RETRACTABLE TECHNOLOGIES INC - Annual Report: 2021 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2021
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 001-16465
Retractable Technologies, Inc.
(Exact name of registrant as specified in its charter)
Texas |
| 75-2599762 |
(State or other jurisdiction of | (I.R.S. Employer | |
511 Lobo Lane | ||
Little Elm, Texas | 75068-5295 | |
(Address of principal executive offices) | (Zip Code) |
972-294-1010
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading Symbol |
| Name of each exchange on which registered |
Common | RVP | NYSE American LLC |
Securities registered pursuant to Section 12(g) of the Act:
Preferred Stock
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ⌧
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ⌧
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ⌧ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ⌧ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer ☐ | Accelerated filer ☐ |
Non-accelerated filer ⌧ | Smaller reporting company ☒ |
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ⌧
The aggregate market value of the common equity held by non-affiliates as of June 30, 2021, was $200,031,281, assuming a closing price of $11.56 and outstanding shares held by non-affiliates of 17,303,744.
APPLICABLE ONLY TO REGISTRANTS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13, or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☐ No ☐
(APPLICABLE ONLY TO CORPORATE REGISTRANTS)
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. As of March 11, 2022, there were 33,123,205 shares of our Common Stock outstanding, excluding treasury shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement filed on an even date herewith for the Annual Meeting of Shareholders to be held May 10, 2022 are incorporated by reference into Part III hereof.
RETRACTABLE TECHNOLOGIES, INC.
FORM 10-K
For the Fiscal Year Ended December 31, 2021
TABLE OF CONTENTS
i
PART I
FORWARD-LOOKING STATEMENT WARNING
Certain statements included by reference in this filing containing the words “could,” “may,” “believes,” “anticipates,” “intends,” “expects,” and similar such words constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Any forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the impact of COVID-19 on all facets of logistics and operations, as well as costs, our ability to scale up production volumes in response to an increase in demand, potential tariffs, our ability to maintain liquidity, our maintenance of patent protection, our ability to maintain favorable third party manufacturing and supplier arrangements and relationships, foreign trade risk, our ability to access the market, production costs, the impact of larger market players, specifically Becton, Dickinson and Company ("BD"), in providing devices to the safety market, and other factors referenced in Item 1A. Risk Factors. Given these uncertainties, undue reliance should not be placed on forward-looking statements.
Item 1. Business.
DESCRIPTION OF BUSINESS
General Development of Business
Retractable Technologies, Inc. was incorporated in Texas in 1994. Our business is the manufacturing and marketing of safety medical products (predominately syringes) for the healthcare industry. We have manufacturing facilities in Little Elm, Texas and use manufacturers in China as well. Our syringes are well-suited for administering vaccinations and our revenues for 2021 materially increased over prior years due to demand during the COVID-19 pandemic. In 2020 and 2021, we increased our revenues by 95.9% and 130.1%, respectively, over the prior years. Our $59.4 million revenues in the fourth quarter of 2021 represent an 85.8% increase over the same quarter in the prior year and a 94.7% increase in units sold. Our principal customer was the U.S. government which purchased products representing 60.3% ($113.7 million) of our revenues in 2021.
We increased our domestic production in 2020 and in 2021, primarily due to the increased demand brought about by the COVID-19 pandemic and resulting U.S. government delivery orders. We have been working to increase our manufacturing capacity in Little Elm, Texas, funded in part by the Technology Investment Agreement ("TIA") with the United States Government Department of Defense, U.S. Army Contracting Command-Aberdeen Proving Ground, Natick Contracting Division & Edgewood Contracting Division (ACC-APG, NCD & ECD) on behalf of the Biomedical Advanced Research and Development Authority (BARDA), as amended (“TIA”). The TIA, as amended, calls for $81.0 million in spending by the U.S. government to purchase additional manufacturing equipment, related ancillary equipment, and an increase in our production facility floorspace. At our own expense, we constructed a new warehouse onsite for housing finished goods and raw materials to be used in the manufacturing process. In addition, we have increased our workforce significantly to meet the increased production needs and to administer the expansion of our facilities and the increase in manufacturing equipment, as well as to provide support personnel. The expansion efforts represent a significant commitment in terms of financial and technical resources.
Description of Business
Our dominant revenue-generating products are our injection devices (syringes and needles). Such products are marketed under the VanishPoint®, Patient Safe®, and EasyPoint® brands. We have only one reporting segment. Most of our products incorporate a feature whereby our needles retract which is a safety feature designed to protect healthcare workers from needlestick injuries. Our VanishPoint® 1mL syringes meet the criteria set by pharmaceutical manufacturers for low dead space, which results in a reduction of wasted medication caused by residual medication remaining in the syringe after a dose has been administered. In some instances, the low dead space allows for additional doses to be obtained from a medication vial.
2
In 2021, the U.S. government was a significant customer due to efforts to vaccinate the U.S. population against COVID-19. On May 1, 2020, we received an order from the Department of Health and Human Services to supply certain automated safety syringes for $83.8 million, plus $10 million in expedited freight costs. The period of performance ended March 2022. In February 2021, we received a new contract from the Department of Health and Human Services for additional safety syringes. As amended, the contract represented a total of $147 million in revenues and freight costs plus additional reimbursable freight costs of $6 million. As of December 31, 2021, we recorded total sales of $113.7 million to the U.S. government, representing 60.3% of our overall revenues for 2021.
During 2021, we also continued to provide products to our existing and new private healthcare customers. Our growth in sales in 2021 was predominantly driven by demand for syringes for COVID-19 vaccines and flu vaccines.
Our goal is to become a leading provider of safety medical products. Our principal products were designed to protect healthcare workers, patients, and others from needlestick injuries, cross-contamination through reuse, and reduce disposal costs.
VanishPoint® syringe sales have historically comprised most of our sales. VanishPoint® syringe sales were 85.3%, 84.0%, and 93.6% of our revenues in 2019, 2020, and 2021. EasyPoint® products accounted for 5.1% of sales in 2021.
We currently have under development additional safety products that add to or build upon our current product line offering. Notwithstanding the foregoing, our primary focus over the last year has centered on providing existing products to meet demand related to COVID-19 vaccinations.
Our products are sold to and used by healthcare providers primarily in the U.S. (with 11.1% of revenues in 2021 generated from sales outside the U.S.).
In years not dominated by direct sales to the U.S. government, representatives of group purchasing organizations (“GPOs”) and purchasing representatives (rather than the end-users of the product) make the vast majority of decisions relating to the purchase of medical supplies. The GPOs and larger manufacturers often enter into contracts which can prohibit or limit entry in the marketplace by competitors.
We distribute our products throughout the U.S. through general line and specialty distributors. We also use international distributors. We have developed a national direct marketing network in order to market our products to health care customers and their purchaser representatives.
Sources and Availability of Raw Materials
Our product components, including needle adhesives and packaging materials, are purchased from various suppliers. There is no current scarcity of such materials or such suppliers.
Intellectual Property
Intellectual property rights, particularly patent rights, are material to our business. The patent rights are jointly owned by the Company and Thomas J. Shaw, our founder and CEO, and have varying expiration dates. Under the terms of an exclusive license agreement that has been in effect since 1995, the Company is exclusively licensed to use the patent rights held by Mr. Shaw, and Mr. Shaw generally receives a 5% royalty on gross sales of products subject to the license and he receives 50% of the royalties paid to the Company by certain sublicensees of the technology subject to the license.
Recent and expected modifications to our VanishPoint® syringes will effectively cause the modified VanishPoint® syringes products to have extended patent expiration dates. Following the expiration of patents related to the old design, competitors may attempt to copy aspects of such prior design, but not the current design. Patents related to recent modifications to the VanishPoint® syringes and core technology of the VanishPoint® syringes will expire during the years 2028 through 2032. Other patent applications covering inventions applicable to the VanishPoint® syringes are pending.
The Company has unexpired patents which relate to the EasyPoint® technology and other products as well.
3
The Company has registered the following trade names and trademarks for our products: VanishPoint®, EasyPoint®, Patient Safe®, VanishPoint® logos, RT and design, the VanishPoint® and design, the spot design and the Company slogans “The New Standard for Safety” ® and “We Make Safety Safe” ®.
Seasonality
Historically, unit sales have increased during the flu season. Seasonal trends have been less pronounced due to demand related to the COVID-19 vaccine.
Dependence on Customers
Although our business has historically derived significant percentages of its revenues from a few customers, we do not believe that the loss of any one of these customers would have a material adverse effect on our business. The U.S. government was a significant customer from mid-2020 through the end of 2021 in connection with its purchase of syringes for the COVID-19 vaccine.
Government Contracts
In 2020, we entered into a material contract with the U.S. government providing a significant grant and accepted the $83.8 million order under an existing contract for the sale of syringes. In February 2021, we and the Department of Health and Human Services entered into a new contract, and it placed another material order with us for syringes. All such contracts may be terminated by the U.S. government but, given that the 2020 order has been filled and the 2021 orders are nearing completion, we do not believe termination (or renegotiation) is likely.
Government Approval and Government Regulations
Compliance with government regulations represents an important part of our business. As a manufacturer of medical devices and operating under the TIA, we are subject to stringent regulatory requirements. In addition, we are also subject to maintain systems to monitor and report our findings to various regulatory bodies. We are also subject to audit by those bodies and/or third parties acting as proxies to verify our compliance with such regulations. The cost of compliance can be significant in terms of financial and human resource commitments. These costs are ongoing and may become more significant if the regulatory landscape changes.
The development, manufacture, marketing, sale, promotion, and distribution of our products are subject to government regulation by the U.S. Food and Drug Administration (FDA) and similar international regulatory agencies. Regulation by various international, federal and state agencies address the development and approval to market medical products, as well as approval and supervision of manufacturing, labeling, packaging, supply chains, distribution and record-keeping.
For all products manufactured for sale in the domestic market, we have given notice of intent to market to the FDA, and the devices were shown to be substantially equivalent to the predicate devices for the stated intended use. For all products manufactured for sale in the domestic market and foreign market, we hold a Quality Management System certification to ISO 13485:2016. Additionally, for all products manufactured for sale into the applicable countries, we hold a Quality Management System certification in compliance with the Medical Device Single Audit Program (MDSAP). For all products manufactured for sale into European Union countries, we hold a Full Quality Assurance System certification to Directive 93/42/EEC Annex II (excluding section 4). All of these certifications are issued by our notified body, BSI, and are reviewed annually.
Compliance with domestic and international laws and regulations may affect our business. Among other effects, health care regulations and significant changes thereto may substantially increase the time, difficulty, and costs incurred in developing, obtaining, and maintaining approval to market, and marketing newly developed and existing products. We expect this regulatory environment will continue to require effort and investment to ensure compliance. Failure to comply could delay the release of a new product or result in regulatory and enforcement actions, the seizure or recall of a product,
4
the suspension or revocation of the authority necessary for a product’s production and sale, and other civil or criminal sanctions including fines and penalties.
The regulation of data privacy and security, and the protection of the confidentiality of certain personal information (including patient health information, financial information, and other sensitive personal information), is increasing. For example, the European Union, various other countries, and various U.S. states (e.g., California) have enacted stricter data protection laws that contain enhanced financial penalties for noncompliance. Similarly, the U.S. Department of Health and Human Services has issued rules governing the use, disclosure, and security of protected health information, and the FDA has issued further guidance concerning cybersecurity for medical devices. In addition, certain countries have issued or are considering “data localization” laws, which limit companies’ ability to transfer protected data across country borders. Failure to comply with data privacy and security laws and regulations can result in business disruption and enforcement actions, which could include civil or criminal penalties.
The sale of medical products is subject to laws and regulations pertaining to health care fraud and abuse, including state and federal anti-kickback, anti-self-referral, and false claims laws in the United States.
We will continue to comply with applicable regulations of all countries in which our products are registered for sale.
We believe that we do not incur material costs in connection with compliance with environmental laws.
Competitive Conditions
Major domestic competitors include BD and Medtronic Minimally Invasive Therapies (“Medtronic,” formerly known as Covidien). Terumo Medical Corp., Smiths Medical, and B Braun are additional competitors with smaller market shares. BD and Medtronic have controlling U.S. market share; greater financial resources; larger and more established sales, marketing, and distribution organizations; and greater market influence, including long-term and/or exclusive contracts. Additionally, BD may be able to use its resources to improve its products through research or acquisitions or develop new products which may compete with our products.
We compete primarily on the basis of healthcare worker and patient safety, product performance, and quality. We believe our competitive advantages include, but are not limited to, our leadership in quality and innovation. We believe our products continue to be the most effective safety devices in today’s market. Our VanishPoint® 1mL syringes meet the criteria set by pharmaceutical manufacturers for low dead space, which results in a reduction of wasted medication caused by residual medication remaining in the syringe after a dose has been administered. In some instances, the low dead space allows for additional doses to be obtained from a medication vial. Our syringe products include passive safety activation, require less disposal space, and are activated while in the patient, reducing exposure to the contaminated needle. Our price per unit is competitive or even lower than the competition once all the costs incurred during the life cycle of a syringe are considered. Such life cycle costs include disposal costs, testing and treatment costs for needlestick injuries, and treatment for contracted illnesses resulting from needlestick injuries.
EasyPoint® retractable needles offer unique safety benefits not found in other commercially available safety needles. Manually activated safety needles that compete with EasyPoint® must be removed from the patient, exposing the contaminated needle prior to activation of the manual safety mechanism. EasyPoint® needles allow for activation of the automated retraction mechanism while the needle is still in the patient, reducing exposure to the contaminated needle and effectively reducing the risk of needlestick injuries. EasyPoint® retractable needles are compatible with Luer-fitting syringes, including pre-filled syringes. In addition, EasyPoint® retractable needles may be activated with fluid in the syringe, making it applicable for aspiration procedures such as blood collection.
Employees
As of March 11, 2022, we had 235 employees. 233 of such employees were full time employees. We provide equal employment opportunities to all employees and applicants for employment without regard to race, color, religion, gender, national origin, age, disability, marital status, ancestry, veteran status, workers’ compensation status or any other
5
characteristic protected by federal, state, or local law. We have adopted a policy of zero tolerance for any form of unlawful discrimination or retaliation. In 2021, we increased wages considerably, particularly for our entry-level employees, in order to compete for labor.
Available Information
We make available, free of charge on our website (www.retractable.com), our Form 10-K Annual Report and Form 10-Q Quarterly Reports and Current Reports on Form 8-K (and any amendments to such reports) as soon as reasonably practical after such reports are filed.
Item 1A. Risk Factors.
You should carefully consider the following material risks facing us. If any of these risks occur, our business, results of operations, or financial condition could be materially affected.
We Are Challenged by Uncertainties in Obtaining and Enforcing Intellectual Property Rights
Our main competitive strength is our technology. We are dependent on patent rights, and if the patent rights are invalidated or circumvented, our business would be adversely affected. Patent protection is considered, in the aggregate, to be of material importance in the design, development, and marketing of our products.
VanishPoint® syringes comprised 93.6% of sales in 2021. When the patents of the VanishPoint® syringes and other products expire, we may experience a significant and rapid loss of sales, and our competitive position in the marketplace may weaken if other competitors use our technology. Such occurrences could have a material adverse effect on profitability.
We do not maintain patent or trademark protection in all foreign countries, but, where possible, have taken steps to protect our patents and trademarks in those countries where we market our products or where we believe other manufacturers are most likely to attempt to replicate our technology. Our lack of patent and trademark protection in certain foreign countries heightens the risk that our designs may be copied by a competitor in those countries.
We Are Vulnerable to New Technologies
Because we have a narrow focus on particular product lines and technology (currently, predominantly retractable needle products), we are vulnerable to the development of superior competing products and to changes in technology which could eliminate or reduce the need for our products. If a superior technology is created, the demand for our products could greatly diminish.
Our Competitors Have Greater Resources
Our competitors have greater financial resources, larger and more established sales and marketing and distribution organizations, and greater market influence, including long-term contracts. These competitors may be able to use these resources to improve their products through research and acquisitions or develop new products, which may compete more effectively with our products. If our competitors choose to use their resources to create products superior to ours, we may be unable to sell our products and our ability to continue operations would be weakened.
Operations May Be Affected by Foreign Trade Policy
We are subject to risks associated with foreign trade policy. In 2021, we used Chinese manufacturers to produce 92% of our products. However, in accordance with the requirements of the TIA, we are currently working to expand our U.S. manufacturing facility.
