Lunai Bioworks Inc. - Annual Report: 2021 (Form 10-K)
United states
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL report under section 13 Or 15(d) of the securities exchange act of 1934
For the fiscal year ended June 30, 2021
☐ TRANSITION report under section 13 Or 15(d) of the securities exchange act of 1934
For the transition period from
Commission file number 000-54478
| ENOCHIAN BIOSCIENCES INC. |
| (Name of registrant in its charter) |
| Delaware | 45-2559340 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
Employer Identification No.) | |
| 2080 Century Park East Suite 906 Los Angeles, CA | 90067-2012 | |
| (Address of principal executive offices) | (Zip Code) |
+1(305) 918-1980
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered | ||
| Common Stock, par value $0.0001 per share | ENOB | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $0.0001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the last 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically, if any, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ☐ Yes ☒ No
On December 31, 2020, the aggregate market value of the voting and non-voting common equity held by non-affiliates was $82,506,916.
As of September 24, 2021, the number of shares outstanding of the registrant’s common stock, par value $0.0001 per share (the “Common Stock”) was .
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2021 Annual Meeting of stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K or will be filed by amendment.
CONTENTS
| i |
Cautionary Language Regarding Forward-Looking Statements and Industry Data
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding the plans and objectives of management for future operations and market trends and expectations. Forward-looking statements can be identified by the fact that they do not relate strictly to historical or current facts. Forward-looking statements are based upon our current assumptions, expectations and beliefs concerning future developments and their potential effect on our business. In some cases, you can identify forward-looking statements by the following words: “may,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “approximately,” “estimate,” “predict,” “project,” “potential” or the negative of these terms or other comparable terminology, although the absence of these words does not necessarily mean that a statement is not forward-looking.
Forward-looking statements include, but are not limited to, statements concerning:
| ● | Our potentially continuous incurrence of losses as a pre-clinical-stage biotechnology company with no products that have achieved regulatory approval; | |
| ● | Our ability to generate revenue if we fail to develop marketable product; | |
| ● | Our dependence on substantial additional financing to support the research, development, licensing, manufacture, and marketing of product candidates and products, and the possibility that unforeseen operational costs will arise; | |
| ● | The dilutive effect on stockholders’ ownerships interests of the Company raising capital through an equity issuance in connection with future equity financing or equity debt agreements; | |
| ● | Our dependence on the services of experts, including Dr. Serhat Gümrükcü, and third parties to research and develop product candidates in cooperation with our employees, officers, scientific advisory board, and research institutions; | |
| ● | The difficulty or impossibility of predicting future clinical trial results and regulatory outcomes of our products based upon our pre-clinical or earlier clinical trial performance; | |
| ● | The application of heightened regulatory and commercial scrutiny to our gene, cell, and immunotherapy products given their novel nature and concomitant potential for actual or perceived safety issues; | |
| ● | Our ability to compete in a rapidly developing field, and the potential impact to our financial condition, product marketability, and operational capacities of a competitor receiving regulatory approval before us, or a competitor developing a more advanced or efficacious therapy than our product; | |
| ● | Potential delays or total failures of third parties, such as universities, non-profits, and clinical research centers, to perform obligations on which our product research and development rely; | |
| ● | Potential interruption or delay of our and our third-party contractors’ business operations due to COVID-19, which may prevent the timely initiation and conclusion of pre-clinical studies; | |
| ● | The impact on our competitive position, business operations, and financial condition of implementation of amended healthcare laws and regulations related to healthcare pricing and reimbursement; | |
| ● | The dependence of our research and development platform on intellectual property licensed from licensors, and the severe adverse impact to our business operations of a disruption of one of our licensing relationships; | |
| ● | The potential monetary costs of defending our intellectual property rights in a dispute, and the possibility that an intellectual property dispute will not be settled in our favor; | |
| ● | The possibility that our patents and patent applications, even if unchallenged, will not sufficiently protect or provide exclusive use of our intellectual property, which could jeopardize our ability to commercialize our product and dissuade companies from subsequently collaborating with us; |
| ii |
| ● | The negative impact to our competitive position and the value of our technology of our failure to protect trade secrets through the use of non-disclosure and confidentiality agreements, or the unavailability of adequate recourse for breach of such agreements; | |
| ● | The fluctuation and volatility of the market price of our Common Stock due to its limited public market, and the possibility that these issues will compound and strain our stockholders’ ability to resell their Common Stock; | |
| ● | The ability of our principal stockholders and management, through their ownership of a majority of our outstanding Common Stock, to exert significant control over matters requiring stockholder approval; | |
| ● | Our significant dependence on sophisticated management with highly technical expertise to oversee business operations, and our ability to attract and retain qualified personnel to sustain growth; | |
| ● | Our ability to adapt to future growth by training an expanding employee base and shifting away from reliance on third-party contractors; | |
| ● | The risk of liability arising from claims of environmental damage, personal injury, and property damages in connection with our research and development activities, including those that involve the use of hazardous materials; | |
| ● | The possibility that enforcement actions to suspend or severely restrict our business operations will be brought against the Company for our failure to comply with laws or regulations and the potential costs of defending against such actions; | |
| ● | Our reliance on adequate maintenance of the security and integrity of our information technology systems to effectively operate our business; and |
Such other factors as discussed throughout Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and in Part I, Item 1A. Risk Factors herein.
A forward-looking statement is neither a prediction nor a guarantee of future events or circumstances, and those future events or circumstances may not occur. You should not place undue reliance on forward-looking statements, which speak only as of the date of this Annual Report. Forward-looking statements involve known and unknown risks, uncertainties, and other factors, including without limitation the risks and uncertainties described below the heading “Item 1.A. Risk Factors” in this report, that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations and assumptions that involve numerous risks and uncertainties. Our plans and objectives are based, in part, on assumptions involving the continued expansion of our business. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond our control. This is especially emphasized by the anticipated impacts from the COVID-19 pandemic on the Company, including the related effects to our business operations, results of operations, cash flows, and financial position. Although we believe that our assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and, therefore, there can be no assurance that the forward-looking statements included in this Annual Report will prove to be accurate. Given these risks and uncertainties, you should not rely on forward-looking statements as a prediction of actual results. Any or all of the forward-looking statements contained in this Annual Report and any other public statement made by us, including by our management, may turn out to be incorrect. We are including this cautionary note to make applicable and take advantage of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 for forward-looking statements. We expressly disclaim any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Information regarding market and industry statistics contained in this report is included based on information available to us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. Except as required by U.S. federal securities laws, we have no obligation to update forward-looking information to reflect actual results or changes in assumptions or other factors that could affect those statements.
| iii |
PART I
Unless otherwise indicated or the context otherwise requires, all references in this prospectus to “we,” “us,” “our” or the “Company” are to Enochian BioSciences Inc., a Delaware corporation (“Registrant”), together with its wholly owned subsidiaries, Enochian Biopharma, Inc., a Delaware corporation (“Enochian Biopharma”) Enochian Biosciences Denmark ApS, a Danish limited company, organized under the Danish Act on Limited Companies of the Kingdom of Denmark (“DanDrit Denmark”), and Enochian Technology, Inc., a Nevada corporation (“Enochian Technology”).
Our Business
We are a pre-clinical stage biotechnology company committed to using our genetically modified cell, gene, and immune therapy technologies to potentially prevent or cure HIV, HBV, influenza and coronavirus infections, as well as to provide potentially long-term or life-long cancer remission in some of the deadliest cancers.
Over the past several years, Enochian BioSciences has expanded the pipeline from a single potential cure for HIV (autologous transplantation with gene-modified cells; ENOB-HV-01) to three additional potential cures for HIV, a potential cure for Hepatitis B Virus (HBV), potential inhaled treatment and prophylaxis/prevention of all variants of SARS-CoV-1 and -2 (the cause of the COVID-19 pandemic), and potential cures for many solid tumors, beginning with pancreatic cancer.
Our integrated platforms encompass innovative interventions in gene and immune therapies that provide hope for cures or life-long remissions for devastating diseases. Our platforms can potentially streamline and accelerate pre-clinical, regulatory, clinical, and production pathways. Because of the relative ease of administration, our potentially groundbreaking interventions could be used throughout the world.
Platforms:
Hijack RNA
Our novel approach tricks the virus into sending suicide signals to the infected cell instead of turning the cell into a virus factory. The technology is delivered by vectors that would allow it to rapidly treat an infection, or to lay and wait in ambush until a cell becomes infected, which would prevent (prophylaxis, similar to taking drugs to protect from becoming infected with malaria or HIV) future infections.
The delivery mechanism can be adapted for aerosol (e.g., respiratory infections like the virus that causes COVID-19 and influenza), intravenous or other delivery mechanisms (e.g. for Hepatitis B Virus and HIV).
Allogeneic Cell Therapy
The human immune system is designed to recognize “self” and destroy “otherness” or “non-self”, such as bacteria, viruses and cancer cells.
Alloreactivity (reacting against another person’s cells) is the most powerful response the immune system generates. Several of our technologies take advantage of the alloreactivity to hyperstimulate a person’s immune response to better attack a chronic infection (e.g. HIV) or solid tumors. In certain treatments (e.g. HIV and cancer), cells taken from healthy donors are sometimes genetically modified to further boost the immune system to seek and kill diseases.
*********
| 1 |
In addition to those platforms, Enochian BioSciences has an innovative approach to remove cells from a person living with HIV and genetically modify them so they cannot be infected with HIV. The unique innovation is an additional genetic alteration to increase the ability of those cells to survive and expand when they are given back to the same person (autologous transplantation).
To date, our operations have been funded by sales of our securities and the issuance of debt. We have never generated any sales revenue, and we expect this to continue until our therapies or products are approved for marketing in the United States and/or Europe. Even if we are successful in having our therapies or products approved for sale in the United States and/or Europe, we cannot guarantee that a market for the therapies or products will develop. We may never be profitable.
Respiratory Diseases
In June 2021, Enochian BioSciences acquired the exclusive license to a potential pan-SARS-Cornavirus-1 and-2 (SARS-CoV) and pan-Influenza inhaled treatment and prophylaxis.
SARS-CoV-2 has caused the most devastating global pandemic in a century – COVID-19. Using the Hijack RNA platform technology, in vitro and in vivo results showing rapid killing of infected cells but not uninfected cells were presented at the important Conference on Retroviruses and Opportunistic Infections in March 2021. Since that time, there has been substantial progress with a Pre-IND (as defined below) submission expected in the near term.
Influenza has caused dozens of major global pandemics; the most notable in 1918 that killed 50 to 100 million people. There was an H1N1 threat as recently as 2009. The Hijack RNA also has shown promising in vitro results that were presented at the Annual Conference of the American Society of Gene and Cell Therapy (ASGCT) in May 2020.
Two leading scientists and public health experts were announced as members of our newly formed Scientific Advisory Board focused on respiratory diseases on August 30, 2021.
Human Immunodeficiency Virus (HIV), and Acquired Immunodeficiency Syndrome (AIDS)
HIV attacks the human immune system, specifically killing off CD4+ cells, or T cells, which play a central, controlling role in the immune system. Left untreated, HIV dramatically reduces the number of T cells in the body, devastates the immune system, leading to AIDS, a condition where the immune system cannot fight off life-threatening infections and cancers.
Currently there are over 30 antiretroviral drugs, or ART, approved by the U.S. Food and Drug Administration (“FDA”) to treat HIV but these drugs are expensive, require daily adherence, and can have significant side effects over time. In addition, on a global basis, as many as 1 million people, including persons in high-income countries, continue to die each year from HIV/AIDS due to drug-resistant HIV or lack of access to treatment. To date, there are no treatments that can eliminate the reservoir of immune cells that are infected with HIV from the body. Consequently, treatment for HIV is life-long.
There have been several efforts to cure HIV by re-engineering a person’s own T-cells so that these cells no longer express a special protein (C-C chemokine co-receptor type 5 or CCR5), which HIV uses to gain entry to them. A naturally occurring mutation that blocks expression of CCR5 on T cells occurs in ~1% of persons living in or from Northern Europe with no known adverse effects. The “Berlin patient”, and more recently the “London patient” were HIV-positive persons who developed cancer and were treated with a bone marrow transplant with cells donated from persons with this naturally occurring mutation of CCR5. The Berlin and London patients seem to have been effectively cured from HIV providing proof-of-concept that HIV can be cured. However, because the transplanted cells come from another person, such transplants carry high risk and can result in death in a significant proportion of patients. Given the success with these two patients, several researchers and companies have attempted to replicate this experience by genetically modifying T cells of HIV-positive patients to render them unable to be infected by HIV and then returning them to the patient. Because the transplanted cells are from the same person, the risks to the patient are much lower. The uptake, or engraftment of the modified T cells, however, has not been optimal, leading to failure to achieve a cure. In addition, the transplant pre-treatment that has been used is bone marrow-destroying chemotherapy, which wipes out the patient’s immune system and can have long-term side effects including the risk of developing cancer.
| 2 |
ENOB-HV-01 is a novel, proprietary approach with the potential to overcome the failures of recent efforts to develop a cure for HIV. The intervention provides gene-modified T cells with a competitive advantage over non-modified cells in the HIV-positive person, with the potential to significantly increase engraftment; and avoids the need for chemotherapy that substantially depletes the bone marrow and could potentially be given as an outpatient treatment. The Company met with the FDA INTERACT team on June 2, 2020. INTERACT is the first available FDA interaction and is a key step in the process towards a potential Investigational New Drug Application (IND) to study First-in-Human products potentially leading to marketing authorization via Biologics License Application (BLA). The FDA Center for Biologics Evaluation and Research (CBER) has numerous INTERACT requests and grants meetings that are deemed appropriate for this early FDA engagement. The Enochian management team considered the meeting to be successful with strong alignment between Enochian BioSciences’s approach to developing ENOB-HV-01 and the comments of the FDA reviewers.
Initial scientific findings from a mouse study on the ENOB-HV-01 approach were presented at the annual ASCGT conference in May 2020. Additional in vitro and in vivo studies are ongoing and/or planned. We hope to make a Pre-IND submission to the US FDA by end of 2021 or early 2022.
We are also developing ENOB-HV-11 and ENOB-HV-12 that will utilize a novel cellular- and immunotherapy approach that could potentially provide both preventative and therapeutic vaccines for HIV. A non-human primate study is in process and on schedule. Preliminary results could potentially be available by the end of 2021 or early 2022.
Our co-founder and inventor, Dr. Serhat Gumrukcu, who is also the Director of Seraph Research Institute (SRI), submitted Pre-IND for ENOB-HV-21 an innovative treatment of Natural Killer (NK) and Gamma Delta T-Cells (GDT) collected from another person. It is believed that the GDT cells, a small subset of immune cells that can be infected with HIV, could be a key factor in controlling the virus. The initial scientific findings were presented during the ASCGT Conference this past May. Written comments on the submission are expected this Fall. Enochian BioSciences has an exclusive license to use the underlying patent to develop HV-21 for the prevention, treatment, and/or amelioration of and/or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans.
We are in the development phase of additional product candidates related to our HIV pipeline. ENOB-HV-31, which is an in vivo gene therapy, and ENOB-HV-32, which is a peptide drug for packaging and distribution.
Hepatitis B (HBV)
Despite the availability of an effective vaccine and treatment that can control infection if it is taken daily for life, hepatitis B virus (HBV) is the world’s most common serious liver infection. While vaccines are increasingly required for children, many adults have not been vaccinated. Life-long treatment can be difficult for certain people and access can be limited.
In that regard, HBV remains the leading cause of liver cancer and the second leading cause of cancer deaths in the world. Two billion people have been infected with HBV, approximately 350 million have chronic HBV infection, and nearly one million people die every year.
Current efforts to develop novel treatment or cure largely focus on approaches to deplete the pool of a certain type of HBV DNA. Enochian BioSciences has collaborated with SRI to develop an innovative approach to co-opt HBV polymerase, a key expanding factor that the virus needs to reproduce itself, to induce the death of liver cells infected with the virus.
The initial in vitro and in vivo work was presented at the biannual HEP DART meeting in December of 2019, where it was selected as one of the best new therapies/novel strategies. Additional data was presented at the annual ASCGT conference in May 2020. A proof-of-concept, in vivo cure study is in advanced stages. A Pre-IND request was accepted. FDA comments are expected in the near term.
| 3 |
On July 27, 2020, Enochian BioSciences announced the creation of an HBV Scientific Advisory Board comprised of distinguished leaders in HBV disease, treatment and cure. On August 23, 2021, we announced the addition of a third leading expert with substantial experience in HBV clinical trials.
Cancer
Based on learning from peer-reviewed publications of Phase I/IIa trials, we have designed an innovative therapeutic vaccination platform that could potentially be used to induce life-long remissions from some of the deadliest solid tumors. Initial preclinical in vitro studies have been encouraging. We initially plan to target pancreatic cancer, triple-negative breast cancer, glioblastoma, and renal cell carcinoma. The platform might also allow for non-specific immune enhancement that could have impact against a broad array of solid tumors. As with HIV, our approach would potentially allow for outpatient therapy without ablating or significantly impairing the patient’s immune system, as many current approaches require.
Through a collaboration with a leader in the field of pancreatic cancer, our first cancer-related therapeutic target, we are developing the pipeline with in vitro and in vivo proof-of-concept studies to evaluate the potential to induce long-term remission or cure. Results are expected in late 2021or early 2022.
Our Product Candidates
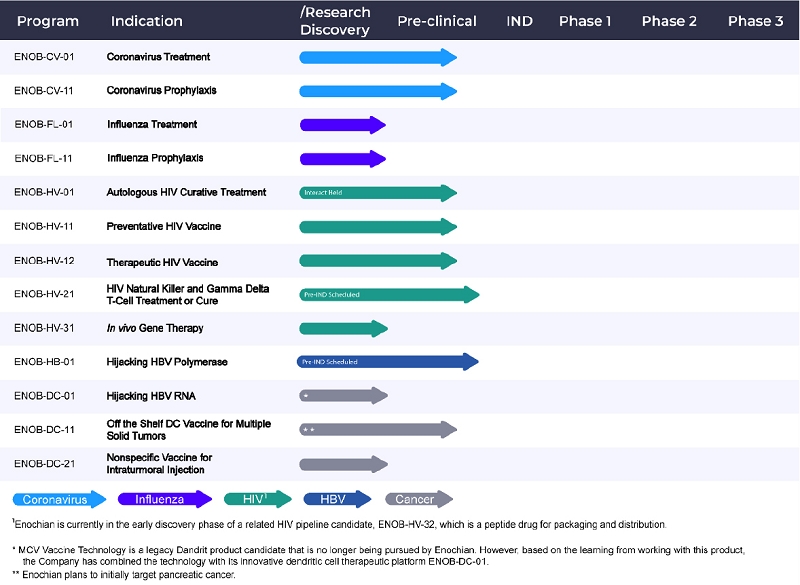
| 4 |
ENOB-HV-01: Autologous Transplant with Genetically Modified Cells:
FDA INTERACT Meeting Held February 2020 - Advanced Pre-Clinical Stage
It has been proven that gene editing to knock down the expression of CCR5 — a door HIV needs to enter and kill CD4+ T cells — in autologous human stem cells (HSC) combined with transplantation can lead to a cure of HIV. However, the approaches currently available require an expensive and risky ablation of the immune system. Even with that drastic intervention, an insufficient number of gene-modified cells survive to achieve durable control of HIV.
We have pioneered a novel approach that we believe will allow sufficient engraftment of the gene-modified HSC to eliminate the need for Antiretroviral Treatment (ART.)
In a transgenic mouse model, the technology increased engraftment in bone marrow by 163%. For context, a 10% increase is often thought to be successful.
Management considered the FDA INTERACT Meeting to be successful and in alignment with Enochian’s experimental plan. Additional in vitro and in vivo experiments are underway. Pre-IND submission is possible by the first part of 2022 with human studies potentially beginning in 2022.
ENOB-HV-11 and 12: Preventive and Therapeutic Vaccines
Allogeneic Cell Therapy Platform - Advanced Pre-Clinical Stage; Non-Human Primate Studies Begun
Boosting a person’s immune system through vaccination can lead to protection from HIV infection in people who are not living with HIV. In persons living with HIV who are controlling the spread of virus with antiretroviral (ARV) treatment, boosting the immune system in a different way than the virus already has through infection, could allow control of HIV after stopping ARVs.
Enochian BioSciences’ technology uses the powerful induction of an immune response created by cells from another person potentially to induce such a response. Based on promising in vitro results, a study in non-human primates was begun by the renowned HIV and cancer researcher Dr. Hans Peter Kiem of the Fred Hutchinson Cancer Research Center, Seattle, Washington.
Preliminary results are expected by the end of 2021 or early 2022. If successful, human studies could potentially begin in 2022.
ENOB-HV-21: Immunotherapy with Allogeneic NK/GDT Cells
Allogeneic Cell Therapy Platform -Pre-IND Scheduled - Advanced Pre-Clinical with Human Data through a Collaboration
On June 14, Enochian BioSciences announced that the FDA has accepted a Pre-IND request for a potential functional cure or treatment of HIV. Written comments are expected this Fall.
Dr. Serhat Gumrukcu, co-founder and inventor of Enochian BioSciences, and Director of SRI, submitted the Pre-IND. The request was based on the results of a 54-year old man living with HIV who had failed to suppress the virus with antiviral therapy. The patient subsequently achieved viral control for 360 days with an innovative treatment of Natural Killer (NK) and Gamma Delta T cells (GDT) collected from another person. During the entire period, no antiviral drugs were given. It is believed that the GDT cells, a small subset of immune cells that can be infected with HIV, could be a key factor in controlling the virus.
| 5 |
This innovative cellular therapy could be an important approach to achieve a “functional cure” of HIV, potentially allowing persons with the virus to stop antiviral treatment for extended periods. The Pre-IND submission requested that the novel strategy be extended to persons with HIV who have achieved suppression of the virus with antiviral treatment. Enochian BioSciences has an exclusive license to use the underlying patent to develop HV-21 for the prevention, treatment, and/or amelioration of and/or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans.
ENOB-HV-31: In Vivo Gene Therapy
Hijack RNA Platform - Early Pre-Clinical
HIV is an RNA virus. Based on the Hijack RNA Platform, an approach to “seek and kill” HIV-infected cells has been developed. In vivo and in vitro studies should begin in the near term.
ENOB-HB-01: Potential Cure for HBV
Hijack RNA Platform - Advanced Pre-Clinical
Current efforts to develop a novel treatment or cure largely focus on approaches to deplete the pool of a certain type of HBV DNA. Enochian has collaborated with SRI to develop an innovative approach to co-opt HBV polymerase to induce the death of liver cells infected with the virus.
ENOB-CV-01: Aerosol/Inhaled Treatment Potentially for All Coronaviruses that Cause Human Disease
Hijack RNA Platform - Pre-IND Submission Expected in Near-term - Advanced Pre-Clinical
The technology tricks – or hijacks – the polymerase of Coronaviruses to trigger infected cells to commit suicide instead of becoming virus-producing factories. The code recognized by the Hijack RNA is similar in SARS-CoV-1 and all variants of SARS-CoV-2 (that cause COVID-19) as well as those known to cause 20 percent of common colds.
