SURMODICS INC - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended September 30, 2022
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from ________ to ________
|
Commission File Number 0-23837
Surmodics, Inc.
(Exact name of Registrant as specified in its Charter)
Minnesota |
41-1356149 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
9924 West 74th Street Eden Prairie, Minnesota |
55344 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (952) 500-7000
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.05 par value |
|
SRDX |
|
Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ No ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such
files). Yes ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☒ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
☐ |
Emerging growth company |
☐ |
|
|
|
|
|
|
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant as of March 31, 2022 was approximately $613 million (based on the closing price of the Registrant’s Common Stock on such date).
The number of shares of Registrant’s Common Stock outstanding as of November 18, 2022 was 14,040,000.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for the Registrant’s 2023 Annual Meeting of Shareholders are incorporated by reference into Part III.
TABLE OF CONTENTS
|
Page |
|
3 |
||
|
|
|
Part I |
||
|
||
Item 1. |
5 |
|
|
19 |
|
Item 1A. |
21 |
|
Item 1B. |
32 |
|
Item 2. |
32 |
|
Item 3. |
32 |
|
Item 4. |
32 |
|
|
|
|
Part II |
||
|
||
Item 5. |
33 |
|
Item 6. |
34 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
35 |
Item 7A. |
48 |
|
Item 8. |
49 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
83 |
Item 9A. |
83 |
|
Item 9B. |
83 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
84 |
|
|
|
Part III |
||
|
||
Item 10. |
84 |
|
Item 11. |
84 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
84 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
84 |
Item 14. |
84 |
|
|
|
|
Part IV |
||
|
||
Item 15. |
85 |
|
Item 16. |
88 |
|
|
|
|
89 |
||
2
Forward-looking Statements
Certain statements contained in this Form 10-K, or in other reports of the Company and other written and oral statements made from time to time by the Company, do not relate strictly to historical or current facts. As such, they are considered “forward-looking statements” that provide current expectations or forecasts of future events. These forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, the expected results of clinical studies, future clinical studies, and their potential timing; our strategies for growth, including our ability to sign new license agreements, conduct clinical evaluations, complete process and manufacturing validations, and bring new products to market; planned limited market evaluations for our products; the development of future products and their anticipated attributes; regulatory submissions and approvals; our intent to pursue certain regulatory actions, including to expand the field of use for our thrombectomy products; the potential impact of U.S. Food and Drug Administration (“FDA”) communications; our initiations for product evaluation activities; potential future milestone payments related to our SurVeil™ drug-coated balloon (“DCB”); revenue potential related to the potential commercial launch of the SurVeil DCB; future commercialization of our other DCB products; potential partnership opportunities for our DCB products; future revenue growth, our longer-term valuation-creation strategy, and our future potential; plans for future clinical investment in new products; potential future disease rates; future opportunities and goals related to new product offerings; future gross margins and operating expenses; estimated future amortization expense; expectations regarding operating expenses and interest expense; recognition of unrecognized compensation costs; anticipated patent expirations and their potential impacts on our royalties revenue; potential future customer actions; research and development plans and expenses, including the estimated cost associated with the TRANSCEND clinical trial; anticipated cash requirements; future cash flow and sources of funding, and their ability together with existing cash, cash equivalents, and investments to provide liquidity sufficient to meet our cash needs and fund our operations and planned capital expenditures for the next twelve months; future property and equipment investment levels; expectations regarding declaring or paying dividends; plans regarding our securities investments and the potential impact of interest rate fluctuations; expectations regarding the maturity of debt; the impact of potential lawsuits or claims; where our manufacturing activities will take place for various categories of products; the impact of potential change in raw material prices, sources of raw materials and our ability to manufacture raw materials ourselves; the impact of Abbott and Medtronic, as well as other significant customers; our ability to recognize the expected benefits of our acquisitions; our strategic transformation to become a provider of vascular intervention medical device products; future income tax expense (benefit), including from the Coronavirus Aid, Relief and Economic Security Act (the "CARES Act"); the future impact of off-balance sheet arrangements and contractual obligations; and the impact of the adoption of new accounting pronouncements. Without limiting the foregoing, words or phrases such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “possible,” “project,” “will” and similar terminology, generally identify forward-looking statements. Forward-looking statements may also represent challenging goals for us. These statements, which represent our expectations or beliefs concerning various future events, are based on current expectations that involve a number of risks and uncertainties that could cause actual results to differ materially from those of such forward-looking statements. We caution that undue reliance should not be placed on such forward-looking statements, which speak only as of the date made. Some of the factors which could cause results to differ from those expressed in any forward-looking statement are set forth under “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K. We disclaim any intent or obligation to update publicly these forward-looking statements, whether because of new information, future events or otherwise.
Although it is not possible to create a comprehensive list of all factors that may cause actual results to differ from our forward-looking statements, such factors include, among others:
3
Many of these factors are outside our control and knowledge and could result in increased volatility in period-to-period results. Investors are advised not to place undue reliance upon our forward-looking statements and to consult any further disclosures by us on this subject in our filings with the SEC.
4
PART I ITEM 1. BUSINESS. OVERVIEW |
|
Surmodics, Inc. (referred to as “Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of performance coating technologies for intravascular medical devices and chemical and biological components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics also develops and commercializes highly differentiated vascular intervention medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface modification and drug-delivery coating technologies, along with its device design, development and manufacturing capabilities. The Company’s mission is to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minnesota.
SURMODICS’ REPORTABLE SEGMENTS:
MEDICAL DEVICE |
|
IN VITRO DIAGNOSTICS (“IVD”) |
Manufacture of performance coatings, including surface modification coating technologies to improve access, deliverability and predictable deployment of medical devices and drug-delivery coating technologies to provide site-specific drug-delivery from the surface of a medical device, with end markets that include coronary, peripheral, neuro-vascular and structural heart, among others. Manufacture of vascular intervention medical devices, including drug-coated balloons, mechanical thrombectomy devices, and radial access balloon catheters and guide sheaths. |
|
Manufacture of chemical and biological components used in in vitro diagnostic immunoassay and molecular tests within the diagnostic and biomedical research markets. Component products include protein stabilizers, substrates, surface coatings and antigens. |
SURMODICS’ PRIMARY REVENUE SOURCES:
PRODUCT SALES |
|
ROYALTIES & LICENSE FEES |
|
RESEARCH & DEVELOPMENT |
• IVD chemical and biological components, including: protein stabilizers, substrates, surface coatings and antigens to the diagnostic and biomedical research markets (IVD segment) • Performance coating reagents, the chemicals used in performance coatings by licensees (Medical Device segment) • Vascular intervention medical devices and related products to original equipment manufacturer suppliers and distributors, as well as directly to healthcare providers (Medical Device segment) |
|
• Performance coating royalties from licensing of our proprietary performance coating technologies to medical device manufacturers (Medical Device segment) • SurVeil™ DCB license fees associated with exclusive worldwide commercialization rights pursuant to our Development and Distribution Agreement with Abbott Vascular, Inc. (Medical Device segment) |
|
• Commercial development feasibility services and contract coating services (Medical Device segment) • Commercial development services (IVD segment) |
Revenue fluctuates from quarter to quarter depending on, among other factors: our customers’ success in selling products incorporating our technologies; the occurrence of milestone events under our development contracts; the timing of introductions of licensed products by our customers and proprietary products by us and our distributors; the timing of introductions of products that compete with our, and our customers’, products; the number and activity level associated with customer development projects; the number and terms of new license agreements that are finalized; and the value of reagent chemicals, medical device and diagnostic products sold to our customers.
5
The information below provides an overview of the principal products, services and markets for each of our two reportable segments. The discussion of other aspects of our business, including patents and proprietary rights, significant customers, manufacturing, government regulation, and our human capital, applies to our business in general, and we describe material segment information within these sections where relevant.
MEDICAL DEVICE SEGMENT |
Our Medical Device segment consists of two interrelated product platforms:
OVERVIEW: VASCULAR INTERVENTION MEDICAL DEVICES |
MEDICAL DEVICE SEGMENT |
Our strategy is to develop a portfolio of highly differentiated medical devices for vascular interventional treatment. We invest in the development and commercialization of devices that serve large, under-penetrated markets; address unmet clinical needs; improve clinical outcomes for patients; and reduce procedure costs. Our pipeline of vascular intervention medical device products under development and recently commercialized includes the following primary platforms:
In addition to these primary platforms, our device manufacturing operations include:
In addition, we leverage our proprietary balloon catheter technology to deliver contract-manufactured balloon catheter products to original equipment manufacturers (“OEMs”) on a limited scale.
We commercialize device products using two strategies:
For all of our products under development, as further described under the caption “Government Regulation” below, the expected timing and potential success of regulatory approval and commercialization for the products pending regulatory approval can vary greatly given the significant uncertainty inherent in the product development and regulatory approval processes.
6
Vascular Intervention Medical Devices – Drug Coated Balloons (“DCBs”) |
MEDICAL DEVICE SEGMENT |
We have leveraged our performance coating technologies to successfully develop multiple DCB devices for use in vascular interventions for the treatment of PAD. DCBs are used by physicians to expand the diameter (lumen) of a narrowed vessel, thus improving or restoring blood flow. The drug coating helps to prevent the vessel from narrowing again (restenosis) after treatment. PAD is a serious and under-diagnosed circulatory condition caused by build-up of arterial plaque, most commonly in the legs. Over 8 million Americans are affected by PAD, which increases risk of coronary artery disease, heart attack and stroke, and can impair the ability to walk. If left untreated, PAD can lead to gangrene and limb amputation.
The following is a brief description of each of these devices and their stage of clinical development, with additional information about each device provided further below.
Our DCB products are required to go through clinical studies in order for us to obtain regulatory approval or clearance to market the product in the U.S. Each clinical study includes one or more primary endpoints, which measure the effectiveness and/or safety of a device based on the product’s ability to achieve one or more pre-specified outcomes. Primary endpoints are selected based on the proposed intended use of the medical device. A pivotal trial is a definitive study designed to gather evidence to evaluate the safety and effectiveness of a product prior to its marketing.
SurVeil DCB. Our SurVeil product is a paclitaxel-coated DCB to treat PAD in the upper leg (superficial femoral artery). The SurVeil DCB is a next-generation device that utilizes best-in-class technology for the treatment of PAD, including a proprietary paclitaxel drug-excipient formulation for a durable balloon coating manufactured using an innovative process to improve coating uniformity. The design of the SurVeil DCB is intended to provide more uniform drug distribution, better efficiency of drug transfer, and fewer downstream particulates and downstream embolization. Abbott has exclusive worldwide commercialization rights for the SurVeil DCB under a Development and Distribution Agreement (the “Abbott Agreement”), as further discussed below.
The development of our SurVeil DCB started in fiscal 2016 and has been a major component of our vascular intervention product strategy. Below is a summary of our clinical and regulatory progress related to the SurVeil DCB.
7
In January 2021, we announced the TRANSCEND 12-month pivotal clinical trial met both the primary safety and primary efficacy endpoints, and the SurVeil DCB was found to be non-inferior in those endpoints to the Medtronic IN.PACT® Admiral® DCB, while delivering a substantially lower drug dose.
In November 2022, we announced TRANSCEND 24-month data demonstrated comparable, sustained clinical outcomes between the SurVeil DCB and IN.PACT® Admiral® DCB cohorts through 24 months. Functional outcomes for treated patients also demonstrated continuous improvement at the two-year point.
In October 2022, we submitted a complete response to FDA comments on our PMA application for the SurVeil DCB, including certain additional data requested by the Agency. Unless and until FDA approval has been obtained, our SurVeil DCB may not be offered for commercial sale in the U.S.
Abbott Agreement. In fiscal 2018, we entered into the Abbott Agreement, which provided Abbott with exclusive worldwide commercialization rights for the SurVeil DCB. Pursuant to the terms of the Abbott Agreement, the Company has received, as of September 30, 2022, upfront and milestone payments totaling $60.8 million. The Company may receive an additional $30 million contingent milestone payment, pursuant to the terms of the Abbott Agreement, upon PMA of our SurVeil DCB by the FDA. The milestone payment is reduced to $27 million if PMA is received after December 31, 2022, but before June 30, 2023, and to $24 million if PMA is received on or after June 30, 2023, pursuant to the terms of the Abbott Agreement.
Surmodics is responsible for conducting all necessary clinical trials and other activities required to achieve U.S. and E.U. regulatory clearances for the SurVeil DCB, including completion of the ongoing TRANSCEND pivotal clinical trial. Expenses related to these activities are paid by Surmodics. Abbott and Surmodics participate on a joint development committee charged with providing guidance on the Company’s clinical and regulatory activities related to the SurVeil DCB product. Upon commercial launch of the SurVeil DCB by Abbott, Surmodics will be responsible for manufacturing clinical and commercial quantities of the product and will realize revenue from product sales to Abbott, as well as a share of profits resulting from sales to third parties.
Paclitaxel Long-term Mortality Signal. On March 15, 2019, the FDA issued a communication (the “FDA communication”) to healthcare providers about the potential for increased long-term mortality after use of paclitaxel-coated balloons and paclitaxel-eluting stents (collectively “paclitaxel-coated products”) to treat PAD in the femoropopliteal artery. The FDA communication updated a previous notification from the FDA on the same topic, which was in response to meta-analysis of randomized trials published in the Journal of the American Heart Association in December 2018. Subsequently, in August 2019, the FDA issued an update on the use of paclitaxel devices to treat PAD that recommended that physicians discuss the risks and benefits of all available treatment options with their patients. The original meta-analysis that triggered the FDA communication has been criticized for flaws in its methodology. Since that meta-analysis was published, there has been ample data published or presented from large, observational datasets, subgroup analyses from randomized controlled trials, and long-term follow-up from the pivotal paclitaxel-coated products randomized controlled trials, none of which replicated an association between paclitaxel-coated products and mortality. Further, no clear mechanism relating paclitaxel to death has been described and a dose-response relationship between paclitaxel and mortality has been established. Nevertheless, the August 2019 FDA recommendations remain in place. The FDA communication and the potential long-term mortality signal related to the use of paclitaxel-coated devices may adversely affect market acceptance of our paclitaxel-coated DCB products and any revenue we may realize from the commercialization of the SurVeil DCB if the FDA grants approval for the product.
8
Sundance DCB. Our sirolimus-coated Sundance DCB is used for the treatment of below-the-knee PAD, including CLI. CLI is estimated to impact between 2.1 million and 3.8 million Americans, a number that could grow to between 2.4 million and 4.7 million by 2030. Rates of amputation and death are significant for CLI patients, and there are currently no drug-delivery devices approved to treat the condition in the U.S.
Sirolimus has potent anti-inflammatory and anti-proliferative effects to inhibit cell division, without creating vascular toxicity, and has a proven history of safety and efficacy in vascular anatomy. We leveraged our expertise in performance coatings in the innovative design of our Sundance DCB, which in pre-clinical benchtop and animal testing has shown clear advantages over competitive technologies, including superior drug coating durability, higher levels of drug transfer, and a unique ability to achieve sustained therapeutic levels in the tissue.
Below is a summary of our clinical and regulatory progress related to the Sundance DCB.
We are in the process of identifying and evaluating potential partnership opportunities to complete the required pivotal clinical trial, seek regulatory approval and, if approved, commercialize the Sundance DCB.
Avess DCB. Our paclitaxel-coated Avess DCB is used for the treatment of AV fistulae commonly used to deliver hemodialysis in patients with ESRD. It is estimated that approximately 800,000 U.S. patients and nearly five million patients worldwide live with ESRD. In the U.S., an estimated 70% of dialysis patients eventually receive dialysis via AV fistula. Stenosis in AV fistulae is a common problem, and preserving fistula patency is a contributor to a reduction of related significant Medicare system cost, as well as patient satisfaction.
Our Avess DCB includes a proprietary drug-excipient formulation for the balloon coating and is manufactured using a proprietary process to improve coating uniformity. Pre-clinical data for our Avess DCB has shown a three to five times higher target tissue drug concentration, a more evenly distributed and durable drug effect, and lower incidence of downstream drug concentrations compared to control DCBs. In fiscal 2019, we commenced and completed enrollment in a first in-human, 12-patient clinical study of our Avess DCB. In fiscal 2020, initial study results were received and demonstrated promising early safety data and performance insights, with greater than 90% of treated patients free from revascularization at six months.
In fiscal 2021, we completed design verification for the full matrix of balloon sizes for the base balloon catheter for our Avess DCB and began the process validation work on the base catheter. Additionally, the FDA has provided high-level feedback on Avess DCB pivotal clinical trial design considerations. We plan to evaluate our strategy for further clinical investment in the Avess DCB based on the experience we gain from the PMA application process for SurVeil DCB.
Vascular Intervention Medical Devices – Mechanical Thrombectomy |
MEDICAL DEVICE SEGMENT |
We have successfully developed, internally and through acquisitions, two FDA 510(k) cleared mechanical thrombectomy devices for the non-surgical removal of thrombi and emboli (clots) from the peripheral vasculature (legs). We believe that the ease of use, intuitive design and efficient performance of our thrombectomy products make these devices viable first-line treatment options for interventionalists.
9
Our thrombectomy devices represent a core offering within our vascular intervention product strategy, providing the opportunity for:
PAO is the blocking of arteries by clots or plaque, is a peripheral vascular condition commonly associated with CLI. Often, these arterial clots require surgical intervention and have proven difficult to remove with currently available medical device technologies. Depending on the age and magnitude of the occlusion and the viability of the threatened limb, existing treatments for this condition may include catheter directed thrombolysis, surgical embolectomy, and/or percutaneous mechanical thrombectomy. In cases in which the occlusion has caused irreversible damage to the limb, acute limb ischemia can result in the amputation of a lower extremity.
VTE is blood clots in the veins and is an under-diagnosed and serious, yet treatable, medical condition that can cause disability and death. VTE includes deep vein thrombosis (“DVT”), which occurs when a blood clot forms in a deep vein, usually in the lower leg, thigh, or pelvis, and pulmonary embolism (“PE”), which occurs when a clot breaks loose and travels through the bloodstream to the lungs. VTE affects approximately 1.2 million U.S. patients each year, of which approximately 800,000 are affected by DVT. The current standard of care for treating VTE is conservative medical management with anticoagulant drugs designed to prevent further blood clotting. While anticoagulation remains the most widespread therapy for DVT, interventional treatment has demonstrated the potential for better outcomes in select patients.
We believe our proprietary Pounce arterial and venous thrombectomy devices provide physicians with the opportunity to treat PAD and VTE in a more effective, cost-efficient manner than currently available treatments. The devices offer innovative designs that may reduce the need for the use of thrombolytics. Thrombolytics are often associated with complications, which can include bleeding complications, longer hospital stays and higher cost of treatment. Our Pounce arterial and venous thrombectomy devices are designed to reduce procedure time, efficiently remove large volumes of clot, and eliminate the need for additional external capital equipment, thereby providing an easy-to-use, on-the-table, single-session solution for clinicians.
Pounce Arterial Thrombectomy. Our Pounce Arterial Thrombectomy System, which received FDA 510(k) clearance in fiscal 2020, is a mechanical thrombectomy device intended for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature. The device consists of three components: a 5 Fr basket delivery catheter, a basket wire, and a funnel assembly. After the basket wire is delivered distal to the location of the thrombus, two nitinol self-expanding baskets are deployed to collect and entrain the clot into a funnel-shaped nitinol wire mesh. With the clot entrained, the funnel assembly is then collapsed into a 7 Fr procedure guide sheath through which the clot is withdrawn and removed from the body. Physician feedback indicates the Pounce Arterial Thrombectomy System is capable of achieving positive outcomes with minimal blood loss and with minimal use of thrombolytics. The device offers an intuitive, grab-and-go design to simplify setup and reduce the physician’s learning curve.
Pounce Venous Thrombectomy. Our Pounce Venous Thrombectomy System, which received FDA 510(k) clearance in fiscal 2021, is a mechanical thrombectomy catheter for use in venous vascular beds that is specifically designed to remove large, mixed-morphology blood clots commonly found with VTE. The Pounce Venous Thrombectomy System has also received CE Mark approval, which is a prerequisite for commercialization in the E.U. The device’s dual-action technology features a constant spring tension basket, which provides optimal wall apposition over a range of vessel diameters, to engage and collect the clot, while the motor-driven Archimedes screw macerates and removes the collected clot. As with our Pounce arterial device, the Pounce Venous Thrombectomy System is intuitive and approachable to facilitate widespread adoption, with a low learning curve for the physician.
We acquired the venous thrombectomy device technology with our fiscal 2021 acquisition of Vetex Medical Limited (“Vetex”), which was privately held and is based in Galway, Ireland. We acquired Vetex with an upfront cash payment of $39.9 million. Additional payments of up to $7 million, of which $3.5 million of which are guaranteed, may be made upon achievement of certain product development and regulatory milestones.
Limited market evaluations for the Pounce Venous Thrombectomy System are planned for fiscal 2023 to obtain physician feedback across a variety of cases and clinical conditions. The real-world feedback obtained through these evaluations will help inform any potential design enhancements that could benefit physicians and patients, while optimizing commercial success.
The FDA requires specific indications for devices to be marketed for treatment of certain aspects of VTE, such as DVT and PE. The Pounce Venous Thrombectomy System is indicated for mechanical de-clotting and controlled and selected infusion of physician specified fluids, including thrombolytics, in the peripheral vasculature. The device currently is not indicated for the treatment of DVT or PE. We intend to pursue development and regulatory actions that would expand the field of use for our thrombectomy products, which may include specific indications, and which may include DVT and PE.
10
Vascular Intervention Medical Devices – Radial Access |
MEDICAL DEVICE SEGMENT |
We have successfully developed and secured FDA 510(k) regulatory clearance for our Sublime™ portfolio of devices designed for vascular intervention via radial (wrist) access that can also be used via femoral (thigh) access. Our Sublime devices are used to access and treat narrowed arteries both above and below the knee, commonly associated with PAD. During fiscal 2022, we established a direct salesforce and commenced commercial sales of our Sublime device portfolio to hospitals and clinics. These radial access devices include:
Our Sublime device portfolio is unique in that each of these devices are purpose built for above- and below-the-knee peripheral interventions that can employ both a conventional transfemoral approach and a transradial approach. Our Sublime guide sheath performance is enhanced by our latest generation hydrophilic coating. We believe that radial access procedures offer significant benefits by improving patient comfort, reducing recovery and ambulation times, and potentially lowering access site complications. Our Sublime device portfolio meets an unmet clinical need by providing the longer, lower-profile devices that are robust enough to deliver treatment from the wrist all the way to the pedal loop in the foot.
We believe the Sublime device portfolio is uniquely positioned to lead the market for dedicated devices that facilitate a radial-to-peripheral approach. Below are a few of the unique advantages of our Sublime devices.
OVERVIEW: PERFORMANCE COATINGS |
MEDICAL DEVICE SEGMENT |
Surmodics’ industry-leading performance coatings are used in a minimally invasive procedure every minute of every day. Surmodics’ surface-enhancing performance coatings add differentiated value to more than 150 medical, biotechnology and pharmaceutical product families worldwide. Our customers use Surmodics’ performance coatings to enable, optimize and differentiate their device products. These performance coatings include:
Surmodics generates royalties revenue by licensing our performance coating technologies to medical device manufacturers, product revenue from sales to licensees of the chemical reagents used in coatings, and R&D revenue from commercial development feasibility services and contract coating services.
11
Our performance coatings are differentiated by their flexibility, durability and ease of use. In terms of flexibility, coatings can be applied to many kinds of surfaces and can immobilize a variety of chemical, pharmaceutical and biological agents. Additionally, the surface modification process can be tailored to provide customers with the ability to improve their devices’ performance by choosing the specific coating properties desired for particular applications. Our performance coating technologies can also be combined to deliver multiple surface-enhancing characteristics on the same device.
The continuing trend toward minimally invasive surgical procedures, which often employ catheter-based delivery technologies, has increased the demand for hydrophilic (i.e., lubricious or slippery) coatings and other coating technologies, including drug-delivery coatings. For example, stents, particularly drug-eluting stents, have significantly reduced the need for repeat intravascular procedures or more invasive cardiac bypass surgery. Transcatheter heart valve repair or replacement via a minimally invasive catheter-based system has enabled the treatment of patients suffering from heart valve disease who are too ill to undergo open-heart surgery.
Hydrophilic Coatings. Our proprietary PhotoLinkTM coating technology (“PhotoLink Technology”) is a versatile, easily applied, coating technology that modifies medical device surfaces by creating covalent bonds between device surfaces and a variety of chemical agents. PhotoLink Technology can impart many performance-enhancing characteristics, such as advanced lubricity (slipperiness or smoothness) and hemocompatibility (preventing blood clot formation), when bound onto surfaces of medical devices or other biological materials without materially changing the dimensions or other physical properties of devices.
PhotoLink Technology reagents can be applied to a range of substrates. The coating formulations are easily applied to the material surface by a variety of methods including, but not limited to, dipping, spraying, roll-coating and ink-jetting. We continue to expand our proprietary reagent portfolio for use by our customers. These reagents enable our customers to develop novel surface features for their devices, satisfying the expanding healthcare industry requirements. We are also continually working to expand the list of materials that are compatible with our surface modification and device drug-delivery reagents. Additionally, we develop coating processes and coating equipment to meet the device quality, manufacturing throughput, and cost requirements of our customers.
The PhotoLink Technology coating process is relatively simple to use and is easily integrated into the customer’s manufacturing operations. In addition, the process does not subject the coated products to harsh chemical or temperature conditions, produces no hazardous byproducts, and does not require lengthy processing or curing time. Further, coatings incorporating the PhotoLink Technology are generally compatible with accepted sterilization processes, so the surface attributes are not lost when the medical device is sterilized.
The latest generation of our Photolink Technology, our SereneTM hydrophilic coating platform, optimizes lubricity and durability, while significantly reducing particulates generation. This latest generation, PhotoLink Technology-enabled coating has demonstrated excellent lubricity on a wide range of substrates and has been used on FDA-cleared coronary, peripheral and structural heart devices.
Drug-delivery Coatings. Our device drug-delivery coating technologies allow therapeutic drugs to be incorporated within our proprietary polymer matrices to provide controlled, site-specific release of the drug into the surrounding environment. The drug release can be tuned to elute quickly (within minutes to a few days) or slowly (from several months to over a year), illustrating the wide range of release profiles that can be achieved with our coating systems. On a wide range of devices, drug-eluting coatings can help improve device performance, increase patient safety, and enable innovative new treatments. DCBs are a typical example of short-term use drug-delivery devices. An example of longer-term drug-delivery devices is drug eluting stents. We work with companies in the medical device and biotechnology industries to develop specialized coatings that allow for the controlled release of drugs from device surfaces. We see at least three primary areas with strong future potential:
12
Performance Coatings – Licensing Arrangements |
MEDICAL DEVICE SEGMENT |
We commercialize our performance coating technologies primarily through licensing arrangements with medical device manufacturers. We believe this approach allows us to focus our resources on further developing new technologies and expanding our licensing activities. Many of our technologies have been designed to allow manufacturers to implement them easily into their own manufacturing processes so customers can control production and quality internally without the need to send their products to a contract manufacturer. We generate the largest proportion of our revenue through licensing arrangements. Royalties revenue represented 30%, 29% and 30% of our total revenue in fiscal 2022, 2021 and 2020, respectively. Revenue from these licensing arrangements typically includes royalties based on a percentage of licensees’ product sales, minimum royalties and milestone payments. In both fiscal 2022 and 2021, we saw double-digit year-over-year growth in revenue associated with our latest generation Serene hydrophilic coating technology driven by customer product launches and resulting market share increases associated with the customer device applications that incorporate this latest generation coating technology. The increase in revenue associated with our Serene hydrophilic coating technology offset decreases in revenue associated with our fourth-generation PhotoLink hydrophilic coating technology as our licenses for the fourth-generation technology transitioned from higher patent royalty rates to lower know-how royalty rates when the patents for the fourth-generation technology expired, which generally occurred in the first quarter of our fiscal 2020.
The licensing process for our performance coating technologies begins with the customer specifying a desired product feature to be created, such as lubricity or drug delivery. Because each device and coating application is unique, we routinely conduct a feasibility study to qualify each new potential product application, often generating commercial development revenue. Feasibility studies can range in duration from several months to a year. After we complete a feasibility study, our customers cannot market their product until they receive regulatory approval. As further described under the caption “Government Regulation,” the regulatory approval process varies in each country and ranges from several months to four or more years. At any time prior to a customer’s commercial launch, a license agreement may be executed granting the licensee rights to use our technology. We often support our customers by providing coating assistance for parts required in animal tests and human clinical trials. Typically, we complete a technology transfer to most customers which enables those customers to apply the coating at their own facilities. We also generate revenue from reagent chemical product sales to licensees for use in their coating processes, as well as from providing contract coating services.
License agreement terms are generally for a specified number of years or our patent’s life, whichever is longer, although a license generally may be terminated by the licensee for any reason with advance written notice. In cases where the royalty obligation extends beyond the life of the applicable patent, it is because the license also includes rights to our know-how or other proprietary rights. Under these circumstances, the royalty obligation typically continues at a reduced royalty rate for a specified number of years, generally tied to the date on which the licensee’s medical device product was first sold.
Our license agreements may include certain license fees and/or milestone payments. Substantially all our licensed performance coatings technology applications are nonexclusive, allowing us to license each technology to multiple customers. Moreover, even exclusive performance coatings technology licenses generally are limited to a specific “field of use,” allowing us the opportunity to further license technology to other customers. The royalty rate on a substantial number of the coatings agreements has traditionally been in the range of two to three percent, but there are certain contracts with lower or higher rates. In certain agreements, our royalty is based on an agreed-upon amount per unit. License fees, milestone payments, and royalty rates are based on various factors, including the licensed product’s or technology’s stage of development, the perceived value of our technology to the customer’s product, the size of the potential market, and whether the arrangement is exclusive or nonexclusive. Our agreements often incorporate a minimum royalty to be paid by the licensee. Royalty payments generally commence one quarter after the customer’s actual product sales occur because of the delay in reporting sales by our licensees. We estimate and recognize sales-based royalties revenue from our performance coating licensees in the same quarter that the underlying customer product sale occurs.
We have over 150 licensed product classes (customer products utilizing Surmodics technology) already in the market generating royalties and greater than 100 customer product classes incorporating our technology in various stages of pre-commercialization.
Under our performance coating technology license agreements, the responsibility for securing regulatory approval for and ultimately commercializing these products rests with our customers. Our reliance on our customers in this regard and the potential risks to our operations as a result are discussed in “Risk Factors” in Part I, Item IA of this Annual Report on Form 10-K. Moreover, we are often contractually obligated to keep the details concerning our customers’ R&D efforts (including the timing of expected regulatory filings, approvals and market introductions) confidential.
