Tilray Brands, Inc. - Annual Report: 2019 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-38594
TILRAY, INC.
(Exact name of Registrant as specified in its Charter)
|
Delaware |
82-4310622 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
1100 Maughan Road Nanaimo, BC |
V9X IJ2 |
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (844) 845-7291
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
Class 2 Common Stock, $0.0001 par value per share |
|
TLRY |
|
The Nasdaq Stock Market LLC The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☒ NO ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☒ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
☐ |
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of the Registrant’s Class 2 Common Stock on The Nasdaq Stock Market on June 28, 2019, was approximately $1.03 billion.
As of March 2, 2020 there were 16,666,667 shares of the Registrant’s Class 1 Common Stock, par value of $0.0001 per share, and 87,390,113 shares of the Registrant’s Class 2 Common Stock, par value $0.0001 per share, issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates certain information by reference from the definitive proxy statement to be filed by the registrant in connection with the 2020 Annual Meeting of Stockholders (the “Proxy Statement”). The Proxy Statement will be filed by the registrant with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the year ended December 31, 2019.
Table of Contents
|
|
|
Page |
|
PART I |
|
|
|
Item 1. |
1 |
|
|
Item 1A. |
17 |
|
|
Item 1B. |
45 |
|
|
Item 2. |
45 |
|
|
Item 3. |
45 |
|
|
Item 4. |
46 |
|
|
|
|
|
|
PART II |
|
|
|
Item 5. |
47 |
|
|
Item 6. |
49 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
50 |
|
Item 7A. |
75 |
|
|
Item 8. |
F-1 |
|
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
76 |
|
Item 9A. |
76 |
|
|
Item 9B. |
81 |
|
|
|
|
|
|
PART III |
|
|
|
Item 10. |
82 |
|
|
Item 11. |
82 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
82 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
82 |
|
Item 14. |
82 |
|
|
|
|
|
|
PART IV |
|
|
|
Item 15. |
83 |
|
|
Item 16 |
86 |
In this Annual Report on Form 10-K, “we,” “our,” “us,” “Tilray,” and “the Company” refer to Tilray, Inc. and, where appropriate, its consolidated subsidiaries. This report contains references to our trademarks and trade names and to trademarks and trade names belonging to other entities. Solely for convenience, trademarks and trade names referred to in this report may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trademarks or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
i
PART I
Special Note Regarding Forward-Looking Statements
Some of the information contained in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or “forward -looking information” within the meaning of Canadian securities laws. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “will,” “would” or the negative or plural of these words or similar expressions or variations. Such forward-looking statements and forward-looking information are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by the forward-looking statements or forward-looking information. Factors that could cause or contribute to such differences include, but are not limited to, those identified in this Annual Report on Form 10-K and those discussed in the section titled “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K and in our other SEC and Canadian public filings. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based on information available to us as of the date of this Annual Report on Form 10-K and while we believe that information provides a reasonable basis for these statements, that information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely on these statements. You should not rely upon forward-looking statements or forward-looking information as predictions of future events. Furthermore, such forward-looking statements or forward-looking information speak only as of the date of this report. Except as required by law, we undertake no obligation to update any forward-looking statements or forward-looking information to reflect events or circumstances after the date of such statements.
Item 1. Business.
Our Vision
Our vision is to build the world’s most trusted and valued cannabis and hemp company.
We are pioneering the future of medical, wellness and adult-use cannabis and hemp research, cultivation, processing and distribution globally, and we are one of the leading suppliers of adult-use cannabis in Canada and a leading supplier of hemp products in North America.
Our Beliefs
Our founders started the Company with the belief that patients and consumers should have safe access and a reliable supply of quality-tested pure, precise and predictable cannabis products.
Our Company is anchored around three core beliefs:
|
• |
Medical cannabis is a mainstream medicine consumed by mainstream patients — similarly, we believe adult-use cannabis and hemp products are mainstream products consumed by mainstream consumers; |
|
• |
We are witnessing a global paradigm shift with regard to cannabis and hemp, and because of this shift, the transformation of a multibillion dollar industry from a state of prohibition to a state of legalization; and |
|
• |
As this transformation occurs, trusted global brands, backed by multinational supply chains, will shape the future of our industry and earn the confidence of patients, consumers, healthcare practitioners and governments around the world. |
1
Our Company
We have supplied high-quality medical cannabis products to tens of thousands of patients in fifteen countries spanning five continents across the world through our subsidiaries in Australia, Canada, Germany, Latin America and Portugal and through agreements with established pharmaceutical distributors. We cultivate medical and adult-use cannabis in Canada and medical cannabis in Europe.
We operate only in countries where cannabis or hemp-derived cannabinoids are legal, by which we mean the activities in those countries are permitted under all applicable federal and state or provincial and territory laws.
We have been an early leader in the development of the global medical cannabis market. We were one of the first companies to be licensed by Health Canada to cultivate and sell medical cannabis in Canada, and one of the first companies to become a licensed dealer of medical cannabis in Canada. These licenses allow us to produce and sell medical cannabis in Canada, to develop new and innovative cannabis products and to export medical cannabis products to other countries in accordance with applicable laws. The cannabis industry is expanding rapidly in Canada, with more than 280 current licenses, though only a few were licensed earlier than us. Our medical cannabis products have been made available or used in clinical trials in Argentina, Australia, Canada, Chile, Croatia, Cyprus, the Czech Republic, Germany, Israel, Ireland, New Zealand, South Africa, Switzerland, United States, and United Kingdom. While there are other Licensed Producers operating in multiple countries, including some licensed in Canada, and other non-cannabis companies expanding into the cannabis market internationally, we were the first company to legally export medical cannabis from North America to Africa, Australia, Europe, Israel and Latin America, and we were among the first companies to be licensed to cultivate and process medical cannabis in two countries, Canada and Portugal. We have successfully recruited an international advisory board consisting of world-renowned policy leaders and business leaders, to advise on our global expansion and add to our growing network of experts in their specific field of expertise.
Our Company is led by a team of visionary entrepreneurs, experienced operators and cannabis industry experts as well as PhD scientists, horticulturists and extraction specialists who apply the latest scientific knowledge and technology to deliver quality-controlled, rigorously tested cannabis products on a large scale. We have made significant investments to establish Tilray as a scientifically rigorous cannabis brand, committed to quality and excellence. Recognizing the opportunity associated with growing and producing cannabis on a large scale, we have invested capital to develop innovative cultivation practices, proprietary product formulations and automated production processes. We have also invested in clinical trials and recruited a Medical Advisory Board comprised of highly accomplished researchers and physicians. We were the first cannabis company with a North American production facility to be Good Manufacturing Practices, or GMP, certified in accordance with European Medicines Agency, or EMA, standards. An internationally recognized standard, GMP certification is the primary quality standard that pharmaceutical manufacturers must meet in their production and manufacturing processes.
We are committed to establishing a diverse team as we continue to grow. We are proud to have one of the first women-majority boards in the cannabis industry. Diversity is a priority for our company and we intend to seek out talented people from a variety of backgrounds to join our leadership team.
We believe our growth to date is a result of our global strategy, our multinational supply chain and distribution network and our methodical commitment to research, innovation, quality and operational excellence. We believe that recognized and trusted brands distributed through multinational supply chains will be best positioned to become global market leaders. Our strategy is to build these brands by consistently producing high-quality, differentiated products on a large scale.
2
Business Segments
We report our operating results in two segments: (i) Cannabis (licensed), and (ii) Hemp (unlicensed). The business segments reflect how our operations are managed, how resources are allocated, how operating performance is evaluated by senior management and the structure of our internal financial reporting. We report total revenue, inclusive of excise duties, in two reportable segments, by product category and product channel, as follows:
Revenue by product channel
|
|
Year Ended |
|
% of |
|
Year Ended |
|
% of |
|
Year Ended |
|
% of |
|
||||||
|
(in thousands of United States dollars) |
December 31, 2019 |
|
Total revenue |
|
December 31, 2018 |
|
Total revenue |
|
December 31, 2017 |
|
Total revenue |
|
||||||
|
Cannabis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adult-use |
$ |
55,763 |
|
|
33 |
% |
$ |
3,521 |
|
|
8 |
% |
$ |
— |
|
|
— |
|
|
Canada - medical |
|
12,556 |
|
|
8 |
% |
|
18,052 |
|
|
42 |
% |
|
19,642 |
|
|
96 |
% |
|
International - medical |
|
13,378 |
|
|
8 |
% |
|
2,912 |
|
|
7 |
% |
|
896 |
|
|
4 |
% |
|
Bulk |
|
25,450 |
|
|
15 |
% |
|
18,645 |
|
|
43 |
% |
|
— |
|
|
— |
|
|
Total cannabis revenue |
$ |
107,147 |
|
|
64 |
% |
$ |
43,130 |
|
|
100 |
% |
$ |
20,538 |
|
|
100 |
% |
|
Hemp |
|
59,832 |
|
|
36 |
% |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Total revenue |
$ |
166,979 |
|
|
100 |
% |
$ |
43,130 |
|
|
100 |
% |
$ |
20,538 |
|
|
100 |
% |
Revenue by product category
|
|
Year Ended |
|
% of |
|
Year Ended |
|
% of |
|
Year Ended |
|
% of |
|
||||||
|
(in thousands of United States dollars) |
December 31, 2019 |
|
Total revenue |
|
December 31, 2018 |
|
Total revenue |
|
December 31, 2017 |
|
Total revenue |
|
||||||
|
Dried cannabis |
$ |
82,753 |
|
|
50 |
% |
$ |
21,674 |
|
|
50 |
% |
$ |
16,260 |
|
|
79 |
% |
|
Cannabis extracts |
|
24,139 |
|
|
14 |
% |
|
21,179 |
|
|
49 |
% |
|
3,965 |
|
|
19 |
% |
|
Hemp products |
|
59,832 |
|
|
36 |
% |
|
— |
|
|
0 |
% |
|
— |
|
|
0 |
% |
|
Accessories and other |
|
255 |
|
|
0 |
% |
|
277 |
|
|
1 |
% |
|
313 |
|
|
2 |
% |
|
Total revenue |
$ |
166,979 |
|
|
100 |
% |
$ |
43,130 |
|
|
100 |
% |
$ |
20,538 |
|
|
100 |
% |
Revenue for the year December 31, 2019 included $13.1 million of excise duties (2018 - $1.2 million, 2017 – nil). Two customers accounted for 13% each of revenue for the year ended December 31, 2019. One customer accounted for 24% of our revenue for the year ended December 31, 2018. No one customer accounted for greater than 10% of our revenue for the year ended December 31, 2017.
3
Cannabis
We are a leader in the legal licensed cannabis segment, which includes Canadian Adult-Use, Canadian Medical, International Medical as well as bulk sales. We have a number of brands in these categories.
Hemp
We are a leader in the unlicensed hemp products segment, which includes hemp foods and cannabidiol (“CBD”) products. Our hemp food products are available in 20 countries and our CBD products are currently available in certain states in the United States.
Our Opportunity
We are approaching our industry from a long-term, global perspective and see opportunities to:
Build global brands that lead, legitimize and define the future of cannabis and hemp. Historically, cannabis has been an unbranded product. As the legal cannabis and hemp industries emerge in more countries around the world, we see an opportunity to create a broad-based portfolio of differentiated brands brought to market in a professional manner, that appeal to a diverse set of patients and consumers. We believe that we have the ability to develop dominant global brands and that as we develop these brands, we will expand the addressable market for our products. We believe our business has the potential to disrupt the pharmaceutical, alcohol, tobacco and functional food and beverages industries because the emergence of the legal cannabis and hemp industries may result in a shift of discretionary income and/or a change in consumer preferences in favor of cannabis and hemp products versus other products. Recognizing the potential of this disruption, several companies in these sectors have already formed partnerships or made investments to gain exposure to the legal cannabis industry, including Sandoz AG, Anheuser-Busch InBev (“AB InBev”), Apotex Inc., Altria Group, Inc., Constellation Brands, Inc. and Imperial Brands PLC. In addition, several alcohol companies have noted in regulatory filings that legal cannabis could have an adverse impact on their business, including AB InBev, Boston Beer Company, and Molson Coors Brewing Company. We further believe that many patients rely on medical cannabis as a substitute to opioids and other narcotics, which has been validated by our annual patient study and peer-reviewed academic research which has demonstrated that the legalization of cannabis has coincided with a decline in the use of prescription drugs. Lastly, we believe that functional food and beverages, that is, products containing or enhanced with vitamins, caffeine, electrolytes, probiotics and other additives and ingredients, will see increased competition from products containing cannabinoids, such as CBD. For example, we believe that many consumers will choose cannabinoid-enhanced beverages in favor of sports drinks or energy drinks.
Invest in markets where cannabis and hemp products are federally legal or are expected to be federally legal. Our goal is to increase our total addressable market size as countries continue to legalize cannabis for medical access and adult-use access globally. To date, 41 countries have formally legalized medical cannabis programs for either research or patient access and two countries, including Canada, have implemented adult-use access for cannabis. The Agriculture Improvement Act of 2018 the “Farm Bill”), was passed into law in the United States during December 2018, which permits the cultivation of hemp and the production of hemp-derived CBD and other cannabinoids. Combined with the growing global acceptance of hemp and hemp-derived CBD products, we believe there is a significant market opportunity in hemp and hemp-derived CBD products globally. We expect to monitor, identify and selectively invest in compelling opportunities that will strengthen our leadership position as demonstrated by our acquisition of Manitoba Harvest in February 2019.
Develop innovative products and form factors that change the way the world consumes cannabis and hemp. We believe the future of the cannabis and hemp industries will primarily be in non-combustible products that will offer patients and consumers alternatives to smoking. We see an opportunity to partner with established pharmaceutical, food, beverage and consumer product companies to develop new non-combustible form factors that will appeal to consumers who are not interested in smoking cannabis, including our beverage research partnership with AB InBev. By developing new, non-combustible products, we believe we will expand our addressable market.
Expand the availability of pure, precise and predictable medical cannabis products for patients in need around the world. Since 2014, we have seen significant increases in demand from patients and governments for pharmaceutical-grade cannabis products. We are well-positioned to expand availability of these products to more patients in more countries as medical cannabis is increasingly recognized as a viable treatment option for patients suffering from a variety of diseases and conditions. Importantly, most European countries have required that all
4
medical products sold be sourced from GMP-certified facilities. As such, GMP-certified producers, such as us, are well-positioned to establish market share in the European medical cannabis market. Outside of our Company, we believe there are very few GMP-certified Licensed Producers.
Foster mainstream acceptance of the therapeutic potential of medical cannabis and cannabinoid-based medicines. We see an opportunity to significantly expand the global market for medical cannabis products by conducting clinical research into the safety and efficacy of medical cannabis for a diverse range of conditions. By generating clinical data demonstrating the safety and efficacy of medical cannabis and cannabinoid-based medicines for various conditions, we see an opportunity to significantly expand and dominate the global medical cannabis market.
Our Strengths
We are a global pioneer with a multinational supply chain and distribution network. We were the first cannabis producer to export medical cannabis from North America and legally import cannabis into the European Union, or the EU. We have licenses to cultivate cannabis in Canada and Portugal. Our products have been made available in fifteen countries spanning five continents, which we believe is more than any other Licensed Producer. To achieve our goal of becoming a global cannabis leader, we have signed agreements with established global industry leaders including:
|
• |
In January 2018, we entered into a supply agreement with Shoppers Drug Mart Inc. (“Shoppers Drug Mart”), Canada’s largest pharmacy chain with more than 1,200 pharmacies. |
|
• |
In December 2018, we entered into a global framework agreement with Sandoz AG, a global leader in generic pharmaceuticals and biosimilars and part of the Novartis group, to increase availability of high quality medical cannabis products across the world. This was an evolution of the existing collaboration agreement with Sandoz Canada and under the framework agreement, Sandoz AG and Tilray will work together to develop and commercialize non-smokable and non-combustible medical cannabis products. |
|
• |
In December 2018, we entered into a research partnership with AB InBev, the world’s leading brewer to research non-alcoholic beverages containing THC and CBD in Canada. AB InBev’s participation is through Labatt Breweries of Canada and Tilray’s participation is through High Park Company, which is a Canadian adult-use subsidiary. These two companies expect to invest up to $50 million each, for a total of up to $100 million in aggregate, in the joint venture. This project was commercialized to form Fluent Beverage Company which launched CBD beverages in December 2019. |
|
• |
In February 2019, we acquired FHF Holdings Ltd. (“Manitoba Harvest”), which is the world’s largest hemp food company with a retail network of approximately 16,000 stores across North America, including Costco, Amazon, and Wal-Mart. |
|
• |
In October 2019, High Park, our adult-use subsidiary in Canada, announced a partnership with Cannfections, a leader in the confectionery space with 85 years of experience developing and producing the world’s most celebrated confectionery brands, allowing us to expedite innovation and new products to market. |
We have entered into agreements to supply adult-use cannabis to eleven provinces and territories. We have been expanding our product offerings and formats since the date of adult-use legalization in Canada, and we intend to continue to increase our distribution of best-in-class brands and products to the Canadian adult-use market.
We have a scientifically rigorous medical cannabis brand approved by governments to supply patients and researchers on five continents. Governments in fifteen countries have issued permits allowing our medical cannabis products to be imported from Canada and/or Portugal for distribution to patients. We believe governments have approved the importation of our products in part because of our reputation for being a scientifically rigorous medical cannabis company known for delivering safe, high-quality products. We are committed to advancing scientific knowledge about the therapeutic potential of cannabis, as demonstrated by our success receiving federal authorizations to supply cannabinoid products to clinical trials in Australia, the United States and Canada and by recruiting a Medical Advisory Board comprised of highly accomplished researchers and physicians specializing in autism, epilepsy, cancer and dermatology.
We have secured the exclusive rights to produce and distribute a broad-based portfolio of certain adult-use brands and products to Canadian consumers for the adult-use market. The brand licensing agreement between a wholly owned subsidiary of ours and Docklight LLC (“Docklight”), a former wholly owned subsidiary of
5
Privateer Holdings, Inc., provides us with intellectual property that we believe will give us a competitive advantage for the adult-use market in Canada. The brand licensing agreement includes the rights to recognized brand names and proprietary product formulations for a wide range of products.
We have a track record for continuing to innovate within our industry. We believe our commitment to research and innovation at this early stage of our industry’s development differentiates us and gives us a competitive advantage. We have invested significant capital to develop innovative cultivation practices and facilities and proprietary product formulations.
We have developed a rigorous, proprietary production process to ensure consistency and quality as we increase the scale of our operations globally. We pride ourselves on consistently delivering high-quality products with precise chemical compositions. We were the first cannabis company with a North American production facility to be GMP-certified in accordance with EMA standards. We believe GMP certification provides regulators and health care providers in countries new to medical cannabis with confidence that our products are a safe, high-quality choice.
We have a highly experienced management team. We believe our management team is one of the most knowledgeable and experienced in the cannabis industry. We recognize that our industry is in the early stages of its development and that we are taking a long-term, global view towards its development. Our management team has significant experience evaluating potential transactions, partnerships and other growth opportunities, and we pride ourselves on making investment decisions that we believe will allow us to grow our business over the long term. We have continued to identify and acquire talent from leading global companies to join our team. We are confident that our team has the diversity and depth of experience to propel Tilray into a global leadership position.
Our Growth Strategy
We aspire to build the world’s most trusted and valuable global cannabis and hemp company through the following key strategies:
Expanding our production capacity in North America and Europe to meet current and expected long-term demand growth. To capitalize on the market opportunity in North America and globally, we are investing to expand our production capacity and to automate certain cultivation, processing and packaging processes to gain efficiencies as we increase the scale of our operations.
Partnering with established distributors and retailers. As the industry evolves, we believe that the distribution of medical cannabis will increasingly mirror the distribution of other pharmaceutical products. Likewise, we believe the distribution of adult-use cannabis and hemp products will increasingly mirror the distribution of other consumer packaged goods. To efficiently and rapidly increase our scale, we are partnering with established distributors and retailers globally.
Developing a differentiated portfolio of brands and products to appeal to diverse sets of patients and consumers. We have established Tilray as a global pioneer shaping the future of the medical cannabis industry by developing a portfolio of high-quality medical cannabis and cannabinoid-based products ranging from dried flower to capsules to oils to well-defined clinical preparations. We will continue to invest in a differentiated portfolio of brands and products to appeal to a wide variety of patients and consumers. We recently developed and launched non-combustible products that offer an alternative to smoking, which we believe will account for the majority of products on the market over the long term. These products include beverages, vape products and edibles.
Expanding the addressable medical market by investing in clinical research and winning the trust of regulators, researchers and physicians in countries new to medical cannabis. We are expanding our addressable medical market by working collaboratively with regulators to implement safe access programs for patients. We provide clinical data to physicians and researchers on the safety and efficacy of medical cannabis to foster mainstream acceptance and enhance our reputation.
Maintaining a rigorous and relentless focus on operational excellence and product quality. We have strategically invested ahead of our growth in our operations, including cultivation, manufacturing and multichannel distribution. In doing so, we have developed a quality management system that enables us to meet the requirements of regulatory agencies in the markets where we export products, while consistently delivering high-quality products. As we continue to grow, we have the opportunity to leverage these investments while maintaining the highest level of safety and quality.
6
Continued innovation within our industry. We have at least fifty filed patents in the fields of cannabis processing technology, formulation, composition delivery system, and treatment methods. Our business partnerships have expanded to include partnerships with global, pharmaceutical companies, consumer product goods companies, distributors, and renowned research and development companies. We believe our growing partnerships with established companies will differentiate us and position us to become a dominant leader in product and process innovation and brand development. We also continue to establish partnerships with leading research institutions and our clinical trials continue to generate safety and efficacy data that can inform treatment decisions, lead to the development of new products, position us to register medicines for market authorization, and enable us to obtain insurance reimbursement where feasible.
Our Brands and Products
Our brand and product strategy centers on developing a broad-based portfolio of differentiated cannabis and hemp brands and products designed to appeal to diverse sets of patients and consumers. These brands and products have been tailored to comply with requirements introduced under local regulations, such as the inclusion of health warnings on labels and restrictions on marketing, and will continue to be adapted as regulators permit a broader range of form factors and revises its labeling and packaging requirements accordingly. Since 2010, members of our management team have been conducting research in more than a dozen countries by consulting third-party industry databases with market and consumer insights data available in various cannabis markets around the world, by commissioning proprietary third-party research and by licensing intellectual property from established cannabis brands.
Our Medical Brand: Tilray
The Tilray brand is designed to appeal to the global medical market by offering a wide range of high-quality, pharmaceutical-grade medical cannabis and cannabinoid-based products. We offer our products to patients, physicians, clinics, pharmacies, governments, hospitals and researchers for commercial purposes, compassionate access and clinical research.
We believe patients choose Tilray because we are a trusted, scientifically rigorous brand known for producing pure, precise and predictable medical-grade products. We have successfully grown over 50 cultivars of cannabis and developed a wide variety of extract products and formulations. Our global portfolio of medical cannabis products includes the following form factor platforms:
|
• |
whole flower; |
|
• |
ground flower; |
|
• |
full-spectrum oil drops and capsules; |
|
• |
purified oil drops medical vape pens; and |
|
• |
clinical compounds. |
Each form factor platform is divided into different product categories that correspond with the particular chemical composition of each product based on the concentration of two active ingredients: THC and CBD. For instance, our whole flower and full-spectrum oil drops and capsules are available in categories THC-Dominant, CBD-Dominant and THC and CBD Balanced.
Our product line focuses on active ingredients and standardized, well-defined preparation methods. We use formulations and delivery formats that are intended to allow for consistent and measured dosing, and we test all our products for potency and purity. Each of our commercial products are developed with comprehensive analysis and thorough documentation. We follow detailed and rigorous documentation standards not only for our own internal purposes but also because this type of documentation is required by researchers, regulators, importers and distributors.
7
We take a scientific approach to our medical-use product development, which we believe gives us credibility and respect in the medical community. We produce products that are characterized by well-defined and reproducible cannabinoid and terpene content, formulated for stable pharmacokinetic profiles, which are customizable in a variety of formulations and available in capsule or liquid forms. We continue to conduct extensive research and development activities as well as develop and promote new products for medical use. We are also currently working with established pharmaceutical companies, such as Sandoz Canada, a division of Novartis, to develop non-combustible, co-branded products for sale in pharmacies when regulations permit.
Our Adult-Use Brands
Our wholly owned subsidiary designed to cultivate, produce, sell and distribute adult-use cannabis brands and products, High Park Company, developed and launched new brands for the adult-use market in Canada which are wholly owned by us, such as CANACA™, Yukon Rove™ and Dubon™, The Batch/La Batch™ and Chowie Wowie™. We have also secured the exclusive rights from Docklight to produce and distribute a broad-based portfolio of certain adult-use brands and products in Canada. The brand licensing agreement includes the rights to recognized brands and proprietary product formulations for a wide range of products.
We currently produce and distribute these brands and products to Canadian consumers through High Park Company, formed to serve the adult-use market in Canada, and have introduced additional brands and products inclusive of vapes, beverages and edibles and have additional innovative products in our pipeline.
Our portfolio of brands and products have been specifically adapted, and our marketing activities carefully structured, to enable us to develop our brands in an effective and compliant manner.
Retail Strategy and Brands
We have the foundation in place to be a leader in the adult-use cannabis market with High Park Company. In October 2018, when the Canadian government federally legalized adult-use cannabis, High Park Company launched a number of cannabis products under various brands in the country’s largest markets, including Ontario, Quebec and British Columbia. Our understanding of the adult-use consumer is informed by extensive research, including post-adult use legalization focus groups across the country including Toronto, Vancouver and Quebec City.
We have established our portfolio and pricing strategies to compete for what we believe to be the largest adult-use consumer segments of the addressable market.
We also believe we have industry-leading customer service, supported by trained, multilingual customer service representatives available 24 hours a day, seven days a week from our Canadian call center.
The brands launched across Canada as part of Phase 1 legalizations includes:
|
|
o |
Canaca – A brand that proudly builds on its homegrown heritage with cannabis whole flower, pre-rolls, oil products and pure cannabis vapes handcrafted by and for Canadian cannabis enthusiasts. Our plants are sourced in BC and expertly cultivated in Ontario for homegrown, down-to-earth quality that’s enjoyed across Canada. |
|
|
o |
Irisa – A women’s wellness brand created with modern health and wellness seekers in mind. Irisa products include cannabis oil drops designed to naturally integrate with consumers’ self-care rituals. |
|
|
o |
Grail – A super-premium cannabis brand that offers discerning connoisseurs a collection of sought-after cultivars and top-shelf products. |
|
|
o |
Dubon – “the good stuff”, a vibrantly Québécois cannabis brand and champion of inspired, creative living. Dubon offers master-crafted cannabis cultivars as whole flower and pre-rolls, exclusively available in Québec. |
|
|
o |
Yukon Rove – A cannabis brand born “wild and free” with the unique spirit of Northern-Canada. Yukon Rove offers an assortment of local favorite cultivars from in whole flower and pre-rolls, exclusively in the Yukon territory. |
8
|
|
o |
The Batch - A no-frills cannabis value brand focused on delivering quality cannabis flower and pre-rolls at competitive prices. The Batch categorizes its product offering by potency rather than cultivar, allowing us to offer quality cannabis at prices that beat the illicit market. |
The brands launched in December 2019 as part of Phase 2 legalization that began in December 2019, include:
|
|
o |
Marley Natural - Crafted with deep respect for wellness and the positive potential of the herb. Marley Natural pure cannabis oil vape products are currently available nationwide in Canada. |
|
|
o |
Chowie Wowie - A new edibles brand bringing the ‘wow’ with perfectly crafted fusions of flavor offered in an array of reliably dosed cannabis-infused chocolates and gummies in THC and CBD varieties. Chowie Wowie cannabis infused milk-chocolates are currently available across Canada. |
|
|
o |
Everie - Fluent, High Park’s joint venture with Labatt Breweries of Canada, introduced Everie, their debut brand of non-alcoholic CBD-infused beverages, with 98% pure CBD isolate and all natural flavors. Everie has launched ready-to-brew teas nationwide in Canada and expect to roll-out further products in 2020. |
The brands expected to launch in 2020 as part of the Phase 2 legalization that began in December 2019 include:
|
|
o |
Goodship - Makers of damn fine edibles, create the industry’s most delectable cannabis-infused baked goods, chocolates and confections. |
|
|
o |
Rmdy. - A CBD-rich wellness brand formulated for wellness seekers will roll-out a variety of edibles and non-combustible cannabis products including mints, melts and all-in-one pens. |
High Park Company launched a physical and online retail presence in October 2018 with product available for sale in British Columbia, Yukon, North West Territories, Saskatchewan, Ontario, Quebec and Prince Edward Island. In March 2019, High Park Company has expanded its presence to include retail access in Alberta and Manitoba. In June 2019, High Park launched retail availability in Nova Scotia and New Brunswick. As a result of this provincial roll out plan High Park Company products are available in 11 of 13 provinces and territories across Canada and will continue to expand its brand and product offering.
Retail stores in Canada fall under two key banners:
|
|
1) |
Government-operated retail with highly regulated trade practices in British Columbia (hybrid), Quebec, New Brunswick, Nova Scotia, Prince Edward Island, Yukon, North West Territories. |
|
|
2) |
Privately-operated retail in British Columbia (hybrid), Alberta, Saskatchewan, Manitoba, Ontario and Newfoundland. |
Supporting the national coverage of retail in Canada, High Park Company has deployed a sales organization with the purpose of driving awareness, trial and sell-through across all government and privately-operated accounts.
Our Operations
We are building a multinational supply chain and distribution network to capitalize on the global medical cannabis market and the adult-use market in Canada.
|
• |
Tilray Seattle Regional Office – Seattle, Washington. Members of our senior leadership team are based in Seattle, along with our finance, legal, and information systems staff. |
9
|
• |
Tilray North America Campus – Nanaimo, British Columbia. We believe that Tilray Nanaimo is one of the world’s most sophisticated, technologically advanced licensed cannabis production facilities based on the amount of capital we have invested, the amount of data we have generated about how to grow cannabis well and the standard operating procedures we have created to ensure maximum yield and product quality. Tilray Nanaimo is a 60,000-square foot facility. It houses approximately 40,000 plants in 33 cultivation rooms, five manufacturing and processing rooms and three laboratories, including an advanced extraction laboratory, all of which allow us to produce more than 50 distinct cannabis strains and various cannabis extract products. The primary purpose of Tilray Nanaimo is to continue to serve the Canadian medical market and the global medical export market for the near term. Tilray Nanaimo is licensed by Health Canada and is GMP-certified by multiple EU recognized health regulators, or Competent Authorities. It also features a patient and physician service center that is open 24 hours a day, seven days a week and staffed with support personnel who speak multiple languages, delivering what we believe to be the best customer service in the industry. At this facility we complete each step of the production process including housing mother stock, cutting clones, cultivating pre-vegetative, vegetative and flowering plants; harvesting and curing plants; securing product in the vault; trimming product; extracting cannabinoids from harvested products; analyzing products in our lab; and packaging and shipping. |
|
• |
Tilray & High Park Toronto Regional Office – Toronto, Ontario. Members of our senior leadership team are based in Toronto, along with our finance, legal, sales and marketing staff. |
|
• |
Tilray European Union Regional Office – Berlin, Germany. Our executive, finance, sales, marketing, operations and regulatory support staff for the EU are located in Berlin, Germany. |
|
• |
Tilray Australia and New Zealand Regional Office – Sydney, Australia. Our sales, marketing and operations team focused on Australia and New Zealand are based in Sydney. We have signed two government contracts with the largest states in Australia: New South Wales and Victoria to supply medical cannabis to children suffering from pediatric epilepsy. Our products are available in three major hospitals in Victoria, as well as other hospitals and pharmacies throughout Australia and New Zealand. |
|
• |
Tilray European Union Campus – Cantanhede, Portugal. In July 2017, the Portuguese National Authority of Medicines and Health Products (INFARMED) awarded Tilray a license to cultivate, import and export bulk medical cannabis. The 2.7 million square-foot campus includes an outdoor cultivation plot which was first harvested in the fall of 2018, a greenhouse with a first harvest completed in February 2019, and GMP-certified manufacturing facility. Tilray Portugal will serve as our primary supply source for patients in the EU that have access to cannabis-derived products. To date we have supplied Germany and Israel from our EU campus. Locating cultivation and manufacturing operations in the EU results in easier and more cost-effective production and distribution. Although each EU member state has its own health and drugs regulatory body, these entities have ongoing cooperation mechanisms that promote similar, though not equal, treatment for medical cannabis, which we believe will facilitate cannabis product sales from Portugal into other European countries. |
10
|
• |
High Park Farms – Enniskillen, Ontario. We have repurposed over 626,000 square feet of existing non-cannabis greenhouses on a 100-acre site in Enniskillen, to serve as High Park Farms. We entered into a three-year lease agreement in October 2017 with an option to extend for three years. We also have a purchase option on the property, which is exercisable at any time during the term of the lease, including the renewal term. The renovation of the greenhouse for flower production and construction of the 40,000-square foot processing facility was completed and licensed by Health Canada on April 15, 2018. The facility currently cultivates and processes products for the Canadian adult-use market. |
|
• |
High Park Processing Facility – London, Ontario. We entered a 10-year lease in February 2018 for a 56,000-square foot processing facility in London. We have exercised the option to purchase the property in December 2022. This facility handles all post-harvest processing from cannabis harvested at the High Park Farms and High Park Gardens. The High Park Processing Facility received a processing license in January 2019 and sales license in April 2019 from Health Canada. We are capable of producing a range of products at this facility including edibles, beverages, capsules, vaporizer oils, vape pens, tinctures, sprays, topicals, pre-rolls and dried flower products. In November 2019, we entered a 10-year lease for a 78,000 square-foot warehousing and processing facility in London with two 5-year renewal options and options to purchase. We anticipate licensing and operating the facility in the third quarter of 2020. |
|
• |
High Park Gardens – Leamington, Ontario. In February 2019, we acquired a 662,000 square-foot greenhouse cultivation facility, of which 270,000 square-feet are currently licensed by Health Canada and being utilized as operational cultivation space. |
|
• |
Manitoba Harvest Processing – Ste. Agathe, Manitoba. In February 2019, we acquired Manitoba Harvest which owns and operates a 35,000 square-foot hemp seed processing facility. |
|
• |
Manitoba Harvest Packaging – Winnipeg, Manitoba. In February 2019, we acquired Manitoba Harvest which leases and operates a 15,000 square-foot hemp seed packaging facility. |
|
• |
Manitoba Harvest Corporate Offices –Minneapolis, Minnesota. Our sales, marketing and senior leadership team focused on United States and Canada hemp foods and CBD distribution through major health and wellness retailers. |
|
• |
Smith & Sinclair Corporate Offices –London, U.K. and New York, USA. Smith & Sinclair, which develops and distributes alcohol-infused confections and edibles, operates its global business from London. Smith & Sinclair’s new subsidiary, Pollen, which creates and distributes high quality CBD products operates primarily outside of the United States. |
Total Global Production and Processing Capacity
Our total production area is 3.6 million square feet as of January 2020. We believe that the maximum potential development of the parcels we currently own or lease would be 8.1 million square feet.
Sales and Distribution
Pharmaceutical distribution and pharmacy supply agreements. We work with established pharmaceutical distributors and pharmacy suppliers to sell our products around the world.
|
• |
In Canada, we have entered into a definitive agreement to supply Shoppers Drug Mart, the largest pharmacy chain in Canada, with our cannabis products. Shoppers Drug Mart is currently distributing our products under its license to sell cannabis products for medical purposes. We believe we are one of four Licensed Producers who have entered into supply agreements with Shoppers Drug Mart. Additionally, we have signed a collaboration agreement with Sandoz Canada, a division of Novartis, to market our non-combustible products to health care practitioners and pharmacists and to co-develop new cannabis products. |
|
• |
In Germany, our products are distributed via multiple wholesalers, including Noweda, a cooperative comprised of approximately 9,000 pharmacists with a network of 16,000 pharmacies throughout Germany and one of the largest wholesalers of pharmaceutical products in Germany, to fulfill prescriptions of our medical cannabis products across Germany. |
11
|
• |
Elsewhere around the world, we have formed partnerships with distributors in multiple countries. Our medical cannabis products are currently available in fifteen countries, including Argentina, Australia, Chile, Croatia, Cyprus, the Czech Republic, Israel, New Zealand, South Africa and the United Kingdom. We have also entered into a global framework agreement with Sandoz AG, pursuant to which we have the option to work with Sandoz AG to develop and commercialize non-smokable and non-combustible medical cannabis products internationally. |
Adult-use supply agreements. High Park Company launched a physical and online retail presence in October 2018 with product available for sale in British Columbia, Yukon, North West Territories, Saskatchewan, Ontario, Quebec and Prince Edward Island. In March of 2019, High Park Company expanded its presence to include retail access in Alberta and Manitoba. High Park products are available in 11 of 13 provinces and territories across Canada and will continue to expand its brand and product offering.
Direct-to-patient (“DTP”). In Canada, medical cannabis patients order from us primarily through our e-commerce platform or over the phone. In Canada, medical cannabis is and will continue to be delivered by secured courier or other methods permitted by the Cannabis Regulations. The DTP channel accounts for the majority of our medical sales.
Wholesale. In Canada, we are also authorized under the Cannabis Regulations to wholesale bulk and finished cannabis products to other licensees under the Cannabis Regulations (“Licensed Producers”). The bulk wholesale sales and distribution channel requires minimal selling, general, administrative and fulfillment costs. We intend to pursue these wholesale sales channels as a part of our adult-use and medical-use growth strategies in Canada.
Our Commitment to Research and Innovation
We believe that our strength as a medical brand is rooted in our commitment to research and development. Our research and development program focuses on developing innovative products, including novel delivery systems and precisely formulated cannabinoid products, and on the creation and improvement of methods, processes and technologies that allow us to efficiently manufacture such products on a large scale.
Patents and proprietary programs. Our commitment to innovation is a core tenet. We have at least fifty filed patents in the fields of cannabis processing technology, formulation, composition delivery system, and treatment methods. We have developed a number of innovative and proprietary programs designed to improve efficiency and overall product quality, including: a micro-propagation program that allows for the mass production of disease-free cannabis plants; methods and formulations to improve cannabinoid bioavailability and stability; a delivery platform to allow for the quick and efficient delivery of cannabinoids in formulation; the fast preservation methods that allow for improved smell, texture and flavor of cannabis products; an integrated pest management system; proprietary plant trimming machines to minimize manufacturing waste and software improvements to optimize manufacturing, inventory and distribution processes.
Trademarks and trade dress. We invest heavily in our growing trademark portfolio and hold at least 70 approved or registered trademarks in a variety of countries, including Canada, the United States, the EU, Australia, Israel and several countries in South America and Asia. We also have at least 110 additional trademarks filed and pending in several countries throughout the world. In addition, as a result of our brand licensing agreement with a former Privateer Holdings subsidiary, we have exclusive access in Canada to a number of strong marks, both registered and applied-for, including Marley Natural and Goodship.
12
Observational research program. We have implemented an extensive observational research program which includes large-scale prospective and cross-sectional studies in order to gather pre-clinical evidence on medical cannabis patient patterns of use, and the impact of that use on sleep, pain, mental health, quality of life, and the use of opioids/prescription drugs, alcohol, tobacco and other substances. These studies include a biennial national Canadian Cannabis Patient Survey (“CCPS”), the Tilray Observational Patient Study (“TOPS”), and the Medical Cannabis in Older Patients Study (“MCOPS”). This research takes place in partnership with Canadian and United States academic institutions, and has provided insight into the use of cannabis in the treatment of headaches/migraines, anxiety, and problematic substance use, and has led to a number of publications in high ranking academic journals, including the following:
|
• |
Lucas, P., & Walsh, Z. (2017). Medical cannabis access, use, and substitution for prescription opioids and other substances: A survey of authorized medical cannabis patients. International Journal of Drug Policy, 42, 30–35. |
|
• |
Baron, E. P., Lucas, P., Eades, J., & Hogue, O. (2018). Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. The Journal of Headache and Pain, 19(1), 37. |
|
• |
Lucas, P., Baron, E. P., & Jikomes, N. (2019). Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduction Journal, 16(1), 9. |
|
• |
Turna, J., Simpson, W., Patterson, B., Lucas, P., & Van Ameringen, M. (2019). Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. Journal of Psychiatric Research, 111, 134–139. |
Clinical trials. Participation in clinical trials is a differentiating element of our research and development program. We believe that the development of clinical data on the use of well-characterized and properly defined cannabinoid products will increase mainstream acceptance within the medical community. As such, we have developed techniques that achieve pharmaceutical-grade Active Pharmaceutical Ingredients (“APIs”) extracted from the cannabis plant to allow Tilray to partner with select academic research partners on trials that meet regulatory agency standards. Our participation in clinical studies includes R&D on the investigational study drug to generate the Chemistry and Manufacturing Controls (“CMC”) documentation required by regulatory agencies, collation of the CMC sections our investigational study drugs, as well as providing assistance in designing the protocol and determining the formulation of the study drug. In some cases, we provide funding for the study itself and/or pharmacokinetic data on the specific study drug. Although some trials, such as the chemotherapy-induced nausea and vomiting, or CINV, trial described below, are undertaken with an aim toward market authorization, most of the trials we participate in serve to generate early phase data that can be used to support patent filings, basic prescribing data for physicians, and signals of efficacy to narrow our focus for future clinical trials. We leverage our research by educating physicians about the unique benefits of cannabis-based medicines in various treatments, which we believe promotes the Tilray brand as the most trusted medical brand in the industry. Our Medical Advisory Board, consisting of experts in a variety of areas, participates in the clinical trial selection process and provides us with additional credibility as a clinical trial participant.
Clinical trials are typically conducted in phases, with Phase I establishing the safety and pharmacokinetics of the investigational study drug, Phase II further providing a signal for the drug’s efficacy and Phase III establishing statistical significance for the treatment of the disease or symptom being studied over the placebo. Below is a list of the clinical trials in which we are currently involved.
13
Clinical Trials
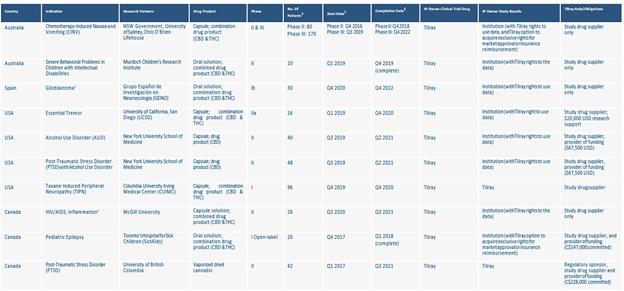
1 See the section titled “Risk Factors”
2 Regulatory approval pending
Regulatory Environment
Canadian Medical and Adult-Use
Medical and adult-use cannabis in Canada is regulated under the Cannabis Regulations (“CR”), promulgated under the Cannabis Act. Both the CR and the Cannabis Act were adopted in October 2018, superseding earlier regulations that permitted commercial distribution and home cultivation of medical cannabis. Health Canada, a federal government entity, is the oversight and regulatory body for cannabis licenses in Canada. The following are the highlights of the legislation:
|
• |
allows individuals over the age of 18 to purchase, possess and cultivate limited amounts of cannabis for adult-use purposes; each province is also being permitted to adopt its own laws governing the distribution, sale and consumption of cannabis and cannabis accessory products within the province, and those laws may set lower maximum permitted quantities for individuals and higher age requirements; |
|
• |
promotion, packaging and labelling of cannabis is strictly regulated. For example, promotion is largely restricted to the place of sale and legally age-gated environments, and promotions that appeal to underage individuals are prohibited; |
|
• |
currently, limited classes of cannabis, including dried cannabis and oils, are permitted for sale into the medical and adult-use markets. |
|
• |
other non-combustible form-factors, including edibles, topicals, and extracts (both ingested and inhaled), are permitted in the adult-use and medical market as of December 17, 2019; |
|
• |
export is restricted to medical cannabis, cannabis for scientific purposes and industrial hemp; and |
|
• |
sale of medical cannabis occurs largely on a direct-to-patient basis, while sale of adult-use cannabis occurs through retail-distribution models established by provincial and territorial governments. |
The retail-distribution models for adult-use cannabis vary nationwide:
|
• |
Quebec, New Brunswick, Nova Scotia and Prince Edward Island have adopted a government-run model for retail and distribution; |
14
|
• |
Ontario, British Columbia, Alberta, Manitoba and Newfoundland have adopted a hybrid model with some aspects, including distribution and online retail being government-run while allowing for private retail; |
|
• |
Saskatchewan has announced a fully private system; |
|
• |
the three northern territories of Yukon, Northwest Territories and Nunavut have adopted a model that mirrors their government-run liquor distribution model. |
All provinces and territories have secured supply agreements from Licensed Producers for their respective markets, and we are fulfilling adult-use supply agreements and purchase orders from various jurisdictions, consisting of: Quebec, Ontario, British Columbia, Prince Edward Island, Saskatchewan, Manitoba, Alberta, Nova Scotia, New Brunswick, Northwest Territories, and the Yukon.
United States Regulation of Hemp
Hemp products are subject to state and federal regulation in respect of the production, distribution and sale of products intended for human ingestion or topical application. Hemp is categorized as Cannabis sativa L., a subspecies of the cannabis genus. Numerous unique, chemical compounds are extractable from Hemp, including THC and CBD. These cannabinoids are responsible for a range of potential psychological and physiological effects. Hemp, as defined in the 2018 Farm Bill, is distinguishable from marijuana, which also comes from the Cannabis sativa L. subspecies, by its absence of more than trace amounts (0.3% or less) of the psychoactive compound THC. Although international standards vary, other countries, such as Canada, have used the same THC potency standards to define Hemp.
The 2018 Farm Bill preserves the authority and jurisdiction of the FDA, under the FD&C Act, to regulate the manufacture, marketing, and sale of food, drugs, dietary supplements, and cosmetics, including products that contain Hemp extracts and derivatives, such as CBD. As a result, the FD&C Act will continue to apply to Hemp-derived food, drugs, dietary supplements, cosmetics, and devices introduced, or prepared for introduction, into interstate commerce. As a producer and marketer of Hemp-derived products, the Company must comply with the FDA regulations applicable to manufacturing and marketing of certain products, including food, dietary supplements, and cosmetics.
As a result of the 2018 Farm Bill, federal law now provides that CBD derived from Hemp is not a controlled substance; however, CBD derived from Hemp could still be considered a controlled substance under applicable state law. States take varying approaches to regulating the production and sale of Hemp and Hemp-derived CBD. While some states explicitly authorize and regulate the production and sale of Hemp-derived CBD or otherwise provide legal protection for authorized individuals to engage in commercial Hemp activities, other states maintain drug laws that do not distinguish between marijuana and Hemp and/or Hemp-derived CBD, resulting in Hemp being classified as a controlled substance under certain state laws.
European Union Medical Use
While each country in the EU has its own laws and regulations, there are many commonalities in how the medical cannabis markets for EU countries are developing. For example, to ensure quality and safe products for patients, many EU countries only permit the import and sale of medical cannabis when the manufacturer can demonstrate certification by a Competent Authority of compliance with GMP standards.
The EU requires adherence to GMP standards for the manufacture of active substances and medicinal products, including cannabis products. Under the system for certification of GMP adopted in the EU, a Competent Authority of any EU member state may conduct an inspection at a drug manufacturing site and, if the GMP standards are met, a certificate of GMP compliance is issued to the manufacturer for specific elements of the manufacturing process being carried on at that site.
Each country in the EU will generally recognize a GMP certificate issued by any Competent Authority within the EU as evidence of compliance with GMP standards. Certificates of GMP compliance issued by a Competent Authority in another country outside of the EU will also be recognized if that country has a mutual recognition agreement with the EU.
15
Competitive Conditions
As of February 2020, more than 308 licenses were issued by Health Canada. Health Canada licenses are limited to individual properties. As such, if a Licensed Producer seeks to commence production at a new site, it must apply to Health Canada for a new license. As of January 2020, the current points of distributions are also limited, with only roughly 735 stores open across Canada. As the demand for legal cannabis increases and retail distribution points increase, we believe that new competitors will enter the market. The principal competitive factors on which we compete with other Licensed Producers are the quality, consistency and variety of cannabis products, brand recognition and physician familiarity.
In addition, we expect more countries will pass regulation allowing for medical cannabis use. We expect this to translate to increased competition internationally.
Employees
As of December 31, 2019, we employed 1,646 total employees, located in Canada, Germany, Portugal, Ireland the United States, Australia and Czech Republic, including 1,068 employees in research, product development, engineering and operations and logistics, 298 employees in general and administrative and 248 employees in sales and marketing. We consider relations with our employees to be good and have never experienced work stoppage. Apart from certain employees in Portugal, none of our employees are represented by a labor union or subject to a collective bargaining agreement. In Portugal, some of our employees are subject to a government-mandated collective bargaining agreement, which grants affected employees certain additional benefits beyond those required by the local labor code.
Our Company
Tilray, Inc. was incorporated in Delaware in January 2018. Prior to January 2018, we operated our business under Decatur Holdings, BV, a Dutch private limited liability company (“Decatur”), which was formed in March 2016. Decatur was incorporated under the laws of the Netherlands on March 8, 2016 as a wholly owned subsidiary of Privateer Holdings, Inc. to hold a 100% ownership interest in our direct and indirect subsidiaries through which we operated our business. Privateer Holdings, Inc. transferred 100% of its equity interest in Decatur to Tilray, Inc. on January 25, 2018 and Decatur was dissolved on December 27, 2018.
Website Access
Our website address is www.tilray.com. We make available, free of charge on our website, our Annual Report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to these reports as soon as reasonably practicable after filing such reports with, or furnishing them to, the Securities and Exchange Commission (“SEC”). Such reports are also available at www.sec.gov. Information contained on our website is not incorporated by reference in, or otherwise part of, this Annual Report on Form 10-K or any of our other filings with the SEC.
16
Item 1A. Risk Factors.
Careful consideration should be given to the following risk factors, in addition to the other information set forth in this Annual Report on Form 10-K and in other documents that we file with the SEC or publicly in Canada, in evaluating our company and our business. Investing in our securities involves a high degree of risk. If any of the following risks actually occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. Additional risks and uncertainties not currently known to us or that we currently consider to not be material may also materially and adversely affect our company and our business.
Risks Related to Medical Cannabis Business
We are dependent upon regulatory approvals and licenses for our ability to grow, process, package, store, sell and export medical cannabis and other products derived therefrom, and these regulatory approvals are subject to ongoing compliance requirements, reporting obligations and fixed terms requiring renewal.
Our ability to grow, process, package, store and sell dried cannabis, cannabis oil and capsules, and other classes of cannabis, including both oil and capsules, for medical purposes in Canada is dependent on our current Health Canada licenses under the Cannabis Regulations, or “CR”, covering our production facility and patient call center at our Tilray North America Campus in Nanaimo, British Columbia, or Tilray Nanaimo. These licenses allow us to produce cannabis in bulk and finished forms at Tilray Nanaimo and to sell and distribute such cannabis in Canada. They also allow us to import and export medical cannabis in bulk and finished form to and from specified jurisdictions around the world, subject to obtaining, for each specific shipment, an export approval from Health Canada and an import approval (or no objection notice) from the applicable regulatory authority in the country to or from which the export or import is being made. The CR licenses for Tilray Nanaimo are valid for fixed periods and will need to be renewed at the end of such periods.
We also hold licenses under the CR covering our facilities in Enniskillen, London, and Leamington, Ontario which we use to service the adult-use market and support the medical market as needed. These licenses allow us to produce, sell, and distribute cannabis and/or cannabis products in Canada. These licenses are valid for fixed periods and will need to be renewed at the end of such periods.
Our ability to operate in our facility at our Tilray European Union Campus located in Cantanhede, Portugal, or Tilray Portugal, is dependent on our current authorization for the cultivation, import and export of cannabis and our Good Manufacturing Practices, or GMP, certification by the Portuguese National Authority of Medicines and Health Products, or INFARMED, for manufacture of cannabis as an active pharmaceutical ingredient, and is dependent on our current authorization for the manufacture of finished cannabis products and GMP certification for manufacture of cannabis as a finished medicinal product. Our current authorization for cultivation, import and export of cannabis is valid for a single growing season at a time and notification to INFARMED is needed to renew the license for subsequent growing seasons. All licenses are subject to ongoing compliance and reporting requirements and renewal.