In the event that we become unable to purchase such product from our Chinese manufacturers, we would need to find an alternate manufacturer for the blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL
6
autodisable syringe, and 2mL, 5mL, and 10mL syringes and we would increase domestic production for the 1mL and 3mL syringes. Even with increased domestic production, we may not be able to avoid a disruption in supply.
Trade protection measures, including tariffs, and/or changes to import or export requirements could materially adversely impact our operations. We cannot predict the impact of potential changes to U.S. foreign trade policy. Additionally, we derive 11.1% of our revenues from international sales. International sales, particularly in emerging market countries, are further subject to a variety of regulatory, economic, and political risks as well.
We Are Controlled by One Shareholder
Thomas J. Shaw, our President and Chief Executive Officer, has investment or voting power over a total of 46.9% of the outstanding Common Stock as of March 11, 2022. Mr. Shaw therefore has the ability to direct our operations and financial affairs and significant influence to elect members of our Board of Directors. His interests may not always coincide with the Company’s interests or the interests of other stockholders. This concentration of ownership, for example, may have the effect of delaying, deferring, or preventing a change in control, impeding a merger, consolidation, takeover, or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us, which in turn could materially adversely affect the market price of our Common Stock. Mr. Shaw’s rights under the Technology License Agreement, as the owner of the technology we produce, present similar conflicts of interest.
Defensive Measures to Deter Hostile Takeovers
On November 16, 2021, we and Mr. Shaw entered into the Third Amendment to Technology License Agreement (the “Amendment”). The Amendment expands the scope of the Technology License Agreement and provides additional protection to the parties in the event of a Hostile Takeover, as defined by the Amendment. Under the Amendment, under certain conditions, Mr. Shaw is granted the unilateral right to terminate the Technology License Agreement or cancel or convert a license thereunder from exclusive to nonexclusive following a Hostile Takeover.
Additionally, as a public Texas corporation, we are generally prohibited from entering into a business combination with a person who acquires twenty percent or more of our stock for three years unless either: (1) the combination or acquisition is pre-approved by our Board; or (2) the combination is approved by affirmative vote of the shareholders of at least two-thirds of the outstanding voting shares entitled to vote, excluding the affiliated shareholder. As such, independent of the rights granted to Mr. Shaw under the Amendment, as owner of 46.9% of our stock and Chairman of the Board, Mr. Shaw has considerable influence on all business combination decisions.
Any Disruption in our Suppliers’ Operations or Timely Availability of Shipments From our Third-Party Freight Carriers, Could Disrupt our Ability to Provide Product to our Customers in a Timely Manner
Our operations are dependent upon timely delivery of finished goods from our Chinese manufacturers and timely delivery of sufficient quantities of components and raw materials for domestic manufacturing. The COVID-19 pandemic has adversely impacted worldwide supply chains and disruptions and delays in the ability of our third-party freight carriers to transport goods continues to be a challenge.
Any continued delays with freight carriers could cause us to not be able to meet customer demand, which could materially and adversely affect our results of operations and cash flows.
Inflationary Price Pressures and Uncertain Availability of Commodities, Raw Materials, Utilities, Labor or Other Inputs Used by us and our Suppliers, or Instability in Logistics and Related Costs, Could Negatively Impact our Profitability
Increases in the price of commodities, raw materials, utilities, labor or other inputs that we or our suppliers use in manufacturing and supplying products, components and parts, along with logistics and other related costs, may lead to higher production and shipping costs for our products, parts, and components. Further, increasing global demand for, and uncertain supply of, such materials could disrupt our or our suppliers’ ability to obtain such materials in a timely manner
7
to meet our supply needs and/or could lead to increased costs. A material increase in the cost of inputs to our production could lead to higher costs for our products and could negatively impact our operating results.
Our Stock Has Recently Experienced Significant Price Fluctuation
Our stock price experienced significant fluctuation during 2021 and may continue to be unpredictable. Our stock price fluctuated in 2021 from a high in February of $21.50 per share to a low price in December of $6.57. As of March 11, 2022, the stock price was $4.52 per share. Noting that the stock appeared undervalued at the then-current price of $10 per share, we entered into a one-year repurchase plan effective June 2021 for the purchase of up to $10 million of our Common Stock. Under the plan, 899,899 shares were purchased as of March 11, 2022 for an aggregate purchase price of approximately $7.2 million. Our stock repurchase history may be accessed at retractable.com/stock-repurchase.
We Face Inherent Product Liability Risks
As a manufacturer and provider of safety needle products, we face an inherent business risk of exposure to product liability claims. Additionally, our success depends on the quality, reliability, and safety of our products and defects in our products could damage our reputation. If a product liability claim is made and damages are in excess of our product liability coverage, our competitive position could be weakened by the amount of money we could be required to pay to compensate those injured by our products. In the event of a recall, we have recall insurance.
Our Business May Be Affected by Changes in the Health Care Regulatory Environment
In the U.S. and internationally, government authorities may enact changes in regulatory requirements, reform existing reimbursement programs, and/or make changes to patient access to health care, all of which could adversely affect the demand for our products and/or put downward pressure on our prices. Future healthcare rulemaking could affect our business. We cannot predict the timing or impact of any future rulemaking or changes in the law.
We May Experience Losses in Our Investment Account
Our investment portfolio is subject to market risk. As a result, the value and liquidity of our cash equivalents and marketable securities could fluctuate substantially. Likewise, our other income and expenses could vary materially depending on gains or losses realized on the sale or exchange of investments and other factors. Increased volatility in the financial markets and overall economic uncertainty could increase the risk that actual amounts realized on our investments may differ from the fair values currently assigned to them. Because 12.7% of our current assets are invested in the market, fluctuations in market values could have a material adverse impact on our business, financial condition, results of operations, or cash flows.
Health Crises Could Have an Adverse Effect on Our Business
Particularly during 2020, several states and local jurisdictions imposed, and others in the future may impose, “shelter-in-place” orders, quarantines, executive orders and similar government orders and restrictions for their residents to control the spread of COVID-19. Although our manufacturing facility has continued to operate during the 2020-2022 COVID-19 pandemic, we continue to monitor the evolving situation and cannot guarantee that the situation would be the same for any future pandemic. In the future, we may elect or be required to close temporarily which would result in a disruption in our activities and operations. Our supply chain, including transportation channels, may be impacted by any such restrictions as well. Any such disruption could impact our sales and operating results.
Widespread health crises also negatively affect economies which could affect demand for our products. With significant sales directly to the U.S. government, our risk was somewhat mitigated for the 2021 year. However, in the event of a resurgence of this disease or in the case of any future pandemic, there is no guarantee that revenues from syringes needed for vaccines would offset the effects to our business of a global economic decline.
Health systems and other healthcare providers in our markets that provide procedures that use our products have suffered financially and operationally and may not be able to return to pre-pandemic levels of operations. Travel and
8
import restrictions may also disrupt our ability to manufacture or distribute our devices. Any import or export or other cargo restrictions related to our products or the raw materials used to manufacture our products could restrict our ability to manufacture and ship products and harm our business, financial condition, and results of operations.
Our key personnel and other employees could still be affected by COVID-19 or any future pandemic, which could affect our ability to operate efficiently.
Disruption of Critical Information Systems or Material Breaches in the Security of Our Systems Could Harm Our Business, Customer Relations, and Financial Condition
Information technology helps us operate efficiently, interface with customers and suppliers, maintain financial accuracy and efficiency, and accurately produce our financial statements. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology infrastructure, we could be subject to transaction errors, processing inefficiencies, the loss of customers, business disruptions, or the loss of or damage to intellectual property through security breach. If our data management systems do not effectively collect, store, process, and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies, or human error, our ability to effectively plan, forecast, and execute our business plan and comply with applicable laws and regulations will be impaired, perhaps materially. Any such impairment could materially and adversely affect our financial condition, results of operations, cash flows, and the timeliness with which we report our internal and external operating results. Third parties may attempt to fraudulently induce employees or customers into giving away sensitive information, which may in turn be used to access our information technology systems. In addition, unauthorized persons may attempt to hack into our systems to obtain our confidential or proprietary information or confidential information we hold on behalf of third parties. If the unauthorized persons successfully hack into or interfere with our system, we may experience a negative impact to our business and reputation. We have programs in place to detect, contain, and respond to data security incidents, and we make ongoing improvements to our systems in order to minimize vulnerabilities, in accordance with industry and regulatory standards. However, we may not be able to anticipate and prevent these intrusions or mitigate them when and if they occur. We also rely on external vendors to supply and/or support certain aspects of our information technology systems. The systems of these external vendors may contain defects in design or manufacture or other problems that could unexpectedly compromise information security of our own systems, and we are dependent on these third parties to deploy appropriate security programs to protect their systems. It is possible for such vulnerabilities to remain undetected for an extended period, including several years or longer. The costs to us to eliminate or alleviate network security problems, bugs, viruses, worms, ransomware and other malicious software programs, and security vulnerabilities could be significant. Our efforts to address these problems may not be successful and could result in unexpected interruptions, delays, cessation of service, and harm to our business operations. Depending on the type of breach, we could also be exposed to a risk of loss or litigation and potential liability, which could have a material adverse impact on our business, financial condition, results of operations, or cash flows.
Illegal Distribution and Sale by Third Parties of Counterfeit Versions of Our Products Could Have a Negative Impact
Third parties may illegally distribute and sell counterfeit versions of our products which do not meet our rigorous manufacturing and testing standards. Our reputation and business could suffer harm as a result. In addition, diversion of products into other channels may result in reduced revenues.
General Risk Factors
We face risk factors common to other U.S. businesses. We could be subject to complex and costly regulation. Our business could suffer if we or our suppliers encounter manufacturing problems or disruptions to transportation channels. We could be subject to risks associated with doing business outside of the U.S, including risks associated with global economic, regulatory, or political changes, or health crises. Current or worsening economic conditions may adversely affect our business and financial condition.
Item 1B. Unresolved Staff Comments.
Not applicable and none.
9
Item 2. Properties.
Our headquarters are located at 511 Lobo Lane, on 35 acres, which we own, overlooking Lake Lewisville in Little Elm, Texas. The headquarters are in good condition and houses our administrative offices and manufacturing facility. The manufacturing facility produced approximately 8% of the units that were manufactured in 2021. As a result of recent expansions, we expect to have significant additional domestic production capacity.
A loan in the original principal amount of approximately $4,210,000 is secured by our land and buildings. See Note 8 to our financial statements for more information.
In the opinion of Management, the property and equipment are suitable for their intended use and are adequately covered by an insurance policy.
Item 3. Legal Proceedings.
Please refer to Note 10 to the financial statements for a complete description of all legal proceedings.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities.
MARKET INFORMATION
Our Common Stock has been listed on the NYSE American (or its predecessor entities) under the symbol “RVP” since May 4, 2001. The closing market price on March 11, 2022 was $4.52 per share.
SHAREHOLDERS
As of March 11, 2022, there were 34,023,104 shares of Common Stock issued, of which 899,899 shares were held in treasury. There were 159 shareholders of record, not including Cede & Co. participants or beneficial owners thereof.
DIVIDENDS
We have not ever declared or paid any dividends on the Common Stock. We have no current plans to pay any cash dividends on the Common Stock.
10
EQUITY COMPENSATION PLAN INFORMATION
The following table sets forth information relating to our equity compensation plans as of December 31, 2021:
Equity Compensation Plan Information
Weighted | Number of securities | ||||||
average exercise | remaining available for | ||||||
Number of securities | price of | future issuance under | |||||
to be issued upon | outstanding | equity compensation | |||||
exercise of | options, | plans (excluding | |||||
outstanding options, | warrants and | securities reflected in | |||||
warrants and rights | rights | column(a)) | |||||
Plan category |
| (a) |
| (b) |
| (c) | |
Equity compensation plans approved by security holders |
| 1,523,050 | $ | 11.76 |
| 650,000 | |
Total |
| 1,523,050 | $ | 11.76 |
| 650,000 | |
STOCK PERFORMANCE GRAPH
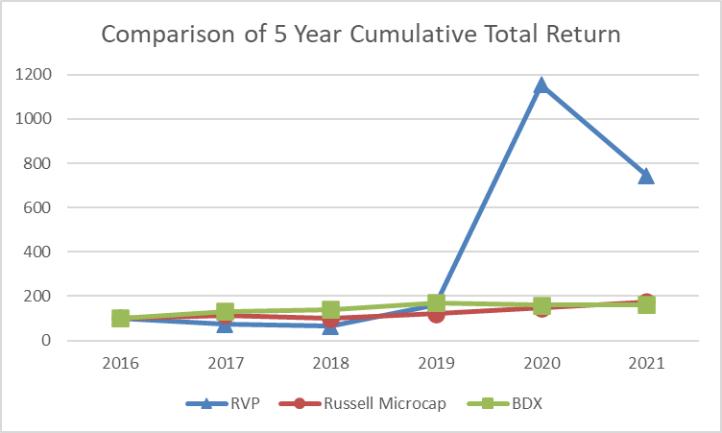
The following graph compares the cumulative total return for our Common Stock (RVP) from December 31, 2016 to December 31, 2021, to the total returns for the Russell Microcap® and Becton, Dickinson and Company (or “BDX”), a peer issuer. The graph assumes an investment of $100 in the aforementioned equities as of December 31, 2016, and that all dividends are reinvested.
UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Not applicable.
11
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
ISSUER PURCHASES OF EQUITY SECURITIES
|
| Total Number of | Approximate | |||||||
Total | Shares Purchased as | Dollar Value of | ||||||||
Number of | Average Price | Part of Publicly | Shares that May Yet Be | |||||||
Shares | Paid Per | Announced Plans or | Purchased Under the | |||||||
Period | Purchased | Share | Programs | Plans or Programs | ||||||
October 1, 2021 through October 31, 2021 | 86,340 | $ | 9.83 | 86,340 | $ | 6,311,709 | ||||
November 1, 2021 through November 30, 2021 | 91,342 |
| $ | 8.84 |
| 91,342 | $ | 5,504,151 | ||
December 1, 2021 through December 31, 2021 | 105,666 | $ | 7.26 | 105,666 | $ | 4,737,499 | ||||
Total | 283,348 | $ | 8.55 | 283,348 | ||||||
(1)These shares were purchased pursuant to our Common Stock repurchase plan structured to comply with Rules 10b5-1 and 10b-18 under the Securities Exchange Act of 1934, announced on June 7, 2021. On June 4, 2021, the Board of Directors authorized the repurchase of up to $10 million of Common Stock subject to Rule 10b-18 limitations as well as certain market value constraints specified in the plan. Notwithstanding the terms of the plan, the exact dollar amount and number of shares which may be purchased pursuant to the plan is difficult to predict. The plan will expire on June 18, 2022 at the latest.
Item 6. Reserved.
Not required.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.
FORWARD-LOOKING STATEMENT WARNING
Certain statements included by reference in this filing containing the words “could,” “may,” “believes,” “anticipates,” “intends,” “expects,” and similar such words constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act. Any forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the impact of COVID-19 on all facets of logistics and operations, as well as costs, our ability to scale up production volumes in response to an increase in demand, potential tariffs, our ability to maintain liquidity, our maintenance of patent protection, our ability to maintain favorable third party manufacturing and supplier arrangements and relationships, foreign trade risk, our ability to access the market, production costs, the impact of larger market players, specifically Becton, Dickinson and Company ("BD"), in providing devices to the safety market, and other factors referenced in Item 1A. Risk Factors. Given these uncertainties, undue reliance should not be placed on forward-looking statements.
Overview
We have been manufacturing and marketing our products since 1997. VanishPoint® syringes comprised 93.6% of our sales in 2021. EasyPoint® products accounted for 5.1% of sales in 2021. We also manufacture and market a blood collection tube holder, IV safety catheter, and VanishPoint® Blood Collection Set.
Our products have been and continue to be distributed nationally and internationally through numerous distributors. Some of our popular syringe products provide low dead-space. Low dead-space syringes reduce residual
12
medication remaining in the syringe after the dose has been administered. In some instances, the low dead-space allows for additional doses of medication to be obtained from the vials.
On May 1, 2020, we were awarded a delivery order under an existing contract by the Department of Health and Human Services of the United States to supply automated retraction safety syringes for COVID-19 vaccination efforts, which order was in the amount of $83.8 million plus $10 million in expedited freight costs. The period of performance for this order ended in March 2022.