13
Our licensing agreements generally require us to keep our customers’ identities confidential, unless they approve of such disclosure. Licensed customers that allow the use of their name include: Abbott Laboratories and Abbott Vascular, Inc., Boston Scientific Corporation (“Boston Scientific”), Cook Medical, Cordis Corporation, Covidien PLC (a subsidiary of Medtronic), Edwards Lifesciences Corporation, Evalve, Inc. (a subsidiary of Abbott), ev3 Inc. (a subsidiary of Medtronic), Medtronic, OrbusNeich Medical, Inc., and Spectranetics Corporation (a subsidiary of Koninklijke Philips N.V.).
Performance Coatings – R&D Services for Customers |
MEDICAL DEVICE SEGMENT |
For our medical device coatings customers, we have distinct, specifically-dedicated R&D facilities and personnel to support delivery of R&D services. We work with our customers to integrate the best possible surface modification and device drug-delivery technologies with their products, not only to meet their performance requirements, but also to perform services quickly so that the product may reach the market ahead of the competition. To quickly solve problems that might arise during the development and optimization process, we offer extensive capabilities in analytical chemistry and surface characterization within our R&D organization. Our state-of-the-art instrumentation and extensive experience allow us to test the purity of coating reagents, to monitor the elution rate of drug from coatings, to measure coating thickness and smoothness, and to map the distribution of chemicals throughout coatings. We believe our capabilities in this area exceed those of our competitors. Our R&D staff support our business development staff and business units in performing feasibility studies, as well as providing technical assistance to existing and potential customers. These services, which generate research, development and other revenue, include optimizing the relevant technologies for specific customer applications; supporting clinical trials; training customers; and integrating our technologies and know-how into customer manufacturing operations.
Competition |
MEDICAL DEVICE SEGMENT |
We are developing and commercializing differentiated vascular intervention medical devices that integrate our performance coatings, catheter, balloon and other proprietary technologies. This high degree of differentiation is strategically designed to capture market share in a highly competitive, dynamic industry. Our vascular intervention products compete with the global leaders in the vascular medical device market. We believe our vascular intervention products will be competitive on the basis of their safety and efficacy as a result of the innovative design and differentiated coating and device design technology. We believe these innovations will enable our vascular intervention products to demonstrate improvements in patient outcomes through reduced invasiveness compared to other devices used for comparable procedures.
We believe that the intense competition within the medical device market creates opportunities for our performance coating technologies as medical device manufacturers seek to differentiate their products through new enhancements or to remain competitive with enhancements offered by other manufacturers. Because a significant portion of our revenue depends on royalties derived from our customers’ medical device product sales incorporating our performance coating technologies, we are also affected by competition within the markets for such devices. As we typically license our performance coating technologies on a non-exclusive basis, we benefit by offering our technologies to multiple competing manufacturers of a device. However, competition in the medical device market could also have an adverse effect on us. While we seek to license our coatings products to established manufacturers, in certain cases, our performance coatings licensees may compete directly with larger, dominant manufacturers with extensive product lines and greater sales, marketing and distribution capabilities.
We also are unable to control other factors that may impact commercialization of our vascular intervention products and licensees with medical devices that utilize our performance coatings, such as regulatory approval, marketing and sales efforts of our customers and licensees, and competitive pricing pressures within the particular market. Many of our existing and potential competitors have greater financial, technical and marketing resources than we have.
The ability of performance coating technologies to improve the performance of medical devices and drugs and to enable new product categories has resulted in increased competition in these markets. Some of our competitors offer device drug-delivery technologies, while others specialize in lubricious or hemocompatible coating technology. Some of these companies target cardiovascular, peripheral or other medical device applications. In addition, because of the many product possibilities afforded by performance coatings, many of the large medical device manufacturers have developed, or are engaged in efforts to develop, internal competency in the area of performance coatings, including drug-delivery technologies.
14
We differentiate ourselves from our performance coatings competitors by providing what we believe is a high value-added approach. We have a proven track record of our customers successfully navigating the regulatory approval process with devices utilizing our enabling technology. We believe that the primary factors customers consider in choosing a particular technology include performance (e.g., flexibility, ability to fine tune drug elution profiles, biocompatibility), ease of manufacturing, time-to-market, intellectual property protection, ability to produce multiple products from a single process, compliance with manufacturing regulations, ability to manufacture clinical and commercial products, customer service and total cost of goods (including manufacturing process labor). We believe our technologies deliver exceptional performance in these areas, allowing us to compete favorably with respect to these factors. With respect to our licensed performance coating technologies, we believe that the cost and time required to obtain the necessary regulatory approvals significantly reduces the likelihood of a customer changing the manufacturing process it uses once a device or drug has been approved for sale.
R&D Strategy |
MEDICAL DEVICE SEGMENT |
Our significant R&D investments over the past several years reflect our ongoing commitment to strengthen our proprietary product pipeline and broaden our capacity for medical device R&D activities. In fiscal 2022, 2021 and 2020, consolidated R&D expense as a percentage of consolidated revenue was 51%, 45% and 53%, respectively. In fiscal 2022, R&D expense was largely associated with our investments in vascular intervention product development; clinical trials for DCBs; and in R&D and regulatory infrastructure, facilities and personnel. R&D expenses primarily consist of research, development, clinical and regulatory activities related to the design, development and commercialization of our products, as well as costs associated with our contract coating services R&D services revenue.
We intend to continue our development efforts to expand our proprietary medical device offerings, including advancing our performance coating technologies to better meet these needs across multiple medical markets and to capture more of the final product value. We anticipate R&D expenses will continue to be significant in fiscal 2023 and beyond, primarily related to medical device product development, including continued investment in our Pounce and Sublime platforms.
To strengthen our licensing business model, R&D personnel and facilities for our vascular intervention products are fully segregated from those for our performance coatings to preserve confidential information of our coatings customers (licensees). In our Medical Device segment, we conduct R&D in multiple facilities. Two of those separate facilities are located in Eden Prairie, Minnesota. Our R&D facilities are as follows:
We have robust procedures to ensure that we protect our coatings customers’ (licensees) intellectual property and avoid conflicts of interest. R&D personnel have specific roles and are part of distinct teams, clearly segregated between: (i) performance coatings technologies R&D, including customer development to support our licensing partnership model and (ii) internal R&D activities to further advance our vascular intervention product portfolio. Our procedures include strict restrictions for physical access to customers’ products and records and limitations on computer file access based on an R&D team member’s role.
15
IN VITRO DIAGNOSTICS SEGMENT |
Surmodics’ In Vitro Diagnostics (“IVD”) segment provides leading in vitro diagnostic companies with the critical components for developing sensitive, reproducible immunoassays to enable our customers’ diagnostic tests to detect the absence or presence of disease. We develop, manufacture and sell chemical and biological components for in vitro diagnostic immunoassay tests and molecular diagnostic tests for the diagnostic and biomedical research markets.
Our portfolio of IVD chemical and biological component products includes:
Our IVD products address the following customer needs:
Customer R&D. The sales cycle for our IVD products generally begins when an IVD company initiates the process to develop a new, or improve a current, diagnostic test. During product development, these companies seek to source the test’s critical components with reagents that it produces internally or with reagents from a supplier, such as Surmodics.
16
As IVD tests are developed and various reagents are tested, companies will generally seek to optimize the sensitivity (false negative reductions), specificity (false positive reductions), speed (time from sample to results), convenience (ideally as few steps as possible), and cost effectiveness. Upon regulatory approval or clearance, the customer’s diagnostic test can be sold in the marketplace. It may take several years after approval or clearance for the test to achieve peak market share and optimize Surmodics’ revenue.
New Product R&D. Our R&D efforts to grow our IVD business segment include identifying and addressing unmet needs that exist in the global IVD marketplace. Our pipeline of IVD products includes components for immunoassay and molecular diagnostic applications, such as new protein stabilizers, detection technologies, accessory reagents and surface coatings that have the potential to add greater sensitivity, specificity, speed, convenience, and lower cost for IVD test manufacturers.
Competition. The diagnostics market is highly fragmented. In the product lines in which we compete, we face an array of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. Some of our competitors have substantially more capital resources, marketing experience, R&D resources and production facilities than we do. We believe that our products compete on performance, stability (shelf life), sensitivity (lower levels detected, faster results), consistency and price. We believe that our continued competitive success will depend on our ability to gain market share, to develop or acquire new proprietary products, obtain patent or other protection for our products and successfully market our products directly or through partners.
OTHER FACTORS IMPACTING OUR OPERATIONS |
Patents and Proprietary Rights |
OTHER FACTORS IMPACTING OUR OPERATIONS |
Patents and other forms of proprietary rights are an essential part of Surmodics’ business. We aggressively pursue patent protection covering the proprietary technologies that we consider strategically important to our business. In addition to seeking patent protection in the U.S., we also generally file patent applications in European countries and, on a selective basis, other foreign countries. We strategically manage our patent portfolio in a manner designed to ensure that we have valid and enforceable patent rights protecting our technological innovations. As of September 30, 2022, Surmodics owned or had exclusive rights to 152 issued U.S. patents and 338 issued international patents. As of the same date, we also owned or had exclusive rights to 57 U.S. pending patent applications and 92 foreign pending patent applications.
We have licensed our PhotoLink Technology on a non-exclusive basis to a number of our customers for use in a variety of medical device surface applications, including those described above. In particular, we have 34 issued U.S. patents, three pending U.S. patent applications, 79 issued international patents, and 11 pending international patent applications protecting various aspects of these technologies, including compositions, methods of manufacture and methods of coating devices. The expiration dates for these patents and anticipated expiration dates of the patent applications range from fiscal 2025 to 2039. These patents and patent applications represent distinct families, with each family generally covering a successive generation of the technology, including improvements that enhance coating performance, manufacturability, or other important features desired by our customers. For additional details, refer to the caption “Performance Coatings – Licensing Arrangements” within this section of this Annual Report on Form 10-K.
We also rely upon trade secrets, trademarks and other un-patented proprietary technologies. We seek to maintain the confidentiality of such information by requiring employees, consultants and other parties to sign confidentiality agreements and by limiting access by parties outside the Company to such information. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of this information, or that others will not be able to independently develop such information. Additionally, there can be no assurance that any agreements regarding confidentiality and non-disclosure will not be breached, or, in the event of any breach, that adequate remedies would be available to us.
Significant Customers |
OTHER FACTORS IMPACTING OUR OPERATIONS |
Revenue from Abbott and Medtronic represented approximately 11% and 13%, respectively, of our consolidated revenue for fiscal 2022. Revenue from these customers was generated from multiple products and fields of use, including revenue from the Abbott Agreement, substantially all of which were recognized in our Medical Device segment. No other customer accounted for more than 10% of our consolidated revenue in fiscal 2022.
With respect to our Medical Device segment, revenue from Abbott and Medtronic represented approximately 15% and 18%, respectively, of our Medical Device segment revenue for fiscal 2022, and revenue from one additional customer represented approximately 12% of our Medical Device segment revenue for fiscal 2022. No other customer accounted for greater than 6% of Medical Device segment revenue for fiscal 2022.
17
With respect to our IVD segment, revenue from two customers represented approximately 17% and 12%, respectively, of our IVD segment revenue for fiscal 2022. No other customer accounted for greater than 9% of IVD segment revenue for fiscal 2022.
Manufacturing |
OTHER FACTORS IMPACTING OUR OPERATIONS |
We manufacture the reagent chemicals used in our performance coatings and our IVD products in one of our Eden Prairie, Minnesota facilities. In certain limited circumstances, we also provide contract manufacturing services for our customers, including, for example, coating their medical devices that are intended for pre-clinical and clinical development (including human clinical trials), and products that are sold for commercial use by our customers. We manufacture PTA balloon catheters and microcatheters in our Ballinasloe, Ireland facility, which offers a suite of capabilities, including balloon forming, extrusion, coating, braiding and assembly of finished products. We manufacture our vascular intervention products, Pounce and Sublime, in our Ireland and U.S. facilities. At our Ballinasloe, Ireland manufacturing facility, we perform a limited volume of contract manufacturing of medical devices for our customers.
We attempt to maintain multiple sources of supply for the key raw materials used to manufacture our products. We do, however, purchase some raw materials from single sources, but we believe that additional sources of supply are readily available. Further, to the extent additional sources of supply are not readily available, we believe that we could manufacture such raw materials.
We follow quality management procedures in accordance with applicable regulations and guidance for the development and manufacture of materials and device, biotechnology or combination products that support clinical trials and commercialization. In order to meet our customers’ needs in this area, all of our manufacturing facilities in Eden Prairie, Minnesota and Ballinasloe, Ireland are certified to ISO 13485 and registered with the U.S. FDA as “Contract Manufacturers.” In addition, one of our manufacturing facilities and our warehouse facility in Eden Prairie, Minnesota are certified to ISO 9001.
Government Regulation |
OTHER FACTORS IMPACTING OUR OPERATIONS |
Medical device and in vitro diagnostic products are required to undergo regulatory review processes that are governed by the FDA and other international regulatory authorities. The process of regulatory review and approval is often prolonged, expensive and uncertain. New medical devices can only be marketed in the U.S. after a pre-market notification for 510(k) clearance or a PMA by the FDA. These processes can take anywhere from several months (e.g., for medical device products seeking regulatory approval under the 510(k) clearance process) to several years (e.g., for medical device products seeking regulatory approval under the PMA application process). In the E.U., regulatory approval is signified by the CE Mark, which is generally granted by one of several competent authorities and is based on the submission of a design dossier, a manufacturer validation assessment, a third-party assessment, and review of the design dossier by a “Notified Body.” In 2017, the E.U. authorized a new medical device regulation. The new regulation, which imposes significant additional pre-market and post-market requirements, became effective for devices submitted for CE Mark after May 2021. Medical devices granted CE Mark prior to May 2021 may continue to be sold until May 2024 or until the CE Mark expires, whichever comes first, providing there are no significant changes to the design or intended use of the device.
For our customers’ products that incorporate our performance coating and IVD technologies, the burden of securing regulatory approval typically rests with the customer, as the medical device manufacturer. For our vascular intervention products, including the SurVeil DCB, the burden of securing regulatory approval rests on us, unless we contract with other organizations to pursue such approval.
In support of our customers’ and our own regulatory filings, we maintain various confidential Device Master Files with the FDA and provide technical information to other regulatory agencies outside the U.S. regarding the nature, chemical structure and biocompatibility of our reagents. Our licensees generally do not have direct access to these files. However, they may, with our permission, reference these files in their various regulatory submissions to these agencies. This approach allows regulatory agencies to understand the details of our technologies without our having to share this highly confidential information with our customers.
U.S. legislation allows companies, prior to obtaining FDA clearance or approval to market a medical product in the U.S., to manufacture medical products in the U.S. and export them for sale in international markets. This generally allows us to realize earned royalties sooner and may result in opportunities to market our vascular intervention products in other countries. However, sales of medical products outside the U.S. are subject to international requirements that vary from country to country. The time required to obtain approval for sale internationally may be longer or shorter than that required by the FDA.
Human Capital |
OTHER FACTORS IMPACTING OUR OPERATIONS |
As of September 30, 2022, we had 447 employees, of which 132 were employed outside the U.S., primarily in manufacturing and R&D functions. We are not a party to any collective bargaining agreements.
18
Our success depends upon our ability to retain and attract highly qualified management and technical personnel. Talent management is critical to our ability to execute on our long-term growth strategy. Through our history of technological innovation, we appreciate the importance of retention, growth and development of our employees. We are committed to an inclusive culture which values equality, opportunity, and respect. In support of our inclusive culture, we believe we offer competitive compensation and benefits, including an annual pay gap assessment; provide respectful workplace training to strengthen employee understanding; and strive to recruit a diverse talent pool across all levels of the organization. We are focused on the engagement and empowerment of our employees through demonstration of our foundational values, which we refer to as the five Cs: we have courage to face challenges with determination, honesty and resourcefulness; candor to speak openly and respectfully; collaboration that recognizes teamwork as the key to success; camaraderie that is genuine and supportive; and commitment to our cause.
SEC FILINGS |
We file annual reports, quarterly reports, proxy statements, and other documents with the SEC under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The public may obtain any documents that we file with the SEC at http://www.sec.gov.
We make available, free of charge, copies of our annual report on Form 10-K, proxy statement, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act on our website, www.surmodics.com, as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the SEC. We are not including the information on our website as a part of, or incorporating it by reference into, our Form 10-K.
EXECUTIVE OFFICERS |
As of November 18, 2022, the names, ages and positions of the Company’s executive officers were as follows:
Name |
|
Age |
|
Position |
Gary R. Maharaj |
|
59 |
|
President and Chief Executive Officer |
Timothy J. Arens |
|
55 |
|
Senior Vice President of Finance and Information Technology and Chief Financial Officer |
Charles W. Olson |
|
58 |
|
Senior Vice President and President, Medical Device Coatings |
Teryl L.W. Sides |
|
53 |
|
Senior Vice President and President, Vascular Interventions |
Joseph J. Stich |
|
57 |
|
Senior Vice President Human Resources and President, In Vitro Diagnostics |
Gordon S. Weber |
|
59 |
|
Senior Vice President of Legal, General Counsel and Secretary |
Gary R. Maharaj joined the Company in December 2010 as President and Chief Executive Officer and was also appointed to the Surmodics Board of Directors at such time. Prior to joining Surmodics, Mr. Maharaj served as President and Chief Executive Officer of Arizant Inc., a provider of patient temperature management systems in hospital operating rooms, from 2006 to 2010. Previously, Mr. Maharaj served in several senior-level management positions for Augustine Medical, Inc. (predecessor to Arizant Inc.) from 1996 to 2006, including Vice President of Marketing, and Vice President of Research and Development. During his 34 years in the medical device industry, Mr. Maharaj has also served in various management and research positions for the orthopedic implant and rehabilitation divisions of Smith & Nephew, PLC.
Timothy J. Arens joined the Company in February 2007 as Director, Business Development and became Senior Director of Financial Planning and Analysis and General Manager, In Vitro Diagnostics in October 2010. He was promoted to Vice President of Finance and Interim Chief Financial Officer in August 2011 and in February 2013 became Vice President Corporate Development and Strategy. In May 2018, Mr. Arens was named interim Vice President of Finance and Chief Financial Officer for a second time and in February 2019 he was named Vice President of Finance and Chief Financial Officer. In April 2020, he was promoted to Senior Vice President of Finance and Information Technology and Chief Financial Officer. Prior to joining Surmodics, Mr. Arens was employed at St. Jude Medical, Inc., a medical technology company, from 2003 to 2007, in positions of increasing responsibility related to business development and strategic planning functions.
19
Charles W. Olson joined the Company in July 2001 as Market Development Manager, was promoted in December 2002 to Director, Business Development, named General Manager of the Hydrophilic Technologies business unit in April 2004, and promoted to Vice President and General Manager, Hydrophilic Technologies in October 2004. In April 2005, the position of Vice President, Sales was added to his responsibilities. In November 2008, Mr. Olson was named Vice President of our Cardiovascular business unit, in October 2010, he was named Senior Vice President and General Manager, Medical Device, and in August 2016 he was named Senior Vice President of Commercial and Business Development, Medical Devices. In May 2022, Mr. Olson was named Senior Vice President and President, Medical Device Coatings. Prior to joining Surmodics, Mr. Olson was employed as General Manager at Minnesota Extrusion from 1998 to 2001 and at Lake Region Manufacturing in project management and technical sales from 1993 to 1998.
Teryl L.W. Sides joined the Company in November 2018 as Senior Vice President and Chief Marketing Officer. In April 2020, Ms. Sides was promoted to Senior Vice President of Product Development and Chief Marketing Officer. In May 2022, Ms. Sides was named Senior Vice President and President, Vascular Interventions. Before joining Surmodics, Ms. Sides served as Founder and Chief Executive Officer of Projectory, a consulting firm that provides strategic marketing services to medical technology clients, ranging from start-ups to global businesses, from 2011 to 2018. Prior to joining Projectory, Ms. Sides was the Vice President of Marketing and Product Development for Arizant, Inc. from 1998 to 2011.
Joseph J. Stich joined the Company in March 2010 as Vice President of Marketing, Corporate Development and Strategy. In August 2011, Mr. Stich became Vice President, Business Operations and General Manager In Vitro Diagnostics. In September 2013, Mr. Stich’s role was adjusted to Vice President and General Manager, In Vitro Diagnostics. In April 2020, Mr. Stich was promoted to Senior Vice President and General Manager of Human Resources and In Vitro Diagnostics. In May 2022, Mr. Stich was named Senior Vice President Human Resources and President, In Vitro Diagnostics. Prior to joining Surmodics, Mr. Stich was Vice President of Corporate Development for Abraxis BioScience, LLC, a biotechnology company focused on oncology therapeutics, from 2009 to 2010. Prior to joining Abraxis, he was a Vice President for MGI Pharma, Inc., a biopharmaceutical company, from 2005 to 2009. Mr. Stich’s prior experience also includes serving as President/COO of Pharmaceutical Corp. of America (a subsidiary of Publicis Healthcare Specialty Group), and positions of increasing responsibility in sales and marketing at Sanofi-Aventis Pharmaceuticals.
Gordon S. Weber joined the Company in May 2020 as Senior Vice President of Legal, General Counsel and Secretary. Prior to joining Surmodics, Mr. Weber served as the Founder and President of Sapere Aude, LLC, a consulting firm, from 2018 to 2020. From 2017 to 2018, Mr. Weber served as Vice President, General Counsel and Secretary of CHF Solutions, Inc., which manufactures and markets ultrafiltration systems for patients suffering from fluid overload. Mr. Weber served as Vice President, General Counsel and Secretary of Vascular Solutions, Inc., a medical device company focused on products treating coronary and peripheral vascular disease, from 2013 until the company was acquired by Teleflex Incorporated in 2017. Mr. Weber practiced law for 13 years with Faegre & Benson LLP (now Faegre Drinker Biddle & Reath LLP), where he was Partner. Mr. Weber began his career with the accounting firm now known as KPMG and has served as Corporate Controller for Osmonics, Inc., an NYSE-listed manufacturer of fluid filtration equipment.
The executive officers of the Company are elected by and serve at the discretion of the Board of Directors. None of our executive officers are related to any other executive officer or any of our directors.
20
ITEM 1A. RISK FACTORS.
RISKS RELATING TO OUR BUSINESS, STRATEGY AND INDUSTRY
We had a net loss for our 2022 fiscal year, expect to incur net losses in the future, and may not be able return to or sustain profitability.
We incurred a net loss of $27.3 million in our fiscal year ended September 30, 2022 and expect to continue to have net losses in the future. We expect to continue to incur significant sales and marketing, research and development, regulatory and other expenses as we expand our commercialization efforts to increase adoption of our products, expand existing relationships with our customers, obtain regulatory clearances or approvals for our planned or future products, conduct clinical trials on our existing and planned or future products, and develop new products or add new features to our existing products. We expect to continue to incur losses in the future, which may fluctuate significantly from period to period. If our revenue declines or fails to grow at a rate faster than increases in our operating expenses, we will not be able to return to and maintain profitability in future periods. We cannot ensure that we will return to profitability or that, if we do become profitable, we will be able to sustain profitability.
The loss of, or significant reduction in business from, one or more of our major customers could significantly reduce our revenue, earnings or other operating results.
A significant portion of our revenue is derived from a relatively small number of customers. Two of our customers each provided more than 10% of our revenue in fiscal 2022. Revenue from Medtronic and Abbott represented approximately 13% and 11%, respectively, of our total revenue for fiscal 2022 and was generated from multiple products and fields of use. The loss of Medtronic, Abbott, or any of our other large customers, or reductions in business from them, could have a material adverse effect on our business, financial condition, results of operations, and cash flow. There can be no assurance that revenue from any customer will continue at their historical levels. If we cannot broaden our customer base, we will continue to depend on a small number of customers for a significant portion of our revenue.
The long-term success of our business may suffer if we are unable to maintain and expand our licensing base, including with customers who may perceive our vascular intervention products as competing with their products.
We intend to continue pursuing a strategy of licensing our performance coating technologies that impart lubricity, pro-healing and biocompatibility characteristics, as well as drug-delivery capabilities (together, “performance coatings” or “performance coating technologies”) to a diverse array of medical device companies, thereby expanding the commercialization opportunities for our technologies. A significant portion of our revenue is derived from customer devices used in connection with procedures in cardiovascular, peripheral vascular, neurovascular, structural heart and other applications. As a result, our business is susceptible to adverse trends in procedures. We may also be subject to adverse trends in specific markets such as the cardiovascular industry, including declines in procedures using our customers’ products as well as declines in average selling prices from which we earn royalties. Further, some of our performance coating technology customers may consider the vascular intervention products that we sell directly to healthcare providers to be competitive with their products.
Our success will depend, in part, on our ability to retain existing performance coatings technology customers and to attract new licensees, to enter into agreements for additional applications with existing licensees, and to develop technologies for use in new applications. There can be no assurance that we will be able to identify, develop and adapt our technologies for new applications in a timely and cost-effective manner; that new license agreements will be executed on terms favorable to us; that new applications will be accepted by customers in our target markets; or that products incorporating newly licensed technology, including new applications, will gain regulatory approval, be commercialized or gain market acceptance. Delays or failures in these efforts could have an adverse effect on our business, financial condition and operating results. In addition, we cannot be sure that existing or potential customers will not avoid using our performance coating technologies because they perceive our vascular intervention products to be a competitive threat, which could have an adverse effect on our business, financial condition and operating results.
Our success depends on our ability to effectively develop and market our products against those of our competitors.
We operate in highly competitive and quickly evolving fields, and new developments are expected to continue at a rapid pace. Our success depends, in part, upon our ability to maintain competitive positions in the development of technologies and products in the fields of surface modification, device drug delivery, medical device products and diagnostics. Our performance coating technologies compete with technologies developed by a number of other companies. In addition, many medical device manufacturers have developed, are engaged in efforts to develop, or through common ownership are or may become affiliates of companies that have developed, performance coating technologies for use on their own or affiliates’ products, particularly in the area of drug delivery. With respect to commercialization of our vascular intervention medical device products, we have faced, and expect to continue to face, competitive pressures, including pricing pressure, from larger OEM suppliers, as well as larger medical device companies that produce similar products. Some of our existing and potential competitors (especially medical device manufacturers pursuing coating solutions through their own R&D efforts) have greater financial and technical resources, as well as production and marketing capabilities, than us. Further, even if we are successful in our plans to develop new medical device products, the commercialization of
21
these products may be dependent upon a commercial partner to effectively market and sell our products to end users. Competitors may succeed in developing competing technologies or obtaining governmental approval for products before us. Products incorporating our competitors’ technologies may gain market acceptance more rapidly than products using our technologies. Furthermore, there can be no assurance that new products or technologies developed by others, or the emergence of new industry standards, will not render our products or technologies or licensees’ products incorporating our technologies uncompetitive or obsolete. Any new technologies that make our performance coatings, medical device platforms or In Vitro Diagnostics technologies less competitive or obsolete would have a material adverse effect on our business, financial condition and results of operations. Competition in the diagnostics market is highly fragmented, and in the product lines in which we compete, we face an array of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. Some of our competitors have substantially more capital resources, marketing experience, R&D resources and production facilities than we do.
We may not be successful in implementing our vascular intervention product development and commercialization strategy.
Since fiscal 2013, we have been focused on a key growth strategy to develop and commercialize vascular intervention products. Our aim is to provide customers with highly differentiated products that address unmet clinical needs. To date, we have commercialized our vascular intervention products through collaborations with other medical device companies and through our own direct sales channel in the United States. We may seek to expand the commercialization of these products through existing customers, third-party distributors, or other distribution channels.
Successfully implementing our vascular intervention product strategy places substantial demands on our resources and requires, among other things:
There is no assurance that we will be able to successfully implement our vascular intervention product strategy, which could negatively impact our ability to realize an acceptable return on the investments we are making in connection with this strategy and may result in an adverse impact on our business and financial results.
We anticipate that increased operating expenses related to the development and direct-sale commercialization of medical device products will have an adverse impact on our near-term operating results and financial position, and they may not be effective.
In fiscal 2022, we established a field sales team to sell our radial access and thrombectomy medical device products directly to healthcare providers in the United States. Our SG&A expenses increased by 53% in fiscal 2022, over the prior year, primarily due to personnel and other investments in our direct sales initiatives. We currently expect SG&A expense to increase further in fiscal 2023 reflecting the full-year impact of direct-sales and support personnel who were hired throughout fiscal 2022. In addition, our R&D expense increased by 8% in fiscal 2022, over the prior year, partially due to increases in staffing levels and expenses related to our medical device products.
Because we expect the increased operating expenses related to direct sales of our medical device products to exceed any related increases in revenues in fiscal 2023, we anticipate that the increased expenses will adversely impact our operating results and cash flow during the year, which is likely to have an adverse effect on our financial position. Accordingly, we may seek additional sources of funds, including additional borrowing against our credit facility, to fund our continued investment in the development and direct sale of our medical device products. Such funds may not be available on favorable terms, if at all.
In addition to the operating expenses associated with product development and direct-sale commercialization activities, such activities are subject to risks of failure that are inherent in the development and commercialization of new medical technologies or products
22
and establishment of a new sales force. There can be no assurance that we will be successful in developing new technologies or products, or in commercializing any such technologies or products through direct sales, or otherwise. Even if we are successful in developing and commercializing new technologies or products, there can be no assurance that gross profits from their sales will exceed our operating expenses related to their development and commercialization.
Our credit agreement contains covenants that restrict our business and financing activities. All of our assets secure our obligations under the credit agreement and may be subject to foreclosure.
On October 14, 2022, we entered into a secured revolving credit facility and secured term loan facilities pursuant to a Credit, Security and Guaranty Agreement (the “MidCap Credit Agreement”) with Mid Cap Funding IV Trust, as agent, and MidCap Financial Trust, as term loan servicer and the lenders from time to time party thereto (together “MidCap”). The MidCap Credit Agreement provides for availability under a secured revolving line of credit of up to $25 million, which may be drawn upon until maturity, subject to a borrowing base, and up to $100 million ($25 million of which is at the sole discretion of MidCap) in term loans, which may be drawn upon in increments of at least $10 million until December 31, 2024. The line of credit and term loans mature on October 1, 2027. We borrowed $30 million upon the closing of the credit facility, consisting of $5 million drawn on the line of credit and $25 million of term loans.
