Premier, Inc. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For The Fiscal Year Ended June 30, 2022
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OF 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For The Transition Period From _______ To _______
Commission File Number 001-36092
Premier, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 35-2477140 | ||||||||||
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | ||||||||||
13034 Ballantyne Corporate Place | 28277 | ||||||||||
Charlotte, | North Carolina | (Zip Code) | |||||||||
| (Address of principal executive offices) | |||||||||||
Registrant's telephone number, including area code: (704) 357-0022
_____________________________________________________________________
Securities Registered Pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbols | Name of Each Exchange on Which Registered | ||||||
| Class A Common Stock, $0.01 Par Value | PINC | NASDAQ Global Select Market | ||||||
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☒ | Accelerated filer | ☐ | |||||||||||
Non-accelerated filer | ☐ | Smaller reporting company | ☐ | |||||||||||
Emerging growth company | ☐ | |||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the Registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the Class A common stock held by non-affiliates of the Registrant as of the last business day of the Registrant's most recently completed second fiscal quarter was approximately $4,893.5 million. For purposes of the foregoing calculation only, executive officers and directors of the registrant have been deemed to be affiliates.
As of August 11, 2022, there were 118,066,513 shares of the Registrant's Class A common stock, par value $0.01 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The Registrant's definitive proxy statement for its 2022 Annual Meeting of Stockholders to be held on or about December 2, 2022 is incorporated by reference into Part III hereof to the extent described herein.
2
PREMIER, INC
FORM 10-K
TABLE OF CONTENTS
| Page | ||||||||
PART I | ||||||||
| ITEM 1. | ||||||||
| ITEM 1A. | ||||||||
| ITEM 1B. | ||||||||
| ITEM 2. | ||||||||
| ITEM 3. | ||||||||
| ITEM 4. | ||||||||
| PART II | ||||||||
| ITEM 5. | ||||||||
| ITEM 6. | ||||||||
| ITEM 7. | ||||||||
| ITEM 7A. | ||||||||
| ITEM 8. | ||||||||
| ITEM 9. | ||||||||
| ITEM 9A. | ||||||||
| ITEM 9B. | ||||||||
| PART III | ||||||||
| ITEM 10. | ||||||||
| ITEM 11. | ||||||||
| ITEM 12. | ||||||||
| ITEM 13. | ||||||||
| ITEM 14. | ||||||||
| PART IV | ||||||||
| ITEM 15. | ||||||||
| ITEM 16. | FORM 10-K SUMMARY | |||||||
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements made in this annual report for the fiscal year ended June 30, 2022 for Premier, Inc. (this “Annual Report”) that are not statements of historical or current facts, such as those under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from historical results or from any future results or projections expressed or implied by such forward-looking statements. In addition to statements that explicitly describe such risks and uncertainties, readers are urged to consider statements in conditional or future tenses or that include terms such as “believes,” “belief,” “expects,” “estimates,” “intends,” “anticipates” or “plans” to be uncertain and forward-looking. Forward-looking statements may include comments as to our beliefs and expectations regarding future events and trends affecting our business and are necessarily subject to uncertainties, many of which are outside our control. Factors that could cause actual results to differ materially from those indicated in any forward-looking statement include, but are not limited to:
•the impact of the continuing financial and operational uncertainty due to the coronavirus pandemic or other pandemics and associated supply chain disruptions and inflation;
•global economic and political instability and conflicts, such as the conflict between Russia and Ukraine, could adversely affect our business, financial condition or results of operations, including issues such as rising inflation and global supply-chain disruption;
•competition which could limit our ability to maintain or expand market share within our industry;
•consolidation in the healthcare industry;
•potential delays recognizing or increasing revenue if the sales cycle or implementation period takes longer than expected;
•the impact to our business if members of our group purchasing organization (“GPO”) programs reduce activity levels or terminate or elect not to renew their contracts on substantially similar terms or at all;
•the rate at which the markets for our software as a service (“SaaS”) or licensed-based clinical analytics products and services develop;
•the dependency of our members on payments from third-party payors;
•our reliance on administrative fees that we receive from GPO suppliers;
•our ability to maintain third-party provider and strategic alliances or enter into new alliances;
•our ability to timely offer new and innovative products and services;
•the portion of revenues we receive from our largest members;
•risks and expenses related to future acquisition opportunities and integration of previous or future acquisitions;
•financial and operational risks associated with non-controlling investments in other businesses or other joint ventures that we do not control, particularly early-stage companies;
•pending and potential litigation;
•our reliance on Internet infrastructure, bandwidth providers, data center providers and other third parties and our own systems for providing services to our users;
•data loss or corruption due to failures or errors in our systems and service disruptions at our data centers, or breaches or failures of our security measures;
•the financial, operational and reputational consequences of cyber-attacks or other data security breaches that disrupt our operations or result in the dissemination of proprietary or confidential information about us or our members or other third parties;
•our ability to use, disclose, de-identify or license data and to integrate third-party technologies;
•our use of “open source” software;
•our dependency on contract manufacturing facilities located in various parts of the world;
4
•inventory risk we face in the event of a potential material decline in demand or price for the personal protective equipment or other products we may have purchased at elevated market prices or fixed prices;
•our ability to attract, hire, integrate and retain key personnel;
•adequate protection of our intellectual property and potential claims against our use of the intellectual property of third parties;
•potential sales and use tax liability in certain jurisdictions;
•changes in tax laws that materially impact our tax rate, income tax expense, anticipated tax benefits, deferred tax assets, cash flows and profitability;
•our indebtedness and our ability to obtain additional financing on favorable terms, including our ability to renew or replace our existing long-term credit facility at maturity;
•fluctuation of our quarterly cash flows, revenues and results of operations;
•changes and uncertainty in the political, economic or regulatory environment affecting healthcare organizations, including with respect to the status of the Patient Protection and Affordable Care Act, as amended by the Healthcare and Education Reconciliation Act of 2010 and pandemic-related public health and reimbursement measures;
•our compliance with complex international, federal and state laws, rules and regulations governing financial relationships among healthcare providers and the submission of false or fraudulent healthcare claims;
•interpretation and enforcement of current or future antitrust laws and regulations;
•compliance with complex federal, state and international privacy, security and breach notification laws;
•compliance with current or future laws, rules or regulations relating to information blocking provisions of the 21st Century Cures Act issued by the Office of the National Coordinator for Health Information Technology (the “ONC Rules”) that may cause our certified Health Information Technology products to be regulated by the ONC Rules;
•compliance with current or future laws, rules or regulations adopted by the Food and Drug Administration applicable to our software applications that may be considered medical devices;
•the impact of payments required under notes payable to former limited partners related to the early termination of the Unit Exchange and Tax Receivable Acceleration Agreements (the “Unit Exchange Agreements”) issued in connection with our August 2020 Restructuring on our overall cash flow and our ability to fully realize the expected tax benefits to match such fixed payment obligations under those notes payable;
•provisions in our certificate of incorporation and bylaws and provisions of Delaware and other applicable laws that discourage or prevent strategic transactions, including a takeover of us;
•failure to maintain an effective system of internal controls over financial reporting or an inability to remediate any weaknesses identified and the related costs of remediation;
•the impact on the price of our Class A common stock if we cease paying dividends or reduce dividend payments from current levels;
•the number of shares of Class A common stock repurchased by us pursuant to any then existing Class A common stock repurchase program and the timing of any such repurchases;
•the number of shares of Class A common stock eligible for sale after the issuance of Class A common stock in our August 2020 Restructuring and the potential impact of such sales; and
•the risk factors discussed under the heading “Risk Factors” in Item 1A herein.
More information on potential factors that could affect our financial results is included from time to time in the “Cautionary Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” or similarly captioned sections of this Annual Report and our other periodic and current filings made from time to time with the Securities and Exchange Commission (“SEC”), which are available on our website at http://investors.premierinc.com/. You should not place undue reliance on any of our forward-looking statements which speak only as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information or future events or otherwise. Furthermore, we cannot guarantee future results, events, levels of activity, performance or achievements.
5
Market Data and Industry Forecasts and Projections
We use market data and industry forecasts and projections throughout this Annual Report and in particular, under Item 1. Business. We have obtained the market data from certain publicly available sources of information, including industry publications. We believe the data others have compiled are reliable, but we have not independently verified the accuracy of this information. While we are not aware of any misstatements regarding the industry data presented herein, forecasts and projections involve risks and uncertainties and are subject to change based on various factors, including those discussed under Item 1A. Risk Factors of this Annual Report. You should not place undue reliance on any such market data or industry forecasts and projections. We undertake no obligation to publicly update or revise any such market data or industry forecasts and projections, whether as a result of new information, future events or otherwise.
Trademarks, Trade Names and Service Marks
This Annual Report includes trademarks, trade names and service marks that we either own or license, such as but not limited to “Acurity,” “ASCEND,” “ASCENDriveTM,” “Conductiv,” “Contigo Health,” “Essensa,” “Health Design Plus,” “Innovatix,” “InterSecttaTM,” “KIINDOTM,” “PINC AITM,” “Premier,” “PremierPro,” “ProvideGx,” “QUEST,” “RemitraTM,” “STOCKD,” “SURPASS,” “S2S Global” and “TheraDoc” which are protected under applicable intellectual property laws. Solely for convenience, trademarks, trade names and service marks referred to in this Annual Report may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks, trade names and service marks. This Annual Report also may contain trademarks, trade names and service marks of other parties, and we do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
Certain Definitions
For periods prior to August 11, 2020, references to “member owners” are references to participants in our GPO programs that were also limited partners of Premier Healthcare Alliance L.P. (“Premier LP”), sometimes referred to as “LPs,” that held Class B common units of Premier LP and shares of our Class B common stock.
For periods on or after August 11, 2020, references to “members” are references to health systems and other customers that utilize any of our programs or services, some of which were formerly member owners.
References to the “August 2020 Restructuring” are references to our corporate restructuring on August 11, 2020 in which we (i) eliminated our dual-class ownership structure, through an exchange under which member owners converted their Class B common units in Premier LP and corresponding Class B common shares of Premier, Inc. into our Class A common stock, on a one-for-one basis, and (ii) exercised our right to terminate the Tax Receivable Agreement (the “TRA”) by providing all former limited partners a notice of termination and the amount of the expected payment to be made to each limited partner pursuant to the early termination provisions of the TRA with a determination date of August 10, 2020. For additional information and details regarding the August 2020 Restructuring, see our 2021 Annual Report.
References to the “Subsidiary Reorganization” are references to an internal legal organization of our corporate subsidiaries in December 2021 for the purpose of simplifying our subsidiary reporting structure. For additional information and details regarding the Subsidiary Reorganization, see our Quarterly Report for the period ended December 31, 2021.
References to “Prior Premier GP” are references to our former wholly owned subsidiary Premier Services, LLC, which was merged with and into Premier, Inc, with Premier, Inc. being the surviving entity as part of the Subsidiary Reorganization.
6
PART I
Item 1. Business
The following discussion should be read in conjunction with our audited consolidated financial statements and accompanying notes thereto included elsewhere in this Annual Report on Form 10-K. The following discussion includes certain forward-looking statements. For a discussion of important factors which could cause actual results to differ materially from the results referred to in the historical information and the forward-looking statements presented herein, see “Item 1A. Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” contained in this Annual Report.
Our Company
Premier, Inc. (“Premier”, the “Company”, “we”, “us” or “our”), a publicly held, for-profit corporation, incorporated in Delaware on May 14, 2013, is a leading healthcare improvement company, uniting an alliance of U.S. hospitals, health systems and other providers and organizations to transform healthcare. We partner with hospitals, health systems, physicians, employers, product suppliers, service providers, and other healthcare providers and organizations with the common goal of improving and innovating in the clinical, financial and operational areas of their businesses to meet the demands of a rapidly evolving healthcare industry. With integrated data and analytics, collaboratives, supply chain services, consulting and other services, Premier enables healthcare providers to deliver better care and outcomes at a lower cost. We believe that we play a critical role in the rapidly evolving healthcare industry, collaborating with members and other customers to co-develop long-term innovative solutions that reinvent and improve the way care is delivered to patients nationwide. We deliver value through a comprehensive technology-enabled platform that offers critical supply chain services, clinical, financial, operational and value-based care software as a service (“SaaS”) as well as clinical and enterprise analytics licenses, consulting services, performance improvement collaborative programs, third-party administrator services, access to our centers of excellence program and digital invoicing and payment processes for healthcare product suppliers and service providers and continue to expand our capabilities to more fully address and coordinate care improvement and standardization in the employer, payor and life sciences markets. We also provide services to other businesses including food service, schools and universities.
As a healthcare alliance, our mission, products and services, and long-term strategy have been developed in partnership with hospitals, health systems, physicians and other healthcare providers and organizations. We believe that this partnership-driven business model creates a relationship between our members and us that is characterized by aligned incentives and mutually beneficial collaboration. This relationship affords us access to critical de-identified proprietary data and encourages member participation in the development and introduction of new products and services. Our interaction with our members provides us additional insights into the latest challenges confronting the healthcare industry and innovative best practices that we can share broadly across the healthcare industry, including throughout our membership. This model has enabled us to develop size and scale, data and analytics assets, expertise and customer engagement required to accelerate innovation, provide differentiated solutions and facilitate growth.
We seek to address challenges facing healthcare providers through our comprehensive suite of solutions that we believe:
•improve the efficiency and effectiveness of the healthcare supply chain;
•deliver improvement in cost, quality and safety;
•innovate and enable success in emerging healthcare delivery and payment models to manage the health of populations;
•utilize data and analytics to drive increased connectivity, and clinical, financial and operational improvement; and
•through employers, payors and life sciences, expand the capabilities within these markets to improve healthcare.
Our business model and solutions are designed to provide our members and other customers access to scale efficiencies while focusing on optimization of information resources and cost containment, provide actionable intelligence derived from anonymized data provided by our members and included in our data warehouse, mitigate the risk of innovation, and disseminate best practices that will help our member organizations and other customers succeed in their transformation to higher quality and more cost-effective healthcare.
We deliver our integrated platform of solutions that address the areas of clinical intelligence, margin improvement and value-based care through two business segments: Supply Chain Services and Performance Services. The Supply Chain Services segment includes our group purchasing organization (“GPO”) program, supply chain co-management, purchased services and direct sourcing activities. The Performance Services segment consists of three sub-brands: PINC AITM, Contigo Health® and RemitraTM. PINC AI is the Company’s technology and services platform with offerings that help optimize performance in three main areas – clinical intelligence, margin improvement and value-based care. PINC AI utilizes advanced analytics to identify improvement opportunities, consulting services for clinical and operational design, and workflow solutions to hardwire
7
sustainable change in the provider, life sciences and payer markets. Contigo Health is the Company’s direct-to-employer business, which provides third party administrator services and management of health benefit programs that allow employers to contract directly with healthcare providers as well as partners with healthcare providers to provide employers access to a specialized care network through Contigo Health’s centers of excellence program. Remitra is the Company’s digital invoicing and payables business which provides financial support services to healthcare product suppliers and service providers.
Fiscal 2022 Developments
In fiscal year 2022, the U.S. and global economies experienced unprecedented challenges resulting from the ongoing consequences and impact of the COVID-19 pandemic, including supply chain bottlenecks and escalating inflation. These challenges were exacerbated by the Russia-Ukraine war which has led to further supply chain disruptions, rising energy costs and further inflationary impacts. These challenges have impacted our business as discussed below.
COVID-19 Pandemic, Variants Thereof, Recurrences or Similar Pandemics
The novel coronavirus (“COVID-19”) global pandemic and its variants continue to create challenges throughout the United States and the rest of the world. The full extent to which the COVID-19 pandemic may impact our business, operating results, financial condition and liquidity will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19 and variants thereof, the continued actions to contain it or treat its impact, including the success of COVID-19 vaccination programs, or recurrences of COVID-19 variants thereof or similar pandemics. As discussed in detail under “Item 1A. Risk Factors” below, as a result of the COVID-19 pandemic and potential future pandemic outbreaks, we face material risks including, but not limited to the following:
•The impact of the COVID-19 pandemic and any variants thereof and associated supply chain disruptions and inflation could result in a prolonged recession or depression in the United States or globally that could harm the banking system, limit or delay demand for many products and services and cause other foreseen and unforeseen events and circumstances, all of which could negatively impact us.
•We experienced and may continue to experience demand uncertainty from both material increases and decreases in demand and pricing for personal protective equipment (“PPE”), drugs and other supplies directly related to treating and preventing the spread of COVID-19 and any variants thereof as well as a decline in demand and pricing for many supplies and services not related to COVID-19.
•Labor shortages and the resulting increases to cost of labor are a continued challenge to the healthcare providers we serve and could negatively affect our business.
•While some of our hospital customers have increased access to their facilities for non-patients, including our field teams, consultants and other professionals, there are many that are still not allowing onsite access outside of their staff. Hospital imposed travel restrictions are also impacting some customers’ ability to participate in face-to-face events with us, such as committee meetings and conferences.
•The global supply chain has been materially disrupted due to personnel shortages associated with ongoing COVID-19 rates of infection, stay-at-home orders, border closings, rapidly escalating shipping costs, raw material availability and material logistical delays due to port congestion.
•We have and may continue to receive requests for contract modifications, payment waivers and deferrals, payment reductions or amended payment terms from our contract counterparties. Inflation in such contract prices may impact member utilization of items and services available through our GPO contracts, with uncertain impact on our net administrative fees revenue and direct sourcing revenue. In addition, several pharmacy suppliers have exercised force majeure clauses related to failure to supply clauses in their contracts with us.
•In response to COVID-19 and variants thereof, federal, state and local governments are issuing new rules, regulations, changing reimbursement eligibility rules, orders and advisories on a regular basis. These government actions can impact us, our members, other customers and suppliers.
Russia-Ukraine War
In February 2022, Russia invaded Ukraine. As military activity continues and sanctions, export controls and other measures are imposed against Russia, Belarus and specific areas of Ukraine, the war is increasingly affecting the global economy and financial markets, as well as exacerbating ongoing economic challenges, including issues such as rising inflation and energy costs and global supply-chain disruption. We continue to monitor the impacts of the Russia-Ukraine war on macroeconomic conditions and prepare for any implications that the war may have on member demand, our suppliers’ ability to deliver products, cybersecurity risks and our liquidity and access to capital. See “Risk Factors — Risks Related to Our Business Operations” below.
8
Impact of Inflation
The U.S. economy is experiencing the highest rates of inflation since the 1980s. Historically, we have not experienced significant inflation risk in our business arising from fluctuations in market prices across our diverse product portfolio. However, our ability to raise our selling prices depends on market conditions and there may be periods during which we are unable to fully recover increases in our costs. In fiscal year 2022, our GPO business was not materially impacted by pricing inflation as we used our members’ aggregated purchasing power to negotiate firm prices in many of our contracts. In our direct sourcing business, we were able to partially offset increases in cost through temporary adjustments to selling prices and through various cost reduction initiatives while ensuring our products remain competitively priced. See “Risk Factors — Risks Related to Our Business Operations” below.
Industry Overview
According to data from the Centers for Medicare & Medicaid Services (“CMS”), healthcare expenditures are a large component of the U.S. economy and are expected to grow by an average of 5.1% per year for the period 2021-2030, reaching 19.6% of gross domestic product, or GDP, by 2030. According to data from the 2020 American Hospital Association’s Annual Survey, published in the 2022 edition of the AHA Hospital Statistics™, there were more than 5,100 U.S. community hospitals with approximately 789,400 staffed beds in the United States. Of these acute care facilities, approximately 3,500 were part of either multi-hospital or diversified single hospital systems, meaning they were owned, leased, sponsored or contract managed by a central organization. Based upon 2021 reporting from the United States Department of Labor and healthcare industry sources, in addition to U.S. hospitals, there were approximately 817,000 facilities and providers across the continuum of care in the United States. These facilities include primary/ambulatory care and post-acute care providers.
Healthcare Supply Chain Services Industry
According to CMS data, total spending on hospital services in the United States is projected to be $1.4 trillion, or approximately 32% of total healthcare expenditures, in calendar year 2022. Expenses associated with the hospital supply chain, such as supplies as well as operational and capital expenditures, typically represent a material portion of a hospital’s budget. With continued reimbursement rate pressure across government and managed care payors, a transitioning payment model from fee-for-service to value-based payment, and national health expenditures representing a material portion of the economy, healthcare providers are examining all sources of cost savings, with supply chain spending a key area of focus. We believe opportunities to drive cost out of the healthcare supply chain include improved pricing for medical supplies, pharmaceuticals, purchased services, facilities expenditures, food service supplies, and information technology, as well as appropriate resource utilization, mitigating pharmaceuticals and medical device shortages and increased operational efficiency.
From origination at the supplier to final consumption by the provider or patient, healthcare products pass through an extensive supply chain incorporating manufacturers, wholesalers, distributors, GPOs, pharmacy benefit managers, and retail, long-term care and integrated pharmacies, among others. In response to the national focus on health spending and managing healthcare costs, supply chain participants are seeking more convenient and cost-efficient ways to deliver products to patients and providers. We believe that improvements to the healthcare supply chain to bring it on par with other industries that have more sophisticated supply chain management can drive out material inefficiencies and cost.
Healthcare Performance Services Industry
State and federal budget pressures stemming from increased deficit spending and employer and consumer demands for lower costs, and the need for improved quality and outcomes have generated greater focus among healthcare providers on cost management, quality and safety, and value-based care. As a result, the Department of Health and Human Services (“HHS”) has embarked on an aggressive effort over the past three administrations to move from fee-for-service to alternative payment models (“APMs”). APMs, such as accountable care organizations (“ACOs”), capitated and bundled payment arrangements, make healthcare providers more accountable for cost and quality goals. This movement was advanced further with the bipartisan enactment of the Medicare Access and CHIP Reauthorization Act, which created incentives for physicians to move to APMs. Even with the possibility of changes to the ACA, this movement has and will likely continue given the strong bipartisan support for these models. Over the long-term, health systems will need to continually monitor performance and manage costs, while demonstrating high levels of quality and implementing new care delivery models.
We expect information technology to continue to play a key enabling role in workflow efficiency and cost reduction, performance improvement and care delivery transformation across the healthcare industry. In particular, the trends toward value-based payment models and healthcare require more sophisticated business intelligence, expanded data sets and technology solutions. To achieve higher-quality outcomes and control total cost of care, providers exhibit a strong and continuing need for more comprehensive data and analytic capabilities to help them understand their current and future performance, identify opportunities for improvement and manage value-based care risk. We expect demand for data
9
management and data analytics products to complement the focus on electronic health record adoption. Similarly, our consulting services business is growing in the areas of business model strategy and redesign, process and margin improvement, labor productivity, non-labor cost management, clinical integration and change management.
Our Membership
Our current membership base includes many of the country’s most progressive and forward-thinking healthcare organizations. The participation of these organizations in our membership provides us additional insights into the latest challenges confronting the industry we serve and innovative best practices that we can share broadly throughout our membership. We continually seek to add new members that are at the forefront of innovation in the healthcare industry. At June 30, 2022, our members included more than 4,400 U.S. hospitals and health systems and approximately 250,000 other providers and organizations. Over 430 individuals, representing approximately 140 of our U.S. hospital members, sit on 29 of our strategic and sourcing committees, and as part of these committees, use their industry expertise to advise on ways to improve the development, quality and value of our products and services. In addition, at June 30, 2022, four senior executives from our U.S. hospital member systems served on our Board of Directors providing valuable and unique insights into the challenges faced by hospitals and hospital systems and the innovations necessary to address these challenges. No individual member or member systems accounted for more than 5% of our net revenue for the fiscal years ended June 30, 2022 and 2021. Total GPO purchasing volume by all members participating in our GPO was more than $82 billion and $69 billion for the calendar years 2021 and 2020, respectively.
The following table sets forth certain information with respect to retention rates for members participating in our GPO in the Supply Chain Services segment and renewal rates for our SaaS informatics products subscriptions and licenses in the Performance Services segment for the fiscal years shown:
| Year Ended June 30, | |||||||||||||||||||||||
| 2022 | 2021 | 2020 | 3 Year Average | ||||||||||||||||||||
GPO retention rate (a)(b) | 97% | 94% | 99% | 97% | |||||||||||||||||||
SaaS institutional renewal rate (c) | 96% | 96% | 95% | 96% | |||||||||||||||||||
_________________________________
(a)The GPO retention rate is calculated based upon the aggregate purchasing volume among all members participating in our GPO for such fiscal year less the annualized GPO purchasing volume for departed members for such fiscal year, divided by the aggregate purchasing volume among all members participating in our GPO for such fiscal year.
(b)Fiscal 2021 GPO retention rate decreased primarily as a result of amendments to GPO participation agreements, effective July 1, 2020, and the August 2020 Restructuring.
(c)The SaaS institutional renewal rate is calculated based upon the total number of members that have SaaS or license revenue in a given period that also have revenue in the corresponding prior year period divided by the total number of members that have SaaS or license revenue in the same period of the prior year.
Our Business Segments
We deliver our integrated platform of solutions that address the areas of clinical intelligence, margin improvement and value-based care and manage our business through two business segments: Supply Chain Services and Performance Services. Refer to Note 19 - Segments to the accompanying audited consolidated financial statements for further information. We have no significant foreign operations or revenues.
Supply Chain Services
Our Supply Chain Services segment assists our members and other customers in managing their non-labor expense and capital spend through a combination of products, services and technologies, including one of the largest national healthcare GPO programs in the United States serving acute, non-acute and non-healthcare sites, and providing supply chain co-management, purchased services and direct sourcing activities. Membership in our GPO also provides access to certain SaaS informatics products related to the supply chain and the opportunity to participate in our ASCEND® and SURPASS® performance groups. Our Supply Chain Services segment consists of the following products and solutions:
Group Purchasing. Our portfolio of over 3,000 contracts with over 1,460 suppliers provides our members with access to a wide range of products and services, including medical and surgical products, pharmaceuticals, laboratory supplies, capital equipment, information technology, facilities and construction, food and nutritional products and purchased services (such as clinical engineering and workforce solutions). We use our members’ aggregate purchasing power to negotiate pricing discounts and improved contract terms with suppliers. Contracted suppliers pay us administrative fees based on the purchase volume of goods and services sold to our members under the contracts we have negotiated. We also partner with other organizations, including regional GPOs, to extend our network base to their members.
10
Our contract portfolio is designed to offer our members a flexible solution comprised of multi-sourced supplier contracts, as well as pre-commitment and/or single-sourced contracts that offer higher discounts. Our multi-sourced contracts offer pricing tiers based on purchasing volume and/or commitment and multiple suppliers for many products and services. Our pre-commitment contracts require that a certain amount of our members commit in advance to a specified amount or percentage of purchasing volume before we enter into a contract with a particular supplier. Our single-source contracts are entered into with a specified supplier, and through this exclusive relationship, allow us to contract for products that meet our members’ specifications. In the case of pre-commitment contracts, we provide the particular supplier with a list of members that have pre-committed to a specified amount or percentage of purchasing volume and the supplier directly handles the tracking and monitoring of fulfillment of such purchasing volume. In the case of single and multi-sourced contracts, we negotiate and execute the contracts with suppliers on behalf of our members and make such contracts available to our members to access. The utilization of such single and multi-sourced contracts is determined by each particular member with assistance from our field force. Since there are no specific fulfillment requirements needed in our single and multi-source contracts in order to obtain certain pricing levels, each particular member and supplier agree on the appropriate pricing tier based on expected purchasing volume with tracking and ongoing validation of such purchasing volume provided by the supplier. The flexibility provided by our expansive contract portfolio allows us to effectively address the varying needs of our members and the significant number of factors that influence and dictate these needs, including overall size, service mix, and the degree of integration between hospitals in a healthcare system.
We continually innovate our GPO programs and supply chain platforms while targeting multiple markets, including acute and non-acute care and non-healthcare site settings. In addition to our core base of approximately 4,400 acute care healthcare providers, our Premier Continuum of Care Program, one of the largest in the United States, which covers over 80 classes of trade, had approximately 250,000 active members as of June 30, 2022, which represents an increase of approximately 25,000 members, or 11%, over fiscal year 2021. A number of these members in our Premier Continuum of Care Program are affiliated, owned, leased, or managed by our members and received a revenue share from us based upon our collected gross administrative fees on their members’ purchases.
Our Premier Continuum of Care Program includes the following:
Premier Continuum of Care - Non-Acute Care. This program includes direct members, group affiliates and healthcare provider offices owned, leased or managed by health systems. Key classes of trade include long-term care dispensing pharmacies, skilled nursing and assisted living facilities, home infusion providers, home health providers and surgery centers. Premier Continuum of Care members have access to most of our GPO supplier contracts, including, but not limited to, pharmaceuticals, medical and surgical supplies, facilities, food and nutritional products and other purchased services.
Premier Business and Industry - Non-Healthcare. This program includes direct members and group affiliates. Key classes of trade include non-healthcare entities, such as education (e.g. K-12 schools, colleges and universities), hospitality, recreation (e.g. stadiums, parks and fairgrounds), and employee food programs. Our Business and Industry members have access to most of our GPO supplier contracts, including administrative services, facilities, food service, and informational services.
The Premier Continuum of Care Program provides business operations and technology to ensure members and other customers are connected to agreements and receiving proper contracted pricing.
Supply Chain Co-Management. We manage and co-manage the supply chain operations for members to drive down costs through processes, including value analysis, product standardization and strategic resource allocation and improved operational efficiency.
Purchased Services Contracts. Our purchased services contracts business, which is separate from the purchased services under our national contract portfolio, includes Conductiv, Inc. (“Conductiv”) and Conductiv Contracts, LLC (“Conductiv Contracts”). Conductiv is a SaaS provider of technology solutions and expert services that enable hospitals and other organizations to analyze, benchmark and source purchased service contracts independent of any existing GPO affiliation. Combined with our purchased services spend data and our performance improvement technology suite, we are able to be a single source provider for healthcare margin improvement. Conductiv Contracts is a regionally focused group purchasing organization independent of any existing GPO affiliation that exclusively focuses on purchased services contracting.
Direct Sourcing. Our direct sourcing business, SVS, LLC d/b/a S2S Global (“S2S Global”), helps our members and other customers access a diverse product portfolio and helps provide transparency to manufacturing costs and competitive pricing. Through our consolidated subsidiary, S2S Global, we facilitate the development of product specifications with our members and other customers, source or contract manufacture the products to member specifications and sell products directly to our members, other customers or distributors. By engaging with our members and other customers at the
11
beginning of the sourcing process to define product specifications and then sourcing, or contract manufacturing, products to meet the exact needs of our members, we eliminate the need for unnecessary product features and specifications that may typically be included by suppliers and result in higher prices for our members without providing incremental value. Therefore, our direct sourcing activities benefit our members and other customers by providing them with an expanding portfolio of medical products through more efficient means, and with greater cost transparency, than if such products were purchased from other third-party suppliers. We market our direct sourcing activities to our members primarily under the PREMIERPRO® brand.
Supply Chain Resiliency Program. In partnership with our members, we have created a program designed to promote domestic and geographically diverse manufacturing and ensure a robust and resilient supply chain for essential medical products. The program is intended to provide a means to invest in or partner with businesses that can supply shortage products, co-fund the development of affordable products that address specific market needs and create strategic sourcing contracts to ensure continuous supply for our members and customers. We believe this program is most successful when we are able to partner with our members through investments or long-term purchasing commitments on these initiatives.
Our Supply Chain Resiliency Program includes, but is not limited to, the following:
PRAM Holdings, LLC. We formed PRAM Holdings, LLC (“PRAM”) in 2020 in partnership with our members to invest in Prestige Ameritech Ltd. (“Prestige”), a domestic manufacturer of masks and other PPE, whereby our members obtain a direct domestic source to critical PPE.
DePre Holdings, LLC. We formed DePre Holdings, LLC (“DPH”) in 2021 in partnership with our members to invest in DePre, LLC (“DePre”), a joint venture between DPH and DeRoyal Industries Inc., a global medical manufacturer, whereby our members obtain a direct source dedicated to the domestic production of isolation gowns.
ExPre Holdings, LLC. We formed ExPre Holdings, LLC (“ExPre”) in 2022 in partnership with our members to invest in Exela Holdings, Inc. (“Exela”), a domestic manufacturer of proprietary and generic sterile injectable products, whereby our members obtain a direct source to certain critical pharmaceutical products.
SaaS Informatics Products. Members of our GPO have access to certain SaaS informatics products related to the supply chain and have the ability to purchase additional elements that are discussed in more detail below under “Our Business Segments - Performance Services”.
Performance Groups. Our Performance Groups are highly committed purchasing programs, which enable members to benefit from coordinated purchasing decisions and maintain standardization across their facilities. Our Performance Groups include the ASCEND® and the SURPASS® Performance Groups.
ASCEND® Performance Group. Our ASCEND Performance Group, which was rebranded as ASCENDriveTM as of July 1, 2022, has developed a process to aggregate purchasing data for our members, enabling such members to benefit from committed group purchases within the Performance Group. Through our ASCEND Performance Group, members receive group purchasing programs, tiers and prices specifically negotiated for them and knowledge sharing with other member participants. As of June 30, 2022, approximately 1,300 U.S. hospital members, which represent over 113,000 hospital beds, participated in the ASCEND Performance Group. These hospital member participants have identified approximately $712.0 million in additional savings as compared to their U.S. hospital peers not participating in the ASCEND Performance Group since its inception in 2009. For calendar year 2021, these member participants had approximately $21.3 billion in annual supply chain purchasing spend.
SURPASS® Performance Group. Our SURPASS Performance Group builds upon and complements our legacy ASCEND Performance Group and drives even greater savings for members at a correspondingly higher level of commitment. The SURPASS Performance Group brings together our most committed members that are able to coordinate purchasing decisions, review utilization and achieve and maintain standardization across their facilities. The SURPASS Performance Group utilizes our PACER (Partnership for the Advancement of Comparative Effectiveness Review) methodology, which brings together clinically led cohorts to make evidence-based decisions about physician and clinician preference items with the goal of materially reducing the total cost of care. As of June 30, 2022, a group of 23 members representing approximately 470 acute care sites and 9,700 alternate sites participate in our SURPASS Performance Group. These hospital member participants have identified over $206.0 million in additional savings via their efforts in more than 150 categories. The SURPASS Performance Group has another 30 potential categories slated for the coming year as well as select initiatives related to utilization and standardization. For calendar year 2021, these member participants had approximately $11.2 billion in annual supply chain purchasing spend.
12
E-Commerce Platform. Our E-Commerce platform, STOCKDTM, is part of our multi-channel supply chain strategy, which provides a digital sales channel where members and other customers can purchase products under our PREMIERPRO brand as well as utilize limited savings programs from Premier GPO suppliers utilizing an e-commerce platform.
PROVIDEGXTM Program. The PROVIDEGX program identifies high-quality supply sources for drugs that are on or may be at risk of being added to the national drug shortage list or that are vulnerable to pricing volatility. The PROVIDEGX program is the next step in our ongoing effort to help facilitate the availability of high-quality pharmaceutical products, including drugs for which there may be supply challenges.
Performance Services
Our Performance Services segment consists of three sub-brands: PINC AI, Contigo Health and Remitra. Each serves different markets but are all united in our vision to optimize provider performance and accelerate industry innovation for better, smarter healthcare. Our PINC AI platform enables us to better reflect our current product offerings and strategy to expand and incorporate artificial intelligence (“AI”) across our portfolio of solutions. This platform further enables connectivity and scale between providers, the life sciences industry and payors, including large employers, to help lower the cost and improve the quality of care. We believe that we house one of the largest clinical, operational and financial datasets in the United States which enables actionable insight and real-world evidence needed to accelerate healthcare improvements. We currently incorporate AI into prior authorization between payors and providers and clinical intelligence through the decision support process which helps key healthcare stakeholders improve the quality, efficiency and value of healthcare delivery. Using our data and scale, we seek to expand our AI capabilities, grow our overall portfolio of solutions and provide our members and customers with technologically advanced products so they can provide better, smarter healthcare.
PINC AI:
With a broad provider network, advanced analytics, and the incorporation and desired expansion of AI-powered technology backed by our large dataset, we believe PINC AI has the ability to accelerate ingenuity in healthcare.
PINC AI helps optimize provider performance in three main areas – clinical intelligence, margin improvement and value-based care – using advanced analytics to identify improvement opportunities, consulting services for clinical and operational design and workflow solutions to hardwire sustainable change.
Clinical intelligence solutions help drive greater clinical effectiveness and efficiency across the care continuum by:
•Surfacing analytics and peer benchmarking on hard-to-find, high-value quality improvement areas, helping providers improve care delivery;
•Delivering real-time clinical surveillance to help providers drive faster, more informed decisions around patient safety, including ongoing infection prevention (like COVID-19), antimicrobial stewardship, and reduction of hospital acquired conditions;
•Using AI-enabled clinical decision support integrated into the provider workflow (EHR) to support evidence-based decisions by providers at the point of care, and improve prior authorization automation;
•Operating the QUEST Collaborative, which works to develop quality, safety and cost metrics with a consistency and standardization. We believe participation in the QUEST Collaborative better prepares providers to deal with evolving and uncertain healthcare reform requirements and differentiate on care delivery in their markets; and
•Providing life sciences services through Premier Applied Science® for the development of research, real-world evidence and clinical trials innovation for medical device, diagnostic and pharmaceutical companies.
Margin improvement solutions help lower total costs and improve provider operating margins by:
•Surfacing analytics and peer benchmarking on hard-to-find, supply savings and workforce management opportunities that lower costs without impacting quality;
•Optimizing workforce management with integrated financial reporting and budgeting across the continuum of care;
•Providing savings through an enterprise resource planning solution built specifically for healthcare;
13
•Deploying consulting services to deliver clinically integrated, margin improvement transformation throughout a health system; and
•Providing management services to insurance programs to assist U.S. hospital and healthcare system members with liability and benefits insurance services, along with risk management services to improve their quality, patient safety and financial performance while lowering costs.
Value-based care solutions help health systems implement effective models of care to succeed in new, value-based payment arrangements by:
•Surfacing analytics and peer benchmarking to help identify hard-to-find, population-based improvement opportunities necessary to take financial risk and succeed in value-based care;
•Optimizing and managing the Physician enterprise to rationalize medical group investment via revenue enhancement, cost reduction strategies and implementation of sustainable evidence-based practices; and
•Participating in the Population Health Management, Bundled Payment and Physician Enterprise Collaboratives, for the opportunity to share value-based care and payment developmental strategies, programs and best practices.
The data yielded through PINC AI is de-identified and aggregated in what we believe to be the nation’s leading comprehensive database, representing over 20 years of data from more than 1,000 hospitals spanning multiple therapeutic areas. A research team including clinicians, epidemiologists, health economists, health services researchers, statisticians and other subject matter experts leverage the dataset to deliver real world evidence, in partnership with Life Science innovators. Studies, test methods, strategies and tools created can promote the adoption and integration of evidence-based practices to help improve outcomes and the quality and effectiveness of care.
Contigo Health:
Contigo Health creates new ways for clinicians, health systems and employers to work together supporting a common goal for all stakeholders: to help increase access to high-quality care, enhance employee engagement, control costs and get employees back to work and life faster. Contigo Health delivers comprehensive services for optimizing employee health benefits, including:
•The Contigo Health Employer Centers of Excellence Network, which through partnerships with some of the nation’s top clinicians, helps to provide care through access to the highest quality outcomes for a bundled cost;
•The Contigo Health Sync Health Plan Administration, which empowers self-funded employers with a flexible approach to employee benefits to help improve access to quality care, achieve cost savings and improve member satisfaction; and
•The Contigo Health Network, which is expected to provide health systems with the ability to sell and participate in employer-focused products.
Remitra:
Remitra provides health systems and suppliers cost management solutions with our cloud-based procure-to-pay technology designed to support greater efficiencies in the procurement process through automated purchasing and payment solutions.
•Remitra’s Procure-to-Pay platform, a cloud-based platform which powers supplier and provider networks and uses optical character recognition to automate invoicing and payables. Remitra seeks to streamline financial processes, reduce errors and fraud, unlock cost and labor efficiencies and become a leading digital invoicing and payables platform for all of healthcare, agnostic of ERP, GPO or treasury partner.
•Remitra’s Cash Flow Optimizer platform, a financial solution for suppliers and providers which leverages Remitra’s cloud-based procure to pay platform and provides opportunities for financial improvements including a reduction in days sales outstanding, on-time payments, improved working capital and a potential reduction over time of allowance of credit losses associated with bad debt.
Pricing and Contracts
We generate revenue from our Supply Chain Services segment through administrative fees received from suppliers based on the total dollar volume of goods and services purchased by our members and other customers in connection with our GPO programs, service fees from supply chain co-management, subscription fees from purchased services and through product sales
14
in connection with our direct sourcing activities. We generate revenue from our Performance Services segment through our three sub-brands: PINC AI, Contigo Health and Remitra.
Supply Chain Services
Our GPO generates revenue through administrative fees received from contracted suppliers for a percentage of the purchase price of goods and services, including purchased services activities, sold to members under negotiated supplier contracts. Pursuant to the terms of GPO participation agreements entered into by the members, our members currently receive revenue share based upon purchasing by such member’s owned, leased, managed and affiliated facilities through our GPO supplier contracts.
The majority of our current GPO participation agreements with our members have terms that commenced in July 2020 and primarily range from five to seven years. Generally, our GPO participation agreements may not be terminated except for cause or in the event of a change of control of the GPO member. The GPO member can terminate the GPO participation agreement at the end of the then-current term by notifying Premier LP of the member’s decision not to renew. Our GPO participation agreements generally provide for liquidated damages in the event of a termination not otherwise permitted under the agreement. Due to competitive market conditions, we have experienced, and expect to continue to experience requests, at times, to provide existing and prospective members increases in revenue share on incremental and/or overall purchasing volume.
In our supply chain co-management activities, we earn revenue in the form of a service fee for services performed under the supply chain management contracts. Service fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed.
In our purchased services activities, we generate revenue through administrative fees, as described above, and subscription fees. Subscription fees, which we generate through our SaaS-based products, are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation.
In our direct sourcing activities, we earn revenue from product sales, including sales from aggregated purchases of certain products, as well as, in some cases, service or licensing fees. Products are sold to our members and other customers through direct shipment and distributor and wholesale channels. Products are also sold to regional medical-surgical distributors and other non-healthcare industries (i.e., foodservice). We have contracts with our members and other customers that buy products through our direct shipment option, which usually do not provide a guaranteed purchase or volume commitment requirement.
Performance Services
Performance Services revenue consists of revenue generated through our three sub-brands: PINC AI, Contigo Health and Remitra. The main sources of revenue under PINC AI are (i) subscription agreements to our SaaS-based clinical analytics products, (ii) enterprise analytics licensing revenue, (iii) professional fees for our consulting services and (iv) other miscellaneous revenue including annual subscriptions to our performance improvement collaboratives, insurance management service fees and commissions from insurance carriers for sponsored insurance programs. Contigo Health’s main sources of revenue are third party administrator fees and fees from the centers of excellence program and Remitra’s main source of revenue is fees from healthcare product suppliers and service providers.
PINC AI:
SaaS-based clinical analytics products subscriptions include the right to access our proprietary hosted technology on a SaaS basis, training and member support to deliver improvements in cost management, margin improvement, quality and safety, value-based care and provider analytics. Pricing varies by application and size of the healthcare system. Clinical analytics products subscriptions are generally three- to five-year agreements with automatic renewal clauses and annual price escalators that typically do not allow for early termination. These agreements do not allow for physical possession of the software. Subscription fees are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation. Implementation involves the completion of data preparation services that are unique to each member's data set and, in certain cases, the installation of member site-specific software, in order to access and transfer member data into our hosted SaaS-based clinical analytics products. Implementation is generally 60 to 240 days following contract execution before the SaaS-based clinical analytics products can be fully utilized by the member.
Enterprise analytics licenses include term licenses that range from three to ten years and offer clinical analytics products, improvements in cost management, quality and safety, value-based care and provider analytics. Pricing varies by application and size of healthcare system. Revenue on licensing is recognized upon delivery of the license and revenue from hosting and maintenance is recognized ratably over the life of the contract.
15
Professional fees for consulting services are sold under contracts, the terms of which vary based on the nature of the engagement. These services typically include general consulting, report-based consulting and cost savings initiatives. Fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed or when deliverables are provided. In situations where the contracts have significant contract performance guarantees or member acceptance provisions, revenue recognition occurs when the fees are fixed and determinable and all contingencies, including any refund rights, have been satisfied. Fees are based either on the savings that are delivered or a fixed fee.
Revenue from performance improvement collaboratives that support our offerings in cost management, quality and safety and value-based care is recognized over the service period as the services are provided, which is generally one year.
Insurance management service fees are recognized in the period in which such services are provided. Commissions from insurance carriers for sponsored insurance programs are earned by acting as an intermediary in the placement of effective insurance policies. Under this arrangement, revenue is recognized at a point in time on the effective date of the associated policies when control of the policy transfers to the customer and is constrained for estimated early terminations.
Contigo Health:
Contigo Health revenue consists of third party administrator fees and fees from the centers of excellence program. Third party administrator fees consist of integrated fees for the processing of self-insured health care plan claims. Third party administrator fees are invoiced to customers monthly and typically collected in that period. Revenue is recognized in the period in which the services have been provided. Fees from the centers of excellence program consist of administrative fees for access to a specialized care network of proven healthcare providers. Centers of excellence fees are invoiced to customers a month in arrears and typically collected in that period. Revenue is recognized in the period in which the services have been provided.
Remitra
Revenue for Remitra primarily consists of fees from healthcare product suppliers and service providers. Fees for services are invoiced to our customers monthly and typically collected in the following period. For fixed fee contracts, revenue is recognized in the period in which the services have been provided. For variable rate contracts, revenue is recognized as customers are invoiced. Additional revenue consists of fees from check replacement services which consist of monthly rebates from bank partners.
Revenue Concentration
Our customers consist of members and other healthcare businesses and non-healthcare businesses such as food service, schools and universities. Our top five customers generated revenue of approximately 21% and 28% of our consolidated net revenues for the years ended June 30, 2022 and 2021, respectively. For the fiscal year ended June 30, 2021, revenue generated from our largest customer, a non-healthcare customer in the Supply Chain Services segment, was approximately 15% of our consolidated net revenues for the year ended June 30, 2021. The significant increase in revenue concentration and revenue generated from our largest customer was due to the greater than normal purchases of products through our direct sourcing business by such customer primarily as of result of the COVID-19 pandemic.
Other than the aforementioned customer, no other customers accounted for more than 10% of our net revenue during each of the years ended June 30, 2022 and 2021.
Intellectual Property
We offer our members a range of products to which we have appropriate intellectual property rights, including online services, best practices content, databases, electronic tools, web-based applications, performance metrics, business methodologies, proprietary algorithms, software products and consulting services deliverables. We own and control a variety of trade secrets, confidential information, trademarks, trade names, copyrights, domain names and other intellectual property rights that, in the aggregate, are of material importance to our business.
We protect our intellectual property by relying on federal, state and common law rights, as well as contractual arrangements. We are licensed to use certain technology and other intellectual property rights owned and controlled by others, and, similarly, other companies are licensed to use certain technology and other intellectual property rights owned and controlled by us.
16
Research and Development
Our research and development (“R&D”) expenditures primarily consist of our strategic investment in internally developed software to develop new and enhance existing SaaS- and license-based products offerings and new product development in the areas of cost management, quality and safety and value-based care. From time to time, we may experience fluctuations in our research and development expenditures, including capitalized software development costs, across reportable periods due to the timing of our software development life cycles, with new product features and functionality, new technologies and upgrades to our service offerings.
Information Technology and Cybersecurity Risk Management
We rely on digital technology to conduct our business operations and engage with our members and business partners. The technology we, our members, and business partners use grows more complex over time as do threats to our business operations from cyber intrusions, denial of service attacks, manipulation and other cyber misconduct. Through a risk management approach that continually assesses and improves our Information Technology (IT) and cybersecurity risk deterrence capabilities, our Information Security and Risk Management groups have formed a functional collaboration to provide leadership and oversight when managing IT and cybersecurity risks.
Through a combination of Governance, Risk and Compliance (GRC) resources, we (i) proactively monitor IT controls to better ensure compliance with legal and regulatory requirements, (ii) assess adherence by third parties we partner with to ensure that the appropriate risk management standards are met, (iii) ensure essential business functions remain available during a business disruption, and (iv) monitor and continually develop and update response plans to address potential weaknesses and IT or cyber incidents should they occur. Our GRC resources are designed to prioritize IT and cybersecurity risks areas, identify solutions that minimize such risks, pursue optimal outcomes and maintain compliance with contractual obligations. We also maintain an operational security function that has a real time 24x7x365 response capability that triages incident management and triggers impact mitigation protocols. These capabilities allow us to apply best practices and reduce exposure in the case of a security incident. For more information regarding the risks associated with these matters, see “Item 1A. Risk Factors-We could suffer a loss of revenue and increased costs, exposure to material liability, reputational harm, and other serious negative consequences if we sustain cyber-attacks or other data security breaches that disrupt our operations or result in the dissemination of proprietary or confidential information about us or our members or other third parties.”
Competition
The markets for our products and services in both our Supply Chain Services segment and Performance Services segment are fragmented, highly competitive and characterized by rapidly evolving technology and product standards, user needs and the frequent introduction of new products and services. We have experienced and expect to continue to experience intense competition from a number of companies.
Our Supply Chain Services segment’s competitors primarily compete with our group purchasing and direct sourcing activities. Our group purchasing business competes with other large GPOs such as HealthTrust Purchasing Group (a subsidiary of HCA Holdings, Inc.), Managed Health Care Associates, Inc. and Vizient, Inc. In addition, we compete against certain healthcare provider-owned GPOs and on-line retailers in this segment. Our direct sourcing business competes primarily with private label offerings/programs, product manufacturers, and distributors, such as Cardinal Health, Inc., McKesson Corporation, Medline Industries, Inc. and Owens & Minor, Inc.
