Wave Life Sciences Ltd. - Quarter Report: 2022 March (Form 10-Q)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2022
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______ to ______
Commission File Number: 001-37627
WAVE LIFE SCIENCES LTD.
(Exact name of registrant as specified in its charter)
Singapore (State or other jurisdiction of incorporation or organization) |
|
Not applicable (I.R.S. Employer Identification No.) |
|
|
|
7 Straits View #12-00, Marina One East Tower Singapore (Address of principal executive offices) |
|
018936 (Zip Code) |
+65 6236 3388
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading symbol |
Name of each exchange on which registered |
$0 Par Value Ordinary Shares |
WVE |
The Nasdaq Global Market |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of ‘‘large accelerated filer,’’ ‘‘accelerated filer,’’ ‘‘smaller reporting company,’’ and ‘‘emerging growth company’’ in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
|
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of outstanding ordinary shares of the registrant as of May 9, 2022 was 61,249,222.
WAVE LIFE SCIENCES LTD.
QUARTERLY REPORT ON FORM 10-Q
TABLE OF CONTENTS
2
As used in this Quarterly Report on Form 10-Q, unless otherwise stated or the context otherwise indicates, references to “Wave,” the “Company,” “we,” “our,” “us” or similar terms refer to Wave Life Sciences Ltd. and our wholly-owned subsidiaries.
Special Note Regarding Forward-Looking Statements
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that relate to future events or to our future operations or financial performance. Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statement. In some cases, forward-looking statements are identified by the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “future,” “goals,” “intend,” “likely,” “may,” “might,” “ongoing,” “objective,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “strategy,” “target,” “will” and “would” or the negative of these terms, or other comparable terminology intended to identify statements about the future, although not all forward-looking statements contain these identifying words. Forward-looking statements include statements, other than statements of historical fact, about, among other things: our ability to fund our future operations; our financial position, revenues, costs, expenses, uses of cash and capital requirements; our need for additional financing or the period for which our existing cash resources will be sufficient to meet our operating requirements; the success, progress, number, scope, cost, duration, timing or results of our research and development activities, preclinical studies and clinical trials, including the timing for initiation or completion of or availability of results from any preclinical studies and clinical trials or for submission, review or approval of any regulatory filing; the timing of, and our ability to, obtain and maintain regulatory approvals for any of our product candidates; the potential benefits that may be derived from any of our product candidates; our strategies, prospects, plans, goals, expectations, forecasts or objectives; the success of our collaborations with third parties; any payment that our collaboration partners may make to us; our ability to identify and develop new product candidates; our intellectual property position; our commercialization, marketing and manufacturing capabilities and strategy; our ability to develop sales and marketing capabilities; our ability to identify, recruit and retain key personnel; our financial performance; developments and projections relating to our competitors in the industry; our liquidity and working capital requirements; the expected impact of new accounting standards; and our expectations regarding the impact of COVID-19, and variants thereof on our business, including on our research and development activities, preclinical studies and clinical trials, supply of drug product, and workforce.
Although we believe that we have a reasonable basis for each forward-looking statement contained in this report, we caution you that these statements are based on our estimates or projections of the future that are subject to known and unknown risks and uncertainties and other important factors that may cause our actual results, level of activity, performance or achievements expressed or implied by any forward-looking statement to differ. These risks, uncertainties and other factors include, among other things, our critical accounting policies; the ability of our preclinical studies to produce data sufficient to support the filing of global clinical trial applications and the timing thereof; our ability to continue to build and maintain the company infrastructure and personnel needed to achieve our goals; the clinical results and timing of our programs, which may not support further development of our product candidates; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials; our effectiveness in managing current and future clinical trials and regulatory processes; the success of our platform in identifying viable candidates; the continued development and acceptance of nucleic acid therapeutics as a class of drugs; our ability to demonstrate the therapeutic benefits of our stereopure candidates in clinical trials, including our ability to develop candidates across multiple therapeutic modalities; our ability to obtain, maintain and protect intellectual property; our ability to enforce our patents against infringers and defend our patent portfolio against challenges from third parties; our ability to fund our operations and to raise additional capital as needed; competition from others developing therapies for similar uses; the severity and duration of the COVID-19 pandemic; the COVID-19 pandemic, and variants thereof, may negatively impact the conduct of, and the timing of enrollment, completion and reporting with respect to, our clinical trials; any other impacts on our business as a result of or related to the COVID-19 pandemic, as well as other risks and uncertainties under the caption “Risk Factors” contained in this Quarterly Report on Form 10-Q and in other filings we make with the Securities and Exchange Commission.
3
Each forward-looking statement contained in this report is based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. As a result of these factors, we cannot assure you that the forward-looking statements in this report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, these statements should not be regarded as representations or warranties by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. We caution you not to place undue reliance on any forward-looking statement.
In addition, any forward-looking statement in this report represents our views only as of the date of this report and should not be relied upon as representing our views as of any subsequent date. We anticipate that subsequent events and developments may cause our views to change. Although we may elect to update these forward-looking statements publicly at some point in the future, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
The Wave Life Sciences Ltd. and Wave Life Sciences Pte. Ltd. names, the Wave Life Sciences mark, PRISM and the other registered and pending trademarks, trade names and service marks of Wave Life Sciences Ltd. appearing in this Form 10-Q are the property of Wave Life Sciences Ltd. This Form 10-Q also contains additional trade names, trademarks and service marks belonging to Wave Life Sciences Ltd. and to other companies. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. Solely for convenience, the trademarks and trade names in this Form 10-Q are referred to without the ® and ™ symbols, but such reference should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
4
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED BALANCE SHEETS
(In thousands, except share amounts)
|
|
March 31, 2022 |
|
|
December 31, 2021 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
61,713 |
|
|
$ |
150,564 |
|
Short-term investments |
|
|
50,000 |
|
|
|
— |
|
Prepaid expenses |
|
|
6,940 |
|
|
|
6,584 |
|
Other current assets |
|
|
5,730 |
|
|
|
5,416 |
|
Total current assets |
|
|
124,383 |
|
|
|
162,564 |
|
Long-term assets: |
|
|
|
|
|
|
||
Property and equipment, net |
|
|
21,046 |
|
|
|
22,266 |
|
Operating lease right-of-use assets |
|
|
17,594 |
|
|
|
18,378 |
|
Restricted cash |
|
|
3,651 |
|
|
|
3,651 |
|
Other assets |
|
|
685 |
|
|
|
148 |
|
Total long-term assets |
|
|
42,976 |
|
|
|
44,443 |
|
Total assets |
|
$ |
167,359 |
|
|
$ |
207,007 |
|
Liabilities, Series A preferred shares and shareholders’ equity (deficit) |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
9,853 |
|
|
$ |
7,281 |
|
Accrued expenses and other current liabilities |
|
|
7,087 |
|
|
|
14,861 |
|
Current portion of deferred revenue |
|
|
36,426 |
|
|
|
37,098 |
|
Current portion of operating lease liability |
|
|
5,120 |
|
|
|
4,961 |
|
Total current liabilities |
|
|
58,486 |
|
|
|
64,201 |
|
Long-term liabilities: |
|
|
|
|
|
|
||
Deferred revenue, net of current portion |
|
|
76,567 |
|
|
|
77,479 |
|
Operating lease liability, net of current portion |
|
|
23,617 |
|
|
|
24,955 |
|
Other liabilities |
|
|
868 |
|
|
|
— |
|
Total long-term liabilities |
|
$ |
101,052 |
|
|
$ |
102,434 |
|
Total liabilities |
|
$ |
159,538 |
|
|
$ |
166,635 |
|
Series A preferred shares, no par value; 3,901,348 shares |
|
$ |
7,874 |
|
|
$ |
7,874 |
|
Shareholders’ equity (deficit): |
|
|
|
|
|
|
||
Ordinary shares, no par value; 60,859,968 and 59,841,116 shares |
|
$ |
751,229 |
|
|
$ |
749,851 |
|
Additional paid-in capital |
|
|
91,951 |
|
|
|
87,980 |
|
Accumulated other comprehensive income |
|
|
95 |
|
|
|
181 |
|
Accumulated deficit |
|
|
(843,328 |
) |
|
|
(805,514 |
) |
Total shareholders’ equity (deficit) |
|
$ |
(53 |
) |
|
$ |
32,498 |
|
Total liabilities, Series A preferred shares and shareholders’ equity (deficit) |
|
$ |
167,359 |
|
|
$ |
207,007 |
|
The accompanying notes are an integral part of the unaudited consolidated financial statements.
