by our board of directors and provide for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, and performance cash awards. Upon adoption of the 2020 Plan, shares that remained available for grant under the 2012 Plan and shares that were subject to outstanding awards under the 2012 Plan were included in the authorized shares available for grant under the 2020 Plan. Further, upon adoption of the 2020 Plan, the Company no longer grants new equity awards under the 2012 Plan.
As of December 31, 2021, there were an aggregate of shares of common stock reserved under the 2020 Plan, including shares subject to outstanding stock options and shares subject to unvested restricted stock units (“RSUs”) and shares available for future grants. RSUs granted by the Company to employees vest annually over 3 years after the grant date, stock options granted by the Company to employees generally vest annually over 4 years after the grant date and, in the instance of stock options, expire within ten years of issuance.
Restricted Stock Units
RSUs are issued under the 2020 Plan or as inducement grants issued outside of the plan to new employees. RSUs are generally subject to graded vesting and the satisfaction of service requirements. Upon vesting, each outstanding RSU will be exchanged for one share of the Company’s common stock. Employee RSU recipients may elect to net share settle upon vesting, in which case the Company pays the employee’s income taxes due upon vesting and withholds a number of shares of equal value. The fair value of the RSUs awarded are based upon the Company’s closing stock price at the grant date and are expensed over the requisite service period.
| F-16 |
The following table summarizes the activity of the Company’s RSUs for the year ended December 31, 2021:
| Number of Shares | Weighted- Average Grant Date Fair Value Per Share | |||||||
| Unvested units at December 31, 2020 | 9,699 | $ | 19.97 | |||||
| Granted | 360,750 | 2.94 | ||||||
| Vested | (3,348 | ) | 22.67 | |||||
| Forfeited | – | – | ||||||
| Unvested units at December 31, 2021 | 367,101 | $ | 3.21 | |||||
Stock-based compensation expense related to RSUs was $ and $ for the years ended December 31, 2021 and 2020, respectively.
The aggregate fair value of awards that vested during the year ended December 31, 2021 was $, which represents the market value of the Company’s common stock on the date that the RSUs vested.
As of December 31, 2021, the compensation expense for all unvested RSUs in the amount of approximately $ will be recognized in the Company’s results of operations over a weighted average period of years.
Stock Options
Stock options are issued under the 2020 Plan or as inducement grants issued outside of the plan to new employees. Stock options are generally subject to graded vesting and the satisfaction of service requirements. Upon the exercise of a stock option, the Company issues new shares and delivers them to the recipient. The Company does not expect to repurchase shares to satisfy stock option exercises.
The Company uses the Black-Scholes option-pricing model to determine the fair value of all its option grants. The risk-free interest rate used for each grant was based upon the yield on zero-coupon U.S. Treasury securities with a term similar to the expected life of the related option. The Company’s expected stock price volatility assumption is based upon the Company’s own implied volatility. As the Company has limited stock option exercise information, the expected life assumption used for option grants is based upon the simplified method provided for under ASC 718. The dividend yield assumption is based upon the fact that the Company has never paid cash dividends and presently has no intention of paying cash dividends.
The Company did not grant stock options during the years ended December 31, 2021 and December 31, 2020. The following table summarizes the activity of the Company’s stock option plan for the year ended December 31, 2021:
| Total Number of Shares | Weighted- Average Exercise Price Per Share | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||||
| Balance at December 31, 2020 | 2,570 | $ | 3,334.06 | |||||||||||||
| Granted | – | – | ||||||||||||||
| Exercised | – | – | ||||||||||||||
| Cancelled | (71 | ) | 946.27 | |||||||||||||
| Balance at December 31, 2021 | 2,499 | $ | 3,401.90 | years | $ | – | ||||||||||
| Exercisable at December 31, 2021 | 2,153 | $ | 3,932.78 | years | $ | – | ||||||||||
| F-17 |
Stock-based compensation expense related to stock options for the years ended December 31, 2021 and 2020 was $ and $, respectively.
