Viracta Therapeutics, Inc. - Annual Report: 2019 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Year Ended December 31, 2019
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 000-51531
SUNESIS PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
94-3295878 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification Number) |
395 Oyster Point Boulevard, Suite 400
South San Francisco, California 94080
(Address of principal executive offices, including zip code)
(650) 266-3500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class: |
Trading Symbol: |
Name of Each Exchange on Which Registered: |
|
Common Stock, par value $0.0001 per share |
SNSS |
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 (the “Exchange Act”) during the preceding 12 months (or for such period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2.) Yes ☐ No ☒
The aggregate market value of common stock held by non-affiliates of the registrant, based on the closing sales price for such stock on June 30, 2019, as reported by The Nasdaq Stock Market, was approximately $46,416,000. The calculation of the aggregate market value of voting and non-voting stock excludes certain shares of the registrant’s common stock held by current executive officers, directors and stockholders that the registrant has concluded are affiliates of the registrant. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
The total number of shares outstanding of the registrant’s common stock, $0.0001 par value per share, as of March 4, 2020, was approximately 111,393,000.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement, to be filed with the Securities and Exchange Commission pursuant to Regulation 14A in connection with the 2020 Annual Meeting of Stockholders of Sunesis Pharmaceuticals, Inc. (hereinafter referred to as “Proxy Statement”) are incorporated by reference in Part III of this report. Such Proxy Statement will be filed with the Securities and Exchange Commission not later than 120 days after the conclusion of the registrant’s year ended December 31, 2019.
TABLE OF CONTENTS
|
|
|
|
|
Page No. |
|
|
|
|
|
|
|
ITEM 1. |
|
|
3 |
|
|
ITEM 1A. |
|
|
15 |
|
|
ITEM 1B. |
|
|
33 |
|
|
ITEM 2. |
|
|
33 |
|
|
ITEM 3. |
|
|
33 |
|
|
ITEM 4. |
|
|
33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ITEM 5. |
|
|
34 |
|
|
ITEM 6. |
|
|
34 |
|
|
ITEM 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
35 |
|
ITEM 7A. |
|
|
44 |
|
|
ITEM 8. |
|
|
45 |
|
|
ITEM 9. |
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
68 |
|
ITEM 9A. |
|
|
68 |
|
|
ITEM 9B. |
|
|
68 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ITEM 10. |
|
|
69 |
|
|
ITEM 11. |
|
|
69 |
|
|
ITEM 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
69 |
|
ITEM 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
70 |
|
ITEM 14. |
|
|
70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ITEM 15. |
|
|
71 |
|
|
ITEM 16. |
|
|
71 |
|
|
|
|
|
72 |
|
|
|
|
|
76 |
2
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report, including the information we incorporate by reference, contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995, which involve risks, uncertainties and assumptions. All statements, other than statements of historical facts, are “forward-looking statements” for purposes of these provisions, including without limitation any statements relating to our expectations for gaining marketing approval in the United States, including the continued development and commercialization of vecabrutinib (formerly SNS-062), SNS-510, and other product candidates, the timing of our Phase 1b/2 trial of vecabrutinib, presenting clinical data and initiating clinical trials, our future research and development activities, including clinical testing and the costs and timing thereof, the potential of our existing product candidates to lead to the development of commercial products, our ability to receive potential milestone or royalty payments under license and collaboration agreements and the timing of receipt of those payments, including those related to TAK 580 and vosaroxin, sufficiency of our cash resources, our ability to raise additional funding when needed, any statements concerning anticipated regulatory activities or licensing or collaborative arrangements , and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “anticipates,” “believe,” “continue,” “could,” “estimates,” “expects,” “intend,” “look forward,” “may,” “seeks,” “plans,” “potential,” or “will” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under “Risk Factors,” and elsewhere in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. All forward-looking statements included in this report are based on information available to us on the date of this report, and we assume no obligation to update any forward-looking statements contained in this report.
In this report, “Sunesis,” the “Company,” “we,” “us,” and “our” refer to Sunesis Pharmaceuticals, Inc. and its wholly-owned subsidiaries, except where it is made clear that the term refers only to the parent company.
General
Sunesis is a biopharmaceutical company focused on the development of novel targeted inhibitors for the treatment of hematologic and solid cancers. Our primary activities since incorporation have been conducting research and development internally and through corporate collaborators, in-licensing and out-licensing pharmaceutical compounds and technology, conducting clinical trials, and raising capital.
Our lead program is vecabrutinib, a selective non-covalent inhibitor of Bruton’s Tyrosine Kinase (“BTK”) with activity against both wild-type and C481S-mutated BTK, the most common mutation associated with resistance to covalent BTK inhibitors. Ibrutinib was the first BTK inhibitor approved for the treatment of chronic lymphocytic leukemia (“CLL”), mantle cell lymphoma (“MCL”), and other B-cell malignancies. Ibrutinib is the market leader in CLL, marketed by Johnson & Johnson and AbbVie Inc. (“AbbVie”), with approximately $5 billion in net revenues in 2018. The C481 mutation has been seen in patients who developed resistance to ibrutinib and to acalabrutinib, another covalent BTK inhibitor that is approved for treatment of CLL and MCL.
Vecabrutinib is being studied in a Phase 1b/2 clinical trial to assess safety and activity in patients with CLL and other advanced B-cell malignancies after two or more prior therapies, including ibrutinib or another covalent BTK inhibitor where approved for the disease. We completed the safety evaluation period for the 400 mg cohort and thus far vecabrutinib has a favorable safety profile. We have not seen any Grade 3 or higher drug-related adverse events in cohorts above 50mg. The seventh cohort, testing 500 mg twice daily, is now open. We have prepared a Phase 2 portion to further explore clinical activity and safety in CLL patients, including those with and without BTK C481 mutations. We will start the Phase 2 only after seeing a sufficient efficacy signal in the ongoing Phase 1b. Vecabrutinib was developed as a result of a collaboration agreement with Biogen MA Inc. (“Biogen”), and we must pay a royalty on sales of vecabrutinib when and if approved and commercialized.
We are developing SNS-510, a PDK1 inhibitor licensed from Millennium Pharmaceuticals, Inc. (“Takeda Oncology”), a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited. In October 2019, at the 2019 AACR -NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, we presented the results of studies characterizing SNS-510 in multiple preclinical pharmacology models, including evaluation in the Eurofins Oncopanel™, a panel of 320 genomically profiled cancer cell lines from diverse tissue origins, and in vivo studies in FLT3-mutated and wild-type AML xenograft models. These studies indicated that CDKN2A-mutated tumors are particularly sensitive to SNS-510, supporting a potential role in combination with CDK4/6 inhibitors to address resistance and improve activity. SNS-510 also demonstrated potent, pathway-mediated antitumor
3
activity in the AML models. We are conducting an Investigational New Drug (“IND”)-enabling program for SNS-510 and plan to file an IND by the end of 2020.
In December 2019, we consented to Takeda Oncology’s assignment of TAK-580 to DOT Therapeutics-1, Inc. (“DOT-1”), and we entered into a license agreement with DOT-1 (the “DOT-1 License Agreement) to grant DOT-1 a worldwide, exclusive license of TAK-580. Pursuant to this agreement, we received a $2.0 million upfront payment from DOT-1. The agreement also includes up to $57.0 million in potential pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580, when and if approved and commercialized.
In December 2019, we entered into an agreement to license vosaroxin to Denovo Biopharma, LLC (“Denovo”), pursuant to which Sunesis received a $200,000 upfront payment and is eligible to receive up to $57.0 million in potential regulatory and commercial milestones, and double-digit royalties on future sales of vosaroxin, when and if approved and commercialized (the “Denovo License Agreement”).
Our Strategy
We plan to continue to build Sunesis into a leading biopharmaceutical company focused on the development and commercialization of new targeted oncology therapeutics by:
|
|
• |
exploring the safety and efficacy of vecabrutinib as a potential treatment for B-cell malignancies, including for CLL patients who have relapsed following treatment with a covalent BTK inhibitor; |
|
|
• |
completing IND-enabling activities and filing an IND for SNS-510 in 2020; and |
|
|
• |
continuing to expand and refine our oncology-focused pipeline through further licensing or collaboration arrangements. |
Development Pipeline
The following chart summarizes our development pipeline and partnered programs:
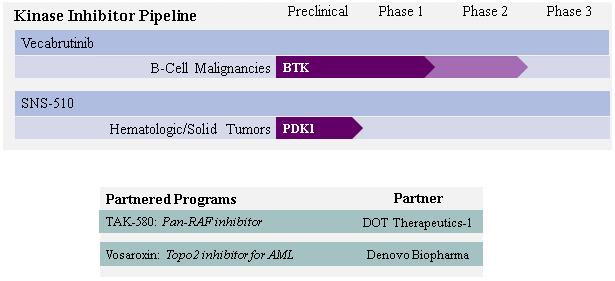
4
Vecabrutinib is a selective, reversible, non-covalent BTK inhibitor. BTK mediates signaling through the B-cell receptor, and is critical for adhesion, migration, proliferation, and survival of normal and malignant B-lineage lymphoid cells. BTK has been well validated as a target for treatment of B-cell malignancies, with the covalent BTK inhibitors Imbruvica® (ibrutinib), Calquence® (acalabrutinib), and Brukinsa™ (zanabrutinib) approved for relapsed/refractory mantle cell lymphoma. Ibrutinib and acalabrutinib are also approved for CLL, and ibrutinib is also approved for Waldenström’s macroglobulinemia, chronic graft versus host disease (“cGVHD"), and marginal zone lymphoma. Covalent BTK inhibitors, including ibrutinib, acalabrutinib, and zanabrutinib, form an irreversible bond with cysteine residue 481 (C481) in the BTK kinase domain. This cysteine may mutate to a serine (“C481S”) or another amino acid and this is associated with disease progression and resistance to further treatment with covalent BTK inhibitors. Resistance to covalent BTK inhibitors is a growing problem, with a seminal 2017 paper in the Journal of Clinical Oncology by Woyach et al finding nearly 30% of Ibrutinib-treated patients had developed resistance by year 5. Additional studies by the European Research Initiative on CLL and the French Innovative Leukemia Organization Group have also identified the BTK C481S mutation in more than half of ibrutinib-relapsed patients. Collectively these studies provide further evidence of resistance to covalent BTK inhibitors as a significant and growing problem. Vecabrutinib has shown potent inhibitory activity in vitro against both wild type and C481S-mutated BTK and may provide a potential solution to resistance to covalent BTK inhibitors.
In addition to vecabrutinib’s non-covalent inhibition of BTK, vecabrutinib inhibits interleukin-2 inducible kinase (“ITK”). Inhibition of ITK may improve anti-tumor T-cell activity, and inhibition of both BTK and ITK contributes to ibrutinib’s activity in cGVHD and also the potential improvement in response when combined with chimeric antigen receptor T (“CAR-T”) cell therapies. Notably, vecabrutinib does not inhibit epidermal growth factor receptor, a kinase target associated with skin and gastrointestinal toxicities. Vecabrutinib’s distinct kinase selectivity profile and favorable pharmacokinetics indicate the potential for vecabrutinib to become a differentiated treatment for B-cell malignancies.
We are studying vecabrutinib in a Phase 1b/2 trial in adults with B-cell malignancies, including relapsed/refractory CLL. The study is now open at 11 leading clinical sites. Preliminary safety, clinical activity, pharmacokinetics, and pharmacodynamics from vecabrutinib’s Phase 1b/2 study were presented at ASH 2019. Vecabrutinib showed sustained exposure over the dosing interval with both exposure and median steady-state minimum blood plasma concentration (Cmin) increasing with dose. Vecabrutinib’s pharmacodynamic effects (reductions in chemokines CCL3 and CCL4) increased with dose in CLL patients and indicate increased impact on BTK signaling as dose is escalated. Three out of the five patients treated at the 300 mg dose level experience stable disease, including one patient with a 40% reduction in tumor size. The most common treatment emergent adverse events (“TEAEs”) included anemia, headache, and night sweats. Headache and nausea were the two most common drug-related TEAEs. The preliminary safety profile of vecabrutinib is acceptable and dose escalation in the study is continuing, with patients now being treated in the 300, 400, and 500 mg cohorts.
SNS-510
SNS-510 is a selective inhibitor of 3-phosphoinositide-dependent kinase 1 (“PDK1”) that was discovered under a research collaboration agreement between Biogen and Sunesis and in-licensed from Takeda Oncology in 2014, as described below. PDK1 is a master kinase that mediates PI3K/AKT(PKB) signaling through its plekstrin homology (PH) domain, and also regulates other pathways independent of phosphoinositide (“PI”) through its PDK1-interacting fragment (“PIF”) pocket. The PI-independent kinases modulated by PDK1 include SGK, PKC, IKK and RSK (S6K) that are critical for growth factor signal transduction. These pathways are involved in cell growth, differentiation, survival and migration and are frequently dysregulated in cancers.
SNS-510 is a potent inhibitor of both active and inactive conformations of PDK1 and binds deep in the adaptive pocket, affecting both PH-domain and PIF-pocket interactions. As a result, SNS-510 blocks both PI-dependent and PI-independent pathways. These activities were demonstrated in vitro and in vivo in preclinical models of hematologic and solid tumor cancers. SNS-510 was recently profiled in Eurofins’ Oncopanel® of 320 genomically profiled cancer cell lines derived from 19 different tissues. SNS-510 showed strong and consistent anti-proliferative activity in the majority of cell lines from diverse tissue origins. Univariate analysis comparing drug response data and genomics data for the OncoPanel cell lines revealed that the presence of mutations or deletions in the Cyclin Dependent Kinase Inhibitor 2A (“CDKN2A”) gene was the genomic feature most significantly associated with sensitivity to SNS-510. CDKN2A aberrations are common in human cancer, and the gene encodes tumor suppressor proteins that regulate TP53 and RB1 pathways. One key gene product is p16/INK4, which regulates cyclin-dependent kinase 4 (“CDK4”). In a 2017 Cancer Research article, Jansen et al. reported that PDK1 is overexpressed in acquired resistance to CDK4/CDK6 inhibitors and that combination of a PDK1 inhibitor with ribociclib, a CDK4/6 inhibitor, can ameliorate this resistance in preclinical models. CDKN2A alterations may prove to be useful biomarkers for sensitivity to SNS-510 supporting investigation of a number of potential indications and combinations.
5
Several PI3K inhibitors are approved in hematologic malignancies and one is approved for PI3KCA-mutated breast cancers; AKT inhibitors are also in late stage development with promising results in combination with other agents in breast cancer. These successes highlight the important role inhibition of the PI3K/AKT signaling axis can play in the treatment of cancer. Inhibition of PDK1 by inhibitors, such as SNS-510, that block both PI-dependent and PI-independent signaling may be more effective anticancer agents than inhibitors that target only PI3K/AKT. No PDK1 inhibitor has reached advanced development or approval to date. SNS-510 is a first-in-class therapeutic with the potential for broad spectrum single agent and combination activity in both solid tumor and hematologic malignancies. We are moving SNS-510 through an IND-enabling program with the goal of filing an IND towards the end of 2020.
TAK-580 (formerly MLN2480)
The Raf kinases (A-Raf, B-Raf and C-Raf) are key regulators of cell proliferation and survival within the MAPK pathway. Pan-RAF inhibitors such as TAK-580 are able to regulate MAPK pathway activation that are driven by RAF-monomer signaling, such as BRAF V600 mutations, and are uniquely positioned to also inhibit RAF dimer signaling, which can drive cancers with RAS mutations, non-V600 BRAF mutations, and RAF fusions.
In February 2018, an investigator-sponsored trial was initiated evaluating TAK-580 in Pediatric Low-Grade Glioma (PLGG), for which we believe the scientific rationale is compelling. PLGG accounts for nearly 30% of pediatric brain cancer, and Fusion-RAF proteins are present in a large proportion of these pediatric tumors. There is a significant unmet need for these children. The trial is ongoing.
TAK-580 had its origins in a collaboration agreement between Sunesis and Biogen. In March 2011, Biogen’s rights to this program were exclusively assigned to Takeda Oncology. In December 2019, we consented to Takeda Oncology’s assignment of TAK-580 to DOT-1, a venture capital-funded biopharmaceutical company. We entered into a concurrent DOT-1 License Agreement to grant DOT-1 a worldwide, exclusive license of TAK-580. Pursuant to the DOT-1 License Agreement, we received a $2.0 million upfront payment from DOT-1. The DOT-1 License Agreement also includes up to $57.0 million in potential pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580, when and if approved and commercialized.
Vosaroxin
Vosaroxin is an anti-cancer quinolone derivative that intercalates DNA and inhibits topoisomerase II, an enzyme critical for cell replication, resulting in replication-dependent, site-selective DNA damage, G2 arrest and apoptosis. In December 2019, Sumitomo assigned its worldwide rights to vosaroxin to Sunesis (the “Sumitomo Assignment”). Also in December 2019, we entered into an agreement to license vosaroxin to Denovo, pursuant to which we received a $200,000 upfront payment and are eligible to receive up to $57.0 million in potential regulatory and commercial milestones, and double-digit royalties on future sales of vosaroxin, when and if approved and commercialized.
License, Collaboration and Royalty Agreements
Vecabrutinib and SNS-510 Licensing and Collaboration Agreements
Overview
In August 2004, we entered into the original collaboration agreement with Biogen (the “Biogen OCA”) to discover, develop and commercialize small molecule inhibitors of the human protein Raf kinase, including family members Raf-1, A-Raf, B-Raf and C-Raf, (collectively “Raf”), and up to five additional targets that play a role in oncology and immunology indications such as BTK and PDK1.
In June 2008, the parties agreed to terminate the research term and related funding. In March 2011, as part of a series of agreements among Sunesis, Biogen and Takeda Oncology, we entered into: (a) an amended and restated collaboration agreement with Biogen (the “Biogen 1st ARCA”); (b) a license agreement with Takeda Oncology (the “Takeda Agreement”); and (c) a termination and transition agreement among Sunesis, Biogen and Takeda Oncology (the “Termination and Transition Agreement”).
The Termination and Transition Agreement provided for the termination of Biogen’s exclusive rights under the Biogen OCA to all discovery programs under such agreement other than for small molecule inhibitors of the human protein BTK and the permitted assignment to Takeda Oncology of all related Biogen collaboration assets and rights to Raf kinase and the human protein PDK1.
Biogen
In December 2013, we entered into a second amended and restated collaboration agreement with Biogen, which amended and restated the Biogen 1st ARCA, to provide us with an exclusive worldwide license to develop, manufacture and commercialize vecabrutinib, a BTK inhibitor synthesized under the Biogen 1st ARCA, solely for oncology indications. During the third quarter of 2017, we made a milestone payment of $2.5 million to Biogen upon the dosing of the first patient in a Phase 1b/2 study to assess the safety and activity of vecabrutinib in patients with advanced B-cell malignancies after two or more prior therapies, including ibrutinib
6
or other covalent BTK inhibitors, and including patients with BTK C481 mutations. We may also be required to make royalty payments on product sales of vecabrutinib.
Takeda Oncology
Under the Takeda Agreement, we granted exclusive licenses to products against two oncology, Raf and PDK1, under substantially the same terms as under the Biogen OCA.
In January 2014, we entered into an amended and restated license agreement with Takeda Oncology (the “Amended Takeda Agreement”), to provide us with an exclusive worldwide license to develop and commercialize preclinical inhibitors of PDK1. In December 2019, we partitioned the Amended Takeda Agreement into two separate agreements: (i) an amended and restated license agreement for PDK (the “PDK Agreement”), and (ii) an amended and restated license agreement for RAF (the “Millennium RAF Agreement”). Pursuant to the PDK Agreement, we may in the future be required to make up to $9.2 million in pre-commercialization milestone payments depending on our development of PDK1 inhibitors and royalty payments depending on related product sales.
TAK-580 License Agreements
In December 2019, we consented to Takeda Oncology’s assignment of the Millennium RAF Agreement to DOT-1, a venture capital-funded biopharmaceutical company. Concurrent with this assignment, we entered into the DOT-1 License Agreement to grant DOT-1 a worldwide, exclusive license of TAK-580. Pursuant to the DOT-1 License Agreement, we received a $2.0 million upfront payment from DOT-1. The agreement includes up to $57.0 million in potential pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580, when and if approved and commercialized.
Vosaroxin License Agreements
To facilitate the outlicensing of vosaroxin to Denovo, in December 2019 we entered the Sumitomo Assignment, providing for Sumitomo to assign to Sunesis certain data, information, know-how, and patents relating to vosaroxin and related compounds. Sunesis made a one-time payment to Sumitomo in the amount of $150,000 in connection with the assignment. In December 2019, we also entered into the Denovo Agreement to license vosaroxin intellectual property to Denovo, pursuant to which Sunesis received a $200,000 upfront payment and is eligible to receive up to $57.0 million in regulatory and commercial milestones and double-digit royalties on future sales of vosaroxin, when and if approved and commercialized.
Manufacturing
We rely on, and we expect to continue to rely on, a limited number of third-party contract manufacturers for the production of clinical and commercial quantities of all of our active pharmaceutical ingredient (“API”), including vecabrutinib and SNS-510 and the finished drug product (“FDP”) incorporating the APIs. Vecabrutinib API and FDP are manufactured under master services agreements. SNS-510 API and FDP are manufactured under research and development agreements.
We currently rely on one contract manufacturer for vecabrutinib API and two for FDP. Three lots of API and FDP have been manufactured at a clinical scale. Scale-up to commercial scale has not been done. The cost to manufacture vecabrutinib at large scale is being determined.
Competition
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching, developing and marketing products designed to address the treatment of cancer, including B-cell malignancies. Many of our competitors have significantly greater financial, manufacturing, marketing and drug development resources than we do. Large pharmaceutical companies in particular have extensive experience in the clinical testing of, obtaining regulatory approvals for, and marketing drugs.
With respect to vecabrutinib, we are aware of a number of companies that are or may be pursuing different product candidates that inhibit C481S-mutated BTK, including Aptose Biosciences Inc., Merck & Co., Inc. through their acquisition of ArQule, Inc., and Eli Lilly and Company through their acquisition of Loxo Oncology, Inc. Moreover, other approved drugs that may compete to treat covalent BTK inhibitor-resistant patients, including patients with C481S-mutated BTK include: AbbVie’s Bcl-2 inhibitor venetoclax, Gilead Sciences, Inc. (“Gilead”)’s idelalisib PI3K inhibitor, TG Therapeutics, Inc.’s umbralisib PI3K inhibitor, Verastem’s duvelisib PI3K inhibitor, and CAR-T cell therapies such as Novartis tisagenlecleucel and Gilead’s axicabtagene ciloleucel.
In addition, numerous companies e continue to develop and market covalent inhibitors of wild-type BTK, including AbbVie, AstraZeneca PLC, BeiGene, Ltd., EMD Merck, Gilead, Principia Biopharma Inc., and others in oncology and non-oncology
7
indications. Other drugs that may compete to treat ibrutinib-refractory patients, including patients with C481S-mutated BTK in monotherapy or in combination, include: AbbVie’s Bcl-2 inhibitor venetoclax, Gilead’s idelalisib PI3K kinase inhibitor, TG Therapeutics, Inc.’s umbralisib PI3K inhibitor, Verastem’s duvelisib PI3K inhibitor, and CAR-T cell therapies such as Novartis tisagenlecleucel and Gilead’s axicabtagene ciloleucel.
Intellectual Property
We believe that patent protection is very important to our business and that our future success depends in part on our ability to obtain patents protecting vecabrutinib, SNS-510, or future drug candidates, if any. Historically, we have patented a wide range of technology, inventions and improvements related to our business. When appropriate, we seek orphan drug status and/or data exclusivity in the United States and their equivalents in other relevant jurisdictions, to the maximum extent that the respective laws will permit at such time. For an approved medicine, such designation may, in the European Union, provide ten years of marketing exclusivity in all member countries, or seven years of market exclusivity in the U.S.
Vecabrutinib Patent Assets
U.S. Patent Nos. 8,785,440 B2 and 9,249,146 B2 cover a genus of compounds encompassing vecabrutinib and methods of use thereof, respectively, with expiry in 2030. Counterpart applications and patents are held in the U.S., Europe, and other countries, with expiry in 2030.
U.S. Patent No. 9,394,277 B2 covers a subgenus of compounds including vecabrutinib, and methods of use thereof. Counterpart applications and patents are held in the U.S., Europe, and other countries, with expiry in 2033.
U.S. Patent App. No. 16/319,506 covers a vecabrutinib succinic acid complex form and methods of use thereof. Counterpart applications are pending in Europe and other countries. The applications if granted would be expected to expire in 2037.
As of December 31, 2019, we own, co-own or have rights to approximately 82 granted U.S. and foreign patents, and approximately 48 pending U.S. and foreign applications, pertaining to vecabrutinib and compositions and uses thereof. As noted above, the expiries of these granted patents and patents that may be granted range from 2030 to 2037.
SNS-510 Patent Assets
U.S. Patent No. 9,546,165 B2 and U.S. Patent No. 10,030,016, cover a genus of compounds including SNS-510, and methods of use thereof, respectively. Counterpart applications and patents are held in the U.S., Europe, and other countries, with expiry in 2030.
U.S. Patent App. No. 15/770,369 covers methods of using SNS-510. Counterpart applications are pending Europe and other countries. The applications if granted would be expected to expire in 2036.