5
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share amounts)
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Revenue |
|
$ |
1,750 |
|
|
$ |
— |
|
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
|
27,470 |
|
|
|
33,393 |
|
General and administrative |
|
|
12,374 |
|
|
|
10,078 |
|
Total operating expenses |
|
|
39,844 |
|
|
|
43,471 |
|
Loss from operations |
|
|
(38,094 |
) |
|
|
(43,471 |
) |
Other income, net: |
|
|
|
|
|
|
||
Dividend income and interest income, net |
|
|
26 |
|
|
|
11 |
|
Other income, net |
|
|
254 |
|
|
|
996 |
|
Total other income, net |
|
|
280 |
|
|
|
1,007 |
|
Loss before income taxes |
|
|
(37,814 |
) |
|
|
(42,464 |
) |
Income tax provision |
|
|
— |
|
|
|
— |
|
Net loss |
|
$ |
(37,814 |
) |
|
$ |
(42,464 |
) |
Net loss per share attributable to ordinary |
|
$ |
(0.62 |
) |
|
$ |
(0.86 |
) |
Weighted-average ordinary shares used in |
|
|
60,516,616 |
|
|
|
49,101,606 |
|
|
|
|
|
|
|
|
||
Other comprehensive loss: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(37,814 |
) |
|
$ |
(42,464 |
) |
Foreign currency translation |
|
|
(86 |
) |
|
|
(120 |
) |
Comprehensive loss |
|
$ |
(37,900 |
) |
|
$ |
(42,584 |
) |
The accompanying notes are an integral part of the unaudited consolidated financial statements.
6
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED STATEMENTS OF SERIES A PREFERRED SHARES AND SHAREHOLDERS’ EQUITY (DEFICIT)
(In thousands, except share amounts)
|
|
Series A |
|
|
|
Ordinary Shares |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Deficit |
|
|
Equity |
|
||||||||
Balance at December 31, 2020 |
|
|
3,901,348 |
|
|
$ |
7,874 |
|
|
|
|
48,778,678 |
|
|
$ |
694,085 |
|
|
$ |
71,573 |
|
|
$ |
389 |
|
|
$ |
(683,269 |
) |
|
$ |
82,778 |
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
844,796 |
|
|
|
8,028 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8,028 |
|
Share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
4,063 |
|
|
|
— |
|
|
|
— |
|
|
|
4,063 |
|
Vesting of RSUs |
|
|
— |
|
|
|
— |
|
|
|
|
155,184 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Option exercises |
|
|
— |
|
|
|
— |
|
|
|
|
31,957 |
|
|
|
200 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
200 |
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
44,036 |
|
|
|
336 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
336 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(120 |
) |
|
|
— |
|
|
|
(120 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(42,464 |
) |
|
|
(42,464 |
) |
Balance at March 31, 2021 |
|
|
3,901,348 |
|
|
$ |
7,874 |
|
|
|
|
49,854,651 |
|
|
$ |
702,649 |
|
|
$ |
75,636 |
|
|
$ |
269 |
|
|
$ |
(725,733 |
) |
|
$ |
52,821 |
|
|
|
Series A |
|
|
|
Ordinary Shares |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Deficit |
|
|
Equity (Deficit) |
|
||||||||
Balance at December 31, 2021 |
|
|
3,901,348 |
|
|
$ |
7,874 |
|
|
|
|
59,841,116 |
|
|
$ |
749,851 |
|
|
$ |
87,980 |
|
|
$ |
181 |
|
|
$ |
(805,514 |
) |
|
$ |
32,498 |
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
458,092 |
|
|
|
1,167 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,167 |
|
Share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
3,971 |
|
|
|
— |
|
|
|
— |
|
|
|
3,971 |
|
Vesting of RSUs |
|
|
— |
|
|
|
— |
|
|
|
|
468,226 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Option exercises |
|
|
— |
|
|
|
— |
|
|
|
|
15,000 |
|
|
|
37 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
37 |
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
77,534 |
|
|
|
174 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
174 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(86 |
) |
|
|
— |
|
|
|
(86 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(37,814 |
) |
|
|
(37,814 |
) |
Balance at March 31, 2022 |
|
|
3,901,348 |
|
|
$ |
7,874 |
|
|
|
|
60,859,968 |
|
|
$ |
751,229 |
|
|
$ |
91,951 |
|
|
$ |
95 |
|
|
$ |
(843,328 |
) |
|
$ |
(53 |
) |
The accompanying notes are an integral part of the consolidated financial statements.
7
WAVE LIFE SCIENCES LTD.
UNAUDITED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Cash flows from operating activities |
|
|
|
|
|
|
||
Net loss |
|
$ |
(37,814 |
) |
|
$ |
(42,464 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|||||||
Amortization of right-of-use assets |
|
|
784 |
|
|
|
512 |
|
Depreciation of property and equipment |
|
|
1,730 |
|
|
|
1,948 |
|
Share-based compensation expense |
|
|
3,971 |
|
|
|
4,063 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Prepaid expenses |
|
|
(356 |
) |
|
|
4 |
|
Other assets |
|
|
(851 |
) |
|
|
(1,715 |
) |
Accounts payable |
|
|
2,490 |
|
|
|
(466 |
) |
Accrued expenses and other current liabilities |
|
|
(7,932 |
) |
|
|
(5,310 |
) |
Deferred revenue |
|
|
(1,584 |
) |
|
|
— |
|
Operating lease liabilities |
|
|
(1,179 |
) |
|
|
(880 |
) |
Other non-current liabilities |
|
|
868 |
|
|
|
(67 |
) |
Net cash used in operating activities |
|
|
(39,873 |
) |
|
|
(44,375 |
) |
Cash flows from investing activities |
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
(208 |
) |
|
|
(108 |
) |
Purchase of short-term investments |
|
|
(50,000 |
) |
|
|
— |
|
Net cash used in investing activities |
|
|
(50,208 |
) |
|
|
(108 |
) |
Cash flows from financing activities |
|
|
|
|
|
|
||
Proceeds from issuance of ordinary shares pursuant to the |
|
|
1,105 |
|
|
|
8,105 |
|
Proceeds from the exercise of share options |
|
|
37 |
|
|
|
200 |
|
Proceeds from the ESPP |
|
|
174 |
|
|
|
336 |
|
Net cash provided by financing activities |
|
|
1,316 |
|
|
|
8,641 |
|
Effect of foreign exchange rates on cash, cash equivalents and restricted cash |
|
|
(86 |
) |
|
|
(120 |
) |
Net decrease in cash, cash equivalents and restricted cash |
|
|
(88,851 |
) |
|
|
(35,962 |
) |
Cash, cash equivalents and restricted cash, beginning of period |
|
|
154,215 |
|
|
|
188,148 |
|
Cash, cash equivalents and restricted cash, end of period |
|
$ |
65,364 |
|
|
$ |
152,186 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
||
At-the-market offering costs in accounts payable at period end |
|
$ |
— |
|
|
$ |
77 |
|
The accompanying notes are an integral part of the unaudited consolidated financial statements.
8
Wave Life Sciences Ltd.
Notes to Unaudited Consolidated Financial Statements
1. THE COMPANY
Organization
Wave Life Sciences Ltd. (together with its subsidiaries, “Wave” or the “Company”) is a clinical-stage genetic medicines company committed to delivering life-changing treatments for people battling devastating diseases. PRISM, Wave’s proprietary discovery and drug development platform, enables the precise design, optimization and production of novel stereopure oligonucleotides. Wave has built a genetic toolkit comprised of multiple therapeutic modalities, including RNase-H mediated silencing, RNAi, splicing, and RNA base editing, all of which leverage learnings and optimizations from the PRISM platform and allow Wave to design built-for-purpose molecules to optimally address disease biology.
The Company was incorporated in Singapore on July 23, 2012 and has its principal U.S. office in Cambridge, Massachusetts. The Company was incorporated with the purpose of combining two commonly held companies, Wave Life Sciences USA, Inc. (“Wave USA”), a Delaware corporation (formerly Ontorii, Inc.), and Wave Life Sciences Japan, Inc. (“Wave Japan”), a company organized under the laws of Japan (formerly Chiralgen., Ltd.), which occurred on September 13, 2012. On May 31, 2016, Wave Life Sciences Ireland Limited (“Wave Ireland”) was formed as a wholly-owned subsidiary of Wave Life Sciences Ltd. On April 3, 2017, Wave Life Sciences UK Limited (“Wave UK”) was formed as a wholly-owned subsidiary of Wave Life Sciences Ltd.
The Company’s primary activities since inception have been developing and evolving PRISM to design, develop and commercialize oligonucleotide therapeutics, advancing the Company’s differentiated neurology portfolio, as well as exploring other therapeutic areas of interest, building the Company’s research, development and manufacturing capabilities, advancing programs into the clinic, furthering clinical development of such clinical-stage programs, building the Company’s intellectual property, and assuring adequate capital to support these activities.
Liquidity
Since its inception, the Company has not generated any product revenue and has incurred recurring net losses. To date, the Company has primarily funded its operations through private placements of debt and equity securities, public offerings of its ordinary shares and collaborations with third parties. Until the Company can generate significant revenue from product sales, if ever, the Company expects to continue to finance operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Adequate additional financing may not be available to the Company on acceptable terms, or at all. The inability to raise capital as and when needed would have a negative impact on the Company’s financial condition and ability to pursue its business strategy.