As of December 31, 2021, the compensation expense for all unvested stock options in the amount of approximately $ will be recognized in the Company’s results of operations over a weighted average period of years.
There is no income tax benefit as the Company is currently operating at a loss and an actual income tax benefit may not be realized.
Compensation Expense Related to Equity Awards
The following table sets forth total stock-based compensation expense for the years ended December 31, 2021 and 2020, in thousands:
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Research and development | $ | 117 | $ | 22 | ||||
| General and administrative | 363 | 114 | ||||||
| Total stock-based compensation | $ | 480 | $ | 136 | ||||
13. Income Taxes
The provisions for income taxes for the years ended December 31, 2021 and 2020 are as follows, in thousands:
| Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Current | ||||||||
| Federal | $ | – | $ | – | ||||
| State | – | – | ||||||
| Total current | – | – | ||||||
| Deferred | ||||||||
| Federal | (3,016 | ) | 9,780 | |||||
| State | (1,329 | ) | 5,548 | |||||
| Total deferred | (4,345 | ) | 15,328 | |||||
| Valuation allowance | 4,345 | (15,328 | ) | |||||
| Total provision for income taxes | $ | – | $ | – | ||||
Reconciliation of the effective income tax rate to the U.S. statutory rate is as follows:
| Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Federal statutory rate | 21.0% | 21.0% | ||||||
| State income taxes, net of federal benefit | 8.5 | 6.7 | ||||||
| Non-deductible expenses | 0.2 | (0.5 | ) | |||||
| Income tax credits | 4.3 | 2.1 | ||||||
| Valuation allowance | (34.0 | ) | (29.3 | ) | ||||
| Effective tax rate | 0.0% | 0.0% | ||||||
| F-18 |
The Company recognizes deferred tax assets and liabilities to reflect the tax effects of temporary differences between the tax basis of assets or liabilities and their reported amounts in the financial statements in accordance with ASC 740. These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled.
ASC 740 requires that a valuation allowance be established when management determines that it is more likely than not that all or a portion of a deferred asset will not be realized. The Company evaluates the realizability of its net deferred income tax assets and valuation allowances as necessary, at least on an annual basis. During this evaluation, the Company reviews its forecasts of income in conjunction with other positive and negative evidence surrounding the realizability of its deferred income tax assets to determine if a valuation allowance is required. Adjustments to the valuation allowance will increase or decrease the Company’s income tax provision or benefit.
The components of the Company’s deferred tax assets are as follows, in thousands:
| Years Ending December 31, | ||||||||
| 2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 10,150 | $ | 6,355 | ||||
| Tax credit carryforwards | 946 | |||||||
| Stock-based compensation | 1,363 | 1,374 | ||||||
| Licensing deduction deferral | 2,018 | 2,376 | ||||||
| Lease liability | 79 | 111 | ||||||
| Other timing differences | 169 | 196 | ||||||
| Deferred tax assets | 14,725 | 10,412 | ||||||
| Deferred tax liabilities: | ||||||||
| Right of use asset | (76 | ) | (108 | ) | ||||
| Deferred tax liability | (76 | ) | (108 | ) | ||||
| Valuation allowance | (14,649 | ) | (10,304 | ) | ||||
| Net deferred tax asset | $ | – | $ | – | ||||
The Company evaluated the realizability of its net deferred tax assets and concluded that, in accordance with the applicable accounting standards, that it is more likely than not that the Company may not realize the benefit of all of its deferred tax assets. Accordingly, a valuation allowance against 100% of its deferred tax assets has been recorded. Significant judgment is required in the evaluation of deferred tax benefits and differences in future results from our estimates could result in material differences in the realization of these assets.