We intend to apply for a sale license for cannabis products under the CR for our facility in Leamington, Ontario. Any future medical cannabis production facilities that we operate in Canada will also be subject to separate licensing requirements under the CR. Although we believe that we will meet the requirements of the CR for future renewals of our existing licenses, and grants of permits under such licenses, and to obtain corresponding licenses for future facilities in Canada, there can be no assurance that existing licenses will be renewed or new licenses obtained on the same or similar terms as our existing licenses, nor can there be any assurance that Health Canada will continue to issue import or export permits on the same terms or on the same timeline, or that other countries will allow, or continue to allow, imports or exports.
Further, we are subject to ongoing inspections by Health Canada and INFARMED to monitor our compliance with their licensing requirements. Our existing licenses and any new licenses that we may obtain in the future in Canada or other jurisdictions may be revoked or restricted at any time in the event that we are found not to be in compliance. Should we fail to comply with the applicable regulatory requirements or with conditions set out under our licenses, should our licenses not be renewed when required, be renewed on different terms, or be revoked, we may not be able to continue producing or distributing medical cannabis in Canada or other jurisdictions or to export medical cannabis outside of Canada or Portugal. In addition, we may be subject to enforcement proceedings resulting from a failure to comply with applicable regulatory requirements in Canada or other jurisdictions, which could result in damage awards, a suspension of our existing approvals, a withdrawal of our existing approvals, the
17
denial of the renewal of our existing approvals or any future approvals, recalls of products, product seizures, the imposition of future operating restrictions on our business or operations or the imposition of civil, regulatory or criminal fines or penalties against us, our officers and directors and other parties. These enforcement actions could delay or entirely prevent us from continuing the production, testing, marketing, sale or distribution of our medical products and divert management’s attention and resources away from our business operations.
The laws, regulations and guidelines generally applicable to the medical cannabis industry in Canada and other countries may change in ways that impact our ability to continue our business as currently conducted or proposed to be conducted.
The successful execution of our medical cannabis business objectives is contingent upon compliance with all applicable laws and regulatory requirements in Canada and other jurisdictions, including the requirements of the CR in Canada, and obtaining all other required regulatory approvals for the sale, import and export of our medical cannabis products. The commercial medical cannabis industry is a relatively new industry in Canada and the CR is a regime that has only been in effect in its current form since October 2018. The effect of Health Canada’s administration, application and enforcement of the regime established by the CR on us and our business in Canada, or the administration, application and enforcement of the laws of other countries by the appropriate regulators in those countries, may significantly delay or impact our ability to participate in the Canadian medical cannabis market or medical cannabis markets outside Canada, to develop medical cannabis products and produce and sell these medical cannabis products.
Further, Health Canada or the regulatory authorities in other countries in which we operate or to which we export our medical cannabis products may change their administration, interpretation or application of the applicable regulations or their compliance or enforcement procedures at any time. Any such changes could require us to revise our ongoing compliance procedures, requiring us to incur increased compliance costs and expend additional resources. There is no assurance that we will be able to comply or continue to comply with applicable regulations.
Any failure on our part to comply with applicable regulations could prevent us from being able to carry on our business.
Health Canada inspectors routinely assess Tilray Nanaimo, High Park Farms, High Park Processing Facility, and High Park Gardens for compliance with applicable regulatory requirements. Our Tilray Portugal facilities have also been inspected for compliance by applicable regulators following completion of the construction and will be subject to certain ongoing inspections and audits once licensing is complete. Furthermore, the import of our products into other jurisdictions, such as Germany, Israel and Australia, is subject to the regulatory requirements of the respective jurisdiction. Any failure by us to comply with the applicable regulatory requirements could require extensive changes to our operations; result in regulatory or agency proceedings or investigations, increased compliance costs, damage awards, civil or criminal fines or penalties or restrictions on our operations; and harm our reputation or give rise to material liabilities or a revocation of our licenses and other permits. There can be no assurance that any pending or future regulatory or agency proceedings, investigations or audits will not result in substantial costs, a diversion of management’s attention and resources or other adverse consequences to us and our business.
Our ability to produce and sell our medical products in, and export our medical products to, other jurisdictions outside of Canada is dependent on compliance with additional regulatory and other requirements.
We are required to obtain and maintain certain permits, licenses or other approvals from regulatory agencies in countries and markets outside of Canada in which we operate, or to which we export, to produce or export to, and sell our medical products in, these countries, including, in the case of certain countries, the ability to demonstrate compliance with GMP standards. Our current certification of compliance with GMP standards for production at Tilray Nanaimo and any other GMP certification that we may receive in the future subject us, or will in the future subject us, to extensive ongoing compliance reviews to ensure that we continue to maintain compliance with GMP standards. There can be no assurance that we will be able to continue to comply with these standards.
18
The continuation or expansion of our international operations depends on our ability to renew or secure necessary permits, licenses and other approvals. An agency’s denial of or delay in issuing or renewing a permit, license or other approval, or revocation or substantial modification of an existing permit, license or approval, could prevent us from continuing our operations in, marketing efforts in, or exporting to countries other than Canada. For example, Tilray Nanaimo’s current certification of GMP compliance must be renewed via re-inspection prior to October 2020, and our failure to maintain such certification, or to comply with applicable industry quality assurance standards or receive similar regulatory certifications at any of our other facilities, may prevent us from continuing the expansion of our international operations. In addition, the export and import of medical cannabis is subject to United Nations treaties establishing country-by-country national estimates and our export and import permits are subject to these estimates which could limit the amount of medical cannabis we can export to any particular country.
The long-term effect of the legalization of adult-use cannabis in Canada on the medical cannabis industry is unknown (including recently amended Canadian cannabis regulations, or Cannabis 2.0), and may have a significant negative effect upon our medical cannabis business if our existing or future medical use customers decide to purchase products available in the adult-use market instead of purchasing medical use products from us.
In June 2018, the government of Canada passed Bill C-45, or the Cannabis Act, the Canadian federal legislation allowing individuals over the age of 18 to legally purchase, process and cultivate limited amounts of cannabis for adult use in Canada. The Cannabis Act and accompanying regulations, the CR, became effective on October 17, 2018. On October 17, 2019, the CR was further amended to permit the sale of new classes of cannabis through both adult-use and medical channels, which classes became available starting December 16, 2019. Individuals who previously relied upon the medical cannabis market to supply their medical cannabis and cannabis-based products may cease this reliance, and instead turn to the adult-use cannabis market to supply their cannabis and cannabis-based products. Factors that may influence this decision include the availability of product in each market, the price of medical cannabis products in relation to similar adult-use cannabis products, and the ease with which each market can be accessed in the individual provinces and territories of Canada. The impact of adult-use cannabis on the medical market is not yet fully understood as the market is still in a state of flux. In addition, new form factors have just been legalized and the degree to which these products will be made available on the medical market versus adult use is not yet known.
A decrease in the overall size of the medical cannabis market as a result of the legal adult-use market in Canada may reduce our medical sales and revenue prospects in Canada. Moreover, the CR regulation of cannabis for medical purposes is expected to be reviewed in light of the adult-use market. The effect on our business, and the medical cannabis market in general, of such a review is uncertain.
There has been limited study on the effects of medical cannabis and future clinical research studies may lead to conclusions that dispute or conflict with our understanding and belief regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis.
Research regarding the medical benefits, viability, safety, efficacy and dosing of cannabis or isolated cannabinoids (such as CBD and THC) remains in relatively early stages. There have been few clinical trials on the benefits of cannabis or isolated cannabinoids conducted by us or by others.
Future research and clinical trials may draw opposing conclusions to statements contained in the articles, reports and studies we have relied on or could reach different or negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing or other facts and perceptions related to medical cannabis, which could adversely affect social acceptance of cannabis and the demand for our products.
Tilray Nanaimo, Manitoba Harvest, High Park Farms, High Park Gardens, High Park Processing Facility and Tilray Portugal are integral to our business and adverse changes or developments affecting any of these facilities may have an adverse impact on us.
Currently, our activities and resources are primarily focused on the operation of Tilray Nanaimo, Manitoba Harvest, High Park Farms, High Park Gardens, Tilray Portugal and our current licenses under the CR are specific to Tilray Nanaimo, High Park Farms, High Park Gardens and our High Park Processing Facility. Adverse changes or developments affecting these facilities, including, but not limited to, disease or infestation of our crops, a fire, an explosion, a power failure, a natural disaster or a material failure of our security infrastructure, could reduce or
19
require us to entirely suspend our production of cannabis. A significant failure of our site security measures and other facility requirements, including any failure to comply with regulatory requirements under the CR, could have an impact on our ability to continue operating under our Health Canada licenses and our prospects of renewing our Health Canada licenses, and could also result in a suspension or revocation of these Health Canada licenses. As we produce much of our medical cannabis products in Tilray Nanaimo, any event impacting our ability to continue production at Tilray Nanaimo, or requiring us to delay production, would prevent us from continuing to operate our business until operations at Tilray Nanaimo could be resumed, or until we were able to commence production at another facility.
We expect to expand Tilray Nanaimo, High Park Farms, our High Park Processing Facility, and our Tilray Portugal facilities. We are also contemplating expanding our High Park Gardens facility. We expect that expanded and additional facilities will significantly increase our cultivation, growing, processing and distribution capacity; however, development impediments such as construction delays or cost over-runs in respect to the development of these facilities, howsoever caused, could delay or prevent our ability to produce cannabis at these facilities. It is also possible that the final costs of the major equipment contemplated by our capital expenditure program relating to the development of our High Park Farms, our High Park Processing Facility and Tilray Portugal may be significantly greater than anticipated, in which circumstance we may be required to curtail, or extend the timeframes for completing, such capital expenditure plans which would reduce our production capacity.
If we are unsuccessful in scaling operations at our facilities, we may become increasingly reliant on third-party cannabis suppliers, likely at a higher price than our own cost to produce, which would have a negative impact on gross profit margins.
The medical cannabis industry and market are relatively new, and this industry and market may not continue to exist or develop as anticipated or we may ultimately be unable to succeed in this industry and market.
We are operating our current business in a relatively new medical cannabis industry and market, and our success depends on our ability to attract and retain patients. In addition to being subject to general business risks applicable to a business involving an agricultural product and a regulated consumer product, we need to continue to build brand awareness of our Tilray brand in the medical cannabis industry and make significant investments in our business strategy and production capacity. These investments include introducing new products into the markets in which we operate, adopting quality assurance protocols and procedures, building our international presence and undertaking regulatory compliance efforts. These activities may not promote our medical products as effectively as intended, or at all, and we expect that our competitors will undertake similar investments to compete with us for market share. Competitive conditions, consumer preferences, regulatory conditions, patient requirements, healthcare practitioner prescribing practices, and spending patterns in this industry and market are relatively unknown and may have unique characteristics that differ from other existing industries and markets and that cause our efforts to further our business to be unsuccessful or to have undesired consequences. As a result, we may not be successful in our efforts to attract and retain patients or to develop new medical cannabis products and produce and distribute these medical cannabis products to the markets in which we operate or to which we export in time to be effectively commercialized, or these activities may require significantly more resources than we currently anticipate in order to be successful.
We compete for market share with other companies, including other producers licensed by Health Canada, some of which have longer operating histories and more financial resources and manufacturing and marketing experience than we have.
We face, and we expect to continue to face, intense competition from Licensed Producers and other potential competitors, some of which have longer operating histories and more financial resources and manufacturing and marketing experience than we have. In addition, it is possible that the medical cannabis industry will undergo consolidation, creating larger companies with financial resources, manufacturing and marketing capabilities and product offerings that are greater than ours. As a result of this competition, we may be unable to maintain our operations or develop them as currently proposed, on terms we consider acceptable, or at all.
20
There are currently hundreds of applications for Licensed Producer status being processed by Health Canada. The number of licenses granted and the number of Licensed Producers ultimately authorized by Health Canada could have an adverse impact on our ability to compete for market share in Canada’s medical cannabis industry. We expect to face additional competition from new market entrants that are granted licenses under the CR or existing license holders that are not yet active in the industry. If a significant number of new licenses are granted by Health Canada, we may experience increased competition for market share and may experience downward price pressure on our medical cannabis products as new entrants increase production.
In addition, the CR permits patients in Canada to produce a limited amount of cannabis for their own medical purposes or to designate a person to produce a limited amount of cannabis on their behalf for such purposes. Widespread reliance upon this allowance could reduce the current or future consumer demand for our medical cannabis products.
If the number of users of cannabis for medical purposes in Canada increases, the demand for products will increase. This could result in the competition in the medical cannabis industry becoming more intense as current and future competitors begin to offer an increasing number of diversified medical cannabis products. Conversely, if there is a contraction in the medical market for cannabis in Canada, competition for market share may increase. To remain competitive, we intend to continue to invest in research and development and sales and patient support; however, we may not have sufficient resources to maintain research and development and sales and patient support efforts on a competitive basis.
In addition to the foregoing, the legal landscape for medical cannabis use is changing internationally. We have operations outside of Canada, which may be affected as other countries develop, adopt and change their medical cannabis laws. Increased international competition, including competition from suppliers in other countries who may be able to produce at lower cost, and limitations placed on us by Canadian or other regulations, might lower the demand for our medical cannabis products on a global scale.
The illicit supply of cannabis and cannabis-based products may reduce our sales and impede our ability to succeed in the medical and adult-use cannabis markets.
In addition to competition from Licensed Producers and those able to produce cannabis legally without a license, we also face competition from unlicensed and unregulated market participants, including illegal dispensaries and illicit market suppliers selling cannabis and cannabis-based products in Canada.
Despite the legalization of medical and adult-use cannabis in Canada, illicit market operations remain abundant and are a substantial competitor to our business. In addition, illegal dispensaries and illicit market participants may be able to (i) offer products with higher concentrations of active ingredients that are either expressly prohibited or impracticable to produce under current Canadian regulations, (ii) brand products more explicitly, and (iii) describe/discuss intended effects of products. As these illicit market participants do not comply with the regulations governing the medical and adult-use cannabis industry in Canada, their operations may also have significantly lower costs.
As a result of the competition presented by the illicit market for cannabis, any unwillingness by consumers currently utilizing these unlicensed distribution channels to begin purchasing from licensed retailers for any reason or any inability or unwillingness of law enforcement authorities to enforce laws prohibiting the unlicensed cultivation and sale of cannabis and cannabis-based products could (i) result in the perpetuation of the illicit market for cannabis, (ii) adversely affect our market share and (iii) adversely impact the public perception of cannabis use and licensed cannabis producers and dealers, all of which would have a materially adverse effect on our business, operations and financial condition.
Risks Related to Adult-Use Cannabis
The adult-use cannabis industry, and the regulations governing this industry (included recently amended Canadian regulations, or Cannabis 2.0), may develop in a way that is significantly different from our current expectations, resulting in our decreased ability, or inability, to compete in this market and industry.
There is no assurance that the adult-use cannabis industry, and the regulations governing this industry, will continue to develop as anticipated. There are and will be significant restrictions on the marketing, branding, product formats, product composition, packaging, and distribution channels allowed under the Cannabis Act, which may
21
reduce the value of certain of our products and brands or negatively impact our ability to compete with other companies in the adult-use cannabis market in Canada. For instance, adult-use legislation includes a requirement for health warnings on product packaging, the limited ability to use logos and branding (only one brand name and one brand element per package), restrictions on packaging itself, and restrictions on types and avenues of marketing; further, Cannabis 2.0 regulations (which came into force on October 17, 2019) govern the production and sale of new classes or forms of cannabis products (including vapes and, edibles,), and impose considerable restrictions on product composition, labeling, and packaging in addition to being subject to similar marketing restrictions as existing form factors. Additional marketing and product composition restrictions have been imposed by some provinces and territories and are subject to changing interpretation without notice. Provincial or other legislation containing additional restrictions, such as a complete ban on marketing, may impact our ability to do so. Such additional restrictions may impair our ability to develop our adult-use brands, and a complete ban on marketing or additional product restrictions imposed under future regulations, may make it uneconomic or unfeasible for us to introduce our entire portfolio of brands and products into the Canadian market, which means that we will be unable to reap the full benefit of the exclusive rights we have secured to such brands and products or launch new products. Further, each province and territory of Canada has the ability to separately regulate the distribution of cannabis within such province or territory, and the rules (including associated regulations) adopted by these provinces or territories vary significantly. Furthermore, some provinces and territories impose significant restrictions on our ability to merchandise products; for example, some provinces impose restrictions on investment in retailers or distributors and their employees as well as in our ability to negotiate for preferential retail space or in-store marketing. Such variance may make participation in the adult-use cannabis market uneconomic or of limited economic benefit for us in those provinces or territories and could result in significant additional compliance or other costs and limitations on our ability to compete successfully in each such market.
Cannabis 2.0 allows for new and untested Cannabis products and form factors, and we may ultimately be unsuccessful in developing and offering these new products in our Canadian markets.
Cannabis 2.0 regulations permit Licensed Producers to develop new cannabis form factors, including CBD- and THC-infused drinks, edibles and non-flower products. We have and will continue to develop strategic partnerships to participate in these new product market opportunities with partners who can provide complementary product development and support capabilities. Strategic initiatives around new products involve significant investment of management time and resources in order to successfully execute and maintain, for novel products that may not generate sufficient market demand. Additionally, there can be no guarantee that such new product offerings, even if successfully developed, will have unit economics that generate an appropriate return on investment. Cannabis 2.0 could result in diversions of management attention, a strain on existing financial and other resources or a lack of product demand for our newly developed form factors, any of which could have a material adverse effect on our business, results of operations and financial condition.
Any failure on our part to comply with supplier standards established by provincial or territorial distributors could prevent us from accessing certain markets in Canada.
Government-run provincial and territorial distributors in Canada require suppliers to meet certain service and business standards, and routinely assess for compliance with such standards. Any failure by us to comply with such standards could result in our being downgraded or disqualified as a supplier, and would severely impede or eliminate our ability to access certain markets within Canada.
The adult-use cannabis market in Canada is continuing to develop and may experience supply fluctuations resulting in revenue and price decreases.
As a result of the legalization of adult cannabis use in Canada, the demand for cannabis may dramatically increase. Licensed Producers, and others licensed to produce cannabis under the Cannabis Act, may not be able to produce enough cannabis to meet adult-use demand. This may result in lower than expected sales and revenues and may result in increased competition for sales and sources of supply. This competition may adversely affect our adult-use business and there is no guarantee that we will be able to supply or acquire the supply, on commercially reasonable terms or at all, to meet the demand for medical and adult-use cannabis.
22
In response to this surge in demand for cannabis, we and other cannabis producers in Canada may produce more cannabis than is needed to satisfy the collective demand of the Canadian medical and adult-use markets, and we may be unable to export that oversupply into other markets where cannabis use is fully legal under all federal and state or provincial laws. Additionally, the Canadian market may experience increased supply fluctuations as new form factors and products become available. As a result, the available supply of cannabis could exceed demand, resulting in a significant decline in the market price for cannabis. If this were to occur, there is no assurance that we would be able to generate sufficient revenue from the sale of adult-use cannabis to result in profitability.
In connection with the amended Canadian adult-use regulations which became effective October 17, 2019 and permitted new classes of cannabis on December 16, 2019, we will now offer cannabis-only vape products in Canada. The vape market is a niche market that remains subject to a great deal of uncertainty and is still evolving. Recent negative public sentiment and regulatory scrutiny of vaporizing in the United States may cause Health Canada to further limit usage and diminish Canadian consumer demand for our cannabis vape products.
Cannabis vape products in Canada are regulated under the Cannabis Act and the CR. Although this legislation sets clear rules and standards for the manufacture, composition, packaging, and marketing of cannabis vape products, these rules and standards predate the spate of vaping-related health issues that have recently arisen in the United States. These issues and accompanying negative public sentiment may prompt Health Canada or individual provinces/territories to further limit or defer industry’s ability to sell cannabis vape products, and may also diminish consumer demand for such products. There can be no assurance that we will be able to meet any additional compliance requirements or regulatory restrictions, or remain competitive in face of unexpected changes in market conditions.
Vaping, electronic cigarettes and related products were recently developed and therefore the scientific community has not had a sufficient period of time to study the long-term health effects of their use. Currently, there is no way of knowing whether these products are safe for their intended use and the medical community is still studying these products’ health effects. If the scientific community were to determine conclusively that use of any or all of these products poses long-term health risks, market demand for these products and their use could materially decline. Such a determination could also lead to litigation and significant regulation. Loss of demand for our product, product liability claims and increased regulation stemming from unfavorable scientific studies on cannabis vaping products could have a material adverse effect on our business, results of operations and financial condition.
The adult-use cannabis industry and market in Canada is subject to many of the same risks as the medical cannabis industry and market, including risks related to our need for regulatory approvals, the early status and uncertain growth of this industry and the competition we expect to face in this industry.
The adult-use cannabis industry and market in Canada is subject to certain risks that are unique to this industry, as well as the risks that are currently applicable to the medical cannabis industry, which are described under the heading above titled “Risk Factors-Risks Related to our Medical Cannabis Business and the Medical Cannabis Industry.”
If any of these shared risks occur, our business, financial condition, results of operations and prospects could be adversely affected in a number of ways, including by our not being able to successfully compete in the adult-use cannabis industry and by our being subject to fines, damage awards and other penalties as a result of regulatory infractions or other claims brought against us.
We may be unsuccessful in competing in the legal adult-use cannabis market in Canada.
Our Canadian adult-use business faces enhanced competition from other Licensed Producers and those individuals and corporations who are licensed under the Cannabis Act to participate in the adult-use cannabis industry.
As previously noted, there are hundreds of applications being processed for licenses under the CR. Moreover, the Cannabis Act allows individuals to cultivate, propagate, harvest and distribute up to four cannabis plants per household, provided that each plant meets certain requirements. If we are unable to effectively compete with other suppliers to the adult-use cannabis market, or a significant number of individuals take advantage of the ability to cultivate and use their own cannabis, our success in the adult-use business may be limited and may not fulfill the expectations of management.
23
We will also face competition from existing Licensed Producers and other producers licensed under the Cannabis Act. Certain of these competitors have significantly greater financial, production, marketing, research and development and technical and human resources than we do. As a result, our competitors may be more successful than us in gaining market penetration and market share. Our commercial opportunity in the adult-use market could be reduced or eliminated if our competitors produce and commercialize products for the adult-use market that, among other things, are safer, more effective, more convenient or less expensive than the products that we may produce, have greater sales, marketing and distribution support than our products, enjoy enhanced timing of market introduction and perceived effectiveness advantages over our products and receive more favorable publicity than our products. If our adult-use products do not achieve an adequate level of acceptance by the adult-use market, we may not generate sufficient revenue from these products, and our adult-use business may not become profitable.
There may be industry consolidation of one or more competitors, which could increase the competitive advantage of certain competitors and reduce overall market share opportunities. Additionally, Canadian provincial regulations are continuing to evolve, and individual provinces have imposed new regulations around expiry dates and age of consumption, thereby further reducing the size of our total addressable market. Increased consolidation and new and disparate provincial regulations could have a material effect on our business and results of operations.
General Business Risks and Risks Related to Our Financial Condition and Operations
We have a limited operating history and a history of net losses, and we may not achieve or maintain profitability in the future.
We began operating in 2014 and have yet to generate a profit. We generated net losses of $321.2 million, $67.7 million and $7.8 million for 2019, 2018 and 2017, respectively. Our accumulated deficit was $430.1 million as of December 31, 2019. We intend to continue to expend significant funds to increase our growing capacity, complete strategic mergers and acquisitions, invest in research and development, expand our marketing and sales operations to increase our base of registered patients and meet the compliance requirements as a public company.
Our efforts to grow our business may be more costly than we expect and we may not be able to increase our revenue enough to offset higher operating expenses. We may incur significant losses in the future for a number of reasons, including as a result of unforeseen expenses, difficulties, complications and delays, the other risks described in this Annual Report on Form 10-K and other unknown events. The amount of future net losses will depend, in part, on the growth of our future expenses and our ability to generate revenue. If we continue to incur losses in the future, the net losses and negative cash flows incurred to date, together with any such future losses, will have an adverse effect on our stockholders’ equity and working capital. Because of the numerous risks and uncertainties associated with producing cannabis products, as outlined herein, we are unable to accurately predict when, or if, we will be able to achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. If we are unable to achieve and sustain profitability, the market price of our Class 2 common stock may significantly decrease and our ability to raise capital, expand our business or continue our operations may be impaired.
We are exposed to risks relating to the laws of various countries as a result of our international operations.
We currently conduct operations in multiple countries and plan to expand these operations. As a result of our operations, we are exposed to various levels of political, economic, legal and other risks and uncertainties associated with operating in or exporting to these jurisdictions. These risks and uncertainties include, but are not limited to, changes in the laws, regulations and policies governing the production, sale and use of cannabis and cannabis-based products, political instability, instability at the United Nations level, currency controls, fluctuations in currency exchange rates and rates of inflation, labor unrest, changes in taxation laws, regulations and policies, restrictions on foreign exchange and repatriation and changing political conditions and governmental regulations relating to foreign investment and the cannabis business more generally.
Changes, if any, in the laws, regulations and policies relating to the advertising, production, sale and use of cannabis and cannabis-based products or in the general economic policies in these jurisdictions, or shifts in political attitude related thereto, may adversely affect the operations or profitability of our international operations in these countries. As we explore novel business models, such as global co-branded products, cannabinoid clinics and cannabis retail, international regulations will become increasingly challenging to manage. Specifically, our operations may be affected in varying degrees by government regulations with respect to, but not limited to,
24
restrictions on advertising, production, price controls, export controls, controls on currency remittance, increased income taxes, restrictions on foreign investment, land and water use restrictions and government policies rewarding contracts to local competitors or requiring domestic producers or vendors to purchase supplies from a particular jurisdiction. Failure to comply strictly with applicable laws, regulations and local practices could result in additional taxes, costs, civil or criminal fines or penalties or other expenses being levied on our international operations, as well as other potential adverse consequences such as the loss of necessary permits or governmental approvals.
Furthermore, although we have begun production at Tilray Portugal with a view toward facilitating exports of our cannabis products to countries in the EU (or, as permissible, elsewhere) from Portugal rather than from Canada, there is no assurance that these EU (or non-EU) countries will authorize the import of our cannabis products from Portugal, or that Portugal will authorize or continue to authorize such exports, or that such exports will provide us with advantages over our current EU export strategy. Each country in the EU (or elsewhere) may impose restrictions or limitations on imports that require the use of, or confer significant advantages upon, producers within that particular country. As a result, we may be required to establish production facilities similar to Tilray Portugal in one or more countries in the EU (or elsewhere) where we wish to distribute our cannabis products in order to take advantage of the favorable legislation offered to producers in these countries.
We plan to expand our business and operations into jurisdictions outside of the current jurisdictions where we conduct business, and there are risks associated with doing so.
We plan in the future to expand our operations and business into jurisdictions outside of the jurisdictions where we currently carry on business. There can be no assurance that any market for our products will develop in any such foreign jurisdiction. We may face new or unexpected risks or significantly increase our exposure to one or more existing risk factors, including economic instability, new competition, changes in laws and regulations, including the possibility that we could be in violation of these laws and regulations as a result of such changes, and the effects of competition. These factors may limit our capability to successfully expand our operations in, or export our products to, those other jurisdictions.
Our business is subject to a variety of United States and foreign laws, many of which are unsettled and still developing and which could subject us to claims or otherwise harm our business.
We are subject to a variety of state and federal laws in the United States, Canada and elsewhere. In the United States, despite cannabis having been legalized at the state level for medical use in many states and for adult-use in a number of states, cannabis meeting the statutory definition of “marihuana” continues to be categorized as a Schedule I controlled substance under the federal Controlled Substances Act, or the CSA, and subject to the Controlled Substances Import and Export Act, or the CSIEA. Hemp and marijuana both originate from the Cannabis sativa plant and CBD is a constituent of both. “Marihuana” or “marijuana” is defined in the CSA as a Schedule I controlled substance whereas “Hemp” is essentially any parts of the Cannabis sativa plant that has not been determined to be marijuana. Pursuant to the Agriculture Improvement Act of 2018, or the Farm Bill, “hemp,” or cannabis and cannabis derivatives containing no more than 0.3% of tetrahydrocannabinol (“THC”), is now excluded from the statutory definition of “marijuana” and, as such, is no longer a Schedule I controlled substance under the CSA. Our activity in the United States is limited to (a) certain corporate and administrative services, including accounting, legal and creative services, (b) supply of study drug for clinical trials under DEA and FDA authorization, and (c) participation in the market for hemp and hemp-derived products containing CBD in compliance with the Farm Bill; except as described above, we do not produce or distribute cannabis products in the United States. Therefore, we believe that we are not currently subject to the CSA or CSIEA.
We have commercialized in the United States a variety of hemp products, which might include certain cannabinoids including CBD, but would exclude THC at amounts more than 0.3%. While the Farm Bill exempted hemp and hemp derived products from the CSA, any such product commercialization will be subject to various laws, including the Farm Bill, the Federal Food, Drug and Cosmetic Act, or the FD&CA, the Dietary Supplement Health and Education Act, or DSHEA, applicable state and/or local laws, and FDA regulations. The FDA has stated in guidance and other public statements that it is prohibited to sell a food, beverage or dietary supplement to which THC or CBD has been added. While the FDA does not have a formal policy of enforcement discretion with respect to any products with added CBD, the agency has stated that its primary focus for enforcement centers on products that put the health and safety of consumers at risk, such as those claiming to prevent, diagnose, mitigate, treat, or cure diseases in the absence of requisite approvals. While the agency’s enforcement to date has therefore focused on
25
products containing CBD and that make drug-like claims, there is the risk that the FDA could expand its enforcement activities and require us to alter our marketing for our hemp-derived CBD products or cease distributing them altogether. Nevertheless, the regulation of hemp and CBD in the United States has been a constantly evolving and changing landscape, with changes in federal and state laws and regulation occurring on a frequent basis. Violations of applicable FDA and other laws could result in warning letters, significant fines, penalties, administrative sanctions, injunctions, convictions or settlements arising from civil proceedings.
We are further subject to a variety of laws and regulations in the United States, Canada and elsewhere that prohibit money laundering, including the Proceeds of Crime and Terrorist Financing Act (Canada) and the Money Laundering Control Act (United States), as amended, and the rules and regulations thereunder and any related or similar rules, regulations or guidelines issued, administered or enforced by governmental authorities in the United States, Canada or any other jurisdiction in which we have business operations or to which we export. Although we believe that none of our activities implicate any applicable money laundering statutes, in the event that any of our business activities, any dividends or distributions therefrom, or any profits or revenue accruing thereby are found to be in violation of money laundering statutes, such transactions may be viewed as proceeds of crime under one or more of the statutes described above or any other applicable legislation, and any persons, including such United States-based investors, found to be aiding and abetting us in such violations could be subject to liability. Any violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction and involve significant costs and expenses, including legal fees. We could also suffer severe penalties, including criminal and civil penalties, disgorgement and other remedial measures.
We are required to comply concurrently with federal, state or provincial, and local laws in each jurisdiction where we operate or to which we export our products.
Various federal, state or provincial and local laws govern our business in the jurisdictions in which we operate or propose to operate, or to which we export or propose to export our products, including laws and regulations relating to health and safety, conduct of operations and the production, management, transportation, storage and disposal of our products and of certain material used in our operations. Compliance with these laws and regulations requires concurrent compliance with complex federal, provincial or state and local laws. These laws change frequently and may be difficult to interpret and apply. Compliance with these laws and regulations requires the investment of significant financial and managerial resources, and a determination that we are not in compliance with these laws and regulations could harm our brand image and business. Moreover, it is impossible for us to predict the cost or effect of such laws, regulations or guidelines upon our future operations. Changes to these laws or regulations could negatively affect our competitive position within our industry and the markets in which we operate, and there is no assurance that various levels of government in the jurisdictions in which we operate will not pass legislation or regulation that adversely impacts our business.
United States regulations relating to hemp-derived CBD products are unclear and rapidly evolving.
Our participation in the market for hemp-derived CBD products in the United States and elsewhere may require us to employ novel approaches to existing regulatory pathways. Although the passage of the Farm Bill in December 2018 legalized the cultivation of hemp in the United States to produce products containing CBD and other non-THC cannabinoids, it remains unclear how the FDA will regulate this industry, and whether and when the FDA will propose or implement new or additional regulations. On May 31, 2019, the FDA held a public hearing to obtain scientific data and information about the safety, manufacturing, product quality, marketing, labeling, and sale of products containing cannabis or cannabis-derived compounds, including CBD. The FDA has also formed an internal working group to evaluate the potential pathways to market for CBD products. It remains unclear how CBD products will be regulated by the agency going forward.
26
In addition, such products may be subject to regulation at the state or local levels. While the Farm Bill created a pathway under which hemp and its derivatives are exempted from the definition of marijuana and, therefore, no longer at risk for deemed a Schedule I controlled substance under the CSA and would be protected from interference in interstate commerce, notwithstanding the ongoing implementation of those provisions, state and local authorities have issued their own restrictions on the cultivation or sale of hemp or hemp-derived CBD. This includes laws that ban the cultivation or possession of hemp or any other plant of the cannabis genus and derivatives thereof, such as CBD. State regulators may take enforcement action against food and dietary supplement products that contain CBD, or enact new laws or regulations that prohibit or limit the sale of such products. Unforeseen regulatory obstacles or compliance costs may hinder our ability to successfully compete in the market for such products.
We may seek to enter into strategic alliances, or expand the scope of currently existing relationships, with third parties that we believe will have a beneficial impact on us, and there are risks that such strategic alliances or expansions of our currently existing relationships may not enhance our business in the desired manner.
We currently have, and may expand or reduce the scope of, and may in the future enter into, strategic alliances with third parties that we believe will complement or augment our existing business. Examples of such strategic alliances include our agreement with Sandoz, joint venture with AB InBev and partnership with ABG Intermediate Holdings 2, LLC (“ABG”). Our ability to complete further strategic alliances is dependent upon, and may be limited by, among other things, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance our business and may involve risks that could adversely affect us, including the investment of significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. We may become dependent on our strategic partners and actions by such partners could harm our business. Future strategic alliances could result in the incurrence of debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will continue to achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, or at all.
We may not be able to successfully identify and execute future acquisitions, dispositions or other equity transactions or to successfully manage the impacts of such transactions on our operations.
Material acquisitions, dispositions and other strategic transactions involve a number of risks, including: (i) the potential disruption of our ongoing business; (ii) the distraction of management away from the ongoing oversight of our existing business activities; (iii) incurring additional indebtedness; (iv) the anticipated benefits and cost savings of those transactions not being realized fully, or at all, or taking longer to realize than anticipated; (v) an increase in the scope and complexity of our operations and (vi) the loss or reduction of control over certain of our assets. Material acquisitions have been and may continue to be material to our business strategy. There is no guarantee that acquisitions, such as High Park Gardens and Manitoba Harvest, will be accretive.
On December 12, 2019, Privateer Holdings, Inc. merged with and into a wholly owned subsidiary of Tilray (the “Downstream Merger”). We incurred and may continue to incur substantial costs and expenses relating directly to the Downstream Merger, including fees and expenses payable to financial advisors, other professional fees and expenses, insurance premium costs, fees and costs relating to regulatory filings and notices, SEC filing fees, printing and mailing costs and other transaction-related costs, fees and expenses. These fees and expenses could have a significant effect on our business, financial condition and results of operation. We have also been subject to demands and a complaint related to the Downstream Merger. Responding to such actions could divert management’s attention away from our business operations and result in substantial costs.
The existence of one or more material liabilities of an acquired company that are unknown to us at the time of acquisition could result in our incurring those liabilities. A strategic transaction may result in a significant change in the nature of our business, operations and strategy, and we may encounter unforeseen obstacles or costs in implementing a strategic transaction or integrating any acquired business into our operations.
We are subject to risks inherent in an agricultural business, including the risk of crop failure.
We grow cannabis, which is an agricultural process. As such, our business is subject to the risks inherent in the agricultural business, including risks of crop failure presented by weather, insects, plant diseases and similar agricultural risks. Although we currently grow our products indoors under climate controlled conditions, we are
27
developing outdoor operations and there can be no assurance that natural elements, such as insects and plant diseases, will not entirely interrupt our production activities or have an adverse effect on our business.
We depend on significant customers for a substantial portion of our revenue. If we fail to retain or expand our customer relationships or if this significant customer were to terminate its relationship with us or reduce its purchases, our revenue could decline significantly.
Two customers accounted 13% each of our revenue, respectively, for the year ended December 31, 2019. We had one customer that accounted for 24% of our revenue for 2018. No one customer accounted for greater than 10% of our revenue in 2017. We believe that our operating results for the foreseeable future will continue to depend on sales to a small number of customers. These customers have no purchase commitments and may cancel, change or delay purchases with little or no notice or penalty. As a result of this customer concentration, our revenue could fluctuate materially and could be materially and disproportionately impacted by purchasing decisions of these customers or any other significant customer. In the future, these customers may decide to purchase less product from us than they have in the past, may alter purchasing patterns at any time with limited notice, or may decide not to continue to purchase our products at all, any of which could cause our revenue to decline materially and materially harm our financial condition and results of operations. If we are unable to diversify our customer base, we will continue to be susceptible to risks associated with customer concentration.
We may be unable to attract or retain key personnel with sufficient experience in the cannabis industry, and we may be unable to attract, develop and retain additional employees required for our development and future success.
Our success is largely dependent on the performance of our management team and certain employees and our continuing ability to attract, develop, motivate and retain highly qualified and skilled employees. Qualified individuals are in high demand, and we may incur significant costs to attract and retain them. The loss of the services of any key personnel, or an inability to attract other suitably qualified persons when needed, could prevent us from executing on our business plan and strategy, and we may be unable to find adequate replacements on a timely basis, or at all. We do not currently maintain key-person insurance on the lives of any of our key personnel.
Further, each director and officer, as well as certain additional key personnel, of a company that holds a license is subject to the requirement to obtain and maintain a security clearance from Health Canada under the CR. Moreover, under the CR, an individual with security clearance must be physically present on site when other individuals are conducting activities with cannabis. Under the CR and the Cannabis Act, a security clearance is valid for a limited time and must be renewed before the expiry of a current security clearance. There is no assurance that any of our existing personnel who presently or may in the future require a security clearance will be able to obtain or renew such clearances or that new personnel who require a security clearance will be able to obtain one. A failure by an individual in a key operational position to maintain or renew his or her security clearance could result in a reduction or complete suspension of our operations. In addition, if an individual in a key operational position leaves us, and we are unable to find a suitable replacement who is able to obtain a security clearance required by the CR in a timely manner, or at all, we may not be able to conduct our operations at planned production volume levels or at all. In addition, the CR requires us to designate a qualified individual in charge who is responsible for supervising activities relating to the production of study drug for clinical trials, which individual must meet certain educational and security clearance requirements. If our current designated qualified person in charge fails to maintain his security clearance, or if our current designated qualified person in charge leaves us and we are unable to find a suitable replacement who meets these requirements, we may no longer be able to continue our clinical trial activities.
Increased labor costs, potential organization of our workforce, employee strikes and other labor-related disruption may adversely affect our operations.
Apart from certain employees in Portugal, none of our employees are represented by a labor union or subject to a collective bargaining agreement. In Portugal, some of our employees are subject to a government-mandated collective bargaining agreement, which grants affected employees certain additional benefits beyond those required by the local labor code. We cannot assure you that our labor costs going forward will remain competitive because in the future our workforce may organize and labor agreements may be put in place that have significantly higher labor rates and company obligations; at the same time, our competitors may maintain significantly lower
28
labor costs, thereby reducing or eliminating our comparative advantages vis-à-vis one or more of our competitors or the larger industry; additionally, our labor costs may increase in connection with our growth.
Significant interruptions in our access to certain supply chains for key inputs such as raw materials, electricity, water and other utilities may impair our cannabis growing operations.
Our business is dependent on a number of key inputs and their related costs (certain of which are sourced in other countries and on different continents), including raw materials, supplies and equipment related to our operations, as well as electricity, water and other utilities. Recently, the Wuhan coronavirus has spread across the world, and it may develop into a pandemic. We operate global manufacturing facilities, and have dispersed suppliers and customers. If a disease spreads sufficiently to cause a pandemic (or to cause the fear of a pandemic to rise) or governments regulate or restrict the flow of labor or products, the Company's operations, suppliers, customers and distribution channels could be severely impacted. Such a pandemic could also have an adverse impact on consumer demand for our products and prices for our raw materials. Any significant interruption, price increase or negative change in the availability or economics of the supply chain for key inputs and, in particular, rising or volatile energy costs could curtail or preclude our ability to continue production. In addition, our operations would be significantly affected by a prolonged power outage.
Our ability to compete and grow cannabis is dependent on us having access, at a reasonable cost and in a timely manner, to skilled labor, equipment, parts and components. No assurances can be given that we will be successful in maintaining our required supply of labor, equipment, parts and components.
Fluctuations in cannabinoid prices relative to contracted prices with third party suppliers could negatively impact our earnings.
A portion of our results of operations and financial condition, as well as the selling prices for our products, are dependent upon cannabinoid supply contracts. As part of our normal course operations, we periodically enter into large and medium-to-long-term supply contracts with third-party growers. Production and pricing of cannabinoids are determined by constantly changing market forces of supply and demand over which we have limited or no control. The market for cannabis biomass is particularly volatile compared to other commoditized markets due to the relatively nascent maturity of the industry in which we operate. Furthermore, the lack of centralized data and large variations in product quality make it difficult to establish a “spot price” for cannabinoids, and develop an effective price hedging strategy. Accordingly, supply contracts with any term may prove to be costly in the future to the extent cannabinoid prices decrease dramatically or at a faster rate than anticipated. Furthermore, supply contracts typically include minimum purchase requirements which could force us to buy significant quantities of product at non-competitive prices in a rapidly changing market.
If we are unable to price our products competitively as a result of committed supply contracts that do not reflect current or future market prices, then our profitability, financial condition and results of operations could be materially and adversely affected.
We may not be able to transport our cannabis products to consumers in a safe and efficient manner.
Due to our direct-to-consumer shipping model for medical cannabis in Canada, we depend on fast and efficient third-party transportation services to distribute our medical cannabis products. We also use such services to transfer bulk shipments to provinces and territories for further distribution to consumers. Any prolonged disruption of third-party transportation services, such as the ongoing Canada Post labor disruptions, could have a material adverse effect on our sales volumes or satisfaction with our services. Rising costs associated with third-party transportation services used by us to ship our products may also adversely impact our profitability, and more generally our business, financial condition and results of operations.
The security of our products during transportation to and from our facilities is of the utmost concern. A breach of security during transport or delivery could result in the loss of high-value product and forfeiture of import and export approvals, since such approvals are shipment specific. Any failure to take steps necessary to ensure the safekeeping of our cannabis could also have an impact on our ability to continue supplying provinces and territories, to continue operating under our existing licenses, to renew or receive amendments to our existing licenses or to receive required new licenses.
29
Our cannabis products may be subject to recalls for a variety of reasons, which could require us to expend significant management and capital resources.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, adulteration, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. Although we have detailed procedures in place for testing finished cannabis products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits, whether frivolous or otherwise. If any of the cannabis products produced by us are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. As a result of any such recall, we may lose a significant amount of sales and may not be able to replace those sales at an acceptable gross profit or at all. In addition, a product recall may require significant management attention or damage our reputation and goodwill or that of our products or brands.
We have experienced product recalls in the past. For example, in April 2019, we commenced a recall of one lot of prerolls supplied to the Canadian adult-use market due to labeling error. In each of our prior recalls, we were able to complete the recall or withdrawal; however, there is no assurance that such incidents will not result in regulatory action or civil lawsuits, whether frivolous or otherwise, or an adverse effect on our reputation or goodwill, or that of our products or brands.
Additionally, product recalls may lead to increased scrutiny of our operations by Health Canada or other regulatory agencies, requiring further management attention, increased compliance costs and potential legal fees, fines, penalties and other expenses. Any product recall affecting the cannabis industry more broadly, whether or not involving us, could also lead consumers to lose confidence in the safety and security of the products sold by Licensed Producers generally, including products sold by us.
We may be subject to product liability claims or regulatory action if our products are alleged to have caused significant loss or injury. This risk is exacerbated by the fact that cannabis use may increase the risk of serious adverse side effects.
As a manufacturer and distributor of products which are ingested by humans, we face the risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused loss or injury. We may be subject to these types of claims due to allegations that our products caused or contributed to injury or illness, failed to include adequate instructions for use or failed to include adequate warnings concerning possible side effects or interactions with other substances. This risk is exacerbated by the fact that cannabis use may increase the risk of developing schizophrenia and other psychoses, symptoms for individuals with bipolar disorder, and other side effects. Previously unknown adverse reactions resulting from human consumption of cannabis products alone or in combination with other medications or substances could also occur. In addition, the manufacture and sale of cannabis products, like the manufacture and sale of any ingested product, involves a risk of injury to consumers due to tampering by unauthorized third parties or product contamination. We have in the past recalled, and may again in the future have to recall, certain of our cannabis products as a result of potential contamination and quality assurance concerns. A product liability claim or regulatory action against us could result in increased costs and could adversely affect our reputation and goodwill with our patients and consumers generally. There can be no assurance that we will be able to maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could result in us becoming subject to significant liabilities that are uninsured and also could adversely affect our commercial arrangements with third parties.
We rely on third-party distributors to distribute our products, and those distributors may not perform their obligations.
We rely on third-party distributors, including pharmaceutical distributors, courier services, and government agencies, and may in the future rely on other third parties, to distribute our products. If these distributors do not successfully carry out their contractual duties, if there is a delay or interruption in the distribution of our products, such as the Canada Post labor disruptions previously experienced, or if these third parties damage our products, it could negatively impact our revenue from product sales. Any damage to our products, such as product spoilage,
30
could expose us to potential product liability, damage our reputation and the reputation of our brands or otherwise harm our business.
We, or the cannabis industry more generally, may receive unfavorable publicity or become subject to negative consumer or investor perception.
We believe that the cannabis industry is highly dependent upon positive consumer and investor perception regarding the benefits, safety, efficacy and quality of the cannabis distributed to consumers. The perception of the cannabis industry and cannabis products, currently and in the future, may be significantly influenced by scientific research or findings, regulatory investigations, litigation, political statements, media attention and other publicity (whether or not accurate or with merit) both in Canada and in other countries relating to the consumption of cannabis products, including unexpected safety or efficacy concerns arising with respect to cannabis products or the activities of industry participants. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the cannabis market or any particular cannabis product or will be consistent with earlier publicity. Adverse future scientific research reports, findings and regulatory proceedings that are, or litigation, media attention or other publicity that is, perceived as less favorable than, or that questions, earlier research reports, findings or publicity (whether or not accurate or with merit) could result in a significant reduction in the demand for our cannabis products. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis, or our products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could adversely affect us. This adverse publicity could arise even if the adverse effects associated with cannabis products resulted from consumers’ failure to use such products legally, appropriately or as directed.
Certain events or developments in the cannabis industry more generally may impact our reputation.
Damage to our reputation can result from the actual or perceived occurrence of any number of events, including any negative publicity, whether true or not. As a producer and distributor of cannabis, which is a controlled substance in Canada that has previously been commonly associated with various other narcotics, violence and criminal activities, there is a risk that our business might attract negative publicity. There is also a risk that the actions of other Licensed Producers or of other companies and service providers in the cannabis industry may negatively affect the reputation of the industry as a whole and thereby negatively impact our reputation. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share negative opinions and views in regards to our activities and the cannabis industry in general, whether true or not.
We do not ultimately have direct control over how we or the cannabis industry is perceived by others. Reputational issues may result in decreased investor confidence, increased challenges in developing and maintaining community relations and present an impediment to our overall ability to advance our business strategy and realize on our growth prospects.
Licensed Producers are constrained by law in their ability to market their products in Canada.
The development of our business and operating results may be hindered by applicable restrictions on sales and marketing activities imposed by Health Canada. The regulatory environment in Canada limits our ability to compete for market share in a manner similar to other industries. All products we distribute into the Canadian adult-use market must comply with requirements under Canadian legislation, including with respect to product formats, product packaging, product composition and marketing activities around such products. As such, our portfolio of brands and products has been specifically adapted, and our marketing activities carefully structured, to enable us to develop our brands in an effective and compliant manner. If we are unable to effectively market our cannabis products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for our cannabis products, then our sales and operating results could be adversely affected.
31
If we are not able to comply with all safety, health and environmental regulations applicable to our operations and industry, we may be held liable for any breaches of those regulations.
Safety, health and environmental laws and regulations affect nearly all aspects of our operations, including product development, working conditions, waste disposal, emission controls, the maintenance of air and water quality standards and land reclamation, and, with respect to environmental laws and regulations, impose limitations on the generation, transportation, storage and disposal of solid and hazardous waste. Continuing to meet GMP standards, which we follow voluntarily, requires satisfying additional standards for the conduct of our operations and subjects us to ongoing compliance inspections in respect of these standards. Compliance with safety, health and environmental laws and regulations can require significant expenditures, and failure to comply with such safety, health and environmental laws and regulations may result in the imposition of fines and penalties, the temporary or permanent suspension of operations, the imposition of clean-up costs resulting from contaminated properties, the imposition of damages and the loss of or refusal of governmental authorities to issue permits or licenses to us or to certify our compliance with GMP standards. Exposure to these liabilities may arise in connection with our existing operations, our historical operations and operations that we may undertake in the future. We could also be held liable for worker exposure to hazardous substances and for accidents causing injury or death. There can be no assurance that we will at all times be in compliance with all safety, health and environmental laws and regulations notwithstanding our attempts to comply with such laws and regulations.
Changes in applicable safety, health and environmental standards may impose stricter standards and enforcement, increased fines and penalties for non-compliance, more stringent environmental assessments of proposed projects and a heightened degree of responsibility for companies and their officers, directors and employees. We are not able to determine the specific impact that future changes in safety, health and environmental laws and regulations may have on our industry, operations and/or activities and our resulting financial position; however, we anticipate that capital expenditures and operating expenses will increase in the future as a result of the implementation of new and increasingly stringent safety, health and environmental laws and regulations. Further changes in safety, health and environmental laws and regulations, new information on existing safety, health and environmental conditions or other events, including legal proceedings based upon such conditions or an inability to obtain necessary permits in relation thereto, may require increased compliance expenditures by us.
We may not be able to obtain adequate insurance coverage in respect of the risks our business faces, the premiums for such insurance may not continue to be commercially justifiable or there may be coverage limitations and other exclusions which may result in such insurance not being sufficient to cover potential liabilities that we face.
We currently have insurance coverage, including product liability insurance, protecting many, but not all, of our assets and operations. Our insurance coverage is subject to coverage limits and exclusions and may not be available for the risks and hazards to which we are exposed. In addition, no assurance can be given that such insurance will be adequate to cover our liabilities, including potential product liability claims, or will be generally available in the future or, if available, that premiums will be commercially justifiable. If we were to incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, we may be exposed to material uninsured liabilities that could impede our liquidity, profitability or solvency.
We may become subject to liability arising from any fraudulent or illegal activity by our employees, contractors, consultants and others.
We are exposed to the risk that our employees, independent contractors, consultants, service providers and licensors may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional undertakings of unauthorized activities, or reckless or negligent undertakings of authorized activities, in each case on our behalf or in our service that violate: (i) government regulations, specifically Health Canada regulations; (ii) manufacturing standards; (iii) Canadian federal and provincial healthcare laws and regulations; (iv) laws that require the true, complete and accurate reporting of financial information or data; (v) United States federal laws banning the possession, sale or importation of cannabis into the United States and prohibiting the financing of activities outside the United States that are unlawful under Canadian or other foreign laws or (vi) the terms of our agreements with insurers. In particular, we could be exposed to class action and other litigation, increased Health Canada inspections and related sanctions, the loss of current GMP compliance certifications or the inability to obtain future GMP compliance certifications, lost sales and revenue or reputational damage as a result of prohibited activities that are undertaken in the growing or production process of our products without our knowledge or permission and contrary to our internal policies, procedures and operating requirements.
32
We cannot always identify and prevent misconduct by our employees and other third parties, including service providers and licensors, and the precautions taken by us to detect and prevent this activity may not be effective in controlling unknown, unanticipated or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from such misconduct. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal or administrative penalties, damages, monetary fines and contractual damages, reputational harm, diminished profits and future earnings or curtailment of our operations.
We may experience breaches of security at our facilities or loss as a result of the theft of our products.