Modeled on the approach taken by operation Warp Speed, production, distribution, and reimbursement potential partners have been identified. Based on promising experiments in mice, a Pre-IND application is expected in the near term.
ENOB-CV-11: Aerosol/Inhaled Prophylaxis Potentially for All Coronaviruses that Cause Human Disease
Hijack RNA Platform - Pre-IND Submission Expected in Near-term - Advanced Pre-Clinical
The technology is delivered by an AAV particle or nanoparticle, allowing it to wait in ambush for a cell to become infected or until the cells die due to natural turnover. Because target cells of the Coronavirus – respiratory epithelial cells - live up to 6 to 20 months in human airways, it is possible that a single inhalation could protect against infection for relatively long periods of time.
The code recognized by the Hijack RNA is similar in SARS-CoV-1 and all variants of SARS-CoV-2 (that cause COVID-19) as well as those known to cause 20 percent of common colds. Therefore, a single inhaled dose every 6 to 20 months could potentially prevent all variants of Coronaviruses. Based on promising experiments in mice, a Pre-IND application is expected in the near term.
ENOB-FL-01: Aerosol/Inhaled Treatment Potentially for All Influenza Viruses
Hijack RNA Platform - Early Pre-Clinical
| 6 |
Influenza viruses are also RNA viruses. Therefore, a similar approach to that for Coronaviruses has been developed. The code targeted by the Hijack RNA is similar in all pandemic variants since 1918. In vitro experiments demonstrated rapid killing of infected cells without damage to uninfected cells.
ENOB-FL-11: Aerosol/Inhaled Prophylaxis Potentially for All Influenza Viruses
Hijack RNA Platform - Early Pre-Clinical
The approach taken will be similar to that described for ENOB-CV-11 above.
ENOB-DC-11: Genetically modified Allogeneic Dendritic Cells as Potential Cure
Allogeneic Cell Therapy Platform - Moderately Advanced Pre-Clinical
As noted in the Allogeneic Cell Therapy Platform Section, allogeneic cells are a potent stimulant to the immune system. Our technology genetically modifies dendritic cells, the conductor of the immune system, to enhance their already powerful ability to activate and orchestrate the immune response to tumors. Combined with fragments of, or fragments from, specific tumors, preliminary in vitro data is promising.
Enochian BioSciences has initiated a collaboration with Dr. Anahid Jewett from UCLA to study further the in vitro and in vivo effectiveness of the approach in pancreatic cancer. The survival rate in pancreatic cancer is only 5 to 10 percent at 5 years. Dr. Jewett created an innovative pancreatic cancer mouse model to study the impact of potential therapy. Results are expected by the end of 2021 with potential Pre-IND submission by the end of the year. The technology is a platform in itself that could potentially be used for other solid tumors.
Collaborations
We have established strategic partnerships with leading scientists and centers, such as The Scripps Institute, Fred Hutchinson Cancer Research Center, the Texas Biomedical Research Institute, the University of California, Los Angeles and The Hepatitis B Foundation and Baruch S. Blumberg Institute, for several of our programs. We will continue to pursue partnerships when appropriate with selected philanthropic, pharmaceutical and biotechnology companies to fund internal research and development activities, and to assist in product development and commercialization. We are applying our technology platform to several commercial applications in which our products provide us and our strategic partners and collaborators with potential technical, competitive and economic advantages.
Our Intellectual Property
Patents and licenses are important to our business. Our strategy is to file license patent applications to protect technology, inventions and improvements to inventions that we consider important for the development of our business. We rely on a combination of patent, copyright, trademark, and trade secret laws, as well as continuing technological innovations, proprietary knowledge, and various third party agreements, including, without limitation, confidentiality agreements, materials transfer agreements, research agreements and licensing agreements, to establish and protect our proprietary rights. We aim to take advantage of all of the intellectual property rights that are available to us and seek protection of those rights so that we can fully exploit our innovations.
We also protect our proprietary information by requiring our employees, consultants, contractors and other advisors to execute nondisclosure and assignment of invention agreements upon commencement of their respective employment or engagement. Our patent filings are discussed briefly below.
| 7 |
Pharmaceutical composition for inducing an immune response in a human or animal (2001 Denmark (DK), 2002 PCT)
This patent family, owned by the Company, is directed to certain melanoma cell lines and the use of an allogenic melanoma cell lysate (MCL)-pulsed autologous dendritic cell vaccine expressing at least one of six MAGE-A antigens to induce an immune response. This patent has been granted in: Europe, USA, China, Australia, Singapore, Russia, and Hong Kong and is pending in Japan. The issued patents relating to ENOB-DB-01 (previously known as “MCV”) begin to expire in November 2022, subject to any applicable patent term extension, patent term adjustment, or supplementary protection certificates that may be available in a country or jurisdiction.
Protocol for generating dendritic cells (2005 DK, 2008 PCT)
This patent family is directed to the generation of dendritic cells based on a blood sample by culturing monocytes at reduced temperatures. Dendritic cells exposed to tumor antigens followed by treatment with T(h) 1-polarizing differentiation signals have paved the way for the development of dendritic cell-based cancer vaccines. Issued claims are directed to a method of generating immature dendritic cells under certain temperature settings, which by further activation has been shown to give a high yield of homogeneous and fully matured dendritic cells. The patent expiration date is December 2026 subject to any applicable patent term extension, patent term adjustment, or supplementary protection certificates that may be available in a country or jurisdiction. This patent has been issued in the USA, Canada, China, Eurasia, Russia, Europe, Israel, Mexico, Malaysia, and New Zealand. This patent is owned by the Company and was not licensed from third parties.
Trade Secrets and Proprietary Know-How
In addition to intellectual property protected by patents and copyrights, we have trade secrets and proprietary know-how relating to our products, production processes, and future strategies.
In-Licensed Technology
On February 16, 2018, Enochian Biopharma, the Registrant’s wholly owned subsidiary, entered into a License Agreement (the “HIV License Agreement”) with Weird Science, LLC (“Weird Science”). The License Agreement contains, among other things, the following terms: (a) a perpetual, fully paid-up, royalty-free, sublicensable, and exclusive (including to the exclusion of Weird Science) worldwide license from Weird Science to Enochian Biopharma to use Weird Science’s intellectual property and technology for the prevention, treatment, and/or amelioration of and/or therapy for HIV in humans, and research and development exclusively relating to HIV in humans (the “Field”) worldwide; (b) a nonexclusive, royalty-free, sublicensable license from Enochian Biopharma to Weird Science to use the Enochian Technology to commercialize products outside of the Field worldwide; (c) a nonexclusive, royalty-free license from Enochian Biopharma to Weird Science to use the results of a study with syngeneic and humanized mice models outside the Field and, at Weird Science’s own expense, to prosecute patents relating to the results of the study, which Weird Science will own, and (d) a perpetual, fully paid-up, royalty-free, sublicensable, and sole and exclusive (including to the exclusion of Weird Science) worldwide license from Weird Science to Enochian Biopharma (which will be part of the license described in (a) above) to use patent applications and patents related to the study results disclosed in (d) above solely in the Field, and to make, have made, use, sell, offer to sell and import inventions claimed in such patent applications and patents solely in the Field. Our current product candidates covered by this license include ENOB-HV-01: Autologous HIV Curative Treatment, ENOB-HV-11: Preventative HIV Vaccine; ENOB-HV-12: Therapeutic HIV Vaccine, (See Note 8 in the Financial Statements)
| 8 |
On January 31, 2020, the Company entered into a Statement of Work and License Agreement (the “HBV License Agreement”) by and among the Company, G Tech Bio, LLC “(G-Tech”), and G Health Research Foundation, a not for profit entity organized under the laws of California doing business as Seraph Research Institute (“SRI”), whereby the Company acquired a perpetual, sublicensable, exclusive license (the “HBV License”) for a treatment under development (aimed to treat Hepatitis B Virus (HBV) infections in accordance with its agreement in principle with G-Tech and SRI announced by the Company on November 25, 2019. The HBV License Agreement states that in consideration for the HBV License, the Company shall provide cash funding for research costs and equipment and certain other in-kind funding related to the Treatment over a 24-month period. The Company paid an upfront payment of $1.2 million on February 6, 2020. Our current product candidate under this license is ENOB-HB-01 Hijacking HBV Polymerase. (See Note 8 in the Financial Statements)
On April 18, 2021, the Company entered into a Statement of Work and License Agreement (the “Influenza and Coronavirus Indications Agreement”) by and among G Tech and SRI, whereby the Company acquired a perpetual, sublicensable, exclusive license (the “Development License”) to research, develop, and commercialize certain formulations which are aimed at preventing and treating pan-coronavirus or the potential combination of the pan-coronavirus and pan-influenza, including the SARS-coronavirus that causes COVID-19 and pan-influenza (the “Prevention and Treatment”). The Influenza and Coronavirus Indications License Agreement states that in consideration for the Development License, the Company shall provide cash funding for research costs and equipment and certain other in-kind funding related to the Prevention and Treatment over a 24-month period. The Company paid an upfront payment of $10 million on June 18, 2021. Our current product candidates under this license are ENOB-CV-01: Coronavirus Treatment, ENOB-CV-11: Coronavirus Prophylaxis, ENOB-FL-01: Influenza Treatment, and ENOB-FL-11: Influenza Prophylaxis. (See Note 8 in the Financial Statements)
On August 25, 2021, the Company entered into an ALC Patent License and Research Funding Agreement in the HIV Field (the “ALC License Agreement”) with Dr. Gumrukcu and SRI whereby Dr. Gumrukcu granted the Company an exclusive, worldwide, perpetual, fully paid-up, royalty-free license, with the right to sublicense, his proprietary technology subject to a U.S. patent application, to make, use, offer to sell, sell or import products for use solely for the prevention, treatment, amelioration of or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans; provided Dr. Gumrukcu retained the right to conduct HIV research in the field. Pursuant to the ALC License Agreement, the Company granted a non-exclusive license back to Dr. Gumrukcu and SRI, under any patents or other intellectual property owned or controlled by the Company, to the extent arising from the ALC License, to make, use offer to sell, sell or import products for use in the diagnosis, prevention, treatment, amelioration or therapy of any (i) HIV Comorbidities and (ii) any other diseases or conditions outside the HIV Field. The Company made an initial payment to SRI of $600,000 and agreed to fund future HIV research conducted by Dr. Gumrukcu and SRI, as mutually agreed to by the parties. Our current product candidate under this license is ENOB-HV-21: HIV Natural Killer and Gamma Delta T Cell Treatment or Cure.
Competition
The biotechnology and pharmaceutical industries, including in the field of gene therapy, are characterized by rapidly advancing technologies, intense competition and a strong emphasis on intellectual property. While we believe that our technology platforms, strong intellectual property portfolio and scientific expertise in the gene therapy field provide us with competitive advantages, we face potential competition from many different sources, including larger and better-funded pharmaceutical and biotechnology companies, new market entrants and new technologies.
We are aware of several companies focused on other methods for editing genes and regulating gene expression, and a limited number of commercial and academic groups pursuing the development of gene regulation and genome editing technology. The field of applied gene regulation and genome editing is highly competitive, and we expect competition to persist and intensify in the future from several different sources, including pharmaceutical and biotechnology companies; academic and research institutions; and government agencies.
| 9 |
Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA approval, or commercializing competitive products before us. If we commence commercial product sales, we may be competing against companies with greater marketing and manufacturing capabilities, areas in which we have limited or no experience. In addition, any product candidate that we successfully develop may compete with existing products that have long histories of safe and effective use.
The competitive landscape that we are facing is as follows:
Gene therapy companies developing gene-based products in clinical trials. uniQure N.V.’s product for lipoprotein lipase deficiency and GlaxoSmithKline plc’s, or GSK, product for severe combined immunodeficiency due to adenosine deaminase deficiency are approved in Europe. No other gene therapy products have yet been approved. Our competitors in this category may include, but not be limited to, Sangamo Therapeutics, Inc., uniQure N.V., bluebird bio, Inc., Regenxbio Inc., Shire, Pfizer Pharmaceutical, and GlaxoSmithKline.
Cell therapy companies developing cell-based products. Our competitors in this category may include Novartis AG, Adaptimmune Therapeutics PLC, Atara Biotherapeutics, Inc., bluebird bio, Inc., Cellectis S.A., Juno Therapeutics, Inc., Kite Pharma, and Iovance Biotechnologies, Inc.
For ENOB-HV-01, we are aware of two companies developing a gene therapy for HIV/AIDS: Sangamo and American Gene Technology.
For ENOB-HV-11 and ENOB-HV-12, we are aware of a few biotech companies developing an HIV vaccine such as Geovax, Biosantech SA, and FIT Biotech, among a few others.
For ENO-DC-11, the competitive landscape is more complex.
Immunotherapy is an active area of research and a number of immune-related products have been identified in recent years that are alleged to modulate the immune system. Many of these products utilize dendritic cells, a form of immune cell that presents cancer target peptides to T cells and that can in turn result in T cell activation. More recently, bi-specific antibodies and checkpoint inhibitors (for instance PD-1/PD-L1 antibodies) have been identified as having utility in the treatment of cancer. Bi-specific antibodies commonly target both the cancer peptide and the T cell receptors (“TCR”), thus bringing both cancer cells and T cells into close proximity to maximize the chance of TCR binding and hence an immune response to the cancer cells. Checkpoint inhibitors on the other hand work by targeting receptors that inhibit T cell effectiveness and proliferation and essentially activate T cells. Other immunotherapies that are being actively investigated include antibody-drug complexes, TCR-mimic antibodies, oncolytic viruses, and cancer vaccines. A variety of cell-based autologous and allogeneic approaches are also being researched and developed.
CAR-T in solid tumors
In addition to hematological malignancies, there are a growing number of pharmaceutical, biotechnology, and academic institutions researching and developing autologous and allogeneic chimeric antigen receptor T cell (“CAR-T”) therapies in the solid tumor setting. These CAR-T cell therapies are at a variety of stages of preclinical and clinical development, as well as directed towards a broad target spectrum. Two Car-T therapies have been approved for treatment of leukemia.
CARsand TCR-mimics targeting peptide-HLA complexes
Most CAR-T therapies in development are directed towards antigen targets. However, competitors are also developing a CAR-T that selectively binds to the peptide-HLA (pHLA) complex (the natural binding site for endogenous TCR). Furthermore, competitors are also looking at pHLA antibodies or TCR mimic antibodies that can either be engineered in T cells or developed as standalone antibody therapies in cancer indications (including solid tumors).
| 10 |
TCR Tcells
Competitors are developing TCR T cells (including affinity engineered T cells) that are directed towards a multitude of targets. Juno Therapeutics has developed an engineered TCR therapeutic candidate where the end TCR is purported to have enhanced affinity through stem-cell selection.
Other cell-based approaches
In addition to all the adoptive cell therapy approaches above, our competitors are also investigating the potential of GammaDelta T cell, CAR-NK cell, NK cell, NKT cell and CTLs either in a preclinical or clinical setting (both hematologic malignancies and solid tumors). In addition, Bristol Myers Squibb’s Abraxane is used for pancreatic cancer.
For ENOB-CV-01 and -11; SARS-CoV:
Treatment: There are currently several products with FDA EUA for treatment of COVID including, but not limited to Remdesivir (Gilead) and monoclonal antibodies (Elly Lily and Regeneron). In addition, other system anti-virals are in advanced clinical trials. SaNOtize Research and Development Corp. has reported data on an aerosolized therapy and its intention to seek EUA at least in Canada and Europe. Virpax Pharmaceuticals, Inc. has announced the conclusion of a Pre-IND with the FDA for an aerosolized treatment.
Prevention: Four vaccines have received FDA EUA (Pfizer, Moderna, Johnson and Johnson and Novavax). One, Pfizer has received full approval. Several companies have announced plans for, and/or are conducting clinical trials of aerosolized vaccines.
For ENOB-FL-01 and -11; Influenza:
Treatment: Current treatments are largely to manage symptoms. Tamiflu is approved for use within the first few days of symptoms.
Prevention: Annual vaccines are approved for use. Several companies have announced plans to develop new vaccines, e.g. mRNA.
For ENOB-HBV-01:
There is an approved vaccine to prevent HBV infection. In addition, several approved combination antivirals can suppress replication, but do not cure HBV. Several companies are pursuing cures, mostly targeting the depletion of ccc-DNA.
Manufacturing
Our intent is to rely on contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs), to help develop and produce our preclinical and clinical product candidates in accordance with FDA and EMA mandated regulations, also known as current good manufacturing practices, (“cGMPs”). We employ a technical operations staff in the areas of process development, analytical development, quality control, quality assurance, project management, and manufacturing, which will facilitate appropriate oversight of our CMOs, support of our regulatory filings and execution of clinical trials.
Government Regulation
FDA Review and Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. Any products we develop will require regulatory review and allowance to proceed prior to conducting clinical trials and additional regulatory approvals prior to commercialization. In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act (FDCA) and the Public Health Service Act (PHSA) and their implementing regulations govern, among other things, biopharmaceutical testing, manufacturing, safety, efficacy, labeling, storage, recordkeeping, advertising, and other promotional practices.
| 11 |
Obtaining FDA approval is a costly and time-consuming process. Generally, FDA approval requires that preclinical studies be conducted in the laboratory and in animal model systems to gain preliminary information on efficacy and to identify any major safety concerns. The results of these studies are then submitted as a part of an IND, which the FDA must review and allow before human clinical trials can start. The IND includes a detailed description of the proposed clinical investigations. An independent Institutional Review Board (“IRB”) must also review and approve the clinical protocol and each clinical site.
A company must submit an IND for each investigational medical product and specific indication(s), and must conduct clinical studies to demonstrate the safety and efficacy of the product necessary to obtain FDA approval. The FDA receives reports on the progress of each phase of clinical testing and may require the modification, suspension, or termination of clinical trials if an unwarranted risk is presented to patients.
Obtaining FDA approval prior to marketing a biopharmaceutical product in the United States typically requires several phases of clinical trials to demonstrate the safety and efficacy of the product candidate. Clinical trials are the means by which experimental treatments are tested in humans and are conducted following preclinical testing. Clinical trials may be conducted within the United States or in foreign countries. If clinical trials are conducted in foreign countries, the products under development as well as the trials are subject to regulations of the FDA and/or its regulatory counterparts in the other countries. Upon successful completion of clinical trials, approval to market the treatment for a particular patient population may be requested from the FDA in the United States and/or its counterparts in other countries.
Clinical trials for therapeutic products are normally conducted in three phases. Phase 1 clinical trials are typically conducted with a small number of patients to evaluate safety, determine a safe dosage range, identify side effects, and, if possible, gain early evidence of effectiveness. Phase 2 clinical trials are conducted with a larger group of patients to evaluate effectiveness of an investigational product for a defined patient population, and to determine common short-term side effects and risks associated with the drug. Phase 3 clinical trials involve large scale, multi-center, comparative trials that are conducted to evaluate the overall benefit-risk relationship of the investigational product and to provide an adequate basis for product labeling. In some special cases where the efficacy testing of a product may present a special challenge to testing in humans, such as in the case of a vaccine to protect healthy humans from a life-threatening disease that is not a naturally occurring threat, effectiveness testing may be required in animals. For certain advanced therapies that meet eligibility criteria for expedited program Designations, clinical development may be expedited.
Clinical trials involve the administration of the biologic product candidate to healthy volunteers or patients under the supervision of qualified investigators which generally are physicians not employed by, or under, the control of the trial sponsor. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted and monitored in accordance with the FDA’s regulations comprising the Good Clinical Practice (“GCP”) requirements, and any additional requirements for the protection of human research subjects and their health information including the requirement that all research subjects provide informed consent.
Further, each clinical trial must be reviewed and approved by an IRB at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers items such as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent that must be signed by each clinical trial subject, or their legal representative, reviews and approves the study protocol, and must monitor the clinical trial until completed. Clinical trials involving recombinant DNA also must be reviewed by an institutional biosafety committee, or IBC, a local institutional committee that reviews and oversees basic and clinical research that utilizes recombinant DNA at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment.
| 12 |
After completion of clinical trials of a new product, FDA marketing approval must be obtained. If the product is regulated as a biologic, a Biologics License Application, or BLA, is required. If the product is classified as a new drug, a New Drug Application, or NDA is required. The NDA or BLA must include results of product development activities, preclinical studies, and clinical trials in addition to detailed chemistry, manufacturing and control information.
Applications submitted to the FDA are subject to an unpredictable and potentially prolonged approval process. Despite good-faith communication and collaboration between the applicant and the FDA during the development process, the FDA may ultimately decide, upon final review of the data, that the application does not satisfy its criteria for approval or requires additional product development or further preclinical or clinical studies. Even if FDA regulatory approval(s) are obtained, a marketed product is subject to continual review, and later discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
Before marketing approval can be secured for a product, the facility in which the product is manufactured must be inspected by the FDA and must comply with the FDA’s current Good Manufacturing Practices, (“cGMP”) regulations. In addition, after marketing approval is secured, the manufacturing facility must be inspected periodically for cGMP compliance by FDA inspectors, and, if the facility is located in California, by inspectors from the Food and Drug Branch of the California Department of Health Services.
Sponsors of clinical trials are required to register, and report results for, all controlled, clinical investigations, other than Phase 1 investigations, of a product subject to FDA regulation. Trial registration may require public disclosure of certain confidential commercial development data.
The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. FDA sanctions could include, among other actions, refusal to approve pending applications, withdrawal of an approval, a clinical he hold, warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on our business, financial condition, results of operations and cash flows
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a product available for this type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting an NDA or BLA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan designation subsequently receives the first FDA approval for such product for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market a product containing the same active moiety for the same use or indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. A product is clinically superior if it is safer, more effective or makes a major contribution to patient care. Any claims of clinical superiority could require a head-to-head clinical trial between such drugs. Competitors may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. If a product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity.
| 13 |
Other Healthcare Laws and Compliance Regulations
Although we currently do not have any products on the market, we may also be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. In the United States, among other things, the research, manufacturing, distribution, sale and promotion of pharmaceutical and biological products are potentially subject to regulation and enforcement by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (“CMS”), other divisions of the United States Department of Health and Human Services (e.g., the Office of Inspector General), the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety and Health Administration, the Environmental Protection Agency, state Attorneys General and other state and local government agencies. Our current and future business activities, including for example, sales, marketing and scientific/educational grant programs, must comply with health care regulatory laws, as applicable, including, without limitation:
| ● | the federal anti-kickback statute, which is a criminal statute that makes it a felony for individuals or entities to knowingly and willfully offer or pay, or to solicit or receive, direct or indirect remuneration, in order to induce the purchase, order, lease, or recommending of items or services, or the referral of patients for services, that are reimbursed under a federal health care program, including Medicare and Medicaid; | |
| ● | the federal False Claims Act, which prohibits, among other things, individuals and entities from knowingly submitting, or causing to be submitted, false or fraudulent claims for payment of government funds, with penalties that include three times the government’s damages plus civil penalties for each false claim; in addition, the False Claims Act permits a person with knowledge of fraud, referred to as a qui tam plaintiff, to file a lawsuit on behalf of the government against the person or business that committed the fraud, and, if the action is successful, the qui tam plaintiff is rewarded with a percentage of the recovery; | |
| ● | federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; | |
| ● | the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; | |
| ● | the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical suppliers to report annually to CMS information related to payments and other transfers of value to physicians, other healthcare professionals and teaching hospitals, and ownership and investment interests held by physicians and other healthcare professionals and their immediate family members; and | |
| ● | state and foreign law equivalents of each of the above federal laws, such as state anti-kickback and false claims laws which may impose more strict requirements than federal law and may apply to items or services reimbursed by any payor (including commercial insurers and cash-paying patients); state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare professionals and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare professionals or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
| 14 |
If our operations are found to be in violation of any of such laws or any other governmental laws or regulations that apply, they may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations, exclusion from participation in federal and state healthcare programs, additional program integrity obligations, individual imprisonment, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of product approvals, refusal to permit us to enter into supply contracts, including government contracts, contractual damages, reputational harm, administrative burdens, diminished profits, and future earnings, any of which could have a material adverse effect on our business, financial condition, result of operations, and cash flows. These additional healthcare regulations could affect our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors.