The Department of Health and Human Services awarded us another contract on February 12, 2021 to supply low dead-space safety syringes for COVID-19 vaccination efforts. The base price for the contract and purchase order was $54.2 million for the five-month base period of performance (February 15, 2021 to July 14, 2021). We received notice that the contract would be extended for seven additional months beyond the base period of performance with a total contract price during such period of approximately $92.8 million plus an additional $6 million in air freight costs. To date, we have received a commitment to exercise the first four option periods which extended through the end of December 2021. The remaining three periods are open but have not yet - and may not - materialize. For each period, the freight reimbursement cost is included in total overall contract value and is estimated at approximately 25% of the overall price.
Our sales under both of the foregoing orders from the U.S. government were $113.7 million during the year ended December 31, 2021, representing 60.3% of our total sales for the year. Both of the above-mentioned orders as well as the TIA from the U.S. government are material events particular to the COVID-19 pandemic and may not be indicative of future operations. We have not received new orders beyond the 2021 order option periods. While we continue to work with the Department of Health and Human Services, significant new orders are uncertain. In the absence of new U.S. government orders and/or an increase in our domestic orders, we would expect our revenues to be materially affected. The addition of manufacturing equipment and facilities will greatly increase our production capacity. If future orders are not placed by the U.S. government and orders from new and existing customers do not materialize, we would have significant excess productive capabilities.
Effective July 1, 2020, we entered into a TIA with the United States Government Department of Defense, U.S. Army Contracting Command-Aberdeen Proving Ground, Natick Contracting Division & Edgewood Contracting Division (ACC-APG, NCD & ECD) on behalf of the Biomedical Advanced Research and Development Authority (BARDA) for $53.7 million in government funding for expanding our domestic production of needles and syringes to meet ongoing and future U.S. COVID-19 medical countermeasures demands. Effective May 12, 2021, we entered into an amendment to the TIA providing an additional $27.4 million in funding to add 12,500 square feet of controlled environment and two additional assembly lines to increase our existing domestic manufacturing capabilities. The amended completion date is August 29, 2022. As of March 8, 2022, we have negotiated contracts for the purchase of automated assembly equipment, molds, and molding equipment, as well as portions of auxiliary equipment, for approximately $63.8 million. We have also received a temporary certificate of occupancy for the $6.7 million 27,800 square foot controlled environment which was funded by the U.S. government under the original agreement. In addition, we have substantially completed the additional controlled environment space required under the May 12, 2021 amendment. Finally, we have received the certificate of occupancy for the new $5.9 million 55,000 square foot warehouse which is our financial responsibility.
As a result of the COVID-19 pandemic, we have implemented certain safety precautions at our facility to reduce the risk of the potential spread of the novel coronavirus. All of our employees are required to be vaccinated. We continue to monitor the evolving situation and will work to further mitigate risks to staff and to customers. We are continuing to evaluate the ever-changing circumstances surrounding this pandemic as it relates to our ability to continue to source materials and products, maintain a workforce, and operate our business effectively and efficiently. We have faced and continue to deal with the logistical challenges of sourcing raw materials and finished goods, particularly finished goods from China. We utilize multiple transportation providers to ensure we can meet our delivery schedules, but we are subject to the global supply chain and its complexities. To date, the freight challenges have neither caused a loss of customers nor a cessation of production.
On April 17, 2020, we entered into the PPP Loan in the principal amount of $1.4 million in favor of Independent Bank pursuant to the Paycheck Protection Program (the “PPP”) of the Coronavirus Aid, Relief, and Economic Security Act, administered by the U.S. Small Business Administration (“SBA”). On May 13, 2021, we were informed that the SBA
13
granted our request for loan forgiveness for the entire original principal amount and accrued interest, for a total of $1.4 million.
We have entered into an agreement to expand our existing administrative offices by 14,000 square feet. We currently expect that the cost of expansion will be approximately $5.6 million. The expected substantial completion date for the new office space is October 2022. To date, we have spent approximately $1 million.
As detailed in Note 4 to the financial statements, we held $13.3 million in debt and equity securities as of December 31, 2021, which represented 12.7% of our current assets. We continually monitor our invested balances.
In response to, among other factors, the global COVID-19 pandemic, our delivery orders from the U.S. government, and the TIA, employee headcount and related salary and benefits costs have increased significantly. As of December 31, 2021, the Company employed approximately 239 full-time, part-time, and temporary employees. This represents approximately a 44.0% increase in our workforce since December 31, 2020.
On March 16, 2021, the Board approved the 2021 Stock Option Plan (the “Plan”) and set aside and reserved 2,000,000 shares of Common Stock for issuance pursuant to the Plan. The Plan was approved by the shareholders at the May 11, 2021 shareholder meeting. The Plan provides for the granting of incentive stock options and non-qualified stock options at a price equal to at least 100% of the fair market value of the Company’s Common Stock as of the date of grant. Participants in the Plan may include employees, consultants, and non-employee Directors. On March 16, 2021, the Compensation and Benefits Committee approved option grants to purchase 1,000,000, 250,000, and 100,000 shares of Common Stock to our chief executive officer, general counsel, and chief financial officer, respectively. These shares will vest in their entirety three years from the grant date.
On March 16, 2021, the Compensation and Benefits Committee modified the annual salaries of our chief executive officer, general counsel, and chief financial officer to $1,000,000, $400,000, and $300,000, respectively. Such salaries were retroactively effective as of January 1, 2021. On March 16, 2021, the Compensation and Benefits Committee also approved issuances of cash bonuses of $300,000, $100,000, and $100,000 to our chief executive officer, general counsel, and chief financial officer, respectively.
In addition to periodic quarterly payments of dividends to preferred shareholders as detailed in Note 12 to the financial statements, on June 4, 2021, the Board of Directors approved payment to Class B Convertible Preferred shareholders of all current dividends, dividends in arrears, as well as dividends still owed to shareholders who converted their preferred stock in the past in the total amount of $5.1 million.
Effective June 4, 2021, we entered into a repurchase plan (the “Plan”) for the purchase of up to $10 million of our Common Stock. Under the Plan, open market purchases of our Common Stock commenced June 18, 2021 and 899,899 shares were purchased as of March 11, 2022 for an aggregate purchase price of approximately $7.2 million.
Historically, unit sales have increased during the flu season. Seasonal trends have been less pronounced due to demand related to the COVID-19 vaccine.
Product purchases from our Chinese manufacturers have enabled us to increase manufacturing capacity with little capital outlay and have provided a competitive manufacturing cost. In 2021, our Chinese manufacturers produced approximately 92% of our products. In the event that we become unable to purchase products from our Chinese manufacturers, we would need to find an alternate manufacturer for the blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and we would increase domestic production for the 1mL and 3mL syringes and EasyPoint® needles.
In 1995, we entered into a license agreement with Thomas J. Shaw for the exclusive right to manufacture, market, and distribute products utilizing his patented automated retraction technology and other patented technology. This technology is the subject of various patents and patent applications owned by Mr. Shaw. The license agreement generally provides for quarterly payments of a 5% royalty fee on gross sales of products subject to the license and he receives fifty percent (50%) of the royalties paid to the Company by certain sublicensees of the technology subject to the license.
14
We have experienced significant cost pressure with respect to transportation costs, particularly freight costs for importing products from our overseas manufacturers. These costs contribute significantly to the cost of manufactured products and have significantly reduced our gross margins for the last half of 2021. In addition, we have experienced an increase in raw materials costs, principally the cost of petroleum-based plastics used in our molded components. Although we experienced certain cost increases in raw materials, those costs primarily affected our domestic manufacturing because the finished goods we purchased from China (being 92% of our products) did not change in price during 2021. Other factors that could affect our unit costs include increases in tariffs, costs by third party manufacturers, and changing production volumes. Increases in such costs may not be recoverable through price increases of our products.
RESULTS OF OPERATIONS
The following discussion may contain trend information and other forward-looking statements that involve a number of risks and uncertainties. Our actual future results could differ materially from our historical results of operations and those discussed in any forward-looking statements. All period references are to our fiscal years ended December 2021 and 2020. Dollar amounts have been rounded for ease of reading.
Comparison of Year Ended
December 31, 2021 and Year Ended December 31, 2020
Domestic sales, including sales to the U.S. government, accounted for 88.9% and 90.2% of the revenues in 2021 and 2020, respectively. Domestic revenues increased 127.0% principally due to increased volumes primarily attributable to orders from the U.S. government. Domestic unit sales increased 111.3%. Domestic unit sales were 83.7% of total unit sales for 2021. Domestic unit sales excluding U.S. government orders rose approximately 28.6%. International revenues increased 158.5% due to an increase in orders from existing international customers, particularly in North (excluding the U.S.) and South America. Our international orders may be subject to significant fluctuation over time. Overall unit sales increased 116.9% and our overall revenues increased by 130.1%. Other than sales to the U.S. government, our increased sales are predominantly attributable to existing customers as well as several new smaller customers who do not operate as distributors. Our sales to the U.S. government were approximately $114 million in 2021.
Cost of manufactured product increased 107.5% principally due to an increase in unit volumes. Royalty expense increased 106.7% due to increased gross sales. Gross profit margins increased from 45.2% in 2020 to 50.6% in 2021 principally due to an overall increase in units sold to the U.S. government, accompanied by freight cost reimbursements.
Operating expenses increased 75.9% from the prior year. This is substantially due to increased headcount and other employee-related expenses, as well as consulting expenses. These increases are due to the growth in order volume and expansion activities required by the TIA. Included in the increased employee expenses were bonuses and retroactive salary increases for the named executive officers of approximately $650 thousand, $2.2 million in other employee bonuses, and $3.7 million of share-based compensation expense. Sales and marketing expenses increased due to employee bonuses and an increase of GPO fees on the basis of the increase in sales.
Income from operations was $72.6 million in 2021 compared to income from operations of $24.1 million in 2020. The increase was due to the increase in net revenues and resulting gross profit, primarily driven by the orders from the U.S. government.
Interest and other income decreased 65.5% from the prior year principally due to unrealized losses from our investments. Interest expense for year ended December 31, 2021 decreased by approximately 12.7% from the prior year. The decrease is primarily attributable to the expiration of certain operating leases and an overall decrease in our loan and private stock purchase installment payments.
For the year ended December 31, 2021, we recorded a provision for income taxes of $18.9 million. For a detailed description of the determination and components of calculating the provision, please refer to Note 11 of the financial statements.
15
A comparison of the results of operations for the years ended December 31, 2020 and December 31, 2019 is omitted from this discussion. Such comparison was included in our Annual Report on Form 10-K filed with the SEC on March 31, 2021 in Item 7 of Part II thereof.
LIQUIDITY AND CAPITAL RESOURCES
Discussion of Statement of Cash Flow Items
Cash flow from operations was $32.8 million in 2021, principally due to our net income for the year. The increase in cash was offset by an increase in accounts receivable, largely driven by orders from the U.S. government. There was also an increase in inventory. Additionally, we have recorded deferred taxes of $9.2 million which is material to the adjustments to total cash flow from operations. The deferred tax asset represents amounts available to reduce income taxes payable on taxable income in future years. The determination and calculation of such asset is further discussed in Note 11 of the financial statements.
Cash used by investing activities was $63.0 million for the year ended December 31, 2021 due primarily to the purchase of property, plant and equipment and the purchase of equity securities. The $58.4 million impact to cash from the purchase of fixed assets primarily reflects down payments on orders for certain assets as discussed in Note 22 to the financial statements. Of the $58.4 million, $11.1 million was spent on additional assembly equipment and a new warehouse outside the TIA reimbursement provisions. In 2021, we increased our invested cash position by $4.7 million.
Cash provided by financing activities was $41.8 million for the year ended December 31, 2021. This was primarily due to proceeds from the government under the TIA for down payments on our orders for fixed assets, but was offset by the repurchase of both preferred and Common Stock and the payment of dividends. Our repurchase of common stock in the amount of $5.3 million was a significant use of cash in 2021. As stated herein in Item 5 of Part II, the exact dollar amount to be purchased under the plan prior to the plan’s termination cannot be predicted, though the total repurchase amount is capped at $10 million.
Internal Sources of Liquidity
We have historically funded operations primarily from the proceeds from revenues, private placements, litigation settlements, and loans. We expect to fund operations going forward from revenues, cash reserves, and investments available for sale if the need to access those funds arises. We do not, and historically have not, utilized lines of credit to fund operations.
Margins
The mix of domestic and international sales affects the average sales price of our products. Generally, the higher the ratio of domestic sales to international sales, the higher the average sales price will be. Some international sales of our products are shipped directly from China to the customer. The number of units produced by us versus manufactured in China can have a significant effect on the carrying costs of Inventory as well as Cost of sales. Generally, an overall increase in units sold can positively affect our margins. The cost of raw materials used in manufacturing and transportation costs can also significantly affect our margins. We will continue to evaluate the appropriate mix of products manufactured domestically and those manufactured in China to achieve economic benefits as well as to maintain our domestic manufacturing capability.
Cash Requirements
We believe we will have adequate means to meet our short-term needs to fund operations for at least 12 months. Besides cash reserves and expected income from operations, we also have access to our investments which may be liquidated in the event that we need to access the funds for operations. Expected short-term uses of cash include payroll and benefits, royalty expense, inventory purchases, contractual obligations, capital expenditures, payment of income taxes, repurchase of shares, quarterly preferred stock dividends, and other operational priorities. Our long-term plans involving material cash requirements for capital expenditures are detailed in this section below under “Capital Resources” and our
16
year-end liabilities are detailed in our financial statements, including Notes 7 through 9 to the financial statements. We believe we will have adequate means to meet our currently foreseeable long-term liquidity needs.
Contracts with the U.S. Government
As discussed above, we were awarded a material delivery order by the Department of Health and Human Services of the United States in the total amount of approximately $83.8 million, plus certain expedited freight expenses. In February 2021, we received another material contract from the Department of Health and Human Services for additional safety syringes representing expected revenues and reimbursable freight costs of $54.2 million for a five-month period ending July 14, 2021 and approximately $92.8 million (plus an additional $6 million in air freight costs) for seven monthly option periods. To date, we have received a commitment to exercise the first four option periods which extended through the end of December 2021. As previously stated, we have not received additional orders beyond the first four option periods. While we continue to work with the Department of Health and Human Services, significant future orders are uncertain.
As discussed above, we entered into a TIA with the U.S. government for a total value of approximately $81.0 million in government funding for expanding our domestic production of needles and syringes. As of March 8, 2022, we have received approximately $67.2 million for down payments on the purchase of certain fixed assets. As of March 8, 2022, we have contributed approximately $5.9 million towards the completion of the new 55,000 square foot warehouse as a portion of the cost sharing agreement. The Company will continue to fund the expansion efforts primarily through providing the necessary workforce to implement the addition of new assets, as well as provide the ongoing necessary support.
External Sources of Liquidity
We received a PPP Loan in the principal amount of $1.4 million. On May 13, 2021, we were informed that the entire original principal amount of $1.4 million would be forgiven.
We consider our investment portfolio a source of liquidity as well. As of December 31, 2021, $13.3 million was invested in third party securities.
Capital Resources
Since the execution of the TIA on July 1, 2020, we have begun construction for significant expansion to our facilities. As of March 8, 2022, we have received a temporary certificate of occupancy for the approximately 27,800 square feet of additional controlled environment within existing properties and a certificate of occupancy for the 55,000 square feet of new warehouse space. We have substantially completed an additional 12,500 square feet of controlled environment space. As of March 8, 2022, we have negotiated contracts for the purchase of automated assembly equipment, molds, and molding equipment, as well as portions of auxiliary equipment, under the original TIA and the modification for approximately $63.8 million. To fund the purchase of the automated assembly equipment, auxiliary equipment, and construction of the controlled environment, we are reimbursed by the U.S. government according to the terms in the TIA. The TIA also allows us to request an advance of funds for larger purchases when necessary. The expenditures which are not reimbursable from the U.S. government under the TIA are funded with cash from operations. The capital assets funded by us under the TIA include the construction of the new warehouse as well as certain accessory equipment.
We have entered into an agreement to expand our existing administrative offices by 14,000 square feet. We currently expect that the cost of expansion will be approximately $5.6 million which we will fund from cash from operations. The expected substantial completion date for the new office space is October 2022. To date, we have spent approximately $1 million.