Our Performance Services segment’s competitors compete with our three sub-brands: PINC AI, Contigo Health and Remitra. The primary competitors of PINC AI range from smaller niche companies to large, well-financed and technologically sophisticated entities. Our primary competitors for PINC AI include (i) information technology providers such as Allscripts Healthcare Solutions, Inc., Change Healthcare, Epic Systems Corporation, Health Catalyst, Inc., IBM Corporation, Infor, Inc. and Oracle Corporation, and (ii) consulting and outsourcing firms such as Deloitte & Touche LLP, Evolent Health, Inc., Healthagen, LLC (a subsidiary of Aetna, Inc.), Huron Consulting, Inc., Guidehouse Consulting, Inc., Optum, Inc. (a subsidiary of UnitedHealth Group, Inc.) and Vizient, Inc. The primary competitors for Contigo Health include AmeriBen, Meritan Health, UMR, WebTPA and Benefit and Risk Management Services for our third party administrative services product, and Carrum Health, Bridge Health, Edison Healthcare, AccessHope and MSK Direct for our Centers of Excellence product. The primary competitors for Remitra include Global Healthcare Exchange, LLC and Prodigo Solutions, Inc. for our digital invoicing product and Coupa Software Inc. and Taulia for our digital payables product.
With respect to our products and services across both segments, we compete on the basis of several factors, including breadth, depth and quality of product and service offerings, ability to deliver clinical, financial and operational performance improvements through the use of products and services, quality and reliability of services, ease of use and convenience, brand
17
recognition and the ability to integrate services with existing technology. With respect to our products and services across both of our business segments, we also compete on the basis of price.
Government Regulation
General
The healthcare industry is highly regulated by federal and state authorities and is subject to changing legal, political, economic and regulatory influences. Factors such as changes in reimbursement policies for healthcare expenses, consolidation in the healthcare industry, regulation, litigation and general economic conditions affect the purchasing practices, operations and the financial health of healthcare organizations. In particular, changes in laws and regulations affecting the healthcare industry, such as increased regulation of the purchase and sale of medical products, or restrictions on permissible discounts and other financial arrangements, could require us to make unplanned and costly modifications to our products and services, and may result in delays or cancellations of orders or a reduction of funds and demand for our products and services.
We are subject to numerous risks arising from governmental oversight and regulation. You should carefully review the following discussion and the risks discussed under “Item 1A. Risk Factors” for a more detailed discussion.
Affordable Care Act
The Patient Protection and Affordable Care Act (“ACA”) is a sweeping law designed to expand access to affordable health insurance, control healthcare spending and improve healthcare quality. The law includes provisions to tie Medicare provider reimbursement to healthcare quality and incentives, mandatory compliance programs, enhanced transparency disclosure requirements, increased funding and initiatives to address fraud and abuse and incentives to state Medicaid programs to promote community-based care as an alternative to institutional long-term care services. In addition, the law created of an innovation center to test and scale new APMs and ACOs. These programs are creating fundamental changes in the delivery of healthcare. Likewise, many states have adopted or are considering changes in healthcare policies in part due to state budgetary shortfalls. Ongoing uncertainty regarding implementation of certain aspects of the ACA makes it difficult to predict the impact the ACA or state law proposals may have on our business. While certain aspects of the ACA remain subject to uncertainty in implementation, in a shift from the ACA’s treatment under the previous administration, which sought to repeal the ACA and eliminate many of its key provision by any means possible, the Biden administration has promoted and expressed support for the ACA. Moreover, in June 2021, the U.S. Supreme Court dismissed, for lack of standing, a challenge to the ACA brought by the Trump administration and a group of state Attorneys General thereby leaving the ACA intact. The Biden administration has identified that it will seek to undo certain of the restrictions placed on the ACA under the Trump administration, which may result in further changes to and re-broadening of formerly limited provisions. Any future changes may ultimately impact the provisions of the ACA or other laws or regulations that either currently affect, or may in the future affect, our business. We believe it is important to note that most of the controversy related to the ACA relates to coverage expansion and not the issues related to quality improvement and cost reduction.
U.S. Food and Drug Administration Regulation
The U.S. Food and Drug Administration (“FDA”) extensively regulates, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of pharmaceuticals and medical devices. To the extent that functionality or intended use in one or more of our current or future software products causes the software to be regulated as a medical device under existing or future FDA laws or regulations including the 21st Century Cures Act, which addresses, among other issues, the patient safety concerns generated by cybersecurity risks to medical devices and the interoperability between medical devices, we could be required to:
•register our company and list our FDA-regulated products with the FDA;
•obtain pre-market approval establishing the safety and efficacy of our regulated products or clearance from the FDA based on demonstration of substantial equivalence to a legally marketed device before marketing our regulated products;
•obtain an investigational device exemption (“IDE”) prior to conducting clinical trials with the regulated products;
•obtain FDA approval by demonstrating the safety and effectiveness of the regulated products prior to commercial marketing;
•submit to pre-market approval or post-market inspections by the FDA; and
•comply with various FDA regulations, including the agency’s quality system regulation, complaint handling and medical device reporting regulations, requirements for medical device modifications, increased rigor of the secure development life cycle in the development of medical devices and the interoperability of medical devices and
18
electronic health records, requirements for clinical investigations or post-market studies, corrections and removal reporting regulations, and post-market surveillance regulations.
A new medical device must be cleared or approved by FDA through the pre-market approval (“PMA”) or 510(k) clearance. For medical devices that require a PMA, clinical studies performed under an IDE will become part of a PMA for a medical device.
Once a medical device product requiring a PMA is identified for development, it enters the feasibility study stage. For significant risk devices, including devices that are substantially important in diagnosing, curing, mitigating or treating disease or in preventing impairment to human health, sponsors must submit an investigational plan to the FDA as part of the IDE. The IDE automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. An IDE sponsor typically must submit results of feasibility studies to FDA to receive approval to proceed with a pivotal study. A pivotal study is generally intended as the primary clinical support for a marketing application.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with good clinical practice (“GCP”) regulations. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IDE, and progress reports detailing the results of the clinical trials must be submitted at least annually. In addition, timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. Medical devices typically rely on one or a few pivotal studies. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board (“IRB”). An IRB responsible for the research conducted at each institution participating in the clinical trial must review and approve each protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative, monitor the study until completed and otherwise comply with IRB regulations.
The FDA, the IRB, or we could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits or a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's requirements or if the device has been associated with unexpected serious harm to patients.
During the development of a new medical device, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IDE and before a PMA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end of feasibility studies to plan for their pivotal trial or trials for a medical device.
Appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life. Before approving a PMA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in full compliance with Current Good Manufacturing Practices (“cGMP”) requirements and adequate to assure consistent production of the product within required specifications.
Manufacturers and others involved in the manufacture and distribution of FDA regulated products must also register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process. Any product manufactured by or imported from a facility that has not registered, whether foreign or domestic, is deemed misbranded under the Federal Food, Drug, and Cosmetic Act (“FDCA”) (21 U.S.C. § 301 et seq.).
Establishments may be subject to periodic unannounced inspections by government authorities to ensure compliance with cGMP and other laws. Manufacturers may have to provide, on request, electronic or physical records regarding their establishments. Delaying, denying, limiting, or refusing inspection by the FDA may lead to a product being deemed to be adulterated.
U.S. Review and Approval Processes for Medical Devices
Unless an exemption applies, medical devices commercially distributed in the United States require either premarket notification, or 510(k) clearance, or approval of a PMA application from the FDA. The FDA classifies medical devices into one of three classes. Class I devices, considered to have the lowest risk, are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable
19
portions of the FDA’s Quality System Regulation (“QSR”) facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (“General Controls”). Class II devices are subject to the FDA's General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device (“Special Controls”). Manufacturers of most Class II and some Class I devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA, requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. The submission of a 510(k) or PMA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances.
510(k) Clearance Pathway for Medical Devices
When a 510(k) clearance is required, an applicant is required to submit a 510(k) application demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of PMAs. By regulation, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance may take longer. Typically, the FDA’s response after reviewing a 510(k) application is a request for additional data or clarification. Depending on the complexity of the application and the amount of data required, the process may be lengthened by several months or more. If additional data, including clinical data, are needed to support our claims, the 510(k) application process may be significantly lengthened.
If the FDA issues an order declaring the device to be Not Substantially Equivalent (“NSE”), the device is placed into a Class III or PMA category. At that time, a manufacturer can request a de novo classification of the product. De novo generally applies where there is no predicate device and the FDA believes the device is sufficiently safe so that no PMA should be required. The request must be in writing and sent within 30 days from the receipt of the NSE determination. The request should include a description of the device, labeling for the device, reasons for the recommended classification and information to support the recommendation. The de novo process has a 60-day review period. If the FDA classifies the device into Class II, a company will then receive an approval order to market the device. This device type can then be used as a predicate device for future 510(k) submissions. However, if the FDA subsequently determines that the device will remain in the Class III category, the device cannot be marketed until the manufacturer has obtained an approved PMA.
Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device, requires a new 510(k) clearance and may even, in some circumstances, require a PMA if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer's decision. If the FDA were to disagree with a manufacturer's determination that changes did not require a new 510(k) submission, it could require the manufacturer to cease marketing and distribution or recall the modified device until 510(k) clearance or PMA approval is obtained. If the FDA requires the manufacturer to seek 510(k) clearance or PMA approval for any modifications, the manufacturer may be required to cease marketing or recall the modified device, if already in distribution, until 510(k) clearance or PMA approval is obtained.
Premarket Approval (PMA) Pathway for Medical Devices
While we believe that if any functionality in one or more of our current or future software products causes the software to be regulated as a medical device, our software products will be subject to the 510(k) clearance pathway, FDA could evaluate our product under the PMA pathway if it believes the device component raises sufficiently complex or novel scientific issues.
A PMA application must be submitted to the FDA if the device cannot be cleared through the 510(k) process, or is not otherwise exempt from the FDA’s premarket clearance and approval requirements. A PMA application must generally be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling, to demonstrate to the FDA's satisfaction the safety and effectiveness of the device for its intended use. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel's recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturer or third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR. Once a PMA is approved, the FDA may require that certain conditions of approval be met, such as conducting a post-market clinical trial.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA
20
supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. Such trials generally require an application for an IDE, which is approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating, or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject.
Post-Approval Regulation of Medical Devices
After a product is placed on the market, numerous regulatory requirements continue to apply. In addition to the requirements below, adverse event reporting regulations require that manufacturers report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Additional regulatory requirements include:
•product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
•QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, validation, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;
•labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved, or off-label use or indication;
•clearance of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared or approved devices;
•notice or approval of product or manufacturing process modifications or deviations that affect the safety or effectiveness of one of our cleared or approved devices;
•post-approval restrictions or conditions, including post-approval study commitments;
•post-market surveillance regulations, which apply, when necessary, to protect the public health or to provide additional safety and effectiveness data for the device;
•the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
•during a public health emergency, notification of permanent discontinuation of a device or a supply disruption due to an interruption in the manufacturing of a device, and the reasons for such discontinuance or supply disruption;
•regulations pertaining to voluntary recalls; and
•notices of corrections or removals.
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the U.S. Federal Trade Commission, or FTC, and by state regulatory and enforcement authorities. Promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. Furthermore, under the federal U.S. Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. In addition, manufacturers are required to meet regulatory requirements in countries outside the United States, which can change rapidly with relatively short notice. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved or uncleared use, it could request that we modify our training or promotional materials or be subjected to regulatory or enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take enforcement actions against us if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in material fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
Failure by us or by our third-party manufacturers and suppliers to comply with all applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in sanctions including, but not limited to:
•untitled letters, warning letters, fines, injunctions, consent decrees and civil monetary penalties;
•customer notifications or repair, replacement, refunds, recall, detention or seizure of our products;
•operating restrictions or partial suspension or total shutdown of production;
•refusing or delaying requests for 510(k) clearance or PMA approvals of new products or modified products;
21
•withdrawing 510(k) clearances or PMA approvals that have already been granted;
•refusing to grant export approval for our products; or
•criminal prosecution.
Civil and Criminal Fraud and Abuse Laws
We are subject to federal and state laws and regulations designed to protect patients, governmental healthcare programs and private health plans from fraudulent and abusive activities. These laws include anti-kickback restrictions and laws prohibiting the submission of false or fraudulent claims. These laws are complex and broadly worded, and their application to our specific products, services and relationships may not be clear and may be applied to our business in ways that we do not anticipate. Federal and state regulatory and law enforcement authorities have over time increased enforcement activities with respect to Medicare and Medicaid fraud and abuse regulations and other reimbursement laws and rules. These laws and regulations include:
Anti-Kickback Laws. The federal Anti-Kickback Statute prohibits the knowing and willful offer, payment, solicitation or receipt of remuneration, directly or indirectly, in return for the referral of patients or arranging for the referral of patients, or in return for the recommendation, arrangement, purchase, lease or order of items or services that are covered, in whole or in part, by a federal healthcare program such as Medicare or Medicaid. The definition of "remuneration" has been broadly interpreted to include anything of value such as gifts, discounts, rebates, waiver of payments or providing anything at less than its fair market value. Many states have adopted similar prohibitions against kickbacks and other practices that are intended to influence the purchase, lease or ordering of healthcare items and services regardless of whether the item or service is covered under a governmental health program or private health plan. Certain statutory and regulatory safe harbors exist that protect specified business arrangements from prosecution under the Anti-Kickback Statute if all elements of an applicable safe harbor are met, however these safe harbors are narrow and often difficult to comply with. Congress has appropriated an increasing amount of funds in recent years to support enforcement activities aimed at reducing healthcare fraud and abuse.
The U.S. Department of Health and Human Services, or HHS, created certain safe harbor regulations which, if fully complied with, assure parties to a particular arrangement covered by a safe harbor that they will not be prosecuted under the Anti-Kickback Statute. We attempt to structure our group purchasing services, pricing discount arrangements with suppliers, and revenue share arrangements with applicable members to meet the terms of the safe harbor for GPOs set forth at 42 C.F.R. § 1001.952(j) and the discount safe harbor set forth at 42 C.F.R. § 1001.952(h). Although full compliance with the provisions of a safe harbor ensures against prosecution under the Anti-Kickback Statute, failure of a transaction or arrangement to fit within a safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the Anti-Kickback Statute will be pursued. From time to time, HHS, through its Office of Inspector General, makes formal and informal inquiries, conducts investigations and audits the business practices of GPOs, including our GPO, the result of which could be new rules, regulations or in some cases, a formal enforcement action.
To help ensure regulatory compliance with HHS rules and regulations, our members that report their costs to Medicare are required under the terms of the Premier Group Purchasing Policy to appropriately reflect all elements of value received in connection with our IPO, including under the various agreements entered into in connection therewith, on their cost reports. We are required to furnish applicable reports to such members setting forth the amount of such value, to assist their compliance with such cost reporting requirements. There can be no assurance that the HHS Office of Inspector General or the U.S. Department of Justice, or DOJ, will concur that these actions satisfy their applicable rules and regulations.
False Claims Act. Our business is also subject to numerous federal and state laws that forbid the submission or “causing the submission” of false or fraudulent information or the failure to disclose information in connection with the submission and payment of claims for reimbursement to Medicare, Medicaid or other governmental healthcare programs or private health plans. In particular, the False Claims Act, or FCA, prohibits a person from knowingly presenting or causing to be presented a false or fraudulent claim for payment or approval by an officer, employee or agent of the United States. In addition, the FCA prohibits a person from knowingly making, using, or causing to be made or used a false record or statement material to such a claim. Violations of the FCA may result in treble damages, material monetary penalties, and other collateral consequences including, potentially, exclusion from participation in federally funded healthcare programs. A claim that includes items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA.
Privacy and Security Laws. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, contains substantial restrictions and requirements with respect to the use and disclosure of certain individually identifiable health information, referred to as “protected health information.” The HIPAA Privacy Rule prohibits a covered entity or a business associate (essentially, a third party engaged to assist a covered entity with enumerated operational and/or compliance functions) from using or disclosing protected health information unless the use or disclosure is validly authorized by the individual or is specifically required or permitted under the HIPAA Privacy Rule and only if certain complex requirements are met. In addition
22
to following these complex requirements, covered entities and business associates must also meet additional compliance obligations set forth in the HIPAA Privacy Rule. The HIPAA Security Rule establishes administrative, organizational, physical and technical safeguards to protect the privacy, integrity and availability of electronic protected health information maintained or transmitted by covered entities and business associates. The HIPAA Security Rule requirements are intended to mandate that covered entities and business associates regularly re-assess the adequacy of their safeguards in light of changing and evolving security risks. Finally, the HIPAA Breach Notification Rule requires that covered entities and business associates, under certain circumstances, notify patients/beneficiaries, media outlets and HHS when there has been an improper use or disclosure of protected health information.
Our self-funded health benefit plan and our healthcare provider members (provided that these members engage in HIPAA-defined standard electronic transactions with health plans, which will be all or the vast majority) are directly regulated by HIPAA as “covered entities.” Additionally, because most of our U.S. hospital members disclose protected health information to us so that we may use that information to provide certain data analytics, benchmarking, consulting or other operational and compliance services to these members, we are a “business associate” of those members. In these cases, in order to provide members with services that involve the use or disclosure of protected health information, HIPAA requires us to enter into “business associate agreements” with our covered entity members. Such agreements must, among other things, provide adequate written assurances:
(i)as to how we will use and disclose the protected health information within certain allowable parameters established by HIPAA,
(ii)that we will implement reasonable and appropriate administrative, organizational, physical and technical safeguards to protect such information from impermissible use or disclosure,
(iii)that we will enter into similar agreements with our agents and subcontractors that have access to the information,
(iv)that we will report breaches of unsecured protected health information, security incidents and other inappropriate uses or disclosures of the information, and
(v)that we will assist the covered entity with certain of its duties under HIPAA.
With the enactment of the Health Information Technology for Economic and Clinical Health, or HITECH Act, the privacy and security requirements of HIPAA were modified and expanded. The HITECH Act applies certain of the HIPAA privacy and security requirements directly to business associates of covered entities. Prior to this change, business associates had contractual obligations to covered entities but were not subject to direct enforcement by the federal government. In 2013, HHS released final rules implementing the HITECH Act changes to HIPAA. These amendments expanded the protection of protected health information by, among other things, imposing additional requirements on business associates, further restricting the disclosure of protected health information in certain cases when the disclosure is part of a remunerated transaction, and modifying the HIPAA Breach Notification Rule, which has been in effect since September 2009, to create a rebuttable presumption that an improper use or disclosure of protected health information under certain circumstances requires notice to affected patients/beneficiaries, media outlets and HHS.
Transaction Requirements. HIPAA also mandates format, data content and provider identifier standards that must be used in certain electronic transactions, such as claims, payment advice and eligibility inquiries. Although our systems are fully capable of transmitting transactions that comply with these requirements, some payors and healthcare clearinghouses with which we conduct business may interpret HIPAA transaction requirements differently than we do or may require us to use legacy formats or include legacy identifiers as they make the transition to full compliance. In cases where payors or healthcare clearinghouses require conformity with their interpretations or require us to accommodate legacy transactions or identifiers as a condition of successful transactions, we attempt to comply with their requirements, but may be subject to enforcement actions as a result. In 2009, CMS published a final rule adopting updated standard code sets for diagnoses and procedures known as ICD-10 code sets and changing the formats to be used for electronic transactions subject to the ICD-10 code sets, known as Version 5010. All healthcare providers are required to comply with Version 5010 and use the ICD-10 code sets.
Other Federal and State Laws. In addition to our obligations under HIPAA there are other federal laws that impose specific privacy and security obligations, above and beyond HIPAA, for certain types of health information and impose additional sanctions and penalties. These rules are not preempted by HIPAA. Most states have enacted patient and/or beneficiary confidentiality laws that protect against the disclosure of confidential medical information, and many states have adopted or are considering adopting further legislation in this area, including privacy safeguards, security standards, data security breach notification requirements, and special rules for so-called “sensitive” health information, such as mental health, genetic testing results, or Human Immunodeficiency Virus, or HIV, status. These state laws, if more stringent than HIPAA requirements, are not preempted by the federal requirements, and we are required to comply with them as well.
23
We are unable to predict what changes to HIPAA or other federal or state laws or regulations might be made in the future or how those changes could affect our business or the associated costs of compliance.
Antitrust Laws
The Sherman Antitrust Act and related federal and state antitrust laws are complex laws that prohibit contracts in restraint of trade or other activities that are designed to or that have the effect of reducing competition in the market. The federal antitrust laws promote fair competition in business and are intended to create a level playing field so that both small and large companies are able to compete in the market. In their 1996 Statements of Antitrust Enforcement Policy in Health Care, or the Healthcare Statements, the DOJ and the Federal Trade Commission, or FTC, set forth guidelines specifically designed to help GPOs gauge whether a particular purchasing arrangement may raise antitrust concerns and established an antitrust safety zone for joint purchasing arrangements among healthcare providers. Under this antitrust safety zone, the DOJ and FTC will not challenge, except in extraordinary circumstances, joint purchasing arrangements among healthcare providers that meet two basic conditions: (i) the purchases made by the healthcare providers account for less than 35% of the total sales of the purchased product or service in the relevant market; and (ii) the cost of the products and services purchased jointly account for less than 20% of the total revenues from all products and services sold by each competing participant in the joint purchasing arrangement.
We have attempted to structure our contracts and pricing arrangements in accordance with the Healthcare Statements and believe that our GPO supplier contracts and pricing discount arrangements should not be found to violate the antitrust laws. No assurance can be given that enforcement authorities will agree with this assessment. In addition, private parties also may bring suit for alleged violations under the U.S. antitrust laws. From time to time, the group purchasing industry comes under review by Congress and other governmental bodies with respect to antitrust laws, the scope of which includes, among other things, the relationships between GPOs and their members, distributors, manufacturers and other suppliers, as well as the services performed and payments received in connection with GPO programs.
Congress, the DOJ, the FTC, the U.S. Senate or another state or federal entity could at any time open a new investigation of the group purchasing industry, or develop new rules, regulations or laws governing the industry, that could adversely impact our ability to negotiate pricing arrangements with suppliers, increase reporting and documentation requirements, or otherwise require us to modify our arrangements in a manner that adversely impacts our business. We may also face private or government lawsuits alleging violations arising from the concerns articulated by these governmental factors or alleging violations based solely on concerns of individual private parties.
Health IT Certification Program
In 2009, Congress included in the American Recovery and Reinvestment Act a program to incentivize the adoption of health information technology by hospitals and ambulatory providers who participate in the Medicare and Medicaid programs. Congress further modified the incentive program for ambulatory providers under the Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”). Any developer of health information technology seeking to offer a product to assist hospitals or ambulatory health care providers to meet the requirements of these programs must obtain certification under the applicable certification criteria established by the Office of the National Coordinator for Health Information Technology (“ONC”). There are two types of certification for health information developers seeking to participate in the certification program: 1) certification to all the certification criteria required to meet the definition of a “2015 Edition Base EHR” or 2) certification as a Health IT Module, meeting specific certification criteria. Meeting the certification criteria as a “2015 Edition Base EHR” allows a developer of health information technology to offer a product that has all the capabilities needed for a hospital or an ambulatory provider to meet the requirements of the health IT incentive programs. A Health IT Module provides a specific set of capabilities. Hospitals or ambulatory providers seeking to avoid potential payment reductions must either implement a 2015 Base EHR using a single product, or multiple Health IT Modules that together have all of the capabilities of a 2015 Base EHR.
We currently have two products that are certified as Health IT Modules. To retain our certification, we must: 1) meet applicable conditions of certification and maintenance of certification requirements established by ONC; 2) pass testing conducted by an ONC-Authorized Testing Laboratory pursuant to test procedures developed by ONC; and 3) obtain certification from an ONC-Authorized Certification Body. ONC’s conditions of certification and maintenance of certification requirements include communication restrictions that largely prevent us from limiting our customer's ability to communicate about the usability, interoperability, security or user experiences relating to our Health IT Modules. These regulations require us to review and modify current contract terms or inform customers that offending contract terms we previously entered into are no longer effective. We are also required to develop and execute a real-world testing plan, which would require us to demonstrate to our ONC-Authorized Certification Body that our Health IT Modules operate as designed when implemented in the field. Failure to properly implement these requirements could result in our two products losing their status as Health IT Modules, which could jeopardize the utility of the products for our customers. We work closely with our selected ONC-Authorized Testing Laboratory
24
and ONC-Authorized Certification Body to meet these and other requirements of Health IT Certification Program. We are unable to predict what changes to the certification program might be made in the future or how those changes could affect our business or the associated costs of compliance.
ERISA and Laws Impacting Employer Group Health Plans
Many of the clients we serve sponsor employer group health plans, which are subject to the Employee Retirement Income Security Act of 1974 (ERISA), the Internal Revenue Code, Medicare Secondary Payer statute, HIPAA privacy, and in some cases, state insurance laws. While compliance for these various rules falls on the employer-sponsor of the health plan, in some cases, compliance is delegated to a vendor, such as us. We protect ourselves from liability for these client health plans by virtue of contractual provisions insulating us from exposure and responsibility for the employer-sponsor's legal obligations.
Governmental Audits
Because we act as a GPO for healthcare providers that participate in governmental programs, our group purchasing services have in the past and may again in the future be subject to periodic surveys and audits by governmental entities or contractors for compliance with Medicare and Medicaid standards and requirements. We will continue to respond to these government reviews and audits but cannot predict what the outcome of any future audits may be or whether the results of any audits could materially or negatively impact our business, our financial condition or results of operations.
Corporate Compliance Department
We execute and maintain a compliance and ethics program that is designed to assist us and our employees in conducting operations and activities ethically with the highest level of integrity and in compliance with applicable laws and regulations and, if violations occur, to promote early detection and prompt resolution. These objectives are achieved through education, monitoring, disciplinary action and other remedial measures we believe to be appropriate. We provide all of our employees with education that has been developed to communicate our standards of conduct, compliance policies and procedures as well as policies for monitoring, reporting and responding to compliance issues. We also provide all of our employees with a third-party toll-free number and Internet website address in order to report any compliance or privacy concerns. In addition, our Chief Ethics & Compliance Officer individually, and along with the Audit and Compliance Committee of the Board of Directors, helps oversee compliance and ethics matters across our business operations.
Human Capital Management
Our employees are our most critical assets. The success and growth of our business depends on our ability to attract, reward, retain, and develop diverse, talented, and high-performing employees at all levels of our organization, while sustaining an environment of anti-discrimination that ensures equal access to opportunities. To succeed in an ever-changing and competitive labor market, we have developed human capital management strategies, objectives and measures that drive recruitment and retention, support business performance, advance innovation, foster employee development and support our Mission — to improve the health of our communities, our Vision — to lead the transformation to high quality, cost effective healthcare, and our Values — integrity, passion for performance, innovation and a focus on people.
Our Mission, Vision and Values, together with our human capital strategies, objectives and measures, form a framework advanced through the following programs and initiatives:
25
Support Employees’ Financial, Health, and Social Well-Being •Competitive, reasonable, and equitable compensation programs designed to align pay and performance and attract and retain employees who are passionate about our mission and exemplify our values. •Annual and long-term incentives designed to drive business and individual achievement. •Comprehensive, competitive, and innovative health and welfare and retirement benefits to support our employees’ physical and financial health. •Employee Stock Purchase Plan and equity compensation to provide financial value, align employees’ interests with those of our shareholders and drive talent retention. •Comprehensive benefits and well-being programs to support all aspects of employee well-being, including physical, emotional, financial and social health. •Generous time off programs. •Social Responsibility Programs including paid Annual Volunteer Afternoon, volunteering hours and matching gifts to give back to the communities in which we serve. •Flexible work environments - including remote and hybrid work options where possible - and enabled technology to enhance employee experience and connectedness in both virtual and in-person settings. •Robust and adaptive COVID-19 response to support the health and safety of our staff. Recognize Employees’ Performance and Contributions •Premier Individual and Team Values Awards to recognize employees who best exemplify Premier’s core values. •Susan D. DeVore President’s Award to recognize the significant career accomplishments of select employees. •Shirley T. Wang Wellness Warrior Award to recognize employees’ commitment to and passion for well-being. •Values in Action online portal to encourage employees in real time to publicly recognize and reward their peers for performance, innovation, focus on people and integrity. | Promote a Diverse, Equitable and Inclusive Workplace •Council on Diversity, Equity, Inclusion and Belonging. •Network of executive-sponsored, employee-led Employee Resource Groups (“ERGs”) designed to build community and foster belonging and advancement of business strategy and employee experience through sharing of diverse thought and perspective. Groups include W.O.M.E.N, Military Veterans, Black Professionals, LGBTQ+, Asian Employees, Latin, Asian Indian Professionals, Disabled Employees and Generations and their Allies groups. We also have a Field Services Advisory Council ERG comprised of employees dedicated to supporting our members. •Regular and ongoing review of compensation equity. •Mentoring and networking programs. •Recruiting outreach to drive diverse representation within our communities. •Continuous listening strategies including semi-annual People First employee engagement survey to seek feedback on a variety of topics to continuously improve our human resources programs, practices and employee experience. Create Opportunities to Grow and Develop •Comprehensive technology-enabled learning and development programs to foster connections, leadership competency and team and individual development. •Leadership and Management development programs. •Performance Management program including a formal, quarterly employee performance feedback cadence to drive high performance and reward excellence. •Enterprise talent planning and career pathing. •Tuition reimbursement program to support continuing education. Company Recognition •World’s Most Ethical Company by the Ethisphere Institute for the 15th consecutive year. •2020 Golden Peacock Award for Global Excellence in Corporate Governance. •Healthiest Employers of Charlotte by Charlotte Business Journal (1st place). •2021 Healthiest 100 Workplaces in America (49th place). •2021 Cigna Well-Being Award (Honorable Culture). •LinkedIn’s 2021 Top Companies in Charlotte. •2021 Prism International Diversity Impact Award for Top 25 National ERGs | ||||
26
Employees
As of June 30, 2022, we employed approximately 2,600 people, all in the United States. We also engage contractors and consultants. Additionally, we regularly track and report internally on key talent metrics including workforce demographics, talent pipeline, diversity data and the engagement of our employees. None of our employees are working under a collective bargaining arrangement.
We conduct sales through our embedded field force, our dedicated national sales team, our Premier consultants, and our Continuum of Care team, collectively comprised of approximately 600 employees as of June 30, 2022.
Our field force works closely with our U.S. hospital members and other members to target new opportunities by developing strategic and operational plans to drive cost management and quality and safety improvement initiatives. As of June 30, 2022, our field force was deployed to seven geographic regions and several strategic/affinity members across the United States. This field force works at our member sites to identify and recommend best practices for both supply chain and clinical integration cost savings opportunities. The regionally deployed field force is augmented by a national team of subject matter specialists who focus on key areas such as lab, surgery, cardiology, orthopedics, imaging, pharmacy, information technology and construction. Our field force assists our members in growing and supporting their Continuum of Care facilities.
Our national sales team provides national sales coverage for establishing initial member relationships and works with our field force to increase sales to existing members. Our regional sales teams are aligned with the seven regions in our field force model.
Our Premier consulting team identifies and targets consulting engagements and wrap-around services for our major SaaS-based clinical analytics products and our GPO to enhance the member value from these programs.
Our Continuum of Care team provides service to these classes of trade and serve a dual role of both enhancing contract penetration (selling current members additional contracts) as well as bringing on new providers to the program.
Available Information
We file or furnish, as applicable, annual, quarterly and current reports, proxy statements and other information with the SEC. You may access these reports and other information without charge at a website maintained by the SEC. The address of this site is https://www.sec.gov. In addition, our website address is www.premierinc.com. We make available through our website the documents identified above, free of charge, promptly after we electronically file such material with, or furnish it to, the SEC.
We also provide information about our company through: Twitter (https://twitter.com/premierha), Facebook (https://www.facebook.com/premierhealthcarealliance), LinkedIn (https://www.linkedin.com/company/6766), YouTube (https://www.youtube.com/user/premieralliance), and Instagram (https://instagram.com/premierha).
Except as specifically indicated otherwise, the information available on our website, the SEC’s website and the social media outlets identified above, is not and shall not be deemed a part of this Annual Report.
27
Item 1A. Risk Factors
Our business, operations, and financial position are subject to various risks. Before making an investment in our Class A common stock or other securities we may have outstanding from time to time, you should carefully consider the following risks, as well as the other information contained in this Annual Report. Any of the risks described below could materially harm our business, financial condition, results of operations and prospects, and as a result, the value of an investment in our Class A common stock or other securities we may have outstanding from time to time could decline, and you may lose part or all of such investment value. This section does not describe all risks that are or may become applicable to us, our industry or our business, and it is intended only as a summary of certain material risk factors. Some statements in this Annual Report, including certain statements in the following risk factors, constitute forward-looking statements. See the section titled “Cautionary Note Regarding Forward-Looking Statements” for a discussion of such statements and their limitations. More detailed information concerning other risks or uncertainties we face, as well as the risk factors described below, is contained in other sections of this Annual Report.
Risk Factors Summary
The following is a summary of the risk factors that could adversely affect our Company and the value of an investment in our Company’s securities.
Risks Related to our Business Operations
•Continuing uncertain economic conditions, including inflation and the risk of a global recession could impair our ability to forecast and may harm our business, operating results, including our revenue growth and profitability, financial condition and cash flows.
•We may continue to face financial and operational uncertainty due to the COVID-19 pandemic, variants thereof, or other pandemics and associated supply chain disruptions.
•We may face financial and operational uncertainty due to global economic and political instability and conflicts, such as the conflict between Russia and Ukraine.
•We face risks related to competition and consolidation in the healthcare industry.
•We may experience delays recognizing or increasing revenue if the sales cycle or implementation period takes longer than expected.
•The risk of loss of one or more of our larger members which could reduce activity levels or that members elect to terminate or not to renew their contracts.
•The markets for our software as a service (“SaaS”) or licensed-based products and services may develop more slowly than we expect, or we may convert more SaaS-based products to license-based products, which could adversely affect our revenue, growth rates and our ability to maintain or increase our profitability.
•Our members are highly dependent on payments from third-party payors, such as Medicare and Medicaid, the denial or reduction of which could adversely affect demand for our products and services.
•We rely on administrative fees that we receive from GPO suppliers.
•Our growth may be affected by our ability to offer new and innovative products and services as well as our ability to maintain third-party provider and strategic alliances or enter into new alliances.
•We face risks and expenses related to future acquisition opportunities and integration of acquisitions, as well as risks associated with non-controlling investments in other businesses or joint ventures.
•We rely on Internet infrastructure, bandwidth providers, data center providers and other third parties and face risks related to data loss or corruption and cyber-attacks or other data security breaches.
•We depend on our ability to use, disclose, de-identify or license data and to integrate third-party technologies.
•We face risks related to our use of “open source” software.
•We face risks associated with our reliance on contract manufacturing facilities located in various parts of the world.
•We may face inventory risk for (i) the personal protective equipment or other products we may purchase at elevated prices during a supply shortage, and (ii) items we purchase in bulk or pursuant to fixed price purchase commitments if we cannot sell such inventory at or above our cost.
•We depend on our ability to attract, hire, integrate and retain key personnel.
•We face risks related to our current and future indebtedness, including our existing long-term credit facility.
•We experience fluctuation in our quarterly cash flows, revenues and results of operations.
28
Regulatory Risks
•We are subject to changes and uncertainty in the legal, political, economic and regulatory environment affecting healthcare organizations.
•We must comply with complex international, federal and state laws and regulations governing financial relationships among healthcare providers and the submission of false or fraudulent healthcare claims, antitrust and employee benefit laws and regulations and privacy, security and breach notification laws.
•Certain of our software products may be subject to regulation regarding health information technology and medical devices.
Legal and Tax-Related Risks
•We are subject to litigation from time to time, including the pending shareholder derivative action against certain of our current and former officers and directors.
•We must adequately protect our intellectual property, and we face potential claims against our use of the intellectual property of third parties.
•We face tax risks, including potential sales and use, franchise and income tax liability in certain jurisdictions, future changes in tax laws and potential material tax disputes.
Risks Related to our Corporate Structure
•We are obligated to make payments under our Unit Exchange and Tax Receivable Acceleration Agreements, and we may not realize all of the expected tax benefits corresponding to the termination of our prior Tax Receivable Agreement.
•Provisions in our certificate of incorporation and bylaws and provisions of Delaware law may impede or prevent strategic transactions, including a takeover of the company.
•We are required to maintain an effective system of internal controls over financial reporting and remediate any material weaknesses and significant deficiencies identified.
•We face risks related to our Class A common stock, including potentially dilutive issuances and uncertainty regarding future dividend payments and stock repurchases.
For a more complete discussion of the material risks facing our business, see below.
Risks Related to Our Business Operations
Continued uncertain economic conditions, including inflation and the risk of a global recession could impair our ability to forecast and may harm our business, operating results, including our revenue growth and profitability, financial condition and cash flows.
Continued global economic uncertainty, political conditions and fiscal challenges in the U.S. and abroad, such as inflation and potential economic recession, have, among other things, limited our ability to forecast future demand for our products and services, contributed to increased periodic volatility in customer demand, impacted availability of supplies and could constrain future access to capital for our suppliers, customers and partners. The impacts of these circumstances are global and pervasive, and the timing and nature of any ultimate resolution of these matters remain highly uncertain. Adverse macroeconomic conditions, including inflation, slower growth or recession, new or increased trade sanctions, tariffs or other barriers to global trade, changes to fiscal and monetary policy and higher interest rates, could materially adversely impact the demand for our products and our operating results. In particular, in fiscal 2022, we experienced inflationary pressure and other constraints in our supply chain. Consequently, these concerns have challenged our business and we expect them to continue to challenge our business for the foreseeable future, which could cause harm to our operating results. Such conditions may result in the failure to meet our forecasted financial expectations and to achieve historical levels of revenue growth.
Our financial condition and results of operations for fiscal year 2023 and beyond may continue to be materially and adversely affected by the coronavirus (“COVID-19”) pandemic, reoccurrences of COVID-19 or variants thereof, or similar pandemics, or other future widespread public health epidemics.
The COVID-19 pandemic spread throughout the United States and the rest of the world beginning in early 2020. In addition to those who were directly affected, millions more have been affected by government and voluntary efforts around the world to slow the spread of the pandemic through quarantines, travel restrictions, business shut-downs, heightened border security and other measures. While the health consequences for the U.S. population have been mitigated to some degree by the availability of vaccines and therapeutics to treat COVID-19 infections, adverse economic impacts continue both domestically and
29
internationally, including the potential for new and extended government imposed lock-downs, border restrictions and transportation and other bottlenecks.
As a result of the COVID-19 pandemic, variants thereof, and potential future pandemic outbreaks, we face material risks including, but not limited to:
•Overall economic and capital markets decline. The impact of the COVID-19 pandemic and variants thereof and associated supply chain disruptions could result in a prolonged recession or depression in the United States or globally that could harm the banking system, limit demand for many products and services and cause other foreseen and unforeseen events and circumstances, all of which could negatively impact us. The continued spread of COVID-19 and variants thereof has led to and could continue to lead to severe disruption and volatility in the United States and global capital markets, which could increase our cost of capital and adversely affect our ability to access the capital markets in the future. In addition, trading prices on the public stock market, as well as that of our Class A common stock, have been highly volatile as a result of the COVID-19 pandemic.
•Changes in the demand for our products and services. We experienced and may continue to experience demand uncertainty from both material increases and decreases in demand and pricing for our products and services as a result of the COVID-19 pandemic. There was a material increase in demand and pricing for personal protective equipment (“PPE”), drugs and other supplies directly related to treating and preventing the spread of COVID-19 and variants thereof during fiscal 2020 and 2021. In the second half of fiscal 2022, demand and pricing for PPE, drugs and other supplies decreased resulting in a decline in revenue relative to the previous two years. Patients, hospitals and other medical facilities continued to defer some elective procedures and routine medical visits due to ongoing and continuing uncertainty from COVID-19 outbreaks or variants or as a result of restrictive government orders or advisories. While demand for many supplies and services not related to COVID-19 may continue to decline into fiscal 2023, rolling shortages of products and drugs needed for routine procedures, such as, contrast media and syringes, could have an impact on demand for hospital services and the financial conditions of providers, particularly those forced to procure such products through resellers.
•Increased labor costs. Labor shortages and the resulting increases to the cost of labor are a continued challenge to the health care providers we serve. Limited availability of staff resources and rolling staff shortages may continue to impair the ability of existing staff to manage product and service procurement. While our non-acute and non-healthcare business such as education and hospitality customers, experienced a rebound in fiscal year 2022, the recovery in the business may be hampered by future COVID-19 variants or outbreaks, which are highly uncertain and cannot be accurately predicted.
•Limited access to our members’ facilities that impacts our ability to fulfill our contractual requirements. While some of our hospital customers have increased access to their facilities for non-patients, including our field teams, consultants and other professionals, there are many that still are not allowing onsite access outside of their staff. Hospital imposed travel restrictions are also impacting some customers’ ability to participate in face-to-face events with us, such as committee meetings and conferences, which limits our ability to build on customer relationships. The long-term continuation, or any future recurrence of these circumstances may negatively impact the ability of our employees to effectively deliver existing or sell new products and services to our members and could negatively affect our performance of our existing contracts.
•Materials and personnel shortages and disruptions in supply chain, including manufacturing and shipping. The global supply chain has been materially disrupted due to personnel shortages associated with ongoing COVID-19 rates of infection, stay-at-home orders, border closings, rapidly escalating shipping costs, raw material availability and material logistical delays due to port congestion. Borders closings, lock-down orders and other restrictions in response to COVID-19, particularly regarding China, have impacted and continue to impact our access to products for our members. Staffing or personnel shortages due to shelter-in-place orders and quarantines, or other public health measures, have impacted and, in the future, may impact us and our members, other customers or suppliers. In addition, due to unprecedented demand during the COVID-19 pandemic, there have been widespread shortages in certain product categories. If the supply chain for materials used in the products purchased by our members through our GPO or products contract manufactured through our direct sourcing business continue to be adversely impacted by the COVID-19 pandemic, our supply chain may continue to be disrupted. Failure of our suppliers, contract manufacturers, distributors, contractors and other business partners to meet their obligations to our members, other customers or to us, or material disruptions in their ability to do so due to their own financial or operational difficulties, may adversely impact our operations.
•Requests for contract modifications, payment deferrals or exercises of force majeure clauses. We have and may continue to receive requests for contract modifications, payment waivers and deferrals, payment reductions or amended payment terms from our contract counterparties. We have and may continue to receive requests to delay service or payment on performance service contracts. In addition, we have and may continue to receive requests from our suppliers for
30
increases to their contracted prices, and such requests may be implemented in the future. Inflation in such contract prices may impact member utilization of items and services available through our GPO contracts, which could adversely impact our net administrative fees revenue and direct sourcing revenue. In addition, several pharmacy suppliers have exercised force majeure clauses related to failure to supply clauses in their contracts with us because they are unable to obtain raw materials for manufacturing from India and China. The standard failure to supply language in our contracts contains financial penalties to suppliers if they are unable to supply products, which such suppliers may not be able to pay. In addition, we may not be able to source products from alternative suppliers on commercially reasonable terms, or at all.
•Managing the evolving regulatory environment. In response to COVID-19 pandemic and variants thereof, federal, state and local governments are issuing new rules, regulations, changing reimbursement eligibility rules, orders and advisories on a regular basis. These government actions can impact us and our members, customers and suppliers.
The ultimate impact of COVID-19, variants thereof, recurrences, or similar pandemics on our business, results of operations, financial condition and cash flows is dependent on future developments, including the duration of any pandemic and the related length of its impact on the United States and global economies and their healthcare systems, which are uncertain and cannot be predicted at this time. The impact of the COVID-19 pandemic, variants thereof, recurrences, or future similar pandemics may also exacerbate many of the other risks described in this “Risk Factors” section. Despite our efforts to manage these impacts, their ultimate impact depends on factors beyond our knowledge or control, including the duration and severity of any variants or outbreaks and actions taken to contain its spread and mitigate its public health effects. The foregoing and other continued disruptions in our business as a result of the COVID-19 pandemic, variants thereof, recurrences or similar pandemics could result in a material adverse effect on our business, results of operations, financial condition, cash flows, prospects and the trading prices of our securities in the future.
We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical instability due to the ongoing military conflict between Russia and Ukraine. Our business, financial condition and results of operations may be materially and adversely affected by any negative impact on the global economy and capital markets resulting from the conflict in Ukraine or any other geopolitical tensions.
U.S. and global markets are experiencing volatility and disruption following the escalation of geopolitical tensions and the start of the military conflict between Russia and Ukraine. On February 24, 2022, Russian troops began a full-scale military invasion of Ukraine. In response, many nations, including the United States, imposed economic, financial and other sanctions on Russia, certain of its allies, and certain individuals. Although the length and impact of the ongoing military conflict and associated sanctions regime is highly unpredictable, the conflict in Ukraine has, and may continue to lead to market disruptions, including significant volatility in commodity prices, energy, credit and capital markets, as well as supply chain interruptions. We are continuing to monitor the situation in Ukraine and prepare for any implications on our business. In addition, Russian military actions and the resulting sanctions could adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets, potentially making it more difficult for us to obtain additional capital.
We face intense competition, which could limit our ability to maintain or expand market share within our industry and harm our business and operating results.
The market for products and services in each of our operating segments is fragmented, intensely competitive and characterized by rapidly evolving technology and product standards, dynamic user needs and the frequent introduction of new products and services. We face intense competition from a number of companies, including the companies listed under “Item 1 - Business - Competition.” The primary competitors for our Supply Chain Services segment compete with our group purchasing and direct sourcing activities. Our group purchasing business competes with other national and regional GPOs, including in certain cases GPOs owned by healthcare providers, distributors and wholesalers. Our direct sourcing business competes primarily with private label offerings and programs, product manufacturers and distributors.
The competitors in our Performance Services segment compete with our three sub-brands: PINC AI, Contigo Health and Remitra. The primary competitors of PINC AI range from smaller niche companies to large, well-financed and technologically sophisticated entities, and include information technology providers and consulting and outsourcing firms. The primary competitors for Contigo Health are smaller niche and larger well-financed healthcare and insurance companies. The primary competitors for Remitra are smaller niche and larger technology companies and financial institutions.
With respect to our products and services in both segments, we compete on the basis of several factors, including breadth, depth and quality of our product and service offerings, ability to deliver clinical, financial and operational performance improvement through the use of our products and services, quality and reliability of services, ease of use and convenience, brand recognition and the ability to integrate services with existing technology. Some of our competitors have larger scale, benefit from greater name recognition, and have substantially greater financial, technical and marketing resources. Other of our competitors have proprietary technology that differentiates their product and service offerings from our offerings. As a result of these competitive
31
advantages, our competitors and potential competitors may be able to respond more quickly to market forces, undertake more extensive marketing campaigns for their brands, products and services and make more attractive offers to our current members and customers and potential new members and customers.
We also compete on the basis of price, primarily in our Supply Chain Services business. We may be subject to pricing pressures as a result of, among other things, competition within the industry, consolidation of healthcare industry participants, practices of managed care organizations, changes in laws and regulations applicable to our business operations, government action affecting reimbursement, financial stress experienced by our members and customers, and increased revenue share obligations to members. In our Supply Chain Services segment, competitive pressure is likely to result in increases in revenue share obligations, some of which may be material, particularly as our current GPO participation agreements approach renewal or if a member undergoes a change of control that triggers a termination right, or as new GPO members join our GPO programs. Material increases in revenue share obligations to existing or new GPO members could adversely impact our business, financial condition and results of operations. In this competitive environment, we cannot be certain that we will be able to retain our current GPO members or expand our member base on historical terms, favorable terms or at all, and the failure to do so may adversely impact our business, financial condition and results of operations. Furthermore, if pricing of our other products and services experiences material downward pressure, our business will be less profitable, and our results of operations will be adversely affected.
Our Performance Services business also competes, to an extent, on the basis of price and to a greater extent on features and functionality of the solutions we offer through our PINC AI, Contigo Health and Remitra brands.
Moreover, we expect that competition will continue to increase as a result of consolidation in both the healthcare information technology and healthcare services industries. If one or more of our competitors or potential competitors were to merge or partner with another of our competitors, or if new competitors were to enter the healthcare space, the change in the competitive landscape could also adversely affect our ability to compete effectively and could harm our business, financial condition, and results of operations.
Consolidation in the healthcare industry could have a material adverse effect on our business, financial condition and results of operations.
Many healthcare industry participants are consolidating to create larger and more integrated healthcare delivery systems with greater market power. We expect legal, regulatory and economic conditions to lead to additional consolidation in the healthcare industry in the future. As consolidation accelerates, the economies of scale of our members’ organizations may grow. If a member experiences sizable growth following consolidation, it may determine that it no longer needs to rely on us and may reduce its demand for our products and services. Some of these large and growing healthcare systems and non-acute care providers may choose to contract directly with suppliers for certain supply categories, and some suppliers may seek to contract directly with the healthcare providers rather than with GPOs such as ours. In connection with any consolidation, our members may move their business to another GPO, particularly when the acquiring hospital or hospital system is a member of a competing GPO or where the post-acquisition management of our member is aligned with a competing GPO. In addition, as healthcare providers consolidate to create larger and more integrated healthcare delivery systems with greater market power, these providers may try to use their market power to negotiate materially increased revenue share obligations and fee reductions for our products and services across both of our business segments. Finally, consolidation may also result in the acquisition or future development by our members of products and services that compete with our products and services. Any of these potential results of consolidation could have a material adverse effect on our business, financial condition, and results of operations.
32
We may experience material delays in recognizing revenue or increasing revenue, or be required to reverse prior revenue recognition, if the sales cycle or implementation period with potential new members takes longer than anticipated or our related project estimates are not accurate.
A key element of our strategy is to market the various products and services in our Supply Chain Services and Performance Services segments directly to healthcare providers and to increase the number of our products and services utilized by existing members. The evaluation and purchasing process is often lengthy and involves material technical evaluation and commitment of personnel by these organizations. Further, the evaluation process depends on a number of factors, many of which we may not be able to control, including potential new members’ internal approval processes, budgetary constraints for technology spending, member concerns about implementing new procurement methods and strategies and other timing effects. In addition, the contract or software implementation process for new products or services can take six months or more and, accordingly, delay our ability to recognize revenue from the sale of such products or services. If we experience an extended or delayed implementation cycle in connection with the sale of additional products and services to existing or new members, it could have a material adverse effect on our business, financial condition and results of operations. In addition, we are required to use estimates to determine revenue recognition for performance-based consulting engagements. These estimates are based on a number of inputs from management regarding project timing, milestone and goal achievement and expected completion dates, each of which may change during the course of the engagement and could result in either delayed revenue recognition or revenue reversals resulting in out of period revenue adjustments, which could have a material adverse effect on our results of operations. In addition, changes in accounting standards that impact revenue recognition as well as conversion of SaaS-based products to licensed-based products, as discussed in the below risk factor “The markets for our SaaS- or licensed-based products and services may develop more slowly than we expect, or we may convert more SaaS-based products to license-based products, which could adversely affect our revenue, growth rates and our ability to maintain or increase our profitability” could adversely impact our ability to recognize revenue consistent with our historical practices and could have a material adverse effect on our business, financial condition and results of operations.