Moreover, the introduction of legislation, implementation of new regulations, or enforcement of existing regulations that have a negative impact on the commercial prospects for the types of products we are developing could negatively impact our share price and our ability to raise capital.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidate that receives regulatory approval. In the United States and markets in other countries, sales of our product candidates, if approved, will depend, in part, on the extent to which third-party payors provide coverage and establish adequate reimbursement levels.
In the United States, third-party payors include federal and state healthcare programs, government authorities, private managed care providers, private health insurers and other organizations. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. Such payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all the FDA-approved drugs for a particular indication. Third-party payor coverage may be more limited than the purposes for which the product is approved by the FDA or foreign regulatory authorities. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product.
Moreover, the process for determining whether a third-party payor will provide coverage for a drug product may be separate from the process for setting the price of a drug product or for establishing the reimbursement rate that such a payor will pay for the drug product. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved or that the product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale, and distribution. There may be significant delays in obtaining reimbursement for approved products, and reimbursement rates may fluctuate over time or vary according to the use of the product or clinical setting in which a product is used. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of products from countries where they may be sold at lower prices than in the United States.
Further, third-party payers are increasingly challenging the price of medical products and services, and there is increasing pressure on biotechnology companies to reduce healthcare costs. If purchasers or users of our products are not able to obtain adequate reimbursement for the cost of using our products, they may forego or reduce their use. Significant uncertainty exists as to the reimbursement status of newly approved healthcare products, and whether adequate third-party coverage will be available. Our inability to promptly obtain coverage and profitable payment rates from both government funded and private payors for future products we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize potential products, and our overall financial condition.
| 15 |
Healthcare Reform
In March 2010, former President Obama Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “Affordable Care Act”),, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States, and significantly affected the pharmaceutical industry. The Affordable Care Act contains a number of provisions, including those governing enrollments in federal healthcare programs, reimbursement adjustments and fraud and abuse changes. Additionally, the Affordable Care Act increases the minimum level of Medicaid rebates payable by manufacturers of brand name drugs; requires collection of rebates for drugs paid by Medicaid managed care organizations; requires manufacturers to participate in a coverage gap discount program, under which they must agree to offer point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted, including aggregate reductions of Medicare payments to providers and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, proposing to encourage importation from other countries and bulk purchasing. We cannot predict what healthcare reform initiatives may be adopted in the future.
We also are subject to various federal, state and local laws, regulations, and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used in connection with our research. The extent of government regulation that might result from any future legislation or administrative action cannot be accurately predicted.
| 16 |
Foreign Corrupt Practices Act
Our business activities may be subject to the Foreign Corrupt Practices Act, or FCPA, and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, the health care providers who prescribe pharmaceuticals are employed by their government, and the purchasers of pharmaceuticals are government entities; therefore, our dealings with these prescribers and purchasers are subject to regulation under the FCPA. There is no certainty that all of our employees, agents, suppliers, manufacturers, contractors, or collaborators, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, the closing down of facilities, including those of our suppliers and manufacturers, requirements to obtain export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries as well as difficulties in manufacturing or continuing to develop our products, and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and financial condition.
Employees
As of June 30, 2021, we had 11 full-time employees. We believe that we have good relations with our employees.
Corporate Information
On February 16, 2018, we completed our acquisition of Enochian Biopharma pursuant to an acquisition agreement, dated January 12, 2018, by and among the Registrant, its wholly owned subsidiary DanDrit Acquisition Sub, Inc., Enochian Biopharma and Weird Science (the “Acquisition Agreement”), with Enochian Biopharma surviving as a wholly owned subsidiary of the Registrant. As consideration for the acquisition, the stockholders of Enochian Biopharma received (i) 18,081,962 shares of Common Stock and (ii) the right to receive Contingent Shares pro rata upon the exercise or conversion of warrants, which were outstanding at closing (See Note 1 to the Financial Statements).
We trade on the NASDAQ Capital Market under the ticker “ENOB.”
Our website is http://www.enochianbio.com. We make available free of charge, on or through our internet site, our annual, quarterly, and current reports and any amendments to those reports filed or furnished pursuant to Section 13(a) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information contained in our website is not part of, nor incorporated by reference into, this report.
| 17 |
Item 1A. Risk Factors
RISK FACTORS
Investing in our common stock involves a high degree of risks. Investors should carefully consider all of the risk factors and uncertainties described below, in addition to the other information contained in this Annual Report on Form 10-K, including the section of this report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes, before investing in our common stock.
The risks described below may not be the only ones relating to our Company and additional risks that we currently believe are immaterial may also affect us. If any of these risks, including those described below, materialize, our business, competitive position, reputation, financial condition, results of operations, cash flows and future prospects could be seriously harmed. In these circumstances, the market price of our common stock could decline, and investors may lose all or a part of their investment.
Risks Related to Our Financial Results and Capital Needs
We have incurred substantial losses since our inception and anticipate that we will continue to incur substantial and increasing losses for the foreseeable future.
We are a pre-clinical-stage biotechnology company. Investment in biotechnology related to genetically modified cells is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate will fail to prove effective, gain regulatory approval or become commercially viable. We do not have any products approved by regulatory authorities and have not generated any revenues from product sales or otherwise to date, and have incurred significant research, development and other expenses related to our ongoing operations and expect to continue to incur such expenses. As a result, we have not been profitable and have incurred significant operating losses in every reporting period since our inception. For the years ended June 30, 2021 and 2020, respectively, we reported a net loss of $26.7 million and $11.4 million. We had an accumulated deficit of $90.9 million and $64.2 million as of June 30, 2021 and 2020, respectively.
We do not expect to generate revenues for the foreseeable future. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses to increase as we continue to research, develop and seek regulatory approvals for our product candidates and any additional product candidates we may acquire, in-license or develop, and potentially begin to commercialize product candidates that may achieve regulatory approval. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If any of our product candidates fails in clinical studies or does not gain regulatory approval, or if approved, fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. We anticipate that our expenses will increase in the future as we continue to invest in research and development of our existing product candidates, investigate and potentially acquire new product candidates and expand our manufacturing and commercialization activities.
| 18 |
We are a pre-clinical biotechnology company and may never be able to successfully develop marketable products or generate any revenue. We have a very limited relevant operating history upon which an evaluation of our performance and prospects can be made. There is no assurance that our future operations will result in profits. If we cannot generate sufficient revenues, we may suspend or cease operations.
We are an early stage biotechnology company and have not generated any revenues to date. All of our product candidates are in the discovery stage or pre-clinical development stage. Moreover, we cannot be certain that our research and development efforts will be successful or, if successful, that our potential treatments will ever be approved for sales to generate commercial revenues. Our pipeline includes cell, gene and immunotherapy involving genetically modified cells targeted to treat HIV, Hepatitis B, pan-coronavirus and influenza, and cancer, and we rely on third parties under contract in the development of product candidates in our pipeline. There is no guarantee that we will be able to manage and fund the development of a pipeline with multiple target conditions, nor that third parties will meet their obligations to us in connection with our research and development. We and certain third parties, on which we rely, have no relevant operating history upon which an evaluation of our performance and prospects can be made. We are subject to all of the business risks associated with a new enterprise, including, but not limited to, risks of unforeseen capital requirements, failure of treatments either in non-clinical testing or in clinical trials, failure to establish business relationships, failure of our third parties to meet their obligations to us and competitive disadvantages against larger and more established companies. If we fail to become profitable, we may suspend or cease operations.
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercialization efforts.
We expect to expend substantial resources for the foreseeable future to continue the pre-clinical development of our cell, gene and immunotherapy product candidates, and the advancement and potential expansion of our pre-clinical research pipeline. We also expect to continue to expend resources for the development and manufacturing of product candidates and the technology we have licensed or have a right to license from our licensors. These expenditures will include costs associated with research and development, potentially acquiring or licensing new product candidates or technologies, conducting pre-clinical and clinical studies and potentially obtaining regulatory approvals and manufacturing products, as well as marketing and selling products approved for sale, if any. Under the terms of certain of our license agreements, we are obligated to make payments upon the achievement of certain development, regulatory and commercial milestones. We will also need to make significant expenditures to develop a commercial organization capable of sales, marketing and distribution for any products, if any that we intend to sell ourselves in the markets in which we choose to commercialize on our own. In addition, other unanticipated costs may arise. Because the design and outcome of our ongoing, planned and anticipated pre-clinical and clinical studies is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates.
| 19 |
Our future capital requirements depend on many factors, including:
| ● | the costs and payments associated with license agreements for our product and technologies; | |
| ● | the costs of conducting pre-clinical and clinical studies and the cost of manufacturing our product candidates | |
| ● | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates, if clinical studies are successful, including any costs from post-market requirements; | |
| ● | the cost of commercialization activities for our product candidates, if any of these product candidates is approved for sale, including marketing, sales and distribution costs; | |
| ● | our ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and | |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any. |
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical studies or other development activities for one or more of our product candidates or delay, limit, reduce or terminate our establishment of sales, marketing and distribution capabilities or other activities that may be necessary to commercialize our product candidates.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies.
Until such time as we can generate substantial product revenues, we may attempt to finance our cash needs through equity offerings, debt financings, government and/or other third-party grants or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, our investors’ ownership interest will be diluted. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more clinical research or development programs, which would adversely impact our potential revenues, results of operations and financial condition.
Risks Related to the Development of Our Product Candidates
We are highly dependent on the services of third parties to conduct research and development of our pipeline, and our failure to maintain the services of such third parties could harm our business
We are highly dependent on third parties working in conjunction with our officers, employees, scientific advisory board and research institutions in the research and development of product candidates in our pipeline. Many of the techniques utilized in the development of our product candidates have been developed by Dr. Serhat Gumrukcu, and we rely on the services of Dr. Gumrukcu, and of G-Tech Bio LLC and SRI, in the continued development of our pipeline. Our future performance will depend on our ability to retain the services of Dr. Gumrukcu, G-Tech Bio LLC and SRI. The loss of the services of any of the foregoing, or of any of our key employees or scientific advisory board members could impede the achievement of our research, development, regulatory approvals and commercialization objectives.
| 20 |
The results of pre-clinical studies or earlier clinical studies are not necessarily predictive of future results, and if we fail to demonstrate efficacy in our pre-clinical studies and/or clinical trials in the future our future business prospects, financial condition and operating results will be materially adversely affected.
The success of our research and development efforts will depend upon our ability to demonstrate the efficacy of the treatments in our pipeline in pre-clinical studies, as well as in clinical trials following IND approval by the FDA. Pre-clinical studies involve testing potential product candidates in appropriate non-human disease models to demonstrate efficacy and safety.
Success in pre-clinical studies does not ensure that later clinical studies will generate adequate data to demonstrate the efficacy and safety of an investigational drug. Currently, several of our product candidates, including ENOB-HV-01, our autologous HIV curative treatment, ENOB-HV-11, our preventative HIV vaccine, ENOB-HV-12, our therapeutic HIV vaccine and ENOB-HB-01, our coopting HBV polymerase, our co-opting of virus machinery for SARS-Coronaviruses, including the cause of COVID-19, and Influenza viruses are all currently in various stages of pre-clinical development with ongoing and planned pre-clinical studies in conjunction with research institutions and third parties. We presented pre-clinical data on ENOB-HV-01 in May 2020 at the annual conference of the American Society of Gene and Cell Therapy, and participated in an INTERACT meeting with the FDA related to ENOB-HB-01 in June 2020. Additionally, we presented results on ENOB-HB-01 and ENOB-FL-01 in May 2020 at the annual conference of the American Society of Gene and Cell Therapy, and SARS-Coronavirus at the Conference on Retroviruses and Opportunistic Infections in March 2021. Despite preliminary data we believe is positive, this does not guarantee that any of these products will proceed to the clinical stage or to approval for commercial use. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience than us, have suffered significant setbacks in clinical studies, even after seeing promising results in earlier preclinical studies or clinical studies.
Regulatory agencies evaluate these data carefully before they will approve clinical testing in humans. If certain non-clinical data reveals potential safety issues or the results are inconsistent with an expectation of the potential product candidates’ efficacy in humans, the regulatory agencies may require additional more rigorous testing before allowing human clinical trials. This additional testing will increase program expenses and extend timelines. We may decide to suspend further testing on our product if, in the judgment of our management and advisors, the pre-clinical test results do not support further development.
| 21 |
Our novel gene, cell and immunotherapy product candidates and new therapeutic approaches could result in heightened regulatory scrutiny, delays in clinical development or delays in or our inability to achieve regulatory approval or commercialization of our product candidates.
Our future success is dependent on the successful development of novel gene, cell and immunotherapy product candidates. Because these programs, particularly our pipeline of allogeneic T-cell product candidates that are bioengineered from sick patients, represent a new approach to immunotherapy for the treatment of cancer and other diseases, developing and commercializing our product candidates subject us to a number of challenges.
Moreover, actual or perceived safety issues, including adoption of new therapeutics or novel approaches to treatment, may adversely influence the willingness of subjects to participate in clinical studies, or if approved by applicable regulatory authorities, of physicians to subscribe to the novel treatment mechanics. The FDA or other applicable regulatory authorities may ask for specific post-market requirements, and additional information informing benefits or risks of our products may emerge at any time prior to or after regulatory approval.
We face significant competition in an environment of rapid technological change and the possibility that our competitors may achieve regulatory approval before us or develop therapies that are more advanced or effective than ours, which may adversely affect our financial condition and our ability to successfully market or commercialize our product candidates.
The development of treatments in the fields of HIV, Hepatitis B, COVID-19 and Influenza prevention and treatment and cancer is highly competitive and many pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations may pursue the research and development of technologies, drugs or other therapeutic products for the treatment of some or all of the diseases we are targeting. Nearly all of our competitors have greater capital resources, larger overall research and development staffs and facilities, and a longer history in drug discovery and development, obtaining regulatory approval and pharmaceutical product manufacturing and marketing than we do. Techniques in gene, cell and immunotherapy are subject to rapid technological change and development and is significantly affected by existing rival products and medical procedures, new product introductions and the market activities of other participants. With additional resources, our competitors may be able to respond to the rapid and significant technological changes faster than we can. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies. We may also face competition from products, which have already been approved and accepted by the medical community for the treatment of these same indications. If we are unable to compete effectively with any existing products, new treatment methods and new technologies, we may be unable to commercialize therapeutic products that we may develop in the future, which could adversely impact potential revenues, results of operations and financial condition or lead to abandonment of product candidates in our pipeline.
Our reliance on third parties, such as university laboratories, contract manufacturing organizations and contract or clinical research organizations, may result in delays in completing, or a failure to complete, non-clinical testing or clinical trials if they fail to perform under our agreements with them.
In the course of the development of our pipeline, we have and expect to continue to engage university laboratories, non-profit organizations, independent contractors, other biotechnology companies or contract or clinical manufacturing organizations to conduct and manage research and development, pre-clinical and clinical studies and to manufacture materials for us to be used in pre-clinical and clinical testing. Due to engagements with these organizations, many important aspects of our research have been and will be out of our direct control. If any of these organizations we may engage in the future, fail to perform their obligations under our agreements with them or fail to perform non-clinical testing and/or clinical trials in a satisfactory manner, we may face delays in completing our clinical trials, as well as commercialization of any of our product candidates. Furthermore, any loss or delay in obtaining contracts with such entities may also delay the completion of our clinical trials, regulatory filings and the potential market approval of our product candidates.
Business interruptions resulting from the coronavirus disease 2019 (COVID-19) outbreak or similar public health crises could cause a disruption of the development of our product candidates and adversely impact our business.
Public health crises such as pandemics or similar outbreaks could adversely impact our business. In December 2019, a new strain of coronavirus surfaced in Wuhan, China and has reached multiple other regions and countries, including Los Angeles where our primary office and laboratory facilities are located. The COVID-19 pandemic continues to evolve, and to date has led to the implementation of various mitigation responses, including government-imposed quarantines, travel restrictions and other public health safety measures, as well as leading to reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries. COVID-19 may cause delays in our research activities. COVID-19 has not materially affected our operations to date, it has caused delays in the conduct of experiments due to limitations of various organizations, in particular those conducting experiments related to COVID-19. There have also been increases in the cost to conduct animal studies due to staffing and other limitations. The full extent to which COVID-19 may impact our operations or those of our third-party partners, including research organizations and suppliers will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, effectiveness of vaccines, additional or modified government actions, new information that will emerge concerning the severity and impact of COVID-19 and the actions to contain COVID-19 or address its impact in the short and long term, among others.
| 22 |
Additionally, timely initiation and completion of planned preclinical studies is dependent upon the availability of, for example, preclinical study sites, university researchers and investigators, regulatory agency personnel, and materials, which may be adversely affected by global health matters, such as pandemics. We plan to conduct preclinical studies in geographies that are currently being affected by COVID-19.
Further, in the event that governmental authorities were to further modify current restrictions, our employees conducting research and development activities may not be able to access our laboratory offices, and our core activities may be significantly limited or curtailed, possibly for an extended period of time.
Changes in healthcare law and implementing regulations, including government restrictions on pricing and reimbursement, as well as healthcare policy, may negatively impact our ability to generate revenues.
In the United States and some foreign jurisdictions, there have been a number of proposed legislative and regulatory changes related to the healthcare system that could affect our ability to profitably sell or commercialize our product candidates for which we obtain marketing approval in the future. The potential pricing and reimbursement environment for our product candidates may change in the future and become more challenging due to, among other reasons, policies advanced by the current or any new presidential administration, federal agencies, healthcare legislation passed by Congress, or fiscal challenges faced by all levels of government health administration authorities, or by similar changes in foreign countries. The implementation of any such changes could have a material adverse effect on our competitive position, business, financial condition, results of operations, and prospects, including our share price and ability to raise capital.
We have limited experience in drug development and may not be able to successfully develop any drugs, which would cause us to cease operations.
We have never successfully developed a new drug and brought it to market. Our management and clinical teams have experience in drug development but they may not be able to successfully develop any drugs. Our ability to achieve revenues and profitability in our business will depend on, among other things, our ability to develop products internally or to obtain rights to them from others on favorable terms; complete laboratory testing and human studies; obtain and maintain necessary intellectual property rights to our products; successfully complete regulatory review to obtain requisite governmental agency approvals; enter into arrangements with third parties to manufacture our products on our behalf; and enter into arrangements with third parties to provide sales and marketing functions. If we are unable to achieve these objectives we will be forced to cease operations and you will lose all of your investment.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would harm our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could harm our business.
| 23 |
The COVID-19 pandemic has also resulted in the FDA imposing preventive measures, including postponements of non-U.S. manufacturing and product inspections. If global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Our gene therapy product candidates are still in development and will require extensive clinical testing before we are prepared to submit an application for marketing approval to regulatory authorities. We cannot predict with any certainty if or when we might submit any such application for regulatory approval for our product candidates or whether any such application will be approved by the applicable regulatory authority in our target markets. Human clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For instance, regulatory authorities may not agree with our proposed endpoints for any clinical trials of our gene therapy product candidates, which may delay the commencement of our clinical trials. The clinical trial process is also time-consuming. We estimate that clinical trials of our product candidates will take at least several years to complete.
Clinical trials are expensive, time-consuming, difficult to design and implement, and involve an uncertain outcome.
Our product candidates are still in development and will require extensive clinical testing before we are prepared to submit an application for marketing approval to regulatory authorities. We cannot predict with any certainty if or when we might submit any such application for regulatory approval for our product candidates or whether any such application will be approved by the applicable regulatory authority in our target markets. Human clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For instance, regulatory authorities may not agree with our proposed endpoints for any clinical trials of our product candidates, which may delay the commencement of our clinical trials. The clinical trial process is also time-consuming. We estimate that clinical trials of our product candidates will take at least several years to complete.
A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials, and in the regulatory approval process. In addition, the design of a clinical trial, such as endpoints, inclusion and exclusion criteria, statistical analysis plans, data access protocols and trial sizing, can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our product candidates could be harmed, and our ability to generate revenues may be delayed. In addition, any delays in our clinical trials could increase our costs, cause a drop in our stock price, slow down the approval process and jeopardize our ability to commence product sales and generate revenues. Further, disruptions caused by the COVID-19 pandemic may increase the likelihood that we encounter such difficulties or delays in commencing or completing clinical trials. Any of these occurrences may harm our business, financial condition and results of operations.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials, and even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Patient enrollment and retention in clinical trials depends on many factors, including the size of the patient population, the nature of the trial protocol, the effectiveness of our patient recruitment efforts, delays in enrollment due to travel or quarantine policies, or other factors, related to COVID-19, the existing body of safety and efficacy data with respect to the study candidate, the perceived risks and benefits of gene therapy approaches for the treatment of certain diseases, the number and nature of competing existing treatments for our target indications, the number and nature of ongoing trials for other product candidates in development for our target indications, perceived risk of the delivery procedure, patients with pre-existing conditions that preclude their participation in any trial, the proximity of patients to clinical sites and the eligibility criteria for the study. Furthermore, the results we have reported in clinical trials to date and any other results we may report in clinical trials of any of our gene therapy product candidates in the future may make it difficult or impossible to recruit and retain patients in other clinical trials of those gene therapy product candidates. Similarly, negative results reported by our competitors about their product candidates may negatively affect patient recruitment in our clinical trials. Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our gene therapy product candidates or could render further development impossible. In addition, we expect to rely on clinical trial sites to ensure proper and timely conduct of our future clinical trials and, while we intend to enter into agreements governing their services, we will be limited in our ability to control their actual performance.
| 24 |
Risks Related to Our Intellectual Property
We have licensed a significant portion of our intellectual property from our licensors. If we breach any of our license agreements with these licensors, or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We hold rights under license agreements with our licensors, including Weird Science and G-Tech, , SRI and Dr. Serhat Gumrukcu that are important to our business. Our research and development platform is built, in part, around patent rights licensed from such licensors. Under our existing license agreements, we are subject to various obligations, including diligence obligations with respect to development and commercialization activities, provision of support with respect to development of licensed intellectual property, prosecution of intellectual property protection, payment obligations upon achievement of certain milestones and royalties on product sales. In spite of our efforts, our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If any of these licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products identical to ours and we may be required to cease our development and commercialization of product candidates covered by any such licenses. Any of the foregoing could have a material adverse effect on our competitive position, business, financial condition, results of operations, and prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
| ● | the scope of rights granted under license agreements and other interpretation-related issues; |
| ● |
payment obligations due to licensors under license agreements and other disputes related to the obligations for payment related to intellectual property protection;
| |
| ● | the extent to which our product candidates, technology and processes infringe on intellectual property of a licensor that is not subject to a licensing agreement; |
| ● | the sublicensing of patent and other rights under our collaborative development relationships; |
| ● | our diligence obligations under license agreements and what activities satisfy those diligence obligations; |
| ● | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us; and |
| ● | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations.