Because of the nature of our products and the limited legal channels for distribution, as well as the concentration of inventory in our facilities, we are subject to the risk of theft of our products and other security breaches. A security breach at any one of our facilities could result in a significant loss of available products, expose us to additional liability under applicable regulations and to potentially costly litigation or increase expenses relating to the resolution and future prevention of similar thefts, any of which could have an adverse effect on our business, financial condition and results of operations.
We may be subject to risks related to our information technology systems, including the risk that we may be the subject of a cyber-attack and the risk that we may be in non-compliance with applicable privacy laws.
We have entered into agreements with third parties for hardware, software, telecommunications and other information technology, or IT, services in connection with our operations. Our operations depend, in part, on how well we and our vendors protect networks, equipment, IT systems and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism, theft, malware, ransomware and phishing attacks. Any of these and other events could result in IT system failures, delays or increases in capital expenses. Our operations also depend on the timely maintenance, upgrade and replacement of networks, equipment and IT systems and software, as well as preemptive expenses to mitigate the risks of failures. The failure of IT systems or a component of IT systems could, depending on the nature of any such failure, adversely impact our reputation and results of operations.
There are a number of laws protecting the confidentiality of certain patient health information and other personal information, including patient records, and restricting the use and disclosure of that protected information. In particular, the privacy rules under the Personal Information Protection and Electronics Documents Act (Canada), or the PIPEDA, the European Unions’ General Data Protection Regulation (“GDPR”), and similar laws in other jurisdictions, protect medical records and other personal health information by limiting their use and disclosure to the minimum level reasonably necessary to accomplish the intended purpose. We collect and store personal information about our consumers and are responsible for protecting that information from privacy breaches. A privacy breach may occur through a procedural or process failure, an IT malfunction or deliberate unauthorized intrusions. Theft of data for competitive purposes, particularly patient lists and preferences, is an ongoing risk whether perpetrated through employee collusion or negligence or through deliberate cyber-attack. Moreover, if we are found to be in violation of the privacy or security rules under PIPEDA or other laws protecting the confidentiality of patient health information, including as a result of data theft and privacy breaches, we could be subject to sanctions and civil or criminal penalties, which could increase our liabilities and harm our reputation.
As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any information security vulnerabilities. While we have implemented security resources to protect our data security and information technology systems, such measures may not prevent such events. Significant disruption to our information technology system or breaches of data security could have a material adverse effect on our business financial condition and results of operations.
33
We may be unable to sustain our revenue growth and development.
Our revenue has grown in recent years. Our ability to sustain this growth will depend on a number of factors, many of which are beyond our control, including, but not limited to, the availability of sufficient capital on suitable terms, changes in laws and regulations respecting the production and distribution of cannabis products, competition from other Licensed Producers, the size of the black market, the size of the Canadian adult-use market, and our ability to produce sufficient volumes of our cannabis-based products to meet demand. Regulatory changes in the United States, Germany and Canada may continue to attract market entrants, therefore diluting our potential opportunity and early-mover advantage. In addition, we are subject to a variety of business risks generally associated with developing companies. Future development and expansion could place significant strain on our management personnel and likely will require us to recruit additional management personnel, and there is no assurance that we will be able to do so.
We may be unable to expand our operations quickly enough to meet demand or manage our operations beyond their current scale.
There can be no assurance that we will be able to manage our expanding operations, including any acquisitions, effectively, that we will be able to sustain or accelerate our growth or that such growth, if achieved, will result in profitable operations, that we will be able to attract and retain sufficient management personnel necessary for continued growth or that we will be able to successfully make strategic investments or acquisitions.
Demand for cannabis-based products is dependent on a number of social, political and economic factors that are beyond our control. There is no assurance that an increase in existing demand will occur, that we will benefit from any such demand increase or that our business will remain profitable even in the event of such an increase in demand. If we are unable to achieve or sustain profitability, the value of our Class 2 common stock and the notes may significantly decrease.
The cannabis industry continues to face significant funding challenges, and we may not be able to secure adequate or reliable sources of funding required to operate our business or increase our production to meet consumer demand for our products.
The continued development of our business will require significant additional financing, and there is no assurance that we will obtain the financing necessary to be able to achieve our business objectives. Our ability to obtain additional financing will depend on investor demand, our performance and reputation, market conditions and other factors. Our inability to raise such capital could result in the delay or indefinite postponement of our current business objectives or in our inability to continue to carry on our business. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favorable to us.
In addition, from time to time, we may enter into transactions to acquire assets or the capital stock or other equity interests of other entities. Our continued growth may be financed, wholly or partially, with debt, which may increase our debt levels above industry standards. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. Debt financings may also contain provisions that, if breached, may entitle lenders or their agents to accelerate the repayment of loans or realize a first priority security over our significant operating assets, and there is no assurance that we would be able to repay such loans in such an event or prevent the enforcement of security granted pursuant to any such debt financing.
Our senior secured credit facility contains covenant restrictions that may limit our ability to operate our business.
34
On February 28, 2020, we entered into a senior secured credit facility with Bridging Finance Inc. in an aggregate principal amount of $59.6 million (C$79.8 million) (the “Senior Facility”). The Senior Facility contains, and any of our other future debt agreements may contain, covenant restrictions that limit our ability to operate our business, including restrictions on our ability to, among other things, incur additional debt or issue guarantees, create additional liens, repurchase stock or make other restricted payments, and make certain voluntary prepayments of specified debt. As a result of these covenants, our ability to respond to changes in business and economic conditions and engage in beneficial transactions, including to obtain additional financing as needed, may be restricted. Furthermore, our failure to comply with our debt covenants could result in a default under our debt agreements, which could permit the holders to accelerate our obligation to repay the debt. If any of our debt is accelerated, we may not have sufficient funds available to repay it.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt.
As of December 31, 2019, we had $475 million in aggregate principal indebtedness (refer to Note 13 to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K).
On February 28, 2020, we entered into the Senior Facility with an aggregate principal amount of $59.6 million (C$79.8 million). Our substantial consolidated indebtedness may increase our vulnerability to any generally adverse economic and industry conditions. We and our subsidiaries may, subject to the limitations in the terms of our existing and future indebtedness, incur additional debt, secure existing or future debt or recapitalize our debt. Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our current and future indebtedness, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business has not generated positive cash flow from operations. If this continues into the future, we may not have sufficient cash flows to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our current and future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
We incur increased costs as a result of operating as a public company and our management is required to devote substantial time to new compliance initiatives.
As a public company, we have incurred and will incur significant legal, accounting and other expenses that we did not incur prior to our IPO. In addition, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and rules implemented by the SEC and the Nasdaq Global Select Market, impose various requirements on public companies, including requirements to file annual, quarterly and event-driven reports with respect to our business and financial condition and operations and establish and maintain effective disclosure and financial controls and corporate governance practices. Effective January 1, 2020, we became a “large accelerated filer” under SEC reporting rules and, and are required to file our annual report and quarterly reports more quickly than we previously had been required to file them, which may require us to dedicate additional resources to the timely filing of such reports. In addition, pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404, we are required to furnish a report by our management on our Internal Controls over Financial Reporting (“ICFR”), which must be accompanied by an attestation report on ICFR issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we have documented and evaluated our ICFR, which has been both costly and challenging. We expect our costs to increase substantially in order to comply with these additional and more burdensome requirements. Our existing management team has and will continue to devote a substantial amount of time to these compliance initiatives, and we may need to hire additional personnel to assist us with complying with these requirements. Moreover, these rules and regulations have increased and will continue to increase our legal and financial compliance costs and will make some activities more time consuming and costly.
Management may not be able to successfully implement adequate internal controls over financial reporting.
Management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Rules 13a-15(f) and 15d(f) under the Exchange Act, internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with United States Generally Accepted
35
Accounting Principles (“U.S. GAAP”). Our management and other personnel have limited experience operating a public company, which may result in a failure of our ICFR and Disclosure Controls and Procedures (“DCP”) necessary to ensure timely and accurate reporting of operational and financial results. Due to inherent limitations, our internal control over financial reporting may not prevent or detect all misstatements, including the possibility of human error, the circumvention or overriding of controls, or fraud.
A material weakness is a deficiency, or combination of control deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
As of December 31, 2019 we identified material weaknesses in two components of internal control as defined by COSO 2013 (Control Environment and Control Activities).
We did not maintain an effective control environment based on the criteria established in the COSO framework. We have identified deficiencies in the principles associated with the control environment of the COSO framework. Specifically, these control deficiencies constitute material weaknesses, either individually or in the aggregate, relating to: (i) appropriate organizational structure, reporting lines, and authority and responsibilities in pursuit of objectives, (ii) our commitment to attract, develop, and retain competent individuals, and (iii) holding individuals accountable for their internal control related responsibilities.
As of December 31, 2019, we did not maintain an effective control environment to allow for the accurate and timely filing of our financial statements primarily attributable to the following factor:
|
• |
We did not have a sufficient complement of accounting and financial reporting personnel with an appropriate level of knowledge, US GAAP proficiency, experience and training commensurate with our financial reporting requirements. |
We did not fully design and implement effective control activities based on the criteria established in the COSO framework. We have identified deficiencies in the principles associated with the control activities component of the COSO framework. Specifically, these control deficiencies constitute material weaknesses, either individually or in the aggregate, relating to: (i) Selecting and developing control activities that contribute to the mitigation of risks to the achievement of objectives to acceptable levels, (ii) deploying control activities through policies that establish what is expected and procedures that put policies into action.
We did not have effective controls in response to the risks of material misstatement. This material weakness is primarily attributable to the following factors:
|
• |
We did not have an adequate process or appropriate controls in place to support the accurate reporting of our financial results and disclosures on our Form 10-K. |
|
• |
We did not have effective controls over the completeness and accuracy of key spreadsheets and reports used in financial reporting. |
|
• |
We did not have adequate review procedures around the recording of manual entries. |
Due to the existence of the above material weaknesses, management, including the CEO and CFO, has concluded that our internal control over financial reporting was not effective as of December 31, 2019. These material weaknesses create a reasonable possibility that a material misstatement to the consolidated financial statements will not be prevented or detected on a timely basis.
36
Conflicts of interest may arise between us and our directors and officers as a result of other business activities undertaken by such individuals.
We may be subject to various potential conflicts of interest because some of our directors and executive officers may be engaged in a range of business activities. In addition, our directors and executive officers are permitted under their applicable agreements with us to devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to us and subject to any contractual restrictions restricting such activities. These business interests could require the investment of significant time and attention by our executive officers and directors. In some cases, our executive officers and directors, including our Chief Executive Officer and President, Brendan Kennedy and board member, Michael Auerbach, may have fiduciary obligations associated with business interests that interfere with their ability to devote time to our business and affairs, which could adversely affect our operations. Please refer to “Part II, Item 8. Note 22 – Related-Party Transactions” to our financial statements appearing elsewhere in this Annual Report on Form 10-K for further details.
Third parties with whom we do business may perceive themselves as being exposed to reputational risk as a result of their relationship with us.
The parties with whom we do business, or would like to do business, may perceive that they are exposed to reputational risk as a result of our business activities relating to cannabis, which could hinder our ability to establish or maintain business relationships. These perceptions relating to the cannabis industry may interfere with our relationship with service providers, particularly in the financial services industry.
Tax and accounting requirements may change in ways that are unforeseen to us and we may face difficulty or be unable to implement or comply with any such changes.
We are subject to numerous tax and accounting requirements, and changes in existing accounting or taxation rules or practices, or varying interpretations of current rules or practices, could have a significant adverse effect on our financial results, the manner in which we conduct our business or the marketability of any of our products. We currently have international operations and plan to expand such operations in the future. These operations, and any expansion thereto, will require us to comply with the tax laws and regulations of multiple jurisdictions, which may vary substantially. Complying with the tax laws of these jurisdictions can be time consuming and expensive and could potentially subject us to penalties and fees in the future if we were to fail to comply.
Because a significant portion of our sales are generated in Canada, fluctuations in foreign currency exchange rates could harm our results of operations.
The reporting currency for our financial statements is the United States dollar. We derive a significant portion of our revenue and incur a significant portion of our operating costs in Canada, and changes in exchange rates between the Canadian dollar and the United States dollar may have a significant, and potentially adverse, effect on our results of operations. Our primary risk of loss regarding foreign currency exchange rate risk is caused by fluctuations in the exchange rates between the United States dollar and the Canadian dollar, although as we expand internationally, we will be subject to additional foreign currency exchange risks. Because we recognize revenue in Canada in Canadian dollars, if the Canadian dollar weakens against the United States dollar it would have a negative impact on our Canadian operating results upon the translation of those results into U.S. dollars for the purposes of consolidation. In addition, a weakening of the Canadian dollar against the United States dollar would make it more difficult for us to meet our obligations under the convertible notes. We have not historically engaged in hedging transactions and do not currently contemplate engaging in hedging transactions to mitigate foreign exchange risks. As we continue to recognize gains and losses in foreign currency transactions, depending upon changes in future currency rates, such gains or losses could have a significant, and potentially adverse, effect on our results of operations.
We may have exposure to greater than anticipated tax liabilities, which could seriously harm our business.
Our income tax obligations are based on our corporate operating structure and third-party and intercompany arrangements, including the manner in which we develop, value and use our intellectual property and
37
the valuations of our intercompany transactions. The tax laws applicable to our international business activities, including the laws of the United States, Canada and other jurisdictions, are subject to change and uncertain interpretation. The taxing authorities of the jurisdictions in which we operate may challenge our methodologies for valuing developed technology, intercompany arrangements or transfer pricing, which could increase our worldwide effective tax rate and the amount of taxes that we pay and seriously harm our business. Taxing authorities may also determine that the manner in which we operate our business is not consistent with how we report our income, which could increase our effective tax rate and the amount of taxes that we pay and could seriously harm our business. In addition, our future income taxes could fluctuate because of earnings being lower than anticipated in jurisdictions that have lower statutory tax rates and higher than anticipated in jurisdictions that have higher statutory tax rates, by changes in the valuation of our deferred tax assets and liabilities or by changes in tax laws, regulations or accounting principles. We are subject to regular review and audit by United States federal and state and foreign tax authorities. Any adverse outcome from a review or audit could seriously harm our business. In addition, determining our worldwide provision for income taxes and other tax liabilities requires significant judgment by management, and there are many transactions where the ultimate tax determination is uncertain. Although we believe that the amounts recorded in our financial statements are reasonable, the ultimate tax outcome relating to such amounts may differ for such period or periods and may seriously harm our business.
The long-term effect of United States tax reform could adversely affect our business and financial condition.
On December 22, 2017, the legislation commonly referred to as the Tax Cuts and Jobs Act was enacted, which contains significant changes to United States tax law, including, but not limited to, a reduction in the corporate tax rate, limitation of the tax deduction for interest expense (with certain exceptions), limitation of the deduction for net operating losses arising after 2017 to 80% of current year taxable income and elimination of carryback of such net operating losses, one-time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, immediate deductions for certain new investments instead of deductions for depreciation expense over time, modifying or repealing many business deductions and credits, deemed repatriation of certain intangible related income and a transition to a new quasi-territorial system of taxation. Notwithstanding the reduction in the corporate income tax rate, our business and financial condition could be adversely affected in future periods by the overall impact of the Tax Act. In addition, the Tax Act could be amended or subject to technical correction, possibly with retroactive effect, which could change the financial impacts that were recorded at December 31, 2019, or are expected to be recorded in future periods. Additionally, further guidance may be forthcoming from the Financial Accounting Standards Board and SEC, as well as regulations, interpretations and rulings from federal and state tax agencies, which could result in additional impacts, possibly with retroactive effect. Any such changes or potential additional impacts could adversely affect our business and financial condition. We will continue to examine and assess the impact this tax reform legislation may have on our business.
As a result of an investment in our securities, you could be prevented from entering the United States or become subject to a lifetime ban on entry into the United States.
United States Customs and Border Protection (“CBP”) has confirmed that border agents may seek to permanently ban any foreign visitor who admits to working or investing in the cannabis industry, or admits to having used cannabis, even though adult-use cannabis is now legal in Canada. CBP confirmed that investing even in publicly-traded cannabis companies is considered facilitation of illicit drug trade under CBP policy. This policy is limited to citizens of foreign countries and not citizens of the United States. Therefore, as a result of an investment in our securities, if you are not a citizen of the United States, you could be prevented from entering the United States or could become subject to a lifetime ban on entry into the United States.
Risks Related to our Intellectual Property
We may be subject to risks related to the protection and enforcement of our intellectual property rights, or intellectual property we license from others, and may become subject to allegations that we or our licensors are in violation of intellectual property rights of third parties.
The ownership, licensing and protection of trademarks, patents and intellectual property rights are significant aspects of our future success. Unauthorized parties may attempt to replicate or otherwise obtain and use our products and technology. Policing the unauthorized use of our current or future trademarks, patents or other
38
intellectual property rights now or in the future could be difficult, expensive, time consuming and unpredictable, as may be enforcing these rights against the unauthorized use by others. Identifying the unauthorized use of intellectual property rights is difficult as we may be unable to effectively monitor and evaluate the products being distributed by our competitors, including parties such as unlicensed dispensaries and black-market participants, and the processes used to produce such products. In addition, in any infringement proceeding, some or all of our trademarks, patents or other intellectual property rights or other proprietary know-how, and that which we license from others, or arrangements or agreements seeking to protect the same for our benefit, may be found invalid, unenforceable, anti-competitive or not infringed or may be interpreted narrowly and such proceeding could put existing intellectual property applications at risk of not being issued.
In addition, other parties may claim that our products, or those that we license from others, infringe on their proprietary or patent protected rights. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources and legal fees, result in injunctions or temporary restraining orders or require the payment of damages. As well, we may need to obtain licenses from third parties who allege that we have infringed on their lawful rights. Such licenses may not be available on terms acceptable to us, or at all. In addition, we may not be able to obtain or utilize on terms that are favorable to us, or at all, licenses or other rights with respect to intellectual property that we do not own.
We also rely on certain trade secrets, technical know-how and proprietary information that are not protected by patents to maintain our competitive position. Our trade secrets, technical know-how and proprietary information, which are not protected by patents, may become known to or be independently developed by competitors, which could adversely affect us.
We license some intellectual property rights, and the failure of the owner of such intellectual property to properly maintain or enforce the intellectual property underlying such licenses could have a material adverse effect on our business, financial condition and performance.
We are party to a number of licenses, including with entities formerly affiliated with the former Privateer Holdings, that give us rights to use third-party intellectual property that is necessary or useful to our business. Our success will depend, in part, on the ability of the licensor to maintain and enforce its licensed intellectual property, in particular, those intellectual property rights to which we have secured exclusive rights. Without protection for the intellectual property we have licensed, other companies might be able to offer substantially similar products for sale or utilize substantially similar processes, which could have a material adverse effect on us.
Any of our licensors may allege that we have breached our license agreement, whether with or without merit, and accordingly seek to terminate our license. If successful, this could result in our loss of the right to use the licensed intellectual property, which could adversely affect our ability to commercialize our products or services, as well as have a material adverse effect on us.
We may not realize the full benefit of the clinical trials or studies that we participate in because the terms of some of our agreements to participate do not give us full rights to the resulting intellectual property, the ability to acquire full rights to that intellectual property on commercially reasonable terms or the ability to prevent other parties from using that intellectual property.
Although we have participated in several clinical trials, we are not the sponsor of many of these trials and, as such, do not have full control over the design, conduct and terms of the trials. In some cases, for instance, we are only the provider of a cannabis study drug for a trial that is designed and initiated by an independent investigator within an academic institution. In such cases, we are often not able to acquire rights to all the intellectual property generated by the trials. Although the terms of all clinical trial agreements entered into by us provide us with, at a minimum, ownership of intellectual property relating directly to the study drug being trialed ( e.g. intellectual property relating to use of the study drug), and ownership of intellectual property that does not relate directly to the study drug is often retained by the institution. As such, we are vulnerable to any dispute among the investigator, the institution and us with respect to classification and therefore ownership of any particular piece of intellectual property generated during the trial. Such a dispute may affect our ability to make full use of intellectual property generated by a clinical trial.
Where intellectual property generated by a trial is owned by the institution, we are often granted a right of first negotiation to obtain an exclusive license to such intellectual property. If we exercise such a right, there is a risk
39
that the parties will fail to come to an agreement on the license, in which case such intellectual property may be licensed to other parties or commercialized by the institution.
We may not realize the full benefit of our licenses if the licensed material has less market appeal than expected, or if restrictions on packaging and marketing hinder our ability to realize value from our licenses, and our licenses may not be profitable to us.
An integral part of our Canadian adult-use cannabis business strategy involves obtaining territorially exclusive licenses to produce products using various brands and images. As a licensee of brand-based properties, we have no assurance that a particular brand or property will translate into a successful adult-use cannabis product. Additionally, a successful brand may not continue to be successful or maintain a high level of sales. As well, the popularity of licensed properties may not result in popular products or the success of the properties with the public. Promotion, packaging and labelling of adult-use cannabis is strictly regulated. These restrictions may further hinder our ability to benefit from our licenses. Acquiring or renewing licenses may require the payment of minimum guaranteed royalties that we consider to be too high to be profitable, which may result in losing licenses we currently hold when they become renewable under their terms or missing business opportunities for new licenses. If we are unable to acquire or maintain successful licenses on advantageous terms, or to derive sufficient revenue from sales of licensed products, our adult-use business may not be successful.
Risks Related to Ownership of Our Securities
Holders of Class 2 common stock have limited voting rights as compared to holders of Class 1 common stock. We cannot predict the impact that our capital structure and concentrated control by former Privateer Holdings stockholders may have on the market price of our Class 2 common stock.
Following consummation of the Downstream Merger, Brendan Kennedy (our Chief Executive Officer and President and a director), Michael Blue and Christian Groh, including individual and affiliated entities, beneficially own or control approximately 75% of the voting power of our capital stock. Class 1 common stock, held entirely by such individuals and affiliated entities, has 10 votes per share, resulting in such individuals and affiliated entities controlling a majority of the voting power of all outstanding shares of our capital stock and control of all matters that may be submitted to our stockholders for approval as long as they hold at least approximately 10% of all outstanding shares of our capital stock. Generally, a transfer by these individuals and entities of the Class 1 common stock they hold would cause a conversion of such shares into Class 2 common stock (including, if there is a transfer of Class 1 common stock, or entering into a binding agreement with respect to the power to vote or direct the voting of such shares). However, a transfer to certain entities controlled by such individuals, such as estate planning entities, would not result in a conversion and these individuals would continue to hold Class 1 common stock the superior voting rights of 10 votes per share. This concentrated control reduces other stockholders’ ability to influence corporate matters and, as a result, we may take actions that our stockholders other than Messrs. Kennedy, Blue and Groh do not view as beneficial. Further, the concentration of the ownership of our Class 1 common stock may prevent or delay the consummation of change of control transactions that stockholders other than or Messrs. Kennedy, Blue and Groh may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. As a result, the market price of our Class 2 common stock could be adversely affected.
Additionally, while other companies listed on United States stock exchanges have publicly traded classes of stock with limited voting rights, we cannot predict whether this structure, combined with concentrated control by Messrs. Kennedy, Blue and Groh will result in a lower trading price or greater fluctuations in the trading price of our Class 2 common stock as compared to the market price were we to have a single class of common stock, or will result in adverse publicity or other adverse consequences.
40
The price of our Class 2 common stock in public markets has experienced and may experience significant fluctuations.
The market price for our Class 2 common stock, and the market price of stock of other companies operating in the cannabis industry, has been extremely volatile. For example, during the year ended December 31, 2019, the trading price of our Class 2 common stock has fluctuated between a low sales price of $15.57and a high sales price of $106.00 per share, demonstrating an unusual degree of volatility even relative to other cannabis companies during the same time period. The market price of our Class 2 common stock may continue to be volatile and subject to wide fluctuations in response to numerous factors, many of which are beyond our control, including the following: (i) actual or anticipated fluctuations in our quarterly results of operations; (ii) recommendations by securities research analysts; (iii) changes in the economic performance or market valuations of other issuers that investors deem comparable to us; (iv) the addition or departure of our executive officers or other key personnel; (v) the release or expiration of lock-up or other transfer restrictions on our common stock, including as it relates to the Downstream Merger; (vi) sales or perceived sales, or the expectation of future sales, of our common stock; (vii) significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors; (viii) news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in the cannabis industry or our target markets; and (ix) the impact of the Downstream Merger.
Future sales or distributions of our securities, including by former Privateer Holdings stockholders who received shares of our common stock in the Downstream Merger, could cause the market price for our Class 2 common stock to fall significantly.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the market perception that the holders of a large number of shares of our Class 2 common stock, or shares of our Class 1 common stock which are convertible into Class 2 common stock on a one-for-one basis, intend to sell our Class 2 common stock, could significantly reduce the market price of our Class 2 common stock.
Pursuant to the Downstream Merger, former Privateer Holdings stockholders who received shares of our common stock in the Downstream Merger entered into a lock-up agreement. Each Privateer Holdings equity holder who received shares of our stock in the Downstream Merger is subject to a lock-up allowing for the sale of such shares only under certain circumstances over a two-year period. During the first year following the closing of the Downstream Merger, unless otherwise approved by us, shares will be released only pursuant to certain offerings or sales arranged by and at our discretion. We may also determine to release shares from the lock-up in the absence of an offering or arranged sale if we determine it to be in the Company’s best interest. At the end of the first year, to the extent not already released at our discretion as a result of the aforementioned offerings or sales or otherwise, 50 percent of the total shares subject to the lock-up will be released, or approximately 37.5 million shares. Over the course of the second year following closing, the remaining shares will be subject to a staggered release in four equal quarterly increments, which we also could choose to waive to allow earlier release in our discretion.
We cannot predict the effect, if any, that future public sales of these securities or the availability of these securities for sale will have on the market price of our Class 2 common stock. Shares held by former Privateer stockholders represent approximately 75 million shares or 73% of our currently outstanding shares and, therefore, a significant overhang on our stock. If 50 percent of the former Privateer Holdings stockholders are released on the one-year anniversary of the Downstream Merger or a significant portion were released earlier by us, it could put significant downward pricing pressure on our stock. If the market price of our Class 2 common stock were to drop as a result, this might impede our ability to raise additional capital and might cause our remaining stockholders to lose all or part of their investment.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, our stock price and trading volume could decline.
The trading market for our Class 2 common stock depends, in part, on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If one or more of the securities or industry analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
41
We may not have the ability to raise the funds necessary to settle conversions of the convertible notes in cash or to repurchase the convertible notes upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion or repurchase of the convertible notes.
Holders of the convertible notes have the right to require us to repurchase their convertible notes upon the occurrence of a fundamental change at a fundamental change repurchase price equal to 100% of the principal amount of the convertible notes to be repurchased, plus accrued and unpaid interest, if any. In addition, upon conversion of the convertible notes, unless we elect to deliver solely shares of our Class 2 common stock to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the convertible notes being converted. However, we may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of convertible notes surrendered. In addition, our ability to repurchase the convertible notes or to pay cash upon conversions of the convertible notes may be limited by law, by regulatory authority or by agreements governing our future indebtedness. Our failure to repurchase convertible notes at a time when the repurchase is required by the indenture or to pay any cash payable on future conversions of the convertible notes as required by the indenture would constitute a default under the indenture. A default under the indenture or the fundamental change itself could also lead to a default under agreements governing our existing or future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the convertible notes or make cash payments upon conversions thereof.
The conditional conversion feature of the convertible notes, if triggered, may adversely affect our financial condition and operating results.
In the event the conditional conversion feature of the convertible notes is triggered, holders of convertible notes will be entitled to convert the convertible notes at any time during specified periods at their option. If one or more holders elect to convert their convertible notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our Class 2 common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders of convertible notes do not elect to convert their convertible notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the convertible notes as a current rather than long-term liability, which would result in a material reduction of our net working capital.
42
Holders of our Class 2 common stock may be subject to dilution resulting from future offerings of common stock by us.
We may raise additional funds in the future by issuing common stock or equity-linked securities. Holders of our securities have no preemptive rights in connection with such further issuances. Our board of directors has the discretion to determine if an issuance of our capital stock is warranted, the price at which such issuance is to be effected and the other terms of any future issuance of capital stock. In addition, additional common stock will be issued by us in connection with the exercise of options or grant of other equity awards granted by us. Such additional equity issuances could, depending on the price at which such securities are issued, substantially dilute the interests of the holders of our existing securities.
Conversion of the convertible notes may dilute the ownership interest of our stockholders or may otherwise depress the price of our Class 2 common stock.
The conversion of some or all of the convertible notes may dilute the ownership interests of our stockholders. Upon conversion of the convertible notes, we have the option to pay or deliver, as the case may be, cash, shares of our Class 2 common stock, or a combination of cash and shares of our Class 2 common stock. If we elect to settle our conversion obligation in shares of our Class 2 common stock or a combination of cash and shares of our Class 2 common stock, any sales in the public market of our Class 2 common stock issuable upon such conversion could adversely affect prevailing market prices of our Class 2 common stock. In addition, the existence of the convertible notes may encourage short selling by market participants because the conversion of the convertible notes could be used to satisfy short positions, or anticipated conversion of the convertible notes into shares of our Class 2 common stock could depress the price of our Class 2 common stock.
It is not anticipated that any dividends will be paid to holders of our Class 2 common stock for the foreseeable future, if any.
No dividends on our Class 2 common stock have been paid to date. We anticipate that, for the foreseeable future, we will retain future earnings and other cash resources for the operation and development of our business. The payment of any future dividends will be at the discretion of our board of directors after taking into account many factors, including our earnings, operating results, financial condition and current and anticipated cash needs.
Provisions in our corporate charter documents could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our Class 2 common stock, thereby depressing the market price of our Class 2 common stock. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. Among others, these provisions include the following:
|
• |
our board of directors is divided into three classes with staggered three-year terms which may delay or prevent a change of our management or a change in control; |
|
• |
our board of directors has the right to elect directors to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
|
• |
our stockholders may not act by written consent or call special stockholders’ meetings; as a result, a holder, or holders, controlling a majority of our capital stock would not be able to take certain actions other than at annual stockholders’ meetings or special stockholders’ meetings called by the board of directors, the chairman of the board or our chief executive officer; |
|
• |
our certificate of incorporation prohibits cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
43
|
• |
stockholders must provide advance notice and additional disclosures in order to nominate individuals for election to the board of directors or to propose matters that can be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company; and |
|
• |
our board of directors may issue, without stockholder approval, shares of undesignated preferred stock; the ability to issue undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
Certain jurisdictions may take positions adverse to investments in, or investors themselves, in cannabis companies.
Certain jurisdictions may prohibit or restrict its citizens or residents from investing in or transacting with companies involved in the cannabis industry, even if such companies only conduct business in jurisdictions where cannabis is legal. For example, if an investor in the United Kingdom profits from an investment in a cannabis producer or supplier, such investment may technically violate the United Kingdom Proceeds of Crime Act 2002. Similar prohibitions or restrictions may apply in other jurisdictions where cannabis has not been legalized. In addition, such prohibitions and restriction may limit your ability to receive dividends if such dividends were to be declared in the future. However, no dividends on our Class 2 common stock have been paid to date and we do not anticipate that, for the foreseeable future, we will pay dividends on our Class 2 common stock.
Certain provisions in the indenture governing the convertible notes may delay or prevent an otherwise beneficial takeover attempt of us.
Certain provisions in the indenture governing the convertible notes may make it more difficult or expensive for a third party to acquire us. For example, the indenture governing the convertible notes requires us to repurchase the convertible notes for cash upon the occurrence of a fundamental change and, in certain circumstances, to increase the relevant conversion rate for a holder that converts its convertible notes in connection with a make-whole fundamental change. A takeover of us may trigger the requirement that we repurchase the convertible notes and/or increase the conversion rate, which could make it more costly for a potential acquirer to engage in such takeover. Such additional costs may have the effect of delaying or preventing a takeover of us that would otherwise be beneficial to investors.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware and the federal district courts of the United States of America will be the exclusive forums for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for:
|
• |
any derivative action or proceeding brought on our behalf; |
|
• |
any action asserting a breach of fiduciary duty; |
|
• |
any action asserting a claim against us arising under the Delaware General Corporation Law, our amended and restated certificate of incorporation or our amended and restated bylaws; and |
|
• |
any action asserting a claim against us that is governed by the internal-affairs doctrine. |
Our amended and restated certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. This exclusive forum provision would not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
44
Our restated certificate of incorporation also provides that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to this choice of forum provision. It is possible that a court of law could rule that the choice of forum provision contained in our restated certificate of incorporation is inapplicable or unenforceable if it is challenged in a proceeding or otherwise.
These exclusive-forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find the exclusive-forum provision in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving the dispute in other jurisdictions, which could seriously harm our business.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our headquarters is located in Nanaimo, British Columbia. Our Nanaimo campus is comprised of one manufacturing and R&D facility which we own and one leased building of office space. We also have five manufacturing locations owned or leased, located in Enniskillen, Leamington and London, Ontario, as well as in Ste. Agathe and Winnipeg, Manitoba. In Cantanhede, Portugal, we own one manufacturing location and land adjacent to this facility for future expansion. We also have leased space in Seattle, Washington, Minneapolis, Minnesota, Toronto, Ontario and Berlin, Germany to be used for general corporate and administrative purposes. We believe that our facilities and committed leased space are currently adequate to meet our needs. As we continue to expand our operations, we may need to lease additional or alternative facilities.
Item 3. Legal Proceedings.
420 Investments Ltd. Litigation
On February 21, 2020, 420 Investments Ltd., as Plaintiff (“420”), filed a lawsuit against Tilray Inc. and High Park Shops Inc. (“High Park”), as Defendants, in Calgary, Alberta in the Court of Queen’s Bench of Alberta. In August 2019, Tilray and High Park entered into an Arrangement Agreement with 420 and others. Pursuant to the Arrangement Agreement, High Park was to acquire the securities of 420. In February 2020, Tilray and High Park gave notice of termination of the Arrangement Agreement. The Plaintiff alleges that the termination was unlawful and without merit and further alleges that the Defendants had no legal basis to terminate. The Plaintiff alleges that the Defendants did not meet their contractual and good faith obligations under the Arrangement Agreement. The Plaintiff seeks an order of specific performance (compelling the closing of the Arrangement Agreement). Alternatively, in the absence of specific performance, the Plaintiff seeks damages in the stated amount of C$120 million, plus C$20 million in aggravated damages. Tilray’s and High Park’s Statement of Defense is due March 21, 2020, and no trial date has been set.
Braun Litigation
On February 27, 2020, stockholders Braun and Noorian filed a class action and derivative complaint in the Delaware Court of Chancery styled Braun v. Kennedy, C.A. No. 2020-0137. The suit named Brendan Kennedy, Christian Groh, Michael Blue, Maryscott Greenwood, Michael Auerbach, and Privateer Evolution, LLC (as successor to Privateer Holdings, Inc.) as defendants and Tilray as a nominal defendant. The complaint asserts claims for breach of fiduciary duty against Kennedy, Groh, Blue, and Privateer Evolution, LLC for alleged breaches of fiduciary duty in their capacity as Tilray’s controlling stockholders and against Kennedy, Greenwood, and Auerbach for alleged breaches of fiduciary duties in their capacities as directors and/or officers of Tilray in connection with the Downstream Merger.
The complaint alleges that the Privateer Defendants breached their fiduciary duties by causing Tilray to enter into the Downstream Merger and Tilray’s Board to approve that Downstream Merger, and that Defendants Kennedy, Greenwood, and Auerbach breached their fiduciary duties as directors by approving the Downstream Merger. Plaintiffs allege that the Downstream Merger gave the Privateer Defendants hundreds of millions of dollars
45
of tax savings in which Tilray did not share equally and that it unfairly transferred and extended Kennedy, Blue, and Groh’s control over Tilray.
We believe we have meritorious defenses to these matters and will continue to vigorously defend against them, but there are no assurances as to their outcome at this time. An adverse judgment or award against the Company in these cases could result in an event of default under the terms of the Senior Facility or the convertible notes.
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. We are not currently a party to any other legal proceedings other than described above, the outcome of which, if determined adversely to us, would individually or in the aggregate have a material adverse effect on our business, financial condition, results of operations or prospects.
Item 4. Mine Safety Disclosures.
Not applicable.
46
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our Class 2 common stock is traded on the Nasdaq Global Select Market under the symbol “TLRY.”
Holders
As of March 2, 2020, there were approximately 569 holders of record of our Class 2 common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have never declared or paid dividends on our Class 2 common stock. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. Any declared dividends will be declared on both our Class 1 common stock and Class 2 common stock at the same rate per share. We do not intend to declare or pay cash dividends on our Class 2 common stock in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors subject to applicable laws and will depend upon, among other factors, our results of operations, financial condition, contractual restrictions and capital requirements. Because a significant portion of our operations is conducted through our wholly owned subsidiaries, our ability to pay dividends depends in part on our receipt of cash dividends from such subsidiaries, which may further restrict our ability to pay dividends as a result of the laws of their jurisdiction of organization or covenants under any future outstanding indebtedness such subsidiaries incur. Our future ability to pay cash dividends on our Class 2 common stock is limited by the terms of the Senior Facility and cannot be paid without the consent of Bridging Finance Inc., as well as any future debt or preferred securities.
The equity plan compensation information called for by Item 201(d) of Regulation S-K will be set forth under the heading “Equity Compensation Plan Information” in the Company’s 2020 Proxy Statement.
Recent sales of unregistered securities; use of proceeds from registered securities.
Each issuance of common stock described below, unless otherwise noted, were exempt from registration under Section 4(2) of the Securities Act 1933 in transactions by an issuer not involving a public offering.
In connection with consummation of the previously disclosed Profit Participation Agreement and Payment Agreement with ABG Intermediate Holdings 2, LLC (“ABG”) on January 14, 2019, pursuant to which we purchased from ABG participation rights in up to 49% of the net (i.e. post-expense) royalties from cannabis products bearing brands currently within the ABG portfolio that ABG receives from the exploitation of certain ABG brands in connection with the development, marketing and sale of cannabis-related products, we issued 840,107 shares of Class 2 Common Stock in March 2019.
On June 12, 2019, the Company issued 28,361 shares of Class 2 Common Stock in exchange for a minority investment in a Canadian cannabis retailer that provides for collaboration between the two companies in the sale and distribution of the Company’s High Park portfolio of branded cannabis products.
On July 12, 2019, the Company issued 79,289 shares of Class 2 Common Stock in connection with the acquisition of Smith & Sinclair Ltd., which crafts edible candies, cocktails and fragrances in the United Kingdom and enables the Company to develop CBD-infused edibles for distribution in Canada, the United States and Europe.
On September 13, 2019, the Company issued 128,670 shares of Class 2 Common Stock in exchange for a minority investment in a Canadian cannabis retailer that provides for collaboration between the two companies in the sale and distribution of the Company’s High Park portfolio of branded cannabis products.
47
On September 19, 2019, the Company issued 63,747 shares of Class 2 Common Stock as a portion of the purchase consideration for a 50% equity interest in a cannabis edibles manufacturer, pursuant to which the Company and such manufacturer will develop and manufacture cannabis products for phase two of adult-use legalization in Canada.
On September 20, 2019, the Company issued 161,632 shares of Class 2 Common Stock in exchange for a convertible note issued by a specialized equipment company.
On October 15, 2019 and October 22, 2019, the Company issued 2,147 shares and 2,006 shares of its Class 2 common stock, respectively, in satisfaction of certain performance milestones in connection with its previously disclosed acquisition of Natura Naturals Holdings Inc.
On August 28, 2019, the Company issued 899,306 shares of Class 2 Common Stock to FHF Holdings Ltd. as a portion of the purchase price consideration in connection with the previously disclosed acquisition of Manitoba Harvest. These securities were issued as exempt securities under Section 3(a)(10) of the Securities Act of 1933.
Stock Performance Graph
The following graph reflects the cumulative total return to our stockholders during the period from July 19, 2018 through December 31, 2019 in comparison to the indicated indexes. The results assume that $100 was invested on July 19, 2018 in our Class 2 common stock and each of the indicated indexes.
|
|
|
July 18, |
|
|
December 31, |
|
||||||
|
|
|
2018 |
|
|
2018 |
|
|
2019 |
|
|||
|
Tilray Inc. |
|
$ |
100.00 |
|
|
$ |
414.94 |
|
|
$ |
100.76 |
|
|
Nasdaq Composite |
|
$ |
100.00 |
|
|
$ |
84.94 |
|
|
$ |
116.15 |
|
|
Horizons Marijuana Life Sciences Index |
|
$ |
100.00 |
|
|
$ |
86.32 |
|
|
$ |
57.12 |
|
This information under “Stock Performance Graph” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of Tilray under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation language in those filings.
48
Item 6. Selected Financial Data.
The following selected financial data should be read together with our financial statements and the related notes appearing elsewhere in this Annual Report on Form 10-K and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Annual Report on Form 10-K.
|
|
|
Years ended December 31, |
|
|||||||||||||
|
|
|
2019(1) |
|
|
2018 |
|
|
2017 |
|
|
2016 |
|
||||
|
|
|
(in thousands of United States dollars, except for share and per share data) |
|
|||||||||||||
|
Earnings data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue (inclusive of excise duties of $13,136, $1,200, $0 and $0 respectively) |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
20,538 |
|
|
$ |
12,644 |
|
|
Operating loss |
|
$ |
(301,702 |
) |
|
$ |
(57,650 |
) |
|
$ |
(7,498 |
) |
|
$ |
(7,049 |
) |
|
Net loss |
|
$ |
(321,169 |
) |
|
$ |
(67,723 |
) |
|
$ |
(7,809 |
) |
|
$ |
(7,883 |
) |
|
Net loss per share - basic and diluted |
|
$ |
(3.20 |
) |
|
$ |
(0.82 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.11 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
896,330 |
|
|
$ |
656,667 |
|
|
$ |
53,948 |
|
|
$ |
33,093 |
|
|
Total liabilities, less current portion |
|
$ |
518,632 |
|
|
$ |
433,077 |
|
|
$ |
8,579 |
|
|
$ |
8,576 |
|
|
Total stockholders' equity (deficit) |
|
$ |
285,271 |
|
|
$ |
197,653 |
|
|
$ |
(4,852 |
) |
|
$ |
2,528 |
|
|
(1) |
Effective January 1, 2019, we adopted new accounting pronouncements as described in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 2, “New accounting pronouncements recently adopted”. Prior year balances remain unchanged. |
49
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with the financial information and the notes thereto included in Part II, Item 8 of this Form 10-K in this Annual Report for the fiscal year ended December 31, 2019 (“Annual Report”). Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or “forward -looking information” within the meaning of Canadian securities laws. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “will,” “would” or the negative or plural of these words or similar expressions or variations. Such forward-looking statements and forward-looking information are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by the forward-looking statements or forward-looking information. Factors that could cause or contribute to such differences include, but are not limited to, those identified in this Annual Report on Form 10-K and those discussed in the section titled “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K and in our other SEC and Canadian public filings. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based on information available to us as of the date of this Annual Report on Form 10-K and while we believe that information provides a reasonable basis for these statements, that information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely on these statements. You should not rely upon forward-looking statements or forward-looking information as predictions of future events. Furthermore, such forward-looking statements or forward-looking information speak only as of the date of this report. Except as required by law, we undertake no obligation to update any forward-looking statements or forward-looking information to reflect events or circumstances after the date of such statements.
Amounts are presented in thousands of United States dollars, except for per share data or as otherwise noted. The Canadian dollar (“C$”) equivalents presented are derived using the average exchange rate during the reporting period. Amounts are individually converted by multiplying the United States dollar to Canadian dollar rate to determine the Canadian dollar amount.
Overview
Our vision is to build the world’s most trusted and valuable cannabis and hemp company. We are pioneering the future of medical, wellness and adult-use cannabis and hemp research, cultivation, processing and distribution globally, and we are one of the leading suppliers of adult-use cannabis in Canada and a leading supplier of hemp products in North America.
We have supplied high-quality cannabis products to tens of thousands of patients in fifteen countries spanning five continents through our subsidiaries in Australia, Canada, Germany, Latin America and Portugal, and through agreements with established pharmaceutical distributors. We cultivate medical and adult-use cannabis in Canada and medical cannabis in Europe. We operate only in countries where cannabis or hemp-derived cannabinoids are legal, by which we mean the activities in those countries are permitted under all applicable federal and state or provincial and territory laws.
We are witnessing a global paradigm shift for cannabis and hemp, and as a result of this shift, the transformation of a multibillion dollar industry from a state of prohibition to a state of legalization. Medical cannabis is now authorized at the national or federal level in forty-one countries. The legal market for medical cannabis is still in its early stages and we believe the number of countries with legalized regimes will continue to increase. We believe that as this transformation occurs, trusted global brands with multinational supply chains will become market leaders by earning the confidence of patients, doctors, governments and adult consumers around the world.
50
We are a leader in the Canadian adult-use market. We have entered into agreements to supply certain provinces and territories with our adult-use products for sale through the distribution systems they have established. Adult-use legalization occurred in Canada on October 17, 2018 and on October 17, 2019, the Canadian adult-use regulations were amended to permit the sale of new class of cannabis including edibles, beverages and vape products.
We introduced our phase two products, which we refer to as Cannabis 2.0 products, in December 2019. The new additions included new confectionery brand Chowie Wowie™; new wellness brand Rmdy.™; new beverage brand Everie, developed by Fluent (High Park Company’s joint venture with Labatt Breweries of Canada), and brings to the Canadian market, all-in-one-vape-pens and cartridges U.S. brand Marley Natural™, and confectionary brand Goodship™. An assortment of our Cannabis 2.0 products shipped on December 16, 2019. We expect the adult-use market to represent a higher proportion of our revenues as new consumers participate in, and previously illicit consumers adopt, Canada’s framework for the sale of cannabis.
We welcomed Manitoba Harvest to our portfolio of companies on February 28, 2019. Manitoba Harvest is the world’s largest hemp food manufacturer and a leader in the natural foods industry, producing, manufacturing, marketing and distributing a broad-based portfolio of hemp-based (cannabis) consumer products sold in over 16,000 stores at major retailers across the United States and Canada. Manitoba Harvest also launched a line of CBD products in the United States in 2019, which are available in over 500 locations.
We continue to develop strategic alliances, such as our collaboration with Sandoz to increase the availability of high quality medical cannabis products, and our joint venture with Anheuser-Busch InBev (“AB InBev”), through its subsidiary Labatt Breweries of Canada, to research non-alcohol beverages containing tetrahydrocannabinol (“THC”) and cannabidiol (“CBD”), demonstrating our continuing commitment to pioneer the development of a professional, transparent, and well-regulated cannabis industry. In the third quarter of 2019, we partnered with Cannfections Group Inc., a leader in the confectionery space with 85 years of experience developing and producing the world’s favorite confectionary brands, to further build our product and manufacturing capacity of confectionery cannabis products and expedite innovation and new products to market.
On January 14, 2019, we entered into a Profit Participation Arrangement with ABG Intermediate Holdings 2, LLC (“ABG”) where we purchased: (i) participation rights in up to 49% of the net (i.e. post-expense) cannabis revenues from certain existing ABG brands into perpetuity, (ii) guaranteed minimum receipt of $10 million annually for ten years (prorated based on total consideration paid to ABG) in quarterly payments for participation rights, (iii) preferred supplier rights of all cannabinoid ingredients for products under cannabis-related licenses of certain existing ABG brands into perpetuity, (iv) preferred royalty rates for us to license and develop cannabis products for brands currently within the ABG portfolio, and (v) first negotiation and matching rights related to participation rights in net cannabis revenues for any additional brands acquired by ABG after entering into the Profit Participation Arrangement. As consideration for this arrangement, we paid to date approximately $33 million in cash and 1,680,214 shares of Class 2 common stock. We also agreed to pay approximately $83 million, in a combination of Class 2 common stock and up to $17 million in cash at ABG’s election, upon certain triggers relating to the regulatory status of THC in the United States, or receipt of $5 million in participation rights distributions from cannabis products containing THC outside the United States, in accordance with terms outlined in the arrangement. Further information can be found in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 4, “ABG Profit Participation Arrangement”. On January 24, 2020, we entered into an amendment related to the ABG Profit Participation Arrangement (refer to “Subsequent events”).
51
On February 15, 2019, we acquired Natura Naturals Holdings Inc. (“Natura”), a licensed cultivator under the Cannabis Act specializing in greenhouse cultivation. Our acquisition of Natura increases our capacity to supply high-quality branded cannabis products to the Canadian market. The purchase price of approximately $54 million consists of approximately $15 million in cash and 180,332 shares of Class 2 common stock issued on closing, approximately $20 million contingent consideration based on production levels, and effective settlement of pre-existing debt and previously held interest. We have paid $4.45 million in Class 2 common stock in relation to contingent consideration on December 2, 2019. Production levels for the remaining period were not expected to be achieved and as a result the fair value is nil at December 31, 2019. Further information can be found in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 3, “Business Combinations.”
On February 28, 2019, we acquired FHF Holdings Ltd. (“Manitoba Harvest”), a developer and distributor of a diverse portfolio of hemp-based natural food and wellness products that enables us to expand into the growing CBD product market in the United States. The purchase price of approximately $310 million consists of approximately $115 million in cash and 1,209,946 shares of Class 2 common stock issued on closing, approximately $37 million in cash and approximately $32 million in Class 2 common stock issued six months after closing, and approximately $29 million contingent consideration based on gross branded CBD product sales in the United States in 2019. Manitoba Harvest did not earn the $29 million contingent consideration as its CBD sales for 2019 did not achieve the thresholds. Further information can be found in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 3, “Business Combinations”.
On July 11, 2019, we acquired Smith & Sinclair Ltd. (“S&S”), which crafts edible candies, fragrances and creative consumables in the United Kingdom and enables us to develop CBD-infused edibles and beverages as well as alcohol-infused edibles for distribution in Canada, United States and Europe. The purchase consideration includes approximately $2 million in cash and 79,289 shares of Class 2 common stock issued on closing, and approximately $2 million contingent consideration based on revenue as well as the launch of CBD product in the United States and Europe or THC product in Canada by milestones in 2019 and 2020. The contingent consideration has been remeasured to $420 at December 31, 2019. Further information can be found in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 3, “Business Combinations”.
On August 28, 2019, we signed a definitive agreement to acquire 420 Investments Ltd. (“420”), an adult-use cannabis retail operator in Alberta, Canada. The purchase price consisted of $53 million in shares of Class 2 common stock on closing and up to $30 million of contingent consideration subject to the achievement of certain performance milestones by 420. On February 4, 2020, we served 420 with a notice of breach and a notice of termination pursuant to the Arrangement Agreement due to a material adverse change under such agreement.
On February 21, 2020, 420 Investments Ltd., as Plaintiff (“420”), filed a lawsuit against Tilray Inc. and High Park Shops Inc. (“High Park”), as Defendants, in Calgary, Alberta in the Court of Queen’s Bench of Alberta. Plaintiff alleges unlawful termination of the merger agreement, and seeks specific performance of the transactions, or, alternatively, monetary damages in the amount of C$120 million, plus C$20 million in aggravated damages. We believe that this claim is without merit, and we intend to defend this case vigorously, but there are no assurances as to its outcome at this early stage.
Business Segments
We report our operating results in two segments: (i) Cannabis (licensed), and (ii) Hemp (unlicensed). The business segments reflect how our operations are managed, how resources are allocated, how operating performance is evaluated by senior management and the structure of our internal financial reporting. Our Cannabis segment sales consists of adult-use, medical and bulk sales of cannabis under regulated licenses and sold to retail, wholesale, pharmacy, government, and direct to patient. Our Hemp segment sales consist of hemp seed, hemp foods, board spectrum hemp extract containing CBD that are sold in an unlicensed operation and sold to retail, wholesale and direct to consumers.
We evaluate the financial results of these segments focusing primarily on segment revenue and gross profit or loss. We utilize segment revenue, gross profit and segment income (loss) from operations because we believe they provide useful information for effectively allocating our resources between segments, evaluating the health of our business segments based on metrics that management can actively influence and gauging our investments.
52
Key Operating Metrics
We use the following key operating metrics to evaluate our business and operations, measure our performance, identify trends affecting our business, project our future performance and make strategic decisions.