If members of our GPO programs reduce activity levels or terminate or elect not to renew their contracts, our revenue and results of operations may decrease materially.
We have GPO participation agreements with all of our GPO members. Our GPO participation agreements may generally be terminated for cause or in the event of a change of control of the GPO member. In addition, the GPO member can terminate the GPO participation agreement at the end of the then-current term by notifying us of the member’s decision not to renew. Although we renewed most of our then existing GPO participation agreements primarily for terms of five to seven years at the beginning of fiscal 2021, there can be no assurance that our GPO members will extend or renew their GPO participation agreements on the same or similar economic terms at the end of the term of the agreement, or at all, or that the GPO members will not terminate their GPO participation agreements for cause or due to a change of control of the GPO member. Failure of our GPO members to maintain, extend or renew their GPO participation agreements on the same or similar economic terms, or at all, may have a material adverse impact on our business, financial condition and results of operations.
Our success in retaining member participation in our GPO programs depends upon our reputation, strong relationships with such GPO members and our ability to deliver consistent, reliable and high-quality products and services, and a failure in any of these areas may result in the loss of GPO members. Some of our GPO competitors offer higher revenue share arrangements compared to our average arrangements. Our ability to retain and expand participation in our GPO programs depends upon our ability to provide overall value to GPO members, including competitive revenue share arrangements, in an economically competitive environment. In addition, GPO members may seek to modify or elect not to renew their contracts due to factors that are beyond our control and are unrelated to our performance, including a change of control of the GPO member, changes in their strategies or business plans, changes in their supply chain personnel or management, or economic conditions in general. When contracts are reduced by modification or not renewed for any reason, we lose the anticipated future revenue associated with such contracts and, consequently, our revenue and results of operations may decrease materially.
Historically, we have enjoyed a strong strategic alignment with our GPO members, in many cases as a result of such GPO members being significant equity owners of both us and Premier LP. As a result of the August 2020 Restructuring, our former member-owners’ equity holdings in Premier LP were canceled and converted into shares of our Class A common stock which is publicly traded on the NASDAQ Global Select Market (“NASDAQ”) under the ticker symbol “PINC.” Furthermore, former member-owners who received shares of our Class A common stock as part of the August 2020 Restructuring are free to sell those shares at any time. Any material reduction in our member-owners’ equity holdings in us could result in reduced alignment between us and such member-owners, which may make it more difficult to retain these GPO members or to ensure that they extend or renew their GPO participation agreements on the same or similar economic terms, or at all, the failure of which may have a material adverse impact on our business, financial condition and results of operations.
33
We derive a material portion of our revenues from our largest members and certain other customers and the sudden loss of one or more of these members or customers could materially and adversely affect our business, financial condition and results of operations.
Our top five customers generated revenue of approximately 21% and 28% of our consolidated net revenues for the fiscal years ended June 30, 2022 and 2021. The sudden loss of any material customer or a number of smaller customers that are participants in our group purchasing programs, or utilize any of our programs or services, or a material change in revenue share or other economic terms we have with such customers could materially and adversely affect our business, financial condition and results of operations.
The markets for our SaaS- or licensed-based products and services may develop more slowly than we expect, or we may convert more SaaS-based products to license-based products, which could adversely affect our revenue, growth rates and our ability to maintain or increase our profitability.
Our success will depend on the willingness of existing and potential new customers to increase their use of our SaaS- or licensed-based products and services as well as our ability to sell license-based products to existing and potential new customers at rates sufficient to offset the loss of SaaS-based product sales. Fluctuating member demand for SaaS- or license-based products that materially alter our mix of SaaS- and licensed-based product sales and conversion of SaaS-based products to license-based products can result in volatility of revenue and lower growth rates in any given year which could materially adversely affect our business, financial condition and results of operations. Furthermore, many companies have invested substantial resources to integrate established enterprise software into their businesses and therefore may be reluctant or unwilling to switch to our products and services and some companies may have concerns regarding the risks associated with the security and reliability of the technology delivery model associated with these services. If companies do not perceive the benefits of our products and services, then the market for these products and services may not expand as much or develop as quickly as we expect, which would materially adversely affect our business, financial condition and results of operations.
Our members are highly dependent on payments from third-party healthcare payors, including Medicare, Medicaid and other government-sponsored programs, and reductions or changes in third-party reimbursement could adversely affect these members and consequently our business.
Our members derive a substantial portion of their revenue from third-party private and governmental payors, including Medicare, Medicaid and other government sponsored programs. Our sales and profitability depend, in part, on the extent to which coverage of and reimbursement for our products and services our members purchase or otherwise obtain through us is available to our members from governmental health programs, private health insurers, managed care plans and other third-party payors. These third-party payors are increasingly using their enhanced bargaining power to secure discounted reimbursement rates and may impose other requirements that adversely impact our members’ ability to obtain adequate reimbursement for our products and services. If third-party payors do not approve our products and services for reimbursement or fail to reimburse for them adequately, our members may suffer adverse financial consequences which, in turn, may reduce the demand for and ability to purchase our products or services.
In addition, government actions or changes in laws or regulations could limit government spending generally for the Medicare and Medicaid programs, limit payments to healthcare providers and increase emphasis on financially accountable payment programs such as accountable care organizations, bundled payments and capitated primary care that could have an adverse impact on our members and, in turn, on our business, financial condition and results of operations.
We rely on the administrative fees we receive from our GPO suppliers, and the failure to maintain contracts with these GPO suppliers could have a generally negative effect on our relationships with our members and could adversely affect our business, financial condition and results of operations.
Historically, we have derived a substantial amount of our revenue from the administrative fees that we receive from our GPO suppliers. We maintain contractual relationships with these suppliers which provide products and services to our members at reduced costs and which pay us administrative fees based on the dollars spent by our members for such products and services. Our contracts with these GPO suppliers generally may be terminated upon 90 days’ notice. A termination of any relationship or agreement with a GPO supplier would result in the loss of administrative fees pursuant to our arrangement with that supplier, which could adversely affect our business, financial condition and results of operations. In addition, if we lose a relationship with a GPO supplier we may not be able to negotiate similar arrangements for our members with other suppliers on the same terms and conditions or at all, which could damage our reputation with our members and adversely impact our ability to maintain our member agreements or expand our membership base and could have a material adverse effect on our business, financial condition and results of operations.
34
In addition, CMS, which administers the Medicare and federal aspects of state Medicaid programs, has issued complex rules requiring pharmaceutical manufacturers to calculate and report drug pricing for multiple purposes, including the limiting of reimbursement for certain drugs. These rules generally exclude from the pricing calculation administrative fees paid by pharmaceutical manufacturers to GPOs to the extent that such fees meet CMS’s “bona fide service fee” definition. There can be no assurance that CMS will continue to allow exclusion of GPO administrative fees from the pricing calculation, which could negatively affect the willingness of pharmaceutical manufacturers to pay administrative fees to us, which could have a material adverse effect on our member retention, business, financial condition and results of operations.
If we are unable to maintain our relationships with third-party providers or maintain or enter into new strategic alliances, we may be unable to grow our current base business.
Our business strategy includes entering into and maintaining strategic alliances and affiliations with leading service providers. These companies may pursue relationships with our competitors, develop or acquire products and services that compete with our products and services, experience financial difficulties, be acquired by one of our competitors or other third party or exit the healthcare industry, any of which may adversely affect our relationship with them. In addition, in many cases, these companies may terminate their relationships with us for any reason with limited or no notice. If existing relationships with third-party providers or strategic alliances are adversely impacted or are terminated or we are unable to enter into relationships with leading healthcare service providers and other GPOs, we may be unable to maintain or increase our industry presence or effectively execute our business strategy.
If we are not able to timely offer new and innovative products and services, we may not remain competitive and our revenue and results of operations may suffer.
Our success depends on providing products and services within our Supply Chain Services and Performance Services segments that healthcare providers use to improve clinical, financial and operational performance. Information technology providers and other competitors are incorporating enhanced analytical tools and functionality and otherwise developing products and services that may become viewed as more efficient or appealing to our members. If we cannot adapt to rapidly evolving industry standards, technology, member and other customers’ needs, including changing regulations and provider reimbursement policies, we may be unable to anticipate changes in our current and potential new members’ and other customers’ requirements that could make our existing technology, products or service offerings obsolete. We must continue to invest material resources in research and development or acquisitions in order to enhance our existing products and services, maintain or improve our product category rankings and introduce new high-quality products and services that members and potential new members and customers will want. If our enhanced existing or new products and services are not responsive to the needs of our members or potential new members and customers, are not appropriately timed with market opportunity or are not effectively brought to market, we may lose existing members and be unable to obtain new members and customers, which could have a material adverse effect on our business, financial condition or results of operations.
Our acquisition activities could result in operating difficulties, dilution, unrecoverable costs and other negative consequences, any of which may adversely impact our financial condition and results of operations.
Our business strategy includes growth through acquisitions of additional businesses and assets. Future acquisitions may not be completed on preferred terms and acquired assets or businesses may not be successfully integrated into our operations or provide anticipated financial or operational benefits. Any acquisitions we complete will involve risks commonly encountered in acquisitions of businesses or assets. Such risks include, among other things:
•failing to integrate the operations and personnel of the acquired businesses in an efficient, timely manner, which can be exacerbated by pandemics, such as COVID-19;
•failure of a selling party to produce all material information during the pre-acquisition due diligence process, or to meet their obligations under post-acquisition agreements;
•potential liabilities of or claims against an acquired company or its assets, some of which may not become known until after the acquisition;
•an acquired company’s lack of compliance with applicable laws and governmental rules and regulations, and the related costs and expenses necessary to bring such company into compliance;
•an acquired company’s general information technology controls or their legacy third-party providers may not be sufficient to prevent unauthorized access or transactions, cyber-attacks or other data security breaches;
•managing the potential disruption to our ongoing business;
•distracting management focus from our existing core businesses;
•encountering difficulties in identifying and acquiring products, technologies, or businesses that will help us execute our business strategy;
35
•entering new markets in which we have little to no experience;
•impairing relationships with employees, members, and strategic partners;
•failing to implement or remediate controls, procedures and policies appropriate for a public company at acquired companies lacking such financial, disclosure or other controls, procedures and policies, potentially resulting in a material weakness in our internal controls over financial reporting;
•unanticipated changes in market or industry practices that adversely impact our strategic and financial expectations of an acquired company, assets or business and require us to write-off or dispose of such acquired company, assets, or business;
•the amortization of purchased intangible assets;
•incurring expenses associated with an impairment of all or a portion of goodwill and other intangible assets due to the failure of certain acquisitions to realize expected benefits; and
•diluting the share value and voting power of existing stockholders.
In addition, anticipated benefits of our previous and future acquisitions may not materialize. Future acquisitions or dispositions of under-performing businesses could result in the incurrence of debt, material exit costs, contingent liabilities or amortization expenses, impairments or write-offs of goodwill and other intangible assets, any of which could harm our business, financial condition and results of operations. In addition, expenses associated with potential acquisitions, including, among others, due diligence costs, legal, accounting, technology and financial advisory fees, travel and internal resources utilization, can be material. These expenses may be incurred regardless of whether any potential acquisition is completed. In instances where acquisitions are not ultimately completed, these expenses typically cannot be recovered or offset by the anticipated financial benefits of a successful acquisition. As we pursue our business strategy and evaluate opportunities, these expenses may adversely impact our results of operations and earnings per share.
Numerous potential acquisition targets that had previously expressed an interest in commencing strategic discussions with us prior to or early into the COVID-19 pandemic delayed or deferred indefinitely their exploration of strategic alternatives. Our ability to execute our growth strategy may be materially impacted if COVID-19 variants or future pandemics, or general market conditions, materially reduce the number of target companies willing to evaluate strategic alternatives and start a process for the sale of part or all of their equity or assets.
Our business and growth strategies also include non-controlling investments in other businesses and joint ventures. In the event the companies or joint ventures we invest in do not perform as well as expected, we could experience the loss of some or all of the value of our investment, which loss could adversely impact our financial condition and results of operations.
Although we conduct accounting, financial, legal and business due diligence prior to making investments, we cannot guarantee that we will discover all material issues that may affect a particular target business, or that factors outside the control of the target business and outside of our control will not later arise. Occasionally, current and future investments are, and will be, made on a non-controlling basis, in which case we have limited ability to influence the financial or business operations of the companies in which we invest. To the extent we invest in a financially underperforming or unstable company or an entity in its development stage that does not successfully mature, we may lose the value of our investment. We have in the past and may in the future be required to write down or write off our investment or recognize impairment or other charges that could adversely impact our financial condition or results of operations and our stock price. Even though these charges may be non-cash items and not have a material impact on our liquidity, the fact that we report charges of this nature could contribute to negative market perceptions about us and our business strategy and our Class A common stock.
We rely on Internet infrastructure, bandwidth providers, data center providers and other third parties and our own systems for providing services to our users, and any failure or interruption in the services provided by these third parties or our own systems, including from a cyber or other catastrophic event, could expose us to litigation and negatively impact our relationships with users, adversely affecting our brand, our business and our financial performance.
Our ability to deliver our Performance Services segment products is dependent on the development and maintenance of the infrastructure of the Internet and other telecommunications services by third parties. This includes maintenance of a reliable network backbone with the necessary speed, data capacity and security for providing reliable Internet access and services and reliable telephone, Wi-Fi, facsimile and pager systems. We have experienced and expect that we will experience in the future interruptions and delays in these services and availability from time to time. We rely on internal systems as well as third-party suppliers, including bandwidth and telecommunications equipment providers, to provide our services. We have also migrated some of our data center operations to third-party data-hosting facilities. We do not maintain redundant systems or facilities for some of these services. In the event of a material cyber-attack or catastrophic event with respect to one or more of these providers, systems or facilities, we may experience an extended period of system unavailability, which could negatively impact our relationship with users. To operate without interruption, both we and our service providers must guard against:
36
•damage from fire, power loss, and other natural disasters;
•communications failures;
•software and hardware errors, failures, and crashes;
•cyber-attacks, viruses, worms, malware, ransomware and other malicious software programs;
•security breaches and computer viruses and similar disruptive problems; and
•other potential interruptions.
Any disruption in the network access, telecommunications or co-location services provided by our third-party providers or any failure of or by these third-party providers or our own systems to handle current or higher volume of use could materially harm our business. We exercise limited control over these third-party suppliers, which increases our vulnerability to problems with services they provide. Any errors, failures, interruptions or delays experienced in connection with these third-party technologies and information services or our own systems could negatively impact our relationships with users and adversely affect our business and financial performance and could expose us to third-party liabilities, some of which may not be adequately insured.
Data loss or corruption due to failures or errors in our systems and service disruptions at our data centers may adversely affect our reputation and relationships with existing members, which could have a negative impact on our business, financial condition and results of operations.
Because of the large amount of data that we collect and manage, it is possible that hardware failures or errors in our systems could result in data loss or corruption or cause the information that we collect to be incomplete or contain inaccuracies that our members regard as material. Complex software such as ours may contain errors or failures that are not detected until after the software is introduced or updates and new versions are released. Despite testing by us, from time to time we have discovered defects or errors in our software, and such defects or errors may be discovered in the future. Any defects or errors could expose us to risk of liability to members and the government and could cause delays in the introduction of new products and services, result in increased costs and diversion of development resources, require design modifications, decrease market acceptance or member satisfaction with our products and services or cause harm to our reputation.
Furthermore, our members might use our software together with products from other companies. As a result, when problems occur, it might be difficult to identify the source of the problem. Even when our software does not cause these problems, the existence of these errors might cause us to incur material costs, divert the attention of our technical personnel from our product development efforts, impact our reputation and lead to material member relations problems.
Moreover, our data centers and service provider locations store and transmit critical member data that is essential to our business. While these locations are chosen for their stability, failover capabilities and system controls, we do not directly control the continued or uninterrupted availability of every location. In addition to the services we provide from our offices, we have migrated the majority of our data center operations to a third-party data-hosting facility. Data center facilities are vulnerable to damage or interruption from natural disasters, fires, power loss, telecommunications failures, acts of terrorism, acts of war, and similar events. They are also subject to break-ins, sabotage, intentional acts of vandalism, cyber-attacks and similar misconduct. Despite precautions taken at these facilities, the occurrence of a natural disaster or an act of terrorism, could result in a decision to close the facilities without adequate notice or other unanticipated problems, which could cause lengthy interruptions in our service. These service interruption events could impair our ability to deliver services or deliverables or cause us to fail to achieve service levels required in agreements with our members, which could negatively affect our ability to retain existing members and attract new members.
If our cyber and other security measures are breached or fail and unauthorized access to a member’s data is obtained, or our members fail to obtain proper permission for the use and disclosure of information, our services may be perceived as not being secure, members may curtail or stop using our services and we may incur material liabilities.
Our services involve the web-based storage and transmission of members’ proprietary information, personal information of employees and protected health information of patients. From time to time we may detect vulnerabilities in our systems, which, even if not resulting in a security breach, may reduce member confidence and require substantial resources to address. If our security measures are breached or fail as a result of third-party action, employee error, malfeasance, insufficiency, defective design or otherwise, someone may be able to obtain unauthorized access to member or patient data. As a result, our reputation could be damaged, our business may suffer, and we could face damages for contract breach, penalties and fines for violation of applicable laws or regulations and material costs for notification to affected individuals, remediation and efforts to prevent future occurrences.
In addition to our cyber and other security measures, we rely upon our members as users of our system for key activities to promote security of the system and the data within it. On occasion, our members have failed to perform these activities. Failure of members to perform these activities may result in claims against us that could expose us to material expense and harm our
37
reputation. In addition, our members may authorize or enable third parties to access their data or the data of their patients on our systems. Because we do not control such access, we cannot ensure the complete propriety of that access or integrity or security of such data in our systems. In addition, although our development infrastructure is based in the United States, we outsource development work for a portion of our products and services to persons outside the United States, particularly India. We cannot guarantee that the cyber and other security measures and regulatory environment of our foreign partners are as robust as in the United States. Any breach of our security by our members or foreign partners could have a material adverse effect on our business, financial condition and results of operations.
Additionally, we require our members to provide necessary notices and to obtain necessary permissions and waivers for use and disclosure of the information that we receive. If our members do not obtain necessary permissions and waivers, then our use and disclosure of information that we receive from them or on their behalf may be limited or prohibited by state, federal, or international privacy laws or other laws. Any such failure to obtain proper permissions and waivers could impair our functions, processes and databases that reflect, contain or are based upon such data and may prevent use of such data. Moreover, we may be subject to claims or liability for use or disclosure of information by reason of our lack of a valid notice, permission or waiver. These claims or liabilities could subject us to unexpected costs and adversely affect our business, financial condition and results of operations.
We could suffer a loss of revenue and increased costs, exposure to material liability, reputational harm, and other serious negative consequences if we are subject to cyber-attacks or other data security breaches that disrupt our operations or result in the dissemination of proprietary or confidential information about us or our members or other third parties.
We manage and store proprietary information and sensitive or confidential data relating to our operations. We may be subject to cyber-attacks on and breaches of the information technology systems we use for these purposes. Experienced computer programmers and hackers may be able to penetrate our network security and misappropriate or compromise our confidential information or that of third parties, create system disruptions, or cause shutdowns. Computer programmers and hackers also may be able to develop and deploy viruses, worms, malware, ransomware and other malicious software programs that attack our systems or products or otherwise exploit security vulnerabilities of our systems or products. In addition, sophisticated hardware and operating system software and applications that we produce or procure from third parties may contain defects in design or manufacture, including “bugs” and other problems that could unexpectedly interfere with the operation of our systems.
We expend material capital to protect against the threat of security breaches, including cyber-attacks, viruses, worms, malware, ransomware and other malicious software programs. Substantial additional expenditures may be required before or after a cyber-attack or breach to mitigate in advance or to alleviate any problems caused by cyber-attacks and breaches, including unauthorized access to or theft of personal or patient data and protected health information stored in our information systems and the introduction of computer viruses, worms, malware, ransomware and other malicious software programs to our systems. Our remediation efforts may not be successful and could result in interruptions, delays or cessation of service and loss of existing or potential members.
While we provide our domestic and foreign employees and contractors training and regular reminders on important measures they can take to prevent breaches, we often identify attempts to gain unauthorized access to our systems. Given the rapidly evolving nature and proliferation of cyber threats, there can be no assurance our training and network security measures or other controls will detect, prevent or remediate security or data breaches in a timely manner or otherwise prevent unauthorized access to, damage to, or interruption of our systems and operations. For example, it has been widely reported that many well-organized international interests, in certain cases with the backing of sovereign governments, are targeting the theft of patient information through the use of advance persistent threats. In recent years, a number of hospitals have reported being the victim of ransomware attacks in which they lost access to their systems, including clinical systems, during the course of the attacks. We are likely to face attempted attacks in the future. Accordingly, we may be vulnerable to losses associated with the improper functioning, security breach or unavailability of our information systems as well as any systems used in acquired operations.
Breaches of our security measures and the unapproved use or disclosure of proprietary information or sensitive or confidential data about us or our members or other third parties could expose us, our members or other affected third parties to a risk of loss or misuse of this information, result in litigation, governmental inquiry and potential liability for us, damage our brand and reputation or otherwise harm our business. Furthermore, we are exposed to additional risks because we rely in certain capacities on third-party data management providers whose possible security problems and security vulnerabilities are beyond our control.
We may experience cyber-security and other breach incidents that remain undetected for an extended period. Because techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are not recognized until launched, we may be unable to anticipate these techniques or to implement adequate preventative measures to stop or mitigate any potential damage in a timely manner. Given the increasing cyber security threats in the healthcare industry, there can be no assurance we will not experience business interruptions; data loss, ransom, misappropriation or corruption; theft or misuse of
38
proprietary or patient information; or litigation and investigation related to any of those, any of which could have a material adverse effect on our financial position and results of operations and harm our business reputation.
Any restrictions on our use of, or ability to license, data, or our failure to license data and integrate third-party technologies, could have a material adverse effect on our business, financial condition and results of operations.
We depend upon licenses from third parties, most of which are non-exclusive, for some of the technology and data used in our applications, and for some of the technology platforms upon which these applications are built and operate. We also obtain a portion of the data that we use from government entities and public records and from our members for specific member engagements. We cannot assure that our licenses for information will allow us to use that information for all potential or contemplated applications and products. In addition, if our members revoke their consent for us to maintain, use, de-identify and share their data, our data assets could be degraded.
In the future, data providers could withdraw their data from us or restrict our usage due to competitive reasons or because of new legislation or judicial interpretations restricting use of the data currently used in our products and services. In addition, data providers could fail to adhere to our quality control standards in the future, causing us to incur additional expense to appropriately utilize the data. If a substantial number of data providers were to withdraw or restrict their data, or if they fail to adhere to our quality control standards, and if we are unable to identify and contract with suitable alternative data suppliers and integrate these data sources into our service offerings, our ability to provide products and services to our members would be materially and adversely impacted, resulting in a material adverse effect on our business, financial condition and results of operations.
We also integrate into our proprietary applications and use third-party software to maintain and enhance, among other things, content generation and delivery, and to support our technology infrastructure. Some of this software is proprietary and some is open source. Our use of third-party technologies exposes us to increased risks, including, but not limited to, risks associated with the integration of new technology into our solutions, the diversion of our resources from development of our own proprietary technology and our inability to generate revenue from licensed technology sufficient to offset associated acquisition and maintenance costs. These technologies may not be available to us in the future on commercially reasonable terms or at all and could be difficult to replace once integrated into our own proprietary applications. Our inability to obtain, maintain or comply with any of these licenses could delay development until equivalent technology can be identified, licensed and integrated, which would harm our business, financial condition and results of operations.
Most of our third-party licenses are non-exclusive and our competitors may obtain the right to access any of the technology covered by these licenses to compete directly with us. Our use of third-party technologies exposes us to increased risks, including, but not limited to, risks associated with the integration of new technology into our solutions, the diversion of our resources from development of our own proprietary technology and our inability to generate revenue from licensed technology sufficient to offset associated acquisition and maintenance costs. In addition, if our data suppliers choose to discontinue support of the licensed technology in the future, we might not be able to modify or adapt our own solutions.
Our use of “open source” software could adversely affect our ability to sell our products and subject us to possible litigation.
The products or technologies acquired, licensed or developed by us may incorporate so-called “open source” software, and we may incorporate open source software into other products in the future. There is little or no legal precedent governing the interpretation of many of the terms of certain of these licenses, and therefore the potential impact of these terms on our business is unknown and may result in unanticipated obligations or litigation regarding our products and technologies. For example, we may be subjected to certain conditions, including requirements that we offer our products that use particular open source software at no cost to the user, that we make available the source code for modifications or derivative works we create based upon, incorporating or using the open source software, and/or that we license such modifications or derivative works under the terms of the particular open source license. In addition, if we combine our proprietary software with open source software in a certain manner, under some open source licenses we could be required to release the source code of our proprietary software, which could substantially help our competitors develop products that are similar to or better than ours. If an author or other party that distributes such open source software were to allege that we had not complied with the conditions of one or more of these licenses, we could be required to incur material legal costs defending ourselves against such allegations and could be subject to material damages.
Our direct sourcing activities depend on contract manufacturing facilities located in various parts of the world, and any physical, financial, regulatory, environmental, labor or operational disruption or product quality issues could result in a reduction in sales volumes, the incurrence of substantial expenditures and the loss of product availability.
As part of our direct sourcing activities, we contract with manufacturing facilities in various parts of the world, including facilities in Bangladesh, Cambodia, China, India, Malaysia, Sri Lanka, Taiwan, Thailand and Vietnam. Operations at and
39
securing products from these manufacturing facilities could be curtailed or partially or completely shut down as the result of a number of circumstances, most of which are outside of our control, such as unscheduled maintenance, power conservation/shortages, an earthquake, hurricane, flood, tsunami or other natural disaster, material labor strikes or work stoppages, government implementation of export limitations or freezes, port or other shipping delays, political unrest or pandemics, such as COVID-19. We are also subject to some of these risks with manufacturers we contract with in the United States. Any material curtailment of production at these facilities, or production issue resulting in a substandard product, could result in litigation or governmental inquiry or materially reduced revenues and cash flows in our direct sourcing activities. In addition, our business practices in international markets are subject to the requirements of the U.S. Foreign Corrupt Practices Act of 1977, as amended, any violation of which could subject us to material fines, criminal sanctions and other penalties. We expect all of our contracted manufacturing facilities to comply with all applicable laws, including labor, safety and environmental laws, and to otherwise meet our standards of conduct. Our ability to find manufacturing facilities that uphold these standards is a challenge, especially with respect to facilities located outside the United States. We also are subject to the risk that one or more of these manufacturing facilities will engage in business practices in violation of our standards or applicable laws, which could damage our reputation and adversely impact our business and results of operations.
A material portion of the manufacturing for our direct sourcing activities is conducted in China. As a result, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China as well as trade disputes between China and the United States and the potential imposition of bilateral tariffs. In addition, during the COVID-19 pandemic, China imposed export restrictions and new regulatory requirements on PPE and other medical equipment needed by our member hospitals. The imposition of tariffs or export restrictions on products imported by us from China could require us to (i) increase prices to our members or (ii) locate suitable alternative manufacturing capacity or relocate our operations from China to other countries. In the event we are unable to increase our prices or find alternative manufacturing capacity or relocate to an alternative base of operation outside of China on favorable terms, we would likely experience higher manufacturing costs and lower gross margins, which could have an adverse effect on our business and results of operations. The Chinese economy differs from the economies of most developed countries in many respects, including the degree of government involvement, the level of development, the growth rate, the control of foreign exchange, access to financing and the allocation of resources.
Additionally, the facilities in Malaysia with which we contract are particularly susceptible to labor shortages, labor disputes and interruptions, rising labor costs as a result of minimum wage laws, scheduling and overtime requirements and forced or child labor.
Validation of our direct sourcing suppliers around the world can be challenging and our vetting process may not eliminate all associated risks, particularly since the information shared is largely dependent on the supplier level of transparency. If one or more of the manufacturing facilities we contract with engage in business practices in violation of our standards or applicable laws, we could experience damage to our reputation and suffer an adverse impact our business, results of operations and reputation.
We may have inventory risk for (i) the PPE or other product inventory we purchase at elevated market prices, and (ii) items we purchase in bulk or pursuant to fixed price purchase commitments if we are unable to sell such inventory at or above our cost. As a result, we may experience a material adverse effect on our business, financial condition and results of operations.
As part of our efforts to satisfy PPE demands of our GPO members during the COVID-19 pandemic, we purchased PPE product inventory in forward buys at then current global market prices, which were elevated due to the volatility of global market prices for PPE products. In addition, as we strive to create a healthier global supply chain with more diversification in the country of origin, including a focus on supporting PPE and medical product manufacturing in the United States with our domestic sourcing initiative, we may source more of our products from US-based or near shore manufacturers, which may come at a higher acquisition cost than sourcing from Asia or other lower cost countries. From time to time, we also purchase other items as part of bulk purchases to resell to our members. If we are unable to sell the PPE or other products for more than our inventory cost, we could experience a material adverse effect on our business, financial condition and results of operations. In addition, if our GPO members are unwilling to pay higher prices for products made in the United States, or if they choose to buy lower cost products manufactured in lower cost countries, now or in the future, this may impact our customer growth and results of operations if we have to lower prices to compete or sell our higher-cost inventory.
If we lose key personnel or if we are unable to attract, hire, integrate and retain key personnel, our business would be harmed.
Our future success depends in part on our ability to attract, hire, integrate and retain key personnel, including our executive officers and other highly skilled technical, managerial, editorial, sales, marketing and customer service professionals. Competition for such personnel is intense and the labor market has tightened considerably as a consequence of the COVID-19 pandemic. We have from time to time in the past experienced, and we expect to continue to experience in the future, difficulty
40
in hiring and retaining highly skilled employees with appropriate qualifications. We cannot be certain of our ability to identify, hire and retain adequately qualified personnel, if we lose key personnel unexpectedly. In addition, to the extent we lose an executive officer or senior manager, we may incur increased expenses in connection with the hiring, promotion or replacement of these individuals and the transition of leadership and critical knowledge. Failure to identify, hire and retain necessary key personnel could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Capital Structure and Liquidity
We may need to obtain additional financing which may not be available or may be on unfavorable terms and result in dilution to, or a diminution of the rights of, our stockholders and cause a decrease in the price of our Class A common stock.
We may need to raise additional funds in order to, among other things:
•finance unanticipated working capital requirements;
•develop or enhance our technological infrastructure and our existing products and services;
•fund strategic relationships;
•respond to competitive pressures; and
•acquire complementary businesses, assets, technologies, products or services.
Additional financing may not be available on terms favorable to us, or at all. If adequate funds are not available or are not available on acceptable terms, our ability to fund our expansion strategy, take advantage of unanticipated opportunities, develop or enhance technology or services or otherwise respond to competitive pressures would be materially limited. If we raise additional funds by issuing equity or convertible debt securities, our then-existing stockholders may be diluted and holders of these newly issued securities may have rights, preferences or privileges senior to those of our then-existing stockholders. The issuance of these securities may cause a material decrease in the trading price of our Class A common stock or the value of your investment in us.
If we cannot refinance or replace our existing credit facility at maturity, it could have a material adverse effect on our ability to fund our ongoing cash requirements. Current or future indebtedness could adversely affect our business and our liquidity position.
We have a five-year $1 billion unsecured revolving credit facility. The Credit Facility also provides us the ability to incur incremental term loans and request an increase in the revolving commitments under the credit facility, up to an additional aggregate of $350.0 million, subject to the approval of the lenders under the credit facility. As of June 30, 2022, we had $150.0 million outstanding under this credit facility. Our current credit facility matures on November 9, 2023 and any outstanding indebtedness would be payable on or before that date. If we are not able to refinance or replace our existing credit facility at or before maturity or do so on acceptable terms, it would have a material adverse effect on our ability to fund our ongoing working capital requirements, business strategies, acquisitions and related business investments, future cash dividend payments, if any, or repurchases of Class A common stock under any then existing or future stock repurchase programs, if any.
Our indebtedness may increase from time to time in the future for various reasons, including fluctuations in operating results, capital expenditures and potential acquisitions. Any indebtedness we incur and restrictive covenants contained in the agreements related thereto could:
•make it difficult for us to satisfy our obligations, including making interest payments on our other debt obligations;
•limit our ability to obtain additional financing to operate our business;
•require us to dedicate a substantial portion of our cash flow to payments on our debt, reducing our ability to use our cash flow to fund capital expenditures and working capital and other general operational requirements;
•limit our flexibility to execute our business strategy and plan for and react to changes in our business and the healthcare industry;
•place us at a competitive disadvantage relative to some of our competitors that have less debt than us;
•limit our ability to pursue acquisitions; and
•increase our vulnerability to general adverse economic and industry conditions, including changes in interest rates or a downturn in our business or the economy.
41
The occurrence of any one of these events could cause us to incur increased borrowing costs and thus have a material adverse effect on our cost of capital, business, financial condition and results of operations or cause a material decrease in our liquidity and impair our ability to pay amounts due on our indebtedness.
Our unsecured revolving credit facility contains, among other things, restrictive covenants that will limit our and our subsidiaries’ ability to finance future operations or capital needs or to engage in other business activities. The credit facility restricts, among other things, our ability and the ability of our subsidiaries to incur additional indebtedness or issue guarantees, create liens on our assets, make distributions on or redeem equity interests, make investments, transfer or sell properties or other assets, and engage in mergers, consolidations or acquisitions. Furthermore, the credit facility includes cross-default provisions and requires us to meet specified financial ratios and tests. In addition, any debt securities we may issue or indebtedness we incur in the future may have similar or more restrictive financial or operational covenants that may limit our ability to execute our business strategies or operate our Company.
Our quarterly revenues and results of operations have fluctuated in the past and may continue to fluctuate in the future which could adversely affect the value of our Class A common stock, our revenues and our liquidity.
Fluctuations in our quarterly results of operations may be due to a number of factors, some of which are not within our control, including:
•our ability to offer new and innovative products and services;
•regulatory changes, including changes in healthcare laws;
•unforeseen legal expenses, including litigation and settlement costs;
•the purchasing and budgeting cycles of our members;
•the lengthy sales cycles for our products and services, which may cause material delays in generating revenues or an inability to generate revenues;
•pricing pressures with respect to our future sales;
•the timing and success of new product and service offerings by us or by our competitors;
•the timing of enterprise license agreements;
•member decisions regarding renewal or termination of their contracts, especially those involving our larger member relationships;
•the amount and timing of costs related to the maintenance and expansion of our business, operations and infrastructure;
•the amount and timing of costs related to the development, adaptation, acquisition, or integration of acquired technologies or businesses;
•the financial condition of our current and potential new members;
•general economic and market conditions and economic conditions specific to the healthcare industry; and
•the impact of COVID-19 and future pandemics on the economy and healthcare industry.
Our quarterly results of operations may vary materially in the future and period-to-period comparisons of our results of operations may not be meaningful. You should not rely on the results of one quarter as an indication of future performance. If our quarterly results of operations fall below the expectations of securities analysts or investors, the price of the Class A common stock could decline substantially. In addition, any adverse impacts on the Class A common stock may harm the overall reputation of our organization, cause us to lose members and impact our ability to raise additional capital in the future.
Risks Related to Healthcare and Employee Benefit Regulation
The healthcare industry is highly regulated. Any material changes in the political, economic or regulatory environment that affect the GPO business or the purchasing practices and operations of healthcare organizations, or that lead to consolidation in the healthcare industry, could reduce the funds available to providers to purchase our products and services or otherwise require us to modify our services.
Our business, financial condition and results of operations depend upon conditions affecting the healthcare industry generally and hospitals and health systems particularly, as well as our ability to increase the number of programs and services that we sell to our members and other customers. The life sciences and healthcare industry is highly regulated by federal and state authorities and is subject to changing political, economic and regulatory influences. Factors such as changes in reimbursement policies for healthcare expenses, consolidation in the healthcare industry, regulation, litigation and general economic conditions affect the purchasing practices, operations and the financial health of healthcare organizations. In particular, changes in regulations affecting the healthcare industry, such as increased regulation of the purchase and sale of medical products, tariffs,
42
new quality measurement and payment models, data privacy and security, government price controls, modification or elimination of applicable regulatory safe harbors, regulation of third-party administrators or restrictions on permissible discounts and other financial arrangements, could require us to make unplanned modifications of our products and services, result in delays or cancellations of orders or reduce funds and demand for our products and services.
In March 2010, President Barack Obama signed into law the Patient Protection and Affordable Care Act (“ACA”). The ACA is a sweeping measure designed to expand access to affordable health insurance, control healthcare spending and improve healthcare quality. In addition, many states have adopted or are considering changes in healthcare laws or policies in part due to state budgetary shortfalls. The ACA set the industry moving in a clear direction on access to health insurance, payment, quality and cost management.
With the election of President Joe Biden, as well the 2021 U.S. Supreme Court decision upholding the ACA, there appears to be greater certainty and a continuation of the policies and directions set forth in the ACA. While these developments will create greater certainty regarding the continued existence of the ACA and its reforms to the health insurance and healthcare market, healthcare will continue to be a highly partisan and contentious area. This environment is creating risks for healthcare providers and our business that could adversely affect our business and financial performance.
If we fail to comply with complex federal and state laws and regulations governing financial relationships among healthcare providers and submission of false or fraudulent claims to government healthcare programs, we may be subject to civil and criminal penalties or loss of eligibility to participate in government healthcare programs.
Anti-Kickback Regulations
We are subject to federal and state laws and regulations designed to protect patients, government healthcare programs and private health plans from fraudulent and abusive activities. These laws include anti-kickback restrictions and laws prohibiting the submission of false or fraudulent claims. These laws are complex, and their application to our specific products, services and relationships may not be clear and may be applied to our business in ways that we do not anticipate. Federal and state regulatory and law enforcement authorities have over time increased enforcement activities with respect to Medicare and Medicaid fraud and abuse regulations and other reimbursement laws and rules. From time to time, we and others in the healthcare industry have received inquiries or requests to produce documents in connection with such activities. We could be required to expend material time and resources to comply with these requests, and the attention of our management team could be diverted to these efforts. Furthermore, if we are found to be in violation of any federal or state fraud and abuse laws, we could be subject to civil and criminal penalties and we could be excluded from participating in federal and state healthcare programs such as Medicare and Medicaid. The occurrence of any of these events could materially harm our business, financial performance and financial condition.
Provisions in Title XI of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibit the knowing and willful offer, payment, solicitation or receipt of remuneration, directly or indirectly, in return for the referral of patients or arranging for the referral of patients, or in return for the recommendation, arrangement, purchase, lease or order of items or services that are covered, in whole or in part, by a federal healthcare program such as Medicare or Medicaid. The definition of “remuneration” has been broadly interpreted to include anything of value such as gifts, discounts, rebates, waiver of payments or providing anything at less than its fair market value. Many states have adopted similar prohibitions against kickbacks and other practices that are intended to influence the purchase, lease or ordering of healthcare items and services regardless of whether the item or service is covered under a governmental health program or private health plan. Although certain statutory and regulatory safe harbors exist, these safe harbors are narrow and often difficult to comply with. Congress has appropriated an increasing amount of funds in recent years to support enforcement activities aimed at reducing healthcare fraud and abuse. We cannot assure you that our arrangements will be protected by such safe harbors or that such increased enforcement activities will not directly or indirectly have an adverse effect on our business, financial condition or results of operations. Any determination by a state or federal agency that any of our activities violate any of these laws could subject us to civil or criminal penalties, could require us to change or terminate some portions of our operations or business or could disqualify us from providing services to healthcare providers doing business with government programs and, thus, could have a material adverse effect on our business, financial condition and results of operations.
CMS has provided specific guidance on the proper treatment on Medicare cost reports of revenue distributions received from GPOs, including us. To assist our members that report their costs to Medicare to comply with these guidelines, such members are required under the terms of the Premier Group Purchasing Policy to appropriately reflect all elements of value received in connection with our IPO, including under agreements entered into in connection therewith, on their cost reports. We furnish applicable reports to such members setting forth the amount of such value, to assist their compliance with such cost reporting requirements. Any determination by a state or federal agency that the provision of such elements of value violate any of these laws could subject us to civil or criminal penalties, could require us to change or terminate some portions of our operations or
43
business, or could disqualify us from providing services to healthcare providers doing business with government programs, and, thus could have a material adverse effect on our business, financial condition and results of operations.
We periodically receive and respond to questions from government agencies on various matters, and we responded to an informal request in July 2014 from the United States Department of Health and Human Services (“HHS”) Office of Inspector General to analyze and discuss how the GPO participation agreements comply with the discount safe harbor to the Anti-Kickback Statute. We have had no further correspondence or interaction, oral or written, with the HHS Office of Inspector General regarding Anti-Kickback Statute compliance since that time. There is no safe harbor to the Anti-Kickback Statute that is applicable in its entirety across all of the agreements with our members, and no assurance can be given that the HHS Office of Inspector General or other regulators or enforcement authorities will agree with our assessment. Any determination by a state or federal agency that the terms, agreements and related communications with members, or our relationships with our members violates the Anti-Kickback Statute or any other federal or state laws could subject us to civil or criminal penalties, could require us to change or terminate some portions of our operations or business and could disqualify us from providing services to healthcare providers doing business with government programs and, thus, result in a material adverse effect on our business, financial condition and results of operations.
False Claims Regulations
Our business is also subject to numerous federal and state laws that forbid the submission or “causing the submission” of false or fraudulent information or the failure to disclose information in connection with the submission and payment of claims for reimbursement to Medicare, Medicaid, other federal healthcare programs or private health plans. In particular, the False Claims Act, or FCA, prohibits a person from knowingly presenting or causing to be presented a false or fraudulent claim for payment or approval by an officer, employee or agent of the United States. In addition, the FCA prohibits a person from knowingly making, using, or causing to be made or used a false record or statement material to such a claim. Violations of the FCA may result in treble damages, material monetary penalties and other collateral consequences, potentially including exclusion from participation in federally funded healthcare programs. The minimum and maximum per claim monetary damages for FCA violations occurring on or after November 2, 2015 and assessed after May 9, 2022 are from $12,537 to $25,076 per claim, respectively, and will be periodically readjusted for inflation. If enforcement authorities find that we have violated the FCA, it could have a material adverse effect on our business, financial condition and results of operations. Pursuant to the ACA, a claim that includes items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA.
These laws and regulations may change rapidly and it is frequently unclear how they apply to our business. Errors created by our products or consulting services that relate to entry, formatting, preparation or transmission of claim or cost report information by our members may be determined or alleged to be in violation of these laws and regulations. Any failure of our businesses or our products or services to comply with these laws and regulations, or the assertion that any of our relationships with suppliers or members violated the Anti-Kickback Statute and therefore caused the submission of false or fraudulent claims, could (i) result in substantial civil or criminal liability, (ii) adversely affect demand for our services, (iii) invalidate all or portions of some of our member contracts, (iv) require us to change or terminate some portions of our business, (v) require us to refund portions of our services fees, (vi) cause us to be disqualified from serving members doing business with government payors, and (vii) have a material adverse effect on our business, financial condition and results of operations.
ERISA Regulatory Compliance
As a threshold matter, the obligation for compliance with the Employee Retirement Income Security Act of 1974, as amended, (“ERISA”), the Internal Revenue Code (the “Code”), the ACA, the Heath Insurance Portability and Accountability Act (together with its amendments related to the Health Information Technology for Economic and Clinical Health Act, “HIPAA”), the Mental Health Parity and Addiction Equity Act, the Newborns’ and Mothers’ Health Protection Act, the Women’s Health and Cancer Rights Act, the Consolidated Omnibus Budget Reconciliation Act (“COBRA”), the Genetic Information Nondiscrimination Act of 2008, and other laws governing self-funded group health plans (collectively “Employee Benefit Laws”) generally rests with our clients as plan sponsors to whom we provide third party administrative (“TPA”) services). That is, employers/clients that sponsor group health plans generally bear the obligation of complying with Employee Benefit Laws, rather than entities, like us, that provide TPA services related to the group health plans. In certain cases, however, TPAs to ERISA plans can become “co-fiduciaries” with their clients and, therefore, can be liable for ERISA compliance in a limited capacity. We could become a co-fiduciary either by (1) entering a contractual obligation to be an ERISA fiduciary or (2) by acting as an ERISA fiduciary based on functions performed. Under ERISA, fiduciary status flows from actions, and TPAs who exercise certain functions, including any discretionary authority or discretionary responsibility over plan administration or exercise any authority or control respecting management or disposition of plan assets are generally “functional fiduciaries” with respect to (and limited to) the functions performed by the TPA that trigger fiduciary status.
44
We undertake no express liability under ERISA for our clients’ ERISA-governed plans in our template contracts. However, deviations from this standard language contained in final contracts could subject us to liability for breaches of fiduciary duty under ERISA (and related claims, such as ERISA prohibited transactions).
If current or future antitrust laws and regulations are interpreted or enforced in a manner adverse to us or our business, we may be subject to enforcement actions, penalties and other material limitations on our business.
We are subject to federal and state laws and regulations designed to protect competition which, if enforced in a manner adverse to us or our business, could have a material adverse effect on our business, financial condition and results of operations. Over the last decade or so, the group purchasing industry has been the subject of multiple reviews and inquiries by the U.S. Senate and its members with respect to antitrust laws. Additionally, the U.S. General Accounting Office, or GAO, has published several reports examining GPO pricing, contracting practices, activities and fees. We and several other operators of GPOs have responded to GAO inquiries in connection with the development of such reports. No assurance can be given regarding any further inquiries or actions arising or resulting from these examinations and reports, or any related impact on our business, financial condition or results of operations.
Congress, the DOJ, the Federal Trade Commission, or FTC, the U.S. Senate or another state or federal entity could at any time open a new investigation of the group purchasing industry, or develop new rules, regulations or laws governing the industry, that could adversely impact our ability to negotiate pricing arrangements with suppliers, increase reporting and documentation requirements, or otherwise require us to modify our arrangements in a manner that adversely impacts our business, financial condition and results of operations. We may also face private or government lawsuits alleging violations arising from the concerns articulated by these governmental factors or alleging violations based solely on concerns of individual private parties.
If we are found to be in violation of the antitrust laws, we could be subject to civil and criminal penalties or damages. The occurrence of any of these events could materially harm our business, financial condition and results of operations.
Complex international, federal and state, as well as international, privacy, security and breach notification laws may increase the costs of operation and expose us to civil and criminal government sanctions and third-party civil litigation.
We must comply with extensive federal and state requirements regarding the use, retention, security and re-disclosure of patient/beneficiary healthcare information. The Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations that have been issued under it, which we refer to collectively as HIPAA, contain substantial restrictions and complex requirements with respect to the use and disclosure of certain individually identifiable health information, referred to as “protected health information.” The HIPAA Privacy Rule prohibits a covered entity or a business associate (essentially, a third party engaged to assist a covered entity with enumerated operational and/or compliance functions) from using or disclosing protected health information unless the use or disclosure is validly authorized by the individual or is specifically required or permitted under the HIPAA Privacy Rule and only if certain complex requirements are met. The HIPAA Security Rule establishes administrative, organizational, physical and technical safeguards to protect the privacy, integrity and availability of electronic protected health information maintained or transmitted by covered entities and business associates. The HIPAA Breach Notification Rule requires that covered entities and business associates, under certain circumstances, notify patients/beneficiaries and HHS when there has been an improper use or disclosure of protected health information.
Our self-funded health benefit plan, the Premier, Inc. Health & Welfare Plan, and our healthcare provider members (provided that these members engage in HIPAA-defined standard electronic transactions with health plans, which will be all or the vast majority) are directly regulated by HIPAA as “covered entities.” Most of our U.S. hospital members disclose protected health information to us so that we may use that information to provide certain data analytics, benchmarking, consulting or other operational and compliance services to these members and accordingly, we are a “business associate” of those members and are required to protect such health information under HIPAA. With the enactment of the HITECH Act of 2009 and subsequent promulgation of the HIPAA Omnibus Rule in March 2013, the privacy and security requirements of HIPAA were modified and expanded, and, by way of example, further restrict the disclosure of protected health information by business associates and covered entities for marketing purposes or as part of a sale of the information to a third party, and require notification of affected individuals in the event of a breach. The Breach Notification Rule, included within the HIPAA Omnibus Rule, creates a rebuttable presumption that any acquisition, access, use or disclosure of protected health information not permitted under the Privacy Rule requires notice to affected patients/beneficiaries and HHS.
Any failure or perceived failure of our products or services to meet HIPAA standards and related regulatory requirements could expose us to certain notification, penalty and/or enforcement risks, damage our reputation and adversely affect demand for our products and services and force us to expend material capital, research and development and other resources to modify our products or services to address the privacy and security requirements of our members and HIPAA.