U.S. Patent App. No. 16/185,793 and International Patent App. No. PCT/US2018/060111 cover certain pharmaceutical compositions including SNS-510 and method of use thereof. The applications if granted would be expected to expire in 2038. The International application enters the national stage in May 2020.
As of December 31, 2019, we own, co-own or have rights to approximately 69 granted U.S. and foreign patents, and approximately 33 pending U.S. and foreign applications, pertaining to SNS-510 and compositions and uses thereof. As noted above, the expiries of these granted patents and patents that may be granted range from 2030 to 2038.
General
While it is possible that patent term restoration and/or supplemental patent certificates could be available for some of these or other patents we own or control through licenses after possible approval of commercial product, we cannot guarantee that such additional protection will be obtained, and the expiration dates described here do not include such term restoration.
Our commercial success depends on our ability to operate without infringing patents and proprietary rights of third parties. We cannot determine with certainty whether patents or patent applications of other parties may materially affect our ability to conduct our business. The existence of third-party patent applications and patents could significantly reduce the coverage of patents owned by or licensed to us and limit our ability to obtain meaningful patent protection. If patents containing competitive or conflicting claims are issued to third parties and these claims are ultimately determined to be valid, we may be enjoined from pursuing research, development or commercialization of vecabrutinib, SNS-510, TAK-580, or future drug candidates, if any, or be required to obtain licenses to such patents or to develop or obtain alternative technology.
8
We also rely on trade secrets to protect our technology, especially in situations or jurisdictions in which we believe patent protection may not be appropriate or obtainable. However, trade secrets are difficult to maintain and do not protect technology against independent developments made by third parties. We seek to protect our proprietary information by requiring our employees, consultants, contractors and other advisers to execute nondisclosure and assignment of invention agreements upon commencement of their employment or engagement. Agreements with our employees also prevent them from bringing the proprietary rights of third parties to us. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials. We seek to protect our company name and the names of our products and technologies by obtaining trademark registrations, as well as common law rights in trademarks and service marks, in the United States and in other countries.
Government Regulation
The Food and Drug Administration (“FDA”), state and local regulatory agencies and non-US regulatory authorities impose substantial legal requirements, directives and guidelines upon any clinical investigation of a new drug. These agencies regulate activities including current Good Manufacturing Practice (“cGMP”), quality assurance and control testing, documentation, approval, storage, packaging, labeling, distribution, Good Clinical Practice (“GCP”), safety, efficacy, advertising and promotion of our product candidates and any future drug candidates we may develop, if any. The application of these regulatory frameworks to the development, approval and commercialization of our drug candidates will take several years to accomplish, if at all, and involve the expenditure of substantial resources.
In the United States, the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act, as amended, and implements regulations. The process required by the FDA before any of our drug candidates may be marketed in the U.S. generally involves the following:
|
|
• |
completion of extensive preclinical laboratory tests, in vivo preclinical studies and formulation studies; |
|
|
• |
satisfactory manufacturing at facilities which the product candidate is produced under cGMP regulations; |
|
|
• |
performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product candidate for each proposed indication conducted under GCP; |
|
|
• |
submission to the FDA of an IND application, which must become effective before clinical trials begin; |
|
|
• |
submission of a New Drug Application (“NDA”) to the FDA; |
|
|
• |
satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the product candidate is produced to assess compliance with cGMP regulations; and |
|
|
• |
FDA review and approval of the NDA, including proposed labeling (package insert information) and promotional materials, prior to any commercial marketing, sale or shipment of the drug. |
The testing and approval process require substantial time, effort and financial resources, and we cannot be certain that approvals will be granted on a timely basis, if at all.
Preclinical Testing and INDs
Preclinical tests include laboratory evaluation of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals. Laboratories that comply with the FDA GLP regulations must conduct preclinical safety tests. The results of preclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND application to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin.
Clinical Trials
Clinical trials involve the administration of an investigational drug to healthy volunteers or to patients under the supervision of a qualified principal investigator. Clinical trials are conducted in accordance with the FDA’s Protection of Human Subjects regulations and GCP, under protocols that detail the objectives of the study, the parameters to be used to monitor safety, and the efficacy criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND application.
In addition, each clinical study must be conducted under the auspices of an independent institutional review board (“IRB”), at each institution where the study will be conducted. Each IRB will consider, among other things, ethical factors, the safety of human
9
subjects and the possible liability of the institution. The FDA, an IRB, or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP requirements and regulations for informed consent.
Clinical trials are typically conducted in three sequential phases, which may overlap, sometimes followed by a fourth phase:
|
|
• |
Phase 1 clinical trials are initially conducted in a limited population to test the drug candidate for safety (adverse effects), dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in patients, such as cancer patients. In some cases, particularly in cancer trials, a sponsor may decide to conduct what is referred to as a “Phase 1b” evaluation, which is a safety-focused, multiple ascending dose Phase 1 clinical trial, often conducted in patients. |
|
|
• |
Phase 2 clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the drug candidate for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials. In some cases, a sponsor may decide to conduct what is referred to as a “Phase 2b” evaluation, which is a second, confirmatory Phase 2 clinical trial that could, if positive and accepted by the FDA, serve as a pivotal clinical trial in the approval of a drug candidate. |
|
|
• |
Phase 3 clinical trials are commonly referred to as pivotal trials. When Phase 2 clinical trials demonstrate that a drug candidate has potential activity in a disease or condition and has an acceptable safety profile, Phase 3 clinical trials are undertaken to further evaluate clinical efficacy and to further test for safety in an expanded patient population at multiple, geographically dispersed clinical trial sites. |
|
|
• |
Phase 4 (post-marketing) clinical trials may be required by the FDA in some cases. The FDA may conditionally approve an NDA for a drug candidate on a sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and/or efficacy after NDA approval. Such post-approval trials are typically referred to as Phase 4 clinical trials. |
New Drug Applications
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to a substantial application fee under the Prescription Drug User Fee Act (“PDUFA”), and the sponsor of an approved NDA is also subject to annual program fees, which are typically increased annually.
The FDA conducts a preliminary review of all NDAs within the first 60 days after submission before accepting them for filing to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review of NDAs. Under these goals, the FDA has committed to review most such applications for non-priority products within 10 months of filing, and most applications for priority review products, that is, drugs that the FDA determines represent a significant improvement over existing therapy, within six months of filing. The review process may be extended by the FDA for three additional months to consider certain information or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drugs or products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. In addition, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP and integrity of the clinical data submitted.
The testing and approval process requires substantial time, effort and financial resources, and each may take many years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to develop our product candidates and secure necessary governmental approvals, which could delay or preclude us from marketing our products.
10
After the FDA’s evaluation of the NDA and inspection of the manufacturing facilities, the FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval and refuse to approve the NDA. Even if the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including Risk Evaluation and Mitigation Strategies, or REMs, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Orphan Drug Designation
The United States Orphan Drug Act promotes the development of products that demonstrate promise for the diagnosis and treatment of diseases or conditions that affect fewer than 200,000 people in the United States. Upon receipt of orphan drug designation from the FDA, the sponsor is eligible for tax credits for qualified clinical trial expenses, the ability to apply for annual grant funding, waiver of PDUFA application fee, and upon approval, the potential for seven years of market exclusivity for the orphan-designated product for the orphan-designated indication.
In the European Union, orphan status is available for therapies addressing conditions that affect five or fewer out of 10,000 people and provides for the potential for 10 years of marketing exclusivity in Europe for the orphan-designated product for the orphan-designated indication. The marketing exclusivity period can be reduced to six years if, at the end of the fifth year, available evidence establishes that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Other Regulatory Requirements
Any drugs manufactured or distributed by us, or our current or potential future licensees or collaboration partners, if any, pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping requirements and reporting of adverse experiences associated with the drug. Drug manufacturers and their subcontractors are required to register with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Physicians may prescribe legally available drugs for uses that are not described in the drug’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties, including cancer therapy. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Healthcare Law and Regulation
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws restrict our business activities, including certain marketing practices. These laws include, without limitation, anti-kickback laws, false claims laws, data privacy and security laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers.
The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for either the referral of an individual, or the purchasing, leasing, ordering or
11
arranging for the purchase, lease or order of any healthcare item, good, facility or service reimbursable under Medicare, Medicaid or other federal healthcare programs.
Federal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, prohibit any person or entity from, among other things, knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid.
The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity.
Additionally, the federal Physician Payments Sunshine Act, created under the Affordable Care Act (“ACA”), and its implementing regulations, require certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to annually report to the Centers for Medicare & Medicaid Services (“CMS”), information related to certain payments or other transfers of value provided to physicians, as defined by such law, and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members.
The majority of states also have statutes or regulations similar to the aforementioned federal laws, some of which are broader in scope and apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Additionally, we may be subject to state laws that require pharmaceutical companies to comply with the federal government’s and/or pharmaceutical industry’s voluntary compliance guidelines, state laws that require drug manufacturers to report information related to payments and other transfers of value to healthcare providers or marketing expenditures, as well as state and local laws requiring the registration of pharmaceutical sales representatives. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. These laws may affect our sales, marketing and other promotional activities by imposing administrative and compliance burdens.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to a wide range of sanctions and penalties, potentially significant criminal and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, integrity obligations, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. We are unable to predict whether we would be subject to actions under these laws or the impact of such actions. However, the cost of defending any such claims, as well as any sanctions imposed, could adversely affect our financial performance and disrupt our business operations.
Coverage and Reimbursement
Sales of pharmaceutical products, when and if approved for marketing, depend significantly on the availability of third-party coverage and adequate reimbursement. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services, and significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we obtain regulatory approval. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In addition, significant uncertainty exists as to the coverage and reimbursement status of newly approved healthcare products. We may need to conduct expensive clinical studies to demonstrate the comparative cost-effectiveness of our products. The product candidates that we develop may not be considered cost-effective. It is time consuming and expensive for us to seek reimbursement from third-party payors. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor not to
12
cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition. Coverage and adequate reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Health Reform
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives, such as the ACA. Among the provisions of the ACA of importance to our business are that it: created an annual fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; increased the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; extended a manufacturer’s Medicaid rebate liability; expanded eligibility criteria for Medicaid programs; and created a new Medicare Part D coverage gap discount program. There remain judicial and Congressional challenges to certain aspects of the ACA, as well as efforts by the Trump administration to repeal or replace certain aspects of the ACA. Since January 2017, President Trump signed two Executive Orders and other directives to delay the implementation of certain provisions of the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the ACA such as removing penalties, starting January 1, 2019, for not complying with the ACA’s individual mandate to carry health insurance and eliminating the implementation of certain ACA-mandated fees. In addition, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminates the health insurer tax. On December 14, 2018, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Cuts and Jobs Act of 2017. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. It is unclear how this decision, future decisions, subsequent appeals, and other efforts to repeal and replace the ACA will impact the ACA and our business.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes included the Budget Control Act of 2011, which caused aggregate reductions to Medicare payments to providers of up to 2% per fiscal year effective April 1, 2013 which, following passage of the Bipartisan Budget Act of 2015, as well as other legislative amendments to the statute, will stay in effect through 2029 unless additional Congressional action is taken. Additionally, the American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several types of providers.
There has also been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. For example, at the federal level, the Trump administration’s budget proposal for fiscal year 2020 contained further drug price control measures that could be enacted during the budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. Further, the Trump administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. The Department of Health and Human Services (“HHS”), has solicited feedback on some of these measures and, at the same, has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage plans the option to use step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. While some of these and other measures may require additional authorization to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs.
Foreign Regulation
In addition to regulations in the U.S., we are subject to foreign regulations governing clinical trials and commercial sales and distribution of or our drug candidates. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those
13
countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, permission to conduct clinical research is granted by the Competent Authority of each European Member State (“MS”), and the applicable Ethics Committees (“EC”), through the submission of a Clinical Trial Application. An EC in the European Union serves the same function as an IRB in the United States. The review times vary by MS but may not exceed 60 days. The EC has a maximum of 60 days to give its opinion on the acceptability of the Clinical Trial Application to both the governing MS and the sponsor applicant. If the application is deemed acceptable, the MS informs the applicant (or does not within the 60-day window inform the applicant of non-acceptance) and we may proceed with the clinical trial.
To obtain a marketing authorization of a drug in the European Union, we must submit a marketing authorization application (an “MAA”) under the centralized procedure. The centralized procedure provides for the grant of a single marketing authorization from the European Commission following a favorable opinion by the Committee for Medicinal Products for Human Use (the “CHMP”) of the European Medicines Agency (the “EMA”) that is valid in the European Economic Area (the “EEA”), which includes all European Union member states, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for medicines produced by specified biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products and products with a new active substance indicated for the treatment of specified diseases. Under the centralized procedure the maximum timeframe for the evaluation of an MAA by the EMA is 210 days, excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP.
In the EEA, the EMA’s Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the European Union and for which no satisfactory method of diagnosis, prevention, or treatment has been authorized (or the product would be a significant benefit to those affected). Additionally, designation is granted for products intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the medicinal product. A European Union orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity is granted following medicinal product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
In the EEA, marketing authorization applications for new medicinal products not authorized have to include the results of studies conducted in the pediatric population, in compliance with a pediatric investigation plan (“PIP”), agreed with the EMA’s Pediatric Committee (“PDCO”). The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the drug for which marketing authorization is being sought. The PDCO can grant a deferral of the obligation to implement some or all of the measures of the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults. Further, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data is not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once a marketing authorization is obtained for a pediatric indication in all Member States of the European Union and study results are included in the product information, even when negative, the product is eligible for six months’ supplementary protection certificate extension. For orphan-designated medicinal products, the 10-year period of market exclusivity is extended to 12 years.
In addition to regulations in the United States and the European Union, we will be subject to a variety of other foreign regulations governing clinical trials and commercial distribution of our product candidates. Our ability to sell drugs will also depend on the availability of reimbursement from government and private insurance companies.
Research and Development Expenses
We expect to continue to incur significant development expenses related to the development of vecabrutinib and our other drug candidates.
Environment
We have made, and will continue to make, expenditures for environmental compliance and protection. We do not expect that such expenditures will have a material effect on our capital expenditures or results of operations in the foreseeable future.
14
As of December 31, 2019, our workforce consisted of 24 full-time employees, of which 12 are engaged in research and development and 12 are engaged in general and administrative, medical affairs and commercial planning functions. We have no collective bargaining agreements with our employees, and we have not experienced any work stoppages.
Corporate Background
We were incorporated in Delaware in February 1998. Our offices are headquartered at 395 Oyster Point Boulevard, Suite 400, South San Francisco, California 94080, and our telephone number is (650) 266-3500. Our website address is www.sunesis.com. Information contained in, or accessible through, our website is not incorporated by reference into and does not form a part of this report.
Available Information
Our website is located at www.sunesis.com. The contents of our website are not intended to be incorporated by reference into this Annual Report on Form 10-K or in any other report or document we file with the Securities and Exchange Commission (the “SEC”), and any references to our websites are intended to be inactive textual references only. The following filings are available through our website as soon as reasonably practicable after we file them with the SEC: Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, as well as any amendments to such reports and all other filings pursuant to Section 13(a) or 15(d) of the Securities Act. These filings are also available for download free of charge on our investor relations website.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below and all information contained in this Annual Report on Form 10-K, as each of these risks could adversely affect our business, operating results and financial conditions. If any of the possible adverse events described below actually occurs, we may be unable to conduct our business as currently planned and our financial condition and operating results could be adversely affected. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. In addition, the trading price of our common stock could decline due to the occurrence of any of these risks, and you may lose all or part of your investment. Please see “Special Note Regarding Forward-Looking Statements.”
Risks Related to Our Business
We need to raise substantial additional funding to continue the development of vecabrutinib, SNS-510, and any other future programs.
We will need to raise substantial additional capital to:
|
|
• |
fund additional nonclinical and clinical trials of vecabrutinib prior to any regulatory filing for approval; |
|
|
• |
fund preclinical and clinical development of SNS-510, including any potential milestone payments to Takeda Oncology; |
|
|
• |
expand our development activities; |
|
|
• |
implement additional internal systems and infrastructure; and |
|
|
• |
build or access commercialization and additional manufacturing capabilities and supplies. |
Our future funding requirements and sources will depend on many factors, including but not limited to the:
|
|
• |
rate of progress and cost of our clinical trials; |
|
|
• |
need for additional or expanded clinical trials; |
|
|
• |
timing, economic and other terms of any licensing, collaboration or other similar arrangement into which we may enter; |
|
|
• |
costs and timing of seeking and obtaining European Medicines Agency (the “EMA”), U.S. Food and Drug Administration (the “FDA”) or other regulatory approvals; |
|
|
• |
extent of our other development activities, including our other clinical programs and in-license agreements; |
|
|
• |
costs associated with building or accessing commercialization and additional manufacturing capabilities and supplies; |
15
|
|
• |
costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
|
• |
effect of competing technological and market developments; |
|
|
• |
costs of supporting our arrangements with Denovo, DOT-1, or any potential future licensees or partners. |
Until we can generate a sufficient amount of licensing, collaboration or product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through equity issuances, debt arrangements, one or more possible licenses, collaborations or other similar arrangements with respect to development and/or commercialization rights to vecabrutinib, SNS-510, or our other development programs, or a combination of the above. Any issuance of convertible debt securities, preferred stock or common stock may be at a discount from the then-current trading price of our common stock. If we issue additional common or preferred stock or securities convertible into common or preferred stock, our stockholders will experience additional dilution, which may be significant. Further, we do not know whether additional funding will be available on acceptable terms, or at all. If we are unable to raise substantial additional funding on acceptable terms, or at all, we will be forced to delay or reduce the scope of our vecabrutinib, SNS-510, or other development programs.
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We may not ever achieve or sustain profitability.
We are not profitable and have incurred losses in each year since our inception in 1998. Our net losses for the years ended December 31, 2019, 2018 and 2017 were $23.3 million, $26.6 million and $35.5 million, respectively. As of December 31, 2019, we had an accumulated deficit of $682.8 million. We do not currently have any products that have been approved for marketing, and we expect to incur significant losses for the foreseeable future as we continue to incur substantial development and general and administrative expenses related to our operations. We have prioritized development funding on kinase inhibitors with a focus on vecabrutinib. We have a limited number of products that are still in the early stages of development and will require significant additional investment. Our losses, among other things, have caused and will continue to cause our stockholders’ equity and working capital to decrease.
To date, we have derived substantially all of our revenue from license and collaboration agreements. We currently have two agreements, the DOT-1 License Agreement and the Denovo Agreement, both of which include certain pre-commercialization event-based payments and royalty payments. We cannot predict if our collaborators will continue development or whether we will receive any such payments under these agreements in the foreseeable future, or at all.
We are unable to predict when we will generate revenue from the sale of products, if at all. In the absence of additional sources of capital or partnering opportunity, which may not be available to us on acceptable terms, or at all, the development of vecabrutinib or future product candidates may be reduced in scope, delayed or terminated. If our product candidates or those of our collaborators fail in clinical trials or do not gain regulatory approval, or if our future products do not achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
There is substantial doubt about our ability to continue as a going concern.
We adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40) effective December 31, 2016, which requires us to make certain disclosures if we conclude that there is substantial doubt about our ability to continue as a going concern within one year from the date our financial statements contained in this Annual Report on Form 10-K are available to be issued.
We have incurred significant losses and negative cash flows from operations since our inception, and as of December 31, 2019, had cash and cash equivalents, restricted cash, and marketable securities totaling $34.6 million and an accumulated deficit of $682.8 million. We expect our cash and cash equivalents, and marketable securities of $29.1 million, which excludes restricted cash of $5.5 million, as of December 31, 2019 are not sufficient to support our operations for a period of twelve months from the date our financial statements contained in this Annual Report on Form 10-K are available to be issued. We will require additional financing to fund working capital, repay debt and pay our obligations as they come due. Additional financing might include one or more offerings and one or more of a combination of equity securities, debt arrangements or partnership or licensing collaborations. However, there can be no assurance that we will be successful in acquiring additional funding at levels sufficient to fund our operations or on terms favorable to us. These conditions raise substantial doubt about our ability to continue as a going concern for a period of one year from the date our financial statements contained in this Annual Report on Form 10-K are available to be issued. If we are unsuccessful in our efforts to raise additional financing in the near term, we will be required to significantly reduce or cease operations. The accompanying
16
financial statements have been prepared assuming we will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts of liabilities that may result from uncertainty related to our ability to continue as a going concern.
The development of vecabrutinib, SNS-510, or other product candidates could be halted or significantly delayed for various reasons; our clinical trials for vecabrutinib, SNS-510, or other product candidates may not lead to regulatory approval.
Our product candidates are vulnerable to the risks of failure inherent in the drug development process. Failure can occur at any stage of the development process, and successful preclinical studies and early clinical trials do not ensure that later clinical trials will be successful. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials.
Our product candidates may experience toxicities that lead to a maximum tolerated dose that is not effective, or they may fail to demonstrate efficacy at the doses tested. If this were the case for vecabrutinib, for example, such a result would delay or prevent further development, which would severely and adversely affect our financial results, business and business prospects.
We do not know whether our current or any future clinical trials with vecabrutinib, SNS-510, or any of our product candidates will be completed on schedule, or at all, or whether our ongoing or planned clinical trials will begin or progress on the time schedule we anticipate. The commencement and completion of future clinical trials could be substantially delayed or prevented by several factors, including:
|
|
• |
delays or failures to raise additional funding; |
|
|
• |
delays or failures in obtaining regulatory approval to commence a clinical trial; |
|
|
• |
delays or failures in obtaining approval from independent IRBs or ECs to conduct a clinical trial at prospective sites; or |
|
|
• |
delays or failures in reaching acceptable clinical trial agreement terms or clinical trial protocols with prospective sites. |
|
|
• |
delays or failures in obtaining sufficient clinical materials, including any of our product and any drugs to be tested in combination with our products; |
|
|
• |
failure of third parties such as Contract Research Organizations and medical institutions to perform their contractual duties and obligations; |
|
|
• |
slower than expected rates of patient recruitment and enrollment; |
|
|
• |
failure of patients to complete the clinical trial; |
|
|
• |
delays or failures in reaching the number of events pre-specified in the trial design; |
|
|
• |
the need to expand the clinical trial; |
|
|
• |
unforeseen safety issues; |
|
|
• |
lack of efficacy during clinical trials; |
|
|
• |
inability or unwillingness of patients or clinical investigators to follow our clinical trial protocols; and |
|
|
• |
inability to monitor patients adequately during or after treatment. |
Additionally, our clinical trials may be suspended or terminated at any time by the FDA, other regulatory authorities, or ourselves for reasons such as change in protocol. Any failure to complete or significant delay in completing clinical trials for our product candidates could harm our financial results and the commercial prospects for our product candidates.
We rely on a limited number of third parties to supply us with our Active Pharmaceutical Ingredient (“API”) and Finished Drug Product (“FDP”). If we fail to obtain sufficient quantities of these materials, the development and potential commercialization of vecabrutinib, SNS-510 and future products, if any, could be halted or significantly delayed.
We currently rely on contract manufacturers for all API and FDP. Additional third-party contract manufacturing organizations are relied on to manufacture key starting materials and intermediates required in the manufacture of API. We have limited
17
manufacturing experience, and we have not yet scaled-up to commercial scale. The cost to manufacture at commercial scale may materially exceed the cost of clinical-stage manufacturing.
If our third-party API or FDP manufacturers are unable or unwilling to produce the API or FDP we require, we would need to establish arrangements with one or more alternative suppliers. However, establishing a relationship with an alternative supplier would likely delay our ability to produce API or FDP in a timely manner. Our ability to replace an existing manufacturer would also be challenging and time consuming because the number of potential manufacturers is limited and the FDA, EMA or other corresponding state agencies must approve any replacement manufacturer before it can be approved as a commercial supplier. Such approval would require new testing, stability programs and compliance inspections. It may be difficult or impossible for us to identify and engage a reliable replacement manufacturer on acceptable terms in a timely manner, or at all. We expect to continue to depend on third-party contract manufacturers for all our API and FDP needs for the foreseeable future.
Our products require precise and high quality manufacturing processes. In addition to process impurities, the failure of our contract manufacturers to achieve and maintain high manufacturing standards in compliance with cGMP regulations could result in other manufacturing errors leading to patient injury or death, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery. Although contract manufacturers are subject to ongoing periodic unannounced inspection by the FDA, EMA or other corresponding state agencies to ensure strict compliance with cGMP and other applicable government regulations and corresponding foreign standards, any such performance failures on the part of a contract manufacturer could result in the delay or prevention of filing or approval of marketing applications for our products, cost overruns or other problems that could seriously harm our business. This would deprive us of potential product revenue and result in additional losses.
The stability of API and FDP is also a key risk, as we must demonstrate that products continue to meet product specifications over time. There can be no assurances that future lots will meet stability requirements and if they do not, development and commercialization of our products may be delayed.
The failure to enroll patients for clinical trials may cause delays in developing vecabrutinib or other product candidates.
We may encounter delays if we are unable to enroll enough patients to complete clinical trials of vecabrutinib or other product candidates. Patient enrollment depends on many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites, the number and nature of competing treatments and ongoing clinical trials of competing drugs for the same indication, and the eligibility criteria for the trial. In a Phase 1 dose escalation, slots are assigned to sites to avoid over-enrolling. After allocating a slot to a patient, patients may be unable to commence the study if eligibility criteria are not met or they withdraw consent. Patients participating in our trials may come off study due to progressive disease, adverse events, or they or their physician may choose to discontinue study participation.