The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
If we do not obtain required intellectual property licenses or rights, we could encounter delays in our product development efforts while we attempt to design around other patents or even be prohibited from developing, manufacturing or selling products requiring these rights or licenses. There is also a risk that legal disputes may arise as to the rights to technology developed in collaboration with other parties, all with attendant risk, distraction, expense, and lack of predictability.
If we are unable to obtain and maintain sufficient intellectual property protection for our product candidates, or if the scope of the intellectual property protection is not sufficiently broad, our ability to commercialize our product candidates successfully and to compete effectively may be adversely affected.
We rely upon a combination of patents, trademarks, trade secrets and confidentiality agreements – either that we own or possess or that are owned or possessed by our licensors that are licensed to us – to protect the intellectual property related to our technology and product candidates. When we refer to “our” technologies, inventions, patents, provisional patents, patent applications or other intellectual property rights, we are referring to both the rights that we own or possess as well as those that we license, many of which are critical to our intellectual property protection and our business. For example, the product candidates and platform technology we have licensed from our licensors are protected primarily by patent or patent applications of our licensors that we have licensed and as confidential know-how and trade secrets. If the intellectual property that we rely on is not adequately protected, competitors may be able to use our technologies and erode or negate any competitive advantage we may have.
| 25 |
The patentability of inventions and the validity, enforceability and scope of patents in the biotechnology field is uncertain because it involves complex legal, scientific and factual considerations, and it has in recent years been the subject of significant litigation. Moreover, the standards applied by the U.S. Patent and Trademark Office, or USPTO, and non-U.S. patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology patents.
There is no assurance that all potentially relevant prior art relating to our patents and patent applications is known to us or has been found in the instances where searching was done. We may be unaware of prior art that could be used to invalidate an issued patent or prevent a pending patent application from issuing as a patent. There also may be prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim of one of our patents or patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of such claim. We also may not be able to obtain full patent protection from provisional patents for which we have sought or will seek further patent protection. As a consequence of these and other factors, our patent applications may fail to result in issued patents with claims that cover our product candidates in the U.S. or in other countries.
Even if patents have issued or do successfully issue from patent applications, and even if these patents cover our product candidates, third parties may challenge the validity, enforceability or scope thereof, which may result in these patents being narrowed, invalidated or held to be unenforceable. No assurance can be given that if challenged, our patents would be declared by a court to be valid or enforceable.
Even if unchallenged, our patents and patent applications or other intellectual property rights may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. The possibility exists that others will develop products on an independent basis which have the same effect as our product candidates and which do not infringe our patents or other intellectual property rights, or that others will design around the claims of patents that we have had issued that cover our product candidates. If the breadth or strength of protection provided by our patents and patent applications with respect to our product candidates is threatened, it could jeopardize our ability to commercialize our product candidates and dissuade companies from collaborating with us.
We may also desire to seek a license from a third party who owns intellectual property that may be useful for providing exclusivity for our product candidates, or for providing the ability to develop and commercialize a product candidate in an unrestricted manner. There is no guarantee that we will be able to obtain a license from such a third party on commercially reasonable terms, or at all.
In addition, the United States Patent and Trademark Office (USPTO) and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
We and our licensors have filed a number of patent applications covering our product candidates or methods of using or making those product candidates. We cannot offer any assurances about which, if any, patents will be issued with respect to these pending patent applications, the breadth of any such patents that are ultimately issued or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Because patent applications in the U.S. and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file any patent application related to a product candidate. We or our licensors may also become involved in proceedings regarding our patents, including patent infringement lawsuits, interference or derivation proceedings, oppositions, and inter partes and post-grant review proceedings before the USPTO, the European Patent Office and other non-U.S. patent offices.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be negatively impacted and our business would be harmed.
In addition to the protection afforded by patents we hold rights to, we also rely on trade secret protection for certain aspects of our intellectual property. However, trade secrets are difficult to protect. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, consultants, independent contractors, advisors, contract manufacturers, suppliers and other third parties. We also enter into confidentiality and invention or patent assignment agreements with employees and certain consultants. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we might not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. Further, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, it could have a material adverse effect on our business, financial condition, results of operations, and prospects.
| 26 |
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our success will depend in part on our ability to commercialize our product candidates without infringing the proprietary rights of others. Much of the intellectual property utilized in our product candidates is licensed from our licensors, who hold patents and provisional patents in their names. We have not conducted extensive freedom of use patent searches and no assurance can be given that patents do not exist or could be issued which would have an adverse effect on our ability to market our technology or maintain our competitive position with respect to our technology. We also cannot be sure that patents or provisional patents filed by others are valid or will be upheld if challenged. It is possible that there are additional patents that may cover certain other aspects of technology used in our product candidates that is not covered by our licensed intellectual property. If our licensed technology or other subject matter are claimed under other United States patents or other international patents or are otherwise protected by third party proprietary rights, we or our licensors may be subject to infringement actions. In such event, we may challenge the validity of such patents or other proprietary rights or we may be required to obtain licenses from such companies in order to develop, manufacture or market our technology. There can be no assurances that we would be successful in a challenge or be able to obtain such licenses or that such licenses, if available, could be obtained on commercially reasonable terms. Furthermore, the failure to succeed in a challenge, develop a commercially viable alternative or obtain needed licenses could have significant adverse consequences to the development of our pipeline. Adverse consequences include delays in marketing some or all of our product candidates based on our technology or the inability to proceed with the development, manufacture or sale of products requiring such licenses. If we defend ourselves against charges of patent infringement or to protect our proprietary rights against third parties, substantial costs will be incurred regardless of whether we are successful. Such proceedings are typically protracted with no certainty of success. An adverse outcome could subject us to significant liabilities to third parties and force us to curtail or cease the research and development of our technology.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Additionally, parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
| 27 |
Risks Related to our Common Stock
Our stock price has been and will likely continue to be volatile and may decline regardless of our operating performance.
Our stock price has fluctuated in the past and can be expected to be volatile in the future. From July 1, 2020 through June 30, 2021, the reported sale price of our Common Stock has fluctuated between $6.79 and $2.82 per share. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may experience losses on their investment in our Common Stock. The market price of our Common Stock may be influenced by many factors, including the following:
| ● | the success of competitive products or technologies; | |
| ● | regulatory actions with respect to our product candidates or products or our competitors’ product candidates or products; | |
| ● | actual or anticipated changes in our growth rate relative to our competitors; | |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; | |
| ● | results of clinical studies of our product candidates or those of our competitors; | |
| ● | regulatory or legal developments in the U.S. and other countries; | |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; | |
| ● | the recruitment or departure of key personnel; | |
| ● | the level of expenses related to any of our product candidates or clinical development programs; | |
| ● | the results of our efforts to in-license or acquire additional product candidates or products; | |
| ● | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; | |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; | |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; | |
| ● | inconsistent trading volume levels of our shares; | |
| ● | announcement or expectation of additional financing efforts; | |
| ● | sales of our Common Stock by us, our insiders or our other stockholders; | |
| ● | market conditions in the pharmaceutical and biotechnology sectors; | |
| ● | general economic, industry and market conditions; and | |
| ● | the other risks described in this “Risk Factors” section. |
In addition, the stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have experienced significant volatility that has often been unrelated to the operating performance of particular companies.
| 28 |
Our principal stockholders and management own a significant percentage of our stock and could exert significant control over matters subject to stockholder approval.
Our executive officers, directors, affiliates, and entities they control own approximately 62.9% of our outstanding Common Stock and voting power. These stockholders, should they act in concert, could determine the outcome of all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our Common Stock. The interests of our significant stockholders who are also affiliates may not always coincide with the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their Common Stock, and might affect the market price for our Common Stock.
Sales of a substantial number of shares of our Common Stock in the public market could cause our stock price to fall.
A significant portion of our Common Stock is held in restricted form, and consequentially a minority of our outstanding Common Stock actively trades in the public markets. Sales of a substantial number of such shares of our Common Stock in the public market could occur at any time. While a large majority of such shares are unregistered and subject to volume restrictions on sale pursuant to Rule 144 under the Securities Act, these restrictions could be lifted if any of our stockholders ceased to be bound by such restrictions. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our Common Stock.
Trading of our Common Stock may be volatile and sporadic, which could depress the market price of our Common Stock and make it difficult for our stockholders to resell their shares.
There is currently a limited market for our Common Stock and the volume of our Common Stock traded on any day may vary significantly from one period to another. Trading in our stock is often thin and characterized by wide fluctuations in trading prices, due to many factors that may have little to do with our operations or business prospects. The availability of buyers and sellers represented by this volatility could lead to a market price for our Common Stock that is unrelated to operating performance. There is no assurance that a sufficient market will develop in the stock, in which case it could be difficult for our stockholders to resell their stock.
We have incurred and will continue to incur increased costs as a result of being a public company and our management expects to devote substantial time to public company compliance programs.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses. We are subject to the reporting requirements of the Exchange Act, which require, among other things, that we file with the SEC annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and The NASDAQ Stock Market to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. As a Smaller Reporting Company and Non-accelerated Filer, we are able to take advantage of certain accommodations afforded to such companies, including being exempt from the requirement to conduct an audit of our internal controls. In the event we no longer qualify as a Smaller Reporting Company and Non-accelerated Filer, we will lose such accommodations, which could involve significant costs that could affect our operations. Changes in reporting requirements, the current political environment and the potential for future regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
The rules and regulations applicable to public companies have substantially increased our legal and financial compliance costs and make some activities more time-consuming and costly. To the extent these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be the sole source of potential gain for our stockholders.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. As a result, capital appreciation, if any, of our Common Stock will be the sole source of gain for our stockholders for the foreseeable future.
Future sales and issuances of our Common Stock or rights to purchase Common Stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell substantial amounts of Common Stock or securities convertible into or exchangeable for Common Stock in one or more transactions at prices and in a manner we determine from time to time. These future issuances of Common Stock or Common Stock-related securities, together with the exercise of outstanding options or warrants, and any additional shares that may be issued in connection with acquisitions or licenses, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our Common Stock. Pursuant to our equity incentive plans, our compensation committee is authorized to grant equity-based incentive awards to our employees, non-employee directors and consultants. Future grants of RSUs, options and other equity awards and issuances of Common Stock under our equity incentive plans will result in dilution and may have an adverse effect on the market price of our Common Stock.
| 29 |
Some terms of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Our Certificate of Incorporation, and our Bylaws, as well as Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include terms that:
| ● | permit our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate; |
| ● | provide that all vacancies on our board of directors, including as a result of newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; | |
| ● | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; and | |
| ● | not provide for cumulative voting rights, thereby allowing the holders of a majority of the shares of Common Stock entitled to vote in any election of directors to elect all of the directors standing for election. |
Any of the factors listed above may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management.
In addition, because we are incorporated in Delaware, we are governed by Section 203 of the Delaware General Corporation Law, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any term of our Certificate of Incorporation or Bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock and could also affect the price that some investors are willing to pay for our Common Stock.
Risks Related To Our Business Operations and Managing Growth
If our operations require a full time Chief Medical Officer, and we are not able to hire a full time Chief Medical Officer to manage our clinical operations or if our current Chief Executive Officer, Chief Financial Officer or key scientific personnel cease to serve, our business will be harmed.
Currently, our management team is led by Dr. Mark Dybul, the Chief Executive Officer and Luisa Puche, our Chief Financial Officer. If Dr. Dybul or Ms. Puche should cease to serve, our business operations may suffer. Additionally, we may in the future require a Chief Medical Officer, and if we are unable to hire a Chief Medical Officer, our business operations and the continued development of our product candidates may suffer.
In addition, we are dependent on our continued ability to attract, retain and motivate highly qualified additional management and scientific personnel. If we are not able to retain our management and to attract, on acceptable terms, additional qualified personnel necessary for the continued development of our business, we might not be able to sustain our operations or grow.
We have limited corporate infrastructure and may experience difficulties in managing growth.
As of June 30, 2021, we had only 11 full time employees and we rely on third-party contractors for the provision of professional, scientific, regulatory and other services. As our development and commercialization plans and strategies develop, we may need additional managerial, scientific, operational, financial, and other resources. Our management may need to divert a disproportionate amount of its attention away from our day-to-day operations and devote a substantial amount of time to managing these growth activities. We might not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational inefficiencies, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our current and potential future product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and grow revenue could be reduced and we might not be able to implement our business strategy. Our future financial performance, our ability to commercialize product candidates, develop a scalable infrastructure and compete effectively will depend, in part, on our ability to effectively manage any future growth.
If we, our service providers, or third parties fail to comply with environmental and health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
If we, our service providers, or any third parties engaged in development of our product candidates fail to comply with laws regulating the protection of the environment, health and animal and human safety, we could be subject to enforcement actions and our business prospects could be adversely affected.
| 30 |
Our research and development activities, and the research and development activities of our service providers and any third parties engaged in development of our product candidates, may involve the use of hazardous materials and chemicals or other regulated activities. In conjunction with our service providers and other third parties, we are also engaged in pre-clinical studies using live animals and samples of infectious diseases. Failure to adequately handle and dispose of hazardous materials or to meet various standards imposed by federal, state, local or foreign regulators could lead to liabilities for resulting damages, which could be substantial. We also may be subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of bio-hazardous materials.
If we, our service providers, or any third parties engaged in development of our product candidates fail to comply with applicable federal, state, local or foreign laws or regulations, we could be subject to enforcement actions, which could adversely affect our ability to develop, market and sell our product candidates successfully and could harm our reputation and lead to reduced acceptance of our product candidates. These enforcement actions may include:
| ● | restrictions on, or prohibitions against, marketing our product candidates; |
| ● | restrictions on importation of our product candidates; |
| ● | suspension of review or refusal to approve new or pending applications; |
| ● | suspension or withdrawal of product approvals; |
| ● | product seizures; |
| ● | injunctions; and |
| ● | civil and criminal penalties and fines. |
We rely upon information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cyber security incidents, could harm our ability to operate our business effectively.
Our business operations could suffer in the event of system failure. Despite the implementation of security measures, our internal computer systems and those of our contract research organizations, and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of formulas or data from completed or ongoing or planned pre-clinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and further development of our product candidates could be delayed.
Our business plan may lead to the initiation of one or more product development programs, the discontinuation of one or more development programs, or the execution of one or more transactions that you do not agree with or that you do not perceive as favorable to your investment.
We are pursuing a strategy to leverage our clinical experience and expertise for the clinical development and regulatory approval of our gene therapy product candidates. As part of our ongoing business strategy, we continue to explore potential opportunities to acquire or license new product candidates and to collaborate on our existing products in development. We cannot be certain that our product candidates will be successfully developed, or that the early clinical trial results of these product candidates will be predictive of future clinical trial results. We may determine at any time that one or more of our in-licensed product candidates is not suitable for continued development due to cost, feasibility of obtaining regulatory approvals or any other reason, and may terminate the related license.
| 31 |
This business plan requires us to be successful in a number of challenging, uncertain and risky activities, including pursuing development of our gene therapy product candidates in indications for which we have limited or no human clinical data, designing and executing a nonclinical and/or clinical development program for our product candidates, building internal or outsourced gene therapy capabilities, converting early stage gene therapy research efforts into clinical development opportunities, identifying additional promising new assets for development that are available for acquisition or in-license and that fit our strategic focus and identifying potential partners to collaborate on our products. We may not be successful at one or more of the activities required for us to execute this business plan. In addition, we may consider other strategic alternatives, such as mergers, acquisitions, divestitures, joint ventures, partnerships and collaborations. We cannot be sure when or if any type of transaction will result. Even if we pursue a transaction, such transaction may not be consistent with our stockholders’ expectations or may not ultimately be favorable for our stockholders, either in the shorter or longer term.
Our growth prospects and the future value of our Company are primarily dependent on the progress of our ongoing and planned development programs for our product candidates as well as the outcome of our ongoing business development efforts and pipeline [expansion activities/progression], together with the amount of our remaining available cash. The development of our product candidates and the outcome of our ongoing business development efforts and pipeline [expansion activities/progression] are highly uncertain. We expect to continue to reassess and make changes to our existing development programs and pipeline strategy. Our plans for our development programs may be affected by the results of competitors’ clinical trials of product candidates addressing our current target indications, and our business development efforts and pipeline [expansion activities/progression] may also be affected by the results of competitors’ ongoing research and development efforts. We may modify, expand or terminate some or all of our development programs, clinical trials or collaborative research programs at any time as a result of new competitive information or as the result of changes to our product pipeline or business development strategy.
If serious adverse events or other undesirable side effects or safety concerns attributable to our product candidates occur, they may adversely affect or delay our clinical development and commercialization of some or all of our product candidates.
Undesirable side effects or safety concerns caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt our clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval. Although no treatment-related serious adverse events (“SAEs”) were observed in any clinical trials of any of our product candidates to date, if treatment-related SAEs or other undesirable side effects or safety concerns, or unexpected characteristics attributable to our product candidates are observed in any future clinical trials, they may adversely affect or delay our clinical development and commercialization of the effected product candidate, and the occurrence of these events could have a material adverse effect on our business and financial prospects. Results of our future clinical trials could reveal a high and unacceptable severity and prevalence of adverse side effects. In such an event, our trials could be suspended or terminated and the FDA or other regulatory agency could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims.
Additionally, if any of our product candidates receives marketing approval and we or others later identify undesirable or unacceptable side effects or safety concerns caused by these product candidates, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw, suspend, or limit approvals of such product and require us to take them off the market; | |
| ● | regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies; | |
| ● | regulatory authorities may require a medication guide outlining the risks of such side effects for distribution to patients, or that we implement a REMS or REMS-like plan to ensure that the benefits of the product outweigh its risks; | |
| ● | we may be required to change the way a product is distributed or administered, conduct additional clinical trials or change the labeling of a product; | |
| ● | we may be required to conduct additional post-marketing studies or surveillance; | |
| ● | we may be subject to limitations on how we may promote the product; | |
| ● | sales of the product may decrease significantly; | |
| ● | we may be subject to regulatory investigations, | |
| ● | government enforcement actions, litigation or product liability claims; and | |
| ● | our products may become less competitive or our reputation may suffer. |
| 32 |
Any of these events could prevent us or any collaborators from achieving or maintaining market acceptance of our product candidates or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating revenue from the sale of our product candidates.
We have no manufacturing experience, and the failure to comply with all applicable manufacturing regulations and requirements could have a materially adverse effect on our business.
We have never manufactured products in the highly regulated environment of pharmaceutical manufacturing, and our team has limited experience in the manufacture of drug therapies. There are numerous regulations and requirements that must be maintained to obtain licensure and permitting required prior to the commencement of manufacturing, as well as additional requirements to continue manufacturing pharmaceutical products. In addition, we do not have the resources at this time to acquire or lease suitable facilities. If we or our CMO fail to comply with regulations, to obtain the necessary licenses and knowhow or to obtain the requisite financing in order to comply with all applicable regulations and to own or lease the required facilities in order to manufacture our products, we could be forced to cease operations, which would cause you to lose all of your investment.
In addition, the FDA and other regulatory authorities require that product candidates and drug products be manufactured according to cGMP. Any failure by our third-party manufacturers to comply with cGMP could lead to a shortage of our product candidates. In addition, such failure could be the basis for action by the FDA to withdraw approval, if granted to us, and for other regulatory enforcement action, including Warning Letters, product seizure, injunction or other civil or criminal penalties.
Product candidates that we develop may have to compete with other products and product candidates for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing for us and willing to do so. If we need to find another source of drug substance or drug product manufacturing for our product candidates, we may not be able to identify, or reach agreement with, commercial-scale manufacturers on commercially reasonably terms, or at all. If third parties that we engage in the future to manufacture a product for commercial sale or for our clinical trials, should cease to continue to do so for any reason, we likely would experience significant delays in obtaining sufficient quantities of product for us to meet commercial demand or to advance our clinical trials while we identify and qualify replacement suppliers. If for any reason we are unable to obtain adequate supplies of any product candidate that we develop, or the drug substances used to manufacture it, it will be more difficult for us to compete effectively, generate revenue, and further develop our products. In addition, if we are unable to assure a sufficient quantity of the drug for patients with rare diseases or conditions, we may lose any Orphan Drug exclusivity to which the product otherwise would be entitled.
We may, in the future, choose to seek FDA Orphan Drug designation for one or more of our current or future CNS product candidates. Even if we obtain Orphan Drug designation from the FDA for a product candidate, there are limitations to the exclusivity afforded by such designation.
In the U.S., the company that first obtains FDA approval for a designated orphan drug for the specified rare disease or condition receives orphan drug marketing exclusivity for that drug for a period of seven years. This orphan drug exclusivity prevents the FDA from approving another application, including a full NDA to market the same drug for the same orphan indication, except in very limited circumstances, including when the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the drug in question. To obtain Orphan Drug status for a drug that shares the same active moiety as an already approved drug, it must be demonstrated to the FDA that the drug is safer or more effective than the approved orphan designated drug, or that it makes a major contribution to patient care. In addition, a designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the U.S. may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition or if another drug with the same active moiety is determined to be safer, more effective, or represents a major contribution to patient care.
1B. Unresolved Staff Comments
There are no unresolved SEC staff comments.
| 33 |
Item 2. Properties
The Company currently leases the following properties:
| Location | Use | Terms | ||
| 5901 W. Olympic
Blvd, Suite 419 Los Angeles, CA 90036 |
Physical office space | On November 13, 2017, the Company entered into a Lease Agreement for a term of five years and two months from November 1, 2017. The Leased Premises consist of approximately 2,325 rentable square feet. The base rent for such leased premises increases by 3% each year over the term, and ranges from approximately $8,719 per month for the first year to $10,107 per month for the two months of the sixth year. The Company was entitled to $70,800 in tenant improvement allowance in the form of free rent applied over 10 months in equal installments from January 2018. | ||
| 2080 Century
Park East, Suite 906 Los Angeles, CA 90067 |
Headquarters | The Company entered into a Lease Agreement on June 19, 2018 for our corporate headquarters located at Century City Medical Plaza. We have a ten-year lease that was for approximately 2,453 square feet at this location. In February 2019, we extended our corporate headquarters to encompass the adjoining suite for approximately 1,101 square feet, bringing the total workspace to 3,554 square feet. The new base rent for this leased premises increases by 3% each year over the term, and ranges from $17,770 per month as of the date of the amendment until the end of the first year to $ 23,186 per month for the tenth year. The additional suite was in the form of an amendment to the original lease and will expire on the same date as the original lease. The Company was entitled to a total of $148,168 in contributions toward tenant improvements for both spaces. |
Item 3. Legal Proceedings
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. We are not currently a party to any legal proceeding that we believe would have a material adverse effect on our business, financial condition or operating results.