CRITICAL ACCOUNTING ESTIMATES
We are responsible for developing estimates for amounts reported as assets and liabilities, and revenues and expenses in conformity with U.S. generally accepted accounting principles (“GAAP”). Those estimates require that we
17
develop assumptions of future events based on past experience and expectations of economic factors. Among the more critical estimates management makes is the estimate for customer rebates. The amount reported as a contractual allowance for rebates involves examination of past historical trends related to our sales to customers and the related credits issued once contractual obligations of the customers have been met. The establishment of a liability for future claims of rebates against sales in the current period requires that we have an understanding of the relevant sales with respect to product categories, sales distribution channels, and the likelihood of contractual obligations being satisfied. We examine the results of estimates against actual results historically and use the determination to further develop our basis for assumptions in future periods, as well as the accuracy of past estimates. While we believe that we have sufficient historical data, and a firm basis for establishing reserves for contractual obligations, there is an inherent risk that our estimates and the underlying assumptions may not reflect actual future results. In the event that these estimates and/or assumptions are incorrect, adjustments to our reserves may have a material impact on future results.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Not applicable to smaller reporting companies.
18
Item 8. Financial Statements and Supplementary Data.
RETRACTABLE TECHNOLOGIES, INC.
FINANCIAL STATEMENTS AND
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
DECEMBER 31, 2021, 2020, and 2019
F-1
RETRACTABLE TECHNOLOGIES, INC.
INDEX TO FINANCIAL STATEMENTS
| Page | |
|
|
|
Report of Independent Registered Public Accounting Firm (Moss Adams LLP, Dallas, TX, PCAOB ID No. 659) |
| F-3 |
|
|
|
Financial Statements: |
|
|
|
|
|
| F-5 | |
Statements of Operations for the years ended December 31, 2021, 2020, and 2019 |
| F-6 |
|
|
|
Statements of Changes in Stockholders’ Equity for the years ended December 31, 2021, 2020, and 2019 |
| F-7 |
|
|
|
Statements of Cash Flows for the years ended December 31, 2021, 2020, and 2019 |
| F-9 |
|
|
|
| F-10 | |
|
|
|
Financial Statement Schedule: |
|
|
|
|
|
| 21 |
F-2
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of
Retractable Technologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Retractable Technologies, Inc. (the Company) as of December 31, 2021 and 2020, the related statements of operations, changes in stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2021, and the related notes and schedules (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue Recognition – Rebates
As described in Note 2 to the financial statements, the Company’s estimated contractual pricing allowances for rebates at December 31, 2021 is $6.2 million. The Company recognizes revenue when it has satisfied all performance obligations to the customer. Under certain contracts, revenue is recorded on the basis of sales price to distributors, less contractual pricing allowances. Contractual pricing allowances consist of: (i) rebates granted to distributors who provide tracking reports which show, among other things, the facility that purchased the products, and (ii) a provision for estimated contractual pricing allowances for products for which the Company has not received tracking reports. Once rebates are issued they are applied against the customer’s receivable balance.
F-3
We identified management’s estimates of contractual pricing allowances for rebates as a critical audit matter because our evaluation of the Company’s methods and assumptions used in estimating the contractual pricing allowances involved especially challenging auditor judgment and required a high degree of audit effort.
The primary procedures we performed to address this critical audit matter included:
| ● | Testing management’s process for determining the estimates of contractual pricing allowances for rebates by performing the following procedures: |
| o | Obtaining an understanding of management’s process for estimating the contractual pricing allowances for rebates. |
| o | Testing management’s analysis for clerical accuracy. |
| o | Testing the completeness, accuracy, and reliability of underlying data used by management in the estimate. |
| o | Evaluating the reasonableness of significant assumptions used by management. |
| ● | Comparing rebates issued after period end with the estimated amounts as of period end as part of a retrospective review. |
| ● | Developing an independent expectation of contractual pricing allowances for rebates as of the period end based on historical trends in sales to distributors and compared such expectation to the Company’s estimate. |
/s/ Moss Adams LLP
Dallas, Texas
March 31, 2022
We have served as the Company’s auditor since 2016.
F-4
RETRACTABLE TECHNOLOGIES, INC.
BALANCE SHEETS
| December 31, 2021 |
| December 31, 2020 | |||
ASSETS | ||||||
Current assets: | ||||||
Cash and cash equivalents | $ | 29,162,913 | $ | 17,566,682 | ||
Accounts receivable, net of allowance for doubtful accounts of $352,217 and $205,822 |
| 34,859,505 |
| 21,131,841 | ||
Receivable from Technology Investment Agreement (TIA) | 5,924,136 | 11,779,078 | ||||
Investments in debt and equity securities, at fair value | 13,268,986 | 8,081,833 | ||||
Inventories |
| 20,589,919 |
| 10,234,646 | ||
Other current assets |
| 701,969 |
| 684,317 | ||
Total current assets |
| 104,507,428 |
| 69,478,397 | ||
Property, plant, and equipment, net |
| 87,925,651 |
| 30,816,504 | ||
Deferred tax asset | 13,865,834 | 4,631,206 | ||||
Other assets |
| 5,675 |
| 44,567 | ||
Total assets | $ | 206,304,588 | $ | 104,970,674 | ||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||
Current liabilities: | ||||||
Accounts payable | $ | 20,404,573 | $ | 16,256,444 | ||
Current portion of long-term debt |
| 289,114 |
| 1,030,763 | ||
Accrued compensation |
| 1,056,656 |
| 826,762 | ||
Dividends payable |
| 1,438,371 |
| 49,091 | ||
Accrued royalties to shareholder |
| 3,450,684 |
| 1,973,781 | ||
Other accrued liabilities |
| 3,725,527 |
| 3,398,904 | ||
Income taxes payable |
| 4,959,878 |
| 4,365,770 | ||
Total current liabilities |
| 35,324,803 |
| 27,901,515 | ||
Other long-term liabilities | 69,996,330 | 24,478,697 | ||||
Long-term debt, net of current maturities |
| 1,814,194 |
| 2,710,337 | ||
Total liabilities |
| 107,135,327 |
| 55,090,549 | ||
Commitments and contingencies – see Note 10 | ||||||
Stockholders’ equity: | ||||||
Preferred stock, $1 par value: | ||||||
Class B; authorized: 5,000,000 shares | ||||||
Series II, Class B convertible; outstanding: 156,200 shares at December 31, 2021 and 2020 (liquidation preference of $1,952,500) |
| 156,200 |
| 156,200 | ||
Series III, Class B convertible; outstanding: 76,245 and 106,745 shares at December 30, 2021 and 2020, respectively (liquidation preference of $953,063) |
| 76,245 |
| 106,745 | ||
Common Stock, no par value; authorized: 100,000,000 shares; 34,013,104 issued and 33,484,935 outstanding and 33,957,204 and outstanding at December 31, 2021 and 2020, respectively |
| — |
| — | ||
Additional paid-in capital |
| 63,024,888 |
| 59,285,401 | ||
Retained earnings (accumulated deficit) |
| 41,182,429 |
| (9,668,221) | ||
Common stock in treasury – at cost: 528,169 and 0 shares at December 31, 2021 and 2020, respectively | (5,270,501) | — | ||||
Total stockholders’ equity |
| 99,169,261 |
| 49,880,125 | ||
Total liabilities and stockholders’ equity | $ | 206,304,588 | $ | 104,970,674 | ||
See accompanying notes to financial statements
F-5
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF OPERATIONS
Years Ended December 31, | |||||||||
| 2021 |
| 2020 |
| 2019 | ||||
Sales, net | $ | 188,382,454 | $ | 81,862,453 | $ | 41,797,179 | |||
Cost of sales: | |||||||||
Cost of manufactured product |
| 81,711,840 |
| 39,377,794 |
| 24,209,401 | |||
Royalty expense to shareholder |
| 11,318,093 |
| 5,476,306 |
| 3,449,822 | |||
Total cost of sales |
| 93,029,933 |
| 44,854,100 |
| 27,659,223 | |||
Gross profit |
| 95,352,521 |
| 37,008,353 |
| 14,137,956 | |||
Operating expenses: | |||||||||
Sales and marketing |
| 4,477,651 |
| 4,061,904 |
| 4,217,863 | |||
Research and development |
| 901,381 |
| 574,527 |
| 516,095 | |||
General and administrative |
| 17,378,301 |
| 8,301,169 |
| 6,432,158 | |||
Total operating expenses |
| 22,757,333 |
| 12,937,600 |
| 11,166,116 | |||
Income from operations |
| 72,595,188 |
| 24,070,753 |
| 2,971,840 | |||
Gain on forgiveness of PPP loan | 1,377,652 | — | — | ||||||
Other income - TIA | 425,158 | — | — | ||||||
Interest and other income |
| 779,996 |
| 2,262,758 |
| 351,166 | |||
Interest expense |
| (227,183) |
| (260,264) |
| (166,897) | |||
Income before income taxes |
| 74,950,811 |
| 26,073,247 |
| 3,156,109 | |||
Provision for income taxes |
| 18,886,570 |
| 1,850,234 |
| 7,875 | |||
Net income |
| 56,064,241 |
| 24,223,013 |
| 3,148,234 | |||
Preferred Stock dividend requirements |
| (241,703) |
| (573,868) |
| (702,618) | |||
Deemed contribution on extinguishment of preferred stock | — | 2,975,708 | — | ||||||
Net income applicable to common shareholders | $ | 55,822,538 | $ | 26,624,853 | $ | 2,445,616 | |||
| |||||||||
Basic earnings per share | $ | 1.65 | $ | 0.80 | $ | 0.07 | |||
Diluted earnings per share | $ | 1.63 | $ | 0.80 | $ | 0.07 | |||
Weighted average common shares outstanding: | |||||||||
Basic |
| 33,870,819 |
| 33,169,307 |
| 32,672,475 | |||
Diluted |
| 34,244,699 |
| 33,300,654 |
| 32,672,475 | |||
See accompanying notes to financial statements
F-6
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Series I Class B | Series II Class B | Series III Class B | Series IV Class B | Series V Class B | Common | |||||||||||||||||||||||||
| Shares |
| Amount |
| Shares |
| Amount |
| Shares |
| Amount |
| Shares |
| Amount |
| Shares |
| Amount |
| Shares |
| Amount | |||||||
Balance as of December 31, 2018 |
| 98,500 | $ | 98,500 |
| 171,200 | $ | 171,200 |
| 129,245 | $ | 129,245 |
| 342,500 | $ | 342,500 |
| 40,000 | $ | 40,000 |
| 32,666,454 | $ | — | ||||||
Conversion of Preferred Stock into Common Stock | (2,500) | (2,500) | — | — | — | — | — | — | (6,000) | (6,000) | 8,500 | — | ||||||||||||||||||
Dividends |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — | ||||||
Net income |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
| — | ||||||
Balance as of December 31, 2019 | 96,000 |
| 96,000 | 171,200 | 171,200 | 129,245 |
| 129,245 | 342,500 |
| 342,500 | 34,000 |
| 34,000 | 32,674,954 |
| — | |||||||||||||
Exchange of Preferred Stock for Common Stock | — | — | — | — | (22,500) | (22,500) | (342,500) | (342,500) | (34,000) | (34,000) | 754,000 | — | ||||||||||||||||||
Conversion of Preferred Stock into Common Stock | (81,700) | (81,700) | (15,000) | (15,000) | — | — | — | — | — | — | 96,700 | — | ||||||||||||||||||
Stock Option Exercises | — | — | — | — | — | — | — | — | — | — | 431,550 | — | ||||||||||||||||||
Redemption | (14,300) | (14,300) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Dividends | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Net income | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Balance as of December 31, 2020 | — | — | 156,200 | 156,200 | 106,745 | 106,745 | — | — | — | — | 33,957,204 | — | ||||||||||||||||||
Conversion of Preferred Stock into Common Stock | — | — | — | — | (30,500) | (30,500) | — | — | — | — | 30,500 | — | ||||||||||||||||||
Stock Option Exercises | — | — | — | — | — | — | — | — | — | — | 25,400 | — | ||||||||||||||||||
Dividends | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Stock Option Compensation | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Repurchase of Common Stock - at cost | — | — | — | — | — | — | — | — | — | — | (528,169) | — | ||||||||||||||||||
Net income | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||
Balance as of December 31, 2021 | — | $ | — | 156,200 | $ | 156,200 | 76,245 | $ | 76,245 | — | $ | — | — | $ | — | 33,484,935 | $ | — | ||||||||||||
See accompanying notes to financial statements
F-7
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Additional |
| Retained Earnings/ |
|
| |||||||
Paid-in | (Accumulated | Treasury | ||||||||||
Capital | Deficit) | Stock | Total | |||||||||
Balance as of December 31, 2018 | $ | 61,871,756 | $ | (37,039,468) |
| — | $ | 25,613,733 | ||||
Conversion of Preferred Stock into Common Stock | 8,500 | — | — | |||||||||
Dividends | (219,512) | — | — | (219,512) | ||||||||
Net income | — | 3,148,234 | — | 3,148,234 | ||||||||
Balance as of December 31, 2019 | 61,660,744 | (33,891,234) | — | 28,542,455 | ||||||||
Exchange of Preferred Stock for Common Stock | (3,090,672) | — | — | (3,489,672) | ||||||||
Conversion of Preferred Stock into Common Stock | 96,700 | — | — | — | ||||||||
Stock Option Exercises | 922,512 | — | — | 922,512 | ||||||||
Redemption | (92,950) | — | — | (107,250) | ||||||||
Dividends | (210,933) | — | — | (210,933) | ||||||||
Net income | — | 24,223,013 | — | 24,223,013 | ||||||||
Balance as of December 31, 2020 | 59,285,401 | (9,668,221) | — | 49,880,125 | ||||||||
Conversion of Preferred Stock into Common Stock | 30,500 | — | — | — | ||||||||
Stock Option Exercises | 48,600 | — | — | 48,600 | ||||||||
Dividends | — | (5,213,591) | — | (5,213,591) | ||||||||
Stock Option Compensation | 3,660,387 | — | — | 3,660,387 | ||||||||
Repurchase of Common Stock - at cost | — | — | (5,270,501) | (5,270,501) | ||||||||
Net income | — | 56,064,241 | — | 56,064,241 | ||||||||
Balance as of December 31, 2021 | $ | 63,024,888 | $ | 41,182,429 | $ | (5,270,501) | $ | 99,169,261 | ||||
See accompanying notes to financial statements
F-8
RETRACTABLE TECHNOLOGIES, INC.