Other companies, including companies in our industry, may calculate key operating metrics with similar names differently which may reduce their usefulness as comparative measures.
|
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|
|
2018 vs 2017 Change |
|
|||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
Qty/$ |
|
|
% |
|
|
Qty/$ |
|
|
% |
|
|||||||
|
Kilogram equivalents sold- cannabis |
|
|
35,380 |
|
|
|
6,478 |
|
|
|
3,024 |
|
|
|
28,902 |
|
|
|
446 |
% |
|
|
3,454 |
|
|
|
114 |
% |
|
Kilograms harvested - cannabis |
|
|
50,144 |
|
|
|
11,022 |
|
|
|
6,779 |
|
|
|
39,122 |
|
|
|
355 |
% |
|
|
4,243 |
|
|
|
63 |
% |
|
Thousand units sold - hemp products |
|
|
7,826 |
|
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
|
N/A |
|
|
N/A |
|
||||
|
Average net selling price per gram - cannabis |
|
$ |
7.90 |
|
|
$ |
6.63 |
|
|
$ |
6.52 |
|
|
$ |
1.27 |
|
|
|
19 |
% |
|
$ |
0.11 |
|
|
|
2 |
% |
|
Average cost per gram sold - cannabis |
|
$ |
2.36 |
|
|
$ |
3.73 |
|
|
$ |
2.84 |
|
|
$ |
(1.37 |
) |
|
|
(37 |
)% |
|
$ |
0.89 |
|
|
|
31 |
% |
|
Average gross selling price per unit -hemp products |
|
$ |
7.65 |
|
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
|
N/A |
|
|
N/A |
|
||||
Kilogram equivalents sold - cannabis. We sell two product categories: (1) dried cannabis, which includes whole flower and ground flower, and (2) cannabis extracts, which includes full-spectrum and purified oil drops and capsules. Cannabis extracts are converted to flower equivalent grams based on the type and number of dried cannabis grams required to produce extracted cannabis in the form of cannabis oils. This conversion ratio is based on the amount of active cannabinoids in the products rather than the volume of oil. For example, our 40mL oil drops are converted to five gram equivalents.
Total kilogram equivalents sold increased during 2019 compared to 2018, primarily due to increased adult-use, bulk and international medical sales. Total kilogram equivalents sold increased for 2018 from 2017, primarily due to increased bulk, adult-use, and international medical sales.
Kilograms harvested - cannabis. Kilograms harvested represents the weight of dried whole plants post-harvest, drying and curing. This operating metric is used to measure the production efficiency of our facilities and production team.
Total kilograms harvested increased during 2019 compared to 2018 by 355% primarily due to additional operational capacity through ramp up of new production facilities and the acquisition of Natura.
The High Park Farms facility, with 13 acres of greenhouse space, has been steadily increasing production and harvest yields since its first harvest in July 2018. High Park Farms has had consistent harvest yields every month during 2019.
It is our expectation that harvest quantities will continue to increase in 2020 with the improvement of operational efficiencies as our operational processes mature and capital expansion plans progress. Our current production and manufacturing footprint in Canada is approximately 1.0 million square feet. In addition, we announced increases for international export capacity with a new outdoor cultivation site in Portugal with Esporão, adding 20 hectares of outdoor cultivation space in Alentejo, Portugal to our existing 5 hectares of indoor and outdoor cultivation and 70,000 square feet of manufacturing, processing and research space at our European Union (EU) Campus in Cantanhede, Portugal, which expands our total footprint to 3.6 million square feet worldwide.
Total kilograms harvested increased for 2018 from 2017, primarily due to the additional operational capacity provided by our new facility High Park Farms brought into operations in 2018.
Thousand units sold – hemp. As a result of the acquisition of Manitoba Harvest, we sell hemp products such as shelled hemp seed, ground hemp, broad spectrum hemp extract containing CBD and hemp seed oil that are tracked by individual units.
This is our first financial year reporting hemp product sales and we have no sales data for 2018 or 2017.
Average net selling price per gram - cannabis. The average net selling price per gram is an indicator that shows our pricing trends over time on a gram equivalent basis and is impacted by sales mix, channel and product type. We exclude revenue associated with hemp products, accessories and freight sales to arrive at cannabis-related
53
revenue. We calculate average net selling price per gram by dividing cannabis-related revenue by kilogram equivalents sold. As Cannabis 2.0 products become a larger percentage of our mix, we may change this operating metric from per gram to unit measures, as the Cannabis 2.0 products include more value-added activities and the cannabis inputs will be a lower portion of the overall cost and value of the products.
The average net selling price per gram increased during 2019 compared to 2018 due to a shift in distribution channels and product mix. Since legalization, adult-use products increased to 51% of total revenue. Adult-use products are sold directly to wholesalers, which have lower sales price per gram and higher sales volume compared to medical channel sales. We expect our average selling price to increase over time as a result of two factors: 1) an increase in our sales mix of international medical cannabis due to GMP certifications at our Portugal facility and 2) an increase in new form factors for the Canadian adult use market that generally have higher price points. Shipments of the new form factors, including edible, beverage and vape products, began on December 16, 2019.
The average net selling price per gram increased for year ended December 31, 2018 from 2017, due to shift in mix demand of our products. In 2018 there was significant revenue growth for our extract products compared to dried flower. We introduced several new extract products which increased extract revenue from 20% in 2017 to 50% of cannabis-related revenue in 2018.
To determine the Canadian dollar average net selling price per gram range above, revenue and costs are converted using the average exchange rate during the reporting period. All input costs are individually converted by multiplying the United States dollar to Canadian dollar rate to determine the Canadian dollar amount.
Average cost per gram sold - cannabis. The average cost per gram sold measures the efficiency in our cultivation, manufacturing and fulfillment operations. We deduct hemp products, inventory valuation adjustments and the cost of sales related to accessories from total cost of sales to arrive at cannabis-related cost of sales. Cannabis-related cost of sales is then divided by total kilogram equivalents sold to calculate the average cost per gram sold. As Cannabis 2.0 products become a larger percentage of our mix, we may change this operating metric from per gram to unit measures, as the Cannabis 2.0 products include other input costs that can be a great portion of the unit cost than the cannabis ingredients.
The average cost per gram sold decreased during 2019 compared to 2018 primarily as a result of improved harvest quantities. In 2018, all the product sold were primarily from Tilray Canada, a GMP indoor grow facility, compared to three greenhouses in operation in 2019. Our greenhouse operations have lower overhead costs compared to indoor operations, driving improvement in our cost per gram. Moreover, from the third quarter of 2019 onwards, we had full operations for the High Park Processing Facility, with higher output and lower manufacturing costs compared to the temporary operation at High Park Farm used previously. Improvement in production costs in 2019 resulted in a 37% decrease in our average cost per gram from $3.73 per gram in 2018. We expect that this will continue to decrease.
The average cost per gram sold increased for 2018 from 2017, primarily due to sourcing product from other Licensed Producers as well as launching of our new cultivation facilities that were scaling up during 2018.
Average gross selling price per unit – hemp. The average gross selling price per unit is an indicator that shows our pricing trends over time on a unit basis for our hemp products and is impacted by sales mix, channel and product type. We exclude revenue associated with cannabis, accessories and freight sales to arrive at hemp product-related revenue. We calculate average gross selling price per unit by dividing hemp product-related revenue by units sold.
This is our first financial year reporting hemp product activity and we have no sales data for 2018 or 2017.
54
Factors Impacting our Business
We believe that our future success will primarily depend on the following factors:
Global medical market expansion. We believe that we have a significant opportunity to capitalize on cannabis markets globally as medical cannabis becomes legal in more markets. Medical cannabis is now authorized at the national or federal level in over 41 countries, and more than half of these countries have legalized or introduced significant reforms to their cannabis-use laws to broaden the scope of permitted use since the beginning of 2015. Over the past three years, we have established regional offices in Portugal, Germany, Australia and Chile, and have invested significant resources in personnel, partnerships and in-country sales and marketing to build the foundation for new and existing export channels. Our products have been made available in 15 countries, and we will continue to explore market expansion opportunities as more countries legalize medical cannabis.
Adult-use legalization in Canada. The legalization of adult-use cannabis in Canada represented a significant opportunity for us, and the expansion of the adult-use cannabis market on December 16, 2019 to include new form factors (edibles, beverages and vape products) represents another significant opportunity. We have invested, and will continue to invest, significant resources into production capacity, brand development, business development and corporate infrastructure so that we can serve the current and future adult-use market in Canada.
Expanding Household Penetration. We acquired the Manitoba Harvest business in February 2019, which is a leading provider of hemp seeds and related food products sold through over 16,000 locations in United States and Canada. The household penetration of hemp seed products is approximately 5% in Canada and about 1.5% penetration in the United States. The hemp seed products had been available in Canada for a longer period compared to the United States and we believe that creating awareness of the wellness benefits of the products provides an opportunity to increase household penetration of the products. Additionally, the household penetration of broad spectrum hemp oil containing CBD in the United States is at its early stages and we believe there is significant opportunity to expand penetration of this new product category.
Expanding capacity. At this early stage of the industry, we believe that it is beneficial to be vertically integrated and control our entire production process to generate consistency and quality on a large scale. As we expand into new and existing markets, we will need to invest significant resources into cultivation and production facilities, which may require us to raise additional capital.
New product innovation. We believe there is a significant market opportunity for non-combustible products as global medical markets mature. In certain developed cannabis markets, non-combustible products have surpassed dried flower on a market share basis. In 2019, 2018 and 2017, dried flower sales comprised 78%, 53% and 79% of cannabis-related revenue, respectively. We believe our success will depend on our ability to continually develop, introduce and expand non-combustible products and brands, which we believe will have higher gross profits compared to combustible products.
Critical Accounting Policies and Significant Judgments and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). A detailed discussion of our significant accounting policies can be found in Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 2, “Summary of Significant Accounting Policies”, and the impact and risks associated with our accounting policies are discussed throughout this Form 10‑K and in the Notes to the Consolidated Financial Statements. We have identified certain policies and estimates as critical to our business operations and the understanding of our past or present results of operations related to (i) revenue recognition, (ii) valuation of inventory, (iii) impairment of goodwill and indefinite life intangible assets, (iv) stock-based compensation, (v) business combinations and goodwill and (vi) leases. These policies and estimates are considered critical because they had a material impact, or they have the potential to have a material impact, on our consolidated financial statements and because they require us to make significant judgments, assumptions or estimates. We believe that the estimates, judgments and assumptions made when accounting for the items described below were reasonable, based on information available at the time they were made. Actual results could differ materially from these estimates.
55
|
|
(i) |
Revenue recognition |
On January 1, 2019, we adopted ASC 606, Revenue from Contracts with Customers (“ASC 606”), using the modified retrospective method to all contracts not completed as of January 1, 2019. Prior period amounts continue to be reported in accordance with pre-adoption standards. Refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7 of our 2018 Annual Report on Form 10-K for a discussion over critical accounting policies and significant judgments and estimates relating to revenue recognition for the periods prior to adoption of ASC 606.
Revenue is recognized when the control of the promised goods, through performance obligation, is transferred to the customer in an amount that reflects the consideration we expect to be entitled to in exchange for the performance obligations. We generate substantially all of our revenue from the sale of cannabis and hemp products through contracts with customers. Cannabis and hemp products are sold through various distribution channels. Revenue is recognized when the control of the goods is transferred to the customer, which occurs at a point in time, typically upon delivery to or receipt by the customer, depending on shipping terms. In determining the transaction price for the sale of goods, we consider the effects of variable consideration. Some contracts for the sale of goods may provide customers with a right of return, volume discount, bonuses for volume/quality achievement, or sales allowances. In addition, we may provide in certain circumstances, a retrospective price reduction to a customer based primarily on inventory movement. These items give rise to variable consideration. We use historical evidence, current information and forecasts to estimate the variable consideration. The requirements in ASC 606 on constraining estimates are applied to determine the amount of the variable consideration.
|
|
(ii) |
Valuation of inventory |
Inventory is comprised of raw materials, work-in-progress and finished goods. Cannabis and hemp costs include expenditures directly related to the manufacturing process as well as suitable portions of related production overheads, based on normal operating capacity. Refer to Part II, Item 8 of this Form 10-K in the Notes to Consolidated Financial Statements in Note 2, “Summary of Significant Accounting Policies” for further details on our inventory cost policy. At the end of each reporting period, we perform an assessment of inventory and record inventory valuation adjustments for excess and obsolete inventories based on our estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. A reserve is estimated to ensure the inventory balance at the end of the year reflects our estimates of product we expect to sell in the next year. Changes in the regulatory structure, lack of retail distribution locations or lack of consumer demand could result in future inventory reserves.
|
|
(iii) |
Impairment of goodwill and indefinite life intangible assets |
Goodwill and indefinite life intangible assets are tested for impairment annually, or more frequently when events or circumstances indicate that impairment may have occurred. As part of the impairment evaluation, we may elect to perform an assessment of qualitative factors. If this qualitative assessment indicates that it is more likely than not that the fair value of the indefinite-lived intangible asset or the reporting unit (for goodwill) is less than its carrying value, a quantitative impairment test to compare the fair value to the carrying value. An impairment charge is recorded if the carrying value exceeds the fair value. The assessment of whether an indication of impairment exists is performed at the end of each reporting period and requires the application of judgment, historical experience, and external and internal sources of information. We make estimates in determining the future cash flows and discount rates in the quantitative impairment test to compare the fair value to the carrying value.
|
|
(iv) |
Stock-based compensation |
We measure and recognize compensation expenses for stock options and restricted stock units (“RSUs”) to employees and non-employees on a straight-line basis over the vesting period based on their grant date fair values. We estimate the fair value of stock options on the date of grant using the Black-Scholes option pricing model. The fair value of RSUs is based on the share price as at the date of grant. For stock options and RSUs granted in 2018, prior to the Company’s initial public offering, the fair value of common stock at the date of grant was determined by the Board of Directors with assistance from third-party valuation specialists. We estimate forfeitures at the time of grant and revise these estimates in subsequent periods if actual forfeitures differ from those estimates.
56
Determining the estimated fair value of at the grant date requires judgment in determining the appropriate valuation model and assumptions, including the fair value of common shares on the grant date, risk-free rate, volatility rate, annual dividend yield and the expected term. Volatility is estimated by using the historical volatility of Tilray and, for periods prior to the Company’s initial public offering, other companies that we consider comparable and have trading and volatility history.
|
|
(v) |
Business combinations and goodwill |
We use judgment in applying the acquisition method of accounting for business combinations and estimates to value identifiable assets and liabilities at the acquisition date. Estimates are used to determine cash flow projections, including the period of future benefit, and future growth and discount rates, among other factors. The values allocated to the acquired assets and liabilities assumed affect the amount of goodwill recorded on acquisition. Fair value is typically estimated using an income approach, which is based on the present value of future discounted cash flows. Significant estimates in the discounted cash flow model include the discount rate, rate of future revenue growth and profitability of the acquired business and working capital effects. The discount rate considers the relevant risk associated with the business-specific characteristics and the uncertainty related to the ability to achieve projected cash flows. These estimates and the resulting valuations require significant judgment. Management engages third party experts to assist in the valuation of material acquisitions.
|
|
(vi) |
Leases |
On January 1, 2019, we adopted ASC 842, Leases (“ASC 842”), using the modified retrospective method which provides a method for recording existing leases at adoption using the effective date as its date of initial application. We also applied the practical expedient which allows entities to elect not to recast comparative periods presented. As a result of the adoption of ASC 842 on January 1, 2019, we have changed our accounting policy for leases. We consider the lease accounting policy under ASC 842 to be critical because the adoption has a material impact in our consolidated financial statements and requires us to make significant judgments, estimates and assumptions.
ASC 842 requires leases to be accounted for using a right-of-use model, which recognizes that, at the date of commencement, a lessee has a financial obligation to make lease payments to the lessor for the right to use the underlying asset during the lease term. The lessee recognizes a corresponding right-of-use asset related to this right. The most significant impact is the recognition of right-of-use assets and lease liabilities for operating leases, while the accounting for finance leases remains substantially unchanged.
We apply judgment in determining whether a contract contains a lease and if a lease is classified as an operating lease or a finance lease. We determine the lease term as the non-cancellable term of the lease, which may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. We have several lease contracts that include extension and termination options. We apply judgment in evaluating whether it is reasonably certain whether or not to exercise the option to renew or terminate the lease and estimate the lease term applicable to lease contracts. That is, we consider all relevant factors that create an economic incentive to exercise a renewal or termination. After the commencement date, we reassess the lease term if there is a significant event or change in circumstance that is within our control and affects our ability to exercise or not to exercise the option to renew or terminate. We also apply judgment in allocating the consideration in a contract between lease and non-lease components. We consider whether we can benefit from the right-of-use asset either on its own or together with other resources and whether the asset is highly dependent on or highly interrelated with another right-of-use asset.
Right of use assets and liabilities are recognized at the commencement date based on the present value of the lease payments over the term. As most of our leases do not provide an implicit rate, the incremental borrowing rate is used based on the information available at commencement date in determining the present value of lease payments. We make estimates in determining the incremental borrowing rates.
Transactions with Related Parties
Downstream merger
The Company was a wholly owned subsidiary of Privateer Holdings, Inc. (“Privateer Holdings”) prior to our Series A preferred stock financing and initial public offering. On December 12, 2019, we closed the merger of
57
Privateer Holdings, with and into a wholly owned subsidiary of Tilray pursuant to an Agreement and Plan of Merger and Reorganization with Privateer Holdings (the “Downstream Merger”). Prior to the close of the Downstream Merger on December 12, 2019, Privateer Holdings held more than 10% of our outstanding shares of Class 2 common stock and held 100% of our Class 1 common stock.
Pursuant to the Downstream Merger, all of Privateer Holdings capital stock outstanding of 58,333,333 shares of Tilray Class 2 common stock and 16,666,667 shares of Tilray Class 1 common stock immediately prior to the effective time of the Downstream Merger were cancelled and automatically converted solely into the right to receive the applicable portion of an aggregate shares of Tilray Class 2 common stock and shares of Tilray Class 1 common stock, inclusive of shares of Tilray Class 2 common stock held in escrow for contingent release to Privateer Holdings stockholders, issuable as consideration in Downstream Merger. In connection with the Downstream Merger, we exchanged the shares held by Privateer Holdings and issued the same value of shares to the underlying Privateer Holdings shareholders at a conversion rate of 1.07290. We did not pay any cash consideration in connection with the Downstream Merger and there was no impact on the balance sheets or statements of net loss and comprehensive loss. In accordance with our Related-Persons Transactions Policy, the Audit Committee of the Board of Directors, comprised solely of the independent directors, approved the Downstream Merger.
Acquisition of Smith & Sinclair Ltd. (“S&S”)
Pursuant to the Subversive Capital Alliance Agreement dated May 15, 2018 between Privateer Holdings and Subversive Capital, LLC (“Subversive”) as agent for Privateer Holdings, Subversive held 5,530 shares of S&S at a cost of £347.96 per share (£1,924 in aggregate) on July 11, 2019. Subversive is a company controlled by Michael Auerbach, who is a member of our Board of Directors. On July 11, 2019, the Subversive Capital Alliance Agreement was terminated in connection with our acquisition of S&S and we paid £1,924 in cash to Subversive for the 5,530 shares, which represented approximately 30% ownership of S&S and only the original cost basis in such shares. The cash paid to Subversive as part of the purchase consideration for the acquisition of S&S reflected no gain on its investment, thereby eliminating any economic conflict of interest or appearance thereof. In accordance with our Related-Persons Transactions Policy, the Audit Committee of the Board of Directors, comprised solely of the independent directors, approved the acquisition of S&S and the payment to Subversive.
Recent Accounting Pronouncements Not Yet Adopted
Refer to Note 2 included in Part II, Item 8 of this Form 10-K for a description of recent accounting pronouncements not yet adopted related to financial instruments, disclosure framework, income taxes and investments. We are currently evaluating the effect of adopting recent accounting pronouncements on our financial statements.
Components of Results of Operations
Revenue - cannabis
Revenue is comprised of sales to patients through the medical program under the Cannabis Regulations, wholesale of bulk and finished product to other Licensed Producers under the Cannabis Regulations, wholesale of finished product to provinces and provincially regulated distributors under the Cannabis Act and applicable provincial legislation, and export sales to third-party distributors, hospitals, pharmacies and patients. Our products currently include: whole flower, ground flower, broad-spectrum cannabis oils and capsules, purified cannabis oils and capsules and accessories. Revenue is net of incentives, after discounts, returns and allowances for our assurance program and veterans coverage program.
58
Revenue - hemp
Revenue is comprised of sales to retailers, wholesalers or direct to consumers of finished product and export sales to third-party distributors or retailers. Our products currently include hemp: seeds, protein powder, oil, granola, bars, milk, and broad spectrum hemp extract containing CBD in tincture and capsule form.
Cost of sales - cannabis
Cost of sales is mainly comprised of three categories: pre-harvest, post-harvest and shipment and fulfillment. Pre-harvest costs include labor and direct materials to grow cannabis, which includes water, electricity, nutrients, integrated pest management, growing supplies and allocated overhead. Post-harvest costs include costs associated with drying, trimming, blending, extraction, purification, quality testing and allocated overhead. Shipment and fulfillment costs include the costs of packaging, labelling, courier services and allocated overhead. Total cost of sales also includes cost of sales associated with accessories and inventory adjustments.
Cost of sales - hemp
Cost of sales is mainly comprised of three categories: seeds, packaging and co-packing. Seed costs include commodity cost from farmers, genetic seed cost to provide and manage contracted farmers, hulling and processing costs, including labor and overhead. Packaging costs include packaging materials, labor and overhead to running machinery. Co-packing cost are generally for products not manufactured by us directly and would include the all costs to product the products. Total cost of sales also includes cost of sales associated with managing the plants and inventory adjustments.
General and administrative expenses
General and administrative expenses consist of costs incurred in our corporate offices, primarily related to personnel costs, which include salaries, variable compensation and benefits. General and administrative expenses also include audit, legal, tax and professional fees and governance costs associated with operating as a public company. Other expenses in this category include general support services and commercialization costs associated with the expansion of our business in North America, Europe, Latin America and Asia Pacific. Also included in general and administrative expenses is tax equalization expenses for cross-border executives on stock benefits.
Sales and marketing expenses
Sales and marketing expenses primarily consist of personnel-related costs, including salaries, benefits, commissions for our employees engaged in physician and patient support, customer service and public relations. Sales and marketing expenses also include business development costs to support patient, physician, distributor, hospital, pharmacy and government relationships. Costs also include the development of branding, marketing, packaging and educational materials for adult-use market.
Research and development expenses
Research and development expenses consist of new product development, clinical trial expenses, study drug production, patient studies and surveys, pharmacokinetic studies, consultants and legal expenses. Research and development expenses also include process and systems engineering in both production and manufacturing aspects.
59
Depreciation and amortization expenses
Depreciation and amortization expenses represents the depreciation and amortization recognized on the Company’s tangible general office space and equipment and intangible assets during the year.
Impairment of assets
Impairment of assets represents impairment of indefinite-lived intangible assets and loans receivable.
Stock-based compensation expenses
Stock-based compensation expenses consists of non-cash costs for the fair value of compensation charges related to stock options and RSUs that are issued to employees, directors and consultants and amortized over the expected life of the instrument.
Acquisition-related (income) expenses, net
Acquisition-related (income) expenses, net represents transaction costs incurred during acquisitions and change in fair value of contingent consideration.
Loss from equity method investments
Loss from equity method investments represents the Company’s share of losses from the investments in entities over which the Company has significant influence but not a controlling financial interest and are accounted for using the equity method.
Foreign exchange (gain) loss, net
Foreign exchange gains and losses represent the gains or losses resulting from foreign currency transactions. Revenues and expenses denominated in foreign currencies were translated into United States dollars at the monthly average exchange rate for the period.
Interest expenses, net
Interest expenses, net is related to loans from convertible notes, interest on lease liabilities and other finance liabilities, and for prior years, a third-party mortgage on our Tilray Canada Ltd. property and Privateer Holdings debt facilities.
Finance income from ABG
Finance income from ABG represents interest income from ABG Profit Participation Arrangement recognized using the effective interest rate method in relation to the portion of the loan relating to cash paid to ABG.
Loss on disposal of property and equipment
Loss on disposal of property and equipment includes the difference between proceeds received and the net book value of disposed property and equipment.
Other income, net
Other income, net includes realized and unrealized gains and losses on equity investments measured at fair value (beginning January 1, 2019 after the adoption of ASU 2016-01), realized gains and losses on debt securities classified as available-for-sale, and other miscellaneous non-operating income and expenses.
60
Income taxes
We are subject to income taxes in the jurisdictions where we operate or otherwise have a taxable presence. Consequently, income tax expenses are driven by the allocation of taxable income to those jurisdictions. Activities performed in each jurisdiction impact the magnitude and timing of taxable events.
Results of Operations
Financial data is expressed in thousands of United States dollars.
Consolidated Statements of Net Loss Data
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Revenue (inclusive of excise duties of $13,136, $1,200 and $0, respectively) |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
20,538 |
|
|
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
121,892 |
|
|
|
24,294 |
|
|
|
8,544 |
|
|
Inventory valuation adjustments |
|
|
68,583 |
|
|
|
4,561 |
|
|
|
617 |
|
|
Gross (loss) profit |
|
|
(23,496 |
) |
|
|
14,275 |
|
|
|
11,377 |
|
|
General and administrative expenses |
|
|
81,968 |
|
|
|
29,461 |
|
|
|
7,499 |
|
|
Sales and marketing expenses |
|
|
61,084 |
|
|
|
15,366 |
|
|
|
7,164 |
|
|
Research and development expenses |
|
|
6,558 |
|
|
|
4,264 |
|
|
|
3,171 |
|
|
Stock-based compensation expenses |
|
|
31,842 |
|
|
|
20,988 |
|
|
|
139 |
|
|
Depreciation and amortization expenses |
|
|
11,607 |
|
|
|
1,598 |
|
|
|
902 |
|
|
Impairment of assets |
|
|
112,070 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition-related (income) expenses, net |
|
|
(31,427 |
) |
|
|
248 |
|
|
|
— |
|
|
Loss from equity method investments |
|
|
4,504 |
|
|
|
— |
|
|
|
— |
|
|
Operating loss |
|
|
(301,702 |
) |
|
|
(57,650 |
) |
|
|
(7,498 |
) |
|
Foreign exchange (gain) loss, net |
|
|
(5,944 |
) |
|
|
7,234 |
|
|
|
(1,363 |
) |
|
Interest expenses, net |
|
|
34,690 |
|
|
|
9,110 |
|
|
|
1,686 |
|
|
Finance income from ABG |
|
|
(764 |
) |
|
|
— |
|
|
|
— |
|
|
Loss on disposal of property and equipment |
|
|
2,436 |
|
|
|
190 |
|
|
|
— |
|
|
Other income, net |
|
|
(2,501 |
) |
|
|
(2,010 |
) |
|
|
(12 |
) |
|
Loss before income taxes |
|
|
(329,619 |
) |
|
|
(72,174 |
) |
|
|
(7,809 |
) |
|
Deferred income tax recoveries |
|
|
(8,847 |
) |
|
|
(4,485 |
) |
|
|
— |
|
|
Current income tax expenses |
|
|
397 |
|
|
|
34 |
|
|
|
— |
|
|
Net loss |
|
$ |
(321,169 |
) |
|
$ |
(67,723 |
) |
|
$ |
(7,809 |
) |
|
Other Financial Data |
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted EBITDA(1) |
|
$ |
(89,829 |
) |
|
$ |
(28,291 |
) |
|
$ |
(4,889 |
) |
|
(1) |
Adjusted EBITDA is a non-GAAP financial measure. For information on how we define and calculate Adjusted EBITDA, and a reconciliation of net loss to Adjusted EBITDA, refer to “Non-GAAP Financial Measures”. |
61
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
(as a percentage of revenue) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales - product costs |
|
|
73 |
% |
|
|
56 |
% |
|
|
42 |
% |
|
Cost of sales - inventory valuation adjustments |
|
|
41 |
% |
|
|
11 |
% |
|
|
3 |
% |
|
Gross (loss) profit |
|
|
(14 |
%) |
|
|
33 |
% |
|
|
55 |
% |
|
General and administrative expenses |
|
|
49 |
% |
|
|
68 |
% |
|
|
37 |
% |
|
Sales and marketing expenses |
|
|
37 |
% |
|
|
36 |
% |
|
|
35 |
% |
|
Research and development expenses |
|
|
4 |
% |
|
|
10 |
% |
|
|
15 |
% |
|
Stock-based compensation expenses |
|
|
19 |
% |
|
|
49 |
% |
|
|
1 |
% |
|
Depreciation and amortization expenses |
|
|
7 |
% |
|
|
4 |
% |
|
|
4 |
% |
|
Impairment of assets |
|
|
67 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
Acquisition-related (income) expenses, net |
|
|
(19 |
%) |
|
|
1 |
% |
|
|
0 |
% |
|
Loss from equity method investments |
|
|
3 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
Operating loss |
|
|
(181 |
%) |
|
|
(134 |
%) |
|
|
(37 |
%) |
|
Foreign exchange (gain) loss, net |
|
|
(4 |
%) |
|
|
17 |
% |
|
|
(7 |
%) |
|
Interest expenses, net |
|
|
21 |
% |
|
|
21 |
% |
|
|
8 |
% |
|
Finance income from ABG |
|
|
(0 |
%) |
|
|
0 |
% |
|
|
0 |
% |
|
Loss on disposal of property and equipment |
|
|
1 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
Other income, net |
|
|
(1 |
%) |
|
|
(5 |
%) |
|
|
(0 |
%) |
|
Loss before income taxes |
|
|
(197 |
%) |
|
|
(167 |
%) |
|
|
(38 |
%) |
|
Deferred income tax recoveries |
|
|
(5 |
%) |
|
|
(10 |
%) |
|
|
0 |
% |
|
Current income tax expenses |
|
|
0 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
Net loss |
|
|
(192 |
%) |
|
|
(157 |
%) |
|
|
(38 |
%) |
|
Other Financial Data |
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted EBITDA¹ |
|
|
(54 |
)% |
|
|
(66 |
)% |
|
|
(24 |
)% |
|
(1) |
Adjusted EBITDA is a non-GAAP financial measure. For information on how we define and calculate Adjusted EBITDA, and a reconciliation of net loss to Adjusted EBITDA, refer to “Non-GAAP Financial Measures”. |
Revenue
We evaluate revenue by product channel and category.
Revenue by product channel
(in thousands of United States dollars)
|
|
|
For the year ended December 31, |
|
|
For the year ended December 31, |
|||||||||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
$ Change |
|
|
% Change |
|
|
2018 |
|
|
2017 |
|
|
$ Change |
|
|
% Change |
|||||||
|
Cannabis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adult-use |
|
$ |
55,763 |
|
|
$ |
3,521 |
|
|
$ |
52,242 |
|
|
|
1484 |
% |
|
$ |
3,521 |
|
|
$ |
— |
|
|
$ |
3,521 |
|
|
N/A |
|
Canada - medical |
|
|
12,556 |
|
|
|
18,052 |
|
|
|
(5,496 |
) |
|
|
(30 |
)% |
|
|
18,052 |
|
|
|
19,642 |
|
|
|
(1,590 |
) |
|
N/A |
|
International - medical |
|
|
13,378 |
|
|
|
2,912 |
|
|
|
10,466 |
|
|
|
359 |
% |
|
|
2,912 |
|
|
|
896 |
|
|
$ |
2,016 |
|
|
N/A |
|
Bulk |
|
|
25,450 |
|
|
|
18,645 |
|
|
|
6,805 |
|
|
|
36 |
% |
|
|
18,645 |
|
|
|
— |
|
|
|
18,645 |
|
|
N/A |
|
Total cannabis revenue |
|
|
107,147 |
|
|
|
43,130 |
|
|
|
64,017 |
|
|
|
148 |
% |
|
|
43,130 |
|
|
|
20,538 |
|
|
|
22,592 |
|
|
N/A |
|
Hemp |
|
|
59,832 |
|
|
|
— |
|
|
|
59,832 |
|
|
N/A |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
Total revenue |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
123,849 |
|
|
|
287 |
% |
|
$ |
43,130 |
|
|
$ |
20,538 |
|
|
$ |
22,592 |
|
|
N/A |
|
Excise duties included in revenue |
|
$ |
13,136 |
|
|
$ |
1,200 |
|
|
$ |
11,936 |
|
|
N/A |
|
|
$ |
1,200 |
|
|
$ |
— |
|
|
$ |
1,200 |
|
|
N/A |
|
N/A: Not a meaningful percentage.
62
Revenue. Revenue increased 3.9 times to $167.0 million (C$220.9 million) for 2019 from $43.1 million (C$56.4 million) for 2018. The increase was driven by $64 million in the Cannabis segment and the addition of the hemp segment, through the acquisition of Manitoba Harvest in 2019 providing $59.8 million in revenues in 2019.
Revenue increased 2.1 times to $43.1 million (C$56.4 million) in 2018 from $20.5 million (C$26.6 million) in 2017. The increase was driven by the January 2018 launch of high CBD oil drops, which helped drive extract sales in Canada. Our extract products revenue was $21.2 million (C$26.4 million) in 2018, and $4.0 million (C$5.2 million) in 2017.
Cannabis. Cannabis segment revenue increased 148% to $107.1 million (C$138.1 million) from $43.1 million (C$56.4 million) for 2018. The increase was primarily driven by the Canadian adult-use market, which began in October of 2018, the acceleration of international medical sales and to a lesser extent an increase in bulk sales to other licensed producers. This growth was slightly offset by a decline in Canadian medical sales, which were the result of supply constraints in the first half of 2019. We expect continued growth in these channels in 2020, excluding bulk sales, which is expected to decline in 2020, primarily due to increased industry supply of cannabis oils. As all revenue in 2018 related to the Cannabis segment, refer to the overall revenue analysis above for the change from 2017 to 2018.
Hemp. Hemp segment revenues began upon the acquisition of Manitoba Harvest on February 28, 2019 and contributed $59.8 million (C$79.2 million) in revenues in 2019. Manitoba Harvest also launched a line of broad spectrum hemp oil including CBD in the United States in 2019, which are available in over 500 locations. We expect continued growth in the Hemp segment driven by increases in household penetration as well as increased placement of the broader hemp product portfolio in 2020. This is our first financial year reporting hemp product activity and we have no revenue data for 2018 or 2017.
Revenue by product category
(in thousands of United States dollars)
|
|
|
For the year ended December 31, |
|
|
For the year ended December 31, |
|
||||||||||||||||||||||||||
|
Test |
|
2019 |
|
|
2018 |
|
|
$ Change |
|
|
% Change |
|
|
2018 |
|
|
2017 |
|
|
$ Change |
|
|
% Change |
|
||||||||
|
Dried cannabis |
|
|
82,753 |
|
|
|
21,674 |
|
|
|
61,079 |
|
|
|
282 |
% |
|
|
21,674 |
|
|
|
16,260 |
|
|
|
5,414 |
|
|
|
33 |
% |
|
Cannabis extracts |
|
|
24,139 |
|
|
|
21,179 |
|
|
|
2,960 |
|
|
|
14 |
% |
|
|
21,179 |
|
|
|
3,965 |
|
|
|
17,214 |
|
|
|
434 |
% |
|
Hemp products |
|
|
59,832 |
|
|
|
— |
|
|
|
59,832 |
|
|
N/A |
|
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
|||
|
Accessories and other |
|
|
255 |
|
|
|
277 |
|
|
|
(22 |
) |
|
|
(8 |
)% |
|
|
277 |
|
|
|
313 |
|
|
|
(36 |
) |
|
|
(12 |
)% |
|
Total revenue |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
123,849 |
|
|
|
287 |
% |
|
$ |
43,130 |
|
|
$ |
20,538 |
|
|
$ |
22,592 |
|
|
|
110 |
% |
|
Excise duties included in revenue |
|
$ |
13,136 |
|
|
$ |
1,200 |
|
|
$ |
11,936 |
|
|
N/A |
|
|
$ |
1,200 |
|
|
$ |
— |
|
|
$ |
1,200 |
|
|
N/A |
|
||
N/A: Not a meaningful percentage.
We additionally analyze our sales mix by dried cannabis, extracts, hemp and accessories. Dried cannabis represented 77% of cannabis revenue mix for 2019 and 50% for 2018. The increase in dried cannabis was driven by the adult-use market legalization for a full year, which only allowed a limited number of form factors in 2019. Cannabis extracts represented 23% of cannabis revenue mix in 2019 compared to 49% in 2018. Extracts generally provide for higher margins and the reduction in mix was primarily due to legalization of adult-use cannabis in Canada for a full year in 2019, which limited extract products based on the regulatory framework. We expect extract products to increase as a percent of overall sales in future years, as the regulatory framework allows for more extract derivative products to be sold beginning in December 2019. Hemp products represented 36% of revenues in 2019 for the first year, driven by our acquisition of Manitoba Harvest in February 2019. We expect our cannabis products to grow at a faster rate than our other product categories due to the development of the Canadian adult-use market as well as growing international medical markets.
Dried cannabis represented 50% of cannabis revenue mix for 2018 and 79% for 2017 and extracts represented 49% and 19%, respectively for 2018 and 2017. The change in product mix was due to a significant increase in extract product capacities by the company for the medical market, which allowed extract products to grow at a faster rate than the dried cannabis growth rate.
63
Cost of sales and gross margin – Cannabis
(in thousands of United States dollars)
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|
|
2018 vs 2017 Change |
|
|||||||||||||||||||
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
$ |
|
|
% |
|
|
$ |
|
|
% |
|
|||||||
|
Cost of sales - product costs |
$ |
85,917 |
|
|
$ |
24,294 |
|
|
$ |
8,544 |
|
|
$ |
61,622 |
|
|
|
254 |
% |
|
$ |
15,750 |
|
|
|
184 |
% |
|
Cost of sales - inventory valuation adjustments |
|
63,532 |
|
|
|
4,561 |
|
|
|
617 |
|
|
|
58,971 |
|
|
N/A |
|
|
|
3,944 |
|
|
N/A |
|
||
|
Total Cannabis cost of sales |
$ |
149,449 |
|
|
$ |
28,855 |
|
|
$ |
9,161 |
|
|
$ |
120,593 |
|
|
N/A |
|
|
$ |
19,694 |
|
|
N/A |
|
||
|
Gross profit |
$ |
(42,302 |
) |
|
$ |
14,275 |
|
|
$ |
11,377 |
|
|
$ |
(56,577 |
) |
|
N/A |
|
|
$ |
2,898 |
|
|
|
25 |
% |
|
|
Gross profit (excluding inventory valuation adjustments)(1) |
|
21,231 |
|
|
|
18,836 |
|
|
|
11,994 |
|
|
|
2,395 |
|
|
N/A |
|
|
|
6,842 |
|
|
|
57 |
% |
|
|
Gross margin percentage |
|
(39 |
%) |
|
|
33 |
% |
|
|
55 |
% |
|
|
(72 |
%) |
|
N/A |
|
|
|
(22 |
%) |
|
|
(40 |
%) |
|
|
Gross margin percentage (excluding inventory valuation adjustments)(1) |
|
20 |
% |
|
|
44 |
% |
|
|
58 |
% |
|
|
(24 |
%) |
|
N/A |
|
|
|
(15 |
%) |
|
|
(25 |
%) |
|
N/A: Not a meaningful percentage.
|
(1) |
Gross profit (excluding inventory valuation adjustments) and gross margin percentage (excluding inventory valuation adjustments) are non-GAAP financial measures. For information on how we define and calculate these non-GAAP financial measures, refer to “Non-GAAP Financial Measures”. |
Cost of sales. Cost of sales increased in 2019 from the comparable period in 2018 primarily due to greater sales, the addition of our acquisition and start-up of Natura, the start-up of High Park Farms and Portugal cultivation facilities. Additionally, we purchased third-party cannabis supply at higher prices than we are able to produce ourselves. We incurred inventory valuation adjustments primarily for cannabis oil products, which did not have the sell through opportunity, as many cannabis derivative products were not available for sale under the regulatory framework until December 2019, resulting in a significant accumulation of cannabis oil and cannabis by-product to be converted into oil. The total inventory valuation adjustment of $63.5 million (C$82.6 million) reflects our estimate of excess product based on current sales forecasts, which have been reduced from previous estimates due to the slower than expected transition of the Canadian adult use market than expected. We do not expect material future inventory valuation adjustments; however, changes in the regulatory structure or lack of retail distribution locations or lack of consumer demand could result in future inventory valuation adjustments.
Cost of sales increased in 2018 from the comparable period in 2017 primarily due to increased sales, a shift towards a mix of high THC and high CBD cultivars that have lower yields along with procurement of third-party supply. In mid-2018, we had our initial harvest of product at our High Park Farms facility and manufactured product.
Gross margin. Gross margin of (39%) in 2019 decreased from the comparable period in 2018 primarily due to inventory valuation adjustments. Excluding inventory valuation adjustments, gross margin was 20%, which was impacted by the change in product mix from 2018 as well as the need to purchase high priced third-party supply for the Canadian adult-use market. We expect third-party supply pricing will continue to reduce in 2020, plus we expect to benefit from reduced costs at our own facilities that were scaling in 2019, which are expected to result in future gross margin improvements.
Gross margin percentage decreased in 2018 from the comparable period in 2017 primarily due to our post-harvest costs per gram increasing due to procurement of third-party supply and low yields and low through put during the scaling of new facilities.
64
Cost of sales and gross margin – Hemp
(in thousands of United States dollars)
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|||||||||||||
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
$ |
|
|
% |
||||
|
Cost of sales - product costs |
$ |
35,976 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
35,976 |
|
|
N/A |
|
Cost of sales - inventory valuation adjustments |
|
5,051 |
|
|
|
— |
|
|
|
— |
|
|
|
5,051 |
|
|
N/A |
|
Total Hemp cost of sales |
$ |
41,026 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
41,026 |
|
|
N/A |
|
Gross profit |
$ |
18,806 |
|
|
|
— |
|
|
|
— |
|
|
$ |
18,806 |
|
|
N/A |
|
Gross profit (excluding inventory valuation adjustments and purchase accounting value step-up)(1) |
|
25,898 |
|
|
|
— |
|
|
|
— |
|
|
|
25,898 |
|
|
N/A |
|
Gross margin percentage |
|
31 |
% |
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
|
Gross margin percentage (excluding inventory valuation adjustments and purchase accounting value step-up)(1) |
|
43 |
% |
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
N/A: Not a meaningful percentage.
|
(1) |
Gross profit (excluding inventory valuation adjustments and purchase accounting value step-up) and gross margin percentage (excluding inventory valuation adjustments and purchase accounting value step-up) are non-GAAP financial measures. For information on how we define and calculate these non-GAAP financial measures, refer to “Non-GAAP Financial Measures”. |
Cost of sales. Cost of sales were $41 million in 2019 which are comprised of cost of production for our products. We incurred inventory valuation adjustments primarily for certain CBD inventory and some protein powder in the amount of $5.1 million (C$6.6 million). The development of the United States CBD market has progress at a slower pace than expected due to the lack of clarity from the United States Food and Drug Administration, that indicated that it will take some time for them to complete the regulatory framework for CBD products. Additionally, we reported non-cash charge due to the one-time purchase accounting step-up in inventory value in the amount of $2.0 million for 2019. We do not expect material future inventory valuation adjustments; however, changes in the regulatory structure or lack of consumer demand could result in future inventory valuation adjustments.
This is our first financial year reporting hemp product activity and we have no cost of sales data for 2018 or 2017.
Gross margin. Gross margin was 31% in 2019 and excluding inventory valuation adjustments and purchase accounting inventory step-up, gross margin was 43%. We expect gross margins to increase to the 43% - 45% range in 2020.
This is our first financial year reporting hemp product activity and we have no gross margin data for 2018 or 2017.
65
Operating expenses
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|
|
|
2018 vs 2017 Change |
|
|||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
$ |
|
|
% |
|
|
|
$ |
|
|
% |
|
|||||||
|
General and administrative expenses |
|
$ |
81,968 |
|
|
$ |
29,461 |
|
|
$ |
7,499 |
|
|
$ |
52,507 |
|
|
|
178 |
% |
|
|
$ |
21,962 |
|
|
|
293 |
% |
|
Sales and marketing expenses |
|
|
61,084 |
|
|
|
15,366 |
|
|
|
7,164 |
|
|
|
45,718 |
|
|
|
298 |
|
|
|
|
8,202 |
|
|
|
114 |
|
|
Research and development expenses |
|
|
6,558 |
|
|
|
4,264 |
|
|
|
3,171 |
|
|
|
2,294 |
|
|
|
54 |
|
|
|
|
1,093 |
|
|
|
34 |
|
|
Stock-based compensation expenses |
|
|
31,842 |
|
|
|
20,988 |
|
|
|
139 |
|
|
|
10,854 |
|
|
|
52 |
|
|
|
|
20,849 |
|
|
N/A |
|
|
|
Depreciation and amortization expenses |
|
|
11,607 |
|
|
|
1,598 |
|
|
|
902 |
|
|
|
10,009 |
|
|
N/A |
|
|
|
|
696 |
|
|
N/A |
|
||
|
Impairment of assets |
|
|
112,070 |
|
|
|
— |
|
|
|
— |
|
|
|
112,070 |
|
|
N/A |
|
|
|
|
— |
|
|
|
— |
|
|
|
Acquisition-related (income) expenses, net |
|
|
(31,427 |
) |
|
|
248 |
|
|
|
— |
|
|
|
(31,675 |
) |
|
N/A |
|
|
|
|
248 |
|
|
N/A |
|
||
|
Loss from equity method investments |
|
|
4,504 |
|
|
|
— |
|
|
|
— |
|
|
|
4,504 |
|
|
N/A |
|
|
|
|
— |
|
|
|
— |
|
|
|
Total |
|
$ |
278,206 |
|
|
$ |
71,925 |
|
|
$ |
18,875 |
|
|
$ |
206,281 |
|
|
|
287 |
% |
|
|
$ |
53,050 |
|
|
|
281 |
% |
|
(as a percentage of revenue) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses |
|
|
49 |
% |
|
|
68 |
% |
|
|
37 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing expenses |
|
|
37 |
% |
|
|
36 |
% |
|
|
35 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
4 |
% |
|
|
10 |
% |
|
|
15 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation expenses |
|
|
19 |
% |
|
|
49 |
% |
|
|
1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization expenses |
|
|
7 |
% |
|
|
4 |
% |
|
|
4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of assets |
|
|
67 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition-related (income) expenses, net |
|
|
(19 |
%) |
|
|
1 |
% |
|
|
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from equity method investments |
|
|
3 |
% |
|
|
0 |
% |
|
|
0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
167 |
% |
|
|
167 |
% |
|
|
92 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N/A: Not a meaningful comparison
General and administrative. General and administrative expenses increased in 2019 and 2018 as compared to prior years due to costs incurred for the startup of the operations of our subsidiaries High Park Farms, Ltd., High Park Holdings, Ltd. and Tilray Portugal Unipessoal, Lda., higher employee costs to support a larger business from the acquisition of Manitoba Harvest, increases in professional fees related to legal, audit, human resources and IT services to support our growth, and public company costs. Moreover, during the year ended 2019, we incurred $6.59 million in non-recurring costs. We expect continued increase in general and administrative expenses as we build out our global infrastructure. In 2019, we also incurred $8.4 million related to tax equalization expenses for cross-border executives on stock benefits.
General and administrative expenses increased in 2018 and 2017 as compared to prior years primarily due to increases in professional fees related to legal, audit and human resources, IT services to support our growth, public company costs and expansion plans and costs incurred for the startup of the operations of our subsidiaries High Park Farms, Ltd., High Park Holdings, Ltd. and Tilray Portugal Unipessoal, Lda.
Sales and marketing. Sales and marketing expenses increased in 2019 from the comparable period in 2018 primarily due to the acquisition of Manitoba Harvest, development of our Canadian adult-use sales and marketing team, and the development of our European leadership team, as we expand our international presence. In addition, High Park developed a comprehensive portfolio of new brands and products for next phase of the adult-use market. The expanded broad-based portfolio includes innovative cannabis products and formats, including edibles, vape products and CBD beverages. We expect continued increase in sales and marketing expenses as we launch new products.
Sales and marketing expenses increased in 2018 from the comparable period in 2017 primarily due to development of our Canadian adult-use sales and marketing team and the increase in headcount in Tilray Deutschland GmbH.
Research and development. Research and development expenses increased year over year in 2019 and 2018 as compared to the prior years, primarily due to our continued support in advancing cannabinoid-based science to
66
further understand the potential benefits of medical cannabis as a treatment. During the year ended December 31, 2019, we supported two new clinical research studies with New York University School of Medicine. The two studies will test the efficacy of CBD to treat patients suffering from alcohol use disorder (“AUD”) and patients suffering from AUD comorbid with post-traumatic stress disorder (“PTSD”). We expect our research and development expense to increase as we pursue more clinical trial opportunities and continue to invest in developing non-combustible delivery formats and formulations.
Research and development expenses increased in 2018 compared to 2017, primarily due to an increase of new product initiatives and the production of drugs for clinical trials.
Stock-based compensation expenses. Stock-based compensation expenses increased in 2019 as compared to 2018 primarily due to the issuance of stock options and restricted stock units granted under the 2018 Equity Incentive Plan for more employees to support a larger business.
Stock-based compensation expenses increased in 2018 as compared to 2017 primarily due to the issuance of stock options, restricted stock units and certain IPO contingency triggers related to performance-based awards granted under the 2018 Equity Incentive Plan.
Depreciation and Amortization. Depreciation and amortization expenses increased in 2019 compared to 2018 primarily due to increased investment in new cultivation and production facilities as well as investment in acquisitions, resulting in greater fixed assets as well as intangible assets. We expected continued increases in depreciation and amortization as we continue to invest in capital projects to expand our capacity.
Depreciation and amortization increased in 2018 from the comparable period in 2017 due to capital expenditures for expansion of cultivation and production assets.
Impairment of assets. An impairment of $112.1 million was recognized in 2019 primarily due to the analysis of future cash flows for our ABG Profit Participation Agreement, which have been reduced due to the delayed clarity from the FDA regarding CBD products in the United States. The impairment conclusion was made in connection with the preparation and review of the financial statements included in this Annual Report on Form 10-K.
Acquisition-related (income) expenses, net. Acquisition-related (income) expenses resulted in income in 2019 from the comparable period expenses in 2018 due to a change in fair value of contingent consideration for the acquisitions of Manitoba Harvest, Natura and S&S based on actual results to date and forecasts for the remainder of the earn-out periods. The reductions in the fair value of contingent consideration offset acquisition-related expenses incurred during the year.
Non-operating income and expenses
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|
|
|
2018 vs 2017 Change |
||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
$ |
|
|
% |
|
|
|
$ |
|
|
% |
||||||
|
Foreign exchange (gain) loss, net |
|
$ |
(5,944 |
) |
|
$ |
7,234 |
|
|
$ |
(1,363 |
) |
|
$ |
(13,178 |
) |
|
|
-182 |
% |
|
|
$ |
8,597 |
|
|
N/A |
|
Interest expenses, net |
|
|
34,690 |
|
|
|
9,110 |
|
|
|
1,686 |
|
|
|
25,580 |
|
|
|
281 |
% |
|
|
|
7,424 |
|
|
N/A |
|
Finance income from ABG |
|
|
(764 |
) |
|
|
— |
|
|
|
— |
|
|
|
(764 |
) |
|
N/A |
|
|
|
|
— |
|
|
N/A |
|
|
Loss on disposal of property and equipment |
|
|
2,436 |
|
|
|
190 |
|
|
|
— |
|
|
|
2,246 |
|
|
N/A |
|
|
|
|
190 |
|
|
N/A |
|
|
Other income, net |
|
|
(2,501 |
) |
|
|
(2,010 |
) |
|
|
(12 |
) |
|
|
(491 |
) |
|
|
24 |
% |
|
|
|
(1,998 |
) |
|
N/A |
|
Total |
|
$ |
27,917 |
|
|
$ |
14,524 |
|
|
$ |
311 |
|
|
$ |
13,393 |
|
|
|
92 |
% |
|
|
$ |
14,213 |
|
|
N/A |
Foreign exchange (gain) loss, net. Foreign exchange in 2019 was a $5.9 million gain compared to $7.2 million loss in 2018. As we hold a significant portion of balances in Canadian dollars, the appreciation of foreign exchange rates between Canadian dollars and United States dollars drove the foreign exchange gain in 2019.
Foreign exchange in 2018 was $7.2 million loss compared to $1.4 million gain in 2017. The loss in 2018 was driven by significantly larger cash balances held in Canadian currency and the rapid decline in Canadian currency compared to United States currency.