The results of preclinical studies and clinical trials may not satisfy the requirements of the FDA, EMA or other regulatory agencies.
Prior to receiving approval to commercialize vecabrutinib, SNS-510, or future product candidates in Europe, the United States or in other territories, we must demonstrate with substantial evidence from well-controlled clinical trials, to the satisfaction of the FDA, EMA and other regulatory authorities, that such product candidates are safe and effective for their intended uses. The results from preclinical studies and clinical trials can be interpreted in different ways. Even if we believe preclinical or clinical data from preclinical studies and clinical trials are promising, such data may not be sufficient to support approval by the FDA, EMA and other regulatory authorities. Results in preclinical studies may not be predictive of results in human clinical trials and early stage human clinical trials may not be predictive of results in later, larger trials.
Our product candidates, the methods used to deliver them or their dosage levels may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following any regulatory approval.
Undesirable side effects caused by our product candidates, their delivery methods or dosage levels could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authority. As a result of safety or toxicity issues that we may experience in our clinical trials, we may not receive approval to market any product candidates, which could prevent us from ever generating revenues or achieving profitability. Results of our trials could reveal an unacceptably high severity and incidence of side effects, or side effects outweighing the benefits of our product candidates. In such an event, our trials could be suspended or terminated, and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product
18
candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims.
Additionally, if any of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result, including that:
|
|
• |
we may be forced to suspend marketing of that product; |
|
|
• |
regulatory authorities may withdraw or change their approvals of that product; |
|
|
• |
regulatory authorities may require additional warnings on the label or limit access of that product to selective specialized centers with additional safety reporting and with requirements that patients be geographically close to these centers for all or part of their treatment; |
|
|
• |
we may be required to conduct post-marketing studies; |
|
|
• |
we may be required to change the way the product is administered; |
|
|
• |
we could be sued and held liable for harm caused to subjects or patients; and |
|
|
• |
our reputation may suffer. |
Any of these events could diminish the usage or otherwise limit the commercial success of our product candidates and prevent us from achieving or maintaining market acceptance of the affected product candidate, if approved by applicable regulatory authorities.
We may not be able to obtain or maintain orphan drug exclusivity for our product candidates.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States.
Generally, if a product with an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the EMA or the FDA from approving another marketing application for the same drug for that time period. The applicable period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not be maintained or effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve a new drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or fail to meet expected deadlines, we may be unable to obtain regulatory approval for, or commercialize, vecabrutinib or other product candidates.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct our planned and existing clinical trials for vecabrutinib and other product candidates. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical trial protocols or for any other reason, we may need to enter into new arrangements with alternative third parties and our clinical trials may be extended, delayed or terminated or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the product candidate being tested in such trials.
19
We may expand our development capabilities in the future, and any difficulties hiring or retaining key personnel or managing this growth could disrupt our operations.
We are highly dependent on the principal members of our development staff. We may expand our research and development capabilities in the future by increasing expenditures in these areas, hiring additional employees and potentially expanding the scope of our current operations. Future growth will require us to continue to implement and improve our managerial, operational and financial systems and continue to retain, recruit and train additional qualified personnel, which may impose a strain on our administrative and operational infrastructure. The competition for qualified personnel in the biopharmaceutical field is intense. We are highly dependent on our continued ability to retain, attract and motivate highly qualified management and specialized personnel required for clinical development. Due to our limited resources, we may not be able to effectively manage any expansion of our operations or recruit and train additional qualified personnel. If we are unable to retain key personnel or manage our growth effectively, we may not be able to implement our business plan.
If we are sued for infringing intellectual property rights of third parties, litigation will be costly and time consuming and could prevent us from developing or commercializing vecabrutinib, SNS-510, or other product candidates.
Our commercial success depends on not infringing the patents and other proprietary rights of third parties and not breaching any collaboration or other agreements we have entered into with regard to our technologies and product candidates. If a third party asserts that we, our licensors, collaboration partners, or any employees thereof have misappropriated their intellectual property, or otherwise claim that we, our licensors, or collaboration partners are using technology claimed in issued and unexpired patents, or other proprietary rights, owned or controlled by the third party, even if the technology is regarded as our own intellectual property, we may need to obtain a license, enter into litigation to challenge the validity or enforceability of the patents or other rights or incur the risk of litigation in the event that a third party asserts that we infringe its patents or have misappropriated other rights.
If a third party asserts that we infringe its patents or other proprietary rights, we could face a number of challenges that could seriously harm our competitive position, including:
|
|
• |
infringement and other intellectual property claims, which would be costly and time consuming to litigate, whether or not the claims have merit, and which could delay the regulatory approval process and divert management’s attention from our business; |
|
|
• |
substantial damages for past infringement, which we may have to pay if a court determines that vecabrutinib, SNS-510, or any future product candidates infringe a third party’s patent or other proprietary rights; |
|
|
• |
a court order prohibiting us from selling or licensing vecabrutinib, SNS-510, or any future product candidates unless a third-party licenses relevant patent or other proprietary rights to us, which it is not required to do; and |
|
|
• |
if a license is available from a third-party, we may have to pay substantial royalties or grant cross-licenses to our patents or other proprietary rights. |
If our competitors develop and market products that are more effective, safer or more popular than vecabrutinib, SNS-510, or other product candidates, or obtain marketing approval sooner than ours, our commercial opportunities will be negatively impacted.
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching, developing and marketing products designed to address the treatment of cancer, including B-cell malignancies. Many of our competitors, such as Eli Lilly and Company and Merck & Co., Inc., have significantly greater financial, manufacturing, marketing and drug development resources than we do. Large pharmaceutical companies in particular have extensive experience in the clinical testing of, obtaining regulatory approvals for, and marketing drugs.
We expect competition during the development and commercialization of all of our products in all of their potential future indications. Competition is likely to increase as additional products are developed and approved in various patient populations. If our competitors market products that are more effective, safer, and/or less expensive than our future products, if any, or that reach the market sooner we may not achieve commercial success or substantial market penetration. In addition, the biopharmaceutical industry is characterized by rapid change. Products developed by our competitors may render any of our future product candidates obsolete.
20
Our proprietary rights may not adequately protect vecabrutinib, SNS-510, or future product candidates, if any.
We use patents, trade secrets, trademarks, service marks, and marketing exclusivity administered by regulatory authorities to protect our products from generic copies of our products. Our ability to build and maintain our proprietary position for any future drug candidates will depend on our success in obtaining effective patent claims and enforcing granted claims. The patent positions of biopharmaceutical companies like ours are generally uncertain and involve complex legal and factual questions for which some important legal principles remain unresolved. No consistent policy regarding the breadth of patent claims has emerged to date in the United States. The patent situation outside the United States is even more uncertain. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Even if patents are issued, they may not be sufficient to protect vecabrutinib, SNS-510, or other product candidates. The patents we own or license and those that may be issued in the future may be opposed, challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages.
We apply for patents covering both our technologies and product candidates, as we deem appropriate. However, we may fail to apply for patents on important technologies or product candidates in a timely fashion, throughout the world, or at all. Our existing patents and any future patents we obtain may not be sufficiently broad, valid, enforceable, or extend globally in order to prevent others from practicing our technologies or from developing competing products and technologies. Further, obtaining and maintaining patent protection relies on compliance with various procedural requirements imposed by governmental patent agencies, including, for example, mandatory document submissions and fee payments. Failure to comply with these requirements may reduce or eliminate opportunities for, or rights to, patent protection. In addition, we generally do not exclusively control the patent prosecution of subject matter that we license to or from others. Accordingly, in such cases we are unable to exercise the same degree of control over this intellectual property as we would over our own. Similarly, we do not always exclusively control patent prosecution due to contractual and other legal obligations to our licensors and collaborations partners. Moreover, the patent positions of biopharmaceutical companies are highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. As a result, the scope, validity and enforceability of patents in addition to the related cost, can vary from country to country, and can change depending on changes in national and international law, and as such, cannot be predicted with certainty. In addition, we do not know whether:
|
|
• |
we, our licensors or our collaboration partners were the first to make the inventions covered by each of our issued patents and pending patent applications; |
|
|
• |
we, our licensors or our collaboration partners were the first to file patent applications for these inventions; |
|
|
• |
others will independently develop similar or alternative technologies or duplicate any of our technologies; |
|
|
• |
any of our, our licensors’ or our collaboration partners’ pending patent applications will result in issued patents; |
|
|
• |
any of our, our licensors’ or our collaboration partners’ patents will be valid or enforceable; |
|
|
• |
because of differences in patent laws of countries, any patent granted in one country or region will be granted in another, or, if so, have the same or a different scope; |
|
|
• |
any patents issued to us, our licensors or our collaboration partners will provide us with any competitive advantages, or will be challenged by third parties; |
|
|
• |
we will develop additional proprietary technologies that are patentable; |
|
|
• |
we, our licensors, or our collaboration partners will be subject to claims challenging the inventorship, ownership, or rights to claim priority with regard to our patents and other intellectual property; or |
|
|
• |
any patents or other proprietary rights of third parties will have an adverse effect on our business. |
We may need to commence or defend administrative proceedings or litigation to enforce or to determine the scope and validity of any patents issued to us or to determine the scope and validity of third-party proprietary rights. Litigation would result in substantial costs, even if the eventual outcome is favorable to us. An adverse outcome in a proceeding or litigation affecting proprietary rights we own or have licensed could present significant risk of competition for drug candidates that we market or seek to develop. Any adverse outcome in a proceeding or litigation affecting third party proprietary rights could subject us to significant liabilities to third parties and could require us to seek licenses of the disputed rights from third parties or to cease using the technology if such licenses are unavailable.
There can be no assurance that the trademarks or service marks we use or register will protect our company name or any products or technologies that we develop and commercialize, that our trademarks, service marks, or trademark registrations will be enforceable against third parties, or that our trademarks and service marks will not interfere with or infringe trademark rights of third parties. We may need to commence litigation to enforce our trademarks and service marks or to determine the scope and validity of
21
our or a third party’s trademark rights. Litigation would result in substantial costs, even if the eventual outcome is favorable to us. An adverse outcome in litigation could subject us to significant liabilities to third parties and require us to seek licenses of the disputed rights from third parties or to cease using the trademarks or service marks if such licenses are unavailable.
We also rely on trade secrets to protect some of our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain and enforce. While we use reasonable efforts to protect our trade secrets, our or our collaboration partners’ employees, consultants, contractors or scientific and other advisors, or those of our licensors or collaborators, may unintentionally or willfully disclose our proprietary information to competitors. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. In addition, foreign courts are sometimes less willing than U.S. courts to protect trade secrets. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secret protection against them and our business could be harmed.
There can be no assurance that the confidentiality and other agreements we put in place with employees, consultants, and partners will provide meaningful protection, that these agreements will not be breached, that we will have an adequate remedy for any such breach, or that our trade secrets will not otherwise become known or independently developed by a third party.
We do not know whether the patent term for any drug candidate or product will offer protection for an adequate or profitable amount of time. We do not know whether patent term extensions and data exclusivity periods will be available in the future for any or all of the patent rights we own or have licensed. While it is possible that patent term restoration and/or supplemental patent certificates would be available for some of the patents we own or control through licenses, we cannot guarantee that such additional protection will be obtained, and the expiration dates described here do not include such term restoration. However, patent expiration dates described here for U.S. patents may reflect patent term adjustments by the United States Patent and Trademark Office or terminal disclaimers over related patents or patent applications. Our obligation to pay royalties to licensors may extend beyond the patent expiration, which would further erode the profitability of our products.
Intellectual property rights may not address all potential threats to our competitive position for at least the reasons described above and below.
We may not realize the potential benefits of our licensing arrangements for products such as vosaroxin and TAK-580 and may not receive any future milestones or royalty payments.
There can be no assurance that products we out-license, such as vosaroxin to Denovo and TAK-580 to DOT-1, will be successfully developed and commercialized. The product(s) may fail in development, or our partner(s) may elect to discontinue development and/or terminate their agreement(s) with us. In this case, we may also incur some costs to wind down our activities related to the product in question. Completing development of the product could require significant resources. If we cannot find another partner and do not undertake development on our own, there will be no possibility of any future upside from the product.
We may fail to make timely milestone or royalty payments under our agreements, triggering remedies that would be adverse to us.
Under our license agreements we have certain milestone obligations, such as the remaining development milestones payable to Takeda Oncology for our development of PDK1, and royalty obligations, such as the royalty payable to Biogen for vecabrutinib. As another example, we are required to pay RPI Finance Trust (“RPI”), an entity related to Royalty Pharma, a specified percentage of any consideration we receive for vosaroxin. If we do not make timely payments, our partners may seek remedies.
Any future workforce and expense reductions may have an adverse impact on our internal programs, our ability to hire and retain key personnel and may be distracting to management.
We have previously implemented workforce reductions. Depending on our need for additional funding and expense control, we may be required to implement further workforce and expense reductions in the future. Further workforce and expense reductions could result in reduced progress on our internal programs. In addition, employees, whether or not directly affected by a reduction, may seek future employment with our business partners or competitors. Although our employees are required to sign a confidentiality agreement at the time of hire, the confidentiality of certain proprietary information and knowledge may not be maintained in the course of any such future employment. Further, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled personnel. We may have difficulty retaining and attracting such personnel as a result of a perceived risk of future workforce and expense reductions. In addition, the implementation of expense reduction programs may result in the diversion of efforts of our executive management team and other key employees, which could adversely affect our business.
22
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers.
Many of our employees were previously employed at biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that we or our employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
A loss of key personnel or the work product of current or former personnel could hamper or prevent our ability to commercialize vecabrutinib, SNS-510, and other product candidates, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We may lose key employees or have difficulty hiring employees to fill key roles.
A loss of key personnel or difficulty in hiring employees to fill key roles could slow or prevent our ability to develop and commercialize our products. For example, we have been operating with an interim Chief Executive Officer since 2018. If we have difficulty hiring a Chief Executive Officer, it may adversely impact our future prospects.
We depend on various consultants and advisors for the success and continuation of our development efforts.
We work extensively with various consultants and advisors, who provide advice and/or services in various business and development functions, including clinical development, operations and strategy, clinical and nonclinical pharmacology, regulatory matters, biostatistics, legal and finance. The potential success of our drug development programs depends, in part, on continued collaborations with certain of these consultants and advisors. Our consultants and advisors are not our employees and may have commitments and obligations to other entities that may limit their availability to us. We do not know if we will be able to maintain such relationships or that such consultants and advisors will not enter into other arrangements with competitors, any of which could have a detrimental impact on our development objectives and our business.
If conflicts of interest, or a failure or dispute of reporting or diligence efforts arise between our current or future licensees or collaboration partners, if any, and us, any of them may act in their self-interest, which may be adverse to our interests.
If a conflict of interest arises between us and one or more of our current or potential future licensees or collaboration partners, if any, they may act in their own self-interest or otherwise in a way that is not in the interest of our company or our stockholders. Biogen, Takeda Oncology, Denovo, DOT-1, or potential future licensees or collaboration partners, if any, are conducting or may conduct product development efforts within the disease area that is the subject of a license or collaboration with our company. In current or potential future licenses or collaborations, if any, we have agreed or may agree not to conduct, independently or with any third party, any research that is competitive with the research conducted under our licenses or collaborations. Our licensees or collaboration partners, however, may develop, either alone or with others, products in related fields that are competitive with the product candidates that are the subject of these licenses or collaborations. Competing products, either developed by our licensees or collaboration partners or to which our licensees or collaboration partners have rights, may result in their withdrawal of support for a product candidate covered by the license or collaboration agreement.
If one or more of our current or potential future licensees or collaboration partners, if any, were to breach or terminate their license or collaboration agreements with us or otherwise fail to perform their obligations thereunder in a timely manner, the preclinical or clinical development or commercialization of the affected product candidates could be delayed or terminated. We do not know whether our licensees or collaboration partners will pursue alternative technologies or develop alternative product candidates, either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases targeted by licenses or collaboration agreements with our company.
We and our current collaboration partners have certain reporting and diligence obligations to each other, and failure to report, or disagreement over the impact of information reported, or a lack of diligent efforts, or dispute of the impact of the efforts, may be adverse to our interests, the development of the product candidates and could lead to an ultimate withdrawal or dispute of the rights to a product candidate covered by the license or collaboration agreement.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure may create uncertainty regarding compliance matters. New or changed laws, regulations and standards are subject to varying interpretations in many cases. As a result, their application in practice may evolve over time. We are committed to maintaining high standards of corporate
23
governance and public disclosure. Complying with evolving interpretations of new or changed legal requirements may cause us to incur higher costs as we revise current practices, policies and procedures, and may divert management time and attention from potential revenue-generating activities to compliance matters. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, our reputation may also be harmed. Further, our board members, chief executive officer and chief financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified board members and executive officers, which could harm our business. Our Directors and Officers insurance provides certain coverage to our board members and executive officers, but the cost of coverage may be prohibitively expensive or not provide enough coverage.
Raising funds through lending arrangements or revenue participation agreements may restrict our operations or produce other adverse results.
In April 2019, we used the proceeds of the term loan agreement with Silicon Valley Bank (“SVB” and such agreement with SVB, the “SVB Loan Agreement”) plus cash on hand to repay our remaining obligations in the amount of $5.9 million under our existing loan agreement with Western Alliance Bank and Solar Capital Ltd and Western Alliance, as Collateral Agent, as amended. Our obligations under the SVB Loan Agreement are secured by a first priority security interest in cash held at an account with SVB (the “Collateral Account”). We are obligated to maintain sufficient cash in the Collateral Account at all times in an amount greater than the outstanding balance of the borrowings.
The SVB Loan Agreement contains customary affirmative and negative covenants which, among other things, limit the Company’s ability to (i) incur additional indebtedness, (ii) pay dividends or make certain distributions, (iii) dispose of its assets, grant liens or encumber its assets or (iv) fundamentally alter the nature of its business. These covenants are subject to a number of exceptions and qualifications. The SVB Loan Agreement also contains customary events of default, including among other things, our failure to make any principal or interest payments when due, the occurrence of certain bankruptcy or insolvency events or its breach of the covenants under the SVB Loan Agreement. Upon the occurrence of an event of default, SVB may, among other things, accelerate our obligations under the SVB Loan Agreement.
We are exposed to risks related to foreign currency exchange rates.
Some of our costs and expenses are denominated in foreign currencies. When the U.S. dollar weakens against the Euro or British pound, the U.S. dollar value of the foreign currency denominated expense increases, and when the U.S. dollar strengthens against the Euro or British pound, the U.S. dollar value of the foreign currency denominated expense decreases. Consequently, changes in exchange rates, and in particular a weakening of the U.S. dollar, may adversely affect our results of operations. We have and may continue to purchase certain European currencies or highly-rated investments denominated in such currencies to manage the risk of future movements in foreign exchange rates that would affect such payables, in accordance with our investment policy. However, there is no guarantee that the related gains and losses will substantially offset each other, and we may be subject to significant exchange gains or losses as currencies fluctuate from quarter to quarter.
Our facilities are located near known earthquake fault zones, and the occurrence of an earthquake or other catastrophic disaster, or interruption by man-made problems such as network security breaches, viruses or terrorism, could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facilities are located in the San Francisco Bay Area near known earthquake fault zones and are vulnerable to significant damage from earthquakes. We are also vulnerable to damage from other types of disasters, including fires, floods, power loss, communications failures and other catastrophic events, such as the ongoing Coronavirus epidemic. Despite the implementation of network security measures, our networks also may be vulnerable to computer viruses, break-ins and similar disruptions. We rely on information technology systems to operate our business and to communicate among our workforce and with third parties. If any disruption were to occur, whether caused by a natural disaster or by manmade problems, our ability to operate our business at our facilities may be seriously or completely impaired and our data could be lost or destroyed.
24
Our systems are potentially vulnerable to data security breaches, whether by employees or others, that may expose sensitive data to unauthorized persons. If we are unable to prevent such data security breaches or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial loss and other regulatory penalties because of lost or misappropriated information. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. Moreover, the prevalent use of mobile devices that access confidential information increases the risk of data security breaches, which could lead to the loss of confidential information, trade secrets or other intellectual property. While we have implemented security measures to protect our data security and information technology systems, such measures may not prevent such events. Such disruptions and breaches of security could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Industry
The regulatory approval process is expensive, time consuming and uncertain and may prevent us from obtaining approval for the commercialization of our product candidates.
The research, testing, manufacturing, selling and marketing of product candidates are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, and regulations differ from country to country. Neither we nor our present or potential future collaboration or licensing partners, if any, are permitted to market our product candidates in the United States or Europe until we receive approval of an MAA or NDA for these respective territories, or in any other country without the equivalent marketing approval from such country. We have not received marketing approval for vecabrutinib in any jurisdiction. In addition, failure to comply with FDA, EMA, and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including warning letters, civil and criminal penalties, injunctions, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending MAAs, NDAs, supplements to approved MAAs, NDAs or their equivalents in other territories.
Regulatory approval of an MAA or NDA or their equivalent in other territories is not guaranteed, and the approval process is expensive, uncertain and may take several years. Furthermore, the development process for oncology products may take longer than in other therapeutic areas. Regulatory authorities have substantial discretion in the drug approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon clinical trials or to repeat or perform additional preclinical studies and clinical trials. The number of preclinical studies and clinical trials that will be required for marketing approval varies depending on the drug candidate, the disease or condition that the drug candidate is designed to address, and the regulations applicable to any particular drug candidate.
The FDA, EMA or other foreign regulatory authority can delay, limit or deny approval of a drug candidate for many reasons, including:
|
|
• |
the drug candidate may not be deemed safe or effective; |
|
|
• |
regulatory officials may not find the data from preclinical studies and clinical trials sufficient; |
|
|
• |
the FDA, EMA or other foreign regulatory authority might not approve our or our third-party manufacturers’ processes or facilities; or |
|
|
• |
the FDA, EMA or other foreign regulatory authority may change its approval policies or adopt new regulations. |
We may be subject to costly claims related to our clinical trials and may not be able to obtain adequate insurance.
Because we conduct clinical trials in humans, we face the risk that the use of vecabrutinib, SNS-510, or other product candidates, if any, will result in adverse side effects. We cannot predict the possible harms or side effects that may result from our clinical trials. Although we have clinical trial liability insurance, our insurance may be insufficient to cover any such events. We do not know whether we will be able to continue to obtain clinical trial coverage on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limit of, our insurance coverage. There is also a risk that third parties that we have agreed to indemnify could incur liability. Any litigation arising from our clinical trials, even if we were ultimately successful, would consume substantial amounts of our financial and managerial resources and may create adverse publicity.
25
Even if we receive regulatory approval to sell vecabrutinib, SNS-510, or other product candidates, the market may not be receptive.
Even if one of our product candidates obtains regulatory approval, it may not gain market acceptance among physicians, patients, healthcare payors and/or the medical community. We believe that the degree of market acceptance will depend on a number of factors, including:
|
|
• |
the timing of market introduction of competitive products; |
|
|
• |
the efficacy of our product; |
|
|
• |
the prevalence and severity of any side effects; |
|
|
• |
the potential advantages or disadvantages over alternative treatments; |
|
|
• |
the strength of marketing and distribution support; |
|
|
• |
the price of the product, both in absolute terms and relative to alternative treatments; and |
|
|
• |
the availability of reimbursement from health maintenance organizations and other third-party payors. |
If vecabrutinib, SNS-510, or other product candidates fail to achieve market acceptance, due to unacceptable side effects or any other reasons, we may not be able to generate significant revenue or to achieve or sustain profitability.
Even if we receive regulatory approval for vecabrutinib, SNS-510, or any other future product candidate, we will be subject to ongoing FDA, EMA and other regulatory obligations and continued regulatory review, which may result in significant additional expense and limit our ability to commercialize vecabrutinib, SNS-510, or any other future product candidate.
Any regulatory approvals that we or our potential future collaboration partners receive for vecabrutinib, SNS-510, or our future product candidates, if any, may also be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing trials. In addition, even if approved, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and recordkeeping for any product will be subject to extensive and ongoing regulatory requirements. The subsequent discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the product, and could include withdrawal of the product from the market.
The FDA and other agencies, including the Department of Justice (“DOJ”), closely regulate and monitor the post-approval marketing and promotion of products to ensure that they are marketed and distributed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA and DOJ impose stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing. Violations of the Federal Food, Drug, and Cosmetic Act and other statutes, including the False Claims Act (the “FCA”), relating to the promotion and advertising of prescription drugs may lead to investigations and enforcement actions alleging violations of federal and state health care fraud and abuse laws and state consumer protection laws.
Regulatory policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States, Europe or other territories. If we are not able to maintain regulatory compliance, we might not be permitted to market our future products and we may not achieve or sustain profitability. Other penalties for failing to comply with regulatory requirements include restrictions on such products, manufacturers or manufacturing processes; restrictions on the labeling or marketing of a product; restrictions on distribution or use of a product; requirements to conduct post-marketing studies or clinical trials; warning letters or untitled letters; withdrawal of the products from the market; refusal to approve pending applications or supplements to approved applications that we submit; recall of products; damage to relationships with any potential collaborators; unfavorable press coverage and damage to our reputation; fines, restitution or disgorgement of profits or revenues; suspension or withdrawal of marketing approvals; refusal to permit the import or export of our products; product seizure; injunctions or the imposition of civil or criminal penalties; and litigation involving patients using our products. Additionally, failure to comply with the European Union’s requirements regarding the protection of personal information also can lead to significant penalties and sanctions.