STATEMENTS OF CASH FLOWS
Years Ended December 31, | ||||||||||
| 2021 |
| 2020 |
| 2019 | |||||
Cash flows from operating activities | ||||||||||
Net income | $ | 56,064,241 | $ | 24,223,013 | $ | 3,148,234 | ||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||
Depreciation and amortization |
| 1,257,417 |
| 832,069 |
| 852,080 | ||||
Net unrealized gain on investments | (513,529) | (1,870,010) | (129,315) | |||||||
Realized gains on investments | — | (162,595) | (7,925) | |||||||
Accreted interest | 109,019 | — | — | |||||||
Deferred taxes | (9,234,628) | (4,631,206) | — | |||||||
Provision for doubtful accounts |
| 150,000 |
| 59,440 |
| — | ||||
Share-based compensation | 3,660,387 | — | — | |||||||
Gain on forgiveness of PPP loan | (1,377,652) | — | ||||||||
Loss on disposal of assets | — | 33,140 | — | |||||||
(Increase) decrease in operating assets: | ||||||||||
Accounts receivable |
| (13,877,663) |
| (14,626,910) |
| (1,652,015) | ||||
Inventories |
| (10,355,273) |
| (2,784,054) |
| 94,502 | ||||
Other current assets |
| (17,652) |
| (49,116) |
| 9,602 | ||||
Income taxes receivable | — | 100,785 | 100,937 | |||||||
Other assets | 38,892 | 43,748 | 77,541 | |||||||
Increase (decrease) in operating liabilities: | ||||||||||
Accounts payable |
| 4,148,128 |
| 11,248,840 |
| (362,072) | ||||
Accrued liabilities |
| 2,147,705 |
| 2,232,059 |
| 55,150 | ||||
Income taxes payable |
| 594,107 |
| 4,347,826 |
| 7,919 | ||||
Net cash provided by operating activities |
| 32,793,499 |
| 18,997,029 |
| 2,194,638 | ||||
|
|
| ||||||||
Cash flows from investing activities | ||||||||||
Purchase of property, plant, and equipment |
| (58,366,563) |
| (21,049,656) |
| (632,078) | ||||
Purchase of debt and equity securities | (4,748,624) | (2,242,897) | (7,360,398) | |||||||
Proceeds from the sales of debt and equity securities | 75,000 | 3,965,329 | 2,712,134 | |||||||
Net cash used by investing activities |
| (63,040,187) |
| (19,327,224) |
| (5,280,342) | ||||
Cash flows from financing activities | ||||||||||
Repayments of long-term debt |
| (274,791) |
| (260,894) |
| (407,014) | ||||
Proceeds of long-term debt |
| — |
| 1,363,000 |
| — | ||||
Proceeds from Technology Investment Agreement (TIA) | 52,366,282 | 10,636,822 | — | |||||||
Proceeds from the exercise of stock options |
| 48,600 |
| 922,512 |
| — | ||||
Payment of preferred stock redemption price payable | (101,250) | — | — | |||||||
Payment of preferred stock repurchase payable | (1,101,110) | (482,670) | — | |||||||
Payment of preferred stock dividends |
| (3,824,311) |
| (216,642) |
| (219,825) | ||||
Repurchase of common stock | (5,270,501) | — | — | |||||||
Net cash provided (used) by financing activities |
| 41,842,919 |
| 11,962,128 |
| (626,839) | ||||
|
| |||||||||
Net increase (decrease) in cash and cash equivalents |
| 11,596,231 |
| 11,631,933 |
| (3,712,543) | ||||
Cash and cash equivalents at: |
|
| ||||||||
Beginning of year |
| 17,566,682 |
| 5,934,749 |
| 9,647,292 | ||||
End of year | $ | 29,162,913 | $ | 17,566,682 | $ | 5,934,749 | ||||
Supplemental schedule of cash flow information: | ||||||||||
Interest paid | $ | 118,163 | $ | 260,264 | $ | 166,897 | ||||
Income taxes paid | $ | 27,124,342 | $ | 2,106,000 | $ | — | ||||
Supplemental schedule of noncash investing and financing activities: | ||||||||||
Preferred dividends declared, not paid | $ | 1,438,371 | $ | 49,091 | $ | 54,800 | ||||
Conversion of preferred stock to common stock | $ | 30,500 | $ | 96,700 | $ | 8,500 | ||||
Amounts receivable under Technology Investment Agreement | $ | 5,924,136 | $ | 11,779,078 | $ | — | ||||
Redemption price payable | $ | 6,000 | $ | 107,250 | $ | — | ||||
Preferred stock repurchase payable | $ | 2,132,948 | $ | 3,007,002 | $ | — | ||||
See accompanying notes to financial statements
F-9
NOTES TO FINANCIAL STATEMENTS
1. BUSINESS OF THE COMPANY AND BASIS OF PRESENTATION
Business of the Company
Retractable Technologies, Inc. (the “Company”) was incorporated in Texas on May 9, 1994, and designs, develops, manufactures, and markets safety syringes and other safety medical products for the healthcare profession. The Company began to develop its manufacturing operations in 1995. The Company’s manufacturing and administrative facilities are located in Little Elm, Texas. The Company’s products are the VanishPoint® 0.5mL insulin syringe; 1mL tuberculin, insulin, and allergy antigen syringes; 0.5mL, 1mL, 2mL, 3mL, 5mL, and 10mL syringes; the small diameter tube adapter; the blood collection tube holder; the allergy tray; the IV safety catheter; the Patient Safe® syringes; the Patient Safe® Luer Cap; the VanishPoint® Blood Collection Set; and the EasyPoint® needle, as well as a standard 3mL syringe packaged with an EasyPoint® needle. The Company also sells VanishPoint® autodisable syringes in the international market in addition to the Company’s other products.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Accounting estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires Management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ significantly from those estimates. The amount reported as a contractual allowance for rebates involves examination of past historical trends related to sales to customers and the related credits issued once contractual obligations of the customers have been met. The establishment of a liability for future claims of rebates against sales in the current period requires that the Company has an understanding of the relevant sales with respect to product categories, sales distribution channels, and the likelihood of contractual obligations being satisfied.
Cash and cash equivalents
For purposes of reporting cash flows, cash and cash equivalents include cash, money market accounts, and investments with original maturities of three months or less.
Accounts receivable
The Company records trade receivables when revenue is recognized. No product has been consigned to customers. The Company’s allowance for doubtful accounts is primarily determined by review of specific trade receivables. Those accounts that are doubtful of collection are included in the allowance. This provision is reviewed to determine the adequacy of the allowance for doubtful accounts. Trade receivables are charged off when there is certainty as to their being uncollectible. Trade receivables are considered delinquent when payment has not been made within contract terms.
The Company requires certain customers to make a prepayment prior to beginning production or shipment of their order. Customers may apply such prepayments to their outstanding invoices or pay the invoice and continue to carry forward the deposit for future orders. Such amounts are included in Other accrued liabilities on the Balance Sheets and are shown in Note 7, Other Accrued Liabilities.
The Company records an allowance for estimated returns as a reduction to Accounts receivable and Gross sales. Historically, returns have been insignificant.
F-10
Receivable from Technology Investment Agreement (TIA)
The amounts set forth as Receivable from Technology Investment Agreement (TIA) represent amounts receivable under a contractual agreement under the TIA. The amounts may represent advance requests or reimbursement requests for expenditures the Company makes or has made under its obligations with the federal government. For further explanation, please refer to Note 22 – Technology Investment Agreement.
Inventories
Inventories are valued at the lower of cost or net realizable value, with cost being determined using actual average cost. The Company compares the average cost to the net realizable value and records the lower value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Management considers such factors as the amount of inventory on hand and in the distribution channel, estimated time to sell such inventory, the shelf life of inventory, and current market conditions when determining excess or obsolete inventories. Once inventory items are deemed to be either excess or obsolete, they are written down to their net realizable value.
Investments in debt and equity securities
The Company holds high-grade exchange-traded and closed-end funds (ETFs), mutual funds, equity securities, and debt securities as investments. These assets are readily marketable and are carried at fair value as of the date of the Balance Sheets. Net unrealized and realized gains or losses on investments in debt and equity securities are reflected as a component of Interest and other income. Realized gains or losses on investments in debt and equity securities are recognized using the specific identification method.
Property, plant, and equipment
Property, plant, and equipment are stated at cost. Expenditures for maintenance and repairs are charged to operations as incurred. Cost includes major expenditures for improvements and replacements which extend useful lives or increase capacity and interest cost associated with significant capital additions. Gains or losses from disposals are included in Interest and other income.
The Company's property, plant, and equipment primarily consist of buildings, land, assembly equipment, molding machines, molds, office equipment, furniture, and fixtures. Depreciation and amortization are calculated using the straight-line method over the following useful lives:
Production equipment |
| 3 to 13 years |
Office furniture and equipment |
| 3 to 10 years |
Buildings |
| 39 years |
Building improvements |
| 15 years |
Long-lived assets
The Company assesses the recoverability of long-lived assets using an assessment of the estimated undiscounted future cash flows related to such assets. In the event that assets are found to be carried at amounts which are in excess of estimated gross future cash flows, the assets will be adjusted for impairment to a level commensurate with fair value determined using a discounted cash flow analysis or appraised values of the underlying assets.
Fair value measurements
For assets and liabilities that are measured using quoted prices in active markets, total fair value is the published market price per unit multiplied by the number of units held without consideration of transaction costs. Assets and liabilities that are measured using significant other observable inputs are valued by reference to similar assets or liabilities, adjusted for contract restrictions and other terms specific to that asset or liability. For these items, a
F-11
significant portion of fair value is derived by reference to quoted prices of similar assets or liabilities in active markets. For all remaining assets and liabilities, fair value is derived using a fair value model, such as a discounted cash flow model or Black-Scholes model.
Financial instruments
The Company estimates the fair value of financial instruments through the use of public market prices, quotes from financial institutions, and other available information. Judgment is required in interpreting data to develop estimates of fair value and, accordingly, amounts are not necessarily indicative of the amounts that could be realized in a current market exchange. Short-term financial instruments, including cash and cash equivalents, accounts receivable, accounts payable, and other liabilities, consist primarily of instruments without extended maturities, the fair value of which, based on Management’s estimates, equals their recorded values. Investments in equity securities consist primarily of individual equity securities, exchange-traded and closed-end funds and mutual funds and are reported at their fair value based upon quoted prices in active markets. Investments in certificates of deposit (CD) with original maturities of greater than three months are reported at their estimated fair value based upon the duration of the CD and the interest rate earned on the CD versus current interest rates of similar duration CDs. The fair value of long-term liabilities, based on Management’s estimates, approximates their reported values.
Concentration risks
The Company’s financial instruments exposed to concentrations of credit risk consist primarily of cash, cash equivalents, certificates of deposit, exchange-traded and closed-end funds, mutual funds, equity securities, and accounts receivable. Cash balances, some of which exceed federally insured limits, are maintained in financial institutions; however, Management believes the institutions are of high credit quality. The majority of accounts receivable are due from companies that are well-established entities. The Company assesses market risk in debt and equity securities through consultation with its outside investment advisors. Management is responsible for directing investment activity based on current economic conditions. In 2021, a significant portion of the Company's sales were to the U.S. government, which Management does not consider a credit risk. As a consequence, Management considers any exposure from concentrations of credit risks to be limited.
The following table reflects our significant customers in 2021, 2020, and 2019:
Years Ended December 31, |
| ||||||||||
| 2021 |
| 2020 |
| 2019 |
| |||||
Number of significant customers |
|
| 1 |
| 2 |
| 3 | ||||
Aggregate dollar amount of net sales to significant customers | $ | 113.7 million | $ | 41.6 million | $ | 19.0 million | |||||
Percentage of net sales to significant customers | 60.3 | % | 50.6 | % | 45.6 | % | |||||
The Company increased its allowance for doubtful accounts by $150 thousand in 2021.
The Company manufactures some of its products in Little Elm, Texas, as well as utilizing manufacturers in China. The Company obtained roughly 92% of its products in 2021 from its Chinese manufacturers. Purchases from Chinese manufacturers aggregated 85.2% and 82.6% of products in 2020 and 2019, respectively. In the event that the Company becomes unable to purchase products from its Chinese manufacturers, the Company would need to find an alternate manufacturer for its blood collection set, IV catheter, Patient Safe® syringe, 0.5mL insulin syringe, 0.5mL autodisable syringe, and 2mL, 5mL, and 10mL syringes and would increase domestic production for the 1mL and 3mL syringes and EasyPoint® needles.
Revenue recognition
The Company recognizes revenue when control of performance obligations passes to the customer, generally when the product ships. Payments from customers with approved credit terms are typically due 30 days from the invoice
F-12
date. Under certain contracts, revenue is recorded on the basis of sales price to distributors, less contractual pricing allowances. Contractual pricing allowances consist of: (i) rebates granted to distributors who provide tracking reports which show, among other things, the facility that purchased the products, and (ii) a provision for estimated contractual pricing allowances for products for which the Company has not received tracking reports. When rebates are issued, they are applied against the customer’s receivable balance. Distributors receive a rebate for the difference between the Wholesale Acquisition Cost and the appropriate contract price as reflected on a tracking report provided by the distributor to the Company. If product is sold by a distributor to an entity that has no contract, there is a standard rebate (lower than a contracted rebate) given to the distributor. One of the purposes of the rebate is to encourage distributors to submit tracking reports to the Company. The provision for contractual pricing allowances is recognized in the period the related sales are recognized and is reviewed at the end of each quarter and adjusted for changes in levels of products for which there is no tracking report. Additionally, if it becomes clear that tracking reports will not be provided by individual distributors, the provision is further adjusted. The estimated contractual allowance is included in Accounts payable in the Balance Sheets and deducted from Revenues in the Statements of Operations. Accounts payable included estimated contractual allowances for $6.2 million and $3.4 million as of December 31, 2021 and 2020, respectively. The terms and conditions of contractual pricing allowances are governed by contracts between the Company and its distributors. Revenue for shipments directly to end-users is recognized when title and risk of ownership pass from the Company. End-users do not receive any contractual allowances on their purchases. Any product shipped or distributed for evaluation purposes is expensed.
The Company provides product warranties that: i) the products are fit for medical use as generally defined within the boundaries of United States FDA approval; ii) the products are not defective; and iii) the products will conform to the descriptions set forth in their respective labeling, provided that they are used in accordance with such labeling and the Company’s written directions for use. The Company has historically not incurred significant warranty claims.
The Company’s domestic return policy provides that a customer may return incorrect shipments within 10 days following arrival at the distributor’s facility. In all such cases, the distributor must obtain an authorization code from the Company and affix the code to the returned product.
The Company’s domestic return policy also generally provides that a customer may return product that is overstocked. Overstocking returns are limited to two times in each 12-month period up to 1% of distributor’s total purchase of products for the prior 12-month period. All product overstocks and returns are subject to inspection and acceptance by the Company. The Company has not historically incurred significant returns.
The Company’s international distribution agreements generally do not provide for any returns.
The Company requires certain customers to pay in advance of product shipment. Such prepayments from customers are recorded in Other accrued liabilities and are generally recognized as revenue upon shipment of the product.
The Company periodically recognizes revenue from licensing agreements. If the Company licenses its products for sale and the customers of the sublicensee are not known to the Company, the Company is obligated to pay Thomas J. Shaw, the owner of certain patented technology, fifty percent (50%) of such revenue pursuant to the terms of the Technology License Agreement between the Company and Mr. Shaw.
F-13
Disaggregated information of revenue recognized from contracts with customers and licensing fees recognized are as follows:
For the year ended December 31, 2021: | |||||||||||||||
|
| Blood |
|
|
| Total | |||||||||
Collection | EasyPoint® | Other | Product | ||||||||||||
Geographic Segment | Syringes | Products | Needles | Products | Sales | ||||||||||
U.S. sales (excluding U.S. government) | $ | 42,770,403 | $ | 2,171,680 | $ | 8,892,712 | $ | 53,341 | $ | 53,888,136 | |||||
Sales to U.S. government | 113,662,440 | — | — | — | 113,662,440 | ||||||||||
North and South America sales (excluding U.S.) |
| 14,345,874 |
| 4,800 |
| 100,848 |
| 109,714 |
| 14,561,236 | |||||
Other international sales |
| 5,551,592 |
| 71,670 |
| 642,880 |
| 4,500 |
| 6,270,642 | |||||
Total | $ | 176,330,309 | $ | 2,248,150 | $ | 9,636,440 | $ | 167,555 | $ | 188,382,454 | |||||
For the year ended December 31, 2020: | |||||||||||||||
|
| Blood |
|
|
| Total | |||||||||
Collection | EasyPoint® | Other | Product | ||||||||||||
Geographic Segment | Syringes | Products | Needles | Products | Sales | ||||||||||
U.S. sales (excluding U.S. government) | $ | 30,446,858 | 2,116,108 | 9,542,122 | 64,375 | $ | 42,169,463 | ||||||||
Sales to U.S. government | 31,634,343 | — | — | — | 31,634,343 | ||||||||||
North and South America sales (excluding U.S.) |
| 5,733,116 | 8,450 | 86,816 | 1,064,768 |
| 6,893,150 | ||||||||
Other international sales |
| 917,478 | 239,329 | 235 | 8,455 |
| 1,165,497 | ||||||||
Total | $ | 68,731,795 | $ | 2,363,887 | $ | 9,629,173 | $ | 1,137,598 | $ | 81,862,453 | |||||
For the year ended December 31, 2019: | |||||||||||||||
|
| Blood |
|
|
| Total | |||||||||
Collection | EasyPoint® | Other | Product | ||||||||||||
Geographic Segment | Syringes | Products | Needles | Products | Sales | ||||||||||
U.S. sales | $ | 26,722,414 | $ | 2,130,767 | $ | 2,970,374 | $ | 74,369 | $ | 31,897,924 | |||||
North and South America sales (excluding U.S.) |
| 7,863,796 |
| 6,313 |
| 7,996 |
| 370,885 |
| 8,248,990 | |||||
Other international sales |
| 1,052,217 |
| 578,617 |
| 635 |
| 18,796 |
| 1,650,265 | |||||
Total | $ | 35,638,427 | $ | 2,715,697 | $ | 2,979,005 | $ | 464,050 | $ | 41,797,179 | |||||
Income taxes
The Company evaluates tax positions taken or expected to be taken in a tax return for recognition in the financial statements based on whether it is “more-likely-than-not” that a tax position will be sustained based upon the technical merits of the position. Measurement of the tax position is based upon the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement.
The Company provides for deferred income taxes through utilizing an asset and liability approach for financial accounting and reporting based on the tax effects of differences between the financial statement and tax bases of assets and liabilities, based on enacted rates expected to be in effect when such differences reverse in future periods. Deferred tax assets are periodically reviewed for realizability. In prior periods, the Company established a valuation allowance for its net deferred tax asset as future taxable income which could not be reasonably assured. During the quarter ended June 30, 2020, the Company released its valuation allowance based on available evidence supporting that its deferred tax assets will be realized in full.