The MidCap Credit Agreement contains covenants that limit our ability to engage in certain transactions. Subject to limited exceptions, these covenants limit our ability to, among other things:
These provisions impose significant operating and financial restrictions on us and may limit our ability to compete effectively, take advantage of new business opportunities, or take other actions that may be in our, or our shareholders’, best interests.
In addition to the other covenants under the MidCap Credit Agreement, we must maintain minimum core net revenue levels tested quarterly if term loans exceed $25.0 million.
The MidCap Credit Agreement contains customary indemnification obligations and customary events of default, including, among other things:
• non-payment; • breach of warranty; • non-performance of covenants and obligations; • default on other indebtedness; • certain judgments; |
• change of control; • bankruptcy and insolvency; • impairment of security; • regulatory matters; and • material adverse effect. |
Our obligations under the MidCap Credit Agreement are secured by all our existing and future acquired assets, including intellectual property and real estate.
Our inability to comply with any of the provisions of the MidCap Credit Agreement could result in a default under it. If such a default occurs, the lenders may elect to declare all borrowings outstanding, together with accrued interest and other fees, to be immediately due and payable, and it would have the right to terminate any commitments to provide further funds. If we are unable to repay outstanding borrowings when due, the lender also has the right under the MidCap Credit Agreement to proceed against the collateral granted to it to secure the indebtedness under the MidCap Credit Agreement. The occurrence of any of these events could have a material adverse effect on our business, financial condition, results of operations and liquidity.
We expect our interest expense to increase, and we may draw on our term loan availability to preserve our access to capital, both of which may adversely impact our financial results.
In our fiscal 2022, we incurred approximately $0.6 million of interest expense on our outstanding debt of $10 million with our prior lender, based on a weighted average annual interest rate on the debt of 3.96% for the year. As of October 14, 2022, we borrowed $5
23
million on our revolving credit line with MidCap at an annual interest rate equal to 3.00% plus the greater of Term SOFR (as defined in the MidCap Credit Agreement) or 1.50%, which represented an annual interest rate of 6.04% as of such date. We also borrowed $25 million of term loans with MidCap as of October 14, 2022. We entered into a five-year interest rate swap transaction on October 14, 2022 with Wells Fargo Bank, N.A. that fixed the annual interest rate on the $25 million of term loans at 10.205%. The combination of greater outstanding debt and higher interest rates will cause our interest expense to increase in our fiscal 2023 and beyond, which will adversely impact our cash flow and financial results.
Under the MidCap Credit Agreement, we may borrow up to an additional $75 million ($25 million of which is at the sole discretion of MidCap) in term loans, which may be drawn upon in increments of at least $10 million prior to December 31, 2024. Since we cannot draw upon the term loans between December 31, 2024 and when they mature on October 1, 2027, we may, in order to preserve our access to this source of capital, elect to draw upon the term loans prior to when we would need the proceeds to fund our operations. Such borrowing may cause our interest expense to increase further and adversely impact our financial results and cash flow after such borrowing.
We may seek to prepay our borrowings under the MidCap Credit Agreement before its maturity, which would subject us to early termination fees and may lead us to raise capital on unfavorable terms.
Subject to certain limitations, the term loans under our MidCap Credit Agreement have a prepayment fee for payments made prior to the maturity date equal to 3.0% of the prepaid principal amount for the first year following the closing date of the MidCap Credit Agreement, 2.0% of the prepaid principal amount for the second year following the closing date, and 1.0% of the prepaid principal amount for the third year following the closing date and thereafter. In addition, if the revolving credit facility under the MidCap Credit Agreement is terminated in whole or in part prior to the maturity date, we must pay a prepayment fee equal to 3.0% of the terminated commitment amount for the first year following the closing date of the MidCap Credit Agreement, 2.0% of the terminated commitment amount for the second year following the closing date and 1.0% of the terminated commitment amount for the third year following the closing date and thereafter. We also are required to pay a full exit fee at the time of maturity or full prepayment equal to 2.5% of the aggregate principal amount of the term loans made pursuant to the MidCap Credit Agreement and a partial exit fee at the time of any partial prepayment event equal to 2.5% of the amount prepaid.
To obtain more favorable interest rates or credit terms, or for other financial or strategic reasons, we may seek to prepay our borrowings under the MidCap Credit Agreement. To do so, we may seek to raise additional capital through equity offerings or debt financings and such additional financing may not be available to us on acceptable terms, or at all. Further, any additional equity or debt financing transaction may contain terms that are not favorable to us or our shareholders. For example, if we raise funds by issuing equity or equity-linked securities, the issuance of such securities could result in dilution to our shareholders. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common stock. Further, the issuance of additional equity securities by us, or the possibility of such issuance, may cause the market price of our common stock to decline.
In addition, the terms of debt securities issued or borrowings could impose significant restrictions on our operations including restrictive covenants, such as limitations on our ability to, among other things, dispose of assets, effect certain mergers, incur debt, grant liens, pay dividends and distributions on capital stock, make investments and acquisitions, and enter into transactions with affiliates, and other operating restrictions that could adversely affect our ability to conduct our business.
If we enter into asset transactions, collaborations or licensing arrangements to raise capital, we may be required to accept unfavorable terms, such as the relinquishment or licensing of certain technologies or products that we otherwise might seek to develop or commercialize ourselves, or reserve for future potential arrangements when we might otherwise be able to achieve more favorable terms.
Failure to successfully commercialize the Pounce™ venous thrombectomy product obtained in the acquisition of Vetex Medical Limited may limit our growth and adversely impact our operating results, balance sheet, cash flows and liquidity.
On July 2, 2021, we completed the acquisition of all outstanding shares of Vetex Medical Limited (“Vetex”). Vetex holds a Food and Drug Administration 510(k) clearance, E.U. CE Mark, and portfolio of patents related to the Pounce venous mechanical thrombectomy catheter product (the “Pounce Venous Thrombectomy Catheter”). However, Vetex had not initiated commercial production or established commercialization of the product prior to the acquisition. We acquired Vetex with an upfront cash payment of $39.9 million and are obligated to pay additional installments totaling $3.5 million in fiscal 2024 through fiscal 2027. An additional $3.5 million in payments are contingent upon the achievement of certain product development and regulatory milestones within a contingency period ending in fiscal 2027. As of the acquisition date, we recognized $28 million in intangible assets, $3 million in deferred tax liabilities and $19 million in goodwill related to the acquisition.
We began limited market evaluations of the Pounce Venous Thrombectomy Catheter in June of 2022. For us to realize the anticipated benefits of the Vetex acquisition, we must successfully establish commercial manufacturing for the Pounce Venous Thrombectomy Catheter, and successfully develop and execute a commercialization strategy for the product. If we are unsuccessful, or encounter delays or cost overruns, in establishing commercial manufacturing for the Pounce Venous Thrombectomy Catheter, or if potential customers do not adopt the Pounce Venous Thrombectomy Catheter at sufficient levels to make it a commercial success, our operating
24
results, cash flows and liquidity may be adversely impacted. Further, the goodwill and intangible assets that we recognized related to the acquisition may become impaired if the financial performance of the Pounce Venous Thrombectomy Catheter does not meet our expectations, which could negatively affect our balance sheet.
Concerns over a study that reported a mortality signal associated with paclitaxel-coated products may adversely affect market acceptance of our paclitaxel-coated DCB products and our potential revenues from them.
On March 15, 2019, the FDA issued a communication (the “FDA communication”) to healthcare providers about the potential for increased long-term mortality after use of paclitaxel-coated balloons and paclitaxel-eluting stents (collectively “paclitaxel-coated products”) to treat PAD in the femoropopliteal artery. The FDA communication updated a previous notification from the FDA on the same topic, which was in response to meta-analysis of randomized trials published in the Journal of the American Heart Association in December 2018. Subsequently, in August 2019, the FDA issued an update on the use of paclitaxel devices to treat PAD that recommended that physicians discuss the risks and benefits of all available treatment options with their patients. The FDA communication and the potential long-term mortality signal related to the use of paclitaxel-coated devices may adversely affect market acceptance of our paclitaxel-coated DCB products and the revenue we may realize from the commercialization of the SurVeil DCB if the FDA grants premarket approval (“PMA”) for the product.
Failure to effectively utilize our limited ability to make acquisitions, to accurately financially model the impact of acquisitions, or to integrate acquired businesses or technologies into our operations successfully may limit our growth and adversely impact operating results, cash flows and liquidity.
The MidCap Credit Agreement defines acquisitions broadly and limits our ability to pay for acquisitions to (i) an aggregate of $10 million in cash consideration over the five-year term of the MidCap Credit Agreement, and (ii) consideration consisting of noncash equity interests. Acquisitions of complementary businesses or technologies can be important potential catalysts for our revenue growth. Our limited ability to make cash acquisitions may prevent us from making acquisitions that would enhance our business and revenues. It also may cause us to use equity interests as consideration for larger acquisitions, which would dilute the equity interest of our existing shareholders.
Our identification of suitable acquisition candidates involves risks inherent in assessing the technology, value, strengths, weaknesses, overall risks and profitability, if any, of acquisition candidates. We may not be able to identify suitable acquisition candidates, or we may be unable to execute acquisitions due to competition from buyers with more resources.
Our ability to realize the anticipated benefits of a potential acquisition depends, in part, on the accuracy of our financial model of the timing and magnitude of cash flows, expenses and revenues related to the acquired business. If the expectations reflected in our financial models for acquisitions are not realized, our operating results, cash flows and liquidity may be materially adversely affected.
The process of integrating acquired businesses into our operations could pose numerous and significant risks. In addition, future acquisitions may cause large one-time expenses or create goodwill or other intangible assets that could result in future significant asset impairment charges. In addition, if we acquire entities that have not yet commercialized products, but rather are developing technologies for future commercialization, our earnings per share may fluctuate as we expend significant funds for continued R&D efforts necessary to commercialize such acquired technology.
Our failure to expand our management systems and controls to support anticipated growth or integrate acquisitions could seriously harm our operating results and business.
Our operations are expanding, and we expect this trend to continue as we execute our business strategy. Executing our business strategy has placed significant demands on management and our administrative, development, operational, information technology, manufacturing, financial and personnel resources. Accordingly, our future operating results will depend on the ability of our officers and other key employees to continue to implement and improve our operational, development, customer support and financial control systems, and effectively expand, train and manage our employee base. Otherwise, we may not be able to manage our growth successfully.
Goodwill or other assets on our balance sheet may become impaired or require valuation reserves, which could have a material adverse effect on our operating results.
As of September 30, 2022, we had $68.9 million of goodwill and intangible assets on our consolidated balance sheet. As required by the accounting guidance, we evaluate at least annually the potential impairment of our goodwill. Testing for impairment of goodwill involves the determination of the fair value of our reporting units. The estimation of fair values involves a high degree of judgment and subjectivity in the assumptions used. We also evaluate other assets on our balance sheet, including definite-lived intangible assets, whenever events or changes in circumstances indicate that their carrying value may not be recoverable. Our estimate of the fair value of the assets may be based on fair value appraisals or discounted cash flow models using various inputs. Future impairment charges could materially adversely affect our results of operations.
25
In the fourth quarter of our fiscal 2022, we recognized $10.2 million of non-cash income tax expense related to the establishment of a full valuation reserve against our U.S. deferred tax assets. While we do not have any unreserved U.S. deferred tax assets remaining on our balance sheet, we do have significant amounts of goodwill and other intangible assets on our balance sheet, which could be subject to future impairment charges.
The COVID-19 pandemic had adverse effects on our business and results of operations, and it or other global health concerns could seriously harm our business, results of operations, and financial condition.
Early in the COVID-19 pandemic, U.S. healthcare providers limited non-emergent elective medical procedures other than high acuity treatments in order to conserve personal protective equipment and limit exposure to COVID-19. The reduction in elective medical procedures resulted in reductions in the use of certain medical devices, which in turn reduced the licensing revenue that we recognized from impacted devices that incorporate our technologies. Throughout the pandemic, we incurred additional operating expenses to facilitate our employees working from home when possible, provide personal protective equipment, enhance cleaning and sanitation procedures, and modify workspaces to reduce the potential for disease transmission. We also suspended operations for limited periods in limited production work areas when employees were identified as having COVID-19.
We cannot predict the duration or scope of the COVID-19 pandemic or whether or when other global health concerns may emerge. In response to COVID-19 resurgences or other global health concerns, we, governments, businesses, and healthcare providers may take actions that could have material adverse effects on our business, results of operations, cash flows, financial condition, and capital investments.
Our business could be adversely affected by global economic conditions.
Prolonged economic uncertainties or downturns could adversely affect our business, financial condition, and results of operations. Negative conditions in the general economy in either the United States or abroad, including conditions resulting from financial and credit market fluctuations, increased inflation and interest rates, changes in economic policy, trade uncertainty, including changes in tariffs, sanctions, international treaties, and other trade restrictions, the occurrence of a natural disaster or global public health crisis, such as the COVID-19 pandemic, or armed conflicts, such as between Russia and Ukraine, impact corporate spending in general and negatively affect the growth of our business.
These conditions could make it difficult for us and our customers to forecast and plan future business activities accurately and could cause our customers to reevaluate or delay their decisions to license our technologies, purchase our products or enter into R&D arrangements with us. A substantial downturn affecting our customers may cause them to react to worsening conditions by reducing their capital expenditures in general or by specifically reducing their spending on medical devices and technologies. We cannot predict the timing, strength, or duration of any economic slowdown, downturn, instability, or recovery, generally or within any particular industry or geography. Any downturn of the general economy or industries in which we operate would adversely affect our business, financial condition, and results of operations.
We recognize revenue in accordance with complex accounting standards, and changes in circumstances or interpretations may lead to accounting adjustments. Failure to implement these standards properly might impact the effectiveness of our internal control over financial reporting or impact the reliability of our financial reporting.
Our revenue recognition policies involve application of complex accounting standards, including the determination of when control is transferred to the customer and the allocation of the transaction price to multiple performance obligations. Our compliance with such accounting standards often involves management’s judgment regarding whether the criteria set forth in the standards have been met such that we can recognize as revenue the amounts that we expect to receive as payment for our products or services. We base our judgments on assumptions that we believe to be reasonable under the circumstances. However, these judgments, or the assumptions underlying them, may change over time. In addition, the SEC or the Financial Accounting Standards Board (“FASB”) may issue new positions or revised guidance on the treatment of complex accounting matters. Changes in circumstances or third-party guidance could cause our judgments to change with respect to our interpretations of these complex standards, and transactions recorded, including revenue recognized, for one or more prior reporting periods, could be adversely affected. In addition, failure to implement these standards properly could impact the effectiveness of our internal control over financial reporting or impact the reliability of our financial reporting, which could cause investors to lose confidence in our reported financial information and have a negative effect on the trading price of our stock.
Our business includes foreign operations, which exposes us to certain risks related to fluctuations in U.S. dollar and foreign currency exchange rates.
We report our consolidated financial statements in U.S. dollars. In a period where the U.S. dollar is strengthening or weakening relative to the Euro, our revenue and expenses denominated in the Euro are translated into U.S. dollars at a lower or higher value than they would be in an otherwise constant currency exchange rate environment. As our foreign operations expand, the effects may become material to our consolidated financial statements.
26
Changes in product mix and increased manufacturing costs could cause our product gross margin percentage to fluctuate or decline in the future.
Changes in our product mix and increases in manufacturing costs could cause our gross profit percentage to fluctuate or decline in the future. These factors, together with the scale-up of our manufacturing operations, particularly in Ireland, adversely affected our gross margin percentage for the last fiscal year and these factors will likely continue to affect our gross profit percentage in fiscal 2023 and beyond.
RISKS RELATING TO OUR OPERATIONS AND RELIANCE ON THIRD PARTIES
We rely on third parties to market, distribute and sell most products incorporating our coating and device technologies, as well as certain of our vascular intervention products.
A principal element of our business strategy is to enter into licensing arrangements with medical device and other companies that manufacture products incorporating our technologies. We derived 36%, 45%, and 43% of our revenue from royalties and license fees (including related to our SurVeil DCB) under such licensing arrangements in fiscal 2022, 2021 and 2020, respectively. The revenue that we derive from such arrangements depends upon our ability, or our licensees’ ability, to successfully develop, obtain regulatory approval for, manufacture (if applicable), market, and sell products incorporating our technologies. Many of these factors are outside of our control. Our failure, or the failure of our licensees, to meet these requirements could have a material adverse effect on our business, financial condition and results of operations.
Additionally, a licensee could modify their product in such a way that it no longer incorporates our technology. Moreover, under our standard license agreements, licensees can terminate the license for any reason upon 90 days’ prior written notice. Existing and potential licensees have no obligation to deal exclusively with us and may pursue parallel development or licensing of competing technologies on their own or with third parties. A decision by a licensee to terminate its relationship with us could have a material adverse effect on our business, financial condition and results of operations.
In fiscal 2018, we entered into an agreement with Abbott whereby Abbott will have exclusive worldwide commercialization rights for the SurVeil DCB. Upon receipt of U.S. regulatory approval for the SurVeil DCB, Abbott has the right to purchase commercial units from us and we will realize revenue from product sales to Abbott at an agreed-upon transfer price, as well as a share of net profits resulting from third-party product sales by Abbott. Upon receipt of U.S. regulatory approval, we will rely on Abbott to effectively market and sell the SurVeil DCB. If Abbott is unable or unwilling to effectively market and sell the SurVeil DCB, it could have a have material adverse effect on our business, financial condition and results of operations.
We have not produced our SurVeil DCB on a commercial scale. If the FDA grants PMA for the product, we may encounter challenges in scaling up our production of it, which could have an adverse impact on our operating results.
If the FDA grants PMA of our SurVeil DCB, we expect Abbott to launch commercialization of the product in the U.S. We will be responsible for manufacturing commercial quantities of the product. The SurVeil DCB is a highly complicated drug/device combination product that we have never manufactured on a commercial scale. It is not uncommon for there to be low yields, inefficiencies, or production issues when the manufacturing processes for a complicated product are ramped up to commercial scale. Any production issues related to our SurVeil DCB could have material adverse effects on our revenues and operating results.
A portion of our IVD business relies on distribution agreements and relationships with various third parties, and any adverse change in those relationships could result in a loss of revenue and harm that business.
We sell many of our IVD products outside of the U.S. through distributors. Some of our distributors also sell our competitors’ products. If they favor our competitors’ products for any reason, they may fail to market our products as effectively or to devote resources necessary to provide significant sales, which would cause our results to suffer. Additionally, we serve as the exclusive distributor in the U.S., Canada and Puerto Rico for DIARECT GmbH (Part of BBI Solutions) for its recombinant and native antigens. The success of these arrangements with these third parties depends, in part, on the continued adherence to the terms of our agreements with them. Any disruption in these arrangements will adversely affect our financial condition and results of operations.
We rely on our customers to accurately report and make payments under our license agreements with them.
We rely on our performance coatings technology customers to determine whether the products that they sell are royalty-bearing and, if so, to report and pay the amount of royalties owed to us under our agreements with them. The majority of our performance coatings technology license agreements with our customers give us the right to audit their records to verify the accuracy of their reports to us. However, these audits can be expensive, time-consuming and possibly detrimental to our ongoing business relationships with our customers. Inaccuracies in customer royalty reports have resulted in, and could result in, additional overpayments or underpayments of royalties, which could have a material adverse effect on our business, financial condition and results of operations.
27
We currently have limited or no redundancy in our manufacturing facilities for certain products, and we may lose revenue and be unable to maintain our customer relationships if we lose our production capacity.
We manufacture all of our performance coating reagents (and provide coating manufacturing services for certain customers) and our IVD products at one of our Eden Prairie, Minnesota facilities. We also manufacture balloon catheter products at our facility in Ballinasloe, Ireland and catheter-based medical devices in limited quantities in one of our facilities in Eden Prairie, Minnesota. If we receive the necessary regulatory approvals, we plan to manufacture our SurVeil DCB both in our Ireland facility and in our manufacturing facility in Eden Prairie, Minnesota. If our existing production facilities become incapable of manufacturing products for any reason, we may be unable to meet production requirements, we may lose revenue and we may not be able to maintain our relationships with our customers, including certain of our licensees. In addition, because most of our customers use our performance coating reagents to manufacture their own products that generate royalty revenue for us, failure by us to supply these reagents could result in decreased royalty revenue, as well as decreased revenue from our performance coating product sales. Without our existing production facilities, we would have no other means of manufacturing products until we were able to restore the manufacturing capability at these facilities or develop one or more alternative manufacturing facilities. Although we carry business interruption insurance to cover lost revenue and profits in an amount we consider adequate, this insurance does not cover all possible situations. In addition, our business interruption insurance would not compensate us for the loss of opportunity and potential adverse impact on relations with our existing customers resulting from our inability to produce products for them.
We may face product liability claims related to participation in clinical trials or the use or misuse of our products.
The development and sale of medical devices and component products involves inherent risks of product liability claims. For medical device products that incorporate our performance coating technologies, most of the licenses provide us with indemnification against such claims. However, there can be no assurance that product liability claims will not be filed against us for such products, or for medical device products that we manufacture as part of our vascular intervention product strategy, that parties indemnifying us will have the financial ability to honor their indemnification obligations, or that such manufacturers will not seek indemnification or other relief from us for any such claims. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, significant liabilities and diversion of our management’s time, attention and resources. We have obtained a level of liability insurance coverage that we believe is appropriate to our activities, however, we cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all. Furthermore, we do not expect to be able to obtain insurance covering our costs and losses as a result of any recall of products or devices incorporating our technologies because of alleged defects, whether such recall is instituted by us, by a customer, or is required by a regulatory agency. A product liability claim, recall or other claim with respect to uninsured liabilities, or for amounts in excess of insured liabilities, could have a material adverse effect on our business, financial condition and results of operations.
Our revenue will be harmed if we experience disruptions in our supply chain.
Supply chains across many industries have experienced delays and disruptions due to a wide variety of factors including labor and materials shortages and a lack of transportation capacity. A disruption in the supply of even a minor competent of a product can have a major impact on the production and delivery of that product. Further, we currently purchase some of the components we use to manufacture reagents from sole suppliers. If any of our suppliers becomes unwilling to supply components to us, experiences an interruption in its production, or is otherwise unable to provide us, on a timely basis or at all, with sufficient material to manufacture our reagents and other products, we will experience production interruptions. If we lose our sole supplier of any particular reagent component or are otherwise unable to procure all components required for our reagent manufacturing for an extended period of time, we may lose the ability to manufacture the reagents our customers require to commercialize products incorporating our technology. This could result in lost royalties and product sales, which would harm our financial results. Adding suppliers to our approved vendor list may require significant time and resources. We routinely attempt to maintain multiple suppliers of each of our significant materials, so we will have alternative suppliers, if necessary. However, if the number of suppliers of a material is reduced, or if we are otherwise unable to obtain our material requirements on a timely basis and on favorable terms, our operations may be harmed.
We depend upon key personnel and may not be able to attract or retain qualified personnel in the future.
Our success depends upon our ability to retain and attract highly qualified management and technical personnel. We face intense competition for such qualified personnel. We do not maintain key person insurance, and we generally do not enter into employment agreements, except with certain executive officers. Although we have non-compete agreements with most employees, there can be no assurance that such agreements will be enforceable. The loss of the services of one or more key employees or the failure to attract and retain additional qualified personnel could have a material adverse effect on our business, financial condition and results of operations.
28
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
We collect and store sensitive data, including intellectual property, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information of our customers and employees, on our networks. The secure maintenance of this information is critical to our operations and business strategy, and our customers expect that we will securely maintain their information. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers resulting from employee error, malfeasance or other disruptions. Any information technology breach could compromise our networks and the information stored on them could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under personal privacy laws and regulatory penalties, disrupt our operations and the services that we provide to our customers, damage our reputation and cause a loss of confidence in our products and services, any of which could adversely affect our business and competitive position.
Our information systems, and those of third-party suppliers with whom we contract, require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information technology, evolving systems and regulatory standards, and changing threats. These systems could be vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees, third-party vendors and/or business partners, or from cyber-attacks by malicious third parties. We also are subject to other cyber-attacks, including state-sponsored cyber-attacks, industrial espionage, insider threats, computer denial-of-service attacks, computer viruses, ransomware and other malware, payment fraud or other cyber incidents. Any significant breakdown, intrusion, breach, interruption, corruption or destruction of these systems could have a material adverse effect on our business and reputation and could materially adversely affect our results of operations and financial condition.
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
We may not be able to obtain, maintain or protect proprietary rights necessary for the commercialization of our technologies.
Our success depends, in large part, on our ability to obtain and maintain patents and trade secrets. We have been granted U.S. and foreign patents and have U.S. and foreign patent applications pending related to our proprietary technologies. There can be no assurance that any pending patent application will be approved, that we will develop additional proprietary technologies that are patentable, that any patents issued will provide us with competitive advantages or will not be challenged or invalidated by third parties, that the patents of others will not prevent the commercialization of products incorporating our technologies, or that others will not independently develop similar technologies or design around our patents. Furthermore, because we generate a significant amount of our revenue through licensing arrangements, the loss or expiration of patent protection for our licensed technologies will result in a reduction of the revenue derived from these arrangements, which may have a material adverse effect on our business, cash flow, results of operations, financial position and prospects.
We may become involved in expensive and unpredictable patent litigation or other intellectual property proceedings which could result in liability for damages or impair our development and commercialization efforts.
Our commercial success also will depend, in part, on our ability to avoid infringing patent or other intellectual property rights of third parties. There has been substantial litigation regarding patent and other intellectual property rights in the medical device and pharmaceutical industries, and intellectual property litigation may be used against us as a means of gaining a competitive advantage. Intellectual property litigation is complex, time consuming and expensive, and the outcome of such litigation is difficult to predict. If we were found to be infringing any third-party patent or other intellectual property right, we could be required to pay significant damages, alter our products or processes, obtain licenses from others, which we may not be able to do on commercially reasonable terms, if at all, or cease commercialization of our products and processes. Any of these outcomes could have a material adverse effect on our business, financial condition and results of operations.
Patent litigation or certain other administrative proceedings may also be necessary to enforce our patents or to determine the scope and validity of third-party proprietary rights. These activities could result in substantial cost to us, even if the eventual outcome is favorable to us. An adverse outcome from any such litigation or interference proceeding could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using our technology. Any action to defend or prosecute intellectual property would be costly and result in significant diversion of the efforts of our management and technical personnel, regardless of outcome, and could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to keep our trade secrets confidential, our technology and proprietary information may be used by others to compete against us.
We rely significantly upon proprietary technology, information, processes and know-how that are not subject to patent protection. We seek to protect this information through trade secret or confidentiality agreements with our employees, consultants, potential licensees, or other parties as well as through other security measures. There can be no assurance that these agreements or any security
29
measure will provide meaningful protection for our un-patented proprietary information. In addition, our trade secrets may otherwise become known or be independently developed by competitors. If we determine that our proprietary rights have been misappropriated, we may seek to enforce our rights which would draw upon our financial resources and divert the time and efforts of our management, and could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to convince our customers to adopt our advanced generation of hydrophilic coating technologies, our royalty revenue may decrease, and the expiration of the patent family protecting this technology has and will continue to result in a reduction of the royalty revenue associated with existing license agreements.
In our Medical Device segment, we have licensed our PhotoLink hydrophilic technology to a number of our customers for use in a variety of medical device surface applications. We have several U.S. and international issued patents and pending U.S. and international patent applications protecting various aspects of these technologies, including compositions, methods of manufacture, and methods of coating devices. The anticipated expiration dates of the patents range from fiscal 2026 to 2039. These patents and patent applications represent distinct families, with each family generally covering a successive generation of the technology, including improvements that enhance coating performance, manufacturability, or other important features desired by our customers.
Our fourth-generation PhotoLink technology was protected by a family of patents that expired in the first quarter of fiscal 2020 in all countries where patent coverage existed for the technology, except in Japan, where the relevant patent expired in the first quarter of fiscal 2021. Of the license agreements using our fourth-generation technologies, most continue to generate royalty revenue beyond patent expiration, but at a reduced royalty rate.
While we are actively working to encourage and support our customers’ adoption of our advanced generations of our hydrophilic coating technology, there can be no assurance that they will do so, or that those customers that have adopted, or will adopt, our hydrophilic coating technology will sell products utilizing our technology which will generate earned royalty revenue for us.
If we or any of our licensees breach any of the agreements under which we have in-licensed intellectual property from others, we could be deprived of important intellectual property rights and future revenue.
We are a party to various agreements through which we have in-licensed or otherwise acquired rights to certain technologies that are important to our business. In exchange for the rights granted to us under these agreements, we have agreed to meet certain research, development, commercialization, sublicensing, royalty, indemnification, insurance or other obligations. If we or one of our licensees fails to comply with these obligations set forth in the relevant agreement through which we have acquired rights, we may be unable to effectively use, license, or otherwise exploit the relevant intellectual property rights and may be deprived of current or future revenue that is associated with such intellectual property.
RISKS RELATING TO CLINICAL AND REGULATORY MATTERS
The FDA has requested additional data, and may continue to make such requests, in its review of the premarket approval application for our SurVeil DCB, which may delay FDA action on the application and have an adverse impact on our operating results and cash flows.
In June 2021, we submitted the fourth and final module of the PMA application to the FDA related to our SurVeil DCB. In its subsequent comments on the PMA application, the FDA requested additional testing data in order to evaluate the product and its unique technologies. In October 2022, we submitted a complete response, including additional testing data, to the FDA’s comments on our PMA application for the SurVeil DCB. The FDA may request additional information, including test data, related to our most recent submission in support of the PMA application.
As we previously have disclosed, we expect to receive a $27 million milestone payment under the Abbott Agreement following FDA approval of our PMA application, if it ultimately is granted. Further, we expect Abbott to begin commercialization of the SurVeil DCB following such approval, if granted. There can be no assurance that the SurVeil DCB will receive FDA approval. If FDA approval of the SurVeil DCB is delayed or denied, our operating results and cash flows may be materially adversely impacted.
The development of new products and enhancement of existing products requires significant research and development and regulatory approvals, which may require clinical trials, all of which may be very expensive and time-consuming and may not result in commercially viable products.
The development of new products and enhancement of existing products requires significant investment in research and development and regulatory approvals. Regulators may require successful clinical trials prior to granting approvals for new or enhanced products.
There can be no assurance that any products now in development, or that we may seek to develop or refine in the future, will achieve technological feasibility, obtain regulatory approval or gain market acceptance. If we are unable to obtain regulatory approval for new products or enhanced products, our ability to successfully compete in the markets in which we participate may be materially adversely impacted. A delay in the development or approval of new products and technologies may also adversely impact the timing of when these products contribute to our future revenue and earnings growth.