Item 4. Mine Safety Disclosures.
Not applicable.
| 34 |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information and Holders of our Common Stock
Our Common Stock trades on the Nasdaq Capital Market under the symbol “ENOB”.
As of September 28, 2021, the Company had 52,219,661 shares of Common Stock issued and outstanding and approximately 227 stockholders of record. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Recent Sales of Unregistered Securities
From March 18, 2021 through June 9, 2021, the Company issued 408,164 shares of Common Stock at a price of $3.92 per share pursuant to a private placement for total proceeds to the Registrant of $1,600,000.
Company Purchases of Equity Securities
[None]
Dividends
The Company has not declared or paid any cash dividends on its Common Stock and does not intend to declare or pay any cash dividend in the foreseeable future. The payment of dividends, if any, is within the discretion of the Board and will depend on the Company’s earnings, if any, its capital requirements and financial condition and such other factors as the Board may consider.
Item 6. [Reserved]
| 35 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements, and the related notes to those statements included elsewhere in this report. In addition to the historical financial information, the following discussion and analysis contain forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements.
Our Business
Enochian BioSciences, Inc. is a biopharmaceutical company focused on developing innovative platforms for gene-modified cellular and immune therapies to potentially cure and treat deadly diseases. The company’s gene-modified cell and immune therapy platforms can potentially be applied to multiple indications, including HIV/AIDS, Hepatitis B, all Corona and Influenza viruses, and Oncology.
To date, our operations have been funded by sales of our securities and debt financing. We have never generated any sales revenue and we expect this to continue until our therapies or products are approved for marketing in the United States and/or Europe. Even if we are successful in having our therapies or products approved for sale in the United States and/or Europe, we cannot guarantee that a market for the therapies or products will develop. We may never be profitable.
Recent Developments
On August 11, 2021, and with an effective date of July 1, 2021, the Company and Dr. Dybul entered into an Employment Agreement, the form of which was approved and recommended by the Board on October 30, 2019 and approved by stockholders of the Company with a majority of the voting power of all shares of the Company’s capital stock entitled to vote on the matter on October 31, 2019. The material terms of the Employment Agreement are as set forth in the Company’s Information Statement filed with the Securities and Exchange Commission on November 12, 2019.
COVID-19
The COVID-19 pandemic continues to evolve, and to date has led to the implementation of various mitigation responses, including government-imposed quarantines, travel restrictions and other public health safety measures, as well as leading to reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries. COVID-19 may cause delays in our research activities. To date, it has not materially affected our operations; however it has caused delays in the conduct of experiments due to limitations of various organizations, in particular those conducting experiments related to COVID-19. There have also been increases in the cost to conduct animal studies due to staffing and other limitations.
The full extent to which the COVID-19 pandemic may impact our business and operations is subject to future developments, which are uncertain and difficult to predict. Further quarantines, shelter-in-place or similar restrictions and other actions taken or imposed by foreign, federal, state and local governments could adversely impact our or our partners’ clinical, research and development, regulatory and manufacturing operations or timelines.
We continue to monitor the impact of the COVID-19 pandemic on our business and operations and will seek to adjust our activities as appropriate. In addition, the pandemic could result in significant and prolonged disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect the financial resources available to us.
| 36 |
RESULTS OF OPERATIONS
Year ended June 30, 2021 compared to the year ended June 30, 2020.
The following table sets forth our revenues, expenses and net income for the years ended June 30, 2021 and 2020. The financial information below is derived from our audited consolidated financial statements included elsewhere in this Annual Report.
| For the Year Ended | ||||||||||||||||
| June 30, | Increase/(Decrease) | |||||||||||||||
| 2021 | 2020 | $ | % | |||||||||||||
| Operating Expenses | ||||||||||||||||
| General and administrative expenses | $ | 7,740,130 | $ | 7,120,835 | $ | 619,295 | 9 | % | ||||||||
| Research and development expenses | 15,538,122 | 4,694,349 | 10,843,773 | 231 | % | |||||||||||
| Depreciation and amortization | 123,535 | 108,584 | 14,951 | 14 | % | |||||||||||
| Total Operating Expenses | 23,401,787 | 11,923,768 | 11,478,019 | 96 | % | |||||||||||
| LOSS FROM OPERATIONS | (23,401,787 | ) | (11,923,768 | ) | (11,478,019 | ) | (96 | )% | ||||||||
| Other Income (Expenses) | ||||||||||||||||
| Change in fair value of contingent consideration | (3,048,033 | ) | 274,566 | (3,322,599 | ) | (1,210 | )% | |||||||||
| Interest expense | (379,608 | ) | (104,280 | ) | (275,328 | ) | 264 | % | ||||||||
| Gain (Loss) on currency transactions | (32,634 | ) | 146,828 | (179,462 | ) | (122 | )% | |||||||||
| Gain on settlement | — | 140,000 | (140,000 | ) | (100 | )% | ||||||||||
| Interest income | 13,179 | 50,296 | (37,117 | )) | (74 | )% | ||||||||||
| Total Other Income (Expenses) | (3,447,096 | ) | 507,410 | (3,954,506 | ) | (779 | )% | |||||||||
| Loss Before Income Taxes | (26,848,883 | ) | (11,416,358 | ) | (15,432,525 | ) | 135 | % | ||||||||
| Income Tax Benefit | 125,276 | — | 125,276 | 100 | % | |||||||||||
| NET LOSS | $ | (26,723,607 | ) | $ | (11,416,358 | ) | $ | (15,307,249 | ) | 134 | % | |||||
| 37 |
| For the Year Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Net Loss | $ | (26,723,607 | ) | $ | (11,416,358 | ) | ||
| Other Comprehensive Income (Loss) | ||||||||
| Foreign Currency Translation Gain (Loss), Net of Taxes | 30,582 | (143,234 | ) | |||||
| Comprehensive Loss | $ | (26,693,025 | ) | $ | (11,559,592 | ) | ||
Revenues
We are a pre-revenue, pre-clinical biotechnology company. We have never generated revenues and have incurred losses since inception. We do not anticipate earning any revenues until our therapies or products are approved for marketing and sale.
Operating Expenses
Our operating expenses for the years ended June 30, 2021 and 2020 were $23,401,787 and $11,923,768, respectively, representing an increase of $11,478,019, or 96%. The largest contributor to the increase in operating expenses for the year ended June 30, 2021, was the increase in research and development expenses of $10,843,773 in connection with the continued growth in our research and development efforts, and an increase in general and administrative expenses of $619,295.
General and administrative expenses for the years ended June 30, 2021 and 2020 were $7,740,130 and $7,120,835, respectively, representing an increase of $619,295, or 9%. General and administrative expenses include audit fees, non-cash compensation expenses, consulting expenses, board compensation, filing fees, corporate taxes, security expenses, legal fees, office leases, insurance, patent fees, salaries, investor relations, travel expenses and other general and administrative expenses. The increase in general and administrative expenses are primarily related to increases of $416,076 in stock-based compensation, $254,512 in salaries, $179,363 related to insurance, and $99,880 in investor relations, partially offset by decreases in legal and security fees and laboratory costs totaling approximately $420,862.
Research and development expenses for the years ended June 30, 2021, and June 30, 2020, were $15,538,122 and $4,694,349, respectively, representing an increase of $10,843,773 or 231%. Overall research and development expenses remained consistent with the exception of the $10 million upfront fees related to the Coronavirus and Influenza License Agreement discussed above, and approximately $657,000 in collaboration partner costs in the development of our product lines ENOB-HV-12 and DC-11 in the year ended June 30, 2021.
Other Income (Expense)
Net other income (expense) for the years ended June 30, 2021 and 2020 was $(3,447,096) and $507,410, respectively, representing an increase of 3,954,506 or 779%. The increase in other expense was due primarily to the change in the fair value of contingent consideration of $3,322,000, which is impacted by contingent shares issued during the period and the mark to market adjustments on the remaining contingent consideration liability, as well as an increase in interest expense of approximately $275,000.
Net Loss
Net loss for the years ended June 30, 2021 and June 30, 2020 was $26,723,607 and $11,416,358, respectively, representing an increase in net loss of $15,307,249, or 134%. The increase in net loss was primarily due to the upfront fees of $10 million related to the Coronavirus and Influenza License Agreement recorded in research and development costs, an approximate increase in the change in fair value of contingent consideration of $3,332,599 and $619,295 increase in general and administrative expenses.
| 38 |
Liquidity and Capital Resources
We have historically satisfied our capital and liquidity requirements through funding from shareholders, the sale of our Common Stock and warrants, and debt financing. We have never generated any sales revenue to support our operations and we expect this to continue until our therapies or products are approved for marketing in the United States and/or Europe. Even if we are successful in having our therapies or products approved for sale in the United States and/or Europe, we cannot guarantee that a market for the therapies or products will develop. We may never be profitable.
At this time, we believe we have sufficient liquidity and access to committed funds to fund our operations for the next twelve months. We may need additional funds for (a) the purchase of equipment, (b) increases in personnel, and, (c) research and development, specifically to advance towards an Investigational New Drug Application (IND) following Pre-IND readouts from the FDA for ENOB CV-01, ENOB-HB-01, ENOB-HV-01, and ENOB-HV-21. We will also require additional funding to continue our research and development of ENOB-HV11/12 and ENOB-DC11 and ENOB-FL-01 and -11 and ENOB-CV-11, to fund the Coronavirus and Influenza Indications License Agreement in furtherance of the treatment related to all coronaviruses, and for possible future strategic acquisitions of businesses, products or technologies complementary to our business. If additional funds are required, we may raise such funds from time to time through public or private sales of our equity or debt securities. Such financing may not be available on acceptable terms, or at all, and our failure to raise capital when needed could materially adversely affect our growth plans and our financial condition and results of operations.
As of June 30, 2021, the Company had $20,664,410 in cash and working capital of $19,013,100 as compared to $8,696,361 in cash and working capital of $7,606,411 as of June 30, 2020. The increase in cash of $11,968,049 is primarily due to the proceeds from the various equity raises throughout the year that totaled approximately $33,066,148, partially offset by the cost of operations primarily related to research and development costs of $15,500,000 (see Note 8 of the Financial Statements.)
Equity
On July 8, 2020, we entered into a purchase agreement (the “LPC Purchase Agreement”) with Lincoln Park Capital Fund, LLC, (“LPC”), pursuant to which LPC is committed to buy, and we have the right, but not the obligation, to sell to LPC up to an aggregate of $20,000,000 of our Common Stock, subject to certain limitations and conditions set forth in the LPC Purchase Agreement, including a limitation on the number of shares of Common Stock we can put to LPC and the pricing parameters for the sales. For the year ended June 30, 2021, the Company issued 200,000 shares of Common Stock for proceeds of $1,221,350 (see Note 7 of the Financial Statements.)
Pursuant to a private placement offering, the Company issued 1,275,719 shares of Common Stock resulting in proceeds of $5,000,800. The Company effected the issuances of the shares of Common Stock from March 15, 2021 to June 9, 2021. The private placement was made directly by the Company in reliance upon Regulation S of the Securities Act of 1933. No underwriter or placement agent was engaged by the Company for this private placement (see Note 7 of the Financial Statements.)
On June 14, 2021, the Company and certain institutional investors entered into a securities purchase agreement (the “Purchase Agreement”), pursuant to which the Company agreed to sell to such investors an aggregate of 3,866,668 shares of Common Stock, in a registered direct offering, for gross proceeds of approximately $29 million (the “Financing”). The purchase price for each share of Common Stock was $7.50. The Company agreed not to issue or enter into any agreement to issue Common Stock from June 14, 2021 until ninety (90) days after the closing of the Financing. The Company entered into a letter agreement dated June 14, 2021 (the “Letter Agreement”) with H.C. Wainwright & Co., LLC, as exclusive placement agent (the “Placement Agent”), pursuant to which the Placement Agent agreed to act as the exclusive placement agent for the Financing. The Company agreed to pay the Placement Agent an aggregate fee equal to 7.0% of the gross proceeds raised in the Financing. The Company also agreed to pay the Placement Agent certain expenses. The Company paid $2,090,000 in commissions and incurred offering expenses, and issuance costs of $66,011, resulting in net proceeds of $26,843,999 in connection with the Financing. The Financing closed on June 16, 2021 (see Note 7 of the Financial Statements.)
Warrant Exercises
On July 3, 2019, certain of our warrant holders exercised warrants to purchase 500,000 shares of Common Stock for total proceeds to the Company of $1,000,000. For the year ended June 30, 2021, the Company issued 63,122 shares of Common Stock for total proceeds of $82,056 upon the exercise of warrants.
Debt
On February 6, 2020, the Company issued two Convertible Notes (the “Convertible Notes”) to an existing stockholder of the Company each with a face value amount of $600,000, convertible into shares of Common Stock. The holder did not exercise the conversion feature that expired on February 6, 2021. The outstanding principal amount of the Convertible Notes is due and payable on February 6, 2023. Interest on the Convertible Notes commenced accruing on the date of issuance at six percent (6%) per annum, computed on the basis of twelve 30-day months, and is compounded monthly on the final day of each calendar month based upon the principal and all accrued and unpaid interest outstanding as of such compound date. The interest is payable in cash on a semi-annual basis. For the years ended June 30, 2021 and 2020, the interest expense amounted to $72,967 and $30,302, respectively (see Note 5 to the Financial Statements.)
On March 30, 2020 (the “Issuance Date”), the Company issued a Promissory Note in the principal amount of $5,000,000 (the “Unsecured Note”) to Paseco APS (the “Holder”), a Danish limited company and an existing stockholder of the Company. The principal amount of the Unsecured Note will be payable on November 30, 2021, and bears interest at a fixed rate of 6% per annum, which was prepaid by the Company in full on the date of issuance through the issuance of 188,485 shares of the Company’s Common Stock based on the closing market price on that date, valued at $501,370. On February 11, 2021, the Company and the Holder amended the original Unsecured Note to extend the maturity date to November 30, 2022. The Company prepaid in full all accrued interest from November 30, 2021 to the new maturity date November 30, 2022, through the issuance of 74,054 shares of Common Stock based on the closing market price on that date, valued at $299,178. (See Note 5 to the Financial Statements).
| 39 |
Cash Flows
Year ended June 30, 2021 compared to the year ended June 30, 2020
Following is a summary of the Company’s cash flows provided by (used by) operating, investing, and financing activities:
| For the Year Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Net Cash Used by Operating Activities | $ | (20,610,723 | ) | $ | (10,459,422 | ) | ||
| Net Cash Used by Investing Activities | (48,892 | ) | (184,463 | ) | ||||
| Net Cash Provided by Financing Activities | 32,601,553 | 7,200,000 | ||||||
| Effect of exchange rates on cash | 26,111 | (141,978 | ) | |||||
| Net Increase (Decrease) in Cash | $ | 11,968,049 | $ | (3,585,863 | ) | |||
At June 30, 2021, we had cash and cash equivalents of $20,664,410, an increase of $11,968,049, when compared to the June 30, 2020 balance of $8,696,361. This increase was primarily due to cash provided by financing activities, primarily from equity sale transactions, partially offset by cash used in operating activities.
We plan to use our cash and cash equivalents to fund research and development, specifically to open an Investigational New Drug Application (IND) (the first step in the drug review process by the FDA) for ENOB CV-01, ENOB-HB01, ENOB-HV01, and ENOB-HV21, to continue our research and development of ENOB-HV11/12 and ENOB-DC11, and to fund the Coronavirus and Influenza Indications License Agreement in furtherance of the Treatment, and possible future strategic acquisitions of businesses, products or technologies complementary to our business. These activities will require an increase in staffing and possible additional property, plant and equipment purchases to support the expected growth.
Cash used by operating activities represents the cash receipts and disbursements related to all of our activities other than investing and financing activities. Operating cash flow is derived by adjusting our net income for non-cash items and changes in operating assets and liabilities. Net cash used by operating activities for the years ended June 30, 2021 and 2020 was $20,610,723 and $10,459,422, respectively, representing an increase of $10,151,301. The increase is primarily related to the increase in our net loss as adjusted for non-cash items, partially offset by changes in our operating assets and liabilities of $932,133.
Net cash used by investing activities for the years ended June 30, 2021 and 2020 was $48,892 and $184,463, respectively, representing a decrease of $135,571. The decrease is primarily due to lower costs incurred in purchases of equipment in the current year.
Net cash provided by financing activities for the years ended June 30, 2021 and 2020 was $32,601,553 and $7,200,000, respectively, representing an increase of $25,401,553. The net cash provided by financing activities in the current year consists primarily of $26,843,998 of net proceeds from the issuance of Common Stock as part of a direct offering, $5,000,800 of proceeds from the issuance of Common Stock through a private placement, and $1,221,350 proceeds from issuance of Common Stock related to equity line draws. The prior year net cash from financing activities primarily consisted of $1,200,000 related to a convertible note payable, $5,000,000 related to a long-term note payable, and $1,000,000 related to warrants exercised.
| 40 |
Off-Balance Sheet Arrangements
As of June 30, 2021, and 2020, we had no off-balance sheet arrangements. We are not aware of any material transactions, which are not disclosed in our consolidated financial statements.
Significant Accounting Policies and Critical Accounting Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses. On an on-going basis, we evaluate our critical accounting policies and estimates. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable in the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions and conditions. Our most critical accounting estimates are detailed below, and our significant accounting policies are more fully described in Note 1 of the accompanying consolidated financial statements.
Intangible Assets - The Company has both Definite and Indefinite life intangible assets.
Definite life intangible assets relate to patents. The Company accounts for definite life intangible assets in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 350, Goodwill and Other Intangible Assets. Intangible assets are recorded at cost. Patent costs capitalized consist of costs incurred to acquire the underlying patent. If it is determined that a patent will not be issued, the related remaining capitalized patent costs are charged to expense. Definite life intangible assets are amortized on a straight-line basis over their estimated useful life. The estimated useful life of patents is twenty years from the date of application.
Indefinite life intangible assets include license agreements and goodwill acquired in a business combination. The Company accounts for indefinite life intangible assets in accordance with ASC 350, License agreement costs represent the fair value of the license agreement on the date acquired and are tested annually for impairment.
Goodwill - Goodwill is not amortized but is evaluated for impairment annually as of June 30 or whenever events or changes in circumstances indicate the carrying value may not be recoverable.
Impairment of Goodwill and Indefinite Lived Intangible Assets – We test for goodwill impairment at the reporting unit level, which is one level below the operating segment level. Our detailed impairment testing involves comparing the fair value of each reporting unit to its carrying value, including goodwill. Fair value reflects the price a market participant would be willing to pay in a potential sale of the reporting unit and is based on discounted cash flows or relative market-based approaches. If the carrying value of the reporting unit exceeds its fair value, we record an impairment loss for such excess. The carrying value of IPR&D and goodwill at June 30, 2021, were $154,824,000 and $11,640,000, respectively.
For indefinite-lived intangible assets, such as licenses acquired as an IPR&D asset, on an annual basis we determine the fair value of the asset and record an impairment less, if any, for the excess of the carrying value of the asset over its fair value. The fair value analysis performed on the license agreement, and the annual fair value analysis performed on goodwill supported that both indefinite life intangible assets are not impaired as of June 30, 2021 (see Note 3.)
Fair Value of Financial Instruments - The Company accounts for fair value measurements for financial assets and financial liabilities in accordance with FASB ASC Topic 820. Under the authoritative guidance, fair value is defined as the exit price, representing the amount that would either be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the guidance established a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
| ● | Level 1. Observable inputs such as quoted prices in active markets for identical assets or liabilities; |
| ● | Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and |
| ● | Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
| 41 |
There were no assets that use Level 1, 2 or 3 inputs, nor any liabilities that use Level 1 or 2 inputs as of June 30, 2021.
Liabilities that use Level 3 inputs held as of June 30, 2021 consisted of a contingent consideration liability related to the February 16, 2018 acquisition of Enochian BioPharma Inc. (the “Acquisition”). As consideration for the Acquisition, the stockholders of Enochian Biopharma received (i) 18,081,962 shares of common stock, and (ii) the right to receive contingent shares pro rata upon the exercise of warrants, which were outstanding at closing. The contingent consideration liability was recorded at fair value of $21,516,000 at the time of the Acquisition and is subsequently remeasured to fair value at each reporting date. At June 30, 2021, 1,350,000 contingent shares are issuable in connection with the Acquisition.
The fair value of the contingent consideration liability is estimated using an option-pricing model. The key inputs to the model are all contractual or observable with the exception being volatility, which is computed based on the value of the Company’s underlying stock. The key inputs to valuing the contingent consideration liability on the date of acquisition and as of June 30, 2021 include the Company’s stock price, the exercise price of the warrants of $1.30 per share, the risk-free rate, the expected volatility of the Company’s common stock and the digital call rate. The fair value measurements are highly sensitive to changes in these inputs and significant changes in these inputs could result in a significantly higher or lower fair value.
Stock-Based Compensation - The Company has granted stock options, restricted share units (“RSUs”) and warrants to certain employees, officers, directors and consultants. The Company accounts for options in accordance with the provisions of FASB ASC Topic 718, Compensation – Stock Compensation. Stock based compensation costs for the vesting of options and RSUs granted to certain employees, officers, directors and consultants for the years ended June 30, 2021 and 2020 were $1,444,798 and $1,028,721, respectively (see Note 7 to the Financial Statements).
The Company recognizes compensation costs for stock option awards to employees, officers and directors based on their grant-date fair value. The value of each stock option is estimated on the date of grant using the Black-Scholes option-pricing model. The weighted-average assumptions used to estimate the fair values of the stock options granted using the Black-Scholes option-pricing model are the expected term of the award, the underlying stock price volatility, the risk-free interest rate and the expected dividend yield.
The Company records stock-based compensation for services received from non-employees in accordance with ASC 718, Compensation—Stock Compensation Non-Employees. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to consultants and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued and are recognized over the consultants’ required service period, which is generally the vesting period (see Note 7 to the Financial Statements.)