The coverage and reimbursement status of newly approved drugs is uncertain and may be impacted by current and future legislation, and failure to obtain adequate coverage and reimbursement could limit our ability to market our product candidates and decrease our ability to generate revenue.
There is significant uncertainty related to the third-party coverage and reimbursement of newly approved drugs both nationally and internationally. The commercial success of our future products, if any, in both domestic and international markets depends on
26
whether third-party coverage and reimbursement is available for the ordering of our future products by the medical profession for use by their patients. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to manage healthcare costs by limiting both coverage and the level of reimbursement of new drugs and, as a result, they may not cover or provide adequate payment for our future products. These payors may not view our future products as cost-effective, and reimbursement may not be available to consumers or may not be sufficient to allow our future products to be marketed on a competitive basis.
Likewise, in the United States and some foreign jurisdictions, there have been a number of legislative or regulatory efforts to control or reduce healthcare costs or reform government healthcare programs that could result in lower prices or rejection of our future products. Such efforts have resulted in several recent United States congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products.
27
For example, at the federal level, the Trump administration’s budget proposal for fiscal year 2020 contained further drug price control measures that could be enacted during the budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. Further, the Trump administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. The Department of Health and Human Services (“HHS”), has solicited feedback on some of these measures and, at the same, has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage plans the option to use step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. While some of these and other measures may require additional authorization to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. Changes in coverage and reimbursement policies or healthcare cost containment initiatives that may limit or restrict reimbursement for our future products may reduce any future product revenue.
Additionally, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”), was enacted, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. In the years since its enactment, there have been, and continue to be, significant developments in, and continued legislative activity around, attempts to repeal or repeal and replace the ACA. While Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the ACA such as removing penalties, starting January 1, 2019, for not complying with the ACA’s individual mandate to carry health insurance and delaying the implementation of certain ACA-mandated fees. In addition, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminates the health insurer tax. On December 14, 2018, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the TCJA. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. It is unclear how this decision, future decisions, subsequent appeals, and other efforts to repeal and replace the ACA will impact the ACA and our business and operations.
The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
Our relationships with healthcare providers, clinical investigators, and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which, in the event of a violation, could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, clinical investigators, and third-party payors will play a primary role in the recommendation and prescription of any drug candidates for which we obtain marketing approval. Our current and future arrangements with healthcare providers, clinical investigators and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. Restrictions under applicable state, federal and foreign healthcare laws and regulations include the following:
|
|
• |
The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for either the referral of an individual, or the purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item, good, facility or service reimbursable under Medicare, Medicaid or other federal healthcare programs; |
|
|
• |
Federal false claims laws, including the civil FCA, and civil monetary penalties laws, prohibit any person or entity from, among other things, knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid; |
|
|
• |
HIPAA prohibits, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services; |
28
|
|
things, makes HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity; created four new tiers of civil monetary penalties; amended HIPAA to make civil and criminal penalties directly applicable to business associates; and gave state attorneys general new authority to file civil actions to enforce the federal HIPAA laws; |
|
|
• |
the federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to annually report to CMS information related to certain payments or other transfers of value provided to physicians, as defined by such law, and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members; and |
|
|
• |
analogous local, state and foreign laws and regulations, such as state anti-kickback and false claims laws, transparency statutes, and privacy and security laws. Such laws may be broader than the federal law, including that they may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by third party payors, including private insurers. There also are an increasing number of state laws that require manufacturers to file reports with states regarding drug pricing and marketing information, tracking and reporting of gifts, compensation, other remuneration and items of value provided to health care professionals and health care entities, or marketing expenditures; require pharmaceutical companies to, among other things, establish and implement commercial compliance programs or codes of conducts; and/or require a pharmaceutical company’s sales representatives to be registered or licensed by the state or local governmental entity. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to a wide range of sanctions and penalties, including potentially significant criminal, and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, integrity obligations, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. We are unable to predict whether we would be subject to actions under these laws or the impact of such actions. However, the cost of defending any such claims, as well as any sanctions imposed, could adversely affect our financial performance and disrupt our business operations.
Foreign governments often impose strict price controls, which may adversely affect our future profitability.
If we or a potential future collaboration partner obtain approval in one or more foreign jurisdictions, we or the potential future collaboration partner will be subject to rules and regulations in those jurisdictions. In some foreign countries, particularly in the European Union, prescription drug pricing is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug candidate. To obtain reimbursement or pricing approval in some countries, we or a potential future collaboration partner may be required to conduct a clinical trial that compares the cost-effectiveness of our products to other available therapies. If reimbursement is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
We may incur significant costs complying with environmental laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We, through third-party contractors, use hazardous chemicals and radioactive and biological materials in our business and are subject to a variety of federal, state, regional and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials. Although we believe our safety procedures for handling and disposing of these materials and waste products comply with these laws and regulations, we cannot eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of hazardous materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could significantly exceed our insurance coverage, which is limited for pollution cleanup and contamination.
Our ability to use net operating loss carryforwards to offset future taxable income, and our ability to use tax credit carryforwards, may be subject to certain limitations.
29
Our ability to use our federal and state net operating losses (“NOLs”), to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon our generation of future taxable income, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our NOLs.
As of December 31, 2019, we reported U.S. federal and state NOLs of approximately $463.4 million and $310.7 million, respectively. Our federal NOLs generated prior to 2018 will continue to be governed by the NOL tax rules as they existed prior to the adoption of the 2017 Tax Cut and Jobs Act (the “Tax Act”), which means that generally they will expire 20 years after they were generated if not used prior thereto. $423.1 million of our $463.4 million federal NOLs are subject to the 20 years expirations and a portion will continue to expire each year until 2037. Many states have similar laws, and our state NOLs will begin to expire in 2028. Accordingly, these federal and state NOLs could expire unused and be unavailable to offset future income tax liabilities. Under the Tax Act, federal NOLs incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal NOL’s is limited to 80% of current year taxable income. It is uncertain if and to what extent various states will conform to the Tax Act.
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, our ability to utilize these NOLs and other tax attributes, such as federal tax credits, in any taxable year may be limited if we have experienced an “ownership change.” Generally, a Section 382 ownership change occurs if one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a three-year testing period. Similar rules may apply under state tax laws. Any such material limitation or expiration of our NOLs may harm our future operating results by effectively increasing our future tax obligations.
Risks Related to Our Common Stock
The price of our common stock may continue to be volatile, and the value of an investment in our common stock may decline.
In 2019, our common stock traded as low as $0.20 and as high as $1.77. Factors that could cause continued volatility in the market price of our common stock include, but are not limited to:
|
|
• |
all the other risks mentioned herein, including but not limited to our ability to raise additional capital to fund our operations and complete our clinical development plans, compliance with government regulations, the safety and efficacy of our products, and our ability to protect our intellectual property; |
|
|
• |
announcements relating to restructuring and other operational changes; |
|
|
• |
market conditions in the pharmaceutical, biopharmaceutical and biotechnology sectors; |
|
|
• |
changes in the structure of healthcare payment systems; |
|
|
• |
issuance of new or changed securities analysts’ reports or recommendations; |
|
|
• |
announcements relating to our arrangements with Biogen, Takeda Oncology, Denovo, DOT-1, or RPI; |
|
|
• |
actual and anticipated fluctuations in our quarterly operating results; |
|
|
• |
deviations in our operating results from the estimates of analysts; |
|
|
• |
litigation or public concern about the safety of future products, if any; |
|
|
• |
failure to develop or sustain an active and liquid trading market for our common stock; |
|
|
• |
short-selling or manipulation of our common stock by investors; |
|
|
• |
sales of our common stock by our officers, directors or significant stockholders; and |
|
|
• |
additions or departures of key personnel. |
Our failure to meet the continued listing requirements of The Nasdaq Stock Market LLC could result in a delisting of our common stock.
Our common stock is listed on The Nasdaq Stock Market LLC, which imposes, among other requirements a minimum bid requirement. Our common stock traded for less than $1.00 for 30 consecutive trading days, and we received notice of this from the Listing Qualifications Staff of The Nasdaq Stock Market LLC on July 9, 2019. Under Nasdaq Listing Rule 5810(c)(3)(A)(the “Rule”), we were granted a 180 calendar day grace period, or until January 6, 2020, to regain compliance with the minimum bid price requirement. Subsequently, we requested and on January 7, 2020 we received a second 180-calendar day extension, or until July 6,
30
2020, to demonstrate compliance. The minimum bid price requirement will be met if our common stock has a closing bid price of at least $1.00 per share for a minimum of 10 consecutive business days during the 180 calendar day grace period. We will consider a reverse stock split to regain compliance. There can be no assurance that we will be able to regain compliance.
If we do not regain compliance with the Rule by July 6, 2020, the Staff will provide written notification to us that our common stock will be subject to delisting. At that time, we may appeal the Staff’s delisting determination to a Nasdaq Hearings Panel, or the Panel. Our common stock would remain listed pending the Panel’s decision. There can be no assurance that, if we do appeal the delisting determination by the Staff to the Panel, that such appeal would be successful.
The delisting of our common stock from Nasdaq may make it more difficult for us to raise capital on favorable terms in the future, or at all. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. Further, if our common stock were to be delisted from The Nasdaq Capital Market, our common stock would cease to be recognized as a covered security and we would be subject to additional regulation in each state in which we offer our securities. Moreover, there is no assurance that any actions that we take to restore our compliance with the Nasdaq minimum bid requirement would stabilize the market price or improve the liquidity of our common stock, prevent our common stock from falling below the Nasdaq minimum bid price required for continued listing again, or prevent future non-compliance with Nasdaq’s listing requirements.
There can be no assurance that we will continue to meet the minimum bid price requirement, or any other requirement in the future. If we fail to meet the minimum bid price requirement, or other applicable Nasdaq listing requirements, including maintaining minimum levels of stockholders’ equity or market values of our common stock, our common stock could be delisted. If our common stock were to be delisted, the liquidity of our common stock would be adversely affected, and the market price of our common stock could decrease.
Unless our common stock continues to be listed on a national securities exchange it will become subject to the so-called “penny stock” rules that impose restrictive sales practice requirements.
If we are unable to maintain the listing of our common stock on Nasdaq or another national securities exchange, our common stock could become subject to the so-called “penny stock” rules if the shares have a market value of less than $5.00 per share. The SEC has adopted regulations that define a penny stock to include any stock that has a market price of less than $5.00 per share, subject to certain exceptions, including an exception for stock traded on a national securities exchange. The SEC regulations impose restrictive sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. An accredited investor generally is a person whose individual annual income exceeded $200,000, or whose joint annual income with a spouse exceeded $300,000 during the past two years and who expects their annual income to exceed the applicable level during the current year, or a person with net worth in excess of $1.0 million, not including the value of the investor’s principal residence and excluding mortgage debt secured by the investor’s principal residence up to the estimated fair market value of the home, except that any mortgage debt incurred by the investor within 60 days prior to the date of the transaction shall not be excluded from the determination of the investor’s net worth unless the mortgage debt was incurred to acquire the residence. For transactions covered by this rule, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. This means that if we are unable maintain the listing of our common stock on a national securities exchange, the ability of stockholders to sell their common stock in the secondary market could be adversely affected.
If a transaction involving a penny stock is not exempt from the SEC’s rule, a broker-dealer must deliver a disclosure schedule relating to the penny stock market to each investor prior to a transaction. The broker-dealer also must disclose the commissions payable to both the broker-dealer and its registered representative, current quotations for the penny stock, and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the customer’s account and information on the limited market in penny stocks.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of our company, even if the acquisition would be beneficial to our stockholders, and could make it more difficult to change management.
Provisions of our amended and restated certificate of incorporation and amended and restated bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders might otherwise consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
31
|
|
• |
a prohibition on stockholder action through written consent; |
|
|
• |
limitations on our stockholders’ ability to call special meetings of stockholders; |
|
|
• |
an advance notice requirement for stockholder proposals and nominations; and |
|
|
• |
the authority of our board of directors to issue preferred stock with such terms as our board of directors may determine. |
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of our company.
Provisions in our charter documents and provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our common stock.
We have never paid dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We do not anticipate paying any cash dividends on our capital stock in the foreseeable future. In addition, under the terms of the SVB Loan Agreement, we are precluded from paying cash dividends without the prior written consent of SVB. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
We are at risk of securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced greater than average stock price volatility in recent years. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock in one or more transactions at prices and in a manner we determine from time to time, including pursuant to our Controlled Equity OfferingSM sales agreement, with Cantor Fitzgerald & Co., Common Stock Purchase Agreement with Aspire Capital Fund, LLC, or any similar arrangements into which we may enter. These future issuances of common stock or common stock-related securities, together with the exercise of outstanding options and any additional shares issued in connection with acquisitions or in-licenses, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
Pursuant to our equity incentive plans, our compensation committee is authorized to grant equity-based incentive awards to our employees, non-employee directors and consultants. Future grants of RSUs, options and other equity awards and issuances of common stock under our equity incentive plans will result in dilution and may have an adverse effect on the market price of our common stock.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us and our business. In the event securities or industry analysts who cover us downgrade our stock or publish unfavorable research about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of
32
our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
None.
Our corporate headquarters is currently located at 395 Oyster Point Boulevard in South San Francisco, California. The lease was entered into in January 2014 and was amended several times since 2014. The lease was last amended in December 2017 to extend the expiration date to June 30, 2021. We have an option to extend the lease for two additional years.
From time to time, we may be involved in routine legal proceedings, as well as demands, claims and threatened litigation, which arise in the normal course of our business. The ultimate outcome of any litigation is uncertain and unfavorable outcomes could have a negative impact on our results of operations and financial condition. Regardless of outcome, litigation can have an adverse impact on us because of the defense costs, diversion of management resources and other factors.
We believe there is no litigation pending that could, individually or in the aggregate, have a material adverse effect on our results of operations or financial condition.
Not applicable.
33
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is listed on The Nasdaq Stock Market under the symbol “SNSS.”
As of March 4, 2020, there were approximately 130 holders of record of our common stock. In addition, we believe that a significant number of beneficial owners of our common stock hold their shares in nominee or in “street name” accounts through brokers.
Dividend Policy
We have never paid cash dividends on our common stock. We do not anticipate paying any cash dividends on our capital stock in the foreseeable future. While subject to periodic review, the current policy of our board of directors is to retain cash and investments primarily to provide funds for our future growth.
Recent Sales of Unregistered Securities
There were no unregistered sales of equity securities by us during the year ended December 31, 2019.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information under this item.
34
The following discussion and analysis of our financial condition as of December 31, 2019 and results of operations for the year ended December 31, 2019 should be read together with our consolidated financial statements and related notes included elsewhere in this report.
This discussion and analysis contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995, which involve risks, uncertainties and assumptions. All statements, other than statements of historical facts, are “forward-looking statements” for purposes of these provisions, including without limitation any statements relating to our expectations for gaining marketing approval in the United States, including the continued development and commercialization of vecabrutinib (formerly SNS-062), SNS-510, and other product candidates, the timing of our Phase 1b/2 trial of vecabrutinib, presenting clinical data and initiating clinical trials, our future research and development activities, including clinical testing and the costs and timing thereof, the potential of our existing product candidates to lead to the development of commercial products, our ability to receive potential milestone or royalty payments under license and collaboration agreements and the timing of receipt of those payments, including those related to TAK 580 and vosaroxin, sufficiency of our cash resources, our ability to raise additional funding when needed, any statements concerning anticipated regulatory activities or licensing or collaborative arrangements, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “anticipates,” “believe,” “continue,” “estimates,” “expects,” “intend,” “look forward,” “may,” “could,” “seeks,” “plans,” “potential,” or “will” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under “Risk Factors,” and elsewhere in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. All forward-looking statements included in this report are based on information available to us on the date of this report, and we assume no obligation to update any forward-looking statements contained in this report.
In this report, “Sunesis,” the “Company,” “we,” “us,” and “our” refer to Sunesis Pharmaceuticals, Inc. and its wholly-owned subsidiaries, except where it is made clear that the term refers only to the parent company.
35
Sunesis is a biopharmaceutical company focused on the development of novel targeted inhibitors for the treatment of hematologic and solid cancers. Our primary activities since incorporation have been conducting research and development internally and through corporate collaborators, in-licensing and out-licensing pharmaceutical compounds and technology, conducting clinical trials, and raising capital.
Our lead program is vecabrutinib, a selective non-covalent inhibitor of Bruton’s Tyrosine Kinase (“BTK”) with activity against both wild-type and C481S-mutated BTK, the most common mutation associated with resistance to covalent BTK inhibitors. Ibrutinib was the first BTK inhibitor approved for the treatment of chronic lymphocytic leukemia (“CLL”), mantle cell lymphoma (“MCL”), and other B-cell malignancies. Ibrutinib is the market leader in CLL, marketed by Johnson & Johnson and AbbVie Inc. (“AbbVie”), with approximately $5 billion in net revenues in 2018. The C481 mutation has been seen in patients who developed resistance to ibrutinib and to acalabrutinib, another covalent BTK inhibitor that is approved for treatment of CLL and MCL.
Vecabrutinib is being studied in a Phase 1b/2 clinical trial to assess safety and activity in patients with CLL and other advanced B-cell malignancies after two or more prior therapies, including ibrutinib or another covalent BTK inhibitor where approved for the disease. We completed the safety evaluation period for the 400 mg cohort and thus far vecabrutinib has a favorable safety profile. We have not seen any Grade 3 or higher drug-related adverse events in cohorts above 50mg. The seventh cohort, testing 500 mg twice daily, is now open. We have prepared a Phase 2 portion to further explore clinical activity and safety in CLL patients, including those with and without BTK C481 mutations. We will start the Phase 2 only after seeing a sufficient efficacy signal in the ongoing Phase 1b. Vecabrutinib was developed as a result of a collaboration agreement with Biogen MA Inc. (“Biogen”), and we must pay a royalty on sales of vecabrutinib when and if approved and commercialized.
We are developing SNS-510, a PDK1 inhibitor licensed from Millennium Pharmaceuticals, Inc. (“Takeda Oncology”), a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited. In October 2019, at the 2019 AACR -NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, we presented the results of studies characterizing SNS-510 in multiple preclinical pharmacology models, including evaluation in the Eurofins Oncopanel™, a panel of 320 genomically profiled cancer cell lines from diverse tissue origins, and in vivo studies in FLT3-mutated and wild-type AML xenograft models. These studies indicated that CDKN2A-mutated tumors are particularly sensitive to SNS-510, supporting a potential role in combination with CDK4/6 inhibitors to address resistance and improve activity. SNS-510 also demonstrated potent, pathway-mediated antitumor activity in the AML models. We are conducting an IND-enabling program for SNS-510 and plan to file an IND by the end of 2020.
In December 2019, we consented to Takeda Oncology’s assignment of TAK-580 to DOT Therapeutics-1, Inc. (“DOT-1”), and we entered into a license agreement with DOT-1 (the “DOT-1 License Agreement) to grant DOT-1 a worldwide, exclusive license of TAK-580. Pursuant to this agreement, we received a $2.0 million upfront payment from DOT-1. The agreement also includes up to $57.0 million in potential pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580, when and if approved and commercialized.
In December 2019, we entered into an agreement to license vosaroxin to Denovo Biopharma, LLC (“Denovo”), pursuant to which Sunesis received a $200,000 upfront payment and is eligible to receive up to $57.0 million in potential regulatory and commercial milestones, and double-digit royalties on future sales of vosaroxin, when and if approved and commercialized (the “Denovo License Agreement”).
Recent Financial History
Underwritten Offerings
In July 2019, we completed underwritten public offerings of (i) 38,333,717 shares of our common stock at a price to the public of $0.60 for each share of common stock, including the full exercise of the underwriters’ option to purchase 5,000,050 additional shares of common stock to cover over-allotments, and (ii) 8,333 shares of our non-voting Series F Convertible Preferred Stock (“Series F Stock”) at a price to the public of $600.00 for each share of Series F Stock. Gross proceeds from the sale were approximately $28.0 million and net proceeds were approximately $26.1 million.
In January 2019, we completed underwritten public offerings of (i) 23,000,000 shares of our common stock at a price to the public of $0.50 for each share of common stock, and (ii) 17,000 shares of our non-voting Series E Convertible Preferred Stock (“Series E Stock”), at a price to the public of $500.00 for each share of Series E Stock. Gross proceeds from the sale were $20.0 million and net proceeds were approximately $18.5 million.
Debt Refinancing
In April 2019, we entered into a term loan agreement with Silicon Valley Bank (“SVB” and such agreement, the “SVB Loan Agreement”), pursuant to which we borrowed $5.5 million and used the proceeds of the SVB Loan Agreement plus cash on hand to
36
repay our remaining obligations in the amount of $5.9 million under our existing loan agreement (the “Loan Agreement and Amendments”) with Western Alliance Bank and Solar Capital Ltd and Western Alliance, as Collateral Agent.
The maturity date of the SVB Loan Agreement is December 1, 2022. Under the terms of the SVB Loan Agreement, we are required to make interest-only payments through December 31, 2020 on the borrowings at a floating rate equal to the greater of 3.25% or the Prime Rate as defined in the SVB Loan Agreement minus 2.25%, followed by an amortization period of 24 months of equal monthly payments of principal plus interest amounts until paid in full. In addition to and not in substitution for the regular monthly payments of principal plus accrued interest, we are required to make a final payment equal to 4% of the original principal amount of the borrowings (the “Final Payment Fee”). Additionally, we may prepay all, but not less than all of the borrowings at any time upon 30 days’ prior notice to SVB. Any such prepayment would require, in addition to payment of principal and accrued interest as well as the Final Payment Fee, a prepayment fee, in the amount of (a) $165,000 if the prepayment occurs prior to the 1st anniversary of April 26, 2019, or the Effective Date (as defined in the SVB Loan Agreement); (b) $110,000 if the prepayment occurs on or after the 1st anniversary of the Effective Date but prior to the 2nd anniversary of the Effective Date; or (c) $55,000 if the prepayment occurs on or after the 2nd anniversary of the Effective Date.
Aspire Common Stock Purchase Agreement
In June 2018, we entered into the Common Stock Purchase Agreement (the “CSPA”) with Aspire Capital Fund, LLC (“Aspire”), pursuant to which we could issue and sell shares of our common stock having an aggregate gross sales price of up to $15.5 million. Upon execution of the CSPA, we sold to Aspire 228,311 shares of common stock at a price of $2.19 per share, for total proceeds of $0.5 million. In addition, Aspire committed to purchasing up to an additional $15 million of common shares, at our request, from time to time during a 24-month period at prices based on the market price at the time of each sale. Under the CSPA, on any trading day selected by us on which the closing price of our common stock is equal to or greater than $0.25 per share, we have the right, in our sole discretion, to present Aspire with a purchase notice directing Aspire to purchase up to 200,000 shares of common stock per business day, at a purchase price equal to the lesser of:
|
|
a) |
the lowest sale price of common stock on the purchase date; or |
|
|
b) |
the arithmetic average of the three lowest closing sale prices during the 10 consecutive business days ending on the trading day immediately preceding the purchase date. |
We shall also have the right to require Aspire to purchase up to an additional 30% of the trading volume of the shares for the next business day at a purchase price (the “VWAP Purchase Price”), equal to the lesser of: (i) the closing sale price of the shares on the purchase date, or (ii) ninety-seven percent (97%) of the next business day’s volume weighted average price (each such purchase, a “VWAP Purchase”). We shall have the right, in our sole discretion, to determine a maximum number of shares and set a minimum market price threshold for each VWAP Purchase. We can only require a VWAP Purchase if we have also submitted a regular purchase on the notice date for the VWAP Purchase. There are no limits on the number of VWAP Purchases that we may require.
There are no trading volume requirements or restrictions under the CSPA, and we will control the timing and amount of sales. Aspire has no right to require any sales by us, but is obligated to make purchases from us as directed by us in accordance with the CSPA. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. The CSPA may be terminated by us at any time, at our discretion, without any cost to us. Aspire has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of common stock during any time prior to the termination of the CSPA. Any proceeds we receive under the CSPA are expected to be used for working capital and general corporate purposes. We cannot request Aspire to purchase more than 2,000,000 shares per business day.
As consideration for Aspire’s obligation under the CSPA, we issued 212,329 shares of common stock to Aspire as a commitment fee. This $0.4 million commitment fee and $0.1 million in other transaction costs were recorded in June 2018 as costs of equity financing, within additional paid-in capital. During 2019, no shares were issued under the CSPA, with Aspire. Aspire’s remaining purchase commitment was $10.9 million as of December 31, 2019.
Cantor Controlled Equity Offering
In August 2011, we entered into a Controlled Equity OfferingSM sales agreement (the “Sales Agreement”), with Cantor Fitzgerald & Co. (“Cantor”), as agent and/or principal, pursuant to which we could issue and sell shares of common stock. The Sales Agreement, as amended, provides for an aggregate gross sales of $45.0 million. We will pay Cantor a commission of up to 3.0% of the gross proceeds from any common stock sold under the Sales Agreement, as amended.