As of December 31, 2021, the Company had federal net operating loss carryforwards of approximately $42,900,000 and state net operating loss carryforwards of approximately $18,100,000 to reduce future taxable income. The utilization of the federal carryforwards as an available offset to future taxable income is subject to limitations under federal income tax laws. Under current federal income tax law, federal net operating losses incurred in tax years beginning after December 31, 2017 may be carried forward indefinitely, but are limited to 80% of taxable income. As of December 31, 2021, approximately $42,579,000 of our federal net operating loss carryforwards will carryforward indefinitely, while the remaining federal net operating loss of $321,000 will begin to expire in , unless previously utilized. The Company’s state tax loss carryforwards will begin to expire in , unless previously utilized. In addition, the Company had federal and state research and development credits of approximately $573,000 and $472,000, respectively, which will begin to expire in . Based on an assessment of all available evidence, the Company has concluded that it is more likely than not that these net operating loss carryforwards and credits will not be realized and, as a result, a full deferred income tax valuation allowance has been recorded against these assets.
Ownership changes may limit the amount of net operating loss carryforwards or research and development tax credit carryforwards that can be utilized to offset future taxable income or tax liability. In general, an ownership change, as defined by Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”), results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% over a three-year period. If the Company has experienced a change of control, utilization of the net operating loss carryforwards or research and development tax credit carryforwards would be subject to an annual limitation under Section 382 and 383 of the Code. Any limitation may result in expiration of a portion of the net operating loss carryforwards or research and development tax credit carryforwards before utilization.
During 2021, the Company completed an assessment of the available net operating loss and tax credit carryforwards under Section 382 and 383 and determined that the Company underwent six ownership changes during the period from 2012 to 2021. As a result, net operating loss and tax credit carryforwards attributable to the pre-ownership changes are subject to substantial annual limitations under Section 382 and 383 of Code due to the ownership changes. The Company has adjusted its net operating loss and tax credit carryforwards to address the impact of the ownership changes. This resulted in a reduction of available federal and state net operating loss carryforwards of approximately $55,000,000 and $70,000,000, respectively, which related to the years ended December 31, 2020 and prior. This also resulted in a reduction of federal and state tax credit carryforwards of approximately $1,400,000 and $700,000, respectively, related to the years ended December 31, 2020 and prior. Accordingly, the net operating loss and tax credit carryforwards presented above for the year ending December 31, 2020 were reduced by $15,952,000 and $2,017,000, respectively, with a corresponding reduction to the valuation allowance of $17,969,000.
| F-19 |
The recognition and measurement of benefits related to the Company’s tax positions requires significant judgment, as uncertainties often exist with respect to new laws, new interpretations of existing laws, and rulings by taxing authorities. The Company follows a more-likely-than not threshold for financial statement recognition and measurement of a tax position taken, or expected to be taken in a tax return. The guidance relates to, amongst other things, classification, accounting for interest and penalties associated with tax positions, and disclosure requirements. Any interest and penalties accrued related to uncertain tax positions are recorded as tax expense. Differences between actual results and the Company’s assumptions or changes in the Company’s assumptions in future periods are recorded in the period they become known. The Company has not recorded any uncertain tax positions as of December 31, 2021 or 2020. The Company does not believe there will be any material changes in its unrecognized tax positions over the next 12 months. The Company has not incurred any interest or penalties. In the event that the Company is assessed interest or penalties at some point in the future, they will be classified in the financial statements as general and administrative expenses.
The Company files income tax returns in the United States, Massachusetts and New Jersey. The Company is subject to tax examinations for federal and state purposes for tax years 2013 through 2021.
14. Collaboration and License Agreements
In March 2021, the Company entered into a clinical development collaboration with AgonOx, Inc. (“AgonOx”), a private company developing a pipeline of novel immunotherapy drugs targeting key regulators of the immune response to cancer. Under the collaboration, the companies will develop a T cell-based therapy using PH-762 and AgonOx’s “double positive” TIL (“DP TIL”) technology. Per the terms of the clinical development agreement, AgonOx will receive financial support from Phio to conduct a clinical trial with AgonOx’s DP TIL technology and PH-762. Phio will be entitled to certain future development milestones and sales-based royalty payments from AgonOx’s DP TIL technology. The Company did not provide financial support to AgonOx, nor has Phio received milestone or sales-based royalty payments from AgonOx under this agreement in 2021.