Recently Enacted Accounting Standards
For a description of accounting changes and recent accounting standards, including the expected dates of adoption and estimated effects, if any, on our consolidated financial statements, see “Note 1: Recent Accounting Pronouncements” in the financial statements included elsewhere in this Annual Report.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
The Registrant is a smaller reporting company and is not required to provide this information.
| 42 |
Item 8. Financial Statements and Supplementary Data
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
Index to Consolidated Financial Statements
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Enochian Biosciences, Inc.:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Enochian Biosciences, Inc. (“the Company”) as of June 30, 2021, and 2020, the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the years in the two-year period ended June 30, 2021 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2021, and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended June 30, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) related to accounts or disclosures that are material to the financial statements and (2) involved especially challenging, subjective, or complex judgements. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Indefinite-Lived Intangible Asset Impairment Assessment
Critical Audit Matter Description
The Company has an indefinite-lived intangible asset related to an acquired license treated as an in-process research and development asset (“IPR&D”). As of June 30, 2021, the carrying value of the asset is $154,824,000. To assess the carrying value of the IPR&D asset for impairment, management estimated the fair value of IPR&D annually on its elected assessment date of June 30, 2021, using a multi-period excess earnings method, which is a specific discounted cash flow method. The determination of the fair value requires management to make significant estimates including, but not limited to, the discount rate used in the model, the total addressable market for each potential drug, market penetration assumptions, and for the estimated timing of commercialization of the drugs. Changes in these assumptions could have a significant impact on the fair value of the IPR&D and a significant change in fair value could cause a significant impairment.
| F-2 |
How the Critical Audit Matter was Addressed in the Audit
We identified the impairment testing of the IPR&D asset as a critical audit matter because of the significant estimates and assumptions management makes related to the addressable market, market penetration estimates, commercialization dates of the drugs and selection of the discount rate to determine the fair value of the goodwill. This required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate the selection of the discount rate, the reasonableness of the total addressable market and market penetration for each drug, and the reasonableness of management’s forecast of the commercialization dates for IPR&D. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Our audit procedures related to the following:
| ● | Testing management’s process for developing the fair value of the IPR&D. | |
| ● | Evaluating the reasonableness and consistency of the selected valuation methodology and assumptions utilized by the Company. | |
| ● | Testing the mathematical accuracy of the model used by management. | |
| ● | Testing the completeness and accuracy of underlying data used in the fair value estimate. | |
| ● | Evaluating the significant assumptions provided by management or developed by the third-party valuation specialist related to total addressable market, market penetration, commercialization forecasts, and discount rate to discern whether they are reasonable considering (i) current and past performance of the entity; (ii) their consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. |
In addition, professionals with specialized skill and knowledge were utilized by the Firm to assist in the performance of these procedures.
Goodwill Impairment Assessment
Critical Audit Matter Description
As of June 30, 2021, the carrying value of goodwill was $11,640,000. As described in note 1 to the consolidated financial statements, the Company tests goodwill for impairment annually at the reporting unit level, or more frequently if events or circumstances indicate it is more likely than not that the fair value of a reporting unit is less than it’s carrying amount. To assess the carrying value of the goodwill for impairment, management estimated the fair value of goodwill on its elected assessment date of June 30, 2021, using a discounted cash flow model. The determination of the fair value requires management to make significant estimates including, but not limited to, the discount rate used in the model, the total addressable market for each potential drug, market penetration assumptions, and for the estimated timing of commercialization of the drugs.
How the Critical Audit Matter was Addressed in the Audit
We identified the evaluation of the impairment analysis for goodwill as a critical audit matter because of the significant estimates and assumptions management makes related to the addressable market, market penetration, commercialization dates of the drugs and selection of the discount rate to determine the fair value of the goodwill. This required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate the selection of the discount rate, the reasonableness of the total addressable market and market penetration forecasts for each drug, and the reasonableness of management’s forecast of the commercialization dates for the drugs. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Our audit procedures related to the following:
| ● | Testing management’s process for developing the fair value of the reporting unit. | |
| ● | Evaluating the reasonableness and consistency of the selected valuation methodology and assumptions utilized by the Company. | |
| ● | Testing the mathematical accuracy of the model used by management. | |
| ● | Testing the completeness and accuracy of underlying data used in the fair value estimate. | |
| ● | Evaluating the significant assumptions provided by management or developed by the third-party valuation specialist related to total addressable market, market penetration, commercialization forecasts, and discount rate to discern whether they are reasonable considering (i) current and past performance of the entity; (ii) their consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. |
In addition, professionals with specialized skill and knowledge were utilized by the Firm to assist in the performance of these procedures.
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company’s auditor since 2018.
Draper, UT
September 24, 2021
| F-3 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 20,664,410 | $ | 8,696,361 | ||||
| Other receivables | 1,640 | 1,982 | ||||||
| Prepaid expenses | 232,943 | 242,866 | ||||||
| Total Current Assets | 20,898,993 | 8,941,209 | ||||||
| Property and equipment, net | 719,364 | 778,118 | ||||||
| OTHER ASSETS | ||||||||
| Definite life intangible assets, net | 65,906 | 77,323 | ||||||
| Indefinite life intangible assets | 154,824,000 | 154,824,000 | ||||||
| Goodwill | 11,640,000 | 11,640,000 | ||||||
| Deposits and other assets | 20,984 | 137,550 | ||||||
| Operating lease rights-of-use assets | 1,435,978 | 1,703,859 | ||||||
| Total Other Assets | 167,986,868 | 168,382,732 | ||||||
| TOTAL ASSETS | $ | 189,605,225 | $ | 178,102,059 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (CONTINUED)
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| LIABILITIES | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable – trade | $ | 320,559 | $ | 592,877 | ||||
| Accrued expenses | 1,182,323 | 470,636 | ||||||
| Other current liabilities | 90,602 | |||||||
| Current portion of operating lease liabilities | 292,409 | 271,285 | ||||||
| Total Current Liabilities | 1,885,893 | 1,334,798 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Contingent consideration liability | 6,037,945 | 3,182,434 | ||||||
| Convertible notes payable | 1,200,000 | 1,200,000 | ||||||
| Notes payable, net | 4,579,114 | 4,580,787 | ||||||
| Operating lease liabilities, net of current portion | 1,239,334 | 1,531,779 | ||||||
| Total Non-Current Liabilities | 14,942,286 | 11,829,798 | ||||||
| Commitments and Contingencies | ||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||
| Preferred stock, $ par value; shares authorized; shares issued and outstanding | ||||||||
| Common stock, par value $, shares authorized, shares issued and outstanding at June 30, 2021; shares issued and outstanding at June 30, 2020 | 5,222 | 4,650 | ||||||
| Additional paid-in capital | 265,580,356 | 230,497,225 | ||||||
| Accumulated deficit | (90,911,805 | ) | (64,188,198 | ) | ||||
| Accumulated other comprehensive (loss) | (10,834 | ) | (41,416 | ) | ||||
| Total Stockholders’ Equity | 174,662,939 | 166,272,261 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | 189,605,225 | $ | 178,102,059 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Years Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Operating Expenses | ||||||||
| General and administrative | $ | 7,740,130 | $ | 7,120,835 | ||||
| Research and development | 15,538,122 | 4,694,349 | ||||||
| Depreciation and amortization | 123,535 | 108,584 | ||||||
| Total Operating Expenses | 23,401,787 | 11,923,768 | ||||||
| LOSS FROM OPERATIONS | (23,401,787 | ) | (11,923,768 | ) | ||||
| Other Income (Expenses) | ||||||||
| Change in fair value of contingent consideration | (3,048,033 | ) | 274,566 | |||||
| Interest expense | (379,608 | ) | (104,280 | ) | ||||
| Gain (loss) on currency transactions | (32,634 | ) | 146,828 | |||||
| Gain on settlement of debt | 140,000 | |||||||
| Interest income | 13,179 | 50,296 | ||||||
| Total Other Income (Expenses) | (3,447,096 | ) | 507,410 | |||||
| Loss Before Income Taxes | (26,848,883 | ) | (11,416,358 | ) | ||||
| Income Tax Benefit | 125,276 | |||||||
| NET LOSS | $ | (26,723,607 | ) | $ | (11,416,358 | ) | ||
| BASIC AND DILUTED NET LOSS PER COMMON SHARE | $ | (0.57 | ) | $ | (0.25 | ) | ||
| WEIGHTED AVERAGE NUMBER OF SHARES OF COMMON STOCK OUTSTANDING - BASIC AND DILUTED | 47,167,262 | 46,330,743 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| For the Years Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Net Loss | $ | (26,723,607 | ) | $ | (11,416,358 | ) | ||
| Other Comprehensive Income (Loss) | ||||||||
| Foreign currency translation, net of taxes | 30,582 | (143,234 | ) | |||||
| Other Comprehensive Loss | $ | (26,693,025 | ) | $ | (11,599,592 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
For the Years Ended June 30, 2021 and June 30, 2020
| # of Shares | Common Stock | Additional Paid-In Capital | Accumulated Deficit | Accumulated Other Comprehensive Income (Loss) | Total | |||||||||||||||||||
| Balance July 1, 2019 | 45,273,924 | $ | 4,527 | $ | 225,765,432 | $ | (52,771,840 | ) | $ | 101,818 | $ | 173,099,937 | ||||||||||||
| Stock issued pursuant to warrants exercised | 500,000 | 50 | 999,950 | 1,000,000 | ||||||||||||||||||||
| Contingent shares issued pursuant to acquisition agreement | 500,000 | 50 | 2,209,950 | 2,210,000 | ||||||||||||||||||||
| Restricted shares converted to shares for services rendered | 30,000 | 3 | 143,997 | 144,000 | ||||||||||||||||||||
| Shares issued in lieu of interest on notes payable | 188,485 | 19 | 493,173 | 493,192 | ||||||||||||||||||||
| Shares issued for fully vested RSUs | 5,000 | 1 | (1 | ) | ||||||||||||||||||||
| Stock-based compensation | 884,724 | 884,724 | ||||||||||||||||||||||
| Net Loss | — | (11,416,358 | ) | (11,416,358 | ) | |||||||||||||||||||
| Foreign currency translation loss | — | (143,234 | ) | (143,234 | ) | |||||||||||||||||||
| Balance June 30, 2020 | 46,497,409 | $ | 4,650 | $ | 230,497,225 | $ | (64,188,198 | ) | $ | (41,416 | ) | $ | 166,272,261 | |||||||||||
| Issuance of commitment shares related to LPC purchase agreement | 139,567 | 14 | (14 | ) | ||||||||||||||||||||
| Stock issued pursuant to warrants exercised | 63,122 | 6 | 82,050 | 82,056 | ||||||||||||||||||||
| Contingent shares issued pursuant to acquisition agreement | 63,122 | 6 | 192,516 | 192,522 | ||||||||||||||||||||
| Shares issued pursuant to 2021 private placement | 1,275,719 | 128 | 5,000,672 | 5,000,800 | ||||||||||||||||||||
| Shares issued in lieu of interest on $5 million notes payable extension | 74,054 | 7 | 298,171 | 298,178 | ||||||||||||||||||||
| Shares issued pursuant to LPC equity agreement | 200,000 | 20 | 1,221,330 | 1,221,350 | ||||||||||||||||||||
| Shares issued pursuant to direct offering, net of issuance costs | 3,866,668 | 387 | 26,843,612 | 26,843,999 | ||||||||||||||||||||
| Shares issued for fully vested RSUs | 5,000 | |||||||||||||||||||||||
| Restricted shares converted to shares for services rendered | 35,000 | 4 | 146,996 | 147,000 | ||||||||||||||||||||
| Stock-based Compensation | — | 1,297,798 | 1,297,798 | |||||||||||||||||||||
| Net Loss | — | (26,723,607 | ) | (26,723,607 | ) | |||||||||||||||||||
| Foreign currency translation gain | — | 30,582 | 30,582 | |||||||||||||||||||||
| Balance June 30, 2021 | 52,219,661 | $ | 5,222 | $ | 265,580,356 | $ | (90,911,805 | ) | $ | (10,834 | ) | $ | 174,662,939 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-8 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (26,723,607 | ) | $ | (11,416,358 | ) | ||
| ADJUSTMENTS TO RECONCILE NET LOSS TO NET CASH USED IN OPERATING ACTIVITIES : | ||||||||
| Depreciation and amortization | 123,534 | 108,583 | ||||||
| Change in fair value of contingent consideration | 3,048,033 | (274,566 | ) | |||||
| Non-cash stock-based compensation expense | 1,444,798 | 1,028,724 | ||||||
| Amortization of right-of-use assets | 267,881 | 257,685 | ||||||
| Amortization of discount on note payable | 296,505 | 73,979 | ||||||
| Gain on settlement of debt | (140,000 | ) | ||||||
| Changes in assets and liabilities: | ||||||||
| Other receivables | 342 | 18,812 | ||||||
| Prepaid expenses/deposits | 733,739 | (50,897 | ) | |||||
| Accounts payable | (272,318 | ) | 59,314 | |||||
| Accounts payable non-trade | (100,000 | ) | ||||||
| Other current liabilities | 30,004 | |||||||
| Operating lease liabilities | (271,321 | ) | (251,231 | ) | ||||
| Accrued expenses | 711,687 | 226,533 | ||||||
| NET CASH USED BY OPERATING ACTIVITIES | (20,610,723 | ) | (10,459,422 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | (48,892 | ) | (184,463 | ) | ||||
| NET CASH USED BY INVESTING ACTIVITIES | (48,892 | ) | (184,463 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from convertible notes payable | 1,200,000 | |||||||
| Proceeds from notes payable | 5,272,700 | |||||||
| Repayments of finance agreement | (546,651 | ) | ||||||
| Repayment of notes payable | (272,700 | ) | ||||||
| Proceeds from exercise of warrants | 82,056 | 1,000,000 | ||||||
| Proceeds from 2021 private placement | 5,000,800 | |||||||
| Proceeds from direct offering, net of issuance costs | 26,843,998 | |||||||
| Proceeds from LPC equity agreement | 1,221,350 | |||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 32,601,553 | 7,200,000 | ||||||
| Effect of exchange rates on cash | 26,111 | (141,978 | ) | |||||
| NET INCREASE (DECREASE) IN CASH | 11,968,049 | (3,585,863 | ) | |||||
| CASH, BEGINNING OF PERIOD | 8,696,361 | 12,282,224 | ||||||
| CASH, END OF PERIOD | $ | 20,664,410 | $ | 8,696,361 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-9 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | ||||||||
| Cash Paid during the year for: | ||||||||
| Interest | $ | 89,224 | $ | |||||
| Income Taxes | $ | 37 | $ | |||||
| SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Contingent Shares issued pursuant to Acquisition Agreement | $ | 192,522 | $ | 2,210,000 | ||||
| Right-of-use assets in exchange for operating lease liabilities upon adoption of ASC 842-Leases | $ | $ | 2,054,295 | |||||
| Shares issued in lieu of interest expense on note payable | $ | (298,178 | ) | $ | (493,192 | ) | ||
| Finance agreement entered into in exchange for prepaid assets | $ | 607,250 | $ |
The accompanying notes are an integral part of these consolidated financial statements.
| F-10 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business– Enochian BioSciences Inc., formerly DanDrit Biotech USA, Inc. (“Enochian”, or “Registrant”, and together with its subsidiaries, the “Company”, “we” or “us”) engages in the research and development of pharmaceutical and biological products for the human treatment of HIV, HBV, influenza and coronavirus infections, and cancer with the intent to manufacture said products.
Basis of Presentation- The Company prepares consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and follows the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”).
Consolidation - For the years ended June 30, 2021 and 2020, the consolidated financial statements include the accounts and operations of the Registrant, and its wholly owned subsidiaries. All material inter-company transactions and accounts have been eliminated in the consolidation.
Accounting Estimates - The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimated. Significant estimates include the fair value and potential impairment of intangible assets, the fair value of the contingent consideration liability, and fair value of equity instruments issued.
Subsidiaries- Enochian Biopharma Inc. (“Enochian Biopharma”) was incorporated on May 19, 2017 in Delaware and is a 100% owned subsidiary of the Registrant. Enochian Biopharma owns a perpetual, fully paid-up, royalty-free, sublicensable, and sole and exclusive worldwide license to research, develop, use, sell, have sold, make, have made, offer for sale, import and otherwise commercialize certain intellectual property in cellular therapies for the prevention, treatment, amelioration of and/or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans. As of June 30, 2021 and June 30, 2020, 1,350,000 and 1,438,122 shares of Common Stock, respectively, remain contingently issuable in connection with the acquisition of Enochian BioPharma in February 2018 (the “Contingent Shares”).
Enochian Biosciences Denmark ApS, a Danish corporation was incorporated on April 1, 2001 (“Enochian Denmark”). On February 12, 2014, in accordance with the terms and conditions of a Share Exchange Agreement, the Company acquired Enochian Denmark and it became a 100% owned subsidiary of the Registrant subject to 185,053 shares of common stock of the Registrant held in escrow according to Danish law (the “Escrow Shares”). As of June 30, 2021, there are , Escrow Shares remaining (see Note 7).
COVID-19 Update
The COVID-19 pandemic continues to evolve, and to date has led to the implementation of various mitigation responses, including government-imposed quarantines, travel restrictions and other public health safety measures, as well as leading to reported adverse impacts on healthcare resources, facilities and providers across the United States and in other countries. COVID-19 may cause delays in our research activities. To date, it has not materially affected our operations; however, it has caused delays in the conduct of experiments due to limitations of various organizations, in particular those conducting experiments related to COVID-19. There have also been increases in the cost to conduct animal studies due to staffing and other limitations.
The full extent to which the COVID-19 pandemic may impact our business and operations is subject to future developments, which are uncertain and difficult to predict. Further quarantines, shelter-in-place or similar restrictions and other actions taken or imposed by foreign, federal, state and local governments could adversely impact our or our partners’ clinical, research and development, regulatory and manufacturing operations or timelines.
| F-11 |
We continue to monitor the impact of the COVID-19 pandemic on our business and operations and will seek to adjust our activities as appropriate. In addition, the pandemic could result in significant and prolonged disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect the financial resources available to us.
Functional Currency and Foreign Currency Translation - The functional currency of Enochian Denmark is the Danish Kroner (“DKK”). The Company is reporting currency is the U.S. Dollar for the purpose of these financial statements. The Company’s consolidated balance sheet accounts are translated into U.S. dollars at the period-end exchange rates and all revenue and expenses are translated into U.S. dollars at the average exchange rates prevailing during the years ended June 30, 2021 and 2020. Translation gains and losses are deferred and accumulated as a component of other comprehensive income in stockholders’ equity. Transaction gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are included in the statement of operations as incurred.
Cash and Cash Equivalents - The Company considers all highly liquid debt instruments purchased with a maturity of three months or less to be cash equivalents. The Company’s cash balances at June 30, 2021, and 2020, are $20,664,410 and $8,696,361, respectively. The Company had balances held in financial institutions in Denmark and in the United States in excess of federally insured amounts at June 30, 2021 and 2020 of $20,287,212, and $8,160,271, respectively.
Property and Equipment - Property and equipment are stated at cost. Expenditures for major renewals and betterments that extend the useful lives of property and equipment are capitalized, and depreciated upon being placed in service. Expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is computed for financial statement purposes on a straight-line basis over the estimated useful lives of the assets, which range from four to ten years (see Note 2).
Intangible Assets - The Company has both Definite and Indefinite life intangible assets.
Definite life intangible assets relate to patents. The Company accounts for definite life intangible assets in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 350, Goodwill and Other Intangible Assets. Intangible assets are recorded at cost. Patent costs capitalized consist of costs incurred to acquire the underlying patent. If it is determined that a patent will not be issued, the related remaining capitalized patent costs are charged to expense. Definite life intangible assets are amortized on a straight-line basis over their estimated useful life. The estimated useful life of patents is twenty years from the date of application.
Indefinite life intangible assets include license agreements and goodwill acquired in a business combination. The Company accounts for indefinite life intangible assets in accordance with ASC 350, License agreement costs represent the fair value of the license agreement on the date acquired and are tested annually for impairment.
| F-12 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Goodwill - Goodwill is not amortized but is evaluated for impairment annually as of June 30 or whenever events or changes in circumstances indicate the carrying value may not be recoverable.
Impairment of Goodwill and Indefinite Lived Intangible Assets – We test for goodwill impairment at the reporting unit level, which is one level below the operating segment level. Our detailed impairment testing involves comparing the fair value of each reporting unit to its carrying value, including goodwill. Fair value reflects the price a market participant would be willing to pay in a potential sale of the reporting unit and is based on discounted cash flows or relative market-based approaches. If the carrying value of the reporting unit exceeds its fair value, we record an impairment loss for such excess. The carrying value of IPR&D and goodwill at June 30, 2021, were $154,824,000 and $11,640,000, respectively.
For indefinite-lived intangible assets, such as licenses acquired as an IPR&D asset, on an annual basis we determine the fair value of the asset and record an impairment less, if any, for the excess of the carrying value of the asset over its fair value. The fair value analysis performed on the license agreement, and the annual fair value analysis performed on goodwill supported that both indefinite life intangible assets are not impaired as of June 30, 2021 (see Note 3.)
Impairment of Long-Lived Assets - Long-lived assets, such as property, plant, and equipment and definite life intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life.
Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and would no longer be depreciated. The depreciable basis of assets that are impaired and continue in use are their respective fair values.
Leases - In accordance with ASC Topic 842, the Company determined the initial classification and measurement of its right-of-use assets and lease liabilities at the lease commencement date and thereafter. The lease terms include any renewal options and termination options that the Company is reasonably assured to exercise, if applicable. The present value of lease payments is determined by using the implicit interest rate in the lease, if that rate is readily determinable; otherwise, the Company develops an incremental borrowing rate based on the information available at the commencement date in determining the present value of the future payments.
Rent expense for operating leases is recognized on a straight-line basis, unless the operating lease right-of-use assets have been impaired, over the reasonably assured lease term based on the total lease payments and is included in general and administrative expenses in the consolidated statements of operations. For operating leases that reflect impairment, the Company will recognize the amortization of the operating lease right-of-use assets on a straight-line basis over the remaining lease term with rent expense still included in general and administrative expenses in the consolidated statements of operations.
The Company has elected the practical expedient to not separate lease and non-lease components. The Company’s non-lease components are primarily related to property maintenance, insurance and taxes, which vary based on future outcomes, and thus are recognized in general and administrative expenses when incurred (see Note 4.)
Research and Development Expenses - The Company expenses research and development costs incurred in formulating, improving, validating and creating alternative or modified processes related to and expanding the use of the HIV, HBV, Coronaviruses and Oncology therapies and technologies for use in the prevention, treatment, amelioration of and/or therapy for HIV, HBV, Coronaviruses and Oncology. Research and development expenses for the year ended June 30, 2021 and 2020 amounted to $15,538,122 and $4,694,349, respectively.
Income Taxes - The Company accounts for income taxes in accordance with FASB ASC Topic 740 Accounting for Income Taxes, which requires an asset and liability approach for accounting for income taxes (see Note 6.)
| F-13 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair Value of Financial Instruments - The Company accounts for fair value measurements for financial assets and financial liabilities in accordance with FASB ASC Topic 820, Fair Value Measurements. Under the authoritative guidance, fair value is defined as the exit price, representing the amount that would either be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the guidance established a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
| ● | Level 1. Observable inputs such as quoted prices in active markets for identical assets or liabilities; |
| ● | Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and |
| ● | Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
There were no assets that use Level 1, 2 or 3 inputs, nor any liabilities that use Level 1 or 2 inputs as of June 30, 2021.
Liabilities that use Level 3 inputs held as of June 30, 2021 consisted of a contingent consideration liability related to the February 16, 2018 acquisition of Enochian BioPharma (the “Acquisition”). As consideration for the Acquisition, the stockholders of Enochian Biopharma received (i) shares of Common Stock, and (ii) the right to receive contingent shares pro rata upon the exercise of warrants, which were outstanding at closing. The contingent consideration liability was recorded at fair value of $21,516,000 at the time of the Acquisition and is subsequently remeasured to fair value at each reporting date. At June 30, 2021, contingent shares are issuable in connection with the Acquisition of Enochian Biopharma.