30
Delays in clinical studies are common and have many causes, and any significant delay in clinical studies being conducted by us could result in delays in obtaining regulatory approvals and jeopardize the ability to proceed to commercialization of our products.
We have conducted clinical studies on DCB products, some of which are ongoing. We may conduct additional clinical studies on our DCB or other products. There are risks involved in such clinical studies, including that they may fail to enroll a sufficient number of patients for a variety of reasons or fail to be completed on schedule, if at all. Clinical studies for any of our products could be delayed or terminated for a variety of reasons, including, but not limited to:
If the initiation or completion of any of the ongoing or planned clinical studies for our products is delayed for any of the above or other reasons, the regulatory approval process would be delayed and the ability to commercialize and commence sales of our products could be materially harmed. Additionally, clinical study delays may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. Any of these events could have a material adverse effect on our business, financial condition and results of operations.
We cannot be sure that clinical studies of our products will be successful, or that their results will be adequate to obtain or maintain regulatory approvals.
We cannot be sure that the endpoints or safety profile of any clinical trial will be met. In addition, we cannot be sure that any clinical trial that is successful will support regulatory approval of the product subject to the trial. We may expend significant financial and human capital resources on clinical trials. If they fail to achieve their endpoints, or support regulatory approvals, it could have a material adverse effect on our business, financial condition and results of operations.
Healthcare policy changes may have a material adverse effect on us.
Healthcare costs have risen significantly during the past decade. There have been and continue to be proposals by legislators, regulators and third-party payers to reduce healthcare expenditures. Certain proposals, if implemented, would impose limitations on the prices our customers will be able to charge for our products, or the amounts of reimbursement available for their products from governmental agencies or third-party payers, or otherwise negatively impact pricing and reimbursement. Because a significant portion of our revenue is currently derived from royalties on products that constitute a percentage of our customer’s product’s selling price, these limitations could have an adverse effect on our revenue.
Healthcare reform continues to be a prominent political topic. We cannot predict what healthcare programs and regulations may ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the U.S. or internationally may have on our business.
Vascular intervention medical devices and other products incorporating our technologies are subject to increasing scrutiny and regulations, including extensive approval/clearance processes and manufacturing requirements. Any adverse regulatory and/or enforcement action (for us or our licensees) may materially affect our financial condition and business operations.
Our products and our business activities are subject to complex regulatory regimes both in the U.S. and internationally. Additionally, certain state governments and the federal government have enacted legislation aimed at increasing transparency of industry interactions with healthcare providers. Any failure to comply with these legal and regulatory requirements could impact our business. In addition, we will continue to devote substantial human capital and financial resources to further developing and implementing policies, systems and processes to comply with enhanced legal and regulatory requirements, which may impact our business and results of operations. We anticipate that governmental authorities will continue to scrutinize our industry closely, and that additional regulation may increase compliance and legal costs, exposure to litigation, and other adverse effects to our operations.
To varying degrees, the FDA and comparable agencies outside the U.S. require us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our products. Our compliance with these laws and regulations takes significant human capital and financial resources; involves stringent testing and surveillance; involves attention to any needed product improvements (such as modifications, repairs, or replacements); and may include significant limitations of the uses of our products.
31
Changes in existing regulations or adoption of new governmental regulations or policies could prevent or delay regulatory approval of products incorporating our technologies or subject us to additional regulation. Failure or delay by us or our licensees in obtaining FDA, E.U., and other necessary regulatory approval or clearance, or the loss of previously obtained approvals, could have a material adverse effect on our business, financial condition and results of operations.
RISKS RELATING TO OUR SECURITIES
Our stock price has been volatile and may continue to be volatile.
The trading price of our common stock has been, and may continue to be, highly volatile, in large part attributable to developments and circumstances related to factors identified in “Forward-looking Statements” and “Risk Factors.” Our common stock price may rise or fall sharply at any time based on announcement regarding regulatory actions, our operations or our financial performance; as a result of sales executed by significant holders of our stock; because of short positions taken by investors from time to time in our stock; or due to factors unrelated to our performance, including industry-specific or general economic conditions. In addition, in the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were to become involved in securities litigation, it could subject us to substantial costs, divert resources and the attention of management from our business and harm our business, results of operations, financial condition and reputation. These factors may materially and adversely affect the market price of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 2. PROPERTIES.
Our principal operations are located in Eden Prairie, a suburb of Minneapolis, Minnesota, where we own a building that has approximately 64,000 square feet of space utilized by our Corporate, Medical Device and IVD reportable segments. We also own a 45,000 square foot building in Ballinasloe, Ireland dedicated to our Medical Device segment. We lease a warehouse in Eden Prairie through December 2025. We lease a 90,000 square foot facility in Eden Prairie through April 2028, which is primarily used by our Medical Device segment for operations, R&D, and redundant manufacturing capacity. We lease office space in Galway, Ireland through April 2024 dedicated to our Medical Device segment. We own an undeveloped parcel of land adjacent to our principal facility in Eden Prairie, which we may use to accommodate our growth needs. The Midcap Credit Agreement requires that all of our owned real property, including the properties set forth above, be subject to mortgages securing our obligations under the Midcap Credit Agreement.
ITEM 3. LEGAL PROCEEDINGS.
See the discussion of “Litigation” in Note 2 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K, which is incorporated herein by reference.
ITEM 4. MINE SAFETY DISCLOSURES.
Not Applicable.
32
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our stock is traded on the Nasdaq Global Select Market under the symbol “SRDX.”
Our transfer agent is:
Broadridge Corporate Issuer Solutions, Inc.
P.O. Box 1342
Brentwood, NY 11717
1-877-830-4936
According to the records of our transfer agent, as of November 18, 2022, there were 269 holders of record of our common stock.
We have never declared or paid any dividends on our common stock. We currently intend to retain future earnings, if any, for the operation and expansion of our business and to repurchase shares of our common stock under the repurchase authorization described below, if appropriate, and therefore we do not anticipate declaring or paying cash dividends in the foreseeable future. The declaration and payment by Surmodics of future dividends, if any, on our common stock will be at the sole discretion of the Board of Directors and will depend on our anticipated earnings, financial condition, capital requirements and other factors that the Board of Directors deems relevant. In addition, the MidCap Credit Agreement restricts our ability to pay dividends.
On November 6, 2015, the Company’s Board of Directors authorized it to repurchase up to an additional $20.0 million (“fiscal 2016 authorization”) of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, accelerated share repurchase (“ASR”) transactions, tender offers or by any combination of such methods. The share repurchase program does not have a fixed expiration date.
On November 5, 2014, the Company’s Board of Directors authorized it to repurchase up to $30.0 million (“fiscal 2015 authorization”) of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, ASR transactions, tender offers or by any combination of such methods. An aggregate of $20.0 million of the fiscal 2015 authorization was utilized in fiscal 2015, with an additional $4.7 million utilized in fiscal 2017. The share repurchase program does not have a fixed expiration date.
The Company has an aggregate of $25.3 million available for future common stock purchases under the current authorizations. The MidCap Credit Agreement restricts our ability to purchase our common stock.
Issuer Repurchases of Equity Securities
The following table presents the information with respect to purchases made by or on behalf of Surmodics, Inc. or any “affiliated purchaser” (as defined in Rule 10b-18(a)(3) under the Securities Exchange Act of 1934), of our common stock during the fourth quarter of fiscal 2022:
|
|
Total Number of |
|
|
Average Price Paid |
|
|
Total Number of Shares Purchased as Part of Publicly Announced Programs |
|
|
Maximum Dollar Value of Shares that May Yet Be Purchased Under the Programs |
|
||||
Period: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
July 1 – 31, 2022 |
|
|
188 |
|
|
$ |
28.00 |
|
|
|
— |
|
|
$ |
25,300,000 |
|
August 1 – 31, 2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25,300,000 |
|
September 1 – 30, 2022 |
|
|
343 |
|
|
|
29.62 |
|
|
|
— |
|
|
|
25,300,000 |
|
Total |
|
|
531 |
|
|
|
29.04 |
|
|
|
— |
|
|
|
|
|
33
Stock Performance Chart
The following chart compares the cumulative total shareholder return on the Company’s Common Stock with the cumulative total return on the Nasdaq US Benchmark Total Return Index (our broad equity market index) and the Nasdaq Medical Supplies Total Return Index (our published industry index). The comparisons assume $100 was invested on September 30, 2017 and assume reinvestment of dividends.
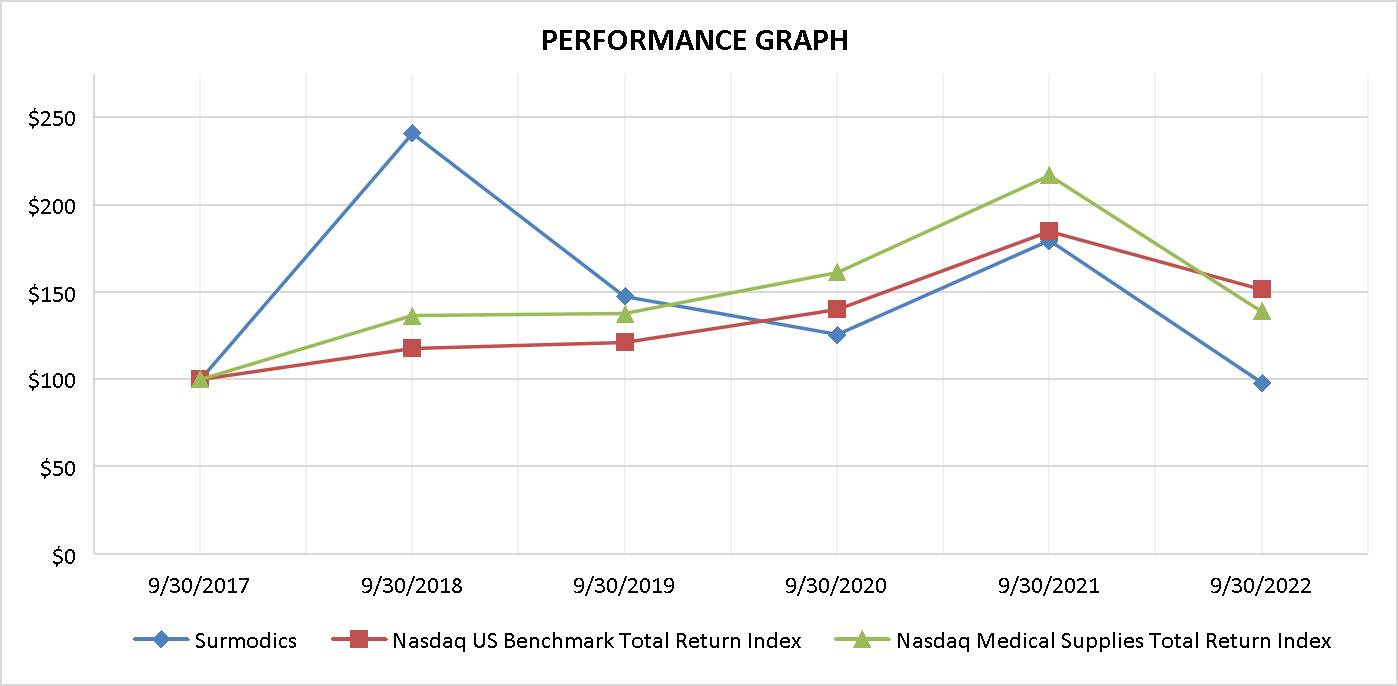
$100 investment in stock or index |
|
Ticker |
|
9/30/2017 |
|
|
9/30/2018 |
|
|
9/30/2019 |
|
|
9/30/2020 |
|
|
9/30/2021 |
|
|
9/30/2022 |
|
||||||
Surmodics |
|
SRDX |
|
$ |
100.00 |
|
|
$ |
240.81 |
|
|
$ |
147.55 |
|
|
$ |
125.52 |
|
|
$ |
179.35 |
|
|
$ |
98.06 |
|
Nasdaq US Benchmark Total Return Index |
|
NQUSBT |
|
|
100.00 |
|
|
|
117.79 |
|
|
|
121.29 |
|
|
|
140.05 |
|
|
|
184.89 |
|
|
|
151.60 |
|
Nasdaq Medical Supplies Total Return Index |
|
NQUSB20102015T |
|
|
100.00 |
|
|
|
136.45 |
|
|
|
137.57 |
|
|
|
161.28 |
|
|
|
216.78 |
|
|
|
139.08 |
|
ITEM 6. [RESERVED].
34
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis provide information management believes is useful in understanding the operating results, cash flows and financial condition of Surmodics. The following discussion should be read together with our audited consolidated financial statements and related notes appearing elsewhere in this report. Any discussion and analysis regarding our future financial condition and results of operations are forward-looking statements that involve risks, uncertainties and assumptions, as more fully identified in “Forward-looking Statements” and “Risk Factors.” Our actual future financial condition and results of operations may differ materially from those anticipated in the forward-looking statements.
Overview
Surmodics, Inc. (referred to as “Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of performance coating technologies for intravascular medical devices and chemical and biological components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics develops and commercializes highly differentiated vascular intervention medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface modification and drug-delivery coating technologies, along with its device design, development and manufacturing capabilities. The Company’s mission is to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minnesota.
Vascular Intervention Medical Device Platforms
Within our Medical Device segment, we develop and manufacture our own proprietary vascular intervention medical device products, which leverage our expertise in performance coating technologies, product design and engineering capabilities. We believe our strategy of developing our own medical device products has increased, and will continue to increase, our relevance in the medical device industry. This strategy is key to our future growth and profitability, providing us with the opportunity to capture more revenue and operating margin with vascular intervention device products than we would by licensing our device-enabling technologies.
Highlighted below are select medical device products within our development pipeline that are a focus for development and commercialization efforts. For both our thrombectomy and radial access platforms, we are pursuing commercialization via a direct sales strategy leveraging a small team of experienced sales professionals and clinical specialists. Beginning in fiscal 2022, we began to see modest, but meaningful and growing revenue associated with the adoption, utilization and sales of our Pounce™ and Sublime™ platform products.
Pounce Thrombectomy Platform
We have successfully developed, internally and through acquisitions, two U.S. Food and Drug Administration ("FDA" or the “Agency”) 510(k)-cleared mechanical thrombectomy devices for the non-surgical removal of thrombi and emboli (clots) from the peripheral vasculature (legs). In addition to FDA clearance, our Pounce Venous Thrombectomy System has received the Conformité Européenne Mark (“CE Mark”) approval prerequisite for commercialization in the European Union (”E.U.”). We believe that the ease of use, intuitive design and efficient performance of our thrombectomy products make these devices viable first-line treatment options for interventionalists. These devices include:
Sublime Radial Access Platform
We have successfully developed and secured FDA 510(k) regulatory clearance for a suite of devices that enable vascular intervention via radial (wrist) access for which commercial sales began in the first quarter of fiscal 2022. These devices include:
35
Drug-coated Balloon Platform
Surmodics’ drug-coated balloons (“DCBs”) are designed for vascular interventions to treat PAD, a condition that causes a narrowing of the blood vessels supplying the extremities.
The SurVeil DCB has the necessary regulatory approval for commercialization in the E.U., and timing of commercialization in the E.U. is at the discretion of our exclusive distribution partner, Abbott. In fiscal 2021, the TRANSCEND pivotal clinical trial of our SurVeil DCB met both the primary safety and primary efficacy endpoints and was found to be non-inferior to the control device in those endpoints.
In June 2021, we submitted the fourth and final module of our application to the FDA for premarket approval (“PMA”) of our SurVeil DCB, including certain long-term vital status data required by the FDA. The Agency provided us with comments on our PMA application and requested certain additional testing data. In October 2022, we submitted a complete response, including additional testing data, to the Agency’s comments on the PMA. The FDA may request additional information, including testing data, related to our most recent submission in support of the PMA application. Receipt of PMA from the FDA, if granted, would be expected to fulfill the requirements for a $30 million milestone payment pursuant to the Abbott Agreement (if PMA received by December 31, 2022), $27 million (if PMA received after December 31, 2022, but before June 30, 2023), or $24 million (if PMA received on or after June 30, 2023).
For more information regarding our product development and commercialization strategy, see Part I, Item 1 of this Annual Report on Form 10-K.
CARES Act Employee Retention Credit
In fiscal 2021, a benefit of $3.6 million was recorded to reduce operating costs and expenses as a result of our eligibility for the employee retention credit under the provisions of the Coronavirus Aid, Relief and Economic Security Act (the "CARES Act") enacted in March 2020. This $3.6 million benefit in fiscal 2021 reflects anticipated reimbursement of personnel expenses we incurred in fiscal 2021 and 2020 and provided a $0.5 million benefit to product costs, a $2.2 million benefit to research and development (“R&D”) expense, and a $0.9 million benefit to selling, general and administrative (“SG&A”) expense.
36
Results of Operations
Fiscal Years Ended September 30, 2022, 2021 and 2020
Revenue. Fiscal 2022 revenue was $100.0 million, a $5.2 million or 5% decrease from fiscal 2021 revenue. Fiscal 2021 revenue was $105.1 million, a $10.3 million or 11% increase from fiscal 2020 revenue. The following is a summary of revenue streams within each reportable segment.
|
Fiscal Year |
|
|
Increase/(Decrease) |
|
|
Increase/(Decrease) |
|
|||||||||||||||||||
(Dollars in thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|
2022 vs. 2021 |
|
|
2021 vs. 2020 |
|
|||||||||||||
Medical Device |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Product sales |
$ |
27,930 |
|
|
$ |
21,777 |
|
|
$ |
21,608 |
|
|
$ |
6,153 |
|
|
|
28 |
% |
|
$ |
169 |
|
|
|
1 |
% |
Royalties |
|
30,267 |
|
|
|
30,781 |
|
|
|
28,614 |
|
|
|
(514 |
) |
|
|
(2 |
)% |
|
|
2,167 |
|
|
|
8 |
% |
License fees |
|
5,981 |
|
|
|
16,275 |
|
|
|
12,020 |
|
|
|
(10,294 |
) |
|
|
(63 |
)% |
|
|
4,255 |
|
|
|
35 |
% |
Research, development and other |
|
8,211 |
|
|
|
9,420 |
|
|
|
9,159 |
|
|
|
(1,209 |
) |
|
|
(13 |
)% |
|
|
261 |
|
|
|
3 |
% |
Medical Device Revenue |
|
72,389 |
|
|
|
78,253 |
|
|
|
71,401 |
|
|
|
(5,864 |
) |
|
|
(7 |
)% |
|
|
6,852 |
|
|
|
10 |
% |
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Product sales |
|
26,691 |
|
|
|
24,701 |
|
|
|
22,709 |
|
|
|
1,990 |
|
|
|
8 |
% |
|
|
1,992 |
|
|
|
9 |
% |
Research, development and other |
|
871 |
|
|
|
2,182 |
|
|
|
754 |
|
|
|
(1,311 |
) |
|
|
(60 |
)% |
|
|
1,428 |
|
|
|
189 |
% |
In Vitro Diagnostics Revenue |
|
27,562 |
|
|
|
26,883 |
|
|
|
23,463 |
|
|
|
679 |
|
|
|
3 |
% |
|
|
3,420 |
|
|
|
15 |
% |
Total Revenue |
$ |
99,951 |
|
|
$ |
105,136 |
|
|
$ |
94,864 |
|
|
$ |
(5,185 |
) |
|
|
(5 |
)% |
|
$ |
10,272 |
|
|
|
11 |
% |
Medical Device. Revenue in our Medical Device segment was $72.4 million in fiscal 2022, a 7% decrease from $78.3 million in fiscal 2021, primarily driven by lower license fees revenue, partly offset by broad-based product sales growth.
Abbott Agreement license fee revenue is recognized as costs are incurred on a proportional basis to total expected costs for the TRANSCEND pivotal clinical trial. The percentage of costs incurred relative to total estimated costs for the TRANSCEND pivotal clinical trial of our SurVeil DCB was approximately 85%, 76% and 65% as of September 30, 2022, 2021 and 2020, respectively. We estimate this percentage will be approximately 92% by the end of fiscal 2023, with the remaining 8% of costs incurred and revenue recognized over the subsequent final two years of the TRANSCEND trial follow-up and clinical reporting period.
Future license fee revenue related to the Abbott Agreement will depend primarily on whether and when we receive the final milestone payment associated with receipt of the PMA for the SurVeil DCB. Receipt of PMA from the FDA, if granted, would be expected to fulfill the requirements for a milestone payment of up to $30 million. The milestone payment is reduced to $27 million if PMA is received after December 31, 2022 but before June 30, 2023, and to $24 million if PMA is received on or after June 30, 2023, pursuant to the terms of the Abbott Agreement. The potential revenue during fiscal 2023 associated with the $30 million, $27 million or $24 million milestone payment would be approximately $27 million, $25 million or $22 million, respectively.
In fiscal 2021, revenue in our Medical Device segment was $78.3 million, a 10% increase from $71.4 million in fiscal 2020, primarily driven by increased royalties and license fees revenue.
37
In Vitro Diagnostics. Revenue in our IVD segment was $27.6 million in fiscal 2022, a 3% increase from $26.9 million in fiscal 2021, driven primarily by broad-based product sales growth, partly offset by lower R&D and other revenue.
In fiscal 2021, revenue in our IVD segment was $26.9 million, a 15% increase from $23.5 million in fiscal 2020, driven primarily by increased sales volume of our distributed antigen products and customer development projects.
Product sales, product costs, product gross profit, product gross margin, and operating costs and expenses were as follows:
|
Fiscal Year |
|
|
Increase/(Decrease) |
|
|
Increase/(Decrease) |
|
|||||||||||||||||||
(Dollars in thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|
2022 vs. 2021 |
|
|
2021 vs. 2020 |
|
|||||||||||||
Product sales |
$ |
54,621 |
|
|
$ |
46,478 |
|
|
$ |
44,317 |
|
|
$ |
8,143 |
|
|
|
18 |
% |
|
$ |
2,161 |
|
|
|
5 |
% |
Product costs |
|
20,342 |
|
|
|
17,177 |
|
|
|
15,317 |
|
|
|
3,165 |
|
|
|
18 |
% |
|
|
1,860 |
|
|
|
12 |
% |
Product gross profit (1) |
|
34,279 |
|
|
|
29,301 |
|
|
|
29,000 |
|
|
|
4,978 |
|
|
|
17 |
% |
|
|
301 |
|
|
|
1 |
% |
% Product gross margin (2) |
|
62.8 |
% |
|
|
63.0 |
% |
|
|
65.4 |
% |
|
|
(0.2 |
) |
ppt |
|
|
|
(2.4 |
) |
ppt |
|
||||
Research and development |
|
50,609 |
|
|
|
46,734 |
|
|
|
50,188 |
|
|
|
3,875 |
|
|
|
8 |
% |
|
|
(3,454 |
) |
|
|
(7 |
)% |
% Total revenue |
|
51 |
% |
|
|
45 |
% |
|
|
53 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Selling, general and administrative |
|
46,935 |
|
|
|
30,677 |
|
|
|
28,392 |
|
|
|
16,258 |
|
|
|
53 |
% |
|
|
2,285 |
|
|
|
8 |
% |
% Total revenue |
|
47 |
% |
|
|
29 |
% |
|
|
30 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Acquired intangible asset amortization |
|
4,150 |
|
|
|
2,793 |
|
|
|
2,218 |
|
|
|
1,357 |
|
|
|
49 |
% |
|
|
575 |
|
|
|
26 |
% |
Acquisition transaction, integration and other costs |
|
— |
|
|
|
1,049 |
|
|
|
— |
|
|
|
(1,049 |
) |
|
|
|
|
|
1,049 |
|
|
|
|
||
Contingent consideration expense |
|
12 |
|
|
|
3 |
|
|
|
— |
|
|
|
9 |
|
|
|
|
|
|
3 |
|
|
|
|
||
38
Product gross margins. Product gross margins were 62.8%, 63.0% and 65.4% in fiscal 2022, 2021 and 2020, respectively.
Research and development expense. R&D expense was $50.6 million, $46.7 million and $50.2 million in fiscal 2022, 2021 and 2020, respectively.
Selling, general and administrative expense. SG&A expense was $46.9 million, $30.7 million and $28.4 million in fiscal 2022, 2021 and 2020, respectively.
Acquired intangible asset amortization. We have previously acquired certain intangible assets through business combinations, which are amortized over periods ranging from six to 14 years. The year-over-year increase in expense from amortization of the Vetex developed technology acquired in the fourth quarter of fiscal 2021 was $1.5 million and $0.5 million in fiscal 2022 and fiscal 2021, respectively.
Acquisition transaction, integration and other costs. In fiscal 2021, we incurred $1.0 million in legal, accounting and other due diligence costs specifically related to the acquisition of Vetex.
Contingent consideration expense. We have contingent consideration obligations related to business combinations. Expense (gain) recognized is related to changes in the probability and timing of achieving certain contractual milestones, as well as accretion expense for the passage of time. In fiscal 2022 and 2021, contingent consideration expense consisted of accretion for liabilities associated with the fiscal 2021 Vetex acquisition.
39
Other expense. Major classifications of other expense were as follows:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Interest expense |
$ |
(598 |
) |
|
$ |
(310 |
) |
|
$ |
(133 |
) |
Foreign exchange gain (loss) |
|
103 |
|
|
|
(170 |
) |
|
|
(248 |
) |
Investment income, net |
|
99 |
|
|
|
123 |
|
|
|
656 |
|
Loss on strategic investments and other |
|
— |
|
|
|
— |
|
|
|
(478 |
) |
Other expense |
$ |
(396 |
) |
|
$ |
(357 |
) |
|
$ |
(203 |
) |
Interest expense increased in fiscal 2022 and 2021 relative to the respective prior year due to rising interest rates and utilization of our revolving credit facility. Refer to “Liquidity and Capital Resources” for further discussion of financing arrangements and expectations for fiscal 2023 interest expense.
Foreign currency exchange gains (losses) result primarily from the impact of U.S. dollar to Euro exchange rate fluctuations on certain intercompany transactions and balances. Investment income, net declined in fiscal 2022 and 2021 relative to the respective prior year due to the decline in the balance of available-for-sale investments. In fiscal 2020, we recognized a $0.5 million impairment loss on our strategic investment in ViaCyte, Inc. to reduce the carrying value to zero.
Income tax (expense) benefit. We reported income tax expense of $(4.8) million in fiscal 2022, income tax expense of $(2.1) million in fiscal 2021, and income tax benefit of $2.6 million in fiscal 2020. Our effective tax rate was (21)%, 33% and 177% in fiscal 2022, 2021 and 2020, respectively. Recurring items cause our effective tax rate to differ from the U.S. federal statutory rate of 21%, including U.S. federal and Irish R&D credits, Irish and U.S. state tax rates, and excess tax benefits associated with stock-based compensation. In addition, the following items had a significant impact on reported tax (expense) benefit:
Segment Operating Results
Operating results for each of our reportable segments were as follows:
|
Fiscal Year |
|
|
Increase/(Decrease) |
|
||||||||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|
2022 vs. 2021 |
|
|
2021 vs. 2020 |
|
|||||
Operating (loss) income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Medical Device |
$ |
(22,923 |
) |
|
$ |
4,683 |
|
|
$ |
(3,246 |
) |
|
$ |
(27,606 |
) |
|
$ |
7,929 |
|
In Vitro Diagnostics |
|
13,073 |
|
|
|
13,770 |
|
|
|
11,771 |
|
|
|
(697 |
) |
|
|
1,999 |
|
Total segment operating (loss) income |
|
(9,850 |
) |
|
|
18,453 |
|
|
|
8,525 |
|
|
|
(28,303 |
) |
|
|
9,928 |
|
Corporate |
|
(12,247 |
) |
|
|
(11,750 |
) |
|
|
(9,776 |
) |
|
|
(497 |
) |
|
|
(1,974 |
) |
Total operating (loss) income |
$ |
(22,097 |
) |
|
$ |
6,703 |
|
|
$ |
(1,251 |
) |
|
$ |
(28,800 |
) |
|
$ |
7,954 |
|
40
Medical Device. Our Medical Device business reported an operating loss of $(22.9) million in fiscal 2022, compared to operating income of $4.7 million in fiscal 2021, representing (32)% and 6% of Medical Device revenue in fiscal 2022 and 2021, respectively.
In fiscal 2021, our Medical Device business reported operating income of $4.7 million, compared to an operating loss of $(3.2) million in fiscal 2020, representing 6% and (5)% of Medical Device revenue in fiscal 2021 and 2020, respectively.
In Vitro Diagnostics. Our IVD business reported operating income of $13.1 million in fiscal 2022, a decrease of 5% or $0.7 million compared to fiscal 2021. IVD operating income was 47% and 51% of revenue in fiscal 2022 and 2021, respectively. In fiscal 2022, R&D and other revenue decreased $1.3 million year-over-year due to the completion of a customer development program. In fiscal 2021, IVD operating income included a $0.5 million benefit associated with the employee retention credit under the CARES Act. These decreases were partly offset by a $1.1 million year-over-year increase in IVD product gross profit in fiscal 2022. IVD product gross margins were 66.5% and 67.5% for fiscal 2022 and 2021, respectively. The prior year product gross profit includes a $0.2 million benefit associated with the employee retention credit under the CARES Act. Fiscal 2022 gross margin was unfavorably impacted by a shift in revenue mix towards distributed antigen products with relatively lower gross margins, partly offset by the favorable impact of leverage on revenue growth.
41
In fiscal 2021, our IVD business reported operating income of $13.8 million in fiscal 2021, an increase of 17% or $2.0 million compared to fiscal 2020. IVD operating income was 51% and 50% of revenue in fiscal 2021 and 2020, respectively. R&D and other revenue increased $1.4 million year-over-year in fiscal 2021 from customer development project opportunities. In fiscal 2021, IVD operating income included a $0.5 million benefit associated with the employee retention credit under the CARES Act. IVD product gross profit increased $0.9 million year-over-year in fiscal 2021, and product gross margins were 67.5% and 69.4% for fiscal 2021 and 2020, respectively. Fiscal 2021 product gross margins were favorably impacted by leverage on revenue growth and a $0.2 million benefit associated with the employee retention credit under the CARES Act. This was more than offset by a shift in revenue mix towards distributed antigen products with relatively lower gross margins.