As of March 31, 2022, the Company had cash, cash equivalents and short-term investments of $111.7 million. The Company expects that its existing cash, cash equivalents and short-term investments will be sufficient to fund its operations for at least the next twelve months. The Company has based this expectation on assumptions that may prove to be incorrect, and the Company may use its available capital resources sooner than it currently expects. If the Company’s anticipated operating results are not achieved in future periods, planned expenditures may need to be further reduced in order to extend the time period over which the then-available resources would be able to fund the Company’s operations. In addition, the Company may elect to raise additional funds before it needs them if the conditions for raising capital are favorable due to market conditions or strategic considerations, even if the Company expects it has sufficient funds for its current or future operating plans.
Risks and Uncertainties
The Company is subject to risks common to companies in the biotechnology industry including, but not limited to, new technological innovations, protection of proprietary technology, maintaining internal manufacturing capabilities, dependence on key personnel, compliance with government regulations and the need to obtain additional financing. The Company’s therapeutic programs will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval, prior to commercialization of any product candidates. These efforts require significant amounts of additional capital, adequate personnel infrastructure and extensive compliance-reporting capabilities. There can be no assurance that the Company’s research and development efforts will be successful, that adequate protection for the Company’s intellectual property will be obtained, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from product sales. The Company operates in an environment of rapid change in technology and substantial competition from pharmaceutical and biotechnology companies.
9
Basis of Presentation
The Company has prepared the accompanying consolidated financial statements in conformity with generally accepted accounting principles in the United States (“U.S. GAAP”) and in U.S. dollars.
2. SIGNIFICANT ACCOUNTING POLICIES
The significant accounting policies described in the Company’s audited financial statements as of and for the year ended December 31, 2021, and the notes thereto, which are included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission (“SEC”) on March 3, 2022, as amended (the “2021 Annual Report on Form 10-K”), have had no material changes during the three months ended March 31, 2022, except as described below.
Fair Value of Financial Instruments
The Company is required to disclose information on all assets and liabilities reported at fair value that enables an assessment of the inputs used in determining the reported fair values. The fair value hierarchy is a hierarchy of inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the financial instrument based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the financial instrument and are developed based on the information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported fair value of the investments and is not a measure of the investment credit quality. The hierarchy defines three levels of valuation inputs:
Level 1—Unadjusted quoted prices in active markets that are accessible at the measurement date of identical, unrestricted assets.
Level 2—Quoted prices for similar assets, or inputs that are observable, either directly or indirectly, for substantially the full term through corroboration with observable market data. Level 2 includes investments valued at quoted prices adjusted for legal or contractual restrictions specific to the security.
Level 3—Pricing inputs are unobservable for the asset, that is, inputs that reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing the asset. Level 3 includes private investments that are supported by little or no market activity.
Cash and Cash Equivalents
The Company considers all highly liquid securities with original final maturities of three months or less from the date of purchase to be cash equivalents. Cash and cash equivalents are comprised of funds in cash and money market accounts.
Short-Term Investments
The Company considers all time deposits with original maturities of more than three months from the date of purchase to be short-term investments.
Concentration of Credit Risk
Cash, cash equivalents, restricted cash and short-term investments are financial instruments that potentially subject the Company to concentration of credit risk. The Company uses several financial institutions to maintain its cash, cash equivalents, restricted cash and short-term investments, all of which are high quality, accredited financial institutions and, accordingly, such funds are subject to minimal credit risk. The Company has not experienced any losses in such accounts and management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. The Company has no financial instruments with off-balance sheet risk of loss.
10
Unaudited Interim Financial Data
The accompanying interim consolidated balance sheet as of March 31, 2022, the related interim consolidated statements of operations and comprehensive loss for the three months ended March 31, 2022 and 2021, the consolidated statements of Series A preferred shares and shareholders’ equity (deficit) for the three months ended March 31, 2022 and 2021, the consolidated statements of cash flows for the three months ended March 31, 2022 and 2021, and the related interim information contained within the notes to the unaudited consolidated financial statements have been prepared in accordance with the rules and regulations of the SEC for interim financial information. Accordingly, they do not include all of the information and the notes required by U.S. GAAP for complete financial statements. The financial data and other information disclosed in these notes related to the three months ended March 31, 2022 and 2021 are unaudited. In the opinion of management, the unaudited interim consolidated financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair presentation of the Company’s financial position and results of operations for the three months ended March 31, 2022 and 2021. The results of operations for the interim periods are not necessarily indicative of the results to be expected for the year ending December 31, 2022 or any other interim period or future year or period.
3. FAIR VALUE MEASUREMENTS
The following table sets forth the Company’s financial assets that are measured at fair value on a recurring basis:
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
|
(in thousands) |
|
|||||||||||||
March 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents |
|
$ |
61,713 |
|
|
$ |
61,713 |
|
|
$ |
— |
|
|
$ |
— |
|
Short-term investments |
|
|
50,000 |
|
|
|
— |
|
|
|
50,000 |
|
|
|
— |
|
Restricted cash |
|
|
3,651 |
|
|
|
3,651 |
|
|
|
— |
|
|
|
— |
|
Total |
|
$ |
115,364 |
|
|
$ |
65,364 |
|
|
$ |
50,000 |
|
|
$ |
— |
|
December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents |
|
$ |
150,564 |
|
|
$ |
150,564 |
|
|
$ |
— |
|
|
$ |
— |
|
Short-term investments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Restricted cash |
|
|
3,651 |
|
|
|
3,651 |
|
|
|
— |
|
|
|
— |
|
Total |
|
$ |
154,215 |
|
|
$ |
154,215 |
|
|
$ |
— |
|
|
$ |
— |
|
There have been no transfers between fair value levels during the three months ended March 31, 2022.
Cash, cash equivalents and restricted cash are Level 1 assets which are comprised of funds held in checking and money market accounts. Short-term investments are Level 2 assets which are comprised of time deposits with original maturities of more than three months. The Company determined that the fair value of its short-term investments is $50.0 million as of March 31, 2022, which approximates the carrying value of the term deposits. There were no short-term investments as of December 31, 2021, as the term deposits that constitute the Company’s short-term investments were purchased during the three months ended March 31, 2022. The carrying amounts of accounts payable and accrued expenses approximate their fair values due to their short-term maturities.
4. SHARE-BASED COMPENSATION
The Wave Life Sciences Ltd. 2021 Equity Incentive Plan (the “2021 Plan”) was approved by the Company’s shareholders and went into effect on August 10, 2021. The 2021 Plan serves as the successor to the Wave Life Sciences Ltd. 2014 Equity Incentive Plan, as amended (the “2014 Plan”), such that outstanding awards granted under the 2014 Plan continue to be governed by the terms of the 2014 Plan, but no awards may be made under the 2014 Plan after August 10, 2021. The aggregate number of ordinary shares authorized for issuance of awards under the 2021 Plan is 5,450,000 ordinary shares, plus the number of ordinary shares underlying any awards under the 2014 Plan that are forfeited, cancelled or otherwise terminated (other than by exercise or withheld by the Company to satisfy any tax withholding obligation) on or after August 10, 2021.
The 2021 Plan authorizes (and the 2014 Plan previously authorized) the Company’s board of directors or a committee of the board of directors to, among other things, grant non-qualified share options, restricted awards, which include restricted shares and restricted share units (“RSUs”), and performance awards to eligible employees and directors of the Company.
Options generally vest over periods of to four years, and any options that are forfeited or cancelled are available to be granted again. The contractual life of options is generally or ten years from the grant date. RSUs can be time-based or performance-based. Time-based RSUs generally vest over a period of to four years. The vesting of performance-based RSUs is contingent on the achievement of certain performance milestones. Any RSUs that are forfeited are available to be granted again.
In March 2021, the compensation committee of the Company’s board of directors approved an amendment and restatement of the Company’s outstanding 2019 performance-based RSUs to add an additional milestone to the existing milestones. In 2021, the Company also granted performance-based RSUs with the same terms to certain employees who did not receive the 2019
11
performance-based RSUs. This modification did not result in any incremental expense. The Company did not recognize any expense related to the performance-based RSUs during the three months ended March 31, 2022, as the related milestones were not considered probable of achievement as of March 31, 2022.
During the three months ended March 31, 2022, the Company granted 1,989,025 options and 24,025 time-based RSUs to employees.
As of March 31, 2022, 1,286,637 ordinary shares remained available for future grant under the 2021 Plan.
The Wave Life Sciences Ltd. 2019 Employee Share Purchase Plan (“ESPP”) allows all full-time and certain part-time employees to purchase the Company’s ordinary shares at a discount to fair market value. Eligible employees may enroll in a six-month offering period beginning on or about January 15th and July 15th every year. Shares are purchased at a price equal to 85% of the lower of the fair market value of the Company’s ordinary shares on the first business day or the last business day of an offering period. During the three months ended March 31, 2022, 77,534 ordinary shares were issued under the ESPP. As of March 31, 2022, there were 804,940 ordinary shares available for issuance under the ESPP.