67
Interest expenses, net. Interest expenses, net in 2019 was $34.7 million compared to $9.1 million in 2018. The increase in expense in 2019 from 2018 was primarily due to the addition of the $475 million in convertible notes that were issued in October 2018. In 2018 interest expense was related to loans from a third-party mortgage on Tilray Canada, Ltd. and Privateer Holdings debt facilities.
Interest expense in 2018 was $9.1 million compared to $1.7 million in 2017. The increase in expense in 2018 from 2017 was primarily due to the addition of the $475 million in convertible notes that were issued in October 2018. In 2017, interest expense was related to loans from a third-party mortgage on Tilray Canada, Ltd. and Privateer Holdings debt facilities.
Finance income from ABG. Finance income from ABG represents interest income from ABG Profit Participation Arrangement which was entered into in 2019.
Loss on disposal of property and equipment.Loss on disposal of property and equipment in 2019 was $2.4 million compared to $0.2 million in 2018. The loss in 2019 was due to discontinued construction of certain facilities.
Other income, net. Other income, net increased in 2019 compared to 2018 due to gains on the sale of short-term investments during the year ended December 31, 2019, offset by unrealized losses on equity investments recorded at fair value. In 2018, prior to the adoption of ASU 2016-01, unrealized gains and losses on equity investments recorded at fair value were recorded to other comprehensive income.
Other income, net increased by $2.0 million in 2018 compared to 2017 as we did not hold any short-term and long-term investments in 2017.
Net loss and Adjusted EBITDA(1)
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|
2019 vs 2018 Change |
|
|
2018 vs 2017 Change |
|
|||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
$ |
|
|
% |
|
|
$ |
|
|
% |
|
|||||||
|
Net loss |
|
$ |
(321,169 |
) |
|
$ |
(67,723 |
) |
|
$ |
(7,809 |
) |
|
$ |
(253,446 |
) |
|
|
374 |
% |
|
$ |
(59,914 |
) |
|
|
767 |
% |
|
Adjusted EBITDA(1) |
|
$ |
(89,829 |
) |
|
$ |
(28,291 |
) |
|
$ |
(4,889 |
) |
|
$ |
(61,538 |
) |
|
|
218 |
% |
|
$ |
(23,402 |
) |
|
|
479 |
% |
|
(1) |
Adjusted EBITDA is a non-GAAP financial measure. For information on how we define and calculate Adjusted EBITDA, and a reconciliation of net loss to Adjusted EBITDA, refer to “Non-GAAP Financial Measures |
Net loss increased in 2019 from the comparable periods in 2018 and 2017 primarily due to the impairment of assets, inventory valuation adjustments, an increase in operating expenses related to continued growth, the expansion of our international teams, interest related to our convertible notes, and the results of the Manitoba Harvest and Natura businesses acquired.
Adjusted earnings before interest, tax and depreciation (“Adjusted EBITDA”) decreased in 2019 from 2018 and 2017 primarily due increase in operating expenses related to continued growth as well as expansion and development into new markets.
Non-GAAP Financial Measures
To supplement our financial statements, which are prepared and presented in accordance with United States generally accepted accounting principles, or GAAP, we use certain measures, as described below, to understand and evaluate our operating performance. These measures, which may be different than similarly titled measures used by other companies, is presented to help investors’ overall understanding of our financial performance and should not be considered a substitute for, or superior to, the financial information prepared and presented in accordance with GAAP.
68
Adjusted EBITDA
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Adjusted EBITDA reconciliation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(321,169 |
) |
|
$ |
(67,723 |
) |
|
$ |
(7,809 |
) |
|
Inventory valuation adjustments |
|
|
68,583 |
|
|
|
4,561 |
|
|
|
617 |
|
|
Depreciation and amortization expenses |
|
|
15,849 |
|
|
|
3,562 |
|
|
|
1,853 |
|
|
Stock-based compensation expenses |
|
|
31,842 |
|
|
|
20,988 |
|
|
|
139 |
|
|
Other stock-based compensation related expenses |
|
|
8,411 |
|
|
|
— |
|
|
|
— |
|
|
Impairment of assets |
|
|
112,070 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition-related (income) expenses, net |
|
|
(31,427 |
) |
|
|
248 |
|
|
|
— |
|
|
Loss from equity method investments |
|
|
4,504 |
|
|
|
— |
|
|
|
— |
|
|
Foreign exchange (gain) loss, net |
|
|
(5,944 |
) |
|
|
7,234 |
|
|
|
(1,363 |
) |
|
Interest expenses, net |
|
|
34,690 |
|
|
|
9,110 |
|
|
|
1,686 |
|
|
Finance income from ABG |
|
|
(764 |
) |
|
|
— |
|
|
|
— |
|
|
Loss on disposal of property and equipment |
|
|
2,436 |
|
|
|
190 |
|
|
|
— |
|
|
Other income, net |
|
|
(2,501 |
) |
|
|
(2,010 |
) |
|
|
(12 |
) |
|
Amortization of inventory step-up |
|
|
2,041 |
|
|
|
— |
|
|
|
— |
|
|
Deferred income tax recoveries |
|
|
(8,847 |
) |
|
|
(4,485 |
) |
|
|
— |
|
|
Current income tax expenses |
|
|
397 |
|
|
|
34 |
|
|
|
— |
|
|
Adjusted EBITDA |
|
$ |
(89,829 |
) |
|
$ |
(28,291 |
) |
|
$ |
(4,889 |
) |
Adjusted EBITDA should not be considered in isolation from, or as a substitute for, net loss. There are a number of limitations related to the use of Adjusted EBITDA as compared to net loss, the closest comparable GAAP measure. Adjusted EBITDA excludes:
|
• |
Non-cash inventory valuation adjustments; |
|
• |
Non-cash depreciation and amortization expenses and, although these are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future; |
|
• |
Stock-based compensation expenses, which has been, and will continue to be for the foreseeable future, a significant recurring expense in our business and an important part of our compensation strategy; |
|
• |
Other stock-based compensation expenses included within general and administrative expenses, relating to tax equalization expenses for cross-border executives on stock benefits. |
|
• |
Non-cash impairment charges, as the charges are not expected to be a recurring business activity; |
|
• |
Acquisition and integration expenses and changes in the fair value of contingent consideration, which vary significantly by transaction and are excluded to evaluate ongoing operating results; |
|
• |
Non-cash loss from equity method investments; |
|
• |
Non-cash foreign exchange gains or losses, which accounts for the effect of both realized and unrealized foreign exchange transactions. Unrealized gains or losses represent foreign exchange revaluation of foreign denominated monetary assets and liabilities; |
|
• |
Interest expenses, finance income from ABG, loss on disposal of property and equipment and other income, net, to reflect ongoing operating activities; |
|
• |
Amortization of purchase accounting step-up in inventory value included in costs of sales - product costs; and |
|
• |
Current and deferred income tax expenses and recoveries, which could be a significant recurring expense or recovery in our business in the future and reduce or increase cash available to us. |
69
Gross profit (excluding inventory valuation adjustments)
Gross profit (excluding inventory valuation adjustments) is a non-GAAP measure calculated in the Cannabis segment. It is calculated as revenue less cost of sales, adjusted to add back inventory valuation adjustments.
Gross margin percentage (excluding inventory valuation adjustments)
Gross margin percentage (excluding inventory valuation adjustments) is a non-GAAP measure calculated in the Cannabis segment calculated as the gross profit (excluding inventory valuation adjustments), as defined above, divided by revenue.
Gross profit (excluding inventory valuation adjustments and purchase accounting value step-up)
Gross profit (excluding inventory valuation adjustments and purchase accounting value step-up) is a non-GAAP measure calculated in the Hemp segment. It is calculated as revenue less cost of sales, adjusted to add back inventory valuation adjustments and purchase accounting value step-up of $2.0 million for the year ending December 31, 2019 (2018 and 2017 - $0).
Gross margin percentage (excluding inventory valuation adjustments and purchase accounting value step-up)
Gross margin percentage (excluding inventory valuation adjustments and purchase accounting value step-up) is a non-GAAP measure calculated in the Hemp segment calculated as the gross profit (excluding inventory valuation adjustments and purchase accounting value step-up), as defined above, divided by revenue.
Income Taxes
Provision for income taxes, effective tax rate and statutory federal income tax rate for 2019, 2018 and 2017 were as follows:
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Provision for income taxes |
|
$ |
(8,450 |
) |
|
$ |
(4,451 |
) |
|
$ |
— |
|
|
Effective tax rate |
|
|
2.57 |
% |
|
|
6.17 |
% |
|
|
0.00 |
% |
|
Statutory federal income tax rate |
|
|
21.00 |
% |
|
|
21.00 |
% |
|
|
35.00 |
% |
On December 22, 2017, the United States enacted the Tax Cuts and Jobs Act (the “Act”), which significantly changed United States tax law. The Act lowered the United States statutory federal income tax rate from 35% to 21% effective January 1, 2018.
The Company’s effective tax rate for 2019 was lower than the 2019 United States tax rate primarily due to minimal taxes in foreign tax jurisdictions and no United States current taxes due to net operation losses. Income tax benefit in 2019 was $8.5 million compared to $4.5 million in 2018. The increase in tax benefit in 2019 from 2018 was primarily due to tax attributes related to acquisitions in 2019.
The Company’s effective tax rate for 2018 was lower than the 2018 United States tax rate primarily due to minimal taxes in foreign tax jurisdictions and no United States current taxes due to net operation losses. Income tax benefit in 2018 was $4.5 million compared to $0 in 2017. The increase in tax benefit in 2018 from 2017 was primarily due to the recognition in 2018 of deferred taxable differences that will reverse in future years resulting in recognition of tax benefit from operating losses.
As of December 31, 2019, we had United States net operating loss carryforwards of approximately $27 million that can be carried forward indefinitely and limited in annual use to 80% of current year taxable income. We have Canadian net operating loss carry-forwards of approximately $199 million that can be carried forward 20 years and begin to expire in 2028. We believe that it is more-likely-than-not that the benefit from certain United States and foreign net operating loss carryforwards will not be realized. In recognition of this risk, the change in the total valuation allowance was an increase of $37 million and $6 million for the years ended December 31, 2019 and 2018,
70
respectively. We continually evaluate the amount of the valuation allowance, if any, by assessing the realizability of deferred tax assets.
Liquidity and Capital Resources
As at December 31, 2019, we had cash and cash equivalents of $97 million, which were held for working capital and general corporate purposes.
In February and March 2018, we issued 7,794,042 shares of Series A preferred stock at $7.10 per share (C$8.90 per share) in exchange for cash gross proceeds of approximately $55.0 million (C$69.1 million) from third-party institutional investors. On our IPO, all shares of the outstanding Series A preferred stock automatically converted into 7,794,042 shares of Class 2 common stock on a one-for-one basis.
In July 2018, we completed our IPO, whereby 10,350,000 shares of our Class 2 common stock were sold at a price of $17.00 per share (C$22.45 per share), which included 1,350,000 shares sold pursuant to the underwriters’ option to purchase additional shares. We received net proceeds of $163.7 million after deducting the underwriting discount.
In October 2018, we entered an indenture relating to the issuance of $475.0 million aggregate principal amount of 5.00% convertible notes, which included $25.0 million pursuant to the underwriters’ option to purchase an additional aggregate principal amount. Net proceeds from the issuance were approximately $460.134 million, after deducting the initial purchasers’ commissions.
In September 2019, we entered into a sales agreement with Cowen and Company, LLC that enables us to issue and sell shares of Class 2 common stock from time to time up to up to an aggregate offering price of $400.0 million through an “at-the-market” equity offering program. During the year ended December 31, 2019, we issued 5,396,501 shares of Class 2 common stock for gross proceeds of approximately $113.5 million under the program.
On February 28, 2020, we entered into a senior secured credit facility with Bridging Finance Inc. in an aggregate principal amount of $59.6 million (C$79.8 million) (the “Senior Facility”). Further information can be found in “Subsequent Events” below.
Our primary need for liquidity is to fund working capital requirements, capital expenditures, debt service obligations and for general corporate purposes. Our ability to fund operations and make planned capital expenditures and debt service obligations depends on future operating performance and cash flows, which are subject to prevailing economic conditions and financial, business and other factors.
Our financial statements, in Part II, Item 8 of this Form 10-K, have been prepared on a going-concern basis, which assumes that we will continue to be in operation for the foreseeable future and, accordingly, will be able to realize our assets and discharge our liabilities in the normal course of operations as they come due. Further information can be found in Part II, Item 8 of this Form 10-K, in the Notes to Consolidated Financial Statements in Note 2, “Summary of Significant Accounting Policies.”
Current management forecasts and related assumptions illustrate that the Company can adequately manage the operational needs of the business with the additional Senior Facility of $59.6 million (C$79.8 million) secured on February 28, 2020 and as necessary, through accessing capital from the at-the market program with available authorized funding of $271.7 million or other equity offerings. However, given that there can be no guarantee that the Company may be able to raise equity financing under the at-the-market program when, if and as needed, additional evaluation of management’s plans and forecasts have been assessed to consider the ability to meet the Company’s contractual commitments and obligations. Should there be constraints on access to capital under the at-the-market program or other equity offerings, the Company can manage cash-outflows through reduced capital expenditures and managing the operational expenses of the business that pertain to future investments that are discretionary in nature and can be adequately managed.
71
The following table sets forth the major components of our statements of cash flows for the periods presented:
(in thousands of United States dollars)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Net cash used in operating activities |
|
$ |
(258,065 |
) |
|
$ |
(46,248 |
) |
|
$ |
(6,003 |
) |
|
Net cash used in investing activities |
|
|
(253,181 |
) |
|
|
(98,620 |
) |
|
|
(11,815 |
) |
|
Net cash provided by financing activities |
|
|
114,700 |
|
|
|
630,998 |
|
|
|
12,235 |
|
|
Effect of foreign currency translation |
|
|
6,082 |
|
|
|
(1,198 |
) |
|
|
375 |
|
|
Cash and cash equivalents, beginning of year |
|
|
487,255 |
|
|
|
2,323 |
|
|
|
7,531 |
|
|
Cash and cash equivalents, ending of year |
|
|
96,791 |
|
|
|
487,255 |
|
|
|
2,323 |
|
|
Increase (decrease) in cash and cash equivalents |
|
$ |
(390,464 |
) |
|
$ |
484,932 |
|
|
$ |
(5,208 |
) |
Cash flows from operating activities
The changes in net cash used by operating activities in 2019 compared to 2018 primarily related to changes in working capital fluctuations and changes in non-cash expenses, all of which are highly variable. The changes in net cash used by operating activities in 2018 compared to 2017 was primarily due to an increase in operating costs to expand cultivation facilities, enter new markets and public company costs.
Cash flows from investing activities
The change in net cash used in investing activities in 2019 compared to 2018 primarily related to our acquisitions of Manitoba Harvest, Natura and S&S, investment in the ABG Profit Participation Arrangement, and purchase of property and equipment related to our expansion projects in Canada and Portugal. The changes in net cash used in investing activities in 2018 compared to 2017 was primarily due to an increase in investments purchased using proceeds from the convertible notes and IPO as well as capital expenditures for expansion of cultivation and production assets.
Cash flows from financing activities
The change in net cash provided by financing activities in 2019 compared to 2018 primarily related to proceeds from our at-the-market equity offering program, exercise of stock options, and ABG Profit Participation Arrangement. The changes in net cash provided by financing activities in 2018 compared to 2017 includes net proceeds from our convertible notes, Series A preferred stock financing, IPO and repayment of debt facilities.
The table below sets out the cash and cash equivalents, short term investments and inventory:
(in thousands of United States dollars)
|
|
|
As at December 31, |
|
|
As at December 31, |
|
||
|
|
|
2019 |
|
|
2018 |
|
||
|
Cash and cash equivalents |
|
$ |
96,791 |
|
|
$ |
487,255 |
|
|
Short-term investments |
|
|
— |
|
|
|
30,335 |
|
|
Inventory |
|
|
87,861 |
|
|
|
16,211 |
|
We primarily financed our operations through the issuance of common stock, sale of convertible notes and revenue generating activities. We believe that our existing cash will be sufficient to meet our working capital requirements.
We manage our liquidity risk by preparing budgets and cash forecasts to ensure we have sufficient funds to meet obligations. In managing working capital, we may limit the amount of our cash needs by selling inventory at wholesale rates, pursuing additional financing sources and managing the timing of capital expenditures. While we believe we have sufficient cash to meet working capital requirements in the short term, we may need additional sources of capital and/or financing, to meet planned growth requirements and to fund construction activities at our cultivation and processing facilities.
72
Subsequent Events
During the month of January 2020, we issued 274,044 shares of Class 2 common stock for gross proceeds of approximately $14.8 million under the at-the-market equity offering program.
On January 24, 2020, we entered into (i) an Amended and Restated Profit Participation Agreement (the “A&R Profit Participation Agreement”) with ABG, which amended and restated in its entirety the Profit Participation Agreement, dated January 14, 2019, and (ii) the First Amendment to Payment Agreement with ABG (the “Payment Agreement Amendment”), which amends the Payment Agreement, dated January 14, 2019. We agreed with ABG that Tilray will no longer have any obligation to pay the additional consideration with an aggregate value of $83.3 million in cash or in shares of Class 2 common stock, In addition, we will not be entitled to any guaranteed minimum participation rights and beginning January 1, 2020 through December 31, 2028 and we agreed that we will not be entitled to any participation rights until such participation rights with respect to each contract year exceeds $10 million, and in the event the participation rights are achieved, we will be entitled to the full 49% participation rights.
The impact of the A&R Profit Participation Agreement will result in a write-off of the ABG finance receivable of $7.0 million which will be recorded through the statements of net loss and comprehensive loss and $28.9 million through accumulated deficit in January 2020.
During the month of February 2020, we restrucuted our global organization to meet the needs of the current industry environment. As a result, we incurred $0.7 million in restructuring costs.
On February 28, 2020, we (“the Borrower”) entered into a credit agreement for a senior secured credit facility in a maximum aggregate principal amount of $59.6 million (C$79.8 million) (the “Senior Facility”). Transaction fees incurred on the Senior Facility are $4.5 million. The Senior Facility consists of a 2-year $59.6 million (C$79.8 million) senior secured term loan facility, of which $49.7 million (C$66.5 million) was drawn at the closing, and of which $9.9 million (C$13.3 million) may be drawn at any point 90 days following closing at the Borrower’s election. The Senior Facility will bear interest on the outstanding principal balance at an annual rate equal to the Canadian prime rate plus 8.05%, calculated based on the daily outstanding balance of the Senior Facility calculated and compounded monthly, not in advance and with no deemed reinvestment of monthly payments. The Senior Facility contains certain affirmative and negative covenants. The operational covenant includes a minimum unrestricted cash threshold of $29.9 million (C$40.0 million) for capital expenditures and investments.
Contractual Obligations and Commitments
Lease commitments
We lease various facilities, under non-cancelable finance and operating leases, which expire at various dates through September 2027.
Maturities of lease liabilities:
|
Year ending December 31, |
|
Operating Leases |
|
|
Finance Leases |
|
||
|
2020 |
|
$ |
3,493 |
|
|
$ |
1,083 |
|
|
2021 |
|
|
3,276 |
|
|
|
1,083 |
|
|
2022 |
|
|
2,897 |
|
|
|
7,333 |
|
|
2023 |
|
|
2,824 |
|
|
|
15,677 |
|
|
2024 |
|
|
2,436 |
|
|
|
— |
|
|
Thereafter |
|
|
7,861 |
|
|
|
— |
|
|
Total lease payments |
|
|
22,787 |
|
|
|
25,176 |
|
|
Imputed interest |
|
|
5,059 |
|
|
|
11,024 |
|
|
Obligations recognized |
|
$ |
17,728 |
|
|
$ |
14,152 |
|
73
Purchase commitments
The following table reflects our future non-cancellable minimum contractual commitments as at December 31, 2019:
|
|
|
Total |
|
|
2020 |
|
|
|
|
2021 |
|
|
|
|
2022 |
|
|
|
|
2023 |
|
|
|
|
2024 |
|
|
|
|
Thereafter |
|
|||||||
|
Purchase commitments |
|
$ |
132,743 |
|
|
$ |
131,010 |
|
|
|
|
$ |
1,657 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
- |
|
|
|
|
$ |
- |
|
|
Total |
|
$ |
132,743 |
|
|
$ |
131,010 |
|
|
|
|
$ |
1,657 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
- |
|
|
|
|
$ |
- |
|
As a result of changing industry dynamics, we are currently in the process of re-negotiating the terms of several supply agreements, including quantities and pricing, related to CBD, cannabis extracts/oils, and hemp flower. The re-negotiations are ongoing and there can be no assurance that terms satisfactory to us can be reached on a timely basis, or at all. The failure of re-negotiations could result in us being contractually obligated to purchase significant amounts of products, some of which may be priced above then-current market prices, or litigation against us, or interruption of the supply of inputs for the manufacturing of our products, all of which could have a material adverse effect on our business, results of operations, financial condition, liquidity and prospects. In addition, any litigation or arbitration resulting in an adverse judgment or award against us could result in a default under our Senior Facility and convertible notes.
In 2018, we signed an agreement with Rose Lifescience Inc. ("Rose") for distribution and marketing of product in Quebec in exchange for a minimum fee of $0.4 million per annum for an initial term of five years. We agreed to purchase the lesser of 2,000 kg per year or 40% of the production of Cannabis at a rate of 115% of cost of goods sold from the Rose facility. As the purchase commitment is an undeterminable variable amount, it is excluded from the above schedule.
In 2018, we entered into a Product and Trademark License Agreement with Docklight LLC, a related party, to use certain intellectual property rights in exchange for payment of royalty depending upon specified percentage of licensed product net sales. As the purchase commitment is an undeterminable variable amount, it is excluded from the above schedule.
Other commitments
We have payments on the ABG finance liability and convertible notes as follows:
|
|
|
Total |
|
|
2020 |
|
|
|
|
2021 |
|
|
|
|
2022 |
|
|
|
|
2023 |
|
|
|
|
2024 |
|
|
|
|
Thereafter |
|
|||||||
|
ABG finance liability |
|
$ |
8,500 |
|
|
$ |
1,000 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
Convertible notes |
|
|
475,000 |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
475,000 |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Total |
|
$ |
483,500 |
|
|
$ |
1,000 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
476,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
Contingencies
In the normal course of business, we may receive inquiries or become involved in legal disputes regarding various litigation matters. In the opinion of management, any potential liabilities resulting from such claims would not have a material adverse effect on our consolidated financial statements.
Off-Balance Sheet Arrangements
We did not have many off-balance sheet arrangements during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
74
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
Interest rate risk is the risk that the value or yield of fixed-income investments may decline if interest rates change. Fluctuations in interest rates may impact the level of income and expense recorded on our cash equivalent, short-term investments, convertible notes and the market value of all interest-earning assets, other than those which possess a short term to maturity. A 1% change in the interest rate in effect on December 31, 2019 would not have a material effect on i) fair value of our cash equivalents and short-term investments as the majority of the portfolio have a maturity date of three-months or less, and ii) interest income as interest income is not a significant component of the Company’s earnings and cash flow. In addition, the convertible notes bear interest at a fixed rate of 5% and are not publicly traded. Therefore, fair value of the convertible notes and interest expense is not affected by changes in the market interest rates.
Equity Price Risks
As of December 31, 2019, we held long-term equity investments at fair value and equity investments under the measurement alternative. These investment in equities were acquired as part of our strategic transactions.
Accordingly, the changes in fair values of investment in equities measured at fair value or under the measurement alternative are recognized through other income, net in the statements of net loss and comprehensive loss. Based on the fair value of investment in equities held as of December 31, 2019, a hypothetical decrease of 10% in the prices for these companies would reduce the fair values of the investments and result in unrealized loss recorded in other comprehensive income by $2.4 million.
Foreign Currency Risk
Our consolidated financial statements are expressed in United States dollars, but we have net assets and liabilities denominated in Canadian dollars, Euro, Australian dollars and Chilean dollars. As a result, we are exposed to foreign currency translation gains and losses. Revenue and expenses of all foreign operations are translated into United States dollars at the foreign currency exchange rates that approximate the rates in effect at the dates when such items are recognized. Appreciating foreign currencies relative to the United States dollar will adversely impact operating income and net earnings, while depreciating foreign currencies relative to the United States dollar will have a positive impact.
A 10% change in the exchange rates for the foreign currencies would affect the carrying value of net assets by approximately $12.5 million as of December 31, 2019, with a corresponding impact to accumulated other comprehensive income. We have not historically engaged in hedging transactions and do not currently contemplate engaging in hedging transactions to mitigate foreign exchange risks. As we continue to recognize gains and losses in foreign currency transactions, depending upon changes in future currency rates, such gains or losses could have a significant, and potentially adverse, effect on our results of operations.
75
Item 8. Financial Statements and Supplementary Data.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
F-2 |
|
|
|
|
|
Consolidated Balance Sheets as of December 31, 2019 and 2018 |
F-7 |
|
|
|
|
F-8 |
|
|
|
|
|
F-9 |
|
|
|
|
|
Consolidated Statements of Cash Flows for the Years ended December 31, 2019, 2018 and 2017 |
F-10 |
|
|
|
|
F-11 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Tilray Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Tilray Inc. and subsidiaries (the "Company") as of December 31, 2019 and 2018, the related consolidated statements of net loss and comprehensive loss, and changes in stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 2, 2020, expressed an adverse opinion on the Company's internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 2 to the financial statements, effective January 1, 2019, the Company adopted ASU 2016-02, Leases, codified as ASC 842 Leases, as amended, using the modified retrospective approach.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Inventory – Cannabis Costing — Refer to Notes 2 and 5 to the financial statements
Critical Audit Matter Description
Inventory is comprised of raw materials, finished goods and work-in-progress for cannabis and hemp products. Cost includes expenditures directly related to the manufacturing process as well as suitable portions of related production overheads, based on normal operating capacity. Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. For cannabis inventory, costs include pre-harvest, post-harvest, shipment and fulfillment, as well as related accessories.
The nature of the process for cannabis inventory costing is manual and requires management to use complex
F-2
spreadsheet models updated monthly (“models”) to calculate a month by month continuity of the cost of inventory. In addition, the models need to take into account a variety of inputs and source data in order to calculate cost. Auditing the cost of inventory required an increased extent of audit effort.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the cost of cannabis inventory included the following, among others:
|
|
• |
Evaluated the complex spreadsheet models and the inputs to such models used to calculate the cost of cannabis inventory by: |
|
|
o |
Evaluating the incorporation of the source data into the models, testing the formulas used and testing the computational accuracy. |
|
|
o |
Testing purchases used in the models to third party source documentation. |
|
|
o |
Testing production costs used in the models to actual costs incurred. |
|
|
o |
Performing independent calculations of key inputs used in the models and comparing to inputs used by management. |
|
|
o |
Testing management’s allocation of indirect costs between inventory products by assessing the appropriateness of the allocation method, recalculating the allocations and on a sample basis testing the underlying allocations by tracing to source documents. |
|
|
o |
Testing production quantities used in the models by physically observing and verifying inventory quantities. |
|
|
• |
As a result of the Company’s material weaknesses identified by the Company in two components of Internal Control – Integrated Framework (2013) issued by COSO, we increased the extent of inventory physical observations and verifications, increased the extent of testing where sampling methodology was used, and utilized third party source documents in the performance of our testing procedures. |
ABG Profit Participation Arrangement – Recognition of Loan — Refer to Notes 2, 4, 6, and 26 to the financial statements
Critical Audit Matter Description
On January 14, 2019, the Company entered into a Profit Participation Arrangement (“the Arrangement”) with ABG Intermediate Holdings 2, LLC (“ABG”) that offers the Company various rights and licenses. Since the Arrangement conveys a right for the Company to receive guaranteed minimum cash from ABG over ten years, it meets the definition of a loan pursuant to ASC 310, Receivables. The portion of the loan relating to cash paid to ABG is recorded within prepayments and other current assets (current portion) and in ABG finance receivable and other assets (non-current portion). The portion of the loan relating to shares issued is recorded within additional paid-in capital. Subsequent to December 31, 2019, on January 24, 2020, the Company entered into a new agreement with ABG, which amended and restated in its entirety the Agreement dated January 14, 2019.
There are many components embedded in the Arrangement that resulted in management making judgments on the accounting treatment of the guaranteed minimum in particular (1) determining whether the right to receive the guaranteed minimum was a loan or other form of asset and (2) determining whether a portion should be recorded in equity or should the arrangement be shown entirely as a financial asset. In addition, there was also subjectivity in management’s determination of the interest rate used to calculate the fair value of the loan. Auditing management’s judgments of the accounting treatment of the loan, and management’s determination of the interest rate used to calculate the fair value of the loan required a high degree of subjectivity. This resulted in an increased extent of audit effort, including the need to involve fair value specialists and professionals in our firm with expertise in financial instruments
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to management’s judgments of the accounting treatment of the guaranteed minimum, in particular (1) determining whether the right to receive the guaranteed minimum was a loan or other form of asset and (2) determining whether a portion should be recorded in equity or should the arrangement be shown entirely as a
F-3
financial asset and management’s determination of the interest rate used to calculate the fair value of the loan, included the following among others:
|
|
• |
With the assistance of professionals in our firm with expertise in financial instruments assessed management’s judgments of the accounting treatment of the guaranteed minimum, in particular (1) determining whether the right to receive the guaranteed minimum was a loan or other form of asset and (2) determining whether a portion should be recorded in equity or should the arrangement be shown entirely as a financial asset by: |
|
|
o |
Assessing the information in the Arrangement to understand and evaluate that all components were identified; |
|
|
o |
Evaluating management’s judgments related to the accounting treatment of the loan by analyzing against various aspects of GAAP, including conceptual framework and guidance. |
|
|
• |
With the assistance of fair value specialists, evaluated management’s determination of the interest rate used to calculate the fair value of the loan by: |
|
|
o |
Independently calculating the interest rate by obtaining a list of outstanding loans for ABG including the interest rate and interest spread issued for each debt instrument as well as the benchmark interest risk-free rate curve as at each of the loan issue dates. |
|
|
• |
Assessed the January 24, 2020 agreement to determine if it had an impact on the December 31, 2019 financial statements. |
Indefinite-lived intangible assets – Rights Under the ABG Profit Participation Agreement— Refer to Notes 2, 4 and 10 to the financial statements
Critical Audit Matter Description
The Company has an indefinite-lived intangible, rights under the ABG Profit Participation Arrangement (“ABG participation rights”) that is tested for impairment annually or more frequently when events or circumstances indicate that impairment may have occurred. If the carrying value of the indefinite-lived intangible asset exceeds its fair value an impairment charge is recorded. At the reporting date, the Company determined the fair value of the ABG participation rights was below the carrying value. The decline in fair value of the ABG participation rights is attributable to deferred regulatory clarity for sales of CBD products in the United States. The Company recorded an impairment charge of $103 million in the 4th quarter.
In determining the fair value of the ABG participation rights for the purpose of the impairment test using a discounted cash flow approach, the judgments and assumptions with the highest degree of impact and subjectivity are the discount rate and forecasted rate of future CBD related revenue growth for ABG. Auditing management’s assumptions used in the ABG participation rights impairment analysis required a high degree of auditor judgment and an increased extent of audit effort, including the need to involve fair value specialists.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the determination of the discount rate and forecasted CBD related revenue for ABG in the determination of the fair value of the ABG participation rights included the following among others:
|
|
• |
Compared the forecasted CBD related revenue for certain ABG brands to actual CBD related revenue and other relevant information. |
|
|
• |
Confirmed the forecasted CBD revenue with the counter party to the contract and agreed it to management’s model. |
|
|
• |
With the assistance of fair value specialists; |
|
|
o |
Evaluated the rate of future revenue growth to assess the reasonableness against market expectations and, |
|
|
o |
Evaluated the reasonableness of the discount rate by (1) testing the source information underlying the determination of the discount rate and testing the mathematical accuracy of the calculation and |
F-4
|
|
(2) developing a range of independent estimates and comparing those to the discount rate selected by management. |
Acquisition of Manitoba Harvest – Refer to Notes 2, 3 and 10 to the financial statements
Critical Audit Matter Description
The Company completed the acquisition of all issued and outstanding shares of FHF Holdings Ltd. (“Manitoba Harvest”) on February 28, 2019.The purchase price was allocated to the assets acquired and liabilities assumed based on their respective fair values, including intangible assets of trademarks, developed technology and customer relationships. Management estimated the fair value of the intangible assets using a discounted cash flow approach. To estimate fair value, management is required to make estimates, and assumptions on the discount rate, rate of future revenue growth and profitability of the acquired business and working capital effects.
Given the fair value determination of the intangible assets acquired for Manitoba Harvest required management to make significant estimates and assumptions related to the determination of the rate of future revenue growth and the discount rate. Performing audit procedures to evaluate the reasonableness of these estimates and assumptions required a high degree of auditor judgment and an increased extent of audit effort, including the need to involve fair value specialists.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the determination of the rate of future revenue growth and the discount rate used to determine the fair value of the intangible assets acquired included the following, among others:
|
|
• |
Assessed the reasonableness of the rate of future revenue growth by comparing the projections to historical results and certain peer companies. |
|
|
• |
Evaluated whether the rate of future revenue growth was consistent with evidence obtained in other areas of the audit. |
|
|
• |
With the assistance of fair value specialists; |
|
|
o |
Evaluated the reasonableness of the discount rate by (1) testing the source information underlying the determination of the discount rate and testing the mathematical accuracy of the calculation and (2) developing a range of independent estimates and comparing those to the discount rate selected by management. |
Basis of Presentation and Going Concern – Disclosure — Refer to Note 2 to the financial statements
Critical Audit Matter Description
The financial statements of the Company are prepared on a going concern basis, which assumes that the Company will continue in operation for the foreseeable future and, accordingly, will be able to realize its assets and discharge its liabilities in the normal course of operations. For the fiscal years ended December 31, 2019, 2018 and 2017, the Company reported net losses of $321,169, $67,723 and $7,809, respectively. The Company has contractual obligations such as non-cancelable minimum purchase commitments, interest and lease payments (collectively “obligations”). Currently management’s forecasts and related assumptions illustrate their ability to meet the obligations through management of expenditures and, if necessary, accessing additional funding from the at-the-market program or other equity financing. Should there be constraints on the ability to access capital under the at-the-market program or other equity financing, the Company can manage cash outflows to meet the obligations through reductions in capital expenditures and other operating expenditures.
Management made judgments to conclude that it is probable that the Company’s plans will be effectively implemented and will provide the necessary cash flows to fund the Company’s obligations as they become due. Specifically, the judgments with the highest degree of impact and subjectivity in determining it is probable that the Company’s plans will be effectively implemented included the revenue growth and gross margin assumptions underlying its forecast operating cash flows, its ability to reduce capital expenditures and other operating expenditures and its ability to access funding from the at-the-market program. Auditing the judgments made by management required a high degree of auditor judgment and an increased extent of audit effort.
F-5
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the Company’s disclosure and the management’s ability to effectively implement its plan included the following, among others:
|
|
• |
Tested key assumptions underlying management’s forecast operating cash flows, including revenue growth and gross margin assumptions. |
|
|
• |
Evaluated the reasonableness of management’s forecast operating cash flows by comparing the forecasts to industry and analyst reports. |
|
|
• |
Evaluated the probability that the Company will be able to access funding from the at-the-market program by assessing the terms of the program and Company’s history of using the program. |
|
|
• |
Evaluated the probability that the Company will be able to reduce capital expenditures and other operating expenditures if required. |
|
|
• |
Assessed management’s plans in the context of other audit evidence obtained during the audit to determine whether it supported or contradicted the conclusion reached by management. |
/s/ Deloitte LLP
Chartered Professional Accountants
Vancouver, Canada
March 2, 2020
We have served as the Company's auditor since 2017.
F-6
TILRAY, INC.
Consolidated Balance Sheets
(in thousands of United States dollars, except for share and per share data)
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
96,791 |
|
|
$ |
487,255 |
|
|
Short-term investments |
|
|
— |
|
|
|
30,335 |
|
|
Accounts receivable, net of allowance for doubtful accounts of $2,015 and $292, respectively |
|
|
36,202 |
|
|
|
16,525 |
|
|
Inventory |
|
|
87,861 |
|
|
|
16,211 |
|
|
Prepayments and other current assets |
|
|
38,173 |
|
|
|
3,976 |
|
|
Total current assets |
|
|
259,027 |
|
|
|
554,302 |
|
|
Property and equipment, net |
|
|
184,217 |
|
|
|
80,214 |
|
|
Operating lease, right-of-use assets |
|
|
17,514 |
|
|
|
— |
|
|
Intangible assets, net |
|
|
228,828 |
|
|
|
4,486 |
|
|
Goodwill |
|
|
163,251 |
|
|
|
— |
|
|
Equity method investments |
|
|
11,448 |
|
|
|
— |
|
|
Other investments |
|
|
24,184 |
|
|
|
16,911 |
|
|
ABG finance receivable and other assets |
|
|
7,861 |
|
|
|
754 |
|
|
Total assets |
|
$ |
896,330 |
|
|
$ |
656,667 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
|
39,125 |
|
|
|
10,649 |
|
|
Accrued expenses and other current liabilities |
|
|
50,829 |
|
|
|
14,818 |
|
|
Accrued obligations under finance lease |
|
|
— |
|
|
|
470 |
|
|
Accrued obligations under operating lease |
|
|
2,473 |
|
|
|
— |
|
|
Total current liabilities |
|
|
92,427 |
|
|
|
25,937 |
|
|
Accrued obligations under finance lease |
|
|
14,152 |
|
|
|
8,286 |
|
|
Accrued obligations under operating lease |
|
|
15,255 |
|
|
|
— |
|
|
ABG finance liability |
|
|
5,566 |
|
|
|
— |
|
|
Deferred tax liability |
|
|
53,363 |
|
|
|
4,424 |
|
|
Convertible notes, net of issuance costs |
|
|
430,210 |
|
|
|
420,367 |
|
|
Other liabilities |
|
|
86 |
|
|
|
— |
|
|
Total liabilities |
|
$ |
611,059 |
|
|
$ |
459,014 |
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (refer to Note 17) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity |
|
|
|
|
|
|
|
|
|
Class 1 common stock ($0.0001 par value, 250,000,000 shares authorized; 16,666,667 shares issued and outstanding) |
|
|
2 |
|
|
|
2 |
|
|
Class 2 common stock ($0.0001 par value; 500,000,000 shares authorized; 86,114,558 and 76,504,200 shares issued and outstanding, respectively) |
|
|
9 |
|
|
|
8 |
|
|
Additional paid-in capital |
|
|
705,671 |
|
|
|
302,057 |
|
|
Accumulated other comprehensive income |
|
|
9,719 |
|
|
|
3,763 |
|
|
Accumulated deficit |
|
|
(430,130 |
) |
|
|
(108,177 |
) |
|
Total stockholders' equity |
|
$ |
285,271 |
|
|
$ |
197,653 |
|
|
Total liabilities and stockholders' equity |
|
$ |
896,330 |
|
|
$ |
656,667 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
TILRAY, INC.
Consolidated Statements of Net Loss and Comprehensive Loss
(in thousands of United States dollars, except for share and per share data)
|
|
|
Years ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Revenue (inclusive of excise duties of $13,136, $1,200 and $0, respectively) |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
20,538 |
|
|
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
121,892 |
|
|
|
24,294 |
|
|
|
8,544 |
|
|
Inventory valuation adjustments |
|
|
68,583 |
|
|
|
4,561 |
|
|
|
617 |
|
|
Gross (loss) profit |
|
|
(23,496 |
) |
|
|
14,275 |
|
|
|
11,377 |
|
|
General and administrative expenses |
|
|
81,968 |
|
|
|
29,461 |
|
|
|
7,499 |
|
|
Sales and marketing expenses |
|
|
61,084 |
|
|
|
15,366 |
|
|
|
7,164 |
|
|
Research and development expenses |
|
|
6,558 |
|
|
|
4,264 |
|
|
|
3,171 |
|
|
Stock-based compensation expenses |
|
|
31,842 |
|
|
|
20,988 |
|
|
|
139 |
|
|
Depreciation and amortization expenses |
|
|
11,607 |
|
|
|
1,598 |
|
|
|
902 |
|
|
Impairment of assets |
|
|
112,070 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition-related (income) expenses, net |
|
|
(31,427 |
) |
|
|
248 |
|
|
|
— |
|
|
Loss from equity method investments |
|
|
4,504 |
|
|
|
— |
|
|
|
— |
|
|
Operating loss |
|
|
(301,702 |
) |
|
|
(57,650 |
) |
|
|
(7,498 |
) |
|
Foreign exchange (gain) loss, net |
|
|
(5,944 |
) |
|
|
7,234 |
|
|
|
(1,363 |
) |
|
Interest expenses, net |
|
|
34,690 |
|
|
|
9,110 |
|
|
|
1,686 |
|
|
Finance income from ABG |
|
|
(764 |
) |
|
|
— |
|
|
|
— |
|
|
Loss on disposal of property and equipment |
|
|
2,436 |
|
|
|
190 |
|
|
|
— |
|
|
Other income, net |
|
|
(2,501 |
) |
|
|
(2,010 |
) |
|
|
(12 |
) |
|
Loss before income taxes |
|
|
(329,619 |
) |
|
|
(72,174 |
) |
|
|
(7,809 |
) |
|
Deferred income tax recoveries |
|
|
(8,847 |
) |
|
|
(4,485 |
) |
|
|
— |
|
|
Current income tax expenses |
|
|
397 |
|
|
|
34 |
|
|
|
— |
|
|
Net loss |
|
|
(321,169 |
) |
|
|
(67,723 |
) |
|
|
(7,809 |
) |
|
Net loss per share - basic and diluted |
|
$ |
(3.20 |
) |
|
$ |
(0.82 |
) |
|
$ |
(0.10 |
) |
|
Weighted average shares used in computation of net loss per share - basic and diluted |
|
|
100,455,677 |
|
|
|
83,009,656 |
|
|
|
75,000,000 |
|
|
Net loss |
|
|
(321,169 |
) |
|
|
(67,723 |
) |
|
|
(7,809 |
) |
|
Foreign currency translation gain, net |
|
|
5,174 |
|
|
|
662 |
|
|
|
282 |
|
|
Unrealized loss on investments |
|
|
(21 |
) |
|
|
(765 |
) |
|
|
— |
|
|
Other comprehensive income (loss) |
|
|
5,153 |
|
|
|
(103 |
) |
|
|
282 |
|
|
Comprehensive loss |
|
$ |
(316,016 |
) |
|
$ |
(67,826 |
) |
|
$ |
(7,527 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
TILRAY, INC.
Consolidated Statements of Stockholders’ Equity (Deficit)
(in thousands of United States dollars, except for share and per share data)
|
|
|
|
Preferred shares |
|
|
Common stock |
|
|
Additional |
|
|
Accumulated other |
|
|
|
|
|
|
Total stockholders' |
|
|||||||||||||
|
|
|
|
Number of shares |
|
|
Amount |
|
|
Number of shares |
|
|
Amount |
|
|
paid-in capital |
|
|
comprehensive income |
|
|
Accumulated deficit |
|
|
equity (deficit) |
|
||||||||
|
Balance at December 31, 2016 |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
31,589 |
|
|
$ |
3,584 |
|
|
$ |
(32,645 |
) |
|
$ |
2,528 |
|
|
Contributions |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8 |
|
|
|
— |
|
|
|
— |
|
|
|
8 |
|
|
Stock-based compensation expenses |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
139 |
|
|
|
— |
|
|
|
— |
|
|
|
139 |
|
|
Foreign currency translation gain |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
282 |
|
|
|
— |
|
|
|
282 |
|
|
Net loss |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7,809 |
) |
|
|
(7,809 |
) |
|
Balance at December 31, 2017 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
31,736 |
|
|
|
3,866 |
|
|
|
(40,454 |
) |
|
|
(4,852 |
) |
|
Shares issued for preferred shares, net of issuance costs |
|
|
|
7,794,042 |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
52,558 |
|
|
|
— |
|
|
|
— |
|
|
|
52,560 |
|
|
Conversion of preferred shares |
|
|
|
(7,794,042 |
) |
|
|
(2 |
) |
|
|
7,794,042 |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Common stock issuance, net of issuance costs |
|
|
|
— |
|
|
|
— |
|
|
|
85,350,000 |
|
|
|
8 |
|
|
|
160,784 |
|
|
|
— |
|
|
|
— |
|
|
|
160,792 |
|
|
Stock-based compensation expenses |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
20,988 |
|
|
|
— |
|
|
|
— |
|
|
|
20,988 |
|
|
Other comprehensive loss |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(103 |
) |
|
|
— |
|
|
|
(103 |
) |
|
Deferred tax liability related to convertible notes, net of issuance costs |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8,809 |
) |
|
|
— |
|
|
|
— |
|
|
|
(8,809 |
) |
|
Issuance of shares for Alef acquisition |
|
|
|
— |
|
|
|
— |
|
|
|
26,825 |
|
|
|
— |
|
|
|
2,855 |
|
|
|
— |
|
|
|
— |
|
|
|
2,855 |
|
|
Equity component related to issuance of convertible notes, net of issuance costs |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
41,945 |
|
|
|
— |
|
|
|
— |
|
|
|
41,945 |
|
|
Net loss |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(67,723 |
) |
|
|
(67,723 |
) |
|
Balance at December 31, 2018 |
|
|
|
— |
|
|
|
— |
|
|
|
93,170,867 |
|
|
|
10 |
|
|
|
302,057 |
|
|
|
3,763 |
|
|
|
(108,177 |
) |
|
|
197,653 |
|
|
Cumulative effect adjustment from transition to ASU 2016-01 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
803 |
|
|
|
(803 |
) |
|
|
— |
|
|
Cumulative effect adjustment from transition to ASC 842 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
19 |
|
|
|
19 |
|
|
Shares issued for Natura acquisition |
|
|
|
— |
|
|
|
— |
|
|
|
180,332 |
|
|
|
— |
|
|
|
15,099 |
|
|
|
— |
|
|
|
— |
|
|
|
15,099 |
|
|
Shares issued for Natura contingent consideration |
|
|
|
— |
|
|
|
— |
|
|
|
238,826 |
|
|
|
— |
|
|
|
4,450 |
|
|
|
— |
|
|
|
— |
|
|
|
4,450 |
|
|
Shares issued for Manitoba Harvest acquisition |
|
|
|
— |
|
|
|
— |
|
|
|
2,109,252 |
|
|
|
— |
|
|
|
128,710 |
|
|
|
— |
|
|
|
— |
|
|
|
128,710 |
|
|
Shares issued for ABG Profit Participation Arrangement |
|
|
|
— |
|
|
|
— |
|
|
|
1,680,214 |
|
|
|
— |
|
|
|
125,097 |
|
|
|
— |
|
|
|
— |
|
|
|
125,097 |
|
|
ABG finance receivable, net of finance income of $2,700 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(27,553 |
) |
|
|
— |
|
|
|
— |
|
|
|
(27,553 |
) |
|
Shares issued for common stock at-the-market, net of issuance costs |
|
|
|
— |
|
|
|
— |
|
|
|
5,396,501 |
|
|
|
1 |
|
|
|
111,072 |
|
|
|
— |
|
|
|
— |
|
|
|
111,073 |
|
|
Shares issued for investments |
|
|
|
— |
|
|
|
— |
|
|
|
550,646 |
|
|
|
— |
|
|
|
10,551 |
|
|
|
— |
|
|
|
— |
|
|
|
10,551 |
|
|
Shares issued for S & S acquisition |
|
|
|
— |
|
|
|
— |
|
|
|
79,289 |
|
|
|
— |
|
|
|
3,189 |
|
|
|
— |
|
|
|
— |
|
|
|
3,189 |
|
|
Shares issued under stock-based compensation plans |
|
|
|
— |
|
|
|
— |
|
|
|
1,575,455 |
|
|
|
— |
|
|
|
506 |
|
|
|
— |
|
|
|
— |
|
|
|
506 |
|
|
Shares issued for employee compensation |
|
|
|
— |
|
|
|
— |
|
|
|
11,868 |
|
|
|
— |
|
|
|
651 |
|
|
|
— |
|
|
|
— |
|
|
|
651 |
|
|
Stock-based compensation expenses |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
31,842 |
|
|
|
— |
|
|
|
— |
|
|
|
31,842 |
|
|
Downstream merger |
|
|
|
— |
|
|
|
— |
|
|
|
(2,212,025 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Other comprehensive income |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,153 |
|
|
|
— |
|
|
|
5,153 |
|
|
Net loss |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(321,169 |
) |
|
|
(321,169 |
) |
|
Balance at December 31, 2019 |
|
|
|
— |
|
|
|
— |
|
|
|
102,781,225 |
|
|
$ |
11 |
|
|
$ |
705,671 |
|
|
$ |
9,719 |
|
|
$ |
(430,130 |
) |
|
$ |
285,271 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-9
TILRAY, INC.
Consolidated Statements of Cash Flows
(in thousands of United States dollars, except for per share data)
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(321,169 |
) |
|
$ |
(67,723 |
) |
|
$ |
(7,809 |
) |
|
Adjusted for the following items: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventory valuation adjustments |
|
|
68,583 |
|
|
|
384 |
|
|
|
204 |
|
|
Depreciation and amortization expenses |
|
|
15,849 |
|
|
|
3,562 |
|
|
|
1,853 |
|
|
Impairment of assets |
|
|
112,070 |
|
|
|
— |
|
|
|
— |
|
|
Stock-based compensation expenses |
|
|
31,842 |
|
|
|
20,988 |
|
|
|
139 |
|
|
Gain on sale of short-term investment |
|
|
(2,631 |
) |
|
|
— |
|
|
|
— |
|
|
Change in fair value of contingent consideration |
|
|
(46,914 |
) |
|
|
— |
|
|
|
— |
|
|
Loss from equity method investments |
|
|
4,504 |
|
|
|
— |
|
|
|
— |
|
|
Loss from equity investments measured at fair value |
|
|
939 |
|
|
|
6 |
|
|
|
— |
|
|
Interest on debt securities |
|
|
(149 |
) |
|
|
— |
|
|
|
— |
|
|
Deferred taxes |
|
|
(8,847 |
) |
|
|
(4,485 |
) |
|
|
— |
|
|
Amortization of discount on convertible notes |
|
|
9,843 |
|
|
|
2,180 |
|
|
|
— |
|
|
Foreign currency (gain) loss |
|
|
(5,944 |
) |
|
|
6,477 |
|
|
|
(1,363 |
) |
|
Accretion related to obligations under finance leases |
|
|
367 |
|
|
|
— |
|
|
|
— |
|
|
Non-cash interest expenses |
|
|
— |
|
|
|
5,669 |
|
|
|
693 |
|
|
Provision for doubtful accounts |
|
|
1,723 |
|
|
|
285 |
|
|
|
— |
|
|
Loss (gain) on disposal of property and equipment |
|
|
2,436 |
|
|
|
(2 |
) |
|
|
11 |
|
|
Changes in non-cash working capital: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(14,820 |
) |
|
|
(16,512 |
) |
|
|
(507 |
) |
|
Taxes receivable |
|
|
(5,196 |
) |
|
|
101 |
|
|
|
(1,187 |
) |
|
Inventory |
|
|
(102,643 |
) |
|
|
(9,226 |
) |
|
|
(3,295 |
) |
|
Prepayments and other current assets |
|
|
(46,212 |
) |
|
|
(2,588 |
) |
|
|
(433 |
) |
|
Accounts payable |
|
|
20,003 |
|
|
|
5,218 |
|
|
|
4,728 |
|
|
Accrued expenses and other current liabilities |
|
|
28,215 |
|
|
|
9,418 |
|
|
|
963 |
|
|
Other liabilities |
|
|
86 |
|
|
|
— |
|
|
|
— |
|
|
Net cash used in operating activities |
|
|
(258,065 |
) |
|
|
(46,248 |
) |
|
|
(6,003 |
) |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Business combinations, net of cash acquired |
|
|
(163,889 |
) |
|
|
— |
|
|
|
— |
|
|
Investment in ABG Profit Participation Arrangement |
|
|
(33,333 |
) |
|
|
— |
|
|
|
— |
|
|
Investment in equity method investees |
|
|
(14,201 |
) |
|
|
— |
|
|
|
— |
|
|
Change in deposits and other assets |
|
|
(2,689 |
) |
|
|
— |
|
|
|
(397 |
) |
|
Purchases of short-term and other investments |
|
|
(1,350,666 |
) |
|
|
(319,373 |
) |
|
|
— |
|
|
Proceeds from sales and maturities of short-term investments |
|
|
1,383,632 |
|
|
|
274,497 |
|
|
|
— |
|
|
Purchases of property and equipment |
|
|
(73,741 |
) |
|
|
(50,198 |
) |
|
|
(10,910 |
) |
|
Proceeds from disposal of property and equipment |
|
|
6,581 |
|
|
|
713 |
|
|
|
23 |
|
|
Purchases of intangible assets |
|
|
(4,875 |
) |
|
|
(4,259 |
) |
|
|
(531 |
) |
|
Net cash used in investing activities |
|
|
(253,181 |
) |
|
|
(98,620 |
) |
|
|
(11,815 |
) |
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from at-the market equity offering, net of costs |
|
|
111,073 |
|
|
|
— |
|
|
|
— |
|
|
Proceeds from ABG Profit Participation Arrangement |
|
|
4,187 |
|
|
|
— |
|
|
|
— |
|
|
Payment of ABG finance liability |
|
|
(500 |
) |
|
|
— |
|
|
|
— |
|
|
Payment under Privateer Holdings debt facilities |
|
|
— |
|
` |
|
(36,940 |
) |
|
|
— |
|
|
Advances under Privateer Holdings debt and construction facilities |
|
|
— |
|
|
|
3,453 |
|
|
|
12,434 |
|
|
Proceeds from Preferred Shares - Series A, net of transaction costs |
|
|
— |
|
|
|
52,560 |
|
|
|
— |
|
|
Proceeds from exercise of stock options |
|
|
5,458 |
|
|
|
— |
|
|
|
— |
|
|
Payment on the settlement of stock options |
|
|
(5,014 |
) |
|
|
— |
|
|
|
— |
|
|
Payment of mortgage debt |
|
|
— |
|
|
|
(9,136 |
) |
|
|
— |
|
|
Payment of obligations under finance lease |
|
|
(504 |
) |
|
|
— |
|
|
|
(199 |
) |
|
Proceeds from issuance of convertible notes, net of issuance costs |
|
|
— |
|
|
|
460,269 |
|
|
|
— |
|
|
Proceeds from issuance of common stock pursuant to IPO, net |
|
|
— |
|
|
|
160,792 |
|
|
|
— |
|
|
Net cash provided by financing activities |
|
|
114,700 |
|
|
|
630,998 |
|
|
|
12,235 |
|
|
Effect of foreign currency translation on cash and cash equivalents |
|
|
6,082 |
|
|
|
(1,198 |
) |
|
|
375 |
|
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Decrease) increase in cash and cash equivalents |
|
|
(390,464 |
) |
|
|
484,932 |
|
|
|
(5,208 |
) |
|
Cash and cash equivalents, beginning of period |
|
|
487,255 |
|
|
|
2,323 |
|
|
|
7,531 |
|
|
Cash and cash equivalents, end of period |
|
$ |
96,791 |
|
|
$ |
487,255 |
|
|
$ |
2,323 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-10
TILRAY, INC.