Corporate. The Corporate category includes expenses for administrative corporate functions, such as executive management, corporate accounting, legal, information technology, human resources and Board of Directors related fees and expenses, which we do not fully allocate to the Medical Device and IVD segments. Corporate also includes expenses, such as acquisition-related costs and litigation, which are not specific to a segment and thus not allocated to our reportable segments. The unallocated Corporate expense operating loss was $(12.2) million, $(11.8) million and $(9.8) million in fiscal 2022, 2021 and 2020, respectively. The year-over-year increase in Corporate expense in fiscal 2022 of $0.5 million, or 4%, was primarily related to compensation and facilities expenses. In fiscal 2021, the year-over-year increase in Corporate expense of $2.0 million, or 20%, was primarily driven by $1.0 million in Vetex acquisition transaction, integration and other costs and increased compensation expenses, partly offset by a $0.5 million benefit associated with the fiscal 2021 employee retention credit.
Cash Flow Operating Results
The following is a summary of cash flow results:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Cash (used in) provided by: |
|
|
|
|
|
|
|
|
|||
Operating activities |
$ |
(17,223 |
) |
|
$ |
15,389 |
|
|
$ |
14,010 |
|
Investing activities |
|
6,230 |
|
|
|
(25,238 |
) |
|
|
(9,066 |
) |
Financing activities |
|
(375 |
) |
|
|
10,227 |
|
|
|
(4,648 |
) |
Effect of exchange rates on changes in cash and cash equivalents |
|
(787 |
) |
|
|
(10 |
) |
|
|
128 |
|
Net change in cash and cash equivalents |
$ |
(12,155 |
) |
|
$ |
368 |
|
|
$ |
424 |
|
Operating Activities. Cash (used in) provided by operating activities totaled $(17.2) million, $15.4 million and $14.0 million in fiscal 2022, 2021 and 2020, respectively. During fiscal 2022, 2021 and 2020, we reported net (loss) income of $(27.3) million, $4.2 million and $1.1 million, respectively. Net changes in operating assets and liabilities (reduced) increased cash flows from operating activities by $(12.3) million, $(4.9) million and $1.1 million in fiscal 2022, 2021 and 2020, respectively.
Significant changes in operating assets and liabilities affecting cash flows during fiscal 2022, 2021 and 2020 included:
42
Investing Activities. Cash provided by (used in) investing activities was $6.2 million, $(25.2) million and $(9.1) million in fiscal 2022, 2021 and 2020, respectively.
Financing Activities. Cash (used in) provided by financing activities totaled $(0.4) million, $10.2 million and $(4.6) million in fiscal 2022, 2021 and 2020, respectively.
Liquidity and Capital Resources
As of September 30, 2022, working capital totaled $25.5 million, a decrease of $14.9 million from September 30, 2021. We define working capital as current assets minus current liabilities. Cash and cash equivalents and available-for-sale investments totaled $19.0 million as of September 30, 2022, a decrease of $21.9 million from $40.9 million as of September 30, 2021.
Subject to the terms of the Abbott Agreement, the Company is to receive a milestone payment under the Abbott Agreement if the SurVeil DCB receives PMA. The amount of the milestone payment is $30 million upon PMA of our SurVeil DCB (if PMA is received prior to December 31, 2022), $27 million (if PMA is received after December 31, 2022 but prior to June 30, 2023), or $24 million (if PMA is received on or after June 30, 2023), pursuant to the terms of the Abbott Agreement.
The Company proactively manages its access to capital to support liquidity and continued growth. On October 14, 2022, Surmodics entered into a new, five-year secured credit agreement with MidCap Funding IV Trust, as agent, and MidCap Financial Trust, as term loan servicer and the lenders from time to time party thereto (together “MidCap”), comprised of up to $100 million in term loans ($25 million of which is at the sole discretion of MidCap) and a $25 million revolving credit facility. The Company drew $25 million on the term loan and $5 million on the revolving credit facility at close. These proceeds were partially used to retire the Company’s existing $25 million revolving credit facility with Bridgewater Bank, of which $10 million was outstanding. Upon closing, the Company’s cash balance increased by $19.5 million. Additional draws on the term loan may be made in increments of at least $10 million, up to a total of $50 million through December 31, 2024. A second tranche of up to $25 million may be available through December 31, 2024 at MidCap’s sole discretion. Availability to draw on the five-year, $25 million revolving credit facility is based on a borrowing base consisting primarily of the Company’s inventory and receivable balances. The credit agreement calls for interest-only payments on the term loan over the first four years, which can be extended to five years if certain criteria are met. The revolving credit facility matures in five years. The Company has also entered into an interest rate swap arrangement with Wells Fargo, whereby the initial borrowing on term loan’s variable base rate was fixed at 10.205% per annum for the five-year loan term. The revolving credit facility has an annual interest rate equal to 3.00% plus the greater of Term SOFR (as defined in the credit agreement) or 1.50%. The Company expects total interest expense under the credit agreement to be approximately $3.4 million in fiscal 2023.
As of September 30, 2022, the Company’s shelf registration statement with the SEC allows the Company to offer potentially up to $200 million in debt securities, common stock, preferred stock, warrants, and other securities or any such combination of such securities in amounts, at prices, and on terms announced if and when the securities are ever offered.
43
In fiscal 2023, we anticipate an increase in SG&A expenditures of between $12.0 million and $13.5 million, as well as an increase in capital expenditures. We expect that increasing SG&A expenditures in fiscal 2023 will exceed any associated increases in revenues, and therefore will reduce our cash flow from operations. We also anticipate R&D expenses will continue to be significant in fiscal 2023, primarily related to medical device product development, including continued investment in our Pounce and Sublime product platforms. We believe that our existing cash and cash equivalents and available-for-sale investments, which totaled $19.0 million as of September 30, 2022, together with cash flow from operations and our revolving credit facility and term loans, will provide liquidity sufficient to meet our cash needs and fund our operations and planned capital expenditures for fiscal 2023. There can be no assurance, however, that our business will continue to generate cash flows at historic levels.
Beyond fiscal 2023, our cash requirements will depend extensively on the timing of market introduction and extent of market acceptance of products in our medical device product portfolio, including our SurVeil DCB if PMA is received. Our long-term cash requirements also will be significantly impacted by the level of our investment in commercialization of our vascular intervention device products and whether we make future corporate transactions. We cannot accurately predict our long-term cash requirements at this time. We may seek additional sources of liquidity and capital resources, including through borrowing, debt or equity financing or corporate transactions to generate cashflow. There can be no assurance that such transactions will be available to us on favorable terms, if at all.
Below is a summary of short-term and long-term anticipated cash requirements under contractual obligations existing as of September 30, 2022.
|
September 30, 2022 |
|
|||||||||
(In thousands) |
Total |
|
|
Fiscal 2023 |
|
|
After Fiscal 2023 |
|
|||
Operating leases (1) |
$ |
6,438 |
|
|
$ |
1,172 |
|
|
$ |
5,266 |
|
Asset acquisition & business combination obligations (2) |
|
5,500 |
|
|
|
1,000 |
|
|
|
4,500 |
|
Clinical trial CRO obligations (3) |
|
4,497 |
|
|
|
2,135 |
|
|
|
2,362 |
|
Total gross value |
$ |
16,435 |
|
|
$ |
4,307 |
|
|
$ |
12,128 |
|
For additional information regarding the above obligations, see Notes 2, 11 and 12 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
As of September 30, 2022, we did not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures, or capital resources that is material to investors.
Share Purchase Activity
Our Board of Directors has authorized the repurchase of up to an additional $25.3 million of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, accelerated share repurchase transactions, tender offers or by any combination of such methods. The authorization has no fixed expiration date. However, our credit agreement with MidCap prohibits us from acquiring outstanding shares of the Company’s common stock.
44
Customer Concentrations
Revenue from customers that equaled or exceeded 10% of total revenue was as follows:
|
Fiscal Year |
|
|||||||||
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Abbott |
|
11 |
% |
|
|
21 |
% |
|
|
19 |
% |
Medtronic |
|
13 |
% |
|
|
13 |
% |
|
|
14 |
% |
Our licensed technologies provide royalties and license fee revenue. We have agreements with a diverse base of customers, and certain customers have multiple products using our technology. Abbott and Medtronic plc (“Medtronic”) are our largest customers. Abbott has several separately licensed products, including the SurVeil DCB license, which generate royalties and license fee revenue for Surmodics. Revenue from the SurVeil DCB license represented 6%, 15% and 13% of total revenue for fiscal 2022, 2021 and 2020, respectively. Apart from the SurVeil DCB license, Abbott has several separately licensed products which generate revenue for Surmodics, none of which represented more than 3% of total revenue for fiscal 2022. Medtronic has several separately licensed products that generate royalties revenue for Surmodics, none of which represented more than 5% of our total revenue for fiscal 2022.
Our licensing agreements with many of our customers, including most of our significant customers, cover many licensed products that each separately generates royalties revenue. This structure reduces the potential risk to our operations that may result from reduced sales (or the termination of a license) of a single product for any specific customer.
New Accounting Pronouncements
Information regarding new accounting pronouncements is included in Note 2 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”). The preparation of these consolidated financial statements is based in part on the application of significant accounting policies, many of which require management to make estimates and assumptions; see Notes 1 and 2 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K. Actual results may differ from these estimates and such differences could materially impact our financial condition and results of operations.
Critical accounting estimates are those that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on our financial condition and results of operations. They require the application of management’s most challenging subjective or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Critical accounting estimates involve judgments and uncertainties that are sufficiently likely to result in materially different results under different assumptions and conditions. We believe the following are critical areas in the application of our accounting estimates that currently affect our financial condition and results of operations.
Revenue Recognition
We license technology to medical device manufacturers (third parties) and collect royalties based on the greater of the contractual percentage of a customer’s sales of products incorporating our licensed technologies or minimum contractual royalties. Sales-based royalties revenue is recognized as our license customers sell products containing our technologies, which is generally reported to us a quarter after those sales occur. This requires us to estimate the revenue earned on these arrangements and record it prior to our customers reporting the underlying sales to us. Sales-based royalties are estimated using the most-likely amount method based on historical sales information, adjusted for known changes, such as product launches and patent expirations. We also consider macroeconomic factors affecting the medical device market. These inputs require significant management judgement and are updated quarterly. Minimum royalty fees are recognized through the non-cancellable period, which is generally 90 days, but can be up to one year. Revenue related to contingent milestones is recognized upon the achievement of the milestone, provided collectability is assured. Customer advances are accounted for as a liability (deferred revenue) until all criteria for revenue recognition have been met.
45
Revenue associated with our license and development agreement with Abbott is recognized as the clinical and regulatory activities are performed and control is transferred which is measured based on actual costs incurred relative to the expected total cost of the underlying activities, which consist of the TRANSCEND clinical trial. A significant component of the cost of this trial is the cost of our outsourced clinical trial CRO consultants, which are estimated based on executed statements of work, project budgets, and patient enrollment and follow-up timing, among other things. Costs related to the clinical and regulatory activities are expensed in the period incurred. A significant change to the Company’s estimate of the costs to complete the TRANSCEND clinical trial could have a material effect on the Company’s results of operations. The total expected cost of the trial is a significant management estimate and is reviewed and assessed each reporting period. The current portion of deferred revenue on the consolidated balance sheet represents the amount of deferred revenue that is expected to be recognized over the next year, based on estimated costs to be incurred. The estimate of future revenue from the Abbott Agreement will continue to be monitored and adjusted based on estimates in effect each period-end. For further disclosures related to revenue recognition, see Notes 2, 3 and 4 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
Goodwill and Definite-lived Intangible Assets
Our estimates associated with the annual test of goodwill for impairment, as well as the as-needed assessment of the recoverability of definite-lived intangible assets, are considered critical due to the amount of these assets recorded on our consolidated balance sheets and the judgment required.
We record all assets and liabilities acquired in business acquisitions at fair value, including goodwill and other intangible assets. The initial recognition of goodwill and other intangible assets requires management to make subjective judgments concerning estimates of how the acquired assets will perform in the future using valuation methods including discounted cash flow analysis.
Goodwill represents the excess of the purchase price of an acquired business over the fair value assigned to the assets purchased and liabilities assumed. Goodwill is not amortized but is subject, at a minimum, to annual tests for impairment in accordance with accounting guidance for goodwill. The carrying amount of goodwill is evaluated annually, and between annual evaluations if events occur or circumstances change indicating that it is more likely than not that the fair value of a reporting unit is less than its carrying amount.
Our reporting units are the Medical Device and In Vitro Diagnostics reportable segments. Inherent in the determination of fair value of the reporting units are certain estimates and judgments, including the interpretation of current economic indicators and market valuations, as well as management’s strategic plans with regard to its operations. When utilizing a quantitative assessment, we determine fair value at the reporting unit level based on a combination of an income approach and market approach. The income approach is based on estimated future cash flows, discounted at a rate that approximates the cost of capital of a market participant, while the market approach is based on sales and/or earnings multiples of similar companies. These approaches use significant estimates and assumptions, including projected future cash flows and the timing of those cash flows, discount rates reflecting risks inherent in future cash flows, perpetual growth rates, and determination of appropriate market comparables.
We perform our annual assessment of goodwill for impairment as of July 1st of each fiscal year. Goodwill was not impaired in either reporting unit based on the outcome of the fiscal 2022 annual impairment test, which utilized a quantitative assessment. No goodwill impairment charges were recorded in fiscal 2022, 2021 and 2020.
With respect to definite-lived intangible assets, we periodically evaluate whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of such assets. If such events or circumstances indicate that the carrying amount of these assets may not be recoverable, management would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected future cash flows (undiscounted and without interest charges) were less than the carrying amount of the assets, we would recognize an impairment charge to reduce such assets to their fair value. In fiscal 2022, 2021 and 2020, no impairment charges were recorded related to our definite-lived intangible assets.
46
Income Taxes
Significant judgment is required in evaluating our tax positions and in determining income tax expense (benefit), deferred tax assets and liabilities, and any valuation allowance recorded against our deferred tax assets. We evaluate the recoverability of deferred tax assets based on available evidence. This process involves significant management judgment about assumptions that are subject to change from period to period based on changes in tax laws or variances between future projected operating performance and actual results. Under GAAP, we establish a valuation allowance for deferred tax assets if we determine, based on available evidence at the time the determination is made, that it is more likely than not (defined as a likelihood of more than 50%) that all or a portion of the deferred tax assets will not be realized. In making this determination, we evaluate all positive and negative evidence as of the end of each reporting period. Future adjustments (either increases or decreases) to the deferred tax asset valuation allowance are determined based upon changes in the expected realization of the net deferred tax assets. In fiscal 2022, we recorded a non-cash charge to income tax expense of $10.2 million that resulted from the establishment of a full valuation allowance against U.S. net deferred tax assets as of September 30, 2022. The realization of the deferred tax assets ultimately depends on the existence of sufficient taxable income or tax liability in either the carry-back or carry-forward periods under the tax law. Due to significant estimates used to establish the valuation allowance and the potential for changes in facts and circumstances, it is reasonably possible that we will be required to record additional adjustments to the valuation allowance in future reporting periods that could have a material effect on our results of operations.
We establish reserves for uncertain tax positions when, despite our belief that our tax return positions are fully supportable, we believe that certain positions are likely to be challenged and that we may or may not prevail. Under GAAP, if we determine that a tax position is more likely than not of being sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement. We presume that all tax positions will be examined by a taxing authority with full knowledge of all relevant information. The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations. We regularly monitor our tax positions and tax liabilities. We reevaluate the technical merits of our tax positions and recognize an uncertain tax benefit, or derecognize a previously recorded tax benefit, when there is: (i) a completion of a tax audit, (ii) effective settlement of an issue, (iii) a change in applicable tax law including a tax case or legislative guidance, or (iv) the expiration of the applicable statute of limitations. Significant judgment is required in accounting for tax reserves. Although we believe that we have adequately provided for liabilities resulting from tax assessments by taxing authorities, positions taken by these tax authorities could have a material impact on our results of operations.
Business Acquisitions
We account for acquired businesses using the acquisition method of accounting which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective fair values. The judgments made in determining the estimated fair value assigned to each class of assets acquired and liabilities assumed, as well as asset lives, can materially impact our results of operations. Accordingly, for significant items, we typically engage a third-party valuation firm. There are several methods that can be used to determine the fair value of assets acquired and liabilities assumed in a business combination. For intangible assets, we historically have utilized the income method. The income method starts with a forecast of all of the expected future net cash flows attributable to the subject intangible asset. These cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the cash flow streams. Some of the more significant estimates and assumptions inherent in the income method (or other methods) include the projected future cash flows (including timing) and the discount rate reflecting the risks inherent in the future cash flows. Estimating the useful life of an intangible asset also requires judgment. For example, different types of intangible assets will have different useful lives, influenced by the nature of the asset, competitive environment and rate of change in the industry. All of these judgments and estimates can significantly impact the determination of the amortization period of the intangible asset, and thus net income. Contingent consideration liabilities are remeasured to fair value each reporting period using discount rates, probabilities of payment and projected payment dates. Increases or decreases in the fair value of the contingent consideration liability can result from changes in the timing or likelihood of achieving value-enhancing milestones and changes in discount periods and rates. Projected contingent payment amounts are discounted back to the current period using a discount cash flow model. For further disclosures related to acquisitions and contingent consideration, see Notes 2, 5 and 12 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
47
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our investment policy requires investments with high credit quality issuers and limits the amount of credit exposure to any one issuer. Our investments consist principally of interest-bearing corporate debt securities with varying maturity dates, which generally are less than one year. Because of the credit criteria of our investment policies, the primary market risk associated with these investments is interest rate risk. As of September 30, 2022, we did not hold any available-for-sale debt securities. Therefore, interest rate fluctuations relating to investments would have an insignificant impact on our results of operations or cash flows. Our policy also allows the Company to hold a substantial portion of funds in cash and cash equivalents, which are defined as financial instruments with original maturities of three months or less and may include money market instruments, certificates of deposit, repurchase agreements and commercial paper instruments.
Loans under the Midcap credit agreement bear interest at floating rates tied to Term SOFR. As a result, changes in Term SOFR can affect our results of operation and cash flows to the extent we do not have effective interest rate swap arrangements in place. On October 14, 2022, we entered into a five-year interest rate swap transaction with Wells Fargo Bank, N.A. with respect to $25.0 million of notional value of the term loans funded under the MidCap credit agreement. The interest rate swap transaction fixes at 4.455% the one-month Term SOFR portion of interest rate under the $25.0 million initial Term Loan funded such that the interest rate on the initial Term Loan will be 10.205% through its maturity. We have no other swap arrangements in place for any other loans under the Midcap credit agreement.
Management believes that a reasonable change in raw material prices would not have a material impact on future earnings or cash flows because the Company’s inventory exposure is not material.
We are exposed to increasing Euro currency risk with respect to our manufacturing operations in Ireland. In a period where the U.S. dollar is strengthening or weakening relative to the Euro, our revenue and expenses denominated in Euro currency are translated into U.S. dollars at a lower or higher value than they would be in an otherwise constant currency exchange rate environment. All sales transactions are denominated in U.S. dollars or Euros. We generate royalties revenue from the sale of customer products in foreign jurisdictions. Royalties generated in foreign jurisdictions by customers are converted and paid in U.S. dollars per contractual terms. Substantially all of our purchasing transactions are denominated in U.S. dollars or Euros. To date, we have not entered into any foreign currency forward exchange contracts or other derivative financial instruments to hedge the effects of adverse fluctuations in foreign currency exchange rates.
48
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
TABLE OF CONTENTS
|
Page (s) |
Reports of Independent Registered Public Accounting Firm (PCAOB ID No. 34) |
50 to 52 |
53 |
|
54 |
|
55 |
|
56 |
|
57 to 58 |
|
59 to 82 |
49
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of Surmodics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Surmodics, Inc. and subsidiaries (the "Company") as of September 30, 2022 and 2021, the related consolidated statements of operations, comprehensive (loss) income, shareholders' equity, and cash flows, for each of the three years in the period ended September 30, 2022, and the related notes and the financial statement schedule listed in the Table of Contents at Item 15 (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended September 30, 2022, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of September 30, 2022, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated November 23, 2022, expressed an unqualified opinion on the Company's internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Royalties and license fees – Sales-based Royalty Estimates — Refer to Note 2 of the financial statements
Critical Audit Matter Description
Royalty revenue consists of sales-based royalties earned under licenses of performance coating technologies. Performance obligations under these licenses, which consist of the right to use the Company’s proprietary technology, are satisfied at a point in time corresponding with delivery of the underlying technology rights to the customer, which is generally upon transfer of the licensed technology to the customer. Sales-based royalty revenue represents variable consideration under the license agreements and is recognized in the period a customer sells products incorporating the Company’s licensed technologies. The Company estimates sales-based royalty revenue earned but unpaid at each reporting period using the expected value method based on historical sales information, adjusted for known changes such as product launches and patent expirations. The Company also considers macroeconomic factors affecting the medical device market. These inputs require significant management judgment.
50
Given the significant judgments made by management relating to the inputs used in the expected value method to estimate the sales-based royalties earned under licenses of performance coating technologies, auditing such inputs required an increased extent of audit effort and a high degree of auditor judgment when performing audit procedures and evaluating the results of those procedures.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the sales-based royalty estimates under licenses of performance coating technologies included the following, among others:
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
November 23, 2022
We have served as the Company's auditor since 2002.
51
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of Surmodics, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Surmodics, Inc. and subsidiaries (the “Company”) as of September 30, 2022, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2022, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended September 30, 2022, of the Company and our report dated November 23, 2022, expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
November 23, 2022
52
Surmodics, Inc. and Subsidiaries
Consolidated Balance Sheets
As of September 30,
(In thousands, except per share data) |
2022 |
|
|
2021 |
|
||
ASSETS |
|
|
|
|
|
||
Current Assets: |
|
|
|
|
|
||
Cash and cash equivalents |
$ |
18,998 |
|
|
$ |
31,153 |
|
Available-for-sale securities |
|
— |
|
|
|
7,717 |
|
Accounts receivable, net of allowances of $81 and $119 as of |
|
10,452 |
|
|
|
9,169 |
|
Contract assets — royalties and license fees |
|
7,116 |
|
|
|
7,091 |
|
Inventories, net |
|
11,819 |
|
|
|
6,760 |
|
Income tax receivable |
|
2,438 |
|
|
|
1,912 |
|
Prepaids and other |
|
6,764 |
|
|
|
6,453 |
|
Total Current Assets |
|
57,587 |
|
|
|
70,255 |
|
Property and equipment, net |
|
27,148 |
|
|
|
30,090 |
|
Available-for-sale securities |
|
— |
|
|
|
2,002 |
|
Deferred income taxes |
|
— |
|
|
|
5,867 |
|
Intangible assets, net |
|
28,145 |
|
|
|
37,054 |
|
Goodwill |
|
40,710 |
|
|
|
45,606 |
|
Other assets |
|
4,769 |
|
|
|
3,718 |
|
Total Assets |
$ |
158,359 |
|
|
$ |
194,592 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
||
Current Liabilities: |
|
|
|
|
|
||
Accounts payable |
$ |
3,136 |
|
|
$ |
1,783 |
|
Accrued liabilities: |
|
|
|
|
|
||
Compensation |
|
8,929 |
|
|
|
8,480 |
|
Accrued other |
|
5,854 |
|
|
|
4,905 |
|
Short-term borrowings |
|
10,000 |
|
|
|
10,000 |
|
Deferred revenue |
|
4,160 |
|
|
|
4,647 |
|
Total Current Liabilities |
|
32,079 |
|
|
|
29,815 |
|
Deferred revenue, less current portion |
|
5,088 |
|
|
|
10,301 |
|
Deferred income taxes |
|
2,027 |
|
|
|
2,742 |
|
Other long-term liabilities |
|
10,773 |
|
|
|
11,649 |
|
Total Liabilities |
|
49,967 |
|
|
|
54,507 |
|
|
|
|
|
|
|||
Stockholders’ Equity: |
|
|
|
|
|
||
Series A preferred stock — $.05 par value, 450 shares authorized; no shares issued and outstanding |
|
|
|
|
|
||
Common stock — $.05 par value, 45,000 shares authorized; 14,029 and 13,899 shares issued and outstanding, as of September 30, 2022 and 2021, respectively |
|
701 |
|
|
|
695 |
|
Additional paid-in capital |
|
28,774 |
|
|
|
21,598 |
|
Accumulated other comprehensive (loss) income |
|
(9,874 |
) |
|
|
1,727 |
|
Retained earnings |
|
88,791 |
|
|
|
116,065 |
|
Total Stockholders’ Equity |
|
108,392 |
|
|
|
140,085 |
|
Total Liabilities and Stockholders’ Equity |
$ |
158,359 |
|
|
$ |
194,592 |
|
The accompanying notes are an integral part of these consolidated financial statements.
53
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Operations
For the Fiscal Year Ended September 30,
(In thousands, except per share data) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Revenue: |
|
|
|
|
|
|
|
|
|||
Product sales |
$ |
54,621 |
|
|
$ |
46,478 |
|
|
$ |
44,317 |
|
Royalties and license fees |
|
36,248 |
|
|
|
47,056 |
|
|
|
40,634 |
|
Research, development and other |
|
9,082 |
|
|
|
11,602 |
|
|
|
9,913 |
|
Total revenue |
|
99,951 |
|
|
|
105,136 |
|
|
|
94,864 |
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|||
|
20,342 |
|
|
|
17,177 |
|
|
|
15,317 |
|
|
Research and development |
|
50,609 |
|
|
|
46,734 |
|
|
|
50,188 |
|
Selling, general and administrative |
|
46,935 |
|
|
|
30,677 |
|
|
|
28,392 |
|
Acquired intangible asset amortization |
|
4,150 |
|
|
|
2,793 |
|
|
|
2,218 |
|
Acquisition transaction, integration and other costs |
|
— |
|
|
|
1,049 |
|
|
|
— |
|
Contingent consideration expense |
|
12 |
|
|
|
3 |
|
|
|
— |
|
Total operating costs and expenses |
|
122,048 |
|
|
|
98,433 |
|
|
|
96,115 |
|
Operating (loss) income |
|
(22,097 |
) |
|
|
6,703 |
|
|
|
(1,251 |
) |
Other expense: |
|
|
|
|
|
|
|
|
|||
Interest expense |
|
(598 |
) |
|
|
(310 |
) |
|
|
(133 |
) |
Foreign exchange gain (loss) |
|
103 |
|
|
|
(170 |
) |
|
|
(248 |
) |
Investment income, net |
|
99 |
|
|
|
123 |
|
|
|
656 |
|
Loss on strategic investments and other |
|
— |
|
|
|
— |
|
|
|
(478 |
) |
Other expense |
|
(396 |
) |
|
|
(357 |
) |
|
|
(203 |
) |
(Loss) income before income taxes |
|
(22,493 |
) |
|
|
6,346 |
|
|
|
(1,454 |
) |
Income tax (expense) benefit |
|
(4,781 |
) |
|
|
(2,109 |
) |
|
|
2,577 |
|
Net (loss) income |
$ |
(27,274 |
) |
|
$ |
4,237 |
|
|
$ |
1,123 |
|
|
|
|
|
|
|
|
|
|
|||
Basic net (loss) income per share |
$ |
(1.96 |
) |
|
$ |
0.31 |
|
|
$ |
0.08 |
|
Diluted net (loss) income per share |
$ |
(1.96 |
) |
|
$ |
0.30 |
|
|
$ |
0.08 |
|
|
|
|
|
|
|
|
|
|
|||
Weighted average number of shares outstanding: |
|
|
|
|
|
|
|
|
|||
Basic |
|
13,916 |
|
|
|
13,765 |
|
|
|
13,552 |
|
Diluted |
|
13,916 |
|
|
|
13,989 |
|
|
|
13,812 |
|
The accompanying notes are an integral part of these consolidated financial statements.
54
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Comprehensive (Loss) Income
For the Fiscal Year Ended September 30,
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Net (loss) income |
$ |
(27,274 |
) |
|
$ |
4,237 |
|
|
$ |
1,123 |
|
Other comprehensive (loss) income: |
|
|
|
|
|
|
|
|
|||
Net changes related to available-for-sale securities, net of tax |
|
(1 |
) |
|
|
1 |
|
|
|
(10 |
) |
Foreign currency translation adjustments |
|
(11,600 |
) |
|
|
(1,448 |
) |
|
|
2,788 |
|
Other comprehensive (loss) income |
|
(11,601 |
) |
|
|
(1,447 |
) |
|
|
2,778 |
|
Comprehensive (loss) income |
$ |
(38,875 |
) |
|
$ |
2,790 |
|
|
$ |
3,901 |
|
The accompanying notes are an integral part of these consolidated financial statements.
55
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Equity
For the Fiscal Years Ended September 30, 2022, 2021 and 2020
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
Total |
|
||||||
|
Common Stock |
|
|
Paid-In |
|
|
Comprehensive |
|
|
Retained |
|
|
Stockholders’ |
|
|||||||||
(In thousands) |
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Earnings |
|
|
Equity |
|
||||||
Balance at September 30, 2019 |
|
13,504 |
|
|
$ |
675 |
|
|
$ |
10,740 |
|
|
$ |
396 |
|
|
$ |
110,705 |
|
|
$ |
122,516 |
|
Net income |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,123 |
|
|
|
1,123 |
|
Other comprehensive income |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,778 |
|
|
|
— |
|
|
|
2,778 |
|
Issuance of common stock |
|
149 |
|
|
|
8 |
|
|
|
492 |
|
|
|
— |
|
|
|
— |
|
|
|
500 |
|
Common stock options exercised, net |
|
64 |
|
|
|
3 |
|
|
|
1,112 |
|
|
|
— |
|
|
|
— |
|
|
|
1,115 |
|
Purchase of common stock to pay |
|
(45 |
) |
|
|
(2 |
) |
|
|
(2,428 |
) |
|
|
— |
|
|
|
— |
|
|
|
(2,430 |
) |
Stock-based compensation |
|
— |
|
|
|
— |
|
|
|
5,453 |
|
|
|
— |
|
|
|
— |
|
|
|
5,453 |
|
Balance at September 30, 2020 |
|
13,672 |
|
|
|
684 |
|
|
|
15,369 |
|
|
|
3,174 |
|
|
|
111,828 |
|
|
|
131,055 |
|
Net income |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,237 |
|
|
|
4,237 |
|
Other comprehensive loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,447 |
) |
|
|
— |
|
|
|
(1,447 |
) |
Issuance of common stock |
|
100 |
|
|
|
5 |
|
|
|
614 |
|
|
|
— |
|
|
|
— |
|
|
|
619 |
|
Common stock options exercised, net |
|
146 |
|
|
|
7 |
|
|
|
2,502 |
|
|
|
— |
|
|
|
— |
|
|
|
2,509 |
|
Purchase of common stock to pay |
|
(19 |
) |
|
|
(1 |
) |
|
|
(2,750 |
) |
|
|
— |
|
|
|
— |
|
|
|
(2,751 |
) |
Stock-based compensation |
|
— |
|
|
|
— |
|
|
|
5,863 |
|
|
|
— |
|
|
|
— |
|
|
|
5,863 |
|
Balance at September 30, 2021 |
|
13,899 |
|
|
|
695 |
|
|
|
21,598 |
|
|
|
1,727 |
|
|
|
116,065 |
|
|
|
140,085 |
|
Net loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(27,274 |
) |
|
|
(27,274 |
) |
Other comprehensive loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(11,601 |
) |
|
|
— |
|
|
|
(11,601 |
) |
Issuance of common stock |
|
124 |
|
|
|
6 |
|
|
|
826 |
|
|
|
— |
|
|
|
— |
|
|
|
832 |
|
Common stock options exercised, net |
|
27 |
|
|
|
1 |
|
|
|
413 |
|
|
|
— |
|
|
|
— |
|
|
|
414 |
|
Purchase of common stock to pay |
|
(21 |
) |
|
|
(1 |
) |
|
|
(1,120 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,121 |
) |
Stock-based compensation |
|
— |
|
|
|
— |
|
|
|
7,057 |
|
|
|
— |
|
|
|
— |
|
|
|
7,057 |
|
Balance at September 30, 2022 |
|
14,029 |
|
|
$ |
701 |
|
|
$ |
28,774 |
|
|
$ |
(9,874 |
) |
|
$ |
88,791 |
|
|
$ |
108,392 |
|
The accompanying notes are an integral part of these consolidated financial statements.