F-14
Earnings per share
The Company computes basic earnings per share (“EPS”) by dividing net earnings for the period (adjusted for any cumulative dividends for the period) by the weighted average number of common shares outstanding during the period. Diluted EPS includes the determinants of basic EPS and, in addition, reflects the dilutive effect, if any, of the common stock deliverable pursuant to stock options and/or common stock issuable upon the conversion of convertible preferred stock.
The calculation of diluted EPS under the treasury stock method included the following shares in 2021, 2020, and 2019:
Years Ended December 31, | ||||||
| 2021 |
| 2020 |
| 2019 | |
Common Stock underlying issued and outstanding stock options | 141,435 |
| 131,347 | 639,300 | ||
Common stock issuable upon the conversion of convertible preferred shares | 232,445 |
| — |
| — | |
373,880 |
| 131,347 |
| 639,300 | ||
In 2020 and 2019, preferred stock was excluded from the calculation of diluted EPS because the effect was antidilutive.
The potential dilution, if any, is shown on the following schedule:
Years Ended December 31, | ||||||||||
| 2021 |
| 2020 |
| 2019 | |||||
Net income | $ | 56,064,241 | $ | 24,223,013 | $ | 3,148,234 | ||||
Preferred stock dividend requirements |
| (241,703) |
| (573,868) |
| (702,618) | ||||
Deemed contribution on extinguishment of preferred stock | — | 2,975,708 | — | |||||||
Income applicable to common shareholders | $ | 55,822,538 | $ | 26,624,853 | $ | 2,445,616 | ||||
Average common shares outstanding |
| 33,870,819 |
| 33,169,307 |
| 32,672,475 | ||||
Average common and common equivalent shares outstanding — assuming dilution |
| 34,244,699 |
| 33,300,654 |
| 32,672,475 | ||||
Basic earnings per share | $ | 1.65 | $ | 0.80 | $ | 0.07 | ||||
Diluted earnings per share | $ | 1.63 | $ | 0.80 | $ | 0.07 | ||||
The FASB Codification 260-10-S99-2, Effect on the Calculation of Earnings per Share for the Redemption or Induced Conversion of Preferred Stock, requires the gain or loss on extinguishment of equity-classified preferred stock to be included in the net income per common stockholder used to calculate earnings per share (similar to the treatment of dividends paid on preferred stock). The difference between (1) the fair value of the consideration transferred to the holders of the preferred stock and (2) the carrying amount of the preferred stock (net of issuance costs) is subtracted from (or added to) net income to arrive at income available to common stockholders in the calculation of earnings per share.
The Company has determined to apply this guidance to its accounting treatment of the preferred stock transactions described in Note 20.
Shipping and handling costs
The Company classifies shipping and handling costs as part of Cost of sales in the Statements of Operations.
F-15
Share-based Compensation
The Company’s share-based payments are accounted for using the Black-Scholes fair value method. The Company records share-based compensation expense on a straight-line basis over the requisite service period. The Company incurred share-based compensation costs of $3.7 million which were classified as General and administrative expenses.
Self-insured employee benefit costs
The Company self-insures certain health insurance benefits for its employees under certain policy limits. The Company has additional coverage provided by an insurance company for any individual with claims in excess of $100,000 and/or total plan claims in excess of $1.6 million for the plan year.
Research and development costs
Research and development costs are expensed as incurred.
Leases
The Company determines if an arrangement is a lease at inception. Operating and finance leases are included in Other assets, Other accrued liabilities, and Other long-term liabilities on the Balance Sheets. Right-of-use ("ROU") assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As the Company's leases do not provide an implicit rate, the incremental borrowing rate based on information available at the commencement date was used in determining the present value of lease payments.
The operating lease ROU asset also includes any lease payments made and excludes lease incentives. Lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term. Leases with an initial term of twelve months or less are not recorded on the Balance Sheets; however, rent expense is recognized on a straight-line basis over the lease term.
Technology Investment Agreement (TIA)
Effective July 1, 2020, the Company entered into a Technology Investment Agreement ("TIA") with the United States Government Department of Defense, U.S. Army Contracting Command-Aberdeen Proving Ground, Natick Contracting Division & Edgewood Contracting Division (ACC-APG, NCD & ECD) on behalf of the Biomedical Advanced Research and Development Authority (BARDA), as amended, for $81,029,518 in government funding for expanding the Company's domestic production of needles and syringes. Pursuant to the terms of the TIA, the Company is expected to make significant additions to its facilities which should allow the Company to increase domestic production. As reimbursements are received from the U.S. government for such expenditures, the Company records a deferred liability. The deferred liability will be systematically amortized as a gain over the life of the related property, plant, and equipment as to offset the related depreciation expense of the assets acquired. The amortization will be presented separately from the depreciation expense on the Statements of Operations.
Recently Adopted Pronouncements
The Company adopted Financial Accounting Standards Board ("FASB") Accounting Standards Update ("ASU") 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” as well as subsequent clarifying amendments on January 1, 2020. Among other things, these amendments require the measurement of all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. Many of the loss estimation
F-16
techniques applied previously will still be permitted, although the inputs to those techniques will change to reflect the full amount of expected credit losses. The adoption of ASU 2016-13, as well as the Targeted Transition Relief as provided by ASU 2019-05, “Financial Instruments – Credit Losses (Topic 326) – Targeted Transition Relief” did not have a significant impact on the Company’s financial statements.
The Company adopted ASU 2018-15, “Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That is a Service Contract (a Consensus of the FASB Emerging Issues Task Force)" on January 1, 2020. This amendment requires that implemented costs incurred in a hosting arrangement that is a service contract should be accounted for in accordance with ASC 350-40 Internal-Use Software. Accordingly, costs incurred during the preliminary project and post-implementation stages are expensed and costs associated with the application development phase are capitalized. The amendment also requires that capitalized costs be amortized over the term of the hosting arrangement and that capitalized costs should be evaluated for impairment. The adoption of this ASU did not have a significant impact on the Company's financial statements or disclosures.
In August 2018, the FASB issued ASU 2018-13 "Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement." The amendment modifies, among other things, disclosure requirements on fair value measurements and eliminates certain disclosures related to transfers and valuation levels of Level 3 fair value measurements. Additionally, the amendment requires disclosure of changes in unrealized gains and losses in other comprehensive income for Level 3 fair value measurements and certain qualitative factors related to significant unobservable inputs used in Level 3 valuations. The amendment was effective for annual periods beginning after December 15, 2019 and interim periods within the annual period. The adoption of ASU 2018-13 did not have a significant effect on the Company's financial statements, as the Company does not currently have any investments classified as Level 3 fair value measurements.
In December 2019, the FASB issued ASU 2019-12, “Income Taxes: Simplifying the Accounting for Income Taxes”. The new standard is intended to simplify the accounting for income taxes by eliminating certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The new guidance also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard is effective for annual periods beginning after December 15, 2020 and interim periods within the annual period, with early adoption permitted. Adoption of the standard requires certain changes primarily be made prospectively, with some changes to be made retrospectively. The Company has determined that the adoption of ASU 2019-12 did not have a material impact on its financial statements.
Recently Issued Pronouncements
In March 2020, the FASB issued ASU No. 2020-04, “Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting”, to ease the potential burden in accounting for reference rate reform. The new guidance provides optional expedients for contracts that reference LIBOR, if certain criteria are met, that can be applied through December 31, 2022. As reference rate reform is still an ongoing process, the Company will continue to evaluate the timing and potential impact of adoption for optional expedients when deemed necessary.
In November 2021, the FASB issued ASU 2021-10, “Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance”. The new standard is intended to provide increased transparency by requiring business entities to disclose information about certain types of government assistance they receive in the notes to the financial statements. ASU 2021-10 also adds a new Topic – ASC 832, Government Assistance – to the FASB’s Codification. Included in the disclosures under the guidance are the nature of the transaction including the nature of the assistance being given, the accounting policies being used to account for the transaction and other provisions of relevance. The guidance is effective for annual periods beginning after December 15, 2021, with early adoption permitted. The Company has determined that the guidance will not have a material impact on its financial statements as such disclosures surrounding the TIA, including the accounting policies used to account for the agreement have been in place since its inception.
F-17
3. INVENTORIES
Inventories consist of the following:
| December 31, 2021 |
| December 31, 2020 | |||
Raw materials | $ | 4,402,828 | $ | 1,358,552 | ||
Finished goods | 16,187,091 | 8,876,094 | ||||
$ | 20,589,919 | $ | 10,234,646 | |||
4. FAIR VALUE OF FINANCIAL INSTRUMENTS
ASC 820, “Fair Value Measurements”, defines fair value, establishes a framework for measuring fair value and requires additional disclosures regarding certain fair value measurements. ASC 820 establishes a three-tier hierarchy for measuring fair value, as follows:
| ● | Level 1 – quoted market prices in active markets for identical assets and liabilities |
| ● | Level 2 – inputs other than quoted prices that are directly or indirectly observable |
| ● | Level 3 - unobservable inputs where there is little or no market activity |
The following tables summarize the values of assets designated as Investments in debt and equity securities:
December 31, 2021 | ||||||||||||
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |||||
Equity securities | $ | 9,112,607 | $ | — | $ | — | $ | 9,112,607 | ||||
Mutual funds and exchange traded funds | 4,156,379 | — | — | 4,156,379 | ||||||||
$ | 13,268,986 | $ | — | $ | — | $ | 13,268,986 | |||||
December 31, 2020 | ||||||||||||
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |||||
Equity securities | $ | 3,990,533 | $ | — | $ | — | $ | 3,990,533 | ||||
Mutual funds and exchange traded funds | 4,013,956 | — | — | 4,013,956 | ||||||||
Certificates of deposit |
| — |
| 77,344 |
| — |
| 77,344 | ||||
$ | 8,004,489 | $ | 77,344 | $ | — | $ | 8,081,833 | |||||
The Company holds high-grade ETFs, mutual funds, individual equity stocks, and debt securities as investments. These assets are readily marketable and are carried at fair value as of the date of the Balance Sheets. The Company intends to hold these assets for possible future operating requirements.
The following table summarizes gross unrealized gains and losses from Investments in debt and equity securities:
December 31, 2021 | ||||||||||||
Gross Unrealized | Aggregate | |||||||||||
| Cost |
| Gains |
| Losses |
| Fair Value | |||||
Equity securities | $ | 6,729,245 | $ | 2,383,362 | $ | — | $ | 9,112,607 | ||||
Mutual funds and exchange traded funds | 4,018,488 | 137,891 | — | 4,156,379 | ||||||||
$ | 10,747,733 | $ | 2,521,253 | $ | — | $ | 13,268,986 | |||||
F-18
December 31, 2020 | ||||||||||||
Gross Unrealized | Aggregate | |||||||||||
| Cost |
| Gains |
| Losses |
| Fair Value | |||||
Equity securities | $ | 2,098,144 | $ | 1,892,389 | $ | — | $ | 3,990,533 | ||||
Mutual funds and exchange traded funds | 3,909,364 | 104,592 | — | 4,013,956 | ||||||||
Certificates of deposit |
| 75,000 |
| 2,344 |
| — |
| 77,344 | ||||
$ | 6,082,508 | $ | 1,999,325 | $ | — | $ | 8,081,833 | |||||
Unrealized gains on investments in debt and equity securities were $2,521,253 and $1,999,325 for the years ended December 31, 2021 and 2020, respectively.
5. PROPERTY, PLANT, AND EQUIPMENT
Property, plant, and equipment consist of the following:
December 31, | ||||||
| 2021 |
| 2020 | |||
Land | $ | 261,893 | $ | 261,893 | ||
Buildings and building improvements |
| 24,364,084 |
| 11,593,952 | ||
Production equipment |
| 34,112,766 |
| 20,290,331 | ||
Office furniture and equipment |
| 4,089,362 |
| 3,630,455 | ||
Construction in progress |
| 52,681,005 |
| 21,365,915 | ||
| 115,509,110 |
| 57,142,546 | |||
Accumulated depreciation |
| (27,583,459) |
| (26,326,042) | ||
$ | 87,925,651 | $ | 30,816,504 | |||
Depreciation expense for the years ended December 31, 2021, 2020, and 2019 was $1,257,417; $832,069; and $851,673, respectively.
6. LICENSE AGREEMENT
In 1995, the Company entered into a license agreement with the Chief Executive Officer of the Company, Thomas J. Shaw, for the exclusive right to manufacture, market, and distribute products utilizing automated retraction technology, which agreement has been amended. This technology is the subject of various patents and patent applications owned by Mr. Shaw. The license agreement provides for quarterly payments of a 5% royalty fee on gross sales. Additionally, if the Company sublicenses the technology and the sublicensee’s customers are not known to the Company, then Mr. Shaw shall be entitled to receive from the Company fifty percent (50)% of the royalties actually paid to the Company by such sublicensee. The royalty fee expense is recognized in the period in which it is earned. Royalty fees of $11,318,093; $5,476,306; and $3,449,822; are included in Cost of sales for the years ended December 31, 2021, 2020, and 2019, respectively. Royalties payable under this agreement aggregated $3,450,684 and $1,973,781 at December 31, 2021, and 2020, respectively. Gross sales upon which royalties are based were $226,294,765; $109,526,118; and $67,529,783 for 2021, 2020, and 2019, respectively.
On November 16, 2021, the Company and Mr. Shaw entered into the Third Amendment to Technology License Agreement (the “Amendment”). The Amendment expands the scope of the Technology License Agreement and provides additional protection to the parties in the event of a Hostile Takeover, as defined by the Amendment. Under the Amendment, under certain conditions, Mr. Shaw is granted the unilateral right to terminate the Technology License Agreement or cancel or convert a license thereunder from exclusive to nonexclusive following a Hostile Takeover.
F-19
7. OTHER ACCRUED LIABILITIES
Other accrued liabilities consist of the following:
| December 31, 2021 |
| December 31, 2020 | |||
Prepayments from customers | $ | 2,339,530 | $ | 1,686,868 | ||
Accrued professional fees | 185,515 | 331,204 | ||||
Current portion – preferred stock repurchase |
| 1,098,282 |
| 1,092,282 | ||
Other accrued expenses |
| 102,200 |
| 288,550 | ||
Total | $ | 3,725,527 | $ | 3,398,904 | ||
8. LONG-TERM DEBT
Long-term debt consists of the following:
December 31, | ||||||
| 2021 |
| 2020 | |||
Loan from American First National Bank. Maturity date is April 10, 2028. The loan, in the original amount of $4,209,608, provided funding for the expansion of the warehouse, additional office space, and a new Controlled Environment. The loan is secured by the Company’s land and buildings. The interest rate is equal to prime rate plus 0.25%, not to be less than 5.0%. The interest rate was 5.0% at December 31, 2021. | $ | 2,103,308 | $ | 2,378,100 | ||
Loan from Independent Bank pursuant to the Paycheck Protection Program. This loan was forgiven in May 2021. | — | 1,363,000 | ||||
| 2,103,308 |
| 3,741,100 | |||
Less: current portion |
| (289,114) |
| (1,030,763) | ||
$ | 1,814,194 | $ | 2,710,337 | |||
The fair value of long-term liabilities, based on Management’s estimates, approximates their reported values.
The aggregate maturities of long-term debt as of December 31, 2021, are as follows:
2022 |
| $ | 289,114 |
2023 | 304,116 | ||
2024 | 319,682 | ||
2025 | 336,485 | ||
2026 | 353,944 | ||
Thereafter | 499,967 | ||
$ | 2,103,308 |
9. OTHER LONG-TERM LIABILITIES
Other long-term liabilities consist of the following:
| December 31, 2021 |
| December 31, 2020 | |||
Technology Investment Agreement (TIA) |
| $ | 68,955,664 |
| $ | 22,444,324 |
Stock repurchase |
| 1,040,666 |
| 2,034,373 | ||
Total | $ | 69,996,330 | $ | 24,478,697 | ||
The TIA provides for reimbursement to the Company for the purchase of equipment and supplies related to the expansion of the Company’s domestic production of needles and syringes. Under the TIA, reimbursable amounts will
F-20
be reflected as a liability until the time its deferred income can be systematically amortized over a period matching the useful life of the purchased assets.