45
In addition to our obligations under HIPAA, there are other federal laws that include specific privacy and security obligations, above and beyond HIPAA, for certain types of health information and impose additional sanctions and penalties. These rules are not preempted by HIPAA. All 50 states, the District of Columbia, Guam, Puerto Rico and the Virgin Islands have enacted legislation requiring notice to individuals of security breaches of information involving personal health information, which is not uniformly defined amongst the breach notification laws. Organizations must review each state’s definitions, mandates and notification requirements and timelines to appropriately prepare and notify affected individuals and government agencies, including the attorney general in many states, in compliance with such state laws. Further, most states have enacted patient and/or beneficiary confidentiality laws that protect against the disclosure of confidential medical information, and many states have adopted or are considering adopting further legislation in this area, including privacy safeguards, security standards and special rules for so-called “sensitive” health information, such as mental health, genetic testing results, HIV status and biometric data. These state laws, if more stringent than HIPAA requirements, are not preempted by the federal requirements, and we are required to comply with them as well. The federal government also regulates the confidentiality of substance use disorder treatment records. These regulations, promulgated under 42 C.F.R. Part 2, apply to federally supported substance use disorder treatment programs and lawful holders of substance use disorder treatment records as a result of an individual consenting to their disclosure to such record holders. We may be considered a lawful holder of treatment records protected 42 C.F.R. Part 2 and therefore have responsibilities to protect such treatment records in ways that go beyond the HIPAA requirements. For example, we may be restricted from disclosing substance use disorder treatment records in response to requests from law enforcement agencies without first receiving a court order, or we may be prohibited from disclosing such records to third parties to whom we could typically disclose protected health information under HIPAA. We may be required to develop additional policies and procedures to address the requirements of 42 C.F.R. Part 2 and more stringent state laws, and we cannot guarantee that we have all such policies and procedures in place.
On June 28, 2018, California passed the California Consumer Privacy Act (“CCPA”), which imposes material changes in data privacy regulation in response to consumer demand for better protection of personal data and privacy. CCPA imposes consumer protections that are comparable to the European Union’s General Data Protection Regulation (“GDPR”) and took effect on January 1, 2020. In the wake of the CCPA’s passage, approximately 22 other states have either introduced, proposed or passed similar privacy legislation. Virginia was the second state to create sweeping consumer data privacy protections through the passage of the Consumer Data Protection Act (“CDPA”) which will go into effect on January 1, 2023. On June 8, 2021, Colorado passed the Colorado Privacy Act (“CPA”) which will go into effect on July 1, 2023. These consumer data privacy laws are similar in nature while maintaining specific unique requirements and definitions that require close analysis and application of each law to our business practices and related data protections. Similar proposals are also being considered at the federal level. The CCPA, the most stringent of the state privacy laws, applies to a wide range of businesses that handle Californians’ personal information and is not limited in scope to entities that have physical operations in California. It applies to for-profit entities “doing business” in the state that either: (i) have a gross annual revenue in excess of $25 million; or (ii) annually buy, receive for commercial purposes, sell or share for commercial purposes personal information of 50,000 or more California consumers, households or devices; or, (iii) derive 50% or more of their annual revenues from selling California consumers’ personal information. CCPA broadens the definition of personal information to include data elements not previously considered under any U.S. law, and we believe that we have taken the steps necessary to comply with new requirements governing the collection, use and sharing of personal information, including updating the disclosures in our privacy notices, establishing processes for responding to consumer rights requests, observing restrictions on data monetization practices, revisiting relationships and, where necessary, revising our agreements with vendors that handle personal information on our behalf. Violations of the CCPA are subject to enforcement by the California Attorney General’s office, which can seek civil penalties of $2,500 for each violation or $7,500 for each intentional violation after notice and a 30-day opportunity to cure have been provided. Enforcement activities under the CCPA by the Attorney General became effective July 1, 2020.
The implementation of GDPR on May 25, 2018, a regulation in European Union (“EU”) law on data protection and privacy for all individuals within the EU and the European Economic Area (“EEA”), can affect our obligations on the receipt, storage and use of personally identifiable information (Personal Data) attributed to individuals residing in the EU and EEA. GDPR applies to all enterprises, regardless of location, that are doing business in the EU, or that collect and analyze data tied to EU and EEA residents in connection with goods/services offered to such individuals. Some of our products and solutions are accessible internationally and such services collect Personal Data attributed to EU and EEA individuals when they engage in the use of our products and solutions. GDPR requires stringent technical and security controls surrounding the storage, use and disclosure of Personal Data, including the right to revoke consent to use, maintain, share or identify the individual through their Personal Data. GDPR is a regulation, not a directive; therefore, it does not require national governments to pass any enabling legislation and is directly binding and applicable. Sanctions under GDPR for violations of certain provisions range from a warning in writing to €20 million or up to 4% of the annual worldwide turnover of the preceding financial year for that organization, whichever is greater.
46
We are unable to predict what changes to HIPAA, the GDPR, the CCPA, CDP, CPA or other federal or state laws or regulations might be made in the future or how those changes could affect the demand for our products and services, our business or the associated costs of compliance.
Failure to comply with any of the federal and state standards regarding patient privacy, identity theft prevention and detection and data security may subject us to penalties, including civil monetary penalties and, in some circumstances, criminal penalties. In addition, such failure may materially injure our reputation and adversely affect our ability to retain members and attract new members and, accordingly, adversely affect our financial performance.
New requirements related to the interoperability of health information technology promulgated by the Office of the National Coordinator for Health Information Technology and enforced by the HHS Office of Inspector General could increase the costs of operation and expose us to civil government sanctions.
On May 1, 2020, the Office of the National Coordinator (“ONC”) for Health Information Technology promulgated final regulations under the authority of the 21st Century Cures Act (“ONC Rules”) to impose new conditions to obtaining and maintaining certification of certified health information technology and prohibit certain actors - developers of certified health information technology, health information networks, health information exchanges and health care providers - from engaging in activities that are likely to interfere with the access, exchange or use of electronic health information (information blocking). The final regulations further defined exceptions for activities that are permissible, even though they may have the effect of interfering with the access, exchange or use of electronic health information. The information subject to the information blocking restrictions is limited to electronic individually identifiable health information to the extent that it would be included in a designated record set. Until October 6, 2022, the information subject to the information blocking restrictions is further limited to the data elements represented in the United States Core Data for Interoperability standard.
Under the ONC Rules, we are considered a “health IT developer” because of the government certifications we hold in our TheraDoc and eCQM solutions. As such, we have evaluated and assessed the applicability of the ONC Rules to our TheraDoc and eCQM solutions, and we have determined that the ONC Rules currently do not apply to the data we hold on TheraDoc and eCQM solutions because the data is not part of any designated record set. Further, our customers contractually agree that the data that we maintain and process on behalf of our customers does not qualify as a designated record set. We will continue to assess our products and services to discern whether or not they fall under the purview of the ONC Rules. On April 24, 2020, the HHS Office of Inspector General published a proposed rule to incorporate its new civil monetary penalty authority for activities that constitute information blocking. When finalized, the HHS Office of Inspector General may impose information blocking penalties against developers of certified health information technology, health information networks or health information exchanges of up to $1 million per violation. The HHS Office of Inspector General proposed that its civil monetary penalty authority for information blocking will begin 60 days after it issues a final rule and has indicated that it intends to issue a final rule in September 2022. Any application of ONC Rules or similar regulations to our business could adversely affect our financial results by increasing our operating costs, slowing our time to market for our solutions, and making it uneconomical to offer some products.
If we become subject to regulation by the Food and Drug Administration because the functionality in one or more of our software applications causes the software to be regulated as a medical device, our financial results may be adversely impacted due to increased operating costs or delayed commercialization of regulated software products.
The Food and Drug Administration (“FDA”) has the authority to regulate products that meet the definition of a medical device under the Federal Food, Drug, and Cosmetic Act. To the extent that functionality or intended use in one or more of our current or future software products causes the software to be regulated as a medical device under existing or future FDA laws or regulations including the 21st Century Cures Act, which addresses, among other issues, the patient safety concerns generated by cybersecurity risks to medical devices and the interoperability between medical devices, we could be required to:
•register our company and list our FDA-regulated products with the FDA;
•obtain pre-market clearance from the FDA based on demonstration of substantial equivalence to a legally marketed device before marketing our regulated products;
•obtain FDA approval by demonstrating the safety and effectiveness of the regulated products prior to marketing;
•submit to inspections by the FDA; and
•comply with various FDA regulations, including the agency’s quality system regulation, compliant handling and medical device reporting regulations, requirements for medical device modifications, increased rigor of the secure development life cycle in the development of medical devices and the interoperability of medical devices and electronic health records, requirements for clinical investigations, corrections and removal reporting regulations, and post-market surveillance regulations.
47
The FDA can impose extensive requirements governing pre- and post-market activities, such as clinical investigations involving the use of a regulated product, as well as conditions relating to clearance or approval, labeling and manufacturing of a regulated product. In addition, the FDA can impose extensive requirements governing development controls and quality assurance processes. Any application of FDA regulations to our business could adversely affect our financial results by increasing our operating costs, slowing our time to market for regulated software products, subjecting us to additional government oversight and regulatory inspections and making it uneconomical to offer some software products.
Legal and Tax-Related Risks
We are subject to litigation from time to time, which could have a material adverse effect on our business, financial condition and results of operations.
We participate in businesses and activities that are subject to substantial litigation. We are from time to time involved in litigation, which may include claims relating to contractual disputes, product liability, torts or personal injury, employment, antitrust, intellectual property or other commercial or regulatory matters. Additionally, if current or future government regulations are interpreted or enforced in a manner adverse to us or our business, specifically those with respect to antitrust or healthcare laws, we may be subject to enforcement actions, penalties, damages and other material limitations on our business.
Furthermore, as a public company, we may become subject to stockholder inspection demands under Delaware law, and derivative or other similar litigation that can be expensive, divert human and financial capital to less productive uses, and result in potential reputational damage. The August 2020 Restructuring resulted in (i) the announcement of several investigations by private law firms of possible securities law violations; (ii) stockholder inspection demands seeking to investigate possible breaches of fiduciary duties; and (iii) the filing of a shareholder derivative complaint on March 4, 2022, captioned City of Warren General Employees’ Retirement System v. Michael Alkire, et al., Case No. 2022-0207-JTL. The complaint, purportedly brought on behalf of Premier, was filed in the Delaware Court of Chancery against our current and former Chief Executive Officers and current and certain former directors. We are named as a nominal defendant in the complaint. The lawsuit alleges that the named officers and directors breached their fiduciary duties and committed corporate waste by approving agreements between Premier and certain of the former LPs that provided for accelerated payments as consideration for the early termination of the tax receivable agreement (“TRA”) with such LPs. (See “Item 3. Legal Proceedings”). In the event that the City of Warren General Employees’ Retirement System case, or any of the other matters referenced above that results in formal litigation, ultimately result in an adverse judgment, we may experience an adverse impact on our financial condition, results of operations or stock price.
From time to time, we have been named as a defendant in class action antitrust lawsuits brought by suppliers or purchasers of medical products. Typically, these lawsuits have alleged the existence of a conspiracy among manufacturers of competing products, distributors and/or operators of GPOs, including us, to deny the plaintiff access to a market for certain products, to raise the prices for products and/or to limit the plaintiff’s choice of products to buy. No assurance can be given that we will not be subjected to similar actions in the future or that any such existing or future matters will be resolved in a manner satisfactory to us or which will not harm our business, financial condition or results of operations.
We may become subject to additional litigation or governmental investigations in the future. These claims may result in material defense costs or may compel us to pay material fines, judgments or settlements, which, if uninsured, could have a material adverse effect on our business, financial condition, results of operations and cash flows. In addition, certain litigation matters could adversely impact our commercial reputation, which is critical for attracting and retaining customers, suppliers and member participation in our GPO programs. Further, stockholder and other litigation may result in adverse investor perception of our company, negatively impact our stock price and increase our cost of capital.
Failure to protect our intellectual property and claims against our use of the intellectual property of third parties could cause us to incur unanticipated expense and prevent us from providing our products and services, which could adversely affect our business, financial condition and results of operations.
Our success depends in part upon our ability to protect our core technology and intellectual property. To accomplish this, we rely on a combination of intellectual property rights, including trade secrets, copyrights and trademarks, as well as customary contractual and confidentiality protections and internal policies applicable to employees, contractors, members and business partners. These protections may not be adequate, however, and we cannot assure you that they will prevent misappropriation of our intellectual property. In addition, parties that gain access to our intellectual property might fail to comply with the terms of our agreements and policies and we may not be able to enforce our rights adequately against these parties. The disclosure to, or independent development by, a competitor of any trade secret, know-how or other technology not protected by a patent could materially and adversely affect any competitive advantage we may have over such competitor. The process of enforcing our intellectual property rights through legal proceedings would likely be burdensome and expensive and our ultimate success
48
cannot be assured. Our failure to adequately protect our intellectual property and proprietary rights could adversely affect our business, financial condition and results of operations.
In addition, we could be subject to claims of intellectual property infringement, misappropriation or other intellectual property violations as our applications’ functionalities overlap with competitive products, and third parties may claim that we do not own or have rights to use all intellectual property used in the conduct of our business or acquired by us. We could incur substantial costs and diversion of management resources defending any such claims. Furthermore, a party making a claim against us could secure a judgment awarding substantial damages as well as injunctive or other equitable relief that could effectively block our ability to provide products or services. Such claims also might require indemnification of our members at material expense.
A number of our contracts with our members contain indemnity provisions whereby we indemnify them against certain losses that may arise from third-party claims that are brought in connection with the use of our products.
Our exposure to risks associated with the protection and use of intellectual property may be increased as a result of acquisitions, as we have limited visibility into the development process of acquired entities or businesses with respect to their technology or the care taken by acquired entities or businesses to safeguard against infringement risks. In addition, third parties may make infringement and similar or related claims after we have acquired technology that had not been asserted prior to our acquisition thereof.
If we are required to collect sales and use taxes on the products and services we sell in certain jurisdictions or online, we may be subject to tax liability for past sales, future sales may decrease and our financial condition may be materially and adversely affected.
Sales tax is currently not imposed on the administrative fees we collect in connection with our GPO programs. If sales tax were imposed in the future on such fees, the profitability of our GPO programs may be materially and adversely affected.
Rules and regulations applicable to sales and use tax vary materially by tax jurisdiction. In addition, the applicability of these rules given the nature of our products and services is subject to change.
We may lose sales or incur material costs should various tax jurisdictions be successful in imposing sales and use taxes on a broader range of products and services than those currently so taxed, including products and services sold online. A successful assertion by one or more taxing authorities that we should collect sales or other taxes on the sale of our solutions could result in substantial tax liabilities for past and future sales, decrease our ability to compete and otherwise harm our business.
If one or more taxing authorities determines that taxes should have, but have not, been paid with respect to our products and services, including products and services sold online, we may be liable for past taxes in addition to taxes going forward. Liability for past taxes may also include very substantial interest and penalty charges. If we are required to collect and pay back taxes (and the associated interest and penalties) and if our members fail or refuse to reimburse us for all or a portion of these amounts, we will have incurred unplanned costs that may be substantial. Moreover, imposition of such taxes on our services going forward will effectively increase the cost of such services to our members and may adversely affect our ability to retain existing members or to gain new members in the areas in which such taxes are imposed.
Changes in tax laws could materially impact our effective tax rate, income tax expense, anticipated tax benefits, deferred tax assets, cash flows and profitability.
Continued economic and political conditions in the United States could result in changes in U.S. tax laws beyond those enacted in connection with the TCJA on December 22, 2017 and the Coronavirus Aid, Relief, and Economic Security Act (“CARES”) on March 27, 2020. Further changes to U.S. tax laws could impact how U.S. corporations are taxed. Although we cannot predict whether or in what form such changes will pass, if enacted into law, they could have a material impact on our effective tax rate, income tax expense, ability to fully realize anticipated tax benefits that correspond to our fixed payment obligations associated with the acceleration of our TRA, deferred tax assets, results of operations, cash flows and profitability.
A loss of a major tax dispute could result in a higher tax rate on our earnings, which could result in a material adverse effect on our financial condition and results of operations.
Income tax returns that we file are subject to review and examination. We recognize the benefit of income tax positions we believe are more likely than not to be sustained upon challenge by a tax authority. If any tax authority successfully challenges our positions or if we lose a material tax dispute, our effective tax rate on our earnings could increase substantially and result in a material adverse effect on our financial condition.
49
Risks Related to Our Corporate Structure
Payments required under the Unit Exchange and Tax Receivable Acceleration Agreements will reduce the amount of overall cash flow that would otherwise be available to us. In addition, we may not be able to realize all or a portion of the expected tax benefits that correspond to our fixed payment obligations associated with the acceleration of our TRA.
We entered into Unit Exchange and Tax Receivable Acceleration Agreements, effective as of July 1, 2020 (the “Unit Exchange Agreements”), with a substantial majority of our member-owners. Pursuant to the terms of the Unit Exchange Agreements, we elected to terminate the TRA upon payment to the member-owners of the discounted present value of the tax benefit payments otherwise owed to them over a 15-year period under the TRA. As a result of the acceleration and termination of the TRA, we are obligated to pay our member-owners approximately $472.6 million in aggregate. Of that amount, an aggregate of $299.0 million remains payable in equal quarterly installments through the quarter ending June 30, 2025. Due to the payments required under the Unit Exchange Agreements, our overall cash flow and discretionary funds will be reduced, which may limit our ability to execute our business strategies or deploy capital for preferred use. In addition, if we do not have available capital on hand or access to adequate funds to make these required payments, our financial condition would be materially adversely impacted.
The payments required upon termination of the TRA are based upon the present value of all forecasted future payments that would have otherwise been made under the TRA. These payments are fixed obligations of ours and could ultimately exceed the actual tax benefits that we realize. Additionally, if our actual taxable income were insufficient or there were adverse changes in applicable law or regulations, we may be unable to realize all or a portion of these expected benefits and our cash flows and stockholders’ equity could be negatively affected.
Our certificate of incorporation and bylaws and provisions of Delaware law may discourage or prevent strategic transactions, including a takeover of our company, even if such a transaction would be beneficial to our stockholders.
Provisions contained in our certificate of incorporation and bylaws and provisions of the Delaware General Corporation Law, or DGCL, could delay or prevent a third party from entering into a strategic transaction with us, even if such a transaction would benefit our stockholders. For example, our certificate of incorporation and bylaws:
•divide our Board of Directors into three classes with staggered three-year terms, which may delay or prevent a change of our management or a change in control;
•authorize our Board of Directors to issue “blank check” preferred stock in order to increase the aggregate number of outstanding shares of capital stock and thereby make a takeover more difficult and expensive;
•do not permit cumulative voting in the election of directors, which would otherwise allow less than a majority of stockholders to elect director candidates;
•do not permit stockholders to take action by written consent;
•provide that special meetings of the stockholders may be called only by or at the direction of the Board of Directors, the chair of our Board or the chief executive officer;
•require advance notice to be given by stockholders of any stockholder proposals or director nominees;
•require a super-majority vote of the stockholders to amend our certificate of incorporation; and
•allow our Board of Directors to make, alter or repeal our bylaws but only allow stockholders to amend our bylaws upon the approval of 662/3% or more of the voting power of all of the outstanding shares of our capital stock entitled to vote.
In addition, we are subject to the provisions of Section 203 of the DGCL which limits, subject to certain exceptions, the right of a corporation to engage in a business combination with a holder of 15% or more of the corporation’s outstanding voting securities or certain affiliated persons.
These restrictions could limit stockholder value by impeding the sale of our company and discouraging potential takeover attempts that might otherwise be financially beneficial to our stockholders.
50
Risks Related to Our Class A Common Stock
If we fail to maintain an effective system of integrated internal controls, we may not be able to report our financial results accurately, we may determine that our prior financial statements are not reliable, or we may be required to expend material financial and personnel resources to remediate any weaknesses, any of which could have a material adverse effect on our business, financial condition and results of operations.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be evaluated frequently. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and attestations of the effectiveness of internal controls by independent auditors. Maintaining effective internal controls has been and will continue to be costly and may divert management’s attention.
We have identified material weaknesses in our internal controls over financial reporting in the past. Our future evaluation of our internal controls over financial reporting may identify additional material weaknesses that may cause us to (i) be unable to report our financial information on a timely basis or (ii) determine that our previously issued financial statements should no longer be relied upon because of a material error in such financial statements, and thereby result in adverse regulatory consequences, including sanctions by the SEC, violations of NASDAQ listing rules or stockholder litigation. In the event that we identify a material weakness in our internal control over financial reporting, we may need to amend previously reported financial statements and will be required to implement a remediation plan to address the identified weakness, which will likely result in our expending material financial and personnel resources to remediate the identified weakness. There also could be a negative reaction in the financial markets due to a loss of investor confidence in us and the reliability of our financial statements. Confidence in the reliability of our financial statements also could suffer if we or our independent registered public accounting firm were to report a material weakness in our internal controls over financial reporting. The occurrence of any of these events could materially adversely affect our business, financial condition and results of operations and could also lead to a decline in the price of our Class A common stock.
There can be no assurance we will pay dividends on our Class A common stock at current levels or at all, and failure to pay any such dividends could have a material adverse impact on our stock price and your investment in Premier.
Since September 2020, we have paid quarterly cash dividends on our Class A common stock. The continued payment of dividends and the rate of any such dividends will be at the discretion of our Board of Directors after taking into account various factors, including our business, operating results and financial condition, current and anticipated capital requirements and cash needs, plans for expansion and any legal or contractual limitations on our ability to pay dividends. If we cease paying dividends, we could experience a material adverse impact on our stock price and your investment may materially decline, and as a result, capital appreciation in the price of our Class A common stock, if any, may be your only source of gain on an investment in our Class A common stock.
Our future issuance of common stock, preferred stock, limited partnership units or debt securities could have a dilutive effect on our common stockholders and adversely affect the market value of our Class A common stock.
In the future, we could issue a material number of shares of Class A common stock, which could dilute our existing stockholders materially and have a material adverse effect on the market price for the shares of our Class A common stock. Furthermore, the future issuance of shares of preferred stock with voting rights may adversely affect the voting power of our common stockholders, either by diluting the voting power of our common stock if the preferred stock votes together with the common stock as a single class or by giving the holders of any such preferred stock the right to block an action on which they have a separate class vote even if the action were approved by the holders of our common stock. The future issuance of shares of preferred stock with dividend or conversion rights, liquidation preferences or other economic terms favorable to the holders of preferred stock could adversely affect the market price for our Class A common stock by making an investment in the Class A common stock less attractive. In addition to potential equity issuances described above, we also may issue debt securities that would rank senior to shares of our Class A common stock.
Upon our liquidation, holders of our preferred shares, if any, and debt securities and instruments will receive a distribution of our available assets before holders of shares of our Class A common stock. We are not required to offer any such additional debt or equity securities to existing stockholders on a preemptive basis. Therefore, additional issuances of our Class A common stock, directly or through convertible or exchangeable securities, warrants or options, will dilute the holders of shares of our existing Class A common stock and such issuances, or the anticipation of such issuances, may reduce the market price of shares of our Class A common stock. Any preferred shares, if issued, would likely have a preference on distribution payments, periodically or upon liquidation, which could limit our ability to make distributions to holders of shares of our Class A common stock. Because our decision to issue debt or equity securities or otherwise incur debt in the future will depend on market
51
conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future capital raising efforts.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
As of June 30, 2022, we occupy our Charlotte, North Carolina headquarters under a long-term lease which expires in 2026 and includes options for us, at our discretion, to renew the lease for up to 15 years in total beyond that date. We also lease or sublease nine smaller facilities across five states. We believe that our headquarters, as well as our smaller leased facilities, are suitable for our use and are, in all material respects, adequate for our present and expected needs. In connection with COVID-19 and related temporary closures, we continue to evaluate our real estate needs.
We generally conduct the operations of our Supply Chain Services segment and our Performance Services segment across our property locations. See Note 18 - Commitments and Contingencies to the accompanying audited consolidated financial statements for more information about our operating leases.
Item 3. Legal Proceedings
We operate businesses that are subject to substantial litigation from time to time. We are periodically involved in litigation, arising in the ordinary course of business or otherwise, which from time to time may include claims relating to contractual disputes, product liability, tort or personal injury, employment, antitrust, intellectual property or other commercial or regulatory matters. If current or future government regulations are interpreted or enforced in a manner adverse to us or our business, including without limitation those with respect to antitrust or healthcare laws, we may be subject to enforcement actions, penalties, damages and material limitations on our business.
From time to time we have been named as a defendant in class action antitrust lawsuits brought by suppliers or purchasers of medical products. Typically, these lawsuits have alleged the existence of a conspiracy among manufacturers of competing products, distributors and/or operators of GPOs, including us, to deny the plaintiff access to a market for certain products, to raise the prices for products and/or limit the plaintiff’s choice of products to buy. We believe that we have at all times conducted our business affairs in an ethical and legally compliant manner and have successfully resolved all such actions. No assurance can be given that we will not be subjected to similar actions in the future or that any such existing or future matters will be resolved in a manner satisfactory to us or which will not harm our business, financial condition or results of operations.
On March 4, 2022, a shareholder derivative complaint captioned City of Warren General Employees’ Retirement System v. Michael Alkire, et al., Case No. 2022-0207-JTL, purportedly brought on behalf of Premier, was filed in the Delaware Court of Chancery against our current and former Chief Executive Officers and current and certain former directors. We are named as a nominal defendant in the complaint. The lawsuit alleges that the named officers and directors breached their fiduciary duties and committed corporate waste by approving agreements between Premier and certain of the former LPs that provided for accelerated payments as consideration for the early termination of the TRA with such LPs. The complaint asserts that the aggregate early termination payment amounts of $473.5 million exceeded the alleged value of the tax assets underlying the TRA by approximately $225.0 million.
The complaint seeks unspecified damages, costs and expenses, including attorney fees, and declaratory and other equitable relief. Since the lawsuit is purportedly brought on behalf of Premier, and we are only a nominal defendant, the alleged damages were allegedly suffered by us. We and the individual defendants deny the allegations in the complaint and intend to vigorously defend the litigation. In light of the fact that the lawsuit is in an early stage and the claims do not specify an amount of damages, we cannot predict the ultimate outcome of the suit.
Additional information relating to certain legal proceedings in which we are involved is included in Note 18 - Commitments and Contingencies, to the accompanying audited consolidated financial statements, which is incorporated herein by reference.
Item 4. Mine Safety Disclosures
Not Applicable.
52
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our Class A common stock is publicly traded on the NASDAQ Global Select Market (“NASDAQ”) under the ticker symbol “PINC.”
Based on the records of our Class A common stock transfer agent, as of August 11, 2022, there were 118,066,513 shares of our Class A common stock issued and outstanding, held by 82 holders of record. Because a substantial portion of our Class A common stock is held by brokers and other institutions on behalf of shareholders, we are unable to estimate the total number of beneficial owners currently holding our Class A common stock.
Dividend Policy
During fiscal year 2022, our Board of Directors declared regular quarterly cash dividends of $0.20 per share on our outstanding shares of Class A common stock, which were paid on September 15, 2021, December 15, 2021, March 15, 2022 and June 15, 2022.
On August 4, 2022, our Board of Directors declared a quarterly cash dividend of $0.21 per share, payable on September 15, 2022 to stockholders of record on September 1, 2022.
The actual declaration of any future cash dividends, and the setting of record and payment dates as well as the per share amounts, will be at the discretion of our Board of Directors each quarter after consideration of various factors, including our results of operations, financial condition and capital requirements, earnings, general business conditions, restrictions imposed by our current credit facility and any future financing arrangements, legal restrictions on the payment of dividends and other factors our Board of Directors deems relevant. We currently expect quarterly dividends to continue to be paid on or about December 15, March 15, June 15 and September 15, respectively.
Recent Sales of Unregistered Securities
All sales of unregistered securities during the fiscal year ended June 30, 2022 have been previously reported in filings with the SEC.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 201(d) of Regulation S-K is provided under “Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters—Equity Compensation Plan Information”, incorporated herein by reference.
Purchase of Equity Securities
On August 5, 2021, our Board of Directors authorized the repurchase of up to $250.0 million of our outstanding Class A common stock during fiscal year 2022 through open market purchases or privately negotiated transactions. As of June 30, 2022, we had completed our stock repurchase program and repurchased 6.4 million shares of Class A common stock at an average price of $38.88 per share for a total purchase price of $250.0 million. No shares of Class A common stock were repurchased during the three months ended June 30, 2022.
Company Stock Performance
The performance graph below shows a five-year comparison of the total cumulative return, assuming reinvestment of all dividends, had $100 been invested at the close of business on June 30, 2017, in each of:
•our Class A common stock;
•the NASDAQ Composite stock index (“NASDAQ Composite Index”);
•a customized peer group of companies previously used by us (the “Prior Peer Group”); and
•a customized peer group of 12 companies selected by us that we believe is better aligned with our company (the “Peer Group”).
We have used the Peer Group, a group selected in good faith and used by our compensation committee of the Board of Directors (“compensation committee”) for peer comparison benchmarking purposes because we believe this group provides an accurate representation of our peers. Our compensation committee reviewed and, in consultation with its independent
53
consultant, selected the companies in our fiscal year 2022 Peer Group in April 2022. Our compensation committee determined it appropriate to reconfigure our peer group to a more representative group of appropriately sized companies that reflect our diverse and growing business model. As the companies in our Peer Group change, our compensation committee will continue to review and reconfigure our Peer Group as it deems necessary in consultation with its independent consultant.
The Peer Group graph line consists of the following 12 companies: Allscripts Healthcare Solutions Inc., AMN Healthcare Services, Inc., ASGN Inc., Change Healthcare Inc., Evolent Health, Inc., FTI Consulting Inc., Huron Consulting Group Inc., Mednax Inc., Omnicell Inc., Owens & Minor Inc., Patterson Companies, Inc. and R1 RCM Inc. Compared to our the Prior Peer Group, our current Peer Group includes: Change Healthcare Inc., Evolent Health, Inc., and R1 RCM Inc. which our compensation committee believed were similar in size and business operations to us and excludes Cerner Corp., Hill-Rom Holdings Inc., HMS Holdings Corp., Magellan Health, Inc., and NextGen Healthcare, Inc. HMS Holdings Corp. was included in the initial Prior Peer Group but was excluded from the graph below because it was acquired during our fiscal year 2021.
The information contained in the performance graph below shall not be deemed “soliciting material” or to be “filed” with the SEC nor shall such information be deemed incorporated by reference into any future filing under the Securities Act or the Exchange Act except to the extent we specifically incorporate it by reference into such filing.
The comparisons in the graph below are based upon historical data and are not indicative of, nor intended to forecast, future performance of our common stock. Research Data Group, Inc. provided the data for the indices presented below. We assume no responsibility for the accuracy of the indices’ data, but we are not aware of any reason to doubt its accuracy.
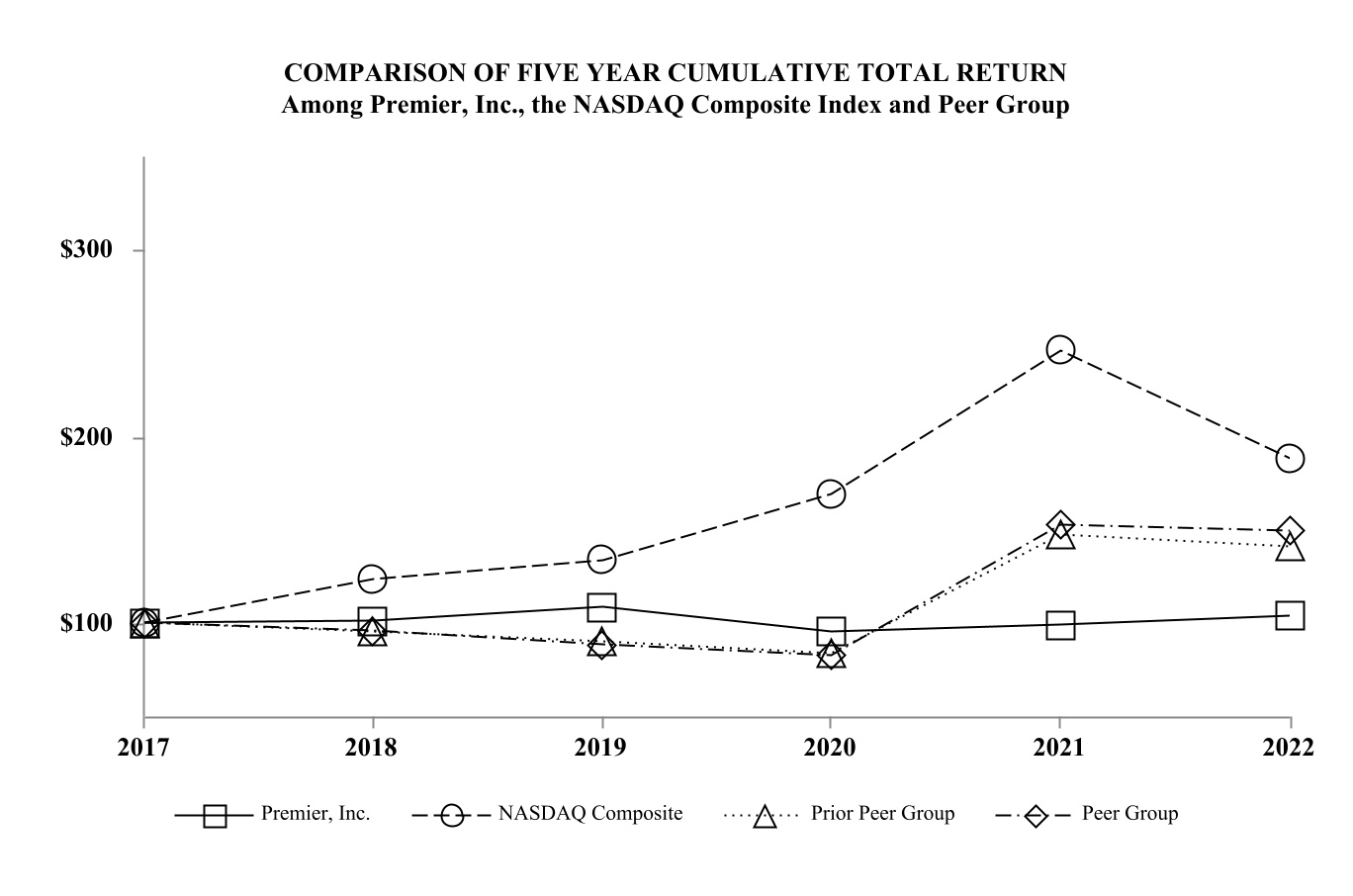
Value of Investment as of June 30(a): | ||||||||||||||||||||
| Company/Index Name | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | ||||||||||||||
Premier, Inc. Class A Common Stock | $ | 100.00 | $ | 101.06 | $ | 108.64 | $ | 95.22 | $ | 98.84 | $ | 103.58 | ||||||||
NASDAQ Composite Index | $ | 100.00 | $ | 123.60 | $ | 133.22 | $ | 169.11 | $ | 245.60 | $ | 188.07 | ||||||||
| Prior Peer Group | $ | 100.00 | $ | 95.12 | $ | 90.20 | $ | 83.90 | $ | 147.06 | $ | 140.73 | ||||||||
| Peer Group | $ | 100.00 | $ | 95.63 | $ | 88.55 | $ | 82.78 | $ | 152.77 | $ | 149.44 | ||||||||
54
_________________________________
(a)Assumes $100 invested on June 30, 2017, including reinvestment of dividends for periods from 2017-2022. We began paying cash dividends in September 2020.
We will neither make nor endorse any predictions as to future stock performance or whether the trends depicted in the graph above will continue or change in the future. The stock price performance included in this graph is not necessarily indicative of future stock price performance.
Item 6. Reserved
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with our audited consolidated financial statements and the notes thereto included elsewhere in this Annual Report. This discussion is designed to provide the reader with information that will assist in understanding our consolidated financial statements, the changes in certain key items in those financial statements from year to year, and the primary factors that accounted for those changes, as well as how certain accounting principles affect our consolidated financial statements. In addition, the following discussion includes certain forward-looking statements. For a discussion of important factors, including the continuing development of our business and other factors which could cause actual results to differ materially from the results referred to in the forward-looking statements, see “Item 1A. Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” contained in this Annual Report. Unless otherwise indicated, information in Management’s Discussion and Analysis of Financial Condition and Results of Operations has been retrospectively adjusted to reflect continuing operations for all periods presented. See Note 4 - Discontinued Operations and Exit Activities to the audited consolidated financial statements included in this Annual Report for further information.
Business Overview
Our Business
Premier, Inc. (“Premier”, the “Company”, “we”, or “our”) is a leading healthcare improvement company, uniting an alliance of U.S. hospitals, health systems and other providers and organizations to transform healthcare. We partner with hospitals, health systems, physicians, employers, product suppliers, service providers, and other healthcare providers and organizations with the common goal of improving and innovating in the clinical, financial and operational areas of their businesses to meet the demands of a rapidly evolving healthcare industry. We deliver value through a comprehensive technology-enabled platform that offers critical supply chain services, clinical, financial, operational and value-based care software-as-a-service (“SaaS”) as well as clinical and enterprise analytics licenses, consulting services, performance improvement collaborative programs, third-party administrator services, access to our centers of excellence program and digital invoicing and payment processes for healthcare product suppliers and service providers and continue to expand our capabilities to more fully address and coordinate care improvement and standardization in the employer, payor and life sciences markets. We also provide services to other businesses including food service, schools and universities.
We generated net revenue, net income and Adjusted EBITDA (a financial measure not determined in accordance with generally accepted accounting principles (“Non-GAAP”)) for the periods presented as follows (in thousands):
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net revenue | $ | 1,432,901 | $ | 1,721,152 | |||||||
| Net income | 268,318 | 304,584 | |||||||||
| Non-GAAP Adjusted EBITDA | 498,682 | 473,230 | |||||||||
See “Our Use of Non-GAAP Financial Measures” and “Results of Operations” below for a discussion of our use of Non-GAAP Adjusted EBITDA and a reconciliation of net income to Non-GAAP Adjusted EBITDA.
Our Business Segments
Our business model and solutions are designed to provide our members and other customers access to scale efficiencies while focusing on optimization of information resources and cost containment, provide actionable intelligence derived from anonymized data in our data warehouse provided by our members, mitigate the risk of innovation, and disseminate best practices that will help our member organizations and other customers succeed in their transformation to higher quality and more cost-effective healthcare. We deliver our integrated platform of solutions that address the areas of clinical intelligence, margin improvement and value-based care through two business segments: Supply Chain Services and Performance Services.
55
Segment net revenue was as follows (in thousands):
| Year Ended June 30, | % of Net Revenue | ||||||||||||||||||||||||||||||||||
| Net revenue: | 2022 | 2021 | Change ($) | Change (%) | 2022 | 2021 | |||||||||||||||||||||||||||||
| Supply Chain Services | $ | 1,031,946 | $ | 1,343,634 | $ | (311,688) | (23) | % | 72 | % | 78 | % | |||||||||||||||||||||||
| Performance Services | 400,983 | 377,518 | 23,465 | 6 | % | 28 | % | 22 | % | ||||||||||||||||||||||||||
| Segment net revenue | $ | 1,432,929 | $ | 1,721,152 | $ | (288,223) | (17) | % | 100 | % | 100 | % | |||||||||||||||||||||||
Our Supply Chain Services segment includes one of the largest healthcare group purchasing organization programs (“GPO”) in the United States, serving acute, non-acute and non-healthcare sites and providing supply chain co-management, purchased services and direct sourcing activities. We generate revenue in our Supply Chain Services segment from administrative fees received from suppliers based on the total dollar volume of goods and services purchased by our members and other customers, service fees from supply chain co-management, subscription fees from purchased services and through product sales in connection with our direct sourcing activities.
Our Performance Services segment consists of three sub-brands: PINC AITM, our technology and services platform with offerings that help optimize performance in three main areas – clinical intelligence, margin improvement and value-based care – using advanced analytics to identify improvement opportunities, consulting services for clinical and operational design, and workflow solutions to hardwire sustainable change in the provider, life sciences and payor markets; Contigo Health®, our direct-to-employer business which provides third party administrator services and management of health benefit programs that allow employers to contract directly with healthcare providers as well as partners with healthcare providers to provide employers access to a specialized care network through Contigo Health’s centers of excellence program; and RemitraTM, our digital invoicing and payables business which provides financial support services to healthcare product suppliers and service providers. Each sub-brand serves different markets but are all united in our vision to optimize provider performance and accelerate industry innovation for better, smarter healthcare. For additional information, please see “Performance Services” above.
Acquisitions and Divestitures
Acquisition of Invoice Delivery Services, LP Assets
On March 1, 2021, we acquired, through our indirect, wholly owned subsidiary, Premier IDS, LLC, substantially all the assets and assumed certain liabilities of Invoice Delivery Services, LP (“IDS”) for an adjusted purchase price of $80.7 million, subject to certain adjustments, of which $80.0 million was paid at closing with borrowings under our Credit Facility (as defined in Note 10 - Debt and Notes Payable to the accompanying audited consolidated financial statements). IDS has been integrated within Premier under the brand name Remitra and is reported as part of the Performance Services segment. See Note 3 - Business Acquisitions to the accompanying audited consolidated financial statements for further information.
Market and Industry Trends and Outlook
We expect that certain trends and economic or industrywide factors will continue to affect our business, both in the short- and long-term. We have based our expectations described below on assumptions made by us and on information currently available to us. To the extent our underlying assumptions about, or interpretation of, available information prove to be incorrect, our actual results may vary materially from our expected results. See “Cautionary Note Regarding Forward-Looking Statements” and “Risk Factors.”
Trends in the U.S. healthcare market affect our revenues and costs in the Supply Chain Services and Performance Services segments. The trends we see affecting our current healthcare business include the impact of the implementation of current or future healthcare legislation, particularly the potential for the Affordable Care Act (“ACA”) to be materially altered by Congress, through regulatory action by government agencies, or in the event of a change of party control in Congress. Actions related to the ACA could be disruptive for Premier and our customers, impacting revenue, reporting requirements, payment reforms, shift in care to the alternate site market and increased data availability and transparency. To meet the demands of this environment, there will be increased focus on scale and cost containment and healthcare providers will need to measure and report on and bear financial risk for outcomes. Over the long-term, we believe these trends will result in increased demand for our Supply Chain Services and Performance Services solutions in the areas of cost management, quality and safety, and value-based care; however, there are uncertainties and risks that may affect the actual impact of these anticipated trends, expected demand for our services or related assumptions on our business. See “Cautionary Note Regarding Forward-Looking Statements” for more information.
56
COVID-19 Pandemic, Variants Thereof, Recurrences or Similar Pandemics
In addition to the trends in the U.S. healthcare market discussed above, we face known and unknown uncertainties arising from the outbreak of the novel coronavirus (“COVID-19”) and the resulting global pandemic and financial and operational uncertainty, including its impact on the overall economy, our sales, operations and supply chains, our members and other customers, workforce and suppliers, and countries. As a result of the COVID-19 pandemic, variants thereof, and potential future pandemic outbreaks, we face significant risks including, but not limited to:
•Overall economic and capital markets decline. The impact of the COVID-19 pandemic and variants thereof and associated supply chain disruptions could result in a prolonged recession or depression in the United States or globally that could harm the banking system, limit demand for many products and services and cause other foreseen and unforeseen events and circumstances, all of which could negatively impact us. The continued spread of COVID-19 and variants thereof has led to and could continue to lead to severe disruption and volatility in the United States and global capital markets, which could increase our cost of capital and adversely affect our ability to access the capital markets in the future. In addition, trading prices on the public stock market, as well as that of our Class A common stock, have been highly volatile as a result of the COVID-19 pandemic.
•Changes in the demand for our products and services. We experienced and may continue to experience demand uncertainty from both material increases and decreases in demand and pricing for our products and services as a result of the COVID-19 pandemic. There was a material increase in demand and pricing for personal protective equipment (“PPE”), drugs and other supplies directly related to treating and preventing the spread of COVID-19 and variants thereof during fiscal 2020 and 2021. In the second half of fiscal 2022, demand and pricing for PPE, drugs and other supplies decreased resulting in a decline in revenue relative to the previous two years. Patients, hospitals and other medical facilities continued to defer some elective procedures and routine medical visits due to ongoing and continuing uncertainty from COVID-19 outbreaks or variants or as a result of restrictive government orders or advisories. While demand for many supplies and services not related to COVID-19 may continue to decline into fiscal 2023, rolling shortages of products and drugs needed for routine procedures, such as, contrast media and syringes, could have an impact on demand for hospital services and the financial conditions of providers, particularly those forced to procure such products through resellers.
•Increased labor costs. Labor shortages and the resulting increases to the cost of labor are a continued challenge to the health care providers we serve. Limited availability of staff resources and rolling staff shortages may continue to impair the ability of existing staff to manage product and service procurement. While our non-acute and non-healthcare business such as education and hospitality customers, experienced a rebound in fiscal year 2022, the recovery in the business may be hampered by future COVID-19 variants or outbreaks, which are highly uncertain and cannot be accurately predicted.
•Limited access to our members’ facilities that impacts our ability to fulfill our contractual requirements. While some of our hospital customers have increased access to their facilities for non-patients, including our field teams, consultants and other professionals, there are many that still are not allowing onsite access outside of their staff. Hospital imposed travel restrictions are also impacting some customers’ ability to participate in face-to-face events with us, such as committee meetings and conferences, which limits our ability to build on customer relationships. The long-term continuation, or any future recurrence of these circumstances may negatively impact the ability of our employees to effectively deliver existing or sell new products and services to our members and could negatively affect our performance of our existing contracts.
•Materials and personnel shortages and disruptions in supply chain, including manufacturing and shipping. The global supply chain has been materially disrupted due to personnel shortages associated with ongoing COVID-19 rates of infection, stay-at-home orders, border closings, rapidly escalating shipping costs, raw material availability and material logistical delays due to port congestion. Borders closings, lock-down orders and other restrictions in response to COVID-19, particularly regarding China, have impacted and continue to impact our access to products for our members. Staffing or personnel shortages due to shelter-in-place orders and quarantines, or other public health measures, have impacted and, in the future, may impact us and our members, other customers or suppliers. In addition, due to unprecedented demand during the COVID-19 pandemic, there have been widespread shortages in certain product categories. If the supply chain for materials used in the products purchased by our members through our GPO or products contract manufactured through our direct sourcing business continue to be adversely impacted by the COVID-19 pandemic, our supply chain may continue to be disrupted. Failure of our suppliers, contract manufacturers, distributors, contractors and other business partners to meet their obligations to our members, other customers or to us, or material disruptions in their ability to do so due to their own financial or operational difficulties, may adversely impact our operations.
•Requests for contract modifications, payment deferrals or exercises of force majeure clauses. We have and may continue to receive requests for contract modifications, payment waivers and deferrals, payment reductions or amended
57
payment terms from our contract counterparties. We have and may continue to receive requests to delay service or payment on performance service contracts. In addition, we have and may continue to receive requests from our suppliers for increases to their contracted prices, and such requests may be implemented in the future. Inflation in such contract prices may impact member utilization of items and services available through our GPO contracts, which could adversely impact our net administrative fees revenue and direct sourcing revenue. In addition, several pharmacy suppliers have exercised force majeure clauses related to failure to supply clauses in their contracts with us because they are unable to obtain raw materials for manufacturing from India and China. The standard failure to supply language in our contracts contains financial penalties to suppliers if they are unable to supply products, which such suppliers may not be able to pay. In addition, we may not be able to source products from alternative suppliers on commercially reasonable terms, or at all.
•Managing the evolving regulatory environment. In response to COVID-19 pandemic and variants thereof, federal, state and local governments are issuing new rules, regulations, changing reimbursement eligibility rules, orders and advisories on a regular basis. These government actions can impact us and our members, customers and suppliers.
The ultimate impact of COVID-19, variants thereof, recurrences, or similar pandemics on our business, results of operations, financial condition and cash flows is dependent on future developments, including the duration of any pandemic and the related length of its impact on the United States and global economies, which are uncertain and cannot be predicted at this time. The impact of the COVID-19 pandemic, variants thereof, recurrences, or future similar pandemics may also exacerbate many of the other risks described in this “Item 1A. Risk Factors” section. Despite our efforts to manage these impacts, their ultimate impact depends on factors beyond our knowledge or control, including the duration and severity of any outbreak and actions taken to contain its spread and mitigate its public health effects. The foregoing and other continued disruptions in our business as a result of the COVID-19 pandemic, variants thereof, recurrences or similar pandemics could result in a material adverse effect on our business, results of operations, financial condition, cash flows, prospects and the trading prices of our securities in the near-term and through fiscal 2022 and beyond.
Russia-Ukraine War
In February 2022, Russia invaded Ukraine. As military activity proceeds and sanctions, export controls and other measures are imposed against Russia, Belarus and specific areas of Ukraine, the war is increasingly affecting the global economy and financial markets, as well as exacerbating ongoing economic challenges, including rising inflation and global supply-chain disruption. We will continue to monitor the impacts of the Russia-Ukraine war on macroeconomic conditions and continually assess the effect these matters may have on member demand, our suppliers’ ability to deliver products, cybersecurity risks and our liquidity and access to capital. See “Risk Factors”.
Critical Accounting Policies and Estimates
Below is a discussion of our critical accounting policies and estimates. These and other significant accounting policies are set forth under Note 2 - Significant Accounting Policies to the accompanying audited consolidated financial statements for more information.
Business Combinations
We account for acquisitions of a business using the acquisition method. All the assets acquired, liabilities assumed, contractual contingencies and contingent consideration are generally recognized at their fair value on the acquisition date. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Acquisition-related costs are recorded as expenses in the Consolidated Statements of Income and Comprehensive Income.
Several valuation methods may be used to determine the fair value of assets acquired and liabilities assumed. For intangible assets, we typically use the income method. This method starts with a forecast of all of the expected future net cash flows for each asset. These cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the cash flow streams. Some of the more significant estimates and assumptions inherent in the income method or other methods include the amount and timing of projected future cash flows, the discount rate selected to measure the risks inherent in the future cash flows and the assessment of the asset's life cycle and the competitive trends impacting the asset, including consideration of any technical, legal, regulatory or economic barriers to entry. Determining the useful life of an intangible asset also requires judgment as different types of intangible assets will have different useful lives and certain assets may even be considered to have indefinite useful lives.
Goodwill
Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses. We perform our annual goodwill impairment testing on the first day of the last fiscal quarter of our fiscal year unless impairment indicators are present, which could require an interim impairment test.
58
Under accounting rules, we may elect to perform a qualitative assessment to determine if an impairment is more likely than not to have occurred. This qualitative assessment requires an evaluation of any excess of fair value over the carrying value for a reporting unit and significant judgment regarding potential changes in valuation inputs, including a review of our most recent long-range projections, analysis of operating results versus the prior year, changes in market values, changes in discount rates and changes in terminal growth rate assumptions. If it is determined that an impairment is more likely than not to exist, then we are required to perform a quantitative assessment to determine whether or not goodwill is impaired and to measure the amount of goodwill impairment, if any.