Subsequent to March 31, 2022, the Company determined that a performance-based RSU milestone was achieved and consequently 50% of the outstanding performance-based RSUs vested, which resulted in the issuance of 384,646 ordinary shares. During the three months ending June 30, 2022, the Company plans to record share-based compensation expense of approximately $3.8 million, which represents all of the expense related to the achievement of this performance-based RSU milestone.
5. COLLABORATION AGREEMENTS
Takeda Collaboration and Equity Agreements
In February 2018, Wave USA and Wave UK entered into a global strategic collaboration (the “Takeda Collaboration”) with Takeda Pharmaceutical Company Limited (“Takeda”), pursuant to which Wave USA, Wave UK and Takeda agreed to collaborate on the research, development and commercialization of oligonucleotide therapeutics for disorders of the Central Nervous System (“CNS”). The Takeda Collaboration provides the Company with at least $230.0 million in committed cash and Takeda with the option to co-develop and co-commercialize the Company’s CNS development programs in (1) Huntington’s disease (“HD”); (2) amyotrophic lateral sclerosis (“ALS”) and frontotemporal dementia (“FTD”); and (3) the Company’s discovery-stage program targeting ATXN3 for the treatment of spinocerebellar ataxia 3 (“SCA3”) (collectively, “Category 1 Programs”). In addition, the Takeda Collaboration provided Takeda the right to exclusively license multiple preclinical programs for CNS disorders, including Alzheimer’s disease and Parkinson’s disease (collectively, “Category 2 Programs”). In April 2018, the Takeda Collaboration became effective and Takeda paid the Company $110.0 million as an upfront payment. Takeda also agreed to fund the Company’s research and preclinical activities in the amount of $60.0 million during the four-year research term and to reimburse the Company for any collaboration-budgeted research and preclinical expenses incurred by the Company that exceed that amount.
Simultaneously with Wave USA and Wave UK’s entry into the collaboration and license agreement with Takeda (the “Takeda Collaboration Agreement”), the Company entered into a share purchase agreement with Takeda (the “Takeda Equity Agreement,” and together with the Takeda Collaboration Agreement, the “Takeda Agreements”) pursuant to which it agreed to sell to Takeda 1,096,892 of its ordinary shares at a purchase price of $54.70 per share. In April 2018, the Company closed the Takeda Equity Agreement and received aggregate cash proceeds of $60.0 million. The Company did not incur any material costs in connection with the issuance of shares.
With respect to Category 1 Programs, the Company will be responsible for researching and developing products and companion diagnostics for Category 1 Programs through completion of the first proof of mechanism study for such products. Takeda will have an exclusive option for each target and all associated products and companion diagnostics for such target, which it may exercise at any time through completion of the proof of mechanism study. If Takeda exercises this option, the Company will receive an opt-in payment and will lead manufacturing and joint clinical co-development activities and Takeda will lead joint co-commercial activities in the United States and all commercial activities outside of the United States. Global costs and potential profits will be shared 50:50 and the Company will be eligible to receive development and commercial milestone payments. In addition to its 50% profit share, the Company is eligible to receive option exercise fees and development and commercial milestone payments for each of the Category 1 Programs.
With respect to Category 2 Programs, the Company granted Takeda the right to exclusively license multiple preclinical programs during a four-year research term (subject to limited extension for programs that were initiated prior to the expiration of the research term, in accordance with the Takeda Collaboration Agreement) (“Category 2 Research Term”). During that term, the Takeda Collaboration provided that the parties may collaborate on preclinical programs for up to six targets at any one time. The Company was responsible for researching and preclinically developing products and companion diagnostics directed to the agreed upon targets through completion of Investigational New Drug application (“IND”)-enabling studies in the first major market country. Thereafter, Takeda would have an exclusive worldwide license to develop and commercialize products and companion diagnostics directed to such targets, subject to the Company’s retained rights to lead manufacturing activities for products directed to such targets. Takeda agreed to fund the Company’s research and preclinical activities in the amount of $60.0 million during the research term and
12
reimburse the Company for any collaboration-budgeted research and preclinical expenses incurred by the Company that exceeded that amount. The Company was also eligible to receive tiered high single-digit to mid-teen royalties on Takeda’s global commercial sales of products from each Category 2 Program.
Under the Takeda Collaboration Agreement, each party granted to the other party specific intellectual property licenses to enable the other party to perform its obligations and exercise its rights under the Takeda Collaboration Agreement, including license grants to enable each party to conduct research, development and commercialization activities pursuant to the terms of the Takeda Collaboration Agreement.
The term of the Takeda Collaboration Agreement commenced on April 2, 2018 and, unless terminated earlier, will continue until the date on which: (i) with respect to each Category 1 Program target for which Takeda does not exercise its option, the expiration or termination of the development program with respect to such target; (ii) with respect to each Category 1 Program target for which Takeda exercises its option, the date on which neither party is researching, developing or manufacturing any products or companion diagnostics directed to such target; or (iii) with respect to each Category 2 Program target, the date on which royalties are no longer payable with respect to products directed to such target.
Takeda may terminate the Takeda Collaboration Agreement for convenience on 180 days’ notice, in its entirety or on a target-by-target basis. Subject to certain exceptions, each party has the right to terminate the Takeda Collaboration Agreement on a target-by-target basis if the other party, or a third party related to such party, challenges the patentability, enforceability or validity of any patents within the licensed technology that cover any product or companion diagnostic that is subject to the Takeda Collaboration Agreement. In the event of any material breach of the Takeda Collaboration Agreement by a party, subject to cure rights, the other party may terminate the Takeda Collaboration Agreement in its entirety if the breach relates to all targets or on a target-by-target basis if the breach relates to a specific target. In the event that Takeda and its affiliates cease development, manufacturing and commercialization activities with respect to compounds or products subject to the Takeda Collaboration Agreement and directed to a particular target, the Company may terminate the Takeda Collaboration Agreement with respect to such target. Either party may terminate the Takeda Collaboration Agreement for the other party’s insolvency. In certain termination circumstances, the Company would receive a license from Takeda to continue researching, developing and manufacturing certain products, and companion diagnostics.
The Takeda Collaboration is managed by a joint steering committee (“JSC”) in which both parties are represented equally. The JSC is tasked with overseeing the scientific progression of each Category 1 Program and, prior to the Amendment (discussed below), the Category 2 Programs.
The Company assessed this arrangement in accordance with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”) and concluded that the contract counterparty, Takeda, is a customer for Category 1 Programs prior to Takeda exercising its option, and for Category 2 Programs during the Category 2 Research Term. The Company identified the following material promises under the arrangement: (1) the non-exclusive, royalty-free research and development license for each Category 1 Program; (2) the research and development services for each Category 1 Program through completion of the first proof of mechanism study; (3) the exclusive option to license, co-develop and co-commercialize each Category 1 Program; (4) the right to exclusively license the Category 2 Programs; and (5) the research and preclinical development services of the Category 2 Programs through completion of IND-enabling studies. The research and development services for each Category 1 Program were determined to not be distinct from the research and development license and should therefore be combined into a single performance obligation for each Category 1 Program. The research and preclinical development services for the Category 2 Programs were determined to not be distinct from the exclusive licenses for the Category 2 Programs and should therefore be combined into a single performance obligation.
Additionally, the Company determined that the exclusive option for each Category 1 Program was priced at a discount, and, as such, provide material rights to Takeda, representing three separate performance obligations. Based on these assessments, the Company identified seven performance obligations in the Takeda Collaboration Agreement: (1) research and development services through completion of the first proof of mechanism and non-exclusive research and development license for HD; (2) research and development services through completion of the first proof of mechanism and non-exclusive research and development license for ALS and FTD; (3) research and development services through completion of the first proof of mechanism and non-exclusive research and development license for SCA3; (4) the material right provided for the exclusive option to license, co-develop and co-commercialize HD; (5) the material right provided for the exclusive option to license, co-develop and co-commercialize ALS and FTD; (6) the material right provided for the exclusive option to license, co-develop and co-commercialize SCA3; and (7) the research and preclinical development services and right to exclusively license the Category 2 Programs.
At the outset of the arrangement, the transaction price included the $110.0 million upfront consideration received and the $60.0 million of committed research and preclinical funding for the Category 2 Programs. The Company determined that the Takeda Collaboration Agreement did not contain a significant financing component. The option exercise fees to license, co-develop and co-commercialize each Category 1 Program that may be received are excluded from the transaction price until each customer option is exercised. The potential milestone payments were excluded from the transaction price, as all milestone amounts were fully constrained at the inception of the Takeda Collaboration Agreement. The Company will reevaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur, if necessary, will adjust its estimate of the transaction price.