The stock repurchase liability represents the long-term portion, at net present value, of $3,303,330 gross payable by the Company to former preferred shareholders as a result of private stock purchases in 2020 of 320,333 shares of Class B Series IV preferred stock and 25,000 shares of Class B Series V preferred stock. The purchase price is payable in three annual installments of $1,101,110.
10. COMMITMENTS AND CONTINGENCIES
On November 7, 2019, the Company filed a lawsuit in the 44th District Court of Dallas County, Texas (No. DC-19-17946) against Locke Lord, LLP and Roy Hardin in connection with their legal representation of the Company in its previous litigation against Becton, Dickinson and Company ("BD"). The Company alleges that the defendants breached their fiduciary duties, committed malpractice, and were negligent in their representation of the Company. The Company seeks actual and exemplary damages, disgorgement, costs, and interest. On October 6, 2020, the Court dismissed Locke Lord, LLP and Mr. Hardin’s motion to dismiss. Such order was affirmed on April 20, 2021 by the Court of Appeals, Fifth District of Texas at Dallas. On March 23, 2022, Locke Lord, LLP and Mr. Hardin filed a motion for partial summary judgment regarding the Company’s cause of action for breach of fiduciary duty. A jury trial date of January 30, 2023 has been set for this case.
11. INCOME TAXES
The provision (benefit) for income taxes consists of the following:
For the Years Ended December 31, | |||||||||
| 2021 |
| 2020 |
| 2019 | ||||
Current tax provision (benefit) | |||||||||
Federal | $ | 20,041,644 | $ | 4,431,590 | $ | — | |||
State |
| 8,079,555 |
| 2,049,850 |
| 7,875 | |||
Total current provision (benefit) |
| 28,121,199 |
| 6,481,440 |
| 7,875 | |||
Deferred tax provision (benefit) | |||||||||
Federal |
| (6,719,211) |
| (3,428,399) |
| — | |||
State |
| (2,515,418) |
| (1,202,807) |
| — | |||
Total deferred tax provision (benefit) |
| (9,234,629) |
| (4,631,206) |
| — | |||
Total income tax provision (benefit) | $ | 18,886,570 | $ | 1,850,234 | $ | 7,875 | |||
The Company has state net operating losses of $2.0 million as of December 31, 2021 which will begin to expire in 2030. The Company fully utilized its $23.3 million in tax benefits attributable to federal net operating losses as of December 31, 2020.
Utilization of the state net operating loss carry forwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by state rules that are similar to the Internal Revenue Code of 1986, as amended.
Deferred taxes are provided for those items reported in different periods for income tax and financial reporting purposes. The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities are presented below:
F-21
December 31, | ||||||
| 2021 |
| 2020 | |||
Deferred tax assets | ||||||
Net operating loss carry forwards | $ | 130,643 | $ | 198,675 | ||
Accrued expenses and reserves |
| 1,298,083 |
| 824,920 | ||
Employee stock option expense |
| 958,530 |
| 15,188 | ||
Nonemployee stock option expense | 8,887 | 8,515 | ||||
Inventories |
| 118,201 |
| 98,748 | ||
Deferred income – TIA contract | 18,199,768 | 5,675,617 | ||||
Deferred tax assets |
| 20,714,112 |
| 6,821,663 | ||
Deferred tax liabilities | ||||||
Unrealized gains/losses | (665,960) | (508,197) | ||||
Property, plant, and equipment |
| (6,182,318) |
| (1,682,260) | ||
Deferred tax liabilities |
| (6,848,278) |
| (2,190,457) | ||
Net deferred tax assets | $ | 13,865,834 | $ | 4,631,206 | ||
Deferred income tax calculations reflect the effects of temporary differences between the carrying amounts of assets and liabilities and their tax bases, as well as from net operating loss carry forwards, and are stated at the U.S. tax rate of 21%. Deferred income tax assets represent amounts available to reduce income taxes payable on taxable income in future years.
Deferred tax assets are periodically reviewed for realizability. In prior periods, the Company established a valuation allowance for its net deferred tax asset as future taxable income which could not be reasonably assured. The Company determined that no valuation allowance is needed for the years ended December 21, 2021 and 2020. The valuation allowance of $5.0 million was fully released during the year ended December 31, 2020.
Under the Tax Cuts and Jobs Act, net operating losses incurred after December 31, 2017 can only offset 80% of taxable income. However, these net operating losses may be carried forward indefinitely instead of limited to twenty years under previous tax law. Carryback of these losses is no longer permitted.
The CARES Act temporarily removed the 80% of taxable income limitation to allow NOL carryforwards to fully offset income. For tax years beginning before 2021, the Company can take an NOL deduction equal to 100% of taxable income. For tax years beginning after 2021, the Company can take: (1) a 100% deduction of NOLs arising in tax years prior to 2018, and (2) a deduction limited to 80% of modified taxable income for NOLs arising in tax years after 2017.
A reconciliation of income taxes based on the federal statutory rate and the effective income tax rate is summarized as follows:
December 31, |
| |||||||
| 2021 |
| 2020 |
| 2019 |
| ||
U.S. statutory federal tax rate |
|
| 21.0 | % | 21.0 | % | 21.0 | % |
State tax, net of federal tax |
|
| 6.4 |
| 4.2 |
| 2.0 |
|
Change in valuation allowance |
|
| — |
| (19.2) |
| (35.6) |
|
Valuation Allowance |
|
| — |
| (0.6) |
| — |
|
Stock options | (0.1) | — | — | |||||
PPP loan | (0.4) | — | — | |||||
Return-to-provision and other |
|
| (1.7) |
| 1.7 |
| 12.9 |
|
Effective tax rate |
|
| 25.2 | % | 7.1 | % | 0.3 | % |
The Company files income tax returns in the U.S. federal jurisdiction and in various state and local jurisdictions. The Company’s federal income tax returns for all tax years ended on or after December 31, 2019, remain subject to examination by the Internal Revenue Service. The Company’s state and local income tax returns are subject to
F-22
examination by the respective state and local authorities over various statutes of limitations, most ranging from to five years from the date of filing.
12. DIVIDENDS
The Board declared and the Company paid cash dividends to Series I and Series II Class B Preferred Shareholders within one month of the end of each quarter in 2019 and 2020, resulting in cumulative annual payments of: $48,625, and $171,200 to Series I and Series II preferred shareholders, respectively, in 2019; and $48,000, and $168,642 to Series I and Series II preferred shareholders, respectively, in 2020. One payment of $10,041, and $39,050 was made to Series I and Series II preferred shareholders, respectively, in January 2021. A payment of $39,050 was paid in April 2021 to Series II preferred shareholders.
In June 2021, the Board of Directors approved payments to its Series II, Series III, and former Series IV and Series V Class B Preferred Shareholders in the cumulative amount of $5,056,945 representing all current dividends, dividends in arrears, as well as dividends still owed to shareholders who converted their preferred stock in the past. Of this amount, $39,050 was declared and paid to Series II Class B Convertible Preferred shareholders. To Series III Class B Convertible Preferred shareholders, $4,086,704 was declared, covering amounts in arrears from the date of purchase though the date of conversion or June 30, 2021, whichever is applicable. To former Series IV Class B Convertible Preferred shareholders, $101,475 was declared, covering amounts in arrears from the date of purchase though the date of conversion. To former Series V Class B Convertible Preferred shareholders, $829,716 was declared, covering amounts in arrears from the date of purchase though the date of conversion. The dividends were paid on July 22, 2021 to all shareholders who had been contacted and confirmed as the rightful owner entitled to payment. The Company has not yet established contact with all former shareholders, most of whom converted their shares prior to 2001. As of March 31, 2022, the Company is continuing its efforts to establish contact with approximately 90 former shareholders who are entitled to approximately $1.4 million.
A payment of $39,050 was paid in both October 2021 and January 2022 to Series II preferred shareholders. Series III preferred shareholders were paid $39,495 in January 2022.
13. STOCKHOLDERS’ EQUITY
Preferred Stock
The Company is authorized to issue 5,000,000 shares of Preferred Stock Class A with a par value of One Dollar ($1.00) per share; 5,000,000 shares of Preferred Stock Class B with a par value of One Dollar ($1.00) per share; and 5,000,000 shares of Preferred Stock Class C with a par value of One Dollar ($1.00) per share.
The Company has one class of Preferred Stock outstanding: Class B Convertible Preferred Stock (“Class B Stock”). The Class B Stock has two series: Series II and Series III. Series I, Series IV, and Series V were cancelled by Board resolution effective March 16, 2021.
The Class B Series II and III stock had 156,200 and 76,245 shares outstanding, respectively, at December 31, 2021. The remaining 4,767,555 authorized shares have not been assigned a series.
Series II Class B Stock
There were 156,200 shares of $1 par value Series II Class B Stock outstanding at December 31, 2021 and 2020. Holders of Series II Class B Stock are entitled to receive a cumulative annual dividend of $1.00 per share, payable quarterly if declared by the Board of Directors. Holders of Series II Class B Stock generally have no voting rights until dividends are in arrears and unpaid for twelve consecutive quarters. In such case, the holders of Series II Class B Stock have the right to elect of the Board of Directors of the Company. The Company paid dividends of $156,200 in 2021 and $168,642 in 2020. At December 31, 2021, no dividends were in arrears.
F-23
Series II Class B Stock is redeemable at the option of the Company at a price of $15.00 per share plus all unpaid dividends. Each share of Series II Class B Stock may, at the option of the stockholder, be converted to one share of Common Stock. No shares were converted in 2021 and 15,000 shares were converted into Common Stock in 2020. In the event of voluntary or involuntary dissolution, liquidation, or winding up of the Company, holders of Series II Class B Stock then outstanding are entitled to $12.50 per share, plus all unpaid dividends, prior to any distributions to holders of Series III Class B Stock or Common Stock.
Series III Class B Stock
There were 76,245 shares and 106,745 shares of $1 par value Series III Class B Stock outstanding at December 31, 2021 and 2020, respectively. Holders of Series III Class B Stock are entitled to receive a cumulative annual dividend of $1.00 per share, payable quarterly if declared by the Board of Directors. The Company paid dividends of $3,245,693 in 2021, including dividend amounts owed to shareholders who converted their preferred stock in the past. No dividends were paid on the Series III Class B Stock in 2020. At December 31, 2021, no dividends were in arrears.
Series III Class B Stock is redeemable at the option of the Company at a price of $15.00 per share, plus all unpaid dividends. Each share of Series III Class B Stock may, at the option of the stockholder, be converted to one share of Common Stock. 30,500 shares were converted into Common Stock in 2021. No shares were converted in 2020. 22,500 shares were exchanged for Common Stock in private transactions in 2020. Please see Note 20 for a description of private exchange transactions in 2020. In the event of voluntary or involuntary dissolution, liquidation, or winding up of the Company, holders of Series III Class B Stock then outstanding are entitled to $12.50 per share, plus all unpaid dividends, after distribution obligations to Series II Class B Stock have been satisfied and prior to any distributions to holders of Common Stock.
Common stock
The Company is authorized to issue 100,000,000 shares of no par value Common Stock, of which 33,484,935 and 33,957,204 shares were outstanding at December 31, 2021 and 2020, respectively. At December 31, 2021, 528,169 shares were held as treasury stock and were not deemed outstanding. Additionally, as of December 31, 2021, a total of 405,495 shares of Common Stock were issuable upon the conversion of Preferred Stock and the exercise of stock options.
14. TREASURY STOCK
In June 2021, the Company approved a stock repurchase plan as described by Note 23. The Company accounts for the purchased shares under the cost method as Common Stock Held in Treasury – at cost, which represents the cost of the shares and the cost of acquiring the shares through the Company’s broker.
15. RELATED PARTY TRANSACTIONS
The Company has a license agreement with the Chief Executive Officer of the Company. See Note 6.
16. STOCK OPTIONS
Stock options
Options for the purchase of 3,649,508 shares of Common Stock have been issued under the 2008 Stock Option Plan. Options for the purchase of 173,050 shares under the 2008 Stock Option Plan were outstanding as of December 31, 2021. No shares are available for future issuance under the 2008 Stock Option Plan, which expired July 25, 2018.
Options for the purchase of 1,350,000 shares of Common Stock are issued and outstanding under the 2021 Stock Option Plan and none have vested. 650,000 shares are available for future issuance under the 2021 Stock Option Plan.
F-24
The Compensation and Benefits Committee administers the Company’s stock option plans.
Stock option exercises
Stock options were exercised by the Company’s employees and directors during 2021, and, consequently, a total of 25,400 shares of Common Stock were issued for an aggregate payment to the Company of $48,600 to exercise such options.
Director, officer, and employee options
A summary of Director, officer, and employee options granted and outstanding under the 2008 Stock Option Plan is presented below:
Years Ended December 31, | |||||||||||||||
2021 | 2020 | 2019 | |||||||||||||
|
| Weighted |
|
| Weighted |
|
| Weighted | |||||||
Average | Average | Average | |||||||||||||
Exercise | Exercise | Exercise | |||||||||||||
Shares | Price | Shares | Price | Shares | Price | ||||||||||
Outstanding at beginning of period |
| 199,450 | $ | 2.05 |
| 639,300 | $ | 2.12 |
| 1,300,303 | $ | 1.57 | |||
Granted |
| — | $ | — |
| — | $ | — |
| — | $ | — | |||
Exercised |
| (25,400) | $ | 1.91 |
| (431,550) | $ | (2.14) |
| — | $ | — | |||
Forfeited |
| (1,000) | $ | 2.75 |
| (8,300) | $ | (2.75) |
| (661,003) | $ | (1.05) | |||
Outstanding at end of period |
| 173,050 | $ | 2.06 |
| 199,450 | $ | 2.05 |
| 639,300 | $ | 2.12 | |||
Exercisable at end of period |
| 173,050 | $ | 2.06 |
| 199,450 | $ | 2.05 |
| 639,300 | $ | 2.12 | |||
No options were issued in 2021, 2020, or 2019 under the 2008 Stock Option Plan to employees or non-employee directors.
The following table summarizes information about Director, officer, and employee options outstanding under the 2008 Stock Option Plan at December 31, 2021:
Weighted Average | |||||||
Exercise Prices |
| Shares Outstanding |
| Remaining Contractual Life |
| Shares Exercisable | |
$ | 1.05 |
| 70,000 |
| 4.99 |
| 70,000 |
$ | 2.75 |
| 103,050 |
| 4.70 |
| 103,050 |
In the year ended December 31, 2021, three officers were granted options for the purchase of a total of 1,350,000 shares under the 2021 Stock Option Plan. All options were outstanding, but none were exercisable at the end of 2021. The fair value of the 2021 grant is $10.21 per share using the Black-Scholes option pricing model with a risk-free rate of $1.20 and a volatility factor of 92.66%. The options were granted on March 16, 2021, have a ten-year term, and may vest in their entirety three years from the grant date.
Non-employee options
In 2021 and 2020, there were no non-employee options outstanding. In 2019, non-employees forfeited 57,500 shares underlying options exercisable at $0.81 under the 2008 Stock Option Plan.
Stock-based Compensation
The Company recorded $3,660,387 in stock-based compensation expense in 2021. Assuming there are no changes to the stock options granted under the 2021 Stock Option Plan, the Company expects to recognize $4,595,400,
F-25
$4,595,400, and $935,013 in stock-based compensation expense in 2022, 2023, and 2024, respectively. No stock-based compensation expense was recorded in 2020 or 2019.
The total intrinsic value of outstanding and exercisable stock options with exercise prices lower than the closing market price was $842,349 and $1,733,856 at December 31, 2021 and December 31, 2020, respectively.
Options Pricing Models – Assumptions
The expected life is based on the Company’s historical experience with option exercise trends. The assumptions for expected volatility are based on a calculation of volatility over the five-years preceding the grant date. Risk-free interest rates are set using grant-date U.S. Treasury yield curves. In its calculations, the Company assumed no dividends. The Company elected a policy to account for forfeitures as they occur, rather than on an estimated basis.
17. 401(k) PLAN
The Company implemented an employee savings and retirement plan (the “401(k) Plan”) in 2005 that is intended to be a tax-qualified plan covering substantially all employees. The 401(k) Plan is available to all employees on the first day of the month after 90 days of service. Under the terms of the 401(k) Plan, employees may elect to contribute up to 88% of their compensation, or the statutory prescribed limit, if less. The Company may, at its discretion, match employee contributions. For 2021, 2020, and 2019, the Company matched each participant’s elective deferrals up to 2% of the participant’s compensation for the pay period. The total match was $204,032; $162,008; and $117,917 in 2021, 2020, and 2019, respectively.