The fair value of the contingent consideration liability is estimated using an option-pricing model. The key inputs to the model are all contractual or observable with the exception being volatility, which is computed, based on the Company’s underlying stock. The key inputs to valuing the contingent consideration liability on the date of acquisition and as of June 30, 2021, include the Company’s stock price on the valuation date of $; the exercise price of the warrants of $, the risk-free rate of %, the expected volatility of the Company’s Common Stock of %, and the digital call rate of 90%. Fair Value measurements are highly sensitive to changes in these inputs and significant changes in these inputs could result in a significantly higher or lower fair value.
Unless otherwise disclosed, the fair value of the Company’s financial instruments including cash, accounts receivable, prepaid expenses, accounts payable, accrued expenses, and notes payable approximate their recorded values due to their short-term nature.
The following table sets forth the liabilities at June 30, 2021 and 2020, which are recorded on the balance sheet at fair value on a recurring basis by level of input within the fair value hierarchy. As required, these are classified based on the lowest level of input that is significant to the fair value measurement:
| Fair Value Measurements at Reporting Date Using | ||||||||||||
| Quoted Prices in Active Markets for Identical Assets Inputs | Significant Other Observable Inputs | Significant Other Unobservable Inputs | ||||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||
| Contingent Consideration Liability at June 30, 2021 | $ | $ | $ | 6,037,945 | ||||||||
| The roll forward of the contingent consideration liability is as follows: | ||||||||||||
| Balance June 30, 2020 | 3,182,434 | |||||||||||
| Contingent Shares issued pursuant to the Acquisition Agreement | (192,522 | ) | ||||||||||
| Fair value adjustment | 3,048,033 | |||||||||||
| Balance June 30, 2021 | $ | $ | $ | 6,037,945 | ||||||||
| F-14 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Stock Options and Warrants - The Company has granted stock options to certain employees, officers and directors that were subsequently converted to Grant Warrants. During the years presented in the accompanying consolidated financial statements, the Company has granted stock options and warrants. The Company accounts for options and warrants in accordance with the provisions of FASB ASC Topic 718, Compensation – Stock Compensation. Stock-based compensation costs related to employee compensation and consulting fees for the years ended June 30, 2021 and 2020 were $ and $, respectively (see Note 7).
Stock-Based Compensation —The Company records stock-based compensation for services received from non-employees in accordance with ASC 718. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued for goods or services and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued and are recognized over the consultants’ required service period, which is generally the vesting period. For the year ended June 30, 2021, the Company issued shares at a value of $147,000. For the year ended June 30, 2020, the Company issued shares at a value of $144,000 (see Note 7.)
Recent Accounting Pronouncements - The Company adopted ASU 2018-13, Fair Value Measurement (Topic 820), Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurements, which amends certain disclosures requirements over fair value measurements. Under the new guidance, entities will no longer be required to disclose the amount of and reasons for transfers between Level 1 and Level 2 inputs of the fair value hierarchy or valuation processes for Level 3 inputs in fair value measurements. However, public companies will be required to disclose the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements, and related changes in unrealized gains and losses included in other comprehensive income. The Company adopted this guidance on July 1, 2020, and there was no material impact to its consolidated financial statement disclosures (see Note 1-Fair Value of Financial Instruments for more information about the Company’s fair value classifications.)
New Accounting Pronouncements Not Yet Adopted - Recent accounting pronouncements issued by the FASB do not or are not believed by management to have a material impact on the Company’s present or future consolidated financial statements.
| F-15 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 - PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at June 30, 2021 and 2020:
| Useful Life | June 30, 2021 | June 30, 2020 | ||||||||||
| Lab equipment and instruments | 4-7 | $ | 583,421 | $ | 534,527 | |||||||
| Leasehold improvements | 10 | 224,629 | 224,629 | |||||||||
| Furniture, fixtures and equipment | 4-7 | 171,975 | 171,975 | |||||||||
| Total | 980,025 | 931,131 | ||||||||||
| Less accumulated depreciation | (260,661 | ) | (153,013 | ) | ||||||||
| Net Property and Equipment | $ | 719,364 | $ | 778,118 | ||||||||
Depreciation expense amounted to $107,647 and $93,861 for the years ended June 30, 2021 and 2020, respectively.
| F-16 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 — INTANGIBLE ASSETS AND GOODWILL
At June 30, 2021 and 2020, definite-life intangible assets, net of accumulated amortization, consisted of patents on the Company’s products and processes of $65,906 and $77,323, respectively. The patents are recorded at cost and amortized over twenty years from the date of application. Amortization expense for the year ended June 30, 2021 and 2020 was $15,888 and $14,772, respectively.
At June 30, 2021 and 2020, indefinite life intangible assets consisted of a license agreement classified as In-Process Research and Development (“IPR&D”) intangible assets, which are not amortizable until the intangible asset provides economic benefit, and goodwill.
At June 30, 2021 and 2020, definite-life and indefinite-life intangible assets consisted of the following:
| Useful Life | June 30, 2020 | Period Change | Effect of Currency Translation | June 30, 2021 | ||||||||||||||
| Definite Life Intangible Assets | ||||||||||||||||||
| Patents | 20 Years | $ | 299,175 | $ | — | 16,940 | $ | 316,115 | ||||||||||
| Less Accumulated Amortization | (221,852 | ) | (15,888 | ) | (12,469 | ) | (250,209 | ) | ||||||||||
| Net Definite-Life Intangible Assets | $ | 77,323 | $ | (15,888 | ) | $ | 4,471 | $ | 65,906 | |||||||||
| Indefinite Life Intangible Assets | ||||||||||||||||||
| License Agreement | $ | 154,824,000 | $ | — | $ | — | $ | 154,824,000 | ||||||||||
| Goodwill | 11,640,000 | — | — | 11,640,000 | ||||||||||||||
| Total Indefinite Life Intangible Assets | $ | 166,464,000 | $ | — | $ | — | $ | 166,464,000 | ||||||||||
Expected future amortization expense is as follows:
| Years ended June 30, | |||||
| 2022 | $ | 15,154 | |||
| 2023 | 15,154 | ||||
| 2024 | 15,154 | ||||
| 2025 | 15,154 | ||||
| 2026 | 5,290 | ||||
| Thereafter | |||||
| Total | $ | 65,906 | |||
During February 2018, the Company acquired a License Agreement (as licensee) to the HIV therapy being developed as ENOB-HV-01 which consists of a perpetual, fully paid-up, royalty-free, sub-licensable, and sole and exclusive worldwide license to research, develop, use, sell, have sold, make, have made, offer for sale, import and otherwise commercialize certain intellectual property in cellular therapies for the prevention, treatment, amelioration of and/or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans. Because the HIV License Agreement is considered an IPR&D intangible asset, it is classified as an indefinite life asset that is tested annually for impairment.
Impairment – Following the fourth quarter of each year, management performs its annual test of impairment of intangible assets by performing a quantitative assessment and determines if it is more than likely than not that, the fair value of the asset is greater than or equal to the carrying value of the asset. The results of the quantitative assessment supported Management’s conclusion that an impairment adjustment was not required as of June 30, 2021.
| F-17 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 — LEASES
Operating Leases — On November 13, 2017, the Registrant entered into a lease agreement for a term of five years and two months from November 1, 2017 with Plaza Medical Office Building, LLC, pursuant to which the Registrant agreed to lease approximately 2,325 rentable square feet (the “Plaza Lease”). The base rent for the Plaza Lease increases by 3% each year, and ranges from approximately $8,719 per month, for the first year to $10,107 per month for the two months of the sixth year. The equalized monthly lease payment for the term of the lease is $7,862. The Registrant was entitled to $70,800 in tenant improvement allowance in the form of free rent applied over 10 months in equal installments beginning in January 2018.
On June 19, 2018, the Registrant entered into a lease agreement for a term of ten years from September 1, 2018 with Century City Medical Plaza Land Co., Inc., pursuant to which the Company agreed to lease approximately 2,453 rentable square feet. On February 20, 2019, the Registrant entered into an Addendum to the original lease agreement with an effective date of December 1, 2019, where it expanded the lease area to include another 1,101 square feet for a total rentable 3,554 square feet. The base rent increases by 3% each year, and ranges from $17,770 per month as of the date of the amendment to $23,186 per month for the tenth year. The equalized monthly lease payment for the term of the lease is $20,050. The Company was entitled to $148,168 in contributions toward tenant improvements.
The Company identified and assessed the following significant assumptions in recognizing the right-of-use asset and corresponding liabilities:
Expected lease term — The expected lease term includes both contractual lease periods and, when applicable, cancelable option periods when it is reasonably certain that the Company would exercise such options. The Company’s leases have remaining lease terms between 19 months and 74 months. As of June 30, 2021, the weighted-average remaining term is 5.65 years.
Incremental borrowing rate — The Company’s lease agreements do not provide an implicit rate. As the Company does not have any external borrowings for comparable terms of its leases, the Company estimated the incremental borrowing rate based on the U.S. Treasury Yield Curve rate that corresponds to the length of each lease. This rate is an estimate of what the Company would have to pay if borrowing on a collateralized basis over a similar term in an amount equal to the lease payments in a similar economic environment. As of June 30, 2021, the weighted-average discount rate is 4%.
Lease and non-lease components — In certain cases the Company is required to pay for certain additional charges for operating costs, including insurance, maintenance, taxes, and other costs incurred, which are billed based on both usage and as a percentage of the Company’s share of total square footage. The Company determined that these costs are non-lease components and they are not included in the calculation of the lease liabilities because they are variable. Payments for these variable, non-lease components are considered variable lease costs and are recognized in the period in which the costs are incurred.
For the years ended June 30, 2021, and 2020, the lease expenses charged to general and administrative expenses amounted to $339,094, and $395,528, respectively.
Below are the lease commitments for the next 5 years:
| Years Ending June 30 | Lease Expense | |||
| 2022 | $ | 348,495 | ||
| 2023 | 298,305 | |||
| 2024 | 246,004 | |||
| 2025 | 253,384 | |||
| 2026 | 260,985 | |||
| Thereafter | 313,836 | |||
| Less imputed interest | (189,266 | ) | ||
| Total | $ | 1,531,743 | ||
| F-18 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 — NOTES PAYABLE
Convertible Notes Payable- On February 6, 2020, the Company issued two Convertible Notes (the “Convertible Notes”) to an existing stockholder of the Company each with a face value amount of $600,000, convertible into shares of Common Stock, $ par value per share. The outstanding principal amount of the Convertible Notes is due and payable on February 6, 2023. Interest on the Convertible Notes commenced accruing on the date of issuance at six percent (6%) per annum, computed on the basis of twelve 30-day months, and is compounded monthly on the final day of each calendar month based upon the principal and all accrued and unpaid interest outstanding as of such compound date. The interest is payable in cash on a semi-annual basis.
The holder of the Convertible Notes had the right at any time prior to the date that is twelve months from issuance to convert all or any part of the outstanding and unpaid principal and all unpaid interest into shares of the Company’s Common Stock. The conversion price is equal to $12.00 per share of Common Stock. The holder did not exercise the conversion feature that expired on February 6, 2021. The Company evaluated the Convertible Notes in accordance with ASC 470-20 and identified that they each contain an embedded conversion feature that shall not be bifurcated from the host document (i.e., the Convertible Notes) as they are not deemed to be readily convertible into cash. All proceeds received from the issuance have been recognized as a liability on the balance sheet. The Convertible Notes balance as of June 30, 2021 and June 30, 2020, was $1,200,000. For the year ended June 30, 2021 and 2020, the Company recorded accrued interest in the amount of $24,181 and $30,302, respectively, which is included in accrued expenses. For the years ended June 30, 2021 and 2020, the interest expense related to the Convertible Notes amounted to $72,967 and $30,302, respectively.
Note Payable- On March 30, 2020 (the “Issuance Date”), the Company issued a Promissory Note in the principal amount of $5,000,000 (the “Unsecured Note”) to Paseco APS, a Danish limited company and an existing stockholder of the Company. The principal amount of the Unsecured Note was originally payable on November 30, 2021 (the “Maturity Date”). The Unsecured Note bore interest at a fixed rate of 6% per annum, computed based on the number of days between the Issuance Date and the Maturity Date, which was prepaid by the Company in full on the Issuance Date through the issuance of shares of the Company’s Common Stock based on the closing market price on that date for a total value of $501,370. The Company evaluated the Unsecured Note and PIK interest in accordance with ASC 470-Debt and ASC 835-Interest, respectively. Pursuant to ASC 470-20, proceeds received from the issuance are to be recognized at their relative fair value, thus the liability is shown net of the corresponding discount of $493,192, which is the relative fair value of the shares issued for the PIK interest on the closing date using the effective interest method. The discount of $493,192 will be accreted over the life of the Unsecured Note.
On February 11, 2021, the Company entered into an amendment to the Unsecured Note in the principal amount of $5,000,000 that extends the Maturity Date out to November 30, 2022. All other terms of the Unsecured Note remain the same. The change in Maturity Date required an additional year of interest at the fixed rate of 6% per annum, which was prepaid by the Company in full on the date of the amendment through the issuance of 74,054 shares of the Company’s Common Stock based on the closing market price on that date for a total value of $298,178. For the year ended June 30, 2021 and 2020, discount amortization of $296,506 and $73,979 was charged to interest expense. The Unsecured Note balance, net of discount at June 30, 2021 is $4,579,114.
Finance Agreement- On December 4, 2020, the Company entered into a premium finance agreement (“Agreement”) with a principal amount of $607,250 and an interest rate of 4.99% per annum. The repayment of the Agreement will be made in nine equal monthly installments of $62,077. The remaining balance at June 30, 2021 is $60,598. The amount is reflected in other current liabilities. Interest expense related to the Agreement for the year ended June 30, 2021 totaled $10,135.
As of June 30, 2021 and 2020, the Company recorded a total of $24,181 and $30,302 of accrued interest, respectively. Total interest expense recorded for the years ended June 30, 2021 and 2020, was $379,608 and $104,280, respectively.
| F-19 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 — INCOME TAXES
The Company accounts for income taxes in accordance with FASB ASC Topic 740, Accounting for Income Taxes; which requires the Company to provide a net deferred tax asset or liability equal to the expected future tax benefit or expense of temporary reporting differences between book and tax accounting and any available operating loss or tax credit carryforwards. The amount of and ultimate realization of the benefits from the deferred tax assets for income tax purposes is dependent, in part, upon the tax laws in effect, the Company’s future earnings, and other future events, the effects of which cannot be determined.
As of June 30, 2021 and 2020, the Company had net operating loss carryforwards of approximately $51,327,066 and $29,247,919, respectively, giving rise to deferred tax assets of $13,532,665 and $6,944,248, respectively. The net operating loss carryforwards generated prior to January 1, 2018, expire over various dates from 2031 to 2036. All subsequent net operating loss carryforwards are indefinite.
The Company files Danish and U.S. income tax returns and these returns are generally no longer subject to tax examinations for years prior to 2008 for the Danish tax returns and 2012 for the U.S. tax returns.
The temporary differences, tax credits and carry forwards gave rise to the following deferred tax asset (liabilities) at June 30, 2021 and 2020:
| June 30 | ||||||||
| 2021 | 2020 | |||||||
| Excess of tax over book depreciation of fixed assets | $ | (6,100 | ) | $ | (17,628 | ) | ||
| Excess of tax over book depreciation of patents | 5,449 | 2,654 | ||||||
| Stock/options compensation | 1,192,741 | 761,613 | ||||||
| Depreciation and amortization | 81,140 | 44,278 | ||||||
| Net Operating Loss Carryforwards | 13,536,884 | 6,944,248 | ||||||
| Change in tax rate | 4,218 | |||||||
| Valuation allowance | (14,810,114 | ) | (7,739,383 | ) | ||||
| Total Deferred Tax Assets (Liabilities) | $ | $ | ||||||
In accordance with prevailing accounting guidance, the Company is required to recognize and disclose any income tax uncertainties. The guidance provides a two-step approach to recognizing and measuring tax benefits and liabilities when realization of the tax position is uncertain. The first step is to determine whether the tax position meets the more- likely -than -not condition for recognition, and the second step is to determine the amount to be recognized based on the cumulative probability that exceeds 50%. The amount of and ultimate realization of the benefits from the deferred tax assets for income tax purposes is dependent, in part, upon the tax laws in effect, the Company’s future earnings, and other future events, the effects of which can be difficult to determine and can only be estimated. Management estimates that it is more likely than not that the Company will not generate adequate net profits to use the deferred tax assets; and consequently, a valuation allowance was recorded for all deferred tax assets.
A reconciliation of income tax expense at the federal statutory rate to income tax expense at the Company’s effective rate is as follows for the year ended June 30, 2021 and 2020:
| June 30 | ||||||||
| 2021 | 2020 | |||||||
| Computed tax at expected statutory rate | $ | (7,070,732 | ) | $ | (2,828,885 | ) | ||
| Non-US income taxed at different rates | (125,276 | ) | ||||||
| Non-deductible expenses / other items | ||||||||
| Valuation allowance | 7,070,732 | 2,828,885 | ||||||
| Income Tax Expense (Benefit) | $ | (125,276 | ) | $ | ||||
The components of income tax expense (benefit) from continuing operations for the year ended June 30, 2021 and 2020 consisted of the following:
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| Current Tax Expense | ||||||||
| Danish income tax (benefit) | $ | (125,276 | ) | $ | ||||
| Total Current Tax Expense (Benefit) | $ | (125,276 | ) | $ | ||||
| Deferred Income Tax Expense (Benefit) | ||||||||
| Excess of tax over book depreciation of fixed assets | $ | (6,100 | ) | $ | (17,628 | ) | ||
| Excess of tax over book depreciation of patents | 5,449 | 2,654 | ||||||
| Stock/options compensation | 1,192,741 | 761,613 | ||||||
| Depreciation and amortization | 81,140 | 44,278 | ||||||
| Net Operating Loss Carryforwards | 13,536,884 | 6,944,248 | ||||||
| Change in tax rate | 4,218 | |||||||
| Change in the valuation allowance | (14,810,114 | ) | (7,739,383 | ) | ||||
| Total Deferred Tax Expense | $ | $ | ||||||
Deferred income tax expense (benefit) results primarily from the reversal of temporary timing differences between tax and financial statement income.
| F-20 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 — STOCKHOLDERS’ EQUITY
Preferred Stock — The Company has authorized shares of Preferred Stock, par value $per share. At June 30, 2021 and 2020, there were zero shares issued and outstanding.
Common Stock — The Company has authorized shares of Common Stock, par value $ per share. At June 30, 2021 and 2020, there were and shares issued and outstanding, respectively.
Voting — Holders of Common Stock are entitled to one vote for each share held of record on each matter submitted to a vote of stockholders, including the election of directors, and do not have any right to cumulate votes in the election of directors.
Dividends — Holders of Common Stock are entitled to receive ratably such dividends as the Company’s Board of Directors from time to time may declare out of funds legally available.
Liquidation Rights — In the event of any liquidation, dissolution or winding-up of the affairs of the Company, after payment of all of our debts and liabilities, the holders of Common Stock will be entitled to share ratably in the distribution of any of our remaining assets.
Purchase Agreement with Lincoln Park Capital
On July 8, 2020, we entered into a purchase agreement (the “Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”), pursuant to which the Company may sell and issue to Lincoln Park, and Lincoln Park is obligated to purchase, up to $20,000,000 of shares of our Common Stock from time to time through August 1, 2023.
Under the Purchase Agreement, we may direct Lincoln Park, at our sole discretion subject to certain conditions, to purchase up to shares of Common Stock on any business day (a “Regular Purchase”). The amount of a Regular Purchase may be increased under certain circumstances up to shares of Common Stock, provided, that Lincoln Park’s committed obligation for Regular Purchases on any business day shall not exceed $1,000,000. In the event we direct the purchase of the full amount allowed for a Regular Purchase on any given business day, we may also direct Lincoln Park to purchase additional amounts as accelerated and additional accelerated purchases. The purchase price of shares of Common Stock related to the future funding will be based on the then prevailing market prices of such shares at the time of sales as described in the Purchase Agreement.
Our sale of shares of Common Stock to Lincoln Park subsequent to the date of the Purchase Agreement is limited to 12,016,457 shares of Common Stock, representing 19.99% of the shares of the Common Stock outstanding on the date of the Purchase Agreement unless (i) stockholder approval is obtained, (ii) the average price of all applicable sales to Lincoln Park under the Purchase Agreement equals or exceeds the lower of (A) the closing price of the Common Stock on the Nasdaq Capital Market immediately preceding the date of the Purchase Agreement or (B) the average of the closing prices on the Nasdaq Capital Market for the five Business Days immediately preceding the date of the Purchase Agreement or (iii) to the extent it would cause Lincoln Park to beneficially own more than 9.99% of the Company’s outstanding shares of Common Stock at any given time.
In consideration for entering into the Purchase Agreement, we issued shares of Common Stock to Lincoln Park as a commitment fee on July 21, 2020.
In June 2021, we issued shares of Common Stock to Lincoln Park under the Purchase Agreement for a purchase price of $1,221,350.
| F-21 |
Private Placement
Pursuant to a private placement offering, the Company issued Common Stock resulting in proceeds of $5,000,800. The Company effected the issuances of the shares of Common Stock from March 15, 2021 to June 9, 2021. The private placements was made directly by the Company. No underwriter or placement agent was engaged by the Company for this private placement.
Purchase Agreement Pursuant to Registered Direct Offering
On June 14, 2021, the Company and certain institutional investors entered into a securities purchase agreement (the “Registered Direct Purchase Agreement”), pursuant to which the Company agreed to sell to such investors an aggregate of 3,866,668 shares of Common Stock, in a registered direct offering, for gross proceeds of approximately $29 million (the “Financing”). The purchase price for each share of Common Stock was $7.50. Pursuant to the Registered Direct Purchase Agreement, the Company agreed not to issue or enter into any agreement to issue Common Stock from June 14, 2021 until ninety (90) days after the closing of the Financing. H.C. Wainwright & Co., LLC, acted as the exclusive placement agent (the “Placement Agent”.) The Financing closed on June 16, 2021.
The Company agreed to pay the Placement Agent an aggregate fee equal to 7.0% of the gross proceeds raised in the Financing. The Company also agreed to pay the Placement Agent certain expenses. The Company paid $2,090,000 in commissions and incurred offering expenses, and issuance costs of $66,011, resulting in net proceeds of $26,843,998 in connection with the Financing.
Common Stock Issuances
On June 30, 2021, the Company issued shares of Common Stock related to restricted share units that vested on January 7, 2021. These shares were expensed during the period.
On June 16, 2021, the Company issued shares of Common Stock at a price of $per share pursuant to the Registered Direct Purchase Agreement for total proceeds to the Company of $26,843,998 net of $2,156,012 of issuance costs.