As part of its business, the Company enters into licensing agreements with third parties that often require milestone and royalty payments based on the progress of the asset through development stages. Milestone payments may be required, for example, upon approval of the product for marketing by a regulatory agency. In certain agreements, the Company is required to make royalty payments based upon a percentage of the sales of the products licensed pursuant to such agreements.
The expenditures required under these arrangements may be material individually in the event that the Company develops product candidates covered by the intellectual property licensed under any such arrangement, and in the unlikely event that milestones for multiple products covered by these arrangements were reached in the same period, the aggregate charge to expense could be material to the results of operations. In addition, these arrangements often give the Company discretion to unilaterally terminate development of the product, which would allow the Company to avoid making the contingent payments; however, the Company is unlikely to cease development if the compound successfully achieves clinical testing objectives.
Advirna LLC. We have entered into an agreement with Advirna LLC (“Advirna”), pursuant to which Advirna assigned to us its existing patent and technology rights related to the INTASYL™ technology. Under the terms of the agreement, in April 2012, the Company issued to Advirna shares of common stock equal to 5% of the Company’s fully-diluted shares outstanding at the time of issuance. In exchange, the Company is also obligated to pay Advirna an annual maintenance fee and a milestone payment upon the issuance of the first patent with valid claims covering the assigned technology, which was paid in 2014. Additionally, we will be required to pay low single-digit royalties to Advirna on any licensing revenue received by us with respect to future licensing of the assigned Advirna patent and technology rights. To date, royalties owed to Advirna under the agreement have been minimal. We also granted back to Advirna a license under the assigned patent and technology rights for fields of use outside human therapeutics.
Our rights under the Advirna agreement will expire upon the later of: (i) the expiration of the last-to-expire of the “patent rights” (as defined therein) included in the Advirna agreement; or (ii) the abandonment of the last-to-be abandoned of such patents, unless earlier terminated in accordance with the provisions of the agreement.
| F-20 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (who is also acting as our principal financial officer) and our Principal Accounting Officer, evaluated the effectiveness of disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this report to ensure that information that we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms.
Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives. We believe that a control system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the control system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. Based on the evaluation of our disclosure controls and procedures as of the end of the period covered by this report, management, with the participation of our Chief Executive Officer (who is also acting as our principal financial officer) and our Principal Accounting Officer, concluded that our disclosure controls and procedures were effective as of such date.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
There are inherent limitations in the effectiveness of any system of internal control, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal controls can provide only reasonable assurances with respect to financial statement preparation. Further, because of changes in conditions, the effectiveness of internal control may vary over time.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2021. In making this assessment, management used the criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on this assessment, management, with the participation of our Chief Executive Officer (who is also acting as our principal financial officer) and our Principal Accounting Officer, concluded that, as of December 31, 2021, our internal control over financial reporting was effective.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K provides only management’s report. As a smaller reporting company, we are not required to provide an attestation report by our independent registered public accounting firm regarding our internal control over financial reporting.