18. BUSINESS SEGMENT
The following is a summary of the Company’s sales and long-lived assets by geography:
| 2021 |
| 2020 |
| 2019 | |||||
U.S. sales (excluding U.S. government) | $ | 53,888,136 | $ | 42,169,463 | $ | 31,897,924 | ||||
Sales to U.S. government | 113,662,440 | 31,634,343 | — | |||||||
North and South America sales (excluding U.S.) |
| 14,561,236 |
| 6,893,150 |
| 8,248,990 | ||||
Other international sales |
| 6,270,642 |
| 1,165,497 |
| 1,650,265 | ||||
Total sales | $ | 188,382,454 | $ | 81,862,453 | $ | 41,797,179 | ||||
| December 31, 2021 |
| December 31, 2020 | ||||
Long-lived assets | |||||||
U.S. | $ | 83,695,991 | $ | 30,751,259 | |||
International | 4,229,660 | 65,245 | |||||
Total | $ | 87,925,651 | $ | 30,816,504 | |||
The Company does not operate in separate reportable segments. Shipments to international customers generally require a prepayment either by wire transfer or an irrevocable confirmed letter of credit. The Company does extend credit to international customers on some occasions depending upon certain criteria, including, but not limited to, the credit worthiness of the customer, the stability of the country, banking restrictions, and the size of the order. All transactions are in U.S. currency.
19. LEASES
The Company has no finance leases and its operating leases for a warehouse and equipment terminated on August 15, 2021. The Company has chosen not to renew the terminated leases. The ROU asset value was determined based on the lease liability adjusted for lease incentives received. Lease expense has been recognized on a straight-line basis
F-26
over the lease term. Certain costs incidental to the use of the property were separate from the minimum rent payment and were not considered in the determination of the lease liability and ROU asset. The Company elected the policy to not separate lease from non-lease components if they are combined with the minimum rent payment. The option periods were not included in the determination of the lease liability and right-of-use asset.
The operating lease cost component of the lease expense was $38,892 and $103,312 for the years ended December 31, 2021 and 2020, respectively. The cash paid for amounts included in the measurement of lease liabilities as a component of cash flows related to leases was $32,272 and $106,101 for the years ended December 31, 2021 and 2020, respectively.
Assets and liabilities associated with these leases included in the Balance Sheets are as follows:
| December 31, 2021 |
| December 31, 2020 | |||
OPERATING LEASES |
|
|
|
| ||
$ | — | $ | 38,892 | |||
$ | — | $ | 38,892 | |||
| — |
| — | |||
Total operating lease liabilities | $ | — | $ | 38,892 | ||
20. PRIVATE EXCHANGES AND REDEMPTION
Private Exchanges of Preferred Stock for Common Stock
In 2020, the Company entered into several agreements with shareholders to purchase its outstanding Class B Convertible Preferred Stock. The consideration for these purchases consisted of both cash and Common Stock. In addition, in each such transaction, the preferred shareholder counterparty waived all rights to unpaid dividends in arrears. In total, 22,500 shares of Series III Class B Convertible Preferred Stock, 342,500 shares of Series IV Class B Convertible Preferred Stock, and 34,000 shares of Series V Class B Convertible Preferred Stock were purchased by the Company. The aggregate cash consideration equaled $3,786,000, of which $482,670 was paid in 2020 with the rest payable over a three-year period. Two of such equal installment payments were paid in February 2021 and February 2022 in the amount of $1,101,110 each. The aggregate stock consideration was 754,000 shares of Common Stock. As a result of the transactions, $7,642,049 in unpaid dividends in arrears were waived, as measured from the effective date of each transaction.
Redemption of Class B Series I Preferred Stock
The Company caused a redemption of its Class B Series I Preferred Stock on December 31, 2020 pursuant to the terms of the Certificate of Designation for such series of preferred stock which required a redemption price of $7.50 per share. Pursuant to such redemption, all shares of the Class B Series I Preferred Stock existing on December 31, 2020 (14,300 shares) were cancelled.
21. PAYCHECK PROTECTION PROGRAM LOAN
On April 17, 2020, the Company entered into a promissory note in the principal amount of $1,363,000 (the “PPP Loan”) in favor of Independent Bank pursuant to the Paycheck Protection Program (the “PPP”) of the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”), administered by the U.S. Small Business Administration (“SBA”). The PPP Loan’s original maturity date was April 17, 2022 with an interest rate of 1.0% per annum. The PPP Loan had a prepayment option with no prepayment penalties. The PPP Loan was unsecured and was a non-recourse obligation. On May 13, 2021, the Company was informed that the SBA granted its request for loan forgiveness for the entire original principal and accrued interest, for a total of $1,377,652. No payments were made prior to receiving forgiveness.
F-27
22. TECHNOLOGY INVESTMENT AGREEMENT
Effective July 1, 2020, the Company entered into the TIA with the U.S. government. The principal purpose of the TIA is to fund the expansion of the Company's manufacturing capacity for hypodermic safety needles and corresponding syringes in response to the worldwide COVID-19 global pandemic. The award is an expenditure-type TIA, whereby the U.S. government will make payments to the Company for the Company's expenditures for equipment and supplies in carrying out the expansion of the Company's domestic production. The Company's contributions under the terms of the TIA to enhance domestic capacity of pandemic-essential technology include providing facilities, technical expertise, labor, and maintenance of the TIA-funded equipment for a ten-year term.
As of December 31, 2021, the Company had negotiated contracts for the purchase of automated assembly equipment, molds, and molding equipment, as well as portions of auxiliary equipment, for approximately $46.3 million. The Company has received a temporary certificate of occupancy for the approximately 27,800 square foot controlled environment and a certificate of occupancy for the approximately 55,000 square foot new warehouse space. The final cost of the controlled environment within existing properties is $6.7 million. The new warehouse space final cost is $5.9 million. The cost of the controlled environment was funded by the U.S. government under the TIA, while the cost of the new warehouse was funded by the Company. A May 2021 amendment to the TIA required further expansion and new assembly lines. As of December 31, 2021, the Company had issued purchase orders for approximately $17.1 million for the purchase of additional production and ancillary equipment in connection with the foregoing amendment.
23. STOCK REPURCHASE PLAN
The Company entered into a repurchase plan (the “Plan”) dated June 4, 2021 with an independent broker for the purchase of up to $10 million of the Company’s Common Stock. Under the Plan, open market purchases of the Company’s Common Stock commenced June 18, 2021 and 528,169 shares were purchased in the year ended December 31, 2021 for an aggregate purchase price of $5.3 million. These treasury share purchases are accounted for under the cost method and are included as a component of treasury stock in the Company’s balance sheets. The Plan terminates on the earliest of: June 18, 2022, the completion of all purchases contemplated by the Plan, termination by either party, the existence of a legal or regulatory restriction, certain fundamental business transactions, liquidation or reorganization, or failure of the Company to adhere to the representations and warranties in the Plan. The Plan is structured to comply with Rules 10b5-1 and 10b-18 under the Securities Exchange Act of 1934. The purchases under the Plan are subject to Rule 10b-18 limitations as well as certain price and market volume constraints specified in the Plan. As of March 11, 2022, 371,730 shares had been purchased since January 1, 2022 for an aggregate purchase price of $2.0 million.
F-28
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There were no reportable disagreements with accountants on accounting and financial disclosures.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
Pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934 (the "Exchange Act"), Management, with the participation of our President, Chairman, and Chief Executive Officer, Thomas J. Shaw (the “CEO”), and our Vice President and Chief Financial Officer, John W. Fort III (the “CFO”), acting in their capacities as our principal executive and financial officers, evaluated the effectiveness of our disclosure controls and procedures, as defined in Rule 13a-15(e) under the Exchange Act. The term disclosure controls and procedures means controls and other procedures that are designed to ensure that information required to be disclosed by us in our periodic reports is: i) recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission's (the “SEC”) rules and forms; and ii) accumulated and communicated to our Management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Based upon this evaluation, the CEO and CFO concluded that, as of December 31, 2021, our disclosure controls and procedures were effective.
Management's Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over our financial reporting as defined in Rule 13a-15(f) under the Exchange Act. The term internal control over financial reporting means a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board of Directors, Management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets; (ii) provide reasonable assurance that our transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of Management and Directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements. Management used the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission to evaluate the effectiveness of our internal control over financial reporting as required by paragraph (c) of Rule 13a-15 under the Exchange Act. Management, with the participation of our CEO and CFO, concluded that our internal control over financial reporting as of December 31, 2021, was effective. No material weaknesses in our internal control over financial reporting were identified by Management.
Our Management, including the CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all instances of fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting during the fourth quarter of 2021 or subsequent to December 31, 2021 which has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
19
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information in the sections “Proposal – The Election of Three Class 2 Directors” and “Corporate Governance” in the 2022 proxy statement is incorporated herein by reference.
Item 11. Executive Compensation.
The information in the section “Compensation” in the 2022 proxy statement is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information in the section “Security Ownership” in the 2022 proxy statement is incorporated herein by reference. See also Item 5 of Part II of this Annual Report for Equity Compensation Plan Information.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information in the section “Corporate Governance” in the 2022 proxy statement is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services.
The information in the section “Accounting Matters” in the 2022 proxy statement is incorporated herein by reference.
20
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) (1) All financial statements: See Retractable Technologies, Inc. Index to Financial Statements on Page F-2.
(2) Those financial statement schedules required to be filed by Item 8 of this form, and by paragraph (b) below. Schedule II-Schedule of Valuation and Qualifying Accounts for the years ended December 31, 2021, 2020, and 2019:
| Balance at |
|
|
| ||||||||
beginning | Balance at | |||||||||||
of period | Additions | Deductions | end of period | |||||||||
Provision for Inventories | ||||||||||||
Fiscal year ended 2019 | $ | 297,208 | $ | — | $ | — | $ | 297,208 | ||||
Fiscal year ended 2020 | $ | 297,208 | $ | — | $ | — | $ | 297,208 | ||||
Fiscal year ended 2021 | $ | 297,208 | $ | — | $ | — | $ | 297,208 | ||||
Provision for Accounts Receivable |
|
|
|
| ||||||||
Fiscal year ended 2019 | $ | 149,665 | $ | — | $ | 3,283 | $ | 146,382 | ||||
Fiscal year ended 2020 | $ | 146,382 | $ | 125,000 | $ | 65,560 | $ | 205,822 | ||||
Fiscal year ended 2021 | $ | 205,822 | $ | 150,000 | $ | 3,605 | $ | 352,217 | ||||
Deferred Tax Valuation |
|
|
|
| ||||||||
Fiscal year ended 2019 | $ | 6,151,398 | $ | — | $ | 1,121,560 | $ | 5,029,838 | ||||
Fiscal year ended 2020 | $ | 5,029,838 | $ | — | $ | 5,029,838 | $ | — | ||||
Fiscal year ended 2021 | $ | — | $ | — | $ | — | $ | — | ||||
Provision for Rebates |
| (A) |
| (B) |
| (C) | ||||||
Fiscal year ended 2019 | $ | 4,586,847 | $ | 24,212,830 | $ | 24,526,108 | $ | 4,273,569 | ||||
Fiscal year ended 2020 | $ | 4,273,569 | $ | 26,104,612 | $ | 26,566,256 | $ | 3,811,925 | ||||
Fiscal year ended 2021 | $ | 3,811,925 | $ | 36,230,028 | $ | 33,403,838 | $ | 6,638,115 | ||||
| (A) | Represents estimated rebates deducted from gross revenues. |
| (B) | Represents rebates credited to the distributor and charge offs against the allowance. |
| (C) | Includes $6,209,708; $3,435,352; and $3,586,726 in Accounts payable for 2021, 2020, and 2019, respectively. |
(3) Exhibits:
The following exhibits are filed herewith or incorporated herein by reference to exhibits previously filed with the SEC.
21
(b) Exhibits
Exhibit |
| Description of Document | |
3(i) |
| ||
|
|
| |
3(ii) |
| ||
|
|
| |
4(i) |
| ||
|
|
| |
4(vi) |
| ||
|
|
| |
10.1 |
| Employment Agreement between RTI and Thomas J. Shaw dated as of January 1, 2008(5) | |
|
|
| |
10.2 |
| Technology License Agreement between Thomas J. Shaw and RTI dated the 23rd day of June, 1995(6) | |
|
|
| |
10.3 |
| ||
|
|
| |
10.4 |
| ||
|
|
| |
10.5 | |||
10.6 |
| Retractable Technologies, Inc. First Amended 2008 Stock Option Plan(10) | |
|
| ||
10.7 |
| Voting Agreement Between Thomas J. Shaw and Suzanne August dated November 8, 2006 (11) | |
|
|
| |
10.8 |
| Technology Investment Agreement between RTI and U.S. Department of Defense dated July 1, 2020(12) | |
10.9 |
| ||
|
|
| |
10.10 |
| ||
|
|
| |
14 |
| Retractable Technologies, Inc. Code of Business Conduct and Ethics(15) | |
|
|
| |
31.1 |
| ||
|
|
| |
31.2 |
| ||
|
|
| |
32 |
| ||
|
|
| |
101 |
| The following materials from this report, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) Balance Sheets as of December 31, 2021, and 2020, (ii) the Statements of Operations for the years ended December 31, 2021, 2020, and 2019, (iii) the Statements of Changes in Stockholders’ Equity for the years ended December 31, 2021, 2020, and 2019, (iv) the Statements of Cash Flows for the years ended December 31, 2021, 2020, and 2019, and (v) Notes to Financial Statements.(19) | |
104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded in the Inline XBRL document and included in Exhibit 101). | ||
(1)Incorporated herein by reference to RTI’s Form 10-K filed on March 31, 2021
(2)Incorporated herein by reference to RTI’s Form 8-K filed on May 13, 2010
(3)Incorporated herein by reference to RTI’s Form 10-K filed on March 31, 2021
(4)Filed herewith
22
(5)Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2008
(6)Incorporated herein by reference to RTI’s Registration Statement on Form 10-SB filed on June 23, 2000
(7)Incorporated herein by reference to RTI’s Form 10-K filed on March 31, 2009
(8)Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2012
(9)Incorporated herein by reference to RTI’s Form 8-K filed on November 18, 2021
(10)Incorporated herein by reference to RTI's Form 10-Q filed on November 14, 2014
(11)Incorporated herein by reference to RTI’s Schedule TO filed on October 17, 2008
(12)Incorporated herein by reference to RTI’s Form 10-Q filed on November 16, 2020
(13)Incorporated herein by reference to RTI’s Form 8-K filed October 5, 2020
(14)Incorporated herein by reference to RTI’s Schedule 14A filed March 31, 2021
(15)Incorporated herein by reference to RTI’s Form 8-K filed on August 17, 2020
(16)Filed herewith
(17)Filed herewith
(18)Filed herewith
(19)Filed herewith
(c)Excluded Financial Statement Schedules: None
Item 16. Form 10-K Summary.
None.
23
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| RETRACTABLE TECHNOLOGIES, INC. |
|
| (Registrant) |
|
|
|
| By: | /s/ Thomas J. Shaw |
|
| THOMAS J. SHAW |
|
| CHAIRMAN, PRESIDENT, AND |
|
| CHIEF EXECUTIVE OFFICER |
|
|
|
|
| March 31, 2022 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ John W. Fort III |
| JOHN W. FORT III |
| VICE PRESIDENT, CHIEF FINANCIAL OFFICER, PRINCIPAL ACCOUNTING OFFICER, TREASURER, AND DIRECTOR |
|
|
| March 31, 2022 |
|
|
| /s/ Amy Mack |
| AMY MACK |
| DIRECTOR |
|
|
| March 31, 2022 |
|
|
| /s/ Marco Laterza |
| MARCO LATERZA |
| DIRECTOR |
|
|
| March 31, 2022 |
|
|
| /s/ Walter O. Bigby, Jr. |
| WALTER O. BIGBY, JR. |
| DIRECTOR |
|
|
| March 31, 2022 |
|
|
| /s/ Darren E. Findley |
| DARREN E. FINDLEY |
| DIRECTOR |
|
|
| March 31, 2022 |
24
Similar companies
See also STRYKER CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also BECTON DICKINSON & CO - Annual report 2022 (10-K 2022-09-30) Annual report 2023 (10-Q 2023-06-30)
See also 3M CO - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also BOSTON SCIENTIFIC CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-03-31)
See also RESMED INC - Annual report 2023 (10-K 2023-06-30) Annual report 2023 (10-Q 2023-09-30)