On June 14, 2021, the Company issued shares of Common Stock at an average price of $ per share pursuant to the Purchase Agreement with Lincoln Park for total proceeds to the Company of $1,018,500.
On June 11, 2021, the Company issued shares of Common Stock at an average price of $ per share pursuant to the Purchase Agreement with Lincoln Park for total proceeds to the Company of $202,850.
From March 18, 2021 through June 9, 2021, the Company issued shares of Common Stock at a price of $ per share pursuant to a private placement for total proceeds to the Company of $5,000,800.
On February 18, 2021, there were restricted share units issued that immediately vested and were converted into shares of Common Stock in exchange for consulting services valued at $147,000.
On February 11, 2021, the Company issued shares of Common Stock valued at $298,178 based on the closing price on that date, issued in lieu of prepaid interest related to an amendment that extended the maturity date of the Unsecured Note to November 30, 2022 (see Note 5).
On December 14, 2020, the Company issued shares of Common Stock valued at the price of $1.30 strike price per share pursuant to the exercise of vested warrants for total proceeds of $82,056.
On December 14, 2020, the Company issued shares of Common Stock valued at the price of $3.05 per share in connection with the acquisition of Enochian Biopharma Inc. This non-cash transaction impacted stockholders’ equity in the amount of $192,522.
| F-22 |
On March 30, 2020, the Company issued shares valued at $501,370 based on the closing share price on that date, in lieu of prepaid interest related to the $5 million in principal of the Unsecured Note, which is recorded against the Unsecured Note at its computed relative fair value of $493,192 (see Note 5).
On January 9, 2020, the Company issued shares of Common Stock related to restricted share units that vested on January 7, 2020. These shares were expensed during the period.
On December 27, 2019, there were restricted share units (RSUs) issued with immediate vesting were converted into shares of Common Stock in exchange for consulting services valued at $144,000.
On July 3, 2019, the Registrant issued shares of Common Stock valued at the strike price of $2.00 per share pursuant to the exercise of vested grant warrants for total proceeds of $1.0 million.
On July 3, 2019, the Registrant issued shares of Common Stock valued at the price of $4.42 per share in connection with the acquisition of Enochian Biopharma. This non-cash transaction impacted stockholders’ equity in the amount of $2.2 million.
Acquisition of Enochian Biopharma / Contingently issuable shares
On February 16, 2018, the acquisition of Enochian Biopharma was completed. As part of the acquisition, the stockholders of Enochian Biopharma received (i) 18,081,962 shares of Common Stock, and (ii) the right to receive Contingent Shares of Common Stock pro rata upon the exercise or conversion of warrants, which were outstanding at closing. As of June 30, 2021, 1,350,000 Contingent Shares are potentially issuable (see Note 1).
Acquisition of Enochian Denmark
At June 30, 2021 and 2020, the Company maintained a reserve of and Escrow Shares, respectively, all of which are reflected as issued and outstanding in the accompanying financial statements. The Escrow Shares are reserved to acquire the shares of Enochian Denmark held by non-consenting shareholders of Enochian Denmark on both June 30, 2021 and 2020, in accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark. There have been shares of Common Stock issued to non-consenting shareholders of Enochian Denmark as of June 30, 2021. During the year ended June 30, 2021, the Company issued shares of Common Stock to such non-consenting shareholders of Enochian Denmark with no impact on the number of outstanding shares as all Escrow Shares are reflected as issued and outstanding.
| F-23 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 — STOCKHOLDERS’ EQUITY (continued)
Stock-based Compensation
The Company recognizes compensation costs for stock option awards to employees based on their grant-date fair value. The value of each stock option is estimated on the date of grant using the Black-Scholes option-pricing model. In the year ended June 30, 2021, the weighted-average assumptions used to estimate the fair values of the stock options granted using the Black-Scholes option-pricing model are as follows:
| Enochian Biosciences Inc. | ||||
| Expected term (in years) | – | |||
| Volatility | – % | |||
| Risk free interest rate | %- % | |||
| Dividend yield | % | |||
The Company recognized stock-based compensation expense related to all equity instruments of $ and $ for the years ended June 30, 2021 and 2020, respectively. At June 30, 2021, the Company had approximately $ of unrecognized compensation cost related to non-vested options.
Grant Warrants
In October of 2017, the Company issued warrants to APE Invest A/S and N.E. Nielsen, and in January 2018, the Registrant issued a warrant to Eric Leire (each a “Grant Warrant” and collectively the “Grant Warrants”) for an aggregate of Grant Warrants to purchase shares of Common Stock. During the year ended June 30, 2020, there were 500,000 Grant Warrants exercised at the strike price of $2.00 per share, for total proceeds of $1,000,000. As of June 30, 2021, all Grant Warrants have been exercised.
Plan Options
On February 6, 2014, the Board adopted the Company’s 2014 Equity Incentive Plan (the “2014 Plan”), and the Company had reserved shares of Common Stock for issuance in accordance with the terms of the 2014 Plan.
On October 30, 2019, the Board approved and on October 31, 2019, the Company’s stockholders adopted Enochian’s 2019 Equity Incentive Plan (the “2019 Plan”), which replaced the 2014 Plan. The 2019 Plan authorized options to be awarded to not exceed the sum of (1) 6,000,000 new shares, and (2) the number of shares available for the grant of awards as of the effective date under the 2014 Plan plus any options related to awards that expire, are terminated, surrendered, or forfeited for any reason without issuance of shares under the 2014 Plan after the effective date of the 2019 Plan.
Pursuant to the 2019 Plan, the Company granted options to purchase shares to employees with a three-year vesting period during the year ended June 30, 2021. For the year ended June 30, 2020, the Company granted options to purchase shares with a three-year vesting period under the 2019 Plan. Options are exercisable at the market price of the Company’s Common Stock on the date of the grant.
During the years ended June 30, 2021, and 2020 the Company granted options to purchase and shares, respectively, to the Board of Directors and Scientific Advisory Board Members with a one-year vesting period. Options are exercisable at the market price of the Company’s Common Stock on the date of the grant. To date the Company has granted options under the Plan (“Plan Options”) to purchase shares of Common Stock.
The Company issued options to purchase 15,000 shares with immediate vesting for services rendered with a Black-Scholes value of $27,990, during the year ended June 30, 2021.
| F-24 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
A summary of the Plan Options outstanding at June 30, 2021 is presented below:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||||||
| Exercise Price Ranges | Number Outstanding | Weighted Average Remaining Contractual Life (years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Remaining Contractual Life (years) | Weighted Average Exercise Price | ||||||||||||||||||||||
| $ | – | 288,543 | $ | 3.24 | 106,940 | $ | 2.98 | |||||||||||||||||||||
| $ | – | 506,495 | $ | 6.13 | 451,999 | $ | 6.18 | |||||||||||||||||||||
| $ | – | 534,115 | $ | 7.97 | 534,115 | $ | 7.97 | |||||||||||||||||||||
| Total | 1,329,153 | $ | 6.24 | 1,093,053 | $ | 6.74 | ||||||||||||||||||||||
A summary of changes since July 1, 2020 are presented below:
| Weighted Average | Weighted Average | Weighted Average | |||||||||||||||
| Shares | Exercise Price | Remaining Life | Intrinsic Value | ||||||||||||||
| Outstanding at July 1, 2020 | 1,105,442 | $ | 6.78 | $ | 107,931 | ||||||||||||
| Granted | 231,209 | 3.65 | |||||||||||||||
| Exercised | |||||||||||||||||
| Forfeited | |||||||||||||||||
| Expired | (7,499 | ) | |||||||||||||||
| Outstanding at June 30, 2021 | 1,329,153 | $ | 6.24 | $ | 511,239 | ||||||||||||
| Exercisable at June 30, 2021 | 1,093,053 | $ | 6.74 | $ | 233,670 | ||||||||||||
At June 30, 2021, the Company has exercisable Plan Options. The total intrinsic value of options exercisable at June 30, 2021 was $. Intrinsic value is measured using the fair market value at the date of exercise (for shares exercised) and at June 30, 2021 (for outstanding options), less the applicable exercise price.
| F-25 |
Common Stock Purchase Warrants
A summary of the warrants outstanding at June 30, 2021, and changes in the warrants in the year ended June 30, 2021 are presented below:
| Weighted Average | Weighted Average | ||||||||||||
| Underlying Shares | Exercise Price | Remaining Life | |||||||||||
| Outstanding at July 1, 2020 | 1,438,122 | $ | 1.42 | ||||||||||
| Granted | |||||||||||||
| Exercised | (63,122 | ) | 1.30 | ||||||||||
| Cancelled/Expired | (25,000 | ) | |||||||||||
| Outstanding at June 30, 2021 | 1,350,000 | $ | 1.30 | ||||||||||
| Exercisable at June 30, 2021 | 1,350,000 | $ | 1.30 | ||||||||||
| Outstanding | Equivalent Shares Exercisable | |||||||||||||||||||||
| Exercise Prices | Underlying Shares | Weighted Average Remaining Contractual Life (years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | |||||||||||||||||
| $ | 1.30 | 1,350,000 | $ | 1.30 | 1,350,000 | $ | 1.30 | |||||||||||||||
The exercise price of certain warrants and the number of shares underlying the warrants are subject to adjustment for stock dividends, subdivisions of the outstanding shares of Common Stock and combinations of the outstanding shares of Common Stock. For so long as the warrants remain outstanding, we are required to keep reserved from our authorized and unissued shares of Common Stock a sufficient number of shares to provide for the issuance of the shares underlying the warrants.
Restricted Stock Units (RSUs)
The Company recognized stock-based compensation expense related to RSUs of $ and $ for the years ended June 30, 2021 and 2020, respectively. On June 30, 2020, the Company issued shares of Common Stock related to restricted share units that vested on January 7, 2021. At June 30, 2021, the Company had approximately $ of unrecognized compensation cost related to restricted stock units.
A summary of Restricted Stock Units outstanding at June 30, 2020 and changes in the RSUs in the year ended June 30, 2021 are presented below:
| Shares | Weighted Average Issuance Price | Weighted Average Remaining Life | Weighted Average Intrinsic Value | ||||||||||||||
| Outstanding at July 1, 2020 | 10,000 | $ | 6.15 | $ | |||||||||||||
| Granted | 35,000 | ||||||||||||||||
| Exercised | (40,000 | ) | |||||||||||||||
| Cancelled/Expired | |||||||||||||||||
| Outstanding at June 30, 2021 | 5,000 | $ | 6.15 | $ | |||||||||||||
| Restricted Stock Units Outstanding | ||||||||||||
| Shares | Weighted Average Remaining Contractual Life (years) | Weighted Average Issuance Price | ||||||||||
| 5,000 | $ | 6.15 | ||||||||||
| F-26 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 — COMMITMENTS AND CONTINGENCIES
Consulting Agreements - On July 9, 2018, the Company entered into a consulting agreement with G-Tech Bio, LLC, a California limited liability company (“G-Tech”) to assist the Company with the development of the gene therapy and cell therapy modalities for the prevention, treatment, amelioration of HIV in humans, and with the development of a genetically enhanced Dendritic Cell for use as a wide spectrum platform for various diseases (including but not limited to cancers and infectious diseases) (the “G-Tech Agreement”). G-Tech was entitled to consulting fees for 20 months, a monthly consulting fee of not greater than $130,000 per month. Upon the completion of the 20 months, the monthly consulting fee of $25,000 continued for scientific consulting and knowledge transfer on existing HIV experiments, and will continue until the services are no longer rendered or the agreement is terminated G Tech is controlled by certain members of Weird Science. For the years ended June 30, 2021 and 2020, $275,000 and $1,125,000, respectively, was charged to research and development expenses in the accompanying consolidated statements of operations related to this consulting agreement.
On January 31, 2020, the Company entered into a Statement of Work and License Agreement (the “HBV License Agreement”) by and among the Company, G Tech, and G Health Research Foundation, a not for profit entity organized under the laws of California doing business as Seraph Research Institute (“SRI”), whereby the Company acquired a perpetual, sublicensable, exclusive license (the “HBV License”) for a treatment under development (the “Treatment”) aimed to treat Hepatitis B Virus (HBV) infections.
The HBV License Agreement states that in consideration for the HBV License, the Company shall provide cash funding for research costs and equipment and certain other in-kind funding related to the Treatment over a 24 month period, and provides for an up-front payment of $1.2 million within 7 days of January 31, 2020, along with additional payments upon the occurrence of certain benchmarks in the development of the technology set forth in the HBV License Agreement, in each case subject to the terms of the HBV License Agreement. Additionally, the HBV License Agreement provides for cooperation related to the development of intellectual property related to the Treatment and for a 2% royalty to G Tech on any net sales that may occur under the HBV License. On February 6, 2020, the Company paid the $1.2 million up-front payment. The HBV License Agreement contains customary representations, warranties and covenants of the parties with respect to the development of the Treatment and the HBV License.
The cash funding for research costs pursuant to the HBV License Agreement consists of monthly payments amounting to $144,500 that cover scientific staffing resources to complete the project as well as periodic payments for materials and equipment needed to complete the project. During the years ended June 30, 2021 and 2020, the Company paid a total of $2,409,000 and $1,022,000, respectively for scientific staffing resources, R&D and IND Enabling studies.
On April 18, 2021, the Company entered into a Statement of Work and License Agreement (the “License Agreement”), by and among the Company, G Tech and SRI, whereby the Company acquired a perpetual sublicensable, exclusive license (the “Development License”) to research, develop, and commercialize certain formulations which are aimed at preventing and treating pan-coronavirus or the potential combination of the pan-coronavirus and pan-influenza, including the SARS-coronavirus that causes COVID-19 and pan-influenza (the “Prevention and Treatment”).
The License Agreement was entered into pursuant to the existing Framework Agreement between the parties dated November 15, 2019. The License Agreement states that in consideration for the Development License, the Company shall provide cash funding for research costs and equipment and certain other in-kind funding related to the Prevention and Treatment over a 24-month period. Additionally, the License Agreement provides for an up-front payment of $10 million and a $760,000 payment for expenditures to date prior to the effective date related to research towards the Prevention and Treatment within 60 days of April 18, 2021. The License Agreement provides for additional payments upon the occurrence of certain benchmarks in the development of the technology set forth in the License Agreement, in each case subject to the terms of the License Agreement.
| F-27 |
The License Agreement provides for cooperation related to the development of intellectual property related to the Prevention and Treatment and for a 3% royalty to G Tech on any net sales that may occur under the License Agreement. As of June 30, 2021, the Company paid the $10 million up-front payment, and the $760,000 related to prior research costs.
G Tech is controlled by Dr. Serhat Gümrükcü and Anderson Wittekind, shareholders of the Company, and SRI is controlled by Dr. Serhat Gümrükcü.
Shares held for non-consenting shareholders – In connection with the share exchange upon the acquisition of Enochian Denmark certain shareholders of DanDrit Denmark had not been identified or did not consent to the exchange of shares. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, the non-consenting Shareholders that did not exchange their DanDrit Denmark equity interests for shares of the Company, will be entitled to receive up to 185,053 shares of Common Stock of the Company that each such non-consenting Shareholder would have been entitled to receive if such shareholder had consented to the Share Exchange. During the year ended June 30, 2021, the Company issued shares of Common Stock to such non-consenting shareholders of DanDrit Denmark. The remaining shares have been reflected as issued and outstanding in the accompanying financial statements.
Service Agreements - The Company maintains employment agreements with other staff in the ordinary course of business.
Contingencies - The Company is from time to time involved in routine legal and administrative proceedings and claims of various types. While any proceedings or claim contains an element of uncertainty, management does not expect a material impact on our results of operations or financial position from such proceedings or claims.
| F-28 |
ENOCHIAN BIOSCIENCES INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 — RELATED PARTY TRANSACTIONS
On July 9, 2018, the Company entered into a consulting agreement with G-Tech to assist the Company with the development of the gene therapy and autologous and allogenic cell therapy modalities for the prevention, treatment, amelioration of HIV in humans, and with the development of a genetically enhanced Allogenic Dendritic Cell for use as a wide spectrum platform for various diseases (including but not limited to cancers and infectious diseases) (see Note 8.)
On January 31, 2020, the Company entered into the HBV License Agreement by and among the Company, G Tech and SRI, whereby the Company acquired the HBV License for the Treatment (see Note 8.)
On April 18, 2020 the Company entered into the Flu-CoV License Agreement by and among the Company, G Tech and SRI, whereby the Company acquired the Development License to research, develop, and commercialize certain formulations, which are aimed at preventing and treating pan-coronavirus and pan-influenza, including the SARS-coronavirus that causes COVID-19 and pan-influenza (see Note 8.)
NOTE 10 — SUBSEQUENT EVENTS
On August 11, 2021, and with an effective date of July 1, 2021, the Company and Dr. Dybul entered into an Employment Agreement, the form of which was approved and recommended by the Board on October 30, 2019 and approved by the stockholders of the Company with a majority of the voting power of all shares of the Company’s capital stock entitled to vote on the matter on October 31, 2019. The material terms of the Employment Agreement are as set forth in the Company’s Information Statement filed with the Commission on November 12, 2019.
On August 25, 2021, the Company entered into an ALC Patent License and Research Funding Agreement in the HIV Field (the “ALC License Agreement”) with Dr. Gumrukcu and SRI whereby Dr. Gumrukcu granted the Company an exclusive, worldwide, perpetual, fully paid-up, royalty-free license, with the right to sublicense, his proprietary technology subject to a U.S. patent application, to make, use, offer to sell, sell or import products for use solely for the prevention, treatment, amelioration of or therapy exclusively for HIV in humans, and research and development exclusively relating to HIV in humans; provided Dr. Gumrukcu retained the right to conduct HIV research in the field. Pursuant to the ALC License Agreement, the Company granted a non-exclusive license back to Dr. Gumrukcu and SRI, under any patents or other intellectual property owned or controlled by the Company, to the extent arising from the ALC License, to make, use, offer to sell, sell or import products for use in the diagnosis, prevention, treatment, amelioration or therapy of any (i) HIV Comorbidities and (ii) any other diseases or conditions outside the HIV Field. The Company made an initial payment to SRI of $600,000 and agreed to fund future HIV research conducted by Dr. Gumrukcu and SRI, as mutually agreed to by the parties. On September 10, 2021, pursuant to the ALC License Agreement, the Company paid the initial payment of $600,000.
| F-29 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our Principal Executive Officer and Principal Financial Officer (the “Certifying Officers”) are responsible for establishing and maintaining disclosure controls and procedures for the Company. The Certifying Officers have designed such disclosure controls and procedures to ensure that material information is made known to the Certifying Officers, particularly during the period in which this Report was prepared.
The Certifying Officers conducted a review of the Company’s “disclosure controls and procedures” (as defined in the Exchange Act, Rules 13a-15(e) and 15-d-15(e)) as of the end of the period covered by this Annual Report (the “Evaluation Date”). Based upon that evaluation, the Certifying Officers concluded that, as of June 30, 2021, our disclosure controls and procedures were not effective in ensuring that the information we were required to disclose in reports that we file or submit under the SEC Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
Management Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Management used the “Internal Control over Financial Reporting Integrated Framework” issued by the Committee of Sponsoring Organizations (“COSO”) to conduct a review of the Company’s internal controls over financial reporting. As of June 30, 2021, Management concluded that internal controls over financial reporting were not effective, based on COSO’s framework. The deficiencies are attributed to the fact that the Company does not have an adequate number of people to whom it can segregate accounting tasks within the Company so as to ensure segregation of duties between those people who approve and issue payment from those people who are responsible for recording and reconciling such transactions within the Company’s accounting system. These control deficiencies will be monitored, and attention will be given to this matter as we continue to accelerate through our current growth stage.
This Annual Report does not include an attestation report from the Company’s registered public accounting firm regarding internal controls over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to the rules of the SEC that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the period covered by this report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Not Applicable.
| 43 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item 10 will be included under the captions “Directors and Executive Officers”, “Information as to Nominees and Other Directors”, “Information Regarding Meetings and Committees of the Board”, “Compliance with Section 16(a) of the Exchange Act”, “Code of Ethics”, “Corporate Governance” and as otherwise set forth in the Company’s 2021 Proxy Statement and is incorporated herein by reference or, alternatively will be included, by amendment to this Form 10-K under cover of Form 10-K/A no later than 120-days after the end of our fiscal year covered by this report.
Item 11. Executive Compensation
This information will be contained in our definitive proxy statement for our upcoming Annual Meeting of Shareholders, to be filed with the SEC no later than 120 days after the end of our fiscal year covered by this report, and incorporated herein by reference or, alternatively, will be included by amendment to this Form 10-K under cover of Form 10-K/A no later than the end of such 120-day period.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
This information will be contained in our definitive proxy statement for our upcoming Annual Meeting of Shareholders, to be filed with the SEC no later than 120 days after the end of our fiscal year covered by this report, and incorporated herein by reference or, will be included alternatively, by amendment to this Form 10-K under cover of Form 10-K/A no later than the end of such 120-day period.
Item 13. Certain Relationships and Related Transactions and Director Independence
This information will be contained in our definitive proxy statement for our upcoming Annual Meeting of Shareholders, to be filed with the SEC no later than 120 days after the end of our fiscal year covered by this report, and incorporated herein by reference or, alternatively, will be included by amendment to this Form 10-K under cover of Form 10-K/A no later than the end of such 120-day period.
Item 14. Principal Accounting Fees and Services
This information will be contained in our definitive proxy statement for our upcoming Annual Meeting of Shareholders, to be filed with the SEC no later than 120 days after the end of our fiscal year covered by this report, and incorporated herein by reference or, alternatively, will be included by amendment to this Form 10-K under cover of Form 10-K/A no later than the end of such 120-day period.
| 44 |
PART IV
Item 15. Exhibits, Financial Statement Schedules
| 45 |
| * | Provided herewith. | ||
| ** | Furnished herewith. | ||
| 46 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: September 24, 2021 | ENOCHIAN BIOSCIENCES INC. | |
| By: | /s/ Mark Dybul | |
| Mark Dybul | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| By: | /s/ Luisa Puche | |
| Luisa Puche | ||
| Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Dr. Mark Dybul | Chief Executive Officer | September 24, 2021 | ||
| Dr. Mark Dybul | (Principal Executive Officer) | |||
| /s/ Luisa Puche | Chief Financial Officer | September 24, 2021 | ||
| Luisa Puche | (Principal Financial and Accounting Officer) | |||
| /s/ René Sindlev | Director and Chairman of the Board | September 24, 2021 | ||
| René Sindlev | ||||
| /s/ Henrik Grønfeldt-Sørensen | Director | September 24, 2021 | ||
| Henrik Grønfeldt-Sørensen | ||||
| /s/ Carl Sandler | Director | September 24, 2021 | ||
| Carl Sandler | ||||
| /s/ Gregg Alton | Director | September 24, 2021 | ||
| Gregg Alton |
| /s/ Jayne McNicol | Director | September 24, 2021 | ||
| Ms. Jayne McNicol |
| /s/ James Sapirstein | Director | September 24, 2021 | ||
| James Sapirstein | ||||
| /s/ Carol Brosgart | Director | September 24, 2021 | ||
| Carol Brosgart |
47
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-10-01)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