| 41 |
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting that occurred during the year ended December 31, 2021 that have materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
Our certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for the following types of actions or proceedings: any derivative action or proceeding brought on behalf of the Company, any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders, any action asserting a claim against the Company arising pursuant to any provision of the Delaware General Corporation Law or the Company’s certificate of incorporation or bylaws, or any action asserting a claim against the Company governed by the internal affairs doctrine. Despite the fact that our certificate of incorporation provides for this exclusive forum provision to be applicable to the fullest extent permitted by applicable law, Section 27 of the Securities Act and Exchange Act, creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder and Section 22 of the Securities Act, creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. As a result, this provision of our certificate of incorporation would not apply to claims brought to enforce a duty or liability created by the Securities Act, Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
| 42 |
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item will be incorporated by reference to the information contained in our Definitive Proxy Statement to be filed no later than 120 days after the fiscal year end of December 31, 2021.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item will be incorporated by reference to the information contained in our Definitive Proxy Statement to be filed no later than 120 days after the fiscal year end of December 31, 2021.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item will be incorporated by reference to the information contained in our Definitive Proxy Statement to be filed no later than 120 days after the fiscal year end of December 31, 2021.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item will be incorporated by reference to the information contained in our Definitive Proxy Statement to be filed no later than 120 days after the fiscal year end of December 31, 2021.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this Item will be incorporated by reference to the information contained in our Definitive Proxy Statement to be filed no later than 120 days after the fiscal year end of December 31, 2021.
| 43 |
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Financial Statements
Our consolidated financial statements are set forth in Item 8 to this Annual Report on Form 10-K.
Financial Statement Schedules
Certain schedules are omitted because they are not applicable, or are not required by smaller reporting companies.
Exhibits
| Exhibit | Incorporated by Reference Herein | ||||||||
| Number | Description | Form | Date | ||||||
| 3.1 | Amended and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. | Current Report on Form 8-K (File No. 001-36304) | November 19, 2018 | ||||||
| 3.2 | Certificate of Amendment to the Amendment and Restated Certificate of Incorporation of Phio Pharmaceuticals Corp. | Current Report on Form 8-K (File No. 001-36304) | January 14, 2020 | ||||||
| 3.3 | Amended and Restated Bylaws of Phio Pharmaceutical Corp. | Current Report on Form 8-K (File No. 001-36304) | October 13, 2020 | ||||||
| 4.1 | Form of Warrant. | Current Report on Form 8-K (File No. 001-36304) | April 11, 2018 | ||||||
| 4.2 | Form of Placement Agent Warrant. | Current Report on Form 8-K (File No. 001-36304) | April 11, 2018 | ||||||
| 4.3 | Form of Warrant. | Amendment No. 1 to the Registration Statement on Form S-1 (File No. 333-221173) | September 28, 2018 | ||||||
| 4.4 | Form of Underwriter Warrant. | Current Report on Form 8-K (File No. 001-36304) | October 5, 2018 | ||||||
| 4.5 | Form of Placement Agent Warrant. | Current Report on Form 8-K (File No. 001-36304) | November 19, 2019 |
| 44 |
| 4.6 | Form of Warrant | Current Report on Form 8-K (File No. 001-36304) | February 6, 2020 | ||||||
| 4.7 | Form of Warrant. | Current Report on Form 8-K (File No. 001-36304) | February 13, 2020 | ||||||
| 4.8 | Form of Underwriter Warrant. | Current Report on Form 8-K (File No. 001-36304) | February 13, 2020 | ||||||
| 4.9 | Form of Warrant. | Current Report on Form 8-K (File No. 001-36304) | April 2, 2020 | ||||||