37
During 2019, we sold an aggregate of 0.4 million shares of common stock under the Sales Agreement, as amended, at an average price of approximately $1.19 per share for net proceeds of $0.5 million, after deducting Cantor’s commission. As of December 31, 2019, $43.1 million of common stock remained available to be sold under the Sales Agreement, as amended, subject to certain conditions.
Capital Requirements
We have incurred significant losses in each year since our inception. As of December 31, 2019, we had cash and cash equivalents, restricted cash, and marketable securities of $34.6 million and an accumulated deficit of $682.8 million. We expect to continue to incur significant losses for the foreseeable future as we continue the development of our kinase inhibitor pipeline, with a focus on our BTK inhibitor vecabrutinib. We have a limited number of product candidates that are still in the early stages of development and will require significant additional future investment.
We expect our current cash and cash equivalents, and marketable securities of $29.1 million, which excludes restricted cash of $5.5 million, as of December 31, 2019, are not sufficient to support our operations for a period of twelve months from the date the financial statements are available to be issued. We will require additional financing to fund working capital, repay debt and pay our obligations as they come due. Additional financing might include one or more offerings and one or more of a combination of equity securities, debt arrangements or partnership or licensing collaborations. However, there can be no assurance that we will be successful in acquiring additional funding at levels sufficient to fund our operations or on terms favorable to us. These conditions raise substantial doubt about our ability to continue as a going concern for a period of one year from the date these financial statements are available to be issued. If we are unsuccessful in our efforts to raise additional financing in the near term, we will be required to significantly reduce or cease operations. The accompanying financial statements have been prepared assuming we will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts of liabilities that may result from uncertainty related to our ability to continue as a going concern.
Critical Accounting Policies and the Use of Estimates
The accompanying discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements and the related disclosures, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires our management to make estimates, assumptions and judgments that affect the amounts reported in our financial statements and accompanying notes, including reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as revenue and expenses during the reporting periods. We evaluate our estimates, assumptions and judgments on an ongoing basis. We base our estimates on historical experience and on various other assumptions we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Management has discussed the development, selection and disclosure of these estimates with the Audit Committee of our Board of Directors. Actual results could differ materially from these estimates under different assumptions or conditions.
Our significant accounting policies are more fully described in Note 2 to our consolidated financial statements included elsewhere in this report. We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our consolidated financial statements.
Revenue Recognition
On January 1, 2018, the Company adopted Topic 606, Revenue from Contracts with Customers (“Topic 606”) using the modified retrospective method applied to those contracts which were not completed as of January 1, 2018. Results for reporting periods beginning after January 1, 2018 are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with the Company’s historic accounting under Topic 605, Revenue Recognition (“Topic 605”).
Adoption of the new standard did not result in any change to our opening retained earnings as of January 1, 2018 as no cumulative impact to the adoption of ASC 606 was noted as a result of our assessment of the comparative revenue recognized since inception of the contracts under the new revenue standard ASC 606 and historic standard ASC 605. We are applying the practical exemption allowed under ASC 606 and does not disclose the value of variable consideration that is a sale-based royalty promised in exchange for a license of intellectual property. The adoption of the new standard resulted in changes to our accounting policies for revenue recognition as detailed below:
38
Our contract revenues consist of license revenue primarily generated through agreements with strategic partners for the development and commercialization of our product candidates. The terms of the agreement typically include non-refundable upfront fees, payments based upon achievement of milestones and royalties on net product sales. We have both fixed and variable consideration. Non-refundable upfront fees are considered fixed, while milestone payments are identified as variable consideration.
In determining the appropriate amount of revenue to be recognized as we fulfill our obligations under these agreements, we perform the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and (v) recognition of revenue when (or as) we satisfy each performance obligation.
Licenses of intellectual property: If the license to our intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, we recognize revenues from non-refundable, up-front fees allocated to the license when the license is transferred to the customer, and the customer can use and benefit from the license. For licenses that are bundled with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. We evaluate the measure of progress each reporting period and, if necessary, adjust the measure of performance and related revenue recognition.
Milestone payments: At the inception of each arrangement that includes milestone payments, we evaluate whether the milestones are considered probable of being achieved and estimate the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the value of the associated milestone is included in the transaction price. Milestone payments that are not within our control are not included in the transaction price until they become probable of being achieved.
Royalties: For arrangements that include sales-based royalties and the license is deemed to be the predominant item to which the royalties relate, we recognize revenue at the later of (a) when the related sales occur, or (b) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, we have not recognized any royalty revenue resulting from any of our licensing arrangements.
Clinical Trial Accounting
We record accruals for estimated clinical trial costs, which include payments for work performed by contract research organizations (“CROs”), and participating clinical trial sites. These costs are generally a significant component of research and development expense. Costs incurred for setting up clinical trial sites for participation in trials are generally non-refundable, and are expensed as incurred, with any refundable advances related to enrollment of the first patient recorded as prepayments and assessed for recoverability on a quarterly basis. Costs related to patient enrollment are accrued as patients progress through the clinical trial, including amortization of any first-patient prepayments. This amortization generally matches when the related services are rendered, however, these cost estimates may or may not match the actual costs incurred by the CROs or clinical trial sites, and if we have incomplete or inaccurate information, our clinical trial accruals may not be accurate. The difference between accrued expenses based on our estimates and actual expenses has not been significant to date.
Leases
We determine if an arrangement is or contains a lease at inception. In determining whether an arrangement is a lease, we consider whether (1) explicitly or implicitly identified assets have been deployed in the arrangement and (2) we obtain substantially all of the economic benefits from the use of that underlying asset and direct how and for what purpose the asset is used during the term of the contract.
Right-of-Use (“ROU”), assets represent our right to use an underlying asset for the lease term, and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized based on the present value of lease payments over the lease term. When an implicit rate is not readily determinable, we use our incremental borrowing rate based on the information available at commencement date for new leases or effective date for existing leases, in determining the present value of lease payments.
Leases may contain initial periods of free rent and/or periodic escalations. When such items are included in a lease agreement, we record rent expense on a straight-line basis over the initial term of a lease. The difference between the rent payment and the straight-line rent expense is recorded as a deferred rent liability. We expense any additional payments under its operating leases for taxes, insurance, or other operating expenses as incurred.
39
We have not generated any revenue from the sale of commercial products. Our current and past revenue have been generated through license and collaboration agreements. We cannot predict if our licensees will continue development or whether we will receive any additional event-based payments or royalties from these agreements in the foreseeable future, or at all.
Overview of Operating Expenses
Research and development expense. Research and development expense consists primarily of clinical trial costs, which include: payments for work performed by our contract research organizations, clinical trial sites, labs and other clinical service providers and for drug packaging, storage and distribution; drug manufacturing costs, which include costs for producing drug substance and drug product, and for stability and other testing; personnel costs, including non-cash stock-based compensation; other outside services and consulting costs; and payments under license agreements. We expense all research and development costs as they are incurred.
The table below sets forth our research and development expense by program for each period presented:
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
|
|
|
|
|
|
|
|
|
|
Vecabrutinib |
|
$ |
14,014 |
|
|
$ |
12,923 |
|
|
SNS-510 |
|
|
908 |
|
|
|
701 |
|
|
Vosaroxin |
|
|
490 |
|
|
|
991 |
|
|
Total |
|
$ |
15,412 |
|
|
$ |
14,615 |
|
We are currently focused on the development of vecabrutinib for the treatment of B-cell malignancies and our other product candidate, SNS-510, for the treatment of solid tumor and hematologic malignancies. Research and development costs typically increase as product development candidates move from early stage to later stage, larger clinical trials. As a result, our research and development costs may increase in the future. Due to the above uncertainties and other risks inherent in the development process, we are unable to estimate the costs we will incur in the development of our product candidates in the future.
If we engage a development or commercialization partner for our development programs, or if, in the future, we acquire additional product candidates, our research and development expenses could be significantly affected. We cannot predict whether future licensing or collaborative arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements. We anticipate expenditure associated with vosaroxin to diminish as result of out-licensing of vosaroxin to Denovo, and continuing expenditures associated with advancing the vecabrutinib and SNS-510 programs in 2020 and beyond.
General and administrative expense. General and administrative expense consists primarily of personnel costs for the related employees, including non-cash stock-based compensation; outside service costs, including fees paid to external legal advisors, marketing consultants and our independent registered public accounting firm; facilities expenses; and other administrative costs.
Liquidity and Capital Resources
Sources of Liquidity
We have incurred significant losses in each year since our inception. As of December 31, 2019, we had cash and cash equivalents, restricted cash, and marketable securities of $34.6 million and an accumulated deficit of $682.8 million. We expect to continue to incur significant losses for the foreseeable future. Our products are still in the early stages of approval and will require significant additional investment.
We expect our current cash and cash equivalents, and marketable securities of $29.1 million, which excludes restricted cash of $5.5 million, as of December 31, 2019, are not sufficient to support our operations for a period of twelve months beyond the date the financial statements are available to be issued. We will require additional financing to fund working capital, repay debt and pay our obligations as they come due, so substantial doubt exists about our ability to continue as a going concern. Additional financing might include one or more of a combination of offerings of equity securities or debt arrangements or partnerships or licensing collaborations. However, there can be no assurance that we will be successful in acquiring additional funding at levels sufficient to fund our operations or on terms favorable to us.
Our cash and cash equivalents, restricted cash, and marketable securities was $34.6 million as of December 31, 2019, compared to $13.7 million as of December 31, 2018. The increase of $20.9 million was primarily due to $45.1 million of net proceeds from the
40
issuance of common and preferred stock, and $5.5 million of proceeds from the SVB Loan Agreement, partially offset by $22.2 million net cash used in operating activities and $7.5 million principal payment on the Loan Agreement and Amendments.
In July 2019, we completed underwritten public offerings of (i) 38,333,717 shares of our common stock at a price to the public of $0.60 for each share of common stock, and (ii) 8,333 shares of Series F Stock at a price to the public of $600.00 for each share of Series F Stock. Gross proceeds from the sale were approximately $28.0 million and net proceeds were approximately $26.1 million.
In January 2019, we completed underwritten public offerings of (i) 23,000,000 shares of our common stock at a price to the public of $0.50 for each share of common stock, and (ii) 17,000 shares of our Series E Stock, at a price to the public of $500 for each share of Series E Stock. Gross proceeds from the sale were $20.0 million and net proceeds were approximately $18.5 million.
In April 2019, we entered into a term loan agreement with Silicon Valley Bank, or SVB Loan Agreement, pursuant to which we borrowed $5.5 million and used the proceeds of the SVB Loan Agreement plus cash on hand to repay our remaining obligations in the amount of $5.9 million under our Loan Agreement and Amendments.
The maturity date of the SVB Loan Agreement is December 1, 2022. Under the terms of the SVB Loan Agreement, we are required to make interest-only payments through December 31, 2020 on the borrowings at a floating rate equal to the greater of 3.25% or the Prime Rate as defined in the SVB Loan Agreement minus 2.25%, followed by an amortization period of 24 months of equal monthly payments of principal plus interest amounts until paid in full. In addition to and not in substitution for the regular monthly payments of principal plus accrued interest, we are required to make a final payment equal to 4% of the original principal amount of the borrowings, or Final Payment Fee. Additionally, we may prepay all, but not less than all of the borrowings at any time upon 30 days’ prior notice to Silicon Valley Bank, or SVB. Any such prepayment would require, in addition to payment of principal and accrued interest as well as the Final Payment Fee, a prepayment fee, in the amount of (a) $165,000 if the prepayment occurs prior to the 1st anniversary of April 26, 2019, or the Effective Date; (b) $110,000 if the prepayment occurs on or after the 1st anniversary of the Effective Date but prior to the 2nd anniversary of the Effective Date; or (c) $55,000 if the prepayment occurs on or after the 2nd anniversary of the Effective Date.
41
As of December 31, 2019, the remaining purchase commitment for Aspire under the CSPA was $10.9 million. In 2019, we sold 0.4 million shares of common stock under the Sales Agreement, as amended, with Cantor, for net proceeds of $0.5 million. As of December 31, 2019, $43.1 million of common stock remains available to be sold under the Sales Agreement, as amended, subject to certain conditions.
If we become unable to continue as a going concern, we may have to liquidate our assets, and might realize significantly less than the values at which they are carried on our consolidated financial statements, and stockholders may lose all or part of their investment in our common stock. Other than raising additional funds from investors or business partners, management cannot identify conditions or events to mitigate the substantial doubt that exists about our ability to continue as a going concern.
Cash Flows
Operating activities
Net cash used in operating activities was $22.2 million in 2019, compared to $24.4 million in 2018. Net cash used in the 2019 period resulted primarily from the net loss of $23.3 million and changes in operating assets and liabilities of $0.7 million, offset by net adjustments for non-cash items of $1.8 million. Net cash used in the 2018 period resulted primarily from the net loss of $26.6 million and changes in operating assets and liabilities of $0.6 million, offset by net adjustments for non-cash items of $2.8 million.
Investing activities
Net cash used by investing activities was $16.3 million in 2019, compared to net cash provided by investing activities of $4.8 million in 2018. Net cash used in investing activities in 2019 consists of purchase of marketable securities and net cash provided in 2018 consisted of proceeds from maturities of marketable securities.
Financing activities
Net cash provided by financing activities was $43.0 million in 2019, compared to $6.3 million in 2018. Net cash provided in 2019 resulted primarily from $45.1 million net proceeds from issuance of common and preferred stock, and $5.5 million proceeds from the SVB Loan Agreement, offset by $7.5 million principal payment on the Loan Agreement and Amendments. Net cash provided in 2018 resulted primarily from issuances of common stock through the Sales Agreement with Cantor and CSPA with Aspire of $6.0 million and net proceeds of $0.3 million from the exercise of stock options under our 2011 Equity Incentive Plan and purchases under our 2011 Employee Stock Purchase Plan.
Operating Cash Requirements
We have incurred significant operating losses and negative cash flows from operations since our inception. As of December 31, 2019, we had cash and cash equivalents, restricted cash, and marketable securities of $34.6 million and cash used in operating activities of $22.2 million for 2019.
We expect to continue to incur substantial operating losses in the future. We will not receive any product revenue until a product candidate has been approved by the FDA, EMA, or similar regulatory agencies in other countries, and has been successfully commercialized, if ever. We will need to raise substantial additional funding to complete the development and potential commercialization of any of our development programs. Additionally, we may evaluate in-licensing and acquisition opportunities to gain access to new drugs or drug targets that would fit with our strategy. Any such transaction would likely increase our funding needs in the future.
Our future funding requirements will depend on many factors, including but not limited to:
|
|
• |
the rate of progress and cost of our clinical trials; |
|
|
• |
the timing, economic and other terms of any licensing, collaboration or other similar arrangement into which we may enter; |
|
|
• |
the costs and timing of seeking and obtaining FDA, EMA, or other regulatory approvals; |
|
|
• |
the costs associated with building or accessing commercialization and additional manufacturing capabilities and supplies; |
|
|
• |
the costs of acquiring or investing in businesses, product candidates and technologies, if any; |
|
|
• |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
42
|
|
• |
the costs, if any, of supporting our arrangements with Denovo and DOT-1. |
Our failure to raise significant additional capital in the future would force us to delay or reduce the scope of our vecabrutinib and other development programs, potentially including any additional clinical trials or subsequent regulatory filings in the United States or Europe, and/or limit or cease our operations. Any one of the foregoing would have a material adverse effect on our business, financial condition and results of operations.
Results of Operations
Years Ended December 31, 2019 and 2018
Revenue. Total revenue was $2.1 million in 2019 compared to $0.2 million in 2018. Revenue in both periods was derived from license agreements. The increase of $1.9 million in 2019 was primarily due to revenue recognized from the upfront payments received under the license agreements with DOT-1 and Denovo.
Research and development expense. Research and development expense was $15.4 million in 2019 as compared to $14.6 million in 2018, primarily relating to the vecabrutinib and the vosaroxin development programs in each year, respectively. The increase of $0.8 million in 2019 was primarily due to a $1.8 million increase in professional services and clinical expenses related to the preparation for the Phase 2 portion of our ongoing clinical trial for vecabrutinib, offset by a $1.0 million decrease in salary and personnel expenses due to lower headcount.
General and administrative expense. General and administrative expense was $9.9 million in 2019 compared to $11.3 million in 2018. The decrease of $1.4 million in 2019 was primarily due to a $1.1 million decrease in salary and personnel expenses due to lower headcount and stock-based compensation and a $0.8 million decrease in professional services expenses due to lower legal and vosaroxin patent expenses. The decreases in the comparable periods were partially offset by a $0.3 increase in director and officer insurance premiums.
Interest expense. Interest expense was $0.5 million in 2019 compared to $1.2 million in 2018. The decrease in 2019 was primarily due the lower interest paid due to the lower interest rate on the lower principal amount under the SVB Loan Agreement.
Other income, net. Net other income was $0.5 million in 2019 as compared to $0.2 million in 2018. Other income was primarily comprised of interest income from short term investments, and the increase of $0.3 million in 2019 was primarily due to increase in marketable securities investments.
Income Taxes
Deferred tax assets or liabilities may arise from differences between the tax basis of assets or liabilities and their basis for financial reporting. Deferred tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Our policy is to recognize interest charges and penalties in other income (expense), net in the statements of operations and comprehensive loss.
Since inception, we have incurred operating losses and, accordingly, have not recorded a provision for income taxes for any of the periods presented. As of December 31, 2019, we had net operating loss carry-forwards for federal and state income tax purposes of $463.4 million and $310.7 million, respectively. We also had federal and state research and development tax credit carry-forwards of $8.8 million and $7.8 million, respectively. If not utilized, the federal net operating loss and tax credit carry-forwards will begin to expire in 2020 and the state net operating loss carry-forwards expire beginning in 2028. The state research and development tax credit carry-forwards do not expire. Utilization of these net operating loss and tax credit carry-forwards may be subject to a substantial annual limitation due to ownership change rules under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”). The limitations are applicable if an “ownership change,” as defined in the Code, is deemed to have occurred or occurs in the future. The annual limitation may result in the expiration of net operating loss and credit carry-forwards before they can be utilized.
43
Off-Balance Sheet Arrangements
Since our inception, we have not had any off-balance sheet arrangements or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or variable interest entities, which are typically established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information under this item.
44
Index to Consolidated Financial Statements
|
|
|
Page |
|
|
46 |
|
|
|
47 |
|
|
Consolidated Statements of Operations and Comprehensive Loss |
|
48 |
|
|
49 |
|
|
|
50 |
|
|
|
51 |
45
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Sunesis Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Sunesis Pharmaceuticals, Inc. (the Company) as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
The Company's Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management's evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Adoption of New Accounting Standard
As discussed in Note 2 to the consolidated financial statements, the Company changed its method of accounting for leases as a result of the adoption of Accounting Standards Update (ASU) No. 2016-02, Leases (Topic 842), using the modified retrospective transition method effective January 1, 2019.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1998
Salt Lake City, Utah
March 10, 2020
46
(In thousands, except per share amounts)
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
12,761 |
|
|
$ |
13,696 |
|
|
Restricted cash |
|
|
5,500 |
|
|
|
— |
|
|
Marketable securities |
|
|
16,364 |
|
|
|
— |
|
|
Prepaids and other current assets |
|
|
1,697 |
|
|
|
1,504 |
|
|
Total current assets |
|
|
36,322 |
|
|
|
15,200 |
|
|
Property and equipment, net |
|
|
3 |
|
|
|
11 |
|
|
Operating lease - right-of-use asset |
|
|
817 |
|
|
|
— |
|
|
Other assets |
|
|
98 |
|
|
|
113 |
|
|
Total assets |
|
$ |
37,240 |
|
|
$ |
15,324 |
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
791 |
|
|
$ |
1,393 |
|
|
Accrued clinical expense |
|
|
521 |
|
|
|
500 |
|
|
Accrued compensation |
|
|
985 |
|
|
|
943 |
|
|
Other accrued liabilities |
|
|
1,109 |
|
|
|
1,091 |
|
|
Notes payable |
|
|
5,465 |
|
|
|
7,396 |
|
|
Operating lease liability - current |
|
|
545 |
|
|
|
— |
|
|
Total current liabilities |
|
|
9,416 |
|
|
|
11,323 |
|
|
Other liabilities |
|
|
9 |
|
|
|
8 |
|
|
Operating lease liability - long term |
|
|
272 |
|
|
|
— |
|
|
Total liabilities |
|
|
9,697 |
|
|
|
11,331 |
|
|
Commitments and contingencies (Note 8) |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Convertible preferred stock, $0.0001 par value; 10,000 shares authorized as of December 31, 2019; 20 and 18 shares issued and outstanding as of December 31, 2019 and 2018, respectively |
|
|
11,769 |
|
|
|
20,998 |
|
|
Common stock, $0.0001 par value; 400,000 shares authorized as of December 31, 2019; 111,393 and 37,474 shares issued and outstanding as of December 31, 2019 and 2018, respectively |
|
|
11 |
|
|
|
4 |
|
|
Additional paid-in capital |
|
|
698,562 |
|
|
|
642,460 |
|
|
Accumulated other comprehensive income |
|
|
1 |
|
|
|
— |
|
|
Accumulated deficit |
|
|
(682,800 |
) |
|
|
(659,469 |
) |
|
Total stockholders’ equity |
|
|
27,543 |
|
|
|
3,993 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
37,240 |
|
|
$ |
15,324 |
|
See accompanying notes to consolidated financial statements.
47
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except per share amounts)
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Revenue: |
|
|
|
|
|
|
|
|
|
License and other revenue |
|
$ |
2,073 |
|
|
$ |
237 |
|
|
Total revenues |
|
|
2,073 |
|
|
|
237 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
15,412 |
|
|
|
14,615 |
|
|
General and administrative |
|
|
9,949 |
|
|
|
11,332 |
|
|
Total operating expenses |
|
|
25,361 |
|
|
|
25,947 |
|
|
Loss from operations |
|
|
(23,288 |
) |
|
|
(25,710 |
) |
|
Interest expense |
|
|
(514 |
) |
|
|
(1,154 |
) |
|
Other income, net |
|
|
472 |
|
|
|
249 |
|
|
Net loss |
|
|
(23,330 |
) |
|
|
(26,615 |
) |
|
Unrealized gain on available-for-sale securities |
|
|
1 |
|
|
|
7 |
|
|
Comprehensive loss |
|
$ |
(23,329 |
) |
|
$ |
(26,608 |
) |
|
Basic and diluted loss per common share: |
|
|
|
|
|
|
|
|
|
Net loss: |
|
$ |
(23,330 |
) |
|
$ |
(26,615 |
) |
|
Shares used in computing net basic and diluted loss per common share: |
|
|
87,129 |
|
|
|
35,582 |
|
|
Net loss per common share: |
|
$ |
(0.27 |
) |
|
$ |
(0.75 |
) |
See accompanying notes to consolidated financial statements.
48
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
Total |
|
||
|
|
|
Convertible |
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Comprehensive |
|
|
|
|
|
|
Stock |
|
||||||||
|
|
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid-In |
|
|
Income |
|
|
Accumulated |
|
|
holders’ |
|
||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
(Loss) |
|
|
Deficit |
|
|
Equity |
|
||||||||
|
Balance as of December 31, 2017 |
|
|
18 |
|
|
$ |
20,966 |
|
|
|
34,291 |
|
|
$ |
3 |
|
|
$ |
633,436 |
|
|
$ |
(7 |
) |
|
$ |
(632,854 |
) |
|
$ |
21,544 |
|
|
Adjustment to issuance cost related to common stock, preferred stock, and warrants issued in prior year |
|
|
— |
|
|
|
32 |
|
|
|
— |
|
|
|
— |
|
|
|
94 |
|
|
|
— |
|
|
|
— |
|
|
|
126 |
|
|
Issuance of $6,068 of common stock through controlled equity offering facilities, net of issuance costs of $77 |
|
|
— |
|
|
|
— |
|
|
|
3,009 |
|
|
|
1 |
|
|
|
5,990 |
|
|
|
— |
|
|
|
— |
|
|
|
5,991 |
|
|
Issuance of common stock from vesting of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
21 |
|
|
|
— |
|
|
|
83 |
|
|
|
— |
|
|
|
— |
|
|
|
83 |
|
|
Issuance of common stock under employee stock purchase plans |
|
|
— |
|
|
|
— |
|
|
|
104 |
|
|
|
— |
|
|
|
139 |
|
|
|
— |
|
|
|
— |
|
|
|
139 |
|
|
Issuance of common stock pursuant to stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
49 |
|
|
|
— |
|
|
|
164 |
|
|
|
— |
|
|
|
— |
|
|
|
164 |
|
|
Stock-based compensation expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,554 |
|
|
|
— |
|
|
|
— |
|
|
|
2,554 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(26,615 |
) |
|
|
(26,615 |
) |
|
Unrealized gain on available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
7 |
|
|
|
— |
|
|
|
7 |
|
|
Balance as of December 31, 2018 |
|
|
18 |
|
|
|
20,998 |
|
|
|
37,474 |
|
|
|
4 |
|
|
|
642,460 |
|
|
|
— |
|
|
|
(659,469 |
) |
|
|
3,993 |
|
|
Issuance of $38,000 of common stock, $10,000 preferred stock, net of issuance cost of $3,400 |
|
|
25 |
|
|
|
12,533 |
|
|
|
61,333 |
|
|
|
6 |
|
|
|
32,065 |
|
|
|
— |
|
|
|
— |
|
|
|
44,604 |
|
|
Issuance of common stock upon conversion of preferred stock |
|
|
(23 |
) |
|
|
(21,762 |
) |
|
|
11,950 |
|
|
|
1 |
|
|
|
21,761 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of $473 of common stock through controlled equity offering facilities, net of issuance costs of $9 |
|
|
— |
|
|
|
— |
|
|
|
398 |
|
|
|
— |
|
|
|
464 |
|
|
|
— |
|
|
|
— |
|
|
|
464 |
|
|
Issuance of common stock from vesting of restricted stock awards |
|
|
— |
|
|
|
— |
|
|
|
104 |
|
|
|
— |
|
|
|
54 |
|
|
|
— |
|
|
|
— |
|
|
|
54 |
|
|
Issuance of common stock under employee stock purchase plans |
|
|
— |
|
|
|
— |
|
|
|
134 |
|
|
|
— |
|
|
|
63 |
|
|
|
— |
|
|
|
— |
|
|
|
63 |
|
|
Stock-based compensation expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,695 |
|
|
|
— |
|
|
|
— |
|
|
|
1,695 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(23,331 |
) |
|
|
(23,331 |
) |
|
Unrealized gain on available-for-sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
1 |
|
|
Balance as of December 31, 2019 |
|
|
20 |
|
|
$ |
11,769 |
|
|
|
111,393 |
|
|
$ |
11 |
|
|
$ |
698,562 |
|
|
$ |
1 |
|
|
$ |
(682,800 |
) |
|
$ |
27,543 |
|
See accompanying notes to consolidated financial statements.