A goodwill impairment charge is recognized for the amount by which the reporting unit’s carrying amount exceeds its fair value. We determine the fair value of a reporting unit using a discounted cash flow analysis as well as market-based approaches. Determining fair value requires the exercise of significant judgment, including judgment about appropriate discount rates, perpetual growth rates and the amount and timing of expected future cash flows. The cash flows employed in the discounted cash flow analyses are based on the most recent budget and long-term forecast. The discount rates used in the discounted cash flow analyses are intended to reflect the risks inherent in the future cash flows of the respective reporting units. The market comparable approach estimates fair value using market multiples of various financial measures compared to a set of comparable public companies and recent comparable transactions.
Our most recent annual impairment testing as of April 1, 2022 consisted of a quantitative assessment and did not result in any goodwill impairment charges.
Revenue Recognition
We account for a contract with a customer when the contract is committed, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of collection.
Revenue is recognized when, or as, control of a promised product or service transfers to a customer, in an amount that reflects the consideration to which we expect to be entitled in exchange for transferring those products or services. If the consideration promised in a contract includes a variable amount, we estimate the amount to which we expect to be entitled using either the expected value or most likely amount method. Our contracts may include terms that could cause variability in the transaction price, including, for example, revenue share, rebates, discounts, and variable fees based on performance.
We only include estimated amounts of consideration in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. These estimates require management to make complex, difficult or subjective judgments, and to make estimates about the effect of matters inherently uncertain. As such, we may not be able to reliably estimate variable fees based on performance in certain long-term arrangements due to uncertainties that are not expected to be resolved for a long period of time or when our experience with similar types of contracts is limited. Estimates of variable consideration and the determination of whether to include estimated amounts of consideration in the transaction price are based on information (historical, current and forecasted) that is reasonably available to us, taking into consideration the type of customer, the type of transaction and the specific facts and circumstances of each arrangement. Additionally, management performs periodic analyses to verify the accuracy of estimates for variable consideration.
Although we believe that our approach in developing estimates and reliance on certain judgments and underlying inputs is reasonable, actual results could differ which may result in exposure of increases or decreases in revenue that could be material.
Performance Obligations
A performance obligation is a promise to transfer a distinct good or service to a customer. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. Contracts may have a single performance obligation as the promise to transfer individual goods or services is not separately identifiable from other promises, and therefore, not distinct, while other contracts may have multiple performance obligations, most commonly due to the contract covering multiple deliverable arrangements (licensing fees, subscription fees, professional fees for consulting services, etc.).
Net Administrative Fees Revenue
Net administrative fees revenue is a single performance obligation earned through a series of distinct daily services and includes maintaining a network of members to participate in the group purchasing program and providing suppliers efficiency in contracting and access to our members. Revenue is generated through administrative fees received from suppliers and is included in service revenue in the accompanying Consolidated Statements of Income and Comprehensive Income.
59
Through our GPO programs, we aggregate member purchasing power to negotiate pricing discounts and improve contract terms with suppliers. Contracted suppliers pay us administrative fees which generally represent 1% to 3% of the purchase price of goods and services sold to members under the contracts we have negotiated. Administrative fees are variable consideration and are recognized as earned based upon estimated purchases by our members utilizing analytics based on historical member spend and updates for current trends and expectations. Administrative fees are estimated due to the difference in timing of when a member purchases on a supplier contract and when we receive the purchasing information. Member and supplier contracts substantiate persuasive evidence of an arrangement. We do not take title to the underlying equipment or products purchased by members through our GPO supplier contracts. Administrative fee revenue receivable is included in contract assets in the accompanying Consolidated Balance Sheets.
Generally, we pay a revenue share to members equal to a percentage of gross administrative fees, which is estimated according to the members’ contractual agreements with us using a portfolio approach based on historical revenue fee share percentages and adjusted for current or anticipated trends. Revenue share is recognized as a reduction to gross administrative fees revenue to arrive at a net administrative fees revenue, and the corresponding revenue share liability is included in revenue share obligations in the accompanying Consolidated Balance Sheets.
Products Revenue
Direct sourcing generates revenue primarily through products sold to our members, other customers or distributors. Revenue is recognized once control of products has been transferred to the customer and is recorded net of discounts and rebates offered to customers. Discounts and rebates are estimated based on contractual terms and historical trends.
Software Licenses, Other Services and Support Revenue
We generate software licenses, other services and support revenue through Performance Services and Supply Chain Services.
Within Performance Services, which provides technology with wrap-around service offerings, revenue is generated through our three sub-brands: PINC AI, Contigo Health and Remitra. The main sources of revenue under PINC AI consists of SaaS-based clinical analytics products subscriptions, enterprise analytics licenses, professional fees for consulting services and other miscellaneous revenue including performance improvement collaboratives, insurance management service fees and commissions from insurance carriers for sponsored insurance programs. Contigo Health’s main sources of revenue are third-party administrator fees and fees from the centers of excellence program. Remitra’s main source of revenue is fees from healthcare product suppliers and service providers.
PINC AI
SaaS-based Products Subscriptions. SaaS-based clinical analytics subscriptions include the right to access our proprietary hosted technology on a SaaS basis, training and member support to deliver improvements in cost management, margin improvement, quality and safety, value-based care and provider analytics. SaaS arrangements create a single performance obligation for each subscription within the contract in which the nature of the obligation is a stand-ready obligation, and each day of service meets the criteria for over time recognition. Pricing varies by application and size of healthcare system. Clinical analytics products subscriptions are generally three- to five-year agreements with automatic renewal clauses and annual price escalators that typically do not allow for early termination. These agreements do not allow for physical possession of the software. Subscription fees are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation. Implementation involves the completion of data preparation services that are unique to each member’s data set and, in certain cases, the installation of member site-specific software, in order to access and transfer member data into our hosted SaaS-based clinical analytics products. Implementation is generally 60 to 240 days following contract execution before the SaaS-based clinical analytics products can be fully utilized by the member.
Software Licenses. Enterprise analytics licenses include term licenses that range from three to ten years and offer clinical analytics products, improvements in cost management, quality and safety, value-based care and provider analytics. Pricing varies by application and size of healthcare system. Revenue on licensing is recognized upon delivery of the license, and revenue from hosting and maintenance is recognized ratably over the life of the contract.
Consulting Services. Professional fees for consulting services are sold under contracts, the terms of which vary based on the nature of the engagement. These services typically include general consulting, report-based consulting and cost savings initiatives. Promised services under such consulting engagements are typically not considered distinct and are regularly combined and accounted for as one performance obligation. Fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed or when deliverables are provided. In situations where the contracts have significant contract performance guarantees, the performance guarantees are estimated
60
and accounted for as a form of variable consideration when determining the transaction price. In the event that guaranteed savings levels are not achieved, we may have to perform additional services at no additional charge in order to achieve the guaranteed savings or pay the difference between the savings that were guaranteed and the actual achieved savings. Occasionally, our entitlement to consideration is predicated on the occurrence of an event such as the delivery of a report for which client acceptance is required. However, except for event-driven point-in-time transactions, the majority of services provided within this service line are delivered over time due to the continuous benefit provided to our customers.
Consulting arrangements can require significant estimates for the transaction price and estimated number of hours within an engagement. These estimates are based on the expected value which is derived from outcomes from historical contracts that are similar in nature and forecasted amounts based on anticipated savings for the new agreements. The transaction price is generally constrained until the target transaction price becomes more certain.
Other Miscellaneous Revenue. Revenue from performance improvement collaboratives that support our offerings in cost management, quality and safety, and value-based care is recognized over the service period as the services are provided, which is generally one year. Performance improvement collaboratives revenue is considered one performance obligation and is generated by providing customers access to online communities whereby data is housed and available for analytics and benchmarking.
Insurance management service fees are recognized in the period in which such services are provided. Commissions from insurance carriers for sponsored insurance programs are earned by acting as an intermediary in the placement of effective insurance policies. Under this arrangement, revenue is recognized at a point in time on the effective date of the associated policies when control of the policy transfers to the customer and is constrained for estimated early terminations.
Contigo Health
Contigo Health revenue consists of third party administrator fees and fees from the centers of excellence program. Third party administrator fees consist of integrated fees for the processing of self-insured health care plan claims. Third party administrator fees are invoiced to customers monthly and typically collected in that period. Revenue is recognized in the period in which the services have been provided. Fees from the centers of excellence program consist of administrative fees for access to a specialized care network of proven healthcare providers. Centers of excellence fees are invoiced to customers a month in arrears and typically collected in that period. Revenue is recognized in the period in which the services have been provided.
Remitra
Revenue for Remitra primarily consists of fees from healthcare product suppliers and service providers. Fees for services are invoiced to our customers monthly and typically collected in the following period. For fixed fee contracts, revenue is recognized in the period in which the services have been provided. For variable rate contracts, revenue is recognized as customers are invoiced. Additional revenue consists of fees from check replacement services which consist of monthly rebates from bank partners.
Within Supply Chain Services, revenue is generated through supply chain co-management and SaaS-based purchased services activities.
Supply Chain Co-Management. Supply chain co-management activities generate revenue in the form of a service fee for services performed under the supply chain management contracts. Service fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed.
Purchased Services. Purchased services generate revenue through subscription fees for SaaS-based products. Subscription fees are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation.
Multiple Deliverable Arrangements
We enter into agreements where the individual deliverables discussed above, such as SaaS subscriptions and consulting services, are bundled into a single service arrangement. These agreements are generally provided over a time period ranging from approximately three months to five years after the applicable contract execution date. Revenue, including both fixed and variable consideration, is allocated to the individual performance obligations within the arrangement based on the stand-alone selling price when it is sold separately in a stand-alone arrangement.
61
Software Development Costs
Costs associated with internally developed computer software that are incurred in the preliminary project stage are expensed as incurred. During the development stage and once the project has reached technological feasibility, direct consulting costs and payroll and payroll-related costs for employees that are directly associated with each project are capitalized. Capitalized software costs are included in property and equipment, net in the accompanying Consolidated Balance Sheets. Capitalized costs are amortized on a straight-line basis over the estimated useful lives of the related software applications of up to five years and amortization is included in cost of revenue or selling, general and administrative expenses in the accompanying Consolidated Statements of Income and Comprehensive Income, based on the software’s end use. Replacements and major improvements are capitalized, while maintenance and repairs are expensed as incurred. Some of the more significant estimates and assumptions inherent in this process involve determining the stages of the software development project, the direct costs to capitalize and the estimated useful life of the capitalized software.
Income Taxes
We account for income taxes under the asset and liability approach. Deferred tax assets or liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities as measured by the enacted tax rates as well as net operating losses and credit carryforwards, which will be in effect when these differences reverse. We provide a valuation allowance against net deferred tax assets when, based upon the available evidence, it is more likely than not that the deferred tax assets will not be realized.
We prepare and file tax returns based on interpretations of tax laws and regulations. Our tax returns are subject to examination by various taxing authorities in the normal course of business. Such examinations may result in future tax, interest and penalty assessments by these taxing authorities.
In determining our tax expense for financial reporting purposes, we establish a reserve when there are transactions, calculations, and tax filing positions for which the tax determination is uncertain, and it is more likely than not that such positions would not be sustained upon examinations.
We adjust tax reserve estimates periodically based on the changes in facts and circumstances, such as ongoing examinations by, and settlements with, varying taxing authorities, as well as changes in tax laws, regulations and interpretations. The consolidated tax expense of any given year includes adjustments to prior year income tax reserve and related estimated interest charges that are considered appropriate. Our policy is to recognize, when applicable, interest and penalties on uncertain income tax positions as part of income tax expense.
New Accounting Standards
New accounting standards that we have recently adopted as well as those that have been recently issued but not yet adopted by us are included in Note 2 - Significant Accounting Policies to the accompanying audited consolidated financial statements, which is incorporated herein by reference.
Key Components of Our Results of Operations
Net Revenue
Net revenue consists of services and software licenses revenue, which includes net administrative fees revenue and software licenses, other services and support revenue, and products revenue.
Supply Chain Services
Supply Chain Services revenue is comprised of:
•net administrative fees revenue which consists of GPO net administrative fees (gross administrative fees received from suppliers, reduced by the amount of revenue share paid to members);
•software licenses, other services and support revenue which consist of supply chain co-management and purchased services revenue; and
•products revenue which consists of direct sourcing sales.
The success of our Supply Chain Services revenue streams are influenced by our ability to negotiate favorable contracts with suppliers and members, the number of members that utilize our GPO supplier contracts and the volume of their purchases, the impact of changes in the defined allowable reimbursement amounts determined by Medicare, Medicaid and other managed care plans and the number of members and other customers that purchase products through our direct sourcing activities and the impact of competitive pricing. Refer to “Impact of Inflation” within “Liquidity and Capital Resources” section of Item 7 -
62
Management’s Discussion and Analysis of Financial Condition and Results of Operations for discussion of inflation and its impact on our Supply Chain Services’ businesses.
Performance Services
Performance Services revenue is comprised of the following software licenses, other services and support revenue:
•health care information technology license and SaaS-based clinical, margin improvement and value-based care products subscriptions, license fees, professional fees for consulting services, performance improvement collaborative and other service subscriptions and insurance services management fees and commissions from endorsed commercial insurance programs under our PINC AI technology and services platform;
•third-party administrator fees and fees from the centers of excellence program for Contigo Health; and
•fees from healthcare product suppliers and service providers for Remitra.
Our Performance Services growth will depend upon the expansion of our PINC AI technology and services platform to new and existing members and other customers, expansion of our Contigo Health and Remitra businesses to new and existing members, renewal of existing subscriptions to our SaaS and licensed software products, our ability to sell enterprise analytics licenses to new and existing customers at rates sufficient to offset the loss of recurring SaaS-based revenue due to the conversion to an enterprise analytics license and expansion into new markets.
Cost of Revenue
Cost of revenue consists of cost of services and software licenses revenue and cost of products revenue.
Cost of services and software licenses revenue includes expenses related to employees, consisting of compensation and benefits, and outside consultants who directly provide services related to revenue-generating activities, including consulting services to members and other customers, third-party administrator services and implementation services related to our SaaS and licensed software products along with associated amortization of certain capitalized contract costs. Amortization of contract costs represent amounts that have been capitalized and reflect the incremental costs of obtaining and fulfilling a contract including costs related to implementing SaaS informatics tools. Cost of services and software licenses revenue also includes expenses related to hosting services, related data center capacity costs, third-party product license expenses and amortization of the cost of internally developed software applications.
Cost of products revenue consists of purchase and shipment costs for direct sourced medical and commodity products and is influenced by the manufacturing and transportation costs associated with direct sourced medical and commodity products. Refer to “Impact of Inflation” within “Liquidity and Capital Resources” section of Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations for discussion of inflation and its impact on our Supply Chain Services’ businesses.
Operating Expenses
Operating expenses includes selling, general and administrative expenses, research and development expenses and amortization of purchased intangible assets.
Selling, general and administrative expenses are directly associated with selling and administrative functions and support of revenue-generating activities including expenses to support and maintain our software-related products and services. Selling, general and administrative expenses primarily consist of compensation and benefits related costs, travel-related expenses, business development expenses, including costs for business acquisition opportunities, non-recurring strategic initiative and financial restructuring-related expenses, indirect costs such as insurance, professional fees and other general overhead expenses, and amortization of certain contract costs. Amortization of contract costs represent amounts, including sales commissions, that have been capitalized and reflect the incremental costs of obtaining and fulfilling a contract.
Research and development expenses consist of employee-related compensation and benefit expenses and third-party consulting fees of technology professionals, net of capitalized labor, incurred to develop our software-related products and services prior to reaching technological feasibility.
Amortization of purchased intangible assets includes the amortization of all identified intangible assets.
Other Income (Expense), Net
Other income (expense), net, includes equity in net income of unconsolidated affiliates that is generated from our equity method investments. Our equity method investments primarily consist of our interests in FFF Enterprises, Inc. (“FFF”), Exela Holdings, Inc. (“Exela”), and Prestige Ameritech Ltd. (“Prestige”) (see Note 5 - Investments). Other income (expense), net, also includes
63
the fiscal 2021 change in fair value of our FFF Put and Call Rights and the fiscal year 2022 gain recognized due to the termination of the FFF Put Right and derecognition of the associated liability (see Note 6 - Fair Value Measurements), interest income and expense, realized and unrealized gains or losses on deferred compensation plan assets, gains or losses on the disposal of assets, and any impairment on our assets or held-to-maturity investments.
Income Tax Expense (Benefit)
See Note 16 - Income Taxes for discussion of income tax expense.
Net Income Attributable to Non-Controlling Interest
We recognize net income attributable to non-controlling interest for non-Premier ownership in our consolidated subsidiaries which hold interest in our equity method investments. At June 30, 2022, we recognized net income attributable to non-controlling interest for the 74%, 79% and 85% interest held in PRAM Holdings, LLC (“PRAM”), DePre Holdings, LLC (“DePre”) and ExPre Holdings, LLC (“ExPre”), respectively, by member health systems or their affiliates. PRAM, DePre and ExPre are investments we made as part of our long-term supply chain resiliency program to promote domestic and geographically diverse manufacturing and to help ensure a robust and resilient supply chain for essential medical products.
As of June 30, 2022, we owned 93% of the equity interest in Contigo Health and recognized net income attributable to non-controlling interest for the 7% of equity held by certain customers of Contigo Health.
In addition to our non-controlling interest for non-Premier ownership in PRAM and DePre, for the year ended June 30, 2021, we recognized net income attributable to the limited partners of Premier LP through the date of the August 2020 Restructuring.
Our Use of Non-GAAP Financial Measures
The other key business metrics we consider are EBITDA, Adjusted EBITDA, Segment Adjusted EBITDA, Adjusted Net Income, Adjusted Earnings per Share and Free Cash Flow, which are all Non-GAAP financial measures.
We define EBITDA as net income before income or loss from discontinued operations, net of tax, interest and investment income or expense, net, income tax expense, depreciation and amortization and amortization of purchased intangible assets. We define Adjusted EBITDA as EBITDA before merger and acquisition-related expenses and non-recurring, non-cash or non-operating items and including equity in net income of unconsolidated affiliates. For all Non-GAAP financial measures, we consider non-recurring items to be income or expenses and other items that have not been earned or incurred within the prior two years and are not expected to recur within the next two years. Such items include certain strategic initiative and financial restructuring-related expenses. Non-operating items include gains or losses on the disposal of assets and interest and investment income or expense.
We define Segment Adjusted EBITDA as the segment’s net revenue less cost of revenue and operating expenses directly attributable to the segment excluding depreciation and amortization, amortization of purchased intangible assets, merger and acquisition-related expenses, and non-recurring or non-cash items, and including equity in net income of unconsolidated affiliates. Operating expenses directly attributable to the segment include expenses associated with sales and marketing, general and administrative, and product development activities specific to the operation of each segment. General and administrative corporate expenses that are not specific to a particular segment are not included in the calculation of Segment Adjusted EBITDA. Segment Adjusted EBITDA also excludes any income and expense that has been classified as discontinued operations.
We define Adjusted Net Income as net income attributable to Premier (i) excluding income or loss from discontinued operations, net, (ii) excluding income tax expense, (iii) excluding the impact of adjustment of redeemable limited partners’ capital to redemption amount, (iv) excluding the effect of non-recurring or non-cash items, including certain strategic initiative and financial restructuring-related expenses, (v) assuming, for periods prior to our August 2020 Restructuring, the exchange of all the Class B common units for shares of Class A common stock, which resulted in the elimination of non-controlling interest in Premier LP and (vi) reflecting an adjustment for income tax expense on Non-GAAP net income before income taxes at our estimated annual effective income tax rate, adjusted for unusual or infrequent items. We define Adjusted Earnings per Share as Adjusted Net Income divided by diluted weighted average shares (see Note 13 - Earnings Per Share).
We define Free Cash Flow as net cash provided by operating activities from continuing operations less distributions and TRA payments to limited partners for periods prior to our August 2020 Restructuring, early termination payments to certain former limited partners that elected to execute a Unit Exchange and Tax Receivable Acceleration Agreement (“Unit Exchange Agreement”) in connection with our August 2020 Restructuring and purchases of property and equipment. Free Cash Flow does not represent discretionary cash available for spending as it excludes certain contractual obligations such as debt repayments.
Adjusted EBITDA and Free Cash Flow are supplemental financial measures used by us and by external users of our financial statements and are considered to be indicators of the operational strength and performance of our business. Adjusted EBITDA
64
and Free Cash Flow measures allow us to assess our performance without regard to financing methods and capital structure and without the impact of other matters that we do not consider indicative of the operating performance of our business. More specifically, Segment Adjusted EBITDA is the primary earnings measure we use to evaluate the performance of our business segments.
We use Adjusted EBITDA, Segment Adjusted EBITDA, Adjusted Net Income and Adjusted Earnings per Share to facilitate a comparison of our operating performance on a consistent basis from period to period that, when viewed in combination with our results prepared in accordance with GAAP, provides a more complete understanding of factors and trends affecting our business. We believe Adjusted EBITDA and Segment Adjusted EBITDA assist our Board of Directors, management and investors in comparing our operating performance on a consistent basis from period to period because they remove the impact of earnings elements attributable to our asset base (primarily depreciation and amortization), certain items outside the control of our management team, e.g. taxes, other non-cash items (such as impairment of intangible assets, purchase accounting adjustments and stock-based compensation), non-recurring items (such as strategic initiative and financial restructuring-related expenses) and income and expense that has been classified as discontinued operations from our operating results. We believe Adjusted Net Income and Adjusted Earnings per Share assist our Board of Directors, management and investors in comparing our net income and earnings per share on a consistent basis from period to period because these measures remove non-cash (such as impairment of intangible assets, purchase accounting adjustments and stock-based compensation) and non-recurring items (such as strategic initiative and financial restructuring-related expenses), and eliminate the variability of non-controlling interest that primarily resulted from member owner exchanges of Class B common units for shares of Class A common stock. We believe Free Cash Flow is an important measure because it represents the cash that we generate after payment of tax distributions to limited partners prior to our August 2020 Restructuring, payments to certain former limited partners that elected to execute a Unit Exchange Agreement in connection with our August 2020 Restructuring and capital investment to maintain existing products and services and ongoing business operations, as well as development of new and upgraded products and services to support future growth. Our Free Cash Flow allows us to enhance stockholder value through acquisitions, partnerships, joint ventures, investments in related businesses and debt reduction.
Despite the importance of these Non-GAAP financial measures in analyzing our business, determining compliance with certain financial covenants in our Credit Facility, measuring and determining incentive compensation and evaluating our operating performance relative to our competitors, EBITDA, Adjusted EBITDA, Segment Adjusted EBITDA, Adjusted Net Income, Adjusted Earnings per Share and Free Cash Flow are not measurements of financial performance under GAAP, may have limitations as analytical tools and should not be considered in isolation from, or as an alternative to, net income, net cash provided by operating activities, or any other measure of our performance derived in accordance with GAAP.
Some of the limitations of the EBITDA, Adjusted EBITDA and Segment Adjusted EBITDA measures include that they do not reflect: our capital expenditures or our future requirements for capital expenditures or contractual commitments; changes in, or cash requirements for, our working capital needs; the interest expense or the cash requirements to service interest or principal payments under our Credit Facility; income tax payments we are required to make; and any cash requirements for replacements of assets being depreciated or amortized. In addition, EBITDA, Adjusted EBITDA, Segment Adjusted EBITDA and Free Cash Flow are not measures of liquidity under GAAP, or otherwise, and are not alternatives to cash flows from operating activities.
Some of the limitations of the Adjusted Net Income and Adjusted Earnings per Share measures are that they do not reflect income tax expense or income tax payments we are required to make. In addition, Adjusted Net Income and Adjusted Earnings per Share are not measures of profitability under GAAP.
We also urge you to review the reconciliation of these Non-GAAP financial measures included elsewhere in this Annual Report. To properly and prudently evaluate our business, we encourage you to review the consolidated financial statements and related notes included elsewhere in this Annual Report and to not rely on any single financial measure to evaluate our business. In addition, because the EBITDA, Adjusted EBITDA, Segment Adjusted EBITDA, Adjusted Net Income, Adjusted Earnings per Share and Free Cash Flow measures are susceptible to varying calculations, such Non-GAAP financial measures may differ from, and may therefore not be comparable to, similarly titled measures used by other companies.
Non-recurring and non-cash items excluded in our calculation of Adjusted EBITDA, Segment Adjusted EBITDA and Adjusted Net Income consist of stock-based compensation, acquisition- and disposition-related expenses, strategic initiative and financial restructuring-related expenses, gain or loss on FFF Put and Call Rights, income and expense that has been classified as discontinued operations and other reconciling items. More information about certain of the more significant items follows below.
Income tax expense on adjusted income
Adjusted Net Income, a Non-GAAP financial measure as defined below in “Our Use of Non-GAAP Financial Measures”, is calculated net of taxes based on our estimated annual effective tax rate for federal and state income tax, adjusted for unusual or
65
infrequent items, as we are a consolidated group for tax purposes with all of our subsidiaries’ activities included. Prior to the August 2020 Restructuring, Adjusted Net Income was calculated as if we were one consolidated group for tax purposes. The tax rate used to compute the Adjusted Net Income was 26% and 22% for the years ended June 30, 2022 and 2021, respectively. The 22% tax rate in fiscal year 2021 was primarily due to the benefit from the valuation allowance release as a result of the August 2020 Restructuring. In fiscal year 2022, the tax rate increased to 26% as a result of an increase in non-GAAP adjusted income before income taxes and a lesser benefit from the valuation allowance release as a result of the Subsidiary Reorganization as compared to fiscal 2021.
As a result of the Subsidiary Reorganization, one of our consolidated subsidiaries is expected to have sufficient income to utilize its net operating loss and research and development credit carryforwards. During the first quarter of fiscal 2022, we assessed the future realization of our deferred tax assets as a result of our plan to complete the Subsidiary Reorganization by the end of the second quarter of fiscal year 2022. On December 1, 2021, we completed the Subsidiary Reorganization. We reassessed the valuation allowance release as of June 30, 2022. In fiscal year 2022, we released $32.3 million of deferred tax valuation allowance primarily related to finite-lived net operating losses and research and development credit carryforwards. As a result of the Subsidiary Reorganization, we have offset ordinary income of $3.1 million during fiscal year 2022. The remaining $29.2 million of valuation allowance related to finite-lived net operating losses and research and development credit carryforwards is expected to be released and utilized in future periods.
Stock-based compensation
In addition to non-cash employee stock-based compensation expense, this item includes non-cash stock purchase plan expense of $0.6 million and $0.5 million for the years ended June 30, 2022 and 2021, respectively (see Note 14 - Stock-Based Compensation to the accompanying consolidated financial statements).
Acquisition- and disposition-related expenses
Acquisition-related expenses include legal, accounting and other expenses related to acquisition activities and gains and losses on the change in fair value of earn-out liabilities. Disposition-related expenses include severance and retention benefits and financial advisor fees and legal fees related to disposition activities.
Strategic initiative and financial restructuring-related expenses
Strategic initiative and financial restructuring-related expenses include legal, accounting and other expenses related to strategic initiative and financial restructuring-related activities.
Gain or loss on FFF Put and Call Rights
See Note 6 - Fair Value Measurements to the accompanying consolidated financial statements.
Impairment of assets
Impairment of assets relates to impairment of long-lived assets.
Other reconciling items
Other reconciling items includes, but is not limited to, gains and losses on disposals of long-lived assets and imputed interest on notes payable to former limited partners.
66
Results of Operations for the Years Ended June 30, 2022 and 2021
The following table presents our results of operations for the fiscal years presented (in thousands, except per share data):
| Year Ended June 30, | |||||||||||||||||||||||
| 2022 | 2021 | ||||||||||||||||||||||
| Amount | % of Net Revenue | Amount | % of Net Revenue | ||||||||||||||||||||
| Net revenue: | |||||||||||||||||||||||
| Net administrative fees | $ | 601,128 | 42 | % | $ | 572,700 | 34 | % | |||||||||||||||
| Software licenses, other services and support | 438,267 | 31 | % | 404,330 | 23 | % | |||||||||||||||||
| Services and software licenses | 1,039,395 | 73 | % | 977,030 | 57 | % | |||||||||||||||||
| Products | 393,506 | 27 | % | 744,122 | 43 | % | |||||||||||||||||
| Net revenue | 1,432,901 | 100 | % | 1,721,152 | 100 | % | |||||||||||||||||
| Cost of revenue: | |||||||||||||||||||||||
| Services and software licenses | 183,984 | 13 | % | 170,773 | 10 | % | |||||||||||||||||
| Products | 363,878 | 25 | % | 713,045 | 41 | % | |||||||||||||||||
| Cost of revenue | 547,862 | 38 | % | 883,818 | 51 | % | |||||||||||||||||
| Gross profit | 885,039 | 62 | % | 837,334 | 49 | % | |||||||||||||||||
| Operating expenses | 624,966 | 44 | % | 580,417 | 34 | % | |||||||||||||||||
| Operating income | 260,073 | 18 | % | 256,917 | 15 | % | |||||||||||||||||
| Other income (expense), net | 66,827 | 5 | % | (6,276) | — | % | |||||||||||||||||
| Income before income taxes | 326,900 | 23 | % | 250,641 | 15 | % | |||||||||||||||||
| Income tax expense (benefit) | 58,582 | 4 | % | (53,943) | (3) | % | |||||||||||||||||
| Net income | 268,318 | 19 | % | 304,584 | 18 | % | |||||||||||||||||
| Net income attributable to non-controlling interest | (2,451) | — | % | (17,062) | (1) | % | |||||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | nm | (26,685) | (2) | % | ||||||||||||||||||
| Net income attributable to stockholders | $ | 265,867 | 19 | % | $ | 260,837 | 15 | % | |||||||||||||||
| Earnings per share attributable to stockholders: | |||||||||||||||||||||||
| Basic | $ | 2.21 | $ | 2.24 | |||||||||||||||||||
| Diluted | $ | 2.19 | $ | 2.22 | |||||||||||||||||||
For the following Non-GAAP financial measures and reconciliations of our performance derived in accordance with GAAP to the Non-GAAP financial measures, refer to “Our Use of Non-GAAP Financial Measures” for further information regarding items excluded in our calculation of Adjusted EBITDA, Segment Adjusted EBITDA, Non-GAAP Adjusted Net Income and Non-GAAP Adjusted Earnings Per Share.
The following table provides certain Non-GAAP financial measures for the fiscal years presented (in thousands, except per share data).
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | ||||||||||||||||
| Certain Non-GAAP Financial Data: | Amount | % of Net Revenue | Amount | % of Net Revenue | |||||||||||||
| Adjusted EBITDA | $ | 498,682 | 35% | $ | 473,230 | 27% | |||||||||||
| Non-GAAP Adjusted Net Income | 302,738 | 21% | 305,974 | 18% | |||||||||||||
| Non-GAAP Adjusted Earnings Per Share | 2.49 | nm | 2.48 | nm | |||||||||||||
67
The following table provides the reconciliation of net income to Adjusted EBITDA and the reconciliation of income before income taxes to Segment Adjusted EBITDA (in thousands).
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net income | $ | 268,318 | $ | 304,584 | |||||||
| Interest expense, net | 11,142 | 11,964 | |||||||||
| Income tax expense (benefit) | 58,582 | (53,943) | |||||||||
| Depreciation and amortization | 85,171 | 76,309 | |||||||||
| Amortization of purchased intangible assets | 43,936 | 44,753 | |||||||||
| EBITDA | 467,149 | 383,667 | |||||||||
| Stock-based compensation | 46,809 | 35,915 | |||||||||
| Acquisition- and disposition-related expenses | 11,453 | 18,095 | |||||||||
| Strategic initiative and financial restructuring-related expenses | 18,005 | 6,990 | |||||||||
| (Gain) loss on FFF Put and Call Rights | (64,110) | 27,352 | |||||||||
| Impairment of assets | 18,829 | — | |||||||||
Other reconciling items, net (a) | 547 | 1,211 | |||||||||
| Adjusted EBITDA | $ | 498,682 | $ | 473,230 | |||||||
| Income before income taxes | $ | 326,900 | $ | 250,641 | |||||||
| Equity in net income of unconsolidated affiliates | (23,505) | (21,073) | |||||||||
| Interest expense, net | 11,142 | 11,964 | |||||||||
| (Gain) loss on FFF Put and Call Rights | (64,110) | 27,352 | |||||||||
| Other expense (income), net | 9,646 | (11,967) | |||||||||
| Operating income | 260,073 | 256,917 | |||||||||
| Depreciation and amortization | 85,171 | 76,309 | |||||||||
| Amortization of purchased intangible assets | 43,936 | 44,753 | |||||||||
| Stock-based compensation | 46,809 | 35,915 | |||||||||
| Acquisition- and disposition-related expenses | 11,453 | 18,095 | |||||||||
| Strategic initiative and financial restructuring-related expenses | 18,005 | 6,990 | |||||||||
| Equity in net income of unconsolidated affiliates | 23,505 | 21,073 | |||||||||
| Deferred compensation plan (expense) income | (9,401) | 12,745 | |||||||||
| Impairment of assets | 18,829 | — | |||||||||
| Other reconciling items, net | 302 | 433 | |||||||||
| Adjusted EBITDA | $ | 498,682 | $ | 473,230 | |||||||
| Segment Adjusted EBITDA: | |||||||||||
| Supply Chain Services | $ | 500,854 | $ | 467,868 | |||||||
| Performance Services | 126,938 | 132,225 | |||||||||
| Corporate | (129,110) | (126,863) | |||||||||
| Adjusted EBITDA | $ | 498,682 | $ | 473,230 | |||||||
_________________________________
(a)Other reconciling items, net is primarily attributable to loss on disposal of long-lived assets.
68
The following table provides the reconciliation of net income attributable to stockholders to Non-GAAP Adjusted Net Income and the reconciliation of the numerator and denominator for earnings per share attributable to stockholders to Non-GAAP Adjusted Earnings per Share for the years presented (in thousands).
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net income attributable to stockholders | $ | 265,867 | $ | 260,837 | |||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | 26,685 | |||||||||
| Net income attributable to non-controlling interest | 2,451 | 17,062 | |||||||||
| Income tax expense (benefit) | 58,582 | (53,943) | |||||||||
| Amortization of purchased intangible assets | 43,936 | 44,753 | |||||||||
| Stock-based compensation | 46,809 | 35,915 | |||||||||
| Acquisition- and disposition-related expenses | 11,453 | 18,095 | |||||||||
| Strategic initiative and financial restructuring-related expenses | 18,005 | 6,990 | |||||||||
| (Gain) loss on FFF Put and Call Rights | (64,110) | 27,352 | |||||||||
| Impairment of assets | 18,829 | — | |||||||||
Other reconciling items, net (a) | 7,284 | 8,529 | |||||||||
| Non-GAAP adjusted income before income taxes | 409,106 | 392,275 | |||||||||
Income tax expense on adjusted income before income taxes (b) | 106,368 | 86,301 | |||||||||
| Non-GAAP Adjusted Net Income | $ | 302,738 | $ | 305,974 | |||||||
| Reconciliation of denominator for earnings per share attributable to stockholders to Non-GAAP Adjusted Earnings per Share | |||||||||||
| Weighted average: | |||||||||||
| Basic weighted average shares outstanding | 120,220 | 116,527 | |||||||||
| Dilutive securities | 1,448 | 1,005 | |||||||||
| Weighted average shares outstanding - diluted | 121,668 | 117,532 | |||||||||
Class B shares outstanding (c) | — | 5,638 | |||||||||
| Non-GAAP weighted average shares outstanding - diluted | 121,668 | 123,170 | |||||||||
_________________________________
(a)Other reconciling items, net is primarily attributable to loss on disposal of long-lived assets and imputed interest on notes payable to former limited partners.
(b)Reflects income tax expense at an estimated effective income tax rate of 26% and 22% of non-GAAP adjusted net income before income taxes for the years ended June 30, 2022 and 2021, respectively.
(c)For the year ended June 30, 2021, the effect of 5.6 million Class B common shares were excluded from the GAAP diluted weighted average shares outstanding as they had an anti-dilutive effect. On a non-GAAP basis, the effect of 5.6 million Class B common shares were included in the non-GAAP diluted weighted average shares outstanding for the year ended June 30, 2021.
69
The following table provides the reconciliation of basic earnings per share attributable to stockholders to Non-GAAP Adjusted Earnings per Share for the periods presented.
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Basic earnings per share attributable to stockholders | $ | 2.21 | $ | 2.24 | |||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | 0.23 | |||||||||
| Net income attributable to non-controlling interest | 0.02 | 0.15 | |||||||||
| Income tax expense (benefit) | 0.49 | (0.46) | |||||||||
| Amortization of purchased intangible assets | 0.37 | 0.38 | |||||||||
| Stock-based compensation | 0.39 | 0.31 | |||||||||
| Acquisition- and disposition-related expenses | 0.10 | 0.16 | |||||||||
| Strategic initiative and financial restructuring-related expenses | 0.15 | 0.06 | |||||||||
| (Gain) loss on FFF Put and Call Rights | (0.53) | 0.23 | |||||||||
| Impairment of assets | 0.16 | — | |||||||||
Other reconciling items, net (a) | 0.06 | 0.07 | |||||||||
Impact of corporation taxes (b) | (0.88) | (0.74) | |||||||||
Impact of dilutive shares (c) | (0.05) | (0.15) | |||||||||
| Non-GAAP Adjusted Earnings Per Share | $ | 2.49 | $ | 2.48 | |||||||
_________________________________
(a)Other reconciling items, net is primarily attributable to loss on disposal of long-lived assets and imputed interest on notes payable to former limited partners.
(b)Reflects income tax expense at an estimated effective income tax rate of 26% and 22% of non-GAAP adjusted net income before income taxes for the years ended June 30, 2022 and 2021, respectively. The change in the estimated effective income tax is as a result of the Subsidiary Reorganization.
(c)Reflects impact of dilutive shares on a non-GAAP basis, primarily attributable to the assumed conversion of all Class B common units for the year ended June 30, 2021 for Class A common stock.
Consolidated Results - Comparison of the Years Ended June 30, 2022 to 2021
The variances in the material factors contributing to the changes in the consolidated results are discussed further in “Segment Results” below.
Net Revenue
Net revenue decreased by $288.3 million, or 17%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to a decrease of $350.6 million in products revenue. This decrease was partially offset by increases of $34.0 million in software licenses, other services and support revenue and $28.4 million in net administrative fees revenue.
Cost of Revenue
Cost of revenue decreased by $335.9 million, or 38%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to a decrease of $349.1 million in cost of products revenue partially offset by an increase of $13.2 million in cost of services and software licenses revenue.
Operating Expenses
Operating expenses increased by $44.6 million, or 8%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 due to increases of $44.6 million in selling, general and administrative expenses and $0.9 million in research and development expenses partially offset by an decrease of $0.9 million in amortization of intangible assets.
Other Income (Expense), Net
Other income (expense), net increased by $73.1 million during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to the current year gain on the FFF Put Right as a result of the termination and corresponding derecognition of the FFF Put Right liability on July 29, 2021 compared to the loss on the FFF put and call rights in the prior period (see Note 6 - Fair Value Measurements to the accompanying consolidated financial statements for further information). The increase was partially offset by a deferred compensation plan expense.
70
Income Tax Expense (Benefit)
We recorded an income tax expense of $58.6 million for the year ended June 30, 2022 compared to an income tax benefit of $53.9 million for the year ended June 30, 2021. The income tax expense and benefit resulted in effective tax rates of 18% and (22)% for the years ended June 30, 2022 and 2021, respectively. The change in the effective tax rate is primarily attributable to the prior year’s one-time deferred tax benefit associated with the remeasurement of the deferred tax asset and valuation allowance release as a result of the August 2020 Restructuring (see Note 16 - Income Taxes to the accompanying consolidated financial statements for further information).
Net Income Attributable to Non-Controlling Interest
Net income attributable to non-controlling interest decreased by $14.6 million during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to the August 2020 Restructuring, whereby net income attributable to non-controlling interest in Premier LP was not recorded after August 11, 2020.
Adjusted EBITDA
Adjusted EBITDA, a Non-GAAP financial measure as defined in “Our Use of Non-GAAP Financial Measures”, increased by $25.5 million, or 5%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 driven by an increase of $33.0 million in Supply Chain Services partially offset by decreases of $5.3 million and $2.2 million in Performance Services and Corporate Adjusted EBITDA, respectively.
71
Segment Results
Supply Chain Services
The following table presents our results of operations and Adjusted EBITDA, a Non-GAAP financial measure, in the Supply Chain Services segment for the fiscal years presented (in thousands):
| Year Ended June 30, | |||||||||||||||||||||||
| 2022 | 2021 | Change | |||||||||||||||||||||
| Net revenue: | |||||||||||||||||||||||
| Net administrative fees | $ | 601,128 | $ | 572,700 | $ | 28,428 | 5 | % | |||||||||||||||
| Software licenses, other services and support | 37,312 | 26,812 | 10,500 | 39 | % | ||||||||||||||||||
| Services and software licenses | 638,440 | 599,512 | 38,928 | 6 | % | ||||||||||||||||||
| Products | 393,506 | 744,122 | (350,616) | (47) | % | ||||||||||||||||||
| Net revenue | 1,031,946 | 1,343,634 | (311,688) | (23) | % | ||||||||||||||||||
| Cost of revenue: | |||||||||||||||||||||||
| Services and software licenses | 14,869 | 4,238 | 10,631 | 251 | % | ||||||||||||||||||
| Products | 363,878 | 713,045 | (349,167) | (49) | % | ||||||||||||||||||
| Cost of revenue | 378,747 | 717,283 | (338,536) | (47) | % | ||||||||||||||||||
| Gross profit | 653,199 | 626,351 | 26,848 | 4 | % | ||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Selling, general and administrative | 212,436 | 195,094 | 17,342 | 9 | % | ||||||||||||||||||
| Research and development | 397 | 164 | 233 | 142 | % | ||||||||||||||||||
| Amortization of intangibles | 32,428 | 32,342 | 86 | — | % | ||||||||||||||||||
| Operating expenses | 245,261 | 227,600 | 17,661 | 8 | % | ||||||||||||||||||
| Operating income | 407,938 | 398,751 | 9,187 | 2 | % | ||||||||||||||||||
| Depreciation and amortization | 22,996 | 4,731 | |||||||||||||||||||||
| Amortization of purchased intangible assets | 32,428 | 32,342 | |||||||||||||||||||||
| Acquisition- and disposition-related expenses | 1,915 | 10,938 | |||||||||||||||||||||
| Equity in net income of unconsolidated affiliates | 22,869 | 20,854 | |||||||||||||||||||||
| Impairment of assets | 12,695 | — | |||||||||||||||||||||
| Other reconciling items, net | 13 | 252 | |||||||||||||||||||||
| Segment Adjusted EBITDA | $ | 500,854 | $ | 467,868 | $ | 32,986 | 7 | % | |||||||||||||||
Net Revenue
Supply Chain Services segment revenue decreased by $311.7 million, or 23%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 driven by a decrease of $350.6 million in products revenue, which was partially offset by increases of $28.4 million and $10.5 million in net administrative fees and software licenses, other services and support revenue, respectively.
Net Administrative Fees Revenue
Net administrative fees revenue increased $28.4 million, or 5%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, driven by an increase in the demand for supplies and services, increased utilization of our contracts by our existing members, the addition of new categories and suppliers and the addition of new members to our contract portfolio. These increases in net administrative fees revenue were partially offset by an increase in revenue share paid to members and the departure of members from our contract portfolio.
Products Revenue
Products revenue decreased by $350.6 million, or 47%, during the year ended June 30, 2022 compared to the year ended June 30, 2021. The decrease was primarily driven by lower demand for and pricing of PPE and other high demand supplies as a result of the state of the COVID-19 pandemic, which was partially offset by growth in commodity products under our
72
PREMIERPRO® brand. As the COVID-19 pandemic continues to subside and become more manageable, we expect further stabilization of the market for some of these products and, accordingly, a decrease in period-over-period products revenue.
Software Licenses, Other Services and Support Revenue
Software licenses, other services and support revenue increased by $10.5 million, or 39%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to an increase in supply chain co-management fees and SaaS-based purchased services revenue.
Cost of Revenue
Supply Chain Services segment cost of revenue decreased by $338.5 million, or 47%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily attributable to the decrease in products revenue of $349.2 million due to the prior year increase in demand as well as fluctuations in product costs partially offset by escalating transportation costs due to continued global supply chain issues. In addition, cost of services and software licenses revenue increased by $10.6 million primarily due to an increase in depreciation and amortization expense as well as the aforementioned increase in software licenses, other services and support revenue. As the COVID-19 pandemic continues to subside and become more manageable, we expect further stabilization of the market for some of these products and, accordingly, a decrease in period-over-period cost of products revenue.
Operating Expenses
Operating expenses increased by $17.7 million, or 8%, during the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase was primarily due to an increase in selling, general and administrative expenses of $17.3 million driven by increases in depreciation and amortization expenses and personnel costs as well as the impairment of property and equipment (see Note 8 - Supplemental Balance Sheet Information) partially offset by a decrease in acquisition- and disposition-related expenses.
Segment Adjusted EBITDA
Segment Adjusted EBITDA in the Supply Chain Services segment increased by $33.0 million, or 7%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to the aforementioned increase in net administrative fees revenue and favorable product mix in our direct sourcing business.
73
Performance Services
The following table presents our results of operations and Adjusted EBITDA in the Performance Services segment for the fiscal years presented (in thousands):
| Year Ended June 30, | |||||||||||||||||||||||
| 2022 | 2021 | Change | |||||||||||||||||||||
| Net revenue: | |||||||||||||||||||||||
| Software licenses, other services and support | |||||||||||||||||||||||
| SaaS-based products subscriptions | $ | 193,586 | $ | 198,512 | $ | (4,926) | (2) | % | |||||||||||||||
| Consulting services | 64,087 | 58,851 | 5,236 | 9 | % | ||||||||||||||||||
| Software licenses | 65,621 | 56,157 | 9,464 | 17 | % | ||||||||||||||||||
| Other | 77,689 | 63,998 | 13,691 | 21 | % | ||||||||||||||||||
| Net revenue | 400,983 | 377,518 | 23,465 | 6 | % | ||||||||||||||||||
| Cost of revenue: | |||||||||||||||||||||||
| Services and software licenses | 169,116 | 166,535 | 2,581 | 2 | % | ||||||||||||||||||
| Cost of revenue | 169,116 | 166,535 | 2,581 | 2 | % | ||||||||||||||||||
| Gross profit | 231,867 | 210,983 | 20,884 | 10 | % | ||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Selling, general and administrative | 170,677 | 146,005 | 24,672 | 17 | % | ||||||||||||||||||
| Research and development | 3,754 | 3,174 | 580 | 18 | % | ||||||||||||||||||
| Amortization of intangibles | 11,508 | 12,411 | (903) | (7) | % | ||||||||||||||||||
| Operating expenses | 185,939 | 161,590 | 24,349 | 15 | % | ||||||||||||||||||
| Operating income | 45,928 | 49,393 | (3,465) | (7)% | |||||||||||||||||||
| Depreciation and amortization | 53,166 | 62,980 | |||||||||||||||||||||
| Amortization of purchased intangible assets | 11,508 | 12,411 | |||||||||||||||||||||
| Acquisition- and disposition-related expenses | 9,538 | 7,157 | |||||||||||||||||||||
| Equity in net income of unconsolidated affiliates | 636 | 219 | |||||||||||||||||||||
| Impairment of assets | 6,134 | — | |||||||||||||||||||||
| Other reconciling items, net | 28 | 65 | |||||||||||||||||||||
| Segment Adjusted EBITDA | $ | 126,938 | $ | 132,225 | $ | (5,287) | (4) | % | |||||||||||||||
Net Revenue
Net revenue in our Performance Services segment increased by $23.5 million, or 6%, during the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase was primarily driven by growth of $9.5 million and $5.2 million in software licenses and consulting services revenue, respectively, under our PINC AI platform as well as growth of $13.7 million in other net revenue which includes the growth in Contigo Health and incremental revenue and growth from our Remitra business. These increases in net revenue were partially offset by a decrease in SaaS-based products subscriptions revenue due to the conversion of SaaS-based products to licensed-based products.
Cost of Revenue
Cost of services and software licenses revenue in our Performance Services segment increased by $2.6 million, or 2%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to an increase in personnel costs related to growth in our Contigo Health business and incremental expenses associated with our Remitra business.
Operating Expenses
Performance Services segment operating expenses increased by $24.3 million, or 15%, during the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase was primarily due to an increase in selling, general and administrative expenses of $24.7 million driven by increases in personnel costs and professional fees associated with a decrease in capitalized labor costs and intangible asset impairment (see Note 9 - Goodwill and Intangible Assets) as well as increase in acquisition- and disposition-related expenses. These increases were partially offset by a decrease in depreciation and amortization expense.
74
Segment Adjusted EBITDA
Segment Adjusted EBITDA in the Performance Services segment decreased by $5.3 million, or 4%, during the year ended June 30, 2022 compared to the year ended June 30, 2021, primarily due to the aforementioned increases in cost of revenue and operating expenses partially offset by the aforementioned increase in net revenue.
Corporate
The following table summarizes corporate expenses and Adjusted EBITDA for the fiscal years presented (in thousands):
| Year Ended June 30, | |||||||||||||||||||||||
| 2022 | 2021 | Change | |||||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Selling, general and administrative | $ | 193,794 | $ | 191,227 | $ | 2,567 | 1 | % | |||||||||||||||
| Operating expenses | 193,794 | 191,227 | 2,567 | 1 | % | ||||||||||||||||||
| Operating loss | (193,794) | (191,227) | (2,567) | 1 | % | ||||||||||||||||||
| Depreciation and amortization | 9,009 | 8,598 | |||||||||||||||||||||
| Stock-based compensation | 46,809 | 35,915 | |||||||||||||||||||||
| Strategic initiative and financial restructuring-related expenses | 18,005 | 6,990 | |||||||||||||||||||||
| Deferred compensation plan (expense) income | (9,401) | 12,745 | |||||||||||||||||||||
| Other reconciling items, net | 262 | 116 | |||||||||||||||||||||
| Adjusted EBITDA | $ | (129,110) | $ | (126,863) | $ | (2,247) | 2 | % | |||||||||||||||
Operating Expenses
Corporate operating expenses increased by $2.6 million, or 1%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to increases in stock-based compensation expense as a result of higher achievement of performance share awards, professional fees related to strategic initiative and financial restructuring-related activities and employee-related expenses, including employee travel and meeting expenses as travel and meeting limitations due to the COVID-19 pandemic began to subside. These increases were partially offset by deferred compensation plan expense in the current year compared to deferred compensation income in the prior year due to market changes.