| 4.10 | Form of Common Stock Warrant. | Current Report on Form 8-K (File No. 001-36304) | January 25, 2021 |
||||||
| 4.11 | Form of Placement Agent Warrant. | Current Report on Form 8-K (File No. 001-36304) | February 17, 2021 | ||||||
| 4.12 | Description of Securities Registered Pursuant to Section 12(b) of the Securities Exchange Act of 1934.** | Annual Report on Form 10-K (File. 001-36304) |
March 26, 2020 | ||||||
| 10.1 | Patent and Technology Assignment Agreement between RXi Pharmaceuticals Corporation (formerly RNCS, Inc.) and Advirna, LLC, effective as of September 24, 2011. | Registration Statement on Form S-1 (File No. 333-177498) | October 25, 2011 | ||||||
| 10.2 | Phio Pharmaceuticals Corp. 2020 Long Term Incentive Plan.* | Registration Statement on Form S-8 (File No. 333-251670) | December 23, 2020 | ||||||
| 10.3 | Form of Restricted Stock Unit Award under the Company’s 2020 Long Term Incentive Plan.* | Annual Report on Form 10-K (File. 001-36304) | March 25, 2021 | ||||||
| 10.4 | Phio Pharmaceuticals Corp. 2012 Long Term Incentive Plan.* | Quarterly Report on Form 10-Q (File No. 001-36304) | November 12, 2019 | ||||||
| 10.5 | Form of Restricted Stock Unit Award under the Company’s 2012 Long Term Incentive Plan.* | Amendment No. 2 to the Registration Statement on Form S-1 (File No. 333-177498) | December 29, 2011 | ||||||
| 10.6 | Form of Incentive Stock Option Award under the Company’s 2012 Long Term Incentive Plan, as amended.* | Registration Statement on Form S-1 (File No. 333-191236) | September 18, 2013 | ||||||
| 10.7 | Form of Non-Qualified Stock Option Award under the Company’s 2012 Long Term Incentive Plan, as amended.* | Registration Statement on Form S-1 (File No. 333-191236) | September 18, 2013 | ||||||
| 45 |
| 46 |
| 23.1 | Consent of BDO USA, LLP, an Independent Registered Public Accounting Firm.** | ||||||||
| 31.1 | Sarbanes-Oxley Act Section 302 Certification of Principal Executive Officer and Principal Financial Officer.** | ||||||||
| 32.1 | Sarbanes-Oxley Action Section 906 Certification of Principal Executive Officer and Principal Financial Officer.** | ||||||||
| 101.INS | Inline XBRL Instance Document.* | ||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document.* | ||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document.* | ||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document.* | ||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document.* | ||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document.* | ||||||||
| 104 | The cover page for this report, formatted in Inline XBRL (included in Exhibit 101).* |
_________________
| * | Indicates a management contract or compensatory plan or arrangement. | ||
| ** | Filed herewith. | ||
| + | Confidential treatment has been requested or granted for certain portions which have been blanked out in the copy of the exhibit filed with the Securities and Exchange Commission. The omitted information has been filed separately with the Securities and Exchange Commission. | ||
| ITEM 16. | FORM 10-K SUMMARY |
None.
| 47 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PHIO PHARMACEUTICALS CORP. | ||
| By: |
/s/ Gerrit Dispersyn | |
| Gerrit Dispersyn, Dr. Med. Sc. | ||
|
President and Chief Executive Officer (as Principal Executive and Financial Officer) | ||
| Date: March 22, 2022 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signatures | Title | Date |
|
/s/ Gerrit Dispersyn Gerrit Dispersyn, Dr. Med. Sc. |
President, Chief Executive Officer and Director (Principal Executive Officer and Principal Financial Officer) |
March 22, 2022 |
|
/s/ Caitlin Kontulis Caitlin Kontulis |
Vice President of Finance and Administration and Secretary (Principal Accounting Officer) |
March 22, 2022 |
|
/s/ Robert J. Bitterman Robert J. Bitterman |
Director | March 22, 2022 |
|
/s/ Geert Cauwenbergh Geert Cauwenbergh, Dr. Med. Sc. |
Director | March 22, 2022 |
|
/s/ H. Paul Dorman H. Paul Dorman |
Director | March 22, 2022 |
|
/s/ Robert L. Ferrara Robert L. Ferrara |
Director |
March 22, 2022 |
|
/s/ Jonathan E. Freeman Jonathan E. Freeman, Ph.D. |
Director | March 22, 2022 |
|
/s/ Curtis A. Lockshin Curtis A. Lockshin, Ph.D. |
Director | March 22, 2022 |
| 48 |
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-10-01)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