49
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(23,330 |
) |
|
$ |
(26,615 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Stock-based compensation expense |
|
|
1,749 |
|
|
|
2,637 |
|
|
Accretion of investment discounts and depreciation |
|
|
(70 |
) |
|
|
9 |
|
|
Amortization of debt discount and debt issuance costs |
|
|
116 |
|
|
|
192 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Prepaids and other assets |
|
|
(294 |
) |
|
|
1,063 |
|
|
Accounts payable |
|
|
(602 |
) |
|
|
(304 |
) |
|
Accrued clinical expense |
|
|
21 |
|
|
|
(267 |
) |
|
Accrued compensation |
|
|
42 |
|
|
|
(497 |
) |
|
Other accrued liabilities |
|
|
183 |
|
|
|
(622 |
) |
|
Net cash used in operating activities |
|
|
(22,185 |
) |
|
|
(24,404 |
) |
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchases of marketable securities |
|
|
(20,035 |
) |
|
|
— |
|
|
Sale and maturities of marketable securities |
|
|
3,750 |
|
|
|
4,780 |
|
|
Net cash (used in) provided by investing activities |
|
|
(16,285 |
) |
|
|
4,780 |
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from notes payable, net of issuance cost |
|
|
5,453 |
|
|
|
— |
|
|
Principal payments on notes payable |
|
|
(7,500 |
) |
|
|
— |
|
|
Proceeds from issuance of convertible preferred stock offering, net |
|
|
12,533 |
|
|
|
— |
|
|
Proceeds from issuance of common stock, net |
|
|
32,022 |
|
|
|
— |
|
|
Proceeds from issuance of common stock through controlled equity offering facilities, net |
|
|
464 |
|
|
|
6,040 |
|
|
Proceeds from exercise of stock options and stock purchase rights |
|
|
63 |
|
|
|
303 |
|
|
Net cash provided by financing activities |
|
|
43,035 |
|
|
|
6,343 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
4,565 |
|
|
|
(13,281 |
) |
|
Cash, cash equivalents and restricted cash at beginning of period |
|
|
13,696 |
|
|
|
26,977 |
|
|
Cash, cash equivalents and restricted cash at end of period |
|
$ |
18,261 |
|
|
$ |
13,696 |
|
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ |
695 |
|
|
$ |
790 |
|
|
Supplemental disclosure of non-cash investing and financing activities |
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock |
|
$ |
21,762 |
|
|
$ |
— |
|
|
Right-of-use asset obtained in exchange for lease obligation |
|
$ |
1,362 |
|
|
$ |
— |
|
|
Commitment shares issued as cost of equity financing |
|
$ |
— |
|
|
$ |
448 |
|
|
Legal expenses accrued as cost of equity financing, net of adjustments |
|
$ |
— |
|
|
$ |
39 |
|
See accompanying notes to consolidated financial statements.
50
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Company Overview
Description of Business
Sunesis Pharmaceuticals, Inc. (“Sunesis” or the “Company”) is a biopharmaceutical company focused on the development of novel targeted inhibitors for the treatment of hematologic and solid cancers. The Company’s primary activities since incorporation have been conducting research and development internally and through corporate collaborators, in-licensing and out-licensing pharmaceutical compounds and technology, conducting clinical trials, and raising capital.
The Company’s lead program is vecabrutinib, a selective non-covalent inhibitor of Bruton’s Tyrosine Kinase (“BTK”) with activity against both wild-type and C481S-mutated BTK, the most common mutation associated with resistance to covalent BTK inhibitors. Ibrutinib was the first BTK inhibitor approved for the treatment of chronic lymphocytic leukemia (“CLL”), mantle cell lymphoma (“MCL”), and other B-cell malignancies. The C481 mutation has been seen in patients who developed resistance to ibrutinib and to acalabrutinib, another covalent BTK inhibitor recently approved for treatment of CLL and MCL.
Vecabrutinib is being studied in a Phase 1b/2 clinical trial to assess safety and activity in patients with CLL and other advanced B-cell malignancies after two or more prior therapies, including ibrutinib or another covalent BTK inhibitor where approved for the disease. The Company has completed the safety evaluation period for the 400 mg cohort, and the seventh cohort, testing 500 mg twice daily, is now being studied.
The Company is developing SNS-510, a PDK1 inhibitor that it in-licensed from Millennium Pharmaceuticals, Inc. (“Takeda Oncology”), a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited. In October 2019, the Company presented the results of studies assessing SNS-510 in multiple preclinical cancer models.
In December 2019, the Company consented to Takeda Oncology’s assignment of TAK-580 to DOT Therapeutics-1, Inc. (“DOT-1”), and the Company entered into a license agreement with DOT-1 to grant DOT-1 a worldwide, exclusive license of TAK-580. Pursuant to this agreement, the Company received a $2.0 million upfront payment from DOT-1 and is eligible to receive up to $57.0 million in pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580.
In December 2019, Sumitomo Dainippon Pharma Co., Ltd. (“Sumitomo”) assigned to Sunesis worldwide rights to vosaroxin. The Company entered into an agreement to license vosaroxin to Denovo Biopharma, LLC (“Denovo”), pursuant to which Sunesis received a $200,000 upfront payment and is eligible to receive up to $57.0 million in regulatory and commercial milestones, and double-digit royalties on future sales of vosaroxin.
Liquidity and Going Concern
The Company has incurred significant losses and negative cash flows from operations since its inception, and as of December 31, 2019, had cash and cash equivalents, restricted cash, and marketable securities totaling $34.6 million and an accumulated deficit of $682.8 million.
The Company expects to continue to incur significant losses for the foreseeable future as it continues development of its kinase inhibitor pipeline, including its BTK inhibitor, vecabrutinib. The Company has prioritized development funding on its kinase inhibitor portfolio with a focus on vecabrutinib. The Company has product candidates that are still in the early stages of development and will require significant additional investment.
The Company’s cash and cash equivalents, restricted cash, and marketable securities are not sufficient to support its operations for a period of twelve months from the date these consolidated financial statements are available to be issued. These factors raise substantial doubt about its ability to continue as a going concern. The Company will require additional financing to fund working capital, repay debt and pay its obligations as they come due. Additional financing might include one or more offerings and one or more of a combination of equity securities, debt arrangements or partnership or licensing collaborations. However, there can be no assurance that the Company will be successful in acquiring additional funding at levels sufficient to fund its operations or on terms favorable to the Company. If the Company is unsuccessful in its efforts to raise additional financing in the near term, the Company will be required to significantly reduce or cease operations. The principal payments due under the SVB Loan Agreement (as defined in Note 7) have been classified as a current liability as of December 31, 2019 due to the considerations discussed above and the assessment that the material adverse change clause under the SVB Loan Agreement is not within the Company's control. The SVB
51
Loan Agreement also contains customary events of default, including among other things, the Company’s failure to make principal or interest payments when due, the occurrence of certain bankruptcy or insolvency events or its breach of the covenants under the SVB Loan Agreement. Upon the occurrence of an event of default (as defined in Note 7), SVB may, among other things, accelerate the Company’s obligations under the SVB Loan Agreement. The Company has not been notified of an event of default by SVB as of the date of the filing of this Form 10-K. The accompanying consolidated financial statements have been prepared assuming the Company will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
Concentrations of Credit Risk
In accordance with its investment policy, the Company invests cash that is not currently being used for operational purposes. The policy allows for the purchase of low risk debt securities issued by: (a) the United States and certain European governments and government agencies, and (b) highly rated banks and corporations, denominated in U.S. dollars, Euros, or British pounds, subject to certain concentration limits. The policy limits maturities of securities purchased to no longer than 24 months and the weighted average maturity of the portfolio to 12 months. Management believes these guidelines ensure both the safety and liquidity of any investment portfolio the Company may hold.
Financial instruments that potentially subject the Company to concentrations of credit risk generally consist of cash and cash equivalents, restricted cash and marketable securities. The Company is exposed to credit risk in the event of default by the institutions holding its cash and cash equivalents, restricted cash and any marketable securities to the extent of the amounts recorded in the balance sheets.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”).
Adopted Accounting Pronouncements
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230: Classification of Certain Cash Receipts and Cash Payments). This guidance addresses specific cash flow issues with the objective of reducing the diversity in practice for the treatment of these issues. The areas identified include: debt prepayment or debt extinguishment costs; settlement of zero-coupon debt instruments; contingent consideration payments made after a business combination; proceeds from the settlement of insurance claims; proceeds from the settlement of corporate-owned life insurance policies; distributions received from equity method investees; beneficial interests in securitization transactions; and application of the predominance principle with respect to separately identifiable cash flows. The guidance will generally be applied retrospectively and is effective for fiscal years beginning after December 15, 2018, and interim periods within fiscal years beginning after December 15, 2019. Early adoption is permitted. The Company adopted this ASU during the quarter ended March 31, 2019. The adoption of this ASU did not have a significant impact on its condensed financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASC 842”). ASC 842 is aimed at making leasing activities more transparent and comparable and requires substantially all leases be recognized by lessees on their balance sheet as a right-of-use asset and corresponding lease liability, including leases currently accounted for as operating leases. ASC 842 is effective for Sunesis’ interim and annual reporting periods during the year ending December 31, 2019, and all annual and interim reporting periods thereafter. In July 2018, the FASB issued ASU 2018-10, Codification Improvements to Topic 842, Leases, ASU 2018-11, Leases (Topic 842): Targeted Improvements and issued ASU 2019-01, Leases (Topic 842): Codification Improvements in March 2019. These pronouncements have the same effective date as the new leases standard and provide additional guidance, clarification and practical expedients to reduce the cost and complexity of applying the new standard. The Company adopted the new guidance on January 1, 2019 using the modified retrospective method at the effective date.
The Company has elected the package of practical expedients permitted under ASC 842. Accordingly, the Company accounted for its existing operating leases as operating leases under the new guidance, without reassessing (a) whether the contracts contain a lease under ASC Topic 842, (b) whether classification of the operating leases would be different in accordance with ASC Topic 842, or (c) whether the unamortized initial direct costs before transition adjustments would have met the definition of initial direct costs in ASC Topic 842 at lease commencement. In addition, the Company made an accounting policy election to combine the lease and non-lease components and the short-term lease practical expedients allowed under ASC 842. As a result of the adoption of ASC 842, the Company recognized on January 1, 2019 (a) a lease liability of approximately $1,362,000, which represents the present value of the remaining lease payments of approximately $1,434,000, discounted using the Company’s incremental borrowing rate of 4.0%, and (b)
52
a right-of-use (“ROU”) asset equal to the lease liability of approximately $1,362,000. Once recorded, the Company also evaluates the right-of-use asset for impairment as part of an asset group, following the principles of ASC 360, Property, Plant, and Equipment. The adoption of the new standard resulted in changes to the Company’s accounting policies for leases.
In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. The amendments in this ASU expand the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. This new guidance is effective for the Company in fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. Early adoption is permitted. On January 1, 2019, the Company adopted this new guidance and the measurement of equity-classified nonemployee awards will be fixed at the grant date. Upon adoption, the Company applied the new guidance to equity-classified nonemployee awards for which a measurement date has not been established and compared the cumulative amounts that were recorded for its nonemployee share-based payments through the end of December 31, 2018 to the cumulative amounts that should be recognized at the adoption date to calculate the transition adjustment. On January 1, 2019, the Company recognized the transition adjustment as an adjustment to retained earnings, which had no material impact on the Company’s consolidated financial statements or related footnote disclosures.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments, which will require a reporting entity to use a new forward-looking impairment model for most financial assets that generally will result in the earlier recognition of allowances for losses. For available-for-sale debt securities with unrealized losses, credit losses will be recognized as allowances rather than as reductions in amortized cost. Entities will apply the guidance as a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is adopted. In April 2019, the FASB issued ASU 2019-04, Codification Improvements to Topic 326, Financial Instruments—Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments, to increase stakeholders’ awareness of the amendments and to expedite improvements to the Codification. In May 2019, the FASB issued ASU 2019-05, Financial Instruments—Credit Losses, Topic 326, providing an option to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost basis. These ASUs do not change the core principle of the guidance in ASU 2016-13. Instead these amendments are intended to clarify and improve operability of certain topics. In November 2019, FASB issued ASU 2019-10, Financial Instruments—Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842): Effective Dates and ASU 2019-11, Codification Improvements to Topic 326, Financial Instruments—Credit Losses. ASC 2019-10 defers the effective dates of the new credit losses standard for all entities except SEC filers that are not smaller reporting companies to fiscal years beginning after 15 December 2022, including interim periods within those fiscal years. ASU 2019-11 clarifies and addresses stakeholders’ specific issues about certain aspects of the amendments in ASU 2016-13. The standard will be effective for the Company for annual periods beginning after December 15, 2022, with early adoption permitted beginning in 2019. The Company does not expect the adoption of this standard will have a material impact on its financial statements and accompanying footnotes.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement. The amendments in this ASU modify the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement. Various disclosure requirements have been removed, including the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, the policy for timing of transfers between levels, the valuation processes for Level 3 fair value measurements held at the end of the reporting period. The ASU also modified various disclosure requirements and added some disclosure requirements for Level 3 fair value measurements. The amendments in this ASU are effective for the Company on January 1, 2020. The additional disclosures on changes in unrealized gains and losses, the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements, and the narrative description of measurement uncertainty should be applied prospectively for only the most recent interim or annual period presented in the initial fiscal year of adoption. All other amendments should be applied retrospectively to all periods presented upon their effective date. An entity is permitted to early adopt any removed or modified disclosures upon issuance of this ASU and delay adoption of the additional disclosures until their effective date. The Company does not expect the adoption of this ASU will have a material impact on its financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. The amendments in this ASU simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of and simplify GAAP for other areas of Topic 740 by clarifying and amending existing guidance. The amendments in this ASU are effective for the Company on January 1, 2021. The Company is currently evaluating the impact that the adoption of ASU 2019-12 will have on its financial statements and accompanying footnotes.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, Sunesis Europe Limited, a United Kingdom corporation, and Sunesis Pharmaceuticals (Malta) Ltd., a Malta corporation. All intercompany balances and transactions have been eliminated in consolidation.
53
Management has determined that the Company operates as a single reportable segment.
Significant Estimates and Judgments
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the Company’s consolidated financial statements and accompanying notes thereto. Actual results could differ materially from these estimates. Estimates, assumptions and judgments made by management include those related to the valuation of marketable securities, equity and related instruments, revenue recognition, stock-based compensation and clinical trial accounting.
Cash Equivalents and Marketable Securities
The Company considers all highly liquid securities with original maturities of three months or less from the date of purchase to be cash equivalents, which generally consist of money market funds, repurchase agreements, and corporate debt securities. Restricted cash consists of amounts pledged as collateral for term loan agreement as contractually required by the lender. Marketable securities consist of securities with original maturities of greater than three months, which may include U.S. and European government obligations and corporate debt securities.
Management determines the appropriate classification of securities at the time of purchase and reevaluates such designation at each balance sheet date. The Company generally classifies its entire investment portfolio as available-for-sale. The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company classifies all investments as short-term, even though the stated maturity may be more than one year from the current balance sheet date. Available-for-sale debt securities are carried at fair value, with unrealized gains and losses reported in accumulated other comprehensive income (loss), which is a separate component of stockholders’ equity except for unrealized loss determined to be other than temporary, which would be recorded within Other income, net.
The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion is included in other income, net in the statements of operations and comprehensive loss. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities, if any, are also recorded to other income, net. The cost of securities sold is based on the specific-identification method.
Invoices for certain services provided to the Company are denominated in foreign currencies. To manage the risk of future movements in foreign exchange rates that would affect such amounts, the Company may purchase certain European currencies or highly-rated investments denominated in those currencies, subject to similar criteria as for other investments defined in the Company’s investment policy. There is no guarantee that the related gains and losses will substantially offset each other, and the Company may be subject to significant exchange gains or losses as currencies fluctuate from quarter to quarter. As of December 31, 2019 and December 31, 2018, the Company held investments denominated in Euros with an aggregate fair value of zero, and $0.8 million, respectively. Any cash, cash equivalent and short-term investment balances denominated in foreign currencies are recorded at their fair value based on the current exchange rate as of each balance sheet date. The resulting exchange gains or losses and those from amounts payable for services originally denominated in foreign currencies are both recorded in other income, net in the statements of operations and comprehensive loss.
Fair Value Measurements
The Company measures cash equivalents and marketable securities at fair value on a recurring basis using the following hierarchy to prioritize valuation inputs, in accordance with applicable GAAP:
|
|
Level 1 - |
Observable input such as quoted prices (unadjusted) in active markets for identical assets and liabilities that can be accessed at the measurement date; |
|
|
Level 2 - |
inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly for the asset or liability. These include quoted prices for similar assets or liabilities in active markets; and |
|
|
Level 3 - |
unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s Level 2 valuations of marketable securities are generally derived from independent pricing services based upon quoted prices in active markets for similar securities, with prices adjusted for yield and number of days to maturity, or based on industry models using data inputs, such as interest rates and prices that can be directly observed or corroborated in active markets.
54
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3, if any. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The carrying amounts of the Company’s financial instruments, including cash, prepayments, accounts payable, accrued liabilities, deferred revenue, and notes payable approximated their fair value as of December 31, 2019 and December 31, 2018.
Leases
The Company determines if an arrangement is or contains a lease at inception. In determining whether an arrangement is a lease, the Company considers whether (1) explicitly or implicitly identified assets have been deployed in the arrangement and (2) the Company obtains substantially all of the economic benefits from the use of that underlying asset and directs how and for what purpose the asset is used during the term of the contract.
ROU assets represent the Company’s right to use an underlying asset for the lease term, and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized based on the present value of lease payments over the lease term. When an implicit rate is not readily determinable, the Company uses its incremental borrowing rate based on the information available at commencement date for new leases or effective date for existing leases, in determining the present value of lease payments.
Leases may contain initial periods of free rent and/or periodic escalations. When such items are included in a lease agreement, the Company records rent expense on a straight-line basis over the initial term of a lease. The difference between the rent payment and the straight-line rent expense is recorded as a deferred rent liability. The Company expenses any additional payments under its operating leases for taxes, insurance or other operating expenses as incurred
Accounting for Notes Payable
The accounting for certain fees and expenses related to the SVB Loan Agreement (see Note 8) is as follows. The debt issuance cost is being accounted for as a debt discount and classified within notes payable on the Company’s balance sheet. The debt discount is being amortized as interest expense over the term of the loan using the effective interest method. The final payment is being accreted as interest expense over the term of the loans using the effective interest method.
Revenue Recognition
On January 1, 2018, the Company adopted Topic 606, Revenue from Contracts with Customers (“Topic 606”) using the modified retrospective method applied to those contracts which were not completed as of January 1, 2018. Results for reporting periods beginning after January 1, 2018 are presented under Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with the Company’s historic accounting under Topic 605, Revenue Recognition (“Topic 605”).
Adoption of the new standard did not result in any change to the Company’s opening retained earnings as of January 1, 2018 as no cumulative impact to the adoption of ASC 606 was noted as a result of the Company’s assessment of the comparative revenue recognized since inception of the contracts under the new revenue standard ASC 606 and historic standard ASC 605. The Company is applying the practical exemption allowed under ASC 606 and does not disclose the value of variable consideration that is a sale-based royalty promised in exchange for a license of intellectual property. The adoption of the new standard resulted in changes to the Company’s accounting policies for revenue recognition as detailed below:
The Company’s contracts consist license, milestone and royalty payments primarily generated through agreements with strategic partners for the development and commercialization of the Company’s product candidates. The terms of the agreement typically include non-refundable upfront fees, payments based upon achievement of milestones and royalties on net product sales. The Company has both fixed and variable consideration. Non-refundable upfront fees are considered fixed, while milestone and royalty payments are identified as variable consideration.
In determining the appropriate amount of revenue to be recognized as it fulfills its performance obligations under its agreements, the Company performs the following steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations based on estimated selling prices; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation.
55
Licenses of intellectual property: If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenues from non-refundable, up-front fees allocated to the license when the license is transferred to the customer, and the customer can use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Event-based or milestone payments: At the inception of each arrangement that includes event-based or milestone payments, the Company evaluates whether the events or milestones are considered probable of being achieved and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the value of the associated event-based or milestone payments is included in the transaction price. Event-based or milestone payments that are not within the control of the Company are not included in the transaction price until they become probable of being achieved.
Royalties: For arrangements that include sales-based royalties and the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (a) when the related sales occur, or (b) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any royalty revenue resulting from any of its licensing arrangements.
Research and Development
Research and development expense consists primarily of: (a) clinical trial costs, which include payments for work performed by contract research organizations (“CROs”), clinical trial sites, labs and other clinical service providers, and for drug packaging, storage and distribution; (b) drug manufacturing costs, which include costs for producing drug substance and drug product, and for stability and other testing; (c) personnel costs for related permanent and temporary employees; (d) other outside services and consulting costs; and (e) payments under license agreements. All research and development costs are expensed as they are incurred.
Clinical Trial Accounting
The Company records accruals for estimated clinical trial costs, which include payments for work performed by CROs and participating clinical trial sites. These costs are generally a significant component of research and development expense. Costs incurred for setting up clinical trial sites for participation in trials are generally non-refundable, and are expensed as incurred, with any refundable advances related to enrollment of the first patient recorded as prepayments and assessed for recoverability on a quarterly basis. Costs related to patient enrollment are accrued as patients progress through the clinical trial, including amortization of any first-patient prepayments. This amortization generally matches when the related services are rendered, however, these cost estimates may or may not match the actual costs incurred by the CROs or clinical trial sites, and if the Company has incomplete or inaccurate information, the clinical trial accruals may not be accurate. The difference between accrued expenses based on the Company’s estimates and actual expenses have not been significant to date.
Warrants for Shares of Common Stock
The Company accounts for warrants for shares of common stock as equity instruments in the accompanying balance sheets at their fair value on the date of issuance because such warrants are indexed to the Company’s common stock and no cash settlement is required except for (i) liquidation of the Company, or (ii) a change in control in which the common stockholders also receive cash.
Stock-Based Compensation
The Company grants options to purchase common stock to its employees, directors and consultants under its stock option plans. Under the Company’s Employee Stock Purchase Plan, eligible employees can also purchase shares of the Company’s common stock at 85% of the lower of the fair market value of the Company’s common stock at the beginning of a 12-month offering period or at the end of one of the two related six-month purchase periods.
The Company values these share-based awards using the Black-Scholes option valuation model (the “Black-Scholes model”). The determination of fair value of share-based payment awards on the date of grant using the Black-Scholes model is affected by the Company’s stock price as well as assumptions regarding a number of subjective variables. These variables include, but are not limited to, the expected stock price volatility over the term of the awards and actual and projected employee stock option exercise behaviors. The Company accounts for forfeitures of share-based payment awards as they occur.
56
Transactions that are denominated in a foreign currency are translated into U.S. dollars at the current exchange rate on the transaction date. Any foreign currency-denominated monetary assets and liabilities are subsequently remeasured at current exchange rates as of each balance sheet date, with gains or losses on foreign exchange recognized in other income, net in the statements of operations and comprehensive loss.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the differences between the tax basis of assets and liabilities and their basis for financial reporting. Deferred tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. The Company’s policy is to recognize interest charges and penalties in other income, net in the statements of operations and comprehensive loss.
3. Loss per Common Share
Basic loss per common share is calculated by dividing net loss by the weighted-average number of common shares outstanding for the period. Diluted net loss per common share is computed by dividing (a) net loss, less any anti-dilutive amounts recorded during the period, by (b) the weighted-average number of common shares outstanding for the period plus dilutive potential common shares as determined using the treasury stock method for options and warrants to purchase common stock.