Adjusted EBITDA
Adjusted EBITDA decreased by $2.2 million, or 2%, during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to an increase in employee-related expenses, including employee travel and meeting expenses.
75
Results of Operations for the Years Ended June 30, 2021 and 2020
A discussion of changes in our results of operations from fiscal year 2020 to fiscal year 2021 has been omitted from this Annual Report but may be found in “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” of our Form 10-K for the fiscal year ended June 30, 2021, filed with the SEC on August 17, 2021, which is available free of charge on the SECs website at www.sec.gov and our website at http://investors.premierinc.com.
Off-Balance Sheet Arrangements
As of June 30, 2022, we did not have any off-balance sheet arrangements.
Liquidity and Capital Resources
Our principal source of cash has been primarily cash provided by operating activities. From time to time we have used, and expect to use in the future, borrowings under our Credit Facility (as defined in Note 10 - Debt and Notes Payable to the accompanying consolidated financial statements for more information) as a source of liquidity. Our primary cash requirements include operating expenses, working capital fluctuations, revenue share obligations, tax payments, capital expenditures, dividend payments on our Class A common stock, if and when declared, repurchases of Class A common stock pursuant to stock repurchase programs in place from time to time, acquisitions and related business investments, and general corporate activities. Our capital expenditures typically consist of internally developed software costs, software purchases and computer hardware purchases.
As of June 30, 2022 and 2021, we had cash and cash equivalents of $86.1 million and $129.1 million, respectively. As of June 30, 2022 and 2021, there was $150.0 million and $75.0 million, respectively, of outstanding borrowings under our Credit Facility. During the year ended June 30, 2022, we borrowed $325.0 million and repaid $250.0 million of borrowings under the Credit Facility, which were used to partially fund the $250.0 million share repurchase program and other general corporate purposes.
We expect cash generated from operations and borrowings under our Credit Facility to provide us with adequate liquidity to fund our anticipated working capital requirements, revenue share obligations, tax payments, capital expenditures, dividend payments on our Class A common stock, if and when declared, and repurchases of Class A common stock pursuant to stock repurchase programs in place from time to time. Our capital requirements depend on numerous factors, including funding requirements for our product and service development and commercialization efforts, our information technology requirements and the amount of cash generated by our operations. We believe that we have adequate capital resources at our disposal to fund currently anticipated capital expenditures, business growth and expansion, and current and projected debt service requirements. However, strategic growth initiatives will likely require the use of one or a combination of various forms of capital resources including available cash on hand, cash generated from operations, borrowings under our Credit Facility and other long-term debt and, potentially, proceeds from the issuance of additional equity or debt securities.
On August 4, 2022, our Board of Directors declared a cash dividend of $0.21 per share, payable on September 15, 2022 to stockholders of record on September 1, 2022.
Discussion of Cash Flows for the Years Ended June 30, 2022 and 2021
A summary of net cash flows follows (in thousands):
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net cash provided by (used in): | |||||||||||
| Operating activities | $ | 444,234 | $ | 407,402 | |||||||
| Investing activities | (139,440) | (174,568) | |||||||||
| Financing activities | (347,789) | (202,997) | |||||||||
| Effect of exchange rate changes on cash flows | (3) | — | |||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (42,998) | $ | 29,837 | |||||||
Net cash provided by operating activities increased by $36.8 million for the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase in cash provided by operating activities was primarily due to the net increase in cash from our direct sourcing business of $132.3 million driven by a higher cash inflows from the collection of accounts receivable and reduction in inventory purchases from fiscal year 2021. The increase in cash was partially offset by an increase of $75.8 million in payments of operating expenses and $20.0 million in miscellaneous payments including taxes and interest.
76
Net cash used in investing activities decreased by $35.1 million for the year ended June 30, 2022 compared to the year ended June 30, 2021. The decrease in cash used in investing activities was primarily due to higher cash outlay in the prior year for the fiscal 2021 acquisition of IDS compared to cash paid in the current year for investments in Exela and Qventus, Inc. The decrease was partially offset by a net increase in purchases of property and equipment and other investing activities.
Net cash used in financing activities increased by $144.8 million for the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase in net cash used in financing activities was primarily driven by $250.0 million for repurchases of Class A common stock under the fiscal 2022 stock repurchase program and an increase of $48.5 million in payments made on notes payable driven by an increase in early termination payments to certain former limited partners that elected to execute a Unit Exchange Agreement in connection with the August 2020 Restructuring as quarterly payments commenced during the quarter ended March 31, 2021. The increase in net cash used in financing activities was partially offset by an increase of $75.0 million in net proceeds under our Credit Facility, an increase of $28.4 million in proceeds from the issuance of Class A common stock in connection with the exercise of outstanding stock options, a reduction of $34.2 million in distributions to limited partners of Premier LP and payments to limited partners of Premier LP related to tax receivable agreements as both distributions and payments were eliminated in connection with the August 2020 Restructuring and an increase of $19.2 million in other financing activities. The increase in other financing activities is primarily driven by proceeds from member health systems that acquired membership interests in ExPre.
Discussion of Non-GAAP Free Cash Flow for the Years Ended June 30, 2022 and 2021
We define Non-GAAP Free Cash Flow as net cash provided by operating activities from continuing operations less distributions and TRA payments to limited partners for periods prior to our August 2020 Restructuring, early termination payments to certain former limited partners that elected to execute a Unit Exchange Agreement in connection with our August 2020 Restructuring and purchases of property and equipment. Non-GAAP Free Cash Flow does not represent discretionary cash available for spending as it excludes certain contractual obligations such as debt repayments under our Credit Facility. A summary of Non-GAAP Free Cash Flow and reconciliation to net cash provided by operating activities for the periods presented follows (in thousands):
| Year Ended June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Net cash provided by operating activities | $ | 444,234 | $ | 407,402 | |||||||
| Purchases of property and equipment | (87,440) | (88,876) | |||||||||
Early termination payments to certain former limited partners that elected to execute a Unit Exchange Agreement (a) | (95,948) | (44,024) | |||||||||
| Distributions to limited partners of Premier LP | — | (9,949) | |||||||||
| Payments to limited partners of Premier LP related to tax receivable agreements | — | (24,218) | |||||||||
| Non-GAAP Free Cash Flow | $ | 260,846 | $ | 240,335 | |||||||
_________________________________
(a) Early termination payments to certain former limited partners that elected to execute a Unit Exchange Agreement in connection with our August 2020 Restructuring are presented in our Consolidated Statements of Cash Flows under “Payments made on notes payable”. During the year ended June 30, 2022, we paid $102.7 million to members including imputed interest of $6.7 million which is included in net cash provided by operating activities. During the year ended June 30, 2021, we paid $51.3 million to members including imputed interest of $7.3 million which is included in net cash provided by operating activities. See Note 10 - Debt and Notes Payable to the accompanying audited consolidated financial statements for further information.
Non-GAAP Free Cash Flow increased by $20.5 million for the year ended June 30, 2022 compared to the year ended June 30, 2021. The increase in Non-GAAP Free Cash Flow was driven by the aforementioned increase of $36.8 million in net cash provided by operating activities and no distributions to limited partners of Premier LP or payments to limited partners of Premier LP related to tax receivable agreements during the year ended June 30, 2022 as both were eliminated in connection with the August 2020 Restructuring. These increases in Non-GAAP Free Cash Flow were partially offset by an increase of $51.9 million in early termination payments to certain former limited partners in connection with the August 2020 Restructuring.
See “Our Use of Non-GAAP Financial Measures” above for additional information regarding our use of Non-GAAP Free Cash Flow.
77
Contractual Obligations
The following table presents our contractual obligations as of June 30, 2022 (in thousands):
| Payments Due by Period | |||||||||||||||||
| Contractual Obligations | Total | Less Than 1 Year | 1-3 Years | 3-5 Years | Greater Than 5 Years | ||||||||||||
Notes payable to former limited partners (a) | $ | 308,055 | $ | 102,685 | $ | 205,370 | $ | — | $ | — | |||||||
Other notes payable (b) | 5,333 | 3,053 | 2,280 | — | — | ||||||||||||
Operating lease obligations (c) | 47,027 | 12,131 | 24,568 | 10,328 | — | ||||||||||||
Deferred consideration (d) | 60,000 | 30,000 | 30,000 | — | — | ||||||||||||
| Total contractual obligations | $ | 420,415 | $ | 147,869 | $ | 262,218 | $ | 10,328 | $ | — | |||||||
_________________________________
(a)Notes payable to former limited partners represent the amount of the expected payment to be made to each former limited partner pursuant to the early termination provisions of the TRA (each such amount an “Early Termination Payment”). See Note 10 - Debt and Notes Payable to the accompanying consolidated financial statements for more information.
(b)Other notes payable are non-interest bearing and generally have stated maturities of three to five years from the date of issuance. See Note 10 - Debt and Notes Payable to the accompanying consolidated financial statements for more information.
(c)Future contractual obligations for leases represent future minimum payments under noncancelable operating leases primarily for office space. See Note 18 - Commitments and Contingencies to the accompanying consolidated financial statements for more information.
(d)Deferred consideration to be paid pursuant to the purchase agreement for the acquisition of substantially all of the assets and certain liabilities of Acurity, Inc. and Nexera, Inc. in fiscal year 2020.
Credit Facility
Outstanding borrowings under the Credit Facility (as defined in Note 10 - Debt and Notes Payable to the accompanying consolidated financial statements for more information) bear interest on a variable rate structure with borrowings bearing interest at either the London Interbank Offered Rate (“LIBOR”) plus an applicable margin ranging from 1.000% to 1.500% or the prime lending rate plus an applicable margin ranging from 0.000% to 0.500%. We pay a commitment fee ranging from 0.100% to 0.200% for unused capacity under the Credit Facility. At June 30, 2022, the interest rate on outstanding borrowings under the Credit Facility was 2.178% and the commitment fee was 0.100%.
The Credit Facility contains customary representations and warranties as well as customary affirmative and negative covenants. We were in compliance with all such covenants at June 30, 2022. The Credit Facility also contains customary events of default, including a cross-default of any indebtedness or guarantees in excess of $75.0 million. If any event of default occurs and is continuing, the administrative agent under the Credit Facility may, with the consent, or shall, at the request of a majority of the lenders under the Credit Facility, terminate the commitments and declare all of the amounts owed under the Credit Facility to be immediately due and payable.
Proceeds from borrowings under the Credit Facility may generally be used to finance ongoing working capital requirements, including permitted acquisitions, repurchases of Class A common stock pursuant to stock repurchase programs, in place from time to time, dividend payments, if and when declared, and other general corporate activities. At June 30, 2022, we had outstanding borrowings of $150.0 million under the Credit Facility with $849.9 million of available borrowing capacity after reductions for outstanding borrowings and outstanding letters of credit.
The above summary does not purport to be complete, and is subject to, and qualified in its entirety by reference to, the complete text of the Credit Facility, as amended, which is filed as Exhibit 10.1 in our quarterly report for the period ended December 31, 2021. See also Note 10 - Debt and Notes Payable to the accompanying condensed consolidated financial statements.
Cash Dividends
In each of September 15, 2021, December 15, 2021, March 15, 2022 and June 15, 2022, we paid a cash dividend of $0.20 per share on outstanding shares of Class A common stock. On August 4, 2022, our Board of Directors declared a cash dividend of $0.21 per share, payable on September 15, 2022 to stockholders of record on September 1, 2022.
We currently expect quarterly dividends to continue to be paid on or about December 15, March 15, June 15 and September 15, respectively. However, the actual declaration of any future cash dividends, and the setting of record and payment dates as well as the per share amounts, will be at the discretion of our Board of Directors each quarter after consideration of various factors, including our results of operations, financial condition and capital requirements, earnings, general business conditions, restrictions imposed by our current credit facility and any future financing arrangements, legal restrictions on the payment of dividends and other factors our Board of Directors deems relevant.
78
Stock Repurchase Program
On August 5, 2021, our Board of Directors authorized the repurchase of up to $250.0 million of our outstanding Class A common stock during fiscal year 2022 through open market purchases or privately negotiated transactions. At June 30, 2022, we had completed our stock purchase program and purchased approximately 6.4 million shares of Class A common stock at an average price of $38.88 per share for a total purchase price of $250.0 million.
Fiscal 2022 Developments
In fiscal year 2022, the U.S. and global economies experienced unprecedented challenges resulting from the ongoing consequences of the COVID-19 pandemic, including supply chain bottlenecks and escalating inflation. These challenges were exacerbated by the Russia-Ukraine war which has led to further supply chain disruptions and rising energy costs and led to further inflationary impacts. These challenges have impacted our business as discussed below.
COVID-19 Pandemic, Variants Thereof, Recurrences or Similar Pandemics
The COVID-19 global pandemic and its variants continue to create challenges throughout the United States and the rest of the world. The full extent to which the COVID-19 pandemic may impact our business, operating results, financial condition and liquidity in the future will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning COVID-19 and variants thereof, the continued actions to contain it or treat its impact, including the success of COVID-19 vaccination programs, or recurrences of COVID-19, variants thereof or similar pandemics. As discussed in detail under “Item 1A. Risk Factors”, as a result of the COVID-19 pandemic and potential future pandemic outbreaks, we face material risks including but not limited to the following:
•The impact of the COVID-19 pandemic and any variants thereof and associated supply chain disruptions and inflation could result in a prolonged recession or depression in the United States or globally that could harm the banking system, limit or delay demand for many products and services and cause other foreseen and unforeseen events and circumstances, all of which could negatively impact us.
•We experienced and may continue to experience demand uncertainty from both material increases and decreases in demand and pricing for personal protective equipment (“PPE”), drugs and other supplies directly related to treating and preventing the spread of COVID-19 and any variants thereof as well as a decline in demand and pricing for many supplies and services not related to COVID-19.
•Labor shortages and the resulting increases to cost of labor are a continued challenge to the healthcare providers we serve and could negatively affect our business.
•While some of our hospital customers have increased access to their facilities for non-patients, including our field teams, consultants and other professionals, there are many that are still not allowing onsite access outside of their staff. Hospital imposed travel restrictions are also impacting some customers’ ability to participate in face-to-face events with us, such as committee meetings and conferences.
•The global supply chain has been materially disrupted due to personnel shortages associated with ongoing COVID-19 rates of infection, stay-at-home orders, border closings, rapidly escalating shipping costs, raw material availability and material logistical delays due to port congestion.
•We have and may continue to receive requests for contract modifications, payment waivers and deferrals, payment reductions or amended payment terms from our contract counterparties. Inflation in such contract prices may impact member utilization of items and services available through our GPO contracts, with uncertain impact on our net administrative fees revenue and direct sourcing revenue. In addition, several pharmacy suppliers have exercised force majeure clauses related to failure to supply clauses in their contracts with us.
•In response to COVID-19 and variants thereof, federal, state and local governments are issuing new rules, regulations, changing reimbursement eligibility rules, orders and advisories on a regular basis. These government actions can impact us, our members, other customers and suppliers.
Russia-Ukraine War
In February 2022, Russia invaded Ukraine. As military activity continues and sanctions, export controls and other measures are imposed against Russia, Belarus and specific areas of Ukraine, the war is increasingly affecting the global economy and financial markets, as well as exacerbating ongoing economic challenges, including issues such as rising inflation and energy costs and global supply-chain disruption. We continue to monitor the impacts of the Russia-Ukraine war on macroeconomic conditions and prepare for any implications that the war may have on member demand, our suppliers’ ability to deliver products, cybersecurity risks and our liquidity and access to capital. See “Risk Factors — Risks Related to Our Business Operations”.
79
Impact of Inflation
The U.S. economy is experiencing the highest rates of inflation since the 1980s. Historically, we have not experienced significant inflation risk in our business arising from fluctuations in market prices across our diverse product portfolio. However, our ability to raise our selling prices depends on market conditions and there may be periods during which we are unable to fully recover increases in our costs. In fiscal year 2022, our GPO business was largely unaffected by pricing inflation as we used our members’ aggregated purchasing power to negotiate firm prices in many of our contracts. In our Direct Sourcing business, we were able to partially offset increases in cost through temporary adjustments to selling prices and through various cost reduction initiatives while ensuring our products remain competitively priced. See “Risk Factors — Risks Related to Our Business Operations”.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
Our exposure to market risk related primarily to the increase or decrease in the amount of any interest expense we must pay with respect to outstanding debt instruments. At June 30, 2022, we had $150.0 million outstanding borrowings under our Credit Facility. At June 30, 2022, a one-percent increase or decrease in the interest rate charged on outstanding borrowings under the Credit Facility would increase or decrease interest expense over the next 12 months by $1.5 million.
We invest our excess cash in a portfolio of individual cash equivalents. We do not hold any material derivative financial instruments. We do not expect changes in interest rates to have a material impact on our results of operations or financial position. We plan to mitigate default, market and investment risks of our invested funds by investing in low-risk securities.
Foreign Currency Risk.
Substantially all of our financial transactions are conducted in U.S. dollars. We do not have significant foreign operations and, accordingly, do not believe we have market risk associated with foreign currencies.
80
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements and related notes are filed together with this Annual Report. See the index to financial statements under Item 15(a) for a list of financial statements filed with this report, and under this item.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
81
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors of Premier, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Premier, Inc. (the Company) as of June 30, 2022 and 2021, the related consolidated statements of income and comprehensive income, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended June 30, 2022, and the related notes and financial statement schedule listed in the Index at Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at June 30, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of June 30, 2022, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated August 16, 2022 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the account or disclosures to which it relates.
82
| Valuation of Goodwill | |||||
Description of the Matter | At June 30, 2022, the Company’s goodwill was $999.9 million. As discussed in Note 2 to the consolidated financial statements, goodwill is tested for impairment annually at the reporting unit level on the first day of the last fiscal quarter of the fiscal year unless impairment indicators are present which could require an interim impairment test. The Company’s goodwill is initially assigned to its reporting units as of the acquisition date. Auditing management’s annual goodwill impairment test was complex and highly judgmental due to the estimation required to determine the fair value of the reporting units. Fair value is estimated by management based on an income approach using a discounted cash flow model as well as market-based approaches. In particular, the fair value estimates are sensitive to changes in significant assumptions, such as the amount and timing of expected future cash flows, perpetual growth rates, and discount rates, which are affected by expected future market or economic conditions. | ||||
How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s goodwill impairment testing process. For example, we tested controls over management’s review of the significant inputs and assumptions discussed above used in determining the reporting unit fair values. To test the estimated fair value of the Company’s reporting units, our audit procedures included, among others, assessing the methodologies used and testing the significant assumptions discussed above, including the completeness and accuracy of the underlying data used by the Company. For example, we compared the significant assumptions used by management to current industry and economic trends, historical financial results and other relevant factors. We performed sensitivity analyses of significant assumptions to evaluate the change in the fair value of the reporting units resulting from changes in the inputs and assumptions. We assessed the historical accuracy of management’s projections and involved our valuation specialists to assist in our evaluation of the significant assumptions. We also evaluated the reconciliation of the estimated aggregate fair value of the reporting units to the market capitalization of the Company. | ||||
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1991.
Raleigh, North Carolina
August 16, 2022
83
Report of Independent Registered Public Accounting Firm
The Stockholders and Board of Directors of Premier, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Premier, Inc.’s internal control over financial reporting as of June 30, 2022, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Premier, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of June 30, 2022, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the June 30, 2022 consolidated financial statements of the Company and our report dated August 16, 2022 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Raleigh, North Carolina
August 16, 2022
84
| PREMIER, INC. | |||||||||||
Consolidated Balance Sheets | |||||||||||
| (In thousands, except per share data) | |||||||||||
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Assets | |||||||||||
| Cash and cash equivalents | $ | 86,143 | $ | 129,141 | |||||||
Accounts receivable (net of $2,043 and $2,284 allowance for credit losses, respectively) | 114,129 | 141,447 | |||||||||
Contract assets (net of $755 and $0 allowance for credit losses, respectively) | 260,061 | 267,283 | |||||||||
| Inventory | 119,652 | 176,376 | |||||||||
| Prepaid expenses and other current assets | 65,581 | 68,049 | |||||||||
| Total current assets | 645,566 | 782,296 | |||||||||
Property and equipment (net of $578,644 and $518,332 accumulated depreciation, respectively) | 213,379 | 224,271 | |||||||||
Intangible assets (net of $217,582 and $289,912 accumulated amortization, respectively) | 356,572 | 396,642 | |||||||||
| Goodwill | 999,913 | 999,913 | |||||||||
| Deferred income tax assets | 725,032 | 781,824 | |||||||||
| Deferred compensation plan assets | 47,436 | 59,581 | |||||||||
| Investments in unconsolidated affiliates | 215,545 | 153,224 | |||||||||
| Operating lease right-of-use assets | 39,530 | 48,199 | |||||||||
| Other assets | 114,154 | 76,948 | |||||||||
| Total assets | $ | 3,357,127 | $ | 3,522,898 | |||||||
| Liabilities and stockholders' equity | |||||||||||
| Accounts payable | $ | 44,631 | $ | 85,413 | |||||||
| Accrued expenses | 40,968 | 48,144 | |||||||||
| Revenue share obligations | 245,395 | 226,883 | |||||||||
| Accrued compensation and benefits | 93,638 | 100,713 | |||||||||
| Deferred revenue | 30,463 | 34,058 | |||||||||
| Current portion of notes payable to former limited partners | 97,806 | 95,948 | |||||||||
| Line of credit and current portion of long-term debt | 153,053 | 78,295 | |||||||||
| Other current liabilities | 47,183 | 47,330 | |||||||||
| Total current liabilities | 753,137 | 716,784 | |||||||||
| Long-term debt, less current portion | 2,280 | 5,333 | |||||||||
| Notes payable to former limited partners, less current portion | 201,188 | 298,995 | |||||||||
| Deferred compensation plan obligations | 47,436 | 59,581 | |||||||||
| Deferred consideration, less current portion | 28,702 | 56,809 | |||||||||
| Operating lease liabilities, less current portion | 32,960 | 43,102 | |||||||||
| Other liabilities | 42,574 | 112,401 | |||||||||
| Total liabilities | 1,108,277 | 1,293,005 | |||||||||
| Commitments and contingencies (Note 18) | |||||||||||
| Stockholders' equity: | |||||||||||
Class A common stock, $0.01 par value, 500,000,000 shares authorized; 124,481,610 shares issued and 118,052,235 outstanding at June 30, 2022 and 122,533,051 shares issued and outstanding at June 30, 2021 | 1,245 | 1,225 | |||||||||
Treasury stock, at cost; 6,429,375 and 0 shares at June 30, 2022 and 2021, respectively | (250,129) | — | |||||||||
| Additional paid-in capital | 2,166,047 | 2,059,194 | |||||||||
| Retained earnings | 331,690 | 169,474 | |||||||||
| Accumulated other comprehensive income | (3) | — | |||||||||
| Total stockholders' equity | 2,248,850 | 2,229,893 | |||||||||
| Total liabilities and stockholders' equity | $ | 3,357,127 | $ | 3,522,898 | |||||||
See accompanying notes to the consolidated financial statements.
85
PREMIER, INC. | |||||||||||||||||
Consolidated Statements of Income and Comprehensive Income | |||||||||||||||||
(In thousands, except share data) | |||||||||||||||||
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Net revenue: | |||||||||||||||||
| Net administrative fees | $ | 601,128 | $ | 572,700 | $ | 670,593 | |||||||||||
| Software licenses, other services and support | 438,267 | 404,330 | 359,054 | ||||||||||||||
| Services and software licenses | 1,039,395 | 977,030 | 1,029,647 | ||||||||||||||
| Products | 393,506 | 744,122 | 269,945 | ||||||||||||||
| Net revenue | 1,432,901 | 1,721,152 | 1,299,592 | ||||||||||||||
| Cost of revenue: | |||||||||||||||||
| Services and software licenses | 183,984 | 170,773 | 188,275 | ||||||||||||||
| Products | 363,878 | 713,045 | 244,516 | ||||||||||||||
| Cost of revenue | 547,862 | 883,818 | 432,791 | ||||||||||||||
| Gross profit | 885,039 | 837,334 | 866,801 | ||||||||||||||
| Other operating income: | |||||||||||||||||
| Remeasurement of tax receivable agreement liabilities | — | — | 24,584 | ||||||||||||||
| Other operating income | — | — | 24,584 | ||||||||||||||
| Operating expenses: | |||||||||||||||||
| Selling, general and administrative | 576,879 | 532,326 | 459,859 | ||||||||||||||
| Research and development | 4,151 | 3,338 | 2,376 | ||||||||||||||
| Amortization of purchased intangible assets | 43,936 | 44,753 | 55,530 | ||||||||||||||
| Operating expenses | 624,966 | 580,417 | 517,765 | ||||||||||||||
| Operating income | 260,073 | 256,917 | 373,620 | ||||||||||||||
| Equity in net income of unconsolidated affiliates | 23,505 | 21,073 | 12,537 | ||||||||||||||
| Interest and investment loss, net | (11,142) | (11,964) | (11,313) | ||||||||||||||
| Gain (loss) on FFF Put and Call Rights | 64,110 | (27,352) | 4,690 | ||||||||||||||
| Other (expense) income, net | (9,646) | 11,967 | 4,153 | ||||||||||||||
| Other income (expense), net | 66,827 | (6,276) | 10,067 | ||||||||||||||
| Income before income taxes | 326,900 | 250,641 | 383,687 | ||||||||||||||
| Income tax expense (benefit) | 58,582 | (53,943) | 92,561 | ||||||||||||||
| Net income from continuing operations | 268,318 | 304,584 | 291,126 | ||||||||||||||
| Income from discontinued operations, net of tax | — | — | 1,054 | ||||||||||||||
| Net income | 268,318 | 304,584 | 292,180 | ||||||||||||||
| Net income from continuing operations attributable to non-controlling interest | (2,451) | (17,062) | (161,318) | ||||||||||||||
| Net income from discontinued operations attributable to non-controlling interest | — | — | (498) | ||||||||||||||
| Net income attributable to non-controlling interest | (2,451) | (17,062) | (161,816) | ||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | (26,685) | 468,311 | ||||||||||||||
| Net income attributable to stockholders | $ | 265,867 | $ | 260,837 | $ | 598,675 | |||||||||||
86
PREMIER, INC. | |||||||||||||||||
Consolidated Statements of Income and Comprehensive Income | |||||||||||||||||
(In thousands, except share data) | |||||||||||||||||
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Comprehensive income: | |||||||||||||||||
| Net income | $ | 268,318 | $ | 304,584 | $ | 292,180 | |||||||||||
| Comprehensive income attributable to non-controlling interest | (2,451) | (17,062) | (161,816) | ||||||||||||||
| Foreign currency translation loss | (3) | — | — | ||||||||||||||
| Comprehensive income attributable to stockholders | $ | 265,864 | $ | 287,522 | $ | 130,364 | |||||||||||
| Weighted average shares outstanding: | |||||||||||||||||
| Basic | 120,220 | 116,527 | 67,035 | ||||||||||||||
| Diluted | 121,668 | 117,532 | 123,614 | ||||||||||||||
| Earnings per share attributable to stockholders: | |||||||||||||||||
| Basic earnings per share | |||||||||||||||||
| Continuing operations | $ | 2.21 | $ | 2.24 | $ | 8.92 | |||||||||||
| Discontinued operations | — | — | 0.01 | ||||||||||||||
| Basic earnings per share attributable to stockholders | $ | 2.21 | $ | 2.24 | $ | 8.93 | |||||||||||
| Diluted earnings per share | |||||||||||||||||
| Continuing operations | $ | 2.19 | $ | 2.22 | $ | 2.03 | |||||||||||
| Discontinued operations | — | — | 0.01 | ||||||||||||||
| Diluted earnings per share attributable to stockholders | $ | 2.19 | $ | 2.22 | $ | 2.04 | |||||||||||
See accompanying notes to the consolidated financial statements.
87
PREMIER, INC. | ||||||||||||||||||||||||||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||||||||
(In thousands, except share data) | ||||||||||||||||||||||||||||||||
| Class A Common Stock | Class B Common Stock | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Loss | (Accumulated Deficit) Retained Earnings | Total Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
| Balance at June 30, 2019 | 61,938 | $ | 644 | 64,548 | $ | — | 2,419 | $ | (87,220) | $ | — | $ | — | $ | (775,674) | $ | (862,250) | |||||||||||||||
| Balance at July 1, 2019 | 61,938 | 644 | 64,548 | — | 2,419 | (87,220) | — | — | (775,674) | (862,250) | ||||||||||||||||||||||
| Impact of change in accounting principle | — | — | — | — | — | — | — | — | (899) | (899) | ||||||||||||||||||||||
| Adjusted balance at July 1, 2019 | 61,938 | 644 | 64,548 | — | 2,419 | (87,220) | — | — | (776,573) | (863,149) | ||||||||||||||||||||||
| Exchange of Class B units for Class A common stock by member owners | 13,552 | 65 | (13,553) | — | (7,065) | 237,313 | 223,215 | — | — | 460,593 | ||||||||||||||||||||||
| Redemption of limited partners | — | — | (782) | — | — | — | — | — | — | — | ||||||||||||||||||||||
| Increase in additional paid-in capital related to quarterly exchange by member owners, including associated TRA revaluation | — | — | — | — | — | — | 71,568 | — | — | 71,568 | ||||||||||||||||||||||
| Issuance of Class A common stock under equity incentive plan | 703 | 7 | — | — | — | — | 6,654 | — | — | 6,661 | ||||||||||||||||||||||
| Issuance of Class A common stock under employee stock purchase plan | 80 | — | — | — | — | — | 2,832 | — | — | 2,832 | ||||||||||||||||||||||
| Treasury stock | (4,646) | — | — | — | 4,646 | (150,093) | — | — | — | (150,093) | ||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | 20,706 | — | — | 20,706 | ||||||||||||||||||||||
| Repurchase of vested restricted units for employee tax-withholding | — | — | — | — | — | — | (8,530) | — | — | (8,530) | ||||||||||||||||||||||
| Net income | — | — | — | — | — | — | — | — | 292,180 | 292,180 | ||||||||||||||||||||||
| Net income attributable to non-controlling interest in Premier LP | — | — | — | — | — | — | — | — | (161,816) | (161,816) | ||||||||||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | — | — | — | — | — | (177,898) | — | 646,209 | 468,311 | ||||||||||||||||||||||
| Balance at June 30, 2020 | 71,627 | $ | 716 | 50,213 | $ | — | — | $ | — | $ | 138,547 | $ | — | $ | — | $ | 139,263 | |||||||||||||||
| Balance at July 1, 2020 | 71,627 | 716 | 50,213 | — | — | — | 138,547 | — | — | 139,263 | ||||||||||||||||||||||
| Impact of change in accounting principle | — | — | — | — | — | — | — | — | (1,228) | (1,228) | ||||||||||||||||||||||
| Adjusted balance at July 1, 2020 | 71,627 | 716 | 50,213 | — | — | — | 138,547 | — | (1,228) | 138,035 | ||||||||||||||||||||||
| Exchange of Class B common units for Class A common stock by member owners | 70 | 1 | (70) | — | — | — | 2,436 | — | — | 2,437 | ||||||||||||||||||||||
| Increase in additional paid-in capital related to quarterly exchange by member owners, including associated TRA revaluation | — | — | — | — | — | — | 37,319 | — | — | 37,319 | ||||||||||||||||||||||
| Increase in additional paid-in capital related to final exchange by member owners, including TRA termination | — | — | — | — | — | — | 517,526 | — | — | 517,526 | ||||||||||||||||||||||
| Issuance of Class A common stock under equity incentive plan | 598 | 6 | — | — | — | — | 9,350 | — | — | 9,356 | ||||||||||||||||||||||
| Issuance of Class A common stock under employee stock purchase plan | 94 | 1 | — | — | — | — | 3,245 | — | — | 3,246 | ||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | 35,425 | — | — | 35,425 | ||||||||||||||||||||||
| Repurchase of vested restricted units for employee tax-withholding | — | — | — | — | — | — | (3,114) | — | — | (3,114) | ||||||||||||||||||||||
| Net income | — | — | — | — | — | — | — | — | 304,584 | 304,584 | ||||||||||||||||||||||
| Net income attributable to non-controlling interest | — | — | — | — | — | — | 5,217 | — | (17,062) | (11,845) | ||||||||||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | — | — | — | — | — | — | — | (26,685) | (26,685) | ||||||||||||||||||||||
| Reclassification of redeemable limited partners' capital to permanent equity | — | — | — | — | — | — | 1,750,840 | — | 3,767 | 1,754,607 | ||||||||||||||||||||||
| Final exchange of Class B common units for Class A common stock by member owners | 50,144 | 501 | (50,143) | — | — | — | (501) | — | — | — | ||||||||||||||||||||||
| Early termination payments to former member owners | — | — | — | — | — | — | (438,967) | — | — | (438,967) | ||||||||||||||||||||||
Dividends ($0.19 per share) | — | — | — | — | — | — | — | — | (93,584) | (93,584) | ||||||||||||||||||||||
88
PREMIER, INC. | ||||||||||||||||||||||||||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||||||||
(In thousands, except share data) | ||||||||||||||||||||||||||||||||
| Class A Common Stock | Class B Common Stock | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Loss | (Accumulated Deficit) Retained Earnings | Total Stockholders' Equity (Deficit) | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
| Adjustment in additional paid-in capital related to consolidated investment | — | — | — | — | — | — | 318 | — | (318) | — | ||||||||||||||||||||||
| Distribution of investment in unconsolidated affiliate to non-controlling interests | — | — | — | — | — | — | (4,095) | — | — | (4,095) | ||||||||||||||||||||||
| Capital contributions | — | — | — | — | — | — | 1,958 | — | — | 1,958 | ||||||||||||||||||||||
| Non-controlling interest in consolidated investments | — | — | — | — | — | — | 3,690 | — | — | 3,690 | ||||||||||||||||||||||
| Balance at June 30, 2021 | 122,533 | $ | 1,225 | — | $ | — | — | $ | — | $ | 2,059,194 | $ | — | $ | 169,474 | $ | 2,229,893 | |||||||||||||||
| Issuance of Class A common stock under equity incentive plan | 1,843 | 18 | — | — | — | — | 37,748 | — | — | 37,766 | ||||||||||||||||||||||
| Issuance of Class A common stock under employee stock purchase plan | 105 | 2 | — | — | — | — | 3,849 | — | — | 3,851 | ||||||||||||||||||||||
| Treasury Stock | (6,429) | — | — | — | 6,429 | (250,129) | — | — | — | (250,129) | ||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | 46,229 | — | — | 46,229 | ||||||||||||||||||||||
| Repurchase of vested restricted units for employee tax-withholding | — | — | — | — | — | — | (10,866) | — | — | (10,866) | ||||||||||||||||||||||
| Net income | — | — | — | — | — | — | — | — | 268,318 | 268,318 | ||||||||||||||||||||||
| Net income attributable to non-controlling interest | — | — | — | — | — | — | 2,451 | — | (2,451) | — | ||||||||||||||||||||||
| Change in ownership of consolidated entity | — | — | — | — | — | — | 202 | — | (142) | 60 | ||||||||||||||||||||||
Dividends ($0.20 per share) | — | — | — | — | — | — | — | — | (97,082) | (97,082) | ||||||||||||||||||||||
| Distribution of investment in unconsolidated affiliate to non-controlling interests | — | — | — | — | — | — | 4,095 | — | (6,427) | (2,332) | ||||||||||||||||||||||
| Non-controlling interest in consolidated investments | — | — | — | — | — | — | 23,145 | — | — | 23,145 | ||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | — | — | — | (3) | — | (3) | ||||||||||||||||||||||
| Balance at June 30, 2022 | 118,052 | $ | 1,245 | — | $ | — | 6,429 | $ | (250,129) | $ | 2,166,047 | $ | (3) | $ | 331,690 | $ | 2,248,850 | |||||||||||||||
See accompanying notes to the consolidated financial statements.
89
PREMIER, INC. | |||||||||||||||||
Consolidated Statements of Cash Flows | |||||||||||||||||
(In thousands) | |||||||||||||||||
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Operating activities | |||||||||||||||||
| Net income | $ | 268,318 | $ | 304,584 | $ | 292,180 | |||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||||||||
| Income from discontinued operations, net of tax | — | — | (1,054) | ||||||||||||||
| Depreciation and amortization | 129,107 | 121,062 | 152,827 | ||||||||||||||
| Equity in net income of unconsolidated affiliates | (23,505) | (21,073) | (12,537) | ||||||||||||||
| Deferred income taxes | 56,792 | (83,692) | 67,980 | ||||||||||||||
| Stock-based compensation | 46,229 | 35,425 | 20,706 | ||||||||||||||
| Remeasurement of tax receivable agreement liabilities | — | — | (24,584) | ||||||||||||||
| Impairment of assets | 18,829 | — | 8,500 | ||||||||||||||
| (Gain) loss on FFF Put and Call Rights | (64,110) | 27,352 | (4,690) | ||||||||||||||
| Other | 5,803 | 9,358 | 853 | ||||||||||||||
| Changes in operating assets and liabilities, net of the effects of acquisitions: | |||||||||||||||||
| Accounts receivable, inventories, prepaid expenses and other assets | 124,659 | (68,008) | (121,735) | ||||||||||||||
| Contract assets | (47,219) | (51,685) | (8,205) | ||||||||||||||
| Accounts payable, accrued expenses, deferred revenue, revenue share obligations and other liabilities | (70,669) | 134,079 | (30,353) | ||||||||||||||
| Net cash provided by operating activities from continuing operations | 444,234 | 407,402 | 339,888 | ||||||||||||||
| Net cash provided by operating activities from discontinued operations | — | — | 9,636 | ||||||||||||||
| Net cash provided by operating activities | 444,234 | 407,402 | 349,524 | ||||||||||||||
| Investing activities | |||||||||||||||||
| Purchases of property and equipment | (87,440) | (88,876) | (94,397) | ||||||||||||||
| Acquisition of businesses and equity method investments, net of cash acquired | (26,000) | (84,463) | (121,640) | ||||||||||||||
| Investment in unconsolidated affiliates | (16,000) | — | (10,165) | ||||||||||||||
| Other | (10,000) | (1,229) | 3,880 | ||||||||||||||
| Net cash used in investing activities | (139,440) | (174,568) | (222,322) | ||||||||||||||
| Financing activities | |||||||||||||||||
| Payments made on notes payable | (99,243) | (50,713) | (2,419) | ||||||||||||||
| Proceeds from credit facility | 325,000 | 225,000 | 400,000 | ||||||||||||||
| Payments on credit facility | (250,000) | (225,000) | (350,000) | ||||||||||||||
| Cash dividends paid | (96,455) | (92,898) | — | ||||||||||||||
| Repurchase of Class A common stock (held as treasury stock) | (250,129) | — | (150,093) | ||||||||||||||
| Payments on deferred consideration related to acquisition of business | (28,586) | (29,217) | — | ||||||||||||||
| Proceeds from exercise of stock options under equity incentive plan | 37,766 | 9,356 | 6,661 | ||||||||||||||
| Distributions to limited partners of Premier LP | — | (9,949) | (48,904) | ||||||||||||||
| Payments to limited partners of Premier LP related to tax receivable agreements | — | (24,218) | (17,425) | ||||||||||||||
| Other | 13,858 | (5,358) | (6,773) | ||||||||||||||
| Net cash used in financing activities | (347,789) | (202,997) | (168,953) | ||||||||||||||
| Effect of exchange rate changes on cash flows | (3) | — | — | ||||||||||||||
| Net (decrease) increase in cash and cash equivalents | (42,998) | 29,837 | (41,751) | ||||||||||||||
| Cash and cash equivalents at beginning of year | 129,141 | 99,304 | 141,055 | ||||||||||||||
| Cash and cash equivalents at end of period | $ | 86,143 | $ | 129,141 | $ | 99,304 | |||||||||||
See accompanying notes to the consolidated financial statements.
90
PREMIER, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Information presented in the Notes to the Consolidated Financial Statements are as of June 30, 2022 unless otherwise specifically noted.
(1) ORGANIZATION AND BASIS OF PRESENTATION
Organization
Premier, Inc. (“Premier” or the “Company”) is a publicly held, for-profit Delaware corporation located in the United States. The Company is a holding company with no material business operations of its own. Following the Subsidiary Reorganization, the Company’s primary asset is its equity interest in its wholly owned subsidiary Premier Healthcare Solutions, Inc., a Delaware corporation (“PHSI”). The Company conducts substantially all of its business operations through PHSI and its other consolidated subsidiaries, including Premier Healthcare Alliance L.P. (“Premier LP”). The Company, together with its subsidiaries and affiliates, is a leading healthcare performance improvement company that unites hospitals, health systems, physicians, employers, product suppliers, service providers, and other healthcare providers and organizations to improve and innovate in the clinical, financial and operational areas of their businesses to meet the demands of a rapidly evolving healthcare industry and continues to expand its capabilities to more fully address and coordinate care improvement and standardization in the employer, payor and life sciences markets. The Company also provides services to other businesses including food service, schools and universities.
The Company’s business model and solutions are designed to provide its members and other customers access to scale efficiencies, spread the cost of their development, provide actionable intelligence derived from anonymized data in the Company’s enterprise data warehouse, mitigate the risk of innovation and disseminate best practices to help the Company’s members and other customers succeed in their transformation to higher quality and more cost-effective healthcare.
The Company, together with its subsidiaries and affiliates, delivers its integrated platform of solutions through two business segments: Supply Chain Services and Performance Services. See Note 19 - Segments for further information related to the Company’s reportable business segments. The Supply Chain Services segment includes one of the largest healthcare group purchasing organization (“GPO”) programs in the United States, supply chain co-management, purchased services and direct sourcing activities. The Performance Services segment consists of three sub-brands: PINC AITM, the Company’s technology and services platform with offerings that help optimize performance in three main areas – clinical intelligence, margin improvement and value-based care – using advanced analytics to identify improvement opportunities, consulting services for clinical and operational design, and workflow solutions to hardwire sustainable change in the provider, life sciences and payer markets; Contigo Health®, the Company’s direct-to-employer business which provides third party administrator services and management of health benefit programs that allow employers to contract directly with healthcare providers as well as partners with healthcare providers to provide employers access to a specialized care network through Contigo Health’s centers of excellence program; and RemitraTM, the Company’s digital invoicing and payables business which provides financial support services to healthcare product suppliers and service providers.
Principles of Consolidation
The accompanying consolidated financial statements have been prepared pursuant to the rules and regulations of the SEC and in accordance with U.S. generally accepted accounting principles (“GAAP”) and include the assets, liabilities, revenues and expenses of all majority-owned subsidiaries over which the Company exercised control and when applicable, entities for which the Company had a controlling financial interest or was the primary beneficiary. All intercompany transactions have been eliminated upon consolidation. Accordingly, the consolidated financial statements reflect all adjustments that, in the opinion of management, are necessary for a fair presentation of results of operations and financial condition for the periods shown, including normal recurring adjustments.
91
Supplementary Cash Flows Information
The following table presents supplementary cash flows information for the years ended June 30, 2022, 2021 and 2020 (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Supplemental schedule of non-cash investing and financing activities: | |||||||||||||||||
| Non-cash additions to property and equipment | $ | 402 | $ | 755 | $ | 5,000 | |||||||||||
| Accrued dividend equivalents | 963 | 686 | — | ||||||||||||||
| Increase (decrease) in redeemable limited partners' capital for adjustment to fair value, with offsetting decrease (increase) in stockholders' equity | — | 26,685 | (468,311) | ||||||||||||||
| Decrease in redeemable limited partners' capital, with offsetting increase in stockholders' equity related to quarterly exchanges by member owners | — | (2,437) | (460,593) | ||||||||||||||
| Net increase in deferred tax assets related to departures and quarterly exchanges by member owners and other adjustments | — | 331 | 62,776 | ||||||||||||||
| Net increase in deferred tax assets related to final exchange by member owners | — | 284,852 | — | ||||||||||||||
| Reclassification of redeemable limited partners' capital to additional paid in capital | — | 1,754,607 | — | ||||||||||||||
| Decrease in additional paid-in capital related to notes payable to members, net of discounts | — | 438,967 | — | ||||||||||||||
| Net increase in additional paid-in capital related to departures and quarterly exchanges by member owners and other adjustments | — | 37,319 | 71,568 | ||||||||||||||
| Increase in additional paid-in capital related to final exchange by member owners | — | 517,526 | — | ||||||||||||||
Variable Interest Entities
At June 30, 2021, as a result of the August 2020 Restructuring, Premier LP no longer met the definition of a variable interest entity (“VIE”), as defined in Accounting Standards Codification (“ASC”) Topic 810. The results of operations of Premier LP are included in the consolidated financial statements.
At June 30, 2020, Premier LP was a VIE as the limited partners did not have the ability to exercise a substantive removal right with respect to the general partner. The Company, through Premier GP, had the exclusive power and authority to manage the business and affairs of Premier LP, to make all decisions with respect to driving the economic performance of Premier LP, and had both an obligation to absorb losses and a right to receive benefits. As such, the Company was the primary beneficiary of the VIE and consolidated the operations of Premier LP under the Variable Interest Model.
Net income attributable to Premier LP, including income and expense that has been classified as discontinued operations, during the year ended June 30, 2020 was as follows (in thousands):
| Year Ended June 30, 2020 | |||||
| Premier LP net income | $ | 359,978 | |||
Premier LP’s cash flows, including cash flows attributable to discontinued operations, for the year ended June 30, 2020 consisted of the following (in thousands):
| Year Ended June 30, 2020 | |||||
| Net cash provided by (used in): | |||||
| Operating activities | $ | 339,894 | |||
| Investing activities | (222,322) | ||||
| Financing activities | (159,948) | ||||
| Net decrease in cash and cash equivalents | (42,376) | ||||
| Cash and cash equivalents at beginning of year | 131,210 | ||||
| Cash and cash equivalents at end of year | $ | 88,834 | |||
92
Use of Estimates in the Preparation of Financial Statements
The preparation of the Company’s consolidated financial statements in accordance with GAAP requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. Significant estimates are evaluated on an ongoing basis, including but not limited to estimates for net administrative fees revenue, software licenses, other services and support revenue, contract assets, deferred revenue, contract costs, allowances for credit losses, reserves for net realizable value of inventory, obsolete inventory, useful lives of property and equipment, stock-based compensation, deferred tax balances including valuation allowances on deferred tax assets, uncertain tax positions, values of investments not publicly traded, projected future cash flows used in the evaluation of asset impairments, values of put and call rights, values of earn-out liabilities and the allocation of purchase prices. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
(2) SIGNIFICANT ACCOUNTING POLICIES
Business Combinations
The Company accounts for acquisitions of a business using the acquisition method. All of the assets acquired, liabilities assumed, contractual contingencies and contingent consideration are recognized at their fair value on the acquisition date. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. Acquisition costs are recorded as expenses in the Consolidated Statements of Income and Comprehensive Income.
Several valuation methods may be used to determine the fair value of assets acquired and liabilities assumed. For intangible assets, the Company typically uses the income method. This method starts with a forecast of all of the expected future net cash flows for each asset. These cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the cash flow streams. Some of the more significant estimates and assumptions inherent in the income method or other methods include the amount and timing of projected future cash flows, the discount rate selected to measure the risks inherent in the future cash flows and the assessment of the asset’s life cycle and the competitive trends impacting the asset, including consideration of any technical, legal, regulatory or economic barriers to entry. Determining the useful life of an intangible asset also requires judgment as different types of intangible assets will have different useful lives and certain assets may even be considered to have indefinite useful lives.
Cash and Cash Equivalents
Cash and cash equivalents include cash and highly liquid investments with remaining maturities of three months or less at the time of acquisition.
Fair Value of Financial Instruments
The fair value of an asset or liability is based on the assumptions that market participants would use in pricing the asset or liability. Valuation techniques consistent with the market approach, income approach and/or cost approach are used to measure fair value. The Company follows a three-tiered fair value hierarchy when determining the inputs to valuation techniques. The fair value hierarchy prioritizes the inputs to valuation techniques into three broad levels in order to maximize the use of observable inputs and minimize the use of unobservable inputs. The levels of the fair value hierarchy are as follows:
Level 1: consists of financial instruments whose values are based on quoted market prices for identical financial instruments in an active market;
Level 2: consists of financial instruments whose values are determined using models or other valuation methodologies that utilize inputs that are observable either directly or indirectly, including (i) quoted prices for similar assets or liabilities in active markets, (ii) quoted prices for identical or similar assets or liabilities in markets that are not active, (iii) pricing models whose inputs are observable for substantially the full term of the financial instrument and (iv) pricing models whose inputs are derived principally from or corroborated by observable market data through correlation or other means for substantially the full term of the financial instrument; and
Level 3: consists of financial instruments whose values are determined using pricing models that utilize significant inputs that are primarily unobservable, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant management judgment or estimation.
93
Accounts Receivable
Financial instruments, other than marketable securities, that subject the Company to potential concentrations of credit risk consist primarily of the Company’s receivables and contract assets (see below for discussion of contract assets). Receivables consist largely of amounts due from hospital and healthcare system members for services and products. The Company maintains an allowance for expected credit losses. This allowance is an estimate and is regularly evaluated by the Company for adequacy by taking into consideration factors such as past experience, credit quality of the member and other customer base and age of the receivable balances, both individually and in the aggregate. As receivables are generally due within one year, changes to economic conditions are not expected to have a significant impact on our estimate of expected credit losses. However, economic conditions are monitored on a quarterly basis to determine if any adjustments are deemed necessary. Provisions for the allowance for expected credit losses attributable to bad debt are recorded in selling, general and administrative expenses in the accompanying Consolidated Statements of Income and Comprehensive Income. Accounts deemed uncollectible are written off, net of actual recoveries. If circumstances related to specific customers change, the Company’s estimate of the recoverability of receivables could be further adjusted.