56
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
For the Fiscal Year Ended September 30,
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Operating Activities: |
|
|
|
|
|
|
|
|
|||
Net (loss) income |
$ |
(27,274 |
) |
|
$ |
4,237 |
|
|
$ |
1,123 |
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
|
|
|
|
|
|
|
|
|||
Depreciation and amortization |
|
9,142 |
|
|
|
8,017 |
|
|
|
7,263 |
|
Stock-based compensation |
|
7,057 |
|
|
|
5,863 |
|
|
|
5,453 |
|
Noncash lease expense |
|
529 |
|
|
|
308 |
|
|
|
246 |
|
Provision for credit losses |
|
5 |
|
|
|
(11 |
) |
|
|
73 |
|
Deferred taxes |
|
5,268 |
|
|
|
1,651 |
|
|
|
(1,139 |
) |
Payment of contingent consideration obligations in excess of acquisition-date value |
|
— |
|
|
|
— |
|
|
|
(608 |
) |
Loss on strategic investment |
|
— |
|
|
|
— |
|
|
|
479 |
|
Other |
|
326 |
|
|
|
181 |
|
|
|
5 |
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|||
Accounts receivable and contract assets |
|
(1,522 |
) |
|
|
(2,480 |
) |
|
|
3,461 |
|
Inventories |
|
(5,060 |
) |
|
|
(818 |
) |
|
|
(1,377 |
) |
Prepaids and other |
|
(665 |
) |
|
|
(2,391 |
) |
|
|
410 |
|
Accounts payable |
|
1,608 |
|
|
|
264 |
|
|
|
(483 |
) |
Accrued liabilities |
|
132 |
|
|
|
1,406 |
|
|
|
1,847 |
|
Income taxes |
|
(1,069 |
) |
|
|
210 |
|
|
|
(1,558 |
) |
Deferred revenue |
|
(5,700 |
) |
|
|
(1,048 |
) |
|
|
(1,185 |
) |
Net cash (used in) provided by operating activities |
|
(17,223 |
) |
|
|
15,389 |
|
|
|
14,010 |
|
Investing Activities: |
|
|
|
|
|
|
|
|
|||
Purchases of property and equipment |
|
(3,370 |
) |
|
|
(5,279 |
) |
|
|
(3,671 |
) |
Payment for acquisition of intangible assets |
|
— |
|
|
|
(1,000 |
) |
|
|
— |
|
Purchases of available-for-sale securities |
|
— |
|
|
|
(22,723 |
) |
|
|
(59,917 |
) |
Sales and maturities of available-for-sale securities |
|
9,600 |
|
|
|
43,317 |
|
|
|
54,522 |
|
Purchase of business, net of acquired cash |
|
— |
|
|
|
(39,553 |
) |
|
|
— |
|
Net cash provided by (used in) investing activities |
|
6,230 |
|
|
|
(25,238 |
) |
|
|
(9,066 |
) |
Financing Activities: |
|
|
|
|
|
|
|
|
|||
Proceeds from short-term borrowings |
|
— |
|
|
|
10,000 |
|
|
|
— |
|
Issuance of common stock |
|
1,246 |
|
|
|
3,128 |
|
|
|
1,615 |
|
Payments for taxes related to net share settlement of equity awards |
|
(1,121 |
) |
|
|
(2,751 |
) |
|
|
(2,534 |
) |
Payment of deferred financing costs |
|
— |
|
|
|
— |
|
|
|
(137 |
) |
Payments for acquisition of in-process research and development |
|
(500 |
) |
|
|
(150 |
) |
|
|
(1,000 |
) |
Payment of contingent consideration obligations |
|
— |
|
|
|
— |
|
|
|
(2,592 |
) |
Net cash (used in) provided by financing activities |
|
(375 |
) |
|
|
10,227 |
|
|
|
(4,648 |
) |
Effect of exchange rate changes on cash |
|
(787 |
) |
|
|
(10 |
) |
|
|
128 |
|
Net change in cash and cash equivalents |
|
(12,155 |
) |
|
|
368 |
|
|
|
424 |
|
Cash and Cash Equivalents: |
|
|
|
|
|
|
|
|
|||
Beginning of year |
|
31,153 |
|
|
|
30,785 |
|
|
|
30,361 |
|
End of year |
$ |
18,998 |
|
|
$ |
31,153 |
|
|
$ |
30,785 |
|
The accompanying notes are an integral part of these consolidated financial statements.
57
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows (Continued)
For the Fiscal Year Ended September 30,
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Supplemental Information: |
|
|
|
|
|
|
|
|
|||
Cash paid for income taxes |
$ |
416 |
|
|
$ |
160 |
|
|
$ |
30 |
|
Cash paid for interest |
|
415 |
|
|
|
74 |
|
|
|
— |
|
Noncash financing and investing activities: |
|
|
|
|
|
|
|
|
|||
Acquisition of property and equipment and intangible assets, net of refundable credits in other current assets and liabilities |
|
70 |
|
|
|
211 |
|
|
|
1,306 |
|
Right-of-use assets and property and equipment obtained in exchange for new operating lease liabilities |
|
1,725 |
|
|
|
234 |
|
|
|
1,181 |
|
Deferred and contingent consideration assumed in business acquisition |
|
— |
|
|
|
4,071 |
|
|
|
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
58
Surmodics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Organization
Description of Business
Surmodics, Inc. and subsidiaries (referred to as “Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of performance coating technologies for intravascular medical devices and chemical and biological components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics develops and commercializes highly differentiated vascular intervention medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface modification and drug-delivery coating technologies, along with its device design, development and manufacturing capabilities. The Company’s mission is to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minnesota.
Basis of Presentation and Principles of Consolidation
The consolidated financial statements include all accounts and wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”). All intercompany transactions have been eliminated. The Company operates on a fiscal year ending on September 30.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Ultimate results could differ from those estimates.
2. Summary of Significant Accounting Policies and Select Balance Sheet Information
Cash and Cash Equivalents
Cash and cash equivalents consist of financial instruments with maturities of three months or less at the Company’s acquisition date of the security and are stated at cost which approximates fair value and may include money market instruments, certificates of deposit, repurchase agreements and commercial paper instruments.
Accounts Receivable, Net
We grant credit to customers in the normal course of business and maintain an allowance for credit losses. The allowance for credit losses reflects the current estimate of credit losses expected to be incurred over the life of the accounts receivable. We consider various factors in establishing, monitoring and adjusting the allowance for credit losses including the aging of accounts and aging trends, the historical level of charge-offs, and specific exposures related to particular customers. We base our estimates of credit loss reserves on historical experience and adjust, as necessary, to reflect current conditions using reasonable and supportable forecasts not already reflected in the historical loss information.
Investments
As of September 30, 2022 and 2021, investments in available-for-sale debt securities totaled zero and $9.7 million, respectively, on the consolidated balance sheets. As of September 30, 2021, investments consisted of commercial paper and corporate bond securities, were classified as available-for-sale, and were reported at fair value. Interest earned on debt securities, including amortization of premiums and accretion of discounts, is included in investment income, net within other expense. Realized gains and losses from the sales of debt securities, which are included in other expense, are determined using the specific identification method. Investment purchases are accounted for on the date the trade is executed, which may not be the same as the date the transaction is cash settled. Unrealized gains and losses, net of tax, are excluded from the consolidated statements of operations and reported on the consolidated statements of comprehensive (loss) income as well as a separate component of stockholders’ equity on the consolidated balance sheets. For investments in an unrealized loss position, we make the following assessments. If it is more likely than not we will sell the investment before recovery of its amortized cost basis, we write down the security’s amortized cost basis to fair value and reclassify the net unrealized loss from accumulated other comprehensive (loss) income to other expense. If the decline in fair value is deemed to be due to a credit loss, we recognize an allowance for the expected credit loss to reduce the cost basis to fair value, with a corresponding adjustment to other expense.
59
There were no available-for-sale securities as of September 30, 2022. As of September 30, 2021, the amortized cost, unrealized holding gains and losses, and fair value of available-for-sale securities were as follows:
|
September 30, 2021 |
|
|||||||||||||||||||||
|
Valuation |
|
|
Balance Sheet Classification |
|
||||||||||||||||||
(In thousands) |
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
Fair |
|
|
Current Assets |
|
|
Noncurrent Assets |
|
||||||
Commercial paper and corporate bonds |
$ |
9,718 |
|
|
$ |
2 |
|
|
$ |
(1 |
) |
|
$ |
9,719 |
|
|
$ |
7,717 |
|
|
$ |
2,002 |
|
Total |
$ |
9,718 |
|
|
$ |
2 |
|
|
$ |
(1 |
) |
|
$ |
9,719 |
|
|
$ |
7,717 |
|
|
$ |
2,002 |
|
There were no held-to-maturity debt securities as of September 30, 2022 and 2021. There were no realized gains or losses on sales of available-for-sale securities for fiscal 2022, 2021 or 2020.
Inventories
Inventories are principally stated at the lower of cost or net realizable value using the specific identification method and include direct labor, materials and overhead, with cost of product sales determined on a first-in, first-out basis. Inventories consisted of the following components:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Raw materials |
$ |
6,102 |
|
|
$ |
4,165 |
|
Work-in process |
|
1,595 |
|
|
|
1,295 |
|
Finished products |
|
4,122 |
|
|
|
1,300 |
|
Total |
$ |
11,819 |
|
|
$ |
6,760 |
|
Prepaids and Other Assets, Current
Prepaids and other current assets consisted of the following:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Prepaid expenses |
$ |
2,570 |
|
|
$ |
1,712 |
|
Irish research and development credits receivable |
|
753 |
|
|
|
1,164 |
|
CARES Act employee retention credit receivable |
|
3,441 |
|
|
|
3,577 |
|
Prepaids and other |
$ |
6,764 |
|
|
$ |
6,453 |
|
In fiscal 2021, a benefit of $3.6 million was recorded to reduce operating costs and expenses as a result of our eligibility for the employee retention credit under the provisions of the Coronavirus Aid, Relief and Economic Security Act (the "CARES Act") enacted in March 2020. This benefit and corresponding receivable reflected anticipated reimbursement of personnel expenses we incurred in fiscal 2021 and 2020.
Property and Equipment
Property and equipment are stated at cost, less any impairment, and are depreciated using the straight-line method over the estimated useful lives of the assets. The Company recorded depreciation expense of $4.7 million, $4.9 million and $4.8 million in fiscal 2022, 2021 and 2020, respectively.
The September 30, 2022 and 2021 balances in construction-in-progress include the cost of equipment and building improvements not yet placed in service. As assets are placed in service, construction-in-progress is transferred to the specific property and equipment categories and depreciated over the estimated useful lives of the assets. Leasehold improvements are amortized over the shorter of the term of the lease or the estimated useful life of the asset. Expenditures for maintenance and repairs and minor renewals and betterments that do not extend or improve the life of the respective assets are expensed as incurred.
60
Property and equipment consisted of the following components:
|
Useful Life |
|
September 30, |
|
|||||
(Dollars in thousands) |
(Years) |
|
2022 |
|
|
2021 |
|
||
Land |
N/A |
|
$ |
4,409 |
|
|
$ |
4,419 |
|
Laboratory fixtures and equipment |
3 to 10 |
|
|
28,810 |
|
|
|
29,482 |
|
Buildings and improvements |
3 to 20 |
|
|
26,373 |
|
|
|
26,573 |
|
Leasehold improvements |
5 to 10 |
|
|
6,499 |
|
|
|
6,499 |
|
Office furniture and equipment |
3 to 10 |
|
|
9,205 |
|
|
|
8,713 |
|
Construction-in-progress |
|
|
|
3,175 |
|
|
|
2,120 |
|
Less: Accumulated depreciation |
|
|
|
(51,323 |
) |
|
|
(47,716 |
) |
Property and equipment, net |
|
|
$ |
27,148 |
|
|
$ |
30,090 |
|
Intangible Assets
Intangible assets consisted of the following:
|
September 30, 2022 |
|
|||||||||||
(Dollars in thousands) |
Weighted Average Original Life (Years) |
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net |
|
|||
Definite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|||
Customer lists and relationships |
8.9 |
|
$ |
11,354 |
|
|
$ |
(8,827 |
) |
|
$ |
2,527 |
|
Developed technology |
11.9 |
|
|
31,943 |
|
|
|
(7,994 |
) |
|
|
23,949 |
|
Patents and other |
14.1 |
|
|
3,551 |
|
|
|
(2,462 |
) |
|
|
1,089 |
|
Total definite-lived intangible assets |
|
|
|
46,848 |
|
|
|
(19,283 |
) |
|
|
27,565 |
|
Unamortized intangible assets: |
|
|
|
|
|
|
|
|
|
|
|||
Trademarks and trade names |
|
|
|
580 |
|
|
|
— |
|
|
|
580 |
|
Total intangible assets |
|
|
$ |
47,428 |
|
|
$ |
(19,283 |
) |
|
$ |
28,145 |
|
|
September 30, 2021 |
|
|||||||||||
(Dollars in thousands) |
Weighted Average Original Life (Years) |
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net |
|
|||
Definite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|||
Customer lists and relationships |
8.9 |
|
$ |
13,216 |
|
|
$ |
(8,878 |
) |
|
$ |
4,338 |
|
Developed technology |
11.9 |
|
|
36,531 |
|
|
|
(5,652 |
) |
|
|
30,879 |
|
Patents and other |
14.1 |
|
|
3,551 |
|
|
|
(2,294 |
) |
|
|
1,257 |
|
Total definite-lived intangible assets |
|
|
|
53,298 |
|
|
|
(16,824 |
) |
|
|
36,474 |
|
Unamortized intangible assets: |
|
|
|
|
|
|
|
|
|
|
|||
Trademarks and trade names |
|
|
|
580 |
|
|
|
— |
|
|
|
580 |
|
Total intangible assets |
|
|
$ |
53,878 |
|
|
$ |
(16,824 |
) |
|
$ |
37,054 |
|
The Company recorded amortization expense of $4.4 million, $3.1 million and $2.5 million in fiscal 2022, 2021 and 2020, respectively.
Based on the intangible assets in service as of September 30, 2022, estimated amortization expense for future fiscal years is as follows:
(In thousands) |
|
|
|
2023 |
$ |
3,549 |
|
2024 |
|
3,471 |
|
2025 |
|
3,439 |
|
2026 |
|
2,618 |
|
2027 |
|
2,384 |
|
Thereafter |
|
12,104 |
|
Definite-lived intangible assets |
$ |
27,565 |
|
Future amortization amounts presented above are estimates. Actual future amortization expense may be different as a result of future acquisitions, impairments, changes in amortization periods, foreign currency exchange rates or other factors.
61
The Company defines in-process research and development (“IPR&D”) as the value of technology acquired for which the related projects have substance and are incomplete. IPR&D acquired in a business combination is recognized at fair value and is capitalized as an indefinite-lived intangible asset until completion or abandonment of the IPR&D project. Upon completion of the development project (generally when regulatory approval to market the product is obtained), an impairment assessment is performed prior to amortizing the asset over its estimated useful life. In cases where the IPR&D projects are abandoned, the related IPR&D assets are written off. The Company assesses indefinite-lived assets for impairment annually in the fourth quarter and whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired. Similar to the goodwill impairment assessment, the indefinite-lived assets impairment assessment requires the Company to make several estimates about fair value, most of which are based on projected future cash flows.
The Company performs its annual assessment of indefinite-lived intangible assets for impairment as of July 1st of each fiscal year. No impairment charges were recorded in fiscal 2022, 2021 and 2020.
Goodwill
Goodwill in the Medical Device reporting unit represents the gross value from the fiscal 2021 acquisition of Vetex Medical Limited (“Vetex”) and the fiscal 2016 acquisitions of Creagh Medical, Ltd. (“Creagh Medical”) and NorMedix, Inc. (“NorMedix”). Goodwill in the In Vitro Diagnostics reporting unit represents the gross value from the acquisition of BioFX Laboratories, Inc. in 2007. Refer to Note 12 Acquisitions for further disclosures for Vetex.
Changes in the carrying amount of goodwill by segment were as follows:
(In thousands) |
In Vitro |
|
|
Medical Device |
|
|
Total |
|
|||
Goodwill as of September 30, 2020 |
$ |
8,010 |
|
|
$ |
19,175 |
|
|
$ |
27,185 |
|
Acquisition of Vetex Medical Limited |
|
— |
|
|
|
19,089 |
|
|
|
19,089 |
|
Foreign currency translation adjustment |
|
— |
|
|
|
(668 |
) |
|
|
(668 |
) |
Goodwill as of September 30, 2021 |
|
8,010 |
|
|
|
37,596 |
|
|
|
45,606 |
|
Foreign currency translation adjustment |
|
— |
|
|
|
(5,173 |
) |
|
|
(5,173 |
) |
Measurement period adjustments (1) |
|
— |
|
|
|
277 |
|
|
|
277 |
|
Goodwill as of September 30, 2022 |
$ |
8,010 |
|
|
$ |
32,700 |
|
|
$ |
40,710 |
|
Goodwill represents the excess of the purchase price of an acquired business over the fair value assigned to the assets purchased and liabilities assumed. Goodwill is not amortized but is subject, at a minimum, to annual tests for impairment. The carrying amount of goodwill is evaluated annually, and between annual evaluations if events occur or circumstances change indicating that it is more likely than not that the fair value of a reporting unit is less than its carrying amount.
The Company’s reporting units are the Medical Device and In Vitro Diagnostics reportable segments. Inherent in the determination of fair value of the reporting units are certain estimates and judgments, including the interpretation of current economic indicators and market valuations, as well as the Company’s strategic plans with regard to its operations. When utilizing a quantitative assessment, the Company determines fair value at the reporting unit level based on a combination of an income approach and market approach. The income approach is based on estimated future cash flows, discounted at a rate that approximates the cost of capital of a market participant, while the market approach is based on sales and/or earnings multiples of similar companies. These approaches use significant estimates and assumptions, including projected future cash flows and the timing of those cash flows, discount rates reflecting risks inherent in future cash flows, perpetual growth rates, and determination of appropriate market comparables.
The Company performs its annual assessment of goodwill for impairment as of July 1st of each fiscal year. Goodwill was not impaired in either reporting unit based on the outcome of the fiscal 2022 annual impairment test, which utilized a quantitative assessment. No goodwill impairment charges were recorded in fiscal 2022, 2021 and 2020.
62
Other Assets, Noncurrent
Other noncurrent assets consisted of the following:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Operating lease right-of-use assets |
$ |
3,633 |
|
|
$ |
2,435 |
|
Other |
|
1,136 |
|
|
|
1,283 |
|
Other assets, noncurrent |
$ |
4,769 |
|
|
$ |
3,718 |
|
Valuation of Long-lived Assets
The Company periodically evaluates whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of long-lived assets, such as property and equipment, right-of-use assets, and definite-lived intangible assets. If such events or circumstances were to indicate that the carrying amount of these assets may not be recoverable, the Company would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected future cash flows (undiscounted and without interest charges) were less than the carrying amount of the assets, the Company would recognize an impairment charge to reduce such assets to their fair value. In fiscal 2022, 2021 and 2020, no impairment charges were recorded related to the Company’s long-lived assets.
Accrued Other Liabilities
Accrued other liabilities consisted of the following:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Accrued professional fees |
$ |
279 |
|
|
$ |
489 |
|
Accrued clinical study expense |
|
1,425 |
|
|
|
1,667 |
|
Accrued purchases |
|
1,655 |
|
|
|
1,195 |
|
Acquisition of in-process research and development (1) |
|
981 |
|
|
|
494 |
|
|
963 |
|
|
|
518 |
|
|
Other |
|
551 |
|
|
|
542 |
|
Total accrued other liabilities |
$ |
5,854 |
|
|
$ |
4,905 |
|
Other Long-term Liabilities
Other long-term liabilities consisted of the following:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Deferred consideration (1) |
$ |
4,260 |
|
|
$ |
5,106 |
|
Contingent consideration (2) |
|
829 |
|
|
|
817 |
|
Unrecognized tax benefits (3) |
|
1,841 |
|
|
|
2,538 |
|
(4) |
|
3,843 |
|
|
|
3,188 |
|
Other long-term liabilities |
$ |
10,773 |
|
|
$ |
11,649 |
|
63
Revenue Recognition
Revenue is recognized when control of the promised goods or services is transferred to our customers in an amount that reflects the consideration we expect to be entitled to receive in exchange for those goods or services. The Company primarily sells or licenses its products, technologies and services to other medical device and diagnostics companies. Revenue is recorded net of taxes collected from customers, and taxes collected are recorded as current liabilities until remitted to the relevant government authority. The amount of foreign taxes imposed on specific revenue producing transactions that is the responsibility of the Company is expensed as incurred and reported in income tax expense on the consolidated statements of operations. For contracts that have an original duration of one year or less, the Company uses the practical expedient applicable to such contracts and does not adjust the transaction price for the time value of money.
Performance Obligations
We derive our revenue from three primary sources:
Product Sales |
|
Royalties and License Fees |
|
Research, Development and Other |
IVD chemical and biological components, including: protein stabilizers, substrates, surface coatings and antigens to the diagnostic and biomedical research markets (IVD segment) |
|
Performance coating royalties from licensing of our proprietary performance coating technologies to medical device manufacturers (Medical Device segment) |
|
Commercial development feasibility services and contract coating services (Medical Device segment) |
Performance coating reagents, the chemicals used in performance coatings by licensees (Medical Device segment) |
|
SurVeil™ DCB license fees associated with the Abbott Agreement (Medical Device segment) |
|
Commercial development services (IVD segment) |
Vascular intervention medical devices and related products to original equipment manufacturer suppliers and distributors, as well as directly to healthcare providers (Medical Device segment) |
|
|
|
|
The Company recognizes revenue when control is transferred to the customer. The transfer of control varies by revenue classification and is described below. If a contract contains more than one distinct performance obligation, the transaction price is allocated to each performance obligation based on relative standalone selling price.
Product Sales. Revenue from product sales is recognized at the point in time control of the products is transferred, generally upon shipment based upon the standard contract terms. Shipping and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered to be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard warranty policy, and returns are generally not significant. Payment terms for product sales are generally set at 30-45 days after shipment.
Royalties. Royalties revenue consists of sales-based and recurring minimum royalties earned under licenses of our performance coating technologies. Performance obligations under these licenses, which consist of the right to use the Company’s proprietary technology, are satisfied at a point in time corresponding with delivery of the underlying technology rights to the customer, which is generally upon transfer of the licensed technology to the customer. Sales-based royalties revenue represents variable consideration under the license agreements and is recognized in the period a customer sells products incorporating the Company’s licensed technologies. The Company estimates sales-based royalties revenue earned but unpaid at each reporting period using the expected value method based on historical sales information, adjusted for known changes such as product launches and patent expirations. The Company also considers macroeconomic factors affecting the medical device market. The Company's license arrangements also often provide for recurring fees (minimum royalties), which the Company recognizes at the later of the satisfaction of the underlying performance obligation or upon renewal of the contract, which generally occurs on a quarterly basis. Sales-based and minimum royalties are generally due within 45 days after the end of each quarter.
64
License Fees. For distinct license performance obligations, upfront license fees are recognized when the Company satisfies the underlying performance obligation. This generally occurs upon transfer of the right to use the Company’s licensed technology to the customer, with the exception of the license of the Company’s SurVeil™ drug-coated balloon (the “SurVeil DCB”) disclosed below. Certain license arrangements include contingent milestone payments, which are due following achievement by our customers of specified sales or regulatory milestones. Contingent milestone payment terms vary by contract. The Company has generally fulfilled its performance obligation prior to achievement of these milestones. However, because of the uncertainty of the milestone achievement, and/or the dependence on sales of our customers, variable consideration for contingent milestones is fully constrained and excluded from the contract price until the milestone is achieved by our customer, to the extent collectability is reasonably certain.
The Company has a collaborative arrangement contract with Abbott Vascular, Inc. (“Abbott”) disclosed in Note 4 Collaborative Arrangement (the “Abbott Agreement”). As of September 30, 2022, the Company has received payments totaling $60.8 million under the Abbott Agreement and may receive an additional contingent milestone payment upon PMA of our SurVeil DCB of $30 million (if PMA is received prior to December 31, 2022) or $27 million (if PMA is received after December 31, 2022 but prior to June 30, 2023), or $24 million (if PMA is received on or after June 30, 2023), pursuant to the terms of the Abbott Agreement.
The performance obligation identified in the Abbott Agreement includes delivery of our licensed technology and completion of research and development activities, primarily clinical trial activities (together, “R&D and Clinical Activities”). These promises are not distinct performance obligations because the product necessary for completion of the R&D and Clinical Activities is currently only able to be manufactured by the Company due to the exclusive proprietary know-how and certain regulatory requirements associated with the manufacture of the product. The customer, Abbott, simultaneously receives and consumes the benefits of the R&D and Clinical Activities as study data are generated to support regulatory approval submissions. Control is effectively transferred over time as we complete the TRANSCEND clinical study of the SurVeil DCB and related regulatory activities. License fee revenue related to this contract is recognized using the cost-to-cost method which measures progress based on costs incurred to date relative to the expected total cost of the services, as the Company believes this represents a faithful depiction of the satisfaction of its performance obligation. Use of the cost-to-cost method requires significant estimates, including the total cost of the TRANSCEND study, which is expected to be completed over the next three years. Revenue is recorded based on the cost-to-cost completion estimate relative to the transaction price, which is equal to the total upfront fee plus the expected value of the clinical and regulatory milestones.
Revenue from the upfront fee and contingent clinical and regulatory milestone payments, once the underlying contingencies are achieved, is recognized within royalties and license fees on the consolidated statements of operations as the clinical and regulatory activities are performed on a proportional performance basis. Performance is measured based on actual costs incurred relative to the expected total cost of the underlying activities, most notably the completion of the TRANSCEND clinical trial. A significant component of the cost of this trial is the cost of the Company’s outsourced clinical trial clinical research organization (“CRO”) consultants, which is estimated based on executed statements of work, project budgets, and patient enrollment timing, among other factors. A significant change to the Company’s estimate of the costs to complete the TRANSCEND clinical trial could have a material effect on the Company’s results of operations. Significant judgment is used to estimate total revenue and cost at completion for this contract.
To account for the Abbott Agreement, the Company applied the guidance in ASC Topic 808 (Collaborative Arrangements) as the parties are active participants and are exposed to significant risks and rewards dependent on commercial success of the collaborative activity. See Note 4 Collaborative Arrangement for further disclosures related to the Abbott Agreement.
Research and Development. The Company performs research and development (“R&D”) activities as a service to customers, which are typically charged to customers on a time-and-materials basis. Generally, revenue for R&D services is recorded over time as the services are provided to the customer in the amount to which the Company has the right to invoice. These services are generally charged to the customer as they are provided. Payment terms for R&D services are generally set at 30-45 days.
Contract Assets, Deferred Revenue and Remaining Performance Obligations
Contract assets are generally short in duration given the nature of products produced and services provided by the Company. Contract assets consist of sales-based and minimum royalties revenue earned for which unconditional right to payment does not exist as of the balance sheet date. These assets are comprised of estimated sales-based royalties earned, but not yet reported by the Company’s customers, minimum royalties on non-cancellable contracts, and contingent milestones earned, but not yet billable based on the terms of the contract. See Note 3 Revenue for further contract asset disclosures.
The Company records a contract liability, or deferred revenue, when there is an obligation to provide a product or service to the customer, and payment is received or due in advance of performance, or when payment is received for a period outside the contract term. See Note 4 Collaborative Arrangement for further deferred revenue disclosures.
65
Remaining performance obligations include deferred revenue and amounts the Company expects to receive for goods and services that have not yet been delivered or provided under existing, noncancellable contracts. For contracts that have an original duration of one year or less, the Company has elected the practical expedient applicable to such contracts and does not disclose the transaction price for remaining performance obligations at the end of each reporting period or the expecting timing of recognition of related revenue. See Note 4 Collaborative Arrangement for further performance obligation disclosures.
Leases
The Company leases facilities for research, office, manufacturing and warehousing. The Company determines whether a contract is a lease or contains a lease at inception date. Upon commencement, the Company recognizes a right-of-use asset and lease liability based on the net present value of the future minimum lease payments over the lease term at the commencement date. The net present value of future minimum lease payments recorded upon lease commencement is reduced by the discounted value of any leasehold improvement incentives payable to the Company considered to be in-substance fixed payments. The unamortized balance of leasehold improvement incentives in the form of tenant allowances represents the primary difference between the balance of the right-of-use assets and operating lease liabilities. As the Company’s leases typically do not provide an implicit rate, the Company’s lease liabilities are measured on a discounted basis using the Company's incremental borrowing rate. Lease terms used in the recognition of right-of-use assets and lease liabilities include only options to extend the lease that are reasonably certain to be exercised. The consolidated balance sheets do not include recognized assets or liabilities for leases that, at the commencement date, have a term of twelve months or less and do not include an option to purchase the underlying asset that is reasonably certain to be exercised. The Company recognizes such leases on the consolidated statements of operations on a straight-line basis over the lease term.
The Company’s leases include one or more and extend the lease term at the Company’s discretion. These renewal options are not included in right-of-use assets and lease liabilities as they are not reasonably certain of exercise. The Company regularly evaluates renewal options, and when they are reasonably certain to be exercised, the renewal period is included in the lease term.