The following table sets forth the computation of basic and diluted loss per common share for the periods presented (in thousands, except per share amounts):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Numerator: |
|
|
|
|
|
|
|
|
|
Net loss—basic and diluted |
|
$ |
(23,330 |
) |
|
$ |
(26,615 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding—basic and diluted |
|
|
87,129 |
|
|
|
35,582 |
|
|
Net loss per common share: |
|
|
|
|
|
|
|
|
|
Basic and Diluted |
|
$ |
(0.27 |
) |
|
$ |
(0.75 |
) |
The following table represents the potential common shares issuable pursuant to outstanding securities as of the related period end dates that were excluded from the computation of diluted loss per common share because their inclusion would have had an anti-dilutive effect (in thousands):
|
|
|
As of December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Warrants to purchase shares of common stock |
|
|
218 |
|
|
|
218 |
|
|
Convertible preferred stock |
|
|
19,714 |
|
|
|
6,331 |
|
|
Options to purchase shares of common stock |
|
|
5,156 |
|
|
|
4,160 |
|
|
Outstanding securities not included in calculations |
|
|
25,088 |
|
|
|
10,709 |
|
57
Financial Assets
The following tables summarize the estimated fair value of the Company’s financial assets measured on a recurring basis as of the dates indicated, which were comprised solely of available-for-sale marketable securities with remaining contractual maturities of one year or less (in thousands):
|
|
|
|
|
|
|
|
|
Gross |
|
|
Gross |
|
|
|
|
|
||
|
|
|
Valuation |
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
Estimated Fair |
|
||||
|
December 31, 2019 |
|
Input Level |
|
Cost |
|
|
Gains |
|
|
Losses |
|
|
Value |
|
||||
|
Money market funds |
|
Level 1 |
|
$ |
3,495 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,495 |
|
|
U.S. Treasury securities |
|
Level 1 |
|
|
1,594 |
|
|
|
1 |
|
|
|
— |
|
|
|
1,595 |
|
|
Repurchase agreements |
|
Level 2 |
|
|
5,000 |
|
|
|
— |
|
|
|
— |
|
|
|
5,000 |
|
|
U.S. corporate debt obligations |
|
Level 2 |
|
|
5,155 |
|
|
|
— |
|
|
|
— |
|
|
|
5,155 |
|
|
U.S. commercial paper |
|
Level 2 |
|
|
11,412 |
|
|
|
— |
|
|
|
— |
|
|
|
11,412 |
|
|
Total available-for-sale securities |
|
|
|
|
26,656 |
|
|
|
1 |
|
|
|
— |
|
|
|
26,657 |
|
|
Less amounts classified as cash equivalents |
|
|
|
|
(10,293 |
) |
|
|
— |
|
|
|
— |
|
|
|
(10,293 |
) |
|
Amounts classified as marketable securities |
|
|
|
$ |
16,363 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
16,364 |
|
|
|
|
|
|
|
|
|
|
Gross |
|
|
Gross |
|
|
|
|
|
||
|
|
|
Valuation |
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
Estimated Fair |
|
||||
|
December 31, 2018 |
|
Input Level |
|
Cost |
|
|
Gains |
|
|
Losses |
|
|
Value |
|
||||
|
Money market funds |
|
Level 1 |
|
$ |
10,845 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
10,845 |
|
|
Total available-for-sale securities |
|
|
|
|
10,845 |
|
|
|
— |
|
|
|
— |
|
|
|
10,845 |
|
|
Less amounts classified as cash equivalents |
|
|
|
|
(10,845 |
) |
|
|
— |
|
|
|
— |
|
|
|
(10,845 |
) |
|
Amounts classified as marketable securities |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
There were no available-for-sale securities in an unrealized loss position as of December 31, 2019.
No significant facts or circumstances have arisen to indicate that there has been any deterioration in the creditworthiness of the issuers of these securities. There were no realized gains or losses on the available-for-sale securities in the years ended December 31, 2019 and 2018. There were no sales of available-for-sale debt securities in the years ended December 31, 2019 and 2018.
5. Other Accrued Liabilities
Other accrued liabilities as of December 31 were as follows (in thousands):
|
|
|
2019 |
|
|
2018 |
|
||
|
Accrued outside services |
|
$ |
690 |
|
|
$ |
556 |
|
|
Accrued professional services |
|
|
220 |
|
|
|
251 |
|
|
Accrued interest |
|
|
57 |
|
|
|
284 |
|
|
Deferred revenue |
|
|
120 |
|
|
|
— |
|
|
Other accruals |
|
|
22 |
|
|
|
— |
|
|
Total other accrued liabilities |
|
$ |
1,109 |
|
|
$ |
1,091 |
|
58
Biogen Idec
The first amended and restated collaboration agreement with Biogen Idec MA, Inc. (the “Biogen 1st ARCA”) amended and restated the collaboration agreement with Biogen (the “Biogen OCA”), to provide for the discovery, development and commercialization of small molecule BTK inhibitors. Under this agreement, the Company no longer has research obligations, but licenses granted to Biogen with respect to the research collaboration under the Biogen OCA (other than the licenses transferred to Takeda Oncology under the Takeda Agreement) remain in effect. In December 2018, the Company entered into a settlement agreement with Biogen whereas Biogen will no longer be obligated to pay future event-based payments or royalty payments to the Company.
In December 2013, the Company entered into a second amended and restated collaboration agreement with Biogen, to provide the Company with an exclusive worldwide license to develop, manufacture and commercialize vecabrutinib, a BTK inhibitor synthesized under the Biogen 1st ARCA, solely for oncology indications. During the third quarter of 2017, the Company made a milestone payment of $2.5 million to Biogen upon the dosing of the first patient in a Phase 1b/2 study to assess the safety and activity of vecabrutinib in patients with advanced B-cell malignancies after two or more prior therapies, including ibrutinib or other covalent BTK inhibitor for those patients with malignancies for which a BTK inhibitor is approved, and including patients with BTK C481 mutations. The payment was recorded in the research and development expenses line item in the consolidated statement of operations. The Company may also be required to make tiered royalty payments based on percentages of net sales of vecabrutinib, if any, in the mid-single-digits.
Takeda Oncology
In March 2011, Takeda Oncology purchased and exclusively licensed Biogen’s rights to a PDK1 inhibitor program and a pan-Raf inhibitor program which were both originally developed through a collaboration agreement between Sunesis and Biogen. In January 2014, the Company entered into an amended and restated license agreement with Takeda Oncology (the “Amended Takeda Agreement”), to provide the Company with an exclusive worldwide license to develop and commercialize preclinical inhibitors of PDK1. In December 2019, the Company partitioned the Amended Takeda Agreement into two separate agreements: (i) an amended and restated license agreement for PDK (the “PDK Agreement”), and (ii) an amended and restated license agreement for RAF (the “Millennium RAF Agreement”). Pursuant to the PDK Agreement, the Company may in the future be required to make up to $9.2 million in pre-commercialization milestone payments depending on its development of PDK1 inhibitors and tiered royalty payments depending on related product sales, if any, beginning in the mid-single-digits and not to exceed the low-teens.
DOT-1
In December 2019, Takeda Oncology assigned the Millennium RAF Agreement to DOT-1, a venture capital-funded biopharmaceutical company. The Company entered into a concurrent license agreement with DOT-1. Pursuant to this agreement, the Company received a $2.0 million upfront payment from DOT-1 to grant DOT-1 a worldwide, exclusive license of TAK-580. The agreement also includes up to $57.0 million in pre-commercialization, event-based milestone payments and royalty payments on future sales of TAK-580. The Company recognized the $2.0 million upfront payment as revenue upon inception of the contract as identified performance obligation has been satisfied. As of December 31, 2019, all future event-based payments and royalty payments are considered fully constrained variable considerations and therefore, no contract assets have been recorded and no revenue have been recognized on these variable considerations.
Denovo
In December 2019, the Company entered into the Denovo License Agreement, pursuant to which Sunesis licensed vosaroxin intellectual property to Denovo, received an upfront payment of $0.2 million, and is eligible to receive up to $57.0 million in regulatory and commercial milestones payments and double-digit royalty payments on future sales of vosaroxin. The Company recognized $0.1 million of the upfront payment as revenue in 2019 when the identified performance obligation has been satisfied and the remaining $0.1 million has been recorded as deferred revenue as part of other accrued liabilities on the Company’s consolidated balance sheet as of December 31, 2019. As of December 31, 2019, all future event-based payments and royalty payments are considered fully constrained variable considerations and therefore, no contract assets have been recorded and no revenue have been recognized on these variable considerations.
59
In April 2019, the Company entered into a term loan agreement with Silicon Valley Bank (the “SVB Loan Agreement”), pursuant to which the Company borrowed $5.5 million. The Company used the proceeds of the SVB Loan Agreement plus cash on hand to repay its remaining obligations in the amount of $5.9 million under its existing loan agreement with Western Alliance Bank and Solar Capital Ltd (the “Loan Agreement and Amendments”). The maturity date of the SVB Loan Agreement is December 1, 2022. Under the terms of the SVB Loan Agreement, the Company is required to make interest-only payments through December 31, 2020 on the borrowings at a floating rate equal to the greater of 3.25% or the Prime Rate as defined in the SVB Loan Agreement minus 2.25%, followed by an amortization period of 24 months of equal monthly payments of principal plus interest amounts until paid in full. In addition to and not in substitution for the regular monthly payments of principal plus accrued interest, the Company is required to make a final payment equal to 4% of the original principal amount of the borrowings (the “Final Payment Fee”). Additionally, the Company may prepay all, but not less than all, of the borrowings at any time upon 30 days’ prior notice to Silicon Valley Bank (“SVB”). Any such prepayment would require, in addition to payment of principal and accrued interest as well as the Final Payment Fee, a prepayment fee, in the amount of (a) $165,000 if the prepayment occurs prior to the 1st anniversary of April 26, 2019, or the Effective Date; (b) $110,000 if the prepayment occurs on or after the 1st anniversary of the Effective Date but prior to the 2nd anniversary of the Effective Date; or (c) $55,000 if the prepayment occurs on or after the 2nd anniversary of the Effective Date.
The Company’s obligations under the SVB Loan Agreement are secured by a first priority security interest in cash held at an account with SVB (the “Collateral Account”). The Company is obligated to maintain sufficient cash in the Collateral Account at all times in an amount equal to or greater than the outstanding balance of the borrowings. The Company has classified the Collateral Account as restricted cash on its consolidated balance sheets as of December 31, 2019.
The SVB Loan Agreement contains customary affirmative and negative covenants which, among other things, limit the Company’s ability to (i) incur additional indebtedness, (ii) pay dividends or make certain distributions, (iii) dispose of its assets, grant liens or encumber its assets or (iv) fundamentally alter the nature of its business. These covenants are subject to a number of exceptions and qualifications. The SVB Loan Agreement also contains customary events of default, including among other things, the Company’s failure to make any principal or interest payments when due, the occurrence of certain bankruptcy or insolvency events or a material adverse change, or its breach of the covenants under the SVB Loan Agreement. Upon the occurrence of an event of default, SVB may, among other things, accelerate the Company’s obligations under the SVB Loan Agreement. The Company was in compliance with all applicable covenants set forth in the SVB Loan Agreement as of December 31, 2019. The principal payments due under the SVB Loan Agreement have been classified as a current liability at December 31, 2019 due to the considerations discussed in Note 1 and the assessment that the material adverse change clause under the SVB Loan Agreement is not within the Company's control. The Company has not been notified of an event of default by the Lenders as of the date of the filing of this Annual Report Form 10-K.
Aggregate future minimum payments due under the SVB Loan Agreement as of December 31, 2019 were as follows (in thousands):
|
Year ending December 31, |
|
Total |
|
|
|
2020 |
|
|
179 |
|
|
2021 |
|
|
2,888 |
|
|
2022 |
|
|
3,018 |
|
|
Total minimum payments |
|
|
6,085 |
|
|
Less amount representing interest |
|
|
(585 |
) |
|
Total notes payable as of December 31, 2019 |
|
|
5,500 |
|
|
Less unamortized debt discount and issuance costs |
|
|
(35 |
) |
|
Less current portion of notes payable |
|
|
(5,465 |
) |
|
Non-current portion of notes payable |
|
$ |
— |
|
8. Commitments and Contingencies
From time to time, the Company may be involved in legal proceedings, as well as demands, claims and threatened litigation, which arise in the normal course of its business or otherwise. The ultimate outcome of any litigation is uncertain and unfavorable outcomes could have a negative impact on the Company’s results of operations and financial condition. Regardless of outcome, litigation can have an adverse impact on the Company because of the defense costs, diversion of management resources and other factors. The Company is not currently involved in any material legal proceedings.
60
Underwritten Offerings
In July 2019, the Company completed underwritten public offerings of (i) 38,333,717 shares of its common stock at a price to the public of $0.60 for each share of common stock, including the full exercise of the underwriters’ option to purchase 5,000,050 additional shares of common stock to cover over-allotments, and (ii) 8,333 shares of its non-voting Series F Convertible Preferred Stock (“Series F Stock”) at a price to the public of $600.00 for each share of Series F Stock. Gross proceeds from the sale were approximately $28.0 million and net proceeds were approximately $26.1 million. Each share of non-voting Series F Stock is convertible into 1,000 shares of the Company’s common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would own more than 9.98% of the total number of shares of the Company’s common stock then outstanding; provided, however, that a holder may, upon written notice, elect to increase or decrease this percentage (not to exceed the limits under Nasdaq Marketplace Rule 5635(b), to the extent applicable).
In January 2019, the Company completed underwritten public offerings of (i) 23,000,000 shares of its common stock at a price to the public of $0.50 for each share of common stock, and (ii) 17,000 shares of its non-voting Series E Convertible Preferred Stock (“Series E Stock”) at a price to the public of $500.00 for each share of Series E Stock. Gross proceeds from the sale were $20.0 million and net proceeds were approximately $18.5 million. Each share of non-voting Series E Stock is convertible into 1,000 shares of the Company’s common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would own more than 9.98% of the total number of shares of the Company’s common stock then outstanding; provided, however, that a holder may, upon written notice, elect to increase or decrease this percentage (not to exceed the limits under Nasdaq Marketplace Rule 5635(b), to the extent applicable).
Preferred Stock
The Company has 10,000,000 shares of authorized preferred stock available for issuance in one or more series. Upon issuance, the Company can determine the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. There were 19,714 and 17,697 shares of preferred stock outstanding as of December 31, 2019 and 2018, respectively. These shares are non-voting Series D, Series E, and Series F Convertible Preferred Stock at a price of $2,000, $500, and $600 per share, respectively. Each share of non-voting Series D Stock, Series E Stock, and Series F Stock is convertible into 1,000 shares of common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would own more than 9.98% of the total number of shares of common stock then outstanding. In the event of the Company’s liquidation, dissolution, or winding up, holders of Series D, Series E, and Series F Stock will receive a payment equal to $0.0001 per share of Series D, Series E, and Series F Stock before any proceeds are distributed to the holders of Common Stock. Shares of Series D, Series E, and Series F Stock will generally have no voting rights, except as required by law and except that the consent of holders of a majority of the outstanding Series D and Series E Stock will be required to amend the terms of the Series D, Series E, and Series F Stock. Shares of the Series D, Series E, and Series F Stock will not be entitled to receive any dividends, unless and until specifically declared by the Company’s board of directors, and will rank:
|
|
• |
senior to all of the Company’s Common Stock; |
|
|
• |
senior to any class or series of the Company’s capital stock hereafter created specifically ranking by its terms junior to the Series D, Series E, and Series F Stock; |
|
|
• |
on parity with any class or series of the Company’s capital stock hereafter created specifically ranking by its terms on parity with the Series D, Series E, and Series F Stock; |
|
|
• |
junior to any class or series of the Company’s capital stock hereafter created specifically ranking by its terms senior to the Series D, Series E, and Series F Stock; in each case, as to distributions of assets upon the Company’s liquidation, dissolution or winding up whether voluntarily or involuntarily. |
During the year ended December 31, 2019, the Company issued a total of 11,950,165 shares of its common stock upon conversion of 13,639 shares of its non-voting Series B Convertible Preferred Stock, 1,558 shares of its non-voting Series C Convertible Preferred Stock, 1,119 shares of its Series D Stock, and 7,000 shares of Series E Stock. No shares of non-voting Series B or Series C Convertible Preferred Stock remain outstanding after the conversion. 1,381 shares of Series D Stock, 10,000 shares of Series E Stock, and 8,333 shares of Series F Stock remain outstanding after the conversion.
Common Stock
Holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders of the Company. Subject to the preferences that may be applicable to any outstanding shares of preferred stock, the holders of common stock are
61
entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors. Under the terms of the Loan Agreement with the Lenders, the Company is precluded from paying cash dividends without the prior written consent of the Lenders.
Controlled Equity Offerings
In August 2011, the Company entered into a Controlled Equity OfferingSM sales agreement (the “Sales Agreement”), with Cantor Fitzgerald & Co. (“Cantor”), as agent and/or principal, pursuant to which the Company could issue and sell shares of its common stock. The most recent amendment to the Sales Agreement, made in November, 2017, provides for an increase in the aggregate gross sales under the Sales Agreement to $45.0 million. The Company will pay Cantor a commission of up to 3.0% of the gross proceeds from any common stock sold through the Sales Agreement, as amended.
During the years ended December 31, 2019 and 2018, the Company sold 0.4 million shares and 0.6 million shares, respectively, of common stock under the Sales Agreement, as amended, at an average price of approximately $1.19 per share and $2.38 per share, respectively, for gross and net proceeds of $0.5 million and $1.4 million, respectively, after deducting Cantor’s commission. As of December 31, 2019, $43.1 million of common stock remained available to be sold under this facility.
Aspire Common Stock Purchase Agreement
In June 2018, the Company entered into a Common Stock Purchase Agreement (the “CSPA”) with Aspire Capital Fund, LLC (“Aspire”), pursuant to which the Company could issue and sell shares of its common stock having an aggregate gross sales price of up to $15.5 million. Upon execution of the CSPA, the Company sold to Aspire 228,311 shares of common stock at a price of $2.19 per share, for total proceeds of $0.5 million. In addition, Aspire committed to purchasing up to an additional $15.0 million of common shares, at the Company’s request, from time to time during a 24-month period at prices based on the market price at the time of each sale. Under the CSPA, on any trading day selected by the Company on which the closing price of its common stock is equal to or greater than $0.25 per share, the Company has the right, in its sole discretion, to present Aspire with a purchase notice directing Aspire to purchase up to 200,000 shares of common stock per business day, at a purchase price equal to the lesser of:
|
|
a) |
the lowest sale price of common stock on the purchase date; or |
|
|
b) |
the arithmetic average of the three lowest closing sale prices during the 10 consecutive business days ending on the trading day immediately preceding the purchase date. |
The Company also has the right to require Aspire to purchase up to an additional 30% of the trading volume of the shares for the next business day at a purchase price (the “VWAP Purchase Price”), equal to the lesser of: (i) the closing sale price of the shares on the purchase date, or (ii) ninety-seven percent (97%) of the next business day’s volume weighted average price (each such purchase, a “VWAP Purchase”). The Company shall have the right, in its sole discretion, to determine a maximum number of shares and set a minimum market price threshold for each VWAP Purchase. The Company can only require a VWAP Purchase if the Company has also submitted a regular purchase on the notice date for the VWAP Purchase. There are no limits on the number of VWAP purchases that the Company may require.
There are no trading volume requirements or restrictions under the CSPA, and the Company will control the timing and amount of sales. Aspire has no right to require any sales by the Company, but is obligated to make purchases from the Company as directed by the Company in accordance with the CSPA There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. The CSPA may be terminated by the Company at any time, at its discretion, without any cost to the Company. Aspire has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of common stock during any time prior to the termination of the CSPA. Any proceeds from the Company receives under the CSPA are expected to be used for working capital and general corporate purposes. The Company cannot request Aspire to purchase more than 2,000,000 shares per business day.
As consideration for Aspire’s obligation under the CSPA, the Company issued 212,329 shares of common stock to Aspire as a commitment fee. This $0.4 million commitment fee and $0.1 million in other transaction costs were recorded in June 2018 as costs of equity financing, within additional paid-in capital. The Company also entered into a Registration Rights Agreement with Aspire. During the year ended December 31, 2018, the Company issued to Aspire a total of 2,390,640 shares for total net proceeds of $4.6 million. The shares were issued at an average price of $2.20 per share, excluding the 212,329 commitment shares issued. No shares were issued to Aspire during the year ended December 31, 2019. Aspire’s remaining purchase commitment was $10.9 million as of December 31, 2019.
62
The Company grants options to purchase shares of its common stock primarily to: (i) new employees, of which 25% of the shares subject to such options become exercisable on the first anniversary of the vesting commencement date, and 1/48th of the shares subject to such options become exercisable each month over the remainder of the four-year vesting period, (ii) existing employees with various vesting schedules over three to four years, (iii) new non-employee members of the board of directors, of which 1/24th of the shares subject to such options become exercisable each month following the date of grant over a two-year vesting period, and (iv) continuing non-employee members of the board of directors, of which 1/12th of the shares subject to such options become exercisable each month following the date of grant over a one-year vesting period.
On March 15, 2011, the Company’s Board of Directors adopted, and on June 3, 2011, the Company’s stockholders approved, the 2011 Equity Incentive Plan (the “2011 Plan”). The 2011 Plan is intended as the successor to and continuation of the Company’s 1998 Stock Plan, 2001 Stock Plan, 2005 Equity Incentive Award Plan and 2006 Employment Commencement Incentive Plan (collectively, the “Prior Plans”). No additional stock awards will be granted under the Prior Plans.
The number of shares of common stock available for issuance under the 2011 Plan automatically increases on January 1st of each year for a period of 10 years commencing on January 1, 2012 by an amount equal to: (i) 4.0% of the Company’s outstanding shares of common stock on December 31st of the preceding calendar year, or (ii) a lesser amount determined by the Board of Directors. On January 1, 2019 and 2018, in accordance with the above, the number of shares of common stock available for issuance under the 2011 Plan was increased by 1,498,960 and 1,371,308 shares, respectively.
During the year ended December 31, 2019, options to purchase 1,715,518 shares of the Company’s common stock were granted under the 2011 Plan. As of December 31, 2019, there were 1,154,817 shares available for future grants under the 2011 Plan.
Employee Stock Purchase Plans
On March 5, 2011, the Company’s Board of Directors adopted, and on June 3, 2011, the Company’s stockholders approved, the 2011 Employee Stock Purchase Plan (the “2011 ESPP”).
The 2011 ESPP permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. Eligible employees can purchase shares of the Company’s common stock at 85% of the lower of the fair market value of the common stock at (i) the beginning of a 12-month offering period, or (ii) at the end of one of the two related 6-month purchase periods. No participant in the 2011 ESPP may be issued or transferred shares of common stock valued at more than $25,000 per calendar year.
The number of shares of common stock available for issuance under the 2011 ESPP automatically increases on January 1st of each year for a period of 10 years commencing on January 1, 2012 by an amount equal to: (i) 1.0% of the Company’s outstanding shares of common stock on December 31st of the preceding calendar year, or (ii) a lesser amount determined by the Board of Directors. On January 1, 2019, in accordance with the above, the number of shares of common stock available for issuance under the 2011 ESPP was increased by 336,000 shares.
A total of 133,322 and 104,099 shares were issued under the 2011 ESPP during the year ended December 31, 2019 and December 31, 2018, respectively. As of December 31, 2019, there were 266,983 shares available for future issuance under the ESPP.
Warrants
Warrants to purchase shares of the Company’s common stock outstanding as of December 31, 2019 were as follows (in thousands, except per share amounts):
|
|
|
|
|
|
|
Exercise Price |
|
|
|
|
|
Date Issued |
|
Shares |
|
|
Per Share |
|
|
Expiration |
||
|
February 2015 |
|
|
10 |
|
|
$ |
13.32 |
|
|
February 2020 |
|
March 2016 |
|
|
208 |
|
|
$ |
3.25 |
|
|
March 2021 |
|
Total warrants outstanding and exercisable |
|
|
218 |
|
|
|
|
|
|
|
Warrants to purchase 10,245 shares of the Company’s common stock expired unexercised as of February 27, 2020.