Notes to Consolidated Financial Statements
(in thousands of United States dollars, except for per share data)
1. Description of Business and Summary
Tilray, Inc., a Delaware corporation, and its wholly owned subsidiaries (collectively “Tilray”, the “Company”, “we”, “our”, or “us”), is pioneering the future of medical cannabis research, cultivation, processing and distribution globally, and is one of the leading suppliers of adult-use cannabis in Canada. The Company also markets and distributes food products from hemp seed, offering a broad range of natural and organic food products and ingredients that are sold through retailers and websites globally.
Prior to January 2018, the Company operated its business under Decatur Holdings, B.V. (“Decatur”), which was formed in March 2016. Decatur was incorporated under the laws of the Netherlands on March 8, 2016 as a wholly owned subsidiary of Privateer Holdings, Inc. (“Privateer Holdings”). On January 25, 2018, Privateer Holdings transferred the equity interest in Decatur to Tilray. Decatur was subsequently dissolved on December 27, 2018. The transfers of the equity interests were between entities under common control and were recorded at their carrying amounts. The consolidated financial statements of the Company (“the financial statements”) are prepared, on a continuity of interest basis, reflecting the historical financial information of Decatur prior to January 25, 2018.
2. Summary of Significant Accounting Policies
Basis of presentation and going concern
The accompanying financial statements reflect the accounts of the Company. The financial statements were prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the United States Securities and Exchange Commission (“SEC”).
These financial statements have been prepared on a going concern basis, which assumes that the Company will continue in operation for the foreseeable future and, accordingly, will be able to realize its assets and discharge its liabilities in the normal course of operations as they come due. The Company’s ability to continue as a going concern is dependent upon obtaining additional financing to meet anticipated cash needs for working capital and capital expenditures through the next twelve months.
For the fiscal year ended December 31, 2019 the Company reported a consolidated net loss of $321,169 and a net loss of $67,723 and $7,809 for the year ending December 31, 2018 and December 31, 2017, respectively.
For the years ended December 31, 2019, 2018 and 2017, the Company had negative cash flows used in operating activities of $258,065, $46,248 and $6,003, respectively. The Company had net cash outflows for the year ended December 31, 2019 of $390,464.
As at December 31, 2019 and 2018, the Company had working capital of $166,600 and $528,365 respectively, reflecting a decrease in cash of $361,765 for the year ending December 31, 2019.
Current management forecasts and related assumptions support the view that the Company can adequately manage the operational needs of the business with the additional financing of $59,600 secured on February 28, 2020 (refer to Note 26) and as necessary, through accessing capital from the at-the-market program with available funding of $271,687 (refer to Note 14 and Note 26) or other equity financings. However, due to uncertainties the Company may face in raising additional equity financing in the future, an additional evaluation of management’s plans and forecasts was conducted to assess the Company’s ability to meet their contractual commitments and obligations over the next twelve months.
These management forecasts and assumptions support the Company’s ability to meet its contractual obligations such as non-cancelable minimum purchase commitments for inventory of $132,743 (refer to Note 17), payment of interest on the 5% convertible notes of $23,750 (refer to Note 13), payment of interest on the additional financing (refer to Note 26) and the Company’s lease commitments of $4,576 (refer to Note 17).
Should there be constraints on access to capital under the at-the-market program, the Company can manage cash-outflows through reduced capital expenditures and managing the operational expenses of the business that pertain to future investments that are discretionary in nature. Accordingly, the Company has concluded that it is
F-11
probable that it is able to implement plans that would effectively mitigate the conditions and events that raise substantial doubt about the entity’s ability to continue as a going concern for the next twelve months.
These financial statements do not include any adjustments to the carrying amount and classification of reported assets, liabilities, revenues or expenses that might be necessary should the Company not be successful with the aforementioned initiatives. Any such adjustments could be material.
These financial statements reflect all adjustments, which, in the opinion of management, are necessary for a fair presentation of the Company’s financial position and results of operations.
The statements of net loss and comprehensive loss for the years ended December 31, 2018 and 2017 were reclassified to conform to the current period’s presentation. Cost of sales, which was formerly presented as a single line item, is now broken out between product costs and inventory valuation adjustments. Depreciation and amortization expenses as well as acquisition-related (income) expenses, net, which were formerly presented as part of general and administrative expenses, are now presented separately. Loss on disposal of property and equipment is presented seperately from other income, net.
Large accelerated filer status
The Company is now a large accelerated filer and as a result, the Company complies with new and revised accounting standards applicable to public companies for the year ended December 31, 2019. All new accounting pronouncements recently adopted as described below were adopted in the forth quarter of 2019 with an effective date of January 1, 2019. Quarterly financial information presented in the December 31, 2019 financial statements reflect the new and revised accounting standards and therefore do not mirror the 2019 interim period condensed consolidated financial statements.
Basis of consolidation
These financial statements include the accounts of the following entities wholly owned by the Company as of December 31, 2019:
|
Name of entity |
Date of formation |
Place of incorporation |
|
Natura Naturals Inc. |
May 31, 1985 |
Canada |
|
Tilray, Inc. |
July 8, 2005 |
United States |
|
Manitoba Harvest USA LLC |
February 8, 2010 |
United States |
|
Tilray Canada, Ltd. |
September 6, 2013 |
Canada |
|
Dorada Ventures, Ltd. |
October 18, 2013 |
Canada |
|
Smith & Sinclair Ltd. |
June 1, 2014 |
United Kingdom |
|
FHF Holdings Ltd. |
July 15, 2015 |
Canada |
|
High Park Farms Ltd. |
February 19, 2016 |
Canada |
|
Tilray Deutschland GmbH |
November 3, 2016 |
Germany |
|
Pardal Holdings, Lda. |
April 5, 2017 |
Portugal |
|
Tilray Portugal Unipessoal, Lda. |
April 20, 2017 |
Portugal |
|
Tilray Australia New Zealand Pty. Ltd. |
May 9, 2017 |
Australia |
|
Tilray Ventures Ltd. |
June 6, 2017 |
Ireland |
|
Manitoba Harvest Japan K.K. |
August 29, 2017 |
Japan |
|
High Park Holdings, Ltd. |
February 8, 2018 |
Canada |
|
Fresh Hemp Foods Ltd. |
May 7, 2018 |
Canada |
|
Natura Naturals Holdings Inc. |
May 17, 2018 |
Canada |
|
National Cannabinoid Clinics Pty Ltd. |
September 19, 2018 |
Australia |
|
Tilray Latin America SpA |
November 19, 2018 |
Chile |
|
Tilray Portugal II, Lda. |
December 11, 2018 |
Portugal |
|
High Park Gardens Inc. |
February 7, 2019 |
Canada |
|
High Park Shops Inc. |
August 15, 2019 |
Canada |
|
Privateer Evolution, LLC |
December 12, 2019 |
United States |
The entities listed above are wholly owned by the Company and have been formed or acquired to support the intended operations of the Company and all intercompany transactions and balances have been eliminated in the financial statements of the Company.
During the year ended December 31, 2019 the following entities have been added as a result of business combinations: Natura Naturals Inc., Manitoba Harvest USA LLC, Smith and Sinclair Ltd., FHF Holdings Ltd.,
F-12
Mantitoba Harvest Japan K.K., Fresh Hemp Foods Ltd., Natura Naturals Holdings Inc. Refer to Note 3 for further details on business combinations.
On December 12, 2019, the Company closed the merger of Privateer Holdings, with and into a wholly owned subsidiary of the Company pursuant to the Agreement and Plan of Merger and Reorganization with Privateer Holdings (the “Downstream Merger”). As a result, Privateer Evolution, LLC, previously named Down River Merger Sub, LLC, has been added to the wholly owned entities for the year ended December 31, 2019.
The financial statements also include variable interest entities (“VIE”). A VIE is a legal entity that does not have sufficient equity at risk to finance its activities without additional subordinated financial support, is structured such that equity investors lack the ability to make significant decisions relating to the entity’s operations through voting rights, or do not substantively participate in the gains and losses of the entity. Upon inception of a contractual agreement, the Company performs an assessment to determine whether the arrangement contains a variable interest in a legal entity and whether that legal entity is a VIE. The primary beneficiary has both the power to direct the activities of the VIE that most significantly impact the entity’s economic performance and the obligation to absorb losses or the right to receive benefits from the VIE entity that could potentially be significant to the VIE. Where the Company concludes that it is the primary beneficiary of a VIE, the Company consolidates the accounts of that VIE. When the Company is not the primary beneficiary, the VIE is accounted for using the equity method and is included in Equity method investments within the balance sheets. At December 31, 2019, 2018 and 2017, the Company had no consolidated VIEs. Refer to Note 7 for the Company’s VIEs accounted for using the equity method.
The Company regularly reviews and reconsiders previous conclusions regarding whether the Company is the primary beneficiary of a VIE. The Company also reviews and reconsiders previous conclusions regarding whether the Company holds a variable interest in a potential VIE, the status of an entity as a VIE, and whether the Company is required to consolidate such a VIE in the financial statements when a change occurs.
New accounting pronouncements recently adopted
Financial instruments
On January 1, 2019, the Company adopted FASB ASU No. 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities (“ASU 2016-01”), which updates certain aspects of the recognition, measurement, presentation and disclosure of financial instruments. Most prominent among the changes in the standard is the requirement for changes in the fair value of equity investments, with certain exceptions, to be recognized through net income rather than other comprehensive income.
The Company adopted the standard effective January 1, 2019. Adoption of the standard was applied using a modified retrospective approach through a cumulative effect adjustment from accumulated other comprehensive income to accumulated deficit as of the effective date in the amount of $803. The Company elected to measure equity investments without readily determinable fair values using the measurement alternative, at cost with adjustments for observable changes in price or impairments. The cumulative effect adjustment included any previously held unrealized gains and losses held in accumulated other comprehensive income related to the Company’s equity investments carried at fair value, other than those measured using the measurement alternative, which is applied prospectively.
Leases
In February 2016, the FASB issued ASU 2016-02, Leases, codified as ASC 842 Leases (“ASC 842”). ASC 842 requires leases to be accounted for using a right-of-use model, which recognizes that, at the date of commencement, a lessee has a financial obligation to make lease payments to the lessor for the right to use the underlying asset during the lease term. The lessee recognizes a corresponding right-of-use asset related to this right. Prior to adopting ASC 842, the Company followed the lease accounting guidance as issued in ASC 840, Leases (“ASC 840”) under which the Company classified its leases as operating or capital leases based on evaluation of certain criteria of the lease agreement. Effective January 1, 2019, the Company adopted ASC 842 using the modified retrospective approach, which provides a method for recording existing leases at adoption using the effective date as its date of initial application. The Company also applied the practical expedient which provides an additional transition method which allows entities to elect not to recast comparative periods presented. The Company has
F-13
elected this practical expedient in the adoption of the ASC 842. Lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected remaining lease term.
The Company elected the package of practical expedients provided by ASC 842, which allowed the Company to forgo reassessing the following upon adoption of the new standard: (1) whether contracts contain leases for any expired or existing contracts, (2) the lease classification for any expired or existing leases, and (3) initial direct costs for any existing or expired leases. In addition, the Company elected an accounting policy to exclude from the balance sheet the right-of-use assets and lease liabilities related to short-term leases, which are those leases with a lease term of twelve months or less that do not include an option to purchase the underlying asset that the Company is reasonably certain to exercise.
The standard has a material impact in the Company’s balance sheets, but does not have an impact in the statements of net loss and comprehensive loss. The most significant impact is the recognition of right-of-use assets and lease liabilities for operating leases, while the accounting for finance leases remains substantially unchanged. As of the date of implementation on January 1, 2019, the impact of the adoption of ASC 842 resulted in the recognition of a right-of-use asset and lease liability on the Company’s balance sheet of $3,276 and $3,257, respectively, with a cumulative effect adjustment of $19 to accumulated deficit.
Revenue
On January 1, 2019, the Company adopted ASU 2014-09, Revenue from Contracts with Customers and all subsequent amendments to the ASU, codified as ASC 606 Revenue from Contracts with Customers (collectively, “ASC 606”), which amended revenue recognition principles and provides a single, comprehensive set of criteria for revenue recognition. ASC 606 applies to all contracts with customers except for contracts that are within the scope of other standards.
ASC 606 provides a five-step framework through which revenue is recognized when control of promised goods or services is transferred to a customer at an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. To determine revenue recognition for arrangements that the Company concludes are within the scope of ASC 606, management performs the following five steps: (i) identifies the contract(s) with a customer; (ii) identifies the performance obligations in the contract (s); (iii) determines the transaction price, including whether there are any constraints on variable consideration; (iv) allocates the transaction price to the performance obligations; and (v) recognizes revenue when (or as) the Company satisfies a performance obligation.
The Company adopted ASC 606 using the modified retrospective method to all contracts not completed as of January 1, 2019. Results for reporting periods beginning after January 1, 2019 are presented under ASC 606 while prior period amounts continue to be reported in accordance with pre-adoption standards. The adoption of ASC 606 did not result in a change to the accounting for any of the in-scope revenue contracts; as such, no cumulative effect adjustment was recorded.
Accounting for nonemployee share-based compensation
In June 2018, the FASB issued ASU 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”), to simplify the accounting for share-based payments to nonemployees by aligning it with the accounting for share-based payments to employees, with certain exceptions. The provisions of this standard specify that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. The Company adopted the provisions of ASU 2018-07 using a modified retrospective approach on January 1, 2019, which affected the method used to value the stock options and RSUs granted to consultants and advisors. Prior to the adoption of ASU 2018-07, stock options and RSUs were revalued at each reporting period. Pursuant to the requirements of ASU 2018-07 and under the provisions of Topic 718, these stock options and RSUs are now valued at the grant date fair value, consistent with the method the Company uses to value stock options and RSUs to employees. Adoption of the standard resulted in no cumulative effect adjustment.
F-14
Use of estimates and significant judgments
The preparation of the Company’s financial statements requires management to make estimates, assumptions and judgments that affect the reported amounts of revenue, expenses, assets, liabilities, accompanying disclosures and the disclosure of contingent liabilities. These estimates and judgments are subject to change based on experience and new information which could result in outcomes that require a material adjustment to the carrying amounts of assets or liabilities affecting future periods. Estimates and judgments are assessed on an ongoing basis. Revisions to estimates are recognized prospectively.
Examples of key estimates in these financial statements include cash flows and discount rates used in accounting for business combinations including contingent consideration, the value of Class 2 common shares with transfer restrictions, asset impairment including estimated future cash flows and fair values, imputed interest for loans receivable, the allowance for doubtful accounts receivable and loans receivables, provisions for prepayments and other current assets, inventory valuation adjustments that contemplate the market value of, and demand for inventory, estimated useful lives of property and equipment and intangible assets, valuation allowance on deferred income tax assets, determining the fair value of financial instruments, fair value of stock-based compensation, estimated variable consideration on contracts with customers, sales return estimates, the fair value of the convertible notes and equity component and the classification, incremental borrowing rates and lease terms applicable to lease contracts.
Financial statement areas that require significant judgments are as follows:
Variable interest entities - The Company assesses all variable interests in entities and uses judgment when determining if the Company is the primary beneficiary. Other qualitative factors that are considered include decision-making responsibilities, the VIE capital structure, risk and rewards sharing, contractual agreements with the VIE, voting rights and the level of involvement of other parties.
Contingent consideration – Contingent consideration is subject to measurement uncertainty as the financial impact will only be confirmed by the outcome of a future event. The assessment of contingent consideration involves a significant amount of judgment, including determining a reliable estimate of the amount of cash outflow required to settle the obligation based on significant unobservable inputs as well as estimates around the probability and timing of satisfying the future events on which the contingent consideration is based.
Asset impairment – Asset impairment tests require the allocation of assets to asset groups, where appropriate, which requires significant judgment and interpretation with respect to the integration between the assets and shared resources. Asset impairment tests require the determination of whether there is an indication of impairment. The assessment of whether an indication of impairment exists is performed at the end of each reporting period and requires the application of judgment, historical experience, and external and internal sources of information.
Leases – The Company applies judgment in determining whether a contract contains a lease and if a lease is classified as an operating lease or a finance lease. The Company determines the lease term as the non-cancellable term of the lease, which may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
The Company has several lease contracts that include extension and termination options. The Company applies judgment in evaluating whether it is reasonably certain whether or not to exercise the option to renew or terminate the lease. That is, it considers all relevant factors that create an economic incentive for it to exercise either the renewal or termination. After the commencement date, the Company reassesses the lease term if there is a significant event or change in circumstances that is within its control and affects its ability to exercise or not to exercise the option to renew or to terminate (e.g., construction of significant leasehold improvements or significant customization to the leased asset).
The Company also applies judgment in allocating the consideration in a contract between lease and non-lease components. It considers whether the Company can benefit from the right-of-use asset either on its own or together with other resources and whether the asset is highly dependent on or highly interrelated with another right-of-use asset.
Foreign currency
These financial statements are presented in the United States dollar (“USD”), which is the Company’s reporting currency. Functional currencies for the entities in these financial statements are their respective local
F-15
currencies, including USD, Canadian dollar (“CAD”), Australian dollar, Chilean Peso, Great Britain Pound, Japanese Yen and Euro.
The assets and liabilities of each of the Company’s subsidiaries are translated to USD at the foreign exchange rate in effect at the balance sheet date. Certain transactions affecting the stockholders’ equity (deficit) are translated at historical foreign exchange rates. The statements of net loss and comprehensive loss and statements of cash flows are translated to USD applying the average foreign exchange rate in effect during the reporting period. The resulting translation adjustments are included in other comprehensive loss.
The Company’s monetary assets and liabilities denominated in foreign currencies are translated to the functional currency by applying the foreign exchange rate in effect at the balance sheet date. Revenues and expenses are translated using the average foreign exchange rate in effect during the reporting period. Realized and unrealized foreign currency differences are recognized in the statements of net loss and comprehensive loss.
Net loss per share
Basic net loss per share is computed by dividing reported net loss by the weighted average number of common shares outstanding for the reported period. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock of the Company during the reporting period. Diluted net loss per share is computed by dividing net loss by the sum of the weighted average number of common shares and the number of potential dilutive common share equivalents outstanding during the period. Potential dilutive common share equivalents consist of the incremental common shares issuable upon the exercise of vested share options and the incremental shares issuable upon conversion of the convertible notes. Potential dilutive common share equivalents consist of stock options, restricted stock units (“RSUs”) and restricted stock awards.
In computing diluted earnings per share, common share equivalents are not considered in periods in which a net loss is reported, as the inclusion of the common share equivalents would be anti-dilutive. As of December 31, 2019, there were 10,532,988 common share equivalents with potential dilutive impact (2018 - 7,902,263, 2017 - none). Since the Company is in a net loss for all periods presented in these financial statements, there is no difference between the Company’s basic and diluted net loss per share for the periods presented.
Cash and cash equivalents
Cash and cash equivalents are comprised of cash and highly liquid investments that are readily convertible into known amounts of cash with original maturities of three months or less.
Cash and cash equivalents include amounts held in USD, CAD, Euro, Australian dollar, Chilean Peso, Great Britain Pound, Japanese Yen, corporate bonds, commercial paper, treasury bills and money market funds.
Investments
As a result of the adoption of ASU 2016-01 on January 1, 2019, the Company has changed its accounting policy for investments. Investments consist of debt securities and equity investments. Debt securities consists of convertible debt securities. Equity investments generally consist of securities that represent ownership interests in an entity for which the Company does not have a controlling financial interest.
Debt securities
Debt securities are classified as available-for-sale and are recorded at fair value. Unrealized gains and losses during the year, net of the related tax effect, are excluded from income and reflected in other comprehensive income (loss), and the cumulative effect is reported as a separate component of shareholders’ equity until realized. Debt securities are impaired when a decline in fair value is determined to be other-than-temporary. If the cost of an investment exceeds its fair value, the Company evaluates, among other factors, general market conditions, credit quality of debt instrument issuers, and the duration and extent to which the fair value is less than cost. Once a decline in fair value is determined to be other-than-temporary, an impairment charge is recorded in the statements of net loss and a new cost basis for the investment is established. The Company also evaluates whether there is a plan to sell the security or it is more likely than not that the Company will be required to sell the security before recovery. If neither of the conditions exist, then only the portion of the impairment loss attributable to credit loss is recorded in the statements of net loss and the remaining amount is recorded in other comprehensive income (loss).
F-16
Equity investments
Investments in entities over which the Company does not have a controlling financial interest or significant influence are accounted for at fair value. Equity investments without readily determinable fair values are measured at cost with adjustments for observable changes in price or impairments (referred to as the “measurement alternative”). In applying the measurement alternative, the Company performs a qualitative assessment on a quarterly basis and recognizes an impairment if there are sufficient indicators that the fair value of the equity investments are less than carrying values. Changes in value are recorded in other income, net.
Investments in entities over which the Company does not have a controlling financial interest but has significant influence, are accounted for using the equity method, with the Company’s share of earnings or losses reported in earnings or losses from equity method investments on the statements of net loss and comprehensive loss. Equity method investments are recorded at cost, plus the Company’s share of undistributed earnings or losses, and impairment, if any, within Equity method investments on the balance sheets.
The Company assesses investments in equity method investments if there is reason to believe an impairment may have occurred including, but not limited to, ongoing operating losses, projected decreases in earnings, increases in the weighted-average cost of capital, or significant business disruptions. The significant assumptions used to estimate fair value include revenue growth and profitability, capital spending, depreciation and taxes, foreign currency exchange rates, and discount rate. By their nature, these projections and assumptions are uncertain. If it is determined that the current fair value of an equity method investment is less than the carrying value of the investment, the Company will assess if the shortfall is of a temporary or permanent nature and write down the investment to its fair value if it is concluded the impairment is other than temporary.
Accounting policy related to periods prior to the adoption of ASU 2016-01
Investments consist of treasury bills and equity securities. Equity securities generally consist of securities that represent ownership interests in an enterprise for which do not have significant influence or a controlling financial interest. The Company’s investments are classified as available-for-sale securities or as a cost method investment.
Available-for-sale securities
Securities classified as available-for-sale are recorded at fair value. Unrealized gains and losses during the year, net of the related tax effect applicable to available-for-sale, are excluded from income and reflected in other comprehensive income, and the cumulative effect is reported as a separate component of shareholders’ equity until realized. If a decline in fair value is deemed to be other-than-temporary, the investment is written down to its fair value and the amount of the write-down is recorded as other-than-temporary impairment loss in the statements of net loss and comprehensive loss. Any portion of such decline related to the securities that are not held-to-maturity and is believed to arise from factors other than credit is recorded as a component of other comprehensive income rather than against income.
Net realized gains and losses on investments are determined in accordance with the specific identification method.
Cost method investments
Equity securities for which the fair value is not readily determinable are carried at cost. Distributions from the equity security are recognized as income dividend when received.
An impairment charge is recorded if the carrying amount of the investment exceeds its fair value and determined to be other-than-temporary.
Business combinations and goodwill
The Company accounts for business combinations using the acquisition method in accordance with ASC 805, Business Combinations, which requires recognition of assets acquired and liabilities assumed, including contingent assets and liabilities, at their respective fair values on the date of acquisition. Any excess of the purchase consideration over the net fair value of tangible and identified intangible assets acquired less liabilities assumed is
F-17
recorded as goodwill. The costs of business acquisitions, including fees for accounting, legal, professional consulting and valuation specialists, are expensed as incurred within acquisition-related (income) expenses, net. Purchase price allocations may be preliminary and, during the measurement period not to exceed one year from the date of acquisition, changes in assumptions and estimates that result in adjustments to the fair value of assets acquired and liabilities assumed are recorded in the period the adjustments are determined.
For business combinations achieved in stages, the Company’s previously held interest in the acquiree is remeasured at its acquisition date fair value, with the resulting gain or loss recorded in the statements of net loss and comprehensive loss. For a pre-existing relationship between the Company and the acquiree that is not extinguished on the business combination, such a relationship is considered effectively settled as part of the business combination even if it is not legally cancelled. At the acquisition date, it becomes an intercompany relationship and is eliminated upon consolidation.
The estimated fair value of acquired assets and assumed liabilities are determined primarily using a discounted cash flow approach, with estimated cash flows discounted at a rate that the Company believes a market participant would determine to be commensurate with the inherent risks associated with the asset and related estimated cash flow streams. Contingent consideration in a business combination is remeasured at fair value each reporting period until the contingency is resolved and any change in fair value from either the passage of time or events occurring after the acquisition date, is recorded within acquisition-related (income) expenses, net on the statements of net loss and comprehensive loss.
Fair value measurements
The carrying value of the Company’s accounts receivable, accounts payable, accrued expenses and other current liabilities approximate their fair value due to their short-term nature. Debt securities classified as available-for-sale are recorded at fair value based on publicly available market information or other estimates determined by management. Equity investments (excluding equity method investments) are recorded at fair value using quoted market prices or broker or dealer quotations, or using the measurement alternative for equity investments without readily determinable fair values. The fair value for equity investments measured using the measurement alternative is determined based on valuation techniques using the best information available, and may include quoted market prices, market comparables, and discounted cash flow projections. Contingent consideration is measured at fair value on a recurring basis based on discounted cash flow projections.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability if market participants would take those characteristics into account when pricing the asset or liability at the measurement date.
Inventory
Inventory is comprised of raw materials, finished goods and work-in-progress. Cost includes expenditures directly related to the manufacturing process as well as suitable portions of related production overheads, based on normal operating capacity.
Cannabis: Inventory cost includes pre-harvest, post-harvest and shipment and fulfillment, as well as related accessories. Pre-harvest costs include labor and direct materials to grow cannabis, which includes water, electricity, nutrients, integrated pest management, growing supplies and allocated overhead. Post-harvest costs include costs associated with drying, trimming, blending, extraction, purification, quality testing and allocated overhead. Shipment and fulfillment costs include the costs of packaging, labelling, courier services and allocated overhead.
Hemp: Inventory cost includes seeds, packaging and co-packing. Seed costs include commodity cost from farmers, genetic seed cost to provide and manage contracted farmers, hulling and processing costs, including labor and overhead. Packaging costs include packaging materials, labor and overhead to running machinery. Co-packing cost are generally for products not manufactured by the Company directly and would include the all costs to produce the products.
Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company
F-18
performs an assessment of inventory and records write-downs for excess and obsolete inventories based on the Company’s estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. Actual inventory losses may differ from management’s estimates and such differences could be material to the Company’s balance sheets, statements of net loss and comprehensive loss and statements of cash flows.
Property and equipment
Property and equipment are recorded at cost net of accumulated depreciation and impairment, if any. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. The estimated useful life of buildings ranges from twenty to twenty-five years and the estimated useful life of property and equipment, other than buildings, ranges from three to fifteen years. Land is not depreciated. Leasehold improvements are depreciated over the lesser of the asset’s estimated useful life or the remaining lease term.
When assets are retired or disposed of, the cost and accumulated depreciation are removed from the respective accounts and any related gain or loss is recognized. Maintenance and repairs are charged to expenses as incurred. Significant expenditures, which extend the useful lives of assets or increase productivity, are capitalized. When significant parts of an item of property and equipment have different useful lives, they are accounted for as separate items or components of property and equipment.
Construction-in-process includes construction progress payments, deposits, engineering costs, interest expense on long-term construction projects and other costs directly related to the construction of the facilities. Expenditures are capitalized during the construction period and construction in progress is transferred to the relevant class of property and equipment when the assets are available for use, at which point the depreciation of the asset commences.
The estimated useful lives are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
Capitalization of interest
Interest incurred relating to the construction or expansion of facilities is capitalized to the construction in progress. The Company ceases the capitalization of interest when construction activities are substantially completed and the facility is available for commercial use
Intangible assets
Intangible assets include intangible assets acquired as part of business combinations, asset acquisitions and other business transactions. The Company records intangible assets at cost, net of accumulated amortization and accumulated impairment losses, if any. Cost is measured based on the fair values of cash consideration paid and equity interests issued. The cost of an intangible asset acquired is its acquisition date fair value.
The Company capitalizes certain internal-use software development costs, consisting primarily of contractor costs and employee salaries and benefits allocated to the software. Capitalization of costs incurred in connection with internally developed software commences when both the preliminary project stage is completed and management has authorized further funding for the project, based on a determination that it is probable the project will be completed and used to perform the function intended. Capitalization of costs ceases no later than the point at which the project is substantially complete and ready for its intended use. All other costs are expensed as incurred. Amortization is calculated on a straight-line basis over three years. Costs incurred for enhancements that are expected to result in additional functionalities are capitalized.
Amortization of definite life intangible assets is calculated on a straight-line basis over the estimated useful lives of the assets as follows:
|
Patents |
|
4 years |
|
Customer relationships |
|
14 to 16 years |
|
Developed technology |
|
10 years |
|
Websites |
|
3 years |
|
Definite life trademarks and licenses |
|
Term of agreements |
F-19
When there is no foreseeable limit on the period of time over which an intangible asset is expected to contribute to the cash flows of the Company, an intangible asset is determined to have an indefinite life. Indefinite life intangible assets are not amortized, but tested for impairment annually or more frequently when indicators of impairment exist. If the carrying value of an individual indefinite-lived intangible asset exceeds its fair value, such individual indefinite-life intangible asset is impaired by the amount of the excess.
The estimated useful lives are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
Impairment of long-lived assets
The Company reviews long-lived assets, including property and equipment and definite life intangible assets, for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. In order to determine if assets have been impaired, assets are grouped and tested at the lowest level for which identifiable independent cash flows are available (“asset group”). An impairment loss is recognized when the sum of projected undiscounted cash flows is less than the carrying value of the asset group. The measurement of the impairment loss to be recognized is based on the difference between the fair value and the carrying value of the asset group. Fair value can be determined using a market approach, income approach or cost approach. The reversal of impairment losses is prohibited.
Impairment of goodwill and indefinite life intangible assets
Goodwill and indefinite life intangible assets are tested for impairment annually, or more frequently when events or circumstances indicate that impairment may have occurred. As part of the impairment evaluation, the Company may elect to perform an assessment of qualitative factors. If this qualitative assessment indicates that it is more likely than not that the fair value of the indefinite-lived intangible asset or the reporting unit (for goodwill) is less than its carrying value, a quantitative impairment test to compare the fair value to the carrying value. An impairment charge is recorded if the carrying value exceeds the fair value.
Leases
As a result of the adoption of ASC 842 on January 1, 2019, the Company has changed its accounting policy for leases. The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right‐of‐use (“ROU”) assets and accrued obligations under operating lease (current and non-current) in the balance sheets. Finance lease ROU assets are included in property and equipment, net and accrued obligations under finance lease (current and non-current) in the balance sheets.
ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. ROU assets are classified as a finance lease or an operating lease. A finance lease is a lease in which 1) ownership of the property transfers to the lessee by the end of the lease term; 2) the lease grants the lessee an option to purchase the underlying asset that the lessee is reasonably certain to exercise; 3) the lease is for a major part of the remaining economic life of the underlying asset; 4) The present value of the sum of the lease payments and any residual value guaranteed by the lessee that is not already included in the lease payments equals or exceeds substantially all of the fair value; or 5) the underlying asset is of such a specialized nature that it is expected to have no alternative use to the lessor at the end of the lease term. The Company classifies a lease as an operating lease when it does not meet any one of these criteria.
ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, the incremental borrowing rate is used based on the information available at commencement date in determining the present value of lease payments. The Company uses the implicit rate when readily determinable. The ROU assets also include any lease payments made and excludes lease incentives. The lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
For finance leases, lease expenses are the sum of interest on the lease obligations and amortization of the ROU assets, resulting in a front-loaded expense pattern. The expenses form part of facility costs which are included in product costs within cost of sales within the statements of net loss and comprehensive loss. ROU assets are amortized based on the lesser of the lease term and the useful life of the leased asset according to the property and
F-20
equipment accounting policy. If ownership of the ROU assets transfers to the Company at the end of the lease term or if the Company is reasonably certain to exercise a purchase option, amortization is calculated using the estimated useful life of the leased asset, according to the property and equipment accounting policy. For operating leases, the lease expenses are generally recognized on a straight-line basis over the lease term and recorded to general and administrative expenses in the statements of net loss and comprehensive loss.
The Company has elected to apply the practical expedient, for each class of underlying asset, except real estate leases, to not separate non-lease components from the associated lease components of the lessee’s contract and account for both components as a single lease component. Additionally, for certain equipment leases, the Company applies a portfolio approach to effectively account for the operating lease ROU assets and liabilities.
The Company has elected not to recognize ROU assets and lease liabilities for short-term leases that have a lease term of 12 months or less that do not include an option to purchase the underlying asset that the Company is reasonably certain to exercise. Short-term leases include real estate and vehicles and are not significant in comparison to the Company’s overall lease portfolio. The Company continues to recognize the lease payments associated with these leases as expenses on a straight-line basis over the lease term.
Accounting policy related to periods prior to the adoption of ASC 842
The Company enters into various leases in conducting its business. At the inception of each lease, the Company evaluates the lease agreement to determine whether the lease is an operating or capital lease. A capital lease is a lease in which 1) ownership of the property transfers to the lessee by the end of the lease term; 2) the lease contains a bargain purchase option; 3) the lease term is equal to 75% or more of the economic life of the leased property; or 4) the present value of the minimum lease payment at the inception of the lease term equals or exceeds 90% of the fair value of the leased property.
An asset and a corresponding liability are established at inception for capital leases. The capital lease assets are included in property and equipment and the capital lease obligations are included in accrued obligations under finance lease. Operating lease payments are recognized as expenses on a straight-line basis over the lease term.
Convertible notes
The Company accounts for its convertible notes with a cash conversion feature in accordance with ASC 470-20, Debt with Conversion and Other Options (“ASC 470-20”), which requires the liability and equity components of convertible debt instruments that may be settled in cash upon conversion, including partial cash settlement, to be separately accounted for in a manner that reflects the issuer’s nonconvertible debt borrowing rate. The initial proceeds from the sale of convertible notes are allocated between a liability component and an equity component in a manner that reflects interest expense at the rate of similar nonconvertible debt that could have been issued at such time. The equity component represents the excess initial proceeds received over the fair value of the liability component of the notes as of the date of issuance. The resulting debt discount is amortized over the period during which the convertible notes are expected to be outstanding as additional non-cash interest expenses.
Upon repurchase of convertible debt instruments, ASC 470-20 requires the issuer to allocate total settlement consideration, inclusive of transaction costs, amongst the liability and equity components of the instrument based on the fair value of the liability component immediately prior to repurchase. The difference between the settlement consideration allocated to the liability component and the net carrying value of the liability component, including unamortized debt issuance costs, would be recognized as gain (loss) on extinguishment of debt in the statements of net loss and comprehensive loss. The remaining settlement consideration allocated to the equity component would be recognized as a reduction of additional paid-in capital in the balance sheets.
Revenue recognition
As a result of the adoption of ASC 606 on January 1, 2019, the Company has changed its accounting policy for revenue recognition. Revenue is recognized when control of the promised goods or services, through performance obligations by the Company, is transferred to the customer in an amount that reflects the consideration it expects to be entitled to in exchange for the performance obligations.
F-21
The Company generates substantially all of its revenue from the sale of cannabis and hemp products through contracts with customers. Cannabis and hemp products are sold through various distribution channels. Revenue is recognized when the control of the goods is transferred to the customer, which occurs at a point in time, typically upon delivery to or receipt by the customer, depending on shipping terms.
Sales taxes collected from customers are remitted to the appropriate taxing jurisdictions and are excluded from sales revenue as the Company considers itself a pass-through conduit for collecting and remitting sales taxes. Excise duties that are both imposed on and concurrent with a specific revenue-producing transaction, that are collected by the Company from a customer are included in revenue. Freight revenues on all product sales, when applicable, are also recognized, on a consistent manner, at a point in time. The term between invoicing and when payment is due is not significant and the period between when the entity transfers the promised good or service to the customer and when the customer pays for that good or service is one year or less.
The Company considers whether there are other promises in the contracts that are separate performance obligations to which a portion of the transaction price needs to be allocated. In determining the transaction price for the sale of goods, the Company considers the effects of variable consideration and the existence of significant financing components (if any).
|
(i) |
Variable consideration |
Some contracts for the sale of goods may provide customers with a right of return, volume discount, bonuses for volume/quality achievement, or sales allowance. In addition, the Company may provide in certain circumstances, a retrospective price reduction to a customer based primarily on inventory movement. These items give rise to variable consideration. The Company uses the expected value method to estimate the variable consideration because this method best predicts the amount of variable consideration to which the Company will be entitled. The Company uses historical evidence, current information and forecasts to estimate the variable consideration. The requirements in ASC 606 on constraining estimates of variable consideration are applied to determine the amount of variable consideration that can be included in the transaction price. The Company reduces revenue and recognizes a contract liability equal to the amount expected to be refunded to the customer in the form of a future rebate or credit for a retrospective price reduction, representing its obligation to return the customer’s consideration. The estimate is updated at each reporting period.
|
(ii) |
Significant financing component |
The Company may receive short-term advances from its customers. Using the practical expedient in ASC 606, the Company does not adjust the promised amount of consideration for the effects of a significant financing component if the Company expects, at contract inception, that the period between when the Company transfers a promised good to a customer and when the customer pays for that good or service will be one year or less. The Company has not, nor expects to receive long-term advances from customers.
|
(iii) |
Contract balance |
Contract assets
A contract asset is the right to consideration in exchange for goods or services transferred to the customer. If the Company performs by transferring goods to a customer before the customer pays consideration or before payment is due, a contract asset is recognized for the earned consideration.
Accounts receivable
A receivable represents the Company’s right to an amount of consideration that is unconditional (i.e., only the passage of time is required before payment of the consideration).
F-22
Contract liabilities
A contract liability is the obligation to transfer goods or services to a customer for which the Company has received consideration from the customer. If a customer pays consideration before the Company transfers goods or services, a contract liability is recognised when the payment is made. Contract liabilities are recognised as revenue when the Company performs under the contract.
Right of return assets
Right of return assets represent the Company’s right to recover the goods expected to be returned by customers. The asset is measured at the former carrying amount of the inventory, less any expected costs to recover the goods, including any potential decreases in the value of the returned goods. The Company updates the measurement of the asset recorded for any revisions to its expected level of returns, as well as any additional decreases in the value of the returned products.
Refund liabilities
A refund liability is the obligation to refund some or all of the consideration received (or receivable) from the customer and is measured at the amount the Company ultimately expects it will have to return to the customer. The Company updates its estimates of refund liabilities (and the corresponding change in the transaction price) at each reporting period. Refer to above accounting policy on variable consideration.
Accounting policy related to periods prior to the adoption of ASC 606
The Company recognizes revenue as earned when the following four criteria have been met: (i) when persuasive evidence of an arrangement exists, (ii) the product has been delivered to a customer, (iii) the sales price is fixed or determinable, and (iv) collection is reasonably assured. Revenue is recognized net of sales incentives and returns, after discounts for the assurance program, veterans coverage program and compassionate programs.
Direct-to-patient sales are recognized when the products are shipped to the customers. Bulk and adult-use sales under wholesale agreements are recognized based on the shipping terms of the agreements. Export sales under pharmaceutical distribution and pharmacy supply agreements are recognized when products are delivered to the end customers or patients.
Customer loyalty awards are accounted for as a separate component of the sales transaction in which they are granted. A portion of the consideration received in a transaction that includes the issuance of an award is deferred until the awards are ultimately redeemed. The allocation of the consideration to the award is based on an evaluation of the award’s estimated fair value at the date of the transaction. The customer loyalty program was discontinued in September 2017 and all customer loyalty awards expired as at December 31, 2017.
Cost of sales
Cost of sales represents costs directly related to manufacturing and distribution of the Company’s products. Primary costs include raw materials, packaging, direct labor, overhead, shipping and handling and the depreciation of manufacturing equipment and production facilities. Manufacturing overhead and related expenses include salaries, wages, employee benefits, utilities, maintenance and property taxes. Cost of sales also includes inventory valuation adjustments. The Company recognizes the cost of sales as the associated revenues are recognized.
Stock-based compensation
The Company measures and recognizes compensation expense for stock options and RSUs to employees and non-employees on a straight-line basis over the vesting period based on their grant date fair values. Prior to the adoption of ASU 2018-07 on January 1, 2019, the fair value of stock options and RSUs to non-employees were re-measured at each reporting date until one of either of the counterparty’s commitment to perform is established or until the performance is complete. The Company estimates the fair value of stock options on the date of grant using the Black-Scholes option pricing model.
The fair value of RSUs is based on the share price as at date of grant. For stock options and RSUs granted in 2018, prior to the Company’s initial public offering, the fair value of common stock at the date of grant was
F-23
determined by the Board of Directors with assistance from third-party valuation specialists. The Company estimates forfeitures at the time of grant and revises these estimates in subsequent periods if actual forfeitures differ from those estimates.
For performance-based stock options and RSUs, the Company records compensation expense over the estimated service period adjusted for a probability factor of achieving the performance-based milestones. At each reporting date, the Company assesses the probability factor and records compensation expense accordingly, net of estimated forfeitures.
Fully vested, non-forfeitable equity instruments issued to parties other than employees are measured on the date they are issued where there is no specific performance required by the grantee to retain those equity instruments. Stock-based payment transactions with non-employees are measured at the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. Where fully vested, non-forfeitable equity instruments are granted to parties other than employees in exchange for notes or financing receivable, the note or receivable is presented in additional paid-in capital on the balance sheets.
Income taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Management makes an assessment of the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
The Company recognizes uncertain income tax positions at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Changes in recognition or measurement are reflected in the period in which judgment occurs.
Accounting changes – segmented reporting
With the acquisition of FHF Holdings Ltd. (“Manitoba Harvest”) on February 28, 2019, the Company began realigning its management structure along with major product categories and determined the process was sufficiently advanced on October 1, 2019 to identify two operating and reportable segments: Cannabis and Hemp. The Company performed a goodwill impairment test immediately before and after the change. The goodwill impairment tests did not result in impairment. Prior period amounts contained in the financial statements have been adjusted to conform to the new segment presentation (refer to Note 25).
New accounting pronouncements not yet adopted
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires the measurement of current expected credit losses for financial assets held at the reporting date based on historical experience, current conditions and reasonable and supportable forecasts. Adoption of ASU 2016-13 will require financial institutions and other organizations to use forward-looking information to better formulate their credit loss estimates. In addition, the ASU amends the accounting for credit losses on available for sale debt securities and purchased financial assets with credit deterioration. This update will be effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company expects to implement the provisions of ASU 2016-13 as of January 1, 2020. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements.
In August 2018, the FASB issued ASU 2018-13, Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement (Topic 820). ASU 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements. ASU 2018-13 is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements.
F-24
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements.
In January 2020, the FASB issued ASU 2020-01, Investments - Equity Securities (Topic 321), Investments - Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) (“ASU 2020-01”), which is intended to clarify the interaction of the accounting for equity securities under Topic 321 and investments accounted for under the equity method of accounting in Topic 323 and the accounting for certain forward contracts and purchased options accounted for under Topic 815. ASU 2020-01 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements.
3. Business Combinations
Acquisition of Manitoba Harvest
On February 28, 2019, the Company completed the acquisition of all issued and outstanding shares of Manitoba Harvest. Manitoba Harvest develops and distributes a diverse portfolio of hemp-based natural food and wellness products and enables the Company to expand into the growing cannabidiol (“CBD”) product market in the United States.
Subsequent to the acquisition date, the Company revised the preliminary purchase price of the Manitoba Harvest acquisition to include working capital adjustments of $280 related to the acquisition. The Company also revised the preliminary allocation of the purchase price to assets acquired and liabilities assumed at the acquisition date, resulting in a $1,112 decrease in goodwill. The Company completed the final purchase price allocation for Manitoba Harvest. The goodwill of $126,881, assigned to the Hemp reportable segment (refer to Note 11), is attributable to factors such as market share, reputation with customers and vendors, and the skilled workforce of Manitoba Harvest. Goodwill is not deductible for tax purposes. The gross contractual amount of receivables as at the date of acquisition was $6,340, of which approximately $133 was not expected to be collected.
The financial results of Manitoba Harvest are included in the Company’s financial statements since acquisition close. The statements of net loss and comprehensive loss include revenue of $58,029 and net loss of $14,441 of Manitoba Harvest for the year ended December 31, 2019, respectively. The Company incurred acquisition costs of $1,328 for the acquisition of Manitoba Harvest.
Acquisition of Natura
On February 15, 2019, the Company acquired the remaining 97% issued and outstanding shares of Natura Naturals Holdings Inc. (“Natura”). Natura is licensed to cultivate and produce medical cannabis, expanding the Company’s capacity to supply high-quality branded cannabis products to the Canadian market.
The Company revised the preliminary allocation of the purchase price to assets acquired and liabilities assumed at the acquisition date, resulting in a $2,340 increase in goodwill. The Company completed the final purchase price allocation. The goodwill of $29,314, assigned to the Cannabis reportable segment (refer to Note 11), is attributable to factors such as strong supply chain, quality of products and the skilled workforce of Natura. Goodwill is not deductible for tax purposes.
The financial results of Natura are included in the Company’s financial statements since acquisition close. The statements of net loss and comprehensive loss include revenue of $14,544 and net loss of $125 for the year ended December 31, 2019, respectively. The Company incurred acquisition costs of $824 for the acquisition of Natura.
F-25
Acquisition of S&S
On July 11, 2019, the Company acquired all issued and outstanding shares of Smith & Sinclair Ltd. (“S&S”), which crafts edible candies, fragrances and creative consumables in the United Kingdom and enables the Company to develop CBD-infused edibles and beverages as well as alcohol-infused edibles for distribution in Canada, United States and Europe. The financial results of S&S are included in the Company’s financial statements since acquisition close. The goodwill of $4,932 is assigned to the Hemp reportable segment (refer to Note 11). The statements of net loss and comprehensive loss include revenue of $1,633 and net loss of $2,774 for the year ended December 31, 2019, respectively.
F-26
The final allocations of the purchase price to assets acquired and liabilities assumed on the respective acquisition dates of Manitoba Harvest, Natura and S&S are as follows:
|
|
|
Manitoba Harvest |
|
|
Natura |
|
|
S&S |
|
|||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
5,534 |
|
|
|
169 |
|
|
|
137 |
|
|
Accounts receivable |
|
|
6,207 |
|
|
|
109 |
|
|
|
264 |
|
|
Inventory |
|
|
15,331 |
|
|
|
3,482 |
|
|
|
195 |
|
|
Prepayments and other current assets |
|
|
1,030 |
|
|
|
166 |
|
|
|
125 |
|
|
Property and equipment |
|
|
23,581 |
|
|
|
17,435 |
|
|
|
138 |
|
|
Intangible assets(1)(2)(3) |
|
|
195,966 |
|
|
|
10,494 |
|
|
|
2,418 |
|
|
Goodwill |
|
|
126,881 |
|
|
|
29,314 |
|
|
|
4,932 |
|
|
Total assets |
|
|
374,530 |
|
|
|
61,169 |
|
|
|
8,209 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
|
4,973 |
|
|
|
3,280 |
|
|
|
220 |
|
|
Accrued expenses and other current liabilities |
|
|
4,911 |
|
|
|
876 |
|
|
|
89 |
|
|
Deferred tax liability |
|
|
54,393 |
|
|
|
2,781 |
|
|
|
459 |
|
|
Total liabilities |
|
|
64,277 |
|
|
|
6,937 |
|
|
|
768 |
|
|
Net assets acquired |
|
$ |
310,253 |
|
|
$ |
54,232 |
|
|
$ |
7,441 |
|
Intangible assets include:
|
(1) |
Manitoba Harvest: trademarks - $54,688, developed technology - $6,988 and customer relationships - $134,290 |
|
(2) |
Natura: licenses - $10,494 |
|
(3) |
S&S: trademarks - $1,670, patent - $690 and website - $58 |
The final purchase price of the Manitoba Harvest, Natura and S&S acquisitions are calculated as follows:
|
|
|
Manitoba Harvest |
|
|
Natura |
|
|
S&S |
|
|||
|
Cash paid on closing |
|
$ |
114,566 |
|
|
$ |
15,253 |
|
|
$ |
2,420 |
|
|
Cash paid six months after closing |
|
|
37,490 |
|
|
|
— |
|
|
|
— |
|
|
Class 2 common stock issued on closing(1)(2)(5) |
|
|
96,844 |
|
|
|
15,099 |
|
|
|
3,189 |
|
|
Class 2 common stock issued six months after closing (1) |
|
|
31,866 |
|
|
|
— |
|
|
|
— |
|
|
Working capital adjustment |
|
|
280 |
|
|
|
— |
|
|
|
— |
|
|
Contingent consideration |
|
|
29,207 |
|
|
|
20,007 |
|
|
|
1,812 |
|
|
Fair value of previously held interest (3) |
|
|
— |
|
|
|
1,565 |
|
|
|
— |
|
|
Effective settlement of pre-existing debt (4) |
|
|
— |
|
|
|
2,308 |
|
|
|
— |
|
|
Subscription rights |
|
|
— |
|
|
|
— |
|
|
|
20 |
|
|
Total fair value of consideration transferred |
|
|
310,253 |
|
|
|
54,232 |
|
|
|
7,441 |
|
|
(1) |
For the acquisition of Manitoba Harvest, 1,209,946 shares of Class 2 common stock were issued on closing and 899,306 shares of Class 2 common stock were issued six months after closing. |
|
(2) |
For the acquisition of Natura, 180,332 shares of Class 2 common stock were issued on closing. |
|
(3) |
The fair value of the Company’s previously held interest in Natura on the acquisition date was determined based on the fair value of total consideration transferred and reflected book value on the acquisition date. |
|
(4) |
The Company held C$3,000 convertible debt of Natura at the acquisition date. On acquisition, this debt and related accrued interest was effectively settled. |
|
(5) |
For the acquisition of S&S, 79,289 shares of Class 2 common stock were issued on closing. |
Supplemental pro forma information
The unaudited pro forma information for the periods set forth below gives effect to the acquisitions of Manitoba Harvest, Natura and S&S as if the acquisitions had occurred as of January 1, 2018. This pro forma information is presented for informational purposes only and is not necessarily indicative of the results of operations that actually would have been achieved had the acquisitions been consummated as of that time:
F-27
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Revenue |
|
$ |
178,885 |
|
|
$ |
107,786 |
|
|
Net loss |
|
|
(325,760 |
) |
|
|
(74,444 |
) |
|
Net loss per share - basic and diluted |
|
|
(3.24 |
) |
|
|
(0.90 |
) |
Acquisition-related (income) expenses, net
Acquisition-related (income) expenses, net for the years ended December 31 2019 and 2018 are comprised of the following items:
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Acquisition and integration expenses |
|
$ |
15,487 |
|
|
$ |
248 |
|
|
Change in fair value of contingent consideration |
|
|
(46,914 |
) |
|
|
— |
|
|
Total |
|
$ |
(31,427 |
) |
|
$ |
248 |
|
4. ABG Profit Participation Arrangement
On January 14, 2019, the Company entered into a Profit Participation Arrangement (“ABG Arrangement”) with ABG Intermediate Holdings 2, LLC (“ABG”) that offers the Company: (i) participation rights in up to 49% of the net (i.e. post-expense) cannabis revenues from certain existing ABG brands in perpetuity, (ii) guaranteed minimum receipt of $10,000 annually for ten years (prorated based on total consideration paid to ABG) in quarterly payments for participation rights, (iii) preferred supplier rights of all cannabinoid ingredients for products under cannabis-related licenses of certain existing ABG brands in perpetuity, (iv) preferred royalty rates for the Company to license and develop cannabis products for certain existing ABG brands, and (v) first negotiation and matching rights related to participation rights in net cannabis revenues for any additional brands acquired by ABG after entering into the Profit Participation Arrangement (collectively referred to as “Rights Under The ABG Profit Participation Arrangement”).