13
The Company allocated the transaction price to the performance obligations on a relative standalone selling price basis. For the performance obligations associated with the research and development services through completion of the first proof of mechanism and non-exclusive research and development license for HD; the research and development services through completion of the first proof of mechanism and non-exclusive research and development license for ALS and FTD; the research and development services through completion of the first proof of mechanism and non-exclusive research and development license for SCA3; and the research and preclinical development services and right to exclusively license the Category 2 Programs, the Company determined the standalone selling price using estimates of the costs to perform the research and development services, including expected internal and external costs for services and supplies, adjusted to reflect a profit margin. The total estimated cost of the research and development services reflected the nature of the services to be performed and the Company’s best estimate of the length of time required to perform the services. For the performance obligations associated with the material right provided for the exclusive option to license, co-develop and co-commercialize HD; the material right provided for the exclusive option to license, co-develop and co-commercialize ALS and FTD; and the material right provided for the exclusive option to license, co-develop and co-commercialize SCA3, the Company estimated the standalone fair value of the option to license each Category 1 Program utilizing an adjusted market assessment approach, and determined that any standalone fair value in excess of the amounts to be paid by Takeda associated with each option represented a material right.
Revenue associated with the research and development services for each Category 1 Program performance obligation is being recognized as the research and development services are provided using an input method, according to the costs incurred on each Category 1 Program and the total costs expected to be incurred to satisfy each Category 1 Program performance obligation. Revenue associated with the research and preclinical development services for the Category 2 Programs performance obligation is being recognized as the research and preclinical development services are provided using an input method, according to the costs incurred on Category 2 Programs and the total costs expected to be incurred to satisfy the performance obligation. The transfer of control for these performance obligations occurs over time and, in management’s judgment, this input method is the best measure of progress towards satisfying the performance obligations. The amount allocated to the material right for each Category 1 Program option will be recognized on the date that Takeda exercises each respective option, or immediately as each option expires unexercised. The amounts received that have not yet been recognized as revenue are recorded in deferred revenue on the Company’s consolidated balance sheet.
On October 15, 2021, Wave USA, Wave UK and Takeda entered into the Second Amendment to the Takeda Collaboration Agreement (the “Amendment”), which discontinued the Category 2 component of the Takeda Collaboration. Pursuant to the Amendment, Takeda agreed to pay the Company an additional $22.5 million as full payment for reimbursable Category 2 Program collaboration-budgeted research and preclinical expenses. The Category 1 Programs under the Takeda Collaboration Agreement remain in effect and are unchanged by the Amendment.
Through March 31, 2022, the Company had recognized revenue of approximately $79.5 million as collaboration revenue under the Takeda Collaboration Agreement. During the three months ended March 31, 2022, the Company recognized revenue of $1.6 million under the Takeda Collaboration Agreement. No revenue was recognized under the Takeda Collaboration Agreement during the three months ended March 31, 2021 based on the revenue recognition standard.
The aggregate amount of the transaction price allocated to the Company’s unsatisfied and partially unsatisfied performance obligations and recorded in deferred revenue as of December 31, 2021 was $114.6 million, of which $37.1 million was included in current liabilities. The aggregate amount of the transaction price allocated to the Company’s unsatisfied and partially unsatisfied performance obligations and recorded in deferred revenue at March 31, 2022 is $113.0 million, of which $36.4 million is included in current liabilities. The Company expects to recognize revenue for the portion of the deferred revenue that relates to the research and development services for each Category 1 Program as costs are incurred, over the remaining research term. The Company expects to recognize revenue for the portion of the deferred revenue that relates to the material right for each Category 1 Program option upon Takeda’s exercise of such option, or immediately as each option expires unexercised.
14
6. NET LOSS PER ORDINARY SHARE
The Company applies the two-class method to calculate its basic and diluted net loss per share attributable to ordinary shareholders, as its Series A preferred shares are participating securities. The two-class method is an earnings allocation formula that treats a participating security as having rights to earnings that otherwise would have been available to ordinary shareholders.
Basic loss per share is computed by dividing net loss attributable to ordinary shareholders by the weighted-average number of ordinary shares outstanding.
The Company’s potentially dilutive shares, which include outstanding share options to purchase ordinary shares, RSUs, and Series A preferred shares, are considered to be ordinary share equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following ordinary share equivalents, presented based on amounts outstanding at each period end, were excluded from the calculation of diluted net loss per share attributable to ordinary shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
As of March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Options to purchase ordinary shares |
|
|
8,760,336 |
|
|
|
4,588,951 |
|
RSUs |
|
|
1,420,568 |
|
|
|
2,022,268 |
|
Series A preferred shares |
|
|
3,901,348 |
|
|
|
3,901,348 |
|
Additionally, for the periods presented, the two-class method does not impact the net loss per ordinary share as the Company was in a net loss position for each of the periods presented and holders of Series A preferred shares do not participate in losses.
7. INCOME TAXES
During the three months ended March 31, 2022 and 2021, the Company recorded no income tax provision. The Company maintained a full valuation allowance for the three months ended March 31, 2022 and 2021 in all jurisdictions due to uncertainty regarding future taxable income.
8. GEOGRAPHIC DATA
Substantially all of the Company’s long-lived assets were located in the United States as of March 31, 2022 and December 31, 2021.
9. RELATED PARTIES
The Company had the following related party transaction for the periods presented in the accompanying consolidated financial statements:
10. ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities consist of the following:
|
|
March 31, 2022 |
|
|
December 31, 2021 |
|
||
|
|
(in thousands) |
|
|||||
Accrued compensation |
|
$ |
3,459 |
|
|
$ |
10,181 |
|
Accrued expenses related to CROs and CMOs |
|
|
3,152 |
|
|
|
3,571 |
|
Accrued expenses and other current liabilities |
|
|
476 |
|
|
|
1,109 |
|
Total accrued expenses and other current liabilities |
|
$ |
7,087 |
|
|
$ |
14,861 |
|
15
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q and in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission (“SEC”) on March 3, 2022, as amended (the “2021 Annual Report on Form 10-K”). Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Quarterly Report on Form 10-Q or the “Risk Factor” section of our 2021 Annual Report on Form 10-K, our actual results could differ materially from the results described in, or implied by, these forward-looking statements.
Overview
We are a clinical-stage genetic medicines company committed to delivering life-changing treatments for people battling devastating diseases. Using PRISM™, our proprietary discovery and drug development platform that enables the precise design, optimization and production of novel stereopure oligonucleotides, we aspire to develop best-in-class medicines that target the transcriptome to treat genetically defined diseases with a high degree of unmet need.
We are developing oligonucleotides that target ribonucleic acid (“RNA”) and harness existing cellular machinery to reduce the expression of disease-promoting RNA or proteins, restore the production of functional proteins, or modulate protein expression. By intervening at the RNA level, we have the potential to address diseases that have historically been difficult to treat with small molecules or biologics, while retaining the ability to titrate dose, modulate duration of effect, and avoid risk of permanent off-target genetic changes and other challenges associated with DNA editing or gene therapy approaches. Oligonucleotides have additional advantages as a therapeutic class, including the ability to access multiple tissue types and the ability to modulate the frequency of dosing to ensure broad distribution within tissues over time. Oligonucleotides also have well-established manufacturing processes and validated test methods based on decades of improvements, as well as established regulatory, access and reimbursement pathways.
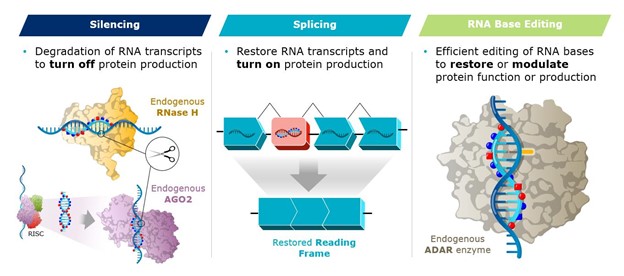
Our approach is based on the scientific insight that the biological machinery necessary to address genetic diseases already exists in human cells and can be controlled with the right tools. We have built a genetic toolkit comprised of multiple therapeutic modalities, including RNase-H mediated silencing, RNAi, splicing, and RNA base editing, all of which leverage learnings and optimizations from our PRISM platform and allow us to design built-for-purpose molecules to optimally address disease biology.
Our A-to-I RNA base editing oligonucleotides (“AIMers”) represent our newest therapeutic modality. AIMers are designed to correct single base mutations on RNA transcript, thereby avoiding permanent changes to the genome that occur with DNA-targeting approaches. Rather than using an exogenous editing enzyme, AIMers recruit proteins that exist in the body, called ADAR (adenosine deaminases acting on RNA) enzymes, which naturally possess the ability to change an adenine (A) to an inosine (I), which cells read as guanine (G). This approach enables simplified delivery and avoids the risk of irreversible off-target effects with DNA-targeting approaches. AIMers are short in length, fully chemically modified, and use novel chemistry, including proprietary PN backbone modifications and control of stereochemistry, which make them distinct from other ADAR-mediated editing approaches.
16
Our PRISM platform is built on the recognition that a significant opportunity exists to tune the pharmacological properties of oligonucleotide therapeutics by leveraging three key features of these molecules: sequence, chemistry, and stereochemistry. Our unique ability to control stereochemistry, which is a reality of chemically modified oligonucleotides, provides the resolution necessary to optimize pharmacological profiles.