As of September 30, 2022, operating lease maturities were as follows:
(In thousands) |
|
|
|
2023 |
$ |
1,172 |
|
2024 |
|
1,210 |
|
2025 |
|
1,214 |
|
2026 |
|
1,132 |
|
2027 |
|
1,135 |
|
Thereafter |
|
575 |
|
Total expected operating lease payments |
|
6,438 |
|
Less: Imputed interest |
|
(1,632 |
) |
Total operating lease liabilities |
$ |
4,806 |
|
Operating lease cost was $1.1 million, $0.8 million and $0.6 million for fiscal 2022, 2021 and 2020, respectively. Cash paid for operating lease liabilities approximated operating lease cost for fiscal 2022, 2021 and 2020. As of September 30, 2022, the weighted average remaining lease term for operating leases was 5.3 years, and the weighted average discount rate used to determine operating lease liabilities was 3.9%.
Stock-based Compensation
We measure the cost of employee services received in exchange for the award of equity instruments based on the fair value of the award at the date of grant. Share-based payments are expensed based on their grant-date fair values on a straight-line basis over the requisite service period of the total award, less estimated forfeitures based on historical experience. Shares awarded under the Company’s stock-based compensation plans, with the exception of restricted stock awards, are not considered issued or outstanding common stock of the Company until they vest and the shares are released. New awards and forfeitures of unvested restricted stock result in an increase (decrease), respectively, in common stock issued and outstanding.
66
Research and Development
R&D expenses include costs associated with the design, development, testing, enhancement and regulatory approval of the Company’s products. R&D expenses include employee compensation (including stock-based compensation), internal and external costs associated with our regulatory compliance and quality assurance functions, the costs of product used in development and clinical trials, consulting expenses, and facilities overhead. The Company also incurs significant R&D expenses to operate clinical trials. R&D costs are expensed as incurred.
Certain R&D costs are related to customer contracts, and the related revenue is recognized as described in “Revenue Recognition” in this Note 2. Costs associated with customer-related R&D include specific project direct labor and materials expenses, as well as an allocation of overhead costs based on direct labor costs.
Clinical Trial Costs. The Company sponsors clinical trials intended to obtain the necessary clinical data required to obtain approval from various regulatory agencies to market medical devices developed by the Company. Costs associated with clinical trials include trial design and management expenses, clinical site reimbursements and third-party fees, among other costs. The Company’s clinical trials are administered by third-party CROs. These CROs generally bill monthly for certain services performed, as well as upon achievement of certain milestones. The Company monitors patient enrollment, the progress of clinical studies, and related activities through internal reviews of data reported to the Company by the CROs and correspondence with the CROs. We periodically evaluate our estimates to determine if adjustments are necessary or appropriate based on information received. These estimates often require significant judgement on the part of the Company’s management.
Government Funding. In prior fiscal years, the Company has been eligible to receive reimbursement for certain qualifying R&D expenditures under a grant from the Industrial Development Agency of Ireland (“IDA”). Reimbursements are recognized as a reduction of R&D expense when there is reasonable assurance that the funding will be received and conditions associated with the funding are met. In fiscal 2020, the Company recorded $0.8 million in reimbursements from IDA grants as a reduction of R&D expense.
Litigation
From time to time, the Company may become involved in various legal actions involving its operations, products and technologies, including intellectual property and employment disputes. The outcomes of these legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the claimants may seek damages as well as other relief, including injunctions barring the sale of products that are the subject of the lawsuit, which if granted, could require significant expenditures or result in lost revenue. The Company records a liability on the consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate, the minimum amount of the range is accrued. If a loss is possible but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded.
Income Taxes
We record a tax (expense) benefit for the anticipated tax consequences of the reported results of operations. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income (loss) in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in earnings in the period that includes the enactment date of such change.
Deferred tax assets represent amounts available to reduce income taxes payable on taxable income in future years. Such assets arise from net operating losses and tax credits and are primarily a result of temporary differences between the financial reporting and tax bases of assets and liabilities. We evaluate the recoverability of deferred tax assets by assessing all available evidence, both positive and negative, to determine whether, based on the weight of that evidence, a valuation allowance for deferred tax assets is needed. A valuation allowance is established if it is more likely than not (defined as a likelihood of more than 50%) that all or a portion of deferred tax assets will not be realized. The determination of whether a valuation allowance should be established, as well as the amount of such allowance, requires significant judgment and estimates, including estimates of future earnings.
67
In evaluating the realizability of our net deferred tax assets, we perform an assessment each reporting period of both positive and negative evidence, including (i) the existence of three-year cumulative U.S. pre-tax losses adjusted for permanent adjustments; (ii) our forecast of future earnings; and (iii) future reversal of taxable temporary differences and carryforwards. We apply judgment to consider the relative impact of negative and positive evidence, and the weight given to negative and positive evidence is commensurate with the extent to which such evidence can be objectively verified. Objective historical evidence, such as cumulative three-year pre-tax losses adjusted for permanent adjustments, is given greater weight than subjective positive evidence such as forecasts of future earnings. The more objective negative evidence that exists limits our ability to consider other, potentially positive, subjective evidence, such as our future earnings projections. Due to significant estimates used to establish the valuation allowance and the potential for changes in facts and circumstances, it is reasonably possible that we will be required to record adjustments to the valuation allowance in future reporting periods that could have a material effect on our results of operations.
Net (Loss) Income Per Share Data
Basic net (loss) income per common share is calculated by dividing net (loss) income by the weighted average number of common shares outstanding during the period. Diluted net (loss) income per common share is computed by dividing net (loss) income by the weighted average number of common and common equivalent shares outstanding during the period. The Company’s potentially dilutive common shares are those that result from dilutive common stock options and non-vested stock relating to restricted stock awards and restricted stock units. However, these items have been excluded from the calculation of diluted net loss per share for fiscal 2022 as their effect was anti-dilutive as a result of the net loss incurred for this period. Therefore, diluted weighted average number of shares outstanding and diluted net loss per share were the same as basic weighted average number of shares outstanding and basic net loss per share for fiscal 2022.
The following table presents the denominator for the computation of diluted weighted average shares outstanding:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Basic weighted average shares outstanding |
|
13,916 |
|
|
|
13,765 |
|
|
|
13,552 |
|
Dilutive effect of outstanding stock options, non-vested restricted stock, and non-vested restricted stock units |
|
— |
|
|
|
224 |
|
|
|
260 |
|
Diluted weighted average shares outstanding |
|
13,916 |
|
|
|
13,989 |
|
|
|
13,812 |
|
The calculation of weighted average diluted shares outstanding excluded outstanding common stock options associated with the right to purchase less than 0.1 million shares for both fiscal 2021 and 2020 as their inclusion would have had an antidilutive effect on diluted net income per share for those periods.
Business Combinations
For acquisitions accounted for as business combinations, we record assets and liabilities acquired at their respective fair values as of the acquisition date. Contingent consideration is recognized at fair value as of the acquisition date, and changes in fair value are recognized in earnings until settlement. Acquisition-related transaction costs are expensed as incurred.
Currency Translation
The Company’s reporting currency is the U.S. dollar. Assets and liabilities of non-U.S. dollar functional currency subsidiaries are translated into U.S. dollars at the period-end exchange rates, and revenue and expenses are translated at the average quarterly exchange rates during the period. The net effect of these translation adjustments on the consolidated financial statements is recorded as a foreign currency translation adjustment, a component of accumulated other comprehensive (loss) income on the consolidated balance sheets. Realized foreign currency transaction gains and losses are included in other expense on the consolidated statements of operations.
68
New Accounting Pronouncements
Accounting Standards Recently Adopted
Credit Losses. In June 2016, the Financial Accounting Standards Board (“FASB”) issued ASU 2016-13, Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Statements. This ASU requires a financial asset (or a group of financial assets) measured at an amortized cost basis to be presented at the net amount expected to be collected. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial asset(s) to present the net carrying value at the amount expected to be collected on the financial asset. Effective in fiscal 2021 (October 1, 2020), we adopted this guidance using the modified retrospective method. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
Income Taxes. In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, which eliminates certain exceptions related to the approach for intraperiod tax allocation and to the methodology for calculating taxes during the quarters, as well as clarifies the accounting for enacted changes in tax laws. Effective in fiscal 2021 (October 1, 2020), we adopted this guidance using a prospective approach. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
No other new accounting pronouncement issued or effective has had, or is expected to have, a material impact on the Company’s consolidated financial statements.
3. Revenue
The following is a disaggregation of revenue within each reportable segment:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Medical Device |
|
|
|
|
|
|
|
|
|||
Product sales |
$ |
27,930 |
|
|
$ |
21,777 |
|
|
$ |
21,608 |
|
Royalties |
|
30,267 |
|
|
|
30,781 |
|
|
|
28,614 |
|
License fees |
|
5,981 |
|
|
|
16,275 |
|
|
|
12,020 |
|
Research, development and other |
|
8,211 |
|
|
|
9,420 |
|
|
|
9,159 |
|
Medical Device revenue |
|
72,389 |
|
|
|
78,253 |
|
|
|
71,401 |
|
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|||
Product sales |
|
26,691 |
|
|
|
24,701 |
|
|
|
22,709 |
|
Research, development and other |
|
871 |
|
|
|
2,182 |
|
|
|
754 |
|
In Vitro Diagnostics revenue |
|
27,562 |
|
|
|
26,883 |
|
|
|
23,463 |
|
Total Revenue |
$ |
99,951 |
|
|
$ |
105,136 |
|
|
$ |
94,864 |
|
Contract assets totaled $7.1 million as of each of September 30, 2022 and 2021 on the consolidated balance sheets. Fluctuations in the balance of contract assets result primarily from changes in sales-based and minimum royalties earned, but not collected at each balance sheet date due to payment timing and contractual changes in the normal course of business. For discussion of contract liability (deferred revenue) balances and remaining performance obligations, see Note 4 Collaborative Arrangement.
Revenue from customers that equaled or exceeded 10% of total revenue was as follows:
|
Fiscal Year |
|
|||||||||
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Abbott |
|
11 |
% |
|
|
21 |
% |
|
|
19 |
% |
Medtronic |
|
13 |
% |
|
|
13 |
% |
|
|
14 |
% |
4. Collaborative Arrangement
On February 26, 2018, the Company entered into an agreement with Abbott whereby Abbott has exclusive worldwide commercialization rights for Surmodics' SurVeil DCB to treat the superficial femoral artery (the “Abbott Agreement”). A premarket approval (“PMA”) application for the SurVeil DCB was being evaluated by the U.S. Food and Drug Administration (“FDA”) as of September 30, 2022.
69
Surmodics is responsible for conducting all necessary clinical trials and other activities required to achieve U.S. regulatory clearance for the SurVeil DCB, including completion of the ongoing TRANSCEND pivotal clinical trial. Abbott and Surmodics participate on a joint development committee charged with providing guidance on the Company’s clinical and regulatory activities with regard to the SurVeil DCB product. Upon receipt of U.S. regulatory approval for our SurVeil DCB, Abbott will have the right to purchase commercial units from the Company, and Surmodics will realize revenue from product sales to Abbott at an agreed-upon transfer price, as well as a share of net profits resulting from third-party product sales by Abbott.
As of September 30, 2022, the Company has received payments totaling $60.8 million under the Abbott Agreement, which consist of the following: (i) $25 million upfront fee in fiscal 2018, (ii) $10 million milestone payment in fiscal 2019 upon completion of enrollment in the TRANSCEND clinical trial, (iii) $10.8 million milestone payment in fiscal 2020 upon receipt of Conformité Européenne Mark (“CE Mark”) approval prerequisite for commercialization of the SurVeil DCB in the European Union, and (iv) $15 million milestone payment in fiscal 2021 upon receipt by Abbott of the clinical study report and related materials from the TRANSCEND pivotal trial that demonstrated the primary safety and primary clinical endpoints were non-inferior to the control device. As of September 30, 2022, the Company may receive an additional contingent milestone payment of up to $30 million upon PMA of our SurVeil DCB. The milestone payment is reduced to $27 million (if PMA is received after December 31, 2022 but before June 30, 2023), and to $24 million (if PMA is received on or after June 30, 2023), pursuant to the terms of the Abbott Agreement. As of September 30, 2022, consideration from this potential regulatory milestone was fully excluded from the contract price (i.e., deemed fully constrained) due to the high level of uncertainty of achievement as of September 30, 2022.
Revenue recognized from the Abbott Agreement totaled $5.7 million, $16.0 million and $12.0 million in fiscal 2022, 2021 and 2020, respectively. As of September 30, 2022, the Company had recognized total license fee revenue of $51.6 million from the Abbott Agreement. Revenue recognized from the Abbott Agreement, which was included in the respective beginning of fiscal year balances of deferred revenue on the consolidated balance sheets, totaled $5.7 million, $4.7 million and $5.0 million for fiscal 2022, 2021 and 2020, respectively. As of September 30, 2022 and 2021, total deferred revenue from the upfront and milestone payments received of $9.2 million and $14.9 million, respectively, was recorded on the consolidated balance sheets.
As of September 30, 2022, the estimated revenue expected to be recognized in future periods totaled approximately $9.2 million related to performance obligations that are unsatisfied for executed contracts with an original duration of one year or more. These remaining performance obligations relate to the Abbott Agreement, exclude the potential contingent milestone payment under the Abbott Agreement, and are expected to be recognized over the next three years as the services, which are primarily comprised of the R&D and Clinical Activities performance obligation in the Abbott Agreement, are completed. As of September 30, 2022, we expect to recognize approximately $4.1 million of these remaining performance obligations as revenue within one year, with the remaining $5.1 million over the subsequent, final two years of the TRANSCEND trial follow-up and clinical reporting period.
See Note 2 for further information regarding revenue recognition for the Abbott Agreement.
5. Fair Value Measurements
In determining the fair value of financial assets and liabilities, we utilize market data or other assumptions that we believe market participants would use in pricing the asset or liability in the principal or most advantageous market and adjust for non-performance and/or other risk associated with the company as well as counterparties, as appropriate. When considering market participant assumptions in fair value measurements, the following fair value hierarchy distinguishes between observable and unobservable inputs, which are categorized in one of the following levels:
Level 1 — Quoted (unadjusted) prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability.
Level 3 — Unobservable inputs to the valuation methodology that are supported by little or no market activity and that are significant to the measurement of the fair value of the assets or liabilities. Level 3 assets and liabilities include those with fair value measurements that are determined using pricing models, discounted cash flow methodologies or similar valuation techniques, as well as significant management judgment or estimation. In valuing Level 3 assets and liabilities, we are required to maximize the use of quoted market prices and minimize the use of unobservable inputs.
The hierarchy gives the highest priority to Level 1, as this level provides the most reliable measure of fair value, while giving the lowest priority to Level 3.
70
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Assets and liabilities measured at fair value on a recurring basis by level of the fair value hierarchy were as follows:
|
September 30, 2022 |
|
|||||||||||||
(In thousands) |
Quoted Prices in |
|
|
Significant |
|
|
Significant |
|
|
Total Fair |
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
||||
Cash equivalents (1) |
$ |
— |
|
|
$ |
2,035 |
|
|
$ |
— |
|
|
$ |
2,035 |
|
Total assets |
$ |
— |
|
|
$ |
2,035 |
|
|
$ |
— |
|
|
$ |
2,035 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
||||
Contingent consideration (2) |
$ |
— |
|
|
$ |
— |
|
|
$ |
829 |
|
|
$ |
829 |
|
Total liabilities |
$ |
— |
|
|
$ |
— |
|
|
$ |
829 |
|
|
$ |
829 |
|
|
September 30, 2021 |
|
|||||||||||||
(In thousands) |
Quoted Prices in |
|
|
Significant |
|
|
Significant |
|
|
Total Fair |
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
||||
Cash equivalents (1) |
$ |
— |
|
|
$ |
5,308 |
|
|
$ |
— |
|
|
$ |
5,308 |
|
Available-for-sale investments (1) |
|
— |
|
|
|
9,719 |
|
|
|
— |
|
|
|
9,719 |
|
Total assets |
$ |
— |
|
|
$ |
15,027 |
|
|
$ |
— |
|
|
$ |
15,027 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
||||
Contingent consideration (2) |
$ |
— |
|
|
$ |
— |
|
|
$ |
817 |
|
|
$ |
817 |
|
Total liabilities |
$ |
— |
|
|
$ |
— |
|
|
$ |
817 |
|
|
$ |
817 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Contingent consideration liabilities are remeasured to fair value each reporting period using discount rates, probabilities of payment and projected payment dates. Increases or decreases in the fair value of the contingent consideration liability can result from changes in the timing or likelihood of achieving milestones and changes in discount periods and rates. Projected contingent payment amounts are discounted back to the current period using a discount cash flow model. Interest accretion and fair value adjustments associated with contingent consideration liabilities are reported in contingent consideration expense (gain) on the consolidated statements of operations.
71
Changes in the contingent consideration liabilities measured at fair value using Level 3 inputs were as follows:
(In thousands) |
|
|
|
Contingent consideration liability at September 30, 2020 |
$ |
— |
|
Additions |
|
814 |
|
Fair value adjustments |
|
— |
|
Settlements |
|
— |
|
Interest accretion |
|
3 |
|
Foreign currency translation |
|
— |
|
Contingent consideration liability at September 30, 2021 |
|
817 |
|
Additions |
|
— |
|
Fair value adjustments |
|
— |
|
Settlements |
|
— |
|
Interest accretion |
|
12 |
|
Foreign currency translation |
|
— |
|
Contingent consideration liability at September 30, 2022 |
$ |
829 |
|
Assets and Liabilities Measured at Fair Value on a Non-recurring Basis
We measure certain assets at fair value on a non-recurring basis, primarily goodwill, intangible assets, and long-lived assets. These assets were initially measured and recognized at amounts equal to the fair value determined as of the date of acquisition or purchase and are subject to changes in value only for foreign currency translation and impairment. See Note 2 for additional information on impairment assessments and related Level 3 inputs for goodwill, indefinite-lived intangible assets and long-lived assets.
Assets and Liabilities Not Measured at Fair Value
Certain financial instruments are not measured at fair value but are recorded at carrying amounts approximating fair value based on their short-term nature. The carrying value of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximated fair value as of September 30, 2022 and 2021.
6. Debt
On September 14, 2020, the Company entered into a secured revolving credit facility pursuant to a Loan and Security Agreement, which was amended by a First Amendment on July 2, 2021 and by a Second Amendment on March 7, 2022 (as amended, the "Loan Agreement") with Bridgewater Bank (“Bridgewater”). The Loan Agreement provided for availability under a secured revolving line of credit of up to $25 million (the "Bridgewater Revolving Credit Facility"). The outstanding balance on the Bridgewater Revolving Credit Facility was $10.0 million as of each of September 30, 2022 and 2021.
As of September 30, 2022, the Bridgewater Revolving Credit Facility was scheduled to mature on September 14, 2023. The Company's obligations under the Loan Agreement were secured by substantially all of the Company’s and its material subsidiaries' assets, other than intellectual property, real estate and foreign assets, including equity in foreign subsidiaries. The Company also pledged the stock of certain of its subsidiaries to secure such obligations. Interest under the Loan Agreement accrued at a rate per annum equal to the greater of (i) 3.25% and (ii) the 90-day interest rate yield for U.S. Government Treasury Securities plus 2.75%. A facility fee was payable on unused commitments at a rate of 0.075% quarterly. As of September 30, 2022 and 2021, the weighted average interest rate on outstanding borrowings on the Bridgewater Revolving Credit Facility was 6.1% and 3.3%, respectively.
The Loan Agreement contained affirmative and negative covenants customary for a facility of its type which, among other things, required the Company to meet certain financial tests, including (i) minimum liquidity, (ii) minimum current ratio, (iii) minimum quarterly revenue, and (iv) minimum tangible net worth. The Loan Agreement also contained covenants which, among other things, limited the Company's ability to incur additional debt, make certain investments, create or permit certain liens, create or permit restrictions on the ability of subsidiaries to pay dividends or make other distributions, consolidate or merge, and engage in other activities customarily restricted in such agreements, in each case subject to exceptions permitted by the Loan Agreement. The Loan Agreement also contained customary events of default, the occurrence of which would permit Bridgewater to terminate its commitment and accelerate the Bridgewater Revolving Credit Facility.
See Note 14 Subsequent Events for information on financing arrangements subsequent to September 30, 2022.
72
7. Stockholders’ Equity
Repurchase of Common Stock
Shares are repurchased from time to time to support the Company’s stock-based compensation programs and to return capital to stockholders, and depend upon many factors, including the Company’s results of operations, financial condition, capital requirements and contractual restrictions. The Company accounts for repurchases of common stock using the par value method.
On November 6, 2015, and on November 5, 2014, the Company’s Board of Directors authorized the repurchase of up to $20.0 million and $30.0 million, respectively, of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, accelerated share repurchase transactions, tender offers or by any combination of such methods. The authorizations have no fixed expiration date. As of September 30, 2022, $25.3 million remained available to the Company for the purchase of its common stock under outstanding authorizations.
8. Stock-based Compensation Plans
The Company has stock-based compensation plans under which it grants stock options, restricted stock awards, restricted stock units and deferred stock units. Stock-based compensation expense was reported as follows on the consolidated statements of operations:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Product costs |
$ |
234 |
|
|
$ |
122 |
|
|
$ |
119 |
|
Research and development |
|
1,424 |
|
|
|
1,298 |
|
|
|
896 |
|
Selling, general and administrative |
|
5,399 |
|
|
|
4,443 |
|
|
|
4,438 |
|
Total stock-based compensation expense |
$ |
7,057 |
|
|
$ |
5,863 |
|
|
$ |
5,453 |
|
As of September 30, 2022, approximately $10.5 million of total unrecognized compensation costs related to non-vested awards is expected to be recognized over a weighted average period of approximately 2.3 years.
Under the amended 2019 Equity Incentive Plan (“2019 Plan”), the Company is authorized to issue 1,900,000 shares, plus the number of shares pursuant to any awards granted under the 2009 Equity Incentive Plan (“2009 Plan”) that were outstanding on the effective date of the 2019 Plan that expire, are cancelled or forfeited, or are settled for cash. As of September 30, 2022, there were approximately 845,000 shares available for future equity awards under the 2019 Plan, including stock options, restricted stock, restricted stock units and deferred stock units.
Stock Option Awards
The Company grants non-qualified stock options at fair market value on the grant date to certain key employees and members of the Board. The Company uses the Black-Scholes option pricing model to determine the fair value of stock options as of the date of each grant. Weighted average stock option fair value assumptions and the weighted average grant date fair value of stock options granted were as follows:
|
Fiscal Year |
|
|||||||||
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Stock option fair value assumptions: |
|
|
|
|
|
|
|
|
|||
Risk-free interest rate |
|
1.49 |
% |
|
|
0.40 |
% |
|
|
1.41 |
% |
Expected life (years) |
4.6 |
|
|
4.6 |
|
|
4.6 |
|
|||
Expected volatility |
|
43 |
% |
|
|
43 |
% |
|
|
39 |
% |
Dividend yield |
|
— |
% |
|
|
— |
% |
|
|
— |
% |
Weighted average grant date fair value of stock options granted |
$ |
15.96 |
|
|
$ |
14.71 |
|
|
$ |
14.13 |
|
The risk-free interest rate assumption is based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the awards. The expected life of options granted is determined based on the Company’s experience. Expected volatility is based on the Company’s stock price movement over a period approximating the expected term. Based on management’s judgment, dividend yields are expected to be zero for the expected life of the options.
73
With respect to members of the Board, non-qualified stock options generally become exercisable on a monthly pro-rata basis within the one-year period following the date of grant. With respect to employees, non-qualified stock options generally become exercisable at a 25% rate on each of the first anniversaries following the grant date. Non-qualified stock options generally expire in seven years or upon, or shortly after termination of employment or service as a Board member. The stock-based compensation expense table above includes stock option expenses recognized related to these awards, which totaled $3.4 million, $2.8 million and $2.5 million in fiscal 2022, 2021 and 2020, respectively.
As of September 30, 2022, the aggregate intrinsic value of the option shares outstanding was $0.6 million, and the aggregate intrinsic value of option shares exercisable was $0.6 million. As of September 30, 2022, the weighted average remaining contractual life of options outstanding and options exercisable was 4.5 years and 3.2 years, respectively. The total pre-tax intrinsic value of options exercised was $1.0 million, $7.1 million and $2.0 million in fiscal 2022, 2021 and 2020, respectively. The intrinsic value represents the difference between the exercise price and the fair market value of the Company’s common stock on the last day of the respective fiscal year end.
Stock option activity was as follows:
(In thousands, except per share data) |
Number of |
|
|
Weighted |
|
||
Options outstanding at September 30, 2019 |
|
871 |
|
|
$ |
32.18 |
|
Granted |
|
299 |
|
|
|
41.06 |
|
Exercised |
|
(125 |
) |
|
|
22.89 |
|
Forfeited and expired |
|
(105 |
) |
|
|
41.69 |
|
Options outstanding at September 30, 2020 |
|
940 |
|
|
|
35.18 |
|
Granted |
|
274 |
|
|
|
40.95 |
|
Exercised |
|
(248 |
) |
|
|
24.22 |
|
Forfeited and expired |
|
(44 |
) |
|
|
44.58 |
|
Options outstanding at September 30, 2021 |
|
922 |
|
|
|
39.39 |
|
Granted |
|
342 |
|
|
|
42.10 |
|
Exercised |
|
(45 |
) |
|
|
21.24 |
|
Forfeited and expired |
|
(58 |
) |
|
|
43.99 |
|
Options outstanding at September 30, 2022 |
|
1,161 |
|
|
|
40.66 |
|
Options vested and exercisable at September 30, 2022 |
|
546 |
|
|
$ |
39.44 |
|
Restricted Stock Awards
The Company has entered into restricted stock agreements with certain key employees, covering the issuance of common stock (“Restricted Stock”). Restricted Stock generally vests at a 33% rate on each of the first anniversaries following the grant date. Restricted Stock is released to employees if they are employed by the Company at the end of the vesting period. Restricted Stock is valued based on the market value of the shares as of the date of grant with the value allocated to expense evenly over the vesting period. The stock-based compensation expense table above includes Restricted Stock expenses recognized related to these awards, which totaled $2.7 million, $2.2 million and $2.0 million in fiscal 2022, 2021 and 2020, respectively.
74
Restricted Stock activity was as follows:
(In thousands, except per share data) |
Number of |
|
|
Weighted Average |
|
||
Unvested restricted stock awards at September 30, 2019 |
|
90 |
|
|
$ |
43.69 |
|
Granted |
|
67 |
|
|
|
41.40 |
|
Vested |
|
(43 |
) |
|
|
38.74 |
|
Forfeited |
|
(14 |
) |
|
|
44.76 |
|
Unvested restricted stock awards at September 30, 2020 |
|
100 |
|
|
|
44.16 |
|
Granted |
|
71 |
|
|
|
38.83 |
|
Vested |
|
(48 |
) |
|
|
44.07 |
|
Forfeited |
|
(4 |
) |
|
|
40.45 |
|
Unvested restricted stock awards at September 30, 2021 |
|
119 |
|
|
|
41.14 |
|
Granted |
|
99 |
|
|
|
42.35 |
|
Vested |
|
(55 |
) |
|
|
42.98 |
|
Forfeited |
|
(5 |
) |
|
|
41.83 |
|
Unvested restricted stock awards at September 30, 2022 |
|
158 |
|
|
$ |
41.24 |
|
Restricted Stock Units and Deferred Stock Units
The Company has entered into restricted stock unit agreements with certain key employees in foreign jurisdictions and members of the Board, covering the issuance of common stock (“RSUs”). With respect to employees, RSUs generally vest at a 33% rate on each of the first anniversaries following the grant date, and RSUs are settled in shares and issued to the employees if they are employed by the Company at the end of the vesting period. With respect to members of the Board, RSUs vest on a monthly pro-rata basis within the one-year period following the date of grant, and RSUs are settled in shares and generally issued upon termination of service as a Board member. RSUs are valued based on the market value of the shares as of the date of grant with the value allocated to expense evenly over the vesting period. The Company awarded approximately 14,000, 17,000 and 18,000 RSUs in fiscal 2022, 2021 and 2020, respectively. As of September 30, 2022 and 2021, outstanding RSUs (including unvested units and vested units not yet settled) totaled approximately 65,000 and 61,000 units, respectively, with a weighted average grant date fair value per unit of $33.14 and $33.45, respectively. The stock-based compensation table above includes RSU expenses recognized related to these awards, which totaled $0.5 million, $0.5 million and $0.6 million in fiscal 2022, 2021 and 2020, respectively.
Directors may elect to receive their annual fees for services to the Board in deferred stock units (“DSUs”). DSUs are fully vested and expensed upon grant at the market value of the shares on the grant date. DSUs are settled in shares and issued to the Director upon termination of service as a Board member. As of September 30, 2022 and 2021, outstanding, fully vested DSUs totaled approximately 36,000 and 34,000 units, respectively, with a weighted average grant date fair value per unit of $30.97 and $30.32, respectively. The stock-based compensation expense table above includes DSU expenses recognized related to these awards, which totaled $0.1 million per year in each of fiscal 2022, 2021 and 2020.
1999 Employee Stock Purchase Plan
Under the amended 1999 Employee Stock Purchase Plan (“ESPP”), the Company is authorized to issue up to 600,000 shares of common stock. All full-time and part-time U.S. employees can elect to have up to 10% of their annual compensation withheld, with an annual limit of $25,000, to purchase the Company’s common stock at purchase prices defined within the provisions of the ESPP. ESPP share awards are valued based on the value of the discount feature plus the fair value of the optional features as of the date of grant using the Black-Scholes valuation model. The value of these share awards is allocated to expense evenly over each six-month purchase period. Employee contributions to the ESPP included in accrued liabilities on the consolidated balance sheets totaled $0.1 million as of both September 30, 2022 and 2021. The stock-based compensation expense table above includes expenses recognized related to the ESPP, which totaled $0.3 million, $0.2 million and $0.2 million for fiscal 2022, 2021 and 2020, respectively.