As consideration for this arrangement, the Company issued 840,107 shares of Class 2 common stock and paid $20,000 in cash in January 2019, paid $13,333 in cash in February 2019, and issued 840,107 shares of Class 2 common stock in March 2019 (refer to Note 14). Under the terms of the ABG Arrangement, the Company shall pay $83,333, in a combination of Class 2 common stock and up to $16,667 in cash at ABG’s election, upon certain triggers relating to the regulatory status of tetrahydrocannabinol (“THC”) in the United States or receipt of $5,000 in participation rights distributions from cannabis products containing THC outside the United States, in accordance with terms outlined in the ABG Arrangement. The Company will record a liability related to this contingent payment when the triggers are met and the consideration becomes payable.
Since the ABG Arrangement conveys a right for the Company to receive guaranteed minimum cash from ABG over ten years, it meets the definition of a loan pursuant to ASC 310, Receivables. As of December 31, 2019 $671 was recorded in prepayments and other current assets and $6,653 in ABG finance receivable and other assets for the current and non-current portions of the loans relating to cash paid to ABG. The portion of the loans relating to shares issued to ABG of $30,253 is recorded within additional paid-in capital as of December 31, 2019. The allocation of the loans between the asset and equity portions was determined on a relative fair value basis. As the loans have no stated interest rate, fair value was determined using the present value of the expected cash flows at a 12% discount rate, which reflects an appropriate market rate for each loan at the time it was issued. Interest on the loan is calculated using the effective interest rate method and recognized in finance income from ABG Profit Participation Arrangement on the statements of net loss and comprehensive loss for the portion of the loan relating to cash paid to ABG, and in additional paid-in capital on the balance sheet for the portion relating to shares issued to ABG.
As of December 31, 2019, the Company has intangible assets with indefinite life in the amount of $16,765 for the Rights under the ABG Profit Participation Arrangement (refer to Note 10). The intangible assets were impaired at December 31, 2019 in the amount of $102,601, recorded to impairment of assets in the statements of net loss and comprehensive loss, as a result of deferred regulatory clarity for sales of CBD products in the United States, resulting in more conservative estimates of future cash flows related to the Company’s Rights under the ABG Profit Participation Arrangement.
F-28
The original cost of the Rights under the ABG Profit Participation Arrangement were calculated using the fair value of the cash paid and shares issued, less the fair value attributable to the loan described above.
The Company entered into a Trademark License Agreement with ABG on April 1, 2019 for the use of Prince trademark (“ABG Prince Agreement”). Under the ABG Prince Agreement, the Company’s right to use the Prince trademark on products that contain CBD sold in the European Union. The ABG Prince Agreement matures December 31, 2025 with certain extension periods available to the Company.
Under the ABG Prince Agreement, the Company pays a royalty on actual product sales in addition to a guaranteed minimum royalty payment (“GMR”) of $500 on April 1, 2019, October 1, 2019, January 1, 2020 and July 1, 2020, with subsequent quarterly payments of $375 commencing January 1, 2021 until maturity of the ABG Prince Agreement.
At inception of the ABG Prince Agreement, the Company recorded an intangible asset of $7,117 in trademarks and licenses within intangible assets (refer to Note 10) with an offsetting ABG finance liability on the balance sheets. The trademark intangible asset and the ABG finance liability were recognized based on the discounted cash flows of the GMR using an effective interest rate of 9%.
The current portion of the ABG finance liability is recorded in accrued expenses and other current liabilities on the balance sheets (refer to Note 12).
Interest expenses recognized is $448 for the period and is recorded in interest expenses, net on the statements of net loss and comprehensive loss.
On January 24, 2020, the Company entered into an amendment related to the ABG Profit Participation Arrangement (refer to Note 26).
5. Inventory
Inventory is comprised of the following items:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Raw materials |
|
$ |
15,926 |
|
|
$ |
2,132 |
|
|
Work-in-process |
|
|
53,973 |
|
|
|
12,812 |
|
|
Finished goods |
|
|
17,962 |
|
|
|
1,267 |
|
|
Total |
|
$ |
87,861 |
|
|
$ |
16,211 |
|
Inventory is written down for any obsolescence, spoilage and excess inventory or when the net realizable value of inventory is less than the carrying value. Inventory valuation adjustments included in cost of sales on the statements of net loss and comprehensive loss is comprised of the following:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Raw materials |
|
$ |
788 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Work-in-process |
|
|
61,302 |
|
|
|
4,561 |
|
|
|
617 |
|
|
Finished goods |
|
|
6,493 |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
68,583 |
|
|
$ |
4,561 |
|
|
$ |
617 |
|
F-29
During the year ended December 31, 2019, cannabis products were written down by $49,378 primarily as a result of an accumulation of oil and cannabis by-product to be converted into oil, as regulations did not allow the sale of these products until December 2019. In addition, hemp products were written down by $3,880 primarily due to the fact the United States CBD market has progressed at a slower pace than expected due to the lack of clarity from the United States Food and Drug Administration, which is responsible for establishing the regulatory framework for CBD products. Also included in inventory valuation adjustments in cost of sales is $15,325 relating to a loss on advance payment on future purchases of inventory to secure supply (refer to Note 6).
6. Prepayments and Other Current Assets
Prepayments and other current assets are comprised of the following items:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Deposits |
|
$ |
25,490 |
|
|
$ |
1,511 |
|
|
Prepayments |
|
|
5,847 |
|
|
|
1,496 |
|
|
Taxes receivable |
|
|
6,165 |
|
|
|
969 |
|
|
ABG finance receivable - current |
|
|
671 |
|
|
|
— |
|
|
Total |
|
$ |
38,173 |
|
|
$ |
3,976 |
|
Deposits include advance payments on future purchases of inventory to secure supply. During the year ended December 31, 2019, the Company determined that certain suppliers are unable to provide an amount of inventory equivalent to the prepayment. As a result, deposits have been written down by $14,154 and $1,171, for Cannabis and Hemp, respectively, totaling $15,325 recorded in inventory valuation adjustments in the statements of net loss and comprehensive loss (refer to Note 5).
7. Investments
Short-term investments
The Company’s short-term investments consist of debt securities classified as available-for-sale investments. All short-term investments have contractual maturities of one year or less. As at December 31, 2019, there were no short-term debt securities remaining and therefore total unrealized gains and losses recognized to accumulated other comprehensive loss during the year ended December 31, 2019 was nil. Gross realized gains on the sale of short-term investments recognized in other income, net was $2,631.
Other investments
Long-term investments are comprised of the following items:
|
|
|
December 31, 2019 |
|
|
|
Equity investments measured at fair value |
|
$ |
4,183 |
|
|
Equity investments under measurement alternative |
|
$ |
14,954 |
|
|
Debt securities classified as available-for-sale method |
|
$ |
5,047 |
|
|
Total other investments |
|
$ |
24,184 |
|
The Company’s equity investments at fair value consist of publicly traded shares and warrants held by the Company. The Company’s equity investments under measurement alternative include equity investments without readily determinable fair values. The Company’s debt securities under available-for-sale method consists of convertible debt instruments with interest rates ranging from 10% – 12% and with contractual maturities in 2022.
For the year ended December 31, 2019, there was no realized gain or loss recognized related to equity investments at fair value. Unrealized losses recognized in other income, net during the year ended December 31, 2019 on equity investments still held at December 31, 2019 is $939. There were no impairments or adjustments to equity investments under the measurement alternative.
Unrealized gains of $17 in accumulated other comprehensive income at December 31, 2019 relates to the long-term available-for-sale debt securities.
F-30
Equity method investments
On December 31, 2018, the Company entered into a joint venture with Anheuser-Busch InBev (“AB InBev”) to research and develop non-alcohol beverages containing cannabis. Under the terms of the arrangement, the Company and AB InBev each have 50% ownership and 50% voting interest in the Plain Vanilla Research Limited Partnership (“Fluent”), headquartered in Canada. The Company has determined that Fluent is a VIE, but the Company is not the primary beneficiary as the Company does not have the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance. Accordingly, the Company does not consolidate the financial statements of Fluent and accounts for this investment using the equity method of accounting. At the date of initial investment there was no difference in the carrying value of the investment and the proportional interest in the underlying equity in the net assets of Fluent. At December 31, 2019 the maximum exposure to loss is limited to the Company’s equity investment in the joint venture.
The Company has made capital contributions of $12,000 to Fluent during the year ended December 31, 2019. In addition, the Company had purchased $4,300 of equipment which was subsequently sold to Fluent at the net book value of $4,300 during the year ended December 31, 2019.
The Company provides production support services to Fluent on a cost recovery basis. During the year ended December 31, 2019, total fees charged were $388, which are included in accounts receivable at December 31, 2019.
On September 19, 2019, the Company entered into a joint venture with Cannfections Group Inc. (“Cannfections”) to develop and manufacture confectionary cannabis products. Under the terms of the arrangement, the Company and Cannfections each have 50% ownership and 50% voting interest. At the date of initial investment, there was no difference in the carrying value of the investment and the proportional interest in the underlying equity in the net assets of Cannfections. During the year ended December 31, 2019, the Company contributed $3,600 to the joint venture, consisting of $1,901 of cash and $1,699 of Class 2 common stock.
The Company’s ownership interests in its equity method investments as of December 31, 2019 were as follows:
|
|
|
Approximate |
|
|
Carrying value |
|
|
Loss from equity method investments Year ended |
|
|||
|
|
|
ownership % |
|
|
December 31, 2019 |
|
|
December 31, 2019 |
|
|||
|
Investment in Fluent |
|
50% |
|
|
$ |
7,836 |
|
|
$ |
4,437 |
|
|
|
Investment in Cannfections |
|
50% |
|
|
$ |
3,612 |
|
|
$ |
67 |
|
|
|
Total equity method investments |
|
|
|
|
|
$ |
11,448 |
|
|
$ |
4,504 |
|
F-31
Summary financial information for the equity method investments on an aggregated basis was as follows:
|
|
|
December 31, 2019 |
|
|
|
Current assets |
|
$ |
13,942 |
|
|
Non current assets |
|
$ |
4,987 |
|
|
Current liabilities |
|
$ |
1,561 |
|
|
Non current liabilities |
|
$ |
— |
|
|
|
|
Year ended |
|
|
|
|
|
December 31, 2019 |
|
|
|
Revenues |
|
$ |
113 |
|
|
Gross profit |
|
$ |
78 |
|
|
Net loss |
|
$ |
(9,008 |
) |
Disclosures related to periods prior to the adoption of ASU 2016-01
The Company’s short-term investments are classified as available-for-sale investments and the long-term investments are classified as either available-for-sale or cost method investments.
The following table summarizes the unrealized gains and losses and estimated fair value of our short-term investments as of December 31, 2018:
|
|
|
Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair value |
|
||||
|
Treasury bills |
|
$ |
30,367 |
|
|
$ |
32 |
|
|
$ |
64 |
|
|
$ |
30,335 |
|
|
Total |
|
$ |
30,367 |
|
|
$ |
32 |
|
|
$ |
64 |
|
|
$ |
30,335 |
|
Short-term investments consist of treasury bills, which are deemed to be low risk based on their credit ratings from the major rating agencies. All short-term investments have contractual maturities of one year or less.
The following table summarizes the unrealized gains and losses and estimated fair value of our long-term investments as of December 31, 2018:
|
|
|
Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair value |
|
||||
|
Investment in equities |
|
$ |
17,714 |
|
|
$ |
— |
|
|
$ |
803 |
|
|
$ |
16,911 |
|
|
Total |
|
$ |
17,714 |
|
|
$ |
— |
|
|
$ |
803 |
|
|
$ |
16,911 |
|
Investment in equities are reported in long-term investments on the balance sheets. The following table provides a summary of the classification of investment in equities:
|
|
|
December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Investments in equities under available-for-sale method |
|
$ |
1,845 |
|
|
$ |
— |
|
|
Investment in equities under the cost method |
|
|
15,066 |
|
|
|
— |
|
|
Total investment in equities |
|
$ |
16,911 |
|
|
$ |
— |
|
Total unrealized loss recognized to other comprehensive income related to the long-term available-for-sale equity securities during the year ended December 31, 2018 was $803.
As at December 31, 2017, the Company did not hold any short-term and long-term investments.
F-32
8. Property and Equipment, Net
Property and equipment, net consisted of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Land |
|
|
6,417 |
|
|
$ |
4,498 |
|
|
Buildings and leasehold improvements |
|
|
109,172 |
|
|
|
51,111 |
|
|
Laboratory and manufacturing equipment |
|
|
31,173 |
|
|
|
6,131 |
|
|
Office and computer equipment |
|
|
2,659 |
|
|
|
970 |
|
|
ROU assets under finance lease |
|
|
14,753 |
|
|
|
9,661 |
|
|
Construction-in-process |
|
|
37,160 |
|
|
|
15,343 |
|
|
|
|
|
201,334 |
|
|
|
87,714 |
|
|
Less: accumulated depreciation |
|
|
(17,117 |
) |
|
|
(7,500 |
) |
|
Total |
|
$ |
184,217 |
|
|
$ |
80,214 |
|
For the year ended December 31, 2019, total depreciation on property and equipment was $9,282 (2018 - $3,410 and 2017 – $1,457). Depreciation expenses included in cost of sales relating to manufacturing equipment and production facilities for the year ended December 31, 2019 is $4,242 (2018 – $1,964 and 2017 – $1,303). Depreciation expenses related to general office space and equipment of $1,783 (2018 – $149, 2017 - $95) is included in depreciation and amortization expenses. The remaining depreciation is capitalized in the cost of inventory.
The Company had $119,184 in property and equipment additions during the year ended December 31, 2019 (2018 – $44,451). Additions to building and leasehold improvements primarily relate to the Company’s acquisitions of Manitoba Harvest, Natura and S&S (refer to Note 3). Additions also include a non-cash finance lease asset of $4,617 (2018 - $114) and for the year ended December 31, 2019, there is $652 (2018 – $158 and 2017 - $34) of capitalized interest included in construction-in-progress.
Additions to construction-in-process primarily relate to the ongoing construction of the Company’s London, Ontario and Portugal facilities. The Company has discontinued the construction of certain facilities resulting in a loss of $2,436 recorded to loss on disposal of property and equipment in the statements of net loss and comprehensive loss.
9. Leases
The Company has operating and finance leases for facilities and certain equipment. Operating and finance leases have remaining weighted-average remaining lease terms of 9 years and 4 years, respectively, as at December 31, 2019, some of which include options to extend the leases for up to 10 years and some of which include options to terminate the leases within 1 year.
Components of lease expenses
|
|
|
December 31, |
|
|
|
|
|
2019 |
|
|
|
Finance lease cost |
|
|
|
|
|
Amortization of ROU assets |
|
$ |
588 |
|
|
Interest on lease liabilities |
|
|
370 |
|
|
Operating lease expenses(1) |
|
|
2,519 |
|
|
Short term lease expenses(1) |
|
|
256 |
|
|
Sublease income(2) |
|
|
(230 |
) |
|
Total lease expenses |
|
$ |
3,503 |
|
|
(1) |
Included in general and administrative expenses |
|
(2) |
Included in other income, net |
F-33
Supplemental cash flow information related to leases
|
|
|
December 31, |
|
|
|
|
|
2019 |
|
|
|
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
Operating cash flows from operating leases |
|
|
2,312 |
|
|
Operating cash flows from finance leases |
|
|
336 |
|
|
Financing cash flows from finance leases |
|
|
504 |
|
|
Non-cash additions to ROU assets and lease liabilities |
|
|
|
|
|
Operating leases |
|
|
16,043 |
|
|
Finance leases |
|
|
4,617 |
|
Other information about lease amounts recognized in the financial statements
|
|
|
December 31, |
|
|
|
|
|
2019 |
|
|
|
Weighted-average remaining lease term (years) – operating leases |
|
|
|
|
|
Weighted-average remaining lease term (years) – finance leases |
|
|
|
|
|
Weighted-average discount rate – operating leases |
|
|
5.73 |
% |
|
Weighted-average discount rate – finance leases |
|
|
8.42 |
% |
Refer to Note 17 for lease commitments.
Disclosures related to periods prior to the adoption of ASC 842
At December 31, 2018, the Company leased various facilities, under non-cancelable capital and operating leases, which expire at various dates through September 2027. Under the terms of the operating lease agreements, the Company is responsible for certain insurance and maintenance expenses. The Company recorded rent expenses on a straight-line basis over the terms of the underlying leases. Rent expenses for the year ended December 31, 2018 was $745 (2017 – $175).
At December 31, 2018, aggregate future minimum rental payments under all non-cancelable capital and operating leases were as follows:
|
|
|
Operating Leases |
|
|
Capital Leases |
|
||
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2018 |
|
|
2018 |
|
||
|
2019 |
|
$ |
916 |
|
|
$ |
733 |
|
|
2020 |
|
|
857 |
|
|
|
733 |
|
|
2021 |
|
|
727 |
|
|
|
733 |
|
|
2022 |
|
|
589 |
|
|
|
733 |
|
|
2023 |
|
|
510 |
|
|
|
183 |
|
|
Thereafter |
|
|
1,372 |
|
|
|
— |
|
|
|
|
$ |
4,971 |
|
|
$ |
3,115 |
|
The supplemental cash flow information of the Company’s leases prior to the adoption of ASC 842 was as follows:
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Non-cash financing activities |
|
|
|
|
|
|
|
|
|
Capital lease obligation |
|
|
— |
|
|
$ |
8,958 |
|
|
Non-cash investing |
|
|
|
|
|
|
|
|
|
Addition to property and equipment under capital lease |
|
|
114 |
|
|
$ |
8,958 |
|
F-34
10. Intangible Assets
Intangible assets are comprised of the following items:
|
|
|
December 31, |
|
|||||||||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
||||||||||||||||||||||
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Impairment |
|
|
Net |
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Net |
|
|||||||
|
Definite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patent |
|
|
716 |
|
|
|
99 |
|
|
|
— |
|
|
|
617 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Customer relationships |
|
|
135,953 |
|
|
|
7,132 |
|
|
|
— |
|
|
|
128,821 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Developed technology |
|
|
7,074 |
|
|
|
590 |
|
|
|
— |
|
|
|
6,484 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Websites |
|
|
5,157 |
|
|
|
3,331 |
|
|
|
— |
|
|
|
1,826 |
|
|
|
3,755 |
|
|
|
2,253 |
|
|
|
1,502 |
|
|
Trademarks and licenses |
|
|
9,135 |
|
|
|
925 |
|
|
|
— |
|
|
|
8,210 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
|
158,035 |
|
|
|
12,077 |
|
|
|
— |
|
|
|
145,958 |
|
|
|
3,755 |
|
|
|
2,253 |
|
|
|
1,502 |
|
|
Indefinite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cultivation license |
|
|
10,689 |
|
|
|
— |
|
|
|
— |
|
|
|
10,689 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Alef license |
|
|
4,086 |
|
|
|
— |
|
|
|
4,086 |
|
|
|
— |
|
|
|
2,984 |
|
|
|
— |
|
|
|
2,984 |
|
|
Trademarks |
|
|
55,416 |
|
|
|
— |
|
|
|
— |
|
|
|
55,416 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Rights under ABG Profit Participation Arrangement |
|
|
119,366 |
|
|
|
— |
|
|
|
102,601 |
|
|
|
16,765 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
|
189,557 |
|
|
|
— |
|
|
|
106,687 |
|
|
|
82,870 |
|
|
|
2,984 |
|
|
|
— |
|
|
|
2,984 |
|
|
Total intangible assets |
|
$ |
347,592 |
|
|
$ |
12,077 |
|
|
$ |
106,687 |
|
|
$ |
228,828 |
|
|
$ |
6,739 |
|
|
$ |
2,253 |
|
|
$ |
4,486 |
|
As of December 31, 2019, there are no intangible assets not yet available for use (December 31, 2018 – $3,027).
Intangible asset additions during the year ended December 31, 2019 primarily related to customer relationships, developed technology and trademarks as part of the acquisition of Manitoba Harvest, cultivation license and supply contract as part of the acquisition of Natura and trademarks as part of the acquisition of S & S (refer to Note 3). Moreover, indefinite-lived rights under the ABG Profit Participation Arrangement and definite-lived trademarks under the ABG Prince Agreement were acquired during the year ended December 31, 2019 (refer to Note 4).
Amortization expenses for intangibles was $9,824, $374, and $549 in 2019, 2018, and 2017, respectively. Expected future amortization expenses for intangible assets as of December 31, 2019 are as follows: 2020 – $11,674; 2021 – $11,328; 2022 - $10,757; 2023 - $10,428, 2024 – $10,423; and thereafter – $91,348.
In the fourth quarter of fiscal 2019, the Company decided not to pursue cannabis cultivation in Chile and as a result the Alef license was impaired by the entire value of $4,086 which was recorded in impairment of assets on the statements of net loss and comprehensive loss.
In connection with the preparation and review of these financial statements, the Company determined that the fair value (Level 3) of Rights under ABG Profit participation Arrangement was below the carrying value. The decline in fair value of Rights under ABG Profit participation Arrangement is primarily due to deferred regulatory clarity for sales of CBD products in the United States, resulting in a reduced estimate of future cash flows related to the Company’s Rights under the ABG Profit Participation Arrangement. As a result, the Company incurred a non-cash impairment charge of $102,601 presented in impairment of assets in the accompanying statements of net loss and comprehensive loss (refer to Note 4).
11. Goodwill
The following table shows the change in carrying amount of goodwill:
|
|
|
Hemp |
|
|
Cannabis |
|
|
Total |
|
|||
|
Goodwill - January 1, 2019 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Acquisition of Manitoba Harvest |
|
|
126,881 |
|
|
|
— |
|
|
|
126,881 |
|
|
Acquisition of Natura |
|
|
— |
|
|
|
29,314 |
|
|
|
29,314 |
|
|
Acquisition of S & S |
|
|
4,932 |
|
|
|
— |
|
|
|
4,932 |
|
|
Foreign currency translation adjustment |
|
|
1,501 |
|
|
|
623 |
|
|
|
2,124 |
|
|
Goodwill - December 31, 2019 |
|
$ |
133,314 |
|
|
$ |
29,937 |
|
|
$ |
163,251 |
|
F-35
12. Accounts Payable, Accrued Expenses and Other Current Liabilities
Accounts payable, accrued expenses and other current liabilities are comprised of the following items:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Accounts payable - trade |
|
$ |
39,057 |
|
|
$ |
9,716 |
|
|
Accounts payable - related parties |
|
|
68 |
|
|
|
933 |
|
|
Total accounts payable |
|
$ |
39,125 |
|
|
$ |
10,649 |
|
|
|
|
|
|
|
|
|
|
|
|
Accrued payroll and employment related withholding taxes |
|
|
24,765 |
|
|
|
3,278 |
|
|
Other accrued expenses and current liabilities |
|
|
17,032 |
|
|
|
5,673 |
|
|
Accrued interest on convertible notes |
|
|
5,938 |
|
|
|
5,302 |
|
|
ABG finance liability - current |
|
|
1,500 |
|
|
|
— |
|
|
Accrued legal and professional fees |
|
|
1,174 |
|
|
|
565 |
|
|
Contingent consideration for acquisitions |
|
|
420 |
|
|
|
— |
|
|
Total accrued expenses and other current liabilities |
|
$ |
50,829 |
|
|
$ |
14,818 |
|
The acquisition of Manitoba Harvest (refer to Note 3) included contingent consideration whereby the Company may pay a maximum of $37,129 payable in shares of Class 2 common stock, based on the gross branded CBD product sales in the United States for the period from January 1, 2019 to December 31, 2019. The estimated fair value of contingent consideration at the purchase date was $29,207. CBD sales for 2019 did not achieve the thresholds and as a result the fair value is at December 31, 2019. The adjustment to fair value is recorded to acquisition-related (income) expenses, net.
The acquisition of Natura (refer to Note 3) included contingent consideration whereby the Company issued promissory notes with an aggregate principal amount of $20,007. The ultimate payment amounts are based on production levels of consumer grade dry finished cannabis flower from Natura facilities during four periods from February 1, 2019 to January 31, 2020 and are payable in shares of Class 2 common stock. The Company has paid $4,450 in Class 2 common stock on December 2, 2019. Production levels for the remaining period were not expected to be achieved and as a result the fair value is at December 31, 2019. The adjustment to fair value is recorded to acquisition-related (income) expenses, net.
The acquisition of S&S (refer to Note 3) included contingent consideration with an aggregate principal of $1,812 which has been remeasured to $420 at December 31, 2019. The adjustment to fair value is recorded to acquisition-related (income) expenses, net.
13. Convertible notes
In October 2018 the Company issued convertible notes with a face value of $475,000. The net proceeds from the offering were approximately $460,134, after deducting commissions and other fees incurred.
The convertible notes bear interest at a rate of 5.00% per annum, payable semi-annually in arrears on April 1 and October 1 of each year, beginning on April 1, 2019. Additional interest may accrue on the convertible notes in specified circumstances. The convertible notes will mature on October 1, 2023, unless earlier repurchased, redeemed or converted. There are no principal payments required over the five year term of the convertible notes, except in the case of redemption or events of defaults.
The convertible notes are governed by an Indenture between the Company, as issuer, and GLAS Trust Company LLC, as trustee. The convertible notes are the Company’s general unsecured obligations and rank senior in right of payment to all of the Company’s indebtedness that is expressly subordinated in right of payment to the notes; equal in right of payment with any of the Company’s unsecured indebtedness that is not so subordinated; effectively junior in right of payment to any of Company’s secured indebtedness to the extent of the value of the assets securing such indebtedness; and structurally junior to all indebtedness and other liabilities (including trade payables but excluding intercompany obligations) of the Company’s current or future subsidiaries.
The Indenture includes customary covenants and sets forth certain events of default after which the convertible notes may be declared immediately due and payable, including certain types of bankruptcy or insolvency involving the Company.
F-36
To the extent the Company so elects, the sole remedy for an event of default relating to certain failures by the Company to comply with certain reporting covenants in the Indenture will, for the first 365 days after such event of default, consist exclusively of the right to receive additional interest on the notes. Upon conversion, the Company will pay or deliver, as the case may be, cash, shares of our common stock or a combination of cash and shares of the Company’s common stock, at the Company’s election (the “cash conversion option”). The initial conversion rate for the convertible notes is 5.9735 shares of common stock per one thousand dollar principal amount of notes, which is equivalent to an initial conversion price of approximately $167.41 per share of common stock, which represents approximately 2,837 shares of common stock, based on the $475,000 aggregate principal amount of convertible notes outstanding as of December 31, 2019. Throughout the term of the convertible notes, the conversion rate may be adjusted upon the occurrence of certain events.
Prior to the close of business on the business day immediately preceding April 1, 2023, the convertible notes will be convertible only under the specified circumstances. On or after April 1, 2023 until the close of business on the business day immediately preceding the maturity date, holders may convert all or any portion of their convertible notes, in multiples of one thousand dollar principal amount, at the option of the holder regardless of the forementioned circumstances.
As a result of the cash conversion option, the Company separately accounts for the value of the embedded conversion option as a component of equity. The value of the embedded conversion option is the residual of the net proceeds of the issuance, less the estimated fair value of the debt without the conversion feature, and amounted to $57,595 at issuance. The estimated fair value of the debt without the conversion feature, was determined using the expected cash flows of the convertible notes discounted by the estimated interest rate of similar nonconvertible debt; the debt discount is being amortized as additional non-cash interest expenses over the term of the convertible notes using the interest method with an effective interest rate of 8% per annum. The equity component is not remeasured as long as it continues to meet the conditions for equity classification.
As of December 31, 2019, the convertible notes are not yet convertible. The convertible notes will become convertible upon the satisfaction of the above circumstances. In accounting for the transaction costs related to the issuance of the convertible notes, the Company allocated the total amount of offering costs incurred to the debt and equity components based on their relative values. Transaction costs attributable to the convertible notes totaling $13,467, are being amortized as non-cash interest expenses over the term of the convertible notes, and offering costs attributable to the equity component, totaling $1,398, were recorded within stockholders’ equity (deficit). The remaining unamortized debt discount related to the convertible notes of $34,219 as of December 31, 2019 will be accreted over the remaining term of the convertible notes, which is approximately 45 months.
As at December 31, 2019, the Company was in compliance with all the covenants set forth under the Indenture.
The following table sets forth the net carrying amount of the convertible notes:
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
||
|
5.00% convertible notes |
|
$ |
475,000 |
|
|
$ |
475,000 |
|
|
Unamortized discount |
|
|
(34,219 |
) |
|
|
(41,687 |
) |
|
Unamortized transaction costs |
|
|
(10,571 |
) |
|
|
(12,946 |
) |
|
Net carrying amount |
|
$ |
430,210 |
|
|
$ |
420,367 |
|
The following table sets forth total interest expenses recognized related to the convertible notes:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Contractual coupon interest |
|
$ |
23,750 |
|
|
$ |
5,302 |
|
|
Amortization of discount |
|
|
7,468 |
|
|
|
2,152 |
|
|
Amortization of direct issue costs |
|
|
2,375 |
|
|
|
28 |
|
|
Total |
|
$ |
33,593 |
|
|
$ |
7,482 |
|
F-37
14. Stockholders’ Equity
Common and preferred stock
The Company’s certificate of incorporation authorized the Company to issue the following classes of shares with the following par value and voting rights as of December 31, 2019. The liquidation and dividend rights are identical among Class 1 common stock and Class 2 common stock, and all classes of common stock share equally in our earnings and losses.
|
|
|
Par Value |
|
|
Authorized |
|
|
Voting Rights |
||
|
Class 1 common stock |
|
$ |
0.0001 |
|
|
|
250,000,000 |
|
|
10 votes for each share |
|
Class 2 common stock |
|
$ |
0.0001 |
|
|
|
500,000,000 |
|
|
1 vote for each share |
|
Preferred stock |
|
$ |
0.0001 |
|
|
|
10,000,000 |
|
|
N/A |
In connection with the ABG Profit Participation arrangement (refer to Note 4), the Company issued 840,107 shares of Class 2 common stock in January 2019 at a deemed issuance price of $79.35 per share and 840,107 shares of Class 2 common stock in March 2019 at a deemed issuance price of $79.35 per share. Given that the shares of Class 2 common stock issued to ABG were not registered with the SEC and subject to transfer restrictions, the fair values of the issuances were $89.13 and $59.77 per share, respectively, as recorded in the statements of stockholders’ equity (deficit) (refer to Note 4).
In February 2019, the Company issued 180,332 shares of Class 2 common stock at a deemed issuance price of $83.73 per share in connection with the closing of the Natura acquisition (refer to Note 3). On December 2, 2019, the Company issued 238,826 Class 2 common stock in relation to contingent consideration (refer to Note 3 and Note 12).
In connection with the acquisition of Manitoba Harvest, the Company issued 1,209,946 shares of Class 2 common stock in March 2019 at a deemed issuance price of $80.04 per share on closing and 899,306 shares of Class 2 common stock in August 2019 at a deemed issuance price of $35.48 per share as share consideration six months after close (refer to Note 3).
In connection with the acquisition of S&S, the Company issued 79,289 shares of Class 2 common stock in July 2019 at a deemed issuance price of $40.22 per share on closing (refer to Note 3).
On September 10, 2019, the Company entered into a sales agreement with Cowen and Company, LLC pursuant to which the Company can issue and sell, through a sales agent, shares of Class 2 common stock from time to time up to up to an aggregate offering price of $400 million through an “at-the-market” equity offering program.
For the year ended December 31, 2019 the Company issued a total of 5,396,501 shares of Class 2 common stock for gross proceeds of $113,543 (net proceeds of $111,073 after issuance costs) under the at-the-market program.
Pursuant to the Downstream Merger, all of Privateer Holdings capital stock outstanding of 58,333,333 shares of Tilray Class 2 common stock and 16,666,667 shares of Tilray Class 1 common stock immediately prior to the effective time of the Downstream Merger, were cancelled and automatically converted solely into the right to receive the applicable portion of an aggregate shares of Tilray Class 2 common stock and shares of Tilray Class 1 common stock, inclusive of shares of Tilray Class 2 common stock held in escrow for contingent release to Privateer Holdings stockholders, issuable as consideration in Downstream Merger. In connection with the Downstream Merger, the Company exchanged the shares held by Privateer Holdings and issued the same value of shares to the underlying Privateer Holdings shareholders at a conversion rate of 1.07290. The Company did not pay any cash consideration in connection with the Downstream Merger and there was no impact on the statements of balance sheets or statements of net loss and comprehensive loss.
15. Stock-Based Compensation
Original Stock Option Plan
Certain employees and other service providers of the Company participate in the equity-based compensation plan of Privateer Holdings, Inc (the “Original Plan”) under the terms and valuation method detailed below. For the year ended December 31, 2019, the total stock-based compensation expenses associated with the
F-38
Original Plan was $469 (December 31, 2018 – $359 and 2017 – $139). There were no new grants under the Original Plan for the year ended December 31, 2019.
The Original Plan was assumed by the Company on December 12, 2019 as a result of the Downstream Merger and as a result, at December 31, 2019, the Original Plan has 3,134,431 shares of Tilray common stock reserved for issuance. All outstanding options are subject to the terms of the Original Plan until exercised, terminated or expired by their terms. Stock options granted under the Original Plan are either incentive stock options or nonqualified stock options. Prior to the Downstream Merger, stock options and shares of Privateer Holdings common stock issued under the Original Plan were determined by the Board of Directors of Privateer Holdings and were not issued at less than 100% of the fair value of the shares on the date of the grant. Fair value was determined by the Board of Directors of Privateer Holdings. Stock options generally vested over a period of four years and expire, if not exercised, 10 years from the date of grant. Shares of Privateer Holdings common stock were issued in exchange for services based on the fair value of the services or the fair value of the Privateer Holdings common stock at the time of grant, as determined by the Board of Directors of Privateer Holdings. Prior to the Downstream Merger, the compensation expenses under the Original Plan was allocated from Privateer Holdings to Tilray employees who held options under the Original Plan.
As a result of the Downstream Merger, the Company also assumed 692,843 stock options under the Original Plan (refer to Note 14), together with additional Privateer Holdings stock options not previously allocated to Tilray, which were converted at a conversion rate of 1.07290 into 3,134,431 of Tilray stock options outstanding under the Original Plan until exercised. Of the 3,134,431 options issued as part of the Downstream Merger, 2,404,000 shares were fully vested and all performance obligations related to the options had been performed. The stock options assumed as a result of the Downstream Merger were not remeasured as the fair value of the stock options before and after the Downstream Merger was the same.
The fair value of each stock option to employees granted under the Original Plan was estimated on the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions:
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Expected stock option life |
|
|
— |
|
|
5.15 years |
|
|
5.84 years |
|
||
|
Expected volatility |
|
|
— |
|
|
|
48.82 |
% |
|
|
56.23 |
% |
|
Risk-free interest rate |
|
|
— |
|
|
|
2.35 |
% |
|
|
2.01 |
% |
|
Expected dividend yield |
|
|
— |
|
|
|
- |
% |
|
|
- |
% |
The expected life of the stock options represented the period of time stock options were expected to be outstanding and was estimated considering vesting terms and employees’ historical exercise and post-vesting employment termination behavior. Expected volatility was based on historical volatilities of public companies operating in a similar industry to Privateer Holdings. The risk-free rate is based on the United States Treasury yield curve in effect at the time of grant. The expected dividend yield was determined based on the stock option’s exercise price and expected annual dividend rate at the time of grant.
Stock option activity for the Company up to and including the Downstream Merger under the Original Plan
|
|
|
Stock Options |
|
|
Weighted- average exercise price |
|
|
Weighted- average remaining contractual term (years) |
|
|
Aggregate intrinsic value |
|
||||
|
Balance December 31, 2018 |
|
|
592,594 |
|
|
$ |
4.14 |
|
|
|
|
|
|
$ |
989 |
|
|
Allocated to Tilray |
|
|
143,794 |
|
|
|
4.21 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(25,751 |
) |
|
|
3.95 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(9,527 |
) |
|
|
4.25 |
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
(8,267 |
) |
|
|
3.46 |
|
|
|
|
|
|
|
|
|
|
Converted with Downstream Merger |
|
|
(692,843 |
) |
|
|
4.22 |
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2019 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
Vested and expected to vest, December 31, 2019 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
Vested and exercisable, December 31, 2019 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
F-39
The weighted-average fair values of all stock options granted in 2019, 2018 and 2017 were $0, $3.05 and $1.79, respectively. The total intrinsic values of stock options exercised in 2019, 2018 and 2017 were $350 , $176 and $19, respectively. As of December 31, 2019, the total remaining unrecognized compensation expenses related to non-vested stock options amounted to $0 (2018 - $557), which will be amortized over the weighted-average remaining requisite service period of approximately 0 years (2018 - 1.1 years). The total fair values of stock options vested in 2019, 2018 and 2017 were $669, $276 and $145, respectively.
Downstream Merger time-based stock option activity
|
|
|
Stock Options |
|
|
Weighted- average exercise price |
|
|
Weighted- average remaining contractual term (years) |
|
|
Aggregate intrinsic value |
|
||||
|
Balance December 31, 2018 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
Assumed on Downstream Merger |
|
|
3,134,431 |
|
|
|
2.99 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(107,359 |
) |
|
|
1.81 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
— |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
(13,068 |
) |
|
|
9.04 |
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2019 |
|
|
3,014,004 |
|
|
$ |
3.04 |
|
|
|
|
|
|
$ |
44,108 |
|
|
Vested and expected to vest, December 31, 2019 |
|
|
2,992,598 |
|
|
$ |
2.98 |
|
|
|
|
|
|
$ |
43,717 |
|
|
Vested and exercisable, December 31, 2019 |
|
|
2,733,170 |
|
|
$ |
2.77 |
|
|
|
|
|
|
$ |
40,423 |
|
The weighted-average fair values of time-based stock options assumed on the Downstream Merger in 2019 was $2.37 per share. The total intrinsic values of these stock options exercised in 2019 was $1,686. As of December 31, 2019, the total remaining unrecognized compensation expenses related to non-vested stock options amounted to $921, which will be amortized over the weighted-average remaining requisite service period of approximately 0.8 year. The total fair value of stock options vested in 2019 was $2,789 .
New Stock Option and Restricted Stock Unit Plan
The Company adopted the 2018 Equity Incentive Plan (the “2018 EIP”) as amended and approved by stockholders in May 2018 under the terms and valuation methods detailed in our Annual Financial Statements. The 2018 EIP authorizes the award of stock options, restricted stock units (“RSUs”) and stock appreciation rights (“SARs”) to employees, including officers, non-employee directors and consultants and the employees and consultants of our affiliates. Shares subject to awards granted under the 2018 EIP that expire or terminate without being exercised in full, or that are paid out in cash rather than in shares, do not reduce the number of shares available for issuance under the 2018 EIP. Additionally, shares become available for future grant under the 2018 EIP if they were issued under the 2018 EIP and if the Company repurchases them or they are forfeited. This includes shares used to pay the exercise price of an award or to satisfy the tax withholding obligations related to an award. The maximum number of shares of common stock subject to stock awards granted under the 2018 EIP or otherwise during any one calendar year to any non-employee director, taken together with any cash fees paid by the Company to such non-employee director during such calendar year for service on the Board of Directors, will not exceed five hundred thousand dollars in total value, calculating the value of any such stock awards based on the grant date fair value of such stock awards for financial reporting purposes, or, with respect to the calendar year in which a nonemployee director is first appointed or elected to our Board of Directors, one million dollars.
Stock options represent the right to purchase shares of our Class 2 common stock on the date of exercise at a stated exercise price. The exercise price of a stock option generally must be at least equal to the fair market value of our shares of Class 2 common stock on the date of grant. The Company’s compensation committee may provide for stock options to be exercised only as they vest or to be immediately exercisable with any shares issued on exercise being subject to the Company’s right of repurchase that lapses as the shares vest. The maximum term of stock options granted under the 2018 EIP is ten years.
F-40
RSUs represent a right to receive Class 2 common stock or their cash equivalent for each RSU that vests, which vesting may be based on time or achievement of performance conditions. Unless otherwise determined by our compensation committee at the time of grant, vesting will cease on the date the participant no longer provides services to the Company and unvested shares will be forfeited. If an RSU has not been forfeited, then on the date specified in the RSUs, the Company will deliver to the holder a number of whole shares of Class 2 common stock, cash or a combination of shares of our Class 2 common stock and cash. Additionally, dividend equivalents may be credited in respect of shares covered by the RSUs. Any additional shares covered by the RSU credited by reason of such dividend equivalents will be subject to all of the same terms and conditions of the underlying RSU agreement to which they relate. The RSUs generally vest over a 3-or-4 year period. The fair value of RSUs are based on the share price as at date of grant.
SARs provide for a payment, or payments, in cash or shares of Class 2 common stock to the holder based upon the difference between the fair market value of shares of our Class 2 common stock on the date of exercise and the stated exercise price. The maximum term of SARs granted under the 2018 EIP is ten years. No SARs were issued to date.
The 2018 EIP permits the grant of performance-based stock and cash awards. The performance goals may be based on company-wide performance or performance of one or more business units, divisions, affiliates or business segments and may be either absolute or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. The length of any performance period, the performance goals to be achieved during the performance period, and the measure of whether and to what degree such performance goals have been attained will be conclusively determined by the Board of Directors.
As of May 21, 2018, 9,199,338 shares of Class 2 common stock had been reserved for issuance under the 2018 EIP. The number of shares of Class 2 common stock reserved for issuance under the 2018 EIP will automatically increase on January 1 of each calendar year, for a period of not more than ten years, starting on January 1, 2019 and ending on and including January 1, 2027, in an amount equal to 4% of the total number of shares of our common stock outstanding on December 31 of the prior calendar year, or a lesser number of shares determined by our Board of Directors. The shares reserved include only the outstanding shares related to stock options and RSUs, and excludes stock options outstanding under the Original Plan. The number of shares reserved for issuance under the 2018 EIP is 12,926,172, effective as of January 1, 2019.
For the year ended December 31, 2019, the total stock-based compensation expenses associated with the 2018 EIP was $31,373 (December 31, 2018 - $20,629).
The fair value of each stock option granted to employees under the 2018 EIP is estimated on the grant date using the Black-Scholes option pricing model with the following weighted average assumptions:
|
|
|
Assumptions 2019 |
|
|
Assumptions 2018 |
|
||
|
Expected stock option life (years) |
|
8.97 years |
|
|
5.79 years |
|
||
|
Expected volatility |
|
|
61.33 |
% |
|
|
58.54 |
% |
|
Risk-free interest rate |
|
|
2.10 |
% |
|
|
2.92 |
% |
|
Expected dividend yield |
|
|
- |
% |
|
|
|
|
The expected life of the award is estimated using the simplified method since the Company does not have adequate historical exercise data to estimate the expected term. Expected volatility is based on historical volatilities of public companies operating in a similar industry to the Company. A forfeiture rate is estimated at the time of grant to reflect the amount of awards that are granted but are expected to be forfeited by the award holder prior to vesting. The estimated forfeiture rate applied to these amounts is derived from management’s estimate of the future stock option forfeiture behavior over the expected life of the awards. The risk-free rate is based on the United States Treasury yield curve in effect at the time of grant.
F-41
Stock option and RSU activity for the Company under the 2018 EIP is as follows:
Time-based stock option activity
|
|
|
Stock Options |
|
|
Weighted- average exercise price |
|
|
Weighted- average remaining contractual term (years) |
|
|
Aggregate intrinsic value |
|
||||
|
Balance December 31, 2018 |
|
|
6,015,791 |
|
|
$ |
13.54 |
|
|
|
|
|
|
$ |
342,916 |
|
|
Granted |
|
|
10,000 |
|
|
|
70.25 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(621,363 |
) |
|
|
7.76 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(91,048 |
) |
|
|
30.16 |
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
(6,250 |
) |
|
|
7.76 |
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2019 |
|
|
5,307,130 |
|
|
$ |
14.04 |
|
|
|
|
|
|
$ |
44,297 |
|
|
Vested and expected to vest, December 31, 2019 |
|
|
5,072,605 |
|
|
$ |
13.80 |
|
|
|
|
|
|
$ |
42,537 |
|
|
Vested and exercisable, December 31, 2019 |
|
|
2,512,513 |
|
|
$ |
11.91 |
|
|
|
|
|
|
$ |
21,840 |
|
The weighted-average fair values of time-based stock options granted in 2019 was $40.11 per share (2018 - $7.74). The total intrinsic values of these stock options exercised in 2019 and 2018 were $29,655 and $0 respectively. As of December 31, 2019, the total remaining unrecognized compensation expenses related to non-vested stock options amounted to $23,649 (2018 - $38,250), which will be amortized over the weighted-average remaining requisite service period of approximately 1.9 years (2018 - 2.8 years). The total fair value of stock options vested in 2019 were $16,708 (2018 - $5,508).
Performance-based stock option activity
|
|
|
Stock Options |
|
|
Weighted- average exercise price |
|
|
Weighted- average remaining contractual term (years) |
|
|
Aggregate intrinsic value |
|
||||
|
Balance December 31, 2018 |
|
|
600,000 |
|
|
$ |
7.76 |
|
|
|
|
|
|
$ |
37,668 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(80,000 |
) |
|
|
7.76 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2019 |
|
|
520,000 |
|
|
$ |
7.76 |
|
|
|
|
|
|
$ |
4,872 |
|
|
Vested and expected to vest, December 31, 2019 |
|
|
520,000 |
|
|
$ |
7.76 |
|
|
|
|
|
|
$ |
4,872 |
|
|
Vested and exercisable, December 31, 2019 |
|
|
520,000 |
|
|
$ |
7.76 |
|
|
|
|
|
|
$ |
4,872 |
|
The weighted-average fair values of all performance-based stock options granted in 2019 was $0 per share (2018 - $4.15). The total intrinsic values of stock options exercised in 2019 and 2018 were $5,054 and $0 respectively. As of December 31, 2019, the total remaining unrecognized compensation expenses related to non-vested stock options amounted to $0 (2018 - $593), which will be amortized over the weighted-average remaining requisite service period of approximately 0 years (2018 - 0.6 years). The total fair value of stock options vested in 2019 were $1,246 (2018 - $1,246).
F-42
Time-based RSU activity
|
|
|
Time-based RSUs |
|
|
Weighted-average grant-date fair value per share |
|
||
|
Non-vested December 31, 2018 |
|
|
237,222 |
|
|
$ |
49.86 |
|
|
Granted |
|
|
1,370,703 |
|
|
|
41.00 |
|
|
Vested |
|
|
(122,289 |
) |
|
|
38.16 |
|
|
Forfeited |
|
|
(62,244 |
) |
|
|
56.35 |
|
|
Non-vested December 31, 2019 |
|
|
1,423,392 |
|
|
$ |
42.05 |
|
As of December 31, 2019, there was approximately $41,898 (2018 - $10,336) of total unrecognized compensation cost related to non-vested time-based RSUs that will be recognized as expenses over a weighted-average period of 2.3 years (2018 - 3.2 years). The total intrinsic values of time-based RSUs vested in 2019 and 2018 were $3,446, and $0 respectively. The total fair value of time-based RSUs vested in 2019 were $4,667 (2018 - $0)
Performance-based RSU activity
|
|
|
Performance- based RSUs |
|
|
Weighted-average grant-date fair value per share |
|
||
|
Non-vested December 31, 2018 |
|
|
1,050,000 |
|
|
$ |
7.76 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
Vested |
|
|
(784,375 |
) |
|
$ |
7.76 |
|
|
Forfeited |
|
|
— |
|
|
|
— |
|
|
Non-vested December 31, 2019 |
|
|
265,625 |
|
|
$ |
7.76 |
|
As of December 31, 2019, there was approximately $330 (2018 - $1,882) of total unrecognized compensation cost related to non-vested performance-based RSUs that will be recognized as expenses over a weighted-average period of 1.0 year (2018 - 1.7 years). The total intrinsic values of performance-based RSUs vested in 2019 and 2018 were $46,423 and $0 respectively. The total fair value of performance-based RSUs vested in 2019 were $6,087 (2018 - $ 0).
16. Accumulated Other Comprehensive Income (“AOCI”)
The components of AOCI, net of tax, were as follows:
|
|
|
Foreign Currency Translation Adjustments |
|
|
Unrealized (loss) gain on cash equivalents and investments |
|
||
|
Balance as at January 1, 2018 |
|
$ |
3,866 |
|
|
$ |
— |
|
|
Other comprehensive income (loss) |
|
|
662 |
|
|
|
(765 |
) |
|
Balance as at December 31, 2018 |
|
|
4,528 |
|
|
|
(765 |
) |
|
Cumulative effect adjustment from transition to ASU 2016-01 |
|
|
— |
|
|
|
803 |
|
|
Other comprehensive income (loss) |
|
|
5,174 |
|
|
|
(21 |
) |
|
Balance as at December 31, 2019 |
|
$ |
9,702 |
|
|
$ |
17 |
|
F-43
17. Commitments and Contingencies
Legal proceedings
In the normal course of business, the Company may become involved in legal disputes regarding various litigation matters. In the opinion of management, any potential liabilities resulting from such claims would not have a material effect on the financial statements.
Lease commitments
The Company leases various facilities, under non-cancelable finance and operating leases, which expire at various dates through September 2027.
Maturities of lease liabilities:
|
Year ending December 31, |
|
Operating Leases |
|
|
Finance Leases |
|
||
|
2020 |
|
$ |
3,493 |
|
|
$ |
1,083 |
|
|
2021 |
|
|
3,276 |
|
|
|
1,083 |
|
|
2022 |
|
|
2,897 |
|
|
|
7,333 |
|
|
2023 |
|
|
2,824 |
|
|
|
15,677 |
|
|
2024 |
|
|
2,436 |
|
|
|
— |
|
|
Thereafter |
|
|
7,861 |
|
|
|
— |
|
|
Total lease payments |
|
|
22,787 |
|
|
|
25,176 |
|
|
Imputed interest |
|
|
5,059 |
|
|
|
11,024 |
|
|
Obligations recognized |
|
$ |
17,728 |
|
|
$ |
14,152 |
|
Purchase commitments
The following table reflects the Company’s future non-cancellable minimum purchase commitments for inventory as of December 31, 2019:
|
|
|
Total |
|
|
2020 |
|
|
|
|
2021 |
|
|
|
|
2022 |
|
|
|
|
2023 |
|
|
|
|
2024 |
|
|
|
|
Thereafter |
|
|||||||
|
Purchase commitments |
|
$ |
132,743 |
|
|
$ |
131,010 |
|
|
|
|
$ |
1,657 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
- |
|
|
|
|
$ |
- |
|
|
Total |
|
$ |
132,743 |
|
|
$ |
131,010 |
|
|
|
|
$ |
1,657 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
38 |
|
|
|
|
$ |
- |
|
|
|
|
$ |
- |
|
As a result of changing industry dynamics, the Company is currently in the process of re-negotiating the terms of several supply agreements, including quantities and pricing, related to CBD, cannabis extracts/oils, and hemp flower. The re-negotiations are ongoing and there can be no assurance that terms satisfactory to the Company can be reached on a timely basis, or at all.