PRISM enables us to design stereopure oligonucleotides, which are comprised of molecules with atoms precisely and purposefully arranged in three-dimensional orientations at each linkage. These differ from the mixture-based oligonucleotides currently on the market or in development by others. Additionally, to mitigate pharmacological risks and potential manufacturing challenges, our approach focuses on designing short, chemically modified oligonucleotides without the need for complex delivery vehicles or engineered exogenous enzymes.
Our work in developing stereopure oligonucleotides has enabled the continued evolution of PRISM and our drug discovery process of selecting genetically defined targets, identifying a sequence and applying the therapeutic modality we determine is best suited for the disease biology. We use our PRISM platform engine to screen candidates and optimize the pharmacologic profile based on predefined design principles, which reflect a deep understanding of how the interplay among oligonucleotide sequence, chemistry and backbone stereochemistry impacts key pharmacological properties. Through continued exploration of these interactions using iterative analysis of in vitro and in vivo outcomes and machine learning-driven predictive modeling, we also continue to refine our design principles that we deploy across subsequent programs. We continue to invest in PRISM to further evolve and apply the expanding capabilities and promise of our unique platform.
In August 2020, we publicly introduced our novel PN backbone chemistry modifications, which have been shown preclinically to increase potency, distribution, and durability of effect across various modalities. PN chemistry has been incorporated into all our current clinical, preclinical, and discovery-stage programs.
We have a robust and diverse pipeline of PN-modified, stereopure oligonucleotides, including our clinical silencing and splicing programs, as well as our AIMers. Our lead clinical development programs are designed to treat genetic diseases within the central nervous system (“CNS”), including amyotrophic lateral sclerosis (“ALS”), frontotemporal dementia (“FTD”), Huntington’s disease (“HD”), and Duchenne muscular dystrophy (“DMD”). These programs include:
With RNA base editing, our initial focus is on using GalNAc-conjugated AIMers to treat hepatic diseases and our lead program is designed to treat alpha-1antitrypsin deficiency (“AATD”). We expect to select an AATD AIMer development candidate and initiate IND-enabling toxicology studies in the third quarter of 2022.
When we built Wave, we recognized the growing momentum in RNA therapeutics and anticipated the value in having an internal current good manufacturing practices (“cGMP”) manufacturing facility. This capability provides us with increased control and visibility of our drug substance supply chain, thereby accelerating transitions from discovery through clinical development, and the continued ability to innovate in oligonucleotide manufacturing. Our team includes experts in oligonucleotide manufacturing that have successfully delivered clinical supply for six global studies at Wave to date. With our existing manufacturing facility, we are evaluating using our additional capacity to support the clinical supply of innovative oligonucleotides for new partners.
17
Our Current Programs

Additional details regarding our lead therapeutic programs are set forth below.
Neurology
WVE-004: In ALS and FTD, we are advancing WVE-004, which uses our novel PN chemistry and preferentially targets the transcripts containing the hexanucleotide G4C2 expansion in the C9orf72 gene. In C9 BAC transgenic mice, WVE-004 led to substantial reductions in repeat-containing C9orf72 transcripts and dipeptide repeat (“DPR”) proteins that are sustained for at least six months, without disrupting total C9orf72 protein expression.
In 2021, we initiated our FOCUS-C9 trial, which is a global, multicenter, randomized, double-blind, placebo-controlled Phase 1b/2a clinical trial to assess the safety and tolerability of intrathecal doses of WVE-004 for patients with C9-ALS and/or C9-FTD. Additional objectives include measurement of polyGP proteins in the cerebrospinal fluid (“CSF”), plasma and CSF pharmacokinetics and exploratory biomarker and clinical endpoints. The FOCUS-C9 trial is designed to be adaptive with dose level and dosing frequency being guided by an independent committee. Preclinical models that have established pharmacologic activity have informed the starting dose for this trial.
18
In April 2022, we announced a positive update to the ongoing FOCUS-C9 trial that was driven by the observation of potent, durable reductions of poly(GP) dipeptide repeat proteins in CSF with low, single doses of WVE-004. Poly(GP) is a key C9-ALS/C9-FTD disease biomarker that, when reduced in CSF, indicates WVE-004’s engagement of target in the brain and spinal cord.
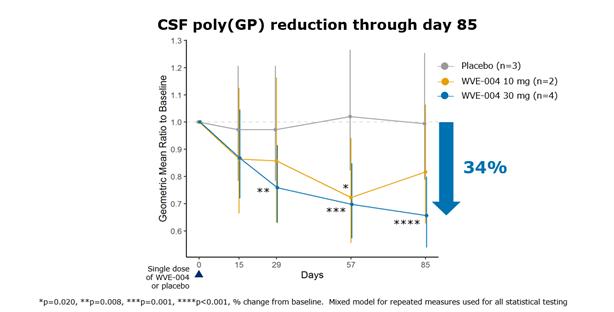
We expect to share additional single and multidose data from the FOCUS-C9 clinical trial throughout 2022. We expect to use these data to optimize dose level and frequency, as well as enable discussions with regulatory authorities regarding the next phase of development later in 2022. Planning is underway for expected initiation of an open-label extension clinical trial in mid-2022.
19
WVE-003: In HD, we are currently advancing WVE-003, a stereopure antisense oligonucleotide designed to selectively target an undisclosed single nucleotide polymorphism (“SNP”), “mHTT SNP3”, associated with the disease-causing mutant huntingtin (“mHTT”) mRNA transcript within the HTT gene. WVE-003 incorporates our novel PN chemistry, as well as learnings from our first-generation HD programs. Targeting mRNA with SNP3 allows us to lower expression of transcript from the mutant allele, while leaving the healthy transcript relatively intact, thereby preserving wild-type (healthy) huntingtin (“wtHTT”) protein, which is important for neuronal function. Our allele-selective approach may also enable us to address the pre-manifest, or asymptomatic, HD patient population in the future. In preclinical studies, WVE-003 showed dose-dependent and selective reduction of mHTT mRNA in vitro, potent and durable knockdown of mHTT mRNA and protein in vivo. A pharmacokinetic-pharmacodynamic (“PK-PD”) model for WVE-003 based on preclinical data predicts that WVE-003 may attain sufficient concentrations to engage mHTT transcript in both the cortex and striatum and decrease expression of the mHTT protein.
The SELECT-HD trial is a multicenter, randomized, double-blind, placebo-controlled Phase 1b/2a clinical trial to assess the safety and tolerability of intrathecally administered WVE-003 for patients with early manifest HD. Additional objectives include measurement of mHTT and wtHTT protein and exploratory pharmacokinetic, pharmacodynamic, clinical and magnetic resonance imaging (“MRI”) endpoints. The SELECT-HD trial is designed to be adaptive, with dose level and dosing frequency being guided by an independent committee. Preclinical models that have established pharmacologic activity have informed the starting dose for this trial. In September 2021, we announced the initiation of dosing in the SELECT-HD trial. We expect to share clinical data in 2022 to provide further insight into the clinical effects of PN chemistry and enable decision making for WVE-003.
WVE-N531: In DMD, we are advancing WVE-N531, which is designed to target exon 53 within the dystrophin gene. WVE-N531 is designed to cause the cellular splicing machinery to skip over this exon during pre-mRNA processing, which restores the dystrophin mRNA reading frame and enables production of truncated, but functional dystrophin protein. Exon-skipping produces dystrophin from the endogenous dystrophin gene (not micro or mini dystrophin expressed from a vector), under the control of native gene-regulatory elements, resulting in normal temporospatial expression. WVE-N531 is both our first splicing candidate and our first systemically administered candidate incorporating PN chemistry to be assessed in the clinic.
In September 2021, we announced the initiation of dosing in an open-label clinical trial evaluating WVE-N531 as a treatment for boys with DMD who are amenable to exon 53 skipping. We expect to share clinical data, including muscle biopsies, in 2022 to provide further insight into the clinical effects of PN chemistry and enable decision making for WVE-N531.
Hepatic
We are initially focused on developing AIMers to address genetic hepatic diseases. Our AIMers are relatively short and stable (fully chemically modified), which enables us to leverage clinically-proven N-acetyl galactosamine (“GalNAc”)-mediated delivery to hepatocytes with subcutaneous dosing.
Alpha-1 antitrypsin deficiency (“AATD”): Our AATD program is the first to leverage our novel RNA editing capability that uses GalNAc-conjugated AIMers (RNA base editing oligonucleotides) and endogenous ADAR enzymes by correcting the single RNA base mutation, ADAR editing may provide an ideal approach for increasing circulating levels of wild-type AAT protein and reducing aggregation in the liver, thus simultaneously addressing both the lung and liver manifestations of the disease. We expect to select an AATD AIMer development candidate and initiate IND-enabling toxicology studies in the third quarter of 2022.
Continuing Impacts of COVID-19
We continue to closely monitor developments related to COVID-19, which was declared a pandemic by the World Health Organization on March 11, 2020. In response to this global pandemic, we have concentrated our efforts on the health and safety of our employees and patients, while maintaining business continuity and honoring our commitment to deliver life-changing treatments for people battling devastating diseases.