75
9. Income Taxes
Income taxes on the consolidated statements of operations consisted of the following:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Current (benefit) expense: |
|
|
|
|
|
|
|
|
|||
U.S. Federal |
$ |
(510 |
) |
|
$ |
263 |
|
|
$ |
(1,570 |
) |
U.S. State |
|
(143 |
) |
|
|
108 |
|
|
|
42 |
|
International |
|
166 |
|
|
|
87 |
|
|
|
90 |
|
Total current (benefit) expense |
|
(487 |
) |
|
|
458 |
|
|
|
(1,438 |
) |
Deferred expense (benefit): |
|
|
|
|
|
|
|
|
|||
U.S. Federal |
|
5,200 |
|
|
|
1,851 |
|
|
|
(1,336 |
) |
U.S. State |
|
515 |
|
|
|
(62 |
) |
|
|
197 |
|
International |
|
(447 |
) |
|
|
(138 |
) |
|
|
— |
|
Total deferred expense (benefit) |
|
5,268 |
|
|
|
1,651 |
|
|
|
(1,139 |
) |
Total income tax expense (benefit) |
$ |
4,781 |
|
|
$ |
2,109 |
|
|
$ |
(2,577 |
) |
The difference between amounts calculated at the statutory U.S. federal income tax rate of 21% and the Company’s effective tax rate was as follows:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Amount at statutory U.S. federal income tax rate |
$ |
(4,724 |
) |
|
$ |
1,333 |
|
|
$ |
(305 |
) |
Change because of the following items: |
|
|
|
|
|
|
|
|
|||
State income taxes, net of federal benefit |
|
(897 |
) |
|
|
(273 |
) |
|
|
(551 |
) |
Foreign and state rate differential |
|
628 |
|
|
|
596 |
|
|
|
212 |
|
U.S. federal and foreign R&D credits |
|
(1,511 |
) |
|
|
(920 |
) |
|
|
(1,571 |
) |
Valuation allowance change (1) |
|
10,978 |
|
|
|
1,059 |
|
|
|
825 |
|
Stock-based compensation (2) |
|
481 |
|
|
|
(544 |
) |
|
|
(81 |
) |
U.S. Federal and state rate change |
|
— |
|
|
|
(35 |
) |
|
|
17 |
|
Tax reserve change |
|
(123 |
) |
|
|
(150 |
) |
|
|
609 |
|
Foreign-derived income deduction |
|
— |
|
|
|
— |
|
|
|
(88 |
) |
Impact of CARES Act (3) |
|
— |
|
|
|
735 |
|
|
|
(1,700 |
) |
Acquisition-related transaction costs |
|
— |
|
|
|
187 |
|
|
|
— |
|
Other |
|
(51 |
) |
|
|
121 |
|
|
|
56 |
|
Income tax expense (benefit) |
$ |
4,781 |
|
|
$ |
2,109 |
|
|
$ |
(2,577 |
) |
76
Excess tax benefits related to stock-based compensation expense are recorded within income tax (expense) benefit on the consolidated statements of operations and totaled $0.2 million, $0.9 million and $0.4 million for fiscal 2022, 2021 and 2020, respectively.
The components of deferred income taxes, net, consisted of the following and resulted from differences in the recognition of transactions for income tax and financial reporting purposes:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
Depreciable assets |
$ |
(3,995 |
) |
|
$ |
(5,106 |
) |
Deferred revenue |
|
2,103 |
|
|
|
2,130 |
|
Accruals and reserves |
|
1,615 |
|
|
|
1,572 |
|
Stock-based compensation |
|
2,443 |
|
|
|
1,997 |
|
Impaired strategic investments |
|
1,787 |
|
|
|
1,782 |
|
NOL carryforwards (1) |
|
6,379 |
|
|
|
4,319 |
|
U.S. Federal and state R&D credits (2) |
|
4,465 |
|
|
|
3,066 |
|
Other |
|
848 |
|
|
|
618 |
|
Valuation allowance |
|
(17,672 |
) |
|
|
(7,253 |
) |
Deferred taxes, net |
$ |
(2,027 |
) |
|
$ |
3,125 |
|
As of September 30, 2022 and 2021, valuation allowances against deferred tax assets, net, totaled $17.7 million and $7.3 million, respectively. Deferred tax assets represent amounts available to reduce income taxes payable on taxable income in future years. Such assets arise from net operating loss and tax credits and are primarily a result of temporary differences between the financial reporting and tax bases of assets and liabilities. We evaluate the recoverability of deferred tax assets by assessing all available evidence, both positive and negative, to determine whether, based on the weight of that evidence, a valuation allowance for deferred tax assets is needed. A valuation allowance is established if it is more likely than not (defined as a likelihood of more than 50%) that all or a portion of deferred tax assets will not be realized. The determination of whether a valuation allowance should be established, as well as the amount of such allowance, requires significant judgment and estimates, including estimates of future earnings.
In evaluating the realizability of our net deferred tax assets, we perform an assessment each reporting period of both positive and negative evidence. As of September 30, 2022, we identified negative evidence that included the existence of three-year cumulative U.S. pre-tax losses adjusted for permanent adjustments and short-term future losses. As of September 30, 2022, we identified positive evidence that included (i) our forecast of long-term future earnings; and (ii) future reversal of taxable temporary differences and carryforwards.
We apply judgment to consider the relative impact of negative and positive evidence and the weight given to negative and positive evidence is commensurate with the extent to which such evidence can be objectively verified. Objective historical evidence, such as cumulative three-year pre-tax losses adjusted for permanent adjustments, is given greater weight than subjective positive evidence such as forecasts of future earnings. The more objective negative evidence that exists limits our ability to consider other, potentially positive, subjective evidence, such as our future earnings projections. Based on our evaluation of all available positive and negative evidence, and by placing greater weight on the objective negative evidence associated with our three-year cumulative U.S. pre-tax loss adjusted for permanent adjustments, we determined, as of September 30, 2022, that it is more likely than not that our net U.S. deferred tax assets will not be realized. Accordingly, in fiscal 2022, we recorded a full valuation allowance against our net U.S. deferred tax assets as of September 30, 2022, resulting in a non-cash charge to income tax expense of $10.2 million in fiscal 2022. Due to significant estimates used to establish the valuation allowance and the potential for changes in facts and circumstances, it is reasonably possible that we will be required to record additional adjustments to the valuation allowance in future reporting periods that could have a material effect on our results of operations.
77
Unrecognized tax benefits are the differences between a tax position taken, or expected to be taken in a tax return, and the benefit recognized for accounting purposes pursuant to accounting guidance. The following is a reconciliation of the changes in unrecognized tax benefits, excluding interest and penalties:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Unrecognized tax benefits, beginning balance |
$ |
2,887 |
|
|
$ |
2,871 |
|
|
$ |
2,323 |
|
Increases in tax positions for prior years |
|
53 |
|
|
|
15 |
|
|
|
58 |
|
Decreases in tax positions for prior years |
|
(35 |
) |
|
|
(8 |
) |
|
|
(1 |
) |
Increases in tax positions for current year |
|
519 |
|
|
|
458 |
|
|
|
664 |
|
Settlements with taxing authorities |
|
— |
|
|
|
— |
|
|
|
— |
|
Lapse of the statute of limitations |
|
(631 |
) |
|
|
(449 |
) |
|
|
(173 |
) |
Unrecognized tax benefits, ending balance |
$ |
2,793 |
|
|
$ |
2,887 |
|
|
$ |
2,871 |
|
The total amount of unrecognized tax benefits excluding interest and penalties that, if recognized, would affect the effective tax rate was $2.5 million and $2.7 million as of September 30, 2022 and 2021, respectively. As of September 30, 2022, the Company does not expect the liability for unrecognized tax benefits to change significantly in the next 12 months and has classified the above balances on the consolidated balance sheets in other noncurrent liabilities. Interest and penalties related to unrecognized tax benefits are recorded in income tax expense on the consolidated statements of operations. As of September 30, 2022 and 2021, the gross amount accrued for interest and penalties on unrecognized tax benefits was $0.3 million and $0.4 million, respectively.
The Company files income tax returns, including returns for its subsidiaries, in the U.S. federal jurisdiction and in various state jurisdictions, as well as several non-U.S. jurisdictions. Uncertain tax positions are related to tax years that remain subject to examination. The Internal Revenue Service commenced an examination of the Company’s fiscal 2019 U.S. federal tax return during fiscal 2022; the examination has not been completed. U.S. federal income tax returns for years prior to fiscal 2019 are no longer subject to examination by federal tax authorities. For tax returns for U.S. state and local jurisdictions, the Company is no longer subject to examination for tax years generally before fiscal 2012. For tax returns for non-U.S. jurisdictions, the Company is no longer subject to income tax examination for years prior to 2018. Additionally, the Company has been indemnified of liability for any taxes relating to Creagh Medical, NorMedix and Vetex for periods prior to the respective acquisition dates, pursuant to the terms of the related share purchase agreements. As of September 30, 2022 and 2021, there were no undistributed earnings in foreign subsidiaries.
10. Defined Contribution Plans
The Company has a 401(k) retirement and savings plan for the benefit of qualifying U.S. employees, and a defined contribution Personal Retirement Savings Account plan for the benefit of qualifying Ireland employees. For eligible U.S. employees, effective January 1, 2022, the Company makes matching contributions of up to 4% of eligible compensation; prior to January 1, 2022, the Company made matching contributions of up to 3% of eligible compensation on employee contributions of up to 6% of eligible compensation. For eligible Ireland employees, the Company makes contributions of up to 8% of eligible compensation on employee contributions of up to 6% of eligible compensation. Expense recognized for Company contributions to defined contribution plans totaled $1.7 million, $1.1 million and $1.0 million in fiscal 2022, 2021 and 2020, respectively.
11. Commitments and Contingencies
Clinical Trials. The Company has engaged CRO consultants to assist with the administration of its ongoing clinical trials. The Company has executed separate contracts with two CROs for services rendered in connection with the TRANSCEND pivotal clinical trial for the SurVeil DCB, including pass-through expenses paid by the CROs, of up to approximately $30 million in the aggregate. As of September 30, 2022, an estimated $5 million remains to be paid on these contracts, which may vary depending on actual pass-through expenses incurred to execute the trial. The Company estimates that the total cost of the TRANSCEND clinical trial will be in the range of $37 million to $40 million from inception to completion. In the event the Company were to terminate any trial, it may incur certain financial penalties, which would become payable to the CRO for costs to wind down the terminated trial.
Asset Acquisitions. In fiscal 2018, the Company acquired certain intellectual property assets of Embolitech, LLC (the “Embolitech Transaction”). As part of the Embolitech Transaction, the Company paid the sellers $5.0 million in fiscal 2018, $1.0 million in fiscal 2020, $1.0 million in fiscal 2021 and $0.5 million in fiscal 2022. The Company is obligated to pay additional installments totaling $2.0 million in fiscal 2023 through fiscal 2024. These payments may be accelerated upon the occurrence of certain sales and regulatory milestones. An additional $1.0 million payment is contingent upon the achievement of a certain regulatory milestone within a contingency period ending in 2033.
78
Business Combinations. See Note 12 Acquisitions for disclosure of the fiscal 2021 acquisition of Vetex and associated deferred and contingent consideration liabilities.
12. Acquisitions
Vetex Medical Limited
On July 2, 2021, Surmodics acquired all of the outstanding shares of Vetex Medical Limited (“Vetex”). Vetex, which was formerly privately held and is based in Galway, Ireland, develops and manufactures medical devices focused on venous clot removal solutions. The transaction expanded Surmodics’ thrombectomy portfolio with a second FDA 510(k)-cleared device, a mechanical venous thrombectomy device. The acquisition was accounted for as a business combination. The acquired assets, liabilities and operating results of Vetex have been included on our consolidated financial statements within the Medical Device segment from the date of acquisition.
Surmodics acquired Vetex with an upfront cash payment of $39.9 million funded using cash on hand and $10.0 million from the revolving credit facility in place during the period. The Company is obligated to pay additional installments totaling $3.5 million in fiscal 2024 through fiscal 2027. These payments may be accelerated upon the occurrence of certain product development and regulatory milestones. An additional $3.5 million in payments is contingent upon the achievement of certain product development and regulatory milestones within a contingency period ending in fiscal 2027.
The acquisition date fair value of purchase consideration was as follows:
(In thousands) |
|
|
|
Consideration paid at closing |
$ |
39,985 |
|
Deferred consideration |
|
3,257 |
|
Contingent consideration |
|
814 |
|
Total purchase consideration |
|
44,056 |
|
Less: Cash acquired |
|
(432 |
) |
Total purchase consideration, net of cash acquired |
$ |
43,624 |
|
The fair value of contingent consideration was derived using a discounted cash flow approach based on Level 3 inputs. See Note 5 Fair Value Measurements for additional disclosures regarding contingent consideration.
The final allocation of purchase consideration as of the acquisition date was as follows:
(In thousands) |
|
|
|
Asset (Liability) |
|
|
|
Current assets |
$ |
18 |
|
Property and equipment |
|
37 |
|
Intangible assets |
|
27,600 |
|
Other non-current assets |
|
37 |
|
Accrued compensation |
|
(236 |
) |
Other accrued liabilities |
|
(111 |
) |
Deferred income taxes |
|
(3,087 |
) |
Net assets acquired |
|
24,258 |
|
Goodwill |
|
19,366 |
|
Total purchase consideration, net of cash acquired |
$ |
43,624 |
|
During the third quarter of fiscal 2022, the Company recorded measurement adjustments to provisional amounts previously recognized, which resulted in a $0.3 million increase in goodwill and a corresponding decrease in net identifiable assets acquired. The Company finalized the accounting for the Vetex acquisition in the third quarter of fiscal 2022.
Acquired intangible assets consist of developed technology. We used the income approach, specifically the discounted cash flow method and the incremental cash flow approach using Level 3 inputs, to derive the fair value of the developed technology. The developed technology is amortized on a straight-line basis over its estimated useful life of 12 years. The amortization of the acquired intangible assets is tax deductible.
79
The goodwill recorded from the Vetex acquisition is a result of expected synergies from integrating the Vetex business into the Company’s Medical Device segment and from acquiring and retaining the existing Vetex workforce. The goodwill is not deductible for tax purposes.
In the year of acquisition, fiscal 2021, we reported zero revenue and $(0.9) million net loss from Vetex in our consolidated statements of operations. In addition, in fiscal 2021, we recognized $1.0 million in acquisition transaction, integration and other costs related to the Vetex acquisition on the consolidated statements of operations.
The pro forma impact of business combinations during fiscal years 2021 and 2020 was not significant, neither individually nor in the aggregate, to the consolidated results of the Company.
13. Reportable Segment Information
Reportable segments are components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, who is the Company’s Chief Executive Officer, in deciding how to allocate resources and in assessing performance. We operate two reportable segments:
Segment revenue, operating (loss) income, and depreciation and amortization were as follows:
|
Fiscal Year |
|
|||||||||
(In thousands) |
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Revenue: |
|
|
|
|
|
|
|
|
|||
Medical Device |
$ |
72,389 |
|
|
$ |
78,253 |
|
|
$ |
71,401 |
|
In Vitro Diagnostics |
|
27,562 |
|
|
|
26,883 |
|
|
|
23,463 |
|
Total revenue |
$ |
99,951 |
|
|
$ |
105,136 |
|
|
$ |
94,864 |
|
|
|
|
|
|
|
|
|
|
|||
Operating (loss) income: |
|
|
|
|
|
|
|
|
|||
Medical Device |
$ |
(22,923 |
) |
|
$ |
4,683 |
|
|
$ |
(3,246 |
) |
In Vitro Diagnostics |
|
13,073 |
|
|
|
13,770 |
|
|
|
11,771 |
|
Total segment operating (loss) income |
|
(9,850 |
) |
|
|
18,453 |
|
|
|
8,525 |
|
Corporate |
|
(12,247 |
) |
|
|
(11,750 |
) |
|
|
(9,776 |
) |
Total operating (loss) income |
$ |
(22,097 |
) |
|
$ |
6,703 |
|
|
$ |
(1,251 |
) |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization: |
|
|
|
|
|
|
|
|
|||
Medical Device |
$ |
8,368 |
|
|
$ |
7,224 |
|
|
$ |
6,223 |
|
In Vitro Diagnostics |
|
355 |
|
|
|
395 |
|
|
|
483 |
|
Corporate |
|
419 |
|
|
|
398 |
|
|
|
557 |
|
Total depreciation and amortization |
$ |
9,142 |
|
|
$ |
8,017 |
|
|
$ |
7,263 |
|
The Corporate category includes expenses that are not fully allocated to the Medical Device and In Vitro Diagnostics segments. These Corporate costs are related to administrative corporate functions, such as executive management, corporate accounting, information technology, legal, human resources and Board of Directors. Corporate may also include expenses, such as acquisition-related costs and litigation, which are not specific to a segment and thus not allocated to the reportable segments.
Asset information by segment is not presented because the Company does not provide its chief operating decision maker assets by segment, as the data is not readily available.
80
Revenue by geographic region was as follows:
|
Fiscal Year |
|
|||||||||
|
2022 |
|
|
2021 |
|
|
2020 |
|
|||
Domestic |
|
74 |
% |
|
|
79 |
% |
|
|
78 |
% |
Foreign |
|
26 |
% |
|
|
21 |
% |
|
|
22 |
% |
Long-lived assets by country, including property and equipment and intangible assets net of accumulated depreciation and amortization, respectively, were as follows:
|
September 30, |
|
|||||
(In thousands) |
2022 |
|
|
2021 |
|
||
U.S. |
$ |
24,788 |
|
|
$ |
25,920 |
|
Ireland |
|
30,505 |
|
|
|
41,224 |
|
14. Subsequent Events
MidCap Credit Agreement
On October 14, 2022, the Company entered into a secured revolving credit facility and secured term loan facilities pursuant to a Credit, Security and Guaranty Agreement (the “MidCap Credit Agreement”) with Mid Cap Funding IV Trust, as agent, and MidCap Financial Trust, as term loan servicer and the lenders from time to time party thereto.
The MidCap Credit Agreement provides for availability under a secured revolving line of credit of up to $25.0 million (the “Midcap Revolving Credit Facility”), and proceeds may be used for transaction fees and for working capital needs and general corporate purposes. Availability under the Midcap Revolving Credit Facility is subject to a borrowing base.
The MidCap Credit Agreement also provides for up to $75.0 million in term loans (the “Term Loans”), consisting of a $25.0 million Tranche 1 (“Tranche 1”) and a $50.0 million Tranche 2 (“Tranche 2”), which may be drawn in increments of at least $10.0 million. In addition, after the closing and prior to December 31, 2024, the Term Loan lenders may, in their sole discretion, fund an additional tranche of Term Loans of up to $25.0 million upon the written request of the Company. Upon closing, the Company borrowed $25.0 million of Tranche 1, borrowed $5.0 million on the Midcap Revolving Credit Facility, and used approximately $10.0 million of the proceeds to repay borrowings under the Bridgewater Revolving Credit Facility, and intends to use the remaining proceeds to fund working capital needs and other general corporate purposes. Until December 31, 2024, the Company will be eligible to borrow Tranche 2 at the Company’s option upon meeting certain conditions set forth in the MidCap Credit Agreement, including having no less than $60.0 million of rolling-four-quarter core net revenue as of the end of the prior fiscal quarter.
Pursuant to the MidCap Credit Agreement, the Company provided a first priority security interest in all existing and future acquired assets, including intellectual property and real estate, owned by the Company. The MidCap Credit Agreement contains certain covenants that limit the Company’s ability to engage in certain transactions. Subject to certain limited exceptions, these covenants limit the Company’s ability to, among other things:
The MidCap Credit Agreement also contains customary indemnification obligations and customary events of default, including, among other things, (i) non-payment, (ii) breach of warranty, (iii) non-performance of covenants and obligations, (iv) default on other indebtedness, (v) judgments, (vi) change of control, (vii) bankruptcy and insolvency, (viii) impairment of security, (ix) termination of a pension plan, (x) regulatory matters, and (xi) material adverse effect.
In addition, the Company must maintain minimum core net revenue levels tested quarterly to the extent that Term Loans advanced under the MidCap Credit Agreement exceed $25.0 million. In the event of default under the MidCap Credit Agreement, the Company would be required to pay interest on principal and all other due and unpaid obligations at the current rate in effect plus 2%.
81
The Midcap Revolving Credit Facility and the Term Loans mature on October 1, 2027. The Midcap Revolving Credit Facility bears interest at an annual rate equal to 3.00% plus the greater of Term SOFR (as defined in the MidCap Credit Agreement) or 1.50%, and the Term Loans bear interest at an annual rate equal to 5.75% plus the greater of Term SOFR or 1.50%. The Company is required to make monthly interest payments on the Midcap Revolving Credit Facility with the entire principal payment due at maturity. The Company is required to make 48 monthly interest payments on the Term Loans beginning on November 1, 2022 (the “Interest-Only Period”). If the Company is in covenant compliance at the end of the Interest-Only Period, the Company will have the option to extend the Interest-Only Period through maturity with the entire principal payment due at maturity. If the Company is not in covenant compliance at the end of the Interest-Only Period, the Company is required to make 12 months of straight-line amortization payments with the entire principal amount due at maturity.
Subject to certain limitations, the Term Loans have a prepayment fee for payments made prior to the maturity date equal to 3.0% of the prepaid principal amount for the first year following the closing date of the MidCap Credit Agreement, 2.0% of the prepaid principal amount for the second year following the closing date and 1.0% of the prepaid principal amount for the third year following the closing date and thereafter. In addition, if the Midcap Revolving Credit Facility is terminated in whole or in part prior to the maturity date, the Company must pay a prepayment fee equal to 3.0% of the terminated commitment amount for the first year following the closing date of the MidCap Credit Agreement, 2.0% of the terminated commitment amount for the second year following the closing date of the MidCap Credit Agreement and 1.0% of the terminated commitment amount for the third year following the closing date and thereafter. The Company is also required to pay a full exit fee at the time of maturity or full prepayment event equal to 2.5% of the aggregate principal amount of the Term Loans made pursuant to the MidCap Credit Agreement and a partial exit fee at the time of any partial prepayment event equal to 2.5% of the amount prepaid. The Company also is obligated to pay customary origination fees at the time of each funding of the Term Loans and a customary annual administrative fee based on the amount borrowed under the Term Loan, due on an annual basis. The customary fees on the Midcap Revolving Credit Facility include (i) an origination fee based on the commitment amount, which was paid on the closing date, (ii) an annual collateral management fee based on the outstanding balance of the Midcap Revolving Credit Facility, payable monthly in arrears and (iii) an unused line fee based on the average unused portion of the Midcap Revolving Credit Facility, payable monthly in arrears. The Company must also maintain a minimum balance of no less than 20% of availability under the Midcap Revolving Credit Facility or a minimum balance fee applies.
Interest Rate Swap
On October 14, 2022, the Company entered into a 5-year interest rate swap transaction with Wells Fargo Bank, N.A. with respect to $25.0 million of notional value of the Term Loans funded as Tranche 1 under the MidCap Credit Agreement. The interest rate swap transaction will effectively fix at 4.455% the one-month term SOFR portion of interest rate under the Term Loans funded as Tranche 1 such that the fixed interest rate per annum on the swapped $25.0 million notional value of such Term Loan will be 10.205% through its maturity.
82
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
The Company maintains disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are designed to ensure that information required to be disclosed in our reports filed under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and no evaluation can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected.
The Company’s management, under the supervision and with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, referred to collectively herein as the Certifying Officers, carried out an evaluation of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of September 30, 2022, the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, the Certifying Officers concluded that the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) were effective as of September 30, 2022, as designed and implemented to ensure that information required to be disclosed by the Company in reports that it files under the Exchange Act is recorded, processed, summarized and reported within the time period specified in the Securities Exchange Commission rules and forms, and to ensure that information required to be disclosed by the Company in the reports the Company files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its Certifying Officers, as appropriate, to allow timely decisions regarding required disclosures.
a. Management’s Annual Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f) and 15d-15(f). The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our consolidated financial statements.
Management evaluated the design and operating effectiveness of the Company’s internal control over financial reporting based on the criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation, management concluded that internal control over financial reporting was effective as of September 30, 2022.
The Company’s independent registered public accounting firm, Deloitte & Touche LLP, who audited the consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the effectiveness of the Company’s internal control over financial reporting as of September 30, 2022. This report states that internal control over financial reporting was effective and appears in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
b. Changes in Internal Control Over Financial Reporting. There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the quarter ended September 30, 2022 that materially affected, or are reasonable likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
83
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not Applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information required by Item 10 relating to directors, our audit committee, the nature of changes, if any, to procedures by which our shareholders may recommend nominees for directors, our code of ethics and compliance with Section 16(a) of the Exchange Act will appear in the Company’s Proxy Statement for its 2023 Annual Meeting of Shareholders and is incorporated herein by reference. The information required by Item 10 relating to executive officers appears in Part I, Item 1 of this Annual Report on Form 10-K.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by Item 11 will appear in the Company’s Proxy Statement for its 2023 Annual Meeting of Shareholders and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by Item 12 will appear in the Company’s Proxy Statement for its 2023 Annual Meeting of Shareholders and is incorporated herein by reference.
Equity Compensation Plan Information
The following table provides information related to the Company’s equity compensation plans in effect as of September 30, 2022:
Plan Category |
(a) |
|
|
|
(b) |
|
|
|
(c) |
|
|||
Equity compensation plans approved by shareholders |
|
1,261,049 |
|
(1) |
$ |
37.42 |
|
(1) |
|
967,063 |
|
||
Equity compensation plans not approved by shareholders |
|
— |
|
|
N/A |
|
|
|
— |
|
|||
Total |
|
1,261,049 |
|
|
$ |
37.42 |
|
|
|
967,063 |
|
||
(1) Excludes shares that may be issued under the Company’s amended and restated 1999 Employee Stock Purchase Plan.
The information required by Item 13 will appear in the Company’s Proxy Statement for its 2023 Annual Meeting of Shareholders and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by Item 14 will appear in the Company’s Proxy Statement for its 2023 Annual Meeting of Shareholders and is incorporated herein by reference.
84
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) 1. Financial Statements
The following consolidated financial statements are set forth in Part II, Item 8:
Reports of Independent Registered Public Accounting Firm
Consolidated Statements of Operations
Consolidated Statements of Comprehensive (Loss) Income
Consolidated Statements of Stockholders’ Equity
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
2. Financial Statement Schedules
Schedule II —Valuation and Qualifying Accounts for fiscal years ended September 30, 2022, 2021 and 2020. All other schedules are omitted because they are inapplicable, not required, or the information is in the consolidated financial statements or related notes.
Surmodics, Inc.
Schedule II – Valuation and Qualifying Accounts
(In thousands) |
|
Balance at |
|
|
Additions: |
|
|
Deductions: |
|
|
|
Balance at |
|
||||
Allowance for credit losses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Fiscal year ended September 30, 2020 |
|
$ |
200 |
|
|
$ |
73 |
|
|
$ |
(143 |
) |
(a) |
|
$ |
130 |
|
Fiscal year ended September 30, 2021 |
|
|
130 |
|
|
|
(11 |
) |
|
|
— |
|
(a) |
|
|
119 |
|
Fiscal year ended September 30, 2022 |
|
|
119 |
|
|
|
5 |
|
|
|
(43 |
) |
(a) |
|
|
81 |
|
3. Exhibits
Exhibit |
|
Description |
|
||
|
||
|
||
|
||
|
85
Exhibit |
|
Description |
|
||
|
||
|
||
10.1* |
|
|
10.2* |
|
|
10.3* |
|
|
10.4* |
|
|
10.5* |
|
|
10.6* |
|
|
10.7* |
|
|
10.8* |
|
|
10.9* |
|
|
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
86
Exhibit |
|
Description |
|
||
10.19** |
|
|
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
|
||
21† |
|
|
23† |
|
|
|
Power of Attorney (included on signature page of this Form 10-K). |
87
Exhibit |
|
Description |
31.1† |
|
|
31.2† |
|
|
32.1† |
|
|
32.2† |
|
|
101.INS† |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File as its XBRL tags are embedded within the inline XBRL document. |
101.SCH† |
|
Inline XBRL Taxonomy Extension Schema. |
101.CAL† |
|
Inline XBRL Taxonomy Extension Calculation Linkbase. |
101.DEF† |
|
Inline XBRL Taxonomy Extension Definition Linkbase. |
101.LAB† |
|
Inline XBRL Taxonomy Extension Label Linkbase. |
101.PRE† |
|
Inline XBRL Taxonomy Extension Presentation Linkbase. |
104† |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101). |
* Management contract or compensatory plan or arrangement.
† Filed herewith.
** Portions of this document, which have been separately filed with the Securities and Exchange Commission, have been omitted pursuant to a request for confidential treatment.
ITEM 16. FORM 10-K SUMMARY.
None.
88
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
SURMODICS, INC. |
||
|
|
|
By: |
|
/s/ Gary R. Maharaj |
|
|
Gary R. Maharaj |
|
|
President and Chief Executive Officer |
Dated: November 23, 2022
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant, in the capacities, and on the dates indicated.
(Power of Attorney)
Each person whose signature appears below authorizes GARY R. MAHARAJ or TIMOTHY J. ARENS, and constitutes and appoints said persons as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any or all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, authorizing said persons and granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all said attorneys-in-fact and agents, or his substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
|
|
|
/s/ Gary R. Maharaj Gary R. Maharaj |
|
President and Chief Executive Officer (principal executive officer) and Director |
|
November 23, 2022 |
|
|
|
|
|
/s/ Timothy J. Arens Timothy J. Arens |
|
Senior Vice President of Finance and Chief Financial Officer (principal financial officer) |
|
November 23, 2022 |
|
|
|
|
|
/s/ John D. Manders |
|
Vice President of Finance and Corporate Controller |
|
November 23, 2022 |
John D. Manders /s/ Susan E. Knight Susan E. Knight |
|
(principal accounting officer) Chairman of the Board of Directors |
|
November 23, 2022 |
|
|
|
|
|
/s/ José H. Bedoya José H. Bedoya |
|
Director |
|
November 23, 2022 |
|
|
|
|
|
/s/ David R. Dantzker, M.D. David R. Dantzker, M.D. |
|
Director |
|
November 23, 2022 |
|
|
|
|
|
/s/ Ronald B. Kalich Ronald B. Kalich |
|
Director |
|
November 23, 2022 |
|
|
|
|
|
/s/ Lisa Wipperman Heine |
|
Director |
|
November 23, 2022 |
Lisa Wipperman Heine |
|
|
|
|
89
Similar companies
See also STRYKER CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also BECTON DICKINSON & CO - Annual report 2022 (10-K 2022-09-30) Annual report 2023 (10-Q 2023-06-30)
See also 3M CO - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also BOSTON SCIENTIFIC CORP - Annual report 2022 (10-K 2022-12-31) Annual report 2024 (10-Q 2024-03-31)
See also RESMED INC - Annual report 2023 (10-K 2023-06-30) Annual report 2023 (10-Q 2023-09-30)