Contract Assets
Supply Chain Services contract assets represents estimated member and other customer purchases on supplier contracts for which administrative fees have been earned, but not collected. Historically, the Company has not recognized a provision for contract assets. Performance Services contract assets represents revenue earned for services provided but which the Company is not contractually able to bill as of the end of the respective reporting period. Under ASC Topic 326, the Company includes Performance Services’ contract assets in the reserving process and assess the risk of loss similar to the methodology of the Company’s receivables, since the contract assets are reclassified to receivables when the Company becomes entitled to payment. Accordingly, a reserve is applied upon recognition of the contract asset. Certain contract assets are due for periods greater than one year and changes to economic conditions may have an impact on these receivables. The Company monitors economic conditions on a quarterly basis to determine if changes to the reserve are deemed necessary.
Inventory
Inventory consisting of finished goods, primarily medical products, are stated at the lower of cost or net realizable values on an average cost basis. The Company performs periodic assessments to determine the existence of obsolete, slow-moving and unusable inventory and records necessary provisions to reduce such inventory to net realizable value.
Property and Equipment, Net
Property and equipment is recorded at cost, net of accumulated depreciation. Expenditures for major additions and improvements are capitalized and minor replacements, maintenance and repairs are charged to expense as incurred. When property and equipment is retired or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the Consolidated Statements of Income and Comprehensive Income for the respective period. Depreciation is calculated over the estimated useful lives of the related assets using the straight-line method. Capitalized modifications to leased properties are amortized using the straight-line method over the shorter of the lease term or the assets’ estimated useful lives. See Note 8 - Supplemental Balance Sheet Information.
Costs associated with internally developed computer software that are incurred in the preliminary project stage are expensed as incurred. During the development stage and once the project has reached technological feasibility, direct consulting costs and payroll and payroll-related costs for employees that are directly associated with each project are capitalized. Capitalized software costs are included in property and equipment, net in the accompanying Consolidated Balance Sheets. Capitalized costs are amortized on a straight-line basis over the estimated useful lives of the related software applications of up to five years and amortization is included in cost of revenue or selling, general and administrative expenses in the accompanying Consolidated Statements of Income and Comprehensive Income, based on the software’s end use. Replacements and major improvements are capitalized, while maintenance and repairs are expensed as incurred. Some of the more significant estimates and assumptions inherent in this process involve determining the stages of the software development project, the direct costs to capitalize and the estimated useful life of the capitalized software. The Company capitalized costs related to internally developed software of $48.7 million and $77.0 million during the years ended June 30, 2022 and 2021, respectively.
The Company reviews the carrying value of property and equipment for impairment whenever events and circumstances indicate that the carrying value of an asset or asset group may not be recoverable from the estimated cash flows expected to result from its use and eventual disposition. In cases where the undiscounted cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of the asset or asset group. The factors considered by the Company in performing this assessment include current and projected operating results, trends
94
and prospects, the manner in which the asset or asset group is used, and the effects of obsolescence, demand, competition and other economic factors.
Intangible Assets
Definite-lived intangible assets consist primarily of member relationships, technology, customer relationships, trade names and non-compete agreements, and are amortized on a straight-line basis over their estimated useful lives. See Note 9 - Goodwill and Intangible Assets.
The Company reviews the carrying value of definite-lived intangible assets subject to amortization for impairment whenever events and circumstances indicate that the carrying value of the intangible asset subject to amortization may not be recoverable from the estimated cash flows expected to result from its use and eventual disposition. In cases where the undiscounted cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of the intangible asset subject to amortization on the measurement date. The factors considered by the Company in performing this assessment include current and projected operating results, trends and prospects, the manner in which the definite-lived intangible asset is used, and the effects of obsolescence, demand and competition, as well as other economic factors.
Goodwill
Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses. The Company performs its annual goodwill impairment testing on the first day of the last fiscal quarter of its fiscal year unless impairment indicators are present which could require an interim impairment test.
Under accounting rules, the Company may elect to perform a qualitative assessment to determine if an impairment is more likely than not to have occurred. This qualitative assessment requires an evaluation of any excess of fair value over the carrying value for a reporting unit and significant judgment regarding potential changes in valuation inputs, including a review of the Company’s most recent long-range projections, analysis of operating results versus the prior year, changes in market values, changes in discount rates and changes in terminal growth rate assumptions. If it is determined that an impairment is more likely than not to exist, then the Company is required to perform a quantitative assessment to determine whether or not goodwill is impaired and to measure the amount of goodwill impairment, if any.
A goodwill impairment charge is recognized for the amount by which the reporting unit's carrying amount exceeds its fair value. The Company determines the fair value of a reporting unit using a discounted cash flow analysis as well as market-based approaches. Determining fair value requires the exercise of significant judgment, including judgment about appropriate discount rates, perpetual growth rates and the amount and timing of expected future cash flows. The cash flows employed in the discounted cash flow analyses are based on the most recent budget and long-term forecast. The discount rates used in the discounted cash flow analyses are intended to reflect the risks inherent in the future cash flows of the respective reporting units. The market comparable approach estimates fair value using market multiples of various financial measures compared to a set of comparable public companies and recent comparable transactions.
The Company’s most recent annual impairment testing as of April 1, 2022 consisted of a quantitative assessment and did not result in any goodwill impairment charges.
Contract Costs
Contract costs represent amounts the Company has capitalized and reflect the incremental costs of obtaining and fulfilling a contract, which include sales commissions and costs related to implementing SaaS informatics tools. For commissions on new contracts, these costs are amortized over the life of the expected relationship with the customer for the respective performance obligation. For renewals, commissions are amortized over the contract life with the customer. Implementation costs are amortized on a straight-line basis, once the tool is implemented, over the life of the expected relationship with the customer for the respective performance obligation, which is consistent with the transfer of services to the customer to which the implementation relates. The Company’s contract costs are included in other assets in the Consolidated Balance Sheets, while the associated amortization related to sales commissions is included in selling, general and administrative expenses and the associated amortization related to implementation costs is included in cost of revenue in the Consolidated Statements of Income and Comprehensive Income.
Deferred Revenue
Deferred revenue consists of unrecognized revenue related to advanced customer invoicing or member payments received prior to fulfillment of the Company’s revenue recognition criteria. Substantially all deferred revenue consists of deferred subscription fees and deferred consulting fees. Subscription fees for Company-hosted SaaS applications are deferred until the customer’s
95
unique data records have been incorporated into the underlying software database, or until customer site-specific software has been implemented and the customer has access to the software. Deferred consulting fees arise upon invoicing to customers prior to services being performed.
Deferred Compensation Plan Assets and Related Liabilities
The Company maintains a non-qualified deferred compensation plan for the benefit of eligible employees. This plan is designed to permit employee deferrals in excess of certain tax limits and provides for discretionary employer contributions in excess of the tax limits applicable to the Company’s 401(k) plan. The amounts deferred are invested in assets at the direction of the employee. Company assets designated to pay benefits under the plan are held by a rabbi trust and are subject to the general creditors of the Company.
The assets, classified as trading securities, and liabilities of the rabbi trust are recorded at fair value and are accounted for as assets and liabilities of the Company. The assets of the rabbi trust are designated to fund the deferred compensation liabilities owed to current and former employees. The deferred compensation plan contains both current and non-current assets. The current portion of the deferred compensation plan assets is comprised of estimated amounts to be paid within one year to departed participants following separation from the Company. The current portion, $5.3 million and $5.5 million at June 30, 2022 and 2021, respectively, is included in prepaid expenses and other current assets in the accompanying Consolidated Balance Sheets. The corresponding current portion of deferred compensation plan liabilities is included in other current liabilities in the accompanying Consolidated Balance Sheets at June 30, 2022 and 2021. The non-current portion of the deferred compensation plan assets, $47.4 million and $59.6 million at June 30, 2022 and 2021, respectively, is included in long-term assets in the accompanying Consolidated Balance Sheets. The corresponding non-current portion of deferred compensation plan liabilities is included in long-term liabilities in the accompanying Consolidated Balance Sheets at June 30, 2022 and 2021. Realized and unrealized (losses) and gains of $(9.4) million, $12.7 million and $3.9 million on plan assets as of the years ended June 30, 2022, 2021 and 2020, respectively, are included in other (expense) income, net in the accompanying Consolidated Statements of Income and Comprehensive Income. Deferred compensation expense from the change in the corresponding liability of $(9.4) million, $12.7 million and $3.9 million, respectively, is included in selling, general and administrative expense in the accompanying Consolidated Statements of Income and Comprehensive Income for the years ended June 30, 2022, 2021 and 2020, respectively.
Leases
The Company enters into lease contracts in which the Company is the lessee, substantially all of which are related to office space leased in various buildings used for general corporate purposes. The terms of these non-cancelable operating leases typically require the Company to pay rent and a share of operating expenses and real estate taxes, generally with an inflation-based rent increase included. The Company's lease agreements do not contain any material residual value guarantees or material restrictive covenants.
Operating lease right-of-use assets and operating lease liabilities are recognized based on the present value of future minimum lease payments over the lease term beginning at the commencement date. Operating lease right-of-use assets are adjusted for lease incentives, deferred rent and initial direct costs, if incurred. The Company’s leases generally do not include an implicit rate; therefore, the Company determined the present value of future minimum lease payments using an incremental borrowing rate based on information available as of July 1, 2019, the transition date. The related lease expense is recognized on a straight-line basis over the lease term.
Redeemable Limited Partners’ Capital
The fair value of redeemable limited partners’ capital at July 31, 2020 was reclassified from temporary equity in the mezzanine section of the Consolidated Balance Sheets to additional paid-in capital as a component of permanent equity. Prior to July 31, 2020, the Company recorded redeemable limited partners’ capital as temporary equity in the mezzanine section of the Consolidated Balance Sheets at the redemption amount, which represented the greater of the book value or redemption amount of Class B common units per the Amended and Restated Limited Partnership Agreement at the reporting date.
Revenue Recognition
The Company accounts for a contract with a customer when the contract is committed, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of collection.
Revenue is recognized when, or as, control of a promised product or service transfers to a customer, in an amount that reflects the consideration to which the Company expects to be entitled in exchange for transferring those products or services. If the consideration promised in a contract includes a variable amount, the Company estimates the amount to which it expects to be entitled using either the expected value or most likely amount method. The Company’s contracts may include terms that could
96
cause variability in the transaction price, including, for example, revenue share, rebates, discounts, and variable fees based on performance.
The Company only includes estimated amounts of consideration in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. These estimates require management to make complex, difficult or subjective judgments, and to make estimates about the effect of matters inherently uncertain. As such, the Company may not be able to reliably estimate variable fees based on performance in certain long-term arrangements due to uncertainties that are not expected to be resolved for a long period of time or when the Company’s experience with similar types of contracts is limited. Estimates of variable consideration and the determination of whether to include estimated amounts of consideration in the transaction price are based on information (historical, current and forecasted) that is reasonably available to the Company, taking into consideration the type of customer, the type of transaction and the specific facts and circumstances of each arrangement. Additionally, management performs periodic analyses to verify the accuracy of estimates for variable consideration.
Although the Company believes that its approach in developing estimates and reliance on certain judgments and underlying inputs is reasonable, actual results could differ which may result in exposure of increases or decreases in revenue that could be material.
Performance Obligations
A performance obligation is a promise to transfer a distinct good or service to a customer. A contract's transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. Contracts may have a single performance obligation as the promise to transfer individual goods or services is not separately identifiable from other promises, and therefore, not distinct, while other contracts may have multiple performance obligations, most commonly due to the contract covering multiple deliverable arrangements (licensing fees, subscription fees, professional fees for consulting services, etc.).
Net Administrative Fees Revenue
Net administrative fees revenue is a single performance obligation earned through a series of distinct daily services and includes maintaining a network of members to participate in the group purchasing program and providing suppliers efficiency in contracting and access to the Company’s members. Revenue is generated through administrative fees received from suppliers and is included in service revenue in the accompanying Consolidated Statements of Income and Comprehensive Income.
The Company, through its GPO programs, aggregates member purchasing power to negotiate pricing discounts and improve contract terms with suppliers. Contracted suppliers pay the Company administrative fees which generally represent 1% to 3% of the purchase price of goods and services sold to members under the contracts the Company has negotiated. Administrative fees are variable consideration and are recognized as earned based upon estimated purchases by the Company’s members utilizing analytics based on historical member spend and updates for current trends and expectations. Administrative fees are estimated due to the difference in timing of when a member purchases on a supplier contract and when the Company receives the purchasing information. Member and supplier contracts substantiate persuasive evidence of an arrangement. The Company does not take title to the underlying equipment or products purchased by members through its GPO supplier contracts. Administrative fee revenue receivable is included in contract assets in the accompanying Consolidated Balance Sheets.
Generally, the Company pays a revenue share to members equal to a percentage of gross administrative fees, which is estimated according to the members’ contractual agreements with the Company using a portfolio approach based on historical revenue fee share percentages and adjusted for current or anticipated trends. Revenue share is recognized as a reduction to gross administrative fees revenue to arrive at a net administrative fees revenue, and the corresponding revenue share liability is included in revenue share obligations in the accompanying Consolidated Balance Sheets.
Products Revenue
Direct sourcing generates revenue primarily through products sold to the Company’s members, other customers or distributors. Revenue is recognized once control of products has been transferred to the customer and is recorded net of discounts and rebates offered to customers. Discounts and rebates are estimated based on contractual terms and historical trends.
Software Licenses, Other Services and Support Revenue
The Company generates software licenses, other services and support revenue through Performance Services and Supply Chain Services.
97
Within Performance Services, which provides technology with wrap-around service offerings, revenue consists of revenue generated through three sub-brands: PINC AI, Contigo Health and Remitra. The main sources of revenue under PINC AI consists of SaaS-based clinical analytics products subscriptions, enterprise analytics licenses, professional fees for consulting services and other miscellaneous revenue including performance improvement collaboratives, insurance management service fees and commissions from group-sponsored insurance programs. Contigo Health’s main sources of revenue are third-party administrator fees and fees from the centers of excellence program. Remitra’s main source of revenue fees from healthcare product suppliers and service providers.
PINC AI:
SaaS-based Products Subscriptions: SaaS-based clinical analytics subscriptions include the right to access the Company’s proprietary hosted technology on a SaaS basis, training and member support to deliver improvements in cost management, margin improvement, quality and safety, value-based care and provider analytics. SaaS arrangements create a single performance obligation for each subscription within the contract in which the nature of the obligation is a stand-ready obligation, and each day of service meets the criteria for over time recognition. Pricing varies by application and size of healthcare system. Clinical analytics products subscriptions are generally - to five-year agreements with automatic renewal clauses and annual price escalators that typically do not allow for early termination. These agreements do not allow for physical possession of the software. Subscription fees are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation. Implementation involves the completion of data preparation services that are unique to each member’s data set and, in certain cases, the installation of member site-specific software, in order to access and transfer member data into the Company’s hosted SaaS-based clinical analytics products. Implementation is generally 60 to 240 days following contract execution before the SaaS-based clinical analytics products can be fully utilized by the member.
Software Licenses: Enterprise analytics licenses include term licenses that range from to ten years and offer clinical analytics products, improvements in cost management, quality and safety, value-based care and provider analytics. Pricing varies by application and size of healthcare system. Revenue on licensing is recognized upon delivery of the license, and revenue from hosting and maintenance is recognized ratably over the life of the contract.
Consulting Services: Professional fees for consulting services are sold under contracts, the terms of which vary based on the nature of the engagement. These services typically include general consulting, report-based consulting and cost savings initiatives. Promised services under such consulting engagements are typically not considered distinct and are regularly combined and accounted for as one performance obligation. Fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed or when deliverables are provided. In situations where the contracts have significant contract performance guarantees, the performance guarantees are estimated and accounted for as a form of variable consideration when determining the transaction price. In the event that guaranteed savings levels are not achieved, the Company may have to perform additional services at no additional charge in order to achieve the guaranteed savings or pay the difference between the savings that were guaranteed and the actual achieved savings. Occasionally, the Company’s entitlement to consideration is predicated on the occurrence of an event such as the delivery of a report for which client acceptance is required. However, except for event-driven point-in-time transactions, the majority of services provided within this service line are delivered over time due to the continuous benefit provided to the Company’s customers.
Consulting arrangements can require significant estimates for the transaction price and estimated number of hours within an engagement. These estimates are based on the expected value which is derived from outcomes from historical contracts that are similar in nature and forecasted amounts based on anticipated savings for the new agreements. The transaction price is generally constrained until the target transaction price becomes more certain.
Other Miscellaneous Revenue: Revenue from performance improvement collaboratives that support the Company’s offerings in cost management, quality and safety, and value-based care is recognized over the service period as the services are provided, which is generally one year. Performance improvement collaboratives revenue is considered one performance obligation and is generated by providing customers access to online communities whereby data is housed and available for analytics and benchmarking.
Insurance management service fees are recognized in the period in which such services are provided. Commissions from insurance carriers for sponsored insurance programs are earned by acting as an intermediary in the placement of effective insurance policies. Under this arrangement, revenue is recognized at a point in time on the effective date of the associated policies when control of the policy transfers to the customer and is constrained for estimated early terminations.
98
Contigo Health:
Contigo Health revenue consists of third-party administrator fees and fees from the centers of excellence program. Third party administrator fees consist of integrated fees for the processing of self-insured health care plan claims. Third party administrator fees are invoiced to customers monthly and typically collected in that period. Revenue is recognized in the period in which the services have been provided. Fees from the centers of excellence program consist of administrative fees for access to a specialized care network of proven healthcare providers. Centers of excellence fees are invoiced to customers a month in arrears and typically collected in that period. Revenue is recognized in the period in which the services have been provided.
Remitra:
Revenue for Remitra primarily consists of fees from healthcare product suppliers and service providers. Fees for services are invoiced to our customers monthly and typically collected in the following period. For fixed fee contracts, revenue is recognized in the period in which the services have been provided. For variable rate contracts, revenue is recognized as customers are invoiced. Additional revenue consists of fees from check replacement services which consist of monthly rebates from bank partners.
Within Supply Chain Services, revenue is generated through supply chain co-management and SaaS-based purchased services activities.
Supply Chain Co-Management. Supply chain co-management activities generate revenue in the form of a service fee for services performed under the supply chain management contracts. Service fees are billed as stipulated in the contract, and revenue is recognized on a proportional performance method as services are performed.
Purchased Services. Purchased services generate revenue through subscription fees for SaaS-based products. Subscription fees are typically billed on a monthly basis and revenue is recognized as a single deliverable on a straight-line basis over the remaining contractual period following implementation.
Multiple Deliverable Arrangements
The Company enters into agreements where the individual deliverables discussed above, such as SaaS subscriptions and consulting services, are bundled into a single service arrangement. These agreements are generally provided over a time period ranging from approximately three months to five years after the applicable contract execution date. Revenue, including both fixed and variable consideration, is allocated to the individual performance obligations within the arrangement based on the stand-alone selling price when it is sold separately in a stand-alone arrangement.
Cost of Revenue and Operating Expenses
Cost of Revenue
Cost of services and software licenses revenue includes expenses related to employees (including compensation and benefits) and outside consultants who directly provide services related to revenue-generating activities, including consulting services to members and capitalized implementation services related to SaaS informatics products. Cost of services and software licenses revenue also includes expenses related to hosting services, related data center capacity costs, third-party product license expenses and amortization of the cost of internally developed software.
Cost of product revenue consists of purchase and shipment costs for direct sourced medical products.
Operating Expenses
Selling, general and administrative expenses consist of expenses directly associated with selling and administrative employees and indirect expenses associated with employees that primarily support revenue generating activities (including compensation and benefits) and travel-related expenses, as well as occupancy and other indirect expenses, insurance expenses, professional fees, and other general overhead expenses.
Research and development expenses consist of employee-related compensation and benefits expenses, and third-party consulting fees of technology professionals, incurred to develop, support and maintain the Company’s software-related products and services.
Amortization of purchased intangible assets includes the amortization of all identified definite-lived intangible assets resulting from acquisitions.
99
Advertising Costs
Advertising costs are expensed as incurred. Advertising costs are reflected in selling, general and administrative expenses in the accompanying Consolidated Statements of Income and Comprehensive Income and were $6.5 million, $4.8 million and $5.0 million for the years ended June 30, 2022, 2021 and 2020, respectively.
Income Taxes
The Company accounts for income taxes under the asset and liability approach. Deferred tax assets or liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities as measured by the enacted tax rates as well as net operating losses and credit carryforwards, which will be in effect when these differences reverse. The Company provides a valuation allowance against net deferred tax assets when, based upon the available evidence, it is more likely than not that the deferred tax assets will not be realized.
The Company prepares and files tax returns based on interpretations of tax laws and regulations. The Company’s tax returns are subject to examination by various taxing authorities in the normal course of business. Such examinations may result in future tax, interest and penalty assessments by these taxing authorities.
In determining the Company’s tax expense for financial reporting purposes, the Company establishes a reserve when there are transactions, calculations, and tax filing positions for which the tax determination is uncertain, and it is more likely than not that such positions would not be sustained upon examinations.
The Company adjusts its tax reserve estimates periodically based on the changes in facts and circumstances, such as ongoing examinations by, and settlements with, varying taxing authorities, as well as changes in tax laws, regulations and interpretations. The consolidated tax expense of any given year includes adjustments to prior year income tax reserve and related estimated interest charges that are considered appropriate. The Company’s policy is to recognize, when applicable, interest and penalties on uncertain income tax positions as part of income tax expense.
Comprehensive Income
Comprehensive income includes all changes in stockholders’ deficit during a period from non-owner sources. Net income and other comprehensive income are reported, net of their related tax effect, to arrive at comprehensive income.
Basic and Diluted Earnings per Share (“EPS”)
Basic EPS is calculated by dividing net income attributable to stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted EPS assumes the conversion, exercise or issuance of all potentially issuable dilutive shares of Class A common stock, unless the effect of such inclusion would result in the reduction of a loss or the increase in income per share. Diluted EPS is computed by dividing net income attributable to stockholders by the weighted average number of shares of common stock increased by the dilutive effects of potentially issuable dilutive shares of Class A common stock during the period. The number of potential common shares outstanding is determined in accordance with the treasury stock method.
Recently Issued Accounting Standards Not Yet Adopted
In October 2021, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2021-08 Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers, (“ASU 2021-08”), which requires that an acquirer recognize and measure contract assets and contract liabilities acquired in a business combination in accordance with Topic 606, Revenue from Contracts with Customers. ASU 2021-08 will be effective for the Company for the fiscal year beginning July 1, 2023. Early adoption is permitted including adoption in interim periods. The Company is currently evaluating the impact of the adoption of the new standard on its consolidated financial statements and related disclosures.
(3) BUSINESS ACQUISITIONS
Acquisition of Invoice Delivery Services, LP Assets
On March 1, 2021, the Company acquired, through its indirect, wholly owned subsidiary Premier IDS, LLC, substantially all the assets and assumed certain liabilities of Invoice Delivery Services, LP (“IDS”) for an adjusted purchase price of $80.7 million, subject to certain adjustments, of which $80.0 million was paid at closing with borrowings under the Company’s Credit Facility (as defined in Note 10 - Debt and Notes Payable).
100
The Company accounted for the IDS acquisition as a business combination whereby the purchase price was allocated to tangible and intangible assets acquired and liabilities assumed based on their fair values. The total fair value assigned to intangible assets acquired was $22.4 million, consisting primarily of developed technology.
The IDS acquisition resulted in the recognition of $57.7 million of goodwill based on the purchase price paid in the acquisition compared to the fair value of the assets acquired. The IDS acquisition was considered an asset acquisition for income tax purposes. Accordingly, the Company expects tax goodwill to be deductible for tax purposes. The purchase price allocation was finalized during the three months ended March 31, 2022. IDS has been integrated within Premier under the brand name Remitra and is reported as part of the Performance Services segment.
Pro forma results of operations for the acquisition have not been presented because the effects on revenue and net income were not material to the Company’s historic consolidated financial statements.
(4) DISCONTINUED OPERATIONS AND EXIT ACTIVITIES
In connection with the sale of certain assets and wind down and exit from the specialty pharmacy business, the Company met the criteria for classifying certain assets and liabilities of its specialty pharmacy business as a discontinued operation as of June 30, 2019. Prior to its classification as a discontinued operation, the specialty pharmacy business was included as part of the Supply Chain Services segment.
As of June 30, 2020, the Company had completed the wind down and exit from the specialty pharmacy business and had no net income or loss from discontinued operations for the years ended June 30, 2022 and 2021.
The following table summarizes the major components of net income from discontinued operations for the year ended June 30, 2020 (in thousands):
| Year Ended June 30, 2020 | |||||
| Operating income from discontinued operations | $ | — | |||
| Net gain on disposal and impairment of assets | (1,697) | ||||
| Income from discontinued operations before income taxes | 1,697 | ||||
| Income tax expense | 643 | ||||
| Income from discontinued operations, net of tax | 1,054 | ||||
| Net income from discontinued operations attributable to non-controlling interest in Premier LP | (498) | ||||
| Net income from discontinued operations attributable to stockholders | $ | 556 | |||
(5) INVESTMENTS
Investments in Unconsolidated Affiliates
The Company’s investments in unconsolidated affiliates consisted of the following (in thousands):
| Carrying Value | Equity in Net Income | ||||||||||||||||||||||||||||
| June 30, | Year Ended June 30, | ||||||||||||||||||||||||||||
| 2022 | 2021 | 2022 | 2021 | 2020 | |||||||||||||||||||||||||
| FFF | $ | 137,162 | $ | 120,548 | $ | 16,614 | $ | 11,344 | $ | 12,299 | |||||||||||||||||||
| Exela | 27,733 | — | 1,733 | — | — | ||||||||||||||||||||||||
| Prestige | 15,597 | 14,478 | 4,303 | 8,856 | — | ||||||||||||||||||||||||
| Qventus | 16,000 | — | — | — | — | ||||||||||||||||||||||||
| Other investments | 19,053 | 18,198 | 855 | 873 | 238 | ||||||||||||||||||||||||
| Total investments | $ | 215,545 | $ | 153,224 | $ | 23,505 | $ | 21,073 | $ | 12,537 | |||||||||||||||||||
The Company, through its indirect, wholly owned subsidiary Premier Supply Chain Improvement, Inc. (“PSCI”), held a 49% interest in FFF Enterprises, Inc. (“FFF”) through its ownership of stock of FFF at June 30, 2022 and 2021. On July 29, 2021, the FFF shareholders’ agreement was amended resulting in the termination of the FFF Put Right, which had previously provided the majority shareholder of FFF a right to require the Company to purchase such shareholder’s interest in FFF, on an all or nothing basis, on or after April 15, 2023 (“FFF Put Right”). The termination of the FFF Put Right resulted in the derecognition of the FFF Put Right liability and the recognition of a corresponding non-cash gain of $64.1 million in the
101
accompanying Consolidated Statements of Income and Comprehensive Income (see Note 6 - Fair Value Measurements for additional information).
The Company, through its consolidated subsidiary, ExPre Holdings, LLC (“ExPre”), held an approximate 6% interest in Exela Holdings, Inc. (“Exela”) through its ownership of Exela Class A common stock at June 30, 2022. At June 30, 2022, the Company owned approximately 15% of the membership interest of ExPre, with the remainder of the membership interests held by 11 member health systems or their affiliates.
The Company, through its consolidated subsidiary, PRAM Holdings, LLC (“PRAM”), held an approximate 20% interest in Prestige Ameritech Ltd. (“Prestige”) through its ownership of Prestige limited partnership units at June 30, 2022. The Company owns approximately 26% of the membership interest of PRAM, with the remainder of the membership interests held by 16 member health systems.
The Company accounts for its investments in FFF, Exela and Prestige using the equity method of accounting and includes the investment as part of the Supply Chain Services segment.
On January 31, 2022, the Company, through PHSI, purchased an approximate 7% interest in Qventus, Inc. (“Qventus”) through its ownership of Qventus Series C preferred stock. The Company accounts for its investment in Qventus at initial cost less impairments, if any, plus or minus any observable changes in fair value. The Company includes Qventus as part of the Performance Services segment.
Unconsolidated Significant Subsidiaries
In accordance with Rules 3-09 and 4-08(g) of Regulation S-X, the Company must determine which of its unconsolidated investments, if any, are considered “significant subsidiaries.” In evaluating these investments, there are three tests utilized to determine if any unconsolidated subsidiaries are considered significant subsidiaries: the investment test, the asset test and the income test. Rule 3-09 of Regulation S-X requires the Company to include separate audited financial statements of any unconsolidated majority-owned subsidiary (unconsolidated subsidiaries in which the Company owns greater than 50% of the voting securities) in an annual report if any of the three tests exceed 20%. Rule 4-08(g) of Regulation S-X requires summarized financial information of unconsolidated subsidiaries in an annual report if any of the three tests exceeds 10%, and summarized financial information in a quarterly report if any of the three tests exceeds 20% pursuant to Rule 10-01(b)(1) of Regulation S-X.
As of June 30, 2022, 2021, and 2020 the Company held one unconsolidated investment whose assets represented greater than 10% of its total assets.
The following table shows summarized unaudited financial information for FFF, which met the 10% asset test for the years ended June 30, 2022 and 2021 (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Total current assets | $ | 841,555 | $ | 635,642 | |||||||
| Total non-current assets | 103,298 | 86,783 | |||||||||
| Total current liabilities | 463,863 | 348,477 | |||||||||
| Total non-current liabilities | 325,693 | 251,866 | |||||||||
| Non-controlling equity | 76,096 | 59,820 | |||||||||
The following table shows summarized unaudited results of operations information for FFF, which met the 10% asset test for the years ended June 30, 2022, 2021, and 2020 (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Revenue | $ | 2,728,855 | $ | 2,047,494 | $ | 1,990,282 | |||||||||||
| Gross profit | 150,980 | 122,890 | 108,733 | ||||||||||||||
| Income from operations | 55,379 | 41,643 | 35,624 | ||||||||||||||
| Net income | 33,215 | 23,841 | 22,565 | ||||||||||||||
| Net income attributable to non-controlling interest | 16,275 | 11,682 | 11,057 | ||||||||||||||
102
(6) FAIR VALUE MEASUREMENTS
Recurring Fair Value Measurements
The following table represents the Company's financial assets and liabilities, which are measured at fair value on a recurring basis (in thousands):
| Fair Value of Financial Assets and Liabilities | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||
| June 30, 2022 | ||||||||||||||
| Cash equivalents | $ | 75 | $ | 75 | $ | — | $ | — | ||||||
| Deferred compensation plan assets | 52,718 | 52,718 | — | — | ||||||||||
| Total assets | 52,793 | 52,793 | — | — | ||||||||||
| Earn-out liabilities | 22,789 | — | — | 22,789 | ||||||||||
| Total liabilities | $ | 22,789 | $ | — | $ | — | $ | 22,789 | ||||||
| June 30, 2021 | ||||||||||||||
| Cash equivalents | $ | 75 | $ | 75 | $ | — | $ | — | ||||||
| Deferred compensation plan assets | 65,051 | 65,051 | — | — | ||||||||||
| Total assets | 65,126 | 65,126 | — | — | ||||||||||
| Earn-out liabilities | 24,249 | — | — | 24,249 | ||||||||||
| FFF put right | 64,110 | — | — | 64,110 | ||||||||||
| Total liabilities | $ | 88,359 | $ | — | $ | — | $ | 88,359 | ||||||
Deferred compensation plan assets consisted of highly liquid mutual fund investments, which were classified as Level 1. The current portion of deferred compensation plan assets ($5.3 million and $5.5 million at June 30, 2022 and 2021, respectively) was included in prepaid expenses and other current assets in the accompanying Consolidated Balance Sheets.
Financial Instruments Measured at Fair Value on a Recurring Basis Using Significant Unobservable Inputs (Level 3)
FFF Put and Call Rights
On July 29, 2021, the FFF shareholders’ agreement was amended resulting in the termination of the FFF Put Right and the derecognition of the FFF Put Right liability.
In the event of a Key Man Event (generally defined in the shareholders’ agreement as the resignation, termination for cause, death or disability of the majority shareholder), the Company has a call right that requires the majority shareholder to sell its remaining interest in FFF to the Company, and is exercisable at any time within the later of 180 calendar days after the date of a Key Man Event (the “Call Right”, together with the FFF Put Right, the “Put and Call Rights”). As of June 30, 2022 and 2021, the Call Right had zero value. In the event that either of these rights are exercised, the purchase price for the additional interest in FFF will be at a per share price equal to the earnings before interest, taxes, depreciation and amortization (“FFF EBITDA”) over the twelve calendar months prior to the purchase date multiplied by a market adjusted multiple, adjusted for any outstanding debt and cash and cash equivalents, divided by the number of shares of FFF common stock then outstanding (“Equity Value per Share”).
At June 30, 2021, the fair values of the Put and Call Rights were determined using a Monte Carlo simulation in a risk-neutral framework based on the Equity Value per Share calculation using unobservable inputs, which included the estimated FFF Put and Call Rights’ expiration dates, the forecast of the FFF EBITDA and enterprise value over the option period, forecasted movements in the overall market and the likelihood of a Key Man Event. FFF’s enterprise value over the option period was valued utilizing expected annual FFF EBITDA and revenue growth rates, among other assumptions. The resulting FFF enterprise value was an assumption utilized in the valuation of the Put and Call Rights.
103
The Company utilized the following assumptions to estimate the fair value of FFF Put and Call Rights at June 30, 2021:
| June 30, 2021 | |||||
| Annual FFF EBITDA growth rate | 2.5-10.8% | ||||
| Annual revenue growth rate | 2.5-6.3% | ||||
| Correlation | 80.0 | % | |||
| Weighted average cost of capital | 14.0 | % | |||
| Asset volatility | 30.0 | % | |||
| Credit spread | 0.8 | % | |||
The significant assumptions using the Monte Carlo simulation approach for valuation of the Put and Call Rights are:
(i)Annual FFF EBITDA growth rate: The forecasted FFF EBITDA growth range over six years;
(ii)Annual revenue growth rate: The forecasted revenue growth range over six years;
(iii)Correlation: The estimated correlation between future Business Enterprise Value and FFF EBITDA;
(iv)Weighted average cost of capital: The expected rate paid to security holders to finance debt and equity;
(v)Asset volatility: Based on the asset volatility of guideline public companies in the healthcare industry; and
(vi)Credit spread: Based on term-matched BBB yield curve.
At June 30, 2021, the Company recorded the FFF Put and Call Rights within long-term other liabilities and long-term other assets, respectively, within the accompanying Consolidated Balance Sheets. Net changes in the fair values of the FFF Put and Call Rights, including the gain recorded as a result of the termination of the FFF Put Right, were recorded within other (expense) income, net in the accompanying Consolidated Statements of Income and Comprehensive Income.
Earn-out liabilities
An earn-out liability was established in connection with the acquisition of substantially all of the assets and certain liabilities of Acurity, Inc. and Nexera, Inc. (the “Acurity and Nexera asset acquisition”) in February 2020. The earn-out liability was classified as Level 3 of the fair value hierarchy.
The earn-out liability arising from expected earn-out payments related to the Acurity and Nexera asset acquisition was measured on the acquisition date using a probability-weighted expected payment model and are remeasured periodically due to changes in management’s estimates of the number of transferred member renewals and market conditions. In determining the fair value of the contingent liabilities, management reviews the current results of the acquired business, along with projected results for the remaining earn-out period, to calculate the expected earn-out payment to be made based on the contractual terms set out in the acquisition agreement. The Acurity and Nexera earn-out liability utilized a credit spread of 1.6% and 0.9% at June 30, 2022 and 2021, respectively. As of June 30, 2022 and 2021, the undiscounted range of outcomes is between $0 and $30.0 million. A significant decrease in the probability could result in a significant decrease in the value of the earn-out liability. The fair value of the Acurity and Nexera earn-out liability at June 30, 2022 and 2021 was $22.8 million and $24.2 million, respectively.
Acurity and Nexera Earn-out
| As of June 30, | |||||||||||
| Input assumptions | 2022 | 2021 | |||||||||
| Probability of transferred member renewal percentage < 50% | 5.0 | % | 5.0 | % | |||||||
| Probability of transferred member renewal percentage between 50% and 65% | 10.0 | % | 10.0 | % | |||||||
| Probability of transferred member renewal percentage between 65% and 80% | 25.0 | % | 25.0 | % | |||||||
| Probability of transferred member renewal percentage > 80% | 60.0 | % | 60.0 | % | |||||||
| Credit spread | 1.6 | % | 0.9 | % | |||||||
104
A reconciliation of the FFF Put Right and earn-out liabilities is as follows (in thousands):
| Beginning Balance | Settlements | Ending Balance | ||||||||||||
| Year Ended June 30, 2022 | ||||||||||||||
| Earn-out liabilities | $ | 24,249 | $ | — | $ | (1,460) | $ | 22,789 | ||||||
FFF put right (a) | 64,110 | (64,110) | — | — | ||||||||||
| Total Level 3 liabilities | $ | 88,359 | $ | (64,110) | $ | (1,460) | $ | 22,789 | ||||||
| Year Ended June 30, 2021 | ||||||||||||||
Earn-out liabilities (b) | 33,151 | (13,733) | 4,831 | 24,249 | ||||||||||
| FFF put right | 36,758 | — | 27,352 | 64,110 | ||||||||||
| Total Level 3 liabilities | $ | 69,909 | $ | (13,733) | $ | 32,183 | $ | 88,359 | ||||||
_________________________________
(a)Settlements for the year ended June 30, 2022 includes non-cash gain recognized as a result of the termination of the FFF Put Right and the derecognition of the FFF Put Right liability.
(b)Settlements for the year ended June 30, 2021, includes earnout liabilities from previous acquisitions which were earned and paid during the period.
(c)A gain on level 3 liability balances will decrease the liability ending balance and a loss on level 3 liability balance will increase the liability ending balance.
Non-Recurring Fair Value Measurements
During the year ended June 30, 2021, the Company recorded notes payable to former limited partners as a result of the August 2020 Restructuring. Although these notes are non-interest bearing, they include a Level 2 input associated with the implied interest rate of 1.8% and are calculated as of August 11, 2020. (see Note 10 - Debt and Notes Payable).
During the year ended June 30, 2022, no non-recurring fair value measurements were required relating to the measurement of goodwill and intangible assets for impairment.
Financial Instruments For Which Fair Value Only is Disclosed
The fair values of non-interest bearing notes payable, classified as Level 2, were less than their carrying value by $0.1 million at both June 30, 2022 and 2021, based on assumed market interest rates of 1.6% for both periods.
Other Financial Instruments
The fair values of cash, accounts receivable, accounts payable, accrued liabilities, and the Credit Facility (as defined in Note 10 - Debt and Notes Payable) approximated carrying value due to the short-term nature of these financial instruments.
(7) CONTRACT BALANCES
Contract Assets, Deferred Revenue and Revenue Share Obligations
The timing of revenue recognition, billings and cash collections results in accounts receivables, contract assets (unbilled receivables) and deferred revenue on the Consolidated Balance Sheets. Contract assets increased by $47.2 million during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to the acceleration of revenue recognition from licensing contracts in Performance Services and increased gross administrative fees driven by higher members’ purchases. The acceleration of revenue recognition from licensing contracts represents performance obligations that have been satisfied prior to customer invoicing offset by the timing of invoicing related to certain cost management consulting services and performance-based engagements where revenue is recognized as work is performed. Revenue share obligations increased by $18.5 million during the year ended June 30, 2022 compared to the year ended June 30, 2021 primarily due to the underlying revenue share arrangements which include a higher average revenue fee share percentage.
Revenue recognized during the year ended June 30, 2022 that was included in the opening balance of deferred revenue at June 30, 2021 was $25.4 million, which is a result of satisfying performance obligations within the Performance Services segment.
105
Performance Obligations
A performance obligation is a promise to transfer a distinct good or service to a customer. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. Contracts may have a single performance obligation as the promise to transfer individual goods or services is not separately identifiable from other promises and, therefore, not distinct, while other contracts may have multiple performance obligations, most commonly due to the contract covering multiple phases or deliverable arrangements (licensing fees, SaaS subscription fees, maintenance and support fees, and professional fees for consulting services).
Net revenue of $5.3 million was recognized during the year ended June 30, 2022 from performance obligations that were satisfied or partially satisfied on or before June 30, 2021. The net revenue recognized was driven by an increase of $4.8 million in net administrative fees revenue related to under-forecasted cash receipts received in the current period and an increase of $0.5 million associated with revised forecasts from underlying contracts that include variable consideration components as well as additional fluctuations due to input method contracts which occur in the normal course of business.
The reduction to net revenue recognized during the year ended June 30, 2021 from performance obligations that were satisfied or partially satisfied on or before June 30, 2020 was $2.9 million. The reduction in net revenue recognized was driven by $3.3 million associated with revised forecasts from underlying contracts that include variable consideration components as well as additional fluctuations due to input method contracts which occur in the normal course of business partially offset by $0.4 million of net administrative fees revenue related to under-forecasted cash receipts received in the current period.
Remaining performance obligations represent the portion of the transaction price that has not yet been satisfied or achieved. As of June 30, 2022, the aggregate amount of the transaction price allocated to remaining performance obligations was $752.7 million. The Company expects to recognize 40% of the remaining performance obligations over the next 12 months and an additional 25% over the following 12 months, with the remainder recognized thereafter.
Contract Costs
The Company capitalizes the incremental costs of obtaining and fulfilling a contract, which include costs associated with implementing SaaS informatics tools and sales commissions. At June 30, 2022, the Company had $22.9 million in capitalized contract costs, including $10.7 million related to implementation costs and $12.2 million related to sales commissions. The Company recognized $8.9 million of related amortization expense for the year ended June 30, 2022.
At June 30, 2021, the Company had $21.7 million in capitalized contract costs, including $10.2 million related to implementation costs and $11.5 million related to sales commissions. The Company recognized $7.6 million of related amortization expense for the year ended June 30, 2021.
106
(8) SUPPLEMENTAL BALANCE SHEET INFORMATION
Accounts Receivable, Net
Trade accounts receivable consisted of amounts due from hospital and healthcare system members as well as non-healthcare customers services and products. Managed services receivable consisted of amounts receivable related to fees for services provided to members to support contract negotiation and administration, claims data, rebate processing and evaluation of pharmacy formulary and utilization.
Accounts receivable, net consisted of the following (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Trade accounts receivable | $ | 114,214 | $ | 131,246 | |||||||
| Managed services receivable | 1,422 | 11,972 | |||||||||
| Other | 536 | 513 | |||||||||
| Total accounts receivable | 116,172 | 143,731 | |||||||||
| Allowance for credit losses | (2,043) | (2,284) | |||||||||
| Accounts receivable, net | $ | 114,129 | $ | 141,447 | |||||||
Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
| June 30, | ||||||||||||||
| Useful life | 2022 | 2021 | ||||||||||||
| Capitalized software | 2-5 years | $ | 705,319 | $ | 653,515 | |||||||||
| Computer hardware | 3-5 years | 60,399 | 62,930 | |||||||||||
| Furniture and other equipment | 5 years | 7,097 | 7,097 | |||||||||||
| Leasehold improvements | Lesser of estimated useful life or term of lease | 19,208 | 19,061 | |||||||||||
| Total property and equipment | 792,023 | 742,603 | ||||||||||||
| Accumulated depreciation and amortization | (578,644) | (518,332) | ||||||||||||
| Property and equipment, net | $ | 213,379 | $ | 224,271 | ||||||||||
Depreciation and amortization expense related to property and equipment was $85.2 million, $76.3 million and $97.3 million for the years ended June 30, 2022, 2021 and 2020, respectively. Unamortized capitalized software costs were $177.6 million and $178.4 million at June 30, 2022 and 2021, respectively.
During the year ended June 30, 2022, the Company incurred an impairment of long-lived assets of $12.7 million associated with capitalized software assets in the Supply Chain Services segment as the carrying value of the assets was not recoverable. The Company did not incur a material loss on disposal or impairment of long-lived assets during the years ended June 30, 2021 and 2020.
Other Long-Term Assets
Other long-term assets consisted of the following (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Contract assets, less current portion | $ | 54,441 | $ | — | |||||||
| Acurity prepaid contract administrative fee share, less current portion | 29,099 | 48,498 | |||||||||
| Capitalized contract costs | 22,894 | 21,686 | |||||||||
Other (a) | 7,720 | 6,764 | |||||||||
| Total other long-term assets | $ | 114,154 | $ | 76,948 | |||||||
107
_________________________________
(a)Includes deferred loan costs, net of $0.9 million and $1.5 million as of June 30, 2022 and 2021, respectively.
Pursuant to the Acurity and Nexera asset acquisition, the Company capitalized one-time rebates pursuant to the purchase agreement with Acurity, Inc. as prepaid contract administrative fee share.
Contract costs include capitalized sales commissions and implementation costs. See Note 7 - Contract Balances for further information.
The Company recorded $0.6 million in amortization expense on deferred loan costs for each of the years ended June 30, 2022, 2021 and 2020. Amortization expense on deferred loan costs was recognized based on the straight-line method, which approximates the effective interest method, and was included in interest expense, net in the Consolidated Statements of Income and Comprehensive Income.
Other Long-Term Liabilities
Other long-term liabilities consisted of the following (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Earn-out liability, less current portion | $ | 22,789 | $ | 24,249 | |||||||
| Reserve for uncertain tax positions | 18,799 | 18,524 | |||||||||
| FFF Put Right | — | 64,110 | |||||||||
| Other | 986 | 5,518 | |||||||||
| Total other long-term liabilities | $ | 42,574 | $ | 112,401 | |||||||
On July 29, 2021, the Company executed an amendment to the FFF shareholders’ agreement which resulted in the termination of the Put Right (see Note 5 - Investments and Note 6 - Fair Value Measurements).
Earn-out liabilities were established in connection with the Acurity and Nexera asset acquisition. See Note 6 - Fair Value Measurements for further information.
(9) GOODWILL AND INTANGIBLE ASSETS
Goodwill
At June 30, 2022 and 2021, the Company had goodwill balances recorded at Supply Chain Services and Performance Services of $388.5 million and $611.4 million, respectively.
Intangible Assets, Net
Intangible assets, net consisted of the following (in thousands):
| June 30, | ||||||||||||||
| Useful Life | 2022 | 2021 | ||||||||||||
| Member relationships | 14.7 years | $ | 386,100 | $ | 386,100 | |||||||||
| Technology | 7.2 years | 98,017 | 186,017 | |||||||||||
| Customer relationships | 10.4 years | 47,830 | 70,830 | |||||||||||
| Trade names | 6.9 years | 17,210 | 24,610 | |||||||||||
| Non-compete agreements | 5.2 years | 17,315 | 11,315 | |||||||||||
Other (a) | 10.2 years | 7,682 | 7,682 | |||||||||||
| Total intangible assets | 574,154 | 686,554 | ||||||||||||
| Accumulated amortization | (217,582) | (289,912) | ||||||||||||
| Total intangible assets, net | $ | 356,572 | $ | 396,642 | ||||||||||
_________________________________
(a)Includes a $1.0 million indefinite-lived asset.
108
The net carrying value of intangible assets by segment was as follows (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Supply Chain Services | $ | 301,611 | $ | 334,038 | |||||||
Performance Services (a) | 54,961 | 62,604 | |||||||||
| Total intangible assets, net | $ | 356,572 | $ | 396,642 | |||||||
_________________________________
(a)Includes a $1.0 million indefinite-lived asset that was acquired through the HDP acquisition.
The Company reviews the carrying value of definite-lived intangible assets subject to amortization for impairment whenever events and circumstances indicate that the carrying value of the intangible asset subject to amortization may not be recoverable. During the year ended June 30, 2022, the carrying value of $4.4 million in customer relationships and $1.7 million in trade names in the Performance Services segment were not recoverable and the Company recorded an impairment on assets of $6.1 million in the accompanying Consolidated Statements of Income and Comprehensive Income. During the years ended June 30, 2021 and 2020, no impairment of assets was recorded in the accompanying Consolidated Statements of Income and Comprehensive Income.
Intangible asset amortization expense was $43.9 million, $44.8 million and $55.5 million for the years ended June 30, 2022, 2021 and 2020, respectively.
The estimated amortization expense for each of the next five fiscal years and thereafter is as follows (in thousands):
| 2023 | $ | 40,714 | |||
| 2024 | 39,543 | ||||
| 2025 | 38,135 | ||||
| 2026 | 36,920 | ||||
| 2027 | 34,268 | ||||
| Thereafter | 165,992 | ||||
| Total amortization expense | $ | 355,572 | |||
109
(10) DEBT AND NOTES PAYABLE
Long-term debt and notes payable consisted of the following (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Credit Facility | $ | 150,000 | $ | 75,000 | |||||||
| Notes payable to members, net of discount | 298,994 | 394,943 | |||||||||
| Other notes payable | 5,333 | 8,628 | |||||||||
| Total debt and notes payable | 454,327 | 478,571 | |||||||||
| Less: current portion | (250,859) | (174,243) | |||||||||
| Total long-term debt and notes payable | $ | 203,468 | $ | 304,328 | |||||||
Credit Facility
PHSI, along with its consolidated subsidiaries, Premier LP and PSCI, as Co-Borrowers, Prior Premier GP and certain domestic subsidiaries of the Co-Borrowers, as guarantors, entered into an unsecured Credit Facility, dated as of November 9, 2018 (the “Credit Facility”). The Credit Facility has a maturity date of November 9, 2023, subject to up to two one-year extensions at the request of the Co-Borrowers and approval of a majority of the lenders under the Credit Facility. The Credit Facility provides for borrowings of up to $1.0 billion with (i) a $50.0 million sub-facility for standby letters of credit and (ii) a $100.0 million sub-facility for swingline loans. The Credit Facility also provides that Co-Borrowers may from time to time (i) incur incremental term loans and (ii) request an increase in the revolving commitments under the Credit Facility, together up to an aggregate $350.0 million, subject to the approval of the lenders providing such term loans or revolving commitment increases. The Credit Facility includes an unconditional and irrevocable guaranty of all obligations under the Credit Facility by Premier GP, certain domestic subsidiaries of Premier GP and future guarantors, if any, Premier, Inc. is not a guarantor under the Credit Facility.