63
Shares of the Company’s common stock reserved for future issuance as of December 31, 2019 were as follows (in thousands):
|
|
|
Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
Available |
|
|
|
|
|
|
Total |
|
||
|
|
|
for Future |
|
|
Outstanding |
|
|
Shares |
|
|||
|
|
|
Grant |
|
|
Securities |
|
|
Reserved |
|
|||
|
Warrants |
|
|
— |
|
|
|
218 |
|
|
|
218 |
|
|
Convertible preferred stock |
|
|
— |
|
|
|
19,714 |
|
|
|
19,714 |
|
|
Stock option plans |
|
|
1,155 |
|
|
|
5,156 |
|
|
|
6,311 |
|
|
Employee stock purchase plan |
|
|
267 |
|
|
|
— |
|
|
|
267 |
|
|
Total reserved shares of common stock |
|
|
1,422 |
|
|
|
25,088 |
|
|
|
26,510 |
|
10. Stock-Based Compensation
Overview
Employee stock-based compensation expense is calculated based on the grant-date fair value of awards ultimately expected to vest and recognized under the straight-line attribution method, assuming that all stock-based awards will vest. The following table summarizes stock-based compensation expense related to the Company’s stock-based awards for the periods indicated (in thousands):
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Research and development |
|
$ |
513 |
|
|
$ |
581 |
|
|
General and administrative |
|
|
816 |
|
|
|
903 |
|
|
Employee stock-based compensation expense |
|
|
1,329 |
|
|
|
1,484 |
|
|
Non-employee stock-based compensation expense |
|
|
420 |
|
|
|
1,153 |
|
|
Total stock-based compensation expense |
|
$ |
1,749 |
|
|
$ |
2,637 |
|
Fair Value of Awards
The Company determines the fair value of stock-based awards on the grant date using the Black-Scholes model, which is impacted by the Company’s stock price, as well as assumptions regarding a number of subjective variables. The following table summarizes the weighted-average assumptions used as inputs to the Black-Scholes model, and resulting weighted-average and total estimated grant date fair values of employee stock options granted during the periods indicated:
|
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
|
2019 |
|
|
2018 |
|
||||||||||
|
|
|
Employees |
|
|
Consultants |
|
|
Employees |
|
|
Consultants |
|
||||
|
Assumptions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected term (years) |
|
|
4.4 |
|
|
|
4.2 |
|
|
|
4.3 |
|
|
|
9.8 |
|
|
Expected volatility |
|
|
108.9 |
% |
|
|
114.2 |
% |
|
|
126.0 |
% |
|
|
117.6 |
% |
|
Risk-free interest rate |
|
|
1.9 |
% |
|
|
1.7 |
% |
|
|
2.6 |
% |
|
|
2.8 |
% |
|
Expected dividend yield |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
Fair value: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average estimated grant date fair value per share |
|
$ |
0.67 |
|
|
$ |
0.56 |
|
|
$ |
0.96 |
|
|
$ |
1.19 |
|
|
Options granted (in thousands) |
|
|
1,098 |
|
|
|
617 |
|
|
|
1,204 |
|
|
|
418 |
|
|
Total estimated grant date fair value (in thousands) |
|
$ |
735 |
|
|
$ |
292 |
|
|
$ |
1,161 |
|
|
$ |
497 |
|
The estimated fair value of stock options that vested in the years ended December 31, 2019 and 2018 was $1.6 million and $2.0 million, respectively. The Company based its assumptions for the expected term on historical cancellation and exercise data, and the contractual term and vesting terms of the awards. Expected volatility is based on historical volatility of the Company’s common stock. The Company does not anticipate paying any cash dividends in the foreseeable future, and therefore uses an expected dividend yield of zero.
64
The following table summarizes stock option activity for the Company’s stock option plans in the periods presented (in thousands, except per share amounts):
|
|
|
|
|
|
|
Weighted |
|
|
Weighted |
|
|
|
|
|
||
|
|
|
|
|
|
|
Average |
|
|
Average |
|
|
|
|
|
||
|
|
|
Number |
|
|
Exercise |
|
|
Remaining |
|
|
Aggregate |
|
||||
|
|
|
of |
|
|
Price Per |
|
|
Contractual |
|
|
Intrinsic |
|
||||
|
|
|
Shares |
|
|
Share |
|
|
Term (Years) |
|
|
Value |
|
||||
|
Outstanding as of December 31, 2018 |
|
|
4,160 |
|
|
$ |
3.66 |
|
|
|
|
|
|
|
|
|
|
Options granted |
|
|
1,716 |
|
|
$ |
1.00 |
|
|
|
|
|
|
|
|
|
|
Options exercised |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Options forfeited or expired |
|
|
(720 |
) |
|
$ |
3.27 |
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2019 |
|
|
5,156 |
|
|
$ |
2.73 |
|
|
|
8.19 |
|
|
$ |
— |
|
|
Vested and expected to vest as of December 31, 2019 |
|
|
5,156 |
|
|
$ |
2.73 |
|
|
|
8.19 |
|
|
$ |
— |
|
|
Exercisable as of December 31, 2019 |
|
|
3,151 |
|
|
$ |
3.56 |
|
|
|
7.73 |
|
|
$ |
— |
|
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (i.e., the difference between the Company’s closing stock price on the last trading day of the period and the exercise price, multiplied by the number of in-the-money options) that would have been received by option holders if they had exercised all their options on December 31, 2019.
The intrinsic value of options exercised during each of the years ended December 31, 2019 and 2018 was zero and less than $0.1 million, respectively. As the Company believes it is probable that no stock option related tax benefits will be realized, the Company does not record any net tax benefits related to exercised options.
Total estimated unrecognized stock-based compensation cost related to unvested stock options was $2.0 million as of December 31, 2019, which is expected to be recognized over the respective vesting terms of each award. The weighted average term of the unrecognized stock-based compensation expense is 2.3 years.
11. Income Taxes
Loss before the provision for income taxes consisted of the following (in thousands):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
U.S. operations |
|
$ |
(23,330 |
) |
|
$ |
(21,132 |
) |
|
Foreign operations |
|
|
— |
|
|
|
(5,483 |
) |
|
Loss before provision for income taxes |
|
$ |
(23,330 |
) |
|
$ |
(26,615 |
) |
No provision for income taxes was recorded in the periods presented due to tax losses incurred in each period. The income tax provision differs from the amount computed by applying the statutory income tax rate of 21% to pre-tax loss as follows (in thousands):
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2019 |
|
|
2018 |
|
|
||
|
Tax (benefit) at statutory federal rate |
|
|
21.0 |
|
% |
|
21.0 |
|
% |
|
State tax (benefit), net of federal benefit |
|
|
7.1 |
|
|
|
4.6 |
|
|
|
Foreign tax rate differential |
|
|
— |
|
|
|
(4.3 |
) |
|
|
Permanent differences |
|
|
(0.6 |
) |
|
|
(0.6 |
) |
|
|
Research and development credits |
|
|
1.3 |
|
|
|
1.0 |
|
|
|
Change in valuation allowance |
|
|
(20.9 |
) |
|
|
(15.9 |
) |
|
|
Provision-to-return |
|
|
— |
|
|
|
(0.7 |
) |
|
|
Expired NOLs, research and development credits, and other carryfowards |
|
|
(6.9 |
) |
|
|
(2.2 |
) |
|
|
Non-qualified stock option cancellations |
|
|
(1.0 |
) |
|
|
(2.9 |
) |
|
|
Effective tax rate |
|
|
— |
|
% |
|
— |
|
% |
65
Deferred income taxes reflect the net tax effects of loss and credit carry-forwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets for federal and state income taxes are as follows (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Federal and state net operating loss carry-forwards |
|
$ |
119,015 |
|
|
$ |
114,254 |
|
|
Federal and state research credit carry-forwards |
|
|
15,140 |
|
|
|
14,885 |
|
|
Capitalized research costs |
|
|
6,081 |
|
|
|
6,134 |
|
|
Stock-based compensation |
|
|
3,996 |
|
|
|
4,002 |
|
|
Lease liabilities |
|
|
152 |
|
|
|
— |
|
|
Property and equipment |
|
|
77 |
|
|
|
79 |
|
|
Accrued liabilities |
|
|
86 |
|
|
|
143 |
|
|
Gross deferred tax assets |
|
|
144,547 |
|
|
|
139,497 |
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
|
Right-of-use assets |
|
|
(152 |
) |
|
|
— |
|
|
Gross deferred tax liabilities |
|
|
(152 |
) |
|
|
— |
|
|
Net deferred tax assets |
|
|
144,395 |
|
|
|
139,497 |
|
|
Valuation allowance |
|
|
(144,395 |
) |
|
|
(139,497 |
) |
|
Deferred tax assets, net of valuation allowance |
|
$ |
— |
|
|
$ |
— |
|
The Company’s unrecognized tax benefits relate to research and development tax credits claimed on the Company’s tax returns. The research and development tax credits have not been utilized, are fully offset by a valuation allowance, and currently have no tax expense impact and no related interest and penalties has been accrued. The Company does not anticipate the unrecognized tax benefits position will significantly change over the next twelve months.
A reconciliation of the Company’s beginning and ending amount of unrecognized tax benefits is follows (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Unrecognized tax benefits at beginning of period |
|
$ |
1,812 |
|
|
$ |
1,769 |
|
|
Increases related to current year tax positions |
|
|
58 |
|
|
|
43 |
|
|
Decreases related to prior year tax positions |
|
|
(23 |
) |
|
|
— |
|
|
Unrecognized tax benefits at the end of period |
|
$ |
1,847 |
|
|
$ |
1,812 |
|
The Company has recorded a full valuation allowance against its net deferred tax assets due to the uncertainty as to whether such assets will be realized. The valuation allowance increased by approximately $4.9 million in the year ended December 31, 2019 primarily due to the generation of current year net operating losses and research and development credits claimed.
As of December 31, 2019, the Company had federal net operating loss carry-forwards of $463.4 million and federal research and development tax credit carry-forwards of $9.8 million. If not utilized, the federal net operating loss and tax credit carry-forwards will begin to expire 2020. As of December 31, 2019, the Company had state net operating loss carry-forwards of $310.7 million, which expire beginning in 2028, and state research and development tax credit carry-forwards of $8.7 million, which do not expire. In addition, the use of net operating loss and tax credit carryforwards may be limited under Section 382 of the Internal Revenue Code in certain situations where changes occur in the stock ownership of a company. In the event that the Company has had a change in ownership, utilization of the carryforwards could be restricted.
The Company recognizes the financial statement effect of tax positions when it is more likely than not that the tax positions will be sustained upon examination by the appropriate taxing authorities. As of December 31, 2019 and 2018, the Company had unrecognized tax benefits of $1.8 million.
66
The Company files U.S. federal and California tax returns. The Company’s wholly owned subsidiaries, Sunesis Europe Limited and Sunesis Pharmaceuticals (Malta) Ltd., are currently not required to file tax returns. To date, neither the Company nor any of its subsidiaries have been audited by the Internal Revenue Service, any state income tax authority or tax authority in the related jurisdictions. Due to net operating loss carry-forwards, substantially all of the Company’s tax years remain open to federal tax examination.
12. Guarantees and Indemnification
As permitted under Delaware law and in accordance with the Company’s Bylaws, the Company indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The indemnification agreements with the Company’s officers and directors terminate upon termination of their employment, but the termination does not affect claims for indemnification relating to events occurring prior to the effective date of termination. The maximum amount of potential future indemnification is unlimited; however, the Company’s officer and director insurance policy reduces the Company’s exposure and may enable the Company to recover a portion of any future amounts paid. The Company believes that the fair value of these indemnification agreements is minimal. In addition, in the ordinary course of business the Company enters into agreements, such as licensing agreements, clinical trial agreements and certain services agreements, containing standard indemnifications provisions. The Company believes that the likelihood of an adverse judgment related to such indemnification provisions is remote. Accordingly, the Company has not recorded any liabilities for any of these agreements as of December 31, 2019.
13. Leases
The Company’s operating lease obligations as of December 31, 2019 relate solely to the leasing of office space in a building at 395 Oyster Point Boulevard in South San Francisco, California, which is currently the Company’s headquarters. The lease was entered into in January 2014 and was amended several times since 2014. The lease was last amended in December 2017 to extend the expiration date to June 30, 2021, with an option to extend the lease for two additional years. The Company did not assume the option to extend the lease term for two additional years in its determination of the lease term as the exercise of the option was not reasonably certain when the lease was last amended in December 2017. The remaining lease term as of December 31, 2019 was 1.5 years.
The cash paid for operating lease liability was $0.6 million and the ROU asset obtained in exchange for new operating lease liability was $1.4 million, for the year ended December 31, 2019.
Maturity of lease liability is as follows (in thousands):
|
Through December 31, |
|
Payments |
|
|
|
2020 |
|
$ |
579 |
|
|
2021 |
|
|
294 |
|
|
Total rental payments |
|
|
873 |
|
|
Less imputed interest |
|
|
(56 |
) |
|
Present value of lease liability |
|
$ |
817 |
|
The Company recognizes rent expense on a straight-line basis. The Company recorded rent expense of $0.6 million and $0.4 million for the year ended December 31, 2019 and 2018, respectively.
67
Not applicable.
Disclosure Controls and Procedures
Based on our evaluation as of December 31, 2019, the Company’s interim Chief Executive Officer and Chief Financial Officer, with the participation of management, have concluded that, subject to the limitations described below, our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act) were effective at the reasonable assurance level to ensure the information required to be disclosed by us in reports that we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States.
Under the supervision and with the participation of our management, including our interim Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2019. Management based its assessment on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) in Internal Control—Integrated Framework. Based on this evaluation, our management concluded that as of December 31, 2019, our internal control over financial reporting was effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls
Our disclosure controls and procedures provide our interim Chief Executive Officer and Chief Financial Officer with only reasonable assurances that our disclosure controls and procedures will achieve their objectives. However, our management, including our interim Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting can or will prevent all human error. A control system, no matter how well designed and implemented, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Furthermore, the design of a control system must reflect the fact that there are internal resource constraints, and the benefit of controls must be weighed relative to their corresponding costs. Because of the limitations in all control systems, no evaluation of controls can provide complete assurance that all control issues and instances of error, if any, within our company are detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur due to human error or mistake. Additionally, controls, no matter how well designed, could be circumvented by the individual acts of specific persons within the organization. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated objectives under all potential future conditions.
None.
68
Certain information required by Part III is omitted from this report because we will file with the SEC a definitive proxy statement pursuant to Regulation 14A, or the Proxy Statement, not later than 120 days after the year ended December 31, 2019, and certain information included therein is incorporated herein by reference.
Information responsive to this item regarding directors and director nominees, executive officers, the board of directors and its committees, and certain corporate governance matters is incorporated herein by reference to the information set forth under the captions “Election of Nominees to the Board of Directors,” “Information About the Board of Directors and Corporate Governance” and “Certain Information with Respect to Executive Officers” in our definitive Proxy Statement.
Code of Business Conduct & Ethics
We have adopted a Code of Business Conduct & Ethics which applies to all of our directors, officers and employees. A copy of our Code of Business Conduct & Ethics can be found on our website, www.sunesis.com, in the section titled “Investors & Media” under the subsection titled “Corporate Governance”. Information found on our website is not incorporated by reference into this report. In addition, we intend to promptly disclose (1) the nature of any amendment to our Code of Business Conduct & Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our Code of Business Conduct & Ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
All additional information required by this Item 10 will be set forth in our definitive Proxy Statement and is incorporated in this report by reference.
Information responsive to this item is incorporated herein by reference to the information set forth under the captions “Executive Compensation and Related Information” and “Information About the Board of Directors and Corporate Governance” in our definitive Proxy Statement.
Ownership of Sunesis Securities
Information responsive to this item is incorporated herein by reference to the information set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” in our definitive Proxy Statement.
69
Equity Compensation Plan Information
The following table provides certain information with respect to our equity compensation plans in effect as of December 31, 2019:
|
|
|
(A) |
|
|
|
(B) |
|
|
(C) |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
Number of Securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Remaining Available for |
|
|
|
|
|
|
Number of Securities |
|
|
|
|
|
|
|
Future Issuance Under |
|
|
||
|
|
|
to be Issued upon |
|
|
|
Weighted Average |
|
|
Equity Compensation Plans |
|
|
|||
|
|
|
Exercise of |
|
|
|
Exercise Price of |
|
|
(Excluding Securities |
|
|
|||
|
Plan Category |
|
Outstanding Options |
|
|
|
Outstanding Options |
|
|
Reflected in Column A) |
|
|
|||
|
Equity Compensation Plans Approved by Stockholders(1) |
|
|
5,155,609 |
|
(2) |
|
$ |
2.73 |
|
|
|
1,421,800 |
|
(3) |
|
Equity Compensation Plans Not Approved by Stockholders |
|
|
— |
|
|
|
$ |
— |
|
|
|
— |
|
|
|
Total |
|
|
5,155,609 |
|
|
|
$ |
2.73 |
|
|
|
1,421,800 |
|
|
|
(1) |
Includes securities issuable under our 2011 Equity Incentive Plan, or 2011 Plan, and 2011 Employee Stock Purchase Plan, or ESPP. |
|
(2) |
Excludes purchase rights currently accruing under the ESPP. Offering periods under the ESPP are 12-month periods, which are comprised of two six-month purchase periods. Eligible employees may purchase shares of common stock at a price equal to 85% of the lower of the fair market value of the common stock at the beginning of each offering period or the end of each semi-annual purchase period. No participant in the ESPP may be issued or transferred shares of common stock valued at more than $25,000 per calendar year. |
|
(3) |
Includes (i) 1,154,817 shares of common stock available for issuance under our 2011 Plan and (ii) 266,983 shares of common stock available for issuance under our ESPP. Beginning in 2012, the number of shares of common stock reserved under the 2011 Plan automatically increases on January 1st of each year by an amount equal to: (i) 4.0% of our shares of common stock outstanding on December 31st of the preceding calendar year, or (ii) a lesser amount determined by our Board of Directors. The number of shares of common stock reserved under our ESPP automatically increases on January 1st of each year by an amount equal to: (i) 1.0% of our shares of common stock outstanding on December 31st of the preceding calendar year, or (ii) a lesser amount determined by our Board of Directors. |
Information responsive to this item is incorporated herein by reference to the information set forth under the captions “Certain Relationships and Related Party Transactions” and “Information About the Board of Directors and Corporate Governance” in our definitive Proxy Statement.
Information responsive to this item is incorporated herein by reference to the information set forth under the caption “Independent Registered Public Accounting Firm” in our definitive Proxy Statement.
70
Exhibits and Financial Statement Schedules:
(a)(1) Financial Statements
|
|
|
Page |
|
|
46 |
|
|
|
47 |
|
|
Consolidated Statements of Operations and Comprehensive Loss |
|
48 |
|
|
49 |
|
|
|
50 |
|
|
|
51 |
(a)(2) Financial Statement Schedules
All financial statement schedules are omitted because they are not applicable, or the information is included in the financial statements or notes thereto.
(a)(3) Exhibits
A list of exhibits filed with this report or incorporated herein by reference is found in the Exhibit Index below:
71
|
|
|
|
|
Incorporated By Reference |
|
|
|
|
||||||||||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
|
Filing Date |
|
|
Filed Herewith |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.1 |
|
Amended and Restated Certificate of Incorporation of the Registrant |
|
|
10-K/A |
|
|
000-51531 |
|
|
3.1 |
|
|
|
5/23/2007 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.2 |
|
|
|
8-K |
|
|
000-51531 |
|
|
3.2 |
|
|
|
12/11/2007 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.3 |
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation |
|
|
8-K |
|
|
000-51531 |
|
|
3.1 |
|
|
|
9/7/2016 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.4 |
|
Certificate of Designation of Series D Convertible Preferred Stock |
|
|
8-K |
|
|
000-51531 |
|
|
3.1 |
|
|
|
10/26/2017 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.5 |
|
Certificate of Designation of Series E Convertible Preferred Stock |
|
|
8-K |
|
|
000-51531 |
|
|
3.1 |
|
|
|
1/22/2019 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.6 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
3.11 |
|
|
|
8/8/2018 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
3.7 |
|
Certificate of Designation of the Series F Convertible Preferred Stock of the Registrant |
|
|
8-K |
|
|
000-51531 |
|
|
3.1 |
|
|
|
7/12/2019 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.1 |
|
|
|
10-K |
|
|
000-51531 |
|
|
4.2 |
|
|
|
3/29/2011 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.2 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
12/16/2015 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.3 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
10/19/2016 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.4 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
10/26/2017 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.5 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.2 |
|
|
|
10/26/2017 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.6 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
6/25/2018 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.7 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
1/22/2019 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
4.8 |
|
|
|
8-K |
|
|
000-51531 |
|
|
4.1 |
|
|
|
7/12/2019 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.1 |
|
|
|
10-Q/A |
|
|
000-51531 |
|
|
10.4 |
|
|
|
6/30/2011 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.2 |
|
|
|
10-Q/A |
|
|
000-51531 |
|
|
10.5 |
|
|
|
6/30/2011 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.3 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
10.6 |
|
|
|
5/12/2011 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.4* |
|
Sunesis Pharmaceuticals, Inc. 2011 Employee Stock Purchase Plan |
|
|
S-8 |
|
|
333-174732 |
|
|
99.2 |
|
|
|
6/6/2011 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.5 |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
8/11/2011 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.6* |
|
Forms of Stock Option Grant Notice and Option Agreement under the 2011 Equity Incentive Plan |
|
|
10-K |
|
|
000-51531 |
|
|
10.57 |
|
|
|
3/14/2012 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.7* |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.58 |
|
|
|
3/14/2012 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
72
|
|
|
|
|
Incorporated By Reference |
|
|
|
|
||||||||||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
|
Filing Date |
|
|
Filed Herewith |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
4/10/2013 |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.9† |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.18 |
|
|
|
3/7/2019 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.10† |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.47 |
|
|
|
3/6/2014 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.11 |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.48 |
|
|
|
3/6/2014 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.12 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
10.1 |
|
|
|
8/05/2014 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.13 |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.44 |
|
|
|
3/12/2015 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.14 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
10.5 |
|
|
|
11/5/2015 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.15 |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
3/12/2015 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.16 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
10.4 |
|
|
|
5/9/2016 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.17 |
|
Warrant, dated March 31, 2016, issued to Western Alliance Bank |
|
|
10-Q |
|
|
000-51531 |
|
|
10.5 |
|
|
|
5/9/2016 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.18 |
|
|
|
10-Q |
|
|
000-51531 |
|
|
10.4 |
|
|
|
7/29/2016 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.19* |
|
Amended and Restated Non-Employee Director Compensation Policy |
|
|
10-Q |
|
|
000-51531 |
|
|
10.2 |
|
|
|
5/8/2017 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.20* |
|
|
|
DEF 14A |
|
|
000-51531 |
|
|
Appendix A |
|
|
|
4/20/2017 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.21 |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
11/7//2017 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
73
|
|
|
|
|
Incorporated By Reference |
|
|
|
|
||||||||||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
|
Filing Date |
|
|
Filed Herewith |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
10-K |
|
|
000-51531 |
|
|
10.42 |
|
|
|
3/9/2018 |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.23 |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.43 |
|
|
|
3/9/2018 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.24* |
|
|
|
10-K |
|
|
000-51531 |
|
|
10.45 |
|
|
|
3/9/2018 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.25 |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
6/25/2018 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.26* |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
2/4/2019 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.27 |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
4/29/2019 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.28* |
|
|
|
8-K |
|
|
000-51531 |
|
|
10.1 |
|
|
|
2/14/2020 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
10.29† |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
21.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
23.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
24.1 |
|
Power of Attorney (included on Signature page) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
31.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
31.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
32.1# |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
74
|
|
|
|
|
Incorporated By Reference |
|
|
|
|
||||||||||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
|
Filing Date |
|
|
Filed Herewith |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
* |
Management contract, compensatory plan or arrangement. |
|
† |
Portions of the exhibit have been omitted pursuant to a request for confidential treatment. The omitted information has been filed separately with the Securities and Exchange Commission. |
|
# |
In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release Nos. 33-8238 and 34-47986, Final Rule; Management’s Reports on Internal Control over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the Certification furnished in Exhibit 32.1 hereto is deemed to accompany this Form 10-K and will not be filed for purposes of Section 18 of the Exchange Act. Such certification will not be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference. |
75
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, Sunesis Pharmaceuticals, Inc. has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 10, 2020.
|
SUNESIS PHARMACEUTICALS, INC. |
||
|
|
|
|
|
By: |
|
/S/ WILLIAM P. QUINN |
|
|
|
William P. Quinn |
|
|
|
Chief Financial Officer, Senior Vice President, Finance and Corporate Development |
|
|
|
|
POWER OF ATTORNEY KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dayton Misfeldt and William P. Quinn, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/S/ JAMES W. YOUNG, PH.D. |
|
Chairman of the Board |
|
March 10, 2020 |
|
James W. Young, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/S/ DAYTON MISFELDT |
|
Interim Chief Executive Officer and Director (Principal Executive Officer) |
|
March 10, 2020 |
|
Dayton Misfeldt |
|
|
|
|
|
|
|
|
|
|
|
/S/ WILLIAM P. QUINN |
|
Chief Financial Officer, Senior Vice President, Finance and Corporate Development (Principal Financial Officer and Principal Accounting Officer) |
|
March 10, 2020 |
|
William P. Quinn |
|
|
|
|
|
|
|
|
|
|
|
/S/ STEVE CARCHEDI |
|
Director |
|
March 10, 2020 |
|
Steve Carchedi |
|
|
|
|
|
|
|
|
|
|
|
/S/ STEVEN B. KETCHUM, PH.D |
|
Director |
|
March 10, 2020 |
|
Steven B. Ketchum, Ph. D. |
|
|
|
|
|
|
|
|
|
|
|
/S/ NICOLE ONETTO |
|
Director |
|
March 10, 2020 |
|
Nicole Onetto |
|
|
|
|
|
|
|
|
|
|
|
/S/ HOMER L. PEARCE, PH.D. |
|
Director |
|
March 10, 2020 |
|
Homer L. Pearce, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/S/ DAVID C. STUMP, M.D. |
|
Director |
|
March 10, 2020 |
|
David C. Stump, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/S/ H. Ward wolff |
|
Director |
|
March 10, 2020 |
|
H. Ward Wolff |
|
|
|
|
76
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-10-01)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