Our on-site activities continue with protocols for safely accessing and working within our facilities, including a mandatory COVID-19 vaccination policy that we implemented in October 2021. While we continue to conduct research and development activities, including our ongoing clinical trials, the COVID-19 pandemic has impacted, and may continue to impact, certain of our early-stage discovery efforts and clinical trials. We are working with our clinical investigators, research and development vendors, and supply chain vendors to continually assess and take steps to mitigate the potential impact of COVID-19 on our manufacturing operations and research and development activities.
We will continue to closely monitor the COVID-19 situation as we evolve our business continuity plans. Given the global risks and uncertainties associated with COVID-19, our business, results of operations, and prospects could be materially adversely affected. For additional information, see “Item 1A. Risk Factors” in the 2021 Annual Report on Form 10-K.
20
Financial Operations Overview
We have never been profitable, and since our inception, we have incurred significant operating losses. Our net loss was $37.8 million and $42.5 million for the three months ended March 31, 2022 and 2021, respectively. As of March 31, 2022 and December 31, 2021, we had an accumulated deficit of $843.3 million and $805.5 million, respectively. We expect to incur significant expenses and operating losses for the foreseeable future.
Revenue
We have not generated any product revenue since our inception and do not expect to generate any revenue from the sale of products for the foreseeable future. Under the revenue recognition standard, we recognize collaboration revenue under the Takeda Collaboration Agreement (as defined in Note 5 in the notes to the unaudited consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q, “Note 5”), which became effective in April 2018.
Operating Expenses
Our operating expenses since inception have consisted primarily of research and development expenses and general and administrative expenses.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our discovery efforts, and the development of our product candidates, which include:
We recognize research and development costs as incurred. We recognize external development costs based on an evaluation of the progress to completion of specific tasks using information provided to us by our vendors. Payments for these activities are based on the terms of the individual agreements, which may differ from the pattern of costs incurred, and are reflected in our financial statements as prepaid or accrued expenses.
Our primary research and development focus since inception has been the development of our proprietary discovery and drug development platform, PRISM. We are using PRISM, which includes our novel PN backbone chemistry modifications, to design, develop and commercialize a broad pipeline of nucleic acid therapeutic candidates that target RNA using silencing, splicing, and ADAR editing.
21
Our research and development expenses consist primarily of expenses related to our CROs, CMOs, consultants, other external vendors and fees paid to global regulatory agencies to conduct our clinical trials, in addition to compensation-related expenses, internal manufacturing expenses, facility-related expenses and other general operating expenses. These expenses are incurred in connection with research and development efforts and our preclinical studies and clinical trials. We track certain external expenses on a program-by-program basis. However, we do not allocate compensation-related expenses, internal manufacturing expenses, equipment repairs and maintenance expense, facility-related expenses or other operating expenses to specific programs. These expenses, which are not allocated on a program-by-program basis, are included in the “PRISM (including ADAR editing) and other research and development expenses” category along with other external expenses related to our discovery and development programs, as well as platform development and identification of potential drug discovery candidates.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect to continue to incur significant research and development expenses in the foreseeable future as we continue to manage our existing clinical trials, initiate additional clinical trials for certain product candidates, pursue later stages of clinical development for certain product candidates, maintain our manufacturing capabilities and continue to discover and develop additional product candidates in multiple therapeutic areas.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation-related expenses, including salaries, bonuses, share-based compensation and other related benefits costs for personnel in our executive, finance, corporate, legal and administrative functions, as well as compensation-related expenses for our board of directors. General and administrative expenses also include legal fees; expenses associated with being a public company; professional fees for accounting, auditing, tax and consulting services; insurance costs; travel expenses; other operating costs; and facility-related expenses.
Other Income, Net
Other income, net consists primarily of refundable tax credits from tax authorities. We recognize refundable tax credits when there is reasonable assurance that we will comply with the requirements of the refundable tax credit and that the refundable tax credit will be received.
Income Taxes
We are a Singapore multi-national company subject to taxation in the United States and various other jurisdictions.
Critical Accounting Policies and Significant Judgments and Estimates
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparation of our financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amount of assets, liabilities, revenue, costs and expenses and related disclosures.
Our significant accounting policies, judgments and estimates are described in Note 2 in the notes to the audited consolidated financial statements included in the 2021 Annual Report on Form 10-K, as well as in Note 2 in the notes to the unaudited consolidated financial statements included in this Quarterly Report on Form 10-Q. We believe that our revenue recognition policy, particularly (a) assessing the number of performance obligations; (b) determining the transaction price; (c) allocating the transaction price to the performance obligations in the contract; and (d) determining the pattern over which performance obligations are satisfied, including estimates to complete performance obligations, and the assumptions and estimates used in our analysis of contracts with CROs and CMOs to estimate the contract expense, involve a greater degree of judgment, and therefore we consider them to be our critical accounting policies. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions and conditions.
22
Results of Operations
Comparison of the three months ended March 31, 2022 and 2021
|
|
Three Months Ended March 31, |
|
|
|
|
||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|||
|
|
(in thousands) |
|
|||||||||
Revenue |
|
$ |
1,750 |
|
|
$ |
— |
|
|
$ |
1,750 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Research and development |
|
|
27,470 |
|
|
|
33,393 |
|
|
|
(5,923 |
) |
General and administrative |
|
|
12,374 |
|
|
|
10,078 |
|
|
|
2,296 |
|
Total operating expenses |
|
|
39,844 |
|
|
|
43,471 |
|
|
|
(3,627 |
) |
Loss from operations |
|
|
(38,094 |
) |
|
|
(43,471 |
) |
|
|
5,377 |
|
Total other income, net |
|
|
280 |
|
|
|
1,007 |
|
|
|
(727 |
) |
Loss before income taxes |
|
|
(37,814 |
) |
|
|
(42,464 |
) |
|
|
4,650 |
|
Income tax provision |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Net loss |
|
$ |
(37,814 |
) |
|
$ |
(42,464 |
) |
|
$ |
4,650 |
|
Revenue
Revenue for the three months ended March 31, 2022 was $1.8 million, which was mainly due to revenue earned under the Takeda Collaboration Agreement (as defined in Note 5) for performing research and development services. No revenue was recognized under the Takeda Collaboration Agreement for the three months ended March 31, 2021, based on the revenue recognition standard.
Research and Development Expenses
|
|
Three Months Ended March 31, |
|
|
|
|
||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|||
|
|
(in thousands) |
|
|||||||||
ALS and FTD programs |
|
$ |
1,739 |
|
|
$ |
3,440 |
|
|
$ |
(1,701 |
) |
HD programs |
|
|
2,193 |
|
|
|
8,450 |
|
|
|
(6,257 |
) |
DMD programs |
|
|
425 |
|
|
|
(200 |
) |
|
|
625 |
|
PRISM (including ADAR editing) and |
|
|
23,113 |
|
|
|
21,703 |
|
|
|
1,410 |
|
Total research and development expenses |
|
$ |
27,470 |
|
|
$ |
33,393 |
|
|
$ |
(5,923 |
) |
Research and development expenses were $27.5 million for the three months ended March 31, 2022, compared to $33.4 million for the three months ended March 31, 2021. The decrease of $5.9 million was due to the following:
General and Administrative Expenses
General and administrative expenses were $12.4 million for the three months ended March 31, 2022, as compared to $10.1 million for the three months ended March 31, 2021. The increase of $2.3 million was primarily due to increases in compensation-related expenses, as well as increases in professional services expenses and other general and administrative operating expenses.
23
Other Income, Net
Other income, net for the three months ended March 31, 2022 and 2021, was $0.3 million and $1.0 million, respectively. Other income, net for both periods primarily consisted of income related to refundable tax credits from tax authorities.
Income Tax Provision
During the three months ended March 31, 2022 and 2021, we recorded no income tax provision. We maintained a full valuation allowance for the three months ended March 31, 2022 and 2021 in all jurisdictions due to uncertainty regarding future taxable income.
Liquidity and Capital Resources
Since our inception, we have not generated any product revenue and have incurred recurring net losses. To date, we have primarily funded our operations through public offerings of our ordinary shares, collaborations with third parties and private placements of debt and equity securities. Through March 31, 2022, we have received an aggregate of approximately $955.7 million in net proceeds from these transactions, consisting of $565.4 million in net proceeds from public offerings of our ordinary shares, $301.0 million from our collaborations and $89.3 million in net proceeds from private placements of our debt and equity securities.
As of March 31, 2022, we had cash, cash equivalents and short-term investments totaling $111.7 million, restricted cash of $3.7 million for our leased premises in Cambridge, Massachusetts and Lexington, Massachusetts, and an accumulated deficit of $843.3 million. Our operating lease commitments as of March 31, 2022 total $35.7 million, of which $5.4 million is related to payments in 2022 and $30.3 million is related to payments beyond 2022.
We expect that our existing cash, cash equivalents and short-term investments will be sufficient to fund our operations for at least the next twelve months. We have based this expectation on assumptions that may prove to be incorrect, and we may use our available capital resources sooner than we currently expect. In addition, we may elect to raise additional funds before we need them if the conditions for raising capital are favorable due to market conditions or strategic considerations, even if we expect we have sufficient funds for our current or future operating plans.