In 2018, the Company signed an agreement with Rose Lifescience Inc. ("Rose") for distribution and marketing of product in Quebec in exchange for a minimum fee of $384 per annum for an initial term of five years. The Company has agreed to purchase the lesser of 2,000 Kg per year or 40% of the production of Cannabis at a rate of 115% of cost of goods sold from the Rose facility. As the purchase commitment is an undeterminable variable amount, it is excluded from the above schedule.
In 2018, the Company entered into a Product and Trademark License Agreement with Docklight LLC, a related party (refer to Note 22), to use certain intellectual property rights in exchange for payment of royalty depending upon specified percentage of licensed product net sales. As the purchase commitment is an undeterminable variable amount, it is excluded from the above schedule.
F-44
Other commitments
The Company has payments on the ABG finance liability (refer to Note 4) and convertible notes (refer to Note 13) as follows:
|
|
|
Total |
|
|
2020 |
|
|
|
|
2021 |
|
|
|
|
2022 |
|
|
|
|
2023 |
|
|
|
|
2024 |
|
|
|
|
Thereafter |
|
|||||||
|
ABG finance liability |
|
$ |
8,500 |
|
|
$ |
1,000 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
Convertible notes |
|
|
475,000 |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
475,000 |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Total |
|
$ |
483,500 |
|
|
$ |
1,000 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
476,500 |
|
|
|
|
$ |
1,500 |
|
|
|
|
$ |
1,500 |
|
18. Revenue from Contracts with Customers
The Company reports two segments: cannabis and hemp, in accordance with ASC 280 Segment Reporting. The Company generates revenues from the cannabis and hemp segments through contracts with customers, each with a single performance obligation, being the sale of products. The Company determines that revenue information disclosed in business segment information in Note 25 disaggregates revenue into categories that depict how the nature, amount, timing and uncertainty of revenue and cash flows are affected by economic factors.
For certain long-term arrangements, the Company has performance obligations for goods it has not yet delivered. For these arrangements, the Company does not have a right to bill for the undelivered goods. The Company has determined that any unbilled consideration relates entirely to the value of undelivered goods. Accordingly, the Company has not recognized revenue, and has elected not to disclose amounts, related to these undelivered goods. As of December 31, 2019, other than accounts receivable, net of allowance for doubtful debts, the Company has no contract balances in the balance sheets.
There are no applicable disclosures related to periods prior to the adoption of ASC 606.
19. General and Administrative Expenses
General and administrative expenses are comprised of the following items:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Salaries and benefits |
|
$ |
39,565 |
|
|
$ |
11,721 |
|
|
$ |
3,717 |
|
|
Professional fees |
|
|
21,189 |
|
|
|
7,557 |
|
|
|
1,715 |
|
|
Travel expenses |
|
|
4,565 |
|
|
|
2,031 |
|
|
|
287 |
|
|
Other expenses |
|
|
16,649 |
|
|
|
8,152 |
|
|
|
1,780 |
|
|
Total |
|
$ |
81,968 |
|
|
$ |
29,461 |
|
|
$ |
7,499 |
|
20. Income Taxes
For financial reporting purposes, loss before income taxes includes the following components:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
United States |
|
$ |
(156,010 |
) |
|
$ |
(42,418 |
) |
|
|
— |
|
|
Canada |
|
|
(151,736 |
) |
|
|
(25,333 |
) |
|
|
(7,411 |
) |
|
Portugal |
|
|
(11,781 |
) |
|
|
(2,208 |
) |
|
|
— |
|
|
Other countries |
|
|
(10,092 |
) |
|
|
(2,215 |
) |
|
|
(398 |
) |
|
Total |
|
$ |
(329,619 |
) |
|
$ |
(72,174 |
) |
|
$ |
(7,809 |
) |
F-45
The (recoveries) expenses for income taxes consists of:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
151 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Canada |
|
|
112 |
|
|
|
— |
|
|
|
— |
|
|
Other countries |
|
|
134 |
|
|
|
34 |
|
|
|
— |
|
|
Total |
|
|
397 |
|
|
|
34 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
(4,390 |
) |
|
$ |
(4,485 |
) |
|
$ |
— |
|
|
Canada |
|
|
(3,383 |
) |
|
|
— |
|
|
|
— |
|
|
Other countries |
|
|
(1,074 |
) |
|
|
— |
|
|
|
— |
|
|
Total |
|
|
(8,847 |
) |
|
|
(4,485 |
) |
|
|
— |
|
|
Total |
|
$ |
(8,450 |
) |
|
$ |
(4,451 |
) |
|
$ |
— |
|
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Loss before income taxes: |
|
$ |
(329,619 |
) |
|
$ |
(72,174 |
) |
|
$ |
(7,809 |
) |
|
Income tax benefits at statutory rate |
|
|
(69,220 |
) |
|
|
(15,157 |
) |
|
|
(2,733 |
) |
|
Tax impact of foreign operations |
|
|
(9,193 |
) |
|
|
(1,864 |
) |
|
|
675 |
|
|
Foreign exchange and other |
|
|
1,015 |
|
|
|
1,399 |
|
|
|
(480 |
) |
|
Non-deductible expenses |
|
|
483 |
|
|
|
5,331 |
|
|
|
61 |
|
|
Changes in enacted rates |
|
|
(3 |
) |
|
|
— |
|
|
|
(288 |
) |
|
Utilization of losses not previously recognized |
|
|
— |
|
|
|
— |
|
|
|
(9 |
) |
|
Stock based and other compensation |
|
|
2,113 |
|
|
|
— |
|
|
|
— |
|
|
Change in valuation allowance |
|
|
66,355 |
|
|
|
5,840 |
|
|
|
2,774 |
|
|
Income tax benefits, net |
|
$ |
(8,450 |
) |
|
$ |
(4,451 |
) |
|
$ |
— |
|
The following table summarizes the components of deferred tax:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Deferred assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss carryforwards - United States |
|
$ |
5,843 |
|
|
$ |
4,173 |
|
|
$ |
— |
|
|
Operating loss carryforwards - Canada |
|
|
59,755 |
|
|
|
13,723 |
|
|
|
8,297 |
|
|
Operating loss carryforwards - Other Countries |
|
|
5,158 |
|
|
|
607 |
|
|
|
148 |
|
|
Property and equipment |
|
|
— |
|
|
|
2,510 |
|
|
|
183 |
|
|
Currently nondeductible interest |
|
|
4,915 |
|
|
|
— |
|
|
|
— |
|
|
Outside basis difference |
|
|
21,546 |
|
|
|
— |
|
|
|
— |
|
|
Deferred financing costs |
|
|
208 |
|
|
|
27 |
|
|
|
37 |
|
|
Investment tax credits and related pool balance |
|
|
180 |
|
|
|
57 |
|
|
|
57 |
|
|
Other |
|
|
931 |
|
|
|
— |
|
|
|
8 |
|
|
Total Deferred tax assets |
|
|
98,536 |
|
|
|
21,097 |
|
|
|
8,730 |
|
|
Less valuation allowance |
|
|
(84,337 |
) |
|
|
(14,433 |
) |
|
|
(8,601 |
) |
|
Net deferred tax assets |
|
|
14,199 |
|
|
|
6,664 |
|
|
|
129 |
|
|
Deferred tax liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment |
|
|
(5,800 |
) |
|
|
(2,328 |
) |
|
|
— |
|
|
Intangible assets |
|
|
(54,814 |
) |
|
|
(289 |
) |
|
|
(129 |
) |
|
Deferred financing costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Equity portion of convertible notes |
|
|
(6,948 |
) |
|
|
(8,471 |
) |
|
|
— |
|
|
Total deferred tax liabilities |
|
|
(67,562 |
) |
|
|
(11,088 |
) |
|
|
(129 |
) |
|
Net deferred tax liability |
|
$ |
(53,363 |
) |
|
$ |
(4,424 |
) |
|
$ |
— |
|
F-46
Effective January 1, 2018, the United States tax law provides a deduction for the foreign-source portion of dividends received from specified foreign corporations. As such, the Company does not maintain an indefinite reinvestment assertion on unremitted foreign earnings and has recorded a deferred tax liability, as necessary, for any estimated foreign, federal, or state tax liabilities associated with a future repatriation of foreign earnings.
At December 31, 2019, the Company had United States net operating loss carryforwards of approximately $27,000 that can be carried forward indefinitely and limited in annual use to 80% of the current year taxable income. The Company has Canadian net operating loss carry-forwards of approximately $223,000 that can be carried forward 20 years and begin to expire in 2028. Management believes that it is more-likely-than-not that the benefit from certain United States and foreign net operating loss carryforwards will not be realized. In recognition of this risk, the Company has provided a valuation allowance on the deferred tax assets relating to these carryforwards. The net change in the total valuation allowance was an increase of $66,355 and $5,840 for the years ended December 31, 2019 and 2018, respectively.
The Company recognizes the financial statement impact of a tax position only after determining that the relevant tax authority would more-likely-than-not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest impact that has a greater than fifty percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
The total amount of gross unrecognized tax benefits was $86, $0, and $0 as of December 31, 2019, 2018 and 2017 respectively. There is a reasonable possibility that the Company’s unrecognized tax benefits will change within twelve months due to audit settlements or the expiration of statute of limitations, but the Company does not expect the change to be material to the financial statements.
The Company recognizes interest and, if applicable, penalties (not included in the “unrecognized tax benefits” table above) for any uncertain tax positions. Interest and penalties are recorded as a component of income tax expenses. In the years ended December 31, 2019, 2018 and 2017, the Company recorded approximately $0, $0 and $0, respectively, of interest and penalty expenses related to uncertain tax positions. As of December 31, 2019 and 2018, the Company had a cumulative balance of accrued interest and penalties on unrecognized tax positions of $0 and $0, respectively.
The Company and its subsidiaries are subject to United States federal income tax as well as the income tax of multiple state and foreign jurisdictions. The Company is not currently under audit in any jurisdiction for any period. Major jurisdictions where there are wholly owned subsidiaries of Tilray, Inc. which require income tax filings include the Canada, Portugal, Germany, Australia, United Kingdom, and Chile. The earliest periods open for review by local taxing authorities are fiscal years 2015 for Canada, 2017 for Portugal, 2016 for Germany, 2017 for Australia, 2014 for the U.K. and 2018 for Chile. Within the next four fiscal quarters, the statute of limitations will begin to close on fiscal year 2016 Canadian income tax returns.
21. Supplemental Cash Flow Information(1)
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Cash paid for interest |
|
$ |
28,206 |
|
|
$ |
1,189 |
|
|
$ |
1,157 |
|
|
Cash paid for income taxes |
|
|
145 |
|
|
|
— |
|
|
|
— |
|
|
Non-cash financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock |
|
|
— |
|
|
|
2 |
|
|
|
— |
|
|
Non-cash investing |
|
|
|
|
|
|
|
|
|
|
|
|
|
Alef acquisition |
|
|
— |
|
|
|
2,855 |
|
|
|
— |
|
|
Acquisition of Manitoba Harvest |
|
|
158,197 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition of Natura |
|
|
38,979 |
|
|
|
— |
|
|
|
— |
|
|
Acquisition of S&S |
|
|
5,021 |
|
|
|
— |
|
|
|
— |
|
|
Investment in ABG Profit Participation Arrangement, net of receivable |
|
|
97,544 |
|
|
|
— |
|
|
|
— |
|
|
Purchases of investments |
|
|
10,551 |
|
|
|
— |
|
|
|
— |
|
|
(1) |
For supplemental cash flow information related to leases, refer to Note 9. |
F-47
22. Related-Party Transactions
The Company was a wholly owned subsidiary of Privateer Holdings prior to the Company’s Series A preferred stock financing and initial public offering. Prior to the close of the Downstream Merger on December 12, 2019 (refer to Note 14), Privateer Holdings held more than 10% of the Company’s outstanding shares of Class 2 common stock and held 100% of the Company’s Class 1 common stock.
In the normal course of business, the Company entered into related party transactions with Privateer Holdings and its subsidiaries, including charges for services provided by executives and employees of Privateer Holdings. Transactions disclosed below with Privateer Holdings relate to the period up to December 12, 2019. Transactions disclosed below that were previously subsidiaries of Privateer Holdings continue to be related parties as they remain under common control.
Privateer Holdings
Pursuant to the Subversive Capital Alliance Agreement dated May 15, 2018 between Privateer Holdings and Subversive Capital, LLC (“Subversive”) as agent for Privateer Holdings, Subversive held 5,530 shares of S&S at a cost of £347.96 per share (£1,924 in aggregate) on July 11, 2019. Subversive is a company controlled by Michael Auerbach, who is a member of the Company’s Board of Directors. On July 11, 2019, the Subversive Capital Alliance Agreement was terminated in connection with the Company’s acquisition of S&S and the Company paid £1,924 in cash to Subversive for the 5,530 shares, which represented approximately 30% ownership of S&S and only the original cost basis in such shares. The cash paid to Subversive as part of the purchase consideration for the acquisition of S&S reflected no gain on its investment, thereby eliminating any economic conflict of interest or appearance thereof. In accordance with the Company’s Related-Persons Transactions Policy, the Audit Committee of the Board of Directors, comprised solely of the independent directors, approved the acquisition of S&S and the payment to Subversive.
During the second quarter of 2019, the Company assumed a real estate operating lease upon assignment from Privateer Holdings. In connection with this lease, the Company reimbursed Privateer Holdings $2,070 for leasehold improvements at cost and $1,000 for the security deposit held by the landlord at cost, recorded within property and equipment and deposits and other assets, respectively, on the balance sheets as of December 31, 2019.
Management services charged by Privateer Holdings for services performed include management services, support services, business development services and research and development services recorded in operating expenses for the year ended December 31, 2019 in the amounts of $1,054 (2018 – $3,878). Depending on the nature of the services performed, these expenses are included within general and administrative expenses, sales and marketing expenses or research and development expenses in the statements of net loss and comprehensive loss. Pursuant to the Company’s agreement with Privateer Holdings entered in February 2018 and terminated in February 2019, personnel compensation was charged at cost plus a 3.0% markup and other services at cost. As of December 31, 2019, no amounts are recorded within accounts payable for management services due to Privateer Holdings (December 31, 2018 – $3,878).
Previously Privateer Holdings’ subsidiaries
Leafly Holdings, Inc. (“Leafly”) operational expenses
The Company pays on behalf of Leafly, previously a wholly owned subsidiary of Privateer Holdings, certain operational expenses and vice-versa. These payments are then recharged to the company that incurred the expenses. During the year ended December 31, 2019, operational expenses of $272 was recorded within general and administrative expenses in the statements of net loss and comprehensive loss. Payments made during the year ended December 31, 2018 were deemed immaterial.
Docklight LLC (“Docklight”) royalty and management services
The Company pays Docklight, previously a wholly owned subsidiary of Privateer Holdings, a royalty fee for using their branding on company products. Additionally, the Company receives management services from Docklight, for which the Company is charged management fees. During the year ended December 31, 2019, fees and services of $176 were recorded within general and administrative expenses in the statements of net loss and comprehensive loss. Payments made during the year ended December 31, 2018 were deemed immaterial. During the year ended December 31, 2019, the Company sold $165 of Hemp CBD Isolate to Docklight. Refer to Note 17 for purchase commitments with Docklight.
F-48
Other Related Parties
Ten Eleven management fees
In February 2019, the Company entered into a management agreement with Ten Eleven Management LLC doing business as Privateer Management (“Ten Eleven”), pursuant to which Ten Eleven provides the Company with certain general administrative and corporate services on an as-requested basis for a monthly service fee. Prior to the Downstream Merger on December 12, 2019, the owners of Ten Eleven collectively held more than 10% of the Company’s outstanding shares of Class 1 common stock indirectly through investment in Privateer Holdings. Subsequent to the Downstream Merger, the owners of Ten Eleven own the shares of Tilray directly. In February 2020, the Company extended the agreement with Ten Eleven to continue services until March 2020. During the year ended December 31, 2019, management services of $275 was recorded within general and administrative expenses in the statements of net loss and comprehensive loss. No amounts were recorded for the comparative periods in 2018.
Fluent and Cannfections
The Company has joint venture arrangements with a 50% ownership and voting interest in each Fluent and Cannfections. Refer to Note 7 for details over transactions with these entities for the year ended December 31, 2019.
23. Financial Instruments
Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company’s cash and cash equivalents, accounts receivable and short-term investments.
The Company’s cash and cash equivalents are deposited in major financial institutions in Canada, Australia, Portugal, Germany, Netherlands and the United States. To date, the Company has not experienced any losses on its cash deposits. Accounts receivable are unsecured and the Company does not require collateral from its customers.
The Company is also exposed to credit risk from the potential default by any of its counterparties on its financial assets.
The Company evaluates the collectability of its accounts receivable and provides an allowance for potential credit losses as necessary. As at December 31, 2019 and December 31, 2018, the Company is not exposed to any significant credit risk related to counterparty performance of outstanding accounts receivable. Allowance for doubtful accounts at December 31, 2019 is $2,015 (2018 - $292).
During the year ended December 31, 2019, the Company had advanced and subsequently written off $5,383, recorded in impairment of assets in the statements of net loss and comprehensive loss, relating to a working capital loan which is not expected to be collected.
Foreign currency risk
As the Company conducts its business in many areas of the world involving transactions denominated in a variety of currencies, the Company is exposed to foreign currency risk. A significant portion of the Company’s assets, revenue, and expenses are denominated in the Canadian dollar. A 10% change in the exchange rates for the Canadian dollar would affect the carrying value of net assets by approximately $12,457 as of December 31, 2019 (2018 - $2,817), with a corresponding impact to accumulated other comprehensive income. For the year ended December 31, 2019, the Company had foreign currency gain of $5,944 (2018 – loss of $7,234, 2017 – gain of $1,363).
Liquidity risk
The Company’s objective is to have sufficient liquidity to meet its liabilities when due. The Company monitors its cash balances and cash flows generated from operations to meet its requirements. As at December 31, 2019 and December 31, 2018, the most significant financial liabilities are accounts payable, accrued expenses and other current liabilities, and convertible notes.
F-49
24. Fair Value Measurement
The Company complies with ASC 820, Fair Value Measurements, for its financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually. In general, fair values determined by Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are observable such as quoted prices, interest rates and yield curves. Fair values determined by Level 3 inputs are unobservable data points for the asset or liability, and includes situations where there is little, if any, market activity for the asset or liability.
The following tables present information about the Company’s assets that are measured at fair value on a recurring basis as of December 31, 2019 and 2018 indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
|
|
|
Quoted prices |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in active |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
markets for |
|
|
Other |
|
|
Significant |
|
|
|
|
|
|||
|
|
|
identical |
|
|
observable |
|
|
unobservable |
|
|
|
|
|
|||
|
|
|
assets |
|
|
inputs |
|
|
inputs |
|
|
|
|
|
|||
|
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|
Total |
|
||||
|
December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity investments measured at fair value |
|
|
4,183 |
|
|
|
— |
|
|
|
— |
|
|
|
4,183 |
|
|
Debt securities classified as available-for-sale |
|
|
727 |
|
|
|
— |
|
|
|
4,320 |
|
|
|
5,047 |
|
|
Acquisition-related contingent consideration |
|
|
— |
|
|
|
— |
|
|
|
420 |
|
|
|
420 |
|
|
Total recurring fair value measurements |
|
$ |
4,910 |
|
|
$ |
— |
|
|
$ |
4,740 |
|
|
$ |
9,650 |
|
|
|
|
Quoted prices |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in active |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
markets for |
|
|
Other |
|
|
Significant |
|
|
|
|
|
|||
|
|
|
identical |
|
|
observable |
|
|
unobservable |
|
|
|
|
|
|||
|
|
|
assets |
|
|
inputs |
|
|
inputs |
|
|
|
|
|
|||
|
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|
Total |
|
||||
|
December 31, 2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents |
|
$ |
203,761 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
203,761 |
|
|
Investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term investments - debt securities |
|
|
30,335 |
|
|
|
— |
|
|
|
— |
|
|
|
30,335 |
|
|
Equity investments measured at fair value |
|
|
1,163 |
|
|
|
682 |
|
|
|
— |
|
|
|
1,845 |
|
|
Total recurring fair value measurements |
|
$ |
235,259 |
|
|
$ |
682 |
|
|
$ |
— |
|
|
$ |
235,941 |
|
Items measured at fair value on a recurring basis
The Company’s financial assets and liabilities required to be measured on a recurring basis are its short-term investments – debt securities, equity investments measured at fair value, debt securities classified as available-for-sale and acquisition-related contingent consideration.
Debt securities classified as available-for-sale (including short-term investments) and equity investments recorded at fair value: The estimated fair value is determined using quoted market prices or broker or dealer quotations.
F-50
Acquisition-related contingent consideration: Contingent consideration is recorded within accrued expenses and other current liabilities and primarily reflects the consideration for: (i) the acquisition of Manitoba Harvest payable in shares of Class 2 common stock contingent on revenues earned in 2019, (ii) the acquisition of Natura payable in shares of Class 2 common stock contingent on production levels, and (iii) the acquisition of S&S. For Manitoba Harvest acquisition, the estimated fair value of the contingent consideration was valued using a probability-weighted discounted cash flow model based on internal forecasts and the estimated cost of debt for the Company. For Natura acquisition, the estimated fair value of the contingent consideration on the acquisition date was valued using a discounted cash flow analysis based on internal forecast projected using a Monte Carlo simulation model, an expected quarterly production distribution function, and a weighted average cost of capital adjusted to account for revenue risk derived as of the date of acquisition. Significant increases (decreases) in the volatility of revenue levels or in any of the probabilities of achievement of specified milestones, or decreases (increases) in the discount rate would result in a significantly higher (lower) fair value, respectively, and commensurate changes to contingent consideration. The contingent consideration is reassessed and adjusted to fair value at each reporting date through acquisition-related (income) expense, net (refer to Note 3 and 12).
The opening balances of assets and liabilities categorized within Level 3 of the fair value hierarchy measured at fair value on a recurring basis are reconciled to the closing balances as follows:
|
|
|
Debt securities classified as available-for- sale |
|
|
Acquisition- related contingent consideration |
|
||
|
Opening balance as at January 1, 2019 |
|
$ |
— |
|
|
$ |
— |
|
|
Additions and settlements |
|
|
|
|
|
|
|
|
|
Additions |
|
|
4,171 |
|
|
|
51,026 |
|
|
Settlements |
|
|
— |
|
|
|
(4,450 |
) |
|
Total gains or losses for the period: |
|
|
— |
|
|
|
— |
|
|
Included in net loss |
|
|
— |
|
|
|
— |
|
|
Interest expenses, net |
|
|
149 |
|
|
|
— |
|
|
Acquisition-related (income) expenses, net |
|
|
— |
|
|
|
(46,914 |
) |
|
Foreign currency translation gain, net |
|
|
— |
|
|
|
758 |
|
|
Closing balance as at December 31, 2019 |
|
$ |
4,320 |
|
|
$ |
420 |
|
Items measured at fair value on a non-recurring basis
The Company's non-financial assets, such as prepayments and other current assets, ABG finance receivable, long lived assets, including property and equipment and intangible assets, are measured at fair value when there is an indicator of impairment and are recorded at fair value only when an impairment charge is recognized. In connection with an evaluation of such non-financial assets during the year ended December 31, 2019, the carrying values of prepayments and intangible assets were concluded to exceed their fair values. As a result, the Company recorded impairment charges that incorporates fair value measurements based on Level 3 inputs (refer to Note 6 and Note 10).
The estimated fair value of cash and cash equivalents, accounts receivable, net, accounts payable, and accrued expenses and other current liabilities at December 31, 2019 and 2018 approximate their carrying amount due to short term nature of these instruments.
F-51
25. Business Segment Information
As of October 1, 2019, the Company has two operating segments based on major product categories: Cannabis and Hemp. These operating segments are also the Company’s reportable segments. Historical financial information has been recast to reflect the current segment structure.
The Cannabis segment cultivates, processes and distributes medical and adult-use cannabis products in a variety of formats, as well as related accessories, on a global basis. The Hemp segment cultivates, processes and distributes a diverse portfolio of hemp-based natural food and wellness products within North America.
The results of each segment are regularly reviewed by the Company’s Chief Executive Officer, who is the Company’s chief operating decision maker, to assess the performance of the segment and make decisions regarding the allocation of resources. The Company’s chief operating decision maker uses revenue and gross profit as the measure of segment profit or loss. The accounting policies of each segment are the same as those set out under the summary of significant accounting policies in Note 2. There are no intersegment sales or transfers.
|
|
|
Year ended December 31, |
|
|||||||||||||||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||||||||||||||
|
|
|
Revenue |
|
|
Gross profit |
|
|
Revenue |
|
|
Gross profit |
|
|
Revenue |
|
|
Gross profit |
|
||||||
|
Cannabis |
|
$ |
107,147 |
|
|
$ |
(42,302 |
) |
|
$ |
43,130 |
|
|
$ |
14,275 |
|
|
$ |
20,538 |
|
|
$ |
11,377 |
|
|
Hemp |
|
$ |
59,832 |
|
|
$ |
18,806 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total |
|
$ |
166,979 |
|
|
$ |
(23,496 |
) |
|
$ |
43,130 |
|
|
$ |
14,275 |
|
|
$ |
20,538 |
|
|
$ |
11,377 |
|
No asset information is provided for the segments because the Company’s chief operating decision maker does not receive asset information by segment on a regular basis.
Total revenue and gross profit for the reportable segments is equal to the Company’s consolidated revenue and gross profit.
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Gross profit for the segments |
|
$ |
(23,496 |
) |
|
$ |
14,275 |
|
|
$ |
11,377 |
|
|
General and administrative expenses |
|
|
(81,968 |
) |
|
|
(29,461 |
) |
|
|
(7,499 |
) |
|
Sales and marketing expenses |
|
|
(61,084 |
) |
|
|
(15,366 |
) |
|
|
(7,164 |
) |
|
Research and development expenses |
|
|
(6,558 |
) |
|
|
(4,264 |
) |
|
|
(3,171 |
) |
|
Depreciation and amortization expenses |
|
|
(11,607 |
) |
|
|
(1,598 |
) |
|
|
(902 |
) |
|
Stock-based compensation expense |
|
|
(31,842 |
) |
|
|
(20,988 |
) |
|
|
(139 |
) |
|
Impairment of assets |
|
|
(112,070 |
) |
|
|
— |
|
|
|
— |
|
|
Acquisition-related income (expense), net |
|
|
31,427 |
|
|
|
(248 |
) |
|
|
— |
|
|
Loss from equity method investments |
|
|
(4,504 |
) |
|
|
— |
|
|
|
— |
|
|
Foreign exchange (loss) gain, net |
|
|
5,944 |
|
|
|
(7,234 |
) |
|
|
1,363 |
|
|
Interest expense, net |
|
|
(34,690 |
) |
|
|
(9,110 |
) |
|
|
(1,686 |
) |
|
Finance income from ABG |
|
|
764 |
|
|
|
— |
|
|
|
— |
|
|
Loss on disposal of property and equipment |
|
|
(2,436 |
) |
|
|
(190 |
) |
|
|
|
|
|
Other income, net |
|
|
2,501 |
|
|
|
2,010 |
|
|
|
12 |
|
|
Loss before income taxes |
|
$ |
(329,619 |
) |
|
$ |
(72,174 |
) |
|
$ |
(7,809 |
) |
Sources of revenue were as follows:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Dried cannabis |
|
$ |
82,753 |
|
|
$ |
21,674 |
|
|
$ |
16,260 |
|
|
Cannabis extracts |
|
|
24,139 |
|
|
|
21,179 |
|
|
|
3,965 |
|
|
Hemp products |
|
|
59,832 |
|
|
|
— |
|
|
|
— |
|
|
Accessories and other |
|
|
255 |
|
|
|
277 |
|
|
|
313 |
|
|
Total |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
20,538 |
|
F-52
Channels of revenue were as follows:
|
|
Year Ended December 31, |
|
|||||||
|
|
2019 |
|
2018 |
|
2017 |
|
|||
|
Cannabis |
|
|
|
|
|
|
|
|
|
|
Adult-use |
$ |
55,763 |
|
$ |
3,521 |
|
$ |
— |
|
|
Canada - medical |
|
12,556 |
|
|
18,052 |
|
|
19,642 |
|
|
International - medical |
|
13,378 |
|
|
2,912 |
|
|
896 |
|
|
Bulk |
|
25,450 |
|
|
18,645 |
|
|
— |
|
|
Total Cannabis revenue |
$ |
107,147 |
|
$ |
43,130 |
|
$ |
20,538 |
|
|
Hemp |
|
59,832 |
|
|
— |
|
|
— |
|
|
Total |
$ |
166,979 |
|
$ |
43,130 |
|
$ |
20,538 |
|
F-53
Revenue attributed to geographic region based on the location of the customer was as follows:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Canada |
|
$ |
130,291 |
|
|
$ |
40,209 |
|
|
$ |
19,775 |
|
|
United States |
|
|
23,516 |
|
|
|
— |
|
|
|
— |
|
|
Other countries |
|
|
13,172 |
|
|
|
2,921 |
|
|
|
763 |
|
|
Total |
|
$ |
166,979 |
|
|
$ |
43,130 |
|
|
$ |
20,538 |
|
Long-lived assets consisting of property and equipment, net of accumulated depreciation, attributed to geographic regions based on their physical location were as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Canada |
|
$ |
144,065 |
|
|
$ |
64,687 |
|
|
Portugal |
|
|
36,908 |
|
|
|
15,455 |
|
|
United States |
|
|
3,171 |
|
|
|
— |
|
|
Other countries |
|
|
73 |
|
|
|
72 |
|
|
Total |
|
$ |
184,217 |
|
|
$ |
80,214 |
|
Major Customers
Two customers accounted for 13% each of revenue for the year ended December 31, 2019. One customer accounted for 24% of the Company’s revenue for the year ended December 31, 2018. No one customer accounted for greater than 10% of the Company’s revenue for the year ended December 31, 2017.
Two customers accounted for 20% and 10%, respectively, of the Company’s accounts receivable balance as of December 31, 2019. Two customers accounted for 30% and 16%, respectively, of the Company’s accounts receivable balance as of December 31, 2018. No one customer accounted for greater than 10% of the Company’s accounts receivable as of December 31, 2017.
26. Subsequent Events
During the month of January 2020, we issued 274,044 shares of Class 2 common stock for gross proceeds of approximately $14,770 under the at-the-market equity offering program.
On January 24, 2020, the Company entered into (i) an Amended and Restated Profit Participation Agreement (the “A&R Profit Participation Agreement”) with ABG, which amended and restated in its entirety the Profit Participation Agreement, dated January 14, 2019, and (ii) the First Amendment to Payment Agreement with ABG (the “Payment Agreement Amendment”), which amends the Payment Agreement, dated January 14, 2019. The Company and ABG agreed that Tilray will no longer have any obligation to pay the additional consideration with an aggregate value of $83,333 in cash or in shares of Class 2 common stock, In addition, the Company will not be entitled to any guaranteed minimum participation rights and beginning January 1, 2020 through December 31, 2028, the Company agreed that it will not be entitled to any participation rights until such participation rights with respect to each contract year exceeds $10,000, and in the event the participation rights are achieved, the Company will be entitled to the full 49% participation rights.
The impact of the A&R Profit Participation Agreement will result in a write-off of the ABG finance receivable of $7,030 which will be recorded through the statement of net loss and comprehensive loss and $28,900 through accumulated deficit in January 2020.
During the month of February 2020, the Company restructured its global organization to meet the needs of the current industry environment. As a result, the Company incurred $650 in restructuring costs.
On February 28, 2020, the Company (“the Borrower”) entered into a credit agreement for a senior secured credit facility in a maximum aggregate principal amount of $59,600 (the “Senior Facility”). Transaction fees incurred on the Senior Facility are $4,500. The Senior Facility consists of a 2-year $59,600 senior secured term loan
F-54
facility, of which $49,700 was drawn at the closing, and of which $9,900 may be drawn at any point 90 days following closing at the Borrower’s election. The Senior Facility will bear interest on the outstanding principal balance at an annual rate equal to the Canadian prime rate plus 8.05%, calculated based on the daily outstanding balance of the Senior Facility calculated and compounded monthly, not in advance and with no deemed reinvestment of monthly payments. The Senior Facility contains certain affirmative and negative covenants. The operational covenant includes a minimum unrestricted cash threshold of $29,868 for capital expenditures and investments.
27. Quarterly Financial Data (unaudited)
The following table contains selected quarterly data for 2019 and 2018. The information should be read in conjunction with the Company’s financial statements and related notes included elsewhere in this report. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
|
|
|
Three months ended |
|
|||||||||||||
|
|
|
March 31, |
|
|
June 30, |
|
|
September 30, |
|
|
December 31, |
|
||||
|
2019¹ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
23,038 |
|
|
$ |
45,904 |
|
|
$ |
51,101 |
|
|
$ |
46,936 |
|
|
Gross profit |
|
|
5,385 |
|
|
|
12,273 |
|
|
|
15,853 |
|
|
|
(57,007 |
) |
|
Operating loss |
|
|
(28,332 |
) |
|
|
(32,961 |
) |
|
|
(23,785 |
) |
|
|
(216,624 |
) |
|
Net loss |
|
|
(29,369 |
) |
|
|
(36,301 |
) |
|
|
(36,351 |
) |
|
|
(219,148 |
) |
|
Net loss per share—basic and diluted² |
|
$ |
(0.31 |
) |
|
$ |
(0.37 |
) |
|
$ |
(0.37 |
) |
|
$ |
(2.14 |
) |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
7,808 |
|
|
$ |
9,744 |
|
|
$ |
10,047 |
|
|
$ |
15,531 |
|
|
Gross profit |
|
|
3,896 |
|
|
|
4,177 |
|
|
|
3,068 |
|
|
|
3,134 |
|
|
Operating loss |
|
|
(3,740 |
) |
|
|
(10,990 |
) |
|
|
(20,012 |
) |
|
|
(22,908 |
) |
|
Net loss |
|
|
(5,181 |
) |
|
|
(12,833 |
) |
|
|
(18,699 |
) |
|
|
(31,010 |
) |
|
Net loss per share—basic and diluted² |
|
$ |
(0.07 |
) |
|
$ |
(0.17 |
) |
|
$ |
(0.21 |
) |
|
$ |
(0.33 |
) |
|
¹ |
In the fourth quarter of 2019, the Company adopted ASU 2016-01, ASC 842, ASC 606 and ASU 2018-07. Each interim period in 2019 has been recast to reflect the effects of this adoption. Refer to Note 2 for further discussion of the new accounting pronouncements recently adopted. |
|
² |
Earnings per share for the four quarters combined may not equal earnings per share for the year due to rounding. |
F-55
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Management, with the participation of our Chief Executive Officer and Chief Financial Officer (the Company’s principal executive officer and principal financial officer, respectively), evaluated the effectiveness of the Company’s disclosure controls and procedures as of December 31, 2019. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, (or “DCPs”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. DCPs include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based on the evaluation of the Company’s DCPs as of December 31, 2019, the Company’s Chief Executive Officer and Chief Financial Officer concluded that, as a result of the material weaknesses in the Company’s internal control described below, as of such date, the Company’s DCPs were not effective.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Rules 13a‐15(f) and 15d(f) under the Exchange Act, internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of the Company’s financial reporting and the preparation of financial statements for external purposes in accordance with United States Generally Accepted Accounting Policies (“U.S. GAAP”). Due to inherent limitations, the Company's internal control over financial reporting may not prevent or detect all misstatements, including the possibility of human error, the circumvention or overriding of controls, or fraud. Effective internal control can provide only reasonable assurance with respect to the preparation and fair presentation of financial statements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management of the Company, under the supervision and participation of the CEO and CFO, conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019 based on the criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
As permitted by SEC guidance, management has excluded from its assessment the internal control over financial reporting at FHF Holdings Ltd. (“Manitoba Harvest”), for which control was acquired on February 28, 2019, and Natura Naturals Holdings Inc. (“Natura Naturals”), for which control was acquired on February 15, 2019. The financial statements of these entities constitute, in aggregate, 50% of total assets, 44% of revenues and 6% of net loss of the consolidated financial statement amounts as of and for the year ended December 31, 2019.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis by the Company’s internal controls.
As a result of management’s evaluation of the effectiveness of the Company's internal control over financial reporting, management concluded that as of December 31, 2019, the company had material weaknesses
76
relating to two components of the COSO framework. These material weaknesses are summarized below, and remediation efforts are outlined in the “Remediation of Material Weaknesses in Internal Control over Financial Reporting” section below.
Material Weaknesses in Internal Control
As of December 31, 2019, the Company identified material weaknesses as of December 31, 2019 in two components of internal control as defined by COSO 2013 (Control Environment and Control Activities).
Control environment: The Company did not maintain an effective control environment based on the criteria established in the COSO framework. The Company has identified deficiencies in the principles associated with the control environment of the COSO framework. Specifically, these control deficiencies constitute material weaknesses, either individually or in the aggregate, relating to: (i) appropriate organizational structure, reporting lines, and authority and responsibilities in pursuit of objectives, (ii) our commitment to attract, develop, and retain competent individuals, and (iii) holding individuals accountable for their internal control related responsibilities.
As of December 31, 2019, the Company did not maintain an effective control environment primarily attributable to the following factor:
|
• |
The Company did not have a sufficient complement of accounting and financial reporting personnel with an appropriate level of knowledge, U.S. GAAP proficiency, experience and training commensurate with our financial reporting requirements. |
Control activities: The Company did not fully design and implement effective control activities based on the criteria established in the COSO framework. The Company has identified deficiencies in the principles associated with the control activities component of the COSO framework. Specifically, these control deficiencies constitute material weaknesses, either individually or in the aggregate, relating to: (i) Selecting and developing control activities that contribute to the mitigation of risks to the achievement of objectives to acceptable levels and (ii) deploying control activities through policies that establish what is expected and procedures that put policies into action.
The Company did not have effective controls in response to the risks of material misstatement. This material weakness is primarily attributable to the following factors:
|
• |
The Company did not have an adequate process or appropriate controls in place to support the accurate reporting of our financial results and disclosures on our Form 10‐K. |
|
• |
The Company did not have effective controls over the completeness and accuracy of key spreadsheets and reports used in financial reporting. |
|
• |
The Company did not have adequate review procedures around the recording of manual entries. |
Due to the existence of the above material weaknesses, management, including the CEO and CFO, has concluded that the Company’s internal control over financial reporting was not effective as of December 31, 2019. These material weaknesses create a reasonable possibility that a material misstatement to the consolidated financial statements will not be prevented or detected on a timely basis.
Deloitte LLP, an independent registered public accounting firm, has audited the Company’s Financial Statements for the fiscal year ended December 31, 2019 and has included its attestation report on management's assessment of the Company’s internal control over financial reporting.
Remediation of Material Weaknesses in Internal Control over Financial Reporting
While the Company believes it has improved its organizational capabilities, the material weaknesses remain unremediated as of December 31, 2019 and the Company’s remediation activities are continuing to take place in 2020. Additionally, although the Company implemented control enhancements in the third and fourth quarters of 2019, there was insufficient time to demonstrate full remediation of monthly and quarterly controls by December 31, 2019.
77
The Company continues to strengthen our internal control over financial reporting and are committed to ensuring that such controls are designed and operating effectively. The Company is implementing process and control improvements to address the above material weaknesses as follows:
|
• |
The Company has supplemented existing accounting resources with external advisors to assist with performing technical accounting activities. In addition, the Company is enhancing the review controls over the application of GAAP and accounting measurements for significant accounts, transactions and related financial statement disclosures and enhancing existing controls that support management’s assertions with respect to the completeness, accuracy and validity of complex accounting measurements on a timely basis. The Company plans to hire full time employees with technical accounting expertise and public company experience, as needed. |
|
• |
The Company began the process of implementing additional consolidation and financial close related controls and automating manual processes, each of which is expected to increase the efficiency of processing transactions, produce accurate and timely information in order to address various operational and compliance needs and reduce our reliance on end‐user spreadsheets. |
|
• |
The Company is designing and implementing procedures and controls to appropriately identify and assess changes made to master data that could significantly impact data integrity and the internal control framework, including but not limited to maintaining customer and vendor master files, perpetual inventory records, and inventory cycle counts. |
|
• |
The Company began the process of formalizing procedures to ensure appropriate internal communications between the accounting department and other operating departments necessary to support the proper functioning of internal controls. |
Management has made significant progress with the Company’s remediation plans and will continue to take measures in 2020 to remediate these material weaknesses. In addition, under the direction of the Audit Committee of the Board of Directors, management will continue to review and make necessary changes to the overall design of the Company’s internal control environment, as well as to refine policies and procedures to improve the overall effectiveness of internal control over financial reporting of the Company.
The material weaknesses in the Company’s internal control over financial reporting will not be considered remediated until the remediated controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively. The Company is working to have these material weaknesses remediated as soon as possible. No system of controls, no matter how well designed and operated, can provide absolute assurance that the objectives of the system of controls will be met, and no evaluation of controls can provide absolute assurance that all control deficiencies or material weaknesses have been or will be detected. There is no assurance that the remediation will be fully effective. As described above, these material weaknesses have not been remediated as of the filing date of this Form 10‐K. If these remediation efforts do not prove effective and control deficiencies and material weaknesses persist or occur in the future, the accuracy and timing of the Company’s financial reporting may be materially and adversely affected.
Changes in Internal Controls over Financial Reporting
Other than those described above, there have been no changes in the Company’s internal control over financial reporting (as defined in Rule 13a‐15(f) and 15d‐5(f) under the Exchange Act) during the quarter and year ended December 31, 2019, that have materially affected, or that are reasonably likely to materially affect, the Company’s internal control over financial reporting.
78
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Tilray, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Tilray, Inc. and subsidiaries (the “Company”) as of December 31, 2019, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, because of the effect of the material weaknesses identified below on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2019, of the Company and our report dated March 2, 2020, expressed an unqualified opinion on those financial statements, included an explanatory paragraph regarding the Company’s adoption of ASU 2016-02, Leases, codified as ASC 842 Leases, as amended, using the modified retrospective approach.
As described in Management’s Report on Internal Control over Financial Reporting, management excluded from its assessment the internal control over financial reporting at FHF Holdings Ltd. (“Manitoba Harvest”), for which control was acquired on February 28, 2019 and Natura Naturals Holdings Inc. (“Natura Naturals”), for which control was acquired on February 15, 2019. The financial statements of these entities constitute, in in aggregate 50% of total assets, 44% of revenues and 6% of net loss of the consolidated financial statement amounts as of and for the year ended December 31, 2019. Accordingly, our audit did not include the internal control over financial reporting at Manitoba Harvest and at Natura Naturals.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become
79
inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Material Weaknesses
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management's assessment: a) Control Environment - control deficiencies constituting material weaknesses, either individually or in the aggregate, relating to: (i) appropriate organizational structure, reporting lines, and authority and responsibilities in pursuit of objectives, (ii) our commitment to attract, develop, and retain competent individuals, and (iii) holding individuals accountable for their internal control related responsibilities. b) Control Activities - control deficiencies constituting material weaknesses, either individually or in the aggregate, relating to: i) Selecting and developing control activities that contribute to the mitigation of risks to the achievement of objectives to acceptable levels, (ii) deploying control activities through policies that establish what is expected and procedures that put policies into action. These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the consolidated financial statements as of and for the year ended December 31, 2019, of the Company, and this report does not affect our report on such financial statements.
/s/ Deloitte LLP
Chartered Professional Accountants
Vancouver, Canada
March 2, 2020
80
Item 9B. Other Information.
On February 28, 2020, we (“the Borrower”) entered into a credit agreement for a senior secured credit facility in a maximum aggregate principal amount of $59.6 million (C$79.8 million) (the “Senior Facility”). Transaction fees incurred on the Senior Facility are $4.5 million. The Senior Facility consists of a 2-year $59.6 million (C$79.8 million) senior secured term loan facility, of which $49.7 million (C$66.5 million) was drawn at the closing, and of which $9.9 million (C$13.3 million) may be drawn at any point 90 days following closing at the Borrower’s election. The Senior Facility will bear interest on the outstanding principal balance at an annual rate equal to the Canadian prime rate plus 8.05%, calculated based on the daily outstanding balance of the Senior Facility calculated and compounded monthly, not in advance and with no deemed reinvestment of monthly payments. The Senior Facility contains certain affirmative and negative covenants. The operational covenant includes a minimum unrestricted cash threshold of $29.9 million (C$40.0 million) for capital expenditures and investments.
The proceeds from the Senior Facility will be used for general corporate purposes, including working capital or such other reasonable business purposes not otherwise prohibited by the Senior Facility. The forgoing summary of the terms and conditions of the Senior Facility is qualified in its entirety by reference to the full text of the Senior Facility, which is attached to this Annual Report on Form 10-K as Exhibit 10.25.
81
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
|
(1) |
The information required by this Item concerning our executive officers and our directors and nominees for director, including information with respect to our audit committee and audit committee financial expert, may be found under the section entitled “Proposal No. 1 Election of Directors,” “Information Regarding the Board of Directors and Corporate Governance,” and “Executive Officers” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
|
(2) |
The information required by this Item concerning our code of ethics may be found under the section entitled “Information Regarding the Board of Directors and Corporate Governance” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
|
(3) |
The information required by this Item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 may be found in the section entitled “Delinquent Section 16(a) Reports” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
Item 11. Executive Compensation.
The information required by this Item may be found under the sections entitled “Director Compensation,” “Executive Compensation” and “Equity Compensation Plan Information” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
(1) |
The information required by this Item with respect to security ownership of certain beneficial owners and management may be found under the section entitled “Security Ownership of Certain Beneficial Owners and Management” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
|
(2) |
The information required by this Item with respect to securities authorized for issuance under our equity compensation plans may be found under the sections entitled “Equity Compensation Plan Information” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
|
(1) |
The information required by this Item concerning related party transactions may be found under the section entitled “Transactions with Related Persons” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
|
(2) |
The information required by this Item concerning director independence may be found under the sections entitled “Information Regarding the Board of Directors and Corporate Governance—Independence of the Board of Directors” and “Information Regarding the Board of Directors and Corporate Governance—Information Regarding Committees of the Board of Directors” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference. |
Item 14. Principal Accounting Fees and Services.
The information required by this Item may be found under the section entitled “Proposal No. 3 - Ratification of Appointment of Independent Registered Public Accounting Firm” appearing in the 2020 Proxy Statement. Such information is incorporated herein by reference.
82
PART IV
Item 15. Exhibits, Financial Statement Schedules.
|
(a) |
The following documents are filed as part of this report: |
|
(1) |
Financial Statements and Report of Independent Registered Public Accounting Firm |
|
(2) |
Financial Statement Schedules |
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
|
(3) |
Exhibits are incorporated herein by reference or are filed with this report as indicated below (numbered in accordance with Item 601 of Regulation S-K). |
|
(b) |
Exhibits |
The exhibits listed below on the Exhibit Index are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
83
|
|
|
|
|
Incorporate by Reference |
|
|
|||||||
|
Exhibit No. |
|
Description of Document |
|
Schedule Form |
|
File Number |
|
Exhibit |
|
Filing Date |
|
File Herewith |
|
|
2.1* |
|
|
8-K |
|
001-38594 |
|
2.1 |
|
1/25/2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2* |
|
|
8-K |
|
001-38594 |
|
2.2 |
|
2/25/2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3* |
|
|
8-K |
|
001-38594 |
|
2.3 |
|
3/4/2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.4* |
|
|
8-K |
|
001-38594 |
|
2.1 |
|
9/10/2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation, as currently in effect |
|
8-K |
|
001-38594 |
|
3.1 |
|
12/17/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
|
S-1 |
|
333-225741 |
|
3.4 |
|
7/9/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
Indenture, dated October 10, 2018, between the Registrant and GLAS Trust Company LLC |
|
8-K |
|
001-38594 |
|
4.1 |
|
10/10/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
Form of 5.00% Convertible Senior Note due 2023 (included in Exhibit 4.1) |
|
8-K |
|
001-38594 |
|
4.2 |
|
10/10/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3 |
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1+ |
|
|
S-1 |
|
333-225741 |
|
10.2 |
|
7/9/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2+ |
|
|
S-1 |
|
333-225741 |
|
10.3 |
|
7/9/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3+ |
|
|
S-1 |
|
333-225741 |
|
10.4 |
|
7/9/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4+ |
|
Privateer Holdings Inc. 2011 Equity Incentive Plan as amended |
|
S-8 |
|
333-235581 |
|
99.1 |
|
12/19/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5+ |
|
|
S-8 |
|
333-235581 |
|
99.2 |
|
12/19/2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6 |
|
Form of Indemnity Agreement by and between the Registrant and its directors and officers |
|
S-1 |
|
333-225741 |
|
10.5 |
|
7/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7+ |
|
Employment Agreement by and between the Registrant and Brendan Kennedy dated May 30, 2018 |
|
S-1 |
|
333-225741 |
|
10.6 |
|
6/20/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8+ |
|
Employment Agreement by and between the Registrant and Mark Castaneda dated May 30, 2018 |
|
S-1 |
|
333-225741 |
|
10.7 |
|
6/20/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9+ |
|
Employment Agreement by and between the Registrant and Edward Wood Pastorius, Jr. dated May 30, 2018 |
|
S-1 |
|
333-225741 |
|
10.8 |
|
6/20/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10 |
|
|
S-1 |
|
333-225741 |
|
10.9 |
|
6/20/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11 |
|
|
S-1 |
|
333-225741 |
|
10.10 |
|
6/20/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12 |
|
|
S-1 |
|
333-225741 |
|
10.11 |
|
6/20/2018 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
84
85
|
|
|
|
|
Incorporate by Reference |
|
|
|||||||
|
Exhibit No. |
|
Description of Document |
|
Schedule Form |
|
File Number |
|
Exhibit |
|
Filing Date |
|
File Herewith |
|
|
31.2 |
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1** |
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
The following financial statements from the Company's Annual Report on Form 10-K for the year ended December 31, 2019, formatted in Inline XBRL: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Net Loss and Comprehensive Loss , (iii) Consolidated Statements of Stockholders' Equity (Deficit), (iv) Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements, tagged as blocks of text and including detailed tags. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ Indicates management contract or compensatory plan.
* Schedules and certain other information have been omitted pursuant to Item 601(b)(2) of Regulations S-K. The registrant will furnish copies of any such schedules to the Securities and Exchange Commission upon request.
** Document has been furnished, is not deemed filed and is not to be incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, irrespective of any general incorporation language contained in any such filing.
† Registrant has omitted portions of the referenced exhibit pursuant to a request for confidential treatment under Rule 406 promulgated under the Securities Act.
Item 16. Form 10-K Summary
None.
86
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
Tilray, Inc. |
|
|
|
|
|
|
|
Date: March 2, 2020 |
|
By: |
/s/ Brendan Kennedy |
|
|
|
|
Brendan Kennedy |
|
|
|
|
President, Chief Executive Officer and Director |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
|
Name |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Brendan Kennedy |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 2, 2020 |
|
Brendan Kennedy |
|
|
|
|
|
|
|
|
|
|
|
/s/ Mark Castaneda |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 2, 2020 |
|
Mark Castaneda |
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael Auerbach |
|
Director |
|
March 2, 2020 |
|
Michael Auerbach |
|
|
|
|
|
|
|
|
|
|
|
/s/ Rebekah Dopp |
|
Director |
|
March 2, 2020 |
|
Rebekah Dopp |
|
|
|
|
|
|
|
|
|
|
|
/s/ Maryscott Greenwood |
|
Director |
|
March 2, 2020 |
|
Maryscott Greenwood |
|
|
|
|
|
|
|
|
|
|
|
/s/ Christine St.Clare |
|
Director |
|
March 2, 2020 |
|
Christine St.Clare |
|
|
|
|
87
Similar companies
See also CannTrust Holdings Inc.See also SNDL Inc.
See also Curaleaf Holdings, Inc.
See also Definium Therapeutics, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Trulieve Cannabis Corp. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