Outstanding borrowings under the Credit Facility bear interest on a variable rate structure with borrowings bearing interest at either London Interbank Offered Rate (“LIBOR”) plus an applicable margin ranging from 1.000% to 1.500% or the prime lending rate plus an applicable margin ranging from 0.000% to 0.500%. At June 30, 2022, the weighted average interest rate on outstanding borrowings under the Credit Facility was 2.178%. The Co-Borrowers are required to pay a commitment fee ranging from 0.100% to 0.200% per annum on the actual daily unused amount of commitments under the Credit Facility. At June 30, 2022, the commitment fee was 0.100%. The Credit Facility contains customary representations and warranties as well as customary affirmative and negative covenants, including, among others, limitations on liens, indebtedness, fundamental changes, dispositions, restricted payments and investments. Premier GP was in compliance with all such covenants at June 30, 2022. The Credit Facility also contains customary events of default, including cross-defaults of any indebtedness or guarantees in excess of $75.0 million. If any event of default occurs and is continuing, the administrative agent under the Credit Facility may, with the consent, or shall, at the request of a majority of the lenders under the Credit Facility, terminate the commitments and declare all of the amounts owed under the Credit Facility to be immediately due and payable.
Proceeds from borrowings under the Credit Facility may generally be used to finance ongoing working capital requirements, including permitted acquisitions, repurchases of Class A common stock pursuant to any then-existing stock repurchase programs, dividend payments, if and when declared, and other general corporate activities. During the year ended June 30, 2022, the Company borrowed $325.0 million and repaid $250.0 million of borrowings under the Credit Facility. The Company had $150.0 million in outstanding borrowings under the Credit Facility at June 30, 2022 with $849.9 million of available borrowing capacity after reductions for outstanding borrowings and outstanding letters of credit.
During the year ended June 30, 2022, interest expense on borrowings under the Credit Facility was $2.8 million and interest paid during the period was $2.6 million. During the year ended June 30, 2021 interest expense on borrowings under the Credit Facility and interest paid during the period was $2.2 million
Notes Payable
Notes Payable to Former Limited Partners
At June 30, 2022, the Company had $299.0 million of notes payable to former limited partners, net of discounts on notes payable of $9.1 million, of which $97.8 million was recorded to current portion of notes payable to former limited partners in the accompanying Consolidated Balance Sheets. At June 30, 2021, the Company had $394.9 million of notes payable to former limited partners, net of discounts on notes payable of $15.8 million, of which $95.9 million was recorded to current portion of notes payable to former limited partners in the accompanying Consolidated Balance Sheets. The notes payable to former limited
110
partners were issued in connection with the early termination of the TRA as part of the August 2020 Restructuring. Although the notes payable to former limited partners are non-interest bearing, pursuant to GAAP requirements, they were recorded net of imputed interest at a fixed annual rate of 1.8%. During the year ended June 30, 2022, the Company paid $102.7 million to members including imputed interest of $6.7 million. During the year ended June 30, 2021, the Company paid $51.3 million to members including imputed interest of $7.3 million.
Other
At June 30, 2022 and 2021, the Company had $5.3 million and $8.6 million in other notes payable, respectively, of which $3.1 million and $3.3 million, respectively, were included in current portion of long-term debt in the accompanying Consolidated Balance Sheets. Other notes payable do not bear interest and generally have stated maturities of to five years from their date of issuance.
Future minimum principal payments on the notes as of June 30, 2022 are as follows (in thousands):
| 2023 | $ | 105,738 | |||
| 2024 | 104,231 | ||||
| 2025 | 103,419 | ||||
| 2026 | — | ||||
| 2027 | — | ||||
| Total principal payments | $ | 313,388 | |||
(11) REDEEMABLE LIMITED PARTNERS' CAPITAL
The fair value of redeemable limited partners’ capital was reclassified from temporary equity in the mezzanine section of the Consolidated Balance Sheets to additional paid in capital as a component of permanent equity at July 31, 2020. As a result, there were no adjustments to the fair value of redeemable limited partners’ capital for the year ended June 30, 2022.
For the years ended June 30, 2021 and 2020, the Company recorded adjustments of $(26.7) million and $468.3 million, respectively, to the fair value of redeemable limited partners’ capital as an adjustment of redeemable limited partners’ capital to redemption amount in the accompanying Consolidated Statements of Income and Comprehensive Income. Subsequent to July 31, 2020, there were no adjustments to the fair value of redeemable limited partners’ capital recorded in the accompanying Consolidated Statements of Income and Comprehensive Income.
111
The table below provides a summary of the changes in the redeemable limited partners’ capital for the years ended June 30, 2021 and 2020 (in thousands). There were no changes in redeemable limited partners’ capital for the year ended June 30, 2022.
| Receivables From Limited Partners | Redeemable Limited Partners’ Capital | Total Redeemable Limited Partners’ Capital | |||||||||||||||
| June 30, 2019 | $ | (1,204) | $ | 2,524,474 | $ | 2,523,270 | |||||||||||
| Distributions applied to receivables from limited partners | 209 | — | 209 | ||||||||||||||
| Redemption of limited partners | — | (1,372) | (1,372) | ||||||||||||||
| Net income attributable to non-controlling interest in Premier LP | — | 161,816 | 161,816 | ||||||||||||||
| Non-controlling interest due to acquisition | — | 9,004 | 9,004 | ||||||||||||||
| Distributions to limited partners | — | (43,714) | (43,714) | ||||||||||||||
| Exchange of Class B common units for Class A common stock by member owners | — | (460,593) | (460,593) | ||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | (468,311) | (468,311) | ||||||||||||||
| June 30, 2020 | (995) | 1,721,304 | 1,720,309 | ||||||||||||||
| Distributions applied to receivables from limited partners | 141 | — | 141 | ||||||||||||||
| Net income attributable to non-controlling interest in Premier LP | — | 11,845 | 11,845 | ||||||||||||||
| Distributions to limited partners | — | (1,936) | (1,936) | ||||||||||||||
| Exchange of Class B common units for Class A common stock by member owners | — | (2,437) | (2,437) | ||||||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | — | 26,685 | 26,685 | ||||||||||||||
| Reclassification to permanent equity | 854 | (1,755,461) | (1,754,607) | ||||||||||||||
| June 30, 2021 | $ | — | $ | — | $ | — | |||||||||||
(12) STOCKHOLDERS' EQUITY
As of June 30, 2022, there were 118,052,235 shares of the Company’s Class A common stock, par value $0.01 per share, outstanding.
On August 5, 2021, the Company’s Board of Directors authorized the repurchase of up to $250.0 million of our outstanding Class A common stock during fiscal year 2022 through open market purchases or privately negotiated transactions. As of June 30, 2022, the Company completed its stock repurchase program and purchased approximately 6.4 million shares of Class A common stock at an average price of $38.88 per share for a total purchase price of $250.0 million.
Holders of Class A common stock are entitled to (i) one vote for each share held of record on all matters submitted to a vote of stockholders, (ii) receive dividends, when and if declared by the Board of Directors out of funds legally available, subject to any statutory or contractual restrictions on the payment of dividends and subject to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock or any class of series of stock having a preference over or the right to participate with the Class A common stock with respect to the payment of dividends or other distributions and (iii) receive pro rata, based on the number of shares of Class A common stock held, the remaining assets available for distribution upon the dissolution or liquidation of Premier, after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any.
The Company paid quarterly cash dividends of $0.20 per share on outstanding shares of Class A common stock to stockholders on each of September 15, 2021, December 15, 2021, March 15, 2022 and June 15, 2022. On August 4, 2022, the Board of Directors declared a quarterly cash dividend of $0.21 per share, payable on September 15, 2022 to stockholders of record on September 1, 2022.
(13) EARNINGS PER SHARE
Basic earnings per share is computed by dividing net income attributable to stockholders by the weighted average number of shares of common stock outstanding for the period. Net income attributable to stockholders includes the adjustment recorded in the period to reflect redeemable limited partners’ capital at the redemption amount, which was due to the exchange benefit obtained by former limited partners through the ownership of Class B common units, which were canceled in conjunction with the August 2020 Restructuring. Except when the effect would be anti-dilutive, the diluted earnings per share calculation, which is calculated using the treasury stock method, includes the impact of all potentially issuable dilutive shares of Class A common
112
stock and the effect of the assumed redemption of Class B common units through the issuance of Class A common shares.
The following table provides a reconciliation of the numerator and denominator used for basic and diluted earnings per share (in thousands, except per share amounts):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Numerator for basic earnings per share: | |||||||||||||||||
Net income from continuing operations attributable to stockholders (a) | $ | 265,867 | $ | 260,837 | $ | 598,119 | |||||||||||
| Net income from discontinued operations attributable to stockholders | — | — | 556 | ||||||||||||||
| Net income attributable to stockholders | $ | 265,867 | $ | 260,837 | $ | 598,675 | |||||||||||
| Numerator for diluted earnings per share: | |||||||||||||||||
Net income from continuing operations attributable to stockholders (a) | $ | 265,867 | $ | 260,837 | $ | 598,119 | |||||||||||
| Adjustment of redeemable limited partners’ capital to redemption amount | — | — | (468,311) | ||||||||||||||
| Net income from continuing operations attributable to non-controlling interest | — | — | 161,318 | ||||||||||||||
| Net income from continuing operations | 265,867 | 260,837 | 291,126 | ||||||||||||||
Tax effect on Premier, Inc. net income (b) (c) | — | — | (40,154) | ||||||||||||||
| Adjusted net income from continuing operations attributable to stockholders | $ | 265,867 | $ | 260,837 | $ | 250,972 | |||||||||||
| Net income from discontinued operations attributable to stockholders | $ | — | $ | — | $ | 556 | |||||||||||
| Net income from discontinued operations attributable to non-controlling interest | — | — | 498 | ||||||||||||||
| Adjusted net income from discontinued operations attributable to stockholders | $ | — | $ | — | $ | 1,054 | |||||||||||
| Adjusted net income attributable to stockholders | $ | 265,867 | $ | 260,837 | $ | 252,026 | |||||||||||
| Denominator for earnings per share: | |||||||||||||||||
Basic weighted average shares outstanding (d) | 120,220 | 116,527 | 67,035 | ||||||||||||||
Effect of dilutive securities: (e) | |||||||||||||||||
| Stock options | 206 | 301 | 329 | ||||||||||||||
| Restricted stock | 510 | 376 | 248 | ||||||||||||||
| Performance share awards | 732 | 328 | 67 | ||||||||||||||
| Class B shares outstanding | — | — | 55,935 | ||||||||||||||
| Diluted weighted average shares and assumed conversions | 121,668 | 117,532 | 123,614 | ||||||||||||||
| Basic earnings per share: | |||||||||||||||||
| Basic earnings per share from continuing operations | $ | 2.21 | $ | 2.24 | $ | 8.92 | |||||||||||
| Basic earnings per share from discontinued operations | — | — | 0.01 | ||||||||||||||
| Basic earnings per share attributable to stockholders | $ | 2.21 | $ | 2.24 | $ | 8.93 | |||||||||||
| Diluted earnings per share: | |||||||||||||||||
| Diluted earnings per share from continuing operations | $ | 2.19 | $ | 2.22 | $ | 2.03 | |||||||||||
| Diluted earnings per share from discontinued operations | — | — | 0.01 | ||||||||||||||
| Diluted earnings per share attributable to stockholders | $ | 2.19 | $ | 2.22 | $ | 2.04 | |||||||||||
_________________________________
113
(a)Net income from continuing operations attributable to stockholders was calculated as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Net income from continuing operations | $ | 268,318 | $ | 304,584 | $ | 291,126 | |||||||||||
| Net income from continuing operations attributable to non-controlling interest | (2,451) | (17,062) | (161,318) | ||||||||||||||
| Adjustment of redeemable limited partners’ capital to redemption amount | — | (26,685) | 468,311 | ||||||||||||||
| Net income from continuing operations attributable to stockholders | $ | 265,867 | $ | 260,837 | $ | 598,119 | |||||||||||
(b)For the years ended June 30, 2022 and 2021, the tax expense related to Premier, Inc. retaining the portion of net income from continuing operations attributable to income from non-controlling interest was calculated as a component of the income tax provision for the years ended June 30, 2022 and 2021.
(c)For the year ended June 30, 2020, tax effect on Premier, Inc. net income represents income tax expense related to Premier, Inc. retaining the portion of net income attributable to income from non-controlling interest in Premier LP for the purpose of diluted earnings per share.
(d)Weighted average number of common shares used for basic earnings per share excludes the impact of all potentially issuable dilutive shares of Class A common stock and Class B shares outstanding for the years ended June 30, 2021 and 2020. For the year ended June 30, 2022, there were no Class B shares outstanding as all of the issued and outstanding Class B shares were canceled in conjunction with the August 2020 Restructuring.
(e)For the year ended June 30, 2022, the effect of 0.6 million stock options and restricted stock units was excluded from diluted weighted average shares outstanding as it had an anti-dilutive effect.
For the year ended June 30, 2021, the effect of 1.8 million stock options and restricted stock units and 5.6 million Class B common units were excluded from diluted weighted average shares outstanding as they had an anti-dilutive effect and the effect of less than 0.1 million performance share awards was excluded from diluted weighted average shares outstanding as the awards had not satisfied the applicable performance criteria at the end of the period.
For the year ended June 30, 2020, the effect of 0.8 million stock options and restricted stock units were excluded from diluted weighted average shares outstanding as they had an anti-dilutive effect.
(14) STOCK-BASED COMPENSATION
Stock-based compensation expense is recognized over the requisite service period, which generally equals the stated vesting period. The associated deferred tax benefit was calculated at a rate of 25% for the years ended June 30, 2022 and 2020 and 26% for the year ended June 30, 2021, which represents the expected effective income tax rate at the time of the compensation expense deduction and differs from the Company’s current effective income tax rate. See Note 16 - Income Taxes for further information.
Stock-based compensation expense and the resulting deferred tax benefits were as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Pre-tax stock-based compensation expense | $ | 46,229 | $ | 35,425 | $ | 20,706 | |||||||||||
Deferred tax benefit (a) | 8,787 | 6,167 | 3,014 | ||||||||||||||
| Total stock-based compensation expense, net of tax | $ | 37,442 | $ | 29,258 | $ | 17,692 | |||||||||||
_________________________________
(a)For the years ended June 30, 2022 and 2021, the deferred tax benefit was reduced by $3.0 million attributable to stock-based compensation expense that is nondeductible for tax purposes pursuant to Section 162(m) as amended by the Tax Cuts and Jobs Act of 2017.
Premier 2013 Equity Incentive Plan
The Premier 2013 Equity Incentive Plan, as amended and restated (and including any further amendments thereto, the “2013 Equity Incentive Plan”) provides for grants of up to 14.8 million shares of Class A common stock, all of which are eligible to be issued as non-qualified stock options, incentive stock options, stock appreciation rights, restricted stock, restricted stock units or performance share awards. As of June 30, 2022, there were approximately 4.7 million shares available for grant under the 2013 Equity Incentive Plan.
114
The following table includes information related to restricted stock, performance share awards and stock options for the year ended June 30, 2022:
| Restricted Stock | Performance Share Awards | Stock Options | ||||||||||||||||||||||||
| Number of Awards | Weighted Average Fair Value at Grant Date | Number of Awards | Weighted Average Fair Value at Grant Date | Number of Options | Weighted Average Exercise Price | |||||||||||||||||||||
| Outstanding at June 30, 2021 | 990,301 | $ | 35.27 | 1,731,002 | $ | 35.56 | 2,163,006 | $ | 30.32 | |||||||||||||||||
| Granted | 658,389 | 37.74 | 651,392 | 37.22 | — | — | ||||||||||||||||||||
| Vested/exercised | (295,854) | 40.16 | (588,142) | 43.74 | (1,252,486) | 30.21 | ||||||||||||||||||||
| Forfeited | (151,706) | 33.96 | (215,457) | 32.18 | (14,166) | 36.77 | ||||||||||||||||||||
| Outstanding at June 30, 2022 | 1,201,130 | $ | 35.59 | 1,578,795 | $ | 33.66 | 896,354 | $ | 30.38 | |||||||||||||||||
| Stock options outstanding and exercisable at June 30, 2022 | 896,354 | $ | 30.38 | |||||||||||||||||||||||
Restricted stock units and restricted stock awards issued and outstanding generally vest over a three-year period for employees and a one-year period for directors. Performance share awards issued and outstanding generally vest over a three-year period if performance targets are met. Stock options have a term of ten years from the date of grant. Vested stock options will generally expire either twelve months after an employee’s termination with the Company or 90 days after an employee’s termination with the Company, depending on the termination circumstances. Stock options generally vest in equal annual installments over three years.
Unrecognized stock-based compensation expense at June 30, 2022 was as follows (in thousands). At June 30, 2022, there was no unrecognized stock-based compensation expense for outstanding stock options.
| Unrecognized Stock-Based Compensation Expense | Weighted Average Amortization Period | ||||||||||
| Restricted stock | $ | 22,082 | 1.9 years | ||||||||
| Performance share awards | 20,553 | 1.6 years | |||||||||
| Total unrecognized stock-based compensation expense | $ | 42,635 | 1.8 years | ||||||||
The aggregate intrinsic value of stock options at June 30, 2022 was as follows (in thousands):
| Intrinsic Value of Stock Options | |||||
| Outstanding and exercisable | $ | 4,768 | |||
| Exercised during the year ended June 30, 2022 | 9,765 | ||||
(15) POST-RETIREMENT BENEFITS
The Company maintains a defined contribution 401(k) retirement savings plan which covers employees who meet certain age and service requirements. This plan allows for employee contributions of up to 30% and matching employer contributions of up to 4% of the total contributions, not to exceed certain limits. The Company’s 401(k) expense related to such matching of employee contributions was $12.1 million, $11.2 million and $10.1 million for the years ended June 30, 2022, 2021 and 2020, respectively.
The Company also maintains a non-qualified deferred compensation plan for the benefit of eligible employees. This plan is designed to permit employee deferrals in excess of certain tax limits and provides for discretionary employer contributions in excess of certain tax limits.
(16) INCOME TAXES
At the consummation of the Subsidiary Reorganization on December 1, 2021, the Company recorded a one-time deferred tax benefit of $33.5 million, primarily driven by deferred tax remeasurement due to tax rate changes and a valuation allowance release.
115
Significant components of consolidated income tax expense (benefit) are as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Current: | |||||||||||||||||
| Federal | $ | 864 | $ | 22,356 | $ | 11,394 | |||||||||||
| State | 926 | 7,393 | 12,545 | ||||||||||||||
| Total current tax expense | 1,790 | 29,749 | 23,939 | ||||||||||||||
| Deferred: | |||||||||||||||||
| Federal | 49,335 | 22,165 | 35,768 | ||||||||||||||
| State | 7,457 | (105,857) | 32,854 | ||||||||||||||
| Total deferred tax expense (benefit) | 56,792 | (83,692) | 68,622 | ||||||||||||||
| Total income tax expense (benefit) | $ | 58,582 | $ | (53,943) | $ | 92,561 | |||||||||||
The reconciliation between the Company’s income tax expense (benefit) and taxes computed at the federal statutory tax rate of 21.0% for fiscal years ended June 30, 2022, 2021 and 2020, is as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Tax at federal statutory rate | $ | 68,649 | $ | 52,635 | $ | 80,814 | |||||||||||
| Partnership income not subject to tax | (701) | (4,375) | (40,154) | ||||||||||||||
| State taxes (net of federal benefit) | 14,138 | 9,880 | 7,072 | ||||||||||||||
| Remeasurement adjustments and other permanent items | 8,118 | 7,124 | (1,570) | ||||||||||||||
| Change in valuation allowance | (31,361) | (25,328) | 12,472 | ||||||||||||||
| Deferred tax remeasurement | (242) | (113,213) | 34,447 | ||||||||||||||
| Uncertain tax position | 842 | 1,293 | 7,472 | ||||||||||||||
| Change in tax status | — | 19,514 | — | ||||||||||||||
| Other | (861) | (1,473) | (7,992) | ||||||||||||||
| Total income tax expense (benefit) | $ | 58,582 | $ | (53,943) | $ | 92,561 | |||||||||||
| Effective tax rate | 17.9 | % | (21.5) | % | 24.1 | % | |||||||||||
The fiscal year 2022 effective tax rate of 17.9% differs from the statutory income tax rate of 21.0% largely driven by the aforementioned one-time deferred tax remeasurement and valuation allowance release as a result of the Subsidiary Reorganization.
The fiscal year 2021 effective tax rate of (21.5)% differs from the statutory income tax rate of 21.0% primarily driven by the consummation of the merger on August 11, 2020. The Company simplified its tax structure, resulting in the Company and its subsidiaries forming one consolidated filing group for federal income tax purposes. As a result, the Company recorded a one-time deferred tax benefit of $108.8 million, primarily driven by deferred tax remeasurement due to tax rate changes and a valuation allowance release.
The fiscal year 2020 effective tax rate of 24.1% differs from the statutory income tax rate of 21.0% primarily due to the remeasurement of deferred tax assets and liabilities as a result of a change to the State of North Carolina income tax law, partially offset by Premier LP income which is not subject to federal, state and local income taxes.
116
Deferred Income Taxes
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities as of June 30, 2022 and 2021 are presented below (in thousands):
| June 30, | |||||||||||
| 2022 | 2021 | ||||||||||
| Deferred tax asset | |||||||||||
| Purchased intangible assets and depreciation | $ | 631,415 | $ | 689,810 | |||||||
| Stock compensation | 15,125 | 16,943 | |||||||||
| Accrued expenses | 49,161 | 41,474 | |||||||||
| Net operating losses and credits | 50,742 | 66,782 | |||||||||
| Other | 5,787 | 22,513 | |||||||||
| Total deferred tax assets | 752,230 | 837,522 | |||||||||
| Valuation allowance for deferred tax assets | (4,552) | (35,913) | |||||||||
| Net deferred tax assets | 747,678 | 801,609 | |||||||||
| Deferred tax liability | |||||||||||
| Other liabilities | (22,646) | (19,785) | |||||||||
| Net deferred tax asset | $ | 725,032 | $ | 781,824 | |||||||
As of June 30, 2022 and 2021, the Company had net deferred tax assets of $725.0 million and $781.8 million, respectively. The decrease is largely attributable to deferred tax assets recorded in connection with the final exchange of Class B common units pursuant to the August 2020 Restructuring.
At June 30, 2022, the Company had federal and state net operating loss carryforwards of $156.3 million and $130.4 million, respectively, primarily attributable to PHSI and PSCI. The resulting federal and state deferred tax assets are $32.7 million and $7.3 million, respectively. The federal and state net operating loss carryforwards generated prior to fiscal year 2019 expire between the years ending June 30, 2022 through June 30, 2038 while the net operating losses generated in fiscal year 2019 and beyond can be carried forward indefinitely, until utilized. A valuation allowance was established for federal and state losses as the Company believes it is more likely than not that a portion of these losses will not be realized in the near future.
At June 30, 2022, the Company had federal research and development credit carryforwards of $12.4 million. The federal credit carryforwards expire at various times between the years ended June 30, 2023 through June 30, 2040, until utilized. As a result of the Subsidiary Reorganization, the Company believes it is more likely than not that the federal and state credit carryforwards will be realized in the near future, so the previously recorded valuation allowance was released during the year ended June 30, 2022.
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and income tax purposes. The Company assessed the future realization of the tax benefit of its existing deferred tax assets and concluded that it is more likely than not that a portion of the deferred tax assets will not be realized in the future. As a result, the Company recorded a valuation allowance of $4.6 million against its deferred tax assets at June 30, 2022. The valuation allowance decreased by $31.3 million from the $35.9 million valuation allowance recorded as of June 30, 2021. The decrease is primarily driven by the utilization of the net operating loss carryforward as a result of the aforementioned Subsidiary Reorganization.
117
Unrecognized Tax Benefits
The Company recognizes income tax benefits for those income tax positions determined more likely than not to be sustained upon examination, based on the technical merits of the positions. The reserve for uncertain income tax positions is included in other liabilities in the Consolidated Balance Sheets. A reconciliation of the beginning and ending gross amounts of the Company’s uncertain tax position reserves for the years ended June 30, 2022, 2021 and 2020 are as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Beginning of year balance | $ | 16,704 | $ | 15,596 | $ | 8,266 | |||||||||||
| Increases in prior period tax positions | 120 | 111 | 7,734 | ||||||||||||||
| Decreases in prior period tax positions | (63) | — | (48) | ||||||||||||||
| Reductions on settlements and lapse in statute of limitations | (21) | (27) | (2,276) | ||||||||||||||
| Increases in current period tax positions | 384 | 1,024 | 1,920 | ||||||||||||||
| End of year balance | $ | 17,124 | $ | 16,704 | $ | 15,596 | |||||||||||
If the Company were to recognize the benefits of these uncertain tax positions, the income tax provision would be impacted by $15.6 million, $14.8 million and $12.8 million, including interest and penalties and net of the federal and state benefit for income taxes, for the years ended June 30, 2022, 2021 and 2020, respectively. The Company recognizes interest and penalties accrued on uncertain income tax positions as part of the income tax provision. The amount of accrued interest and penalties was $4.4 million, $4.1 million, and $2.5 million at 2022, 2021 and 2020, respectively.
Federal tax returns for tax years June 30, 2018 through 2021 remain open as of June 30, 2022. The Company is subject to ongoing state and local examinations for various periods. Activity related to these examinations did not have a material impact on the Company’s financial position or results of operations.
The Company made cash tax payments of $3.1 million and $44.0 million during the years ended June 30, 2022 and 2021, respectively.
(17) RELATED PARTY TRANSACTIONS
The Company’s 49% ownership share of net income of FFF, which was acquired on July 26, 2016, included in equity in net income of unconsolidated affiliates in the accompanying Consolidated Statements of Income and Comprehensive Income was $16.6 million, $11.3 million and $12.3 million for the years ended June 30, 2022, 2021 and 2020, respectively. The Company maintains group purchasing agreements with FFF and receives administrative fees for purchases made by the Company’s members and other customers pursuant to those agreements. Net administrative fees revenue recorded from purchases under those agreements was $6.3 million, $6.0 million and $7.4 million during the years ended June 30, 2022, 2021 and 2020, respectively.
(18) COMMITMENTS AND CONTINGENCIES
Operating Leases
Operating lease expense was $10.1 million, $10.8 million and $12.3 million for the years ended June 30, 2022, 2021 and 2020, respectively. As of June 30, 2022, the weighted average remaining lease term was 3.8 years and the weighted average discount rate was 4%.
118
Future minimum lease payments under noncancelable operating leases with initial lease terms in excess of one year were as follows (in thousands):
| 2023 | $ | 12,131 | |||
| 2024 | 12,267 | ||||
| 2025 | 12,301 | ||||
| 2026 | 9,005 | ||||
| 2027 | 1,323 | ||||
| Total future minimum lease payments | 47,027 | ||||
| Less: imputed interest | 3,445 | ||||
| $ | 43,582 | ||||
_________________________________
(a)As of June 30, 2022, total operating lease liabilities included $10.6 million within other liabilities, current in the Consolidated Balance Sheets.
Other Matters
The Company is not currently involved in any litigation it believes to be material. The Company is periodically involved in litigation, arising in the ordinary course of business or otherwise, which from time to time may include stockholder derivative or other similar litigation, claims relating to commercial, product liability, tort and personal injury, employment, antitrust, intellectual property, or other regulatory matters. If current or future government regulations, including but not limited to those with respect to antitrust or healthcare laws, are interpreted or enforced in a manner adverse to the Company or its business, the Company may be subject to regulatory inquiries or investigations, enforcement actions, penalties and other material limitations which could have a material adverse effect on the Company’s business, financial condition and results of operations.
(19) SEGMENTS
The Company delivers its solutions and manages its business through two reportable business segments, the Supply Chain Services segment and the Performance Services segment. The Supply Chain Services segment includes the Company’s GPO, supply chain co-management, purchased services and direct sourcing activities. The Performance Services segment consists of three sub-brands: PINC AI, the Company’s technology and services platform; Contigo Health, the Company’s direct-to-employer business; and Remitra, the Company’s digital invoicing and payables business.
The following table presents disaggregated revenue by reportable business segment and underlying source (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Net revenue: | |||||||||||||||||
| Supply Chain Services | |||||||||||||||||
| Net administrative fees | $ | 601,128 | $ | 572,700 | $ | 670,593 | |||||||||||
| Software licenses, other services and support | 37,312 | 26,812 | 12,225 | ||||||||||||||
| Services and software licenses | 638,440 | 599,512 | 682,818 | ||||||||||||||
| Products | 393,506 | 744,122 | 269,945 | ||||||||||||||
Total Supply Chain Services (a)(b) | 1,031,946 | 1,343,634 | 952,763 | ||||||||||||||
| Performance Services | |||||||||||||||||
| Software licenses, other services and support | |||||||||||||||||
| SaaS-based products subscriptions | 193,586 | 198,512 | 203,390 | ||||||||||||||
| Consulting services | 64,087 | 58,851 | 56,936 | ||||||||||||||
| Software licenses | 65,621 | 56,157 | 42,556 | ||||||||||||||
| Other | 77,689 | 63,998 | 43,947 | ||||||||||||||
Total Performance Services (a) | 400,983 | 377,518 | 346,829 | ||||||||||||||
| Total segment net revenue | 1,432,929 | 1,721,152 | 1,299,592 | ||||||||||||||
Eliminations (a) | (28) | — | — | ||||||||||||||
| Net revenue | $ | 1,432,901 | $ | 1,721,152 | $ | 1,299,592 | |||||||||||
_________________________________
119
(a)Includes intersegment revenue that is eliminated in consolidation. Intersegment revenue is not separately identified in Segments as the amounts are not material.
(b)Consolidated net revenues for the fiscal year ended June 30, 2021 includes revenue generated from our largest customer, a non-healthcare customer, which accounted for approximately 15% of our consolidated net revenues. The significant increase in revenue generated from our largest customer in the fiscal year ended June 30, 2021 is due to the increase in products revenue primarily as of result of the COVID-19 pandemic.
Additional segment information related to depreciation and amortization expense, capital expenditures and total assets was as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
Depreciation and amortization expense (a): | 2022 | 2021 | 2020 | ||||||||||||||
| Supply Chain Services | $ | 55,424 | $ | 37,073 | $ | 25,968 | |||||||||||
| Performance Services | 64,674 | 75,391 | 118,556 | ||||||||||||||
| Corporate | 9,009 | 8,598 | 8,303 | ||||||||||||||
| Total depreciation and amortization expense | $ | 129,107 | $ | 121,062 | $ | 152,827 | |||||||||||
| Capital expenditures: | |||||||||||||||||
| Supply Chain Services | $ | 29,677 | $ | 10,408 | $ | 7,143 | |||||||||||
| Performance Services | 51,298 | 72,068 | 78,231 | ||||||||||||||
| Corporate | 6,465 | 6,400 | 9,023 | ||||||||||||||
| Total capital expenditures | $ | 87,440 | $ | 88,876 | $ | 94,397 | |||||||||||
| Year Ended June 30, | |||||||||||
| Total assets: | 2022 | 2021 | |||||||||
| Supply Chain Services | $ | 1,406,108 | $ | 1,550,300 | |||||||
| Performance Services | 1,054,687 | 1,043,608 | |||||||||
| Corporate | 896,336 | 928,939 | |||||||||
| Total assets | 3,357,131 | 3,522,847 | |||||||||
Eliminations (b) | (4) | 51 | |||||||||
| Total assets, net | $ | 3,357,127 | $ | 3,522,898 | |||||||
_________________________________
(a)Includes amortization of purchased intangible assets.
(b)Includes eliminations of intersegment transactions which occur during the ordinary course of business.
The Company uses Segment Adjusted EBITDA (a financial measure not determined in accordance with generally accepted accounting principles (“Non-GAAP”)) as its primary measure of profit or loss to assess segment performance and to determine the allocation of resources. The Company also uses Segment Adjusted EBITDA to facilitate the comparison of the segment operating performance on a consistent basis from period to period. The Company defines Segment Adjusted EBITDA as the segment’s net revenue less cost of revenue and operating expenses directly attributable to the segment excluding depreciation and amortization, amortization of purchased intangible assets, merger and acquisition-related expenses, and non-recurring or non-cash items, and including equity in net income of unconsolidated affiliates. Operating expenses directly attributable to the segment include expenses associated with sales and marketing, general and administrative, and product development activities specific to the operation of each segment. General and administrative corporate expenses that are not specific to a particular segment are not included in the calculation of Segment Adjusted EBITDA. Segment Adjusted EBITDA also excludes any income and expense that has been classified as discontinued operations.
For more information on Segment Adjusted EBITDA and the use of Non-GAAP financial measures, see “Our Use of Non-GAAP Financial Measures” within Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
120
A reconciliation of income before income taxes to the unaudited Segment Adjusted EBITDA, a Non-GAAP financial measure, is as follows (in thousands):
| Year Ended June 30, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Income before income taxes | $ | 326,900 | $ | 250,641 | $ | 383,687 | |||||||||||
Equity in net income of unconsolidated affiliates (a) | (23,505) | (21,073) | (12,537) | ||||||||||||||
| Interest expense, net | 11,142 | 11,964 | 11,313 | ||||||||||||||
(Gain) loss on FFF put and call rights (b) | (64,110) | 27,352 | (4,690) | ||||||||||||||
| Other expense (income), net | 9,646 | (11,967) | (4,153) | ||||||||||||||
| Operating income | 260,073 | 256,917 | 373,620 | ||||||||||||||
| Depreciation and amortization | 85,171 | 76,309 | 97,297 | ||||||||||||||
| Amortization of purchased intangible assets | 43,936 | 44,753 | 55,530 | ||||||||||||||
Stock-based compensation (c) | 46,809 | 35,915 | 21,132 | ||||||||||||||
| Acquisition- and disposition-related expenses | 11,453 | 18,095 | 19,319 | ||||||||||||||
| Strategic initiative and financial restructuring-related expenses | 18,005 | 6,990 | 4,228 | ||||||||||||||
Remeasurement of tax receivable agreement liabilities (d) | — | — | (24,584) | ||||||||||||||
Equity in net income of unconsolidated affiliates (a) | 23,505 | 21,073 | 12,537 | ||||||||||||||
Deferred compensation plan (expense) income (e) | (9,401) | 12,745 | 3,904 | ||||||||||||||
| Impairment of assets | 18,829 | — | — | ||||||||||||||
| Other reconciling items, net | 302 | 433 | 1,057 | ||||||||||||||
| Adjusted EBITDA | $ | 498,682 | $ | 473,230 | $ | 564,040 | |||||||||||
| Segment Adjusted EBITDA: | |||||||||||||||||
Supply Chain Services (f) | $ | 500,854 | $ | 467,868 | $ | 570,298 | |||||||||||
Performance Services (f) | 126,938 | 132,225 | 111,282 | ||||||||||||||
| Corporate | (129,110) | (126,863) | (117,540) | ||||||||||||||
| Adjusted EBITDA | $ | 498,682 | $ | 473,230 | $ | 564,040 | |||||||||||
_________________________________
(a)Refer to Note 5 - Investments for further information.
(b)Refer to Note 6 - Fair Value Measurements for more information.
(c)Represents non-cash employee stock-based compensation expense and stock purchase plan expense of $0.6 million, $0.5 million and $0.4 million for the years ended June 30, 2022, 2021 and 2020, respectively.
(d)The adjustments to TRA liabilities for the year ended June 30, 2020 are primarily attributable to decreases in the Premier, Inc. effective tax rate related to state tax liabilities and the TCJA, respectively.
(e)Represents realized and unrealized gains and losses and dividend income on deferred compensation plan assets.
(f)Includes intersegment revenue which is eliminated in consolidation.
121
(20) QUARTERLY FINANCIAL DATA (UNAUDITED)
The following tables present unaudited summarized financial data by quarter for the years ended June 30, 2022 and 2021 (in thousands, except per share data):
| First | Second | Third | Fourth | |||||||||||
| Fiscal Year 2022 | Quarter | Quarter | Quarter | Quarter | ||||||||||
| Net revenue | $ | 365,147 | $ | 379,215 | $ | 347,833 | $ | 340,706 | ||||||
| Gross profit | 211,976 | 236,500 | 212,477 | 224,086 | ||||||||||
| Net income | 121,306 | 77,232 | 39,069 | 30,711 | ||||||||||
| Net loss (income) attributable to non-controlling interest | 698 | (1,687) | (654) | (808) | ||||||||||
| Net income attributable to stockholders | 122,004 | 75,545 | 38,415 | 29,903 | ||||||||||
| Weighted average shares outstanding: | ||||||||||||||
| Basic | 122,945 | 121,181 | 118,697 | 118,001 | ||||||||||
| Diluted | 124,573 | 122,473 | 119,813 | 119,760 | ||||||||||
| Earnings per share attributable to stockholders: | ||||||||||||||
| Basic | $ | 0.99 | $ | 0.62 | $ | 0.32 | $ | 0.25 | ||||||
| Diluted | $ | 0.97 | $ | 0.62 | $ | 0.32 | $ | 0.25 | ||||||
| First | Second | Third | Fourth | |||||||||||
| Fiscal Year 2021 | Quarter | Quarter | Quarter | Quarter | ||||||||||
| Net revenue | $ | 346,887 | $ | 422,827 | $ | 469,923 | $ | 481,515 | ||||||
| Gross profit | 194,709 | 210,983 | 211,807 | 219,835 | ||||||||||
| Net income | 157,528 | 44,904 | 51,444 | 50,708 | ||||||||||
| Net income attributable to non-controlling interest | (11,845) | (935) | (3,123) | (1,159) | ||||||||||
| Adjustment of redeemable limited partners' capital to redemption amount | (26,685) | — | — | — | ||||||||||
| Net income attributable to stockholders | 118,998 | 43,969 | 48,321 | 49,549 | ||||||||||
| Weighted average shares outstanding: | ||||||||||||||
| Basic | 99,575 | 122,127 | 122,254 | 122,341 | ||||||||||
| Diluted | 100,130 | 122,919 | 123,116 | 124,055 | ||||||||||
| Earnings per share attributable to stockholders: | ||||||||||||||
| Basic | $ | 1.20 | $ | 0.36 | $ | 0.40 | $ | 0.41 | ||||||
| Diluted | $ | 1.19 | $ | 0.36 | $ | 0.39 | $ | 0.40 | ||||||
122
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) that are designed to ensure that information required to be disclosed in our reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosures. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives.
As of the end of the period covered by this Annual Report, our chief executive officer and chief financial officer carried out an evaluation of the effectiveness of our disclosure controls and procedures. Based upon this evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective as of June 30, 2022.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on its financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Our chief executive officer and chief financial officer conducted an assessment of the effectiveness of our internal control over financial reporting as of June 30, 2022. In making this assessment, the chief executive officer and chief financial officer used the criteria set forth in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, the COSO framework. Based upon this evaluation, our chief executive officer and chief financial officer concluded that, as of June 30, 2022, our internal control over financial reporting was effective.
The effectiveness of our internal control over financial reporting as of June 30, 2022 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act) during the quarter ended June 30, 2022, that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
Item 9B. Other Information
None.
123
PART III
We expect to file a definitive proxy statement relating to our 2022 Annual Meeting of Stockholders with the SEC pursuant to Regulation 14A, not later than 120 days after the end of our most recent fiscal year. Accordingly, certain information required by Part III of this Annual Report has been omitted under General Instruction G(3) to Form 10-K. Only the information from the definitive proxy statement that specifically addresses disclosure requirements of Items 10-14 below is incorporated by reference.
Item 10. Directors, Executive Officers and Corporate Governance
We will provide information that is responsive to this Item 10 in our definitive proxy statement for our 2022 Annual Meeting of Stockholders or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report, in either case under the captions “Item 1 - Election of Directors,” “Corporate Governance and Board Structure,” “Delinquent Section 16(a) Reports” and “Executive Officers,” and possibly elsewhere therein. That information is incorporated in this Item 10 by reference.
Code of Ethics
We maintain a Corporate Code of Conduct for all of our employees and officers, including the principal executive officer, principal financial officer, and principal accounting officer or controller, or persons performing similar functions, and, where applicable, to directors. In addition, the Board of Directors is subject to a separate Board Code of Ethics and Board Conflict of Interest Policy (collectively, the “Board Codes”). The Corporate Code of Conduct, along with the Board Codes, can be found on our Investor Relations website at investors.premierinc.com under “Corporate Governance-Governance Documents.” A copy of the Corporate Code of Conduct is available to any stockholder who requests it by writing to Investor Relations, Premier, Inc., 13034 Ballantyne Corporate Place, Charlotte, North Carolina 28277. We will disclose any substantive amendments to, or waivers (for directors or executive officers) from, certain provisions (relating to one or more elements of Item 4.06(b) of Regulation S-K) of the Corporate Code of Conduct and Board Codes on our website promptly following the date of such amendment or waiver.
Our website and information contained on it or incorporated in it are not intended to be incorporated in this Annual Report or other filings with the SEC.
Item 11. Executive Compensation
We will provide information that is responsive to this Item 11 in our definitive proxy statement for our 2022 Annual Meeting of Stockholders or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report, in either case under the captions “Executive Compensation” and “Corporate Governance and Board Structure,” and possibly elsewhere therein. That information is incorporated in this Item 11 by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
We will provide information that is responsive to this Item 12 in our definitive proxy statement for our 2022 Annual Meeting of Stockholders or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report, in either case under the caption “Security Ownership of Certain Beneficial Owners and Management” and possibly elsewhere therein. That information is incorporated in this Item 12 by reference.
Equity Compensation Plan Information
We have granted equity awards to employees and directors under the Amended and Restated Premier, Inc. 2013 Equity Incentive Plan, which initially was approved by our stockholders prior to our IPO and was approved most recently by our stockholders in December 2018. The following table sets forth certain information as of June 30, 2022 concerning the shares of Class A common stock authorized for issuance under this equity incentive plan. No shares of Class B common stock are authorized for issuance under this plan, and we have no equity compensation plans under which shares may be issued that have not been approved by our stockholders.
124
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in first column) (c) | ||||||||
| Equity compensation plans approved by security holders: | |||||||||||
| Amended and Restated Premier, Inc. 2013 Equity Incentive Plan | 3,676,279 | $30.38 | 4,669,729 | ||||||||
| Equity compensation plans not approved by security holders | n/a | n/a | n/a | ||||||||
| Total | 3,676,279 | $30.38 | 4,669,729 | ||||||||
_________________________________
(a)Assumes restricted stock unit (RSU), performance share (PSA) and stock option awards are paid at target. Actual shares awarded may be higher or lower based upon actual performance over the measurement period. For more detailed information, see Note 14 - Stock-Based Compensation to our Consolidated Financial Statements.
(b)This calculation only reflects outstanding stock option awards.
(c)As of June 30, 2022, reflects shares reserved for future grants of stock options, RSUs, RSAs, PSAs and/or other equity awards. Any shares withheld to satisfy tax withholding obligations or tendered to pay the exercise price of an option shall again be available for grant under the terms of the plan.
Item 13. Certain Relationships and Related Transactions, and Director Independence
We will provide information that is responsive to this Item 13 in our definitive proxy statement for our 2022 Annual Meeting of Stockholders or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report, in either case under the captions “Related Person Transactions,” and “Corporate Governance and Board Structure,” and possibly elsewhere therein. That information is incorporated in this Item 13 by reference.
Item 14. Principal Accounting Fees and Services
We will provide information that is responsive to this Item 14 in our definitive proxy statement for our 2022 Annual Meeting of Stockholders or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report, in either case under the caption “Item 2 - Ratification of Appointment of Independent Registered Public Accounting Firm,” and possibly elsewhere therein. That information is incorporated in this Item 14 by reference.
125
PART IV
Item 15. Exhibits and Financial Statement Schedules
Documents as part of this Report:
(a) (1) The following consolidated financial statements are filed herewith in Item 8 of Part II above.
(i) Report of Independent Registered Public Accounting Firm
(ii) Consolidated Balance Sheets
(iii) Consolidated Statements of Income and Comprehensive Income
(iv) Consolidated Statements of Stockholders' Equity (Deficit)
(v) Consolidated Statements of Cash Flows
(vi) Notes to Consolidated Financial Statements
(2) Financial Statement Schedule
Schedule II Valuation and Qualifying Accounts
| (in thousands) | Beginning Balance | Additions/(Reductions) to Expense or Other Accounts | Deductions | Ending Balance | |||||||||||||||||||
| Year ended June 30, 2022 | |||||||||||||||||||||||
| Allowance for credit losses | $ | 2,284 | 1,244 | 730 | $ | 2,798 | |||||||||||||||||
| Deferred tax assets valuation allowance | 35,913 | (31,361) | — | 4,552 | |||||||||||||||||||
| Year ended June 30, 2021 | |||||||||||||||||||||||
| Allowance for credit losses | $ | 731 | 1,883 | 330 | $ | 2,284 | |||||||||||||||||
| Deferred tax assets valuation allowance | 61,241 | (25,328) | — | 35,913 | |||||||||||||||||||
| Year ended June 30, 2020 | |||||||||||||||||||||||
| Allowance for credit losses | $ | 739 | 669 | 677 | $ | 731 | |||||||||||||||||
| Deferred tax assets valuation allowance | 48,769 | 12,472 | — | 61,241 | |||||||||||||||||||
All other supplemental schedules are omitted because of the absence of conditions under which they are required.
(3) Exhibits
The exhibits listed in the accompanying Exhibit Index at the end of this Item 15 are filed as a part of this report.
(b) Exhibits
See Exhibit Index at the end of this Item 15.
(c) Separate Financial Statements and Schedule
None.
126
EXHIBIT INDEX
| Exhibit No. | Description | |||||||
| 3.1 | ||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 4.1.1 | ||||||||
| 10.1 | ||||||||
| 10.2 | ||||||||
| 10.3 | ||||||||
| 10.3.1 | ||||||||
| 10.4 | ||||||||
| 10.5 | ||||||||
| 10.6 | Premier, Inc. Annual Incentive Compensation Plan, amended and restated effective August 5, 2020 (Incorporated by reference to Exhibit 10.8 of our Annual Report on Form 10-K filed on August 25, 2020)+ | |||||||
| 10.7 | ||||||||
| 10.8 | ||||||||
| 10.9 | ||||||||
| 10.10 | ||||||||
| 10.11 | ||||||||
| 10.12 | ||||||||
| 10.13 | ||||||||
| 10.14 | ||||||||
| 10.15 | ||||||||
127
| Exhibit No. | Description | |||||||
| 10.16 | ||||||||
| 10.17 | ||||||||
| 10.18 | ||||||||
| 10.19 | ||||||||
| 10.20 | ||||||||
| 10.21 | ||||||||
| 10.22 | ||||||||
| 21 | ||||||||
| 23 | ||||||||
| 24 | Power of Attorney (included on the signature page hereof)* | |||||||
| 31.1 | ||||||||
| 31.2 | ||||||||
| 32.1 | ||||||||
| 32.2 | ||||||||
| 101.INS | XBRL Instance Document* | |||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document* | |||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document* | |||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document* | |||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document* | |||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document* | |||||||
* Filed herewith
+ Indicates a management contract or compensatory plan or arrangement
‡ Furnished herewith
Our SEC file number for documents filed with the SEC pursuant to the Securities Exchange Act of 1934, as amended, is 001-36092. The SEC file number for our Registration Statement on Form S-1 is 333-190828.
Item 16. Form 10-K Summary
We have elected not to provide a summary.
128
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PREMIER, INC. | |||||||||||
| By: | /s/ MICHAEL J. ALKIRE | ||||||||||
| Name: | Michael J. Alkire | ||||||||||
| Title: | President and Chief Executive Officer | ||||||||||
| Date: | August 16, 2022 | ||||||||||
POWER OF ATTORNEY
Each person whose signature appears below hereby severally constitutes and appoints each of Craig S. McKasson and David L. Klatsky his/her true and lawful attorney-in-fact and agent with full power of substitution and re-substitution, for him/her in his/her name, place and stead, in any and all capacities, to sign any and all amendments to this report and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to each such attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he/she might or could do in person, hereby ratifying and confirming all that each said attorney-in-fact and agent or his substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Capacity | Date | |||||||||||||||
| /s/ MICHAEL J. ALKIRE | President and Chief Executive Officer and Director (principal executive officer) | ||||||||||||||||
Michael J. Alkire | August 16, 2022 | ||||||||||||||||
| /s/ CRAIG S. MCKASSON | Chief Administrative and Financial Officer and Senior Vice President (principal financial and accounting officer) | ||||||||||||||||
| Craig S. McKasson | August 16, 2022 | ||||||||||||||||
| /s/ JOHN T. BIGALKE | |||||||||||||||||
| John T. Bigalke | Director | August 16, 2022 | |||||||||||||||
| /s/ HELEN M. BOUDREAU | |||||||||||||||||
| Helen M. Boudreau | Director | August 16, 2022 | |||||||||||||||
| /s/ JODY R. DAVIDS | |||||||||||||||||
| Jody R. Davids | Director | August 16, 2022 | |||||||||||||||
| /s/ PETER S. FINE | |||||||||||||||||
| Peter S. Fine | Director | August 16, 2022 | |||||||||||||||
| /s/ MARC D. MILLER | |||||||||||||||||
Marc D. Miller | Director | August 16, 2022 | |||||||||||||||
| /s/ MARVIN R. O’QUINN | |||||||||||||||||
Marvin R. O'Quinn | Director | August 16, 2022 | |||||||||||||||
129
| /s/ TERRY D. SHAW | |||||||||||||||||
Terry D. Shaw | Director | August 16, 2022 | |||||||||||||||
| /s/ RICHARD J. STATUTO | |||||||||||||||||
Richard J. Statuto | Director | August 16, 2022 | |||||||||||||||
| /s/ ELLEN C. WOLF | |||||||||||||||||
Ellen C. Wolf | Director | August 16, 2022 | |||||||||||||||
130
Similar companies
See also GARTNER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also R1 RCM Inc. /DE - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (NT 10-Q 2023-09-30)
See also TERMINIX GLOBAL HOLDINGS INC - Annual report 2021 (10-K 2021-12-31) Annual report 2022 (10-Q 2022-06-30)
See also Evolent Health, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also FRANKLIN COVEY CO - Annual report 2023 (10-K 2023-08-31) Annual report 2023 (10-Q 2023-05-31)