Until we can generate significant revenue from product sales, if ever, we expect to continue to finance our operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. In May 2019, we filed a shelf registration statement on Form S-3ASR with the SEC pursuant to which we registered for sale an indeterminate amount of any combination of our ordinary shares, debt securities, warrants, rights and/or units from time to time and at prices and on terms that we may determine. Our shelf registration statement on Form S-3ASR also included a prospectus covering up to an aggregate of $250.0 million in ordinary shares that we may issue and sell from time to time, through Jefferies LLC (“Jefferies”) acting as our sales agent, pursuant to the open market sales agreement that we entered into with Jefferies in May 2019, as amended in March 2020 and March 2022 (the “Sales Agreement”), for our “at-the-market” equity program. Since we no longer qualified as a “well-known seasoned issuer” at the time of the filing of our Annual Report on Form 10-K for the year ended December 31, 2019, we previously amended the shelf registration statement to register for sale up to $500.0 million of any combination of our ordinary shares, debt securities, warrants, rights and/or units from time to time and at prices and on terms that we may determine, including the $250.0 million in ordinary shares that we may issue and sell from time to time pursuant to our “at-the-market” equity program. This registration statement, which we refer to as the “2019 Form S-3,” remained effective until our 2022 Form S-3 (as defined below) was declared effective on May 4, 2022, after which time we may no longer offer or sell any securities under the 2019 Form S-3. During the three months ended March 31, 2022, the Company sold 458,092 ordinary shares under its at-the-market equity program for aggregate net proceeds of $1.2 million.
On March 3, 2022, we filed a new universal shelf registration on Form S-3 with the SEC, which was declared effective by the SEC on May 4, 2022, pursuant to which we registered for sale up to $500.0 million of any combination of our ordinary shares, debt securities, warrants, rights and/or units from time to time and at prices and on terms that we may determine, which we refer to as the “2022 Form S-3.” The 2022 Form S-3 includes a prospectus covering up to approximately $132.0 million in ordinary shares that have not yet been issued or sold under our Sales Agreement with Jefferies. As of May 11, 2022, we have $500.0 million in securities available for issuance under the 2022 Form S-3, including approximately $132.0 million in ordinary shares available for issuance under our at-the-market equity program.
Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We will need to generate significant revenue to achieve profitability, and we may never do so.
24
Cash Flows
The following table summarizes our cash flow activity:
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
(in thousands) |
|
|||||
Net cash used in operating activities |
|
$ |
(39,873 |
) |
|
$ |
(44,375 |
) |
Net cash used in investing activities |
|
|
(50,208 |
) |
|
|
(108 |
) |
Net cash provided by financing activities |
|
|
1,316 |
|
|
|
8,641 |
|
Effect of foreign exchange rates on cash, cash equivalents and restricted cash |
|
|
(86 |
) |
|
|
(120 |
) |
Net decrease in cash, cash equivalents and restricted cash |
|
$ |
(88,851 |
) |
|
$ |
(35,962 |
) |
Operating Activities
During the three months ended March 31, 2022, operating activities used $39.9 million of cash, primarily due to our net loss of $37.8 million.
During the three months ended March 31, 2021, operating activities used $44.4 million of cash, primarily due to our net loss of $42.5 million.
Investing Activities
During the three months ended March 31, 2022, investing activities used $50.2 million of cash, of which $50.0 million related to purchases of short-term investments and $0.2 million related to purchases of property and equipment.
During the three months ended March 31, 2021, investing activities used $0.1 million of cash, related to purchases of property and equipment.
Financing Activities
During the three months ended March 31, 2022, net cash provided by financing activities was $1.3 million, which was primarily due to the net proceeds from sales of ordinary shares under our at-the-market equity program.
During the three months ended March 31, 2021, net cash provided by financing activities was $8.6 million, which was primarily due to the net proceeds from sales of ordinary shares under our at-the-market equity program.
Funding Requirements
We expect to continue to incur significant expenses in connection with our ongoing research and development activities and our internal cGMP manufacturing activities. Furthermore, we anticipate that our expenses will continue to vary if and as we:
25
We may experience delays or encounter issues with any of the above, including but not limited to failed studies, complex results, safety issues or other regulatory challenges.
Because of the numerous risks and uncertainties associated with the development of drug candidates and because the extent to which we may enter into collaborations with third parties for development of product candidates is unknown, we are unable to estimate the amounts of future capital outlays and operating expenses associated with completing the research and development for our therapeutic programs. Our future capital requirements for our therapeutic programs will depend on many factors, including:
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our product revenue, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if ever. Accordingly, we will need to obtain substantial additional funds to achieve our business objectives.
Adequate additional funds may not be available to us on acceptable terms when we need them, or at all. We do not currently have any committed external source of funds, except for possible future payments from Takeda under the Takeda Collaboration Agreement. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. Additional debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends and may require the issuance of warrants, which could potentially dilute our shareholders’ ownership interests.
If we raise additional funds through collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development programs or any future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
26
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily the result of fluctuations in interest rates and foreign exchange rates, as well as, to a lesser extent, inflation and capital market risk.
Interest Rate Risk
We are exposed to interest rate risk in the ordinary course of our business. Our cash and cash equivalents are held in readily available checking and money market accounts. Our short-term investments are comprised of term deposits which have fixed interest rates.
Foreign Currency Exchange Rate Risk
Due to our operations outside of the United States, we are exposed to market risk related to changes in foreign currency exchange rates. Historically, we have not hedged our foreign currency exposure. For the three months ended March 31, 2022 and 2021, changes in foreign currency exchange rates did not have a material impact on our business, financial condition, results of operations or cash flows.
Inflation Risk
We do not believe that inflation had a material effect on our business, financial condition, results of operations or cash flows for the three months ended March 31, 2022 and 2021.
Capital Market Risk
We currently have no product revenues and depend on funds raised through other sources. One possible source of funding is through further equity offerings. Our ability to raise funds in this manner depends upon capital market forces affecting our share price, including impacts of the COVID-19 pandemic on the capital markets.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of March 31, 2022. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to its management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of March 31, 2022, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation of such internal control required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the three months ended March 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
27
PART II – OTHER INFORMATION
Item 1. Legal Proceedings
We are not currently a party to any material legal proceedings.
Item 1A. Risk Factors
In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors discussed under the caption “Risk Factors” that appear in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on March 3, 2022, as amended.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Recent Sales of Unregistered Equity Securities
None.
Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the three months ended March 31, 2022.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
28
Item 6. Exhibits
Exhibit Number |
|
Exhibit Description |
|
Filed with this Report |
|
Incorporated by Reference herein from Form or Schedule |
|
Filing Date |
|
SEC File/Reg. Number |
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
Description of Securities of the Registrant and Comparison of Shareholder Rights |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
|
|
|
Form 8-K (Exhibit 10.1) |
|
03/03/2022 |
|
001-37627 |
|
|
|
|
|
|
|
|
|
|
|
|
10.2† |
|
|
|
|
Form 10-Q (Exhibit 10.1) |
|
05/09/2018 |
|
001-37627 |
|
|
|
|
|
|
|
|
|
|
|
|
10.3 |
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4†† |
|
|
|
|
Form 10-K (Exhibit 10.3.2) |
|
03/03/2022 |
|
001-37627 |
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
Rule 13a-14(a)/15d-14(a) Certification of Principal Executive Officer |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
Rule 13a-14(a)/15d-14(a) Certification of Principal Financial Officer |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32* |
|
Section 1350 Certifications of Principal Executive Officer and Principal Financial Officer |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – The instance document does not appear in the interactive data file because its Inline XBRL tags are embedded within the Inline XBRL document. |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted in Inline XBRL and contained in Exhibit 101) |
|
X |
|
|
|
|
|
|
(*) The certifications attached as Exhibit 32 that accompany this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Wave Life Sciences Ltd. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of such Form 10-Q), irrespective of any general incorporation language contained in such filing.
(†) Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
29
(††) Certain confidential portions of this Exhibit were omitted by means of marking such portions with brackets (“[***]”) because the identified confidential portions (i) are not material and (ii) would be competitively harmful if publicly disclosed.
30
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
WAVE LIFE SCIENCES LTD. |
||
|
|
|
|
Date: May 12, 2022 |
By: |
|
/s/ Paul B. Bolno, M.D., MBA |
|
|
|
Paul B. Bolno, M.D., MBA |
|
|
|
President and Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
|
|
|
|
Date: May 12, 2022 |
By: |
|
/s/ Kyle Moran |
|
|
|
Kyle Moran |
|
|
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
31
Similar companies
See also JOHNSON & JOHNSON - Annual report 2023 (10-K 2023-01-01) Annual report 2023 (10-Q 2023-10-01)See also ELI LILLY & Co - Annual report 2024 (10-K 2024-12-31) Annual report 2023 (10-Q 2023-09-30)
See also PFIZER INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-10-01)
See also AbbVie Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also NOVO NORDISK A S
